
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
THIABENDAZOLE
IDENTITY
Chemical name
2-(4'-thiazolyl) benzimidazole
Synonyms
Thibenzole (R), Tecto (R), Mertect (R), TBZ
Structural formula
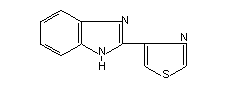 Molecular Weight 201.3
Other information on identity and properties
A stable, white crystalline powder. Solubility in water at pH 2.2 =
3.84 percent. Solubility decreases at higher or lower pH values. Very
soluble in dimethyl formamide, alcohols and acetone. Soluble in
chlorinated hydrocarbons, esters and ether.
No change in ultraviolet absorption occurs in samples stored eight
days at 100°C. Heating at 220°C at atmospheric pressure has no
apparent effect on its antifungal properties.
Technical grade contains not less than 97 percent thiabendazole. There
are no related impurities in the technical grade. Melting point is
296-303°C. Pure products melt at 304-305°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Following a dose of 100 mg/kg body-weight, thiabendazole labelled with
14C in the benzene ring was rapidly absorbed from the
gastrointestinal tract of rats, with a maximum concentration found
from two to three hours after initial treatment. Radioactivity
gradually disappeared from the blood, and approximately 92 percent of
a dose of 25 mg/kg and 80 percent of a 100 mg/kg dose was excreted in
the urine and faeces within three days. Most of the drug and its
metabolites were excreted within the first 24 hours. Of the
metabolites, 50 percent appeared as the glucuronide of
5-hydroxythiabendazole (II) and 40 percent as the sulfate ester of the
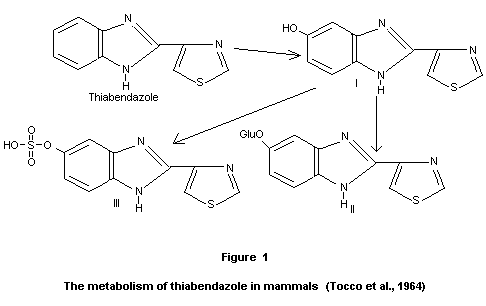
same aglycone (III) (see Fig 1). Traces of unchanged thiabendazole and
5-hydroxythiabendazole were also evident (Robinson, 1965a).
Dogs given a single oral dose of 50 mg/kg body-weight of 14C-labelled
thiabendazole were found to have maximum plasma levels within two
hours. Excretion was essentially complete in eight days with
approximately 35 percent of the dose appearing in the urine and 47
percent appearing in the faeces (Robinson 1965a).
Sixteen male humans were administered thiabendazole at dosages of 1 to
2 grams per person in the form of tablets, wafers, capsules or
suspension. The material was rapidly absorbed, with peak plasma
concentrations observed about one hour after treatment. Plasma drug
levels declined rapidly thereafter and reached essentially zero values
between 24 and 48 hours. Thiabendazole and its metabolic products were
excreted rapidly in the urine and faeces in 48 hours, which accounted
for 87 to 100 percent of the dose. Approximately half of the material
in the urine was associated with compounds identified as the
glucuronide and sulfate esters of 5-hydroxythiabendazole (II and III).
In plasma, both unchanged thiabendazole and free
5-hydroxythiabendazole (I) were also found. At the higher dosage
levels, maximum drug levels in plasma appeared at three hours
following dosing. These studies were done utilizing 14C-labelled and
unlabelled thiabendazole (Robinson, 1965a; Tocco et al., 1966).
Information is also available on the metabolism of thiabendazole in
sheep goats and cattle. In cows, about 0.1 percent of an oral dose was
detectable in the milk within 60 hours. Details of this work are given
in the section entitled "Fate of residues, In animals".
Based upon all the metabolism studies, the pathway for thiabendazole
metabolism is represented by Fig. 1.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Mouse
A multi-generation reproduction study for five generations utilized 25
male and 25 female mice given diets containing thiabendazole at
concentrations of 0, 200, 1000 and 5000 ppm. When the mice attained
the age of eight weeks, they were mated and continued on the same
diet. The young from these matings, when they were weaned, were
maintained on the test diet and mated when they reached the age of
eight weeks. This procedure continued for five complete reproductive
cycles. No effects were noted at 200 ppm. At 1000 ppm, slight decrease
in the weights of weanlings was observed in all five generations. At
5000 ppm, the number of mice born and weaned per litter were reduced,
and a marked reduction in the average weanling weight of the young was
observed (Robinson, 1965a).
Rat
A three-generation, two-litter reproduction study (10 males and 10
females per group) at dosages of 0, 20, 40 and 80 mg/kg body-weight
per day showed no adverse effect on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight, but not growth
rate, and decreased food consumption in the male rats at all dosage
levels in the F1 and F2 generations. Slight decreases in final
body-weights and food consumption at the 80 m/kg dose level in the F1
and F2 generation females was also observed (Vogin,1968).
Two groups of rats (20 male and 20 female) were placed on a diet
containing 0 or 500 ppm thiabendazole. There were no abnormalities
among the young from either mating attributable to thiabendazole at
500 ppm. It was concluded that no evidence of teratogenesis is present
in the data from this particular study (Johnson, 1964).
Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg body weight at 2
1/2 to 8 weeks prior to lambing without any effect on the birth rate,
growth or viability of the lambs. This dose had no effect on the ewes
throughout the test interval or on viability of the lambs to six weeks
of age. Miscellaneous tests using 39 ewes produced 29 weanling lambs
after treatment with thiabendazole at 400 mg/kg, whereas, from a
control group of 22 ewes, 13 lambs were weaned. Ewes tolerated a
single dose of 18 grams per animal (equivalent to 235 to 335 mg/kg)
three to ten days after lambing without any effect on the weight or
food intake of the ewes or survival growth of the lambs up to weaning
time of six weeks (Robinson, 1965a).
Special studies on cardiovascular and respiratory effects
Oral doses of thiabendazole (4000 mg/kg) produced no striking acute
pharmacological effects on blood pressure or respiration in either
cats or dogs. Similarly, there were no alterations in the
electrocardiogram (Robinson, 1965a, 1965b).
Special studies on eye irritation
Other than a very slight erythema observed for approximately one hour
after application of 1 ml of thiabendazole as a 12 percent suspension
in sodium carboxymethyl cellulose or 10 mg of the dry powder to the
conjunctival sac of two rabbits, no evidence of irritation was noted
(Robinson, 1965a, 1965b).
Molecular Weight 201.3
Other information on identity and properties
A stable, white crystalline powder. Solubility in water at pH 2.2 =
3.84 percent. Solubility decreases at higher or lower pH values. Very
soluble in dimethyl formamide, alcohols and acetone. Soluble in
chlorinated hydrocarbons, esters and ether.
No change in ultraviolet absorption occurs in samples stored eight
days at 100°C. Heating at 220°C at atmospheric pressure has no
apparent effect on its antifungal properties.
Technical grade contains not less than 97 percent thiabendazole. There
are no related impurities in the technical grade. Melting point is
296-303°C. Pure products melt at 304-305°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Following a dose of 100 mg/kg body-weight, thiabendazole labelled with
14C in the benzene ring was rapidly absorbed from the
gastrointestinal tract of rats, with a maximum concentration found
from two to three hours after initial treatment. Radioactivity
gradually disappeared from the blood, and approximately 92 percent of
a dose of 25 mg/kg and 80 percent of a 100 mg/kg dose was excreted in
the urine and faeces within three days. Most of the drug and its
metabolites were excreted within the first 24 hours. Of the
metabolites, 50 percent appeared as the glucuronide of
5-hydroxythiabendazole (II) and 40 percent as the sulfate ester of the
same aglycone (III) (see Fig 1). Traces of unchanged thiabendazole and
5-hydroxythiabendazole were also evident (Robinson, 1965a).
Dogs given a single oral dose of 50 mg/kg body-weight of 14C-labelled
thiabendazole were found to have maximum plasma levels within two
hours. Excretion was essentially complete in eight days with
approximately 35 percent of the dose appearing in the urine and 47
percent appearing in the faeces (Robinson 1965a).
Sixteen male humans were administered thiabendazole at dosages of 1 to
2 grams per person in the form of tablets, wafers, capsules or
suspension. The material was rapidly absorbed, with peak plasma
concentrations observed about one hour after treatment. Plasma drug
levels declined rapidly thereafter and reached essentially zero values
between 24 and 48 hours. Thiabendazole and its metabolic products were
excreted rapidly in the urine and faeces in 48 hours, which accounted
for 87 to 100 percent of the dose. Approximately half of the material
in the urine was associated with compounds identified as the
glucuronide and sulfate esters of 5-hydroxythiabendazole (II and III).
In plasma, both unchanged thiabendazole and free
5-hydroxythiabendazole (I) were also found. At the higher dosage
levels, maximum drug levels in plasma appeared at three hours
following dosing. These studies were done utilizing 14C-labelled and
unlabelled thiabendazole (Robinson, 1965a; Tocco et al., 1966).
Information is also available on the metabolism of thiabendazole in
sheep goats and cattle. In cows, about 0.1 percent of an oral dose was
detectable in the milk within 60 hours. Details of this work are given
in the section entitled "Fate of residues, In animals".
Based upon all the metabolism studies, the pathway for thiabendazole
metabolism is represented by Fig. 1.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Mouse
A multi-generation reproduction study for five generations utilized 25
male and 25 female mice given diets containing thiabendazole at
concentrations of 0, 200, 1000 and 5000 ppm. When the mice attained
the age of eight weeks, they were mated and continued on the same
diet. The young from these matings, when they were weaned, were
maintained on the test diet and mated when they reached the age of
eight weeks. This procedure continued for five complete reproductive
cycles. No effects were noted at 200 ppm. At 1000 ppm, slight decrease
in the weights of weanlings was observed in all five generations. At
5000 ppm, the number of mice born and weaned per litter were reduced,
and a marked reduction in the average weanling weight of the young was
observed (Robinson, 1965a).
Rat
A three-generation, two-litter reproduction study (10 males and 10
females per group) at dosages of 0, 20, 40 and 80 mg/kg body-weight
per day showed no adverse effect on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight, but not growth
rate, and decreased food consumption in the male rats at all dosage
levels in the F1 and F2 generations. Slight decreases in final
body-weights and food consumption at the 80 m/kg dose level in the F1
and F2 generation females was also observed (Vogin,1968).
Two groups of rats (20 male and 20 female) were placed on a diet
containing 0 or 500 ppm thiabendazole. There were no abnormalities
among the young from either mating attributable to thiabendazole at
500 ppm. It was concluded that no evidence of teratogenesis is present
in the data from this particular study (Johnson, 1964).
Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg body weight at 2
1/2 to 8 weeks prior to lambing without any effect on the birth rate,
growth or viability of the lambs. This dose had no effect on the ewes
throughout the test interval or on viability of the lambs to six weeks
of age. Miscellaneous tests using 39 ewes produced 29 weanling lambs
after treatment with thiabendazole at 400 mg/kg, whereas, from a
control group of 22 ewes, 13 lambs were weaned. Ewes tolerated a
single dose of 18 grams per animal (equivalent to 235 to 335 mg/kg)
three to ten days after lambing without any effect on the weight or
food intake of the ewes or survival growth of the lambs up to weaning
time of six weeks (Robinson, 1965a).
Special studies on cardiovascular and respiratory effects
Oral doses of thiabendazole (4000 mg/kg) produced no striking acute
pharmacological effects on blood pressure or respiration in either
cats or dogs. Similarly, there were no alterations in the
electrocardiogram (Robinson, 1965a, 1965b).
Special studies on eye irritation
Other than a very slight erythema observed for approximately one hour
after application of 1 ml of thiabendazole as a 12 percent suspension
in sodium carboxymethyl cellulose or 10 mg of the dry powder to the
conjunctival sac of two rabbits, no evidence of irritation was noted
(Robinson, 1965a, 1965b).
 Special studies on dermal irritation
There was no evidence of a significant degree of irritation when 0.5
gm thiabendazole was applied in cold cream at concentrations of 10 and
50 percent (w/w) for 24 hours to intact rabbit skin (Robinson, 1965a,
1965b).
Acute toxicity
LD50 doses for various animal species are given in Table I.
Toxic signs observed following administration of large doses of
thiabendazole by oral or intraperitoneal routes were generally similar
and consisted of lethargy and stupor. The intravenous administration
of a large dose of the hydrochloride produced narcosis. Death appeared
to be due to respiratory failure. Rabbits that survived large doses
initially lost weight but recovered after several days (Robinson,
1965a).
Short-term studies
Calf
Five female Holstein calves (seven months old) were fed a diet
containing 0, 320, 1 000, 3 200 or 10 000 ppm of thiabendazole for a
14-week test period. Calves tolerated 3 200 ppm thiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg body-weight. At the 10 000 ppm thiabendazole level, the
calves grew normally for the first two weeks, but in the following 12
weeks the weight gain was about half that observed for the controls.
Gross examination at the time of autopsy revealed no pathological
condition, and histological examination of several tissues showed no
change as resulting from the incorporation of thiabendazole in the
diet (Robinson, 1965a).
Chicken
Groups of 0.5 week old male White Leghorn chicks (10 to 20 per group)
were fed thiabendazole in the diet at dosage levels ranging from 1 to
10 000 ppm for a period of 2 1/2 weeks. This level corresponded to a
mean daily intake of thiabendazole ranging from 0.1 mg/kg to 1 200
mg/kg. A gradual decrease in growth appeared to occur with
concentrations of 100 ppm thiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg per day. Gross pathological
examination at autopsy of the chicks on a dietary level of 4 000 ppm
thiabendazole showed them to be normal except for a smaller size
(Robinson, 1965a).
Dog
Groups comprising two male and two female dogs were orally
administered thiabendazole daily for periods of over two years at
doses of 0, 20, 100 and 200 mg/kg body-weight/day. No clinical signs
of morbidity were observed; food and water consumption were normal;
blood and urine chemistry were normal; a slight retardation in
body-weight gain at the 200 mg/kg level was observed, which was
accompanied by a slight reduction in total erythrocyte count. At this
level, changes in haemoglobin concentration and haematocrit were also
observed. No gross pathological observations were noted at the
conclusion of this test, and organ-weights appeared normal.
TABLE I
Thiabendazole toxicity in various animal species
LD50
Animal Route Form Solvent (mg/kg body-weight) Reference
Mouse oral HCl Water 2 400 (Robinson 1965a, 1965b)
ip HCl Water 430 " " "
iv HCl Water 150 " " "
oral Base CMC 2% 1 3 810 " " "
Rat oral HCl Water 3 600 " " "
ip HCl Water 1 850 " " "
iv HCl Water 130 " " "
oral Base CMC 2% 3 330 " " "
Rabbit oral Base CMC 2% 3 850 " "
Sheep drench HCl Water 2 000 " "
Goat drench HCl Water >2 000 " "
1 CMC = carboxy methyl cellulose
A significant haemosiderosis was present in dogs at the 100 and 200
mg/kg level in the spleen, liver, lymph nodes and bone-marrow. This
was not associated with an increase in serum haemoglobin. No
significant toxicological effects were noted at 20 mg/kg/day
(Robinson, 1965a, 1965b).
Groups of dogs (three male and three female) were orally administered
thiabendazole for two years at doses of 0, 20, 50 and 125 mg/kg
body-weight/day. At the 125 mg/kg/day level, two of six dogs died. One
of these dogs had marked liver cirrhosis, seminal tubular
degeneration, bone marrow atrophy and degenerative renal changes.
Slight to moderate reduction in haemoglobin and packed cell volume
occurred. Elevated blood urea nitrogen, serum alkaline phosphatase and
serum glutamic oxal oacetic transaminase were evident. Urinary albumin
was seen more frequently than in controls. Inspissated bile entwined
in the gall bladder villi was also observed in one dog. One dog of
this group was sacrificed and was found to have pulmonary arteritis of
parasitic origin. At 50 mg/kg/day, growth of male dogs was slightly
depressed. All six dogs given thiabendazole at 50 mg/kg survived the
study, and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa in
one. Five of six dogs survived thiabendazole at 20 mg/kg for the term
of the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes in
all treated dogs (Woodard et al., 1964).
Pig
Five groups of four barrows each were given a diet containing 0, 320,
1 000, 3 200 and 10 000 ppm thiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects for
the 14-week period. This concentration corresponded to an intake of 15
mg/kg body-weight per day. Concentration of 10 000 ppm thiabendazole
in the diet caused reduced weight gains and reduced food consumption
with no mortality observed. Gross pathology examination revealed no
abnormal conditions (Robinson, 1965a).
Rat
Rats (10 males and 10 females per group) were administered
thiabendazole once daily at dosage levels of 0, 100, 400, 800, 1 200
and 1 600 mg/k-g body-weight per day. During the 30-day experimental
period, rats in the 800 mg/kg per day group decreased their food
intake and gradually lost weight. They were slightly less active, had
ruffled fur and became slightly flaccid. Three males and three females
died during the course of the experiment. All of the rats in the 1 200
and 1 600 mg/kg groups died during the 30-day test period. Rats dosed
at 100 and 400 mg/kg appeared normal throughout the test period,
although a slight depression in body-weight gain was noted in males at
100 mg/kg per day and in females at 400 mg/kg per day. Haematologic
studies on rats receiving 800 mg/kg per day showed a mild neutrophilia
with concurrent lymphopenia. There was also a suggestion of a decrease
in red blood cell elements. No gross anatomical changes were noted in
rats dosed with 100 mg/kg per day, whereas male and female rats
treated with 400 mg/kg per day showed thymic involution. A slight
enlargement of the liver was also noted in females at 400 mg/kg per
day. Animals surviving 800 mg/kg per day showed gross signs indicating
starvation where normal body fat depot and subcutaneous fat appeared
to be depleted. Liver and adrenals were slightly enlarged in males and
females and the thymus involuted. Microscopic pathology showed bone
marrow hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg (Robinson, 1965a, 1965b).
Rats (30 males and 30 females per group) were administered
thiabendazole suspended in 1.0 percent methocel daily for 180 days at
doses of 0, 12.5, 25, 50, 100, 200 and 400 mg/kg body-weight. All of
the animals survived throughout the duration of the experiment. At 400
mg/kg, a depression in body-weight was observed with both sexes. At
200 mg/kg per day, the male rats showed a slight depression in weight
gain. No clinical signs of toxicity were observable at levels below
100 mg/kg. Haematological studies at 400 mg/kg per day indicated a
slight suggestion of a fall of the red blood cell elements which was
not evident at levels below this dose. Biochemical blood studies and
urinalysis were normal at all dosage levels with a slight polyurea in
both male and female rats at 400 mg/kg, which was apparently the
result of a slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and females at
400 mg/kg per day and in females at 200 mg/kg per day. There was an
increase in liver size at doses of 100 mg/kg and above in males and at
25 mg/kg per day and above in females. Males dosed with 200 and 400
mg/kg and females at 400 mg/kg showed an apparent increase in kidney
weight. Microscopic examination of animals at 100 mg/kg showed a small
incidence of haemosiderosis of the thymus. At 200 and 400 mg/kg, these
examinations showed considerably more haemosiderosis of the thymus and
colloid depletion in the thyroid (Robinson, 1965a, 1965b).
Sheep
Thirty weanling wethers were employed in a 16-week study at doses of
0, 10, 50, 100, 200, 400 and 800 mg/kg body-weight per day.
Thiabendazole was administered in gelatin capsules. Two of the four
sheep dosed at 100 mg/kg did not survive the test period. However,
because of infection, thiabendazole may not have been directly related
to the cause of their death. All the animals treated at 200 mg/kg per
day and above died before the end of the test interval. No significant
effects on blood biochemistry or urinalysis were observed. Doses of 50
mg/kg and above affected body-weight gain, after approximately 120
days on the test. Doses of 10 mg/kg had no effect. Food consumption
was unaffected at doses of 10 mg/kg per day, but a minor decrease was
noted at 50 and 100 mg/kg per day and a significant decrease at 200
mg/kg per day and above. No gross pathological changes were observed
at doses below 200 mg/kg per day. Starvation and loss of body-weight
complicated the interpretation of data concerning organ-weights at 200
mg/kg and above. At 200 to 800 mg/kg, a moderate hypoplasia of the
bone marrow with replacement by adipose tissue was observed. Loss of
colloid in thyroid was evident at 800 mg/kg, as well as lymphoid
atrophy at the higher dose levels (Robinson, 1965a).
Five groups of two ewe lambs (10 to 12 weeks old) and eight wether
lambs (10 to 19 weeks old) were fed diets containing 0, 100, 320, 1
000 and 3 200 ppm thiabendazole for periods up to 50 weeks. Sheep 10
weeks of age tolerated 1 000 ppm thiabendazole in the diet for the
duration of the experiment. (This dose corresponded to 30 to 50 mg/kg
body-weight per day.) The sheep tolerated 3 200 ppm in their diet for
the first 14 weeks but thereafter lost weight in the final stages of
the experiment (Robinson, 1965a).
Long-term studies
Rat
Groups of rats (35 of each sex) were fed dietary levels adjusted to
provide 0, 10, 40 or 160 mg/kg body-weight per day of thiabendazole
for up to two years. At the 160 mg/kg level, a reduction of weight
gain of about 25 percent, with concomitant reduction in food
consumption and slightly reduced haemoglobin and microhaematocrit
values, were the only changes seen attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidents or location of neoplasms and histopathological examination
of the tissues. No effect on food consumption or haemograms were seen
at either the 10 or 40 mg/kg levels. Very slight depression in growth
rate, of questionable significance, was observed in the male rats at
40 mg/kg but not at 10 mg/kg. At the 10 mg/kg level, the mean absolute
thyroid weights of the male rats (including the individual thyroid
weights from five out of eight animals tested) were heavier than the
mean thyroid weight of the controls. This effect was not observed in
the rats given 40 or 160 mg/kg (Woodard et al., 1964).
OBSERVATIONS IN MAN
Human subjects have ingested a single oral dose of 2 g (approximately
30 mg/kg body-weight) of thiabendazole in studies designed to
ascertain the metabolic fate (see "Biochemical aspects"). No
information was given on the toxicological effects, if any (Robinson,
1965a).
COMMENTS
Metabolic studies indicate a rapid absorption of thiabendazole from
the gastrointestinal tract of rats, dogs, sheep and goats with
excretion in urine and faeces complete within 3-8 days. In cows,
approximately 0.1 percent of an oral dose was detectable in milk
within 60 hours. In excreta of man and all animals studied,
metabolites include 5-hydroxythiabendazole, free and conjugated as a
glucuronide and sulfate ester, as well as traces of unchanged
thiabendazole. Slight differences in man and other animals were noted
in quantitative recovery of the various compounds. A common effect for
several animal species at high dose levels of thiabendazole is
retarded growth.
Short-term studies in rate, sheep, pigs, calves and chickens have been
carried out with oral administration or with incorporation of
thiabendazole in the diet for periods of 4-50 weeks. At higher doses
in rats, thymus involution, colloid depletion in the thyroid and
increase in liver and kidney size was observed. Loss of the colloid in
thyroid of sheep administered 200 mg/kg body-weight was also evident.
Oral administration of 12.5 mg/kg for 180 days did not cause
significant toxicological changes in rats.
In a two-year dog study a no-effect level of 20 mg/kg body-weight was
demonstrated. A two-year study in rats showed heavier thyroid glands
at 10 mg/kg body-weight/day in the male animals; this effect, however,
was not dose dependent.
A five-generation reproduction study in mice showed no adverse effects
at 200 ppm level. In a three-generation study in rats, only decreased
body-weight in relation to decreased food consumption could be
observed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 20 mg/kg body-weight/day
Mouse: 200 ppm in the diet, equivalent to 30 mg/kg body-weight/day
Rat: 10 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.05 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Administration to animals
Thiabendazole is widely used as a broad spectrum anthelmintic for
sheep, cattle, horses, pigs and goats. It is also administered as a
vermifuge directly to man. Following the announcement by Brown (1961)
of the chemotherapeutic properties of benzimidazoles, many
investigators report the efficacy of thiabendazole as an anthelmintic.
Gordon (1964) generally reported on its properties. Since that time
very large numbers of sheep and cattle have been treated with
thiabendazole suspensions. In sheep a dose of 44 mg/kg is adequate in
moot circumstances. Dosing may be repeated as frequently as once per
month, depending upon the level of reinfestation. In cattle a dose of
88 mg/kg is usually employed and in pigs 50-100 mg/kg.
Usually the dose is administered by drenching (oral administration of
an aqueous suspension) but for convenience it is possible to
administer via the feed when dealing with individual animals. At the
present time, economic considerations probably preclude the use of
thiabendazole as a feed additive.
Pre-harvest treatment of plants
Thiabendazole has been evaluated and has proved effective for use as a
fungicide on a number of crops including apples, pears, peaches,
apricots and bananas but, as far as is known, pre-harvest treatments
have not yet been adopted commercially. Preliminary results of residue
trials indicate that the residues from such pre-harvest uses will vary
considerably due to the systemic action of thiabendazole and the
ability of plants to take it up from the soil (Wernke, 1969).
Post-harvest treatment
Bananas
Scott (1967), Burden (1967), Anon (1967, 1969), Cuille (1968) and
Shillingsford (1970) have shown that thiabendazole is highly effective
for control of post-harvest fungal rots of bananas, when the picked
fruit is dipped in a suspension of thiabendazole containing from 100-1
000 ppm. A dip containing 200 ppm (0.02%) appears to be the most
widely used. Post-harvest spray treatments have also proved effective
in experimental use, but it is not known whether these are yet used in
practice.
Thiabendazole is most soluble in acid solution and can be rendered
soluble by treatment with lactic acid. Considerable experimental work
was carried out with lactic acid solutions as fruit dips, but in spite
of the convenience, the acidic solution gave rise to corrosion
problems in packing houses and during transport and resulted in
decomposition of the thiabendazole. Such solutions have been abandoned
in favour of micro-fine powder suspensions which require minimal
agitation to maintain uniform concentrations.
After the bunches of bananas have been picked, they are usually washed
in water or a solution of sodium hypochlorite. The bunches are then
separated into hands or clusters of 10-12 fruit which is allowed to
"bleed" in water for approximately 15-30 minutes. This facilitates the
removal of natural latex which, if allowed to run on the fruit, will
result in a visual disfigurement. The wet fruit is then dipped for 2-4
minutes in a suspension containing 200 ppm thiabendazole before being
drained for packing into cartons. No visible residue results from the
treatment.
Citrus
Crivelli (1966) and his associates in Italy first demonstrated that
Penicillium decay of oranges could be prevented by dipping fruit in
solutions containing 750-1 000 ppm thiabendazole dissolved in lactic
acid. Confirmation by other laboratories indicated that concentrations
of 200 to 500 ppm are adequate to give excellent control of
Penicillium decay. Brown (1967), Eckert (1968), Harding (1968),
McCormack (1968), Seberry (1968) show the efficacy of thiabendazole in
controlling Penicillium, and Seberry (1968) gives data showing
reduction in stem end rot caused by Diaporthe citri after dipping
oranges in suspensions of 500-1 000 ppm. Spraying or dipping citrus
fruits in 0.5 to 1.0 percent thiabendazole suspensions, without
subsequent rinsing, prevents sporulation of Penicillium upon the
surface of decaying fruits. Oranges so treated have a natural
appearance with no odour or visible residue.
The most efficient methods for commercial application of thiabendazole
appear to be as picking bin drenches, processing line spray or as an
additive to water emulsion waxes. Thiabendazole is registered in
Australia and U.S.A. as a post-harvest spray or dip treatment of
citrus fruit with suspension containing up to 5 000 ppm thiabendazole.
A rinse is not required, but rinsing does not appear to significantly
reduce the efficacy of a dip treatment.
Sporulation of Penicillium on the surface of decayed fruit causes
superficial soiling of sound fruits adjacent to decayed fruits. This
soiling can completely ruin all the fruit in a whole container with
only one or two decayed fruits. For this reason, a post-harvest
fungicide treatment must control both primary decay (prevent infection
of fruit) and reduce Penicillium sporulation on the surface of those
fruits which decay due to deep primary infections. Diphenyl and
sodium-o-phenylphenate, which have been used as standard treatments
for many years, have serious deficiencies. Thiabendazole on citrus wax
is used as a means of controlling fungus diseases referred to as green
mould, blue mould and stem end rot. This treatment is effective in
controlling both primary decay and sporulation.
Thiabendazole/citrus wax treatments are used commercially in Israel
and on an experimental basis in U.S.A. Aqueous emulsions of waxes
designed for application to citrus have been in use for many years.
Thiabendazole is suspended in sufficient wax emulsion to yield a
concentration of up to 0.5% w/w thiabendazole (5000 ppm). The prepared
emulsion is used without dilution in waxing equipment designed to
apply citrus wax evenly to freshly washed citrus. In this equipment,
the wax containing the thiabendazole is applied through nozzles to the
citrus as it revolves on horsehair brushes below. The nozzle is
attached to a chain to move back and forth across the width of the
waxer at a right angle to the flow of the citrus passing along the
conveyer. The amount of wax applied is governed by the size of the
nozzle and the pressure at which it is applied. These are equilibrated
with the flow of the citrus so that 1 litre of emulsion will coat ca.
