
PARATHION (addendum)
First draft prepared by
M.S. Morrow,
US Environmental Protection Agency,
Washington DC, USA
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive and developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Parathion is a broad-spectrum organophosphorus pesticide which
was last evaluated toxicologically in 1967 (Annex I, reference 8). An
ADI of 0-0.005 mg/kg bw was established on the basis of cholinesterase
inhibition in a long-term feeding study in rats. Parathion was
re-evaluated at the present Meeting within the periodic review
programme of the CCPR. Data generated since the previous review are
summarized in this monograph addendum.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Parathion, C10H14O5, is readily absorbed after administration
by all routes.
The biokinetic behaviour of 14C-parathion was investigated in
male rats given oral or intravenous doses ranging from 0.1 to
5.0 mg/kg bw; females received labelled parathion only by the oral
route at a dose of 1.0 mg/kg bw. The parathion was almost completely
absorbed from the gastrointestinal tract after oral administration,
and > 99% of the administered compound was eliminated in the urine
and faeces within 48 h. In males, faecal elimination accounted for 6
and 8% of orally administered doses of 1 and 5 mg/kg, respectively.
Only 11% of the 14C label was present in the body 1 h after oral
treatment and only 0.09% after two days (Weber et al., 1980).
14C-Parathion was administered through a stomach needle to
female ICR mice at a dose of 1 mg/kg bw, and the amount of radiolabel
was determined in the gastrointestinal tract and selected organs. By
1 h, 57% of the radiolabel had been absorbed. The half-life for
penetration was 33.3 ± 2.4 min (Ahdaya et al., 1981).
14C-Parathion was administered either intravenously at a dose
of 0.87 mg, as single topical applications of 4 mg/cm2 for seven
days or as multiple topical applications of 4 mg/cm2 for 15 days, to
four female rhesus monkeys. Urine was collected and assayed for
radiolabel. After intravenous administration, renal excretion was
essentially complete, parathion being eliminated primarily in the
urine (75 ± 11%). The amount of the 14C dose that was absorbed was
not determined after the single topical dose; after multiple topical
applications, the amount of radiolabelled material absorbed was
43 ± 14% after the first dose and 38 ± 8% after the eighth dose
(Bucks et al., 1990).
Percutaneous absorption of parathion by excised porcine skin was
increased by high relative humidity and elevated temperature. Lower
doses appeared to be more sensitive to environmental changes,
suggesting that dose, temperature, and humidity have independent,
variable effects on percutaneous absorption of parathion (Chang &
Riviere, 1991).
After subcutaneous injection of labelled parathion to mice at a
dose of 0.3 ng/g bw, the label was absorbed slowly from the
subcutaneous tissue, a major part remaining at the injection site
throughout the observation period. The amount of radiolabel in the
cardiovascular system was low, but it accumulated in the salivary
glands, brown fat, liver, kidney, and adipose tissue. Radiolabel was
also present in neural tissue and in the intestinal walls, thyroid,
spleen, and lungs. Excretion occurred primarily through the kidneys
(Fredriksson & Bigelow, 1961).
Maximal blood levels were seen 15 min after administration of
parathion as an aerosol into the trachea of rats (Nye & Donough,
1976).
Intravenous administration of parathion into the tail vein of
rats resulted in parathion and unspecified metabolites in the liver,
kidney, and plasma. Parathion and the metabolite paraoxon were also
found in small amounts in brain and fat. The peak distribution in
tissues occurred 1-2 h after injection (Gagne & Brodeur, 1972).
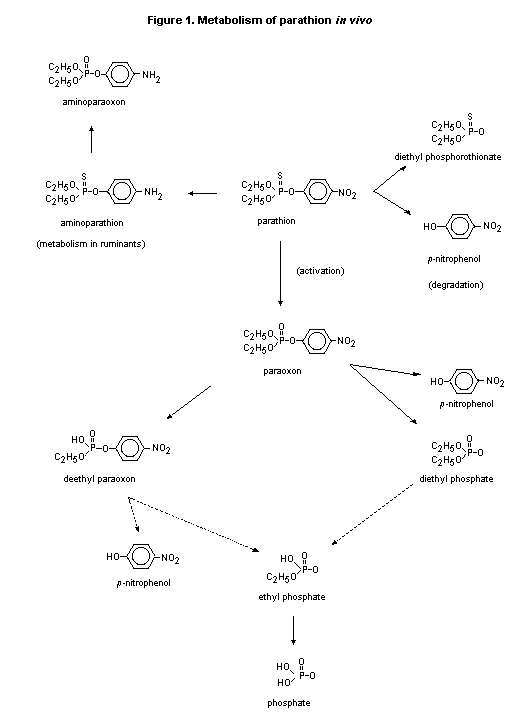
(b) Biotransformation
Two hypotheses have been put forward for the metabolism of
parathion. One is based on a requirement for two separate enzyme
systems; the other is based on a one-enzyme system of metabolism.
Interestingly, the spectrum of metabolites is the same in the two
hypotheses.
Neal (1967) demonstrated that parathion is metabolized in vitro
to paraoxon and diethyl phosphorothionate when incubated with liver
miscrosomes from rats and mice. Both metabolism to paraoxon by the
activation pathway and metabolism to diethyl phosphorothionate by a
degradation pathway require NADPH and oxygen. Differential inhibition
of the formation of diethyl phosphorothionate and paraoxon in the
presence of mixed-function oxidase inhibition indicated the presence
of either two separate enzyme systems or two different binding sites
for parathion that have a common electron transport pathway. The
separate enzyme theory was supported by Nakatsugawa et al. (1969),
who found that diethyl phosphorothionate is produced by separate liver
fractions -- one involving microsomal enzymes and the other an enzyme
that requires reduced glutathione. Evidence for the single-enzyme
hypotheses was provided by Kamataki & Neal (1976) in a study
in vitro in which parathion was incubated with a reconstituted
mixed-function oxidase enzyme system from rabbits. The authors
concluded that the metabolites of parathion, paraoxon, diethyl
phosphorothionate, and diethylphosphate, are formed by different
reactions from a common intermediate.
In a study in rats, activation and degradation were found to be
the two major pathways in the metabolism of parathion (Figure 1). The
metabolite paraoxon was generated after administration of parathion
and was further metabolized by liver esterases, so that the half-life
was 62 times shorter than that of parathion. This short half-life
provides a rationale for the fact that paraoxon has not been found to
accumulate after administration of parathion (Eigenberg et al.,
1983).
The metabolism of parathion in rats appears to be related to age
and sex, adult males metabolizing parathion more rapidly than females
and weanlings. In cattle, rumen microorganisms are believed to be
responsible for the reduction of parathion and paraoxon and for the
production of aminoparathion and aminoparaoxon. Aminoparathion was
also detected in the liver, blood, urine, and bile of humans who
ingested parathion (Chan et al., 1983).
Parathion is excreted primarily in the urine. Urinary metabolites
identified in rats include diethylphosphate, diethyl phosphoro-
thionate, de-ethyl paraoxon, de-ethyl parathion, and para-nitro-
phenol.
Two lactating, pregnant goats were fed diets containing
14C-labelled parathion (purity, 97%) at a level equivalent to
96.9 ppm for five consecutive days and were sacrificed 6 h after the
last dose. The amount of radiolabel in milk, liver, kidney, renal fat,
and muscle was determined by high-performance liquid chromatography
and ranged from 82.6% in milk to 971% in renal fat. Metabolites of
parathion in the milk and tissues were also identified by
high-performance liquid chromatography: para-acetamidophenol was
present in all the matrices examined, except milk; para-amino-
parathion was found in all matrices except muscle; parathion and
para-acetamidoparaoxon were present in all matrices; and
para-nitrophenol was detected at low levels in muscle and kidney
(Chen, 1990).
Fifteen white Leghorn laying hens were given capsules containing
1.5 mg 14C-parathion for six consecutive days. Five control birds
were available. All birds were sacrificed 6 h after the sixth dose.
Extractable radiolabel represented 52.5% in thigh muscle to 85.5% in
eggs. The metabolites para-nitrophenyl phosphate, para-acet-
amidophenol, O-ethyl- para-nitrophenyl phosphorothioate, parathion,
and para-nitrophenol were present in all of the matrices examined.
Paraoxon was present in small quantities in the liver and kidney, and
para-aminophenol was present in small quantities in eggs and fat.
para-Acetamidoparaoxon was present in all matrices except eggs. The
proposed metabolic pathways in hens involve desulfuration to oxygen
analogues, reduction of the nitro group to an amino group,
N-acetylation of the amino group, and hydrolysis of substituted phenyl
phosphates. (Chen, 1987, 1988, 1990).
(c) Effects on enzymes and other biochemical parameters
Parathion was found to pass through a maternal reservoir and
enter the fetal compartment after perfusion, resulting in about 50%
inhibition of the acetylcholinesterase activity of fetal tissue
(Benjaminov et al., 1992). It has been suggested that the metabolism
of parathion by liver microsomes is indicative of the induction of the
mixed-function oxidase system in the liver (Davis, 1975).
 2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of parathion are summarized in
Table 1.
The toxicity of parathion was not potentiated by simultaneous
oral administration of methamidophos (Flucke & Kimmerle, 1977),
temaron, or parathion methyl (Gröning, 1975). Additive acute toxicity
was reported for each of these chemicals in combination with
parathion.
(b) Short-term toxicity
Mice
Groups of five CD-1 mice of each sex received parathion at
dietary levels of 100, 200, or 400 ppm for 29 days. All of the animals
at the high dose either died or were sacrificed in a moribund
condition during the first 11 days of dosing. The mortality and
moribundity in the group at the medium dose was similar to that at the
high dose, with the exception of one surviving male. Clinical signs of
toxicity included tremors, decreased activity, and emaciation in all
treated groups. The mean body weights of males and females at the low
dose were 7-10% lower than those of controls, but food consumption
exceeded that of controls. No gross pathological changes that could be
attributed to the administration of parathion were reported in animals
at the low dose. Gastric erosion was seen in males at the two higher
doses and lung congestion in females at the high dose. There was no
NOAEL in view of the presence of cholinergic symptoms at 100 ppm,
equivalent to 15 mg/kg bw per day (Ramundo, 1979).
Groups of 15 male and 15 female Charles River COBS (ICR derived)
CD-1 mice received diets containing parathion (purity, 95.11%) at
doses of 0, 15, 50, or 100 ppm for three months. All animals survived
except for one male at the low dose and one female at the high dose.
The body weights of males at the high dose were significantly lower
than those of controls from the first week of the study until
termination. Sporadic, unsustained decreases in body weight were
reported for males at the middle dose and in females at the high dose,
and the differences in body weight were significant in weeks 1-5 and
8-9. At all other intervals, body weight was similar to controls. No
treatment-associated gross or microscopic lesions were reported.
Cholinesterase activity was not monitored in this study. The NOAEL was
50 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of the
reported decreases in body weight in males receiving 100 ppm
(Daly, 1980).
Table 1. Acute toxicity of parathion
Species Sex Route LD50 or LC50, Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male Oral 22 98 Carr & Cuthbert (1986)
Female 2
Rat Male Oral 7 NR Auletta (1984)
Female 2.6
Rat Male, female Oral 6.85 NR Owens (1976)
Rat Male Oral 13.7 NR Heimann (1982)
Rat Male, female Dermal 73 98 Carr & Cuthbert (1986)
Rabbit Male Dermal 910 NR Daly (1984b)
Female 1400
Rat Male, female Inhalation (4-h) 0.03 98 Greenough & McDonald (1986)
Rat Male Inhalation (1-h) 245 97.8 Thyssen (1972)
Female 71
Rat Male Inhalation (4-h) 77-91 97.8 Thyssen (1972)
Female 24
NR, not reported
Rats
Groups of 10 male and 10 female Wistar rats were exposed by
inhalation by head-nose to a parathion (purity, 98%) aerosol at
concentrations of 0.25, 0.92, or 3.9 mg/m3 for up to 6 h per day for
15 days. Animals of each sex at 0.92 mg/m3 showed inhibition of
plasma and erythrocyte cholinesterase activity in comparison with mean
baseline values; at the highest dose, plasma, erythrocyte, and brain
cholinesterase activities were reduced. Animals receiving the highest
dose also showed nonspecific, transient behavioural changes; females
had muscular tremors and increased mortality. With the exception of
increased organ congestion in females at tile high dose, there were no
gross or histopathological lesions that could be associated with the
administration of parathion. The NOAEL was 0.25 mg/m3 (Fauluhn,
1984).
Groups of 20 Sprague-Dawley rats of each sex were fed diets
containing parathion (purity, 95.11%) at 0, 2.5, 25, or 75 ppm for
three months. Animals were observed for clinical signs of toxicity,
and body weights and food consumption were recorded weekly. Clinical
chemical and haematological parameters and plasma and erythrocyte
cholinesterase activities were determined before the administration of
parathion and at designated intervals during the study. Brain
acetylcholinesterase activity was determined in 10 males and 10
females before assignment to the treatment groups and in groups of 10
animals of each sex per group at three months. Urinalysis was
conducted at one and three months of the study. Nine of the females at
the high dose died or were sacrificed during the study. Anogenital
staining, tremors, and emaciation were reported in females at the high
dose, which also had elevated levels of serum aspartate and alanine
aminotransferases and alkaline phosphatase and decreased haemoglobin
and haematocrit. None of these alterations in clinical pathology could
be attributed to parathion, since they were within the normal
biological ranges.
Decreases in plasma and erythrocyte cholinesterases were reported
in all treated animals. At 2.5 ppm, a statistically significant
decrease in erythrocyte acetylcholinesterase was reported in females,
amounting to 64% of the activity in controls at one month, 73% of that
at two months, and 44% of that at three months. At 25 ppm, a 20%
inhibition of plasma and erythrocyte cholinesterase activities was
seen in treated animals in comparison with controls at all collection
times. Brain acetylcholinesterase activity was significantly inhibited
in females but not males at the middle dose. At 75 ppm, there was
significant inhibition of erythrocyte and plasma cholinesterase
activities in males at two and three months and in females at all
intervals. Brain acetylcholinesterase activity was 40% of that of
controls in females and 38% in males after the third month of
treatment. The NOAEL was 2.5 ppm, equal to 0.18 mg/kg bw per day, on
the basis of a significant depression in brain acetylcholinesterase in
females (Daly, 1980b).
Rabbits
Parathion formulated with water and cremaphor was administered to
male and female New Zealand white rabbits at 0, 0.1, or 2 mg/kg bw per
day for 15 days at a volume of 0.5 ml/kg bw on shaved backs or flanks
and was left in contact with the skin for 6 h per exposure. No dermal
irritation and no compound-related effects on clinical behaviour,
clinical signs, or gross or microscopic pathological appearance were
reported. Plasma, erythrocyte, and brain cholinesterase activities
were all inhibited at 2 mg/kg bw per day; however, no cholinergic
symptoms were reported. The NOAEL was 0.1 mg/kg bw per day (Mihail &
Gröning, 1981).
Dogs
Groups of two beagle dogs of each sex were fed diets containing
parathion at 0, 1.5, 3.0, or 6 mg/kg bw per day for 14 days and were
observed for clinical signs of toxicity, mortality, food intake,
weight gain, and gross pathological lesions. All animals survived the
study. Food intake was significantly lower in males at the high dose
than in controls. There was a dose-related increase in the incidence
of vomiting in all groups receiving parathion, and after two weeks
animals at the two higher doses had lower body-weight gain and feed
efficiency than controls. No gross lesions were seen in any of the
treated dogs. Cholinesterase was not monitored in this study, but
appeared to have been affected on the basis of the clinical symptom of
vomiting. There was no NOAEL because of the presence of signs of
cholinergic poisoning at the lowest dose (Tegeris & Underwood, 1977).
Groups of 16 male and 16 female beagle dogs were randomly
assigned to groups designated to receive dietary levels of parathion
(purity, 94.4%) at 0, 0.3,1, or 3 mg/kg bw per day for 90 days.
Animals were observed daily for clinical signs of toxicity and for
survival. Clinical chemical and haematological parameters were
determined before treatment and at predetermined intervals during the
90-day period. Blood and plasma cholinesterase levels were determined
during the study, and brain cholinesterase was measured after
sacrifice. Organ weights were recorded and gross and microscopic
pathological changes were evaluated. Plasma cholinesterase activity
was significantly reduced in all treated groups in comparison with
controls; erythrocyte acetylcholinesterase activity was significantly
inhibited in all females and in males at the two higher doses. With
the exception of the effects on plasma and erythrocyte cholinesterase
activity, there were no alterations in clinical chemistry, and
haematological and gross and microscopic pathological parameters, body
weights, and food intake were also unaffected. No compound-associated
effects were reported on brain acetylcholinesterase activity. Soft
stools were observed in all groups, but the frequency and severity of
this finding could not be associated with increasing dietary levels of
parathion. There was no NOAEL (Underwood, 1978).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female B6C3F1 mice received diets
containing parathion at 0, 60, 100, or 140 ppm for 18 months. There
was no compound-related effect on survival. The body weights of males
at the highest dose were 14% lower than those of controls. Clinical
signs of toxicity reflecting the cholinergic properties of the
material were reported in all treated animals during the first month
of the study, including laboured breathing, pallor, hypoactivity, and
tremors. The increased incidence was dose-related. At 18 months, the
inhibition of plasma and erythrocyte cholinesterase was > 20% of the
control values in all groups. Brain acetylcholinesterase activity was
inhibited in males and females at the high dose, to 87% at day 10 and
85% at 18 months in males and 82% at day 10 and 76% at 18 months in
females. Brain:kidney weight ratios were significantly lower than
those of controls in female mice receiving 140 ppm; decreases were
also reported in males receiving 100 and 140 ppm. Alveolar-bronchiolar
adenomas were seen in 16/50 male mice receiving 60 ppm but not at the
next two doses. There was no NOAEL, as signs of cholinergic poisoning
were seen at all doses (Page & Heath, 1991).
Rats
Groups of 60 male and 60 female Sprague-Dawley (CrlCD:BR) rats
received diets containing parathion (purity, 95.11%) at 0, 0.5, 5, or
50 ppm for up to 28 months. The mean body weights of animals receiving
50 ppm were significantly decreased throughout the study, the
decreases ranging from 10 to 16% in males and 8 to 25% in females.
There was no corresponding decrease in food consumption. Males and
females at the high dose had slightly lowered haemoglobin and
haematocrit in comparison with controls. Significantly lower values
were reported in females at the high dose at 6, 12, and 18 months;
however, the effect does not appear to be of biological significance
as the values are within the normal range for this strain. Plasma
cholinesterase activity was reduced in males and females at the two
higher doses throughout the study. Erythrocyte acetylcholinesterase
values were reduced in animals of each sex but not to statistically or
biologically significant levels. Brain acetylcholinesterase activity
was significantly reduced in males (22% of controls) and females (18%
of controls) at the highest dose.
Treatment was associated with increased incidences of tremors,
alopecia, anogenital staining, and abnormal gait in females at the
high dose, which also had elevated alkaline phosphatase activity and
increased blood urea nitrogen; however, the reported values are within
the normal range for this strain. There were no effects on organ
weight and no gross lesions associated with administration of the test
material. The incidence of retinal degeneration was increased in
females at the high dose, and the severity of peripheral neuropathy
characterized by myelin sheath degeneration in the proximal sciatic
nerve and myelin corrugations, demyelinated lengths, and myelin
ovoids was increased in males at the high dose. There was no
treatment-associated carcinogenicity. The systemic NOAEL was 5 ppm
(equivalent to 0.25 mg/kg bw per day), and the LOAEL was 50 ppm
(2.5 mg/kg bw per day) on the basis of effects on brain, plasma, and
erythrocyte cholinesterase activity, retinal degeneration, and
peripheral neuropathy (Daly, 1984c).
Groups of 50 Wistar rats of each sex received diets containing
parathion (purity, 96.7%) at doses of 0, 2, 8, or 32 ppm for two
years; a further 15 rats of each sex received the dietary
concentrations for 12 months. Animals at the highest dose had poor
general condition and chromodacryorrhoea, and females also had
tremors, hair loss, and head tilt at greater frequency than controls;
however, the increase was not statistically significant. Females at
the highest dose experienced 36% mortality, in comparison with 22% for
controls. Plasma and erythrocyte cholinesterase activities were
inhibited by > 20% of the control level in animals of each sex at 8
or 32 ppm, the inhibition being greater at the highest dose; brain
acetylcholinesterase activity was also statistically and biologically
lower than that in controls at the highest dose. There was no
compound-related effect on urinary or haematological parameters. The
body weights of animals of each sex at the highest dose were decreased
in comparison with controls; however, there were no apparent effects
on organ weights.
Histologically, there was a nonsignificantly increased incidence
of proliferative lesions in the exocrine pancreas in males receiving 8
or 32 ppm. At 32 ppm, there were also adverse effects on the retina,
as indicated by structural degeneration and retinal atrophy. No
treatment-related oncogenic effects were reported. No significant
effects on peripheral nerves were reported at the highest dose. The
systemic NOAEL in this strain was 8 ppm (equivalent to 0.4 mg/kg bw
per day), and the LOAEL was 32 ppm (equivalent to 1.6 mg/kg bw per
day), on the basis of inhibition of brain, plasma, and erythrocyte
cholinesterase activity (Eiben, 1987).
(d) Reproductive and developmental toxicity
Rats
Groups of 24 female Sprague-Dawley rats in which mating was
confirmed by vaginal smear were given parathion (purity, 95.1.1%) by
gavage on days 6-19 of gestation at doses of 0, 0.25, 1.0, or
1.5 mg/kg bw per day. On day 20, all animals were sacrificed, the
uteri were removed, and the animals were subjected to a complete gross
post-mortem examination and examination of the reproductive system.
The numbers of live and dead fetuses, late and early resorptions,
implantation sites, and corpora lutea were recorded. Each fetus was
examined grossly to determine whether malformations were present;
one-half of each litter was evaluated for soft-tissue defects and the
other for skeletal defects. The mortality rate was increased in dams
at the high dose, which also had significantly lower corrected body
weights than controls. These animals had an increased incidence of
mucoid nasal discharge at day 15 and an increased incidence of dry
nasal discharge on day 20. No compound-related effects on fetal
development were reported. The NOAEL was 1.5 mg/kg bw per day, and the
NOAEL for maternal toxicity was 1.0 mg/kg bw per day (Schroeder,
1983).
Groups of 25 Wistar rats were fed parathion (purity, 98.8%) in a
Cremophor emulsion at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on
days 6-15 of gestation. Caesarean sections were performed on day 20.
Thirteen of the dams at the high dose died as a result of circulatory
and/or respiratory collapse, and 10 of these were found to have red
lungs at necropsy. Clinical signs of toxicity in the animals at the
high dose included tremors, rough coats, light stools, sunken flanks,
and reduced water intake. A slight, nonsignificant decrease in
body-weight gain was reported in animals receiving the highest dose.
