
WHO Pesticide Residues Series, No. 1
1971 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The evaluations contained in these monographs were prepared by the
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues that met
in Geneva from 22 to 29 November 1971.1
World Health Organization
Geneva
1972
1 Pesticide Residues in Food: Report of the 1971 Joint Meeting of
the FAO Working Party of Experts on Pesticide Residues and the WHO
Expert Committee on Pesticide Residues, Wld Hlth Org. techn. Rep.
Ser., No. 502; FAO Agricultural Studies, 1972, No. 88.
These monographs are also issued by the Food and Agriculture
Organization of the United Nations, Rome, as document AGP-1971/M/9/1.
FAO and WHO 1972
FENTHION
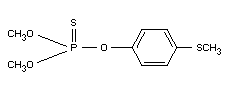
Chemical names:
1. dimethyl 3-methyl-4-methylthiophenyl
phosphorothionate
2. O,O-dimethyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate
Synonyms: (R)Lebaycid, (R)Baycid, (R)Baytex, (R)Entex,
(R)Tiguvon
BAY 29 493, S 1752
Formula:
 Other information on identity and properties
The pure active ingredient is a colourless liquid and the technical
active ingredient is a brownish viscous liquid. The active ingredient
has a boiling point of 87°C at 0.01 mm Hg. It is practically insoluble
in water, and readily soluble in most organic solvents. It has a very
low vapour pressure and slight volatility.
Impurities in the technical active ingredient
active ingredient (FAO-method No. 79/l/m/1.2)* 95-97%
O-methyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate <0.5%
5-hydroxy-2-methylmercapto-toluol <0.5%
O,S-dimethyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate 0.5-1.0%
O,O,O,O-tetramethyl-dithiopyrophosphate <0.5%
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate 5-7%
5-methoxy-2-methylmercapto-toluol <0.8%
O,O,O-trimethyl-thiophosphate <0.5%
Water max. 0.15%
* By this analytical method the impurity
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate
is determined together with the active ingredient.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
The first studies on mammals (rats) were reported by Brady and Arthur
(1961) who used a p32-labelled compound. Within a few hours of
applying the compound, large amounts of p32-activity was found in
the tissues including the bones, evidence that fenthion was rapidly
degraded by rats. Fenthion or its oxidative metabolites were, however,
not stored in tissues even when rats received daily doses of 10 mg/kg
by the intraperitoneal route for 10 consecutive days. At 1.5 hours,
following a single i.p. injection of 200 mg/kg, the following
acetonitrile-soluble residues were observed:
liver 29.5 ppm
muscle 9.6 ppm
skin 6.1 ppm
kidney 26.9 ppm
heart 9.6 ppm
The tissues from rats treated orally with 100 mg/kg (single dose)
contained less than 0.01 ppm chloroform-soluble residues at three days
after treatment, except for the liver (0.2 ppm); the blood, brain and
fat of these animals contained no detectable acetonitrile-soluble
residues, either. The orally treated animals eliminated 86% of the
activity in the excreta by seven days after treatment. One to four per
cent. of the activity in urine and faeces was chloroform-soluble.
As a result of partial starvation, oxidative metabolism in rabbits was
increased as evidenced by greater concentrations of fenthion-oxygen
analogue in blood. The peak of radioactive substances in blood of
normal rabbits following oral or subcutaneous administration of 35S
fenthion was observed in six to nine hours following treatment while
in starved rabbits the peak value was obtained one hour after
treatment (Begum, 1967).
Biotransformation
Fenthion is activated through an enzymatic oxidation, in both plants
and animals, to more active anti-cholinesterase compounds. The molar
I50 fenthion for rat brain cholinesterase is 1.3 × 10-4m. (Dubois
and Kinoshita, 1964). Following intraperitoneal administration of
32p-labelled fenthion approximately 75% of the administered dose was
recovered within three days in rat urine (60%) and faeces (15%). After
oral administration 86% of the dose was excreted in urine (45%) and
faeces (40%) with the majority excreted in three days (Brady and
Arthur, 1961). Starvation of rabbits had no effect on elimination of
fenthion (or metabolites) following oral or subcutaneous acute
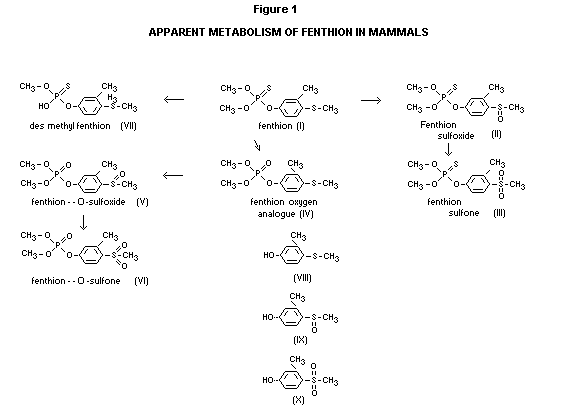
administration (Begum, 1967). Five metabolites (see Figure I) were
isolated from rat urine and characterized as the sulfoxide (II) and
sulfone (III) of the phosphorothioate (I), the oxygen analogue (IV) of
the parent compound and its corresponding sulfoxide (V) and sulfone
(VI) derivatives.
In rabbits the major urinary metabolites were fenthion-sulfoxide (II),
fenthion-O-sulfoxide (V) and sulfone (VI) (Begum, 1967). The metabolic
scheme appears as shown in Figure I.
Effects on enzymes and other biochemical parameters
Fenthion and its metabolites are typical organophosphorus
anticholinesterase agents. Typical of this class of agents the oxygen
analogue is the most potent enzyme inhibitor of all the metabolites
containing a phosphorus triester configuration (Francis and Barnes,
1963)). Fenthion is unusual in that clinically and biochemically a
prolonged effect following a single dose is manifest (recovery of
blood and brain enzyme levels is very slow (Brady and Arthur, 1961).
This effect may be as a result of inhibition of cholinesterase by
metabolites which are released at different rates from storage in the
body (Francis and Barnes, 1963), or a possible potentiation of its own
antiesterase effects by selectively inhibiting phosphatase activity
(Brady and Arthur, 1961), which is responsible for hydrolysis of the
phosphate ester. Cholinesterase inhibitor cannot be reactivated by
2-PAM, TMB-4 or P-2-S in vivo indicating that fenthion may inhibit
cholinesterase in a manner differing from many of the other
organophosphate esters. (Dubois, 1960; Dubois and Kinoshita, 1964;
Francis and Barnes, 1963).
Other information on identity and properties
The pure active ingredient is a colourless liquid and the technical
active ingredient is a brownish viscous liquid. The active ingredient
has a boiling point of 87°C at 0.01 mm Hg. It is practically insoluble
in water, and readily soluble in most organic solvents. It has a very
low vapour pressure and slight volatility.
Impurities in the technical active ingredient
active ingredient (FAO-method No. 79/l/m/1.2)* 95-97%
O-methyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate <0.5%
5-hydroxy-2-methylmercapto-toluol <0.5%
O,S-dimethyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate 0.5-1.0%
O,O,O,O-tetramethyl-dithiopyrophosphate <0.5%
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate 5-7%
5-methoxy-2-methylmercapto-toluol <0.8%
O,O,O-trimethyl-thiophosphate <0.5%
Water max. 0.15%
* By this analytical method the impurity
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate
is determined together with the active ingredient.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
The first studies on mammals (rats) were reported by Brady and Arthur
(1961) who used a p32-labelled compound. Within a few hours of
applying the compound, large amounts of p32-activity was found in
the tissues including the bones, evidence that fenthion was rapidly
degraded by rats. Fenthion or its oxidative metabolites were, however,
not stored in tissues even when rats received daily doses of 10 mg/kg
by the intraperitoneal route for 10 consecutive days. At 1.5 hours,
following a single i.p. injection of 200 mg/kg, the following
acetonitrile-soluble residues were observed:
liver 29.5 ppm
muscle 9.6 ppm
skin 6.1 ppm
kidney 26.9 ppm
heart 9.6 ppm
The tissues from rats treated orally with 100 mg/kg (single dose)
contained less than 0.01 ppm chloroform-soluble residues at three days
after treatment, except for the liver (0.2 ppm); the blood, brain and
fat of these animals contained no detectable acetonitrile-soluble
residues, either. The orally treated animals eliminated 86% of the
activity in the excreta by seven days after treatment. One to four per
cent. of the activity in urine and faeces was chloroform-soluble.
As a result of partial starvation, oxidative metabolism in rabbits was
increased as evidenced by greater concentrations of fenthion-oxygen
analogue in blood. The peak of radioactive substances in blood of
normal rabbits following oral or subcutaneous administration of 35S
fenthion was observed in six to nine hours following treatment while
in starved rabbits the peak value was obtained one hour after
treatment (Begum, 1967).
Biotransformation
Fenthion is activated through an enzymatic oxidation, in both plants
and animals, to more active anti-cholinesterase compounds. The molar
I50 fenthion for rat brain cholinesterase is 1.3 × 10-4m. (Dubois
and Kinoshita, 1964). Following intraperitoneal administration of
32p-labelled fenthion approximately 75% of the administered dose was
recovered within three days in rat urine (60%) and faeces (15%). After
oral administration 86% of the dose was excreted in urine (45%) and
faeces (40%) with the majority excreted in three days (Brady and
Arthur, 1961). Starvation of rabbits had no effect on elimination of
fenthion (or metabolites) following oral or subcutaneous acute
administration (Begum, 1967). Five metabolites (see Figure I) were
isolated from rat urine and characterized as the sulfoxide (II) and
sulfone (III) of the phosphorothioate (I), the oxygen analogue (IV) of
the parent compound and its corresponding sulfoxide (V) and sulfone
(VI) derivatives.
In rabbits the major urinary metabolites were fenthion-sulfoxide (II),
fenthion-O-sulfoxide (V) and sulfone (VI) (Begum, 1967). The metabolic
scheme appears as shown in Figure I.
Effects on enzymes and other biochemical parameters
Fenthion and its metabolites are typical organophosphorus
anticholinesterase agents. Typical of this class of agents the oxygen
analogue is the most potent enzyme inhibitor of all the metabolites
containing a phosphorus triester configuration (Francis and Barnes,
1963)). Fenthion is unusual in that clinically and biochemically a
prolonged effect following a single dose is manifest (recovery of
blood and brain enzyme levels is very slow (Brady and Arthur, 1961).
This effect may be as a result of inhibition of cholinesterase by
metabolites which are released at different rates from storage in the
body (Francis and Barnes, 1963), or a possible potentiation of its own
antiesterase effects by selectively inhibiting phosphatase activity
(Brady and Arthur, 1961), which is responsible for hydrolysis of the
phosphate ester. Cholinesterase inhibitor cannot be reactivated by
2-PAM, TMB-4 or P-2-S in vivo indicating that fenthion may inhibit
cholinesterase in a manner differing from many of the other
organophosphate esters. (Dubois, 1960; Dubois and Kinoshita, 1964;
Francis and Barnes, 1963).
 TOXICOLOGICAL STUDIES
Special studies
(a) Acute toxicity of the metabolites
Compound Acute LD50 (mg/kg) 150 (M)c
Orala i.p.b
I. Fenthion 220 325 >5 × 10-4
II. Fenthion sulfoxide 125 250 4.5 × 10-5
III. Fenthion sulfone 125 250 4.7 × 10-4
IV. Oxygen analogue 125 26 2.65 × 10-6
V. O-Sulfoxide 50 22 4.8 × 10-5
IV. O-Sulfone 30 9 3.2 × 10-5
VIII. 4-(methylthio)m-cresol 6500d
IX. 4-methyl(thiosulfoxide)
m-cresol 3500d
X. 4-methyl(thiosulfone)
m-cresol 7000d
a Male rats - according to Francis and Barnes, 1963.
b Female rats - according to Dubois and Kinoshita, 1964.
c 150 = Molar concentration resulting in 50% inhibition of
human RBC Cholinesterase (Francis and Barnes, 1963).
d Female rats according to Nelson, 1967.
(b) Reproduction
Except for a slight growth depression at the highest level in the
diet, fenthion at levels of 0, 3, 15 and 75 ppm for three generations
(two litters per generation), produced no adverse effect on rat
reproduction (Loser, 1969). Microscopic examination of the tissues of
the F3b generation did not reveal any significant abnormality (Spicer,
1971).
(c) Potentiation
Fenthion potentiates the acute intraperitoneal toxicity of malathion,
dioxathion, and coumaphos in rats. Intraperitoneal administration of
13 other organophosphate or carbamate insecticides did not result in a
greater than additive effect when administered with fenthion (Dubois
and Kinoshita, 1964). Dietary combination of fenthion with coumaphos
neither of which alone affected cholinesterase when fed to dogs for
six weeks, was found to potentiate the anticholinesterase activity of
serum and red blood cells. Less evident potentiation was observed when
fenthion and malathion were fed and when fenthion was fed in
combination with dioxathion no potentiation was noted (Doull et al.,
1962), Fenthion (2 ppm) and malathion (100 ppm) resulted in moderate
erythrocyte and serum cholinesterase activity inhibition (30-40%).
When fenthion (2 ppm) and dioxathion (3 ppm) was fed cholinesterase
activity was not depressed. When fenthion (2 ppm) and coumaphos
(2 ppm) was fed to dogs, significant inhibition (75%) of serum
cholinesterase and moderate inhibition (30%) of erythrocyte
cholinesterase was evident. Oral administration to rats of a 75:25
mixture of fenthion and dichlorvos did not result in a greater than
theoretically additive toxicity (Kimmerle, 1967b).
(d) Neurotoxicity
No evidence for delayed neurological disruption in hens was evident
when fenthion was administered orally at a single dose of 25 mg/kg
(Kimmerle, 1965a). When chickens were fed up to 100 ppm in the diet
for 30 days, clinical examination of the animals and histological
examination of nerve tissue indicated no demyelinating effect from
fenthion (Kimmerle, 1965b; Dieckmann, 1971).
(e) Antidotes
A number of antidotes which are commonly used for organophosphorous
poisoning have been shown to be relatively inactive when used
following fenthion intoxication. Studies with atropine, 2-PAM
Toxogonin P-2-S, and TMB-4 (Dubois and Kinoshita, 1964; Francis and
Barnes, 1963; and Dubois, 1960; Lorke and Kimmerle, 1969) administered
alone and in combination showed that these materials did not
successfully alleviate the parasympathomimetic signs of
organophosphate poisoning. When BH-G (Merck; Darmstadt, Germany) was
administered three to four times in combination with atropine, the
LD50 value increased from 250 to 440 mg/kg (Kimmerle, 1963).
Toxogonin was not effective as a cholinesterase reactivator following
oral intoxication of dogs by fenthion (Hahn and Henschler, 1969).
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Mouse (M) Oral 150 Francis and Barnes, 1963
227 Dubois, 1968
(F) 190 Francis and Barnes, 1963
225 Dubois, 1968
(F) i.p. 150 Dubois and Kinoshita, 1964
(M) 125 Dubois and Kinoshita, 1964
(Continued)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat (F) Oral 245-310 (Dubois and Kinoshita, 1964
(Gaines, 1960
615 Francis and Barnes, 1963
(M) 175-470 (Dubois and Kinoshita, 1964
(Klimmer, 1963
(Gaines, 1969
(Francis and Barnes, 1963
(M) i.p. 325 Dubois and Kinoshita, 1964
(F) 260 Dubois and Kinoshita, 1964
(M) 152 Klimmer, 1963
(M) Dermal 330-650 Gaines, 1969
Klimmer, 1963
Francis and Barnes, 1963
(F) 330-500 Dubois and Kinoshita, 1964
Gaines, 1969
Francis and Barnes, 1963
Guinea-pig
(M) Oral 1000 Francis and Barnes, 1963
260 Dubois and Kinoshita, 1964
i.p. 310 Dubois and Kinoshita, 1964
Rabbit (M) Oral 150-175 Francis and Barnes, 1963
Dermal 150 Klimmer, 1963
Duck Oral 15 Dubois and Doull, 1960
1-2 Keith and Mulla, 1966
Chicken 30 Dubois and Doull, 1960
28 Sherman and Ross, 1961
30-40 Francis and Barnes, 1963
Calf Approx. 40 McGrath, 1961
Various solvents used to solubilize fenthion in combination with water
or ethanol had no significant effect on the acute oral LD50
(Kimmerle, 1967a).
Fenthion is an organophosphorus insecticide of intermediate toxicity
to mammals although it displays considerable differences in its
toxicity to various species, e.g. fowls are very sensitive (Keith and
Mulla, 1966).
The rate of absorption through the skin of rabbits is slow. When
fenthion was applied to a cotton plug and placed in a rabbit ear for
four hours, swelling occurred. When it was left for 24 hours mortality
resulted (Kimmerle, 1960).
Female rats tolerated a daily one hour inhalation challenge of 0.163
mg/l air for 30 days with cholinesterase depression but no mortality.
At 0.415 mg/l air all animals were dead within 10 days (Dilley and
Doull, 1961a).
Exposure by inhalation daily for nine days, six hours per day at 210
mg/M3 (initial concentration in a static inhalation chamber),
resulted in signs of poisoning, but no mortality in cats, guinea pigs,
rabbits and rats. Cholinesterase, which was severely depressed,
recovered in three weeks (Klimmer, 1963). Fenthion exhibits a
relatively low degree of acute mammalian toxicity. In only one
instance was a sex difference in susceptibility noted and this is in
contrast to the generally noted resistance of males to most other
phosphorothioates (Dubois and Kinoshita, 1964). The signs of poisoning
are typical of the central and peripheral cholinergic effects of
organophosphorus esters with a gradual onset of the symptoms. The
symptoms in humans and other animals include tremors, lacrymation,
salivation, vomiting, diarrhoea and other signs of cholinergic
stimulation.
Short-term studies
Duck
Mallard ducks fed 25 ppm of fenthion in the diet for six weeks became
emaciated and could not fly or walk; after two days on normal food,
recovery was evident. A dietary level of 5 ppm produced no adverse
clinical reaction (Keith and Mulla, 1966).
Rat
Dermal administration of fenthion at 14.5 and 25 mg/kg for 60 days to
rats resulted in 40% mortality in the higher dosed group and no
mortality in the lower dosed group. However, blood cholinesterase
levels were depressed to about 20% of normal at the lower treatment
level (Dubois, 1961). Cholinesterase was depressed and mortality was
absent when fenthion was applied to rats dermally for 12 days at 2.9
mg/kg (Dubois and Puchala, 1960).
Rats tolerated daily intraperitoneal administration of 10 mg/kg
fenthion for 60 days with no mortality. At 20 mg/kg, 80% of the
treated animals died within 20 days (Dubois and Kinoshita, 1964).
Mortality (12 dead of 30 rats tested) occurred following daily oral
administration of approximately 25 mg/kg (1/10 LD50) for 75 days
(six days per week). Signs of poisoning were transient disappearing
shortly after dosing (Klimmer, 1963).
In a preliminary study, male rats were orally administered fenthion
five days a week for 13 weeks at a dose of 30 mg/kg/day. Mortality
occurred in approximately 30% of the rats over the course of the
experiment and cholinesterase activity was depressed between 80-90% of
normal. At the conclusion of the experiment cholinesterase recovery
was very slow - up to 40 days (Kimmerle, 1961).
Rats (22 male and 22 female per group) were fed dietary levels of
fenthion at 0.25, 0.50, 2.5, and 5.0 mg/kg/day for three months.
Cholinesterase activity depression was evident at 0.5 mg/kg in red
blood cells, serum, liver and heart at all testing intervals. At the
lowest feeding level (0.25 mg/kg) the inhibition (approx. 10-20%) did
not progressively increase with time indicating lack of cumulative
effects. Mortality in females was evident at 5.0 mg/kg. The animals
died manifesting muscarinic and nicotinic effects. Body-weight gain
was reduced in males at 0.25 mg/kg and above while in females it was
evident only at 2.5 mg/kg. Behavioural effects were noted
(piloerection) at 0.5 mg/kg and above (especially in females). This
effect decreased with time and disappeared by week seven. Organ
weights were all distinctly lower than the controls but as the
body-weight was also reduced the organ to body-weight ratio did not
appear to be affected. Histological examination showed the testis to
have reduced spermatogenesis and atrophic prostate glands at the
highest feeding levels (2.5 and 5.0 mg/kg). The ovary was not affected
(Shimamoto and Hattori, 1969).
Rats (six groups of 12 male and 12 female) were fed for 16 weeks on
diets containing 0, 2, 3, 5, 25 and 100 ppm. Cholinesterase depression
was evident at 25 ppm and absent at 5 ppm. No adverse effects were
noted in food consumption, weight gain or gross and microscopic
examination of tissues (Doull et al., 1961).
Rats (six groups of 25 male and 25 female) were fed for one year on
diets containing 0, 2, 3, 5, 25 and 100 ppm of fenthion. There was no
evidence of significant changes in growth rate, food consumption,
general appearance and gross of microscopic examination of tissues.
Survival of male rats at 25 ppm was slightly depressed. Cholinesterase
examinations indicated depression at the 5 ppm level and above with
3 ppm showing no adverse enzyme effects. A mild extramedullary
haematopoiesis was observed in controls and all dosage levels and
haemosideresis was evident in the spleen of the rats at 100 ppm levels
(Doull et al., 1963a).
Dog
Dogs (four groups of two males and two females per group) were fed
fenthion at 0, 2, 5 and 50 ppm for 12 weeks. Growth was not affected
at any dietary level. Erythrocyte cholinesterase activity was
depressed at 50 ppm while serum cholinesterase was depressed at 5 ppm
and above. Little if any depression of the serum cholinesterase was
evident before five weeks, after which it progressively decreased to
about 40% inhibition (Doull et al., 1961).
Dogs (four groups of two males and two females) were fed fenthion at
0, 2, 5, and 50 ppm in the diet for one year. There was no effect of
fenthion on food consumption or growth over the test interval.
Erythrocyte and serum cholinesterase were significantly depressed at
50 ppm with the serum also depressed at 5 ppm. An increase in the
weight of the spleen, which was not dose dependent, was evident in all
of the treated animals. Microscopic examinations of the tissues showed
splenic congestion and some decrease in the cellularity of the red
pulp was evident at all dose levels fed. Extramedullary haematopoiesis
and haemosideresis was also observed in the spleen. Microscopic
examination of other tissues did not reveal any significant change
(Doull et al., 1963b).
Long-term studies
No data available.
Observations in man
Fenthion has been widely used in many parts of the world for control
of household pests, mosquitos, etc. Cholinesterase studies conducted
on individuals in areas treated by WHO for malaria eradication have
shown that very slight plasma cholinesterase depression occurs when
exaggerated spray schedules were followed. The plasma cholinesterase
levels were depressed for up to six weeks after spraying (Elliot and
Barnes, 1963). It was also evident that the children in the population
(less than seven years old) were more susceptible to the
anticholinesterase effects (Taylor, 1963). A man who ingested two
ounces of fenthion (Entex)(R) recovered from severe
organophosphorous poisoning after being in critical condition for the
first six days after poisoning. Recovery was slow, lasting up to 30
days. Cholinesterase measurements showed that at 22 days after
poisoning the cholinesterase activity was still depressed (Pickering,
1966). In humans, the signs of poisoning appear rapidly beginning with
blurred vision, unsteady gait and slurred speech. After 72 hours of
emergency treatment following an unknown quantity of fenthion, a man
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation. The patient began to recover
only after 11 days of treatment which included atropine, PAM and
toxogonin (Dean et al., 1967). In another case, 45 minutes after
ingestion of 30 ml of fenthion, a man was in a comatose state with
pale skin, cyanotic mucous membranes, slow regular heart beat, no
peripheral blood pressure and no reactions to pain or light
stimulation on the pupils. Recovery took six days (von Clarmann and
Geldmacher-von-Mallinkradt, 1966).
Comments
Fenthion is slowly absorbed, metabolized by a complex series of
reactions and excreted. It does not appear to accumulate in the body.
In most instances the unhydrolysed metabolites containing a phosphorus
atom are more toxic than the parent compound. The signs of
intoxication from a single oral dose develop slowly, persist for a
considerable period of time and are not readily alleviated by atropine
or by most common oxime reactivators. Short-term studies in the rat
and dog suggest that cholinesterase inhibition is the most sensitive
criterion of biological effect. Anti-spermatogenesis in one rat study
was not confirmed in other studies or in a reproduction study. The
occurrence of an effect on the spleen in both rats and dogs was not
considered to constitute evidence of significant toxicity. Of
particular concern was the slow onset of symptoms of cholinergic
stimulation, differences in susceptibility to acutely toxic doses in
rodents and birds, the long lasting cholinesterase depression and the
apparent lack of an effective antidote.
Because of the apparently unusual effect on cholinesterase activity
and the lack of long-term feeding studies only a temporary acceptable
daily intake for man was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 3 ppm in the diet equivalent to 0.15 mg/kg per day.
Dog - 2 ppm in the diet equivalent to 0.05 mg/kg per day.
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolites and possible products of decomposition
A list of theoretically possible metabolites and primary products of
hydrolysis is given [text missing]. These compounds are numbered and the
numbers are used throughout this part of the text; thus (2).
Use pattern
Fenthion is an insecticide with a broad spectrum of activity. It is
chiefly effective against Diptera, cereal bugs, rice stem borers. The
main uses are as follows:
50% field crops (rice, cereals, sugar beets, etc.)
30% fruit and vine (i.e. pome and stone fruit, citrus, olives)
20% other uses (i.e. ornamentals, public health, animal health)
It is registered in 31 countries for use on crops.
Application to plants
The following formulations are available for application to crops:
50 Emulsifiable Concentrate (550 g/l)
1000 ULV (1000 g/l)
40% Wettable Powder
25% Wettable Powder
3% Dust
Pre-harvest treatments
The recommended application rates/concentrations for the major crops
are as follows:
Sugar cane 0.5-0.75 kg/ha
Rice 0.04-0.075% and 0.6-1.2 kg/ha, resp.
Field corn 0.75-1.0 kg/ha
Beets 0.3-1.5 kg/ha
Pome and stone 0.05-0.075%
fruit
Citrus fruits 0.05-0.075% (or bait application, see olives)
Pistachio 0.05%
Cotton 0.25-2.0 kg/ha
Olives 0.05% bait application at 0.15 kg + 0.3 kg
bait/20 litres of water for aerial
application or 0.25-0.3 kg + 0.75-1.0 kg
bait/100 litres of water for ground machine
application,
Coffee 0.05-0.075%
Cocoa 0.05%
Vegetables 0.05-0.075%, peas up to 0.1%
Vines 0.75-1.0 kg/ha
Corn 0.75-1.0 kg/ha
The above-mentioned concentrations and rates of application refer to
the active ingredient.
Post-harvest treatments
No recommendations are made for post-harvest treatments.
Application to animals
For use as an animal health product, fenthion is applied in the
following formulations, concentrations and dosage rates and by the
given methods for the control of ectoparasites and endoparasites on
domestic animals and livestock.
(1) 25% emulsifiable concentrate
(2) 2 or 3% solution (pour-on)
(3) 0.25%-5% dust (for experimental use)
(4) 0.5-1% in oil (back-rubber)
(5) 10% water-miscible feed premix (for experimental use)
(6) Injectable solution
(7) 0.32% Molasse-block (for experimental use)
Fenthion EC is used at concentrations of 0.025-0.25% as a dip, spray
and wash treatment for the control of ticks, biting and sucking lice,
flies, keds, hornflies, mange and cattle grubs on horses, sheep,
cattle, dogs and poultry.
Fenthion 2 or 3% (pour-on) is used at dosages of 4-45 mg/kg for the
control of cattle grubs, ticks, biting and sucking lice, flies and
fleas on cattle.
Fenthion Dust is used at concentrations of 0.25-5% for the control of
ticks, hornflies, biting and sucking lice, flies, fleas and keds on
horses, cattle, sheep, dogs and poultry.
Fenthion in oil is used in the form of back-rubbers as a
self-treatment for the control of hornflies, biting and sucking lice,
flies and fleas on cattle.
Fenthion 10% water-miscible and 1% premix is applied at dosages of
1.2-30 mg/kg for the control of cattle grubs, hornflies, flies, fleas,
biting and suckling lice, nose bots and endoparasites on cattle and
sheep.
Fenthion (injectable) is used at dosages of 4.4-15 mg/kg for the
control of demodicosis, heart worms, Ancylostoma spp. and
Uncinaria spp.in dogs.
Fenthion is registered for the use on animals in the following
countries: Australia, Austria, Belgium, Germany, Great Britain,
Ireland, Italy, New Zealand, Spain, Switzerland, United States of
America, Yugoslavia.
Other uses
Fenthion is widely used for the control of insects affecting public
health, especially mosquitos and flies where a combination of high
potency, persistence and stability when applied to surfaces of
buildings, ditches, swamps, etc.
These applications are not likely to give rise to contamination of
food.
Residues resulting from supervised trials
On plants
Detailed residue data are available from many countries, including
England, Germany, Greece, Italy, New Zealand, South Africa, Spain,
Switzerland and the United States of America on many important crops
and have been deposited with FAO. The results showing the residue
levels remaining after application of fenthion according to registered
use patterns are reflected in the following typical examples:
TABLE
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Apples England 1´ lb/acre EC 50 3 3.12 1.74 0.72 0 (84)
S. Africa 0.05% WP 25 1 2.1 1.4
Switzerland 0.05% EC 50 1 1.5 1.35 0.6 0.65 0.15(35)
Grapes S. Africa 1 kg/ha D 5 1 1.15 0.15 0.05 <0.05
Peaches S. Africa 4 oz/100 gal EC 50 1 2.1 0.9
S. Africa 8 oz/100 gal EC 50 1 3.2 1.6
S. Africa 8 oz/100 gal EC 50 1 2.3 0.9 0.25
Oranges Spain 300 g/ha ULV 1 0.1 0.1 N.D.
Oranges whole Spain 0.025% WP 1 0.55 0.5 0.3
skin Spain 0.025% WP 1 2.4 2.4 1.4
pulp Spain 0.025% WP 1 0.2 0.05 <0.05
whole Spain 300 g/ha EC 50 1 0.13 0.13 0.03 0.04 0.03(54)
skin Spain 300 g/ha EC 50 1 0.42 0.42 0.05 0.05 0.04(54)
Cherries Switzerland 0.05% EC 50 1 1.65 1.05 0.45 0.3
Gemany 0.05% EC 50 1 4.75 0.95 0.55
Gemany 0.025% EC 50 1 2.4 0.8 0.35
Peas whole pod England 0.125% EC 50 2 4.52 0.85 0.38 0.02
peas only England 0.125% EC 50 2 <0.02 0.23 <0.02
pod England 0.025% EC 50 2 1.1 0.5 0.4 <0.1
peas England 0.025% EC 50 2 <0.02 0.1 0.05 0.05
Squash S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02 <0.02
S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02
Lettuce Gemany 0.05% EC 50 2 3.85 3.46 2.46 0.42 0.05
Gemany 0.05% EC 50 1 3.2 2.3 1.45 0.3
Gemany 0.15% EC 50 1 10.2 3.9 2.75 0.8
Cabbage Germany 0.05% EC 50 2 1.16 0.37 0.0
Germany 0.05% EC 50 2 2.18 1.37 0.24
TABLE (Continued)
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Sugar (roots) Germany 0.05% EC 50 2 8.09 1.23 0.82 0.15 0.0
beet (tops) Germany 0.05% EC 50 2 0.50 0.16
Wheat Spain 0.65 kg/ha EC 50 1 0.02 0.02 <0.01(63)
New Zealand 0.7 kg/ha EC 50 1 0.4 0.2(32)
Rice USA 1.6 oz/acre EC 50 3 <0.05
USA 2.4 oz/acre EC 50 3 <0.05(33)
USA 1.6 oz/acre EC 50 1 0.48 <0.01
Olives Turkey 0.06% EC 50 1 0.39(34)
Greece 0.04% EC 50 4 0.15(36)
Greece 0.04% EC 50 4 0.75(70)
Italy 0.05% EC 50 1 1.3 0.8(32)
On forage crops
Extensive trials have been reported (Chemagro Reports 24 890; 24 891;
24 915; Mulla et al.) to show that following registered uses of
fenthion on alfalfa (0.1-0.5 kg/ha) residues on treated alfalfa range
up to 6 ppm after 7 days, but by the fourteenth day these levels have
declined to less than 2 ppm. In one typical experiment alfalfa was
treated close to harvest with fenthion at 0.11 kg/ha and residues were
18; 1.3; 1.7 and 0.7 ppm at 0; 2; 4; and 8 days after treatment.
The levels in corn and grass forage are somewhat lower than in
alfalfa.
The occurrence of residues in milk and meat following the feeding of
such treated forage is discussed under the heading uptake of the
compound with feed.
On animals
Tissues and organs
Details of many trials made to determine the level and fate of
residues of fenthion in animal tissues, milk and eggs were available
and were deposited with FAO. A selection of typical results has been
reviewed to show the level of residues resulting from approved
applications of fenthion for the control of ectoparasites of cattle,
sheep and poultry and from the ingestion of fenthion residues on
animal feeds and fodder.
A single backline treatment with a 2% pour-on (6.25 mg of fenthion/kg)
produced the following residues (Chemagro, 1965b):
Days after application (ppm)
1 3 7 14 28
Brain <0.1 n.d. n.d. n.d. -
Heart <0.1 n.d. n.d. n.d. -
Liver 0.2 n.d. n.d. n.d. -
Kidney <0.1 n.d. n.d. n.d. -
Loin steak approx. 0.1 <0.1 0.1 n.d. n.d.
Round steak <0.1 n.d. <0.1 n.d. n.d.
Flank steak 0.2 0.1 <0.1 n.d. n.d.
Omental fat 0.8 0.5 0.2 <0.1 n.d.
Back fat 0.7 0.4 1.5 n.d. n.d.
Renal fat 0.9 0.5 1.2 approx. 0.1 n.d.
Averages for 3 animals
Backline treatment of cattle with a 3% pour-on (9.4 mg of fenthion/kg)
resulted in the formation of the following residues in the tissues
(Chemagro, 1968b):
Days after application (ppm)
1 3 7 28
Brain 0.08 <0.01 <0.01 <0.01
Heart 0.10 - 0.07 <0.02
Liver 0.14 0.01 0.01 <0.01
Kidney 0.15 0.06 0.10 0.02
Loin steak 0.18 0.05 0.07 0.01
Round steak 0.12 0.02 0.10 <0.01
Flank steak 0.19 0.08 0.21 0.01
Omental fat 0.19 0.14 0.31 0.02
Renal fat 0.18 0.54 0.38 0.03
Back fat 0.19 0.83 0.62 0.09
The tabulated values represent the averages for three animals, and
comprise the totality of the non-ionic residues.
Another experiment with a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg, at first showed that the residues were only
slight (Chemagro, 1967e) but after 28 days they were of the same order
of magnitude as in the first-mentioned experiment (Chemagro, 1968b):
Days after application (ppm)
7 14 28 42
Brain 0.01 - 0.01 -
Heart <0.01 - 0.02 -
Liver 0.01 <0.01 0.01 -
Kidney 0.01 <0.01 <0.01 -
Loin steak <0.01 - 0.01 -
Round steak <0.01 - <0.01 -
Flank steak <0.01 - 0.02 -
Omental fat 0.10 - 0.07 <0.01
Renal fat 0.12 - 0.15 <0.01
Back fat 0.13 - 0.07 <0.01
Averages for 3 animals
The same organs and tissues were examined also 45 days after topical
application to the skin using a 20% "Spotton solution" (5.8 mg of
fenthion/kg); they were all found to be free of residues (Chemagro,
1970).
Similar results were obtained in an experiment using a spray
application (1 gal of 0.25% a.i. per animal). No residues were found
(Chemagro, 1965c) in brain after three days; in liver after seven
days; in heart and kidney after 14 days; in steaks after 14 days; in
fat after 28 days.
Following three spray applications of a 0.1% fenthion solution to the
point of run-off, at intervals of two weeks, no detectable residues
(less than 0.05 ppm) were present in brain, heart, liver and steaks
after 28 days; omental fat contained on average 0.1 ppm, renal fat
contained 0.05-0.1 ppm, and back fat contained 0.05 ppm. All fat
samples were free of residues after 44 days (Chemagro, 1967d).
Following daily backrubber application (1% a.i.) for four weeks, no
residues were detected in the organs and tissues after the final
application (Chemagro, 1965d).
Elimination in milk
The first results on the possible elimination of fenthion and its
metabolites in milk were reported in the studies of Knowles & Arthur
(1966) (see "Tissues and Organs").