800-900 kg of citrus fruit.
Other fruit
Bondoux (1967) reports good results from soaking apples in dilute
suspension of thiabendazole. Fripp (1969) showed thiabendazole
outstanding for control of brown rot (Sclerotinia fructicola) of
peaches. It is known that many other investigators are working with
thiabendazole to control post-harvest losses of a wide variety of
fruits, including cherries and pineapples, but as yet the available
data are not sufficient to enable proposals to be made for tolerances.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Bananas
Residue trials were carried out in Honduras. Green bananas treated
according to recommended practice were shipped under commercial
conditions at a temperature of 14°-15°C. Analysis was made 8 days
after treatment in the case of green bananas and 15 days in the case
of ripe fruit.
A review of the residue data showed that at the recommended treatment
rate of 200 ppm, a maximum of 0.18 ppm thiabendazole was found in the
banana pulp (see Table II), while a maximum of 1.24 ppm thiabendazole
was present in the peel (see Table III), when calculated on a whole
fruit basis. Therefore, at the recommended rate of 200 ppm, a maximum
of 1.4 ppm thiabendazole is possible in the whole unpeeled banana.
Pulp and peel residues generally increase slightly with an increase in
treatment rate, (see Table II) while the lower treatment rate (100
ppm) results in somewhat lower residue levels of thiabendazole in pulp
and peel (see Tables II and III). At the 100 ppm treatment level, an
average of 0.018 ppm and 0.049 ppm was found in ripe banana pulp from
two trials. This increased to an average of 0.068 ppm - 0.110 ppm at
the 200 ppm treatment rate. Residues appear to level off at the 200
ppm treatment rate, since ripe banana pulp contained an average of
0.126 ppm and 0.094 ppm at the 400 ppm treatment rate.
TABLE II
Thiabendazole residues (ppm) in pulp of green and ripe bananas 1
Treatment GREEN RIPE
Average, ppm Range, ppm Average, ppm Range, ppm
Control 1 0.0095 0.005-0.01 0.011 0.01-0.02
2 0.013 0.01 -0.03 0.024 0.01-0.04
100 ppm 1 0.030 0.01 -0.05 0.018 0.01-0.03
2 0.037 0.03 -0.05 0.049 0.03-0.07
200 ppm 1 0.074 0.04 -0.10 0.068 0.05-0.09
2 0.067 0.05 -0.08 0.110 0.05-0.18
400 ppm 1 0.173 0.05 -0.31 0.126 0.10-0.18
2 0.126 0.07 -0.17 0.094 0.07-0.13
1 Results on treated bananas are not corrected for results on control bananas.
TABLE III
Thiabendazole residues in peel of green and ripe bananas 1
Treatment GREEN RIPE
Average Range Average Range
(ppm) (ppm) (ppm) (ppm)
Control 1 0.020 0.013-0.042 0.040 0.024-0.059
2
100 ppm 1 0.39 0.33 -0.52 0.55 0.39 -0.68
2
200 ppm 1 0.12 0.97 -1.21 0.17 1.12 -1.24
2
400 ppm 1 2.24 2.01 -2.64 2.73 2.61 -2.80
2
1 Results on treated bananas are not corrected for results on control bananas.
The analytical method used had a sensitivity of 0.05 ppm with blank
values for untreated banana peel and pulp in the range of 0.01 - 0.04
ppm. The recovery of thiabendazole added to banana pulp and banana
peel ranged from 74.3 percent to 90.2 percent with an average of 87.3
percent.
In Australia, experiments were conducted by the Department Agriculture
to investigate the use of thiabendazole as a banana dip under
conditions of commercial usage; 100 cases (250 kg) of fruit was
treated at each of four concentrations of thiabendazole suspension.
The results were as follows:
TABLE IV
Whole fruit residues produced by various treatment rates
Treatment On Peel In Peel In Pulp Total
Rate Variety Average Range Average Range Average Range Residue
ppm ppm ppm ppm ppm ppm ppm ppm
0 Orange 0.076 0.00-0.33 0.008 0.00-0.03 0.00 0.00-0.01 0.09
Lemon 0.02 0.00-0.03 0.004 0.00-0.02 0.00 0.00-0.01 0.02
Grapefruit 0.01 0.01-0.02 0.01 0.01-0002 0.01 0.00-0.02 0.03
5 000 Orange 0.72 0.33-1.28 0.072 0.00-0.25 0.01 0.00-0.04 0.73
Lemon 0.31 0.16-0.74 0.045 0.00-0.13 0.005 0.00-0.01 0.36
Grapefruit 0.47 0.27-0.72 0.20 0.14-0.4? 0.02 0.00-0.04 0.66
10 000 Orange 1.25 0.77-2.22 0.098 0.01-0.19 0.01 0.00-0.01 1.36
Lemon 0.95 0.31-1.96 0.10 0.00-0.19 0.005 0.00-0.01 1.06
Grapefruit 0.98 0.66-1.08 0.29 0.23-0.39 0.04 0.01-0.07 1.28
Dip concentration Thiabendazole residues (whole fruit)
0.1% (1000 ppm) 2 - 5 ppm
0.05% (500 ppm) 1.5 - 2.5 ppm
0.025% (250 ppm) 0.8 - 2 ppm
0.0125% (125 ppm) 0.3 - 1.5 ppm
Trials at another research station yielded the following results:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 (200 ppm) 0.03 0.7
0.04 (400 ppm) 0.07 1.5
0.08 (800 ppm) 0.11 4.1
0.16 (1600 ppm) 0.14 6.2
0.32 (3200 ppm) 0.20 12.0
Limit of detection was 0.04 ppm.
Citrus fruits
Residue trials were conducted in conjunction with the Florida Citrus
Experiment Station on oranges, grapefruit and lemons. The following
products were assayed before and after processing: whole unwashed
fruit, single strength juice, finisher pulp, citrus oil, molasses,
dried citrus pulp. The fruit was processed according to typical
practice, including a two-minute flood treatment with a suspension
containing 0, 5 000 and 10 000 ppm thiabendazole. The fruit was then
waxed according to commercial practice.
The detection sensitivity of the analytical method used was 0.03 ppm
for the pulp and 0.40 ppm for the peel with blank values for untreated
pulp and peel in the range 0.00 - 0.02 ppm and 0.00 - 0.40 ppm
respectively.
A review of the whole fruit residue data, which is summarized in Table
IV, shows that at the application rate of 5 000 ppm, a maximum of 0.04
ppm thiabendazole is found in the pulp of the citrus, while a maximum
of 1.28 ppm and 0.41 ppm thiabendazole is present on and in the peel
respectively. This is the maximum residue found in pulp or peel of all
oranges, lemons or grapefruit assayed. Therefore, at the treatment
rate of 5 000 ppm, a maximum of 1.73 ppm is possible on the whole
unpeeled fruit.
In all cases, the peel residues increase with increasing treatment
rates. However, in the case of pulp assays, the level of residue is at
or below the sensitivity of the assay method with no indication of
increases with higher dosages. These results are corroborated by
radioactive studies reported later, which show that the level of
residues in the pulp is approximately equivalent to the assay
sensitivity and appears to result from inadvertent contamination
during peeling or sectioning. The result of assays on dried citrus
pulp (summarized on Table V) indicated the maximum residues range from
1.55 ppm in lemons to 2.87 ppm in the case of grapefruit, with dried
orange pulp being 2.61 ppm.
From yet another research station, it was reported that dipping green
bananas in a suspension containing 800 ppm thiabendazole resulted in
residues of 3.3 ppm in the whole fruit and 0.4 ppm in the pulp.
FATE OF RESIDUES
In animals
Radioactive thiabendazole administered orally to sheep at a dose of 50
to 100 mg/kg body-weight or to goats at a single dose of 150 mg/kg was
rapidly absorbed and metabolized to its 5-hydroxy derivative and
excreted as the aglycone and glucuronide and sulfate ester. Peak
concentrations in plasma occur approximately four hours after
administration, with 90 percent of the radioactivity eliminated in the
excreta in three to four days. Thiabandazole and small concentrations
of two unknown metabolites were observed (Robinson, 1965a; Tocco et
al., 1964).
Residues observed with eight sheep receiving 14C-or 35S-labelled
thiabendazole at 50 mg/kg are given in Table VI, where concentrations
reported as zero are equal to or less than a detection limit of 0.06
ppm.
TABLE V
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Finisher pulp Orange 0.02
Lemon 0.01
Grapefruit 0.01
Single strength Orange 0.01
juice Lemon 0.01
Grapefruit 0.02
Pulp, dry Orange 2.54
Lemon 1.4
Grapefruit 2.53
TABLE V (cont'd)
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Oil Orange 1.05
Lemon 0.16
Grapefruit 1.38
Molasses Orange 1.77
Lemon Not assessed
Grapefruit 1.97
Trials conducted in Australia at the Citrus Wastage Research
Laboratory showed the following results when oranges were dipped in
thiabendazole suspensions under commercial conditions:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 N.D.1 0.49
0.04 N.D. 0.21
0.08 N.D. 0.52
0.10 N.D. 0.39
0.16 N.D. 0.73
0.32 N.D. 1.68
1 N.D. = not detected (less than 0.04 ppm)
Analytical studies in lactating goats showed that although
thiabendazole was absorbed rapidly, less than 1% of the dose was
excreted in their milk within 24 hours after administration of 150
mg/kg body-weight. Peak concentrations were found in the milk within
24 hours, and no thiabendazole or its metabolites were detectable in
the milk four days later (Robinson, 1965a; Tocco et al., 1965).
Studies with goats given single oral dosages of tritium-labelled
thiabendazole at 150 mg/kg, demonstrated that the compound was
absorbed rapidly, reaching peak concentrations in the plasma about 4
hours after administration. The compound was rapidly excreted in the
urine and faeces. Residues of thiabendazole were not detectable in any
of the 15 tissues from goats examined 30 days after treatment.
Thiabendazole is less rapidly excreted by pigs than by several other
animals. Following a single dose of 50 mg/kg body-weight,
approximately 76 percent of the dose was found in the excrete within
about two weeks (Robinson, 1965a; Tocco et al., 1965). Thiabendazole
(14C-labelled) administered to cattle at doses of 50 mg/kg and 200
mg/kg body-weight was rapidly excreted. Plasma levels reached a
maximum in four to seven hours, after which they decreased rapidly,
with no residual thiabendazole or metabolites detectable in tissues 30
days after administration of the lower dose. Traces of radioactivity
were demonstrable in calves two months following doses of 150 and 200
mg/kg. Three fluorometrically detectable metabolites were observed,
corresponding to 5-hydroxythiabendazole and its sulfate and
glucuronide conjugate. Approximately 0.1 percent of an oral dose of 3,
5 or 10 grams per 100 lb was secreted in the milk as thiabendazole and
its metabolites within 60 hours after the animals were dosed. The
highest concentration appeared within 24 hours. Over 99 percent of the
compound was metabolites, and residues were not detectable 2 1/2 days
after the cows were administered the compound (Robinson, 1965a; Tocco
et al., 1965).
TABLE VI
Thiabendazole residues in sheep
Sample 6 hours 5 hours 8 days1/ 16 days 24 days 30 days1/
Abomasum 5.1 0.0 0.15 0.0 0.0 0.0
Blood 7.7 0.0 0.0 0.0 0.0 0.0
Brain 1.0 0.09 0.14 0.0 0.0 0.0
Caecum 34.4 0.0 0.08 0.0 0.0 0.0
Fat 2.8 0.0 0.04 0.0 0.0 0.0
Heart 2.7 0.15 0.14 0.08 0.0 0.0
Kidney 13.9 0.28 0.22 0.0 0.0 0.0
Large intestine 4.6 0.0 0.10 0.0 0.0 0.0
Liver 9.6 0.62 0.62 0.15 0.0 0.0
Lung 2.4 0.0 0.04 0.08 0.0 0.0
Muscle 2.0 0.09 0.06 0.13 0.0 0.0
Pancreas 2.6 0.18 0.13 0.0 0.0 0.0
Skin 3.2 0.0 0.19 0.0 0.0 0.0
Small intestine 33.6 0.0 0.16 0.0 0.0 0.0
Spleen 3.4 0.0 0.0 0.0 0.0 0.0
1/ Two animals slaughtered at each of these intervals. Results given in
table are means of the two values.
Table VII shows thiabendazole residues found in calves treated at 110
mg/kg. Results are the total of thiabendazole and its metabolites
expressed in ppm, determined fluorometrically.
TABLE VII
Thiabendazole residues in calves
Days No.
Post of Muscle Liver Kidney
Treatment Group Animals (ppm) (ppm) (ppm)
Control 3 0.13(0.09-0.17) 0.13(0.13-0.14) 0.12(0.10-0.15)
3 Treated 4 0.12(0.05-0.17) 0.17(0.15-0.19) 0.16(0.13-0.18)
7 " 4 0.10(0.03-0.19) 0.17(0.15-0.19) 0.12(0.10-0.13)
14 " 4 0.08(0.06-0.12) 0.15(0.14-0.18) 0.15(0.07-0.35)
It may be noted that in no instance does the average value exceed the control level by
more than 0.05 ppm.
In plants
Bananas
There is nothing to suggest that thiabendazole residues in bananas
undergo any change during application, shipping, storage or ripening.
Citrus
Radioactive thiabendazole 14C was employed to determine the uptake of
thiabendazole by Valencia oranges after dipping in 0.1% suspension,
before and after storage under conditions simulating customary
shipping and use practices (Merck, 1969; Rosenblum, 1970). Peal and
edible pulp were analysed separately. Peel was divided into inner and
outer portions. At the end of the maximum storage period, which
extended for two weeks at 10°C followed by an additional two weeks at
21°C, peel was assayed by the reverse isotope dilution method for
intact thiabendazole as an estimate of the stability of the latter in
the fruit.
The pulp was essentially free of thiabendazole even after four weeks
of storage. The small apparent residues recorded are probably due to
uncontrollable contamination from the peel during sectioning of the
orange for analysis. Peel representing 25% of the whole fruit
contained approximately 50 ppm of thiabendazole (on weight of peel).
All the thiabendazole applied as a suspension was still present as
intact thiabendazole after the four weeks of storage.
In several instances the stability of the sorbed thiabendazole - 14C
was investigated by extraction and by thin layer chromatography, with
and without prior addition of unlabelled carrier compound. Since the
radioactivity of the pulp did not increase with temperature or time of
storage above the initial value prior to storage, it may be concluded
that the thiabendazole content of the pulp due to diffusion of
absorbed fungicide into the interior during the four weeks of storage
is less than or equal to a detection limit of 0.01 ppm. Diffusion from
the surface of the fruit into the peel is appreciable an from 7-20% of
the measurable activity is observed in the inner peel. This diffusion
does not, however, extend to the pulp at the interior of the fruit.
Because of the widespread practice of waxing citrus after washing and
cleaning, the application of thiabendazole in the aqueous wax emulsion
is a convenient, practicable and efficient method of conferring
protection against fungal spoilage. Trials carried out in California
under controlled conditions in commercial packing houses resulted in
acceptable protection. The residues found on the treated fruit ranged
up to 4.3 ppm, and the results are set out in Table VIII. It is not
anticipated that the penetration into the peel will be any greater
when the treatment is applied via wax emulsion that it would from a
flooding, dipping or spraying treatment.
TABLE VIII
Residues of thiabendazole on whole fruit following application to citrus
of thiabendazole in wax emulsion
Storage Time
Variety Treatment Interval Residue
Temp. Humidity (days) (ppm)
Valencia
Oranges 0.3% TBZ in wax 21-24°C 90% 8 1.25
" 0.5% TBZ in wax 21-24°C 90% 8 2.16
" 0.3% TBZ in wax 21-24°C 90% 10 1.76
" 0.4% TBZ in wax 21-24°C 90% 10 2.20
" 0.5% TBZ in wax 21-24°C 90% 10 2.43
" 0.35% TBZ in wax 21-24°C 90% 10 2.15
" 0.5% TBZ in wax 21-24°C 90% 10 3.91
" 5267 ppm TBZ in wax 9-20°C 90% 31 4.3
Evidence of Residue in Food In Commerce
Shipments of commercially processed grapefruit which had been treated
by dipping in thiabendazole before being exported from U.S.A. were
sampled in Europe and systematically analysed for thiabendazole
residues. Ten fruits were homogenized from each shipment and analysis
was carried out on the homogenate. The results which are set out in
Table IX are slightly lower than those obtained from controlled
experiments carried out in Florida. It is notable that the residue on
grapefruit treated by flooding in a bath containing 3 000 ppm
thiabendazole are approximately twice as high as those found in fruit
treated in a similar manner in a bath containing 1 000 ppm
thiabendazole.
In order to determine the fruit-to-fruit variation in any one
consignment, nine separate fruit were taken from a consignment and
these were analysed individually. There was close agreement between
the results (range 0.26-0.55 ppm - standard deviation 0.09 ppm) with
the mean agreeing closely with the result obtained by analysis of a
homogenized sample of the same consignment.
TABLE IX
Residues of thiabendazole on whole citrus exported from U.S.A. and
analysed in Europe
Shipment Variety Treatment Residue on whole fruit (ppm) 1/
No. Rate Average Range
1 Grapefruit 1 000 ppm 0.22 0.18 - 0.25
3 000 ppm 0.44 0.32 - 0.63
2 " 1 000 ppm 0.23 0.20 - 0.29
3 000 ppm 0.57 0.46 - 0.86
3 " 1 000 ppm 0.17 0.14 - 0.18
1 000 ppm 0.13 0.10 - 0.16
3 000 ppm 0.27 0.23 - 0.30
4 " 1 000 ppm 0.17 0.15 - 0.19
1 000 ppm 0.18 0.16 - 0.21
3 000 ppm 0.24 0.21 - 0.28
5 " 1 000 ppm 0.39 0.37 - 0.42
1 000 ppm 0.33 0.21 - 0.28
3 000 ppm 0.86 0.72 - 0.96
1/ Variation in thiabendazole residue levels between individual fruits
Analysis of composite sample = 0.39 ppm
Analysis of individual fruits
0.53 0.37 0.38 Mean = 0.39 ppm
0.38 0.55 0.30 Standard Deviation 0.09 ppm
0.26 0.36 0.34 Range 0.26 - 0.55
Shipments of a variety of citrus exported from Israel to Europe were
sampled and analysed to determine the fruit-to-fruit variation.
Samples were taken of citrus prepared and packed by 13 separate
packing houses. In this case, the thiabendazole was applied as a
suspension in the wax emulsion used to treat the fruit after washing
and prior to packing in cartons. The wax contained 0.3% (3 000 ppm)
thiabendazole. The results given in Table X show a much higher level
of residues than those found following dipping. The fruit-to-fruit
variation was considerably greater and there was a significant
variation between different packing houses. Table XI shows results of
analysis of individual fruits from 7 packing houses in Israel
following treatment with 0.3% thiabendazole in wax emulsion.
TABLE X
Thiabendazole residues on Israeli citrus following application
of 0.3% thiabendazole in citrus wax
Packing No. of Fruit Average Standard Range
House Analysed ppm Deviation ppm
1 20 1.79 0.31 1.21 - 2.36
2 20 0.71 0.20 0.43 - 1.13
3 10 2.65 1.10 1.51 - 4.81
4 10 4.41 1.10 2.76 - 5.89
5 10 4.76 1.2 3.39 - 6.40
6 10 3.89 1.7 0.95 - 7.35
7 10 4.75 1.0 3.37 - 5.73
8 10 2.97 0.69 2.12 - 4.19
9 10 3.34 0.53 2.45 - 4.28
10 10 1.47 0.45 0.92 - 2.24
11 10 3.86 0.81 2.96 - 5.56
12 10 1.12 0.53 0.59 - 1.48
13 10 4.06 0.83 2.98 - 5.44
TABLE XI
Distribution of results of analysis of individual citrus
fruits treated with 0.3% thiabendazole in wax and analysed
one week after application
Range of residue levels Number of fruits Percent
less than 1.0 ppm 24 10
1.0 - 1.9 64 26
2.0 - 2.9 59 23
3.0 - 3.9 41 18
4.0 - 4.9 22 9
5.0 - 5.9 25 10
above 6 ppm 10 4
Total 245 100
Samples of oranges treated in South Africa by dipping in a suspension
containing 0.5% (5 000 ppm) thiabendazole which were analysed after
arrival in the United Kingdom contained between 0.28 ppm and 0.35 ppm
thiabendazole. The lower residue levels were found on fruit which had
received a water rinse after dipping. The higher residues were on
unrinsed fruit. Fruit dipped in suspensions at 43°C were found to have
residues of 0.80 ppm thiabendazole, twice the level found in those
dipped cold.
A further twenty samples representing various treatment levels, times,
temperatures and after-treatments showed residues ranging from 0.2 ppm
to 1.62 ppm. The lower levels were found in fruit treated at 1 000 ppm
and the higher level in fruit treated at 5 000 ppm. After-treatment
with citrus wax tended to reduce the residue levels to 0.15 ppm and
0.5 ppm, respectively.
METHODS OF RESIDUE ANALYSIS
Citrus
The method developed for the determination of residues in and on
citrus and bananas has been published by the U.S. Food and Drug
Administration (1969). The whole fruit is rinsed with ethyl acetate to
remove wax and thiabendazole from the surface. The pulp and peel are
homogenized with water. The thiabendazole is extracted from the
homogenates with ethyl acetate. The ethyl acetate solutions from
rinse, pulp and peel are combined. The solutions are purified by a
series of extraction procedures. The final solution in 0.1N
hydrochloric acid is measured spectrophotofluorometrically.
For routine testing of treated citrus, it is advisable to homogenize
about 10 fruits in a large chopper and to take a sub-sample of 60g of
puree for analysis. This is blended with an equal weight of sodium
acetate/sodium chloride solution, and from the slurry a 20 g sample is
taken for analysis. A number of minor variations have been introduced
into the extraction procedure to deal with various citrus fruits, the
separate components of the fruit and citrus products. When analysing
whole fruit, the method is sensitive to 0.1 ppm, but with pulp the
sensitivity is estimated to be 0.03 ppm and with peel 0.4 ppm. Blank
values for untreated pulp and peel range from 0.00-0.02 ppm and
0.00-0.40 ppm, respectively.
After purification, the extract is dissolved in 0.1N hydrochloric acid
and the fluorescence of the final acid solution is read on an
Amico-Bowman spectrophotofluorometer having an excitation wavelength
set at approximately 300mu and the mission wavelength set at 360mu.
Other instruments may operate more effectively at slightly different
wavelengths. The method is suitable for regulatory purposes but
requires a suitable spectrophotofluorometer.
Where a suitable spectrophotofluorometer is not available, the method
of Szalkowski (1965) has been modified by Gilbert (1969) to yield a
reliable colorimetric method with a sensitivity of 0.08 ppm and
recoveries between 93% and 110%. The blank varies between 0.02 and
0.04 ppm for both pulp and whole fruit. The thiabendazole is extracted
from homogenized fruit with acidic methanol and interference removed
with an alkaline wash followed by alumina column cleanup. The
thiabendazole is reduced to form hydrogen sulphide which complexes
with p-phenylene diamine. This complex in oxidized with ferric
solution to give a blue thiazine dye, which is determined
colorimetrically.
Bananas
The method described for the determination of thiabendazole in citrus
has been adapted to determine residues in banana pulp and peel by the
U.S. Food and Drug Administration (1969b).
The thiabendazole in extracted into ethyl acetate from a Ph 4.5
buffered suspension of ten grams of the banana pulp or peel. The
combined ethyl acetate extract is washed twice with 0.05N sodium
hydroxide. Then the thiabendazole is returned to an aqueous phase by
extraction with 0.1N hydrochloric acid and determined by fluorescence,
the method described for citrus. The sensitivity of this method is
0.05 ppm.
Animal tissues
The spectrophotofluorometric method has been used successfully for the
determination of thiabendazole and its 5-hydroxy derivative in animal
tissue. For liver and kidney tissue a preliminary digestion with
enzyme in necessary to convert the metabolite conjugates to 5-hydroxy
thiabendazole. The sensitivity is about 0.1 ppm.
Milk samples from animals receiving thiabendazole therapy or being fed
on citrus residues containing thiabendazole may be analysed for
thiabendazole by spectrophotofluorometric method using 1-5 ml samples.
For milk, as little an 0.05 ppm may be recovered.
NATIONAL TOLERANCES
Country Commodity Tolerance,ppm
U.S.A. Bananas 3
Banana pulp 0.4
Citrus 2
Citrus from wax treatment 6 (proposed)
Citrus pulp (dried) 8
Edible tissues of cattle,
sheep, goats and pigs 0.1
Milk (negligible residue) 0.05
(Cont'd)
Country Commodity Tolerance,ppm
Canada Bananas 3
Banana pulp 0.4
Citrus 2
Australia Bananas 3
Banana pulp 0.4
Citrus 2
Meat of cattle, sheep, 0.2
goats and pigs
Milk 0.05
France Bananas 3
Banana pulp 0.4
Citrus (provisional) 6
Netherlands Citrus (whole) 6
Citrus with diphenyl 3
Sweden Banana (whole) 3
Banana (pulp) 0.4
Citrus (whole) 6
E.E.C. Bananas (whole) 6
Citrus (whole) 6
Germany Citrus 6
Bananas 3
Belgium Bananas 3
Citrus 6
New Zealand Bananas 3
Citrus 2
Norway Citrus 6
Bananas 3
Finland Citrus 6
Italy Citrus (when used alone) 6
Citrus (when used with
other antifungals) 3
Bananas 3
APPRAISAL
Thiabendazole is a particularly effective fungicide for the
post-harvest treatment of bananas and citrus. It has been used very
extensively since 1960-62 as an anthelmintic for sheep, cattle, pigs
and horses and later as a vermifuge in human beings.
Post-harvest dips containing 200 ppm thiabendazole protect bananas
against the most important fungal rots. Treatment of green bananas
during picking and packing operations ensures that the fruit may be
shipped to distant markets with minimum loss or deterioration.
Likewise post-harvest sprays, dips and wax coatings containing
thiabendazole at concentrations of 1 000 to 3 000 ppm have proved
outstandingly useful. Diphenyl impregnated wraps and interlinings
have been used for many years to reduce Penicillium sporulation, and
sodium ortho-phenylphenate dips have been used against stem and
rots. Both treatments have many disadvantages and objections, and it
is anticipated that thiabendazole will provide a more acceptable
alternative to both treatments.
Data on the level of residues in whole fruit, peel, pulp and processed
citrus products, as well as in green and ripe bananas, were available
from U.S.A., West Indies, Australia, Israel and Europe. Results of
residue analysis of commercial citrus shipments to Europe were also
available and were not significantly different from those resulting
from supervised trials.
Thiabendazole has high stability on and in fruit and under conditions
of use no metabolites or degradation products are likely to occur. The
residue levels do not decline significantly in storage.
Several methods of residue analysis are available. The most convenient
and sensitive method employs spectrophotofluorometric measurement of
the thiabendazole in dilute hydrochloric acid solution. The method may
be adapted to determine thiabendazole residues in a variety of fruit
products and in animal tissues and milk. The sensitivity varies with
the substrate but ranges from 0.01 ppm to 0.04 ppm. This sensitivity
is adequate for the level of residues likely to occur and the proposed
tolerances. The method is suitable for regulatory purposes and is
specific to thiabendazole. de Vos and Bosma examined the possibility
of using gas chromatography with sulphur specific detector; they
showed that it was possible to determine thiabendazole residues to a
limit of 0.1 ppm by using the ethyl acetate extract of raw fruit for
injection into the chromatograph. They found that the method using
concentrated crude ethyl acetate extracts was satisfactory, but it led
to rapid fouling of the chromatographic column.
The administration of thiabendazole to sheep and cattle as an
anthelmintic gives rise to low level transient residues in several
edible tissues especially kidney and liver, but these residues are
eliminated within three days. Lactating dairy animals receiving a
therapeutic dose of thiabendazole excrete detectable amounts in the
milk for 24 hours post treatment. However, animals with severe
helminthiasis and liable to be dosed with thiabendazole are rarely
suitable for slaughter or for production of milk for human
consumption. Furthermore, it would be very unusual to treat more than
a small proportion of a herd at any one time and thereby to exceed the
detectable level of about 0.05 ppm in meat or milk. Under these
circumstances it does not appear necessary to recommend a tolerance
for thiabendazole residues in meat or milk.
RECOMMENDATIONS FOR TOLERANCES
TOLERANCES
The following tolerances are recommended for thiabendazole:
Crop Residues ppm
bananas, whole 3
banana pulp 0.4
citrus, whole 6
FURTHER WORK OR INFORMATION
DESIRABLE
Studies to determine the effect of thiabendazole on the thyroid gland.
