
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
DIQUAT
IDENTITY
Chemical name
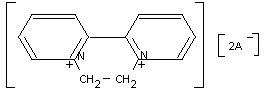
9,10 - Dihydro - 8a, 10a - diazoniaphenanthrene ion
1,1' - Ethylene - 2,2' - bipyridylium ion
6,7 - Dihydrodipyridol (1, 2a : 2', 1' - c) - pyrazidinium ion
Synonyms
FB 2(R), Reglone(R), Aquacide(R), Dextrone(R)
Structural formula
 Other relevant chemical properties
Diquat is available only as a salt, generally as the dibromide. The
cationic portion of the molecule is the active ingredient. Diquat
dibromide exists as the monohydrate and forms white to yellow crystals
which decompose above 300°C. The technical material is available only
as a dark reddish-brown aqueous solution. Solubility in water at 20°C
is 700 g/litre: it is slightly soluble in alcoholic and hydroxylic
solvents and practically insoluble in non-polar organic solvents,
stable in acid or neutral solutions and unstable under alkaline
conditions. One electron reduction by zinc or sodium dithionite yields
the green free-radical which in the presence of atmospheric oxygen
reverts to diquat. The single electron reduction is completely
reversible, and the redox potential of - 349 mV is independent of pH.
Further reduction yields polyhydrobipyridyl derivatives.
Solutions of the free radical exhibit a sharp absorption peak at 379
nm which has a greater intensity than that of the unreduced diquat at
310 nm. Concentrated aqueous solutions corrode steel, tinplate,
galvanised iron and aluminium.
Purity
Technical, 95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Dogs administered orally 10-15 mg 14C-labelled diquat dibromide
excreted 29-32 percent in the urine and 51-62 percent in the faeces in
72 hours. In the first 24 hours, 25-28 percent of the dose was
excreted in the urine (Swan, 1960).
Following oral doses of 5-10 mg/kg to rats, diquat dibromide was
eliminated entirely with 96-101 percent recovery within four days, the
levels in the urine were 4-6 percent and in the faeces 90-96 percent
(Daniel and Gage, 1966). Following subcutaneous doses of 5 or 6 mg/kg
to rats, diquat dibromide was eliminated again within four days with
90-98 percent recovery-in urine 88-98 percent and in faeces 0-2
percent. In all studies, the bulk of the residues excreted following
oral administration was in the faeces, whereas after a subcutaneous
dose most was in the urine, indicating that absorption from the gut
was relatively poor. Studies where chemical rather than radiological
measurements were made showed that following an oral dose of 30-40
mg/kg to rats, only 4-7 percent of the dose was recovered in the urine
and 11-42 percent in the faeces after 48 hours (Swan, 1960).
The percentage excretion of an oral dose in the urine, calculated from
a chemical method of analysis, was lower than that obtained from a
radio-analytical method of analysis, suggesting that a proportion of
the dose appeared in a form other than diquat. Following a
subcutaneous dose, the radiochemical and chemical analyses gave
results which were closer than the values following an oral dose,
indicating possible absorption from the gut of microbial degradation
products following oral administration, rather than actual animal
metabolism (Swan, 1960).
In vitro experiments using suspensions of gut material suggested
that microbial breakdown of diquat was responsible for the low
recoveries by the chemical assay where only the parent ion was
measured (Swan, 1960).
Effects on enzymes and other biochemical parameters
Although there is no direct evidence in mammals to support the view,
it is tempting to assume that the ability of bipyridyls to be reduced
and re-oxidized with the production of free radicals is linked with
their toxic effects, as has been suggested to be the case in plants.
Gage (1968) has shown that free radicals can be produced from diquat
incubated anaerobically in the presence of NADPH and microsomes
derived from rat liver. Diquat also increased the respiration of liver
mitochondrial fragments. This action has been related to the activity
of flavo-protein dehydrogenases. Purified lipoamide dehydrogenase from
pig heart was able to reduce diquat to the free radical in the
presence of NADH. Rees (1969) has shown that a fresh extract of bovine
lens, incubated anaerobically with diquat, can catalyze similar
reactions. From these experiments, there can be little doubt that
flavo-proteins of animal tissues in the presence of their
co-substrates can reduce the bipyridyls, although aerobically the
equilibrium concentration of the free radical is probably extremely
low.
The property that diquat has of undergoing cyclic reduction and
oxidation might suggest that it could interfere in electron-transport
processes, diverting electrons from the system and reducing oxygen to
water. Gage (1968) found that the resting respiration of mitochondria
was almost unaffected by diquat, probably because of its inability to
penetrate the mitochondrial membrane.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females) were examined for
reproduction and teratogenic effects of diquat dichloride at 0, 125
and 500 ppm in the diet (Griffiths et al., 1966). This experiment
examined the effects of dietary feeding of diquat to males only,
females only and both males and females at 500 ppm and males and
females at 125 ppm. Growth of the parent rats was moderately retarded
at 500 ppm, and all parents developed cataracts. No effect on
reproduction or occurrence of terata was observed. At 125 ppm, no
effect on the growth of parents was noted. Lens opacities did not
occur. Of all offspring produced (1637), one female at 500 ppm had a
unilateral cataract.
A single intraperitoneal injection of diquat (7 mg/kg from day six
through 14 of gestation) produced a high incidence of retarded growth
of sternum and auditory ossicles, as well as marked weight reduction
in rat embryos. A higher dose of 14 mg/kg interrupted most
pregnancies, and in the rats that reached term, the embryonic effects
were more pronounced (Khera and Whitta, 1968).
Special studies for mutagenicity
Diquat was screened for mutagenic activity in Drosophila
melanogaster using the Muller-5 test to detect recessive lethality
on the X chromosome. Compared to a spontaneous mutation rate of 0.14
percent, the rate was 0.11 percent after treatment with diquat, which
is comparable with the control value (Benes and Sram, 1969).
Special studies on cataractogenicity
The initial cataractogenic effects in rats of diquat appear to be
reversible, as was noted in a study where rats were fed a diet
containing 500 ppm diquat. This dose was shown to produce cataracts in
three to six months. After feeding continuously for periods of several
days to eight weeks, the rats were given a normal diquat-free diet for
the remainder of one year. Cataracts did not develop in any of these
rats, indicating that continuous prolonged exposure to diquat was
necessary for the formation of cataracts in the rat. Temporary
exposure to this known cataractogen does not lead to irreversible
damage (Clark and Hurst, 1970).
Since it is known that some forms of cataract are influenced by light
and that the toxicity of diquat to plants is dependent upon light,
experiments were undertaken to study the effect of light on cataract
formation in rats. Rats were fed 500 ppm diquat in the dark for three
months and controls were fed the same diet under light conditions.
After three months feeding on these diets, both groups of animals
showed an equal number of cataracts, suggesting that light does not
influence the development of cataract formation (Clark and Hurst,
1970).
Ascorbic acid (200 mg/ml) in the drinking water of rats receiving
diquat (500 ppm) in the diet did not influence the development of
cataracts (Clark and Hurst, 1970.)
See also "Short" and "Long-term Studies" and "Reproduction". LD50
values for diquat in various species are summarized in Table I.
A level of 90 ppm diquat at 18°C killed 50 percent of a population of
rainbow trout within 48 hours. At 70 ppm, 50 percent mortality was
observed in 48 hours (Swan, 1960).
A single dermal application of 10 or 20 mg of diquat to rats as an
aqueous solution produced a slight reversible erythema in the treated
area (Cooke and Gage, 1956).
Acute dermal administration of diquat at doses of 500 and 1000 mg/kg
to rabbits resulted in toxic signs within 48-72 hours at 1000 mg/kg
and no abnormalities at 500 mg/kg (Swan, 1963b).
Installation of 10 mg diquat of a 10 percent aqueous solution into the
conjuctival sac of rabbits produced no effect (Cook and Gage, 1956).
Short-term studies
Rat
Three groups of rats (four males and four females) were exposed to
diquat in aerosol form at levels of 0.5, 1.06 and 2 µg/litre for 15
daily six-hour exposure periods. At the 2 µg/litre level, weight gain
was slightly reduced. No such effect was noted at the 1.06 and 0.5
µg/litre levels. Histopathological examination of the lungs showed
slight irritation with peribronchial lymphoid hyperplasia,
perivascular oedema and macrophages in the alveoli at the 2 µg/litre
level (Gage, 1967).
TABLE I
LD50 values of diquat in various species
Salt LD50
Species Route Form (mg Reference
cation/kg)
Mouse Oral Cl 125 Swan, 1963a
Br 125 Clark and Hurst, 1970
Rat Oral Cl 302 Swan, 1962
Br 215-235 Clark and Hurst, 1970
Swan, 1960
s.c. Cl 11 Clark and Hurst, 1970
Br 11-20 Clark and Hurst, 1970
Cook and Gage, 1956
Guinea Pig Oral Br 100 Clark and Hurst, 1970
Rabbit Oral Br 100 Swan, 1960
Clark and Hurst, 1970
Dermal Br 400 Swan, 1963b
Cookson and McElligott, 1966
i.p. Br 15 " " "
Hen Oral Br 215-430 Swan, 1960
Br 200-400 Clark and Hurst, 1970
Dog Oral Br 100-200 Clark and Hurst, 1970
Cow Oral Br approx. 30 Walley, 1962
Clark and Hurst, 1970
Subcutaneous administration of 1 mg diquat/kg body-weight to rats for
21 days produced no toxic effects; post-mortem examination showed no
organic damage (Cooke and Gage, 1956).
Rabbit
Groups of rabbits (three males and three females) were administered
diquat percutaneously at doses of 0, 20, 40, 80 and 160 mg diquat
ion/kg body-weight for 20 days. At 20 mg/kg, no deaths occurred,
whereas, at 40 mg/kg and above, from 66 to 100 percent of the animals
died.
Microscopic examination revealed vacuolar changes in the distal
convoluted renal tubules with occasional necrosis of cells. These
changes were preceded by weight loss and muscular weakness (Cookson
and McElligott, 1966).
Groups of five female and five male rabbits were administered up to 20
daily percutaneous doses of diquat at O, 3.13, 6.25, 12.5 and 25
mg/kg. The calculated LD50 was 7.9 mg/kg (5.7 mg ion/kg). Signs of
poisoning included: local hyperaemic and subcutaneous oedema;
increasing sloughing of surface layers of skin followed by scab
formation. These effects were reversible at the 6.25 mg/kg dose after
treatment was concluded. Other signs included weakness, incoordination
and lethargy. In many animals, ulceration of the gastric mucosa was
observed at post-mortem indicating that oral contamination had
presumably occurred (Swan, 1963b).
Dog
Groups of dogs (three males and three females) were fed diets
containing 0, 16, 32, 68, 200 and 600 ppm diquat dichloride for
periods up to four years. At dosages of 600 and 200 ppm, bilateral
opacities of the lens were observed at ten and 15 months,
respectively. Dietary levels of 68 ppm did not affect the lens within
the four year interval tested. No effects were observed on growth,
tumour formation, food consumption, blood chemistry and gross and
microscopic pathology. The dose of 68 ppm (equivalent to 50 ppm diquat
ion) is the no-effect level in dogs with regard to cataract formation
(Hurst, 1966).
Sheep and calf
Five groups of two sheep (one male and one female, eight months old)
and three groups of one calf each were given diquat at doses of 0, 1,
5, 10 and 20 ppm and 0, 5 and 20 ppm, respectively, in their drinking
water for one month. These levels caused no toxicological effect over
the trial period as evidenced by growth, food consumption and visual
observation (Sarfaty, 1963).
Cow
Oral administration of diquat for five days at 10 mg/kg body-weight to
a cow resulted in death within 15 days preceded by severe signs of
poison, including dullness, inappetence anaemia, increased heart rate.
At post-mortem, heart and kidney infarcts and intestinal catarrh were
found. Administration of 5 mg diquat/kg daily for fourteen days to a
cow (in the diet for two days, then drenched) resulted in increased
inappetence after two days, slight haemorrhage as noted by blood in
the faeces and temporary impairment of vision (Walley, 1962).
Comparative
Groups of four male and four female mice and guinea pigs, two female
rabbits and a male dog, exposed to 15 daily six-hour treatments with
diquat in aerosol form at 1.06 mg/litre, showed no adverse effects
(Gage, 1967).
Long-term studies
Rat
Groups of rats (25 males and 25 females per group) were fed diquat
dichloride for two years at doses of 0.125, 250, 500 or 1000 ppm in
the diet. After 56 days, the 1000 ppm level was discontinued because
of lack of growth and mortality in males and females. At 125 ppm, a
partial lens opacity (cataract) was seen at 207 days. All males and 19
out of 21 females were affected by 657 days. At 250 and 500 ppm, lens
opacities were produced in all animals within 155 and 124 days,
respectively. At 500 ppm, a reduction of female body-weight was
apparent after 20 weeks and in males after five weeks. No such effects
were noted at 250 and 125 ppm. No adverse effects were reported in
survival, blood chemistry, tumour, incidence and gross and microscopic
pathology (Swan, 1962; Goater et al., 1964).
Seven groups of rats (25 males and 25 females/group; controls had 75
and 75 females) were fed diquat dichloride at levels of 0,10, 50, 100,
250, 500 or 1000 ppm for two years. Growth depression was observed in
the males at 1000 ppm. Lens opacity was observed at doses of 50 ppm
and above in both males and females (no such effect was noted at the
10 ppm level). Growth, food consumption survival, tumour formation,
behaviour, haematological and urine analyses and gross and microscopic
examination revealed no effects differing from the controls. A
no-effect level based upon cataract formation is 10 ppm, equivalent to
7.2 ppm diquat cation (Kohn et al., 1965a, b and c).
OBSERVATIONS IN MAN
Damage and discolouration of fingernails caused by exposure to
concentrated solutions of diquat were observed in three instances. The
cause of the damage is unknown but presumably is of a local nature.
All three patients had frequent exposure to the concentrated chemical
without taking precautions to prevent contamination of the skin. The
cause of the nail damage was unknown, but it seemed probable that the
chemical reached the nail matrix by entering the nail fold and
stimulated infection, interfering with the formation of the nail from
the matrix. The damage is presumed to be local and not as result of
ingestion because of asymmetry of the lesions and the fact that the
toenails were unaffected. A curious colour change and softening of the
nail at the base are characteristic. In some instances, the nail was
shed and was not regrown (Samman and Johnston, 1969).
Of 42 reported exposures of man varying from one to 75 individual
exposures, four incidents of dermal abnormalities were reported. These
include rashes, blisters and a transient skin discolouration. In
almost all instances, these incidents were attributable to the
concentrated commercial preparation (Anonymous, 1966).
Poisoning cases in humans with bipyridyl compounds have demonstrated
the acute toxic effects of these compounds. In one case, the
accidental ingestion of a small quantity of diquat led to diarrhoea
and oral ulceration. After forced diuresis, the man recovered and was
released from care. It was observed that the urine contained traces of
diquat as long as eleven days after ingestion (Oreopoulos, 1969).
COMMENT
In biochemical studies in rats and dogs, it has been observed that
after oral administration, the major part of the dose is excreted in
the faeces. Following a subcutaneous dose, most residue was found in
the urine, indicating that absorption from the gut was relatively
poor. A small proportion of the material found in urine after oral
administration was other than the parent compound, due possibly to
absorption of microbial degradation products rather than actual animal
metabolism.
Diquat is a cataractogenic compound, as has been demonstrated in
lont-term rat and in dog experiments at relatively low levels of 2.5
and 5 mg/kg body-weight, respectively. Transient exposure of rats to
diquat does not lead to the formation of cataracts.
