
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
PARATHION
Chemical name
Diethyl 4-nitrophenylphosphorothionate; O,O diethyl
O-p-nitrophenyl phosphorothioate; O,O diethyl
O-p-nitrophenyl thionophosphate
Synonyms
Thiophos; Niram; SNP; Paraphos
Empirical formula
C10H14O5NSP
Structural formula
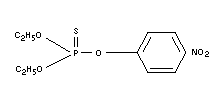 BIOLOGICAL DATA
Biochemical aspects
Parathion is readily absorbed by all the routes of
administration. It is a powerful inhibitor of cholinesterase in
vivo. In vitro, pure parathion had little effect on the enzyme
(Aldridge & Davison, 1952). In mammals and plants parathion is readily
oxidized to a powerful cholinesterase inhibitor, paraoxon, which is
the active form of parathion (Heath, 1961). In mice given
subcutaneously 32P labelled parathion, the highest radioactivity
appeared in the salivary glands and cervical brown fat within 4 hours
of injection. Liver, kidney and adipose tissues showed high uptake of
radioactivity and fairly high activity was found in gastric and
intestinal walls, thyroid, spleen and lungs. Less activity was noted
in the central nervous system, musculature and bone marrow
(Frederiksson & Bigelow, 1961). Parathion is finally broken down to
p-nitrophenol, with traces of p-aminophenol (Gardocki & Hazleton,
1951) and these are excreted in the urine. The final product depends
on the species (Heath, 1961).
The molar I50 for paraoxon against sheep erythrocyte
cholinesterase was 2.0 × 10-8 (30 min.; 37°C) (Aldridge & Davison,
1952).
In vitro studies on human liver enzyme indicated that parathion
could inhibit the non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 6.0-25.0* Frawley et al., 1952
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Mouse, male Intraperitoneal 10.4-11.4 Engbaek & Jensen, 1951
Rat, female Oral 1.75-5.0* Deichmann et al., 1952
Edson & Noakes, 1960
Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Rat, male Oral 5.0-30.0* Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Rat Intraperitoneal 5.5 DuBois & Coon, 1952
Guinea-pig Oral 9.3-32.0* Frawley et al., 1952
Hazleton & Holland, 1950
Guinea-pig Intraperitoneal 12.0 Klimmer & Pfaff, 1955
Rabbit Oral 10.0 American Cyanamid Company, 1955
Chicken Intraperitoneal 8.7 American Cyanamid Company, 1955
Dog Oral 3.0-5.0 Hazleton & Holland, 1950
(lethal dose)
Dog Intraperitoneal 12.0-20.0 DuBois et al., 1949
* Differences caused by the use of different vehicles.
Man. An oral dose of 3-5 mg/kg is usually fatal to man (Erdmann
& Lendle, 1960).
Short-term studies
Rat. Groups each of 5 male rats were placed on diets containing
1, 5 and 25 ppm of parathion for 2 weeks. The lowest level that caused
inhibition of the erythrocyte cholinesterase activity (the most
sensitive to parathion) was 5 ppm (Frawley et al., 1952). 1 ppm did
not give inhibition. Groups of 10 female rats were fed for 16 weeks
diets containing 1, 5, 25 and 125 ppm of parathion. At 125 ppm, severe
toxic symptoms, growth suppression and death occurred. At 25 ppm
slight muscular fibrillation was seen in the first 2 weeks. At 5 and 1
ppm no ill-effects were observed on general behaviour and growth.
Erythrocyte cholinesterase activity was severely inhibited at 5 ppm
and 25 ppm and above. No inhibition was observed at 1 ppm (Edson &
Noakes, 1960).
Concentrations of 0.05, 0.5 and 5.0 ppm of parathion was fed
for a period of 12 weeks to 4 groups of 20 rats. Of the initial 20
rats, after 1, 4 and 8 weeks, 4 animals, and after 12 weeks 8 animals,
were killed to determine the cholinesterase activity in brain,
erythrocytes and plasm. In this experiment 0.5 ppm was the highest
ineffective level in respect of toxic effects or blood cholinesterase
inhibition (Edson, 1957).
Dog. Groups each of one male and one female dog were placed for
24 weeks on diets containing 1, 2 and 5 ppm of parathion, so that the
average daily intake was 0.021, 0.047 and 0.117 mg/kg body-weight
respectively. The 2 and 5 ppm levels caused about 60% and 70%
inhibition of plasm cholinesterase activity and the 1 ppm level caused
a small but significant inhibition. For erythrocyte cholinesterase
activity only the 5 ppm level produced significant depression. The
recovery of plasma activity at all levels was complete within 4 weeks
after return to a control diet, and was frequently followed by a
transient elevation above the pre-treatment activity. The recovery
rate for erythrocyte activity was slower, requiring 11 weeks (Frawley
& Fuyat, 1957).
Dogs were given 100 ppm of parathion in the diet for 9 weeks.
In addition to a very strong inhibition of plasm and erythrocyte
cholinesterase activity, a change in the protein content of the serum
was noticed. The albumin content decreased from 48% to 34% and the
ß-globulin content increased from 13.5% to 23.3% (Williams et al.,
1958).
Pig. Parathion was fed to groups each of 2 pigs for periods of
33 to 122 days. The diets contained 0.2, 1 and 5 ppm. After 33 days
the 0.2 ppm concentration was increased to 25 ppm, and after 40 days
the 1 ppm concentration to 100 ppm. The highest ineffective dose in
respect of toxic effects or depression of blood cholinesterase
activity was 1.0 mg/kg body-weight/day (Edson, 1957).
Man. A group of 5 subjects was fed parathion 3 mg/day for 28
days, 4.5 mg/day for 28 days and 6 mg/day for 43 days. Three and 4.5
mg did not produce any significant depression of erythrocyte or plasm
cholinesterase activity, but 6 mg produced a depression of 10-15% of
both plasm and erythrocyte cholinesterase activity. Depression of
plasm cholinesterase activity persisted two weeks after administration
of the drug was discontinued, whereas erythrocyte cholinesterase
activity continued to be depressed for 43 days. No side-effects were
observed (Moeller & Rider, 1961).