The concentration of acetonitrile-soluble materials, which represented
predominantly fenthion or its oxidation products, was at its maximum
six hours after dermal treatment (0.44 ppm) and 20 hours after
intramuscular treatment (0.80 ppm). After 14 days, the residues in the
milk amounted to less than 0.001 ppm (dermal) and 0.014 ppm (i.m.). Of
the total activity administered, 1.1% was eliminated (as 32P) in the
milk within 14 days after dermal treatment, and 2.2% was eliminated in
the milk within 20 days after i.m. treatment.
A daily backrubber application of 50 ml of a 1% fenthion solution for
seven days resulted in acetonitrile-soluble residues of 0.06-0.36 ppm
during treatment. Five days after treatment, these residues were below
0.01 ppm (Chemagro, 1963).
Backline application of a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg., and, in a comparison experiment, of a three
times higher concentration produced the following residues (in ppm) in
the milk (Chemagro, 1969):
Days after application 9.4 mg/kg 28 mg/kg
0.4 0.81 2.82
1 0.33 0.66
2 0.13 0.15
3 0.047 0.056
5 0.011 0.017
7 0.04(?) 0.003
10 0.003 <0.002
14 <0.002 <0.002
21 <0.002 <0.002
An experiment on six animals (single backline application with
3% pour-on, 9.4 mg/kg) provided a good picture of the range of
residues in the milk of the individual cows (Chemagro, 1968a).
Residues in the milk (ppm)
Days after application Range Average
1 0.055 - 0.106 0.072
2 0.129 - 0.167 0.150
3 0.012 - 0.023 0.019
7 <0.005 <0.005
Daily backrubber treatment of cattle with 1% a.i., equivalent to
approximately 0.5 g a.i. per animal and day, for a period of four
weeks caused no formation of residues in the milk at the end of the
treatment period (Chemagro, 1967a).
The results presented here by and large agree with the data of
Möllhoff (1970) who reports that following application of 10 mg of
fenthion/kg (2% pour-on) the milk was free of residues after seven
days and the edible tissues were free of residues after 13 days. All
these observations can be accounted for by the physical properties of
fenthion, on the one hand, and its metabolites, on the other hand. The
oxidation products have a higher polarity. As a result of these
properties, the penetration of fenthion through the skin is favourably
influenced by the lipophilic properties of the parent compound, on the
one hand, whereas, on the other hand, compounds (2) to (6), which are
formed following penetration by metabolic processes, undergo faster
degradation and elimination than unchanged fenthion would.
Shillam et al. (1971) applied 125 ml fenthion as a 2% solution in
liquid paraffin to each of three cows (equivalent to 2.5 ml fenthion
per animal) and determined the level of fenthion in the milk. The cows
were in low production (approximately 20 lbs milk per day when milked
twice daily).
It was found that milk drawn 15 hours post application contained 0.14
ppm fenthion (0.08, 0.14, 0.20 ppm) but milk drawn 48 hours post
treatment contained no measurable amount of fenthion (less than 0.005
PPM). Pasteurization of the 15 hour milk reduced the fenthion residue
level to 0.04 ppm. There was a further decline in the residue level
when the pasteurized milk was analysed after 48 hours storage at 4°C.
Shipp (1970) reported that the excretion of fenthion in milk reached a
peak 12 hours after treating cows with 2% fenthion solution at the
rate of 100 ml per cow (2 g per head). The concentration found in milk
from four separate cows ranged from 1.1 ppm to 1.7 ppm in the
butterfat of milk drawn 12 hours after application. The level had
declined to 0.7-1.1 ppm at 24 hours and 0.2-0.3 ppm at 36 hours. At 48
hours the residue level was less than 0.1 ppm in the fat of milk.
Uptake of the compound together with feed
A feed supplement treatment of two cows (two daily applications of
0.72 mg of fenthion/kg in capsules) for 14 days resulted in residues
of 0.02-0.36 ppm in the milk during treatment; two days
post-treatment these residues were less than 0.01 ppm (Chemagro,
1963).
Administration of fenthion-containing feed to cattle for six days (2.5
mg of fenthion/kg) produced no detectable residues (less than 0.1 ppm)
in the following organs: brain, heart, liver, kidney, steaks (loin,
round, flank), fat (omental, renal, back) (Chemagro, 1965a). Daily
administration of 5 ppm of fenthion in the feed for four weeks
produced no residues in the milk at the end of the experiment
(Chemagro, 1966d). Administration of 10 ppm of fenthion in the feed
for seven days (0.5 mg/kg/day) likewise resulted in no residues in the
milk on the final day of the experiment (Chemagro, 1966a).
Bowman et al. (1970) investigated:
(a) whether residues of fenthion and its metabolites are secreted in
the milk or excreted in the urine and faeces of cows fed silage
made from corn treated with fenthion, and
(b) whether residues of fenthion in silage affect the physiological
activity of lactating cows.
The corn was sprayed with (A) 0.56, (B) 1.12, and (C) 2.24 kg of
fenthion/hectare and ensiled in tower silos one day post-treatment.
The ensiled corn was allowed to age 88 days before the silages were
fed to the cows during a 56-day study. The residues of fenthion in
corn and corn silage after field treatment at the three rates were as
follows (in ppm):
A B C
day of treatment (forage) 4.3 17.6 34.6
day after treatment (forage) 1.7 3.5 12.0
ensiled 14 days (silage) 1.4 4.3 10.0
ensiled 56 days (silage) 1.0 2.1 8.9
ensiled 71 days (silage) 0.82 2.5 9.6
silage fed first week 0.44 1.3 5.9
silage fed third week 0.55 1.7 6.0
silage fed fifth week 0.55 1.6 5.0
silage fed eighth week 0.62 1.3 5.1
In all cases, compound (2) accounted for most of the residue.
One of the silages was assigned to each group of four cows. The
animals ingested averages of (A) 0.03, (B) 0.07, and (C) 0.3 mg of
residues/kg/day. No residues were detected in the milk from (A) and
(B). Only low levels (maximum of 0.014 ± 0.003 ppm) were found in the
milk from cows fed silage from corn treated at a rate of 2.24 kg of
fenthion/hectare (C). Also, one week after feeding was terminated, no
residues could be found in the milk in group (C).
Only slight amounts of residues occurred also in the faeces and urine
of the animals (expressed in ppm):
Days fed treated feed A B C
26 faeces 0.003 0.015 0.09
urine 0.004 0.016 0.12
55 faeces 0.014 0.03 0.16
urine 0.004 0.014 0.16
No residues were detected in urine and faeces one week after feeding
was terminated.
The effect of diet on the metabolism of fenthion in laboratory
animals (rabbits and rats) was studied by Begum (1968) 35S-labelled
fenthion (Y position) was used in these experiments. When this
compound was administered orally or subcutaneously to healthy,
well-fed animals (group A), the peak concentration of the
radioactivity appeared in the blood six to nine hours after treatment.
The peak was observed in lean, half-starved animals (group B) one hour
after treatment. There was more oxidized fenthion in the blood of
group B rabbits than in the blood of group A rabbits. In both groups
of animals, fenthion was eliminated primarily in the urine, and to a
lesser extent in the faeces. There was a higher percentage of
radioactive materials (mostly hydrolyzed products) excreted in the
urine and faeces by group B animals. Apparently there was a more rapid
breakdown and elimination from the system of lean animals than from
fat ones.
Experiments with sheep
Sheep which were treated with a 2% pour-on (60 mg of fenthion/kg)
showed no residues in brain, heart, liver, kidney, loin steak,
shoulder, leg and fat (omental, renal, back) 44 days after the
application (Chemagro, 1966c). Sheep treated with 50% powder applied
as an oral drench at a dose level of 30 mg/kg also displayed no
residues in the above-mentioned organs and tissues 14 days after the
application (Chemagro, 1966b).
When sheep were treated by dipping them in a 500 ppm solution of
fenthion, only very slight residues appeared (Möllhoff, 1970). No
residues were detected in the organs; in meat, residues were
detectable only at seven days after treatment in one out of two
animals (0.1 ppm). The concentration was highest in fat (up to 0.35
ppm on the seventh day). When the same formulation was injected
intravenously in two sheep at a rate of 5 mg of fenthion/kg 12 hours
before slaughter, the highest residues again appeared in the fat
(1.6 ppm) whilst in meat the residues amounted to a maximum of 0.7 ppm
(Möllhoff, 1970).
Experiments on poultry
Fenthion is not eliminated in hen eggs. Hens were fed on a diet
containing 2 ppm of fenthion for four weeks; at the end of the feeding
period, there was no trace of residues in the eggs (Chemagro, 1967b).
The tissues of six hens (giblets, muscle and fat) were also free of
residues at the end of the feeding experiment (only exception: giblets
contained 0.08 ppm in one out of six hens; fat contained 0.07 ppm in
one out of six hens (Chemagro, 1967c)).
FATE OF RESIDUES
The behaviour of the compound fenthion in living systems, like that of
all pesticides which contain a thioether moiety, is determined by its
readiness to form sulfoxides and sulfones. As an S-alkyl isomerization
can also be reckoned with under thermal influences, the following
non-ionic metabolites of fenthion (1) are theoretically to be
expected:
TOXICOLOGICAL STUDIES
Special studies
(a) Acute toxicity of the metabolites
Compound Acute LD50 (mg/kg) 150 (M)c
Orala i.p.b
I. Fenthion 220 325 >5 × 10-4
II. Fenthion sulfoxide 125 250 4.5 × 10-5
III. Fenthion sulfone 125 250 4.7 × 10-4
IV. Oxygen analogue 125 26 2.65 × 10-6
V. O-Sulfoxide 50 22 4.8 × 10-5
IV. O-Sulfone 30 9 3.2 × 10-5
VIII. 4-(methylthio)m-cresol 6500d
IX. 4-methyl(thiosulfoxide)
m-cresol 3500d
X. 4-methyl(thiosulfone)
m-cresol 7000d
a Male rats - according to Francis and Barnes, 1963.
b Female rats - according to Dubois and Kinoshita, 1964.
c 150 = Molar concentration resulting in 50% inhibition of
human RBC Cholinesterase (Francis and Barnes, 1963).
d Female rats according to Nelson, 1967.
(b) Reproduction
Except for a slight growth depression at the highest level in the
diet, fenthion at levels of 0, 3, 15 and 75 ppm for three generations
(two litters per generation), produced no adverse effect on rat
reproduction (Loser, 1969). Microscopic examination of the tissues of
the F3b generation did not reveal any significant abnormality (Spicer,
1971).
(c) Potentiation
Fenthion potentiates the acute intraperitoneal toxicity of malathion,
dioxathion, and coumaphos in rats. Intraperitoneal administration of
13 other organophosphate or carbamate insecticides did not result in a
greater than additive effect when administered with fenthion (Dubois
and Kinoshita, 1964). Dietary combination of fenthion with coumaphos
neither of which alone affected cholinesterase when fed to dogs for
six weeks, was found to potentiate the anticholinesterase activity of
serum and red blood cells. Less evident potentiation was observed when
fenthion and malathion were fed and when fenthion was fed in
combination with dioxathion no potentiation was noted (Doull et al.,
1962), Fenthion (2 ppm) and malathion (100 ppm) resulted in moderate
erythrocyte and serum cholinesterase activity inhibition (30-40%).
When fenthion (2 ppm) and dioxathion (3 ppm) was fed cholinesterase
activity was not depressed. When fenthion (2 ppm) and coumaphos
(2 ppm) was fed to dogs, significant inhibition (75%) of serum
cholinesterase and moderate inhibition (30%) of erythrocyte
cholinesterase was evident. Oral administration to rats of a 75:25
mixture of fenthion and dichlorvos did not result in a greater than
theoretically additive toxicity (Kimmerle, 1967b).
(d) Neurotoxicity
No evidence for delayed neurological disruption in hens was evident
when fenthion was administered orally at a single dose of 25 mg/kg
(Kimmerle, 1965a). When chickens were fed up to 100 ppm in the diet
for 30 days, clinical examination of the animals and histological
examination of nerve tissue indicated no demyelinating effect from
fenthion (Kimmerle, 1965b; Dieckmann, 1971).
(e) Antidotes
A number of antidotes which are commonly used for organophosphorous
poisoning have been shown to be relatively inactive when used
following fenthion intoxication. Studies with atropine, 2-PAM
Toxogonin P-2-S, and TMB-4 (Dubois and Kinoshita, 1964; Francis and
Barnes, 1963; and Dubois, 1960; Lorke and Kimmerle, 1969) administered
alone and in combination showed that these materials did not
successfully alleviate the parasympathomimetic signs of
organophosphate poisoning. When BH-G (Merck; Darmstadt, Germany) was
administered three to four times in combination with atropine, the
LD50 value increased from 250 to 440 mg/kg (Kimmerle, 1963).
Toxogonin was not effective as a cholinesterase reactivator following
oral intoxication of dogs by fenthion (Hahn and Henschler, 1969).
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Mouse (M) Oral 150 Francis and Barnes, 1963
227 Dubois, 1968
(F) 190 Francis and Barnes, 1963
225 Dubois, 1968
(F) i.p. 150 Dubois and Kinoshita, 1964
(M) 125 Dubois and Kinoshita, 1964
(Continued)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat (F) Oral 245-310 (Dubois and Kinoshita, 1964
(Gaines, 1960
615 Francis and Barnes, 1963
(M) 175-470 (Dubois and Kinoshita, 1964
(Klimmer, 1963
(Gaines, 1969
(Francis and Barnes, 1963
(M) i.p. 325 Dubois and Kinoshita, 1964
(F) 260 Dubois and Kinoshita, 1964
(M) 152 Klimmer, 1963
(M) Dermal 330-650 Gaines, 1969
Klimmer, 1963
Francis and Barnes, 1963
(F) 330-500 Dubois and Kinoshita, 1964
Gaines, 1969
Francis and Barnes, 1963
Guinea-pig
(M) Oral 1000 Francis and Barnes, 1963
260 Dubois and Kinoshita, 1964
i.p. 310 Dubois and Kinoshita, 1964
Rabbit (M) Oral 150-175 Francis and Barnes, 1963
Dermal 150 Klimmer, 1963
Duck Oral 15 Dubois and Doull, 1960
1-2 Keith and Mulla, 1966
Chicken 30 Dubois and Doull, 1960
28 Sherman and Ross, 1961
30-40 Francis and Barnes, 1963
Calf Approx. 40 McGrath, 1961
Various solvents used to solubilize fenthion in combination with water
or ethanol had no significant effect on the acute oral LD50
(Kimmerle, 1967a).
Fenthion is an organophosphorus insecticide of intermediate toxicity
to mammals although it displays considerable differences in its
toxicity to various species, e.g. fowls are very sensitive (Keith and
Mulla, 1966).
The rate of absorption through the skin of rabbits is slow. When
fenthion was applied to a cotton plug and placed in a rabbit ear for
four hours, swelling occurred. When it was left for 24 hours mortality
resulted (Kimmerle, 1960).
Female rats tolerated a daily one hour inhalation challenge of 0.163
mg/l air for 30 days with cholinesterase depression but no mortality.
At 0.415 mg/l air all animals were dead within 10 days (Dilley and
Doull, 1961a).
Exposure by inhalation daily for nine days, six hours per day at 210
mg/M3 (initial concentration in a static inhalation chamber),
resulted in signs of poisoning, but no mortality in cats, guinea pigs,
rabbits and rats. Cholinesterase, which was severely depressed,
recovered in three weeks (Klimmer, 1963). Fenthion exhibits a
relatively low degree of acute mammalian toxicity. In only one
instance was a sex difference in susceptibility noted and this is in
contrast to the generally noted resistance of males to most other
phosphorothioates (Dubois and Kinoshita, 1964). The signs of poisoning
are typical of the central and peripheral cholinergic effects of
organophosphorus esters with a gradual onset of the symptoms. The
symptoms in humans and other animals include tremors, lacrymation,
salivation, vomiting, diarrhoea and other signs of cholinergic
stimulation.
Short-term studies
Duck
Mallard ducks fed 25 ppm of fenthion in the diet for six weeks became
emaciated and could not fly or walk; after two days on normal food,
recovery was evident. A dietary level of 5 ppm produced no adverse
clinical reaction (Keith and Mulla, 1966).
Rat
Dermal administration of fenthion at 14.5 and 25 mg/kg for 60 days to
rats resulted in 40% mortality in the higher dosed group and no
mortality in the lower dosed group. However, blood cholinesterase
levels were depressed to about 20% of normal at the lower treatment
level (Dubois, 1961). Cholinesterase was depressed and mortality was
absent when fenthion was applied to rats dermally for 12 days at 2.9
mg/kg (Dubois and Puchala, 1960).
Rats tolerated daily intraperitoneal administration of 10 mg/kg
fenthion for 60 days with no mortality. At 20 mg/kg, 80% of the
treated animals died within 20 days (Dubois and Kinoshita, 1964).
Mortality (12 dead of 30 rats tested) occurred following daily oral
administration of approximately 25 mg/kg (1/10 LD50) for 75 days
(six days per week). Signs of poisoning were transient disappearing
shortly after dosing (Klimmer, 1963).
In a preliminary study, male rats were orally administered fenthion
five days a week for 13 weeks at a dose of 30 mg/kg/day. Mortality
occurred in approximately 30% of the rats over the course of the
experiment and cholinesterase activity was depressed between 80-90% of
normal. At the conclusion of the experiment cholinesterase recovery
was very slow - up to 40 days (Kimmerle, 1961).
Rats (22 male and 22 female per group) were fed dietary levels of
fenthion at 0.25, 0.50, 2.5, and 5.0 mg/kg/day for three months.
Cholinesterase activity depression was evident at 0.5 mg/kg in red
blood cells, serum, liver and heart at all testing intervals. At the
lowest feeding level (0.25 mg/kg) the inhibition (approx. 10-20%) did
not progressively increase with time indicating lack of cumulative
effects. Mortality in females was evident at 5.0 mg/kg. The animals
died manifesting muscarinic and nicotinic effects. Body-weight gain
was reduced in males at 0.25 mg/kg and above while in females it was
evident only at 2.5 mg/kg. Behavioural effects were noted
(piloerection) at 0.5 mg/kg and above (especially in females). This
effect decreased with time and disappeared by week seven. Organ
weights were all distinctly lower than the controls but as the
body-weight was also reduced the organ to body-weight ratio did not
appear to be affected. Histological examination showed the testis to
have reduced spermatogenesis and atrophic prostate glands at the
highest feeding levels (2.5 and 5.0 mg/kg). The ovary was not affected
(Shimamoto and Hattori, 1969).
Rats (six groups of 12 male and 12 female) were fed for 16 weeks on
diets containing 0, 2, 3, 5, 25 and 100 ppm. Cholinesterase depression
was evident at 25 ppm and absent at 5 ppm. No adverse effects were
noted in food consumption, weight gain or gross and microscopic
examination of tissues (Doull et al., 1961).
Rats (six groups of 25 male and 25 female) were fed for one year on
diets containing 0, 2, 3, 5, 25 and 100 ppm of fenthion. There was no
evidence of significant changes in growth rate, food consumption,
general appearance and gross of microscopic examination of tissues.
Survival of male rats at 25 ppm was slightly depressed. Cholinesterase
examinations indicated depression at the 5 ppm level and above with
3 ppm showing no adverse enzyme effects. A mild extramedullary
haematopoiesis was observed in controls and all dosage levels and
haemosideresis was evident in the spleen of the rats at 100 ppm levels
(Doull et al., 1963a).
Dog
Dogs (four groups of two males and two females per group) were fed
fenthion at 0, 2, 5 and 50 ppm for 12 weeks. Growth was not affected
at any dietary level. Erythrocyte cholinesterase activity was
depressed at 50 ppm while serum cholinesterase was depressed at 5 ppm
and above. Little if any depression of the serum cholinesterase was
evident before five weeks, after which it progressively decreased to
about 40% inhibition (Doull et al., 1961).
Dogs (four groups of two males and two females) were fed fenthion at
0, 2, 5, and 50 ppm in the diet for one year. There was no effect of
fenthion on food consumption or growth over the test interval.
Erythrocyte and serum cholinesterase were significantly depressed at
50 ppm with the serum also depressed at 5 ppm. An increase in the
weight of the spleen, which was not dose dependent, was evident in all
of the treated animals. Microscopic examinations of the tissues showed
splenic congestion and some decrease in the cellularity of the red
pulp was evident at all dose levels fed. Extramedullary haematopoiesis
and haemosideresis was also observed in the spleen. Microscopic
examination of other tissues did not reveal any significant change
(Doull et al., 1963b).
Long-term studies
No data available.
Observations in man
Fenthion has been widely used in many parts of the world for control
of household pests, mosquitos, etc. Cholinesterase studies conducted
on individuals in areas treated by WHO for malaria eradication have
shown that very slight plasma cholinesterase depression occurs when
exaggerated spray schedules were followed. The plasma cholinesterase
levels were depressed for up to six weeks after spraying (Elliot and
Barnes, 1963). It was also evident that the children in the population
(less than seven years old) were more susceptible to the
anticholinesterase effects (Taylor, 1963). A man who ingested two
ounces of fenthion (Entex)(R) recovered from severe
organophosphorous poisoning after being in critical condition for the
first six days after poisoning. Recovery was slow, lasting up to 30
days. Cholinesterase measurements showed that at 22 days after
poisoning the cholinesterase activity was still depressed (Pickering,
1966). In humans, the signs of poisoning appear rapidly beginning with
blurred vision, unsteady gait and slurred speech. After 72 hours of
emergency treatment following an unknown quantity of fenthion, a man
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation. The patient began to recover
only after 11 days of treatment which included atropine, PAM and
toxogonin (Dean et al., 1967). In another case, 45 minutes after
ingestion of 30 ml of fenthion, a man was in a comatose state with
pale skin, cyanotic mucous membranes, slow regular heart beat, no
peripheral blood pressure and no reactions to pain or light
stimulation on the pupils. Recovery took six days (von Clarmann and
Geldmacher-von-Mallinkradt, 1966).
Comments
Fenthion is slowly absorbed, metabolized by a complex series of
reactions and excreted. It does not appear to accumulate in the body.
In most instances the unhydrolysed metabolites containing a phosphorus
atom are more toxic than the parent compound. The signs of
intoxication from a single oral dose develop slowly, persist for a
considerable period of time and are not readily alleviated by atropine
or by most common oxime reactivators. Short-term studies in the rat
and dog suggest that cholinesterase inhibition is the most sensitive
criterion of biological effect. Anti-spermatogenesis in one rat study
was not confirmed in other studies or in a reproduction study. The
occurrence of an effect on the spleen in both rats and dogs was not
considered to constitute evidence of significant toxicity. Of
particular concern was the slow onset of symptoms of cholinergic
stimulation, differences in susceptibility to acutely toxic doses in
rodents and birds, the long lasting cholinesterase depression and the
apparent lack of an effective antidote.
Because of the apparently unusual effect on cholinesterase activity
and the lack of long-term feeding studies only a temporary acceptable
daily intake for man was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 3 ppm in the diet equivalent to 0.15 mg/kg per day.
Dog - 2 ppm in the diet equivalent to 0.05 mg/kg per day.
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolites and possible products of decomposition
A list of theoretically possible metabolites and primary products of
hydrolysis is given [text missing]. These compounds are numbered and the
numbers are used throughout this part of the text; thus (2).
Use pattern
Fenthion is an insecticide with a broad spectrum of activity. It is
chiefly effective against Diptera, cereal bugs, rice stem borers. The
main uses are as follows:
50% field crops (rice, cereals, sugar beets, etc.)
30% fruit and vine (i.e. pome and stone fruit, citrus, olives)
20% other uses (i.e. ornamentals, public health, animal health)
It is registered in 31 countries for use on crops.
Application to plants
The following formulations are available for application to crops:
50 Emulsifiable Concentrate (550 g/l)
1000 ULV (1000 g/l)
40% Wettable Powder
25% Wettable Powder
3% Dust
Pre-harvest treatments
The recommended application rates/concentrations for the major crops
are as follows:
Sugar cane 0.5-0.75 kg/ha
Rice 0.04-0.075% and 0.6-1.2 kg/ha, resp.
Field corn 0.75-1.0 kg/ha
Beets 0.3-1.5 kg/ha
Pome and stone 0.05-0.075%
fruit
Citrus fruits 0.05-0.075% (or bait application, see olives)
Pistachio 0.05%
Cotton 0.25-2.0 kg/ha
Olives 0.05% bait application at 0.15 kg + 0.3 kg
bait/20 litres of water for aerial
application or 0.25-0.3 kg + 0.75-1.0 kg
bait/100 litres of water for ground machine
application,
Coffee 0.05-0.075%
Cocoa 0.05%
Vegetables 0.05-0.075%, peas up to 0.1%
Vines 0.75-1.0 kg/ha
Corn 0.75-1.0 kg/ha
The above-mentioned concentrations and rates of application refer to
the active ingredient.
Post-harvest treatments
No recommendations are made for post-harvest treatments.
Application to animals
For use as an animal health product, fenthion is applied in the
following formulations, concentrations and dosage rates and by the
given methods for the control of ectoparasites and endoparasites on
domestic animals and livestock.
(1) 25% emulsifiable concentrate
(2) 2 or 3% solution (pour-on)
(3) 0.25%-5% dust (for experimental use)
(4) 0.5-1% in oil (back-rubber)
(5) 10% water-miscible feed premix (for experimental use)
(6) Injectable solution
(7) 0.32% Molasse-block (for experimental use)
Fenthion EC is used at concentrations of 0.025-0.25% as a dip, spray
and wash treatment for the control of ticks, biting and sucking lice,
flies, keds, hornflies, mange and cattle grubs on horses, sheep,
cattle, dogs and poultry.
Fenthion 2 or 3% (pour-on) is used at dosages of 4-45 mg/kg for the
control of cattle grubs, ticks, biting and sucking lice, flies and
fleas on cattle.
Fenthion Dust is used at concentrations of 0.25-5% for the control of
ticks, hornflies, biting and sucking lice, flies, fleas and keds on
horses, cattle, sheep, dogs and poultry.
Fenthion in oil is used in the form of back-rubbers as a
self-treatment for the control of hornflies, biting and sucking lice,
flies and fleas on cattle.
Fenthion 10% water-miscible and 1% premix is applied at dosages of
1.2-30 mg/kg for the control of cattle grubs, hornflies, flies, fleas,
biting and suckling lice, nose bots and endoparasites on cattle and
sheep.
Fenthion (injectable) is used at dosages of 4.4-15 mg/kg for the
control of demodicosis, heart worms, Ancylostoma spp. and
Uncinaria spp.in dogs.
Fenthion is registered for the use on animals in the following
countries: Australia, Austria, Belgium, Germany, Great Britain,
Ireland, Italy, New Zealand, Spain, Switzerland, United States of
America, Yugoslavia.
Other uses
Fenthion is widely used for the control of insects affecting public
health, especially mosquitos and flies where a combination of high
potency, persistence and stability when applied to surfaces of
buildings, ditches, swamps, etc.
These applications are not likely to give rise to contamination of
food.
Residues resulting from supervised trials
On plants
Detailed residue data are available from many countries, including
England, Germany, Greece, Italy, New Zealand, South Africa, Spain,
Switzerland and the United States of America on many important crops
and have been deposited with FAO. The results showing the residue
levels remaining after application of fenthion according to registered
use patterns are reflected in the following typical examples:
TABLE
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Apples England 1´ lb/acre EC 50 3 3.12 1.74 0.72 0 (84)
S. Africa 0.05% WP 25 1 2.1 1.4
Switzerland 0.05% EC 50 1 1.5 1.35 0.6 0.65 0.15(35)
Grapes S. Africa 1 kg/ha D 5 1 1.15 0.15 0.05 <0.05
Peaches S. Africa 4 oz/100 gal EC 50 1 2.1 0.9
S. Africa 8 oz/100 gal EC 50 1 3.2 1.6
S. Africa 8 oz/100 gal EC 50 1 2.3 0.9 0.25
Oranges Spain 300 g/ha ULV 1 0.1 0.1 N.D.
Oranges whole Spain 0.025% WP 1 0.55 0.5 0.3
skin Spain 0.025% WP 1 2.4 2.4 1.4
pulp Spain 0.025% WP 1 0.2 0.05 <0.05
whole Spain 300 g/ha EC 50 1 0.13 0.13 0.03 0.04 0.03(54)
skin Spain 300 g/ha EC 50 1 0.42 0.42 0.05 0.05 0.04(54)
Cherries Switzerland 0.05% EC 50 1 1.65 1.05 0.45 0.3
Gemany 0.05% EC 50 1 4.75 0.95 0.55
Gemany 0.025% EC 50 1 2.4 0.8 0.35
Peas whole pod England 0.125% EC 50 2 4.52 0.85 0.38 0.02
peas only England 0.125% EC 50 2 <0.02 0.23 <0.02
pod England 0.025% EC 50 2 1.1 0.5 0.4 <0.1
peas England 0.025% EC 50 2 <0.02 0.1 0.05 0.05
Squash S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02 <0.02
S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02
Lettuce Gemany 0.05% EC 50 2 3.85 3.46 2.46 0.42 0.05
Gemany 0.05% EC 50 1 3.2 2.3 1.45 0.3
Gemany 0.15% EC 50 1 10.2 3.9 2.75 0.8
Cabbage Germany 0.05% EC 50 2 1.16 0.37 0.0
Germany 0.05% EC 50 2 2.18 1.37 0.24
TABLE (Continued)
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Sugar (roots) Germany 0.05% EC 50 2 8.09 1.23 0.82 0.15 0.0
beet (tops) Germany 0.05% EC 50 2 0.50 0.16
Wheat Spain 0.65 kg/ha EC 50 1 0.02 0.02 <0.01(63)
New Zealand 0.7 kg/ha EC 50 1 0.4 0.2(32)
Rice USA 1.6 oz/acre EC 50 3 <0.05
USA 2.4 oz/acre EC 50 3 <0.05(33)
USA 1.6 oz/acre EC 50 1 0.48 <0.01
Olives Turkey 0.06% EC 50 1 0.39(34)
Greece 0.04% EC 50 4 0.15(36)
Greece 0.04% EC 50 4 0.75(70)
Italy 0.05% EC 50 1 1.3 0.8(32)
On forage crops
Extensive trials have been reported (Chemagro Reports 24 890; 24 891;
24 915; Mulla et al.) to show that following registered uses of
fenthion on alfalfa (0.1-0.5 kg/ha) residues on treated alfalfa range
up to 6 ppm after 7 days, but by the fourteenth day these levels have
declined to less than 2 ppm. In one typical experiment alfalfa was
treated close to harvest with fenthion at 0.11 kg/ha and residues were
18; 1.3; 1.7 and 0.7 ppm at 0; 2; 4; and 8 days after treatment.
The levels in corn and grass forage are somewhat lower than in
alfalfa.
The occurrence of residues in milk and meat following the feeding of
such treated forage is discussed under the heading uptake of the
compound with feed.
On animals
Tissues and organs
Details of many trials made to determine the level and fate of
residues of fenthion in animal tissues, milk and eggs were available
and were deposited with FAO. A selection of typical results has been
reviewed to show the level of residues resulting from approved
applications of fenthion for the control of ectoparasites of cattle,
sheep and poultry and from the ingestion of fenthion residues on
animal feeds and fodder.
A single backline treatment with a 2% pour-on (6.25 mg of fenthion/kg)
produced the following residues (Chemagro, 1965b):
Days after application (ppm)
1 3 7 14 28
Brain <0.1 n.d. n.d. n.d. -
Heart <0.1 n.d. n.d. n.d. -
Liver 0.2 n.d. n.d. n.d. -
Kidney <0.1 n.d. n.d. n.d. -
Loin steak approx. 0.1 <0.1 0.1 n.d. n.d.
Round steak <0.1 n.d. <0.1 n.d. n.d.
Flank steak 0.2 0.1 <0.1 n.d. n.d.
Omental fat 0.8 0.5 0.2 <0.1 n.d.
Back fat 0.7 0.4 1.5 n.d. n.d.
Renal fat 0.9 0.5 1.2 approx. 0.1 n.d.
Averages for 3 animals
Backline treatment of cattle with a 3% pour-on (9.4 mg of fenthion/kg)
resulted in the formation of the following residues in the tissues
(Chemagro, 1968b):
Days after application (ppm)
1 3 7 28
Brain 0.08 <0.01 <0.01 <0.01
Heart 0.10 - 0.07 <0.02
Liver 0.14 0.01 0.01 <0.01
Kidney 0.15 0.06 0.10 0.02
Loin steak 0.18 0.05 0.07 0.01
Round steak 0.12 0.02 0.10 <0.01
Flank steak 0.19 0.08 0.21 0.01
Omental fat 0.19 0.14 0.31 0.02
Renal fat 0.18 0.54 0.38 0.03
Back fat 0.19 0.83 0.62 0.09
The tabulated values represent the averages for three animals, and
comprise the totality of the non-ionic residues.
Another experiment with a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg, at first showed that the residues were only
slight (Chemagro, 1967e) but after 28 days they were of the same order
of magnitude as in the first-mentioned experiment (Chemagro, 1968b):
Days after application (ppm)
7 14 28 42
Brain 0.01 - 0.01 -
Heart <0.01 - 0.02 -
Liver 0.01 <0.01 0.01 -
Kidney 0.01 <0.01 <0.01 -
Loin steak <0.01 - 0.01 -
Round steak <0.01 - <0.01 -
Flank steak <0.01 - 0.02 -
Omental fat 0.10 - 0.07 <0.01
Renal fat 0.12 - 0.15 <0.01
Back fat 0.13 - 0.07 <0.01
Averages for 3 animals
The same organs and tissues were examined also 45 days after topical
application to the skin using a 20% "Spotton solution" (5.8 mg of
fenthion/kg); they were all found to be free of residues (Chemagro,
1970).
Similar results were obtained in an experiment using a spray
application (1 gal of 0.25% a.i. per animal). No residues were found
(Chemagro, 1965c) in brain after three days; in liver after seven
days; in heart and kidney after 14 days; in steaks after 14 days; in
fat after 28 days.
Following three spray applications of a 0.1% fenthion solution to the
point of run-off, at intervals of two weeks, no detectable residues
(less than 0.05 ppm) were present in brain, heart, liver and steaks
after 28 days; omental fat contained on average 0.1 ppm, renal fat
contained 0.05-0.1 ppm, and back fat contained 0.05 ppm. All fat
samples were free of residues after 44 days (Chemagro, 1967d).
Following daily backrubber application (1% a.i.) for four weeks, no
residues were detected in the organs and tissues after the final
application (Chemagro, 1965d).
Elimination in milk
The first results on the possible elimination of fenthion and its
metabolites in milk were reported in the studies of Knowles & Arthur
(1966) (see "Tissues and Organs").
The concentration of acetonitrile-soluble materials, which represented
predominantly fenthion or its oxidation products, was at its maximum
six hours after dermal treatment (0.44 ppm) and 20 hours after
intramuscular treatment (0.80 ppm). After 14 days, the residues in the
milk amounted to less than 0.001 ppm (dermal) and 0.014 ppm (i.m.). Of
the total activity administered, 1.1% was eliminated (as 32P) in the
milk within 14 days after dermal treatment, and 2.2% was eliminated in
the milk within 20 days after i.m. treatment.
A daily backrubber application of 50 ml of a 1% fenthion solution for
seven days resulted in acetonitrile-soluble residues of 0.06-0.36 ppm
during treatment. Five days after treatment, these residues were below
0.01 ppm (Chemagro, 1963).
Backline application of a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg., and, in a comparison experiment, of a three
times higher concentration produced the following residues (in ppm) in
the milk (Chemagro, 1969):
Days after application 9.4 mg/kg 28 mg/kg
0.4 0.81 2.82
1 0.33 0.66
2 0.13 0.15
3 0.047 0.056
5 0.011 0.017
7 0.04(?) 0.003
10 0.003 <0.002
14 <0.002 <0.002
21 <0.002 <0.002
An experiment on six animals (single backline application with
3% pour-on, 9.4 mg/kg) provided a good picture of the range of
residues in the milk of the individual cows (Chemagro, 1968a).
Residues in the milk (ppm)
Days after application Range Average
1 0.055 - 0.106 0.072
2 0.129 - 0.167 0.150
3 0.012 - 0.023 0.019
7 <0.005 <0.005
Daily backrubber treatment of cattle with 1% a.i., equivalent to
approximately 0.5 g a.i. per animal and day, for a period of four
weeks caused no formation of residues in the milk at the end of the
treatment period (Chemagro, 1967a).
The results presented here by and large agree with the data of
Möllhoff (1970) who reports that following application of 10 mg of
fenthion/kg (2% pour-on) the milk was free of residues after seven
days and the edible tissues were free of residues after 13 days. All
these observations can be accounted for by the physical properties of
fenthion, on the one hand, and its metabolites, on the other hand. The
oxidation products have a higher polarity. As a result of these
properties, the penetration of fenthion through the skin is favourably
influenced by the lipophilic properties of the parent compound, on the
one hand, whereas, on the other hand, compounds (2) to (6), which are
formed following penetration by metabolic processes, undergo faster
degradation and elimination than unchanged fenthion would.