Intraperitoneal administration of 7 mg/kg diquat to pregnant rats
resulted in embryotoxic effects. In a three-generation rat
reproduction study, 500 ppm diquat resulted in no effect on
reproduction.
In man, the acute and dermal problems associated with accidental or
suicidal ingestion and dermal contamination appear to be of primary
concern. Cataract formation has not been observed in man as a result
of exposure to diquat, nevertheless studies in the prophylaxis and
treatment of diquat-induced cataracts in mammals were considered
urgent.
Non-reversible cataractogenic effects in rats and dogs at relatively
low oral levels, 50 ppm (2.5 mg/kg) and 200 ppm (5
mg/kg),reapectively, and a potential embryonic effect make it
advisable to establish only a temporary acceptable daily intake for
man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg body-weight/day
(corresponds to 0.36 mg diquat ion/kg body-weight/day)
Dog: 68 ppm in the diet, equivalent to 1.7 mg/kg body-weight/day
(corresponds to 1.22 mg diquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.0025 mg/kg body-weight as diquat dichloride (0-0.002 mg/kg
body-weight expressed as diquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Diquat is used world wide for the following purposes; desiccation of
beans, peas, sugar beet, sorghum, maize, rice etc; potato haulm
destruction; pre-emergence weed control in carrots, onions, hops,
vines and sugar-cane flower control; control of submerged and some
floating weeds in still water or streams, defoliant and desiccant for
cotton; desiccation of red and white clover, sunflower, linseed and
rape for seed; desiccant for barley and wheat for stock feed purposes.
FATE OF RESIDUES
Photodecomposition
The photodecomposition of diquat resulted in disappearance of
radioactivity with both the ring and ethylene bridge radioactivity
labelled indicating the occurrence of volatile breakdown products.
Approximately 75% of the material was lost with 96 hours of sunlight
exposure. The photo-chemical breakdown of diquat gives
1,2,3,4-tetrahydro-1-oxo-pyridyl 1,2-a-5-pyrazinium bromide.
Other relevant chemical properties
Diquat is available only as a salt, generally as the dibromide. The
cationic portion of the molecule is the active ingredient. Diquat
dibromide exists as the monohydrate and forms white to yellow crystals
which decompose above 300°C. The technical material is available only
as a dark reddish-brown aqueous solution. Solubility in water at 20°C
is 700 g/litre: it is slightly soluble in alcoholic and hydroxylic
solvents and practically insoluble in non-polar organic solvents,
stable in acid or neutral solutions and unstable under alkaline
conditions. One electron reduction by zinc or sodium dithionite yields
the green free-radical which in the presence of atmospheric oxygen
reverts to diquat. The single electron reduction is completely
reversible, and the redox potential of - 349 mV is independent of pH.
Further reduction yields polyhydrobipyridyl derivatives.
Solutions of the free radical exhibit a sharp absorption peak at 379
nm which has a greater intensity than that of the unreduced diquat at
310 nm. Concentrated aqueous solutions corrode steel, tinplate,
galvanised iron and aluminium.
Purity
Technical, 95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Dogs administered orally 10-15 mg 14C-labelled diquat dibromide
excreted 29-32 percent in the urine and 51-62 percent in the faeces in
72 hours. In the first 24 hours, 25-28 percent of the dose was
excreted in the urine (Swan, 1960).
Following oral doses of 5-10 mg/kg to rats, diquat dibromide was
eliminated entirely with 96-101 percent recovery within four days, the
levels in the urine were 4-6 percent and in the faeces 90-96 percent
(Daniel and Gage, 1966). Following subcutaneous doses of 5 or 6 mg/kg
to rats, diquat dibromide was eliminated again within four days with
90-98 percent recovery-in urine 88-98 percent and in faeces 0-2
percent. In all studies, the bulk of the residues excreted following
oral administration was in the faeces, whereas after a subcutaneous
dose most was in the urine, indicating that absorption from the gut
was relatively poor. Studies where chemical rather than radiological
measurements were made showed that following an oral dose of 30-40
mg/kg to rats, only 4-7 percent of the dose was recovered in the urine
and 11-42 percent in the faeces after 48 hours (Swan, 1960).
The percentage excretion of an oral dose in the urine, calculated from
a chemical method of analysis, was lower than that obtained from a
radio-analytical method of analysis, suggesting that a proportion of
the dose appeared in a form other than diquat. Following a
subcutaneous dose, the radiochemical and chemical analyses gave
results which were closer than the values following an oral dose,
indicating possible absorption from the gut of microbial degradation
products following oral administration, rather than actual animal
metabolism (Swan, 1960).
In vitro experiments using suspensions of gut material suggested
that microbial breakdown of diquat was responsible for the low
recoveries by the chemical assay where only the parent ion was
measured (Swan, 1960).
Effects on enzymes and other biochemical parameters
Although there is no direct evidence in mammals to support the view,
it is tempting to assume that the ability of bipyridyls to be reduced
and re-oxidized with the production of free radicals is linked with
their toxic effects, as has been suggested to be the case in plants.
Gage (1968) has shown that free radicals can be produced from diquat
incubated anaerobically in the presence of NADPH and microsomes
derived from rat liver. Diquat also increased the respiration of liver
mitochondrial fragments. This action has been related to the activity
of flavo-protein dehydrogenases. Purified lipoamide dehydrogenase from
pig heart was able to reduce diquat to the free radical in the
presence of NADH. Rees (1969) has shown that a fresh extract of bovine
lens, incubated anaerobically with diquat, can catalyze similar
reactions. From these experiments, there can be little doubt that
flavo-proteins of animal tissues in the presence of their
co-substrates can reduce the bipyridyls, although aerobically the
equilibrium concentration of the free radical is probably extremely
low.
The property that diquat has of undergoing cyclic reduction and
oxidation might suggest that it could interfere in electron-transport
processes, diverting electrons from the system and reducing oxygen to
water. Gage (1968) found that the resting respiration of mitochondria
was almost unaffected by diquat, probably because of its inability to
penetrate the mitochondrial membrane.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females) were examined for
reproduction and teratogenic effects of diquat dichloride at 0, 125
and 500 ppm in the diet (Griffiths et al., 1966). This experiment
examined the effects of dietary feeding of diquat to males only,
females only and both males and females at 500 ppm and males and
females at 125 ppm. Growth of the parent rats was moderately retarded
at 500 ppm, and all parents developed cataracts. No effect on
reproduction or occurrence of terata was observed. At 125 ppm, no
effect on the growth of parents was noted. Lens opacities did not
occur. Of all offspring produced (1637), one female at 500 ppm had a
unilateral cataract.
A single intraperitoneal injection of diquat (7 mg/kg from day six
through 14 of gestation) produced a high incidence of retarded growth
of sternum and auditory ossicles, as well as marked weight reduction
in rat embryos. A higher dose of 14 mg/kg interrupted most
pregnancies, and in the rats that reached term, the embryonic effects
were more pronounced (Khera and Whitta, 1968).
Special studies for mutagenicity
Diquat was screened for mutagenic activity in Drosophila
melanogaster using the Muller-5 test to detect recessive lethality
on the X chromosome. Compared to a spontaneous mutation rate of 0.14
percent, the rate was 0.11 percent after treatment with diquat, which
is comparable with the control value (Benes and Sram, 1969).
Special studies on cataractogenicity
The initial cataractogenic effects in rats of diquat appear to be
reversible, as was noted in a study where rats were fed a diet
containing 500 ppm diquat. This dose was shown to produce cataracts in
three to six months. After feeding continuously for periods of several
days to eight weeks, the rats were given a normal diquat-free diet for
the remainder of one year. Cataracts did not develop in any of these
rats, indicating that continuous prolonged exposure to diquat was
necessary for the formation of cataracts in the rat. Temporary
exposure to this known cataractogen does not lead to irreversible
damage (Clark and Hurst, 1970).
Since it is known that some forms of cataract are influenced by light
and that the toxicity of diquat to plants is dependent upon light,
experiments were undertaken to study the effect of light on cataract
formation in rats. Rats were fed 500 ppm diquat in the dark for three
months and controls were fed the same diet under light conditions.
After three months feeding on these diets, both groups of animals
showed an equal number of cataracts, suggesting that light does not
influence the development of cataract formation (Clark and Hurst,
1970).
Ascorbic acid (200 mg/ml) in the drinking water of rats receiving
diquat (500 ppm) in the diet did not influence the development of
cataracts (Clark and Hurst, 1970.)
See also "Short" and "Long-term Studies" and "Reproduction". LD50
values for diquat in various species are summarized in Table I.
A level of 90 ppm diquat at 18°C killed 50 percent of a population of
rainbow trout within 48 hours. At 70 ppm, 50 percent mortality was
observed in 48 hours (Swan, 1960).
A single dermal application of 10 or 20 mg of diquat to rats as an
aqueous solution produced a slight reversible erythema in the treated
area (Cooke and Gage, 1956).
Acute dermal administration of diquat at doses of 500 and 1000 mg/kg
to rabbits resulted in toxic signs within 48-72 hours at 1000 mg/kg
and no abnormalities at 500 mg/kg (Swan, 1963b).
Installation of 10 mg diquat of a 10 percent aqueous solution into the
conjuctival sac of rabbits produced no effect (Cook and Gage, 1956).
Short-term studies
Rat
Three groups of rats (four males and four females) were exposed to
diquat in aerosol form at levels of 0.5, 1.06 and 2 µg/litre for 15
daily six-hour exposure periods. At the 2 µg/litre level, weight gain
was slightly reduced. No such effect was noted at the 1.06 and 0.5
µg/litre levels. Histopathological examination of the lungs showed
slight irritation with peribronchial lymphoid hyperplasia,
perivascular oedema and macrophages in the alveoli at the 2 µg/litre
level (Gage, 1967).
TABLE I
LD50 values of diquat in various species
Salt LD50
Species Route Form (mg Reference
cation/kg)
Mouse Oral Cl 125 Swan, 1963a
Br 125 Clark and Hurst, 1970
Rat Oral Cl 302 Swan, 1962
Br 215-235 Clark and Hurst, 1970
Swan, 1960
s.c. Cl 11 Clark and Hurst, 1970
Br 11-20 Clark and Hurst, 1970
Cook and Gage, 1956
Guinea Pig Oral Br 100 Clark and Hurst, 1970
Rabbit Oral Br 100 Swan, 1960
Clark and Hurst, 1970
Dermal Br 400 Swan, 1963b
Cookson and McElligott, 1966
i.p. Br 15 " " "
Hen Oral Br 215-430 Swan, 1960
Br 200-400 Clark and Hurst, 1970
Dog Oral Br 100-200 Clark and Hurst, 1970
Cow Oral Br approx. 30 Walley, 1962
Clark and Hurst, 1970
Subcutaneous administration of 1 mg diquat/kg body-weight to rats for
21 days produced no toxic effects; post-mortem examination showed no
organic damage (Cooke and Gage, 1956).
Rabbit
Groups of rabbits (three males and three females) were administered
diquat percutaneously at doses of 0, 20, 40, 80 and 160 mg diquat
ion/kg body-weight for 20 days. At 20 mg/kg, no deaths occurred,
whereas, at 40 mg/kg and above, from 66 to 100 percent of the animals
died.
Microscopic examination revealed vacuolar changes in the distal
convoluted renal tubules with occasional necrosis of cells. These
changes were preceded by weight loss and muscular weakness (Cookson
and McElligott, 1966).
Groups of five female and five male rabbits were administered up to 20
daily percutaneous doses of diquat at O, 3.13, 6.25, 12.5 and 25
mg/kg. The calculated LD50 was 7.9 mg/kg (5.7 mg ion/kg). Signs of
poisoning included: local hyperaemic and subcutaneous oedema;
increasing sloughing of surface layers of skin followed by scab
formation. These effects were reversible at the 6.25 mg/kg dose after
treatment was concluded. Other signs included weakness, incoordination
and lethargy. In many animals, ulceration of the gastric mucosa was
observed at post-mortem indicating that oral contamination had
presumably occurred (Swan, 1963b).
Dog
Groups of dogs (three males and three females) were fed diets
containing 0, 16, 32, 68, 200 and 600 ppm diquat dichloride for
periods up to four years. At dosages of 600 and 200 ppm, bilateral
opacities of the lens were observed at ten and 15 months,
respectively. Dietary levels of 68 ppm did not affect the lens within
the four year interval tested. No effects were observed on growth,
tumour formation, food consumption, blood chemistry and gross and
microscopic pathology. The dose of 68 ppm (equivalent to 50 ppm diquat
ion) is the no-effect level in dogs with regard to cataract formation
(Hurst, 1966).
Sheep and calf
Five groups of two sheep (one male and one female, eight months old)
and three groups of one calf each were given diquat at doses of 0, 1,
5, 10 and 20 ppm and 0, 5 and 20 ppm, respectively, in their drinking
water for one month. These levels caused no toxicological effect over
the trial period as evidenced by growth, food consumption and visual
observation (Sarfaty, 1963).
Cow
Oral administration of diquat for five days at 10 mg/kg body-weight to
a cow resulted in death within 15 days preceded by severe signs of
poison, including dullness, inappetence anaemia, increased heart rate.
At post-mortem, heart and kidney infarcts and intestinal catarrh were
found. Administration of 5 mg diquat/kg daily for fourteen days to a
cow (in the diet for two days, then drenched) resulted in increased
inappetence after two days, slight haemorrhage as noted by blood in
the faeces and temporary impairment of vision (Walley, 1962).
Comparative
Groups of four male and four female mice and guinea pigs, two female
rabbits and a male dog, exposed to 15 daily six-hour treatments with
diquat in aerosol form at 1.06 mg/litre, showed no adverse effects
(Gage, 1967).
Long-term studies
Rat
Groups of rats (25 males and 25 females per group) were fed diquat
dichloride for two years at doses of 0.125, 250, 500 or 1000 ppm in
the diet. After 56 days, the 1000 ppm level was discontinued because
of lack of growth and mortality in males and females. At 125 ppm, a
partial lens opacity (cataract) was seen at 207 days. All males and 19
out of 21 females were affected by 657 days. At 250 and 500 ppm, lens
opacities were produced in all animals within 155 and 124 days,
respectively. At 500 ppm, a reduction of female body-weight was
apparent after 20 weeks and in males after five weeks. No such effects
were noted at 250 and 125 ppm. No adverse effects were reported in
survival, blood chemistry, tumour, incidence and gross and microscopic
pathology (Swan, 1962; Goater et al., 1964).
Seven groups of rats (25 males and 25 females/group; controls had 75
and 75 females) were fed diquat dichloride at levels of 0,10, 50, 100,
250, 500 or 1000 ppm for two years. Growth depression was observed in
the males at 1000 ppm. Lens opacity was observed at doses of 50 ppm
and above in both males and females (no such effect was noted at the
10 ppm level). Growth, food consumption survival, tumour formation,
behaviour, haematological and urine analyses and gross and microscopic
examination revealed no effects differing from the controls. A
no-effect level based upon cataract formation is 10 ppm, equivalent to
7.2 ppm diquat cation (Kohn et al., 1965a, b and c).
OBSERVATIONS IN MAN
Damage and discolouration of fingernails caused by exposure to
concentrated solutions of diquat were observed in three instances. The
cause of the damage is unknown but presumably is of a local nature.