Five groups of 4 subjects were given parathion orally at
dosages of 0 mg/day, 0.6 mg/day for 4 weeks followed by 9 weeks at
4.8 mg daily, 1.2 mg/daily, 2.4 mg/daily and 7.2 mg/daily for 6 weeks.
Only in the latter group the whole blood cholinesterase was reduced,
declining to 67% of the controls at the end of the 6 weeks. At this
time red cell and plasma cholinesterases were respectively 84% and 63%
of the controls. Whole blood cholinesterase was restored to 87% of
control activity 28 days after withdrawal of parathion (Edson, 1964).
Four male and 4 female subjects were fed 0.003, 0.010, 0.025
and 0.050 mg/kg body-weight of parathion for successive periods each
of 3 weeks. A significant increase in the mean plasma cholinesterase
activity without an accompanying change in the erythrocyte
cholinesterase activity occurred early in the feeding programs, but
the level declined to control values when doses of 0.050 mg/kg
body-weight were reached (Rider et al., 1958).
Long-term studies
Rat. In a one-year toxicity test groups of 36 male and 36
female rats were maintained on diets containing 10, 20, 50, 75 and 100
ppm of parathion. At 50 ppm and above growth retardation, toxic
symptom and death occurred. Histological examination of animals dying
with toxic symptoms revealed lesions of the salivary glands, pancreas
and thymus. No other lesions attributable to parathion were found. At
20 ppm and less no ill-effects were observed on growth, behaviour,
mortality rate or histology. In breeding experiments performed with
the animals from all groups the second generation of rats fed at 10
ppm produced normal litters but the young frequently died (Barnes &
Denz, 1951).
In a 2-year study, groups of 8-10 male rats were fed diets
containing parathion at the levels of 50 and 100 ppm, corresponding to
average daily amounts of 1 and 2 mg per rat. At 100 ppm a slight
retardation of growth and toxic symptoms were observed for the first
few weeks. No growth reduction was present at 50 ppm. No ill-effects
on food consumption, mortality rate, blood picture or gross pathology
were observed. No histological changes attributable to parathion were
observed. On chemical analysis no parathion was found in brain, liver,
kidney, lung, abdominal fat or whole blood. In groups of 10-16 male
rats placed on diets containing 10 and 25 ppm parathion for 2 years no
ill-effects were observed on general condition, food consumption,
growth and survival. Groups of 6-9 female rats were fed with diets
containing 10 and 50 ppm of parathion for 2 years. There was no
evidence of toxicity. All females in the control and 10 ppm groups,
and all except one of the 50 ppm group, produced living litters
(Hazleton & Holland, 1950).
In a 2-year study, groups of rats were maintained on diets
containing 10, 25 and 100 ppm of parathion. No morbid anatomical
effects were seen at any level (Lehman, 1952).
Comments on experimental studies reported
The acute and short-term studies are extensive and cover a wide
range of animal species. Long-term studies were also carried out in
the rat. Experiments in the dog, though limited, suggest that this
species is more sensitive than the rat to the anticholinesterase
activity of parathion. Man and the rat are equally sensitive to the
anticholinesterase action of parathion. Erythrocyte cholinesterase
activity is a most sensitive indicator of this action of parathion and
a wide margin exists between the highest dose without action on
cholinesterase activity and the lowest dose needed to cause clinical
effect. The figures for rat and man thus provide a basis for
evaluation.
EVALUATION
Level causing no significant toxicological effect
Rat. The maximum level having no effect on cholinesterase
activity in the rat was 1 ppm, equivalent to 0.05 mg/kg body-weight.
Man. 0.05 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.005 mg/kg body-weight
Further studies considered desirable
Reproduction studies in the rat.
REFERENCES
Aldridge, W. N. & Davison, A. N. (1952) Biochem. J., 52, 663
American Cyanamid Company New York (1955) Report on Parathion
Barnes, J. M. & Denz, F. A. (1951) J. Hyg. (Lond.), 49, 430
Deichmann W. B. Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 44
DuBois, K. P. Doull, J., Salerno, P. R. & Coon, J. M. (1949) J.
Pharmacol. exp. Ther., 95, 79
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. (1957) Proceedings of the fourth International Congress
of Crop Protection Hamburg, 1625; (quoted by Barnes, J. M. (1957)
Adv. Pest. Control Res., 1, 1)
Edson, E. F. & Noakes, D. N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E. F. (1964) Fd. Cosmet. Toxicol., 2, 311
Engbaek, L. & Jensen, O. S. (1951) Acta pharmacol. (Kbh.), 7, 189
Erdmann, W. D. & Lendle, L. (1960) Ergebnisse inn. Med.
Kinderheilk., 10, 104
Frawley, J. P., Hagan, E. C. & Fitzhugh, O. G. (1952) J. Pharmacol.
exp. Ther., 105, 156
Frawley, J. P. & Fuyat, H. N. (1957) J. Agr. Food Chem., 5, 347
Frederiksson, T. & Bigelow, J. K. (1961) Arch. environm. Hlth, 2,
663
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gardocki, J. F. & Hazleton, L. W. (1951) J. Amer. pharm. Ass., 40,
491
Hazleton, L. W. & Holland, E. G. (1950) Advances in Chemistry,
Series 1, 31
Heath, D. F. (1961) Organophosphorus Poisons, Pergamon Press
Klimmer, O. R. & Pfaff, W. (1955) Arzneimittel-Forsch., 5, 626
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20 (Pt 1) 434
Rider, J. A., Moeller, H. C., Swader, J. & Weilerstein, R. W. (1958)
A.M.A. Arch. industr. Hyg., 18, 442
Williams, M. W. Fitzhugh, O. G. & Cook, J. W. (1958) J. Pharmacol.
