
DIQUAT
First draft prepared by
T.C. Marrs
Department of Health, London, United Kingdom
EXPLANATION
Diquat was previously evaluated by the Joint Meeting in 1970,
1972 and 1977 (Annex 1, references 14, 18 and 28). An ADI of 0-
0.008 mg diquat ion/kg bw was allocated in 1977. This monograph
summarizes new or not previously reviewed data on diquat, as well as
relevant data from previous monographs and monograph addenda on this
pesticide.
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Using unlabelled diquat or 14C-labelled diquat, Daniel &
Henson (1960) demonstrated that diquat was absorbed to a small
extent when administered to rats orally in aqueous solution. In all
species examined, diquat was poorly absorbed from the
gastrointestinal tract, the small part absorbed being principally
eliminated via the urine.
In a study in rats, 14C-labelled diquat dibromide (5 or 10 mg
ion/kg bw) or dichloride (22 or 24 mg ion/kg bw) was administered by
gavage; the dibromide was also administered subcutaneously at doses
of 5 or 6 mg ion/kg bw. Most of the radioactivity appeared in the
excreta within 48 hours, irrespective of the route of
administration. After oral administration, most of the diquat was
found in urine. Measurements of diquat in excreta suggested that
biotransformation had occurred after oral adminstration, presumably
by gut flora, as little evidence of biliary excretion was observed
(Daniel & Gage, 1966).
In another study in Wistar rats, 14C-diquat was administered
as a single oral dose (45 mg ion/kg bw) in aqueous solution by
gavage; 6% and 89% were excreted in urine and faeces, respectively,
over 4 days but mainly in the first 24 hours. After subcutaneous
administration of 10 mg ion/kg bw, 87% and 5% were excreted in the
urine and faeces respectively within 4 days (Mills, 1976).
14C-Diquat as the dibromide was administered orally to Wistar
to rats at a dose of 1 mg ion/kg bw. A mean of 90% and 94% of the
administered radioactivity was recovered in the faeces within 24
hours and 168 hours, respectively. Urinary excretion after 168
hours was 3% of the administered dose (Johnston et al., 1990a).
In another study, a single oral dose of 14C-labelled diquat
dibromide (> 97% purity) was administered to Wistar rats at a dose
of 100 mg diquat ion/kg bw. About 73% of the radioactivity was
recovered in faeces by 48 hours and 86% by 168 hours. Urinary
excretion was 5% of the dose over 168 hours. There was no evidence
of retention of label in tissues at 168 hours (Johnston et al.,
1990b).
In a study using animals with cannulated bile ducts, 15 mg
ion/kg bw diquat dichloride was injected i.p. in rats and 5 mg
ion/kg bw in guinea-pigs and rabbits. Some of the 14C-labelled
dose of diquat was excreted in the bile (1.4% in the rat, 4.8% in
the guinea-pig and 2.9% in the rabbit) (Hughes et al., 1973).
In lactating cows, very little (< 2%) radioactivity occurred
in milk after administration of 14C-diquat and < 5% was found in
the urine (Stevens & Walley, 1966). In a further study in a cow,
14C-diquat was administered in the diet at a concentration of 30
ppm for 7 days. Faeces contained 91% of the dose mostly as
unchanged diquat. Only 0.4% was excreted in urine. Traces (0.004%)
of label were found in milk (Leahy et al., 1976). In a further
study in cows, 14C-labelled diquat and its photodegradation
products were fed with barley. The vast majority of the dose was
excreted in the faeces within 10 days, 0.4% in urine and a low level
in milk (Hemingway et al., 1974).
In a single goat, 96% of the label from a single oral dose of
14C-diquat (7 mg/kg bw) was excreted within 7 days. The vast
majority of the label was found in faeces (94%). Only 0.0175% was
detected in milk (Griggs & Davis, 1975).
Diquat is poorly absorbed through human skin in vitro (Scott
& Corrigan, 1990; Scott et al., 1991) but skin of rats, mice,
rabbits and guinea-pigs are more permeable (Scott & Corrigan, 1990).
Diquat is also very poorly absorbed through human skin in vivo
(Feldmann & Maibach, 1974). Unlike paraquat, diquat is not
selectively taken up by the lungs (see below).
Biotransformation
Results obtained by Daniel & Gage (1966) led to the conclusion
that a substantial proportion of orally administered diquat was
metabolized by gut flora. However this was based on poor recovery
of diquat from the faeces and it is probable that gut flora
metabolism was overestimated.
After administration of 14C-diquat to Wistar rats in aqueous
solution by gavage (45 mg ion/kg bw), the major excreted product was
diquat in both urine (5% of dose) and faeces (> 57% of dose).
Diquat monopyridone was the main metabolite mainly in faeces (5% of
dose), but a minor one in the urine. Following subcutaneous
injection (10 mg ion/kg bw), 75% of the dose was present in the
urine as diquat, about 3% as diquat monopyridone and 6% as the
dipyridone. Studies in vitro suggested that the caecal microflora
of the rat can metabolize diquat to the monopyridone (Mills, 1976).
After oral administration of 14C-diquat to rats (strain not
stated) at 100 mg/kg bw, the major excreted component was diquat in
both urine and faeces. In urine, diquat comprised 75-80% of the
radioactivity, while about 1% (collectively) was picolinic acid,
diquat dipyridone and diquat monopyridone. In faeces, only diquat
was observed except in females where another small component was
found (Williams et al., 1991).
In another study in Wistar rats, in which a single oral dose of
14C-labelled diquat dibromide (> 97% purity) was administered at
100 mg ion/kg bw. The major labelled component in both faeces and
urine was diquat (Johnston et al., 1991).
Hughes et al. (1973) showed that rabbits metabolized 18% of
an i.p. 14C-labelled dose of diquat. Approximately 3% of the dose
was excreted in the bile as an unidentified metabolite.
In lactating cows fed 14C-labelled diquat, most of the small
amount of diquat in the milk appeared as metabolites (Stevens &
Walley, 1966). Urine also largely contained breakdown products. In
another study in a cow fed straw containing 14C-diquat and its
degradation products, residues present at low levels in the milk
were diquat, diquat monopyridone, 1,2,3,4-tetrahydro-1-oxo-pyrido
(1,2a)-5-pyrizinium salt (TOPPS), picolinic acid and picolinamide
(Hemingway et al., 1974).
In a goat, diquat was the major component in the faeces.
Diquat monopyridone was also detected. The main compounds observed
in urine in the first day were diquat monopyridone (2-4%) and diquat
(20%). In milk, 22% of the radioactivity was present as diquat, 13%
as TOPPS and 7% as diquat monopyridone (Griggs & Davis, 1975). In
another study, straw containing diquat and its degradation products
was fed to goats. The main residues present in the milk were diquat
and TOPPS, but both were present at extremely low levels (Hemingway
et al., 1973). In studies in sheep and cattle fed silage
containing diquat, residues were not detected in meat or milk: 40-
45% of the ingested diquat was excreted in the faeces and less than
10% in the urine (Black et al., 1966). The authors hypothesized
that the balance was biotransformed in the gut.
The metabolism of diquat in animals is presented in Figure 1
where I = diquat ion, II = diquat monopyridone, III = 1,2,3,4-
tetrahydro-1-oxo-pyrido (1,2a)-5-pyrizinium salt (TOPPS); IV =
picolinamide, V = picolinic acid and VI = diquat dipyridone.
Effects on enzymes and other biochemical parameters
Unlike paraquat, diquat is not actively taken up by lung slices
(Rose et al., 1975; Kurisaki & Sato, 1979), and higher
concentrations of diquat are necessary for stimulating production of
CO2 from glucose in rat lung slices (Rose et al., 1976). The
difference in accumulation of diquat and paraquat by lungs is
responsible for the major difference in toxicity between the two
compounds (Rose & Smith 1977, Sharp et al., 1972). Lung toxicity
is not characteristic of diquat poisoning (Smith & Rose, 1977).
However, there are analogies between the two compounds at the
cellular level and it is likely that the cytotoxicity of diquat is
caused by radical formation (Baldwin et al., 1975). Hepatocytes
from old rats were reported to be more susceptible to diquat-induced
cytotoxicity than those from young rats (Rikans & Cai, 1993; Rikans
et al., 1993). Diquat is readily reduced to form a green-coloured
free radical which, in aerobic environments, is oxidized by
molecular oxygen generating the superoxide anion radical and diquat.
Rose et al. (1974) reported that diquat (and paraquat)
increased the response of the rat adrenal cortex to ACTH. However
it was later reported, on the basis of studies in vitro in the rat
adrenal and in vivo in rats, that increased adrenal
steroidogenesis was caused by ACTH release from the adenohypophysis
(Crabtree & Rose, 1976).
In rats treated orally with diquat at 540 µmol/kg bw, decreased
clearance of inulin, aminohippuric acid and N-methyl nicotinamide
were noted. A haemoconcentration was also observed and Lock (1979)
hypothesized that this resulted from redistribution of fluid into
the gut lumen, consequent alteration in renal haemodynamics and
reduction in renal excretory function. Diquat was also toxic to
renal tubular cells, producing proteinuria and glycosuria in the rat
(Lock & Ishmael, 1979)
In some long-term studies with diquat, cataractogenesis was
observed. The mechanism is not clear since despite low levels of
ascorbic acid in the lens, feeding ascorbic acid did not prevent
cataract formation (Pirie & Rees, 1970). Diquat free radicals may
be formed in the lens under certain circumstances. The role of OH
and H202 produced by reaction of diquat free radical with 02 may
be important in generating cataracts (Bhuyan & Bhuyan, 1991).
Toxicological studies
Acute toxicity studies
The results of acute toxicity studies of diquat are presented
in Table 1. Diquat has been classified by WHO as moderately
hazardous (WHO, 1992).
Short-term toxicity studies
Rats
A 90-day feeding study in rats was carried out using groups of
12 male and 12 female Alpk:APfSD rats and technical diquat
dibromide, containing 26.9% (w/v) diquat ion. The rats received 0,
20, 100 or 500 ppm diquat. The clinical condition of the animals,
including body-weight gain and food consumption were monitored
during the study and the eyes were examined ophthalmoscopically.
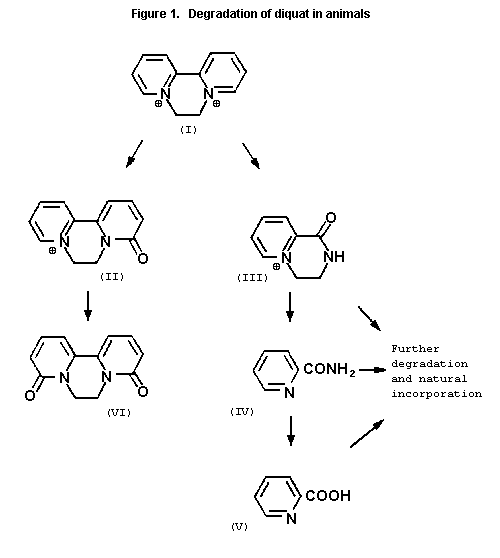 Table 1. Acute toxicity of diquat (dibromide unless otherwise stated)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Mouse Alderley Park M p.o. 125 Clark & Hurst (1970)
(106-146)
p.o. 170 WHO (1984)
Rat Alderley Park F p.o. 231 Clark & Hurst (1970)
(194-274)
Wistar M p.o. 231 Pritchard (1986)
Alpk:AP (211-254.5)
Wistar M p.o. 214 McCall & Robinson (1990a)
Alpk:APfSD (180-271)
Wistar F p.o. 222 McCall & Robinson (1990a)
Alpk: APfSD (203-241)
Alderley Park M s.c.1 11 Clark & Hurst (1970)
(5-15)
Alderley Park F s.c.1 10 Clark & Hurst (1970)
(6-14)
Alderley Park F s.c. 11 Clark & Hurst (1970)
(9-12)
Wistar (Alpk:AP) M p.c.2 > 400 Southwood (1987a)
Wistar Alpk:AP F p.c.2 > 400 Southwood (1987a)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Alderley Park ? p.c. 50-100 Parkinson (1974a)
albino
Sprague-Dawley M inh3 121 µg/l Bruce (1985)
CD (0.3-63 300)
Sprague-Dawley F inh3 132 µg/l Bruce (1985)
CD (0.3-141 560)
Rabbit ? F p.o. 101 Clark & Hurst (1970)
(72-138)
? F p.c. > 400 Clark & Hurst (1970)
New Zealand ? p.c. 50-100 Parkinson (1974b)
White
Guinea-pig ? F p.o. 100 Clark & Hurst (1970)
Dogs ? F p.o. 100-200 Clark & Hurst (1970)
Hens ? F p.o. 200-400 Clark & Hurst (1970)
Cattle ? ? p.o. 30 Walley (1987)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Cattle ? F p.o. 30 Clark & Hurst (1970)
Monkey Cynomolgus ? p.o.1 100-300 Cobb & Grimshaw (1979)
Notes
1) diquat dichloride
2) administered as 21.2% diquat ion
3) whole body 240 min exposure to aerosol
Urine and blood were examined during the study. At autopsy, blood
was taken for clinical chemistry and haematology and selected organs
weighed and examined histologically. Animals receiving diquat at a
concentration of 500 ppm showed a marked reduction in weight gain,
food consumption and utilization. Cataract formation was also
observed at this dose at 8 weeks and at autopsy, corneal opacity was
observed in 7/12 males and 4/12 females; at histological
examination, cataract was seen in 12/12 males and 11/12 females.
Additionally, a low incidence of focal inflammation of the tongue
and epithelium of the palate was observed. Reduced plasma protein
was seen at 500 ppm, possibly caused by low food intake. Absolute,
but not relative, organ weights were reduced, almost certainly a
reflection of poor food intake. Treatment-related effects were not
seen at 100 ppm. The NOAEL was 100 ppm, equal to 8.5 and 9.2 mg
ion/kg bw/day in males and females, respectively (Hodge, 1989a).
Dogs
A one-year study in dogs was carried out using diquat dibromide
technical (26.7 w/v ion) added to the feed. Groups of 4 male and 4
female beagles received diquat at doses of 0, 0.5, 2.5 or 12.5 mg
ion/kg bw/day. Clinical condition, body weight and food consumption
were monitored throughout. Ophthalmoscopy, haematology and clinical
chemistry were carried out. The animals were sacrificed and
autopsied at 52 weeks and a range of organs examined and processed
for histological examination. There was a small but statistically
significant decrease in body-weight gain in the high-dose groups of
both sexes, somewhat greater in the females. No such decrease was
seen at the lower doses. There was a statistically significant
decrease in WBC and neutrophil count in males of all treatment
groups at a single time point (week 4) which was ascribed to raised
counts in one control. There were decreased platelet counts in top
dose females at 4, 26 and 52 weeks. Raised plasma chloride levels
observed in the top dose animals were attributed to bromide ion
interference. Plasma triglyceride in the males given 12.5 mg ion/kg
bw/day were higher than in the controls throughout the study; at 4
and 26 weeks these increases were statistically significant.
Statistically significant increases in relative and absolute kidney
weight were observed in both sexes at 12.5 mg ion/kg bw/day. There
were decreases in absolute and relative adrenal weight in all
treatment groups in the males, which were statistically significant
only in the case of relative weights. Additionally, there was a
decrease in the absolute and relative weight of the epididymides in
all test groups compared to the controls; this finding was only
statistically significant for absolute weights in the 2.5 mg ion/kg
bw/day group and for relative weights in the top dose group.
Changes in organ weights did not correspond to any histopathological
changes. Cataract was seen in all the top dose animals by the end
of the study, both by ophthalmoscopy and histologically and in 2/4
females receiving diquat at a dose of 2.5 mg ion/kg bw/day; however
in the latter group only one was confirmed by histopathological
examination. Inflammatory changes were seen at the top dose in the
large intestine, consisting of reduction in mucosal thickness, loss
and abnormality of mucosal glands, epithelial hyperplasia in crypts
and increased goblet cell activity. The NOAEL was 0.5 mg ion/kg
bw/day based upon lens opacity in females at the next dose (Hopkins,
1990).
Long-term toxicity/carcinogenicity studies
Mice
In an 80-week feeding study, groups of CD-1 mice (60/sex) were
fed diquat dibromide (98.5% pure) at dietary concentrations of 30,
150 or 500 ppm. The highest dietary concentration was reduced to
400 ppm after 3 weeks and to 300 ppm after 5 weeks; these changes
were made because of lethal toxicity and the decedent mice were
replaced. Not all groups were started simultaneously: one control
group and the two lower test groups were started about three months
before the other control groups and the highest dosage group. In
general the test material had little clinical effect, except that
marked toxicity, evidenced by huddling, hypoactivity, and an
ungroomed appearance, were observed for the first few weeks at 500
ppm. Clinical recovery appeared complete by the eighth week.
Survival was not affected by treatment. Growth rates were reduced
at the highest dietary concentration and at 150 ppm. At 30 ppm,
there were no effects on body-weight gain. There was an increased
frequency of hepatic vacuolation in livers at 150 ppm (males) and at
the highest dose in both sexes. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day (Ashby, 1987).
In a 2-year study, groups of 60 male and 60 female mice
(C57BL/10JfCD-1/Alpk) were fed a diet containing 0, 30, 100 or 300
ppm diquat. The test material was technical grade diquat dibromide,
containing 26.7% diquat ion w/v. Haematological examination of tail
vein blood samples was carried out on all animals at 53 and 79
weeks. Fuller haematology was carried out on blood obtained by
cardiac puncture at 104 weeks. Various tissues were taken at
autopsy and processed for histological examination. There was
marked toxicity at the highest dose, shown by statistically
significant reductions in body-weight gain in both sexes, small and
sometimes statistically significant reductions in food consumption,
eye discharge and mild nephropathy. Changes in certain
haematological parameters at 300 ppm were also seen, namely
statistically significantly decreased neutrophil count and increased
lymphocyte count in both sexes at weeks 53 and 79. There was also a
significant increase in total WBC at 2 years in males. Relative
kidney weights were increased significantly in males and females.
At 100 ppm there were statistically significant reductions in body-
weight gain in both sexes, particularly in the males later in the
study; there were also some changes in haematology (lymphocytosis
and neutropenia) which, although statistically significant were
probably unimportant, and relative kidney weights were increased
significantly in males. No treatment-related carcinogenic effects
were seen in the study (there were reductions in certain tumour
incidences at 300 ppm) and the incidence of cataract was not related
to the test material. The NOAEL was 30 ppm, equal to 3.6 mg ion/kg
bw/day (males) and 4.8 mg ion/kg bw/day (females) (Hodge, 1992).
Rats
In a two-year feeding study, groups of Wistar-derived rats
(35/sex) received diquat dibromide (100% pure = 53.6% ion) at
dietary concentrations of 0, 15, 25 or 75 ppm. Weight gain and food
consumption did not differ significantly between groups. A
significantly increased incidence of cataracts was observed at 75
ppm. The NOAEL was 25 ppm, equivalent to 1.3 mg ion/kg bw/day
(Rogerson & Broad, 1978).
In another 2-year study, diquat dibromide (technical grade) was
administered in the diet to groups of Sprague-Dawley rats (60/sex)
at dietary concentrations of 0, 5, 15, 75 or 375 ppm. Additionally,
satellite groups of 10 males and 10 females received diquat at the
same dietary concentrations and were killed at 1 year.
Ophthalmoscopy was carried out before dosing and periodically during
the study. Haematological and biochemical variables were measured
on 10 animals from each main group before dosing, and at 26, 52, 78
and 104 weeks, and in five satellite animals at week 52. A
reduction in food consumption and utilization efficacy was observed
at 375 ppm. After 26 weeks, there was a reduction in MCV and in
haemoglobin in females and males, respectively. Minimal reductions
in red blood cell parameters were observed in males at 15 ppm and
above, at 52 and 78 weeks. Other changes were prolonged activated
partial thromboplastin times at 75 and 375 ppm in females at 52 and
104 weeks and at 52 weeks in the 15 ppm females. Blood urea
nitrogen (BUN) was elevated at 52 weeks at 75 and 375 ppm and at 78
weeks in the 375 ppm females. At 52 weeks, the 75 and 375 ppm
groups exhibited lower total protein and albumen levels. On
ophthalmoscopic examination, lenticular opacities were seen in the
75 and 375 ppm groups at 13 weeks and the findings progressed at
subsequent examinations. Ophthalmoscopy at 104 weeks showed severe
lens opacities at 375 ppm in all surviving rats, while less severe
ones were seen at 75 ppm. At 15 ppm, a single instance of cataract
was seen in each sex. Histological examination of the eyes
postmortem, showed advanced cataractogenesis in all animals at 375
ppm and in about 80% of the 75 ppm group. There was a low
prevalence of cataract at 15 ppm: cataract-type changes being seen
in 0/22 controls and 3/22 at 15 ppm (males) and 0/22 controls and
2/20 at 15 ppm (females). Small differences were seen in
nephropathy in arteritis and in aneurism formation in males at the
highest dose. Elevations reported as statistically significant were
seen in the incidence of benign phenochromocytomas at 75 ppm with no
real evidence of a trend and in combined thyroid parafollicular cell
adenomas and carcinomas at 5 ppm, both in males but numbers were
very small. There was evidence of a trend for thyroid follicular
cell adenoma; however these figures appear to have been much
influenced by the frequency of the tumour at 375 ppm (in males the
number of tumours out of the number of thyroid glands examined in
each groups were 2/24, 1/32, 1/25, 0/25 and 3/32 for the control, 5,
15, 75 and 375 ppm groups respectively; none were observed in
females). There was a significant increase in the number of females
with multiple neoplasia and with malignant neoplasms at 5 and 75
ppm. No other change attributable to the test material was seen.
The LOAEL was 15 ppm (equal to 0.58 and 0.72 mg ion/kg bw/day for
males and females) based upon cataractogenesis. The NOAEL was 5
ppm, equal to 0.19 mg ion/kg bw/day (males) and 0.24 mg ion/kg
bw/day (females) (Colley et al., 1985).
Reproduction studies
Rats
A three-generation study was conducted in rats by Fletcher et
al. (1972) using diquat dibromide monohydrate (100% pure).
Wistar-derived rats were divided into 3 groups, each containing 12
males and 24 females. One group received standard diet while the
other two groups received diquat dibromide in aqueous solution at
dietary concentrations of 0, 125 or 500 ppm when they were
approximately 35-days old. Thereafter the three groups and their
progeny remained on the diet throughout the study. Body weights and
food consumption were recorded weekly and the animals physically
examined daily. The F0 animals were mated after 100-days feeding,
one male and two females being housed together for this purpose.
Where pregnancy had not occurred in three weeks, the male was
replaced by another from the same group. Litters were examined
within 20 hours of delivery (F1a). At 21 days, each litter was
counted, weighed sexed and subjected to autopsy. Second matings
were arranged after a 10-day rest period. From the progeny (F1b),
12 males and 24 females were reared and continued on the diet from
weaning, the remainder being killed and examined. At 100 days, the
F1b animals were mated for the production of the second generation
(F2). The F2 generation was treated in the same way as the F1,
the F2a litters being examined and autopsied at 21 days and the F2b
providing the breeders for production of the F3 generation. The
F3a litters were treated as before and the F3b animals were also
autopsied at 21 days and detailed examination, including
histological examination was carried out postmortem. Rats treated
with diquat (500 ppm) developed lens opacity from about 125 days.
The proportion exhibiting this abnormality increased and at 300 days
about 50% were affected. Opacity was not seen at the lower dose.
There was also a statistically significant reduction in weight gain
at 500 ppm, at 15 weeks in both sexes of F0 parents. Similar
reductions (after weaning) were found in the subsequent generations.
In the second generation, significantly reduced body weights were
seen in the 125 ppm females at weaning and the final week before
mating but not at other weighings. There were 6 fewer pregnancies
in the 500 ppm group of F1 animals. Atrophy of the seminiferous
tubules was observed in parents and progeny of each generation.
Although more common in the 500 ppm groups, there was no clear dose-
response relationship. This study did not exhibit an NOAEL since
there was decreased weight gain in F0 and F1 animals at the lowest
dose, but the effects observed at this dose (125 ppm, equivalent to
6.3 mg ion/kg bw/day) were trivial (Fletcher et al., 1972).
A multigeneration study was conducted using technical grade
diquat dibromide, containing diquat ion 26.7% w/v. Groups of
Alpk:APfSD rats (30/sex) were fed diets containing 0, 16, 80 or 400
ppm diquat. After 12 weeks the animals were mated and allowed to
rear the litters that resulted (F1a). The process was repeated
with 30 male and 30 female parents/group selected from the F1a
litter, these F1 parents being mated 11 weeks after selection. The
dose received by the top dose F1 rats was reduced after 4 weeks to
240 ppm. The animals were examined daily and mouths examined
weekly. Animals were weighed weekly in the premating period. After
mating the males were weighed monthly and the females were weighed
on days 1, 8, 15 and 20 of pregnancy and days 1, 5, 11, 16 and 22 of
lactation. All animals were weighed at the terminal kill. Food
consumption was recorded weekly until mating, and weekly in the
females during pregnancy and lactation. Ophthalmoscopic examination
of the eyes was carried out on the controls and top dose group at 12
weeks and on all the F0 animals at termination (24 weeks). A
similar examination was carried out on the F1 rats 4 weeks after
selection, at 11 weeks before mating and at termination (21 weeks).
Diquat had no effect on fertility in either sex. Decreased body-
weight gain was seen at the top dose in both adults (F0 and F1)
and pups. Inflammatory lesions in the mouth, particularly
ulceration of the hard palate was observed at 400 ppm in F0 rats
and F1 pups and adults. Cataracts, first seen at week 12 in F0
females and at week 13 in males and 2 weeks earlier in each sex in
the F1a and F1b, were also observed in the F1 adults. Although
cataract formation was mostly confined to the 240 ppm group, a low
incidence was seen at 80 ppm in the F1 female parents (3/30 at
premating ophthalmoscopic examination and 4/30 at examination before
termination of the study). At the terminal histopathological
examination, cataract was seen in the highest dose group only of F0
and F1 parents. There was an increase in pathological changes in
the renal tract in the F1 and F2 pups. Reproductive toxicity
(reduced pup weight gain) was observed at 400 ppm. The NOAEL was 16
ppm (equivalent to 0.8 mg/kg bw/day) based upon a low incidence of
partial cataract formation at 80 ppm (Hodge, 1990).