No developmental effects were reported. The number of fetal
malformations was higher in the group at 1 mg/kg bw per day, but this
increase was driven by the increased incidence of malformations in one
litter, and there were no significant differences in the litter
incidence of fetal malformations. The NOAEL for maternal toxicity was
0.3 mg/kg bw per day, and the NOAEL for developmental toxicity was
1.0 mg/kg bw per day (Renhof, 1984).
A two-generation study of reproductive toxicity was conducted in
Sprague-Dawley rats given diets containing parathion (purity, 95.1%)
at doses of 0, 0.5, 5, or 25 ppm. Animals in the F0 generation
received the test material daily for 14 weeks before mating and
throughout mating, gestation, and lactation. Animals selected as F1
parents received parathion 18 weeks before mating and throughout the
mating, gestation, and lactation periods. F0 animals were sacrificed
after the F1 generation was weaned, and F1 animals were sacrificed
about five weeks after the F2 generation had been weaned; F2
animals were sacrificed at weaning. Tremors were observed in F0
females at the high dose, and the mean body weights of these animals
were reduced throughout the premating period and during gestation and
lactation. Tremors were also reported in F1 females at the high dose
during lactation. Slight, nonsignificant reductions in body weight
were reported in animals of each sex in the F1 generation at the
high dose. On day 21 of the lactation period, reduced mean body
weights were seen in F1 pups of each sex at 25 ppm and in F1
females at 5 ppm. The reduction was not statistically significant but
was reproduced in F2 pups of each sex at 25 ppm. The decrease in
mean pup weight may not have been a primary reproductive effect but an
effect associated with the systemic toxicity of the compound. The
NOAEL for reproductive toxicity was 25 ppm, equivalent to 1.25 mg/kg
bw per day, and that for parental toxicity was 5 ppm, equivalent to
0.25 mg/kg bw per day (Daly, 1982).
In another two-generation study, groups of 28 female
Sprague-Dawley rats were given parathion (purity, 96.7%) at dietary
levels of 0, 1, 10, or 20 ppm. F0 animals received the compound for
10 weeks before mating, and animals selected as F1 parents were
exposed for 11 weeks before mating. Toxicity was seen in F0 and F1
animals of each sex at 10 and 20 ppm, manifested as significant
reductions in plasma, erythrocyte, and/or brain cholinesterase
activities. At 10 ppm, plasma cholinesterase activity was inhibited by
> 20% in comparison with controls; inhibition of erythrocyte
acetylcholinesterase activity was not as pronounced. Brain
acetylcholinesterase activity was < 90% of control values in F0 and
F1 animals of each sex, and the reduction was considered to be
biologically significant. At 20 ppm, brain acetylcholinesterase and
plasma cholinesterase activities were markedly inhibited in females of
both generations. The body weights of dams at lactation were decreased
in both generations at 20 ppm from day 7 to day 21 in the F0
maternal animals and on day 21 in the F1 maternal animals. There
were no compound-related effects on reproductive parameters in
parental animals. In the F1 litters, reduced body weights and
reduced body-weight gains were reported at the highest dose, and
postnatal pup deaths were nonsignificantly increased on lactation days
14-21. Similar findings were not found for the F2 litters. The
parental NOAEL was 1 ppm, equivalent to 0.05 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase; the perinatal NOAEL
was 10 ppm, equivalent to 1 mg/kg bw per day, on the basis of adverse
effects on body weight of offspring at 20 ppm (Neeper-Bradley, 1990).
Rabbits
Groups of 15 female Himalayan rabbits received parathion (purity,
98.8%) by gavage at doses of 0, 0.03, 0.1, or 0.3 mg/kg bw per day on
days 6-18 of gestation. All animals were sacrificed on day 29, and the
fetuses were removed. There were no effects on maternal animals or
offspring. The NOAEL for both maternal and developmental toxicity was
0.3 mg/kg bw per day (Renhof, 1985).
(e) Genotoxicity
Studies of the genotoxicity of parathion are summarized in
Table 2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.5 ml of a test material containing parathion on an epilated area of
the skin for 4 h. Mild skin irritation, characterized by very slight
erythema and very slight oedema, occurred within 1 h. All signs of
irritation had subsided by 72 h (Carr & Cuthbert, 1986).
In another study in New Zealand white rabbits, the compound was
classified as a mild irritant, with a primary irritation index of 0.92
(Pauluhn, 1983).
The scores obtained with the Magnusson-Klegman technique in
Bor:SPF, DPHW guinea-pigs indicated that parathion is not a
sensitizing agent (Heimann, 1986).
(ii) Neurotoxicity
Groups of five beagle dogs of each sex were given parathion
(purity, 98%) for six months in gelatin capsules at doses of 0,
0.0024, 0.0079, or 0.7937 mg/kg bw per day. Plasma, erythrocyte,
brain, and retinal cholinesterase activities were determined, and
ocular assessments were made using slit lamp examinations,
retinoscopic examinations, and electroretinograms. Intra-ocular
pressure and pupillary status were also assessed. No treatment-related
effects were reported, and hence no functional ocular impairment was
observed. Plasma and erythrocyte cholinesterase activities were
inhibited in animals of each sex at the two highest doses. Brain
acetylcholinesterase activity was inhibited in the pons but not in the
cerebellum of male beagles at the highest dose. Retinal cholinesterase
was also significantly decreased animals of each sex at the highest
dose, but the decrease was not associated with morphological changes
in the eye. The NOAEL was 0.0079 mg/kg bw per day on the basis of
inhibition of brain and retinal acetylcholinesterase activities
(Atkinson, 1991a).
Groups of female Sprague-Dawley rats received parathion (purity,
98%) in the diet at levels designed to achieve 0.04, 0.4, or 4 mg/kg
bw per day, for three months. Control rats received the basal diet. At
4 mg/kg bw, decreases were seen in body weight (29%), plasma
cholinesterase (87-95%), and brain acetylcholinesterase (83.5%).
Electroretinograms revealed an increase in the latency and a decrease
in the amplitude of the b-wave. At 0.4 mg/kg bw, the plasma
cholinesterase levels were 37-42% lower than those of controls and the
latency and amplitude of the b-wave were affected in the same way as
in rats receiving 4 mg/kg bw. No morphological changes were seen in
animals at 0.4 or 4 mg/kg bw. The ocular changes observed at the two
highest doses were considered not to be significant toxicologically,
as there was wide variation in the individual and group mean values
for b-wave latency and amplitude. Furthermore, no adverse effects were
seen by electron microscopy. The NOAEL was 0.4 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase (Atkinson, 1991b).
In the 26-month carcinogenicity study conducted in Sprague-Dawley
rats, peripheral neuropathy in males was associated with the
administration of parathion at a dietary level of 50 ppm (2.5 mg/kg bw
per day). Fibres teased from the sciatic nerves of males at the high
dose showed an increased percentage of myelin corrugations and
increased demyelination (Daly, 1984a).
The potential of parathion to produce organophosphorus-induced
delayed neurotoxicity has been investigated in vivo in animals and
in vitro in human neuroblastoma cell lines. Parathion did not induce
delayed neurotoxicity in hens after subchronic exposure. Similar
conclusions were reached in a study in CD-1 mice, in which parathion
was administered orally for 30 days at a dose of 6.75 mg/kg bw per
day. There was no delayed neurotoxicity, and brain neurotoxic
esterases were not inhibited (Solimon et al., 1982).
3. Observations in humans
Parathion was given to human volunteers at doses ranging from
3 mg/day for 28 days to 6 mg/day for 43 days, and plasma and
erythrocyte cholinesterase levels were monitored. The highest dose
induced immediate depression of both types of cholinesterase, to
levels 10-15% of baseline values determined before treatment, which
persisted for two weeks after treatment was discontinued; erythrocyte
acetylcholinesterase activity was inhibited for up to 43 days after
the last dose (Moeller & Rider, 1961).
Five men received parathion at 7.5 mg/day for five days. Plasma
cholinesterase was more sensitive than erythrocyte acetylcholine-
sterase activity to the inhibitory effects of parathion, with an
average decrease of 28% on day 16 after treatment (Rider et al.,
1958).
Table 2. Results of tests for the genotoxicity of parathion
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 3.15-3150 µg/plate 98.9 Negative Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA100, < 10 000 µg/plate 97-98 Negative Lawlor & Wagner (1988)
TA1535, TA1537, TA1538
hprt mutation Chinese hamster ovary cells 0.4-1.0 µl/litre 96.7 Negative Harbell (1989)
hprt mutation Chinese hamster ovary cells 0.03-0.2 µl/litre 97-98 Suspect Yang (1988)
Unscheduled DNA Human WI-38 cell line 10-5 µl/ml NR Positive Simmon et al. (1979)
synthesis 10-6 µl/ml Negativea
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2µl/ml 97-98 Negative Curren (1988)
synthesis
DNA damage E coli K12 and W-3110 625-10 000 µg/plate 98 Negative Herbold (1985)
Cytogenetic damage Human lymphocytes 9.8, 20, 39 µg/mlb 98 Negative Jensen (1991)
78, 156, 313 µg/mla
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw orally 98.8 Negative Herbold (1986)
Dominant lethal mutation Mouse 10 mg/kg bw orally NR Negative Degraeve et al. (1979)
Micronucleus formation Mouse bone marrow 2 × 5, 2 × 10 mg/kg bw 95.9 Negative Herbold (1982)
orally
Micronucleus formation Mouse bone marrow 10-65 mg/kg bw 97-98 Negative Putman (1988)
NR, not reported
a With metabolic activation
b Without metabolic activation
Comments
Parathion is readily absorbed from the respiratory and digestive
tracts and is excreted primarily in the urine. Two hypotheses have
been proposed for its metabolism; however, the metabolic spectrum is
the same in both. Parathion is metabolized to paraoxon and diethyl
phosphorothioic acid. Paraoxon is further metabolized, and following
its oral administration to rats diethyl phosphate, diethyl
phosphorothioate, de-ethyl paraoxon, and para-nitrophenol were
identified in the urine. In cattle, ruminal microorganisms are
believed to be responsible for the production of aminoparathion and
aminoparaoxon.
Parathion is extremely hazardous when given orally (LD50 =
2 mg/kg bw) or by inhalation (4-h LC50 = 0.03 mg/litre) and
moderately hazardous when given dermally (LD50 = 73 mg/kg bw). The
compound has been characterized in studies in laboratory animals as a
mild dermal and ocular irritant and a s a non-sensitizing agent. When
it was administered with other organophosphate pesticides, its toxic
effects were not potentiated. WHO has classified parathion as
'extremely hazardous'.
In a three-week study in rabbits treated dermally, the NOAEL was
0.1 mg/kg bw per day on the basis of depression of plasma,
erythrocyte, and brain cholinesterase activities at 2 mg/kg bw per
day. In a three-week study by inhalation in rats, the NOAEL was
0.9 mg/litre on the basis of decreases in brain, plasma, and
erythrocyte cholinesterase activity. In a 14-day study in dogs,
parathion was administered orally at doses of 0, 1.5, 3, or 6 mg/kg bw
per day. There was no NOAEL, as clinical cholinergic signs were
observed at the lowest dose tested. Cholinesterase activity was not
monitored in this study. In a 90-day study in dogs at doses of 0, 0.3,
1, or 3 mg/kg bw per day, the NOAEL was 3 mg/kg bw per day.
Cholinesterase activity was not measured.
In a 29-day study in mice at doses of 0, 100, 200, or 400 ppm
(equivalent to 15, 30, and 60 mg/kg bw per day), clinical signs of
toxicity were reported in all groups. Cholinesterase activity was not
determined, and there was no NOAEL. In a 90-day study in mice at
dietary concentrations of 0, 15, 50, or 100 ppm, the NOAEL was 50 ppm
(equivalent to 7.5 mg/kg bw per day) on the basis of decreased body
weights of males. Cholinesterase activity was not monitored. When
parathion was administered to rats for 90 days at dietary
concentrations of 0, 2.5, 25, or 75 ppm, the NOAEL was 2.5 ppm (equal
to 0.2 mg/kg bw per day), on the basis of depression of brain
acetylcholinesterase.
In a two-year study in rats, parathion was not associated with
carcinogenicity when administered at dietary concentrations of 0, 0.5,
5, or 50 ppm. The NOAEL for systemic toxicity was 5 ppm (equivalent to
0.25 mg/kg bw per day) on the basis of decreased brain, plasma, and
erythrocyte cholinesterase activity, retinal atrophy, and increased
severity of degenerative changes in the sciatic nerve. In another
study in rats, given dietary levels of 0, 2, 8, or 32 ppm for two
years, there was again no evidence of carcinogenicity. The NOAEL for
systemic toxicity was 8 ppm (equivalent to 0.4 mg/kg bw per day) on
the basis of decreases in brain acetylcholinesterase activity and
retinal atrophy. No effects on sciatic nerves were reported at the
highest dose tested.
In mice receiving dietary concentrations of parathion at 0, 60,
100, or 140 ppm for 18 months, there was no NOAEL, as cholinergic
signs were seen at all doses.
Two studies of developmental toxicity were conducted in rats. In
the first study, parathion was administered by gavage at doses of 0,
0.25, 1, or 1.5 mg/kg bw on gestation days 6-19. The NOAEL for
maternal toxicity was 1 mg/kg bw per day on the basis of increased
mortality, and the NOAEL for developmental toxicity was 1.5 mg/kg bw
per day. In the second study, parathion was administered by gavage at
doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on gestation days 6-15.
The NOAEL for developmental toxicity was 1 mg/kg bw per day and that
for maternal toxicity was 0.3 mg/kg bw per day on the basis of
increased mortality and clinical signs of toxicity.
Two studies of developmental toxicity were conducted in rabbits.
In the first study, parathion was administered by gavage on gestation
days 7-19 at 1,4, or 16 mg/kg bw per day. The NOAEL for developmental
toxicity was 16 mg/kg bw per day, and the NOAEL for maternal toxicity
was 4 mg/kg bw per day on the basis of decreased body-weight gain. In
the second study, parathion was administered by gavage on days 6-18 of
gestation at 0, 0.03, 0.1, or 0.3 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 0.3 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats, doses
of 0, 0.5, 5, or 25 ppm were administered in the diet. Dams at the
highest dose had tremors, and a reduction in body weight was seen
during premating, gestation, and lactation. The NOAEL for reproductive
toxicity was 25 ppm (equivalent to 1.2 mg/kg bw per day); the NOAEL
for maternal toxicity was 5 ppm (equivalent to 0.25 mg/kg bw per day)
on the basis of the observation of tremors in F0 and F1 females.
In the second study, parathion was administered at dietary
concentrations of 0, 1, 10 or 20 ppm. The NOAEL for reproductive
toxicity was 20 ppm (equivalent to 1 mg/kg bw per day); the NOAEL for
perinatal toxicity was 10 ppm (equivalent to 1 mg/kg bw per day) on
the basis of reduced body weights; and the NOAEL for maternal toxicity
was 1 ppm (equivalent to 0.05 mg/kg bw per day) on the basis of
decreased brain acetylcholinesterase activity.
Special studies were conducted to assess the ocular toxicity of
parathion. When parathion was administered to dogs at doses of 0,
0.002, 0.008, or 0.8 mg/kg bw per day for six months, no functional
impairment of the eye was observed. The NOAEL was 0.008 mg/kg bw per
day on the basis of depression of brain and retinal acetylcholine-
sterase activity. In a three-month study of toxicity in female rats,
parathion was administered at levels of 0, 0.04, 0.4, or 4 mg/kg bw
per day. The NOAEL was 0.4 mg/kg bw per day on the basis of depression
of brain acetylcholinesterase activity. No significant effects on
ocular toxicity were reported at any dose.
Parathion was not associated with organophosphorus-induced
delayed neurotoxicity in hens, but it induced demyelination in the
peripheral nerves of rats at a dietary level of 50 ppm (equivalent to
2.5 mg/kg bw per day). The NOAEL was 0.25 mg/kg bw per day.
In a study conducted earlier in humans, an NOAEL of 7.5 mg/day
was determined on the basis of lack of effect on erythrocyte
acetylcholinesterase.
Parathion has been adequately tested for genotoxicity in a range
of tests in vitro and in vivo. The Meeting concluded that
parathion is not genotoxic.
An ADI of 0-0.004 mg/kg bw was established on the basis of an
NOAEL of 0.4 mg/kg bw per day in the two-year study in rats for
retinal atrophy and inhibition of brain acetylcholinesterase at the
higher dose. A safety factor of 100 was used. Lower NOAELs in animals,
based only on inhibition of erythrocyte or brain acetylcholinesterase,
were not considered relevant because of the availability of an NOAEL
for erythrocyte acetyl-cholinesterase inhibition in humans, which was
0.1 mg/kg bw per day.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (90-day study of
toxicity)
Rat: 1 ppm, equivalent to 0.05 mg/kg bw per day (study of
reproductive toxicity)
2.5 ppm, equal to 0.18 mg/kg bw per day (90-day study of
toxicity)
8 ppm, equivalent to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Dog: 0.008 mg/kg bw per day (six-month study of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.01 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 7.5 mg/day (highest
oral dose), corresponding to about 0.1 mg/kg bw per day, in humans.
This was based on the absence of inhibition of erythrocyte
acetylcholinesterase.
Studies that would provide information useful for continued evaluation
of the compound
Further observation in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion
Exposure Relevant route, study type, species Results, remarks
Short-term ( 1-7 days) Skin, irritation, rabbit Irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Non-sensitizing
Oral, toxicity, rat LD50 = 2 mg/kg bw
Dermal, toxicity, rat LD50 = 73 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.03 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 1 mg/kg bw per day, based on
decreased brain acetylcholinesterase. No
dermal effect
Repeated inhalation, 21 days, toxicity, rat NOAEL = 0.92 mg/m3 based on decreased
brain acetylcholinesterase
Repeated oral, reproductive toxicity, rat NOAEL = 0.05 mg/kg bw per day for
maternal toxicity, decreased brain
cholinesterase; NOAEL = 1 mg/kg bw per
day for reproductive toxicity; NOAEL =
1 mg/kg bw per day for perinatal toxicity
based on reduced body weight
Repeated oral, developmental, NOAEL = 1 mg/ kg bw per day for developmental
toxicity, rat toxicity; NOAEL = 0.3 mg/kg per
day for maternal toxicity based on increased
mortality and cholinergic signs
Repeated oral, developmental, NOAEL >0.3 mg/kg bw per day for
toxicity, rabbit maternal and developmental toxicity
Long-term (> one year) Repeated oral, two years, toxicity and NOAEL = 0.25 mg/kg bw per day based on
carcinogenicity, rat lowered brain acetylcholinesterase. No
carcinogenicity
References
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice.
Pestic. Biochem. Physiol., 16, 38-46.
Atkinson, J.E. (1991a) A six month oral study of ethyl parathion in
dogs with specific emphasis on ocular effects. Unpublished report
No. 89-3439 prepared by Biodynamics, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Atkinson, J.E. (1991b) A three month oral study in rats via the diet
with ethyl parathion to investigate ocular effects and cholin
activity. Unpublished report No. 89-3469 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Auletta, C.S. (1984) Acute oral toxicity in rats -- Acute dermal
toxicity in rabbits. Unpublished report No. 4997-84, 5505-84
prepared by Biodynamics Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Benjaminov, O., Hoffer, E., Taitelman, U., Urbak, B. & Brandis, L.
(1992) Parathion transfer and acetycholme activity in
an in vitro perfused human placenta. Vet. Hum. Toxicol.,
34, 10-12.
Bucks, D.A.W., Hinz, R.S., Sarason, R., Maibach, H.I. & Guy, R.H.
(1990) In vivo percutaneous absorption of chemicals -- A multiple
dose study in rhesus monkeys. Chem. Toxicol., 28, 129.
Carr, S.M.A. & Cuthbert, J.A. (1986) Ethyl parathion 98% technical
acute toxicity tests. Unpublished report No. 234117 prepared by
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Chan, L.T.F., Crowley, R.J. & Geyer, R. (1983) Detection and analysis
of aminoparathion in human postmortem specimens.
J. Forensic Sci., 28, 122-127.
Chang, S.K. & Riviere, J.E. (1991) Percutaneous absorption of
parathion in vitro in porcine skin: Effects of dose, temperature,
humidity and perfusate composition on absorptive flux.
Fundam. Appl. Toxicol., 17, 494-504.
Cheng, T. (1990) Ethyl parathion -- Nature of the residues in
livestock -- Laying hens. Unpublished report No. HLA 6222-100,
prepared by Hazleton Laboratories America, Inc. Submitted to WHO
by Cheminova Agro AS, Lemvig, Denmark.
Cheng, T (1987, 1988, 1990) Ethyl parathion -- Nature of the residue
in livestock -- Lactating goats. Unpublished report
No. HLA 6222-101, prepared by Hazleton Laboratories America, Inc.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepotocytes. Unpublished report prepared by Microbiological
Associates, Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1980a) A three month feed study of ethyl parathion in
mice. Unpublished report No. 77-2052 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1980b) A three month feeding study of ethyl parathion in
rats. Unpublished report No. 77-2054 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of ethyl
parathion in rats. Unpublished report No. 80-2457 prepared by
Biodynamics Inc., East Millstone, New Jersey, USA Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1984a) Acute oral toxicity study in rats. Unpublished
report No. 4997-84, prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984b) Acute dermal toxicity study in rabbits. Unpublished
report No. 5505-84 prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984c) A two year chronic feeding study of ethyl parathion
in rats. Unpublished report No. 77-2055 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Davis, J.E. (1975) The metabolism of parathion by liver microsomal
mixed-function oxidases. Dissertation, University of Miami,
Florida, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Degraeve, N., Moutschen, J., Moutschen-Dahmen, M., Gilot-Delhalle, J.,
Colizzi, A., Houbrechts, N. & Cholett, M.C. (1979) Genetic
effects of organophosphate insecticides in mouse (Abstract).
Mutat. Res., 64, 131.
Eiben, R. (1987) Parathion study for chronic toxicity and
cancerogenicity in Wistar rats. Unpublished report No. 16305 from
Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Eigenberg, D.A., Pazdernik, T.L. & Doull, J. (1983) Hernofusion and
pharmacokinetic studies with parathion and parathionoxon in the
rat and dog. Drug Metab. Disposition, 11, 366-370.
Flucke, W. & Kimmerle, G. (1977) Studies of oral acute toxicity of
simultaneously administered methamidophos and parathion ethyl.
Unpublished report from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Fredricksson, T. & Bigelow, J.K. (1-961) Tissue distribution of 32P
labeled parathion. Arch. Environ. Health, 2, 663-667.
Gagne, J. & Brodeur, J. (1972) Metabolic studies on the mechanisms of
increased susceptibility of weanling rats to parathion.
Can. J. Physiol. Pharmacol., 50, 902-915.
