
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
CAPTAFOL
IDENTITY
Chemical name
N-(1,1,2,2-tetrachloroethylthio)-3a,4,7,7a-tetrahydrophthalimide
N-(1,1,2,2-tetrachloroethylthio)cyclohex-4-ene-1,2-dicarboximide
N-(1,1,2,2-tetrachloroethylmercapto)-4-cyclohexene-1,2-dicarboximide
N-(1,1,2,2-tetrachloroethylsulphenyl)-cis-delta-
4-cyclohexene-1,2-diocarboximide
N-(1,1,2,2-tetrachloroethyl) thiotetrahydrophthalamide
Synonyms
Difolatan(R)
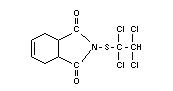
Structural formula
 Other relevant chemical properties
The pure material is a white crystalline solid; m.p. 160 to 161°C. It
is practically insoluble in water (less than 1 ppm) and slightly
soluble in most organic solvents. The technical material is a
recrystallized product of 98 percent purity; the impurities consist of
0.5-1.5 percent toluene, 0.5-1.5 percent tetrahydrophthalimide (THPI),
and 0.1-0.2 percent unknown chlorinated substances. It is a light tan
powder with a characteristic odour. It is formulated as 80 percent
wettable powder (Ortho Difolatan 80 W) and as flowable water
suspension (4 lbs per U.S. gallon). Captafol is stable except under
alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Captafol (1), on hydrolysis, yields tetrahydrophthalimide (II),
chloride ion, dichloroacetic acid (III) and inorganic sulphur in
various oxidation states. In the presence of sulfhydryl compounds at
neutral or slightly alkaline pH, captafol is rapidly degraded to
tetrahydrophthalimide and chloride ion and no organochlorine compound
is formed. This reaction with sulfhydryl compounds is much faster than
the hydrolytic reaction and it may be the dominant reaction in a
biological system where sulfhydryl groups are present. It is also well
known that the -N-S- bond in organic compounds is easily subject to
certain types of chemical attack, and two mechanisms for the cleavage
of this bond in captafol have been proposed; either nucleophilic
attack by a sulfhydryl compound or the slower hydrolytic reaction
(Kohn, 1965; Berteau et al., 1966).
Other relevant chemical properties
The pure material is a white crystalline solid; m.p. 160 to 161°C. It
is practically insoluble in water (less than 1 ppm) and slightly
soluble in most organic solvents. The technical material is a
recrystallized product of 98 percent purity; the impurities consist of
0.5-1.5 percent toluene, 0.5-1.5 percent tetrahydrophthalimide (THPI),
and 0.1-0.2 percent unknown chlorinated substances. It is a light tan
powder with a characteristic odour. It is formulated as 80 percent
wettable powder (Ortho Difolatan 80 W) and as flowable water
suspension (4 lbs per U.S. gallon). Captafol is stable except under
alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Captafol (1), on hydrolysis, yields tetrahydrophthalimide (II),
chloride ion, dichloroacetic acid (III) and inorganic sulphur in
various oxidation states. In the presence of sulfhydryl compounds at
neutral or slightly alkaline pH, captafol is rapidly degraded to
tetrahydrophthalimide and chloride ion and no organochlorine compound
is formed. This reaction with sulfhydryl compounds is much faster than
the hydrolytic reaction and it may be the dominant reaction in a
biological system where sulfhydryl groups are present. It is also well
known that the -N-S- bond in organic compounds is easily subject to
certain types of chemical attack, and two mechanisms for the cleavage
of this bond in captafol have been proposed; either nucleophilic
attack by a sulfhydryl compound or the slower hydrolytic reaction
(Kohn, 1965; Berteau et al., 1966).
 Shay rats were fed captafol at levels of 60 and 600 ppm in the diet.
At several intervals the stomach contents were analysed. Captafol
degraded rapidly, the reaction having a half-life of about three
hours. Both tetrahydrophthalimide and tetrahydrophthalic acid were
detected. No dichloroacetic acid was detected at the low dose level
but small amounts of it were found at the 600 ppm level, the maximum,
found 0.5 hours after feeding, being about 1 percent of the original
dose given (Leary, 1966).
Rats, dogs and monkeys were fed carbon14-carbonyl-labelled captafol
and radioactivity measurements were performed on expired carbon
dioxide, faeces. urine and various tissues. Almost 80 percent of the
dose given was excreted within 36 hours, mainly via the urine with
smaller amounts in the faeces and none in the expired carbon dioxide.
Less than 0.5 percent of the dose remained in the liver, heart,
kidneys, blood, muscle or fat. The rate of excretion of carbon14 was
almost identical for all three species. Unchanged captafol accounted
for the majority of the radioactivity in the faeces, but no captafol
was detected in blood, tissues or urine. The primary metabolite,
tetralydrophthalimide was detected in blood, faeces and urine although
the major portion of the activity in blood and urine was due to other,
more water-soluble metabolites. The epoxide of captafol was not
detected in blood, urine or faeces and it was concluded the
epoxidation is not a metabolic route (Dye, 1969).
Captafol differs from captan only in the nature of the halogenated
group attached to the sulphur. Both compounds give the same
decomposition product tetrahydrophthalimide. Therefore, all the
information on the metabolism of captan relative to the imide portion
of the molecule is considered to be applicable to captafol (Dye,
1969). (See the monograph on captan).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed captafol at 0, 50 (raised
to 100 after first generation), 250 (raised to 500 after first
generation), and 1000 ppm in the diet in a three-generation
reproduction study. There were no adverse effects on body-weight gain,
mortality, or organ-weights of parental animals or on reproductive
performance, fertility and lactation indices, litter-size, or number
of stillbirths in any test group. Pup survival in the test groups at
various intervals in the lactation period was not significantly
different from the control group. Weanling body-weights in the 1000
ppm group were depressed in both males and females in the first and
third generations. Weanlings of the second generation showed only
slight weight depression. This effect on weanling weights did not
occur at the lower test levels (even after they were raised to 100 and
500 ppm). Gross examinations and histopathology carried out on
parental animals and on weanlings in the 0 and 1000 ppm groups
revealed no changes that could be attributed to captafol (Kennedy et
al. 1966).
Special studies on teratogenicity
Chicken-egg
Captafol was injected, in dimethylsulfoxide solution, into either the
yolk or air cell of fresh fertile eggs at levels from 3-20 mg/kg
egg-weight. The eggs were incubated and non-viable embryos and hatched
chicks were examined for gross abnormalities. In a total of 270 eggs
treated with captafol, the incidence of malformations was 6.67 percent
compared to the control value of 1.6 percent for 1500 eggs injected
with dimethylsulfoxide alone. In the same experiment the metabolite
tetrahydrophthalimide was also injected. Of 1025 eggs the incidence of
malformation was 4.78 percent. The epoxy-derivative of captafol
("captafol-epoxide") was also tested for its effect on chicken eggs.
Of 115 eggs into which this compound was injected. the incidence of
malformation was 15.05 percent. In all cases the malformations
consisted mainly of micromelia, amelia and phocomelia (Verrett et al.,
1969).
Monkey
Groups of seven pregnant Rhesus monkeys were given oral doses of 6.25,
12.5 or 25 mg of technical captafol per kg of body-weight from
gestation day 22 through 32. Another group of seven pregnant females
received 10 mg/kg body-weight of thalidomide, on days 25, 26 and 27.
Three additional females ware treated with 15 consecutive doses of
captafol at 12.5 mg/kg body-weight later in gestation (66-80, 81-959
and 86-100 days). All doses were administered by gastric intubation of
a suspension in 0.9 percent gelatin solution in the morning before
feeding. All the females used had produced at least one live, normal
baby prior to this study. Pregnancies were terminated by Caesarean
section at approximately the 84th day of gestation. The three monkeys
receiving captafol later in gestation were allowed to proceed to term
and deliver their young unaided. Careful examination of 19 foetuses
from the captafol-treated monkeys, including gross observation, X-ray
and skeletal examination following alizarin red S staining, revealed
no abnormalities. Internal structural formation, observed both grossly
and by evaluation of organ-weight and organ to body-weight ratio data,
was normal. Foetal mortality (resorption or abortion) occurred in the
sixth week of pregnancy in two of the seven animals receiving 25 mg/kg
of captafol. In the group given thalidomide teratogenic activity (limb
malformations) was observed in five of the seven foetuses. The three
monkeys allowed to proceed to term delivered three normal babies, as
indicated by external and X-ray examination (Kennedy et al., 1968b).
Rabbit
Groups of 10 pregnant Dutch Belted rabbits received daily oral doses
of 0 or 75 mg/kg body-weight of technical captafol. A third group was
given thalidomide at 75 mg/kg body-weight. Dosing, administered by
gelatin capsule, began on day 6 and ended on day 16 of the gestation
period (the day of conception being day 0). On day 28, each doe was
sacrificed and the foetuses removed by Caesarean section. The rabbits
given captafol lost weight over the period of treatment. One doe in
this group aborted eight young, another showed one resorption site.
Three does in the thalidomide group showed evidence of resorption. Of
the viable young, 86 percent from the group given captafol survived a
six hour incubation (37°C) period compared with 100 percent in the
control group. No abnormalities were seen among 74 young in the group
given captafol. Animals in the group given thalidomide showed
significant teratogenic effects (Ives and Calandra, 1965; Kennedy et
al., 1968a).
Groups of 10 pregnant New Zealand albino rabbits were given technical
captafol (98.9 percent purity) at doses of 0, 37.5, 75, 112.5 or 150
mg/kg body-weight on gestation day 6 through 18 inclusive. A positive
control group was given 75 mg/kg body-weight of thalidomide. All doses
were administered via gelatin capsule. On day 29 each doe was
sacrificed and the young were removed by Caesarean section, weighed
and observed for abnormalities. At the lowest level tested, there was
no maternal mortality and the animals gained weight, although not as
much as the controls. Resorption sites occurred in 2 of the 10 females
(three sites). Examination of 62 foetuses from this group revealed no
abnormalities. Body-weights and 24-hour incubator survival were
comparable to those of the control group. At all the higher
dose-levels, toxic effects were seen in the mothers. Deaths occurred
in each group and resorption sites were prevalent in the survivors.
However, all young delivered in all three groups were free of gross
teratologic effects and survived the 24-hour incubation period.
Body-weights were lower than those of controls. In the group given
thalidomide, 32 of 55 foetuses showed abnormalities (Jackson at al.,
1967; Kennedy et al., 1968a).
Rat
A group of nine pregnant female rats was given doses of 100 mg/kg
body-weight/day of captafol orally from day 6 to day 15 of gestation
and another group of five pregnant rats was given 500 mg/kg from day 8
to day 10. Examination of 180 foetuses revealed no gross malformations
(Kennedy et al., 1968a).
Special studies on the metabolite, tetrahydrophthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted rabbits received 0 or 75 mg/kg
body-weight of tetrahydrophthalimide, the hydrolytic metabolite of
captafol, from day 6 to 16 inclusive of the gestation period. A
treated control group was given 75 mg/kg bodyweight of thalidomide
over the same period. The doses were given orally via gelatin capsule.
On day 28 each doe was sacrificed, the young were removed by Caesarean
section and examined for abnormalities. Examination of 57 embryos from
the test group revealed no external or skeletal abnormalities.
Fourteen embryos of a total of 44 in the group given thalidomide had
skeletal abnormalities. There was an increase in the occurrence of
resorption sites in the test group compared with the controls. Five of
the ten females exhibited one to three resorption sites (a total of
nine) although the number of viable young in the group was not reduced
(Palazzolo at al., 1966; Kennedy at al., 1968a).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat oral 62001/ Palazzolo et al., 1965a
Rat oral 50002/ Palazzolo et al., 1965b
(continued)
LD50
Animal Route mg/kg body-weight References
Rat oral 25002/ Palazzolo et al., 1964
Rabbit dermal 154002/ Palazzolo et al., 1964
1/ Corn oil solution
2/ Aqueous suspension
Short-term studies
Dog
Groups of two male and two female dogs were given daily doses of 0,
10, 30, 100 or 300 mg/kg body-weight of captafol over a two year
period. The material was given in gelatin capsules immediately after
each day's feeding. At the two highest dose levels there was a
decreased body-weight gain over the period of the experiment. Emesis
and loose stools occurred quite frequently in both these groups during
the first four weeks but only occasionally thereafter. These effects
were not seen at the two lower levels. Increased absolute liver and
kidney-weights and liver and kidney to bodyweight ratios were seen in
all animals at the 30, 100 and 300 mg/kg levels. Alterations In other
organ-weights appeared unrelated to the administration of the test
material. Haematologic studies revealed a mild anaemia at the
termination of the study in the dogs given 100 and 300 mg/kg per day.
Histopathology, blood chemistry, urinalysis and liver-function tests
revealed no adverse effects that could be attributed to the
administration of captafol. The 10 mg/kg dose level appeared to cause
no significant effects (Cervenka at al., 1964).
Rabbit
Four groups of eight rabbits were given dermal applications of
captafol at levels of 0, 500, 1000 and 2000 mg/kg body-weight/day for
20 days. Half the animals in each group were treated on abraded skin
areas, the other half on intact skin. There was a marked adverse
effect on body-weight, even at the lowest level tested. Deaths
occurred at all test levels and the 20-day dermal LD50 was found to
be 1100 mg/kg body-weight/day (for the 80 percent wettable powder).
The only effects seen in gross and microscopic examinations were in
the skin at the application site (Palazzolo et al., 1964).
Long-term studies
Rat
In a two year study with captafol added to the diet at 0 (70 males and
70 females), 250, 500, 1500 and 5000 ppm (35 females and 35 males at
each test level), there was growth depression at the 1500 and 5000 ppm
levels. Mortality was increased in the 5000 ppm group, with no males
left alive after 23 months. A lymphocyte to neutrophile shift was
observed in the surviving males of this group after 21 months. There
was an increase in the liver to body-weight ratio at the 500, 1500 and
5000 ppm levels at 12 months. An increase in this ratio was also seen
in males at 250 ppm. At the end of the experiment there was no longer
a significant difference at the two lower tent levels. Significant
increases were also observed in organ weight and organ to bodyweight
ratios for kidney and adrenal of rats fed at 1500 and 5000 ppm.
Histopathology revealed liver changes characterized by degeneration of
hepatic cells, vacuolization, incipient fat alteration, and
infiltration by mononuclear cells. Kidney changes were characterized
by alterations in proximal and distal tubular cells; many giant forms
with large irregular nuclei being present. These changes in liver and
kidney were only seen in rate fed the two highest dose levels. No
other histopathological changes were associated with the
administration of captafol. No effects on tumour incidence were
observed (Kohn et al., 1964).
COMMENT
Captafol appears to be metabolized rapidly and in a similar way in
rats, dogs and monkeys. The primary metabolite and other metabolites
have been identified in excretion studies. Information is, however,
incomplete on the nature of the metabolites in animal tissues, as well
as on the absorption and distribution of captafol and its metabolites
after oral administration. A two-year study in dogs indicated that a
dose level of 10 mg/kg body-weight/day was without significant adverse
effect. In the long-term study in rate at the lowest level tested (250
ppm), an increase in the liver to body-weight ratio was evident at 12
months but not at 24 months. For this reason a definite no-effect
level has not been established in that species. Teratogenicity studies
in mammalian species produced evidence of embryotoxic effects at the
lowest level tested but there was no indication of malformation of the
foetuses. The nature of the reported histopathological effects upon
the kidney and liver observed in the two year study in rats fed high
dose levels of captafol is of some concern.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 10 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.05 mg/kg body-weight/day
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captafol is used to control fungus diseases.
Rates of application and recommended intervals between treatment and
harvest are (Dye, 1969):
Fruits - 0.05 to 0.2% a.i., applied at bloom, petal
fall, shuck split and 10 to 14 day intervals
up to harvest.
Melons - 1.3 to 2.7 kg a.i./ha applied as needed at 7
day intervals up to harvest.
Tomatoes and
cucumbers - 1.1. to 2.7 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Potatoes - 0.10 to 1.8 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Post-harvest treatments
Captafol is recommended for post-harvest use on peaches, cherries,
plums, and nectarines (Ogawa at al., 1964).
Other uses
In-furrow spray application is recommended for the control of seedling
diseases on cotton. Captafol has been used to control
Helminthosporium heveae in rubber (Turner, 1969).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data of captafol are from treatments made under commercial
conditions in the U.S.A. (Dye, 1969). The tests made with peaches and
watermelons indicate that the captafol is present as a surface residue
and is not systemically translocated into the flesh of the fruit. The
initial residue of captafol is reduced by one half generally within a
week or two. Two types of applications are recommended: dormant and
blossom, and seasonal foliar. The residue data are given separately
(Table I).