REFERENCES
Anon. (1967) Control of squirter disease of bananas by thiabendazole.
Agricultural Gazette of New South Wales, 78:604
Anon. (1969) New treatments reduce fruit wastage - Rural Research in
CSIRO, 67:7-12
AOAC. (1966) Analysis of thiabendazole in feeds. J.A.O.A.C.
49:238-239 and 312
Bondoux, P. (1967) Acac. Agric. France, Compt. Rend. Hebd. Seances,
53: 1314-1323
Brown, G.E., McCormack, A.A. and Smoot, J.J. (1967) Thiabendazole as a
post-harvest fungicide for Florida citrus fruit. Plant Disease
Reporter, 52:95-97
Brown, H.D., et al. (1961) Journal of American Chemical Society,
83:1764
Burden, O.J. (1967) Studies on grown rot on bananas. Queensland
Agricultural Journal, March, p. 186
Crivelli, G. (1966) Part V Tests with the fungistat thiabendazole
against Penicillium of oranges (in Italian). Il. Freddo, 20:25-29
Cuille, J. and Bur-Ravault, L. (1968) Fruits, (Paris), 23:351-356
Eckert, J.W., Kolbenz, M.J. and Kraght, A.J. (1968) Proc.
International Citrus Symposium, Vol 3, Riverside, Cal., 13 March 1968
Fripp, Ivonne J. and Dettman, E. Belinda. (1969) Thiabendazole as a
post-harvest treatment against Sclerotinia fructicola in dessert
peaches. Australian Journal of Experimental Agriculture and Animal
Husbandry, 9:9-11
Gilbert, W.S. and Wright, Julia. (1969) Method for determining
thiabendazole residues on oranges
Gordon, H. McL. Studies of anthelmintics for sheep - thiabendazole.
Australian Veterinary Journal, 40(1):9-18
Harding, P.R. Jr. (1968) Plant Disease Reporter, 52: 623-625
Johnson, C.D. (1964) Evaluation of teratogenic potential in the rat.
Unpublished Report (April 1964) from Woodard Research Corporation
through Merck Institute for Therapeutic Research to FDA
McCormack, A.A. and Brown, G.E. (1968) Thiabendazole, an experimental
fungicide for fresh citrus fruit. Proc. Florida State Horticultural
Soc., 80:232-237
Merck and Company, Inc. (1969) Rahway, New Jersey, U.S.A.
thiabendazole residues in bananas and citrus. Submission to U.S. Food
and Drug Administration
Merck and Company, Inc. (1970) Rahway, New Jersey, U.S.A.
Thiabendazole residues in citrus following application in citrus wax.
Submission to U.S. Food and Drug Administration
Robinson, H.J., Stoerk, H.C. and Graessle, A.E. (1965b) Studies on the
toxicological and pharmacological properties of thiabendazole.
Toxicol. appl. Pharmacol., 7:53-63
Rosenblum, C. and Meriwether, H.T. (1970) Determination by the
radioactive indicator Method of the retention and stability of
thiabendazole in treated Valencia orange. Journal of Radioanalytical
Chemistry, (in press)
Scott, K.J., and Roberts, E.A. (1967) Control in bananas of black end
rot caused by Gloeosporium musarium. Australian Journal of
Experimental Agriculture and Animal Husbandry, 7:283-286
Seberry, J.A., and Baldwin, R.A. (1968) Thiabendazole and
2-amino-butane as post-harvest fungicides for citrus. Australian
Journal of Experimental Agriculture and Animal Husbandry, 8:440-443
Seberry, J.A. (1968) Proc. Int. Citrus Symposium, Riverside, Calif.,
13 March 1968
Shillingsford, C.A. (1970) Banana fruit rot control in Jamaica.
P.A.N.S.,16(1)
Szalkowski and Kanora. (1965) Spectrophotometric determination of TBZ
in feed. JAOAC,48(2):288-295
Tocco D.J., Buhs, R.P., Brown, H.D., Matzuk, A.R., Mertel, H.E.,
Harman, R.E. and Trenner, N.R. (1964) The metabolic fate of
thiabendazole in sheep. J. med. Chem., 7:399-405
Tocco, D.J., Egerton, J.R., Bowers, W., Christensen, V.W. and
Rosenblum, C.J. (1965) Absorption, metabolism and elimination of
thiabendazole in farm animals and a method for its estimation in
biological materials. J. Pharmacol. exp. Therap., 149:263-271
Tocco, D.J., Rosenblum C., Martin. C.M. and Robinson, H.G. (1966)
Absorption, metabolism and excretion of thiabendazole in man and
laboratory animals. Toxicol. appl. Pharmacol., 9:31-39
U.S. Food and Drug Administration. (1969) Thiabendazole residues in
citrus fruits. Pesticide analytical manual, Vol II, Section 120.242
U.S. Food and Drug Administration. (1969b) Thiabendazole residues in
banana peel and pulp. Method A. Pesticide analytical manual, Vol II,
Section 120.242.
Vogin, E.E. (1968) Multigeneration reproduction and lactation studies
with thiabendazole. Unpublished reports (Dec. 1967 and March 1968).
Food and Drug Research Laboratories, through Merck Institute for
Therapeutic Research to FDA
de Vos, R.H. and M.P. Bosma. (1970) Residues of thiabendazole in
citrus fruit. Report 3199. TNO Central Instituut voor
Voldingsonderzoek, Ziest, Netherlands
Weinke, K.E., Lauber, J.J., Greenwald, B.W. and Preiser, F.A. (1969)
Thiabendazole, a new systemic fungicide. Proc. 5th Brit. Insectic.
Fungic. Conference
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1964) Safety evaluation
by oral administration to dogs and rats for 104 weeks. Unpublished
Report (April 1964). Woodard Research Corporation through Merck
Institute for Therapeutic Research to FDA
Special studies on dermal irritation
There was no evidence of a significant degree of irritation when 0.5
gm thiabendazole was applied in cold cream at concentrations of 10 and
50 percent (w/w) for 24 hours to intact rabbit skin (Robinson, 1965a,
1965b).
Acute toxicity
LD50 doses for various animal species are given in Table I.
Toxic signs observed following administration of large doses of
thiabendazole by oral or intraperitoneal routes were generally similar
and consisted of lethargy and stupor. The intravenous administration
of a large dose of the hydrochloride produced narcosis. Death appeared
to be due to respiratory failure. Rabbits that survived large doses
initially lost weight but recovered after several days (Robinson,
1965a).
Short-term studies
Calf
Five female Holstein calves (seven months old) were fed a diet
containing 0, 320, 1 000, 3 200 or 10 000 ppm of thiabendazole for a
14-week test period. Calves tolerated 3 200 ppm thiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg body-weight. At the 10 000 ppm thiabendazole level, the
calves grew normally for the first two weeks, but in the following 12
weeks the weight gain was about half that observed for the controls.
Gross examination at the time of autopsy revealed no pathological
condition, and histological examination of several tissues showed no
change as resulting from the incorporation of thiabendazole in the
diet (Robinson, 1965a).
Chicken
Groups of 0.5 week old male White Leghorn chicks (10 to 20 per group)
were fed thiabendazole in the diet at dosage levels ranging from 1 to
10 000 ppm for a period of 2 1/2 weeks. This level corresponded to a
mean daily intake of thiabendazole ranging from 0.1 mg/kg to 1 200
mg/kg. A gradual decrease in growth appeared to occur with
concentrations of 100 ppm thiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg per day. Gross pathological
examination at autopsy of the chicks on a dietary level of 4 000 ppm
thiabendazole showed them to be normal except for a smaller size
(Robinson, 1965a).
Dog
Groups comprising two male and two female dogs were orally
administered thiabendazole daily for periods of over two years at
doses of 0, 20, 100 and 200 mg/kg body-weight/day. No clinical signs
of morbidity were observed; food and water consumption were normal;
blood and urine chemistry were normal; a slight retardation in
body-weight gain at the 200 mg/kg level was observed, which was
accompanied by a slight reduction in total erythrocyte count. At this
level, changes in haemoglobin concentration and haematocrit were also
observed. No gross pathological observations were noted at the
conclusion of this test, and organ-weights appeared normal.
TABLE I
Thiabendazole toxicity in various animal species
LD50
Animal Route Form Solvent (mg/kg body-weight) Reference
Mouse oral HCl Water 2 400 (Robinson 1965a, 1965b)
ip HCl Water 430 " " "
iv HCl Water 150 " " "
oral Base CMC 2% 1 3 810 " " "
Rat oral HCl Water 3 600 " " "
ip HCl Water 1 850 " " "
iv HCl Water 130 " " "
oral Base CMC 2% 3 330 " " "
Rabbit oral Base CMC 2% 3 850 " "
Sheep drench HCl Water 2 000 " "
Goat drench HCl Water >2 000 " "
1 CMC = carboxy methyl cellulose
A significant haemosiderosis was present in dogs at the 100 and 200
mg/kg level in the spleen, liver, lymph nodes and bone-marrow. This
was not associated with an increase in serum haemoglobin. No
significant toxicological effects were noted at 20 mg/kg/day
(Robinson, 1965a, 1965b).
Groups of dogs (three male and three female) were orally administered
thiabendazole for two years at doses of 0, 20, 50 and 125 mg/kg
body-weight/day. At the 125 mg/kg/day level, two of six dogs died. One
of these dogs had marked liver cirrhosis, seminal tubular
degeneration, bone marrow atrophy and degenerative renal changes.
Slight to moderate reduction in haemoglobin and packed cell volume
occurred. Elevated blood urea nitrogen, serum alkaline phosphatase and
serum glutamic oxal oacetic transaminase were evident. Urinary albumin
was seen more frequently than in controls. Inspissated bile entwined
in the gall bladder villi was also observed in one dog. One dog of
this group was sacrificed and was found to have pulmonary arteritis of
parasitic origin. At 50 mg/kg/day, growth of male dogs was slightly
depressed. All six dogs given thiabendazole at 50 mg/kg survived the
study, and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa in
one. Five of six dogs survived thiabendazole at 20 mg/kg for the term
of the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes in
all treated dogs (Woodard et al., 1964).
Pig
Five groups of four barrows each were given a diet containing 0, 320,
1 000, 3 200 and 10 000 ppm thiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects for
the 14-week period. This concentration corresponded to an intake of 15
mg/kg body-weight per day. Concentration of 10 000 ppm thiabendazole
in the diet caused reduced weight gains and reduced food consumption
with no mortality observed. Gross pathology examination revealed no
abnormal conditions (Robinson, 1965a).
Rat
Rats (10 males and 10 females per group) were administered
thiabendazole once daily at dosage levels of 0, 100, 400, 800, 1 200
and 1 600 mg/k-g body-weight per day. During the 30-day experimental
period, rats in the 800 mg/kg per day group decreased their food
intake and gradually lost weight. They were slightly less active, had
ruffled fur and became slightly flaccid. Three males and three females
died during the course of the experiment. All of the rats in the 1 200
and 1 600 mg/kg groups died during the 30-day test period. Rats dosed
at 100 and 400 mg/kg appeared normal throughout the test period,
although a slight depression in body-weight gain was noted in males at
100 mg/kg per day and in females at 400 mg/kg per day. Haematologic
studies on rats receiving 800 mg/kg per day showed a mild neutrophilia
with concurrent lymphopenia. There was also a suggestion of a decrease
in red blood cell elements. No gross anatomical changes were noted in
rats dosed with 100 mg/kg per day, whereas male and female rats
treated with 400 mg/kg per day showed thymic involution. A slight
enlargement of the liver was also noted in females at 400 mg/kg per
day. Animals surviving 800 mg/kg per day showed gross signs indicating
starvation where normal body fat depot and subcutaneous fat appeared
to be depleted. Liver and adrenals were slightly enlarged in males and
females and the thymus involuted. Microscopic pathology showed bone
marrow hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg (Robinson, 1965a, 1965b).
Rats (30 males and 30 females per group) were administered
thiabendazole suspended in 1.0 percent methocel daily for 180 days at
doses of 0, 12.5, 25, 50, 100, 200 and 400 mg/kg body-weight. All of
the animals survived throughout the duration of the experiment. At 400
mg/kg, a depression in body-weight was observed with both sexes. At
200 mg/kg per day, the male rats showed a slight depression in weight
gain. No clinical signs of toxicity were observable at levels below
100 mg/kg. Haematological studies at 400 mg/kg per day indicated a
slight suggestion of a fall of the red blood cell elements which was
not evident at levels below this dose. Biochemical blood studies and
urinalysis were normal at all dosage levels with a slight polyurea in
both male and female rats at 400 mg/kg, which was apparently the
result of a slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and females at
400 mg/kg per day and in females at 200 mg/kg per day. There was an
increase in liver size at doses of 100 mg/kg and above in males and at
25 mg/kg per day and above in females. Males dosed with 200 and 400
mg/kg and females at 400 mg/kg showed an apparent increase in kidney
weight. Microscopic examination of animals at 100 mg/kg showed a small
incidence of haemosiderosis of the thymus. At 200 and 400 mg/kg, these
examinations showed considerably more haemosiderosis of the thymus and
colloid depletion in the thyroid (Robinson, 1965a, 1965b).
Sheep
Thirty weanling wethers were employed in a 16-week study at doses of
0, 10, 50, 100, 200, 400 and 800 mg/kg body-weight per day.
Thiabendazole was administered in gelatin capsules. Two of the four
sheep dosed at 100 mg/kg did not survive the test period. However,
because of infection, thiabendazole may not have been directly related
to the cause of their death. All the animals treated at 200 mg/kg per
day and above died before the end of the test interval. No significant
effects on blood biochemistry or urinalysis were observed. Doses of 50
mg/kg and above affected body-weight gain, after approximately 120
days on the test. Doses of 10 mg/kg had no effect. Food consumption
was unaffected at doses of 10 mg/kg per day, but a minor decrease was
noted at 50 and 100 mg/kg per day and a significant decrease at 200
mg/kg per day and above. No gross pathological changes were observed
at doses below 200 mg/kg per day. Starvation and loss of body-weight
complicated the interpretation of data concerning organ-weights at 200
mg/kg and above. At 200 to 800 mg/kg, a moderate hypoplasia of the
bone marrow with replacement by adipose tissue was observed. Loss of
colloid in thyroid was evident at 800 mg/kg, as well as lymphoid
atrophy at the higher dose levels (Robinson, 1965a).
Five groups of two ewe lambs (10 to 12 weeks old) and eight wether
lambs (10 to 19 weeks old) were fed diets containing 0, 100, 320, 1
000 and 3 200 ppm thiabendazole for periods up to 50 weeks. Sheep 10
weeks of age tolerated 1 000 ppm thiabendazole in the diet for the
duration of the experiment. (This dose corresponded to 30 to 50 mg/kg
body-weight per day.) The sheep tolerated 3 200 ppm in their diet for
the first 14 weeks but thereafter lost weight in the final stages of
the experiment (Robinson, 1965a).
Long-term studies
Rat
Groups of rats (35 of each sex) were fed dietary levels adjusted to
provide 0, 10, 40 or 160 mg/kg body-weight per day of thiabendazole
for up to two years. At the 160 mg/kg level, a reduction of weight
gain of about 25 percent, with concomitant reduction in food
consumption and slightly reduced haemoglobin and microhaematocrit
values, were the only changes seen attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidents or location of neoplasms and histopathological examination
of the tissues. No effect on food consumption or haemograms were seen
at either the 10 or 40 mg/kg levels. Very slight depression in growth
rate, of questionable significance, was observed in the male rats at
40 mg/kg but not at 10 mg/kg. At the 10 mg/kg level, the mean absolute
thyroid weights of the male rats (including the individual thyroid
weights from five out of eight animals tested) were heavier than the
mean thyroid weight of the controls. This effect was not observed in
the rats given 40 or 160 mg/kg (Woodard et al., 1964).
OBSERVATIONS IN MAN
Human subjects have ingested a single oral dose of 2 g (approximately
30 mg/kg body-weight) of thiabendazole in studies designed to
ascertain the metabolic fate (see "Biochemical aspects"). No
information was given on the toxicological effects, if any (Robinson,
1965a).
COMMENTS
Metabolic studies indicate a rapid absorption of thiabendazole from
the gastrointestinal tract of rats, dogs, sheep and goats with
excretion in urine and faeces complete within 3-8 days. In cows,
approximately 0.1 percent of an oral dose was detectable in milk
within 60 hours. In excreta of man and all animals studied,
metabolites include 5-hydroxythiabendazole, free and conjugated as a
glucuronide and sulfate ester, as well as traces of unchanged
thiabendazole. Slight differences in man and other animals were noted
in quantitative recovery of the various compounds. A common effect for
several animal species at high dose levels of thiabendazole is
retarded growth.
Short-term studies in rate, sheep, pigs, calves and chickens have been
carried out with oral administration or with incorporation of
thiabendazole in the diet for periods of 4-50 weeks. At higher doses
in rats, thymus involution, colloid depletion in the thyroid and
increase in liver and kidney size was observed. Loss of the colloid in
thyroid of sheep administered 200 mg/kg body-weight was also evident.
Oral administration of 12.5 mg/kg for 180 days did not cause
significant toxicological changes in rats.
In a two-year dog study a no-effect level of 20 mg/kg body-weight was
demonstrated. A two-year study in rats showed heavier thyroid glands
at 10 mg/kg body-weight/day in the male animals; this effect, however,
was not dose dependent.
A five-generation reproduction study in mice showed no adverse effects
at 200 ppm level. In a three-generation study in rats, only decreased
body-weight in relation to decreased food consumption could be
observed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 20 mg/kg body-weight/day
Mouse: 200 ppm in the diet, equivalent to 30 mg/kg body-weight/day
Rat: 10 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.05 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Administration to animals
Thiabendazole is widely used as a broad spectrum anthelmintic for
sheep, cattle, horses, pigs and goats. It is also administered as a
vermifuge directly to man. Following the announcement by Brown (1961)
of the chemotherapeutic properties of benzimidazoles, many
investigators report the efficacy of thiabendazole as an anthelmintic.
Gordon (1964) generally reported on its properties. Since that time
very large numbers of sheep and cattle have been treated with
thiabendazole suspensions. In sheep a dose of 44 mg/kg is adequate in
moot circumstances. Dosing may be repeated as frequently as once per
month, depending upon the level of reinfestation. In cattle a dose of
88 mg/kg is usually employed and in pigs 50-100 mg/kg.
Usually the dose is administered by drenching (oral administration of
an aqueous suspension) but for convenience it is possible to
administer via the feed when dealing with individual animals. At the
present time, economic considerations probably preclude the use of
thiabendazole as a feed additive.
Pre-harvest treatment of plants
Thiabendazole has been evaluated and has proved effective for use as a
fungicide on a number of crops including apples, pears, peaches,
apricots and bananas but, as far as is known, pre-harvest treatments
have not yet been adopted commercially. Preliminary results of residue
trials indicate that the residues from such pre-harvest uses will vary
considerably due to the systemic action of thiabendazole and the
ability of plants to take it up from the soil (Wernke, 1969).
Post-harvest treatment
Bananas
Scott (1967), Burden (1967), Anon (1967, 1969), Cuille (1968) and
Shillingsford (1970) have shown that thiabendazole is highly effective
for control of post-harvest fungal rots of bananas, when the picked
fruit is dipped in a suspension of thiabendazole containing from 100-1
000 ppm. A dip containing 200 ppm (0.02%) appears to be the most
widely used. Post-harvest spray treatments have also proved effective
in experimental use, but it is not known whether these are yet used in
practice.
Thiabendazole is most soluble in acid solution and can be rendered
soluble by treatment with lactic acid. Considerable experimental work
was carried out with lactic acid solutions as fruit dips, but in spite
of the convenience, the acidic solution gave rise to corrosion
problems in packing houses and during transport and resulted in
decomposition of the thiabendazole. Such solutions have been abandoned
in favour of micro-fine powder suspensions which require minimal
agitation to maintain uniform concentrations.
After the bunches of bananas have been picked, they are usually washed
in water or a solution of sodium hypochlorite. The bunches are then
separated into hands or clusters of 10-12 fruit which is allowed to
"bleed" in water for approximately 15-30 minutes. This facilitates the
removal of natural latex which, if allowed to run on the fruit, will
result in a visual disfigurement. The wet fruit is then dipped for 2-4
minutes in a suspension containing 200 ppm thiabendazole before being
drained for packing into cartons. No visible residue results from the
treatment.
Citrus
Crivelli (1966) and his associates in Italy first demonstrated that
Penicillium decay of oranges could be prevented by dipping fruit in
solutions containing 750-1 000 ppm thiabendazole dissolved in lactic
acid. Confirmation by other laboratories indicated that concentrations
of 200 to 500 ppm are adequate to give excellent control of
Penicillium decay. Brown (1967), Eckert (1968), Harding (1968),
McCormack (1968), Seberry (1968) show the efficacy of thiabendazole in
controlling Penicillium, and Seberry (1968) gives data showing
reduction in stem end rot caused by Diaporthe citri after dipping
oranges in suspensions of 500-1 000 ppm. Spraying or dipping citrus
fruits in 0.5 to 1.0 percent thiabendazole suspensions, without
subsequent rinsing, prevents sporulation of Penicillium upon the
surface of decaying fruits. Oranges so treated have a natural
appearance with no odour or visible residue.
The most efficient methods for commercial application of thiabendazole
appear to be as picking bin drenches, processing line spray or as an
additive to water emulsion waxes. Thiabendazole is registered in
Australia and U.S.A. as a post-harvest spray or dip treatment of
citrus fruit with suspension containing up to 5 000 ppm thiabendazole.
A rinse is not required, but rinsing does not appear to significantly
reduce the efficacy of a dip treatment.
Sporulation of Penicillium on the surface of decayed fruit causes
superficial soiling of sound fruits adjacent to decayed fruits. This
soiling can completely ruin all the fruit in a whole container with
only one or two decayed fruits. For this reason, a post-harvest
fungicide treatment must control both primary decay (prevent infection
of fruit) and reduce Penicillium sporulation on the surface of those
fruits which decay due to deep primary infections. Diphenyl and
sodium-o-phenylphenate, which have been used as standard treatments
for many years, have serious deficiencies. Thiabendazole on citrus wax
is used as a means of controlling fungus diseases referred to as green
mould, blue mould and stem end rot. This treatment is effective in
controlling both primary decay and sporulation.
Thiabendazole/citrus wax treatments are used commercially in Israel
and on an experimental basis in U.S.A. Aqueous emulsions of waxes
designed for application to citrus have been in use for many years.
Thiabendazole is suspended in sufficient wax emulsion to yield a
concentration of up to 0.5% w/w thiabendazole (5000 ppm). The prepared
emulsion is used without dilution in waxing equipment designed to
apply citrus wax evenly to freshly washed citrus. In this equipment,
the wax containing the thiabendazole is applied through nozzles to the
citrus as it revolves on horsehair brushes below. The nozzle is
attached to a chain to move back and forth across the width of the
waxer at a right angle to the flow of the citrus passing along the
conveyer. The amount of wax applied is governed by the size of the
nozzle and the pressure at which it is applied. These are equilibrated
with the flow of the citrus so that 1 litre of emulsion will coat ca.
800-900 kg of citrus fruit.
Other fruit
Bondoux (1967) reports good results from soaking apples in dilute
suspension of thiabendazole. Fripp (1969) showed thiabendazole
outstanding for control of brown rot (Sclerotinia fructicola) of
peaches. It is known that many other investigators are working with
thiabendazole to control post-harvest losses of a wide variety of
fruits, including cherries and pineapples, but as yet the available
data are not sufficient to enable proposals to be made for tolerances.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Bananas
Residue trials were carried out in Honduras. Green bananas treated
according to recommended practice were shipped under commercial
conditions at a temperature of 14°-15°C. Analysis was made 8 days
after treatment in the case of green bananas and 15 days in the case
of ripe fruit.
A review of the residue data showed that at the recommended treatment
rate of 200 ppm, a maximum of 0.18 ppm thiabendazole was found in the
banana pulp (see Table II), while a maximum of 1.24 ppm thiabendazole
was present in the peel (see Table III), when calculated on a whole
fruit basis. Therefore, at the recommended rate of 200 ppm, a maximum
of 1.4 ppm thiabendazole is possible in the whole unpeeled banana.
Pulp and peel residues generally increase slightly with an increase in
treatment rate, (see Table II) while the lower treatment rate (100
ppm) results in somewhat lower residue levels of thiabendazole in pulp
and peel (see Tables II and III). At the 100 ppm treatment level, an
average of 0.018 ppm and 0.049 ppm was found in ripe banana pulp from
two trials. This increased to an average of 0.068 ppm - 0.110 ppm at
the 200 ppm treatment rate. Residues appear to level off at the 200
ppm treatment rate, since ripe banana pulp contained an average of
0.126 ppm and 0.094 ppm at the 400 ppm treatment rate.
TABLE II
Thiabendazole residues (ppm) in pulp of green and ripe bananas 1
Treatment GREEN RIPE
Average, ppm Range, ppm Average, ppm Range, ppm
Control 1 0.0095 0.005-0.01 0.011 0.01-0.02
2 0.013 0.01 -0.03 0.024 0.01-0.04
100 ppm 1 0.030 0.01 -0.05 0.018 0.01-0.03
2 0.037 0.03 -0.05 0.049 0.03-0.07
200 ppm 1 0.074 0.04 -0.10 0.068 0.05-0.09
2 0.067 0.05 -0.08 0.110 0.05-0.18
400 ppm 1 0.173 0.05 -0.31 0.126 0.10-0.18
2 0.126 0.07 -0.17 0.094 0.07-0.13
1 Results on treated bananas are not corrected for results on control bananas.
TABLE III
Thiabendazole residues in peel of green and ripe bananas 1
Treatment GREEN RIPE
Average Range Average Range
(ppm) (ppm) (ppm) (ppm)
Control 1 0.020 0.013-0.042 0.040 0.024-0.059
2
100 ppm 1 0.39 0.33 -0.52 0.55 0.39 -0.68
2
200 ppm 1 0.12 0.97 -1.21 0.17 1.12 -1.24
2
400 ppm 1 2.24 2.01 -2.64 2.73 2.61 -2.80
2
1 Results on treated bananas are not corrected for results on control bananas.
The analytical method used had a sensitivity of 0.05 ppm with blank
values for untreated banana peel and pulp in the range of 0.01 - 0.04
ppm. The recovery of thiabendazole added to banana pulp and banana
peel ranged from 74.3 percent to 90.2 percent with an average of 87.3
percent.
In Australia, experiments were conducted by the Department Agriculture
to investigate the use of thiabendazole as a banana dip under
conditions of commercial usage; 100 cases (250 kg) of fruit was
treated at each of four concentrations of thiabendazole suspension.
The results were as follows:
TABLE IV
Whole fruit residues produced by various treatment rates
Treatment On Peel In Peel In Pulp Total
Rate Variety Average Range Average Range Average Range Residue
ppm ppm ppm ppm ppm ppm ppm ppm
0 Orange 0.076 0.00-0.33 0.008 0.00-0.03 0.00 0.00-0.01 0.09
Lemon 0.02 0.00-0.03 0.004 0.00-0.02 0.00 0.00-0.01 0.02
Grapefruit 0.01 0.01-0.02 0.01 0.01-0002 0.01 0.00-0.02 0.03
5 000 Orange 0.72 0.33-1.28 0.072 0.00-0.25 0.01 0.00-0.04 0.73
Lemon 0.31 0.16-0.74 0.045 0.00-0.13 0.005 0.00-0.01 0.36
Grapefruit 0.47 0.27-0.72 0.20 0.14-0.4? 0.02 0.00-0.04 0.66
10 000 Orange 1.25 0.77-2.22 0.098 0.01-0.19 0.01 0.00-0.01 1.36
Lemon 0.95 0.31-1.96 0.10 0.00-0.19 0.005 0.00-0.01 1.06
Grapefruit 0.98 0.66-1.08 0.29 0.23-0.39 0.04 0.01-0.07 1.28
Dip concentration Thiabendazole residues (whole fruit)
0.1% (1000 ppm) 2 - 5 ppm
0.05% (500 ppm) 1.5 - 2.5 ppm
0.025% (250 ppm) 0.8 - 2 ppm
0.0125% (125 ppm) 0.3 - 1.5 ppm
Trials at another research station yielded the following results:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 (200 ppm) 0.03 0.7
0.04 (400 ppm) 0.07 1.5
0.08 (800 ppm) 0.11 4.1
0.16 (1600 ppm) 0.14 6.2
0.32 (3200 ppm) 0.20 12.0
Limit of detection was 0.04 ppm.
Citrus fruits
Residue trials were conducted in conjunction with the Florida Citrus
Experiment Station on oranges, grapefruit and lemons. The following
products were assayed before and after processing: whole unwashed
fruit, single strength juice, finisher pulp, citrus oil, molasses,
dried citrus pulp. The fruit was processed according to typical
practice, including a two-minute flood treatment with a suspension
containing 0, 5 000 and 10 000 ppm thiabendazole. The fruit was then
waxed according to commercial practice.