Greenough, R.J. & McDonald, P. (1986) Ethyl ara 98% technical: Acute
inhalation toxicity study in rats. Unpublished report
No. 632295 prepared by Inveresk Research International,
Musselburgh, United Kingdom. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Gröning, P. (1975) Tamaron-Special toxicity studies (potentiation).
Unpublished report prepared by Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Harbell, J.W. (1989) CHO/HGPRT confirmatory mutation assay with ethyl
parathion? Unpublished report prepared by Microbiological
Assocites Inc., Musselburgh, United Kingdom. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1982) E-605-ethyl study for acute oral toxicity.
Unpublished report No. 11230 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1986) E-605-ethyl study for skin sensitizing effect on
guinea pigs. Unpublished report No. 14999 from Bayer AG.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1982) Parathion (E-605-ethyl active ingredient) --
Micronucleus test on mouse to evaluate for mutagenic effect.
Unpublished report No. 10777 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1985) E-605 (c.n. parathion) Pol A1-test on E-coli to
evaluate for potential DNA damage. Unpublished report
No. 10777 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Herbold, B.A. (1986) E-605 (c.m. Parathion) -- Dominant lethal test on
male mouse to evaluate for mutagenic effect. Unpublished report
No. 14224 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Jensen, J.C. (1991) Mutagenicity test: In vitro mammalian cytogenetic
test in human lymphocytes performed with parathion-ethyl, Batch
No. 70818-01. Unpublished report prepared by Scaatox. Submitted
to WHO by Cheminova Agro AS, Lemvig, Denmark.
Kamataki, T. & Neal, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothionate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfer
atom. Mol. Pharmacol., 12, 933-944.
Lawlor, T.E. & Wagner, V.O. (1988) Ethyl parathion (97-98% technical)
-- Salmonella/mammalian-microsome plate incorporation
mutagenicity assay (Ames test) with a confirmatory assay.
Unpublished report No. USAE 605220388 prepared by Microbiological
Associates Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Mihail, F. & Gröning, P. (1981) E-605. Ethylactive ingredient/subacute
cutaneous toxicity study on rabbits. Unpublished report No 10342
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Moeller, H.S. & Rider, J.A. (1961) Studies on the anticholinesterase
effect of parathion and methyl parathion in humans (Abstract).
Fed Proc., 20, 434.
Nakatsugawa, T., Tolman, N.U. & Dahm, P.A. (1969) Degradation of
parathion in the rat. Biochem. 18, 1103-1114.
Neal, R.A. (1967) Studies on the metabolism of diethyl 4-nitrophenyl
phosphorothionate (parathion) in vitro. Biochem. J.,
103, 183-191.
Neeper-Bradley, T.L. (1990) Two-generation reproduction study of ethyl
parathion technical administered in the diet of CD (Sprague
Dawley) rats. Unpublished report from Busky Run Research Center,
Pennsylvania, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Nye, D.E. & Donough, H.W. (1976) Fate of insecticides administered
endotracheally to rats. Bull. Environ. Contam. Toxicol.,
15, 291-296.
Oesch, F. (1977) Ames test for Folidol E-605 (parathion). Unpublished
report No. E605301177. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Owens, E.J. (1976) The effect of ethyl parathion in the rat and dog
after acute and subacute inhalation and oral administration.
Technical report No. AMRL TR-76-1215 from Aerospace Medical
Research Laboratories, pp. 203-222. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Page, J.G. & Heath, G.E. (1991) Carcinogenicity study of ethyl
parathion administered by dosed feed to B6C3F1 mice.
Unpublished report No. E605210291 from Southern Research
Institute, Alabama, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Pauluhn, J. (1983) E605-Ethyl/Study for dermal and mucous membrane
irritant effects. Unpublished report No. 11401 from Bayer AG
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Pauluhn, J. (1984) E605 (c.m. parathion) -- Study for subacute
inhalative toxicity in the rat (exposure 15 × 6 hours).
Unpublished report No. 12395 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Putman, D.L. (1988) Micronucleus cytogenetic assay in mice.
Unpublished report No. T4772.122 prepared by Microbiological
Associates, Inc., USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Ramundo, J. (1979) A 4 week pilot study in mice with ethyl parathion.
Unpublished report No. 77-2051 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS. Lemvig, Denmark.
Renhof, M. (1984) Parathion -- Study for embryotoxic effects in rats
after oral administration. Unpublished report No. 12909 from
Bayer AG. Submitted to WHO by Cheminova Agro AS. Lemvig, Denmark.
Renhof, M. (1985) Parathion -- Study for embryotoxic effects on
rabbits after oral administration. Unpublished report No. 13288
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark
Rider, J.A., Moeller, H.C., Swader, J.I. & Weilerstein, R.W. (1958)
The effect of parathion on human red blood cell and plasma
cholinesterase. Arch. Ind. Health, 18, 442-445.
Schroeder (1983) A teratogenicity study in rats with ethyl parathion.
Unpublished report No. 82-2644 prepared by Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Simmon, V.F., Mitchell, R.D. & Jorgenson, T.A. (1976) In vitro
mutagenic studies on twenty pesticides. Toxicol. Appl.
Pharmacol., 37, 109.
Soliman, S.A., Farmer, J. & Curley, A. (1982) Is delayed neurotoxicity
a property of all organophosphorus compounds? A study with a
model compound: parathion. Toxicology, 23, 267-279.
Tegeris, A.S. & Underwood, P.C. (1977) Fourteen day feeding study in
the dog. Ethyl parathion. Unpublished report No. 77-113 prepared
by Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Thyssen, J. (1972) E-605-Ethyl -- Study for acute inhalation toxicity.
Unpublished report No. 8209 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Underwood, P.C. (1978) Ethyl parathion: Ninety day feeding to dogs.
Unpublished report No. USAE 65060378 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Weber, H., Patzchke, K. & Wegner, L.A. (1980) Parathion C14 (benzene
ring-labelled compound): Biokinetic studies in rats. Unpublished
report No. PH8820 from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Yang, L.L. (1988) Ethyl parathion (97/98% technical) CHO/HGPRT
mutation assay. Unpublished report No. USAE 605280388 from
Microbiological Associates Inc., USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of parathion are summarized in
Table 1.
The toxicity of parathion was not potentiated by simultaneous
oral administration of methamidophos (Flucke & Kimmerle, 1977),
temaron, or parathion methyl (Gröning, 1975). Additive acute toxicity
was reported for each of these chemicals in combination with
parathion.
(b) Short-term toxicity
Mice
Groups of five CD-1 mice of each sex received parathion at
dietary levels of 100, 200, or 400 ppm for 29 days. All of the animals
at the high dose either died or were sacrificed in a moribund
condition during the first 11 days of dosing. The mortality and
moribundity in the group at the medium dose was similar to that at the
high dose, with the exception of one surviving male. Clinical signs of
toxicity included tremors, decreased activity, and emaciation in all
treated groups. The mean body weights of males and females at the low
dose were 7-10% lower than those of controls, but food consumption
exceeded that of controls. No gross pathological changes that could be
attributed to the administration of parathion were reported in animals
at the low dose. Gastric erosion was seen in males at the two higher
doses and lung congestion in females at the high dose. There was no
NOAEL in view of the presence of cholinergic symptoms at 100 ppm,
equivalent to 15 mg/kg bw per day (Ramundo, 1979).
Groups of 15 male and 15 female Charles River COBS (ICR derived)
CD-1 mice received diets containing parathion (purity, 95.11%) at
doses of 0, 15, 50, or 100 ppm for three months. All animals survived
except for one male at the low dose and one female at the high dose.
The body weights of males at the high dose were significantly lower
than those of controls from the first week of the study until
termination. Sporadic, unsustained decreases in body weight were
reported for males at the middle dose and in females at the high dose,
and the differences in body weight were significant in weeks 1-5 and
8-9. At all other intervals, body weight was similar to controls. No
treatment-associated gross or microscopic lesions were reported.
Cholinesterase activity was not monitored in this study. The NOAEL was
50 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of the
reported decreases in body weight in males receiving 100 ppm
(Daly, 1980).
Table 1. Acute toxicity of parathion
Species Sex Route LD50 or LC50, Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male Oral 22 98 Carr & Cuthbert (1986)
Female 2
Rat Male Oral 7 NR Auletta (1984)
Female 2.6
Rat Male, female Oral 6.85 NR Owens (1976)
Rat Male Oral 13.7 NR Heimann (1982)
Rat Male, female Dermal 73 98 Carr & Cuthbert (1986)
Rabbit Male Dermal 910 NR Daly (1984b)
Female 1400
Rat Male, female Inhalation (4-h) 0.03 98 Greenough & McDonald (1986)
Rat Male Inhalation (1-h) 245 97.8 Thyssen (1972)
Female 71
Rat Male Inhalation (4-h) 77-91 97.8 Thyssen (1972)
Female 24
NR, not reported
Rats
Groups of 10 male and 10 female Wistar rats were exposed by
inhalation by head-nose to a parathion (purity, 98%) aerosol at
concentrations of 0.25, 0.92, or 3.9 mg/m3 for up to 6 h per day for
15 days. Animals of each sex at 0.92 mg/m3 showed inhibition of
plasma and erythrocyte cholinesterase activity in comparison with mean
baseline values; at the highest dose, plasma, erythrocyte, and brain
cholinesterase activities were reduced. Animals receiving the highest
dose also showed nonspecific, transient behavioural changes; females
had muscular tremors and increased mortality. With the exception of
increased organ congestion in females at tile high dose, there were no
gross or histopathological lesions that could be associated with the
administration of parathion. The NOAEL was 0.25 mg/m3 (Fauluhn,
1984).
Groups of 20 Sprague-Dawley rats of each sex were fed diets
containing parathion (purity, 95.11%) at 0, 2.5, 25, or 75 ppm for
three months. Animals were observed for clinical signs of toxicity,
and body weights and food consumption were recorded weekly. Clinical
chemical and haematological parameters and plasma and erythrocyte
cholinesterase activities were determined before the administration of
parathion and at designated intervals during the study. Brain
acetylcholinesterase activity was determined in 10 males and 10
females before assignment to the treatment groups and in groups of 10
animals of each sex per group at three months. Urinalysis was
conducted at one and three months of the study. Nine of the females at
the high dose died or were sacrificed during the study. Anogenital
staining, tremors, and emaciation were reported in females at the high
dose, which also had elevated levels of serum aspartate and alanine
aminotransferases and alkaline phosphatase and decreased haemoglobin
and haematocrit. None of these alterations in clinical pathology could
be attributed to parathion, since they were within the normal
biological ranges.
Decreases in plasma and erythrocyte cholinesterases were reported
in all treated animals. At 2.5 ppm, a statistically significant
decrease in erythrocyte acetylcholinesterase was reported in females,
amounting to 64% of the activity in controls at one month, 73% of that
at two months, and 44% of that at three months. At 25 ppm, a 20%
inhibition of plasma and erythrocyte cholinesterase activities was
seen in treated animals in comparison with controls at all collection
times. Brain acetylcholinesterase activity was significantly inhibited
in females but not males at the middle dose. At 75 ppm, there was
significant inhibition of erythrocyte and plasma cholinesterase
activities in males at two and three months and in females at all
intervals. Brain acetylcholinesterase activity was 40% of that of
controls in females and 38% in males after the third month of
treatment. The NOAEL was 2.5 ppm, equal to 0.18 mg/kg bw per day, on
the basis of a significant depression in brain acetylcholinesterase in
females (Daly, 1980b).
Rabbits
Parathion formulated with water and cremaphor was administered to
male and female New Zealand white rabbits at 0, 0.1, or 2 mg/kg bw per
day for 15 days at a volume of 0.5 ml/kg bw on shaved backs or flanks
and was left in contact with the skin for 6 h per exposure. No dermal
irritation and no compound-related effects on clinical behaviour,
clinical signs, or gross or microscopic pathological appearance were
reported. Plasma, erythrocyte, and brain cholinesterase activities
were all inhibited at 2 mg/kg bw per day; however, no cholinergic
symptoms were reported. The NOAEL was 0.1 mg/kg bw per day (Mihail &
Gröning, 1981).
Dogs
Groups of two beagle dogs of each sex were fed diets containing
parathion at 0, 1.5, 3.0, or 6 mg/kg bw per day for 14 days and were
observed for clinical signs of toxicity, mortality, food intake,
weight gain, and gross pathological lesions. All animals survived the
study. Food intake was significantly lower in males at the high dose
than in controls. There was a dose-related increase in the incidence
of vomiting in all groups receiving parathion, and after two weeks
animals at the two higher doses had lower body-weight gain and feed
efficiency than controls. No gross lesions were seen in any of the
treated dogs. Cholinesterase was not monitored in this study, but
appeared to have been affected on the basis of the clinical symptom of
vomiting. There was no NOAEL because of the presence of signs of
cholinergic poisoning at the lowest dose (Tegeris & Underwood, 1977).
Groups of 16 male and 16 female beagle dogs were randomly
assigned to groups designated to receive dietary levels of parathion
(purity, 94.4%) at 0, 0.3,1, or 3 mg/kg bw per day for 90 days.
Animals were observed daily for clinical signs of toxicity and for
survival. Clinical chemical and haematological parameters were
determined before treatment and at predetermined intervals during the
90-day period. Blood and plasma cholinesterase levels were determined
during the study, and brain cholinesterase was measured after
sacrifice. Organ weights were recorded and gross and microscopic
pathological changes were evaluated. Plasma cholinesterase activity
was significantly reduced in all treated groups in comparison with
controls; erythrocyte acetylcholinesterase activity was significantly
inhibited in all females and in males at the two higher doses. With
the exception of the effects on plasma and erythrocyte cholinesterase
activity, there were no alterations in clinical chemistry, and
haematological and gross and microscopic pathological parameters, body
weights, and food intake were also unaffected. No compound-associated
effects were reported on brain acetylcholinesterase activity. Soft
stools were observed in all groups, but the frequency and severity of
this finding could not be associated with increasing dietary levels of
parathion. There was no NOAEL (Underwood, 1978).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female B6C3F1 mice received diets
containing parathion at 0, 60, 100, or 140 ppm for 18 months. There
was no compound-related effect on survival. The body weights of males
at the highest dose were 14% lower than those of controls. Clinical
signs of toxicity reflecting the cholinergic properties of the
material were reported in all treated animals during the first month
of the study, including laboured breathing, pallor, hypoactivity, and
tremors. The increased incidence was dose-related. At 18 months, the
inhibition of plasma and erythrocyte cholinesterase was > 20% of the
control values in all groups. Brain acetylcholinesterase activity was
inhibited in males and females at the high dose, to 87% at day 10 and
85% at 18 months in males and 82% at day 10 and 76% at 18 months in
females. Brain:kidney weight ratios were significantly lower than
those of controls in female mice receiving 140 ppm; decreases were
also reported in males receiving 100 and 140 ppm. Alveolar-bronchiolar
adenomas were seen in 16/50 male mice receiving 60 ppm but not at the
next two doses. There was no NOAEL, as signs of cholinergic poisoning
were seen at all doses (Page & Heath, 1991).
Rats
Groups of 60 male and 60 female Sprague-Dawley (CrlCD:BR) rats
received diets containing parathion (purity, 95.11%) at 0, 0.5, 5, or
50 ppm for up to 28 months. The mean body weights of animals receiving
50 ppm were significantly decreased throughout the study, the
decreases ranging from 10 to 16% in males and 8 to 25% in females.
There was no corresponding decrease in food consumption. Males and
females at the high dose had slightly lowered haemoglobin and
haematocrit in comparison with controls. Significantly lower values
were reported in females at the high dose at 6, 12, and 18 months;
however, the effect does not appear to be of biological significance
as the values are within the normal range for this strain. Plasma
cholinesterase activity was reduced in males and females at the two
higher doses throughout the study. Erythrocyte acetylcholinesterase
values were reduced in animals of each sex but not to statistically or
biologically significant levels. Brain acetylcholinesterase activity
was significantly reduced in males (22% of controls) and females (18%
of controls) at the highest dose.
Treatment was associated with increased incidences of tremors,
alopecia, anogenital staining, and abnormal gait in females at the
high dose, which also had elevated alkaline phosphatase activity and
increased blood urea nitrogen; however, the reported values are within
the normal range for this strain. There were no effects on organ
weight and no gross lesions associated with administration of the test
material. The incidence of retinal degeneration was increased in
females at the high dose, and the severity of peripheral neuropathy
characterized by myelin sheath degeneration in the proximal sciatic
nerve and myelin corrugations, demyelinated lengths, and myelin
ovoids was increased in males at the high dose. There was no
treatment-associated carcinogenicity. The systemic NOAEL was 5 ppm
(equivalent to 0.25 mg/kg bw per day), and the LOAEL was 50 ppm
(2.5 mg/kg bw per day) on the basis of effects on brain, plasma, and
erythrocyte cholinesterase activity, retinal degeneration, and
peripheral neuropathy (Daly, 1984c).
Groups of 50 Wistar rats of each sex received diets containing
parathion (purity, 96.7%) at doses of 0, 2, 8, or 32 ppm for two
years; a further 15 rats of each sex received the dietary
concentrations for 12 months. Animals at the highest dose had poor
general condition and chromodacryorrhoea, and females also had
tremors, hair loss, and head tilt at greater frequency than controls;
however, the increase was not statistically significant. Females at
the highest dose experienced 36% mortality, in comparison with 22% for
controls. Plasma and erythrocyte cholinesterase activities were
inhibited by > 20% of the control level in animals of each sex at 8
or 32 ppm, the inhibition being greater at the highest dose; brain
acetylcholinesterase activity was also statistically and biologically
lower than that in controls at the highest dose. There was no
compound-related effect on urinary or haematological parameters. The
body weights of animals of each sex at the highest dose were decreased
in comparison with controls; however, there were no apparent effects
on organ weights.
Histologically, there was a nonsignificantly increased incidence
of proliferative lesions in the exocrine pancreas in males receiving 8
or 32 ppm. At 32 ppm, there were also adverse effects on the retina,
as indicated by structural degeneration and retinal atrophy. No
treatment-related oncogenic effects were reported. No significant
effects on peripheral nerves were reported at the highest dose. The
systemic NOAEL in this strain was 8 ppm (equivalent to 0.4 mg/kg bw
per day), and the LOAEL was 32 ppm (equivalent to 1.6 mg/kg bw per
day), on the basis of inhibition of brain, plasma, and erythrocyte
cholinesterase activity (Eiben, 1987).
(d) Reproductive and developmental toxicity
Rats
Groups of 24 female Sprague-Dawley rats in which mating was
confirmed by vaginal smear were given parathion (purity, 95.1.1%) by
gavage on days 6-19 of gestation at doses of 0, 0.25, 1.0, or
1.5 mg/kg bw per day. On day 20, all animals were sacrificed, the
uteri were removed, and the animals were subjected to a complete gross
post-mortem examination and examination of the reproductive system.
The numbers of live and dead fetuses, late and early resorptions,
implantation sites, and corpora lutea were recorded. Each fetus was
examined grossly to determine whether malformations were present;
one-half of each litter was evaluated for soft-tissue defects and the
other for skeletal defects. The mortality rate was increased in dams
at the high dose, which also had significantly lower corrected body
weights than controls. These animals had an increased incidence of
mucoid nasal discharge at day 15 and an increased incidence of dry
nasal discharge on day 20. No compound-related effects on fetal
development were reported. The NOAEL was 1.5 mg/kg bw per day, and the
NOAEL for maternal toxicity was 1.0 mg/kg bw per day (Schroeder,
1983).
Groups of 25 Wistar rats were fed parathion (purity, 98.8%) in a
Cremophor emulsion at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on
days 6-15 of gestation. Caesarean sections were performed on day 20.
Thirteen of the dams at the high dose died as a result of circulatory
and/or respiratory collapse, and 10 of these were found to have red
lungs at necropsy. Clinical signs of toxicity in the animals at the
high dose included tremors, rough coats, light stools, sunken flanks,
and reduced water intake. A slight, nonsignificant decrease in
body-weight gain was reported in animals receiving the highest dose.
No developmental effects were reported. The number of fetal
malformations was higher in the group at 1 mg/kg bw per day, but this
increase was driven by the increased incidence of malformations in one
litter, and there were no significant differences in the litter
incidence of fetal malformations. The NOAEL for maternal toxicity was
0.3 mg/kg bw per day, and the NOAEL for developmental toxicity was
1.0 mg/kg bw per day (Renhof, 1984).
A two-generation study of reproductive toxicity was conducted in
Sprague-Dawley rats given diets containing parathion (purity, 95.1%)
at doses of 0, 0.5, 5, or 25 ppm. Animals in the F0 generation
received the test material daily for 14 weeks before mating and
throughout mating, gestation, and lactation. Animals selected as F1
parents received parathion 18 weeks before mating and throughout the
mating, gestation, and lactation periods. F0 animals were sacrificed
after the F1 generation was weaned, and F1 animals were sacrificed
about five weeks after the F2 generation had been weaned; F2
animals were sacrificed at weaning. Tremors were observed in F0
females at the high dose, and the mean body weights of these animals
were reduced throughout the premating period and during gestation and
lactation. Tremors were also reported in F1 females at the high dose
during lactation. Slight, nonsignificant reductions in body weight
were reported in animals of each sex in the F1 generation at the
high dose. On day 21 of the lactation period, reduced mean body
weights were seen in F1 pups of each sex at 25 ppm and in F1
females at 5 ppm. The reduction was not statistically significant but
was reproduced in F2 pups of each sex at 25 ppm. The decrease in
mean pup weight may not have been a primary reproductive effect but an
effect associated with the systemic toxicity of the compound. The
NOAEL for reproductive toxicity was 25 ppm, equivalent to 1.25 mg/kg
bw per day, and that for parental toxicity was 5 ppm, equivalent to
0.25 mg/kg bw per day (Daly, 1982).
In another two-generation study, groups of 28 female
Sprague-Dawley rats were given parathion (purity, 96.7%) at dietary
levels of 0, 1, 10, or 20 ppm. F0 animals received the compound for
10 weeks before mating, and animals selected as F1 parents were
exposed for 11 weeks before mating. Toxicity was seen in F0 and F1
animals of each sex at 10 and 20 ppm, manifested as significant
reductions in plasma, erythrocyte, and/or brain cholinesterase
activities. At 10 ppm, plasma cholinesterase activity was inhibited by
> 20% in comparison with controls; inhibition of erythrocyte
acetylcholinesterase activity was not as pronounced. Brain
acetylcholinesterase activity was < 90% of control values in F0 and
F1 animals of each sex, and the reduction was considered to be
biologically significant. At 20 ppm, brain acetylcholinesterase and
plasma cholinesterase activities were markedly inhibited in females of
both generations. The body weights of dams at lactation were decreased
in both generations at 20 ppm from day 7 to day 21 in the F0
maternal animals and on day 21 in the F1 maternal animals. There
were no compound-related effects on reproductive parameters in
parental animals. In the F1 litters, reduced body weights and
reduced body-weight gains were reported at the highest dose, and
postnatal pup deaths were nonsignificantly increased on lactation days
14-21. Similar findings were not found for the F2 litters. The
parental NOAEL was 1 ppm, equivalent to 0.05 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase; the perinatal NOAEL
was 10 ppm, equivalent to 1 mg/kg bw per day, on the basis of adverse
effects on body weight of offspring at 20 ppm (Neeper-Bradley, 1990).
