
FAO/PL:1969/M/17/1
WHO/FOOD ADD./70.38
1969 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 8 - 15 December 1969.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1970
THIOMETON
IDENTITY
Chemical name
S-[2-(ethylthio)ethyl] dimethyl phosphorothiclothionate
Synonyms
S-[2-(ethylthio)ethyl] 0,0-dimethyl phosphorodithioate, Ecatin(R),
Intration (Czechoslovakia)
Structural formula
CH3O S
\ "
P-S-CH2CH2-S-CH2CH3
/
CH3O
Other relevant chemical properties
20
Properties of thiometon - boiling point 110°C/0.1 mm; d4 1.209;
colourless oil with characteristic odour; soluble in most organic
solvents, less soluble in petroleum ether, 200 mg/l at 25°C in water;
stable in apolar solvents, unstable pure or under alkaline conditions.
Formulations - 20 and 25 percent by weight emulsifiable concentrates
and 2 percent by weight dust. Stability of formulations with full
activity in at least two years when stored below 40°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
No information is available on the metabolism of thiometon in animals.
Oxidative metabolites have been found in plants treated with thiometon
(Jucker, 1958). See also the section entitled "RESIDUES IN FOOD AND
THEIR EVALUATION".
Effect on enzymes and other biochemical parameters
The anti-cholinesterase properties of thiometon have been demonstrated
from in vitro experiments with guinea-pig serum cholinesterase.
Concentrations of 10-4 molar and 10-3 molar of the pure compound gave
inhibitions of 54 percent and 85 percent respectively (Klotzsche,
1958).
A single oral dose of 125 mg/kg body-weight of thiometon was
administered to adult and seven week old chickens. Symptoms of
poisoning appeared after three hours in all birds and death followed
within eight hours. There was almost complete depletion of
cholinesterase when the first signs of poisoning appeared. A
significant increase in ascorbic acid and glucose concentration in the
blood as well as a decrease in adrenal cholesterol and liver glycogen
also occurred (Madejski and Juszkiewicz, 1966).
Two groups, each comprising 16 egg-laying hens, were given a single
dose of 0 or 250 mg/kg of thiometon by gavage. Symptoms of poisoning
in the test group appeared after three to four hours followed by death
after approximately seven hours. At the terminal stage the treated
birds displayed complete inhibition of cholinesterase activity in red
cells, plasma and liver, and a highly significant decrease of
cholinesterase, activity in brain, heart and adrenals associated with
increasing sinus bradycardia. Increase in blood ascorbic acid and
glucose occurred with no significant changes in the blood cholesterol
level. Distinct electrocardiographic changes were recorded in the
poisoned birds (Juszkiewicz and Rakalska, 1968).
Special studies on neurotoxicity
Two groups, each of four chickens, were given 35 mg/kg body-weight of
thiometon by intramuscular injection. One of the groups was protected
against acute anti-cholinesterase poisoning with atropine and
pralidoxime. The unprotected chickens died, while the protected group,
and a further group given atropine and pralidoxime alone, did not
develop any signs of neurotoxicity during an observation period of 29
days. A positive control group given triorthocresylphosphate displayed
definite signs of paralysis (Sandoz, 1968a).
Special studies on potentiation
Groups of male and female rats were given thiometon by stomach tube in
combination with the following organophosphorus compounds: diazinon,
dimethoate, formothion, malathion, parathion and phosphamidon. There
was no indication of potentiation except possibly in the female rats
given the thiometon-parathion combination, and in this case, the
effect if any, was extremely slight (Klotzsche, 1967).
Special studies on reproduction
After six months of administering thiometon in the 12-month experiment
in rats, described under "Short-term studies", 10 male and 10 female
animals from each dose-level and from the control group were mated and
the off-spring observed for 30 days. The number of pairs producing
off-spring were five (controls), seven (1 mg/kg), three (2 mg/kg), one
(6 mg/kg), and two (18 mg/kg). Based upon these limited numbers of
animals it was concluded that there was no difference between the
controls and the groups given 1 and 2 mg/kg of thiometon as regards
litter size and development of the young animals over 30 days (Sandoz,
1968a).
Groups of rats (15 males and 15 females) were administered by stomach
tube 1 mg/kg body-weight of thiometon daily through three generations.
The second litter from each generation served as parents to establish
the next generation. In the F2b generation a dose-level of 2 mg/kg
body-weight was administered as well as the 1 mg/kg level. It is
claimed that no adverse effects on the parents or on their progeny
were found with respect to growth, mortality, pathology and
reproduction performances (Sandoz, 1968a; Thomann and Klotzsche,
1969).
Special studies on teratogenicity
Groups of 10 female rabbits were given 1 and 5 mg/kg body-weight of
thiometon by stomach tube from days 6 to 18 of pregnancy. Several
groups served as controls. After sacrifice on day 29 of pregnancy the
foetuses were recovered by Caesarean section and no difference was
found between the treated groups and the four control groups examined
with respect to the number of implantations, live and dead foetuses,
embryonic and foetal deaths, resorptions and the number of
malformations. There were also no appreciable differences between the
foetal and placental weights in the control and test groups (Sandoz,
1968a; Thomann and Klotzsche, 1969).
Acute toxicity
LD50 mg/kg
Animal Route body-weight References
Mouse (mixed) oral 65 Sandoz, 1968b
Rat (M) oral 225 (pure compound) Klotzsche, 1958
Rat (M) oral 100-120 Sandoz, 1968b
Rat (F) oral 120-125 Sandoz, 1968b
Rat (M) i.v. 27.5 Sandoz, 1968b
Rat (F) i.v. 35.5 Sandoz, 1968b
Guinea pig oral 261 Sandoz, 1968b
Rabbit (M) oral 95 Sandoz, 1968b
Rabbit (M) i.v. 22 Sandoz, 1968b
Cat oral 36 Sandoz, 1968b
Except where otherwise stated the LD50 values were obtained from the
formulated product Ekatin 25 percent and are reported on an active
ingredient basis.