Other studies on reproduction parameters
An antifertility effect on male mice was noted during the
dominant lethal study of Pasi et al. (1974). Diquat at doses up
to 10 ppm in the diet of hens for 6 weeks did not affect food
consumption, egg production and hatchability. Residues of diquat
were not found in eggs or in hens' tissues (Edwards & Smith, 1975).
Special studies on embryo/fetotoxicity
Mice
In a study in Swiss-Webster and Sprague-Dawley (CD) mice, a
single dose of 15 mg/kg bw diquat was given i.p. to groups of three
animals at day 7-21 of gestation. Nine maternal deaths out of 45
animals occurred. The percentage dead plus resorbed fetuses was
57%. A NOAEL could not be determined in this study (Bus et al.,
1975).
Groups of 20 female CFLP mice were administered diquat i.p. (11
mg/kg bw Reglone) on day 9 of gestation or diquat (2.7 mg/kg bw/day
Reglone) on days 9-12 of gestation. Both groups showed increased
fetal loss or resorption compared to controls. Skeletal
abnormalities were observed in the test group embryos. A NOAEL
could not be determined in this study (Selypes et al., 1980).
Rats
Diquat was administered in the diet to pregnant Sprague-Dawley
rats, from days 1-20 of pregnancy at 0, 125 or 500 ppm diquat ion.
There were 18 control rats, and 20 in each of the test groups.
Animals were killed at day 20, fetuses removed and the uteri
examined. All rats remained in good condition throughout the study
but food consumption and body-weight gain were reduced significantly
at 500 ppm. There was no adverse effect on implantations, mean
number of fetuses, litter weight or sex ratio. Mean fetal weight
was significantly lower in the 500 ppm group. A dose-related
increase in subcutaneous fetal haemorrhages compared to the controls
was observed. A NOAEL could not be determined in this study (Moore
& Wilson, 1973).
Diquat containing 26.2 % ion w/v was administered by gavage to
groups of 8 female rats (Wistar derived Alpk:APfSD) at doses of 0,
4, 12, 24 or 40 mg ion/kg bw/day in deionized water from days 7-16
of gestation. Controls received deionized water. On day 22 the
females were killed and the uteri examined for live fetuses and
intrauterine deaths. At a dose of 40 mg ion/kg bw/day the mothers
showed reduced weight gain and changes in clinical condition
(piloerection, urinary incontinence and gasping) and 4/24 animals at
24 mg ion/kg bw/day showed milder signs. Some fetotoxicity was seen
at 40 mg ion/kg bw/day (reduced fetal weight gain), but not at the
lower doses, whereas maternal toxicity, as indicated by dose-related
reduced food consumption and body-weight gain, was observed at all
doses. Although there was a statistically non-significant increase
in pre- and post-implantation loss at 24 mg ion/kg bw/day, this was
not observed at the other three doses. No fetal abnormality was
observed. For fetal toxicity, the LOAEL was 40 mg ion/kg bw/day and
the NOAEL 24 mg ion/kg bw/day. The NOAEL for maternal toxicity
could not be determined in this study (Milburn, 1989).
Groups of 24 female rats were given diquat by gavage in
deionized water (26.2 % ion w/v) at doses of 4, 12 or 40 mg ion/kg
bw/day from days 7-16 of gestation. Maternal toxicity was seen at
40 mg ion/kg bw/day as reduced weight gain and food consumption.
Significant reductions in fetal weight, litter weight and gravid
uterine weight as well as fetal defects in ossification were seen at
40 mg ion/kg bw/day. Minor evidence of reduced ossification were
seen at the lower doses but these were considered not to be of
biological significance. For both maternal and fetotoxicity the
LOAEL was 40 mg ion/kg bw/day and the NOAEL 12 mg ion/kg bw/day
(Wickramatne, 1989).
Rabbits
Groups of up to 20 female mated Dutch rabbits were dosed orally
with diquat dibromide (100% pure = 53.6% ion) at levels of 1.3, 2.5
or 5.0 mg ion/kg bw/day from days 1-28 of gestation (day of mating =
day 0). The material was administered in "Dispersol OG" (a 10%
solution of ricinoleic glycerides with glycerol and polyglycerols),
which was also used as control. An additional 4 does were allowed
to litter and the eyes of the offspring examined for cataracts. The
rabbits were sacrificed at day 29 and the uteri examined for live
fetuses and intrauterine deaths. The fetuses were weighed and
examined for gross abnormality and about half processed for skeletal
examination and half for soft tissue examination. There was a
reduction in maternal weight gain at 5.0 mg ion/kg bw/day, which was
not statistically significant. There was no evidence of any effect
on embryonic or fetal development. The NOAEL was 2.5 mg ion/kg
bw/day based on mild maternal toxicity at the highest dose (Hodge,
1987). A feature of this study was the poor pregnancy rate in all
groups which necessitated mating of extra animals to achieve
acceptable numbers of pregnancies per group.
In a second study, groups of 7 or 8 female New Zeeland white
rabbits were administered diquat dibromide technical by gavage in
deionized water (26.2% ion w/v) at doses of 0, 1, 3, 7 or 10 mg
ion/kg bw/day from days 7-19 of gestation. Controls received water.
On day 30 of gestation, the females were killed and the uteri
examined for live fetuses and intrauterine deaths. Doses of 3 mg
ion/kg bw/day or above were associated with maternal toxicity as
manifested by weight loss or reduced weight gain and reduced food
intake. No evidence of fetotoxicity was observed. The NOAEL was 1
mg ion/kg bw/day based upon maternal toxicity (Hodge, 1989b).
In a third study, groups of 20 New Zeeland white rabbits were
administered by gavage diquat dibromide technical (26.2% ion w/v) in
deionized water, at doses of 0, 1, 3 or 10 mg ion/kg bw/day from
days 7-19 of gestation. On day 30 of gestation the females were
killed and the uteri examined for live fetuses and intrauterine
deaths. The fetuses were weighed, examined externally and for
visceral abnormalities, eviscerated and stained for skeletal
abnormalities. Doses of 10 mg ion/kg bw/day caused maternal
toxicity as shown by weight loss and reduced food intake; five
animals from this group were sacrificed early in extremis.
Effects on body weight and food consumption, although less severe,
were also present at 3 mg ion/kg bw/day. Some evidence of
fetotoxicity was observed at 10 mg/kg bw/day (mottled and friable
livers and small increase in minor skeletal defects at 3 and 10 mg
ion/kg bw/day, in the form of partially ossified sternabrae). The
elevation in the proportion of fetuses with minor skeletal defect
was significant at 3 and 10 mg ion/kg bw/day; there was a non-
significant increase at the lowest dose group. The NOAEL was 1 mg
ion/kg bw/day based on maternal toxicity (reduced weight gain and
food consumption) and skeletal effects in the fetuses at doses of 3
mg ion/kg bw/day (Hodge, 1989c).
Special studies on genotoxicity
Based on the results of the genotoxicity assays given in Table
2, the Meeting concluded that diquat was not genotoxic.
Toxicity of metabolites
In Wistar-derived rats, diquat dipyridone and monopyridine were
less toxic than diquat when given subcutaneously (Parkinson, 1974b;
Crabtree, 1976).
The results of acute and genotoxicity studies with TOPPS are
given in Tables 3 and 4, respectively.
Other animal studies
Diquat dichloride and dibromide are both moderate skin
irritants to the skin of the rat and mildly irritant to the rabbit
eye (Parkinson, 1974). Orally administered diquat increases
secretion into the gut lumen (Crabtree et al., 1977; Rawlings et
al., 1992). In a study to detect any other major actions of
diquat in laboratory animals, single doses of up to 280 mg/kg bw
were administered orally to rats. Effects such as CNS depression
and increased gut motility were observed at perilethal doses only
(Allen & Brammer, 1990).
Table 2. Results of genotoxicity assays on diquat
Test system Test object Concentration of diquat Purity Results Reference
In vitro
Ames test S. typhimurium 0.01-50 100% -ve Shirasu et al. (1979)
(5) (strains TA1535, µg/plate
1537, 1538, 98, 100)
Ames test S. typhimurium 0.00256-100 100% -ve Callander (1986a)
(5) (strains TA1535, µg/plate
1537, 1538, 98, ± S9
100
Ames test S. typhimurium 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
(5) (strains TA1535, with S9, ion (technical)
1537, 1538 0.1-50 µg/plate
98, 100) without S9
Reverse E. coli WP2 hcr 0.01-50 µg/plate 100% -ve Shirasu et al. (1979)
mutation (5)
Reverse E. coli WP2 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
mutation (5) uvrA pKM101 with S9, ion (technical)
0.1-50 µg/plate
without S9
Mammalian Mouse lymphoma 6.25-100 µg/ml 100% -ve Cross (1986a)
cell (5) L5178Y TK+/-
Mammalian Mouse lymphoma 6.25-100 µg/ml 25.8% w/w -ve Cross (1986b)
cell (5) L5178Y TK+/- ion (technical)
Table 2 (contd)
Test system Test object Concentration of diquat Purity Results Reference
Cytogenetics Human lymphocytes 26.7, 107, 267(2), 100% +ve (4) Wildgoose et al. (1986)
(1) 534.8(3) µg/ml as ion
Cytogenetics Human lymphocytes 12.9-129 µg/ml 25.8% w/w +ve (4) Richardson et al. (1986)
(1) ion (technical)
Rec-assay B. subtilis 2-200 µg/disc 100% -ve Shirasu et al. (1979)
H17, M45
In vivo
Cytogenetics Rat bone marrow 4.4, 9.5, 14, 100% -ve Anderson et al. (1980)
(Wistar-derived mg/kg bw/day
Alderley Park) for 5 days orally
Cytogenetics Mouse bone marrow 0.73, 3.6, 7.3, 22 100% -ve Selypes et al. (1980)
(CFLP) mg/kg bw ip
90 mg/kg/bw/po
Micro-nucleus Mouse bone marrow 0, 62.5, 100 mg/kg 25.8% w/w -ve Sheldon et al. (1986)
C57BL/6J/Alpk bw ion (technical)
Dominant Mouse (CD-1) 0.1-10 mg ion/kg 28.6% w/w -ve Anderson et al. (1976)
Lethal Test bw/day for 5 days ion (technical)
UDS Rat hepatocyte 225, 450, 900 25.8% w/w -ve Trueman et al. (1987)
in vivo mg/kg ion ion (technical)
1) ± S9
2) Donor 1 MTD
3) Donor 2 MTD
4) Clastogenic only at doses causing cytotoxicity
5) with and without metabolic activation
Table 3. Acute toxicity of TOPPS
Species Strain Sex Route LD50 (mg/kg bw) References
Rat Wistar Alpk:A M PO 2449 Southwood (1987b)
(2000-3000)
Wistar Alpk:A F PO 2942 Southwood (1987b)
(2000-5000)
Table 4. Results of genotoxicity assays on TOPPS
Test system Test object Concentration of TOPPS Purity Results Reference
Ames (1) S. typhimurium 0.1-5 mg/plate 98% -ve Ohta (1987)
TA1535, TA1537,
98, 100
E. coli 0.1-5 mg/plate 98% -ve Ohta (1987)
WP uvrA
Rec Assay (1) B. subtilis 0.2-10 mg/disc 98% -ve Ohta (1987)
(1) With or without metabolic activation.
Special studies on cataractogenesis
Pirie & Rees (1970) fed Wistar albino rats a diet containing
0.05 or 0.075% diquat dibromide. Lens opacity was produced after 4-
8 months at both doses. Ascorbic acid content of the lens, but
unusually not GSH content, decreased during development of cataract.
Radioactivity appeared in the lens after intraperitoneal injection
of 14C-labelled diquat. Pirie et al. (1970) suggested that free-
radical formation might be responsible for cataract formation.
Observations in humans
Vanholder et al. (1981) reviewed several cases of poisoning
with diquat. In one case, initial signs and symptoms were
abdominal. Later oliguria and coma developed. Shock supervened
followed by cardiac arrest. In another case progression was slower
but renal failure occurred and eventually led to anuria. Despite
haemodialysis, death occurred from ventricular fibrillation, and
renal failure was observed. Unlike poisoning with paraquat, diquat
does not cause lung fibrosis. Cataracts have not been observed in
humans (IPCS, 1984) and more recent investigations have not shown
cataract formation in those engaged in diquat manufacture or
formulation (Bonsall, 1990).
Two patients were splashed in the eyes with a preparation
containing both paraquat and diquat. In both cases the corneal
epithelium was damaged and healing was slow (Nirei et al., 1993).
In a recent report, a child survived what was initially
believed to be a fatal dose of diquat, apparently with no sequelae
(Buckley & MacKiernan, 1991, 1992). A recent case report describes
a patient who developed Parkinsonism a few days after exposure to
diquat (Sechi et al., 1992). The extent of exposure of the
patient to the pesticide was not known and thus the significance of
this isolated observation in a 72-year old man is questionable.
COMMENTS
When administered orally 14C-diquat is poorly absorbed from
the gastrointestinal tract of rats, cows and goats and mainly
eliminated vis the faeces during the first 24 hours, the small part
absorbed being principally eliminated via the urine. The total
percentages of administered doses eliminated via the faeces were 94,
91 and 94 for the rat, cow and goat, respectively; 3.1% and 0.4%
were eliminated in the urine of the rat and the cow, respectively,
and very small percentages of radioactivity were found in cow's and
goat's milk (0.004% and 0.0175%), respectively.
After oral administration of 14C-diquat to rats (45 mg ion/kg
bw), the major excreted product was diquat in both urine (5% of
dose) and faeces (> 57% of dose): diquat monopyridone was the main
metabolite in the faeces (5% of dose), but a minor one in the urine.
In another oral study in rats (100 mg ion/kg bw), a small amount of
diquat dipyridone and picolinic acid was found in addition to the
monopyridone. After subcutaneous injection (10 mg ion/kg bw) in the
rat, 75% of the dose was present in the urine as diquat, about 3% as
the monopyridone and 6% as the dipyridone.
Unlike paraquat, diquat is not actively taken up by lung
slices, and lung toxicity is not characteristic of diquat poisoning.
The acute oral toxicity of diquat varies with species, but is
between 125 and 250 mg ion/kg bw in rodents. It is classified by
WHO as moderately hazardous.
In a 90-day feeding study in rats, using dietary concentrations
of 0, 20, 100 or 500 ppm, the NOAEL was 100 ppm, equal to 8.5 mg
ion/kg bw/day, based upon reduction in body-weight gain, food
consumption and reduced plasma protein at the next higher dose.
In a one-year feeding study, dogs received doses of 0, 0.5, 2.5
or 12.5 mg/kg bw/day. The NOAEL was 0.5 mg ion/kg bw/day based upon
lens opacity in females at the next dose.
Two long-term toxicity/carcinogenicity studies were conducted
in mice. The first (80 weeks) used dietary concentrations of diquat
ion of 0, 30, 150 or 500 ppm. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day, based upon reduced growth rates at the next
higher dose together with hepatic vacuolation in males. In a 2-year
study in mice, in which dietary concentrations of 0, 30, 100 or 300
ppm were used, the NOAEL was 30 ppm, equal to 3.6 mg ion/kg bw/day,
based on reduction in body-weight gain and increased relative kidney
weights at the next higher dose. There was no evidence of
carcinogenicity in mice.
Two 2-year feeding studies in rats have been conducted. In the
first study, diquat dibromide was administered in the diet at
concentrations of 0, 5, 15, 75 or 375 ppm. The NOAEL was 5 ppm,
equal to 0.19 mg ion/kg bw/day, based upon cataract formation in the
15 ppm group. In the second study, dietary concentrations of 0, 15,
25 or 75 ppm diquat ion were used. The NOAEL was 25 ppm (equivalent
to 1.3 mg ion/kg bw/day), based on cataract formation at the next
higher dose. There was no evidence of carcinogenicity in rats.
Numerous teratogenicity studies have been conducted. NOAELs
could not be determined in two mouse studies. There were three
teratogenicity studies in rats; in the first study dietary
concentrations of 0, 125 or 500 ppm diquat ion were used. A dose-
related increase in subcutaneous fetal haemorrhages compared to the
controls was observed. A NOAEL could not be derived from this
study. In the second study, diquat was administered at oral doses
of 0, 4, 12, 24 or 40 mg ion/kg bw/day. For fetal toxicity, the
NOAEL was 24 mg ion/kg bw/day, but maternal toxicity was observed in
all test groups (reduced weight gain and food consumption). In the
third study, diquat was administered by gavage at doses of 0, 4, 12
or 40 mg ion/kg bw/day. The NOAEL for both maternal and fetal
toxicity was 12 mg ion/kg bw/day, based in the case of the dams on
reduced body weight and food consumption and in the case of the
fetuses on reduced fetal weight and defects in fetal ossification at
the highest dose.
In a study in rabbits, diquat was given orally at doses of 0,
1.3, 2.5 or 5.0 mg ion/kg bw/day. There was no evidence of any
effects on embryonic or fetal development. The NOAEL was 2.5 mg
ion/kg bw/day based on mild maternal toxicity at the highest dose.
In a second study in rabbits, doses of 0, 1, 3, 7 or 10 mg ion/kg
bw/day were administered by gavage. Doses of 3 mg ion/kg bw/day or
above were associated with maternal toxicity as manifested by weight
loss or reduced weight gain and reduced food intake. No evidence of
fetotoxicity was observed. The NOAEL was 1 mg ion/kg bw/day based
upon maternal toxicity. In a third study in rabbits, doses of 0, 1,
3 or 10 mg ion/kg bw/day diquat were given by gavage. The NOAEL was
1 mg ion/kg bw/day based upon maternal toxicity (reduced weight gain
and food consumption) and skeletal effects in the fetuses at doses
of 3 mg ion/kg bw/day.
Two multigeneration reproduction studies were conducted in
rats. In the first study, diquat was given at dietary
concentrations of 0, 125 or 500 ppm. This study did not exhibit an
NOAEL, since there was decreased weight gain in F0 and F1 animals
at the lowest dose, but the effects observed at this dose (125 ppm,
equivalent to 6.3 mg ion/kg bw/day) were trivial. In the second
study, rats were fed diquat at dietary concentrations of 0, 16, 80
or 400 ppm. The NOAEL was 16 ppm (equivalent to 0.8 mg/kg bw/day)
based upon a low incidence of partial cataract formation at 80 ppm.
Diquat has been adequately tested in a series of genotoxicity
assays in vitro and in vivo. Chromosomal aberrations were
induced in vitro but there was no other evidence of genotoxicity.
The Meeting concluded that diquat was not genotoxic.
An ADI of 0-0.002 mg/kg bw was established based upon a NOAEL
of 0.19 mg ion/kg bw/day identified in a two-year study in rats,
using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 30 ppm, equal to 3.6 mg ion/kg bw/day
(two-year study)
Rat: 5 ppm, equal to 0.19 mg ion/kg bw/day
(two-year study)
12 mg ion/kg bw/day (teratogenicity study)
16 ppm, equivalent to 1.6 mg ion/kg bw/day
(multigeneration reproduction study)
Rabbit: 1 mg/kg bw/day (teratogenicity study)
Dog: 0.5 mg ion/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.002 mg ion/kg bw
Studies which will provide information in the continued evaluation
of the compound
Observations in humans.
REFERENCES
Anderson, D., McGregor, D.B. & Purchase, I.F.H. (1976). Dominant
lethal studies with paraquat and diquat in male CD-1 mice. Mutation
Research, 40: 349-358.
Anderson, D., Richardson, C.R., Howard, C.A., Banham, P., Hart, D. &
Weight, T.M. (1980). Unpublished report. Diquat: a cytogenetic study
in the rat. Report CTL/P/366. Imperial Chemical Industries PLC,
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Allen, S.L. & Brammer, A. (1990). Unpublished report. Diquat:
pharmacological evaluation. Report No CTL/P/2842*(revised). ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Ashby, R. (1987). Unpublished report. Diquat dibromide monohydrate:
evaluation of potential carcinogenicity in dietary adminstration to
mice for 80 weeks. Final Report CTL/C/3761 (LSR 87/ILY001/375). Life
Sciences Research Ltd, Eye, Suffolk, England. Supplied to WHO by
Zeneca. Vols 1 & 2.
Baldwin, R.C., Pasi, A., McGregor, J.T. & Hine, C.H. (1975). The
rates of radical formation from the dipyridilium herbicides
paraquat, diquat, and morphamquat in homogenates of rat lung,
kidney, and liver: an inhibitory effect of carbon monoxide. Toxicol
Appl Pharmacol., 32: 298-304.
Bhuyan, D.K. & Bhuyan, K.C. (1991). Oxy radicals in the eye tissues
of rabbits after diquat in vivo. Free Radic Res Commun. 12-13
Pt.2: 621-627.
Black, W.J.M., Calderbank, A., Douglas, G. & McKenna, R.H. (1966).
Residues in herbage and silage and feeding experiments following the
use of diquat as a dessicant. J Sci Food Agric., 17: 506-509.
Bonsall, J.L. (1990). Letter to F Tripet, Stauffer Chemicals,
Switzerland dated October 9 1990.
Bruce, E.D. (1985). Unpublished report. The acute inhalation
toxicity of diquat water weed killer (SX-1574) in rats. Report
CTL/C/2701 (SOCAL 2353). Chevron Environmental Health Center,
Richmond, California. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Buckley, D.A. & McKiernan, J. (1991/1992). Survival after ingestion
of a fatal dose of diquat (letter). Irish Med. J., 84: 134.
Bus, J.S., Preache, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
Sharp, C.W. & Gibson, J.E. (1975). Fetal Toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl Pharmacol.,
33: 450-460.
Callander, R.D. (1986a). Unpublished report. Diquat dibromide: an
evaluation in the Salmonella mutagenicity assay. Report
CTL/P/1413. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Callander, R.D. (1986b). Unpublished report. Diquat dibromide
(technical): an evaluation in the Salmonella mutagenicity assay.
Report CTL/P/1463. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Clark, D.G. & Hurst, E.W. (1970). The toxicity of diquat. Brit J
Industr Med., 27: 51-55.
Cobb, L.M. & Grimshaw, P. (1979). Acute toxicity of oral diquat
(1,1'-ethylene-2,2'-bipyridinium) in cynomolgus monkeys. Toxicology
Appl Pharmacol. 51: 277-282.
Colley, J., Warren, S., Heywood, R., Street, A.E., Almond, R.H. &
Gopinath, C. (1985). Unpublished report. Diquat dibromide evaluation
of potential carcinogenicity and chronic toxicity by prolonged
dietary administration to rats. Report ICI 406/83763. Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, England. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. (1976). Unpublished report. Comparison of the
subcutaneous toxicity of diquat, diquat monopyridone and diquat
dipyridone. Report CTL/P/351. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. & Rose, M.S. (1976). Unpublished report. Early
effects of diquat on plasma corticosteroid concentrations in rats.
Report CTL/R/380. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C., Lock, E.A. & Rose, M.S. (1977). Effects of diquat on
the gastrointestinal tract of rats. Toxicology Appl Pharmacol.,
41: 585-595.
Cross, M.F. (1986a). Unpublished report. Diquat dibromide:
assessment of mutagenic potential using L5178Y mouse lymphoma cells.
Report CTL/P/1554. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Cross, M.F. (1986b). Unpublished report. Diquat dibromide
(technical): assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Report CTL/P/1602. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Daniel, J.W. & Gage, J.C. (1966). Absorption and excretion of diquat
and paraquat in rats. Brit J Industr Med., 23: 133-136.
Daniel, J.W. & Henson, A.F. (1960). Unpublished report. The
absorption and excretion of the herbicide K.8483 in rats. Report
IHR/137. Industrial Hygiene Research Laboratories and Akers Research
Laboratories. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Edwards, M.J. & Smith, D.C. (1975). Unpublished report. Diquat:
residue transfer and hatchability study in laying hens. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Feldmann, K.J. & Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicol appl Pharmacol,,
28: 126-132.
Fletcher, K., Griffiths, D. & Kinch, D.A. (1972). Unpublished
report. Diquat dibromide: three-generation reproduction study in
rats. Report HO/IH/R/331A. Imperial Chemical Industries Ltd
Industrial Hygiene Research Laboratories. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Griggs, R.E. & Davis, J.A. (1975). Unpublished report. Diquat:
excretion and metabolism in a goat. Report AR 2585 A. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Griggs, R.E. (1973).
Unpublished report. Metabolism of diquat and its photoproducts in
goats. Report AR 2448 B. ICI Plant Protection Ltd. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Burgess (1974).
Unpublished report. Diquat: metabolism of diquat and its
photoproducts in a cow. Report AR 2448 B. ICI Plant Protection Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1987). Unpublished report. Diquat dibromide:
teratogenic studies in the rabbit. Report OH/CTL/P114B. Imperial
Chemical Industries PLC, Alderley Park, Macclesfield, Cheshire, UK.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989a). Unpublished report. Diquat: 90 day feeding
study in the rat. Report no CTL/P/1832 (revised). Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, UK. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989b). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2196. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989c). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2379. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1990). Unpublished report. Diquat: multigeneration
study in the rat. Report CTL/P/2462. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1992). Unpublished report. Diquat: two year feeding
study in mice. Report CTL/P/3409. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hopkins, M.N. (1990). Unpublished report. Diquat: 1 year feeding
study in dogs. Report CTL/P/2596. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hughes, R.D., Millburn, P. & Williams, R.T. (1973). Biliary
excretion of some diquaternary ammonium cations in the rat, guinea-
pig and rabbit. Biochem J., 136: 979-984.
IPCS (1984). Environmental Health Criteria 39 paraquat and diquat.
International Programme on Chemical Safety, WHO, Geneva.
Johnston, A.M., Jones, C., McCullum, J., Mutch, P.J. & Scott, G.
(1990a). The elimination of 14C-diquat in the rat following single
dose oral administration (low level). Report no CTL/C/2554. Inveresk
Research International, Tranent, EH33 2NE, Scotland. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Mutch, P.J. & Scott, G. (1990b). The elimination of
14C-diquat in the rat following single dose oral administration
(high dose level). Report CTL/C/2555. Inveresk Research
International, Tranent, EH33 2NE, Scotland. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Jones, C., McCallam, J. & Scott, G. (1991).
Unpublished report. The disposition of 14C-diquat in the rat.
Report CTL/C/2553. Inveresk Research International, Tranent, EH33
2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Kurisaki, E. & Sato, H. (1979). Tissue distribution of paraquat and
diquat after oral administration in rats. Forensic Science, 14:
165-170.
Leahy, J.P., Gatehouse, D.M., Carpenter, P.K. & Benwell, M. (1976).