Shillam et al. (1971) applied 125 ml fenthion as a 2% solution in
liquid paraffin to each of three cows (equivalent to 2.5 ml fenthion
per animal) and determined the level of fenthion in the milk. The cows
were in low production (approximately 20 lbs milk per day when milked
twice daily).
It was found that milk drawn 15 hours post application contained 0.14
ppm fenthion (0.08, 0.14, 0.20 ppm) but milk drawn 48 hours post
treatment contained no measurable amount of fenthion (less than 0.005
PPM). Pasteurization of the 15 hour milk reduced the fenthion residue
level to 0.04 ppm. There was a further decline in the residue level
when the pasteurized milk was analysed after 48 hours storage at 4°C.
Shipp (1970) reported that the excretion of fenthion in milk reached a
peak 12 hours after treating cows with 2% fenthion solution at the
rate of 100 ml per cow (2 g per head). The concentration found in milk
from four separate cows ranged from 1.1 ppm to 1.7 ppm in the
butterfat of milk drawn 12 hours after application. The level had
declined to 0.7-1.1 ppm at 24 hours and 0.2-0.3 ppm at 36 hours. At 48
hours the residue level was less than 0.1 ppm in the fat of milk.
Uptake of the compound together with feed
A feed supplement treatment of two cows (two daily applications of
0.72 mg of fenthion/kg in capsules) for 14 days resulted in residues
of 0.02-0.36 ppm in the milk during treatment; two days
post-treatment these residues were less than 0.01 ppm (Chemagro,
1963).
Administration of fenthion-containing feed to cattle for six days (2.5
mg of fenthion/kg) produced no detectable residues (less than 0.1 ppm)
in the following organs: brain, heart, liver, kidney, steaks (loin,
round, flank), fat (omental, renal, back) (Chemagro, 1965a). Daily
administration of 5 ppm of fenthion in the feed for four weeks
produced no residues in the milk at the end of the experiment
(Chemagro, 1966d). Administration of 10 ppm of fenthion in the feed
for seven days (0.5 mg/kg/day) likewise resulted in no residues in the
milk on the final day of the experiment (Chemagro, 1966a).
Bowman et al. (1970) investigated:
(a) whether residues of fenthion and its metabolites are secreted in
the milk or excreted in the urine and faeces of cows fed silage
made from corn treated with fenthion, and
(b) whether residues of fenthion in silage affect the physiological
activity of lactating cows.
The corn was sprayed with (A) 0.56, (B) 1.12, and (C) 2.24 kg of
fenthion/hectare and ensiled in tower silos one day post-treatment.
The ensiled corn was allowed to age 88 days before the silages were
fed to the cows during a 56-day study. The residues of fenthion in
corn and corn silage after field treatment at the three rates were as
follows (in ppm):
A B C
day of treatment (forage) 4.3 17.6 34.6
day after treatment (forage) 1.7 3.5 12.0
ensiled 14 days (silage) 1.4 4.3 10.0
ensiled 56 days (silage) 1.0 2.1 8.9
ensiled 71 days (silage) 0.82 2.5 9.6
silage fed first week 0.44 1.3 5.9
silage fed third week 0.55 1.7 6.0
silage fed fifth week 0.55 1.6 5.0
silage fed eighth week 0.62 1.3 5.1
In all cases, compound (2) accounted for most of the residue.
One of the silages was assigned to each group of four cows. The
animals ingested averages of (A) 0.03, (B) 0.07, and (C) 0.3 mg of
residues/kg/day. No residues were detected in the milk from (A) and
(B). Only low levels (maximum of 0.014 ± 0.003 ppm) were found in the
milk from cows fed silage from corn treated at a rate of 2.24 kg of
fenthion/hectare (C). Also, one week after feeding was terminated, no
residues could be found in the milk in group (C).
Only slight amounts of residues occurred also in the faeces and urine
of the animals (expressed in ppm):
Days fed treated feed A B C
26 faeces 0.003 0.015 0.09
urine 0.004 0.016 0.12
55 faeces 0.014 0.03 0.16
urine 0.004 0.014 0.16
No residues were detected in urine and faeces one week after feeding
was terminated.
The effect of diet on the metabolism of fenthion in laboratory
animals (rabbits and rats) was studied by Begum (1968) 35S-labelled
fenthion (Y position) was used in these experiments. When this
compound was administered orally or subcutaneously to healthy,
well-fed animals (group A), the peak concentration of the
radioactivity appeared in the blood six to nine hours after treatment.
The peak was observed in lean, half-starved animals (group B) one hour
after treatment. There was more oxidized fenthion in the blood of
group B rabbits than in the blood of group A rabbits. In both groups
of animals, fenthion was eliminated primarily in the urine, and to a
lesser extent in the faeces. There was a higher percentage of
radioactive materials (mostly hydrolyzed products) excreted in the
urine and faeces by group B animals. Apparently there was a more rapid
breakdown and elimination from the system of lean animals than from
fat ones.
Experiments with sheep
Sheep which were treated with a 2% pour-on (60 mg of fenthion/kg)
showed no residues in brain, heart, liver, kidney, loin steak,
shoulder, leg and fat (omental, renal, back) 44 days after the
application (Chemagro, 1966c). Sheep treated with 50% powder applied
as an oral drench at a dose level of 30 mg/kg also displayed no
residues in the above-mentioned organs and tissues 14 days after the
application (Chemagro, 1966b).
When sheep were treated by dipping them in a 500 ppm solution of
fenthion, only very slight residues appeared (Möllhoff, 1970). No
residues were detected in the organs; in meat, residues were
detectable only at seven days after treatment in one out of two
animals (0.1 ppm). The concentration was highest in fat (up to 0.35
ppm on the seventh day). When the same formulation was injected
intravenously in two sheep at a rate of 5 mg of fenthion/kg 12 hours
before slaughter, the highest residues again appeared in the fat
(1.6 ppm) whilst in meat the residues amounted to a maximum of 0.7 ppm
(Möllhoff, 1970).
Experiments on poultry
Fenthion is not eliminated in hen eggs. Hens were fed on a diet
containing 2 ppm of fenthion for four weeks; at the end of the feeding
period, there was no trace of residues in the eggs (Chemagro, 1967b).
The tissues of six hens (giblets, muscle and fat) were also free of
residues at the end of the feeding experiment (only exception: giblets
contained 0.08 ppm in one out of six hens; fat contained 0.07 ppm in
one out of six hens (Chemagro, 1967c)).
FATE OF RESIDUES
The behaviour of the compound fenthion in living systems, like that of
all pesticides which contain a thioether moiety, is determined by its
readiness to form sulfoxides and sulfones. As an S-alkyl isomerization
can also be reckoned with under thermal influences, the following
non-ionic metabolites of fenthion (1) are theoretically to be
expected:
 X Y Z
S S O = (1) = fenthion
S SO O = (2)
S SO2 O = (3)
O S O = (4)
O SO O = (5)
O SO2 O = (6)
O S S = (7)
O SO S = (8)
O SO2 S = (9)
(See also the metabolic chart Figure 1, under "Biochemical Aspects").
Isomerization to the P-S-aryl forms has not been observed so far.
Of all these compounds, O-desmethyl forms are conceivable as
metabolites. Whenever they are discussed, we shall mark them with a
letter a), for example the compound will be marked 1a), and so on.
X Y Z
S S O = (1) = fenthion
S SO O = (2)
S SO2 O = (3)
O S O = (4)
O SO O = (5)
O SO2 O = (6)
O S S = (7)
O SO S = (8)
O SO2 S = (9)
(See also the metabolic chart Figure 1, under "Biochemical Aspects").
Isomerization to the P-S-aryl forms has not been observed so far.
Of all these compounds, O-desmethyl forms are conceivable as
metabolites. Whenever they are discussed, we shall mark them with a
letter a), for example the compound will be marked 1a), and so on.
 The primary hydrolytic products that are theoretically to be expected
are as follows:
(10) (CH3O)2 P (S) OH (16) HO-C7H6-S-CH3
(11) (CH3O) P (S) (OH)2 (17) HO-C7H7-SO-CH3
(12) P (S) (OH)3 (18) HO-C7H6-SO2-CH3
(13) (CH3O)2 P (O) OH
(14) (CH3O) P (O) (OH)2
(15) P (O) (OH)3
In animals
Knowles and Arthur (1966) applied fenthion dermally to two dairy cows
each weighing 360 kg at a rate of approximately 13 mg/kg per cow, and
treated two other lactating cows (each weighing 410 kg) by the
intramuscular route with approximately 8.5 mg of fenthion/kg per cow.
In the urine the peak concentration of radioactive materials occurred
on the first day following either method of treatment. The total ppm
of P32 materials from intramuscular treatment decreased from 33 ppm
at one day to 1.6 ppm by 21 days. More than 95% of the radioactive
materials eliminated in the urine consisted of hydrolytic products.
The total P32-materials eliminated in the faeces peaked two days
after both types of treatment. The cumulative percentage of the
administered dose was about 4%. The peak concentration of
acetonitrile-soluble radioactivity occurred one day after
intramuscular treatment and three days after dermal treatment. The
hair of the two cows treated dermally contained about 2000 ppm
radioactive fenthion equivalents immediately after treatment. These
residues declined to 16 ppm by 14 days after treatment. Chloroform to
water partition data indicated that fenthion underwent little change
on the hair to water-soluble components. The radioactivity in the
blood after both types of treatment reached a peak during the first 24
hours. Between 10 and 38% of the P32-materials partitioned into
chloroform but no detectable chloroform-soluble P32-materials were
present in the blood at seven days after either treatment.
At 14 days after dermal treatment and 21 days after intramuscular
treatment the cows were killed, and portions of liver, left and right
sirloin steak, left and right round steak, T-bone steak, omental fat,
and left and right subcutaneous fat were removed for analysis. None of
these tissues contained acetonitrilesoluble radioactive materials
following dermal treatment; all tissues contained less than 0.05 ppm
of total radioactivity with the exception of the liver which contained
0.44 ppm. The situation was vastly different in the tissues of the
intramuscularly treated animals. The tissues from the left side (site
of injection) of the cows contained considerably more P32-materials
than those from the right side (e.g. left round steak contained 1.03
ppm total radioactive materials, right round steak contained 0.11
ppm). The acetonitrile-soluble compounds amounted to a maximum of 0.36
ppm in the left steaks (less than 0.1 ppm in the right steaks); 0.2
ppm in the left subcutaneous fat and 0.08 ppm in the right
subcutaneous fat; 0.05 ppm in T-bone steak; 0.15 ppm in omental fat;
and 0.76 ppm in liver. The injection sites contained high residues in
both cases: skin 0.49 ppm total activity (0.08 ppm
acetonitrile-soluble), injection site 164 ppm (76 pp.
acetonitrile-soluble). The amount of the applied P32-materials
remaining in the skin after dermal treatment was, however, only 0.14%.
A similar calculation for the percentage remaining on the hair gave a
figure of 0.73%.
Following dermal and intramuscular treatment of cows with fenthion
(Knowles and Arthur, 1966), fenthion constituted more than 50% of the
non-ionic residues for three days after dermal and seven days after
i.m. treatment in the milk. The remainder was composed chiefly of
(3) + (5) + (6) and (2) [+ (4) ?]. In the urine, fenthion accounted
for only a small percentage of the chloroform-soluble radioactivity.
Some of (4) and/or (2) was present, but in most cases more than 70% of
the activity consisted of (3) and/or (5) and/or (6). The hydrolytic
products were composed of almost equal proportions of (10) and (13),
and a small portion consisted of an unidentified compound (probably a
O-desmethyl form of fenthion or one of its metabolites). More than 50%
of the acetonitrile-soluble radioactive materials in the faeces
consisted of the parent compound. The remainder was accounted for by
the other metabolites just as in urine. The composition of the
hydrolytic products was also very similar to that found in urine. In
the tissues of the animals slaughtered 14 days after dermal treatment
and 21 days after i.m. treatment, more than 50% of the radioactive
acetonitrile-soluble materials was chromatographed as fenthion, but
oxidation products were also present. At the injection site, (12) was
the predominant product of the water-soluble materials.
The silage feeding experiments performed by Bowman et al. (1970)
referred to earlier, also provide certain indications of the
transformation of fenthion metabolites in the cow. For example, the
residue (total of six ppm) in the feed fed in the fourth week and in
the resultant milk samples had the following composition:
Compound Feed Milk
(1) 8% -
(2) 89% <2%
(3) 2% <2%
(4) - -
(5) 1% 97%
(6) - -
Compound (5) accounted for 95% of the metabolites present in the
urine. Those present in the faeces consisted almost exclusively of
compound (1) (fenthion). Unfortunately the authors do not provide any
data on the occurrence of metabolites in the blood of the animals;
however, the cholinesterase activity of the animals of group C was
significantly depressed during the feeding experiment but otherwise
there were no changes in performance.
In plants
The transformation of fenthion in plants is basically similar to that
in animals. The first studies performed to investigate transformation
of fenthion in plants were made by Brady and Arthur (1961). Cotton
plants were sprayed with an emulsion of P32-labelled fenthion at a
rate of 2 kg of fenthion per hectare. The plants were growing under
field conditions. Three days after spraying, 189 ppm fenthion
equivalents were present on or in the leaves, but only 14 ppm were
chloroform-soluble materials. The respective residues after 14 days
were 71/5 ppm. Compound (3) was tentatively identified as by far the
most prevalent metabolite (60-80%), followed in quantity by unchanged
fenthion (28% after three days, 13% after 14 days). Compounds
(5) + (6) appeared in smaller percentages (less than 10%). The
hydrolytic products found were chiefly (10) and small amounts of (13)
and an unknown compound.
Experiments with P32-labelled fenthion on beans, in which the
metabolites were separated by paper-chromatography (Niessen et al.,
1962a) and quantitatively determined (Niesson et al., 1962b), produced
the following results:
The labelled parent compound contained 94.2% of (1), 4.5% of (7), 0.7%
of (2) and 0.6% of ionic compounds. Bean plants (Phaseolus vulgaris)
were either briefly dipped in a 0.2% emulsion of this compound by
their stem, or placed in the emulsion by their roots. The
chloroform-soluble extracts had the following composition (ppm):
Days (1) (2) (3) (5) (6) (7) (8)
1/4 100 16 - 1.2 - 2.8 1.5
2 27 25 2.1 3.5 0.06 - 0.4
5 4.7 7.2 1.4 1.5 0.12 - 0.09
8 1.6 4.7 1.4 1.2 0.11 - 0.05
After eight days, only 1.6% of the parent compound was present; (2)
was the principal metabolite. The impurity (7) was no longer
detectable from the second day; however, it is also oxidized to the
sulfoxide (8). The total residue decreased in the eight-day period
from 122 ppm to a level of 9 ppm. The rate at which these
transformations take place depends upon the temperature.
Oxidation by plant enzymes preferably takes place at the thiono sulfur
atom, while oxidation at the methylmercapto group seems chiefly to be
a photosensitized reaction. The compound has a certain systemic
action. (7) has a much stronger systemic action, but it is not found
in the plant.
Several experiments on glass plates confirmed that the formation of
sulfoxide and sulfone is a light-induced reaction whilst (7) is formed
from (1) and (8) is formed from (2) due to the influence of heat.
These findings were later confirmed by Metcalf et al. (1963).
At the same time as the studies of Niessen et al. (1962b) were
conducted, Japanese research workers investigated the behaviour of
P32-labelled fenthion in rice, tea and cabbage (Fukuda et al. 1962,
Tomizawa et al., 1962). The neutral metabolites were separated by
paper chromatography and the water-soluble metabolites were separated
by ion exchange chromatography. Immediately after the treatment, the
deposit of fenthion on the rice plants amounted to between 110 and 150
ppm on the leaf blade and to between 18 and 28 ppm in the leaf sheath.
(2) and (3) were the principal metabolites in the leaf blade and
sheath. The accumulation of fenthion metabolites in ears and grains
was examined in one variety which was treated a few days before
heading. Four weeks after application, the distribution of
radioactivity was as follows (ppm):
Total Chloroform-extractable
Husk 2.4 n.d.
Bran 60 0.9
Polished rice 5.7 0.1
The composition of the metabolite mixture in the ears 12 days after
the application was as follows: 45% (2); 20% (7?); 18% (3); 17%
unidentified. The water-soluble metabolites in the grains 14 days
after the application were composed chiefly of (1a), plus a few
percent. (less than 10) of (10), (13) and (12) and/or (15).
The amounts of fenthion deposit on tea and cabbage after the
application were as follows: 43 ppm on young tea leaves, 90 ppm on old
tea leaves and 46 ppm on cabbage leaves. (2) and (3) were the
principal metabolites also in these experiments. The appearance of
O-desmethyl fenthion observed by the Japanese research workers seems
astonishing and was never again reported by any other observers.
In 1963, Metcalf et al. published qualitative results which they
obtained on cotton. In these studies, too, (2) and (3) were the
principal metabolites.
With the improvement of analytical techniques, a better insight was
gained into the metabolism of fenthion in plants. Leuck and Bowman
(1968) treated Coastal bermudagrass and ensilage corn at rates of 0.56
kg/hectare, 1.12 kg/hectare and 2.24 kg/hectare (a.i.). The ppm
residues determined in Coastal bermudagrass treated at the highest
application rate were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 64 71 1.0 1.8 n.d.
7 0.31 10 4.0 2.0 0.34
14 0.07 2.1 2.3 0.48 0.18
21 0.03 0.59 1.3 0.08 0.11
The corresponding residue levels for corn were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 0.41 26 0.22 0.63 <0.005
7 0.04 2.4 0.68 1.03 0.16
14 0.01 0.42 0.24 0.11 0.09
21 <0.002 0.34 0.22 <0.02 0.04
(4) was not detected (except on day 0 in bermudagrass when its level
was 0.06 ppm). (2) and (3) were again the principal metabolites.
The total residues were as follows (expressed in ppm):
Days Bermudagrass Corn
post-treatment 0.56 kg/ha 1.12 kg/ha 0.56 kg/ha 1.12 kg/ha
7 3.3 8.5 0.65 1.95
14 1.3 3.2 0.15 0.4
21 0.5 1.3 0.1 0.2
These findings were very largely confirmed by a study performed by
Bowman et al. (1968), in which nine procedures for removal of
phosphorus insecticides and their metabolites were compared. This
study also provides an impressive picture of the differing effect of
various extraction procedures. Soxhlet extraction with 10% methanol in
chloroform proved to be the best one.
The fate of phenolic hydrolytic products of fenthion in plants was not
further studied. However, findings have been published by Wendel and
Bull (1970) in respect of GC-6506 (dimethyl 4-methylthiophenyl
phosphate). From some of the metabolites, substituted phenols could be
liberated by hydrolysis with ß-glucosidase (Bull and Stokes, 1970).
The predominant products were the glucosides of phenol-sulfoxide and
phenol-sulfone. The glycone portions of the conjugates are further
altered, possibly by the formation of a ß-gentiobiside.
In soils
The half-life of fenthion and its metabolites in soil is between 14
and 40 days; only in one out of six experiments was a half-life of
more than 30 days recorded. The experiments used various formulations
including granules and emulsifiable solutions. (Chemagro - Summary
under soils in main submission).
In storage and processing
Under frozen storage (-18 to -23°C), fenthion residues were shown to
be stable in alfalfa for 28 weeks and in cattle fat for 11 weeks
(Chemagro, 1966e,f). In liver from cattle, a decrease of 20% seemed to
occur between the twelfth and twenty-sixth week of storage (Chemagro,
1966f).
Fenthion proved to be stable for four weeks at -18°C in cattle brain,
heart, liver, kidney, steak and fat over a period of four weeks; (6)
decreased slightly in brain, heart, steak and fat, by 30% in kidney,
and by 80% in liver in six weeks (Olson, 1966).
Following treatment of corn with fenthion at rates of 0.56, 1.12 and
2.24 kg per hectare (Boman and Beroza, 1969), the plants treated at
the highest rate contained 39 ppm of residues (1) + (2) + (3) + (5),
in a ratio of 1.8 : 96.5 : 0.75 : 0.97. At 70 days after ensiling
(which took place on the day after application), the residues amounted
to 14.6 ppm (17 : 74 : 7.8 : 1.1); at 141 days, they amounted to 6.85
ppm (9.7 : 85 : 4.7 : 0.5). The predominant metabolite throughout the
whole period was (2). In another silage experiment with corn, the
residues were consistently shown to be at a lower oxidation stage than
were those in the field samples from which the silage was prepared
(Leuck and Boman, 1968).
The relatively high persistence of the residues in silage and the
occurrence of (2) as the principal metabolite were again confirmed
later (Bowman et al., 1970).
Residues in canned and preserved peaches persist to a certain extent,
especially when maintained at low temperatures (refrigerator) (Pigatti
et al., 1967).
Evidence of residues in commerce or at consumption
Krause and Kirchhoff (1969) examined 78 market samples of different
fruits and vegetables of domestic and foreign origin for residues of
20 organophosphorus insecticides including fenthion. Residues of
fenthion were not found in any of the samples.
Total diet studies were carried out in England and Wales, in 1966/67,
with altogether 66 samples of total diet. Each sample comprised seven
sub-samples consisting of cereals, meats, fish, fats, fruits and
preserves, root vegetables, green vegetables and milk. Fenthion and
its immediate oxidation products could have been detected by the
method employed, but they were not detected (Abbott et al., 1970).
Methods of residue analysis
Although the present-day gas chromatographic methods can be carried
out largely without clean-up, it is nevertheless necessary in some
cases to eliminate interfering substances. In this, consideration must
be given to the greatly differing polarity of fenthion residues.
Chemagro (1963) has published, for example, the following data on
partition:
n-hexane water n-hexane
Compound acetonitrile chloroform water
(1) 1 : 8.4 1 : 1720 1 : 0.016
(5) 1 : 300 1 : 88 1 :
(6) 1 : 87 1 : 15 1 : 78
Bowman and Beroza (1968) give the following p-values (=fractional
amount in nonpolar phase):
n-hexane n-hexane n-hexane
Compound 20% acetonitrile 40% acetonitrile water
in water in water
(1) 0.98 0.92 1.00
(2) 0.18 0.03 0.50
(3) 0.61 0.12 0.94
(4) 0.65 0.18 0.92
(5) 0 0 0
(6) 0 0 0.01
Regarding the extraction of residues, attention is drawn to the study
of Bowman et al. (1968) already referred to earlier.
The first methods for the determination of fenthion and its
metabolites were based on determination of total phosphorus (Frehse et
al., 1962a for plant material; Frehse et al., 1962b for olives (oil);
Adam (1967) for olives). In 1966, the first methods were published for
the determination of fenthion in plant and animal tissues (Anderson et
al., 1966) and milk (Katague, 1966), based on oxidation of the various
compounds to the sulfone (6) which in turn was hydrolyzed to the
corresponding phenol. The phenol was brominated and acetylated prior
to detection measurement by electron-capture GLC. The methods were
sensitive to 0.1 ppm (0.01 ppm for milk) but unavoidably very
complicated and time-consuming.
Therefore, Chemagro began in 1967 to develop methods using the
thermionic phosphorus detector: for soil (Olson 1967a), rice grain
(Olson 1967b), eggs and milk (Olson 1968), and animal tissues
(Thornton 1967).
These methods are based on oxidation of the residues to (6). The
sensitivities are below 0.1 ppm for soil and rice, and about 0.005 ppm
for eggs and milk. GLG conditions are:
(a) soil: 11 in × 3 mm i.d. column, packed with 6% DC-200 and 1% QF-1
on 80/100 mesh Gas Chrom Q, 230°C;
(b) other material: 16 in × 3 mm i.d. column, packed with 10% DC-200
and 0.2% QF-1 on 80/100 mesh Gas Chrom Q, 210°C, retention time
3.5 minutes.
Forty-five organophosphorus insecticides were examined for possible
interference with the rice method (Olson, 1967b); no interferences
were noted for any of the compounds except (R) Dasanit (Olson,
1967c).
Bowman and Beroza (1968) used a different principle of determination.
Extracts from corn, grass and milk were separated by liquid
chromatography into three fractions which then were analysed by GLC
with a flame photometric detector: 90 cm × 4 mm i.d, column, packed
with 10% DC-200 on 80/100 mesh Gas Chrom Q, 210°C for the two
fractions (1) + (3) and (2) + (4) + (6); a 45 cm column is used for
(5) (retention times (1) to (6) : 1.75, 4.2, 4.05, 1.45, 2.3 minutes).
Ten per cent. QF-1 (50 cm), 1% Carbowax 20 M (50 cm) and 5% DEGS
(50 cm) can also be used as the liquid phase. A complete separation of
all six compounds was achieved by using liquid chromatography with a
silica gel column and different elution systems. Later, Bowman and
Beroza (1969) also used the oxidation method with m-chloroperbenzoic
acid for the same substrates: 90 cm × 4 mm i.d. column packed with 10%
OV-101 on 80/100 mesh Gas Chrom Q, 215°C. With this column, the
metabolites can also be determined singly (retention times of
0.7 - 2.75 minutes, 2.3 minutes for (6)); it was found that the
results of the single metabolite determinations those of the total
determination. The recoveries were above 90%. Excessive amounts of the
oxidation mixture are removed on an alumina column.
Further studies performed by Bowman and Beroza (1970) show that 2.4
metre × 4 mm i.d. columns, packed with 5% OV-101 or OV-210 on Gas
Chrom Q are also suitable for determination of the compounds (1) to
(6).
Examples of national tolerances
Country Crop Tolerance Safety
in ppm Interval
in days
Algeria General 15
Australia Fruit, vegetables 2 7
Meat of cattle 1 1
Milk and milk products 2 1
Austria General 35
Belgium Fruit, vegetables excluding
potatoes 0.3 21
Brazil General 14
Fruit 1.0
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
Bulgaria Sugar beets 14
Cherries, cereals 14
Canada Beef cattle N.R.
Finland General 14
France General 15
Olives 21
Germany Fruit 14
Vegetables, field & fodder
crops 10
Israel Fruit 21
Miscellaneous (e.g. dry onions
and cucurbits) 30
Italy General 20
Olives 30
Morocco General 15
Olives 30
Netherlands Fruit, vegetables excluding
potatoes 0.3 -
New Zealand General 21
Norway General 14
Poland Fruit, vegetables, root crops 30
Early cherries 14
Portugal General 14
Olives (as oil and preserves) 42
Russia Cereals, cottonseed oil 0.35
Spain Fruit and olives 30
South Africa General 2.0
Apricots, peaches, apples,
pears, grapes 10
Subtropical crops & cucurbits 7
Deciduous fruit 10
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
United States Alfalfa (fresh), grass 5.0
of America Alfalfa (hay), grass hay 18.0
Animal products 0.1
Milk 0.01
Yugoslavia General 0.5
N.R. = registered for use on a no-residue basis.
Appraisal
Fenthion is an organophosphorus insecticide with a broad spectrum of
activity used against insects infesting field crops, fruit crops,
vineyards, olives, cotton, insects of public health concern and
ectoparasites of domestic animals. It is especially useful for control
of fruit flies in many crops where its ability to penetrate plant
tissues allows for destruction of larvae within the fruit. The
concentrations/rate of use ranges from 0.5-2 kg/ha in the case of
field crops and cotton and 0.05-0.1% solution on horticultural crops.
Animals are treated with 0.25%-5% dust, 0.025%-0.25% solutions as dips
or sprays and 2%-3% solutions in oil as pour-on preparations or back
rubbers.
The residue data available to the meeting were obtained from many
different countries, reflecting typical registered uses under a wide
range of climatic and ecological conditions. Considerable information
is available about the fate of fenthion residues in foods of plant and
animal origin and extensive studies have been carried out on the
nature of the degradation products formed under a variety of
influences.
Little information is available about residues of fenthion in foods in
commerce.
The literature includes a number of methods of residue analysis based
on gas-chromatographic procedures. The greatly differing polarity of
the various fenthion metabolites has been investigated and
recommendations for the most appropriate extraction procedures have
been made.
In order that all biologically active metabolites may be determined
simultaneously the analytical procedure has been modified to provide
for oxidation of the residues of the various compounds to the sulfone
which is hydrolyzed to the corresponding phenol. The phenol is
brominated and acetylated prior to measurement by electron-captive
gas/liquid chromatography. The limit of detection is 0.1 ppm for most
foods and 0.01 ppm for milk.
Procedures which are less complicated and less time consuming have
been developed using the thermionic phosphorus detector following
oxidation to the sulfone. The limit of detection is below 0.1 ppm for
soil and grain, and about 0.005 ppm for eggs, milk and animal tissues.
These procedures appear suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The temporary tolerances are for fenthion and its major metabolites,
determined separately or together and expressed as fenthion.
Apples, peaches, cherries, lettuce, 2 ppm
fat of meat
Cabbage, cauliflower, olives, 1 ppm
olive oil
Grapes, oranges, peas, meat 0.5 ppm
Squash 0.2 ppm
Wheat, rice, milk products (fat basis) 0.1 ppm
Milk (whole) 0.05 ppm
Further work or information
Required before 30 June 1975
1. Adequate two-year feeding studies in the dog and in one rodent
species.
2. Establishment of the sequence of metabolic changes in man and
laboratory animals in order to elucidate the mechanism of long
lasting cholinesterase inhibition.
Desirable
Information on the frequency and level of fenthion residues in food
commodities in commerce.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adam, N. Chr. (1967) Méthode de détermination des résidue de Lebaycid
dans l'huile d'olive et lea olives. Ann. Inst. Phytopath. Benaki, N.S.
8: 78-84
Anderson, R. J., Thornton, J. S., Anderson, C. A. and Katague, D. B,
(1966) Determination of fenthion residues in plant and animal tissues
by electron-capture gas chromatography. J. Agr. Food Chem. 14:
619-622
Begum, A. (1968) Effect of diet on metabolism of fenthion in animals.
Ph.D. thesis, Auburn University, Auburn, Alabama. Dissert, Abstr.,
Sect. B, 28: 4165
Bowman, M. C. and Beroza, M. (1968) Determination of fenthion and five
of its metabolites in corn, grass and milk. J. Agr. Food Chem. 16:
399-402
Bowman, M. C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton and phorate in corn, milk, grass and
faeces, J.A.O.A.C. 52: 1231-1237
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J.A.O.A.C. 53: 499-508
Bowman, M. C., Beroza, M. and Leuck, D. B. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites from
field treated crops. J. Agr. Food Chem. 16: 796-802
Bowman, M. C., Leuck, D. B., Johnson, J. C., jr and Knox, F. E. (1970)
Residues in corn silage and effect of feeding dairy cows the treated
silage. J. econ. Entomol. 63: 1523-1528
Brady, U. E., jr and Arthur, B. W. (1961) Metabolism of O,o-dimethyl
O-[4-(methylthio)-m-toly] phosphorothioate by white rats. J. econ.
Entomol, 54: 1232-1236
Bull, D. L. and Stokes, R. A. (1970) Metabolism of dimethyl
p-(methylthio) phenyl phosphate in animals and plants. J. Agr. Food
Chem. 18: 1134-1138
Chemagro Milk and tissue residues resulting from backline or oral
treatment of Cattle with Bayer 29493, p. 32
1963 Report No. 10. 946, December 3
1965a Report No. 16. 551, July 27 (revised January 10, 1968)
1965b Report No. 16. 990, October 21
1965c Report No. 17. 015, October 28
1965d Report No. 17. 062, November 3
1966a Report No. 17. 445, January 19 (revised April 28, 1966)
1966b Report No. 17. 969, April 21
1966c Report No. 18. 009, May 3
1966d Report No. 18. 067, May 12
1966e Report No. 18. 179, May 20
1966f Report No. 18. 191, May 24
1967a Report No. 20. 310, April 18
1967b Report No. 20. 738, June 20
1967c Report No. 20. 798, July 5
1967d Report No. 21. 113, September 13
1967e Report No. 21. 504, November 13
1968a Report No. 21. 796, January 25
1968b Report No. 22. 647, May 15
1969 Report No. 24. 266, February 14 (revised April 2, 1969)
1970 Report No. 28. 719, December 3
Dean, G., Coxon, J. and Brereton, D. (1967) Poisoning by an
organo-phosphorus compound. A case report. So. African Med. J.,
1017-19
Dieckmann, W. (1971) Neurotoxizctatsunter-suchungen an
Huhnern-Histopathologie. Unpublished report of Farbenfabriken Bayer
A.G.
Dilley, J. and Doull, J. (1961a) Chronic inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dilley, J. and Doull, J. (1961b) Acute inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. (1960) The absence of antidote activity by 2-PAM and
TMB-4 against acute poisoning by Bayer 29493. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. (1961) Effects of repeated dermal application of Bayer
29493 on rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1962) Acute oral toxicity of a sample of Bayer 29493 to
female rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Dubois, K. P. and Doull, J. (1960) The acute toxicity of Bayer 29493
to chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. and Puchala, E. (1960) Influence of Bayer 29493 on the
cholinesterase activity of the blood of rats. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. and Kinoshita, F. (1964) Acute toxicity and
anti-cholinesterase action of O,O-dimethyl O-4-(methylthio)-m-tolyl
phosphorothioate (DMTP; Baytex) and related compounds. Tox. Appl.
Pharmacol. 6: 86-95
Doull, J., Root, M. and Cowan, J. (1961) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Doull, J., Root, M. and Cowan, J. (1962) Effect of adding Bayer 29493
in combination with other cholinergic insecticides to the diet of male
and female dogs. Unpublished report from Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Cowan, N. J., Vesselinovitch, D., Fitch, F. W.
(1963a) and Meskauskas, J. Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Root, M., Cowan, J. and Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. and
Meskauskas, J. (1961) The effects of feeding diets containing Bayer
29493 to rats for a period of 16 weeks. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Elliott, R. and Barnes, J. M. (1963) Organophosphorus insecticides for
the control of mosquitos in Nigeria. Bull. Wld Hlth Org. 28: 35-54
Francis, J. I, and Barnes, J. M. (1963) Studies on the mammalian
toxicity of fenthion. Bull. Wld. Hlth. Org. 29: 205-12
Frehse, H. (1970) Ruckstande van pflanzenschutz mittein in nahrung und
um welt in chemie der pflanzenschutz und schadlingshenamp-fungsmittel.
Band 2-Wegler, R., Ed. Springer-Verlag, 1970
Frehse, H., Niessen, H. and Tietz, H. (1962a) Method of determining
residues of the insecticide Lebaycid(R) in plant material.
Pflanzenschutz-Nachr Bayer 15: 148-159
Frehse, H., Niessen, H. and Tietz, H. (1962b) Method of determining
residues of the insecticide Lebaycid(R) in olives and olive oil.
Pflanzenschutz-Nachr Bayer 15: 160-165
Fukuda, H., Masuda, T., Miyahara, Y. and Tomizawa Ch. (1962) Fate of
O,O-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on rice plant. Japan. J. appl. Entomol. Zool. 6: 230-236
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14: 515-34
Hahn, H. L. and Hensehler, D. (1969) The ability of phosphorylated
cholinesterases to be reactivated by obidoxime chloride (Toxogonin)
in vivo. Arch. Toxikol. 24: 147-63
Katague, D. B. (1966) Determination of fenthion residues in milk by
electron-capture gas chromatography. Chemagro Corp., Research
Department, Report No. 17, 887
Keith, J. O, and Mulla, M. S. (1966) Relative toxicity of five
organo-phosphorus mosquito larvicides to mallard ducks. J. Wildlife
Management 30: 553-63
Kimmerle, G. (1960) Re: Active substance S1752. Unpublished report
submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1961) Subchronische oral versuche bei ratten mit
S-1752-Wirkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1963) Product BH6 and S1752 Poisoning. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965a) Nourotoxic studies with Bayer 29493. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965b) Neurotoxische untersuch-ungen mit
S-1752-Werkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1966) Langdavernde Inhalatronsversuche bei hunden mit
dem Baytex-Werkstoff (S-1752)
Kimmerle, G. (1967a) Abhangigkeit der akuten oralen toxizitat bei
ratten vom losungsmittel. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. (1967b) Potenzierung von DDVP und S-1752. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Klimmer, O. R. (1963) Toxicological testing of Bayer 29493.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Knowles, C. O. and Arthur, B. W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of radiolabelled
fenthion to dairy cows. J. econ. Entomol. 59: 1346-52
Krause, Ch. and Kirchhoff, J. (1969) Organophosphatrückstande auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen
Nachrichtenbl. Deut. Pflanzenschutzdienstes (Braunschweig), 21:
81-84
Leuck, D. B. and Bowman, M. C. (1968) Residues of fenthion and five of
its metabolites their persistence on corn and grass forage, J. econ.