The animals displayed the same symptoms as in poisoning with other
organophosphorus compounds. The symptoms started with tremors, later
convulsions, the formation of red lachrymal fluid and difficulties in
breathing. The onset of symptoms were usually delayed for 60-90
minutes after oral administration (Sandoz, 1968a).
Short-term studies
Dog
Groups of dogs (two males and one female) were initially treated
orally with 0, 1, 2, 10 and 50 mg/kg body-weight of thiometon in
gelatine capsules for six months for an unspecified number of days per
week. All dogs in the 50 mg/kg group died within one week. In the 10
mg/kg group, one animal died and the dose was decreased to 5 mg/kg for
the two remaining dogs. Food consumption, body-weight, haematology,
urinalysis and blood enzymes were comparable in the 1, 2 and 5 mg/kg
groups and in the control group. Serum cholinesterase activity was
slightly, but not definitely, lower in the test animals, while the red
cell cholinesterase activity was markedly lower from the second week
onward when compared to the controls. Brain cholinesterase measured in
the cerebellum of the sacrificed animals showed no inhibition in the 1
mg/kg group, a definite inhibition in the 2 mg/kg group and a marked
inhibition in the 5 mg/kg group. No specific pathological changes
attributable to thiometon were found by histological examination of
all the dogs in the experiment (Sandoz, 1968a, Sandoz, 1969).
Rat
Groups of rats each containing 25 males and 25 females were given
daily doses by stomach tube of 0, 2, 6 and 18 mg/kg body-weight of
thiometon for 12 months. Food intake, haematology, (limited number of
animals) urinalysis and biochemical serum enzyme tests were comparable
in the test and control groups. Females from all experimental groups
showed the same weight gain as the controls, while the male rats given
6 mg/kg showed a slight and with 18 mg/kg a marked decrease in weight
gain. Serum and red blood cell cholinesterase determined in animals
after monthly interim sacrifices showed fluctuating values both in the
experimental and control groups. Both serum and red cell
cholinesterase displayed a distinct depression in the 2 mg/kg, 6 mg/kg
and 18 mg/kg groups. In the rats given 1 mg/kg there was a possible
depression of serum cholinesterase and a definite depression of the
red cell cholinesterase activity. Upon examination of 10 animals of
each sex from each group, the average organ and body-weights were
comparable in experimental and control groups and no changes in
gross- or histopathology attributable to thiometon were found (Sandoz,
1968a; Sandoz, 1969).
Groups, each initially containing five male rats, were given oral
doses of 5, 10, 15 and 20 mg/kg body-weight of thiometon for three
months. All the animals showed mild to severe symptoms of poisoning.
In the surviving five rats (four from the 5 mg/kg and one from the 10
mg/kg group) the symptoms disappeared after 10 weeks of receiving
thiometon and histological examination did not reveal specific
pathological changes. These animals were sacrificed after 13 weeks
(Sandoz, 1968a; Klotzsche, 1958).
Groups of rats (five males and five females) were given 0, 0.08, 0.8
and 8 mg/l of thiometon in their drinking water for six months.
Water-intake, weight-gain, haematology and urinalysis were comparable
in experimental and control groups. Other groups, containing five male
rats, were treated with thiometon six times a week for six months with
doses of 0, 0.0008, 0.008 and 0.8 mg/kg of body-weight using a stomach
tube. In the rats given 0.008 and 0.08 mg/kg an effect on conditional
reflexes and slight, but not definite, changes in haematology and
urinalysis were apparent (Cabejszek et al., 1967).
Long-term studies
No Information available.
COMMENT
No information on metabolic studies in animals and no observations in
man are available. No long-term studies have been reported, the
12-month study in the rat not being of sufficient duration for
consideration as a long-term study, and the experiment in dogs was of
only six months' duration. In these short-term studies, the lowest
dose tested, namely 1 mg/kg, displayed marked inhibition of
erythrocyte cholinesterase activity and slight depression of serum
cholinesterase in two species of animal. Therefore, a no-effect level
could not be established. Inadequate information is available on the
composition of the technical product. For these reasons it is not
possible to establish an acceptable daily intake for man at this time.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Thiometon is a phosphorus-containing systemic insecticide and
acaricide with activities as a contact and stomach poison. It has no
ovicidal action. It is applied as an emulsifiable concentrate or dust
to control aphids, red spider mites and saw flies on a wide variety of
crops including cereals, cotton, sugarbeets, oil crops, forage, fruit,
field crops, vegetables, coffee, tea, and tobacco. Protection when
properly applied lasts 10-20 days depending on season, crop, and
susceptibility of the pests. The standard concentration for high
volume spray application is 0.1%. In low-volume applications thiometon
per unit area or per tree remains the same. Amounts sprayed are 100 to
600 g/ha. The usual methods of application are used; under certain
conditions when rapid uptake or translocation is possible the
formulated insecticide may be applied as a drench.
Formulations of thiometon can be combined with most fungicides
excluding alkaline ones. However, separate applications are
preferable.
The insecticide has been used in agricultural areas in Europe, South
America, Africa, Asia, and Australia. Thiometon and its formulations
have been registered in over 50 countries. Waiting periods after
treatment are two weeks in Great Britain, three weeks in Australia,
four weeks in Belgium, Denmark, Netherlands, Sweden, and Switzerland,
and five weeks in Austria. Waiting periods before harvest more aptly
depend on individual circumstances.
Pre-harvest treatments
Rate of spraying the insecticide on vegetables, bananas, cotton,
coffee, tea, citrus, and sugarbeets is 0.025 percent and on stone
fruits and vines 0.025-0.04 percent. On beets, cereals, and potatoes
125-250 g/ha is applied. Application on beets and potatoes is repeated
in 10-14 days to prevent virus infection. A 0.05 percent drench is
also used on vegetables and hops.
Post-harvest treatments
None
Other uses
Used on ornamentals, tobacco, and some other plants where sucking
insects are a problem.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Maximum residues following treatment of various crops are given in
Table I. Residues after normal application generally decrease to less
than 1 ppm after 10 days and, depending upon the crop and
environmental factors (rainfall, temperature), disappear in two to
four weeks.
