
FENTHION
First draft prepared by
S. Ma
Health Evaluation Division, Pest Management Regulatory Agency, Health
Canada, Ottawa, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, red excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal red ocular irritation and dermal sensitization
Potentiation
Cholinesterase inhibition
Delayed neurotoxicity
Studies of metabolites
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Fenthion was previously evaluated by the Joint Meeting in 1971,
1975, 1978, 1979, and 1980 (Annex I, references 16, 24, 30, 32, and
34). An ADI of 0-0.001 mg/kg bw was allocated in 1980, based on a
NOAEL of 0.09 mg/kg bw per day (3 ppm) for acetylcholinesterase
depression in a two-year feeding study in dogs. The compound was
re-examined at the present Meeting within the CCPR periodic review
programme. This monograph summarizes pertinent new (since 1980) data
as well as relevant data from the previous monographs and monograph
addenda on fenthion.
Evaluation for acceptable daily intake
1. Biochemical aspects
The toxicokinetics of fenthion has been extensively studied after
oral and topical administration to various animal species, using
radiolabelled test material. Summaries of the most relevant and most
recent data are presented below.
(a) Absorption, distribution, and excretion
The first studies on the metabolism of fenthion in rats were
reported by Brady and Arthur (1961), who used a 32p-labelled
compound. Within a few hours of oral or intraperitoneal administration
of the compound, large amounts of 32p label were found in tissues
including the bone, suggesting rapid absorption and translocation of
fenthion. Three days after administration of a single oral dose of
100 mg/kg bw, the tissue concentration of chloroform-soluble residues
was < 0.01 mg/kg bw; there were no detectable acetonitrile-soluble
residues except in the liver (0.2 mg/kg bw). Tissue retention of
32P-labelled residues remained low in rats that received 10 daily
doses of unlabelled fenthion at 10 mg/kg bw intraperitoneally before
injection of a single dose of 200 mg/kg bw radiolabelled compound.
Seven days later, 86% of the orally administered 32p radiolabel had
been eliminated in the excreta (46% in urine and 40% in faeces),
the majority being excreted within the first three days. After an
intraperitoneal injection, about 75% of the administered radioactivity
was recovered in the excreta (60% in urine and 15% in faeces) within
three days.
In a more recent study (Puhl & Hurley, 1982), four groups of five
male and five female Wistar rats were fasted for 16-24 h and then
given a single dose of [ring-1-14C]-fenthion (purity, 98-99%), either
intravenously at 2 mg/kg bw or by gavage at 10 or 100 mg/kg bw. One
group of animals was given 14 daily doses of unlabelled fenthion at
10 mg/kg bw (purity, 97.2%) before receiving a single oral dose
of [ring-1-14C]-fenthion at 10 mg/kg bw. Orally administered
[ring-1-14C]-fenthion was readily absorbed from the gastrointestinal
tract; absorption (96-100% at 72 h) was not dose-dependent over the
dose range tested. [ring-1-14C]-Fenthion was rapidly eliminated,
> 90% of the administered radiolabel being excreted within 48 h.
Urine was the major route of elimination, accounting for 93-97% of the
total label recovered 72 h after treatment. Only 3-6% was recovered in
faeces, and none was found in expired gases. The excretion profiles
were generally similar, regardless of the route of administration,
dose, sex of the rats, or pretreatment with unlabelled fenthion for
14 days. Tissue retention of 14C radiolabel was low: 72 h after
treatment, a mean total of < 1% of the administered dose was
retained. The highest tissue concentrations were found in fat, gonads,
and liver.
Five groups of five or six Wistar rats of each sex were fasted
for about 2 h and then given a single dose of [ring-1-14C]-fenthion
(purity, 98%), either intravenously at 0.125 mg/kg bw or by gavage at
0.3 or 1.5 mg/kg bw. One group was given 14 consecutive daily doses of
0.3 mg/kg bw unlabelled fenthion (purity, 96.5%) before receiving a
single oral dose of 0.3 mg/kg bw [ring-1-14C]-fenthion; a further
group of two male and two female rats was given a single oral dose of
0.3 mg/kg bw 14C-fenthion for measurement of expired carbon dioxide,
and one of three male and three female rats served as untreated
controls. Excretion of the radiolabel was rapid, 75-104% of the dose
being eliminated within 48 h; the mean total excretion over 168 h was
80-107%. The excretion profiles were similar, regardless of sex, dose,
route of administration, or pretreatment with unlabelled fenthion.
Urine was the main route of excretion, accounting for 88-98% of the
total radiolabel excreted; only minor amounts (1-10% of the dose) were
excreted in the faeces, and no label was found in expired carbon
dioxide. Serum cholinesterase activity, used as a measure of exposure
to fenthion, was inhibited to 36-50% of the control value 24 h after a
single oral dose of 1.5 mg/kg bw 14C-fenthion. By 72 h, serum
cholinesterase activity appeared to return to control levels, and it
was unchanged at 168 h. These results reflect the excretion profile of
fenthion in the rats. Tissue retention of radiolabel was very low: the
mean total label recovered in the tissues and carcass at termination
was < 0.5%, and the amount recovered in the tissues alone was either
below the detection limit or < 0.01% in all treated groups. The
tissue residue levels were generally < 1 ppb (Doolittle & Bates,
1993).
One male and one female Duroc pig were given a single dose of at
5 mg/kg bw [ring-1-14C]-fenthion (purity, 99%) by gavage and seven
days later two or three consecutive daily doses of 10 mg/kg bw
14C-fenthion. Elimination of label was rapid, total excretion over
the first 54 h being 90-99% of the administered dose. Urine was the
main route of elimination, accounting for > 80% of the dose within
24 h; only minor amounts (< 10% of the single dose) were excreted in
the faeces. Tissue residue levels declined rapidly, from 2.4-8.6 to
0.15-1.15 ppm, between 6 h (peak blood level) and 30 h after the last
multiple dose, indicating rapid elimination from tissues and a low
tendency for bioaccumulation (Pither, 1979).
A single male pig (Yorkshire cross-breed) was given a single
dermal dose of [ring-1-14C]-fenthion (purity, 97.6%) at 14.4 mg/kg bw
active ingredient, prepared as a formulation for treatment of lice in
pigs and applied uniformly along the animal's backbone. The pig was
sacrificed 18 h after treatment, and skin and selected tissues were
removed and analysed for residues The tissue residue levels were
generally low (0.1-0.8 ppm 14C-fenthion equivalents in muscle, liver,
kidney, and fat), except at the site of application, where
significantly higher levels were measured in hair (1398 ppm), skin
(134 ppm), and subcutaneous fat (3.9 ppm) (Crosby et al., 1991)).
Fenthion was given to lactating dairy cows at dietary levels of
25.5 or 100 ppm for 28 days. The mean concentrations of residues of
fenthion, its sulfoxide and sulfone, and the sulfoxide and sulfone of
the oxygen analogue in the milk over the treatment period were 0.016,
0.049, and 0.099 mg/kg milk, respectively. The mean total residues of
fenthion and its sulfoxide in faeces were 0.042-0.308 mg/kg, and the
mean total residues of the sulfoxide and sulfone of fenthion and its
oxygen analogue in urine were 0.43-1.05 mg/kg. Seven days after the
end of treatment, no residues were detected in milk, faeces, or urine
(Johnson & Bowman, 1972).
Two lactating dairy cows were treated dermally with 32p-labelled
fenthion (a 20% topical treatment for control of lice) at a single
dose of 9 mg/kg bw. The total 32p-radiolabel in blood, milk, urine,
and faeces peaked between the first and second days after treatment.
Over four weeks, 45-55% of the administered dose was recovered in the
urine, 2-2.5% in the faeces, and 1.2-2% in the milk. The residues were
predominantly water-soluble hydrolysis products of fenthion. The
highest level of fenthion and its organo-soluble metabolites in the
milk was 0.1 mg/kg, found on the first day after treatment (Avrahami &
White, 1975)
One lactating dairy cow (Jersey breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 98.2%) at 5.08 mg/kg bw active
ingredient, prepared as a formulation for treatment of lice and horn
flies in beef and non-lactating dairy cattle and applied uniformly
along the animal's backbone. The cow was sacrificed 18 h after
treatment, and urine, milk, skin, and selected tissues were analysed
for residues. The mean residue level in milk 6, 12, or 18 h after
treatment was < 0.05 ppm 14C-fenthion equivalents. The mean
urinary concentration 0-18 h after treatment was 3.9 ppm. The tissue
residues were generally low: 0.1-0.3 ppm in skin, liver, kidney,
muscle, and peritoneal fat; 1.8 ppm in subcutaneous fat; and 2.3 ppm
in hair. At the site of application, significantly higher levels were
measured: 16 215 ppm in hair, 106 ppm in skin, and 6.1 ppm in
subcutaneous fat (Krautter, 1990a).
A single lactating goat was given [phenyl-1-14C]-fenthion
(chemical and radiopurity, > 99%) by gavage in gelatin capsules at
20 mg/kg bw daily for three days. The goat was sacrificed 3.5 h after
the last dose, and the level and nature of the residues were
determined in urine, milk, and edible tissues. The total recovery of
radiolabel 51.5 h after the first dose (3.5 h after the third dose)
was 51.5%:44.1% was excreted in urine, 6.3% in faeces, 0.2% in milk,
and 0.9% as residue in edible tissues. The rate of gastrointestinal
absorption was rapid: the time for an increase of 25-75% in plasma
concentration over the maximal value was 0.96 h, and a plasma peak
level of 7.74 µg/ml was reached about 3 h after the first dose. The
maximal residue level in milk was 2.8-3.4 µg/g 8 h after treatment.
The tissue residue concentrations 3.5 h after the last dose were
judged to be low, the highest being found in kidney (24.1 µg/g), liver
(3.32 µg/g), fat (1.61 µg/g), and muscle (0.62 µg/g) (Weber & Ecker,
1992).
(b) Biotransformation
In studies of rats treated with 14C-labelled fenthion (purity,
> 98%) orally or intravenously, no major differences were seen in
metabolite profiles with route of administration, dose, sex, or
pretreatment with unlabelled fenthion for 14 days. No unchanged parent
compound was detected in the urine and very little (< 2%) in the
faeces. Fourteen urinary metabolites were identified which represented
93-96% of the total recovered label. The major group of metabolites
(about 60% of the total label) was composed of the three phenols
(phenol fenthion, phenol sulfoxide, and phenol sulfone) and their
glucuronide, sulfoxide, and sulfone conjugates. Four demethyl
metabolites were also identified, accounting for about 30% of the
label, while the oxygen analogue sulfoxide constituted only 1-4%. A
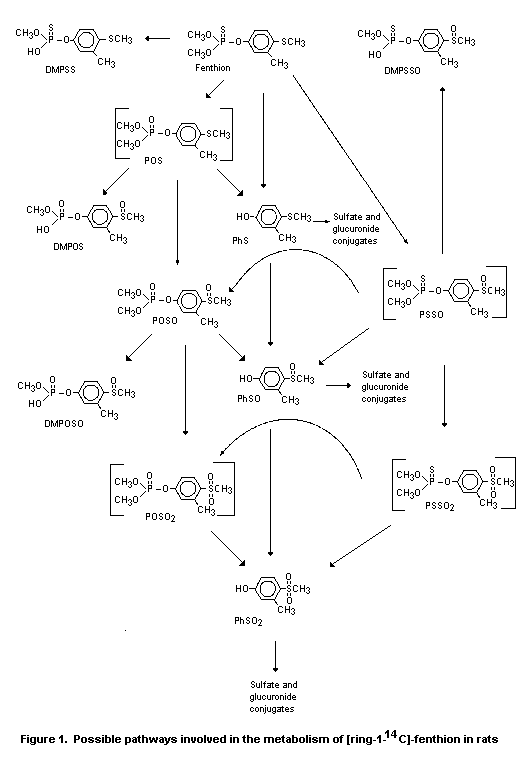
metabolic pathway for fenthion in rats was proposed on the basis of
these results (Figure 1) (Puhl & Hurley, 1982; Doolittle & Bates,
1993).
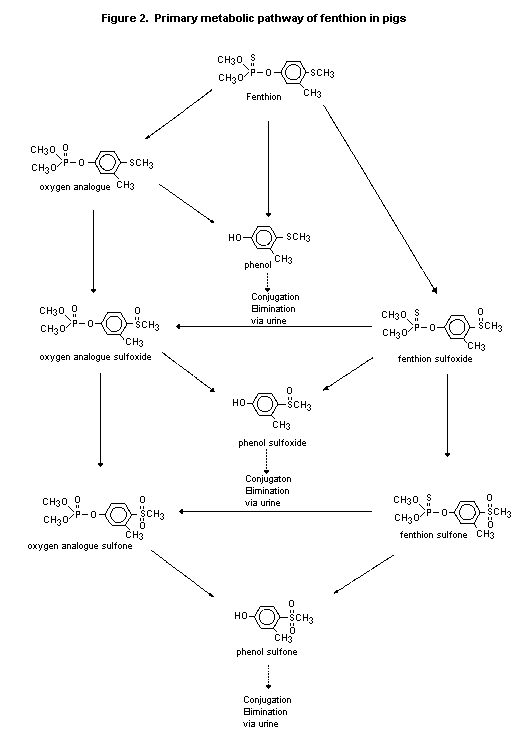
In a study of pigs given a single oral dose of 5 mg/kg bw
14C-labelled fenthion (purity, 99%), followed seven days later by two
or three consecutive daily oral doses of 10 mg/kg bw, the main urinary
metabolites were conjugated phenols (phenol fenthion, phenol sulfoxide
and phenol sulfone). It was proposed that the primary route of
metabolism in the pig is oxidation of the thiomethyl and thiophosphate
moieties to form fenthion sulfoxide and sulfone and oxygen analogue
metabolites, which are then hydrolysed at the P-O-phenyl bond to yield
the corresponding phenols. The phenols are conjugated before
elimination in urine (Figure 2) (Pither, 1979).
One male pig (Yorkshire cross-breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 97.6%) at 14.4 mg/kg bw active
ingredient, prepared as a formulation, and was sacrificed 18 h after
treatment. Analysis of tissue residues showed that unchanged fenthion
was the major component, accounting for > 96% of the residue in all
samples (hair, skin, and subcutaneous fat) collected from the
application site, 69-88% in liver, peritoneal fat, and muscle, and 26%
in kidney. Other minor residue components were fenthion sulfoxide (in
peritoneal fat and muscle; 11-12% of residue) and fenthion sulfone (in
kidney; 7% of residue). An unidentified polar metabolite was found in
liver and kidney, representing 30 and 67%, respectively, of the total
fenthion residues. A number of unknown polar metabolites were also
found in the liver and kidney, representing 30 and 67% of the total
residues, respectively, two of which were identified by high-
performance liquid chromatography in a later study (Krautter, 1990b)
as glucuronide conjugates of phenol sulfoxide and phenol sulfone, the
primary metabolites of fenthion (Crosby et al., 1990).
 Two lactating cows received 32p-fenthion as a single dermal dose
of about 13 mg/kg bw or a single intramuscular dose of about 8.5 mg/kg
bw. Unchanged fenthion constituted > 50% of the nonionic residues in
the milk for three days after the dermal and seven days after the
intramuscular treatment. In urine, > 95% of the radiolabel excreted
was in the form of hydrolytic products; the parent compound accounted
for only a small percentage of the chloroform-soluble residues. In
faeces, unchanged fenthion constituted > 50% of the acetonitrile-
soluble residues. The animals were slaughtered 14 days after the
dermal and 21 days after the intramuscular treatment; > 50% of the
tissue residues appeared as unchanged fenthion, but oxidation products
were also present (Knowles & Arthur, 1966).
One lactating dairy cow (Jersey breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 98.2%) at 5.08 mg/kg bw active
ingredient, as a formulation for treatment of lice and horn flies in
beef and non-lactating dairy cattle, and sacrificed 18 h after
treatment. Urine, milk, skin, and selected tissues were then analysed
for 14C-fenthion residues. Unchanged (parent) fenthion was the major
tissue residue, accounting for > 95% of the residues in all tissue
samples collected from the application site, 71-95% in liver, muscle,
and peritoneal fat, and 51% in kidney. Other minor residue components
identified included fenthion sulfoxide (muscle, 5% of residue) and
fenthion sulfone (liver, 8% of residue). In addition, a number of
unknown polar metabolites were found in the liver and kidney,
representing 22 and 44%, of the total residue, respectively, two of
which were identified by high-performance liquid chromatography in a
later study (Krautter, 1990b) as glucuronide conjugates of phenol
sulfoxide and phenol sulfone, the primary metabolites of fenthion
(Krautter, 1990a).
A single lactating goat received [phenyl-1-14C]-fenthion
(chemical and radiopurity, > 99%) by gavage at 20 mg/kg bw daily for
three consecutive days and was sacrificed 3.5 h after the last dose.
The nature of the residues was determined in urine, milk, and edible
tissues. Phenol fenthion, phenol sulfoxide, and phenol sulfone were
the three major metabolites in all tissues examined. Various
intermediate metabolites in the biodegradation from parent fenthion to
the phenols (demethylated phosphorus-containing compounds with varying
oxidation at the methylsulfur moiety and/or at the phosphoric acid
moiety of the molecule) were also identified. Demethyl fenoxon
sulfoxide and sulfone were especially predominant in muscle, while the
sulfoxide and sulfone of fenthion constituted about 30% of the
residues in fat. Unchanged fenthion was not detected in any tissue
sample. The metabolites excreted in urine were similar to those in the
liver, exception that a higher percentage of phenol sulfide was
present in the urine (Weber & Ecker, 1992).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion are
summarized in Table 1. Technical-grade fenthion is moderately toxic to
mammals (mice, rats, guinea-pigs, and rabbits) when given by oral,
intraperitoneal, dermal, or inhalation routes. It is highly toxic to
arian species (especially to the wild mallard duck) when administered
orally. The clinical symptoms of acute toxicity are consistent with
those of central and peripheral cholinergic intoxication by
organophosphorus esters, including decreased spontaneous activity,
apathy, dyspnoea, ataxia, tremors, convulsions, lacrimation,
salivation, and diarrhoea. The symptoms were reversible in surviving
animals.
(b) Short-term toxicity
Exposure to fenthion by inhalation for 6 h/day daily for nine
days at 0.21 mg/litre (initial concentration in a static inhalation
chamber) resulted in signs of poisoning but no mortality, in rats,
guinea-pigs, rabbits, or cats. Serum and erythrocyte cholinesterase
activities were severely depressed but recovered within three weeks
(Klimmer, 1963).
Mice
Groups of 10 male and 10 female B6C3F1 mice were given E 1752
technical (purity, 98.7%) in the diet at 0 (control diet), 150, 200,
or 250 ppm (equal to 0, 83.2, 117.2, and 140.3 mg/kg bw per day in
males and 0, 83.6, 114.5, and 151.8 mg/kg bw per day in females) for
one month. No NOAEL was seen At the lowest dose, emaciation, tremor,
and decreased body weight were observed in males, reduced water
consumption and decreased spleen weight in animals of each sex, and
reduced liver weight in females. Severe inhibition (68-80%) of brain
and erythrocyte acetylcholinesterase was noted in all animals. At the
two higher doses, additional treatment-related effects seen were a
moribund condition resulting in unscheduled sacrifice of two males per
dose group and emaciation and decreased body weight in females (Leser,
1990).
Rats
Five groups of 22 male and 22 female rats were fed diets
containing technical-grade fenthion (purity not given, in soya bean
oil) at 0, 0.25, 0.5, 2.5, or 5.0 mg/kg bw per day for three months.
The NOAEL was 0.25 mg/kg bw per day, based on depression of
cholinesterase activity in erythrocytes, plasma, heart, liver, and
brain (no raw data) at > 0.5 mg/kg bw per day. At this dose and
above, clinical signs of cholinergic intoxication and significant
Table 1. Acute toxicity of fenthion
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Mouse Male Oral 150 Francis & Barnes (1963)
Female 190
Male Oral 227 Dubois (1968)
Female 225
Male Intraperitoneal 125 Dubois & Kinoshita (1964)
Female 150
Female Intraperitoneal 200-2404 Budreau & Singh (1973a)
Rat Male Oral 175-470 Klimmer (1963); Francis & Barnes (1963);
Female 245-310 Gaines (1969); Dubois & Kinoshita (1964)
Male Oral 405 Eigenberg (1987a)
Female 566
Male Intraperitoneal 260 Dubois & Kinoshita (1964)
Female 325
Male Intraperitoneal 152 Klimmer (1963)
Male Intraperitoneal 330 Krotlinger (1993)
Female 203
Male Dermal 330-650 Klimmer (1963); Francis & Barnes (1963);
Gaines (1969); Dubois & Kinoshita (1964)
Female 330-500
Male Dermal 1680 Mihail (1978)
Female 2830
Male Dermal 586 Bomann (1991)
Female 800
Male, female Inhalation (1-h) > 1.197 Thyssen (1978)
Male Inhalation (4-h) 1.2 Thyssen (1978)
Female 0.8
Male Inhalation (1-h) 1.838 Shiotsuka (1987a)
Female 1.637
Table 1. (con't)
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Male Inhalation (4-h) 0.919 Shiotsuka (1987a)
Female 0.819
Male Inhalation (4-h) 0.507 Shiotsuka (1987b)
Female 0.454
Guinea-pig Male Oral > 1000 Francis & Barnes (1963)
Male Oral 260 Dubois & Kinoshita (1964)
Male Intraperitoneal 310 Dubois & Kinoshita (1964)
Rabbit Male Oral 150-175 Francis & Barnes (1963)
Male Dermal 150 Klimmer (1963)
Male Dermal 150 Lamb & Anderson (1974)
Female 131
Male, female Dermal 667 Eigenberg (1987b)
Chicken NR NR 310 Dubois & Doull (1960)
Male Oral 28 Sherman & Ross (1961)
Female Oral 30-40 Francis & Barnes (1963)
Female Oral 27 Flucke (1986a)
Male Oral 37.5 Singh et al. (1987)
Female Dermal 222 Flucke (1986b)
Duck NR Oral 15 Dubois & Doull (1960)
NR Oral 1-2 Keith & Mulla (1966)
NR, not reported
reductions in food consumption and body weight were evident. At the
two highest doses, decreased spermatogenesis and atrophic prostate
glands were noted in males; the female reproductive system was not
affected. All females at the highest dose died during the second and
third weeks of treatment (Shimamoto & Hattori, 1969).
Six groups of 12 male and 12 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0.2, 3, 5,
25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5.0 mg/kg
bw per day, for 16 weeks. No treatment-related adverse effects were
observed on body weight or body-weight gain or on food consumption;
gross and microscopic examination of tissues also showed no effect,
The NOAEL was 0.25 mg/kg bw per day based on cholinesterase depression
(source of enzyme not specified) at 1.25 mg/kg bw per day (Doull
et al., 1961a).
Six groups of 25 male and 25 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 3,
5, 25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5 mg/kg
bw per day, for one year. Significant inhibition (> 20%) of
erythrocyte acetylcholinesterase activity was noted at > 5 ppm.
Brain acetylcholinesterase activity was significantly depressed
(> 10%) and a higher mortality rate (particularly among males) was
observed at 25 ppm and above. At the highest dose, increased
haemosiderosis was noted in the spleen. The NOAEL was 0.25 mg/kg bw
per day, based on significant inhibition of brain acetylcholinesterase
at higher doses (Doull et al., 1963a),
Four groups of 10 male and 10 female Wistar rats were exposed to
an aerosol of technical-grade fenthion (purity, 98.2%) at nominal
concentrations of 0, 0.003, 0.015, or 0.075 mg/litre, equal to
analytical concentrations of 0, 0.001, 0.003, or 0.016 mg/litre, in a
head-/nose-only inhalation chamber for 6 h/day, five days per week for
three weeks. Exposure did not induce mortality or changes in body
weight, the results of haematology, clinical chemistry, or urinalysis,
gross pathology, or organ weights. At analytical concentrations
> 0.003 mg/litre, clinical symptoms of behavioural disturbance
(inactivity and/or unpreened coats) were observed in females only;
plasma cholinesterase was depressed in animals of each sex.
Significant inhibition of erythrocyte and brain acetylcholinesterase
activities occurred at the highest dose, and treatment-induced
inflammatory changes of the respiratory tract were seen in females.
The NOAEL was 0.001 mg/litre (analytical), based on clinical signs of
intoxication in females at 0.003 mg/litre and depression of brain and
erythrocyte acetylcholinesterase activities at 0.016 mg/litre
(Thyssen, 1979).
Rabbits
Groups of six male and six female New Zealand white rabbits
received dermal applications of technical-grade fenthion (purity,
98.2%) in Cremophos EL (1.5% v/v in distilled water) on intact (three
animals) or abraded (three animals) skin sites at 0, 5, or 25 mg/kg bw
per day for 7 h each day, five days per week for three weeks. The
NOAEL was 5 mg/kg bw per day, based on inhibition of erythrocyte
acetylcholinesterase in animals of each sex at 25 mg/kg bw per day. No
treatment-related effect was observed on general appearance,
behaviour, or body weight or by haematology, clinical chemistry,
urinalysis, gross pathology, organ weight measurement, or
histopathology (Mihail & Schilde, 1979).
Groups of five male and five female HRA:(NZW) specific pathogen-
free rabbits received dermal applications of technical-grade fenthion
(purity, 96.9%) formulated in Cremophos EL (1.5% v/v in distilled
water) on intact skin sites at 0, 5, 50, or 100 mg/kg bw per day for
6 h each day, five days per week for three weeks. The NOAEL was
50 mg/kg bw per day, based on inhibition of brain acetylcholinesterase
in females at 100 mg/kg bw per day. No treatment-related effect was
observed on appearance, behaviour, or body weight, or by haematology,
clinical chemistry, urinalysis, gross pathology, organ weight
measurements, or histopathology (Bailey, 1987).
Groups of five male and five female HRA:(NZW) specific
pathogen-free rabbits received dermal applications of technical-grade
fenthion (purity, 96.9%) formulated in Cremophos EL (1.5% v/v in
distilled water) on intact skin sites at 0, 200, or 400 mg/kg bw per
day for 6 h each day, five days per week for three weeks. The study
was terminated within two weeks of the start of treatment owing to
high mortality. An additional group of five male and five female
rabbits was given a lower dose of 150 mg/kg bw per day under similar
conditions; the original control group was retained. This treatment
did not affect general appearance, behaviour, body weight, or food
consumption, or the results of haematology, clinical chemistry, organ
weight measurement, or gross or histopathology. Plasma, erythrocyte,
and brain cholinesterase activities were depressed in animals of each
sex, with a significant inhibition of > 50% in males and 30% in
females at termination of the study. The LOAEL was 150 mg/kg bw per
day, based on inhibition of brain and erythrocyte acetylcholinesterase
activities (Bailey, 1988).
Dogs
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, or 1.25 mg/kg bw per day) for
12 weeks. The NOAEL was 0.125 mg/kg bw per day, based on significant
(> 20%) inhibition of erythrocyte acetylcholinesterase activity at
50 ppm. At > 5 ppm, plasma cholinesterase was also depressed, but
the effect (an indication of exposure) was not considered to be
toxicologically significant (Doull et al., 1961b).
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, and 1.25 mg/kg bw per day)
for one year. The NOAEL was 0.125 mg/kg bw per day, based on
significant inhibition of erythrocyte (> 20%) and brain (> 10%)
acetylcholinesterase activity at 50 ppm. At > 5 ppm, plasma
cholinesterase was also depressed, but the effect (an indication of
exposure) was not considered to be toxicologically significant. A
slight increase in spleen weight with splenic congestion,
extramedullary haematopoiesis, and haemosiderosis, was also observed
in all treated animals; the effect was not dose-related and not
considered to be adverse (Doull et al., 1963b).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade fenthion (purity, 97%) at 0, 2, 10, or
50 ppm, equal to 0, 0.06, 0.26, or 1.23 mg/kg bw per day in males and
0, 0.06, 0.26, or 1.18 mg/kg bw per day in females, for one year. The
NOAEL was 0.06 mg/kg bw per day, based on toxicologically significant
inhibition (> 10%) of brain acetylcholinesterase activity at 10 ppm
At the highest dose, both erythrocyte and brain acetylcholinesterase
activities were significantly depressed (> 50 and > 30%,
respectively). Plasma cholinesterase activity was inhibited at all
doses tested; but the effect (an indicator of exposure) was not
considered to be toxicologically significant. No other treatment-
related change was observed (Christenson, 1990a).
Groups of four male and four female pure-bred dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 3, 10,
or 30 (weeks 0-64), 50 (weeks 65-67), or 60 (weeks 68-104) ppm (equal
to 0, 0.09, 0.32, and 1.28 mg/kg bw per day in animals of each sex)
for two years. No mortality or clinical sign of toxicity was seen
during the study. No significant treatment-related change in body
weight, ophthalmoscopic, haematological, or urinary parameters, gross
or histopathology of tissues was evident at any dose. The NOAEL was
0.09 mg/kg bw per day, based on significant inhibition of erythrocyte
acetylcholinesterase activity in males (> 20%) and of brain
acetylcholinesterase activity in females (> 10%) at 10 ppm. At the
highest dose, erythrocyte and brain acetylcholinesterase activities
were significantly depressed in animals of each sex. Decreased food
consumption (females) and lowered plasma protein levels (males) were
also observed (Hoffmann & Weischer, 1975).
Monkeys
Four groups of five male and five female rhesus monkeys were
given daily doses of technical-grade fenthion (purity, 98.1%) in corn
oil by gavage at concentrations of 0, 0.02, 0.07, or 0.20 mg/kg bw per
day for two years. Animals were observed daily for general appearance
and clinical signs; body weight and ophthalmological parameters were
recorded monthly, and clinical chemistry, haematology, and urinalyses
were performed at 0, 1, 3, 6, 12, 18, and 23 months. Plasma and
erythrocyte cholinesterase activities were measured weekly for the
first four weeks and monthly thereafter. One animal of each sex at 0
and 0.20 mg/kg bw per day was killed seven months and three weeks
after the beginning of treatment for measurement of brain
acetylcholinesterase and gross and histopathology. All monkeys
underwent necropsy after 23 months, but no histopathology was
performed. Plasma cholinesterase was consistently depressed in animals
of each sex at 0.20 mg/kg bw per day, but the effect (an indicator of
exposure) was not considered to be toxicologically significant. A
significant (> 20%) inhibition of erythrocyte acetylcholinesterase
activity was observed occasionally in animals of each sex (9/26
determinations over the 23-month study period for males and 2/26 for
females) at the highest dose. No effect on brain acetylcholinesterase
was evident in the four animals killed at 7.25 months. Gross
examination revealed abnormally small testes and ovaries in animals at
0.20 mg/kg bw per day; however, in the absence of organ weights and
histopathological data, the toxicological significance of this finding
could not be fully assessed. No other treatment-related adverse
effect was recorded. The NOAEL was 0.07 mg/kg bw per day, based on
inconsistent erythrocyte acetylcholinesterase depression and possible
effects on male and female reproductive organs at 0.20 mg/kg bw per
day (Rosenblum, 1980).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of male and female B6C3F1 mice, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) in the diet at concentrations of 0,10,
or 20 ppm (equivalent to 0, 1.5, or 3.0 mg/kg bw per day) for 103
weeks. All surviving animals were maintained on control diet for up to
one week after the end of treatment before they were sacrificed and
subjected to gross and microscopic examination. Treatment with
fenthion did not result in significant differences in the survival or
growth rate of mice at any dose. Clinical signs of intoxication were
evident four months after the beginning of treatment (LOAEL not
specified). At the end of the study (104 weeks), histopathological
examination revealed a variety of neoplasms, the majority of which
were judged to be unrelated to fenthion administration. An increased
incidence of sarcomas, fibrosarcomas, or rhabdomyosarcomas of the
integumentary system was observed in treated male mice; the incidences
of integumentary sarcomas were 0/25 at 0 ppm, 7/49 at 10 ppm, and 8/48
at 20 ppm (7/435 in historical controls). The effect was considered to
be treatment-related, indicating that technical-grade fenthion is
oncogenic in male mice under the conditions of the study. There was no
NOAEL for systemic toxicity, since the LOAEL for clinical signs was
not specified (US National Cancer Institute, 1979).
Groups of 60 male and 60 female specific-pathogen-free B6C3F1
mice were fed diets containing technical-grade fenthion (purity,
92.3%) at 0, 0.1, 1.0, 5.0, or 25 ppm, equal to 0, 0.03, 0.40, 1.95,
or 9.42 mg/kg bw per day in males and 0, 0.03, 0.47, 2.25, or
10.63 mg/kg bw per day in females, for 102 weeks. Additional groups of
20 mice of each sex were fed the same diets for 50 weeks before
scheduled interim kills. Brain acetylcholinesterase activity was
depressed by > 10% at > 0.1 ppm in males and at > 1.0 ppm in
females, but the effect was not dose-related and was seen in males at
terminal but not interim sacrifice and in females at interim but not
terminal sacrifice. Significant (> 20%) inhibition of erythrocyte
acetylcholinesterase activity occurred only in the males at 25 ppm at
week 54. As the inhibition of brain acetylcholinesterase was only
marginally significant (10-17%) at the three lower doses, there was no
dose-response relationship, and the effect was inconsistent over
treatment intervals, it was judged to be of minimal toxicological
significance at doses > 5.0 ppm. The NOAEL for systemic toxicity
was 1.95 mg/kg bw per day, as brain acetylcholinesterase activity was
clearly depressed at the next highest dose in males at both interim
and terminal sacrifice (29-32%) and in females at interim sacrifice
(26%). At this dose, there were also significant increases in body
weight in animals of each sex, elevated plasma cholesterol levels in
animals of each sex early in the study, and elevated absolute and
relative liver weights of male mice at termination. In the absence of
remarkable histopathological findings, the changes at 25 ppm were
considered not to be toxicologically significant. There was no
increased incidence of any neoplastic or non-neoplastic lesion in the
treated mice at any dose up to and including 9.42 mg/kg bw per day.
Technical-grade fenthion was thus considered not to be oncogenic to
mice under the conditions of the study (Suberg & Leser, 1990).
The observation of an increased incidence of integumentary
sarcomas in male mice (US National Cancer Institute, 1979) at doses of
1.5 and 3.0 mg/kg bw per day was not reproduced in a second study
(Suberg & Leser, 1990) in which doses up to 9.42 mg/kg bw per day were
tested. As the increased tumour incidence in the first study was not
dose-related and was site-and sex-specific and the results of the
second study (conducted with more control animals and more groups for
interim sacrifice) were clearly negative at doses up to three times
those of the first study, technical-grade fenthion would appear not to
be oncogenic in the mouse.
Rats
Groups of male and female Fischer 344 rats, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) at dietary concentrations of 0, 10, or
20 ppm (equivalent to 0, 0.5, or 1.0 mg/kg bw per day) for 103 weeks.
All surviving animals were maintained for one or two weeks after the
end of treatment and were then sacrificed and submitted to gross and
microscopic examination. No treatment-related mortality or behavioural
changes were observed, and the growth rate was comparable in control
and treated groups. Histopathological examination revealed a variety
of neoplastic and non-neoplastic lesions common to rats of this
strain. No increase in the incidence of any type of tumour was
observed in the treated rats at any dose up to and including 20 ppm
(1.0 mg/kg bw per day). Technical-grade fenthion was thus considered
not to be oncogenic to the rat under the conditions of the study (US
National Cancer Institute, 1979).
Groups of male and female specific-pathogen-free Wistar rats, 100
of each sex in the control group and 50 of each sex per dose, were fed
technical-grade fenthion (purity unspecified) in the diet at
concentrations of 0, 3, 15, or 75 ppm, equal to 0, 0.14, 0.72, or
374 mg/kg bw per day in males and 0, 0.19, 0.93, or 4.46 mg/kg bw per
day in females, for 24 months. The rats were weighed weekly during the
first 26 weeks and every two weeks thereafter. Food consumption was
recorded weekly. Haematology, clinical chemistry, liver and kidney
funtional tests, and urinalysis were performed on five rats of each
sex per group at 1, 3, 6, and 12 months, and on 10 rats of each sex
per group at the end of the study (24 months). Plasma and erythrocyte
cholinesterase activities were determined at the end of weeks 1, 2, 4,
8, 13, 26, 52, 78, and 105; brain acetylcholinesterase activity was
not measured. At the end of the study, all surviving rats were
sacrificed and examined macroscopically, and tissues and organs were
removed, weighed, and studied microscopically. Doses of 3 or 15 ppm
did not affect physical appearance, behaviour, growth, or survival
rate, but males at the highest dose had a significantly lower body
weight and both males and females appeared to have increased mortally.
No treatment-related effect was observed by haematology, clinical
chemistry, urinalysis, or gross or microscopic pathology. (It should
be noted that the rats from which blood and urine samples were taken
for analysis during the first year were not identified.) Significant,
dose-dependent depression of plasma and erythrocyte cholinesterase
activities was observed in animals of each sex at 15 and 75 ppm. The
NOAEL for systemic toxicity, based on erythrocyte acetylcholinesterase
inhibition, was 0.14 mg/kg bw per day. The results of histopathology
showed no increase in the incidence of tumours in treated rats at any
dose up to and including 3.74 mg/kg bw per day, the highest dose
tested, indicating that technical-grade fenthion was not oncogenic to
the rat under the conditions of the study (Bomhard & Loser, 1977).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenthion (purity, 97.0%) at concentrations
of 0, 5, 20, or 100 ppm, equal to 0, 0.2, 0.8, or 5.2 mg/kg bw per day
in males and 0, 0.3, 1.3, or 7.3 mg/kg bw per day in females, for two
years. Two additional groups of 20 rats of each sex were fed diets
containing fenthion at concentrations of 0 or 100 ppm for one year
before scheduled interim sacrifice. No NOAEL for systemic toxicity
could be determined, because of statistically (P < 0.05) and
toxicologically significant (> 10%), dose-related inhibition of brain
acetylcholinesterase activity in animals of each sex at all doses,
including the lowest dose of 0.20 mg/kg bw per day. Plasma and
erythrocyte cholinesterase activities were also depressed at all
doses, but toxicologically significant (> 20%) inhibition of
erythrocyte acetylcholinesterase occurred only at > 20 ppm. At the
highest dose, technical-grade fenthion was retinotoxic, especially in
females, and ophthalmological examination revealed an increased
incidence of retinal degeneration and posterior subcapsular cataract
formation in females and focal corneal scarring in animals of each
sex. Histopathological examination revealed a higher incidence of
corneal mineralization and retinal atrophy in females, and corneal
neovascularization and optic nerve atrophy in animals of each sex. The
retinotoxic effect was further confirmed by electroretinographic
examination, with suppression of electroretinograms also in a number
of females at 20 ppm at interim sacrifice at week 75. At 100 ppm,
additional treatment-related effects were an increased incidence of
nonspecific clinical signs (urine staining, tail and paw lesions,
rough coat, and hunched back), lower body-weight gain, and remarkable
histopathological findings including vacuolar degeneration of the
nasolacrimal ducts, mineralized and raised areas in the stomach,
stomach acanthosis, hyperkeratosis (males) and hyperplasia (females),
epididymal vacuolar degeneration, and chronic active inflammation of
the skin of the tail and hindlimbs. A higher incidence of vacuolar
degeneration of the nasolacrimal ducts was also seen in females at
20 ppm. There were no treatment-related oncogenic effects in males or
females at any dose up to and including 5.2 mg/kg bw per day, the
highest dose tested. Technical-grade fenthion was thus considered not
to be oncogenic to the rat under the conditions of the study
(Christenson, 1990b).
(d) Reproductive toxicity
Mice
In a five-generation, two-litter per generation study, two groups
of CD-1 mice received technical-grade fenthion (purity unspecified) in
their drinking-water at concentrations of 0 or 60 ppm (equivalent to
9.0 mg/kg bw per day). The control group comprised 10 males and 14
females, and the treated group comprised 10 males and 22 females.
There was a significant increase in pup mortality during the first
postnatal week, especially in the second, third, and fourth
generations. No treatment-related effect on reproductive performance,
lactation, or growth rate of pups was observed. No histopathological
changes were noted in the liver or kidney of adult males of the third
generation (Budreau & Singh, 1973b).
Rats
In a two-generation, one-litter per generation study in Crl:CD BR
rats, five groups of 30 male and 30 female animals were fed diets
containing technical-grade fenthion (purity, 96.9%) at concentrations
of 0, 1, 2, 14, or 100 ppm, equal to 0, 0.08, 0.16, 1.16, or 8.3 mg/kg
bw per day (based on premating food intake values), for 70 days (F0
and F1 parents) before mating (1:1 ratio) for production of the F1
and F2 litters. No treatment-related effect on mortality, clinical
signs of toxicity, mean body weight, mean food consumption, or gross
pathology was observed in adults of the F0 or F1 generation.
Epididymal weight was increased in F0 males at 100 ppm.
Histopathological examination revealed vacuolation in the lining
ductal epithelial cells of the corpus epididymus in a few F0 and F1
males at 14 ppm and in all at 100 ppm. Brain acetylcholinesterase
activity was significantly (> 10%) depressed in F0 females at all
doses and in F0 males and F1 males and females at the two higher
doses. Inhibition at the two lower doses (-22 and -17%, respectively)
in F0 females was not dose-related and was of a magnitude similar
to those at 14 ppm in F0 males (-19%) and in F1 males (-28%)
and females (-18%). F0 female controls had a mean brain
acetylcholinesterase activity of 2428 mU/g, which was significantly
higher than the other control values observed in the study (2137,
1894, and 1515 mU/g for F0 male, F1 male, and F1 female controls,
respectively). The effect on brain acetylcholinesterase activity seen
in F0 females at 1 and 2 ppm was therefore judged to be of minimal
toxicological significance. At the two higher doses, significant
(> 20%) inhibition of plasma and erythrocyte cholinesterase
activity was seen in all parental adults and pups. Neonatal brain
acetylcholinesterase activity was inhibited only at the highest dose.
The NOAEL for maternal toxicity was 0.16 mg/kg bw per day, based on
consistent inhibition of brain and erythrocyte acetylcholinesterase
activity at 14 ppm. There was no treatment-related effect on
reproductive performance, embryo- or fetotoxicity, litter size, pup
viability, or growth at > 14 ppm. At the highest dose, decreased
fertility, a decreased number of implantation sites per dam, decreased
litter size, an increased number of stillborn pups per litter, a
reduced viability index (days 0-4), and decreased pup body-weight gain
during lactation (days 14 and 21) were observed in both F0 and F1
generations and F1 and F2 litters. The NOAEL for reproductive
toxicity was thus 1.16 mg/kg bw per day (Kowalski et al., 1989).
(e) Developmental toxicity
Rats
Four groups of 20 mated FB30 Long-Evans female rats were given
technical-grade fenthion (purity, 98.1%) in aqueous Cremophor EL
emulsion by gavage at 0, 1, 3, or 10 mg/kg bw per day once daily on
days 6-15 of gestation; the day semen was detected in a vaginal smear
was designated day 0 of gestation. On gestation day 20, all surviving
dams were sacrificed, and the fetuses were delivered by caesarean
section and necropsied. The numbers of implantation sites and of live,
dead, and resorbed fetuses, litter weight, fetal body weight, and
placental weight were recorded, and all fetuses were examined for
external, visceral, and skeletal abnormalities. No treatment-related
mortality, clinical symptoms of maternal toxicity, or disturbance of
intrauterine development were noted at any dose. All fetuses delivered
developed normally. No signs of fetotoxicity were seen, and no
treatment-related malformations were observed in any of the fetuses at
any dose up to and including 10 mg/kg bw per day. The NOAEL for
maternal toxicity, embryo- and fetotoxicity, and teratogenicity was
10 mg/kg bw per day, the highest dose tested (Machemer, 1978a).
Groups of 33 mated Crl:CD BR female rats were given technical-
grade fenthion (purity, 96.5%) in 5% aqueous Emulphor solution by
gavage at doses of 0 (vehicle control), 1, 4.2, or 18 mg/kg bw per day
once daily on days 6-15 of gestation; the day spermatozoa were
detected in a vaginal smear was designated as day 0 of gestation. Five
animals from each group were sacrificed on day 16, and the remaining
28 dams in each group were killed on day 20 of gestation. All dams
were necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. No NOAEL for maternal toxicity
could be determined owing to dose-related, toxicologically
significant inhibition of brain (> 10%) and erythrocyte (> 20%)
acetylcholinesterase activities at all doses tested, including the
lowest dose of 1 mg/kg bw per day, on days 16 and 20 of gestation.