All three patients had frequent exposure to the concentrated chemical
without taking precautions to prevent contamination of the skin. The
cause of the nail damage was unknown, but it seemed probable that the
chemical reached the nail matrix by entering the nail fold and
stimulated infection, interfering with the formation of the nail from
the matrix. The damage is presumed to be local and not as result of
ingestion because of asymmetry of the lesions and the fact that the
toenails were unaffected. A curious colour change and softening of the
nail at the base are characteristic. In some instances, the nail was
shed and was not regrown (Samman and Johnston, 1969).
Of 42 reported exposures of man varying from one to 75 individual
exposures, four incidents of dermal abnormalities were reported. These
include rashes, blisters and a transient skin discolouration. In
almost all instances, these incidents were attributable to the
concentrated commercial preparation (Anonymous, 1966).
Poisoning cases in humans with bipyridyl compounds have demonstrated
the acute toxic effects of these compounds. In one case, the
accidental ingestion of a small quantity of diquat led to diarrhoea
and oral ulceration. After forced diuresis, the man recovered and was
released from care. It was observed that the urine contained traces of
diquat as long as eleven days after ingestion (Oreopoulos, 1969).
COMMENT
In biochemical studies in rats and dogs, it has been observed that
after oral administration, the major part of the dose is excreted in
the faeces. Following a subcutaneous dose, most residue was found in
the urine, indicating that absorption from the gut was relatively
poor. A small proportion of the material found in urine after oral
administration was other than the parent compound, due possibly to
absorption of microbial degradation products rather than actual animal
metabolism.
Diquat is a cataractogenic compound, as has been demonstrated in
lont-term rat and in dog experiments at relatively low levels of 2.5
and 5 mg/kg body-weight, respectively. Transient exposure of rats to
diquat does not lead to the formation of cataracts.
Intraperitoneal administration of 7 mg/kg diquat to pregnant rats
resulted in embryotoxic effects. In a three-generation rat
reproduction study, 500 ppm diquat resulted in no effect on
reproduction.
In man, the acute and dermal problems associated with accidental or
suicidal ingestion and dermal contamination appear to be of primary
concern. Cataract formation has not been observed in man as a result
of exposure to diquat, nevertheless studies in the prophylaxis and
treatment of diquat-induced cataracts in mammals were considered
urgent.
Non-reversible cataractogenic effects in rats and dogs at relatively
low oral levels, 50 ppm (2.5 mg/kg) and 200 ppm (5
mg/kg),reapectively, and a potential embryonic effect make it
advisable to establish only a temporary acceptable daily intake for
man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg body-weight/day
(corresponds to 0.36 mg diquat ion/kg body-weight/day)
Dog: 68 ppm in the diet, equivalent to 1.7 mg/kg body-weight/day
(corresponds to 1.22 mg diquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.0025 mg/kg body-weight as diquat dichloride (0-0.002 mg/kg
body-weight expressed as diquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Diquat is used world wide for the following purposes; desiccation of
beans, peas, sugar beet, sorghum, maize, rice etc; potato haulm
destruction; pre-emergence weed control in carrots, onions, hops,
vines and sugar-cane flower control; control of submerged and some
floating weeds in still water or streams, defoliant and desiccant for
cotton; desiccation of red and white clover, sunflower, linseed and
rape for seed; desiccant for barley and wheat for stock feed purposes.
FATE OF RESIDUES
Photodecomposition
The photodecomposition of diquat resulted in disappearance of
radioactivity with both the ring and ethylene bridge radioactivity
labelled indicating the occurrence of volatile breakdown products.
Approximately 75% of the material was lost with 96 hours of sunlight
exposure. The photo-chemical breakdown of diquat gives
1,2,3,4-tetrahydro-1-oxo-pyridyl 1,2-a-5-pyrazinium bromide.
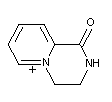 Further degradation of the molecule occurs in the presence of sunlight
presumably to volatile products (Funderburk and Bozarth, 1967; Slade
and Smith, 1967).
In soil
One of the most striking features of diquat is its rapid and complete
inactivation by soil. This inactivation results from a reaction
complex formed between the positively charged diquat cation and the
negatively charged sites on the clay minerals present in the soils.
In animals
Silage made from grass desiccated with diquat (13 ppm) was fed to farm
animals over long periods; no residues were detected in the animal
tissue or milk secreted during this time (Black et al., 1966). The
fate of 14C-diquat administered orally to cattle has been studied.
Three cows received single oral doses of 5 mg diquat dibromide/kg
body-weight and one cow received a single dose of 20 mg/kg. Milk
residues after seven days amounted to 0.001 to 0.015% of the
administered dose with the major residues occurring in three days of
dosing at the lower levels. At the higher dose levels, residues were
evident at all sampling intervals (seven days) with the major amounts
recovered within six days. Residues in urine varied from 0.4 to 2.6%
of the administered dose. Tissue residues, 24 hours after an oral
administration of 11.5 mg diquat dibromide/kg, were found primarily in
kidney (0.7 ppm) and liver (0.2 ppm) with slight residues in several
other tissues (<0.1 ppm). Chemical analysis of the liver and kidney
resulted in 0.01 and less than 0.03 ppm diquat, indicating that major
residues were not diquat but metabolic conversion products (Stevens
and Walley, 1966). Traces of metabolites found in the milk were
believed to arise from breakdown in the gut or in the rumen; Daniel
and Gage (1966) found similar breakdown in the rat. After dosing a
calf with 14C-diquat (5 ppm), less than 0.01 ppm of diquat or its
metabolites were found in the muscle tissue. Because of its use for
aquatic weed control, the fate of diquat in fish has been studied.
Radiotracer studies of the distribution of diquat in fish following
treatment of water indicated that the major residues occurred in the
digestive system, and the only residue was diquat (Funderburk and
Bozarth, 1967).
After exposure of rainbow trout to 1 ppm, diquat residues were found
in the viscera and skin but none in the flesh (Calderbank, 1968).
In plants
The uptake by foliage and extent of subsequent movement are critically
dependent on the environmental conditions. Traces of diquat were
detected in potato tubers after the tops had been killed with the
chemical (Calderbank et al., 1961; Headford et al., 1967). By means of
14C-labelled diquat, it has been shown that this transfer occurs in
the xylem. Smith et al., (1966) confirmed that darkness increased this
long-distance transport of diquat. Experiments with potato plants and
tubers have shown that even if metabolism occurred in the plant no
degradation products were transported to the tubers (Smith, 1967). No
significant loss of diquat residues was found after storage of potato
tubers for up to seven months. It has been assumed that since the
plants die rapidly in bright sunlight, significant quantities of the
breakdown products formed on the surface of dead tissues should not
move from these dead leaves to edible parts of the plant. This has
been partially demonstrated with potatoes, where it has been shown
that diquat residues in tubers from sprayed plants appear as the
unchanged diquat and not the photoderivative.
Diquat taken up from nutrient solution into plants was not metabolized
but remained as the parent compound (Funderburk and Lawrence, 1964).
In water
Diquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds, absorption by soil, silt and
particulate suspended matter, and, to a slight extent, by
photochemical degradation (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance is very variable, depending on the movement
of the water, the presence of mud or suspended matter, and the
strength of sunlight, but treatments within the range of 0.5 - 4
mg/litre in the water have resulted in less than 0.1 mg/litre being
detected in from four to 14 days after application. Decomposition of
the killed weed is rapid, any remaining residue of diquat thus
liberated being subsequently absorbed on the bottom mud. Such residues
in the largely organic muds may be more readily available to bacterial
degradation than when absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
A summary of the diquat residues in food crops, raw and processed in
wheat, flour and bread and also in barley, malt and beer is given in
Tables II, III and IV: (Calderbank, 1968).
METHODS OF RESIDUE ANALYSIS
The residue analysis of diquat is usually carried out colorimetrically
after extraction of the plant or animal tissue with boiling dilute
sulphuric acid, isolation by ion exchange column, elution, reduction
and the absorption of the derived coloured free radical measured at
377 nm. This method of Calderbank et al. (1961), was modified by
Kirston (1966), simplified by Calderbank and Yuen (1966) and adapted
by Black et al. (1966). Limit of sensitivity is about 0.01 ppm, and
the procedure should be suitable for regulatory purposes. Engelhardt
and McKinley (1966) determined the compound by polarography:
Coha and Koljcojic (1969) used a combination of TLC and ring oven.
Funderburk and Lawrence (1963) developed a sensitive bioassay
technique for residues in water using the lesser duckweed (Lemna
minor L.) and also Funderburk et al. (1966) used a thin-layer
chromatographic procedure for examining the photochemical
decomposition products of diquat in solution. Faust and Hunter (1965)
determined diquat in natural surface waters by absorptiometric
measurements at the wavelength of maximum absorption of the unreduced
herbicide (310 nm) following clean-up by ion-exchange.
TABLE II
Summary of residues in food crops (dessiccation uses)
Average Residues
3-21 days after
Rate of application,
CROP Application mg/kg
lb/acre DIQUAT
Barley 0.5-1.0 0.5-4.0
Wheat, rape seed 0.5-1.0 ND-1.3
Maize 0.5-1.2 ND
Rice (with husk) 0.15-0.54 0.7-5.0
Rice (dehusked or polished) 0.15-0.54 ND
Peas, beans, sunflower seed 0.35-1.2 ND-0.2
Sorghum seed 0.25-1.0 0.2-0.8
Cotton (as picked) 0.5-1.0 0.05-0.5
Onions 0.5-2.0 0.02-0.05
Potatoes 0.5-1.5 ND-0.04
Sugar cane juice 0.5-2.0 ND
Seed oils (sesame, sunflower,
rape, cotton) up to 1.2 ND
ND - not detected
TABLE III
Residues in wheat, flour and bread
(Wheat desiccated with diquat at 0.7 lb/acre)
Sample Residues of diquat
(mg/kg)
Wheat (at harvest) 1 (approx)
Wheat (cleaned for milling) 0.6-0.7
White flour 0-0.1
Bread 0.1
Bran 1.2-2.4
TABLE IV
Residues in barley, malt and beer
Intervals between Diquat residues found,
application and harvest mg/kg
(0.68 lb diquat/acre applied) Barley Malt Beer
4 days 4.24 2.05 0.12
5 days 2.35 1.07 0.04
10 days 0.94 0.61 0.02
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
United States of America Sugarcane 0.05
(negligible
residue)
APPRAISAL
Diquat is very widely used as a desiccant - e.g. for rice, clover,
rape, linseed, peas, beans, maize, potato haulm, etc. and as a
pre-emergence herbicide in carrots, onions, hops, vines, etc.; it is
also used for aquatic weed control. Residues are very unlikely to
accrue from soil or pre-emergence applications but can occur following
use for desiccation purposes. Residues in seed from such desiccated
crops vary from negligible in such well-protected seeds as maize to
about 5 ppm in rice (husk). Barley and wheat for stock feed or seed
purposes can contain up to 5 and 2 ppm, respectively, of diquat
following desiccation uses. Residues often occur in treated oil seeds,
such as sunflower, sesame and cotton, but no residues are observed in
the expressed oil. The spectrophotometric procedure should be suitable
for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Rice (with husk) 5 ppm
Rape seed, sorghum seed 2 ppm
Peas, beans, sunflower seed 0.5 ppm
Onions, potatoes, maize, rice (polished) 0.1 ppm
Sesame, sunflower, rape, cotton seed oils 0.1 ppm
Information given also on:
Cotton (as picked) 1 ppm
In cereal grains for stock feed or seed purposes only, the following
levels should also be accepted:
Barley 5 ppm
Wheat 2 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Further studies on the mechanism of cataractogenesis in animals.
2. A three-generation reproduction study where there is exposure to
diquat during the entire duration of the experiment.
DESIRABLE
Clinical studies on factory workers and users of diquat in order to
detect the extent of cataractogenic risk.
REFERENCES
Anonymous. (1966) Unpublished report 29 December 1966 from ICI Ltd.
through Chevron Chemical Co. to FDA
Benes, V. and Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Industr. Med Surg., 38: 442-444
Black, W.J.M., Calderbank, A., Douglas, G. and McKenna, R.H.J. (1966)
Residues in herbage and silage and feeding experiments following the
use of diquat as a desiccant. J.Sci.Fd. Agr., 17: 506-509
Calderbank, A., Morgan, C.B. and Yuen, S.H. (1961) Determination of
diquat residues in potato tubers. Analyst, 86: 569-579
Calderbank, A. and Yuen, S.H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629
Calderbank, A. (1968) The bipyridylium herbicides. Advances in pest
control research, 8: 127-235
Clark, D.G. and Hurst, E.W. (1970) The toxicity of diquat. Brit. J.
Ind. Med., 27: 51-55
Coha, F. and Koljcojic, R. (1969) Combination of thin-layer
chromatography and the ring oven method for the semi-quantitative
estimation of some herbicides. J. Chromatog., 40: 304-307
Cooke, E. and Gage, J.C. (1956) Toxicological report. Unpublished
report TR/35 15 March 1956 for ICI Ltd. through Chevron Chemical Co.
to FDA
Cookson, J.H. and McElligott. (1966) The dermal toxicity of diquat.
Unpublished report IHR. 200 from ICI Ltd. through Chevron Chemical Co.
to FDA
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. Indust. Med. J., 23: 133-136
Engelhardt, J. and McKinley, W.P. (1966) Bypyridylium herbicides.
Polarography of 1,1'-ethylene-2,2'-bipyridylium dibromide. J. Agr. Fd.
Chem., 14: 377-380
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the detection
of aquatic herbicides. J. Am. Water Works Assoc., 57: 1028-1037
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Lawrence, J.M. (1964) Mode of action of diquat
and paraquat. Weeds, 12: 259-264
Funderburk, H.H., Negi, N.S. and Lawrence, J.M. (1966) Photochemical
decomposition of diquat and paraquat. Weeds, 14: 240-243
Funderburk, H.H. and Bozarth. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agr. Fd. Chem., 15: 563-567
Gage, J.C. (1967) The toxicity of diquat aerosols. Unpublished report
IHR/212 (March 1967) from ICI Ltd. through Chevron Chemical Co. to FDA
Gage, J.C. (1968) The action of paraquat and diquat on the respiration
of liver cell fraction. Biochem. J., 109: 757-761
Goater, T.O., Kenyon, A.J. and Hurst, E.W. (1964) The two-year test
with diquat dichloride monohydrate in rats. Unpublished report IHR/165
(Nov. 1964) ICI Ltd.
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with diquat monohydrate in the diet.
Unpublished report IHR/188 (Jan. 1966) from ICI Ltd. through Chevron
Chemical Co. to FDA
Headford, D.W.R. and Douglas, G. (1967) Tuber necrosis following the
desiccation of potato foliage with diquat. Weed Res., 7: 131-144
Hurst, E.W. (1966) A long-term toxicity test with diquat dichloride
monohydrate in dogs. Unpublished report IHR/195 (May 1966) from ICI
Ltd. through Chevron Chemical Co. to FDA
Kirston, W.J. (1966) The determination of diquat residues in potato
tubers. Analyst, 91: 732-738
Khera, K.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965a) Two-year chronic oral
toxicity of diquat - albino rats 1000, 500 and 250 ppm (27 February
1965). Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.D. (1965b) Two-year chronic oral
toxicity of diquat - albino rats 100 and 10 ppm (27 February 1965).
Addendum report - Unpublished report from Industrial Bio-Test Lab.
Inc. through ICI and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965c) Two-year chronic oral
toxicity of diquat - albino rats, 50 ppm (27 February 1965). Addendum
report. Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Oreopoulos, D.G. and McEvoy, J. (1969) Diquat poisoning. Post-Grad.
