
FENTHION
Summary
Identity and physical and chemical properties
Sources of human and environmental exposure
Environmental transport, distribution, and transformation
Environmental levels
Effects on organisms in the laboratory and the field
Identity and physical and chemical properties
Identity
Physical and chemical properties
Sources of human and environmental exposure
Production levels and processes
Uses
Environmental transport, distribution, and transformation
Transport and distribution between media
Dissipation from water
Soil sorption
Soil mobility
Dissipation from soil in the field
Uptake and dissipation from plants
Entry into the food chain
Abiotic transformation
Hydrolytic cleavage and oxidation
Phototransformation
Biotransformation
Water and sediment
Soil
Bioconcentration
Environmental levels
Effects on organisms in the laboratory and the field
Laboratory experiments
Microorganisms
Aquatic organisms
Terrestrial organisms
Field observations
Microorganisms
Aquatic organisms
Terrestrial organisms
Evaluation of effects on the environment
Risk assessment
Use as an avicide
Agricultural use
References
1. Summary
1.1 Identity and physical and chemical properties
Fenthion is an organophosphorus pesticide. The empirical formula
is C10H15O3PS2. The purity of technical-grade fenthion is
generally > 95%; it smells like mercaptans and is a colourless-
to-yellow liquid with a specific gravity of 1.25 and a fairly low
vapour pressure, slightly soluble in water, and very soluble in
various organic solvents. The octanol-water partition coefficient (log
Kow) is 4.8 at 20°C. Henry's law constant indicates no substantial
volatilization from water. Fenthion is not surface active and has no
explosive or oxidizing properties.
Adjuvants in formulations of fenthion, e.g. xylene, emulsifiers,
and diesel oil, may increase sorption to plant surfaces.
1.2 Sources of human and environmental exposure
Fenthion is a systemic organophosphorus pesticide that is used in
both agricultural and nonagricultural areas all over the world. It is
applied to many crops, e.g. rice, cotton, and citrus, and may be
applied in various commercial formulations. Lebaycid is the major
formulation. Application rates up to 2.4 kg/ha have been reported but
are dependent on the type of use. Fenthion is also registered for bird
control and in veterinary use. Environmental exposure may occur
because of deposition due to drift and accidental release. No data
were available on exposure after treatment of cattle or domestic
animals.
1.3 Environmental transport, distribution, and transformation
Fenthion dissipates from water with a half-life < 7 days.
Sediment can be an important sink. Dissipation from water appears to
occur mainly via phototransformation, biotransformation, and sorption
to sediment. No data are available on the possibility of dissipation
via sorption to suspended particles.
The adsorption coefficients (Ks/l) for fenthion in laboratory
experiments vary between 7.7 and 38 dm3/kg, indicating strong
sorption. The mechanism of sorption of fenthion to soil is only
partially understood. It appears to be positively correlated to the
organic matter content, but unequivocal data demonstrating this
correlation are not available.
Fenthion does not leach substantially to shallow groundwater
under laboratory or field conditions simulating a temperate climate.
The Rf values for fenthion do not exceed 0.17. Some transformation
products appear to be more mobile than the parent compound, although
their sorption coefficients are not known. In less reliable laboratory
experiments with aged residues, up to 46% of the applied activity was
recovered in the leachate after two days of leaching through a sandy
soil. The transformation products detected in leachates are
3-methyl-4-methylsulfonyl-phenol, 3-methyl-4-methylsulfinyl-phenol,
fenthion sulfoxide (thiophosphoric acid, O,O-dimethyl- O-[3-methyl-
4-methylsulfinyl-phenyl] ester), and fenthion sulfone (thiophosphoric
acid, O,O-dimethyl- O-[3-methyl-4-methylsulfonyl-phenyl] ester).
These transformation products probably do not leach substantially to
groundwater, as their half-lives are < 15 days, but no field data
are available.
Fenthion dissipates from soil relatively rapidly under aerobic
laboratory conditions simulating a temperate climate, with a half-life
of about 10 days. Some experiments indicate slower dissipation
outdoors (about 30 days) than under controlled laboratory conditions.
Dissipation appears to occur mainly via phototransformation,
biotransformation, and sorption.
The few data on uptake by plants in this report indicate moderate
persistence, of the order of two weeks.
Secondary poisoning of raptorial birds with pests treated with
fenthion may occur within one or a few days after application, as
dissipation appears to be rapid.
Hydrolysis of fenthion in sterile buffers at 25°C is slow, with
half-lives of 56, 41, and 32 days at pH 5, 7, and 9, respectively.
Phototransformation in water or on soil occurs rapidly via oxidation
and hydrolysis; the half-lives in water and on soil under laboratory
conditions are 0.08-4 h and 1- > 4 days, respectively.
Biotransformation in systems with water and sediments and in
systems with soils includes oxidation and hydrolysis. Some methylation
has been reported under anaerobic conditions. In whole water-sediment
systems, the half-life under dark, aerobic conditions appears to equal
that under anaerobic conditions, i.e. 10 days. The half-life of about
10 days in a soil system under dark, aerobic conditions also indicates
rapid transformation. The half-life under anaerobic conditions (> 32
days) indicates a more moderate transformation rate, but the
experiment from which this half-life was derived was unreliable.
Under temperate climatic conditions, it can be assumed that 50%
or more of applied fenthion in soil or natural water with sediment is
degraded to carbon dioxide within six months. Biotransformation in
soil under anaerobic conditions may be an exception, as no unequivocal
test results on this subject are available. Generally, the biotrans-
formation rate in a water-sediment system is lower than that in a soil
system. It should be noted however, that these rough assumptions are
based on only a few laboratory experiments.
The major metabolites in a dark, aerobic water-sediment system at
about 22°C were fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol,
3-methylsulfonyl-phenol, and demethylfenoxon sulfoxide; the major
metabolites in a dark, anaerobic water-sediment system were 3-methyl-
4-methylthio-phenol, 3-methyl-4-methylsulfinyl-phenol, and 3-methyl-
phenol. The major metabolites in a dark, aerobic soil system were
fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol and 3-methyl-4-
methylsulfinyl-phenol.
Fenthion has moderate potential to bioconcentrate in freshwater
fish. Laboratory experiments with bluegill sunfish, channel catfish,
and guppies showed bioconcentration factors up to 226. When fish were
placed in uncontaminated water after exposure in a flow-through system
for 14 days, the residue levels declined within a few days. In an
experiment in bluegill sunfish under flow-through conditions, 70% of
the activity in one fish was recovered in the viscera 11 days after
the start of the experiment. No residual fenthion was detected after a
few weeks of depuration.
1.4 Environmental levels
Few data derived from regular monitoring programmes are available
on the occurrence of fenthion in environmental biota and abiota. The
results of such a program in the Netherlands that was started in 1992
revealed the occurrence of fenthion in 25% of freshwater locations and
8% of saltwater locations at concentrations < 0.12 µg/litre. The
source of the emissions was not clear, as fenthion is allowed in the
Netherlands only as a veterinary chemical and not for treating
agricultural crops. The concentrations of fenthion in wildlife have
been determined only occasionally, and are thus of limited statistical
value.
Data from field experiments simulating common agricultural
practice are also scarce. In field studies of mosquito control in
wetlands and of bird control, the maximal concentration in water was
1.7 µg/litre but dissipated rapidly. In sources of bird food,
0.28 mg/kg was reported in Polia larvae, an important source for an
American songbird, 1.1 mg/kg in seed, 38 mg/kg in plants, and 23 µg
per insect in beetles.
1.5 Effects on organisms in the laboratory and the field
Technical-grade fenthion is toxic or even highly toxic to aquatic
algae under laboratory conditions, with four-day median effective
concentration (EC50) values of 550-1800 µg/litre and four-day
no-observed-effect concentrations (NOECs) of 100-1120 µg/litre. The
fenthion formulations Lebaycid and Baytex are toxic to aquatic algae,
with four-day EC50 values of 1100-> 2000 µg/litre. Few data are
available on the effects of fenthion on aquatic microorganisms in the
field, although in one experiment, phytoplankton were not affected. In
a laboratory experiment, fenthion at concentrations exceeding its
solubility in water did not affect the microorganisms in activated
sludge. Lebaycid did not affect soil respiration or nitrification in a
sandy loam or a silt loam soil under laboratory conditions at 20°C.
Baytex increased the biomass of the aquatic macrophyte, duckweed, at
actual concentrations up to 2.8 mg/litre.
Technical-grade fenthion is highly toxic to aquatic crustaceans,
with a two-day EC50 value of 5.7 µg/litre and a 21-day maximal
acceptable toxicant concentration (MATC) value of 0.042-0.082
µg/litre. Lebaycid is also highly toxic to aquatic crustaceans, with a
21-day NOEC value of 0.018 µg/litre. The few data available on
applications of fenthion in the field at agriculturally recommended
rates indicated that four to five months were required for recovery of
a crustacean population in an artificial outdoor pond after a single
application of fenthion. The adverse effects were due partly, however,
to a harsh winter period. A comparable experiment without a very cold
period showed a recovery period of about two months.
Technical-grade fenthion is moderately to highly toxic to fish,
with four-day median lethal concentration (LC50) values of 0.83-
1.7 mg/litre and an 88-day MATC value of 13-27 µg/litre. Lebaycid is
toxic to fish, with four-day LC50 values of 2.3-4.3 mg/litre. The
transformation products 3-methyl-4-methylsulfinyl-phenol and 3-methyl-
4-methylsulfonyl-phenol are very slightly toxic to freshwater fish,
with four-day LC50 values > 100 mg/litre. The only other aquatic
vertebrate species for which a toxicity value is available is the
amphibian Rana hexadactyla, with a four-day LC50 value of 0.84
µg/litre, indicating high toxicity. A threshold limit value of 7.4
µg/litre for Bufo bufo japonica indicates lower toxicity. One field
experiment in an artificial outdoor pond showed no adverse effects on
fish or tadpoles; however, the application rate in this experiment was
0.22 kg/ha, which is low in comparison with the highest recommended
rate.
No data were available on the effects of fenthion on terrestrial
macrophytes. Technical-grade fenthion is obviously toxic to various
terrestrial insects; e.g. the contact LD50 value for honey bees is
0.16-< 2 µg/bee. Laboratory tests with predatory insects such as
wasps, mites, hoverflies, and green lacewings indicate that fenthion
is highly toxic for these groups. No data were available on oral
toxicity to honey bees. Lebaycid is slightly toxic to earthworms, with
a 14-day NOEC of 100 mg/kg of dry soil.
Field experiments show variable adverse effects on terrestrial
invertebrates, owing to differences in the extent of exposure and in
the sensitivity of species. In a field experiment in Egypt, four
consecutive sprayings of cotton with Lebaycid induced a mortality rate
of up to 96% in two populations of ladybirds. The populations
recovered partially during the 15-day interval between sprayings. The
ladybird Scymnus appeared to be less senstitive to fenthion than
Coccinella in this experiment. In a field experiment in the
Philippines, no substantial effects of fenthion were found on
pest-predatory spiders and hemipterous species. In another field
experiment in Egypt, both the occurrence and absence of adverse
effects on pest-predatory species were observed within the treated
area.
Technical-grade fenthion is also obviously toxic to various
birds. Laboratory experiments showed a moderately toxic to toxic
effect, with an LD50 value of 7.2 mg/kg bw and eight-day LC50
values of 60-1259 mg/kg feed. The main mode of action appears to be
depression of cholinesterase activity. In experiments in which
fenthion was used as an avicide, secondary poisoning of starling
predators was seen after the perches of starlings were smeared with
fenthion.
Many cases of poisoning of wild birds have been reported after
use of fenthion for controlling mosquitos or birds. In some field
experiments, poisoned birds showed depression of cholinesterase
activity in both brain and blood. In most field experiments, the
activity in brain is measured. It is assumed that a depression of >
50% in cholinesterase activity indicates severe exposure to fenthion.
In a field experiment in Kenya in which fenthion was used as an
avicide, cholinesterase activities < 20 µmol/min per g were taken
to indicate exposure, and activities < 10 µmol/min per g indicated
severe sickness or death. Data on cholinesterase activity should be
combined with measurements of fenthion residues in order to establish
a causal relationship between exposure and its effects. The response
of some birds to substantial exposure is to hide themselves in e.g.
thick bushes, and this may lead to underestimation of the number of
affected birds.
Variable effects on wild birds were observed in field experiments
in Wyoming (USA) in which about the same aerial application rate was
used (50 g/ha). In the first experiment, the mortality rates of three
common bird species were treatment-related, and the deaths were
correlated with substantial depressions in cholinesterase activity. In
the second experiment, only the growth rate of nestlings of a common
songbird was significantly decreased in one of two treated plots;
however, the decrease may have had no biological consequences, as the
transient depression in cholinesterase activity in the nestlings was
not significant. No other parameter related to mortality, biomass, or
reproduction was influenced by the treatment. It was notable that
although the abundance of an important feed item -- the caterpillar --
like larvae of Polia -- was decreased by 50%, the feeding behaviour
of the adults was not altered. The percentage of larvae in the feed
collected for the nestlings was also not altered.
No clear treatment-related effects on non-target birds
(free-roaming galliformous and raptorial birds and caged granivorous
doves) were found in outdoor experiments in Kenya in which red-billed
quelea colonies were sprayed aerially with Queletox, except for a
significant depression in plasma cholinesterase activity in 70% of the
raptorial birds analysed; the biological significance of this finding
was not clear.
2. Identity and physical and chemical properties
2.1 Identity
Fenthion is the primary name of an organophosphorus pesticide,
the chemical name of which is thiophosphoric acid, O,O-dimethyl-
O-[3-methyl-4-(methylthio)phenyl] ester according to IUPAC
nomenclature. The Chemical Abstracts name is O,O-dimethyl
O-[3-methyl-4-(methylthio)phenyl]phosphorothioate, and its CAS
registry number is 55-38-9. The empirical formula is C10H15O3PS2,
and the structural formula is:
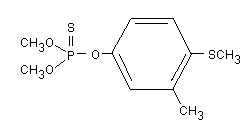 The relative molecular mass of fenthion is 278.3. Technical-grade
fenthion has a purity of > 90%, generally exceeding 95%. Fenthion
is usually formulated as an emulsifiable concentrate. Emulsifiers,
such as Toximul MP-8, and solvents such as xylene, 'fog' (a fine water
spray), and diesel oil, may be added to formulations of fenthion, the
type of adjuvant differing according to the formulation. The addition
of oily materials increases the sorption of fenthion to plant surface
tissues. There is no up-to-date survey of the adjuvants currently used
in formulations.
2.2 Physical and chemical properties
The physical and chemical properties of fenthion are shown in
Table 1. Fenthion is slightly soluble in water at room temperature and
is readily soluble in organic solvents, the solubility in xylene,
acetone, acetonitrile, and dimethylsulfoxide exceeding 250 000
mg/litre. Fenthion is slightly volatile, in view of its vapour
pressure, and is essentially nonvolatile from water, in view of its
Henry's law constant. It is not surface active.
Table 1. Physical and chemical properties of fenthion
Property Remarks
Physical state Liquid
Colour Colourless to yellow brown
Odour Like mercaptans
Boiling-point 284°C Calculated at 1013 hPa
Specific gravity (density) 1.25 At 20°C
Vapour pressure 3.7 × 10-4 Pa Extrapolated at 20°C
7.4 × 10-4 Pa Extrapolated at 25°C
Solubility in water 4.2 mg/litre At 20°C
Solubility in n-hexane 100 000 mg/litre At 20°C
Solubility in n-octanol > 300 000 mg/litre
Henry's law constant 24 × 10-2 Pa . m3/mol Calculated at 20°C (with dimensions)
10 × 10-5 Calculated at 20°C (dimensionless)
Octanol-water partition 4.8 At 20°C
coefficient (log Kow)
Surface tension 70 mN/m
Explosiveness Not explosive
Oxidizing properties No oxidizing properties
Ignition temperature 365°C
Flash-point 170°C
Quantum yield 0.8
Data provided by Bayer AG for fenthion with a purity > 95%
3. Sources of human and environmental exposure
3.1 Production levels and processes
No data were available on world production of fenthion and its
formulations, and there were no data on losses to the environment
during normal production and formulation or accidental losses.
3.2 Uses
Fenthion is a systemic pesticide used primarily against insects
such as lice, ticks, cockroaches, flies, leaf-miners, rice stem
borers, and cereal pests. It can be used to protect livestock,
domestic animals, and various crops such as olives, sugar beet,
cotton, cacao, citrus, coffee, and rice. Agricultural use in the
countries of the European Union is primarily in horticulture and
orchards (Bayer AG, 1995). It is no longer used on cotton crops (Bayer
AG, personal communication). Fenthion is also used against birds such
as the red-billed weaver (Quelea quelea), the European starling
(Sturnus vulgaris), and the rock dove (Columbia livea), which can
destroy crops substantially. Bayer AG does not recommend its use in
urban areas (personal communication). It is also used for mosquito
control in wetlands.
Fenthion is used worldwide. In 1985, 39 417 litres of the
formulation Queletox were sprayed over 23 370 ha to control grain pest
birds in five eastern African countries (Bruggers et al., 1989). No
other quantitative data on the use of fenthion are available. FAO/WHO
(1973) estimated that the main uses of fenthion in 1971 were field
crops (50%), fruit and vines (30%), and other uses such as ornamental
plants, public health, and animal health (20%). In 1971, fenthion was
admitted for use on crops in 31 countries. It is registered for use in
bird control in Canada and the USA.
Fenthion can be applied in various formulations, summarized in
Table 2. This summary is far from complete, and some of these
formulations may have been withdrawn from the market. The major
formulations of fenthion used currently are probably Lebaycid EC50 and
Lebaycid EC500, which contain 535 and 506 g of fenthion per litre,
respectively. Other synonyms for fenthion and its formulations are
Baycid, Entex, BAY 29493, OM-2,51725, sulfidophos, quilitox, and Antex
(FAO/WHO, 1973).
Various other active ingredients can be mixed with fenthion. In
Germany, a mixture with propineb is registered. In Japan, various
mixtures were registered with disulfoton and edinphos, especially for
rice. The application rates of fenthion depend on the formulation and
type of use. Bayer AG (1995) recommends rates of 60-1250 g/ha for
various horticultural and fruit crops. FAO/WHO (1973) recommended
rates up to 2 kg/ha on cotton. Lebaycid is generally applied as a
0.05-0.075% solution in water by spraying. The most commonly used
formulations contain an emulsifiable concentrate, a fogging
concentrate or an ultra-low volume liquid, the last being frequently
used under (sub)tropical conditions to minimize losses by
volatilization. Fenthion can also be applied as granules on rice or as
a dustable powder.
Table 2. Composition of some commercial formulations of fenthion
Name Concentration of Remarks
fenthion (g/litre)
Lebaycid 50EC 535 Also contains 2% of emulgator A, 8% of
emulgator B, and up to 39% xylene
Lebaycid 500EC 506
Rid-A-Bird 1100 110
Tiguvon Not reported
Baytexa Not reported Sprayed with 'fog' or diesel oil
Queletox Not reported
All formulations produced by Bayer AG; data provided by Bayer AG
a Liquid concentrate with 93% pure fenthion, which can be mixed with the
emulsifier Toximul MP-8 and a xylene-type solvent at ratios of 30:3.1:1.7;
can also be mixed with diesel oil (Powell, 1984)
Fenthion can be applied in various ways. For large-scale
treatments, aerial application may be appropriate; small-scale
treatment can be done with e.g. back-pack spraying equipment or behind
vehicles. Aerial application may lead to substantial losses due to
drift by wind, and crops, flora, and fauna may be exposed to
off-target deposits. Such downwind deposits can be assumed to depend
on meteorological conditions, the plant canopy structure, the
application method, including the release height, the droplet size,
the occurrence of overlapping spray swathes, the calibration of the
spraying apparatus, the placement of nozzles on the boom, and the
speed of the aircraft (Seabloom et al., 1973; DeWeese et al.,
1983; Bruggers et al., 1989).
4. Environmental transport, distribution, and transformation
The term 'biotransformation' is used in this monograph in
preference to 'biodegradation', as the first refers to a microbial
transformation process resulting in a smaller or greater molecule,
whereas the latter refers to a smaller molecule only. Fenthion
molecules can either increase or decrease in size. Dissipation is the
decrease in size due to microbial activity, to other chemical
transformation processes, and to transfer to other compartments. As
the metabolism of fenthion is preferably called transformation rather
than degradation, the term half-life is preferred to median
dissipation rate (DT50). Thus, confusion about whether D refers to
degradation or dissipation is avoided. It should become clear from the
context whether the half-life refers to a particular process.
The types of sediment or soil mentioned are within the textural
groupings of the system of the US Department of Agriculture (1951).
Only the results of reliable tests are discussed below. In
general, only studies with an adequate method and description are
listed in the tables. Studies that were considered less or not
reliable are sometimes mentioned in the text in order to confirm or
contradict conclusions based on the results of intrinsically reliable
tests.
4.1 Transport and distribution between media
4.1.1 Dissipation from water
Fenthion dissipates rapidly from water in laboratory experiments
under both aerobic and anaerobic conditions, with half-lives up to
seven days (Eichelberger & Lichtenberg, 1971; Bayer AG, 1988a,b;
O'Neill et al., 1989; see also Table 3). Dissipation under anaerobic
conditions is slightly slower than that under aerobic conditions, but
the rate follows first-order kinetics under either condition. The
half-lives shown in Table 3 were deduced from laboratory experiments
with sediment. Dissipation from water appears to occur primarily by
sorption to sediment, phototransformation, and biotransformation (see
sections 4.2.2 and 4.3).
In an indoor microcosm, the presence of plants in the sediment
did not influence dissipation from the water column (O'Neill et al.,
1989). In salt-marsh water and its associated sediment, half of the
applied technical-grade fenthion was dissipated within 1.5 days.
Whereas the parent compound dissipates rapidly from a water
column, the residues, including transformation products, do not
necessarily do so. In a laboratory experiment with water, sediment,
and fish, the total amount of 14C-fenthion residues in the water
remained fairly constant over 28 days after a single application of
fenthion equivalent to 0.011 kg/ha (ChemAgro, 1975a). Immediately
after application, however, 100% of the recovered residues was soluble
in chloroform, whereas after 28 days 66% was water-soluble, indicating
that the composition had shifted towards more polar residues, probably
by phototransformation and biotransformation.
In outdoor experiments, fenthion also dissipated rapidly from the
water column. In jars containing pond water, with or without the
accompanying sediment, that were buried outdoors in the soil for 16
days in Kansas (USA) in 1971, fenthion dissipated with a half-life of
up to two days (ChemAgro, 1972, 1976a). In one of these experiments,
the dissipation rate in the system with sediment was comparable to
that in the system without sediment. This experiment corroborates the
report of Bayer AG (1988a) that other processes influence dissipation
before accumulation in the sediment, regardless of the presence of
sediment. In the other experiment (ChemAgro, 1976a), sediment was an
important sink, as the sediment-bound residues in silt gradually
increased up to 49% of the applied activity after 46 days. Detection
of fenthion in Dutch surface water at concentrations up to
0.12 µg/litre, however, may indicate that dissipation in the field is
less rapid than that under more controlled conditions (see section
5.1).
4.1.2 Soil sorption
Fenthion binds readily to various soils. In laboratory
experiments in which fenthion was added to aqueous soil suspensions,
the adsorption coefficient Ks/l (= Kd = Kom) was 7.7-38 dm3/kg
in six soil types ranging from sand to silty clay loam (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988). These values indicate
strong sorption, which can be described by the Freundlich equation.
Three additional Ks/l values (6.4, 8.6, and 67 dm3/kg) obtained in
these experiments must be considered inaccurate, as the accompanying
1/n constants deviate substantially from unity (< 0.7 or > 1.1).
The mechanism of sorption of fenthion to soil is only partially
understood, and several factors may be involved. Sorption to organic
matter appears to be important, as soils with the highest organic
matter content showed the highest adsorption coefficients (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988).
In a laboratory experiment with 14C-fenthion, the desorption
coefficients were about two times higher than the sorption coefficents
(ABC Inc., 1988). Although 0.01 mol/litre of calcium chloride were
added to simulate potential desorption in the field, sorption may not
be reversible under outdoor conditions.
Table 3. Biotransformation and dissipation of technical-grade fenthion in water and sediments in the laboratory
Water type Sediment Test Sediment Organic matter Temperature pH Length of Half-life Reference
type (%) in sediment (%) (°C) experiment (days)
(days)
Biotransformation in whole system
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8. 66 9a Bayer
in dark approx. 8 (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 11a,b Bayer
in dark (1988b)
Dissipation from water column
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8.8 66 4a Bayer
in dark (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 7a Bayer
in dark (1988b)
Surface waterc Not Aerobic Not Not 20 Not 8 1.5 O'Neill et al.
reported with reported reported reported (1989)
light-dark
cycle
a Approximate value derived from authors' data by linear regression of the recovered percentages after logarithmic
(In) transformation. Times until 90-99% of the applied fenthion has been transformed were used for calculation.
b Although the half-life under anaerobic conditions equals that under aerobic conditions, the initial transformation
rate under anaerobic conditions is slower
c Water from a salt marsh with 11% salinity
4.1.3 Soil mobility
In view of its Ks/l values, fenthion should be immobile in many
soils, as confirmed in several experiments both in the laboratory and
in the field. In thin-layer chromatography studies, which do not
generate reliable Ks/l values, the Rf values for 14C-fenthion in
five soil types with organic matter contents of 0.6-5.1% were
0.14-0.17 (ChemAgro, 1976b). In less reliable leaching experiments
with columns of 45 cm, in which the leaching time was not reported,
water fluxes of 50, 95, and 490 ml were imposed on a sandy loam with
1.4% organic matter, a silty clay loam with 2% organic matter, and a
silty clay loam with 4.4% organic matter, respectively (ChemAgro,
1972). Fenthion was recovered only in the leachate of the silty clay
loam with a high organic matter content, representing 1.8% of the
recovered fenthion. In these experiments, 64-92% of the recovered
fenthion was found in the upper 3-cm layer of the columns.
Transformation products of fenthion appear to be more mobile than
fenthion itself; however, the only data on the mobility of transform-
ation products are those from leaching experiments with aged residues.
The adsorption coefficients of metabolites have not been reported.
In studies under laboratory or greenhouse conditions with
residues aged for 4-30 days, 1.9-46% of the applied activity was
leached (ChemAgro, 1974, 1975b; Mobay Corp., 1987a). The greatest
amount of leaching was found in a sandy soil with the lowest
percentage of organic matter (0.2%) of all soils tested, with 36%
leaching after four days of aging and 46% after 25 days (Mobay Corp.,
1987a). The columns were 30 cm long, the water flux over two days was
high (51 cm), and the soil that had been aged prior to leaching was a
sandy loam soil. These experiments cannot be considered reliable, as
the type of soil placed on the top of the column was different from
that inside the column, and the amounts of fenthion residues after
aging and before application on the column were not reported. In spite
of the high maximal leaching percentages in sand, more than 50% of the
applied activity was recovered in the upper 12 cm of the columns. Four
transformation products were recovered in the sand column segments and
leachates. Those in the leachate, in decreasing order of magnitude,
were 3-methyl-4-methylsulfonyl-phenol, 3-methyl-4-methylsulfinyl-
phenol, fenthion sulfoxide (thiophosphoric acid, O,O-dimethyl- O-
[3-methyl-4-methylsulfinyl-phenyl] ester), and fenthion sulfone
(thiophosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfonyl-
phenyl] ester). The maximal percentages of recovered activity at the
end of the experiments were 20, 15, 11, and 0.8%, respectively. It can
be concluded that at least the more polar transformation products of
fenthion, which generally remain primarily organosoluble, may leach
into shallow groundwater; however, as the half-lives of these
transformation products are probably < 15 days (see section 4.3.2),
substantial leaching of these products to shallow groundwater is
unlikely.
Table 4. Biotransformation and phototransformation of fenthion in soils
Soil type Test type Moisture Temperature pH Organic Length of Half-life Reference
content (%) (°C) matter (%) experiment (days)
(days)
Biotransformation
Silty loama Laboratory, approx. 30 Not 5.9 3.0 120 10c Mobay Corp.
aerobic reportedb (1978b)
Silty loama Laboratory, approx. 30 Not 5.9 3.0 32 > 32 Mobay Corp.
aerobic reportedb (1978b)
Phototransformation
Sandy loam Field, Not 16-37 5.1 2.4 1.2 1c Mobay Corp.
aerobicd reported (1987b)
Sandy loam Field, Not Not 4.5-7.5 0.8-6.3 4 > 4 Gohre & Miller
aerobic reported reported (1986)
a Silty loam is assumed to have a moisture content of 40% (v:v) at field capacity; moisture content was kept at 75% of the
field capacity
b Possibly room temperature, but not clearly stated
c Approximate value derived from authors' data of by linear regression of the recovered percentages after logarithmic
(In) transformation. As transformation of up to 99% of the applied fenthion was in accordance with first-order
kinetics, the coinciding times were used for calculation.
d A slurry of soil, water, and acetonitrile in a photomodule was exposed to fenthion by pipetting it onto the slurry.
4.1.4 Dissipation from soil in the field
No substantial dissipation of fenthion due to run-off on an 8°
slope was found on a sandy loam or a silty clay loam soil on a fallow,
raked field (ChemAgro, 1972). The silty clay loam site had been
divided into one with a low (2%) and the other with a high (4.4%)
organic matter content. Maximal percentages of 0.9% and 1.2% of the
applied fenthion were recovered in the run-off water as the parent
compound and fenthion sulfoxide, respectively, in the sandy loam and
silty clay loam with the low organic matter contents, corresponding to
concentrations of 0.12 and 0.23 µg/litre, respectively. The lowest
run-off of fenthion residues and the largest amount of fenthion
residues (at the site of application) were seen on soil with the
highest percentage of organic matter, coinciding with the strong
sorption of fenthion on various soils. As the soil and the run-off
water were analysed after weekly irrigation for one month, the
dissipation of fenthion must be low, due mainly to strong sorption
(see also section 4.1.2). In these experiments, the soils were
irrigated for one month with 10-17 cm of water (total), and the rate
of application of fenthion was 11.2 kg/ha. The results indicate that
dissipation of fenthion under outdoor conditions may be slower than
that under controlled laboratory conditions. Thirty days after
application, up to 38 and 55% of the applied fenthion were recovered
at the site of application as the parent compound and fenthion
sulfoxide, respectively. Dissipation in laboratory experiments due to
microbial action is much faster (see Table 4 and section 4.3).
4.1.5 Uptake and dissipation from plants
Fenthion can be taken up by plants, and its ability to penetrate
plant tissues results in destruction of e.g. larvae even within fruit.
Fenthion is reported to persist for some time after being taken up.
The amount of fenthion residues in Bermuda grass (Cynodon dactylon)
and corn (Zea mays) decreased by about 60 and 80% within the first
two weeks after application (FAO/WHO, 1973).
4.1.6 Entry into the food chain
Fenthion is used primarily against insects and birds (see section
3.1.2). As the amount of fenthion found in these species after
ingestion or on them after contact may increase immediately after
application, it may enter the food chain by ingestion or direct
contact with e.g. insectivorous and bird-predatory species. Hunt
et al. (1991) investigated the possibility of secondary poisoning of
the American kestrel (Falco sparverius) through predation on house
sparrows (Passer domesticus) exposed to fenthion by smearing of
their perches with the formulation Rid-A-Bird(R). Exposure of the
kestrels by contact with the sparrows' feet may have contributed
substantially to the observed toxicity. When the contaminated sparrows
were penned with the kestrels for three days, 79% of the kestrels died
from fenthion poisoning within one day, and all were dead within three
days. The contaminated kestrels showed 78-92% depression of brain
cholinesterase activity and 97% of that in plasma, which correlated
with concentrations of fenthion up to 14 µg/g in the gastrointestinal
tract and 19 µg/g in feet. Most of the contaminated sparrows died
within 8 h of exposure and contained fenthion at concentrations of up
to 6 µg/g in the internal carcass, 631 µg/g in the external carcass
(i.e. feathers and skin), and 1152 µg/g in the feet.
The route of exposure before secondary intoxication is often
unclear. In five bald eagles (Haliaeetus leucocephalus) found dead
in Iowa (USA) in 1984, brain cholinesterase activity was depressed by
80-92% (Henny et al., 1987). The remains of piglets found in their
stomachs contained fenthion at concentrations of 0.1-6.8 mg/kg wet
weight. The two possible sources of the fenthion are intentional
primary poisoning of the eagles with contaminated bait or secondary
poisoning from contaminated piglets. This case indicates the
vulnerability of scavenging species like eagles.
4.2 Abiotic transformation
4.2.1 Hydrolytic cleavage and oxidation
Fenthion is transfomed slowly in sterile phosphate buffers, with
half-lives of 56, 41, and 32 days at pH 5, 7, and 9, respectively, at
25°C for 70 days (ChemAgro, 1976c); comparable results were found at 5
and 40°C. These results indicate slower transformation rates under
more acid conditions. This pH-dependent transformation was confirmed
in other studies (eg. Bayer AG, 1983a). In the experiments of ChemAgro
(1976c) at 25°C, the main transformation products were fenthion
sulfoxide, 3-methyl-4-methylsulfinyl-phenol, fenoxon (phosphoric acid,
O,O-dimethyl- O-[3-methyl-4-methylthio-phenyl] ester), fenoxon
sulfone (phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methyl-
sulfonyl-phenyl] ester), fenthion sulfone, and fenoxon sulfoxide
(phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfinyl-
phenyl] ester). The maximal percentages of the administered activity
were 14, 12, 10, 8, 7, and 6%, respectively. The transformation
products indicate both oxidation (fenthion sulfoxide, fenoxon, fenoxon
sulfone, fenthion sulfone, and fenoxon sulfoxide) and hydrolysis
(3-methyl-4-methylsulfinyl-phenol).
A low abiotic transformation rate in the dark was confirmed in
other laboratory experiments (ChemAgro, 1972; Bayer AG, 1983a). In
buffered aqueous solutions, the extrapolated half-lives at 22°C were
223, 200, and 151 days at pH 4, 7, and 9, respectively (Bayer AG,
1983a), and the transformation product was 3-methyl-4-methylthio-
phenol. In aqueous phosphate buffers at 30°C, the experimental
half-lives at pH 5, 7, and 9 were 31, 26, and 24 days, respectively
(ChemAgro, 1972).
4.2.2 Phototransformation
(a) Water
Photochemical transformation may occur rapidly in water under
both laboratory and field conditions. In a sterile aqueous buffer at
pH 5, substantial phototransformation of 13C/14C-1-ring-labelled
fenthion was observed after exposure to artificial light simulating
sunlight for 4 h at 23°C (Mobay Corp., 1987c); the half-life was
0.5 h. Rapid phototransformation in the laboratory was confirmed by
Bayer AG (1983b, 1988c) and ChemAgro (1976b).
The major photochemical transformation products in the study
reported by the Mobay Corp. (1987c) were 3-methyl-4-methylthio-phenol
(maximal totally recovered activity, 23%), fenthion sulfoxide
(maximum, 17%), and 3-methyl-4-methylsulfinyl-phenol (maximum, 15%).
Transformation products found in minor quantities in this experiment
were 3-methyl-4-sulfo-phenol (maximum, 8%), fenoxon sulfone (maximum,
7%), fenoxon sulfoxide (maximum, 6%), and 3-methyl-4-methylsulfonyl-
phenol (maximum, 6%). Fenoxon was also found as a phototransformation
product in other experiments (maximum, 10-11% of the applied activity)
(ChemAgro, 1976b).
The phototransformation pattern is temperature-dependent: at
25°C, the rate of production of fenthion sulfoxide via oxidation is
comparable to that of 3-methyl-4-methylthio-phenol via hydrolysis.
Oxidation to fenoxon sulfoxide is assumed to prevail at higher
temperatures and hydrolysis to 3-methyl-4-methylthio-phenol at lower
temperatures (ChemAgro, 1976d). When 2% acetone was added to an
aqueous solution of fenthion as a photosensitizer before irradiation
with artificial light for 2 h, oxidation via fenthion sulfoxide to
unknown polar transformation products was accelerated. Thus, changes
in temperature and the presence of sensitizers can result in different
mixtures of fenthion transformation products.
Fenthion also undergoes rapid phototransformation outdoors,
although possibly at a somewhat slower rate than in laboratory
experiments. Fenthion in distilled water exposed to sunlight in the
summer was phototransformed with a half-life of 4 h (Bayer AG, 1983b);
in a comparable experiment under laboratory conditions with artificial
light, the half-life was 0.08 h. Fenthion is phototransformed rapidly
in sunlight (lambda > 290 nm), primarily because of its high quantum
yield.
(b) Soil
As fenthion applied to soil appears to be phototransformed
rapidly by sunlight, with half-lives of 1-> 4 days (see Table 4),
phototransformation is an important route of dissipation. In an
outdoor experiment, 13C/14C-1-ring-labelled fenthion mixed with
unlabelled fenthion was exposed for 1.2 days to natural sunlight after
application to a sandy loam at a rate of 53 mg/kg soil (see also Table
4). Fenthion was rapidly phototransformed, and the main photochemical
transformation product was fenthion sulfoxide (maximum, 58% of the
recovered activity); fenoxon sulfoxide, 3-methyl-4-methylsulfinyl-
phenol, and fenthion sulfone were minor transformation products (i.e.
< 10% of the recovered activity). The oxidation path apparently
prevails (Mobay Corp., 1987b). As the temperature during this
experiment rose to about 37°C, a shift to the oxidation rather than to
the hydrolysis pathway seems to have occurred, as in the
phototransformation experiments with water (ChemAgro, 1976d).
(c) Air
Phototransformation occurs rapidly in the troposphere. The
half-life of fenthion in air is estimated to be 1.7 h (Bayer AG,
1994a) on the basis of the assumption that phototransformation occurs
via the reaction of hydroxyl radicals with fenthion. The primary
target of the radicals is probably the P=S bond. Such attacks may
result in secondary oxidation products (e.g. fenoxon), which can be
deposited on soil, water, and vegetation by wet and dry deposition.
4.3 Biotransformation
Biotransformation may contribute to the general processes of
dissipation (see section 4.1). No data from primary sources on the
microorganisms that can metabolize fenthion have been incorporated in
this monograph. It has been reported to be biotransformed to fenoxon
sulfoxide by the soil fungus Rhizopus japonicus (Wallnöfer, 1978,
cited by Mobay Corp., 1978b). Similarly, no data on the biodegrad-
ability of the adjuvants in formulations of fenthion have been
incorporated; however, it can be assumed that the biodegradability of
e.g. 'fog' and diesel oil in a natural environment is slight.
A simplified scheme, showing some common transformation products
in environmental compartments, is presented in Figure 1. The scheme
includes both abiotic and biotic transformation products.
4.3.1 Water and sediment
The relevant laboratory experiments in which biotransformation in
water-sediment systems was studied were summarized in Table 3. The
results indicate that the rate of biotransformation is rapid under
both aerobic and anaerobic conditions: 50% biotransformation of
fenthion (half-life) in a whole water-sediment system occurs within
about 10 days. Experimental conditions other than the availability of
oxygen, e.g. temperature, salinity, the presence of plants, the type
of water, and the type of sediment, probably have less effect on the
transformation rate. In microcosms of salt-marsh water, sediment and
sediment-rooted plants, however, the amount of detritus and the extent
of bioperturbation of the sediment may also influence the biotrans-
formation rate (O'Neill et al., 1989).
In general, sediments appear to function as a major sink for
fenthion under both aerobic and anaerobic conditions. Rapid
partitioning to sediment was seen under aerobic and anaerobic
conditions, as 21-28% of the applied activity was recovered in the
sediment within 1 h after application (Bayer AG, 1988a,b); maxima of
41-80% of the applied activity were recovered in sediment. Under
aerobic conditions, the amount in the sediment increased gradually up
to 62-80% of the applied activity (Bayer AG, 1988a) by the end of the
test at 66 days. Under anaerobic conditions, the maxima were 66-70%
and were reached after 7-14 days (Bayer AG, 1988b). The total amounts
of activity in the sediments decreased thereafter, probably due to
further mineralization to 14C-carbon dioxide (see below). Under
either aerobic or anaerobic incubation, most of the activity in the
sediment shifted from water-and acetone-extractable compounds to
non-extractable (i.e. sediment-bound) residue in the course of the
experiments.
The rate of biotransformation in non-sterile sediment without
plants was best modelled by assuming biotransformation in the upper
1 mm of the sediment (O'Neill et al., 1989). When plants were
present, biotransformation in the upper 7 mm was the best assumption.
In aerobic experiments with water and associated sediments, the
amount of fenthion declines rapidly over time, with transient
increases in various metabolites, an increase in 14C-carbon dioxide,
and an increase in sediment-bound residues (Bayer AG, 1988a,b). Under
anaerobic conditions, a small amount (3-4%) of 14C-methane was
formed. The production of 14C-carbon dioxide appears to be more
rapid under aerobic than anaerobic conditions, but the final amounts
of 14C-carbon dioxide differed substantially with the duration of
the test: 12-15% after 66 days under aerobic conditions and 52% after
120-190 days under anaerobic conditions. As the amount of oxygen was
very low under anaerobic conditions (0-0.4 mg/litre), the oxygen in
the carbon dioxide may have been supplied by nitrates or sulfates. The
redox potential during the experiment varied between -186 and 137 mV.
The main transformation products were demethyl fenoxon sulfoxide
(maximum, 30% of whole water-sediment system after 7-14 days),
fenthion sulfoxide (maximum, 28% after 14 days), 3-methyl-4-methyl-
sulfinyl-phenol (maximum, 13% after 66 days), and 3-methyl-4-methyl-
sulfonyl-phenol (maximum, 11% after 66 days) under aerobic conditions,
The relative molecular mass of fenthion is 278.3. Technical-grade
fenthion has a purity of > 90%, generally exceeding 95%. Fenthion
is usually formulated as an emulsifiable concentrate. Emulsifiers,
such as Toximul MP-8, and solvents such as xylene, 'fog' (a fine water
spray), and diesel oil, may be added to formulations of fenthion, the
type of adjuvant differing according to the formulation. The addition
of oily materials increases the sorption of fenthion to plant surface
tissues. There is no up-to-date survey of the adjuvants currently used
in formulations.