Diquat: metabolism and residue in a cow. ICI Plant Protection
Division. Report No. AR2698A. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Lock, E.A. (1979). The effect of paraquat and diquat on renal
function in the rat. Toxicol Appl Pharmacol., 48: 327-336.
Lock, E.A. & Ishmael, J. (1979). The acute toxic effects of paraquat
and diquat on the rat kidney. Toxicol Appl Pharmacol., 50: 67-76.
McCall, J.C. & Robinson, P. (1990a). Unpublished report. Diquat
dibromide: acute oral toxicity to the rat. Report CTL/P/2999. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
McCall, J.C. & Robinson, P. (1990b). Unpublished report. Diquat
dibromide: acute dermal toxicity to the rat. Report CTL/P/2982. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Milburn, G.M. (1989). Diquat: embryotoxicity study in the rat.
Unpublished report CTL/P/2275. ICI Central Toxicology Laboratory.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Mills, I.H. (1976). Unpublished report. Diquat: disposition and
metabolism in the rat.Report CTL/P/214. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Moore, S. & Wilson, G.J. (1973). Unpublished report. Diquat
dibromide: teratogenicity studies in the rat. Report No HO/IH/P/82B.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey.
Nirei, M., Hayasaka, S., Nagata, M., Tamai, A. & Tawara, T. (1993).
Ocular injury caused by preeglox-l, a herbicide contained paraquat,
diquat and surfactants. Jpn J Ophthalmol., 37: 43-46.
Ohta, T. (1987). Unpublished report. TOPPS: microbial mutagenicity
study. Report CTL/P/253A. Institute of Environmental Toxicology,
Kodaira, Tokyo 187, Japan. Report prepared for ICI Japan Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Parkinson, G.R. (1974a). Unpublished report. Diquat dichloride and
dibromide: comparison of dermal toxicity and local irritancy. Report
No HO/IH/P/116. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Parkinson, G.R. (1974b). Unpublished report. Diquat monopyridone:
acute and subacute oral toxicity. Report No CTL/P/12213. ICI Central
Toxicology Laboratory, Macclesfield. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Pirie, A. & Rees, J.R. (1970). Diquat cataract in the rat. Exptl.
Eye Research, 9: 198-203.
Pirie, A., Rees, J.R. & Holmberg, N.J. (1970). Diquat cataract:
formation of the free radical and its reaction with constituents of
the eye. Exptl. Eye Research, 9: 204-218.
Pritchard, V.K. (1986). Unpublished report. Diquat: acute oral
toxicity to the rat of a 200 g/l formulation ("Reglox"). Report
CTL/P/1525. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rawlings, J.M., Foster, J.R. & Heylings, J.R. (1992). Diquat-induced
intestinal secretion in the anaesthetized rat. Human Exp Toxicol.,
11: 524-529.
Richardson, C.R., Howerd, C.A., Wildgoose, J. (1986). Unpublished
report. Diquat dibromide (technical): a cytogenetic study in human
lymphocytes in vitro. Report CTL/P/1561. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Rikans, L.E. & Cai, Y. (1993). Diquat - induced oxidative damage in
BCNU - pretreated hepatocytes of mature and old rats. Tox Appl.
Pharm., 118: 263-270.
Rikans, L.E., Cai, Y., Kosanke, S.D., Venkataraman, P.S. (1993).
Redox cycling and hepatotoxicity of diquat in aging fischer 344
rats. Drug Metab. Dispos., 21: 605-610.
Rogerson, A. & Broad, R.D. (1978). Unpublished report. Diquat
dibromide: 2 year feeding study in rats - amended report. Report
CTL/P/253A.Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S. & Smith, L.L. (1977). The relevance of paraquat
accumulation by tissues. In: Autor PA Ed. Biochemical Mechanisms of
paraquat toxicity. Academic Press, New York pp 72-91.
Rose, M.S., Lock, E.A., Smith, L.L. & Wyatt, I. (1975). Unpublished
report. Paraquat accumulation: tissue and species specificity.
Report CTL/R/367A. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S., Smith, L.L. & Wyatt, I. (1976). The relevance of pentose
phosphate pathway stimulation in rat lung to the mechanism of
paraquat toxicity. Biochem Pharmacol., 25: 1763-1767.
Rose, M.S., Crabtree, H.C., Fletcher, K. & Wyatt, I. (1974).
Biochemical effects of diquat and paraquat disturbance of the
control of corticosteroid synthesis in rat adrenal and subsequent
effects on the control of liver glycogen synthesis. Biochem J.,
138: 437-443.
Scott, R.C. & Corrigan, M.A. (1990). The in vitro percutaneous
absorption of diquat: a species comparison. Toxicol in vitro, 4:
137-141.
Scott, R.C., Walker, M. & Mawdesley, S.J. (1991). Unpublished
report. Diquat dibromide: in vitro absorption from technical
concentrate ('Reglone 40') and spray strength solution through human
skin. ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Sechi, G.P., Agnetti, V., Piredda, M., Canu, M., Deserra, F., Omar,
H.A., Rosati, G. (1992). Acute and persistent parkinsonism after use
of diquat. Neurology, 42: 261-263.
Selypes, A., Nagymajtényi, L. & Berencsi, G. (1980). Mutagenic and
embryotoxic effects of paraquat and diquat. Bull Environ Contam
Toxicol., 25: 513-518.
Sharp, C.W., Ottlenghi, A. & Posner, H.S. (1972). Correlation of
paraquat toxicity with tissue concentrations and weight loss in the
rat. Toxicol Appl Pharmacol., 22: 241-251.
Sheldon, T., Richardson, C.R. & Shaw, J. (1986). Unpublished report.
Diquat dibromide (technical): an evaluation in the mouse
micronucleus test. No ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Shirasu, Y., Moriya, M. & Toshihiro, T. (1979). Unpublished report.
Mutagenicity testing on diquat in microbial systems. Institute of
Environmental Health Toxicology Division, Japan.
Smith, L.L. & Rose, M.S. (1977). A comparison of the effect of
paraquat and diquat on the water content of rat lung and the
incorporation of thymidine into lung DNA. Toxicology, 8: 223-230.
Southwood, J.(1987a). Unpublished report. Diquat: acute dermal
toxicity to the rat of a 200 g/l formulation. Report CTL/P/1881.
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Southwood, J.(1987b). Unpublished report. Topps acute oral toxicity
to the rat. Report CTL/P/1788. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Stevens, M.A. & Walley, J.K. (1966). Tissue and milk residues
arising from the ingestion of single doses of diquat and paraquat by
cattle. J. Sci. Food Agric., 17: 472-475.
Trueman, R.W., Elliot, B.M. & Barber, G. (1987). Unpublished report.
Diquat dibromide (technical): assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Report
CTL/P/1814. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Vanholder, R., Colardyn, F., de Reuck, J., Praet, M., Lameire, N.&
Ringoir. S. (1981). Diquat intoxication: report of two cases and
review of the literature. Am J Med., 70: 1267-1271.
Walley, J.K. (1987). Unpublished report.Diquat toxicity in cattle.
Industrial Hygiene Research Laboratories Report IHR/167 (3B.1/7).
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wickramatne, G.A. (1989). Unpublished report. Diquat: teratogenicity
study in the rat. Report CTL/P/2331. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Wildgoose, J., Braithwaite, I., Howerd, C.A., Richardson, C.R.
(1986). Unpublished report. Diquat dibromide: a cytogenetic study in
human lymphocytes in vitro. Report CTL/P/1469. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Williams, S.G., Cameron, B.D. & McGuire, G.M. (1991). Unpublished
report. Identification of the major radioactive components in urine
and faeces from rats following single oral administration of [14C]-
diquat. Report CTL/C/2523. Inveresk Research International, Tranent,
EH33 2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Table 1. Acute toxicity of diquat (dibromide unless otherwise stated)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Mouse Alderley Park M p.o. 125 Clark & Hurst (1970)
(106-146)
p.o. 170 WHO (1984)
Rat Alderley Park F p.o. 231 Clark & Hurst (1970)
(194-274)
Wistar M p.o. 231 Pritchard (1986)
Alpk:AP (211-254.5)
Wistar M p.o. 214 McCall & Robinson (1990a)
Alpk:APfSD (180-271)
Wistar F p.o. 222 McCall & Robinson (1990a)
Alpk: APfSD (203-241)
Alderley Park M s.c.1 11 Clark & Hurst (1970)
(5-15)
Alderley Park F s.c.1 10 Clark & Hurst (1970)
(6-14)
Alderley Park F s.c. 11 Clark & Hurst (1970)
(9-12)
Wistar (Alpk:AP) M p.c.2 > 400 Southwood (1987a)
Wistar Alpk:AP F p.c.2 > 400 Southwood (1987a)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Alderley Park ? p.c. 50-100 Parkinson (1974a)
albino
Sprague-Dawley M inh3 121 µg/l Bruce (1985)
CD (0.3-63 300)
Sprague-Dawley F inh3 132 µg/l Bruce (1985)
CD (0.3-141 560)
Rabbit ? F p.o. 101 Clark & Hurst (1970)
(72-138)
? F p.c. > 400 Clark & Hurst (1970)
New Zealand ? p.c. 50-100 Parkinson (1974b)
White
Guinea-pig ? F p.o. 100 Clark & Hurst (1970)
Dogs ? F p.o. 100-200 Clark & Hurst (1970)
Hens ? F p.o. 200-400 Clark & Hurst (1970)
Cattle ? ? p.o. 30 Walley (1987)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Cattle ? F p.o. 30 Clark & Hurst (1970)
Monkey Cynomolgus ? p.o.1 100-300 Cobb & Grimshaw (1979)
Notes
1) diquat dichloride
2) administered as 21.2% diquat ion
3) whole body 240 min exposure to aerosol
Urine and blood were examined during the study. At autopsy, blood
was taken for clinical chemistry and haematology and selected organs
weighed and examined histologically. Animals receiving diquat at a
concentration of 500 ppm showed a marked reduction in weight gain,
food consumption and utilization. Cataract formation was also
observed at this dose at 8 weeks and at autopsy, corneal opacity was
observed in 7/12 males and 4/12 females; at histological
examination, cataract was seen in 12/12 males and 11/12 females.
Additionally, a low incidence of focal inflammation of the tongue
and epithelium of the palate was observed. Reduced plasma protein
was seen at 500 ppm, possibly caused by low food intake. Absolute,
but not relative, organ weights were reduced, almost certainly a
reflection of poor food intake. Treatment-related effects were not
seen at 100 ppm. The NOAEL was 100 ppm, equal to 8.5 and 9.2 mg
ion/kg bw/day in males and females, respectively (Hodge, 1989a).
Dogs
A one-year study in dogs was carried out using diquat dibromide
technical (26.7 w/v ion) added to the feed. Groups of 4 male and 4
female beagles received diquat at doses of 0, 0.5, 2.5 or 12.5 mg
ion/kg bw/day. Clinical condition, body weight and food consumption
were monitored throughout. Ophthalmoscopy, haematology and clinical
chemistry were carried out. The animals were sacrificed and
autopsied at 52 weeks and a range of organs examined and processed
for histological examination. There was a small but statistically
significant decrease in body-weight gain in the high-dose groups of
both sexes, somewhat greater in the females. No such decrease was
seen at the lower doses. There was a statistically significant
decrease in WBC and neutrophil count in males of all treatment
groups at a single time point (week 4) which was ascribed to raised
counts in one control. There were decreased platelet counts in top
dose females at 4, 26 and 52 weeks. Raised plasma chloride levels
observed in the top dose animals were attributed to bromide ion
interference. Plasma triglyceride in the males given 12.5 mg ion/kg
bw/day were higher than in the controls throughout the study; at 4
and 26 weeks these increases were statistically significant.
Statistically significant increases in relative and absolute kidney
weight were observed in both sexes at 12.5 mg ion/kg bw/day. There
were decreases in absolute and relative adrenal weight in all
treatment groups in the males, which were statistically significant
only in the case of relative weights. Additionally, there was a
decrease in the absolute and relative weight of the epididymides in
all test groups compared to the controls; this finding was only
statistically significant for absolute weights in the 2.5 mg ion/kg
bw/day group and for relative weights in the top dose group.
Changes in organ weights did not correspond to any histopathological
changes. Cataract was seen in all the top dose animals by the end
of the study, both by ophthalmoscopy and histologically and in 2/4
females receiving diquat at a dose of 2.5 mg ion/kg bw/day; however
in the latter group only one was confirmed by histopathological
examination. Inflammatory changes were seen at the top dose in the
large intestine, consisting of reduction in mucosal thickness, loss
and abnormality of mucosal glands, epithelial hyperplasia in crypts
and increased goblet cell activity. The NOAEL was 0.5 mg ion/kg
bw/day based upon lens opacity in females at the next dose (Hopkins,
1990).
Long-term toxicity/carcinogenicity studies
Mice
In an 80-week feeding study, groups of CD-1 mice (60/sex) were
fed diquat dibromide (98.5% pure) at dietary concentrations of 30,
150 or 500 ppm. The highest dietary concentration was reduced to
400 ppm after 3 weeks and to 300 ppm after 5 weeks; these changes
were made because of lethal toxicity and the decedent mice were
replaced. Not all groups were started simultaneously: one control
group and the two lower test groups were started about three months
before the other control groups and the highest dosage group. In
general the test material had little clinical effect, except that
marked toxicity, evidenced by huddling, hypoactivity, and an
ungroomed appearance, were observed for the first few weeks at 500
ppm. Clinical recovery appeared complete by the eighth week.
Survival was not affected by treatment. Growth rates were reduced
at the highest dietary concentration and at 150 ppm. At 30 ppm,
there were no effects on body-weight gain. There was an increased
frequency of hepatic vacuolation in livers at 150 ppm (males) and at
the highest dose in both sexes. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day (Ashby, 1987).
In a 2-year study, groups of 60 male and 60 female mice
(C57BL/10JfCD-1/Alpk) were fed a diet containing 0, 30, 100 or 300
ppm diquat. The test material was technical grade diquat dibromide,
containing 26.7% diquat ion w/v. Haematological examination of tail
vein blood samples was carried out on all animals at 53 and 79
weeks. Fuller haematology was carried out on blood obtained by
cardiac puncture at 104 weeks. Various tissues were taken at
autopsy and processed for histological examination. There was
marked toxicity at the highest dose, shown by statistically
significant reductions in body-weight gain in both sexes, small and
sometimes statistically significant reductions in food consumption,
eye discharge and mild nephropathy. Changes in certain
haematological parameters at 300 ppm were also seen, namely
statistically significantly decreased neutrophil count and increased
lymphocyte count in both sexes at weeks 53 and 79. There was also a
significant increase in total WBC at 2 years in males. Relative
kidney weights were increased significantly in males and females.
At 100 ppm there were statistically significant reductions in body-
weight gain in both sexes, particularly in the males later in the
study; there were also some changes in haematology (lymphocytosis
and neutropenia) which, although statistically significant were
probably unimportant, and relative kidney weights were increased
significantly in males. No treatment-related carcinogenic effects
were seen in the study (there were reductions in certain tumour
incidences at 300 ppm) and the incidence of cataract was not related
to the test material. The NOAEL was 30 ppm, equal to 3.6 mg ion/kg
bw/day (males) and 4.8 mg ion/kg bw/day (females) (Hodge, 1992).
Rats
In a two-year feeding study, groups of Wistar-derived rats
(35/sex) received diquat dibromide (100% pure = 53.6% ion) at
dietary concentrations of 0, 15, 25 or 75 ppm. Weight gain and food
consumption did not differ significantly between groups. A
significantly increased incidence of cataracts was observed at 75
ppm. The NOAEL was 25 ppm, equivalent to 1.3 mg ion/kg bw/day
(Rogerson & Broad, 1978).
In another 2-year study, diquat dibromide (technical grade) was
administered in the diet to groups of Sprague-Dawley rats (60/sex)
at dietary concentrations of 0, 5, 15, 75 or 375 ppm. Additionally,
satellite groups of 10 males and 10 females received diquat at the
same dietary concentrations and were killed at 1 year.
Ophthalmoscopy was carried out before dosing and periodically during
the study. Haematological and biochemical variables were measured
on 10 animals from each main group before dosing, and at 26, 52, 78
and 104 weeks, and in five satellite animals at week 52. A
reduction in food consumption and utilization efficacy was observed
at 375 ppm. After 26 weeks, there was a reduction in MCV and in
haemoglobin in females and males, respectively. Minimal reductions
in red blood cell parameters were observed in males at 15 ppm and
above, at 52 and 78 weeks. Other changes were prolonged activated
partial thromboplastin times at 75 and 375 ppm in females at 52 and
104 weeks and at 52 weeks in the 15 ppm females. Blood urea
nitrogen (BUN) was elevated at 52 weeks at 75 and 375 ppm and at 78
weeks in the 375 ppm females. At 52 weeks, the 75 and 375 ppm
groups exhibited lower total protein and albumen levels. On
ophthalmoscopic examination, lenticular opacities were seen in the
75 and 375 ppm groups at 13 weeks and the findings progressed at
subsequent examinations. Ophthalmoscopy at 104 weeks showed severe
lens opacities at 375 ppm in all surviving rats, while less severe
ones were seen at 75 ppm. At 15 ppm, a single instance of cataract
was seen in each sex. Histological examination of the eyes
postmortem, showed advanced cataractogenesis in all animals at 375
ppm and in about 80% of the 75 ppm group. There was a low
prevalence of cataract at 15 ppm: cataract-type changes being seen
in 0/22 controls and 3/22 at 15 ppm (males) and 0/22 controls and
2/20 at 15 ppm (females). Small differences were seen in
nephropathy in arteritis and in aneurism formation in males at the
highest dose. Elevations reported as statistically significant were
seen in the incidence of benign phenochromocytomas at 75 ppm with no
real evidence of a trend and in combined thyroid parafollicular cell
adenomas and carcinomas at 5 ppm, both in males but numbers were
very small. There was evidence of a trend for thyroid follicular
cell adenoma; however these figures appear to have been much
influenced by the frequency of the tumour at 375 ppm (in males the
number of tumours out of the number of thyroid glands examined in
each groups were 2/24, 1/32, 1/25, 0/25 and 3/32 for the control, 5,
15, 75 and 375 ppm groups respectively; none were observed in
females). There was a significant increase in the number of females
with multiple neoplasia and with malignant neoplasms at 5 and 75
ppm. No other change attributable to the test material was seen.
The LOAEL was 15 ppm (equal to 0.58 and 0.72 mg ion/kg bw/day for
males and females) based upon cataractogenesis. The NOAEL was 5
ppm, equal to 0.19 mg ion/kg bw/day (males) and 0.24 mg ion/kg
bw/day (females) (Colley et al., 1985).
Reproduction studies
Rats
A three-generation study was conducted in rats by Fletcher et
al. (1972) using diquat dibromide monohydrate (100% pure).
Wistar-derived rats were divided into 3 groups, each containing 12
males and 24 females. One group received standard diet while the
other two groups received diquat dibromide in aqueous solution at
dietary concentrations of 0, 125 or 500 ppm when they were
approximately 35-days old. Thereafter the three groups and their
progeny remained on the diet throughout the study. Body weights and
food consumption were recorded weekly and the animals physically
examined daily. The F0 animals were mated after 100-days feeding,
one male and two females being housed together for this purpose.
Where pregnancy had not occurred in three weeks, the male was
replaced by another from the same group. Litters were examined
within 20 hours of delivery (F1a). At 21 days, each litter was
counted, weighed sexed and subjected to autopsy. Second matings
were arranged after a 10-day rest period. From the progeny (F1b),
12 males and 24 females were reared and continued on the diet from
weaning, the remainder being killed and examined. At 100 days, the
F1b animals were mated for the production of the second generation
(F2). The F2 generation was treated in the same way as the F1,
the F2a litters being examined and autopsied at 21 days and the F2b
providing the breeders for production of the F3 generation. The
F3a litters were treated as before and the F3b animals were also
autopsied at 21 days and detailed examination, including
histological examination was carried out postmortem. Rats treated
with diquat (500 ppm) developed lens opacity from about 125 days.
The proportion exhibiting this abnormality increased and at 300 days
about 50% were affected. Opacity was not seen at the lower dose.
There was also a statistically significant reduction in weight gain
at 500 ppm, at 15 weeks in both sexes of F0 parents. Similar
reductions (after weaning) were found in the subsequent generations.
In the second generation, significantly reduced body weights were
seen in the 125 ppm females at weaning and the final week before
mating but not at other weighings. There were 6 fewer pregnancies
in the 500 ppm group of F1 animals. Atrophy of the seminiferous
tubules was observed in parents and progeny of each generation.
Although more common in the 500 ppm groups, there was no clear dose-
response relationship. This study did not exhibit an NOAEL since
there was decreased weight gain in F0 and F1 animals at the lowest
dose, but the effects observed at this dose (125 ppm, equivalent to
6.3 mg ion/kg bw/day) were trivial (Fletcher et al., 1972).
A multigeneration study was conducted using technical grade
diquat dibromide, containing diquat ion 26.7% w/v. Groups of
Alpk:APfSD rats (30/sex) were fed diets containing 0, 16, 80 or 400
ppm diquat. After 12 weeks the animals were mated and allowed to
rear the litters that resulted (F1a). The process was repeated
with 30 male and 30 female parents/group selected from the F1a
litter, these F1 parents being mated 11 weeks after selection. The
dose received by the top dose F1 rats was reduced after 4 weeks to
240 ppm. The animals were examined daily and mouths examined
weekly. Animals were weighed weekly in the premating period. After
mating the males were weighed monthly and the females were weighed
on days 1, 8, 15 and 20 of pregnancy and days 1, 5, 11, 16 and 22 of
lactation. All animals were weighed at the terminal kill. Food
consumption was recorded weekly until mating, and weekly in the
females during pregnancy and lactation. Ophthalmoscopic examination
of the eyes was carried out on the controls and top dose group at 12
weeks and on all the F0 animals at termination (24 weeks). A
similar examination was carried out on the F1 rats 4 weeks after
selection, at 11 weeks before mating and at termination (21 weeks).
Diquat had no effect on fertility in either sex. Decreased body-
weight gain was seen at the top dose in both adults (F0 and F1)
and pups. Inflammatory lesions in the mouth, particularly
ulceration of the hard palate was observed at 400 ppm in F0 rats
and F1 pups and adults. Cataracts, first seen at week 12 in F0
females and at week 13 in males and 2 weeks earlier in each sex in
the F1a and F1b, were also observed in the F1 adults. Although
cataract formation was mostly confined to the 240 ppm group, a low
incidence was seen at 80 ppm in the F1 female parents (3/30 at
premating ophthalmoscopic examination and 4/30 at examination before
termination of the study). At the terminal histopathological
examination, cataract was seen in the highest dose group only of F0
and F1 parents. There was an increase in pathological changes in
the renal tract in the F1 and F2 pups. Reproductive toxicity
(reduced pup weight gain) was observed at 400 ppm. The NOAEL was 16
ppm (equivalent to 0.8 mg/kg bw/day) based upon a low incidence of
partial cataract formation at 80 ppm (Hodge, 1990).
Other studies on reproduction parameters
An antifertility effect on male mice was noted during the
dominant lethal study of Pasi et al. (1974). Diquat at doses up
to 10 ppm in the diet of hens for 6 weeks did not affect food
consumption, egg production and hatchability. Residues of diquat
were not found in eggs or in hens' tissues (Edwards & Smith, 1975).
Special studies on embryo/fetotoxicity
Mice
In a study in Swiss-Webster and Sprague-Dawley (CD) mice, a
single dose of 15 mg/kg bw diquat was given i.p. to groups of three
animals at day 7-21 of gestation. Nine maternal deaths out of 45
animals occurred. The percentage dead plus resorbed fetuses was
57%. A NOAEL could not be determined in this study (Bus et al.,
1975).
Groups of 20 female CFLP mice were administered diquat i.p. (11
mg/kg bw Reglone) on day 9 of gestation or diquat (2.7 mg/kg bw/day
Reglone) on days 9-12 of gestation. Both groups showed increased
fetal loss or resorption compared to controls. Skeletal
abnormalities were observed in the test group embryos. A NOAEL
could not be determined in this study (Selypes et al., 1980).
Rats
Diquat was administered in the diet to pregnant Sprague-Dawley
rats, from days 1-20 of pregnancy at 0, 125 or 500 ppm diquat ion.
There were 18 control rats, and 20 in each of the test groups.
Animals were killed at day 20, fetuses removed and the uteri
examined. All rats remained in good condition throughout the study
but food consumption and body-weight gain were reduced significantly
at 500 ppm. There was no adverse effect on implantations, mean
number of fetuses, litter weight or sex ratio. Mean fetal weight
was significantly lower in the 500 ppm group. A dose-related
increase in subcutaneous fetal haemorrhages compared to the controls
was observed. A NOAEL could not be determined in this study (Moore
& Wilson, 1973).
Diquat containing 26.2 % ion w/v was administered by gavage to
groups of 8 female rats (Wistar derived Alpk:APfSD) at doses of 0,
4, 12, 24 or 40 mg ion/kg bw/day in deionized water from days 7-16
of gestation. Controls received deionized water. On day 22 the
females were killed and the uteri examined for live fetuses and
intrauterine deaths. At a dose of 40 mg ion/kg bw/day the mothers
showed reduced weight gain and changes in clinical condition
(piloerection, urinary incontinence and gasping) and 4/24 animals at
24 mg ion/kg bw/day showed milder signs. Some fetotoxicity was seen
at 40 mg ion/kg bw/day (reduced fetal weight gain), but not at the
lower doses, whereas maternal toxicity, as indicated by dose-related
reduced food consumption and body-weight gain, was observed at all
doses. Although there was a statistically non-significant increase
in pre- and post-implantation loss at 24 mg ion/kg bw/day, this was
not observed at the other three doses. No fetal abnormality was
observed. For fetal toxicity, the LOAEL was 40 mg ion/kg bw/day and
the NOAEL 24 mg ion/kg bw/day. The NOAEL for maternal toxicity
could not be determined in this study (Milburn, 1989).
Groups of 24 female rats were given diquat by gavage in
deionized water (26.2 % ion w/v) at doses of 4, 12 or 40 mg ion/kg
bw/day from days 7-16 of gestation. Maternal toxicity was seen at
40 mg ion/kg bw/day as reduced weight gain and food consumption.