TABLE I
Residue data from field trials
Crop Rate of Number of Pre-harvest Captafol
application treatments interval residue
(kg a.i. per ha) (days) (ppm)
Blossom application
Apricots 3.4-8.1 3 102 0.1-0.2
Cherries,
sweet 2.3-10.8 2-3 45-68 0.6-1.4
Plums (0.1-0.3% a.i.) 2-3 131-141 n.d.-0.2
Seasonal foliar application
Cherries,
sour 1.8-3.6 4 20 7-9
Peaches 3.1-4.5 1-13 10-14 2.5-14
Melons 2.0-4.0 5-9 1 0.4-1.8
Cucumbers 1.3-1.8 6-9 1 0.1-0.4
Tomatoes 2.7-5.4 6-11 1 0.4-3.8
FATE OF RESIDUES
General comments
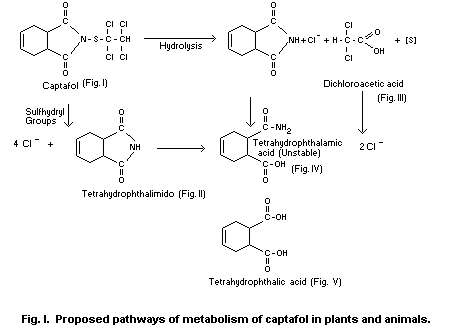
As outlined under 'BIOCHEMICAL ASPECTS', the N-S bond in substances
like captafol, is subject to chemical attack. For captafol, the two
mechanisms proposed (Kohn, 1965) are: (1) nucleophilic attack by
sulfhydryl compounds (Anon., 1965a) acid (2) hydrolysis (Berteau,
1963, and Potter, 1964) (Fig. 1). Both initiative reactions result in
THPI. In addition, in reaction (1) free Cl- ions, inorganic sulphur
compounds, and 2C-fragment are released and in reaction (2) chlorine
is only partly appearing as free ion, the rest being bound to the
2C-moiety. Subsequently, tetrahydrophthalimide decomposes via the
unstable tetrahydrophthalamic acid into tetrahydrophthalic acid.
In the presence of sulfhydryl compounds and at or near neutral pH, the
sulfhydryl reaction (1) is much faster than the hydrolysis (2). At pH
7 and 25°C, the "half-life" of captafol in a homogeneous sulfhydryl
reaction was found to be 4 minutes and in the corresponding hydrolysis
1000 minutes (Anon., 1965a). As the temperature and/or pH rises the
rates of both reactions increases.
In animals
The main routes of degradation of captafol in animals are the same as
in plants (Dye, 1969).
In plants
Due to the extremely low water solubility of captafol the residues of
the parent compound are occurring on the surface of the treated
plants. This is greatly limiting the exposure of captafol to the
degradation reaction by the sulfhydryl compounds of the plant tissue.
As a result, the captafol residues show a high persistence in situ.
In vitro studies on the degradation of captafol by spinach, tomato,
and celery macerates and filtrates (Potter, 1965) have confirmed that
captafol is very readily decomposed by the cell sap of various plants.
There were distinct differences in the degradation rates of various
plants. In spinach the captafol residue (10 ppm) was nearly completely
degraded in one hour, but in tomato in about 24 hours. Boiling did not
alter the degradative capacity of the macerates which indicates that
no essential enzymatic reactions are involved in the degradation of
captafol.
The in situ residues of captafol are found to produce minor amounts
of tetrahydrophthalimide and tetrahydrophthalic acid (Table II). No
dichloroacetic acid has been found. Thus, even though dichloroacetic
acid can be formed from captafol under certain conditions, it is not
found to be present as a residue on crops treated under field
conditions and would, therefore, not be consumed by man (Dye, 1969).
In soils
It has been found that captafol degrades rapidly in soil, the rate
being a function of both the soil type and the initial concentration;
even the longest "half-life" found was only 11 days (Berteau and Pack,
1966a). These studies show that under normal agricultural conditions,
the captafol that is supplied one year, even late in the season, would
be completely degraded by the beginning of the next growing season.
Therefore, there would be no accumulation of captafol in soil from
year to year.
It has been found that in natural, non-sterile soil, captafol
degradation results in only a barely detectable trace of
dichloroacetic acid being formed at short (i.e. one week) intervals.
At longer intervals, none is detectable at a limit of detectability of
0.02 ppm dichloroacetic acid itself is rapidly degraded by natural
soil, being completely degraded within one week. In normal
agricultural practice, therefore, there would be no buildup in the
soil (Berteau and Pack, 1966b).
In sterile soil, the degradation of captafol is associated with a
buildup of dichloroacetic acid and in dichloroacetic acid fortified
soil no loss was observed over a period of one week. The degradation
of the acid in soil is, therefore, definitely biochemical (Berteau and
Pack, 1966b).
The movement of captafol through soil columns by water leaching has
been studied. The results show that captafol does not move
significantly and will not accumulate in water leaching from treated
areas (Berteau and Pack, 1966c).
Carrots and radishes were grown in soils treated with captafol to
determine if any captafol would be taken up by the crop. At a limit of
detectability of 0.05 ppm or better, no captafol residue was found in
either crop (Anon., 1965a).
In storage and processing
All the data available indicate that the captafol residues on fruit
are very stable under commercial storage conditions.
In the studies on dried peaches and prunes, no residues were found in
the fresh fruit, but small residues ware detected in the dried
samples. Apparently, any residue in the fresh fruit was below the
limit of detectability but this residue became detectable as it was
concentrated in the drying operation (Table III) (Dye, 1969).
In the canned peach samples, there was a slight residue of captafol
detected at 1 day interval, but no detectable quantities at subsequent
intervals. The residue of 1.6 ppm of tetrahydrophthalimide in the 1
day peach sample decreased to below the limit of detection at 22 and
150 days. Tetrahydrophthalic acid was barely detectable in canned
peaches, and no detectable residues of dichloroacetic acid were found
in any of the samples studied (Table III) (Dye, 1969).
In the case of tomatoes no detectable residues of tetrahydrophthalic
acid or dichloroacetic acid could be found in any of the canned fruit
samples (Table III) (Dye, 1969).
Because of the nature of the captafol residues they would be readily
removed by washing, blanching, and peeling the fruit. A detailed
evaluation can not be made because of lack of complete information.
According to Chalkov and Vanev (1968), captafol has a suppressing
action on wine yeast. Cabral and Tomaz (1956) reported that it
completely inhibited fermentation of the must from treated grapes.
METHODS OF RESIDUE ANALYSIS
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is electron
capture GLC, the alternate procedure is based on thin-layer
chromatography.
The crop is extracted with benzene. The extract is cleaned-up by
either a column chromatographic method or a thin layer chromatographic
(TLC) method, or a combination of the two. The cleaned-up extract is
then chromatographed by TLC with captafol standards. The plate is
sprayed with N,N-dimethyl-p-phenylenediamine to visualize the spots.
The amount of captafol is determined by visual commission of the
sample spot with the standard spots. The method is a positive
identification of captafol residues. Its sensitivity is about
0.03-0.05 ppm, depending on the crop interferences (Anon., 1966b).
There is a modified column cleanup method which is useful for oily
crops where interferences in the thin-layer chromatography is
encountered from plant extractives (Anon., 1964). Pomerantz and Rose
(1968) have developed a TLC method which differentiates between
captan, folpet, captafol, and their metabolites.
The preferred method employs electron capture GLC for the final
detection and estimation of residues of captafol. Either the column or
TLC cleanup procedure can be used when cleanup is needed (Anon.,
1965b). Twenty-seven pesticides were tested for possible interference
in this GLC method of analysis. It found that none of the pesticides
studied eluted at the same elution time as did captafol under the
conditions employed, and therefore there would be no interference from
any of them. The sensitivity of 0.01 ppm can be obtained by the GLC
method (Kilgore and White, 1967).
The analytical method for the analysis of dichloroacetic acid which is
a demonstrated hydrolysis product of captafol, is based upon
microcoulometric gas chromatography. This method of analysis is both
highly specific and quite free from interfering material. The limit of
detection of this method is approximately 0.05 ppm, or possibly lower.
The sample is macerated and extracted with water. The water extract is
then extracted with ether. The DCAA in the ether layer is esterified
with diazomethane and the analysis completed with the microcoulometric
gas chromatography (Anon., 1965c).
The hydrolysis products of the imide portion of the captafol molecule,
tetrahydrophthalimide and tetrahydrophthalic acid, are detected by a
method which involves the extraction, cleanup, and esterification of
the acid derivatives with diazomethane and the subsequent gas
chromatographic detection of the esters. The detection system used is
hydrogen flame. This method is quite specific but great care must be
taken to eliminate interfering materials which would be detected by
the hydrogen flame. With adequate cleanup on most crops, this method
is sensitive to approximately 0.05 ppm (Anon., 1966c).
TABLE II
Data on captafol residues and its degradation products in various fruits (Anon., 1969
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments (days) Captafol hydrophthalimide acid
Peach 9.0 12 1 3.4 1.0 0.5
7 3.5 1.1 0.3
3.1 4 1 11.7 0.00 0.00
5 12.0 0.00 0.00
10 9.9 0.00 0.00
15 9.3 0.00 0.00
3.1 4 1 20.6 0.00 0.00
5 13.0 0.00 0.00
10 13.0 0.00 0.00
15 10.6 0.00 0.00
Cucumber 1.8 6 1 0.14 0.00 0.06
6 0.10 0.00 0.13
Tomato 2.7 9 1 9.7 0.16 0.00
7 10.1 0.16 0.07
2.7 9 1 5.2 0.27 0.00
7 4.3 0.20 0.00
* No dichloroacetic acid is detected
TABLE III
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
Peaches 7.1 1 fresh 139 0.00
dried 0.14
Plums 4.7-6.2 2 fresh 139 0.00
dried 0.42
15.6-3.9 3 fresh 139 0.00
dried 0.19
Peaches 2.4-3.9 7 canned 1 0.1 1.6 0.0
6 6 0.0 0.1 0.1
5 15 0.0 0.1 0.1
4 22 0.0 0.0 0.1
3 150 0.0 0.0 0.1
Tomatoes 2.7 5 canned ( 1 0.44 0.08 0.00
juice ( 180 0.13 0.00 0.03
2.7 5 canned 1 0.15 0.00 0.00
juice
canned 1 0.68 0.10 0.00
puree
canned 180 0.00 0.05 0.00
juice
canned 180 0.16 0.20 0.00
puree
TABLE III (cont'd)
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
2.7 10 fruit 1 0.48 0.12 0.08
7 0.13 0.06 0.00
canned 1 0.16 0.07 0.00
juice
canned 1 0.23 0.07 0.00
puree
canned 180 0.00 0.00 0.00
juice
canned 180 0.00 0.12 0.00
puree
0.9 canned 1 0.02
concentrate
* No dichloroacetic acid is detected
The GLC method is considered most suitable for regulatory purposes,
but the TLC method could also be applied.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Australia Stone fruit 20
Other fruit and vegetables 5
Canada Cherries (sour) 50
Apricots, nectarines, peaches 30
Tomatoes 15
Cherries (sweet), cucumbers, 5
melons, plums, prunes
Switzerland Grapes, strawberries 5
United States of Cherries (sour) 50
America Apricots, peaches 30
Tomatoes 15
Melons 5
Cherries (sweet), cucumbers, 2
nectarines, plums (fresh prunes)
APPRAISAL
Captafol is used to control fungus diseases on tree fruits, melons,
cucurbits, tomatoes, and potatoes. In the case of peaches, apricots,
nectarines, cherries, plums and prunes, a dormant or blossom
application is recommended. In addition, seasonal foliar applications
are recommended for certain crops. In-furrow spray applications are
recommended for the control of representative diseases on cotton.
Captafol is used for foliage application at a rate of 0.05 to 0.2
percent of active ingredient wettable powder of flowable suspension in
spray. It is quite stable except under alkaline conditions.
Tolerances established in the U.S.A., Canada, Switzerland and
Australia vary from 2 to 50 ppm.
The residue data of captafol available to the meeting are only from
treatments made under commercial conditions in the U.S.A. The initial
residue of captafol is reduced by one half generally within a week or
two. The residue is mainly on the surface of the fruit.
The two major routes of degradation in plants are the same as those in
animals, namely reactions with sulfhydryl compounds and hydrolysis.
Sulfhydryl reaction is more rapid than hydrolysis. Main degradation
products are tetrahydrophthalimide and tetrahydrophthalic acid.
Dichloracetic acid has not been found.
In thermal food processes as well as in macerated plant materials,
captafol is extensively decomposed. Decomposition products,
tetrahydrophthalimide and tetrahydrophthalic acid, may appear in
processed foods at the beginning of the storage period.
Captafol is found to degrade rapidly in natural soils.
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is GLC. The
alternate procedure is based on thinlayer chromatography, the
sensitivity of which is about 0.03-0.05 ppm, depending on the crop
interferences.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Residues resulting from good agricultural practice as follows are
recommended, effective till 1973:
Pre-harvest Captafol residue
Crop Comment interval (days) (ppm)
Melons, whole 1 2
Cucumbers, whole 1 1
Tomatoes 1 5
Peaches 10-14 15
Cherries, sour Foliar application 20 10
Cherries, sweet Blossom application 45-70 2
Apricots Blossom application 100 0.5
Plums Blossom application 130-140 0.2
The data on pineapples, apples and grapes were inadequate for
evaluation.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Studies to elucidate the effects seen in the teratogenicity
experiments.
2. Data from countries ether that the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Data on residue levels in raw agricultural commodities moving in
commerce.
4. Data on the effect of washing, peeling and blanching on the residue
levels of various crops.
5. Elucidation of the histopathology of the kidney and liver in the
rat.
DESIRABLE
1. Metabolic studies to provide further information on the absorption
and distribution after oral administration and to identify the
metabolites found in animal tissues.
2. Collaborative studies to establish a regulatory method for captafol
in the presence of captan and folpet.
REFERENCES
Anon. (1964) Difolatan residue analysis - cleanup procedure for oily
materials. Addendum to RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1965a) The reaction of Difolatan with sulfhydryl compounds.
Chevron Chemical Co. Unpub. Rept.
Anon. (1965b) Difolatan residue analysis by electron capture gas
chromatography. Method RM-6B. Chevron Chemical Co. Unpub. Rept.
Anon. (1965c) Difolatan metabolism - The analysis of residues of
dichloroacetic acid using microcoulometric gas chromatography. Method
RM-6C. Chevron Chemical Co. Unpub. Rept.
Anon. (1966a) Studies on the uptake of Difolatan by root crops grown
in soils treated with Difolatan. Chevron Chemical Co. Unpub. Rept.
Anon. (1966b) Difolatan residue analysis - thin layer chromatographic
method. Method RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1966c) The analysis of residues of Difolatan and two
tetrahydrophthalic acid derivatives. Residue Method RM-6D. Chevron
Chemical Co. Unpub. Rept.
Berteau, P.E. (1963) Difolatan - a preliminary report on its
hydrolysis and related reactions. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E., Pack, D.E., Ospenson, J.N. and Crossley, J. (1966) The
metabolism of Difolatan. Paper presented at the American Chemical
Society, Western Regional Meeting. San Francisco, California, 18
October 1966 [Abstracted in Vortex, (Publication of the California
section, American Chemical Society) 27, insert P. 37 (1966)]
Berteau, P.E. and Pack, D.E. (1966a) The degradation of Difolatan in
soils. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966b) Difolatan degradation in
soil-studies on the formation and decay of dichloroacetic acid.
Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966c) The movement of Difolatan through
soil columns. Chevron Chemical Co. Unpub. Rept.
Cabral, R.V. de G. and Tomes, I.L. (1966) Fungicidal assay against
Botrytis cinerea and the effects of fungicides on must fermentation.
An. Inst. Super. Agron., Univ. Tec. Lisboa 29:279-97 (Chem. Abstr.
70:46363 u, 1969)
Cervenka, H., Key, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of RE 5865-Beagle dogs. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar (Sofia)
17:33-40 (Chem. Abstr. 69:34908 s, 1968)
Dye, D.F. (1969) Difolatan(R). Unpub. Rept. prepared and submitted by
Chevron Chemical Co.
Ives, M. and Calandra, J.C. (1965) Teratogenic study on difolatan.
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Jackson, G.L., Fancher, O.E. and Calandra, J.C. (1967) Rabbit
teratogenic study. Difolatan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1966) Three-generation
reproduction study on difolatan - Albino rats. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968a) An investigation
of the teratogenic potential of captan, folpet and difolatan. Toxicol.
appl. Pharmacol. 13:421-30
Kennedy, G., Fancher, O.E and Calandra, J.C. (1968b) Teratologic
investigation of difolatan in Macaca mulatta (Rhesus monkey). Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kilgore, W.W. and White, E.R. (1967) Determination of Difolatan
residues in fruits by electron-capture gas chromatography. J. Apr.