2.2 Physical and chemical properties
The physical and chemical properties of fenthion are shown in
Table 1. Fenthion is slightly soluble in water at room temperature and
is readily soluble in organic solvents, the solubility in xylene,
acetone, acetonitrile, and dimethylsulfoxide exceeding 250 000
mg/litre. Fenthion is slightly volatile, in view of its vapour
pressure, and is essentially nonvolatile from water, in view of its
Henry's law constant. It is not surface active.
Table 1. Physical and chemical properties of fenthion
Property Remarks
Physical state Liquid
Colour Colourless to yellow brown
Odour Like mercaptans
Boiling-point 284°C Calculated at 1013 hPa
Specific gravity (density) 1.25 At 20°C
Vapour pressure 3.7 × 10-4 Pa Extrapolated at 20°C
7.4 × 10-4 Pa Extrapolated at 25°C
Solubility in water 4.2 mg/litre At 20°C
Solubility in n-hexane 100 000 mg/litre At 20°C
Solubility in n-octanol > 300 000 mg/litre
Henry's law constant 24 × 10-2 Pa . m3/mol Calculated at 20°C (with dimensions)
10 × 10-5 Calculated at 20°C (dimensionless)
Octanol-water partition 4.8 At 20°C
coefficient (log Kow)
Surface tension 70 mN/m
Explosiveness Not explosive
Oxidizing properties No oxidizing properties
Ignition temperature 365°C
Flash-point 170°C
Quantum yield 0.8
Data provided by Bayer AG for fenthion with a purity > 95%
3. Sources of human and environmental exposure
3.1 Production levels and processes
No data were available on world production of fenthion and its
formulations, and there were no data on losses to the environment
during normal production and formulation or accidental losses.
3.2 Uses
Fenthion is a systemic pesticide used primarily against insects
such as lice, ticks, cockroaches, flies, leaf-miners, rice stem
borers, and cereal pests. It can be used to protect livestock,
domestic animals, and various crops such as olives, sugar beet,
cotton, cacao, citrus, coffee, and rice. Agricultural use in the
countries of the European Union is primarily in horticulture and
orchards (Bayer AG, 1995). It is no longer used on cotton crops (Bayer
AG, personal communication). Fenthion is also used against birds such
as the red-billed weaver (Quelea quelea), the European starling
(Sturnus vulgaris), and the rock dove (Columbia livea), which can
destroy crops substantially. Bayer AG does not recommend its use in
urban areas (personal communication). It is also used for mosquito
control in wetlands.
Fenthion is used worldwide. In 1985, 39 417 litres of the
formulation Queletox were sprayed over 23 370 ha to control grain pest
birds in five eastern African countries (Bruggers et al., 1989). No
other quantitative data on the use of fenthion are available. FAO/WHO
(1973) estimated that the main uses of fenthion in 1971 were field
crops (50%), fruit and vines (30%), and other uses such as ornamental
plants, public health, and animal health (20%). In 1971, fenthion was
admitted for use on crops in 31 countries. It is registered for use in
bird control in Canada and the USA.
Fenthion can be applied in various formulations, summarized in
Table 2. This summary is far from complete, and some of these
formulations may have been withdrawn from the market. The major
formulations of fenthion used currently are probably Lebaycid EC50 and
Lebaycid EC500, which contain 535 and 506 g of fenthion per litre,
respectively. Other synonyms for fenthion and its formulations are
Baycid, Entex, BAY 29493, OM-2,51725, sulfidophos, quilitox, and Antex
(FAO/WHO, 1973).
Various other active ingredients can be mixed with fenthion. In
Germany, a mixture with propineb is registered. In Japan, various
mixtures were registered with disulfoton and edinphos, especially for
rice. The application rates of fenthion depend on the formulation and
type of use. Bayer AG (1995) recommends rates of 60-1250 g/ha for
various horticultural and fruit crops. FAO/WHO (1973) recommended
rates up to 2 kg/ha on cotton. Lebaycid is generally applied as a
0.05-0.075% solution in water by spraying. The most commonly used
formulations contain an emulsifiable concentrate, a fogging
concentrate or an ultra-low volume liquid, the last being frequently
used under (sub)tropical conditions to minimize losses by
volatilization. Fenthion can also be applied as granules on rice or as
a dustable powder.
Table 2. Composition of some commercial formulations of fenthion
Name Concentration of Remarks
fenthion (g/litre)
Lebaycid 50EC 535 Also contains 2% of emulgator A, 8% of
emulgator B, and up to 39% xylene
Lebaycid 500EC 506
Rid-A-Bird 1100 110
Tiguvon Not reported
Baytexa Not reported Sprayed with 'fog' or diesel oil
Queletox Not reported
All formulations produced by Bayer AG; data provided by Bayer AG
a Liquid concentrate with 93% pure fenthion, which can be mixed with the
emulsifier Toximul MP-8 and a xylene-type solvent at ratios of 30:3.1:1.7;
can also be mixed with diesel oil (Powell, 1984)
Fenthion can be applied in various ways. For large-scale
treatments, aerial application may be appropriate; small-scale
treatment can be done with e.g. back-pack spraying equipment or behind
vehicles. Aerial application may lead to substantial losses due to
drift by wind, and crops, flora, and fauna may be exposed to
off-target deposits. Such downwind deposits can be assumed to depend
on meteorological conditions, the plant canopy structure, the
application method, including the release height, the droplet size,
the occurrence of overlapping spray swathes, the calibration of the
spraying apparatus, the placement of nozzles on the boom, and the
speed of the aircraft (Seabloom et al., 1973; DeWeese et al.,
1983; Bruggers et al., 1989).
4. Environmental transport, distribution, and transformation
The term 'biotransformation' is used in this monograph in
preference to 'biodegradation', as the first refers to a microbial
transformation process resulting in a smaller or greater molecule,
whereas the latter refers to a smaller molecule only. Fenthion
molecules can either increase or decrease in size. Dissipation is the
decrease in size due to microbial activity, to other chemical
transformation processes, and to transfer to other compartments. As
the metabolism of fenthion is preferably called transformation rather
than degradation, the term half-life is preferred to median
dissipation rate (DT50). Thus, confusion about whether D refers to
degradation or dissipation is avoided. It should become clear from the
context whether the half-life refers to a particular process.
The types of sediment or soil mentioned are within the textural
groupings of the system of the US Department of Agriculture (1951).
Only the results of reliable tests are discussed below. In
general, only studies with an adequate method and description are
listed in the tables. Studies that were considered less or not
reliable are sometimes mentioned in the text in order to confirm or
contradict conclusions based on the results of intrinsically reliable
tests.
4.1 Transport and distribution between media
4.1.1 Dissipation from water
Fenthion dissipates rapidly from water in laboratory experiments
under both aerobic and anaerobic conditions, with half-lives up to
seven days (Eichelberger & Lichtenberg, 1971; Bayer AG, 1988a,b;
O'Neill et al., 1989; see also Table 3). Dissipation under anaerobic
conditions is slightly slower than that under aerobic conditions, but
the rate follows first-order kinetics under either condition. The
half-lives shown in Table 3 were deduced from laboratory experiments
with sediment. Dissipation from water appears to occur primarily by
sorption to sediment, phototransformation, and biotransformation (see
sections 4.2.2 and 4.3).
In an indoor microcosm, the presence of plants in the sediment
did not influence dissipation from the water column (O'Neill et al.,
1989). In salt-marsh water and its associated sediment, half of the
applied technical-grade fenthion was dissipated within 1.5 days.
Whereas the parent compound dissipates rapidly from a water
column, the residues, including transformation products, do not
necessarily do so. In a laboratory experiment with water, sediment,
and fish, the total amount of 14C-fenthion residues in the water
remained fairly constant over 28 days after a single application of
fenthion equivalent to 0.011 kg/ha (ChemAgro, 1975a). Immediately
after application, however, 100% of the recovered residues was soluble
in chloroform, whereas after 28 days 66% was water-soluble, indicating
that the composition had shifted towards more polar residues, probably
by phototransformation and biotransformation.
In outdoor experiments, fenthion also dissipated rapidly from the
water column. In jars containing pond water, with or without the
accompanying sediment, that were buried outdoors in the soil for 16
days in Kansas (USA) in 1971, fenthion dissipated with a half-life of
up to two days (ChemAgro, 1972, 1976a). In one of these experiments,
the dissipation rate in the system with sediment was comparable to
that in the system without sediment. This experiment corroborates the
report of Bayer AG (1988a) that other processes influence dissipation
before accumulation in the sediment, regardless of the presence of
sediment. In the other experiment (ChemAgro, 1976a), sediment was an
important sink, as the sediment-bound residues in silt gradually
increased up to 49% of the applied activity after 46 days. Detection
of fenthion in Dutch surface water at concentrations up to
0.12 µg/litre, however, may indicate that dissipation in the field is
less rapid than that under more controlled conditions (see section
5.1).
4.1.2 Soil sorption
Fenthion binds readily to various soils. In laboratory
experiments in which fenthion was added to aqueous soil suspensions,
the adsorption coefficient Ks/l (= Kd = Kom) was 7.7-38 dm3/kg
in six soil types ranging from sand to silty clay loam (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988). These values indicate
strong sorption, which can be described by the Freundlich equation.
Three additional Ks/l values (6.4, 8.6, and 67 dm3/kg) obtained in
these experiments must be considered inaccurate, as the accompanying
1/n constants deviate substantially from unity (< 0.7 or > 1.1).
The mechanism of sorption of fenthion to soil is only partially
understood, and several factors may be involved. Sorption to organic
matter appears to be important, as soils with the highest organic
matter content showed the highest adsorption coefficients (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988).
In a laboratory experiment with 14C-fenthion, the desorption
coefficients were about two times higher than the sorption coefficents
(ABC Inc., 1988). Although 0.01 mol/litre of calcium chloride were
added to simulate potential desorption in the field, sorption may not
be reversible under outdoor conditions.
Table 3. Biotransformation and dissipation of technical-grade fenthion in water and sediments in the laboratory
Water type Sediment Test Sediment Organic matter Temperature pH Length of Half-life Reference
type (%) in sediment (%) (°C) experiment (days)
(days)
Biotransformation in whole system
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8. 66 9a Bayer
in dark approx. 8 (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 11a,b Bayer
in dark (1988b)
Dissipation from water column
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8.8 66 4a Bayer
in dark (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 7a Bayer
in dark (1988b)
Surface waterc Not Aerobic Not Not 20 Not 8 1.5 O'Neill et al.
reported with reported reported reported (1989)
light-dark
cycle
a Approximate value derived from authors' data by linear regression of the recovered percentages after logarithmic
(In) transformation. Times until 90-99% of the applied fenthion has been transformed were used for calculation.
b Although the half-life under anaerobic conditions equals that under aerobic conditions, the initial transformation
rate under anaerobic conditions is slower
c Water from a salt marsh with 11% salinity
4.1.3 Soil mobility
In view of its Ks/l values, fenthion should be immobile in many
soils, as confirmed in several experiments both in the laboratory and
in the field. In thin-layer chromatography studies, which do not
generate reliable Ks/l values, the Rf values for 14C-fenthion in
five soil types with organic matter contents of 0.6-5.1% were
0.14-0.17 (ChemAgro, 1976b). In less reliable leaching experiments
with columns of 45 cm, in which the leaching time was not reported,
water fluxes of 50, 95, and 490 ml were imposed on a sandy loam with
1.4% organic matter, a silty clay loam with 2% organic matter, and a
silty clay loam with 4.4% organic matter, respectively (ChemAgro,
1972). Fenthion was recovered only in the leachate of the silty clay
loam with a high organic matter content, representing 1.8% of the
recovered fenthion. In these experiments, 64-92% of the recovered
fenthion was found in the upper 3-cm layer of the columns.
Transformation products of fenthion appear to be more mobile than
fenthion itself; however, the only data on the mobility of transform-
ation products are those from leaching experiments with aged residues.
The adsorption coefficients of metabolites have not been reported.
In studies under laboratory or greenhouse conditions with
residues aged for 4-30 days, 1.9-46% of the applied activity was
leached (ChemAgro, 1974, 1975b; Mobay Corp., 1987a). The greatest
amount of leaching was found in a sandy soil with the lowest
percentage of organic matter (0.2%) of all soils tested, with 36%
leaching after four days of aging and 46% after 25 days (Mobay Corp.,
1987a). The columns were 30 cm long, the water flux over two days was
high (51 cm), and the soil that had been aged prior to leaching was a
sandy loam soil. These experiments cannot be considered reliable, as
the type of soil placed on the top of the column was different from
that inside the column, and the amounts of fenthion residues after
aging and before application on the column were not reported. In spite
of the high maximal leaching percentages in sand, more than 50% of the
applied activity was recovered in the upper 12 cm of the columns. Four
transformation products were recovered in the sand column segments and
leachates. Those in the leachate, in decreasing order of magnitude,
were 3-methyl-4-methylsulfonyl-phenol, 3-methyl-4-methylsulfinyl-
phenol, fenthion sulfoxide (thiophosphoric acid, O,O-dimethyl- O-
[3-methyl-4-methylsulfinyl-phenyl] ester), and fenthion sulfone
(thiophosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfonyl-
phenyl] ester). The maximal percentages of recovered activity at the
end of the experiments were 20, 15, 11, and 0.8%, respectively. It can
be concluded that at least the more polar transformation products of
fenthion, which generally remain primarily organosoluble, may leach
into shallow groundwater; however, as the half-lives of these
transformation products are probably < 15 days (see section 4.3.2),
substantial leaching of these products to shallow groundwater is
unlikely.
Table 4. Biotransformation and phototransformation of fenthion in soils
Soil type Test type Moisture Temperature pH Organic Length of Half-life Reference
content (%) (°C) matter (%) experiment (days)
(days)
Biotransformation
Silty loama Laboratory, approx. 30 Not 5.9 3.0 120 10c Mobay Corp.
aerobic reportedb (1978b)
Silty loama Laboratory, approx. 30 Not 5.9 3.0 32 > 32 Mobay Corp.
aerobic reportedb (1978b)
Phototransformation
Sandy loam Field, Not 16-37 5.1 2.4 1.2 1c Mobay Corp.
aerobicd reported (1987b)
Sandy loam Field, Not Not 4.5-7.5 0.8-6.3 4 > 4 Gohre & Miller
aerobic reported reported (1986)
a Silty loam is assumed to have a moisture content of 40% (v:v) at field capacity; moisture content was kept at 75% of the
field capacity
b Possibly room temperature, but not clearly stated
c Approximate value derived from authors' data of by linear regression of the recovered percentages after logarithmic
(In) transformation. As transformation of up to 99% of the applied fenthion was in accordance with first-order
kinetics, the coinciding times were used for calculation.
d A slurry of soil, water, and acetonitrile in a photomodule was exposed to fenthion by pipetting it onto the slurry.
4.1.4 Dissipation from soil in the field
No substantial dissipation of fenthion due to run-off on an 8°
slope was found on a sandy loam or a silty clay loam soil on a fallow,
raked field (ChemAgro, 1972). The silty clay loam site had been
divided into one with a low (2%) and the other with a high (4.4%)
organic matter content. Maximal percentages of 0.9% and 1.2% of the
applied fenthion were recovered in the run-off water as the parent
compound and fenthion sulfoxide, respectively, in the sandy loam and
silty clay loam with the low organic matter contents, corresponding to
concentrations of 0.12 and 0.23 µg/litre, respectively. The lowest
run-off of fenthion residues and the largest amount of fenthion
residues (at the site of application) were seen on soil with the
highest percentage of organic matter, coinciding with the strong
sorption of fenthion on various soils. As the soil and the run-off
water were analysed after weekly irrigation for one month, the
dissipation of fenthion must be low, due mainly to strong sorption
(see also section 4.1.2). In these experiments, the soils were
irrigated for one month with 10-17 cm of water (total), and the rate
of application of fenthion was 11.2 kg/ha. The results indicate that
dissipation of fenthion under outdoor conditions may be slower than
that under controlled laboratory conditions. Thirty days after
application, up to 38 and 55% of the applied fenthion were recovered
at the site of application as the parent compound and fenthion
sulfoxide, respectively. Dissipation in laboratory experiments due to
microbial action is much faster (see Table 4 and section 4.3).
4.1.5 Uptake and dissipation from plants
Fenthion can be taken up by plants, and its ability to penetrate
plant tissues results in destruction of e.g. larvae even within fruit.
Fenthion is reported to persist for some time after being taken up.
The amount of fenthion residues in Bermuda grass (Cynodon dactylon)
and corn (Zea mays) decreased by about 60 and 80% within the first
two weeks after application (FAO/WHO, 1973).
4.1.6 Entry into the food chain
Fenthion is used primarily against insects and birds (see section
3.1.2). As the amount of fenthion found in these species after
ingestion or on them after contact may increase immediately after
application, it may enter the food chain by ingestion or direct
contact with e.g. insectivorous and bird-predatory species. Hunt
et al. (1991) investigated the possibility of secondary poisoning of
the American kestrel (Falco sparverius) through predation on house
sparrows (Passer domesticus) exposed to fenthion by smearing of
their perches with the formulation Rid-A-Bird(R). Exposure of the
kestrels by contact with the sparrows' feet may have contributed
substantially to the observed toxicity. When the contaminated sparrows
were penned with the kestrels for three days, 79% of the kestrels died
from fenthion poisoning within one day, and all were dead within three
days. The contaminated kestrels showed 78-92% depression of brain
cholinesterase activity and 97% of that in plasma, which correlated
with concentrations of fenthion up to 14 µg/g in the gastrointestinal
tract and 19 µg/g in feet. Most of the contaminated sparrows died
within 8 h of exposure and contained fenthion at concentrations of up
to 6 µg/g in the internal carcass, 631 µg/g in the external carcass
(i.e. feathers and skin), and 1152 µg/g in the feet.
The route of exposure before secondary intoxication is often
unclear. In five bald eagles (Haliaeetus leucocephalus) found dead
in Iowa (USA) in 1984, brain cholinesterase activity was depressed by
80-92% (Henny et al., 1987). The remains of piglets found in their
stomachs contained fenthion at concentrations of 0.1-6.8 mg/kg wet
weight. The two possible sources of the fenthion are intentional
primary poisoning of the eagles with contaminated bait or secondary
poisoning from contaminated piglets. This case indicates the
vulnerability of scavenging species like eagles.
4.2 Abiotic transformation
4.2.1 Hydrolytic cleavage and oxidation
Fenthion is transfomed slowly in sterile phosphate buffers, with
half-lives of 56, 41, and 32 days at pH 5, 7, and 9, respectively, at
25°C for 70 days (ChemAgro, 1976c); comparable results were found at 5
and 40°C. These results indicate slower transformation rates under
more acid conditions. This pH-dependent transformation was confirmed
in other studies (eg. Bayer AG, 1983a). In the experiments of ChemAgro
(1976c) at 25°C, the main transformation products were fenthion
sulfoxide, 3-methyl-4-methylsulfinyl-phenol, fenoxon (phosphoric acid,
O,O-dimethyl- O-[3-methyl-4-methylthio-phenyl] ester), fenoxon
sulfone (phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methyl-
sulfonyl-phenyl] ester), fenthion sulfone, and fenoxon sulfoxide
(phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfinyl-
phenyl] ester). The maximal percentages of the administered activity
were 14, 12, 10, 8, 7, and 6%, respectively. The transformation
products indicate both oxidation (fenthion sulfoxide, fenoxon, fenoxon
sulfone, fenthion sulfone, and fenoxon sulfoxide) and hydrolysis
(3-methyl-4-methylsulfinyl-phenol).
A low abiotic transformation rate in the dark was confirmed in
other laboratory experiments (ChemAgro, 1972; Bayer AG, 1983a). In
buffered aqueous solutions, the extrapolated half-lives at 22°C were
223, 200, and 151 days at pH 4, 7, and 9, respectively (Bayer AG,
1983a), and the transformation product was 3-methyl-4-methylthio-
phenol. In aqueous phosphate buffers at 30°C, the experimental
half-lives at pH 5, 7, and 9 were 31, 26, and 24 days, respectively
(ChemAgro, 1972).
4.2.2 Phototransformation
(a) Water
Photochemical transformation may occur rapidly in water under
both laboratory and field conditions. In a sterile aqueous buffer at
pH 5, substantial phototransformation of 13C/14C-1-ring-labelled
fenthion was observed after exposure to artificial light simulating
sunlight for 4 h at 23°C (Mobay Corp., 1987c); the half-life was
0.5 h. Rapid phototransformation in the laboratory was confirmed by
Bayer AG (1983b, 1988c) and ChemAgro (1976b).
The major photochemical transformation products in the study
reported by the Mobay Corp. (1987c) were 3-methyl-4-methylthio-phenol
(maximal totally recovered activity, 23%), fenthion sulfoxide
(maximum, 17%), and 3-methyl-4-methylsulfinyl-phenol (maximum, 15%).
Transformation products found in minor quantities in this experiment
were 3-methyl-4-sulfo-phenol (maximum, 8%), fenoxon sulfone (maximum,
7%), fenoxon sulfoxide (maximum, 6%), and 3-methyl-4-methylsulfonyl-
phenol (maximum, 6%). Fenoxon was also found as a phototransformation
product in other experiments (maximum, 10-11% of the applied activity)
(ChemAgro, 1976b).
The phototransformation pattern is temperature-dependent: at
25°C, the rate of production of fenthion sulfoxide via oxidation is
comparable to that of 3-methyl-4-methylthio-phenol via hydrolysis.
Oxidation to fenoxon sulfoxide is assumed to prevail at higher
temperatures and hydrolysis to 3-methyl-4-methylthio-phenol at lower
temperatures (ChemAgro, 1976d). When 2% acetone was added to an
aqueous solution of fenthion as a photosensitizer before irradiation
with artificial light for 2 h, oxidation via fenthion sulfoxide to
unknown polar transformation products was accelerated. Thus, changes
in temperature and the presence of sensitizers can result in different
mixtures of fenthion transformation products.
Fenthion also undergoes rapid phototransformation outdoors,
although possibly at a somewhat slower rate than in laboratory
experiments. Fenthion in distilled water exposed to sunlight in the
summer was phototransformed with a half-life of 4 h (Bayer AG, 1983b);
in a comparable experiment under laboratory conditions with artificial
light, the half-life was 0.08 h. Fenthion is phototransformed rapidly
in sunlight (lambda > 290 nm), primarily because of its high quantum
yield.
(b) Soil
As fenthion applied to soil appears to be phototransformed
rapidly by sunlight, with half-lives of 1-> 4 days (see Table 4),
phototransformation is an important route of dissipation. In an
outdoor experiment, 13C/14C-1-ring-labelled fenthion mixed with
unlabelled fenthion was exposed for 1.2 days to natural sunlight after
application to a sandy loam at a rate of 53 mg/kg soil (see also Table
4). Fenthion was rapidly phototransformed, and the main photochemical
transformation product was fenthion sulfoxide (maximum, 58% of the
recovered activity); fenoxon sulfoxide, 3-methyl-4-methylsulfinyl-
phenol, and fenthion sulfone were minor transformation products (i.e.
< 10% of the recovered activity). The oxidation path apparently
prevails (Mobay Corp., 1987b). As the temperature during this
experiment rose to about 37°C, a shift to the oxidation rather than to
the hydrolysis pathway seems to have occurred, as in the
phototransformation experiments with water (ChemAgro, 1976d).
(c) Air
Phototransformation occurs rapidly in the troposphere. The
half-life of fenthion in air is estimated to be 1.7 h (Bayer AG,
1994a) on the basis of the assumption that phototransformation occurs
via the reaction of hydroxyl radicals with fenthion. The primary
target of the radicals is probably the P=S bond. Such attacks may
result in secondary oxidation products (e.g. fenoxon), which can be
deposited on soil, water, and vegetation by wet and dry deposition.
4.3 Biotransformation
Biotransformation may contribute to the general processes of
dissipation (see section 4.1). No data from primary sources on the
microorganisms that can metabolize fenthion have been incorporated in
this monograph. It has been reported to be biotransformed to fenoxon
sulfoxide by the soil fungus Rhizopus japonicus (Wallnöfer, 1978,
cited by Mobay Corp., 1978b). Similarly, no data on the biodegrad-
ability of the adjuvants in formulations of fenthion have been
incorporated; however, it can be assumed that the biodegradability of
e.g. 'fog' and diesel oil in a natural environment is slight.
A simplified scheme, showing some common transformation products
in environmental compartments, is presented in Figure 1. The scheme
includes both abiotic and biotic transformation products.
4.3.1 Water and sediment
The relevant laboratory experiments in which biotransformation in
water-sediment systems was studied were summarized in Table 3. The
results indicate that the rate of biotransformation is rapid under
both aerobic and anaerobic conditions: 50% biotransformation of
fenthion (half-life) in a whole water-sediment system occurs within
about 10 days. Experimental conditions other than the availability of
oxygen, e.g. temperature, salinity, the presence of plants, the type
of water, and the type of sediment, probably have less effect on the
transformation rate. In microcosms of salt-marsh water, sediment and
sediment-rooted plants, however, the amount of detritus and the extent
of bioperturbation of the sediment may also influence the biotrans-
formation rate (O'Neill et al., 1989).
In general, sediments appear to function as a major sink for
fenthion under both aerobic and anaerobic conditions. Rapid
partitioning to sediment was seen under aerobic and anaerobic
conditions, as 21-28% of the applied activity was recovered in the
sediment within 1 h after application (Bayer AG, 1988a,b); maxima of
41-80% of the applied activity were recovered in sediment. Under
aerobic conditions, the amount in the sediment increased gradually up
to 62-80% of the applied activity (Bayer AG, 1988a) by the end of the
test at 66 days. Under anaerobic conditions, the maxima were 66-70%
and were reached after 7-14 days (Bayer AG, 1988b). The total amounts
of activity in the sediments decreased thereafter, probably due to
further mineralization to 14C-carbon dioxide (see below). Under
either aerobic or anaerobic incubation, most of the activity in the
sediment shifted from water-and acetone-extractable compounds to
non-extractable (i.e. sediment-bound) residue in the course of the
experiments.
The rate of biotransformation in non-sterile sediment without
plants was best modelled by assuming biotransformation in the upper
1 mm of the sediment (O'Neill et al., 1989). When plants were
present, biotransformation in the upper 7 mm was the best assumption.
In aerobic experiments with water and associated sediments, the
amount of fenthion declines rapidly over time, with transient
increases in various metabolites, an increase in 14C-carbon dioxide,
and an increase in sediment-bound residues (Bayer AG, 1988a,b). Under
anaerobic conditions, a small amount (3-4%) of 14C-methane was
formed. The production of 14C-carbon dioxide appears to be more
rapid under aerobic than anaerobic conditions, but the final amounts
of 14C-carbon dioxide differed substantially with the duration of
the test: 12-15% after 66 days under aerobic conditions and 52% after
120-190 days under anaerobic conditions. As the amount of oxygen was
very low under anaerobic conditions (0-0.4 mg/litre), the oxygen in
the carbon dioxide may have been supplied by nitrates or sulfates. The
redox potential during the experiment varied between -186 and 137 mV.
The main transformation products were demethyl fenoxon sulfoxide
(maximum, 30% of whole water-sediment system after 7-14 days),
fenthion sulfoxide (maximum, 28% after 14 days), 3-methyl-4-methyl-
sulfinyl-phenol (maximum, 13% after 66 days), and 3-methyl-4-methyl-
sulfonyl-phenol (maximum, 11% after 66 days) under aerobic conditions,
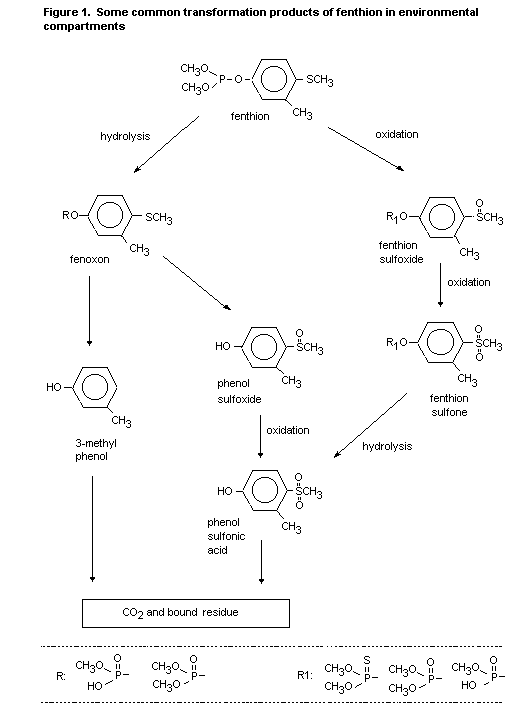 and 3-methyl-4-methylthio-phenol (maximum, 35% after 60 days),
3-methyl-4-methylsulfinyl-phenol (maximum, 26% after 30 days), and
3-methyl-phenol (maximum, 10% after 60 days) under anaerobic
conditions. In these experiments, only the moieties with a phenyl
group were qualified or quantified; the fate of the thiophosphoric
acid moiety is less well described. Eichelberger and Lichtenberg
(1971) assumed that O,O-dimethyl- O-thiophosphoric acid was formed
by hydrolysis.
4.3.2 Soil
Fenthion appears to be biotransformed rapidly in soil under
aerobic conditions and more slowly under anaerobic conditions: the
half-life of 13C/14C-1-ring-labelled fenthion in a silty loam soil
was 10 days under aeobic conditions and more than 32 days under
anaerobic conditions (Mobay Corp., 1978b; see Table 4). The latter
value is not reliable, however, as the anaerobic incubation was
started when about 99% of the parent molecule had been biotransformed
aerobically. These experiments and some important experimental
conditions are summarized in Table 4, which indicates that fenthion is
transformed rapidly under optimal conditions, such as sufficient
oxygen and a moisture content > 75% of the field capacity. Rapid
transformation of fenthion under aerobic conditions was confirmed by
Bayer AG (1974), with half-lives of 1.7 and 0.5 days in a sand and a
sandy loam soil. These results are somewhat unreliable, however, as
the moisture content of the soils during the tests was not reported
and they may have been stored under overly dry conditions. The rate of
biotransformation of fenthion in soils can be described by linear
first-order kinetics, although few studies are available for
verification.
The main metabolites of fenthion under aerobic conditions are
fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol, and 3-methyl-
4-methylsulfonyl-phenol (Mobay Corp., 1978b). In laboratory
experiments, the maximal amounts of these metabolites in silt loam
were 30-33, 15-18, and 30-31%, respectively, of the total activity.
These maxima were reached within 14 days after application at rates of
1-10 mg/kg dry weight. Substantial amounts of these metabolites appear
to be formed microbially, as only fenthion sulfoxide and to a lesser
extent 3-methyl-4-methylsulfonyl-phenol were formed under sterile
conditions in the same soil; they were formed more slowly under
sterile conditions. Some minor metabolites quantified in these
experiments were fenthion sulfone and 1-methoxy-3-methyl-4-methyl-
sulfonyl-benzene, which were found at 4-8 and 4-6%, respectively, of
the total activity within 59 days after application. It should be
noted that the formation of 1-methoxy-3-methyl-4-methylsulfonyl-
benzene indicates methylation.
Under aerobic conditions, the amounts of soil-bound residues
gradually increased up to a plateau of 41% of the total activity 30
days after application and continued until the end of the experiment
after 120 days (Mobay Corp., 1978b). These soil-bound residues were
due mainly to microbial action, as under sterile conditions only 9%
was recovered as soil-bound residues 30 days after application.
Mineralization to carbon dioxide occurs in soil in the laboratory
under both aerobic and anaerobic conditions, although more slowly
under the latter conditions. After application of 13C/14C-1-ring-
labelled fenthion at 1 mg/kg dry weight to a silt loam soil under
aerobic conditions in the laboratory, about 50% 14C-carbon dioxide
evolved within 120 days (Mobay Corp., 1978b). In the same soil under
anaerobic conditions, the mineralization rate was substantially lower
than that under aerobic conditions after aerobic preincubation for 30
days; during 32 days of anaerobic incubation, only 4% of the total
activity was degraded to 14C-carbon dioxide.
4.4 Bioconcentration
Fenthion is a lipophilic compound which is only slightly soluble
in water and has an octanol-water partition coefficient (log Kow) of
4.8. These properties indicate possible bioconcentration, as confirmed
in a laboratory experiment in which fenthion was shown to be
moderately concentrated in fish (ChemAgro, 1975a).
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 14C-fenthion at 0.008-0.12 mg/litre
(actual concentrations) for 14 days, the calculated daily biocon-
centration factors based on the wet weight of the whole fish increased
from 226 by 0.2 days after the start of the test to 400-500 by 4-7
days (ChemAgro, 1975a); after 14 days of exposure, the daily
bioconcentration factors were 200-300. The maximal concentrations of
labelled residues in the whole fish were 58 mg/kg wet weight four days
after the start. When the fish were subsequently exposed to uncontam-
inated water, all of the residues were eliminated within 11 days.
Eleven days after the start of exposure, 62% of the activity recovered
from a dissected fish was water-soluble and 38% chloroform-soluble;
the latter fraction contained fenthion (as 73% of the activity, in the
fraction), fenthion sulfoxide (20%), 3-methyl-4-methylsulfinyl-phenol
(5%), and fenoxon sulfoxide (2%). Seventy percent of the labelled
residues in one exposed fish was recovered in the viscera, but it was
unclear to what extent transformation products had been formed. These
products can be formed in water by photo- and biotransformation (see
above).
Bluegill sunfish (Lepomis macrochirus) and channel catfish
(Ictalurus punctatus) were exposed under static conditions to a
single dose of 14C-ring-labelled fenthion at a concentration of
about 3.7 µg/litre in aquariums containing both water and sediment and
were observed for 28 days. The calculated maximal daily biocon-
centration factors were 118 and 115 after 8 and 24 h for the sunfish
and the catfish, respectively. The maximal amount of labelled residues
in both species was 0.3 mg/kg bw 8-24 h after application. No residues
were recovered in fish after 21 days. The maximal amounts were not
related to the maximal depressions in brain cholinesterase activity,
which were 97% after three days for the sunfish and 76% after six days
for the catfish. There appeared to be a lag of a few days between
exposure and effects. The fish appeared to have recovered 21 days
after application (ChemAgro, 1975a).
De Bruijn & Hermens (1991) confirmed that fenthion is moderately
concentrated in fish. They found a bioconcentration factor of 170 in
guppies (Poecilia reticulata) exposed for 11 days to a mixture of
toxicants, including fenthion. Uptake and elimination were equili-
brated after three days.
5. Environmental levels
The concentrations of fenthion in the environment are summarized
in Table 5. Few measurements are available from regular monitoring
programmes, except in the Netherlands, where fenthion was detected in
various fresh and marine surface waters in 1992. The only published
data on residues of fenthion on vegetation and insects after aerial
application of the pesticide are derived from monitoring after
application of 2.4 kg a.i./ha to two sites for control of weaver birds
(Quelea quelea) in Kenya (Bruggers et al., 1989). The applications
were the first to be used in the area to control birds. The
concentrations of residues on young birds were 44 and 84 µg per bird
on the two sprayed areas, respectively, on day 1 and about 10 µg per
bird cm days 3-4. Raptors that ate the birds were not killed, but some
were debilitated; the concentrations in the raptors were not measured.
Aggregate samples of insects contained fenthion at 7.2 mg/kg; the
highest concentration of residues was found in carabid beetles (23 µg
per beetle). The concentrations on grass were 39 and 28 mg/kg on the
two sprayed areas, respectively, on the first day after spraying and
1.9 and 1.1 mg/kg on day 44. A sample of millet seed spread on a mesh
in a sprayed area contained fenthion at 1.1 mg/kg.
Owing to the scarcity of data from monitoring, Table 5 also shows
some actual measurements in the field from experiments performed with
recommended application rates simulating common agricultural practice.
Only maximal amounts are tabulated as indicative values, and data on
the rate at which fenthion dissipated, when available, are mentioned
in the comments (see also sections 4.1 and 4.13). No data were
available on the occurrence of transformation products.
6. Effects on organisms in the laboratory and the field
6.1 Laboratory experiments
6.1.1 Microorganisms
(a) Water
The acute and chronic toxicity of technical-grade fenthion and a
formulation of fenthion to aquatic microorganisms and invertebrates
are summarized in Tables 6 and 7. Technical-grade fenthion is toxic to
highly toxic, with four-day EC50 values of 550-1800 µg/litre and
four-day NOEC values of 100-1120 µg/litre. One formulation of fenthion
is toxic, with four-day EC50 values of 1500-> 2000 µg/litre. The
toxicity of fenthion to e.g. algae is dependent on the species or
strain tested (Wängberg & Blanck, 1988). High toxicity for aquatic
organisms was confirmed by Saini & Saxena (1986), who reported a
six-day NOEC of 100 µg/litre for the freshwater protozoan Tetrahymena
pyriformis. High toxicity was also reported for some saltwater
microorganisms, with one-day EC50 values of 1000 µg/litre for the
alga Cyclotella nana and 100 µg/litre for the diatom Skeletonema
costatum and a one-day NOEC of 10 µg/litre for Cyclotella nana,
based on production of oxygen (Derby & Ruber, 1971).
The toxicity of fenthion for microorganisms in activated sludge
was tested in a laboratory experiment for 30 min at 20°C (Bayer AG,
1994b). The sludge had been collected in a plant treating domestic
waste. Only at the highest nominal dose of fenthion, 10 000 mg/litre,
was respiration inhibited substantially. The test is of limited value,
however, as the nominal test concentrations exceeded the water
solubility by up to 2380 times.
(b) Soil
No unequivocal indications of deleterious effects of fenthion on
microorganisms in the soil are available. The formulation Lebaycid
inhibits neither processes involved in the nitrogen transformation
cycle nor enzymes involved in microbial activity. It did not affect
soil respiration or nitrification in sandy loam or silt loam soils
after application at the recommended rate, 0.75 kg/ha, or a rate five
times higher, 3.8 kg/ha (Bayer AG, 1989a,b). Soil respiration was
measured over 28 days after amendment with glucose (Bayer AG, 1989a),
and incubation was at 20°C in the dark. The effects on nitrification
were analysed by measuring ammonium, nitrite, and nitrate up to and
including 28 days after application (Bayer AG, 1989b). Only slight,
transient increases in nitrate were observed in the silt loam soil and
decreases in the sandy loam soil. The soils had not been treated with
pesticides for one to two years previously.
Table 5. Maximal concentrations of fenthion in environmental water, soil, sediment, and biota
Sample Location Year Period Concentration Comments Reference
Kenya
Carabid Kulalu 1985 April-May 23 µg/beetle Application rate, 1.5-2.4 kg/ha; Bruggers et al.
beetle Ranch mean in insects, 7.2 mg/kg (1989)
Savannah Kulalu 1985 April-May 38 mg/kg Application rate, 1.5-2.4 kg/ha;
grass Ranch whether based on dry or wet Bruggers et al.
weight not explicitly reported; (1989)
amount decreased to 1.9 and 1.1 mg/kg
after 3 and 4 days, respectively
Millet Kulalu 1985 April-May 1.1 mg/kg Application rate, 1.5--2.4 kg/ha; Bruggers et al.
seed Ranch whether based on dry or wet weight (1989)
not explicitly reported; deliberate
overspraying of artificial plot
Netherlands
Fresh Large rivers 1992 0.01-0.12 Measured in pumping station; fenthion Van Meerendonk et al.
surface and lakes µg/litre detected in 25% of freshwater locations (1994)
water at a mean concentration of
0.022 µg/litre
Marine North Sea, 1992 0.01 µg/litre Fenthion detected in 25% of freshwater Van Meerendonk et al.
surface Waddensea locations at a mean concentration of (1994)
water 0.02 µg/litre
Table 5. (cont'd).
Sample Location Year Period Concentration Comments Reference
United States
Salt-marsh Indian River 1984 September 1.7 µg/litre No fenthion detectable (< 0.01 µg/litre) Wang et al.
surface County, Florida within 24-48 h of aerial application (1987)
water of 0.032 kg a.i./ha
Salt-marsh Indian River 1985 March < 0.01 No explanation for low concentration; Wang et al.
surface County, Florida µg/litre deposition was confirmed, as up to 0.5 (1987)
water µg/litre was detected in dishes with
an aqueous medium; application rate,
0.032 kg a.i./ha
Salt-marsh Indian River 1985 June 0.16 No fenthion detectable (<0.01 µg/litre) Wang et al.
surface County, Florida µg/litre within 24-48 h of aerial application of (1987)
water of 0.032 kg a.i./ha
Polia larvae Laramie, 1979 Summer 0.28 mg/kg 8 h after spraying in field experiment Powell
(bird feed) Wyoming at an application rate of 52 g/ha; no (1984)
fenthion detected 30 h after treatment
Table 6. Acute toxicity of fenthion and two metabolites to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Crustacean
Daphnia magna, Static Technical Reconstituted 8.0 10 20 2 EC50, 0.0057a,b Bayer (1985a)
first instar
Fish
Onchorhynchus mykiss, Continuous Technical Well 8.0-8.1 225-275 12-13 4 LC50, 0.83b,c,d ABC Inc.
0.4 cm, 0.8 g (1986a)
Lepomis macrochirus, Continuous Technical Well 8.1-8.2 225-275 21 4 LC50, 1.7b,c ABC Inc.