Entomol. 61: 1594-1597
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmakol. 263: 237-8
Loser, E. (1969) Generation versuche an Ration. Unpublished report
submitted by Farbenfabriken Bayer A.G.
McGrath, H. B. (1969) Toxicity of Bayer 29493 in calves. Unpublished
report submitted by Farbenfabriken Bayer A.G
Metcalf, R. L., Fukuto, T. R. and Winton, M. Y. (1963) Chemical and
biological behaviour of fenthion residues. Bull. Wld Hlth Org. 29:
219-226
Möllhoff, E. (1970) Determination of trichlorfon and fenthion residues
in animals of different species, Pestic. Sci. 2: 179-181
Nelson, D. L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Niessen, H., Tietz, H. and Frehse, H. (1962a) Papier
chromatographische Trennung aromatischer
Phosphorsäure-ester-Insektizide. J. Chromatog. 9: 111-113
Niessen, H., Tietz, H. and Frehse, H. (1962b) On the occurrence of
biologically active metabolites of the active ingredient S-1752 after
application of Lebaycid(R) Pflanzenschutz-Nachr. Bayer 15: 125-147
Olson, T. J. (1966) The determination of the stability of fenthion and
its oxygen analog sulfon in frozen cattle tissues. Chemagro Corp.,
Research Department, Report No. 19, 289,
Olson, T. J. (1967a) Determination of residues of fenthion in soil by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 20, 324.
Olson, T. J. (1967b) Determination of residues of fenthion in rice
grain by thermionic emission gas chromatography. Chemagro Corp.,
Research Department, Report No. 20, 417 (revised 21 October, 1968)
Olson, T. J. (1967c) A study of the possible interference of other
pesticides with the analytical method for fenthion in rice. Chemagro
Corp., Research Department, Report No. 20, 595
Olson, T. J. (1968) Determination of fenthion in eggs and milk by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 22, 933
Pickering, E. N. (1966) Organic phosphate insecticide poisoning. Can.
J. Med. Tech., p. 174
Pigatti, A., Pigatti, P., Orlando, A., Suplicy, F. O., Sampaio, A. S.
and Rigitano, O. (1967) Persistência de residuos de fenthion am
pêssego, ameixa e maca. Arq. Inst. Biol. (S. Paulo) 34: 275-284
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol Appl. Pharmacol. 3, 512-33
Shillam, K. W. G., Medd, R. K., Roberts, N. L. and Burrows, I. E.
(1971) Residual levels of fenthion in raw and pasteurized milk. Report
of Huntington Research Centre 4225/71/383
Shimamoto, K. and Hattori, K. (1969) Chronic feeding of Baytex
(O, O-dimethyl-o-(4-methylmercapto-3-methyl) phenyl-thiophosphate) in
rats. Acta Med. Univ. Kioto, 40: 163-71
Shipp, E. (1970) Fenthion residues in milk. Report of the Entomology
School, University of New South Wales
Spicer, E. J. F. (1971) Pathology Report of Bay 29493. Generation
study in rats. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull. Wld Hlth Org.,
29: 213-18
Thornton, J. S. (1967) Determination of fenthion residues in animal
tissues by thermionic emission flame gas chromatography. Chemagro
Corp., Research Department, Report No. 20 420 (revised 21 October,
1968)
Tomizawa, Ch., Fukuda, H., Masuda, T. and Miyahara, Y. (1962) Fate of
O,o-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on tea and cabbage leaves. Japan. J. appl. Entomol. Zool., 6:
237-241
von Clarmann, M. and Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and its
demonstration in the gastric contents and urine. Arch. Toxik., 22:
2-11
Wendel, L. E. and Bull, D. L. (1970) Systemic activity and metabolism
of dimethyl p-(methylthio) phenyl phosphate in cotton. J. Agr. Food
Chem., 18: 420-424
The primary hydrolytic products that are theoretically to be expected
are as follows:
(10) (CH3O)2 P (S) OH (16) HO-C7H6-S-CH3
(11) (CH3O) P (S) (OH)2 (17) HO-C7H7-SO-CH3
(12) P (S) (OH)3 (18) HO-C7H6-SO2-CH3
(13) (CH3O)2 P (O) OH
(14) (CH3O) P (O) (OH)2
(15) P (O) (OH)3
In animals
Knowles and Arthur (1966) applied fenthion dermally to two dairy cows
each weighing 360 kg at a rate of approximately 13 mg/kg per cow, and
treated two other lactating cows (each weighing 410 kg) by the
intramuscular route with approximately 8.5 mg of fenthion/kg per cow.
In the urine the peak concentration of radioactive materials occurred
on the first day following either method of treatment. The total ppm
of P32 materials from intramuscular treatment decreased from 33 ppm
at one day to 1.6 ppm by 21 days. More than 95% of the radioactive
materials eliminated in the urine consisted of hydrolytic products.
The total P32-materials eliminated in the faeces peaked two days
after both types of treatment. The cumulative percentage of the
administered dose was about 4%. The peak concentration of
acetonitrile-soluble radioactivity occurred one day after
intramuscular treatment and three days after dermal treatment. The
hair of the two cows treated dermally contained about 2000 ppm
radioactive fenthion equivalents immediately after treatment. These
residues declined to 16 ppm by 14 days after treatment. Chloroform to
water partition data indicated that fenthion underwent little change
on the hair to water-soluble components. The radioactivity in the
blood after both types of treatment reached a peak during the first 24
hours. Between 10 and 38% of the P32-materials partitioned into
chloroform but no detectable chloroform-soluble P32-materials were
present in the blood at seven days after either treatment.
At 14 days after dermal treatment and 21 days after intramuscular
treatment the cows were killed, and portions of liver, left and right
sirloin steak, left and right round steak, T-bone steak, omental fat,
and left and right subcutaneous fat were removed for analysis. None of
these tissues contained acetonitrilesoluble radioactive materials
following dermal treatment; all tissues contained less than 0.05 ppm
of total radioactivity with the exception of the liver which contained
0.44 ppm. The situation was vastly different in the tissues of the
intramuscularly treated animals. The tissues from the left side (site
of injection) of the cows contained considerably more P32-materials
than those from the right side (e.g. left round steak contained 1.03
ppm total radioactive materials, right round steak contained 0.11
ppm). The acetonitrile-soluble compounds amounted to a maximum of 0.36
ppm in the left steaks (less than 0.1 ppm in the right steaks); 0.2
ppm in the left subcutaneous fat and 0.08 ppm in the right
subcutaneous fat; 0.05 ppm in T-bone steak; 0.15 ppm in omental fat;
and 0.76 ppm in liver. The injection sites contained high residues in
both cases: skin 0.49 ppm total activity (0.08 ppm
acetonitrile-soluble), injection site 164 ppm (76 pp.
acetonitrile-soluble). The amount of the applied P32-materials
remaining in the skin after dermal treatment was, however, only 0.14%.
A similar calculation for the percentage remaining on the hair gave a
figure of 0.73%.
Following dermal and intramuscular treatment of cows with fenthion
(Knowles and Arthur, 1966), fenthion constituted more than 50% of the
non-ionic residues for three days after dermal and seven days after
i.m. treatment in the milk. The remainder was composed chiefly of
(3) + (5) + (6) and (2) [+ (4) ?]. In the urine, fenthion accounted
for only a small percentage of the chloroform-soluble radioactivity.
Some of (4) and/or (2) was present, but in most cases more than 70% of
the activity consisted of (3) and/or (5) and/or (6). The hydrolytic
products were composed of almost equal proportions of (10) and (13),
and a small portion consisted of an unidentified compound (probably a
O-desmethyl form of fenthion or one of its metabolites). More than 50%
of the acetonitrile-soluble radioactive materials in the faeces
consisted of the parent compound. The remainder was accounted for by
the other metabolites just as in urine. The composition of the
hydrolytic products was also very similar to that found in urine. In
the tissues of the animals slaughtered 14 days after dermal treatment
and 21 days after i.m. treatment, more than 50% of the radioactive
acetonitrile-soluble materials was chromatographed as fenthion, but
oxidation products were also present. At the injection site, (12) was
the predominant product of the water-soluble materials.
The silage feeding experiments performed by Bowman et al. (1970)
referred to earlier, also provide certain indications of the
transformation of fenthion metabolites in the cow. For example, the
residue (total of six ppm) in the feed fed in the fourth week and in
the resultant milk samples had the following composition:
Compound Feed Milk
(1) 8% -
(2) 89% <2%
(3) 2% <2%
(4) - -
(5) 1% 97%
(6) - -
Compound (5) accounted for 95% of the metabolites present in the
urine. Those present in the faeces consisted almost exclusively of
compound (1) (fenthion). Unfortunately the authors do not provide any
data on the occurrence of metabolites in the blood of the animals;
however, the cholinesterase activity of the animals of group C was
significantly depressed during the feeding experiment but otherwise
there were no changes in performance.
In plants
The transformation of fenthion in plants is basically similar to that
in animals. The first studies performed to investigate transformation
of fenthion in plants were made by Brady and Arthur (1961). Cotton
plants were sprayed with an emulsion of P32-labelled fenthion at a
rate of 2 kg of fenthion per hectare. The plants were growing under
field conditions. Three days after spraying, 189 ppm fenthion
equivalents were present on or in the leaves, but only 14 ppm were
chloroform-soluble materials. The respective residues after 14 days
were 71/5 ppm. Compound (3) was tentatively identified as by far the
most prevalent metabolite (60-80%), followed in quantity by unchanged
fenthion (28% after three days, 13% after 14 days). Compounds
(5) + (6) appeared in smaller percentages (less than 10%). The
hydrolytic products found were chiefly (10) and small amounts of (13)
and an unknown compound.
Experiments with P32-labelled fenthion on beans, in which the
metabolites were separated by paper-chromatography (Niessen et al.,
1962a) and quantitatively determined (Niesson et al., 1962b), produced
the following results:
The labelled parent compound contained 94.2% of (1), 4.5% of (7), 0.7%
of (2) and 0.6% of ionic compounds. Bean plants (Phaseolus vulgaris)
were either briefly dipped in a 0.2% emulsion of this compound by
their stem, or placed in the emulsion by their roots. The
chloroform-soluble extracts had the following composition (ppm):
Days (1) (2) (3) (5) (6) (7) (8)
1/4 100 16 - 1.2 - 2.8 1.5
2 27 25 2.1 3.5 0.06 - 0.4
5 4.7 7.2 1.4 1.5 0.12 - 0.09
8 1.6 4.7 1.4 1.2 0.11 - 0.05
After eight days, only 1.6% of the parent compound was present; (2)
was the principal metabolite. The impurity (7) was no longer
detectable from the second day; however, it is also oxidized to the
sulfoxide (8). The total residue decreased in the eight-day period
from 122 ppm to a level of 9 ppm. The rate at which these
transformations take place depends upon the temperature.
Oxidation by plant enzymes preferably takes place at the thiono sulfur
atom, while oxidation at the methylmercapto group seems chiefly to be
a photosensitized reaction. The compound has a certain systemic
action. (7) has a much stronger systemic action, but it is not found
in the plant.
Several experiments on glass plates confirmed that the formation of
sulfoxide and sulfone is a light-induced reaction whilst (7) is formed
from (1) and (8) is formed from (2) due to the influence of heat.
These findings were later confirmed by Metcalf et al. (1963).
At the same time as the studies of Niessen et al. (1962b) were
conducted, Japanese research workers investigated the behaviour of
P32-labelled fenthion in rice, tea and cabbage (Fukuda et al. 1962,
Tomizawa et al., 1962). The neutral metabolites were separated by
paper chromatography and the water-soluble metabolites were separated
by ion exchange chromatography. Immediately after the treatment, the
deposit of fenthion on the rice plants amounted to between 110 and 150
ppm on the leaf blade and to between 18 and 28 ppm in the leaf sheath.
(2) and (3) were the principal metabolites in the leaf blade and
sheath. The accumulation of fenthion metabolites in ears and grains
was examined in one variety which was treated a few days before
heading. Four weeks after application, the distribution of
radioactivity was as follows (ppm):
Total Chloroform-extractable
Husk 2.4 n.d.
Bran 60 0.9
Polished rice 5.7 0.1
The composition of the metabolite mixture in the ears 12 days after
the application was as follows: 45% (2); 20% (7?); 18% (3); 17%
unidentified. The water-soluble metabolites in the grains 14 days
after the application were composed chiefly of (1a), plus a few
percent. (less than 10) of (10), (13) and (12) and/or (15).
The amounts of fenthion deposit on tea and cabbage after the
application were as follows: 43 ppm on young tea leaves, 90 ppm on old
tea leaves and 46 ppm on cabbage leaves. (2) and (3) were the
principal metabolites also in these experiments. The appearance of
O-desmethyl fenthion observed by the Japanese research workers seems
astonishing and was never again reported by any other observers.
In 1963, Metcalf et al. published qualitative results which they
obtained on cotton. In these studies, too, (2) and (3) were the
principal metabolites.
With the improvement of analytical techniques, a better insight was
gained into the metabolism of fenthion in plants. Leuck and Bowman
(1968) treated Coastal bermudagrass and ensilage corn at rates of 0.56
kg/hectare, 1.12 kg/hectare and 2.24 kg/hectare (a.i.). The ppm
residues determined in Coastal bermudagrass treated at the highest
application rate were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 64 71 1.0 1.8 n.d.
7 0.31 10 4.0 2.0 0.34
14 0.07 2.1 2.3 0.48 0.18
21 0.03 0.59 1.3 0.08 0.11
The corresponding residue levels for corn were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 0.41 26 0.22 0.63 <0.005
7 0.04 2.4 0.68 1.03 0.16
14 0.01 0.42 0.24 0.11 0.09
21 <0.002 0.34 0.22 <0.02 0.04
(4) was not detected (except on day 0 in bermudagrass when its level
was 0.06 ppm). (2) and (3) were again the principal metabolites.
The total residues were as follows (expressed in ppm):
Days Bermudagrass Corn
post-treatment 0.56 kg/ha 1.12 kg/ha 0.56 kg/ha 1.12 kg/ha
7 3.3 8.5 0.65 1.95
14 1.3 3.2 0.15 0.4
21 0.5 1.3 0.1 0.2
These findings were very largely confirmed by a study performed by
Bowman et al. (1968), in which nine procedures for removal of
phosphorus insecticides and their metabolites were compared. This
study also provides an impressive picture of the differing effect of
various extraction procedures. Soxhlet extraction with 10% methanol in
chloroform proved to be the best one.
The fate of phenolic hydrolytic products of fenthion in plants was not
further studied. However, findings have been published by Wendel and
Bull (1970) in respect of GC-6506 (dimethyl 4-methylthiophenyl
phosphate). From some of the metabolites, substituted phenols could be
liberated by hydrolysis with ß-glucosidase (Bull and Stokes, 1970).
The predominant products were the glucosides of phenol-sulfoxide and
phenol-sulfone. The glycone portions of the conjugates are further
altered, possibly by the formation of a ß-gentiobiside.
In soils
The half-life of fenthion and its metabolites in soil is between 14
and 40 days; only in one out of six experiments was a half-life of
more than 30 days recorded. The experiments used various formulations
including granules and emulsifiable solutions. (Chemagro - Summary
under soils in main submission).
In storage and processing
Under frozen storage (-18 to -23°C), fenthion residues were shown to
be stable in alfalfa for 28 weeks and in cattle fat for 11 weeks
(Chemagro, 1966e,f). In liver from cattle, a decrease of 20% seemed to
occur between the twelfth and twenty-sixth week of storage (Chemagro,
1966f).
Fenthion proved to be stable for four weeks at -18°C in cattle brain,
heart, liver, kidney, steak and fat over a period of four weeks; (6)
decreased slightly in brain, heart, steak and fat, by 30% in kidney,
and by 80% in liver in six weeks (Olson, 1966).
Following treatment of corn with fenthion at rates of 0.56, 1.12 and
2.24 kg per hectare (Boman and Beroza, 1969), the plants treated at
the highest rate contained 39 ppm of residues (1) + (2) + (3) + (5),
in a ratio of 1.8 : 96.5 : 0.75 : 0.97. At 70 days after ensiling
(which took place on the day after application), the residues amounted
to 14.6 ppm (17 : 74 : 7.8 : 1.1); at 141 days, they amounted to 6.85
ppm (9.7 : 85 : 4.7 : 0.5). The predominant metabolite throughout the
whole period was (2). In another silage experiment with corn, the
residues were consistently shown to be at a lower oxidation stage than
were those in the field samples from which the silage was prepared
(Leuck and Boman, 1968).
The relatively high persistence of the residues in silage and the
occurrence of (2) as the principal metabolite were again confirmed
later (Bowman et al., 1970).
Residues in canned and preserved peaches persist to a certain extent,
especially when maintained at low temperatures (refrigerator) (Pigatti
et al., 1967).
Evidence of residues in commerce or at consumption
Krause and Kirchhoff (1969) examined 78 market samples of different
fruits and vegetables of domestic and foreign origin for residues of
20 organophosphorus insecticides including fenthion. Residues of
fenthion were not found in any of the samples.
Total diet studies were carried out in England and Wales, in 1966/67,
with altogether 66 samples of total diet. Each sample comprised seven
sub-samples consisting of cereals, meats, fish, fats, fruits and
preserves, root vegetables, green vegetables and milk. Fenthion and
its immediate oxidation products could have been detected by the
method employed, but they were not detected (Abbott et al., 1970).
Methods of residue analysis
Although the present-day gas chromatographic methods can be carried
out largely without clean-up, it is nevertheless necessary in some
cases to eliminate interfering substances. In this, consideration must
be given to the greatly differing polarity of fenthion residues.
Chemagro (1963) has published, for example, the following data on
partition:
n-hexane water n-hexane
Compound acetonitrile chloroform water
(1) 1 : 8.4 1 : 1720 1 : 0.016
(5) 1 : 300 1 : 88 1 :
(6) 1 : 87 1 : 15 1 : 78
Bowman and Beroza (1968) give the following p-values (=fractional
amount in nonpolar phase):
n-hexane n-hexane n-hexane
Compound 20% acetonitrile 40% acetonitrile water
in water in water
(1) 0.98 0.92 1.00
(2) 0.18 0.03 0.50
(3) 0.61 0.12 0.94
(4) 0.65 0.18 0.92
(5) 0 0 0
(6) 0 0 0.01
Regarding the extraction of residues, attention is drawn to the study
of Bowman et al. (1968) already referred to earlier.
The first methods for the determination of fenthion and its
metabolites were based on determination of total phosphorus (Frehse et
al., 1962a for plant material; Frehse et al., 1962b for olives (oil);
Adam (1967) for olives). In 1966, the first methods were published for
the determination of fenthion in plant and animal tissues (Anderson et
al., 1966) and milk (Katague, 1966), based on oxidation of the various
compounds to the sulfone (6) which in turn was hydrolyzed to the
corresponding phenol. The phenol was brominated and acetylated prior
to detection measurement by electron-capture GLC. The methods were
sensitive to 0.1 ppm (0.01 ppm for milk) but unavoidably very
complicated and time-consuming.
Therefore, Chemagro began in 1967 to develop methods using the
thermionic phosphorus detector: for soil (Olson 1967a), rice grain
(Olson 1967b), eggs and milk (Olson 1968), and animal tissues
(Thornton 1967).
These methods are based on oxidation of the residues to (6). The
sensitivities are below 0.1 ppm for soil and rice, and about 0.005 ppm
for eggs and milk. GLG conditions are:
(a) soil: 11 in × 3 mm i.d. column, packed with 6% DC-200 and 1% QF-1
on 80/100 mesh Gas Chrom Q, 230°C;
(b) other material: 16 in × 3 mm i.d. column, packed with 10% DC-200
and 0.2% QF-1 on 80/100 mesh Gas Chrom Q, 210°C, retention time
3.5 minutes.
Forty-five organophosphorus insecticides were examined for possible
interference with the rice method (Olson, 1967b); no interferences
were noted for any of the compounds except (R) Dasanit (Olson,
1967c).
Bowman and Beroza (1968) used a different principle of determination.
Extracts from corn, grass and milk were separated by liquid
chromatography into three fractions which then were analysed by GLC
with a flame photometric detector: 90 cm × 4 mm i.d, column, packed
with 10% DC-200 on 80/100 mesh Gas Chrom Q, 210°C for the two
fractions (1) + (3) and (2) + (4) + (6); a 45 cm column is used for
(5) (retention times (1) to (6) : 1.75, 4.2, 4.05, 1.45, 2.3 minutes).
Ten per cent. QF-1 (50 cm), 1% Carbowax 20 M (50 cm) and 5% DEGS
(50 cm) can also be used as the liquid phase. A complete separation of
all six compounds was achieved by using liquid chromatography with a
silica gel column and different elution systems. Later, Bowman and
Beroza (1969) also used the oxidation method with m-chloroperbenzoic
acid for the same substrates: 90 cm × 4 mm i.d. column packed with 10%
OV-101 on 80/100 mesh Gas Chrom Q, 215°C. With this column, the
metabolites can also be determined singly (retention times of
0.7 - 2.75 minutes, 2.3 minutes for (6)); it was found that the
results of the single metabolite determinations those of the total
determination. The recoveries were above 90%. Excessive amounts of the
oxidation mixture are removed on an alumina column.
Further studies performed by Bowman and Beroza (1970) show that 2.4
metre × 4 mm i.d. columns, packed with 5% OV-101 or OV-210 on Gas
Chrom Q are also suitable for determination of the compounds (1) to
(6).
Examples of national tolerances
Country Crop Tolerance Safety
in ppm Interval
in days
Algeria General 15
Australia Fruit, vegetables 2 7
Meat of cattle 1 1
Milk and milk products 2 1
Austria General 35
Belgium Fruit, vegetables excluding
potatoes 0.3 21
Brazil General 14
Fruit 1.0
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
Bulgaria Sugar beets 14
Cherries, cereals 14
Canada Beef cattle N.R.
Finland General 14
France General 15
Olives 21
Germany Fruit 14
Vegetables, field & fodder
crops 10
Israel Fruit 21
Miscellaneous (e.g. dry onions
and cucurbits) 30
Italy General 20
Olives 30
Morocco General 15
Olives 30
Netherlands Fruit, vegetables excluding
potatoes 0.3 -
New Zealand General 21
Norway General 14
Poland Fruit, vegetables, root crops 30
Early cherries 14
Portugal General 14
Olives (as oil and preserves) 42
Russia Cereals, cottonseed oil 0.35
Spain Fruit and olives 30
South Africa General 2.0
Apricots, peaches, apples,
pears, grapes 10
Subtropical crops & cucurbits 7
Deciduous fruit 10
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
United States Alfalfa (fresh), grass 5.0
of America Alfalfa (hay), grass hay 18.0
Animal products 0.1
Milk 0.01
Yugoslavia General 0.5
N.R. = registered for use on a no-residue basis.
Appraisal
Fenthion is an organophosphorus insecticide with a broad spectrum of
activity used against insects infesting field crops, fruit crops,
vineyards, olives, cotton, insects of public health concern and
ectoparasites of domestic animals. It is especially useful for control
of fruit flies in many crops where its ability to penetrate plant
tissues allows for destruction of larvae within the fruit. The
concentrations/rate of use ranges from 0.5-2 kg/ha in the case of
field crops and cotton and 0.05-0.1% solution on horticultural crops.
Animals are treated with 0.25%-5% dust, 0.025%-0.25% solutions as dips
or sprays and 2%-3% solutions in oil as pour-on preparations or back
rubbers.
The residue data available to the meeting were obtained from many
different countries, reflecting typical registered uses under a wide
range of climatic and ecological conditions. Considerable information
is available about the fate of fenthion residues in foods of plant and
animal origin and extensive studies have been carried out on the
nature of the degradation products formed under a variety of
influences.
Little information is available about residues of fenthion in foods in
commerce.
The literature includes a number of methods of residue analysis based
on gas-chromatographic procedures. The greatly differing polarity of
the various fenthion metabolites has been investigated and
recommendations for the most appropriate extraction procedures have
been made.
In order that all biologically active metabolites may be determined
simultaneously the analytical procedure has been modified to provide
for oxidation of the residues of the various compounds to the sulfone
which is hydrolyzed to the corresponding phenol. The phenol is
brominated and acetylated prior to measurement by electron-captive
gas/liquid chromatography. The limit of detection is 0.1 ppm for most
foods and 0.01 ppm for milk.
Procedures which are less complicated and less time consuming have
been developed using the thermionic phosphorus detector following
oxidation to the sulfone. The limit of detection is below 0.1 ppm for
soil and grain, and about 0.005 ppm for eggs, milk and animal tissues.
These procedures appear suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The temporary tolerances are for fenthion and its major metabolites,
determined separately or together and expressed as fenthion.
Apples, peaches, cherries, lettuce, 2 ppm
fat of meat
Cabbage, cauliflower, olives, 1 ppm
olive oil
Grapes, oranges, peas, meat 0.5 ppm
Squash 0.2 ppm
Wheat, rice, milk products (fat basis) 0.1 ppm
Milk (whole) 0.05 ppm
Further work or information
Required before 30 June 1975
1. Adequate two-year feeding studies in the dog and in one rodent
species.
2. Establishment of the sequence of metabolic changes in man and
laboratory animals in order to elucidate the mechanism of long
lasting cholinesterase inhibition.
Desirable
Information on the frequency and level of fenthion residues in food
commodities in commerce.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adam, N. Chr. (1967) Méthode de détermination des résidue de Lebaycid
dans l'huile d'olive et lea olives. Ann. Inst. Phytopath. Benaki, N.S.
8: 78-84
Anderson, R. J., Thornton, J. S., Anderson, C. A. and Katague, D. B,
(1966) Determination of fenthion residues in plant and animal tissues
by electron-capture gas chromatography. J. Agr. Food Chem. 14:
619-622
Begum, A. (1968) Effect of diet on metabolism of fenthion in animals.
Ph.D. thesis, Auburn University, Auburn, Alabama. Dissert, Abstr.,
Sect. B, 28: 4165
Bowman, M. C. and Beroza, M. (1968) Determination of fenthion and five
of its metabolites in corn, grass and milk. J. Agr. Food Chem. 16:
399-402
Bowman, M. C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton and phorate in corn, milk, grass and
faeces, J.A.O.A.C. 52: 1231-1237
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J.A.O.A.C. 53: 499-508
Bowman, M. C., Beroza, M. and Leuck, D. B. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites from
field treated crops. J. Agr. Food Chem. 16: 796-802
Bowman, M. C., Leuck, D. B., Johnson, J. C., jr and Knox, F. E. (1970)
Residues in corn silage and effect of feeding dairy cows the treated
silage. J. econ. Entomol. 63: 1523-1528
Brady, U. E., jr and Arthur, B. W. (1961) Metabolism of O,o-dimethyl
O-[4-(methylthio)-m-toly] phosphorothioate by white rats. J. econ.
Entomol, 54: 1232-1236
Bull, D. L. and Stokes, R. A. (1970) Metabolism of dimethyl
p-(methylthio) phenyl phosphate in animals and plants. J. Agr. Food
Chem. 18: 1134-1138
Chemagro Milk and tissue residues resulting from backline or oral
treatment of Cattle with Bayer 29493, p. 32
1963 Report No. 10. 946, December 3
1965a Report No. 16. 551, July 27 (revised January 10, 1968)
1965b Report No. 16. 990, October 21
1965c Report No. 17. 015, October 28
1965d Report No. 17. 062, November 3
1966a Report No. 17. 445, January 19 (revised April 28, 1966)
1966b Report No. 17. 969, April 21
1966c Report No. 18. 009, May 3
1966d Report No. 18. 067, May 12
1966e Report No. 18. 179, May 20
1966f Report No. 18. 191, May 24
1967a Report No. 20. 310, April 18
1967b Report No. 20. 738, June 20
1967c Report No. 20. 798, July 5
1967d Report No. 21. 113, September 13
1967e Report No. 21. 504, November 13
1968a Report No. 21. 796, January 25
1968b Report No. 22. 647, May 15
1969 Report No. 24. 266, February 14 (revised April 2, 1969)
1970 Report No. 28. 719, December 3
Dean, G., Coxon, J. and Brereton, D. (1967) Poisoning by an
organo-phosphorus compound. A case report. So. African Med. J.,
1017-19
Dieckmann, W. (1971) Neurotoxizctatsunter-suchungen an
Huhnern-Histopathologie. Unpublished report of Farbenfabriken Bayer
A.G.
Dilley, J. and Doull, J. (1961a) Chronic inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dilley, J. and Doull, J. (1961b) Acute inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. (1960) The absence of antidote activity by 2-PAM and
TMB-4 against acute poisoning by Bayer 29493. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. (1961) Effects of repeated dermal application of Bayer
29493 on rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1962) Acute oral toxicity of a sample of Bayer 29493 to
female rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Dubois, K. P. and Doull, J. (1960) The acute toxicity of Bayer 29493
to chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. and Puchala, E. (1960) Influence of Bayer 29493 on the
cholinesterase activity of the blood of rats. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. and Kinoshita, F. (1964) Acute toxicity and
anti-cholinesterase action of O,O-dimethyl O-4-(methylthio)-m-tolyl
phosphorothioate (DMTP; Baytex) and related compounds. Tox. Appl.
Pharmacol. 6: 86-95
Doull, J., Root, M. and Cowan, J. (1961) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Doull, J., Root, M. and Cowan, J. (1962) Effect of adding Bayer 29493
in combination with other cholinergic insecticides to the diet of male
and female dogs. Unpublished report from Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Cowan, N. J., Vesselinovitch, D., Fitch, F. W.
(1963a) and Meskauskas, J. Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Root, M., Cowan, J. and Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. and
Meskauskas, J. (1961) The effects of feeding diets containing Bayer
29493 to rats for a period of 16 weeks. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Elliott, R. and Barnes, J. M. (1963) Organophosphorus insecticides for
the control of mosquitos in Nigeria. Bull. Wld Hlth Org. 28: 35-54
Francis, J. I, and Barnes, J. M. (1963) Studies on the mammalian
toxicity of fenthion. Bull. Wld. Hlth. Org. 29: 205-12
Frehse, H. (1970) Ruckstande van pflanzenschutz mittein in nahrung und
um welt in chemie der pflanzenschutz und schadlingshenamp-fungsmittel.
Band 2-Wegler, R., Ed. Springer-Verlag, 1970
Frehse, H., Niessen, H. and Tietz, H. (1962a) Method of determining
residues of the insecticide Lebaycid(R) in plant material.
Pflanzenschutz-Nachr Bayer 15: 148-159
Frehse, H., Niessen, H. and Tietz, H. (1962b) Method of determining
residues of the insecticide Lebaycid(R) in olives and olive oil.
Pflanzenschutz-Nachr Bayer 15: 160-165
Fukuda, H., Masuda, T., Miyahara, Y. and Tomizawa Ch. (1962) Fate of
O,O-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on rice plant. Japan. J. appl. Entomol. Zool. 6: 230-236
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14: 515-34
Hahn, H. L. and Hensehler, D. (1969) The ability of phosphorylated
cholinesterases to be reactivated by obidoxime chloride (Toxogonin)
in vivo. Arch. Toxikol. 24: 147-63
Katague, D. B. (1966) Determination of fenthion residues in milk by
electron-capture gas chromatography. Chemagro Corp., Research
Department, Report No. 17, 887
Keith, J. O, and Mulla, M. S. (1966) Relative toxicity of five
organo-phosphorus mosquito larvicides to mallard ducks. J. Wildlife
Management 30: 553-63
Kimmerle, G. (1960) Re: Active substance S1752. Unpublished report
submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1961) Subchronische oral versuche bei ratten mit
S-1752-Wirkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1963) Product BH6 and S1752 Poisoning. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965a) Nourotoxic studies with Bayer 29493. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965b) Neurotoxische untersuch-ungen mit
S-1752-Werkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1966) Langdavernde Inhalatronsversuche bei hunden mit
dem Baytex-Werkstoff (S-1752)
Kimmerle, G. (1967a) Abhangigkeit der akuten oralen toxizitat bei
ratten vom losungsmittel. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. (1967b) Potenzierung von DDVP und S-1752. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Klimmer, O. R. (1963) Toxicological testing of Bayer 29493.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Knowles, C. O. and Arthur, B. W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of radiolabelled
fenthion to dairy cows. J. econ. Entomol. 59: 1346-52
Krause, Ch. and Kirchhoff, J. (1969) Organophosphatrückstande auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen
Nachrichtenbl. Deut. Pflanzenschutzdienstes (Braunschweig), 21:
81-84
Leuck, D. B. and Bowman, M. C. (1968) Residues of fenthion and five of
its metabolites their persistence on corn and grass forage, J. econ.
Entomol. 61: 1594-1597
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmakol. 263: 237-8
Loser, E. (1969) Generation versuche an Ration. Unpublished report
submitted by Farbenfabriken Bayer A.G.
McGrath, H. B. (1969) Toxicity of Bayer 29493 in calves. Unpublished
report submitted by Farbenfabriken Bayer A.G
Metcalf, R. L., Fukuto, T. R. and Winton, M. Y. (1963) Chemical and
biological behaviour of fenthion residues. Bull. Wld Hlth Org. 29:
219-226
Möllhoff, E. (1970) Determination of trichlorfon and fenthion residues
in animals of different species, Pestic. Sci. 2: 179-181
Nelson, D. L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Niessen, H., Tietz, H. and Frehse, H. (1962a) Papier
chromatographische Trennung aromatischer
Phosphorsäure-ester-Insektizide. J. Chromatog. 9: 111-113
Niessen, H., Tietz, H. and Frehse, H. (1962b) On the occurrence of
biologically active metabolites of the active ingredient S-1752 after
application of Lebaycid(R) Pflanzenschutz-Nachr. Bayer 15: 125-147
Olson, T. J. (1966) The determination of the stability of fenthion and
its oxygen analog sulfon in frozen cattle tissues. Chemagro Corp.,
Research Department, Report No. 19, 289,
Olson, T. J. (1967a) Determination of residues of fenthion in soil by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 20, 324.
Olson, T. J. (1967b) Determination of residues of fenthion in rice
grain by thermionic emission gas chromatography. Chemagro Corp.,
Research Department, Report No. 20, 417 (revised 21 October, 1968)
Olson, T. J. (1967c) A study of the possible interference of other
pesticides with the analytical method for fenthion in rice. Chemagro
Corp., Research Department, Report No. 20, 595
Olson, T. J. (1968) Determination of fenthion in eggs and milk by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 22, 933
Pickering, E. N. (1966) Organic phosphate insecticide poisoning. Can.
J. Med. Tech., p. 174
Pigatti, A., Pigatti, P., Orlando, A., Suplicy, F. O., Sampaio, A. S.
and Rigitano, O. (1967) Persistência de residuos de fenthion am
pêssego, ameixa e maca. Arq. Inst. Biol. (S. Paulo) 34: 275-284
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol Appl. Pharmacol. 3, 512-33
Shillam, K. W. G., Medd, R. K., Roberts, N. L. and Burrows, I. E.
(1971) Residual levels of fenthion in raw and pasteurized milk. Report
of Huntington Research Centre 4225/71/383
Shimamoto, K. and Hattori, K. (1969) Chronic feeding of Baytex
(O, O-dimethyl-o-(4-methylmercapto-3-methyl) phenyl-thiophosphate) in
rats. Acta Med. Univ. Kioto, 40: 163-71
Shipp, E. (1970) Fenthion residues in milk. Report of the Entomology
School, University of New South Wales
Spicer, E. J. F. (1971) Pathology Report of Bay 29493. Generation
study in rats. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull. Wld Hlth Org.,
29: 213-18
Thornton, J. S. (1967) Determination of fenthion residues in animal
tissues by thermionic emission flame gas chromatography. Chemagro
Corp., Research Department, Report No. 20 420 (revised 21 October,
1968)
Tomizawa, Ch., Fukuda, H., Masuda, T. and Miyahara, Y. (1962) Fate of
O,o-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on tea and cabbage leaves. Japan. J. appl. Entomol. Zool., 6:
237-241
von Clarmann, M. and Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and its
demonstration in the gastric contents and urine. Arch. Toxik., 22:
2-11
Wendel, L. E. and Bull, D. L. (1970) Systemic activity and metabolism
of dimethyl p-(methylthio) phenyl phosphate in cotton. J. Agr. Food
Chem., 18: 420-424
 Other information on identity and properties
The pure active ingredient is a colourless liquid and the technical
active ingredient is a brownish viscous liquid. The active ingredient
has a boiling point of 87°C at 0.01 mm Hg. It is practically insoluble
in water, and readily soluble in most organic solvents. It has a very
low vapour pressure and slight volatility.