Food Chem. 15:1118-20
Kohn, F.E., Key, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of RE 5865 Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kohn, G.K. (1965) Present status of metabolic fate studies of
Difolatan. Unpub. Rept. of Chevron Chemical Corporation for
presentation at the Orthocide Conference, Amsterdam, 29 November - 5
December 1965
Leary, J.B. (1966) Difolatan: shay rat tests. Unpub. Rept. of Chevron
Chemical Co. (cited by Crossley, 1967 - see the monograph of captan)
Ogawa, J.M., Boyack, G.A., Sandeno, J.L., and Mathre, J.H. (1964)
Control of postharvest fruit decays in relation to residues of
2.6-dichloro-4-nitroaniline and Difolatan. Hilgardia 35:365-73
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1964) Acute toxicity
studies on Difolatan 80W. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965a) Acute oral
toxicity on RE 5865. Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1965b) Acute oral
toxicity on RE 5865 (aqueous suspension). Unpub. Rept. of Industrial
Bio-Test Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study, THPI. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51:1058-62
Potter, J.L. (1964) Studies on the hydrolysis of Difolatan in a
homogeneous aqueous-acetone buffer system under neutral pH conditions.
Chevron Chemical Co. Unpub. Rept.
Potter, J.L. (1965) Preliminary studies on the in vitro degradation of
Difolatan by crop macerates and filtrates. Chevron Chemical Co. Unpub.
Rept.
Turner, P. D. (1969) Evaluation of fungicides for the control of
Helminthosporium heveae on Hevea rubber in Malaysia. I. Laboratory
assessment. Experimental Agriculture 5:33-40
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Annals N.Y. Acad. Sci.
160.334-43
Shay rats were fed captafol at levels of 60 and 600 ppm in the diet.
At several intervals the stomach contents were analysed. Captafol
degraded rapidly, the reaction having a half-life of about three
hours. Both tetrahydrophthalimide and tetrahydrophthalic acid were
detected. No dichloroacetic acid was detected at the low dose level
but small amounts of it were found at the 600 ppm level, the maximum,
found 0.5 hours after feeding, being about 1 percent of the original
dose given (Leary, 1966).
Rats, dogs and monkeys were fed carbon14-carbonyl-labelled captafol
and radioactivity measurements were performed on expired carbon
dioxide, faeces. urine and various tissues. Almost 80 percent of the
dose given was excreted within 36 hours, mainly via the urine with
smaller amounts in the faeces and none in the expired carbon dioxide.
Less than 0.5 percent of the dose remained in the liver, heart,
kidneys, blood, muscle or fat. The rate of excretion of carbon14 was
almost identical for all three species. Unchanged captafol accounted
for the majority of the radioactivity in the faeces, but no captafol
was detected in blood, tissues or urine. The primary metabolite,
tetralydrophthalimide was detected in blood, faeces and urine although
the major portion of the activity in blood and urine was due to other,
more water-soluble metabolites. The epoxide of captafol was not
detected in blood, urine or faeces and it was concluded the
epoxidation is not a metabolic route (Dye, 1969).
Captafol differs from captan only in the nature of the halogenated
group attached to the sulphur. Both compounds give the same
decomposition product tetrahydrophthalimide. Therefore, all the
information on the metabolism of captan relative to the imide portion
of the molecule is considered to be applicable to captafol (Dye,
1969). (See the monograph on captan).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed captafol at 0, 50 (raised
to 100 after first generation), 250 (raised to 500 after first
generation), and 1000 ppm in the diet in a three-generation
reproduction study. There were no adverse effects on body-weight gain,
mortality, or organ-weights of parental animals or on reproductive
performance, fertility and lactation indices, litter-size, or number
of stillbirths in any test group. Pup survival in the test groups at
various intervals in the lactation period was not significantly
different from the control group. Weanling body-weights in the 1000
ppm group were depressed in both males and females in the first and
third generations. Weanlings of the second generation showed only
slight weight depression. This effect on weanling weights did not
occur at the lower test levels (even after they were raised to 100 and
500 ppm). Gross examinations and histopathology carried out on
parental animals and on weanlings in the 0 and 1000 ppm groups
revealed no changes that could be attributed to captafol (Kennedy et
al. 1966).
Special studies on teratogenicity
Chicken-egg
Captafol was injected, in dimethylsulfoxide solution, into either the
yolk or air cell of fresh fertile eggs at levels from 3-20 mg/kg
egg-weight. The eggs were incubated and non-viable embryos and hatched
chicks were examined for gross abnormalities. In a total of 270 eggs
treated with captafol, the incidence of malformations was 6.67 percent
compared to the control value of 1.6 percent for 1500 eggs injected
with dimethylsulfoxide alone. In the same experiment the metabolite
tetrahydrophthalimide was also injected. Of 1025 eggs the incidence of
malformation was 4.78 percent. The epoxy-derivative of captafol
("captafol-epoxide") was also tested for its effect on chicken eggs.
Of 115 eggs into which this compound was injected. the incidence of
malformation was 15.05 percent. In all cases the malformations
consisted mainly of micromelia, amelia and phocomelia (Verrett et al.,
1969).
Monkey
Groups of seven pregnant Rhesus monkeys were given oral doses of 6.25,
12.5 or 25 mg of technical captafol per kg of body-weight from
gestation day 22 through 32. Another group of seven pregnant females
received 10 mg/kg body-weight of thalidomide, on days 25, 26 and 27.
Three additional females ware treated with 15 consecutive doses of
captafol at 12.5 mg/kg body-weight later in gestation (66-80, 81-959
and 86-100 days). All doses were administered by gastric intubation of
a suspension in 0.9 percent gelatin solution in the morning before
feeding. All the females used had produced at least one live, normal
baby prior to this study. Pregnancies were terminated by Caesarean
section at approximately the 84th day of gestation. The three monkeys
receiving captafol later in gestation were allowed to proceed to term
and deliver their young unaided. Careful examination of 19 foetuses
from the captafol-treated monkeys, including gross observation, X-ray
and skeletal examination following alizarin red S staining, revealed
no abnormalities. Internal structural formation, observed both grossly
and by evaluation of organ-weight and organ to body-weight ratio data,
was normal. Foetal mortality (resorption or abortion) occurred in the
sixth week of pregnancy in two of the seven animals receiving 25 mg/kg
of captafol. In the group given thalidomide teratogenic activity (limb
malformations) was observed in five of the seven foetuses. The three
monkeys allowed to proceed to term delivered three normal babies, as
indicated by external and X-ray examination (Kennedy et al., 1968b).
Rabbit
Groups of 10 pregnant Dutch Belted rabbits received daily oral doses
of 0 or 75 mg/kg body-weight of technical captafol. A third group was
given thalidomide at 75 mg/kg body-weight. Dosing, administered by
gelatin capsule, began on day 6 and ended on day 16 of the gestation
period (the day of conception being day 0). On day 28, each doe was
sacrificed and the foetuses removed by Caesarean section. The rabbits
given captafol lost weight over the period of treatment. One doe in
this group aborted eight young, another showed one resorption site.
Three does in the thalidomide group showed evidence of resorption. Of
the viable young, 86 percent from the group given captafol survived a
six hour incubation (37°C) period compared with 100 percent in the
control group. No abnormalities were seen among 74 young in the group
given captafol. Animals in the group given thalidomide showed
significant teratogenic effects (Ives and Calandra, 1965; Kennedy et
al., 1968a).
Groups of 10 pregnant New Zealand albino rabbits were given technical
captafol (98.9 percent purity) at doses of 0, 37.5, 75, 112.5 or 150
mg/kg body-weight on gestation day 6 through 18 inclusive. A positive
control group was given 75 mg/kg body-weight of thalidomide. All doses
were administered via gelatin capsule. On day 29 each doe was
sacrificed and the young were removed by Caesarean section, weighed
and observed for abnormalities. At the lowest level tested, there was
no maternal mortality and the animals gained weight, although not as
much as the controls. Resorption sites occurred in 2 of the 10 females
(three sites). Examination of 62 foetuses from this group revealed no
abnormalities. Body-weights and 24-hour incubator survival were
comparable to those of the control group. At all the higher
dose-levels, toxic effects were seen in the mothers. Deaths occurred
in each group and resorption sites were prevalent in the survivors.
However, all young delivered in all three groups were free of gross
teratologic effects and survived the 24-hour incubation period.
Body-weights were lower than those of controls. In the group given
thalidomide, 32 of 55 foetuses showed abnormalities (Jackson at al.,
1967; Kennedy et al., 1968a).
Rat
A group of nine pregnant female rats was given doses of 100 mg/kg
body-weight/day of captafol orally from day 6 to day 15 of gestation
and another group of five pregnant rats was given 500 mg/kg from day 8
to day 10. Examination of 180 foetuses revealed no gross malformations
(Kennedy et al., 1968a).
Special studies on the metabolite, tetrahydrophthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted rabbits received 0 or 75 mg/kg
body-weight of tetrahydrophthalimide, the hydrolytic metabolite of
captafol, from day 6 to 16 inclusive of the gestation period. A
treated control group was given 75 mg/kg bodyweight of thalidomide
over the same period. The doses were given orally via gelatin capsule.
On day 28 each doe was sacrificed, the young were removed by Caesarean
section and examined for abnormalities. Examination of 57 embryos from
the test group revealed no external or skeletal abnormalities.
Fourteen embryos of a total of 44 in the group given thalidomide had
skeletal abnormalities. There was an increase in the occurrence of
resorption sites in the test group compared with the controls. Five of
the ten females exhibited one to three resorption sites (a total of
nine) although the number of viable young in the group was not reduced
(Palazzolo at al., 1966; Kennedy at al., 1968a).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat oral 62001/ Palazzolo et al., 1965a
Rat oral 50002/ Palazzolo et al., 1965b
(continued)
LD50
Animal Route mg/kg body-weight References
Rat oral 25002/ Palazzolo et al., 1964
Rabbit dermal 154002/ Palazzolo et al., 1964
1/ Corn oil solution
2/ Aqueous suspension
Short-term studies
Dog
Groups of two male and two female dogs were given daily doses of 0,
10, 30, 100 or 300 mg/kg body-weight of captafol over a two year
period. The material was given in gelatin capsules immediately after
each day's feeding. At the two highest dose levels there was a
decreased body-weight gain over the period of the experiment. Emesis
and loose stools occurred quite frequently in both these groups during
the first four weeks but only occasionally thereafter. These effects
were not seen at the two lower levels. Increased absolute liver and
kidney-weights and liver and kidney to bodyweight ratios were seen in
all animals at the 30, 100 and 300 mg/kg levels. Alterations In other
organ-weights appeared unrelated to the administration of the test
material. Haematologic studies revealed a mild anaemia at the
termination of the study in the dogs given 100 and 300 mg/kg per day.
Histopathology, blood chemistry, urinalysis and liver-function tests
revealed no adverse effects that could be attributed to the
administration of captafol. The 10 mg/kg dose level appeared to cause
no significant effects (Cervenka at al., 1964).
Rabbit
Four groups of eight rabbits were given dermal applications of
captafol at levels of 0, 500, 1000 and 2000 mg/kg body-weight/day for
20 days. Half the animals in each group were treated on abraded skin
areas, the other half on intact skin. There was a marked adverse
effect on body-weight, even at the lowest level tested. Deaths
occurred at all test levels and the 20-day dermal LD50 was found to
be 1100 mg/kg body-weight/day (for the 80 percent wettable powder).
The only effects seen in gross and microscopic examinations were in
the skin at the application site (Palazzolo et al., 1964).
Long-term studies
Rat
In a two year study with captafol added to the diet at 0 (70 males and
70 females), 250, 500, 1500 and 5000 ppm (35 females and 35 males at
each test level), there was growth depression at the 1500 and 5000 ppm
levels. Mortality was increased in the 5000 ppm group, with no males
left alive after 23 months. A lymphocyte to neutrophile shift was
observed in the surviving males of this group after 21 months. There
was an increase in the liver to body-weight ratio at the 500, 1500 and
5000 ppm levels at 12 months. An increase in this ratio was also seen
in males at 250 ppm. At the end of the experiment there was no longer
a significant difference at the two lower tent levels. Significant
increases were also observed in organ weight and organ to bodyweight
ratios for kidney and adrenal of rats fed at 1500 and 5000 ppm.
Histopathology revealed liver changes characterized by degeneration of
hepatic cells, vacuolization, incipient fat alteration, and
infiltration by mononuclear cells. Kidney changes were characterized
by alterations in proximal and distal tubular cells; many giant forms
with large irregular nuclei being present. These changes in liver and
kidney were only seen in rate fed the two highest dose levels. No
other histopathological changes were associated with the
administration of captafol. No effects on tumour incidence were
observed (Kohn et al., 1964).
COMMENT
Captafol appears to be metabolized rapidly and in a similar way in
rats, dogs and monkeys. The primary metabolite and other metabolites
have been identified in excretion studies. Information is, however,
incomplete on the nature of the metabolites in animal tissues, as well
as on the absorption and distribution of captafol and its metabolites
after oral administration. A two-year study in dogs indicated that a
dose level of 10 mg/kg body-weight/day was without significant adverse
effect. In the long-term study in rate at the lowest level tested (250
ppm), an increase in the liver to body-weight ratio was evident at 12
months but not at 24 months. For this reason a definite no-effect
level has not been established in that species. Teratogenicity studies
in mammalian species produced evidence of embryotoxic effects at the
lowest level tested but there was no indication of malformation of the
foetuses. The nature of the reported histopathological effects upon
the kidney and liver observed in the two year study in rats fed high
dose levels of captafol is of some concern.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 10 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.05 mg/kg body-weight/day
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captafol is used to control fungus diseases.
Rates of application and recommended intervals between treatment and
harvest are (Dye, 1969):
Fruits - 0.05 to 0.2% a.i., applied at bloom, petal
fall, shuck split and 10 to 14 day intervals
up to harvest.
Melons - 1.3 to 2.7 kg a.i./ha applied as needed at 7
day intervals up to harvest.
Tomatoes and
cucumbers - 1.1. to 2.7 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Potatoes - 0.10 to 1.8 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Post-harvest treatments
Captafol is recommended for post-harvest use on peaches, cherries,
plums, and nectarines (Ogawa at al., 1964).
Other uses
In-furrow spray application is recommended for the control of seedling
diseases on cotton. Captafol has been used to control
Helminthosporium heveae in rubber (Turner, 1969).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data of captafol are from treatments made under commercial
conditions in the U.S.A. (Dye, 1969). The tests made with peaches and
watermelons indicate that the captafol is present as a surface residue
and is not systemically translocated into the flesh of the fruit. The
initial residue of captafol is reduced by one half generally within a
week or two. Two types of applications are recommended: dormant and
blossom, and seasonal foliar. The residue data are given separately
(Table I).
TABLE I
Residue data from field trials
Crop Rate of Number of Pre-harvest Captafol
application treatments interval residue
(kg a.i. per ha) (days) (ppm)
Blossom application
Apricots 3.4-8.1 3 102 0.1-0.2
Cherries,
sweet 2.3-10.8 2-3 45-68 0.6-1.4
Plums (0.1-0.3% a.i.) 2-3 131-141 n.d.-0.2
Seasonal foliar application
Cherries,
sour 1.8-3.6 4 20 7-9
Peaches 3.1-4.5 1-13 10-14 2.5-14
Melons 2.0-4.0 5-9 1 0.4-1.8
Cucumbers 1.3-1.8 6-9 1 0.1-0.4
Tomatoes 2.7-5.4 6-11 1 0.4-3.8
FATE OF RESIDUES
General comments
As outlined under 'BIOCHEMICAL ASPECTS', the N-S bond in substances
like captafol, is subject to chemical attack. For captafol, the two
mechanisms proposed (Kohn, 1965) are: (1) nucleophilic attack by
sulfhydryl compounds (Anon., 1965a) acid (2) hydrolysis (Berteau,
1963, and Potter, 1964) (Fig. 1). Both initiative reactions result in
THPI. In addition, in reaction (1) free Cl- ions, inorganic sulphur
compounds, and 2C-fragment are released and in reaction (2) chlorine
is only partly appearing as free ion, the rest being bound to the
2C-moiety. Subsequently, tetrahydrophthalimide decomposes via the
unstable tetrahydrophthalamic acid into tetrahydrophthalic acid.
In the presence of sulfhydryl compounds and at or near neutral pH, the
sulfhydryl reaction (1) is much faster than the hydrolysis (2). At pH
7 and 25°C, the "half-life" of captafol in a homogeneous sulfhydryl
reaction was found to be 4 minutes and in the corresponding hydrolysis
1000 minutes (Anon., 1965a). As the temperature and/or pH rises the
rates of both reactions increases.
In animals
The main routes of degradation of captafol in animals are the same as
in plants (Dye, 1969).