5.4 cm, 1.9 g (1987)
Onchorhynchus mykiss, Static Lebaycid Tap 7.5-7.9 284 16 4 LC50, 2.3b,d Bayer (1994c)
5.5-6 cm, 1.5 g (adapted)
Leuciscus idus, Static Lebaycid Tap 7.2-7.9 284 21 4 LC50, 4.3b,d Bayer (1994d)
5.5 cm, 1.5 g (adapted
Onchorhynchus mykiss, Static Psx Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Onchorhynchus mykiss, Static Psn Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Lepomis macrochirus, Static Psx Well 7.3-7.6 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Lepomis macrochirus, Static Psn Well 72-7.9 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Psx, 3-methyl-4-methylsulfinyl-phenol; Psn, 3-methyl-4-methylsulfonyl-phenol
a Based on immobilization
b As phototransformation occurred, value is based on a mixture of fenthion residues
c Measured concentrations
d Actual concentration
Table 7. Long-term toxicity of fenthion to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Green algae
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 550a,b Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 1800a,c Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 NOEC, 100a,b Bayer (1985a)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, 1500a,b Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, > 2000a,c Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 NOEC, 1120a,b Bayer (1989c)
subspicatus
Selenastrum Static Baytex Artificial NR 1.5 24 4 EC50, 1100a,c,d,e ABC Inc. (1986b)
capricornutum
Selenastrum Static Baytex Artificial NR 1.5 24 4 NOEC, 700a,c,d,e ABC Inc. (1986b)
capricornutum
Crustaceans
Daphnia magna, Continuous Lebaycid Artificial 7.5-8.3 204 20 21 NOEC, 0.018d,e,f,g TNO (1989)
first instar
Daphnia magna, Continuous Technical Well 8.1-8.3 206-275 20 21 MATC, Bayer (1985b)
first instar 0.042-0.082d,e,h
Table 7. (cont'd).
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Fish
Oncorhynchus Continuous Technical Well 6.6-7.8 29-30 12 88 MATC, 13-27d,e,i SLS Inc. (1988)
mykiss
NR, not reported
a As phototransformation occurred, the value is for a mixture of fenthion residues
b For decrease in biomass
c For inhibition of growth rate
d Measured concentration
e Actual concentration
f All concentrations below the detection limit of 0.1 µg/litre
g For reproduction
h For mortality, growth, and reproduction
i For embryo viability, embryo survival at hatch, survival, and growth of larvae
6.1.2 Aquatic organisms
(a) Plants
Stimulation but no decrease in biomass was observed in an
experiment in which duckweed (Lemna gibba) was exposed in an
artificial medium to the formulation Baytex at measured concentrations
up to 2.8 mg/litre for 14 days at 25°C (Malcolm Pirie, 1987). The
stimulation may be related to hormesis.
(b) Invertebrates
Technical-grade fenthion is highly toxic to the crustacean
Daphnia magna, with a two-day EC50 value of 5.7 µg/litre and a
21-day maximal acceptable toxicant concentration (MATC) of 0.042-0.082
µg/litre. The formulation Lebaycid is also highly toxic to this
species, with a 21 -day NOEC value of 0.018 µg/litre. Static and
chronic treatment of other invertebrate species provide more variable
results, with two- to four-day L(E)C50 values of 0.62-1800 µg/litre
for various freshwater molluscs, insects, and crustaceans, 17- to
32-week NOEC values of 1000-2000 µg/litre for the freshwater mollusc
Lymnea stagnalis, one- to four-day L(E)C50 values of 0.02-550
µg/litre for saltwater molluscs, insects, and crustaceans, and 8- to
20-day NOEC values of 0.037-0079 µg/litre for the saltwater crustacean
Mysidopsis bahia (e.g. Seugé & Bluzat, 1980; Mayer, 1986; Mayer &
Ellersieck, 1986; McKenney, 1986). These tests were performed with
technical- or analytical-grade fenthion.
(c) Vertebrates
The acute and long-term toxicity of technical-grade fenthion, the
metabolites 3-methyl-4-methylsulfinyl-phenol and 3-methyl-4-methyl-
sulfonyl-phenol, and a formulation of fenthion to aquatic vertebrates
is summarized in Tables 6 and 7. Technical-grade fenthion is
moderately to highly toxic to freshwater fish, with four-day LC50
values of 0.83-1.7 mg/litre and an 88-day MATC value of 13-27
µg/litre. The formulation Lebaycid is toxic, with four-day LC50
values of 2.3-4.3 mg/litre. The metabolites 3-methyl-4-methyl-
sulfinyl-phenol and 3-methylsulfonyl-phenol are very slightly toxic to
freshwater fish, with four-day LC50 values exceeding 100 mg/litre.
Other tests show moderate to high toxicity of technical-grade fenthion
(e.g. Korn & Earnest, 1974; Mayer & Ellersieck, 1986; WHO, 1986
[original references not checked], with two- to four-day LC50 values
of 1-3.4 mg/litre for freshwater fish and 0.45-2.5 mg/litre for
saltwater fish.
Fenthion is highly toxic for amphibians: Khangarot et al.
(1985) reported a four-day LC50 value of 0.84 µg/litre for the frog
Rana hexadactyla with a length of 20 mm.
Many sublethal effects have been observed in fish after exposure
to a wide range of concentrations, including bottom disorientation,
loss of equilibrium, discolouration, rapid respiration, bloated
stomach, gulping of air, quiescence, surfacing, and irregular swimming
behaviour (ABC Inc., 1986; SLS Inc., 1988; Bayer AG, 1994c,d).
6.1.3 Terrestrial organisms
(a) Plants
No data were available.
(b) Invertebrates
Technical-grade fenthion is obviously toxic to various
terrestrial insects. Few studies have addressed other terrestrial
invertebrates.
Fenthion appears to be toxic to honey bees (Apis mellifera),
with contact LD50 values of 0.16-< 2 µg/bee (Bayer AG, 1995
[original references not checked]). No data were available on the
toxicity of fenthion for honey bees after oral exposure, but it would
be expected to be toxic. It is also toxic for predators of pests such
as wasps, mites, hoverflies, and green lacewings tested at moderately
high temperatures (22-27°C during the day and night and 16-18°C at
night).
Fenthion is highly toxic to a microhymenopterous wasp,
Trichogramma caoeciae, that parasitizes the eggs of the parasite
Sitrotroga cereallella (Conrad Appel GmbH, 1989). In one experiment,
larvae were exposed to Lebaycid sprayed onto glass plates at a rate of
16 kg/ha as fenthion. All of the larvae died within 24 h. In a second
experiment, host eggs containing pupae did not hatch after they had
been dipped in an aqueous solution of 0.1% Lebaycid.
Fenthion is also highly toxic to the predating mite Phytoseiulus
persimilis Athias-Henriot (von Kniehase & Zoebelein, 1990). Male and
female mites were placed on the leaves of kidney beans (Phaseolus
vulgaris) to produce a small population, and five days later the
leaves were dipped into a solution of 0.075% Lebaycid EC 500 at the
highest recommended application rate. All mites were killed within
seven days. Treatment with 0.015% Lebaycid EC 500 did not kill all of
the mites.
All early-instar larvae of the aphid predating hover fly
(Episyrphus balteatus) died within 24 h after spraying of Lebaycid
500 EC on glass plates at a rate of 0.77 kg/ha as fenthion (University
of Southampton, 1992).
Fenthion was highly toxic to the larvae of the green lacewing
(Chrysoperla carnea) exposed by contact to Lebaycid at 12 kg/ha as
fenthion in a light-dark cycle for three to five days (GAB Biotech-
nologie, GmbH, 1991). The rate of mortality was 100% of larvae and
unhatched imagos and only 7% of controls.
Artificial soil contaminated with Lebaycid 50EC was slightly
toxic to earthworms (Eisenia andrei) (Bayer AG, 1989d), with a
14-day LC50 of 723 mg/kg soil dry weight and a dose-related weight
loss. The 14-day NOEC was thus 100 mg/kg dry weight of soil. As the
vessel containing the worms was lit continuously, some photo-
degradation may have occurred.
(c) Vertebrates
The acute and subacute toxicity of fenthion to birds is
summarized in Table 8.
Technical-grade fenthion is moderately toxic or toxic to birds,
with an LD50 value of 7.2 mg/kg bw and an eight-day LC50 value of
60-1259 mg/kg feed. Five- to 10-day LC50 values of 25-231 mg/kg feed
were reported for the mallard duck (Anas platyrhynchos), the
bobwhite quail (Colinus virginianus), the Japanese quail (Coturnix
japonica), and the domestic chicken (Gallus domestica) (Hill
et al., 1975; Royal Society of Chemistry, 1995). Fenthion appears to
be toxic after dermal application in several bird species, with LD50
values of 1.8 mg/kg bw for the weaver bird (Quelea quelea) and
2.4 mg/kg bw for the house sparrow (Passer domesticus) (Schafer
et al., 1973).
The sublethal effects observed in birds treated with fenthion
experimentally are wing drop, ataxia, salivation, reduced body weight,
tremors, convulsions, diarrhoea, and laboured breathing (Grue, 1982;
Mobay Corp., 1987c,d,e). Necropsy of poisoned birds showed fluid-
filled crops and intestines, mottled spleens (possible due to organ
hypostasis), and oily fluid in crops or gastrointestinal tract,
indicating incomplete absorption of fenthion. Common grackles
(Quiscalus quiscula) found dead after five days' exposure to fenthion
had lost 28-36% of their initial weight, and muscle tissue on the
breastbone and observable fat had decreased substantially (Grue,
1982). The reduced body weights were due to treatment-related
reductions in feed consumption, perhaps indicating that fenthion is a
repellent, probably because of its strong mercaptan odour. The
estimated daily intake remained constant and was thus independent of
the concentrations tested.
Table 8. Acute and subacute toxicity of technical-grade fenthion given to birds for eight days
Species Sex Age Route Result Reference
Mallard duck (Anas platyrhynchos) Not reported 9 days Diet LC50, 1259 mg/kg feed Mobay Corp. (1987c)
Bobwhite quail (Colinus virginianus) Not reported 10 days Diet LC50, 60 mg/kg feed Mobay Corp. (1987d)
Bobwhite quail (Colinus virginianus) Male, female 18 weeks Oral LD50, 7.2 mg/kg bw Mobay Corp. (1987e)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, 57 mg/kg feeda Grue (1982)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, < 30 mg/kg feedb Grue (1982)
a Birds caged outdoors 20-27 July 1978
b Birds caged outdoors 14-21 August 1978
A decrease in cholinesterase activity in the brain and plasma is
an important physiological characteristic of avian poisoning by
fenthion. Common grackles that had been fed fenthion at rates of
25-400 mg/kg feed showed a significant, 80% depression in brain
cholinesterase activity. All birds had died before enzyme analysis
(Grue, 1982).
Fenthion appears to be less acutely toxic to terrestrial mammals
than to birds. Kenaga (1979) reported an LD50 range of 255-298 mg/kg
bw for the rat (Rattus norvegicus), indicating moderate toxicity.
FAO/WHO (1973) reported a three-month LOEC value of 0.25 mg/kg diet
based on growth reduction. The highest dietary levels that caused no
toxicological effect were estimated to be 3 mg/kg feed for rats and
2 mg/kg feed for dogs.
6.2 Field observations
6.2.1 Microorganisms
(a) Water
A single application of fenthion at a rate of 0.22 kg/ha did not
affect phytoplankton in small artificial outdoor ponds with natural
surface water and sediment in experiments performed in Florida (USA)
in 1962-63 (Patterson & von Windeguth, 1964).
(b) Soil
No data were available.
6.2.2 Aquatic organisms
(a) Plants
No data were available.
(b) Invertebrates
The populations of crustaceans and chironomids disappeared within
one week after application of fenthion to small artificial outdoor
ponds with natural surface water and sediment at a rate of 0.22 kg/ha
in experiments performed in Florida in 1962-63 (Patterson & von
Windeguth, 1964). The period required for complete recovery of the
numbers of water fleas and midge larvae was four to five months during
a cold winter period and two months in the absence of a cold period.
There were substantially more oligochaetes in treated than in
untreated ponds. Other aquatic invertebrates such as snails,
ostracods, copepods, and Hydra appeared to be unaffected by the
treatment.
(c) Vertebrates
Application of fenthion to small artificial and outdoor ponds at
a rate of 0.22 kg/ha did not appear to affect fish or tadpoles
(Patterson & von Windeguth, 1964).
6.2.3 Terrestrial organisms
(a) Plants
No data were available
(b) Invertebrates
As fenthion is highly toxic to insects in the laboratory, it can
be expected to be so in the field under certain conditions. Its
toxicity is linked mainly to the extent of exposure. In field
experiments in Assiut (Egypt) between June and August at the end of
the 1960s, the formulation Lebaycid, sprayed at a rate of 2.9 kg/ha
(as fenthion) four times at intervals of 15 days with back-pack
spraying equipment, was shown to be toxic to two pest predatory
coccinellids (Coccinella undecimpunctata and Scymnus interruptus)
in cotton (von Afify et al., 1970). Although the formulation was
generally toxic to imago beetles, there was substantial temporal and
spatial variation. The mortality of Coccinella was 58-79% (percentages
corrected for mortality in controls) within three, days of application
and 61-96% within 10 days of application. The mortality of Scymnus
was 55-83% within three days and 28-80% within 10 days. These figures
show partial recovery, at least of Scymnus; Coccinella may be more
susceptible, or Scymnus may recolonize and re-emerge. There was no
clear indication of increasing adverse effects in either species.
Field experiments in Los Baños (Philippines) in 1979-80 showed no
substantial adverse effects of fenthion on predators of the brown
planthopper (Nilaparvata lugens), a common rice pest in South and
Southeast Asia (Reissig et al., 1982). In these experiments,
fenthion was applied three times to plots of lowland rice at a rate of
0.75 kg/ha per treatment. Both planthoppers and their predators were
sampled one day before and two days after the treatments; the
predators sampled were the spiders Lycosa pseudoannulata, Tetragnatha
sp., and Araneus sp. and the hemipterous Microvelia atrolineata
and Cyrtorhinus lividipennis. The numbers of neither the
planthoppers nor their predators were significantly altered by the
treatments.
Field experiments in Egypt in 1965-66 showed that adverse effects
on pest predatory species may be present and absent in the same area
(Kira et al., 1972). Some predators of borers and aphids in corn
(Zea mays) appeared to increase in number after treatment of crops
with fenthion at a rate of 1.2 kg/ha per treatment, while some
decreased.
The mortality of honey (Apis mellifera) and alkali bees (Nomia
melanderi Cockerell) in outdoor cages, caused by the introduction of
alfalfa leaves sprayed with fenthion as an ultra-low volume liquid
with a hand-sprayer at a rate of 0.9 kg/ha, was decreased by 75% when
the contaminated leaves were brought into the cages 2 h after
application of fenthion instead of immediately afterwards (Johansen
et al., 1983), indicating that fenthion can be applied with minimal
hazard to bees that are not foraging.
(c) Vertebrates
Various incidents of poisoning of birds with fenthion have been
reported. It is generally assumed that a depression in brain
cholinesterase activity > 50% indicates severe exposure to fenthion
(e.g. Henny et al., 1987). In a field experiment in Kenya,
cholinesterase activity < 20 µmol/min per g was considered to
indicate exposure to fenthion and activities < 10 µmol/min per g to
indicate severe sickness or death (Bruggers et al., 1989).
On a wet meadow of 1.8 km2 in a field experiment in Wyoming
(USA) in 1978, 99 birds and 15 mammals were found dead or sick within
16 days after aerial treatment with fenthion at a rate of 47 g/ha
(DeWeese et al., 1983). As mortality is correlated with signifi-
cantly depressed brain cholinesterase activity in three common bird
species, it was assumed that the mortality and sickness were
treatment-related. A lower mortality rate seen on a comparable trial
site may have been due to factors such as different wind speeds and
overlap of spray swathes. The route of fenthion that induced poisoning
was unclear.
The maximal fenthion residues in dead American kestrels (Falco
sparverius) were correlated with cholinesterase activity depressions
of 78-92% in brain and 97% in plasma (Hunt et al., 1991). Within
three days after treatment of sparrow perches with fenthion, all of
the kestrels were dead. As the kestrels tended to hide in bushes as
paralysis advanced, the numbers of contaminated birds may have been
underestimated. The time for a kestrel to catch a sparrow was
positively correlated with the extent of cholinesterase depression in
the kestrel's brain. This indicates that a kestrel exposed to more
potent fenthion residues will have greater cholinesterase depression
or that kestrels with greater cholinesterase depression are less
capable of catching sparrows. There are indications that the more
fenthion sparrows are exposed to, the more sensitive they are to
kestrel predation (Hunt, 1990, cited by Kendall & Akerman, 1992).
Two of four flood-irrigated hay meadows were sprayed with the
formulation Baytex at 52 g/ha (as fenthion) in order to investigate
the effects on the reproduction of red-winged blackbirds (Anglaius
phoeniceus) in an experiment in Wyoming (USA) in June-July 1979
(Powell, 1984). In one of the treated fields, the growth rate of
nestlings was significantly decreased by 15%; however, the biological
interpretation of this effect is difficult, as cholinesterase activity
was not reduced. It appears to be a transient effect, since the
surviving nestlings recovered from the growth reduction. No
significant treatment-related effects on hatching, on fledgling
survival, or on foraging behaviour of adult blackbirds for nestling
care were observed. The latter parameter, investigated by counting the
number of trips per hour and their duration and distance, was
remarkably stable, as the abundance of the main feed type, the
caterpillar-like larva Polia, had been reduced by about 50% within
one to two days after the treatment.
The effect of fenthion on non-target birds was studied after
aerial application against the red-billed weaver bird (Quelea
quelea), a grain pest in Africa (Bruggers et al., 1989). In these
experiments, conducted in April-May 1985, two quelea colonies in a
savannah (Kulalu Ranch, Kenya) of low thornbush were sprayed at a rate
of 1.5-2.4 kg/ha. The brain cholinesterase activities that were
measured in 19 potentially exposed non-target bird species varied
substantially. Raptorial birds that were captured in or around the
quelea colonies, which had been radioequipped and were tracked up to
14 days after the treatment, were apparently not killed by the
treatment, but 70% had substantially depressed cholinesterase activity
in blood plasma, indicating actual exposure. Most raptorial birds were
assumed by the authors to have been exposed to fenthion by ingesting
contaminated quelea birds within one day after treatment. As no dead
radioequipped raptorial birds were recaptured, it may cautiously be
concluded that the risk of dying through secondary poisoning was
limited; however, the biological consequences for raptorial birds of
depressed cholinesterase activities with respect to other end-points
such as growth and reproduction are unclear.
The effects of fenthion on granivorous birds were also studied:
galliform species (Coturnix delegorguei, Gallus gallus, Pterocles
decoratus) and laughing doves (Streptopelia senegalensis) in cages
that were placed 100, 200, 300, and 400 m downwind of the treated
quelea colonies showed no mortality or signs of intoxication during an
observation period of five to seven days.
An additional experiment showed that the possibility of secondary
poisoning from scavenged contaminated carcasses cannot be excluded.
During the first night after the treatment, 24-90% of intentionally,
placed corpses were removed by scavengers such as jackals (Canis
mesomelas), bat-eared foxes (Otocyon megalotis), and baboons
(Papio cyanocephalus). This high rate of scavenging substantially
reduced the numbers of poisoned dead animals that could be collected,
resulting in an underestimation of the actual risks for non-target
birds.
7. Evaluation of effects on the environment
Fenthion is a systemic organophosphorus pesticide used for both
agricultural and nonagricultural purposes. Its major use is in the
control of insect pests (at an application rate of 1.25 kg a.i./ha),
but it is also used as a veterinary product to control external
parasites on domestic animals and to control birds (at an application
rate of 2.4 kg a.i./ha). Sprays for bird control are composed of a
formulation that is not available commercially and are used only under
governmental control. The pesticide is usually formulated as an
emulsifiable concentrate and is applied by ground spraying (80-85%) or
from the air (15-20%). It is also formulated as granules and as a
paste for control of urban birds. It can enter the environment beyond
the intended zone of treatment by spray drift, as residues on
potential food items such as birds, and, presumably, by loss of
surface residues from domestic animals.
Fenthion that enters the environment is adsorbed rapidly and
strongly on soil and sediment by a poorly understood mechanism. The
compound can be considered to be immobile in soil, and the parent
compound is unlikely to leach below the to firstfew centimetres of the
soil profile.
While photodegradation occurs under laboratory conditions, it is
not considered to be a significant route of breakdown in the field.
Hydrolysis occurs slowly and is dependent on pH; the half-lives of
fenthion in sterile buffers range from 30 to 60 days, with slower
degradation at low pH. Fenthion is degraded by microorganisms in
laboratory soil, with a half-life of 10 days; field degradation is
slower, about half of the compound being degraded to carbon dioxide
within six months in temperate climates. Generally, breakdown is
slower in water-sediment systems than in soil. Fenthion has been
measured in surface waters in untreated areas at concentrations up to
0.12 µg/litre. As the properties of fenthion would not result in its
entering the aquatic environment by leaching or run-off, there is no
satisfactory explanation for the aquatic residues found. It does not
bioaccumulate to a significant extent, despite its high solubility in
fat, since its metabolism and depuration in organisms are rapid.
Because fenthion is broken down rapidly in the environment, its
acute toxicity is relevant. Most species are not exposed chronically,
and its binding to soil and sediment particles would be expected to
reduce its bioavailability. Fenthion and formulations are toxic to
highly toxic to microorganisms, with four-day EC50 values of 550 and
1100 µg/litre. No data were available on its acute toxicity to algae,
but it is of low toxicity after long-term exposure. Screening tests
have indicated no phytotoxicity. Its acute and long-term toxicity to
crustaceans is high, with an EC50 value of 5.7 µg/litre and an NOEC
value of 0.018 µg/litre. Fenthion is very toxic to fish, with an
LD50 of 830 µg/litre and an MATC of 20 µg/litre. One study showed
toxicity to larvae at 0.84 µg/litre. Fenthion is moderately toxic to
birds, with an LD50 value of 7.2. µg/kg bw and a five-day LC50
value of 60 µg/kg feed. It is also toxic to honey bees, with a contact
LD50 of 0.16 µg per bee, and is slightly toxic to earthworms, with a
1-h LC50 of 723 µg/kg dry weight.
7.1 Risk assessment
Exposure concentrations have been derived from the results of
monitoring programs or field experiments simulating common agricul-
tural practice and from a simple calculation after spraying. The model
used for calculating the predicted environmental concentration (PEC)
is similar to that used currently by the US Environmental Protection
Agency, the Pesticides Safety Directorate in the United Kingdom, and
the Uniform System for the Evaluation of Substances in the
Netherlands. The method is presented in Figure 2. The data used in the
calculation are those from a two-year monitoring programme in 15
freshwater locations in the Netherlands where fenthion was detected;
however, the sources of these emissions are not clear. No other data,
such as concentrations in surface water, were available. The results
used in the hazard evaluation are those of field experiments of aerial
application of fenthion for mosquito control. No data were available
from field experiments on concentrations in surface water or residues
in sediment.
The concentrations resulting from long-term exposure are assumed
to result from dissipation in accordance with first-order kinetics:
(a) monitoring programme in salt-marsh (see Table 9)
and 3-methyl-4-methylthio-phenol (maximum, 35% after 60 days),
3-methyl-4-methylsulfinyl-phenol (maximum, 26% after 30 days), and
3-methyl-phenol (maximum, 10% after 60 days) under anaerobic
conditions. In these experiments, only the moieties with a phenyl
group were qualified or quantified; the fate of the thiophosphoric
acid moiety is less well described. Eichelberger and Lichtenberg
(1971) assumed that O,O-dimethyl- O-thiophosphoric acid was formed
by hydrolysis.
4.3.2 Soil
Fenthion appears to be biotransformed rapidly in soil under
aerobic conditions and more slowly under anaerobic conditions: the
half-life of 13C/14C-1-ring-labelled fenthion in a silty loam soil
was 10 days under aeobic conditions and more than 32 days under
anaerobic conditions (Mobay Corp., 1978b; see Table 4). The latter
value is not reliable, however, as the anaerobic incubation was
started when about 99% of the parent molecule had been biotransformed
aerobically. These experiments and some important experimental
conditions are summarized in Table 4, which indicates that fenthion is
transformed rapidly under optimal conditions, such as sufficient
oxygen and a moisture content > 75% of the field capacity. Rapid
transformation of fenthion under aerobic conditions was confirmed by
Bayer AG (1974), with half-lives of 1.7 and 0.5 days in a sand and a
sandy loam soil. These results are somewhat unreliable, however, as
the moisture content of the soils during the tests was not reported
and they may have been stored under overly dry conditions. The rate of
biotransformation of fenthion in soils can be described by linear
first-order kinetics, although few studies are available for
verification.
The main metabolites of fenthion under aerobic conditions are
fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol, and 3-methyl-
4-methylsulfonyl-phenol (Mobay Corp., 1978b). In laboratory
experiments, the maximal amounts of these metabolites in silt loam
were 30-33, 15-18, and 30-31%, respectively, of the total activity.
These maxima were reached within 14 days after application at rates of
1-10 mg/kg dry weight. Substantial amounts of these metabolites appear
to be formed microbially, as only fenthion sulfoxide and to a lesser
extent 3-methyl-4-methylsulfonyl-phenol were formed under sterile
conditions in the same soil; they were formed more slowly under
sterile conditions. Some minor metabolites quantified in these
experiments were fenthion sulfone and 1-methoxy-3-methyl-4-methyl-
sulfonyl-benzene, which were found at 4-8 and 4-6%, respectively, of
the total activity within 59 days after application. It should be
noted that the formation of 1-methoxy-3-methyl-4-methylsulfonyl-
benzene indicates methylation.
Under aerobic conditions, the amounts of soil-bound residues
gradually increased up to a plateau of 41% of the total activity 30
days after application and continued until the end of the experiment
after 120 days (Mobay Corp., 1978b). These soil-bound residues were
due mainly to microbial action, as under sterile conditions only 9%
was recovered as soil-bound residues 30 days after application.
Mineralization to carbon dioxide occurs in soil in the laboratory
under both aerobic and anaerobic conditions, although more slowly
under the latter conditions. After application of 13C/14C-1-ring-
labelled fenthion at 1 mg/kg dry weight to a silt loam soil under
aerobic conditions in the laboratory, about 50% 14C-carbon dioxide
evolved within 120 days (Mobay Corp., 1978b). In the same soil under
anaerobic conditions, the mineralization rate was substantially lower
than that under aerobic conditions after aerobic preincubation for 30
days; during 32 days of anaerobic incubation, only 4% of the total
activity was degraded to 14C-carbon dioxide.
4.4 Bioconcentration
Fenthion is a lipophilic compound which is only slightly soluble
in water and has an octanol-water partition coefficient (log Kow) of
4.8. These properties indicate possible bioconcentration, as confirmed
in a laboratory experiment in which fenthion was shown to be
moderately concentrated in fish (ChemAgro, 1975a).
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 14C-fenthion at 0.008-0.12 mg/litre
(actual concentrations) for 14 days, the calculated daily biocon-
centration factors based on the wet weight of the whole fish increased
from 226 by 0.2 days after the start of the test to 400-500 by 4-7
days (ChemAgro, 1975a); after 14 days of exposure, the daily
bioconcentration factors were 200-300. The maximal concentrations of
labelled residues in the whole fish were 58 mg/kg wet weight four days
after the start. When the fish were subsequently exposed to uncontam-
inated water, all of the residues were eliminated within 11 days.
Eleven days after the start of exposure, 62% of the activity recovered
from a dissected fish was water-soluble and 38% chloroform-soluble;
the latter fraction contained fenthion (as 73% of the activity, in the
fraction), fenthion sulfoxide (20%), 3-methyl-4-methylsulfinyl-phenol
(5%), and fenoxon sulfoxide (2%). Seventy percent of the labelled
residues in one exposed fish was recovered in the viscera, but it was
unclear to what extent transformation products had been formed. These
products can be formed in water by photo- and biotransformation (see
above).
Bluegill sunfish (Lepomis macrochirus) and channel catfish
(Ictalurus punctatus) were exposed under static conditions to a
single dose of 14C-ring-labelled fenthion at a concentration of
about 3.7 µg/litre in aquariums containing both water and sediment and
were observed for 28 days. The calculated maximal daily biocon-
centration factors were 118 and 115 after 8 and 24 h for the sunfish
and the catfish, respectively. The maximal amount of labelled residues
in both species was 0.3 mg/kg bw 8-24 h after application. No residues
were recovered in fish after 21 days. The maximal amounts were not
related to the maximal depressions in brain cholinesterase activity,
which were 97% after three days for the sunfish and 76% after six days
for the catfish. There appeared to be a lag of a few days between
exposure and effects. The fish appeared to have recovered 21 days
after application (ChemAgro, 1975a).
De Bruijn & Hermens (1991) confirmed that fenthion is moderately
concentrated in fish. They found a bioconcentration factor of 170 in
guppies (Poecilia reticulata) exposed for 11 days to a mixture of
toxicants, including fenthion. Uptake and elimination were equili-
brated after three days.
5. Environmental levels
The concentrations of fenthion in the environment are summarized
in Table 5. Few measurements are available from regular monitoring
programmes, except in the Netherlands, where fenthion was detected in
various fresh and marine surface waters in 1992. The only published
data on residues of fenthion on vegetation and insects after aerial
application of the pesticide are derived from monitoring after
application of 2.4 kg a.i./ha to two sites for control of weaver birds
(Quelea quelea) in Kenya (Bruggers et al., 1989). The applications
were the first to be used in the area to control birds. The
concentrations of residues on young birds were 44 and 84 µg per bird
on the two sprayed areas, respectively, on day 1 and about 10 µg per
bird cm days 3-4. Raptors that ate the birds were not killed, but some
were debilitated; the concentrations in the raptors were not measured.
Aggregate samples of insects contained fenthion at 7.2 mg/kg; the
highest concentration of residues was found in carabid beetles (23 µg
per beetle). The concentrations on grass were 39 and 28 mg/kg on the
two sprayed areas, respectively, on the first day after spraying and
1.9 and 1.1 mg/kg on day 44. A sample of millet seed spread on a mesh
in a sprayed area contained fenthion at 1.1 mg/kg.
Owing to the scarcity of data from monitoring, Table 5 also shows
some actual measurements in the field from experiments performed with
recommended application rates simulating common agricultural practice.
Only maximal amounts are tabulated as indicative values, and data on
the rate at which fenthion dissipated, when available, are mentioned
in the comments (see also sections 4.1 and 4.13). No data were
available on the occurrence of transformation products.
6. Effects on organisms in the laboratory and the field
6.1 Laboratory experiments
6.1.1 Microorganisms
(a) Water
The acute and chronic toxicity of technical-grade fenthion and a
formulation of fenthion to aquatic microorganisms and invertebrates
are summarized in Tables 6 and 7. Technical-grade fenthion is toxic to
highly toxic, with four-day EC50 values of 550-1800 µg/litre and
four-day NOEC values of 100-1120 µg/litre. One formulation of fenthion
is toxic, with four-day EC50 values of 1500-> 2000 µg/litre. The
toxicity of fenthion to e.g. algae is dependent on the species or
strain tested (Wängberg & Blanck, 1988). High toxicity for aquatic
organisms was confirmed by Saini & Saxena (1986), who reported a
six-day NOEC of 100 µg/litre for the freshwater protozoan Tetrahymena
pyriformis. High toxicity was also reported for some saltwater
microorganisms, with one-day EC50 values of 1000 µg/litre for the
alga Cyclotella nana and 100 µg/litre for the diatom Skeletonema
costatum and a one-day NOEC of 10 µg/litre for Cyclotella nana,
based on production of oxygen (Derby & Ruber, 1971).
The toxicity of fenthion for microorganisms in activated sludge
was tested in a laboratory experiment for 30 min at 20°C (Bayer AG,
1994b). The sludge had been collected in a plant treating domestic
waste. Only at the highest nominal dose of fenthion, 10 000 mg/litre,
was respiration inhibited substantially. The test is of limited value,
however, as the nominal test concentrations exceeded the water
solubility by up to 2380 times.
(b) Soil
No unequivocal indications of deleterious effects of fenthion on
microorganisms in the soil are available. The formulation Lebaycid
inhibits neither processes involved in the nitrogen transformation
cycle nor enzymes involved in microbial activity. It did not affect
soil respiration or nitrification in sandy loam or silt loam soils
after application at the recommended rate, 0.75 kg/ha, or a rate five
times higher, 3.8 kg/ha (Bayer AG, 1989a,b). Soil respiration was
measured over 28 days after amendment with glucose (Bayer AG, 1989a),
and incubation was at 20°C in the dark. The effects on nitrification
were analysed by measuring ammonium, nitrite, and nitrate up to and
including 28 days after application (Bayer AG, 1989b). Only slight,
transient increases in nitrate were observed in the silt loam soil and
decreases in the sandy loam soil. The soils had not been treated with
pesticides for one to two years previously.
Table 5. Maximal concentrations of fenthion in environmental water, soil, sediment, and biota
Sample Location Year Period Concentration Comments Reference
Kenya
Carabid Kulalu 1985 April-May 23 µg/beetle Application rate, 1.5-2.4 kg/ha; Bruggers et al.
beetle Ranch mean in insects, 7.2 mg/kg (1989)
Savannah Kulalu 1985 April-May 38 mg/kg Application rate, 1.5-2.4 kg/ha;
grass Ranch whether based on dry or wet Bruggers et al.
weight not explicitly reported; (1989)
amount decreased to 1.9 and 1.1 mg/kg
after 3 and 4 days, respectively
Millet Kulalu 1985 April-May 1.1 mg/kg Application rate, 1.5--2.4 kg/ha; Bruggers et al.
seed Ranch whether based on dry or wet weight (1989)
not explicitly reported; deliberate
overspraying of artificial plot
Netherlands
Fresh Large rivers 1992 0.01-0.12 Measured in pumping station; fenthion Van Meerendonk et al.
surface and lakes µg/litre detected in 25% of freshwater locations (1994)
water at a mean concentration of
0.022 µg/litre
Marine North Sea, 1992 0.01 µg/litre Fenthion detected in 25% of freshwater Van Meerendonk et al.
surface Waddensea locations at a mean concentration of (1994)
water 0.02 µg/litre
Table 5. (cont'd).
Sample Location Year Period Concentration Comments Reference
United States
Salt-marsh Indian River 1984 September 1.7 µg/litre No fenthion detectable (< 0.01 µg/litre) Wang et al.
surface County, Florida within 24-48 h of aerial application (1987)
water of 0.032 kg a.i./ha
Salt-marsh Indian River 1985 March < 0.01 No explanation for low concentration; Wang et al.
surface County, Florida µg/litre deposition was confirmed, as up to 0.5 (1987)
water µg/litre was detected in dishes with
an aqueous medium; application rate,
0.032 kg a.i./ha
Salt-marsh Indian River 1985 June 0.16 No fenthion detectable (<0.01 µg/litre) Wang et al.
surface County, Florida µg/litre within 24-48 h of aerial application of (1987)
water of 0.032 kg a.i./ha
Polia larvae Laramie, 1979 Summer 0.28 mg/kg 8 h after spraying in field experiment Powell
(bird feed) Wyoming at an application rate of 52 g/ha; no (1984)
fenthion detected 30 h after treatment
Table 6. Acute toxicity of fenthion and two metabolites to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Crustacean
Daphnia magna, Static Technical Reconstituted 8.0 10 20 2 EC50, 0.0057a,b Bayer (1985a)
first instar
Fish
Onchorhynchus mykiss, Continuous Technical Well 8.0-8.1 225-275 12-13 4 LC50, 0.83b,c,d ABC Inc.
0.4 cm, 0.8 g (1986a)
Lepomis macrochirus, Continuous Technical Well 8.1-8.2 225-275 21 4 LC50, 1.7b,c ABC Inc.
5.4 cm, 1.9 g (1987)
Onchorhynchus mykiss, Static Lebaycid Tap 7.5-7.9 284 16 4 LC50, 2.3b,d Bayer (1994c)
5.5-6 cm, 1.5 g (adapted)
Leuciscus idus, Static Lebaycid Tap 7.2-7.9 284 21 4 LC50, 4.3b,d Bayer (1994d)
5.5 cm, 1.5 g (adapted
Onchorhynchus mykiss, Static Psx Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Onchorhynchus mykiss, Static Psn Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Lepomis macrochirus, Static Psx Well 7.3-7.6 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Lepomis macrochirus, Static Psn Well 72-7.9 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Psx, 3-methyl-4-methylsulfinyl-phenol; Psn, 3-methyl-4-methylsulfonyl-phenol
a Based on immobilization
b As phototransformation occurred, value is based on a mixture of fenthion residues
c Measured concentrations
d Actual concentration
Table 7. Long-term toxicity of fenthion to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Green algae
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 550a,b Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 1800a,c Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 NOEC, 100a,b Bayer (1985a)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, 1500a,b Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, > 2000a,c Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 NOEC, 1120a,b Bayer (1989c)
subspicatus
Selenastrum Static Baytex Artificial NR 1.5 24 4 EC50, 1100a,c,d,e ABC Inc. (1986b)
capricornutum
Selenastrum Static Baytex Artificial NR 1.5 24 4 NOEC, 700a,c,d,e ABC Inc. (1986b)
capricornutum
Crustaceans
Daphnia magna, Continuous Lebaycid Artificial 7.5-8.3 204 20 21 NOEC, 0.018d,e,f,g TNO (1989)
first instar
Daphnia magna, Continuous Technical Well 8.1-8.3 206-275 20 21 MATC, Bayer (1985b)
first instar 0.042-0.082d,e,h
Table 7. (cont'd).
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Fish
Oncorhynchus Continuous Technical Well 6.6-7.8 29-30 12 88 MATC, 13-27d,e,i SLS Inc. (1988)
mykiss
NR, not reported
a As phototransformation occurred, the value is for a mixture of fenthion residues
b For decrease in biomass
c For inhibition of growth rate
d Measured concentration
e Actual concentration
f All concentrations below the detection limit of 0.1 µg/litre
g For reproduction
h For mortality, growth, and reproduction
i For embryo viability, embryo survival at hatch, survival, and growth of larvae
6.1.2 Aquatic organisms
(a) Plants
Stimulation but no decrease in biomass was observed in an
experiment in which duckweed (Lemna gibba) was exposed in an
artificial medium to the formulation Baytex at measured concentrations
up to 2.8 mg/litre for 14 days at 25°C (Malcolm Pirie, 1987). The
stimulation may be related to hormesis.
(b) Invertebrates
Technical-grade fenthion is highly toxic to the crustacean
Daphnia magna, with a two-day EC50 value of 5.7 µg/litre and a
21-day maximal acceptable toxicant concentration (MATC) of 0.042-0.082
µg/litre. The formulation Lebaycid is also highly toxic to this
species, with a 21 -day NOEC value of 0.018 µg/litre. Static and
chronic treatment of other invertebrate species provide more variable
results, with two- to four-day L(E)C50 values of 0.62-1800 µg/litre
for various freshwater molluscs, insects, and crustaceans, 17- to
32-week NOEC values of 1000-2000 µg/litre for the freshwater mollusc
Lymnea stagnalis, one- to four-day L(E)C50 values of 0.02-550
µg/litre for saltwater molluscs, insects, and crustaceans, and 8- to
20-day NOEC values of 0.037-0079 µg/litre for the saltwater crustacean
Mysidopsis bahia (e.g. Seugé & Bluzat, 1980; Mayer, 1986; Mayer &
Ellersieck, 1986; McKenney, 1986). These tests were performed with
technical- or analytical-grade fenthion.
(c) Vertebrates
The acute and long-term toxicity of technical-grade fenthion, the
metabolites 3-methyl-4-methylsulfinyl-phenol and 3-methyl-4-methyl-
sulfonyl-phenol, and a formulation of fenthion to aquatic vertebrates
is summarized in Tables 6 and 7. Technical-grade fenthion is
moderately to highly toxic to freshwater fish, with four-day LC50
values of 0.83-1.7 mg/litre and an 88-day MATC value of 13-27
µg/litre. The formulation Lebaycid is toxic, with four-day LC50
values of 2.3-4.3 mg/litre. The metabolites 3-methyl-4-methyl-
sulfinyl-phenol and 3-methylsulfonyl-phenol are very slightly toxic to
freshwater fish, with four-day LC50 values exceeding 100 mg/litre.
Other tests show moderate to high toxicity of technical-grade fenthion
(e.g. Korn & Earnest, 1974; Mayer & Ellersieck, 1986; WHO, 1986
[original references not checked], with two- to four-day LC50 values
of 1-3.4 mg/litre for freshwater fish and 0.45-2.5 mg/litre for
saltwater fish.
Fenthion is highly toxic for amphibians: Khangarot et al.
(1985) reported a four-day LC50 value of 0.84 µg/litre for the frog
Rana hexadactyla with a length of 20 mm.
Many sublethal effects have been observed in fish after exposure
to a wide range of concentrations, including bottom disorientation,
loss of equilibrium, discolouration, rapid respiration, bloated
stomach, gulping of air, quiescence, surfacing, and irregular swimming
behaviour (ABC Inc., 1986; SLS Inc., 1988; Bayer AG, 1994c,d).
6.1.3 Terrestrial organisms
(a) Plants
No data were available.
(b) Invertebrates
Technical-grade fenthion is obviously toxic to various
terrestrial insects. Few studies have addressed other terrestrial
invertebrates.
Fenthion appears to be toxic to honey bees (Apis mellifera),
with contact LD50 values of 0.16-< 2 µg/bee (Bayer AG, 1995
[original references not checked]). No data were available on the
toxicity of fenthion for honey bees after oral exposure, but it would
be expected to be toxic. It is also toxic for predators of pests such
as wasps, mites, hoverflies, and green lacewings tested at moderately
high temperatures (22-27°C during the day and night and 16-18°C at
night).
Fenthion is highly toxic to a microhymenopterous wasp,
Trichogramma caoeciae, that parasitizes the eggs of the parasite
Sitrotroga cereallella (Conrad Appel GmbH, 1989). In one experiment,
larvae were exposed to Lebaycid sprayed onto glass plates at a rate of
16 kg/ha as fenthion. All of the larvae died within 24 h. In a second
experiment, host eggs containing pupae did not hatch after they had
been dipped in an aqueous solution of 0.1% Lebaycid.