exp. Ther., 122(1), 83A
BIOLOGICAL DATA
Biochemical aspects
Parathion is readily absorbed by all the routes of
administration. It is a powerful inhibitor of cholinesterase in
vivo. In vitro, pure parathion had little effect on the enzyme
(Aldridge & Davison, 1952). In mammals and plants parathion is readily
oxidized to a powerful cholinesterase inhibitor, paraoxon, which is
the active form of parathion (Heath, 1961). In mice given
subcutaneously 32P labelled parathion, the highest radioactivity
appeared in the salivary glands and cervical brown fat within 4 hours
of injection. Liver, kidney and adipose tissues showed high uptake of
radioactivity and fairly high activity was found in gastric and
intestinal walls, thyroid, spleen and lungs. Less activity was noted
in the central nervous system, musculature and bone marrow
(Frederiksson & Bigelow, 1961). Parathion is finally broken down to
p-nitrophenol, with traces of p-aminophenol (Gardocki & Hazleton,
1951) and these are excreted in the urine. The final product depends
on the species (Heath, 1961).
The molar I50 for paraoxon against sheep erythrocyte
cholinesterase was 2.0 × 10-8 (30 min.; 37°C) (Aldridge & Davison,
1952).
In vitro studies on human liver enzyme indicated that parathion
could inhibit the non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 6.0-25.0* Frawley et al., 1952
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Mouse, male Intraperitoneal 10.4-11.4 Engbaek & Jensen, 1951
Rat, female Oral 1.75-5.0* Deichmann et al., 1952
Edson & Noakes, 1960
Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Rat, male Oral 5.0-30.0* Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Rat Intraperitoneal 5.5 DuBois & Coon, 1952
Guinea-pig Oral 9.3-32.0* Frawley et al., 1952
Hazleton & Holland, 1950
Guinea-pig Intraperitoneal 12.0 Klimmer & Pfaff, 1955
Rabbit Oral 10.0 American Cyanamid Company, 1955
Chicken Intraperitoneal 8.7 American Cyanamid Company, 1955
Dog Oral 3.0-5.0 Hazleton & Holland, 1950
(lethal dose)
Dog Intraperitoneal 12.0-20.0 DuBois et al., 1949
* Differences caused by the use of different vehicles.
Man. An oral dose of 3-5 mg/kg is usually fatal to man (Erdmann
& Lendle, 1960).
Short-term studies
Rat. Groups each of 5 male rats were placed on diets containing
1, 5 and 25 ppm of parathion for 2 weeks. The lowest level that caused
inhibition of the erythrocyte cholinesterase activity (the most
sensitive to parathion) was 5 ppm (Frawley et al., 1952). 1 ppm did
not give inhibition. Groups of 10 female rats were fed for 16 weeks
diets containing 1, 5, 25 and 125 ppm of parathion. At 125 ppm, severe
toxic symptoms, growth suppression and death occurred. At 25 ppm
slight muscular fibrillation was seen in the first 2 weeks. At 5 and 1
ppm no ill-effects were observed on general behaviour and growth.
Erythrocyte cholinesterase activity was severely inhibited at 5 ppm
and 25 ppm and above. No inhibition was observed at 1 ppm (Edson &
Noakes, 1960).
Concentrations of 0.05, 0.5 and 5.0 ppm of parathion was fed
for a period of 12 weeks to 4 groups of 20 rats. Of the initial 20
rats, after 1, 4 and 8 weeks, 4 animals, and after 12 weeks 8 animals,
were killed to determine the cholinesterase activity in brain,
erythrocytes and plasm. In this experiment 0.5 ppm was the highest
ineffective level in respect of toxic effects or blood cholinesterase
inhibition (Edson, 1957).
Dog. Groups each of one male and one female dog were placed for
24 weeks on diets containing 1, 2 and 5 ppm of parathion, so that the
average daily intake was 0.021, 0.047 and 0.117 mg/kg body-weight
respectively. The 2 and 5 ppm levels caused about 60% and 70%
inhibition of plasm cholinesterase activity and the 1 ppm level caused
a small but significant inhibition. For erythrocyte cholinesterase
activity only the 5 ppm level produced significant depression. The
recovery of plasma activity at all levels was complete within 4 weeks
after return to a control diet, and was frequently followed by a
transient elevation above the pre-treatment activity. The recovery
rate for erythrocyte activity was slower, requiring 11 weeks (Frawley
& Fuyat, 1957).
Dogs were given 100 ppm of parathion in the diet for 9 weeks.
In addition to a very strong inhibition of plasm and erythrocyte
cholinesterase activity, a change in the protein content of the serum
was noticed. The albumin content decreased from 48% to 34% and the
ß-globulin content increased from 13.5% to 23.3% (Williams et al.,
1958).
Pig. Parathion was fed to groups each of 2 pigs for periods of
33 to 122 days. The diets contained 0.2, 1 and 5 ppm. After 33 days
the 0.2 ppm concentration was increased to 25 ppm, and after 40 days
the 1 ppm concentration to 100 ppm. The highest ineffective dose in
respect of toxic effects or depression of blood cholinesterase
activity was 1.0 mg/kg body-weight/day (Edson, 1957).
Man. A group of 5 subjects was fed parathion 3 mg/day for 28
days, 4.5 mg/day for 28 days and 6 mg/day for 43 days. Three and 4.5
mg did not produce any significant depression of erythrocyte or plasm
cholinesterase activity, but 6 mg produced a depression of 10-15% of
both plasm and erythrocyte cholinesterase activity. Depression of
plasm cholinesterase activity persisted two weeks after administration
of the drug was discontinued, whereas erythrocyte cholinesterase
activity continued to be depressed for 43 days. No side-effects were
observed (Moeller & Rider, 1961).