The detection sensitivity of the analytical method used was 0.03 ppm
for the pulp and 0.40 ppm for the peel with blank values for untreated
pulp and peel in the range 0.00 - 0.02 ppm and 0.00 - 0.40 ppm
respectively.
A review of the whole fruit residue data, which is summarized in Table
IV, shows that at the application rate of 5 000 ppm, a maximum of 0.04
ppm thiabendazole is found in the pulp of the citrus, while a maximum
of 1.28 ppm and 0.41 ppm thiabendazole is present on and in the peel
respectively. This is the maximum residue found in pulp or peel of all
oranges, lemons or grapefruit assayed. Therefore, at the treatment
rate of 5 000 ppm, a maximum of 1.73 ppm is possible on the whole
unpeeled fruit.
In all cases, the peel residues increase with increasing treatment
rates. However, in the case of pulp assays, the level of residue is at
or below the sensitivity of the assay method with no indication of
increases with higher dosages. These results are corroborated by
radioactive studies reported later, which show that the level of
residues in the pulp is approximately equivalent to the assay
sensitivity and appears to result from inadvertent contamination
during peeling or sectioning. The result of assays on dried citrus
pulp (summarized on Table V) indicated the maximum residues range from
1.55 ppm in lemons to 2.87 ppm in the case of grapefruit, with dried
orange pulp being 2.61 ppm.
From yet another research station, it was reported that dipping green
bananas in a suspension containing 800 ppm thiabendazole resulted in
residues of 3.3 ppm in the whole fruit and 0.4 ppm in the pulp.
FATE OF RESIDUES
In animals
Radioactive thiabendazole administered orally to sheep at a dose of 50
to 100 mg/kg body-weight or to goats at a single dose of 150 mg/kg was
rapidly absorbed and metabolized to its 5-hydroxy derivative and
excreted as the aglycone and glucuronide and sulfate ester. Peak
concentrations in plasma occur approximately four hours after
administration, with 90 percent of the radioactivity eliminated in the
excreta in three to four days. Thiabandazole and small concentrations
of two unknown metabolites were observed (Robinson, 1965a; Tocco et
al., 1964).
Residues observed with eight sheep receiving 14C-or 35S-labelled
thiabendazole at 50 mg/kg are given in Table VI, where concentrations
reported as zero are equal to or less than a detection limit of 0.06
ppm.
TABLE V
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Finisher pulp Orange 0.02
Lemon 0.01
Grapefruit 0.01
Single strength Orange 0.01
juice Lemon 0.01
Grapefruit 0.02
Pulp, dry Orange 2.54
Lemon 1.4
Grapefruit 2.53
TABLE V (cont'd)
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Oil Orange 1.05
Lemon 0.16
Grapefruit 1.38
Molasses Orange 1.77
Lemon Not assessed
Grapefruit 1.97
Trials conducted in Australia at the Citrus Wastage Research
Laboratory showed the following results when oranges were dipped in
thiabendazole suspensions under commercial conditions:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 N.D.1 0.49
0.04 N.D. 0.21
0.08 N.D. 0.52
0.10 N.D. 0.39
0.16 N.D. 0.73
0.32 N.D. 1.68
1 N.D. = not detected (less than 0.04 ppm)
Analytical studies in lactating goats showed that although
thiabendazole was absorbed rapidly, less than 1% of the dose was
excreted in their milk within 24 hours after administration of 150
mg/kg body-weight. Peak concentrations were found in the milk within
24 hours, and no thiabendazole or its metabolites were detectable in
the milk four days later (Robinson, 1965a; Tocco et al., 1965).
Studies with goats given single oral dosages of tritium-labelled
thiabendazole at 150 mg/kg, demonstrated that the compound was
absorbed rapidly, reaching peak concentrations in the plasma about 4
hours after administration. The compound was rapidly excreted in the
urine and faeces. Residues of thiabendazole were not detectable in any
of the 15 tissues from goats examined 30 days after treatment.
Thiabendazole is less rapidly excreted by pigs than by several other
animals. Following a single dose of 50 mg/kg body-weight,
approximately 76 percent of the dose was found in the excrete within
about two weeks (Robinson, 1965a; Tocco et al., 1965). Thiabendazole
(14C-labelled) administered to cattle at doses of 50 mg/kg and 200
mg/kg body-weight was rapidly excreted. Plasma levels reached a
maximum in four to seven hours, after which they decreased rapidly,
with no residual thiabendazole or metabolites detectable in tissues 30
days after administration of the lower dose. Traces of radioactivity
were demonstrable in calves two months following doses of 150 and 200
mg/kg. Three fluorometrically detectable metabolites were observed,
corresponding to 5-hydroxythiabendazole and its sulfate and
glucuronide conjugate. Approximately 0.1 percent of an oral dose of 3,
5 or 10 grams per 100 lb was secreted in the milk as thiabendazole and
its metabolites within 60 hours after the animals were dosed. The
highest concentration appeared within 24 hours. Over 99 percent of the
compound was metabolites, and residues were not detectable 2 1/2 days
after the cows were administered the compound (Robinson, 1965a; Tocco
et al., 1965).
TABLE VI
Thiabendazole residues in sheep
Sample 6 hours 5 hours 8 days1/ 16 days 24 days 30 days1/
Abomasum 5.1 0.0 0.15 0.0 0.0 0.0
Blood 7.7 0.0 0.0 0.0 0.0 0.0
Brain 1.0 0.09 0.14 0.0 0.0 0.0
Caecum 34.4 0.0 0.08 0.0 0.0 0.0
Fat 2.8 0.0 0.04 0.0 0.0 0.0
Heart 2.7 0.15 0.14 0.08 0.0 0.0
Kidney 13.9 0.28 0.22 0.0 0.0 0.0
Large intestine 4.6 0.0 0.10 0.0 0.0 0.0
Liver 9.6 0.62 0.62 0.15 0.0 0.0
Lung 2.4 0.0 0.04 0.08 0.0 0.0
Muscle 2.0 0.09 0.06 0.13 0.0 0.0
Pancreas 2.6 0.18 0.13 0.0 0.0 0.0
Skin 3.2 0.0 0.19 0.0 0.0 0.0
Small intestine 33.6 0.0 0.16 0.0 0.0 0.0
Spleen 3.4 0.0 0.0 0.0 0.0 0.0
1/ Two animals slaughtered at each of these intervals. Results given in
table are means of the two values.
Table VII shows thiabendazole residues found in calves treated at 110
mg/kg. Results are the total of thiabendazole and its metabolites
expressed in ppm, determined fluorometrically.
TABLE VII
Thiabendazole residues in calves
Days No.
Post of Muscle Liver Kidney
Treatment Group Animals (ppm) (ppm) (ppm)
Control 3 0.13(0.09-0.17) 0.13(0.13-0.14) 0.12(0.10-0.15)
3 Treated 4 0.12(0.05-0.17) 0.17(0.15-0.19) 0.16(0.13-0.18)
7 " 4 0.10(0.03-0.19) 0.17(0.15-0.19) 0.12(0.10-0.13)
14 " 4 0.08(0.06-0.12) 0.15(0.14-0.18) 0.15(0.07-0.35)
It may be noted that in no instance does the average value exceed the control level by
more than 0.05 ppm.
In plants
Bananas
There is nothing to suggest that thiabendazole residues in bananas
undergo any change during application, shipping, storage or ripening.
Citrus
Radioactive thiabendazole 14C was employed to determine the uptake of
thiabendazole by Valencia oranges after dipping in 0.1% suspension,
before and after storage under conditions simulating customary
shipping and use practices (Merck, 1969; Rosenblum, 1970). Peal and
edible pulp were analysed separately. Peel was divided into inner and
outer portions. At the end of the maximum storage period, which
extended for two weeks at 10°C followed by an additional two weeks at
21°C, peel was assayed by the reverse isotope dilution method for
intact thiabendazole as an estimate of the stability of the latter in
the fruit.
The pulp was essentially free of thiabendazole even after four weeks
of storage. The small apparent residues recorded are probably due to
uncontrollable contamination from the peel during sectioning of the
orange for analysis. Peel representing 25% of the whole fruit
contained approximately 50 ppm of thiabendazole (on weight of peel).
All the thiabendazole applied as a suspension was still present as
intact thiabendazole after the four weeks of storage.
In several instances the stability of the sorbed thiabendazole - 14C
was investigated by extraction and by thin layer chromatography, with
and without prior addition of unlabelled carrier compound. Since the
radioactivity of the pulp did not increase with temperature or time of
storage above the initial value prior to storage, it may be concluded
that the thiabendazole content of the pulp due to diffusion of
absorbed fungicide into the interior during the four weeks of storage
is less than or equal to a detection limit of 0.01 ppm. Diffusion from
the surface of the fruit into the peel is appreciable an from 7-20% of
the measurable activity is observed in the inner peel. This diffusion
does not, however, extend to the pulp at the interior of the fruit.
Because of the widespread practice of waxing citrus after washing and
cleaning, the application of thiabendazole in the aqueous wax emulsion
is a convenient, practicable and efficient method of conferring
protection against fungal spoilage. Trials carried out in California
under controlled conditions in commercial packing houses resulted in
acceptable protection. The residues found on the treated fruit ranged
up to 4.3 ppm, and the results are set out in Table VIII. It is not
anticipated that the penetration into the peel will be any greater
when the treatment is applied via wax emulsion that it would from a
flooding, dipping or spraying treatment.
TABLE VIII
Residues of thiabendazole on whole fruit following application to citrus
of thiabendazole in wax emulsion
Storage Time
Variety Treatment Interval Residue
Temp. Humidity (days) (ppm)
Valencia
Oranges 0.3% TBZ in wax 21-24°C 90% 8 1.25
" 0.5% TBZ in wax 21-24°C 90% 8 2.16
" 0.3% TBZ in wax 21-24°C 90% 10 1.76
" 0.4% TBZ in wax 21-24°C 90% 10 2.20
" 0.5% TBZ in wax 21-24°C 90% 10 2.43
" 0.35% TBZ in wax 21-24°C 90% 10 2.15
" 0.5% TBZ in wax 21-24°C 90% 10 3.91
" 5267 ppm TBZ in wax 9-20°C 90% 31 4.3
Evidence of Residue in Food In Commerce
Shipments of commercially processed grapefruit which had been treated
by dipping in thiabendazole before being exported from U.S.A. were
sampled in Europe and systematically analysed for thiabendazole
residues. Ten fruits were homogenized from each shipment and analysis
was carried out on the homogenate. The results which are set out in
Table IX are slightly lower than those obtained from controlled
experiments carried out in Florida. It is notable that the residue on
grapefruit treated by flooding in a bath containing 3 000 ppm
thiabendazole are approximately twice as high as those found in fruit
treated in a similar manner in a bath containing 1 000 ppm
thiabendazole.
In order to determine the fruit-to-fruit variation in any one
consignment, nine separate fruit were taken from a consignment and
these were analysed individually. There was close agreement between
the results (range 0.26-0.55 ppm - standard deviation 0.09 ppm) with
the mean agreeing closely with the result obtained by analysis of a
homogenized sample of the same consignment.
TABLE IX
Residues of thiabendazole on whole citrus exported from U.S.A. and
analysed in Europe
Shipment Variety Treatment Residue on whole fruit (ppm) 1/
No. Rate Average Range
1 Grapefruit 1 000 ppm 0.22 0.18 - 0.25
3 000 ppm 0.44 0.32 - 0.63
2 " 1 000 ppm 0.23 0.20 - 0.29
3 000 ppm 0.57 0.46 - 0.86
3 " 1 000 ppm 0.17 0.14 - 0.18
1 000 ppm 0.13 0.10 - 0.16
3 000 ppm 0.27 0.23 - 0.30
4 " 1 000 ppm 0.17 0.15 - 0.19
1 000 ppm 0.18 0.16 - 0.21
3 000 ppm 0.24 0.21 - 0.28
5 " 1 000 ppm 0.39 0.37 - 0.42
1 000 ppm 0.33 0.21 - 0.28
3 000 ppm 0.86 0.72 - 0.96
1/ Variation in thiabendazole residue levels between individual fruits
Analysis of composite sample = 0.39 ppm
Analysis of individual fruits
0.53 0.37 0.38 Mean = 0.39 ppm
0.38 0.55 0.30 Standard Deviation 0.09 ppm
0.26 0.36 0.34 Range 0.26 - 0.55
Shipments of a variety of citrus exported from Israel to Europe were
sampled and analysed to determine the fruit-to-fruit variation.
Samples were taken of citrus prepared and packed by 13 separate
packing houses. In this case, the thiabendazole was applied as a
suspension in the wax emulsion used to treat the fruit after washing
and prior to packing in cartons. The wax contained 0.3% (3 000 ppm)
thiabendazole. The results given in Table X show a much higher level
of residues than those found following dipping. The fruit-to-fruit
variation was considerably greater and there was a significant
variation between different packing houses. Table XI shows results of
analysis of individual fruits from 7 packing houses in Israel
following treatment with 0.3% thiabendazole in wax emulsion.
TABLE X
Thiabendazole residues on Israeli citrus following application
of 0.3% thiabendazole in citrus wax
Packing No. of Fruit Average Standard Range
House Analysed ppm Deviation ppm
1 20 1.79 0.31 1.21 - 2.36
2 20 0.71 0.20 0.43 - 1.13
3 10 2.65 1.10 1.51 - 4.81
4 10 4.41 1.10 2.76 - 5.89
5 10 4.76 1.2 3.39 - 6.40
6 10 3.89 1.7 0.95 - 7.35
7 10 4.75 1.0 3.37 - 5.73
8 10 2.97 0.69 2.12 - 4.19
9 10 3.34 0.53 2.45 - 4.28
10 10 1.47 0.45 0.92 - 2.24
11 10 3.86 0.81 2.96 - 5.56
12 10 1.12 0.53 0.59 - 1.48
13 10 4.06 0.83 2.98 - 5.44
TABLE XI
Distribution of results of analysis of individual citrus
fruits treated with 0.3% thiabendazole in wax and analysed
one week after application
Range of residue levels Number of fruits Percent
less than 1.0 ppm 24 10
1.0 - 1.9 64 26
2.0 - 2.9 59 23
3.0 - 3.9 41 18
4.0 - 4.9 22 9
5.0 - 5.9 25 10
above 6 ppm 10 4
Total 245 100
Samples of oranges treated in South Africa by dipping in a suspension
containing 0.5% (5 000 ppm) thiabendazole which were analysed after
arrival in the United Kingdom contained between 0.28 ppm and 0.35 ppm
thiabendazole. The lower residue levels were found on fruit which had
received a water rinse after dipping. The higher residues were on
unrinsed fruit. Fruit dipped in suspensions at 43°C were found to have
residues of 0.80 ppm thiabendazole, twice the level found in those
dipped cold.
A further twenty samples representing various treatment levels, times,
temperatures and after-treatments showed residues ranging from 0.2 ppm
to 1.62 ppm. The lower levels were found in fruit treated at 1 000 ppm
and the higher level in fruit treated at 5 000 ppm. After-treatment
with citrus wax tended to reduce the residue levels to 0.15 ppm and
0.5 ppm, respectively.
METHODS OF RESIDUE ANALYSIS
Citrus
The method developed for the determination of residues in and on
citrus and bananas has been published by the U.S. Food and Drug
Administration (1969). The whole fruit is rinsed with ethyl acetate to
remove wax and thiabendazole from the surface. The pulp and peel are
homogenized with water. The thiabendazole is extracted from the
homogenates with ethyl acetate. The ethyl acetate solutions from
rinse, pulp and peel are combined. The solutions are purified by a
series of extraction procedures. The final solution in 0.1N
hydrochloric acid is measured spectrophotofluorometrically.
For routine testing of treated citrus, it is advisable to homogenize
about 10 fruits in a large chopper and to take a sub-sample of 60g of
puree for analysis. This is blended with an equal weight of sodium
acetate/sodium chloride solution, and from the slurry a 20 g sample is
taken for analysis. A number of minor variations have been introduced
into the extraction procedure to deal with various citrus fruits, the
separate components of the fruit and citrus products. When analysing
whole fruit, the method is sensitive to 0.1 ppm, but with pulp the
sensitivity is estimated to be 0.03 ppm and with peel 0.4 ppm. Blank
values for untreated pulp and peel range from 0.00-0.02 ppm and
0.00-0.40 ppm, respectively.
After purification, the extract is dissolved in 0.1N hydrochloric acid
and the fluorescence of the final acid solution is read on an
Amico-Bowman spectrophotofluorometer having an excitation wavelength
set at approximately 300mu and the mission wavelength set at 360mu.
Other instruments may operate more effectively at slightly different
wavelengths. The method is suitable for regulatory purposes but
requires a suitable spectrophotofluorometer.
Where a suitable spectrophotofluorometer is not available, the method
of Szalkowski (1965) has been modified by Gilbert (1969) to yield a
reliable colorimetric method with a sensitivity of 0.08 ppm and
recoveries between 93% and 110%. The blank varies between 0.02 and
0.04 ppm for both pulp and whole fruit. The thiabendazole is extracted
from homogenized fruit with acidic methanol and interference removed
with an alkaline wash followed by alumina column cleanup. The
thiabendazole is reduced to form hydrogen sulphide which complexes
with p-phenylene diamine. This complex in oxidized with ferric
solution to give a blue thiazine dye, which is determined
colorimetrically.
Bananas
The method described for the determination of thiabendazole in citrus
has been adapted to determine residues in banana pulp and peel by the
U.S. Food and Drug Administration (1969b).
The thiabendazole in extracted into ethyl acetate from a Ph 4.5
buffered suspension of ten grams of the banana pulp or peel. The
combined ethyl acetate extract is washed twice with 0.05N sodium
hydroxide. Then the thiabendazole is returned to an aqueous phase by
extraction with 0.1N hydrochloric acid and determined by fluorescence,
the method described for citrus. The sensitivity of this method is
0.05 ppm.
Animal tissues
The spectrophotofluorometric method has been used successfully for the
determination of thiabendazole and its 5-hydroxy derivative in animal
tissue. For liver and kidney tissue a preliminary digestion with
enzyme in necessary to convert the metabolite conjugates to 5-hydroxy
thiabendazole. The sensitivity is about 0.1 ppm.
Milk samples from animals receiving thiabendazole therapy or being fed
on citrus residues containing thiabendazole may be analysed for
thiabendazole by spectrophotofluorometric method using 1-5 ml samples.
For milk, as little an 0.05 ppm may be recovered.
NATIONAL TOLERANCES
Country Commodity Tolerance,ppm
U.S.A. Bananas 3
Banana pulp 0.4
Citrus 2
Citrus from wax treatment 6 (proposed)
Citrus pulp (dried) 8
Edible tissues of cattle,
sheep, goats and pigs 0.1
Milk (negligible residue) 0.05
(Cont'd)
Country Commodity Tolerance,ppm
Canada Bananas 3
Banana pulp 0.4
Citrus 2
Australia Bananas 3
Banana pulp 0.4
Citrus 2
Meat of cattle, sheep, 0.2
goats and pigs
Milk 0.05
France Bananas 3
Banana pulp 0.4
Citrus (provisional) 6
Netherlands Citrus (whole) 6
Citrus with diphenyl 3
Sweden Banana (whole) 3
Banana (pulp) 0.4
Citrus (whole) 6
E.E.C. Bananas (whole) 6
Citrus (whole) 6
Germany Citrus 6
Bananas 3
Belgium Bananas 3
Citrus 6
New Zealand Bananas 3
Citrus 2
Norway Citrus 6
Bananas 3
Finland Citrus 6
Italy Citrus (when used alone) 6
Citrus (when used with
other antifungals) 3
Bananas 3
APPRAISAL
Thiabendazole is a particularly effective fungicide for the
post-harvest treatment of bananas and citrus. It has been used very
extensively since 1960-62 as an anthelmintic for sheep, cattle, pigs
and horses and later as a vermifuge in human beings.
Post-harvest dips containing 200 ppm thiabendazole protect bananas
against the most important fungal rots. Treatment of green bananas
during picking and packing operations ensures that the fruit may be
shipped to distant markets with minimum loss or deterioration.
Likewise post-harvest sprays, dips and wax coatings containing
thiabendazole at concentrations of 1 000 to 3 000 ppm have proved
outstandingly useful. Diphenyl impregnated wraps and interlinings
have been used for many years to reduce Penicillium sporulation, and
sodium ortho-phenylphenate dips have been used against stem and
rots. Both treatments have many disadvantages and objections, and it
is anticipated that thiabendazole will provide a more acceptable
alternative to both treatments.
Data on the level of residues in whole fruit, peel, pulp and processed
citrus products, as well as in green and ripe bananas, were available
from U.S.A., West Indies, Australia, Israel and Europe. Results of
residue analysis of commercial citrus shipments to Europe were also
available and were not significantly different from those resulting
from supervised trials.
Thiabendazole has high stability on and in fruit and under conditions
of use no metabolites or degradation products are likely to occur. The
residue levels do not decline significantly in storage.
Several methods of residue analysis are available. The most convenient
and sensitive method employs spectrophotofluorometric measurement of
the thiabendazole in dilute hydrochloric acid solution. The method may
be adapted to determine thiabendazole residues in a variety of fruit
products and in animal tissues and milk. The sensitivity varies with
the substrate but ranges from 0.01 ppm to 0.04 ppm. This sensitivity
is adequate for the level of residues likely to occur and the proposed
tolerances. The method is suitable for regulatory purposes and is
specific to thiabendazole. de Vos and Bosma examined the possibility
of using gas chromatography with sulphur specific detector; they
showed that it was possible to determine thiabendazole residues to a
limit of 0.1 ppm by using the ethyl acetate extract of raw fruit for
injection into the chromatograph. They found that the method using
concentrated crude ethyl acetate extracts was satisfactory, but it led
to rapid fouling of the chromatographic column.
The administration of thiabendazole to sheep and cattle as an
anthelmintic gives rise to low level transient residues in several
edible tissues especially kidney and liver, but these residues are
eliminated within three days. Lactating dairy animals receiving a
therapeutic dose of thiabendazole excrete detectable amounts in the
milk for 24 hours post treatment. However, animals with severe
helminthiasis and liable to be dosed with thiabendazole are rarely
suitable for slaughter or for production of milk for human
consumption. Furthermore, it would be very unusual to treat more than
a small proportion of a herd at any one time and thereby to exceed the
detectable level of about 0.05 ppm in meat or milk. Under these
circumstances it does not appear necessary to recommend a tolerance
for thiabendazole residues in meat or milk.
RECOMMENDATIONS FOR TOLERANCES
TOLERANCES
The following tolerances are recommended for thiabendazole:
Crop Residues ppm
bananas, whole 3
banana pulp 0.4
citrus, whole 6
FURTHER WORK OR INFORMATION
DESIRABLE
Studies to determine the effect of thiabendazole on the thyroid gland.
REFERENCES
Anon. (1967) Control of squirter disease of bananas by thiabendazole.
Agricultural Gazette of New South Wales, 78:604
Anon. (1969) New treatments reduce fruit wastage - Rural Research in
CSIRO, 67:7-12
AOAC. (1966) Analysis of thiabendazole in feeds. J.A.O.A.C.
49:238-239 and 312
Bondoux, P. (1967) Acac. Agric. France, Compt. Rend. Hebd. Seances,
53: 1314-1323
Brown, G.E., McCormack, A.A. and Smoot, J.J. (1967) Thiabendazole as a
post-harvest fungicide for Florida citrus fruit. Plant Disease
Reporter, 52:95-97
Brown, H.D., et al. (1961) Journal of American Chemical Society,
83:1764
Burden, O.J. (1967) Studies on grown rot on bananas. Queensland
Agricultural Journal, March, p. 186
Crivelli, G. (1966) Part V Tests with the fungistat thiabendazole
against Penicillium of oranges (in Italian). Il. Freddo, 20:25-29
Cuille, J. and Bur-Ravault, L. (1968) Fruits, (Paris), 23:351-356
Eckert, J.W., Kolbenz, M.J. and Kraght, A.J. (1968) Proc.
International Citrus Symposium, Vol 3, Riverside, Cal., 13 March 1968
Fripp, Ivonne J. and Dettman, E. Belinda. (1969) Thiabendazole as a
post-harvest treatment against Sclerotinia fructicola in dessert
peaches. Australian Journal of Experimental Agriculture and Animal
Husbandry, 9:9-11
Gilbert, W.S. and Wright, Julia. (1969) Method for determining
thiabendazole residues on oranges
Gordon, H. McL. Studies of anthelmintics for sheep - thiabendazole.
Australian Veterinary Journal, 40(1):9-18
Harding, P.R. Jr. (1968) Plant Disease Reporter, 52: 623-625
Johnson, C.D. (1964) Evaluation of teratogenic potential in the rat.
Unpublished Report (April 1964) from Woodard Research Corporation
through Merck Institute for Therapeutic Research to FDA
McCormack, A.A. and Brown, G.E. (1968) Thiabendazole, an experimental
fungicide for fresh citrus fruit. Proc. Florida State Horticultural
Soc., 80:232-237
Merck and Company, Inc. (1969) Rahway, New Jersey, U.S.A.
thiabendazole residues in bananas and citrus. Submission to U.S. Food
and Drug Administration
Merck and Company, Inc. (1970) Rahway, New Jersey, U.S.A.
Thiabendazole residues in citrus following application in citrus wax.
Submission to U.S. Food and Drug Administration
Robinson, H.J., Stoerk, H.C. and Graessle, A.E. (1965b) Studies on the
toxicological and pharmacological properties of thiabendazole.
Toxicol. appl. Pharmacol., 7:53-63
Rosenblum, C. and Meriwether, H.T. (1970) Determination by the
radioactive indicator Method of the retention and stability of
thiabendazole in treated Valencia orange. Journal of Radioanalytical
Chemistry, (in press)
Scott, K.J., and Roberts, E.A. (1967) Control in bananas of black end
rot caused by Gloeosporium musarium. Australian Journal of
Experimental Agriculture and Animal Husbandry, 7:283-286
Seberry, J.A., and Baldwin, R.A. (1968) Thiabendazole and
2-amino-butane as post-harvest fungicides for citrus. Australian
Journal of Experimental Agriculture and Animal Husbandry, 8:440-443
Seberry, J.A. (1968) Proc. Int. Citrus Symposium, Riverside, Calif.,
13 March 1968
Shillingsford, C.A. (1970) Banana fruit rot control in Jamaica.
P.A.N.S.,16(1)
Szalkowski and Kanora. (1965) Spectrophotometric determination of TBZ
in feed. JAOAC,48(2):288-295
Tocco D.J., Buhs, R.P., Brown, H.D., Matzuk, A.R., Mertel, H.E.,
Harman, R.E. and Trenner, N.R. (1964) The metabolic fate of
thiabendazole in sheep. J. med. Chem., 7:399-405
Tocco, D.J., Egerton, J.R., Bowers, W., Christensen, V.W. and
Rosenblum, C.J. (1965) Absorption, metabolism and elimination of
thiabendazole in farm animals and a method for its estimation in
biological materials. J. Pharmacol. exp. Therap., 149:263-271
Tocco, D.J., Rosenblum C., Martin. C.M. and Robinson, H.G. (1966)
Absorption, metabolism and excretion of thiabendazole in man and
laboratory animals. Toxicol. appl. Pharmacol., 9:31-39
U.S. Food and Drug Administration. (1969) Thiabendazole residues in
citrus fruits. Pesticide analytical manual, Vol II, Section 120.242
U.S. Food and Drug Administration. (1969b) Thiabendazole residues in
banana peel and pulp. Method A. Pesticide analytical manual, Vol II,
Section 120.242.
Vogin, E.E. (1968) Multigeneration reproduction and lactation studies
with thiabendazole. Unpublished reports (Dec. 1967 and March 1968).
Food and Drug Research Laboratories, through Merck Institute for
Therapeutic Research to FDA
de Vos, R.H. and M.P. Bosma. (1970) Residues of thiabendazole in
citrus fruit. Report 3199. TNO Central Instituut voor
Voldingsonderzoek, Ziest, Netherlands
Weinke, K.E., Lauber, J.J., Greenwald, B.W. and Preiser, F.A. (1969)
Thiabendazole, a new systemic fungicide. Proc. 5th Brit. Insectic.
Fungic. Conference
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1964) Safety evaluation
by oral administration to dogs and rats for 104 weeks. Unpublished
Report (April 1964). Woodard Research Corporation through Merck
Institute for Therapeutic Research to FDA
 Molecular Weight 201.3
Other information on identity and properties
A stable, white crystalline powder. Solubility in water at pH 2.2 =
3.84 percent. Solubility decreases at higher or lower pH values. Very
soluble in dimethyl formamide, alcohols and acetone. Soluble in
chlorinated hydrocarbons, esters and ether.