Fenthion is also highly toxic to the predating mite Phytoseiulus
persimilis Athias-Henriot (von Kniehase & Zoebelein, 1990). Male and
female mites were placed on the leaves of kidney beans (Phaseolus
vulgaris) to produce a small population, and five days later the
leaves were dipped into a solution of 0.075% Lebaycid EC 500 at the
highest recommended application rate. All mites were killed within
seven days. Treatment with 0.015% Lebaycid EC 500 did not kill all of
the mites.
All early-instar larvae of the aphid predating hover fly
(Episyrphus balteatus) died within 24 h after spraying of Lebaycid
500 EC on glass plates at a rate of 0.77 kg/ha as fenthion (University
of Southampton, 1992).
Fenthion was highly toxic to the larvae of the green lacewing
(Chrysoperla carnea) exposed by contact to Lebaycid at 12 kg/ha as
fenthion in a light-dark cycle for three to five days (GAB Biotech-
nologie, GmbH, 1991). The rate of mortality was 100% of larvae and
unhatched imagos and only 7% of controls.
Artificial soil contaminated with Lebaycid 50EC was slightly
toxic to earthworms (Eisenia andrei) (Bayer AG, 1989d), with a
14-day LC50 of 723 mg/kg soil dry weight and a dose-related weight
loss. The 14-day NOEC was thus 100 mg/kg dry weight of soil. As the
vessel containing the worms was lit continuously, some photo-
degradation may have occurred.
(c) Vertebrates
The acute and subacute toxicity of fenthion to birds is
summarized in Table 8.
Technical-grade fenthion is moderately toxic or toxic to birds,
with an LD50 value of 7.2 mg/kg bw and an eight-day LC50 value of
60-1259 mg/kg feed. Five- to 10-day LC50 values of 25-231 mg/kg feed
were reported for the mallard duck (Anas platyrhynchos), the
bobwhite quail (Colinus virginianus), the Japanese quail (Coturnix
japonica), and the domestic chicken (Gallus domestica) (Hill
et al., 1975; Royal Society of Chemistry, 1995). Fenthion appears to
be toxic after dermal application in several bird species, with LD50
values of 1.8 mg/kg bw for the weaver bird (Quelea quelea) and
2.4 mg/kg bw for the house sparrow (Passer domesticus) (Schafer
et al., 1973).
The sublethal effects observed in birds treated with fenthion
experimentally are wing drop, ataxia, salivation, reduced body weight,
tremors, convulsions, diarrhoea, and laboured breathing (Grue, 1982;
Mobay Corp., 1987c,d,e). Necropsy of poisoned birds showed fluid-
filled crops and intestines, mottled spleens (possible due to organ
hypostasis), and oily fluid in crops or gastrointestinal tract,
indicating incomplete absorption of fenthion. Common grackles
(Quiscalus quiscula) found dead after five days' exposure to fenthion
had lost 28-36% of their initial weight, and muscle tissue on the
breastbone and observable fat had decreased substantially (Grue,
1982). The reduced body weights were due to treatment-related
reductions in feed consumption, perhaps indicating that fenthion is a
repellent, probably because of its strong mercaptan odour. The
estimated daily intake remained constant and was thus independent of
the concentrations tested.
Table 8. Acute and subacute toxicity of technical-grade fenthion given to birds for eight days
Species Sex Age Route Result Reference
Mallard duck (Anas platyrhynchos) Not reported 9 days Diet LC50, 1259 mg/kg feed Mobay Corp. (1987c)
Bobwhite quail (Colinus virginianus) Not reported 10 days Diet LC50, 60 mg/kg feed Mobay Corp. (1987d)
Bobwhite quail (Colinus virginianus) Male, female 18 weeks Oral LD50, 7.2 mg/kg bw Mobay Corp. (1987e)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, 57 mg/kg feeda Grue (1982)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, < 30 mg/kg feedb Grue (1982)
a Birds caged outdoors 20-27 July 1978
b Birds caged outdoors 14-21 August 1978
A decrease in cholinesterase activity in the brain and plasma is
an important physiological characteristic of avian poisoning by
fenthion. Common grackles that had been fed fenthion at rates of
25-400 mg/kg feed showed a significant, 80% depression in brain
cholinesterase activity. All birds had died before enzyme analysis
(Grue, 1982).
Fenthion appears to be less acutely toxic to terrestrial mammals
than to birds. Kenaga (1979) reported an LD50 range of 255-298 mg/kg
bw for the rat (Rattus norvegicus), indicating moderate toxicity.
FAO/WHO (1973) reported a three-month LOEC value of 0.25 mg/kg diet
based on growth reduction. The highest dietary levels that caused no
toxicological effect were estimated to be 3 mg/kg feed for rats and
2 mg/kg feed for dogs.
6.2 Field observations
6.2.1 Microorganisms
(a) Water
A single application of fenthion at a rate of 0.22 kg/ha did not
affect phytoplankton in small artificial outdoor ponds with natural
surface water and sediment in experiments performed in Florida (USA)
in 1962-63 (Patterson & von Windeguth, 1964).
(b) Soil
No data were available.
6.2.2 Aquatic organisms
(a) Plants
No data were available.
(b) Invertebrates
The populations of crustaceans and chironomids disappeared within
one week after application of fenthion to small artificial outdoor
ponds with natural surface water and sediment at a rate of 0.22 kg/ha
in experiments performed in Florida in 1962-63 (Patterson & von
Windeguth, 1964). The period required for complete recovery of the
numbers of water fleas and midge larvae was four to five months during
a cold winter period and two months in the absence of a cold period.
There were substantially more oligochaetes in treated than in
untreated ponds. Other aquatic invertebrates such as snails,
ostracods, copepods, and Hydra appeared to be unaffected by the
treatment.
(c) Vertebrates
Application of fenthion to small artificial and outdoor ponds at
a rate of 0.22 kg/ha did not appear to affect fish or tadpoles
(Patterson & von Windeguth, 1964).
6.2.3 Terrestrial organisms
(a) Plants
No data were available
(b) Invertebrates
As fenthion is highly toxic to insects in the laboratory, it can
be expected to be so in the field under certain conditions. Its
toxicity is linked mainly to the extent of exposure. In field
experiments in Assiut (Egypt) between June and August at the end of
the 1960s, the formulation Lebaycid, sprayed at a rate of 2.9 kg/ha
(as fenthion) four times at intervals of 15 days with back-pack
spraying equipment, was shown to be toxic to two pest predatory
coccinellids (Coccinella undecimpunctata and Scymnus interruptus)
in cotton (von Afify et al., 1970). Although the formulation was
generally toxic to imago beetles, there was substantial temporal and
spatial variation. The mortality of Coccinella was 58-79% (percentages
corrected for mortality in controls) within three, days of application
and 61-96% within 10 days of application. The mortality of Scymnus
was 55-83% within three days and 28-80% within 10 days. These figures
show partial recovery, at least of Scymnus; Coccinella may be more
susceptible, or Scymnus may recolonize and re-emerge. There was no
clear indication of increasing adverse effects in either species.
Field experiments in Los Baños (Philippines) in 1979-80 showed no
substantial adverse effects of fenthion on predators of the brown
planthopper (Nilaparvata lugens), a common rice pest in South and
Southeast Asia (Reissig et al., 1982). In these experiments,
fenthion was applied three times to plots of lowland rice at a rate of
0.75 kg/ha per treatment. Both planthoppers and their predators were
sampled one day before and two days after the treatments; the
predators sampled were the spiders Lycosa pseudoannulata, Tetragnatha
sp., and Araneus sp. and the hemipterous Microvelia atrolineata
and Cyrtorhinus lividipennis. The numbers of neither the
planthoppers nor their predators were significantly altered by the
treatments.
Field experiments in Egypt in 1965-66 showed that adverse effects
on pest predatory species may be present and absent in the same area
(Kira et al., 1972). Some predators of borers and aphids in corn
(Zea mays) appeared to increase in number after treatment of crops
with fenthion at a rate of 1.2 kg/ha per treatment, while some
decreased.
The mortality of honey (Apis mellifera) and alkali bees (Nomia
melanderi Cockerell) in outdoor cages, caused by the introduction of
alfalfa leaves sprayed with fenthion as an ultra-low volume liquid
with a hand-sprayer at a rate of 0.9 kg/ha, was decreased by 75% when
the contaminated leaves were brought into the cages 2 h after
application of fenthion instead of immediately afterwards (Johansen
et al., 1983), indicating that fenthion can be applied with minimal
hazard to bees that are not foraging.
(c) Vertebrates
Various incidents of poisoning of birds with fenthion have been
reported. It is generally assumed that a depression in brain
cholinesterase activity > 50% indicates severe exposure to fenthion
(e.g. Henny et al., 1987). In a field experiment in Kenya,
cholinesterase activity < 20 µmol/min per g was considered to
indicate exposure to fenthion and activities < 10 µmol/min per g to
indicate severe sickness or death (Bruggers et al., 1989).
On a wet meadow of 1.8 km2 in a field experiment in Wyoming
(USA) in 1978, 99 birds and 15 mammals were found dead or sick within
16 days after aerial treatment with fenthion at a rate of 47 g/ha
(DeWeese et al., 1983). As mortality is correlated with signifi-
cantly depressed brain cholinesterase activity in three common bird
species, it was assumed that the mortality and sickness were
treatment-related. A lower mortality rate seen on a comparable trial
site may have been due to factors such as different wind speeds and
overlap of spray swathes. The route of fenthion that induced poisoning
was unclear.
The maximal fenthion residues in dead American kestrels (Falco
sparverius) were correlated with cholinesterase activity depressions
of 78-92% in brain and 97% in plasma (Hunt et al., 1991). Within
three days after treatment of sparrow perches with fenthion, all of
the kestrels were dead. As the kestrels tended to hide in bushes as
paralysis advanced, the numbers of contaminated birds may have been
underestimated. The time for a kestrel to catch a sparrow was
positively correlated with the extent of cholinesterase depression in
the kestrel's brain. This indicates that a kestrel exposed to more
potent fenthion residues will have greater cholinesterase depression
or that kestrels with greater cholinesterase depression are less
capable of catching sparrows. There are indications that the more
fenthion sparrows are exposed to, the more sensitive they are to
kestrel predation (Hunt, 1990, cited by Kendall & Akerman, 1992).
Two of four flood-irrigated hay meadows were sprayed with the
formulation Baytex at 52 g/ha (as fenthion) in order to investigate
the effects on the reproduction of red-winged blackbirds (Anglaius
phoeniceus) in an experiment in Wyoming (USA) in June-July 1979
(Powell, 1984). In one of the treated fields, the growth rate of
nestlings was significantly decreased by 15%; however, the biological
interpretation of this effect is difficult, as cholinesterase activity
was not reduced. It appears to be a transient effect, since the
surviving nestlings recovered from the growth reduction. No
significant treatment-related effects on hatching, on fledgling
survival, or on foraging behaviour of adult blackbirds for nestling
care were observed. The latter parameter, investigated by counting the
number of trips per hour and their duration and distance, was
remarkably stable, as the abundance of the main feed type, the
caterpillar-like larva Polia, had been reduced by about 50% within
one to two days after the treatment.
The effect of fenthion on non-target birds was studied after
aerial application against the red-billed weaver bird (Quelea
quelea), a grain pest in Africa (Bruggers et al., 1989). In these
experiments, conducted in April-May 1985, two quelea colonies in a
savannah (Kulalu Ranch, Kenya) of low thornbush were sprayed at a rate
of 1.5-2.4 kg/ha. The brain cholinesterase activities that were
measured in 19 potentially exposed non-target bird species varied
substantially. Raptorial birds that were captured in or around the
quelea colonies, which had been radioequipped and were tracked up to
14 days after the treatment, were apparently not killed by the
treatment, but 70% had substantially depressed cholinesterase activity
in blood plasma, indicating actual exposure. Most raptorial birds were
assumed by the authors to have been exposed to fenthion by ingesting
contaminated quelea birds within one day after treatment. As no dead
radioequipped raptorial birds were recaptured, it may cautiously be
concluded that the risk of dying through secondary poisoning was
limited; however, the biological consequences for raptorial birds of
depressed cholinesterase activities with respect to other end-points
such as growth and reproduction are unclear.
The effects of fenthion on granivorous birds were also studied:
galliform species (Coturnix delegorguei, Gallus gallus, Pterocles
decoratus) and laughing doves (Streptopelia senegalensis) in cages
that were placed 100, 200, 300, and 400 m downwind of the treated
quelea colonies showed no mortality or signs of intoxication during an
observation period of five to seven days.
An additional experiment showed that the possibility of secondary
poisoning from scavenged contaminated carcasses cannot be excluded.
During the first night after the treatment, 24-90% of intentionally,
placed corpses were removed by scavengers such as jackals (Canis
mesomelas), bat-eared foxes (Otocyon megalotis), and baboons
(Papio cyanocephalus). This high rate of scavenging substantially
reduced the numbers of poisoned dead animals that could be collected,
resulting in an underestimation of the actual risks for non-target
birds.
7. Evaluation of effects on the environment
Fenthion is a systemic organophosphorus pesticide used for both
agricultural and nonagricultural purposes. Its major use is in the
control of insect pests (at an application rate of 1.25 kg a.i./ha),
but it is also used as a veterinary product to control external
parasites on domestic animals and to control birds (at an application
rate of 2.4 kg a.i./ha). Sprays for bird control are composed of a
formulation that is not available commercially and are used only under
governmental control. The pesticide is usually formulated as an
emulsifiable concentrate and is applied by ground spraying (80-85%) or
from the air (15-20%). It is also formulated as granules and as a
paste for control of urban birds. It can enter the environment beyond
the intended zone of treatment by spray drift, as residues on
potential food items such as birds, and, presumably, by loss of
surface residues from domestic animals.
Fenthion that enters the environment is adsorbed rapidly and
strongly on soil and sediment by a poorly understood mechanism. The
compound can be considered to be immobile in soil, and the parent
compound is unlikely to leach below the to firstfew centimetres of the
soil profile.
While photodegradation occurs under laboratory conditions, it is
not considered to be a significant route of breakdown in the field.
Hydrolysis occurs slowly and is dependent on pH; the half-lives of
fenthion in sterile buffers range from 30 to 60 days, with slower
degradation at low pH. Fenthion is degraded by microorganisms in
laboratory soil, with a half-life of 10 days; field degradation is
slower, about half of the compound being degraded to carbon dioxide
within six months in temperate climates. Generally, breakdown is
slower in water-sediment systems than in soil. Fenthion has been
measured in surface waters in untreated areas at concentrations up to
0.12 µg/litre. As the properties of fenthion would not result in its
entering the aquatic environment by leaching or run-off, there is no
satisfactory explanation for the aquatic residues found. It does not
bioaccumulate to a significant extent, despite its high solubility in
fat, since its metabolism and depuration in organisms are rapid.
Because fenthion is broken down rapidly in the environment, its
acute toxicity is relevant. Most species are not exposed chronically,
and its binding to soil and sediment particles would be expected to
reduce its bioavailability. Fenthion and formulations are toxic to
highly toxic to microorganisms, with four-day EC50 values of 550 and
1100 µg/litre. No data were available on its acute toxicity to algae,
but it is of low toxicity after long-term exposure. Screening tests
have indicated no phytotoxicity. Its acute and long-term toxicity to
crustaceans is high, with an EC50 value of 5.7 µg/litre and an NOEC
value of 0.018 µg/litre. Fenthion is very toxic to fish, with an
LD50 of 830 µg/litre and an MATC of 20 µg/litre. One study showed
toxicity to larvae at 0.84 µg/litre. Fenthion is moderately toxic to
birds, with an LD50 value of 7.2. µg/kg bw and a five-day LC50
value of 60 µg/kg feed. It is also toxic to honey bees, with a contact
LD50 of 0.16 µg per bee, and is slightly toxic to earthworms, with a
1-h LC50 of 723 µg/kg dry weight.
7.1 Risk assessment
Exposure concentrations have been derived from the results of
monitoring programs or field experiments simulating common agricul-
tural practice and from a simple calculation after spraying. The model
used for calculating the predicted environmental concentration (PEC)
is similar to that used currently by the US Environmental Protection
Agency, the Pesticides Safety Directorate in the United Kingdom, and
the Uniform System for the Evaluation of Substances in the
Netherlands. The method is presented in Figure 2. The data used in the
calculation are those from a two-year monitoring programme in 15
freshwater locations in the Netherlands where fenthion was detected;
however, the sources of these emissions are not clear. No other data,
such as concentrations in surface water, were available. The results
used in the hazard evaluation are those of field experiments of aerial
application of fenthion for mosquito control. No data were available
from field experiments on concentrations in surface water or residues
in sediment.
The concentrations resulting from long-term exposure are assumed
to result from dissipation in accordance with first-order kinetics:
(a) monitoring programme in salt-marsh (see Table 9)
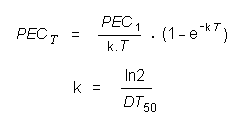
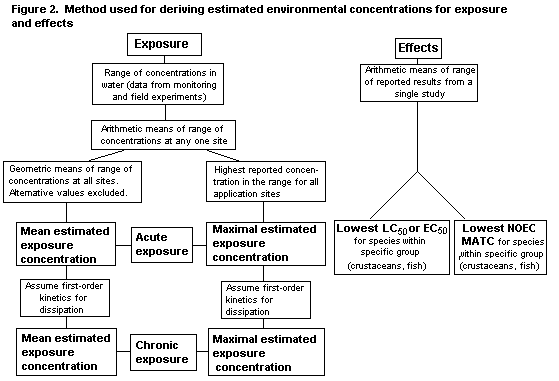 (b) spraying (see Table 10)
(b) spraying (see Table 10)
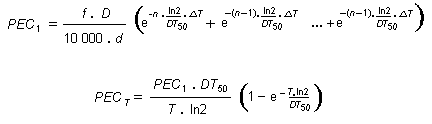 The following data are used in the calculations for situations
(a) and (b):
- water depth, 0.3 m
- 'worst-case' application rate on ornamental plants, three times 1
kg a.i./ha at seven-day interval, taking into account half-life
of seven days: 1.75 kg a.i./ha
- emission factor, 0.05 spray drift at 1 m
- tests periods of four days for algae, 21 days for crustaceans,
and 88 days for fish.
The half-life used is the longest value reported (see Table 3), which
can be considered a realistic 'worst-case' value. The lowest L(E)C50,
and NOEC values for algae, crustaceans, and fish were taken from
Tables 6 and 7.
It should be noted that limited information is available on
concentrations in the environment and toxicity. Only the water
compartment and a few species living in open water have been included.
Data on the toxicity of transformation products for aquatic organisms
were available only for 3-methyl-4-methylsulfinyl-phenol and
3-methylsulfonyl-phenol. The exposure concentrations are calculated
for the times equal to the duration of an experiment; e.g. the 21-day
NOEC for water fleas must be compared with the PEC21 days, calculated
as described above.
Table 9 gives a comparison of the estimated mean and maximal
exposure concentrations in the field with the lowest reported
L(E)C50 and NOEC values for acute and long-term exposure of
organisms in open water. The ratio between exposure and effect
concentration (TER) has been calculated. The Table is meant to serve
as a guide to classifying risk in the field and is not intended for
use in estimating the degree of effect likely to be seen. The
classification 'possible risk' is a simple one based on different
classification phrases for order-of-magnitude segments of the ratios.
Table 9 shows that crustaceans are at possible risk after either
acute or long-term exposure, but fish and algae appear not to be
affected. The few field observations available confirm the risk for
crustaceans (see above), but they are not altogether reliable, as some
relevant test conditions (e.g. physicochemical characteristics of the
water, temperature, actual concentrations of fenthion) are
incompletely described.
Table 10 gives a summary of results obtained for the example of
spraying ornamental plants. Crustaceans are again the organisms at
risk after either acute or long-term exposure. Algae are not affected,
and the effect on fish can be considered low.
It should be noted on the one hand that the risk classifications
shown in Tables 9 and 10 are for realistic 'worst-case' conditions:
the lowest reported values for toxicity were included; the slowest
reported dissipation rate was used; the concentrations used to
estimate mean exposure are only those from sites where fenthion was
actually detected; and the maximal estimated exposure concentration is
that used in aerial application for mosquito control. On the other
hand, only four groups of aquatic organisms are considered (e.g. no
sediment-dwelling organisms were included), adjuvants and transform-
ation products were not taken in to account, and the risks are not
relevant for water temperatures exceeding 22°C.
Table 9. Indications of environmental risk for aquatic organisms due to technical-grade fenthion
Effect Organism Estimated exposure Toxicity End-point Toxicity: Risk
concentration (µg/litre) exposure classification
(µg/litre) ratio
Mean estimated exposure concentration (monitoring programme)
Acute Crustaceans 0.020 EC50 = 5.7 Immobilization 285 Negligible
Acute Fish 0.020 LC50 = 830 Mortality > 1000 Negligible
Acute Amphibia 0.020 LC50 = 0.84 Mortality 42 Low
Chronic Algae 0.017 EC50 = 550 Biomass decrease > 1000 Negligible
Chronic Crustaceans 0.0084 NOEC = 0.018 Reproduction 2.1 Present
Chronic Fish 0.0023 MATC = 20a Reproduction > 1000 Negligible
Maximum estimated exposure concentration (salt-marsh)
Acute Crustaceans 1.7 EC50 = 5.7 Immobilization 3.4 Present
Acute Fish 1.7 LC50 = 830 Mortality 488 Negligible
Acute Amphibia 1.7 LC50 = 0.84 Mortality 0.5 High
Chronic Algae 1.4 EC50 = 550 Biomass decrease 393 Negligible
Chronic Crustaceans 0.71 NOEC = 0.018 Reproduction 0.03 Very high
Chronic Fish 0.2 MATC = 20a Reproduction 100 Negligible
a Mean of 13 and 27 µg/litre (see Table 7)
Table 10. Risk assessment for highest recommended rate of agricultural application based on
predicted environmental concentration
Effect Organism Preicted Toxicity End-point Toxicity: Risk
environmental (µg/litre) exposure classification
concentration ratio
(µg/litre)
Acute Crustaceans 29 EC50 = 5.7 Immobilization 0.2 High
Acute Fish 29 LC50 = 830 Mortality 28.6 Negligible
Acute Amphibia 29 LC50 = 0.84 Mortality 0.03 Very high
Chronic Algae 23.9 EC50 = 550 Biomass decrease 23.0 Negligible
Chronic Crustaceans 12.2 NOEC = 0.018 Reproduction 0.0015 Very high
Chronic Fish 3.3 MATC = 20 Reproduction 6.1 Present
Fenthion kills some beneficial insect predators, although the
results are variable; less effect was seen in the field than in the
laboratory. It is considered not to be hazardous to earthworms at
recommended rates of application. The risk to honey bees is considered
to be negligible provided they are not foraging. Fenthion is highly
acutely toxic to birds and is used as an avicide at high application
rates. Risk was assessed for both avicidal and general agricultural
use.
7.2 Use as an avicide
A single report has been made of secondary poisoning of a bird of
prey after ingestion of poisoned house sparrows. The dietary LC50
for the bobwhite quail is 60 mg/kg diet. On the basis of comparative
figures for body weight and food consumption, the LC50 values for
predatory birds that eat avian prey can be estimated from the
following equations to be 267 mg/kg diet for the European kestrel and
282 mg/kg diet for the sparrowhawk:
[Test species] LD50 [Test species] LD50 (mg/kg diet) × bw (kg)
(mg/kg per day) =
Food consumption (kg)
LC50 (mg/kg dry weight [Test species] LD50 (mg/kg diet) × bw (kg)
diet per day) =
Food consumption (kg)
The total daily intake that leads to death is 4.2 and 4.8 mg for the
two species, respectively. The residue concentrations reported in
house sparrows poisoned by fenthion used as an avicide were 6 µg/g in
carcass, 631 µg/g in feathers and skin, and 1152 µg/g in feet; the
total fenthion content of the carcass would be 166 µg on the basis of
a body weight of 27.7 g. Ingestion of the carcass of a sparrow should
not therefore result in a lethal dose, but ingestion of residues on
feathers and feet during plucking of the prey could result in a lethal
intake. A range of bird species may therefore have comparable
sensitivity to fenthion, and it can be assumed that any bird-eating
raptor would be killed by eating contaminated prey.
7.3 Agricultural use
Use of the vertebrate scheme of the European and Mediterranean
Plant Protection Organisation/Council of Europe and the highest
recommended agricultural application rate of 1.25 kg/ha gave predicted
environmental concentrations of 140 mg/kg for grass, 3.4 mg/kg for
insects, and 3.4 mg/kg for grains. The LC50 and TER values for
various bird species that use these items as food were estimated on
the basis of the data on toxicity for the bobwhite quail and are shown
in Table 11, which shows that use of fenthion at the highest
recommended application rate would be likely to cause deaths among
birds. The most susceptible species are those with a low body weight
that feed on vegetation.
Table 11. Toxicity:exposure ratios for birds after application of fenthion at 1.25 kg/ha
Species Estimated LC50 Predicted environmental Toxicity:exposure Risk
(mg/kg diet) concentration (mg/kg) ratio classification
Common quail (Coturnix coturnix) 211 140 1.5 Present
Greylag goose (Anser anser) 658 140 4.7 Present
Wren (Troglodytes troglodytes) 46 3.4 13.5 Low
Jackdaw (Corvus monedula) 285 3.4 84 Low
Reed bunting (Emberiza shoeniclus) 73 3.4 21.5 Low
Red-legged partridge (Alectroris nifa) 355 3.4 104 Very low
References
ABC Inc. (1986a) Acute flow-through toxicity of Baytex to rainbow
trout. Unpublished report No. 35313 by Analytical Biochemistry
Laboratories Inc., Columbia, Missouri, USA. Submitted to WHO by
Bayer AG.
ABC Inc. (1986b) Acute toxicity of Baytex to Selenastrum
capricornutum. Unpublished report No. 35314 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA.
Submitted to WHO by Bayer AG.
ABC Inc. (1987) Acute flow-through toxicity of Baytex to bluegill
sunfish (Lepomis macrochirus). Unpublished report No. 35597 by
Analytical Biochemistry Laboratories Inc., Columbia, Missouri,
USA. Submitted to WHO by Bayer AG.
ABC Inc. (1988) Soil adsorption/desorption with 14C-Baytex (Project
No. 36998). Unpublished report No. 98450 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA Submitted
to WHO by Bayer AG.
von Afify, A.M., Farghaly, H.T., & Hassanein, M.H. (1970) Freiland-
Untersuchungen über die Wirkung verschiedener Insektizide auf das
Vorkommen von zwei entomophagen Coccinelliden an Baumwolle in
Oberägypten. Schaedlungskd Pflanzensch. Umweltsch., 43, 8-13.
Bayer AG (1974) Verhalten des Pflanzenschutzmittelwirkstoffes im
Boden. Unpublished report No. RR5000/74 from Bayer AG
Pflanzenschutz Anwendungstechnik, Biologische Forschung,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1983a) Note on hydrolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG.
Bayer AG (1983b) Note on photolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG).
Bayer AG (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) by fenthion (technical). Unpublished report
No. 89082 from Bayer Institute of Environmental Biology,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1985b) Acute toxicity of fenthion (technical) to water
fleas. Unpublished report No. HBF/Dm 52 from Bayer Institute of
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988a) Degradation of fenthion ((R)Baytex) in the system
water/sediment (Project No. M 151 0186-2). Unpublished report
No. 3026 from Bayer AG Geschäftsbereich Pflanzenschutz, Institut
für Metabolismusforschung, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1988b) Degradation of crop protectants under anaerobic
conditions in the system water/sediment: fenthion (Project
No. 1520194-2). Unpublished report No. PF-3028 from
Bayer AG Geschäftsbereich Pflanzenschutz, Institut für
Metabolismusforschung, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988c) Method of estimating the direct photodegradation of
organic compounds in water under environmental conditions.
Unpublished report No. 2974 from Bayer AG Geschäftsbereich
Pflanzenschutz, Institut für Metabolismusforschung, Leverkusen,
Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989a) Influence of the commercial product (R)Lebaycid on
soil respiration after amendment with glucose (Study No. E 330
0279-4). Unpublished report No. AJO/71589 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989b) Influence of the commercial product (R)Lebaycid on
nitrification in soils (Study No. E 331 0278-4). Unpublished
report No. BSI/71689 from Bayer Crop Protection Research,
Chemical Product Development, Institute for Environmental
Biology, Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989c) Growth inhibition of green algae (Scenedesmus
subspicatus) by (R)Lebaycid EC 50 (Study No. E 323 0280-8).
Unpublished report No. HBF/A1 68 from Bayer Crop Protection
Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989d) Toxicity of (R)Lebaycid to earthworms (Study
No. E 310 0293-8). Unpublished report No. HBF/Rg 105 from Bayer
Crop Protection Research, Chemical Product Development, Institute
for Environmental Biology, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1994a) Calculation of the chemical lifetime of fenthion in
the troposphere. Unpublished report No. HPO-114 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994b) Studies on the ecological behaviour of Lebaycid N.
Unpublished report from Bayer AG, Institute for Environmental
Analysis and Assessments, Leverkusen, Germany. Submitted to WHO
by Bayer AG).
Bayer AG (1994c) Lebaycid 500 EC-acute toxicity (96 hours) to rainbow
trout in a static test. Unpublished revised study report No. DOM
94003 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994d) Lebaycid 500 EC-acute toxicity (96 hours) to golden
orfe in a static test. Unpublished revised study report No. DOM
94002 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1995) JMPE monograph for fenthion. Unpublished report of
28-03-95 submitted to WHO by Bayer AG.
Bowman & Assoc. (1989) Fenthion-acute toxicity test for freshwater
fish (Project No. FEN89C,D,E & F). Unpublished report No. 73916
from MC Bowman & Assoc., Inc., Mount Ida, Arkansas, USA.
Submitted to WHO by Bayer AG.
Bruggers, R.L., Jaeger, M.M., Keith, J.O., Hegdal, P.L., Bourassa,
J.B., Latigo, A.A. & Gillis, J.N. (1989) Impact of fenthion on
nontarget birds during Quelea control in Kenya. Wildl. Soc.
Bull., 17, 149-160.
ChemAgro (1972) The mobility and persistence of Baytex in soil and
water. Unpublished report No. 31985 from ChemAgro, Research and
Development Department, Kansas City, Missouri, USA Submitted to
WHO by Bayer AG.
ChemAgro (1974) Leaching of aged residues of Baytex-ring-UL-14C in
sandy loam soil. Unpublished report No. 40566 from ChemAgro,
Research and Development Department, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975a) Accumulation and persistence of residues in catfish
and bluegill fish exposed to Baytex-14C. Unpublished report
No. 43455 from ChemAgro Agricultural Division, Mobay Chemical
Corp., Research and Development, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975b) Leaching characteristics of Baytex. Unpublished
report No. 45653 from ChemAgro Agricultural Division, Mobay
Chemical Corp., Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
ChemAgro (1976a) Persistence and fate of Baytex in a natural aquatic
environment. Unpublished report No. 47404 from ChemAgro, Research
and Development Department, Kansas City, Missouri, USA. Submitted
to WHO by Bayer AG.
ChemAgro (1976b) Soil thin-layer mobility of twenty four pesticides
chemicals. Unpublished report No. 51016 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976c) Stability of Baytex in sterile aqueous buffer
solutions. Unpublished report No. 49130 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976d) Photodecomposition of Baytex. Unpublished report
No. 49347 from ChemAgro, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Conrad Appel GmbH (1989) Study on the effect of Lebaycid on
Trichogramma cacoeciae (Study No. 40/89 - 38/89). Unpublished
report from Conrad Appel GmbH, Department Trichogramma,
Darmstadt, Germany. Submitted to WHO by Bayer AG.
De Bruijn, J. & Hermens, J.L.M. (1991) Uptake and elimination kinetics
of organophosphorous pesticides in the guppy (Poecilia
reticulata): Correlations with the octanol/water partitioning
coefficient. Environ. Toxicol. Chem., 10, 791-804.
Derby, S.B. & Ruber, E. (1971) Primary production: Depression of
oxygen evolution in algal cultures by organophosphorus
insecticides. Bull. Environ. Contain. Toxicol., 5, 553-558.
DeWeese, L.R., McEwen, L.C., Settimi, L.A. & Deblinger, R.D. (1983)
Effects on birds of fenthion aerial application for mosquito
control. J. Econ. Entomol., 76, 906-911.
Eichelberger, J.W. & Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5, 541-544.
FAO/WHO (1973) 1971 Evaluations of some pesticide residues in food.
Report of the 1971 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues (WHO Technical Report No. 502), Geneva.
GAB Biotechnologie GmbH (1991) Detection of side effects of Lebaycid
on the green lacewing Chrysoperla carnea Steph. in the
laboratory (Study No. 008/01-Cc). Unpublished report from GAB
Biotechnologie GmbH, Niefern-Öschelbronn, Germany. Submitted to
WHO by Bayer AG.
Gohre, K. & Miller, G.C. (1986) Photooxidation of thioether pesticides
on soil surfaces. J. Agric. Food Chem., 34, 709-713.
Grue, C.E. (1982) Response of common grackles to dietary concen-
trations of four organophosphate pesticides. Arch. Environ.
Contain. Toxicol., 11, 617-626.
Henny, C.J., Kolbe, E.J., Hill, E.F. & Blus, L.J. (1987) Case
histories of bald eagles and other raptors killed by organophos-
phorus insecticides topically applied to livestock. J. Wildl.
Dis., 23, 292-295.
Hill, E.F., Heath, R.G., Sparta, J.W. & Williams, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds (Special
scientific report-wildlife no. 191). Washington DC, US Fish and
Wildlife Service.
Hunt, K.A., Bird, D.M., Mineau, P. & Shutt, L. (1991) Secondary
poisoning hazard of fenthion to American kestrels. Arch.
Environ. Contam. Toxicol., 21, 84-90.
Johansen, C.A., Mayer, D.F., Eves, J.D. & Kious, C.W. (1983)
Pesticides and bees. Environ. Entomol., 12, 1513-1518.
Kenaga, E.E. (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down to Earth, 35, 25-31.
Kendall, R.J. & Akerman, J. (1992) Terrestrial wildlife exposed to
agrochemicals: An ecological risk assessment perspective.
Environ. Toxicol. Chem., 11, 1727-1749.
Khangarot, B.S., Sehgal, A. & Bhasin, M.K. (1985) 'Man and
biosphere'-studies on the Sikkim Himalayas. Part 6: Toxicity of
selected pesticides to frog tadpole Rana hexadactyla (Lesson).
Acta Hydrochim. Hydrobiol., 13, 391-394.
Kira, M.T., Tawfik, M.F.S., & Metwally, S.M.I. (1972) Effect of DDT,
Gusathion and Lebaycid on corn pests and their predators. Bull.
Entomol. Soc. Egypt, 6, 221-230.
von Kniehase, U. & Zoebelein, G. (1990) [Testing the effects of
pesticides on the predator mite Phytoseiulus persimilis
Athias-Henriot by means of a new laboratory method approaching to
the practice.] Anz. Schaedlungskd. Pfianzensch. Umweltsch.,
63, 105-113 (in German).
Korn, S. & Earnest, R. (1974) Acute toxicity of twenty insecticides to
striped bass (Morone saxatilis). Calif. Fish Game, 60, 128-131.
Malcolm Pirnie Inc. (1987) The toxicity of Baytex technical, lot
no. 85RO1461 to Lemna gibba. Unpublished report No. 835 from
Malcolm Pirnie Inc., White Plains, New York, USA. Submitted to
WHO by Bayer AG.
Mayer, F.L. (1986) Acute toxicity handbook of chemicals to estuarine
organisms (Report no. EPA/600/X-86/231). Gulf Breeze, Texas, USA,
Environmental Research Laboratory, US Environmental Protection
Agency.
Mayer, F.L. & Ellersieck, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals (Resource publication 160). Washington DC, US
Department of the Interior, Fish and Wildlife Service.
McKenney, C.L. (1986) Influence of the organophosphate insecticide
fenthion on Mysidopsis bahia exposed during a complete life
cycle. I. Survival, reproduction and age-specific growth.
Dis. Aquat. Org., 1, 131-139.
Mobay Corp. (1978a) Soil adsorption and desorption of Baytex-
ring-1-14C. Unpublished report No. 66756 from Mobay Corp.
Agricultural Division, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1978b) The metabolism of Baytex-ring-1-13,14C on soil.
Unpublished report No. 66758 from Mobay Corp., Agricultural
Division, Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987a) Leaching characteristics of aged soil residues of
Baytex (Project No. BX-02-87). Unpublished report No. 94522 from
Mobay Corp., Agricultural Chemicals Division, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987b) Photodegradation of Baytex on soil (Project
No. BX-08-S). Unpublished report No. 94564 from Mobay Corp.,
Agricultural Chemicals Division, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
Mobay Corp. (1987c) Baytex Technical: Subacute dietary LC50 to
mallard ducks (Study No. 86-175-08). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987d) Baytex Technical: subacute dietary LC50 to
bobwhite quail (Study No. 86-175-09). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987e) Baytex (technical grade-acute LD50 to bobwhite
quail (Study No. 86-015-06). Unpublished report No. 831 from
Mobay Corp., Health, Environment and Safety Corporate Toxicology
Department, Stillwell, Kansas, USA. Submitted to WHO by Bayer AG.
O'Neill, E.J., Cripe, C.R., Mueller, L.H., Connolly, J.P. & Pritchard,
P.H. (1989) Fate of fenthion in salt-marsh environments: II.
Transport and biodegradation in microcosms. Environ. Toxicol.
Chem., 8, 759-768.
Patterson, R.S. & von Windeguth, D.L. (1964) The effects of Baytex on
some aquatic organisms. Mosq. News, 24, 46-49.
Powell, G.V.N. (1984) Reproduction by an altricial songbird, the
red-winged blackbird, in fields treated with the organophosphate
insecticide fenthion. J. Appl. Ecol., 21, 83-95.
Reissig, W.H., Heinrichs, E.A. & Valencia, S.L. (1982) Effects of
insecticides on Nilaparvata lugens and its predators: Spiders,
Microvelia atrolineata, and Cyrtorhinus lividipennis. Environ.
Entomol., 11, 193-199.
Saini, A. & Saxena, D.M. (1986) Effect of organophosphorus
insecticides on the growth of Tetrahymena pyriformis. Arch.
Protistenkd., 131, 143-152.
Schafer, W.E., Jr, Brunton, R.B., Lockyer, N.F & De Grazio, J.W.
(1973) Comparative toxicity of seventeen pesticides to the
quelea, house sparrow and red-winged blackbird. Toxicol. Appl.
Pharmacol., 26, 154-157.
Seabloom, R.W., Pearson, G.L., Oring, L.W. & Reilly, J.R. (1973) An
incident of fenthion mosquito control and subsequent arian
mortality. J. Wildl. Dis., 9, 18-20.
Seugé, J. & Bluzat, R. (1980) [Long-term toxicity of an
organophosphorus insecticide (fenthion) in the mollusc Lymnea
stagnalis.] Hydrobiology, 76, 241-248 (in French).
SLS Inc. (1988) The toxicity of technical grade fenthion (trade name
Baytex) to rainbow trout (Salmo gairdneri) embryos and larvae.
Unpublished report No. 95641 from Springborn Life Sciences Inc.,
Wareham, Massachusetts, USA. Submitted to WHO by Bayer AG.
TNO (1989) Reproduction test with Lebaycid EC 50 and Daphnia magna.
Unpublished report No. R 89/272 from TNO Division of Technology
for Society, Delft, Netherlands. Submitted to WHO by Bayer AG.
University of Southampton (1992) An evaluation of the side-effects of
Lebaycid 500 EC on larvae of the hoverfly Episyrphus balteatus
(Study No. BAY-91-1). Unpublished report from Agrochemical
Evaluation Unit, Department of Biology, University of
Southampton, Southampton, United Kingdom. Submitted to WHO by
Bayer AG.
US Department of Agriculture (1951) Soil Survey/Manual (Agriculture
Handbook No. 18). Washington DC, USA.
Van Meerendonk, J.H., Van Steenwijk, J.M., Phernambucq, A.J.W. &
Barreveld, H.L. (1994) [Tracking traces II. An inventory survey
for hazardous chemicals in the salt and freshwater systems in the
Netherlands] (RIKZ Report No. 94.007), The Hague, Netherlands,
National Institute for Coastal and Marine Management & National
Institute of Inland Water Management (in Dutch).
Wang, T.C., Lenaham, R.A., Tucker, J.W., Jr & Kadlac, T. (1987) Aerial
spray of mosquito adulticides in a salt marsh environment.
Int. Assoc. Water Qual., pp. 113-125.
WHO (1986) Organophosphorus Insecticides: A General Introduction
(Environmental Health Criteria 63). Geneva, International
Programme on Chemical Safety.
The following data are used in the calculations for situations
(a) and (b):
- water depth, 0.3 m
- 'worst-case' application rate on ornamental plants, three times 1
kg a.i./ha at seven-day interval, taking into account half-life
of seven days: 1.75 kg a.i./ha
- emission factor, 0.05 spray drift at 1 m
- tests periods of four days for algae, 21 days for crustaceans,
and 88 days for fish.
The half-life used is the longest value reported (see Table 3), which
can be considered a realistic 'worst-case' value. The lowest L(E)C50,
and NOEC values for algae, crustaceans, and fish were taken from
Tables 6 and 7.
It should be noted that limited information is available on
concentrations in the environment and toxicity. Only the water
compartment and a few species living in open water have been included.
Data on the toxicity of transformation products for aquatic organisms
were available only for 3-methyl-4-methylsulfinyl-phenol and
3-methylsulfonyl-phenol. The exposure concentrations are calculated
for the times equal to the duration of an experiment; e.g. the 21-day
NOEC for water fleas must be compared with the PEC21 days, calculated
as described above.
Table 9 gives a comparison of the estimated mean and maximal
exposure concentrations in the field with the lowest reported
L(E)C50 and NOEC values for acute and long-term exposure of
organisms in open water. The ratio between exposure and effect
concentration (TER) has been calculated. The Table is meant to serve
as a guide to classifying risk in the field and is not intended for
use in estimating the degree of effect likely to be seen. The
classification 'possible risk' is a simple one based on different
classification phrases for order-of-magnitude segments of the ratios.