Plasma cholinesterase was also depressed at > 1 mg/kg bw per day on
day 16 and at 18 mg/kg bw per day on day 20. No other treatment-
related maternal or embro- or fetotoxicity was seen at > 4.2 mg/kg
bw per day. At the highest dose, clinical symptoms of intoxication
(dry or bloody lacrimation, exophthalmia, hypoactivity, tremors,
salivation, and urine-stained peritoneum), decreased mean bodyweight
gain, and reduced food consumption were observed. There was also a
slight increase in fetal skeletal variations, including an increased
incidence of incomplete ossification of the cervical arches, skull
bones, and third, fourth, and sixth sternebrae, and incomplete and/or
unossified metacarpals and metatarsals. These findings represent a
delay in skeletal maturation and were considered not to be adverse or
of toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in the fetuses at any dose up to and
including 18 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity
and teratogenicity was therefore 18 mg/kg bw per day, the highest dose
tested (Kowalski, 1987).
Rabbits
Groups of 17 artificially inseminated American-Dutch female
rabbits were given technical-grade fenthion (purity, 96.5%) in 5%
aqueous Emulphor solution by gavage at doses of 0 (vehicle control),
1, 2.75, or 7.5 mg/kg bw per day once daily on days 6-18 of gestation;
the day of artificial insemination was designated day 0 of gestation.
All surviving animals were sacrificed on gestation day 28 and
necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. The NOAEL for maternal toxicity
was 1 mg/kg bw per day based on toxicologically significant inhibition
of brain (> 10%) and erythrocyte (> 20%) acetylcholinesterase
activities at the two higher doses on gestation day 19 and/or 28. At
> 2.75 mg/kg bw per day, an increased incidence of soft stools was
noted both during and after treatment. At the highest dose, plasma
cholinesterase activity was depressed on gestation day 19 only. There
was also an increased incidence of unossified anterior metacarpals
among the fetuses, but this finding represents a delay in skeletal
maturation and was considered not to be an adverse effect of
toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in any of the fetuses at any dose up to
and including 7.5 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity and teratogenicity was 7.5 mg/kg bw per day, the highest
dose tested (Clemens, 1987).
(f) Genotoxicity
In an assay for dominant lethal mutation, three groups of 50 male
NMRI mice were each given a single oral dose of 0, 10, or 25 mg/kg bw
of technical-grade fenthion (purity unspecified) as a 2% aqueous
emulsion. Each male was then caged with an untreated virgin female for
four days; the same procedure was repeated for a total of 12 matings.
On days 12-16 of gestation, the females were sacrificed, and the
numbers of implantations and of live and dead implants (deciduomata,
resorption sites, and dead embryos) were counted. The high dose
resulted in overt clinical signs of toxicity in males (drowsiness,
ruffled coat, and dilated intestines). Except for increased
preimplantation loss at 25 mg/kg bw in the first two mating periods,
no other treatment-related effects on reproductive performance
(fertility, pre- and post-implantation loss) were observed (Machemer,
1978b).
A battery of studies was conducted with technical-grade fenthion
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were mostly negative; weakly positive results were obtained
in two of five assays for sister chromatid exchange, in one of two
assays for micronucleus formation (at a dose of 150 mg/kg bw), and in
one assay for unscheduled DNA synthesis. Summary results from the US
Environmental Protection Agency Health Effects Research Laboratory
(Simmon et al., 1977) indicated that technical-grade fenthion does
not induce reverse mutation in Salmonella typhimurium TA98, TA100,
TA1535, TA1537, or TA1538 or Escherichia coli WP2 uvrA; enhanced
mitotic recombination in yeast ( Saccharomyces cerevisiae D3);
unscheduled DNA synthesis in human fibroblasts (WI-38 cells);
preferential toxicity (DNA repair-proficient/deficient) in E. coli
(W3110, p3478) or Bacillus subtilis (H17, M45); or sex-linked
recessive lethality in Drosophila melanogaster in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenthion (purity 97-99%) was not irritating to
the skin and was minimally or not irritating to the eyes of New
Zealand white rabbits (Pauluhn, 1985; Eigenberg, 1987c,d).
Technical-grade fenthion (purity 98.5%) did not induce dermal
sensitization in a maximization test (according to the method of
Magnusson and Kligman) in male DHPW guinea-pigs (Flucke, 1987)
(ii) Potentiation
Fenthion potentiated the acute intraperitoneal toxicity of
malathion, dioxathion, and coumaphos in rats, but intraperitoneal
administration of 13 other organophosphate or carbamate insecticides
to rats in combination with fenthion did not result in greater than
additive toxic effects (Dubois & Kinoshita, 1964). Dietary combination
of equitoxic doses (2 mg/kg bw) of fenthion with coumaphos, neither of
which alone affected cholinesterase activity when fed to dogs for six
weeks, was found to potentiate the anticholinesterase activity of
serum and erythrocytes by 75 and 30%, respectively. The potentiation
was less evident with malathion, and no potentiation was noted when
fenthion was fed in combination with dioxathion (Doull et al.,
1962). Oral administration to rats of a single dose of either a 3:1
mixture of fenthion and dichlorvos or a 1:3 mixture of fenthion and
isofenthion did not result in a greater than the theoretical additive
toxic effect (Kimmerle, 1967; Heimann, 1982). In several recent
studies, pretreatment with fenthion significantly potentiated the
acute toxicity of 2- sec-butylphenyl N-methylcarbamate in mice and
dogs. Its acute toxicity in mice was increased 15-fold by a 1-h oral
pretreatment with 30 mg/kg bw fenthion (one-tenth of the LD50).
Table 2. Results of tests for the genotoxicity of technical-grade fenthion
End-point Test system Concentration Results Reference
or dose
IN vitro
Reverse mutation S. typhimurium TA98, 0, 20-12 500 µg/plate Negative Herbold (1987)
TA100, TA1535, TA1537 0, 750-12 000 µg/plate
Reverse mutation S. typhimurium TA98, 0, 8-5000 µg/plate Negative Herbold (1990a)
TA100, TA1535, TA1537
hprt forward mutation Chinese hamster ovary 0, 20.0-100 µg/ml Negative Lehn (1990a)
cells 0, 12.5-75 µg/ml
Chromosomal aberration Chinese hamster ovary 0. 0.02-0.15 µg/ml Negativea Putman & Morris
cells (1989)
Chromosomal aberration Chinese hamster lung cells 0, 23.5-94 µg/ml Negativea Kajiwara (1989)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml Negative at < 20b,c Chen et al.
(1982a)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml (in diffusion Weakly positive Chen et al. (1982b)
chambers) at > 40b
Sister chromatid exchange Human lymphoid cell line 0, 0.02-20 µg/ml (unactivated) Negativea,b Sobti et al.
(1982)
LAZ-007 (of B-cell origin) 0, 20 µg/ml (activated)
Chromosomal aberration, Human lymphocytes 0, 0.5-5.0 µg/ml Weakly positive Rani & Rao (1991)
sister chromatid exchange at > 1.5
Unscheduled DNA Rat primary hepatocytes 0, 10-100 µg/ml Weakly positive Lehn (1990b)
synthesis 0, 5-30 µg/ml at > 5
Table 2. (con't)
End-point Test system Concentration Results Reference
or dose
In vivo
Sister chromatid exchange Rat bone-marrow 0, 10-100 mg/kg bw Negative Bai et al (1990)
lymphoid cells
Micronucleus formation Bor:NMRI mouse bone 0, 150 mg/kg bw Weakly positive Herbold (1990b)
marrow at 150b
a With and without exogenous metabolic activation
b Not confirmed in an independent assay
c Threshold of cytotoxicity
Inhibition of the metabolism or detoxification of 2- sec-butylphenyl
N-methylcarbamate by desulfuration of fenthion may have been
responsible, at least in part, for the synergistic effect (Miyaoka
et al., 1984, 1986, 1987).
(iii) Cholinesterase inhibition
Twelve groups of 10 tasted male Fischer 344 (CDF/Crl/Br) rats
were given single doses of technical-grade fenthion (purity, 97.5%)
orally, dermally, or subcutaneously at 0, 1, 5, or 25 mg/kg bw, and
the effect of the route of administration on plasma, erythrocyte,
and/or brain cholinesterase activity was studied for up to 14 days
after treatment. The NOAEL for all routes of administration was
5 mg/kg bw, based on toxicologically significant inhibition of
erythrocyte (> 20%) and brain (> 10%) acetylcholinesterase
activities at 25 mg/kg bw. Inhibition of plasma and erythrocyte
cholinesterase activity was maximal at 24 h after oral administration
and four days after dermal or subcutaneous administration, suggesting
more efficient absorption by the oral route. On day 14 after
treatment, depression of brain acetylcholinesterase activity was
greatest in the group that were treated subcutaneously; the prolonged
recovery may be associated with a slower release of fenthion into the
circulation when given by this route. The magnitude of cholinesterase
inhibition appeared to be only marginally significant after dermal
administration, suggesting that absorption is less effective by this
route. The rates of absorption were thus oral route > subcutaneous
route > dermal route (Christenson, 1990c).
(iv) Delayed neurotoxicity
Acute delayed neurotoxicity in hens: Groups of 15
atropine-protected, adult laying hens (Lohmann selected Leghorn
strain), five to seven months old, were given two doses of fenthion
(E 1752; purity, 98.5%) in 2% Cremophor EL by gavage at 40 mg/kg bw or
dermally at 200 mg/kg bw, 21 days apart. A positive control group of
five hens was given tri- ortho-cresyl phosphate (purity, 99.1%)
orally as a single dose of 375 mg/kg bw. Six hens given an oral dose
of 5 ml/kg bw 2% Cremophor EL and six untreated hens served as
negative controls. All animals were observed for 21 days after the
second dose before sacrifice, with the exception of the tri- ortho-
cresyl phosphate-treated hens which were killed on day 22 in moribund
condition. At termination, peripheral and central nervous tissues were
removed, fixed, and stained for microscopic examination. Clinical
signs of acute cholinergic intoxication were evident in all
fenthion-treated hens 7-24 h after treatment; recovery occurred within
7-8 days after oral administration and 14-18 days after dermal
application. No clinical symptoms (irreversible impairment of motor
coordination) or neurohistopathological lesions indicative of delayed
neuropathy were observed. The positive controls exhibited symptoms of
delayed nerurotoxicity (abnormal gait, ataxia, and paresis) from day 7
after treatment, which persisted until sacrifice on day 22;
neurohistopathological lesions characteristic of delayed neuropathy
were also evident (Flucke & Kaliner, 1987).
Neuropathy target esterase activity: Groups of nine atropine-
protected, adult hens (Lohmann selected Leghorn strain) were given a
single oral dose of technical-grade fenthion (E 1752; purity, 98.5%)
in 2% Cremophor EL by intubation at doses of 0 (vehicle control) or
40 mg/kg bw. Nine positive controls received tri- ortho-cresyl
phosphate as a single oral dose of 100 mg/kg bw. Neuropathy target
esterase activity in brain and spinal cord was inhibited by 0-14% over
that in vehicle controls 24 and 48 h and seven days after treatment.
Severe inhibition (> 50-90%) was seen in the positive controls
throughout the study (Flucke, 1988a).
Groups of nine atropine-protected adult hens (Lohmann selected
Leghorn strain) were given a single dermal dose of technical-grade
fenthion (E 1752; purity, 98.5%) in 2% Cremophor EL at doses of 0
(vehicle control), 200, or 400 mg/kg bw. A positive control group of
three to nine hens received tri- ortho-cresyl phosphate as a single
dose of 100 mg/kg bw by intubation. Neuropathy target esterase
activity in brain and spinal cord was inhibited by 0-20% over that in
vehicle controls at 24, 48, and 72 h and up to seven days after
treatment. Severe inhibition (> 80%) was seen in the positive
controls 24 and 48 h after treatment, demonstrating the sensitivity of
the test (Flucke, 1988b)
Short-term delayed neurotoxicity in hens: Groups of 10 adult
white Leghorn hens (Gallus gallus) were given technical-grade
fenthion (purity, 96.5%) by gavage in clear corn oil solution at doses
of 0, 1, 2, or 4 mg/kg bw per day for 14 weeks. Tri- ortho-cresyl
phosphate (10-60 mg/kg bw per day) was given as a positive control. No
treatment-related clinical symptoms or increases in the incidence
and/or severity of histopathological lesions of nervous tissues
characteristic of organophosphate-induced delayed neurotoxicity were
evident in the fenthion-treated hens at up to and including the
highest dose. Clinical signs of decreased activity and ataxia were
noted, but predominantly in the first hours after treatment; they were
no longer seen before the next dose, suggesting that they were likely
to have been caused by repetitive, severe acute cholinergic
intoxication and were not related to delayed neurotoxicity. The
positive controls had both toxic symptoms and histopathological
lesions of the nervous tissues typical of delayed neurotoxicity.
Histopathological examination revealed a dose-related increase in the
incidence of muscular hypertrophy or hyperplasia in all muscle layers
of the oesophagus, crop, proventriculus, gizzard, and intestine of all
fenthion-treated hens and glandular and nonglandular epithelial
hyperplasia in the oesophagus, crop, and proventriculus of some at the
middle dose and in most at the high dose. These lesions were probably
due to localized acetylcholinesterase inhibition, with subsequent
overstimulated muscle hypertrophy (Hayes & Ramm, 1988).
A second short-term study, in which technical-grade fenthion was
fed to hens was conducted to determine if the muscular hypertrophy and
hyperplasia observed by Hayes and Ramm (1988) were a direct effect of
fenthion or were due to the route of treatment. Two groups of 10 adult
white Leghorn hens (Gallus gallus domesticus) were given technical-
grade fenthion (purity, 96.9%) at dietary concentrations of 0 or
52 ppm (equivalent to 4 mg/kg bw per day) for 90 days. Minimal to
moderate muscular hypertrophy or hyperplasia was seen in the distal
oesophagus (between the crop and the proventriculus) of all treated
hens, which accounted for 98-99% of the increased thickness (+ 55%, in
comparison with controls) in the oesophageal wall. Hypertrophy or
hyperplasia of the oesophageal glandular components was also seen in
four birds. In addition, treated hens had a statistically significant
(P < 0.05) depression of cholinesterase activity in whole blood
(> 50% in comparison with controls) and tissue from all three
regions of the upper gastrointestinal tract (oesophagus, crop, and
proventriculus: about 70% in comparison with controls). It was
concluded that the muscular hypertrophy and hyperplasia observed
in the fenthion-treated hens was probably due to localized
acetylcholinesterase inhibition with subsequent overstimulation of the
oesophageal smooth muscle layers (Hayes, 1989).
Assessment of the neurotoxicity of fenthion in the literature:
A position paper (Flucke, 1990) which presented and discussed nine
publications dealing with the assessment of neurotoxicity of fenthion
was submitted. The main findings and critical evaluations are
summarized below.
1. In the series of four studies by Farage-Elawar & Francis (1987,
1988a,b; Francis & Farage-Elawar, 1987), the effects of fenthion,
debromoleptophos (which induces organophosphate-induced delayed
neurotoxicity), and fenitrothion (which does not) were compared
in very young chicks. On the basis of the finding that fenthion
produced neurotoxic signs (altered gait) and inhibition (> 50%)
of cholinesterase but little or no inhibition of neuropathy
target esterase, it was postulated that its neurotoxic potential
is not classical organophosphate-induced delayed neurotoxicity. A
critical evaluation of the four publications (by Flucke, 1990,
and the reviewer) revealed that the three organophosphates were
not administered in equitoxic doses, thus rendering direct
comparison of their toxic effects inappropriate, and that the
test animals were malnourished. Under the study conditions, there
was probably prolonged acute cholinergic intoxication due to
severe inhibition of cholinesterases after the lethal or
sublethal doses of debromoleptophos and fenthion, in contrast to
the low dose of fenitrothion (about one-fifth of the LD50),
which would not have induced overt cholinergic symptoms. A
build-up of acetylcholine at the motor end-plates could result in
pronounced, prolonged muscle fasciculation and, secondarily, in
the known necrotizing effects. Young chicks undergoing rapid
growth and muscle development are less likely to compensate
quickly and completely for this type of muscle damage, especially
if they are undernourished. Accordingly, the neurotoxic effects,
which occurred to the same degree with debromoleptophos and
fenthion, could be attributed to primary effects of severe acute
cholinergic intoxication and not to a particular (unknown)
neurotoxic potential of fenthion, as postulated by the authors.
2. Tuler et al. (1988) and Dellinger & Mostrom (1988) examined the
neurotoxic effects of fenthion in dogs. In the first study, the
authors concluded that there was no evidence of delayed
neurotoxicity. Prolonged weekly treatment with fenthion caused
hyperreflexia, proprioceptive deficits, progressive muscle fibre
necrosis, ultrastructural changes in nerve axons, and loss of
small motor units. Fenthion-induced primary neuropathy leading to
secondary myopathy was proposed. In the second study, the authors
concluded that there was no evidence of cholinergic effects and
no effect on vagal tone during the period of fenthion treatment
when maximal inhibition (> 50%) of acetylcholinesterase activity
was measured; however, on day 14 after treatment, the treated
dogs gave a slightly smaller response to an atropine challenge
(in comparison with controls), suggesting possible down-
regulation of the cholinergic receptors.
A critical evaluation of these two publications (by Flucke,
1990 and the reviewer) revealed the following: (i) The fenthion-
treated dogs had severe organophosphate intoxication and that the
clinical symptoms of muscle fasciculation, ataxia, hyperreflexia,
proprioceptive deficits, and hindquarter weakness were likely to
be the result of neuromuscular overstimulation due to severe
acetylcholinesterase inhibition. Reversibility or recovery was
seen in most of the dogs when the dose of fenthion was reduced
from 44 to 22 mg/kg bw or omitted for one week, suggesting the
neuropathy was not irreversible. (ii) Progressive muscle libre
necrosis was evident in the distal parts of the sciatic nerves of
fenthion-treated dogs, with reorganization of the sensory and
motor innervations. As primary impairment of the peripheral
nerves does not result in muscle necrosis but rather in atrophy
of muscle fibres, the results were judged not to be indicative of
a primary neuropathic effect. In addition, receptor down-
regulation can be considered a physiological adaptation process
to maintain internal equilibrium, serving as a protective
mechanism by which the organism normalizes functions that are
impaired by the intake of pharmacodynamic substances.
Accordingly, the small changes noted in the vagal tone of dogs
after chronic administration of fenthion probably represented an
adaptive change and are. not indicative of irreversible
impairment of the neuromuscular junctions. Thus, the studies, of
Tuler et al. (1988) and Dellinger & Mostrom (1988) do not give
evidence of a particular neurotoxic effect of fenthion in dogs.
3. Tuler & Bowen (1989) investigated the neurotoxic effects of
fenthion, paraoxon (an organophosphate), and neostigmine (a
non-organophosphate) on chick embryo nerve cell growth and
ultrastructure in culture. Altered cell membrane integrity and
increased cytoplasmic lipid (vacuole) accumulation were observed
in cells treated with fenthion or paraoxon but not in those
treated with neostigmine. The authors concluded that
organophosphates had direct effects on neuronal cells in culture;
however, the following points should be considered: (i)
Pharmacokinetics plays a special role in intoxication by
organophosphates, in particular thiophosphates, and in
detoxification, both of which occur rapidly and simultaneously.
Fenthion, a thiophosphate, must be converted in vivo to the
active oxygen analogue and would therefore be ineffective as an
inhibitor of esterases in vitro. (ii) The observed effects on
the cell membrane and the cell were not specific to neuronal
cells and can therefore not be compared directly with neurotoxic
effects in vivo. It is therefore concluded that the results of
the study reveal no particular neurotoxic potential of fenthion
in vivo.
4. Misra et al. (1985, 1988) examined clinical and biochemical
changes and neuromuscular function in workers chronically exposed
to fenthion. No clinical signs of peripheral neuropathy or
myopathy and no pathophysiological findings indicative of
irreversible neurological deficits were seen in workers exposed
regularly and repeatedly to fenthion for a mean of 8.5 years.
They showed symptoms typical of acute cholinergic intoxication
and significant inhibition of serum cholinesterase. It is
concluded that the study provides no evidence of a particular or
delayed neurotoxic potential of fenthion in the workers examined.
The literature thus provides no evidence of a particular or
delayed neurotoxic potential but indicates that exposure to fenthion
results in typical organophosphate-induced cholinergic toxicity due to
severe acetylcholinesterase inhibition.
3. Studies of metabolites
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion
metabolites are summarized in Table 3.
Table 3. Acute toxicity of fenthion metabolites
Metabolite LD50 (mg/kg bw) IC50 (mol; 50% inhibition of human
erythrocyte acetylcholinesterase)c
Orala Intraperitonealb
Fenthion 220 325 > 5.0 × 10-4
Fenthion sulfoxide 125 250 4.5 × 10-5
Fenthion sulfone 125 250 4.7 × 10-4
Fenthion oxygen analogue 125 26 2.7 × 10-6
Fenthion O-sulfoxide 50 22 4.8 × 10-5
Fenthion O-sulfone 30 9 3.2 × 10-5
4-(Methylthio)-m-cresol 6500d
4-Methyl(thiosulfoxide)-m-cresol 3500d
4-Methyl(thiosulfone)-m-cresol 7000d
a In male rats (Francis & Barnes, 1963)
b In female rats (Dubois & Kinoshita, 1964)
c Francis & Barnes (1963)
d In female rats (Nelson, 1967)
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR mice were fed diets
containing fenthion sulfoxide (Baycid SO; purity not given) at
concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to 0,
0.45, 1.5, 4.5, 15, or 45 mg/kg bw per day, for four weeks, followed
by a four-week recovery period. Plasma cholinesterase activity was
depressed in animals of each sex at > 3 ppm, but the effect (an
indication of exposure) was not considered to be toxicologically
significant. Brain acetylcholinesterase activity was significantly
inhibited in males at > 3 ppm and in females at > 10 ppm. At the
two highest doses, decreased body weight was noted in animals of each
sex; at the highest dose, tremor was seen in all mice. All of the
treatment-related effects (including cholinesterase depression)
disappeared rapidly during the recovery period. There was no NOAEL.
The LOAEL, based on significant brain acetylcholinesterase inhibition
in males, was 0.45 mg/kg bw per day, the lowest dose tested (Inukai &
Iyatomi, 1981a).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing fenthion sulfoxide (Baycid SO; purity not given)
at concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to
0, 0.15, 0.5, 1.5, 5, or 15 mg/kg bw per day, for four weeks,
followed by a four-week recovery period. Plasma cholinesterase
activity was depressed in females at > 3 ppm, but the effect
(an indication of exposure) was considered not to be toxicologically
significant. No treatment-related adverse effect was observed at
3 ppm. At > 10 ppm, significant, dose-related depression of
acetylcholinesterase activity in brain was seen in animals of each sex
and in erythrocytes in males after four weeks of treatment; in
females, erythrocyte acetylcholinesterase activity was inhibited at
> 30 ppm. At the two highest doses, decreased body weight and food
consumption and increased alkaline phosphatase activity were noted. At
the highest dose, tremor was seen in all rats. All of the treatment-
related effects (including cholinesterase depression) disappeared
rapidly during the recovery period. The NOAEL was 0.15 mg/kg bw per
day, based on significant inhibition of brain (> 10%) and erythrocyte
(> 20%) acetylcholinesterase activities at 0.5 mg/kg bw per day
(Inukai & Iyatomi, 1981b).
4. Observations in humans
Fenthion has been used widely in many parts of the world for
control of e.g. household pests and mosquitos. Studies of individuals
in areas treated for malaria eradication have shown slight plasma
cholinesterase depression when heavy spray schedules were used. The
levels were depressed for up to six weeks after spraying (Elliot &
Barnes, 1963). Children under the age of seven were more susceptible
to the anticholinesterase effect than adults, but no inhibition of
erythrocyte cholinesterase was observed and there were no significant
alterations in normal physiological functions in any individual
examined (Taylor, 1963).
The signs of acute poisoning appear rapidly, beginning with
blurred vision, unsteady gait, and slurred speech. In one reported
case, a man who had taken an unknown quantity of fenthion still
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation after 72 h of emergency
treatment. The patient began to recover only after 11 days of
treatment with antidotes which included atropine, pralidoxime
chloride, and toxogonin (Dean et al., 1967). Another man remained in
a comatose state for 45 min after ingesting a fenthion formulation,
with pale skin, cyanotic mucous membranes, a slow, regular heart beat,
low peripheral blood pressure, and no reactions to pain or light on
the pupils. Recovery took six days (von Clarmann & Geldmacher-von
Mallinkrodt, 1966). A third man, who ingested about 60 g of a fenthion
formulation (Entex), recovered from severe organophosphorous poisoning
after being in a critical condition for the first six days. Recovery
was slow, lasting up to 30 days, and blood cholinesterase activity was
still depressed 22 days after poisoning (Pickering, 1966).
The potential dermal and respiratory exposure of workers to
fenthion during field application by hand-gun power spray equipment,
back-pack hand pressure sprayers, and hand granular dispersal for
mosquito control was studied during two work seasons. Workers exposed
to 3.6-12.3 mg/h dermally (equivalent to 0.5-1.5 mg/kg bw per day) or
< 0.02-0.09 mg/h by inhalation (equivalent to 0.01-0.05 mg/kg bw per
day) had decreased plasma but not erythrocyte cholinesterase activity
(Fytizas-Danielidou, 1971; Wolfe et al., 1974). In two studies of
clinical and biochemical changes, nerve conduction velocity, and
neuromuscular function in 22-24 workers (mean age, 31-32 years)
exposed regularly and repeatedly to fenthion for a mean of 8.2-8.5
years, no clinical signs of peripheral neuropathy or myopathy and
no pathophysiological findings indicative of any irreversible
neurological deficits were seen. The workers showed symptoms typical
of acute cholinergic intoxication and inhibition of serum
cholinesterase, but there was no evidence of delayed neurotoxicity
(Misra et al., 1985, 1988).
In a study of 150 cases of poisoning by anticholinesterase
insecticides, 32 of the patients had consumed fenthion, 48
fenitrothion, and 50 malathion; 20 did not know which agent they had
consumed. Paralytic signs were significantly more frequent after
fenthion than malathion or fenitrothion poisoning (81.2, 30, and 23%,
respectively), and they appeared later and lasted longer. Death
occurred significantly more often after fenthion poisoning, with
mortality rates of 35.5% with fenthion, 4% with malathion, and 2.1%
with fenitrothion. Pulmonary oedema was most common after malathion
poisoning and was not encountered with fenthion. Inhibition of
cholinesterase was most marked after fenthion poisoning, and the
enzyme activity was inhibited by 100% in 18 of the 27 cases studied
(Wadia et al., 1977).
In three groups of four male volunteers given technical-grade
fenthion (purity, 98.1%) in corn oil in gelatin capsules at doses of
0, 0.02, or 0.07 mg/kg bw per day for up to four weeks, no physical or
clinical signs of intoxication were reported, and no abnormal results
were seen by haematology or urinalysis. At 0.07 mg/kg bw per day,
significant depression (> 20%) of plasma cholinesterase was observed,
but no treatment-related effects on erythrocyte acetylcholinesterase
or on other clinical chemistry parameters were observed. The NOAEL was
0.07 mg/kg bw per day, based on the absence of inhibition of
erythrocyte acetylcholinesterase (Griffin et al., 1979).
Comments
A single dose of 14C-radiolabelled fenthion was readily absorbed
and rapidly excreted in the urine and faeces of rats, and no 14C was
detectable in the expired carbon dioxide. About 90% of the radiolabel
was eliminated within 48 h of dosing. The excretion profiles were
similar regardless of sex, dose, or route of administration (oral or
intravenous). Urine was the main route of elimination (> 90% of the
total radiolabel), and only minor amounts were recovered in the
faeces. Little 14C was retained in the tissues, suggesting that
fenthion does not accumulate in the body of rats.
14C-Fenthion was extensively metabolized in rats. No unchanged
parent compound was detected in the urine and very little (< 2%) in
the faeces. The major group of metabolites (accounting for about 60%
of the total recovered 14C) comprised three phenols (fenthion phenol
[4-methylthio- meta-cresol] and its sulfoxide and sulfone) and their
glucuronide and sulfate conjugates. Four demethyl metabolites
(accounting for about 30% of the recovered radiolabel) and the
sulfoxide of fenthion oxon (constituting 1-4%) were also identified.
Fenthion is moderately toxic (LD50 = 50-500 mg/kg body
weight) to mice, rats, guinea-pigs, and rabbits when given orally,
intraperitoneally, dermally, or by inhalation. It is highly toxic to
avian species (especially to the wild mallard duck) when given
orally. The LC50 in rats for a 4-h exposure by inhalation was
0.5-0.9 mg/titre. Fenthion caused cholinergic toxicity with a long
recovery time. It did not irritate rabbit skin and was minimally or
not irritating to the rabbit eye; it did not sensitize guinea-pig
skin. WHO has classified fenthion as highly hazardous.
Fenthion potentiated the acute toxicity of other cholinergic
chemicals, such as malathion, dioxathion, and coumaphos, in rats. In
mice and dogs, pretreatment with fenthion inhibited the metabolism and
detoxification of 2- sec-butylphenyl methylcarbamate, resulting in
significant potentiation of its acute toxicity. There was no evidence
that other cholinergic chemicals potentiate the toxicity of fenthion.
Repeated short-term administration of fenthion (orally to mice,
rats, dogs and monkeys; dermally to rabbits; by inhalation to rats)
and its metabolite fenthion sulfoxide (orally to mice and rats),
resulted primarily in inhibition of cholinesterase. The NOAEL for oral
administration of fenthion, based on toxicologically significant
depression of acetylcholinesterase activity in the brain (> 10%)
and/or erythrocytes (> 20%), was 5 ppm (equivalent to 0.25 mg/kg bw
per day) in rats treated for three months or one year, 3 ppm (equal to
0.09 mg/kg bw per day) in dogs treated for two years, and 0.07 mg/kg
bw per day in monkeys treated for two years The NOAEL for fenthion was
50 mg/kg bw per day when applied dermally to rabbits for 21 days on
the basis of inhibition of brain acetylcholinesterase activity. The
NOAEL for fenthion in rats exposed by inhalation for 21 days was
0.001 mg/litre, on the basis of clinical signs of cholinergic toxicity
and inhibition of brain acetylcholinesterase at higher doses. The
NOAEL for fenthion sulfoxide administered orally to rats for four
weeks was 3 ppm (equivalent to 0.15 mg/kg bw per day) on the basis of
significant inhibition of brain and erythrocyte acetylcholinesterase
activity. No NOAEL was determined for fenthion or its metabolites in
mice; the LOAEL for fenthion was 150 ppm (equal to 83 mg/kg bw per
day) in mice exposed in the diet for four weeks, and the LOAEL for
fenthion sulfoxide in mice similarly exposed was 3 ppm (equivalent to
0.45 mg/kg bw per day).
In a carcinogenicity study, fenthion was administered in the diet
to mice at 0, 0.1, 1, 5 or 25 ppm for 102 weeks. The NOAEL for chronic
toxicity was 5 ppm (equal to 2 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. There was no evidence of carcinogenicity. In
two studies of chronic toxicity and carcinogenicity, rats received
fenthion at dietary concentrations of 0, 3, 15, or 75 ppm or 0, 5,
20, or 100 ppm for 24 months. The NOAEL for chronic toxicity
was 3 ppm (equal to 0.14 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. The compound was toxic to the eyes of rats at
100 ppm (equal to 5.2 mg/kg bw per day), inducing an increased
incidence of retinal atrophy, posterior subcapsular cataract
formation, corneal mineralization, and mineralization and optic nerve
atrophy, especially in females. No ocular toxicity was seen at doses
> 20 ppm (equal to 0.8 mg/kg bw per day). There was no evidence of
carcinogenicity.
In a two-generation study of reproductive toxicity (one litter
per generation), rats were fed diets containing fenthion at levels of
0, 1, 2, 14, or 100 ppm. The NOAEL for systemic toxicity in the parent
generation was 2 ppm (equal to 0.16 mg/kg bw per day) on the basis of
consistent inhibition of brain and erythrocyte acetylcholinesterase.
The NOAEL for reproductive toxicity was 14 ppm (equal to 1.2 mg/kg bw
per day) on the basis of decreased fertility, implantation sites,
litter size, pup viability, and growth at 100 ppm.
Two studies of developmental toxicity were performed in which
rats were exposed by gavage to fenthion at doses of 0, 1, 3, or
10 mg/kg bw per day or 0, 1, 4.2, or 18 mg/kg bw per day on days 6-15
of gestation. There was no NOAEL for maternal toxicity, owing to
toxicologically significant inhibition of brain and erythrocyte
acetylcholinesterase activity at doses > 1 mg/kg bw per day. the
NOAEL for embryo- and fetotoxicity and teratogenicity was 18 mg/kg bw
per day, the highest dose tested.
In a study of developmental toxicity in rabbits, fenthion was
administered by gavage at doses of 0, 1, 2.8, or 7.5 mg/kg bw per day
on days 6-18 of gestation. The NOAEL for maternal toxicity was 1 mg/kg
bw per day, on the basis of toxicologically significant inhibition of
brain and erythrocyte acetylcholinesterase activity at > 2.8 mg/kg
bw per day. The NOAEL for embryo- and fetotoxicity and teratogenicity
was 7.5 mg/kg bw per day, the highest dose tested.
Fenthion has been adequately tested for genotoxicity in a range
of assays in vivo and in vitro. While most showed no significant
response, positive results were obtained in two critical assays. The
Meeting concluded that fenthion is weakly genotoxic.
Fenthion did not cause delayed neuropathy in hens at doses higher
than the LD50.
In a four-week study of male volunteers, the NOAEL was 0.07 mg/kg
bw per day, the highest dose tested, on the basis of no inhibition of
erythrocyte acetylcholinesterase. In two investigations of workers
regularly exposed to fenthion, no evidence of neurotoxicity was
observed.
An ADI of 0-0.007 mg/kg bw was established on the basis of the
NOAEL of 0.07 mg/kg bw per day in the four-week study of human
volunteers, using a safety factor of 10. The ADI provides a margin of
safety of > 100-fold for chronic ocular toxicity and for the
reproductive toxicity observed in rodents.
The available data did not allow the Meeting to establish an
acute reference dose different from the ADI (0-0007 mg/kg bw). It
should be noted, however, that the ADI is derived from a study
of human volunteers in which 9-36% inhibition of plasma cholinesterase
but no inhibition of erythrocyte acetylcholinesterase was found at
the highest dose tested (0.07 mg/kg bw per day for 25 days). In
occupationally exposed workers, about 50% plasma cholinesterase
inhibition was found in the absence of erythrocyte acetyl-
cholinesterase inhibition. It follows that the acute reference
dose is likely to be somewhat higher than the ADI. Data on the
sensitivity to inhibition of plasma cholinesterase and erythrocyte and
brain acetylcholinesterase in vitro by the active metabolites of
fenthion might allow extrapolation to an LOAEL for humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 2.0 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equal to 0.14 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
2 ppm, equal to 0.16 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
14 ppm, equal to 1.2 mg/kg bw per day (two-generation study
of reproductive toxicity)
18 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Rabbit: 6 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
7.5 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 3 ppm, equal to 0.09 mg/kg bw per day (two-year study of
toxicity)
Monkey: 0.07 mg/kg bw per day (two-year study of toxicity)
Human: 0.07 mg/kg bw per day (four-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenthion
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Minimally or not irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Inhalation, 4-h, toxicity, rat LC50 = 0.5--0.9 mg/litre
Oral, toxicity, mouse, rat, guinea-pig, rabbit LD50 = 50-500 mg/kg bw
Oral, dermal, subcutaneous, single doses, NOAEL (all routes) = 5 mg/kg bw per day
cholinesterase activity, rat based on inhibition of brain and
erythrocyte acetylcholinesterase
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 50 mg/kg bw per day for
systemic toxicity on the basis of inhibition
of brain acetylcholinesterase
Repeated inhalation, 21 days, toxicity rat NOAEL = 0.001 mg/litre per day for
systemic toxicity on the basis of clinical
signs of cholinergic toxicity
Repeated dietary, 13-16 weeks, toxicity, rat NOAEL = 0.25 mg/kg bw per day, based
on inhibition of brain acetylcholinesterase
Repeated gavage, developmental toxicity, NOAEL = 1 mg/kg bw per day for
rabbit maternal toxicity on the basis of inhibition
of brain and erythrocyte acetylcholinesterase;
no embryo- or fetotoxic or teratogenic effects
Repeated oral (gelatin capsules), four weeks, NOAEL = 0.07 mg/kg bw per day on the
toxicity, human basis of no inhibition of erythrocyte
acetylcholinesterase
Long-term (> 1 year) Repeated dietary, two years, toxicity, dog NOAEL = 0.09 mg/kg bw per day on the
basis of inhibition of brain and erythrocyte
acetylcholinesterase
References
Avrahami, M. & White, D.A. (1975) Residues in milk of cows after
spot-treatment with 32P-fenthion. New Zealand Exp. Agric., 3,
309-311.
Bai, C.L., Qiao, C., Zhang, W., Chen, Y. & Qu, S. (1990) A study of
the pesticide fenthion: Toxicity, mutagenicity and influence on
tissue enzymes. Biomed. Environ. Sci., 3, 262-275.
Bailey, D.E. (1987) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 938 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bailey, D.E. (1988) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 1031 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bomann, W. (1991) E 1752 (c.n. Fenthion): Study for acute dermal
toxicity in the rat. Unpublished report No. 20221 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Bomhard, E. & Loser, E. (1977) Chronic toxicity study on rats.
Unpublished report No. 6769 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Brady U.E., Jr & Arthur, B.W. (1961) Metabolism of
O,O-dimethyl- O-[4-(methylthio)- m-tolyl] phosphorothioate
by white rats. J. Econ. Entomol., 54, 1232-1236.
Budreau, C.H. & Singh, R.P. (1973a) Teratogenicity and embryotoxicity
of demeton and fenthion in CF1 mouse embryos. Toxicol. Appl.
Pharm., 24, 324-332.
Budreau, C.H. & Singh, R.P. (1973b) Effect of fenthion and dimethoate
on reproduction in the mouse. Toxicol. Appl. Pharmacol., 26,
29-38.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982a) Sister-chromatid
exchanges and cell-cycle delay in Chinese hamster V79 cells
treated with nine organophosphorus compounds (8 pesticides and 1
defoliant). Mutat. Res., 103, 307-313.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982b) Sister-chromatid
exchanges in Chinese hamster cells treated with 17
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990a) Chronic feeding toxicity study of
technical-grade fenthion (Baytex) with dogs. Unpublished report
No. 94863 from Miles, Inc., Agriculture Division, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Christenson, W.R. (1990b) Combined chronic toxicity/oncogenicity study
of technical grade fenthion (Baytex) with rats. Unpublished
report No. 100586 from Mobay Corporation, Corporate Toxicology
Department, Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Christenson, W.R. (1990c) Technical grade fenthion (Baytex): A special
study to examine the effect of the route of acute administration
of technical grade fenthion (Baytex) on cholinesterase activity
in the rat. Unpublished report No. 100573 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
von Clarmann, M. & Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and
its demonstration in the gastric contents and urine. Arch.
Toxikol., 22, 2-11.
Clemens, G.R. (1987) A teratology study in the rabbit with fenthion
(Baytex technical). Unpublished report No. MTD0039 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Crosby, J., Hoglen, N. & Krautter, G. (1990) Nature of residues in
skin and tissues of swine after dermal treatment with
[14C]fenthion. Unpublished Mobay report No. 73984 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dean, G., Coxon, J. & Brereton, D. (1967) Poisoning by an
organophosphorus compound -- A case report. S. Afr. Med. J.,
41, 1017-1019.
Dellinger, J. & Mostrom, M. (1988) Effects of topical fenthion on
blood cholinesterase and vagal tone in dogs. Vet. Hum.
Toxicol., 30, 2,29-234.
Doolittle, K.D. & Bates, N.L (1993) Absorption, distribution and
elimination of 14C-fenthion in rats following a single oral,
repeated oral and single intravenous administration. Unpublished
Miles report No. 74395 from Southwest Bio-Labs, Inc., New Mexico,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. &
Meskauskas, J. (1961a) The effects of feeding diets containing
Bayer 29493 to rats for a period of 16 weeks. Unpublished rayer
Report No. 7899 from Department of Pharmacology, University of
Chicago, Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Doull J., Root, M. & Cowan, J. (1961b) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished Bayer report
No. 8342 from Department of Pharmacology, University of Chicago,
Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M. & Cowan, J. (1962) Effect of adding Bayer 29493 in
combination with other cholinergic insecticides to the diet of
male and female dogs. Unpublished Bayer report No. 9414 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, N.J., Vesselinovitch, D., Fitch, F.M. &
Meskauskas, J. (1963a) Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished Bayer report No. 14658 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, J. & Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
Bayer report No. 10853 from Department of Pharmacology,
University of Chicago, Illinois, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Dubois, K.P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished Mobay report No. 22503
from Department of Pharmacology, University of Chicago, Illinois,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Doull, J. (1960) The acute toxicity of Bayer 29493 to
chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago, Illinois, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Kino-hita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-dimethyl- O-4-
(methylthio- m-tolyl) phosphorotioate (DMTP; Baytex) and
related compounds. Toxicol. Appl. Pharmacol., 6, 86-95.
Eigenberg, D.A. (1987a) Acute oral toxicity of Baytex technical in
albino rats. Unpublished report No. 839 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987b) Acute dermal toxicity of Baytex technical in
albino rabbits. Unpublished report No. 838 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987c) Primary dermal irritation of Baytex technical
in albino rabbits. Unpublished report No. 896 from Mobay
Corporation, Corporate Toxicology Department, Kansas. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987d) Primary eye irritation of Baytex technical in
albino rabbits. Unpublished report No. 817 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Elliot, R. & Barnes, J.M. (1963) Organophosphorus insecticides for the
control of mosquitos in Nigeria. Bull. World Health Org., 28,
35-54.
Farage-Elawar, M. & Francis, B.M.(1987) Acute and delayed effects of
fenthion in young chicks. J. Toxicol. Environ. Health, 21,
455-469.
Farage-Elawar, M. & Francis, B.M. (1988a) Effects of multiple dosing
of fenthion, fentrothion, and desbromoleptophos in young chicks.
J. Toxicol. Environ. Health, 23, 217-228.
Farage-Elawar, M. & Francis, B.M. (1988b) Effects of fenthion,
fentrothion, and desbromoleptophos on gait, acetylcholinesterase
and neurotoxic esterase in young chicks after in ovo exposure.
Toxicology, 49, 253-261.
Flucke, W. (1986a) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute oral toxicity to hens
(Gallus domesticus). Unpublished report No. 15069 from Bayer
AG, Institut für Toxikologie, Wuppertal Germany.
Flucke, W (1986b) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute dermal toxicity to hens
(Gallus domesticus). Unpublished report No. 15113 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1987) E 1753 Technical (c.n fenthion): Study for skin
sensitising effect on guinea pigs (Magnusson and Kligman's
maximization test). Unpublished report No. 15428 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1988a) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following oral administration to hens.
Unpublished report No. 99275 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1988b) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following dermal administration to
hens. Unpublished report No. 99646 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1990) Position paper regarding the literature on
neurotoxicity for the BGA [German Federal Health Agency].
Unpublished report No. 18928 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. & Kaliner, G. (1987) E 1752 (c.n. fenthion, the active
ingredient of Baytex): Acute neurotoxicity studies on hens
following oral and dermal administration. Unpublished report No.
91341 from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Francis, J.I. & Barnes, J.M. (1963) Studies on the mammalian toxicity
of fenthion. Bull. World Health Org., 28, 205-212.
Francis, B.M. & Farage-Elawar, M. (1987) Peripheral and central enzyme
inhibition by fenthion, fenitrothion and desbromoleptophos. In:
Costa, L.G., Galli, C.L. & Murphy, S.D., eds, Toxicology of
Pesticides: Experimental, Clinical and Regulatory Perspectives,
Berlin, Springer Verlag.
Fytizas-Danielidou, R. (1971) Effects of pesticides on reproduction in
white rats. I. Labaycide. In: 23rd International Symposium of
Phytopharmacy, 4 May, 1971.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Griffin, T., Rosenblum, I. & Coulston, F. (1979) Safety evaluation of
fenthion in human volunteers. Unpublished Mobay report No. 68790
from the Institute of Comparative and Human Toxicology and
International Center of Environment Safety, Albany Medical
College, New York, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Hayes, R.H. (1989) Subchronic feeding study with fenthion technical
(Baytex) in hens with specific emphasis on gastrointestinal tract
effects. Unpublished report No. 99273 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Hayes, R.H. & Ramm, W.W. (1988) Subchronic delayed neurotoxicity study
of fenthion technical (Baytex) with hens. Unpublished report No.