Five groups of 4 subjects were given parathion orally at
dosages of 0 mg/day, 0.6 mg/day for 4 weeks followed by 9 weeks at
4.8 mg daily, 1.2 mg/daily, 2.4 mg/daily and 7.2 mg/daily for 6 weeks.
Only in the latter group the whole blood cholinesterase was reduced,
declining to 67% of the controls at the end of the 6 weeks. At this
time red cell and plasma cholinesterases were respectively 84% and 63%
of the controls. Whole blood cholinesterase was restored to 87% of
control activity 28 days after withdrawal of parathion (Edson, 1964).
Four male and 4 female subjects were fed 0.003, 0.010, 0.025
and 0.050 mg/kg body-weight of parathion for successive periods each
of 3 weeks. A significant increase in the mean plasma cholinesterase
activity without an accompanying change in the erythrocyte
cholinesterase activity occurred early in the feeding programs, but
the level declined to control values when doses of 0.050 mg/kg
body-weight were reached (Rider et al., 1958).
Long-term studies
Rat. In a one-year toxicity test groups of 36 male and 36
female rats were maintained on diets containing 10, 20, 50, 75 and 100
ppm of parathion. At 50 ppm and above growth retardation, toxic
symptom and death occurred. Histological examination of animals dying
with toxic symptoms revealed lesions of the salivary glands, pancreas
and thymus. No other lesions attributable to parathion were found. At
20 ppm and less no ill-effects were observed on growth, behaviour,
mortality rate or histology. In breeding experiments performed with
the animals from all groups the second generation of rats fed at 10
ppm produced normal litters but the young frequently died (Barnes &
Denz, 1951).
In a 2-year study, groups of 8-10 male rats were fed diets
containing parathion at the levels of 50 and 100 ppm, corresponding to
average daily amounts of 1 and 2 mg per rat. At 100 ppm a slight
retardation of growth and toxic symptoms were observed for the first
few weeks. No growth reduction was present at 50 ppm. No ill-effects
on food consumption, mortality rate, blood picture or gross pathology
were observed. No histological changes attributable to parathion were
observed. On chemical analysis no parathion was found in brain, liver,
kidney, lung, abdominal fat or whole blood. In groups of 10-16 male
rats placed on diets containing 10 and 25 ppm parathion for 2 years no
ill-effects were observed on general condition, food consumption,
growth and survival. Groups of 6-9 female rats were fed with diets
containing 10 and 50 ppm of parathion for 2 years. There was no
evidence of toxicity. All females in the control and 10 ppm groups,
and all except one of the 50 ppm group, produced living litters
(Hazleton & Holland, 1950).
In a 2-year study, groups of rats were maintained on diets
containing 10, 25 and 100 ppm of parathion. No morbid anatomical
effects were seen at any level (Lehman, 1952).
Comments on experimental studies reported
The acute and short-term studies are extensive and cover a wide
range of animal species. Long-term studies were also carried out in
the rat. Experiments in the dog, though limited, suggest that this
species is more sensitive than the rat to the anticholinesterase
activity of parathion. Man and the rat are equally sensitive to the
anticholinesterase action of parathion. Erythrocyte cholinesterase
activity is a most sensitive indicator of this action of parathion and
a wide margin exists between the highest dose without action on
cholinesterase activity and the lowest dose needed to cause clinical
effect. The figures for rat and man thus provide a basis for
evaluation.
EVALUATION
Level causing no significant toxicological effect
Rat. The maximum level having no effect on cholinesterase
activity in the rat was 1 ppm, equivalent to 0.05 mg/kg body-weight.
Man. 0.05 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.005 mg/kg body-weight
Further studies considered desirable
Reproduction studies in the rat.
REFERENCES
Aldridge, W. N. & Davison, A. N. (1952) Biochem. J., 52, 663
American Cyanamid Company New York (1955) Report on Parathion
Barnes, J. M. & Denz, F. A. (1951) J. Hyg. (Lond.), 49, 430
Deichmann W. B. Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 44
DuBois, K. P. Doull, J., Salerno, P. R. & Coon, J. M. (1949) J.
Pharmacol. exp. Ther., 95, 79
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. (1957) Proceedings of the fourth International Congress
of Crop Protection Hamburg, 1625; (quoted by Barnes, J. M. (1957)
Adv. Pest. Control Res., 1, 1)
Edson, E. F. & Noakes, D. N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E. F. (1964) Fd. Cosmet. Toxicol., 2, 311
Engbaek, L. & Jensen, O. S. (1951) Acta pharmacol. (Kbh.), 7, 189
Erdmann, W. D. & Lendle, L. (1960) Ergebnisse inn. Med.
Kinderheilk., 10, 104
Frawley, J. P., Hagan, E. C. & Fitzhugh, O. G. (1952) J. Pharmacol.
exp. Ther., 105, 156
Frawley, J. P. & Fuyat, H. N. (1957) J. Agr. Food Chem., 5, 347
Frederiksson, T. & Bigelow, J. K. (1961) Arch. environm. Hlth, 2,
663
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gardocki, J. F. & Hazleton, L. W. (1951) J. Amer. pharm. Ass., 40,
491
Hazleton, L. W. & Holland, E. G. (1950) Advances in Chemistry,
Series 1, 31
Heath, D. F. (1961) Organophosphorus Poisons, Pergamon Press
Klimmer, O. R. & Pfaff, W. (1955) Arzneimittel-Forsch., 5, 626
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20 (Pt 1) 434
Rider, J. A., Moeller, H. C., Swader, J. & Weilerstein, R. W. (1958)
A.M.A. Arch. industr. Hyg., 18, 442
Williams, M. W. Fitzhugh, O. G. & Cook, J. W. (1958) J. Pharmacol.
exp. Ther., 122(1), 83A
 BIOLOGICAL DATA
Biochemical aspects
Parathion is readily absorbed by all the routes of
administration. It is a powerful inhibitor of cholinesterase in
vivo. In vitro, pure parathion had little effect on the enzyme
(Aldridge & Davison, 1952). In mammals and plants parathion is readily
oxidized to a powerful cholinesterase inhibitor, paraoxon, which is
the active form of parathion (Heath, 1961). In mice given
subcutaneously 32P labelled parathion, the highest radioactivity
appeared in the salivary glands and cervical brown fat within 4 hours
of injection. Liver, kidney and adipose tissues showed high uptake of
radioactivity and fairly high activity was found in gastric and
intestinal walls, thyroid, spleen and lungs. Less activity was noted
in the central nervous system, musculature and bone marrow
(Frederiksson & Bigelow, 1961). Parathion is finally broken down to
p-nitrophenol, with traces of p-aminophenol (Gardocki & Hazleton,
1951) and these are excreted in the urine. The final product depends
on the species (Heath, 1961).