Table 9 shows that crustaceans are at possible risk after either
acute or long-term exposure, but fish and algae appear not to be
affected. The few field observations available confirm the risk for
crustaceans (see above), but they are not altogether reliable, as some
relevant test conditions (e.g. physicochemical characteristics of the
water, temperature, actual concentrations of fenthion) are
incompletely described.
Table 10 gives a summary of results obtained for the example of
spraying ornamental plants. Crustaceans are again the organisms at
risk after either acute or long-term exposure. Algae are not affected,
and the effect on fish can be considered low.
It should be noted on the one hand that the risk classifications
shown in Tables 9 and 10 are for realistic 'worst-case' conditions:
the lowest reported values for toxicity were included; the slowest
reported dissipation rate was used; the concentrations used to
estimate mean exposure are only those from sites where fenthion was
actually detected; and the maximal estimated exposure concentration is
that used in aerial application for mosquito control. On the other
hand, only four groups of aquatic organisms are considered (e.g. no
sediment-dwelling organisms were included), adjuvants and transform-
ation products were not taken in to account, and the risks are not
relevant for water temperatures exceeding 22°C.
Table 9. Indications of environmental risk for aquatic organisms due to technical-grade fenthion
Effect Organism Estimated exposure Toxicity End-point Toxicity: Risk
concentration (µg/litre) exposure classification
(µg/litre) ratio
Mean estimated exposure concentration (monitoring programme)
Acute Crustaceans 0.020 EC50 = 5.7 Immobilization 285 Negligible
Acute Fish 0.020 LC50 = 830 Mortality > 1000 Negligible
Acute Amphibia 0.020 LC50 = 0.84 Mortality 42 Low
Chronic Algae 0.017 EC50 = 550 Biomass decrease > 1000 Negligible
Chronic Crustaceans 0.0084 NOEC = 0.018 Reproduction 2.1 Present
Chronic Fish 0.0023 MATC = 20a Reproduction > 1000 Negligible
Maximum estimated exposure concentration (salt-marsh)
Acute Crustaceans 1.7 EC50 = 5.7 Immobilization 3.4 Present
Acute Fish 1.7 LC50 = 830 Mortality 488 Negligible
Acute Amphibia 1.7 LC50 = 0.84 Mortality 0.5 High
Chronic Algae 1.4 EC50 = 550 Biomass decrease 393 Negligible
Chronic Crustaceans 0.71 NOEC = 0.018 Reproduction 0.03 Very high
Chronic Fish 0.2 MATC = 20a Reproduction 100 Negligible
a Mean of 13 and 27 µg/litre (see Table 7)
Table 10. Risk assessment for highest recommended rate of agricultural application based on
predicted environmental concentration
Effect Organism Preicted Toxicity End-point Toxicity: Risk
environmental (µg/litre) exposure classification
concentration ratio
(µg/litre)
Acute Crustaceans 29 EC50 = 5.7 Immobilization 0.2 High
Acute Fish 29 LC50 = 830 Mortality 28.6 Negligible
Acute Amphibia 29 LC50 = 0.84 Mortality 0.03 Very high
Chronic Algae 23.9 EC50 = 550 Biomass decrease 23.0 Negligible
Chronic Crustaceans 12.2 NOEC = 0.018 Reproduction 0.0015 Very high
Chronic Fish 3.3 MATC = 20 Reproduction 6.1 Present
Fenthion kills some beneficial insect predators, although the
results are variable; less effect was seen in the field than in the
laboratory. It is considered not to be hazardous to earthworms at
recommended rates of application. The risk to honey bees is considered
to be negligible provided they are not foraging. Fenthion is highly
acutely toxic to birds and is used as an avicide at high application
rates. Risk was assessed for both avicidal and general agricultural
use.
7.2 Use as an avicide
A single report has been made of secondary poisoning of a bird of
prey after ingestion of poisoned house sparrows. The dietary LC50
for the bobwhite quail is 60 mg/kg diet. On the basis of comparative
figures for body weight and food consumption, the LC50 values for
predatory birds that eat avian prey can be estimated from the
following equations to be 267 mg/kg diet for the European kestrel and
282 mg/kg diet for the sparrowhawk:
[Test species] LD50 [Test species] LD50 (mg/kg diet) × bw (kg)
(mg/kg per day) =
Food consumption (kg)
LC50 (mg/kg dry weight [Test species] LD50 (mg/kg diet) × bw (kg)
diet per day) =
Food consumption (kg)
The total daily intake that leads to death is 4.2 and 4.8 mg for the
two species, respectively. The residue concentrations reported in
house sparrows poisoned by fenthion used as an avicide were 6 µg/g in
carcass, 631 µg/g in feathers and skin, and 1152 µg/g in feet; the
total fenthion content of the carcass would be 166 µg on the basis of
a body weight of 27.7 g. Ingestion of the carcass of a sparrow should
not therefore result in a lethal dose, but ingestion of residues on
feathers and feet during plucking of the prey could result in a lethal
intake. A range of bird species may therefore have comparable
sensitivity to fenthion, and it can be assumed that any bird-eating
raptor would be killed by eating contaminated prey.
7.3 Agricultural use
Use of the vertebrate scheme of the European and Mediterranean
Plant Protection Organisation/Council of Europe and the highest
recommended agricultural application rate of 1.25 kg/ha gave predicted
environmental concentrations of 140 mg/kg for grass, 3.4 mg/kg for
insects, and 3.4 mg/kg for grains. The LC50 and TER values for
various bird species that use these items as food were estimated on
the basis of the data on toxicity for the bobwhite quail and are shown
in Table 11, which shows that use of fenthion at the highest
recommended application rate would be likely to cause deaths among
birds. The most susceptible species are those with a low body weight
that feed on vegetation.
Table 11. Toxicity:exposure ratios for birds after application of fenthion at 1.25 kg/ha
Species Estimated LC50 Predicted environmental Toxicity:exposure Risk
(mg/kg diet) concentration (mg/kg) ratio classification
Common quail (Coturnix coturnix) 211 140 1.5 Present
Greylag goose (Anser anser) 658 140 4.7 Present
Wren (Troglodytes troglodytes) 46 3.4 13.5 Low
Jackdaw (Corvus monedula) 285 3.4 84 Low
Reed bunting (Emberiza shoeniclus) 73 3.4 21.5 Low
Red-legged partridge (Alectroris nifa) 355 3.4 104 Very low
References
ABC Inc. (1986a) Acute flow-through toxicity of Baytex to rainbow
trout. Unpublished report No. 35313 by Analytical Biochemistry
Laboratories Inc., Columbia, Missouri, USA. Submitted to WHO by
Bayer AG.
ABC Inc. (1986b) Acute toxicity of Baytex to Selenastrum
capricornutum. Unpublished report No. 35314 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA.
Submitted to WHO by Bayer AG.
ABC Inc. (1987) Acute flow-through toxicity of Baytex to bluegill
sunfish (Lepomis macrochirus). Unpublished report No. 35597 by
Analytical Biochemistry Laboratories Inc., Columbia, Missouri,
USA. Submitted to WHO by Bayer AG.
ABC Inc. (1988) Soil adsorption/desorption with 14C-Baytex (Project
No. 36998). Unpublished report No. 98450 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA Submitted
to WHO by Bayer AG.
von Afify, A.M., Farghaly, H.T., & Hassanein, M.H. (1970) Freiland-
Untersuchungen über die Wirkung verschiedener Insektizide auf das
Vorkommen von zwei entomophagen Coccinelliden an Baumwolle in
Oberägypten. Schaedlungskd Pflanzensch. Umweltsch., 43, 8-13.
Bayer AG (1974) Verhalten des Pflanzenschutzmittelwirkstoffes im
Boden. Unpublished report No. RR5000/74 from Bayer AG
Pflanzenschutz Anwendungstechnik, Biologische Forschung,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1983a) Note on hydrolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG.
Bayer AG (1983b) Note on photolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG).
Bayer AG (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) by fenthion (technical). Unpublished report
No. 89082 from Bayer Institute of Environmental Biology,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1985b) Acute toxicity of fenthion (technical) to water
fleas. Unpublished report No. HBF/Dm 52 from Bayer Institute of
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988a) Degradation of fenthion ((R)Baytex) in the system
water/sediment (Project No. M 151 0186-2). Unpublished report
No. 3026 from Bayer AG Geschäftsbereich Pflanzenschutz, Institut
für Metabolismusforschung, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1988b) Degradation of crop protectants under anaerobic
conditions in the system water/sediment: fenthion (Project
No. 1520194-2). Unpublished report No. PF-3028 from
Bayer AG Geschäftsbereich Pflanzenschutz, Institut für
Metabolismusforschung, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988c) Method of estimating the direct photodegradation of
organic compounds in water under environmental conditions.
Unpublished report No. 2974 from Bayer AG Geschäftsbereich
Pflanzenschutz, Institut für Metabolismusforschung, Leverkusen,
Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989a) Influence of the commercial product (R)Lebaycid on
soil respiration after amendment with glucose (Study No. E 330
0279-4). Unpublished report No. AJO/71589 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989b) Influence of the commercial product (R)Lebaycid on
nitrification in soils (Study No. E 331 0278-4). Unpublished
report No. BSI/71689 from Bayer Crop Protection Research,
Chemical Product Development, Institute for Environmental
Biology, Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989c) Growth inhibition of green algae (Scenedesmus
subspicatus) by (R)Lebaycid EC 50 (Study No. E 323 0280-8).
Unpublished report No. HBF/A1 68 from Bayer Crop Protection
Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989d) Toxicity of (R)Lebaycid to earthworms (Study
No. E 310 0293-8). Unpublished report No. HBF/Rg 105 from Bayer
Crop Protection Research, Chemical Product Development, Institute
for Environmental Biology, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1994a) Calculation of the chemical lifetime of fenthion in
the troposphere. Unpublished report No. HPO-114 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994b) Studies on the ecological behaviour of Lebaycid N.
Unpublished report from Bayer AG, Institute for Environmental
Analysis and Assessments, Leverkusen, Germany. Submitted to WHO
by Bayer AG).
Bayer AG (1994c) Lebaycid 500 EC-acute toxicity (96 hours) to rainbow
trout in a static test. Unpublished revised study report No. DOM
94003 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994d) Lebaycid 500 EC-acute toxicity (96 hours) to golden
orfe in a static test. Unpublished revised study report No. DOM
94002 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1995) JMPE monograph for fenthion. Unpublished report of
28-03-95 submitted to WHO by Bayer AG.
Bowman & Assoc. (1989) Fenthion-acute toxicity test for freshwater
fish (Project No. FEN89C,D,E & F). Unpublished report No. 73916
from MC Bowman & Assoc., Inc., Mount Ida, Arkansas, USA.
Submitted to WHO by Bayer AG.
Bruggers, R.L., Jaeger, M.M., Keith, J.O., Hegdal, P.L., Bourassa,
J.B., Latigo, A.A. & Gillis, J.N. (1989) Impact of fenthion on
nontarget birds during Quelea control in Kenya. Wildl. Soc.
Bull., 17, 149-160.
ChemAgro (1972) The mobility and persistence of Baytex in soil and
water. Unpublished report No. 31985 from ChemAgro, Research and
Development Department, Kansas City, Missouri, USA Submitted to
WHO by Bayer AG.
ChemAgro (1974) Leaching of aged residues of Baytex-ring-UL-14C in
sandy loam soil. Unpublished report No. 40566 from ChemAgro,
Research and Development Department, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975a) Accumulation and persistence of residues in catfish
and bluegill fish exposed to Baytex-14C. Unpublished report
No. 43455 from ChemAgro Agricultural Division, Mobay Chemical
Corp., Research and Development, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975b) Leaching characteristics of Baytex. Unpublished
report No. 45653 from ChemAgro Agricultural Division, Mobay
Chemical Corp., Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
ChemAgro (1976a) Persistence and fate of Baytex in a natural aquatic
environment. Unpublished report No. 47404 from ChemAgro, Research
and Development Department, Kansas City, Missouri, USA. Submitted
to WHO by Bayer AG.
ChemAgro (1976b) Soil thin-layer mobility of twenty four pesticides
chemicals. Unpublished report No. 51016 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976c) Stability of Baytex in sterile aqueous buffer
solutions. Unpublished report No. 49130 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976d) Photodecomposition of Baytex. Unpublished report
No. 49347 from ChemAgro, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Conrad Appel GmbH (1989) Study on the effect of Lebaycid on
Trichogramma cacoeciae (Study No. 40/89 - 38/89). Unpublished
report from Conrad Appel GmbH, Department Trichogramma,
Darmstadt, Germany. Submitted to WHO by Bayer AG.
De Bruijn, J. & Hermens, J.L.M. (1991) Uptake and elimination kinetics
of organophosphorous pesticides in the guppy (Poecilia
reticulata): Correlations with the octanol/water partitioning
coefficient. Environ. Toxicol. Chem., 10, 791-804.
Derby, S.B. & Ruber, E. (1971) Primary production: Depression of
oxygen evolution in algal cultures by organophosphorus
insecticides. Bull. Environ. Contain. Toxicol., 5, 553-558.
DeWeese, L.R., McEwen, L.C., Settimi, L.A. & Deblinger, R.D. (1983)
Effects on birds of fenthion aerial application for mosquito
control. J. Econ. Entomol., 76, 906-911.
Eichelberger, J.W. & Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5, 541-544.
FAO/WHO (1973) 1971 Evaluations of some pesticide residues in food.
Report of the 1971 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues (WHO Technical Report No. 502), Geneva.
GAB Biotechnologie GmbH (1991) Detection of side effects of Lebaycid
on the green lacewing Chrysoperla carnea Steph. in the
laboratory (Study No. 008/01-Cc). Unpublished report from GAB
Biotechnologie GmbH, Niefern-Öschelbronn, Germany. Submitted to
WHO by Bayer AG.
Gohre, K. & Miller, G.C. (1986) Photooxidation of thioether pesticides
on soil surfaces. J. Agric. Food Chem., 34, 709-713.
Grue, C.E. (1982) Response of common grackles to dietary concen-
trations of four organophosphate pesticides. Arch. Environ.
Contain. Toxicol., 11, 617-626.
Henny, C.J., Kolbe, E.J., Hill, E.F. & Blus, L.J. (1987) Case
histories of bald eagles and other raptors killed by organophos-
phorus insecticides topically applied to livestock. J. Wildl.
Dis., 23, 292-295.
Hill, E.F., Heath, R.G., Sparta, J.W. & Williams, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds (Special
scientific report-wildlife no. 191). Washington DC, US Fish and
Wildlife Service.
Hunt, K.A., Bird, D.M., Mineau, P. & Shutt, L. (1991) Secondary
poisoning hazard of fenthion to American kestrels. Arch.
Environ. Contam. Toxicol., 21, 84-90.
Johansen, C.A., Mayer, D.F., Eves, J.D. & Kious, C.W. (1983)
Pesticides and bees. Environ. Entomol., 12, 1513-1518.
Kenaga, E.E. (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down to Earth, 35, 25-31.
Kendall, R.J. & Akerman, J. (1992) Terrestrial wildlife exposed to
agrochemicals: An ecological risk assessment perspective.
Environ. Toxicol. Chem., 11, 1727-1749.
Khangarot, B.S., Sehgal, A. & Bhasin, M.K. (1985) 'Man and
biosphere'-studies on the Sikkim Himalayas. Part 6: Toxicity of
selected pesticides to frog tadpole Rana hexadactyla (Lesson).
Acta Hydrochim. Hydrobiol., 13, 391-394.
Kira, M.T., Tawfik, M.F.S., & Metwally, S.M.I. (1972) Effect of DDT,
Gusathion and Lebaycid on corn pests and their predators. Bull.
Entomol. Soc. Egypt, 6, 221-230.
von Kniehase, U. & Zoebelein, G. (1990) [Testing the effects of
pesticides on the predator mite Phytoseiulus persimilis
Athias-Henriot by means of a new laboratory method approaching to
the practice.] Anz. Schaedlungskd. Pfianzensch. Umweltsch.,
63, 105-113 (in German).
Korn, S. & Earnest, R. (1974) Acute toxicity of twenty insecticides to
striped bass (Morone saxatilis). Calif. Fish Game, 60, 128-131.
Malcolm Pirnie Inc. (1987) The toxicity of Baytex technical, lot
no. 85RO1461 to Lemna gibba. Unpublished report No. 835 from
Malcolm Pirnie Inc., White Plains, New York, USA. Submitted to
WHO by Bayer AG.
Mayer, F.L. (1986) Acute toxicity handbook of chemicals to estuarine
organisms (Report no. EPA/600/X-86/231). Gulf Breeze, Texas, USA,
Environmental Research Laboratory, US Environmental Protection
Agency.
Mayer, F.L. & Ellersieck, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals (Resource publication 160). Washington DC, US
Department of the Interior, Fish and Wildlife Service.
McKenney, C.L. (1986) Influence of the organophosphate insecticide
fenthion on Mysidopsis bahia exposed during a complete life
cycle. I. Survival, reproduction and age-specific growth.
Dis. Aquat. Org., 1, 131-139.
Mobay Corp. (1978a) Soil adsorption and desorption of Baytex-
ring-1-14C. Unpublished report No. 66756 from Mobay Corp.
Agricultural Division, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1978b) The metabolism of Baytex-ring-1-13,14C on soil.
Unpublished report No. 66758 from Mobay Corp., Agricultural
Division, Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987a) Leaching characteristics of aged soil residues of
Baytex (Project No. BX-02-87). Unpublished report No. 94522 from
Mobay Corp., Agricultural Chemicals Division, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987b) Photodegradation of Baytex on soil (Project
No. BX-08-S). Unpublished report No. 94564 from Mobay Corp.,
Agricultural Chemicals Division, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
Mobay Corp. (1987c) Baytex Technical: Subacute dietary LC50 to
mallard ducks (Study No. 86-175-08). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987d) Baytex Technical: subacute dietary LC50 to
bobwhite quail (Study No. 86-175-09). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987e) Baytex (technical grade-acute LD50 to bobwhite
quail (Study No. 86-015-06). Unpublished report No. 831 from
Mobay Corp., Health, Environment and Safety Corporate Toxicology
Department, Stillwell, Kansas, USA. Submitted to WHO by Bayer AG.
O'Neill, E.J., Cripe, C.R., Mueller, L.H., Connolly, J.P. & Pritchard,
P.H. (1989) Fate of fenthion in salt-marsh environments: II.
Transport and biodegradation in microcosms. Environ. Toxicol.
Chem., 8, 759-768.
Patterson, R.S. & von Windeguth, D.L. (1964) The effects of Baytex on
some aquatic organisms. Mosq. News, 24, 46-49.
Powell, G.V.N. (1984) Reproduction by an altricial songbird, the
red-winged blackbird, in fields treated with the organophosphate
insecticide fenthion. J. Appl. Ecol., 21, 83-95.
Reissig, W.H., Heinrichs, E.A. & Valencia, S.L. (1982) Effects of
insecticides on Nilaparvata lugens and its predators: Spiders,
Microvelia atrolineata, and Cyrtorhinus lividipennis. Environ.
Entomol., 11, 193-199.
Saini, A. & Saxena, D.M. (1986) Effect of organophosphorus
insecticides on the growth of Tetrahymena pyriformis. Arch.
Protistenkd., 131, 143-152.
Schafer, W.E., Jr, Brunton, R.B., Lockyer, N.F & De Grazio, J.W.
(1973) Comparative toxicity of seventeen pesticides to the
quelea, house sparrow and red-winged blackbird. Toxicol. Appl.
Pharmacol., 26, 154-157.
Seabloom, R.W., Pearson, G.L., Oring, L.W. & Reilly, J.R. (1973) An
incident of fenthion mosquito control and subsequent arian
mortality. J. Wildl. Dis., 9, 18-20.
Seugé, J. & Bluzat, R. (1980) [Long-term toxicity of an
organophosphorus insecticide (fenthion) in the mollusc Lymnea
stagnalis.] Hydrobiology, 76, 241-248 (in French).
SLS Inc. (1988) The toxicity of technical grade fenthion (trade name
Baytex) to rainbow trout (Salmo gairdneri) embryos and larvae.
Unpublished report No. 95641 from Springborn Life Sciences Inc.,
Wareham, Massachusetts, USA. Submitted to WHO by Bayer AG.
TNO (1989) Reproduction test with Lebaycid EC 50 and Daphnia magna.
Unpublished report No. R 89/272 from TNO Division of Technology
for Society, Delft, Netherlands. Submitted to WHO by Bayer AG.
University of Southampton (1992) An evaluation of the side-effects of
Lebaycid 500 EC on larvae of the hoverfly Episyrphus balteatus
(Study No. BAY-91-1). Unpublished report from Agrochemical
Evaluation Unit, Department of Biology, University of
Southampton, Southampton, United Kingdom. Submitted to WHO by
Bayer AG.
US Department of Agriculture (1951) Soil Survey/Manual (Agriculture
Handbook No. 18). Washington DC, USA.
Van Meerendonk, J.H., Van Steenwijk, J.M., Phernambucq, A.J.W. &
Barreveld, H.L. (1994) [Tracking traces II. An inventory survey
for hazardous chemicals in the salt and freshwater systems in the
Netherlands] (RIKZ Report No. 94.007), The Hague, Netherlands,
National Institute for Coastal and Marine Management & National
Institute of Inland Water Management (in Dutch).
Wang, T.C., Lenaham, R.A., Tucker, J.W., Jr & Kadlac, T. (1987) Aerial
spray of mosquito adulticides in a salt marsh environment.
Int. Assoc. Water Qual., pp. 113-125.
WHO (1986) Organophosphorus Insecticides: A General Introduction
(Environmental Health Criteria 63). Geneva, International
Programme on Chemical Safety.
 The relative molecular mass of fenthion is 278.3. Technical-grade
fenthion has a purity of > 90%, generally exceeding 95%. Fenthion
is usually formulated as an emulsifiable concentrate. Emulsifiers,
such as Toximul MP-8, and solvents such as xylene, 'fog' (a fine water
spray), and diesel oil, may be added to formulations of fenthion, the
type of adjuvant differing according to the formulation. The addition
of oily materials increases the sorption of fenthion to plant surface
tissues. There is no up-to-date survey of the adjuvants currently used
in formulations.
2.2 Physical and chemical properties
The physical and chemical properties of fenthion are shown in
Table 1. Fenthion is slightly soluble in water at room temperature and
is readily soluble in organic solvents, the solubility in xylene,
acetone, acetonitrile, and dimethylsulfoxide exceeding 250 000
mg/litre. Fenthion is slightly volatile, in view of its vapour
pressure, and is essentially nonvolatile from water, in view of its
Henry's law constant. It is not surface active.
Table 1. Physical and chemical properties of fenthion
Property Remarks
Physical state Liquid
Colour Colourless to yellow brown
Odour Like mercaptans
Boiling-point 284°C Calculated at 1013 hPa
Specific gravity (density) 1.25 At 20°C
Vapour pressure 3.7 × 10-4 Pa Extrapolated at 20°C
7.4 × 10-4 Pa Extrapolated at 25°C
Solubility in water 4.2 mg/litre At 20°C
Solubility in n-hexane 100 000 mg/litre At 20°C
Solubility in n-octanol > 300 000 mg/litre
Henry's law constant 24 × 10-2 Pa . m3/mol Calculated at 20°C (with dimensions)
10 × 10-5 Calculated at 20°C (dimensionless)
Octanol-water partition 4.8 At 20°C
coefficient (log Kow)
Surface tension 70 mN/m
Explosiveness Not explosive
Oxidizing properties No oxidizing properties
Ignition temperature 365°C
Flash-point 170°C
Quantum yield 0.8
Data provided by Bayer AG for fenthion with a purity > 95%
3. Sources of human and environmental exposure
3.1 Production levels and processes
No data were available on world production of fenthion and its
formulations, and there were no data on losses to the environment
during normal production and formulation or accidental losses.
3.2 Uses
Fenthion is a systemic pesticide used primarily against insects
such as lice, ticks, cockroaches, flies, leaf-miners, rice stem
borers, and cereal pests. It can be used to protect livestock,
domestic animals, and various crops such as olives, sugar beet,
cotton, cacao, citrus, coffee, and rice. Agricultural use in the
countries of the European Union is primarily in horticulture and
orchards (Bayer AG, 1995). It is no longer used on cotton crops (Bayer
AG, personal communication). Fenthion is also used against birds such
as the red-billed weaver (Quelea quelea), the European starling
(Sturnus vulgaris), and the rock dove (Columbia livea), which can
destroy crops substantially. Bayer AG does not recommend its use in
urban areas (personal communication). It is also used for mosquito
control in wetlands.
Fenthion is used worldwide. In 1985, 39 417 litres of the
formulation Queletox were sprayed over 23 370 ha to control grain pest
birds in five eastern African countries (Bruggers et al., 1989). No
other quantitative data on the use of fenthion are available. FAO/WHO
(1973) estimated that the main uses of fenthion in 1971 were field
crops (50%), fruit and vines (30%), and other uses such as ornamental
plants, public health, and animal health (20%). In 1971, fenthion was
admitted for use on crops in 31 countries. It is registered for use in
bird control in Canada and the USA.
Fenthion can be applied in various formulations, summarized in
Table 2. This summary is far from complete, and some of these
formulations may have been withdrawn from the market. The major
formulations of fenthion used currently are probably Lebaycid EC50 and
Lebaycid EC500, which contain 535 and 506 g of fenthion per litre,
respectively. Other synonyms for fenthion and its formulations are
Baycid, Entex, BAY 29493, OM-2,51725, sulfidophos, quilitox, and Antex
(FAO/WHO, 1973).
Various other active ingredients can be mixed with fenthion. In
Germany, a mixture with propineb is registered. In Japan, various
mixtures were registered with disulfoton and edinphos, especially for
rice. The application rates of fenthion depend on the formulation and
type of use. Bayer AG (1995) recommends rates of 60-1250 g/ha for
various horticultural and fruit crops. FAO/WHO (1973) recommended
rates up to 2 kg/ha on cotton. Lebaycid is generally applied as a
0.05-0.075% solution in water by spraying. The most commonly used
formulations contain an emulsifiable concentrate, a fogging
concentrate or an ultra-low volume liquid, the last being frequently
used under (sub)tropical conditions to minimize losses by
volatilization. Fenthion can also be applied as granules on rice or as
a dustable powder.
Table 2. Composition of some commercial formulations of fenthion
Name Concentration of Remarks
fenthion (g/litre)
Lebaycid 50EC 535 Also contains 2% of emulgator A, 8% of
emulgator B, and up to 39% xylene
Lebaycid 500EC 506
Rid-A-Bird 1100 110
Tiguvon Not reported
Baytexa Not reported Sprayed with 'fog' or diesel oil
Queletox Not reported
All formulations produced by Bayer AG; data provided by Bayer AG
a Liquid concentrate with 93% pure fenthion, which can be mixed with the
emulsifier Toximul MP-8 and a xylene-type solvent at ratios of 30:3.1:1.7;
can also be mixed with diesel oil (Powell, 1984)
Fenthion can be applied in various ways. For large-scale
treatments, aerial application may be appropriate; small-scale
treatment can be done with e.g. back-pack spraying equipment or behind
vehicles. Aerial application may lead to substantial losses due to
drift by wind, and crops, flora, and fauna may be exposed to
off-target deposits. Such downwind deposits can be assumed to depend
on meteorological conditions, the plant canopy structure, the
application method, including the release height, the droplet size,
the occurrence of overlapping spray swathes, the calibration of the
spraying apparatus, the placement of nozzles on the boom, and the
speed of the aircraft (Seabloom et al., 1973; DeWeese et al.,
1983; Bruggers et al., 1989).
4. Environmental transport, distribution, and transformation
The term 'biotransformation' is used in this monograph in
preference to 'biodegradation', as the first refers to a microbial
transformation process resulting in a smaller or greater molecule,
whereas the latter refers to a smaller molecule only. Fenthion
molecules can either increase or decrease in size. Dissipation is the
decrease in size due to microbial activity, to other chemical
transformation processes, and to transfer to other compartments. As
the metabolism of fenthion is preferably called transformation rather
than degradation, the term half-life is preferred to median
dissipation rate (DT50). Thus, confusion about whether D refers to
degradation or dissipation is avoided. It should become clear from the
context whether the half-life refers to a particular process.
The types of sediment or soil mentioned are within the textural
groupings of the system of the US Department of Agriculture (1951).
Only the results of reliable tests are discussed below. In
general, only studies with an adequate method and description are
listed in the tables. Studies that were considered less or not
reliable are sometimes mentioned in the text in order to confirm or
contradict conclusions based on the results of intrinsically reliable
tests.
4.1 Transport and distribution between media
4.1.1 Dissipation from water
Fenthion dissipates rapidly from water in laboratory experiments
under both aerobic and anaerobic conditions, with half-lives up to
seven days (Eichelberger & Lichtenberg, 1971; Bayer AG, 1988a,b;
O'Neill et al., 1989; see also Table 3). Dissipation under anaerobic
conditions is slightly slower than that under aerobic conditions, but
the rate follows first-order kinetics under either condition. The
half-lives shown in Table 3 were deduced from laboratory experiments
with sediment. Dissipation from water appears to occur primarily by
sorption to sediment, phototransformation, and biotransformation (see
sections 4.2.2 and 4.3).
In an indoor microcosm, the presence of plants in the sediment
did not influence dissipation from the water column (O'Neill et al.,
1989). In salt-marsh water and its associated sediment, half of the
applied technical-grade fenthion was dissipated within 1.5 days.
Whereas the parent compound dissipates rapidly from a water
column, the residues, including transformation products, do not
necessarily do so. In a laboratory experiment with water, sediment,
and fish, the total amount of 14C-fenthion residues in the water
remained fairly constant over 28 days after a single application of
fenthion equivalent to 0.011 kg/ha (ChemAgro, 1975a). Immediately
after application, however, 100% of the recovered residues was soluble
in chloroform, whereas after 28 days 66% was water-soluble, indicating
that the composition had shifted towards more polar residues, probably
by phototransformation and biotransformation.
In outdoor experiments, fenthion also dissipated rapidly from the
water column. In jars containing pond water, with or without the
accompanying sediment, that were buried outdoors in the soil for 16
days in Kansas (USA) in 1971, fenthion dissipated with a half-life of
up to two days (ChemAgro, 1972, 1976a). In one of these experiments,
the dissipation rate in the system with sediment was comparable to
that in the system without sediment. This experiment corroborates the
report of Bayer AG (1988a) that other processes influence dissipation
before accumulation in the sediment, regardless of the presence of
sediment. In the other experiment (ChemAgro, 1976a), sediment was an
important sink, as the sediment-bound residues in silt gradually
increased up to 49% of the applied activity after 46 days. Detection
of fenthion in Dutch surface water at concentrations up to
0.12 µg/litre, however, may indicate that dissipation in the field is
less rapid than that under more controlled conditions (see section
5.1).
4.1.2 Soil sorption
Fenthion binds readily to various soils. In laboratory
experiments in which fenthion was added to aqueous soil suspensions,
the adsorption coefficient Ks/l (= Kd = Kom) was 7.7-38 dm3/kg
in six soil types ranging from sand to silty clay loam (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988). These values indicate
strong sorption, which can be described by the Freundlich equation.
Three additional Ks/l values (6.4, 8.6, and 67 dm3/kg) obtained in
these experiments must be considered inaccurate, as the accompanying
1/n constants deviate substantially from unity (< 0.7 or > 1.1).
The mechanism of sorption of fenthion to soil is only partially
understood, and several factors may be involved. Sorption to organic
matter appears to be important, as soils with the highest organic
matter content showed the highest adsorption coefficients (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988).
In a laboratory experiment with 14C-fenthion, the desorption
coefficients were about two times higher than the sorption coefficents
(ABC Inc., 1988). Although 0.01 mol/litre of calcium chloride were
added to simulate potential desorption in the field, sorption may not
be reversible under outdoor conditions.
Table 3. Biotransformation and dissipation of technical-grade fenthion in water and sediments in the laboratory
Water type Sediment Test Sediment Organic matter Temperature pH Length of Half-life Reference
type (%) in sediment (%) (°C) experiment (days)
(days)
Biotransformation in whole system
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8. 66 9a Bayer
in dark approx. 8 (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 11a,b Bayer
in dark (1988b)
Dissipation from water column
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8.8 66 4a Bayer
in dark (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 7a Bayer
in dark (1988b)
Surface waterc Not Aerobic Not Not 20 Not 8 1.5 O'Neill et al.
reported with reported reported reported (1989)
light-dark
cycle
a Approximate value derived from authors' data by linear regression of the recovered percentages after logarithmic
(In) transformation. Times until 90-99% of the applied fenthion has been transformed were used for calculation.
b Although the half-life under anaerobic conditions equals that under aerobic conditions, the initial transformation
rate under anaerobic conditions is slower
c Water from a salt marsh with 11% salinity
4.1.3 Soil mobility
In view of its Ks/l values, fenthion should be immobile in many
soils, as confirmed in several experiments both in the laboratory and
in the field. In thin-layer chromatography studies, which do not
generate reliable Ks/l values, the Rf values for 14C-fenthion in
five soil types with organic matter contents of 0.6-5.1% were
0.14-0.17 (ChemAgro, 1976b). In less reliable leaching experiments
with columns of 45 cm, in which the leaching time was not reported,
water fluxes of 50, 95, and 490 ml were imposed on a sandy loam with
1.4% organic matter, a silty clay loam with 2% organic matter, and a
silty clay loam with 4.4% organic matter, respectively (ChemAgro,
1972). Fenthion was recovered only in the leachate of the silty clay
loam with a high organic matter content, representing 1.8% of the
recovered fenthion. In these experiments, 64-92% of the recovered
fenthion was found in the upper 3-cm layer of the columns.
Transformation products of fenthion appear to be more mobile than
fenthion itself; however, the only data on the mobility of transform-
ation products are those from leaching experiments with aged residues.
The adsorption coefficients of metabolites have not been reported.
In studies under laboratory or greenhouse conditions with
residues aged for 4-30 days, 1.9-46% of the applied activity was
leached (ChemAgro, 1974, 1975b; Mobay Corp., 1987a). The greatest
amount of leaching was found in a sandy soil with the lowest
percentage of organic matter (0.2%) of all soils tested, with 36%
leaching after four days of aging and 46% after 25 days (Mobay Corp.,
1987a). The columns were 30 cm long, the water flux over two days was
high (51 cm), and the soil that had been aged prior to leaching was a
sandy loam soil. These experiments cannot be considered reliable, as
the type of soil placed on the top of the column was different from
that inside the column, and the amounts of fenthion residues after
aging and before application on the column were not reported. In spite
of the high maximal leaching percentages in sand, more than 50% of the
applied activity was recovered in the upper 12 cm of the columns. Four
transformation products were recovered in the sand column segments and
leachates. Those in the leachate, in decreasing order of magnitude,
were 3-methyl-4-methylsulfonyl-phenol, 3-methyl-4-methylsulfinyl-
phenol, fenthion sulfoxide (thiophosphoric acid, O,O-dimethyl- O-
[3-methyl-4-methylsulfinyl-phenyl] ester), and fenthion sulfone
(thiophosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfonyl-
phenyl] ester). The maximal percentages of recovered activity at the
end of the experiments were 20, 15, 11, and 0.8%, respectively. It can
be concluded that at least the more polar transformation products of
fenthion, which generally remain primarily organosoluble, may leach
into shallow groundwater; however, as the half-lives of these
transformation products are probably < 15 days (see section 4.3.2),
substantial leaching of these products to shallow groundwater is
unlikely.
Table 4. Biotransformation and phototransformation of fenthion in soils
Soil type Test type Moisture Temperature pH Organic Length of Half-life Reference
content (%) (°C) matter (%) experiment (days)
(days)
Biotransformation
Silty loama Laboratory, approx. 30 Not 5.9 3.0 120 10c Mobay Corp.
aerobic reportedb (1978b)
Silty loama Laboratory, approx. 30 Not 5.9 3.0 32 > 32 Mobay Corp.
aerobic reportedb (1978b)
Phototransformation
Sandy loam Field, Not 16-37 5.1 2.4 1.2 1c Mobay Corp.
aerobicd reported (1987b)
Sandy loam Field, Not Not 4.5-7.5 0.8-6.3 4 > 4 Gohre & Miller
aerobic reported reported (1986)
a Silty loam is assumed to have a moisture content of 40% (v:v) at field capacity; moisture content was kept at 75% of the
field capacity
b Possibly room temperature, but not clearly stated
c Approximate value derived from authors' data of by linear regression of the recovered percentages after logarithmic
(In) transformation. As transformation of up to 99% of the applied fenthion was in accordance with first-order
kinetics, the coinciding times were used for calculation.
d A slurry of soil, water, and acetonitrile in a photomodule was exposed to fenthion by pipetting it onto the slurry.
4.1.4 Dissipation from soil in the field
No substantial dissipation of fenthion due to run-off on an 8°
slope was found on a sandy loam or a silty clay loam soil on a fallow,
raked field (ChemAgro, 1972). The silty clay loam site had been
divided into one with a low (2%) and the other with a high (4.4%)
organic matter content. Maximal percentages of 0.9% and 1.2% of the
applied fenthion were recovered in the run-off water as the parent
compound and fenthion sulfoxide, respectively, in the sandy loam and
silty clay loam with the low organic matter contents, corresponding to
concentrations of 0.12 and 0.23 µg/litre, respectively. The lowest
run-off of fenthion residues and the largest amount of fenthion
residues (at the site of application) were seen on soil with the
highest percentage of organic matter, coinciding with the strong
sorption of fenthion on various soils. As the soil and the run-off
water were analysed after weekly irrigation for one month, the
dissipation of fenthion must be low, due mainly to strong sorption
(see also section 4.1.2). In these experiments, the soils were
irrigated for one month with 10-17 cm of water (total), and the rate
of application of fenthion was 11.2 kg/ha. The results indicate that
dissipation of fenthion under outdoor conditions may be slower than
that under controlled laboratory conditions. Thirty days after
application, up to 38 and 55% of the applied fenthion were recovered
at the site of application as the parent compound and fenthion
sulfoxide, respectively. Dissipation in laboratory experiments due to
microbial action is much faster (see Table 4 and section 4.3).
4.1.5 Uptake and dissipation from plants
Fenthion can be taken up by plants, and its ability to penetrate
plant tissues results in destruction of e.g. larvae even within fruit.
Fenthion is reported to persist for some time after being taken up.
The amount of fenthion residues in Bermuda grass (Cynodon dactylon)
and corn (Zea mays) decreased by about 60 and 80% within the first
two weeks after application (FAO/WHO, 1973).
4.1.6 Entry into the food chain
Fenthion is used primarily against insects and birds (see section
3.1.2). As the amount of fenthion found in these species after
ingestion or on them after contact may increase immediately after
application, it may enter the food chain by ingestion or direct
contact with e.g. insectivorous and bird-predatory species. Hunt
et al. (1991) investigated the possibility of secondary poisoning of
the American kestrel (Falco sparverius) through predation on house
sparrows (Passer domesticus) exposed to fenthion by smearing of
their perches with the formulation Rid-A-Bird(R). Exposure of the
kestrels by contact with the sparrows' feet may have contributed
substantially to the observed toxicity. When the contaminated sparrows
were penned with the kestrels for three days, 79% of the kestrels died
from fenthion poisoning within one day, and all were dead within three
days. The contaminated kestrels showed 78-92% depression of brain
cholinesterase activity and 97% of that in plasma, which correlated
with concentrations of fenthion up to 14 µg/g in the gastrointestinal
tract and 19 µg/g in feet. Most of the contaminated sparrows died
within 8 h of exposure and contained fenthion at concentrations of up
to 6 µg/g in the internal carcass, 631 µg/g in the external carcass
(i.e. feathers and skin), and 1152 µg/g in the feet.
The route of exposure before secondary intoxication is often
unclear. In five bald eagles (Haliaeetus leucocephalus) found dead
in Iowa (USA) in 1984, brain cholinesterase activity was depressed by
80-92% (Henny et al., 1987). The remains of piglets found in their
stomachs contained fenthion at concentrations of 0.1-6.8 mg/kg wet
weight. The two possible sources of the fenthion are intentional
primary poisoning of the eagles with contaminated bait or secondary
poisoning from contaminated piglets. This case indicates the
vulnerability of scavenging species like eagles.
4.2 Abiotic transformation
4.2.1 Hydrolytic cleavage and oxidation
Fenthion is transfomed slowly in sterile phosphate buffers, with
half-lives of 56, 41, and 32 days at pH 5, 7, and 9, respectively, at
25°C for 70 days (ChemAgro, 1976c); comparable results were found at 5
and 40°C. These results indicate slower transformation rates under
more acid conditions. This pH-dependent transformation was confirmed
in other studies (eg. Bayer AG, 1983a). In the experiments of ChemAgro
(1976c) at 25°C, the main transformation products were fenthion
sulfoxide, 3-methyl-4-methylsulfinyl-phenol, fenoxon (phosphoric acid,
O,O-dimethyl- O-[3-methyl-4-methylthio-phenyl] ester), fenoxon
sulfone (phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methyl-
sulfonyl-phenyl] ester), fenthion sulfone, and fenoxon sulfoxide
(phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfinyl-
phenyl] ester). The maximal percentages of the administered activity
were 14, 12, 10, 8, 7, and 6%, respectively. The transformation
products indicate both oxidation (fenthion sulfoxide, fenoxon, fenoxon
sulfone, fenthion sulfone, and fenoxon sulfoxide) and hydrolysis
(3-methyl-4-methylsulfinyl-phenol).
A low abiotic transformation rate in the dark was confirmed in
other laboratory experiments (ChemAgro, 1972; Bayer AG, 1983a). In
buffered aqueous solutions, the extrapolated half-lives at 22°C were
223, 200, and 151 days at pH 4, 7, and 9, respectively (Bayer AG,
1983a), and the transformation product was 3-methyl-4-methylthio-
phenol. In aqueous phosphate buffers at 30°C, the experimental
half-lives at pH 5, 7, and 9 were 31, 26, and 24 days, respectively
(ChemAgro, 1972).