Med. J., 45: 635-637
Rees, J.R. (1969) Reaction between lens constituents and substances
which cause cataracts. Thesis for B.Sc. Degree, University of Oxford
cited by Conning et al. (1969)
Samman, P.D. and Johnston, E.K. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report from ICI Ltd. through Chevron Chemical Co.
to FDA
Slade, P. and Smith, A.E. (1967) Photochemical degradation of diquat.
Nature, 213: 919-920
Smith, J.M. and Sagar, G.R. (1966) Re-examination of the influence of
light and darkness on the long distance transport of diquat in
Lycopersicon esculentum. Weed Res., 6: 314-321
Smith, A.E. (1967) Residues in potato tubers following haulm
desiccation with 14C-diquat. Bull. Environ. Contam. Toxicol., 169-177
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Fd. Agric., 17: 472-475
Swan, A.A.B (1960) Toxicological report. Unpublished report TR/35
Supplement (7 June 1960) from ICI Ltd. through Chevron Chemical Co. to
FDA
Swan, A.A.B. (1962) Unpublished interim toxicological report No:
TR/375 (5 July 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963a) Diquat-oral toxicity to mice. Unpublished report
(24 January 1963) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963b) The dermal toxicity of diquat dichloride.
Unpublished report TR/540 (20 June 1963) from ICI Ltd. through Chevron
Chemical Co. to FDA
Walley, J.K. (1962) Diquat toxicity in cattle. Unpublished report
IHR/167 (Nov. 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Further degradation of the molecule occurs in the presence of sunlight
presumably to volatile products (Funderburk and Bozarth, 1967; Slade
and Smith, 1967).
In soil
One of the most striking features of diquat is its rapid and complete
inactivation by soil. This inactivation results from a reaction
complex formed between the positively charged diquat cation and the
negatively charged sites on the clay minerals present in the soils.
In animals
Silage made from grass desiccated with diquat (13 ppm) was fed to farm
animals over long periods; no residues were detected in the animal
tissue or milk secreted during this time (Black et al., 1966). The
fate of 14C-diquat administered orally to cattle has been studied.
Three cows received single oral doses of 5 mg diquat dibromide/kg
body-weight and one cow received a single dose of 20 mg/kg. Milk
residues after seven days amounted to 0.001 to 0.015% of the
administered dose with the major residues occurring in three days of
dosing at the lower levels. At the higher dose levels, residues were
evident at all sampling intervals (seven days) with the major amounts
recovered within six days. Residues in urine varied from 0.4 to 2.6%
of the administered dose. Tissue residues, 24 hours after an oral
administration of 11.5 mg diquat dibromide/kg, were found primarily in
kidney (0.7 ppm) and liver (0.2 ppm) with slight residues in several
other tissues (<0.1 ppm). Chemical analysis of the liver and kidney
resulted in 0.01 and less than 0.03 ppm diquat, indicating that major
residues were not diquat but metabolic conversion products (Stevens
and Walley, 1966). Traces of metabolites found in the milk were
believed to arise from breakdown in the gut or in the rumen; Daniel
and Gage (1966) found similar breakdown in the rat. After dosing a
calf with 14C-diquat (5 ppm), less than 0.01 ppm of diquat or its
metabolites were found in the muscle tissue. Because of its use for
aquatic weed control, the fate of diquat in fish has been studied.
Radiotracer studies of the distribution of diquat in fish following
treatment of water indicated that the major residues occurred in the
digestive system, and the only residue was diquat (Funderburk and
Bozarth, 1967).
After exposure of rainbow trout to 1 ppm, diquat residues were found
in the viscera and skin but none in the flesh (Calderbank, 1968).
In plants
The uptake by foliage and extent of subsequent movement are critically
dependent on the environmental conditions. Traces of diquat were
detected in potato tubers after the tops had been killed with the
chemical (Calderbank et al., 1961; Headford et al., 1967). By means of
14C-labelled diquat, it has been shown that this transfer occurs in
the xylem. Smith et al., (1966) confirmed that darkness increased this
long-distance transport of diquat. Experiments with potato plants and
tubers have shown that even if metabolism occurred in the plant no
degradation products were transported to the tubers (Smith, 1967). No
significant loss of diquat residues was found after storage of potato
tubers for up to seven months. It has been assumed that since the
plants die rapidly in bright sunlight, significant quantities of the
breakdown products formed on the surface of dead tissues should not
move from these dead leaves to edible parts of the plant. This has
been partially demonstrated with potatoes, where it has been shown
that diquat residues in tubers from sprayed plants appear as the
unchanged diquat and not the photoderivative.
Diquat taken up from nutrient solution into plants was not metabolized
but remained as the parent compound (Funderburk and Lawrence, 1964).
In water
Diquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds, absorption by soil, silt and
particulate suspended matter, and, to a slight extent, by
photochemical degradation (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance is very variable, depending on the movement
of the water, the presence of mud or suspended matter, and the
strength of sunlight, but treatments within the range of 0.5 - 4
mg/litre in the water have resulted in less than 0.1 mg/litre being
detected in from four to 14 days after application. Decomposition of
the killed weed is rapid, any remaining residue of diquat thus
liberated being subsequently absorbed on the bottom mud. Such residues
in the largely organic muds may be more readily available to bacterial
degradation than when absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
A summary of the diquat residues in food crops, raw and processed in
wheat, flour and bread and also in barley, malt and beer is given in
Tables II, III and IV: (Calderbank, 1968).
METHODS OF RESIDUE ANALYSIS
The residue analysis of diquat is usually carried out colorimetrically
after extraction of the plant or animal tissue with boiling dilute
sulphuric acid, isolation by ion exchange column, elution, reduction
and the absorption of the derived coloured free radical measured at
377 nm. This method of Calderbank et al. (1961), was modified by
Kirston (1966), simplified by Calderbank and Yuen (1966) and adapted
by Black et al. (1966). Limit of sensitivity is about 0.01 ppm, and
the procedure should be suitable for regulatory purposes. Engelhardt
and McKinley (1966) determined the compound by polarography:
Coha and Koljcojic (1969) used a combination of TLC and ring oven.
Funderburk and Lawrence (1963) developed a sensitive bioassay
technique for residues in water using the lesser duckweed (Lemna
minor L.) and also Funderburk et al. (1966) used a thin-layer
chromatographic procedure for examining the photochemical
decomposition products of diquat in solution. Faust and Hunter (1965)
determined diquat in natural surface waters by absorptiometric
measurements at the wavelength of maximum absorption of the unreduced
herbicide (310 nm) following clean-up by ion-exchange.
TABLE II
Summary of residues in food crops (dessiccation uses)
Average Residues
3-21 days after
Rate of application,
CROP Application mg/kg
lb/acre DIQUAT
Barley 0.5-1.0 0.5-4.0
Wheat, rape seed 0.5-1.0 ND-1.3
Maize 0.5-1.2 ND
Rice (with husk) 0.15-0.54 0.7-5.0
Rice (dehusked or polished) 0.15-0.54 ND
Peas, beans, sunflower seed 0.35-1.2 ND-0.2
Sorghum seed 0.25-1.0 0.2-0.8
Cotton (as picked) 0.5-1.0 0.05-0.5
Onions 0.5-2.0 0.02-0.05
Potatoes 0.5-1.5 ND-0.04
Sugar cane juice 0.5-2.0 ND
Seed oils (sesame, sunflower,
rape, cotton) up to 1.2 ND
ND - not detected
TABLE III
Residues in wheat, flour and bread
(Wheat desiccated with diquat at 0.7 lb/acre)
Sample Residues of diquat
(mg/kg)
Wheat (at harvest) 1 (approx)
Wheat (cleaned for milling) 0.6-0.7
White flour 0-0.1
Bread 0.1
Bran 1.2-2.4
TABLE IV
Residues in barley, malt and beer
Intervals between Diquat residues found,
application and harvest mg/kg
(0.68 lb diquat/acre applied) Barley Malt Beer
4 days 4.24 2.05 0.12
5 days 2.35 1.07 0.04
10 days 0.94 0.61 0.02
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
United States of America Sugarcane 0.05
(negligible
residue)
APPRAISAL
Diquat is very widely used as a desiccant - e.g. for rice, clover,
rape, linseed, peas, beans, maize, potato haulm, etc. and as a
pre-emergence herbicide in carrots, onions, hops, vines, etc.; it is
also used for aquatic weed control. Residues are very unlikely to
accrue from soil or pre-emergence applications but can occur following
use for desiccation purposes. Residues in seed from such desiccated
crops vary from negligible in such well-protected seeds as maize to
about 5 ppm in rice (husk). Barley and wheat for stock feed or seed
purposes can contain up to 5 and 2 ppm, respectively, of diquat
following desiccation uses. Residues often occur in treated oil seeds,
such as sunflower, sesame and cotton, but no residues are observed in
the expressed oil. The spectrophotometric procedure should be suitable
for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Rice (with husk) 5 ppm
Rape seed, sorghum seed 2 ppm
Peas, beans, sunflower seed 0.5 ppm
Onions, potatoes, maize, rice (polished) 0.1 ppm
Sesame, sunflower, rape, cotton seed oils 0.1 ppm
Information given also on:
Cotton (as picked) 1 ppm
In cereal grains for stock feed or seed purposes only, the following
levels should also be accepted:
Barley 5 ppm
Wheat 2 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Further studies on the mechanism of cataractogenesis in animals.
2. A three-generation reproduction study where there is exposure to
diquat during the entire duration of the experiment.
DESIRABLE
Clinical studies on factory workers and users of diquat in order to
detect the extent of cataractogenic risk.
REFERENCES
Anonymous. (1966) Unpublished report 29 December 1966 from ICI Ltd.
through Chevron Chemical Co. to FDA
Benes, V. and Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Industr. Med Surg., 38: 442-444
Black, W.J.M., Calderbank, A., Douglas, G. and McKenna, R.H.J. (1966)
Residues in herbage and silage and feeding experiments following the
use of diquat as a desiccant. J.Sci.Fd. Agr., 17: 506-509
Calderbank, A., Morgan, C.B. and Yuen, S.H. (1961) Determination of
diquat residues in potato tubers. Analyst, 86: 569-579
Calderbank, A. and Yuen, S.H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629
Calderbank, A. (1968) The bipyridylium herbicides. Advances in pest
control research, 8: 127-235
Clark, D.G. and Hurst, E.W. (1970) The toxicity of diquat. Brit. J.
Ind. Med., 27: 51-55
Coha, F. and Koljcojic, R. (1969) Combination of thin-layer
chromatography and the ring oven method for the semi-quantitative
estimation of some herbicides. J. Chromatog., 40: 304-307
Cooke, E. and Gage, J.C. (1956) Toxicological report. Unpublished
report TR/35 15 March 1956 for ICI Ltd. through Chevron Chemical Co.
to FDA
Cookson, J.H. and McElligott. (1966) The dermal toxicity of diquat.
Unpublished report IHR. 200 from ICI Ltd. through Chevron Chemical Co.
to FDA
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. Indust. Med. J., 23: 133-136
Engelhardt, J. and McKinley, W.P. (1966) Bypyridylium herbicides.
Polarography of 1,1'-ethylene-2,2'-bipyridylium dibromide. J. Agr. Fd.
Chem., 14: 377-380
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the detection
of aquatic herbicides. J. Am. Water Works Assoc., 57: 1028-1037
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Lawrence, J.M. (1964) Mode of action of diquat
and paraquat. Weeds, 12: 259-264
Funderburk, H.H., Negi, N.S. and Lawrence, J.M. (1966) Photochemical
decomposition of diquat and paraquat. Weeds, 14: 240-243
Funderburk, H.H. and Bozarth. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agr. Fd. Chem., 15: 563-567
Gage, J.C. (1967) The toxicity of diquat aerosols. Unpublished report
IHR/212 (March 1967) from ICI Ltd. through Chevron Chemical Co. to FDA
Gage, J.C. (1968) The action of paraquat and diquat on the respiration
of liver cell fraction. Biochem. J., 109: 757-761
Goater, T.O., Kenyon, A.J. and Hurst, E.W. (1964) The two-year test
with diquat dichloride monohydrate in rats. Unpublished report IHR/165
(Nov. 1964) ICI Ltd.
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with diquat monohydrate in the diet.
Unpublished report IHR/188 (Jan. 1966) from ICI Ltd. through Chevron
Chemical Co. to FDA
Headford, D.W.R. and Douglas, G. (1967) Tuber necrosis following the
desiccation of potato foliage with diquat. Weed Res., 7: 131-144
Hurst, E.W. (1966) A long-term toxicity test with diquat dichloride
monohydrate in dogs. Unpublished report IHR/195 (May 1966) from ICI
Ltd. through Chevron Chemical Co. to FDA
Kirston, W.J. (1966) The determination of diquat residues in potato
tubers. Analyst, 91: 732-738
Khera, K.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965a) Two-year chronic oral
toxicity of diquat - albino rats 1000, 500 and 250 ppm (27 February
1965). Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.D. (1965b) Two-year chronic oral
toxicity of diquat - albino rats 100 and 10 ppm (27 February 1965).
Addendum report - Unpublished report from Industrial Bio-Test Lab.
Inc. through ICI and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965c) Two-year chronic oral
toxicity of diquat - albino rats, 50 ppm (27 February 1965). Addendum
report. Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Oreopoulos, D.G. and McEvoy, J. (1969) Diquat poisoning. Post-Grad.
Med. J., 45: 635-637
Rees, J.R. (1969) Reaction between lens constituents and substances
which cause cataracts. Thesis for B.Sc. Degree, University of Oxford
cited by Conning et al. (1969)
Samman, P.D. and Johnston, E.K. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report from ICI Ltd. through Chevron Chemical Co.
to FDA
Slade, P. and Smith, A.E. (1967) Photochemical degradation of diquat.
Nature, 213: 919-920
Smith, J.M. and Sagar, G.R. (1966) Re-examination of the influence of
light and darkness on the long distance transport of diquat in
Lycopersicon esculentum. Weed Res., 6: 314-321
Smith, A.E. (1967) Residues in potato tubers following haulm
desiccation with 14C-diquat. Bull. Environ. Contam. Toxicol., 169-177
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Fd. Agric., 17: 472-475
Swan, A.A.B (1960) Toxicological report. Unpublished report TR/35
Supplement (7 June 1960) from ICI Ltd. through Chevron Chemical Co. to
FDA
Swan, A.A.B. (1962) Unpublished interim toxicological report No:
TR/375 (5 July 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963a) Diquat-oral toxicity to mice. Unpublished report
(24 January 1963) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963b) The dermal toxicity of diquat dichloride.
Unpublished report TR/540 (20 June 1963) from ICI Ltd. through Chevron
Chemical Co. to FDA
Walley, J.K. (1962) Diquat toxicity in cattle. Unpublished report
IHR/167 (Nov. 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
 Other relevant chemical properties
Diquat is available only as a salt, generally as the dibromide. The
cationic portion of the molecule is the active ingredient. Diquat
dibromide exists as the monohydrate and forms white to yellow crystals
which decompose above 300°C. The technical material is available only
as a dark reddish-brown aqueous solution. Solubility in water at 20°C
is 700 g/litre: it is slightly soluble in alcoholic and hydroxylic
solvents and practically insoluble in non-polar organic solvents,
stable in acid or neutral solutions and unstable under alkaline
conditions. One electron reduction by zinc or sodium dithionite yields
the green free-radical which in the presence of atmospheric oxygen
reverts to diquat. The single electron reduction is completely
reversible, and the redox potential of - 349 mV is independent of pH.
Further reduction yields polyhydrobipyridyl derivatives.