The molar I50 for paraoxon against sheep erythrocyte
cholinesterase was 2.0 × 10-8 (30 min.; 37°C) (Aldridge & Davison,
1952).
In vitro studies on human liver enzyme indicated that parathion
could inhibit the non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 6.0-25.0* Frawley et al., 1952
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Mouse, male Intraperitoneal 10.4-11.4 Engbaek & Jensen, 1951
Rat, female Oral 1.75-5.0* Deichmann et al., 1952
Edson & Noakes, 1960
Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Rat, male Oral 5.0-30.0* Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Rat Intraperitoneal 5.5 DuBois & Coon, 1952
Guinea-pig Oral 9.3-32.0* Frawley et al., 1952
Hazleton & Holland, 1950
Guinea-pig Intraperitoneal 12.0 Klimmer & Pfaff, 1955
Rabbit Oral 10.0 American Cyanamid Company, 1955
Chicken Intraperitoneal 8.7 American Cyanamid Company, 1955
Dog Oral 3.0-5.0 Hazleton & Holland, 1950
(lethal dose)
Dog Intraperitoneal 12.0-20.0 DuBois et al., 1949
* Differences caused by the use of different vehicles.
Man. An oral dose of 3-5 mg/kg is usually fatal to man (Erdmann
& Lendle, 1960).
Short-term studies
Rat. Groups each of 5 male rats were placed on diets containing
1, 5 and 25 ppm of parathion for 2 weeks. The lowest level that caused
inhibition of the erythrocyte cholinesterase activity (the most
sensitive to parathion) was 5 ppm (Frawley et al., 1952). 1 ppm did
not give inhibition. Groups of 10 female rats were fed for 16 weeks
diets containing 1, 5, 25 and 125 ppm of parathion. At 125 ppm, severe
toxic symptoms, growth suppression and death occurred. At 25 ppm
slight muscular fibrillation was seen in the first 2 weeks. At 5 and 1
ppm no ill-effects were observed on general behaviour and growth.
Erythrocyte cholinesterase activity was severely inhibited at 5 ppm
and 25 ppm and above. No inhibition was observed at 1 ppm (Edson &
Noakes, 1960).
Concentrations of 0.05, 0.5 and 5.0 ppm of parathion was fed
for a period of 12 weeks to 4 groups of 20 rats. Of the initial 20
rats, after 1, 4 and 8 weeks, 4 animals, and after 12 weeks 8 animals,
were killed to determine the cholinesterase activity in brain,
erythrocytes and plasm. In this experiment 0.5 ppm was the highest
ineffective level in respect of toxic effects or blood cholinesterase
inhibition (Edson, 1957).
Dog. Groups each of one male and one female dog were placed for
24 weeks on diets containing 1, 2 and 5 ppm of parathion, so that the
average daily intake was 0.021, 0.047 and 0.117 mg/kg body-weight
respectively. The 2 and 5 ppm levels caused about 60% and 70%
inhibition of plasm cholinesterase activity and the 1 ppm level caused
a small but significant inhibition. For erythrocyte cholinesterase
activity only the 5 ppm level produced significant depression. The
recovery of plasma activity at all levels was complete within 4 weeks
after return to a control diet, and was frequently followed by a
transient elevation above the pre-treatment activity. The recovery
rate for erythrocyte activity was slower, requiring 11 weeks (Frawley
& Fuyat, 1957).
Dogs were given 100 ppm of parathion in the diet for 9 weeks.
In addition to a very strong inhibition of plasm and erythrocyte
cholinesterase activity, a change in the protein content of the serum
was noticed. The albumin content decreased from 48% to 34% and the
ß-globulin content increased from 13.5% to 23.3% (Williams et al.,
1958).
Pig. Parathion was fed to groups each of 2 pigs for periods of
33 to 122 days. The diets contained 0.2, 1 and 5 ppm. After 33 days
the 0.2 ppm concentration was increased to 25 ppm, and after 40 days
the 1 ppm concentration to 100 ppm. The highest ineffective dose in
respect of toxic effects or depression of blood cholinesterase
activity was 1.0 mg/kg body-weight/day (Edson, 1957).
Man. A group of 5 subjects was fed parathion 3 mg/day for 28
days, 4.5 mg/day for 28 days and 6 mg/day for 43 days. Three and 4.5
mg did not produce any significant depression of erythrocyte or plasm
cholinesterase activity, but 6 mg produced a depression of 10-15% of
both plasm and erythrocyte cholinesterase activity. Depression of
plasm cholinesterase activity persisted two weeks after administration
of the drug was discontinued, whereas erythrocyte cholinesterase
activity continued to be depressed for 43 days. No side-effects were
observed (Moeller & Rider, 1961).
Five groups of 4 subjects were given parathion orally at
dosages of 0 mg/day, 0.6 mg/day for 4 weeks followed by 9 weeks at
4.8 mg daily, 1.2 mg/daily, 2.4 mg/daily and 7.2 mg/daily for 6 weeks.