4.2.2 Phototransformation
(a) Water
Photochemical transformation may occur rapidly in water under
both laboratory and field conditions. In a sterile aqueous buffer at
pH 5, substantial phototransformation of 13C/14C-1-ring-labelled
fenthion was observed after exposure to artificial light simulating
sunlight for 4 h at 23°C (Mobay Corp., 1987c); the half-life was
0.5 h. Rapid phototransformation in the laboratory was confirmed by
Bayer AG (1983b, 1988c) and ChemAgro (1976b).
The major photochemical transformation products in the study
reported by the Mobay Corp. (1987c) were 3-methyl-4-methylthio-phenol
(maximal totally recovered activity, 23%), fenthion sulfoxide
(maximum, 17%), and 3-methyl-4-methylsulfinyl-phenol (maximum, 15%).
Transformation products found in minor quantities in this experiment
were 3-methyl-4-sulfo-phenol (maximum, 8%), fenoxon sulfone (maximum,
7%), fenoxon sulfoxide (maximum, 6%), and 3-methyl-4-methylsulfonyl-
phenol (maximum, 6%). Fenoxon was also found as a phototransformation
product in other experiments (maximum, 10-11% of the applied activity)
(ChemAgro, 1976b).
The phototransformation pattern is temperature-dependent: at
25°C, the rate of production of fenthion sulfoxide via oxidation is
comparable to that of 3-methyl-4-methylthio-phenol via hydrolysis.
Oxidation to fenoxon sulfoxide is assumed to prevail at higher
temperatures and hydrolysis to 3-methyl-4-methylthio-phenol at lower
temperatures (ChemAgro, 1976d). When 2% acetone was added to an
aqueous solution of fenthion as a photosensitizer before irradiation
with artificial light for 2 h, oxidation via fenthion sulfoxide to
unknown polar transformation products was accelerated. Thus, changes
in temperature and the presence of sensitizers can result in different
mixtures of fenthion transformation products.
Fenthion also undergoes rapid phototransformation outdoors,
although possibly at a somewhat slower rate than in laboratory
experiments. Fenthion in distilled water exposed to sunlight in the
summer was phototransformed with a half-life of 4 h (Bayer AG, 1983b);
in a comparable experiment under laboratory conditions with artificial
light, the half-life was 0.08 h. Fenthion is phototransformed rapidly
in sunlight (lambda > 290 nm), primarily because of its high quantum
yield.
(b) Soil
As fenthion applied to soil appears to be phototransformed
rapidly by sunlight, with half-lives of 1-> 4 days (see Table 4),
phototransformation is an important route of dissipation. In an
outdoor experiment, 13C/14C-1-ring-labelled fenthion mixed with
unlabelled fenthion was exposed for 1.2 days to natural sunlight after
application to a sandy loam at a rate of 53 mg/kg soil (see also Table
4). Fenthion was rapidly phototransformed, and the main photochemical
transformation product was fenthion sulfoxide (maximum, 58% of the
recovered activity); fenoxon sulfoxide, 3-methyl-4-methylsulfinyl-
phenol, and fenthion sulfone were minor transformation products (i.e.
< 10% of the recovered activity). The oxidation path apparently
prevails (Mobay Corp., 1987b). As the temperature during this
experiment rose to about 37°C, a shift to the oxidation rather than to
the hydrolysis pathway seems to have occurred, as in the
phototransformation experiments with water (ChemAgro, 1976d).
(c) Air
Phototransformation occurs rapidly in the troposphere. The
half-life of fenthion in air is estimated to be 1.7 h (Bayer AG,
1994a) on the basis of the assumption that phototransformation occurs
via the reaction of hydroxyl radicals with fenthion. The primary
target of the radicals is probably the P=S bond. Such attacks may
result in secondary oxidation products (e.g. fenoxon), which can be
deposited on soil, water, and vegetation by wet and dry deposition.
4.3 Biotransformation
Biotransformation may contribute to the general processes of
dissipation (see section 4.1). No data from primary sources on the
microorganisms that can metabolize fenthion have been incorporated in
this monograph. It has been reported to be biotransformed to fenoxon
sulfoxide by the soil fungus Rhizopus japonicus (Wallnöfer, 1978,
cited by Mobay Corp., 1978b). Similarly, no data on the biodegrad-
ability of the adjuvants in formulations of fenthion have been
incorporated; however, it can be assumed that the biodegradability of
e.g. 'fog' and diesel oil in a natural environment is slight.
A simplified scheme, showing some common transformation products
in environmental compartments, is presented in Figure 1. The scheme
includes both abiotic and biotic transformation products.
4.3.1 Water and sediment
The relevant laboratory experiments in which biotransformation in
water-sediment systems was studied were summarized in Table 3. The
results indicate that the rate of biotransformation is rapid under
both aerobic and anaerobic conditions: 50% biotransformation of
fenthion (half-life) in a whole water-sediment system occurs within
about 10 days. Experimental conditions other than the availability of
oxygen, e.g. temperature, salinity, the presence of plants, the type
of water, and the type of sediment, probably have less effect on the
transformation rate. In microcosms of salt-marsh water, sediment and
sediment-rooted plants, however, the amount of detritus and the extent
of bioperturbation of the sediment may also influence the biotrans-
formation rate (O'Neill et al., 1989).
In general, sediments appear to function as a major sink for
fenthion under both aerobic and anaerobic conditions. Rapid
partitioning to sediment was seen under aerobic and anaerobic
conditions, as 21-28% of the applied activity was recovered in the
sediment within 1 h after application (Bayer AG, 1988a,b); maxima of
41-80% of the applied activity were recovered in sediment. Under
aerobic conditions, the amount in the sediment increased gradually up
to 62-80% of the applied activity (Bayer AG, 1988a) by the end of the
test at 66 days. Under anaerobic conditions, the maxima were 66-70%
and were reached after 7-14 days (Bayer AG, 1988b). The total amounts
of activity in the sediments decreased thereafter, probably due to
further mineralization to 14C-carbon dioxide (see below). Under
either aerobic or anaerobic incubation, most of the activity in the
sediment shifted from water-and acetone-extractable compounds to
non-extractable (i.e. sediment-bound) residue in the course of the
experiments.
The rate of biotransformation in non-sterile sediment without
plants was best modelled by assuming biotransformation in the upper
1 mm of the sediment (O'Neill et al., 1989). When plants were
present, biotransformation in the upper 7 mm was the best assumption.
In aerobic experiments with water and associated sediments, the
amount of fenthion declines rapidly over time, with transient
increases in various metabolites, an increase in 14C-carbon dioxide,
and an increase in sediment-bound residues (Bayer AG, 1988a,b). Under
anaerobic conditions, a small amount (3-4%) of 14C-methane was
formed. The production of 14C-carbon dioxide appears to be more
rapid under aerobic than anaerobic conditions, but the final amounts
of 14C-carbon dioxide differed substantially with the duration of
the test: 12-15% after 66 days under aerobic conditions and 52% after
120-190 days under anaerobic conditions. As the amount of oxygen was
very low under anaerobic conditions (0-0.4 mg/litre), the oxygen in
the carbon dioxide may have been supplied by nitrates or sulfates. The
redox potential during the experiment varied between -186 and 137 mV.
The main transformation products were demethyl fenoxon sulfoxide
(maximum, 30% of whole water-sediment system after 7-14 days),
fenthion sulfoxide (maximum, 28% after 14 days), 3-methyl-4-methyl-
sulfinyl-phenol (maximum, 13% after 66 days), and 3-methyl-4-methyl-
sulfonyl-phenol (maximum, 11% after 66 days) under aerobic conditions,
The relative molecular mass of fenthion is 278.3. Technical-grade
fenthion has a purity of > 90%, generally exceeding 95%. Fenthion
is usually formulated as an emulsifiable concentrate. Emulsifiers,
such as Toximul MP-8, and solvents such as xylene, 'fog' (a fine water
spray), and diesel oil, may be added to formulations of fenthion, the
type of adjuvant differing according to the formulation. The addition
of oily materials increases the sorption of fenthion to plant surface
tissues. There is no up-to-date survey of the adjuvants currently used
in formulations.
2.2 Physical and chemical properties
The physical and chemical properties of fenthion are shown in
Table 1. Fenthion is slightly soluble in water at room temperature and
is readily soluble in organic solvents, the solubility in xylene,
acetone, acetonitrile, and dimethylsulfoxide exceeding 250 000
mg/litre. Fenthion is slightly volatile, in view of its vapour
pressure, and is essentially nonvolatile from water, in view of its
Henry's law constant. It is not surface active.
Table 1. Physical and chemical properties of fenthion
Property Remarks
Physical state Liquid
Colour Colourless to yellow brown
Odour Like mercaptans
Boiling-point 284°C Calculated at 1013 hPa
Specific gravity (density) 1.25 At 20°C
Vapour pressure 3.7 × 10-4 Pa Extrapolated at 20°C
7.4 × 10-4 Pa Extrapolated at 25°C
Solubility in water 4.2 mg/litre At 20°C
Solubility in n-hexane 100 000 mg/litre At 20°C
Solubility in n-octanol > 300 000 mg/litre
Henry's law constant 24 × 10-2 Pa . m3/mol Calculated at 20°C (with dimensions)
10 × 10-5 Calculated at 20°C (dimensionless)
Octanol-water partition 4.8 At 20°C
coefficient (log Kow)
Surface tension 70 mN/m
Explosiveness Not explosive
Oxidizing properties No oxidizing properties
Ignition temperature 365°C
Flash-point 170°C
Quantum yield 0.8
Data provided by Bayer AG for fenthion with a purity > 95%
3. Sources of human and environmental exposure
3.1 Production levels and processes
No data were available on world production of fenthion and its
formulations, and there were no data on losses to the environment
during normal production and formulation or accidental losses.
3.2 Uses
Fenthion is a systemic pesticide used primarily against insects
such as lice, ticks, cockroaches, flies, leaf-miners, rice stem
borers, and cereal pests. It can be used to protect livestock,
domestic animals, and various crops such as olives, sugar beet,
cotton, cacao, citrus, coffee, and rice. Agricultural use in the
countries of the European Union is primarily in horticulture and
orchards (Bayer AG, 1995). It is no longer used on cotton crops (Bayer
AG, personal communication). Fenthion is also used against birds such
as the red-billed weaver (Quelea quelea), the European starling
(Sturnus vulgaris), and the rock dove (Columbia livea), which can
destroy crops substantially. Bayer AG does not recommend its use in
urban areas (personal communication). It is also used for mosquito
control in wetlands.
Fenthion is used worldwide. In 1985, 39 417 litres of the
formulation Queletox were sprayed over 23 370 ha to control grain pest
birds in five eastern African countries (Bruggers et al., 1989). No
other quantitative data on the use of fenthion are available. FAO/WHO
(1973) estimated that the main uses of fenthion in 1971 were field
crops (50%), fruit and vines (30%), and other uses such as ornamental
plants, public health, and animal health (20%). In 1971, fenthion was
admitted for use on crops in 31 countries. It is registered for use in
bird control in Canada and the USA.
Fenthion can be applied in various formulations, summarized in
Table 2. This summary is far from complete, and some of these
formulations may have been withdrawn from the market. The major
formulations of fenthion used currently are probably Lebaycid EC50 and
Lebaycid EC500, which contain 535 and 506 g of fenthion per litre,
respectively. Other synonyms for fenthion and its formulations are
Baycid, Entex, BAY 29493, OM-2,51725, sulfidophos, quilitox, and Antex
(FAO/WHO, 1973).
Various other active ingredients can be mixed with fenthion. In
Germany, a mixture with propineb is registered. In Japan, various
mixtures were registered with disulfoton and edinphos, especially for
rice. The application rates of fenthion depend on the formulation and
type of use. Bayer AG (1995) recommends rates of 60-1250 g/ha for
various horticultural and fruit crops. FAO/WHO (1973) recommended
rates up to 2 kg/ha on cotton. Lebaycid is generally applied as a
0.05-0.075% solution in water by spraying. The most commonly used
formulations contain an emulsifiable concentrate, a fogging
concentrate or an ultra-low volume liquid, the last being frequently
used under (sub)tropical conditions to minimize losses by
volatilization. Fenthion can also be applied as granules on rice or as
a dustable powder.
Table 2. Composition of some commercial formulations of fenthion
Name Concentration of Remarks
fenthion (g/litre)
Lebaycid 50EC 535 Also contains 2% of emulgator A, 8% of
emulgator B, and up to 39% xylene
Lebaycid 500EC 506
Rid-A-Bird 1100 110
Tiguvon Not reported
Baytexa Not reported Sprayed with 'fog' or diesel oil
Queletox Not reported
All formulations produced by Bayer AG; data provided by Bayer AG
a Liquid concentrate with 93% pure fenthion, which can be mixed with the
emulsifier Toximul MP-8 and a xylene-type solvent at ratios of 30:3.1:1.7;
can also be mixed with diesel oil (Powell, 1984)
Fenthion can be applied in various ways. For large-scale
treatments, aerial application may be appropriate; small-scale
treatment can be done with e.g. back-pack spraying equipment or behind
vehicles. Aerial application may lead to substantial losses due to
drift by wind, and crops, flora, and fauna may be exposed to
off-target deposits. Such downwind deposits can be assumed to depend
on meteorological conditions, the plant canopy structure, the
application method, including the release height, the droplet size,
the occurrence of overlapping spray swathes, the calibration of the
spraying apparatus, the placement of nozzles on the boom, and the
speed of the aircraft (Seabloom et al., 1973; DeWeese et al.,
1983; Bruggers et al., 1989).
4. Environmental transport, distribution, and transformation
The term 'biotransformation' is used in this monograph in
preference to 'biodegradation', as the first refers to a microbial
transformation process resulting in a smaller or greater molecule,
whereas the latter refers to a smaller molecule only. Fenthion
molecules can either increase or decrease in size. Dissipation is the
decrease in size due to microbial activity, to other chemical
transformation processes, and to transfer to other compartments. As
the metabolism of fenthion is preferably called transformation rather
than degradation, the term half-life is preferred to median
dissipation rate (DT50). Thus, confusion about whether D refers to
degradation or dissipation is avoided. It should become clear from the
context whether the half-life refers to a particular process.
The types of sediment or soil mentioned are within the textural
groupings of the system of the US Department of Agriculture (1951).
Only the results of reliable tests are discussed below. In
general, only studies with an adequate method and description are
listed in the tables. Studies that were considered less or not
reliable are sometimes mentioned in the text in order to confirm or
contradict conclusions based on the results of intrinsically reliable
tests.
4.1 Transport and distribution between media
4.1.1 Dissipation from water
Fenthion dissipates rapidly from water in laboratory experiments
under both aerobic and anaerobic conditions, with half-lives up to
seven days (Eichelberger & Lichtenberg, 1971; Bayer AG, 1988a,b;
O'Neill et al., 1989; see also Table 3). Dissipation under anaerobic
conditions is slightly slower than that under aerobic conditions, but
the rate follows first-order kinetics under either condition. The
half-lives shown in Table 3 were deduced from laboratory experiments
with sediment. Dissipation from water appears to occur primarily by
sorption to sediment, phototransformation, and biotransformation (see
sections 4.2.2 and 4.3).
In an indoor microcosm, the presence of plants in the sediment
did not influence dissipation from the water column (O'Neill et al.,
1989). In salt-marsh water and its associated sediment, half of the
applied technical-grade fenthion was dissipated within 1.5 days.
Whereas the parent compound dissipates rapidly from a water
column, the residues, including transformation products, do not
necessarily do so. In a laboratory experiment with water, sediment,
and fish, the total amount of 14C-fenthion residues in the water
remained fairly constant over 28 days after a single application of
fenthion equivalent to 0.011 kg/ha (ChemAgro, 1975a). Immediately
after application, however, 100% of the recovered residues was soluble
in chloroform, whereas after 28 days 66% was water-soluble, indicating
that the composition had shifted towards more polar residues, probably
by phototransformation and biotransformation.
In outdoor experiments, fenthion also dissipated rapidly from the
water column. In jars containing pond water, with or without the
accompanying sediment, that were buried outdoors in the soil for 16
days in Kansas (USA) in 1971, fenthion dissipated with a half-life of
up to two days (ChemAgro, 1972, 1976a). In one of these experiments,
the dissipation rate in the system with sediment was comparable to
that in the system without sediment. This experiment corroborates the
report of Bayer AG (1988a) that other processes influence dissipation
before accumulation in the sediment, regardless of the presence of
sediment. In the other experiment (ChemAgro, 1976a), sediment was an
important sink, as the sediment-bound residues in silt gradually
increased up to 49% of the applied activity after 46 days. Detection
of fenthion in Dutch surface water at concentrations up to
0.12 µg/litre, however, may indicate that dissipation in the field is
less rapid than that under more controlled conditions (see section
5.1).
4.1.2 Soil sorption
Fenthion binds readily to various soils. In laboratory
experiments in which fenthion was added to aqueous soil suspensions,
the adsorption coefficient Ks/l (= Kd = Kom) was 7.7-38 dm3/kg
in six soil types ranging from sand to silty clay loam (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988). These values indicate
strong sorption, which can be described by the Freundlich equation.
Three additional Ks/l values (6.4, 8.6, and 67 dm3/kg) obtained in
these experiments must be considered inaccurate, as the accompanying
1/n constants deviate substantially from unity (< 0.7 or > 1.1).
The mechanism of sorption of fenthion to soil is only partially
understood, and several factors may be involved. Sorption to organic
matter appears to be important, as soils with the highest organic
matter content showed the highest adsorption coefficients (ChemAgro,
1972; Mobay Corp., 1978a; ABC Inc., 1988).
In a laboratory experiment with 14C-fenthion, the desorption
coefficients were about two times higher than the sorption coefficents
(ABC Inc., 1988). Although 0.01 mol/litre of calcium chloride were
added to simulate potential desorption in the field, sorption may not
be reversible under outdoor conditions.
Table 3. Biotransformation and dissipation of technical-grade fenthion in water and sediments in the laboratory
Water type Sediment Test Sediment Organic matter Temperature pH Length of Half-life Reference
type (%) in sediment (%) (°C) experiment (days)
(days)
Biotransformation in whole system
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8. 66 9a Bayer
in dark approx. 8 (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 11a,b Bayer
in dark (1988b)
Dissipation from water column
Pond water Loamy sand Aerobic 10 0.9 approx. 22 6.8-8.8 66 4a Bayer
in dark (1988a)
Pond water Silt loam Anaerobic 0.2 1.8 approx. 22 5.6-7.6 360 7a Bayer
in dark (1988b)
Surface waterc Not Aerobic Not Not 20 Not 8 1.5 O'Neill et al.
reported with reported reported reported (1989)
light-dark
cycle
a Approximate value derived from authors' data by linear regression of the recovered percentages after logarithmic
(In) transformation. Times until 90-99% of the applied fenthion has been transformed were used for calculation.
b Although the half-life under anaerobic conditions equals that under aerobic conditions, the initial transformation
rate under anaerobic conditions is slower
c Water from a salt marsh with 11% salinity
4.1.3 Soil mobility
In view of its Ks/l values, fenthion should be immobile in many
soils, as confirmed in several experiments both in the laboratory and
in the field. In thin-layer chromatography studies, which do not
generate reliable Ks/l values, the Rf values for 14C-fenthion in
five soil types with organic matter contents of 0.6-5.1% were
0.14-0.17 (ChemAgro, 1976b). In less reliable leaching experiments
with columns of 45 cm, in which the leaching time was not reported,
water fluxes of 50, 95, and 490 ml were imposed on a sandy loam with
1.4% organic matter, a silty clay loam with 2% organic matter, and a
silty clay loam with 4.4% organic matter, respectively (ChemAgro,
1972). Fenthion was recovered only in the leachate of the silty clay
loam with a high organic matter content, representing 1.8% of the
recovered fenthion. In these experiments, 64-92% of the recovered
fenthion was found in the upper 3-cm layer of the columns.
Transformation products of fenthion appear to be more mobile than
fenthion itself; however, the only data on the mobility of transform-
ation products are those from leaching experiments with aged residues.
The adsorption coefficients of metabolites have not been reported.
In studies under laboratory or greenhouse conditions with
residues aged for 4-30 days, 1.9-46% of the applied activity was
leached (ChemAgro, 1974, 1975b; Mobay Corp., 1987a). The greatest
amount of leaching was found in a sandy soil with the lowest
percentage of organic matter (0.2%) of all soils tested, with 36%
leaching after four days of aging and 46% after 25 days (Mobay Corp.,
1987a). The columns were 30 cm long, the water flux over two days was
high (51 cm), and the soil that had been aged prior to leaching was a
sandy loam soil. These experiments cannot be considered reliable, as
the type of soil placed on the top of the column was different from
that inside the column, and the amounts of fenthion residues after
aging and before application on the column were not reported. In spite
of the high maximal leaching percentages in sand, more than 50% of the
applied activity was recovered in the upper 12 cm of the columns. Four
transformation products were recovered in the sand column segments and
leachates. Those in the leachate, in decreasing order of magnitude,
were 3-methyl-4-methylsulfonyl-phenol, 3-methyl-4-methylsulfinyl-
phenol, fenthion sulfoxide (thiophosphoric acid, O,O-dimethyl- O-
[3-methyl-4-methylsulfinyl-phenyl] ester), and fenthion sulfone
(thiophosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfonyl-
phenyl] ester). The maximal percentages of recovered activity at the
end of the experiments were 20, 15, 11, and 0.8%, respectively. It can
be concluded that at least the more polar transformation products of
fenthion, which generally remain primarily organosoluble, may leach
into shallow groundwater; however, as the half-lives of these
transformation products are probably < 15 days (see section 4.3.2),
substantial leaching of these products to shallow groundwater is
unlikely.
Table 4. Biotransformation and phototransformation of fenthion in soils
Soil type Test type Moisture Temperature pH Organic Length of Half-life Reference
content (%) (°C) matter (%) experiment (days)
(days)
Biotransformation
Silty loama Laboratory, approx. 30 Not 5.9 3.0 120 10c Mobay Corp.
aerobic reportedb (1978b)
Silty loama Laboratory, approx. 30 Not 5.9 3.0 32 > 32 Mobay Corp.
aerobic reportedb (1978b)
Phototransformation
Sandy loam Field, Not 16-37 5.1 2.4 1.2 1c Mobay Corp.
aerobicd reported (1987b)
Sandy loam Field, Not Not 4.5-7.5 0.8-6.3 4 > 4 Gohre & Miller
aerobic reported reported (1986)
a Silty loam is assumed to have a moisture content of 40% (v:v) at field capacity; moisture content was kept at 75% of the
field capacity
b Possibly room temperature, but not clearly stated
c Approximate value derived from authors' data of by linear regression of the recovered percentages after logarithmic
(In) transformation. As transformation of up to 99% of the applied fenthion was in accordance with first-order
kinetics, the coinciding times were used for calculation.
d A slurry of soil, water, and acetonitrile in a photomodule was exposed to fenthion by pipetting it onto the slurry.
4.1.4 Dissipation from soil in the field
No substantial dissipation of fenthion due to run-off on an 8°
slope was found on a sandy loam or a silty clay loam soil on a fallow,
raked field (ChemAgro, 1972). The silty clay loam site had been
divided into one with a low (2%) and the other with a high (4.4%)
organic matter content. Maximal percentages of 0.9% and 1.2% of the
applied fenthion were recovered in the run-off water as the parent
compound and fenthion sulfoxide, respectively, in the sandy loam and
silty clay loam with the low organic matter contents, corresponding to
concentrations of 0.12 and 0.23 µg/litre, respectively. The lowest
run-off of fenthion residues and the largest amount of fenthion
residues (at the site of application) were seen on soil with the
highest percentage of organic matter, coinciding with the strong
sorption of fenthion on various soils. As the soil and the run-off
water were analysed after weekly irrigation for one month, the
dissipation of fenthion must be low, due mainly to strong sorption
(see also section 4.1.2). In these experiments, the soils were
irrigated for one month with 10-17 cm of water (total), and the rate
of application of fenthion was 11.2 kg/ha. The results indicate that
dissipation of fenthion under outdoor conditions may be slower than
that under controlled laboratory conditions. Thirty days after
application, up to 38 and 55% of the applied fenthion were recovered
at the site of application as the parent compound and fenthion
sulfoxide, respectively. Dissipation in laboratory experiments due to
microbial action is much faster (see Table 4 and section 4.3).
4.1.5 Uptake and dissipation from plants
Fenthion can be taken up by plants, and its ability to penetrate
plant tissues results in destruction of e.g. larvae even within fruit.
Fenthion is reported to persist for some time after being taken up.
The amount of fenthion residues in Bermuda grass (Cynodon dactylon)
and corn (Zea mays) decreased by about 60 and 80% within the first
two weeks after application (FAO/WHO, 1973).
4.1.6 Entry into the food chain
Fenthion is used primarily against insects and birds (see section
3.1.2). As the amount of fenthion found in these species after
ingestion or on them after contact may increase immediately after
application, it may enter the food chain by ingestion or direct
contact with e.g. insectivorous and bird-predatory species. Hunt
et al. (1991) investigated the possibility of secondary poisoning of
the American kestrel (Falco sparverius) through predation on house
sparrows (Passer domesticus) exposed to fenthion by smearing of
their perches with the formulation Rid-A-Bird(R). Exposure of the
kestrels by contact with the sparrows' feet may have contributed
substantially to the observed toxicity. When the contaminated sparrows
were penned with the kestrels for three days, 79% of the kestrels died
from fenthion poisoning within one day, and all were dead within three
days. The contaminated kestrels showed 78-92% depression of brain
cholinesterase activity and 97% of that in plasma, which correlated
with concentrations of fenthion up to 14 µg/g in the gastrointestinal
tract and 19 µg/g in feet. Most of the contaminated sparrows died
within 8 h of exposure and contained fenthion at concentrations of up
to 6 µg/g in the internal carcass, 631 µg/g in the external carcass
(i.e. feathers and skin), and 1152 µg/g in the feet.
The route of exposure before secondary intoxication is often
unclear. In five bald eagles (Haliaeetus leucocephalus) found dead
in Iowa (USA) in 1984, brain cholinesterase activity was depressed by
80-92% (Henny et al., 1987). The remains of piglets found in their
stomachs contained fenthion at concentrations of 0.1-6.8 mg/kg wet
weight. The two possible sources of the fenthion are intentional
primary poisoning of the eagles with contaminated bait or secondary
poisoning from contaminated piglets. This case indicates the
vulnerability of scavenging species like eagles.
4.2 Abiotic transformation
4.2.1 Hydrolytic cleavage and oxidation
Fenthion is transfomed slowly in sterile phosphate buffers, with
half-lives of 56, 41, and 32 days at pH 5, 7, and 9, respectively, at
25°C for 70 days (ChemAgro, 1976c); comparable results were found at 5
and 40°C. These results indicate slower transformation rates under
more acid conditions. This pH-dependent transformation was confirmed
in other studies (eg. Bayer AG, 1983a). In the experiments of ChemAgro
(1976c) at 25°C, the main transformation products were fenthion
sulfoxide, 3-methyl-4-methylsulfinyl-phenol, fenoxon (phosphoric acid,
O,O-dimethyl- O-[3-methyl-4-methylthio-phenyl] ester), fenoxon
sulfone (phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methyl-
sulfonyl-phenyl] ester), fenthion sulfone, and fenoxon sulfoxide
(phosphoric acid, O,O-dimethyl- O-[3-methyl-4-methylsulfinyl-
phenyl] ester). The maximal percentages of the administered activity
were 14, 12, 10, 8, 7, and 6%, respectively. The transformation
products indicate both oxidation (fenthion sulfoxide, fenoxon, fenoxon
sulfone, fenthion sulfone, and fenoxon sulfoxide) and hydrolysis
(3-methyl-4-methylsulfinyl-phenol).
A low abiotic transformation rate in the dark was confirmed in
other laboratory experiments (ChemAgro, 1972; Bayer AG, 1983a). In
buffered aqueous solutions, the extrapolated half-lives at 22°C were
223, 200, and 151 days at pH 4, 7, and 9, respectively (Bayer AG,
1983a), and the transformation product was 3-methyl-4-methylthio-
phenol. In aqueous phosphate buffers at 30°C, the experimental
half-lives at pH 5, 7, and 9 were 31, 26, and 24 days, respectively
(ChemAgro, 1972).
4.2.2 Phototransformation
(a) Water
Photochemical transformation may occur rapidly in water under
both laboratory and field conditions. In a sterile aqueous buffer at
pH 5, substantial phototransformation of 13C/14C-1-ring-labelled
fenthion was observed after exposure to artificial light simulating
sunlight for 4 h at 23°C (Mobay Corp., 1987c); the half-life was
0.5 h. Rapid phototransformation in the laboratory was confirmed by
Bayer AG (1983b, 1988c) and ChemAgro (1976b).
The major photochemical transformation products in the study
reported by the Mobay Corp. (1987c) were 3-methyl-4-methylthio-phenol
(maximal totally recovered activity, 23%), fenthion sulfoxide
(maximum, 17%), and 3-methyl-4-methylsulfinyl-phenol (maximum, 15%).
Transformation products found in minor quantities in this experiment
were 3-methyl-4-sulfo-phenol (maximum, 8%), fenoxon sulfone (maximum,
7%), fenoxon sulfoxide (maximum, 6%), and 3-methyl-4-methylsulfonyl-
phenol (maximum, 6%). Fenoxon was also found as a phototransformation
product in other experiments (maximum, 10-11% of the applied activity)
(ChemAgro, 1976b).
The phototransformation pattern is temperature-dependent: at
25°C, the rate of production of fenthion sulfoxide via oxidation is
comparable to that of 3-methyl-4-methylthio-phenol via hydrolysis.
Oxidation to fenoxon sulfoxide is assumed to prevail at higher
temperatures and hydrolysis to 3-methyl-4-methylthio-phenol at lower
temperatures (ChemAgro, 1976d). When 2% acetone was added to an
aqueous solution of fenthion as a photosensitizer before irradiation
with artificial light for 2 h, oxidation via fenthion sulfoxide to
unknown polar transformation products was accelerated. Thus, changes
in temperature and the presence of sensitizers can result in different
mixtures of fenthion transformation products.
Fenthion also undergoes rapid phototransformation outdoors,
although possibly at a somewhat slower rate than in laboratory
experiments. Fenthion in distilled water exposed to sunlight in the
summer was phototransformed with a half-life of 4 h (Bayer AG, 1983b);
in a comparable experiment under laboratory conditions with artificial
light, the half-life was 0.08 h. Fenthion is phototransformed rapidly
in sunlight (lambda > 290 nm), primarily because of its high quantum
yield.
(b) Soil
As fenthion applied to soil appears to be phototransformed
rapidly by sunlight, with half-lives of 1-> 4 days (see Table 4),
phototransformation is an important route of dissipation. In an
outdoor experiment, 13C/14C-1-ring-labelled fenthion mixed with
unlabelled fenthion was exposed for 1.2 days to natural sunlight after
application to a sandy loam at a rate of 53 mg/kg soil (see also Table
4). Fenthion was rapidly phototransformed, and the main photochemical
transformation product was fenthion sulfoxide (maximum, 58% of the
recovered activity); fenoxon sulfoxide, 3-methyl-4-methylsulfinyl-
phenol, and fenthion sulfone were minor transformation products (i.e.
< 10% of the recovered activity). The oxidation path apparently
prevails (Mobay Corp., 1987b). As the temperature during this
experiment rose to about 37°C, a shift to the oxidation rather than to
the hydrolysis pathway seems to have occurred, as in the
phototransformation experiments with water (ChemAgro, 1976d).
(c) Air
Phototransformation occurs rapidly in the troposphere. The
half-life of fenthion in air is estimated to be 1.7 h (Bayer AG,
1994a) on the basis of the assumption that phototransformation occurs
via the reaction of hydroxyl radicals with fenthion. The primary
target of the radicals is probably the P=S bond. Such attacks may
result in secondary oxidation products (e.g. fenoxon), which can be
deposited on soil, water, and vegetation by wet and dry deposition.
4.3 Biotransformation
Biotransformation may contribute to the general processes of
dissipation (see section 4.1). No data from primary sources on the
microorganisms that can metabolize fenthion have been incorporated in
this monograph. It has been reported to be biotransformed to fenoxon
sulfoxide by the soil fungus Rhizopus japonicus (Wallnöfer, 1978,
cited by Mobay Corp., 1978b). Similarly, no data on the biodegrad-
ability of the adjuvants in formulations of fenthion have been
incorporated; however, it can be assumed that the biodegradability of
e.g. 'fog' and diesel oil in a natural environment is slight.
A simplified scheme, showing some common transformation products
in environmental compartments, is presented in Figure 1. The scheme
includes both abiotic and biotic transformation products.
4.3.1 Water and sediment
The relevant laboratory experiments in which biotransformation in
water-sediment systems was studied were summarized in Table 3. The
results indicate that the rate of biotransformation is rapid under
both aerobic and anaerobic conditions: 50% biotransformation of
fenthion (half-life) in a whole water-sediment system occurs within
about 10 days. Experimental conditions other than the availability of
oxygen, e.g. temperature, salinity, the presence of plants, the type
of water, and the type of sediment, probably have less effect on the
transformation rate. In microcosms of salt-marsh water, sediment and
sediment-rooted plants, however, the amount of detritus and the extent
of bioperturbation of the sediment may also influence the biotrans-
formation rate (O'Neill et al., 1989).
In general, sediments appear to function as a major sink for
fenthion under both aerobic and anaerobic conditions. Rapid
partitioning to sediment was seen under aerobic and anaerobic
conditions, as 21-28% of the applied activity was recovered in the
sediment within 1 h after application (Bayer AG, 1988a,b); maxima of
41-80% of the applied activity were recovered in sediment. Under
aerobic conditions, the amount in the sediment increased gradually up
to 62-80% of the applied activity (Bayer AG, 1988a) by the end of the
test at 66 days. Under anaerobic conditions, the maxima were 66-70%
and were reached after 7-14 days (Bayer AG, 1988b). The total amounts
of activity in the sediments decreased thereafter, probably due to
further mineralization to 14C-carbon dioxide (see below). Under
either aerobic or anaerobic incubation, most of the activity in the
sediment shifted from water-and acetone-extractable compounds to
non-extractable (i.e. sediment-bound) residue in the course of the
experiments.
The rate of biotransformation in non-sterile sediment without
plants was best modelled by assuming biotransformation in the upper
1 mm of the sediment (O'Neill et al., 1989). When plants were
present, biotransformation in the upper 7 mm was the best assumption.
In aerobic experiments with water and associated sediments, the
amount of fenthion declines rapidly over time, with transient
increases in various metabolites, an increase in 14C-carbon dioxide,
and an increase in sediment-bound residues (Bayer AG, 1988a,b). Under
anaerobic conditions, a small amount (3-4%) of 14C-methane was
formed. The production of 14C-carbon dioxide appears to be more
rapid under aerobic than anaerobic conditions, but the final amounts
of 14C-carbon dioxide differed substantially with the duration of
the test: 12-15% after 66 days under aerobic conditions and 52% after
120-190 days under anaerobic conditions. As the amount of oxygen was
very low under anaerobic conditions (0-0.4 mg/litre), the oxygen in
the carbon dioxide may have been supplied by nitrates or sulfates. The
redox potential during the experiment varied between -186 and 137 mV.
The main transformation products were demethyl fenoxon sulfoxide
(maximum, 30% of whole water-sediment system after 7-14 days),
fenthion sulfoxide (maximum, 28% after 14 days), 3-methyl-4-methyl-
sulfinyl-phenol (maximum, 13% after 66 days), and 3-methyl-4-methyl-
sulfonyl-phenol (maximum, 11% after 66 days) under aerobic conditions,
 and 3-methyl-4-methylthio-phenol (maximum, 35% after 60 days),
3-methyl-4-methylsulfinyl-phenol (maximum, 26% after 30 days), and
3-methyl-phenol (maximum, 10% after 60 days) under anaerobic
conditions. In these experiments, only the moieties with a phenyl
group were qualified or quantified; the fate of the thiophosphoric
acid moiety is less well described. Eichelberger and Lichtenberg
(1971) assumed that O,O-dimethyl- O-thiophosphoric acid was formed
by hydrolysis.
4.3.2 Soil
Fenthion appears to be biotransformed rapidly in soil under
aerobic conditions and more slowly under anaerobic conditions: the
half-life of 13C/14C-1-ring-labelled fenthion in a silty loam soil
was 10 days under aeobic conditions and more than 32 days under
anaerobic conditions (Mobay Corp., 1978b; see Table 4). The latter
value is not reliable, however, as the anaerobic incubation was
started when about 99% of the parent molecule had been biotransformed
aerobically. These experiments and some important experimental
conditions are summarized in Table 4, which indicates that fenthion is
transformed rapidly under optimal conditions, such as sufficient
oxygen and a moisture content > 75% of the field capacity. Rapid
transformation of fenthion under aerobic conditions was confirmed by
Bayer AG (1974), with half-lives of 1.7 and 0.5 days in a sand and a
sandy loam soil. These results are somewhat unreliable, however, as
the moisture content of the soils during the tests was not reported
and they may have been stored under overly dry conditions. The rate of
biotransformation of fenthion in soils can be described by linear
first-order kinetics, although few studies are available for
verification.
The main metabolites of fenthion under aerobic conditions are
fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol, and 3-methyl-
4-methylsulfonyl-phenol (Mobay Corp., 1978b). In laboratory
experiments, the maximal amounts of these metabolites in silt loam
were 30-33, 15-18, and 30-31%, respectively, of the total activity.
These maxima were reached within 14 days after application at rates of
1-10 mg/kg dry weight. Substantial amounts of these metabolites appear
to be formed microbially, as only fenthion sulfoxide and to a lesser
extent 3-methyl-4-methylsulfonyl-phenol were formed under sterile
conditions in the same soil; they were formed more slowly under
sterile conditions. Some minor metabolites quantified in these
experiments were fenthion sulfone and 1-methoxy-3-methyl-4-methyl-
sulfonyl-benzene, which were found at 4-8 and 4-6%, respectively, of
the total activity within 59 days after application. It should be
noted that the formation of 1-methoxy-3-methyl-4-methylsulfonyl-
benzene indicates methylation.
Under aerobic conditions, the amounts of soil-bound residues
gradually increased up to a plateau of 41% of the total activity 30
days after application and continued until the end of the experiment
after 120 days (Mobay Corp., 1978b). These soil-bound residues were
due mainly to microbial action, as under sterile conditions only 9%
was recovered as soil-bound residues 30 days after application.
Mineralization to carbon dioxide occurs in soil in the laboratory
under both aerobic and anaerobic conditions, although more slowly
under the latter conditions. After application of 13C/14C-1-ring-
labelled fenthion at 1 mg/kg dry weight to a silt loam soil under
aerobic conditions in the laboratory, about 50% 14C-carbon dioxide
evolved within 120 days (Mobay Corp., 1978b). In the same soil under
anaerobic conditions, the mineralization rate was substantially lower
than that under aerobic conditions after aerobic preincubation for 30
days; during 32 days of anaerobic incubation, only 4% of the total
activity was degraded to 14C-carbon dioxide.
4.4 Bioconcentration
Fenthion is a lipophilic compound which is only slightly soluble
in water and has an octanol-water partition coefficient (log Kow) of
4.8. These properties indicate possible bioconcentration, as confirmed
in a laboratory experiment in which fenthion was shown to be
moderately concentrated in fish (ChemAgro, 1975a).
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 14C-fenthion at 0.008-0.12 mg/litre
(actual concentrations) for 14 days, the calculated daily biocon-
centration factors based on the wet weight of the whole fish increased
from 226 by 0.2 days after the start of the test to 400-500 by 4-7
days (ChemAgro, 1975a); after 14 days of exposure, the daily
bioconcentration factors were 200-300. The maximal concentrations of
labelled residues in the whole fish were 58 mg/kg wet weight four days
after the start. When the fish were subsequently exposed to uncontam-
inated water, all of the residues were eliminated within 11 days.
Eleven days after the start of exposure, 62% of the activity recovered
from a dissected fish was water-soluble and 38% chloroform-soluble;
the latter fraction contained fenthion (as 73% of the activity, in the
fraction), fenthion sulfoxide (20%), 3-methyl-4-methylsulfinyl-phenol
(5%), and fenoxon sulfoxide (2%). Seventy percent of the labelled
residues in one exposed fish was recovered in the viscera, but it was
unclear to what extent transformation products had been formed. These
products can be formed in water by photo- and biotransformation (see
above).
Bluegill sunfish (Lepomis macrochirus) and channel catfish
(Ictalurus punctatus) were exposed under static conditions to a
single dose of 14C-ring-labelled fenthion at a concentration of
about 3.7 µg/litre in aquariums containing both water and sediment and
were observed for 28 days. The calculated maximal daily biocon-
centration factors were 118 and 115 after 8 and 24 h for the sunfish
and the catfish, respectively. The maximal amount of labelled residues
in both species was 0.3 mg/kg bw 8-24 h after application. No residues
were recovered in fish after 21 days. The maximal amounts were not
related to the maximal depressions in brain cholinesterase activity,
which were 97% after three days for the sunfish and 76% after six days
for the catfish. There appeared to be a lag of a few days between
exposure and effects. The fish appeared to have recovered 21 days
after application (ChemAgro, 1975a).
De Bruijn & Hermens (1991) confirmed that fenthion is moderately
concentrated in fish. They found a bioconcentration factor of 170 in
guppies (Poecilia reticulata) exposed for 11 days to a mixture of
toxicants, including fenthion. Uptake and elimination were equili-
brated after three days.
5. Environmental levels
The concentrations of fenthion in the environment are summarized
in Table 5. Few measurements are available from regular monitoring
programmes, except in the Netherlands, where fenthion was detected in
various fresh and marine surface waters in 1992. The only published
data on residues of fenthion on vegetation and insects after aerial
application of the pesticide are derived from monitoring after
application of 2.4 kg a.i./ha to two sites for control of weaver birds
(Quelea quelea) in Kenya (Bruggers et al., 1989). The applications
were the first to be used in the area to control birds. The
concentrations of residues on young birds were 44 and 84 µg per bird
on the two sprayed areas, respectively, on day 1 and about 10 µg per
bird cm days 3-4. Raptors that ate the birds were not killed, but some
were debilitated; the concentrations in the raptors were not measured.