In plants
Due to the extremely low water solubility of captafol the residues of
the parent compound are occurring on the surface of the treated
plants. This is greatly limiting the exposure of captafol to the
degradation reaction by the sulfhydryl compounds of the plant tissue.
As a result, the captafol residues show a high persistence in situ.
In vitro studies on the degradation of captafol by spinach, tomato,
and celery macerates and filtrates (Potter, 1965) have confirmed that
captafol is very readily decomposed by the cell sap of various plants.
There were distinct differences in the degradation rates of various
plants. In spinach the captafol residue (10 ppm) was nearly completely
degraded in one hour, but in tomato in about 24 hours. Boiling did not
alter the degradative capacity of the macerates which indicates that
no essential enzymatic reactions are involved in the degradation of
captafol.
The in situ residues of captafol are found to produce minor amounts
of tetrahydrophthalimide and tetrahydrophthalic acid (Table II). No
dichloroacetic acid has been found. Thus, even though dichloroacetic
acid can be formed from captafol under certain conditions, it is not
found to be present as a residue on crops treated under field
conditions and would, therefore, not be consumed by man (Dye, 1969).
In soils
It has been found that captafol degrades rapidly in soil, the rate
being a function of both the soil type and the initial concentration;
even the longest "half-life" found was only 11 days (Berteau and Pack,
1966a). These studies show that under normal agricultural conditions,
the captafol that is supplied one year, even late in the season, would
be completely degraded by the beginning of the next growing season.
Therefore, there would be no accumulation of captafol in soil from
year to year.
It has been found that in natural, non-sterile soil, captafol
degradation results in only a barely detectable trace of
dichloroacetic acid being formed at short (i.e. one week) intervals.
At longer intervals, none is detectable at a limit of detectability of
0.02 ppm dichloroacetic acid itself is rapidly degraded by natural
soil, being completely degraded within one week. In normal
agricultural practice, therefore, there would be no buildup in the
soil (Berteau and Pack, 1966b).
In sterile soil, the degradation of captafol is associated with a
buildup of dichloroacetic acid and in dichloroacetic acid fortified
soil no loss was observed over a period of one week. The degradation
of the acid in soil is, therefore, definitely biochemical (Berteau and
Pack, 1966b).
The movement of captafol through soil columns by water leaching has
been studied. The results show that captafol does not move
significantly and will not accumulate in water leaching from treated
areas (Berteau and Pack, 1966c).
Carrots and radishes were grown in soils treated with captafol to
determine if any captafol would be taken up by the crop. At a limit of
detectability of 0.05 ppm or better, no captafol residue was found in
either crop (Anon., 1965a).
In storage and processing
All the data available indicate that the captafol residues on fruit
are very stable under commercial storage conditions.
In the studies on dried peaches and prunes, no residues were found in
the fresh fruit, but small residues ware detected in the dried
samples. Apparently, any residue in the fresh fruit was below the
limit of detectability but this residue became detectable as it was
concentrated in the drying operation (Table III) (Dye, 1969).
In the canned peach samples, there was a slight residue of captafol
detected at 1 day interval, but no detectable quantities at subsequent
intervals. The residue of 1.6 ppm of tetrahydrophthalimide in the 1
day peach sample decreased to below the limit of detection at 22 and
150 days. Tetrahydrophthalic acid was barely detectable in canned
peaches, and no detectable residues of dichloroacetic acid were found
in any of the samples studied (Table III) (Dye, 1969).
In the case of tomatoes no detectable residues of tetrahydrophthalic
acid or dichloroacetic acid could be found in any of the canned fruit
samples (Table III) (Dye, 1969).
Because of the nature of the captafol residues they would be readily
removed by washing, blanching, and peeling the fruit. A detailed
evaluation can not be made because of lack of complete information.
According to Chalkov and Vanev (1968), captafol has a suppressing
action on wine yeast. Cabral and Tomaz (1956) reported that it
completely inhibited fermentation of the must from treated grapes.
METHODS OF RESIDUE ANALYSIS
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is electron
capture GLC, the alternate procedure is based on thin-layer
chromatography.
The crop is extracted with benzene. The extract is cleaned-up by
either a column chromatographic method or a thin layer chromatographic
(TLC) method, or a combination of the two. The cleaned-up extract is
then chromatographed by TLC with captafol standards. The plate is
sprayed with N,N-dimethyl-p-phenylenediamine to visualize the spots.
The amount of captafol is determined by visual commission of the
sample spot with the standard spots. The method is a positive
identification of captafol residues. Its sensitivity is about
0.03-0.05 ppm, depending on the crop interferences (Anon., 1966b).
There is a modified column cleanup method which is useful for oily
crops where interferences in the thin-layer chromatography is
encountered from plant extractives (Anon., 1964). Pomerantz and Rose
(1968) have developed a TLC method which differentiates between
captan, folpet, captafol, and their metabolites.
The preferred method employs electron capture GLC for the final
detection and estimation of residues of captafol. Either the column or
TLC cleanup procedure can be used when cleanup is needed (Anon.,
1965b). Twenty-seven pesticides were tested for possible interference
in this GLC method of analysis. It found that none of the pesticides
studied eluted at the same elution time as did captafol under the
conditions employed, and therefore there would be no interference from
any of them. The sensitivity of 0.01 ppm can be obtained by the GLC
method (Kilgore and White, 1967).
The analytical method for the analysis of dichloroacetic acid which is
a demonstrated hydrolysis product of captafol, is based upon
microcoulometric gas chromatography. This method of analysis is both
highly specific and quite free from interfering material. The limit of
detection of this method is approximately 0.05 ppm, or possibly lower.
The sample is macerated and extracted with water. The water extract is
then extracted with ether. The DCAA in the ether layer is esterified
with diazomethane and the analysis completed with the microcoulometric
gas chromatography (Anon., 1965c).
The hydrolysis products of the imide portion of the captafol molecule,
tetrahydrophthalimide and tetrahydrophthalic acid, are detected by a
method which involves the extraction, cleanup, and esterification of
the acid derivatives with diazomethane and the subsequent gas
chromatographic detection of the esters. The detection system used is
hydrogen flame. This method is quite specific but great care must be
taken to eliminate interfering materials which would be detected by
the hydrogen flame. With adequate cleanup on most crops, this method
is sensitive to approximately 0.05 ppm (Anon., 1966c).
TABLE II
Data on captafol residues and its degradation products in various fruits (Anon., 1969
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments (days) Captafol hydrophthalimide acid
Peach 9.0 12 1 3.4 1.0 0.5
7 3.5 1.1 0.3
3.1 4 1 11.7 0.00 0.00
5 12.0 0.00 0.00
10 9.9 0.00 0.00
15 9.3 0.00 0.00
3.1 4 1 20.6 0.00 0.00
5 13.0 0.00 0.00
10 13.0 0.00 0.00
15 10.6 0.00 0.00
Cucumber 1.8 6 1 0.14 0.00 0.06
6 0.10 0.00 0.13
Tomato 2.7 9 1 9.7 0.16 0.00
7 10.1 0.16 0.07
2.7 9 1 5.2 0.27 0.00
7 4.3 0.20 0.00
* No dichloroacetic acid is detected
TABLE III
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
Peaches 7.1 1 fresh 139 0.00
dried 0.14
Plums 4.7-6.2 2 fresh 139 0.00
dried 0.42
15.6-3.9 3 fresh 139 0.00
dried 0.19
Peaches 2.4-3.9 7 canned 1 0.1 1.6 0.0
6 6 0.0 0.1 0.1
5 15 0.0 0.1 0.1
4 22 0.0 0.0 0.1
3 150 0.0 0.0 0.1
Tomatoes 2.7 5 canned ( 1 0.44 0.08 0.00
juice ( 180 0.13 0.00 0.03
2.7 5 canned 1 0.15 0.00 0.00
juice
canned 1 0.68 0.10 0.00
puree
canned 180 0.00 0.05 0.00
juice
canned 180 0.16 0.20 0.00
puree
TABLE III (cont'd)
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
2.7 10 fruit 1 0.48 0.12 0.08
7 0.13 0.06 0.00
canned 1 0.16 0.07 0.00
juice
canned 1 0.23 0.07 0.00
puree
canned 180 0.00 0.00 0.00
juice
canned 180 0.00 0.12 0.00
puree
0.9 canned 1 0.02
concentrate
* No dichloroacetic acid is detected
The GLC method is considered most suitable for regulatory purposes,
but the TLC method could also be applied.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Australia Stone fruit 20
Other fruit and vegetables 5
Canada Cherries (sour) 50
Apricots, nectarines, peaches 30
Tomatoes 15
Cherries (sweet), cucumbers, 5
melons, plums, prunes
Switzerland Grapes, strawberries 5
United States of Cherries (sour) 50
America Apricots, peaches 30
Tomatoes 15
Melons 5
Cherries (sweet), cucumbers, 2
nectarines, plums (fresh prunes)
APPRAISAL
Captafol is used to control fungus diseases on tree fruits, melons,
cucurbits, tomatoes, and potatoes. In the case of peaches, apricots,
nectarines, cherries, plums and prunes, a dormant or blossom
application is recommended. In addition, seasonal foliar applications
are recommended for certain crops. In-furrow spray applications are
recommended for the control of representative diseases on cotton.
Captafol is used for foliage application at a rate of 0.05 to 0.2
percent of active ingredient wettable powder of flowable suspension in
spray. It is quite stable except under alkaline conditions.
Tolerances established in the U.S.A., Canada, Switzerland and
Australia vary from 2 to 50 ppm.
The residue data of captafol available to the meeting are only from
treatments made under commercial conditions in the U.S.A. The initial
residue of captafol is reduced by one half generally within a week or
two. The residue is mainly on the surface of the fruit.
The two major routes of degradation in plants are the same as those in
animals, namely reactions with sulfhydryl compounds and hydrolysis.
Sulfhydryl reaction is more rapid than hydrolysis. Main degradation
products are tetrahydrophthalimide and tetrahydrophthalic acid.
Dichloracetic acid has not been found.
In thermal food processes as well as in macerated plant materials,
captafol is extensively decomposed. Decomposition products,
tetrahydrophthalimide and tetrahydrophthalic acid, may appear in
processed foods at the beginning of the storage period.
Captafol is found to degrade rapidly in natural soils.
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is GLC. The
alternate procedure is based on thinlayer chromatography, the
sensitivity of which is about 0.03-0.05 ppm, depending on the crop
interferences.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Residues resulting from good agricultural practice as follows are
recommended, effective till 1973:
Pre-harvest Captafol residue
Crop Comment interval (days) (ppm)
Melons, whole 1 2
Cucumbers, whole 1 1
Tomatoes 1 5
Peaches 10-14 15
Cherries, sour Foliar application 20 10
Cherries, sweet Blossom application 45-70 2
Apricots Blossom application 100 0.5
Plums Blossom application 130-140 0.2
The data on pineapples, apples and grapes were inadequate for
evaluation.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Studies to elucidate the effects seen in the teratogenicity
experiments.
2. Data from countries ether that the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Data on residue levels in raw agricultural commodities moving in
commerce.
4. Data on the effect of washing, peeling and blanching on the residue
levels of various crops.
5. Elucidation of the histopathology of the kidney and liver in the
rat.
DESIRABLE
1. Metabolic studies to provide further information on the absorption
and distribution after oral administration and to identify the
metabolites found in animal tissues.
2. Collaborative studies to establish a regulatory method for captafol
in the presence of captan and folpet.
REFERENCES
Anon. (1964) Difolatan residue analysis - cleanup procedure for oily
materials. Addendum to RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1965a) The reaction of Difolatan with sulfhydryl compounds.
Chevron Chemical Co. Unpub. Rept.
Anon. (1965b) Difolatan residue analysis by electron capture gas
chromatography. Method RM-6B. Chevron Chemical Co. Unpub. Rept.
Anon. (1965c) Difolatan metabolism - The analysis of residues of
dichloroacetic acid using microcoulometric gas chromatography. Method
RM-6C. Chevron Chemical Co. Unpub. Rept.
Anon. (1966a) Studies on the uptake of Difolatan by root crops grown
in soils treated with Difolatan. Chevron Chemical Co. Unpub. Rept.
Anon. (1966b) Difolatan residue analysis - thin layer chromatographic
method. Method RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1966c) The analysis of residues of Difolatan and two
tetrahydrophthalic acid derivatives. Residue Method RM-6D. Chevron
Chemical Co. Unpub. Rept.
Berteau, P.E. (1963) Difolatan - a preliminary report on its
hydrolysis and related reactions. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E., Pack, D.E., Ospenson, J.N. and Crossley, J. (1966) The
metabolism of Difolatan. Paper presented at the American Chemical
Society, Western Regional Meeting. San Francisco, California, 18
October 1966 [Abstracted in Vortex, (Publication of the California
section, American Chemical Society) 27, insert P. 37 (1966)]
Berteau, P.E. and Pack, D.E. (1966a) The degradation of Difolatan in
soils. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966b) Difolatan degradation in
soil-studies on the formation and decay of dichloroacetic acid.
Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966c) The movement of Difolatan through
soil columns. Chevron Chemical Co. Unpub. Rept.
Cabral, R.V. de G. and Tomes, I.L. (1966) Fungicidal assay against
Botrytis cinerea and the effects of fungicides on must fermentation.
An. Inst. Super. Agron., Univ. Tec. Lisboa 29:279-97 (Chem. Abstr.
70:46363 u, 1969)
Cervenka, H., Key, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of RE 5865-Beagle dogs. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar (Sofia)
17:33-40 (Chem. Abstr. 69:34908 s, 1968)
Dye, D.F. (1969) Difolatan(R). Unpub. Rept. prepared and submitted by
Chevron Chemical Co.
Ives, M. and Calandra, J.C. (1965) Teratogenic study on difolatan.
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Jackson, G.L., Fancher, O.E. and Calandra, J.C. (1967) Rabbit
teratogenic study. Difolatan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1966) Three-generation
reproduction study on difolatan - Albino rats. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968a) An investigation
of the teratogenic potential of captan, folpet and difolatan. Toxicol.
appl. Pharmacol. 13:421-30
Kennedy, G., Fancher, O.E and Calandra, J.C. (1968b) Teratologic
investigation of difolatan in Macaca mulatta (Rhesus monkey). Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kilgore, W.W. and White, E.R. (1967) Determination of Difolatan
residues in fruits by electron-capture gas chromatography. J. Apr.
Food Chem. 15:1118-20
Kohn, F.E., Key, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of RE 5865 Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kohn, G.K. (1965) Present status of metabolic fate studies of
Difolatan. Unpub. Rept. of Chevron Chemical Corporation for
presentation at the Orthocide Conference, Amsterdam, 29 November - 5
December 1965
Leary, J.B. (1966) Difolatan: shay rat tests. Unpub. Rept. of Chevron
Chemical Co. (cited by Crossley, 1967 - see the monograph of captan)
Ogawa, J.M., Boyack, G.A., Sandeno, J.L., and Mathre, J.H. (1964)
Control of postharvest fruit decays in relation to residues of
2.6-dichloro-4-nitroaniline and Difolatan. Hilgardia 35:365-73
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1964) Acute toxicity
studies on Difolatan 80W. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965a) Acute oral
toxicity on RE 5865. Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1965b) Acute oral
toxicity on RE 5865 (aqueous suspension). Unpub. Rept. of Industrial
Bio-Test Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study, THPI. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51:1058-62
Potter, J.L. (1964) Studies on the hydrolysis of Difolatan in a
homogeneous aqueous-acetone buffer system under neutral pH conditions.
Chevron Chemical Co. Unpub. Rept.
Potter, J.L. (1965) Preliminary studies on the in vitro degradation of
Difolatan by crop macerates and filtrates. Chevron Chemical Co. Unpub.
Rept.
Turner, P. D. (1969) Evaluation of fungicides for the control of
Helminthosporium heveae on Hevea rubber in Malaysia. I. Laboratory
assessment. Experimental Agriculture 5:33-40
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Annals N.Y. Acad. Sci.
160.334-43
 Other relevant chemical properties
The pure material is a white crystalline solid; m.p. 160 to 161°C. It
is practically insoluble in water (less than 1 ppm) and slightly
soluble in most organic solvents. The technical material is a
recrystallized product of 98 percent purity; the impurities consist of
0.5-1.5 percent toluene, 0.5-1.5 percent tetrahydrophthalimide (THPI),
and 0.1-0.2 percent unknown chlorinated substances. It is a light tan
powder with a characteristic odour. It is formulated as 80 percent
wettable powder (Ortho Difolatan 80 W) and as flowable water
suspension (4 lbs per U.S. gallon). Captafol is stable except under
alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Captafol (1), on hydrolysis, yields tetrahydrophthalimide (II),
chloride ion, dichloroacetic acid (III) and inorganic sulphur in
various oxidation states. In the presence of sulfhydryl compounds at
neutral or slightly alkaline pH, captafol is rapidly degraded to
tetrahydrophthalimide and chloride ion and no organochlorine compound
is formed. This reaction with sulfhydryl compounds is much faster than
the hydrolytic reaction and it may be the dominant reaction in a
biological system where sulfhydryl groups are present. It is also well
known that the -N-S- bond in organic compounds is easily subject to
certain types of chemical attack, and two mechanisms for the cleavage
of this bond in captafol have been proposed; either nucleophilic
attack by a sulfhydryl compound or the slower hydrolytic reaction
(Kohn, 1965; Berteau et al., 1966).