No change in ultraviolet absorption occurs in samples stored eight
days at 100°C. Heating at 220°C at atmospheric pressure has no
apparent effect on its antifungal properties.
Technical grade contains not less than 97 percent thiabendazole. There
are no related impurities in the technical grade. Melting point is
296-303°C. Pure products melt at 304-305°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Following a dose of 100 mg/kg body-weight, thiabendazole labelled with
14C in the benzene ring was rapidly absorbed from the
gastrointestinal tract of rats, with a maximum concentration found
from two to three hours after initial treatment. Radioactivity
gradually disappeared from the blood, and approximately 92 percent of
a dose of 25 mg/kg and 80 percent of a 100 mg/kg dose was excreted in
the urine and faeces within three days. Most of the drug and its
metabolites were excreted within the first 24 hours. Of the
metabolites, 50 percent appeared as the glucuronide of
5-hydroxythiabendazole (II) and 40 percent as the sulfate ester of the
same aglycone (III) (see Fig 1). Traces of unchanged thiabendazole and
5-hydroxythiabendazole were also evident (Robinson, 1965a).
Dogs given a single oral dose of 50 mg/kg body-weight of 14C-labelled
thiabendazole were found to have maximum plasma levels within two
hours. Excretion was essentially complete in eight days with
approximately 35 percent of the dose appearing in the urine and 47
percent appearing in the faeces (Robinson 1965a).
Sixteen male humans were administered thiabendazole at dosages of 1 to
2 grams per person in the form of tablets, wafers, capsules or
suspension. The material was rapidly absorbed, with peak plasma
concentrations observed about one hour after treatment. Plasma drug
levels declined rapidly thereafter and reached essentially zero values
between 24 and 48 hours. Thiabendazole and its metabolic products were
excreted rapidly in the urine and faeces in 48 hours, which accounted
for 87 to 100 percent of the dose. Approximately half of the material
in the urine was associated with compounds identified as the
glucuronide and sulfate esters of 5-hydroxythiabendazole (II and III).
In plasma, both unchanged thiabendazole and free
5-hydroxythiabendazole (I) were also found. At the higher dosage
levels, maximum drug levels in plasma appeared at three hours
following dosing. These studies were done utilizing 14C-labelled and
unlabelled thiabendazole (Robinson, 1965a; Tocco et al., 1966).
Information is also available on the metabolism of thiabendazole in
sheep goats and cattle. In cows, about 0.1 percent of an oral dose was
detectable in the milk within 60 hours. Details of this work are given
in the section entitled "Fate of residues, In animals".
Based upon all the metabolism studies, the pathway for thiabendazole
metabolism is represented by Fig. 1.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Mouse
A multi-generation reproduction study for five generations utilized 25
male and 25 female mice given diets containing thiabendazole at
concentrations of 0, 200, 1000 and 5000 ppm. When the mice attained
the age of eight weeks, they were mated and continued on the same
diet. The young from these matings, when they were weaned, were
maintained on the test diet and mated when they reached the age of
eight weeks. This procedure continued for five complete reproductive
cycles. No effects were noted at 200 ppm. At 1000 ppm, slight decrease
in the weights of weanlings was observed in all five generations. At
5000 ppm, the number of mice born and weaned per litter were reduced,
and a marked reduction in the average weanling weight of the young was
observed (Robinson, 1965a).
Rat
A three-generation, two-litter reproduction study (10 males and 10
females per group) at dosages of 0, 20, 40 and 80 mg/kg body-weight
per day showed no adverse effect on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight, but not growth
rate, and decreased food consumption in the male rats at all dosage
levels in the F1 and F2 generations. Slight decreases in final
body-weights and food consumption at the 80 m/kg dose level in the F1
and F2 generation females was also observed (Vogin,1968).
Two groups of rats (20 male and 20 female) were placed on a diet
containing 0 or 500 ppm thiabendazole. There were no abnormalities
among the young from either mating attributable to thiabendazole at
500 ppm. It was concluded that no evidence of teratogenesis is present
in the data from this particular study (Johnson, 1964).
Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg body weight at 2
1/2 to 8 weeks prior to lambing without any effect on the birth rate,
growth or viability of the lambs. This dose had no effect on the ewes
throughout the test interval or on viability of the lambs to six weeks
of age. Miscellaneous tests using 39 ewes produced 29 weanling lambs
after treatment with thiabendazole at 400 mg/kg, whereas, from a
control group of 22 ewes, 13 lambs were weaned. Ewes tolerated a
single dose of 18 grams per animal (equivalent to 235 to 335 mg/kg)
three to ten days after lambing without any effect on the weight or
food intake of the ewes or survival growth of the lambs up to weaning
time of six weeks (Robinson, 1965a).
Special studies on cardiovascular and respiratory effects
Oral doses of thiabendazole (4000 mg/kg) produced no striking acute
pharmacological effects on blood pressure or respiration in either
cats or dogs. Similarly, there were no alterations in the
electrocardiogram (Robinson, 1965a, 1965b).
Special studies on eye irritation
Other than a very slight erythema observed for approximately one hour
after application of 1 ml of thiabendazole as a 12 percent suspension
in sodium carboxymethyl cellulose or 10 mg of the dry powder to the
conjunctival sac of two rabbits, no evidence of irritation was noted
(Robinson, 1965a, 1965b).
Molecular Weight 201.3
Other information on identity and properties
A stable, white crystalline powder. Solubility in water at pH 2.2 =
3.84 percent. Solubility decreases at higher or lower pH values. Very
soluble in dimethyl formamide, alcohols and acetone. Soluble in
chlorinated hydrocarbons, esters and ether.
No change in ultraviolet absorption occurs in samples stored eight
days at 100°C. Heating at 220°C at atmospheric pressure has no
apparent effect on its antifungal properties.
Technical grade contains not less than 97 percent thiabendazole. There
are no related impurities in the technical grade. Melting point is
296-303°C. Pure products melt at 304-305°C.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Following a dose of 100 mg/kg body-weight, thiabendazole labelled with
14C in the benzene ring was rapidly absorbed from the
gastrointestinal tract of rats, with a maximum concentration found
from two to three hours after initial treatment. Radioactivity
gradually disappeared from the blood, and approximately 92 percent of
a dose of 25 mg/kg and 80 percent of a 100 mg/kg dose was excreted in
the urine and faeces within three days. Most of the drug and its
metabolites were excreted within the first 24 hours. Of the
metabolites, 50 percent appeared as the glucuronide of
5-hydroxythiabendazole (II) and 40 percent as the sulfate ester of the
same aglycone (III) (see Fig 1). Traces of unchanged thiabendazole and
5-hydroxythiabendazole were also evident (Robinson, 1965a).
Dogs given a single oral dose of 50 mg/kg body-weight of 14C-labelled
thiabendazole were found to have maximum plasma levels within two
hours. Excretion was essentially complete in eight days with
approximately 35 percent of the dose appearing in the urine and 47
percent appearing in the faeces (Robinson 1965a).
Sixteen male humans were administered thiabendazole at dosages of 1 to
2 grams per person in the form of tablets, wafers, capsules or
suspension. The material was rapidly absorbed, with peak plasma
concentrations observed about one hour after treatment. Plasma drug
levels declined rapidly thereafter and reached essentially zero values
between 24 and 48 hours. Thiabendazole and its metabolic products were
excreted rapidly in the urine and faeces in 48 hours, which accounted
for 87 to 100 percent of the dose. Approximately half of the material
in the urine was associated with compounds identified as the
glucuronide and sulfate esters of 5-hydroxythiabendazole (II and III).
In plasma, both unchanged thiabendazole and free
5-hydroxythiabendazole (I) were also found. At the higher dosage
levels, maximum drug levels in plasma appeared at three hours
following dosing. These studies were done utilizing 14C-labelled and
unlabelled thiabendazole (Robinson, 1965a; Tocco et al., 1966).
Information is also available on the metabolism of thiabendazole in
sheep goats and cattle. In cows, about 0.1 percent of an oral dose was
detectable in the milk within 60 hours. Details of this work are given
in the section entitled "Fate of residues, In animals".
Based upon all the metabolism studies, the pathway for thiabendazole
metabolism is represented by Fig. 1.
TOXICOLOGICAL STUDIES
Special studies on reproduction
Mouse
A multi-generation reproduction study for five generations utilized 25
male and 25 female mice given diets containing thiabendazole at
concentrations of 0, 200, 1000 and 5000 ppm. When the mice attained
the age of eight weeks, they were mated and continued on the same
diet. The young from these matings, when they were weaned, were
maintained on the test diet and mated when they reached the age of
eight weeks. This procedure continued for five complete reproductive
cycles. No effects were noted at 200 ppm. At 1000 ppm, slight decrease
in the weights of weanlings was observed in all five generations. At
5000 ppm, the number of mice born and weaned per litter were reduced,
and a marked reduction in the average weanling weight of the young was
observed (Robinson, 1965a).
Rat
A three-generation, two-litter reproduction study (10 males and 10
females per group) at dosages of 0, 20, 40 and 80 mg/kg body-weight
per day showed no adverse effect on reproduction, lactation or
histomorphology in the three generations of rats examined. The only
treatment-related findings were decreased body-weight, but not growth
rate, and decreased food consumption in the male rats at all dosage
levels in the F1 and F2 generations. Slight decreases in final
body-weights and food consumption at the 80 m/kg dose level in the F1
and F2 generation females was also observed (Vogin,1968).
Two groups of rats (20 male and 20 female) were placed on a diet
containing 0 or 500 ppm thiabendazole. There were no abnormalities
among the young from either mating attributable to thiabendazole at
500 ppm. It was concluded that no evidence of teratogenesis is present
in the data from this particular study (Johnson, 1964).
Sheep
Pregnant ewes tolerated a single dose of 400 mg/kg body weight at 2
1/2 to 8 weeks prior to lambing without any effect on the birth rate,
growth or viability of the lambs. This dose had no effect on the ewes
throughout the test interval or on viability of the lambs to six weeks
of age. Miscellaneous tests using 39 ewes produced 29 weanling lambs
after treatment with thiabendazole at 400 mg/kg, whereas, from a
control group of 22 ewes, 13 lambs were weaned. Ewes tolerated a
single dose of 18 grams per animal (equivalent to 235 to 335 mg/kg)
three to ten days after lambing without any effect on the weight or
food intake of the ewes or survival growth of the lambs up to weaning
time of six weeks (Robinson, 1965a).
Special studies on cardiovascular and respiratory effects
Oral doses of thiabendazole (4000 mg/kg) produced no striking acute
pharmacological effects on blood pressure or respiration in either
cats or dogs. Similarly, there were no alterations in the
electrocardiogram (Robinson, 1965a, 1965b).
Special studies on eye irritation
Other than a very slight erythema observed for approximately one hour
after application of 1 ml of thiabendazole as a 12 percent suspension
in sodium carboxymethyl cellulose or 10 mg of the dry powder to the
conjunctival sac of two rabbits, no evidence of irritation was noted
(Robinson, 1965a, 1965b).
 Special studies on dermal irritation
There was no evidence of a significant degree of irritation when 0.5
gm thiabendazole was applied in cold cream at concentrations of 10 and
50 percent (w/w) for 24 hours to intact rabbit skin (Robinson, 1965a,
1965b).
Acute toxicity
LD50 doses for various animal species are given in Table I.
Toxic signs observed following administration of large doses of
thiabendazole by oral or intraperitoneal routes were generally similar
and consisted of lethargy and stupor. The intravenous administration
of a large dose of the hydrochloride produced narcosis. Death appeared
to be due to respiratory failure. Rabbits that survived large doses
initially lost weight but recovered after several days (Robinson,
1965a).
Short-term studies
Calf
Five female Holstein calves (seven months old) were fed a diet
containing 0, 320, 1 000, 3 200 or 10 000 ppm of thiabendazole for a
14-week test period. Calves tolerated 3 200 ppm thiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg body-weight. At the 10 000 ppm thiabendazole level, the
calves grew normally for the first two weeks, but in the following 12
weeks the weight gain was about half that observed for the controls.
Gross examination at the time of autopsy revealed no pathological
condition, and histological examination of several tissues showed no
change as resulting from the incorporation of thiabendazole in the
diet (Robinson, 1965a).
Chicken
Groups of 0.5 week old male White Leghorn chicks (10 to 20 per group)
were fed thiabendazole in the diet at dosage levels ranging from 1 to
10 000 ppm for a period of 2 1/2 weeks. This level corresponded to a
mean daily intake of thiabendazole ranging from 0.1 mg/kg to 1 200
mg/kg. A gradual decrease in growth appeared to occur with
concentrations of 100 ppm thiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg per day. Gross pathological
examination at autopsy of the chicks on a dietary level of 4 000 ppm
thiabendazole showed them to be normal except for a smaller size
(Robinson, 1965a).
Dog
Groups comprising two male and two female dogs were orally
administered thiabendazole daily for periods of over two years at
doses of 0, 20, 100 and 200 mg/kg body-weight/day. No clinical signs
of morbidity were observed; food and water consumption were normal;
blood and urine chemistry were normal; a slight retardation in
body-weight gain at the 200 mg/kg level was observed, which was
accompanied by a slight reduction in total erythrocyte count. At this
level, changes in haemoglobin concentration and haematocrit were also
observed. No gross pathological observations were noted at the
conclusion of this test, and organ-weights appeared normal.
TABLE I
Thiabendazole toxicity in various animal species
LD50
Animal Route Form Solvent (mg/kg body-weight) Reference
Mouse oral HCl Water 2 400 (Robinson 1965a, 1965b)
ip HCl Water 430 " " "
iv HCl Water 150 " " "
oral Base CMC 2% 1 3 810 " " "
Rat oral HCl Water 3 600 " " "
ip HCl Water 1 850 " " "
iv HCl Water 130 " " "
oral Base CMC 2% 3 330 " " "
Rabbit oral Base CMC 2% 3 850 " "
Sheep drench HCl Water 2 000 " "
Goat drench HCl Water >2 000 " "
1 CMC = carboxy methyl cellulose
A significant haemosiderosis was present in dogs at the 100 and 200
mg/kg level in the spleen, liver, lymph nodes and bone-marrow. This
was not associated with an increase in serum haemoglobin. No
significant toxicological effects were noted at 20 mg/kg/day
(Robinson, 1965a, 1965b).
Groups of dogs (three male and three female) were orally administered
thiabendazole for two years at doses of 0, 20, 50 and 125 mg/kg
body-weight/day. At the 125 mg/kg/day level, two of six dogs died. One
of these dogs had marked liver cirrhosis, seminal tubular
degeneration, bone marrow atrophy and degenerative renal changes.
Slight to moderate reduction in haemoglobin and packed cell volume
occurred. Elevated blood urea nitrogen, serum alkaline phosphatase and
serum glutamic oxal oacetic transaminase were evident. Urinary albumin
was seen more frequently than in controls. Inspissated bile entwined
in the gall bladder villi was also observed in one dog. One dog of
this group was sacrificed and was found to have pulmonary arteritis of
parasitic origin. At 50 mg/kg/day, growth of male dogs was slightly
depressed. All six dogs given thiabendazole at 50 mg/kg survived the
study, and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa in
one. Five of six dogs survived thiabendazole at 20 mg/kg for the term
of the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes in
all treated dogs (Woodard et al., 1964).
Pig
Five groups of four barrows each were given a diet containing 0, 320,
1 000, 3 200 and 10 000 ppm thiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects for
the 14-week period. This concentration corresponded to an intake of 15
mg/kg body-weight per day. Concentration of 10 000 ppm thiabendazole
in the diet caused reduced weight gains and reduced food consumption
with no mortality observed. Gross pathology examination revealed no
abnormal conditions (Robinson, 1965a).
Rat
Rats (10 males and 10 females per group) were administered
thiabendazole once daily at dosage levels of 0, 100, 400, 800, 1 200
and 1 600 mg/k-g body-weight per day. During the 30-day experimental
period, rats in the 800 mg/kg per day group decreased their food
intake and gradually lost weight. They were slightly less active, had
ruffled fur and became slightly flaccid. Three males and three females
died during the course of the experiment. All of the rats in the 1 200
and 1 600 mg/kg groups died during the 30-day test period. Rats dosed
at 100 and 400 mg/kg appeared normal throughout the test period,
although a slight depression in body-weight gain was noted in males at
100 mg/kg per day and in females at 400 mg/kg per day. Haematologic
studies on rats receiving 800 mg/kg per day showed a mild neutrophilia
with concurrent lymphopenia. There was also a suggestion of a decrease
in red blood cell elements. No gross anatomical changes were noted in
rats dosed with 100 mg/kg per day, whereas male and female rats
treated with 400 mg/kg per day showed thymic involution. A slight
enlargement of the liver was also noted in females at 400 mg/kg per
day. Animals surviving 800 mg/kg per day showed gross signs indicating
starvation where normal body fat depot and subcutaneous fat appeared
to be depleted. Liver and adrenals were slightly enlarged in males and
females and the thymus involuted. Microscopic pathology showed bone
marrow hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg (Robinson, 1965a, 1965b).
Rats (30 males and 30 females per group) were administered
thiabendazole suspended in 1.0 percent methocel daily for 180 days at
doses of 0, 12.5, 25, 50, 100, 200 and 400 mg/kg body-weight. All of
the animals survived throughout the duration of the experiment. At 400
mg/kg, a depression in body-weight was observed with both sexes. At
200 mg/kg per day, the male rats showed a slight depression in weight
gain. No clinical signs of toxicity were observable at levels below
100 mg/kg. Haematological studies at 400 mg/kg per day indicated a
slight suggestion of a fall of the red blood cell elements which was
not evident at levels below this dose. Biochemical blood studies and
urinalysis were normal at all dosage levels with a slight polyurea in
both male and female rats at 400 mg/kg, which was apparently the
result of a slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and females at
400 mg/kg per day and in females at 200 mg/kg per day. There was an
increase in liver size at doses of 100 mg/kg and above in males and at
25 mg/kg per day and above in females. Males dosed with 200 and 400
mg/kg and females at 400 mg/kg showed an apparent increase in kidney
weight. Microscopic examination of animals at 100 mg/kg showed a small
incidence of haemosiderosis of the thymus. At 200 and 400 mg/kg, these
examinations showed considerably more haemosiderosis of the thymus and
colloid depletion in the thyroid (Robinson, 1965a, 1965b).
Sheep
Thirty weanling wethers were employed in a 16-week study at doses of
0, 10, 50, 100, 200, 400 and 800 mg/kg body-weight per day.
Thiabendazole was administered in gelatin capsules. Two of the four
sheep dosed at 100 mg/kg did not survive the test period. However,
because of infection, thiabendazole may not have been directly related
to the cause of their death. All the animals treated at 200 mg/kg per
day and above died before the end of the test interval. No significant
effects on blood biochemistry or urinalysis were observed. Doses of 50
mg/kg and above affected body-weight gain, after approximately 120
days on the test. Doses of 10 mg/kg had no effect. Food consumption
was unaffected at doses of 10 mg/kg per day, but a minor decrease was
noted at 50 and 100 mg/kg per day and a significant decrease at 200
mg/kg per day and above. No gross pathological changes were observed
at doses below 200 mg/kg per day. Starvation and loss of body-weight
complicated the interpretation of data concerning organ-weights at 200
mg/kg and above. At 200 to 800 mg/kg, a moderate hypoplasia of the
bone marrow with replacement by adipose tissue was observed. Loss of
colloid in thyroid was evident at 800 mg/kg, as well as lymphoid
atrophy at the higher dose levels (Robinson, 1965a).
Five groups of two ewe lambs (10 to 12 weeks old) and eight wether
lambs (10 to 19 weeks old) were fed diets containing 0, 100, 320, 1
000 and 3 200 ppm thiabendazole for periods up to 50 weeks. Sheep 10
weeks of age tolerated 1 000 ppm thiabendazole in the diet for the
duration of the experiment. (This dose corresponded to 30 to 50 mg/kg
body-weight per day.) The sheep tolerated 3 200 ppm in their diet for
the first 14 weeks but thereafter lost weight in the final stages of
the experiment (Robinson, 1965a).
Long-term studies
Rat
Groups of rats (35 of each sex) were fed dietary levels adjusted to
provide 0, 10, 40 or 160 mg/kg body-weight per day of thiabendazole
for up to two years. At the 160 mg/kg level, a reduction of weight
gain of about 25 percent, with concomitant reduction in food
consumption and slightly reduced haemoglobin and microhaematocrit
values, were the only changes seen attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidents or location of neoplasms and histopathological examination
of the tissues. No effect on food consumption or haemograms were seen
at either the 10 or 40 mg/kg levels. Very slight depression in growth
rate, of questionable significance, was observed in the male rats at
40 mg/kg but not at 10 mg/kg. At the 10 mg/kg level, the mean absolute
thyroid weights of the male rats (including the individual thyroid
weights from five out of eight animals tested) were heavier than the
mean thyroid weight of the controls. This effect was not observed in
the rats given 40 or 160 mg/kg (Woodard et al., 1964).
OBSERVATIONS IN MAN
Human subjects have ingested a single oral dose of 2 g (approximately
30 mg/kg body-weight) of thiabendazole in studies designed to
ascertain the metabolic fate (see "Biochemical aspects"). No
information was given on the toxicological effects, if any (Robinson,
1965a).
COMMENTS
Metabolic studies indicate a rapid absorption of thiabendazole from
the gastrointestinal tract of rats, dogs, sheep and goats with
excretion in urine and faeces complete within 3-8 days. In cows,
approximately 0.1 percent of an oral dose was detectable in milk
within 60 hours. In excreta of man and all animals studied,
metabolites include 5-hydroxythiabendazole, free and conjugated as a
glucuronide and sulfate ester, as well as traces of unchanged
thiabendazole. Slight differences in man and other animals were noted
in quantitative recovery of the various compounds. A common effect for
several animal species at high dose levels of thiabendazole is
retarded growth.
Short-term studies in rate, sheep, pigs, calves and chickens have been
carried out with oral administration or with incorporation of
thiabendazole in the diet for periods of 4-50 weeks. At higher doses
in rats, thymus involution, colloid depletion in the thyroid and
increase in liver and kidney size was observed. Loss of the colloid in
thyroid of sheep administered 200 mg/kg body-weight was also evident.
Oral administration of 12.5 mg/kg for 180 days did not cause
significant toxicological changes in rats.
In a two-year dog study a no-effect level of 20 mg/kg body-weight was
demonstrated. A two-year study in rats showed heavier thyroid glands
at 10 mg/kg body-weight/day in the male animals; this effect, however,
was not dose dependent.
A five-generation reproduction study in mice showed no adverse effects
at 200 ppm level. In a three-generation study in rats, only decreased
body-weight in relation to decreased food consumption could be
observed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 20 mg/kg body-weight/day
Mouse: 200 ppm in the diet, equivalent to 30 mg/kg body-weight/day
Rat: 10 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.05 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Administration to animals
Thiabendazole is widely used as a broad spectrum anthelmintic for
sheep, cattle, horses, pigs and goats. It is also administered as a
vermifuge directly to man. Following the announcement by Brown (1961)
of the chemotherapeutic properties of benzimidazoles, many
investigators report the efficacy of thiabendazole as an anthelmintic.
Gordon (1964) generally reported on its properties. Since that time
very large numbers of sheep and cattle have been treated with
thiabendazole suspensions. In sheep a dose of 44 mg/kg is adequate in
moot circumstances. Dosing may be repeated as frequently as once per
month, depending upon the level of reinfestation. In cattle a dose of
88 mg/kg is usually employed and in pigs 50-100 mg/kg.
Usually the dose is administered by drenching (oral administration of
an aqueous suspension) but for convenience it is possible to
administer via the feed when dealing with individual animals. At the
present time, economic considerations probably preclude the use of
thiabendazole as a feed additive.
Pre-harvest treatment of plants
Thiabendazole has been evaluated and has proved effective for use as a
fungicide on a number of crops including apples, pears, peaches,
apricots and bananas but, as far as is known, pre-harvest treatments
have not yet been adopted commercially. Preliminary results of residue
trials indicate that the residues from such pre-harvest uses will vary
considerably due to the systemic action of thiabendazole and the
ability of plants to take it up from the soil (Wernke, 1969).
Post-harvest treatment
Bananas
Scott (1967), Burden (1967), Anon (1967, 1969), Cuille (1968) and
Shillingsford (1970) have shown that thiabendazole is highly effective
for control of post-harvest fungal rots of bananas, when the picked
fruit is dipped in a suspension of thiabendazole containing from 100-1
000 ppm. A dip containing 200 ppm (0.02%) appears to be the most
widely used. Post-harvest spray treatments have also proved effective
in experimental use, but it is not known whether these are yet used in
practice.
Thiabendazole is most soluble in acid solution and can be rendered
soluble by treatment with lactic acid. Considerable experimental work
was carried out with lactic acid solutions as fruit dips, but in spite
of the convenience, the acidic solution gave rise to corrosion
problems in packing houses and during transport and resulted in
decomposition of the thiabendazole. Such solutions have been abandoned
in favour of micro-fine powder suspensions which require minimal
agitation to maintain uniform concentrations.
After the bunches of bananas have been picked, they are usually washed
in water or a solution of sodium hypochlorite. The bunches are then
separated into hands or clusters of 10-12 fruit which is allowed to
"bleed" in water for approximately 15-30 minutes. This facilitates the
removal of natural latex which, if allowed to run on the fruit, will
result in a visual disfigurement. The wet fruit is then dipped for 2-4
minutes in a suspension containing 200 ppm thiabendazole before being
drained for packing into cartons. No visible residue results from the
treatment.
Citrus
Crivelli (1966) and his associates in Italy first demonstrated that
Penicillium decay of oranges could be prevented by dipping fruit in
solutions containing 750-1 000 ppm thiabendazole dissolved in lactic
acid. Confirmation by other laboratories indicated that concentrations
of 200 to 500 ppm are adequate to give excellent control of
Penicillium decay. Brown (1967), Eckert (1968), Harding (1968),
McCormack (1968), Seberry (1968) show the efficacy of thiabendazole in
controlling Penicillium, and Seberry (1968) gives data showing
reduction in stem end rot caused by Diaporthe citri after dipping
oranges in suspensions of 500-1 000 ppm. Spraying or dipping citrus
fruits in 0.5 to 1.0 percent thiabendazole suspensions, without
subsequent rinsing, prevents sporulation of Penicillium upon the
surface of decaying fruits. Oranges so treated have a natural
appearance with no odour or visible residue.
The most efficient methods for commercial application of thiabendazole
appear to be as picking bin drenches, processing line spray or as an
additive to water emulsion waxes. Thiabendazole is registered in
Australia and U.S.A. as a post-harvest spray or dip treatment of
citrus fruit with suspension containing up to 5 000 ppm thiabendazole.
A rinse is not required, but rinsing does not appear to significantly
reduce the efficacy of a dip treatment.
Sporulation of Penicillium on the surface of decayed fruit causes
superficial soiling of sound fruits adjacent to decayed fruits. This
soiling can completely ruin all the fruit in a whole container with
only one or two decayed fruits. For this reason, a post-harvest
fungicide treatment must control both primary decay (prevent infection
of fruit) and reduce Penicillium sporulation on the surface of those
fruits which decay due to deep primary infections. Diphenyl and
sodium-o-phenylphenate, which have been used as standard treatments
for many years, have serious deficiencies. Thiabendazole on citrus wax
is used as a means of controlling fungus diseases referred to as green
mould, blue mould and stem end rot. This treatment is effective in
controlling both primary decay and sporulation.
Thiabendazole/citrus wax treatments are used commercially in Israel
and on an experimental basis in U.S.A. Aqueous emulsions of waxes
designed for application to citrus have been in use for many years.
Thiabendazole is suspended in sufficient wax emulsion to yield a
concentration of up to 0.5% w/w thiabendazole (5000 ppm). The prepared
emulsion is used without dilution in waxing equipment designed to
apply citrus wax evenly to freshly washed citrus. In this equipment,
the wax containing the thiabendazole is applied through nozzles to the
citrus as it revolves on horsehair brushes below. The nozzle is
attached to a chain to move back and forth across the width of the
waxer at a right angle to the flow of the citrus passing along the
conveyer. The amount of wax applied is governed by the size of the
nozzle and the pressure at which it is applied. These are equilibrated
with the flow of the citrus so that 1 litre of emulsion will coat ca.
800-900 kg of citrus fruit.
Other fruit
Bondoux (1967) reports good results from soaking apples in dilute
suspension of thiabendazole. Fripp (1969) showed thiabendazole
outstanding for control of brown rot (Sclerotinia fructicola) of
peaches. It is known that many other investigators are working with
thiabendazole to control post-harvest losses of a wide variety of
fruits, including cherries and pineapples, but as yet the available
data are not sufficient to enable proposals to be made for tolerances.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Bananas
Residue trials were carried out in Honduras. Green bananas treated
according to recommended practice were shipped under commercial
conditions at a temperature of 14°-15°C. Analysis was made 8 days
after treatment in the case of green bananas and 15 days in the case
of ripe fruit.