98296 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Heimann, K.G. (1982) Fenthion and isofenthion: Study for acute
combination toxicity Unpublished report No. 11062 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1987) E 1752 (c.n. Fenthion): Salmonella/microsome
test for point-mutagenic effect. Unpublished report No. 98366
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1990a) E 1752 Salmonella/microsome test. Unpublished
report No. 18713 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Herbold, B.A. (1990b) E 1752 micronucleus test on the mouse.
Unpublished report No. 100025 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Hoffmann, K. & Weischer, C.H. (1975) Fenthion chronic study on dogs
(two-year feeding experiment). Unpublished report No. 5737 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981a) Fensulfoxide: Short-term toxicity
tests on mice (4-week feeding and 4-week recovery tests).
Unpublished report No. 198 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981b) Fensulfoxide: Short-term toxicity
tests on rats (4-week feeding and 4-week recovery tests).
Unpublished report No. 197 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Johnson, J.C. & Bowman, M.C. (1972) Responses from cows fed diets
containing fenthion or fenitrothion. J. Dairy Sci., 55,
777-782.
Kajiwara, Y. (1989) Chromosomal aberration test of fenthion using
cultured mammalian CHL cells. Unpublished report No. T-1824E from
HITA Research Lab., Japan. Submitted to WHO by Bayer AG,
Wuppertal, Germany.
Keith, J.O. & Mulla, M.S. (1966) Relative toxicity of five
organophosphorous mosquito larvicides to mallard ducks.
J. Wildlife Manage., 30, 553-563.
Kimmerle, G. (1967) Potential of DDVP and S 1752. Unpublished letter
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Klimmer, R. (1963) Toxicological testing of Bayer 29493. Unpublished
report from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Knowles, C.O. & Arthur, B.W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of
radiolabelled fenthion to dairy cows. J. Econ. Entomol., 59,
1346-1352.
Kowalski, R.L. (1987) A teratology study with fenthion (Baytex
technical) in the rat. Unpublished report No. MTD0029 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Kowalski, R.L., Clemens, G.R., Jasty, V., Troup, CM. & Hartnagel,
R.E., Jr (1989) A two-generation reproductive study with fenthion
(Baytex) in the rat. Unpublished report No. MTD0133 from Miles
Inc., Toxicology Department, Indiana, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Krautter, G. (1990a) Nature of residues in milk, skin and tissues of a
lactating cow after dermal treatment with [14C]fenthion.
Unpublished Mobay report No. 74012 from Pharmacology and
Toxicology Research Laboratory, Kentucky, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Krautter, G. (1990b) Characterization of polar metabolites in river
and kidney tissues from a cow and pig treated dermally with
[14C] fenthion. Unpublished Mobay report No. 74124 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Krotlinger, F. (1993) E 1752 (c.n. Fenthion): Study for acute
intraperitoneal toxicity in rats. Unpublished report No. 22144
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Lamb, D.W. & Anderson, R.H. (1974) The acute dermal toxicity of
Tiguvon technical to rabbits. Unpublished report No. 41455 from
Chemagro, Bayer GmbH, Leverkusen, Germany. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Lehn, H. (1990a) E 1752 (c.n. Fenthion): Mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished report No. 18740 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Lehn, H. (1990b) E 1752 (c.n. Fenthion): Mutagenicity test on
unscheduled DNA synthesis in rat liver primary cell cultures
in vitro. Unpublished report No. 100572 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Leser, K.H. (1990) E 1752 (Fenthion): Study for cholinesterase
inhibition following high doses of E1752 (administration to
B6C3F1 mice in the diet over a period of about four weeks).
Unpublished report No. 19088 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Machemer, L. (1978a) S 1752 (Fenthion, Lebaycid active ingredient):
Evaluation for embryotoxic and teratogenic effects in orally
dosed rats. Unpublished report No. 7580 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Machemer, L. (1978b) S 1752 (Fenthion, Lebaycid active ingredient):
Dominant lethal study of male mice to test for mutagenic effects.
Unpublished report No. 7449 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Mihail, F. (1978) S 1752 (Lebaycid active ingredient): Determination
of percutaneous toxicity. Unpublished report No. 7604 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Mihail, F. & Schilde, B. (1979) S 1752 (Fenthion, the active
ingredient of Lebaycid and Baytex): Subacute dermal cumulative
toxicity on rabbits. Unpublished report No. 8624 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Misra, U.K., Nag, D., Bhushan, V. & Ray, P.K. (1985) Clinical and
biochemical changes in chronically exposed organophosphate
workers, Toxicol, Lett., 24, 187-193.
Misra, U.K.. Nag, D., Khan, W.A. & Ray, P.K. (1988) A study of nerve
conduction velocity, late responses and neuromuscular synapse
functions in organophosphate workers in India. Arch. Toxicol.,
61, 496-500.
Miyaoka, T., Takahashi, H., Tsuda, S. & Shirasu, Y. (1984)
Potentiation of acute toxicity of 2- sec-butylpheny-
N-methylcarbamate (BPMC) by fenthion in mice. Fundam. Appl.
Toxicol., 4, 802-807.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1986) Mechanism of potentiation
of BPMC toxicity by fenthion pretreatment in mice.
J. Pharmacobio-Dyn., 9, 697-703.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1987) Effect of O,O-dimethyl
O-(3-methyl-4-methylthiophenyl) phosphorothioate (fenthion)
pretreatment on acute toxicity of 2- sec-butylphenyl
N-methylcarbamate (BPMC) in dogs. Jpn. J. Vet. Sci., 49,
173-175.
Nelson, D.L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Pauluhn, J. (1985) E 1752 Technical (c.n. fenthion): Study for
irritant/corrosive effect on skin and eye (rabbit). Unpublished
report No. 13446 from Bayer AG, Institute für Toxikologie,
Wuppertal, Germany.
Pickering, E.N. (1966) Organic phosphate insecticide poisoning.
Can. J. Med. Technol., 28, 174-179.
Pither, K.M. (1979) Metabolism of Baytex in male and female pigs.
Unpublished report No. 68475 from Mobay Corporation, Corporate
Toxicology Department, Kansas, USA. Submitted to WHO by Bayer AG,
Wuppertal Germany.
Puhl, R.J. & Hurley, J.B. (1982) The absorption, excretion and
metabolism of Baytex-ring-1-14C by rats. Unpublished report No.
82227 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report No. 99660 from
Microbiological Associates Inc., Maryland, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Rani, M.V.U & Rao, M.S. (1991) In vivo effect of fenthion on human
lymphocytes. Bull. Environ. Contamin. Toxicol., 47, 316-320.
Rosenblum, I. (1980) A safety evaluation of fenthion (S 1752) in
rhesus monkeys (Macaca mulatta). Unpublished Mobay report No.
68789 from Albany Medical College, New York, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Sherman, M. & Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. Appl. Pharmacol., 3, 521-533.
Shimamoto, K. & Hattori, K. (1969) Chronic feeding of Baytex
( O,O-dimethyl- O-(4-methylmercapto-3-methyl)phenylthiophosphat
e) in rats. Acta Med. Univ. Kyoto, 40, 163-171.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Baytex in rats. Unpublished report No. 944 from
Mobay Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Shiotsuka, R.N. (1987b) Acute inhalation toxicity study with Baytex
technical in rats. Unpublished report No. 820 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1977) Evaluation of
selected pesticides as chemical mutagens: In vitro and in
vivo studies. US Environmental Protection Agency Health Effects
Research Laboratory Document No. EPA-600/1-77-028 from Stanford
Research Institute, California, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Singh, S.P., Sharma, L.D. & Bahga, H.S. (1987) Acute oral toxicity of
fenthion in poultry. Indian J. Anim. Res., 21, 48-50.
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutat. Res., 102,
89-102.
Suberg, H. & Leser, K.H. (1990) E 1752 Oncogenicity study on B6C3F1
mice (feeding study for periods of up to 24 months). Unpublished
report No. 19624 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull.
World Health Org., 28, 213-218.
Thyssen, J. (1978) S 1752 (Lebaycid-active ingredient): Acute
inhalation toxicity studies. Unpublished report No. 7842 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Thyssen, J. (1979) Fenthion (S 1752, the active ingredient of Lebaycid
and Baytex): Subacute inhalation toxicity study on rats.
Unpublished report No. 8383 from Bayer AG Institut für
Toxikologie, Wuppertal, Germany.
Tuler, S.M. & Bowen, J.M. (1989) Toxic effects of organophosphates on
nerve cell growth and ultrastructure in culture. J. Toxicol.
Environ. Health, 27, 209-223.
Tuler, S.M., Febles, D. & Bowen, J.M. (1988) Neuromuscular effects of
chronic exposure to fenthion in dogs and predictive value of
electromyography. Fundam. Appl. Toxicol., 11, 155-168.
US National Cancer Institute (1979) Bioassay of fenthion for possible
carcinogenicity (National Cancer Institute Carcinogenesis
Technical Report Series No. 103; US Department of Health,
Education, and Welfare Publication No. (NIH) 79-1353). Bioassay
conducted by Gulf S. Research Institute, Louisiana, USA, under
contract to NCI and subcontract to Tracor Jitco, Inc., who
prepared the final report.
Wadia, R.S., Bhirud, R.H., Gulavani, A.V. & Amin, R.S. (1977)
Neurological manifestations of three organophosphate poisons.
Indian J. Med. Res., 66, 460-468.
Weber, H. & Ecker, W. (1992) [phenyl-1-14C]Fenthion: Absorption,
distribution, excretion and metabolism in a lactating goat.
Unpublished report No. 3752 from Bayer AG, Institut für
Metabolismusforschung, Leverkusen-Bayerwerk, Germany. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Wolfe, H.R., Armstrong, J.F. & Durham, W.F. (1974) Exposure of
mosquito control workers to fenthion. Mosquito News, 34,
263-267.
Two lactating cows received 32p-fenthion as a single dermal dose
of about 13 mg/kg bw or a single intramuscular dose of about 8.5 mg/kg
bw. Unchanged fenthion constituted > 50% of the nonionic residues in
the milk for three days after the dermal and seven days after the
intramuscular treatment. In urine, > 95% of the radiolabel excreted
was in the form of hydrolytic products; the parent compound accounted
for only a small percentage of the chloroform-soluble residues. In
faeces, unchanged fenthion constituted > 50% of the acetonitrile-
soluble residues. The animals were slaughtered 14 days after the
dermal and 21 days after the intramuscular treatment; > 50% of the
tissue residues appeared as unchanged fenthion, but oxidation products
were also present (Knowles & Arthur, 1966).
One lactating dairy cow (Jersey breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 98.2%) at 5.08 mg/kg bw active
ingredient, as a formulation for treatment of lice and horn flies in
beef and non-lactating dairy cattle, and sacrificed 18 h after
treatment. Urine, milk, skin, and selected tissues were then analysed
for 14C-fenthion residues. Unchanged (parent) fenthion was the major
tissue residue, accounting for > 95% of the residues in all tissue
samples collected from the application site, 71-95% in liver, muscle,
and peritoneal fat, and 51% in kidney. Other minor residue components
identified included fenthion sulfoxide (muscle, 5% of residue) and
fenthion sulfone (liver, 8% of residue). In addition, a number of
unknown polar metabolites were found in the liver and kidney,
representing 22 and 44%, of the total residue, respectively, two of
which were identified by high-performance liquid chromatography in a
later study (Krautter, 1990b) as glucuronide conjugates of phenol
sulfoxide and phenol sulfone, the primary metabolites of fenthion
(Krautter, 1990a).
A single lactating goat received [phenyl-1-14C]-fenthion
(chemical and radiopurity, > 99%) by gavage at 20 mg/kg bw daily for
three consecutive days and was sacrificed 3.5 h after the last dose.
The nature of the residues was determined in urine, milk, and edible
tissues. Phenol fenthion, phenol sulfoxide, and phenol sulfone were
the three major metabolites in all tissues examined. Various
intermediate metabolites in the biodegradation from parent fenthion to
the phenols (demethylated phosphorus-containing compounds with varying
oxidation at the methylsulfur moiety and/or at the phosphoric acid
moiety of the molecule) were also identified. Demethyl fenoxon
sulfoxide and sulfone were especially predominant in muscle, while the
sulfoxide and sulfone of fenthion constituted about 30% of the
residues in fat. Unchanged fenthion was not detected in any tissue
sample. The metabolites excreted in urine were similar to those in the
liver, exception that a higher percentage of phenol sulfide was
present in the urine (Weber & Ecker, 1992).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion are
summarized in Table 1. Technical-grade fenthion is moderately toxic to
mammals (mice, rats, guinea-pigs, and rabbits) when given by oral,
intraperitoneal, dermal, or inhalation routes. It is highly toxic to
arian species (especially to the wild mallard duck) when administered
orally. The clinical symptoms of acute toxicity are consistent with
those of central and peripheral cholinergic intoxication by
organophosphorus esters, including decreased spontaneous activity,
apathy, dyspnoea, ataxia, tremors, convulsions, lacrimation,
salivation, and diarrhoea. The symptoms were reversible in surviving
animals.
(b) Short-term toxicity
Exposure to fenthion by inhalation for 6 h/day daily for nine
days at 0.21 mg/litre (initial concentration in a static inhalation
chamber) resulted in signs of poisoning but no mortality, in rats,
guinea-pigs, rabbits, or cats. Serum and erythrocyte cholinesterase
activities were severely depressed but recovered within three weeks
(Klimmer, 1963).
Mice
Groups of 10 male and 10 female B6C3F1 mice were given E 1752
technical (purity, 98.7%) in the diet at 0 (control diet), 150, 200,
or 250 ppm (equal to 0, 83.2, 117.2, and 140.3 mg/kg bw per day in
males and 0, 83.6, 114.5, and 151.8 mg/kg bw per day in females) for
one month. No NOAEL was seen At the lowest dose, emaciation, tremor,
and decreased body weight were observed in males, reduced water
consumption and decreased spleen weight in animals of each sex, and
reduced liver weight in females. Severe inhibition (68-80%) of brain
and erythrocyte acetylcholinesterase was noted in all animals. At the
two higher doses, additional treatment-related effects seen were a
moribund condition resulting in unscheduled sacrifice of two males per
dose group and emaciation and decreased body weight in females (Leser,
1990).
Rats
Five groups of 22 male and 22 female rats were fed diets
containing technical-grade fenthion (purity not given, in soya bean
oil) at 0, 0.25, 0.5, 2.5, or 5.0 mg/kg bw per day for three months.
The NOAEL was 0.25 mg/kg bw per day, based on depression of
cholinesterase activity in erythrocytes, plasma, heart, liver, and
brain (no raw data) at > 0.5 mg/kg bw per day. At this dose and
above, clinical signs of cholinergic intoxication and significant
Table 1. Acute toxicity of fenthion
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Mouse Male Oral 150 Francis & Barnes (1963)
Female 190
Male Oral 227 Dubois (1968)
Female 225
Male Intraperitoneal 125 Dubois & Kinoshita (1964)
Female 150
Female Intraperitoneal 200-2404 Budreau & Singh (1973a)
Rat Male Oral 175-470 Klimmer (1963); Francis & Barnes (1963);
Female 245-310 Gaines (1969); Dubois & Kinoshita (1964)
Male Oral 405 Eigenberg (1987a)
Female 566
Male Intraperitoneal 260 Dubois & Kinoshita (1964)
Female 325
Male Intraperitoneal 152 Klimmer (1963)
Male Intraperitoneal 330 Krotlinger (1993)
Female 203
Male Dermal 330-650 Klimmer (1963); Francis & Barnes (1963);
Gaines (1969); Dubois & Kinoshita (1964)
Female 330-500
Male Dermal 1680 Mihail (1978)
Female 2830
Male Dermal 586 Bomann (1991)
Female 800
Male, female Inhalation (1-h) > 1.197 Thyssen (1978)
Male Inhalation (4-h) 1.2 Thyssen (1978)
Female 0.8
Male Inhalation (1-h) 1.838 Shiotsuka (1987a)
Female 1.637
Table 1. (con't)
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Male Inhalation (4-h) 0.919 Shiotsuka (1987a)
Female 0.819
Male Inhalation (4-h) 0.507 Shiotsuka (1987b)
Female 0.454
Guinea-pig Male Oral > 1000 Francis & Barnes (1963)
Male Oral 260 Dubois & Kinoshita (1964)
Male Intraperitoneal 310 Dubois & Kinoshita (1964)
Rabbit Male Oral 150-175 Francis & Barnes (1963)
Male Dermal 150 Klimmer (1963)
Male Dermal 150 Lamb & Anderson (1974)
Female 131
Male, female Dermal 667 Eigenberg (1987b)
Chicken NR NR 310 Dubois & Doull (1960)
Male Oral 28 Sherman & Ross (1961)
Female Oral 30-40 Francis & Barnes (1963)
Female Oral 27 Flucke (1986a)
Male Oral 37.5 Singh et al. (1987)
Female Dermal 222 Flucke (1986b)
Duck NR Oral 15 Dubois & Doull (1960)
NR Oral 1-2 Keith & Mulla (1966)
NR, not reported
reductions in food consumption and body weight were evident. At the
two highest doses, decreased spermatogenesis and atrophic prostate
glands were noted in males; the female reproductive system was not
affected. All females at the highest dose died during the second and
third weeks of treatment (Shimamoto & Hattori, 1969).
Six groups of 12 male and 12 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0.2, 3, 5,
25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5.0 mg/kg
bw per day, for 16 weeks. No treatment-related adverse effects were
observed on body weight or body-weight gain or on food consumption;
gross and microscopic examination of tissues also showed no effect,
The NOAEL was 0.25 mg/kg bw per day based on cholinesterase depression
(source of enzyme not specified) at 1.25 mg/kg bw per day (Doull
et al., 1961a).
Six groups of 25 male and 25 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 3,
5, 25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5 mg/kg
bw per day, for one year. Significant inhibition (> 20%) of
erythrocyte acetylcholinesterase activity was noted at > 5 ppm.
Brain acetylcholinesterase activity was significantly depressed
(> 10%) and a higher mortality rate (particularly among males) was
observed at 25 ppm and above. At the highest dose, increased
haemosiderosis was noted in the spleen. The NOAEL was 0.25 mg/kg bw
per day, based on significant inhibition of brain acetylcholinesterase
at higher doses (Doull et al., 1963a),
Four groups of 10 male and 10 female Wistar rats were exposed to
an aerosol of technical-grade fenthion (purity, 98.2%) at nominal
concentrations of 0, 0.003, 0.015, or 0.075 mg/litre, equal to
analytical concentrations of 0, 0.001, 0.003, or 0.016 mg/litre, in a
head-/nose-only inhalation chamber for 6 h/day, five days per week for
three weeks. Exposure did not induce mortality or changes in body
weight, the results of haematology, clinical chemistry, or urinalysis,
gross pathology, or organ weights. At analytical concentrations
> 0.003 mg/litre, clinical symptoms of behavioural disturbance
(inactivity and/or unpreened coats) were observed in females only;
plasma cholinesterase was depressed in animals of each sex.
Significant inhibition of erythrocyte and brain acetylcholinesterase
activities occurred at the highest dose, and treatment-induced
inflammatory changes of the respiratory tract were seen in females.
The NOAEL was 0.001 mg/litre (analytical), based on clinical signs of
intoxication in females at 0.003 mg/litre and depression of brain and
erythrocyte acetylcholinesterase activities at 0.016 mg/litre
(Thyssen, 1979).
Rabbits
Groups of six male and six female New Zealand white rabbits
received dermal applications of technical-grade fenthion (purity,
98.2%) in Cremophos EL (1.5% v/v in distilled water) on intact (three
animals) or abraded (three animals) skin sites at 0, 5, or 25 mg/kg bw
per day for 7 h each day, five days per week for three weeks. The
NOAEL was 5 mg/kg bw per day, based on inhibition of erythrocyte
acetylcholinesterase in animals of each sex at 25 mg/kg bw per day. No
treatment-related effect was observed on general appearance,
behaviour, or body weight or by haematology, clinical chemistry,
urinalysis, gross pathology, organ weight measurement, or
histopathology (Mihail & Schilde, 1979).
Groups of five male and five female HRA:(NZW) specific pathogen-
free rabbits received dermal applications of technical-grade fenthion
(purity, 96.9%) formulated in Cremophos EL (1.5% v/v in distilled
water) on intact skin sites at 0, 5, 50, or 100 mg/kg bw per day for
6 h each day, five days per week for three weeks. The NOAEL was
50 mg/kg bw per day, based on inhibition of brain acetylcholinesterase
in females at 100 mg/kg bw per day. No treatment-related effect was
observed on appearance, behaviour, or body weight, or by haematology,
clinical chemistry, urinalysis, gross pathology, organ weight
measurements, or histopathology (Bailey, 1987).
Groups of five male and five female HRA:(NZW) specific
pathogen-free rabbits received dermal applications of technical-grade
fenthion (purity, 96.9%) formulated in Cremophos EL (1.5% v/v in
distilled water) on intact skin sites at 0, 200, or 400 mg/kg bw per
day for 6 h each day, five days per week for three weeks. The study
was terminated within two weeks of the start of treatment owing to
high mortality. An additional group of five male and five female
rabbits was given a lower dose of 150 mg/kg bw per day under similar
conditions; the original control group was retained. This treatment
did not affect general appearance, behaviour, body weight, or food
consumption, or the results of haematology, clinical chemistry, organ
weight measurement, or gross or histopathology. Plasma, erythrocyte,
and brain cholinesterase activities were depressed in animals of each
sex, with a significant inhibition of > 50% in males and 30% in
females at termination of the study. The LOAEL was 150 mg/kg bw per
day, based on inhibition of brain and erythrocyte acetylcholinesterase
activities (Bailey, 1988).
Dogs
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, or 1.25 mg/kg bw per day) for
12 weeks. The NOAEL was 0.125 mg/kg bw per day, based on significant
(> 20%) inhibition of erythrocyte acetylcholinesterase activity at
50 ppm. At > 5 ppm, plasma cholinesterase was also depressed, but
the effect (an indication of exposure) was not considered to be
toxicologically significant (Doull et al., 1961b).
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, and 1.25 mg/kg bw per day)
for one year. The NOAEL was 0.125 mg/kg bw per day, based on
significant inhibition of erythrocyte (> 20%) and brain (> 10%)
acetylcholinesterase activity at 50 ppm. At > 5 ppm, plasma
cholinesterase was also depressed, but the effect (an indication of
exposure) was not considered to be toxicologically significant. A
slight increase in spleen weight with splenic congestion,
extramedullary haematopoiesis, and haemosiderosis, was also observed
in all treated animals; the effect was not dose-related and not
considered to be adverse (Doull et al., 1963b).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade fenthion (purity, 97%) at 0, 2, 10, or
50 ppm, equal to 0, 0.06, 0.26, or 1.23 mg/kg bw per day in males and
0, 0.06, 0.26, or 1.18 mg/kg bw per day in females, for one year. The
NOAEL was 0.06 mg/kg bw per day, based on toxicologically significant
inhibition (> 10%) of brain acetylcholinesterase activity at 10 ppm
At the highest dose, both erythrocyte and brain acetylcholinesterase
activities were significantly depressed (> 50 and > 30%,
respectively). Plasma cholinesterase activity was inhibited at all
doses tested; but the effect (an indicator of exposure) was not
considered to be toxicologically significant. No other treatment-
related change was observed (Christenson, 1990a).
Groups of four male and four female pure-bred dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 3, 10,
or 30 (weeks 0-64), 50 (weeks 65-67), or 60 (weeks 68-104) ppm (equal
to 0, 0.09, 0.32, and 1.28 mg/kg bw per day in animals of each sex)
for two years. No mortality or clinical sign of toxicity was seen
during the study. No significant treatment-related change in body
weight, ophthalmoscopic, haematological, or urinary parameters, gross
or histopathology of tissues was evident at any dose. The NOAEL was
0.09 mg/kg bw per day, based on significant inhibition of erythrocyte
acetylcholinesterase activity in males (> 20%) and of brain
acetylcholinesterase activity in females (> 10%) at 10 ppm. At the
highest dose, erythrocyte and brain acetylcholinesterase activities
were significantly depressed in animals of each sex. Decreased food
consumption (females) and lowered plasma protein levels (males) were
also observed (Hoffmann & Weischer, 1975).
Monkeys
Four groups of five male and five female rhesus monkeys were
given daily doses of technical-grade fenthion (purity, 98.1%) in corn
oil by gavage at concentrations of 0, 0.02, 0.07, or 0.20 mg/kg bw per
day for two years. Animals were observed daily for general appearance
and clinical signs; body weight and ophthalmological parameters were
recorded monthly, and clinical chemistry, haematology, and urinalyses
were performed at 0, 1, 3, 6, 12, 18, and 23 months. Plasma and
erythrocyte cholinesterase activities were measured weekly for the
first four weeks and monthly thereafter. One animal of each sex at 0
and 0.20 mg/kg bw per day was killed seven months and three weeks
after the beginning of treatment for measurement of brain
acetylcholinesterase and gross and histopathology. All monkeys
underwent necropsy after 23 months, but no histopathology was
performed. Plasma cholinesterase was consistently depressed in animals
of each sex at 0.20 mg/kg bw per day, but the effect (an indicator of
exposure) was not considered to be toxicologically significant. A
significant (> 20%) inhibition of erythrocyte acetylcholinesterase
activity was observed occasionally in animals of each sex (9/26
determinations over the 23-month study period for males and 2/26 for
females) at the highest dose. No effect on brain acetylcholinesterase
was evident in the four animals killed at 7.25 months. Gross
examination revealed abnormally small testes and ovaries in animals at
0.20 mg/kg bw per day; however, in the absence of organ weights and
histopathological data, the toxicological significance of this finding
could not be fully assessed. No other treatment-related adverse
effect was recorded. The NOAEL was 0.07 mg/kg bw per day, based on
inconsistent erythrocyte acetylcholinesterase depression and possible
effects on male and female reproductive organs at 0.20 mg/kg bw per
day (Rosenblum, 1980).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of male and female B6C3F1 mice, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) in the diet at concentrations of 0,10,
or 20 ppm (equivalent to 0, 1.5, or 3.0 mg/kg bw per day) for 103
weeks. All surviving animals were maintained on control diet for up to
one week after the end of treatment before they were sacrificed and
subjected to gross and microscopic examination. Treatment with
fenthion did not result in significant differences in the survival or
growth rate of mice at any dose. Clinical signs of intoxication were
evident four months after the beginning of treatment (LOAEL not
specified). At the end of the study (104 weeks), histopathological
examination revealed a variety of neoplasms, the majority of which
were judged to be unrelated to fenthion administration. An increased
incidence of sarcomas, fibrosarcomas, or rhabdomyosarcomas of the
integumentary system was observed in treated male mice; the incidences
of integumentary sarcomas were 0/25 at 0 ppm, 7/49 at 10 ppm, and 8/48
at 20 ppm (7/435 in historical controls). The effect was considered to
be treatment-related, indicating that technical-grade fenthion is
oncogenic in male mice under the conditions of the study. There was no
NOAEL for systemic toxicity, since the LOAEL for clinical signs was
not specified (US National Cancer Institute, 1979).
Groups of 60 male and 60 female specific-pathogen-free B6C3F1
mice were fed diets containing technical-grade fenthion (purity,
92.3%) at 0, 0.1, 1.0, 5.0, or 25 ppm, equal to 0, 0.03, 0.40, 1.95,
or 9.42 mg/kg bw per day in males and 0, 0.03, 0.47, 2.25, or
10.63 mg/kg bw per day in females, for 102 weeks. Additional groups of
20 mice of each sex were fed the same diets for 50 weeks before
scheduled interim kills. Brain acetylcholinesterase activity was
depressed by > 10% at > 0.1 ppm in males and at > 1.0 ppm in
females, but the effect was not dose-related and was seen in males at
terminal but not interim sacrifice and in females at interim but not
terminal sacrifice. Significant (> 20%) inhibition of erythrocyte
acetylcholinesterase activity occurred only in the males at 25 ppm at
week 54. As the inhibition of brain acetylcholinesterase was only
marginally significant (10-17%) at the three lower doses, there was no
dose-response relationship, and the effect was inconsistent over
treatment intervals, it was judged to be of minimal toxicological
significance at doses > 5.0 ppm. The NOAEL for systemic toxicity
was 1.95 mg/kg bw per day, as brain acetylcholinesterase activity was
clearly depressed at the next highest dose in males at both interim
and terminal sacrifice (29-32%) and in females at interim sacrifice
(26%). At this dose, there were also significant increases in body
weight in animals of each sex, elevated plasma cholesterol levels in
animals of each sex early in the study, and elevated absolute and
relative liver weights of male mice at termination. In the absence of
remarkable histopathological findings, the changes at 25 ppm were
considered not to be toxicologically significant. There was no
increased incidence of any neoplastic or non-neoplastic lesion in the
treated mice at any dose up to and including 9.42 mg/kg bw per day.
Technical-grade fenthion was thus considered not to be oncogenic to
mice under the conditions of the study (Suberg & Leser, 1990).
The observation of an increased incidence of integumentary
sarcomas in male mice (US National Cancer Institute, 1979) at doses of
1.5 and 3.0 mg/kg bw per day was not reproduced in a second study
(Suberg & Leser, 1990) in which doses up to 9.42 mg/kg bw per day were
tested. As the increased tumour incidence in the first study was not
dose-related and was site-and sex-specific and the results of the
second study (conducted with more control animals and more groups for
interim sacrifice) were clearly negative at doses up to three times
those of the first study, technical-grade fenthion would appear not to
be oncogenic in the mouse.
Rats
Groups of male and female Fischer 344 rats, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) at dietary concentrations of 0, 10, or
20 ppm (equivalent to 0, 0.5, or 1.0 mg/kg bw per day) for 103 weeks.
All surviving animals were maintained for one or two weeks after the
end of treatment and were then sacrificed and submitted to gross and
microscopic examination. No treatment-related mortality or behavioural
changes were observed, and the growth rate was comparable in control
and treated groups. Histopathological examination revealed a variety
of neoplastic and non-neoplastic lesions common to rats of this
strain. No increase in the incidence of any type of tumour was
observed in the treated rats at any dose up to and including 20 ppm
(1.0 mg/kg bw per day). Technical-grade fenthion was thus considered
not to be oncogenic to the rat under the conditions of the study (US
National Cancer Institute, 1979).
Groups of male and female specific-pathogen-free Wistar rats, 100
of each sex in the control group and 50 of each sex per dose, were fed
technical-grade fenthion (purity unspecified) in the diet at
concentrations of 0, 3, 15, or 75 ppm, equal to 0, 0.14, 0.72, or
374 mg/kg bw per day in males and 0, 0.19, 0.93, or 4.46 mg/kg bw per
day in females, for 24 months. The rats were weighed weekly during the
first 26 weeks and every two weeks thereafter. Food consumption was
recorded weekly. Haematology, clinical chemistry, liver and kidney
funtional tests, and urinalysis were performed on five rats of each
sex per group at 1, 3, 6, and 12 months, and on 10 rats of each sex
per group at the end of the study (24 months). Plasma and erythrocyte
cholinesterase activities were determined at the end of weeks 1, 2, 4,
8, 13, 26, 52, 78, and 105; brain acetylcholinesterase activity was
not measured. At the end of the study, all surviving rats were
sacrificed and examined macroscopically, and tissues and organs were
removed, weighed, and studied microscopically. Doses of 3 or 15 ppm
did not affect physical appearance, behaviour, growth, or survival
rate, but males at the highest dose had a significantly lower body
weight and both males and females appeared to have increased mortally.
No treatment-related effect was observed by haematology, clinical
chemistry, urinalysis, or gross or microscopic pathology. (It should
be noted that the rats from which blood and urine samples were taken
for analysis during the first year were not identified.) Significant,
dose-dependent depression of plasma and erythrocyte cholinesterase
activities was observed in animals of each sex at 15 and 75 ppm. The
NOAEL for systemic toxicity, based on erythrocyte acetylcholinesterase
inhibition, was 0.14 mg/kg bw per day. The results of histopathology
showed no increase in the incidence of tumours in treated rats at any
dose up to and including 3.74 mg/kg bw per day, the highest dose
tested, indicating that technical-grade fenthion was not oncogenic to
the rat under the conditions of the study (Bomhard & Loser, 1977).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenthion (purity, 97.0%) at concentrations
of 0, 5, 20, or 100 ppm, equal to 0, 0.2, 0.8, or 5.2 mg/kg bw per day
in males and 0, 0.3, 1.3, or 7.3 mg/kg bw per day in females, for two
years. Two additional groups of 20 rats of each sex were fed diets
containing fenthion at concentrations of 0 or 100 ppm for one year
before scheduled interim sacrifice. No NOAEL for systemic toxicity
could be determined, because of statistically (P < 0.05) and
toxicologically significant (> 10%), dose-related inhibition of brain
acetylcholinesterase activity in animals of each sex at all doses,
including the lowest dose of 0.20 mg/kg bw per day. Plasma and
erythrocyte cholinesterase activities were also depressed at all
doses, but toxicologically significant (> 20%) inhibition of
erythrocyte acetylcholinesterase occurred only at > 20 ppm. At the
highest dose, technical-grade fenthion was retinotoxic, especially in
females, and ophthalmological examination revealed an increased
incidence of retinal degeneration and posterior subcapsular cataract
formation in females and focal corneal scarring in animals of each
sex. Histopathological examination revealed a higher incidence of
corneal mineralization and retinal atrophy in females, and corneal
neovascularization and optic nerve atrophy in animals of each sex. The
retinotoxic effect was further confirmed by electroretinographic
examination, with suppression of electroretinograms also in a number
of females at 20 ppm at interim sacrifice at week 75. At 100 ppm,
additional treatment-related effects were an increased incidence of
nonspecific clinical signs (urine staining, tail and paw lesions,
rough coat, and hunched back), lower body-weight gain, and remarkable
histopathological findings including vacuolar degeneration of the
nasolacrimal ducts, mineralized and raised areas in the stomach,
stomach acanthosis, hyperkeratosis (males) and hyperplasia (females),
epididymal vacuolar degeneration, and chronic active inflammation of
the skin of the tail and hindlimbs. A higher incidence of vacuolar
degeneration of the nasolacrimal ducts was also seen in females at
20 ppm. There were no treatment-related oncogenic effects in males or
females at any dose up to and including 5.2 mg/kg bw per day, the
highest dose tested. Technical-grade fenthion was thus considered not
to be oncogenic to the rat under the conditions of the study
(Christenson, 1990b).
(d) Reproductive toxicity
Mice
In a five-generation, two-litter per generation study, two groups
of CD-1 mice received technical-grade fenthion (purity unspecified) in
their drinking-water at concentrations of 0 or 60 ppm (equivalent to
9.0 mg/kg bw per day). The control group comprised 10 males and 14
females, and the treated group comprised 10 males and 22 females.
There was a significant increase in pup mortality during the first
postnatal week, especially in the second, third, and fourth
generations. No treatment-related effect on reproductive performance,
lactation, or growth rate of pups was observed. No histopathological
changes were noted in the liver or kidney of adult males of the third
generation (Budreau & Singh, 1973b).
Rats
In a two-generation, one-litter per generation study in Crl:CD BR
rats, five groups of 30 male and 30 female animals were fed diets
containing technical-grade fenthion (purity, 96.9%) at concentrations
of 0, 1, 2, 14, or 100 ppm, equal to 0, 0.08, 0.16, 1.16, or 8.3 mg/kg
bw per day (based on premating food intake values), for 70 days (F0
and F1 parents) before mating (1:1 ratio) for production of the F1
and F2 litters. No treatment-related effect on mortality, clinical
signs of toxicity, mean body weight, mean food consumption, or gross
pathology was observed in adults of the F0 or F1 generation.
Epididymal weight was increased in F0 males at 100 ppm.
Histopathological examination revealed vacuolation in the lining
ductal epithelial cells of the corpus epididymus in a few F0 and F1
males at 14 ppm and in all at 100 ppm. Brain acetylcholinesterase
activity was significantly (> 10%) depressed in F0 females at all
doses and in F0 males and F1 males and females at the two higher
doses. Inhibition at the two lower doses (-22 and -17%, respectively)
in F0 females was not dose-related and was of a magnitude similar
to those at 14 ppm in F0 males (-19%) and in F1 males (-28%)
and females (-18%). F0 female controls had a mean brain
acetylcholinesterase activity of 2428 mU/g, which was significantly
higher than the other control values observed in the study (2137,
1894, and 1515 mU/g for F0 male, F1 male, and F1 female controls,
respectively). The effect on brain acetylcholinesterase activity seen
in F0 females at 1 and 2 ppm was therefore judged to be of minimal
toxicological significance. At the two higher doses, significant
(> 20%) inhibition of plasma and erythrocyte cholinesterase
activity was seen in all parental adults and pups. Neonatal brain
acetylcholinesterase activity was inhibited only at the highest dose.
The NOAEL for maternal toxicity was 0.16 mg/kg bw per day, based on
consistent inhibition of brain and erythrocyte acetylcholinesterase
activity at 14 ppm. There was no treatment-related effect on
reproductive performance, embryo- or fetotoxicity, litter size, pup
viability, or growth at > 14 ppm. At the highest dose, decreased
fertility, a decreased number of implantation sites per dam, decreased
litter size, an increased number of stillborn pups per litter, a
reduced viability index (days 0-4), and decreased pup body-weight gain
during lactation (days 14 and 21) were observed in both F0 and F1
generations and F1 and F2 litters. The NOAEL for reproductive
toxicity was thus 1.16 mg/kg bw per day (Kowalski et al., 1989).
(e) Developmental toxicity
Rats
Four groups of 20 mated FB30 Long-Evans female rats were given
technical-grade fenthion (purity, 98.1%) in aqueous Cremophor EL
emulsion by gavage at 0, 1, 3, or 10 mg/kg bw per day once daily on
days 6-15 of gestation; the day semen was detected in a vaginal smear
was designated day 0 of gestation. On gestation day 20, all surviving
dams were sacrificed, and the fetuses were delivered by caesarean
section and necropsied. The numbers of implantation sites and of live,
dead, and resorbed fetuses, litter weight, fetal body weight, and
placental weight were recorded, and all fetuses were examined for
external, visceral, and skeletal abnormalities. No treatment-related
mortality, clinical symptoms of maternal toxicity, or disturbance of
intrauterine development were noted at any dose. All fetuses delivered
developed normally. No signs of fetotoxicity were seen, and no
treatment-related malformations were observed in any of the fetuses at
any dose up to and including 10 mg/kg bw per day. The NOAEL for
maternal toxicity, embryo- and fetotoxicity, and teratogenicity was
10 mg/kg bw per day, the highest dose tested (Machemer, 1978a).
Groups of 33 mated Crl:CD BR female rats were given technical-
grade fenthion (purity, 96.5%) in 5% aqueous Emulphor solution by
gavage at doses of 0 (vehicle control), 1, 4.2, or 18 mg/kg bw per day
once daily on days 6-15 of gestation; the day spermatozoa were
detected in a vaginal smear was designated as day 0 of gestation. Five
animals from each group were sacrificed on day 16, and the remaining
28 dams in each group were killed on day 20 of gestation. All dams
were necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. No NOAEL for maternal toxicity
could be determined owing to dose-related, toxicologically
significant inhibition of brain (> 10%) and erythrocyte (> 20%)
acetylcholinesterase activities at all doses tested, including the
lowest dose of 1 mg/kg bw per day, on days 16 and 20 of gestation.
Plasma cholinesterase was also depressed at > 1 mg/kg bw per day on
day 16 and at 18 mg/kg bw per day on day 20. No other treatment-
related maternal or embro- or fetotoxicity was seen at > 4.2 mg/kg
bw per day. At the highest dose, clinical symptoms of intoxication
(dry or bloody lacrimation, exophthalmia, hypoactivity, tremors,
salivation, and urine-stained peritoneum), decreased mean bodyweight
gain, and reduced food consumption were observed. There was also a
slight increase in fetal skeletal variations, including an increased
incidence of incomplete ossification of the cervical arches, skull
bones, and third, fourth, and sixth sternebrae, and incomplete and/or
unossified metacarpals and metatarsals. These findings represent a
delay in skeletal maturation and were considered not to be adverse or
of toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in the fetuses at any dose up to and
including 18 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity
and teratogenicity was therefore 18 mg/kg bw per day, the highest dose
tested (Kowalski, 1987).
Rabbits
Groups of 17 artificially inseminated American-Dutch female
rabbits were given technical-grade fenthion (purity, 96.5%) in 5%
aqueous Emulphor solution by gavage at doses of 0 (vehicle control),
1, 2.75, or 7.5 mg/kg bw per day once daily on days 6-18 of gestation;
the day of artificial insemination was designated day 0 of gestation.
All surviving animals were sacrificed on gestation day 28 and
necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. The NOAEL for maternal toxicity
was 1 mg/kg bw per day based on toxicologically significant inhibition
of brain (> 10%) and erythrocyte (> 20%) acetylcholinesterase
activities at the two higher doses on gestation day 19 and/or 28. At
> 2.75 mg/kg bw per day, an increased incidence of soft stools was
noted both during and after treatment. At the highest dose, plasma
cholinesterase activity was depressed on gestation day 19 only. There
was also an increased incidence of unossified anterior metacarpals
among the fetuses, but this finding represents a delay in skeletal
maturation and was considered not to be an adverse effect of
toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in any of the fetuses at any dose up to
and including 7.5 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity and teratogenicity was 7.5 mg/kg bw per day, the highest
dose tested (Clemens, 1987).
(f) Genotoxicity
In an assay for dominant lethal mutation, three groups of 50 male
NMRI mice were each given a single oral dose of 0, 10, or 25 mg/kg bw
of technical-grade fenthion (purity unspecified) as a 2% aqueous
emulsion. Each male was then caged with an untreated virgin female for
four days; the same procedure was repeated for a total of 12 matings.
On days 12-16 of gestation, the females were sacrificed, and the
numbers of implantations and of live and dead implants (deciduomata,
resorption sites, and dead embryos) were counted. The high dose
resulted in overt clinical signs of toxicity in males (drowsiness,
ruffled coat, and dilated intestines). Except for increased
preimplantation loss at 25 mg/kg bw in the first two mating periods,
no other treatment-related effects on reproductive performance
(fertility, pre- and post-implantation loss) were observed (Machemer,
1978b).
A battery of studies was conducted with technical-grade fenthion
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were mostly negative; weakly positive results were obtained
in two of five assays for sister chromatid exchange, in one of two
assays for micronucleus formation (at a dose of 150 mg/kg bw), and in
one assay for unscheduled DNA synthesis. Summary results from the US
Environmental Protection Agency Health Effects Research Laboratory
(Simmon et al., 1977) indicated that technical-grade fenthion does
not induce reverse mutation in Salmonella typhimurium TA98, TA100,
TA1535, TA1537, or TA1538 or Escherichia coli WP2 uvrA; enhanced
mitotic recombination in yeast ( Saccharomyces cerevisiae D3);
unscheduled DNA synthesis in human fibroblasts (WI-38 cells);
preferential toxicity (DNA repair-proficient/deficient) in E. coli
(W3110, p3478) or Bacillus subtilis (H17, M45); or sex-linked
recessive lethality in Drosophila melanogaster in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenthion (purity 97-99%) was not irritating to
the skin and was minimally or not irritating to the eyes of New
Zealand white rabbits (Pauluhn, 1985; Eigenberg, 1987c,d).
Technical-grade fenthion (purity 98.5%) did not induce dermal
sensitization in a maximization test (according to the method of
Magnusson and Kligman) in male DHPW guinea-pigs (Flucke, 1987)
(ii) Potentiation
Fenthion potentiated the acute intraperitoneal toxicity of
malathion, dioxathion, and coumaphos in rats, but intraperitoneal
administration of 13 other organophosphate or carbamate insecticides
to rats in combination with fenthion did not result in greater than
additive toxic effects (Dubois & Kinoshita, 1964). Dietary combination
of equitoxic doses (2 mg/kg bw) of fenthion with coumaphos, neither of
which alone affected cholinesterase activity when fed to dogs for six
weeks, was found to potentiate the anticholinesterase activity of
serum and erythrocytes by 75 and 30%, respectively. The potentiation
was less evident with malathion, and no potentiation was noted when
fenthion was fed in combination with dioxathion (Doull et al.,
1962). Oral administration to rats of a single dose of either a 3:1
mixture of fenthion and dichlorvos or a 1:3 mixture of fenthion and
isofenthion did not result in a greater than the theoretical additive
toxic effect (Kimmerle, 1967; Heimann, 1982). In several recent
studies, pretreatment with fenthion significantly potentiated the
acute toxicity of 2- sec-butylphenyl N-methylcarbamate in mice and
dogs. Its acute toxicity in mice was increased 15-fold by a 1-h oral
pretreatment with 30 mg/kg bw fenthion (one-tenth of the LD50).