TABLE I
Maximum residues found after treating various crops
Dosage Pre-harvest Maximum residue at end
Crop % Conc. or g/ha interval, days* of interval, ppm*
Apple 0.04% 21, 120 0.09, n.d.
Bean 0.02% 7, 14, 21 0.05, 0.25, 0.15
TABLE I (cont'd)
Maximum residues found after treating various crops
Dosage Pre-harvest Maximum residue at end
Crop % Conc. or g/ha interval, days* of interval, ppm*
Bean 0.025% 1, 7, 14 0.6, 0.15, <0.1
Cabbage 300-800g 106 n.d.
Carrots 0.25% 126 n.d.
Grapes 0.02% 21, 67 0.5, 0.1
Lettuce 0.02% 3, 9, 14 5, 0.5, 0.1
Olives - ** 7, 21 1.0, 0.70***
Olive Oil - ** 7, 21 0.4, 0.10***
Orange Fruit Pulp 0.0375% 45 n.d.
Orange Peel 0.0375% 45 0.9***
Orange Jam 0.0375% (45) n.d.
Peach Fruit 0.03% 7, 21, 28 0.11, 0.12, 0.12
Peanuts 100g 7 0.15
Peers 0.02% 33, 49 0.3, <0.05
Plums 0.02% 10, 31 n.d., n.d.
Potatoes 200g 36 n.d.
Potatoes 250-700g 89, 100 n.d., n.d.
Sugarbeet 200g 43 n.d.
Tea, Green 0.025% 4, 7, 10 0.2, 0.2, 0.1
Tomato 0.025% 7, 14, 21 n.d., n.d., 0.05
TABLE I (cont'd)
Maximum residues found after treating various crops
Dosage Pre-harvest Maximum residue at end
Crop % Conc. or g/ha interval, days* of interval, ppm*
Wine 0.02% (97) n.d.
* When more than one interval is given, the residue values at each interval are in
respective order. n.d. - none detected. Residues are sum of sulfone and sulfoxide
of thiometon with but few exceptions.
** Amount applied not given (Gandolfo et al., 1966)
*** Data insufficient for setting tolerance.
FATE OF RESIDUES
According to Sandoz (1969) thiometon is rapidly converted to
metabolites and in a short time cannot be found in pure form. The
following oxidation products are formed in the living plant:
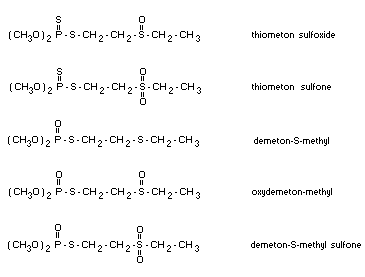 The major metabolites which impart the insecticidal action of
thiometon are said to be the sulfoxide and sulfone of thiometon.
According to Sandoz (1969) the sulfoxide and sulfone of
demeton-S-methyl occur in very small amounts and therefore have much
less significance; despite inclusion in some of the analyses, they are
usually not detected. All metabolites are gradually inactivated by
hydrolysis and after 10-14 days are usually gone.
Data on mammalian metabolism was not provided.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information is available on residues in commerce or at the time of
consumption. Residues in crops are said to be rapidly degraded by high
temperature, especially by cooking.
METHODS OF RESIDUE ANALYSIS
The early residue analyses utilized paper chromatography and more
recent ones paper and thin-layer chromatography. These procedures
require a thorough cleanup, some are only semi-quantitative, and they
have limited sensitivity and specificity. With paper chromatography
recovery is 85-95 percent for thiometon sulfoxide and 70-80 percent
for thiometon sulfone; limit of detection is 0.03-0.05 and 0.06-0.09
ppm, respectively (Faderl 1962; Sandoz, 1969). Thin-layer
chromatography was slightly less sensitive.
In line with today's improved technology a gas chromatographic method
would be the method of choice in terms of accuracy, recovery,
sensitivity, and specificity. Askew et al. (1969) and Ruzicka and
co-workers (1967) utilized the Varian-Aerograph version of the
thermionic detector to analyze compounds related to thiometon, but
reported that oxydemeton-methyl was not detected by gas chromatography
under their conditions. (Oxydemeton-methyl is a metabolite of
thiometon). Bowman et al. (1969) showed that oxydemeton-methyl and its
sulfone may be analyzed by the same flame-photometric gas
chromatographic procedure used for disulfoton (ethyl analogue of
thiometon). By utilizing a temperature of 165°C, they avoid the
decomposition encountered at higher temperatures, and they condition
the analytical column to the extract and compounds being analyzed.
Their method may be adaptable for analysis of residues of thiometon.
Sensitivities are in the order of 0.01 to 0.04 ppm. Specificity in
excellent and virtually no cleanup is needed except for oily crops for
which a simple hexaneacetonitrile portion should suffice.
Another alternative is to oxidize the residues to the sulfone before
gas chromatography. Procedures to accomplish this analysis or that of
related compounds (disulfoton, phorate, and fenthion) have been
described (Bowman and Beroza, 1969). By their procedure oxidation with
m-chloroperbenzoic acid converts all of the metabolites to the
oxygen analogue sulfone for analysis as a single compound. Recoveries
usually were 80-100 percent. The 1968 FAO/WHO report indicates that
oxydemeton-methyl (FAO/WHO, 1969; p. 225), may be oxidized to its
sulfone for analysis by gas chromatography. Recoveries were 74-117
percent for levels between 0.1 and 0.4 ppm. This procedure utilizes
potassium permanganate and if applied to thiometon should produce two
sulfones, one of thiometon and the other of demeton-S-methyl.
Oxidation of the residues to a single compound would probably be the
preferred practical procedure for regulatory purposes and might be
made part of a general scheme for detecting organophosphorus
pesticides.
NATIONAL TOLERANCES
Only the Netherlands and Switzerland have a definite tolerance for
thiometon. It is 0.5 ppm.
APPRAISAL
Thiometon is a systemic organophosphorus insecticide and acaricide
that acts by contact and as a stomach poison. It in used to control
plant lice, mites, and sawflies on a wide variety of crops.