Rabbits
Groups of 15 female Himalayan rabbits received parathion (purity,
98.8%) by gavage at doses of 0, 0.03, 0.1, or 0.3 mg/kg bw per day on
days 6-18 of gestation. All animals were sacrificed on day 29, and the
fetuses were removed. There were no effects on maternal animals or
offspring. The NOAEL for both maternal and developmental toxicity was
0.3 mg/kg bw per day (Renhof, 1985).
(e) Genotoxicity
Studies of the genotoxicity of parathion are summarized in
Table 2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.5 ml of a test material containing parathion on an epilated area of
the skin for 4 h. Mild skin irritation, characterized by very slight
erythema and very slight oedema, occurred within 1 h. All signs of
irritation had subsided by 72 h (Carr & Cuthbert, 1986).
In another study in New Zealand white rabbits, the compound was
classified as a mild irritant, with a primary irritation index of 0.92
(Pauluhn, 1983).
The scores obtained with the Magnusson-Klegman technique in
Bor:SPF, DPHW guinea-pigs indicated that parathion is not a
sensitizing agent (Heimann, 1986).
(ii) Neurotoxicity
Groups of five beagle dogs of each sex were given parathion
(purity, 98%) for six months in gelatin capsules at doses of 0,
0.0024, 0.0079, or 0.7937 mg/kg bw per day. Plasma, erythrocyte,
brain, and retinal cholinesterase activities were determined, and
ocular assessments were made using slit lamp examinations,
retinoscopic examinations, and electroretinograms. Intra-ocular
pressure and pupillary status were also assessed. No treatment-related
effects were reported, and hence no functional ocular impairment was
observed. Plasma and erythrocyte cholinesterase activities were
inhibited in animals of each sex at the two highest doses. Brain
acetylcholinesterase activity was inhibited in the pons but not in the
cerebellum of male beagles at the highest dose. Retinal cholinesterase
was also significantly decreased animals of each sex at the highest
dose, but the decrease was not associated with morphological changes
in the eye. The NOAEL was 0.0079 mg/kg bw per day on the basis of
inhibition of brain and retinal acetylcholinesterase activities
(Atkinson, 1991a).
Groups of female Sprague-Dawley rats received parathion (purity,
98%) in the diet at levels designed to achieve 0.04, 0.4, or 4 mg/kg
bw per day, for three months. Control rats received the basal diet. At
4 mg/kg bw, decreases were seen in body weight (29%), plasma
cholinesterase (87-95%), and brain acetylcholinesterase (83.5%).
Electroretinograms revealed an increase in the latency and a decrease
in the amplitude of the b-wave. At 0.4 mg/kg bw, the plasma
cholinesterase levels were 37-42% lower than those of controls and the
latency and amplitude of the b-wave were affected in the same way as
in rats receiving 4 mg/kg bw. No morphological changes were seen in
animals at 0.4 or 4 mg/kg bw. The ocular changes observed at the two
highest doses were considered not to be significant toxicologically,
as there was wide variation in the individual and group mean values
for b-wave latency and amplitude. Furthermore, no adverse effects were
seen by electron microscopy. The NOAEL was 0.4 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase (Atkinson, 1991b).
In the 26-month carcinogenicity study conducted in Sprague-Dawley
rats, peripheral neuropathy in males was associated with the
administration of parathion at a dietary level of 50 ppm (2.5 mg/kg bw
per day). Fibres teased from the sciatic nerves of males at the high
dose showed an increased percentage of myelin corrugations and
increased demyelination (Daly, 1984a).
The potential of parathion to produce organophosphorus-induced
delayed neurotoxicity has been investigated in vivo in animals and
in vitro in human neuroblastoma cell lines. Parathion did not induce
delayed neurotoxicity in hens after subchronic exposure. Similar
conclusions were reached in a study in CD-1 mice, in which parathion
was administered orally for 30 days at a dose of 6.75 mg/kg bw per
day. There was no delayed neurotoxicity, and brain neurotoxic
esterases were not inhibited (Solimon et al., 1982).
3. Observations in humans
Parathion was given to human volunteers at doses ranging from
3 mg/day for 28 days to 6 mg/day for 43 days, and plasma and
erythrocyte cholinesterase levels were monitored. The highest dose
induced immediate depression of both types of cholinesterase, to
levels 10-15% of baseline values determined before treatment, which
persisted for two weeks after treatment was discontinued; erythrocyte
acetylcholinesterase activity was inhibited for up to 43 days after
the last dose (Moeller & Rider, 1961).
Five men received parathion at 7.5 mg/day for five days. Plasma
cholinesterase was more sensitive than erythrocyte acetylcholine-
sterase activity to the inhibitory effects of parathion, with an
average decrease of 28% on day 16 after treatment (Rider et al.,
1958).
Table 2. Results of tests for the genotoxicity of parathion
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 3.15-3150 µg/plate 98.9 Negative Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA100, < 10 000 µg/plate 97-98 Negative Lawlor & Wagner (1988)
TA1535, TA1537, TA1538
hprt mutation Chinese hamster ovary cells 0.4-1.0 µl/litre 96.7 Negative Harbell (1989)
hprt mutation Chinese hamster ovary cells 0.03-0.2 µl/litre 97-98 Suspect Yang (1988)
Unscheduled DNA Human WI-38 cell line 10-5 µl/ml NR Positive Simmon et al. (1979)
synthesis 10-6 µl/ml Negativea
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2µl/ml 97-98 Negative Curren (1988)
synthesis
DNA damage E coli K12 and W-3110 625-10 000 µg/plate 98 Negative Herbold (1985)
Cytogenetic damage Human lymphocytes 9.8, 20, 39 µg/mlb 98 Negative Jensen (1991)
78, 156, 313 µg/mla
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw orally 98.8 Negative Herbold (1986)
Dominant lethal mutation Mouse 10 mg/kg bw orally NR Negative Degraeve et al. (1979)
Micronucleus formation Mouse bone marrow 2 × 5, 2 × 10 mg/kg bw 95.9 Negative Herbold (1982)
orally
Micronucleus formation Mouse bone marrow 10-65 mg/kg bw 97-98 Negative Putman (1988)
NR, not reported
a With metabolic activation
b Without metabolic activation
Comments
Parathion is readily absorbed from the respiratory and digestive
tracts and is excreted primarily in the urine. Two hypotheses have
been proposed for its metabolism; however, the metabolic spectrum is
the same in both. Parathion is metabolized to paraoxon and diethyl
phosphorothioic acid. Paraoxon is further metabolized, and following
its oral administration to rats diethyl phosphate, diethyl
phosphorothioate, de-ethyl paraoxon, and para-nitrophenol were
identified in the urine. In cattle, ruminal microorganisms are
believed to be responsible for the production of aminoparathion and
aminoparaoxon.
Parathion is extremely hazardous when given orally (LD50 =
2 mg/kg bw) or by inhalation (4-h LC50 = 0.03 mg/litre) and
moderately hazardous when given dermally (LD50 = 73 mg/kg bw). The
compound has been characterized in studies in laboratory animals as a
mild dermal and ocular irritant and a s a non-sensitizing agent. When
it was administered with other organophosphate pesticides, its toxic
effects were not potentiated. WHO has classified parathion as
'extremely hazardous'.
In a three-week study in rabbits treated dermally, the NOAEL was
0.1 mg/kg bw per day on the basis of depression of plasma,
erythrocyte, and brain cholinesterase activities at 2 mg/kg bw per
day. In a three-week study by inhalation in rats, the NOAEL was
0.9 mg/litre on the basis of decreases in brain, plasma, and
erythrocyte cholinesterase activity. In a 14-day study in dogs,
parathion was administered orally at doses of 0, 1.5, 3, or 6 mg/kg bw
per day. There was no NOAEL, as clinical cholinergic signs were
observed at the lowest dose tested. Cholinesterase activity was not
monitored in this study. In a 90-day study in dogs at doses of 0, 0.3,
1, or 3 mg/kg bw per day, the NOAEL was 3 mg/kg bw per day.
Cholinesterase activity was not measured.
In a 29-day study in mice at doses of 0, 100, 200, or 400 ppm
(equivalent to 15, 30, and 60 mg/kg bw per day), clinical signs of
toxicity were reported in all groups. Cholinesterase activity was not
determined, and there was no NOAEL. In a 90-day study in mice at
dietary concentrations of 0, 15, 50, or 100 ppm, the NOAEL was 50 ppm
(equivalent to 7.5 mg/kg bw per day) on the basis of decreased body
weights of males. Cholinesterase activity was not monitored. When
parathion was administered to rats for 90 days at dietary
concentrations of 0, 2.5, 25, or 75 ppm, the NOAEL was 2.5 ppm (equal
to 0.2 mg/kg bw per day), on the basis of depression of brain
acetylcholinesterase.
In a two-year study in rats, parathion was not associated with
carcinogenicity when administered at dietary concentrations of 0, 0.5,
5, or 50 ppm. The NOAEL for systemic toxicity was 5 ppm (equivalent to
0.25 mg/kg bw per day) on the basis of decreased brain, plasma, and
erythrocyte cholinesterase activity, retinal atrophy, and increased
severity of degenerative changes in the sciatic nerve. In another
study in rats, given dietary levels of 0, 2, 8, or 32 ppm for two
years, there was again no evidence of carcinogenicity. The NOAEL for
systemic toxicity was 8 ppm (equivalent to 0.4 mg/kg bw per day) on
the basis of decreases in brain acetylcholinesterase activity and
retinal atrophy. No effects on sciatic nerves were reported at the
highest dose tested.
In mice receiving dietary concentrations of parathion at 0, 60,
100, or 140 ppm for 18 months, there was no NOAEL, as cholinergic
signs were seen at all doses.
Two studies of developmental toxicity were conducted in rats. In
the first study, parathion was administered by gavage at doses of 0,
0.25, 1, or 1.5 mg/kg bw on gestation days 6-19. The NOAEL for
maternal toxicity was 1 mg/kg bw per day on the basis of increased
mortality, and the NOAEL for developmental toxicity was 1.5 mg/kg bw
per day. In the second study, parathion was administered by gavage at
doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on gestation days 6-15.
The NOAEL for developmental toxicity was 1 mg/kg bw per day and that
for maternal toxicity was 0.3 mg/kg bw per day on the basis of
increased mortality and clinical signs of toxicity.
Two studies of developmental toxicity were conducted in rabbits.
In the first study, parathion was administered by gavage on gestation
days 7-19 at 1,4, or 16 mg/kg bw per day. The NOAEL for developmental
toxicity was 16 mg/kg bw per day, and the NOAEL for maternal toxicity
was 4 mg/kg bw per day on the basis of decreased body-weight gain. In
the second study, parathion was administered by gavage on days 6-18 of
gestation at 0, 0.03, 0.1, or 0.3 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 0.3 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats, doses
of 0, 0.5, 5, or 25 ppm were administered in the diet. Dams at the
highest dose had tremors, and a reduction in body weight was seen
during premating, gestation, and lactation. The NOAEL for reproductive
toxicity was 25 ppm (equivalent to 1.2 mg/kg bw per day); the NOAEL
for maternal toxicity was 5 ppm (equivalent to 0.25 mg/kg bw per day)
on the basis of the observation of tremors in F0 and F1 females.
In the second study, parathion was administered at dietary
concentrations of 0, 1, 10 or 20 ppm. The NOAEL for reproductive
toxicity was 20 ppm (equivalent to 1 mg/kg bw per day); the NOAEL for
perinatal toxicity was 10 ppm (equivalent to 1 mg/kg bw per day) on
the basis of reduced body weights; and the NOAEL for maternal toxicity
was 1 ppm (equivalent to 0.05 mg/kg bw per day) on the basis of
decreased brain acetylcholinesterase activity.
Special studies were conducted to assess the ocular toxicity of
parathion. When parathion was administered to dogs at doses of 0,
0.002, 0.008, or 0.8 mg/kg bw per day for six months, no functional
impairment of the eye was observed. The NOAEL was 0.008 mg/kg bw per
day on the basis of depression of brain and retinal acetylcholine-
sterase activity. In a three-month study of toxicity in female rats,
parathion was administered at levels of 0, 0.04, 0.4, or 4 mg/kg bw
per day. The NOAEL was 0.4 mg/kg bw per day on the basis of depression
of brain acetylcholinesterase activity. No significant effects on
ocular toxicity were reported at any dose.
Parathion was not associated with organophosphorus-induced
delayed neurotoxicity in hens, but it induced demyelination in the
peripheral nerves of rats at a dietary level of 50 ppm (equivalent to
2.5 mg/kg bw per day). The NOAEL was 0.25 mg/kg bw per day.
In a study conducted earlier in humans, an NOAEL of 7.5 mg/day
was determined on the basis of lack of effect on erythrocyte
acetylcholinesterase.
Parathion has been adequately tested for genotoxicity in a range
of tests in vitro and in vivo. The Meeting concluded that
parathion is not genotoxic.
An ADI of 0-0.004 mg/kg bw was established on the basis of an
NOAEL of 0.4 mg/kg bw per day in the two-year study in rats for
retinal atrophy and inhibition of brain acetylcholinesterase at the
higher dose. A safety factor of 100 was used. Lower NOAELs in animals,
based only on inhibition of erythrocyte or brain acetylcholinesterase,
were not considered relevant because of the availability of an NOAEL
for erythrocyte acetyl-cholinesterase inhibition in humans, which was
0.1 mg/kg bw per day.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (90-day study of
toxicity)
Rat: 1 ppm, equivalent to 0.05 mg/kg bw per day (study of
reproductive toxicity)
2.5 ppm, equal to 0.18 mg/kg bw per day (90-day study of
toxicity)
8 ppm, equivalent to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Dog: 0.008 mg/kg bw per day (six-month study of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.01 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 7.5 mg/day (highest
oral dose), corresponding to about 0.1 mg/kg bw per day, in humans.
This was based on the absence of inhibition of erythrocyte
acetylcholinesterase.
Studies that would provide information useful for continued evaluation
of the compound
Further observation in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion
Exposure Relevant route, study type, species Results, remarks
Short-term ( 1-7 days) Skin, irritation, rabbit Irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Non-sensitizing
Oral, toxicity, rat LD50 = 2 mg/kg bw
Dermal, toxicity, rat LD50 = 73 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.03 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 1 mg/kg bw per day, based on
decreased brain acetylcholinesterase. No
dermal effect
Repeated inhalation, 21 days, toxicity, rat NOAEL = 0.92 mg/m3 based on decreased
brain acetylcholinesterase
Repeated oral, reproductive toxicity, rat NOAEL = 0.05 mg/kg bw per day for
maternal toxicity, decreased brain
cholinesterase; NOAEL = 1 mg/kg bw per
day for reproductive toxicity; NOAEL =
1 mg/kg bw per day for perinatal toxicity
based on reduced body weight
Repeated oral, developmental, NOAEL = 1 mg/ kg bw per day for developmental
toxicity, rat toxicity; NOAEL = 0.3 mg/kg per
day for maternal toxicity based on increased
mortality and cholinergic signs
Repeated oral, developmental, NOAEL >0.3 mg/kg bw per day for
toxicity, rabbit maternal and developmental toxicity
Long-term (> one year) Repeated oral, two years, toxicity and NOAEL = 0.25 mg/kg bw per day based on
carcinogenicity, rat lowered brain acetylcholinesterase. No
carcinogenicity
References
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice.
Pestic. Biochem. Physiol., 16, 38-46.
Atkinson, J.E. (1991a) A six month oral study of ethyl parathion in
dogs with specific emphasis on ocular effects. Unpublished report
No. 89-3439 prepared by Biodynamics, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Atkinson, J.E. (1991b) A three month oral study in rats via the diet
with ethyl parathion to investigate ocular effects and cholin
activity. Unpublished report No. 89-3469 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Auletta, C.S. (1984) Acute oral toxicity in rats -- Acute dermal
toxicity in rabbits. Unpublished report No. 4997-84, 5505-84
prepared by Biodynamics Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Benjaminov, O., Hoffer, E., Taitelman, U., Urbak, B. & Brandis, L.
(1992) Parathion transfer and acetycholme activity in
an in vitro perfused human placenta. Vet. Hum. Toxicol.,
34, 10-12.
Bucks, D.A.W., Hinz, R.S., Sarason, R., Maibach, H.I. & Guy, R.H.
(1990) In vivo percutaneous absorption of chemicals -- A multiple
dose study in rhesus monkeys. Chem. Toxicol., 28, 129.
Carr, S.M.A. & Cuthbert, J.A. (1986) Ethyl parathion 98% technical
acute toxicity tests. Unpublished report No. 234117 prepared by
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Chan, L.T.F., Crowley, R.J. & Geyer, R. (1983) Detection and analysis
of aminoparathion in human postmortem specimens.
J. Forensic Sci., 28, 122-127.
Chang, S.K. & Riviere, J.E. (1991) Percutaneous absorption of
parathion in vitro in porcine skin: Effects of dose, temperature,
humidity and perfusate composition on absorptive flux.
Fundam. Appl. Toxicol., 17, 494-504.
Cheng, T. (1990) Ethyl parathion -- Nature of the residues in
livestock -- Laying hens. Unpublished report No. HLA 6222-100,
prepared by Hazleton Laboratories America, Inc. Submitted to WHO
by Cheminova Agro AS, Lemvig, Denmark.
Cheng, T (1987, 1988, 1990) Ethyl parathion -- Nature of the residue
in livestock -- Lactating goats. Unpublished report
No. HLA 6222-101, prepared by Hazleton Laboratories America, Inc.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepotocytes. Unpublished report prepared by Microbiological
Associates, Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1980a) A three month feed study of ethyl parathion in
mice. Unpublished report No. 77-2052 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1980b) A three month feeding study of ethyl parathion in
rats. Unpublished report No. 77-2054 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of ethyl
parathion in rats. Unpublished report No. 80-2457 prepared by
Biodynamics Inc., East Millstone, New Jersey, USA Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1984a) Acute oral toxicity study in rats. Unpublished
report No. 4997-84, prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984b) Acute dermal toxicity study in rabbits. Unpublished
report No. 5505-84 prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984c) A two year chronic feeding study of ethyl parathion
in rats. Unpublished report No. 77-2055 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Davis, J.E. (1975) The metabolism of parathion by liver microsomal
mixed-function oxidases. Dissertation, University of Miami,
Florida, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Degraeve, N., Moutschen, J., Moutschen-Dahmen, M., Gilot-Delhalle, J.,
Colizzi, A., Houbrechts, N. & Cholett, M.C. (1979) Genetic
effects of organophosphate insecticides in mouse (Abstract).
Mutat. Res., 64, 131.
Eiben, R. (1987) Parathion study for chronic toxicity and
cancerogenicity in Wistar rats. Unpublished report No. 16305 from
Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Eigenberg, D.A., Pazdernik, T.L. & Doull, J. (1983) Hernofusion and
pharmacokinetic studies with parathion and parathionoxon in the
rat and dog. Drug Metab. Disposition, 11, 366-370.
Flucke, W. & Kimmerle, G. (1977) Studies of oral acute toxicity of
simultaneously administered methamidophos and parathion ethyl.
Unpublished report from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Fredricksson, T. & Bigelow, J.K. (1-961) Tissue distribution of 32P
labeled parathion. Arch. Environ. Health, 2, 663-667.
Gagne, J. & Brodeur, J. (1972) Metabolic studies on the mechanisms of
increased susceptibility of weanling rats to parathion.
Can. J. Physiol. Pharmacol., 50, 902-915.
Greenough, R.J. & McDonald, P. (1986) Ethyl ara 98% technical: Acute
inhalation toxicity study in rats. Unpublished report
No. 632295 prepared by Inveresk Research International,
Musselburgh, United Kingdom. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Gröning, P. (1975) Tamaron-Special toxicity studies (potentiation).
Unpublished report prepared by Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Harbell, J.W. (1989) CHO/HGPRT confirmatory mutation assay with ethyl
parathion? Unpublished report prepared by Microbiological
Assocites Inc., Musselburgh, United Kingdom. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1982) E-605-ethyl study for acute oral toxicity.
Unpublished report No. 11230 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1986) E-605-ethyl study for skin sensitizing effect on
guinea pigs. Unpublished report No. 14999 from Bayer AG.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1982) Parathion (E-605-ethyl active ingredient) --
Micronucleus test on mouse to evaluate for mutagenic effect.
Unpublished report No. 10777 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1985) E-605 (c.n. parathion) Pol A1-test on E-coli to
evaluate for potential DNA damage. Unpublished report
No. 10777 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Herbold, B.A. (1986) E-605 (c.m. Parathion) -- Dominant lethal test on
male mouse to evaluate for mutagenic effect. Unpublished report
No. 14224 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Jensen, J.C. (1991) Mutagenicity test: In vitro mammalian cytogenetic
test in human lymphocytes performed with parathion-ethyl, Batch
No. 70818-01. Unpublished report prepared by Scaatox. Submitted
to WHO by Cheminova Agro AS, Lemvig, Denmark.
Kamataki, T. & Neal, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothionate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfer
atom. Mol. Pharmacol., 12, 933-944.
Lawlor, T.E. & Wagner, V.O. (1988) Ethyl parathion (97-98% technical)
-- Salmonella/mammalian-microsome plate incorporation
mutagenicity assay (Ames test) with a confirmatory assay.
Unpublished report No. USAE 605220388 prepared by Microbiological
Associates Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Mihail, F. & Gröning, P. (1981) E-605. Ethylactive ingredient/subacute
cutaneous toxicity study on rabbits. Unpublished report No 10342
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Moeller, H.S. & Rider, J.A. (1961) Studies on the anticholinesterase
effect of parathion and methyl parathion in humans (Abstract).
Fed Proc., 20, 434.
Nakatsugawa, T., Tolman, N.U. & Dahm, P.A. (1969) Degradation of
parathion in the rat. Biochem. 18, 1103-1114.
Neal, R.A. (1967) Studies on the metabolism of diethyl 4-nitrophenyl
phosphorothionate (parathion) in vitro. Biochem. J.,
103, 183-191.
Neeper-Bradley, T.L. (1990) Two-generation reproduction study of ethyl
parathion technical administered in the diet of CD (Sprague
Dawley) rats. Unpublished report from Busky Run Research Center,
Pennsylvania, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Nye, D.E. & Donough, H.W. (1976) Fate of insecticides administered
endotracheally to rats. Bull. Environ. Contam. Toxicol.,
15, 291-296.