Table 2. Results of tests for the genotoxicity of technical-grade fenthion
End-point Test system Concentration Results Reference
or dose
IN vitro
Reverse mutation S. typhimurium TA98, 0, 20-12 500 µg/plate Negative Herbold (1987)
TA100, TA1535, TA1537 0, 750-12 000 µg/plate
Reverse mutation S. typhimurium TA98, 0, 8-5000 µg/plate Negative Herbold (1990a)
TA100, TA1535, TA1537
hprt forward mutation Chinese hamster ovary 0, 20.0-100 µg/ml Negative Lehn (1990a)
cells 0, 12.5-75 µg/ml
Chromosomal aberration Chinese hamster ovary 0. 0.02-0.15 µg/ml Negativea Putman & Morris
cells (1989)
Chromosomal aberration Chinese hamster lung cells 0, 23.5-94 µg/ml Negativea Kajiwara (1989)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml Negative at < 20b,c Chen et al.
(1982a)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml (in diffusion Weakly positive Chen et al. (1982b)
chambers) at > 40b
Sister chromatid exchange Human lymphoid cell line 0, 0.02-20 µg/ml (unactivated) Negativea,b Sobti et al.
(1982)
LAZ-007 (of B-cell origin) 0, 20 µg/ml (activated)
Chromosomal aberration, Human lymphocytes 0, 0.5-5.0 µg/ml Weakly positive Rani & Rao (1991)
sister chromatid exchange at > 1.5
Unscheduled DNA Rat primary hepatocytes 0, 10-100 µg/ml Weakly positive Lehn (1990b)
synthesis 0, 5-30 µg/ml at > 5
Table 2. (con't)
End-point Test system Concentration Results Reference
or dose
In vivo
Sister chromatid exchange Rat bone-marrow 0, 10-100 mg/kg bw Negative Bai et al (1990)
lymphoid cells
Micronucleus formation Bor:NMRI mouse bone 0, 150 mg/kg bw Weakly positive Herbold (1990b)
marrow at 150b
a With and without exogenous metabolic activation
b Not confirmed in an independent assay
c Threshold of cytotoxicity
Inhibition of the metabolism or detoxification of 2- sec-butylphenyl
N-methylcarbamate by desulfuration of fenthion may have been
responsible, at least in part, for the synergistic effect (Miyaoka
et al., 1984, 1986, 1987).
(iii) Cholinesterase inhibition
Twelve groups of 10 tasted male Fischer 344 (CDF/Crl/Br) rats
were given single doses of technical-grade fenthion (purity, 97.5%)
orally, dermally, or subcutaneously at 0, 1, 5, or 25 mg/kg bw, and
the effect of the route of administration on plasma, erythrocyte,
and/or brain cholinesterase activity was studied for up to 14 days
after treatment. The NOAEL for all routes of administration was
5 mg/kg bw, based on toxicologically significant inhibition of
erythrocyte (> 20%) and brain (> 10%) acetylcholinesterase
activities at 25 mg/kg bw. Inhibition of plasma and erythrocyte
cholinesterase activity was maximal at 24 h after oral administration
and four days after dermal or subcutaneous administration, suggesting
more efficient absorption by the oral route. On day 14 after
treatment, depression of brain acetylcholinesterase activity was
greatest in the group that were treated subcutaneously; the prolonged
recovery may be associated with a slower release of fenthion into the
circulation when given by this route. The magnitude of cholinesterase
inhibition appeared to be only marginally significant after dermal
administration, suggesting that absorption is less effective by this
route. The rates of absorption were thus oral route > subcutaneous
route > dermal route (Christenson, 1990c).
(iv) Delayed neurotoxicity
Acute delayed neurotoxicity in hens: Groups of 15
atropine-protected, adult laying hens (Lohmann selected Leghorn
strain), five to seven months old, were given two doses of fenthion
(E 1752; purity, 98.5%) in 2% Cremophor EL by gavage at 40 mg/kg bw or
dermally at 200 mg/kg bw, 21 days apart. A positive control group of
five hens was given tri- ortho-cresyl phosphate (purity, 99.1%)
orally as a single dose of 375 mg/kg bw. Six hens given an oral dose
of 5 ml/kg bw 2% Cremophor EL and six untreated hens served as
negative controls. All animals were observed for 21 days after the
second dose before sacrifice, with the exception of the tri- ortho-
cresyl phosphate-treated hens which were killed on day 22 in moribund
condition. At termination, peripheral and central nervous tissues were
removed, fixed, and stained for microscopic examination. Clinical
signs of acute cholinergic intoxication were evident in all
fenthion-treated hens 7-24 h after treatment; recovery occurred within
7-8 days after oral administration and 14-18 days after dermal
application. No clinical symptoms (irreversible impairment of motor
coordination) or neurohistopathological lesions indicative of delayed
neuropathy were observed. The positive controls exhibited symptoms of
delayed nerurotoxicity (abnormal gait, ataxia, and paresis) from day 7
after treatment, which persisted until sacrifice on day 22;
neurohistopathological lesions characteristic of delayed neuropathy
were also evident (Flucke & Kaliner, 1987).
Neuropathy target esterase activity: Groups of nine atropine-
protected, adult hens (Lohmann selected Leghorn strain) were given a
single oral dose of technical-grade fenthion (E 1752; purity, 98.5%)
in 2% Cremophor EL by intubation at doses of 0 (vehicle control) or
40 mg/kg bw. Nine positive controls received tri- ortho-cresyl
phosphate as a single oral dose of 100 mg/kg bw. Neuropathy target
esterase activity in brain and spinal cord was inhibited by 0-14% over
that in vehicle controls 24 and 48 h and seven days after treatment.
Severe inhibition (> 50-90%) was seen in the positive controls
throughout the study (Flucke, 1988a).
Groups of nine atropine-protected adult hens (Lohmann selected
Leghorn strain) were given a single dermal dose of technical-grade
fenthion (E 1752; purity, 98.5%) in 2% Cremophor EL at doses of 0
(vehicle control), 200, or 400 mg/kg bw. A positive control group of
three to nine hens received tri- ortho-cresyl phosphate as a single
dose of 100 mg/kg bw by intubation. Neuropathy target esterase
activity in brain and spinal cord was inhibited by 0-20% over that in
vehicle controls at 24, 48, and 72 h and up to seven days after
treatment. Severe inhibition (> 80%) was seen in the positive
controls 24 and 48 h after treatment, demonstrating the sensitivity of
the test (Flucke, 1988b)
Short-term delayed neurotoxicity in hens: Groups of 10 adult
white Leghorn hens (Gallus gallus) were given technical-grade
fenthion (purity, 96.5%) by gavage in clear corn oil solution at doses
of 0, 1, 2, or 4 mg/kg bw per day for 14 weeks. Tri- ortho-cresyl
phosphate (10-60 mg/kg bw per day) was given as a positive control. No
treatment-related clinical symptoms or increases in the incidence
and/or severity of histopathological lesions of nervous tissues
characteristic of organophosphate-induced delayed neurotoxicity were
evident in the fenthion-treated hens at up to and including the
highest dose. Clinical signs of decreased activity and ataxia were
noted, but predominantly in the first hours after treatment; they were
no longer seen before the next dose, suggesting that they were likely
to have been caused by repetitive, severe acute cholinergic
intoxication and were not related to delayed neurotoxicity. The
positive controls had both toxic symptoms and histopathological
lesions of the nervous tissues typical of delayed neurotoxicity.
Histopathological examination revealed a dose-related increase in the
incidence of muscular hypertrophy or hyperplasia in all muscle layers
of the oesophagus, crop, proventriculus, gizzard, and intestine of all
fenthion-treated hens and glandular and nonglandular epithelial
hyperplasia in the oesophagus, crop, and proventriculus of some at the
middle dose and in most at the high dose. These lesions were probably
due to localized acetylcholinesterase inhibition, with subsequent
overstimulated muscle hypertrophy (Hayes & Ramm, 1988).
A second short-term study, in which technical-grade fenthion was
fed to hens was conducted to determine if the muscular hypertrophy and
hyperplasia observed by Hayes and Ramm (1988) were a direct effect of
fenthion or were due to the route of treatment. Two groups of 10 adult
white Leghorn hens (Gallus gallus domesticus) were given technical-
grade fenthion (purity, 96.9%) at dietary concentrations of 0 or
52 ppm (equivalent to 4 mg/kg bw per day) for 90 days. Minimal to
moderate muscular hypertrophy or hyperplasia was seen in the distal
oesophagus (between the crop and the proventriculus) of all treated
hens, which accounted for 98-99% of the increased thickness (+ 55%, in
comparison with controls) in the oesophageal wall. Hypertrophy or
hyperplasia of the oesophageal glandular components was also seen in
four birds. In addition, treated hens had a statistically significant
(P < 0.05) depression of cholinesterase activity in whole blood
(> 50% in comparison with controls) and tissue from all three
regions of the upper gastrointestinal tract (oesophagus, crop, and
proventriculus: about 70% in comparison with controls). It was
concluded that the muscular hypertrophy and hyperplasia observed
in the fenthion-treated hens was probably due to localized
acetylcholinesterase inhibition with subsequent overstimulation of the
oesophageal smooth muscle layers (Hayes, 1989).
Assessment of the neurotoxicity of fenthion in the literature:
A position paper (Flucke, 1990) which presented and discussed nine
publications dealing with the assessment of neurotoxicity of fenthion
was submitted. The main findings and critical evaluations are
summarized below.
1. In the series of four studies by Farage-Elawar & Francis (1987,
1988a,b; Francis & Farage-Elawar, 1987), the effects of fenthion,
debromoleptophos (which induces organophosphate-induced delayed
neurotoxicity), and fenitrothion (which does not) were compared
in very young chicks. On the basis of the finding that fenthion
produced neurotoxic signs (altered gait) and inhibition (> 50%)
of cholinesterase but little or no inhibition of neuropathy
target esterase, it was postulated that its neurotoxic potential
is not classical organophosphate-induced delayed neurotoxicity. A
critical evaluation of the four publications (by Flucke, 1990,
and the reviewer) revealed that the three organophosphates were
not administered in equitoxic doses, thus rendering direct
comparison of their toxic effects inappropriate, and that the
test animals were malnourished. Under the study conditions, there
was probably prolonged acute cholinergic intoxication due to
severe inhibition of cholinesterases after the lethal or
sublethal doses of debromoleptophos and fenthion, in contrast to
the low dose of fenitrothion (about one-fifth of the LD50),
which would not have induced overt cholinergic symptoms. A
build-up of acetylcholine at the motor end-plates could result in
pronounced, prolonged muscle fasciculation and, secondarily, in
the known necrotizing effects. Young chicks undergoing rapid
growth and muscle development are less likely to compensate
quickly and completely for this type of muscle damage, especially
if they are undernourished. Accordingly, the neurotoxic effects,
which occurred to the same degree with debromoleptophos and
fenthion, could be attributed to primary effects of severe acute
cholinergic intoxication and not to a particular (unknown)
neurotoxic potential of fenthion, as postulated by the authors.
2. Tuler et al. (1988) and Dellinger & Mostrom (1988) examined the
neurotoxic effects of fenthion in dogs. In the first study, the
authors concluded that there was no evidence of delayed
neurotoxicity. Prolonged weekly treatment with fenthion caused
hyperreflexia, proprioceptive deficits, progressive muscle fibre
necrosis, ultrastructural changes in nerve axons, and loss of
small motor units. Fenthion-induced primary neuropathy leading to
secondary myopathy was proposed. In the second study, the authors
concluded that there was no evidence of cholinergic effects and
no effect on vagal tone during the period of fenthion treatment
when maximal inhibition (> 50%) of acetylcholinesterase activity
was measured; however, on day 14 after treatment, the treated
dogs gave a slightly smaller response to an atropine challenge
(in comparison with controls), suggesting possible down-
regulation of the cholinergic receptors.
A critical evaluation of these two publications (by Flucke,
1990 and the reviewer) revealed the following: (i) The fenthion-
treated dogs had severe organophosphate intoxication and that the
clinical symptoms of muscle fasciculation, ataxia, hyperreflexia,
proprioceptive deficits, and hindquarter weakness were likely to
be the result of neuromuscular overstimulation due to severe
acetylcholinesterase inhibition. Reversibility or recovery was
seen in most of the dogs when the dose of fenthion was reduced
from 44 to 22 mg/kg bw or omitted for one week, suggesting the
neuropathy was not irreversible. (ii) Progressive muscle libre
necrosis was evident in the distal parts of the sciatic nerves of
fenthion-treated dogs, with reorganization of the sensory and
motor innervations. As primary impairment of the peripheral
nerves does not result in muscle necrosis but rather in atrophy
of muscle fibres, the results were judged not to be indicative of
a primary neuropathic effect. In addition, receptor down-
regulation can be considered a physiological adaptation process
to maintain internal equilibrium, serving as a protective
mechanism by which the organism normalizes functions that are
impaired by the intake of pharmacodynamic substances.
Accordingly, the small changes noted in the vagal tone of dogs
after chronic administration of fenthion probably represented an
adaptive change and are. not indicative of irreversible
impairment of the neuromuscular junctions. Thus, the studies, of
Tuler et al. (1988) and Dellinger & Mostrom (1988) do not give
evidence of a particular neurotoxic effect of fenthion in dogs.
3. Tuler & Bowen (1989) investigated the neurotoxic effects of
fenthion, paraoxon (an organophosphate), and neostigmine (a
non-organophosphate) on chick embryo nerve cell growth and
ultrastructure in culture. Altered cell membrane integrity and
increased cytoplasmic lipid (vacuole) accumulation were observed
in cells treated with fenthion or paraoxon but not in those
treated with neostigmine. The authors concluded that
organophosphates had direct effects on neuronal cells in culture;
however, the following points should be considered: (i)
Pharmacokinetics plays a special role in intoxication by
organophosphates, in particular thiophosphates, and in
detoxification, both of which occur rapidly and simultaneously.
Fenthion, a thiophosphate, must be converted in vivo to the
active oxygen analogue and would therefore be ineffective as an
inhibitor of esterases in vitro. (ii) The observed effects on
the cell membrane and the cell were not specific to neuronal
cells and can therefore not be compared directly with neurotoxic
effects in vivo. It is therefore concluded that the results of
the study reveal no particular neurotoxic potential of fenthion
in vivo.
4. Misra et al. (1985, 1988) examined clinical and biochemical
changes and neuromuscular function in workers chronically exposed
to fenthion. No clinical signs of peripheral neuropathy or
myopathy and no pathophysiological findings indicative of
irreversible neurological deficits were seen in workers exposed
regularly and repeatedly to fenthion for a mean of 8.5 years.
They showed symptoms typical of acute cholinergic intoxication
and significant inhibition of serum cholinesterase. It is
concluded that the study provides no evidence of a particular or
delayed neurotoxic potential of fenthion in the workers examined.
The literature thus provides no evidence of a particular or
delayed neurotoxic potential but indicates that exposure to fenthion
results in typical organophosphate-induced cholinergic toxicity due to
severe acetylcholinesterase inhibition.
3. Studies of metabolites
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion
metabolites are summarized in Table 3.
Table 3. Acute toxicity of fenthion metabolites
Metabolite LD50 (mg/kg bw) IC50 (mol; 50% inhibition of human
erythrocyte acetylcholinesterase)c
Orala Intraperitonealb
Fenthion 220 325 > 5.0 × 10-4
Fenthion sulfoxide 125 250 4.5 × 10-5
Fenthion sulfone 125 250 4.7 × 10-4
Fenthion oxygen analogue 125 26 2.7 × 10-6
Fenthion O-sulfoxide 50 22 4.8 × 10-5
Fenthion O-sulfone 30 9 3.2 × 10-5
4-(Methylthio)-m-cresol 6500d
4-Methyl(thiosulfoxide)-m-cresol 3500d
4-Methyl(thiosulfone)-m-cresol 7000d
a In male rats (Francis & Barnes, 1963)
b In female rats (Dubois & Kinoshita, 1964)
c Francis & Barnes (1963)
d In female rats (Nelson, 1967)
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR mice were fed diets
containing fenthion sulfoxide (Baycid SO; purity not given) at
concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to 0,
0.45, 1.5, 4.5, 15, or 45 mg/kg bw per day, for four weeks, followed
by a four-week recovery period. Plasma cholinesterase activity was
depressed in animals of each sex at > 3 ppm, but the effect (an
indication of exposure) was not considered to be toxicologically
significant. Brain acetylcholinesterase activity was significantly
inhibited in males at > 3 ppm and in females at > 10 ppm. At the
two highest doses, decreased body weight was noted in animals of each
sex; at the highest dose, tremor was seen in all mice. All of the
treatment-related effects (including cholinesterase depression)
disappeared rapidly during the recovery period. There was no NOAEL.
The LOAEL, based on significant brain acetylcholinesterase inhibition
in males, was 0.45 mg/kg bw per day, the lowest dose tested (Inukai &
Iyatomi, 1981a).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing fenthion sulfoxide (Baycid SO; purity not given)
at concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to
0, 0.15, 0.5, 1.5, 5, or 15 mg/kg bw per day, for four weeks,
followed by a four-week recovery period. Plasma cholinesterase
activity was depressed in females at > 3 ppm, but the effect
(an indication of exposure) was considered not to be toxicologically
significant. No treatment-related adverse effect was observed at
3 ppm. At > 10 ppm, significant, dose-related depression of
acetylcholinesterase activity in brain was seen in animals of each sex
and in erythrocytes in males after four weeks of treatment; in
females, erythrocyte acetylcholinesterase activity was inhibited at
> 30 ppm. At the two highest doses, decreased body weight and food
consumption and increased alkaline phosphatase activity were noted. At
the highest dose, tremor was seen in all rats. All of the treatment-
related effects (including cholinesterase depression) disappeared
rapidly during the recovery period. The NOAEL was 0.15 mg/kg bw per
day, based on significant inhibition of brain (> 10%) and erythrocyte
(> 20%) acetylcholinesterase activities at 0.5 mg/kg bw per day
(Inukai & Iyatomi, 1981b).
4. Observations in humans
Fenthion has been used widely in many parts of the world for
control of e.g. household pests and mosquitos. Studies of individuals
in areas treated for malaria eradication have shown slight plasma
cholinesterase depression when heavy spray schedules were used. The
levels were depressed for up to six weeks after spraying (Elliot &
Barnes, 1963). Children under the age of seven were more susceptible
to the anticholinesterase effect than adults, but no inhibition of
erythrocyte cholinesterase was observed and there were no significant
alterations in normal physiological functions in any individual
examined (Taylor, 1963).
The signs of acute poisoning appear rapidly, beginning with
blurred vision, unsteady gait, and slurred speech. In one reported
case, a man who had taken an unknown quantity of fenthion still
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation after 72 h of emergency
treatment. The patient began to recover only after 11 days of
treatment with antidotes which included atropine, pralidoxime
chloride, and toxogonin (Dean et al., 1967). Another man remained in
a comatose state for 45 min after ingesting a fenthion formulation,
with pale skin, cyanotic mucous membranes, a slow, regular heart beat,
low peripheral blood pressure, and no reactions to pain or light on
the pupils. Recovery took six days (von Clarmann & Geldmacher-von
Mallinkrodt, 1966). A third man, who ingested about 60 g of a fenthion
formulation (Entex), recovered from severe organophosphorous poisoning
after being in a critical condition for the first six days. Recovery
was slow, lasting up to 30 days, and blood cholinesterase activity was
still depressed 22 days after poisoning (Pickering, 1966).
The potential dermal and respiratory exposure of workers to
fenthion during field application by hand-gun power spray equipment,
back-pack hand pressure sprayers, and hand granular dispersal for
mosquito control was studied during two work seasons. Workers exposed
to 3.6-12.3 mg/h dermally (equivalent to 0.5-1.5 mg/kg bw per day) or
< 0.02-0.09 mg/h by inhalation (equivalent to 0.01-0.05 mg/kg bw per
day) had decreased plasma but not erythrocyte cholinesterase activity
(Fytizas-Danielidou, 1971; Wolfe et al., 1974). In two studies of
clinical and biochemical changes, nerve conduction velocity, and
neuromuscular function in 22-24 workers (mean age, 31-32 years)
exposed regularly and repeatedly to fenthion for a mean of 8.2-8.5
years, no clinical signs of peripheral neuropathy or myopathy and
no pathophysiological findings indicative of any irreversible
neurological deficits were seen. The workers showed symptoms typical
of acute cholinergic intoxication and inhibition of serum
cholinesterase, but there was no evidence of delayed neurotoxicity
(Misra et al., 1985, 1988).
In a study of 150 cases of poisoning by anticholinesterase
insecticides, 32 of the patients had consumed fenthion, 48
fenitrothion, and 50 malathion; 20 did not know which agent they had
consumed. Paralytic signs were significantly more frequent after
fenthion than malathion or fenitrothion poisoning (81.2, 30, and 23%,
respectively), and they appeared later and lasted longer. Death
occurred significantly more often after fenthion poisoning, with
mortality rates of 35.5% with fenthion, 4% with malathion, and 2.1%
with fenitrothion. Pulmonary oedema was most common after malathion
poisoning and was not encountered with fenthion. Inhibition of
cholinesterase was most marked after fenthion poisoning, and the
enzyme activity was inhibited by 100% in 18 of the 27 cases studied
(Wadia et al., 1977).
In three groups of four male volunteers given technical-grade
fenthion (purity, 98.1%) in corn oil in gelatin capsules at doses of
0, 0.02, or 0.07 mg/kg bw per day for up to four weeks, no physical or
clinical signs of intoxication were reported, and no abnormal results
were seen by haematology or urinalysis. At 0.07 mg/kg bw per day,
significant depression (> 20%) of plasma cholinesterase was observed,
but no treatment-related effects on erythrocyte acetylcholinesterase
or on other clinical chemistry parameters were observed. The NOAEL was
0.07 mg/kg bw per day, based on the absence of inhibition of
erythrocyte acetylcholinesterase (Griffin et al., 1979).
Comments
A single dose of 14C-radiolabelled fenthion was readily absorbed
and rapidly excreted in the urine and faeces of rats, and no 14C was
detectable in the expired carbon dioxide. About 90% of the radiolabel
was eliminated within 48 h of dosing. The excretion profiles were
similar regardless of sex, dose, or route of administration (oral or
intravenous). Urine was the main route of elimination (> 90% of the
total radiolabel), and only minor amounts were recovered in the
faeces. Little 14C was retained in the tissues, suggesting that
fenthion does not accumulate in the body of rats.
14C-Fenthion was extensively metabolized in rats. No unchanged
parent compound was detected in the urine and very little (< 2%) in
the faeces. The major group of metabolites (accounting for about 60%
of the total recovered 14C) comprised three phenols (fenthion phenol
[4-methylthio- meta-cresol] and its sulfoxide and sulfone) and their
glucuronide and sulfate conjugates. Four demethyl metabolites
(accounting for about 30% of the recovered radiolabel) and the
sulfoxide of fenthion oxon (constituting 1-4%) were also identified.
Fenthion is moderately toxic (LD50 = 50-500 mg/kg body
weight) to mice, rats, guinea-pigs, and rabbits when given orally,
intraperitoneally, dermally, or by inhalation. It is highly toxic to
avian species (especially to the wild mallard duck) when given
orally. The LC50 in rats for a 4-h exposure by inhalation was
0.5-0.9 mg/titre. Fenthion caused cholinergic toxicity with a long
recovery time. It did not irritate rabbit skin and was minimally or
not irritating to the rabbit eye; it did not sensitize guinea-pig
skin. WHO has classified fenthion as highly hazardous.
Fenthion potentiated the acute toxicity of other cholinergic
chemicals, such as malathion, dioxathion, and coumaphos, in rats. In
mice and dogs, pretreatment with fenthion inhibited the metabolism and
detoxification of 2- sec-butylphenyl methylcarbamate, resulting in
significant potentiation of its acute toxicity. There was no evidence
that other cholinergic chemicals potentiate the toxicity of fenthion.
Repeated short-term administration of fenthion (orally to mice,
rats, dogs and monkeys; dermally to rabbits; by inhalation to rats)
and its metabolite fenthion sulfoxide (orally to mice and rats),
resulted primarily in inhibition of cholinesterase. The NOAEL for oral
administration of fenthion, based on toxicologically significant
depression of acetylcholinesterase activity in the brain (> 10%)
and/or erythrocytes (> 20%), was 5 ppm (equivalent to 0.25 mg/kg bw
per day) in rats treated for three months or one year, 3 ppm (equal to
0.09 mg/kg bw per day) in dogs treated for two years, and 0.07 mg/kg
bw per day in monkeys treated for two years The NOAEL for fenthion was
50 mg/kg bw per day when applied dermally to rabbits for 21 days on
the basis of inhibition of brain acetylcholinesterase activity. The
NOAEL for fenthion in rats exposed by inhalation for 21 days was
0.001 mg/litre, on the basis of clinical signs of cholinergic toxicity
and inhibition of brain acetylcholinesterase at higher doses. The
NOAEL for fenthion sulfoxide administered orally to rats for four
weeks was 3 ppm (equivalent to 0.15 mg/kg bw per day) on the basis of
significant inhibition of brain and erythrocyte acetylcholinesterase
activity. No NOAEL was determined for fenthion or its metabolites in
mice; the LOAEL for fenthion was 150 ppm (equal to 83 mg/kg bw per
day) in mice exposed in the diet for four weeks, and the LOAEL for
fenthion sulfoxide in mice similarly exposed was 3 ppm (equivalent to
0.45 mg/kg bw per day).
In a carcinogenicity study, fenthion was administered in the diet
to mice at 0, 0.1, 1, 5 or 25 ppm for 102 weeks. The NOAEL for chronic
toxicity was 5 ppm (equal to 2 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. There was no evidence of carcinogenicity. In
two studies of chronic toxicity and carcinogenicity, rats received
fenthion at dietary concentrations of 0, 3, 15, or 75 ppm or 0, 5,
20, or 100 ppm for 24 months. The NOAEL for chronic toxicity
was 3 ppm (equal to 0.14 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. The compound was toxic to the eyes of rats at
100 ppm (equal to 5.2 mg/kg bw per day), inducing an increased
incidence of retinal atrophy, posterior subcapsular cataract
formation, corneal mineralization, and mineralization and optic nerve
atrophy, especially in females. No ocular toxicity was seen at doses
> 20 ppm (equal to 0.8 mg/kg bw per day). There was no evidence of
carcinogenicity.
In a two-generation study of reproductive toxicity (one litter
per generation), rats were fed diets containing fenthion at levels of
0, 1, 2, 14, or 100 ppm. The NOAEL for systemic toxicity in the parent
generation was 2 ppm (equal to 0.16 mg/kg bw per day) on the basis of
consistent inhibition of brain and erythrocyte acetylcholinesterase.
The NOAEL for reproductive toxicity was 14 ppm (equal to 1.2 mg/kg bw
per day) on the basis of decreased fertility, implantation sites,
litter size, pup viability, and growth at 100 ppm.
Two studies of developmental toxicity were performed in which
rats were exposed by gavage to fenthion at doses of 0, 1, 3, or
10 mg/kg bw per day or 0, 1, 4.2, or 18 mg/kg bw per day on days 6-15
of gestation. There was no NOAEL for maternal toxicity, owing to
toxicologically significant inhibition of brain and erythrocyte
acetylcholinesterase activity at doses > 1 mg/kg bw per day. the
NOAEL for embryo- and fetotoxicity and teratogenicity was 18 mg/kg bw
per day, the highest dose tested.
In a study of developmental toxicity in rabbits, fenthion was
administered by gavage at doses of 0, 1, 2.8, or 7.5 mg/kg bw per day
on days 6-18 of gestation. The NOAEL for maternal toxicity was 1 mg/kg
bw per day, on the basis of toxicologically significant inhibition of
brain and erythrocyte acetylcholinesterase activity at > 2.8 mg/kg
bw per day. The NOAEL for embryo- and fetotoxicity and teratogenicity
was 7.5 mg/kg bw per day, the highest dose tested.
Fenthion has been adequately tested for genotoxicity in a range
of assays in vivo and in vitro. While most showed no significant
response, positive results were obtained in two critical assays. The
Meeting concluded that fenthion is weakly genotoxic.
Fenthion did not cause delayed neuropathy in hens at doses higher
than the LD50.
In a four-week study of male volunteers, the NOAEL was 0.07 mg/kg
bw per day, the highest dose tested, on the basis of no inhibition of
erythrocyte acetylcholinesterase. In two investigations of workers
regularly exposed to fenthion, no evidence of neurotoxicity was
observed.
An ADI of 0-0.007 mg/kg bw was established on the basis of the
NOAEL of 0.07 mg/kg bw per day in the four-week study of human
volunteers, using a safety factor of 10. The ADI provides a margin of
safety of > 100-fold for chronic ocular toxicity and for the
reproductive toxicity observed in rodents.
The available data did not allow the Meeting to establish an
acute reference dose different from the ADI (0-0007 mg/kg bw). It
should be noted, however, that the ADI is derived from a study
of human volunteers in which 9-36% inhibition of plasma cholinesterase
but no inhibition of erythrocyte acetylcholinesterase was found at
the highest dose tested (0.07 mg/kg bw per day for 25 days). In
occupationally exposed workers, about 50% plasma cholinesterase
inhibition was found in the absence of erythrocyte acetyl-
cholinesterase inhibition. It follows that the acute reference
dose is likely to be somewhat higher than the ADI. Data on the
sensitivity to inhibition of plasma cholinesterase and erythrocyte and
brain acetylcholinesterase in vitro by the active metabolites of
fenthion might allow extrapolation to an LOAEL for humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 2.0 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equal to 0.14 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
2 ppm, equal to 0.16 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
14 ppm, equal to 1.2 mg/kg bw per day (two-generation study
of reproductive toxicity)
18 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Rabbit: 6 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
7.5 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 3 ppm, equal to 0.09 mg/kg bw per day (two-year study of
toxicity)
Monkey: 0.07 mg/kg bw per day (two-year study of toxicity)
Human: 0.07 mg/kg bw per day (four-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenthion
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Minimally or not irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Inhalation, 4-h, toxicity, rat LC50 = 0.5--0.9 mg/litre
Oral, toxicity, mouse, rat, guinea-pig, rabbit LD50 = 50-500 mg/kg bw
Oral, dermal, subcutaneous, single doses, NOAEL (all routes) = 5 mg/kg bw per day
cholinesterase activity, rat based on inhibition of brain and
erythrocyte acetylcholinesterase
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 50 mg/kg bw per day for
systemic toxicity on the basis of inhibition
of brain acetylcholinesterase
Repeated inhalation, 21 days, toxicity rat NOAEL = 0.001 mg/litre per day for
systemic toxicity on the basis of clinical
signs of cholinergic toxicity
Repeated dietary, 13-16 weeks, toxicity, rat NOAEL = 0.25 mg/kg bw per day, based
on inhibition of brain acetylcholinesterase
Repeated gavage, developmental toxicity, NOAEL = 1 mg/kg bw per day for
rabbit maternal toxicity on the basis of inhibition
of brain and erythrocyte acetylcholinesterase;
no embryo- or fetotoxic or teratogenic effects
Repeated oral (gelatin capsules), four weeks, NOAEL = 0.07 mg/kg bw per day on the
toxicity, human basis of no inhibition of erythrocyte
acetylcholinesterase
Long-term (> 1 year) Repeated dietary, two years, toxicity, dog NOAEL = 0.09 mg/kg bw per day on the
basis of inhibition of brain and erythrocyte
acetylcholinesterase
References
Avrahami, M. & White, D.A. (1975) Residues in milk of cows after
spot-treatment with 32P-fenthion. New Zealand Exp. Agric., 3,
309-311.
Bai, C.L., Qiao, C., Zhang, W., Chen, Y. & Qu, S. (1990) A study of
the pesticide fenthion: Toxicity, mutagenicity and influence on
tissue enzymes. Biomed. Environ. Sci., 3, 262-275.
Bailey, D.E. (1987) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 938 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bailey, D.E. (1988) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 1031 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bomann, W. (1991) E 1752 (c.n. Fenthion): Study for acute dermal
toxicity in the rat. Unpublished report No. 20221 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Bomhard, E. & Loser, E. (1977) Chronic toxicity study on rats.
Unpublished report No. 6769 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Brady U.E., Jr & Arthur, B.W. (1961) Metabolism of
O,O-dimethyl- O-[4-(methylthio)- m-tolyl] phosphorothioate
by white rats. J. Econ. Entomol., 54, 1232-1236.
Budreau, C.H. & Singh, R.P. (1973a) Teratogenicity and embryotoxicity
of demeton and fenthion in CF1 mouse embryos. Toxicol. Appl.
Pharm., 24, 324-332.
Budreau, C.H. & Singh, R.P. (1973b) Effect of fenthion and dimethoate
on reproduction in the mouse. Toxicol. Appl. Pharmacol., 26,
29-38.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982a) Sister-chromatid
exchanges and cell-cycle delay in Chinese hamster V79 cells
treated with nine organophosphorus compounds (8 pesticides and 1
defoliant). Mutat. Res., 103, 307-313.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982b) Sister-chromatid
exchanges in Chinese hamster cells treated with 17
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990a) Chronic feeding toxicity study of
technical-grade fenthion (Baytex) with dogs. Unpublished report
No. 94863 from Miles, Inc., Agriculture Division, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Christenson, W.R. (1990b) Combined chronic toxicity/oncogenicity study
of technical grade fenthion (Baytex) with rats. Unpublished
report No. 100586 from Mobay Corporation, Corporate Toxicology
Department, Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Christenson, W.R. (1990c) Technical grade fenthion (Baytex): A special
study to examine the effect of the route of acute administration
of technical grade fenthion (Baytex) on cholinesterase activity
in the rat. Unpublished report No. 100573 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
von Clarmann, M. & Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and
its demonstration in the gastric contents and urine. Arch.
Toxikol., 22, 2-11.
Clemens, G.R. (1987) A teratology study in the rabbit with fenthion
(Baytex technical). Unpublished report No. MTD0039 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Crosby, J., Hoglen, N. & Krautter, G. (1990) Nature of residues in
skin and tissues of swine after dermal treatment with
[14C]fenthion. Unpublished Mobay report No. 73984 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dean, G., Coxon, J. & Brereton, D. (1967) Poisoning by an
organophosphorus compound -- A case report. S. Afr. Med. J.,
41, 1017-1019.
Dellinger, J. & Mostrom, M. (1988) Effects of topical fenthion on
blood cholinesterase and vagal tone in dogs. Vet. Hum.
Toxicol., 30, 2,29-234.
Doolittle, K.D. & Bates, N.L (1993) Absorption, distribution and
elimination of 14C-fenthion in rats following a single oral,
repeated oral and single intravenous administration. Unpublished
Miles report No. 74395 from Southwest Bio-Labs, Inc., New Mexico,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. &
Meskauskas, J. (1961a) The effects of feeding diets containing
Bayer 29493 to rats for a period of 16 weeks. Unpublished rayer
Report No. 7899 from Department of Pharmacology, University of
Chicago, Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Doull J., Root, M. & Cowan, J. (1961b) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished Bayer report
No. 8342 from Department of Pharmacology, University of Chicago,
Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M. & Cowan, J. (1962) Effect of adding Bayer 29493 in
combination with other cholinergic insecticides to the diet of
male and female dogs. Unpublished Bayer report No. 9414 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, N.J., Vesselinovitch, D., Fitch, F.M. &
Meskauskas, J. (1963a) Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished Bayer report No. 14658 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, J. & Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
Bayer report No. 10853 from Department of Pharmacology,
University of Chicago, Illinois, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Dubois, K.P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished Mobay report No. 22503
from Department of Pharmacology, University of Chicago, Illinois,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Doull, J. (1960) The acute toxicity of Bayer 29493 to
chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago, Illinois, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Kino-hita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-dimethyl- O-4-
(methylthio- m-tolyl) phosphorotioate (DMTP; Baytex) and
related compounds. Toxicol. Appl. Pharmacol., 6, 86-95.
Eigenberg, D.A. (1987a) Acute oral toxicity of Baytex technical in
albino rats. Unpublished report No. 839 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987b) Acute dermal toxicity of Baytex technical in
albino rabbits. Unpublished report No. 838 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987c) Primary dermal irritation of Baytex technical
in albino rabbits. Unpublished report No. 896 from Mobay
Corporation, Corporate Toxicology Department, Kansas. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987d) Primary eye irritation of Baytex technical in
albino rabbits. Unpublished report No. 817 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Elliot, R. & Barnes, J.M. (1963) Organophosphorus insecticides for the
control of mosquitos in Nigeria. Bull. World Health Org., 28,
35-54.
Farage-Elawar, M. & Francis, B.M.(1987) Acute and delayed effects of
fenthion in young chicks. J. Toxicol. Environ. Health, 21,
455-469.
Farage-Elawar, M. & Francis, B.M. (1988a) Effects of multiple dosing
of fenthion, fentrothion, and desbromoleptophos in young chicks.
J. Toxicol. Environ. Health, 23, 217-228.
Farage-Elawar, M. & Francis, B.M. (1988b) Effects of fenthion,
fentrothion, and desbromoleptophos on gait, acetylcholinesterase
and neurotoxic esterase in young chicks after in ovo exposure.
Toxicology, 49, 253-261.
Flucke, W. (1986a) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute oral toxicity to hens
(Gallus domesticus). Unpublished report No. 15069 from Bayer
AG, Institut für Toxikologie, Wuppertal Germany.
Flucke, W (1986b) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute dermal toxicity to hens
(Gallus domesticus). Unpublished report No. 15113 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1987) E 1753 Technical (c.n fenthion): Study for skin
sensitising effect on guinea pigs (Magnusson and Kligman's
maximization test). Unpublished report No. 15428 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1988a) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following oral administration to hens.
Unpublished report No. 99275 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1988b) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following dermal administration to
hens. Unpublished report No. 99646 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1990) Position paper regarding the literature on
neurotoxicity for the BGA [German Federal Health Agency].
Unpublished report No. 18928 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. & Kaliner, G. (1987) E 1752 (c.n. fenthion, the active
ingredient of Baytex): Acute neurotoxicity studies on hens
following oral and dermal administration. Unpublished report No.
91341 from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Francis, J.I. & Barnes, J.M. (1963) Studies on the mammalian toxicity
of fenthion. Bull. World Health Org., 28, 205-212.
Francis, B.M. & Farage-Elawar, M. (1987) Peripheral and central enzyme
inhibition by fenthion, fenitrothion and desbromoleptophos. In:
Costa, L.G., Galli, C.L. & Murphy, S.D., eds, Toxicology of
Pesticides: Experimental, Clinical and Regulatory Perspectives,
Berlin, Springer Verlag.
Fytizas-Danielidou, R. (1971) Effects of pesticides on reproduction in
white rats. I. Labaycide. In: 23rd International Symposium of
Phytopharmacy, 4 May, 1971.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Griffin, T., Rosenblum, I. & Coulston, F. (1979) Safety evaluation of
fenthion in human volunteers. Unpublished Mobay report No. 68790
from the Institute of Comparative and Human Toxicology and
International Center of Environment Safety, Albany Medical
College, New York, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Hayes, R.H. (1989) Subchronic feeding study with fenthion technical
(Baytex) in hens with specific emphasis on gastrointestinal tract
effects. Unpublished report No. 99273 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Hayes, R.H. & Ramm, W.W. (1988) Subchronic delayed neurotoxicity study
of fenthion technical (Baytex) with hens. Unpublished report No.
98296 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Heimann, K.G. (1982) Fenthion and isofenthion: Study for acute
combination toxicity Unpublished report No. 11062 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1987) E 1752 (c.n. Fenthion): Salmonella/microsome
test for point-mutagenic effect. Unpublished report No. 98366
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1990a) E 1752 Salmonella/microsome test. Unpublished
report No. 18713 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Herbold, B.A. (1990b) E 1752 micronucleus test on the mouse.
Unpublished report No. 100025 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Hoffmann, K. & Weischer, C.H. (1975) Fenthion chronic study on dogs
(two-year feeding experiment). Unpublished report No. 5737 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981a) Fensulfoxide: Short-term toxicity
tests on mice (4-week feeding and 4-week recovery tests).
Unpublished report No. 198 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981b) Fensulfoxide: Short-term toxicity
tests on rats (4-week feeding and 4-week recovery tests).
Unpublished report No. 197 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Johnson, J.C. & Bowman, M.C. (1972) Responses from cows fed diets
containing fenthion or fenitrothion. J. Dairy Sci., 55,
777-782.
Kajiwara, Y. (1989) Chromosomal aberration test of fenthion using
cultured mammalian CHL cells. Unpublished report No. T-1824E from
HITA Research Lab., Japan. Submitted to WHO by Bayer AG,
Wuppertal, Germany.
Keith, J.O. & Mulla, M.S. (1966) Relative toxicity of five
organophosphorous mosquito larvicides to mallard ducks.
J. Wildlife Manage., 30, 553-563.
Kimmerle, G. (1967) Potential of DDVP and S 1752. Unpublished letter
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Klimmer, R. (1963) Toxicological testing of Bayer 29493. Unpublished
report from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Knowles, C.O. & Arthur, B.W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of
radiolabelled fenthion to dairy cows. J. Econ. Entomol., 59,
1346-1352.
Kowalski, R.L. (1987) A teratology study with fenthion (Baytex
technical) in the rat. Unpublished report No. MTD0029 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Kowalski, R.L., Clemens, G.R., Jasty, V., Troup, CM. & Hartnagel,
R.E., Jr (1989) A two-generation reproductive study with fenthion
(Baytex) in the rat. Unpublished report No. MTD0133 from Miles
Inc., Toxicology Department, Indiana, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Krautter, G. (1990a) Nature of residues in milk, skin and tissues of a
lactating cow after dermal treatment with [14C]fenthion.
Unpublished Mobay report No. 74012 from Pharmacology and
Toxicology Research Laboratory, Kentucky, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Krautter, G. (1990b) Characterization of polar metabolites in river
and kidney tissues from a cow and pig treated dermally with
[14C] fenthion. Unpublished Mobay report No. 74124 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Krotlinger, F. (1993) E 1752 (c.n. Fenthion): Study for acute
intraperitoneal toxicity in rats. Unpublished report No. 22144
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Lamb, D.W. & Anderson, R.H. (1974) The acute dermal toxicity of
Tiguvon technical to rabbits. Unpublished report No. 41455 from
Chemagro, Bayer GmbH, Leverkusen, Germany. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Lehn, H. (1990a) E 1752 (c.n. Fenthion): Mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished report No. 18740 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Lehn, H. (1990b) E 1752 (c.n. Fenthion): Mutagenicity test on
unscheduled DNA synthesis in rat liver primary cell cultures
in vitro. Unpublished report No. 100572 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Leser, K.H. (1990) E 1752 (Fenthion): Study for cholinesterase
inhibition following high doses of E1752 (administration to
B6C3F1 mice in the diet over a period of about four weeks).
Unpublished report No. 19088 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Machemer, L. (1978a) S 1752 (Fenthion, Lebaycid active ingredient):
Evaluation for embryotoxic and teratogenic effects in orally
dosed rats. Unpublished report No. 7580 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Machemer, L. (1978b) S 1752 (Fenthion, Lebaycid active ingredient):
Dominant lethal study of male mice to test for mutagenic effects.
Unpublished report No. 7449 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Mihail, F. (1978) S 1752 (Lebaycid active ingredient): Determination
of percutaneous toxicity. Unpublished report No. 7604 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Mihail, F. & Schilde, B. (1979) S 1752 (Fenthion, the active
ingredient of Lebaycid and Baytex): Subacute dermal cumulative
toxicity on rabbits. Unpublished report No. 8624 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Misra, U.K., Nag, D., Bhushan, V. & Ray, P.K. (1985) Clinical and
biochemical changes in chronically exposed organophosphate
workers, Toxicol, Lett., 24, 187-193.
Misra, U.K.. Nag, D., Khan, W.A. & Ray, P.K. (1988) A study of nerve
conduction velocity, late responses and neuromuscular synapse
functions in organophosphate workers in India. Arch. Toxicol.,
61, 496-500.
Miyaoka, T., Takahashi, H., Tsuda, S. & Shirasu, Y. (1984)
Potentiation of acute toxicity of 2- sec-butylpheny-
N-methylcarbamate (BPMC) by fenthion in mice. Fundam. Appl.
Toxicol., 4, 802-807.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1986) Mechanism of potentiation
of BPMC toxicity by fenthion pretreatment in mice.