Impurities in the technical active ingredient
active ingredient (FAO-method No. 79/l/m/1.2)* 95-97%
O-methyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate <0.5%
5-hydroxy-2-methylmercapto-toluol <0.5%
O,S-dimethyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate 0.5-1.0%
O,O,O,O-tetramethyl-dithiopyrophosphate <0.5%
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate 5-7%
5-methoxy-2-methylmercapto-toluol <0.8%
O,O,O-trimethyl-thiophosphate <0.5%
Water max. 0.15%
* By this analytical method the impurity
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate
is determined together with the active ingredient.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
The first studies on mammals (rats) were reported by Brady and Arthur
(1961) who used a p32-labelled compound. Within a few hours of
applying the compound, large amounts of p32-activity was found in
the tissues including the bones, evidence that fenthion was rapidly
degraded by rats. Fenthion or its oxidative metabolites were, however,
not stored in tissues even when rats received daily doses of 10 mg/kg
by the intraperitoneal route for 10 consecutive days. At 1.5 hours,
following a single i.p. injection of 200 mg/kg, the following
acetonitrile-soluble residues were observed:
liver 29.5 ppm
muscle 9.6 ppm
skin 6.1 ppm
kidney 26.9 ppm
heart 9.6 ppm
The tissues from rats treated orally with 100 mg/kg (single dose)
contained less than 0.01 ppm chloroform-soluble residues at three days
after treatment, except for the liver (0.2 ppm); the blood, brain and
fat of these animals contained no detectable acetonitrile-soluble
residues, either. The orally treated animals eliminated 86% of the
activity in the excreta by seven days after treatment. One to four per
cent. of the activity in urine and faeces was chloroform-soluble.
As a result of partial starvation, oxidative metabolism in rabbits was
increased as evidenced by greater concentrations of fenthion-oxygen
analogue in blood. The peak of radioactive substances in blood of
normal rabbits following oral or subcutaneous administration of 35S
fenthion was observed in six to nine hours following treatment while
in starved rabbits the peak value was obtained one hour after
treatment (Begum, 1967).
Biotransformation
Fenthion is activated through an enzymatic oxidation, in both plants
and animals, to more active anti-cholinesterase compounds. The molar
I50 fenthion for rat brain cholinesterase is 1.3 × 10-4m. (Dubois
and Kinoshita, 1964). Following intraperitoneal administration of
32p-labelled fenthion approximately 75% of the administered dose was
recovered within three days in rat urine (60%) and faeces (15%). After
oral administration 86% of the dose was excreted in urine (45%) and
faeces (40%) with the majority excreted in three days (Brady and
Arthur, 1961). Starvation of rabbits had no effect on elimination of
fenthion (or metabolites) following oral or subcutaneous acute
administration (Begum, 1967). Five metabolites (see Figure I) were
isolated from rat urine and characterized as the sulfoxide (II) and
sulfone (III) of the phosphorothioate (I), the oxygen analogue (IV) of
the parent compound and its corresponding sulfoxide (V) and sulfone
(VI) derivatives.
In rabbits the major urinary metabolites were fenthion-sulfoxide (II),
fenthion-O-sulfoxide (V) and sulfone (VI) (Begum, 1967). The metabolic
scheme appears as shown in Figure I.
Effects on enzymes and other biochemical parameters
Fenthion and its metabolites are typical organophosphorus
anticholinesterase agents. Typical of this class of agents the oxygen
analogue is the most potent enzyme inhibitor of all the metabolites
containing a phosphorus triester configuration (Francis and Barnes,
1963)). Fenthion is unusual in that clinically and biochemically a
prolonged effect following a single dose is manifest (recovery of
blood and brain enzyme levels is very slow (Brady and Arthur, 1961).
This effect may be as a result of inhibition of cholinesterase by
metabolites which are released at different rates from storage in the
body (Francis and Barnes, 1963), or a possible potentiation of its own
antiesterase effects by selectively inhibiting phosphatase activity
(Brady and Arthur, 1961), which is responsible for hydrolysis of the
phosphate ester. Cholinesterase inhibitor cannot be reactivated by
2-PAM, TMB-4 or P-2-S in vivo indicating that fenthion may inhibit
cholinesterase in a manner differing from many of the other
organophosphate esters. (Dubois, 1960; Dubois and Kinoshita, 1964;
Francis and Barnes, 1963).
Other information on identity and properties
The pure active ingredient is a colourless liquid and the technical
active ingredient is a brownish viscous liquid. The active ingredient
has a boiling point of 87°C at 0.01 mm Hg. It is practically insoluble
in water, and readily soluble in most organic solvents. It has a very
low vapour pressure and slight volatility.
Impurities in the technical active ingredient
active ingredient (FAO-method No. 79/l/m/1.2)* 95-97%
O-methyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate <0.5%
5-hydroxy-2-methylmercapto-toluol <0.5%
O,S-dimethyl-O-(4-methylmercapto-3-methyl-phenyl)-thiophosphate 0.5-1.0%
O,O,O,O-tetramethyl-dithiopyrophosphate <0.5%
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate 5-7%
5-methoxy-2-methylmercapto-toluol <0.8%
O,O,O-trimethyl-thiophosphate <0.5%
Water max. 0.15%
* By this analytical method the impurity
O,O-dimethyl-O-(4,6-bis-methylmercapto-3-methyl-phenyl)-thiophosphate
is determined together with the active ingredient.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Absorption, distribution and excretion
The first studies on mammals (rats) were reported by Brady and Arthur
(1961) who used a p32-labelled compound. Within a few hours of
applying the compound, large amounts of p32-activity was found in
the tissues including the bones, evidence that fenthion was rapidly
degraded by rats. Fenthion or its oxidative metabolites were, however,
not stored in tissues even when rats received daily doses of 10 mg/kg
by the intraperitoneal route for 10 consecutive days. At 1.5 hours,
following a single i.p. injection of 200 mg/kg, the following
acetonitrile-soluble residues were observed:
liver 29.5 ppm
muscle 9.6 ppm
skin 6.1 ppm
kidney 26.9 ppm
heart 9.6 ppm
The tissues from rats treated orally with 100 mg/kg (single dose)
contained less than 0.01 ppm chloroform-soluble residues at three days
after treatment, except for the liver (0.2 ppm); the blood, brain and
fat of these animals contained no detectable acetonitrile-soluble
residues, either. The orally treated animals eliminated 86% of the
activity in the excreta by seven days after treatment. One to four per
cent. of the activity in urine and faeces was chloroform-soluble.
As a result of partial starvation, oxidative metabolism in rabbits was
increased as evidenced by greater concentrations of fenthion-oxygen
analogue in blood. The peak of radioactive substances in blood of
normal rabbits following oral or subcutaneous administration of 35S
fenthion was observed in six to nine hours following treatment while
in starved rabbits the peak value was obtained one hour after
treatment (Begum, 1967).
Biotransformation
Fenthion is activated through an enzymatic oxidation, in both plants
and animals, to more active anti-cholinesterase compounds. The molar
I50 fenthion for rat brain cholinesterase is 1.3 × 10-4m. (Dubois
and Kinoshita, 1964). Following intraperitoneal administration of
32p-labelled fenthion approximately 75% of the administered dose was
recovered within three days in rat urine (60%) and faeces (15%). After
oral administration 86% of the dose was excreted in urine (45%) and
faeces (40%) with the majority excreted in three days (Brady and
Arthur, 1961). Starvation of rabbits had no effect on elimination of
fenthion (or metabolites) following oral or subcutaneous acute
administration (Begum, 1967). Five metabolites (see Figure I) were
isolated from rat urine and characterized as the sulfoxide (II) and
sulfone (III) of the phosphorothioate (I), the oxygen analogue (IV) of
the parent compound and its corresponding sulfoxide (V) and sulfone
(VI) derivatives.
In rabbits the major urinary metabolites were fenthion-sulfoxide (II),
fenthion-O-sulfoxide (V) and sulfone (VI) (Begum, 1967). The metabolic
scheme appears as shown in Figure I.
Effects on enzymes and other biochemical parameters
Fenthion and its metabolites are typical organophosphorus
anticholinesterase agents. Typical of this class of agents the oxygen
analogue is the most potent enzyme inhibitor of all the metabolites
containing a phosphorus triester configuration (Francis and Barnes,
1963)). Fenthion is unusual in that clinically and biochemically a
prolonged effect following a single dose is manifest (recovery of
blood and brain enzyme levels is very slow (Brady and Arthur, 1961).
This effect may be as a result of inhibition of cholinesterase by
metabolites which are released at different rates from storage in the
body (Francis and Barnes, 1963), or a possible potentiation of its own
antiesterase effects by selectively inhibiting phosphatase activity
(Brady and Arthur, 1961), which is responsible for hydrolysis of the
phosphate ester. Cholinesterase inhibitor cannot be reactivated by
2-PAM, TMB-4 or P-2-S in vivo indicating that fenthion may inhibit
cholinesterase in a manner differing from many of the other
organophosphate esters. (Dubois, 1960; Dubois and Kinoshita, 1964;
Francis and Barnes, 1963).
 TOXICOLOGICAL STUDIES
Special studies
(a) Acute toxicity of the metabolites
Compound Acute LD50 (mg/kg) 150 (M)c
Orala i.p.b
I. Fenthion 220 325 >5 × 10-4
II. Fenthion sulfoxide 125 250 4.5 × 10-5
III. Fenthion sulfone 125 250 4.7 × 10-4
IV. Oxygen analogue 125 26 2.65 × 10-6
V. O-Sulfoxide 50 22 4.8 × 10-5
IV. O-Sulfone 30 9 3.2 × 10-5
VIII. 4-(methylthio)m-cresol 6500d
IX. 4-methyl(thiosulfoxide)
m-cresol 3500d
X. 4-methyl(thiosulfone)
m-cresol 7000d
a Male rats - according to Francis and Barnes, 1963.
b Female rats - according to Dubois and Kinoshita, 1964.
c 150 = Molar concentration resulting in 50% inhibition of
human RBC Cholinesterase (Francis and Barnes, 1963).
d Female rats according to Nelson, 1967.
(b) Reproduction
Except for a slight growth depression at the highest level in the
diet, fenthion at levels of 0, 3, 15 and 75 ppm for three generations
(two litters per generation), produced no adverse effect on rat
reproduction (Loser, 1969). Microscopic examination of the tissues of
the F3b generation did not reveal any significant abnormality (Spicer,
1971).
(c) Potentiation
Fenthion potentiates the acute intraperitoneal toxicity of malathion,
dioxathion, and coumaphos in rats. Intraperitoneal administration of
13 other organophosphate or carbamate insecticides did not result in a
greater than additive effect when administered with fenthion (Dubois
and Kinoshita, 1964). Dietary combination of fenthion with coumaphos
neither of which alone affected cholinesterase when fed to dogs for
six weeks, was found to potentiate the anticholinesterase activity of
serum and red blood cells. Less evident potentiation was observed when
fenthion and malathion were fed and when fenthion was fed in
combination with dioxathion no potentiation was noted (Doull et al.,
1962), Fenthion (2 ppm) and malathion (100 ppm) resulted in moderate
erythrocyte and serum cholinesterase activity inhibition (30-40%).
When fenthion (2 ppm) and dioxathion (3 ppm) was fed cholinesterase
activity was not depressed. When fenthion (2 ppm) and coumaphos
(2 ppm) was fed to dogs, significant inhibition (75%) of serum
cholinesterase and moderate inhibition (30%) of erythrocyte
cholinesterase was evident. Oral administration to rats of a 75:25
mixture of fenthion and dichlorvos did not result in a greater than
theoretically additive toxicity (Kimmerle, 1967b).
(d) Neurotoxicity
No evidence for delayed neurological disruption in hens was evident
when fenthion was administered orally at a single dose of 25 mg/kg
(Kimmerle, 1965a). When chickens were fed up to 100 ppm in the diet
for 30 days, clinical examination of the animals and histological
examination of nerve tissue indicated no demyelinating effect from
fenthion (Kimmerle, 1965b; Dieckmann, 1971).
(e) Antidotes
A number of antidotes which are commonly used for organophosphorous
poisoning have been shown to be relatively inactive when used
following fenthion intoxication. Studies with atropine, 2-PAM
Toxogonin P-2-S, and TMB-4 (Dubois and Kinoshita, 1964; Francis and
Barnes, 1963; and Dubois, 1960; Lorke and Kimmerle, 1969) administered
alone and in combination showed that these materials did not
successfully alleviate the parasympathomimetic signs of
organophosphate poisoning. When BH-G (Merck; Darmstadt, Germany) was
administered three to four times in combination with atropine, the
LD50 value increased from 250 to 440 mg/kg (Kimmerle, 1963).
Toxogonin was not effective as a cholinesterase reactivator following
oral intoxication of dogs by fenthion (Hahn and Henschler, 1969).
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Mouse (M) Oral 150 Francis and Barnes, 1963
227 Dubois, 1968
(F) 190 Francis and Barnes, 1963
225 Dubois, 1968
(F) i.p. 150 Dubois and Kinoshita, 1964
(M) 125 Dubois and Kinoshita, 1964
(Continued)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat (F) Oral 245-310 (Dubois and Kinoshita, 1964
(Gaines, 1960
615 Francis and Barnes, 1963
(M) 175-470 (Dubois and Kinoshita, 1964
(Klimmer, 1963
(Gaines, 1969
(Francis and Barnes, 1963
(M) i.p. 325 Dubois and Kinoshita, 1964
(F) 260 Dubois and Kinoshita, 1964
(M) 152 Klimmer, 1963
(M) Dermal 330-650 Gaines, 1969
Klimmer, 1963
Francis and Barnes, 1963
(F) 330-500 Dubois and Kinoshita, 1964
Gaines, 1969
Francis and Barnes, 1963
Guinea-pig
(M) Oral 1000 Francis and Barnes, 1963
260 Dubois and Kinoshita, 1964
i.p. 310 Dubois and Kinoshita, 1964
Rabbit (M) Oral 150-175 Francis and Barnes, 1963
Dermal 150 Klimmer, 1963
Duck Oral 15 Dubois and Doull, 1960
1-2 Keith and Mulla, 1966
Chicken 30 Dubois and Doull, 1960
28 Sherman and Ross, 1961
30-40 Francis and Barnes, 1963
Calf Approx. 40 McGrath, 1961
Various solvents used to solubilize fenthion in combination with water
or ethanol had no significant effect on the acute oral LD50
(Kimmerle, 1967a).
Fenthion is an organophosphorus insecticide of intermediate toxicity
to mammals although it displays considerable differences in its
toxicity to various species, e.g. fowls are very sensitive (Keith and
Mulla, 1966).
The rate of absorption through the skin of rabbits is slow. When
fenthion was applied to a cotton plug and placed in a rabbit ear for
four hours, swelling occurred. When it was left for 24 hours mortality
resulted (Kimmerle, 1960).
Female rats tolerated a daily one hour inhalation challenge of 0.163
mg/l air for 30 days with cholinesterase depression but no mortality.
At 0.415 mg/l air all animals were dead within 10 days (Dilley and
Doull, 1961a).
Exposure by inhalation daily for nine days, six hours per day at 210
mg/M3 (initial concentration in a static inhalation chamber),
resulted in signs of poisoning, but no mortality in cats, guinea pigs,
rabbits and rats. Cholinesterase, which was severely depressed,
recovered in three weeks (Klimmer, 1963). Fenthion exhibits a
relatively low degree of acute mammalian toxicity. In only one
instance was a sex difference in susceptibility noted and this is in
contrast to the generally noted resistance of males to most other
phosphorothioates (Dubois and Kinoshita, 1964). The signs of poisoning
are typical of the central and peripheral cholinergic effects of
organophosphorus esters with a gradual onset of the symptoms. The
symptoms in humans and other animals include tremors, lacrymation,
salivation, vomiting, diarrhoea and other signs of cholinergic
stimulation.
Short-term studies
Duck
Mallard ducks fed 25 ppm of fenthion in the diet for six weeks became
emaciated and could not fly or walk; after two days on normal food,
recovery was evident. A dietary level of 5 ppm produced no adverse
clinical reaction (Keith and Mulla, 1966).
Rat
Dermal administration of fenthion at 14.5 and 25 mg/kg for 60 days to
rats resulted in 40% mortality in the higher dosed group and no
mortality in the lower dosed group. However, blood cholinesterase
levels were depressed to about 20% of normal at the lower treatment
level (Dubois, 1961). Cholinesterase was depressed and mortality was
absent when fenthion was applied to rats dermally for 12 days at 2.9
mg/kg (Dubois and Puchala, 1960).
Rats tolerated daily intraperitoneal administration of 10 mg/kg
fenthion for 60 days with no mortality. At 20 mg/kg, 80% of the
treated animals died within 20 days (Dubois and Kinoshita, 1964).
Mortality (12 dead of 30 rats tested) occurred following daily oral
administration of approximately 25 mg/kg (1/10 LD50) for 75 days
(six days per week). Signs of poisoning were transient disappearing
shortly after dosing (Klimmer, 1963).
In a preliminary study, male rats were orally administered fenthion
five days a week for 13 weeks at a dose of 30 mg/kg/day. Mortality
occurred in approximately 30% of the rats over the course of the
experiment and cholinesterase activity was depressed between 80-90% of
normal. At the conclusion of the experiment cholinesterase recovery
was very slow - up to 40 days (Kimmerle, 1961).
Rats (22 male and 22 female per group) were fed dietary levels of
fenthion at 0.25, 0.50, 2.5, and 5.0 mg/kg/day for three months.
Cholinesterase activity depression was evident at 0.5 mg/kg in red
blood cells, serum, liver and heart at all testing intervals. At the
lowest feeding level (0.25 mg/kg) the inhibition (approx. 10-20%) did
not progressively increase with time indicating lack of cumulative
effects. Mortality in females was evident at 5.0 mg/kg. The animals
died manifesting muscarinic and nicotinic effects. Body-weight gain
was reduced in males at 0.25 mg/kg and above while in females it was
evident only at 2.5 mg/kg. Behavioural effects were noted
(piloerection) at 0.5 mg/kg and above (especially in females). This
effect decreased with time and disappeared by week seven. Organ
weights were all distinctly lower than the controls but as the
body-weight was also reduced the organ to body-weight ratio did not
appear to be affected. Histological examination showed the testis to
have reduced spermatogenesis and atrophic prostate glands at the
highest feeding levels (2.5 and 5.0 mg/kg). The ovary was not affected
(Shimamoto and Hattori, 1969).
Rats (six groups of 12 male and 12 female) were fed for 16 weeks on
diets containing 0, 2, 3, 5, 25 and 100 ppm. Cholinesterase depression
was evident at 25 ppm and absent at 5 ppm. No adverse effects were
noted in food consumption, weight gain or gross and microscopic
examination of tissues (Doull et al., 1961).
Rats (six groups of 25 male and 25 female) were fed for one year on
diets containing 0, 2, 3, 5, 25 and 100 ppm of fenthion. There was no
evidence of significant changes in growth rate, food consumption,
general appearance and gross of microscopic examination of tissues.
Survival of male rats at 25 ppm was slightly depressed. Cholinesterase
examinations indicated depression at the 5 ppm level and above with
3 ppm showing no adverse enzyme effects. A mild extramedullary
haematopoiesis was observed in controls and all dosage levels and
haemosideresis was evident in the spleen of the rats at 100 ppm levels
(Doull et al., 1963a).
Dog
Dogs (four groups of two males and two females per group) were fed
fenthion at 0, 2, 5 and 50 ppm for 12 weeks. Growth was not affected
at any dietary level. Erythrocyte cholinesterase activity was
depressed at 50 ppm while serum cholinesterase was depressed at 5 ppm
and above. Little if any depression of the serum cholinesterase was
evident before five weeks, after which it progressively decreased to
about 40% inhibition (Doull et al., 1961).
Dogs (four groups of two males and two females) were fed fenthion at
0, 2, 5, and 50 ppm in the diet for one year. There was no effect of
fenthion on food consumption or growth over the test interval.
Erythrocyte and serum cholinesterase were significantly depressed at
50 ppm with the serum also depressed at 5 ppm. An increase in the
weight of the spleen, which was not dose dependent, was evident in all
of the treated animals. Microscopic examinations of the tissues showed
splenic congestion and some decrease in the cellularity of the red
pulp was evident at all dose levels fed. Extramedullary haematopoiesis
and haemosideresis was also observed in the spleen. Microscopic
examination of other tissues did not reveal any significant change
(Doull et al., 1963b).
Long-term studies
No data available.
Observations in man
Fenthion has been widely used in many parts of the world for control
of household pests, mosquitos, etc. Cholinesterase studies conducted
on individuals in areas treated by WHO for malaria eradication have
shown that very slight plasma cholinesterase depression occurs when
exaggerated spray schedules were followed. The plasma cholinesterase
levels were depressed for up to six weeks after spraying (Elliot and
Barnes, 1963). It was also evident that the children in the population
(less than seven years old) were more susceptible to the
anticholinesterase effects (Taylor, 1963). A man who ingested two
ounces of fenthion (Entex)(R) recovered from severe
organophosphorous poisoning after being in critical condition for the
first six days after poisoning. Recovery was slow, lasting up to 30
days. Cholinesterase measurements showed that at 22 days after
poisoning the cholinesterase activity was still depressed (Pickering,
1966). In humans, the signs of poisoning appear rapidly beginning with
blurred vision, unsteady gait and slurred speech. After 72 hours of
emergency treatment following an unknown quantity of fenthion, a man
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation. The patient began to recover
only after 11 days of treatment which included atropine, PAM and
toxogonin (Dean et al., 1967). In another case, 45 minutes after
ingestion of 30 ml of fenthion, a man was in a comatose state with
pale skin, cyanotic mucous membranes, slow regular heart beat, no
peripheral blood pressure and no reactions to pain or light
stimulation on the pupils. Recovery took six days (von Clarmann and
Geldmacher-von-Mallinkradt, 1966).
Comments
Fenthion is slowly absorbed, metabolized by a complex series of
reactions and excreted. It does not appear to accumulate in the body.
In most instances the unhydrolysed metabolites containing a phosphorus
atom are more toxic than the parent compound. The signs of
intoxication from a single oral dose develop slowly, persist for a
considerable period of time and are not readily alleviated by atropine
or by most common oxime reactivators. Short-term studies in the rat
and dog suggest that cholinesterase inhibition is the most sensitive
criterion of biological effect. Anti-spermatogenesis in one rat study
was not confirmed in other studies or in a reproduction study. The
occurrence of an effect on the spleen in both rats and dogs was not
considered to constitute evidence of significant toxicity. Of
particular concern was the slow onset of symptoms of cholinergic
stimulation, differences in susceptibility to acutely toxic doses in
rodents and birds, the long lasting cholinesterase depression and the
apparent lack of an effective antidote.
Because of the apparently unusual effect on cholinesterase activity
and the lack of long-term feeding studies only a temporary acceptable
daily intake for man was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 3 ppm in the diet equivalent to 0.15 mg/kg per day.
Dog - 2 ppm in the diet equivalent to 0.05 mg/kg per day.
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolites and possible products of decomposition
A list of theoretically possible metabolites and primary products of
hydrolysis is given [text missing]. These compounds are numbered and the
numbers are used throughout this part of the text; thus (2).
Use pattern
Fenthion is an insecticide with a broad spectrum of activity. It is
chiefly effective against Diptera, cereal bugs, rice stem borers. The
main uses are as follows:
50% field crops (rice, cereals, sugar beets, etc.)
30% fruit and vine (i.e. pome and stone fruit, citrus, olives)
20% other uses (i.e. ornamentals, public health, animal health)
It is registered in 31 countries for use on crops.
Application to plants
The following formulations are available for application to crops:
50 Emulsifiable Concentrate (550 g/l)
1000 ULV (1000 g/l)
40% Wettable Powder
25% Wettable Powder
3% Dust
Pre-harvest treatments
The recommended application rates/concentrations for the major crops
are as follows:
Sugar cane 0.5-0.75 kg/ha
Rice 0.04-0.075% and 0.6-1.2 kg/ha, resp.
Field corn 0.75-1.0 kg/ha
Beets 0.3-1.5 kg/ha
Pome and stone 0.05-0.075%
fruit
Citrus fruits 0.05-0.075% (or bait application, see olives)
Pistachio 0.05%
Cotton 0.25-2.0 kg/ha
Olives 0.05% bait application at 0.15 kg + 0.3 kg
bait/20 litres of water for aerial
application or 0.25-0.3 kg + 0.75-1.0 kg
bait/100 litres of water for ground machine
application,
Coffee 0.05-0.075%
Cocoa 0.05%
Vegetables 0.05-0.075%, peas up to 0.1%
Vines 0.75-1.0 kg/ha
Corn 0.75-1.0 kg/ha
The above-mentioned concentrations and rates of application refer to
the active ingredient.
Post-harvest treatments
No recommendations are made for post-harvest treatments.
Application to animals
For use as an animal health product, fenthion is applied in the
following formulations, concentrations and dosage rates and by the
given methods for the control of ectoparasites and endoparasites on
domestic animals and livestock.
(1) 25% emulsifiable concentrate
(2) 2 or 3% solution (pour-on)
(3) 0.25%-5% dust (for experimental use)
(4) 0.5-1% in oil (back-rubber)
(5) 10% water-miscible feed premix (for experimental use)
(6) Injectable solution
(7) 0.32% Molasse-block (for experimental use)
Fenthion EC is used at concentrations of 0.025-0.25% as a dip, spray
and wash treatment for the control of ticks, biting and sucking lice,
flies, keds, hornflies, mange and cattle grubs on horses, sheep,
cattle, dogs and poultry.
Fenthion 2 or 3% (pour-on) is used at dosages of 4-45 mg/kg for the
control of cattle grubs, ticks, biting and sucking lice, flies and
fleas on cattle.
Fenthion Dust is used at concentrations of 0.25-5% for the control of
ticks, hornflies, biting and sucking lice, flies, fleas and keds on
horses, cattle, sheep, dogs and poultry.
Fenthion in oil is used in the form of back-rubbers as a
self-treatment for the control of hornflies, biting and sucking lice,
flies and fleas on cattle.
Fenthion 10% water-miscible and 1% premix is applied at dosages of
1.2-30 mg/kg for the control of cattle grubs, hornflies, flies, fleas,
biting and suckling lice, nose bots and endoparasites on cattle and
sheep.
Fenthion (injectable) is used at dosages of 4.4-15 mg/kg for the
control of demodicosis, heart worms, Ancylostoma spp. and
Uncinaria spp.in dogs.
Fenthion is registered for the use on animals in the following
countries: Australia, Austria, Belgium, Germany, Great Britain,
Ireland, Italy, New Zealand, Spain, Switzerland, United States of
America, Yugoslavia.
Other uses
Fenthion is widely used for the control of insects affecting public
health, especially mosquitos and flies where a combination of high
potency, persistence and stability when applied to surfaces of
buildings, ditches, swamps, etc.
These applications are not likely to give rise to contamination of
food.
Residues resulting from supervised trials
On plants
Detailed residue data are available from many countries, including
England, Germany, Greece, Italy, New Zealand, South Africa, Spain,
Switzerland and the United States of America on many important crops
and have been deposited with FAO. The results showing the residue
levels remaining after application of fenthion according to registered
use patterns are reflected in the following typical examples:
TABLE
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Apples England 1´ lb/acre EC 50 3 3.12 1.74 0.72 0 (84)
S. Africa 0.05% WP 25 1 2.1 1.4
Switzerland 0.05% EC 50 1 1.5 1.35 0.6 0.65 0.15(35)
Grapes S. Africa 1 kg/ha D 5 1 1.15 0.15 0.05 <0.05
Peaches S. Africa 4 oz/100 gal EC 50 1 2.1 0.9
S. Africa 8 oz/100 gal EC 50 1 3.2 1.6
S. Africa 8 oz/100 gal EC 50 1 2.3 0.9 0.25
Oranges Spain 300 g/ha ULV 1 0.1 0.1 N.D.
Oranges whole Spain 0.025% WP 1 0.55 0.5 0.3
skin Spain 0.025% WP 1 2.4 2.4 1.4
pulp Spain 0.025% WP 1 0.2 0.05 <0.05
whole Spain 300 g/ha EC 50 1 0.13 0.13 0.03 0.04 0.03(54)
skin Spain 300 g/ha EC 50 1 0.42 0.42 0.05 0.05 0.04(54)
Cherries Switzerland 0.05% EC 50 1 1.65 1.05 0.45 0.3
Gemany 0.05% EC 50 1 4.75 0.95 0.55
Gemany 0.025% EC 50 1 2.4 0.8 0.35
Peas whole pod England 0.125% EC 50 2 4.52 0.85 0.38 0.02
peas only England 0.125% EC 50 2 <0.02 0.23 <0.02
pod England 0.025% EC 50 2 1.1 0.5 0.4 <0.1
peas England 0.025% EC 50 2 <0.02 0.1 0.05 0.05
Squash S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02 <0.02
S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02
Lettuce Gemany 0.05% EC 50 2 3.85 3.46 2.46 0.42 0.05
Gemany 0.05% EC 50 1 3.2 2.3 1.45 0.3
Gemany 0.15% EC 50 1 10.2 3.9 2.75 0.8
Cabbage Germany 0.05% EC 50 2 1.16 0.37 0.0
Germany 0.05% EC 50 2 2.18 1.37 0.24
TABLE (Continued)
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Sugar (roots) Germany 0.05% EC 50 2 8.09 1.23 0.82 0.15 0.0
beet (tops) Germany 0.05% EC 50 2 0.50 0.16
Wheat Spain 0.65 kg/ha EC 50 1 0.02 0.02 <0.01(63)
New Zealand 0.7 kg/ha EC 50 1 0.4 0.2(32)
Rice USA 1.6 oz/acre EC 50 3 <0.05
USA 2.4 oz/acre EC 50 3 <0.05(33)
USA 1.6 oz/acre EC 50 1 0.48 <0.01
Olives Turkey 0.06% EC 50 1 0.39(34)
Greece 0.04% EC 50 4 0.15(36)
Greece 0.04% EC 50 4 0.75(70)
Italy 0.05% EC 50 1 1.3 0.8(32)
On forage crops
Extensive trials have been reported (Chemagro Reports 24 890; 24 891;
24 915; Mulla et al.) to show that following registered uses of
fenthion on alfalfa (0.1-0.5 kg/ha) residues on treated alfalfa range
up to 6 ppm after 7 days, but by the fourteenth day these levels have
declined to less than 2 ppm. In one typical experiment alfalfa was
treated close to harvest with fenthion at 0.11 kg/ha and residues were
18; 1.3; 1.7 and 0.7 ppm at 0; 2; 4; and 8 days after treatment.
The levels in corn and grass forage are somewhat lower than in
alfalfa.
The occurrence of residues in milk and meat following the feeding of
such treated forage is discussed under the heading uptake of the
compound with feed.
On animals
Tissues and organs
Details of many trials made to determine the level and fate of
residues of fenthion in animal tissues, milk and eggs were available
and were deposited with FAO. A selection of typical results has been
reviewed to show the level of residues resulting from approved
applications of fenthion for the control of ectoparasites of cattle,
sheep and poultry and from the ingestion of fenthion residues on
animal feeds and fodder.
A single backline treatment with a 2% pour-on (6.25 mg of fenthion/kg)
produced the following residues (Chemagro, 1965b):
Days after application (ppm)
1 3 7 14 28
Brain <0.1 n.d. n.d. n.d. -
Heart <0.1 n.d. n.d. n.d. -
Liver 0.2 n.d. n.d. n.d. -
Kidney <0.1 n.d. n.d. n.d. -
Loin steak approx. 0.1 <0.1 0.1 n.d. n.d.
Round steak <0.1 n.d. <0.1 n.d. n.d.
Flank steak 0.2 0.1 <0.1 n.d. n.d.
Omental fat 0.8 0.5 0.2 <0.1 n.d.
Back fat 0.7 0.4 1.5 n.d. n.d.
Renal fat 0.9 0.5 1.2 approx. 0.1 n.d.
Averages for 3 animals
Backline treatment of cattle with a 3% pour-on (9.4 mg of fenthion/kg)
resulted in the formation of the following residues in the tissues
(Chemagro, 1968b):
Days after application (ppm)
1 3 7 28
Brain 0.08 <0.01 <0.01 <0.01
Heart 0.10 - 0.07 <0.02
Liver 0.14 0.01 0.01 <0.01
Kidney 0.15 0.06 0.10 0.02
Loin steak 0.18 0.05 0.07 0.01
Round steak 0.12 0.02 0.10 <0.01
Flank steak 0.19 0.08 0.21 0.01
Omental fat 0.19 0.14 0.31 0.02
Renal fat 0.18 0.54 0.38 0.03
Back fat 0.19 0.83 0.62 0.09
The tabulated values represent the averages for three animals, and
comprise the totality of the non-ionic residues.
Another experiment with a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg, at first showed that the residues were only
slight (Chemagro, 1967e) but after 28 days they were of the same order
of magnitude as in the first-mentioned experiment (Chemagro, 1968b):
Days after application (ppm)
7 14 28 42
Brain 0.01 - 0.01 -
Heart <0.01 - 0.02 -
Liver 0.01 <0.01 0.01 -
Kidney 0.01 <0.01 <0.01 -
Loin steak <0.01 - 0.01 -
Round steak <0.01 - <0.01 -
Flank steak <0.01 - 0.02 -
Omental fat 0.10 - 0.07 <0.01
Renal fat 0.12 - 0.15 <0.01
Back fat 0.13 - 0.07 <0.01
Averages for 3 animals
The same organs and tissues were examined also 45 days after topical
application to the skin using a 20% "Spotton solution" (5.8 mg of
fenthion/kg); they were all found to be free of residues (Chemagro,
1970).
Similar results were obtained in an experiment using a spray
application (1 gal of 0.25% a.i. per animal). No residues were found
(Chemagro, 1965c) in brain after three days; in liver after seven
days; in heart and kidney after 14 days; in steaks after 14 days; in
fat after 28 days.
Following three spray applications of a 0.1% fenthion solution to the
point of run-off, at intervals of two weeks, no detectable residues
(less than 0.05 ppm) were present in brain, heart, liver and steaks
after 28 days; omental fat contained on average 0.1 ppm, renal fat
contained 0.05-0.1 ppm, and back fat contained 0.05 ppm. All fat
samples were free of residues after 44 days (Chemagro, 1967d).
Following daily backrubber application (1% a.i.) for four weeks, no
residues were detected in the organs and tissues after the final
application (Chemagro, 1965d).
Elimination in milk
The first results on the possible elimination of fenthion and its
metabolites in milk were reported in the studies of Knowles & Arthur
(1966) (see "Tissues and Organs").
The concentration of acetonitrile-soluble materials, which represented
predominantly fenthion or its oxidation products, was at its maximum
six hours after dermal treatment (0.44 ppm) and 20 hours after
intramuscular treatment (0.80 ppm). After 14 days, the residues in the
milk amounted to less than 0.001 ppm (dermal) and 0.014 ppm (i.m.). Of
the total activity administered, 1.1% was eliminated (as 32P) in the
milk within 14 days after dermal treatment, and 2.2% was eliminated in
the milk within 20 days after i.m. treatment.
A daily backrubber application of 50 ml of a 1% fenthion solution for
seven days resulted in acetonitrile-soluble residues of 0.06-0.36 ppm
during treatment. Five days after treatment, these residues were below
0.01 ppm (Chemagro, 1963).
Backline application of a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg., and, in a comparison experiment, of a three
times higher concentration produced the following residues (in ppm) in
the milk (Chemagro, 1969):
Days after application 9.4 mg/kg 28 mg/kg
0.4 0.81 2.82
1 0.33 0.66
2 0.13 0.15
3 0.047 0.056
5 0.011 0.017
7 0.04(?) 0.003
10 0.003 <0.002
14 <0.002 <0.002
21 <0.002 <0.002
An experiment on six animals (single backline application with
3% pour-on, 9.4 mg/kg) provided a good picture of the range of
residues in the milk of the individual cows (Chemagro, 1968a).
Residues in the milk (ppm)
Days after application Range Average
1 0.055 - 0.106 0.072
2 0.129 - 0.167 0.150
3 0.012 - 0.023 0.019
7 <0.005 <0.005
Daily backrubber treatment of cattle with 1% a.i., equivalent to
approximately 0.5 g a.i. per animal and day, for a period of four
weeks caused no formation of residues in the milk at the end of the
treatment period (Chemagro, 1967a).