Significant reductions in fetal weight, litter weight and gravid
uterine weight as well as fetal defects in ossification were seen at
40 mg ion/kg bw/day. Minor evidence of reduced ossification were
seen at the lower doses but these were considered not to be of
biological significance. For both maternal and fetotoxicity the
LOAEL was 40 mg ion/kg bw/day and the NOAEL 12 mg ion/kg bw/day
(Wickramatne, 1989).
Rabbits
Groups of up to 20 female mated Dutch rabbits were dosed orally
with diquat dibromide (100% pure = 53.6% ion) at levels of 1.3, 2.5
or 5.0 mg ion/kg bw/day from days 1-28 of gestation (day of mating =
day 0). The material was administered in "Dispersol OG" (a 10%
solution of ricinoleic glycerides with glycerol and polyglycerols),
which was also used as control. An additional 4 does were allowed
to litter and the eyes of the offspring examined for cataracts. The
rabbits were sacrificed at day 29 and the uteri examined for live
fetuses and intrauterine deaths. The fetuses were weighed and
examined for gross abnormality and about half processed for skeletal
examination and half for soft tissue examination. There was a
reduction in maternal weight gain at 5.0 mg ion/kg bw/day, which was
not statistically significant. There was no evidence of any effect
on embryonic or fetal development. The NOAEL was 2.5 mg ion/kg
bw/day based on mild maternal toxicity at the highest dose (Hodge,
1987). A feature of this study was the poor pregnancy rate in all
groups which necessitated mating of extra animals to achieve
acceptable numbers of pregnancies per group.
In a second study, groups of 7 or 8 female New Zeeland white
rabbits were administered diquat dibromide technical by gavage in
deionized water (26.2% ion w/v) at doses of 0, 1, 3, 7 or 10 mg
ion/kg bw/day from days 7-19 of gestation. Controls received water.
On day 30 of gestation, the females were killed and the uteri
examined for live fetuses and intrauterine deaths. Doses of 3 mg
ion/kg bw/day or above were associated with maternal toxicity as
manifested by weight loss or reduced weight gain and reduced food
intake. No evidence of fetotoxicity was observed. The NOAEL was 1
mg ion/kg bw/day based upon maternal toxicity (Hodge, 1989b).
In a third study, groups of 20 New Zeeland white rabbits were
administered by gavage diquat dibromide technical (26.2% ion w/v) in
deionized water, at doses of 0, 1, 3 or 10 mg ion/kg bw/day from
days 7-19 of gestation. On day 30 of gestation the females were
killed and the uteri examined for live fetuses and intrauterine
deaths. The fetuses were weighed, examined externally and for
visceral abnormalities, eviscerated and stained for skeletal
abnormalities. Doses of 10 mg ion/kg bw/day caused maternal
toxicity as shown by weight loss and reduced food intake; five
animals from this group were sacrificed early in extremis.
Effects on body weight and food consumption, although less severe,
were also present at 3 mg ion/kg bw/day. Some evidence of
fetotoxicity was observed at 10 mg/kg bw/day (mottled and friable
livers and small increase in minor skeletal defects at 3 and 10 mg
ion/kg bw/day, in the form of partially ossified sternabrae). The
elevation in the proportion of fetuses with minor skeletal defect
was significant at 3 and 10 mg ion/kg bw/day; there was a non-
significant increase at the lowest dose group. The NOAEL was 1 mg
ion/kg bw/day based on maternal toxicity (reduced weight gain and
food consumption) and skeletal effects in the fetuses at doses of 3
mg ion/kg bw/day (Hodge, 1989c).
Special studies on genotoxicity
Based on the results of the genotoxicity assays given in Table
2, the Meeting concluded that diquat was not genotoxic.
Toxicity of metabolites
In Wistar-derived rats, diquat dipyridone and monopyridine were
less toxic than diquat when given subcutaneously (Parkinson, 1974b;
Crabtree, 1976).
The results of acute and genotoxicity studies with TOPPS are
given in Tables 3 and 4, respectively.
Other animal studies
Diquat dichloride and dibromide are both moderate skin
irritants to the skin of the rat and mildly irritant to the rabbit
eye (Parkinson, 1974). Orally administered diquat increases
secretion into the gut lumen (Crabtree et al., 1977; Rawlings et
al., 1992). In a study to detect any other major actions of
diquat in laboratory animals, single doses of up to 280 mg/kg bw
were administered orally to rats. Effects such as CNS depression
and increased gut motility were observed at perilethal doses only
(Allen & Brammer, 1990).
Table 2. Results of genotoxicity assays on diquat
Test system Test object Concentration of diquat Purity Results Reference
In vitro
Ames test S. typhimurium 0.01-50 100% -ve Shirasu et al. (1979)
(5) (strains TA1535, µg/plate
1537, 1538, 98, 100)
Ames test S. typhimurium 0.00256-100 100% -ve Callander (1986a)
(5) (strains TA1535, µg/plate
1537, 1538, 98, ± S9
100
Ames test S. typhimurium 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
(5) (strains TA1535, with S9, ion (technical)
1537, 1538 0.1-50 µg/plate
98, 100) without S9
Reverse E. coli WP2 hcr 0.01-50 µg/plate 100% -ve Shirasu et al. (1979)
mutation (5)
Reverse E. coli WP2 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
mutation (5) uvrA pKM101 with S9, ion (technical)
0.1-50 µg/plate
without S9
Mammalian Mouse lymphoma 6.25-100 µg/ml 100% -ve Cross (1986a)
cell (5) L5178Y TK+/-
Mammalian Mouse lymphoma 6.25-100 µg/ml 25.8% w/w -ve Cross (1986b)
cell (5) L5178Y TK+/- ion (technical)
Table 2 (contd)
Test system Test object Concentration of diquat Purity Results Reference
Cytogenetics Human lymphocytes 26.7, 107, 267(2), 100% +ve (4) Wildgoose et al. (1986)
(1) 534.8(3) µg/ml as ion
Cytogenetics Human lymphocytes 12.9-129 µg/ml 25.8% w/w +ve (4) Richardson et al. (1986)
(1) ion (technical)
Rec-assay B. subtilis 2-200 µg/disc 100% -ve Shirasu et al. (1979)
H17, M45
In vivo
Cytogenetics Rat bone marrow 4.4, 9.5, 14, 100% -ve Anderson et al. (1980)
(Wistar-derived mg/kg bw/day
Alderley Park) for 5 days orally
Cytogenetics Mouse bone marrow 0.73, 3.6, 7.3, 22 100% -ve Selypes et al. (1980)
(CFLP) mg/kg bw ip
90 mg/kg/bw/po
Micro-nucleus Mouse bone marrow 0, 62.5, 100 mg/kg 25.8% w/w -ve Sheldon et al. (1986)
C57BL/6J/Alpk bw ion (technical)
Dominant Mouse (CD-1) 0.1-10 mg ion/kg 28.6% w/w -ve Anderson et al. (1976)
Lethal Test bw/day for 5 days ion (technical)
UDS Rat hepatocyte 225, 450, 900 25.8% w/w -ve Trueman et al. (1987)
in vivo mg/kg ion ion (technical)
1) ± S9
2) Donor 1 MTD
3) Donor 2 MTD
4) Clastogenic only at doses causing cytotoxicity
5) with and without metabolic activation
Table 3. Acute toxicity of TOPPS
Species Strain Sex Route LD50 (mg/kg bw) References
Rat Wistar Alpk:A M PO 2449 Southwood (1987b)
(2000-3000)
Wistar Alpk:A F PO 2942 Southwood (1987b)
(2000-5000)
Table 4. Results of genotoxicity assays on TOPPS
Test system Test object Concentration of TOPPS Purity Results Reference
Ames (1) S. typhimurium 0.1-5 mg/plate 98% -ve Ohta (1987)
TA1535, TA1537,
98, 100
E. coli 0.1-5 mg/plate 98% -ve Ohta (1987)
WP uvrA
Rec Assay (1) B. subtilis 0.2-10 mg/disc 98% -ve Ohta (1987)
(1) With or without metabolic activation.
Special studies on cataractogenesis
Pirie & Rees (1970) fed Wistar albino rats a diet containing
0.05 or 0.075% diquat dibromide. Lens opacity was produced after 4-
8 months at both doses. Ascorbic acid content of the lens, but
unusually not GSH content, decreased during development of cataract.
Radioactivity appeared in the lens after intraperitoneal injection
of 14C-labelled diquat. Pirie et al. (1970) suggested that free-
radical formation might be responsible for cataract formation.
Observations in humans
Vanholder et al. (1981) reviewed several cases of poisoning
with diquat. In one case, initial signs and symptoms were
abdominal. Later oliguria and coma developed. Shock supervened
followed by cardiac arrest. In another case progression was slower
but renal failure occurred and eventually led to anuria. Despite
haemodialysis, death occurred from ventricular fibrillation, and
renal failure was observed. Unlike poisoning with paraquat, diquat
does not cause lung fibrosis. Cataracts have not been observed in
humans (IPCS, 1984) and more recent investigations have not shown
cataract formation in those engaged in diquat manufacture or
formulation (Bonsall, 1990).
Two patients were splashed in the eyes with a preparation
containing both paraquat and diquat. In both cases the corneal
epithelium was damaged and healing was slow (Nirei et al., 1993).
In a recent report, a child survived what was initially
believed to be a fatal dose of diquat, apparently with no sequelae
(Buckley & MacKiernan, 1991, 1992). A recent case report describes
a patient who developed Parkinsonism a few days after exposure to
diquat (Sechi et al., 1992). The extent of exposure of the
patient to the pesticide was not known and thus the significance of
this isolated observation in a 72-year old man is questionable.
COMMENTS
When administered orally 14C-diquat is poorly absorbed from
the gastrointestinal tract of rats, cows and goats and mainly
eliminated vis the faeces during the first 24 hours, the small part
absorbed being principally eliminated via the urine. The total
percentages of administered doses eliminated via the faeces were 94,
91 and 94 for the rat, cow and goat, respectively; 3.1% and 0.4%
were eliminated in the urine of the rat and the cow, respectively,
and very small percentages of radioactivity were found in cow's and
goat's milk (0.004% and 0.0175%), respectively.
After oral administration of 14C-diquat to rats (45 mg ion/kg
bw), the major excreted product was diquat in both urine (5% of
dose) and faeces (> 57% of dose): diquat monopyridone was the main
metabolite in the faeces (5% of dose), but a minor one in the urine.
In another oral study in rats (100 mg ion/kg bw), a small amount of
diquat dipyridone and picolinic acid was found in addition to the
monopyridone. After subcutaneous injection (10 mg ion/kg bw) in the
rat, 75% of the dose was present in the urine as diquat, about 3% as
the monopyridone and 6% as the dipyridone.
Unlike paraquat, diquat is not actively taken up by lung
slices, and lung toxicity is not characteristic of diquat poisoning.
The acute oral toxicity of diquat varies with species, but is
between 125 and 250 mg ion/kg bw in rodents. It is classified by
WHO as moderately hazardous.
In a 90-day feeding study in rats, using dietary concentrations
of 0, 20, 100 or 500 ppm, the NOAEL was 100 ppm, equal to 8.5 mg
ion/kg bw/day, based upon reduction in body-weight gain, food
consumption and reduced plasma protein at the next higher dose.
In a one-year feeding study, dogs received doses of 0, 0.5, 2.5
or 12.5 mg/kg bw/day. The NOAEL was 0.5 mg ion/kg bw/day based upon
lens opacity in females at the next dose.
Two long-term toxicity/carcinogenicity studies were conducted
in mice. The first (80 weeks) used dietary concentrations of diquat
ion of 0, 30, 150 or 500 ppm. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day, based upon reduced growth rates at the next
higher dose together with hepatic vacuolation in males. In a 2-year
study in mice, in which dietary concentrations of 0, 30, 100 or 300
ppm were used, the NOAEL was 30 ppm, equal to 3.6 mg ion/kg bw/day,
based on reduction in body-weight gain and increased relative kidney
weights at the next higher dose. There was no evidence of
carcinogenicity in mice.
Two 2-year feeding studies in rats have been conducted. In the
first study, diquat dibromide was administered in the diet at
concentrations of 0, 5, 15, 75 or 375 ppm. The NOAEL was 5 ppm,
equal to 0.19 mg ion/kg bw/day, based upon cataract formation in the
15 ppm group. In the second study, dietary concentrations of 0, 15,
25 or 75 ppm diquat ion were used. The NOAEL was 25 ppm (equivalent
to 1.3 mg ion/kg bw/day), based on cataract formation at the next
higher dose. There was no evidence of carcinogenicity in rats.
Numerous teratogenicity studies have been conducted. NOAELs
could not be determined in two mouse studies. There were three
teratogenicity studies in rats; in the first study dietary
concentrations of 0, 125 or 500 ppm diquat ion were used. A dose-
related increase in subcutaneous fetal haemorrhages compared to the
controls was observed. A NOAEL could not be derived from this
study. In the second study, diquat was administered at oral doses
of 0, 4, 12, 24 or 40 mg ion/kg bw/day. For fetal toxicity, the
NOAEL was 24 mg ion/kg bw/day, but maternal toxicity was observed in
all test groups (reduced weight gain and food consumption). In the
third study, diquat was administered by gavage at doses of 0, 4, 12
or 40 mg ion/kg bw/day. The NOAEL for both maternal and fetal
toxicity was 12 mg ion/kg bw/day, based in the case of the dams on
reduced body weight and food consumption and in the case of the
fetuses on reduced fetal weight and defects in fetal ossification at
the highest dose.
In a study in rabbits, diquat was given orally at doses of 0,
1.3, 2.5 or 5.0 mg ion/kg bw/day. There was no evidence of any
effects on embryonic or fetal development. The NOAEL was 2.5 mg
ion/kg bw/day based on mild maternal toxicity at the highest dose.
In a second study in rabbits, doses of 0, 1, 3, 7 or 10 mg ion/kg
bw/day were administered by gavage. Doses of 3 mg ion/kg bw/day or
above were associated with maternal toxicity as manifested by weight
loss or reduced weight gain and reduced food intake. No evidence of
fetotoxicity was observed. The NOAEL was 1 mg ion/kg bw/day based
upon maternal toxicity. In a third study in rabbits, doses of 0, 1,
3 or 10 mg ion/kg bw/day diquat were given by gavage. The NOAEL was
1 mg ion/kg bw/day based upon maternal toxicity (reduced weight gain
and food consumption) and skeletal effects in the fetuses at doses
of 3 mg ion/kg bw/day.
Two multigeneration reproduction studies were conducted in
rats. In the first study, diquat was given at dietary
concentrations of 0, 125 or 500 ppm. This study did not exhibit an
NOAEL, since there was decreased weight gain in F0 and F1 animals
at the lowest dose, but the effects observed at this dose (125 ppm,
equivalent to 6.3 mg ion/kg bw/day) were trivial. In the second
study, rats were fed diquat at dietary concentrations of 0, 16, 80
or 400 ppm. The NOAEL was 16 ppm (equivalent to 0.8 mg/kg bw/day)
based upon a low incidence of partial cataract formation at 80 ppm.
Diquat has been adequately tested in a series of genotoxicity
assays in vitro and in vivo. Chromosomal aberrations were
induced in vitro but there was no other evidence of genotoxicity.
The Meeting concluded that diquat was not genotoxic.
An ADI of 0-0.002 mg/kg bw was established based upon a NOAEL
of 0.19 mg ion/kg bw/day identified in a two-year study in rats,
using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 30 ppm, equal to 3.6 mg ion/kg bw/day
(two-year study)
Rat: 5 ppm, equal to 0.19 mg ion/kg bw/day
(two-year study)
12 mg ion/kg bw/day (teratogenicity study)
16 ppm, equivalent to 1.6 mg ion/kg bw/day
(multigeneration reproduction study)
Rabbit: 1 mg/kg bw/day (teratogenicity study)
Dog: 0.5 mg ion/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.002 mg ion/kg bw
Studies which will provide information in the continued evaluation
of the compound
Observations in humans.
REFERENCES
Anderson, D., McGregor, D.B. & Purchase, I.F.H. (1976). Dominant
lethal studies with paraquat and diquat in male CD-1 mice. Mutation
Research, 40: 349-358.
Anderson, D., Richardson, C.R., Howard, C.A., Banham, P., Hart, D. &
Weight, T.M. (1980). Unpublished report. Diquat: a cytogenetic study
in the rat. Report CTL/P/366. Imperial Chemical Industries PLC,
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Allen, S.L. & Brammer, A. (1990). Unpublished report. Diquat:
pharmacological evaluation. Report No CTL/P/2842*(revised). ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Ashby, R. (1987). Unpublished report. Diquat dibromide monohydrate:
evaluation of potential carcinogenicity in dietary adminstration to
mice for 80 weeks. Final Report CTL/C/3761 (LSR 87/ILY001/375). Life
Sciences Research Ltd, Eye, Suffolk, England. Supplied to WHO by
Zeneca. Vols 1 & 2.
Baldwin, R.C., Pasi, A., McGregor, J.T. & Hine, C.H. (1975). The
rates of radical formation from the dipyridilium herbicides
paraquat, diquat, and morphamquat in homogenates of rat lung,
kidney, and liver: an inhibitory effect of carbon monoxide. Toxicol
Appl Pharmacol., 32: 298-304.
Bhuyan, D.K. & Bhuyan, K.C. (1991). Oxy radicals in the eye tissues
of rabbits after diquat in vivo. Free Radic Res Commun. 12-13
Pt.2: 621-627.
Black, W.J.M., Calderbank, A., Douglas, G. & McKenna, R.H. (1966).
Residues in herbage and silage and feeding experiments following the
use of diquat as a dessicant. J Sci Food Agric., 17: 506-509.
Bonsall, J.L. (1990). Letter to F Tripet, Stauffer Chemicals,
Switzerland dated October 9 1990.
Bruce, E.D. (1985). Unpublished report. The acute inhalation
toxicity of diquat water weed killer (SX-1574) in rats. Report
CTL/C/2701 (SOCAL 2353). Chevron Environmental Health Center,
Richmond, California. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Buckley, D.A. & McKiernan, J. (1991/1992). Survival after ingestion
of a fatal dose of diquat (letter). Irish Med. J., 84: 134.
Bus, J.S., Preache, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
Sharp, C.W. & Gibson, J.E. (1975). Fetal Toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl Pharmacol.,
33: 450-460.
Callander, R.D. (1986a). Unpublished report. Diquat dibromide: an
evaluation in the Salmonella mutagenicity assay. Report
CTL/P/1413. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Callander, R.D. (1986b). Unpublished report. Diquat dibromide
(technical): an evaluation in the Salmonella mutagenicity assay.
Report CTL/P/1463. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Clark, D.G. & Hurst, E.W. (1970). The toxicity of diquat. Brit J
Industr Med., 27: 51-55.
Cobb, L.M. & Grimshaw, P. (1979). Acute toxicity of oral diquat
(1,1'-ethylene-2,2'-bipyridinium) in cynomolgus monkeys. Toxicology
Appl Pharmacol. 51: 277-282.
Colley, J., Warren, S., Heywood, R., Street, A.E., Almond, R.H. &
Gopinath, C. (1985). Unpublished report. Diquat dibromide evaluation
of potential carcinogenicity and chronic toxicity by prolonged
dietary administration to rats. Report ICI 406/83763. Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, England. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. (1976). Unpublished report. Comparison of the
subcutaneous toxicity of diquat, diquat monopyridone and diquat
dipyridone. Report CTL/P/351. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. & Rose, M.S. (1976). Unpublished report. Early
effects of diquat on plasma corticosteroid concentrations in rats.
Report CTL/R/380. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C., Lock, E.A. & Rose, M.S. (1977). Effects of diquat on
the gastrointestinal tract of rats. Toxicology Appl Pharmacol.,
41: 585-595.
Cross, M.F. (1986a). Unpublished report. Diquat dibromide:
assessment of mutagenic potential using L5178Y mouse lymphoma cells.
Report CTL/P/1554. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Cross, M.F. (1986b). Unpublished report. Diquat dibromide
(technical): assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Report CTL/P/1602. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Daniel, J.W. & Gage, J.C. (1966). Absorption and excretion of diquat
and paraquat in rats. Brit J Industr Med., 23: 133-136.
Daniel, J.W. & Henson, A.F. (1960). Unpublished report. The
absorption and excretion of the herbicide K.8483 in rats. Report
IHR/137. Industrial Hygiene Research Laboratories and Akers Research
Laboratories. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Edwards, M.J. & Smith, D.C. (1975). Unpublished report. Diquat:
residue transfer and hatchability study in laying hens. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Feldmann, K.J. & Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicol appl Pharmacol,,
28: 126-132.
Fletcher, K., Griffiths, D. & Kinch, D.A. (1972). Unpublished
report. Diquat dibromide: three-generation reproduction study in
rats. Report HO/IH/R/331A. Imperial Chemical Industries Ltd
Industrial Hygiene Research Laboratories. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Griggs, R.E. & Davis, J.A. (1975). Unpublished report. Diquat:
excretion and metabolism in a goat. Report AR 2585 A. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Griggs, R.E. (1973).
Unpublished report. Metabolism of diquat and its photoproducts in
goats. Report AR 2448 B. ICI Plant Protection Ltd. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Burgess (1974).
Unpublished report. Diquat: metabolism of diquat and its
photoproducts in a cow. Report AR 2448 B. ICI Plant Protection Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1987). Unpublished report. Diquat dibromide:
teratogenic studies in the rabbit. Report OH/CTL/P114B. Imperial
Chemical Industries PLC, Alderley Park, Macclesfield, Cheshire, UK.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989a). Unpublished report. Diquat: 90 day feeding
study in the rat. Report no CTL/P/1832 (revised). Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, UK. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989b). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2196. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989c). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2379. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1990). Unpublished report. Diquat: multigeneration
study in the rat. Report CTL/P/2462. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1992). Unpublished report. Diquat: two year feeding
study in mice. Report CTL/P/3409. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hopkins, M.N. (1990). Unpublished report. Diquat: 1 year feeding
study in dogs. Report CTL/P/2596. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hughes, R.D., Millburn, P. & Williams, R.T. (1973). Biliary
excretion of some diquaternary ammonium cations in the rat, guinea-
pig and rabbit. Biochem J., 136: 979-984.
IPCS (1984). Environmental Health Criteria 39 paraquat and diquat.
International Programme on Chemical Safety, WHO, Geneva.
Johnston, A.M., Jones, C., McCullum, J., Mutch, P.J. & Scott, G.
(1990a). The elimination of 14C-diquat in the rat following single
dose oral administration (low level). Report no CTL/C/2554. Inveresk
Research International, Tranent, EH33 2NE, Scotland. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Mutch, P.J. & Scott, G. (1990b). The elimination of
14C-diquat in the rat following single dose oral administration
(high dose level). Report CTL/C/2555. Inveresk Research
International, Tranent, EH33 2NE, Scotland. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Jones, C., McCallam, J. & Scott, G. (1991).
Unpublished report. The disposition of 14C-diquat in the rat.
Report CTL/C/2553. Inveresk Research International, Tranent, EH33
2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Kurisaki, E. & Sato, H. (1979). Tissue distribution of paraquat and
diquat after oral administration in rats. Forensic Science, 14:
165-170.
Leahy, J.P., Gatehouse, D.M., Carpenter, P.K. & Benwell, M. (1976).
Diquat: metabolism and residue in a cow. ICI Plant Protection
Division. Report No. AR2698A. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Lock, E.A. (1979). The effect of paraquat and diquat on renal
function in the rat. Toxicol Appl Pharmacol., 48: 327-336.
Lock, E.A. & Ishmael, J. (1979). The acute toxic effects of paraquat
and diquat on the rat kidney. Toxicol Appl Pharmacol., 50: 67-76.
McCall, J.C. & Robinson, P. (1990a). Unpublished report. Diquat
dibromide: acute oral toxicity to the rat. Report CTL/P/2999. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
McCall, J.C. & Robinson, P. (1990b). Unpublished report. Diquat
dibromide: acute dermal toxicity to the rat. Report CTL/P/2982. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Milburn, G.M. (1989). Diquat: embryotoxicity study in the rat.
Unpublished report CTL/P/2275. ICI Central Toxicology Laboratory.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Mills, I.H. (1976). Unpublished report. Diquat: disposition and
metabolism in the rat.Report CTL/P/214. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Moore, S. & Wilson, G.J. (1973). Unpublished report. Diquat
dibromide: teratogenicity studies in the rat. Report No HO/IH/P/82B.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey.
Nirei, M., Hayasaka, S., Nagata, M., Tamai, A. & Tawara, T. (1993).
Ocular injury caused by preeglox-l, a herbicide contained paraquat,
diquat and surfactants. Jpn J Ophthalmol., 37: 43-46.
Ohta, T. (1987). Unpublished report. TOPPS: microbial mutagenicity
study. Report CTL/P/253A. Institute of Environmental Toxicology,
Kodaira, Tokyo 187, Japan. Report prepared for ICI Japan Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Parkinson, G.R. (1974a). Unpublished report. Diquat dichloride and
dibromide: comparison of dermal toxicity and local irritancy. Report
No HO/IH/P/116. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Parkinson, G.R. (1974b). Unpublished report. Diquat monopyridone:
acute and subacute oral toxicity. Report No CTL/P/12213. ICI Central
Toxicology Laboratory, Macclesfield. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Pirie, A. & Rees, J.R. (1970). Diquat cataract in the rat. Exptl.
Eye Research, 9: 198-203.
Pirie, A., Rees, J.R. & Holmberg, N.J. (1970). Diquat cataract:
formation of the free radical and its reaction with constituents of
the eye. Exptl. Eye Research, 9: 204-218.
Pritchard, V.K. (1986). Unpublished report. Diquat: acute oral
toxicity to the rat of a 200 g/l formulation ("Reglox"). Report
CTL/P/1525. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rawlings, J.M., Foster, J.R. & Heylings, J.R. (1992). Diquat-induced
intestinal secretion in the anaesthetized rat. Human Exp Toxicol.,
11: 524-529.
Richardson, C.R., Howerd, C.A., Wildgoose, J. (1986). Unpublished
report. Diquat dibromide (technical): a cytogenetic study in human
lymphocytes in vitro. Report CTL/P/1561. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Rikans, L.E. & Cai, Y. (1993). Diquat - induced oxidative damage in
BCNU - pretreated hepatocytes of mature and old rats. Tox Appl.
Pharm., 118: 263-270.
Rikans, L.E., Cai, Y., Kosanke, S.D., Venkataraman, P.S. (1993).
Redox cycling and hepatotoxicity of diquat in aging fischer 344
rats. Drug Metab. Dispos., 21: 605-610.