Solutions of the free radical exhibit a sharp absorption peak at 379
nm which has a greater intensity than that of the unreduced diquat at
310 nm. Concentrated aqueous solutions corrode steel, tinplate,
galvanised iron and aluminium.
Purity
Technical, 95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Dogs administered orally 10-15 mg 14C-labelled diquat dibromide
excreted 29-32 percent in the urine and 51-62 percent in the faeces in
72 hours. In the first 24 hours, 25-28 percent of the dose was
excreted in the urine (Swan, 1960).
Following oral doses of 5-10 mg/kg to rats, diquat dibromide was
eliminated entirely with 96-101 percent recovery within four days, the
levels in the urine were 4-6 percent and in the faeces 90-96 percent
(Daniel and Gage, 1966). Following subcutaneous doses of 5 or 6 mg/kg
to rats, diquat dibromide was eliminated again within four days with
90-98 percent recovery-in urine 88-98 percent and in faeces 0-2
percent. In all studies, the bulk of the residues excreted following
oral administration was in the faeces, whereas after a subcutaneous
dose most was in the urine, indicating that absorption from the gut
was relatively poor. Studies where chemical rather than radiological
measurements were made showed that following an oral dose of 30-40
mg/kg to rats, only 4-7 percent of the dose was recovered in the urine
and 11-42 percent in the faeces after 48 hours (Swan, 1960).
The percentage excretion of an oral dose in the urine, calculated from
a chemical method of analysis, was lower than that obtained from a
radio-analytical method of analysis, suggesting that a proportion of
the dose appeared in a form other than diquat. Following a
subcutaneous dose, the radiochemical and chemical analyses gave
results which were closer than the values following an oral dose,
indicating possible absorption from the gut of microbial degradation
products following oral administration, rather than actual animal
metabolism (Swan, 1960).
In vitro experiments using suspensions of gut material suggested
that microbial breakdown of diquat was responsible for the low
recoveries by the chemical assay where only the parent ion was
measured (Swan, 1960).
Effects on enzymes and other biochemical parameters
Although there is no direct evidence in mammals to support the view,
it is tempting to assume that the ability of bipyridyls to be reduced
and re-oxidized with the production of free radicals is linked with
their toxic effects, as has been suggested to be the case in plants.
Gage (1968) has shown that free radicals can be produced from diquat
incubated anaerobically in the presence of NADPH and microsomes
derived from rat liver. Diquat also increased the respiration of liver
mitochondrial fragments. This action has been related to the activity
of flavo-protein dehydrogenases. Purified lipoamide dehydrogenase from
pig heart was able to reduce diquat to the free radical in the
presence of NADH. Rees (1969) has shown that a fresh extract of bovine
lens, incubated anaerobically with diquat, can catalyze similar
reactions. From these experiments, there can be little doubt that
flavo-proteins of animal tissues in the presence of their
co-substrates can reduce the bipyridyls, although aerobically the
equilibrium concentration of the free radical is probably extremely
low.
The property that diquat has of undergoing cyclic reduction and
oxidation might suggest that it could interfere in electron-transport
processes, diverting electrons from the system and reducing oxygen to
water. Gage (1968) found that the resting respiration of mitochondria
was almost unaffected by diquat, probably because of its inability to
penetrate the mitochondrial membrane.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females) were examined for
reproduction and teratogenic effects of diquat dichloride at 0, 125
and 500 ppm in the diet (Griffiths et al., 1966). This experiment
examined the effects of dietary feeding of diquat to males only,
females only and both males and females at 500 ppm and males and
females at 125 ppm. Growth of the parent rats was moderately retarded
at 500 ppm, and all parents developed cataracts. No effect on
reproduction or occurrence of terata was observed. At 125 ppm, no
effect on the growth of parents was noted. Lens opacities did not
occur. Of all offspring produced (1637), one female at 500 ppm had a
unilateral cataract.
A single intraperitoneal injection of diquat (7 mg/kg from day six
through 14 of gestation) produced a high incidence of retarded growth
of sternum and auditory ossicles, as well as marked weight reduction
in rat embryos. A higher dose of 14 mg/kg interrupted most
pregnancies, and in the rats that reached term, the embryonic effects
were more pronounced (Khera and Whitta, 1968).
Special studies for mutagenicity
Diquat was screened for mutagenic activity in Drosophila
melanogaster using the Muller-5 test to detect recessive lethality
on the X chromosome. Compared to a spontaneous mutation rate of 0.14
percent, the rate was 0.11 percent after treatment with diquat, which
is comparable with the control value (Benes and Sram, 1969).
Special studies on cataractogenicity
The initial cataractogenic effects in rats of diquat appear to be
reversible, as was noted in a study where rats were fed a diet
containing 500 ppm diquat. This dose was shown to produce cataracts in
three to six months. After feeding continuously for periods of several
days to eight weeks, the rats were given a normal diquat-free diet for
the remainder of one year. Cataracts did not develop in any of these
rats, indicating that continuous prolonged exposure to diquat was
necessary for the formation of cataracts in the rat. Temporary
exposure to this known cataractogen does not lead to irreversible
damage (Clark and Hurst, 1970).
Since it is known that some forms of cataract are influenced by light
and that the toxicity of diquat to plants is dependent upon light,
experiments were undertaken to study the effect of light on cataract
formation in rats. Rats were fed 500 ppm diquat in the dark for three
months and controls were fed the same diet under light conditions.
After three months feeding on these diets, both groups of animals
showed an equal number of cataracts, suggesting that light does not
influence the development of cataract formation (Clark and Hurst,
1970).
Ascorbic acid (200 mg/ml) in the drinking water of rats receiving
diquat (500 ppm) in the diet did not influence the development of
cataracts (Clark and Hurst, 1970.)
See also "Short" and "Long-term Studies" and "Reproduction". LD50
values for diquat in various species are summarized in Table I.
A level of 90 ppm diquat at 18°C killed 50 percent of a population of
rainbow trout within 48 hours. At 70 ppm, 50 percent mortality was
observed in 48 hours (Swan, 1960).
A single dermal application of 10 or 20 mg of diquat to rats as an
aqueous solution produced a slight reversible erythema in the treated
area (Cooke and Gage, 1956).
Acute dermal administration of diquat at doses of 500 and 1000 mg/kg
to rabbits resulted in toxic signs within 48-72 hours at 1000 mg/kg
and no abnormalities at 500 mg/kg (Swan, 1963b).
Installation of 10 mg diquat of a 10 percent aqueous solution into the
conjuctival sac of rabbits produced no effect (Cook and Gage, 1956).
Short-term studies
Rat
Three groups of rats (four males and four females) were exposed to
diquat in aerosol form at levels of 0.5, 1.06 and 2 µg/litre for 15
daily six-hour exposure periods. At the 2 µg/litre level, weight gain
was slightly reduced. No such effect was noted at the 1.06 and 0.5
µg/litre levels. Histopathological examination of the lungs showed
slight irritation with peribronchial lymphoid hyperplasia,
perivascular oedema and macrophages in the alveoli at the 2 µg/litre
level (Gage, 1967).
TABLE I
LD50 values of diquat in various species
Salt LD50
Species Route Form (mg Reference
cation/kg)
Mouse Oral Cl 125 Swan, 1963a
Br 125 Clark and Hurst, 1970
Rat Oral Cl 302 Swan, 1962
Br 215-235 Clark and Hurst, 1970
Swan, 1960
s.c. Cl 11 Clark and Hurst, 1970
Br 11-20 Clark and Hurst, 1970
Cook and Gage, 1956
Guinea Pig Oral Br 100 Clark and Hurst, 1970
Rabbit Oral Br 100 Swan, 1960
Clark and Hurst, 1970
Dermal Br 400 Swan, 1963b
Cookson and McElligott, 1966
i.p. Br 15 " " "
Hen Oral Br 215-430 Swan, 1960
Br 200-400 Clark and Hurst, 1970
Dog Oral Br 100-200 Clark and Hurst, 1970
Cow Oral Br approx. 30 Walley, 1962
Clark and Hurst, 1970
Subcutaneous administration of 1 mg diquat/kg body-weight to rats for
21 days produced no toxic effects; post-mortem examination showed no
organic damage (Cooke and Gage, 1956).
Rabbit
Groups of rabbits (three males and three females) were administered
diquat percutaneously at doses of 0, 20, 40, 80 and 160 mg diquat
ion/kg body-weight for 20 days. At 20 mg/kg, no deaths occurred,
whereas, at 40 mg/kg and above, from 66 to 100 percent of the animals
died.
Microscopic examination revealed vacuolar changes in the distal
convoluted renal tubules with occasional necrosis of cells. These
changes were preceded by weight loss and muscular weakness (Cookson
and McElligott, 1966).
Groups of five female and five male rabbits were administered up to 20
daily percutaneous doses of diquat at O, 3.13, 6.25, 12.5 and 25
mg/kg. The calculated LD50 was 7.9 mg/kg (5.7 mg ion/kg). Signs of
poisoning included: local hyperaemic and subcutaneous oedema;
increasing sloughing of surface layers of skin followed by scab
formation. These effects were reversible at the 6.25 mg/kg dose after
treatment was concluded. Other signs included weakness, incoordination
and lethargy. In many animals, ulceration of the gastric mucosa was
observed at post-mortem indicating that oral contamination had
presumably occurred (Swan, 1963b).
Dog
Groups of dogs (three males and three females) were fed diets
containing 0, 16, 32, 68, 200 and 600 ppm diquat dichloride for
periods up to four years. At dosages of 600 and 200 ppm, bilateral
opacities of the lens were observed at ten and 15 months,
respectively. Dietary levels of 68 ppm did not affect the lens within
the four year interval tested. No effects were observed on growth,
tumour formation, food consumption, blood chemistry and gross and
microscopic pathology. The dose of 68 ppm (equivalent to 50 ppm diquat
ion) is the no-effect level in dogs with regard to cataract formation
(Hurst, 1966).
Sheep and calf
Five groups of two sheep (one male and one female, eight months old)
and three groups of one calf each were given diquat at doses of 0, 1,
5, 10 and 20 ppm and 0, 5 and 20 ppm, respectively, in their drinking
water for one month. These levels caused no toxicological effect over
the trial period as evidenced by growth, food consumption and visual
observation (Sarfaty, 1963).
Cow
Oral administration of diquat for five days at 10 mg/kg body-weight to
a cow resulted in death within 15 days preceded by severe signs of
poison, including dullness, inappetence anaemia, increased heart rate.
At post-mortem, heart and kidney infarcts and intestinal catarrh were
found. Administration of 5 mg diquat/kg daily for fourteen days to a
cow (in the diet for two days, then drenched) resulted in increased
inappetence after two days, slight haemorrhage as noted by blood in
the faeces and temporary impairment of vision (Walley, 1962).
Comparative
Groups of four male and four female mice and guinea pigs, two female
rabbits and a male dog, exposed to 15 daily six-hour treatments with
diquat in aerosol form at 1.06 mg/litre, showed no adverse effects
(Gage, 1967).
Long-term studies
Rat
Groups of rats (25 males and 25 females per group) were fed diquat
dichloride for two years at doses of 0.125, 250, 500 or 1000 ppm in
the diet. After 56 days, the 1000 ppm level was discontinued because
of lack of growth and mortality in males and females. At 125 ppm, a
partial lens opacity (cataract) was seen at 207 days. All males and 19
out of 21 females were affected by 657 days. At 250 and 500 ppm, lens
opacities were produced in all animals within 155 and 124 days,
respectively. At 500 ppm, a reduction of female body-weight was
apparent after 20 weeks and in males after five weeks. No such effects
were noted at 250 and 125 ppm. No adverse effects were reported in
survival, blood chemistry, tumour, incidence and gross and microscopic
pathology (Swan, 1962; Goater et al., 1964).
Seven groups of rats (25 males and 25 females/group; controls had 75
and 75 females) were fed diquat dichloride at levels of 0,10, 50, 100,
250, 500 or 1000 ppm for two years. Growth depression was observed in
the males at 1000 ppm. Lens opacity was observed at doses of 50 ppm
and above in both males and females (no such effect was noted at the
10 ppm level). Growth, food consumption survival, tumour formation,
behaviour, haematological and urine analyses and gross and microscopic
examination revealed no effects differing from the controls. A
no-effect level based upon cataract formation is 10 ppm, equivalent to
7.2 ppm diquat cation (Kohn et al., 1965a, b and c).
OBSERVATIONS IN MAN
Damage and discolouration of fingernails caused by exposure to
concentrated solutions of diquat were observed in three instances. The
cause of the damage is unknown but presumably is of a local nature.
All three patients had frequent exposure to the concentrated chemical
without taking precautions to prevent contamination of the skin. The
cause of the nail damage was unknown, but it seemed probable that the
chemical reached the nail matrix by entering the nail fold and
stimulated infection, interfering with the formation of the nail from
the matrix. The damage is presumed to be local and not as result of
ingestion because of asymmetry of the lesions and the fact that the
toenails were unaffected. A curious colour change and softening of the
nail at the base are characteristic. In some instances, the nail was
shed and was not regrown (Samman and Johnston, 1969).
Of 42 reported exposures of man varying from one to 75 individual
exposures, four incidents of dermal abnormalities were reported. These
include rashes, blisters and a transient skin discolouration. In
almost all instances, these incidents were attributable to the
concentrated commercial preparation (Anonymous, 1966).
Poisoning cases in humans with bipyridyl compounds have demonstrated
the acute toxic effects of these compounds. In one case, the
accidental ingestion of a small quantity of diquat led to diarrhoea
and oral ulceration. After forced diuresis, the man recovered and was
released from care. It was observed that the urine contained traces of
diquat as long as eleven days after ingestion (Oreopoulos, 1969).
COMMENT
In biochemical studies in rats and dogs, it has been observed that
after oral administration, the major part of the dose is excreted in
the faeces. Following a subcutaneous dose, most residue was found in
the urine, indicating that absorption from the gut was relatively
poor. A small proportion of the material found in urine after oral
administration was other than the parent compound, due possibly to
absorption of microbial degradation products rather than actual animal
metabolism.
Diquat is a cataractogenic compound, as has been demonstrated in
lont-term rat and in dog experiments at relatively low levels of 2.5
and 5 mg/kg body-weight, respectively. Transient exposure of rats to
diquat does not lead to the formation of cataracts.
Intraperitoneal administration of 7 mg/kg diquat to pregnant rats
resulted in embryotoxic effects. In a three-generation rat
reproduction study, 500 ppm diquat resulted in no effect on
reproduction.
In man, the acute and dermal problems associated with accidental or
suicidal ingestion and dermal contamination appear to be of primary
concern. Cataract formation has not been observed in man as a result
of exposure to diquat, nevertheless studies in the prophylaxis and
treatment of diquat-induced cataracts in mammals were considered
urgent.
Non-reversible cataractogenic effects in rats and dogs at relatively
low oral levels, 50 ppm (2.5 mg/kg) and 200 ppm (5
mg/kg),reapectively, and a potential embryonic effect make it
advisable to establish only a temporary acceptable daily intake for
man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg body-weight/day
(corresponds to 0.36 mg diquat ion/kg body-weight/day)
Dog: 68 ppm in the diet, equivalent to 1.7 mg/kg body-weight/day
(corresponds to 1.22 mg diquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.0025 mg/kg body-weight as diquat dichloride (0-0.002 mg/kg
body-weight expressed as diquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Diquat is used world wide for the following purposes; desiccation of
beans, peas, sugar beet, sorghum, maize, rice etc; potato haulm
destruction; pre-emergence weed control in carrots, onions, hops,
vines and sugar-cane flower control; control of submerged and some
floating weeds in still water or streams, defoliant and desiccant for
cotton; desiccation of red and white clover, sunflower, linseed and
rape for seed; desiccant for barley and wheat for stock feed purposes.