Only in the latter group the whole blood cholinesterase was reduced,
declining to 67% of the controls at the end of the 6 weeks. At this
time red cell and plasma cholinesterases were respectively 84% and 63%
of the controls. Whole blood cholinesterase was restored to 87% of
control activity 28 days after withdrawal of parathion (Edson, 1964).
Four male and 4 female subjects were fed 0.003, 0.010, 0.025
and 0.050 mg/kg body-weight of parathion for successive periods each
of 3 weeks. A significant increase in the mean plasma cholinesterase
activity without an accompanying change in the erythrocyte
cholinesterase activity occurred early in the feeding programs, but
the level declined to control values when doses of 0.050 mg/kg
body-weight were reached (Rider et al., 1958).
Long-term studies
Rat. In a one-year toxicity test groups of 36 male and 36
female rats were maintained on diets containing 10, 20, 50, 75 and 100
ppm of parathion. At 50 ppm and above growth retardation, toxic
symptom and death occurred. Histological examination of animals dying
with toxic symptoms revealed lesions of the salivary glands, pancreas
and thymus. No other lesions attributable to parathion were found. At
20 ppm and less no ill-effects were observed on growth, behaviour,
mortality rate or histology. In breeding experiments performed with
the animals from all groups the second generation of rats fed at 10
ppm produced normal litters but the young frequently died (Barnes &
Denz, 1951).
In a 2-year study, groups of 8-10 male rats were fed diets
containing parathion at the levels of 50 and 100 ppm, corresponding to
average daily amounts of 1 and 2 mg per rat. At 100 ppm a slight
retardation of growth and toxic symptoms were observed for the first
few weeks. No growth reduction was present at 50 ppm. No ill-effects
on food consumption, mortality rate, blood picture or gross pathology
were observed. No histological changes attributable to parathion were
observed. On chemical analysis no parathion was found in brain, liver,
kidney, lung, abdominal fat or whole blood. In groups of 10-16 male
rats placed on diets containing 10 and 25 ppm parathion for 2 years no
ill-effects were observed on general condition, food consumption,
growth and survival. Groups of 6-9 female rats were fed with diets
containing 10 and 50 ppm of parathion for 2 years. There was no
evidence of toxicity. All females in the control and 10 ppm groups,
and all except one of the 50 ppm group, produced living litters
(Hazleton & Holland, 1950).
In a 2-year study, groups of rats were maintained on diets
containing 10, 25 and 100 ppm of parathion. No morbid anatomical
effects were seen at any level (Lehman, 1952).
Comments on experimental studies reported
The acute and short-term studies are extensive and cover a wide
range of animal species. Long-term studies were also carried out in
the rat. Experiments in the dog, though limited, suggest that this
species is more sensitive than the rat to the anticholinesterase
activity of parathion. Man and the rat are equally sensitive to the
anticholinesterase action of parathion. Erythrocyte cholinesterase
activity is a most sensitive indicator of this action of parathion and
a wide margin exists between the highest dose without action on
cholinesterase activity and the lowest dose needed to cause clinical
effect. The figures for rat and man thus provide a basis for
evaluation.
EVALUATION
Level causing no significant toxicological effect
Rat. The maximum level having no effect on cholinesterase
activity in the rat was 1 ppm, equivalent to 0.05 mg/kg body-weight.
Man. 0.05 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.005 mg/kg body-weight
Further studies considered desirable
Reproduction studies in the rat.
REFERENCES
Aldridge, W. N. & Davison, A. N. (1952) Biochem. J., 52, 663
American Cyanamid Company New York (1955) Report on Parathion
Barnes, J. M. & Denz, F. A. (1951) J. Hyg. (Lond.), 49, 430
Deichmann W. B. Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 44
DuBois, K. P. Doull, J., Salerno, P. R. & Coon, J. M. (1949) J.
Pharmacol. exp. Ther., 95, 79
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. (1957) Proceedings of the fourth International Congress
of Crop Protection Hamburg, 1625; (quoted by Barnes, J. M. (1957)
Adv. Pest. Control Res., 1, 1)
Edson, E. F. & Noakes, D. N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E. F. (1964) Fd. Cosmet. Toxicol., 2, 311
Engbaek, L. & Jensen, O. S. (1951) Acta pharmacol. (Kbh.), 7, 189
Erdmann, W. D. & Lendle, L. (1960) Ergebnisse inn. Med.
Kinderheilk., 10, 104
Frawley, J. P., Hagan, E. C. & Fitzhugh, O. G. (1952) J. Pharmacol.
exp. Ther., 105, 156
Frawley, J. P. & Fuyat, H. N. (1957) J. Agr. Food Chem., 5, 347
Frederiksson, T. & Bigelow, J. K. (1961) Arch. environm. Hlth, 2,
663
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gardocki, J. F. & Hazleton, L. W. (1951) J. Amer. pharm. Ass., 40,
491
Hazleton, L. W. & Holland, E. G. (1950) Advances in Chemistry,
Series 1, 31
Heath, D. F. (1961) Organophosphorus Poisons, Pergamon Press
Klimmer, O. R. & Pfaff, W. (1955) Arzneimittel-Forsch., 5, 626
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20 (Pt 1) 434
Rider, J. A., Moeller, H. C., Swader, J. & Weilerstein, R. W. (1958)
A.M.A. Arch. industr. Hyg., 18, 442
Williams, M. W. Fitzhugh, O. G. & Cook, J. W. (1958) J. Pharmacol.
exp. Ther., 122(1), 83A
BIOLOGICAL DATA
Biochemical aspects
Parathion is readily absorbed by all the routes of
administration. It is a powerful inhibitor of cholinesterase in
vivo. In vitro, pure parathion had little effect on the enzyme
(Aldridge & Davison, 1952). In mammals and plants parathion is readily
oxidized to a powerful cholinesterase inhibitor, paraoxon, which is
the active form of parathion (Heath, 1961). In mice given
subcutaneously 32P labelled parathion, the highest radioactivity
appeared in the salivary glands and cervical brown fat within 4 hours
of injection. Liver, kidney and adipose tissues showed high uptake of
radioactivity and fairly high activity was found in gastric and
intestinal walls, thyroid, spleen and lungs. Less activity was noted
in the central nervous system, musculature and bone marrow
(Frederiksson & Bigelow, 1961). Parathion is finally broken down to
p-nitrophenol, with traces of p-aminophenol (Gardocki & Hazleton,
1951) and these are excreted in the urine. The final product depends
on the species (Heath, 1961).