Rogerson, A. & Broad, R.D. (1978). Unpublished report. Diquat
dibromide: 2 year feeding study in rats - amended report. Report
CTL/P/253A.Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S. & Smith, L.L. (1977). The relevance of paraquat
accumulation by tissues. In: Autor PA Ed. Biochemical Mechanisms of
paraquat toxicity. Academic Press, New York pp 72-91.
Rose, M.S., Lock, E.A., Smith, L.L. & Wyatt, I. (1975). Unpublished
report. Paraquat accumulation: tissue and species specificity.
Report CTL/R/367A. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S., Smith, L.L. & Wyatt, I. (1976). The relevance of pentose
phosphate pathway stimulation in rat lung to the mechanism of
paraquat toxicity. Biochem Pharmacol., 25: 1763-1767.
Rose, M.S., Crabtree, H.C., Fletcher, K. & Wyatt, I. (1974).
Biochemical effects of diquat and paraquat disturbance of the
control of corticosteroid synthesis in rat adrenal and subsequent
effects on the control of liver glycogen synthesis. Biochem J.,
138: 437-443.
Scott, R.C. & Corrigan, M.A. (1990). The in vitro percutaneous
absorption of diquat: a species comparison. Toxicol in vitro, 4:
137-141.
Scott, R.C., Walker, M. & Mawdesley, S.J. (1991). Unpublished
report. Diquat dibromide: in vitro absorption from technical
concentrate ('Reglone 40') and spray strength solution through human
skin. ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Sechi, G.P., Agnetti, V., Piredda, M., Canu, M., Deserra, F., Omar,
H.A., Rosati, G. (1992). Acute and persistent parkinsonism after use
of diquat. Neurology, 42: 261-263.
Selypes, A., Nagymajtényi, L. & Berencsi, G. (1980). Mutagenic and
embryotoxic effects of paraquat and diquat. Bull Environ Contam
Toxicol., 25: 513-518.
Sharp, C.W., Ottlenghi, A. & Posner, H.S. (1972). Correlation of
paraquat toxicity with tissue concentrations and weight loss in the
rat. Toxicol Appl Pharmacol., 22: 241-251.
Sheldon, T., Richardson, C.R. & Shaw, J. (1986). Unpublished report.
Diquat dibromide (technical): an evaluation in the mouse
micronucleus test. No ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Shirasu, Y., Moriya, M. & Toshihiro, T. (1979). Unpublished report.
Mutagenicity testing on diquat in microbial systems. Institute of
Environmental Health Toxicology Division, Japan.
Smith, L.L. & Rose, M.S. (1977). A comparison of the effect of
paraquat and diquat on the water content of rat lung and the
incorporation of thymidine into lung DNA. Toxicology, 8: 223-230.
Southwood, J.(1987a). Unpublished report. Diquat: acute dermal
toxicity to the rat of a 200 g/l formulation. Report CTL/P/1881.
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Southwood, J.(1987b). Unpublished report. Topps acute oral toxicity
to the rat. Report CTL/P/1788. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Stevens, M.A. & Walley, J.K. (1966). Tissue and milk residues
arising from the ingestion of single doses of diquat and paraquat by
cattle. J. Sci. Food Agric., 17: 472-475.
Trueman, R.W., Elliot, B.M. & Barber, G. (1987). Unpublished report.
Diquat dibromide (technical): assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Report
CTL/P/1814. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Vanholder, R., Colardyn, F., de Reuck, J., Praet, M., Lameire, N.&
Ringoir. S. (1981). Diquat intoxication: report of two cases and
review of the literature. Am J Med., 70: 1267-1271.
Walley, J.K. (1987). Unpublished report.Diquat toxicity in cattle.
Industrial Hygiene Research Laboratories Report IHR/167 (3B.1/7).
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wickramatne, G.A. (1989). Unpublished report. Diquat: teratogenicity
study in the rat. Report CTL/P/2331. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Wildgoose, J., Braithwaite, I., Howerd, C.A., Richardson, C.R.
(1986). Unpublished report. Diquat dibromide: a cytogenetic study in
human lymphocytes in vitro. Report CTL/P/1469. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Williams, S.G., Cameron, B.D. & McGuire, G.M. (1991). Unpublished
report. Identification of the major radioactive components in urine
and faeces from rats following single oral administration of [14C]-
diquat. Report CTL/C/2523. Inveresk Research International, Tranent,
EH33 2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
 Table 1. Acute toxicity of diquat (dibromide unless otherwise stated)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Mouse Alderley Park M p.o. 125 Clark & Hurst (1970)
(106-146)
p.o. 170 WHO (1984)
Rat Alderley Park F p.o. 231 Clark & Hurst (1970)
(194-274)
Wistar M p.o. 231 Pritchard (1986)
Alpk:AP (211-254.5)
Wistar M p.o. 214 McCall & Robinson (1990a)
Alpk:APfSD (180-271)
Wistar F p.o. 222 McCall & Robinson (1990a)
Alpk: APfSD (203-241)
Alderley Park M s.c.1 11 Clark & Hurst (1970)
(5-15)
Alderley Park F s.c.1 10 Clark & Hurst (1970)
(6-14)
Alderley Park F s.c. 11 Clark & Hurst (1970)
(9-12)
Wistar (Alpk:AP) M p.c.2 > 400 Southwood (1987a)
Wistar Alpk:AP F p.c.2 > 400 Southwood (1987a)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Alderley Park ? p.c. 50-100 Parkinson (1974a)
albino
Sprague-Dawley M inh3 121 µg/l Bruce (1985)
CD (0.3-63 300)
Sprague-Dawley F inh3 132 µg/l Bruce (1985)
CD (0.3-141 560)
Rabbit ? F p.o. 101 Clark & Hurst (1970)
(72-138)
? F p.c. > 400 Clark & Hurst (1970)
New Zealand ? p.c. 50-100 Parkinson (1974b)
White
Guinea-pig ? F p.o. 100 Clark & Hurst (1970)
Dogs ? F p.o. 100-200 Clark & Hurst (1970)
Hens ? F p.o. 200-400 Clark & Hurst (1970)
Cattle ? ? p.o. 30 Walley (1987)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Cattle ? F p.o. 30 Clark & Hurst (1970)
Monkey Cynomolgus ? p.o.1 100-300 Cobb & Grimshaw (1979)
Notes
1) diquat dichloride
2) administered as 21.2% diquat ion
3) whole body 240 min exposure to aerosol
Urine and blood were examined during the study. At autopsy, blood
was taken for clinical chemistry and haematology and selected organs
weighed and examined histologically. Animals receiving diquat at a
concentration of 500 ppm showed a marked reduction in weight gain,
food consumption and utilization. Cataract formation was also
observed at this dose at 8 weeks and at autopsy, corneal opacity was
observed in 7/12 males and 4/12 females; at histological
examination, cataract was seen in 12/12 males and 11/12 females.
Additionally, a low incidence of focal inflammation of the tongue
and epithelium of the palate was observed. Reduced plasma protein
was seen at 500 ppm, possibly caused by low food intake. Absolute,
but not relative, organ weights were reduced, almost certainly a
reflection of poor food intake. Treatment-related effects were not
seen at 100 ppm. The NOAEL was 100 ppm, equal to 8.5 and 9.2 mg
ion/kg bw/day in males and females, respectively (Hodge, 1989a).
Dogs
A one-year study in dogs was carried out using diquat dibromide
technical (26.7 w/v ion) added to the feed. Groups of 4 male and 4
female beagles received diquat at doses of 0, 0.5, 2.5 or 12.5 mg
ion/kg bw/day. Clinical condition, body weight and food consumption
were monitored throughout. Ophthalmoscopy, haematology and clinical
chemistry were carried out. The animals were sacrificed and
autopsied at 52 weeks and a range of organs examined and processed
for histological examination. There was a small but statistically
significant decrease in body-weight gain in the high-dose groups of
both sexes, somewhat greater in the females. No such decrease was
seen at the lower doses. There was a statistically significant
decrease in WBC and neutrophil count in males of all treatment
groups at a single time point (week 4) which was ascribed to raised
counts in one control. There were decreased platelet counts in top
dose females at 4, 26 and 52 weeks. Raised plasma chloride levels
observed in the top dose animals were attributed to bromide ion
interference. Plasma triglyceride in the males given 12.5 mg ion/kg
bw/day were higher than in the controls throughout the study; at 4
and 26 weeks these increases were statistically significant.
Statistically significant increases in relative and absolute kidney
weight were observed in both sexes at 12.5 mg ion/kg bw/day. There
were decreases in absolute and relative adrenal weight in all
treatment groups in the males, which were statistically significant
only in the case of relative weights. Additionally, there was a
decrease in the absolute and relative weight of the epididymides in
all test groups compared to the controls; this finding was only
statistically significant for absolute weights in the 2.5 mg ion/kg
bw/day group and for relative weights in the top dose group.
Changes in organ weights did not correspond to any histopathological
changes. Cataract was seen in all the top dose animals by the end
of the study, both by ophthalmoscopy and histologically and in 2/4
females receiving diquat at a dose of 2.5 mg ion/kg bw/day; however
in the latter group only one was confirmed by histopathological
examination. Inflammatory changes were seen at the top dose in the
large intestine, consisting of reduction in mucosal thickness, loss
and abnormality of mucosal glands, epithelial hyperplasia in crypts
and increased goblet cell activity. The NOAEL was 0.5 mg ion/kg
bw/day based upon lens opacity in females at the next dose (Hopkins,
1990).
Long-term toxicity/carcinogenicity studies
Mice
In an 80-week feeding study, groups of CD-1 mice (60/sex) were
fed diquat dibromide (98.5% pure) at dietary concentrations of 30,
150 or 500 ppm. The highest dietary concentration was reduced to
400 ppm after 3 weeks and to 300 ppm after 5 weeks; these changes
were made because of lethal toxicity and the decedent mice were
replaced. Not all groups were started simultaneously: one control
group and the two lower test groups were started about three months
before the other control groups and the highest dosage group. In
general the test material had little clinical effect, except that
marked toxicity, evidenced by huddling, hypoactivity, and an
ungroomed appearance, were observed for the first few weeks at 500
ppm. Clinical recovery appeared complete by the eighth week.
Survival was not affected by treatment. Growth rates were reduced
at the highest dietary concentration and at 150 ppm. At 30 ppm,
there were no effects on body-weight gain. There was an increased
frequency of hepatic vacuolation in livers at 150 ppm (males) and at
the highest dose in both sexes. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day (Ashby, 1987).
In a 2-year study, groups of 60 male and 60 female mice
(C57BL/10JfCD-1/Alpk) were fed a diet containing 0, 30, 100 or 300
ppm diquat. The test material was technical grade diquat dibromide,
containing 26.7% diquat ion w/v. Haematological examination of tail
vein blood samples was carried out on all animals at 53 and 79
weeks. Fuller haematology was carried out on blood obtained by
cardiac puncture at 104 weeks. Various tissues were taken at
autopsy and processed for histological examination. There was
marked toxicity at the highest dose, shown by statistically
significant reductions in body-weight gain in both sexes, small and
sometimes statistically significant reductions in food consumption,
eye discharge and mild nephropathy. Changes in certain
haematological parameters at 300 ppm were also seen, namely
statistically significantly decreased neutrophil count and increased
lymphocyte count in both sexes at weeks 53 and 79. There was also a
significant increase in total WBC at 2 years in males. Relative
kidney weights were increased significantly in males and females.
At 100 ppm there were statistically significant reductions in body-
weight gain in both sexes, particularly in the males later in the
study; there were also some changes in haematology (lymphocytosis
and neutropenia) which, although statistically significant were
probably unimportant, and relative kidney weights were increased
significantly in males. No treatment-related carcinogenic effects
were seen in the study (there were reductions in certain tumour
incidences at 300 ppm) and the incidence of cataract was not related
to the test material. The NOAEL was 30 ppm, equal to 3.6 mg ion/kg
bw/day (males) and 4.8 mg ion/kg bw/day (females) (Hodge, 1992).
Rats
In a two-year feeding study, groups of Wistar-derived rats
(35/sex) received diquat dibromide (100% pure = 53.6% ion) at
dietary concentrations of 0, 15, 25 or 75 ppm. Weight gain and food
consumption did not differ significantly between groups. A
significantly increased incidence of cataracts was observed at 75
ppm. The NOAEL was 25 ppm, equivalent to 1.3 mg ion/kg bw/day
(Rogerson & Broad, 1978).
In another 2-year study, diquat dibromide (technical grade) was
administered in the diet to groups of Sprague-Dawley rats (60/sex)
at dietary concentrations of 0, 5, 15, 75 or 375 ppm. Additionally,
satellite groups of 10 males and 10 females received diquat at the
same dietary concentrations and were killed at 1 year.
Ophthalmoscopy was carried out before dosing and periodically during
the study. Haematological and biochemical variables were measured
on 10 animals from each main group before dosing, and at 26, 52, 78
and 104 weeks, and in five satellite animals at week 52. A
reduction in food consumption and utilization efficacy was observed
at 375 ppm. After 26 weeks, there was a reduction in MCV and in
haemoglobin in females and males, respectively. Minimal reductions
in red blood cell parameters were observed in males at 15 ppm and
above, at 52 and 78 weeks. Other changes were prolonged activated
partial thromboplastin times at 75 and 375 ppm in females at 52 and
104 weeks and at 52 weeks in the 15 ppm females. Blood urea
nitrogen (BUN) was elevated at 52 weeks at 75 and 375 ppm and at 78
weeks in the 375 ppm females. At 52 weeks, the 75 and 375 ppm
groups exhibited lower total protein and albumen levels. On
ophthalmoscopic examination, lenticular opacities were seen in the
75 and 375 ppm groups at 13 weeks and the findings progressed at
subsequent examinations. Ophthalmoscopy at 104 weeks showed severe
lens opacities at 375 ppm in all surviving rats, while less severe
ones were seen at 75 ppm. At 15 ppm, a single instance of cataract
was seen in each sex. Histological examination of the eyes
postmortem, showed advanced cataractogenesis in all animals at 375
ppm and in about 80% of the 75 ppm group. There was a low
prevalence of cataract at 15 ppm: cataract-type changes being seen
in 0/22 controls and 3/22 at 15 ppm (males) and 0/22 controls and
2/20 at 15 ppm (females). Small differences were seen in
nephropathy in arteritis and in aneurism formation in males at the
highest dose. Elevations reported as statistically significant were
seen in the incidence of benign phenochromocytomas at 75 ppm with no
real evidence of a trend and in combined thyroid parafollicular cell
adenomas and carcinomas at 5 ppm, both in males but numbers were
very small. There was evidence of a trend for thyroid follicular
cell adenoma; however these figures appear to have been much
influenced by the frequency of the tumour at 375 ppm (in males the
number of tumours out of the number of thyroid glands examined in
each groups were 2/24, 1/32, 1/25, 0/25 and 3/32 for the control, 5,
15, 75 and 375 ppm groups respectively; none were observed in
females). There was a significant increase in the number of females
with multiple neoplasia and with malignant neoplasms at 5 and 75
ppm. No other change attributable to the test material was seen.
The LOAEL was 15 ppm (equal to 0.58 and 0.72 mg ion/kg bw/day for
males and females) based upon cataractogenesis. The NOAEL was 5
ppm, equal to 0.19 mg ion/kg bw/day (males) and 0.24 mg ion/kg
bw/day (females) (Colley et al., 1985).
Reproduction studies
Rats
A three-generation study was conducted in rats by Fletcher et
al. (1972) using diquat dibromide monohydrate (100% pure).
Wistar-derived rats were divided into 3 groups, each containing 12
males and 24 females. One group received standard diet while the
other two groups received diquat dibromide in aqueous solution at
dietary concentrations of 0, 125 or 500 ppm when they were
approximately 35-days old. Thereafter the three groups and their
progeny remained on the diet throughout the study. Body weights and
food consumption were recorded weekly and the animals physically
examined daily. The F0 animals were mated after 100-days feeding,
one male and two females being housed together for this purpose.
Where pregnancy had not occurred in three weeks, the male was
replaced by another from the same group. Litters were examined
within 20 hours of delivery (F1a). At 21 days, each litter was
counted, weighed sexed and subjected to autopsy. Second matings
were arranged after a 10-day rest period. From the progeny (F1b),
12 males and 24 females were reared and continued on the diet from
weaning, the remainder being killed and examined. At 100 days, the
F1b animals were mated for the production of the second generation
(F2). The F2 generation was treated in the same way as the F1,
the F2a litters being examined and autopsied at 21 days and the F2b
providing the breeders for production of the F3 generation. The
F3a litters were treated as before and the F3b animals were also
autopsied at 21 days and detailed examination, including
histological examination was carried out postmortem. Rats treated
with diquat (500 ppm) developed lens opacity from about 125 days.
The proportion exhibiting this abnormality increased and at 300 days
about 50% were affected. Opacity was not seen at the lower dose.
There was also a statistically significant reduction in weight gain
at 500 ppm, at 15 weeks in both sexes of F0 parents. Similar
reductions (after weaning) were found in the subsequent generations.
In the second generation, significantly reduced body weights were
seen in the 125 ppm females at weaning and the final week before
mating but not at other weighings. There were 6 fewer pregnancies
in the 500 ppm group of F1 animals. Atrophy of the seminiferous
tubules was observed in parents and progeny of each generation.
Although more common in the 500 ppm groups, there was no clear dose-
response relationship. This study did not exhibit an NOAEL since
there was decreased weight gain in F0 and F1 animals at the lowest
dose, but the effects observed at this dose (125 ppm, equivalent to
6.3 mg ion/kg bw/day) were trivial (Fletcher et al., 1972).
A multigeneration study was conducted using technical grade
diquat dibromide, containing diquat ion 26.7% w/v. Groups of
Alpk:APfSD rats (30/sex) were fed diets containing 0, 16, 80 or 400
ppm diquat. After 12 weeks the animals were mated and allowed to
rear the litters that resulted (F1a). The process was repeated
with 30 male and 30 female parents/group selected from the F1a
litter, these F1 parents being mated 11 weeks after selection. The
dose received by the top dose F1 rats was reduced after 4 weeks to
240 ppm. The animals were examined daily and mouths examined
weekly. Animals were weighed weekly in the premating period. After
mating the males were weighed monthly and the females were weighed
on days 1, 8, 15 and 20 of pregnancy and days 1, 5, 11, 16 and 22 of
lactation. All animals were weighed at the terminal kill. Food
consumption was recorded weekly until mating, and weekly in the
females during pregnancy and lactation. Ophthalmoscopic examination
of the eyes was carried out on the controls and top dose group at 12
weeks and on all the F0 animals at termination (24 weeks). A
similar examination was carried out on the F1 rats 4 weeks after
selection, at 11 weeks before mating and at termination (21 weeks).
Diquat had no effect on fertility in either sex. Decreased body-
weight gain was seen at the top dose in both adults (F0 and F1)
and pups. Inflammatory lesions in the mouth, particularly
ulceration of the hard palate was observed at 400 ppm in F0 rats
and F1 pups and adults. Cataracts, first seen at week 12 in F0
females and at week 13 in males and 2 weeks earlier in each sex in
the F1a and F1b, were also observed in the F1 adults. Although
cataract formation was mostly confined to the 240 ppm group, a low
incidence was seen at 80 ppm in the F1 female parents (3/30 at
premating ophthalmoscopic examination and 4/30 at examination before
termination of the study). At the terminal histopathological
examination, cataract was seen in the highest dose group only of F0
and F1 parents. There was an increase in pathological changes in
the renal tract in the F1 and F2 pups. Reproductive toxicity
(reduced pup weight gain) was observed at 400 ppm. The NOAEL was 16
ppm (equivalent to 0.8 mg/kg bw/day) based upon a low incidence of
partial cataract formation at 80 ppm (Hodge, 1990).
Other studies on reproduction parameters
An antifertility effect on male mice was noted during the
dominant lethal study of Pasi et al. (1974). Diquat at doses up
to 10 ppm in the diet of hens for 6 weeks did not affect food
consumption, egg production and hatchability. Residues of diquat
were not found in eggs or in hens' tissues (Edwards & Smith, 1975).
Special studies on embryo/fetotoxicity
Mice
In a study in Swiss-Webster and Sprague-Dawley (CD) mice, a
single dose of 15 mg/kg bw diquat was given i.p. to groups of three
animals at day 7-21 of gestation. Nine maternal deaths out of 45
animals occurred. The percentage dead plus resorbed fetuses was
57%. A NOAEL could not be determined in this study (Bus et al.,
1975).
Groups of 20 female CFLP mice were administered diquat i.p. (11
mg/kg bw Reglone) on day 9 of gestation or diquat (2.7 mg/kg bw/day
Reglone) on days 9-12 of gestation. Both groups showed increased
fetal loss or resorption compared to controls. Skeletal
abnormalities were observed in the test group embryos. A NOAEL
could not be determined in this study (Selypes et al., 1980).
Rats
Diquat was administered in the diet to pregnant Sprague-Dawley
rats, from days 1-20 of pregnancy at 0, 125 or 500 ppm diquat ion.
There were 18 control rats, and 20 in each of the test groups.
Animals were killed at day 20, fetuses removed and the uteri
examined. All rats remained in good condition throughout the study
but food consumption and body-weight gain were reduced significantly
at 500 ppm. There was no adverse effect on implantations, mean
number of fetuses, litter weight or sex ratio. Mean fetal weight
was significantly lower in the 500 ppm group. A dose-related
increase in subcutaneous fetal haemorrhages compared to the controls
was observed. A NOAEL could not be determined in this study (Moore
& Wilson, 1973).
Diquat containing 26.2 % ion w/v was administered by gavage to
groups of 8 female rats (Wistar derived Alpk:APfSD) at doses of 0,
4, 12, 24 or 40 mg ion/kg bw/day in deionized water from days 7-16
of gestation. Controls received deionized water. On day 22 the
females were killed and the uteri examined for live fetuses and
intrauterine deaths. At a dose of 40 mg ion/kg bw/day the mothers
showed reduced weight gain and changes in clinical condition
(piloerection, urinary incontinence and gasping) and 4/24 animals at
24 mg ion/kg bw/day showed milder signs. Some fetotoxicity was seen
at 40 mg ion/kg bw/day (reduced fetal weight gain), but not at the
lower doses, whereas maternal toxicity, as indicated by dose-related
reduced food consumption and body-weight gain, was observed at all
doses. Although there was a statistically non-significant increase
in pre- and post-implantation loss at 24 mg ion/kg bw/day, this was
not observed at the other three doses. No fetal abnormality was
observed. For fetal toxicity, the LOAEL was 40 mg ion/kg bw/day and
the NOAEL 24 mg ion/kg bw/day. The NOAEL for maternal toxicity
could not be determined in this study (Milburn, 1989).
Groups of 24 female rats were given diquat by gavage in
deionized water (26.2 % ion w/v) at doses of 4, 12 or 40 mg ion/kg
bw/day from days 7-16 of gestation. Maternal toxicity was seen at
40 mg ion/kg bw/day as reduced weight gain and food consumption.
Significant reductions in fetal weight, litter weight and gravid
uterine weight as well as fetal defects in ossification were seen at
40 mg ion/kg bw/day. Minor evidence of reduced ossification were
seen at the lower doses but these were considered not to be of
biological significance. For both maternal and fetotoxicity the
LOAEL was 40 mg ion/kg bw/day and the NOAEL 12 mg ion/kg bw/day
(Wickramatne, 1989).
Rabbits
Groups of up to 20 female mated Dutch rabbits were dosed orally
with diquat dibromide (100% pure = 53.6% ion) at levels of 1.3, 2.5
or 5.0 mg ion/kg bw/day from days 1-28 of gestation (day of mating =
day 0). The material was administered in "Dispersol OG" (a 10%
solution of ricinoleic glycerides with glycerol and polyglycerols),
which was also used as control. An additional 4 does were allowed
to litter and the eyes of the offspring examined for cataracts. The
rabbits were sacrificed at day 29 and the uteri examined for live
fetuses and intrauterine deaths. The fetuses were weighed and
examined for gross abnormality and about half processed for skeletal
examination and half for soft tissue examination. There was a
reduction in maternal weight gain at 5.0 mg ion/kg bw/day, which was
not statistically significant. There was no evidence of any effect
on embryonic or fetal development. The NOAEL was 2.5 mg ion/kg
bw/day based on mild maternal toxicity at the highest dose (Hodge,
1987). A feature of this study was the poor pregnancy rate in all
groups which necessitated mating of extra animals to achieve
acceptable numbers of pregnancies per group.
In a second study, groups of 7 or 8 female New Zeeland white
rabbits were administered diquat dibromide technical by gavage in
deionized water (26.2% ion w/v) at doses of 0, 1, 3, 7 or 10 mg
ion/kg bw/day from days 7-19 of gestation. Controls received water.
On day 30 of gestation, the females were killed and the uteri
examined for live fetuses and intrauterine deaths. Doses of 3 mg
ion/kg bw/day or above were associated with maternal toxicity as
manifested by weight loss or reduced weight gain and reduced food
intake. No evidence of fetotoxicity was observed. The NOAEL was 1
mg ion/kg bw/day based upon maternal toxicity (Hodge, 1989b).
In a third study, groups of 20 New Zeeland white rabbits were
administered by gavage diquat dibromide technical (26.2% ion w/v) in
deionized water, at doses of 0, 1, 3 or 10 mg ion/kg bw/day from
days 7-19 of gestation. On day 30 of gestation the females were
killed and the uteri examined for live fetuses and intrauterine
deaths. The fetuses were weighed, examined externally and for
visceral abnormalities, eviscerated and stained for skeletal
abnormalities. Doses of 10 mg ion/kg bw/day caused maternal
toxicity as shown by weight loss and reduced food intake; five
animals from this group were sacrificed early in extremis.
Effects on body weight and food consumption, although less severe,
were also present at 3 mg ion/kg bw/day. Some evidence of
fetotoxicity was observed at 10 mg/kg bw/day (mottled and friable
livers and small increase in minor skeletal defects at 3 and 10 mg
ion/kg bw/day, in the form of partially ossified sternabrae). The
elevation in the proportion of fetuses with minor skeletal defect
was significant at 3 and 10 mg ion/kg bw/day; there was a non-
significant increase at the lowest dose group. The NOAEL was 1 mg
ion/kg bw/day based on maternal toxicity (reduced weight gain and
food consumption) and skeletal effects in the fetuses at doses of 3
mg ion/kg bw/day (Hodge, 1989c).