A review of the residue data showed that at the recommended treatment
rate of 200 ppm, a maximum of 0.18 ppm thiabendazole was found in the
banana pulp (see Table II), while a maximum of 1.24 ppm thiabendazole
was present in the peel (see Table III), when calculated on a whole
fruit basis. Therefore, at the recommended rate of 200 ppm, a maximum
of 1.4 ppm thiabendazole is possible in the whole unpeeled banana.
Pulp and peel residues generally increase slightly with an increase in
treatment rate, (see Table II) while the lower treatment rate (100
ppm) results in somewhat lower residue levels of thiabendazole in pulp
and peel (see Tables II and III). At the 100 ppm treatment level, an
average of 0.018 ppm and 0.049 ppm was found in ripe banana pulp from
two trials. This increased to an average of 0.068 ppm - 0.110 ppm at
the 200 ppm treatment rate. Residues appear to level off at the 200
ppm treatment rate, since ripe banana pulp contained an average of
0.126 ppm and 0.094 ppm at the 400 ppm treatment rate.
TABLE II
Thiabendazole residues (ppm) in pulp of green and ripe bananas 1
Treatment GREEN RIPE
Average, ppm Range, ppm Average, ppm Range, ppm
Control 1 0.0095 0.005-0.01 0.011 0.01-0.02
2 0.013 0.01 -0.03 0.024 0.01-0.04
100 ppm 1 0.030 0.01 -0.05 0.018 0.01-0.03
2 0.037 0.03 -0.05 0.049 0.03-0.07
200 ppm 1 0.074 0.04 -0.10 0.068 0.05-0.09
2 0.067 0.05 -0.08 0.110 0.05-0.18
400 ppm 1 0.173 0.05 -0.31 0.126 0.10-0.18
2 0.126 0.07 -0.17 0.094 0.07-0.13
1 Results on treated bananas are not corrected for results on control bananas.
TABLE III
Thiabendazole residues in peel of green and ripe bananas 1
Treatment GREEN RIPE
Average Range Average Range
(ppm) (ppm) (ppm) (ppm)
Control 1 0.020 0.013-0.042 0.040 0.024-0.059
2
100 ppm 1 0.39 0.33 -0.52 0.55 0.39 -0.68
2
200 ppm 1 0.12 0.97 -1.21 0.17 1.12 -1.24
2
400 ppm 1 2.24 2.01 -2.64 2.73 2.61 -2.80
2
1 Results on treated bananas are not corrected for results on control bananas.
The analytical method used had a sensitivity of 0.05 ppm with blank
values for untreated banana peel and pulp in the range of 0.01 - 0.04
ppm. The recovery of thiabendazole added to banana pulp and banana
peel ranged from 74.3 percent to 90.2 percent with an average of 87.3
percent.
In Australia, experiments were conducted by the Department Agriculture
to investigate the use of thiabendazole as a banana dip under
conditions of commercial usage; 100 cases (250 kg) of fruit was
treated at each of four concentrations of thiabendazole suspension.
The results were as follows:
TABLE IV
Whole fruit residues produced by various treatment rates
Treatment On Peel In Peel In Pulp Total
Rate Variety Average Range Average Range Average Range Residue
ppm ppm ppm ppm ppm ppm ppm ppm
0 Orange 0.076 0.00-0.33 0.008 0.00-0.03 0.00 0.00-0.01 0.09
Lemon 0.02 0.00-0.03 0.004 0.00-0.02 0.00 0.00-0.01 0.02
Grapefruit 0.01 0.01-0.02 0.01 0.01-0002 0.01 0.00-0.02 0.03
5 000 Orange 0.72 0.33-1.28 0.072 0.00-0.25 0.01 0.00-0.04 0.73
Lemon 0.31 0.16-0.74 0.045 0.00-0.13 0.005 0.00-0.01 0.36
Grapefruit 0.47 0.27-0.72 0.20 0.14-0.4? 0.02 0.00-0.04 0.66
10 000 Orange 1.25 0.77-2.22 0.098 0.01-0.19 0.01 0.00-0.01 1.36
Lemon 0.95 0.31-1.96 0.10 0.00-0.19 0.005 0.00-0.01 1.06
Grapefruit 0.98 0.66-1.08 0.29 0.23-0.39 0.04 0.01-0.07 1.28
Dip concentration Thiabendazole residues (whole fruit)
0.1% (1000 ppm) 2 - 5 ppm
0.05% (500 ppm) 1.5 - 2.5 ppm
0.025% (250 ppm) 0.8 - 2 ppm
0.0125% (125 ppm) 0.3 - 1.5 ppm
Trials at another research station yielded the following results:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 (200 ppm) 0.03 0.7
0.04 (400 ppm) 0.07 1.5
0.08 (800 ppm) 0.11 4.1
0.16 (1600 ppm) 0.14 6.2
0.32 (3200 ppm) 0.20 12.0
Limit of detection was 0.04 ppm.
Citrus fruits
Residue trials were conducted in conjunction with the Florida Citrus
Experiment Station on oranges, grapefruit and lemons. The following
products were assayed before and after processing: whole unwashed
fruit, single strength juice, finisher pulp, citrus oil, molasses,
dried citrus pulp. The fruit was processed according to typical
practice, including a two-minute flood treatment with a suspension
containing 0, 5 000 and 10 000 ppm thiabendazole. The fruit was then
waxed according to commercial practice.
The detection sensitivity of the analytical method used was 0.03 ppm
for the pulp and 0.40 ppm for the peel with blank values for untreated
pulp and peel in the range 0.00 - 0.02 ppm and 0.00 - 0.40 ppm
respectively.
A review of the whole fruit residue data, which is summarized in Table
IV, shows that at the application rate of 5 000 ppm, a maximum of 0.04
ppm thiabendazole is found in the pulp of the citrus, while a maximum
of 1.28 ppm and 0.41 ppm thiabendazole is present on and in the peel
respectively. This is the maximum residue found in pulp or peel of all
oranges, lemons or grapefruit assayed. Therefore, at the treatment
rate of 5 000 ppm, a maximum of 1.73 ppm is possible on the whole
unpeeled fruit.
In all cases, the peel residues increase with increasing treatment
rates. However, in the case of pulp assays, the level of residue is at
or below the sensitivity of the assay method with no indication of
increases with higher dosages. These results are corroborated by
radioactive studies reported later, which show that the level of
residues in the pulp is approximately equivalent to the assay
sensitivity and appears to result from inadvertent contamination
during peeling or sectioning. The result of assays on dried citrus
pulp (summarized on Table V) indicated the maximum residues range from
1.55 ppm in lemons to 2.87 ppm in the case of grapefruit, with dried
orange pulp being 2.61 ppm.
From yet another research station, it was reported that dipping green
bananas in a suspension containing 800 ppm thiabendazole resulted in
residues of 3.3 ppm in the whole fruit and 0.4 ppm in the pulp.
FATE OF RESIDUES
In animals
Radioactive thiabendazole administered orally to sheep at a dose of 50
to 100 mg/kg body-weight or to goats at a single dose of 150 mg/kg was
rapidly absorbed and metabolized to its 5-hydroxy derivative and
excreted as the aglycone and glucuronide and sulfate ester. Peak
concentrations in plasma occur approximately four hours after
administration, with 90 percent of the radioactivity eliminated in the
excreta in three to four days. Thiabandazole and small concentrations
of two unknown metabolites were observed (Robinson, 1965a; Tocco et
al., 1964).
Residues observed with eight sheep receiving 14C-or 35S-labelled
thiabendazole at 50 mg/kg are given in Table VI, where concentrations
reported as zero are equal to or less than a detection limit of 0.06
ppm.
TABLE V
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Finisher pulp Orange 0.02
Lemon 0.01
Grapefruit 0.01
Single strength Orange 0.01
juice Lemon 0.01
Grapefruit 0.02
Pulp, dry Orange 2.54
Lemon 1.4
Grapefruit 2.53
TABLE V (cont'd)
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Oil Orange 1.05
Lemon 0.16
Grapefruit 1.38
Molasses Orange 1.77
Lemon Not assessed
Grapefruit 1.97
Trials conducted in Australia at the Citrus Wastage Research
Laboratory showed the following results when oranges were dipped in
thiabendazole suspensions under commercial conditions:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 N.D.1 0.49
0.04 N.D. 0.21
0.08 N.D. 0.52
0.10 N.D. 0.39
0.16 N.D. 0.73
0.32 N.D. 1.68
1 N.D. = not detected (less than 0.04 ppm)
Analytical studies in lactating goats showed that although
thiabendazole was absorbed rapidly, less than 1% of the dose was
excreted in their milk within 24 hours after administration of 150
mg/kg body-weight. Peak concentrations were found in the milk within
24 hours, and no thiabendazole or its metabolites were detectable in
the milk four days later (Robinson, 1965a; Tocco et al., 1965).
Studies with goats given single oral dosages of tritium-labelled
thiabendazole at 150 mg/kg, demonstrated that the compound was
absorbed rapidly, reaching peak concentrations in the plasma about 4
hours after administration. The compound was rapidly excreted in the
urine and faeces. Residues of thiabendazole were not detectable in any
of the 15 tissues from goats examined 30 days after treatment.
Thiabendazole is less rapidly excreted by pigs than by several other
animals. Following a single dose of 50 mg/kg body-weight,
approximately 76 percent of the dose was found in the excrete within
about two weeks (Robinson, 1965a; Tocco et al., 1965). Thiabendazole
(14C-labelled) administered to cattle at doses of 50 mg/kg and 200
mg/kg body-weight was rapidly excreted. Plasma levels reached a
maximum in four to seven hours, after which they decreased rapidly,
with no residual thiabendazole or metabolites detectable in tissues 30
days after administration of the lower dose. Traces of radioactivity
were demonstrable in calves two months following doses of 150 and 200
mg/kg. Three fluorometrically detectable metabolites were observed,
corresponding to 5-hydroxythiabendazole and its sulfate and
glucuronide conjugate. Approximately 0.1 percent of an oral dose of 3,
5 or 10 grams per 100 lb was secreted in the milk as thiabendazole and
its metabolites within 60 hours after the animals were dosed. The
highest concentration appeared within 24 hours. Over 99 percent of the
compound was metabolites, and residues were not detectable 2 1/2 days
after the cows were administered the compound (Robinson, 1965a; Tocco
et al., 1965).
TABLE VI
Thiabendazole residues in sheep
Sample 6 hours 5 hours 8 days1/ 16 days 24 days 30 days1/
Abomasum 5.1 0.0 0.15 0.0 0.0 0.0
Blood 7.7 0.0 0.0 0.0 0.0 0.0
Brain 1.0 0.09 0.14 0.0 0.0 0.0
Caecum 34.4 0.0 0.08 0.0 0.0 0.0
Fat 2.8 0.0 0.04 0.0 0.0 0.0
Heart 2.7 0.15 0.14 0.08 0.0 0.0
Kidney 13.9 0.28 0.22 0.0 0.0 0.0
Large intestine 4.6 0.0 0.10 0.0 0.0 0.0
Liver 9.6 0.62 0.62 0.15 0.0 0.0
Lung 2.4 0.0 0.04 0.08 0.0 0.0
Muscle 2.0 0.09 0.06 0.13 0.0 0.0
Pancreas 2.6 0.18 0.13 0.0 0.0 0.0
Skin 3.2 0.0 0.19 0.0 0.0 0.0
Small intestine 33.6 0.0 0.16 0.0 0.0 0.0
Spleen 3.4 0.0 0.0 0.0 0.0 0.0
1/ Two animals slaughtered at each of these intervals. Results given in
table are means of the two values.
Table VII shows thiabendazole residues found in calves treated at 110
mg/kg. Results are the total of thiabendazole and its metabolites
expressed in ppm, determined fluorometrically.
TABLE VII
Thiabendazole residues in calves
Days No.
Post of Muscle Liver Kidney
Treatment Group Animals (ppm) (ppm) (ppm)
Control 3 0.13(0.09-0.17) 0.13(0.13-0.14) 0.12(0.10-0.15)
3 Treated 4 0.12(0.05-0.17) 0.17(0.15-0.19) 0.16(0.13-0.18)
7 " 4 0.10(0.03-0.19) 0.17(0.15-0.19) 0.12(0.10-0.13)
14 " 4 0.08(0.06-0.12) 0.15(0.14-0.18) 0.15(0.07-0.35)
It may be noted that in no instance does the average value exceed the control level by
more than 0.05 ppm.
In plants
Bananas
There is nothing to suggest that thiabendazole residues in bananas
undergo any change during application, shipping, storage or ripening.
Citrus
Radioactive thiabendazole 14C was employed to determine the uptake of
thiabendazole by Valencia oranges after dipping in 0.1% suspension,
before and after storage under conditions simulating customary
shipping and use practices (Merck, 1969; Rosenblum, 1970). Peal and
edible pulp were analysed separately. Peel was divided into inner and
outer portions. At the end of the maximum storage period, which
extended for two weeks at 10°C followed by an additional two weeks at
21°C, peel was assayed by the reverse isotope dilution method for
intact thiabendazole as an estimate of the stability of the latter in
the fruit.
The pulp was essentially free of thiabendazole even after four weeks
of storage. The small apparent residues recorded are probably due to
uncontrollable contamination from the peel during sectioning of the
orange for analysis. Peel representing 25% of the whole fruit
contained approximately 50 ppm of thiabendazole (on weight of peel).
All the thiabendazole applied as a suspension was still present as
intact thiabendazole after the four weeks of storage.
In several instances the stability of the sorbed thiabendazole - 14C
was investigated by extraction and by thin layer chromatography, with
and without prior addition of unlabelled carrier compound. Since the
radioactivity of the pulp did not increase with temperature or time of
storage above the initial value prior to storage, it may be concluded
that the thiabendazole content of the pulp due to diffusion of
absorbed fungicide into the interior during the four weeks of storage
is less than or equal to a detection limit of 0.01 ppm. Diffusion from
the surface of the fruit into the peel is appreciable an from 7-20% of
the measurable activity is observed in the inner peel. This diffusion
does not, however, extend to the pulp at the interior of the fruit.
Because of the widespread practice of waxing citrus after washing and
cleaning, the application of thiabendazole in the aqueous wax emulsion
is a convenient, practicable and efficient method of conferring
protection against fungal spoilage. Trials carried out in California
under controlled conditions in commercial packing houses resulted in
acceptable protection. The residues found on the treated fruit ranged
up to 4.3 ppm, and the results are set out in Table VIII. It is not
anticipated that the penetration into the peel will be any greater
when the treatment is applied via wax emulsion that it would from a
flooding, dipping or spraying treatment.
TABLE VIII
Residues of thiabendazole on whole fruit following application to citrus
of thiabendazole in wax emulsion
Storage Time
Variety Treatment Interval Residue
Temp. Humidity (days) (ppm)
Valencia
Oranges 0.3% TBZ in wax 21-24°C 90% 8 1.25
" 0.5% TBZ in wax 21-24°C 90% 8 2.16
" 0.3% TBZ in wax 21-24°C 90% 10 1.76
" 0.4% TBZ in wax 21-24°C 90% 10 2.20
" 0.5% TBZ in wax 21-24°C 90% 10 2.43
" 0.35% TBZ in wax 21-24°C 90% 10 2.15
" 0.5% TBZ in wax 21-24°C 90% 10 3.91
" 5267 ppm TBZ in wax 9-20°C 90% 31 4.3
Evidence of Residue in Food In Commerce
Shipments of commercially processed grapefruit which had been treated
by dipping in thiabendazole before being exported from U.S.A. were
sampled in Europe and systematically analysed for thiabendazole
residues. Ten fruits were homogenized from each shipment and analysis
was carried out on the homogenate. The results which are set out in
Table IX are slightly lower than those obtained from controlled
experiments carried out in Florida. It is notable that the residue on
grapefruit treated by flooding in a bath containing 3 000 ppm
thiabendazole are approximately twice as high as those found in fruit
treated in a similar manner in a bath containing 1 000 ppm
thiabendazole.
In order to determine the fruit-to-fruit variation in any one
consignment, nine separate fruit were taken from a consignment and
these were analysed individually. There was close agreement between
the results (range 0.26-0.55 ppm - standard deviation 0.09 ppm) with
the mean agreeing closely with the result obtained by analysis of a
homogenized sample of the same consignment.
TABLE IX
Residues of thiabendazole on whole citrus exported from U.S.A. and
analysed in Europe
Shipment Variety Treatment Residue on whole fruit (ppm) 1/
No. Rate Average Range
1 Grapefruit 1 000 ppm 0.22 0.18 - 0.25
3 000 ppm 0.44 0.32 - 0.63
2 " 1 000 ppm 0.23 0.20 - 0.29
3 000 ppm 0.57 0.46 - 0.86
3 " 1 000 ppm 0.17 0.14 - 0.18
1 000 ppm 0.13 0.10 - 0.16
3 000 ppm 0.27 0.23 - 0.30
4 " 1 000 ppm 0.17 0.15 - 0.19
1 000 ppm 0.18 0.16 - 0.21
3 000 ppm 0.24 0.21 - 0.28
5 " 1 000 ppm 0.39 0.37 - 0.42
1 000 ppm 0.33 0.21 - 0.28
3 000 ppm 0.86 0.72 - 0.96
1/ Variation in thiabendazole residue levels between individual fruits
Analysis of composite sample = 0.39 ppm
Analysis of individual fruits
0.53 0.37 0.38 Mean = 0.39 ppm
0.38 0.55 0.30 Standard Deviation 0.09 ppm
0.26 0.36 0.34 Range 0.26 - 0.55
Shipments of a variety of citrus exported from Israel to Europe were
sampled and analysed to determine the fruit-to-fruit variation.
Samples were taken of citrus prepared and packed by 13 separate
packing houses. In this case, the thiabendazole was applied as a
suspension in the wax emulsion used to treat the fruit after washing
and prior to packing in cartons. The wax contained 0.3% (3 000 ppm)
thiabendazole. The results given in Table X show a much higher level
of residues than those found following dipping. The fruit-to-fruit
variation was considerably greater and there was a significant
variation between different packing houses. Table XI shows results of
analysis of individual fruits from 7 packing houses in Israel
following treatment with 0.3% thiabendazole in wax emulsion.
TABLE X
Thiabendazole residues on Israeli citrus following application
of 0.3% thiabendazole in citrus wax
Packing No. of Fruit Average Standard Range
House Analysed ppm Deviation ppm
1 20 1.79 0.31 1.21 - 2.36
2 20 0.71 0.20 0.43 - 1.13
3 10 2.65 1.10 1.51 - 4.81
4 10 4.41 1.10 2.76 - 5.89
5 10 4.76 1.2 3.39 - 6.40
6 10 3.89 1.7 0.95 - 7.35
7 10 4.75 1.0 3.37 - 5.73
8 10 2.97 0.69 2.12 - 4.19
9 10 3.34 0.53 2.45 - 4.28
10 10 1.47 0.45 0.92 - 2.24
11 10 3.86 0.81 2.96 - 5.56
12 10 1.12 0.53 0.59 - 1.48
13 10 4.06 0.83 2.98 - 5.44
TABLE XI
Distribution of results of analysis of individual citrus
fruits treated with 0.3% thiabendazole in wax and analysed
one week after application
Range of residue levels Number of fruits Percent
less than 1.0 ppm 24 10
1.0 - 1.9 64 26
2.0 - 2.9 59 23
3.0 - 3.9 41 18
4.0 - 4.9 22 9
5.0 - 5.9 25 10
above 6 ppm 10 4
Total 245 100
Samples of oranges treated in South Africa by dipping in a suspension
containing 0.5% (5 000 ppm) thiabendazole which were analysed after
arrival in the United Kingdom contained between 0.28 ppm and 0.35 ppm
thiabendazole. The lower residue levels were found on fruit which had
received a water rinse after dipping. The higher residues were on
unrinsed fruit. Fruit dipped in suspensions at 43°C were found to have
residues of 0.80 ppm thiabendazole, twice the level found in those
dipped cold.
A further twenty samples representing various treatment levels, times,
temperatures and after-treatments showed residues ranging from 0.2 ppm
to 1.62 ppm. The lower levels were found in fruit treated at 1 000 ppm
and the higher level in fruit treated at 5 000 ppm. After-treatment
with citrus wax tended to reduce the residue levels to 0.15 ppm and
0.5 ppm, respectively.
METHODS OF RESIDUE ANALYSIS
Citrus
The method developed for the determination of residues in and on
citrus and bananas has been published by the U.S. Food and Drug
Administration (1969). The whole fruit is rinsed with ethyl acetate to
remove wax and thiabendazole from the surface. The pulp and peel are
homogenized with water. The thiabendazole is extracted from the
homogenates with ethyl acetate. The ethyl acetate solutions from
rinse, pulp and peel are combined. The solutions are purified by a
series of extraction procedures. The final solution in 0.1N
hydrochloric acid is measured spectrophotofluorometrically.
For routine testing of treated citrus, it is advisable to homogenize
about 10 fruits in a large chopper and to take a sub-sample of 60g of
puree for analysis. This is blended with an equal weight of sodium
acetate/sodium chloride solution, and from the slurry a 20 g sample is
taken for analysis. A number of minor variations have been introduced
into the extraction procedure to deal with various citrus fruits, the
separate components of the fruit and citrus products. When analysing
whole fruit, the method is sensitive to 0.1 ppm, but with pulp the
sensitivity is estimated to be 0.03 ppm and with peel 0.4 ppm. Blank
values for untreated pulp and peel range from 0.00-0.02 ppm and
0.00-0.40 ppm, respectively.
After purification, the extract is dissolved in 0.1N hydrochloric acid
and the fluorescence of the final acid solution is read on an
Amico-Bowman spectrophotofluorometer having an excitation wavelength
set at approximately 300mu and the mission wavelength set at 360mu.
Other instruments may operate more effectively at slightly different
wavelengths. The method is suitable for regulatory purposes but
requires a suitable spectrophotofluorometer.
Where a suitable spectrophotofluorometer is not available, the method
of Szalkowski (1965) has been modified by Gilbert (1969) to yield a
reliable colorimetric method with a sensitivity of 0.08 ppm and
recoveries between 93% and 110%. The blank varies between 0.02 and
0.04 ppm for both pulp and whole fruit. The thiabendazole is extracted
from homogenized fruit with acidic methanol and interference removed
with an alkaline wash followed by alumina column cleanup. The
thiabendazole is reduced to form hydrogen sulphide which complexes
with p-phenylene diamine. This complex in oxidized with ferric
solution to give a blue thiazine dye, which is determined
colorimetrically.
Bananas
The method described for the determination of thiabendazole in citrus
has been adapted to determine residues in banana pulp and peel by the
U.S. Food and Drug Administration (1969b).
The thiabendazole in extracted into ethyl acetate from a Ph 4.5
buffered suspension of ten grams of the banana pulp or peel. The
combined ethyl acetate extract is washed twice with 0.05N sodium
hydroxide. Then the thiabendazole is returned to an aqueous phase by
extraction with 0.1N hydrochloric acid and determined by fluorescence,
the method described for citrus. The sensitivity of this method is
0.05 ppm.
Animal tissues
The spectrophotofluorometric method has been used successfully for the
determination of thiabendazole and its 5-hydroxy derivative in animal
tissue. For liver and kidney tissue a preliminary digestion with
enzyme in necessary to convert the metabolite conjugates to 5-hydroxy
thiabendazole. The sensitivity is about 0.1 ppm.
Milk samples from animals receiving thiabendazole therapy or being fed
on citrus residues containing thiabendazole may be analysed for
thiabendazole by spectrophotofluorometric method using 1-5 ml samples.
For milk, as little an 0.05 ppm may be recovered.
NATIONAL TOLERANCES
Country Commodity Tolerance,ppm
U.S.A. Bananas 3
Banana pulp 0.4
Citrus 2
Citrus from wax treatment 6 (proposed)
Citrus pulp (dried) 8
Edible tissues of cattle,
sheep, goats and pigs 0.1
Milk (negligible residue) 0.05
(Cont'd)
Country Commodity Tolerance,ppm
Canada Bananas 3
Banana pulp 0.4
Citrus 2
Australia Bananas 3
Banana pulp 0.4
Citrus 2
Meat of cattle, sheep, 0.2
goats and pigs
Milk 0.05
France Bananas 3
Banana pulp 0.4
Citrus (provisional) 6
Netherlands Citrus (whole) 6
Citrus with diphenyl 3
Sweden Banana (whole) 3
Banana (pulp) 0.4
Citrus (whole) 6
E.E.C. Bananas (whole) 6
Citrus (whole) 6
Germany Citrus 6
Bananas 3
Belgium Bananas 3
Citrus 6
New Zealand Bananas 3
Citrus 2
Norway Citrus 6
Bananas 3
Finland Citrus 6
Italy Citrus (when used alone) 6
Citrus (when used with
other antifungals) 3
Bananas 3
APPRAISAL
Thiabendazole is a particularly effective fungicide for the
post-harvest treatment of bananas and citrus. It has been used very
extensively since 1960-62 as an anthelmintic for sheep, cattle, pigs
and horses and later as a vermifuge in human beings.
Post-harvest dips containing 200 ppm thiabendazole protect bananas
against the most important fungal rots. Treatment of green bananas
during picking and packing operations ensures that the fruit may be
shipped to distant markets with minimum loss or deterioration.
Likewise post-harvest sprays, dips and wax coatings containing
thiabendazole at concentrations of 1 000 to 3 000 ppm have proved
outstandingly useful. Diphenyl impregnated wraps and interlinings
have been used for many years to reduce Penicillium sporulation, and
sodium ortho-phenylphenate dips have been used against stem and
rots. Both treatments have many disadvantages and objections, and it
is anticipated that thiabendazole will provide a more acceptable
alternative to both treatments.
Data on the level of residues in whole fruit, peel, pulp and processed
citrus products, as well as in green and ripe bananas, were available
from U.S.A., West Indies, Australia, Israel and Europe. Results of
residue analysis of commercial citrus shipments to Europe were also
available and were not significantly different from those resulting
from supervised trials.
Thiabendazole has high stability on and in fruit and under conditions
of use no metabolites or degradation products are likely to occur. The
residue levels do not decline significantly in storage.
Several methods of residue analysis are available. The most convenient
and sensitive method employs spectrophotofluorometric measurement of
the thiabendazole in dilute hydrochloric acid solution. The method may
be adapted to determine thiabendazole residues in a variety of fruit
products and in animal tissues and milk. The sensitivity varies with
the substrate but ranges from 0.01 ppm to 0.04 ppm. This sensitivity
is adequate for the level of residues likely to occur and the proposed
tolerances. The method is suitable for regulatory purposes and is
specific to thiabendazole. de Vos and Bosma examined the possibility
of using gas chromatography with sulphur specific detector; they
showed that it was possible to determine thiabendazole residues to a
limit of 0.1 ppm by using the ethyl acetate extract of raw fruit for
injection into the chromatograph. They found that the method using
concentrated crude ethyl acetate extracts was satisfactory, but it led
to rapid fouling of the chromatographic column.
The administration of thiabendazole to sheep and cattle as an
anthelmintic gives rise to low level transient residues in several
edible tissues especially kidney and liver, but these residues are
eliminated within three days. Lactating dairy animals receiving a
therapeutic dose of thiabendazole excrete detectable amounts in the
milk for 24 hours post treatment. However, animals with severe
helminthiasis and liable to be dosed with thiabendazole are rarely
suitable for slaughter or for production of milk for human
consumption. Furthermore, it would be very unusual to treat more than
a small proportion of a herd at any one time and thereby to exceed the
detectable level of about 0.05 ppm in meat or milk. Under these
circumstances it does not appear necessary to recommend a tolerance
for thiabendazole residues in meat or milk.
RECOMMENDATIONS FOR TOLERANCES
TOLERANCES
The following tolerances are recommended for thiabendazole:
Crop Residues ppm
bananas, whole 3
banana pulp 0.4
citrus, whole 6
FURTHER WORK OR INFORMATION
DESIRABLE
Studies to determine the effect of thiabendazole on the thyroid gland.
REFERENCES
Anon. (1967) Control of squirter disease of bananas by thiabendazole.
Agricultural Gazette of New South Wales, 78:604
Anon. (1969) New treatments reduce fruit wastage - Rural Research in
CSIRO, 67:7-12
AOAC. (1966) Analysis of thiabendazole in feeds. J.A.O.A.C.