Other relevant chemical properties
The pure material is a white crystalline solid; m.p. 160 to 161°C. It
is practically insoluble in water (less than 1 ppm) and slightly
soluble in most organic solvents. The technical material is a
recrystallized product of 98 percent purity; the impurities consist of
0.5-1.5 percent toluene, 0.5-1.5 percent tetrahydrophthalimide (THPI),
and 0.1-0.2 percent unknown chlorinated substances. It is a light tan
powder with a characteristic odour. It is formulated as 80 percent
wettable powder (Ortho Difolatan 80 W) and as flowable water
suspension (4 lbs per U.S. gallon). Captafol is stable except under
alkaline conditions.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Captafol (1), on hydrolysis, yields tetrahydrophthalimide (II),
chloride ion, dichloroacetic acid (III) and inorganic sulphur in
various oxidation states. In the presence of sulfhydryl compounds at
neutral or slightly alkaline pH, captafol is rapidly degraded to
tetrahydrophthalimide and chloride ion and no organochlorine compound
is formed. This reaction with sulfhydryl compounds is much faster than
the hydrolytic reaction and it may be the dominant reaction in a
biological system where sulfhydryl groups are present. It is also well
known that the -N-S- bond in organic compounds is easily subject to
certain types of chemical attack, and two mechanisms for the cleavage
of this bond in captafol have been proposed; either nucleophilic
attack by a sulfhydryl compound or the slower hydrolytic reaction
(Kohn, 1965; Berteau et al., 1966).
 Shay rats were fed captafol at levels of 60 and 600 ppm in the diet.
At several intervals the stomach contents were analysed. Captafol
degraded rapidly, the reaction having a half-life of about three
hours. Both tetrahydrophthalimide and tetrahydrophthalic acid were
detected. No dichloroacetic acid was detected at the low dose level
but small amounts of it were found at the 600 ppm level, the maximum,
found 0.5 hours after feeding, being about 1 percent of the original
dose given (Leary, 1966).
Rats, dogs and monkeys were fed carbon14-carbonyl-labelled captafol
and radioactivity measurements were performed on expired carbon
dioxide, faeces. urine and various tissues. Almost 80 percent of the
dose given was excreted within 36 hours, mainly via the urine with
smaller amounts in the faeces and none in the expired carbon dioxide.
Less than 0.5 percent of the dose remained in the liver, heart,
kidneys, blood, muscle or fat. The rate of excretion of carbon14 was
almost identical for all three species. Unchanged captafol accounted
for the majority of the radioactivity in the faeces, but no captafol
was detected in blood, tissues or urine. The primary metabolite,
tetralydrophthalimide was detected in blood, faeces and urine although
the major portion of the activity in blood and urine was due to other,
more water-soluble metabolites. The epoxide of captafol was not
detected in blood, urine or faeces and it was concluded the
epoxidation is not a metabolic route (Dye, 1969).
Captafol differs from captan only in the nature of the halogenated
group attached to the sulphur. Both compounds give the same
decomposition product tetrahydrophthalimide. Therefore, all the
information on the metabolism of captan relative to the imide portion
of the molecule is considered to be applicable to captafol (Dye,
1969). (See the monograph on captan).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed captafol at 0, 50 (raised
to 100 after first generation), 250 (raised to 500 after first
generation), and 1000 ppm in the diet in a three-generation
reproduction study. There were no adverse effects on body-weight gain,
mortality, or organ-weights of parental animals or on reproductive
performance, fertility and lactation indices, litter-size, or number
of stillbirths in any test group. Pup survival in the test groups at
various intervals in the lactation period was not significantly
different from the control group. Weanling body-weights in the 1000
ppm group were depressed in both males and females in the first and
third generations. Weanlings of the second generation showed only
slight weight depression. This effect on weanling weights did not
occur at the lower test levels (even after they were raised to 100 and
500 ppm). Gross examinations and histopathology carried out on
parental animals and on weanlings in the 0 and 1000 ppm groups
revealed no changes that could be attributed to captafol (Kennedy et
al. 1966).
Special studies on teratogenicity
Chicken-egg
Captafol was injected, in dimethylsulfoxide solution, into either the
yolk or air cell of fresh fertile eggs at levels from 3-20 mg/kg
egg-weight. The eggs were incubated and non-viable embryos and hatched
chicks were examined for gross abnormalities. In a total of 270 eggs
treated with captafol, the incidence of malformations was 6.67 percent
compared to the control value of 1.6 percent for 1500 eggs injected
with dimethylsulfoxide alone. In the same experiment the metabolite
tetrahydrophthalimide was also injected. Of 1025 eggs the incidence of
malformation was 4.78 percent. The epoxy-derivative of captafol
("captafol-epoxide") was also tested for its effect on chicken eggs.
Of 115 eggs into which this compound was injected. the incidence of
malformation was 15.05 percent. In all cases the malformations
consisted mainly of micromelia, amelia and phocomelia (Verrett et al.,
1969).
Monkey
Groups of seven pregnant Rhesus monkeys were given oral doses of 6.25,
12.5 or 25 mg of technical captafol per kg of body-weight from
gestation day 22 through 32. Another group of seven pregnant females
received 10 mg/kg body-weight of thalidomide, on days 25, 26 and 27.
Three additional females ware treated with 15 consecutive doses of
captafol at 12.5 mg/kg body-weight later in gestation (66-80, 81-959
and 86-100 days). All doses were administered by gastric intubation of
a suspension in 0.9 percent gelatin solution in the morning before
feeding. All the females used had produced at least one live, normal
baby prior to this study. Pregnancies were terminated by Caesarean
section at approximately the 84th day of gestation. The three monkeys
receiving captafol later in gestation were allowed to proceed to term
and deliver their young unaided. Careful examination of 19 foetuses
from the captafol-treated monkeys, including gross observation, X-ray
and skeletal examination following alizarin red S staining, revealed
no abnormalities. Internal structural formation, observed both grossly
and by evaluation of organ-weight and organ to body-weight ratio data,
was normal. Foetal mortality (resorption or abortion) occurred in the
sixth week of pregnancy in two of the seven animals receiving 25 mg/kg
of captafol. In the group given thalidomide teratogenic activity (limb
malformations) was observed in five of the seven foetuses. The three
monkeys allowed to proceed to term delivered three normal babies, as
indicated by external and X-ray examination (Kennedy et al., 1968b).
Rabbit
Groups of 10 pregnant Dutch Belted rabbits received daily oral doses
of 0 or 75 mg/kg body-weight of technical captafol. A third group was
given thalidomide at 75 mg/kg body-weight. Dosing, administered by
gelatin capsule, began on day 6 and ended on day 16 of the gestation
period (the day of conception being day 0). On day 28, each doe was
sacrificed and the foetuses removed by Caesarean section. The rabbits
given captafol lost weight over the period of treatment. One doe in
this group aborted eight young, another showed one resorption site.
Three does in the thalidomide group showed evidence of resorption. Of
the viable young, 86 percent from the group given captafol survived a
six hour incubation (37°C) period compared with 100 percent in the
control group. No abnormalities were seen among 74 young in the group
given captafol. Animals in the group given thalidomide showed
significant teratogenic effects (Ives and Calandra, 1965; Kennedy et
al., 1968a).
Groups of 10 pregnant New Zealand albino rabbits were given technical
captafol (98.9 percent purity) at doses of 0, 37.5, 75, 112.5 or 150
mg/kg body-weight on gestation day 6 through 18 inclusive. A positive
control group was given 75 mg/kg body-weight of thalidomide. All doses
were administered via gelatin capsule. On day 29 each doe was
sacrificed and the young were removed by Caesarean section, weighed
and observed for abnormalities. At the lowest level tested, there was
no maternal mortality and the animals gained weight, although not as
much as the controls. Resorption sites occurred in 2 of the 10 females
(three sites). Examination of 62 foetuses from this group revealed no
abnormalities. Body-weights and 24-hour incubator survival were
comparable to those of the control group. At all the higher
dose-levels, toxic effects were seen in the mothers. Deaths occurred
in each group and resorption sites were prevalent in the survivors.
However, all young delivered in all three groups were free of gross
teratologic effects and survived the 24-hour incubation period.
Body-weights were lower than those of controls. In the group given
thalidomide, 32 of 55 foetuses showed abnormalities (Jackson at al.,
1967; Kennedy et al., 1968a).
Rat
A group of nine pregnant female rats was given doses of 100 mg/kg
body-weight/day of captafol orally from day 6 to day 15 of gestation
and another group of five pregnant rats was given 500 mg/kg from day 8
to day 10. Examination of 180 foetuses revealed no gross malformations
(Kennedy et al., 1968a).
Special studies on the metabolite, tetrahydrophthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted rabbits received 0 or 75 mg/kg
body-weight of tetrahydrophthalimide, the hydrolytic metabolite of
captafol, from day 6 to 16 inclusive of the gestation period. A
treated control group was given 75 mg/kg bodyweight of thalidomide
over the same period. The doses were given orally via gelatin capsule.
On day 28 each doe was sacrificed, the young were removed by Caesarean
section and examined for abnormalities. Examination of 57 embryos from
the test group revealed no external or skeletal abnormalities.
Fourteen embryos of a total of 44 in the group given thalidomide had
skeletal abnormalities. There was an increase in the occurrence of
resorption sites in the test group compared with the controls. Five of
the ten females exhibited one to three resorption sites (a total of
nine) although the number of viable young in the group was not reduced
(Palazzolo at al., 1966; Kennedy at al., 1968a).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat oral 62001/ Palazzolo et al., 1965a
Rat oral 50002/ Palazzolo et al., 1965b
(continued)
LD50
Animal Route mg/kg body-weight References
Rat oral 25002/ Palazzolo et al., 1964
Rabbit dermal 154002/ Palazzolo et al., 1964
1/ Corn oil solution
2/ Aqueous suspension
Short-term studies
Dog
Groups of two male and two female dogs were given daily doses of 0,
10, 30, 100 or 300 mg/kg body-weight of captafol over a two year
period. The material was given in gelatin capsules immediately after
each day's feeding. At the two highest dose levels there was a
decreased body-weight gain over the period of the experiment. Emesis
and loose stools occurred quite frequently in both these groups during
the first four weeks but only occasionally thereafter. These effects
were not seen at the two lower levels. Increased absolute liver and
kidney-weights and liver and kidney to bodyweight ratios were seen in
all animals at the 30, 100 and 300 mg/kg levels. Alterations In other
organ-weights appeared unrelated to the administration of the test
material. Haematologic studies revealed a mild anaemia at the
termination of the study in the dogs given 100 and 300 mg/kg per day.
Histopathology, blood chemistry, urinalysis and liver-function tests
revealed no adverse effects that could be attributed to the
administration of captafol. The 10 mg/kg dose level appeared to cause
no significant effects (Cervenka at al., 1964).
Rabbit
Four groups of eight rabbits were given dermal applications of
captafol at levels of 0, 500, 1000 and 2000 mg/kg body-weight/day for
20 days. Half the animals in each group were treated on abraded skin
areas, the other half on intact skin. There was a marked adverse
effect on body-weight, even at the lowest level tested. Deaths
occurred at all test levels and the 20-day dermal LD50 was found to
be 1100 mg/kg body-weight/day (for the 80 percent wettable powder).
The only effects seen in gross and microscopic examinations were in
the skin at the application site (Palazzolo et al., 1964).
Long-term studies
Rat
In a two year study with captafol added to the diet at 0 (70 males and
70 females), 250, 500, 1500 and 5000 ppm (35 females and 35 males at
each test level), there was growth depression at the 1500 and 5000 ppm
levels. Mortality was increased in the 5000 ppm group, with no males
left alive after 23 months. A lymphocyte to neutrophile shift was
observed in the surviving males of this group after 21 months. There
was an increase in the liver to body-weight ratio at the 500, 1500 and
5000 ppm levels at 12 months. An increase in this ratio was also seen
in males at 250 ppm. At the end of the experiment there was no longer
a significant difference at the two lower tent levels. Significant
increases were also observed in organ weight and organ to bodyweight
ratios for kidney and adrenal of rats fed at 1500 and 5000 ppm.
Histopathology revealed liver changes characterized by degeneration of
hepatic cells, vacuolization, incipient fat alteration, and
infiltration by mononuclear cells. Kidney changes were characterized
by alterations in proximal and distal tubular cells; many giant forms
with large irregular nuclei being present. These changes in liver and
kidney were only seen in rate fed the two highest dose levels. No
other histopathological changes were associated with the
administration of captafol. No effects on tumour incidence were
observed (Kohn et al., 1964).
COMMENT
Captafol appears to be metabolized rapidly and in a similar way in
rats, dogs and monkeys. The primary metabolite and other metabolites
have been identified in excretion studies. Information is, however,
incomplete on the nature of the metabolites in animal tissues, as well
as on the absorption and distribution of captafol and its metabolites
after oral administration. A two-year study in dogs indicated that a
dose level of 10 mg/kg body-weight/day was without significant adverse
effect. In the long-term study in rate at the lowest level tested (250
ppm), an increase in the liver to body-weight ratio was evident at 12
months but not at 24 months. For this reason a definite no-effect
level has not been established in that species. Teratogenicity studies
in mammalian species produced evidence of embryotoxic effects at the
lowest level tested but there was no indication of malformation of the
foetuses. The nature of the reported histopathological effects upon
the kidney and liver observed in the two year study in rats fed high
dose levels of captafol is of some concern.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 10 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.05 mg/kg body-weight/day
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captafol is used to control fungus diseases.
Rates of application and recommended intervals between treatment and
harvest are (Dye, 1969):
Fruits - 0.05 to 0.2% a.i., applied at bloom, petal
fall, shuck split and 10 to 14 day intervals
up to harvest.
Melons - 1.3 to 2.7 kg a.i./ha applied as needed at 7
day intervals up to harvest.
Tomatoes and
cucumbers - 1.1. to 2.7 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Potatoes - 0.10 to 1.8 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Post-harvest treatments
Captafol is recommended for post-harvest use on peaches, cherries,
plums, and nectarines (Ogawa at al., 1964).
Other uses
In-furrow spray application is recommended for the control of seedling
diseases on cotton. Captafol has been used to control
Helminthosporium heveae in rubber (Turner, 1969).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data of captafol are from treatments made under commercial
conditions in the U.S.A. (Dye, 1969). The tests made with peaches and
watermelons indicate that the captafol is present as a surface residue
and is not systemically translocated into the flesh of the fruit. The
initial residue of captafol is reduced by one half generally within a
week or two. Two types of applications are recommended: dormant and
blossom, and seasonal foliar. The residue data are given separately
(Table I).
TABLE I
Residue data from field trials
Crop Rate of Number of Pre-harvest Captafol
application treatments interval residue
(kg a.i. per ha) (days) (ppm)
Blossom application
Apricots 3.4-8.1 3 102 0.1-0.2
Cherries,
sweet 2.3-10.8 2-3 45-68 0.6-1.4
Plums (0.1-0.3% a.i.) 2-3 131-141 n.d.-0.2
Seasonal foliar application
Cherries,
sour 1.8-3.6 4 20 7-9
Peaches 3.1-4.5 1-13 10-14 2.5-14
Melons 2.0-4.0 5-9 1 0.4-1.8
Cucumbers 1.3-1.8 6-9 1 0.1-0.4
Tomatoes 2.7-5.4 6-11 1 0.4-3.8
FATE OF RESIDUES
General comments
As outlined under 'BIOCHEMICAL ASPECTS', the N-S bond in substances
like captafol, is subject to chemical attack. For captafol, the two
mechanisms proposed (Kohn, 1965) are: (1) nucleophilic attack by
sulfhydryl compounds (Anon., 1965a) acid (2) hydrolysis (Berteau,
1963, and Potter, 1964) (Fig. 1). Both initiative reactions result in
THPI. In addition, in reaction (1) free Cl- ions, inorganic sulphur
compounds, and 2C-fragment are released and in reaction (2) chlorine
is only partly appearing as free ion, the rest being bound to the
2C-moiety. Subsequently, tetrahydrophthalimide decomposes via the
unstable tetrahydrophthalamic acid into tetrahydrophthalic acid.