J. Pharmacobio-Dyn., 9, 697-703.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1987) Effect of O,O-dimethyl
O-(3-methyl-4-methylthiophenyl) phosphorothioate (fenthion)
pretreatment on acute toxicity of 2- sec-butylphenyl
N-methylcarbamate (BPMC) in dogs. Jpn. J. Vet. Sci., 49,
173-175.
Nelson, D.L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Pauluhn, J. (1985) E 1752 Technical (c.n. fenthion): Study for
irritant/corrosive effect on skin and eye (rabbit). Unpublished
report No. 13446 from Bayer AG, Institute für Toxikologie,
Wuppertal, Germany.
Pickering, E.N. (1966) Organic phosphate insecticide poisoning.
Can. J. Med. Technol., 28, 174-179.
Pither, K.M. (1979) Metabolism of Baytex in male and female pigs.
Unpublished report No. 68475 from Mobay Corporation, Corporate
Toxicology Department, Kansas, USA. Submitted to WHO by Bayer AG,
Wuppertal Germany.
Puhl, R.J. & Hurley, J.B. (1982) The absorption, excretion and
metabolism of Baytex-ring-1-14C by rats. Unpublished report No.
82227 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report No. 99660 from
Microbiological Associates Inc., Maryland, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Rani, M.V.U & Rao, M.S. (1991) In vivo effect of fenthion on human
lymphocytes. Bull. Environ. Contamin. Toxicol., 47, 316-320.
Rosenblum, I. (1980) A safety evaluation of fenthion (S 1752) in
rhesus monkeys (Macaca mulatta). Unpublished Mobay report No.
68789 from Albany Medical College, New York, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Sherman, M. & Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. Appl. Pharmacol., 3, 521-533.
Shimamoto, K. & Hattori, K. (1969) Chronic feeding of Baytex
( O,O-dimethyl- O-(4-methylmercapto-3-methyl)phenylthiophosphat
e) in rats. Acta Med. Univ. Kyoto, 40, 163-171.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Baytex in rats. Unpublished report No. 944 from
Mobay Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Shiotsuka, R.N. (1987b) Acute inhalation toxicity study with Baytex
technical in rats. Unpublished report No. 820 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1977) Evaluation of
selected pesticides as chemical mutagens: In vitro and in
vivo studies. US Environmental Protection Agency Health Effects
Research Laboratory Document No. EPA-600/1-77-028 from Stanford
Research Institute, California, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Singh, S.P., Sharma, L.D. & Bahga, H.S. (1987) Acute oral toxicity of
fenthion in poultry. Indian J. Anim. Res., 21, 48-50.
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutat. Res., 102,
89-102.
Suberg, H. & Leser, K.H. (1990) E 1752 Oncogenicity study on B6C3F1
mice (feeding study for periods of up to 24 months). Unpublished
report No. 19624 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull.
World Health Org., 28, 213-218.
Thyssen, J. (1978) S 1752 (Lebaycid-active ingredient): Acute
inhalation toxicity studies. Unpublished report No. 7842 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Thyssen, J. (1979) Fenthion (S 1752, the active ingredient of Lebaycid
and Baytex): Subacute inhalation toxicity study on rats.
Unpublished report No. 8383 from Bayer AG Institut für
Toxikologie, Wuppertal, Germany.
Tuler, S.M. & Bowen, J.M. (1989) Toxic effects of organophosphates on
nerve cell growth and ultrastructure in culture. J. Toxicol.
Environ. Health, 27, 209-223.
Tuler, S.M., Febles, D. & Bowen, J.M. (1988) Neuromuscular effects of
chronic exposure to fenthion in dogs and predictive value of
electromyography. Fundam. Appl. Toxicol., 11, 155-168.
US National Cancer Institute (1979) Bioassay of fenthion for possible
carcinogenicity (National Cancer Institute Carcinogenesis
Technical Report Series No. 103; US Department of Health,
Education, and Welfare Publication No. (NIH) 79-1353). Bioassay
conducted by Gulf S. Research Institute, Louisiana, USA, under
contract to NCI and subcontract to Tracor Jitco, Inc., who
prepared the final report.
Wadia, R.S., Bhirud, R.H., Gulavani, A.V. & Amin, R.S. (1977)
Neurological manifestations of three organophosphate poisons.
Indian J. Med. Res., 66, 460-468.
Weber, H. & Ecker, W. (1992) [phenyl-1-14C]Fenthion: Absorption,
distribution, excretion and metabolism in a lactating goat.
Unpublished report No. 3752 from Bayer AG, Institut für
Metabolismusforschung, Leverkusen-Bayerwerk, Germany. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Wolfe, H.R., Armstrong, J.F. & Durham, W.F. (1974) Exposure of
mosquito control workers to fenthion. Mosquito News, 34,
263-267.

 Two lactating cows received 32p-fenthion as a single dermal dose
of about 13 mg/kg bw or a single intramuscular dose of about 8.5 mg/kg
bw. Unchanged fenthion constituted > 50% of the nonionic residues in
the milk for three days after the dermal and seven days after the
intramuscular treatment. In urine, > 95% of the radiolabel excreted
was in the form of hydrolytic products; the parent compound accounted
for only a small percentage of the chloroform-soluble residues. In
faeces, unchanged fenthion constituted > 50% of the acetonitrile-
soluble residues. The animals were slaughtered 14 days after the
dermal and 21 days after the intramuscular treatment; > 50% of the
tissue residues appeared as unchanged fenthion, but oxidation products
were also present (Knowles & Arthur, 1966).
One lactating dairy cow (Jersey breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 98.2%) at 5.08 mg/kg bw active
ingredient, as a formulation for treatment of lice and horn flies in
beef and non-lactating dairy cattle, and sacrificed 18 h after
treatment. Urine, milk, skin, and selected tissues were then analysed
for 14C-fenthion residues. Unchanged (parent) fenthion was the major
tissue residue, accounting for > 95% of the residues in all tissue
samples collected from the application site, 71-95% in liver, muscle,
and peritoneal fat, and 51% in kidney. Other minor residue components
identified included fenthion sulfoxide (muscle, 5% of residue) and
fenthion sulfone (liver, 8% of residue). In addition, a number of
unknown polar metabolites were found in the liver and kidney,
representing 22 and 44%, of the total residue, respectively, two of
which were identified by high-performance liquid chromatography in a
later study (Krautter, 1990b) as glucuronide conjugates of phenol
sulfoxide and phenol sulfone, the primary metabolites of fenthion
(Krautter, 1990a).
A single lactating goat received [phenyl-1-14C]-fenthion
(chemical and radiopurity, > 99%) by gavage at 20 mg/kg bw daily for
three consecutive days and was sacrificed 3.5 h after the last dose.
The nature of the residues was determined in urine, milk, and edible
tissues. Phenol fenthion, phenol sulfoxide, and phenol sulfone were
the three major metabolites in all tissues examined. Various
intermediate metabolites in the biodegradation from parent fenthion to
the phenols (demethylated phosphorus-containing compounds with varying
oxidation at the methylsulfur moiety and/or at the phosphoric acid
moiety of the molecule) were also identified. Demethyl fenoxon
sulfoxide and sulfone were especially predominant in muscle, while the
sulfoxide and sulfone of fenthion constituted about 30% of the
residues in fat. Unchanged fenthion was not detected in any tissue
sample. The metabolites excreted in urine were similar to those in the
liver, exception that a higher percentage of phenol sulfide was
present in the urine (Weber & Ecker, 1992).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion are
summarized in Table 1. Technical-grade fenthion is moderately toxic to
mammals (mice, rats, guinea-pigs, and rabbits) when given by oral,
intraperitoneal, dermal, or inhalation routes. It is highly toxic to
arian species (especially to the wild mallard duck) when administered
orally. The clinical symptoms of acute toxicity are consistent with
those of central and peripheral cholinergic intoxication by
organophosphorus esters, including decreased spontaneous activity,
apathy, dyspnoea, ataxia, tremors, convulsions, lacrimation,
salivation, and diarrhoea. The symptoms were reversible in surviving
animals.
(b) Short-term toxicity
Exposure to fenthion by inhalation for 6 h/day daily for nine
days at 0.21 mg/litre (initial concentration in a static inhalation
chamber) resulted in signs of poisoning but no mortality, in rats,
guinea-pigs, rabbits, or cats. Serum and erythrocyte cholinesterase
activities were severely depressed but recovered within three weeks
(Klimmer, 1963).
Mice
Groups of 10 male and 10 female B6C3F1 mice were given E 1752
technical (purity, 98.7%) in the diet at 0 (control diet), 150, 200,
or 250 ppm (equal to 0, 83.2, 117.2, and 140.3 mg/kg bw per day in
males and 0, 83.6, 114.5, and 151.8 mg/kg bw per day in females) for
one month. No NOAEL was seen At the lowest dose, emaciation, tremor,
and decreased body weight were observed in males, reduced water
consumption and decreased spleen weight in animals of each sex, and
reduced liver weight in females. Severe inhibition (68-80%) of brain
and erythrocyte acetylcholinesterase was noted in all animals. At the
two higher doses, additional treatment-related effects seen were a
moribund condition resulting in unscheduled sacrifice of two males per
dose group and emaciation and decreased body weight in females (Leser,
1990).
Rats
Five groups of 22 male and 22 female rats were fed diets
containing technical-grade fenthion (purity not given, in soya bean
oil) at 0, 0.25, 0.5, 2.5, or 5.0 mg/kg bw per day for three months.
The NOAEL was 0.25 mg/kg bw per day, based on depression of
cholinesterase activity in erythrocytes, plasma, heart, liver, and
brain (no raw data) at > 0.5 mg/kg bw per day. At this dose and
above, clinical signs of cholinergic intoxication and significant
Table 1. Acute toxicity of fenthion
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Mouse Male Oral 150 Francis & Barnes (1963)
Female 190
Male Oral 227 Dubois (1968)
Female 225
Male Intraperitoneal 125 Dubois & Kinoshita (1964)
Female 150
Female Intraperitoneal 200-2404 Budreau & Singh (1973a)
Rat Male Oral 175-470 Klimmer (1963); Francis & Barnes (1963);
Female 245-310 Gaines (1969); Dubois & Kinoshita (1964)
Male Oral 405 Eigenberg (1987a)
Female 566
Male Intraperitoneal 260 Dubois & Kinoshita (1964)
Female 325
Male Intraperitoneal 152 Klimmer (1963)
Male Intraperitoneal 330 Krotlinger (1993)
Female 203
Male Dermal 330-650 Klimmer (1963); Francis & Barnes (1963);
Gaines (1969); Dubois & Kinoshita (1964)
Female 330-500
Male Dermal 1680 Mihail (1978)
Female 2830
Male Dermal 586 Bomann (1991)
Female 800
Male, female Inhalation (1-h) > 1.197 Thyssen (1978)
Male Inhalation (4-h) 1.2 Thyssen (1978)
Female 0.8
Male Inhalation (1-h) 1.838 Shiotsuka (1987a)
Female 1.637
Table 1. (con't)
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Male Inhalation (4-h) 0.919 Shiotsuka (1987a)
Female 0.819
Male Inhalation (4-h) 0.507 Shiotsuka (1987b)
Female 0.454
Guinea-pig Male Oral > 1000 Francis & Barnes (1963)
Male Oral 260 Dubois & Kinoshita (1964)
Male Intraperitoneal 310 Dubois & Kinoshita (1964)
Rabbit Male Oral 150-175 Francis & Barnes (1963)
Male Dermal 150 Klimmer (1963)
Male Dermal 150 Lamb & Anderson (1974)
Female 131
Male, female Dermal 667 Eigenberg (1987b)
Chicken NR NR 310 Dubois & Doull (1960)
Male Oral 28 Sherman & Ross (1961)
Female Oral 30-40 Francis & Barnes (1963)
Female Oral 27 Flucke (1986a)
Male Oral 37.5 Singh et al. (1987)
Female Dermal 222 Flucke (1986b)
Duck NR Oral 15 Dubois & Doull (1960)
NR Oral 1-2 Keith & Mulla (1966)
NR, not reported
reductions in food consumption and body weight were evident. At the
two highest doses, decreased spermatogenesis and atrophic prostate
glands were noted in males; the female reproductive system was not
affected. All females at the highest dose died during the second and
third weeks of treatment (Shimamoto & Hattori, 1969).
Six groups of 12 male and 12 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0.2, 3, 5,
25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5.0 mg/kg
bw per day, for 16 weeks. No treatment-related adverse effects were
observed on body weight or body-weight gain or on food consumption;
gross and microscopic examination of tissues also showed no effect,
The NOAEL was 0.25 mg/kg bw per day based on cholinesterase depression
(source of enzyme not specified) at 1.25 mg/kg bw per day (Doull
et al., 1961a).
Six groups of 25 male and 25 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 3,
5, 25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5 mg/kg
bw per day, for one year. Significant inhibition (> 20%) of
erythrocyte acetylcholinesterase activity was noted at > 5 ppm.
Brain acetylcholinesterase activity was significantly depressed
(> 10%) and a higher mortality rate (particularly among males) was
observed at 25 ppm and above. At the highest dose, increased
haemosiderosis was noted in the spleen. The NOAEL was 0.25 mg/kg bw
per day, based on significant inhibition of brain acetylcholinesterase
at higher doses (Doull et al., 1963a),
Four groups of 10 male and 10 female Wistar rats were exposed to
an aerosol of technical-grade fenthion (purity, 98.2%) at nominal
concentrations of 0, 0.003, 0.015, or 0.075 mg/litre, equal to
analytical concentrations of 0, 0.001, 0.003, or 0.016 mg/litre, in a
head-/nose-only inhalation chamber for 6 h/day, five days per week for
three weeks. Exposure did not induce mortality or changes in body
weight, the results of haematology, clinical chemistry, or urinalysis,
gross pathology, or organ weights. At analytical concentrations
> 0.003 mg/litre, clinical symptoms of behavioural disturbance
(inactivity and/or unpreened coats) were observed in females only;
plasma cholinesterase was depressed in animals of each sex.
Significant inhibition of erythrocyte and brain acetylcholinesterase
activities occurred at the highest dose, and treatment-induced
inflammatory changes of the respiratory tract were seen in females.
The NOAEL was 0.001 mg/litre (analytical), based on clinical signs of
intoxication in females at 0.003 mg/litre and depression of brain and
erythrocyte acetylcholinesterase activities at 0.016 mg/litre
(Thyssen, 1979).
Rabbits
Groups of six male and six female New Zealand white rabbits
received dermal applications of technical-grade fenthion (purity,
98.2%) in Cremophos EL (1.5% v/v in distilled water) on intact (three
animals) or abraded (three animals) skin sites at 0, 5, or 25 mg/kg bw
per day for 7 h each day, five days per week for three weeks. The
NOAEL was 5 mg/kg bw per day, based on inhibition of erythrocyte
acetylcholinesterase in animals of each sex at 25 mg/kg bw per day. No
treatment-related effect was observed on general appearance,
behaviour, or body weight or by haematology, clinical chemistry,
urinalysis, gross pathology, organ weight measurement, or
histopathology (Mihail & Schilde, 1979).
Groups of five male and five female HRA:(NZW) specific pathogen-
free rabbits received dermal applications of technical-grade fenthion
(purity, 96.9%) formulated in Cremophos EL (1.5% v/v in distilled
water) on intact skin sites at 0, 5, 50, or 100 mg/kg bw per day for
6 h each day, five days per week for three weeks. The NOAEL was
50 mg/kg bw per day, based on inhibition of brain acetylcholinesterase
in females at 100 mg/kg bw per day. No treatment-related effect was
observed on appearance, behaviour, or body weight, or by haematology,
clinical chemistry, urinalysis, gross pathology, organ weight
measurements, or histopathology (Bailey, 1987).
Groups of five male and five female HRA:(NZW) specific
pathogen-free rabbits received dermal applications of technical-grade
fenthion (purity, 96.9%) formulated in Cremophos EL (1.5% v/v in
distilled water) on intact skin sites at 0, 200, or 400 mg/kg bw per
day for 6 h each day, five days per week for three weeks. The study
was terminated within two weeks of the start of treatment owing to
high mortality. An additional group of five male and five female
rabbits was given a lower dose of 150 mg/kg bw per day under similar
conditions; the original control group was retained. This treatment
did not affect general appearance, behaviour, body weight, or food
consumption, or the results of haematology, clinical chemistry, organ
weight measurement, or gross or histopathology. Plasma, erythrocyte,
and brain cholinesterase activities were depressed in animals of each
sex, with a significant inhibition of > 50% in males and 30% in
females at termination of the study. The LOAEL was 150 mg/kg bw per
day, based on inhibition of brain and erythrocyte acetylcholinesterase
activities (Bailey, 1988).
Dogs
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, or 1.25 mg/kg bw per day) for
12 weeks. The NOAEL was 0.125 mg/kg bw per day, based on significant
(> 20%) inhibition of erythrocyte acetylcholinesterase activity at
50 ppm. At > 5 ppm, plasma cholinesterase was also depressed, but
the effect (an indication of exposure) was not considered to be
toxicologically significant (Doull et al., 1961b).
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, and 1.25 mg/kg bw per day)
for one year. The NOAEL was 0.125 mg/kg bw per day, based on
significant inhibition of erythrocyte (> 20%) and brain (> 10%)
acetylcholinesterase activity at 50 ppm. At > 5 ppm, plasma
cholinesterase was also depressed, but the effect (an indication of
exposure) was not considered to be toxicologically significant. A
slight increase in spleen weight with splenic congestion,
extramedullary haematopoiesis, and haemosiderosis, was also observed
in all treated animals; the effect was not dose-related and not
considered to be adverse (Doull et al., 1963b).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade fenthion (purity, 97%) at 0, 2, 10, or
50 ppm, equal to 0, 0.06, 0.26, or 1.23 mg/kg bw per day in males and
0, 0.06, 0.26, or 1.18 mg/kg bw per day in females, for one year. The
NOAEL was 0.06 mg/kg bw per day, based on toxicologically significant
inhibition (> 10%) of brain acetylcholinesterase activity at 10 ppm
At the highest dose, both erythrocyte and brain acetylcholinesterase
activities were significantly depressed (> 50 and > 30%,
respectively). Plasma cholinesterase activity was inhibited at all
doses tested; but the effect (an indicator of exposure) was not
considered to be toxicologically significant. No other treatment-
related change was observed (Christenson, 1990a).
Groups of four male and four female pure-bred dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 3, 10,
or 30 (weeks 0-64), 50 (weeks 65-67), or 60 (weeks 68-104) ppm (equal
to 0, 0.09, 0.32, and 1.28 mg/kg bw per day in animals of each sex)
for two years. No mortality or clinical sign of toxicity was seen
during the study. No significant treatment-related change in body
weight, ophthalmoscopic, haematological, or urinary parameters, gross
or histopathology of tissues was evident at any dose. The NOAEL was
0.09 mg/kg bw per day, based on significant inhibition of erythrocyte
acetylcholinesterase activity in males (> 20%) and of brain
acetylcholinesterase activity in females (> 10%) at 10 ppm. At the
highest dose, erythrocyte and brain acetylcholinesterase activities
were significantly depressed in animals of each sex. Decreased food
consumption (females) and lowered plasma protein levels (males) were
also observed (Hoffmann & Weischer, 1975).
Monkeys
Four groups of five male and five female rhesus monkeys were
given daily doses of technical-grade fenthion (purity, 98.1%) in corn
oil by gavage at concentrations of 0, 0.02, 0.07, or 0.20 mg/kg bw per
day for two years. Animals were observed daily for general appearance
and clinical signs; body weight and ophthalmological parameters were
recorded monthly, and clinical chemistry, haematology, and urinalyses
were performed at 0, 1, 3, 6, 12, 18, and 23 months. Plasma and
erythrocyte cholinesterase activities were measured weekly for the
first four weeks and monthly thereafter. One animal of each sex at 0
and 0.20 mg/kg bw per day was killed seven months and three weeks
after the beginning of treatment for measurement of brain
acetylcholinesterase and gross and histopathology. All monkeys
underwent necropsy after 23 months, but no histopathology was
performed. Plasma cholinesterase was consistently depressed in animals
of each sex at 0.20 mg/kg bw per day, but the effect (an indicator of
exposure) was not considered to be toxicologically significant. A
significant (> 20%) inhibition of erythrocyte acetylcholinesterase
activity was observed occasionally in animals of each sex (9/26
determinations over the 23-month study period for males and 2/26 for
females) at the highest dose. No effect on brain acetylcholinesterase
was evident in the four animals killed at 7.25 months. Gross
examination revealed abnormally small testes and ovaries in animals at
0.20 mg/kg bw per day; however, in the absence of organ weights and
histopathological data, the toxicological significance of this finding
could not be fully assessed. No other treatment-related adverse
effect was recorded. The NOAEL was 0.07 mg/kg bw per day, based on
inconsistent erythrocyte acetylcholinesterase depression and possible
effects on male and female reproductive organs at 0.20 mg/kg bw per
day (Rosenblum, 1980).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of male and female B6C3F1 mice, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) in the diet at concentrations of 0,10,
or 20 ppm (equivalent to 0, 1.5, or 3.0 mg/kg bw per day) for 103
weeks. All surviving animals were maintained on control diet for up to
one week after the end of treatment before they were sacrificed and
subjected to gross and microscopic examination. Treatment with
fenthion did not result in significant differences in the survival or
growth rate of mice at any dose. Clinical signs of intoxication were
evident four months after the beginning of treatment (LOAEL not
specified). At the end of the study (104 weeks), histopathological
examination revealed a variety of neoplasms, the majority of which
were judged to be unrelated to fenthion administration. An increased
incidence of sarcomas, fibrosarcomas, or rhabdomyosarcomas of the
integumentary system was observed in treated male mice; the incidences
of integumentary sarcomas were 0/25 at 0 ppm, 7/49 at 10 ppm, and 8/48
at 20 ppm (7/435 in historical controls). The effect was considered to
be treatment-related, indicating that technical-grade fenthion is
oncogenic in male mice under the conditions of the study. There was no
NOAEL for systemic toxicity, since the LOAEL for clinical signs was
not specified (US National Cancer Institute, 1979).
Groups of 60 male and 60 female specific-pathogen-free B6C3F1
mice were fed diets containing technical-grade fenthion (purity,
92.3%) at 0, 0.1, 1.0, 5.0, or 25 ppm, equal to 0, 0.03, 0.40, 1.95,
or 9.42 mg/kg bw per day in males and 0, 0.03, 0.47, 2.25, or
10.63 mg/kg bw per day in females, for 102 weeks. Additional groups of
20 mice of each sex were fed the same diets for 50 weeks before
scheduled interim kills. Brain acetylcholinesterase activity was
depressed by > 10% at > 0.1 ppm in males and at > 1.0 ppm in
females, but the effect was not dose-related and was seen in males at
terminal but not interim sacrifice and in females at interim but not
terminal sacrifice. Significant (> 20%) inhibition of erythrocyte
acetylcholinesterase activity occurred only in the males at 25 ppm at
week 54. As the inhibition of brain acetylcholinesterase was only
marginally significant (10-17%) at the three lower doses, there was no
dose-response relationship, and the effect was inconsistent over
treatment intervals, it was judged to be of minimal toxicological
significance at doses > 5.0 ppm. The NOAEL for systemic toxicity
was 1.95 mg/kg bw per day, as brain acetylcholinesterase activity was
clearly depressed at the next highest dose in males at both interim
and terminal sacrifice (29-32%) and in females at interim sacrifice
(26%). At this dose, there were also significant increases in body
weight in animals of each sex, elevated plasma cholesterol levels in
animals of each sex early in the study, and elevated absolute and
relative liver weights of male mice at termination. In the absence of
remarkable histopathological findings, the changes at 25 ppm were
considered not to be toxicologically significant. There was no
increased incidence of any neoplastic or non-neoplastic lesion in the
treated mice at any dose up to and including 9.42 mg/kg bw per day.
Technical-grade fenthion was thus considered not to be oncogenic to
mice under the conditions of the study (Suberg & Leser, 1990).
The observation of an increased incidence of integumentary
sarcomas in male mice (US National Cancer Institute, 1979) at doses of
1.5 and 3.0 mg/kg bw per day was not reproduced in a second study
(Suberg & Leser, 1990) in which doses up to 9.42 mg/kg bw per day were
tested. As the increased tumour incidence in the first study was not
dose-related and was site-and sex-specific and the results of the
second study (conducted with more control animals and more groups for
interim sacrifice) were clearly negative at doses up to three times
those of the first study, technical-grade fenthion would appear not to
be oncogenic in the mouse.
Rats
Groups of male and female Fischer 344 rats, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) at dietary concentrations of 0, 10, or
20 ppm (equivalent to 0, 0.5, or 1.0 mg/kg bw per day) for 103 weeks.
All surviving animals were maintained for one or two weeks after the
end of treatment and were then sacrificed and submitted to gross and
microscopic examination. No treatment-related mortality or behavioural
changes were observed, and the growth rate was comparable in control
and treated groups. Histopathological examination revealed a variety
of neoplastic and non-neoplastic lesions common to rats of this
strain. No increase in the incidence of any type of tumour was
observed in the treated rats at any dose up to and including 20 ppm
(1.0 mg/kg bw per day). Technical-grade fenthion was thus considered
not to be oncogenic to the rat under the conditions of the study (US
National Cancer Institute, 1979).
Groups of male and female specific-pathogen-free Wistar rats, 100
of each sex in the control group and 50 of each sex per dose, were fed
technical-grade fenthion (purity unspecified) in the diet at
concentrations of 0, 3, 15, or 75 ppm, equal to 0, 0.14, 0.72, or
374 mg/kg bw per day in males and 0, 0.19, 0.93, or 4.46 mg/kg bw per
day in females, for 24 months. The rats were weighed weekly during the
first 26 weeks and every two weeks thereafter. Food consumption was
recorded weekly. Haematology, clinical chemistry, liver and kidney
funtional tests, and urinalysis were performed on five rats of each
sex per group at 1, 3, 6, and 12 months, and on 10 rats of each sex
per group at the end of the study (24 months). Plasma and erythrocyte
cholinesterase activities were determined at the end of weeks 1, 2, 4,
8, 13, 26, 52, 78, and 105; brain acetylcholinesterase activity was
not measured. At the end of the study, all surviving rats were
sacrificed and examined macroscopically, and tissues and organs were
removed, weighed, and studied microscopically. Doses of 3 or 15 ppm
did not affect physical appearance, behaviour, growth, or survival
rate, but males at the highest dose had a significantly lower body
weight and both males and females appeared to have increased mortally.
No treatment-related effect was observed by haematology, clinical
chemistry, urinalysis, or gross or microscopic pathology. (It should
be noted that the rats from which blood and urine samples were taken
for analysis during the first year were not identified.) Significant,
dose-dependent depression of plasma and erythrocyte cholinesterase
activities was observed in animals of each sex at 15 and 75 ppm. The
NOAEL for systemic toxicity, based on erythrocyte acetylcholinesterase
inhibition, was 0.14 mg/kg bw per day. The results of histopathology
showed no increase in the incidence of tumours in treated rats at any
dose up to and including 3.74 mg/kg bw per day, the highest dose
tested, indicating that technical-grade fenthion was not oncogenic to
the rat under the conditions of the study (Bomhard & Loser, 1977).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenthion (purity, 97.0%) at concentrations
of 0, 5, 20, or 100 ppm, equal to 0, 0.2, 0.8, or 5.2 mg/kg bw per day
in males and 0, 0.3, 1.3, or 7.3 mg/kg bw per day in females, for two
years. Two additional groups of 20 rats of each sex were fed diets
containing fenthion at concentrations of 0 or 100 ppm for one year
before scheduled interim sacrifice. No NOAEL for systemic toxicity
could be determined, because of statistically (P < 0.05) and
toxicologically significant (> 10%), dose-related inhibition of brain
acetylcholinesterase activity in animals of each sex at all doses,
including the lowest dose of 0.20 mg/kg bw per day. Plasma and
erythrocyte cholinesterase activities were also depressed at all
doses, but toxicologically significant (> 20%) inhibition of
erythrocyte acetylcholinesterase occurred only at > 20 ppm. At the
highest dose, technical-grade fenthion was retinotoxic, especially in
females, and ophthalmological examination revealed an increased
incidence of retinal degeneration and posterior subcapsular cataract
formation in females and focal corneal scarring in animals of each
sex. Histopathological examination revealed a higher incidence of
corneal mineralization and retinal atrophy in females, and corneal
neovascularization and optic nerve atrophy in animals of each sex. The
retinotoxic effect was further confirmed by electroretinographic
examination, with suppression of electroretinograms also in a number
of females at 20 ppm at interim sacrifice at week 75. At 100 ppm,
additional treatment-related effects were an increased incidence of
nonspecific clinical signs (urine staining, tail and paw lesions,
rough coat, and hunched back), lower body-weight gain, and remarkable
histopathological findings including vacuolar degeneration of the
nasolacrimal ducts, mineralized and raised areas in the stomach,
stomach acanthosis, hyperkeratosis (males) and hyperplasia (females),
epididymal vacuolar degeneration, and chronic active inflammation of
the skin of the tail and hindlimbs. A higher incidence of vacuolar
degeneration of the nasolacrimal ducts was also seen in females at
20 ppm. There were no treatment-related oncogenic effects in males or
females at any dose up to and including 5.2 mg/kg bw per day, the
highest dose tested. Technical-grade fenthion was thus considered not
to be oncogenic to the rat under the conditions of the study
(Christenson, 1990b).
(d) Reproductive toxicity
Mice
In a five-generation, two-litter per generation study, two groups
of CD-1 mice received technical-grade fenthion (purity unspecified) in
their drinking-water at concentrations of 0 or 60 ppm (equivalent to
9.0 mg/kg bw per day). The control group comprised 10 males and 14
females, and the treated group comprised 10 males and 22 females.
There was a significant increase in pup mortality during the first
postnatal week, especially in the second, third, and fourth
generations. No treatment-related effect on reproductive performance,
lactation, or growth rate of pups was observed. No histopathological
changes were noted in the liver or kidney of adult males of the third
generation (Budreau & Singh, 1973b).
Rats
In a two-generation, one-litter per generation study in Crl:CD BR
rats, five groups of 30 male and 30 female animals were fed diets
containing technical-grade fenthion (purity, 96.9%) at concentrations
of 0, 1, 2, 14, or 100 ppm, equal to 0, 0.08, 0.16, 1.16, or 8.3 mg/kg
bw per day (based on premating food intake values), for 70 days (F0
and F1 parents) before mating (1:1 ratio) for production of the F1
and F2 litters. No treatment-related effect on mortality, clinical
signs of toxicity, mean body weight, mean food consumption, or gross
pathology was observed in adults of the F0 or F1 generation.
Epididymal weight was increased in F0 males at 100 ppm.
Histopathological examination revealed vacuolation in the lining
ductal epithelial cells of the corpus epididymus in a few F0 and F1
males at 14 ppm and in all at 100 ppm. Brain acetylcholinesterase
activity was significantly (> 10%) depressed in F0 females at all
doses and in F0 males and F1 males and females at the two higher
doses. Inhibition at the two lower doses (-22 and -17%, respectively)
in F0 females was not dose-related and was of a magnitude similar
to those at 14 ppm in F0 males (-19%) and in F1 males (-28%)
and females (-18%). F0 female controls had a mean brain
acetylcholinesterase activity of 2428 mU/g, which was significantly
higher than the other control values observed in the study (2137,
1894, and 1515 mU/g for F0 male, F1 male, and F1 female controls,
respectively). The effect on brain acetylcholinesterase activity seen
in F0 females at 1 and 2 ppm was therefore judged to be of minimal
toxicological significance. At the two higher doses, significant
(> 20%) inhibition of plasma and erythrocyte cholinesterase
activity was seen in all parental adults and pups. Neonatal brain
acetylcholinesterase activity was inhibited only at the highest dose.
The NOAEL for maternal toxicity was 0.16 mg/kg bw per day, based on
consistent inhibition of brain and erythrocyte acetylcholinesterase
activity at 14 ppm. There was no treatment-related effect on
reproductive performance, embryo- or fetotoxicity, litter size, pup
viability, or growth at > 14 ppm. At the highest dose, decreased
fertility, a decreased number of implantation sites per dam, decreased
litter size, an increased number of stillborn pups per litter, a
reduced viability index (days 0-4), and decreased pup body-weight gain
during lactation (days 14 and 21) were observed in both F0 and F1
generations and F1 and F2 litters. The NOAEL for reproductive
toxicity was thus 1.16 mg/kg bw per day (Kowalski et al., 1989).
(e) Developmental toxicity
Rats
Four groups of 20 mated FB30 Long-Evans female rats were given
technical-grade fenthion (purity, 98.1%) in aqueous Cremophor EL
emulsion by gavage at 0, 1, 3, or 10 mg/kg bw per day once daily on
days 6-15 of gestation; the day semen was detected in a vaginal smear
was designated day 0 of gestation. On gestation day 20, all surviving
dams were sacrificed, and the fetuses were delivered by caesarean
section and necropsied. The numbers of implantation sites and of live,
dead, and resorbed fetuses, litter weight, fetal body weight, and
placental weight were recorded, and all fetuses were examined for
external, visceral, and skeletal abnormalities. No treatment-related
mortality, clinical symptoms of maternal toxicity, or disturbance of
intrauterine development were noted at any dose. All fetuses delivered
developed normally. No signs of fetotoxicity were seen, and no
treatment-related malformations were observed in any of the fetuses at
any dose up to and including 10 mg/kg bw per day. The NOAEL for
maternal toxicity, embryo- and fetotoxicity, and teratogenicity was
10 mg/kg bw per day, the highest dose tested (Machemer, 1978a).
Groups of 33 mated Crl:CD BR female rats were given technical-
grade fenthion (purity, 96.5%) in 5% aqueous Emulphor solution by
gavage at doses of 0 (vehicle control), 1, 4.2, or 18 mg/kg bw per day
once daily on days 6-15 of gestation; the day spermatozoa were
detected in a vaginal smear was designated as day 0 of gestation. Five
animals from each group were sacrificed on day 16, and the remaining
28 dams in each group were killed on day 20 of gestation. All dams
were necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. No NOAEL for maternal toxicity
could be determined owing to dose-related, toxicologically
significant inhibition of brain (> 10%) and erythrocyte (> 20%)
acetylcholinesterase activities at all doses tested, including the
lowest dose of 1 mg/kg bw per day, on days 16 and 20 of gestation.
Plasma cholinesterase was also depressed at > 1 mg/kg bw per day on
day 16 and at 18 mg/kg bw per day on day 20. No other treatment-
related maternal or embro- or fetotoxicity was seen at > 4.2 mg/kg
bw per day. At the highest dose, clinical symptoms of intoxication
(dry or bloody lacrimation, exophthalmia, hypoactivity, tremors,
salivation, and urine-stained peritoneum), decreased mean bodyweight
gain, and reduced food consumption were observed. There was also a
slight increase in fetal skeletal variations, including an increased
incidence of incomplete ossification of the cervical arches, skull
bones, and third, fourth, and sixth sternebrae, and incomplete and/or
unossified metacarpals and metatarsals. These findings represent a
delay in skeletal maturation and were considered not to be adverse or
of toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in the fetuses at any dose up to and
including 18 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity
and teratogenicity was therefore 18 mg/kg bw per day, the highest dose
tested (Kowalski, 1987).
Rabbits
Groups of 17 artificially inseminated American-Dutch female
rabbits were given technical-grade fenthion (purity, 96.5%) in 5%
aqueous Emulphor solution by gavage at doses of 0 (vehicle control),
1, 2.75, or 7.5 mg/kg bw per day once daily on days 6-18 of gestation;
the day of artificial insemination was designated day 0 of gestation.
All surviving animals were sacrificed on gestation day 28 and
necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. The NOAEL for maternal toxicity
was 1 mg/kg bw per day based on toxicologically significant inhibition
of brain (> 10%) and erythrocyte (> 20%) acetylcholinesterase
activities at the two higher doses on gestation day 19 and/or 28. At
> 2.75 mg/kg bw per day, an increased incidence of soft stools was
noted both during and after treatment. At the highest dose, plasma
cholinesterase activity was depressed on gestation day 19 only. There
was also an increased incidence of unossified anterior metacarpals
among the fetuses, but this finding represents a delay in skeletal
maturation and was considered not to be an adverse effect of
toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in any of the fetuses at any dose up to
and including 7.5 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity and teratogenicity was 7.5 mg/kg bw per day, the highest
dose tested (Clemens, 1987).
(f) Genotoxicity
In an assay for dominant lethal mutation, three groups of 50 male
NMRI mice were each given a single oral dose of 0, 10, or 25 mg/kg bw
of technical-grade fenthion (purity unspecified) as a 2% aqueous
emulsion. Each male was then caged with an untreated virgin female for
four days; the same procedure was repeated for a total of 12 matings.
On days 12-16 of gestation, the females were sacrificed, and the
numbers of implantations and of live and dead implants (deciduomata,
resorption sites, and dead embryos) were counted. The high dose
resulted in overt clinical signs of toxicity in males (drowsiness,
ruffled coat, and dilated intestines). Except for increased
preimplantation loss at 25 mg/kg bw in the first two mating periods,
no other treatment-related effects on reproductive performance
(fertility, pre- and post-implantation loss) were observed (Machemer,
1978b).
A battery of studies was conducted with technical-grade fenthion
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were mostly negative; weakly positive results were obtained
in two of five assays for sister chromatid exchange, in one of two
assays for micronucleus formation (at a dose of 150 mg/kg bw), and in
one assay for unscheduled DNA synthesis. Summary results from the US
Environmental Protection Agency Health Effects Research Laboratory
(Simmon et al., 1977) indicated that technical-grade fenthion does
not induce reverse mutation in Salmonella typhimurium TA98, TA100,
TA1535, TA1537, or TA1538 or Escherichia coli WP2 uvrA; enhanced
mitotic recombination in yeast ( Saccharomyces cerevisiae D3);
unscheduled DNA synthesis in human fibroblasts (WI-38 cells);
preferential toxicity (DNA repair-proficient/deficient) in E. coli
(W3110, p3478) or Bacillus subtilis (H17, M45); or sex-linked
recessive lethality in Drosophila melanogaster in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenthion (purity 97-99%) was not irritating to
the skin and was minimally or not irritating to the eyes of New
Zealand white rabbits (Pauluhn, 1985; Eigenberg, 1987c,d).
Technical-grade fenthion (purity 98.5%) did not induce dermal
sensitization in a maximization test (according to the method of
Magnusson and Kligman) in male DHPW guinea-pigs (Flucke, 1987)
(ii) Potentiation
Fenthion potentiated the acute intraperitoneal toxicity of
malathion, dioxathion, and coumaphos in rats, but intraperitoneal
administration of 13 other organophosphate or carbamate insecticides
to rats in combination with fenthion did not result in greater than
additive toxic effects (Dubois & Kinoshita, 1964). Dietary combination
of equitoxic doses (2 mg/kg bw) of fenthion with coumaphos, neither of
which alone affected cholinesterase activity when fed to dogs for six
weeks, was found to potentiate the anticholinesterase activity of
serum and erythrocytes by 75 and 30%, respectively. The potentiation
was less evident with malathion, and no potentiation was noted when
fenthion was fed in combination with dioxathion (Doull et al.,
1962). Oral administration to rats of a single dose of either a 3:1
mixture of fenthion and dichlorvos or a 1:3 mixture of fenthion and
isofenthion did not result in a greater than the theoretical additive
toxic effect (Kimmerle, 1967; Heimann, 1982). In several recent
studies, pretreatment with fenthion significantly potentiated the
acute toxicity of 2- sec-butylphenyl N-methylcarbamate in mice and
dogs. Its acute toxicity in mice was increased 15-fold by a 1-h oral
pretreatment with 30 mg/kg bw fenthion (one-tenth of the LD50).
Table 2. Results of tests for the genotoxicity of technical-grade fenthion
End-point Test system Concentration Results Reference
or dose
IN vitro
Reverse mutation S. typhimurium TA98, 0, 20-12 500 µg/plate Negative Herbold (1987)
TA100, TA1535, TA1537 0, 750-12 000 µg/plate
Reverse mutation S. typhimurium TA98, 0, 8-5000 µg/plate Negative Herbold (1990a)
TA100, TA1535, TA1537
hprt forward mutation Chinese hamster ovary 0, 20.0-100 µg/ml Negative Lehn (1990a)
cells 0, 12.5-75 µg/ml
Chromosomal aberration Chinese hamster ovary 0. 0.02-0.15 µg/ml Negativea Putman & Morris
cells (1989)
Chromosomal aberration Chinese hamster lung cells 0, 23.5-94 µg/ml Negativea Kajiwara (1989)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml Negative at < 20b,c Chen et al.
(1982a)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml (in diffusion Weakly positive Chen et al. (1982b)
chambers) at > 40b
Sister chromatid exchange Human lymphoid cell line 0, 0.02-20 µg/ml (unactivated) Negativea,b Sobti et al.
(1982)
LAZ-007 (of B-cell origin) 0, 20 µg/ml (activated)
Chromosomal aberration, Human lymphocytes 0, 0.5-5.0 µg/ml Weakly positive Rani & Rao (1991)
sister chromatid exchange at > 1.5
Unscheduled DNA Rat primary hepatocytes 0, 10-100 µg/ml Weakly positive Lehn (1990b)
synthesis 0, 5-30 µg/ml at > 5
Table 2. (con't)
End-point Test system Concentration Results Reference
or dose
In vivo
Sister chromatid exchange Rat bone-marrow 0, 10-100 mg/kg bw Negative Bai et al (1990)
lymphoid cells
Micronucleus formation Bor:NMRI mouse bone 0, 150 mg/kg bw Weakly positive Herbold (1990b)
marrow at 150b
a With and without exogenous metabolic activation
b Not confirmed in an independent assay
c Threshold of cytotoxicity
Inhibition of the metabolism or detoxification of 2- sec-butylphenyl
N-methylcarbamate by desulfuration of fenthion may have been
responsible, at least in part, for the synergistic effect (Miyaoka
et al., 1984, 1986, 1987).
(iii) Cholinesterase inhibition
Twelve groups of 10 tasted male Fischer 344 (CDF/Crl/Br) rats
were given single doses of technical-grade fenthion (purity, 97.5%)
orally, dermally, or subcutaneously at 0, 1, 5, or 25 mg/kg bw, and
the effect of the route of administration on plasma, erythrocyte,
and/or brain cholinesterase activity was studied for up to 14 days
after treatment. The NOAEL for all routes of administration was
5 mg/kg bw, based on toxicologically significant inhibition of
erythrocyte (> 20%) and brain (> 10%) acetylcholinesterase
activities at 25 mg/kg bw. Inhibition of plasma and erythrocyte
cholinesterase activity was maximal at 24 h after oral administration
and four days after dermal or subcutaneous administration, suggesting
more efficient absorption by the oral route. On day 14 after
treatment, depression of brain acetylcholinesterase activity was
greatest in the group that were treated subcutaneously; the prolonged
recovery may be associated with a slower release of fenthion into the
circulation when given by this route. The magnitude of cholinesterase
inhibition appeared to be only marginally significant after dermal
administration, suggesting that absorption is less effective by this
route. The rates of absorption were thus oral route > subcutaneous
route > dermal route (Christenson, 1990c).
(iv) Delayed neurotoxicity
Acute delayed neurotoxicity in hens: Groups of 15
atropine-protected, adult laying hens (Lohmann selected Leghorn
strain), five to seven months old, were given two doses of fenthion
(E 1752; purity, 98.5%) in 2% Cremophor EL by gavage at 40 mg/kg bw or
dermally at 200 mg/kg bw, 21 days apart. A positive control group of
five hens was given tri- ortho-cresyl phosphate (purity, 99.1%)
orally as a single dose of 375 mg/kg bw. Six hens given an oral dose
of 5 ml/kg bw 2% Cremophor EL and six untreated hens served as
negative controls. All animals were observed for 21 days after the
second dose before sacrifice, with the exception of the tri- ortho-
cresyl phosphate-treated hens which were killed on day 22 in moribund
condition. At termination, peripheral and central nervous tissues were
removed, fixed, and stained for microscopic examination. Clinical
signs of acute cholinergic intoxication were evident in all
fenthion-treated hens 7-24 h after treatment; recovery occurred within
7-8 days after oral administration and 14-18 days after dermal
application. No clinical symptoms (irreversible impairment of motor
coordination) or neurohistopathological lesions indicative of delayed
neuropathy were observed. The positive controls exhibited symptoms of
delayed nerurotoxicity (abnormal gait, ataxia, and paresis) from day 7
after treatment, which persisted until sacrifice on day 22;
neurohistopathological lesions characteristic of delayed neuropathy
were also evident (Flucke & Kaliner, 1987).