Oesch, F. (1977) Ames test for Folidol E-605 (parathion). Unpublished
report No. E605301177. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Owens, E.J. (1976) The effect of ethyl parathion in the rat and dog
after acute and subacute inhalation and oral administration.
Technical report No. AMRL TR-76-1215 from Aerospace Medical
Research Laboratories, pp. 203-222. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Page, J.G. & Heath, G.E. (1991) Carcinogenicity study of ethyl
parathion administered by dosed feed to B6C3F1 mice.
Unpublished report No. E605210291 from Southern Research
Institute, Alabama, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Pauluhn, J. (1983) E605-Ethyl/Study for dermal and mucous membrane
irritant effects. Unpublished report No. 11401 from Bayer AG
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Pauluhn, J. (1984) E605 (c.m. parathion) -- Study for subacute
inhalative toxicity in the rat (exposure 15 × 6 hours).
Unpublished report No. 12395 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Putman, D.L. (1988) Micronucleus cytogenetic assay in mice.
Unpublished report No. T4772.122 prepared by Microbiological
Associates, Inc., USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Ramundo, J. (1979) A 4 week pilot study in mice with ethyl parathion.
Unpublished report No. 77-2051 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS. Lemvig, Denmark.
Renhof, M. (1984) Parathion -- Study for embryotoxic effects in rats
after oral administration. Unpublished report No. 12909 from
Bayer AG. Submitted to WHO by Cheminova Agro AS. Lemvig, Denmark.
Renhof, M. (1985) Parathion -- Study for embryotoxic effects on
rabbits after oral administration. Unpublished report No. 13288
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark
Rider, J.A., Moeller, H.C., Swader, J.I. & Weilerstein, R.W. (1958)
The effect of parathion on human red blood cell and plasma
cholinesterase. Arch. Ind. Health, 18, 442-445.
Schroeder (1983) A teratogenicity study in rats with ethyl parathion.
Unpublished report No. 82-2644 prepared by Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Simmon, V.F., Mitchell, R.D. & Jorgenson, T.A. (1976) In vitro
mutagenic studies on twenty pesticides. Toxicol. Appl.
Pharmacol., 37, 109.
Soliman, S.A., Farmer, J. & Curley, A. (1982) Is delayed neurotoxicity
a property of all organophosphorus compounds? A study with a
model compound: parathion. Toxicology, 23, 267-279.
Tegeris, A.S. & Underwood, P.C. (1977) Fourteen day feeding study in
the dog. Ethyl parathion. Unpublished report No. 77-113 prepared
by Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Thyssen, J. (1972) E-605-Ethyl -- Study for acute inhalation toxicity.
Unpublished report No. 8209 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Underwood, P.C. (1978) Ethyl parathion: Ninety day feeding to dogs.
Unpublished report No. USAE 65060378 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Weber, H., Patzchke, K. & Wegner, L.A. (1980) Parathion C14 (benzene
ring-labelled compound): Biokinetic studies in rats. Unpublished
report No. PH8820 from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Yang, L.L. (1988) Ethyl parathion (97/98% technical) CHO/HGPRT
mutation assay. Unpublished report No. USAE 605280388 from
Microbiological Associates Inc., USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
 2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of parathion are summarized in
Table 1.
The toxicity of parathion was not potentiated by simultaneous
oral administration of methamidophos (Flucke & Kimmerle, 1977),
temaron, or parathion methyl (Gröning, 1975). Additive acute toxicity
was reported for each of these chemicals in combination with
parathion.
(b) Short-term toxicity
Mice
Groups of five CD-1 mice of each sex received parathion at
dietary levels of 100, 200, or 400 ppm for 29 days. All of the animals
at the high dose either died or were sacrificed in a moribund
condition during the first 11 days of dosing. The mortality and
moribundity in the group at the medium dose was similar to that at the
high dose, with the exception of one surviving male. Clinical signs of
toxicity included tremors, decreased activity, and emaciation in all
treated groups. The mean body weights of males and females at the low
dose were 7-10% lower than those of controls, but food consumption
exceeded that of controls. No gross pathological changes that could be
attributed to the administration of parathion were reported in animals
at the low dose. Gastric erosion was seen in males at the two higher
doses and lung congestion in females at the high dose. There was no
NOAEL in view of the presence of cholinergic symptoms at 100 ppm,
equivalent to 15 mg/kg bw per day (Ramundo, 1979).
Groups of 15 male and 15 female Charles River COBS (ICR derived)
CD-1 mice received diets containing parathion (purity, 95.11%) at
doses of 0, 15, 50, or 100 ppm for three months. All animals survived
except for one male at the low dose and one female at the high dose.
The body weights of males at the high dose were significantly lower
than those of controls from the first week of the study until
termination. Sporadic, unsustained decreases in body weight were
reported for males at the middle dose and in females at the high dose,
and the differences in body weight were significant in weeks 1-5 and
8-9. At all other intervals, body weight was similar to controls. No
treatment-associated gross or microscopic lesions were reported.
Cholinesterase activity was not monitored in this study. The NOAEL was
50 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of the
reported decreases in body weight in males receiving 100 ppm
(Daly, 1980).
Table 1. Acute toxicity of parathion
Species Sex Route LD50 or LC50, Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male Oral 22 98 Carr & Cuthbert (1986)
Female 2
Rat Male Oral 7 NR Auletta (1984)
Female 2.6
Rat Male, female Oral 6.85 NR Owens (1976)
Rat Male Oral 13.7 NR Heimann (1982)
Rat Male, female Dermal 73 98 Carr & Cuthbert (1986)
Rabbit Male Dermal 910 NR Daly (1984b)
Female 1400
Rat Male, female Inhalation (4-h) 0.03 98 Greenough & McDonald (1986)
Rat Male Inhalation (1-h) 245 97.8 Thyssen (1972)
Female 71
Rat Male Inhalation (4-h) 77-91 97.8 Thyssen (1972)
Female 24
NR, not reported
Rats
Groups of 10 male and 10 female Wistar rats were exposed by
inhalation by head-nose to a parathion (purity, 98%) aerosol at
concentrations of 0.25, 0.92, or 3.9 mg/m3 for up to 6 h per day for
15 days. Animals of each sex at 0.92 mg/m3 showed inhibition of
plasma and erythrocyte cholinesterase activity in comparison with mean
baseline values; at the highest dose, plasma, erythrocyte, and brain
cholinesterase activities were reduced. Animals receiving the highest
dose also showed nonspecific, transient behavioural changes; females
had muscular tremors and increased mortality. With the exception of
increased organ congestion in females at tile high dose, there were no
gross or histopathological lesions that could be associated with the
administration of parathion. The NOAEL was 0.25 mg/m3 (Fauluhn,
1984).
Groups of 20 Sprague-Dawley rats of each sex were fed diets
containing parathion (purity, 95.11%) at 0, 2.5, 25, or 75 ppm for
three months. Animals were observed for clinical signs of toxicity,
and body weights and food consumption were recorded weekly. Clinical
chemical and haematological parameters and plasma and erythrocyte
cholinesterase activities were determined before the administration of
parathion and at designated intervals during the study. Brain
acetylcholinesterase activity was determined in 10 males and 10
females before assignment to the treatment groups and in groups of 10
animals of each sex per group at three months. Urinalysis was
conducted at one and three months of the study. Nine of the females at
the high dose died or were sacrificed during the study. Anogenital
staining, tremors, and emaciation were reported in females at the high
dose, which also had elevated levels of serum aspartate and alanine
aminotransferases and alkaline phosphatase and decreased haemoglobin
and haematocrit. None of these alterations in clinical pathology could
be attributed to parathion, since they were within the normal
biological ranges.
Decreases in plasma and erythrocyte cholinesterases were reported
in all treated animals. At 2.5 ppm, a statistically significant
decrease in erythrocyte acetylcholinesterase was reported in females,
amounting to 64% of the activity in controls at one month, 73% of that
at two months, and 44% of that at three months. At 25 ppm, a 20%
inhibition of plasma and erythrocyte cholinesterase activities was
seen in treated animals in comparison with controls at all collection
times. Brain acetylcholinesterase activity was significantly inhibited
in females but not males at the middle dose. At 75 ppm, there was
significant inhibition of erythrocyte and plasma cholinesterase
activities in males at two and three months and in females at all
intervals. Brain acetylcholinesterase activity was 40% of that of
controls in females and 38% in males after the third month of
treatment. The NOAEL was 2.5 ppm, equal to 0.18 mg/kg bw per day, on
the basis of a significant depression in brain acetylcholinesterase in
females (Daly, 1980b).
Rabbits
Parathion formulated with water and cremaphor was administered to
male and female New Zealand white rabbits at 0, 0.1, or 2 mg/kg bw per
day for 15 days at a volume of 0.5 ml/kg bw on shaved backs or flanks
and was left in contact with the skin for 6 h per exposure. No dermal
irritation and no compound-related effects on clinical behaviour,
clinical signs, or gross or microscopic pathological appearance were
reported. Plasma, erythrocyte, and brain cholinesterase activities
were all inhibited at 2 mg/kg bw per day; however, no cholinergic
symptoms were reported. The NOAEL was 0.1 mg/kg bw per day (Mihail &
Gröning, 1981).
Dogs
Groups of two beagle dogs of each sex were fed diets containing
parathion at 0, 1.5, 3.0, or 6 mg/kg bw per day for 14 days and were
observed for clinical signs of toxicity, mortality, food intake,
weight gain, and gross pathological lesions. All animals survived the
study. Food intake was significantly lower in males at the high dose
than in controls. There was a dose-related increase in the incidence
of vomiting in all groups receiving parathion, and after two weeks
animals at the two higher doses had lower body-weight gain and feed
efficiency than controls. No gross lesions were seen in any of the
treated dogs. Cholinesterase was not monitored in this study, but
appeared to have been affected on the basis of the clinical symptom of
vomiting. There was no NOAEL because of the presence of signs of
cholinergic poisoning at the lowest dose (Tegeris & Underwood, 1977).
Groups of 16 male and 16 female beagle dogs were randomly
assigned to groups designated to receive dietary levels of parathion
(purity, 94.4%) at 0, 0.3,1, or 3 mg/kg bw per day for 90 days.
Animals were observed daily for clinical signs of toxicity and for
survival. Clinical chemical and haematological parameters were
determined before treatment and at predetermined intervals during the
90-day period. Blood and plasma cholinesterase levels were determined
during the study, and brain cholinesterase was measured after
sacrifice. Organ weights were recorded and gross and microscopic
pathological changes were evaluated. Plasma cholinesterase activity
was significantly reduced in all treated groups in comparison with
controls; erythrocyte acetylcholinesterase activity was significantly
inhibited in all females and in males at the two higher doses. With
the exception of the effects on plasma and erythrocyte cholinesterase
activity, there were no alterations in clinical chemistry, and
haematological and gross and microscopic pathological parameters, body
weights, and food intake were also unaffected. No compound-associated
effects were reported on brain acetylcholinesterase activity. Soft
stools were observed in all groups, but the frequency and severity of
this finding could not be associated with increasing dietary levels of
parathion. There was no NOAEL (Underwood, 1978).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female B6C3F1 mice received diets
containing parathion at 0, 60, 100, or 140 ppm for 18 months. There
was no compound-related effect on survival. The body weights of males
at the highest dose were 14% lower than those of controls. Clinical
signs of toxicity reflecting the cholinergic properties of the
material were reported in all treated animals during the first month
of the study, including laboured breathing, pallor, hypoactivity, and
tremors. The increased incidence was dose-related. At 18 months, the
inhibition of plasma and erythrocyte cholinesterase was > 20% of the
control values in all groups. Brain acetylcholinesterase activity was
inhibited in males and females at the high dose, to 87% at day 10 and
85% at 18 months in males and 82% at day 10 and 76% at 18 months in
females. Brain:kidney weight ratios were significantly lower than
those of controls in female mice receiving 140 ppm; decreases were
also reported in males receiving 100 and 140 ppm. Alveolar-bronchiolar
adenomas were seen in 16/50 male mice receiving 60 ppm but not at the
next two doses. There was no NOAEL, as signs of cholinergic poisoning
were seen at all doses (Page & Heath, 1991).
Rats
Groups of 60 male and 60 female Sprague-Dawley (CrlCD:BR) rats
received diets containing parathion (purity, 95.11%) at 0, 0.5, 5, or
50 ppm for up to 28 months. The mean body weights of animals receiving
50 ppm were significantly decreased throughout the study, the
decreases ranging from 10 to 16% in males and 8 to 25% in females.
There was no corresponding decrease in food consumption. Males and
females at the high dose had slightly lowered haemoglobin and
haematocrit in comparison with controls. Significantly lower values
were reported in females at the high dose at 6, 12, and 18 months;
however, the effect does not appear to be of biological significance
as the values are within the normal range for this strain. Plasma
cholinesterase activity was reduced in males and females at the two
higher doses throughout the study. Erythrocyte acetylcholinesterase
values were reduced in animals of each sex but not to statistically or
biologically significant levels. Brain acetylcholinesterase activity
was significantly reduced in males (22% of controls) and females (18%
of controls) at the highest dose.
Treatment was associated with increased incidences of tremors,
alopecia, anogenital staining, and abnormal gait in females at the
high dose, which also had elevated alkaline phosphatase activity and
increased blood urea nitrogen; however, the reported values are within
the normal range for this strain. There were no effects on organ
weight and no gross lesions associated with administration of the test
material. The incidence of retinal degeneration was increased in
females at the high dose, and the severity of peripheral neuropathy
characterized by myelin sheath degeneration in the proximal sciatic
nerve and myelin corrugations, demyelinated lengths, and myelin
ovoids was increased in males at the high dose. There was no
treatment-associated carcinogenicity. The systemic NOAEL was 5 ppm
(equivalent to 0.25 mg/kg bw per day), and the LOAEL was 50 ppm
(2.5 mg/kg bw per day) on the basis of effects on brain, plasma, and
erythrocyte cholinesterase activity, retinal degeneration, and
peripheral neuropathy (Daly, 1984c).
Groups of 50 Wistar rats of each sex received diets containing
parathion (purity, 96.7%) at doses of 0, 2, 8, or 32 ppm for two
years; a further 15 rats of each sex received the dietary
concentrations for 12 months. Animals at the highest dose had poor
general condition and chromodacryorrhoea, and females also had
tremors, hair loss, and head tilt at greater frequency than controls;
however, the increase was not statistically significant. Females at
the highest dose experienced 36% mortality, in comparison with 22% for
controls. Plasma and erythrocyte cholinesterase activities were
inhibited by > 20% of the control level in animals of each sex at 8
or 32 ppm, the inhibition being greater at the highest dose; brain
acetylcholinesterase activity was also statistically and biologically
lower than that in controls at the highest dose. There was no
compound-related effect on urinary or haematological parameters. The
body weights of animals of each sex at the highest dose were decreased
in comparison with controls; however, there were no apparent effects
on organ weights.
Histologically, there was a nonsignificantly increased incidence
of proliferative lesions in the exocrine pancreas in males receiving 8
or 32 ppm. At 32 ppm, there were also adverse effects on the retina,
as indicated by structural degeneration and retinal atrophy. No
treatment-related oncogenic effects were reported. No significant
effects on peripheral nerves were reported at the highest dose. The
systemic NOAEL in this strain was 8 ppm (equivalent to 0.4 mg/kg bw
per day), and the LOAEL was 32 ppm (equivalent to 1.6 mg/kg bw per
day), on the basis of inhibition of brain, plasma, and erythrocyte
cholinesterase activity (Eiben, 1987).
(d) Reproductive and developmental toxicity
Rats
Groups of 24 female Sprague-Dawley rats in which mating was
confirmed by vaginal smear were given parathion (purity, 95.1.1%) by
gavage on days 6-19 of gestation at doses of 0, 0.25, 1.0, or
1.5 mg/kg bw per day. On day 20, all animals were sacrificed, the
uteri were removed, and the animals were subjected to a complete gross
post-mortem examination and examination of the reproductive system.
The numbers of live and dead fetuses, late and early resorptions,
implantation sites, and corpora lutea were recorded. Each fetus was
examined grossly to determine whether malformations were present;
one-half of each litter was evaluated for soft-tissue defects and the
other for skeletal defects. The mortality rate was increased in dams
at the high dose, which also had significantly lower corrected body
weights than controls. These animals had an increased incidence of
mucoid nasal discharge at day 15 and an increased incidence of dry
nasal discharge on day 20. No compound-related effects on fetal
development were reported. The NOAEL was 1.5 mg/kg bw per day, and the
NOAEL for maternal toxicity was 1.0 mg/kg bw per day (Schroeder,
1983).
Groups of 25 Wistar rats were fed parathion (purity, 98.8%) in a
Cremophor emulsion at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on
days 6-15 of gestation. Caesarean sections were performed on day 20.
Thirteen of the dams at the high dose died as a result of circulatory
and/or respiratory collapse, and 10 of these were found to have red
lungs at necropsy. Clinical signs of toxicity in the animals at the
high dose included tremors, rough coats, light stools, sunken flanks,
and reduced water intake. A slight, nonsignificant decrease in
body-weight gain was reported in animals receiving the highest dose.
No developmental effects were reported. The number of fetal
malformations was higher in the group at 1 mg/kg bw per day, but this
increase was driven by the increased incidence of malformations in one
litter, and there were no significant differences in the litter
incidence of fetal malformations. The NOAEL for maternal toxicity was
0.3 mg/kg bw per day, and the NOAEL for developmental toxicity was
1.0 mg/kg bw per day (Renhof, 1984).
A two-generation study of reproductive toxicity was conducted in
Sprague-Dawley rats given diets containing parathion (purity, 95.1%)
at doses of 0, 0.5, 5, or 25 ppm. Animals in the F0 generation
received the test material daily for 14 weeks before mating and
throughout mating, gestation, and lactation. Animals selected as F1
parents received parathion 18 weeks before mating and throughout the
mating, gestation, and lactation periods. F0 animals were sacrificed
after the F1 generation was weaned, and F1 animals were sacrificed
about five weeks after the F2 generation had been weaned; F2
animals were sacrificed at weaning. Tremors were observed in F0
females at the high dose, and the mean body weights of these animals
were reduced throughout the premating period and during gestation and
lactation. Tremors were also reported in F1 females at the high dose
during lactation. Slight, nonsignificant reductions in body weight
were reported in animals of each sex in the F1 generation at the
high dose. On day 21 of the lactation period, reduced mean body
weights were seen in F1 pups of each sex at 25 ppm and in F1
females at 5 ppm. The reduction was not statistically significant but
was reproduced in F2 pups of each sex at 25 ppm. The decrease in
mean pup weight may not have been a primary reproductive effect but an
effect associated with the systemic toxicity of the compound. The
NOAEL for reproductive toxicity was 25 ppm, equivalent to 1.25 mg/kg
bw per day, and that for parental toxicity was 5 ppm, equivalent to
0.25 mg/kg bw per day (Daly, 1982).
In another two-generation study, groups of 28 female
Sprague-Dawley rats were given parathion (purity, 96.7%) at dietary
levels of 0, 1, 10, or 20 ppm. F0 animals received the compound for
10 weeks before mating, and animals selected as F1 parents were
exposed for 11 weeks before mating. Toxicity was seen in F0 and F1
animals of each sex at 10 and 20 ppm, manifested as significant
reductions in plasma, erythrocyte, and/or brain cholinesterase
activities. At 10 ppm, plasma cholinesterase activity was inhibited by
> 20% in comparison with controls; inhibition of erythrocyte
acetylcholinesterase activity was not as pronounced. Brain
acetylcholinesterase activity was < 90% of control values in F0 and
F1 animals of each sex, and the reduction was considered to be
biologically significant. At 20 ppm, brain acetylcholinesterase and
plasma cholinesterase activities were markedly inhibited in females of
both generations. The body weights of dams at lactation were decreased
in both generations at 20 ppm from day 7 to day 21 in the F0
maternal animals and on day 21 in the F1 maternal animals. There
were no compound-related effects on reproductive parameters in
parental animals. In the F1 litters, reduced body weights and
reduced body-weight gains were reported at the highest dose, and
postnatal pup deaths were nonsignificantly increased on lactation days
14-21. Similar findings were not found for the F2 litters. The
parental NOAEL was 1 ppm, equivalent to 0.05 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase; the perinatal NOAEL
was 10 ppm, equivalent to 1 mg/kg bw per day, on the basis of adverse
effects on body weight of offspring at 20 ppm (Neeper-Bradley, 1990).
Rabbits
Groups of 15 female Himalayan rabbits received parathion (purity,
98.8%) by gavage at doses of 0, 0.03, 0.1, or 0.3 mg/kg bw per day on
days 6-18 of gestation. All animals were sacrificed on day 29, and the
fetuses were removed. There were no effects on maternal animals or
offspring. The NOAEL for both maternal and developmental toxicity was
0.3 mg/kg bw per day (Renhof, 1985).
(e) Genotoxicity
Studies of the genotoxicity of parathion are summarized in
Table 2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.5 ml of a test material containing parathion on an epilated area of
the skin for 4 h. Mild skin irritation, characterized by very slight
erythema and very slight oedema, occurred within 1 h. All signs of
irritation had subsided by 72 h (Carr & Cuthbert, 1986).
In another study in New Zealand white rabbits, the compound was
classified as a mild irritant, with a primary irritation index of 0.92
(Pauluhn, 1983).
The scores obtained with the Magnusson-Klegman technique in
Bor:SPF, DPHW guinea-pigs indicated that parathion is not a
sensitizing agent (Heimann, 1986).
(ii) Neurotoxicity
Groups of five beagle dogs of each sex were given parathion
(purity, 98%) for six months in gelatin capsules at doses of 0,
0.0024, 0.0079, or 0.7937 mg/kg bw per day. Plasma, erythrocyte,
brain, and retinal cholinesterase activities were determined, and
ocular assessments were made using slit lamp examinations,
retinoscopic examinations, and electroretinograms. Intra-ocular
pressure and pupillary status were also assessed. No treatment-related
effects were reported, and hence no functional ocular impairment was
observed. Plasma and erythrocyte cholinesterase activities were
inhibited in animals of each sex at the two highest doses. Brain
acetylcholinesterase activity was inhibited in the pons but not in the
cerebellum of male beagles at the highest dose. Retinal cholinesterase
was also significantly decreased animals of each sex at the highest
dose, but the decrease was not associated with morphological changes
in the eye. The NOAEL was 0.0079 mg/kg bw per day on the basis of
inhibition of brain and retinal acetylcholinesterase activities
(Atkinson, 1991a).
Groups of female Sprague-Dawley rats received parathion (purity,
98%) in the diet at levels designed to achieve 0.04, 0.4, or 4 mg/kg
bw per day, for three months. Control rats received the basal diet. At
4 mg/kg bw, decreases were seen in body weight (29%), plasma
cholinesterase (87-95%), and brain acetylcholinesterase (83.5%).