In the presence of sulfhydryl compounds and at or near neutral pH, the
sulfhydryl reaction (1) is much faster than the hydrolysis (2). At pH
7 and 25°C, the "half-life" of captafol in a homogeneous sulfhydryl
reaction was found to be 4 minutes and in the corresponding hydrolysis
1000 minutes (Anon., 1965a). As the temperature and/or pH rises the
rates of both reactions increases.
In animals
The main routes of degradation of captafol in animals are the same as
in plants (Dye, 1969).
In plants
Due to the extremely low water solubility of captafol the residues of
the parent compound are occurring on the surface of the treated
plants. This is greatly limiting the exposure of captafol to the
degradation reaction by the sulfhydryl compounds of the plant tissue.
As a result, the captafol residues show a high persistence in situ.
In vitro studies on the degradation of captafol by spinach, tomato,
and celery macerates and filtrates (Potter, 1965) have confirmed that
captafol is very readily decomposed by the cell sap of various plants.
There were distinct differences in the degradation rates of various
plants. In spinach the captafol residue (10 ppm) was nearly completely
degraded in one hour, but in tomato in about 24 hours. Boiling did not
alter the degradative capacity of the macerates which indicates that
no essential enzymatic reactions are involved in the degradation of
captafol.
The in situ residues of captafol are found to produce minor amounts
of tetrahydrophthalimide and tetrahydrophthalic acid (Table II). No
dichloroacetic acid has been found. Thus, even though dichloroacetic
acid can be formed from captafol under certain conditions, it is not
found to be present as a residue on crops treated under field
conditions and would, therefore, not be consumed by man (Dye, 1969).
In soils
It has been found that captafol degrades rapidly in soil, the rate
being a function of both the soil type and the initial concentration;
even the longest "half-life" found was only 11 days (Berteau and Pack,
1966a). These studies show that under normal agricultural conditions,
the captafol that is supplied one year, even late in the season, would
be completely degraded by the beginning of the next growing season.
Therefore, there would be no accumulation of captafol in soil from
year to year.
It has been found that in natural, non-sterile soil, captafol
degradation results in only a barely detectable trace of
dichloroacetic acid being formed at short (i.e. one week) intervals.
At longer intervals, none is detectable at a limit of detectability of
0.02 ppm dichloroacetic acid itself is rapidly degraded by natural
soil, being completely degraded within one week. In normal
agricultural practice, therefore, there would be no buildup in the
soil (Berteau and Pack, 1966b).
In sterile soil, the degradation of captafol is associated with a
buildup of dichloroacetic acid and in dichloroacetic acid fortified
soil no loss was observed over a period of one week. The degradation
of the acid in soil is, therefore, definitely biochemical (Berteau and
Pack, 1966b).
The movement of captafol through soil columns by water leaching has
been studied. The results show that captafol does not move
significantly and will not accumulate in water leaching from treated
areas (Berteau and Pack, 1966c).
Carrots and radishes were grown in soils treated with captafol to
determine if any captafol would be taken up by the crop. At a limit of
detectability of 0.05 ppm or better, no captafol residue was found in
either crop (Anon., 1965a).
In storage and processing
All the data available indicate that the captafol residues on fruit
are very stable under commercial storage conditions.
In the studies on dried peaches and prunes, no residues were found in
the fresh fruit, but small residues ware detected in the dried
samples. Apparently, any residue in the fresh fruit was below the
limit of detectability but this residue became detectable as it was
concentrated in the drying operation (Table III) (Dye, 1969).
In the canned peach samples, there was a slight residue of captafol
detected at 1 day interval, but no detectable quantities at subsequent
intervals. The residue of 1.6 ppm of tetrahydrophthalimide in the 1
day peach sample decreased to below the limit of detection at 22 and
150 days. Tetrahydrophthalic acid was barely detectable in canned
peaches, and no detectable residues of dichloroacetic acid were found
in any of the samples studied (Table III) (Dye, 1969).
In the case of tomatoes no detectable residues of tetrahydrophthalic
acid or dichloroacetic acid could be found in any of the canned fruit
samples (Table III) (Dye, 1969).
Because of the nature of the captafol residues they would be readily
removed by washing, blanching, and peeling the fruit. A detailed
evaluation can not be made because of lack of complete information.
According to Chalkov and Vanev (1968), captafol has a suppressing
action on wine yeast. Cabral and Tomaz (1956) reported that it
completely inhibited fermentation of the must from treated grapes.
METHODS OF RESIDUE ANALYSIS
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is electron
capture GLC, the alternate procedure is based on thin-layer
chromatography.
The crop is extracted with benzene. The extract is cleaned-up by
either a column chromatographic method or a thin layer chromatographic
(TLC) method, or a combination of the two. The cleaned-up extract is
then chromatographed by TLC with captafol standards. The plate is
sprayed with N,N-dimethyl-p-phenylenediamine to visualize the spots.
The amount of captafol is determined by visual commission of the
sample spot with the standard spots. The method is a positive
identification of captafol residues. Its sensitivity is about
0.03-0.05 ppm, depending on the crop interferences (Anon., 1966b).
There is a modified column cleanup method which is useful for oily
crops where interferences in the thin-layer chromatography is
encountered from plant extractives (Anon., 1964). Pomerantz and Rose
(1968) have developed a TLC method which differentiates between
captan, folpet, captafol, and their metabolites.
The preferred method employs electron capture GLC for the final
detection and estimation of residues of captafol. Either the column or
TLC cleanup procedure can be used when cleanup is needed (Anon.,
1965b). Twenty-seven pesticides were tested for possible interference
in this GLC method of analysis. It found that none of the pesticides
studied eluted at the same elution time as did captafol under the
conditions employed, and therefore there would be no interference from
any of them. The sensitivity of 0.01 ppm can be obtained by the GLC
method (Kilgore and White, 1967).
The analytical method for the analysis of dichloroacetic acid which is
a demonstrated hydrolysis product of captafol, is based upon
microcoulometric gas chromatography. This method of analysis is both
highly specific and quite free from interfering material. The limit of
detection of this method is approximately 0.05 ppm, or possibly lower.
The sample is macerated and extracted with water. The water extract is
then extracted with ether. The DCAA in the ether layer is esterified
with diazomethane and the analysis completed with the microcoulometric
gas chromatography (Anon., 1965c).
The hydrolysis products of the imide portion of the captafol molecule,
tetrahydrophthalimide and tetrahydrophthalic acid, are detected by a
method which involves the extraction, cleanup, and esterification of
the acid derivatives with diazomethane and the subsequent gas
chromatographic detection of the esters. The detection system used is
hydrogen flame. This method is quite specific but great care must be
taken to eliminate interfering materials which would be detected by
the hydrogen flame. With adequate cleanup on most crops, this method
is sensitive to approximately 0.05 ppm (Anon., 1966c).
TABLE II
Data on captafol residues and its degradation products in various fruits (Anon., 1969
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments (days) Captafol hydrophthalimide acid
Peach 9.0 12 1 3.4 1.0 0.5
7 3.5 1.1 0.3
3.1 4 1 11.7 0.00 0.00
5 12.0 0.00 0.00
10 9.9 0.00 0.00
15 9.3 0.00 0.00
3.1 4 1 20.6 0.00 0.00
5 13.0 0.00 0.00
10 13.0 0.00 0.00
15 10.6 0.00 0.00
Cucumber 1.8 6 1 0.14 0.00 0.06
6 0.10 0.00 0.13
Tomato 2.7 9 1 9.7 0.16 0.00
7 10.1 0.16 0.07
2.7 9 1 5.2 0.27 0.00
7 4.3 0.20 0.00
* No dichloroacetic acid is detected
TABLE III
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
Peaches 7.1 1 fresh 139 0.00
dried 0.14
Plums 4.7-6.2 2 fresh 139 0.00
dried 0.42
15.6-3.9 3 fresh 139 0.00
dried 0.19
Peaches 2.4-3.9 7 canned 1 0.1 1.6 0.0
6 6 0.0 0.1 0.1
5 15 0.0 0.1 0.1
4 22 0.0 0.0 0.1
3 150 0.0 0.0 0.1
Tomatoes 2.7 5 canned ( 1 0.44 0.08 0.00
juice ( 180 0.13 0.00 0.03
2.7 5 canned 1 0.15 0.00 0.00
juice
canned 1 0.68 0.10 0.00
puree
canned 180 0.00 0.05 0.00
juice
canned 180 0.16 0.20 0.00
puree
TABLE III (cont'd)
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
2.7 10 fruit 1 0.48 0.12 0.08
7 0.13 0.06 0.00
canned 1 0.16 0.07 0.00
juice
canned 1 0.23 0.07 0.00
puree
canned 180 0.00 0.00 0.00
juice
canned 180 0.00 0.12 0.00
puree
0.9 canned 1 0.02
concentrate
* No dichloroacetic acid is detected
The GLC method is considered most suitable for regulatory purposes,
but the TLC method could also be applied.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Australia Stone fruit 20
Other fruit and vegetables 5
Canada Cherries (sour) 50
Apricots, nectarines, peaches 30
Tomatoes 15
Cherries (sweet), cucumbers, 5
melons, plums, prunes
Switzerland Grapes, strawberries 5
United States of Cherries (sour) 50
America Apricots, peaches 30
Tomatoes 15
Melons 5
Cherries (sweet), cucumbers, 2
nectarines, plums (fresh prunes)
APPRAISAL
Captafol is used to control fungus diseases on tree fruits, melons,
cucurbits, tomatoes, and potatoes. In the case of peaches, apricots,
nectarines, cherries, plums and prunes, a dormant or blossom
application is recommended. In addition, seasonal foliar applications
are recommended for certain crops. In-furrow spray applications are
recommended for the control of representative diseases on cotton.
Captafol is used for foliage application at a rate of 0.05 to 0.2
percent of active ingredient wettable powder of flowable suspension in
spray. It is quite stable except under alkaline conditions.
Tolerances established in the U.S.A., Canada, Switzerland and
Australia vary from 2 to 50 ppm.
The residue data of captafol available to the meeting are only from
treatments made under commercial conditions in the U.S.A. The initial
residue of captafol is reduced by one half generally within a week or
two. The residue is mainly on the surface of the fruit.
The two major routes of degradation in plants are the same as those in
animals, namely reactions with sulfhydryl compounds and hydrolysis.
Sulfhydryl reaction is more rapid than hydrolysis. Main degradation
products are tetrahydrophthalimide and tetrahydrophthalic acid.
Dichloracetic acid has not been found.
In thermal food processes as well as in macerated plant materials,
captafol is extensively decomposed. Decomposition products,
tetrahydrophthalimide and tetrahydrophthalic acid, may appear in
processed foods at the beginning of the storage period.
Captafol is found to degrade rapidly in natural soils.
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is GLC. The
alternate procedure is based on thinlayer chromatography, the
sensitivity of which is about 0.03-0.05 ppm, depending on the crop
interferences.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Residues resulting from good agricultural practice as follows are
recommended, effective till 1973:
Pre-harvest Captafol residue
Crop Comment interval (days) (ppm)
Melons, whole 1 2
Cucumbers, whole 1 1
Tomatoes 1 5
Peaches 10-14 15
Cherries, sour Foliar application 20 10
Cherries, sweet Blossom application 45-70 2
Apricots Blossom application 100 0.5
Plums Blossom application 130-140 0.2
The data on pineapples, apples and grapes were inadequate for
evaluation.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Studies to elucidate the effects seen in the teratogenicity
experiments.
2. Data from countries ether that the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Data on residue levels in raw agricultural commodities moving in
commerce.
4. Data on the effect of washing, peeling and blanching on the residue
levels of various crops.
5. Elucidation of the histopathology of the kidney and liver in the
rat.
DESIRABLE
1. Metabolic studies to provide further information on the absorption
and distribution after oral administration and to identify the
metabolites found in animal tissues.
2. Collaborative studies to establish a regulatory method for captafol
in the presence of captan and folpet.
REFERENCES
Anon. (1964) Difolatan residue analysis - cleanup procedure for oily
materials. Addendum to RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1965a) The reaction of Difolatan with sulfhydryl compounds.
Chevron Chemical Co. Unpub. Rept.
Anon. (1965b) Difolatan residue analysis by electron capture gas
chromatography. Method RM-6B. Chevron Chemical Co. Unpub. Rept.
Anon. (1965c) Difolatan metabolism - The analysis of residues of
dichloroacetic acid using microcoulometric gas chromatography. Method
RM-6C. Chevron Chemical Co. Unpub. Rept.
Anon. (1966a) Studies on the uptake of Difolatan by root crops grown
in soils treated with Difolatan. Chevron Chemical Co. Unpub. Rept.
Anon. (1966b) Difolatan residue analysis - thin layer chromatographic
method. Method RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1966c) The analysis of residues of Difolatan and two
tetrahydrophthalic acid derivatives. Residue Method RM-6D. Chevron
Chemical Co. Unpub. Rept.
Berteau, P.E. (1963) Difolatan - a preliminary report on its
hydrolysis and related reactions. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E., Pack, D.E., Ospenson, J.N. and Crossley, J. (1966) The
metabolism of Difolatan. Paper presented at the American Chemical
Society, Western Regional Meeting. San Francisco, California, 18
October 1966 [Abstracted in Vortex, (Publication of the California
section, American Chemical Society) 27, insert P. 37 (1966)]
Berteau, P.E. and Pack, D.E. (1966a) The degradation of Difolatan in
soils. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966b) Difolatan degradation in
soil-studies on the formation and decay of dichloroacetic acid.
Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966c) The movement of Difolatan through
soil columns. Chevron Chemical Co. Unpub. Rept.
Cabral, R.V. de G. and Tomes, I.L. (1966) Fungicidal assay against
Botrytis cinerea and the effects of fungicides on must fermentation.
An. Inst. Super. Agron., Univ. Tec. Lisboa 29:279-97 (Chem. Abstr.
70:46363 u, 1969)
Cervenka, H., Key, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of RE 5865-Beagle dogs. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar (Sofia)
17:33-40 (Chem. Abstr. 69:34908 s, 1968)
Dye, D.F. (1969) Difolatan(R). Unpub. Rept. prepared and submitted by
Chevron Chemical Co.
Ives, M. and Calandra, J.C. (1965) Teratogenic study on difolatan.
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Jackson, G.L., Fancher, O.E. and Calandra, J.C. (1967) Rabbit
teratogenic study. Difolatan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1966) Three-generation
reproduction study on difolatan - Albino rats. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968a) An investigation
of the teratogenic potential of captan, folpet and difolatan. Toxicol.
appl. Pharmacol. 13:421-30
Kennedy, G., Fancher, O.E and Calandra, J.C. (1968b) Teratologic
investigation of difolatan in Macaca mulatta (Rhesus monkey). Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kilgore, W.W. and White, E.R. (1967) Determination of Difolatan
residues in fruits by electron-capture gas chromatography. J. Apr.
Food Chem. 15:1118-20
Kohn, F.E., Key, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of RE 5865 Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kohn, G.K. (1965) Present status of metabolic fate studies of
Difolatan. Unpub. Rept. of Chevron Chemical Corporation for
presentation at the Orthocide Conference, Amsterdam, 29 November - 5
December 1965
Leary, J.B. (1966) Difolatan: shay rat tests. Unpub. Rept. of Chevron
Chemical Co. (cited by Crossley, 1967 - see the monograph of captan)
Ogawa, J.M., Boyack, G.A., Sandeno, J.L., and Mathre, J.H. (1964)
Control of postharvest fruit decays in relation to residues of
2.6-dichloro-4-nitroaniline and Difolatan. Hilgardia 35:365-73
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1964) Acute toxicity
studies on Difolatan 80W. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965a) Acute oral
toxicity on RE 5865. Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1965b) Acute oral
toxicity on RE 5865 (aqueous suspension). Unpub. Rept. of Industrial
Bio-Test Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study, THPI. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51:1058-62
Potter, J.L. (1964) Studies on the hydrolysis of Difolatan in a
homogeneous aqueous-acetone buffer system under neutral pH conditions.
Chevron Chemical Co. Unpub. Rept.
Potter, J.L. (1965) Preliminary studies on the in vitro degradation of
Difolatan by crop macerates and filtrates. Chevron Chemical Co. Unpub.
Rept.
Turner, P. D. (1969) Evaluation of fungicides for the control of
Helminthosporium heveae on Hevea rubber in Malaysia. I. Laboratory
assessment. Experimental Agriculture 5:33-40
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Annals N.Y. Acad. Sci.
160.334-43
Shay rats were fed captafol at levels of 60 and 600 ppm in the diet.
At several intervals the stomach contents were analysed. Captafol
degraded rapidly, the reaction having a half-life of about three
hours. Both tetrahydrophthalimide and tetrahydrophthalic acid were
detected. No dichloroacetic acid was detected at the low dose level
but small amounts of it were found at the 600 ppm level, the maximum,
found 0.5 hours after feeding, being about 1 percent of the original
dose given (Leary, 1966).