Neuropathy target esterase activity: Groups of nine atropine-
protected, adult hens (Lohmann selected Leghorn strain) were given a
single oral dose of technical-grade fenthion (E 1752; purity, 98.5%)
in 2% Cremophor EL by intubation at doses of 0 (vehicle control) or
40 mg/kg bw. Nine positive controls received tri- ortho-cresyl
phosphate as a single oral dose of 100 mg/kg bw. Neuropathy target
esterase activity in brain and spinal cord was inhibited by 0-14% over
that in vehicle controls 24 and 48 h and seven days after treatment.
Severe inhibition (> 50-90%) was seen in the positive controls
throughout the study (Flucke, 1988a).
Groups of nine atropine-protected adult hens (Lohmann selected
Leghorn strain) were given a single dermal dose of technical-grade
fenthion (E 1752; purity, 98.5%) in 2% Cremophor EL at doses of 0
(vehicle control), 200, or 400 mg/kg bw. A positive control group of
three to nine hens received tri- ortho-cresyl phosphate as a single
dose of 100 mg/kg bw by intubation. Neuropathy target esterase
activity in brain and spinal cord was inhibited by 0-20% over that in
vehicle controls at 24, 48, and 72 h and up to seven days after
treatment. Severe inhibition (> 80%) was seen in the positive
controls 24 and 48 h after treatment, demonstrating the sensitivity of
the test (Flucke, 1988b)
Short-term delayed neurotoxicity in hens: Groups of 10 adult
white Leghorn hens (Gallus gallus) were given technical-grade
fenthion (purity, 96.5%) by gavage in clear corn oil solution at doses
of 0, 1, 2, or 4 mg/kg bw per day for 14 weeks. Tri- ortho-cresyl
phosphate (10-60 mg/kg bw per day) was given as a positive control. No
treatment-related clinical symptoms or increases in the incidence
and/or severity of histopathological lesions of nervous tissues
characteristic of organophosphate-induced delayed neurotoxicity were
evident in the fenthion-treated hens at up to and including the
highest dose. Clinical signs of decreased activity and ataxia were
noted, but predominantly in the first hours after treatment; they were
no longer seen before the next dose, suggesting that they were likely
to have been caused by repetitive, severe acute cholinergic
intoxication and were not related to delayed neurotoxicity. The
positive controls had both toxic symptoms and histopathological
lesions of the nervous tissues typical of delayed neurotoxicity.
Histopathological examination revealed a dose-related increase in the
incidence of muscular hypertrophy or hyperplasia in all muscle layers
of the oesophagus, crop, proventriculus, gizzard, and intestine of all
fenthion-treated hens and glandular and nonglandular epithelial
hyperplasia in the oesophagus, crop, and proventriculus of some at the
middle dose and in most at the high dose. These lesions were probably
due to localized acetylcholinesterase inhibition, with subsequent
overstimulated muscle hypertrophy (Hayes & Ramm, 1988).
A second short-term study, in which technical-grade fenthion was
fed to hens was conducted to determine if the muscular hypertrophy and
hyperplasia observed by Hayes and Ramm (1988) were a direct effect of
fenthion or were due to the route of treatment. Two groups of 10 adult
white Leghorn hens (Gallus gallus domesticus) were given technical-
grade fenthion (purity, 96.9%) at dietary concentrations of 0 or
52 ppm (equivalent to 4 mg/kg bw per day) for 90 days. Minimal to
moderate muscular hypertrophy or hyperplasia was seen in the distal
oesophagus (between the crop and the proventriculus) of all treated
hens, which accounted for 98-99% of the increased thickness (+ 55%, in
comparison with controls) in the oesophageal wall. Hypertrophy or
hyperplasia of the oesophageal glandular components was also seen in
four birds. In addition, treated hens had a statistically significant
(P < 0.05) depression of cholinesterase activity in whole blood
(> 50% in comparison with controls) and tissue from all three
regions of the upper gastrointestinal tract (oesophagus, crop, and
proventriculus: about 70% in comparison with controls). It was
concluded that the muscular hypertrophy and hyperplasia observed
in the fenthion-treated hens was probably due to localized
acetylcholinesterase inhibition with subsequent overstimulation of the
oesophageal smooth muscle layers (Hayes, 1989).
Assessment of the neurotoxicity of fenthion in the literature:
A position paper (Flucke, 1990) which presented and discussed nine
publications dealing with the assessment of neurotoxicity of fenthion
was submitted. The main findings and critical evaluations are
summarized below.
1. In the series of four studies by Farage-Elawar & Francis (1987,
1988a,b; Francis & Farage-Elawar, 1987), the effects of fenthion,
debromoleptophos (which induces organophosphate-induced delayed
neurotoxicity), and fenitrothion (which does not) were compared
in very young chicks. On the basis of the finding that fenthion
produced neurotoxic signs (altered gait) and inhibition (> 50%)
of cholinesterase but little or no inhibition of neuropathy
target esterase, it was postulated that its neurotoxic potential
is not classical organophosphate-induced delayed neurotoxicity. A
critical evaluation of the four publications (by Flucke, 1990,
and the reviewer) revealed that the three organophosphates were
not administered in equitoxic doses, thus rendering direct
comparison of their toxic effects inappropriate, and that the
test animals were malnourished. Under the study conditions, there
was probably prolonged acute cholinergic intoxication due to
severe inhibition of cholinesterases after the lethal or
sublethal doses of debromoleptophos and fenthion, in contrast to
the low dose of fenitrothion (about one-fifth of the LD50),
which would not have induced overt cholinergic symptoms. A
build-up of acetylcholine at the motor end-plates could result in
pronounced, prolonged muscle fasciculation and, secondarily, in
the known necrotizing effects. Young chicks undergoing rapid
growth and muscle development are less likely to compensate
quickly and completely for this type of muscle damage, especially
if they are undernourished. Accordingly, the neurotoxic effects,
which occurred to the same degree with debromoleptophos and
fenthion, could be attributed to primary effects of severe acute
cholinergic intoxication and not to a particular (unknown)
neurotoxic potential of fenthion, as postulated by the authors.
2. Tuler et al. (1988) and Dellinger & Mostrom (1988) examined the
neurotoxic effects of fenthion in dogs. In the first study, the
authors concluded that there was no evidence of delayed
neurotoxicity. Prolonged weekly treatment with fenthion caused
hyperreflexia, proprioceptive deficits, progressive muscle fibre
necrosis, ultrastructural changes in nerve axons, and loss of
small motor units. Fenthion-induced primary neuropathy leading to
secondary myopathy was proposed. In the second study, the authors
concluded that there was no evidence of cholinergic effects and
no effect on vagal tone during the period of fenthion treatment
when maximal inhibition (> 50%) of acetylcholinesterase activity
was measured; however, on day 14 after treatment, the treated
dogs gave a slightly smaller response to an atropine challenge
(in comparison with controls), suggesting possible down-
regulation of the cholinergic receptors.
A critical evaluation of these two publications (by Flucke,
1990 and the reviewer) revealed the following: (i) The fenthion-
treated dogs had severe organophosphate intoxication and that the
clinical symptoms of muscle fasciculation, ataxia, hyperreflexia,
proprioceptive deficits, and hindquarter weakness were likely to
be the result of neuromuscular overstimulation due to severe
acetylcholinesterase inhibition. Reversibility or recovery was
seen in most of the dogs when the dose of fenthion was reduced
from 44 to 22 mg/kg bw or omitted for one week, suggesting the
neuropathy was not irreversible. (ii) Progressive muscle libre
necrosis was evident in the distal parts of the sciatic nerves of
fenthion-treated dogs, with reorganization of the sensory and
motor innervations. As primary impairment of the peripheral
nerves does not result in muscle necrosis but rather in atrophy
of muscle fibres, the results were judged not to be indicative of
a primary neuropathic effect. In addition, receptor down-
regulation can be considered a physiological adaptation process
to maintain internal equilibrium, serving as a protective
mechanism by which the organism normalizes functions that are
impaired by the intake of pharmacodynamic substances.
Accordingly, the small changes noted in the vagal tone of dogs
after chronic administration of fenthion probably represented an
adaptive change and are. not indicative of irreversible
impairment of the neuromuscular junctions. Thus, the studies, of
Tuler et al. (1988) and Dellinger & Mostrom (1988) do not give
evidence of a particular neurotoxic effect of fenthion in dogs.
3. Tuler & Bowen (1989) investigated the neurotoxic effects of
fenthion, paraoxon (an organophosphate), and neostigmine (a
non-organophosphate) on chick embryo nerve cell growth and
ultrastructure in culture. Altered cell membrane integrity and
increased cytoplasmic lipid (vacuole) accumulation were observed
in cells treated with fenthion or paraoxon but not in those
treated with neostigmine. The authors concluded that
organophosphates had direct effects on neuronal cells in culture;
however, the following points should be considered: (i)
Pharmacokinetics plays a special role in intoxication by
organophosphates, in particular thiophosphates, and in
detoxification, both of which occur rapidly and simultaneously.
Fenthion, a thiophosphate, must be converted in vivo to the
active oxygen analogue and would therefore be ineffective as an
inhibitor of esterases in vitro. (ii) The observed effects on
the cell membrane and the cell were not specific to neuronal
cells and can therefore not be compared directly with neurotoxic
effects in vivo. It is therefore concluded that the results of
the study reveal no particular neurotoxic potential of fenthion
in vivo.
4. Misra et al. (1985, 1988) examined clinical and biochemical
changes and neuromuscular function in workers chronically exposed
to fenthion. No clinical signs of peripheral neuropathy or
myopathy and no pathophysiological findings indicative of
irreversible neurological deficits were seen in workers exposed
regularly and repeatedly to fenthion for a mean of 8.5 years.
They showed symptoms typical of acute cholinergic intoxication
and significant inhibition of serum cholinesterase. It is
concluded that the study provides no evidence of a particular or
delayed neurotoxic potential of fenthion in the workers examined.
The literature thus provides no evidence of a particular or
delayed neurotoxic potential but indicates that exposure to fenthion
results in typical organophosphate-induced cholinergic toxicity due to
severe acetylcholinesterase inhibition.
3. Studies of metabolites
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion
metabolites are summarized in Table 3.
Table 3. Acute toxicity of fenthion metabolites
Metabolite LD50 (mg/kg bw) IC50 (mol; 50% inhibition of human
erythrocyte acetylcholinesterase)c
Orala Intraperitonealb
Fenthion 220 325 > 5.0 × 10-4
Fenthion sulfoxide 125 250 4.5 × 10-5
Fenthion sulfone 125 250 4.7 × 10-4
Fenthion oxygen analogue 125 26 2.7 × 10-6
Fenthion O-sulfoxide 50 22 4.8 × 10-5
Fenthion O-sulfone 30 9 3.2 × 10-5
4-(Methylthio)-m-cresol 6500d
4-Methyl(thiosulfoxide)-m-cresol 3500d
4-Methyl(thiosulfone)-m-cresol 7000d
a In male rats (Francis & Barnes, 1963)
b In female rats (Dubois & Kinoshita, 1964)
c Francis & Barnes (1963)
d In female rats (Nelson, 1967)
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR mice were fed diets
containing fenthion sulfoxide (Baycid SO; purity not given) at
concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to 0,
0.45, 1.5, 4.5, 15, or 45 mg/kg bw per day, for four weeks, followed
by a four-week recovery period. Plasma cholinesterase activity was
depressed in animals of each sex at > 3 ppm, but the effect (an
indication of exposure) was not considered to be toxicologically
significant. Brain acetylcholinesterase activity was significantly
inhibited in males at > 3 ppm and in females at > 10 ppm. At the
two highest doses, decreased body weight was noted in animals of each
sex; at the highest dose, tremor was seen in all mice. All of the
treatment-related effects (including cholinesterase depression)
disappeared rapidly during the recovery period. There was no NOAEL.
The LOAEL, based on significant brain acetylcholinesterase inhibition
in males, was 0.45 mg/kg bw per day, the lowest dose tested (Inukai &
Iyatomi, 1981a).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing fenthion sulfoxide (Baycid SO; purity not given)
at concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to
0, 0.15, 0.5, 1.5, 5, or 15 mg/kg bw per day, for four weeks,
followed by a four-week recovery period. Plasma cholinesterase
activity was depressed in females at > 3 ppm, but the effect
(an indication of exposure) was considered not to be toxicologically
significant. No treatment-related adverse effect was observed at
3 ppm. At > 10 ppm, significant, dose-related depression of
acetylcholinesterase activity in brain was seen in animals of each sex
and in erythrocytes in males after four weeks of treatment; in
females, erythrocyte acetylcholinesterase activity was inhibited at
> 30 ppm. At the two highest doses, decreased body weight and food
consumption and increased alkaline phosphatase activity were noted. At
the highest dose, tremor was seen in all rats. All of the treatment-
related effects (including cholinesterase depression) disappeared
rapidly during the recovery period. The NOAEL was 0.15 mg/kg bw per
day, based on significant inhibition of brain (> 10%) and erythrocyte
(> 20%) acetylcholinesterase activities at 0.5 mg/kg bw per day
(Inukai & Iyatomi, 1981b).
4. Observations in humans
Fenthion has been used widely in many parts of the world for
control of e.g. household pests and mosquitos. Studies of individuals
in areas treated for malaria eradication have shown slight plasma
cholinesterase depression when heavy spray schedules were used. The
levels were depressed for up to six weeks after spraying (Elliot &
Barnes, 1963). Children under the age of seven were more susceptible
to the anticholinesterase effect than adults, but no inhibition of
erythrocyte cholinesterase was observed and there were no significant
alterations in normal physiological functions in any individual
examined (Taylor, 1963).
The signs of acute poisoning appear rapidly, beginning with
blurred vision, unsteady gait, and slurred speech. In one reported
case, a man who had taken an unknown quantity of fenthion still
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation after 72 h of emergency
treatment. The patient began to recover only after 11 days of
treatment with antidotes which included atropine, pralidoxime
chloride, and toxogonin (Dean et al., 1967). Another man remained in
a comatose state for 45 min after ingesting a fenthion formulation,
with pale skin, cyanotic mucous membranes, a slow, regular heart beat,
low peripheral blood pressure, and no reactions to pain or light on
the pupils. Recovery took six days (von Clarmann & Geldmacher-von
Mallinkrodt, 1966). A third man, who ingested about 60 g of a fenthion
formulation (Entex), recovered from severe organophosphorous poisoning
after being in a critical condition for the first six days. Recovery
was slow, lasting up to 30 days, and blood cholinesterase activity was
still depressed 22 days after poisoning (Pickering, 1966).
The potential dermal and respiratory exposure of workers to
fenthion during field application by hand-gun power spray equipment,
back-pack hand pressure sprayers, and hand granular dispersal for
mosquito control was studied during two work seasons. Workers exposed
to 3.6-12.3 mg/h dermally (equivalent to 0.5-1.5 mg/kg bw per day) or
< 0.02-0.09 mg/h by inhalation (equivalent to 0.01-0.05 mg/kg bw per
day) had decreased plasma but not erythrocyte cholinesterase activity
(Fytizas-Danielidou, 1971; Wolfe et al., 1974). In two studies of
clinical and biochemical changes, nerve conduction velocity, and
neuromuscular function in 22-24 workers (mean age, 31-32 years)
exposed regularly and repeatedly to fenthion for a mean of 8.2-8.5
years, no clinical signs of peripheral neuropathy or myopathy and
no pathophysiological findings indicative of any irreversible
neurological deficits were seen. The workers showed symptoms typical
of acute cholinergic intoxication and inhibition of serum
cholinesterase, but there was no evidence of delayed neurotoxicity
(Misra et al., 1985, 1988).
In a study of 150 cases of poisoning by anticholinesterase
insecticides, 32 of the patients had consumed fenthion, 48
fenitrothion, and 50 malathion; 20 did not know which agent they had
consumed. Paralytic signs were significantly more frequent after
fenthion than malathion or fenitrothion poisoning (81.2, 30, and 23%,
respectively), and they appeared later and lasted longer. Death
occurred significantly more often after fenthion poisoning, with
mortality rates of 35.5% with fenthion, 4% with malathion, and 2.1%
with fenitrothion. Pulmonary oedema was most common after malathion
poisoning and was not encountered with fenthion. Inhibition of
cholinesterase was most marked after fenthion poisoning, and the
enzyme activity was inhibited by 100% in 18 of the 27 cases studied
(Wadia et al., 1977).
In three groups of four male volunteers given technical-grade
fenthion (purity, 98.1%) in corn oil in gelatin capsules at doses of
0, 0.02, or 0.07 mg/kg bw per day for up to four weeks, no physical or
clinical signs of intoxication were reported, and no abnormal results
were seen by haematology or urinalysis. At 0.07 mg/kg bw per day,
significant depression (> 20%) of plasma cholinesterase was observed,
but no treatment-related effects on erythrocyte acetylcholinesterase
or on other clinical chemistry parameters were observed. The NOAEL was
0.07 mg/kg bw per day, based on the absence of inhibition of
erythrocyte acetylcholinesterase (Griffin et al., 1979).
Comments
A single dose of 14C-radiolabelled fenthion was readily absorbed
and rapidly excreted in the urine and faeces of rats, and no 14C was
detectable in the expired carbon dioxide. About 90% of the radiolabel
was eliminated within 48 h of dosing. The excretion profiles were
similar regardless of sex, dose, or route of administration (oral or
intravenous). Urine was the main route of elimination (> 90% of the
total radiolabel), and only minor amounts were recovered in the
faeces. Little 14C was retained in the tissues, suggesting that
fenthion does not accumulate in the body of rats.
14C-Fenthion was extensively metabolized in rats. No unchanged
parent compound was detected in the urine and very little (< 2%) in
the faeces. The major group of metabolites (accounting for about 60%
of the total recovered 14C) comprised three phenols (fenthion phenol
[4-methylthio- meta-cresol] and its sulfoxide and sulfone) and their
glucuronide and sulfate conjugates. Four demethyl metabolites
(accounting for about 30% of the recovered radiolabel) and the
sulfoxide of fenthion oxon (constituting 1-4%) were also identified.
Fenthion is moderately toxic (LD50 = 50-500 mg/kg body
weight) to mice, rats, guinea-pigs, and rabbits when given orally,
intraperitoneally, dermally, or by inhalation. It is highly toxic to
avian species (especially to the wild mallard duck) when given
orally. The LC50 in rats for a 4-h exposure by inhalation was
0.5-0.9 mg/titre. Fenthion caused cholinergic toxicity with a long
recovery time. It did not irritate rabbit skin and was minimally or
not irritating to the rabbit eye; it did not sensitize guinea-pig
skin. WHO has classified fenthion as highly hazardous.
Fenthion potentiated the acute toxicity of other cholinergic
chemicals, such as malathion, dioxathion, and coumaphos, in rats. In
mice and dogs, pretreatment with fenthion inhibited the metabolism and
detoxification of 2- sec-butylphenyl methylcarbamate, resulting in
significant potentiation of its acute toxicity. There was no evidence
that other cholinergic chemicals potentiate the toxicity of fenthion.
Repeated short-term administration of fenthion (orally to mice,
rats, dogs and monkeys; dermally to rabbits; by inhalation to rats)
and its metabolite fenthion sulfoxide (orally to mice and rats),
resulted primarily in inhibition of cholinesterase. The NOAEL for oral
administration of fenthion, based on toxicologically significant
depression of acetylcholinesterase activity in the brain (> 10%)
and/or erythrocytes (> 20%), was 5 ppm (equivalent to 0.25 mg/kg bw
per day) in rats treated for three months or one year, 3 ppm (equal to
0.09 mg/kg bw per day) in dogs treated for two years, and 0.07 mg/kg
bw per day in monkeys treated for two years The NOAEL for fenthion was
50 mg/kg bw per day when applied dermally to rabbits for 21 days on
the basis of inhibition of brain acetylcholinesterase activity. The
NOAEL for fenthion in rats exposed by inhalation for 21 days was
0.001 mg/litre, on the basis of clinical signs of cholinergic toxicity
and inhibition of brain acetylcholinesterase at higher doses. The
NOAEL for fenthion sulfoxide administered orally to rats for four
weeks was 3 ppm (equivalent to 0.15 mg/kg bw per day) on the basis of
significant inhibition of brain and erythrocyte acetylcholinesterase
activity. No NOAEL was determined for fenthion or its metabolites in
mice; the LOAEL for fenthion was 150 ppm (equal to 83 mg/kg bw per
day) in mice exposed in the diet for four weeks, and the LOAEL for
fenthion sulfoxide in mice similarly exposed was 3 ppm (equivalent to
0.45 mg/kg bw per day).
In a carcinogenicity study, fenthion was administered in the diet
to mice at 0, 0.1, 1, 5 or 25 ppm for 102 weeks. The NOAEL for chronic
toxicity was 5 ppm (equal to 2 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. There was no evidence of carcinogenicity. In
two studies of chronic toxicity and carcinogenicity, rats received
fenthion at dietary concentrations of 0, 3, 15, or 75 ppm or 0, 5,
20, or 100 ppm for 24 months. The NOAEL for chronic toxicity
was 3 ppm (equal to 0.14 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. The compound was toxic to the eyes of rats at
100 ppm (equal to 5.2 mg/kg bw per day), inducing an increased
incidence of retinal atrophy, posterior subcapsular cataract
formation, corneal mineralization, and mineralization and optic nerve
atrophy, especially in females. No ocular toxicity was seen at doses
> 20 ppm (equal to 0.8 mg/kg bw per day). There was no evidence of
carcinogenicity.
In a two-generation study of reproductive toxicity (one litter
per generation), rats were fed diets containing fenthion at levels of
0, 1, 2, 14, or 100 ppm. The NOAEL for systemic toxicity in the parent
generation was 2 ppm (equal to 0.16 mg/kg bw per day) on the basis of
consistent inhibition of brain and erythrocyte acetylcholinesterase.
The NOAEL for reproductive toxicity was 14 ppm (equal to 1.2 mg/kg bw
per day) on the basis of decreased fertility, implantation sites,
litter size, pup viability, and growth at 100 ppm.
Two studies of developmental toxicity were performed in which
rats were exposed by gavage to fenthion at doses of 0, 1, 3, or
10 mg/kg bw per day or 0, 1, 4.2, or 18 mg/kg bw per day on days 6-15
of gestation. There was no NOAEL for maternal toxicity, owing to
toxicologically significant inhibition of brain and erythrocyte
acetylcholinesterase activity at doses > 1 mg/kg bw per day. the
NOAEL for embryo- and fetotoxicity and teratogenicity was 18 mg/kg bw
per day, the highest dose tested.
In a study of developmental toxicity in rabbits, fenthion was
administered by gavage at doses of 0, 1, 2.8, or 7.5 mg/kg bw per day
on days 6-18 of gestation. The NOAEL for maternal toxicity was 1 mg/kg
bw per day, on the basis of toxicologically significant inhibition of
brain and erythrocyte acetylcholinesterase activity at > 2.8 mg/kg
bw per day. The NOAEL for embryo- and fetotoxicity and teratogenicity
was 7.5 mg/kg bw per day, the highest dose tested.
Fenthion has been adequately tested for genotoxicity in a range
of assays in vivo and in vitro. While most showed no significant
response, positive results were obtained in two critical assays. The
Meeting concluded that fenthion is weakly genotoxic.
Fenthion did not cause delayed neuropathy in hens at doses higher
than the LD50.
In a four-week study of male volunteers, the NOAEL was 0.07 mg/kg
bw per day, the highest dose tested, on the basis of no inhibition of
erythrocyte acetylcholinesterase. In two investigations of workers
regularly exposed to fenthion, no evidence of neurotoxicity was
observed.
An ADI of 0-0.007 mg/kg bw was established on the basis of the
NOAEL of 0.07 mg/kg bw per day in the four-week study of human
volunteers, using a safety factor of 10. The ADI provides a margin of
safety of > 100-fold for chronic ocular toxicity and for the
reproductive toxicity observed in rodents.
The available data did not allow the Meeting to establish an
acute reference dose different from the ADI (0-0007 mg/kg bw). It
should be noted, however, that the ADI is derived from a study
of human volunteers in which 9-36% inhibition of plasma cholinesterase
but no inhibition of erythrocyte acetylcholinesterase was found at
the highest dose tested (0.07 mg/kg bw per day for 25 days). In
occupationally exposed workers, about 50% plasma cholinesterase
inhibition was found in the absence of erythrocyte acetyl-
cholinesterase inhibition. It follows that the acute reference
dose is likely to be somewhat higher than the ADI. Data on the
sensitivity to inhibition of plasma cholinesterase and erythrocyte and
brain acetylcholinesterase in vitro by the active metabolites of
fenthion might allow extrapolation to an LOAEL for humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 2.0 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equal to 0.14 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
2 ppm, equal to 0.16 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
14 ppm, equal to 1.2 mg/kg bw per day (two-generation study
of reproductive toxicity)
18 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Rabbit: 6 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
7.5 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 3 ppm, equal to 0.09 mg/kg bw per day (two-year study of
toxicity)
Monkey: 0.07 mg/kg bw per day (two-year study of toxicity)
Human: 0.07 mg/kg bw per day (four-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenthion
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Minimally or not irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Inhalation, 4-h, toxicity, rat LC50 = 0.5--0.9 mg/litre
Oral, toxicity, mouse, rat, guinea-pig, rabbit LD50 = 50-500 mg/kg bw
Oral, dermal, subcutaneous, single doses, NOAEL (all routes) = 5 mg/kg bw per day
cholinesterase activity, rat based on inhibition of brain and
erythrocyte acetylcholinesterase
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 50 mg/kg bw per day for
systemic toxicity on the basis of inhibition
of brain acetylcholinesterase
Repeated inhalation, 21 days, toxicity rat NOAEL = 0.001 mg/litre per day for
systemic toxicity on the basis of clinical
signs of cholinergic toxicity
Repeated dietary, 13-16 weeks, toxicity, rat NOAEL = 0.25 mg/kg bw per day, based
on inhibition of brain acetylcholinesterase
Repeated gavage, developmental toxicity, NOAEL = 1 mg/kg bw per day for
rabbit maternal toxicity on the basis of inhibition
of brain and erythrocyte acetylcholinesterase;
no embryo- or fetotoxic or teratogenic effects
Repeated oral (gelatin capsules), four weeks, NOAEL = 0.07 mg/kg bw per day on the
toxicity, human basis of no inhibition of erythrocyte
acetylcholinesterase
Long-term (> 1 year) Repeated dietary, two years, toxicity, dog NOAEL = 0.09 mg/kg bw per day on the
basis of inhibition of brain and erythrocyte
acetylcholinesterase
References
Avrahami, M. & White, D.A. (1975) Residues in milk of cows after
spot-treatment with 32P-fenthion. New Zealand Exp. Agric., 3,
309-311.
Bai, C.L., Qiao, C., Zhang, W., Chen, Y. & Qu, S. (1990) A study of
the pesticide fenthion: Toxicity, mutagenicity and influence on
tissue enzymes. Biomed. Environ. Sci., 3, 262-275.
Bailey, D.E. (1987) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 938 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bailey, D.E. (1988) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 1031 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bomann, W. (1991) E 1752 (c.n. Fenthion): Study for acute dermal
toxicity in the rat. Unpublished report No. 20221 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Bomhard, E. & Loser, E. (1977) Chronic toxicity study on rats.
Unpublished report No. 6769 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Brady U.E., Jr & Arthur, B.W. (1961) Metabolism of
O,O-dimethyl- O-[4-(methylthio)- m-tolyl] phosphorothioate
by white rats. J. Econ. Entomol., 54, 1232-1236.
Budreau, C.H. & Singh, R.P. (1973a) Teratogenicity and embryotoxicity
of demeton and fenthion in CF1 mouse embryos. Toxicol. Appl.
Pharm., 24, 324-332.
Budreau, C.H. & Singh, R.P. (1973b) Effect of fenthion and dimethoate
on reproduction in the mouse. Toxicol. Appl. Pharmacol., 26,
29-38.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982a) Sister-chromatid
exchanges and cell-cycle delay in Chinese hamster V79 cells
treated with nine organophosphorus compounds (8 pesticides and 1
defoliant). Mutat. Res., 103, 307-313.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982b) Sister-chromatid
exchanges in Chinese hamster cells treated with 17
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990a) Chronic feeding toxicity study of
technical-grade fenthion (Baytex) with dogs. Unpublished report
No. 94863 from Miles, Inc., Agriculture Division, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Christenson, W.R. (1990b) Combined chronic toxicity/oncogenicity study
of technical grade fenthion (Baytex) with rats. Unpublished
report No. 100586 from Mobay Corporation, Corporate Toxicology
Department, Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Christenson, W.R. (1990c) Technical grade fenthion (Baytex): A special
study to examine the effect of the route of acute administration
of technical grade fenthion (Baytex) on cholinesterase activity
in the rat. Unpublished report No. 100573 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
von Clarmann, M. & Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and
its demonstration in the gastric contents and urine. Arch.
Toxikol., 22, 2-11.
Clemens, G.R. (1987) A teratology study in the rabbit with fenthion
(Baytex technical). Unpublished report No. MTD0039 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Crosby, J., Hoglen, N. & Krautter, G. (1990) Nature of residues in
skin and tissues of swine after dermal treatment with
[14C]fenthion. Unpublished Mobay report No. 73984 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dean, G., Coxon, J. & Brereton, D. (1967) Poisoning by an
organophosphorus compound -- A case report. S. Afr. Med. J.,
41, 1017-1019.
Dellinger, J. & Mostrom, M. (1988) Effects of topical fenthion on
blood cholinesterase and vagal tone in dogs. Vet. Hum.
Toxicol., 30, 2,29-234.
Doolittle, K.D. & Bates, N.L (1993) Absorption, distribution and
elimination of 14C-fenthion in rats following a single oral,
repeated oral and single intravenous administration. Unpublished
Miles report No. 74395 from Southwest Bio-Labs, Inc., New Mexico,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. &
Meskauskas, J. (1961a) The effects of feeding diets containing
Bayer 29493 to rats for a period of 16 weeks. Unpublished rayer
Report No. 7899 from Department of Pharmacology, University of
Chicago, Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Doull J., Root, M. & Cowan, J. (1961b) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished Bayer report
No. 8342 from Department of Pharmacology, University of Chicago,
Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M. & Cowan, J. (1962) Effect of adding Bayer 29493 in
combination with other cholinergic insecticides to the diet of
male and female dogs. Unpublished Bayer report No. 9414 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, N.J., Vesselinovitch, D., Fitch, F.M. &
Meskauskas, J. (1963a) Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished Bayer report No. 14658 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, J. & Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
Bayer report No. 10853 from Department of Pharmacology,
University of Chicago, Illinois, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Dubois, K.P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished Mobay report No. 22503
from Department of Pharmacology, University of Chicago, Illinois,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Doull, J. (1960) The acute toxicity of Bayer 29493 to
chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago, Illinois, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Kino-hita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-dimethyl- O-4-
(methylthio- m-tolyl) phosphorotioate (DMTP; Baytex) and
related compounds. Toxicol. Appl. Pharmacol., 6, 86-95.
Eigenberg, D.A. (1987a) Acute oral toxicity of Baytex technical in
albino rats. Unpublished report No. 839 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987b) Acute dermal toxicity of Baytex technical in
albino rabbits. Unpublished report No. 838 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987c) Primary dermal irritation of Baytex technical
in albino rabbits. Unpublished report No. 896 from Mobay
Corporation, Corporate Toxicology Department, Kansas. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987d) Primary eye irritation of Baytex technical in
albino rabbits. Unpublished report No. 817 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Elliot, R. & Barnes, J.M. (1963) Organophosphorus insecticides for the
control of mosquitos in Nigeria. Bull. World Health Org., 28,
35-54.
Farage-Elawar, M. & Francis, B.M.(1987) Acute and delayed effects of
fenthion in young chicks. J. Toxicol. Environ. Health, 21,
455-469.
Farage-Elawar, M. & Francis, B.M. (1988a) Effects of multiple dosing
of fenthion, fentrothion, and desbromoleptophos in young chicks.
J. Toxicol. Environ. Health, 23, 217-228.
Farage-Elawar, M. & Francis, B.M. (1988b) Effects of fenthion,
fentrothion, and desbromoleptophos on gait, acetylcholinesterase
and neurotoxic esterase in young chicks after in ovo exposure.
Toxicology, 49, 253-261.
Flucke, W. (1986a) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute oral toxicity to hens
(Gallus domesticus). Unpublished report No. 15069 from Bayer
AG, Institut für Toxikologie, Wuppertal Germany.
Flucke, W (1986b) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute dermal toxicity to hens
(Gallus domesticus). Unpublished report No. 15113 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1987) E 1753 Technical (c.n fenthion): Study for skin
sensitising effect on guinea pigs (Magnusson and Kligman's
maximization test). Unpublished report No. 15428 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1988a) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following oral administration to hens.
Unpublished report No. 99275 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1988b) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following dermal administration to
hens. Unpublished report No. 99646 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1990) Position paper regarding the literature on
neurotoxicity for the BGA [German Federal Health Agency].
Unpublished report No. 18928 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. & Kaliner, G. (1987) E 1752 (c.n. fenthion, the active
ingredient of Baytex): Acute neurotoxicity studies on hens
following oral and dermal administration. Unpublished report No.
91341 from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Francis, J.I. & Barnes, J.M. (1963) Studies on the mammalian toxicity
of fenthion. Bull. World Health Org., 28, 205-212.
Francis, B.M. & Farage-Elawar, M. (1987) Peripheral and central enzyme
inhibition by fenthion, fenitrothion and desbromoleptophos. In:
Costa, L.G., Galli, C.L. & Murphy, S.D., eds, Toxicology of
Pesticides: Experimental, Clinical and Regulatory Perspectives,
Berlin, Springer Verlag.
Fytizas-Danielidou, R. (1971) Effects of pesticides on reproduction in
white rats. I. Labaycide. In: 23rd International Symposium of
Phytopharmacy, 4 May, 1971.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Griffin, T., Rosenblum, I. & Coulston, F. (1979) Safety evaluation of
fenthion in human volunteers. Unpublished Mobay report No. 68790
from the Institute of Comparative and Human Toxicology and
International Center of Environment Safety, Albany Medical
College, New York, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Hayes, R.H. (1989) Subchronic feeding study with fenthion technical
(Baytex) in hens with specific emphasis on gastrointestinal tract
effects. Unpublished report No. 99273 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Hayes, R.H. & Ramm, W.W. (1988) Subchronic delayed neurotoxicity study
of fenthion technical (Baytex) with hens. Unpublished report No.
98296 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Heimann, K.G. (1982) Fenthion and isofenthion: Study for acute
combination toxicity Unpublished report No. 11062 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1987) E 1752 (c.n. Fenthion): Salmonella/microsome
test for point-mutagenic effect. Unpublished report No. 98366
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1990a) E 1752 Salmonella/microsome test. Unpublished
report No. 18713 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Herbold, B.A. (1990b) E 1752 micronucleus test on the mouse.
Unpublished report No. 100025 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Hoffmann, K. & Weischer, C.H. (1975) Fenthion chronic study on dogs
(two-year feeding experiment). Unpublished report No. 5737 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981a) Fensulfoxide: Short-term toxicity
tests on mice (4-week feeding and 4-week recovery tests).
Unpublished report No. 198 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981b) Fensulfoxide: Short-term toxicity
tests on rats (4-week feeding and 4-week recovery tests).
Unpublished report No. 197 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Johnson, J.C. & Bowman, M.C. (1972) Responses from cows fed diets
containing fenthion or fenitrothion. J. Dairy Sci., 55,
777-782.
Kajiwara, Y. (1989) Chromosomal aberration test of fenthion using
cultured mammalian CHL cells. Unpublished report No. T-1824E from
HITA Research Lab., Japan. Submitted to WHO by Bayer AG,
Wuppertal, Germany.
Keith, J.O. & Mulla, M.S. (1966) Relative toxicity of five
organophosphorous mosquito larvicides to mallard ducks.
J. Wildlife Manage., 30, 553-563.
Kimmerle, G. (1967) Potential of DDVP and S 1752. Unpublished letter
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Klimmer, R. (1963) Toxicological testing of Bayer 29493. Unpublished
report from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Knowles, C.O. & Arthur, B.W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of
radiolabelled fenthion to dairy cows. J. Econ. Entomol., 59,
1346-1352.
Kowalski, R.L. (1987) A teratology study with fenthion (Baytex
technical) in the rat. Unpublished report No. MTD0029 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Kowalski, R.L., Clemens, G.R., Jasty, V., Troup, CM. & Hartnagel,
R.E., Jr (1989) A two-generation reproductive study with fenthion
(Baytex) in the rat. Unpublished report No. MTD0133 from Miles
Inc., Toxicology Department, Indiana, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Krautter, G. (1990a) Nature of residues in milk, skin and tissues of a
lactating cow after dermal treatment with [14C]fenthion.
Unpublished Mobay report No. 74012 from Pharmacology and
Toxicology Research Laboratory, Kentucky, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Krautter, G. (1990b) Characterization of polar metabolites in river
and kidney tissues from a cow and pig treated dermally with
[14C] fenthion. Unpublished Mobay report No. 74124 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Krotlinger, F. (1993) E 1752 (c.n. Fenthion): Study for acute
intraperitoneal toxicity in rats. Unpublished report No. 22144
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Lamb, D.W. & Anderson, R.H. (1974) The acute dermal toxicity of
Tiguvon technical to rabbits. Unpublished report No. 41455 from
Chemagro, Bayer GmbH, Leverkusen, Germany. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Lehn, H. (1990a) E 1752 (c.n. Fenthion): Mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished report No. 18740 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Lehn, H. (1990b) E 1752 (c.n. Fenthion): Mutagenicity test on
unscheduled DNA synthesis in rat liver primary cell cultures
in vitro. Unpublished report No. 100572 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Leser, K.H. (1990) E 1752 (Fenthion): Study for cholinesterase
inhibition following high doses of E1752 (administration to
B6C3F1 mice in the diet over a period of about four weeks).
Unpublished report No. 19088 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Machemer, L. (1978a) S 1752 (Fenthion, Lebaycid active ingredient):
Evaluation for embryotoxic and teratogenic effects in orally
dosed rats. Unpublished report No. 7580 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Machemer, L. (1978b) S 1752 (Fenthion, Lebaycid active ingredient):
Dominant lethal study of male mice to test for mutagenic effects.
Unpublished report No. 7449 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Mihail, F. (1978) S 1752 (Lebaycid active ingredient): Determination
of percutaneous toxicity. Unpublished report No. 7604 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Mihail, F. & Schilde, B. (1979) S 1752 (Fenthion, the active
ingredient of Lebaycid and Baytex): Subacute dermal cumulative
toxicity on rabbits. Unpublished report No. 8624 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Misra, U.K., Nag, D., Bhushan, V. & Ray, P.K. (1985) Clinical and
biochemical changes in chronically exposed organophosphate
workers, Toxicol, Lett., 24, 187-193.
Misra, U.K.. Nag, D., Khan, W.A. & Ray, P.K. (1988) A study of nerve
conduction velocity, late responses and neuromuscular synapse
functions in organophosphate workers in India. Arch. Toxicol.,
61, 496-500.
Miyaoka, T., Takahashi, H., Tsuda, S. & Shirasu, Y. (1984)
Potentiation of acute toxicity of 2- sec-butylpheny-
N-methylcarbamate (BPMC) by fenthion in mice. Fundam. Appl.
Toxicol., 4, 802-807.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1986) Mechanism of potentiation
of BPMC toxicity by fenthion pretreatment in mice.
J. Pharmacobio-Dyn., 9, 697-703.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1987) Effect of O,O-dimethyl
O-(3-methyl-4-methylthiophenyl) phosphorothioate (fenthion)
pretreatment on acute toxicity of 2- sec-butylphenyl
N-methylcarbamate (BPMC) in dogs. Jpn. J. Vet. Sci., 49,
173-175.
Nelson, D.L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Pauluhn, J. (1985) E 1752 Technical (c.n. fenthion): Study for
irritant/corrosive effect on skin and eye (rabbit). Unpublished
report No. 13446 from Bayer AG, Institute für Toxikologie,
Wuppertal, Germany.
Pickering, E.N. (1966) Organic phosphate insecticide poisoning.
Can. J. Med. Technol., 28, 174-179.
Pither, K.M. (1979) Metabolism of Baytex in male and female pigs.
Unpublished report No. 68475 from Mobay Corporation, Corporate
Toxicology Department, Kansas, USA. Submitted to WHO by Bayer AG,
Wuppertal Germany.
Puhl, R.J. & Hurley, J.B. (1982) The absorption, excretion and
metabolism of Baytex-ring-1-14C by rats. Unpublished report No.
82227 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report No. 99660 from
Microbiological Associates Inc., Maryland, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Rani, M.V.U & Rao, M.S. (1991) In vivo effect of fenthion on human
lymphocytes. Bull. Environ. Contamin. Toxicol., 47, 316-320.
Rosenblum, I. (1980) A safety evaluation of fenthion (S 1752) in
rhesus monkeys (Macaca mulatta). Unpublished Mobay report No.
68789 from Albany Medical College, New York, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Sherman, M. & Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. Appl. Pharmacol., 3, 521-533.
Shimamoto, K. & Hattori, K. (1969) Chronic feeding of Baytex
( O,O-dimethyl- O-(4-methylmercapto-3-methyl)phenylthiophosphat
e) in rats. Acta Med. Univ. Kyoto, 40, 163-171.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Baytex in rats. Unpublished report No. 944 from
Mobay Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Shiotsuka, R.N. (1987b) Acute inhalation toxicity study with Baytex
technical in rats. Unpublished report No. 820 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1977) Evaluation of
selected pesticides as chemical mutagens: In vitro and in
vivo studies. US Environmental Protection Agency Health Effects
Research Laboratory Document No. EPA-600/1-77-028 from Stanford
Research Institute, California, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Singh, S.P., Sharma, L.D. & Bahga, H.S. (1987) Acute oral toxicity of
fenthion in poultry. Indian J. Anim. Res., 21, 48-50.
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutat. Res., 102,
89-102.
Suberg, H. & Leser, K.H. (1990) E 1752 Oncogenicity study on B6C3F1
mice (feeding study for periods of up to 24 months). Unpublished
report No. 19624 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull.
World Health Org., 28, 213-218.
Thyssen, J. (1978) S 1752 (Lebaycid-active ingredient): Acute
inhalation toxicity studies. Unpublished report No. 7842 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Thyssen, J. (1979) Fenthion (S 1752, the active ingredient of Lebaycid
and Baytex): Subacute inhalation toxicity study on rats.
Unpublished report No. 8383 from Bayer AG Institut für
Toxikologie, Wuppertal, Germany.
Tuler, S.M. & Bowen, J.M. (1989) Toxic effects of organophosphates on
nerve cell growth and ultrastructure in culture. J. Toxicol.
Environ. Health, 27, 209-223.
Tuler, S.M., Febles, D. & Bowen, J.M. (1988) Neuromuscular effects of
chronic exposure to fenthion in dogs and predictive value of
electromyography. Fundam. Appl. Toxicol., 11, 155-168.
US National Cancer Institute (1979) Bioassay of fenthion for possible
carcinogenicity (National Cancer Institute Carcinogenesis
Technical Report Series No. 103; US Department of Health,
Education, and Welfare Publication No. (NIH) 79-1353). Bioassay
conducted by Gulf S. Research Institute, Louisiana, USA, under
contract to NCI and subcontract to Tracor Jitco, Inc., who
prepared the final report.
Wadia, R.S., Bhirud, R.H., Gulavani, A.V. & Amin, R.S. (1977)
Neurological manifestations of three organophosphate poisons.
Indian J. Med. Res., 66, 460-468.
Weber, H. & Ecker, W. (1992) [phenyl-1-14C]Fenthion: Absorption,
distribution, excretion and metabolism in a lactating goat.
Unpublished report No. 3752 from Bayer AG, Institut für
Metabolismusforschung, Leverkusen-Bayerwerk, Germany. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Wolfe, H.R., Armstrong, J.F. & Durham, W.F. (1974) Exposure of
mosquito control workers to fenthion. Mosquito News, 34,
263-267.
Two lactating cows received 32p-fenthion as a single dermal dose
of about 13 mg/kg bw or a single intramuscular dose of about 8.5 mg/kg
bw. Unchanged fenthion constituted > 50% of the nonionic residues in
the milk for three days after the dermal and seven days after the
intramuscular treatment. In urine, > 95% of the radiolabel excreted
was in the form of hydrolytic products; the parent compound accounted
for only a small percentage of the chloroform-soluble residues. In
faeces, unchanged fenthion constituted > 50% of the acetonitrile-
soluble residues. The animals were slaughtered 14 days after the
dermal and 21 days after the intramuscular treatment; > 50% of the
tissue residues appeared as unchanged fenthion, but oxidation products
were also present (Knowles & Arthur, 1966).