Electroretinograms revealed an increase in the latency and a decrease
in the amplitude of the b-wave. At 0.4 mg/kg bw, the plasma
cholinesterase levels were 37-42% lower than those of controls and the
latency and amplitude of the b-wave were affected in the same way as
in rats receiving 4 mg/kg bw. No morphological changes were seen in
animals at 0.4 or 4 mg/kg bw. The ocular changes observed at the two
highest doses were considered not to be significant toxicologically,
as there was wide variation in the individual and group mean values
for b-wave latency and amplitude. Furthermore, no adverse effects were
seen by electron microscopy. The NOAEL was 0.4 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase (Atkinson, 1991b).
In the 26-month carcinogenicity study conducted in Sprague-Dawley
rats, peripheral neuropathy in males was associated with the
administration of parathion at a dietary level of 50 ppm (2.5 mg/kg bw
per day). Fibres teased from the sciatic nerves of males at the high
dose showed an increased percentage of myelin corrugations and
increased demyelination (Daly, 1984a).
The potential of parathion to produce organophosphorus-induced
delayed neurotoxicity has been investigated in vivo in animals and
in vitro in human neuroblastoma cell lines. Parathion did not induce
delayed neurotoxicity in hens after subchronic exposure. Similar
conclusions were reached in a study in CD-1 mice, in which parathion
was administered orally for 30 days at a dose of 6.75 mg/kg bw per
day. There was no delayed neurotoxicity, and brain neurotoxic
esterases were not inhibited (Solimon et al., 1982).
3. Observations in humans
Parathion was given to human volunteers at doses ranging from
3 mg/day for 28 days to 6 mg/day for 43 days, and plasma and
erythrocyte cholinesterase levels were monitored. The highest dose
induced immediate depression of both types of cholinesterase, to
levels 10-15% of baseline values determined before treatment, which
persisted for two weeks after treatment was discontinued; erythrocyte
acetylcholinesterase activity was inhibited for up to 43 days after
the last dose (Moeller & Rider, 1961).
Five men received parathion at 7.5 mg/day for five days. Plasma
cholinesterase was more sensitive than erythrocyte acetylcholine-
sterase activity to the inhibitory effects of parathion, with an
average decrease of 28% on day 16 after treatment (Rider et al.,
1958).
Table 2. Results of tests for the genotoxicity of parathion
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 3.15-3150 µg/plate 98.9 Negative Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA100, < 10 000 µg/plate 97-98 Negative Lawlor & Wagner (1988)
TA1535, TA1537, TA1538
hprt mutation Chinese hamster ovary cells 0.4-1.0 µl/litre 96.7 Negative Harbell (1989)
hprt mutation Chinese hamster ovary cells 0.03-0.2 µl/litre 97-98 Suspect Yang (1988)
Unscheduled DNA Human WI-38 cell line 10-5 µl/ml NR Positive Simmon et al. (1979)
synthesis 10-6 µl/ml Negativea
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2µl/ml 97-98 Negative Curren (1988)
synthesis
DNA damage E coli K12 and W-3110 625-10 000 µg/plate 98 Negative Herbold (1985)
Cytogenetic damage Human lymphocytes 9.8, 20, 39 µg/mlb 98 Negative Jensen (1991)
78, 156, 313 µg/mla
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw orally 98.8 Negative Herbold (1986)
Dominant lethal mutation Mouse 10 mg/kg bw orally NR Negative Degraeve et al. (1979)
Micronucleus formation Mouse bone marrow 2 × 5, 2 × 10 mg/kg bw 95.9 Negative Herbold (1982)
orally
Micronucleus formation Mouse bone marrow 10-65 mg/kg bw 97-98 Negative Putman (1988)
NR, not reported
a With metabolic activation
b Without metabolic activation
Comments
Parathion is readily absorbed from the respiratory and digestive
tracts and is excreted primarily in the urine. Two hypotheses have
been proposed for its metabolism; however, the metabolic spectrum is
the same in both. Parathion is metabolized to paraoxon and diethyl
phosphorothioic acid. Paraoxon is further metabolized, and following
its oral administration to rats diethyl phosphate, diethyl
phosphorothioate, de-ethyl paraoxon, and para-nitrophenol were
identified in the urine. In cattle, ruminal microorganisms are
believed to be responsible for the production of aminoparathion and
aminoparaoxon.
Parathion is extremely hazardous when given orally (LD50 =
2 mg/kg bw) or by inhalation (4-h LC50 = 0.03 mg/litre) and
moderately hazardous when given dermally (LD50 = 73 mg/kg bw). The
compound has been characterized in studies in laboratory animals as a
mild dermal and ocular irritant and a s a non-sensitizing agent. When
it was administered with other organophosphate pesticides, its toxic
effects were not potentiated. WHO has classified parathion as
'extremely hazardous'.
In a three-week study in rabbits treated dermally, the NOAEL was
0.1 mg/kg bw per day on the basis of depression of plasma,
erythrocyte, and brain cholinesterase activities at 2 mg/kg bw per
day. In a three-week study by inhalation in rats, the NOAEL was
0.9 mg/litre on the basis of decreases in brain, plasma, and
erythrocyte cholinesterase activity. In a 14-day study in dogs,
parathion was administered orally at doses of 0, 1.5, 3, or 6 mg/kg bw
per day. There was no NOAEL, as clinical cholinergic signs were
observed at the lowest dose tested. Cholinesterase activity was not
monitored in this study. In a 90-day study in dogs at doses of 0, 0.3,
1, or 3 mg/kg bw per day, the NOAEL was 3 mg/kg bw per day.
Cholinesterase activity was not measured.
In a 29-day study in mice at doses of 0, 100, 200, or 400 ppm
(equivalent to 15, 30, and 60 mg/kg bw per day), clinical signs of
toxicity were reported in all groups. Cholinesterase activity was not
determined, and there was no NOAEL. In a 90-day study in mice at
dietary concentrations of 0, 15, 50, or 100 ppm, the NOAEL was 50 ppm
(equivalent to 7.5 mg/kg bw per day) on the basis of decreased body
weights of males. Cholinesterase activity was not monitored. When
parathion was administered to rats for 90 days at dietary
concentrations of 0, 2.5, 25, or 75 ppm, the NOAEL was 2.5 ppm (equal
to 0.2 mg/kg bw per day), on the basis of depression of brain
acetylcholinesterase.
In a two-year study in rats, parathion was not associated with
carcinogenicity when administered at dietary concentrations of 0, 0.5,
5, or 50 ppm. The NOAEL for systemic toxicity was 5 ppm (equivalent to
0.25 mg/kg bw per day) on the basis of decreased brain, plasma, and
erythrocyte cholinesterase activity, retinal atrophy, and increased
severity of degenerative changes in the sciatic nerve. In another
study in rats, given dietary levels of 0, 2, 8, or 32 ppm for two
years, there was again no evidence of carcinogenicity. The NOAEL for
systemic toxicity was 8 ppm (equivalent to 0.4 mg/kg bw per day) on
the basis of decreases in brain acetylcholinesterase activity and
retinal atrophy. No effects on sciatic nerves were reported at the
highest dose tested.
In mice receiving dietary concentrations of parathion at 0, 60,
100, or 140 ppm for 18 months, there was no NOAEL, as cholinergic
signs were seen at all doses.
Two studies of developmental toxicity were conducted in rats. In
the first study, parathion was administered by gavage at doses of 0,
0.25, 1, or 1.5 mg/kg bw on gestation days 6-19. The NOAEL for
maternal toxicity was 1 mg/kg bw per day on the basis of increased
mortality, and the NOAEL for developmental toxicity was 1.5 mg/kg bw
per day. In the second study, parathion was administered by gavage at
doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on gestation days 6-15.
The NOAEL for developmental toxicity was 1 mg/kg bw per day and that
for maternal toxicity was 0.3 mg/kg bw per day on the basis of
increased mortality and clinical signs of toxicity.
Two studies of developmental toxicity were conducted in rabbits.
In the first study, parathion was administered by gavage on gestation
days 7-19 at 1,4, or 16 mg/kg bw per day. The NOAEL for developmental
toxicity was 16 mg/kg bw per day, and the NOAEL for maternal toxicity
was 4 mg/kg bw per day on the basis of decreased body-weight gain. In
the second study, parathion was administered by gavage on days 6-18 of
gestation at 0, 0.03, 0.1, or 0.3 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 0.3 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats, doses
of 0, 0.5, 5, or 25 ppm were administered in the diet. Dams at the
highest dose had tremors, and a reduction in body weight was seen
during premating, gestation, and lactation. The NOAEL for reproductive
toxicity was 25 ppm (equivalent to 1.2 mg/kg bw per day); the NOAEL
for maternal toxicity was 5 ppm (equivalent to 0.25 mg/kg bw per day)
on the basis of the observation of tremors in F0 and F1 females.
In the second study, parathion was administered at dietary
concentrations of 0, 1, 10 or 20 ppm. The NOAEL for reproductive
toxicity was 20 ppm (equivalent to 1 mg/kg bw per day); the NOAEL for
perinatal toxicity was 10 ppm (equivalent to 1 mg/kg bw per day) on
the basis of reduced body weights; and the NOAEL for maternal toxicity
was 1 ppm (equivalent to 0.05 mg/kg bw per day) on the basis of
decreased brain acetylcholinesterase activity.
Special studies were conducted to assess the ocular toxicity of
parathion. When parathion was administered to dogs at doses of 0,
0.002, 0.008, or 0.8 mg/kg bw per day for six months, no functional
impairment of the eye was observed. The NOAEL was 0.008 mg/kg bw per
day on the basis of depression of brain and retinal acetylcholine-
sterase activity. In a three-month study of toxicity in female rats,
parathion was administered at levels of 0, 0.04, 0.4, or 4 mg/kg bw
per day. The NOAEL was 0.4 mg/kg bw per day on the basis of depression
of brain acetylcholinesterase activity. No significant effects on
ocular toxicity were reported at any dose.
Parathion was not associated with organophosphorus-induced
delayed neurotoxicity in hens, but it induced demyelination in the
peripheral nerves of rats at a dietary level of 50 ppm (equivalent to
2.5 mg/kg bw per day). The NOAEL was 0.25 mg/kg bw per day.
In a study conducted earlier in humans, an NOAEL of 7.5 mg/day
was determined on the basis of lack of effect on erythrocyte
acetylcholinesterase.
Parathion has been adequately tested for genotoxicity in a range
of tests in vitro and in vivo. The Meeting concluded that
parathion is not genotoxic.
An ADI of 0-0.004 mg/kg bw was established on the basis of an
NOAEL of 0.4 mg/kg bw per day in the two-year study in rats for
retinal atrophy and inhibition of brain acetylcholinesterase at the
higher dose. A safety factor of 100 was used. Lower NOAELs in animals,
based only on inhibition of erythrocyte or brain acetylcholinesterase,
were not considered relevant because of the availability of an NOAEL
for erythrocyte acetyl-cholinesterase inhibition in humans, which was
0.1 mg/kg bw per day.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (90-day study of
toxicity)
Rat: 1 ppm, equivalent to 0.05 mg/kg bw per day (study of
reproductive toxicity)
2.5 ppm, equal to 0.18 mg/kg bw per day (90-day study of
toxicity)
8 ppm, equivalent to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Dog: 0.008 mg/kg bw per day (six-month study of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.01 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 7.5 mg/day (highest
oral dose), corresponding to about 0.1 mg/kg bw per day, in humans.
This was based on the absence of inhibition of erythrocyte
acetylcholinesterase.
Studies that would provide information useful for continued evaluation
of the compound
Further observation in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion
Exposure Relevant route, study type, species Results, remarks
Short-term ( 1-7 days) Skin, irritation, rabbit Irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Non-sensitizing
Oral, toxicity, rat LD50 = 2 mg/kg bw
Dermal, toxicity, rat LD50 = 73 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.03 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 1 mg/kg bw per day, based on
decreased brain acetylcholinesterase. No
dermal effect
Repeated inhalation, 21 days, toxicity, rat NOAEL = 0.92 mg/m3 based on decreased
brain acetylcholinesterase
Repeated oral, reproductive toxicity, rat NOAEL = 0.05 mg/kg bw per day for
maternal toxicity, decreased brain
cholinesterase; NOAEL = 1 mg/kg bw per
day for reproductive toxicity; NOAEL =
1 mg/kg bw per day for perinatal toxicity
based on reduced body weight
Repeated oral, developmental, NOAEL = 1 mg/ kg bw per day for developmental
toxicity, rat toxicity; NOAEL = 0.3 mg/kg per
day for maternal toxicity based on increased
mortality and cholinergic signs
Repeated oral, developmental, NOAEL >0.3 mg/kg bw per day for
toxicity, rabbit maternal and developmental toxicity
Long-term (> one year) Repeated oral, two years, toxicity and NOAEL = 0.25 mg/kg bw per day based on
carcinogenicity, rat lowered brain acetylcholinesterase. No
carcinogenicity
References
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice.
Pestic. Biochem. Physiol., 16, 38-46.
Atkinson, J.E. (1991a) A six month oral study of ethyl parathion in
dogs with specific emphasis on ocular effects. Unpublished report
No. 89-3439 prepared by Biodynamics, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Atkinson, J.E. (1991b) A three month oral study in rats via the diet
with ethyl parathion to investigate ocular effects and cholin
activity. Unpublished report No. 89-3469 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Auletta, C.S. (1984) Acute oral toxicity in rats -- Acute dermal
toxicity in rabbits. Unpublished report No. 4997-84, 5505-84
prepared by Biodynamics Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Benjaminov, O., Hoffer, E., Taitelman, U., Urbak, B. & Brandis, L.
(1992) Parathion transfer and acetycholme activity in
an in vitro perfused human placenta. Vet. Hum. Toxicol.,
34, 10-12.
Bucks, D.A.W., Hinz, R.S., Sarason, R., Maibach, H.I. & Guy, R.H.
(1990) In vivo percutaneous absorption of chemicals -- A multiple
dose study in rhesus monkeys. Chem. Toxicol., 28, 129.
Carr, S.M.A. & Cuthbert, J.A. (1986) Ethyl parathion 98% technical
acute toxicity tests. Unpublished report No. 234117 prepared by
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Chan, L.T.F., Crowley, R.J. & Geyer, R. (1983) Detection and analysis
of aminoparathion in human postmortem specimens.
J. Forensic Sci., 28, 122-127.
Chang, S.K. & Riviere, J.E. (1991) Percutaneous absorption of
parathion in vitro in porcine skin: Effects of dose, temperature,
humidity and perfusate composition on absorptive flux.
Fundam. Appl. Toxicol., 17, 494-504.
Cheng, T. (1990) Ethyl parathion -- Nature of the residues in
livestock -- Laying hens. Unpublished report No. HLA 6222-100,
prepared by Hazleton Laboratories America, Inc. Submitted to WHO
by Cheminova Agro AS, Lemvig, Denmark.
Cheng, T (1987, 1988, 1990) Ethyl parathion -- Nature of the residue
in livestock -- Lactating goats. Unpublished report
No. HLA 6222-101, prepared by Hazleton Laboratories America, Inc.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepotocytes. Unpublished report prepared by Microbiological
Associates, Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1980a) A three month feed study of ethyl parathion in
mice. Unpublished report No. 77-2052 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1980b) A three month feeding study of ethyl parathion in
rats. Unpublished report No. 77-2054 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of ethyl
parathion in rats. Unpublished report No. 80-2457 prepared by
Biodynamics Inc., East Millstone, New Jersey, USA Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1984a) Acute oral toxicity study in rats. Unpublished
report No. 4997-84, prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984b) Acute dermal toxicity study in rabbits. Unpublished
report No. 5505-84 prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984c) A two year chronic feeding study of ethyl parathion
in rats. Unpublished report No. 77-2055 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Davis, J.E. (1975) The metabolism of parathion by liver microsomal
mixed-function oxidases. Dissertation, University of Miami,
Florida, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Degraeve, N., Moutschen, J., Moutschen-Dahmen, M., Gilot-Delhalle, J.,
Colizzi, A., Houbrechts, N. & Cholett, M.C. (1979) Genetic
effects of organophosphate insecticides in mouse (Abstract).
Mutat. Res., 64, 131.
Eiben, R. (1987) Parathion study for chronic toxicity and
cancerogenicity in Wistar rats. Unpublished report No. 16305 from
Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Eigenberg, D.A., Pazdernik, T.L. & Doull, J. (1983) Hernofusion and
pharmacokinetic studies with parathion and parathionoxon in the
rat and dog. Drug Metab. Disposition, 11, 366-370.
Flucke, W. & Kimmerle, G. (1977) Studies of oral acute toxicity of
simultaneously administered methamidophos and parathion ethyl.
Unpublished report from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Fredricksson, T. & Bigelow, J.K. (1-961) Tissue distribution of 32P
labeled parathion. Arch. Environ. Health, 2, 663-667.
Gagne, J. & Brodeur, J. (1972) Metabolic studies on the mechanisms of
increased susceptibility of weanling rats to parathion.
Can. J. Physiol. Pharmacol., 50, 902-915.
Greenough, R.J. & McDonald, P. (1986) Ethyl ara 98% technical: Acute
inhalation toxicity study in rats. Unpublished report
No. 632295 prepared by Inveresk Research International,
Musselburgh, United Kingdom. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Gröning, P. (1975) Tamaron-Special toxicity studies (potentiation).
Unpublished report prepared by Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Harbell, J.W. (1989) CHO/HGPRT confirmatory mutation assay with ethyl
parathion? Unpublished report prepared by Microbiological
Assocites Inc., Musselburgh, United Kingdom. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1982) E-605-ethyl study for acute oral toxicity.
Unpublished report No. 11230 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1986) E-605-ethyl study for skin sensitizing effect on
guinea pigs. Unpublished report No. 14999 from Bayer AG.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1982) Parathion (E-605-ethyl active ingredient) --
Micronucleus test on mouse to evaluate for mutagenic effect.
Unpublished report No. 10777 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1985) E-605 (c.n. parathion) Pol A1-test on E-coli to
evaluate for potential DNA damage. Unpublished report
No. 10777 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Herbold, B.A. (1986) E-605 (c.m. Parathion) -- Dominant lethal test on
male mouse to evaluate for mutagenic effect. Unpublished report
No. 14224 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Jensen, J.C. (1991) Mutagenicity test: In vitro mammalian cytogenetic
test in human lymphocytes performed with parathion-ethyl, Batch
No. 70818-01. Unpublished report prepared by Scaatox. Submitted
to WHO by Cheminova Agro AS, Lemvig, Denmark.
Kamataki, T. & Neal, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothionate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfer
atom. Mol. Pharmacol., 12, 933-944.
Lawlor, T.E. & Wagner, V.O. (1988) Ethyl parathion (97-98% technical)
-- Salmonella/mammalian-microsome plate incorporation
mutagenicity assay (Ames test) with a confirmatory assay.
Unpublished report No. USAE 605220388 prepared by Microbiological
Associates Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Mihail, F. & Gröning, P. (1981) E-605. Ethylactive ingredient/subacute
cutaneous toxicity study on rabbits. Unpublished report No 10342
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Moeller, H.S. & Rider, J.A. (1961) Studies on the anticholinesterase
effect of parathion and methyl parathion in humans (Abstract).
Fed Proc., 20, 434.
Nakatsugawa, T., Tolman, N.U. & Dahm, P.A. (1969) Degradation of
parathion in the rat. Biochem. 18, 1103-1114.
Neal, R.A. (1967) Studies on the metabolism of diethyl 4-nitrophenyl
phosphorothionate (parathion) in vitro. Biochem. J.,
103, 183-191.
Neeper-Bradley, T.L. (1990) Two-generation reproduction study of ethyl
parathion technical administered in the diet of CD (Sprague
Dawley) rats. Unpublished report from Busky Run Research Center,
Pennsylvania, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Nye, D.E. & Donough, H.W. (1976) Fate of insecticides administered
endotracheally to rats. Bull. Environ. Contam. Toxicol.,
15, 291-296.
Oesch, F. (1977) Ames test for Folidol E-605 (parathion). Unpublished
report No. E605301177. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Owens, E.J. (1976) The effect of ethyl parathion in the rat and dog
after acute and subacute inhalation and oral administration.
Technical report No. AMRL TR-76-1215 from Aerospace Medical
Research Laboratories, pp. 203-222. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Page, J.G. & Heath, G.E. (1991) Carcinogenicity study of ethyl
parathion administered by dosed feed to B6C3F1 mice.
Unpublished report No. E605210291 from Southern Research
Institute, Alabama, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Pauluhn, J. (1983) E605-Ethyl/Study for dermal and mucous membrane
irritant effects. Unpublished report No. 11401 from Bayer AG
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Pauluhn, J. (1984) E605 (c.m. parathion) -- Study for subacute
inhalative toxicity in the rat (exposure 15 × 6 hours).
Unpublished report No. 12395 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Putman, D.L. (1988) Micronucleus cytogenetic assay in mice.
Unpublished report No. T4772.122 prepared by Microbiological
Associates, Inc., USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Ramundo, J. (1979) A 4 week pilot study in mice with ethyl parathion.
Unpublished report No. 77-2051 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS. Lemvig, Denmark.
Renhof, M. (1984) Parathion -- Study for embryotoxic effects in rats
after oral administration. Unpublished report No. 12909 from
Bayer AG. Submitted to WHO by Cheminova Agro AS. Lemvig, Denmark.
Renhof, M. (1985) Parathion -- Study for embryotoxic effects on
rabbits after oral administration. Unpublished report No. 13288
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark
Rider, J.A., Moeller, H.C., Swader, J.I. & Weilerstein, R.W. (1958)
The effect of parathion on human red blood cell and plasma
cholinesterase. Arch. Ind. Health, 18, 442-445.
Schroeder (1983) A teratogenicity study in rats with ethyl parathion.
Unpublished report No. 82-2644 prepared by Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Simmon, V.F., Mitchell, R.D. & Jorgenson, T.A. (1976) In vitro
mutagenic studies on twenty pesticides. Toxicol. Appl.
Pharmacol., 37, 109.
Soliman, S.A., Farmer, J. & Curley, A. (1982) Is delayed neurotoxicity
a property of all organophosphorus compounds? A study with a
model compound: parathion. Toxicology, 23, 267-279.
Tegeris, A.S. & Underwood, P.C. (1977) Fourteen day feeding study in
the dog. Ethyl parathion. Unpublished report No. 77-113 prepared
by Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Thyssen, J. (1972) E-605-Ethyl -- Study for acute inhalation toxicity.
Unpublished report No. 8209 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Underwood, P.C. (1978) Ethyl parathion: Ninety day feeding to dogs.