Applications by spray or dust (ingredients of product undefined) range
from 0.1 to 0.6 kg/ha and protection usually lasts 10-20 days.
Pre-harvest intervals are two to four weeks. Residue data from
experimental trials in a number of countries are available.
Thiometon itself is rapidly changed to its sulfoxide which is the
principal residue along with the sulfone. The oxygen analogue
sulfoxide and sulfone are said not to occur as residues to any great
extent. Inspection of residues from related compounds (disulfoton,
phorate, fenthion) indicate that the sulfoxides and sulfones of the
oxons are occasionally found in significant amounts. Accordingly
residue analyses for thiometon, its sulfoxide, and sulfone will
suffice when experimental trials indicate that the corresponding
oxygen analogues do not form in significant amounts.
Metabolism studies on animals have not been reported, and residues in
meat and milk have not been determined although parts of plants
treated with thiometon are likely to be used as animal feed. It is not
expected that residues of thiometon will appear in milk or meat since
such residues have not been found after feeding similar compounds such
as oxydemeton-methyl or disulfoton to dairy cows.
The most suitable method for analysis is gas chromatography with a
thermionic or flame-photometric detector. Such a method is likely to
be more accurate, more sensitive (ca. 0.01 ppm), and more specific
than methods currently in use. Evaluation of such a method for
regulatory purposes is suggested.
No data on residues in commerce or in studies of diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient for recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Long-term studies in animals.
2. Cholinesterase inhibition studies to establish a no-effect level.
3. Information on the content of the technical product and assurance
of standard composition.
4. Data on residue levels in way agricultural commodities moving in
commerce and in total diet studies.
5. Data on rate of disappearance during storage, processing and
cooking.
6. Information on composition of technical products, including
impurities.
7. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
DESIRABLE
1. Adequate information on metabolism.
2. Investigation on cholinesterase inhibition in man.
3. A gas chromatographic method for analysis of residues of thiometon
and its metabolites suitable for regulatory purposes.
REFERENCES
Askew, J., Ruzicka, J.R. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C. and Beroza, M. (1969) A rapid gas chromatographic method
for determining residues of the insecticides fenthion, disulfoton,
and phorate in corn, milk, grass and feces. J. Ass. Offic. Anal.
Chem. 52, 1231-7
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetonmethyl and their metabolites in
tobacco plants. J. Ass. Offic. Anal. Chem. 52, 157-62
Cabejszek, I., Rybak, K. and Szulinski, S. (1967) Effects in
warm-blood animals of thiometon (2-ethylthioethyl-O,O-dimethyl
phosphorodithioate) in drinking water (Translated title) Roczn.
Zak. Kig. (Warsz.), 18:257-65
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Lebensm. Hyg. 53, 154-75
FAO/WHO. (1969) 1968 evaluations of none pesticide residues in food.
FAO/PL: 1968/M/9/1; WHO/Food Add. 69.35
Gandolfo, N., Camoni, I., D'Antonio, C., Leoni, V., Ramelli, G.C. and
Sampaolo, A. (1966) Determination of O,O-dimethyl S-ethylmercaptoethyl
phosphorodithioate (thiometon) and its metabolites in olives and
olive oil. Atti simp. Int. Agrochim. 6, 224-33. (Chem. Abstr. 67,
98979g (1967)
Jucker, O. (1958) Thiometon, Verhalten in der Pflanze, Bestimmung von
Spritzrücksänden. Mitt. Lebensmitt. Hyg., 49:299-322
Juszkiewicz, T. and Rakalska, Z. (1968) Biochemical and
electrocardiographic changes in the course of an acute experimental
poisoning of hens with O,O-dimethyl-S-ethylmercaptoethyl
dithiophosphate. (Translated title) Pol. Arch. weteryu., 11:494-506
Klotzsche, C. (1958) Thiometon, ein neuer systemischer
Phosphorsäureester. Mitt. Lebensmitt. Hyg., 49:72-77
Klotzsche, C. (1967) Toxicological investigations on the
potentiation effect of Ekatin (Thiometon). Unpub. Rept. from the
Department of Occupational Hygiene, prepared and submitted by Sandoz
Ltd.
Madejski, Z. and Juszkiewicz, T. (1966) Effects of Ekatin poisoning on
some biochemical indices in chickens (Translated title) Pol. Arch.
weteryn., 10:93-101
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1968a) Thiometon. An organophosphorus systemic insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz Ltd., Basle
Sandoz. (1968b) Thiometon, Unpub. Rept. prepared and submitted by
Sandoz Ltd., Basle
Sandoz. (1969) Ekatin R. Systemisches Insektizid und Akarisid.
Unpub. Rept. prepared and submitted by Sandoz Ltd., Basle Ref. AGRO
SLK No.E-3681
Thomann, G. and Klotzsche, C. Untitled. Unpub. information submitted
by Sandoz Ltd., Basle
The major metabolites which impart the insecticidal action of
thiometon are said to be the sulfoxide and sulfone of thiometon.
According to Sandoz (1969) the sulfoxide and sulfone of
demeton-S-methyl occur in very small amounts and therefore have much
less significance; despite inclusion in some of the analyses, they are
usually not detected. All metabolites are gradually inactivated by
hydrolysis and after 10-14 days are usually gone.
Data on mammalian metabolism was not provided.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information is available on residues in commerce or at the time of
consumption. Residues in crops are said to be rapidly degraded by high
temperature, especially by cooking.
METHODS OF RESIDUE ANALYSIS
The early residue analyses utilized paper chromatography and more
recent ones paper and thin-layer chromatography. These procedures
require a thorough cleanup, some are only semi-quantitative, and they
have limited sensitivity and specificity. With paper chromatography
recovery is 85-95 percent for thiometon sulfoxide and 70-80 percent
for thiometon sulfone; limit of detection is 0.03-0.05 and 0.06-0.09
ppm, respectively (Faderl 1962; Sandoz, 1969). Thin-layer
chromatography was slightly less sensitive.