Rats, dogs and monkeys were fed carbon14-carbonyl-labelled captafol
and radioactivity measurements were performed on expired carbon
dioxide, faeces. urine and various tissues. Almost 80 percent of the
dose given was excreted within 36 hours, mainly via the urine with
smaller amounts in the faeces and none in the expired carbon dioxide.
Less than 0.5 percent of the dose remained in the liver, heart,
kidneys, blood, muscle or fat. The rate of excretion of carbon14 was
almost identical for all three species. Unchanged captafol accounted
for the majority of the radioactivity in the faeces, but no captafol
was detected in blood, tissues or urine. The primary metabolite,
tetralydrophthalimide was detected in blood, faeces and urine although
the major portion of the activity in blood and urine was due to other,
more water-soluble metabolites. The epoxide of captafol was not
detected in blood, urine or faeces and it was concluded the
epoxidation is not a metabolic route (Dye, 1969).
Captafol differs from captan only in the nature of the halogenated
group attached to the sulphur. Both compounds give the same
decomposition product tetrahydrophthalimide. Therefore, all the
information on the metabolism of captan relative to the imide portion
of the molecule is considered to be applicable to captafol (Dye,
1969). (See the monograph on captan).
Special studies on reproduction
Rat
Groups of 8 male and 16 female rats were fed captafol at 0, 50 (raised
to 100 after first generation), 250 (raised to 500 after first
generation), and 1000 ppm in the diet in a three-generation
reproduction study. There were no adverse effects on body-weight gain,
mortality, or organ-weights of parental animals or on reproductive
performance, fertility and lactation indices, litter-size, or number
of stillbirths in any test group. Pup survival in the test groups at
various intervals in the lactation period was not significantly
different from the control group. Weanling body-weights in the 1000
ppm group were depressed in both males and females in the first and
third generations. Weanlings of the second generation showed only
slight weight depression. This effect on weanling weights did not
occur at the lower test levels (even after they were raised to 100 and
500 ppm). Gross examinations and histopathology carried out on
parental animals and on weanlings in the 0 and 1000 ppm groups
revealed no changes that could be attributed to captafol (Kennedy et
al. 1966).
Special studies on teratogenicity
Chicken-egg
Captafol was injected, in dimethylsulfoxide solution, into either the
yolk or air cell of fresh fertile eggs at levels from 3-20 mg/kg
egg-weight. The eggs were incubated and non-viable embryos and hatched
chicks were examined for gross abnormalities. In a total of 270 eggs
treated with captafol, the incidence of malformations was 6.67 percent
compared to the control value of 1.6 percent for 1500 eggs injected
with dimethylsulfoxide alone. In the same experiment the metabolite
tetrahydrophthalimide was also injected. Of 1025 eggs the incidence of
malformation was 4.78 percent. The epoxy-derivative of captafol
("captafol-epoxide") was also tested for its effect on chicken eggs.
Of 115 eggs into which this compound was injected. the incidence of
malformation was 15.05 percent. In all cases the malformations
consisted mainly of micromelia, amelia and phocomelia (Verrett et al.,
1969).
Monkey
Groups of seven pregnant Rhesus monkeys were given oral doses of 6.25,
12.5 or 25 mg of technical captafol per kg of body-weight from
gestation day 22 through 32. Another group of seven pregnant females
received 10 mg/kg body-weight of thalidomide, on days 25, 26 and 27.
Three additional females ware treated with 15 consecutive doses of
captafol at 12.5 mg/kg body-weight later in gestation (66-80, 81-959
and 86-100 days). All doses were administered by gastric intubation of
a suspension in 0.9 percent gelatin solution in the morning before
feeding. All the females used had produced at least one live, normal
baby prior to this study. Pregnancies were terminated by Caesarean
section at approximately the 84th day of gestation. The three monkeys
receiving captafol later in gestation were allowed to proceed to term
and deliver their young unaided. Careful examination of 19 foetuses
from the captafol-treated monkeys, including gross observation, X-ray
and skeletal examination following alizarin red S staining, revealed
no abnormalities. Internal structural formation, observed both grossly
and by evaluation of organ-weight and organ to body-weight ratio data,
was normal. Foetal mortality (resorption or abortion) occurred in the
sixth week of pregnancy in two of the seven animals receiving 25 mg/kg
of captafol. In the group given thalidomide teratogenic activity (limb
malformations) was observed in five of the seven foetuses. The three
monkeys allowed to proceed to term delivered three normal babies, as
indicated by external and X-ray examination (Kennedy et al., 1968b).
Rabbit
Groups of 10 pregnant Dutch Belted rabbits received daily oral doses
of 0 or 75 mg/kg body-weight of technical captafol. A third group was
given thalidomide at 75 mg/kg body-weight. Dosing, administered by
gelatin capsule, began on day 6 and ended on day 16 of the gestation
period (the day of conception being day 0). On day 28, each doe was
sacrificed and the foetuses removed by Caesarean section. The rabbits
given captafol lost weight over the period of treatment. One doe in
this group aborted eight young, another showed one resorption site.
Three does in the thalidomide group showed evidence of resorption. Of
the viable young, 86 percent from the group given captafol survived a
six hour incubation (37°C) period compared with 100 percent in the
control group. No abnormalities were seen among 74 young in the group
given captafol. Animals in the group given thalidomide showed
significant teratogenic effects (Ives and Calandra, 1965; Kennedy et
al., 1968a).
Groups of 10 pregnant New Zealand albino rabbits were given technical
captafol (98.9 percent purity) at doses of 0, 37.5, 75, 112.5 or 150
mg/kg body-weight on gestation day 6 through 18 inclusive. A positive
control group was given 75 mg/kg body-weight of thalidomide. All doses
were administered via gelatin capsule. On day 29 each doe was
sacrificed and the young were removed by Caesarean section, weighed
and observed for abnormalities. At the lowest level tested, there was
no maternal mortality and the animals gained weight, although not as
much as the controls. Resorption sites occurred in 2 of the 10 females
(three sites). Examination of 62 foetuses from this group revealed no
abnormalities. Body-weights and 24-hour incubator survival were
comparable to those of the control group. At all the higher
dose-levels, toxic effects were seen in the mothers. Deaths occurred
in each group and resorption sites were prevalent in the survivors.
However, all young delivered in all three groups were free of gross
teratologic effects and survived the 24-hour incubation period.
Body-weights were lower than those of controls. In the group given
thalidomide, 32 of 55 foetuses showed abnormalities (Jackson at al.,
1967; Kennedy et al., 1968a).
Rat
A group of nine pregnant female rats was given doses of 100 mg/kg
body-weight/day of captafol orally from day 6 to day 15 of gestation
and another group of five pregnant rats was given 500 mg/kg from day 8
to day 10. Examination of 180 foetuses revealed no gross malformations
(Kennedy et al., 1968a).
Special studies on the metabolite, tetrahydrophthalimide
Rabbit (teratogenic study)
Groups of 10 female Dutch Belted rabbits received 0 or 75 mg/kg
body-weight of tetrahydrophthalimide, the hydrolytic metabolite of
captafol, from day 6 to 16 inclusive of the gestation period. A
treated control group was given 75 mg/kg bodyweight of thalidomide
over the same period. The doses were given orally via gelatin capsule.
On day 28 each doe was sacrificed, the young were removed by Caesarean
section and examined for abnormalities. Examination of 57 embryos from
the test group revealed no external or skeletal abnormalities.
Fourteen embryos of a total of 44 in the group given thalidomide had
skeletal abnormalities. There was an increase in the occurrence of
resorption sites in the test group compared with the controls. Five of
the ten females exhibited one to three resorption sites (a total of
nine) although the number of viable young in the group was not reduced
(Palazzolo at al., 1966; Kennedy at al., 1968a).
Acute toxicity
LD50
Animal Route mg/kg body-weight References
Rat oral 62001/ Palazzolo et al., 1965a
Rat oral 50002/ Palazzolo et al., 1965b
(continued)
LD50
Animal Route mg/kg body-weight References
Rat oral 25002/ Palazzolo et al., 1964
Rabbit dermal 154002/ Palazzolo et al., 1964
1/ Corn oil solution
2/ Aqueous suspension
Short-term studies
Dog
Groups of two male and two female dogs were given daily doses of 0,
10, 30, 100 or 300 mg/kg body-weight of captafol over a two year
period. The material was given in gelatin capsules immediately after
each day's feeding. At the two highest dose levels there was a
decreased body-weight gain over the period of the experiment. Emesis
and loose stools occurred quite frequently in both these groups during
the first four weeks but only occasionally thereafter. These effects
were not seen at the two lower levels. Increased absolute liver and
kidney-weights and liver and kidney to bodyweight ratios were seen in
all animals at the 30, 100 and 300 mg/kg levels. Alterations In other
organ-weights appeared unrelated to the administration of the test
material. Haematologic studies revealed a mild anaemia at the
termination of the study in the dogs given 100 and 300 mg/kg per day.
Histopathology, blood chemistry, urinalysis and liver-function tests
revealed no adverse effects that could be attributed to the
administration of captafol. The 10 mg/kg dose level appeared to cause
no significant effects (Cervenka at al., 1964).
Rabbit
Four groups of eight rabbits were given dermal applications of
captafol at levels of 0, 500, 1000 and 2000 mg/kg body-weight/day for
20 days. Half the animals in each group were treated on abraded skin
areas, the other half on intact skin. There was a marked adverse
effect on body-weight, even at the lowest level tested. Deaths
occurred at all test levels and the 20-day dermal LD50 was found to
be 1100 mg/kg body-weight/day (for the 80 percent wettable powder).
The only effects seen in gross and microscopic examinations were in
the skin at the application site (Palazzolo et al., 1964).
Long-term studies
Rat
In a two year study with captafol added to the diet at 0 (70 males and
70 females), 250, 500, 1500 and 5000 ppm (35 females and 35 males at
each test level), there was growth depression at the 1500 and 5000 ppm
levels. Mortality was increased in the 5000 ppm group, with no males
left alive after 23 months. A lymphocyte to neutrophile shift was
observed in the surviving males of this group after 21 months. There
was an increase in the liver to body-weight ratio at the 500, 1500 and
5000 ppm levels at 12 months. An increase in this ratio was also seen
in males at 250 ppm. At the end of the experiment there was no longer
a significant difference at the two lower tent levels. Significant
increases were also observed in organ weight and organ to bodyweight
ratios for kidney and adrenal of rats fed at 1500 and 5000 ppm.
Histopathology revealed liver changes characterized by degeneration of
hepatic cells, vacuolization, incipient fat alteration, and
infiltration by mononuclear cells. Kidney changes were characterized
by alterations in proximal and distal tubular cells; many giant forms
with large irregular nuclei being present. These changes in liver and
kidney were only seen in rate fed the two highest dose levels. No
other histopathological changes were associated with the
administration of captafol. No effects on tumour incidence were
observed (Kohn et al., 1964).
COMMENT
Captafol appears to be metabolized rapidly and in a similar way in
rats, dogs and monkeys. The primary metabolite and other metabolites
have been identified in excretion studies. Information is, however,
incomplete on the nature of the metabolites in animal tissues, as well
as on the absorption and distribution of captafol and its metabolites
after oral administration. A two-year study in dogs indicated that a
dose level of 10 mg/kg body-weight/day was without significant adverse
effect. In the long-term study in rate at the lowest level tested (250
ppm), an increase in the liver to body-weight ratio was evident at 12
months but not at 24 months. For this reason a definite no-effect
level has not been established in that species. Teratogenicity studies
in mammalian species produced evidence of embryotoxic effects at the
lowest level tested but there was no indication of malformation of the
foetuses. The nature of the reported histopathological effects upon
the kidney and liver observed in the two year study in rats fed high
dose levels of captafol is of some concern.
TOXICOLOGICAL EVALUATION
Level causing no significant toxicological effect
Dog: 10 mg/kg body-weight/day
Estimate of temporary acceptable daily intake for man
0-0.05 mg/kg body-weight/day
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
Captafol is used to control fungus diseases.
Rates of application and recommended intervals between treatment and
harvest are (Dye, 1969):
Fruits - 0.05 to 0.2% a.i., applied at bloom, petal
fall, shuck split and 10 to 14 day intervals
up to harvest.
Melons - 1.3 to 2.7 kg a.i./ha applied as needed at 7
day intervals up to harvest.
Tomatoes and
cucumbers - 1.1. to 2.7 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Potatoes - 0.10 to 1.8 kg a.i./ha applied as needed at 7
to 10 day intervals up to harvest.
Post-harvest treatments
Captafol is recommended for post-harvest use on peaches, cherries,
plums, and nectarines (Ogawa at al., 1964).
Other uses
In-furrow spray application is recommended for the control of seedling
diseases on cotton. Captafol has been used to control
Helminthosporium heveae in rubber (Turner, 1969).
RESIDUES RESULTING FROM SUPERVISED TRIALS
The residue data of captafol are from treatments made under commercial
conditions in the U.S.A. (Dye, 1969). The tests made with peaches and
watermelons indicate that the captafol is present as a surface residue
and is not systemically translocated into the flesh of the fruit. The
initial residue of captafol is reduced by one half generally within a
week or two. Two types of applications are recommended: dormant and
blossom, and seasonal foliar. The residue data are given separately
(Table I).
TABLE I
Residue data from field trials
Crop Rate of Number of Pre-harvest Captafol
application treatments interval residue
(kg a.i. per ha) (days) (ppm)
Blossom application
Apricots 3.4-8.1 3 102 0.1-0.2
Cherries,
sweet 2.3-10.8 2-3 45-68 0.6-1.4
Plums (0.1-0.3% a.i.) 2-3 131-141 n.d.-0.2
Seasonal foliar application
Cherries,
sour 1.8-3.6 4 20 7-9
Peaches 3.1-4.5 1-13 10-14 2.5-14
Melons 2.0-4.0 5-9 1 0.4-1.8
Cucumbers 1.3-1.8 6-9 1 0.1-0.4
Tomatoes 2.7-5.4 6-11 1 0.4-3.8
FATE OF RESIDUES
General comments
As outlined under 'BIOCHEMICAL ASPECTS', the N-S bond in substances
like captafol, is subject to chemical attack. For captafol, the two
mechanisms proposed (Kohn, 1965) are: (1) nucleophilic attack by
sulfhydryl compounds (Anon., 1965a) acid (2) hydrolysis (Berteau,
1963, and Potter, 1964) (Fig. 1). Both initiative reactions result in
THPI. In addition, in reaction (1) free Cl- ions, inorganic sulphur
compounds, and 2C-fragment are released and in reaction (2) chlorine
is only partly appearing as free ion, the rest being bound to the
2C-moiety. Subsequently, tetrahydrophthalimide decomposes via the
unstable tetrahydrophthalamic acid into tetrahydrophthalic acid.
In the presence of sulfhydryl compounds and at or near neutral pH, the
sulfhydryl reaction (1) is much faster than the hydrolysis (2). At pH
7 and 25°C, the "half-life" of captafol in a homogeneous sulfhydryl
reaction was found to be 4 minutes and in the corresponding hydrolysis
1000 minutes (Anon., 1965a). As the temperature and/or pH rises the
rates of both reactions increases.
In animals
The main routes of degradation of captafol in animals are the same as
in plants (Dye, 1969).
In plants
Due to the extremely low water solubility of captafol the residues of
the parent compound are occurring on the surface of the treated
plants. This is greatly limiting the exposure of captafol to the
degradation reaction by the sulfhydryl compounds of the plant tissue.
As a result, the captafol residues show a high persistence in situ.
In vitro studies on the degradation of captafol by spinach, tomato,
and celery macerates and filtrates (Potter, 1965) have confirmed that
captafol is very readily decomposed by the cell sap of various plants.
There were distinct differences in the degradation rates of various
plants. In spinach the captafol residue (10 ppm) was nearly completely
degraded in one hour, but in tomato in about 24 hours. Boiling did not
alter the degradative capacity of the macerates which indicates that
no essential enzymatic reactions are involved in the degradation of
captafol.
The in situ residues of captafol are found to produce minor amounts
of tetrahydrophthalimide and tetrahydrophthalic acid (Table II). No
dichloroacetic acid has been found. Thus, even though dichloroacetic
acid can be formed from captafol under certain conditions, it is not
found to be present as a residue on crops treated under field
conditions and would, therefore, not be consumed by man (Dye, 1969).
In soils
It has been found that captafol degrades rapidly in soil, the rate
being a function of both the soil type and the initial concentration;
even the longest "half-life" found was only 11 days (Berteau and Pack,
1966a). These studies show that under normal agricultural conditions,
the captafol that is supplied one year, even late in the season, would
be completely degraded by the beginning of the next growing season.