The results presented here by and large agree with the data of
Möllhoff (1970) who reports that following application of 10 mg of
fenthion/kg (2% pour-on) the milk was free of residues after seven
days and the edible tissues were free of residues after 13 days. All
these observations can be accounted for by the physical properties of
fenthion, on the one hand, and its metabolites, on the other hand. The
oxidation products have a higher polarity. As a result of these
properties, the penetration of fenthion through the skin is favourably
influenced by the lipophilic properties of the parent compound, on the
one hand, whereas, on the other hand, compounds (2) to (6), which are
formed following penetration by metabolic processes, undergo faster
degradation and elimination than unchanged fenthion would.
Shillam et al. (1971) applied 125 ml fenthion as a 2% solution in
liquid paraffin to each of three cows (equivalent to 2.5 ml fenthion
per animal) and determined the level of fenthion in the milk. The cows
were in low production (approximately 20 lbs milk per day when milked
twice daily).
It was found that milk drawn 15 hours post application contained 0.14
ppm fenthion (0.08, 0.14, 0.20 ppm) but milk drawn 48 hours post
treatment contained no measurable amount of fenthion (less than 0.005
PPM). Pasteurization of the 15 hour milk reduced the fenthion residue
level to 0.04 ppm. There was a further decline in the residue level
when the pasteurized milk was analysed after 48 hours storage at 4°C.
Shipp (1970) reported that the excretion of fenthion in milk reached a
peak 12 hours after treating cows with 2% fenthion solution at the
rate of 100 ml per cow (2 g per head). The concentration found in milk
from four separate cows ranged from 1.1 ppm to 1.7 ppm in the
butterfat of milk drawn 12 hours after application. The level had
declined to 0.7-1.1 ppm at 24 hours and 0.2-0.3 ppm at 36 hours. At 48
hours the residue level was less than 0.1 ppm in the fat of milk.
Uptake of the compound together with feed
A feed supplement treatment of two cows (two daily applications of
0.72 mg of fenthion/kg in capsules) for 14 days resulted in residues
of 0.02-0.36 ppm in the milk during treatment; two days
post-treatment these residues were less than 0.01 ppm (Chemagro,
1963).
Administration of fenthion-containing feed to cattle for six days (2.5
mg of fenthion/kg) produced no detectable residues (less than 0.1 ppm)
in the following organs: brain, heart, liver, kidney, steaks (loin,
round, flank), fat (omental, renal, back) (Chemagro, 1965a). Daily
administration of 5 ppm of fenthion in the feed for four weeks
produced no residues in the milk at the end of the experiment
(Chemagro, 1966d). Administration of 10 ppm of fenthion in the feed
for seven days (0.5 mg/kg/day) likewise resulted in no residues in the
milk on the final day of the experiment (Chemagro, 1966a).
Bowman et al. (1970) investigated:
(a) whether residues of fenthion and its metabolites are secreted in
the milk or excreted in the urine and faeces of cows fed silage
made from corn treated with fenthion, and
(b) whether residues of fenthion in silage affect the physiological
activity of lactating cows.
The corn was sprayed with (A) 0.56, (B) 1.12, and (C) 2.24 kg of
fenthion/hectare and ensiled in tower silos one day post-treatment.
The ensiled corn was allowed to age 88 days before the silages were
fed to the cows during a 56-day study. The residues of fenthion in
corn and corn silage after field treatment at the three rates were as
follows (in ppm):
A B C
day of treatment (forage) 4.3 17.6 34.6
day after treatment (forage) 1.7 3.5 12.0
ensiled 14 days (silage) 1.4 4.3 10.0
ensiled 56 days (silage) 1.0 2.1 8.9
ensiled 71 days (silage) 0.82 2.5 9.6
silage fed first week 0.44 1.3 5.9
silage fed third week 0.55 1.7 6.0
silage fed fifth week 0.55 1.6 5.0
silage fed eighth week 0.62 1.3 5.1
In all cases, compound (2) accounted for most of the residue.
One of the silages was assigned to each group of four cows. The
animals ingested averages of (A) 0.03, (B) 0.07, and (C) 0.3 mg of
residues/kg/day. No residues were detected in the milk from (A) and
(B). Only low levels (maximum of 0.014 ± 0.003 ppm) were found in the
milk from cows fed silage from corn treated at a rate of 2.24 kg of
fenthion/hectare (C). Also, one week after feeding was terminated, no
residues could be found in the milk in group (C).
Only slight amounts of residues occurred also in the faeces and urine
of the animals (expressed in ppm):
Days fed treated feed A B C
26 faeces 0.003 0.015 0.09
urine 0.004 0.016 0.12
55 faeces 0.014 0.03 0.16
urine 0.004 0.014 0.16
No residues were detected in urine and faeces one week after feeding
was terminated.
The effect of diet on the metabolism of fenthion in laboratory
animals (rabbits and rats) was studied by Begum (1968) 35S-labelled
fenthion (Y position) was used in these experiments. When this
compound was administered orally or subcutaneously to healthy,
well-fed animals (group A), the peak concentration of the
radioactivity appeared in the blood six to nine hours after treatment.
The peak was observed in lean, half-starved animals (group B) one hour
after treatment. There was more oxidized fenthion in the blood of
group B rabbits than in the blood of group A rabbits. In both groups
of animals, fenthion was eliminated primarily in the urine, and to a
lesser extent in the faeces. There was a higher percentage of
radioactive materials (mostly hydrolyzed products) excreted in the
urine and faeces by group B animals. Apparently there was a more rapid
breakdown and elimination from the system of lean animals than from
fat ones.
Experiments with sheep
Sheep which were treated with a 2% pour-on (60 mg of fenthion/kg)
showed no residues in brain, heart, liver, kidney, loin steak,
shoulder, leg and fat (omental, renal, back) 44 days after the
application (Chemagro, 1966c). Sheep treated with 50% powder applied
as an oral drench at a dose level of 30 mg/kg also displayed no
residues in the above-mentioned organs and tissues 14 days after the
application (Chemagro, 1966b).
When sheep were treated by dipping them in a 500 ppm solution of
fenthion, only very slight residues appeared (Möllhoff, 1970). No
residues were detected in the organs; in meat, residues were
detectable only at seven days after treatment in one out of two
animals (0.1 ppm). The concentration was highest in fat (up to 0.35
ppm on the seventh day). When the same formulation was injected
intravenously in two sheep at a rate of 5 mg of fenthion/kg 12 hours
before slaughter, the highest residues again appeared in the fat
(1.6 ppm) whilst in meat the residues amounted to a maximum of 0.7 ppm
(Möllhoff, 1970).
Experiments on poultry
Fenthion is not eliminated in hen eggs. Hens were fed on a diet
containing 2 ppm of fenthion for four weeks; at the end of the feeding
period, there was no trace of residues in the eggs (Chemagro, 1967b).
The tissues of six hens (giblets, muscle and fat) were also free of
residues at the end of the feeding experiment (only exception: giblets
contained 0.08 ppm in one out of six hens; fat contained 0.07 ppm in
one out of six hens (Chemagro, 1967c)).
FATE OF RESIDUES
The behaviour of the compound fenthion in living systems, like that of
all pesticides which contain a thioether moiety, is determined by its
readiness to form sulfoxides and sulfones. As an S-alkyl isomerization
can also be reckoned with under thermal influences, the following
non-ionic metabolites of fenthion (1) are theoretically to be
expected:
TOXICOLOGICAL STUDIES
Special studies
(a) Acute toxicity of the metabolites
Compound Acute LD50 (mg/kg) 150 (M)c
Orala i.p.b
I. Fenthion 220 325 >5 × 10-4
II. Fenthion sulfoxide 125 250 4.5 × 10-5
III. Fenthion sulfone 125 250 4.7 × 10-4
IV. Oxygen analogue 125 26 2.65 × 10-6
V. O-Sulfoxide 50 22 4.8 × 10-5
IV. O-Sulfone 30 9 3.2 × 10-5
VIII. 4-(methylthio)m-cresol 6500d
IX. 4-methyl(thiosulfoxide)
m-cresol 3500d
X. 4-methyl(thiosulfone)
m-cresol 7000d
a Male rats - according to Francis and Barnes, 1963.
b Female rats - according to Dubois and Kinoshita, 1964.
c 150 = Molar concentration resulting in 50% inhibition of
human RBC Cholinesterase (Francis and Barnes, 1963).
d Female rats according to Nelson, 1967.
(b) Reproduction
Except for a slight growth depression at the highest level in the
diet, fenthion at levels of 0, 3, 15 and 75 ppm for three generations
(two litters per generation), produced no adverse effect on rat
reproduction (Loser, 1969). Microscopic examination of the tissues of
the F3b generation did not reveal any significant abnormality (Spicer,
1971).
(c) Potentiation
Fenthion potentiates the acute intraperitoneal toxicity of malathion,
dioxathion, and coumaphos in rats. Intraperitoneal administration of
13 other organophosphate or carbamate insecticides did not result in a
greater than additive effect when administered with fenthion (Dubois
and Kinoshita, 1964). Dietary combination of fenthion with coumaphos
neither of which alone affected cholinesterase when fed to dogs for
six weeks, was found to potentiate the anticholinesterase activity of
serum and red blood cells. Less evident potentiation was observed when
fenthion and malathion were fed and when fenthion was fed in
combination with dioxathion no potentiation was noted (Doull et al.,
1962), Fenthion (2 ppm) and malathion (100 ppm) resulted in moderate
erythrocyte and serum cholinesterase activity inhibition (30-40%).
When fenthion (2 ppm) and dioxathion (3 ppm) was fed cholinesterase
activity was not depressed. When fenthion (2 ppm) and coumaphos
(2 ppm) was fed to dogs, significant inhibition (75%) of serum
cholinesterase and moderate inhibition (30%) of erythrocyte
cholinesterase was evident. Oral administration to rats of a 75:25
mixture of fenthion and dichlorvos did not result in a greater than
theoretically additive toxicity (Kimmerle, 1967b).
(d) Neurotoxicity
No evidence for delayed neurological disruption in hens was evident
when fenthion was administered orally at a single dose of 25 mg/kg
(Kimmerle, 1965a). When chickens were fed up to 100 ppm in the diet
for 30 days, clinical examination of the animals and histological
examination of nerve tissue indicated no demyelinating effect from
fenthion (Kimmerle, 1965b; Dieckmann, 1971).
(e) Antidotes
A number of antidotes which are commonly used for organophosphorous
poisoning have been shown to be relatively inactive when used
following fenthion intoxication. Studies with atropine, 2-PAM
Toxogonin P-2-S, and TMB-4 (Dubois and Kinoshita, 1964; Francis and
Barnes, 1963; and Dubois, 1960; Lorke and Kimmerle, 1969) administered
alone and in combination showed that these materials did not
successfully alleviate the parasympathomimetic signs of
organophosphate poisoning. When BH-G (Merck; Darmstadt, Germany) was
administered three to four times in combination with atropine, the
LD50 value increased from 250 to 440 mg/kg (Kimmerle, 1963).
Toxogonin was not effective as a cholinesterase reactivator following
oral intoxication of dogs by fenthion (Hahn and Henschler, 1969).
Acute toxicity
Animal Route LD50 Reference
(mg/kg
body-weight)
Mouse (M) Oral 150 Francis and Barnes, 1963
227 Dubois, 1968
(F) 190 Francis and Barnes, 1963
225 Dubois, 1968
(F) i.p. 150 Dubois and Kinoshita, 1964
(M) 125 Dubois and Kinoshita, 1964
(Continued)
Animal Route LD50 Reference
(mg/kg
body-weight)
Rat (F) Oral 245-310 (Dubois and Kinoshita, 1964
(Gaines, 1960
615 Francis and Barnes, 1963
(M) 175-470 (Dubois and Kinoshita, 1964
(Klimmer, 1963
(Gaines, 1969
(Francis and Barnes, 1963
(M) i.p. 325 Dubois and Kinoshita, 1964
(F) 260 Dubois and Kinoshita, 1964
(M) 152 Klimmer, 1963
(M) Dermal 330-650 Gaines, 1969
Klimmer, 1963
Francis and Barnes, 1963
(F) 330-500 Dubois and Kinoshita, 1964
Gaines, 1969
Francis and Barnes, 1963
Guinea-pig
(M) Oral 1000 Francis and Barnes, 1963
260 Dubois and Kinoshita, 1964
i.p. 310 Dubois and Kinoshita, 1964
Rabbit (M) Oral 150-175 Francis and Barnes, 1963
Dermal 150 Klimmer, 1963
Duck Oral 15 Dubois and Doull, 1960
1-2 Keith and Mulla, 1966
Chicken 30 Dubois and Doull, 1960
28 Sherman and Ross, 1961
30-40 Francis and Barnes, 1963
Calf Approx. 40 McGrath, 1961
Various solvents used to solubilize fenthion in combination with water
or ethanol had no significant effect on the acute oral LD50
(Kimmerle, 1967a).
Fenthion is an organophosphorus insecticide of intermediate toxicity
to mammals although it displays considerable differences in its
toxicity to various species, e.g. fowls are very sensitive (Keith and
Mulla, 1966).
The rate of absorption through the skin of rabbits is slow. When
fenthion was applied to a cotton plug and placed in a rabbit ear for
four hours, swelling occurred. When it was left for 24 hours mortality
resulted (Kimmerle, 1960).
Female rats tolerated a daily one hour inhalation challenge of 0.163
mg/l air for 30 days with cholinesterase depression but no mortality.
At 0.415 mg/l air all animals were dead within 10 days (Dilley and
Doull, 1961a).
Exposure by inhalation daily for nine days, six hours per day at 210
mg/M3 (initial concentration in a static inhalation chamber),
resulted in signs of poisoning, but no mortality in cats, guinea pigs,
rabbits and rats. Cholinesterase, which was severely depressed,
recovered in three weeks (Klimmer, 1963). Fenthion exhibits a
relatively low degree of acute mammalian toxicity. In only one
instance was a sex difference in susceptibility noted and this is in
contrast to the generally noted resistance of males to most other
phosphorothioates (Dubois and Kinoshita, 1964). The signs of poisoning
are typical of the central and peripheral cholinergic effects of
organophosphorus esters with a gradual onset of the symptoms. The
symptoms in humans and other animals include tremors, lacrymation,
salivation, vomiting, diarrhoea and other signs of cholinergic
stimulation.
Short-term studies
Duck
Mallard ducks fed 25 ppm of fenthion in the diet for six weeks became
emaciated and could not fly or walk; after two days on normal food,
recovery was evident. A dietary level of 5 ppm produced no adverse
clinical reaction (Keith and Mulla, 1966).
Rat
Dermal administration of fenthion at 14.5 and 25 mg/kg for 60 days to
rats resulted in 40% mortality in the higher dosed group and no
mortality in the lower dosed group. However, blood cholinesterase
levels were depressed to about 20% of normal at the lower treatment
level (Dubois, 1961). Cholinesterase was depressed and mortality was
absent when fenthion was applied to rats dermally for 12 days at 2.9
mg/kg (Dubois and Puchala, 1960).
Rats tolerated daily intraperitoneal administration of 10 mg/kg
fenthion for 60 days with no mortality. At 20 mg/kg, 80% of the
treated animals died within 20 days (Dubois and Kinoshita, 1964).
Mortality (12 dead of 30 rats tested) occurred following daily oral
administration of approximately 25 mg/kg (1/10 LD50) for 75 days
(six days per week). Signs of poisoning were transient disappearing
shortly after dosing (Klimmer, 1963).
In a preliminary study, male rats were orally administered fenthion
five days a week for 13 weeks at a dose of 30 mg/kg/day. Mortality
occurred in approximately 30% of the rats over the course of the
experiment and cholinesterase activity was depressed between 80-90% of
normal. At the conclusion of the experiment cholinesterase recovery
was very slow - up to 40 days (Kimmerle, 1961).
Rats (22 male and 22 female per group) were fed dietary levels of
fenthion at 0.25, 0.50, 2.5, and 5.0 mg/kg/day for three months.
Cholinesterase activity depression was evident at 0.5 mg/kg in red
blood cells, serum, liver and heart at all testing intervals. At the
lowest feeding level (0.25 mg/kg) the inhibition (approx. 10-20%) did
not progressively increase with time indicating lack of cumulative
effects. Mortality in females was evident at 5.0 mg/kg. The animals
died manifesting muscarinic and nicotinic effects. Body-weight gain
was reduced in males at 0.25 mg/kg and above while in females it was
evident only at 2.5 mg/kg. Behavioural effects were noted
(piloerection) at 0.5 mg/kg and above (especially in females). This
effect decreased with time and disappeared by week seven. Organ
weights were all distinctly lower than the controls but as the
body-weight was also reduced the organ to body-weight ratio did not
appear to be affected. Histological examination showed the testis to
have reduced spermatogenesis and atrophic prostate glands at the
highest feeding levels (2.5 and 5.0 mg/kg). The ovary was not affected
(Shimamoto and Hattori, 1969).
Rats (six groups of 12 male and 12 female) were fed for 16 weeks on
diets containing 0, 2, 3, 5, 25 and 100 ppm. Cholinesterase depression
was evident at 25 ppm and absent at 5 ppm. No adverse effects were
noted in food consumption, weight gain or gross and microscopic
examination of tissues (Doull et al., 1961).
Rats (six groups of 25 male and 25 female) were fed for one year on
diets containing 0, 2, 3, 5, 25 and 100 ppm of fenthion. There was no
evidence of significant changes in growth rate, food consumption,
general appearance and gross of microscopic examination of tissues.
Survival of male rats at 25 ppm was slightly depressed. Cholinesterase
examinations indicated depression at the 5 ppm level and above with
3 ppm showing no adverse enzyme effects. A mild extramedullary
haematopoiesis was observed in controls and all dosage levels and
haemosideresis was evident in the spleen of the rats at 100 ppm levels
(Doull et al., 1963a).
Dog
Dogs (four groups of two males and two females per group) were fed
fenthion at 0, 2, 5 and 50 ppm for 12 weeks. Growth was not affected
at any dietary level. Erythrocyte cholinesterase activity was
depressed at 50 ppm while serum cholinesterase was depressed at 5 ppm
and above. Little if any depression of the serum cholinesterase was
evident before five weeks, after which it progressively decreased to
about 40% inhibition (Doull et al., 1961).
Dogs (four groups of two males and two females) were fed fenthion at
0, 2, 5, and 50 ppm in the diet for one year. There was no effect of
fenthion on food consumption or growth over the test interval.
Erythrocyte and serum cholinesterase were significantly depressed at
50 ppm with the serum also depressed at 5 ppm. An increase in the
weight of the spleen, which was not dose dependent, was evident in all
of the treated animals. Microscopic examinations of the tissues showed
splenic congestion and some decrease in the cellularity of the red
pulp was evident at all dose levels fed. Extramedullary haematopoiesis
and haemosideresis was also observed in the spleen. Microscopic
examination of other tissues did not reveal any significant change
(Doull et al., 1963b).
Long-term studies
No data available.
Observations in man
Fenthion has been widely used in many parts of the world for control
of household pests, mosquitos, etc. Cholinesterase studies conducted
on individuals in areas treated by WHO for malaria eradication have
shown that very slight plasma cholinesterase depression occurs when
exaggerated spray schedules were followed. The plasma cholinesterase
levels were depressed for up to six weeks after spraying (Elliot and
Barnes, 1963). It was also evident that the children in the population
(less than seven years old) were more susceptible to the
anticholinesterase effects (Taylor, 1963). A man who ingested two
ounces of fenthion (Entex)(R) recovered from severe
organophosphorous poisoning after being in critical condition for the
first six days after poisoning. Recovery was slow, lasting up to 30
days. Cholinesterase measurements showed that at 22 days after
poisoning the cholinesterase activity was still depressed (Pickering,
1966). In humans, the signs of poisoning appear rapidly beginning with
blurred vision, unsteady gait and slurred speech. After 72 hours of
emergency treatment following an unknown quantity of fenthion, a man
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation. The patient began to recover
only after 11 days of treatment which included atropine, PAM and
toxogonin (Dean et al., 1967). In another case, 45 minutes after
ingestion of 30 ml of fenthion, a man was in a comatose state with
pale skin, cyanotic mucous membranes, slow regular heart beat, no
peripheral blood pressure and no reactions to pain or light
stimulation on the pupils. Recovery took six days (von Clarmann and
Geldmacher-von-Mallinkradt, 1966).
Comments
Fenthion is slowly absorbed, metabolized by a complex series of
reactions and excreted. It does not appear to accumulate in the body.
In most instances the unhydrolysed metabolites containing a phosphorus
atom are more toxic than the parent compound. The signs of
intoxication from a single oral dose develop slowly, persist for a
considerable period of time and are not readily alleviated by atropine
or by most common oxime reactivators. Short-term studies in the rat
and dog suggest that cholinesterase inhibition is the most sensitive
criterion of biological effect. Anti-spermatogenesis in one rat study
was not confirmed in other studies or in a reproduction study. The
occurrence of an effect on the spleen in both rats and dogs was not
considered to constitute evidence of significant toxicity. Of
particular concern was the slow onset of symptoms of cholinergic
stimulation, differences in susceptibility to acutely toxic doses in
rodents and birds, the long lasting cholinesterase depression and the
apparent lack of an effective antidote.
Because of the apparently unusual effect on cholinesterase activity
and the lack of long-term feeding studies only a temporary acceptable
daily intake for man was established for this compound.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat - 3 ppm in the diet equivalent to 0.15 mg/kg per day.
Dog - 2 ppm in the diet equivalent to 0.05 mg/kg per day.
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight.
RESIDUES IN FOOD AND THEIR EVALUATION
Metabolites and possible products of decomposition
A list of theoretically possible metabolites and primary products of
hydrolysis is given [text missing]. These compounds are numbered and the
numbers are used throughout this part of the text; thus (2).
Use pattern
Fenthion is an insecticide with a broad spectrum of activity. It is
chiefly effective against Diptera, cereal bugs, rice stem borers. The
main uses are as follows:
50% field crops (rice, cereals, sugar beets, etc.)
30% fruit and vine (i.e. pome and stone fruit, citrus, olives)
20% other uses (i.e. ornamentals, public health, animal health)
It is registered in 31 countries for use on crops.
Application to plants
The following formulations are available for application to crops:
50 Emulsifiable Concentrate (550 g/l)
1000 ULV (1000 g/l)
40% Wettable Powder
25% Wettable Powder
3% Dust
Pre-harvest treatments
The recommended application rates/concentrations for the major crops
are as follows:
Sugar cane 0.5-0.75 kg/ha
Rice 0.04-0.075% and 0.6-1.2 kg/ha, resp.
Field corn 0.75-1.0 kg/ha
Beets 0.3-1.5 kg/ha
Pome and stone 0.05-0.075%
fruit
Citrus fruits 0.05-0.075% (or bait application, see olives)
Pistachio 0.05%
Cotton 0.25-2.0 kg/ha
Olives 0.05% bait application at 0.15 kg + 0.3 kg
bait/20 litres of water for aerial
application or 0.25-0.3 kg + 0.75-1.0 kg
bait/100 litres of water for ground machine
application,
Coffee 0.05-0.075%
Cocoa 0.05%
Vegetables 0.05-0.075%, peas up to 0.1%
Vines 0.75-1.0 kg/ha
Corn 0.75-1.0 kg/ha
The above-mentioned concentrations and rates of application refer to
the active ingredient.
Post-harvest treatments
No recommendations are made for post-harvest treatments.
Application to animals
For use as an animal health product, fenthion is applied in the
following formulations, concentrations and dosage rates and by the
given methods for the control of ectoparasites and endoparasites on
domestic animals and livestock.
(1) 25% emulsifiable concentrate
(2) 2 or 3% solution (pour-on)
(3) 0.25%-5% dust (for experimental use)
(4) 0.5-1% in oil (back-rubber)
(5) 10% water-miscible feed premix (for experimental use)
(6) Injectable solution
(7) 0.32% Molasse-block (for experimental use)
Fenthion EC is used at concentrations of 0.025-0.25% as a dip, spray
and wash treatment for the control of ticks, biting and sucking lice,
flies, keds, hornflies, mange and cattle grubs on horses, sheep,
cattle, dogs and poultry.
Fenthion 2 or 3% (pour-on) is used at dosages of 4-45 mg/kg for the
control of cattle grubs, ticks, biting and sucking lice, flies and
fleas on cattle.
Fenthion Dust is used at concentrations of 0.25-5% for the control of
ticks, hornflies, biting and sucking lice, flies, fleas and keds on
horses, cattle, sheep, dogs and poultry.
Fenthion in oil is used in the form of back-rubbers as a
self-treatment for the control of hornflies, biting and sucking lice,
flies and fleas on cattle.
Fenthion 10% water-miscible and 1% premix is applied at dosages of
1.2-30 mg/kg for the control of cattle grubs, hornflies, flies, fleas,
biting and suckling lice, nose bots and endoparasites on cattle and
sheep.
Fenthion (injectable) is used at dosages of 4.4-15 mg/kg for the
control of demodicosis, heart worms, Ancylostoma spp. and
Uncinaria spp.in dogs.
Fenthion is registered for the use on animals in the following
countries: Australia, Austria, Belgium, Germany, Great Britain,
Ireland, Italy, New Zealand, Spain, Switzerland, United States of
America, Yugoslavia.
Other uses
Fenthion is widely used for the control of insects affecting public
health, especially mosquitos and flies where a combination of high
potency, persistence and stability when applied to surfaces of
buildings, ditches, swamps, etc.
These applications are not likely to give rise to contamination of
food.
Residues resulting from supervised trials
On plants
Detailed residue data are available from many countries, including
England, Germany, Greece, Italy, New Zealand, South Africa, Spain,
Switzerland and the United States of America on many important crops
and have been deposited with FAO. The results showing the residue
levels remaining after application of fenthion according to registered
use patterns are reflected in the following typical examples:
TABLE
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Apples England 1´ lb/acre EC 50 3 3.12 1.74 0.72 0 (84)
S. Africa 0.05% WP 25 1 2.1 1.4
Switzerland 0.05% EC 50 1 1.5 1.35 0.6 0.65 0.15(35)
Grapes S. Africa 1 kg/ha D 5 1 1.15 0.15 0.05 <0.05
Peaches S. Africa 4 oz/100 gal EC 50 1 2.1 0.9
S. Africa 8 oz/100 gal EC 50 1 3.2 1.6
S. Africa 8 oz/100 gal EC 50 1 2.3 0.9 0.25
Oranges Spain 300 g/ha ULV 1 0.1 0.1 N.D.
Oranges whole Spain 0.025% WP 1 0.55 0.5 0.3
skin Spain 0.025% WP 1 2.4 2.4 1.4
pulp Spain 0.025% WP 1 0.2 0.05 <0.05
whole Spain 300 g/ha EC 50 1 0.13 0.13 0.03 0.04 0.03(54)
skin Spain 300 g/ha EC 50 1 0.42 0.42 0.05 0.05 0.04(54)
Cherries Switzerland 0.05% EC 50 1 1.65 1.05 0.45 0.3
Gemany 0.05% EC 50 1 4.75 0.95 0.55
Gemany 0.025% EC 50 1 2.4 0.8 0.35
Peas whole pod England 0.125% EC 50 2 4.52 0.85 0.38 0.02
peas only England 0.125% EC 50 2 <0.02 0.23 <0.02
pod England 0.025% EC 50 2 1.1 0.5 0.4 <0.1
peas England 0.025% EC 50 2 <0.02 0.1 0.05 0.05
Squash S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02 <0.02
S. Africa 0.065% EC 50 1 0.25 <0.02 <0.02
Lettuce Gemany 0.05% EC 50 2 3.85 3.46 2.46 0.42 0.05
Gemany 0.05% EC 50 1 3.2 2.3 1.45 0.3
Gemany 0.15% EC 50 1 10.2 3.9 2.75 0.8
Cabbage Germany 0.05% EC 50 2 1.16 0.37 0.0
Germany 0.05% EC 50 2 2.18 1.37 0.24
TABLE (Continued)
Crop Country Concentration Formulation No. of Residue at harvest x days after application
Applications
0-1 2-6 7-12 13-20 21-30 >30(days)
Sugar (roots) Germany 0.05% EC 50 2 8.09 1.23 0.82 0.15 0.0
beet (tops) Germany 0.05% EC 50 2 0.50 0.16
Wheat Spain 0.65 kg/ha EC 50 1 0.02 0.02 <0.01(63)
New Zealand 0.7 kg/ha EC 50 1 0.4 0.2(32)
Rice USA 1.6 oz/acre EC 50 3 <0.05
USA 2.4 oz/acre EC 50 3 <0.05(33)
USA 1.6 oz/acre EC 50 1 0.48 <0.01
Olives Turkey 0.06% EC 50 1 0.39(34)
Greece 0.04% EC 50 4 0.15(36)
Greece 0.04% EC 50 4 0.75(70)
Italy 0.05% EC 50 1 1.3 0.8(32)
On forage crops
Extensive trials have been reported (Chemagro Reports 24 890; 24 891;
24 915; Mulla et al.) to show that following registered uses of
fenthion on alfalfa (0.1-0.5 kg/ha) residues on treated alfalfa range
up to 6 ppm after 7 days, but by the fourteenth day these levels have
declined to less than 2 ppm. In one typical experiment alfalfa was
treated close to harvest with fenthion at 0.11 kg/ha and residues were
18; 1.3; 1.7 and 0.7 ppm at 0; 2; 4; and 8 days after treatment.
The levels in corn and grass forage are somewhat lower than in
alfalfa.
The occurrence of residues in milk and meat following the feeding of
such treated forage is discussed under the heading uptake of the
compound with feed.
On animals
Tissues and organs
Details of many trials made to determine the level and fate of
residues of fenthion in animal tissues, milk and eggs were available
and were deposited with FAO. A selection of typical results has been
reviewed to show the level of residues resulting from approved
applications of fenthion for the control of ectoparasites of cattle,
sheep and poultry and from the ingestion of fenthion residues on
animal feeds and fodder.
A single backline treatment with a 2% pour-on (6.25 mg of fenthion/kg)
produced the following residues (Chemagro, 1965b):
Days after application (ppm)
1 3 7 14 28
Brain <0.1 n.d. n.d. n.d. -
Heart <0.1 n.d. n.d. n.d. -
Liver 0.2 n.d. n.d. n.d. -
Kidney <0.1 n.d. n.d. n.d. -
Loin steak approx. 0.1 <0.1 0.1 n.d. n.d.
Round steak <0.1 n.d. <0.1 n.d. n.d.
Flank steak 0.2 0.1 <0.1 n.d. n.d.
Omental fat 0.8 0.5 0.2 <0.1 n.d.
Back fat 0.7 0.4 1.5 n.d. n.d.
Renal fat 0.9 0.5 1.2 approx. 0.1 n.d.
Averages for 3 animals
Backline treatment of cattle with a 3% pour-on (9.4 mg of fenthion/kg)
resulted in the formation of the following residues in the tissues
(Chemagro, 1968b):
Days after application (ppm)
1 3 7 28
Brain 0.08 <0.01 <0.01 <0.01
Heart 0.10 - 0.07 <0.02
Liver 0.14 0.01 0.01 <0.01
Kidney 0.15 0.06 0.10 0.02
Loin steak 0.18 0.05 0.07 0.01
Round steak 0.12 0.02 0.10 <0.01
Flank steak 0.19 0.08 0.21 0.01
Omental fat 0.19 0.14 0.31 0.02
Renal fat 0.18 0.54 0.38 0.03
Back fat 0.19 0.83 0.62 0.09
The tabulated values represent the averages for three animals, and
comprise the totality of the non-ionic residues.
Another experiment with a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg, at first showed that the residues were only
slight (Chemagro, 1967e) but after 28 days they were of the same order
of magnitude as in the first-mentioned experiment (Chemagro, 1968b):
Days after application (ppm)
7 14 28 42
Brain 0.01 - 0.01 -
Heart <0.01 - 0.02 -
Liver 0.01 <0.01 0.01 -
Kidney 0.01 <0.01 <0.01 -
Loin steak <0.01 - 0.01 -
Round steak <0.01 - <0.01 -
Flank steak <0.01 - 0.02 -
Omental fat 0.10 - 0.07 <0.01
Renal fat 0.12 - 0.15 <0.01
Back fat 0.13 - 0.07 <0.01
Averages for 3 animals
The same organs and tissues were examined also 45 days after topical
application to the skin using a 20% "Spotton solution" (5.8 mg of
fenthion/kg); they were all found to be free of residues (Chemagro,
1970).
Similar results were obtained in an experiment using a spray
application (1 gal of 0.25% a.i. per animal). No residues were found
(Chemagro, 1965c) in brain after three days; in liver after seven
days; in heart and kidney after 14 days; in steaks after 14 days; in
fat after 28 days.
Following three spray applications of a 0.1% fenthion solution to the
point of run-off, at intervals of two weeks, no detectable residues
(less than 0.05 ppm) were present in brain, heart, liver and steaks
after 28 days; omental fat contained on average 0.1 ppm, renal fat
contained 0.05-0.1 ppm, and back fat contained 0.05 ppm. All fat
samples were free of residues after 44 days (Chemagro, 1967d).
Following daily backrubber application (1% a.i.) for four weeks, no
residues were detected in the organs and tissues after the final
application (Chemagro, 1965d).
Elimination in milk
The first results on the possible elimination of fenthion and its
metabolites in milk were reported in the studies of Knowles & Arthur
(1966) (see "Tissues and Organs").
The concentration of acetonitrile-soluble materials, which represented
predominantly fenthion or its oxidation products, was at its maximum
six hours after dermal treatment (0.44 ppm) and 20 hours after
intramuscular treatment (0.80 ppm). After 14 days, the residues in the
milk amounted to less than 0.001 ppm (dermal) and 0.014 ppm (i.m.). Of
the total activity administered, 1.1% was eliminated (as 32P) in the
milk within 14 days after dermal treatment, and 2.2% was eliminated in
the milk within 20 days after i.m. treatment.
A daily backrubber application of 50 ml of a 1% fenthion solution for
seven days resulted in acetonitrile-soluble residues of 0.06-0.36 ppm
during treatment. Five days after treatment, these residues were below
0.01 ppm (Chemagro, 1963).
Backline application of a 3% pour-on, equivalent to a dose rate of
9.4 mg of fenthion/kg., and, in a comparison experiment, of a three
times higher concentration produced the following residues (in ppm) in
the milk (Chemagro, 1969):
Days after application 9.4 mg/kg 28 mg/kg
0.4 0.81 2.82
1 0.33 0.66
2 0.13 0.15
3 0.047 0.056
5 0.011 0.017
7 0.04(?) 0.003
10 0.003 <0.002
14 <0.002 <0.002
21 <0.002 <0.002
An experiment on six animals (single backline application with
3% pour-on, 9.4 mg/kg) provided a good picture of the range of
residues in the milk of the individual cows (Chemagro, 1968a).
Residues in the milk (ppm)
Days after application Range Average
1 0.055 - 0.106 0.072
2 0.129 - 0.167 0.150
3 0.012 - 0.023 0.019
7 <0.005 <0.005
Daily backrubber treatment of cattle with 1% a.i., equivalent to
approximately 0.5 g a.i. per animal and day, for a period of four
weeks caused no formation of residues in the milk at the end of the
treatment period (Chemagro, 1967a).
The results presented here by and large agree with the data of
Möllhoff (1970) who reports that following application of 10 mg of
fenthion/kg (2% pour-on) the milk was free of residues after seven
days and the edible tissues were free of residues after 13 days. All
these observations can be accounted for by the physical properties of
fenthion, on the one hand, and its metabolites, on the other hand. The
oxidation products have a higher polarity. As a result of these
properties, the penetration of fenthion through the skin is favourably
influenced by the lipophilic properties of the parent compound, on the
one hand, whereas, on the other hand, compounds (2) to (6), which are
formed following penetration by metabolic processes, undergo faster
degradation and elimination than unchanged fenthion would.
Shillam et al. (1971) applied 125 ml fenthion as a 2% solution in
liquid paraffin to each of three cows (equivalent to 2.5 ml fenthion
per animal) and determined the level of fenthion in the milk. The cows
were in low production (approximately 20 lbs milk per day when milked
twice daily).
It was found that milk drawn 15 hours post application contained 0.14
ppm fenthion (0.08, 0.14, 0.20 ppm) but milk drawn 48 hours post
treatment contained no measurable amount of fenthion (less than 0.005
PPM). Pasteurization of the 15 hour milk reduced the fenthion residue
level to 0.04 ppm. There was a further decline in the residue level
when the pasteurized milk was analysed after 48 hours storage at 4°C.
Shipp (1970) reported that the excretion of fenthion in milk reached a
peak 12 hours after treating cows with 2% fenthion solution at the
rate of 100 ml per cow (2 g per head). The concentration found in milk
from four separate cows ranged from 1.1 ppm to 1.7 ppm in the
butterfat of milk drawn 12 hours after application. The level had
declined to 0.7-1.1 ppm at 24 hours and 0.2-0.3 ppm at 36 hours. At 48
hours the residue level was less than 0.1 ppm in the fat of milk.