Special studies on genotoxicity
Based on the results of the genotoxicity assays given in Table
2, the Meeting concluded that diquat was not genotoxic.
Toxicity of metabolites
In Wistar-derived rats, diquat dipyridone and monopyridine were
less toxic than diquat when given subcutaneously (Parkinson, 1974b;
Crabtree, 1976).
The results of acute and genotoxicity studies with TOPPS are
given in Tables 3 and 4, respectively.
Other animal studies
Diquat dichloride and dibromide are both moderate skin
irritants to the skin of the rat and mildly irritant to the rabbit
eye (Parkinson, 1974). Orally administered diquat increases
secretion into the gut lumen (Crabtree et al., 1977; Rawlings et
al., 1992). In a study to detect any other major actions of
diquat in laboratory animals, single doses of up to 280 mg/kg bw
were administered orally to rats. Effects such as CNS depression
and increased gut motility were observed at perilethal doses only
(Allen & Brammer, 1990).
Table 2. Results of genotoxicity assays on diquat
Test system Test object Concentration of diquat Purity Results Reference
In vitro
Ames test S. typhimurium 0.01-50 100% -ve Shirasu et al. (1979)
(5) (strains TA1535, µg/plate
1537, 1538, 98, 100)
Ames test S. typhimurium 0.00256-100 100% -ve Callander (1986a)
(5) (strains TA1535, µg/plate
1537, 1538, 98, ± S9
100
Ames test S. typhimurium 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
(5) (strains TA1535, with S9, ion (technical)
1537, 1538 0.1-50 µg/plate
98, 100) without S9
Reverse E. coli WP2 hcr 0.01-50 µg/plate 100% -ve Shirasu et al. (1979)
mutation (5)
Reverse E. coli WP2 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
mutation (5) uvrA pKM101 with S9, ion (technical)
0.1-50 µg/plate
without S9
Mammalian Mouse lymphoma 6.25-100 µg/ml 100% -ve Cross (1986a)
cell (5) L5178Y TK+/-
Mammalian Mouse lymphoma 6.25-100 µg/ml 25.8% w/w -ve Cross (1986b)
cell (5) L5178Y TK+/- ion (technical)
Table 2 (contd)
Test system Test object Concentration of diquat Purity Results Reference
Cytogenetics Human lymphocytes 26.7, 107, 267(2), 100% +ve (4) Wildgoose et al. (1986)
(1) 534.8(3) µg/ml as ion
Cytogenetics Human lymphocytes 12.9-129 µg/ml 25.8% w/w +ve (4) Richardson et al. (1986)
(1) ion (technical)
Rec-assay B. subtilis 2-200 µg/disc 100% -ve Shirasu et al. (1979)
H17, M45
In vivo
Cytogenetics Rat bone marrow 4.4, 9.5, 14, 100% -ve Anderson et al. (1980)
(Wistar-derived mg/kg bw/day
Alderley Park) for 5 days orally
Cytogenetics Mouse bone marrow 0.73, 3.6, 7.3, 22 100% -ve Selypes et al. (1980)
(CFLP) mg/kg bw ip
90 mg/kg/bw/po
Micro-nucleus Mouse bone marrow 0, 62.5, 100 mg/kg 25.8% w/w -ve Sheldon et al. (1986)
C57BL/6J/Alpk bw ion (technical)
Dominant Mouse (CD-1) 0.1-10 mg ion/kg 28.6% w/w -ve Anderson et al. (1976)
Lethal Test bw/day for 5 days ion (technical)
UDS Rat hepatocyte 225, 450, 900 25.8% w/w -ve Trueman et al. (1987)
in vivo mg/kg ion ion (technical)
1) ± S9
2) Donor 1 MTD
3) Donor 2 MTD
4) Clastogenic only at doses causing cytotoxicity
5) with and without metabolic activation
Table 3. Acute toxicity of TOPPS
Species Strain Sex Route LD50 (mg/kg bw) References
Rat Wistar Alpk:A M PO 2449 Southwood (1987b)
(2000-3000)
Wistar Alpk:A F PO 2942 Southwood (1987b)
(2000-5000)
Table 4. Results of genotoxicity assays on TOPPS
Test system Test object Concentration of TOPPS Purity Results Reference
Ames (1) S. typhimurium 0.1-5 mg/plate 98% -ve Ohta (1987)
TA1535, TA1537,
98, 100
E. coli 0.1-5 mg/plate 98% -ve Ohta (1987)
WP uvrA
Rec Assay (1) B. subtilis 0.2-10 mg/disc 98% -ve Ohta (1987)
(1) With or without metabolic activation.
Special studies on cataractogenesis
Pirie & Rees (1970) fed Wistar albino rats a diet containing
0.05 or 0.075% diquat dibromide. Lens opacity was produced after 4-
8 months at both doses. Ascorbic acid content of the lens, but
unusually not GSH content, decreased during development of cataract.
Radioactivity appeared in the lens after intraperitoneal injection
of 14C-labelled diquat. Pirie et al. (1970) suggested that free-
radical formation might be responsible for cataract formation.
Observations in humans
Vanholder et al. (1981) reviewed several cases of poisoning
with diquat. In one case, initial signs and symptoms were
abdominal. Later oliguria and coma developed. Shock supervened
followed by cardiac arrest. In another case progression was slower
but renal failure occurred and eventually led to anuria. Despite
haemodialysis, death occurred from ventricular fibrillation, and
renal failure was observed. Unlike poisoning with paraquat, diquat
does not cause lung fibrosis. Cataracts have not been observed in
humans (IPCS, 1984) and more recent investigations have not shown
cataract formation in those engaged in diquat manufacture or
formulation (Bonsall, 1990).
Two patients were splashed in the eyes with a preparation
containing both paraquat and diquat. In both cases the corneal
epithelium was damaged and healing was slow (Nirei et al., 1993).
In a recent report, a child survived what was initially
believed to be a fatal dose of diquat, apparently with no sequelae
(Buckley & MacKiernan, 1991, 1992). A recent case report describes
a patient who developed Parkinsonism a few days after exposure to
diquat (Sechi et al., 1992). The extent of exposure of the
patient to the pesticide was not known and thus the significance of
this isolated observation in a 72-year old man is questionable.
COMMENTS
When administered orally 14C-diquat is poorly absorbed from
the gastrointestinal tract of rats, cows and goats and mainly
eliminated vis the faeces during the first 24 hours, the small part
absorbed being principally eliminated via the urine. The total
percentages of administered doses eliminated via the faeces were 94,
91 and 94 for the rat, cow and goat, respectively; 3.1% and 0.4%
were eliminated in the urine of the rat and the cow, respectively,
and very small percentages of radioactivity were found in cow's and
goat's milk (0.004% and 0.0175%), respectively.
After oral administration of 14C-diquat to rats (45 mg ion/kg
bw), the major excreted product was diquat in both urine (5% of
dose) and faeces (> 57% of dose): diquat monopyridone was the main
metabolite in the faeces (5% of dose), but a minor one in the urine.
In another oral study in rats (100 mg ion/kg bw), a small amount of
diquat dipyridone and picolinic acid was found in addition to the
monopyridone. After subcutaneous injection (10 mg ion/kg bw) in the
rat, 75% of the dose was present in the urine as diquat, about 3% as
the monopyridone and 6% as the dipyridone.
Unlike paraquat, diquat is not actively taken up by lung
slices, and lung toxicity is not characteristic of diquat poisoning.
The acute oral toxicity of diquat varies with species, but is
between 125 and 250 mg ion/kg bw in rodents. It is classified by
WHO as moderately hazardous.
In a 90-day feeding study in rats, using dietary concentrations
of 0, 20, 100 or 500 ppm, the NOAEL was 100 ppm, equal to 8.5 mg
ion/kg bw/day, based upon reduction in body-weight gain, food
consumption and reduced plasma protein at the next higher dose.
In a one-year feeding study, dogs received doses of 0, 0.5, 2.5
or 12.5 mg/kg bw/day. The NOAEL was 0.5 mg ion/kg bw/day based upon
lens opacity in females at the next dose.
Two long-term toxicity/carcinogenicity studies were conducted
in mice. The first (80 weeks) used dietary concentrations of diquat
ion of 0, 30, 150 or 500 ppm. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day, based upon reduced growth rates at the next
higher dose together with hepatic vacuolation in males. In a 2-year
study in mice, in which dietary concentrations of 0, 30, 100 or 300
ppm were used, the NOAEL was 30 ppm, equal to 3.6 mg ion/kg bw/day,
based on reduction in body-weight gain and increased relative kidney
weights at the next higher dose. There was no evidence of
carcinogenicity in mice.
Two 2-year feeding studies in rats have been conducted. In the
first study, diquat dibromide was administered in the diet at
concentrations of 0, 5, 15, 75 or 375 ppm. The NOAEL was 5 ppm,
equal to 0.19 mg ion/kg bw/day, based upon cataract formation in the
15 ppm group. In the second study, dietary concentrations of 0, 15,
25 or 75 ppm diquat ion were used. The NOAEL was 25 ppm (equivalent
to 1.3 mg ion/kg bw/day), based on cataract formation at the next
higher dose. There was no evidence of carcinogenicity in rats.
Numerous teratogenicity studies have been conducted. NOAELs
could not be determined in two mouse studies. There were three
teratogenicity studies in rats; in the first study dietary
concentrations of 0, 125 or 500 ppm diquat ion were used. A dose-
related increase in subcutaneous fetal haemorrhages compared to the
controls was observed. A NOAEL could not be derived from this
study. In the second study, diquat was administered at oral doses
of 0, 4, 12, 24 or 40 mg ion/kg bw/day. For fetal toxicity, the
NOAEL was 24 mg ion/kg bw/day, but maternal toxicity was observed in
all test groups (reduced weight gain and food consumption). In the
third study, diquat was administered by gavage at doses of 0, 4, 12
or 40 mg ion/kg bw/day. The NOAEL for both maternal and fetal
toxicity was 12 mg ion/kg bw/day, based in the case of the dams on
reduced body weight and food consumption and in the case of the
fetuses on reduced fetal weight and defects in fetal ossification at
the highest dose.
In a study in rabbits, diquat was given orally at doses of 0,
1.3, 2.5 or 5.0 mg ion/kg bw/day. There was no evidence of any
effects on embryonic or fetal development. The NOAEL was 2.5 mg
ion/kg bw/day based on mild maternal toxicity at the highest dose.
In a second study in rabbits, doses of 0, 1, 3, 7 or 10 mg ion/kg
bw/day were administered by gavage. Doses of 3 mg ion/kg bw/day or
above were associated with maternal toxicity as manifested by weight
loss or reduced weight gain and reduced food intake. No evidence of
fetotoxicity was observed. The NOAEL was 1 mg ion/kg bw/day based
upon maternal toxicity. In a third study in rabbits, doses of 0, 1,
3 or 10 mg ion/kg bw/day diquat were given by gavage. The NOAEL was
1 mg ion/kg bw/day based upon maternal toxicity (reduced weight gain
and food consumption) and skeletal effects in the fetuses at doses
of 3 mg ion/kg bw/day.
Two multigeneration reproduction studies were conducted in
rats. In the first study, diquat was given at dietary
concentrations of 0, 125 or 500 ppm. This study did not exhibit an
NOAEL, since there was decreased weight gain in F0 and F1 animals
at the lowest dose, but the effects observed at this dose (125 ppm,
equivalent to 6.3 mg ion/kg bw/day) were trivial. In the second
study, rats were fed diquat at dietary concentrations of 0, 16, 80
or 400 ppm. The NOAEL was 16 ppm (equivalent to 0.8 mg/kg bw/day)
based upon a low incidence of partial cataract formation at 80 ppm.
Diquat has been adequately tested in a series of genotoxicity
assays in vitro and in vivo. Chromosomal aberrations were
induced in vitro but there was no other evidence of genotoxicity.
The Meeting concluded that diquat was not genotoxic.
An ADI of 0-0.002 mg/kg bw was established based upon a NOAEL
of 0.19 mg ion/kg bw/day identified in a two-year study in rats,
using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 30 ppm, equal to 3.6 mg ion/kg bw/day
(two-year study)
Rat: 5 ppm, equal to 0.19 mg ion/kg bw/day
(two-year study)
12 mg ion/kg bw/day (teratogenicity study)
16 ppm, equivalent to 1.6 mg ion/kg bw/day
(multigeneration reproduction study)
Rabbit: 1 mg/kg bw/day (teratogenicity study)
Dog: 0.5 mg ion/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.002 mg ion/kg bw
Studies which will provide information in the continued evaluation
of the compound
Observations in humans.
REFERENCES
Anderson, D., McGregor, D.B. & Purchase, I.F.H. (1976). Dominant
lethal studies with paraquat and diquat in male CD-1 mice. Mutation
Research, 40: 349-358.
Anderson, D., Richardson, C.R., Howard, C.A., Banham, P., Hart, D. &
Weight, T.M. (1980). Unpublished report. Diquat: a cytogenetic study
in the rat. Report CTL/P/366. Imperial Chemical Industries PLC,
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Allen, S.L. & Brammer, A. (1990). Unpublished report. Diquat:
pharmacological evaluation. Report No CTL/P/2842*(revised). ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Ashby, R. (1987). Unpublished report. Diquat dibromide monohydrate:
evaluation of potential carcinogenicity in dietary adminstration to
mice for 80 weeks. Final Report CTL/C/3761 (LSR 87/ILY001/375). Life
Sciences Research Ltd, Eye, Suffolk, England. Supplied to WHO by
Zeneca. Vols 1 & 2.
Baldwin, R.C., Pasi, A., McGregor, J.T. & Hine, C.H. (1975). The
rates of radical formation from the dipyridilium herbicides
paraquat, diquat, and morphamquat in homogenates of rat lung,
kidney, and liver: an inhibitory effect of carbon monoxide. Toxicol
Appl Pharmacol., 32: 298-304.
Bhuyan, D.K. & Bhuyan, K.C. (1991). Oxy radicals in the eye tissues
of rabbits after diquat in vivo. Free Radic Res Commun. 12-13
Pt.2: 621-627.
Black, W.J.M., Calderbank, A., Douglas, G. & McKenna, R.H. (1966).
Residues in herbage and silage and feeding experiments following the
use of diquat as a dessicant. J Sci Food Agric., 17: 506-509.
Bonsall, J.L. (1990). Letter to F Tripet, Stauffer Chemicals,
Switzerland dated October 9 1990.
Bruce, E.D. (1985). Unpublished report. The acute inhalation
toxicity of diquat water weed killer (SX-1574) in rats. Report
CTL/C/2701 (SOCAL 2353). Chevron Environmental Health Center,
Richmond, California. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Buckley, D.A. & McKiernan, J. (1991/1992). Survival after ingestion
of a fatal dose of diquat (letter). Irish Med. J., 84: 134.
Bus, J.S., Preache, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
Sharp, C.W. & Gibson, J.E. (1975). Fetal Toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl Pharmacol.,
33: 450-460.
Callander, R.D. (1986a). Unpublished report. Diquat dibromide: an
evaluation in the Salmonella mutagenicity assay. Report
CTL/P/1413. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Callander, R.D. (1986b). Unpublished report. Diquat dibromide
(technical): an evaluation in the Salmonella mutagenicity assay.
Report CTL/P/1463. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Clark, D.G. & Hurst, E.W. (1970). The toxicity of diquat. Brit J
Industr Med., 27: 51-55.
Cobb, L.M. & Grimshaw, P. (1979). Acute toxicity of oral diquat
(1,1'-ethylene-2,2'-bipyridinium) in cynomolgus monkeys. Toxicology
Appl Pharmacol. 51: 277-282.
Colley, J., Warren, S., Heywood, R., Street, A.E., Almond, R.H. &
Gopinath, C. (1985). Unpublished report. Diquat dibromide evaluation
of potential carcinogenicity and chronic toxicity by prolonged
dietary administration to rats. Report ICI 406/83763. Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, England. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. (1976). Unpublished report. Comparison of the
subcutaneous toxicity of diquat, diquat monopyridone and diquat
dipyridone. Report CTL/P/351. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. & Rose, M.S. (1976). Unpublished report. Early
effects of diquat on plasma corticosteroid concentrations in rats.
Report CTL/R/380. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C., Lock, E.A. & Rose, M.S. (1977). Effects of diquat on
the gastrointestinal tract of rats. Toxicology Appl Pharmacol.,
41: 585-595.
Cross, M.F. (1986a). Unpublished report. Diquat dibromide:
assessment of mutagenic potential using L5178Y mouse lymphoma cells.
Report CTL/P/1554. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Cross, M.F. (1986b). Unpublished report. Diquat dibromide
(technical): assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Report CTL/P/1602. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Daniel, J.W. & Gage, J.C. (1966). Absorption and excretion of diquat
and paraquat in rats. Brit J Industr Med., 23: 133-136.
Daniel, J.W. & Henson, A.F. (1960). Unpublished report. The
absorption and excretion of the herbicide K.8483 in rats. Report
IHR/137. Industrial Hygiene Research Laboratories and Akers Research
Laboratories. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Edwards, M.J. & Smith, D.C. (1975). Unpublished report. Diquat:
residue transfer and hatchability study in laying hens. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Feldmann, K.J. & Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicol appl Pharmacol,,
28: 126-132.
Fletcher, K., Griffiths, D. & Kinch, D.A. (1972). Unpublished
report. Diquat dibromide: three-generation reproduction study in
rats. Report HO/IH/R/331A. Imperial Chemical Industries Ltd
Industrial Hygiene Research Laboratories. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Griggs, R.E. & Davis, J.A. (1975). Unpublished report. Diquat:
excretion and metabolism in a goat. Report AR 2585 A. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Griggs, R.E. (1973).
Unpublished report. Metabolism of diquat and its photoproducts in
goats. Report AR 2448 B. ICI Plant Protection Ltd. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Burgess (1974).
Unpublished report. Diquat: metabolism of diquat and its
photoproducts in a cow. Report AR 2448 B. ICI Plant Protection Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1987). Unpublished report. Diquat dibromide:
teratogenic studies in the rabbit. Report OH/CTL/P114B. Imperial
Chemical Industries PLC, Alderley Park, Macclesfield, Cheshire, UK.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989a). Unpublished report. Diquat: 90 day feeding
study in the rat. Report no CTL/P/1832 (revised). Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, UK. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989b). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2196. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989c). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2379. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1990). Unpublished report. Diquat: multigeneration
study in the rat. Report CTL/P/2462. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1992). Unpublished report. Diquat: two year feeding
study in mice. Report CTL/P/3409. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hopkins, M.N. (1990). Unpublished report. Diquat: 1 year feeding
study in dogs. Report CTL/P/2596. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hughes, R.D., Millburn, P. & Williams, R.T. (1973). Biliary
excretion of some diquaternary ammonium cations in the rat, guinea-
pig and rabbit. Biochem J., 136: 979-984.
IPCS (1984). Environmental Health Criteria 39 paraquat and diquat.
International Programme on Chemical Safety, WHO, Geneva.
Johnston, A.M., Jones, C., McCullum, J., Mutch, P.J. & Scott, G.
(1990a). The elimination of 14C-diquat in the rat following single
dose oral administration (low level). Report no CTL/C/2554. Inveresk
Research International, Tranent, EH33 2NE, Scotland. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Mutch, P.J. & Scott, G. (1990b). The elimination of
14C-diquat in the rat following single dose oral administration
(high dose level). Report CTL/C/2555. Inveresk Research
International, Tranent, EH33 2NE, Scotland. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Jones, C., McCallam, J. & Scott, G. (1991).
Unpublished report. The disposition of 14C-diquat in the rat.
Report CTL/C/2553. Inveresk Research International, Tranent, EH33
2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Kurisaki, E. & Sato, H. (1979). Tissue distribution of paraquat and
diquat after oral administration in rats. Forensic Science, 14:
165-170.
Leahy, J.P., Gatehouse, D.M., Carpenter, P.K. & Benwell, M. (1976).
Diquat: metabolism and residue in a cow. ICI Plant Protection
Division. Report No. AR2698A. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Lock, E.A. (1979). The effect of paraquat and diquat on renal
function in the rat. Toxicol Appl Pharmacol., 48: 327-336.
Lock, E.A. & Ishmael, J. (1979). The acute toxic effects of paraquat
and diquat on the rat kidney. Toxicol Appl Pharmacol., 50: 67-76.
McCall, J.C. & Robinson, P. (1990a). Unpublished report. Diquat
dibromide: acute oral toxicity to the rat. Report CTL/P/2999. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
McCall, J.C. & Robinson, P. (1990b). Unpublished report. Diquat
dibromide: acute dermal toxicity to the rat. Report CTL/P/2982. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Milburn, G.M. (1989). Diquat: embryotoxicity study in the rat.
Unpublished report CTL/P/2275. ICI Central Toxicology Laboratory.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Mills, I.H. (1976). Unpublished report. Diquat: disposition and
metabolism in the rat.Report CTL/P/214. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Moore, S. & Wilson, G.J. (1973). Unpublished report. Diquat
dibromide: teratogenicity studies in the rat. Report No HO/IH/P/82B.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey.
Nirei, M., Hayasaka, S., Nagata, M., Tamai, A. & Tawara, T. (1993).
Ocular injury caused by preeglox-l, a herbicide contained paraquat,
diquat and surfactants. Jpn J Ophthalmol., 37: 43-46.
Ohta, T. (1987). Unpublished report. TOPPS: microbial mutagenicity
study. Report CTL/P/253A. Institute of Environmental Toxicology,
Kodaira, Tokyo 187, Japan. Report prepared for ICI Japan Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Parkinson, G.R. (1974a). Unpublished report. Diquat dichloride and
dibromide: comparison of dermal toxicity and local irritancy. Report
No HO/IH/P/116. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Parkinson, G.R. (1974b). Unpublished report. Diquat monopyridone:
acute and subacute oral toxicity. Report No CTL/P/12213. ICI Central
Toxicology Laboratory, Macclesfield. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Pirie, A. & Rees, J.R. (1970). Diquat cataract in the rat. Exptl.
Eye Research, 9: 198-203.
Pirie, A., Rees, J.R. & Holmberg, N.J. (1970). Diquat cataract:
formation of the free radical and its reaction with constituents of
the eye. Exptl. Eye Research, 9: 204-218.
Pritchard, V.K. (1986). Unpublished report. Diquat: acute oral
toxicity to the rat of a 200 g/l formulation ("Reglox"). Report
CTL/P/1525. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rawlings, J.M., Foster, J.R. & Heylings, J.R. (1992). Diquat-induced
intestinal secretion in the anaesthetized rat. Human Exp Toxicol.,
11: 524-529.
Richardson, C.R., Howerd, C.A., Wildgoose, J. (1986). Unpublished
report. Diquat dibromide (technical): a cytogenetic study in human
lymphocytes in vitro. Report CTL/P/1561. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Rikans, L.E. & Cai, Y. (1993). Diquat - induced oxidative damage in
BCNU - pretreated hepatocytes of mature and old rats. Tox Appl.
Pharm., 118: 263-270.
Rikans, L.E., Cai, Y., Kosanke, S.D., Venkataraman, P.S. (1993).
Redox cycling and hepatotoxicity of diquat in aging fischer 344
rats. Drug Metab. Dispos., 21: 605-610.
Rogerson, A. & Broad, R.D. (1978). Unpublished report. Diquat
dibromide: 2 year feeding study in rats - amended report. Report
CTL/P/253A.Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S. & Smith, L.L. (1977). The relevance of paraquat
accumulation by tissues. In: Autor PA Ed. Biochemical Mechanisms of
paraquat toxicity. Academic Press, New York pp 72-91.
Rose, M.S., Lock, E.A., Smith, L.L. & Wyatt, I. (1975). Unpublished
report. Paraquat accumulation: tissue and species specificity.
Report CTL/R/367A. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S., Smith, L.L. & Wyatt, I. (1976). The relevance of pentose
phosphate pathway stimulation in rat lung to the mechanism of
paraquat toxicity. Biochem Pharmacol., 25: 1763-1767.
Rose, M.S., Crabtree, H.C., Fletcher, K. & Wyatt, I. (1974).
Biochemical effects of diquat and paraquat disturbance of the
control of corticosteroid synthesis in rat adrenal and subsequent
effects on the control of liver glycogen synthesis. Biochem J.,
138: 437-443.
Scott, R.C. & Corrigan, M.A. (1990). The in vitro percutaneous
absorption of diquat: a species comparison. Toxicol in vitro, 4:
137-141.
Scott, R.C., Walker, M. & Mawdesley, S.J. (1991). Unpublished
report. Diquat dibromide: in vitro absorption from technical
concentrate ('Reglone 40') and spray strength solution through human
skin. ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Sechi, G.P., Agnetti, V., Piredda, M., Canu, M., Deserra, F., Omar,
H.A., Rosati, G. (1992). Acute and persistent parkinsonism after use
of diquat. Neurology, 42: 261-263.
Selypes, A., Nagymajtényi, L. & Berencsi, G. (1980). Mutagenic and
embryotoxic effects of paraquat and diquat. Bull Environ Contam
Toxicol., 25: 513-518.
Sharp, C.W., Ottlenghi, A. & Posner, H.S. (1972). Correlation of
paraquat toxicity with tissue concentrations and weight loss in the
rat. Toxicol Appl Pharmacol., 22: 241-251.
Sheldon, T., Richardson, C.R. & Shaw, J. (1986). Unpublished report.
Diquat dibromide (technical): an evaluation in the mouse
micronucleus test. No ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Shirasu, Y., Moriya, M. & Toshihiro, T. (1979). Unpublished report.
Mutagenicity testing on diquat in microbial systems. Institute of
Environmental Health Toxicology Division, Japan.
Smith, L.L. & Rose, M.S. (1977). A comparison of the effect of
paraquat and diquat on the water content of rat lung and the
incorporation of thymidine into lung DNA. Toxicology, 8: 223-230.
Southwood, J.(1987a). Unpublished report. Diquat: acute dermal
toxicity to the rat of a 200 g/l formulation. Report CTL/P/1881.
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Southwood, J.(1987b). Unpublished report. Topps acute oral toxicity
to the rat. Report CTL/P/1788. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Stevens, M.A. & Walley, J.K. (1966). Tissue and milk residues
arising from the ingestion of single doses of diquat and paraquat by
cattle. J. Sci. Food Agric., 17: 472-475.
Trueman, R.W., Elliot, B.M. & Barber, G. (1987). Unpublished report.