FATE OF RESIDUES
Photodecomposition
The photodecomposition of diquat resulted in disappearance of
radioactivity with both the ring and ethylene bridge radioactivity
labelled indicating the occurrence of volatile breakdown products.
Approximately 75% of the material was lost with 96 hours of sunlight
exposure. The photo-chemical breakdown of diquat gives
1,2,3,4-tetrahydro-1-oxo-pyridyl 1,2-a-5-pyrazinium bromide.
Other relevant chemical properties
Diquat is available only as a salt, generally as the dibromide. The
cationic portion of the molecule is the active ingredient. Diquat
dibromide exists as the monohydrate and forms white to yellow crystals
which decompose above 300°C. The technical material is available only
as a dark reddish-brown aqueous solution. Solubility in water at 20°C
is 700 g/litre: it is slightly soluble in alcoholic and hydroxylic
solvents and practically insoluble in non-polar organic solvents,
stable in acid or neutral solutions and unstable under alkaline
conditions. One electron reduction by zinc or sodium dithionite yields
the green free-radical which in the presence of atmospheric oxygen
reverts to diquat. The single electron reduction is completely
reversible, and the redox potential of - 349 mV is independent of pH.
Further reduction yields polyhydrobipyridyl derivatives.
Solutions of the free radical exhibit a sharp absorption peak at 379
nm which has a greater intensity than that of the unreduced diquat at
310 nm. Concentrated aqueous solutions corrode steel, tinplate,
galvanised iron and aluminium.
Purity
Technical, 95 percent
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Dogs administered orally 10-15 mg 14C-labelled diquat dibromide
excreted 29-32 percent in the urine and 51-62 percent in the faeces in
72 hours. In the first 24 hours, 25-28 percent of the dose was
excreted in the urine (Swan, 1960).
Following oral doses of 5-10 mg/kg to rats, diquat dibromide was
eliminated entirely with 96-101 percent recovery within four days, the
levels in the urine were 4-6 percent and in the faeces 90-96 percent
(Daniel and Gage, 1966). Following subcutaneous doses of 5 or 6 mg/kg
to rats, diquat dibromide was eliminated again within four days with
90-98 percent recovery-in urine 88-98 percent and in faeces 0-2
percent. In all studies, the bulk of the residues excreted following
oral administration was in the faeces, whereas after a subcutaneous
dose most was in the urine, indicating that absorption from the gut
was relatively poor. Studies where chemical rather than radiological
measurements were made showed that following an oral dose of 30-40
mg/kg to rats, only 4-7 percent of the dose was recovered in the urine
and 11-42 percent in the faeces after 48 hours (Swan, 1960).
The percentage excretion of an oral dose in the urine, calculated from
a chemical method of analysis, was lower than that obtained from a
radio-analytical method of analysis, suggesting that a proportion of
the dose appeared in a form other than diquat. Following a
subcutaneous dose, the radiochemical and chemical analyses gave
results which were closer than the values following an oral dose,
indicating possible absorption from the gut of microbial degradation
products following oral administration, rather than actual animal
metabolism (Swan, 1960).
In vitro experiments using suspensions of gut material suggested
that microbial breakdown of diquat was responsible for the low
recoveries by the chemical assay where only the parent ion was
measured (Swan, 1960).
Effects on enzymes and other biochemical parameters
Although there is no direct evidence in mammals to support the view,
it is tempting to assume that the ability of bipyridyls to be reduced
and re-oxidized with the production of free radicals is linked with
their toxic effects, as has been suggested to be the case in plants.
Gage (1968) has shown that free radicals can be produced from diquat
incubated anaerobically in the presence of NADPH and microsomes
derived from rat liver. Diquat also increased the respiration of liver
mitochondrial fragments. This action has been related to the activity
of flavo-protein dehydrogenases. Purified lipoamide dehydrogenase from
pig heart was able to reduce diquat to the free radical in the
presence of NADH. Rees (1969) has shown that a fresh extract of bovine
lens, incubated anaerobically with diquat, can catalyze similar
reactions. From these experiments, there can be little doubt that
flavo-proteins of animal tissues in the presence of their
co-substrates can reduce the bipyridyls, although aerobically the
equilibrium concentration of the free radical is probably extremely
low.
The property that diquat has of undergoing cyclic reduction and
oxidation might suggest that it could interfere in electron-transport
processes, diverting electrons from the system and reducing oxygen to
water. Gage (1968) found that the resting respiration of mitochondria
was almost unaffected by diquat, probably because of its inability to
penetrate the mitochondrial membrane.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Rat
Six groups of rats (ten males or ten females) were examined for
reproduction and teratogenic effects of diquat dichloride at 0, 125
and 500 ppm in the diet (Griffiths et al., 1966). This experiment
examined the effects of dietary feeding of diquat to males only,
females only and both males and females at 500 ppm and males and
females at 125 ppm. Growth of the parent rats was moderately retarded
at 500 ppm, and all parents developed cataracts. No effect on
reproduction or occurrence of terata was observed. At 125 ppm, no
effect on the growth of parents was noted. Lens opacities did not
occur. Of all offspring produced (1637), one female at 500 ppm had a
unilateral cataract.
A single intraperitoneal injection of diquat (7 mg/kg from day six
through 14 of gestation) produced a high incidence of retarded growth
of sternum and auditory ossicles, as well as marked weight reduction
in rat embryos. A higher dose of 14 mg/kg interrupted most
pregnancies, and in the rats that reached term, the embryonic effects
were more pronounced (Khera and Whitta, 1968).
Special studies for mutagenicity
Diquat was screened for mutagenic activity in Drosophila
melanogaster using the Muller-5 test to detect recessive lethality
on the X chromosome. Compared to a spontaneous mutation rate of 0.14
percent, the rate was 0.11 percent after treatment with diquat, which
is comparable with the control value (Benes and Sram, 1969).
Special studies on cataractogenicity
The initial cataractogenic effects in rats of diquat appear to be
reversible, as was noted in a study where rats were fed a diet
containing 500 ppm diquat. This dose was shown to produce cataracts in
three to six months. After feeding continuously for periods of several
days to eight weeks, the rats were given a normal diquat-free diet for
the remainder of one year. Cataracts did not develop in any of these
rats, indicating that continuous prolonged exposure to diquat was
necessary for the formation of cataracts in the rat. Temporary
exposure to this known cataractogen does not lead to irreversible
damage (Clark and Hurst, 1970).
Since it is known that some forms of cataract are influenced by light
and that the toxicity of diquat to plants is dependent upon light,
experiments were undertaken to study the effect of light on cataract
formation in rats. Rats were fed 500 ppm diquat in the dark for three
months and controls were fed the same diet under light conditions.
After three months feeding on these diets, both groups of animals
showed an equal number of cataracts, suggesting that light does not
influence the development of cataract formation (Clark and Hurst,
1970).
Ascorbic acid (200 mg/ml) in the drinking water of rats receiving
diquat (500 ppm) in the diet did not influence the development of
cataracts (Clark and Hurst, 1970.)
See also "Short" and "Long-term Studies" and "Reproduction". LD50
values for diquat in various species are summarized in Table I.
A level of 90 ppm diquat at 18°C killed 50 percent of a population of
rainbow trout within 48 hours. At 70 ppm, 50 percent mortality was
observed in 48 hours (Swan, 1960).
A single dermal application of 10 or 20 mg of diquat to rats as an
aqueous solution produced a slight reversible erythema in the treated
area (Cooke and Gage, 1956).
Acute dermal administration of diquat at doses of 500 and 1000 mg/kg
to rabbits resulted in toxic signs within 48-72 hours at 1000 mg/kg
and no abnormalities at 500 mg/kg (Swan, 1963b).
Installation of 10 mg diquat of a 10 percent aqueous solution into the
conjuctival sac of rabbits produced no effect (Cook and Gage, 1956).
Short-term studies
Rat
Three groups of rats (four males and four females) were exposed to
diquat in aerosol form at levels of 0.5, 1.06 and 2 µg/litre for 15
daily six-hour exposure periods. At the 2 µg/litre level, weight gain
was slightly reduced. No such effect was noted at the 1.06 and 0.5
µg/litre levels. Histopathological examination of the lungs showed
slight irritation with peribronchial lymphoid hyperplasia,
perivascular oedema and macrophages in the alveoli at the 2 µg/litre
level (Gage, 1967).
TABLE I
LD50 values of diquat in various species
Salt LD50
Species Route Form (mg Reference
cation/kg)
Mouse Oral Cl 125 Swan, 1963a
Br 125 Clark and Hurst, 1970
Rat Oral Cl 302 Swan, 1962
Br 215-235 Clark and Hurst, 1970
Swan, 1960
s.c. Cl 11 Clark and Hurst, 1970
Br 11-20 Clark and Hurst, 1970
Cook and Gage, 1956
Guinea Pig Oral Br 100 Clark and Hurst, 1970
Rabbit Oral Br 100 Swan, 1960
Clark and Hurst, 1970
Dermal Br 400 Swan, 1963b
Cookson and McElligott, 1966
i.p. Br 15 " " "
Hen Oral Br 215-430 Swan, 1960
Br 200-400 Clark and Hurst, 1970
Dog Oral Br 100-200 Clark and Hurst, 1970
Cow Oral Br approx. 30 Walley, 1962
Clark and Hurst, 1970
Subcutaneous administration of 1 mg diquat/kg body-weight to rats for
21 days produced no toxic effects; post-mortem examination showed no
organic damage (Cooke and Gage, 1956).
Rabbit
Groups of rabbits (three males and three females) were administered
diquat percutaneously at doses of 0, 20, 40, 80 and 160 mg diquat
ion/kg body-weight for 20 days. At 20 mg/kg, no deaths occurred,
whereas, at 40 mg/kg and above, from 66 to 100 percent of the animals
died.
Microscopic examination revealed vacuolar changes in the distal
convoluted renal tubules with occasional necrosis of cells. These
changes were preceded by weight loss and muscular weakness (Cookson
and McElligott, 1966).
Groups of five female and five male rabbits were administered up to 20
daily percutaneous doses of diquat at O, 3.13, 6.25, 12.5 and 25
mg/kg. The calculated LD50 was 7.9 mg/kg (5.7 mg ion/kg). Signs of
poisoning included: local hyperaemic and subcutaneous oedema;
increasing sloughing of surface layers of skin followed by scab
formation. These effects were reversible at the 6.25 mg/kg dose after
treatment was concluded. Other signs included weakness, incoordination
and lethargy. In many animals, ulceration of the gastric mucosa was
observed at post-mortem indicating that oral contamination had
presumably occurred (Swan, 1963b).
Dog
Groups of dogs (three males and three females) were fed diets
containing 0, 16, 32, 68, 200 and 600 ppm diquat dichloride for
periods up to four years. At dosages of 600 and 200 ppm, bilateral
opacities of the lens were observed at ten and 15 months,
respectively. Dietary levels of 68 ppm did not affect the lens within
the four year interval tested. No effects were observed on growth,
tumour formation, food consumption, blood chemistry and gross and
microscopic pathology. The dose of 68 ppm (equivalent to 50 ppm diquat
ion) is the no-effect level in dogs with regard to cataract formation
(Hurst, 1966).
Sheep and calf
Five groups of two sheep (one male and one female, eight months old)
and three groups of one calf each were given diquat at doses of 0, 1,
5, 10 and 20 ppm and 0, 5 and 20 ppm, respectively, in their drinking
water for one month. These levels caused no toxicological effect over
the trial period as evidenced by growth, food consumption and visual
observation (Sarfaty, 1963).
Cow
Oral administration of diquat for five days at 10 mg/kg body-weight to
a cow resulted in death within 15 days preceded by severe signs of
poison, including dullness, inappetence anaemia, increased heart rate.
At post-mortem, heart and kidney infarcts and intestinal catarrh were
found. Administration of 5 mg diquat/kg daily for fourteen days to a
cow (in the diet for two days, then drenched) resulted in increased
inappetence after two days, slight haemorrhage as noted by blood in
the faeces and temporary impairment of vision (Walley, 1962).
Comparative
Groups of four male and four female mice and guinea pigs, two female
rabbits and a male dog, exposed to 15 daily six-hour treatments with
diquat in aerosol form at 1.06 mg/litre, showed no adverse effects
(Gage, 1967).
Long-term studies
Rat
Groups of rats (25 males and 25 females per group) were fed diquat
dichloride for two years at doses of 0.125, 250, 500 or 1000 ppm in
the diet. After 56 days, the 1000 ppm level was discontinued because
of lack of growth and mortality in males and females. At 125 ppm, a
partial lens opacity (cataract) was seen at 207 days. All males and 19
out of 21 females were affected by 657 days. At 250 and 500 ppm, lens
opacities were produced in all animals within 155 and 124 days,
respectively. At 500 ppm, a reduction of female body-weight was
apparent after 20 weeks and in males after five weeks. No such effects
were noted at 250 and 125 ppm. No adverse effects were reported in
survival, blood chemistry, tumour, incidence and gross and microscopic
pathology (Swan, 1962; Goater et al., 1964).
Seven groups of rats (25 males and 25 females/group; controls had 75
and 75 females) were fed diquat dichloride at levels of 0,10, 50, 100,
250, 500 or 1000 ppm for two years. Growth depression was observed in
the males at 1000 ppm. Lens opacity was observed at doses of 50 ppm
and above in both males and females (no such effect was noted at the
10 ppm level). Growth, food consumption survival, tumour formation,
behaviour, haematological and urine analyses and gross and microscopic
examination revealed no effects differing from the controls. A
no-effect level based upon cataract formation is 10 ppm, equivalent to
7.2 ppm diquat cation (Kohn et al., 1965a, b and c).
OBSERVATIONS IN MAN
Damage and discolouration of fingernails caused by exposure to
concentrated solutions of diquat were observed in three instances. The
cause of the damage is unknown but presumably is of a local nature.
All three patients had frequent exposure to the concentrated chemical
without taking precautions to prevent contamination of the skin. The
cause of the nail damage was unknown, but it seemed probable that the
chemical reached the nail matrix by entering the nail fold and
stimulated infection, interfering with the formation of the nail from
the matrix. The damage is presumed to be local and not as result of
ingestion because of asymmetry of the lesions and the fact that the
toenails were unaffected. A curious colour change and softening of the
nail at the base are characteristic. In some instances, the nail was
shed and was not regrown (Samman and Johnston, 1969).
Of 42 reported exposures of man varying from one to 75 individual
exposures, four incidents of dermal abnormalities were reported. These
include rashes, blisters and a transient skin discolouration. In
almost all instances, these incidents were attributable to the
concentrated commercial preparation (Anonymous, 1966).
Poisoning cases in humans with bipyridyl compounds have demonstrated
the acute toxic effects of these compounds. In one case, the
accidental ingestion of a small quantity of diquat led to diarrhoea
and oral ulceration. After forced diuresis, the man recovered and was
released from care. It was observed that the urine contained traces of
diquat as long as eleven days after ingestion (Oreopoulos, 1969).
COMMENT
In biochemical studies in rats and dogs, it has been observed that
after oral administration, the major part of the dose is excreted in
the faeces. Following a subcutaneous dose, most residue was found in
the urine, indicating that absorption from the gut was relatively
poor. A small proportion of the material found in urine after oral
administration was other than the parent compound, due possibly to
absorption of microbial degradation products rather than actual animal
metabolism.