In line with today's improved technology a gas chromatographic method
would be the method of choice in terms of accuracy, recovery,
sensitivity, and specificity. Askew et al. (1969) and Ruzicka and
co-workers (1967) utilized the Varian-Aerograph version of the
thermionic detector to analyze compounds related to thiometon, but
reported that oxydemeton-methyl was not detected by gas chromatography
under their conditions. (Oxydemeton-methyl is a metabolite of
thiometon). Bowman et al. (1969) showed that oxydemeton-methyl and its
sulfone may be analyzed by the same flame-photometric gas
chromatographic procedure used for disulfoton (ethyl analogue of
thiometon). By utilizing a temperature of 165°C, they avoid the
decomposition encountered at higher temperatures, and they condition
the analytical column to the extract and compounds being analyzed.
Their method may be adaptable for analysis of residues of thiometon.
Sensitivities are in the order of 0.01 to 0.04 ppm. Specificity in
excellent and virtually no cleanup is needed except for oily crops for
which a simple hexaneacetonitrile portion should suffice.
Another alternative is to oxidize the residues to the sulfone before
gas chromatography. Procedures to accomplish this analysis or that of
related compounds (disulfoton, phorate, and fenthion) have been
described (Bowman and Beroza, 1969). By their procedure oxidation with
m-chloroperbenzoic acid converts all of the metabolites to the
oxygen analogue sulfone for analysis as a single compound. Recoveries
usually were 80-100 percent. The 1968 FAO/WHO report indicates that
oxydemeton-methyl (FAO/WHO, 1969; p. 225), may be oxidized to its
sulfone for analysis by gas chromatography. Recoveries were 74-117
percent for levels between 0.1 and 0.4 ppm. This procedure utilizes
potassium permanganate and if applied to thiometon should produce two
sulfones, one of thiometon and the other of demeton-S-methyl.
Oxidation of the residues to a single compound would probably be the
preferred practical procedure for regulatory purposes and might be
made part of a general scheme for detecting organophosphorus
pesticides.
NATIONAL TOLERANCES
Only the Netherlands and Switzerland have a definite tolerance for
thiometon. It is 0.5 ppm.
APPRAISAL
Thiometon is a systemic organophosphorus insecticide and acaricide
that acts by contact and as a stomach poison. It in used to control
plant lice, mites, and sawflies on a wide variety of crops.
Applications by spray or dust (ingredients of product undefined) range
from 0.1 to 0.6 kg/ha and protection usually lasts 10-20 days.
Pre-harvest intervals are two to four weeks. Residue data from
experimental trials in a number of countries are available.
Thiometon itself is rapidly changed to its sulfoxide which is the
principal residue along with the sulfone. The oxygen analogue
sulfoxide and sulfone are said not to occur as residues to any great
extent. Inspection of residues from related compounds (disulfoton,
phorate, fenthion) indicate that the sulfoxides and sulfones of the
oxons are occasionally found in significant amounts. Accordingly
residue analyses for thiometon, its sulfoxide, and sulfone will
suffice when experimental trials indicate that the corresponding
oxygen analogues do not form in significant amounts.
Metabolism studies on animals have not been reported, and residues in
meat and milk have not been determined although parts of plants
treated with thiometon are likely to be used as animal feed. It is not
expected that residues of thiometon will appear in milk or meat since
such residues have not been found after feeding similar compounds such
as oxydemeton-methyl or disulfoton to dairy cows.
The most suitable method for analysis is gas chromatography with a
thermionic or flame-photometric detector. Such a method is likely to
be more accurate, more sensitive (ca. 0.01 ppm), and more specific
than methods currently in use. Evaluation of such a method for
regulatory purposes is suggested.
No data on residues in commerce or in studies of diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient for recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Long-term studies in animals.
2. Cholinesterase inhibition studies to establish a no-effect level.
3. Information on the content of the technical product and assurance
of standard composition.
4. Data on residue levels in way agricultural commodities moving in
commerce and in total diet studies.
5. Data on rate of disappearance during storage, processing and
cooking.
6. Information on composition of technical products, including
impurities.
7. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
DESIRABLE
1. Adequate information on metabolism.
2. Investigation on cholinesterase inhibition in man.
3. A gas chromatographic method for analysis of residues of thiometon
and its metabolites suitable for regulatory purposes.
REFERENCES
Askew, J., Ruzicka, J.R. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C. and Beroza, M. (1969) A rapid gas chromatographic method
for determining residues of the insecticides fenthion, disulfoton,
and phorate in corn, milk, grass and feces. J. Ass. Offic. Anal.
Chem. 52, 1231-7
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetonmethyl and their metabolites in
tobacco plants. J. Ass. Offic. Anal. Chem. 52, 157-62
Cabejszek, I., Rybak, K. and Szulinski, S. (1967) Effects in
warm-blood animals of thiometon (2-ethylthioethyl-O,O-dimethyl
phosphorodithioate) in drinking water (Translated title) Roczn.
Zak. Kig. (Warsz.), 18:257-65
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Lebensm. Hyg. 53, 154-75
FAO/WHO. (1969) 1968 evaluations of none pesticide residues in food.
FAO/PL: 1968/M/9/1; WHO/Food Add. 69.35
Gandolfo, N., Camoni, I., D'Antonio, C., Leoni, V., Ramelli, G.C. and
Sampaolo, A. (1966) Determination of O,O-dimethyl S-ethylmercaptoethyl
phosphorodithioate (thiometon) and its metabolites in olives and
olive oil. Atti simp. Int. Agrochim. 6, 224-33. (Chem. Abstr. 67,
98979g (1967)
Jucker, O. (1958) Thiometon, Verhalten in der Pflanze, Bestimmung von
Spritzrücksänden. Mitt. Lebensmitt. Hyg., 49:299-322
Juszkiewicz, T. and Rakalska, Z. (1968) Biochemical and
electrocardiographic changes in the course of an acute experimental
poisoning of hens with O,O-dimethyl-S-ethylmercaptoethyl
dithiophosphate. (Translated title) Pol. Arch. weteryu., 11:494-506
Klotzsche, C. (1958) Thiometon, ein neuer systemischer
Phosphorsäureester. Mitt. Lebensmitt. Hyg., 49:72-77
Klotzsche, C. (1967) Toxicological investigations on the
potentiation effect of Ekatin (Thiometon). Unpub. Rept. from the
Department of Occupational Hygiene, prepared and submitted by Sandoz
Ltd.