One lactating dairy cow (Jersey breed) was given a single dermal
dose of [ring-1-14C]-fenthion (purity, 98.2%) at 5.08 mg/kg bw active
ingredient, as a formulation for treatment of lice and horn flies in
beef and non-lactating dairy cattle, and sacrificed 18 h after
treatment. Urine, milk, skin, and selected tissues were then analysed
for 14C-fenthion residues. Unchanged (parent) fenthion was the major
tissue residue, accounting for > 95% of the residues in all tissue
samples collected from the application site, 71-95% in liver, muscle,
and peritoneal fat, and 51% in kidney. Other minor residue components
identified included fenthion sulfoxide (muscle, 5% of residue) and
fenthion sulfone (liver, 8% of residue). In addition, a number of
unknown polar metabolites were found in the liver and kidney,
representing 22 and 44%, of the total residue, respectively, two of
which were identified by high-performance liquid chromatography in a
later study (Krautter, 1990b) as glucuronide conjugates of phenol
sulfoxide and phenol sulfone, the primary metabolites of fenthion
(Krautter, 1990a).
A single lactating goat received [phenyl-1-14C]-fenthion
(chemical and radiopurity, > 99%) by gavage at 20 mg/kg bw daily for
three consecutive days and was sacrificed 3.5 h after the last dose.
The nature of the residues was determined in urine, milk, and edible
tissues. Phenol fenthion, phenol sulfoxide, and phenol sulfone were
the three major metabolites in all tissues examined. Various
intermediate metabolites in the biodegradation from parent fenthion to
the phenols (demethylated phosphorus-containing compounds with varying
oxidation at the methylsulfur moiety and/or at the phosphoric acid
moiety of the molecule) were also identified. Demethyl fenoxon
sulfoxide and sulfone were especially predominant in muscle, while the
sulfoxide and sulfone of fenthion constituted about 30% of the
residues in fat. Unchanged fenthion was not detected in any tissue
sample. The metabolites excreted in urine were similar to those in the
liver, exception that a higher percentage of phenol sulfide was
present in the urine (Weber & Ecker, 1992).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion are
summarized in Table 1. Technical-grade fenthion is moderately toxic to
mammals (mice, rats, guinea-pigs, and rabbits) when given by oral,
intraperitoneal, dermal, or inhalation routes. It is highly toxic to
arian species (especially to the wild mallard duck) when administered
orally. The clinical symptoms of acute toxicity are consistent with
those of central and peripheral cholinergic intoxication by
organophosphorus esters, including decreased spontaneous activity,
apathy, dyspnoea, ataxia, tremors, convulsions, lacrimation,
salivation, and diarrhoea. The symptoms were reversible in surviving
animals.
(b) Short-term toxicity
Exposure to fenthion by inhalation for 6 h/day daily for nine
days at 0.21 mg/litre (initial concentration in a static inhalation
chamber) resulted in signs of poisoning but no mortality, in rats,
guinea-pigs, rabbits, or cats. Serum and erythrocyte cholinesterase
activities were severely depressed but recovered within three weeks
(Klimmer, 1963).
Mice
Groups of 10 male and 10 female B6C3F1 mice were given E 1752
technical (purity, 98.7%) in the diet at 0 (control diet), 150, 200,
or 250 ppm (equal to 0, 83.2, 117.2, and 140.3 mg/kg bw per day in
males and 0, 83.6, 114.5, and 151.8 mg/kg bw per day in females) for
one month. No NOAEL was seen At the lowest dose, emaciation, tremor,
and decreased body weight were observed in males, reduced water
consumption and decreased spleen weight in animals of each sex, and
reduced liver weight in females. Severe inhibition (68-80%) of brain
and erythrocyte acetylcholinesterase was noted in all animals. At the
two higher doses, additional treatment-related effects seen were a
moribund condition resulting in unscheduled sacrifice of two males per
dose group and emaciation and decreased body weight in females (Leser,
1990).
Rats
Five groups of 22 male and 22 female rats were fed diets
containing technical-grade fenthion (purity not given, in soya bean
oil) at 0, 0.25, 0.5, 2.5, or 5.0 mg/kg bw per day for three months.
The NOAEL was 0.25 mg/kg bw per day, based on depression of
cholinesterase activity in erythrocytes, plasma, heart, liver, and
brain (no raw data) at > 0.5 mg/kg bw per day. At this dose and
above, clinical signs of cholinergic intoxication and significant
Table 1. Acute toxicity of fenthion
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Mouse Male Oral 150 Francis & Barnes (1963)
Female 190
Male Oral 227 Dubois (1968)
Female 225
Male Intraperitoneal 125 Dubois & Kinoshita (1964)
Female 150
Female Intraperitoneal 200-2404 Budreau & Singh (1973a)
Rat Male Oral 175-470 Klimmer (1963); Francis & Barnes (1963);
Female 245-310 Gaines (1969); Dubois & Kinoshita (1964)
Male Oral 405 Eigenberg (1987a)
Female 566
Male Intraperitoneal 260 Dubois & Kinoshita (1964)
Female 325
Male Intraperitoneal 152 Klimmer (1963)
Male Intraperitoneal 330 Krotlinger (1993)
Female 203
Male Dermal 330-650 Klimmer (1963); Francis & Barnes (1963);
Gaines (1969); Dubois & Kinoshita (1964)
Female 330-500
Male Dermal 1680 Mihail (1978)
Female 2830
Male Dermal 586 Bomann (1991)
Female 800
Male, female Inhalation (1-h) > 1.197 Thyssen (1978)
Male Inhalation (4-h) 1.2 Thyssen (1978)
Female 0.8
Male Inhalation (1-h) 1.838 Shiotsuka (1987a)
Female 1.637
Table 1. (con't)
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/l air)
Male Inhalation (4-h) 0.919 Shiotsuka (1987a)
Female 0.819
Male Inhalation (4-h) 0.507 Shiotsuka (1987b)
Female 0.454
Guinea-pig Male Oral > 1000 Francis & Barnes (1963)
Male Oral 260 Dubois & Kinoshita (1964)
Male Intraperitoneal 310 Dubois & Kinoshita (1964)
Rabbit Male Oral 150-175 Francis & Barnes (1963)
Male Dermal 150 Klimmer (1963)
Male Dermal 150 Lamb & Anderson (1974)
Female 131
Male, female Dermal 667 Eigenberg (1987b)
Chicken NR NR 310 Dubois & Doull (1960)
Male Oral 28 Sherman & Ross (1961)
Female Oral 30-40 Francis & Barnes (1963)
Female Oral 27 Flucke (1986a)
Male Oral 37.5 Singh et al. (1987)
Female Dermal 222 Flucke (1986b)
Duck NR Oral 15 Dubois & Doull (1960)
NR Oral 1-2 Keith & Mulla (1966)
NR, not reported
reductions in food consumption and body weight were evident. At the
two highest doses, decreased spermatogenesis and atrophic prostate
glands were noted in males; the female reproductive system was not
affected. All females at the highest dose died during the second and
third weeks of treatment (Shimamoto & Hattori, 1969).
Six groups of 12 male and 12 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0.2, 3, 5,
25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5.0 mg/kg
bw per day, for 16 weeks. No treatment-related adverse effects were
observed on body weight or body-weight gain or on food consumption;
gross and microscopic examination of tissues also showed no effect,
The NOAEL was 0.25 mg/kg bw per day based on cholinesterase depression
(source of enzyme not specified) at 1.25 mg/kg bw per day (Doull
et al., 1961a).
Six groups of 25 male and 25 female rats were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 3,
5, 25, or 100 ppm, equivalent to 0, 0.10, 0.15, 0.25, 1.25, or 5 mg/kg
bw per day, for one year. Significant inhibition (> 20%) of
erythrocyte acetylcholinesterase activity was noted at > 5 ppm.
Brain acetylcholinesterase activity was significantly depressed
(> 10%) and a higher mortality rate (particularly among males) was
observed at 25 ppm and above. At the highest dose, increased
haemosiderosis was noted in the spleen. The NOAEL was 0.25 mg/kg bw
per day, based on significant inhibition of brain acetylcholinesterase
at higher doses (Doull et al., 1963a),
Four groups of 10 male and 10 female Wistar rats were exposed to
an aerosol of technical-grade fenthion (purity, 98.2%) at nominal
concentrations of 0, 0.003, 0.015, or 0.075 mg/litre, equal to
analytical concentrations of 0, 0.001, 0.003, or 0.016 mg/litre, in a
head-/nose-only inhalation chamber for 6 h/day, five days per week for
three weeks. Exposure did not induce mortality or changes in body
weight, the results of haematology, clinical chemistry, or urinalysis,
gross pathology, or organ weights. At analytical concentrations
> 0.003 mg/litre, clinical symptoms of behavioural disturbance
(inactivity and/or unpreened coats) were observed in females only;
plasma cholinesterase was depressed in animals of each sex.
Significant inhibition of erythrocyte and brain acetylcholinesterase
activities occurred at the highest dose, and treatment-induced
inflammatory changes of the respiratory tract were seen in females.
The NOAEL was 0.001 mg/litre (analytical), based on clinical signs of
intoxication in females at 0.003 mg/litre and depression of brain and
erythrocyte acetylcholinesterase activities at 0.016 mg/litre
(Thyssen, 1979).
Rabbits
Groups of six male and six female New Zealand white rabbits
received dermal applications of technical-grade fenthion (purity,
98.2%) in Cremophos EL (1.5% v/v in distilled water) on intact (three
animals) or abraded (three animals) skin sites at 0, 5, or 25 mg/kg bw
per day for 7 h each day, five days per week for three weeks. The
NOAEL was 5 mg/kg bw per day, based on inhibition of erythrocyte
acetylcholinesterase in animals of each sex at 25 mg/kg bw per day. No
treatment-related effect was observed on general appearance,
behaviour, or body weight or by haematology, clinical chemistry,
urinalysis, gross pathology, organ weight measurement, or
histopathology (Mihail & Schilde, 1979).
Groups of five male and five female HRA:(NZW) specific pathogen-
free rabbits received dermal applications of technical-grade fenthion
(purity, 96.9%) formulated in Cremophos EL (1.5% v/v in distilled
water) on intact skin sites at 0, 5, 50, or 100 mg/kg bw per day for
6 h each day, five days per week for three weeks. The NOAEL was
50 mg/kg bw per day, based on inhibition of brain acetylcholinesterase
in females at 100 mg/kg bw per day. No treatment-related effect was
observed on appearance, behaviour, or body weight, or by haematology,
clinical chemistry, urinalysis, gross pathology, organ weight
measurements, or histopathology (Bailey, 1987).
Groups of five male and five female HRA:(NZW) specific
pathogen-free rabbits received dermal applications of technical-grade
fenthion (purity, 96.9%) formulated in Cremophos EL (1.5% v/v in
distilled water) on intact skin sites at 0, 200, or 400 mg/kg bw per
day for 6 h each day, five days per week for three weeks. The study
was terminated within two weeks of the start of treatment owing to
high mortality. An additional group of five male and five female
rabbits was given a lower dose of 150 mg/kg bw per day under similar
conditions; the original control group was retained. This treatment
did not affect general appearance, behaviour, body weight, or food
consumption, or the results of haematology, clinical chemistry, organ
weight measurement, or gross or histopathology. Plasma, erythrocyte,
and brain cholinesterase activities were depressed in animals of each
sex, with a significant inhibition of > 50% in males and 30% in
females at termination of the study. The LOAEL was 150 mg/kg bw per
day, based on inhibition of brain and erythrocyte acetylcholinesterase
activities (Bailey, 1988).
Dogs
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, or 1.25 mg/kg bw per day) for
12 weeks. The NOAEL was 0.125 mg/kg bw per day, based on significant
(> 20%) inhibition of erythrocyte acetylcholinesterase activity at
50 ppm. At > 5 ppm, plasma cholinesterase was also depressed, but
the effect (an indication of exposure) was not considered to be
toxicologically significant (Doull et al., 1961b).
Four groups of two male and two female dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 2, 5,
or 50 ppm (equivalent to 0, 0.05, 0.125, and 1.25 mg/kg bw per day)
for one year. The NOAEL was 0.125 mg/kg bw per day, based on
significant inhibition of erythrocyte (> 20%) and brain (> 10%)
acetylcholinesterase activity at 50 ppm. At > 5 ppm, plasma
cholinesterase was also depressed, but the effect (an indication of
exposure) was not considered to be toxicologically significant. A
slight increase in spleen weight with splenic congestion,
extramedullary haematopoiesis, and haemosiderosis, was also observed
in all treated animals; the effect was not dose-related and not
considered to be adverse (Doull et al., 1963b).
Groups of four male and four female beagle dogs were fed diets
containing technical-grade fenthion (purity, 97%) at 0, 2, 10, or
50 ppm, equal to 0, 0.06, 0.26, or 1.23 mg/kg bw per day in males and
0, 0.06, 0.26, or 1.18 mg/kg bw per day in females, for one year. The
NOAEL was 0.06 mg/kg bw per day, based on toxicologically significant
inhibition (> 10%) of brain acetylcholinesterase activity at 10 ppm
At the highest dose, both erythrocyte and brain acetylcholinesterase
activities were significantly depressed (> 50 and > 30%,
respectively). Plasma cholinesterase activity was inhibited at all
doses tested; but the effect (an indicator of exposure) was not
considered to be toxicologically significant. No other treatment-
related change was observed (Christenson, 1990a).
Groups of four male and four female pure-bred dogs were fed diets
containing technical-grade fenthion (purity unspecified) at 0, 3, 10,
or 30 (weeks 0-64), 50 (weeks 65-67), or 60 (weeks 68-104) ppm (equal
to 0, 0.09, 0.32, and 1.28 mg/kg bw per day in animals of each sex)
for two years. No mortality or clinical sign of toxicity was seen
during the study. No significant treatment-related change in body
weight, ophthalmoscopic, haematological, or urinary parameters, gross
or histopathology of tissues was evident at any dose. The NOAEL was
0.09 mg/kg bw per day, based on significant inhibition of erythrocyte
acetylcholinesterase activity in males (> 20%) and of brain
acetylcholinesterase activity in females (> 10%) at 10 ppm. At the
highest dose, erythrocyte and brain acetylcholinesterase activities
were significantly depressed in animals of each sex. Decreased food
consumption (females) and lowered plasma protein levels (males) were
also observed (Hoffmann & Weischer, 1975).
Monkeys
Four groups of five male and five female rhesus monkeys were
given daily doses of technical-grade fenthion (purity, 98.1%) in corn
oil by gavage at concentrations of 0, 0.02, 0.07, or 0.20 mg/kg bw per
day for two years. Animals were observed daily for general appearance
and clinical signs; body weight and ophthalmological parameters were
recorded monthly, and clinical chemistry, haematology, and urinalyses
were performed at 0, 1, 3, 6, 12, 18, and 23 months. Plasma and
erythrocyte cholinesterase activities were measured weekly for the
first four weeks and monthly thereafter. One animal of each sex at 0
and 0.20 mg/kg bw per day was killed seven months and three weeks
after the beginning of treatment for measurement of brain
acetylcholinesterase and gross and histopathology. All monkeys
underwent necropsy after 23 months, but no histopathology was
performed. Plasma cholinesterase was consistently depressed in animals
of each sex at 0.20 mg/kg bw per day, but the effect (an indicator of
exposure) was not considered to be toxicologically significant. A
significant (> 20%) inhibition of erythrocyte acetylcholinesterase
activity was observed occasionally in animals of each sex (9/26
determinations over the 23-month study period for males and 2/26 for
females) at the highest dose. No effect on brain acetylcholinesterase
was evident in the four animals killed at 7.25 months. Gross
examination revealed abnormally small testes and ovaries in animals at
0.20 mg/kg bw per day; however, in the absence of organ weights and
histopathological data, the toxicological significance of this finding
could not be fully assessed. No other treatment-related adverse
effect was recorded. The NOAEL was 0.07 mg/kg bw per day, based on
inconsistent erythrocyte acetylcholinesterase depression and possible
effects on male and female reproductive organs at 0.20 mg/kg bw per
day (Rosenblum, 1980).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of male and female B6C3F1 mice, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) in the diet at concentrations of 0,10,
or 20 ppm (equivalent to 0, 1.5, or 3.0 mg/kg bw per day) for 103
weeks. All surviving animals were maintained on control diet for up to
one week after the end of treatment before they were sacrificed and
subjected to gross and microscopic examination. Treatment with
fenthion did not result in significant differences in the survival or
growth rate of mice at any dose. Clinical signs of intoxication were
evident four months after the beginning of treatment (LOAEL not
specified). At the end of the study (104 weeks), histopathological
examination revealed a variety of neoplasms, the majority of which
were judged to be unrelated to fenthion administration. An increased
incidence of sarcomas, fibrosarcomas, or rhabdomyosarcomas of the
integumentary system was observed in treated male mice; the incidences
of integumentary sarcomas were 0/25 at 0 ppm, 7/49 at 10 ppm, and 8/48
at 20 ppm (7/435 in historical controls). The effect was considered to
be treatment-related, indicating that technical-grade fenthion is
oncogenic in male mice under the conditions of the study. There was no
NOAEL for systemic toxicity, since the LOAEL for clinical signs was
not specified (US National Cancer Institute, 1979).
Groups of 60 male and 60 female specific-pathogen-free B6C3F1
mice were fed diets containing technical-grade fenthion (purity,
92.3%) at 0, 0.1, 1.0, 5.0, or 25 ppm, equal to 0, 0.03, 0.40, 1.95,
or 9.42 mg/kg bw per day in males and 0, 0.03, 0.47, 2.25, or
10.63 mg/kg bw per day in females, for 102 weeks. Additional groups of
20 mice of each sex were fed the same diets for 50 weeks before
scheduled interim kills. Brain acetylcholinesterase activity was
depressed by > 10% at > 0.1 ppm in males and at > 1.0 ppm in
females, but the effect was not dose-related and was seen in males at
terminal but not interim sacrifice and in females at interim but not
terminal sacrifice. Significant (> 20%) inhibition of erythrocyte
acetylcholinesterase activity occurred only in the males at 25 ppm at
week 54. As the inhibition of brain acetylcholinesterase was only
marginally significant (10-17%) at the three lower doses, there was no
dose-response relationship, and the effect was inconsistent over
treatment intervals, it was judged to be of minimal toxicological
significance at doses > 5.0 ppm. The NOAEL for systemic toxicity
was 1.95 mg/kg bw per day, as brain acetylcholinesterase activity was
clearly depressed at the next highest dose in males at both interim
and terminal sacrifice (29-32%) and in females at interim sacrifice
(26%). At this dose, there were also significant increases in body
weight in animals of each sex, elevated plasma cholesterol levels in
animals of each sex early in the study, and elevated absolute and
relative liver weights of male mice at termination. In the absence of
remarkable histopathological findings, the changes at 25 ppm were
considered not to be toxicologically significant. There was no
increased incidence of any neoplastic or non-neoplastic lesion in the
treated mice at any dose up to and including 9.42 mg/kg bw per day.
Technical-grade fenthion was thus considered not to be oncogenic to
mice under the conditions of the study (Suberg & Leser, 1990).
The observation of an increased incidence of integumentary
sarcomas in male mice (US National Cancer Institute, 1979) at doses of
1.5 and 3.0 mg/kg bw per day was not reproduced in a second study
(Suberg & Leser, 1990) in which doses up to 9.42 mg/kg bw per day were
tested. As the increased tumour incidence in the first study was not
dose-related and was site-and sex-specific and the results of the
second study (conducted with more control animals and more groups for
interim sacrifice) were clearly negative at doses up to three times
those of the first study, technical-grade fenthion would appear not to
be oncogenic in the mouse.
Rats
Groups of male and female Fischer 344 rats, 25 of each sex in the
control group and 50 of each sex per dose, were fed technical-grade
fenthion (purity unspecified) at dietary concentrations of 0, 10, or
20 ppm (equivalent to 0, 0.5, or 1.0 mg/kg bw per day) for 103 weeks.
All surviving animals were maintained for one or two weeks after the
end of treatment and were then sacrificed and submitted to gross and
microscopic examination. No treatment-related mortality or behavioural
changes were observed, and the growth rate was comparable in control
and treated groups. Histopathological examination revealed a variety
of neoplastic and non-neoplastic lesions common to rats of this
strain. No increase in the incidence of any type of tumour was
observed in the treated rats at any dose up to and including 20 ppm
(1.0 mg/kg bw per day). Technical-grade fenthion was thus considered
not to be oncogenic to the rat under the conditions of the study (US
National Cancer Institute, 1979).
Groups of male and female specific-pathogen-free Wistar rats, 100
of each sex in the control group and 50 of each sex per dose, were fed
technical-grade fenthion (purity unspecified) in the diet at
concentrations of 0, 3, 15, or 75 ppm, equal to 0, 0.14, 0.72, or
374 mg/kg bw per day in males and 0, 0.19, 0.93, or 4.46 mg/kg bw per
day in females, for 24 months. The rats were weighed weekly during the
first 26 weeks and every two weeks thereafter. Food consumption was
recorded weekly. Haematology, clinical chemistry, liver and kidney
funtional tests, and urinalysis were performed on five rats of each
sex per group at 1, 3, 6, and 12 months, and on 10 rats of each sex
per group at the end of the study (24 months). Plasma and erythrocyte
cholinesterase activities were determined at the end of weeks 1, 2, 4,
8, 13, 26, 52, 78, and 105; brain acetylcholinesterase activity was
not measured. At the end of the study, all surviving rats were
sacrificed and examined macroscopically, and tissues and organs were
removed, weighed, and studied microscopically. Doses of 3 or 15 ppm
did not affect physical appearance, behaviour, growth, or survival
rate, but males at the highest dose had a significantly lower body
weight and both males and females appeared to have increased mortally.
No treatment-related effect was observed by haematology, clinical
chemistry, urinalysis, or gross or microscopic pathology. (It should
be noted that the rats from which blood and urine samples were taken
for analysis during the first year were not identified.) Significant,
dose-dependent depression of plasma and erythrocyte cholinesterase
activities was observed in animals of each sex at 15 and 75 ppm. The
NOAEL for systemic toxicity, based on erythrocyte acetylcholinesterase
inhibition, was 0.14 mg/kg bw per day. The results of histopathology
showed no increase in the incidence of tumours in treated rats at any
dose up to and including 3.74 mg/kg bw per day, the highest dose
tested, indicating that technical-grade fenthion was not oncogenic to
the rat under the conditions of the study (Bomhard & Loser, 1977).
Groups of 50 male and 50 female Fischer 344 rats were fed diets
containing technical-grade fenthion (purity, 97.0%) at concentrations
of 0, 5, 20, or 100 ppm, equal to 0, 0.2, 0.8, or 5.2 mg/kg bw per day
in males and 0, 0.3, 1.3, or 7.3 mg/kg bw per day in females, for two
years. Two additional groups of 20 rats of each sex were fed diets
containing fenthion at concentrations of 0 or 100 ppm for one year
before scheduled interim sacrifice. No NOAEL for systemic toxicity
could be determined, because of statistically (P < 0.05) and
toxicologically significant (> 10%), dose-related inhibition of brain
acetylcholinesterase activity in animals of each sex at all doses,
including the lowest dose of 0.20 mg/kg bw per day. Plasma and
erythrocyte cholinesterase activities were also depressed at all
doses, but toxicologically significant (> 20%) inhibition of
erythrocyte acetylcholinesterase occurred only at > 20 ppm. At the
highest dose, technical-grade fenthion was retinotoxic, especially in
females, and ophthalmological examination revealed an increased
incidence of retinal degeneration and posterior subcapsular cataract
formation in females and focal corneal scarring in animals of each
sex. Histopathological examination revealed a higher incidence of
corneal mineralization and retinal atrophy in females, and corneal
neovascularization and optic nerve atrophy in animals of each sex. The
retinotoxic effect was further confirmed by electroretinographic
examination, with suppression of electroretinograms also in a number
of females at 20 ppm at interim sacrifice at week 75. At 100 ppm,
additional treatment-related effects were an increased incidence of
nonspecific clinical signs (urine staining, tail and paw lesions,
rough coat, and hunched back), lower body-weight gain, and remarkable
histopathological findings including vacuolar degeneration of the
nasolacrimal ducts, mineralized and raised areas in the stomach,
stomach acanthosis, hyperkeratosis (males) and hyperplasia (females),
epididymal vacuolar degeneration, and chronic active inflammation of
the skin of the tail and hindlimbs. A higher incidence of vacuolar
degeneration of the nasolacrimal ducts was also seen in females at
20 ppm. There were no treatment-related oncogenic effects in males or
females at any dose up to and including 5.2 mg/kg bw per day, the
highest dose tested. Technical-grade fenthion was thus considered not
to be oncogenic to the rat under the conditions of the study
(Christenson, 1990b).
(d) Reproductive toxicity
Mice
In a five-generation, two-litter per generation study, two groups
of CD-1 mice received technical-grade fenthion (purity unspecified) in
their drinking-water at concentrations of 0 or 60 ppm (equivalent to
9.0 mg/kg bw per day). The control group comprised 10 males and 14
females, and the treated group comprised 10 males and 22 females.
There was a significant increase in pup mortality during the first
postnatal week, especially in the second, third, and fourth
generations. No treatment-related effect on reproductive performance,
lactation, or growth rate of pups was observed. No histopathological
changes were noted in the liver or kidney of adult males of the third
generation (Budreau & Singh, 1973b).
Rats
In a two-generation, one-litter per generation study in Crl:CD BR
rats, five groups of 30 male and 30 female animals were fed diets
containing technical-grade fenthion (purity, 96.9%) at concentrations
of 0, 1, 2, 14, or 100 ppm, equal to 0, 0.08, 0.16, 1.16, or 8.3 mg/kg
bw per day (based on premating food intake values), for 70 days (F0
and F1 parents) before mating (1:1 ratio) for production of the F1
and F2 litters. No treatment-related effect on mortality, clinical
signs of toxicity, mean body weight, mean food consumption, or gross
pathology was observed in adults of the F0 or F1 generation.
Epididymal weight was increased in F0 males at 100 ppm.
Histopathological examination revealed vacuolation in the lining
ductal epithelial cells of the corpus epididymus in a few F0 and F1
males at 14 ppm and in all at 100 ppm. Brain acetylcholinesterase
activity was significantly (> 10%) depressed in F0 females at all
doses and in F0 males and F1 males and females at the two higher
doses. Inhibition at the two lower doses (-22 and -17%, respectively)
in F0 females was not dose-related and was of a magnitude similar
to those at 14 ppm in F0 males (-19%) and in F1 males (-28%)
and females (-18%). F0 female controls had a mean brain
acetylcholinesterase activity of 2428 mU/g, which was significantly
higher than the other control values observed in the study (2137,
1894, and 1515 mU/g for F0 male, F1 male, and F1 female controls,
respectively). The effect on brain acetylcholinesterase activity seen
in F0 females at 1 and 2 ppm was therefore judged to be of minimal
toxicological significance. At the two higher doses, significant
(> 20%) inhibition of plasma and erythrocyte cholinesterase
activity was seen in all parental adults and pups. Neonatal brain
acetylcholinesterase activity was inhibited only at the highest dose.
The NOAEL for maternal toxicity was 0.16 mg/kg bw per day, based on
consistent inhibition of brain and erythrocyte acetylcholinesterase
activity at 14 ppm. There was no treatment-related effect on
reproductive performance, embryo- or fetotoxicity, litter size, pup
viability, or growth at > 14 ppm. At the highest dose, decreased
fertility, a decreased number of implantation sites per dam, decreased
litter size, an increased number of stillborn pups per litter, a
reduced viability index (days 0-4), and decreased pup body-weight gain
during lactation (days 14 and 21) were observed in both F0 and F1
generations and F1 and F2 litters. The NOAEL for reproductive
toxicity was thus 1.16 mg/kg bw per day (Kowalski et al., 1989).
(e) Developmental toxicity
Rats
Four groups of 20 mated FB30 Long-Evans female rats were given
technical-grade fenthion (purity, 98.1%) in aqueous Cremophor EL
emulsion by gavage at 0, 1, 3, or 10 mg/kg bw per day once daily on
days 6-15 of gestation; the day semen was detected in a vaginal smear
was designated day 0 of gestation. On gestation day 20, all surviving
dams were sacrificed, and the fetuses were delivered by caesarean
section and necropsied. The numbers of implantation sites and of live,
dead, and resorbed fetuses, litter weight, fetal body weight, and
placental weight were recorded, and all fetuses were examined for
external, visceral, and skeletal abnormalities. No treatment-related
mortality, clinical symptoms of maternal toxicity, or disturbance of
intrauterine development were noted at any dose. All fetuses delivered
developed normally. No signs of fetotoxicity were seen, and no
treatment-related malformations were observed in any of the fetuses at
any dose up to and including 10 mg/kg bw per day. The NOAEL for
maternal toxicity, embryo- and fetotoxicity, and teratogenicity was
10 mg/kg bw per day, the highest dose tested (Machemer, 1978a).
Groups of 33 mated Crl:CD BR female rats were given technical-
grade fenthion (purity, 96.5%) in 5% aqueous Emulphor solution by
gavage at doses of 0 (vehicle control), 1, 4.2, or 18 mg/kg bw per day
once daily on days 6-15 of gestation; the day spermatozoa were
detected in a vaginal smear was designated as day 0 of gestation. Five
animals from each group were sacrificed on day 16, and the remaining
28 dams in each group were killed on day 20 of gestation. All dams
were necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. No NOAEL for maternal toxicity
could be determined owing to dose-related, toxicologically
significant inhibition of brain (> 10%) and erythrocyte (> 20%)
acetylcholinesterase activities at all doses tested, including the
lowest dose of 1 mg/kg bw per day, on days 16 and 20 of gestation.
Plasma cholinesterase was also depressed at > 1 mg/kg bw per day on
day 16 and at 18 mg/kg bw per day on day 20. No other treatment-
related maternal or embro- or fetotoxicity was seen at > 4.2 mg/kg
bw per day. At the highest dose, clinical symptoms of intoxication
(dry or bloody lacrimation, exophthalmia, hypoactivity, tremors,
salivation, and urine-stained peritoneum), decreased mean bodyweight
gain, and reduced food consumption were observed. There was also a
slight increase in fetal skeletal variations, including an increased
incidence of incomplete ossification of the cervical arches, skull
bones, and third, fourth, and sixth sternebrae, and incomplete and/or
unossified metacarpals and metatarsals. These findings represent a
delay in skeletal maturation and were considered not to be adverse or
of toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in the fetuses at any dose up to and
including 18 mg/kg bw per day. The NOAEL for embryo- and fetotoxicity
and teratogenicity was therefore 18 mg/kg bw per day, the highest dose
tested (Kowalski, 1987).
Rabbits
Groups of 17 artificially inseminated American-Dutch female
rabbits were given technical-grade fenthion (purity, 96.5%) in 5%
aqueous Emulphor solution by gavage at doses of 0 (vehicle control),
1, 2.75, or 7.5 mg/kg bw per day once daily on days 6-18 of gestation;
the day of artificial insemination was designated day 0 of gestation.
All surviving animals were sacrificed on gestation day 28 and
necropsied; the gravid uterus was removed and examined, and the
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. The NOAEL for maternal toxicity
was 1 mg/kg bw per day based on toxicologically significant inhibition
of brain (> 10%) and erythrocyte (> 20%) acetylcholinesterase
activities at the two higher doses on gestation day 19 and/or 28. At
> 2.75 mg/kg bw per day, an increased incidence of soft stools was
noted both during and after treatment. At the highest dose, plasma
cholinesterase activity was depressed on gestation day 19 only. There
was also an increased incidence of unossified anterior metacarpals
among the fetuses, but this finding represents a delay in skeletal
maturation and was considered not to be an adverse effect of
toxicological significance. No other treatment-related embryo- or
fetotoxic effects were observed, and no treatment-related
malformations were observed in any of the fetuses at any dose up to
and including 7.5 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity and teratogenicity was 7.5 mg/kg bw per day, the highest
dose tested (Clemens, 1987).
(f) Genotoxicity
In an assay for dominant lethal mutation, three groups of 50 male
NMRI mice were each given a single oral dose of 0, 10, or 25 mg/kg bw
of technical-grade fenthion (purity unspecified) as a 2% aqueous
emulsion. Each male was then caged with an untreated virgin female for
four days; the same procedure was repeated for a total of 12 matings.
On days 12-16 of gestation, the females were sacrificed, and the
numbers of implantations and of live and dead implants (deciduomata,
resorption sites, and dead embryos) were counted. The high dose
resulted in overt clinical signs of toxicity in males (drowsiness,
ruffled coat, and dilated intestines). Except for increased
preimplantation loss at 25 mg/kg bw in the first two mating periods,
no other treatment-related effects on reproductive performance
(fertility, pre- and post-implantation loss) were observed (Machemer,
1978b).
A battery of studies was conducted with technical-grade fenthion
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were mostly negative; weakly positive results were obtained
in two of five assays for sister chromatid exchange, in one of two
assays for micronucleus formation (at a dose of 150 mg/kg bw), and in
one assay for unscheduled DNA synthesis. Summary results from the US
Environmental Protection Agency Health Effects Research Laboratory
(Simmon et al., 1977) indicated that technical-grade fenthion does
not induce reverse mutation in Salmonella typhimurium TA98, TA100,
TA1535, TA1537, or TA1538 or Escherichia coli WP2 uvrA; enhanced
mitotic recombination in yeast ( Saccharomyces cerevisiae D3);
unscheduled DNA synthesis in human fibroblasts (WI-38 cells);
preferential toxicity (DNA repair-proficient/deficient) in E. coli
(W3110, p3478) or Bacillus subtilis (H17, M45); or sex-linked
recessive lethality in Drosophila melanogaster in vivo.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Technical-grade fenthion (purity 97-99%) was not irritating to
the skin and was minimally or not irritating to the eyes of New
Zealand white rabbits (Pauluhn, 1985; Eigenberg, 1987c,d).
Technical-grade fenthion (purity 98.5%) did not induce dermal
sensitization in a maximization test (according to the method of
Magnusson and Kligman) in male DHPW guinea-pigs (Flucke, 1987)
(ii) Potentiation
Fenthion potentiated the acute intraperitoneal toxicity of
malathion, dioxathion, and coumaphos in rats, but intraperitoneal
administration of 13 other organophosphate or carbamate insecticides
to rats in combination with fenthion did not result in greater than
additive toxic effects (Dubois & Kinoshita, 1964). Dietary combination
of equitoxic doses (2 mg/kg bw) of fenthion with coumaphos, neither of
which alone affected cholinesterase activity when fed to dogs for six
weeks, was found to potentiate the anticholinesterase activity of
serum and erythrocytes by 75 and 30%, respectively. The potentiation
was less evident with malathion, and no potentiation was noted when
fenthion was fed in combination with dioxathion (Doull et al.,
1962). Oral administration to rats of a single dose of either a 3:1
mixture of fenthion and dichlorvos or a 1:3 mixture of fenthion and
isofenthion did not result in a greater than the theoretical additive
toxic effect (Kimmerle, 1967; Heimann, 1982). In several recent
studies, pretreatment with fenthion significantly potentiated the
acute toxicity of 2- sec-butylphenyl N-methylcarbamate in mice and
dogs. Its acute toxicity in mice was increased 15-fold by a 1-h oral
pretreatment with 30 mg/kg bw fenthion (one-tenth of the LD50).
Table 2. Results of tests for the genotoxicity of technical-grade fenthion
End-point Test system Concentration Results Reference
or dose
IN vitro
Reverse mutation S. typhimurium TA98, 0, 20-12 500 µg/plate Negative Herbold (1987)
TA100, TA1535, TA1537 0, 750-12 000 µg/plate
Reverse mutation S. typhimurium TA98, 0, 8-5000 µg/plate Negative Herbold (1990a)
TA100, TA1535, TA1537
hprt forward mutation Chinese hamster ovary 0, 20.0-100 µg/ml Negative Lehn (1990a)
cells 0, 12.5-75 µg/ml
Chromosomal aberration Chinese hamster ovary 0. 0.02-0.15 µg/ml Negativea Putman & Morris
cells (1989)
Chromosomal aberration Chinese hamster lung cells 0, 23.5-94 µg/ml Negativea Kajiwara (1989)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml Negative at < 20b,c Chen et al.
(1982a)
Sister chromatid exchange Chinese hamster V79 cells 0, 10-80 µg/ml (in diffusion Weakly positive Chen et al. (1982b)
chambers) at > 40b
Sister chromatid exchange Human lymphoid cell line 0, 0.02-20 µg/ml (unactivated) Negativea,b Sobti et al.
(1982)
LAZ-007 (of B-cell origin) 0, 20 µg/ml (activated)
Chromosomal aberration, Human lymphocytes 0, 0.5-5.0 µg/ml Weakly positive Rani & Rao (1991)
sister chromatid exchange at > 1.5
Unscheduled DNA Rat primary hepatocytes 0, 10-100 µg/ml Weakly positive Lehn (1990b)
synthesis 0, 5-30 µg/ml at > 5
Table 2. (con't)
End-point Test system Concentration Results Reference
or dose
In vivo
Sister chromatid exchange Rat bone-marrow 0, 10-100 mg/kg bw Negative Bai et al (1990)
lymphoid cells
Micronucleus formation Bor:NMRI mouse bone 0, 150 mg/kg bw Weakly positive Herbold (1990b)
marrow at 150b
a With and without exogenous metabolic activation
b Not confirmed in an independent assay
c Threshold of cytotoxicity
Inhibition of the metabolism or detoxification of 2- sec-butylphenyl
N-methylcarbamate by desulfuration of fenthion may have been
responsible, at least in part, for the synergistic effect (Miyaoka
et al., 1984, 1986, 1987).
(iii) Cholinesterase inhibition
Twelve groups of 10 tasted male Fischer 344 (CDF/Crl/Br) rats
were given single doses of technical-grade fenthion (purity, 97.5%)
orally, dermally, or subcutaneously at 0, 1, 5, or 25 mg/kg bw, and
the effect of the route of administration on plasma, erythrocyte,
and/or brain cholinesterase activity was studied for up to 14 days
after treatment. The NOAEL for all routes of administration was
5 mg/kg bw, based on toxicologically significant inhibition of
erythrocyte (> 20%) and brain (> 10%) acetylcholinesterase
activities at 25 mg/kg bw. Inhibition of plasma and erythrocyte
cholinesterase activity was maximal at 24 h after oral administration
and four days after dermal or subcutaneous administration, suggesting
more efficient absorption by the oral route. On day 14 after
treatment, depression of brain acetylcholinesterase activity was
greatest in the group that were treated subcutaneously; the prolonged
recovery may be associated with a slower release of fenthion into the
circulation when given by this route. The magnitude of cholinesterase
inhibition appeared to be only marginally significant after dermal
administration, suggesting that absorption is less effective by this
route. The rates of absorption were thus oral route > subcutaneous
route > dermal route (Christenson, 1990c).
(iv) Delayed neurotoxicity
Acute delayed neurotoxicity in hens: Groups of 15
atropine-protected, adult laying hens (Lohmann selected Leghorn
strain), five to seven months old, were given two doses of fenthion
(E 1752; purity, 98.5%) in 2% Cremophor EL by gavage at 40 mg/kg bw or
dermally at 200 mg/kg bw, 21 days apart. A positive control group of
five hens was given tri- ortho-cresyl phosphate (purity, 99.1%)
orally as a single dose of 375 mg/kg bw. Six hens given an oral dose
of 5 ml/kg bw 2% Cremophor EL and six untreated hens served as
negative controls. All animals were observed for 21 days after the
second dose before sacrifice, with the exception of the tri- ortho-
cresyl phosphate-treated hens which were killed on day 22 in moribund
condition. At termination, peripheral and central nervous tissues were
removed, fixed, and stained for microscopic examination. Clinical
signs of acute cholinergic intoxication were evident in all
fenthion-treated hens 7-24 h after treatment; recovery occurred within
7-8 days after oral administration and 14-18 days after dermal
application. No clinical symptoms (irreversible impairment of motor
coordination) or neurohistopathological lesions indicative of delayed
neuropathy were observed. The positive controls exhibited symptoms of
delayed nerurotoxicity (abnormal gait, ataxia, and paresis) from day 7
after treatment, which persisted until sacrifice on day 22;
neurohistopathological lesions characteristic of delayed neuropathy
were also evident (Flucke & Kaliner, 1987).
Neuropathy target esterase activity: Groups of nine atropine-
protected, adult hens (Lohmann selected Leghorn strain) were given a
single oral dose of technical-grade fenthion (E 1752; purity, 98.5%)
in 2% Cremophor EL by intubation at doses of 0 (vehicle control) or
40 mg/kg bw. Nine positive controls received tri- ortho-cresyl
phosphate as a single oral dose of 100 mg/kg bw. Neuropathy target
esterase activity in brain and spinal cord was inhibited by 0-14% over
that in vehicle controls 24 and 48 h and seven days after treatment.
Severe inhibition (> 50-90%) was seen in the positive controls
throughout the study (Flucke, 1988a).
Groups of nine atropine-protected adult hens (Lohmann selected
Leghorn strain) were given a single dermal dose of technical-grade
fenthion (E 1752; purity, 98.5%) in 2% Cremophor EL at doses of 0
(vehicle control), 200, or 400 mg/kg bw. A positive control group of
three to nine hens received tri- ortho-cresyl phosphate as a single
dose of 100 mg/kg bw by intubation. Neuropathy target esterase
activity in brain and spinal cord was inhibited by 0-20% over that in
vehicle controls at 24, 48, and 72 h and up to seven days after
treatment. Severe inhibition (> 80%) was seen in the positive
controls 24 and 48 h after treatment, demonstrating the sensitivity of
the test (Flucke, 1988b)
Short-term delayed neurotoxicity in hens: Groups of 10 adult
white Leghorn hens (Gallus gallus) were given technical-grade
fenthion (purity, 96.5%) by gavage in clear corn oil solution at doses
of 0, 1, 2, or 4 mg/kg bw per day for 14 weeks. Tri- ortho-cresyl
phosphate (10-60 mg/kg bw per day) was given as a positive control. No
treatment-related clinical symptoms or increases in the incidence
and/or severity of histopathological lesions of nervous tissues
characteristic of organophosphate-induced delayed neurotoxicity were
evident in the fenthion-treated hens at up to and including the
highest dose. Clinical signs of decreased activity and ataxia were
noted, but predominantly in the first hours after treatment; they were
no longer seen before the next dose, suggesting that they were likely
to have been caused by repetitive, severe acute cholinergic
intoxication and were not related to delayed neurotoxicity. The
positive controls had both toxic symptoms and histopathological
lesions of the nervous tissues typical of delayed neurotoxicity.
Histopathological examination revealed a dose-related increase in the
incidence of muscular hypertrophy or hyperplasia in all muscle layers
of the oesophagus, crop, proventriculus, gizzard, and intestine of all
fenthion-treated hens and glandular and nonglandular epithelial
hyperplasia in the oesophagus, crop, and proventriculus of some at the
middle dose and in most at the high dose. These lesions were probably
due to localized acetylcholinesterase inhibition, with subsequent
overstimulated muscle hypertrophy (Hayes & Ramm, 1988).
A second short-term study, in which technical-grade fenthion was
fed to hens was conducted to determine if the muscular hypertrophy and
hyperplasia observed by Hayes and Ramm (1988) were a direct effect of
fenthion or were due to the route of treatment. Two groups of 10 adult
white Leghorn hens (Gallus gallus domesticus) were given technical-
grade fenthion (purity, 96.9%) at dietary concentrations of 0 or
52 ppm (equivalent to 4 mg/kg bw per day) for 90 days. Minimal to
moderate muscular hypertrophy or hyperplasia was seen in the distal
oesophagus (between the crop and the proventriculus) of all treated
hens, which accounted for 98-99% of the increased thickness (+ 55%, in
comparison with controls) in the oesophageal wall. Hypertrophy or
hyperplasia of the oesophageal glandular components was also seen in
four birds. In addition, treated hens had a statistically significant
(P < 0.05) depression of cholinesterase activity in whole blood
(> 50% in comparison with controls) and tissue from all three
regions of the upper gastrointestinal tract (oesophagus, crop, and
proventriculus: about 70% in comparison with controls). It was
concluded that the muscular hypertrophy and hyperplasia observed
in the fenthion-treated hens was probably due to localized
acetylcholinesterase inhibition with subsequent overstimulation of the
oesophageal smooth muscle layers (Hayes, 1989).