Diquat is a cataractogenic compound, as has been demonstrated in
lont-term rat and in dog experiments at relatively low levels of 2.5
and 5 mg/kg body-weight, respectively. Transient exposure of rats to
diquat does not lead to the formation of cataracts.
Intraperitoneal administration of 7 mg/kg diquat to pregnant rats
resulted in embryotoxic effects. In a three-generation rat
reproduction study, 500 ppm diquat resulted in no effect on
reproduction.
In man, the acute and dermal problems associated with accidental or
suicidal ingestion and dermal contamination appear to be of primary
concern. Cataract formation has not been observed in man as a result
of exposure to diquat, nevertheless studies in the prophylaxis and
treatment of diquat-induced cataracts in mammals were considered
urgent.
Non-reversible cataractogenic effects in rats and dogs at relatively
low oral levels, 50 ppm (2.5 mg/kg) and 200 ppm (5
mg/kg),reapectively, and a potential embryonic effect make it
advisable to establish only a temporary acceptable daily intake for
man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Rat: 10 ppm in the diet, equivalent to 0.5 mg/kg body-weight/day
(corresponds to 0.36 mg diquat ion/kg body-weight/day)
Dog: 68 ppm in the diet, equivalent to 1.7 mg/kg body-weight/day
(corresponds to 1.22 mg diquat ion/kg body-weight/day)
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE IN MAN
0-0.0025 mg/kg body-weight as diquat dichloride (0-0.002 mg/kg
body-weight expressed as diquat ion)
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Diquat is used world wide for the following purposes; desiccation of
beans, peas, sugar beet, sorghum, maize, rice etc; potato haulm
destruction; pre-emergence weed control in carrots, onions, hops,
vines and sugar-cane flower control; control of submerged and some
floating weeds in still water or streams, defoliant and desiccant for
cotton; desiccation of red and white clover, sunflower, linseed and
rape for seed; desiccant for barley and wheat for stock feed purposes.
FATE OF RESIDUES
Photodecomposition
The photodecomposition of diquat resulted in disappearance of
radioactivity with both the ring and ethylene bridge radioactivity
labelled indicating the occurrence of volatile breakdown products.
Approximately 75% of the material was lost with 96 hours of sunlight
exposure. The photo-chemical breakdown of diquat gives
1,2,3,4-tetrahydro-1-oxo-pyridyl 1,2-a-5-pyrazinium bromide.
 Further degradation of the molecule occurs in the presence of sunlight
presumably to volatile products (Funderburk and Bozarth, 1967; Slade
and Smith, 1967).
In soil
One of the most striking features of diquat is its rapid and complete
inactivation by soil. This inactivation results from a reaction
complex formed between the positively charged diquat cation and the
negatively charged sites on the clay minerals present in the soils.
In animals
Silage made from grass desiccated with diquat (13 ppm) was fed to farm
animals over long periods; no residues were detected in the animal
tissue or milk secreted during this time (Black et al., 1966). The
fate of 14C-diquat administered orally to cattle has been studied.
Three cows received single oral doses of 5 mg diquat dibromide/kg
body-weight and one cow received a single dose of 20 mg/kg. Milk
residues after seven days amounted to 0.001 to 0.015% of the
administered dose with the major residues occurring in three days of
dosing at the lower levels. At the higher dose levels, residues were
evident at all sampling intervals (seven days) with the major amounts
recovered within six days. Residues in urine varied from 0.4 to 2.6%
of the administered dose. Tissue residues, 24 hours after an oral
administration of 11.5 mg diquat dibromide/kg, were found primarily in
kidney (0.7 ppm) and liver (0.2 ppm) with slight residues in several
other tissues (<0.1 ppm). Chemical analysis of the liver and kidney
resulted in 0.01 and less than 0.03 ppm diquat, indicating that major
residues were not diquat but metabolic conversion products (Stevens
and Walley, 1966). Traces of metabolites found in the milk were
believed to arise from breakdown in the gut or in the rumen; Daniel
and Gage (1966) found similar breakdown in the rat. After dosing a
calf with 14C-diquat (5 ppm), less than 0.01 ppm of diquat or its
metabolites were found in the muscle tissue. Because of its use for
aquatic weed control, the fate of diquat in fish has been studied.
Radiotracer studies of the distribution of diquat in fish following
treatment of water indicated that the major residues occurred in the
digestive system, and the only residue was diquat (Funderburk and
Bozarth, 1967).
After exposure of rainbow trout to 1 ppm, diquat residues were found
in the viscera and skin but none in the flesh (Calderbank, 1968).
In plants
The uptake by foliage and extent of subsequent movement are critically
dependent on the environmental conditions. Traces of diquat were
detected in potato tubers after the tops had been killed with the
chemical (Calderbank et al., 1961; Headford et al., 1967). By means of
14C-labelled diquat, it has been shown that this transfer occurs in
the xylem. Smith et al., (1966) confirmed that darkness increased this
long-distance transport of diquat. Experiments with potato plants and
tubers have shown that even if metabolism occurred in the plant no
degradation products were transported to the tubers (Smith, 1967). No
significant loss of diquat residues was found after storage of potato
tubers for up to seven months. It has been assumed that since the
plants die rapidly in bright sunlight, significant quantities of the
breakdown products formed on the surface of dead tissues should not
move from these dead leaves to edible parts of the plant. This has
been partially demonstrated with potatoes, where it has been shown
that diquat residues in tubers from sprayed plants appear as the
unchanged diquat and not the photoderivative.
Diquat taken up from nutrient solution into plants was not metabolized
but remained as the parent compound (Funderburk and Lawrence, 1964).
In water
Diquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds, absorption by soil, silt and
particulate suspended matter, and, to a slight extent, by
photochemical degradation (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance is very variable, depending on the movement
of the water, the presence of mud or suspended matter, and the
strength of sunlight, but treatments within the range of 0.5 - 4
mg/litre in the water have resulted in less than 0.1 mg/litre being
detected in from four to 14 days after application. Decomposition of
the killed weed is rapid, any remaining residue of diquat thus
liberated being subsequently absorbed on the bottom mud. Such residues
in the largely organic muds may be more readily available to bacterial
degradation than when absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
A summary of the diquat residues in food crops, raw and processed in
wheat, flour and bread and also in barley, malt and beer is given in
Tables II, III and IV: (Calderbank, 1968).
METHODS OF RESIDUE ANALYSIS
The residue analysis of diquat is usually carried out colorimetrically
after extraction of the plant or animal tissue with boiling dilute
sulphuric acid, isolation by ion exchange column, elution, reduction
and the absorption of the derived coloured free radical measured at
377 nm. This method of Calderbank et al. (1961), was modified by
Kirston (1966), simplified by Calderbank and Yuen (1966) and adapted
by Black et al. (1966). Limit of sensitivity is about 0.01 ppm, and
the procedure should be suitable for regulatory purposes. Engelhardt
and McKinley (1966) determined the compound by polarography:
Coha and Koljcojic (1969) used a combination of TLC and ring oven.
Funderburk and Lawrence (1963) developed a sensitive bioassay
technique for residues in water using the lesser duckweed (Lemna
minor L.) and also Funderburk et al. (1966) used a thin-layer
chromatographic procedure for examining the photochemical
decomposition products of diquat in solution. Faust and Hunter (1965)
determined diquat in natural surface waters by absorptiometric
measurements at the wavelength of maximum absorption of the unreduced
herbicide (310 nm) following clean-up by ion-exchange.
TABLE II
Summary of residues in food crops (dessiccation uses)
Average Residues
3-21 days after
Rate of application,
CROP Application mg/kg
lb/acre DIQUAT
Barley 0.5-1.0 0.5-4.0
Wheat, rape seed 0.5-1.0 ND-1.3
Maize 0.5-1.2 ND
Rice (with husk) 0.15-0.54 0.7-5.0
Rice (dehusked or polished) 0.15-0.54 ND
Peas, beans, sunflower seed 0.35-1.2 ND-0.2
Sorghum seed 0.25-1.0 0.2-0.8
Cotton (as picked) 0.5-1.0 0.05-0.5
Onions 0.5-2.0 0.02-0.05
Potatoes 0.5-1.5 ND-0.04
Sugar cane juice 0.5-2.0 ND
Seed oils (sesame, sunflower,
rape, cotton) up to 1.2 ND
ND - not detected
TABLE III
Residues in wheat, flour and bread
(Wheat desiccated with diquat at 0.7 lb/acre)
Sample Residues of diquat
(mg/kg)
Wheat (at harvest) 1 (approx)
Wheat (cleaned for milling) 0.6-0.7
White flour 0-0.1
Bread 0.1
Bran 1.2-2.4
TABLE IV
Residues in barley, malt and beer
Intervals between Diquat residues found,
application and harvest mg/kg
(0.68 lb diquat/acre applied) Barley Malt Beer
4 days 4.24 2.05 0.12
5 days 2.35 1.07 0.04
10 days 0.94 0.61 0.02
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
United States of America Sugarcane 0.05
(negligible
residue)
APPRAISAL
Diquat is very widely used as a desiccant - e.g. for rice, clover,
rape, linseed, peas, beans, maize, potato haulm, etc. and as a
pre-emergence herbicide in carrots, onions, hops, vines, etc.; it is
also used for aquatic weed control. Residues are very unlikely to
accrue from soil or pre-emergence applications but can occur following
use for desiccation purposes. Residues in seed from such desiccated
crops vary from negligible in such well-protected seeds as maize to
about 5 ppm in rice (husk). Barley and wheat for stock feed or seed
purposes can contain up to 5 and 2 ppm, respectively, of diquat
following desiccation uses. Residues often occur in treated oil seeds,
such as sunflower, sesame and cotton, but no residues are observed in
the expressed oil. The spectrophotometric procedure should be suitable
for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Rice (with husk) 5 ppm
Rape seed, sorghum seed 2 ppm
Peas, beans, sunflower seed 0.5 ppm
Onions, potatoes, maize, rice (polished) 0.1 ppm
Sesame, sunflower, rape, cotton seed oils 0.1 ppm
Information given also on:
Cotton (as picked) 1 ppm
In cereal grains for stock feed or seed purposes only, the following
levels should also be accepted:
Barley 5 ppm
Wheat 2 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Further studies on the mechanism of cataractogenesis in animals.
2. A three-generation reproduction study where there is exposure to
diquat during the entire duration of the experiment.
DESIRABLE
Clinical studies on factory workers and users of diquat in order to
detect the extent of cataractogenic risk.
REFERENCES
Anonymous. (1966) Unpublished report 29 December 1966 from ICI Ltd.
through Chevron Chemical Co. to FDA
Benes, V. and Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Industr. Med Surg., 38: 442-444
Black, W.J.M., Calderbank, A., Douglas, G. and McKenna, R.H.J. (1966)
Residues in herbage and silage and feeding experiments following the
use of diquat as a desiccant. J.Sci.Fd. Agr., 17: 506-509
Calderbank, A., Morgan, C.B. and Yuen, S.H. (1961) Determination of
diquat residues in potato tubers. Analyst, 86: 569-579
Calderbank, A. and Yuen, S.H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629
Calderbank, A. (1968) The bipyridylium herbicides. Advances in pest
control research, 8: 127-235
Clark, D.G. and Hurst, E.W. (1970) The toxicity of diquat. Brit. J.
Ind. Med., 27: 51-55
Coha, F. and Koljcojic, R. (1969) Combination of thin-layer
chromatography and the ring oven method for the semi-quantitative
estimation of some herbicides. J. Chromatog., 40: 304-307
Cooke, E. and Gage, J.C. (1956) Toxicological report. Unpublished
report TR/35 15 March 1956 for ICI Ltd. through Chevron Chemical Co.
to FDA
Cookson, J.H. and McElligott. (1966) The dermal toxicity of diquat.
Unpublished report IHR. 200 from ICI Ltd. through Chevron Chemical Co.
to FDA
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. Indust. Med. J., 23: 133-136
Engelhardt, J. and McKinley, W.P. (1966) Bypyridylium herbicides.
Polarography of 1,1'-ethylene-2,2'-bipyridylium dibromide. J. Agr. Fd.
Chem., 14: 377-380
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the detection
of aquatic herbicides. J. Am. Water Works Assoc., 57: 1028-1037
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Lawrence, J.M. (1964) Mode of action of diquat
and paraquat. Weeds, 12: 259-264
Funderburk, H.H., Negi, N.S. and Lawrence, J.M. (1966) Photochemical
decomposition of diquat and paraquat. Weeds, 14: 240-243
Funderburk, H.H. and Bozarth. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agr. Fd. Chem., 15: 563-567
Gage, J.C. (1967) The toxicity of diquat aerosols. Unpublished report
IHR/212 (March 1967) from ICI Ltd. through Chevron Chemical Co. to FDA
Gage, J.C. (1968) The action of paraquat and diquat on the respiration
of liver cell fraction. Biochem. J., 109: 757-761
Goater, T.O., Kenyon, A.J. and Hurst, E.W. (1964) The two-year test
with diquat dichloride monohydrate in rats. Unpublished report IHR/165
(Nov. 1964) ICI Ltd.
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with diquat monohydrate in the diet.
Unpublished report IHR/188 (Jan. 1966) from ICI Ltd. through Chevron
Chemical Co. to FDA
Headford, D.W.R. and Douglas, G. (1967) Tuber necrosis following the
desiccation of potato foliage with diquat. Weed Res., 7: 131-144
Hurst, E.W. (1966) A long-term toxicity test with diquat dichloride
monohydrate in dogs. Unpublished report IHR/195 (May 1966) from ICI
Ltd. through Chevron Chemical Co. to FDA
Kirston, W.J. (1966) The determination of diquat residues in potato
tubers. Analyst, 91: 732-738
Khera, K.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965a) Two-year chronic oral
toxicity of diquat - albino rats 1000, 500 and 250 ppm (27 February
1965). Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.D. (1965b) Two-year chronic oral
toxicity of diquat - albino rats 100 and 10 ppm (27 February 1965).
Addendum report - Unpublished report from Industrial Bio-Test Lab.
Inc. through ICI and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965c) Two-year chronic oral
toxicity of diquat - albino rats, 50 ppm (27 February 1965). Addendum
report. Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Oreopoulos, D.G. and McEvoy, J. (1969) Diquat poisoning. Post-Grad.
Med. J., 45: 635-637
Rees, J.R. (1969) Reaction between lens constituents and substances
which cause cataracts. Thesis for B.Sc. Degree, University of Oxford
cited by Conning et al. (1969)
Samman, P.D. and Johnston, E.K. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report from ICI Ltd. through Chevron Chemical Co.
to FDA
Slade, P. and Smith, A.E. (1967) Photochemical degradation of diquat.
Nature, 213: 919-920
Smith, J.M. and Sagar, G.R. (1966) Re-examination of the influence of
light and darkness on the long distance transport of diquat in
Lycopersicon esculentum. Weed Res., 6: 314-321
Smith, A.E. (1967) Residues in potato tubers following haulm
desiccation with 14C-diquat. Bull. Environ. Contam. Toxicol., 169-177
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Fd. Agric., 17: 472-475
Swan, A.A.B (1960) Toxicological report. Unpublished report TR/35
Supplement (7 June 1960) from ICI Ltd. through Chevron Chemical Co. to
FDA
Swan, A.A.B. (1962) Unpublished interim toxicological report No:
TR/375 (5 July 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963a) Diquat-oral toxicity to mice. Unpublished report
(24 January 1963) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963b) The dermal toxicity of diquat dichloride.