Therefore, there would be no accumulation of captafol in soil from
year to year.
It has been found that in natural, non-sterile soil, captafol
degradation results in only a barely detectable trace of
dichloroacetic acid being formed at short (i.e. one week) intervals.
At longer intervals, none is detectable at a limit of detectability of
0.02 ppm dichloroacetic acid itself is rapidly degraded by natural
soil, being completely degraded within one week. In normal
agricultural practice, therefore, there would be no buildup in the
soil (Berteau and Pack, 1966b).
In sterile soil, the degradation of captafol is associated with a
buildup of dichloroacetic acid and in dichloroacetic acid fortified
soil no loss was observed over a period of one week. The degradation
of the acid in soil is, therefore, definitely biochemical (Berteau and
Pack, 1966b).
The movement of captafol through soil columns by water leaching has
been studied. The results show that captafol does not move
significantly and will not accumulate in water leaching from treated
areas (Berteau and Pack, 1966c).
Carrots and radishes were grown in soils treated with captafol to
determine if any captafol would be taken up by the crop. At a limit of
detectability of 0.05 ppm or better, no captafol residue was found in
either crop (Anon., 1965a).
In storage and processing
All the data available indicate that the captafol residues on fruit
are very stable under commercial storage conditions.
In the studies on dried peaches and prunes, no residues were found in
the fresh fruit, but small residues ware detected in the dried
samples. Apparently, any residue in the fresh fruit was below the
limit of detectability but this residue became detectable as it was
concentrated in the drying operation (Table III) (Dye, 1969).
In the canned peach samples, there was a slight residue of captafol
detected at 1 day interval, but no detectable quantities at subsequent
intervals. The residue of 1.6 ppm of tetrahydrophthalimide in the 1
day peach sample decreased to below the limit of detection at 22 and
150 days. Tetrahydrophthalic acid was barely detectable in canned
peaches, and no detectable residues of dichloroacetic acid were found
in any of the samples studied (Table III) (Dye, 1969).
In the case of tomatoes no detectable residues of tetrahydrophthalic
acid or dichloroacetic acid could be found in any of the canned fruit
samples (Table III) (Dye, 1969).
Because of the nature of the captafol residues they would be readily
removed by washing, blanching, and peeling the fruit. A detailed
evaluation can not be made because of lack of complete information.
According to Chalkov and Vanev (1968), captafol has a suppressing
action on wine yeast. Cabral and Tomaz (1956) reported that it
completely inhibited fermentation of the must from treated grapes.
METHODS OF RESIDUE ANALYSIS
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is electron
capture GLC, the alternate procedure is based on thin-layer
chromatography.
The crop is extracted with benzene. The extract is cleaned-up by
either a column chromatographic method or a thin layer chromatographic
(TLC) method, or a combination of the two. The cleaned-up extract is
then chromatographed by TLC with captafol standards. The plate is
sprayed with N,N-dimethyl-p-phenylenediamine to visualize the spots.
The amount of captafol is determined by visual commission of the
sample spot with the standard spots. The method is a positive
identification of captafol residues. Its sensitivity is about
0.03-0.05 ppm, depending on the crop interferences (Anon., 1966b).
There is a modified column cleanup method which is useful for oily
crops where interferences in the thin-layer chromatography is
encountered from plant extractives (Anon., 1964). Pomerantz and Rose
(1968) have developed a TLC method which differentiates between
captan, folpet, captafol, and their metabolites.
The preferred method employs electron capture GLC for the final
detection and estimation of residues of captafol. Either the column or
TLC cleanup procedure can be used when cleanup is needed (Anon.,
1965b). Twenty-seven pesticides were tested for possible interference
in this GLC method of analysis. It found that none of the pesticides
studied eluted at the same elution time as did captafol under the
conditions employed, and therefore there would be no interference from
any of them. The sensitivity of 0.01 ppm can be obtained by the GLC
method (Kilgore and White, 1967).
The analytical method for the analysis of dichloroacetic acid which is
a demonstrated hydrolysis product of captafol, is based upon
microcoulometric gas chromatography. This method of analysis is both
highly specific and quite free from interfering material. The limit of
detection of this method is approximately 0.05 ppm, or possibly lower.
The sample is macerated and extracted with water. The water extract is
then extracted with ether. The DCAA in the ether layer is esterified
with diazomethane and the analysis completed with the microcoulometric
gas chromatography (Anon., 1965c).
The hydrolysis products of the imide portion of the captafol molecule,
tetrahydrophthalimide and tetrahydrophthalic acid, are detected by a
method which involves the extraction, cleanup, and esterification of
the acid derivatives with diazomethane and the subsequent gas
chromatographic detection of the esters. The detection system used is
hydrogen flame. This method is quite specific but great care must be
taken to eliminate interfering materials which would be detected by
the hydrogen flame. With adequate cleanup on most crops, this method
is sensitive to approximately 0.05 ppm (Anon., 1966c).
TABLE II
Data on captafol residues and its degradation products in various fruits (Anon., 1969
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments (days) Captafol hydrophthalimide acid
Peach 9.0 12 1 3.4 1.0 0.5
7 3.5 1.1 0.3
3.1 4 1 11.7 0.00 0.00
5 12.0 0.00 0.00
10 9.9 0.00 0.00
15 9.3 0.00 0.00
3.1 4 1 20.6 0.00 0.00
5 13.0 0.00 0.00
10 13.0 0.00 0.00
15 10.6 0.00 0.00
Cucumber 1.8 6 1 0.14 0.00 0.06
6 0.10 0.00 0.13
Tomato 2.7 9 1 9.7 0.16 0.00
7 10.1 0.16 0.07
2.7 9 1 5.2 0.27 0.00
7 4.3 0.20 0.00
* No dichloroacetic acid is detected
TABLE III
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
Peaches 7.1 1 fresh 139 0.00
dried 0.14
Plums 4.7-6.2 2 fresh 139 0.00
dried 0.42
15.6-3.9 3 fresh 139 0.00
dried 0.19
Peaches 2.4-3.9 7 canned 1 0.1 1.6 0.0
6 6 0.0 0.1 0.1
5 15 0.0 0.1 0.1
4 22 0.0 0.0 0.1
3 150 0.0 0.0 0.1
Tomatoes 2.7 5 canned ( 1 0.44 0.08 0.00
juice ( 180 0.13 0.00 0.03
2.7 5 canned 1 0.15 0.00 0.00
juice
canned 1 0.68 0.10 0.00
puree
canned 180 0.00 0.05 0.00
juice
canned 180 0.16 0.20 0.00
puree
TABLE III (cont'd)
Comparison of captafol residues of fresh and processed fruits (Anon., 1969)
Crop Rate of Pre- Residue*
application harvest (ppm) Tetra-
(kg a.i. No. of interval Tetra- hydrophthalic
per ha) treatments Product (days) Captafol hydrophthalimide acid
2.7 10 fruit 1 0.48 0.12 0.08
7 0.13 0.06 0.00
canned 1 0.16 0.07 0.00
juice
canned 1 0.23 0.07 0.00
puree
canned 180 0.00 0.00 0.00
juice
canned 180 0.00 0.12 0.00
puree
0.9 canned 1 0.02
concentrate
* No dichloroacetic acid is detected
The GLC method is considered most suitable for regulatory purposes,
but the TLC method could also be applied.
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
Australia Stone fruit 20
Other fruit and vegetables 5
Canada Cherries (sour) 50
Apricots, nectarines, peaches 30
Tomatoes 15
Cherries (sweet), cucumbers, 5
melons, plums, prunes
Switzerland Grapes, strawberries 5
United States of Cherries (sour) 50
America Apricots, peaches 30
Tomatoes 15
Melons 5
Cherries (sweet), cucumbers, 2
nectarines, plums (fresh prunes)
APPRAISAL
Captafol is used to control fungus diseases on tree fruits, melons,
cucurbits, tomatoes, and potatoes. In the case of peaches, apricots,
nectarines, cherries, plums and prunes, a dormant or blossom
application is recommended. In addition, seasonal foliar applications
are recommended for certain crops. In-furrow spray applications are
recommended for the control of representative diseases on cotton.
Captafol is used for foliage application at a rate of 0.05 to 0.2
percent of active ingredient wettable powder of flowable suspension in
spray. It is quite stable except under alkaline conditions.
Tolerances established in the U.S.A., Canada, Switzerland and
Australia vary from 2 to 50 ppm.
The residue data of captafol available to the meeting are only from
treatments made under commercial conditions in the U.S.A. The initial
residue of captafol is reduced by one half generally within a week or
two. The residue is mainly on the surface of the fruit.
The two major routes of degradation in plants are the same as those in
animals, namely reactions with sulfhydryl compounds and hydrolysis.
Sulfhydryl reaction is more rapid than hydrolysis. Main degradation
products are tetrahydrophthalimide and tetrahydrophthalic acid.
Dichloracetic acid has not been found.
In thermal food processes as well as in macerated plant materials,
captafol is extensively decomposed. Decomposition products,
tetrahydrophthalimide and tetrahydrophthalic acid, may appear in
processed foods at the beginning of the storage period.
Captafol is found to degrade rapidly in natural soils.
Two methods are reported to be available for the analysis of captafol
residues in plant and animal tissues. The preferred method is GLC. The
alternate procedure is based on thinlayer chromatography, the
sensitivity of which is about 0.03-0.05 ppm, depending on the crop
interferences.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
TEMPORARY TOLERANCES
Residues resulting from good agricultural practice as follows are
recommended, effective till 1973:
Pre-harvest Captafol residue
Crop Comment interval (days) (ppm)
Melons, whole 1 2
Cucumbers, whole 1 1
Tomatoes 1 5
Peaches 10-14 15
Cherries, sour Foliar application 20 10
Cherries, sweet Blossom application 45-70 2
Apricots Blossom application 100 0.5
Plums Blossom application 130-140 0.2
The data on pineapples, apples and grapes were inadequate for
evaluation.
FURTHER WORK OR INFORMATION
REQUIRED (before 30 June 1973)
1. Studies to elucidate the effects seen in the teratogenicity
experiments.
2. Data from countries ether that the United States of America on the
required rates and frequencies of application, pre-harvest
intervals, and the resultant residues.
3. Data on residue levels in raw agricultural commodities moving in
commerce.
4. Data on the effect of washing, peeling and blanching on the residue
levels of various crops.
5. Elucidation of the histopathology of the kidney and liver in the
rat.
DESIRABLE
1. Metabolic studies to provide further information on the absorption
and distribution after oral administration and to identify the
metabolites found in animal tissues.
2. Collaborative studies to establish a regulatory method for captafol
in the presence of captan and folpet.
REFERENCES
Anon. (1964) Difolatan residue analysis - cleanup procedure for oily
materials. Addendum to RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1965a) The reaction of Difolatan with sulfhydryl compounds.
Chevron Chemical Co. Unpub. Rept.
Anon. (1965b) Difolatan residue analysis by electron capture gas
chromatography. Method RM-6B. Chevron Chemical Co. Unpub. Rept.
Anon. (1965c) Difolatan metabolism - The analysis of residues of
dichloroacetic acid using microcoulometric gas chromatography. Method
RM-6C. Chevron Chemical Co. Unpub. Rept.
Anon. (1966a) Studies on the uptake of Difolatan by root crops grown
in soils treated with Difolatan. Chevron Chemical Co. Unpub. Rept.
Anon. (1966b) Difolatan residue analysis - thin layer chromatographic
method. Method RM-6. Chevron Chemical Co. Unpub. Rept.
Anon. (1966c) The analysis of residues of Difolatan and two
tetrahydrophthalic acid derivatives. Residue Method RM-6D. Chevron
Chemical Co. Unpub. Rept.
Berteau, P.E. (1963) Difolatan - a preliminary report on its
hydrolysis and related reactions. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E., Pack, D.E., Ospenson, J.N. and Crossley, J. (1966) The
metabolism of Difolatan. Paper presented at the American Chemical
Society, Western Regional Meeting. San Francisco, California, 18
October 1966 [Abstracted in Vortex, (Publication of the California
section, American Chemical Society) 27, insert P. 37 (1966)]
Berteau, P.E. and Pack, D.E. (1966a) The degradation of Difolatan in
soils. Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966b) Difolatan degradation in
soil-studies on the formation and decay of dichloroacetic acid.
Chevron Chemical Co. Unpub. Rept.
Berteau, P.E. and Pack, D.E. (1966c) The movement of Difolatan through
soil columns. Chevron Chemical Co. Unpub. Rept.
Cabral, R.V. de G. and Tomes, I.L. (1966) Fungicidal assay against
Botrytis cinerea and the effects of fungicides on must fermentation.
An. Inst. Super. Agron., Univ. Tec. Lisboa 29:279-97 (Chem. Abstr.
70:46363 u, 1969)
Cervenka, H., Key, J.H. and Calandra, J.C. (1964) Chronic oral
toxicity of RE 5865-Beagle dogs. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Chalkov, I. and Vanev, S. (1968) Determination of the effect of some
new fungicides, used to control gray rot in grapes under field
conditions, on the enzymic activity of yeasts. Lozarstvo Vinar (Sofia)
17:33-40 (Chem. Abstr. 69:34908 s, 1968)
Dye, D.F. (1969) Difolatan(R). Unpub. Rept. prepared and submitted by
Chevron Chemical Co.
Ives, M. and Calandra, J.C. (1965) Teratogenic study on difolatan.
Unpub. Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Jackson, G.L., Fancher, O.E. and Calandra, J.C. (1967) Rabbit
teratogenic study. Difolatan. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E., and Calandra, J.C. (1966) Three-generation
reproduction study on difolatan - Albino rats. Unpub. Rept. of
Industrial Bio-Test Laboratories submitted by Chevron Chemical Co.
Kennedy, G., Fancher, O.E. and Calandra, J.C. (1968a) An investigation
of the teratogenic potential of captan, folpet and difolatan. Toxicol.
appl. Pharmacol. 13:421-30
Kennedy, G., Fancher, O.E and Calandra, J.C. (1968b) Teratologic
investigation of difolatan in Macaca mulatta (Rhesus monkey). Unpub.
Rept. of Industrial Bio-Test Laboratories submitted by Chevron
Chemical Co.
Kilgore, W.W. and White, E.R. (1967) Determination of Difolatan
residues in fruits by electron-capture gas chromatography. J. Apr.
Food Chem. 15:1118-20
Kohn, F.E., Key, J.H. and Calandra, J.C. (1964) Two-year chronic oral
toxicity of RE 5865 Albino rats. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Kohn, G.K. (1965) Present status of metabolic fate studies of
Difolatan. Unpub. Rept. of Chevron Chemical Corporation for
presentation at the Orthocide Conference, Amsterdam, 29 November - 5
December 1965
Leary, J.B. (1966) Difolatan: shay rat tests. Unpub. Rept. of Chevron
Chemical Co. (cited by Crossley, 1967 - see the monograph of captan)
Ogawa, J.M., Boyack, G.A., Sandeno, J.L., and Mathre, J.H. (1964)
Control of postharvest fruit decays in relation to residues of
2.6-dichloro-4-nitroaniline and Difolatan. Hilgardia 35:365-73
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1964) Acute toxicity
studies on Difolatan 80W. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. (1965a) Acute oral
toxicity on RE 5865. Unpub. Rept. of Industrial Bio-Test Laboratories
submitted by Chevron Chemical Co.
Palazzolo, R.J., Key, J.H. and Calandra, J.C. (1965b) Acute oral
toxicity on RE 5865 (aqueous suspension). Unpub. Rept. of Industrial
Bio-Test Laboratories submitted by Chevron Chemical Co.
Palazzolo, R.J., Fancher, O.E. and Calandra, J.C. (1966) Rabbit
reproduction study, THPI. Unpub. Rept. of Industrial Bio-Test
Laboratories submitted by Chevron Chemical Co.
Pomerantz, I.H. and Ross, R. (1968) Captan and structurally related
compounds: thin-layer and gas-liquid chromatography. J. Assoc. Offic.
Anal. Chem. 51:1058-62
Potter, J.L. (1964) Studies on the hydrolysis of Difolatan in a
homogeneous aqueous-acetone buffer system under neutral pH conditions.
Chevron Chemical Co. Unpub. Rept.
Potter, J.L. (1965) Preliminary studies on the in vitro degradation of
Difolatan by crop macerates and filtrates. Chevron Chemical Co. Unpub.
Rept.
Turner, P. D. (1969) Evaluation of fungicides for the control of
Helminthosporium heveae on Hevea rubber in Malaysia. I. Laboratory
assessment. Experimental Agriculture 5:33-40
Verrett, M.J., Mutchler, M.K., Scott, W.F., Reynaldo, E.F. and
McLaughlin J. (1969) Teratogenic effects of captan and related
compounds in the developing chick embryo. Annals N.Y. Acad. Sci.
160.334-43