49:238-239 and 312
Bondoux, P. (1967) Acac. Agric. France, Compt. Rend. Hebd. Seances,
53: 1314-1323
Brown, G.E., McCormack, A.A. and Smoot, J.J. (1967) Thiabendazole as a
post-harvest fungicide for Florida citrus fruit. Plant Disease
Reporter, 52:95-97
Brown, H.D., et al. (1961) Journal of American Chemical Society,
83:1764
Burden, O.J. (1967) Studies on grown rot on bananas. Queensland
Agricultural Journal, March, p. 186
Crivelli, G. (1966) Part V Tests with the fungistat thiabendazole
against Penicillium of oranges (in Italian). Il. Freddo, 20:25-29
Cuille, J. and Bur-Ravault, L. (1968) Fruits, (Paris), 23:351-356
Eckert, J.W., Kolbenz, M.J. and Kraght, A.J. (1968) Proc.
International Citrus Symposium, Vol 3, Riverside, Cal., 13 March 1968
Fripp, Ivonne J. and Dettman, E. Belinda. (1969) Thiabendazole as a
post-harvest treatment against Sclerotinia fructicola in dessert
peaches. Australian Journal of Experimental Agriculture and Animal
Husbandry, 9:9-11
Gilbert, W.S. and Wright, Julia. (1969) Method for determining
thiabendazole residues on oranges
Gordon, H. McL. Studies of anthelmintics for sheep - thiabendazole.
Australian Veterinary Journal, 40(1):9-18
Harding, P.R. Jr. (1968) Plant Disease Reporter, 52: 623-625
Johnson, C.D. (1964) Evaluation of teratogenic potential in the rat.
Unpublished Report (April 1964) from Woodard Research Corporation
through Merck Institute for Therapeutic Research to FDA
McCormack, A.A. and Brown, G.E. (1968) Thiabendazole, an experimental
fungicide for fresh citrus fruit. Proc. Florida State Horticultural
Soc., 80:232-237
Merck and Company, Inc. (1969) Rahway, New Jersey, U.S.A.
thiabendazole residues in bananas and citrus. Submission to U.S. Food
and Drug Administration
Merck and Company, Inc. (1970) Rahway, New Jersey, U.S.A.
Thiabendazole residues in citrus following application in citrus wax.
Submission to U.S. Food and Drug Administration
Robinson, H.J., Stoerk, H.C. and Graessle, A.E. (1965b) Studies on the
toxicological and pharmacological properties of thiabendazole.
Toxicol. appl. Pharmacol., 7:53-63
Rosenblum, C. and Meriwether, H.T. (1970) Determination by the
radioactive indicator Method of the retention and stability of
thiabendazole in treated Valencia orange. Journal of Radioanalytical
Chemistry, (in press)
Scott, K.J., and Roberts, E.A. (1967) Control in bananas of black end
rot caused by Gloeosporium musarium. Australian Journal of
Experimental Agriculture and Animal Husbandry, 7:283-286
Seberry, J.A., and Baldwin, R.A. (1968) Thiabendazole and
2-amino-butane as post-harvest fungicides for citrus. Australian
Journal of Experimental Agriculture and Animal Husbandry, 8:440-443
Seberry, J.A. (1968) Proc. Int. Citrus Symposium, Riverside, Calif.,
13 March 1968
Shillingsford, C.A. (1970) Banana fruit rot control in Jamaica.
P.A.N.S.,16(1)
Szalkowski and Kanora. (1965) Spectrophotometric determination of TBZ
in feed. JAOAC,48(2):288-295
Tocco D.J., Buhs, R.P., Brown, H.D., Matzuk, A.R., Mertel, H.E.,
Harman, R.E. and Trenner, N.R. (1964) The metabolic fate of
thiabendazole in sheep. J. med. Chem., 7:399-405
Tocco, D.J., Egerton, J.R., Bowers, W., Christensen, V.W. and
Rosenblum, C.J. (1965) Absorption, metabolism and elimination of
thiabendazole in farm animals and a method for its estimation in
biological materials. J. Pharmacol. exp. Therap., 149:263-271
Tocco, D.J., Rosenblum C., Martin. C.M. and Robinson, H.G. (1966)
Absorption, metabolism and excretion of thiabendazole in man and
laboratory animals. Toxicol. appl. Pharmacol., 9:31-39
U.S. Food and Drug Administration. (1969) Thiabendazole residues in
citrus fruits. Pesticide analytical manual, Vol II, Section 120.242
U.S. Food and Drug Administration. (1969b) Thiabendazole residues in
banana peel and pulp. Method A. Pesticide analytical manual, Vol II,
Section 120.242.
Vogin, E.E. (1968) Multigeneration reproduction and lactation studies
with thiabendazole. Unpublished reports (Dec. 1967 and March 1968).
Food and Drug Research Laboratories, through Merck Institute for
Therapeutic Research to FDA
de Vos, R.H. and M.P. Bosma. (1970) Residues of thiabendazole in
citrus fruit. Report 3199. TNO Central Instituut voor
Voldingsonderzoek, Ziest, Netherlands
Weinke, K.E., Lauber, J.J., Greenwald, B.W. and Preiser, F.A. (1969)
Thiabendazole, a new systemic fungicide. Proc. 5th Brit. Insectic.
Fungic. Conference
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1964) Safety evaluation
by oral administration to dogs and rats for 104 weeks. Unpublished
Report (April 1964). Woodard Research Corporation through Merck
Institute for Therapeutic Research to FDA
Special studies on dermal irritation
There was no evidence of a significant degree of irritation when 0.5
gm thiabendazole was applied in cold cream at concentrations of 10 and
50 percent (w/w) for 24 hours to intact rabbit skin (Robinson, 1965a,
1965b).
Acute toxicity
LD50 doses for various animal species are given in Table I.
Toxic signs observed following administration of large doses of
thiabendazole by oral or intraperitoneal routes were generally similar
and consisted of lethargy and stupor. The intravenous administration
of a large dose of the hydrochloride produced narcosis. Death appeared
to be due to respiratory failure. Rabbits that survived large doses
initially lost weight but recovered after several days (Robinson,
1965a).
Short-term studies
Calf
Five female Holstein calves (seven months old) were fed a diet
containing 0, 320, 1 000, 3 200 or 10 000 ppm of thiabendazole for a
14-week test period. Calves tolerated 3 200 ppm thiabendazole in their
diet without observable effects on growth, food intake or general
condition. This concentration corresponded to a mean daily intake of
90 mg/kg body-weight. At the 10 000 ppm thiabendazole level, the
calves grew normally for the first two weeks, but in the following 12
weeks the weight gain was about half that observed for the controls.
Gross examination at the time of autopsy revealed no pathological
condition, and histological examination of several tissues showed no
change as resulting from the incorporation of thiabendazole in the
diet (Robinson, 1965a).
Chicken
Groups of 0.5 week old male White Leghorn chicks (10 to 20 per group)
were fed thiabendazole in the diet at dosage levels ranging from 1 to
10 000 ppm for a period of 2 1/2 weeks. This level corresponded to a
mean daily intake of thiabendazole ranging from 0.1 mg/kg to 1 200
mg/kg. A gradual decrease in growth appeared to occur with
concentrations of 100 ppm thiabendazole in the diet. This dose
corresponded to approximately 13 mg/kg per day. Gross pathological
examination at autopsy of the chicks on a dietary level of 4 000 ppm
thiabendazole showed them to be normal except for a smaller size
(Robinson, 1965a).
Dog
Groups comprising two male and two female dogs were orally
administered thiabendazole daily for periods of over two years at
doses of 0, 20, 100 and 200 mg/kg body-weight/day. No clinical signs
of morbidity were observed; food and water consumption were normal;
blood and urine chemistry were normal; a slight retardation in
body-weight gain at the 200 mg/kg level was observed, which was
accompanied by a slight reduction in total erythrocyte count. At this
level, changes in haemoglobin concentration and haematocrit were also
observed. No gross pathological observations were noted at the
conclusion of this test, and organ-weights appeared normal.
TABLE I
Thiabendazole toxicity in various animal species
LD50
Animal Route Form Solvent (mg/kg body-weight) Reference
Mouse oral HCl Water 2 400 (Robinson 1965a, 1965b)
ip HCl Water 430 " " "
iv HCl Water 150 " " "
oral Base CMC 2% 1 3 810 " " "
Rat oral HCl Water 3 600 " " "
ip HCl Water 1 850 " " "
iv HCl Water 130 " " "
oral Base CMC 2% 3 330 " " "
Rabbit oral Base CMC 2% 3 850 " "
Sheep drench HCl Water 2 000 " "
Goat drench HCl Water >2 000 " "
1 CMC = carboxy methyl cellulose
A significant haemosiderosis was present in dogs at the 100 and 200
mg/kg level in the spleen, liver, lymph nodes and bone-marrow. This
was not associated with an increase in serum haemoglobin. No
significant toxicological effects were noted at 20 mg/kg/day
(Robinson, 1965a, 1965b).
Groups of dogs (three male and three female) were orally administered
thiabendazole for two years at doses of 0, 20, 50 and 125 mg/kg
body-weight/day. At the 125 mg/kg/day level, two of six dogs died. One
of these dogs had marked liver cirrhosis, seminal tubular
degeneration, bone marrow atrophy and degenerative renal changes.
Slight to moderate reduction in haemoglobin and packed cell volume
occurred. Elevated blood urea nitrogen, serum alkaline phosphatase and
serum glutamic oxal oacetic transaminase were evident. Urinary albumin
was seen more frequently than in controls. Inspissated bile entwined
in the gall bladder villi was also observed in one dog. One dog of
this group was sacrificed and was found to have pulmonary arteritis of
parasitic origin. At 50 mg/kg/day, growth of male dogs was slightly
depressed. All six dogs given thiabendazole at 50 mg/kg survived the
study, and their tissues differed from those of the control dogs in
that some liver glycogen depletion was observed in three dogs and
inspissated material was found adhering to the gall bladder mucosa in
one. Five of six dogs survived thiabendazole at 20 mg/kg for the term
of the study. Three of the five showed slight liver glycogen
depletion. A general impression in this study was of an overall
appearance of mild chronic inflammatory degenerative renal changes in
all treated dogs (Woodard et al., 1964).
Pig
Five groups of four barrows each were given a diet containing 0, 320,
1 000, 3 200 and 10 000 ppm thiabendazole for 14 weeks. A
concentration of 320 ppm was tolerated without observable effects for
the 14-week period. This concentration corresponded to an intake of 15
mg/kg body-weight per day. Concentration of 10 000 ppm thiabendazole
in the diet caused reduced weight gains and reduced food consumption
with no mortality observed. Gross pathology examination revealed no
abnormal conditions (Robinson, 1965a).
Rat
Rats (10 males and 10 females per group) were administered
thiabendazole once daily at dosage levels of 0, 100, 400, 800, 1 200
and 1 600 mg/k-g body-weight per day. During the 30-day experimental
period, rats in the 800 mg/kg per day group decreased their food
intake and gradually lost weight. They were slightly less active, had
ruffled fur and became slightly flaccid. Three males and three females
died during the course of the experiment. All of the rats in the 1 200
and 1 600 mg/kg groups died during the 30-day test period. Rats dosed
at 100 and 400 mg/kg appeared normal throughout the test period,
although a slight depression in body-weight gain was noted in males at
100 mg/kg per day and in females at 400 mg/kg per day. Haematologic
studies on rats receiving 800 mg/kg per day showed a mild neutrophilia
with concurrent lymphopenia. There was also a suggestion of a decrease
in red blood cell elements. No gross anatomical changes were noted in
rats dosed with 100 mg/kg per day, whereas male and female rats
treated with 400 mg/kg per day showed thymic involution. A slight
enlargement of the liver was also noted in females at 400 mg/kg per
day. Animals surviving 800 mg/kg per day showed gross signs indicating
starvation where normal body fat depot and subcutaneous fat appeared
to be depleted. Liver and adrenals were slightly enlarged in males and
females and the thymus involuted. Microscopic pathology showed bone
marrow hypoplasia, thymus haemosiderosis and colloid depletion in the
thyroid at 400 mg/kg (Robinson, 1965a, 1965b).
Rats (30 males and 30 females per group) were administered
thiabendazole suspended in 1.0 percent methocel daily for 180 days at
doses of 0, 12.5, 25, 50, 100, 200 and 400 mg/kg body-weight. All of
the animals survived throughout the duration of the experiment. At 400
mg/kg, a depression in body-weight was observed with both sexes. At
200 mg/kg per day, the male rats showed a slight depression in weight
gain. No clinical signs of toxicity were observable at levels below
100 mg/kg. Haematological studies at 400 mg/kg per day indicated a
slight suggestion of a fall of the red blood cell elements which was
not evident at levels below this dose. Biochemical blood studies and
urinalysis were normal at all dosage levels with a slight polyurea in
both male and female rats at 400 mg/kg, which was apparently the
result of a slight increase in water consumption at this dosage level.
Gross pathology showed thymic involution in both males and females at
400 mg/kg per day and in females at 200 mg/kg per day. There was an
increase in liver size at doses of 100 mg/kg and above in males and at
25 mg/kg per day and above in females. Males dosed with 200 and 400
mg/kg and females at 400 mg/kg showed an apparent increase in kidney
weight. Microscopic examination of animals at 100 mg/kg showed a small
incidence of haemosiderosis of the thymus. At 200 and 400 mg/kg, these
examinations showed considerably more haemosiderosis of the thymus and
colloid depletion in the thyroid (Robinson, 1965a, 1965b).
Sheep
Thirty weanling wethers were employed in a 16-week study at doses of
0, 10, 50, 100, 200, 400 and 800 mg/kg body-weight per day.
Thiabendazole was administered in gelatin capsules. Two of the four
sheep dosed at 100 mg/kg did not survive the test period. However,
because of infection, thiabendazole may not have been directly related
to the cause of their death. All the animals treated at 200 mg/kg per
day and above died before the end of the test interval. No significant
effects on blood biochemistry or urinalysis were observed. Doses of 50
mg/kg and above affected body-weight gain, after approximately 120
days on the test. Doses of 10 mg/kg had no effect. Food consumption
was unaffected at doses of 10 mg/kg per day, but a minor decrease was
noted at 50 and 100 mg/kg per day and a significant decrease at 200
mg/kg per day and above. No gross pathological changes were observed
at doses below 200 mg/kg per day. Starvation and loss of body-weight
complicated the interpretation of data concerning organ-weights at 200
mg/kg and above. At 200 to 800 mg/kg, a moderate hypoplasia of the
bone marrow with replacement by adipose tissue was observed. Loss of
colloid in thyroid was evident at 800 mg/kg, as well as lymphoid
atrophy at the higher dose levels (Robinson, 1965a).
Five groups of two ewe lambs (10 to 12 weeks old) and eight wether
lambs (10 to 19 weeks old) were fed diets containing 0, 100, 320, 1
000 and 3 200 ppm thiabendazole for periods up to 50 weeks. Sheep 10
weeks of age tolerated 1 000 ppm thiabendazole in the diet for the
duration of the experiment. (This dose corresponded to 30 to 50 mg/kg
body-weight per day.) The sheep tolerated 3 200 ppm in their diet for
the first 14 weeks but thereafter lost weight in the final stages of
the experiment (Robinson, 1965a).
Long-term studies
Rat
Groups of rats (35 of each sex) were fed dietary levels adjusted to
provide 0, 10, 40 or 160 mg/kg body-weight per day of thiabendazole
for up to two years. At the 160 mg/kg level, a reduction of weight
gain of about 25 percent, with concomitant reduction in food
consumption and slightly reduced haemoglobin and microhaematocrit
values, were the only changes seen attributable to compound
administration. No effects attributable to compound administration
were noted with respect to survival, time of death in non-survivors,
incidents or location of neoplasms and histopathological examination
of the tissues. No effect on food consumption or haemograms were seen
at either the 10 or 40 mg/kg levels. Very slight depression in growth
rate, of questionable significance, was observed in the male rats at
40 mg/kg but not at 10 mg/kg. At the 10 mg/kg level, the mean absolute
thyroid weights of the male rats (including the individual thyroid
weights from five out of eight animals tested) were heavier than the
mean thyroid weight of the controls. This effect was not observed in
the rats given 40 or 160 mg/kg (Woodard et al., 1964).
OBSERVATIONS IN MAN
Human subjects have ingested a single oral dose of 2 g (approximately
30 mg/kg body-weight) of thiabendazole in studies designed to
ascertain the metabolic fate (see "Biochemical aspects"). No
information was given on the toxicological effects, if any (Robinson,
1965a).
COMMENTS
Metabolic studies indicate a rapid absorption of thiabendazole from
the gastrointestinal tract of rats, dogs, sheep and goats with
excretion in urine and faeces complete within 3-8 days. In cows,
approximately 0.1 percent of an oral dose was detectable in milk
within 60 hours. In excreta of man and all animals studied,
metabolites include 5-hydroxythiabendazole, free and conjugated as a
glucuronide and sulfate ester, as well as traces of unchanged
thiabendazole. Slight differences in man and other animals were noted
in quantitative recovery of the various compounds. A common effect for
several animal species at high dose levels of thiabendazole is
retarded growth.
Short-term studies in rate, sheep, pigs, calves and chickens have been
carried out with oral administration or with incorporation of
thiabendazole in the diet for periods of 4-50 weeks. At higher doses
in rats, thymus involution, colloid depletion in the thyroid and
increase in liver and kidney size was observed. Loss of the colloid in
thyroid of sheep administered 200 mg/kg body-weight was also evident.
Oral administration of 12.5 mg/kg for 180 days did not cause
significant toxicological changes in rats.
In a two-year dog study a no-effect level of 20 mg/kg body-weight was
demonstrated. A two-year study in rats showed heavier thyroid glands
at 10 mg/kg body-weight/day in the male animals; this effect, however,
was not dose dependent.
A five-generation reproduction study in mice showed no adverse effects
at 200 ppm level. In a three-generation study in rats, only decreased
body-weight in relation to decreased food consumption could be
observed.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Dog: 20 mg/kg body-weight/day
Mouse: 200 ppm in the diet, equivalent to 30 mg/kg body-weight/day
Rat: 10 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.05 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Administration to animals
Thiabendazole is widely used as a broad spectrum anthelmintic for
sheep, cattle, horses, pigs and goats. It is also administered as a
vermifuge directly to man. Following the announcement by Brown (1961)
of the chemotherapeutic properties of benzimidazoles, many
investigators report the efficacy of thiabendazole as an anthelmintic.
Gordon (1964) generally reported on its properties. Since that time
very large numbers of sheep and cattle have been treated with
thiabendazole suspensions. In sheep a dose of 44 mg/kg is adequate in
moot circumstances. Dosing may be repeated as frequently as once per
month, depending upon the level of reinfestation. In cattle a dose of
88 mg/kg is usually employed and in pigs 50-100 mg/kg.
Usually the dose is administered by drenching (oral administration of
an aqueous suspension) but for convenience it is possible to
administer via the feed when dealing with individual animals. At the
present time, economic considerations probably preclude the use of
thiabendazole as a feed additive.
Pre-harvest treatment of plants
Thiabendazole has been evaluated and has proved effective for use as a
fungicide on a number of crops including apples, pears, peaches,
apricots and bananas but, as far as is known, pre-harvest treatments
have not yet been adopted commercially. Preliminary results of residue
trials indicate that the residues from such pre-harvest uses will vary
considerably due to the systemic action of thiabendazole and the
ability of plants to take it up from the soil (Wernke, 1969).
Post-harvest treatment
Bananas
Scott (1967), Burden (1967), Anon (1967, 1969), Cuille (1968) and
Shillingsford (1970) have shown that thiabendazole is highly effective
for control of post-harvest fungal rots of bananas, when the picked
fruit is dipped in a suspension of thiabendazole containing from 100-1
000 ppm. A dip containing 200 ppm (0.02%) appears to be the most
widely used. Post-harvest spray treatments have also proved effective
in experimental use, but it is not known whether these are yet used in
practice.
Thiabendazole is most soluble in acid solution and can be rendered
soluble by treatment with lactic acid. Considerable experimental work
was carried out with lactic acid solutions as fruit dips, but in spite
of the convenience, the acidic solution gave rise to corrosion
problems in packing houses and during transport and resulted in
decomposition of the thiabendazole. Such solutions have been abandoned
in favour of micro-fine powder suspensions which require minimal
agitation to maintain uniform concentrations.
After the bunches of bananas have been picked, they are usually washed
in water or a solution of sodium hypochlorite. The bunches are then
separated into hands or clusters of 10-12 fruit which is allowed to
"bleed" in water for approximately 15-30 minutes. This facilitates the
removal of natural latex which, if allowed to run on the fruit, will
result in a visual disfigurement. The wet fruit is then dipped for 2-4
minutes in a suspension containing 200 ppm thiabendazole before being
drained for packing into cartons. No visible residue results from the
treatment.
Citrus
Crivelli (1966) and his associates in Italy first demonstrated that
Penicillium decay of oranges could be prevented by dipping fruit in
solutions containing 750-1 000 ppm thiabendazole dissolved in lactic
acid. Confirmation by other laboratories indicated that concentrations
of 200 to 500 ppm are adequate to give excellent control of
Penicillium decay. Brown (1967), Eckert (1968), Harding (1968),
McCormack (1968), Seberry (1968) show the efficacy of thiabendazole in
controlling Penicillium, and Seberry (1968) gives data showing
reduction in stem end rot caused by Diaporthe citri after dipping
oranges in suspensions of 500-1 000 ppm. Spraying or dipping citrus
fruits in 0.5 to 1.0 percent thiabendazole suspensions, without
subsequent rinsing, prevents sporulation of Penicillium upon the
surface of decaying fruits. Oranges so treated have a natural
appearance with no odour or visible residue.
The most efficient methods for commercial application of thiabendazole
appear to be as picking bin drenches, processing line spray or as an
additive to water emulsion waxes. Thiabendazole is registered in
Australia and U.S.A. as a post-harvest spray or dip treatment of
citrus fruit with suspension containing up to 5 000 ppm thiabendazole.
A rinse is not required, but rinsing does not appear to significantly
reduce the efficacy of a dip treatment.
Sporulation of Penicillium on the surface of decayed fruit causes
superficial soiling of sound fruits adjacent to decayed fruits. This
soiling can completely ruin all the fruit in a whole container with
only one or two decayed fruits. For this reason, a post-harvest
fungicide treatment must control both primary decay (prevent infection
of fruit) and reduce Penicillium sporulation on the surface of those
fruits which decay due to deep primary infections. Diphenyl and
sodium-o-phenylphenate, which have been used as standard treatments
for many years, have serious deficiencies. Thiabendazole on citrus wax
is used as a means of controlling fungus diseases referred to as green
mould, blue mould and stem end rot. This treatment is effective in
controlling both primary decay and sporulation.
Thiabendazole/citrus wax treatments are used commercially in Israel
and on an experimental basis in U.S.A. Aqueous emulsions of waxes
designed for application to citrus have been in use for many years.
Thiabendazole is suspended in sufficient wax emulsion to yield a
concentration of up to 0.5% w/w thiabendazole (5000 ppm). The prepared
emulsion is used without dilution in waxing equipment designed to
apply citrus wax evenly to freshly washed citrus. In this equipment,
the wax containing the thiabendazole is applied through nozzles to the
citrus as it revolves on horsehair brushes below. The nozzle is
attached to a chain to move back and forth across the width of the
waxer at a right angle to the flow of the citrus passing along the
conveyer. The amount of wax applied is governed by the size of the
nozzle and the pressure at which it is applied. These are equilibrated
with the flow of the citrus so that 1 litre of emulsion will coat ca.
800-900 kg of citrus fruit.
Other fruit
Bondoux (1967) reports good results from soaking apples in dilute
suspension of thiabendazole. Fripp (1969) showed thiabendazole
outstanding for control of brown rot (Sclerotinia fructicola) of
peaches. It is known that many other investigators are working with
thiabendazole to control post-harvest losses of a wide variety of
fruits, including cherries and pineapples, but as yet the available
data are not sufficient to enable proposals to be made for tolerances.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Bananas
Residue trials were carried out in Honduras. Green bananas treated
according to recommended practice were shipped under commercial
conditions at a temperature of 14°-15°C. Analysis was made 8 days
after treatment in the case of green bananas and 15 days in the case
of ripe fruit.
A review of the residue data showed that at the recommended treatment
rate of 200 ppm, a maximum of 0.18 ppm thiabendazole was found in the
banana pulp (see Table II), while a maximum of 1.24 ppm thiabendazole
was present in the peel (see Table III), when calculated on a whole
fruit basis. Therefore, at the recommended rate of 200 ppm, a maximum
of 1.4 ppm thiabendazole is possible in the whole unpeeled banana.
Pulp and peel residues generally increase slightly with an increase in
treatment rate, (see Table II) while the lower treatment rate (100
ppm) results in somewhat lower residue levels of thiabendazole in pulp
and peel (see Tables II and III). At the 100 ppm treatment level, an
average of 0.018 ppm and 0.049 ppm was found in ripe banana pulp from
two trials. This increased to an average of 0.068 ppm - 0.110 ppm at
the 200 ppm treatment rate. Residues appear to level off at the 200
ppm treatment rate, since ripe banana pulp contained an average of
0.126 ppm and 0.094 ppm at the 400 ppm treatment rate.
TABLE II
Thiabendazole residues (ppm) in pulp of green and ripe bananas 1
Treatment GREEN RIPE
Average, ppm Range, ppm Average, ppm Range, ppm
Control 1 0.0095 0.005-0.01 0.011 0.01-0.02
2 0.013 0.01 -0.03 0.024 0.01-0.04
100 ppm 1 0.030 0.01 -0.05 0.018 0.01-0.03
2 0.037 0.03 -0.05 0.049 0.03-0.07
200 ppm 1 0.074 0.04 -0.10 0.068 0.05-0.09
2 0.067 0.05 -0.08 0.110 0.05-0.18
400 ppm 1 0.173 0.05 -0.31 0.126 0.10-0.18
2 0.126 0.07 -0.17 0.094 0.07-0.13
1 Results on treated bananas are not corrected for results on control bananas.
TABLE III
Thiabendazole residues in peel of green and ripe bananas 1
Treatment GREEN RIPE
Average Range Average Range
(ppm) (ppm) (ppm) (ppm)
Control 1 0.020 0.013-0.042 0.040 0.024-0.059
2
100 ppm 1 0.39 0.33 -0.52 0.55 0.39 -0.68
2
200 ppm 1 0.12 0.97 -1.21 0.17 1.12 -1.24
2
400 ppm 1 2.24 2.01 -2.64 2.73 2.61 -2.80
2
1 Results on treated bananas are not corrected for results on control bananas.
The analytical method used had a sensitivity of 0.05 ppm with blank
values for untreated banana peel and pulp in the range of 0.01 - 0.04
ppm. The recovery of thiabendazole added to banana pulp and banana
peel ranged from 74.3 percent to 90.2 percent with an average of 87.3
percent.
In Australia, experiments were conducted by the Department Agriculture
to investigate the use of thiabendazole as a banana dip under
conditions of commercial usage; 100 cases (250 kg) of fruit was
treated at each of four concentrations of thiabendazole suspension.
The results were as follows:
TABLE IV
Whole fruit residues produced by various treatment rates
Treatment On Peel In Peel In Pulp Total
Rate Variety Average Range Average Range Average Range Residue
ppm ppm ppm ppm ppm ppm ppm ppm
0 Orange 0.076 0.00-0.33 0.008 0.00-0.03 0.00 0.00-0.01 0.09
Lemon 0.02 0.00-0.03 0.004 0.00-0.02 0.00 0.00-0.01 0.02
Grapefruit 0.01 0.01-0.02 0.01 0.01-0002 0.01 0.00-0.02 0.03
5 000 Orange 0.72 0.33-1.28 0.072 0.00-0.25 0.01 0.00-0.04 0.73
Lemon 0.31 0.16-0.74 0.045 0.00-0.13 0.005 0.00-0.01 0.36
Grapefruit 0.47 0.27-0.72 0.20 0.14-0.4? 0.02 0.00-0.04 0.66
10 000 Orange 1.25 0.77-2.22 0.098 0.01-0.19 0.01 0.00-0.01 1.36
Lemon 0.95 0.31-1.96 0.10 0.00-0.19 0.005 0.00-0.01 1.06
Grapefruit 0.98 0.66-1.08 0.29 0.23-0.39 0.04 0.01-0.07 1.28
Dip concentration Thiabendazole residues (whole fruit)
0.1% (1000 ppm) 2 - 5 ppm
0.05% (500 ppm) 1.5 - 2.5 ppm
0.025% (250 ppm) 0.8 - 2 ppm
0.0125% (125 ppm) 0.3 - 1.5 ppm
Trials at another research station yielded the following results:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 (200 ppm) 0.03 0.7
0.04 (400 ppm) 0.07 1.5
0.08 (800 ppm) 0.11 4.1
0.16 (1600 ppm) 0.14 6.2
0.32 (3200 ppm) 0.20 12.0
Limit of detection was 0.04 ppm.
Citrus fruits
Residue trials were conducted in conjunction with the Florida Citrus
Experiment Station on oranges, grapefruit and lemons. The following
products were assayed before and after processing: whole unwashed
fruit, single strength juice, finisher pulp, citrus oil, molasses,
dried citrus pulp. The fruit was processed according to typical
practice, including a two-minute flood treatment with a suspension
containing 0, 5 000 and 10 000 ppm thiabendazole. The fruit was then
waxed according to commercial practice.