Aggregate samples of insects contained fenthion at 7.2 mg/kg; the
highest concentration of residues was found in carabid beetles (23 µg
per beetle). The concentrations on grass were 39 and 28 mg/kg on the
two sprayed areas, respectively, on the first day after spraying and
1.9 and 1.1 mg/kg on day 44. A sample of millet seed spread on a mesh
in a sprayed area contained fenthion at 1.1 mg/kg.
Owing to the scarcity of data from monitoring, Table 5 also shows
some actual measurements in the field from experiments performed with
recommended application rates simulating common agricultural practice.
Only maximal amounts are tabulated as indicative values, and data on
the rate at which fenthion dissipated, when available, are mentioned
in the comments (see also sections 4.1 and 4.13). No data were
available on the occurrence of transformation products.
6. Effects on organisms in the laboratory and the field
6.1 Laboratory experiments
6.1.1 Microorganisms
(a) Water
The acute and chronic toxicity of technical-grade fenthion and a
formulation of fenthion to aquatic microorganisms and invertebrates
are summarized in Tables 6 and 7. Technical-grade fenthion is toxic to
highly toxic, with four-day EC50 values of 550-1800 µg/litre and
four-day NOEC values of 100-1120 µg/litre. One formulation of fenthion
is toxic, with four-day EC50 values of 1500-> 2000 µg/litre. The
toxicity of fenthion to e.g. algae is dependent on the species or
strain tested (Wängberg & Blanck, 1988). High toxicity for aquatic
organisms was confirmed by Saini & Saxena (1986), who reported a
six-day NOEC of 100 µg/litre for the freshwater protozoan Tetrahymena
pyriformis. High toxicity was also reported for some saltwater
microorganisms, with one-day EC50 values of 1000 µg/litre for the
alga Cyclotella nana and 100 µg/litre for the diatom Skeletonema
costatum and a one-day NOEC of 10 µg/litre for Cyclotella nana,
based on production of oxygen (Derby & Ruber, 1971).
The toxicity of fenthion for microorganisms in activated sludge
was tested in a laboratory experiment for 30 min at 20°C (Bayer AG,
1994b). The sludge had been collected in a plant treating domestic
waste. Only at the highest nominal dose of fenthion, 10 000 mg/litre,
was respiration inhibited substantially. The test is of limited value,
however, as the nominal test concentrations exceeded the water
solubility by up to 2380 times.
(b) Soil
No unequivocal indications of deleterious effects of fenthion on
microorganisms in the soil are available. The formulation Lebaycid
inhibits neither processes involved in the nitrogen transformation
cycle nor enzymes involved in microbial activity. It did not affect
soil respiration or nitrification in sandy loam or silt loam soils
after application at the recommended rate, 0.75 kg/ha, or a rate five
times higher, 3.8 kg/ha (Bayer AG, 1989a,b). Soil respiration was
measured over 28 days after amendment with glucose (Bayer AG, 1989a),
and incubation was at 20°C in the dark. The effects on nitrification
were analysed by measuring ammonium, nitrite, and nitrate up to and
including 28 days after application (Bayer AG, 1989b). Only slight,
transient increases in nitrate were observed in the silt loam soil and
decreases in the sandy loam soil. The soils had not been treated with
pesticides for one to two years previously.
Table 5. Maximal concentrations of fenthion in environmental water, soil, sediment, and biota
Sample Location Year Period Concentration Comments Reference
Kenya
Carabid Kulalu 1985 April-May 23 µg/beetle Application rate, 1.5-2.4 kg/ha; Bruggers et al.
beetle Ranch mean in insects, 7.2 mg/kg (1989)
Savannah Kulalu 1985 April-May 38 mg/kg Application rate, 1.5-2.4 kg/ha;
grass Ranch whether based on dry or wet Bruggers et al.
weight not explicitly reported; (1989)
amount decreased to 1.9 and 1.1 mg/kg
after 3 and 4 days, respectively
Millet Kulalu 1985 April-May 1.1 mg/kg Application rate, 1.5--2.4 kg/ha; Bruggers et al.
seed Ranch whether based on dry or wet weight (1989)
not explicitly reported; deliberate
overspraying of artificial plot
Netherlands
Fresh Large rivers 1992 0.01-0.12 Measured in pumping station; fenthion Van Meerendonk et al.
surface and lakes µg/litre detected in 25% of freshwater locations (1994)
water at a mean concentration of
0.022 µg/litre
Marine North Sea, 1992 0.01 µg/litre Fenthion detected in 25% of freshwater Van Meerendonk et al.
surface Waddensea locations at a mean concentration of (1994)
water 0.02 µg/litre
Table 5. (cont'd).
Sample Location Year Period Concentration Comments Reference
United States
Salt-marsh Indian River 1984 September 1.7 µg/litre No fenthion detectable (< 0.01 µg/litre) Wang et al.
surface County, Florida within 24-48 h of aerial application (1987)
water of 0.032 kg a.i./ha
Salt-marsh Indian River 1985 March < 0.01 No explanation for low concentration; Wang et al.
surface County, Florida µg/litre deposition was confirmed, as up to 0.5 (1987)
water µg/litre was detected in dishes with
an aqueous medium; application rate,
0.032 kg a.i./ha
Salt-marsh Indian River 1985 June 0.16 No fenthion detectable (<0.01 µg/litre) Wang et al.
surface County, Florida µg/litre within 24-48 h of aerial application of (1987)
water of 0.032 kg a.i./ha
Polia larvae Laramie, 1979 Summer 0.28 mg/kg 8 h after spraying in field experiment Powell
(bird feed) Wyoming at an application rate of 52 g/ha; no (1984)
fenthion detected 30 h after treatment
Table 6. Acute toxicity of fenthion and two metabolites to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Crustacean
Daphnia magna, Static Technical Reconstituted 8.0 10 20 2 EC50, 0.0057a,b Bayer (1985a)
first instar
Fish
Onchorhynchus mykiss, Continuous Technical Well 8.0-8.1 225-275 12-13 4 LC50, 0.83b,c,d ABC Inc.
0.4 cm, 0.8 g (1986a)
Lepomis macrochirus, Continuous Technical Well 8.1-8.2 225-275 21 4 LC50, 1.7b,c ABC Inc.
5.4 cm, 1.9 g (1987)
Onchorhynchus mykiss, Static Lebaycid Tap 7.5-7.9 284 16 4 LC50, 2.3b,d Bayer (1994c)
5.5-6 cm, 1.5 g (adapted)
Leuciscus idus, Static Lebaycid Tap 7.2-7.9 284 21 4 LC50, 4.3b,d Bayer (1994d)
5.5 cm, 1.5 g (adapted
Onchorhynchus mykiss, Static Psx Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Onchorhynchus mykiss, Static Psn Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Lepomis macrochirus, Static Psx Well 7.3-7.6 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Lepomis macrochirus, Static Psn Well 72-7.9 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Psx, 3-methyl-4-methylsulfinyl-phenol; Psn, 3-methyl-4-methylsulfonyl-phenol
a Based on immobilization
b As phototransformation occurred, value is based on a mixture of fenthion residues
c Measured concentrations
d Actual concentration
Table 7. Long-term toxicity of fenthion to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Green algae
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 550a,b Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 1800a,c Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 NOEC, 100a,b Bayer (1985a)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, 1500a,b Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, > 2000a,c Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 NOEC, 1120a,b Bayer (1989c)
subspicatus
Selenastrum Static Baytex Artificial NR 1.5 24 4 EC50, 1100a,c,d,e ABC Inc. (1986b)
capricornutum
Selenastrum Static Baytex Artificial NR 1.5 24 4 NOEC, 700a,c,d,e ABC Inc. (1986b)
capricornutum
Crustaceans
Daphnia magna, Continuous Lebaycid Artificial 7.5-8.3 204 20 21 NOEC, 0.018d,e,f,g TNO (1989)
first instar
Daphnia magna, Continuous Technical Well 8.1-8.3 206-275 20 21 MATC, Bayer (1985b)
first instar 0.042-0.082d,e,h
Table 7. (cont'd).
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Fish
Oncorhynchus Continuous Technical Well 6.6-7.8 29-30 12 88 MATC, 13-27d,e,i SLS Inc. (1988)
mykiss
NR, not reported
a As phototransformation occurred, the value is for a mixture of fenthion residues
b For decrease in biomass
c For inhibition of growth rate
d Measured concentration
e Actual concentration
f All concentrations below the detection limit of 0.1 µg/litre
g For reproduction
h For mortality, growth, and reproduction
i For embryo viability, embryo survival at hatch, survival, and growth of larvae
6.1.2 Aquatic organisms
(a) Plants
Stimulation but no decrease in biomass was observed in an
experiment in which duckweed (Lemna gibba) was exposed in an
artificial medium to the formulation Baytex at measured concentrations
up to 2.8 mg/litre for 14 days at 25°C (Malcolm Pirie, 1987). The
stimulation may be related to hormesis.
(b) Invertebrates
Technical-grade fenthion is highly toxic to the crustacean
Daphnia magna, with a two-day EC50 value of 5.7 µg/litre and a
21-day maximal acceptable toxicant concentration (MATC) of 0.042-0.082
µg/litre. The formulation Lebaycid is also highly toxic to this
species, with a 21 -day NOEC value of 0.018 µg/litre. Static and
chronic treatment of other invertebrate species provide more variable
results, with two- to four-day L(E)C50 values of 0.62-1800 µg/litre
for various freshwater molluscs, insects, and crustaceans, 17- to
32-week NOEC values of 1000-2000 µg/litre for the freshwater mollusc
Lymnea stagnalis, one- to four-day L(E)C50 values of 0.02-550
µg/litre for saltwater molluscs, insects, and crustaceans, and 8- to
20-day NOEC values of 0.037-0079 µg/litre for the saltwater crustacean
Mysidopsis bahia (e.g. Seugé & Bluzat, 1980; Mayer, 1986; Mayer &
Ellersieck, 1986; McKenney, 1986). These tests were performed with
technical- or analytical-grade fenthion.
(c) Vertebrates
The acute and long-term toxicity of technical-grade fenthion, the
metabolites 3-methyl-4-methylsulfinyl-phenol and 3-methyl-4-methyl-
sulfonyl-phenol, and a formulation of fenthion to aquatic vertebrates
is summarized in Tables 6 and 7. Technical-grade fenthion is
moderately to highly toxic to freshwater fish, with four-day LC50
values of 0.83-1.7 mg/litre and an 88-day MATC value of 13-27
µg/litre. The formulation Lebaycid is toxic, with four-day LC50
values of 2.3-4.3 mg/litre. The metabolites 3-methyl-4-methyl-
sulfinyl-phenol and 3-methylsulfonyl-phenol are very slightly toxic to
freshwater fish, with four-day LC50 values exceeding 100 mg/litre.
Other tests show moderate to high toxicity of technical-grade fenthion
(e.g. Korn & Earnest, 1974; Mayer & Ellersieck, 1986; WHO, 1986
[original references not checked], with two- to four-day LC50 values
of 1-3.4 mg/litre for freshwater fish and 0.45-2.5 mg/litre for
saltwater fish.
Fenthion is highly toxic for amphibians: Khangarot et al.
(1985) reported a four-day LC50 value of 0.84 µg/litre for the frog
Rana hexadactyla with a length of 20 mm.
Many sublethal effects have been observed in fish after exposure
to a wide range of concentrations, including bottom disorientation,
loss of equilibrium, discolouration, rapid respiration, bloated
stomach, gulping of air, quiescence, surfacing, and irregular swimming
behaviour (ABC Inc., 1986; SLS Inc., 1988; Bayer AG, 1994c,d).
6.1.3 Terrestrial organisms
(a) Plants
No data were available.
(b) Invertebrates
Technical-grade fenthion is obviously toxic to various
terrestrial insects. Few studies have addressed other terrestrial
invertebrates.
Fenthion appears to be toxic to honey bees (Apis mellifera),
with contact LD50 values of 0.16-< 2 µg/bee (Bayer AG, 1995
[original references not checked]). No data were available on the
toxicity of fenthion for honey bees after oral exposure, but it would
be expected to be toxic. It is also toxic for predators of pests such
as wasps, mites, hoverflies, and green lacewings tested at moderately
high temperatures (22-27°C during the day and night and 16-18°C at
night).
Fenthion is highly toxic to a microhymenopterous wasp,
Trichogramma caoeciae, that parasitizes the eggs of the parasite
Sitrotroga cereallella (Conrad Appel GmbH, 1989). In one experiment,
larvae were exposed to Lebaycid sprayed onto glass plates at a rate of
16 kg/ha as fenthion. All of the larvae died within 24 h. In a second
experiment, host eggs containing pupae did not hatch after they had
been dipped in an aqueous solution of 0.1% Lebaycid.
Fenthion is also highly toxic to the predating mite Phytoseiulus
persimilis Athias-Henriot (von Kniehase & Zoebelein, 1990). Male and
female mites were placed on the leaves of kidney beans (Phaseolus
vulgaris) to produce a small population, and five days later the
leaves were dipped into a solution of 0.075% Lebaycid EC 500 at the
highest recommended application rate. All mites were killed within
seven days. Treatment with 0.015% Lebaycid EC 500 did not kill all of
the mites.
All early-instar larvae of the aphid predating hover fly
(Episyrphus balteatus) died within 24 h after spraying of Lebaycid
500 EC on glass plates at a rate of 0.77 kg/ha as fenthion (University
of Southampton, 1992).
Fenthion was highly toxic to the larvae of the green lacewing
(Chrysoperla carnea) exposed by contact to Lebaycid at 12 kg/ha as
fenthion in a light-dark cycle for three to five days (GAB Biotech-
nologie, GmbH, 1991). The rate of mortality was 100% of larvae and
unhatched imagos and only 7% of controls.
Artificial soil contaminated with Lebaycid 50EC was slightly
toxic to earthworms (Eisenia andrei) (Bayer AG, 1989d), with a
14-day LC50 of 723 mg/kg soil dry weight and a dose-related weight
loss. The 14-day NOEC was thus 100 mg/kg dry weight of soil. As the
vessel containing the worms was lit continuously, some photo-
degradation may have occurred.
(c) Vertebrates
The acute and subacute toxicity of fenthion to birds is
summarized in Table 8.
Technical-grade fenthion is moderately toxic or toxic to birds,
with an LD50 value of 7.2 mg/kg bw and an eight-day LC50 value of
60-1259 mg/kg feed. Five- to 10-day LC50 values of 25-231 mg/kg feed
were reported for the mallard duck (Anas platyrhynchos), the
bobwhite quail (Colinus virginianus), the Japanese quail (Coturnix
japonica), and the domestic chicken (Gallus domestica) (Hill
et al., 1975; Royal Society of Chemistry, 1995). Fenthion appears to
be toxic after dermal application in several bird species, with LD50
values of 1.8 mg/kg bw for the weaver bird (Quelea quelea) and
2.4 mg/kg bw for the house sparrow (Passer domesticus) (Schafer
et al., 1973).
The sublethal effects observed in birds treated with fenthion
experimentally are wing drop, ataxia, salivation, reduced body weight,
tremors, convulsions, diarrhoea, and laboured breathing (Grue, 1982;
Mobay Corp., 1987c,d,e). Necropsy of poisoned birds showed fluid-
filled crops and intestines, mottled spleens (possible due to organ
hypostasis), and oily fluid in crops or gastrointestinal tract,
indicating incomplete absorption of fenthion. Common grackles
(Quiscalus quiscula) found dead after five days' exposure to fenthion
had lost 28-36% of their initial weight, and muscle tissue on the
breastbone and observable fat had decreased substantially (Grue,
1982). The reduced body weights were due to treatment-related
reductions in feed consumption, perhaps indicating that fenthion is a
repellent, probably because of its strong mercaptan odour. The
estimated daily intake remained constant and was thus independent of
the concentrations tested.
Table 8. Acute and subacute toxicity of technical-grade fenthion given to birds for eight days
Species Sex Age Route Result Reference
Mallard duck (Anas platyrhynchos) Not reported 9 days Diet LC50, 1259 mg/kg feed Mobay Corp. (1987c)
Bobwhite quail (Colinus virginianus) Not reported 10 days Diet LC50, 60 mg/kg feed Mobay Corp. (1987d)
Bobwhite quail (Colinus virginianus) Male, female 18 weeks Oral LD50, 7.2 mg/kg bw Mobay Corp. (1987e)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, 57 mg/kg feeda Grue (1982)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, < 30 mg/kg feedb Grue (1982)
a Birds caged outdoors 20-27 July 1978
b Birds caged outdoors 14-21 August 1978
A decrease in cholinesterase activity in the brain and plasma is
an important physiological characteristic of avian poisoning by
fenthion. Common grackles that had been fed fenthion at rates of
25-400 mg/kg feed showed a significant, 80% depression in brain
cholinesterase activity. All birds had died before enzyme analysis
(Grue, 1982).
Fenthion appears to be less acutely toxic to terrestrial mammals
than to birds. Kenaga (1979) reported an LD50 range of 255-298 mg/kg
bw for the rat (Rattus norvegicus), indicating moderate toxicity.
FAO/WHO (1973) reported a three-month LOEC value of 0.25 mg/kg diet
based on growth reduction. The highest dietary levels that caused no
toxicological effect were estimated to be 3 mg/kg feed for rats and
2 mg/kg feed for dogs.
6.2 Field observations
6.2.1 Microorganisms
(a) Water
A single application of fenthion at a rate of 0.22 kg/ha did not
affect phytoplankton in small artificial outdoor ponds with natural
surface water and sediment in experiments performed in Florida (USA)
in 1962-63 (Patterson & von Windeguth, 1964).
(b) Soil
No data were available.
6.2.2 Aquatic organisms
(a) Plants
No data were available.
(b) Invertebrates
The populations of crustaceans and chironomids disappeared within
one week after application of fenthion to small artificial outdoor
ponds with natural surface water and sediment at a rate of 0.22 kg/ha
in experiments performed in Florida in 1962-63 (Patterson & von
Windeguth, 1964). The period required for complete recovery of the
numbers of water fleas and midge larvae was four to five months during
a cold winter period and two months in the absence of a cold period.
There were substantially more oligochaetes in treated than in
untreated ponds. Other aquatic invertebrates such as snails,
ostracods, copepods, and Hydra appeared to be unaffected by the
treatment.
(c) Vertebrates
Application of fenthion to small artificial and outdoor ponds at
a rate of 0.22 kg/ha did not appear to affect fish or tadpoles
(Patterson & von Windeguth, 1964).
6.2.3 Terrestrial organisms
(a) Plants
No data were available
(b) Invertebrates
As fenthion is highly toxic to insects in the laboratory, it can
be expected to be so in the field under certain conditions. Its
toxicity is linked mainly to the extent of exposure. In field
experiments in Assiut (Egypt) between June and August at the end of
the 1960s, the formulation Lebaycid, sprayed at a rate of 2.9 kg/ha
(as fenthion) four times at intervals of 15 days with back-pack
spraying equipment, was shown to be toxic to two pest predatory
coccinellids (Coccinella undecimpunctata and Scymnus interruptus)
in cotton (von Afify et al., 1970). Although the formulation was
generally toxic to imago beetles, there was substantial temporal and
spatial variation. The mortality of Coccinella was 58-79% (percentages
corrected for mortality in controls) within three, days of application
and 61-96% within 10 days of application. The mortality of Scymnus
was 55-83% within three days and 28-80% within 10 days. These figures
show partial recovery, at least of Scymnus; Coccinella may be more
susceptible, or Scymnus may recolonize and re-emerge. There was no
clear indication of increasing adverse effects in either species.
Field experiments in Los Baños (Philippines) in 1979-80 showed no
substantial adverse effects of fenthion on predators of the brown
planthopper (Nilaparvata lugens), a common rice pest in South and
Southeast Asia (Reissig et al., 1982). In these experiments,
fenthion was applied three times to plots of lowland rice at a rate of
0.75 kg/ha per treatment. Both planthoppers and their predators were
sampled one day before and two days after the treatments; the
predators sampled were the spiders Lycosa pseudoannulata, Tetragnatha
sp., and Araneus sp. and the hemipterous Microvelia atrolineata
and Cyrtorhinus lividipennis. The numbers of neither the
planthoppers nor their predators were significantly altered by the
treatments.
Field experiments in Egypt in 1965-66 showed that adverse effects
on pest predatory species may be present and absent in the same area
(Kira et al., 1972). Some predators of borers and aphids in corn
(Zea mays) appeared to increase in number after treatment of crops
with fenthion at a rate of 1.2 kg/ha per treatment, while some
decreased.
The mortality of honey (Apis mellifera) and alkali bees (Nomia
melanderi Cockerell) in outdoor cages, caused by the introduction of
alfalfa leaves sprayed with fenthion as an ultra-low volume liquid
with a hand-sprayer at a rate of 0.9 kg/ha, was decreased by 75% when
the contaminated leaves were brought into the cages 2 h after
application of fenthion instead of immediately afterwards (Johansen
et al., 1983), indicating that fenthion can be applied with minimal
hazard to bees that are not foraging.
(c) Vertebrates
Various incidents of poisoning of birds with fenthion have been
reported. It is generally assumed that a depression in brain
cholinesterase activity > 50% indicates severe exposure to fenthion
(e.g. Henny et al., 1987). In a field experiment in Kenya,
cholinesterase activity < 20 µmol/min per g was considered to
indicate exposure to fenthion and activities < 10 µmol/min per g to
indicate severe sickness or death (Bruggers et al., 1989).
On a wet meadow of 1.8 km2 in a field experiment in Wyoming
(USA) in 1978, 99 birds and 15 mammals were found dead or sick within
16 days after aerial treatment with fenthion at a rate of 47 g/ha
(DeWeese et al., 1983). As mortality is correlated with signifi-
cantly depressed brain cholinesterase activity in three common bird
species, it was assumed that the mortality and sickness were
treatment-related. A lower mortality rate seen on a comparable trial
site may have been due to factors such as different wind speeds and
overlap of spray swathes. The route of fenthion that induced poisoning
was unclear.
The maximal fenthion residues in dead American kestrels (Falco
sparverius) were correlated with cholinesterase activity depressions
of 78-92% in brain and 97% in plasma (Hunt et al., 1991). Within
three days after treatment of sparrow perches with fenthion, all of
the kestrels were dead. As the kestrels tended to hide in bushes as
paralysis advanced, the numbers of contaminated birds may have been
underestimated. The time for a kestrel to catch a sparrow was
positively correlated with the extent of cholinesterase depression in
the kestrel's brain. This indicates that a kestrel exposed to more
potent fenthion residues will have greater cholinesterase depression
or that kestrels with greater cholinesterase depression are less
capable of catching sparrows. There are indications that the more
fenthion sparrows are exposed to, the more sensitive they are to
kestrel predation (Hunt, 1990, cited by Kendall & Akerman, 1992).
Two of four flood-irrigated hay meadows were sprayed with the
formulation Baytex at 52 g/ha (as fenthion) in order to investigate
the effects on the reproduction of red-winged blackbirds (Anglaius
phoeniceus) in an experiment in Wyoming (USA) in June-July 1979
(Powell, 1984). In one of the treated fields, the growth rate of
nestlings was significantly decreased by 15%; however, the biological
interpretation of this effect is difficult, as cholinesterase activity
was not reduced. It appears to be a transient effect, since the
surviving nestlings recovered from the growth reduction. No
significant treatment-related effects on hatching, on fledgling
survival, or on foraging behaviour of adult blackbirds for nestling
care were observed. The latter parameter, investigated by counting the
number of trips per hour and their duration and distance, was
remarkably stable, as the abundance of the main feed type, the
caterpillar-like larva Polia, had been reduced by about 50% within
one to two days after the treatment.
The effect of fenthion on non-target birds was studied after
aerial application against the red-billed weaver bird (Quelea
quelea), a grain pest in Africa (Bruggers et al., 1989). In these
experiments, conducted in April-May 1985, two quelea colonies in a
savannah (Kulalu Ranch, Kenya) of low thornbush were sprayed at a rate
of 1.5-2.4 kg/ha. The brain cholinesterase activities that were
measured in 19 potentially exposed non-target bird species varied
substantially. Raptorial birds that were captured in or around the
quelea colonies, which had been radioequipped and were tracked up to
14 days after the treatment, were apparently not killed by the
treatment, but 70% had substantially depressed cholinesterase activity
in blood plasma, indicating actual exposure. Most raptorial birds were
assumed by the authors to have been exposed to fenthion by ingesting
contaminated quelea birds within one day after treatment. As no dead
radioequipped raptorial birds were recaptured, it may cautiously be
concluded that the risk of dying through secondary poisoning was
limited; however, the biological consequences for raptorial birds of
depressed cholinesterase activities with respect to other end-points
such as growth and reproduction are unclear.
The effects of fenthion on granivorous birds were also studied:
galliform species (Coturnix delegorguei, Gallus gallus, Pterocles
decoratus) and laughing doves (Streptopelia senegalensis) in cages
that were placed 100, 200, 300, and 400 m downwind of the treated
quelea colonies showed no mortality or signs of intoxication during an
observation period of five to seven days.
An additional experiment showed that the possibility of secondary
poisoning from scavenged contaminated carcasses cannot be excluded.
During the first night after the treatment, 24-90% of intentionally,
placed corpses were removed by scavengers such as jackals (Canis
mesomelas), bat-eared foxes (Otocyon megalotis), and baboons
(Papio cyanocephalus). This high rate of scavenging substantially
reduced the numbers of poisoned dead animals that could be collected,
resulting in an underestimation of the actual risks for non-target
birds.
7. Evaluation of effects on the environment
Fenthion is a systemic organophosphorus pesticide used for both
agricultural and nonagricultural purposes. Its major use is in the
control of insect pests (at an application rate of 1.25 kg a.i./ha),
but it is also used as a veterinary product to control external
parasites on domestic animals and to control birds (at an application
rate of 2.4 kg a.i./ha). Sprays for bird control are composed of a
formulation that is not available commercially and are used only under
governmental control. The pesticide is usually formulated as an
emulsifiable concentrate and is applied by ground spraying (80-85%) or
from the air (15-20%). It is also formulated as granules and as a
paste for control of urban birds. It can enter the environment beyond
the intended zone of treatment by spray drift, as residues on
potential food items such as birds, and, presumably, by loss of
surface residues from domestic animals.
Fenthion that enters the environment is adsorbed rapidly and
strongly on soil and sediment by a poorly understood mechanism. The
compound can be considered to be immobile in soil, and the parent
compound is unlikely to leach below the to firstfew centimetres of the
soil profile.
While photodegradation occurs under laboratory conditions, it is
not considered to be a significant route of breakdown in the field.
Hydrolysis occurs slowly and is dependent on pH; the half-lives of
fenthion in sterile buffers range from 30 to 60 days, with slower
degradation at low pH. Fenthion is degraded by microorganisms in
laboratory soil, with a half-life of 10 days; field degradation is
slower, about half of the compound being degraded to carbon dioxide
within six months in temperate climates. Generally, breakdown is
slower in water-sediment systems than in soil. Fenthion has been
measured in surface waters in untreated areas at concentrations up to
0.12 µg/litre. As the properties of fenthion would not result in its
entering the aquatic environment by leaching or run-off, there is no
satisfactory explanation for the aquatic residues found. It does not
bioaccumulate to a significant extent, despite its high solubility in
fat, since its metabolism and depuration in organisms are rapid.
Because fenthion is broken down rapidly in the environment, its
acute toxicity is relevant. Most species are not exposed chronically,
and its binding to soil and sediment particles would be expected to
reduce its bioavailability. Fenthion and formulations are toxic to
highly toxic to microorganisms, with four-day EC50 values of 550 and
1100 µg/litre. No data were available on its acute toxicity to algae,
but it is of low toxicity after long-term exposure. Screening tests
have indicated no phytotoxicity. Its acute and long-term toxicity to
crustaceans is high, with an EC50 value of 5.7 µg/litre and an NOEC
value of 0.018 µg/litre. Fenthion is very toxic to fish, with an
LD50 of 830 µg/litre and an MATC of 20 µg/litre. One study showed
toxicity to larvae at 0.84 µg/litre. Fenthion is moderately toxic to
birds, with an LD50 value of 7.2. µg/kg bw and a five-day LC50
value of 60 µg/kg feed. It is also toxic to honey bees, with a contact
LD50 of 0.16 µg per bee, and is slightly toxic to earthworms, with a
1-h LC50 of 723 µg/kg dry weight.
7.1 Risk assessment
Exposure concentrations have been derived from the results of
monitoring programs or field experiments simulating common agricul-
tural practice and from a simple calculation after spraying. The model
used for calculating the predicted environmental concentration (PEC)
is similar to that used currently by the US Environmental Protection
Agency, the Pesticides Safety Directorate in the United Kingdom, and
the Uniform System for the Evaluation of Substances in the
Netherlands. The method is presented in Figure 2. The data used in the
calculation are those from a two-year monitoring programme in 15
freshwater locations in the Netherlands where fenthion was detected;
however, the sources of these emissions are not clear. No other data,
such as concentrations in surface water, were available. The results
used in the hazard evaluation are those of field experiments of aerial
application of fenthion for mosquito control. No data were available
from field experiments on concentrations in surface water or residues
in sediment.
The concentrations resulting from long-term exposure are assumed
to result from dissipation in accordance with first-order kinetics:
(a) monitoring programme in salt-marsh (see Table 9)
and 3-methyl-4-methylthio-phenol (maximum, 35% after 60 days),
3-methyl-4-methylsulfinyl-phenol (maximum, 26% after 30 days), and
3-methyl-phenol (maximum, 10% after 60 days) under anaerobic
conditions. In these experiments, only the moieties with a phenyl
group were qualified or quantified; the fate of the thiophosphoric
acid moiety is less well described. Eichelberger and Lichtenberg
(1971) assumed that O,O-dimethyl- O-thiophosphoric acid was formed
by hydrolysis.
4.3.2 Soil
Fenthion appears to be biotransformed rapidly in soil under
aerobic conditions and more slowly under anaerobic conditions: the
half-life of 13C/14C-1-ring-labelled fenthion in a silty loam soil
was 10 days under aeobic conditions and more than 32 days under
anaerobic conditions (Mobay Corp., 1978b; see Table 4). The latter
value is not reliable, however, as the anaerobic incubation was
started when about 99% of the parent molecule had been biotransformed
aerobically. These experiments and some important experimental
conditions are summarized in Table 4, which indicates that fenthion is
transformed rapidly under optimal conditions, such as sufficient
oxygen and a moisture content > 75% of the field capacity. Rapid
transformation of fenthion under aerobic conditions was confirmed by
Bayer AG (1974), with half-lives of 1.7 and 0.5 days in a sand and a
sandy loam soil. These results are somewhat unreliable, however, as
the moisture content of the soils during the tests was not reported
and they may have been stored under overly dry conditions. The rate of
biotransformation of fenthion in soils can be described by linear
first-order kinetics, although few studies are available for
verification.
The main metabolites of fenthion under aerobic conditions are
fenthion sulfoxide, 3-methyl-4-methylsulfinyl-phenol, and 3-methyl-
4-methylsulfonyl-phenol (Mobay Corp., 1978b). In laboratory
experiments, the maximal amounts of these metabolites in silt loam
were 30-33, 15-18, and 30-31%, respectively, of the total activity.
These maxima were reached within 14 days after application at rates of
1-10 mg/kg dry weight. Substantial amounts of these metabolites appear
to be formed microbially, as only fenthion sulfoxide and to a lesser
extent 3-methyl-4-methylsulfonyl-phenol were formed under sterile
conditions in the same soil; they were formed more slowly under
sterile conditions. Some minor metabolites quantified in these
experiments were fenthion sulfone and 1-methoxy-3-methyl-4-methyl-
sulfonyl-benzene, which were found at 4-8 and 4-6%, respectively, of
the total activity within 59 days after application. It should be
noted that the formation of 1-methoxy-3-methyl-4-methylsulfonyl-
benzene indicates methylation.
Under aerobic conditions, the amounts of soil-bound residues
gradually increased up to a plateau of 41% of the total activity 30
days after application and continued until the end of the experiment
after 120 days (Mobay Corp., 1978b). These soil-bound residues were
due mainly to microbial action, as under sterile conditions only 9%
was recovered as soil-bound residues 30 days after application.
Mineralization to carbon dioxide occurs in soil in the laboratory
under both aerobic and anaerobic conditions, although more slowly
under the latter conditions. After application of 13C/14C-1-ring-
labelled fenthion at 1 mg/kg dry weight to a silt loam soil under
aerobic conditions in the laboratory, about 50% 14C-carbon dioxide
evolved within 120 days (Mobay Corp., 1978b). In the same soil under
anaerobic conditions, the mineralization rate was substantially lower
than that under aerobic conditions after aerobic preincubation for 30
days; during 32 days of anaerobic incubation, only 4% of the total
activity was degraded to 14C-carbon dioxide.
4.4 Bioconcentration
Fenthion is a lipophilic compound which is only slightly soluble
in water and has an octanol-water partition coefficient (log Kow) of
4.8. These properties indicate possible bioconcentration, as confirmed
in a laboratory experiment in which fenthion was shown to be
moderately concentrated in fish (ChemAgro, 1975a).
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 14C-fenthion at 0.008-0.12 mg/litre
(actual concentrations) for 14 days, the calculated daily biocon-
centration factors based on the wet weight of the whole fish increased
from 226 by 0.2 days after the start of the test to 400-500 by 4-7
days (ChemAgro, 1975a); after 14 days of exposure, the daily
bioconcentration factors were 200-300. The maximal concentrations of
labelled residues in the whole fish were 58 mg/kg wet weight four days
after the start. When the fish were subsequently exposed to uncontam-
inated water, all of the residues were eliminated within 11 days.
Eleven days after the start of exposure, 62% of the activity recovered
from a dissected fish was water-soluble and 38% chloroform-soluble;
the latter fraction contained fenthion (as 73% of the activity, in the
fraction), fenthion sulfoxide (20%), 3-methyl-4-methylsulfinyl-phenol
(5%), and fenoxon sulfoxide (2%). Seventy percent of the labelled
residues in one exposed fish was recovered in the viscera, but it was
unclear to what extent transformation products had been formed. These
products can be formed in water by photo- and biotransformation (see
above).
Bluegill sunfish (Lepomis macrochirus) and channel catfish
(Ictalurus punctatus) were exposed under static conditions to a
single dose of 14C-ring-labelled fenthion at a concentration of
about 3.7 µg/litre in aquariums containing both water and sediment and
were observed for 28 days. The calculated maximal daily biocon-
centration factors were 118 and 115 after 8 and 24 h for the sunfish
and the catfish, respectively. The maximal amount of labelled residues
in both species was 0.3 mg/kg bw 8-24 h after application. No residues
were recovered in fish after 21 days. The maximal amounts were not
related to the maximal depressions in brain cholinesterase activity,
which were 97% after three days for the sunfish and 76% after six days
for the catfish. There appeared to be a lag of a few days between
exposure and effects. The fish appeared to have recovered 21 days
after application (ChemAgro, 1975a).
De Bruijn & Hermens (1991) confirmed that fenthion is moderately
concentrated in fish. They found a bioconcentration factor of 170 in
guppies (Poecilia reticulata) exposed for 11 days to a mixture of
toxicants, including fenthion. Uptake and elimination were equili-
brated after three days.
5. Environmental levels
The concentrations of fenthion in the environment are summarized
in Table 5. Few measurements are available from regular monitoring
programmes, except in the Netherlands, where fenthion was detected in
various fresh and marine surface waters in 1992. The only published
data on residues of fenthion on vegetation and insects after aerial
application of the pesticide are derived from monitoring after
application of 2.4 kg a.i./ha to two sites for control of weaver birds
(Quelea quelea) in Kenya (Bruggers et al., 1989). The applications
were the first to be used in the area to control birds. The
concentrations of residues on young birds were 44 and 84 µg per bird
on the two sprayed areas, respectively, on day 1 and about 10 µg per
bird cm days 3-4. Raptors that ate the birds were not killed, but some
were debilitated; the concentrations in the raptors were not measured.
Aggregate samples of insects contained fenthion at 7.2 mg/kg; the
highest concentration of residues was found in carabid beetles (23 µg
per beetle). The concentrations on grass were 39 and 28 mg/kg on the
two sprayed areas, respectively, on the first day after spraying and
1.9 and 1.1 mg/kg on day 44. A sample of millet seed spread on a mesh
in a sprayed area contained fenthion at 1.1 mg/kg.
Owing to the scarcity of data from monitoring, Table 5 also shows
some actual measurements in the field from experiments performed with
recommended application rates simulating common agricultural practice.
Only maximal amounts are tabulated as indicative values, and data on
the rate at which fenthion dissipated, when available, are mentioned
in the comments (see also sections 4.1 and 4.13). No data were
available on the occurrence of transformation products.
6. Effects on organisms in the laboratory and the field
6.1 Laboratory experiments
6.1.1 Microorganisms
(a) Water
The acute and chronic toxicity of technical-grade fenthion and a
formulation of fenthion to aquatic microorganisms and invertebrates
are summarized in Tables 6 and 7. Technical-grade fenthion is toxic to
highly toxic, with four-day EC50 values of 550-1800 µg/litre and
four-day NOEC values of 100-1120 µg/litre. One formulation of fenthion
is toxic, with four-day EC50 values of 1500-> 2000 µg/litre. The
toxicity of fenthion to e.g. algae is dependent on the species or
strain tested (Wängberg & Blanck, 1988). High toxicity for aquatic
organisms was confirmed by Saini & Saxena (1986), who reported a
six-day NOEC of 100 µg/litre for the freshwater protozoan Tetrahymena
pyriformis. High toxicity was also reported for some saltwater
microorganisms, with one-day EC50 values of 1000 µg/litre for the
alga Cyclotella nana and 100 µg/litre for the diatom Skeletonema
costatum and a one-day NOEC of 10 µg/litre for Cyclotella nana,
based on production of oxygen (Derby & Ruber, 1971).
The toxicity of fenthion for microorganisms in activated sludge
was tested in a laboratory experiment for 30 min at 20°C (Bayer AG,
1994b). The sludge had been collected in a plant treating domestic
waste. Only at the highest nominal dose of fenthion, 10 000 mg/litre,
was respiration inhibited substantially. The test is of limited value,
however, as the nominal test concentrations exceeded the water
solubility by up to 2380 times.
(b) Soil
No unequivocal indications of deleterious effects of fenthion on
microorganisms in the soil are available. The formulation Lebaycid
inhibits neither processes involved in the nitrogen transformation
cycle nor enzymes involved in microbial activity. It did not affect
soil respiration or nitrification in sandy loam or silt loam soils
after application at the recommended rate, 0.75 kg/ha, or a rate five
times higher, 3.8 kg/ha (Bayer AG, 1989a,b). Soil respiration was
measured over 28 days after amendment with glucose (Bayer AG, 1989a),
and incubation was at 20°C in the dark. The effects on nitrification
were analysed by measuring ammonium, nitrite, and nitrate up to and
including 28 days after application (Bayer AG, 1989b). Only slight,
transient increases in nitrate were observed in the silt loam soil and
decreases in the sandy loam soil. The soils had not been treated with
pesticides for one to two years previously.
Table 5. Maximal concentrations of fenthion in environmental water, soil, sediment, and biota
Sample Location Year Period Concentration Comments Reference
Kenya
Carabid Kulalu 1985 April-May 23 µg/beetle Application rate, 1.5-2.4 kg/ha; Bruggers et al.
beetle Ranch mean in insects, 7.2 mg/kg (1989)
Savannah Kulalu 1985 April-May 38 mg/kg Application rate, 1.5-2.4 kg/ha;
grass Ranch whether based on dry or wet Bruggers et al.
weight not explicitly reported; (1989)
amount decreased to 1.9 and 1.1 mg/kg
after 3 and 4 days, respectively
Millet Kulalu 1985 April-May 1.1 mg/kg Application rate, 1.5--2.4 kg/ha; Bruggers et al.
seed Ranch whether based on dry or wet weight (1989)
not explicitly reported; deliberate
overspraying of artificial plot
Netherlands
Fresh Large rivers 1992 0.01-0.12 Measured in pumping station; fenthion Van Meerendonk et al.
surface and lakes µg/litre detected in 25% of freshwater locations (1994)
water at a mean concentration of
0.022 µg/litre
Marine North Sea, 1992 0.01 µg/litre Fenthion detected in 25% of freshwater Van Meerendonk et al.
surface Waddensea locations at a mean concentration of (1994)
water 0.02 µg/litre
Table 5. (cont'd).
Sample Location Year Period Concentration Comments Reference
United States
Salt-marsh Indian River 1984 September 1.7 µg/litre No fenthion detectable (< 0.01 µg/litre) Wang et al.
surface County, Florida within 24-48 h of aerial application (1987)
water of 0.032 kg a.i./ha
Salt-marsh Indian River 1985 March < 0.01 No explanation for low concentration; Wang et al.
surface County, Florida µg/litre deposition was confirmed, as up to 0.5 (1987)
water µg/litre was detected in dishes with
an aqueous medium; application rate,
0.032 kg a.i./ha
Salt-marsh Indian River 1985 June 0.16 No fenthion detectable (<0.01 µg/litre) Wang et al.
surface County, Florida µg/litre within 24-48 h of aerial application of (1987)
water of 0.032 kg a.i./ha
Polia larvae Laramie, 1979 Summer 0.28 mg/kg 8 h after spraying in field experiment Powell
(bird feed) Wyoming at an application rate of 52 g/ha; no (1984)
fenthion detected 30 h after treatment
Table 6. Acute toxicity of fenthion and two metabolites to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Crustacean
Daphnia magna, Static Technical Reconstituted 8.0 10 20 2 EC50, 0.0057a,b Bayer (1985a)
first instar
Fish
Onchorhynchus mykiss, Continuous Technical Well 8.0-8.1 225-275 12-13 4 LC50, 0.83b,c,d ABC Inc.