Unpublished report TR/540 (20 June 1963) from ICI Ltd. through Chevron
Chemical Co. to FDA
Walley, J.K. (1962) Diquat toxicity in cattle. Unpublished report
IHR/167 (Nov. 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Further degradation of the molecule occurs in the presence of sunlight
presumably to volatile products (Funderburk and Bozarth, 1967; Slade
and Smith, 1967).
In soil
One of the most striking features of diquat is its rapid and complete
inactivation by soil. This inactivation results from a reaction
complex formed between the positively charged diquat cation and the
negatively charged sites on the clay minerals present in the soils.
In animals
Silage made from grass desiccated with diquat (13 ppm) was fed to farm
animals over long periods; no residues were detected in the animal
tissue or milk secreted during this time (Black et al., 1966). The
fate of 14C-diquat administered orally to cattle has been studied.
Three cows received single oral doses of 5 mg diquat dibromide/kg
body-weight and one cow received a single dose of 20 mg/kg. Milk
residues after seven days amounted to 0.001 to 0.015% of the
administered dose with the major residues occurring in three days of
dosing at the lower levels. At the higher dose levels, residues were
evident at all sampling intervals (seven days) with the major amounts
recovered within six days. Residues in urine varied from 0.4 to 2.6%
of the administered dose. Tissue residues, 24 hours after an oral
administration of 11.5 mg diquat dibromide/kg, were found primarily in
kidney (0.7 ppm) and liver (0.2 ppm) with slight residues in several
other tissues (<0.1 ppm). Chemical analysis of the liver and kidney
resulted in 0.01 and less than 0.03 ppm diquat, indicating that major
residues were not diquat but metabolic conversion products (Stevens
and Walley, 1966). Traces of metabolites found in the milk were
believed to arise from breakdown in the gut or in the rumen; Daniel
and Gage (1966) found similar breakdown in the rat. After dosing a
calf with 14C-diquat (5 ppm), less than 0.01 ppm of diquat or its
metabolites were found in the muscle tissue. Because of its use for
aquatic weed control, the fate of diquat in fish has been studied.
Radiotracer studies of the distribution of diquat in fish following
treatment of water indicated that the major residues occurred in the
digestive system, and the only residue was diquat (Funderburk and
Bozarth, 1967).
After exposure of rainbow trout to 1 ppm, diquat residues were found
in the viscera and skin but none in the flesh (Calderbank, 1968).
In plants
The uptake by foliage and extent of subsequent movement are critically
dependent on the environmental conditions. Traces of diquat were
detected in potato tubers after the tops had been killed with the
chemical (Calderbank et al., 1961; Headford et al., 1967). By means of
14C-labelled diquat, it has been shown that this transfer occurs in
the xylem. Smith et al., (1966) confirmed that darkness increased this
long-distance transport of diquat. Experiments with potato plants and
tubers have shown that even if metabolism occurred in the plant no
degradation products were transported to the tubers (Smith, 1967). No
significant loss of diquat residues was found after storage of potato
tubers for up to seven months. It has been assumed that since the
plants die rapidly in bright sunlight, significant quantities of the
breakdown products formed on the surface of dead tissues should not
move from these dead leaves to edible parts of the plant. This has
been partially demonstrated with potatoes, where it has been shown
that diquat residues in tubers from sprayed plants appear as the
unchanged diquat and not the photoderivative.
Diquat taken up from nutrient solution into plants was not metabolized
but remained as the parent compound (Funderburk and Lawrence, 1964).
In water
Diquat applied to water for aquatic weed control purposes quickly
disappears due to uptake by weeds, absorption by soil, silt and
particulate suspended matter, and, to a slight extent, by
photochemical degradation (Calderbank, 1968). No information is
available on the ultimate fate of the chemical in this environment.
The rate of disappearance is very variable, depending on the movement
of the water, the presence of mud or suspended matter, and the
strength of sunlight, but treatments within the range of 0.5 - 4
mg/litre in the water have resulted in less than 0.1 mg/litre being
detected in from four to 14 days after application. Decomposition of
the killed weed is rapid, any remaining residue of diquat thus
liberated being subsequently absorbed on the bottom mud. Such residues
in the largely organic muds may be more readily available to bacterial
degradation than when absorbed to clay minerals in soils.
Evidence of residues in food in commerce or at consumption
A summary of the diquat residues in food crops, raw and processed in
wheat, flour and bread and also in barley, malt and beer is given in
Tables II, III and IV: (Calderbank, 1968).
METHODS OF RESIDUE ANALYSIS
The residue analysis of diquat is usually carried out colorimetrically
after extraction of the plant or animal tissue with boiling dilute
sulphuric acid, isolation by ion exchange column, elution, reduction
and the absorption of the derived coloured free radical measured at
377 nm. This method of Calderbank et al. (1961), was modified by
Kirston (1966), simplified by Calderbank and Yuen (1966) and adapted
by Black et al. (1966). Limit of sensitivity is about 0.01 ppm, and
the procedure should be suitable for regulatory purposes. Engelhardt
and McKinley (1966) determined the compound by polarography:
Coha and Koljcojic (1969) used a combination of TLC and ring oven.
Funderburk and Lawrence (1963) developed a sensitive bioassay
technique for residues in water using the lesser duckweed (Lemna
minor L.) and also Funderburk et al. (1966) used a thin-layer
chromatographic procedure for examining the photochemical
decomposition products of diquat in solution. Faust and Hunter (1965)
determined diquat in natural surface waters by absorptiometric
measurements at the wavelength of maximum absorption of the unreduced
herbicide (310 nm) following clean-up by ion-exchange.
TABLE II
Summary of residues in food crops (dessiccation uses)
Average Residues
3-21 days after
Rate of application,
CROP Application mg/kg
lb/acre DIQUAT
Barley 0.5-1.0 0.5-4.0
Wheat, rape seed 0.5-1.0 ND-1.3
Maize 0.5-1.2 ND
Rice (with husk) 0.15-0.54 0.7-5.0
Rice (dehusked or polished) 0.15-0.54 ND
Peas, beans, sunflower seed 0.35-1.2 ND-0.2
Sorghum seed 0.25-1.0 0.2-0.8
Cotton (as picked) 0.5-1.0 0.05-0.5
Onions 0.5-2.0 0.02-0.05
Potatoes 0.5-1.5 ND-0.04
Sugar cane juice 0.5-2.0 ND
Seed oils (sesame, sunflower,
rape, cotton) up to 1.2 ND
ND - not detected
TABLE III
Residues in wheat, flour and bread
(Wheat desiccated with diquat at 0.7 lb/acre)
Sample Residues of diquat
(mg/kg)
Wheat (at harvest) 1 (approx)
Wheat (cleaned for milling) 0.6-0.7
White flour 0-0.1
Bread 0.1
Bran 1.2-2.4
TABLE IV
Residues in barley, malt and beer
Intervals between Diquat residues found,
application and harvest mg/kg
(0.68 lb diquat/acre applied) Barley Malt Beer
4 days 4.24 2.05 0.12
5 days 2.35 1.07 0.04
10 days 0.94 0.61 0.02
NATIONAL TOLERANCES
Country Crop Tolerance (ppm)
United States of America Sugarcane 0.05
(negligible
residue)
APPRAISAL
Diquat is very widely used as a desiccant - e.g. for rice, clover,
rape, linseed, peas, beans, maize, potato haulm, etc. and as a
pre-emergence herbicide in carrots, onions, hops, vines, etc.; it is
also used for aquatic weed control. Residues are very unlikely to
accrue from soil or pre-emergence applications but can occur following
use for desiccation purposes. Residues in seed from such desiccated
crops vary from negligible in such well-protected seeds as maize to
about 5 ppm in rice (husk). Barley and wheat for stock feed or seed
purposes can contain up to 5 and 2 ppm, respectively, of diquat
following desiccation uses. Residues often occur in treated oil seeds,
such as sunflower, sesame and cotton, but no residues are observed in
the expressed oil. The spectrophotometric procedure should be suitable
for regulatory purposes.
RECOMMENDATIONS
TEMPORARY TOLERANCES
Rice (with husk) 5 ppm
Rape seed, sorghum seed 2 ppm
Peas, beans, sunflower seed 0.5 ppm
Onions, potatoes, maize, rice (polished) 0.1 ppm
Sesame, sunflower, rape, cotton seed oils 0.1 ppm
Information given also on:
Cotton (as picked) 1 ppm
In cereal grains for stock feed or seed purposes only, the following
levels should also be accepted:
Barley 5 ppm
Wheat 2 ppm
FURTHER WORK OR INFORMATION
REQUIRED (before June 1973)
1. Further studies on the mechanism of cataractogenesis in animals.
2. A three-generation reproduction study where there is exposure to
diquat during the entire duration of the experiment.
DESIRABLE
Clinical studies on factory workers and users of diquat in order to
detect the extent of cataractogenic risk.
REFERENCES
Anonymous. (1966) Unpublished report 29 December 1966 from ICI Ltd.
through Chevron Chemical Co. to FDA
Benes, V. and Sram, R. (1969) Mutagenic activity of some pesticides in
Drosophila melanogaster. Industr. Med Surg., 38: 442-444
Black, W.J.M., Calderbank, A., Douglas, G. and McKenna, R.H.J. (1966)
Residues in herbage and silage and feeding experiments following the
use of diquat as a desiccant. J.Sci.Fd. Agr., 17: 506-509
Calderbank, A., Morgan, C.B. and Yuen, S.H. (1961) Determination of
diquat residues in potato tubers. Analyst, 86: 569-579
Calderbank, A. and Yuen, S.H. (1966) An improved method for
determining residues of diquat. Analyst, 91: 625-629
Calderbank, A. (1968) The bipyridylium herbicides. Advances in pest
control research, 8: 127-235
Clark, D.G. and Hurst, E.W. (1970) The toxicity of diquat. Brit. J.
Ind. Med., 27: 51-55
Coha, F. and Koljcojic, R. (1969) Combination of thin-layer
chromatography and the ring oven method for the semi-quantitative
estimation of some herbicides. J. Chromatog., 40: 304-307
Cooke, E. and Gage, J.C. (1956) Toxicological report. Unpublished
report TR/35 15 March 1956 for ICI Ltd. through Chevron Chemical Co.
to FDA
Cookson, J.H. and McElligott. (1966) The dermal toxicity of diquat.
Unpublished report IHR. 200 from ICI Ltd. through Chevron Chemical Co.
to FDA
Daniel, J.W. and Gage, J.C. (1966) Absorption and excretion of diquat
and paraquat in rats. Brit. Indust. Med. J., 23: 133-136
Engelhardt, J. and McKinley, W.P. (1966) Bypyridylium herbicides.
Polarography of 1,1'-ethylene-2,2'-bipyridylium dibromide. J. Agr. Fd.
Chem., 14: 377-380
Faust, S.D. and Hunter, N.E. (1965) Chemical methods for the detection
of aquatic herbicides. J. Am. Water Works Assoc., 57: 1028-1037
Funderburk, H.H. and Lawrence, J.M. (1963) A sensitive method for
determination of low concentrations of diquat and paraquat. Nature,
199: 1011-1012
Funderburk, H.H. and Lawrence, J.M. (1964) Mode of action of diquat
and paraquat. Weeds, 12: 259-264
Funderburk, H.H., Negi, N.S. and Lawrence, J.M. (1966) Photochemical
decomposition of diquat and paraquat. Weeds, 14: 240-243
Funderburk, H.H. and Bozarth. (1967) Review of the metabolism and
decomposition of diquat and paraquat. J. Agr. Fd. Chem., 15: 563-567
Gage, J.C. (1967) The toxicity of diquat aerosols. Unpublished report
IHR/212 (March 1967) from ICI Ltd. through Chevron Chemical Co. to FDA
Gage, J.C. (1968) The action of paraquat and diquat on the respiration
of liver cell fraction. Biochem. J., 109: 757-761
Goater, T.O., Kenyon, A.J. and Hurst, E.W. (1964) The two-year test
with diquat dichloride monohydrate in rats. Unpublished report IHR/165
(Nov. 1964) ICI Ltd.
Griffiths, D., Ponsford, D.C. and Hurst, E.W. (1966) A study of
reproduction in rats treated with diquat monohydrate in the diet.
Unpublished report IHR/188 (Jan. 1966) from ICI Ltd. through Chevron
Chemical Co. to FDA
Headford, D.W.R. and Douglas, G. (1967) Tuber necrosis following the
desiccation of potato foliage with diquat. Weed Res., 7: 131-144
Hurst, E.W. (1966) A long-term toxicity test with diquat dichloride
monohydrate in dogs. Unpublished report IHR/195 (May 1966) from ICI
Ltd. through Chevron Chemical Co. to FDA
Kirston, W.J. (1966) The determination of diquat residues in potato
tubers. Analyst, 91: 732-738
Khera, K.S. and Whitta, L.L. (1968) Embryopathic effects of diquat and
paraquat in the rat. Ind. Med. Surg., 37: 553
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965a) Two-year chronic oral
toxicity of diquat - albino rats 1000, 500 and 250 ppm (27 February
1965). Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.D. (1965b) Two-year chronic oral
toxicity of diquat - albino rats 100 and 10 ppm (27 February 1965).
Addendum report - Unpublished report from Industrial Bio-Test Lab.
Inc. through ICI and Chevron Chemical Co. to FDA
Kohn, F.E., Kay, J.H. and Calandra, J.C. (1965c) Two-year chronic oral
toxicity of diquat - albino rats, 50 ppm (27 February 1965). Addendum
report. Unpublished report from Industrial Bio-Test Lab. Inc. through
ICI Ltd. and Chevron Chemical Co. to FDA
Oreopoulos, D.G. and McEvoy, J. (1969) Diquat poisoning. Post-Grad.
Med. J., 45: 635-637
Rees, J.R. (1969) Reaction between lens constituents and substances
which cause cataracts. Thesis for B.Sc. Degree, University of Oxford
cited by Conning et al. (1969)
Samman, P.D. and Johnston, E.K. (1969) Nail damage associated with
handling of paraquat and diquat. Brit. Med. J., 1: 818-819
Sarfaty, A.B. (1963) Observational trials on toxicity to sheep and
cattle. Unpublished report from ICI Ltd. through Chevron Chemical Co.
to FDA
Slade, P. and Smith, A.E. (1967) Photochemical degradation of diquat.
Nature, 213: 919-920
Smith, J.M. and Sagar, G.R. (1966) Re-examination of the influence of
light and darkness on the long distance transport of diquat in
Lycopersicon esculentum. Weed Res., 6: 314-321
Smith, A.E. (1967) Residues in potato tubers following haulm
desiccation with 14C-diquat. Bull. Environ. Contam. Toxicol., 169-177
Stevens, M.A. and Walley, J.K. (1966) Tissue and milk residues arising
from the ingestion of single doses of diquat and paraquat by cattle.
J. Sci. Fd. Agric., 17: 472-475
Swan, A.A.B (1960) Toxicological report. Unpublished report TR/35
Supplement (7 June 1960) from ICI Ltd. through Chevron Chemical Co. to
FDA
Swan, A.A.B. (1962) Unpublished interim toxicological report No:
TR/375 (5 July 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963a) Diquat-oral toxicity to mice. Unpublished report
(24 January 1963) from ICI Ltd. through Chevron Chemical Co. to FDA
Swan, A.A.B. (1963b) The dermal toxicity of diquat dichloride.
Unpublished report TR/540 (20 June 1963) from ICI Ltd. through Chevron
Chemical Co. to FDA
Walley, J.K. (1962) Diquat toxicity in cattle. Unpublished report
IHR/167 (Nov. 1962) from ICI Ltd. through Chevron Chemical Co. to FDA