Unpublished report No. USAE 65060378 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Weber, H., Patzchke, K. & Wegner, L.A. (1980) Parathion C14 (benzene
ring-labelled compound): Biokinetic studies in rats. Unpublished
report No. PH8820 from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Yang, L.L. (1988) Ethyl parathion (97/98% technical) CHO/HGPRT
mutation assay. Unpublished report No. USAE 605280388 from
Microbiological Associates Inc., USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of parathion are summarized in
Table 1.
The toxicity of parathion was not potentiated by simultaneous
oral administration of methamidophos (Flucke & Kimmerle, 1977),
temaron, or parathion methyl (Gröning, 1975). Additive acute toxicity
was reported for each of these chemicals in combination with
parathion.
(b) Short-term toxicity
Mice
Groups of five CD-1 mice of each sex received parathion at
dietary levels of 100, 200, or 400 ppm for 29 days. All of the animals
at the high dose either died or were sacrificed in a moribund
condition during the first 11 days of dosing. The mortality and
moribundity in the group at the medium dose was similar to that at the
high dose, with the exception of one surviving male. Clinical signs of
toxicity included tremors, decreased activity, and emaciation in all
treated groups. The mean body weights of males and females at the low
dose were 7-10% lower than those of controls, but food consumption
exceeded that of controls. No gross pathological changes that could be
attributed to the administration of parathion were reported in animals
at the low dose. Gastric erosion was seen in males at the two higher
doses and lung congestion in females at the high dose. There was no
NOAEL in view of the presence of cholinergic symptoms at 100 ppm,
equivalent to 15 mg/kg bw per day (Ramundo, 1979).
Groups of 15 male and 15 female Charles River COBS (ICR derived)
CD-1 mice received diets containing parathion (purity, 95.11%) at
doses of 0, 15, 50, or 100 ppm for three months. All animals survived
except for one male at the low dose and one female at the high dose.
The body weights of males at the high dose were significantly lower
than those of controls from the first week of the study until
termination. Sporadic, unsustained decreases in body weight were
reported for males at the middle dose and in females at the high dose,
and the differences in body weight were significant in weeks 1-5 and
8-9. At all other intervals, body weight was similar to controls. No
treatment-associated gross or microscopic lesions were reported.
Cholinesterase activity was not monitored in this study. The NOAEL was
50 ppm (equivalent to 7.5 mg/kg bw per day) on the basis of the
reported decreases in body weight in males receiving 100 ppm
(Daly, 1980).
Table 1. Acute toxicity of parathion
Species Sex Route LD50 or LC50, Purity Reference
(mg/kg bw or (%)
mg/litre air)
Rat Male Oral 22 98 Carr & Cuthbert (1986)
Female 2
Rat Male Oral 7 NR Auletta (1984)
Female 2.6
Rat Male, female Oral 6.85 NR Owens (1976)
Rat Male Oral 13.7 NR Heimann (1982)
Rat Male, female Dermal 73 98 Carr & Cuthbert (1986)
Rabbit Male Dermal 910 NR Daly (1984b)
Female 1400
Rat Male, female Inhalation (4-h) 0.03 98 Greenough & McDonald (1986)
Rat Male Inhalation (1-h) 245 97.8 Thyssen (1972)
Female 71
Rat Male Inhalation (4-h) 77-91 97.8 Thyssen (1972)
Female 24
NR, not reported
Rats
Groups of 10 male and 10 female Wistar rats were exposed by
inhalation by head-nose to a parathion (purity, 98%) aerosol at
concentrations of 0.25, 0.92, or 3.9 mg/m3 for up to 6 h per day for
15 days. Animals of each sex at 0.92 mg/m3 showed inhibition of
plasma and erythrocyte cholinesterase activity in comparison with mean
baseline values; at the highest dose, plasma, erythrocyte, and brain
cholinesterase activities were reduced. Animals receiving the highest
dose also showed nonspecific, transient behavioural changes; females
had muscular tremors and increased mortality. With the exception of
increased organ congestion in females at tile high dose, there were no
gross or histopathological lesions that could be associated with the
administration of parathion. The NOAEL was 0.25 mg/m3 (Fauluhn,
1984).
Groups of 20 Sprague-Dawley rats of each sex were fed diets
containing parathion (purity, 95.11%) at 0, 2.5, 25, or 75 ppm for
three months. Animals were observed for clinical signs of toxicity,
and body weights and food consumption were recorded weekly. Clinical
chemical and haematological parameters and plasma and erythrocyte
cholinesterase activities were determined before the administration of
parathion and at designated intervals during the study. Brain
acetylcholinesterase activity was determined in 10 males and 10
females before assignment to the treatment groups and in groups of 10
animals of each sex per group at three months. Urinalysis was
conducted at one and three months of the study. Nine of the females at
the high dose died or were sacrificed during the study. Anogenital
staining, tremors, and emaciation were reported in females at the high
dose, which also had elevated levels of serum aspartate and alanine
aminotransferases and alkaline phosphatase and decreased haemoglobin
and haematocrit. None of these alterations in clinical pathology could
be attributed to parathion, since they were within the normal
biological ranges.
Decreases in plasma and erythrocyte cholinesterases were reported
in all treated animals. At 2.5 ppm, a statistically significant
decrease in erythrocyte acetylcholinesterase was reported in females,
amounting to 64% of the activity in controls at one month, 73% of that
at two months, and 44% of that at three months. At 25 ppm, a 20%
inhibition of plasma and erythrocyte cholinesterase activities was
seen in treated animals in comparison with controls at all collection
times. Brain acetylcholinesterase activity was significantly inhibited
in females but not males at the middle dose. At 75 ppm, there was
significant inhibition of erythrocyte and plasma cholinesterase
activities in males at two and three months and in females at all
intervals. Brain acetylcholinesterase activity was 40% of that of
controls in females and 38% in males after the third month of
treatment. The NOAEL was 2.5 ppm, equal to 0.18 mg/kg bw per day, on
the basis of a significant depression in brain acetylcholinesterase in
females (Daly, 1980b).
Rabbits
Parathion formulated with water and cremaphor was administered to
male and female New Zealand white rabbits at 0, 0.1, or 2 mg/kg bw per
day for 15 days at a volume of 0.5 ml/kg bw on shaved backs or flanks
and was left in contact with the skin for 6 h per exposure. No dermal
irritation and no compound-related effects on clinical behaviour,
clinical signs, or gross or microscopic pathological appearance were
reported. Plasma, erythrocyte, and brain cholinesterase activities
were all inhibited at 2 mg/kg bw per day; however, no cholinergic
symptoms were reported. The NOAEL was 0.1 mg/kg bw per day (Mihail &
Gröning, 1981).
Dogs
Groups of two beagle dogs of each sex were fed diets containing
parathion at 0, 1.5, 3.0, or 6 mg/kg bw per day for 14 days and were
observed for clinical signs of toxicity, mortality, food intake,
weight gain, and gross pathological lesions. All animals survived the
study. Food intake was significantly lower in males at the high dose
than in controls. There was a dose-related increase in the incidence
of vomiting in all groups receiving parathion, and after two weeks
animals at the two higher doses had lower body-weight gain and feed
efficiency than controls. No gross lesions were seen in any of the
treated dogs. Cholinesterase was not monitored in this study, but
appeared to have been affected on the basis of the clinical symptom of
vomiting. There was no NOAEL because of the presence of signs of
cholinergic poisoning at the lowest dose (Tegeris & Underwood, 1977).
Groups of 16 male and 16 female beagle dogs were randomly
assigned to groups designated to receive dietary levels of parathion
(purity, 94.4%) at 0, 0.3,1, or 3 mg/kg bw per day for 90 days.
Animals were observed daily for clinical signs of toxicity and for
survival. Clinical chemical and haematological parameters were
determined before treatment and at predetermined intervals during the
90-day period. Blood and plasma cholinesterase levels were determined
during the study, and brain cholinesterase was measured after
sacrifice. Organ weights were recorded and gross and microscopic
pathological changes were evaluated. Plasma cholinesterase activity
was significantly reduced in all treated groups in comparison with
controls; erythrocyte acetylcholinesterase activity was significantly
inhibited in all females and in males at the two higher doses. With
the exception of the effects on plasma and erythrocyte cholinesterase
activity, there were no alterations in clinical chemistry, and
haematological and gross and microscopic pathological parameters, body
weights, and food intake were also unaffected. No compound-associated
effects were reported on brain acetylcholinesterase activity. Soft
stools were observed in all groups, but the frequency and severity of
this finding could not be associated with increasing dietary levels of
parathion. There was no NOAEL (Underwood, 1978).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female B6C3F1 mice received diets
containing parathion at 0, 60, 100, or 140 ppm for 18 months. There
was no compound-related effect on survival. The body weights of males
at the highest dose were 14% lower than those of controls. Clinical
signs of toxicity reflecting the cholinergic properties of the
material were reported in all treated animals during the first month
of the study, including laboured breathing, pallor, hypoactivity, and
tremors. The increased incidence was dose-related. At 18 months, the
inhibition of plasma and erythrocyte cholinesterase was > 20% of the
control values in all groups. Brain acetylcholinesterase activity was
inhibited in males and females at the high dose, to 87% at day 10 and
85% at 18 months in males and 82% at day 10 and 76% at 18 months in
females. Brain:kidney weight ratios were significantly lower than
those of controls in female mice receiving 140 ppm; decreases were
also reported in males receiving 100 and 140 ppm. Alveolar-bronchiolar
adenomas were seen in 16/50 male mice receiving 60 ppm but not at the
next two doses. There was no NOAEL, as signs of cholinergic poisoning
were seen at all doses (Page & Heath, 1991).
Rats
Groups of 60 male and 60 female Sprague-Dawley (CrlCD:BR) rats
received diets containing parathion (purity, 95.11%) at 0, 0.5, 5, or
50 ppm for up to 28 months. The mean body weights of animals receiving
50 ppm were significantly decreased throughout the study, the
decreases ranging from 10 to 16% in males and 8 to 25% in females.
There was no corresponding decrease in food consumption. Males and
females at the high dose had slightly lowered haemoglobin and
haematocrit in comparison with controls. Significantly lower values
were reported in females at the high dose at 6, 12, and 18 months;
however, the effect does not appear to be of biological significance
as the values are within the normal range for this strain. Plasma
cholinesterase activity was reduced in males and females at the two
higher doses throughout the study. Erythrocyte acetylcholinesterase
values were reduced in animals of each sex but not to statistically or
biologically significant levels. Brain acetylcholinesterase activity
was significantly reduced in males (22% of controls) and females (18%
of controls) at the highest dose.
Treatment was associated with increased incidences of tremors,
alopecia, anogenital staining, and abnormal gait in females at the
high dose, which also had elevated alkaline phosphatase activity and
increased blood urea nitrogen; however, the reported values are within
the normal range for this strain. There were no effects on organ
weight and no gross lesions associated with administration of the test
material. The incidence of retinal degeneration was increased in
females at the high dose, and the severity of peripheral neuropathy
characterized by myelin sheath degeneration in the proximal sciatic
nerve and myelin corrugations, demyelinated lengths, and myelin
ovoids was increased in males at the high dose. There was no
treatment-associated carcinogenicity. The systemic NOAEL was 5 ppm
(equivalent to 0.25 mg/kg bw per day), and the LOAEL was 50 ppm
(2.5 mg/kg bw per day) on the basis of effects on brain, plasma, and
erythrocyte cholinesterase activity, retinal degeneration, and
peripheral neuropathy (Daly, 1984c).
Groups of 50 Wistar rats of each sex received diets containing
parathion (purity, 96.7%) at doses of 0, 2, 8, or 32 ppm for two
years; a further 15 rats of each sex received the dietary
concentrations for 12 months. Animals at the highest dose had poor
general condition and chromodacryorrhoea, and females also had
tremors, hair loss, and head tilt at greater frequency than controls;
however, the increase was not statistically significant. Females at
the highest dose experienced 36% mortality, in comparison with 22% for
controls. Plasma and erythrocyte cholinesterase activities were
inhibited by > 20% of the control level in animals of each sex at 8
or 32 ppm, the inhibition being greater at the highest dose; brain
acetylcholinesterase activity was also statistically and biologically
lower than that in controls at the highest dose. There was no
compound-related effect on urinary or haematological parameters. The
body weights of animals of each sex at the highest dose were decreased
in comparison with controls; however, there were no apparent effects
on organ weights.
Histologically, there was a nonsignificantly increased incidence
of proliferative lesions in the exocrine pancreas in males receiving 8
or 32 ppm. At 32 ppm, there were also adverse effects on the retina,
as indicated by structural degeneration and retinal atrophy. No
treatment-related oncogenic effects were reported. No significant
effects on peripheral nerves were reported at the highest dose. The
systemic NOAEL in this strain was 8 ppm (equivalent to 0.4 mg/kg bw
per day), and the LOAEL was 32 ppm (equivalent to 1.6 mg/kg bw per
day), on the basis of inhibition of brain, plasma, and erythrocyte
cholinesterase activity (Eiben, 1987).
(d) Reproductive and developmental toxicity
Rats
Groups of 24 female Sprague-Dawley rats in which mating was
confirmed by vaginal smear were given parathion (purity, 95.1.1%) by
gavage on days 6-19 of gestation at doses of 0, 0.25, 1.0, or
1.5 mg/kg bw per day. On day 20, all animals were sacrificed, the
uteri were removed, and the animals were subjected to a complete gross
post-mortem examination and examination of the reproductive system.
The numbers of live and dead fetuses, late and early resorptions,
implantation sites, and corpora lutea were recorded. Each fetus was
examined grossly to determine whether malformations were present;
one-half of each litter was evaluated for soft-tissue defects and the
other for skeletal defects. The mortality rate was increased in dams
at the high dose, which also had significantly lower corrected body
weights than controls. These animals had an increased incidence of
mucoid nasal discharge at day 15 and an increased incidence of dry
nasal discharge on day 20. No compound-related effects on fetal
development were reported. The NOAEL was 1.5 mg/kg bw per day, and the
NOAEL for maternal toxicity was 1.0 mg/kg bw per day (Schroeder,
1983).
Groups of 25 Wistar rats were fed parathion (purity, 98.8%) in a
Cremophor emulsion at doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on
days 6-15 of gestation. Caesarean sections were performed on day 20.
Thirteen of the dams at the high dose died as a result of circulatory
and/or respiratory collapse, and 10 of these were found to have red
lungs at necropsy. Clinical signs of toxicity in the animals at the
high dose included tremors, rough coats, light stools, sunken flanks,
and reduced water intake. A slight, nonsignificant decrease in
body-weight gain was reported in animals receiving the highest dose.
No developmental effects were reported. The number of fetal
malformations was higher in the group at 1 mg/kg bw per day, but this
increase was driven by the increased incidence of malformations in one
litter, and there were no significant differences in the litter
incidence of fetal malformations. The NOAEL for maternal toxicity was
0.3 mg/kg bw per day, and the NOAEL for developmental toxicity was
1.0 mg/kg bw per day (Renhof, 1984).
A two-generation study of reproductive toxicity was conducted in
Sprague-Dawley rats given diets containing parathion (purity, 95.1%)
at doses of 0, 0.5, 5, or 25 ppm. Animals in the F0 generation
received the test material daily for 14 weeks before mating and
throughout mating, gestation, and lactation. Animals selected as F1
parents received parathion 18 weeks before mating and throughout the
mating, gestation, and lactation periods. F0 animals were sacrificed
after the F1 generation was weaned, and F1 animals were sacrificed
about five weeks after the F2 generation had been weaned; F2
animals were sacrificed at weaning. Tremors were observed in F0
females at the high dose, and the mean body weights of these animals
were reduced throughout the premating period and during gestation and
lactation. Tremors were also reported in F1 females at the high dose
during lactation. Slight, nonsignificant reductions in body weight
were reported in animals of each sex in the F1 generation at the
high dose. On day 21 of the lactation period, reduced mean body
weights were seen in F1 pups of each sex at 25 ppm and in F1
females at 5 ppm. The reduction was not statistically significant but
was reproduced in F2 pups of each sex at 25 ppm. The decrease in
mean pup weight may not have been a primary reproductive effect but an
effect associated with the systemic toxicity of the compound. The
NOAEL for reproductive toxicity was 25 ppm, equivalent to 1.25 mg/kg
bw per day, and that for parental toxicity was 5 ppm, equivalent to
0.25 mg/kg bw per day (Daly, 1982).
In another two-generation study, groups of 28 female
Sprague-Dawley rats were given parathion (purity, 96.7%) at dietary
levels of 0, 1, 10, or 20 ppm. F0 animals received the compound for
10 weeks before mating, and animals selected as F1 parents were
exposed for 11 weeks before mating. Toxicity was seen in F0 and F1
animals of each sex at 10 and 20 ppm, manifested as significant
reductions in plasma, erythrocyte, and/or brain cholinesterase
activities. At 10 ppm, plasma cholinesterase activity was inhibited by
> 20% in comparison with controls; inhibition of erythrocyte
acetylcholinesterase activity was not as pronounced. Brain
acetylcholinesterase activity was < 90% of control values in F0 and
F1 animals of each sex, and the reduction was considered to be
biologically significant. At 20 ppm, brain acetylcholinesterase and
plasma cholinesterase activities were markedly inhibited in females of
both generations. The body weights of dams at lactation were decreased
in both generations at 20 ppm from day 7 to day 21 in the F0
maternal animals and on day 21 in the F1 maternal animals. There
were no compound-related effects on reproductive parameters in
parental animals. In the F1 litters, reduced body weights and
reduced body-weight gains were reported at the highest dose, and
postnatal pup deaths were nonsignificantly increased on lactation days
14-21. Similar findings were not found for the F2 litters. The
parental NOAEL was 1 ppm, equivalent to 0.05 mg/kg bw per day, on the
basis of inhibition of brain acetylcholinesterase; the perinatal NOAEL
was 10 ppm, equivalent to 1 mg/kg bw per day, on the basis of adverse
effects on body weight of offspring at 20 ppm (Neeper-Bradley, 1990).
Rabbits
Groups of 15 female Himalayan rabbits received parathion (purity,
98.8%) by gavage at doses of 0, 0.03, 0.1, or 0.3 mg/kg bw per day on
days 6-18 of gestation. All animals were sacrificed on day 29, and the
fetuses were removed. There were no effects on maternal animals or
offspring. The NOAEL for both maternal and developmental toxicity was
0.3 mg/kg bw per day (Renhof, 1985).
(e) Genotoxicity
Studies of the genotoxicity of parathion are summarized in
Table 2.
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Three male and three female New Zealand white rabbits received
0.5 ml of a test material containing parathion on an epilated area of
the skin for 4 h. Mild skin irritation, characterized by very slight
erythema and very slight oedema, occurred within 1 h. All signs of
irritation had subsided by 72 h (Carr & Cuthbert, 1986).
In another study in New Zealand white rabbits, the compound was
classified as a mild irritant, with a primary irritation index of 0.92
(Pauluhn, 1983).
The scores obtained with the Magnusson-Klegman technique in
Bor:SPF, DPHW guinea-pigs indicated that parathion is not a
sensitizing agent (Heimann, 1986).
(ii) Neurotoxicity
Groups of five beagle dogs of each sex were given parathion
(purity, 98%) for six months in gelatin capsules at doses of 0,
0.0024, 0.0079, or 0.7937 mg/kg bw per day. Plasma, erythrocyte,
brain, and retinal cholinesterase activities were determined, and
ocular assessments were made using slit lamp examinations,
retinoscopic examinations, and electroretinograms. Intra-ocular
pressure and pupillary status were also assessed. No treatment-related
effects were reported, and hence no functional ocular impairment was
observed. Plasma and erythrocyte cholinesterase activities were
inhibited in animals of each sex at the two highest doses. Brain
acetylcholinesterase activity was inhibited in the pons but not in the
cerebellum of male beagles at the highest dose. Retinal cholinesterase
was also significantly decreased animals of each sex at the highest
dose, but the decrease was not associated with morphological changes
in the eye. The NOAEL was 0.0079 mg/kg bw per day on the basis of
inhibition of brain and retinal acetylcholinesterase activities
(Atkinson, 1991a).
Groups of female Sprague-Dawley rats received parathion (purity,
98%) in the diet at levels designed to achieve 0.04, 0.4, or 4 mg/kg
bw per day, for three months. Control rats received the basal diet. At
4 mg/kg bw, decreases were seen in body weight (29%), plasma
cholinesterase (87-95%), and brain acetylcholinesterase (83.5%).
Electroretinograms revealed an increase in the latency and a decrease
in the amplitude of the b-wave. At 0.4 mg/kg bw, the plasma
cholinesterase levels were 37-42% lower than those of controls and the
latency and amplitude of the b-wave were affected in the same way as
in rats receiving 4 mg/kg bw. No morphological changes were seen in
animals at 0.4 or 4 mg/kg bw. The ocular changes observed at the two
highest doses were considered not to be significant toxicologically,
as there was wide variation in the individual and group mean values
for b-wave latency and amplitude. Furthermore, no adverse effects were
seen by electron microscopy. The NOAEL was 0.4 mg/kg bw per day on the
basis of inhibition of brain acetylcholinesterase (Atkinson, 1991b).
In the 26-month carcinogenicity study conducted in Sprague-Dawley
rats, peripheral neuropathy in males was associated with the
administration of parathion at a dietary level of 50 ppm (2.5 mg/kg bw
per day). Fibres teased from the sciatic nerves of males at the high
dose showed an increased percentage of myelin corrugations and
increased demyelination (Daly, 1984a).
The potential of parathion to produce organophosphorus-induced
delayed neurotoxicity has been investigated in vivo in animals and
in vitro in human neuroblastoma cell lines. Parathion did not induce
delayed neurotoxicity in hens after subchronic exposure. Similar
conclusions were reached in a study in CD-1 mice, in which parathion
was administered orally for 30 days at a dose of 6.75 mg/kg bw per
day. There was no delayed neurotoxicity, and brain neurotoxic
esterases were not inhibited (Solimon et al., 1982).
3. Observations in humans
Parathion was given to human volunteers at doses ranging from
3 mg/day for 28 days to 6 mg/day for 43 days, and plasma and
erythrocyte cholinesterase levels were monitored. The highest dose
induced immediate depression of both types of cholinesterase, to
levels 10-15% of baseline values determined before treatment, which
persisted for two weeks after treatment was discontinued; erythrocyte
acetylcholinesterase activity was inhibited for up to 43 days after
the last dose (Moeller & Rider, 1961).
Five men received parathion at 7.5 mg/day for five days. Plasma
cholinesterase was more sensitive than erythrocyte acetylcholine-
sterase activity to the inhibitory effects of parathion, with an
average decrease of 28% on day 16 after treatment (Rider et al.,
1958).