Assessment of the neurotoxicity of fenthion in the literature:
A position paper (Flucke, 1990) which presented and discussed nine
publications dealing with the assessment of neurotoxicity of fenthion
was submitted. The main findings and critical evaluations are
summarized below.
1. In the series of four studies by Farage-Elawar & Francis (1987,
1988a,b; Francis & Farage-Elawar, 1987), the effects of fenthion,
debromoleptophos (which induces organophosphate-induced delayed
neurotoxicity), and fenitrothion (which does not) were compared
in very young chicks. On the basis of the finding that fenthion
produced neurotoxic signs (altered gait) and inhibition (> 50%)
of cholinesterase but little or no inhibition of neuropathy
target esterase, it was postulated that its neurotoxic potential
is not classical organophosphate-induced delayed neurotoxicity. A
critical evaluation of the four publications (by Flucke, 1990,
and the reviewer) revealed that the three organophosphates were
not administered in equitoxic doses, thus rendering direct
comparison of their toxic effects inappropriate, and that the
test animals were malnourished. Under the study conditions, there
was probably prolonged acute cholinergic intoxication due to
severe inhibition of cholinesterases after the lethal or
sublethal doses of debromoleptophos and fenthion, in contrast to
the low dose of fenitrothion (about one-fifth of the LD50),
which would not have induced overt cholinergic symptoms. A
build-up of acetylcholine at the motor end-plates could result in
pronounced, prolonged muscle fasciculation and, secondarily, in
the known necrotizing effects. Young chicks undergoing rapid
growth and muscle development are less likely to compensate
quickly and completely for this type of muscle damage, especially
if they are undernourished. Accordingly, the neurotoxic effects,
which occurred to the same degree with debromoleptophos and
fenthion, could be attributed to primary effects of severe acute
cholinergic intoxication and not to a particular (unknown)
neurotoxic potential of fenthion, as postulated by the authors.
2. Tuler et al. (1988) and Dellinger & Mostrom (1988) examined the
neurotoxic effects of fenthion in dogs. In the first study, the
authors concluded that there was no evidence of delayed
neurotoxicity. Prolonged weekly treatment with fenthion caused
hyperreflexia, proprioceptive deficits, progressive muscle fibre
necrosis, ultrastructural changes in nerve axons, and loss of
small motor units. Fenthion-induced primary neuropathy leading to
secondary myopathy was proposed. In the second study, the authors
concluded that there was no evidence of cholinergic effects and
no effect on vagal tone during the period of fenthion treatment
when maximal inhibition (> 50%) of acetylcholinesterase activity
was measured; however, on day 14 after treatment, the treated
dogs gave a slightly smaller response to an atropine challenge
(in comparison with controls), suggesting possible down-
regulation of the cholinergic receptors.
A critical evaluation of these two publications (by Flucke,
1990 and the reviewer) revealed the following: (i) The fenthion-
treated dogs had severe organophosphate intoxication and that the
clinical symptoms of muscle fasciculation, ataxia, hyperreflexia,
proprioceptive deficits, and hindquarter weakness were likely to
be the result of neuromuscular overstimulation due to severe
acetylcholinesterase inhibition. Reversibility or recovery was
seen in most of the dogs when the dose of fenthion was reduced
from 44 to 22 mg/kg bw or omitted for one week, suggesting the
neuropathy was not irreversible. (ii) Progressive muscle libre
necrosis was evident in the distal parts of the sciatic nerves of
fenthion-treated dogs, with reorganization of the sensory and
motor innervations. As primary impairment of the peripheral
nerves does not result in muscle necrosis but rather in atrophy
of muscle fibres, the results were judged not to be indicative of
a primary neuropathic effect. In addition, receptor down-
regulation can be considered a physiological adaptation process
to maintain internal equilibrium, serving as a protective
mechanism by which the organism normalizes functions that are
impaired by the intake of pharmacodynamic substances.
Accordingly, the small changes noted in the vagal tone of dogs
after chronic administration of fenthion probably represented an
adaptive change and are. not indicative of irreversible
impairment of the neuromuscular junctions. Thus, the studies, of
Tuler et al. (1988) and Dellinger & Mostrom (1988) do not give
evidence of a particular neurotoxic effect of fenthion in dogs.
3. Tuler & Bowen (1989) investigated the neurotoxic effects of
fenthion, paraoxon (an organophosphate), and neostigmine (a
non-organophosphate) on chick embryo nerve cell growth and
ultrastructure in culture. Altered cell membrane integrity and
increased cytoplasmic lipid (vacuole) accumulation were observed
in cells treated with fenthion or paraoxon but not in those
treated with neostigmine. The authors concluded that
organophosphates had direct effects on neuronal cells in culture;
however, the following points should be considered: (i)
Pharmacokinetics plays a special role in intoxication by
organophosphates, in particular thiophosphates, and in
detoxification, both of which occur rapidly and simultaneously.
Fenthion, a thiophosphate, must be converted in vivo to the
active oxygen analogue and would therefore be ineffective as an
inhibitor of esterases in vitro. (ii) The observed effects on
the cell membrane and the cell were not specific to neuronal
cells and can therefore not be compared directly with neurotoxic
effects in vivo. It is therefore concluded that the results of
the study reveal no particular neurotoxic potential of fenthion
in vivo.
4. Misra et al. (1985, 1988) examined clinical and biochemical
changes and neuromuscular function in workers chronically exposed
to fenthion. No clinical signs of peripheral neuropathy or
myopathy and no pathophysiological findings indicative of
irreversible neurological deficits were seen in workers exposed
regularly and repeatedly to fenthion for a mean of 8.5 years.
They showed symptoms typical of acute cholinergic intoxication
and significant inhibition of serum cholinesterase. It is
concluded that the study provides no evidence of a particular or
delayed neurotoxic potential of fenthion in the workers examined.
The literature thus provides no evidence of a particular or
delayed neurotoxic potential but indicates that exposure to fenthion
results in typical organophosphate-induced cholinergic toxicity due to
severe acetylcholinesterase inhibition.
3. Studies of metabolites
(a) Acute toxicity
The results of studies of the acute toxicity of fenthion
metabolites are summarized in Table 3.
Table 3. Acute toxicity of fenthion metabolites
Metabolite LD50 (mg/kg bw) IC50 (mol; 50% inhibition of human
erythrocyte acetylcholinesterase)c
Orala Intraperitonealb
Fenthion 220 325 > 5.0 × 10-4
Fenthion sulfoxide 125 250 4.5 × 10-5
Fenthion sulfone 125 250 4.7 × 10-4
Fenthion oxygen analogue 125 26 2.7 × 10-6
Fenthion O-sulfoxide 50 22 4.8 × 10-5
Fenthion O-sulfone 30 9 3.2 × 10-5
4-(Methylthio)-m-cresol 6500d
4-Methyl(thiosulfoxide)-m-cresol 3500d
4-Methyl(thiosulfone)-m-cresol 7000d
a In male rats (Francis & Barnes, 1963)
b In female rats (Dubois & Kinoshita, 1964)
c Francis & Barnes (1963)
d In female rats (Nelson, 1967)
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female ICR mice were fed diets
containing fenthion sulfoxide (Baycid SO; purity not given) at
concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to 0,
0.45, 1.5, 4.5, 15, or 45 mg/kg bw per day, for four weeks, followed
by a four-week recovery period. Plasma cholinesterase activity was
depressed in animals of each sex at > 3 ppm, but the effect (an
indication of exposure) was not considered to be toxicologically
significant. Brain acetylcholinesterase activity was significantly
inhibited in males at > 3 ppm and in females at > 10 ppm. At the
two highest doses, decreased body weight was noted in animals of each
sex; at the highest dose, tremor was seen in all mice. All of the
treatment-related effects (including cholinesterase depression)
disappeared rapidly during the recovery period. There was no NOAEL.
The LOAEL, based on significant brain acetylcholinesterase inhibition
in males, was 0.45 mg/kg bw per day, the lowest dose tested (Inukai &
Iyatomi, 1981a).
Rats
Groups of 10 male and 10 female Sprague-Dawley rats were fed
diets containing fenthion sulfoxide (Baycid SO; purity not given)
at concentrations of 0, 3, 10, 30, 100, or 300 ppm, equivalent to
0, 0.15, 0.5, 1.5, 5, or 15 mg/kg bw per day, for four weeks,
followed by a four-week recovery period. Plasma cholinesterase
activity was depressed in females at > 3 ppm, but the effect
(an indication of exposure) was considered not to be toxicologically
significant. No treatment-related adverse effect was observed at
3 ppm. At > 10 ppm, significant, dose-related depression of
acetylcholinesterase activity in brain was seen in animals of each sex
and in erythrocytes in males after four weeks of treatment; in
females, erythrocyte acetylcholinesterase activity was inhibited at
> 30 ppm. At the two highest doses, decreased body weight and food
consumption and increased alkaline phosphatase activity were noted. At
the highest dose, tremor was seen in all rats. All of the treatment-
related effects (including cholinesterase depression) disappeared
rapidly during the recovery period. The NOAEL was 0.15 mg/kg bw per
day, based on significant inhibition of brain (> 10%) and erythrocyte
(> 20%) acetylcholinesterase activities at 0.5 mg/kg bw per day
(Inukai & Iyatomi, 1981b).
4. Observations in humans
Fenthion has been used widely in many parts of the world for
control of e.g. household pests and mosquitos. Studies of individuals
in areas treated for malaria eradication have shown slight plasma
cholinesterase depression when heavy spray schedules were used. The
levels were depressed for up to six weeks after spraying (Elliot &
Barnes, 1963). Children under the age of seven were more susceptible
to the anticholinesterase effect than adults, but no inhibition of
erythrocyte cholinesterase was observed and there were no significant
alterations in normal physiological functions in any individual
examined (Taylor, 1963).
The signs of acute poisoning appear rapidly, beginning with
blurred vision, unsteady gait, and slurred speech. In one reported
case, a man who had taken an unknown quantity of fenthion still
suffered extreme respiratory difficulty necessitating artificial
ventilation and endotracheal intubation after 72 h of emergency
treatment. The patient began to recover only after 11 days of
treatment with antidotes which included atropine, pralidoxime
chloride, and toxogonin (Dean et al., 1967). Another man remained in
a comatose state for 45 min after ingesting a fenthion formulation,
with pale skin, cyanotic mucous membranes, a slow, regular heart beat,
low peripheral blood pressure, and no reactions to pain or light on
the pupils. Recovery took six days (von Clarmann & Geldmacher-von
Mallinkrodt, 1966). A third man, who ingested about 60 g of a fenthion
formulation (Entex), recovered from severe organophosphorous poisoning
after being in a critical condition for the first six days. Recovery
was slow, lasting up to 30 days, and blood cholinesterase activity was
still depressed 22 days after poisoning (Pickering, 1966).
The potential dermal and respiratory exposure of workers to
fenthion during field application by hand-gun power spray equipment,
back-pack hand pressure sprayers, and hand granular dispersal for
mosquito control was studied during two work seasons. Workers exposed
to 3.6-12.3 mg/h dermally (equivalent to 0.5-1.5 mg/kg bw per day) or
< 0.02-0.09 mg/h by inhalation (equivalent to 0.01-0.05 mg/kg bw per
day) had decreased plasma but not erythrocyte cholinesterase activity
(Fytizas-Danielidou, 1971; Wolfe et al., 1974). In two studies of
clinical and biochemical changes, nerve conduction velocity, and
neuromuscular function in 22-24 workers (mean age, 31-32 years)
exposed regularly and repeatedly to fenthion for a mean of 8.2-8.5
years, no clinical signs of peripheral neuropathy or myopathy and
no pathophysiological findings indicative of any irreversible
neurological deficits were seen. The workers showed symptoms typical
of acute cholinergic intoxication and inhibition of serum
cholinesterase, but there was no evidence of delayed neurotoxicity
(Misra et al., 1985, 1988).
In a study of 150 cases of poisoning by anticholinesterase
insecticides, 32 of the patients had consumed fenthion, 48
fenitrothion, and 50 malathion; 20 did not know which agent they had
consumed. Paralytic signs were significantly more frequent after
fenthion than malathion or fenitrothion poisoning (81.2, 30, and 23%,
respectively), and they appeared later and lasted longer. Death
occurred significantly more often after fenthion poisoning, with
mortality rates of 35.5% with fenthion, 4% with malathion, and 2.1%
with fenitrothion. Pulmonary oedema was most common after malathion
poisoning and was not encountered with fenthion. Inhibition of
cholinesterase was most marked after fenthion poisoning, and the
enzyme activity was inhibited by 100% in 18 of the 27 cases studied
(Wadia et al., 1977).
In three groups of four male volunteers given technical-grade
fenthion (purity, 98.1%) in corn oil in gelatin capsules at doses of
0, 0.02, or 0.07 mg/kg bw per day for up to four weeks, no physical or
clinical signs of intoxication were reported, and no abnormal results
were seen by haematology or urinalysis. At 0.07 mg/kg bw per day,
significant depression (> 20%) of plasma cholinesterase was observed,
but no treatment-related effects on erythrocyte acetylcholinesterase
or on other clinical chemistry parameters were observed. The NOAEL was
0.07 mg/kg bw per day, based on the absence of inhibition of
erythrocyte acetylcholinesterase (Griffin et al., 1979).
Comments
A single dose of 14C-radiolabelled fenthion was readily absorbed
and rapidly excreted in the urine and faeces of rats, and no 14C was
detectable in the expired carbon dioxide. About 90% of the radiolabel
was eliminated within 48 h of dosing. The excretion profiles were
similar regardless of sex, dose, or route of administration (oral or
intravenous). Urine was the main route of elimination (> 90% of the
total radiolabel), and only minor amounts were recovered in the
faeces. Little 14C was retained in the tissues, suggesting that
fenthion does not accumulate in the body of rats.
14C-Fenthion was extensively metabolized in rats. No unchanged
parent compound was detected in the urine and very little (< 2%) in
the faeces. The major group of metabolites (accounting for about 60%
of the total recovered 14C) comprised three phenols (fenthion phenol
[4-methylthio- meta-cresol] and its sulfoxide and sulfone) and their
glucuronide and sulfate conjugates. Four demethyl metabolites
(accounting for about 30% of the recovered radiolabel) and the
sulfoxide of fenthion oxon (constituting 1-4%) were also identified.
Fenthion is moderately toxic (LD50 = 50-500 mg/kg body
weight) to mice, rats, guinea-pigs, and rabbits when given orally,
intraperitoneally, dermally, or by inhalation. It is highly toxic to
avian species (especially to the wild mallard duck) when given
orally. The LC50 in rats for a 4-h exposure by inhalation was
0.5-0.9 mg/titre. Fenthion caused cholinergic toxicity with a long
recovery time. It did not irritate rabbit skin and was minimally or
not irritating to the rabbit eye; it did not sensitize guinea-pig
skin. WHO has classified fenthion as highly hazardous.
Fenthion potentiated the acute toxicity of other cholinergic
chemicals, such as malathion, dioxathion, and coumaphos, in rats. In
mice and dogs, pretreatment with fenthion inhibited the metabolism and
detoxification of 2- sec-butylphenyl methylcarbamate, resulting in
significant potentiation of its acute toxicity. There was no evidence
that other cholinergic chemicals potentiate the toxicity of fenthion.
Repeated short-term administration of fenthion (orally to mice,
rats, dogs and monkeys; dermally to rabbits; by inhalation to rats)
and its metabolite fenthion sulfoxide (orally to mice and rats),
resulted primarily in inhibition of cholinesterase. The NOAEL for oral
administration of fenthion, based on toxicologically significant
depression of acetylcholinesterase activity in the brain (> 10%)
and/or erythrocytes (> 20%), was 5 ppm (equivalent to 0.25 mg/kg bw
per day) in rats treated for three months or one year, 3 ppm (equal to
0.09 mg/kg bw per day) in dogs treated for two years, and 0.07 mg/kg
bw per day in monkeys treated for two years The NOAEL for fenthion was
50 mg/kg bw per day when applied dermally to rabbits for 21 days on
the basis of inhibition of brain acetylcholinesterase activity. The
NOAEL for fenthion in rats exposed by inhalation for 21 days was
0.001 mg/litre, on the basis of clinical signs of cholinergic toxicity
and inhibition of brain acetylcholinesterase at higher doses. The
NOAEL for fenthion sulfoxide administered orally to rats for four
weeks was 3 ppm (equivalent to 0.15 mg/kg bw per day) on the basis of
significant inhibition of brain and erythrocyte acetylcholinesterase
activity. No NOAEL was determined for fenthion or its metabolites in
mice; the LOAEL for fenthion was 150 ppm (equal to 83 mg/kg bw per
day) in mice exposed in the diet for four weeks, and the LOAEL for
fenthion sulfoxide in mice similarly exposed was 3 ppm (equivalent to
0.45 mg/kg bw per day).
In a carcinogenicity study, fenthion was administered in the diet
to mice at 0, 0.1, 1, 5 or 25 ppm for 102 weeks. The NOAEL for chronic
toxicity was 5 ppm (equal to 2 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. There was no evidence of carcinogenicity. In
two studies of chronic toxicity and carcinogenicity, rats received
fenthion at dietary concentrations of 0, 3, 15, or 75 ppm or 0, 5,
20, or 100 ppm for 24 months. The NOAEL for chronic toxicity
was 3 ppm (equal to 0.14 mg/kg bw per day) on the basis of
toxicologically significant inhibition of brain and/or erythrocyte
acetylcholinesterase. The compound was toxic to the eyes of rats at
100 ppm (equal to 5.2 mg/kg bw per day), inducing an increased
incidence of retinal atrophy, posterior subcapsular cataract
formation, corneal mineralization, and mineralization and optic nerve
atrophy, especially in females. No ocular toxicity was seen at doses
> 20 ppm (equal to 0.8 mg/kg bw per day). There was no evidence of
carcinogenicity.
In a two-generation study of reproductive toxicity (one litter
per generation), rats were fed diets containing fenthion at levels of
0, 1, 2, 14, or 100 ppm. The NOAEL for systemic toxicity in the parent
generation was 2 ppm (equal to 0.16 mg/kg bw per day) on the basis of
consistent inhibition of brain and erythrocyte acetylcholinesterase.
The NOAEL for reproductive toxicity was 14 ppm (equal to 1.2 mg/kg bw
per day) on the basis of decreased fertility, implantation sites,
litter size, pup viability, and growth at 100 ppm.
Two studies of developmental toxicity were performed in which
rats were exposed by gavage to fenthion at doses of 0, 1, 3, or
10 mg/kg bw per day or 0, 1, 4.2, or 18 mg/kg bw per day on days 6-15
of gestation. There was no NOAEL for maternal toxicity, owing to
toxicologically significant inhibition of brain and erythrocyte
acetylcholinesterase activity at doses > 1 mg/kg bw per day. the
NOAEL for embryo- and fetotoxicity and teratogenicity was 18 mg/kg bw
per day, the highest dose tested.
In a study of developmental toxicity in rabbits, fenthion was
administered by gavage at doses of 0, 1, 2.8, or 7.5 mg/kg bw per day
on days 6-18 of gestation. The NOAEL for maternal toxicity was 1 mg/kg
bw per day, on the basis of toxicologically significant inhibition of
brain and erythrocyte acetylcholinesterase activity at > 2.8 mg/kg
bw per day. The NOAEL for embryo- and fetotoxicity and teratogenicity
was 7.5 mg/kg bw per day, the highest dose tested.
Fenthion has been adequately tested for genotoxicity in a range
of assays in vivo and in vitro. While most showed no significant
response, positive results were obtained in two critical assays. The
Meeting concluded that fenthion is weakly genotoxic.
Fenthion did not cause delayed neuropathy in hens at doses higher
than the LD50.
In a four-week study of male volunteers, the NOAEL was 0.07 mg/kg
bw per day, the highest dose tested, on the basis of no inhibition of
erythrocyte acetylcholinesterase. In two investigations of workers
regularly exposed to fenthion, no evidence of neurotoxicity was
observed.
An ADI of 0-0.007 mg/kg bw was established on the basis of the
NOAEL of 0.07 mg/kg bw per day in the four-week study of human
volunteers, using a safety factor of 10. The ADI provides a margin of
safety of > 100-fold for chronic ocular toxicity and for the
reproductive toxicity observed in rodents.
The available data did not allow the Meeting to establish an
acute reference dose different from the ADI (0-0007 mg/kg bw). It
should be noted, however, that the ADI is derived from a study
of human volunteers in which 9-36% inhibition of plasma cholinesterase
but no inhibition of erythrocyte acetylcholinesterase was found at
the highest dose tested (0.07 mg/kg bw per day for 25 days). In
occupationally exposed workers, about 50% plasma cholinesterase
inhibition was found in the absence of erythrocyte acetyl-
cholinesterase inhibition. It follows that the acute reference
dose is likely to be somewhat higher than the ADI. Data on the
sensitivity to inhibition of plasma cholinesterase and erythrocyte and
brain acetylcholinesterase in vitro by the active metabolites of
fenthion might allow extrapolation to an LOAEL for humans.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 2.0 mg/kg bw per day (two-year study of
carcinogenicity)
Rat: 3 ppm, equal to 0.14 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
2 ppm, equal to 0.16 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
14 ppm, equal to 1.2 mg/kg bw per day (two-generation study
of reproductive toxicity)
18 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Rabbit: 6 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
7.5 mg/kg bw per day (embryo- and fetotoxicity and
teratogenicity in study of developmental toxicity)
Dog: 3 ppm, equal to 0.09 mg/kg bw per day (two-year study of
toxicity)
Monkey: 0.07 mg/kg bw per day (two-year study of toxicity)
Human: 0.07 mg/kg bw per day (four-week study of toxicity)
Estimate of acceptable daily intake for humans
0-0.007 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenthion
Exposure Relevant route, study type, species Result, remarks
Short-term (1-7 days) Skin, irritation, rabbit Not irritating
Eye, irritation, rabbit Minimally or not irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Inhalation, 4-h, toxicity, rat LC50 = 0.5--0.9 mg/litre
Oral, toxicity, mouse, rat, guinea-pig, rabbit LD50 = 50-500 mg/kg bw
Oral, dermal, subcutaneous, single doses, NOAEL (all routes) = 5 mg/kg bw per day
cholinesterase activity, rat based on inhibition of brain and
erythrocyte acetylcholinesterase
Medium-term (1-26 weeks) Repeated dermal, 21 days, toxicity, rabbit NOAEL = 50 mg/kg bw per day for
systemic toxicity on the basis of inhibition
of brain acetylcholinesterase
Repeated inhalation, 21 days, toxicity rat NOAEL = 0.001 mg/litre per day for
systemic toxicity on the basis of clinical
signs of cholinergic toxicity
Repeated dietary, 13-16 weeks, toxicity, rat NOAEL = 0.25 mg/kg bw per day, based
on inhibition of brain acetylcholinesterase
Repeated gavage, developmental toxicity, NOAEL = 1 mg/kg bw per day for
rabbit maternal toxicity on the basis of inhibition
of brain and erythrocyte acetylcholinesterase;
no embryo- or fetotoxic or teratogenic effects
Repeated oral (gelatin capsules), four weeks, NOAEL = 0.07 mg/kg bw per day on the
toxicity, human basis of no inhibition of erythrocyte
acetylcholinesterase
Long-term (> 1 year) Repeated dietary, two years, toxicity, dog NOAEL = 0.09 mg/kg bw per day on the
basis of inhibition of brain and erythrocyte
acetylcholinesterase
References
Avrahami, M. & White, D.A. (1975) Residues in milk of cows after
spot-treatment with 32P-fenthion. New Zealand Exp. Agric., 3,
309-311.
Bai, C.L., Qiao, C., Zhang, W., Chen, Y. & Qu, S. (1990) A study of
the pesticide fenthion: Toxicity, mutagenicity and influence on
tissue enzymes. Biomed. Environ. Sci., 3, 262-275.
Bailey, D.E. (1987) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 938 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bailey, D.E. (1988) 21-Day dermal toxicity study in rabbits with
Baytex technical. Unpublished Mobay report No. 1031 from Hazelton
Laboratories America, Inc., Virginia, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Bomann, W. (1991) E 1752 (c.n. Fenthion): Study for acute dermal
toxicity in the rat. Unpublished report No. 20221 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Bomhard, E. & Loser, E. (1977) Chronic toxicity study on rats.
Unpublished report No. 6769 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Brady U.E., Jr & Arthur, B.W. (1961) Metabolism of
O,O-dimethyl- O-[4-(methylthio)- m-tolyl] phosphorothioate
by white rats. J. Econ. Entomol., 54, 1232-1236.
Budreau, C.H. & Singh, R.P. (1973a) Teratogenicity and embryotoxicity
of demeton and fenthion in CF1 mouse embryos. Toxicol. Appl.
Pharm., 24, 324-332.
Budreau, C.H. & Singh, R.P. (1973b) Effect of fenthion and dimethoate
on reproduction in the mouse. Toxicol. Appl. Pharmacol., 26,
29-38.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982a) Sister-chromatid
exchanges and cell-cycle delay in Chinese hamster V79 cells
treated with nine organophosphorus compounds (8 pesticides and 1
defoliant). Mutat. Res., 103, 307-313.
Chen, H.H., Sirianni, S.R. & Huang, C.C. (1982b) Sister-chromatid
exchanges in Chinese hamster cells treated with 17
organophosphorus compounds in the presence of a metabolic
activation system. Environ. Mutag., 4, 621-624.
Christenson, W.R. (1990a) Chronic feeding toxicity study of
technical-grade fenthion (Baytex) with dogs. Unpublished report
No. 94863 from Miles, Inc., Agriculture Division, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Christenson, W.R. (1990b) Combined chronic toxicity/oncogenicity study
of technical grade fenthion (Baytex) with rats. Unpublished
report No. 100586 from Mobay Corporation, Corporate Toxicology
Department, Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Christenson, W.R. (1990c) Technical grade fenthion (Baytex): A special
study to examine the effect of the route of acute administration
of technical grade fenthion (Baytex) on cholinesterase activity
in the rat. Unpublished report No. 100573 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
von Clarmann, M. & Geldmacher-von Mallinckrodt, M. (1966) A
successfully treated case of acute oral poisoning by fenthion and
its demonstration in the gastric contents and urine. Arch.
Toxikol., 22, 2-11.
Clemens, G.R. (1987) A teratology study in the rabbit with fenthion
(Baytex technical). Unpublished report No. MTD0039 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Crosby, J., Hoglen, N. & Krautter, G. (1990) Nature of residues in
skin and tissues of swine after dermal treatment with
[14C]fenthion. Unpublished Mobay report No. 73984 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dean, G., Coxon, J. & Brereton, D. (1967) Poisoning by an
organophosphorus compound -- A case report. S. Afr. Med. J.,
41, 1017-1019.
Dellinger, J. & Mostrom, M. (1988) Effects of topical fenthion on
blood cholinesterase and vagal tone in dogs. Vet. Hum.
Toxicol., 30, 2,29-234.
Doolittle, K.D. & Bates, N.L (1993) Absorption, distribution and
elimination of 14C-fenthion in rats following a single oral,
repeated oral and single intravenous administration. Unpublished
Miles report No. 74395 from Southwest Bio-Labs, Inc., New Mexico,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Vesselinovitch, D., Fitch, F., Cowan, J., Root, M. &
Meskauskas, J. (1961a) The effects of feeding diets containing
Bayer 29493 to rats for a period of 16 weeks. Unpublished rayer
Report No. 7899 from Department of Pharmacology, University of
Chicago, Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Doull J., Root, M. & Cowan, J. (1961b) Determination of the safe
dietary level for Bayer 29493 for dogs. Unpublished Bayer report
No. 8342 from Department of Pharmacology, University of Chicago,
Illinois, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M. & Cowan, J. (1962) Effect of adding Bayer 29493 in
combination with other cholinergic insecticides to the diet of
male and female dogs. Unpublished Bayer report No. 9414 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, N.J., Vesselinovitch, D., Fitch, F.M. &
Meskauskas, J. (1963a) Chronic oral toxicity of Bayer 29493 to
male and female rats. Unpublished Bayer report No. 14658 from
Department of Pharmacology, University of Chicago, Illinois, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Doull, J., Root, M., Cowan, J. & Vesselinovitch, D. (1963b) Chronic
oral toxicity of Bayer 29493 to male and female dogs. Unpublished
Bayer report No. 10853 from Department of Pharmacology,
University of Chicago, Illinois, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Dubois, K.P. (1968) Comparison of the acute oral toxicity of Bayer
29493 and Sumithion to mice. Unpublished Mobay report No. 22503
from Department of Pharmacology, University of Chicago, Illinois,
USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Doull, J. (1960) The acute toxicity of Bayer 29493 to
chickens and ducks. Unpublished report from Department of
Pharmacology, University of Chicago, Illinois, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Dubois, K.P. & Kino-hita, F. (1964) Acute toxicity and
anticholinesterase action of O,O-dimethyl- O-4-
(methylthio- m-tolyl) phosphorotioate (DMTP; Baytex) and
related compounds. Toxicol. Appl. Pharmacol., 6, 86-95.
Eigenberg, D.A. (1987a) Acute oral toxicity of Baytex technical in
albino rats. Unpublished report No. 839 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987b) Acute dermal toxicity of Baytex technical in
albino rabbits. Unpublished report No. 838 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987c) Primary dermal irritation of Baytex technical
in albino rabbits. Unpublished report No. 896 from Mobay
Corporation, Corporate Toxicology Department, Kansas. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Eigenberg, D.A. (1987d) Primary eye irritation of Baytex technical in
albino rabbits. Unpublished report No. 817 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Elliot, R. & Barnes, J.M. (1963) Organophosphorus insecticides for the
control of mosquitos in Nigeria. Bull. World Health Org., 28,
35-54.
Farage-Elawar, M. & Francis, B.M.(1987) Acute and delayed effects of
fenthion in young chicks. J. Toxicol. Environ. Health, 21,
455-469.
Farage-Elawar, M. & Francis, B.M. (1988a) Effects of multiple dosing
of fenthion, fentrothion, and desbromoleptophos in young chicks.
J. Toxicol. Environ. Health, 23, 217-228.
Farage-Elawar, M. & Francis, B.M. (1988b) Effects of fenthion,
fentrothion, and desbromoleptophos on gait, acetylcholinesterase
and neurotoxic esterase in young chicks after in ovo exposure.
Toxicology, 49, 253-261.
Flucke, W. (1986a) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute oral toxicity to hens
(Gallus domesticus). Unpublished report No. 15069 from Bayer
AG, Institut für Toxikologie, Wuppertal Germany.
Flucke, W (1986b) E 1752 Technical (c.n. fenthion, the active
ingredient of Baytex): Study of acute dermal toxicity to hens
(Gallus domesticus). Unpublished report No. 15113 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1987) E 1753 Technical (c.n fenthion): Study for skin
sensitising effect on guinea pigs (Magnusson and Kligman's
maximization test). Unpublished report No. 15428 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Flucke, W. (1988a) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following oral administration to hens.
Unpublished report No. 99275 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1988b) E 1752 Technical: Study of the effect on the
neurotoxic esterase (NTE) following dermal administration to
hens. Unpublished report No. 99646 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. (1990) Position paper regarding the literature on
neurotoxicity for the BGA [German Federal Health Agency].
Unpublished report No. 18928 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Flucke, W. & Kaliner, G. (1987) E 1752 (c.n. fenthion, the active
ingredient of Baytex): Acute neurotoxicity studies on hens
following oral and dermal administration. Unpublished report No.
91341 from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Francis, J.I. & Barnes, J.M. (1963) Studies on the mammalian toxicity
of fenthion. Bull. World Health Org., 28, 205-212.
Francis, B.M. & Farage-Elawar, M. (1987) Peripheral and central enzyme
inhibition by fenthion, fenitrothion and desbromoleptophos. In:
Costa, L.G., Galli, C.L. & Murphy, S.D., eds, Toxicology of
Pesticides: Experimental, Clinical and Regulatory Perspectives,
Berlin, Springer Verlag.
Fytizas-Danielidou, R. (1971) Effects of pesticides on reproduction in
white rats. I. Labaycide. In: 23rd International Symposium of
Phytopharmacy, 4 May, 1971.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Griffin, T., Rosenblum, I. & Coulston, F. (1979) Safety evaluation of
fenthion in human volunteers. Unpublished Mobay report No. 68790
from the Institute of Comparative and Human Toxicology and
International Center of Environment Safety, Albany Medical
College, New York, USA. Submitted to WHO by Bayer AG, Wuppertal,
Germany.
Hayes, R.H. (1989) Subchronic feeding study with fenthion technical
(Baytex) in hens with specific emphasis on gastrointestinal tract
effects. Unpublished report No. 99273 from Mobay Corporation,
Corporate Toxicology Department, Kansas, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Hayes, R.H. & Ramm, W.W. (1988) Subchronic delayed neurotoxicity study
of fenthion technical (Baytex) with hens. Unpublished report No.
98296 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Heimann, K.G. (1982) Fenthion and isofenthion: Study for acute
combination toxicity Unpublished report No. 11062 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1987) E 1752 (c.n. Fenthion): Salmonella/microsome
test for point-mutagenic effect. Unpublished report No. 98366
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Herbold, B.A. (1990a) E 1752 Salmonella/microsome test. Unpublished
report No. 18713 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Herbold, B.A. (1990b) E 1752 micronucleus test on the mouse.
Unpublished report No. 100025 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Hoffmann, K. & Weischer, C.H. (1975) Fenthion chronic study on dogs
(two-year feeding experiment). Unpublished report No. 5737 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981a) Fensulfoxide: Short-term toxicity
tests on mice (4-week feeding and 4-week recovery tests).
Unpublished report No. 198 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Inukai, H. & Iyatomi, A. (1981b) Fensulfoxide: Short-term toxicity
tests on rats (4-week feeding and 4-week recovery tests).
Unpublished report No. 197 from Nihon Tokushu Noyaku Seizo K.K.,
Agricultural Chemicals Institute, Japan. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Johnson, J.C. & Bowman, M.C. (1972) Responses from cows fed diets
containing fenthion or fenitrothion. J. Dairy Sci., 55,
777-782.
Kajiwara, Y. (1989) Chromosomal aberration test of fenthion using
cultured mammalian CHL cells. Unpublished report No. T-1824E from
HITA Research Lab., Japan. Submitted to WHO by Bayer AG,
Wuppertal, Germany.
Keith, J.O. & Mulla, M.S. (1966) Relative toxicity of five
organophosphorous mosquito larvicides to mallard ducks.
J. Wildlife Manage., 30, 553-563.
Kimmerle, G. (1967) Potential of DDVP and S 1752. Unpublished letter
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Klimmer, R. (1963) Toxicological testing of Bayer 29493. Unpublished
report from Bayer AG, Institut für Toxikologie, Wuppertal,
Germany.
Knowles, C.O. & Arthur, B.W. (1966) Metabolism of and residues
associated with dermal and intramuscular application of
radiolabelled fenthion to dairy cows. J. Econ. Entomol., 59,
1346-1352.
Kowalski, R.L. (1987) A teratology study with fenthion (Baytex
technical) in the rat. Unpublished report No. MTD0029 from Miles
Laboratories, Inc., Toxicology Department, Indiana, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Kowalski, R.L., Clemens, G.R., Jasty, V., Troup, CM. & Hartnagel,
R.E., Jr (1989) A two-generation reproductive study with fenthion
(Baytex) in the rat. Unpublished report No. MTD0133 from Miles
Inc., Toxicology Department, Indiana, USA. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Krautter, G. (1990a) Nature of residues in milk, skin and tissues of a
lactating cow after dermal treatment with [14C]fenthion.
Unpublished Mobay report No. 74012 from Pharmacology and
Toxicology Research Laboratory, Kentucky, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Krautter, G. (1990b) Characterization of polar metabolites in river
and kidney tissues from a cow and pig treated dermally with
[14C] fenthion. Unpublished Mobay report No. 74124 from
Pharmacology and Toxicology Research Laboratory, Kentucky, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Krotlinger, F. (1993) E 1752 (c.n. Fenthion): Study for acute
intraperitoneal toxicity in rats. Unpublished report No. 22144
from Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Lamb, D.W. & Anderson, R.H. (1974) The acute dermal toxicity of
Tiguvon technical to rabbits. Unpublished report No. 41455 from
Chemagro, Bayer GmbH, Leverkusen, Germany. Submitted to WHO by
Bayer AG, Wuppertal, Germany.
Lehn, H. (1990a) E 1752 (c.n. Fenthion): Mutagenicity study for the
detection of induced forward mutations in the CHO-HGPRT assay
in vitro. Unpublished report No. 18740 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Lehn, H. (1990b) E 1752 (c.n. Fenthion): Mutagenicity test on
unscheduled DNA synthesis in rat liver primary cell cultures
in vitro. Unpublished report No. 100572 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Leser, K.H. (1990) E 1752 (Fenthion): Study for cholinesterase
inhibition following high doses of E1752 (administration to
B6C3F1 mice in the diet over a period of about four weeks).
Unpublished report No. 19088 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Machemer, L. (1978a) S 1752 (Fenthion, Lebaycid active ingredient):
Evaluation for embryotoxic and teratogenic effects in orally
dosed rats. Unpublished report No. 7580 from Bayer AG, Institut
für Toxikologie, Wuppertal, Germany.
Machemer, L. (1978b) S 1752 (Fenthion, Lebaycid active ingredient):
Dominant lethal study of male mice to test for mutagenic effects.
Unpublished report No. 7449 from Bayer AG, Institut für
Toxikologie, Wuppertal, Germany.
Mihail, F. (1978) S 1752 (Lebaycid active ingredient): Determination
of percutaneous toxicity. Unpublished report No. 7604 from Bayer
AG, Institut für Toxikologie, Wuppertal, Germany.
Mihail, F. & Schilde, B. (1979) S 1752 (Fenthion, the active
ingredient of Lebaycid and Baytex): Subacute dermal cumulative
toxicity on rabbits. Unpublished report No. 8624 from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Misra, U.K., Nag, D., Bhushan, V. & Ray, P.K. (1985) Clinical and
biochemical changes in chronically exposed organophosphate
workers, Toxicol, Lett., 24, 187-193.
Misra, U.K.. Nag, D., Khan, W.A. & Ray, P.K. (1988) A study of nerve
conduction velocity, late responses and neuromuscular synapse
functions in organophosphate workers in India. Arch. Toxicol.,
61, 496-500.
Miyaoka, T., Takahashi, H., Tsuda, S. & Shirasu, Y. (1984)
Potentiation of acute toxicity of 2- sec-butylpheny-
N-methylcarbamate (BPMC) by fenthion in mice. Fundam. Appl.
Toxicol., 4, 802-807.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1986) Mechanism of potentiation
of BPMC toxicity by fenthion pretreatment in mice.
J. Pharmacobio-Dyn., 9, 697-703.
Miyaoka, T., Tsuda, S. & Shirasu, Y. (1987) Effect of O,O-dimethyl
O-(3-methyl-4-methylthiophenyl) phosphorothioate (fenthion)
pretreatment on acute toxicity of 2- sec-butylphenyl
N-methylcarbamate (BPMC) in dogs. Jpn. J. Vet. Sci., 49,
173-175.
Nelson, D.L. (1967) The acute oral toxicity of three phenolic
compounds to adult female rats. Unpublished report from Bayer AG,
Institut für Toxikologie, Wuppertal, Germany.
Pauluhn, J. (1985) E 1752 Technical (c.n. fenthion): Study for
irritant/corrosive effect on skin and eye (rabbit). Unpublished
report No. 13446 from Bayer AG, Institute für Toxikologie,
Wuppertal, Germany.
Pickering, E.N. (1966) Organic phosphate insecticide poisoning.
Can. J. Med. Technol., 28, 174-179.
Pither, K.M. (1979) Metabolism of Baytex in male and female pigs.
Unpublished report No. 68475 from Mobay Corporation, Corporate
Toxicology Department, Kansas, USA. Submitted to WHO by Bayer AG,
Wuppertal Germany.
Puhl, R.J. & Hurley, J.B. (1982) The absorption, excretion and
metabolism of Baytex-ring-1-14C by rats. Unpublished report No.
82227 from Mobay Corporation, Corporate Toxicology Department,
Kansas, USA. Submitted to WHO by Bayer AG, Wuppertal, Germany.
Putman, D.L. & Morris, M.J. (1989) Chromosome aberrations in Chinese
hamster ovary (CHO) cells. Unpublished report No. 99660 from
Microbiological Associates Inc., Maryland, USA. Submitted to WHO
by Bayer AG, Wuppertal, Germany.
Rani, M.V.U & Rao, M.S. (1991) In vivo effect of fenthion on human
lymphocytes. Bull. Environ. Contamin. Toxicol., 47, 316-320.
Rosenblum, I. (1980) A safety evaluation of fenthion (S 1752) in
rhesus monkeys (Macaca mulatta). Unpublished Mobay report No.
68789 from Albany Medical College, New York, USA. Submitted to
WHO by Bayer AG, Wuppertal, Germany.
Sherman, M. & Ross, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. Appl. Pharmacol., 3, 521-533.
Shimamoto, K. & Hattori, K. (1969) Chronic feeding of Baytex
( O,O-dimethyl- O-(4-methylmercapto-3-methyl)phenylthiophosphat
e) in rats. Acta Med. Univ. Kyoto, 40, 163-171.
Shiotsuka, R.N. (1987a) Acute one-hour inhalation toxicity study with
technical grade Baytex in rats. Unpublished report No. 944 from
Mobay Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Shiotsuka, R.N. (1987b) Acute inhalation toxicity study with Baytex
technical in rats. Unpublished report No. 820 from Mobay
Corporation, Corporate Toxicology Department, Kansas, USA.
Submitted to WHO by Bayer AG, Wuppertal, Germany.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1977) Evaluation of
selected pesticides as chemical mutagens: In vitro and in
vivo studies. US Environmental Protection Agency Health Effects
Research Laboratory Document No. EPA-600/1-77-028 from Stanford
Research Institute, California, USA. Submitted to WHO by Bayer
AG, Wuppertal, Germany.
Singh, S.P., Sharma, L.D. & Bahga, H.S. (1987) Acute oral toxicity of
fenthion in poultry. Indian J. Anim. Res., 21, 48-50.
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: organophosphates. Mutat. Res., 102,
89-102.
Suberg, H. & Leser, K.H. (1990) E 1752 Oncogenicity study on B6C3F1
mice (feeding study for periods of up to 24 months). Unpublished
report No. 19624 from Bayer AG, Institut für Toxikologie,
Wuppertal, Germany.
Taylor, A. (1963) Observations on human exposure to the
organophosphorus insecticide fenthion in Nigeria. Bull.
World Health Org., 28, 213-218.
Thyssen, J. (1978) S 1752 (Lebaycid-active ingredient): Acute
inhalation toxicity studies. Unpublished report No. 7842 from
Bayer AG, Institut für Toxikologie, Wuppertal, Germany.
Thyssen, J. (1979) Fenthion (S 1752, the active ingredient of Lebaycid
and Baytex): Subacute inhalation toxicity study on rats.
Unpublished report No. 8383 from Bayer AG Institut für
Toxikologie, Wuppertal, Germany.
Tuler, S.M. & Bowen, J.M. (1989) Toxic effects of organophosphates on
nerve cell growth and ultrastructure in culture. J. Toxicol.
Environ. Health, 27, 209-223.
Tuler, S.M., Febles, D. & Bowen, J.M. (1988) Neuromuscular effects of
chronic exposure to fenthion in dogs and predictive value of
electromyography. Fundam. Appl. Toxicol., 11, 155-168.
US National Cancer Institute (1979) Bioassay of fenthion for possible
carcinogenicity (National Cancer Institute Carcinogenesis
Technical Report Series No. 103; US Department of Health,
Education, and Welfare Publication No. (NIH) 79-1353). Bioassay
conducted by Gulf S. Research Institute, Louisiana, USA, under
contract to NCI and subcontract to Tracor Jitco, Inc., who
prepared the final report.
Wadia, R.S., Bhirud, R.H., Gulavani, A.V. & Amin, R.S. (1977)
Neurological manifestations of three organophosphate poisons.
Indian J. Med. Res., 66, 460-468.
Weber, H. & Ecker, W. (1992) [phenyl-1-14C]Fenthion: Absorption,
distribution, excretion and metabolism in a lactating goat.
Unpublished report No. 3752 from Bayer AG, Institut für
Metabolismusforschung, Leverkusen-Bayerwerk, Germany. Submitted
to WHO by Bayer AG, Wuppertal, Germany.
Wolfe, H.R., Armstrong, J.F. & Durham, W.F. (1974) Exposure of
mosquito control workers to fenthion. Mosquito News, 34,
263-267.