Madejski, Z. and Juszkiewicz, T. (1966) Effects of Ekatin poisoning on
some biochemical indices in chickens (Translated title) Pol. Arch.
weteryn., 10:93-101
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1968a) Thiometon. An organophosphorus systemic insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz Ltd., Basle
Sandoz. (1968b) Thiometon, Unpub. Rept. prepared and submitted by
Sandoz Ltd., Basle
Sandoz. (1969) Ekatin R. Systemisches Insektizid und Akarisid.
Unpub. Rept. prepared and submitted by Sandoz Ltd., Basle Ref. AGRO
SLK No.E-3681
Thomann, G. and Klotzsche, C. Untitled. Unpub. information submitted
by Sandoz Ltd., Basle
 The major metabolites which impart the insecticidal action of
thiometon are said to be the sulfoxide and sulfone of thiometon.
According to Sandoz (1969) the sulfoxide and sulfone of
demeton-S-methyl occur in very small amounts and therefore have much
less significance; despite inclusion in some of the analyses, they are
usually not detected. All metabolites are gradually inactivated by
hydrolysis and after 10-14 days are usually gone.
Data on mammalian metabolism was not provided.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information is available on residues in commerce or at the time of
consumption. Residues in crops are said to be rapidly degraded by high
temperature, especially by cooking.
METHODS OF RESIDUE ANALYSIS
The early residue analyses utilized paper chromatography and more
recent ones paper and thin-layer chromatography. These procedures
require a thorough cleanup, some are only semi-quantitative, and they
have limited sensitivity and specificity. With paper chromatography
recovery is 85-95 percent for thiometon sulfoxide and 70-80 percent
for thiometon sulfone; limit of detection is 0.03-0.05 and 0.06-0.09
ppm, respectively (Faderl 1962; Sandoz, 1969). Thin-layer
chromatography was slightly less sensitive.
In line with today's improved technology a gas chromatographic method
would be the method of choice in terms of accuracy, recovery,
sensitivity, and specificity. Askew et al. (1969) and Ruzicka and
co-workers (1967) utilized the Varian-Aerograph version of the
thermionic detector to analyze compounds related to thiometon, but
reported that oxydemeton-methyl was not detected by gas chromatography
under their conditions. (Oxydemeton-methyl is a metabolite of
thiometon). Bowman et al. (1969) showed that oxydemeton-methyl and its
sulfone may be analyzed by the same flame-photometric gas
chromatographic procedure used for disulfoton (ethyl analogue of
thiometon). By utilizing a temperature of 165°C, they avoid the
decomposition encountered at higher temperatures, and they condition
the analytical column to the extract and compounds being analyzed.
Their method may be adaptable for analysis of residues of thiometon.
Sensitivities are in the order of 0.01 to 0.04 ppm. Specificity in
excellent and virtually no cleanup is needed except for oily crops for
which a simple hexaneacetonitrile portion should suffice.
Another alternative is to oxidize the residues to the sulfone before
gas chromatography. Procedures to accomplish this analysis or that of
related compounds (disulfoton, phorate, and fenthion) have been
described (Bowman and Beroza, 1969). By their procedure oxidation with
m-chloroperbenzoic acid converts all of the metabolites to the
oxygen analogue sulfone for analysis as a single compound. Recoveries
usually were 80-100 percent. The 1968 FAO/WHO report indicates that
oxydemeton-methyl (FAO/WHO, 1969; p. 225), may be oxidized to its
sulfone for analysis by gas chromatography. Recoveries were 74-117
percent for levels between 0.1 and 0.4 ppm. This procedure utilizes
potassium permanganate and if applied to thiometon should produce two
sulfones, one of thiometon and the other of demeton-S-methyl.
Oxidation of the residues to a single compound would probably be the
preferred practical procedure for regulatory purposes and might be
made part of a general scheme for detecting organophosphorus
pesticides.
NATIONAL TOLERANCES
Only the Netherlands and Switzerland have a definite tolerance for
thiometon. It is 0.5 ppm.
APPRAISAL
Thiometon is a systemic organophosphorus insecticide and acaricide
that acts by contact and as a stomach poison. It in used to control
plant lice, mites, and sawflies on a wide variety of crops.
Applications by spray or dust (ingredients of product undefined) range
from 0.1 to 0.6 kg/ha and protection usually lasts 10-20 days.
Pre-harvest intervals are two to four weeks. Residue data from
experimental trials in a number of countries are available.
Thiometon itself is rapidly changed to its sulfoxide which is the
principal residue along with the sulfone. The oxygen analogue
sulfoxide and sulfone are said not to occur as residues to any great
extent. Inspection of residues from related compounds (disulfoton,
phorate, fenthion) indicate that the sulfoxides and sulfones of the
oxons are occasionally found in significant amounts. Accordingly
residue analyses for thiometon, its sulfoxide, and sulfone will
suffice when experimental trials indicate that the corresponding
oxygen analogues do not form in significant amounts.
Metabolism studies on animals have not been reported, and residues in
meat and milk have not been determined although parts of plants
treated with thiometon are likely to be used as animal feed. It is not
expected that residues of thiometon will appear in milk or meat since
such residues have not been found after feeding similar compounds such
as oxydemeton-methyl or disulfoton to dairy cows.
The most suitable method for analysis is gas chromatography with a
thermionic or flame-photometric detector. Such a method is likely to
be more accurate, more sensitive (ca. 0.01 ppm), and more specific
than methods currently in use. Evaluation of such a method for
regulatory purposes is suggested.
No data on residues in commerce or in studies of diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient for recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Long-term studies in animals.
2. Cholinesterase inhibition studies to establish a no-effect level.
3. Information on the content of the technical product and assurance
of standard composition.
4. Data on residue levels in way agricultural commodities moving in
commerce and in total diet studies.
5. Data on rate of disappearance during storage, processing and
cooking.
6. Information on composition of technical products, including
impurities.
7. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
DESIRABLE
1. Adequate information on metabolism.
2. Investigation on cholinesterase inhibition in man.
3. A gas chromatographic method for analysis of residues of thiometon
and its metabolites suitable for regulatory purposes.
REFERENCES
Askew, J., Ruzicka, J.R. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C. and Beroza, M. (1969) A rapid gas chromatographic method
for determining residues of the insecticides fenthion, disulfoton,
and phorate in corn, milk, grass and feces. J. Ass. Offic. Anal.
Chem. 52, 1231-7
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetonmethyl and their metabolites in
tobacco plants. J. Ass. Offic. Anal. Chem. 52, 157-62
Cabejszek, I., Rybak, K. and Szulinski, S. (1967) Effects in
warm-blood animals of thiometon (2-ethylthioethyl-O,O-dimethyl
phosphorodithioate) in drinking water (Translated title) Roczn.
Zak. Kig. (Warsz.), 18:257-65
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Lebensm. Hyg. 53, 154-75
FAO/WHO. (1969) 1968 evaluations of none pesticide residues in food.
FAO/PL: 1968/M/9/1; WHO/Food Add. 69.35
Gandolfo, N., Camoni, I., D'Antonio, C., Leoni, V., Ramelli, G.C. and
Sampaolo, A. (1966) Determination of O,O-dimethyl S-ethylmercaptoethyl
phosphorodithioate (thiometon) and its metabolites in olives and
olive oil. Atti simp. Int. Agrochim. 6, 224-33. (Chem. Abstr. 67,
98979g (1967)
Jucker, O. (1958) Thiometon, Verhalten in der Pflanze, Bestimmung von
Spritzrücksänden. Mitt. Lebensmitt. Hyg., 49:299-322
Juszkiewicz, T. and Rakalska, Z. (1968) Biochemical and
electrocardiographic changes in the course of an acute experimental
poisoning of hens with O,O-dimethyl-S-ethylmercaptoethyl
dithiophosphate. (Translated title) Pol. Arch. weteryu., 11:494-506
Klotzsche, C. (1958) Thiometon, ein neuer systemischer
Phosphorsäureester. Mitt. Lebensmitt. Hyg., 49:72-77
Klotzsche, C. (1967) Toxicological investigations on the
potentiation effect of Ekatin (Thiometon). Unpub. Rept. from the
Department of Occupational Hygiene, prepared and submitted by Sandoz
Ltd.
Madejski, Z. and Juszkiewicz, T. (1966) Effects of Ekatin poisoning on
some biochemical indices in chickens (Translated title) Pol. Arch.
weteryn., 10:93-101
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1968a) Thiometon. An organophosphorus systemic insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz Ltd., Basle
Sandoz. (1968b) Thiometon, Unpub. Rept. prepared and submitted by
Sandoz Ltd., Basle
Sandoz. (1969) Ekatin R. Systemisches Insektizid und Akarisid.
Unpub. Rept. prepared and submitted by Sandoz Ltd., Basle Ref. AGRO
SLK No.E-3681
Thomann, G. and Klotzsche, C. Untitled. Unpub. information submitted
by Sandoz Ltd., Basle
The major metabolites which impart the insecticidal action of
thiometon are said to be the sulfoxide and sulfone of thiometon.
According to Sandoz (1969) the sulfoxide and sulfone of
demeton-S-methyl occur in very small amounts and therefore have much
less significance; despite inclusion in some of the analyses, they are
usually not detected. All metabolites are gradually inactivated by
hydrolysis and after 10-14 days are usually gone.
Data on mammalian metabolism was not provided.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information is available on residues in commerce or at the time of
consumption. Residues in crops are said to be rapidly degraded by high
temperature, especially by cooking.
METHODS OF RESIDUE ANALYSIS
The early residue analyses utilized paper chromatography and more
recent ones paper and thin-layer chromatography. These procedures
require a thorough cleanup, some are only semi-quantitative, and they
have limited sensitivity and specificity. With paper chromatography
recovery is 85-95 percent for thiometon sulfoxide and 70-80 percent
for thiometon sulfone; limit of detection is 0.03-0.05 and 0.06-0.09
ppm, respectively (Faderl 1962; Sandoz, 1969). Thin-layer
chromatography was slightly less sensitive.
In line with today's improved technology a gas chromatographic method
would be the method of choice in terms of accuracy, recovery,
sensitivity, and specificity. Askew et al. (1969) and Ruzicka and
co-workers (1967) utilized the Varian-Aerograph version of the
thermionic detector to analyze compounds related to thiometon, but
reported that oxydemeton-methyl was not detected by gas chromatography
under their conditions. (Oxydemeton-methyl is a metabolite of
thiometon). Bowman et al. (1969) showed that oxydemeton-methyl and its
sulfone may be analyzed by the same flame-photometric gas
chromatographic procedure used for disulfoton (ethyl analogue of
thiometon). By utilizing a temperature of 165°C, they avoid the
decomposition encountered at higher temperatures, and they condition
the analytical column to the extract and compounds being analyzed.
Their method may be adaptable for analysis of residues of thiometon.
Sensitivities are in the order of 0.01 to 0.04 ppm. Specificity in
excellent and virtually no cleanup is needed except for oily crops for
which a simple hexaneacetonitrile portion should suffice.
Another alternative is to oxidize the residues to the sulfone before
gas chromatography. Procedures to accomplish this analysis or that of
related compounds (disulfoton, phorate, and fenthion) have been
described (Bowman and Beroza, 1969). By their procedure oxidation with
m-chloroperbenzoic acid converts all of the metabolites to the
oxygen analogue sulfone for analysis as a single compound. Recoveries
usually were 80-100 percent. The 1968 FAO/WHO report indicates that
oxydemeton-methyl (FAO/WHO, 1969; p. 225), may be oxidized to its
sulfone for analysis by gas chromatography. Recoveries were 74-117
percent for levels between 0.1 and 0.4 ppm. This procedure utilizes
potassium permanganate and if applied to thiometon should produce two
sulfones, one of thiometon and the other of demeton-S-methyl.