Table 2. Results of tests for the genotoxicity of parathion
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 3.15-3150 µg/plate 98.9 Negative Oesch (1977)
TA100, TA1537
Reverse mutation S. typhimurium TA100, < 10 000 µg/plate 97-98 Negative Lawlor & Wagner (1988)
TA1535, TA1537, TA1538
hprt mutation Chinese hamster ovary cells 0.4-1.0 µl/litre 96.7 Negative Harbell (1989)
hprt mutation Chinese hamster ovary cells 0.03-0.2 µl/litre 97-98 Suspect Yang (1988)
Unscheduled DNA Human WI-38 cell line 10-5 µl/ml NR Positive Simmon et al. (1979)
synthesis 10-6 µl/ml Negativea
Unscheduled DNA Rat hepatocytes 3 × 10-5-3 × 10-2µl/ml 97-98 Negative Curren (1988)
synthesis
DNA damage E coli K12 and W-3110 625-10 000 µg/plate 98 Negative Herbold (1985)
Cytogenetic damage Human lymphocytes 9.8, 20, 39 µg/mlb 98 Negative Jensen (1991)
78, 156, 313 µg/mla
In vivo
Dominant lethal mutation Mouse 10 mg/kg bw orally 98.8 Negative Herbold (1986)
Dominant lethal mutation Mouse 10 mg/kg bw orally NR Negative Degraeve et al. (1979)
Micronucleus formation Mouse bone marrow 2 × 5, 2 × 10 mg/kg bw 95.9 Negative Herbold (1982)
orally
Micronucleus formation Mouse bone marrow 10-65 mg/kg bw 97-98 Negative Putman (1988)
NR, not reported
a With metabolic activation
b Without metabolic activation
Comments
Parathion is readily absorbed from the respiratory and digestive
tracts and is excreted primarily in the urine. Two hypotheses have
been proposed for its metabolism; however, the metabolic spectrum is
the same in both. Parathion is metabolized to paraoxon and diethyl
phosphorothioic acid. Paraoxon is further metabolized, and following
its oral administration to rats diethyl phosphate, diethyl
phosphorothioate, de-ethyl paraoxon, and para-nitrophenol were
identified in the urine. In cattle, ruminal microorganisms are
believed to be responsible for the production of aminoparathion and
aminoparaoxon.
Parathion is extremely hazardous when given orally (LD50 =
2 mg/kg bw) or by inhalation (4-h LC50 = 0.03 mg/litre) and
moderately hazardous when given dermally (LD50 = 73 mg/kg bw). The
compound has been characterized in studies in laboratory animals as a
mild dermal and ocular irritant and a s a non-sensitizing agent. When
it was administered with other organophosphate pesticides, its toxic
effects were not potentiated. WHO has classified parathion as
'extremely hazardous'.
In a three-week study in rabbits treated dermally, the NOAEL was
0.1 mg/kg bw per day on the basis of depression of plasma,
erythrocyte, and brain cholinesterase activities at 2 mg/kg bw per
day. In a three-week study by inhalation in rats, the NOAEL was
0.9 mg/litre on the basis of decreases in brain, plasma, and
erythrocyte cholinesterase activity. In a 14-day study in dogs,
parathion was administered orally at doses of 0, 1.5, 3, or 6 mg/kg bw
per day. There was no NOAEL, as clinical cholinergic signs were
observed at the lowest dose tested. Cholinesterase activity was not
monitored in this study. In a 90-day study in dogs at doses of 0, 0.3,
1, or 3 mg/kg bw per day, the NOAEL was 3 mg/kg bw per day.
Cholinesterase activity was not measured.
In a 29-day study in mice at doses of 0, 100, 200, or 400 ppm
(equivalent to 15, 30, and 60 mg/kg bw per day), clinical signs of
toxicity were reported in all groups. Cholinesterase activity was not
determined, and there was no NOAEL. In a 90-day study in mice at
dietary concentrations of 0, 15, 50, or 100 ppm, the NOAEL was 50 ppm
(equivalent to 7.5 mg/kg bw per day) on the basis of decreased body
weights of males. Cholinesterase activity was not monitored. When
parathion was administered to rats for 90 days at dietary
concentrations of 0, 2.5, 25, or 75 ppm, the NOAEL was 2.5 ppm (equal
to 0.2 mg/kg bw per day), on the basis of depression of brain
acetylcholinesterase.
In a two-year study in rats, parathion was not associated with
carcinogenicity when administered at dietary concentrations of 0, 0.5,
5, or 50 ppm. The NOAEL for systemic toxicity was 5 ppm (equivalent to
0.25 mg/kg bw per day) on the basis of decreased brain, plasma, and
erythrocyte cholinesterase activity, retinal atrophy, and increased
severity of degenerative changes in the sciatic nerve. In another
study in rats, given dietary levels of 0, 2, 8, or 32 ppm for two
years, there was again no evidence of carcinogenicity. The NOAEL for
systemic toxicity was 8 ppm (equivalent to 0.4 mg/kg bw per day) on
the basis of decreases in brain acetylcholinesterase activity and
retinal atrophy. No effects on sciatic nerves were reported at the
highest dose tested.
In mice receiving dietary concentrations of parathion at 0, 60,
100, or 140 ppm for 18 months, there was no NOAEL, as cholinergic
signs were seen at all doses.
Two studies of developmental toxicity were conducted in rats. In
the first study, parathion was administered by gavage at doses of 0,
0.25, 1, or 1.5 mg/kg bw on gestation days 6-19. The NOAEL for
maternal toxicity was 1 mg/kg bw per day on the basis of increased
mortality, and the NOAEL for developmental toxicity was 1.5 mg/kg bw
per day. In the second study, parathion was administered by gavage at
doses of 0, 0.1, 0.3, or 1 mg/kg bw per day on gestation days 6-15.
The NOAEL for developmental toxicity was 1 mg/kg bw per day and that
for maternal toxicity was 0.3 mg/kg bw per day on the basis of
increased mortality and clinical signs of toxicity.
Two studies of developmental toxicity were conducted in rabbits.
In the first study, parathion was administered by gavage on gestation
days 7-19 at 1,4, or 16 mg/kg bw per day. The NOAEL for developmental
toxicity was 16 mg/kg bw per day, and the NOAEL for maternal toxicity
was 4 mg/kg bw per day on the basis of decreased body-weight gain. In
the second study, parathion was administered by gavage on days 6-18 of
gestation at 0, 0.03, 0.1, or 0.3 mg/kg bw per day. The NOAEL for both
maternal and developmental toxicity was 0.3 mg/kg bw per day.
In a two-generation study of reproductive toxicity in rats, doses
of 0, 0.5, 5, or 25 ppm were administered in the diet. Dams at the
highest dose had tremors, and a reduction in body weight was seen
during premating, gestation, and lactation. The NOAEL for reproductive
toxicity was 25 ppm (equivalent to 1.2 mg/kg bw per day); the NOAEL
for maternal toxicity was 5 ppm (equivalent to 0.25 mg/kg bw per day)
on the basis of the observation of tremors in F0 and F1 females.
In the second study, parathion was administered at dietary
concentrations of 0, 1, 10 or 20 ppm. The NOAEL for reproductive
toxicity was 20 ppm (equivalent to 1 mg/kg bw per day); the NOAEL for
perinatal toxicity was 10 ppm (equivalent to 1 mg/kg bw per day) on
the basis of reduced body weights; and the NOAEL for maternal toxicity
was 1 ppm (equivalent to 0.05 mg/kg bw per day) on the basis of
decreased brain acetylcholinesterase activity.
Special studies were conducted to assess the ocular toxicity of
parathion. When parathion was administered to dogs at doses of 0,
0.002, 0.008, or 0.8 mg/kg bw per day for six months, no functional
impairment of the eye was observed. The NOAEL was 0.008 mg/kg bw per
day on the basis of depression of brain and retinal acetylcholine-
sterase activity. In a three-month study of toxicity in female rats,
parathion was administered at levels of 0, 0.04, 0.4, or 4 mg/kg bw
per day. The NOAEL was 0.4 mg/kg bw per day on the basis of depression
of brain acetylcholinesterase activity. No significant effects on
ocular toxicity were reported at any dose.
Parathion was not associated with organophosphorus-induced
delayed neurotoxicity in hens, but it induced demyelination in the
peripheral nerves of rats at a dietary level of 50 ppm (equivalent to
2.5 mg/kg bw per day). The NOAEL was 0.25 mg/kg bw per day.
In a study conducted earlier in humans, an NOAEL of 7.5 mg/day
was determined on the basis of lack of effect on erythrocyte
acetylcholinesterase.
Parathion has been adequately tested for genotoxicity in a range
of tests in vitro and in vivo. The Meeting concluded that
parathion is not genotoxic.
An ADI of 0-0.004 mg/kg bw was established on the basis of an
NOAEL of 0.4 mg/kg bw per day in the two-year study in rats for
retinal atrophy and inhibition of brain acetylcholinesterase at the
higher dose. A safety factor of 100 was used. Lower NOAELs in animals,
based only on inhibition of erythrocyte or brain acetylcholinesterase,
were not considered relevant because of the availability of an NOAEL
for erythrocyte acetyl-cholinesterase inhibition in humans, which was
0.1 mg/kg bw per day.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 50 ppm, equivalent to 7.5 mg/kg bw per day (90-day study of
toxicity)
Rat: 1 ppm, equivalent to 0.05 mg/kg bw per day (study of
reproductive toxicity)
2.5 ppm, equal to 0.18 mg/kg bw per day (90-day study of
toxicity)
8 ppm, equivalent to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
Dog: 0.008 mg/kg bw per day (six-month study of toxicity)
Estimate of acceptable daily intake for humans
0-0.004 mg/kg bw
Estimate of acute reference dose
An acute reference dose of 0.01 mg/kg bw was derived by applying
the usual 10-fold safety factor to an NOAEL of 7.5 mg/day (highest
oral dose), corresponding to about 0.1 mg/kg bw per day, in humans.
This was based on the absence of inhibition of erythrocyte
acetylcholinesterase.
Studies that would provide information useful for continued evaluation
of the compound
Further observation in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to parathion
Exposure Relevant route, study type, species Results, remarks
Short-term ( 1-7 days) Skin, irritation, rabbit Irritating
Eye, irritation, rabbit Irritating
Skin, sensitization, guinea-pig Non-sensitizing
Oral, toxicity, rat LD50 = 2 mg/kg bw
Dermal, toxicity, rat LD50 = 73 mg/kg bw
Inhalation, toxicity, rat LC50 = 0.03 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 1 mg/kg bw per day, based on
decreased brain acetylcholinesterase. No
dermal effect
Repeated inhalation, 21 days, toxicity, rat NOAEL = 0.92 mg/m3 based on decreased
brain acetylcholinesterase
Repeated oral, reproductive toxicity, rat NOAEL = 0.05 mg/kg bw per day for
maternal toxicity, decreased brain
cholinesterase; NOAEL = 1 mg/kg bw per
day for reproductive toxicity; NOAEL =
1 mg/kg bw per day for perinatal toxicity
based on reduced body weight
Repeated oral, developmental, NOAEL = 1 mg/ kg bw per day for developmental
toxicity, rat toxicity; NOAEL = 0.3 mg/kg per
day for maternal toxicity based on increased
mortality and cholinergic signs
Repeated oral, developmental, NOAEL >0.3 mg/kg bw per day for
toxicity, rabbit maternal and developmental toxicity
Long-term (> one year) Repeated oral, two years, toxicity and NOAEL = 0.25 mg/kg bw per day based on
carcinogenicity, rat lowered brain acetylcholinesterase. No
carcinogenicity
References
Ahdaya, S.M., Monroe, R.J. & Guthrie, F.E. (1981) Absorption and
distribution of intubated insecticides in fasted mice.
Pestic. Biochem. Physiol., 16, 38-46.
Atkinson, J.E. (1991a) A six month oral study of ethyl parathion in
dogs with specific emphasis on ocular effects. Unpublished report
No. 89-3439 prepared by Biodynamics, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Atkinson, J.E. (1991b) A three month oral study in rats via the diet
with ethyl parathion to investigate ocular effects and cholin
activity. Unpublished report No. 89-3469 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Auletta, C.S. (1984) Acute oral toxicity in rats -- Acute dermal
toxicity in rabbits. Unpublished report No. 4997-84, 5505-84
prepared by Biodynamics Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Benjaminov, O., Hoffer, E., Taitelman, U., Urbak, B. & Brandis, L.
(1992) Parathion transfer and acetycholme activity in
an in vitro perfused human placenta. Vet. Hum. Toxicol.,
34, 10-12.
Bucks, D.A.W., Hinz, R.S., Sarason, R., Maibach, H.I. & Guy, R.H.
(1990) In vivo percutaneous absorption of chemicals -- A multiple
dose study in rhesus monkeys. Chem. Toxicol., 28, 129.
Carr, S.M.A. & Cuthbert, J.A. (1986) Ethyl parathion 98% technical
acute toxicity tests. Unpublished report No. 234117 prepared by
Inveresk Research International, Musselburgh, United Kingdom.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Chan, L.T.F., Crowley, R.J. & Geyer, R. (1983) Detection and analysis
of aminoparathion in human postmortem specimens.
J. Forensic Sci., 28, 122-127.
Chang, S.K. & Riviere, J.E. (1991) Percutaneous absorption of
parathion in vitro in porcine skin: Effects of dose, temperature,
humidity and perfusate composition on absorptive flux.
Fundam. Appl. Toxicol., 17, 494-504.
Cheng, T. (1990) Ethyl parathion -- Nature of the residues in
livestock -- Laying hens. Unpublished report No. HLA 6222-100,
prepared by Hazleton Laboratories America, Inc. Submitted to WHO
by Cheminova Agro AS, Lemvig, Denmark.
Cheng, T (1987, 1988, 1990) Ethyl parathion -- Nature of the residue
in livestock -- Lactating goats. Unpublished report
No. HLA 6222-101, prepared by Hazleton Laboratories America, Inc.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Curren, R.D. (1988) Unscheduled DNA synthesis in rat primary
hepotocytes. Unpublished report prepared by Microbiological
Associates, Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1980a) A three month feed study of ethyl parathion in
mice. Unpublished report No. 77-2052 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1980b) A three month feeding study of ethyl parathion in
rats. Unpublished report No. 77-2054 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1982) A two generation reproduction study of ethyl
parathion in rats. Unpublished report No. 80-2457 prepared by
Biodynamics Inc., East Millstone, New Jersey, USA Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Daly, I.W. (1984a) Acute oral toxicity study in rats. Unpublished
report No. 4997-84, prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984b) Acute dermal toxicity study in rabbits. Unpublished
report No. 5505-84 prepared by Biodynamics Inc., East Millstone,
New Jersey, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Daly, I.W. (1984c) A two year chronic feeding study of ethyl parathion
in rats. Unpublished report No. 77-2055 prepared by Biodynamics
Inc., East Millstone, New Jersey, USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Davis, J.E. (1975) The metabolism of parathion by liver microsomal
mixed-function oxidases. Dissertation, University of Miami,
Florida, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Degraeve, N., Moutschen, J., Moutschen-Dahmen, M., Gilot-Delhalle, J.,
Colizzi, A., Houbrechts, N. & Cholett, M.C. (1979) Genetic
effects of organophosphate insecticides in mouse (Abstract).
Mutat. Res., 64, 131.
Eiben, R. (1987) Parathion study for chronic toxicity and
cancerogenicity in Wistar rats. Unpublished report No. 16305 from
Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Eigenberg, D.A., Pazdernik, T.L. & Doull, J. (1983) Hernofusion and
pharmacokinetic studies with parathion and parathionoxon in the
rat and dog. Drug Metab. Disposition, 11, 366-370.
Flucke, W. & Kimmerle, G. (1977) Studies of oral acute toxicity of
simultaneously administered methamidophos and parathion ethyl.
Unpublished report from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Fredricksson, T. & Bigelow, J.K. (1-961) Tissue distribution of 32P
labeled parathion. Arch. Environ. Health, 2, 663-667.
Gagne, J. & Brodeur, J. (1972) Metabolic studies on the mechanisms of
increased susceptibility of weanling rats to parathion.
Can. J. Physiol. Pharmacol., 50, 902-915.
Greenough, R.J. & McDonald, P. (1986) Ethyl ara 98% technical: Acute
inhalation toxicity study in rats. Unpublished report
No. 632295 prepared by Inveresk Research International,
Musselburgh, United Kingdom. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Gröning, P. (1975) Tamaron-Special toxicity studies (potentiation).
Unpublished report prepared by Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Harbell, J.W. (1989) CHO/HGPRT confirmatory mutation assay with ethyl
parathion? Unpublished report prepared by Microbiological
Assocites Inc., Musselburgh, United Kingdom. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1982) E-605-ethyl study for acute oral toxicity.
Unpublished report No. 11230 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Heimann, K.G. (1986) E-605-ethyl study for skin sensitizing effect on
guinea pigs. Unpublished report No. 14999 from Bayer AG.
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1982) Parathion (E-605-ethyl active ingredient) --
Micronucleus test on mouse to evaluate for mutagenic effect.
Unpublished report No. 10777 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Herbold, B.A. (1985) E-605 (c.n. parathion) Pol A1-test on E-coli to
evaluate for potential DNA damage. Unpublished report
No. 10777 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Herbold, B.A. (1986) E-605 (c.m. Parathion) -- Dominant lethal test on
male mouse to evaluate for mutagenic effect. Unpublished report
No. 14224 from Bayer AG. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Jensen, J.C. (1991) Mutagenicity test: In vitro mammalian cytogenetic
test in human lymphocytes performed with parathion-ethyl, Batch
No. 70818-01. Unpublished report prepared by Scaatox. Submitted
to WHO by Cheminova Agro AS, Lemvig, Denmark.
Kamataki, T. & Neal, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothionate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfer
atom. Mol. Pharmacol., 12, 933-944.
Lawlor, T.E. & Wagner, V.O. (1988) Ethyl parathion (97-98% technical)
-- Salmonella/mammalian-microsome plate incorporation
mutagenicity assay (Ames test) with a confirmatory assay.
Unpublished report No. USAE 605220388 prepared by Microbiological
Associates Inc, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Mihail, F. & Gröning, P. (1981) E-605. Ethylactive ingredient/subacute
cutaneous toxicity study on rabbits. Unpublished report No 10342
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark.
Moeller, H.S. & Rider, J.A. (1961) Studies on the anticholinesterase
effect of parathion and methyl parathion in humans (Abstract).
Fed Proc., 20, 434.
Nakatsugawa, T., Tolman, N.U. & Dahm, P.A. (1969) Degradation of
parathion in the rat. Biochem. 18, 1103-1114.
Neal, R.A. (1967) Studies on the metabolism of diethyl 4-nitrophenyl
phosphorothionate (parathion) in vitro. Biochem. J.,
103, 183-191.
Neeper-Bradley, T.L. (1990) Two-generation reproduction study of ethyl
parathion technical administered in the diet of CD (Sprague
Dawley) rats. Unpublished report from Busky Run Research Center,
Pennsylvania, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Nye, D.E. & Donough, H.W. (1976) Fate of insecticides administered
endotracheally to rats. Bull. Environ. Contam. Toxicol.,
15, 291-296.
Oesch, F. (1977) Ames test for Folidol E-605 (parathion). Unpublished
report No. E605301177. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Owens, E.J. (1976) The effect of ethyl parathion in the rat and dog
after acute and subacute inhalation and oral administration.
Technical report No. AMRL TR-76-1215 from Aerospace Medical
Research Laboratories, pp. 203-222. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Page, J.G. & Heath, G.E. (1991) Carcinogenicity study of ethyl
parathion administered by dosed feed to B6C3F1 mice.
Unpublished report No. E605210291 from Southern Research
Institute, Alabama, USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Pauluhn, J. (1983) E605-Ethyl/Study for dermal and mucous membrane
irritant effects. Unpublished report No. 11401 from Bayer AG
Submitted to WHO by Cheminova Agro AS, Lemvig, Denmark.
Pauluhn, J. (1984) E605 (c.m. parathion) -- Study for subacute
inhalative toxicity in the rat (exposure 15 × 6 hours).
Unpublished report No. 12395 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Putman, D.L. (1988) Micronucleus cytogenetic assay in mice.
Unpublished report No. T4772.122 prepared by Microbiological
Associates, Inc., USA. Submitted to WHO by Cheminova Agro AS,
Lemvig, Denmark.
Ramundo, J. (1979) A 4 week pilot study in mice with ethyl parathion.
Unpublished report No. 77-2051 from Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS. Lemvig, Denmark.
Renhof, M. (1984) Parathion -- Study for embryotoxic effects in rats
after oral administration. Unpublished report No. 12909 from
Bayer AG. Submitted to WHO by Cheminova Agro AS. Lemvig, Denmark.
Renhof, M. (1985) Parathion -- Study for embryotoxic effects on
rabbits after oral administration. Unpublished report No. 13288
from Bayer AG. Submitted to WHO by Cheminova Agro AS, Lemvig,
Denmark
Rider, J.A., Moeller, H.C., Swader, J.I. & Weilerstein, R.W. (1958)
The effect of parathion on human red blood cell and plasma
cholinesterase. Arch. Ind. Health, 18, 442-445.
Schroeder (1983) A teratogenicity study in rats with ethyl parathion.
Unpublished report No. 82-2644 prepared by Biodynamics Inc., East
Millstone, New Jersey, USA. Submitted to WHO by Cheminova Agro
AS, Lemvig, Denmark.
Simmon, V.F., Mitchell, R.D. & Jorgenson, T.A. (1976) In vitro
mutagenic studies on twenty pesticides. Toxicol. Appl.
Pharmacol., 37, 109.
Soliman, S.A., Farmer, J. & Curley, A. (1982) Is delayed neurotoxicity
a property of all organophosphorus compounds? A study with a
model compound: parathion. Toxicology, 23, 267-279.
Tegeris, A.S. & Underwood, P.C. (1977) Fourteen day feeding study in
the dog. Ethyl parathion. Unpublished report No. 77-113 prepared
by Pharmacopathics Research Laboratories, Inc., USA. Submitted to
WHO by Cheminova Agro AS, Lemvig, Denmark.
Thyssen, J. (1972) E-605-Ethyl -- Study for acute inhalation toxicity.
Unpublished report No. 8209 from Bayer AG. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
Underwood, P.C. (1978) Ethyl parathion: Ninety day feeding to dogs.
Unpublished report No. USAE 65060378 from Pharmacopathics
Research Laboratories, Inc., USA. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark
Weber, H., Patzchke, K. & Wegner, L.A. (1980) Parathion C14 (benzene
ring-labelled compound): Biokinetic studies in rats. Unpublished
report No. PH8820 from Bayer AG. Submitted to WHO by Cheminova
Agro AS, Lemvig, Denmark.
Yang, L.L. (1988) Ethyl parathion (97/98% technical) CHO/HGPRT
mutation assay. Unpublished report No. USAE 605280388 from
Microbiological Associates Inc., USA. Submitted to WHO by
Cheminova Agro AS, Lemvig, Denmark.