Uptake of the compound together with feed
A feed supplement treatment of two cows (two daily applications of
0.72 mg of fenthion/kg in capsules) for 14 days resulted in residues
of 0.02-0.36 ppm in the milk during treatment; two days
post-treatment these residues were less than 0.01 ppm (Chemagro,
1963).
Administration of fenthion-containing feed to cattle for six days (2.5
mg of fenthion/kg) produced no detectable residues (less than 0.1 ppm)
in the following organs: brain, heart, liver, kidney, steaks (loin,
round, flank), fat (omental, renal, back) (Chemagro, 1965a). Daily
administration of 5 ppm of fenthion in the feed for four weeks
produced no residues in the milk at the end of the experiment
(Chemagro, 1966d). Administration of 10 ppm of fenthion in the feed
for seven days (0.5 mg/kg/day) likewise resulted in no residues in the
milk on the final day of the experiment (Chemagro, 1966a).
Bowman et al. (1970) investigated:
(a) whether residues of fenthion and its metabolites are secreted in
the milk or excreted in the urine and faeces of cows fed silage
made from corn treated with fenthion, and
(b) whether residues of fenthion in silage affect the physiological
activity of lactating cows.
The corn was sprayed with (A) 0.56, (B) 1.12, and (C) 2.24 kg of
fenthion/hectare and ensiled in tower silos one day post-treatment.
The ensiled corn was allowed to age 88 days before the silages were
fed to the cows during a 56-day study. The residues of fenthion in
corn and corn silage after field treatment at the three rates were as
follows (in ppm):
A B C
day of treatment (forage) 4.3 17.6 34.6
day after treatment (forage) 1.7 3.5 12.0
ensiled 14 days (silage) 1.4 4.3 10.0
ensiled 56 days (silage) 1.0 2.1 8.9
ensiled 71 days (silage) 0.82 2.5 9.6
silage fed first week 0.44 1.3 5.9
silage fed third week 0.55 1.7 6.0
silage fed fifth week 0.55 1.6 5.0
silage fed eighth week 0.62 1.3 5.1
In all cases, compound (2) accounted for most of the residue.
One of the silages was assigned to each group of four cows. The
animals ingested averages of (A) 0.03, (B) 0.07, and (C) 0.3 mg of
residues/kg/day. No residues were detected in the milk from (A) and
(B). Only low levels (maximum of 0.014 ± 0.003 ppm) were found in the
milk from cows fed silage from corn treated at a rate of 2.24 kg of
fenthion/hectare (C). Also, one week after feeding was terminated, no
residues could be found in the milk in group (C).
Only slight amounts of residues occurred also in the faeces and urine
of the animals (expressed in ppm):
Days fed treated feed A B C
26 faeces 0.003 0.015 0.09
urine 0.004 0.016 0.12
55 faeces 0.014 0.03 0.16
urine 0.004 0.014 0.16
No residues were detected in urine and faeces one week after feeding
was terminated.
The effect of diet on the metabolism of fenthion in laboratory
animals (rabbits and rats) was studied by Begum (1968) 35S-labelled
fenthion (Y position) was used in these experiments. When this
compound was administered orally or subcutaneously to healthy,
well-fed animals (group A), the peak concentration of the
radioactivity appeared in the blood six to nine hours after treatment.
The peak was observed in lean, half-starved animals (group B) one hour
after treatment. There was more oxidized fenthion in the blood of
group B rabbits than in the blood of group A rabbits. In both groups
of animals, fenthion was eliminated primarily in the urine, and to a
lesser extent in the faeces. There was a higher percentage of
radioactive materials (mostly hydrolyzed products) excreted in the
urine and faeces by group B animals. Apparently there was a more rapid
breakdown and elimination from the system of lean animals than from
fat ones.
Experiments with sheep
Sheep which were treated with a 2% pour-on (60 mg of fenthion/kg)
showed no residues in brain, heart, liver, kidney, loin steak,
shoulder, leg and fat (omental, renal, back) 44 days after the
application (Chemagro, 1966c). Sheep treated with 50% powder applied
as an oral drench at a dose level of 30 mg/kg also displayed no
residues in the above-mentioned organs and tissues 14 days after the
application (Chemagro, 1966b).
When sheep were treated by dipping them in a 500 ppm solution of
fenthion, only very slight residues appeared (Möllhoff, 1970). No
residues were detected in the organs; in meat, residues were
detectable only at seven days after treatment in one out of two
animals (0.1 ppm). The concentration was highest in fat (up to 0.35
ppm on the seventh day). When the same formulation was injected
intravenously in two sheep at a rate of 5 mg of fenthion/kg 12 hours
before slaughter, the highest residues again appeared in the fat
(1.6 ppm) whilst in meat the residues amounted to a maximum of 0.7 ppm
(Möllhoff, 1970).
Experiments on poultry
Fenthion is not eliminated in hen eggs. Hens were fed on a diet
containing 2 ppm of fenthion for four weeks; at the end of the feeding
period, there was no trace of residues in the eggs (Chemagro, 1967b).
The tissues of six hens (giblets, muscle and fat) were also free of
residues at the end of the feeding experiment (only exception: giblets
contained 0.08 ppm in one out of six hens; fat contained 0.07 ppm in
one out of six hens (Chemagro, 1967c)).
FATE OF RESIDUES
The behaviour of the compound fenthion in living systems, like that of
all pesticides which contain a thioether moiety, is determined by its
readiness to form sulfoxides and sulfones. As an S-alkyl isomerization
can also be reckoned with under thermal influences, the following
non-ionic metabolites of fenthion (1) are theoretically to be
expected:
 X Y Z
S S O = (1) = fenthion
S SO O = (2)
S SO2 O = (3)
O S O = (4)
O SO O = (5)
O SO2 O = (6)
O S S = (7)
O SO S = (8)
O SO2 S = (9)
(See also the metabolic chart Figure 1, under "Biochemical Aspects").
Isomerization to the P-S-aryl forms has not been observed so far.
Of all these compounds, O-desmethyl forms are conceivable as
metabolites. Whenever they are discussed, we shall mark them with a
letter a), for example the compound will be marked 1a), and so on.
X Y Z
S S O = (1) = fenthion
S SO O = (2)
S SO2 O = (3)
O S O = (4)
O SO O = (5)
O SO2 O = (6)
O S S = (7)
O SO S = (8)
O SO2 S = (9)
(See also the metabolic chart Figure 1, under "Biochemical Aspects").
Isomerization to the P-S-aryl forms has not been observed so far.
Of all these compounds, O-desmethyl forms are conceivable as
metabolites. Whenever they are discussed, we shall mark them with a
letter a), for example the compound will be marked 1a), and so on.
 The primary hydrolytic products that are theoretically to be expected
are as follows:
(10) (CH3O)2 P (S) OH (16) HO-C7H6-S-CH3
(11) (CH3O) P (S) (OH)2 (17) HO-C7H7-SO-CH3
(12) P (S) (OH)3 (18) HO-C7H6-SO2-CH3
(13) (CH3O)2 P (O) OH
(14) (CH3O) P (O) (OH)2
(15) P (O) (OH)3
In animals
Knowles and Arthur (1966) applied fenthion dermally to two dairy cows
each weighing 360 kg at a rate of approximately 13 mg/kg per cow, and
treated two other lactating cows (each weighing 410 kg) by the
intramuscular route with approximately 8.5 mg of fenthion/kg per cow.
In the urine the peak concentration of radioactive materials occurred
on the first day following either method of treatment. The total ppm
of P32 materials from intramuscular treatment decreased from 33 ppm
at one day to 1.6 ppm by 21 days. More than 95% of the radioactive
materials eliminated in the urine consisted of hydrolytic products.
The total P32-materials eliminated in the faeces peaked two days
after both types of treatment. The cumulative percentage of the
administered dose was about 4%. The peak concentration of
acetonitrile-soluble radioactivity occurred one day after
intramuscular treatment and three days after dermal treatment. The
hair of the two cows treated dermally contained about 2000 ppm
radioactive fenthion equivalents immediately after treatment. These
residues declined to 16 ppm by 14 days after treatment. Chloroform to
water partition data indicated that fenthion underwent little change
on the hair to water-soluble components. The radioactivity in the
blood after both types of treatment reached a peak during the first 24
hours. Between 10 and 38% of the P32-materials partitioned into
chloroform but no detectable chloroform-soluble P32-materials were
present in the blood at seven days after either treatment.
At 14 days after dermal treatment and 21 days after intramuscular
treatment the cows were killed, and portions of liver, left and right
sirloin steak, left and right round steak, T-bone steak, omental fat,
and left and right subcutaneous fat were removed for analysis. None of
these tissues contained acetonitrilesoluble radioactive materials
following dermal treatment; all tissues contained less than 0.05 ppm
of total radioactivity with the exception of the liver which contained
0.44 ppm. The situation was vastly different in the tissues of the
intramuscularly treated animals. The tissues from the left side (site
of injection) of the cows contained considerably more P32-materials
than those from the right side (e.g. left round steak contained 1.03
ppm total radioactive materials, right round steak contained 0.11
ppm). The acetonitrile-soluble compounds amounted to a maximum of 0.36
ppm in the left steaks (less than 0.1 ppm in the right steaks); 0.2
ppm in the left subcutaneous fat and 0.08 ppm in the right
subcutaneous fat; 0.05 ppm in T-bone steak; 0.15 ppm in omental fat;
and 0.76 ppm in liver. The injection sites contained high residues in
both cases: skin 0.49 ppm total activity (0.08 ppm
acetonitrile-soluble), injection site 164 ppm (76 pp.
acetonitrile-soluble). The amount of the applied P32-materials
remaining in the skin after dermal treatment was, however, only 0.14%.
A similar calculation for the percentage remaining on the hair gave a
figure of 0.73%.
Following dermal and intramuscular treatment of cows with fenthion
(Knowles and Arthur, 1966), fenthion constituted more than 50% of the
non-ionic residues for three days after dermal and seven days after
i.m. treatment in the milk. The remainder was composed chiefly of
(3) + (5) + (6) and (2) [+ (4) ?]. In the urine, fenthion accounted
for only a small percentage of the chloroform-soluble radioactivity.
Some of (4) and/or (2) was present, but in most cases more than 70% of
the activity consisted of (3) and/or (5) and/or (6). The hydrolytic
products were composed of almost equal proportions of (10) and (13),
and a small portion consisted of an unidentified compound (probably a
O-desmethyl form of fenthion or one of its metabolites). More than 50%
of the acetonitrile-soluble radioactive materials in the faeces
consisted of the parent compound. The remainder was accounted for by
the other metabolites just as in urine. The composition of the
hydrolytic products was also very similar to that found in urine. In
the tissues of the animals slaughtered 14 days after dermal treatment
and 21 days after i.m. treatment, more than 50% of the radioactive
acetonitrile-soluble materials was chromatographed as fenthion, but
oxidation products were also present. At the injection site, (12) was
the predominant product of the water-soluble materials.
The silage feeding experiments performed by Bowman et al. (1970)
referred to earlier, also provide certain indications of the
transformation of fenthion metabolites in the cow. For example, the
residue (total of six ppm) in the feed fed in the fourth week and in
the resultant milk samples had the following composition:
Compound Feed Milk
(1) 8% -
(2) 89% <2%
(3) 2% <2%
(4) - -
(5) 1% 97%
(6) - -
Compound (5) accounted for 95% of the metabolites present in the
urine. Those present in the faeces consisted almost exclusively of
compound (1) (fenthion). Unfortunately the authors do not provide any
data on the occurrence of metabolites in the blood of the animals;
however, the cholinesterase activity of the animals of group C was
significantly depressed during the feeding experiment but otherwise
there were no changes in performance.
In plants
The transformation of fenthion in plants is basically similar to that
in animals. The first studies performed to investigate transformation
of fenthion in plants were made by Brady and Arthur (1961). Cotton
plants were sprayed with an emulsion of P32-labelled fenthion at a
rate of 2 kg of fenthion per hectare. The plants were growing under
field conditions. Three days after spraying, 189 ppm fenthion
equivalents were present on or in the leaves, but only 14 ppm were
chloroform-soluble materials. The respective residues after 14 days
were 71/5 ppm. Compound (3) was tentatively identified as by far the
most prevalent metabolite (60-80%), followed in quantity by unchanged
fenthion (28% after three days, 13% after 14 days). Compounds
(5) + (6) appeared in smaller percentages (less than 10%). The
hydrolytic products found were chiefly (10) and small amounts of (13)
and an unknown compound.
Experiments with P32-labelled fenthion on beans, in which the
metabolites were separated by paper-chromatography (Niessen et al.,
1962a) and quantitatively determined (Niesson et al., 1962b), produced
the following results:
The labelled parent compound contained 94.2% of (1), 4.5% of (7), 0.7%
of (2) and 0.6% of ionic compounds. Bean plants (Phaseolus vulgaris)
were either briefly dipped in a 0.2% emulsion of this compound by
their stem, or placed in the emulsion by their roots. The
chloroform-soluble extracts had the following composition (ppm):
Days (1) (2) (3) (5) (6) (7) (8)
1/4 100 16 - 1.2 - 2.8 1.5
2 27 25 2.1 3.5 0.06 - 0.4
5 4.7 7.2 1.4 1.5 0.12 - 0.09
8 1.6 4.7 1.4 1.2 0.11 - 0.05
After eight days, only 1.6% of the parent compound was present; (2)
was the principal metabolite. The impurity (7) was no longer
detectable from the second day; however, it is also oxidized to the
sulfoxide (8). The total residue decreased in the eight-day period
from 122 ppm to a level of 9 ppm. The rate at which these
transformations take place depends upon the temperature.
Oxidation by plant enzymes preferably takes place at the thiono sulfur
atom, while oxidation at the methylmercapto group seems chiefly to be
a photosensitized reaction. The compound has a certain systemic
action. (7) has a much stronger systemic action, but it is not found
in the plant.
Several experiments on glass plates confirmed that the formation of
sulfoxide and sulfone is a light-induced reaction whilst (7) is formed
from (1) and (8) is formed from (2) due to the influence of heat.
These findings were later confirmed by Metcalf et al. (1963).
At the same time as the studies of Niessen et al. (1962b) were
conducted, Japanese research workers investigated the behaviour of
P32-labelled fenthion in rice, tea and cabbage (Fukuda et al. 1962,
Tomizawa et al., 1962). The neutral metabolites were separated by
paper chromatography and the water-soluble metabolites were separated
by ion exchange chromatography. Immediately after the treatment, the
deposit of fenthion on the rice plants amounted to between 110 and 150
ppm on the leaf blade and to between 18 and 28 ppm in the leaf sheath.
(2) and (3) were the principal metabolites in the leaf blade and
sheath. The accumulation of fenthion metabolites in ears and grains
was examined in one variety which was treated a few days before
heading. Four weeks after application, the distribution of
radioactivity was as follows (ppm):
Total Chloroform-extractable
Husk 2.4 n.d.
Bran 60 0.9
Polished rice 5.7 0.1
The composition of the metabolite mixture in the ears 12 days after
the application was as follows: 45% (2); 20% (7?); 18% (3); 17%
unidentified. The water-soluble metabolites in the grains 14 days
after the application were composed chiefly of (1a), plus a few
percent. (less than 10) of (10), (13) and (12) and/or (15).
The amounts of fenthion deposit on tea and cabbage after the
application were as follows: 43 ppm on young tea leaves, 90 ppm on old
tea leaves and 46 ppm on cabbage leaves. (2) and (3) were the
principal metabolites also in these experiments. The appearance of
O-desmethyl fenthion observed by the Japanese research workers seems
astonishing and was never again reported by any other observers.
In 1963, Metcalf et al. published qualitative results which they
obtained on cotton. In these studies, too, (2) and (3) were the
principal metabolites.
With the improvement of analytical techniques, a better insight was
gained into the metabolism of fenthion in plants. Leuck and Bowman
(1968) treated Coastal bermudagrass and ensilage corn at rates of 0.56
kg/hectare, 1.12 kg/hectare and 2.24 kg/hectare (a.i.). The ppm
residues determined in Coastal bermudagrass treated at the highest
application rate were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 64 71 1.0 1.8 n.d.
7 0.31 10 4.0 2.0 0.34
14 0.07 2.1 2.3 0.48 0.18
21 0.03 0.59 1.3 0.08 0.11
The corresponding residue levels for corn were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 0.41 26 0.22 0.63 <0.005
7 0.04 2.4 0.68 1.03 0.16
14 0.01 0.42 0.24 0.11 0.09
21 <0.002 0.34 0.22 <0.02 0.04
(4) was not detected (except on day 0 in bermudagrass when its level
was 0.06 ppm). (2) and (3) were again the principal metabolites.
The total residues were as follows (expressed in ppm):
Days Bermudagrass Corn
post-treatment 0.56 kg/ha 1.12 kg/ha 0.56 kg/ha 1.12 kg/ha
7 3.3 8.5 0.65 1.95
14 1.3 3.2 0.15 0.4
21 0.5 1.3 0.1 0.2
These findings were very largely confirmed by a study performed by
Bowman et al. (1968), in which nine procedures for removal of
phosphorus insecticides and their metabolites were compared. This
study also provides an impressive picture of the differing effect of
various extraction procedures. Soxhlet extraction with 10% methanol in
chloroform proved to be the best one.
The fate of phenolic hydrolytic products of fenthion in plants was not
further studied. However, findings have been published by Wendel and
Bull (1970) in respect of GC-6506 (dimethyl 4-methylthiophenyl
phosphate). From some of the metabolites, substituted phenols could be
liberated by hydrolysis with ß-glucosidase (Bull and Stokes, 1970).
The predominant products were the glucosides of phenol-sulfoxide and
phenol-sulfone. The glycone portions of the conjugates are further
altered, possibly by the formation of a ß-gentiobiside.
In soils
The half-life of fenthion and its metabolites in soil is between 14
and 40 days; only in one out of six experiments was a half-life of
more than 30 days recorded. The experiments used various formulations
including granules and emulsifiable solutions. (Chemagro - Summary
under soils in main submission).
In storage and processing
Under frozen storage (-18 to -23°C), fenthion residues were shown to
be stable in alfalfa for 28 weeks and in cattle fat for 11 weeks
(Chemagro, 1966e,f). In liver from cattle, a decrease of 20% seemed to
occur between the twelfth and twenty-sixth week of storage (Chemagro,
1966f).
Fenthion proved to be stable for four weeks at -18°C in cattle brain,
heart, liver, kidney, steak and fat over a period of four weeks; (6)
decreased slightly in brain, heart, steak and fat, by 30% in kidney,
and by 80% in liver in six weeks (Olson, 1966).
Following treatment of corn with fenthion at rates of 0.56, 1.12 and
2.24 kg per hectare (Boman and Beroza, 1969), the plants treated at
the highest rate contained 39 ppm of residues (1) + (2) + (3) + (5),
in a ratio of 1.8 : 96.5 : 0.75 : 0.97. At 70 days after ensiling
(which took place on the day after application), the residues amounted
to 14.6 ppm (17 : 74 : 7.8 : 1.1); at 141 days, they amounted to 6.85
ppm (9.7 : 85 : 4.7 : 0.5). The predominant metabolite throughout the
whole period was (2). In another silage experiment with corn, the
residues were consistently shown to be at a lower oxidation stage than
were those in the field samples from which the silage was prepared
(Leuck and Boman, 1968).
The relatively high persistence of the residues in silage and the
occurrence of (2) as the principal metabolite were again confirmed
later (Bowman et al., 1970).
Residues in canned and preserved peaches persist to a certain extent,
especially when maintained at low temperatures (refrigerator) (Pigatti
et al., 1967).
Evidence of residues in commerce or at consumption
Krause and Kirchhoff (1969) examined 78 market samples of different
fruits and vegetables of domestic and foreign origin for residues of
20 organophosphorus insecticides including fenthion. Residues of
fenthion were not found in any of the samples.
Total diet studies were carried out in England and Wales, in 1966/67,
with altogether 66 samples of total diet. Each sample comprised seven
sub-samples consisting of cereals, meats, fish, fats, fruits and
preserves, root vegetables, green vegetables and milk. Fenthion and
its immediate oxidation products could have been detected by the
method employed, but they were not detected (Abbott et al., 1970).
Methods of residue analysis
Although the present-day gas chromatographic methods can be carried
out largely without clean-up, it is nevertheless necessary in some
cases to eliminate interfering substances. In this, consideration must
be given to the greatly differing polarity of fenthion residues.
Chemagro (1963) has published, for example, the following data on
partition:
n-hexane water n-hexane
Compound acetonitrile chloroform water
(1) 1 : 8.4 1 : 1720 1 : 0.016
(5) 1 : 300 1 : 88 1 :
(6) 1 : 87 1 : 15 1 : 78
Bowman and Beroza (1968) give the following p-values (=fractional
amount in nonpolar phase):
n-hexane n-hexane n-hexane
Compound 20% acetonitrile 40% acetonitrile water
in water in water
(1) 0.98 0.92 1.00
(2) 0.18 0.03 0.50
(3) 0.61 0.12 0.94
(4) 0.65 0.18 0.92
(5) 0 0 0
(6) 0 0 0.01
Regarding the extraction of residues, attention is drawn to the study
of Bowman et al. (1968) already referred to earlier.
The first methods for the determination of fenthion and its
metabolites were based on determination of total phosphorus (Frehse et
al., 1962a for plant material; Frehse et al., 1962b for olives (oil);
Adam (1967) for olives). In 1966, the first methods were published for
the determination of fenthion in plant and animal tissues (Anderson et
al., 1966) and milk (Katague, 1966), based on oxidation of the various
compounds to the sulfone (6) which in turn was hydrolyzed to the
corresponding phenol. The phenol was brominated and acetylated prior
to detection measurement by electron-capture GLC. The methods were
sensitive to 0.1 ppm (0.01 ppm for milk) but unavoidably very
complicated and time-consuming.
Therefore, Chemagro began in 1967 to develop methods using the
thermionic phosphorus detector: for soil (Olson 1967a), rice grain
(Olson 1967b), eggs and milk (Olson 1968), and animal tissues
(Thornton 1967).
These methods are based on oxidation of the residues to (6). The
sensitivities are below 0.1 ppm for soil and rice, and about 0.005 ppm
for eggs and milk. GLG conditions are:
(a) soil: 11 in × 3 mm i.d. column, packed with 6% DC-200 and 1% QF-1
on 80/100 mesh Gas Chrom Q, 230°C;
(b) other material: 16 in × 3 mm i.d. column, packed with 10% DC-200
and 0.2% QF-1 on 80/100 mesh Gas Chrom Q, 210°C, retention time
3.5 minutes.
Forty-five organophosphorus insecticides were examined for possible
interference with the rice method (Olson, 1967b); no interferences
were noted for any of the compounds except (R) Dasanit (Olson,
1967c).
Bowman and Beroza (1968) used a different principle of determination.
Extracts from corn, grass and milk were separated by liquid
chromatography into three fractions which then were analysed by GLC
with a flame photometric detector: 90 cm × 4 mm i.d, column, packed
with 10% DC-200 on 80/100 mesh Gas Chrom Q, 210°C for the two
fractions (1) + (3) and (2) + (4) + (6); a 45 cm column is used for
(5) (retention times (1) to (6) : 1.75, 4.2, 4.05, 1.45, 2.3 minutes).
Ten per cent. QF-1 (50 cm), 1% Carbowax 20 M (50 cm) and 5% DEGS
(50 cm) can also be used as the liquid phase. A complete separation of
all six compounds was achieved by using liquid chromatography with a
silica gel column and different elution systems. Later, Bowman and
Beroza (1969) also used the oxidation method with m-chloroperbenzoic
acid for the same substrates: 90 cm × 4 mm i.d. column packed with 10%
OV-101 on 80/100 mesh Gas Chrom Q, 215°C. With this column, the
metabolites can also be determined singly (retention times of
0.7 - 2.75 minutes, 2.3 minutes for (6)); it was found that the
results of the single metabolite determinations those of the total
determination. The recoveries were above 90%. Excessive amounts of the
oxidation mixture are removed on an alumina column.
Further studies performed by Bowman and Beroza (1970) show that 2.4
metre × 4 mm i.d. columns, packed with 5% OV-101 or OV-210 on Gas
Chrom Q are also suitable for determination of the compounds (1) to
(6).
Examples of national tolerances
Country Crop Tolerance Safety
in ppm Interval
in days
Algeria General 15
Australia Fruit, vegetables 2 7
Meat of cattle 1 1
Milk and milk products 2 1
Austria General 35
Belgium Fruit, vegetables excluding
potatoes 0.3 21
Brazil General 14
Fruit 1.0
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
Bulgaria Sugar beets 14
Cherries, cereals 14
Canada Beef cattle N.R.
Finland General 14
France General 15
Olives 21
Germany Fruit 14
Vegetables, field & fodder
crops 10
Israel Fruit 21
Miscellaneous (e.g. dry onions
and cucurbits) 30
Italy General 20
Olives 30
Morocco General 15
Olives 30
Netherlands Fruit, vegetables excluding
potatoes 0.3 -
New Zealand General 21
Norway General 14
Poland Fruit, vegetables, root crops 30
Early cherries 14
Portugal General 14
Olives (as oil and preserves) 42
Russia Cereals, cottonseed oil 0.35
Spain Fruit and olives 30
South Africa General 2.0
Apricots, peaches, apples,
pears, grapes 10
Subtropical crops & cucurbits 7
Deciduous fruit 10
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
United States Alfalfa (fresh), grass 5.0
of America Alfalfa (hay), grass hay 18.0
Animal products 0.1
Milk 0.01
Yugoslavia General 0.5
N.R. = registered for use on a no-residue basis.
Appraisal
Fenthion is an organophosphorus insecticide with a broad spectrum of
activity used against insects infesting field crops, fruit crops,
vineyards, olives, cotton, insects of public health concern and
ectoparasites of domestic animals. It is especially useful for control
of fruit flies in many crops where its ability to penetrate plant
tissues allows for destruction of larvae within the fruit. The
concentrations/rate of use ranges from 0.5-2 kg/ha in the case of
field crops and cotton and 0.05-0.1% solution on horticultural crops.
Animals are treated with 0.25%-5% dust, 0.025%-0.25% solutions as dips
or sprays and 2%-3% solutions in oil as pour-on preparations or back
rubbers.
The residue data available to the meeting were obtained from many
different countries, reflecting typical registered uses under a wide
range of climatic and ecological conditions. Considerable information
is available about the fate of fenthion residues in foods of plant and
animal origin and extensive studies have been carried out on the
nature of the degradation products formed under a variety of
influences.
Little information is available about residues of fenthion in foods in
commerce.
The literature includes a number of methods of residue analysis based
on gas-chromatographic procedures. The greatly differing polarity of
the various fenthion metabolites has been investigated and
recommendations for the most appropriate extraction procedures have
been made.
In order that all biologically active metabolites may be determined
simultaneously the analytical procedure has been modified to provide
for oxidation of the residues of the various compounds to the sulfone
which is hydrolyzed to the corresponding phenol. The phenol is
brominated and acetylated prior to measurement by electron-captive
gas/liquid chromatography. The limit of detection is 0.1 ppm for most
foods and 0.01 ppm for milk.
Procedures which are less complicated and less time consuming have
been developed using the thermionic phosphorus detector following
oxidation to the sulfone. The limit of detection is below 0.1 ppm for
soil and grain, and about 0.005 ppm for eggs, milk and animal tissues.
These procedures appear suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The temporary tolerances are for fenthion and its major metabolites,
determined separately or together and expressed as fenthion.
Apples, peaches, cherries, lettuce, 2 ppm
fat of meat
Cabbage, cauliflower, olives, 1 ppm
olive oil
Grapes, oranges, peas, meat 0.5 ppm
Squash 0.2 ppm
Wheat, rice, milk products (fat basis) 0.1 ppm
Milk (whole) 0.05 ppm
Further work or information
Required before 30 June 1975
1. Adequate two-year feeding studies in the dog and in one rodent
species.
2. Establishment of the sequence of metabolic changes in man and
laboratory animals in order to elucidate the mechanism of long
lasting cholinesterase inhibition.
Desirable
Information on the frequency and level of fenthion residues in food
commodities in commerce.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adam, N. Chr. (1967) Méthode de détermination des résidue de Lebaycid
dans l'huile d'olive et lea olives. Ann. Inst. Phytopath. Benaki, N.S.
8: 78-84
Anderson, R. J., Thornton, J. S., Anderson, C. A. and Katague, D. B,
(1966) Determination of fenthion residues in plant and animal tissues
by electron-capture gas chromatography. J. Agr. Food Chem. 14:
619-622
Begum, A. (1968) Effect of diet on metabolism of fenthion in animals.
Ph.D. thesis, Auburn University, Auburn, Alabama. Dissert, Abstr.,
Sect. B, 28: 4165
Bowman, M. C. and Beroza, M. (1968) Determination of fenthion and five
of its metabolites in corn, grass and milk. J. Agr. Food Chem. 16:
399-402
Bowman, M. C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton and phorate in corn, milk, grass and
faeces, J.A.O.A.C. 52: 1231-1237
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J.A.O.A.C. 53: 499-508
Bowman, M. C., Beroza, M. and Leuck, D. B. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites from
field treated crops. J. Agr. Food Chem. 16: 796-802
Bowman, M. C., Leuck, D. B., Johnson, J. C., jr and Knox, F. E. (1970)
Residues in corn silage and effect of feeding dairy cows the treated
silage. J. econ. Entomol. 63: 1523-1528
Brady, U. E., jr and Arthur, B. W. (1961) Metabolism of O,o-dimethyl
O-[4-(methylthio)-m-toly] phosphorothioate by white rats. J. econ.
Entomol, 54: 1232-1236
Bull, D. L. and Stokes, R. A. (1970) Metabolism of dimethyl
p-(methylthio) phenyl phosphate in animals and plants. J. Agr. Food
Chem. 18: 1134-1138
Chemagro Milk and tissue residues resulting from backline or oral
treatment of Cattle with Bayer 29493, p. 32
1963 Report No. 10. 946, December 3
1965a Report No. 16. 551, July 27 (revised January 10, 1968)
1965b Report No. 16. 990, October 21
1965c Report No. 17. 015, October 28
1965d Report No. 17. 062, November 3
1966a Report No. 17. 445, January 19 (revised April 28, 1966)
1966b Report No. 17. 969, April 21
1966c Report No. 18. 009, May 3
1966d Report No. 18. 067, May 12
1966e Report No. 18. 179, May 20
1966f Report No. 18. 191, May 24
1967a Report No. 20. 310, April 18
1967b Report No. 20. 738, June 20
1967c Report No. 20. 798, July 5
1967d Report No. 21. 113, September 13
1967e Report No. 21. 504, November 13
1968a Report No. 21. 796, January 25
1968b Report No. 22. 647, May 15
1969 Report No. 24. 266, February 14 (revised April 2, 1969)
1970 Report No. 28. 719, December 3
Dean, G., Coxon, J. and Brereton, D. (1967) Poisoning by an
organo-phosphorus compound. A case report. So. African Med. J.,
1017-19
Dieckmann, W. (1971) Neurotoxizctatsunter-suchungen an
Huhnern-Histopathologie. Unpublished report of Farbenfabriken Bayer
A.G.
Dilley, J. and Doull, J. (1961a) Chronic inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dilley, J. and Doull, J. (1961b) Acute inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. (1960) The absence of antidote activity by 2-PAM and
TMB-4 against acute poisoning by Bayer 29493. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. (1961) Effects of repeated dermal application of Bayer
29493 on rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1962) Acute oral toxicity of a sample of Bayer 29493 to
female rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Dubois, K. P. and Doull, J. (1960) The acute toxicity of Bayer 29493
to chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. and Puchala, E. (1960) Influence of Bayer 29493 on the
cholinesterase activity of the blood of rats. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. and Kinoshita, F. (1964) Acute toxicity and
anti-cholinesterase action of O,O-dimethyl O-4-(methylthio)-m-tolyl
phosphorothioate (DMTP; Baytex) and related compounds. Tox. Appl.
Pharmacol. 6: 86-95
Doull, J., Root, M. and Cowan, J. (1961) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Doull, J., Root, M. and Cowan, J. (1962) Effect of adding Bayer 29493
in combination with other cholinergic insecticides to the diet of male
and female dogs. Unpublished report from Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Cowan, N. J., Vesselinovitch, D., Fitch, F. W.
(1963a) and Meskauskas, J. Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Root, M., Cowan, J. and Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. and
Meskauskas, J. (1961) The effects of feeding diets containing Bayer
29493 to rats for a period of 16 weeks. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Elliott, R. and Barnes, J. M. (1963) Organophosphorus insecticides for
the control of mosquitos in Nigeria. Bull. Wld Hlth Org. 28: 35-54
Francis, J. I, and Barnes, J. M. (1963) Studies on the mammalian
toxicity of fenthion. Bull. Wld. Hlth. Org. 29: 205-12
Frehse, H. (1970) Ruckstande van pflanzenschutz mittein in nahrung und
um welt in chemie der pflanzenschutz und schadlingshenamp-fungsmittel.
Band 2-Wegler, R., Ed. Springer-Verlag, 1970
Frehse, H., Niessen, H. and Tietz, H. (1962a) Method of determining
residues of the insecticide Lebaycid(R) in plant material.
Pflanzenschutz-Nachr Bayer 15: 148-159
Frehse, H., Niessen, H. and Tietz, H. (1962b) Method of determining
residues of the insecticide Lebaycid(R) in olives and olive oil.
Pflanzenschutz-Nachr Bayer 15: 160-165
Fukuda, H., Masuda, T., Miyahara, Y. and Tomizawa Ch. (1962) Fate of
O,O-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on rice plant. Japan. J. appl. Entomol. Zool. 6: 230-236
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14: 515-34
Hahn, H. L. and Hensehler, D. (1969) The ability of phosphorylated
cholinesterases to be reactivated by obidoxime chloride (Toxogonin)
in vivo. Arch. Toxikol. 24: 147-63
Katague, D. B. (1966) Determination of fenthion residues in milk by
electron-capture gas chromatography. Chemagro Corp., Research
Department, Report No. 17, 887
Keith, J. O, and Mulla, M. S. (1966) Relative toxicity of five
organo-phosphorus mosquito larvicides to mallard ducks. J. Wildlife
Management 30: 553-63
Kimmerle, G. (1960) Re: Active substance S1752. Unpublished report
submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1961) Subchronische oral versuche bei ratten mit
S-1752-Wirkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1963) Product BH6 and S1752 Poisoning. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965a) Nourotoxic studies with Bayer 29493. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965b) Neurotoxische untersuch-ungen mit
S-1752-Werkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1966) Langdavernde Inhalatronsversuche bei hunden mit
dem Baytex-Werkstoff (S-1752)
Kimmerle, G. (1967a) Abhangigkeit der akuten oralen toxizitat bei
ratten vom losungsmittel. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. (1967b) Potenzierung von DDVP und S-1752. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Klimmer, O. R. (1963) Toxicological testing of Bayer 29493.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Knowles, C. O. and Arthur, B. W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of radiolabelled
fenthion to dairy cows. J. econ. Entomol. 59: 1346-52
Krause, Ch. and Kirchhoff, J. (1969) Organophosphatrückstande auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen
Nachrichtenbl. Deut. Pflanzenschutzdienstes (Braunschweig), 21:
81-84
Leuck, D. B. and Bowman, M. C. (1968) Residues of fenthion and five of
its metabolites their persistence on corn and grass forage, J. econ.
Entomol. 61: 1594-1597
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmakol. 263: 237-8
Loser, E. (1969) Generation versuche an Ration. Unpublished report
submitted by Farbenfabriken Bayer A.G.
McGrath, H. B. (1969) Toxicity of Bayer 29493 in calves. Unpublished
report submitted by Farbenfabriken Bayer A.G
Metcalf, R. L., Fukuto, T. R. and Winton, M. Y. (1963) Chemical and
biological behaviour of fenthion residues. Bull. Wld Hlth Org. 29:
219-226
Möllhoff, E. (1970) Determination of trichlorfon and fenthion residues
in animals of different species, Pestic. Sci. 2: 179-181
Nelson, D. L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Niessen, H., Tietz, H. and Frehse, H. (1962a) Papier
chromatographische Trennung aromatischer
Phosphorsäure-ester-Insektizide. J. Chromatog. 9: 111-113
Niessen, H., Tietz, H. and Frehse, H. (1962b) On the occurrence of
biologically active metabolites of the active ingredient S-1752 after
application of Lebaycid(R) Pflanzenschutz-Nachr. Bayer 15: 125-147
Olson, T. J. (1966) The determination of the stability of fenthion and
its oxygen analog sulfon in frozen cattle tissues. Chemagro Corp.,
Research Department, Report No. 19, 289,
Olson, T. J. (1967a) Determination of residues of fenthion in soil by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 20, 324.