Diquat dibromide (technical): assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Report
CTL/P/1814. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Vanholder, R., Colardyn, F., de Reuck, J., Praet, M., Lameire, N.&
Ringoir. S. (1981). Diquat intoxication: report of two cases and
review of the literature. Am J Med., 70: 1267-1271.
Walley, J.K. (1987). Unpublished report.Diquat toxicity in cattle.
Industrial Hygiene Research Laboratories Report IHR/167 (3B.1/7).
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wickramatne, G.A. (1989). Unpublished report. Diquat: teratogenicity
study in the rat. Report CTL/P/2331. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Wildgoose, J., Braithwaite, I., Howerd, C.A., Richardson, C.R.
(1986). Unpublished report. Diquat dibromide: a cytogenetic study in
human lymphocytes in vitro. Report CTL/P/1469. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Williams, S.G., Cameron, B.D. & McGuire, G.M. (1991). Unpublished
report. Identification of the major radioactive components in urine
and faeces from rats following single oral administration of [14C]-
diquat. Report CTL/C/2523. Inveresk Research International, Tranent,
EH33 2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Table 1. Acute toxicity of diquat (dibromide unless otherwise stated)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Mouse Alderley Park M p.o. 125 Clark & Hurst (1970)
(106-146)
p.o. 170 WHO (1984)
Rat Alderley Park F p.o. 231 Clark & Hurst (1970)
(194-274)
Wistar M p.o. 231 Pritchard (1986)
Alpk:AP (211-254.5)
Wistar M p.o. 214 McCall & Robinson (1990a)
Alpk:APfSD (180-271)
Wistar F p.o. 222 McCall & Robinson (1990a)
Alpk: APfSD (203-241)
Alderley Park M s.c.1 11 Clark & Hurst (1970)
(5-15)
Alderley Park F s.c.1 10 Clark & Hurst (1970)
(6-14)
Alderley Park F s.c. 11 Clark & Hurst (1970)
(9-12)
Wistar (Alpk:AP) M p.c.2 > 400 Southwood (1987a)
Wistar Alpk:AP F p.c.2 > 400 Southwood (1987a)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Wistar M p.c.2 > 1070 McCall & Robinson (1990b)
Alpk:APfSD
Alderley Park ? p.c. 50-100 Parkinson (1974a)
albino
Sprague-Dawley M inh3 121 µg/l Bruce (1985)
CD (0.3-63 300)
Sprague-Dawley F inh3 132 µg/l Bruce (1985)
CD (0.3-141 560)
Rabbit ? F p.o. 101 Clark & Hurst (1970)
(72-138)
? F p.c. > 400 Clark & Hurst (1970)
New Zealand ? p.c. 50-100 Parkinson (1974b)
White
Guinea-pig ? F p.o. 100 Clark & Hurst (1970)
Dogs ? F p.o. 100-200 Clark & Hurst (1970)
Hens ? F p.o. 200-400 Clark & Hurst (1970)
Cattle ? ? p.o. 30 Walley (1987)
Table 1 (contd)
Species Strain Sex Route LD50/LC50 (mg ion/kg References
bw)/(mg ion/l)
Cattle ? F p.o. 30 Clark & Hurst (1970)
Monkey Cynomolgus ? p.o.1 100-300 Cobb & Grimshaw (1979)
Notes
1) diquat dichloride
2) administered as 21.2% diquat ion
3) whole body 240 min exposure to aerosol
Urine and blood were examined during the study. At autopsy, blood
was taken for clinical chemistry and haematology and selected organs
weighed and examined histologically. Animals receiving diquat at a
concentration of 500 ppm showed a marked reduction in weight gain,
food consumption and utilization. Cataract formation was also
observed at this dose at 8 weeks and at autopsy, corneal opacity was
observed in 7/12 males and 4/12 females; at histological
examination, cataract was seen in 12/12 males and 11/12 females.
Additionally, a low incidence of focal inflammation of the tongue
and epithelium of the palate was observed. Reduced plasma protein
was seen at 500 ppm, possibly caused by low food intake. Absolute,
but not relative, organ weights were reduced, almost certainly a
reflection of poor food intake. Treatment-related effects were not
seen at 100 ppm. The NOAEL was 100 ppm, equal to 8.5 and 9.2 mg
ion/kg bw/day in males and females, respectively (Hodge, 1989a).
Dogs
A one-year study in dogs was carried out using diquat dibromide
technical (26.7 w/v ion) added to the feed. Groups of 4 male and 4
female beagles received diquat at doses of 0, 0.5, 2.5 or 12.5 mg
ion/kg bw/day. Clinical condition, body weight and food consumption
were monitored throughout. Ophthalmoscopy, haematology and clinical
chemistry were carried out. The animals were sacrificed and
autopsied at 52 weeks and a range of organs examined and processed
for histological examination. There was a small but statistically
significant decrease in body-weight gain in the high-dose groups of
both sexes, somewhat greater in the females. No such decrease was
seen at the lower doses. There was a statistically significant
decrease in WBC and neutrophil count in males of all treatment
groups at a single time point (week 4) which was ascribed to raised
counts in one control. There were decreased platelet counts in top
dose females at 4, 26 and 52 weeks. Raised plasma chloride levels
observed in the top dose animals were attributed to bromide ion
interference. Plasma triglyceride in the males given 12.5 mg ion/kg
bw/day were higher than in the controls throughout the study; at 4
and 26 weeks these increases were statistically significant.
Statistically significant increases in relative and absolute kidney
weight were observed in both sexes at 12.5 mg ion/kg bw/day. There
were decreases in absolute and relative adrenal weight in all
treatment groups in the males, which were statistically significant
only in the case of relative weights. Additionally, there was a
decrease in the absolute and relative weight of the epididymides in
all test groups compared to the controls; this finding was only
statistically significant for absolute weights in the 2.5 mg ion/kg
bw/day group and for relative weights in the top dose group.
Changes in organ weights did not correspond to any histopathological
changes. Cataract was seen in all the top dose animals by the end
of the study, both by ophthalmoscopy and histologically and in 2/4
females receiving diquat at a dose of 2.5 mg ion/kg bw/day; however
in the latter group only one was confirmed by histopathological
examination. Inflammatory changes were seen at the top dose in the
large intestine, consisting of reduction in mucosal thickness, loss
and abnormality of mucosal glands, epithelial hyperplasia in crypts
and increased goblet cell activity. The NOAEL was 0.5 mg ion/kg
bw/day based upon lens opacity in females at the next dose (Hopkins,
1990).
Long-term toxicity/carcinogenicity studies
Mice
In an 80-week feeding study, groups of CD-1 mice (60/sex) were
fed diquat dibromide (98.5% pure) at dietary concentrations of 30,
150 or 500 ppm. The highest dietary concentration was reduced to
400 ppm after 3 weeks and to 300 ppm after 5 weeks; these changes
were made because of lethal toxicity and the decedent mice were
replaced. Not all groups were started simultaneously: one control
group and the two lower test groups were started about three months
before the other control groups and the highest dosage group. In
general the test material had little clinical effect, except that
marked toxicity, evidenced by huddling, hypoactivity, and an
ungroomed appearance, were observed for the first few weeks at 500
ppm. Clinical recovery appeared complete by the eighth week.
Survival was not affected by treatment. Growth rates were reduced
at the highest dietary concentration and at 150 ppm. At 30 ppm,
there were no effects on body-weight gain. There was an increased
frequency of hepatic vacuolation in livers at 150 ppm (males) and at
the highest dose in both sexes. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day (Ashby, 1987).
In a 2-year study, groups of 60 male and 60 female mice
(C57BL/10JfCD-1/Alpk) were fed a diet containing 0, 30, 100 or 300
ppm diquat. The test material was technical grade diquat dibromide,
containing 26.7% diquat ion w/v. Haematological examination of tail
vein blood samples was carried out on all animals at 53 and 79
weeks. Fuller haematology was carried out on blood obtained by
cardiac puncture at 104 weeks. Various tissues were taken at
autopsy and processed for histological examination. There was
marked toxicity at the highest dose, shown by statistically
significant reductions in body-weight gain in both sexes, small and
sometimes statistically significant reductions in food consumption,
eye discharge and mild nephropathy. Changes in certain
haematological parameters at 300 ppm were also seen, namely
statistically significantly decreased neutrophil count and increased
lymphocyte count in both sexes at weeks 53 and 79. There was also a
significant increase in total WBC at 2 years in males. Relative
kidney weights were increased significantly in males and females.
At 100 ppm there were statistically significant reductions in body-
weight gain in both sexes, particularly in the males later in the
study; there were also some changes in haematology (lymphocytosis
and neutropenia) which, although statistically significant were
probably unimportant, and relative kidney weights were increased
significantly in males. No treatment-related carcinogenic effects
were seen in the study (there were reductions in certain tumour
incidences at 300 ppm) and the incidence of cataract was not related
to the test material. The NOAEL was 30 ppm, equal to 3.6 mg ion/kg
bw/day (males) and 4.8 mg ion/kg bw/day (females) (Hodge, 1992).
Rats
In a two-year feeding study, groups of Wistar-derived rats
(35/sex) received diquat dibromide (100% pure = 53.6% ion) at
dietary concentrations of 0, 15, 25 or 75 ppm. Weight gain and food
consumption did not differ significantly between groups. A
significantly increased incidence of cataracts was observed at 75
ppm. The NOAEL was 25 ppm, equivalent to 1.3 mg ion/kg bw/day
(Rogerson & Broad, 1978).
In another 2-year study, diquat dibromide (technical grade) was
administered in the diet to groups of Sprague-Dawley rats (60/sex)
at dietary concentrations of 0, 5, 15, 75 or 375 ppm. Additionally,
satellite groups of 10 males and 10 females received diquat at the
same dietary concentrations and were killed at 1 year.
Ophthalmoscopy was carried out before dosing and periodically during
the study. Haematological and biochemical variables were measured
on 10 animals from each main group before dosing, and at 26, 52, 78
and 104 weeks, and in five satellite animals at week 52. A
reduction in food consumption and utilization efficacy was observed
at 375 ppm. After 26 weeks, there was a reduction in MCV and in
haemoglobin in females and males, respectively. Minimal reductions
in red blood cell parameters were observed in males at 15 ppm and
above, at 52 and 78 weeks. Other changes were prolonged activated
partial thromboplastin times at 75 and 375 ppm in females at 52 and
104 weeks and at 52 weeks in the 15 ppm females. Blood urea
nitrogen (BUN) was elevated at 52 weeks at 75 and 375 ppm and at 78
weeks in the 375 ppm females. At 52 weeks, the 75 and 375 ppm
groups exhibited lower total protein and albumen levels. On
ophthalmoscopic examination, lenticular opacities were seen in the
75 and 375 ppm groups at 13 weeks and the findings progressed at
subsequent examinations. Ophthalmoscopy at 104 weeks showed severe
lens opacities at 375 ppm in all surviving rats, while less severe
ones were seen at 75 ppm. At 15 ppm, a single instance of cataract
was seen in each sex. Histological examination of the eyes
postmortem, showed advanced cataractogenesis in all animals at 375
ppm and in about 80% of the 75 ppm group. There was a low
prevalence of cataract at 15 ppm: cataract-type changes being seen
in 0/22 controls and 3/22 at 15 ppm (males) and 0/22 controls and
2/20 at 15 ppm (females). Small differences were seen in
nephropathy in arteritis and in aneurism formation in males at the
highest dose. Elevations reported as statistically significant were
seen in the incidence of benign phenochromocytomas at 75 ppm with no
real evidence of a trend and in combined thyroid parafollicular cell
adenomas and carcinomas at 5 ppm, both in males but numbers were
very small. There was evidence of a trend for thyroid follicular
cell adenoma; however these figures appear to have been much
influenced by the frequency of the tumour at 375 ppm (in males the
number of tumours out of the number of thyroid glands examined in
each groups were 2/24, 1/32, 1/25, 0/25 and 3/32 for the control, 5,
15, 75 and 375 ppm groups respectively; none were observed in
females). There was a significant increase in the number of females
with multiple neoplasia and with malignant neoplasms at 5 and 75
ppm. No other change attributable to the test material was seen.
The LOAEL was 15 ppm (equal to 0.58 and 0.72 mg ion/kg bw/day for
males and females) based upon cataractogenesis. The NOAEL was 5
ppm, equal to 0.19 mg ion/kg bw/day (males) and 0.24 mg ion/kg
bw/day (females) (Colley et al., 1985).
Reproduction studies
Rats
A three-generation study was conducted in rats by Fletcher et
al. (1972) using diquat dibromide monohydrate (100% pure).
Wistar-derived rats were divided into 3 groups, each containing 12
males and 24 females. One group received standard diet while the
other two groups received diquat dibromide in aqueous solution at
dietary concentrations of 0, 125 or 500 ppm when they were
approximately 35-days old. Thereafter the three groups and their
progeny remained on the diet throughout the study. Body weights and
food consumption were recorded weekly and the animals physically
examined daily. The F0 animals were mated after 100-days feeding,
one male and two females being housed together for this purpose.
Where pregnancy had not occurred in three weeks, the male was
replaced by another from the same group. Litters were examined
within 20 hours of delivery (F1a). At 21 days, each litter was
counted, weighed sexed and subjected to autopsy. Second matings
were arranged after a 10-day rest period. From the progeny (F1b),
12 males and 24 females were reared and continued on the diet from
weaning, the remainder being killed and examined. At 100 days, the
F1b animals were mated for the production of the second generation
(F2). The F2 generation was treated in the same way as the F1,
the F2a litters being examined and autopsied at 21 days and the F2b
providing the breeders for production of the F3 generation. The
F3a litters were treated as before and the F3b animals were also
autopsied at 21 days and detailed examination, including
histological examination was carried out postmortem. Rats treated
with diquat (500 ppm) developed lens opacity from about 125 days.
The proportion exhibiting this abnormality increased and at 300 days
about 50% were affected. Opacity was not seen at the lower dose.
There was also a statistically significant reduction in weight gain
at 500 ppm, at 15 weeks in both sexes of F0 parents. Similar
reductions (after weaning) were found in the subsequent generations.
In the second generation, significantly reduced body weights were
seen in the 125 ppm females at weaning and the final week before
mating but not at other weighings. There were 6 fewer pregnancies
in the 500 ppm group of F1 animals. Atrophy of the seminiferous
tubules was observed in parents and progeny of each generation.
Although more common in the 500 ppm groups, there was no clear dose-
response relationship. This study did not exhibit an NOAEL since
there was decreased weight gain in F0 and F1 animals at the lowest
dose, but the effects observed at this dose (125 ppm, equivalent to
6.3 mg ion/kg bw/day) were trivial (Fletcher et al., 1972).
A multigeneration study was conducted using technical grade
diquat dibromide, containing diquat ion 26.7% w/v. Groups of
Alpk:APfSD rats (30/sex) were fed diets containing 0, 16, 80 or 400
ppm diquat. After 12 weeks the animals were mated and allowed to
rear the litters that resulted (F1a). The process was repeated
with 30 male and 30 female parents/group selected from the F1a
litter, these F1 parents being mated 11 weeks after selection. The
dose received by the top dose F1 rats was reduced after 4 weeks to
240 ppm. The animals were examined daily and mouths examined
weekly. Animals were weighed weekly in the premating period. After
mating the males were weighed monthly and the females were weighed
on days 1, 8, 15 and 20 of pregnancy and days 1, 5, 11, 16 and 22 of
lactation. All animals were weighed at the terminal kill. Food
consumption was recorded weekly until mating, and weekly in the
females during pregnancy and lactation. Ophthalmoscopic examination
of the eyes was carried out on the controls and top dose group at 12
weeks and on all the F0 animals at termination (24 weeks). A
similar examination was carried out on the F1 rats 4 weeks after
selection, at 11 weeks before mating and at termination (21 weeks).
Diquat had no effect on fertility in either sex. Decreased body-
weight gain was seen at the top dose in both adults (F0 and F1)
and pups. Inflammatory lesions in the mouth, particularly
ulceration of the hard palate was observed at 400 ppm in F0 rats
and F1 pups and adults. Cataracts, first seen at week 12 in F0
females and at week 13 in males and 2 weeks earlier in each sex in
the F1a and F1b, were also observed in the F1 adults. Although
cataract formation was mostly confined to the 240 ppm group, a low
incidence was seen at 80 ppm in the F1 female parents (3/30 at
premating ophthalmoscopic examination and 4/30 at examination before
termination of the study). At the terminal histopathological
examination, cataract was seen in the highest dose group only of F0
and F1 parents. There was an increase in pathological changes in
the renal tract in the F1 and F2 pups. Reproductive toxicity
(reduced pup weight gain) was observed at 400 ppm. The NOAEL was 16
ppm (equivalent to 0.8 mg/kg bw/day) based upon a low incidence of
partial cataract formation at 80 ppm (Hodge, 1990).
Other studies on reproduction parameters
An antifertility effect on male mice was noted during the
dominant lethal study of Pasi et al. (1974). Diquat at doses up
to 10 ppm in the diet of hens for 6 weeks did not affect food
consumption, egg production and hatchability. Residues of diquat
were not found in eggs or in hens' tissues (Edwards & Smith, 1975).
Special studies on embryo/fetotoxicity
Mice
In a study in Swiss-Webster and Sprague-Dawley (CD) mice, a
single dose of 15 mg/kg bw diquat was given i.p. to groups of three
animals at day 7-21 of gestation. Nine maternal deaths out of 45
animals occurred. The percentage dead plus resorbed fetuses was
57%. A NOAEL could not be determined in this study (Bus et al.,
1975).
Groups of 20 female CFLP mice were administered diquat i.p. (11
mg/kg bw Reglone) on day 9 of gestation or diquat (2.7 mg/kg bw/day
Reglone) on days 9-12 of gestation. Both groups showed increased
fetal loss or resorption compared to controls. Skeletal
abnormalities were observed in the test group embryos. A NOAEL
could not be determined in this study (Selypes et al., 1980).
Rats
Diquat was administered in the diet to pregnant Sprague-Dawley
rats, from days 1-20 of pregnancy at 0, 125 or 500 ppm diquat ion.
There were 18 control rats, and 20 in each of the test groups.
Animals were killed at day 20, fetuses removed and the uteri
examined. All rats remained in good condition throughout the study
but food consumption and body-weight gain were reduced significantly
at 500 ppm. There was no adverse effect on implantations, mean
number of fetuses, litter weight or sex ratio. Mean fetal weight
was significantly lower in the 500 ppm group. A dose-related
increase in subcutaneous fetal haemorrhages compared to the controls
was observed. A NOAEL could not be determined in this study (Moore
& Wilson, 1973).
Diquat containing 26.2 % ion w/v was administered by gavage to
groups of 8 female rats (Wistar derived Alpk:APfSD) at doses of 0,
4, 12, 24 or 40 mg ion/kg bw/day in deionized water from days 7-16
of gestation. Controls received deionized water. On day 22 the
females were killed and the uteri examined for live fetuses and
intrauterine deaths. At a dose of 40 mg ion/kg bw/day the mothers
showed reduced weight gain and changes in clinical condition
(piloerection, urinary incontinence and gasping) and 4/24 animals at
24 mg ion/kg bw/day showed milder signs. Some fetotoxicity was seen
at 40 mg ion/kg bw/day (reduced fetal weight gain), but not at the
lower doses, whereas maternal toxicity, as indicated by dose-related
reduced food consumption and body-weight gain, was observed at all
doses. Although there was a statistically non-significant increase
in pre- and post-implantation loss at 24 mg ion/kg bw/day, this was
not observed at the other three doses. No fetal abnormality was
observed. For fetal toxicity, the LOAEL was 40 mg ion/kg bw/day and
the NOAEL 24 mg ion/kg bw/day. The NOAEL for maternal toxicity
could not be determined in this study (Milburn, 1989).
Groups of 24 female rats were given diquat by gavage in
deionized water (26.2 % ion w/v) at doses of 4, 12 or 40 mg ion/kg
bw/day from days 7-16 of gestation. Maternal toxicity was seen at
40 mg ion/kg bw/day as reduced weight gain and food consumption.
Significant reductions in fetal weight, litter weight and gravid
uterine weight as well as fetal defects in ossification were seen at
40 mg ion/kg bw/day. Minor evidence of reduced ossification were
seen at the lower doses but these were considered not to be of
biological significance. For both maternal and fetotoxicity the
LOAEL was 40 mg ion/kg bw/day and the NOAEL 12 mg ion/kg bw/day
(Wickramatne, 1989).
Rabbits
Groups of up to 20 female mated Dutch rabbits were dosed orally
with diquat dibromide (100% pure = 53.6% ion) at levels of 1.3, 2.5
or 5.0 mg ion/kg bw/day from days 1-28 of gestation (day of mating =
day 0). The material was administered in "Dispersol OG" (a 10%
solution of ricinoleic glycerides with glycerol and polyglycerols),
which was also used as control. An additional 4 does were allowed
to litter and the eyes of the offspring examined for cataracts. The
rabbits were sacrificed at day 29 and the uteri examined for live
fetuses and intrauterine deaths. The fetuses were weighed and
examined for gross abnormality and about half processed for skeletal
examination and half for soft tissue examination. There was a
reduction in maternal weight gain at 5.0 mg ion/kg bw/day, which was
not statistically significant. There was no evidence of any effect
on embryonic or fetal development. The NOAEL was 2.5 mg ion/kg
bw/day based on mild maternal toxicity at the highest dose (Hodge,
1987). A feature of this study was the poor pregnancy rate in all
groups which necessitated mating of extra animals to achieve
acceptable numbers of pregnancies per group.
In a second study, groups of 7 or 8 female New Zeeland white
rabbits were administered diquat dibromide technical by gavage in
deionized water (26.2% ion w/v) at doses of 0, 1, 3, 7 or 10 mg
ion/kg bw/day from days 7-19 of gestation. Controls received water.
On day 30 of gestation, the females were killed and the uteri
examined for live fetuses and intrauterine deaths. Doses of 3 mg
ion/kg bw/day or above were associated with maternal toxicity as
manifested by weight loss or reduced weight gain and reduced food
intake. No evidence of fetotoxicity was observed. The NOAEL was 1
mg ion/kg bw/day based upon maternal toxicity (Hodge, 1989b).
In a third study, groups of 20 New Zeeland white rabbits were
administered by gavage diquat dibromide technical (26.2% ion w/v) in
deionized water, at doses of 0, 1, 3 or 10 mg ion/kg bw/day from
days 7-19 of gestation. On day 30 of gestation the females were
killed and the uteri examined for live fetuses and intrauterine
deaths. The fetuses were weighed, examined externally and for
visceral abnormalities, eviscerated and stained for skeletal
abnormalities. Doses of 10 mg ion/kg bw/day caused maternal
toxicity as shown by weight loss and reduced food intake; five
animals from this group were sacrificed early in extremis.
Effects on body weight and food consumption, although less severe,
were also present at 3 mg ion/kg bw/day. Some evidence of
fetotoxicity was observed at 10 mg/kg bw/day (mottled and friable
livers and small increase in minor skeletal defects at 3 and 10 mg
ion/kg bw/day, in the form of partially ossified sternabrae). The
elevation in the proportion of fetuses with minor skeletal defect
was significant at 3 and 10 mg ion/kg bw/day; there was a non-
significant increase at the lowest dose group. The NOAEL was 1 mg
ion/kg bw/day based on maternal toxicity (reduced weight gain and
food consumption) and skeletal effects in the fetuses at doses of 3
mg ion/kg bw/day (Hodge, 1989c).
Special studies on genotoxicity
Based on the results of the genotoxicity assays given in Table
2, the Meeting concluded that diquat was not genotoxic.
Toxicity of metabolites
In Wistar-derived rats, diquat dipyridone and monopyridine were
less toxic than diquat when given subcutaneously (Parkinson, 1974b;
Crabtree, 1976).
The results of acute and genotoxicity studies with TOPPS are
given in Tables 3 and 4, respectively.
Other animal studies
Diquat dichloride and dibromide are both moderate skin
irritants to the skin of the rat and mildly irritant to the rabbit
eye (Parkinson, 1974). Orally administered diquat increases
secretion into the gut lumen (Crabtree et al., 1977; Rawlings et
al., 1992). In a study to detect any other major actions of
diquat in laboratory animals, single doses of up to 280 mg/kg bw
were administered orally to rats. Effects such as CNS depression
and increased gut motility were observed at perilethal doses only
(Allen & Brammer, 1990).
Table 2. Results of genotoxicity assays on diquat
Test system Test object Concentration of diquat Purity Results Reference
In vitro
Ames test S. typhimurium 0.01-50 100% -ve Shirasu et al. (1979)
(5) (strains TA1535, µg/plate
1537, 1538, 98, 100)
Ames test S. typhimurium 0.00256-100 100% -ve Callander (1986a)
(5) (strains TA1535, µg/plate
1537, 1538, 98, ± S9
100
Ames test S. typhimurium 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
(5) (strains TA1535, with S9, ion (technical)
1537, 1538 0.1-50 µg/plate
98, 100) without S9
Reverse E. coli WP2 hcr 0.01-50 µg/plate 100% -ve Shirasu et al. (1979)
mutation (5)
Reverse E. coli WP2 0.5-100 µg/plate 25.8% w/w -ve Callander (1986b)
mutation (5) uvrA pKM101 with S9, ion (technical)
0.1-50 µg/plate
without S9
Mammalian Mouse lymphoma 6.25-100 µg/ml 100% -ve Cross (1986a)
cell (5) L5178Y TK+/-
Mammalian Mouse lymphoma 6.25-100 µg/ml 25.8% w/w -ve Cross (1986b)
cell (5) L5178Y TK+/- ion (technical)
Table 2 (contd)
Test system Test object Concentration of diquat Purity Results Reference
Cytogenetics Human lymphocytes 26.7, 107, 267(2), 100% +ve (4) Wildgoose et al. (1986)
(1) 534.8(3) µg/ml as ion
Cytogenetics Human lymphocytes 12.9-129 µg/ml 25.8% w/w +ve (4) Richardson et al. (1986)
(1) ion (technical)
Rec-assay B. subtilis 2-200 µg/disc 100% -ve Shirasu et al. (1979)
H17, M45
In vivo
Cytogenetics Rat bone marrow 4.4, 9.5, 14, 100% -ve Anderson et al. (1980)
(Wistar-derived mg/kg bw/day
Alderley Park) for 5 days orally
Cytogenetics Mouse bone marrow 0.73, 3.6, 7.3, 22 100% -ve Selypes et al. (1980)
(CFLP) mg/kg bw ip
90 mg/kg/bw/po
Micro-nucleus Mouse bone marrow 0, 62.5, 100 mg/kg 25.8% w/w -ve Sheldon et al. (1986)
C57BL/6J/Alpk bw ion (technical)
Dominant Mouse (CD-1) 0.1-10 mg ion/kg 28.6% w/w -ve Anderson et al. (1976)
Lethal Test bw/day for 5 days ion (technical)
UDS Rat hepatocyte 225, 450, 900 25.8% w/w -ve Trueman et al. (1987)
in vivo mg/kg ion ion (technical)
1) ± S9
2) Donor 1 MTD
3) Donor 2 MTD
4) Clastogenic only at doses causing cytotoxicity
5) with and without metabolic activation
Table 3. Acute toxicity of TOPPS
Species Strain Sex Route LD50 (mg/kg bw) References
Rat Wistar Alpk:A M PO 2449 Southwood (1987b)
(2000-3000)
Wistar Alpk:A F PO 2942 Southwood (1987b)
(2000-5000)
Table 4. Results of genotoxicity assays on TOPPS
Test system Test object Concentration of TOPPS Purity Results Reference
Ames (1) S. typhimurium 0.1-5 mg/plate 98% -ve Ohta (1987)
TA1535, TA1537,
98, 100
E. coli 0.1-5 mg/plate 98% -ve Ohta (1987)
WP uvrA
Rec Assay (1) B. subtilis 0.2-10 mg/disc 98% -ve Ohta (1987)
(1) With or without metabolic activation.