The molar I50 for paraoxon against sheep erythrocyte
cholinesterase was 2.0 × 10-8 (30 min.; 37°C) (Aldridge & Davison,
1952).
In vitro studies on human liver enzyme indicated that parathion
could inhibit the non-specific esterases to a greater degree than
acetylesterase (Ecobichon & Kalow, 1963).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Mouse Oral 6.0-25.0* Frawley et al., 1952
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Mouse, male Intraperitoneal 10.4-11.4 Engbaek & Jensen, 1951
Rat, female Oral 1.75-5.0* Deichmann et al., 1952
Edson & Noakes, 1960
Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Rat, male Oral 5.0-30.0* Frawley et al., 1952
Gaines, 1960
Hazleton & Holland, 1950
Klimer & Pfaff, 1955
Rat Intraperitoneal 5.5 DuBois & Coon, 1952
Guinea-pig Oral 9.3-32.0* Frawley et al., 1952
Hazleton & Holland, 1950
Guinea-pig Intraperitoneal 12.0 Klimmer & Pfaff, 1955
Rabbit Oral 10.0 American Cyanamid Company, 1955
Chicken Intraperitoneal 8.7 American Cyanamid Company, 1955
Dog Oral 3.0-5.0 Hazleton & Holland, 1950
(lethal dose)
Dog Intraperitoneal 12.0-20.0 DuBois et al., 1949
* Differences caused by the use of different vehicles.
Man. An oral dose of 3-5 mg/kg is usually fatal to man (Erdmann
& Lendle, 1960).
Short-term studies
Rat. Groups each of 5 male rats were placed on diets containing
1, 5 and 25 ppm of parathion for 2 weeks. The lowest level that caused
inhibition of the erythrocyte cholinesterase activity (the most
sensitive to parathion) was 5 ppm (Frawley et al., 1952). 1 ppm did
not give inhibition. Groups of 10 female rats were fed for 16 weeks
diets containing 1, 5, 25 and 125 ppm of parathion. At 125 ppm, severe
toxic symptoms, growth suppression and death occurred. At 25 ppm
slight muscular fibrillation was seen in the first 2 weeks. At 5 and 1
ppm no ill-effects were observed on general behaviour and growth.
Erythrocyte cholinesterase activity was severely inhibited at 5 ppm
and 25 ppm and above. No inhibition was observed at 1 ppm (Edson &
Noakes, 1960).
Concentrations of 0.05, 0.5 and 5.0 ppm of parathion was fed
for a period of 12 weeks to 4 groups of 20 rats. Of the initial 20
rats, after 1, 4 and 8 weeks, 4 animals, and after 12 weeks 8 animals,
were killed to determine the cholinesterase activity in brain,
erythrocytes and plasm. In this experiment 0.5 ppm was the highest
ineffective level in respect of toxic effects or blood cholinesterase
inhibition (Edson, 1957).
Dog. Groups each of one male and one female dog were placed for
24 weeks on diets containing 1, 2 and 5 ppm of parathion, so that the
average daily intake was 0.021, 0.047 and 0.117 mg/kg body-weight
respectively. The 2 and 5 ppm levels caused about 60% and 70%
inhibition of plasm cholinesterase activity and the 1 ppm level caused
a small but significant inhibition. For erythrocyte cholinesterase
activity only the 5 ppm level produced significant depression. The
recovery of plasma activity at all levels was complete within 4 weeks
after return to a control diet, and was frequently followed by a
transient elevation above the pre-treatment activity. The recovery
rate for erythrocyte activity was slower, requiring 11 weeks (Frawley
& Fuyat, 1957).
Dogs were given 100 ppm of parathion in the diet for 9 weeks.
In addition to a very strong inhibition of plasm and erythrocyte
cholinesterase activity, a change in the protein content of the serum
was noticed. The albumin content decreased from 48% to 34% and the
ß-globulin content increased from 13.5% to 23.3% (Williams et al.,
1958).
Pig. Parathion was fed to groups each of 2 pigs for periods of
33 to 122 days. The diets contained 0.2, 1 and 5 ppm. After 33 days
the 0.2 ppm concentration was increased to 25 ppm, and after 40 days
the 1 ppm concentration to 100 ppm. The highest ineffective dose in
respect of toxic effects or depression of blood cholinesterase
activity was 1.0 mg/kg body-weight/day (Edson, 1957).
Man. A group of 5 subjects was fed parathion 3 mg/day for 28
days, 4.5 mg/day for 28 days and 6 mg/day for 43 days. Three and 4.5
mg did not produce any significant depression of erythrocyte or plasm
cholinesterase activity, but 6 mg produced a depression of 10-15% of
both plasm and erythrocyte cholinesterase activity. Depression of
plasm cholinesterase activity persisted two weeks after administration
of the drug was discontinued, whereas erythrocyte cholinesterase
activity continued to be depressed for 43 days. No side-effects were
observed (Moeller & Rider, 1961).
Five groups of 4 subjects were given parathion orally at
dosages of 0 mg/day, 0.6 mg/day for 4 weeks followed by 9 weeks at
4.8 mg daily, 1.2 mg/daily, 2.4 mg/daily and 7.2 mg/daily for 6 weeks.
Only in the latter group the whole blood cholinesterase was reduced,
declining to 67% of the controls at the end of the 6 weeks. At this
time red cell and plasma cholinesterases were respectively 84% and 63%
of the controls. Whole blood cholinesterase was restored to 87% of
control activity 28 days after withdrawal of parathion (Edson, 1964).