Olson, T. J. (1967b) Determination of residues of fenthion in rice
grain by thermionic emission gas chromatography. Chemagro Corp.,
Research Department, Report No. 20, 417 (revised 21 October, 1968)
Olson, T. J. (1967c) A study of the possible interference of other
pesticides with the analytical method for fenthion in rice. Chemagro
Corp., Research Department, Report No. 20, 595
Olson, T. J. (1968) Determination of fenthion in eggs and milk by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 22, 933
Pickering, E. N. (1966) Organic phosphate insecticide poisoning. Can.
J. Med. Tech., p. 174
Pigatti, A., Pigatti, P., Orlando, A., Suplicy, F. O., Sampaio, A. S.
and Rigitano, O. (1967) Persistência de residuos de fenthion am
pêssego, ameixa e maca. Arq. Inst. Biol. (S. Paulo) 34: 275-284
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol Appl. Pharmacol. 3, 512-33
Shillam, K. W. G., Medd, R. K., Roberts, N. L. and Burrows, I. E.
(1971) Residual levels of fenthion in raw and pasteurized milk. Report
of Huntington Research Centre 4225/71/383
Shimamoto, K. and Hattori, K. (1969) Chronic feeding of Baytex
(O, O-dimethyl-o-(4-methylmercapto-3-methyl) phenyl-thiophosphate) in
rats. Acta Med. Univ. Kioto, 40: 163-71
Shipp, E. (1970) Fenthion residues in milk. Report of the Entomology
School, University of New South Wales
Spicer, E. J. F. (1971) Pathology Report of Bay 29493. Generation
study in rats. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull. Wld Hlth Org.,
29: 213-18
Thornton, J. S. (1967) Determination of fenthion residues in animal
tissues by thermionic emission flame gas chromatography. Chemagro
Corp., Research Department, Report No. 20 420 (revised 21 October,
1968)
Tomizawa, Ch., Fukuda, H., Masuda, T. and Miyahara, Y. (1962) Fate of
O,o-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on tea and cabbage leaves. Japan. J. appl. Entomol. Zool., 6:
237-241
von Clarmann, M. and Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and its
demonstration in the gastric contents and urine. Arch. Toxik., 22:
2-11
Wendel, L. E. and Bull, D. L. (1970) Systemic activity and metabolism
of dimethyl p-(methylthio) phenyl phosphate in cotton. J. Agr. Food
Chem., 18: 420-424
The primary hydrolytic products that are theoretically to be expected
are as follows:
(10) (CH3O)2 P (S) OH (16) HO-C7H6-S-CH3
(11) (CH3O) P (S) (OH)2 (17) HO-C7H7-SO-CH3
(12) P (S) (OH)3 (18) HO-C7H6-SO2-CH3
(13) (CH3O)2 P (O) OH
(14) (CH3O) P (O) (OH)2
(15) P (O) (OH)3
In animals
Knowles and Arthur (1966) applied fenthion dermally to two dairy cows
each weighing 360 kg at a rate of approximately 13 mg/kg per cow, and
treated two other lactating cows (each weighing 410 kg) by the
intramuscular route with approximately 8.5 mg of fenthion/kg per cow.
In the urine the peak concentration of radioactive materials occurred
on the first day following either method of treatment. The total ppm
of P32 materials from intramuscular treatment decreased from 33 ppm
at one day to 1.6 ppm by 21 days. More than 95% of the radioactive
materials eliminated in the urine consisted of hydrolytic products.
The total P32-materials eliminated in the faeces peaked two days
after both types of treatment. The cumulative percentage of the
administered dose was about 4%. The peak concentration of
acetonitrile-soluble radioactivity occurred one day after
intramuscular treatment and three days after dermal treatment. The
hair of the two cows treated dermally contained about 2000 ppm
radioactive fenthion equivalents immediately after treatment. These
residues declined to 16 ppm by 14 days after treatment. Chloroform to
water partition data indicated that fenthion underwent little change
on the hair to water-soluble components. The radioactivity in the
blood after both types of treatment reached a peak during the first 24
hours. Between 10 and 38% of the P32-materials partitioned into
chloroform but no detectable chloroform-soluble P32-materials were
present in the blood at seven days after either treatment.
At 14 days after dermal treatment and 21 days after intramuscular
treatment the cows were killed, and portions of liver, left and right
sirloin steak, left and right round steak, T-bone steak, omental fat,
and left and right subcutaneous fat were removed for analysis. None of
these tissues contained acetonitrilesoluble radioactive materials
following dermal treatment; all tissues contained less than 0.05 ppm
of total radioactivity with the exception of the liver which contained
0.44 ppm. The situation was vastly different in the tissues of the
intramuscularly treated animals. The tissues from the left side (site
of injection) of the cows contained considerably more P32-materials
than those from the right side (e.g. left round steak contained 1.03
ppm total radioactive materials, right round steak contained 0.11
ppm). The acetonitrile-soluble compounds amounted to a maximum of 0.36
ppm in the left steaks (less than 0.1 ppm in the right steaks); 0.2
ppm in the left subcutaneous fat and 0.08 ppm in the right
subcutaneous fat; 0.05 ppm in T-bone steak; 0.15 ppm in omental fat;
and 0.76 ppm in liver. The injection sites contained high residues in
both cases: skin 0.49 ppm total activity (0.08 ppm
acetonitrile-soluble), injection site 164 ppm (76 pp.
acetonitrile-soluble). The amount of the applied P32-materials
remaining in the skin after dermal treatment was, however, only 0.14%.
A similar calculation for the percentage remaining on the hair gave a
figure of 0.73%.
Following dermal and intramuscular treatment of cows with fenthion
(Knowles and Arthur, 1966), fenthion constituted more than 50% of the
non-ionic residues for three days after dermal and seven days after
i.m. treatment in the milk. The remainder was composed chiefly of
(3) + (5) + (6) and (2) [+ (4) ?]. In the urine, fenthion accounted
for only a small percentage of the chloroform-soluble radioactivity.
Some of (4) and/or (2) was present, but in most cases more than 70% of
the activity consisted of (3) and/or (5) and/or (6). The hydrolytic
products were composed of almost equal proportions of (10) and (13),
and a small portion consisted of an unidentified compound (probably a
O-desmethyl form of fenthion or one of its metabolites). More than 50%
of the acetonitrile-soluble radioactive materials in the faeces
consisted of the parent compound. The remainder was accounted for by
the other metabolites just as in urine. The composition of the
hydrolytic products was also very similar to that found in urine. In
the tissues of the animals slaughtered 14 days after dermal treatment
and 21 days after i.m. treatment, more than 50% of the radioactive
acetonitrile-soluble materials was chromatographed as fenthion, but
oxidation products were also present. At the injection site, (12) was
the predominant product of the water-soluble materials.
The silage feeding experiments performed by Bowman et al. (1970)
referred to earlier, also provide certain indications of the
transformation of fenthion metabolites in the cow. For example, the
residue (total of six ppm) in the feed fed in the fourth week and in
the resultant milk samples had the following composition:
Compound Feed Milk
(1) 8% -
(2) 89% <2%
(3) 2% <2%
(4) - -
(5) 1% 97%
(6) - -
Compound (5) accounted for 95% of the metabolites present in the
urine. Those present in the faeces consisted almost exclusively of
compound (1) (fenthion). Unfortunately the authors do not provide any
data on the occurrence of metabolites in the blood of the animals;
however, the cholinesterase activity of the animals of group C was
significantly depressed during the feeding experiment but otherwise
there were no changes in performance.
In plants
The transformation of fenthion in plants is basically similar to that
in animals. The first studies performed to investigate transformation
of fenthion in plants were made by Brady and Arthur (1961). Cotton
plants were sprayed with an emulsion of P32-labelled fenthion at a
rate of 2 kg of fenthion per hectare. The plants were growing under
field conditions. Three days after spraying, 189 ppm fenthion
equivalents were present on or in the leaves, but only 14 ppm were
chloroform-soluble materials. The respective residues after 14 days
were 71/5 ppm. Compound (3) was tentatively identified as by far the
most prevalent metabolite (60-80%), followed in quantity by unchanged
fenthion (28% after three days, 13% after 14 days). Compounds
(5) + (6) appeared in smaller percentages (less than 10%). The
hydrolytic products found were chiefly (10) and small amounts of (13)
and an unknown compound.
Experiments with P32-labelled fenthion on beans, in which the
metabolites were separated by paper-chromatography (Niessen et al.,
1962a) and quantitatively determined (Niesson et al., 1962b), produced
the following results:
The labelled parent compound contained 94.2% of (1), 4.5% of (7), 0.7%
of (2) and 0.6% of ionic compounds. Bean plants (Phaseolus vulgaris)
were either briefly dipped in a 0.2% emulsion of this compound by
their stem, or placed in the emulsion by their roots. The
chloroform-soluble extracts had the following composition (ppm):
Days (1) (2) (3) (5) (6) (7) (8)
1/4 100 16 - 1.2 - 2.8 1.5
2 27 25 2.1 3.5 0.06 - 0.4
5 4.7 7.2 1.4 1.5 0.12 - 0.09
8 1.6 4.7 1.4 1.2 0.11 - 0.05
After eight days, only 1.6% of the parent compound was present; (2)
was the principal metabolite. The impurity (7) was no longer
detectable from the second day; however, it is also oxidized to the
sulfoxide (8). The total residue decreased in the eight-day period
from 122 ppm to a level of 9 ppm. The rate at which these
transformations take place depends upon the temperature.
Oxidation by plant enzymes preferably takes place at the thiono sulfur
atom, while oxidation at the methylmercapto group seems chiefly to be
a photosensitized reaction. The compound has a certain systemic
action. (7) has a much stronger systemic action, but it is not found
in the plant.
Several experiments on glass plates confirmed that the formation of
sulfoxide and sulfone is a light-induced reaction whilst (7) is formed
from (1) and (8) is formed from (2) due to the influence of heat.
These findings were later confirmed by Metcalf et al. (1963).
At the same time as the studies of Niessen et al. (1962b) were
conducted, Japanese research workers investigated the behaviour of
P32-labelled fenthion in rice, tea and cabbage (Fukuda et al. 1962,
Tomizawa et al., 1962). The neutral metabolites were separated by
paper chromatography and the water-soluble metabolites were separated
by ion exchange chromatography. Immediately after the treatment, the
deposit of fenthion on the rice plants amounted to between 110 and 150
ppm on the leaf blade and to between 18 and 28 ppm in the leaf sheath.
(2) and (3) were the principal metabolites in the leaf blade and
sheath. The accumulation of fenthion metabolites in ears and grains
was examined in one variety which was treated a few days before
heading. Four weeks after application, the distribution of
radioactivity was as follows (ppm):
Total Chloroform-extractable
Husk 2.4 n.d.
Bran 60 0.9
Polished rice 5.7 0.1
The composition of the metabolite mixture in the ears 12 days after
the application was as follows: 45% (2); 20% (7?); 18% (3); 17%
unidentified. The water-soluble metabolites in the grains 14 days
after the application were composed chiefly of (1a), plus a few
percent. (less than 10) of (10), (13) and (12) and/or (15).
The amounts of fenthion deposit on tea and cabbage after the
application were as follows: 43 ppm on young tea leaves, 90 ppm on old
tea leaves and 46 ppm on cabbage leaves. (2) and (3) were the
principal metabolites also in these experiments. The appearance of
O-desmethyl fenthion observed by the Japanese research workers seems
astonishing and was never again reported by any other observers.
In 1963, Metcalf et al. published qualitative results which they
obtained on cotton. In these studies, too, (2) and (3) were the
principal metabolites.
With the improvement of analytical techniques, a better insight was
gained into the metabolism of fenthion in plants. Leuck and Bowman
(1968) treated Coastal bermudagrass and ensilage corn at rates of 0.56
kg/hectare, 1.12 kg/hectare and 2.24 kg/hectare (a.i.). The ppm
residues determined in Coastal bermudagrass treated at the highest
application rate were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 64 71 1.0 1.8 n.d.
7 0.31 10 4.0 2.0 0.34
14 0.07 2.1 2.3 0.48 0.18
21 0.03 0.59 1.3 0.08 0.11
The corresponding residue levels for corn were as follows:
Days (1) (2) (3) (5) (6)
post-treatment
0 0.41 26 0.22 0.63 <0.005
7 0.04 2.4 0.68 1.03 0.16
14 0.01 0.42 0.24 0.11 0.09
21 <0.002 0.34 0.22 <0.02 0.04
(4) was not detected (except on day 0 in bermudagrass when its level
was 0.06 ppm). (2) and (3) were again the principal metabolites.
The total residues were as follows (expressed in ppm):
Days Bermudagrass Corn
post-treatment 0.56 kg/ha 1.12 kg/ha 0.56 kg/ha 1.12 kg/ha
7 3.3 8.5 0.65 1.95
14 1.3 3.2 0.15 0.4
21 0.5 1.3 0.1 0.2
These findings were very largely confirmed by a study performed by
Bowman et al. (1968), in which nine procedures for removal of
phosphorus insecticides and their metabolites were compared. This
study also provides an impressive picture of the differing effect of
various extraction procedures. Soxhlet extraction with 10% methanol in
chloroform proved to be the best one.
The fate of phenolic hydrolytic products of fenthion in plants was not
further studied. However, findings have been published by Wendel and
Bull (1970) in respect of GC-6506 (dimethyl 4-methylthiophenyl
phosphate). From some of the metabolites, substituted phenols could be
liberated by hydrolysis with ß-glucosidase (Bull and Stokes, 1970).
The predominant products were the glucosides of phenol-sulfoxide and
phenol-sulfone. The glycone portions of the conjugates are further
altered, possibly by the formation of a ß-gentiobiside.
In soils
The half-life of fenthion and its metabolites in soil is between 14
and 40 days; only in one out of six experiments was a half-life of
more than 30 days recorded. The experiments used various formulations
including granules and emulsifiable solutions. (Chemagro - Summary
under soils in main submission).
In storage and processing
Under frozen storage (-18 to -23°C), fenthion residues were shown to
be stable in alfalfa for 28 weeks and in cattle fat for 11 weeks
(Chemagro, 1966e,f). In liver from cattle, a decrease of 20% seemed to
occur between the twelfth and twenty-sixth week of storage (Chemagro,
1966f).
Fenthion proved to be stable for four weeks at -18°C in cattle brain,
heart, liver, kidney, steak and fat over a period of four weeks; (6)
decreased slightly in brain, heart, steak and fat, by 30% in kidney,
and by 80% in liver in six weeks (Olson, 1966).
Following treatment of corn with fenthion at rates of 0.56, 1.12 and
2.24 kg per hectare (Boman and Beroza, 1969), the plants treated at
the highest rate contained 39 ppm of residues (1) + (2) + (3) + (5),
in a ratio of 1.8 : 96.5 : 0.75 : 0.97. At 70 days after ensiling
(which took place on the day after application), the residues amounted
to 14.6 ppm (17 : 74 : 7.8 : 1.1); at 141 days, they amounted to 6.85
ppm (9.7 : 85 : 4.7 : 0.5). The predominant metabolite throughout the
whole period was (2). In another silage experiment with corn, the
residues were consistently shown to be at a lower oxidation stage than
were those in the field samples from which the silage was prepared
(Leuck and Boman, 1968).
The relatively high persistence of the residues in silage and the
occurrence of (2) as the principal metabolite were again confirmed
later (Bowman et al., 1970).
Residues in canned and preserved peaches persist to a certain extent,
especially when maintained at low temperatures (refrigerator) (Pigatti
et al., 1967).
Evidence of residues in commerce or at consumption
Krause and Kirchhoff (1969) examined 78 market samples of different
fruits and vegetables of domestic and foreign origin for residues of
20 organophosphorus insecticides including fenthion. Residues of
fenthion were not found in any of the samples.
Total diet studies were carried out in England and Wales, in 1966/67,
with altogether 66 samples of total diet. Each sample comprised seven
sub-samples consisting of cereals, meats, fish, fats, fruits and
preserves, root vegetables, green vegetables and milk. Fenthion and
its immediate oxidation products could have been detected by the
method employed, but they were not detected (Abbott et al., 1970).
Methods of residue analysis
Although the present-day gas chromatographic methods can be carried
out largely without clean-up, it is nevertheless necessary in some
cases to eliminate interfering substances. In this, consideration must
be given to the greatly differing polarity of fenthion residues.
Chemagro (1963) has published, for example, the following data on
partition:
n-hexane water n-hexane
Compound acetonitrile chloroform water
(1) 1 : 8.4 1 : 1720 1 : 0.016
(5) 1 : 300 1 : 88 1 :
(6) 1 : 87 1 : 15 1 : 78
Bowman and Beroza (1968) give the following p-values (=fractional
amount in nonpolar phase):
n-hexane n-hexane n-hexane
Compound 20% acetonitrile 40% acetonitrile water
in water in water
(1) 0.98 0.92 1.00
(2) 0.18 0.03 0.50
(3) 0.61 0.12 0.94
(4) 0.65 0.18 0.92
(5) 0 0 0
(6) 0 0 0.01
Regarding the extraction of residues, attention is drawn to the study
of Bowman et al. (1968) already referred to earlier.
The first methods for the determination of fenthion and its
metabolites were based on determination of total phosphorus (Frehse et
al., 1962a for plant material; Frehse et al., 1962b for olives (oil);
Adam (1967) for olives). In 1966, the first methods were published for
the determination of fenthion in plant and animal tissues (Anderson et
al., 1966) and milk (Katague, 1966), based on oxidation of the various
compounds to the sulfone (6) which in turn was hydrolyzed to the
corresponding phenol. The phenol was brominated and acetylated prior
to detection measurement by electron-capture GLC. The methods were
sensitive to 0.1 ppm (0.01 ppm for milk) but unavoidably very
complicated and time-consuming.
Therefore, Chemagro began in 1967 to develop methods using the
thermionic phosphorus detector: for soil (Olson 1967a), rice grain
(Olson 1967b), eggs and milk (Olson 1968), and animal tissues
(Thornton 1967).
These methods are based on oxidation of the residues to (6). The
sensitivities are below 0.1 ppm for soil and rice, and about 0.005 ppm
for eggs and milk. GLG conditions are:
(a) soil: 11 in × 3 mm i.d. column, packed with 6% DC-200 and 1% QF-1
on 80/100 mesh Gas Chrom Q, 230°C;
(b) other material: 16 in × 3 mm i.d. column, packed with 10% DC-200
and 0.2% QF-1 on 80/100 mesh Gas Chrom Q, 210°C, retention time
3.5 minutes.
Forty-five organophosphorus insecticides were examined for possible
interference with the rice method (Olson, 1967b); no interferences
were noted for any of the compounds except (R) Dasanit (Olson,
1967c).
Bowman and Beroza (1968) used a different principle of determination.
Extracts from corn, grass and milk were separated by liquid
chromatography into three fractions which then were analysed by GLC
with a flame photometric detector: 90 cm × 4 mm i.d, column, packed
with 10% DC-200 on 80/100 mesh Gas Chrom Q, 210°C for the two
fractions (1) + (3) and (2) + (4) + (6); a 45 cm column is used for
(5) (retention times (1) to (6) : 1.75, 4.2, 4.05, 1.45, 2.3 minutes).
Ten per cent. QF-1 (50 cm), 1% Carbowax 20 M (50 cm) and 5% DEGS
(50 cm) can also be used as the liquid phase. A complete separation of
all six compounds was achieved by using liquid chromatography with a
silica gel column and different elution systems. Later, Bowman and
Beroza (1969) also used the oxidation method with m-chloroperbenzoic
acid for the same substrates: 90 cm × 4 mm i.d. column packed with 10%
OV-101 on 80/100 mesh Gas Chrom Q, 215°C. With this column, the
metabolites can also be determined singly (retention times of
0.7 - 2.75 minutes, 2.3 minutes for (6)); it was found that the
results of the single metabolite determinations those of the total
determination. The recoveries were above 90%. Excessive amounts of the
oxidation mixture are removed on an alumina column.
Further studies performed by Bowman and Beroza (1970) show that 2.4
metre × 4 mm i.d. columns, packed with 5% OV-101 or OV-210 on Gas
Chrom Q are also suitable for determination of the compounds (1) to
(6).
Examples of national tolerances
Country Crop Tolerance Safety
in ppm Interval
in days
Algeria General 15
Australia Fruit, vegetables 2 7
Meat of cattle 1 1
Milk and milk products 2 1
Austria General 35
Belgium Fruit, vegetables excluding
potatoes 0.3 21
Brazil General 14
Fruit 1.0
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
Bulgaria Sugar beets 14
Cherries, cereals 14
Canada Beef cattle N.R.
Finland General 14
France General 15
Olives 21
Germany Fruit 14
Vegetables, field & fodder
crops 10
Israel Fruit 21
Miscellaneous (e.g. dry onions
and cucurbits) 30
Italy General 20
Olives 30
Morocco General 15
Olives 30
Netherlands Fruit, vegetables excluding
potatoes 0.3 -
New Zealand General 21
Norway General 14
Poland Fruit, vegetables, root crops 30
Early cherries 14
Portugal General 14
Olives (as oil and preserves) 42
Russia Cereals, cottonseed oil 0.35
Spain Fruit and olives 30
South Africa General 2.0
Apricots, peaches, apples,
pears, grapes 10
Subtropical crops & cucurbits 7
Deciduous fruit 10
(Continued)
Country Crop Tolerance Safety
in ppm Interval
in days
United States Alfalfa (fresh), grass 5.0
of America Alfalfa (hay), grass hay 18.0
Animal products 0.1
Milk 0.01
Yugoslavia General 0.5
N.R. = registered for use on a no-residue basis.
Appraisal
Fenthion is an organophosphorus insecticide with a broad spectrum of
activity used against insects infesting field crops, fruit crops,
vineyards, olives, cotton, insects of public health concern and
ectoparasites of domestic animals. It is especially useful for control
of fruit flies in many crops where its ability to penetrate plant
tissues allows for destruction of larvae within the fruit. The
concentrations/rate of use ranges from 0.5-2 kg/ha in the case of
field crops and cotton and 0.05-0.1% solution on horticultural crops.
Animals are treated with 0.25%-5% dust, 0.025%-0.25% solutions as dips
or sprays and 2%-3% solutions in oil as pour-on preparations or back
rubbers.
The residue data available to the meeting were obtained from many
different countries, reflecting typical registered uses under a wide
range of climatic and ecological conditions. Considerable information
is available about the fate of fenthion residues in foods of plant and
animal origin and extensive studies have been carried out on the
nature of the degradation products formed under a variety of
influences.
Little information is available about residues of fenthion in foods in
commerce.
The literature includes a number of methods of residue analysis based
on gas-chromatographic procedures. The greatly differing polarity of
the various fenthion metabolites has been investigated and
recommendations for the most appropriate extraction procedures have
been made.
In order that all biologically active metabolites may be determined
simultaneously the analytical procedure has been modified to provide
for oxidation of the residues of the various compounds to the sulfone
which is hydrolyzed to the corresponding phenol. The phenol is
brominated and acetylated prior to measurement by electron-captive
gas/liquid chromatography. The limit of detection is 0.1 ppm for most
foods and 0.01 ppm for milk.
Procedures which are less complicated and less time consuming have
been developed using the thermionic phosphorus detector following
oxidation to the sulfone. The limit of detection is below 0.1 ppm for
soil and grain, and about 0.005 ppm for eggs, milk and animal tissues.
These procedures appear suitable for regulatory purposes.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES OR PRACTICAL
RESIDUE LIMITS
Temporary tolerances
The temporary tolerances are for fenthion and its major metabolites,
determined separately or together and expressed as fenthion.
Apples, peaches, cherries, lettuce, 2 ppm
fat of meat
Cabbage, cauliflower, olives, 1 ppm
olive oil
Grapes, oranges, peas, meat 0.5 ppm
Squash 0.2 ppm
Wheat, rice, milk products (fat basis) 0.1 ppm
Milk (whole) 0.05 ppm
Further work or information
Required before 30 June 1975
1. Adequate two-year feeding studies in the dog and in one rodent
species.
2. Establishment of the sequence of metabolic changes in man and
laboratory animals in order to elucidate the mechanism of long
lasting cholinesterase inhibition.
Desirable
Information on the frequency and level of fenthion residues in food
commodities in commerce.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R. and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pestic. Sci.
1: 10-13
Adam, N. Chr. (1967) Méthode de détermination des résidue de Lebaycid
dans l'huile d'olive et lea olives. Ann. Inst. Phytopath. Benaki, N.S.
8: 78-84
Anderson, R. J., Thornton, J. S., Anderson, C. A. and Katague, D. B,
(1966) Determination of fenthion residues in plant and animal tissues
by electron-capture gas chromatography. J. Agr. Food Chem. 14:
619-622
Begum, A. (1968) Effect of diet on metabolism of fenthion in animals.
Ph.D. thesis, Auburn University, Auburn, Alabama. Dissert, Abstr.,
Sect. B, 28: 4165
Bowman, M. C. and Beroza, M. (1968) Determination of fenthion and five
of its metabolites in corn, grass and milk. J. Agr. Food Chem. 16:
399-402
Bowman, M. C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion, disulfoton and phorate in corn, milk, grass and
faeces, J.A.O.A.C. 52: 1231-1237
Bowman, M. C. and Beroza, M. (1970) GLC retention times of pesticides
and metabolites containing phosphorus and sulphur on four thermally
stable columns. J.A.O.A.C. 53: 499-508
Bowman, M. C., Beroza, M. and Leuck, D. B. (1968) Procedures for
extracting residues of phosphorus insecticides and metabolites from
field treated crops. J. Agr. Food Chem. 16: 796-802
Bowman, M. C., Leuck, D. B., Johnson, J. C., jr and Knox, F. E. (1970)
Residues in corn silage and effect of feeding dairy cows the treated
silage. J. econ. Entomol. 63: 1523-1528
Brady, U. E., jr and Arthur, B. W. (1961) Metabolism of O,o-dimethyl
O-[4-(methylthio)-m-toly] phosphorothioate by white rats. J. econ.
Entomol, 54: 1232-1236
Bull, D. L. and Stokes, R. A. (1970) Metabolism of dimethyl
p-(methylthio) phenyl phosphate in animals and plants. J. Agr. Food
Chem. 18: 1134-1138
Chemagro Milk and tissue residues resulting from backline or oral
treatment of Cattle with Bayer 29493, p. 32
1963 Report No. 10. 946, December 3
1965a Report No. 16. 551, July 27 (revised January 10, 1968)
1965b Report No. 16. 990, October 21
1965c Report No. 17. 015, October 28
1965d Report No. 17. 062, November 3
1966a Report No. 17. 445, January 19 (revised April 28, 1966)
1966b Report No. 17. 969, April 21
1966c Report No. 18. 009, May 3
1966d Report No. 18. 067, May 12
1966e Report No. 18. 179, May 20
1966f Report No. 18. 191, May 24
1967a Report No. 20. 310, April 18
1967b Report No. 20. 738, June 20
1967c Report No. 20. 798, July 5
1967d Report No. 21. 113, September 13
1967e Report No. 21. 504, November 13
1968a Report No. 21. 796, January 25
1968b Report No. 22. 647, May 15
1969 Report No. 24. 266, February 14 (revised April 2, 1969)
1970 Report No. 28. 719, December 3
Dean, G., Coxon, J. and Brereton, D. (1967) Poisoning by an
organo-phosphorus compound. A case report. So. African Med. J.,
1017-19
Dieckmann, W. (1971) Neurotoxizctatsunter-suchungen an
Huhnern-Histopathologie. Unpublished report of Farbenfabriken Bayer
A.G.
Dilley, J. and Doull, J. (1961a) Chronic inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dilley, J. and Doull, J. (1961b) Acute inhalation toxicity of Bayer
29493 to rats and mice. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. (1960) The absence of antidote activity by 2-PAM and
TMB-4 against acute poisoning by Bayer 29493. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. (1961) Effects of repeated dermal application of Bayer
29493 on rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1962) Acute oral toxicity of a sample of Bayer 29493 to
female rats. Unpublished report from Department of Pharmacology,
University of Chicago
Dubois, K. P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Dubois, K. P. and Doull, J. (1960) The acute toxicity of Bayer 29493
to chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago
Dubois, K. P. and Puchala, E. (1960) Influence of Bayer 29493 on the
cholinesterase activity of the blood of rats. Unpublished report from
Department of Pharmacology, University of Chicago
Dubois, K. P. and Kinoshita, F. (1964) Acute toxicity and
anti-cholinesterase action of O,O-dimethyl O-4-(methylthio)-m-tolyl
phosphorothioate (DMTP; Baytex) and related compounds. Tox. Appl.
Pharmacol. 6: 86-95
Doull, J., Root, M. and Cowan, J. (1961) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Doull, J., Root, M. and Cowan, J. (1962) Effect of adding Bayer 29493
in combination with other cholinergic insecticides to the diet of male
and female dogs. Unpublished report from Department of Pharmacology,
University of Chicago
Doull, J., Root, M., Cowan, N. J., Vesselinovitch, D., Fitch, F. W.
(1963a) and Meskauskas, J. Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished report submitted by Farbenfabriken
Bayer A.G.
Doull, J., Root, M., Cowan, J. and Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. and
Meskauskas, J. (1961) The effects of feeding diets containing Bayer
29493 to rats for a period of 16 weeks. Unpublished report submitted
by Farbenfabriken Bayer A.G.
Elliott, R. and Barnes, J. M. (1963) Organophosphorus insecticides for
the control of mosquitos in Nigeria. Bull. Wld Hlth Org. 28: 35-54
Francis, J. I, and Barnes, J. M. (1963) Studies on the mammalian
toxicity of fenthion. Bull. Wld. Hlth. Org. 29: 205-12
Frehse, H. (1970) Ruckstande van pflanzenschutz mittein in nahrung und
um welt in chemie der pflanzenschutz und schadlingshenamp-fungsmittel.
Band 2-Wegler, R., Ed. Springer-Verlag, 1970
Frehse, H., Niessen, H. and Tietz, H. (1962a) Method of determining
residues of the insecticide Lebaycid(R) in plant material.
Pflanzenschutz-Nachr Bayer 15: 148-159
Frehse, H., Niessen, H. and Tietz, H. (1962b) Method of determining
residues of the insecticide Lebaycid(R) in olives and olive oil.
Pflanzenschutz-Nachr Bayer 15: 160-165
Fukuda, H., Masuda, T., Miyahara, Y. and Tomizawa Ch. (1962) Fate of
O,O-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on rice plant. Japan. J. appl. Entomol. Zool. 6: 230-236
Gaines, T. B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol. 14: 515-34
Hahn, H. L. and Hensehler, D. (1969) The ability of phosphorylated
cholinesterases to be reactivated by obidoxime chloride (Toxogonin)
in vivo. Arch. Toxikol. 24: 147-63
Katague, D. B. (1966) Determination of fenthion residues in milk by
electron-capture gas chromatography. Chemagro Corp., Research
Department, Report No. 17, 887
Keith, J. O, and Mulla, M. S. (1966) Relative toxicity of five
organo-phosphorus mosquito larvicides to mallard ducks. J. Wildlife
Management 30: 553-63
Kimmerle, G. (1960) Re: Active substance S1752. Unpublished report
submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1961) Subchronische oral versuche bei ratten mit
S-1752-Wirkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1963) Product BH6 and S1752 Poisoning. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965a) Nourotoxic studies with Bayer 29493. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Kimmerle, G. (1965b) Neurotoxische untersuch-ungen mit
S-1752-Werkstoff. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Kimmerle, G. (1966) Langdavernde Inhalatronsversuche bei hunden mit
dem Baytex-Werkstoff (S-1752)
Kimmerle, G. (1967a) Abhangigkeit der akuten oralen toxizitat bei
ratten vom losungsmittel. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Kimmerle, G. (1967b) Potenzierung von DDVP und S-1752. Unpublished
report submitted by Farbenfabriken Bayer A.G.
Klimmer, O. R. (1963) Toxicological testing of Bayer 29493.
Unpublished report submitted by Farbenfabriken Bayer A.G.
Knowles, C. O. and Arthur, B. W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of radiolabelled
fenthion to dairy cows. J. econ. Entomol. 59: 1346-52
Krause, Ch. and Kirchhoff, J. (1969) Organophosphatrückstande auf
Marktproben von Obst und Gemüse sowie auf Getreideerzeugnissen
Nachrichtenbl. Deut. Pflanzenschutzdienstes (Braunschweig), 21:
81-84
Leuck, D. B. and Bowman, M. C. (1968) Residues of fenthion and five of
its metabolites their persistence on corn and grass forage, J. econ.
Entomol. 61: 1594-1597
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmakol. 263: 237-8
Loser, E. (1969) Generation versuche an Ration. Unpublished report
submitted by Farbenfabriken Bayer A.G.
McGrath, H. B. (1969) Toxicity of Bayer 29493 in calves. Unpublished
report submitted by Farbenfabriken Bayer A.G
Metcalf, R. L., Fukuto, T. R. and Winton, M. Y. (1963) Chemical and
biological behaviour of fenthion residues. Bull. Wld Hlth Org. 29:
219-226
Möllhoff, E. (1970) Determination of trichlorfon and fenthion residues
in animals of different species, Pestic. Sci. 2: 179-181
Nelson, D. L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report submitted by
Farbenfabriken Bayer A.G.
Niessen, H., Tietz, H. and Frehse, H. (1962a) Papier
chromatographische Trennung aromatischer
Phosphorsäure-ester-Insektizide. J. Chromatog. 9: 111-113
Niessen, H., Tietz, H. and Frehse, H. (1962b) On the occurrence of
biologically active metabolites of the active ingredient S-1752 after
application of Lebaycid(R) Pflanzenschutz-Nachr. Bayer 15: 125-147
Olson, T. J. (1966) The determination of the stability of fenthion and
its oxygen analog sulfon in frozen cattle tissues. Chemagro Corp.,
Research Department, Report No. 19, 289,
Olson, T. J. (1967a) Determination of residues of fenthion in soil by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 20, 324.
Olson, T. J. (1967b) Determination of residues of fenthion in rice
grain by thermionic emission gas chromatography. Chemagro Corp.,
Research Department, Report No. 20, 417 (revised 21 October, 1968)
Olson, T. J. (1967c) A study of the possible interference of other
pesticides with the analytical method for fenthion in rice. Chemagro
Corp., Research Department, Report No. 20, 595
Olson, T. J. (1968) Determination of fenthion in eggs and milk by
thermionic emission gas chromatography. Chemagro Corp., Research
Department, Report No. 22, 933
Pickering, E. N. (1966) Organic phosphate insecticide poisoning. Can.
J. Med. Tech., p. 174
Pigatti, A., Pigatti, P., Orlando, A., Suplicy, F. O., Sampaio, A. S.
and Rigitano, O. (1967) Persistência de residuos de fenthion am
pêssego, ameixa e maca. Arq. Inst. Biol. (S. Paulo) 34: 275-284
Sherman, M. and Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol Appl. Pharmacol. 3, 512-33
Shillam, K. W. G., Medd, R. K., Roberts, N. L. and Burrows, I. E.
(1971) Residual levels of fenthion in raw and pasteurized milk. Report
of Huntington Research Centre 4225/71/383
Shimamoto, K. and Hattori, K. (1969) Chronic feeding of Baytex
(O, O-dimethyl-o-(4-methylmercapto-3-methyl) phenyl-thiophosphate) in
rats. Acta Med. Univ. Kioto, 40: 163-71
Shipp, E. (1970) Fenthion residues in milk. Report of the Entomology
School, University of New South Wales
Spicer, E. J. F. (1971) Pathology Report of Bay 29493. Generation
study in rats. Unpublished report submitted by Farbenfabriken Bayer
A.G.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull. Wld Hlth Org.,
29: 213-18
Thornton, J. S. (1967) Determination of fenthion residues in animal
tissues by thermionic emission flame gas chromatography. Chemagro
Corp., Research Department, Report No. 20 420 (revised 21 October,
1968)
Tomizawa, Ch., Fukuda, H., Masuda, T. and Miyahara, Y. (1962) Fate of
O,o-dimethyl O-(3-methyl-4-methylmercaptophenyl) thiophosphate sprayed
on tea and cabbage leaves. Japan. J. appl. Entomol. Zool., 6:
237-241
von Clarmann, M. and Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and its
demonstration in the gastric contents and urine. Arch. Toxik., 22:
2-11
Wendel, L. E. and Bull, D. L. (1970) Systemic activity and metabolism
of dimethyl p-(methylthio) phenyl phosphate in cotton. J. Agr. Food
Chem., 18: 420-424