The detection sensitivity of the analytical method used was 0.03 ppm
for the pulp and 0.40 ppm for the peel with blank values for untreated
pulp and peel in the range 0.00 - 0.02 ppm and 0.00 - 0.40 ppm
respectively.
A review of the whole fruit residue data, which is summarized in Table
IV, shows that at the application rate of 5 000 ppm, a maximum of 0.04
ppm thiabendazole is found in the pulp of the citrus, while a maximum
of 1.28 ppm and 0.41 ppm thiabendazole is present on and in the peel
respectively. This is the maximum residue found in pulp or peel of all
oranges, lemons or grapefruit assayed. Therefore, at the treatment
rate of 5 000 ppm, a maximum of 1.73 ppm is possible on the whole
unpeeled fruit.
In all cases, the peel residues increase with increasing treatment
rates. However, in the case of pulp assays, the level of residue is at
or below the sensitivity of the assay method with no indication of
increases with higher dosages. These results are corroborated by
radioactive studies reported later, which show that the level of
residues in the pulp is approximately equivalent to the assay
sensitivity and appears to result from inadvertent contamination
during peeling or sectioning. The result of assays on dried citrus
pulp (summarized on Table V) indicated the maximum residues range from
1.55 ppm in lemons to 2.87 ppm in the case of grapefruit, with dried
orange pulp being 2.61 ppm.
From yet another research station, it was reported that dipping green
bananas in a suspension containing 800 ppm thiabendazole resulted in
residues of 3.3 ppm in the whole fruit and 0.4 ppm in the pulp.
FATE OF RESIDUES
In animals
Radioactive thiabendazole administered orally to sheep at a dose of 50
to 100 mg/kg body-weight or to goats at a single dose of 150 mg/kg was
rapidly absorbed and metabolized to its 5-hydroxy derivative and
excreted as the aglycone and glucuronide and sulfate ester. Peak
concentrations in plasma occur approximately four hours after
administration, with 90 percent of the radioactivity eliminated in the
excreta in three to four days. Thiabandazole and small concentrations
of two unknown metabolites were observed (Robinson, 1965a; Tocco et
al., 1964).
Residues observed with eight sheep receiving 14C-or 35S-labelled
thiabendazole at 50 mg/kg are given in Table VI, where concentrations
reported as zero are equal to or less than a detection limit of 0.06
ppm.
TABLE V
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Finisher pulp Orange 0.02
Lemon 0.01
Grapefruit 0.01
Single strength Orange 0.01
juice Lemon 0.01
Grapefruit 0.02
Pulp, dry Orange 2.54
Lemon 1.4
Grapefruit 2.53
TABLE V (cont'd)
Citrus byproducts - residues following treatment
(Treatment rate 5 000 ppm)
Byproduct Variety Average residue, ppm
Oil Orange 1.05
Lemon 0.16
Grapefruit 1.38
Molasses Orange 1.77
Lemon Not assessed
Grapefruit 1.97
Trials conducted in Australia at the Citrus Wastage Research
Laboratory showed the following results when oranges were dipped in
thiabendazole suspensions under commercial conditions:
Dip concentration Thiabendazole residues ppm
% Flesh Whole Fruit
0.02 N.D.1 0.49
0.04 N.D. 0.21
0.08 N.D. 0.52
0.10 N.D. 0.39
0.16 N.D. 0.73
0.32 N.D. 1.68
1 N.D. = not detected (less than 0.04 ppm)
Analytical studies in lactating goats showed that although
thiabendazole was absorbed rapidly, less than 1% of the dose was
excreted in their milk within 24 hours after administration of 150
mg/kg body-weight. Peak concentrations were found in the milk within
24 hours, and no thiabendazole or its metabolites were detectable in
the milk four days later (Robinson, 1965a; Tocco et al., 1965).
Studies with goats given single oral dosages of tritium-labelled
thiabendazole at 150 mg/kg, demonstrated that the compound was
absorbed rapidly, reaching peak concentrations in the plasma about 4
hours after administration. The compound was rapidly excreted in the
urine and faeces. Residues of thiabendazole were not detectable in any
of the 15 tissues from goats examined 30 days after treatment.
Thiabendazole is less rapidly excreted by pigs than by several other
animals. Following a single dose of 50 mg/kg body-weight,
approximately 76 percent of the dose was found in the excrete within
about two weeks (Robinson, 1965a; Tocco et al., 1965). Thiabendazole
(14C-labelled) administered to cattle at doses of 50 mg/kg and 200
mg/kg body-weight was rapidly excreted. Plasma levels reached a
maximum in four to seven hours, after which they decreased rapidly,
with no residual thiabendazole or metabolites detectable in tissues 30
days after administration of the lower dose. Traces of radioactivity
were demonstrable in calves two months following doses of 150 and 200
mg/kg. Three fluorometrically detectable metabolites were observed,
corresponding to 5-hydroxythiabendazole and its sulfate and
glucuronide conjugate. Approximately 0.1 percent of an oral dose of 3,
5 or 10 grams per 100 lb was secreted in the milk as thiabendazole and
its metabolites within 60 hours after the animals were dosed. The
highest concentration appeared within 24 hours. Over 99 percent of the
compound was metabolites, and residues were not detectable 2 1/2 days
after the cows were administered the compound (Robinson, 1965a; Tocco
et al., 1965).
TABLE VI
Thiabendazole residues in sheep
Sample 6 hours 5 hours 8 days1/ 16 days 24 days 30 days1/
Abomasum 5.1 0.0 0.15 0.0 0.0 0.0
Blood 7.7 0.0 0.0 0.0 0.0 0.0
Brain 1.0 0.09 0.14 0.0 0.0 0.0
Caecum 34.4 0.0 0.08 0.0 0.0 0.0
Fat 2.8 0.0 0.04 0.0 0.0 0.0
Heart 2.7 0.15 0.14 0.08 0.0 0.0
Kidney 13.9 0.28 0.22 0.0 0.0 0.0
Large intestine 4.6 0.0 0.10 0.0 0.0 0.0
Liver 9.6 0.62 0.62 0.15 0.0 0.0
Lung 2.4 0.0 0.04 0.08 0.0 0.0
Muscle 2.0 0.09 0.06 0.13 0.0 0.0
Pancreas 2.6 0.18 0.13 0.0 0.0 0.0
Skin 3.2 0.0 0.19 0.0 0.0 0.0
Small intestine 33.6 0.0 0.16 0.0 0.0 0.0
Spleen 3.4 0.0 0.0 0.0 0.0 0.0
1/ Two animals slaughtered at each of these intervals. Results given in
table are means of the two values.
Table VII shows thiabendazole residues found in calves treated at 110
mg/kg. Results are the total of thiabendazole and its metabolites
expressed in ppm, determined fluorometrically.
TABLE VII
Thiabendazole residues in calves
Days No.
Post of Muscle Liver Kidney
Treatment Group Animals (ppm) (ppm) (ppm)
Control 3 0.13(0.09-0.17) 0.13(0.13-0.14) 0.12(0.10-0.15)
3 Treated 4 0.12(0.05-0.17) 0.17(0.15-0.19) 0.16(0.13-0.18)
7 " 4 0.10(0.03-0.19) 0.17(0.15-0.19) 0.12(0.10-0.13)
14 " 4 0.08(0.06-0.12) 0.15(0.14-0.18) 0.15(0.07-0.35)
It may be noted that in no instance does the average value exceed the control level by
more than 0.05 ppm.
In plants
Bananas
There is nothing to suggest that thiabendazole residues in bananas
undergo any change during application, shipping, storage or ripening.
Citrus
Radioactive thiabendazole 14C was employed to determine the uptake of
thiabendazole by Valencia oranges after dipping in 0.1% suspension,
before and after storage under conditions simulating customary
shipping and use practices (Merck, 1969; Rosenblum, 1970). Peal and
edible pulp were analysed separately. Peel was divided into inner and
outer portions. At the end of the maximum storage period, which
extended for two weeks at 10°C followed by an additional two weeks at
21°C, peel was assayed by the reverse isotope dilution method for
intact thiabendazole as an estimate of the stability of the latter in
the fruit.
The pulp was essentially free of thiabendazole even after four weeks
of storage. The small apparent residues recorded are probably due to
uncontrollable contamination from the peel during sectioning of the
orange for analysis. Peel representing 25% of the whole fruit
contained approximately 50 ppm of thiabendazole (on weight of peel).
All the thiabendazole applied as a suspension was still present as
intact thiabendazole after the four weeks of storage.
In several instances the stability of the sorbed thiabendazole - 14C
was investigated by extraction and by thin layer chromatography, with
and without prior addition of unlabelled carrier compound. Since the
radioactivity of the pulp did not increase with temperature or time of
storage above the initial value prior to storage, it may be concluded
that the thiabendazole content of the pulp due to diffusion of
absorbed fungicide into the interior during the four weeks of storage
is less than or equal to a detection limit of 0.01 ppm. Diffusion from
the surface of the fruit into the peel is appreciable an from 7-20% of
the measurable activity is observed in the inner peel. This diffusion
does not, however, extend to the pulp at the interior of the fruit.
Because of the widespread practice of waxing citrus after washing and
cleaning, the application of thiabendazole in the aqueous wax emulsion
is a convenient, practicable and efficient method of conferring
protection against fungal spoilage. Trials carried out in California
under controlled conditions in commercial packing houses resulted in
acceptable protection. The residues found on the treated fruit ranged
up to 4.3 ppm, and the results are set out in Table VIII. It is not
anticipated that the penetration into the peel will be any greater
when the treatment is applied via wax emulsion that it would from a
flooding, dipping or spraying treatment.
TABLE VIII
Residues of thiabendazole on whole fruit following application to citrus
of thiabendazole in wax emulsion
Storage Time
Variety Treatment Interval Residue
Temp. Humidity (days) (ppm)
Valencia
Oranges 0.3% TBZ in wax 21-24°C 90% 8 1.25
" 0.5% TBZ in wax 21-24°C 90% 8 2.16
" 0.3% TBZ in wax 21-24°C 90% 10 1.76
" 0.4% TBZ in wax 21-24°C 90% 10 2.20
" 0.5% TBZ in wax 21-24°C 90% 10 2.43
" 0.35% TBZ in wax 21-24°C 90% 10 2.15
" 0.5% TBZ in wax 21-24°C 90% 10 3.91
" 5267 ppm TBZ in wax 9-20°C 90% 31 4.3
Evidence of Residue in Food In Commerce
Shipments of commercially processed grapefruit which had been treated
by dipping in thiabendazole before being exported from U.S.A. were
sampled in Europe and systematically analysed for thiabendazole
residues. Ten fruits were homogenized from each shipment and analysis
was carried out on the homogenate. The results which are set out in
Table IX are slightly lower than those obtained from controlled
experiments carried out in Florida. It is notable that the residue on
grapefruit treated by flooding in a bath containing 3 000 ppm
thiabendazole are approximately twice as high as those found in fruit
treated in a similar manner in a bath containing 1 000 ppm
thiabendazole.
In order to determine the fruit-to-fruit variation in any one
consignment, nine separate fruit were taken from a consignment and
these were analysed individually. There was close agreement between
the results (range 0.26-0.55 ppm - standard deviation 0.09 ppm) with
the mean agreeing closely with the result obtained by analysis of a
homogenized sample of the same consignment.
TABLE IX
Residues of thiabendazole on whole citrus exported from U.S.A. and
analysed in Europe
Shipment Variety Treatment Residue on whole fruit (ppm) 1/
No. Rate Average Range
1 Grapefruit 1 000 ppm 0.22 0.18 - 0.25
3 000 ppm 0.44 0.32 - 0.63
2 " 1 000 ppm 0.23 0.20 - 0.29
3 000 ppm 0.57 0.46 - 0.86
3 " 1 000 ppm 0.17 0.14 - 0.18
1 000 ppm 0.13 0.10 - 0.16
3 000 ppm 0.27 0.23 - 0.30
4 " 1 000 ppm 0.17 0.15 - 0.19
1 000 ppm 0.18 0.16 - 0.21
3 000 ppm 0.24 0.21 - 0.28
5 " 1 000 ppm 0.39 0.37 - 0.42
1 000 ppm 0.33 0.21 - 0.28
3 000 ppm 0.86 0.72 - 0.96
1/ Variation in thiabendazole residue levels between individual fruits
Analysis of composite sample = 0.39 ppm
Analysis of individual fruits
0.53 0.37 0.38 Mean = 0.39 ppm
0.38 0.55 0.30 Standard Deviation 0.09 ppm
0.26 0.36 0.34 Range 0.26 - 0.55
Shipments of a variety of citrus exported from Israel to Europe were
sampled and analysed to determine the fruit-to-fruit variation.
Samples were taken of citrus prepared and packed by 13 separate
packing houses. In this case, the thiabendazole was applied as a
suspension in the wax emulsion used to treat the fruit after washing
and prior to packing in cartons. The wax contained 0.3% (3 000 ppm)
thiabendazole. The results given in Table X show a much higher level
of residues than those found following dipping. The fruit-to-fruit
variation was considerably greater and there was a significant
variation between different packing houses. Table XI shows results of
analysis of individual fruits from 7 packing houses in Israel
following treatment with 0.3% thiabendazole in wax emulsion.
TABLE X
Thiabendazole residues on Israeli citrus following application
of 0.3% thiabendazole in citrus wax
Packing No. of Fruit Average Standard Range
House Analysed ppm Deviation ppm
1 20 1.79 0.31 1.21 - 2.36
2 20 0.71 0.20 0.43 - 1.13
3 10 2.65 1.10 1.51 - 4.81
4 10 4.41 1.10 2.76 - 5.89
5 10 4.76 1.2 3.39 - 6.40
6 10 3.89 1.7 0.95 - 7.35
7 10 4.75 1.0 3.37 - 5.73
8 10 2.97 0.69 2.12 - 4.19
9 10 3.34 0.53 2.45 - 4.28
10 10 1.47 0.45 0.92 - 2.24
11 10 3.86 0.81 2.96 - 5.56
12 10 1.12 0.53 0.59 - 1.48
13 10 4.06 0.83 2.98 - 5.44
TABLE XI
Distribution of results of analysis of individual citrus
fruits treated with 0.3% thiabendazole in wax and analysed
one week after application
Range of residue levels Number of fruits Percent
less than 1.0 ppm 24 10
1.0 - 1.9 64 26
2.0 - 2.9 59 23
3.0 - 3.9 41 18
4.0 - 4.9 22 9
5.0 - 5.9 25 10
above 6 ppm 10 4
Total 245 100
Samples of oranges treated in South Africa by dipping in a suspension
containing 0.5% (5 000 ppm) thiabendazole which were analysed after
arrival in the United Kingdom contained between 0.28 ppm and 0.35 ppm
thiabendazole. The lower residue levels were found on fruit which had
received a water rinse after dipping. The higher residues were on
unrinsed fruit. Fruit dipped in suspensions at 43°C were found to have
residues of 0.80 ppm thiabendazole, twice the level found in those
dipped cold.
A further twenty samples representing various treatment levels, times,
temperatures and after-treatments showed residues ranging from 0.2 ppm
to 1.62 ppm. The lower levels were found in fruit treated at 1 000 ppm
and the higher level in fruit treated at 5 000 ppm. After-treatment
with citrus wax tended to reduce the residue levels to 0.15 ppm and
0.5 ppm, respectively.
METHODS OF RESIDUE ANALYSIS
Citrus
The method developed for the determination of residues in and on
citrus and bananas has been published by the U.S. Food and Drug
Administration (1969). The whole fruit is rinsed with ethyl acetate to
remove wax and thiabendazole from the surface. The pulp and peel are
homogenized with water. The thiabendazole is extracted from the
homogenates with ethyl acetate. The ethyl acetate solutions from
rinse, pulp and peel are combined. The solutions are purified by a
series of extraction procedures. The final solution in 0.1N
hydrochloric acid is measured spectrophotofluorometrically.
For routine testing of treated citrus, it is advisable to homogenize
about 10 fruits in a large chopper and to take a sub-sample of 60g of
puree for analysis. This is blended with an equal weight of sodium
acetate/sodium chloride solution, and from the slurry a 20 g sample is
taken for analysis. A number of minor variations have been introduced
into the extraction procedure to deal with various citrus fruits, the
separate components of the fruit and citrus products. When analysing
whole fruit, the method is sensitive to 0.1 ppm, but with pulp the
sensitivity is estimated to be 0.03 ppm and with peel 0.4 ppm. Blank
values for untreated pulp and peel range from 0.00-0.02 ppm and
0.00-0.40 ppm, respectively.
After purification, the extract is dissolved in 0.1N hydrochloric acid
and the fluorescence of the final acid solution is read on an
Amico-Bowman spectrophotofluorometer having an excitation wavelength
set at approximately 300mu and the mission wavelength set at 360mu.
Other instruments may operate more effectively at slightly different
wavelengths. The method is suitable for regulatory purposes but
requires a suitable spectrophotofluorometer.
Where a suitable spectrophotofluorometer is not available, the method
of Szalkowski (1965) has been modified by Gilbert (1969) to yield a
reliable colorimetric method with a sensitivity of 0.08 ppm and
recoveries between 93% and 110%. The blank varies between 0.02 and
0.04 ppm for both pulp and whole fruit. The thiabendazole is extracted
from homogenized fruit with acidic methanol and interference removed
with an alkaline wash followed by alumina column cleanup. The
thiabendazole is reduced to form hydrogen sulphide which complexes
with p-phenylene diamine. This complex in oxidized with ferric
solution to give a blue thiazine dye, which is determined
colorimetrically.
Bananas
The method described for the determination of thiabendazole in citrus
has been adapted to determine residues in banana pulp and peel by the
U.S. Food and Drug Administration (1969b).
The thiabendazole in extracted into ethyl acetate from a Ph 4.5
buffered suspension of ten grams of the banana pulp or peel. The
combined ethyl acetate extract is washed twice with 0.05N sodium
hydroxide. Then the thiabendazole is returned to an aqueous phase by
extraction with 0.1N hydrochloric acid and determined by fluorescence,
the method described for citrus. The sensitivity of this method is
0.05 ppm.
Animal tissues
The spectrophotofluorometric method has been used successfully for the
determination of thiabendazole and its 5-hydroxy derivative in animal
tissue. For liver and kidney tissue a preliminary digestion with
enzyme in necessary to convert the metabolite conjugates to 5-hydroxy
thiabendazole. The sensitivity is about 0.1 ppm.
Milk samples from animals receiving thiabendazole therapy or being fed
on citrus residues containing thiabendazole may be analysed for
thiabendazole by spectrophotofluorometric method using 1-5 ml samples.
For milk, as little an 0.05 ppm may be recovered.
NATIONAL TOLERANCES
Country Commodity Tolerance,ppm
U.S.A. Bananas 3
Banana pulp 0.4
Citrus 2
Citrus from wax treatment 6 (proposed)
Citrus pulp (dried) 8
Edible tissues of cattle,
sheep, goats and pigs 0.1
Milk (negligible residue) 0.05
(Cont'd)
Country Commodity Tolerance,ppm
Canada Bananas 3
Banana pulp 0.4
Citrus 2
Australia Bananas 3
Banana pulp 0.4
Citrus 2
Meat of cattle, sheep, 0.2
goats and pigs
Milk 0.05
France Bananas 3
Banana pulp 0.4
Citrus (provisional) 6
Netherlands Citrus (whole) 6
Citrus with diphenyl 3
Sweden Banana (whole) 3
Banana (pulp) 0.4
Citrus (whole) 6
E.E.C. Bananas (whole) 6
Citrus (whole) 6
Germany Citrus 6
Bananas 3
Belgium Bananas 3
Citrus 6
New Zealand Bananas 3
Citrus 2
Norway Citrus 6
Bananas 3
Finland Citrus 6
Italy Citrus (when used alone) 6
Citrus (when used with
other antifungals) 3
Bananas 3
APPRAISAL
Thiabendazole is a particularly effective fungicide for the
post-harvest treatment of bananas and citrus. It has been used very
extensively since 1960-62 as an anthelmintic for sheep, cattle, pigs
and horses and later as a vermifuge in human beings.
Post-harvest dips containing 200 ppm thiabendazole protect bananas
against the most important fungal rots. Treatment of green bananas
during picking and packing operations ensures that the fruit may be
shipped to distant markets with minimum loss or deterioration.
Likewise post-harvest sprays, dips and wax coatings containing
thiabendazole at concentrations of 1 000 to 3 000 ppm have proved
outstandingly useful. Diphenyl impregnated wraps and interlinings
have been used for many years to reduce Penicillium sporulation, and
sodium ortho-phenylphenate dips have been used against stem and
rots. Both treatments have many disadvantages and objections, and it
is anticipated that thiabendazole will provide a more acceptable
alternative to both treatments.
Data on the level of residues in whole fruit, peel, pulp and processed
citrus products, as well as in green and ripe bananas, were available
from U.S.A., West Indies, Australia, Israel and Europe. Results of
residue analysis of commercial citrus shipments to Europe were also
available and were not significantly different from those resulting
from supervised trials.
Thiabendazole has high stability on and in fruit and under conditions
of use no metabolites or degradation products are likely to occur. The
residue levels do not decline significantly in storage.
Several methods of residue analysis are available. The most convenient
and sensitive method employs spectrophotofluorometric measurement of
the thiabendazole in dilute hydrochloric acid solution. The method may
be adapted to determine thiabendazole residues in a variety of fruit
products and in animal tissues and milk. The sensitivity varies with
the substrate but ranges from 0.01 ppm to 0.04 ppm. This sensitivity
is adequate for the level of residues likely to occur and the proposed
tolerances. The method is suitable for regulatory purposes and is
specific to thiabendazole. de Vos and Bosma examined the possibility
of using gas chromatography with sulphur specific detector; they
showed that it was possible to determine thiabendazole residues to a
limit of 0.1 ppm by using the ethyl acetate extract of raw fruit for
injection into the chromatograph. They found that the method using
concentrated crude ethyl acetate extracts was satisfactory, but it led
to rapid fouling of the chromatographic column.
The administration of thiabendazole to sheep and cattle as an
anthelmintic gives rise to low level transient residues in several
edible tissues especially kidney and liver, but these residues are
eliminated within three days. Lactating dairy animals receiving a
therapeutic dose of thiabendazole excrete detectable amounts in the
milk for 24 hours post treatment. However, animals with severe
helminthiasis and liable to be dosed with thiabendazole are rarely
suitable for slaughter or for production of milk for human
consumption. Furthermore, it would be very unusual to treat more than
a small proportion of a herd at any one time and thereby to exceed the
detectable level of about 0.05 ppm in meat or milk. Under these
circumstances it does not appear necessary to recommend a tolerance
for thiabendazole residues in meat or milk.
RECOMMENDATIONS FOR TOLERANCES
TOLERANCES
The following tolerances are recommended for thiabendazole:
Crop Residues ppm
bananas, whole 3
banana pulp 0.4
citrus, whole 6
FURTHER WORK OR INFORMATION
DESIRABLE
Studies to determine the effect of thiabendazole on the thyroid gland.
REFERENCES
Anon. (1967) Control of squirter disease of bananas by thiabendazole.
Agricultural Gazette of New South Wales, 78:604
Anon. (1969) New treatments reduce fruit wastage - Rural Research in
CSIRO, 67:7-12
AOAC. (1966) Analysis of thiabendazole in feeds. J.A.O.A.C.
49:238-239 and 312
Bondoux, P. (1967) Acac. Agric. France, Compt. Rend. Hebd. Seances,
53: 1314-1323
Brown, G.E., McCormack, A.A. and Smoot, J.J. (1967) Thiabendazole as a
post-harvest fungicide for Florida citrus fruit. Plant Disease
Reporter, 52:95-97
Brown, H.D., et al. (1961) Journal of American Chemical Society,
83:1764
Burden, O.J. (1967) Studies on grown rot on bananas. Queensland
Agricultural Journal, March, p. 186
Crivelli, G. (1966) Part V Tests with the fungistat thiabendazole
against Penicillium of oranges (in Italian). Il. Freddo, 20:25-29
Cuille, J. and Bur-Ravault, L. (1968) Fruits, (Paris), 23:351-356
Eckert, J.W., Kolbenz, M.J. and Kraght, A.J. (1968) Proc.
International Citrus Symposium, Vol 3, Riverside, Cal., 13 March 1968
Fripp, Ivonne J. and Dettman, E. Belinda. (1969) Thiabendazole as a
post-harvest treatment against Sclerotinia fructicola in dessert
peaches. Australian Journal of Experimental Agriculture and Animal
Husbandry, 9:9-11
Gilbert, W.S. and Wright, Julia. (1969) Method for determining
thiabendazole residues on oranges
Gordon, H. McL. Studies of anthelmintics for sheep - thiabendazole.
Australian Veterinary Journal, 40(1):9-18
Harding, P.R. Jr. (1968) Plant Disease Reporter, 52: 623-625
Johnson, C.D. (1964) Evaluation of teratogenic potential in the rat.
Unpublished Report (April 1964) from Woodard Research Corporation
through Merck Institute for Therapeutic Research to FDA
McCormack, A.A. and Brown, G.E. (1968) Thiabendazole, an experimental
fungicide for fresh citrus fruit. Proc. Florida State Horticultural
Soc., 80:232-237
Merck and Company, Inc. (1969) Rahway, New Jersey, U.S.A.
thiabendazole residues in bananas and citrus. Submission to U.S. Food
and Drug Administration
Merck and Company, Inc. (1970) Rahway, New Jersey, U.S.A.
Thiabendazole residues in citrus following application in citrus wax.
Submission to U.S. Food and Drug Administration
Robinson, H.J., Stoerk, H.C. and Graessle, A.E. (1965b) Studies on the
toxicological and pharmacological properties of thiabendazole.
Toxicol. appl. Pharmacol., 7:53-63
Rosenblum, C. and Meriwether, H.T. (1970) Determination by the
radioactive indicator Method of the retention and stability of
thiabendazole in treated Valencia orange. Journal of Radioanalytical
Chemistry, (in press)
Scott, K.J., and Roberts, E.A. (1967) Control in bananas of black end
rot caused by Gloeosporium musarium. Australian Journal of
Experimental Agriculture and Animal Husbandry, 7:283-286
Seberry, J.A., and Baldwin, R.A. (1968) Thiabendazole and
2-amino-butane as post-harvest fungicides for citrus. Australian
Journal of Experimental Agriculture and Animal Husbandry, 8:440-443
Seberry, J.A. (1968) Proc. Int. Citrus Symposium, Riverside, Calif.,
13 March 1968
Shillingsford, C.A. (1970) Banana fruit rot control in Jamaica.
P.A.N.S.,16(1)
Szalkowski and Kanora. (1965) Spectrophotometric determination of TBZ
in feed. JAOAC,48(2):288-295
Tocco D.J., Buhs, R.P., Brown, H.D., Matzuk, A.R., Mertel, H.E.,
Harman, R.E. and Trenner, N.R. (1964) The metabolic fate of
thiabendazole in sheep. J. med. Chem., 7:399-405
Tocco, D.J., Egerton, J.R., Bowers, W., Christensen, V.W. and
Rosenblum, C.J. (1965) Absorption, metabolism and elimination of
thiabendazole in farm animals and a method for its estimation in
biological materials. J. Pharmacol. exp. Therap., 149:263-271
Tocco, D.J., Rosenblum C., Martin. C.M. and Robinson, H.G. (1966)
Absorption, metabolism and excretion of thiabendazole in man and
laboratory animals. Toxicol. appl. Pharmacol., 9:31-39
U.S. Food and Drug Administration. (1969) Thiabendazole residues in
citrus fruits. Pesticide analytical manual, Vol II, Section 120.242
U.S. Food and Drug Administration. (1969b) Thiabendazole residues in
banana peel and pulp. Method A. Pesticide analytical manual, Vol II,
Section 120.242.
Vogin, E.E. (1968) Multigeneration reproduction and lactation studies
with thiabendazole. Unpublished reports (Dec. 1967 and March 1968).
Food and Drug Research Laboratories, through Merck Institute for
Therapeutic Research to FDA
de Vos, R.H. and M.P. Bosma. (1970) Residues of thiabendazole in
citrus fruit. Report 3199. TNO Central Instituut voor
Voldingsonderzoek, Ziest, Netherlands
Weinke, K.E., Lauber, J.J., Greenwald, B.W. and Preiser, F.A. (1969)
Thiabendazole, a new systemic fungicide. Proc. 5th Brit. Insectic.
Fungic. Conference
Woodard, M.W., Cockrell, K.O. and Woodard, G. (1964) Safety evaluation
by oral administration to dogs and rats for 104 weeks. Unpublished
Report (April 1964). Woodard Research Corporation through Merck
Institute for Therapeutic Research to FDA