Four male and 4 female subjects were fed 0.003, 0.010, 0.025
and 0.050 mg/kg body-weight of parathion for successive periods each
of 3 weeks. A significant increase in the mean plasma cholinesterase
activity without an accompanying change in the erythrocyte
cholinesterase activity occurred early in the feeding programs, but
the level declined to control values when doses of 0.050 mg/kg
body-weight were reached (Rider et al., 1958).
Long-term studies
Rat. In a one-year toxicity test groups of 36 male and 36
female rats were maintained on diets containing 10, 20, 50, 75 and 100
ppm of parathion. At 50 ppm and above growth retardation, toxic
symptom and death occurred. Histological examination of animals dying
with toxic symptoms revealed lesions of the salivary glands, pancreas
and thymus. No other lesions attributable to parathion were found. At
20 ppm and less no ill-effects were observed on growth, behaviour,
mortality rate or histology. In breeding experiments performed with
the animals from all groups the second generation of rats fed at 10
ppm produced normal litters but the young frequently died (Barnes &
Denz, 1951).
In a 2-year study, groups of 8-10 male rats were fed diets
containing parathion at the levels of 50 and 100 ppm, corresponding to
average daily amounts of 1 and 2 mg per rat. At 100 ppm a slight
retardation of growth and toxic symptoms were observed for the first
few weeks. No growth reduction was present at 50 ppm. No ill-effects
on food consumption, mortality rate, blood picture or gross pathology
were observed. No histological changes attributable to parathion were
observed. On chemical analysis no parathion was found in brain, liver,
kidney, lung, abdominal fat or whole blood. In groups of 10-16 male
rats placed on diets containing 10 and 25 ppm parathion for 2 years no
ill-effects were observed on general condition, food consumption,
growth and survival. Groups of 6-9 female rats were fed with diets
containing 10 and 50 ppm of parathion for 2 years. There was no
evidence of toxicity. All females in the control and 10 ppm groups,
and all except one of the 50 ppm group, produced living litters
(Hazleton & Holland, 1950).
In a 2-year study, groups of rats were maintained on diets
containing 10, 25 and 100 ppm of parathion. No morbid anatomical
effects were seen at any level (Lehman, 1952).
Comments on experimental studies reported
The acute and short-term studies are extensive and cover a wide
range of animal species. Long-term studies were also carried out in
the rat. Experiments in the dog, though limited, suggest that this
species is more sensitive than the rat to the anticholinesterase
activity of parathion. Man and the rat are equally sensitive to the
anticholinesterase action of parathion. Erythrocyte cholinesterase
activity is a most sensitive indicator of this action of parathion and
a wide margin exists between the highest dose without action on
cholinesterase activity and the lowest dose needed to cause clinical
effect. The figures for rat and man thus provide a basis for
evaluation.
EVALUATION
Level causing no significant toxicological effect
Rat. The maximum level having no effect on cholinesterase
activity in the rat was 1 ppm, equivalent to 0.05 mg/kg body-weight.
Man. 0.05 mg/kg body-weight per day.
Estimate of acceptable daily intake for man
0-0.005 mg/kg body-weight
Further studies considered desirable
Reproduction studies in the rat.
REFERENCES
Aldridge, W. N. & Davison, A. N. (1952) Biochem. J., 52, 663
American Cyanamid Company New York (1955) Report on Parathion
Barnes, J. M. & Denz, F. A. (1951) J. Hyg. (Lond.), 49, 430
Deichmann W. B. Pugliese, W. & Cassidy, J. (1952) A.M.A. Arch.
industr. Hyg., 5, 44
DuBois, K. P. Doull, J., Salerno, P. R. & Coon, J. M. (1949) J.
Pharmacol. exp. Ther., 95, 79
DuBois, K. P. & Coon, J. M. (1952) A.M.A. Arch. industr. Hyg., 6,
9
Ecobichon, D. J. & Kalow, W. (1963) Canad. J. Biochem., 41, 1537
Edson, E. F. (1957) Proceedings of the fourth International Congress
of Crop Protection Hamburg, 1625; (quoted by Barnes, J. M. (1957)
Adv. Pest. Control Res., 1, 1)
Edson, E. F. & Noakes, D. N. (1960) Toxicol. appl. Pharmacol., 2,
523
Edson, E. F. (1964) Fd. Cosmet. Toxicol., 2, 311
Engbaek, L. & Jensen, O. S. (1951) Acta pharmacol. (Kbh.), 7, 189
Erdmann, W. D. & Lendle, L. (1960) Ergebnisse inn. Med.
Kinderheilk., 10, 104
Frawley, J. P., Hagan, E. C. & Fitzhugh, O. G. (1952) J. Pharmacol.
exp. Ther., 105, 156
Frawley, J. P. & Fuyat, H. N. (1957) J. Agr. Food Chem., 5, 347
Frederiksson, T. & Bigelow, J. K. (1961) Arch. environm. Hlth, 2,
663
Gaines, T. B. (1960) Toxicol. appl. Pharmacol., 2, 88
Gardocki, J. F. & Hazleton, L. W. (1951) J. Amer. pharm. Ass., 40,
491
Hazleton, L. W. & Holland, E. G. (1950) Advances in Chemistry,
Series 1, 31
Heath, D. F. (1961) Organophosphorus Poisons, Pergamon Press
Klimmer, O. R. & Pfaff, W. (1955) Arzneimittel-Forsch., 5, 626
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Moeller, H. C. & Rider, J. A. (1961) Fed. Proc., 20 (Pt 1) 434
Rider, J. A., Moeller, H. C., Swader, J. & Weilerstein, R. W. (1958)
A.M.A. Arch. industr. Hyg., 18, 442
Williams, M. W. Fitzhugh, O. G. & Cook, J. W. (1958) J. Pharmacol.
exp. Ther., 122(1), 83A