Special studies on cataractogenesis
Pirie & Rees (1970) fed Wistar albino rats a diet containing
0.05 or 0.075% diquat dibromide. Lens opacity was produced after 4-
8 months at both doses. Ascorbic acid content of the lens, but
unusually not GSH content, decreased during development of cataract.
Radioactivity appeared in the lens after intraperitoneal injection
of 14C-labelled diquat. Pirie et al. (1970) suggested that free-
radical formation might be responsible for cataract formation.
Observations in humans
Vanholder et al. (1981) reviewed several cases of poisoning
with diquat. In one case, initial signs and symptoms were
abdominal. Later oliguria and coma developed. Shock supervened
followed by cardiac arrest. In another case progression was slower
but renal failure occurred and eventually led to anuria. Despite
haemodialysis, death occurred from ventricular fibrillation, and
renal failure was observed. Unlike poisoning with paraquat, diquat
does not cause lung fibrosis. Cataracts have not been observed in
humans (IPCS, 1984) and more recent investigations have not shown
cataract formation in those engaged in diquat manufacture or
formulation (Bonsall, 1990).
Two patients were splashed in the eyes with a preparation
containing both paraquat and diquat. In both cases the corneal
epithelium was damaged and healing was slow (Nirei et al., 1993).
In a recent report, a child survived what was initially
believed to be a fatal dose of diquat, apparently with no sequelae
(Buckley & MacKiernan, 1991, 1992). A recent case report describes
a patient who developed Parkinsonism a few days after exposure to
diquat (Sechi et al., 1992). The extent of exposure of the
patient to the pesticide was not known and thus the significance of
this isolated observation in a 72-year old man is questionable.
COMMENTS
When administered orally 14C-diquat is poorly absorbed from
the gastrointestinal tract of rats, cows and goats and mainly
eliminated vis the faeces during the first 24 hours, the small part
absorbed being principally eliminated via the urine. The total
percentages of administered doses eliminated via the faeces were 94,
91 and 94 for the rat, cow and goat, respectively; 3.1% and 0.4%
were eliminated in the urine of the rat and the cow, respectively,
and very small percentages of radioactivity were found in cow's and
goat's milk (0.004% and 0.0175%), respectively.
After oral administration of 14C-diquat to rats (45 mg ion/kg
bw), the major excreted product was diquat in both urine (5% of
dose) and faeces (> 57% of dose): diquat monopyridone was the main
metabolite in the faeces (5% of dose), but a minor one in the urine.
In another oral study in rats (100 mg ion/kg bw), a small amount of
diquat dipyridone and picolinic acid was found in addition to the
monopyridone. After subcutaneous injection (10 mg ion/kg bw) in the
rat, 75% of the dose was present in the urine as diquat, about 3% as
the monopyridone and 6% as the dipyridone.
Unlike paraquat, diquat is not actively taken up by lung
slices, and lung toxicity is not characteristic of diquat poisoning.
The acute oral toxicity of diquat varies with species, but is
between 125 and 250 mg ion/kg bw in rodents. It is classified by
WHO as moderately hazardous.
In a 90-day feeding study in rats, using dietary concentrations
of 0, 20, 100 or 500 ppm, the NOAEL was 100 ppm, equal to 8.5 mg
ion/kg bw/day, based upon reduction in body-weight gain, food
consumption and reduced plasma protein at the next higher dose.
In a one-year feeding study, dogs received doses of 0, 0.5, 2.5
or 12.5 mg/kg bw/day. The NOAEL was 0.5 mg ion/kg bw/day based upon
lens opacity in females at the next dose.
Two long-term toxicity/carcinogenicity studies were conducted
in mice. The first (80 weeks) used dietary concentrations of diquat
ion of 0, 30, 150 or 500 ppm. The NOAEL was 30 ppm, equivalent to
4.5 mg ion/kg bw/day, based upon reduced growth rates at the next
higher dose together with hepatic vacuolation in males. In a 2-year
study in mice, in which dietary concentrations of 0, 30, 100 or 300
ppm were used, the NOAEL was 30 ppm, equal to 3.6 mg ion/kg bw/day,
based on reduction in body-weight gain and increased relative kidney
weights at the next higher dose. There was no evidence of
carcinogenicity in mice.
Two 2-year feeding studies in rats have been conducted. In the
first study, diquat dibromide was administered in the diet at
concentrations of 0, 5, 15, 75 or 375 ppm. The NOAEL was 5 ppm,
equal to 0.19 mg ion/kg bw/day, based upon cataract formation in the
15 ppm group. In the second study, dietary concentrations of 0, 15,
25 or 75 ppm diquat ion were used. The NOAEL was 25 ppm (equivalent
to 1.3 mg ion/kg bw/day), based on cataract formation at the next
higher dose. There was no evidence of carcinogenicity in rats.
Numerous teratogenicity studies have been conducted. NOAELs
could not be determined in two mouse studies. There were three
teratogenicity studies in rats; in the first study dietary
concentrations of 0, 125 or 500 ppm diquat ion were used. A dose-
related increase in subcutaneous fetal haemorrhages compared to the
controls was observed. A NOAEL could not be derived from this
study. In the second study, diquat was administered at oral doses
of 0, 4, 12, 24 or 40 mg ion/kg bw/day. For fetal toxicity, the
NOAEL was 24 mg ion/kg bw/day, but maternal toxicity was observed in
all test groups (reduced weight gain and food consumption). In the
third study, diquat was administered by gavage at doses of 0, 4, 12
or 40 mg ion/kg bw/day. The NOAEL for both maternal and fetal
toxicity was 12 mg ion/kg bw/day, based in the case of the dams on
reduced body weight and food consumption and in the case of the
fetuses on reduced fetal weight and defects in fetal ossification at
the highest dose.
In a study in rabbits, diquat was given orally at doses of 0,
1.3, 2.5 or 5.0 mg ion/kg bw/day. There was no evidence of any
effects on embryonic or fetal development. The NOAEL was 2.5 mg
ion/kg bw/day based on mild maternal toxicity at the highest dose.
In a second study in rabbits, doses of 0, 1, 3, 7 or 10 mg ion/kg
bw/day were administered by gavage. Doses of 3 mg ion/kg bw/day or
above were associated with maternal toxicity as manifested by weight
loss or reduced weight gain and reduced food intake. No evidence of
fetotoxicity was observed. The NOAEL was 1 mg ion/kg bw/day based
upon maternal toxicity. In a third study in rabbits, doses of 0, 1,
3 or 10 mg ion/kg bw/day diquat were given by gavage. The NOAEL was
1 mg ion/kg bw/day based upon maternal toxicity (reduced weight gain
and food consumption) and skeletal effects in the fetuses at doses
of 3 mg ion/kg bw/day.
Two multigeneration reproduction studies were conducted in
rats. In the first study, diquat was given at dietary
concentrations of 0, 125 or 500 ppm. This study did not exhibit an
NOAEL, since there was decreased weight gain in F0 and F1 animals
at the lowest dose, but the effects observed at this dose (125 ppm,
equivalent to 6.3 mg ion/kg bw/day) were trivial. In the second
study, rats were fed diquat at dietary concentrations of 0, 16, 80
or 400 ppm. The NOAEL was 16 ppm (equivalent to 0.8 mg/kg bw/day)
based upon a low incidence of partial cataract formation at 80 ppm.
Diquat has been adequately tested in a series of genotoxicity
assays in vitro and in vivo. Chromosomal aberrations were
induced in vitro but there was no other evidence of genotoxicity.
The Meeting concluded that diquat was not genotoxic.
An ADI of 0-0.002 mg/kg bw was established based upon a NOAEL
of 0.19 mg ion/kg bw/day identified in a two-year study in rats,
using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 30 ppm, equal to 3.6 mg ion/kg bw/day
(two-year study)
Rat: 5 ppm, equal to 0.19 mg ion/kg bw/day
(two-year study)
12 mg ion/kg bw/day (teratogenicity study)
16 ppm, equivalent to 1.6 mg ion/kg bw/day
(multigeneration reproduction study)
Rabbit: 1 mg/kg bw/day (teratogenicity study)
Dog: 0.5 mg ion/kg bw/day (one-year study)
Estimate of acceptable daily intake for humans
0-0.002 mg ion/kg bw
Studies which will provide information in the continued evaluation
of the compound
Observations in humans.
REFERENCES
Anderson, D., McGregor, D.B. & Purchase, I.F.H. (1976). Dominant
lethal studies with paraquat and diquat in male CD-1 mice. Mutation
Research, 40: 349-358.
Anderson, D., Richardson, C.R., Howard, C.A., Banham, P., Hart, D. &
Weight, T.M. (1980). Unpublished report. Diquat: a cytogenetic study
in the rat. Report CTL/P/366. Imperial Chemical Industries PLC,
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Allen, S.L. & Brammer, A. (1990). Unpublished report. Diquat:
pharmacological evaluation. Report No CTL/P/2842*(revised). ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca, Agrochemicals,
Fernhurst, Surrey, England.
Ashby, R. (1987). Unpublished report. Diquat dibromide monohydrate:
evaluation of potential carcinogenicity in dietary adminstration to
mice for 80 weeks. Final Report CTL/C/3761 (LSR 87/ILY001/375). Life
Sciences Research Ltd, Eye, Suffolk, England. Supplied to WHO by
Zeneca. Vols 1 & 2.
Baldwin, R.C., Pasi, A., McGregor, J.T. & Hine, C.H. (1975). The
rates of radical formation from the dipyridilium herbicides
paraquat, diquat, and morphamquat in homogenates of rat lung,
kidney, and liver: an inhibitory effect of carbon monoxide. Toxicol
Appl Pharmacol., 32: 298-304.
Bhuyan, D.K. & Bhuyan, K.C. (1991). Oxy radicals in the eye tissues
of rabbits after diquat in vivo. Free Radic Res Commun. 12-13
Pt.2: 621-627.
Black, W.J.M., Calderbank, A., Douglas, G. & McKenna, R.H. (1966).
Residues in herbage and silage and feeding experiments following the
use of diquat as a dessicant. J Sci Food Agric., 17: 506-509.
Bonsall, J.L. (1990). Letter to F Tripet, Stauffer Chemicals,
Switzerland dated October 9 1990.
Bruce, E.D. (1985). Unpublished report. The acute inhalation
toxicity of diquat water weed killer (SX-1574) in rats. Report
CTL/C/2701 (SOCAL 2353). Chevron Environmental Health Center,
Richmond, California. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Buckley, D.A. & McKiernan, J. (1991/1992). Survival after ingestion
of a fatal dose of diquat (letter). Irish Med. J., 84: 134.
Bus, J.S., Preache, M.M., Cagen, S.Z., Posner, H.S., Eliason, B.C.,
Sharp, C.W. & Gibson, J.E. (1975). Fetal Toxicity and distribution
of paraquat and diquat in mice and rats. Toxicol. Appl Pharmacol.,
33: 450-460.
Callander, R.D. (1986a). Unpublished report. Diquat dibromide: an
evaluation in the Salmonella mutagenicity assay. Report
CTL/P/1413. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Callander, R.D. (1986b). Unpublished report. Diquat dibromide
(technical): an evaluation in the Salmonella mutagenicity assay.
Report CTL/P/1463. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Clark, D.G. & Hurst, E.W. (1970). The toxicity of diquat. Brit J
Industr Med., 27: 51-55.
Cobb, L.M. & Grimshaw, P. (1979). Acute toxicity of oral diquat
(1,1'-ethylene-2,2'-bipyridinium) in cynomolgus monkeys. Toxicology
Appl Pharmacol. 51: 277-282.
Colley, J., Warren, S., Heywood, R., Street, A.E., Almond, R.H. &
Gopinath, C. (1985). Unpublished report. Diquat dibromide evaluation
of potential carcinogenicity and chronic toxicity by prolonged
dietary administration to rats. Report ICI 406/83763. Huntingdon
Research Centre plc, Huntingdon, Cambridgeshire, England. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. (1976). Unpublished report. Comparison of the
subcutaneous toxicity of diquat, diquat monopyridone and diquat
dipyridone. Report CTL/P/351. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C. & Rose, M.S. (1976). Unpublished report. Early
effects of diquat on plasma corticosteroid concentrations in rats.
Report CTL/R/380. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Crabtree, H.C., Lock, E.A. & Rose, M.S. (1977). Effects of diquat on
the gastrointestinal tract of rats. Toxicology Appl Pharmacol.,
41: 585-595.
Cross, M.F. (1986a). Unpublished report. Diquat dibromide:
assessment of mutagenic potential using L5178Y mouse lymphoma cells.
Report CTL/P/1554. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Cross, M.F. (1986b). Unpublished report. Diquat dibromide
(technical): assessment of mutagenic potential using L5178Y mouse
lymphoma cells. Report CTL/P/1602. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Daniel, J.W. & Gage, J.C. (1966). Absorption and excretion of diquat
and paraquat in rats. Brit J Industr Med., 23: 133-136.
Daniel, J.W. & Henson, A.F. (1960). Unpublished report. The
absorption and excretion of the herbicide K.8483 in rats. Report
IHR/137. Industrial Hygiene Research Laboratories and Akers Research
Laboratories. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Edwards, M.J. & Smith, D.C. (1975). Unpublished report. Diquat:
residue transfer and hatchability study in laying hens. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Feldmann, K.J. & Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicol appl Pharmacol,,
28: 126-132.
Fletcher, K., Griffiths, D. & Kinch, D.A. (1972). Unpublished
report. Diquat dibromide: three-generation reproduction study in
rats. Report HO/IH/R/331A. Imperial Chemical Industries Ltd
Industrial Hygiene Research Laboratories. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Griggs, R.E. & Davis, J.A. (1975). Unpublished report. Diquat:
excretion and metabolism in a goat. Report AR 2585 A. ICI Plant
Protection Ltd. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Griggs, R.E. (1973).
Unpublished report. Metabolism of diquat and its photoproducts in
goats. Report AR 2448 B. ICI Plant Protection Ltd. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hemingway, R.J., Leahey, J.P., Davis, J.A. & Burgess (1974).
Unpublished report. Diquat: metabolism of diquat and its
photoproducts in a cow. Report AR 2448 B. ICI Plant Protection Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1987). Unpublished report. Diquat dibromide:
teratogenic studies in the rabbit. Report OH/CTL/P114B. Imperial
Chemical Industries PLC, Alderley Park, Macclesfield, Cheshire, UK.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989a). Unpublished report. Diquat: 90 day feeding
study in the rat. Report no CTL/P/1832 (revised). Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, UK. Supplied
to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989b). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2196. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1989c). Unpublished report. Diquat: embryotoxicity
study in the rabbit. Report CTL/P/2379. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1990). Unpublished report. Diquat: multigeneration
study in the rat. Report CTL/P/2462. ICI Central Toxicology
Laboratory, Alderley Park, Macclesfield, Cheshire, UK. Supplied to
WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Hodge, M.C.E. (1992). Unpublished report. Diquat: two year feeding
study in mice. Report CTL/P/3409. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hopkins, M.N. (1990). Unpublished report. Diquat: 1 year feeding
study in dogs. Report CTL/P/2596. ICI Central Toxicology Laboratory,
Alderley Park, Macclesfield, Cheshire, UK. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Hughes, R.D., Millburn, P. & Williams, R.T. (1973). Biliary
excretion of some diquaternary ammonium cations in the rat, guinea-
pig and rabbit. Biochem J., 136: 979-984.
IPCS (1984). Environmental Health Criteria 39 paraquat and diquat.
International Programme on Chemical Safety, WHO, Geneva.
Johnston, A.M., Jones, C., McCullum, J., Mutch, P.J. & Scott, G.
(1990a). The elimination of 14C-diquat in the rat following single
dose oral administration (low level). Report no CTL/C/2554. Inveresk
Research International, Tranent, EH33 2NE, Scotland. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Mutch, P.J. & Scott, G. (1990b). The elimination of
14C-diquat in the rat following single dose oral administration
(high dose level). Report CTL/C/2555. Inveresk Research
International, Tranent, EH33 2NE, Scotland. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Johnston, A.M., Jones, C., McCallam, J. & Scott, G. (1991).
Unpublished report. The disposition of 14C-diquat in the rat.
Report CTL/C/2553. Inveresk Research International, Tranent, EH33
2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Kurisaki, E. & Sato, H. (1979). Tissue distribution of paraquat and
diquat after oral administration in rats. Forensic Science, 14:
165-170.
Leahy, J.P., Gatehouse, D.M., Carpenter, P.K. & Benwell, M. (1976).
Diquat: metabolism and residue in a cow. ICI Plant Protection
Division. Report No. AR2698A. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Lock, E.A. (1979). The effect of paraquat and diquat on renal
function in the rat. Toxicol Appl Pharmacol., 48: 327-336.
Lock, E.A. & Ishmael, J. (1979). The acute toxic effects of paraquat
and diquat on the rat kidney. Toxicol Appl Pharmacol., 50: 67-76.
McCall, J.C. & Robinson, P. (1990a). Unpublished report. Diquat
dibromide: acute oral toxicity to the rat. Report CTL/P/2999. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
McCall, J.C. & Robinson, P. (1990b). Unpublished report. Diquat
dibromide: acute dermal toxicity to the rat. Report CTL/P/2982. ICI
Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Milburn, G.M. (1989). Diquat: embryotoxicity study in the rat.
Unpublished report CTL/P/2275. ICI Central Toxicology Laboratory.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Mills, I.H. (1976). Unpublished report. Diquat: disposition and
metabolism in the rat.Report CTL/P/214. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Moore, S. & Wilson, G.J. (1973). Unpublished report. Diquat
dibromide: teratogenicity studies in the rat. Report No HO/IH/P/82B.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey.
Nirei, M., Hayasaka, S., Nagata, M., Tamai, A. & Tawara, T. (1993).
Ocular injury caused by preeglox-l, a herbicide contained paraquat,
diquat and surfactants. Jpn J Ophthalmol., 37: 43-46.
Ohta, T. (1987). Unpublished report. TOPPS: microbial mutagenicity
study. Report CTL/P/253A. Institute of Environmental Toxicology,
Kodaira, Tokyo 187, Japan. Report prepared for ICI Japan Ltd.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Parkinson, G.R. (1974a). Unpublished report. Diquat dichloride and
dibromide: comparison of dermal toxicity and local irritancy. Report
No HO/IH/P/116. Supplied to WHO by Zeneca Agrochemicals, Fernhurst,
Surrey, England.
Parkinson, G.R. (1974b). Unpublished report. Diquat monopyridone:
acute and subacute oral toxicity. Report No CTL/P/12213. ICI Central
Toxicology Laboratory, Macclesfield. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Pirie, A. & Rees, J.R. (1970). Diquat cataract in the rat. Exptl.
Eye Research, 9: 198-203.
Pirie, A., Rees, J.R. & Holmberg, N.J. (1970). Diquat cataract:
formation of the free radical and its reaction with constituents of
the eye. Exptl. Eye Research, 9: 204-218.
Pritchard, V.K. (1986). Unpublished report. Diquat: acute oral
toxicity to the rat of a 200 g/l formulation ("Reglox"). Report
CTL/P/1525. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rawlings, J.M., Foster, J.R. & Heylings, J.R. (1992). Diquat-induced
intestinal secretion in the anaesthetized rat. Human Exp Toxicol.,
11: 524-529.
Richardson, C.R., Howerd, C.A., Wildgoose, J. (1986). Unpublished
report. Diquat dibromide (technical): a cytogenetic study in human
lymphocytes in vitro. Report CTL/P/1561. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Rikans, L.E. & Cai, Y. (1993). Diquat - induced oxidative damage in
BCNU - pretreated hepatocytes of mature and old rats. Tox Appl.
Pharm., 118: 263-270.
Rikans, L.E., Cai, Y., Kosanke, S.D., Venkataraman, P.S. (1993).
Redox cycling and hepatotoxicity of diquat in aging fischer 344
rats. Drug Metab. Dispos., 21: 605-610.
Rogerson, A. & Broad, R.D. (1978). Unpublished report. Diquat
dibromide: 2 year feeding study in rats - amended report. Report
CTL/P/253A.Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S. & Smith, L.L. (1977). The relevance of paraquat
accumulation by tissues. In: Autor PA Ed. Biochemical Mechanisms of
paraquat toxicity. Academic Press, New York pp 72-91.
Rose, M.S., Lock, E.A., Smith, L.L. & Wyatt, I. (1975). Unpublished
report. Paraquat accumulation: tissue and species specificity.
Report CTL/R/367A. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Rose, M.S., Smith, L.L. & Wyatt, I. (1976). The relevance of pentose
phosphate pathway stimulation in rat lung to the mechanism of
paraquat toxicity. Biochem Pharmacol., 25: 1763-1767.
Rose, M.S., Crabtree, H.C., Fletcher, K. & Wyatt, I. (1974).
Biochemical effects of diquat and paraquat disturbance of the
control of corticosteroid synthesis in rat adrenal and subsequent
effects on the control of liver glycogen synthesis. Biochem J.,
138: 437-443.
Scott, R.C. & Corrigan, M.A. (1990). The in vitro percutaneous
absorption of diquat: a species comparison. Toxicol in vitro, 4:
137-141.
Scott, R.C., Walker, M. & Mawdesley, S.J. (1991). Unpublished
report. Diquat dibromide: in vitro absorption from technical
concentrate ('Reglone 40') and spray strength solution through human
skin. ICI Central Toxicology Laboratory, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Sechi, G.P., Agnetti, V., Piredda, M., Canu, M., Deserra, F., Omar,
H.A., Rosati, G. (1992). Acute and persistent parkinsonism after use
of diquat. Neurology, 42: 261-263.
Selypes, A., Nagymajtényi, L. & Berencsi, G. (1980). Mutagenic and
embryotoxic effects of paraquat and diquat. Bull Environ Contam
Toxicol., 25: 513-518.
Sharp, C.W., Ottlenghi, A. & Posner, H.S. (1972). Correlation of
paraquat toxicity with tissue concentrations and weight loss in the
rat. Toxicol Appl Pharmacol., 22: 241-251.
Sheldon, T., Richardson, C.R. & Shaw, J. (1986). Unpublished report.
Diquat dibromide (technical): an evaluation in the mouse
micronucleus test. No ICI Central Toxicology Laboratory, Alderley
Park, Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Shirasu, Y., Moriya, M. & Toshihiro, T. (1979). Unpublished report.
Mutagenicity testing on diquat in microbial systems. Institute of
Environmental Health Toxicology Division, Japan.
Smith, L.L. & Rose, M.S. (1977). A comparison of the effect of
paraquat and diquat on the water content of rat lung and the
incorporation of thymidine into lung DNA. Toxicology, 8: 223-230.
Southwood, J.(1987a). Unpublished report. Diquat: acute dermal
toxicity to the rat of a 200 g/l formulation. Report CTL/P/1881.
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
Southwood, J.(1987b). Unpublished report. Topps acute oral toxicity
to the rat. Report CTL/P/1788. Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO by
Zeneca Agrochemicals, Fernhurst, Surrey, England.
Stevens, M.A. & Walley, J.K. (1966). Tissue and milk residues
arising from the ingestion of single doses of diquat and paraquat by
cattle. J. Sci. Food Agric., 17: 472-475.
Trueman, R.W., Elliot, B.M. & Barber, G. (1987). Unpublished report.
Diquat dibromide (technical): assessment for the induction of
unscheduled DNA synthesis in rat hepatocytes in vivo. Report
CTL/P/1814. Imperial Chemical Industries PLC, Alderley Park,
Macclesfield, Cheshire, England. Supplied to WHO by Zeneca
Agrochemicals, Fernhurst, Surrey, England.
Vanholder, R., Colardyn, F., de Reuck, J., Praet, M., Lameire, N.&
Ringoir. S. (1981). Diquat intoxication: report of two cases and
review of the literature. Am J Med., 70: 1267-1271.
Walley, J.K. (1987). Unpublished report.Diquat toxicity in cattle.
Industrial Hygiene Research Laboratories Report IHR/167 (3B.1/7).
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Wickramatne, G.A. (1989). Unpublished report. Diquat: teratogenicity
study in the rat. Report CTL/P/2331. Imperial Chemical Industries
PLC, Alderley Park, Macclesfield, Cheshire, England. Supplied to WHO
by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Wildgoose, J., Braithwaite, I., Howerd, C.A., Richardson, C.R.
(1986). Unpublished report. Diquat dibromide: a cytogenetic study in
human lymphocytes in vitro. Report CTL/P/1469. Imperial Chemical
Industries PLC, Alderley Park, Macclesfield, Cheshire, England.
Supplied to WHO by Zeneca Agrochemicals, Fernhurst, Surrey, England.
Williams, S.G., Cameron, B.D. & McGuire, G.M. (1991). Unpublished
report. Identification of the major radioactive components in urine
and faeces from rats following single oral administration of [14C]-
diquat. Report CTL/C/2523. Inveresk Research International, Tranent,
EH33 2NE, Scotland. Supplied to WHO by Zeneca Agrochemicals,
Fernhurst, Surrey, England.