Oxidation of the residues to a single compound would probably be the
preferred practical procedure for regulatory purposes and might be
made part of a general scheme for detecting organophosphorus
pesticides.
NATIONAL TOLERANCES
Only the Netherlands and Switzerland have a definite tolerance for
thiometon. It is 0.5 ppm.
APPRAISAL
Thiometon is a systemic organophosphorus insecticide and acaricide
that acts by contact and as a stomach poison. It in used to control
plant lice, mites, and sawflies on a wide variety of crops.
Applications by spray or dust (ingredients of product undefined) range
from 0.1 to 0.6 kg/ha and protection usually lasts 10-20 days.
Pre-harvest intervals are two to four weeks. Residue data from
experimental trials in a number of countries are available.
Thiometon itself is rapidly changed to its sulfoxide which is the
principal residue along with the sulfone. The oxygen analogue
sulfoxide and sulfone are said not to occur as residues to any great
extent. Inspection of residues from related compounds (disulfoton,
phorate, fenthion) indicate that the sulfoxides and sulfones of the
oxons are occasionally found in significant amounts. Accordingly
residue analyses for thiometon, its sulfoxide, and sulfone will
suffice when experimental trials indicate that the corresponding
oxygen analogues do not form in significant amounts.
Metabolism studies on animals have not been reported, and residues in
meat and milk have not been determined although parts of plants
treated with thiometon are likely to be used as animal feed. It is not
expected that residues of thiometon will appear in milk or meat since
such residues have not been found after feeding similar compounds such
as oxydemeton-methyl or disulfoton to dairy cows.
The most suitable method for analysis is gas chromatography with a
thermionic or flame-photometric detector. Such a method is likely to
be more accurate, more sensitive (ca. 0.01 ppm), and more specific
than methods currently in use. Evaluation of such a method for
regulatory purposes is suggested.
No data on residues in commerce or in studies of diets have been
reported.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
The data were insufficient for recommendations to be made.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake or tolerances can
be established)
1. Long-term studies in animals.
2. Cholinesterase inhibition studies to establish a no-effect level.
3. Information on the content of the technical product and assurance
of standard composition.
4. Data on residue levels in way agricultural commodities moving in
commerce and in total diet studies.
5. Data on rate of disappearance during storage, processing and
cooking.
6. Information on composition of technical products, including
impurities.
7. Data on animal metabolism and residues in meat and milk of animals
consuming agricultural products treated in accordance with good
agricultural practice.
DESIRABLE
1. Adequate information on metabolism.
2. Investigation on cholinesterase inhibition in man.
3. A gas chromatographic method for analysis of residues of thiometon
and its metabolites suitable for regulatory purposes.
REFERENCES
Askew, J., Ruzicka, J.R. and Wheals, B.B. (1969) A general method for
the determination of organophosphorus pesticide residues in river
waters and effluents by gas, thin layer and gel chromatography.
Analyst 94, 275-83
Bowman, M.C. and Beroza, M. (1969) A rapid gas chromatographic method
for determining residues of the insecticides fenthion, disulfoton,
and phorate in corn, milk, grass and feces. J. Ass. Offic. Anal.
Chem. 52, 1231-7
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969) GLC determination of
residues of disulfoton, oxydemetonmethyl and their metabolites in
tobacco plants. J. Ass. Offic. Anal. Chem. 52, 157-62
Cabejszek, I., Rybak, K. and Szulinski, S. (1967) Effects in
warm-blood animals of thiometon (2-ethylthioethyl-O,O-dimethyl
phosphorodithioate) in drinking water (Translated title) Roczn.
Zak. Kig. (Warsz.), 18:257-65
Faderl, N. (1962) Methode zur Bestimmung von Mikromengen organischer
Phosphorinsektizide. Lebensm. Hyg. 53, 154-75
FAO/WHO. (1969) 1968 evaluations of none pesticide residues in food.
FAO/PL: 1968/M/9/1; WHO/Food Add. 69.35
Gandolfo, N., Camoni, I., D'Antonio, C., Leoni, V., Ramelli, G.C. and
Sampaolo, A. (1966) Determination of O,O-dimethyl S-ethylmercaptoethyl
phosphorodithioate (thiometon) and its metabolites in olives and
olive oil. Atti simp. Int. Agrochim. 6, 224-33. (Chem. Abstr. 67,
98979g (1967)
Jucker, O. (1958) Thiometon, Verhalten in der Pflanze, Bestimmung von
Spritzrücksänden. Mitt. Lebensmitt. Hyg., 49:299-322
Juszkiewicz, T. and Rakalska, Z. (1968) Biochemical and
electrocardiographic changes in the course of an acute experimental
poisoning of hens with O,O-dimethyl-S-ethylmercaptoethyl
dithiophosphate. (Translated title) Pol. Arch. weteryu., 11:494-506
Klotzsche, C. (1958) Thiometon, ein neuer systemischer
Phosphorsäureester. Mitt. Lebensmitt. Hyg., 49:72-77
Klotzsche, C. (1967) Toxicological investigations on the
potentiation effect of Ekatin (Thiometon). Unpub. Rept. from the
Department of Occupational Hygiene, prepared and submitted by Sandoz
Ltd.
Madejski, Z. and Juszkiewicz, T. (1966) Effects of Ekatin poisoning on
some biochemical indices in chickens (Translated title) Pol. Arch.
weteryn., 10:93-101
Ruzicka, J., Thomson, J. and Wheals, B.B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatogr. 30, 92-9
Sandoz. (1968a) Thiometon. An organophosphorus systemic insecticide.
Unpub. Rept. on animal toxicology submitted by Sandoz Ltd., Basle
Sandoz. (1968b) Thiometon, Unpub. Rept. prepared and submitted by
Sandoz Ltd., Basle
Sandoz. (1969) Ekatin R. Systemisches Insektizid und Akarisid.
Unpub. Rept. prepared and submitted by Sandoz Ltd., Basle Ref. AGRO
SLK No.E-3681
Thomann, G. and Klotzsche, C. Untitled. Unpub. information submitted
by Sandoz Ltd., Basle