0.4 cm, 0.8 g (1986a)
Lepomis macrochirus, Continuous Technical Well 8.1-8.2 225-275 21 4 LC50, 1.7b,c ABC Inc.
5.4 cm, 1.9 g (1987)
Onchorhynchus mykiss, Static Lebaycid Tap 7.5-7.9 284 16 4 LC50, 2.3b,d Bayer (1994c)
5.5-6 cm, 1.5 g (adapted)
Leuciscus idus, Static Lebaycid Tap 7.2-7.9 284 21 4 LC50, 4.3b,d Bayer (1994d)
5.5 cm, 1.5 g (adapted
Onchorhynchus mykiss, Static Psx Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Onchorhynchus mykiss, Static Psn Well 7.5-7.8 228 12 4 LC50, > 100 Bowmann &
2.5-3 cm, 0.8 g Assoc. (1989)
Lepomis macrochirus, Static Psx Well 7.3-7.6 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Lepomis macrochirus, Static Psn Well 72-7.9 228 22 4 LC50, > 100 Bowmann &
1.4-2 cm, 0.4-0.6 g Assoc. (1989)
Psx, 3-methyl-4-methylsulfinyl-phenol; Psn, 3-methyl-4-methylsulfonyl-phenol
a Based on immobilization
b As phototransformation occurred, value is based on a mixture of fenthion residues
c Measured concentrations
d Actual concentration
Table 7. Long-term toxicity of fenthion to aquatic organisms
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Green algae
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 550a,b Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 EC50, 1800a,c Bayer (1985a)
subspicatus
Scenedesmus Static Technical Artificial 7.8-10.3 24 22-23 4 NOEC, 100a,b Bayer (1985a)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, 1500a,b Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 EC50, > 2000a,c Bayer (1989c)
subspicatus
Scenedesmus Static Lebaycid Artificial 7.7-10.5 24 22-23 4 NOEC, 1120a,b Bayer (1989c)
subspicatus
Selenastrum Static Baytex Artificial NR 1.5 24 4 EC50, 1100a,c,d,e ABC Inc. (1986b)
capricornutum
Selenastrum Static Baytex Artificial NR 1.5 24 4 NOEC, 700a,c,d,e ABC Inc. (1986b)
capricornutum
Crustaceans
Daphnia magna, Continuous Lebaycid Artificial 7.5-8.3 204 20 21 NOEC, 0.018d,e,f,g TNO (1989)
first instar
Daphnia magna, Continuous Technical Well 8.1-8.3 206-275 20 21 MATC, Bayer (1985b)
first instar 0.042-0.082d,e,h
Table 7. (cont'd).
Sample Conditions Compound Water Ph Hardness (mg Temperature Length of Result Reference
CaCO3/litre) (°C) experiment (mg/litre)
(days)
Fish
Oncorhynchus Continuous Technical Well 6.6-7.8 29-30 12 88 MATC, 13-27d,e,i SLS Inc. (1988)
mykiss
NR, not reported
a As phototransformation occurred, the value is for a mixture of fenthion residues
b For decrease in biomass
c For inhibition of growth rate
d Measured concentration
e Actual concentration
f All concentrations below the detection limit of 0.1 µg/litre
g For reproduction
h For mortality, growth, and reproduction
i For embryo viability, embryo survival at hatch, survival, and growth of larvae
6.1.2 Aquatic organisms
(a) Plants
Stimulation but no decrease in biomass was observed in an
experiment in which duckweed (Lemna gibba) was exposed in an
artificial medium to the formulation Baytex at measured concentrations
up to 2.8 mg/litre for 14 days at 25°C (Malcolm Pirie, 1987). The
stimulation may be related to hormesis.
(b) Invertebrates
Technical-grade fenthion is highly toxic to the crustacean
Daphnia magna, with a two-day EC50 value of 5.7 µg/litre and a
21-day maximal acceptable toxicant concentration (MATC) of 0.042-0.082
µg/litre. The formulation Lebaycid is also highly toxic to this
species, with a 21 -day NOEC value of 0.018 µg/litre. Static and
chronic treatment of other invertebrate species provide more variable
results, with two- to four-day L(E)C50 values of 0.62-1800 µg/litre
for various freshwater molluscs, insects, and crustaceans, 17- to
32-week NOEC values of 1000-2000 µg/litre for the freshwater mollusc
Lymnea stagnalis, one- to four-day L(E)C50 values of 0.02-550
µg/litre for saltwater molluscs, insects, and crustaceans, and 8- to
20-day NOEC values of 0.037-0079 µg/litre for the saltwater crustacean
Mysidopsis bahia (e.g. Seugé & Bluzat, 1980; Mayer, 1986; Mayer &
Ellersieck, 1986; McKenney, 1986). These tests were performed with
technical- or analytical-grade fenthion.
(c) Vertebrates
The acute and long-term toxicity of technical-grade fenthion, the
metabolites 3-methyl-4-methylsulfinyl-phenol and 3-methyl-4-methyl-
sulfonyl-phenol, and a formulation of fenthion to aquatic vertebrates
is summarized in Tables 6 and 7. Technical-grade fenthion is
moderately to highly toxic to freshwater fish, with four-day LC50
values of 0.83-1.7 mg/litre and an 88-day MATC value of 13-27
µg/litre. The formulation Lebaycid is toxic, with four-day LC50
values of 2.3-4.3 mg/litre. The metabolites 3-methyl-4-methyl-
sulfinyl-phenol and 3-methylsulfonyl-phenol are very slightly toxic to
freshwater fish, with four-day LC50 values exceeding 100 mg/litre.
Other tests show moderate to high toxicity of technical-grade fenthion
(e.g. Korn & Earnest, 1974; Mayer & Ellersieck, 1986; WHO, 1986
[original references not checked], with two- to four-day LC50 values
of 1-3.4 mg/litre for freshwater fish and 0.45-2.5 mg/litre for
saltwater fish.
Fenthion is highly toxic for amphibians: Khangarot et al.
(1985) reported a four-day LC50 value of 0.84 µg/litre for the frog
Rana hexadactyla with a length of 20 mm.
Many sublethal effects have been observed in fish after exposure
to a wide range of concentrations, including bottom disorientation,
loss of equilibrium, discolouration, rapid respiration, bloated
stomach, gulping of air, quiescence, surfacing, and irregular swimming
behaviour (ABC Inc., 1986; SLS Inc., 1988; Bayer AG, 1994c,d).
6.1.3 Terrestrial organisms
(a) Plants
No data were available.
(b) Invertebrates
Technical-grade fenthion is obviously toxic to various
terrestrial insects. Few studies have addressed other terrestrial
invertebrates.
Fenthion appears to be toxic to honey bees (Apis mellifera),
with contact LD50 values of 0.16-< 2 µg/bee (Bayer AG, 1995
[original references not checked]). No data were available on the
toxicity of fenthion for honey bees after oral exposure, but it would
be expected to be toxic. It is also toxic for predators of pests such
as wasps, mites, hoverflies, and green lacewings tested at moderately
high temperatures (22-27°C during the day and night and 16-18°C at
night).
Fenthion is highly toxic to a microhymenopterous wasp,
Trichogramma caoeciae, that parasitizes the eggs of the parasite
Sitrotroga cereallella (Conrad Appel GmbH, 1989). In one experiment,
larvae were exposed to Lebaycid sprayed onto glass plates at a rate of
16 kg/ha as fenthion. All of the larvae died within 24 h. In a second
experiment, host eggs containing pupae did not hatch after they had
been dipped in an aqueous solution of 0.1% Lebaycid.
Fenthion is also highly toxic to the predating mite Phytoseiulus
persimilis Athias-Henriot (von Kniehase & Zoebelein, 1990). Male and
female mites were placed on the leaves of kidney beans (Phaseolus
vulgaris) to produce a small population, and five days later the
leaves were dipped into a solution of 0.075% Lebaycid EC 500 at the
highest recommended application rate. All mites were killed within
seven days. Treatment with 0.015% Lebaycid EC 500 did not kill all of
the mites.
All early-instar larvae of the aphid predating hover fly
(Episyrphus balteatus) died within 24 h after spraying of Lebaycid
500 EC on glass plates at a rate of 0.77 kg/ha as fenthion (University
of Southampton, 1992).
Fenthion was highly toxic to the larvae of the green lacewing
(Chrysoperla carnea) exposed by contact to Lebaycid at 12 kg/ha as
fenthion in a light-dark cycle for three to five days (GAB Biotech-
nologie, GmbH, 1991). The rate of mortality was 100% of larvae and
unhatched imagos and only 7% of controls.
Artificial soil contaminated with Lebaycid 50EC was slightly
toxic to earthworms (Eisenia andrei) (Bayer AG, 1989d), with a
14-day LC50 of 723 mg/kg soil dry weight and a dose-related weight
loss. The 14-day NOEC was thus 100 mg/kg dry weight of soil. As the
vessel containing the worms was lit continuously, some photo-
degradation may have occurred.
(c) Vertebrates
The acute and subacute toxicity of fenthion to birds is
summarized in Table 8.
Technical-grade fenthion is moderately toxic or toxic to birds,
with an LD50 value of 7.2 mg/kg bw and an eight-day LC50 value of
60-1259 mg/kg feed. Five- to 10-day LC50 values of 25-231 mg/kg feed
were reported for the mallard duck (Anas platyrhynchos), the
bobwhite quail (Colinus virginianus), the Japanese quail (Coturnix
japonica), and the domestic chicken (Gallus domestica) (Hill
et al., 1975; Royal Society of Chemistry, 1995). Fenthion appears to
be toxic after dermal application in several bird species, with LD50
values of 1.8 mg/kg bw for the weaver bird (Quelea quelea) and
2.4 mg/kg bw for the house sparrow (Passer domesticus) (Schafer
et al., 1973).
The sublethal effects observed in birds treated with fenthion
experimentally are wing drop, ataxia, salivation, reduced body weight,
tremors, convulsions, diarrhoea, and laboured breathing (Grue, 1982;
Mobay Corp., 1987c,d,e). Necropsy of poisoned birds showed fluid-
filled crops and intestines, mottled spleens (possible due to organ
hypostasis), and oily fluid in crops or gastrointestinal tract,
indicating incomplete absorption of fenthion. Common grackles
(Quiscalus quiscula) found dead after five days' exposure to fenthion
had lost 28-36% of their initial weight, and muscle tissue on the
breastbone and observable fat had decreased substantially (Grue,
1982). The reduced body weights were due to treatment-related
reductions in feed consumption, perhaps indicating that fenthion is a
repellent, probably because of its strong mercaptan odour. The
estimated daily intake remained constant and was thus independent of
the concentrations tested.
Table 8. Acute and subacute toxicity of technical-grade fenthion given to birds for eight days
Species Sex Age Route Result Reference
Mallard duck (Anas platyrhynchos) Not reported 9 days Diet LC50, 1259 mg/kg feed Mobay Corp. (1987c)
Bobwhite quail (Colinus virginianus) Not reported 10 days Diet LC50, 60 mg/kg feed Mobay Corp. (1987d)
Bobwhite quail (Colinus virginianus) Male, female 18 weeks Oral LD50, 7.2 mg/kg bw Mobay Corp. (1987e)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, 57 mg/kg feeda Grue (1982)
Common grackle (Quiscalus quiscula) Male, female Adult Diet LC50, < 30 mg/kg feedb Grue (1982)
a Birds caged outdoors 20-27 July 1978
b Birds caged outdoors 14-21 August 1978
A decrease in cholinesterase activity in the brain and plasma is
an important physiological characteristic of avian poisoning by
fenthion. Common grackles that had been fed fenthion at rates of
25-400 mg/kg feed showed a significant, 80% depression in brain
cholinesterase activity. All birds had died before enzyme analysis
(Grue, 1982).
Fenthion appears to be less acutely toxic to terrestrial mammals
than to birds. Kenaga (1979) reported an LD50 range of 255-298 mg/kg
bw for the rat (Rattus norvegicus), indicating moderate toxicity.
FAO/WHO (1973) reported a three-month LOEC value of 0.25 mg/kg diet
based on growth reduction. The highest dietary levels that caused no
toxicological effect were estimated to be 3 mg/kg feed for rats and
2 mg/kg feed for dogs.
6.2 Field observations
6.2.1 Microorganisms
(a) Water
A single application of fenthion at a rate of 0.22 kg/ha did not
affect phytoplankton in small artificial outdoor ponds with natural
surface water and sediment in experiments performed in Florida (USA)
in 1962-63 (Patterson & von Windeguth, 1964).
(b) Soil
No data were available.
6.2.2 Aquatic organisms
(a) Plants
No data were available.
(b) Invertebrates
The populations of crustaceans and chironomids disappeared within
one week after application of fenthion to small artificial outdoor
ponds with natural surface water and sediment at a rate of 0.22 kg/ha
in experiments performed in Florida in 1962-63 (Patterson & von
Windeguth, 1964). The period required for complete recovery of the
numbers of water fleas and midge larvae was four to five months during
a cold winter period and two months in the absence of a cold period.
There were substantially more oligochaetes in treated than in
untreated ponds. Other aquatic invertebrates such as snails,
ostracods, copepods, and Hydra appeared to be unaffected by the
treatment.
(c) Vertebrates
Application of fenthion to small artificial and outdoor ponds at
a rate of 0.22 kg/ha did not appear to affect fish or tadpoles
(Patterson & von Windeguth, 1964).
6.2.3 Terrestrial organisms
(a) Plants
No data were available
(b) Invertebrates
As fenthion is highly toxic to insects in the laboratory, it can
be expected to be so in the field under certain conditions. Its
toxicity is linked mainly to the extent of exposure. In field
experiments in Assiut (Egypt) between June and August at the end of
the 1960s, the formulation Lebaycid, sprayed at a rate of 2.9 kg/ha
(as fenthion) four times at intervals of 15 days with back-pack
spraying equipment, was shown to be toxic to two pest predatory
coccinellids (Coccinella undecimpunctata and Scymnus interruptus)
in cotton (von Afify et al., 1970). Although the formulation was
generally toxic to imago beetles, there was substantial temporal and
spatial variation. The mortality of Coccinella was 58-79% (percentages
corrected for mortality in controls) within three, days of application
and 61-96% within 10 days of application. The mortality of Scymnus
was 55-83% within three days and 28-80% within 10 days. These figures
show partial recovery, at least of Scymnus; Coccinella may be more
susceptible, or Scymnus may recolonize and re-emerge. There was no
clear indication of increasing adverse effects in either species.
Field experiments in Los Baños (Philippines) in 1979-80 showed no
substantial adverse effects of fenthion on predators of the brown
planthopper (Nilaparvata lugens), a common rice pest in South and
Southeast Asia (Reissig et al., 1982). In these experiments,
fenthion was applied three times to plots of lowland rice at a rate of
0.75 kg/ha per treatment. Both planthoppers and their predators were
sampled one day before and two days after the treatments; the
predators sampled were the spiders Lycosa pseudoannulata, Tetragnatha
sp., and Araneus sp. and the hemipterous Microvelia atrolineata
and Cyrtorhinus lividipennis. The numbers of neither the
planthoppers nor their predators were significantly altered by the
treatments.
Field experiments in Egypt in 1965-66 showed that adverse effects
on pest predatory species may be present and absent in the same area
(Kira et al., 1972). Some predators of borers and aphids in corn
(Zea mays) appeared to increase in number after treatment of crops
with fenthion at a rate of 1.2 kg/ha per treatment, while some
decreased.
The mortality of honey (Apis mellifera) and alkali bees (Nomia
melanderi Cockerell) in outdoor cages, caused by the introduction of
alfalfa leaves sprayed with fenthion as an ultra-low volume liquid
with a hand-sprayer at a rate of 0.9 kg/ha, was decreased by 75% when
the contaminated leaves were brought into the cages 2 h after
application of fenthion instead of immediately afterwards (Johansen
et al., 1983), indicating that fenthion can be applied with minimal
hazard to bees that are not foraging.
(c) Vertebrates
Various incidents of poisoning of birds with fenthion have been
reported. It is generally assumed that a depression in brain
cholinesterase activity > 50% indicates severe exposure to fenthion
(e.g. Henny et al., 1987). In a field experiment in Kenya,
cholinesterase activity < 20 µmol/min per g was considered to
indicate exposure to fenthion and activities < 10 µmol/min per g to
indicate severe sickness or death (Bruggers et al., 1989).
On a wet meadow of 1.8 km2 in a field experiment in Wyoming
(USA) in 1978, 99 birds and 15 mammals were found dead or sick within
16 days after aerial treatment with fenthion at a rate of 47 g/ha
(DeWeese et al., 1983). As mortality is correlated with signifi-
cantly depressed brain cholinesterase activity in three common bird
species, it was assumed that the mortality and sickness were
treatment-related. A lower mortality rate seen on a comparable trial
site may have been due to factors such as different wind speeds and
overlap of spray swathes. The route of fenthion that induced poisoning
was unclear.
The maximal fenthion residues in dead American kestrels (Falco
sparverius) were correlated with cholinesterase activity depressions
of 78-92% in brain and 97% in plasma (Hunt et al., 1991). Within
three days after treatment of sparrow perches with fenthion, all of
the kestrels were dead. As the kestrels tended to hide in bushes as
paralysis advanced, the numbers of contaminated birds may have been
underestimated. The time for a kestrel to catch a sparrow was
positively correlated with the extent of cholinesterase depression in
the kestrel's brain. This indicates that a kestrel exposed to more
potent fenthion residues will have greater cholinesterase depression
or that kestrels with greater cholinesterase depression are less
capable of catching sparrows. There are indications that the more
fenthion sparrows are exposed to, the more sensitive they are to
kestrel predation (Hunt, 1990, cited by Kendall & Akerman, 1992).
Two of four flood-irrigated hay meadows were sprayed with the
formulation Baytex at 52 g/ha (as fenthion) in order to investigate
the effects on the reproduction of red-winged blackbirds (Anglaius
phoeniceus) in an experiment in Wyoming (USA) in June-July 1979
(Powell, 1984). In one of the treated fields, the growth rate of
nestlings was significantly decreased by 15%; however, the biological
interpretation of this effect is difficult, as cholinesterase activity
was not reduced. It appears to be a transient effect, since the
surviving nestlings recovered from the growth reduction. No
significant treatment-related effects on hatching, on fledgling
survival, or on foraging behaviour of adult blackbirds for nestling
care were observed. The latter parameter, investigated by counting the
number of trips per hour and their duration and distance, was
remarkably stable, as the abundance of the main feed type, the
caterpillar-like larva Polia, had been reduced by about 50% within
one to two days after the treatment.
The effect of fenthion on non-target birds was studied after
aerial application against the red-billed weaver bird (Quelea
quelea), a grain pest in Africa (Bruggers et al., 1989). In these
experiments, conducted in April-May 1985, two quelea colonies in a
savannah (Kulalu Ranch, Kenya) of low thornbush were sprayed at a rate
of 1.5-2.4 kg/ha. The brain cholinesterase activities that were
measured in 19 potentially exposed non-target bird species varied
substantially. Raptorial birds that were captured in or around the
quelea colonies, which had been radioequipped and were tracked up to
14 days after the treatment, were apparently not killed by the
treatment, but 70% had substantially depressed cholinesterase activity
in blood plasma, indicating actual exposure. Most raptorial birds were
assumed by the authors to have been exposed to fenthion by ingesting
contaminated quelea birds within one day after treatment. As no dead
radioequipped raptorial birds were recaptured, it may cautiously be
concluded that the risk of dying through secondary poisoning was
limited; however, the biological consequences for raptorial birds of
depressed cholinesterase activities with respect to other end-points
such as growth and reproduction are unclear.
The effects of fenthion on granivorous birds were also studied:
galliform species (Coturnix delegorguei, Gallus gallus, Pterocles
decoratus) and laughing doves (Streptopelia senegalensis) in cages
that were placed 100, 200, 300, and 400 m downwind of the treated
quelea colonies showed no mortality or signs of intoxication during an
observation period of five to seven days.
An additional experiment showed that the possibility of secondary
poisoning from scavenged contaminated carcasses cannot be excluded.
During the first night after the treatment, 24-90% of intentionally,
placed corpses were removed by scavengers such as jackals (Canis
mesomelas), bat-eared foxes (Otocyon megalotis), and baboons
(Papio cyanocephalus). This high rate of scavenging substantially
reduced the numbers of poisoned dead animals that could be collected,
resulting in an underestimation of the actual risks for non-target
birds.
7. Evaluation of effects on the environment
Fenthion is a systemic organophosphorus pesticide used for both
agricultural and nonagricultural purposes. Its major use is in the
control of insect pests (at an application rate of 1.25 kg a.i./ha),
but it is also used as a veterinary product to control external
parasites on domestic animals and to control birds (at an application
rate of 2.4 kg a.i./ha). Sprays for bird control are composed of a
formulation that is not available commercially and are used only under
governmental control. The pesticide is usually formulated as an
emulsifiable concentrate and is applied by ground spraying (80-85%) or
from the air (15-20%). It is also formulated as granules and as a
paste for control of urban birds. It can enter the environment beyond
the intended zone of treatment by spray drift, as residues on
potential food items such as birds, and, presumably, by loss of
surface residues from domestic animals.
Fenthion that enters the environment is adsorbed rapidly and
strongly on soil and sediment by a poorly understood mechanism. The
compound can be considered to be immobile in soil, and the parent
compound is unlikely to leach below the to firstfew centimetres of the
soil profile.
While photodegradation occurs under laboratory conditions, it is
not considered to be a significant route of breakdown in the field.
Hydrolysis occurs slowly and is dependent on pH; the half-lives of
fenthion in sterile buffers range from 30 to 60 days, with slower
degradation at low pH. Fenthion is degraded by microorganisms in
laboratory soil, with a half-life of 10 days; field degradation is
slower, about half of the compound being degraded to carbon dioxide
within six months in temperate climates. Generally, breakdown is
slower in water-sediment systems than in soil. Fenthion has been
measured in surface waters in untreated areas at concentrations up to
0.12 µg/litre. As the properties of fenthion would not result in its
entering the aquatic environment by leaching or run-off, there is no
satisfactory explanation for the aquatic residues found. It does not
bioaccumulate to a significant extent, despite its high solubility in
fat, since its metabolism and depuration in organisms are rapid.
Because fenthion is broken down rapidly in the environment, its
acute toxicity is relevant. Most species are not exposed chronically,
and its binding to soil and sediment particles would be expected to
reduce its bioavailability. Fenthion and formulations are toxic to
highly toxic to microorganisms, with four-day EC50 values of 550 and
1100 µg/litre. No data were available on its acute toxicity to algae,
but it is of low toxicity after long-term exposure. Screening tests
have indicated no phytotoxicity. Its acute and long-term toxicity to
crustaceans is high, with an EC50 value of 5.7 µg/litre and an NOEC
value of 0.018 µg/litre. Fenthion is very toxic to fish, with an
LD50 of 830 µg/litre and an MATC of 20 µg/litre. One study showed
toxicity to larvae at 0.84 µg/litre. Fenthion is moderately toxic to
birds, with an LD50 value of 7.2. µg/kg bw and a five-day LC50
value of 60 µg/kg feed. It is also toxic to honey bees, with a contact
LD50 of 0.16 µg per bee, and is slightly toxic to earthworms, with a
1-h LC50 of 723 µg/kg dry weight.
7.1 Risk assessment
Exposure concentrations have been derived from the results of
monitoring programs or field experiments simulating common agricul-
tural practice and from a simple calculation after spraying. The model
used for calculating the predicted environmental concentration (PEC)
is similar to that used currently by the US Environmental Protection
Agency, the Pesticides Safety Directorate in the United Kingdom, and
the Uniform System for the Evaluation of Substances in the
Netherlands. The method is presented in Figure 2. The data used in the
calculation are those from a two-year monitoring programme in 15
freshwater locations in the Netherlands where fenthion was detected;
however, the sources of these emissions are not clear. No other data,
such as concentrations in surface water, were available. The results
used in the hazard evaluation are those of field experiments of aerial
application of fenthion for mosquito control. No data were available
from field experiments on concentrations in surface water or residues
in sediment.
The concentrations resulting from long-term exposure are assumed
to result from dissipation in accordance with first-order kinetics:
(a) monitoring programme in salt-marsh (see Table 9)
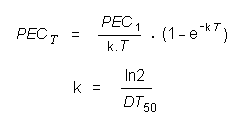
 (b) spraying (see Table 10)
(b) spraying (see Table 10)
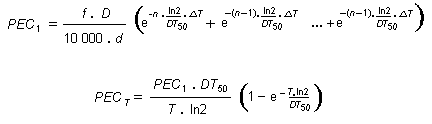 The following data are used in the calculations for situations
(a) and (b):
- water depth, 0.3 m
- 'worst-case' application rate on ornamental plants, three times 1
kg a.i./ha at seven-day interval, taking into account half-life
of seven days: 1.75 kg a.i./ha
- emission factor, 0.05 spray drift at 1 m
- tests periods of four days for algae, 21 days for crustaceans,
and 88 days for fish.
The half-life used is the longest value reported (see Table 3), which
can be considered a realistic 'worst-case' value. The lowest L(E)C50,
and NOEC values for algae, crustaceans, and fish were taken from
Tables 6 and 7.
It should be noted that limited information is available on
concentrations in the environment and toxicity. Only the water
compartment and a few species living in open water have been included.
Data on the toxicity of transformation products for aquatic organisms
were available only for 3-methyl-4-methylsulfinyl-phenol and
3-methylsulfonyl-phenol. The exposure concentrations are calculated
for the times equal to the duration of an experiment; e.g. the 21-day
NOEC for water fleas must be compared with the PEC21 days, calculated
as described above.
Table 9 gives a comparison of the estimated mean and maximal
exposure concentrations in the field with the lowest reported
L(E)C50 and NOEC values for acute and long-term exposure of
organisms in open water. The ratio between exposure and effect
concentration (TER) has been calculated. The Table is meant to serve
as a guide to classifying risk in the field and is not intended for
use in estimating the degree of effect likely to be seen. The
classification 'possible risk' is a simple one based on different
classification phrases for order-of-magnitude segments of the ratios.
Table 9 shows that crustaceans are at possible risk after either
acute or long-term exposure, but fish and algae appear not to be
affected. The few field observations available confirm the risk for
crustaceans (see above), but they are not altogether reliable, as some
relevant test conditions (e.g. physicochemical characteristics of the
water, temperature, actual concentrations of fenthion) are
incompletely described.
Table 10 gives a summary of results obtained for the example of
spraying ornamental plants. Crustaceans are again the organisms at
risk after either acute or long-term exposure. Algae are not affected,
and the effect on fish can be considered low.
It should be noted on the one hand that the risk classifications
shown in Tables 9 and 10 are for realistic 'worst-case' conditions:
the lowest reported values for toxicity were included; the slowest
reported dissipation rate was used; the concentrations used to
estimate mean exposure are only those from sites where fenthion was
actually detected; and the maximal estimated exposure concentration is
that used in aerial application for mosquito control. On the other
hand, only four groups of aquatic organisms are considered (e.g. no
sediment-dwelling organisms were included), adjuvants and transform-
ation products were not taken in to account, and the risks are not
relevant for water temperatures exceeding 22°C.
Table 9. Indications of environmental risk for aquatic organisms due to technical-grade fenthion
Effect Organism Estimated exposure Toxicity End-point Toxicity: Risk
concentration (µg/litre) exposure classification
(µg/litre) ratio
Mean estimated exposure concentration (monitoring programme)
Acute Crustaceans 0.020 EC50 = 5.7 Immobilization 285 Negligible
Acute Fish 0.020 LC50 = 830 Mortality > 1000 Negligible
Acute Amphibia 0.020 LC50 = 0.84 Mortality 42 Low
Chronic Algae 0.017 EC50 = 550 Biomass decrease > 1000 Negligible
Chronic Crustaceans 0.0084 NOEC = 0.018 Reproduction 2.1 Present
Chronic Fish 0.0023 MATC = 20a Reproduction > 1000 Negligible
Maximum estimated exposure concentration (salt-marsh)
Acute Crustaceans 1.7 EC50 = 5.7 Immobilization 3.4 Present
Acute Fish 1.7 LC50 = 830 Mortality 488 Negligible
Acute Amphibia 1.7 LC50 = 0.84 Mortality 0.5 High
Chronic Algae 1.4 EC50 = 550 Biomass decrease 393 Negligible
Chronic Crustaceans 0.71 NOEC = 0.018 Reproduction 0.03 Very high
Chronic Fish 0.2 MATC = 20a Reproduction 100 Negligible
a Mean of 13 and 27 µg/litre (see Table 7)
Table 10. Risk assessment for highest recommended rate of agricultural application based on
predicted environmental concentration
Effect Organism Preicted Toxicity End-point Toxicity: Risk
environmental (µg/litre) exposure classification
concentration ratio
(µg/litre)
Acute Crustaceans 29 EC50 = 5.7 Immobilization 0.2 High
Acute Fish 29 LC50 = 830 Mortality 28.6 Negligible
Acute Amphibia 29 LC50 = 0.84 Mortality 0.03 Very high
Chronic Algae 23.9 EC50 = 550 Biomass decrease 23.0 Negligible
Chronic Crustaceans 12.2 NOEC = 0.018 Reproduction 0.0015 Very high
Chronic Fish 3.3 MATC = 20 Reproduction 6.1 Present
Fenthion kills some beneficial insect predators, although the
results are variable; less effect was seen in the field than in the
laboratory. It is considered not to be hazardous to earthworms at
recommended rates of application. The risk to honey bees is considered
to be negligible provided they are not foraging. Fenthion is highly
acutely toxic to birds and is used as an avicide at high application
rates. Risk was assessed for both avicidal and general agricultural
use.
7.2 Use as an avicide
A single report has been made of secondary poisoning of a bird of
prey after ingestion of poisoned house sparrows. The dietary LC50
for the bobwhite quail is 60 mg/kg diet. On the basis of comparative
figures for body weight and food consumption, the LC50 values for
predatory birds that eat avian prey can be estimated from the
following equations to be 267 mg/kg diet for the European kestrel and
282 mg/kg diet for the sparrowhawk:
[Test species] LD50 [Test species] LD50 (mg/kg diet) × bw (kg)
(mg/kg per day) =
Food consumption (kg)
LC50 (mg/kg dry weight [Test species] LD50 (mg/kg diet) × bw (kg)
diet per day) =
Food consumption (kg)
The total daily intake that leads to death is 4.2 and 4.8 mg for the
two species, respectively. The residue concentrations reported in
house sparrows poisoned by fenthion used as an avicide were 6 µg/g in
carcass, 631 µg/g in feathers and skin, and 1152 µg/g in feet; the
total fenthion content of the carcass would be 166 µg on the basis of
a body weight of 27.7 g. Ingestion of the carcass of a sparrow should
not therefore result in a lethal dose, but ingestion of residues on
feathers and feet during plucking of the prey could result in a lethal
intake. A range of bird species may therefore have comparable
sensitivity to fenthion, and it can be assumed that any bird-eating
raptor would be killed by eating contaminated prey.
7.3 Agricultural use
Use of the vertebrate scheme of the European and Mediterranean
Plant Protection Organisation/Council of Europe and the highest
recommended agricultural application rate of 1.25 kg/ha gave predicted
environmental concentrations of 140 mg/kg for grass, 3.4 mg/kg for
insects, and 3.4 mg/kg for grains. The LC50 and TER values for
various bird species that use these items as food were estimated on
the basis of the data on toxicity for the bobwhite quail and are shown
in Table 11, which shows that use of fenthion at the highest
recommended application rate would be likely to cause deaths among
birds. The most susceptible species are those with a low body weight
that feed on vegetation.
Table 11. Toxicity:exposure ratios for birds after application of fenthion at 1.25 kg/ha
Species Estimated LC50 Predicted environmental Toxicity:exposure Risk
(mg/kg diet) concentration (mg/kg) ratio classification
Common quail (Coturnix coturnix) 211 140 1.5 Present
Greylag goose (Anser anser) 658 140 4.7 Present
Wren (Troglodytes troglodytes) 46 3.4 13.5 Low
Jackdaw (Corvus monedula) 285 3.4 84 Low
Reed bunting (Emberiza shoeniclus) 73 3.4 21.5 Low
Red-legged partridge (Alectroris nifa) 355 3.4 104 Very low
References
ABC Inc. (1986a) Acute flow-through toxicity of Baytex to rainbow
trout. Unpublished report No. 35313 by Analytical Biochemistry
Laboratories Inc., Columbia, Missouri, USA. Submitted to WHO by
Bayer AG.
ABC Inc. (1986b) Acute toxicity of Baytex to Selenastrum
capricornutum. Unpublished report No. 35314 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA.
Submitted to WHO by Bayer AG.
ABC Inc. (1987) Acute flow-through toxicity of Baytex to bluegill
sunfish (Lepomis macrochirus). Unpublished report No. 35597 by
Analytical Biochemistry Laboratories Inc., Columbia, Missouri,
USA. Submitted to WHO by Bayer AG.
ABC Inc. (1988) Soil adsorption/desorption with 14C-Baytex (Project
No. 36998). Unpublished report No. 98450 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA Submitted
to WHO by Bayer AG.
von Afify, A.M., Farghaly, H.T., & Hassanein, M.H. (1970) Freiland-
Untersuchungen über die Wirkung verschiedener Insektizide auf das
Vorkommen von zwei entomophagen Coccinelliden an Baumwolle in
Oberägypten. Schaedlungskd Pflanzensch. Umweltsch., 43, 8-13.
Bayer AG (1974) Verhalten des Pflanzenschutzmittelwirkstoffes im
Boden. Unpublished report No. RR5000/74 from Bayer AG
Pflanzenschutz Anwendungstechnik, Biologische Forschung,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1983a) Note on hydrolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG.
Bayer AG (1983b) Note on photolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG).
Bayer AG (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) by fenthion (technical). Unpublished report
No. 89082 from Bayer Institute of Environmental Biology,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1985b) Acute toxicity of fenthion (technical) to water
fleas. Unpublished report No. HBF/Dm 52 from Bayer Institute of
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988a) Degradation of fenthion ((R)Baytex) in the system
water/sediment (Project No. M 151 0186-2). Unpublished report
No. 3026 from Bayer AG Geschäftsbereich Pflanzenschutz, Institut
für Metabolismusforschung, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1988b) Degradation of crop protectants under anaerobic
conditions in the system water/sediment: fenthion (Project
No. 1520194-2). Unpublished report No. PF-3028 from
Bayer AG Geschäftsbereich Pflanzenschutz, Institut für
Metabolismusforschung, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988c) Method of estimating the direct photodegradation of
organic compounds in water under environmental conditions.
Unpublished report No. 2974 from Bayer AG Geschäftsbereich
Pflanzenschutz, Institut für Metabolismusforschung, Leverkusen,
Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989a) Influence of the commercial product (R)Lebaycid on
soil respiration after amendment with glucose (Study No. E 330
0279-4). Unpublished report No. AJO/71589 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989b) Influence of the commercial product (R)Lebaycid on
nitrification in soils (Study No. E 331 0278-4). Unpublished
report No. BSI/71689 from Bayer Crop Protection Research,
Chemical Product Development, Institute for Environmental
Biology, Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989c) Growth inhibition of green algae (Scenedesmus
subspicatus) by (R)Lebaycid EC 50 (Study No. E 323 0280-8).
Unpublished report No. HBF/A1 68 from Bayer Crop Protection
Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989d) Toxicity of (R)Lebaycid to earthworms (Study
No. E 310 0293-8). Unpublished report No. HBF/Rg 105 from Bayer
Crop Protection Research, Chemical Product Development, Institute
for Environmental Biology, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1994a) Calculation of the chemical lifetime of fenthion in
the troposphere. Unpublished report No. HPO-114 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994b) Studies on the ecological behaviour of Lebaycid N.
Unpublished report from Bayer AG, Institute for Environmental
Analysis and Assessments, Leverkusen, Germany. Submitted to WHO
by Bayer AG).
Bayer AG (1994c) Lebaycid 500 EC-acute toxicity (96 hours) to rainbow
trout in a static test. Unpublished revised study report No. DOM
94003 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994d) Lebaycid 500 EC-acute toxicity (96 hours) to golden
orfe in a static test. Unpublished revised study report No. DOM
94002 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1995) JMPE monograph for fenthion. Unpublished report of
28-03-95 submitted to WHO by Bayer AG.
Bowman & Assoc. (1989) Fenthion-acute toxicity test for freshwater
fish (Project No. FEN89C,D,E & F). Unpublished report No. 73916
from MC Bowman & Assoc., Inc., Mount Ida, Arkansas, USA.
Submitted to WHO by Bayer AG.
Bruggers, R.L., Jaeger, M.M., Keith, J.O., Hegdal, P.L., Bourassa,
J.B., Latigo, A.A. & Gillis, J.N. (1989) Impact of fenthion on
nontarget birds during Quelea control in Kenya. Wildl. Soc.
Bull., 17, 149-160.
ChemAgro (1972) The mobility and persistence of Baytex in soil and
water. Unpublished report No. 31985 from ChemAgro, Research and
Development Department, Kansas City, Missouri, USA Submitted to
WHO by Bayer AG.
ChemAgro (1974) Leaching of aged residues of Baytex-ring-UL-14C in
sandy loam soil. Unpublished report No. 40566 from ChemAgro,
Research and Development Department, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975a) Accumulation and persistence of residues in catfish
and bluegill fish exposed to Baytex-14C. Unpublished report
No. 43455 from ChemAgro Agricultural Division, Mobay Chemical
Corp., Research and Development, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975b) Leaching characteristics of Baytex. Unpublished
report No. 45653 from ChemAgro Agricultural Division, Mobay
Chemical Corp., Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
ChemAgro (1976a) Persistence and fate of Baytex in a natural aquatic
environment. Unpublished report No. 47404 from ChemAgro, Research
and Development Department, Kansas City, Missouri, USA. Submitted
to WHO by Bayer AG.
ChemAgro (1976b) Soil thin-layer mobility of twenty four pesticides
chemicals. Unpublished report No. 51016 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976c) Stability of Baytex in sterile aqueous buffer
solutions. Unpublished report No. 49130 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976d) Photodecomposition of Baytex. Unpublished report
No. 49347 from ChemAgro, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Conrad Appel GmbH (1989) Study on the effect of Lebaycid on
Trichogramma cacoeciae (Study No. 40/89 - 38/89). Unpublished
report from Conrad Appel GmbH, Department Trichogramma,
Darmstadt, Germany. Submitted to WHO by Bayer AG.
De Bruijn, J. & Hermens, J.L.M. (1991) Uptake and elimination kinetics
of organophosphorous pesticides in the guppy (Poecilia
reticulata): Correlations with the octanol/water partitioning
coefficient. Environ. Toxicol. Chem., 10, 791-804.
Derby, S.B. & Ruber, E. (1971) Primary production: Depression of
oxygen evolution in algal cultures by organophosphorus
insecticides. Bull. Environ. Contain. Toxicol., 5, 553-558.
DeWeese, L.R., McEwen, L.C., Settimi, L.A. & Deblinger, R.D. (1983)
Effects on birds of fenthion aerial application for mosquito
control. J. Econ. Entomol., 76, 906-911.
Eichelberger, J.W. & Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5, 541-544.
FAO/WHO (1973) 1971 Evaluations of some pesticide residues in food.
Report of the 1971 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues (WHO Technical Report No. 502), Geneva.
GAB Biotechnologie GmbH (1991) Detection of side effects of Lebaycid
on the green lacewing Chrysoperla carnea Steph. in the
laboratory (Study No. 008/01-Cc). Unpublished report from GAB
Biotechnologie GmbH, Niefern-Öschelbronn, Germany. Submitted to
WHO by Bayer AG.
Gohre, K. & Miller, G.C. (1986) Photooxidation of thioether pesticides
on soil surfaces. J. Agric. Food Chem., 34, 709-713.
Grue, C.E. (1982) Response of common grackles to dietary concen-
trations of four organophosphate pesticides. Arch. Environ.
Contain. Toxicol., 11, 617-626.
Henny, C.J., Kolbe, E.J., Hill, E.F. & Blus, L.J. (1987) Case
histories of bald eagles and other raptors killed by organophos-
phorus insecticides topically applied to livestock. J. Wildl.
Dis., 23, 292-295.
Hill, E.F., Heath, R.G., Sparta, J.W. & Williams, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds (Special
scientific report-wildlife no. 191). Washington DC, US Fish and
Wildlife Service.
Hunt, K.A., Bird, D.M., Mineau, P. & Shutt, L. (1991) Secondary
poisoning hazard of fenthion to American kestrels. Arch.
Environ. Contam. Toxicol., 21, 84-90.
Johansen, C.A., Mayer, D.F., Eves, J.D. & Kious, C.W. (1983)
Pesticides and bees. Environ. Entomol., 12, 1513-1518.
Kenaga, E.E. (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down to Earth, 35, 25-31.
Kendall, R.J. & Akerman, J. (1992) Terrestrial wildlife exposed to
agrochemicals: An ecological risk assessment perspective.
Environ. Toxicol. Chem., 11, 1727-1749.
Khangarot, B.S., Sehgal, A. & Bhasin, M.K. (1985) 'Man and
biosphere'-studies on the Sikkim Himalayas. Part 6: Toxicity of
selected pesticides to frog tadpole Rana hexadactyla (Lesson).
Acta Hydrochim. Hydrobiol., 13, 391-394.
Kira, M.T., Tawfik, M.F.S., & Metwally, S.M.I. (1972) Effect of DDT,
Gusathion and Lebaycid on corn pests and their predators. Bull.
Entomol. Soc. Egypt, 6, 221-230.
von Kniehase, U. & Zoebelein, G. (1990) [Testing the effects of
pesticides on the predator mite Phytoseiulus persimilis
Athias-Henriot by means of a new laboratory method approaching to
the practice.] Anz. Schaedlungskd. Pfianzensch. Umweltsch.,
63, 105-113 (in German).
Korn, S. & Earnest, R. (1974) Acute toxicity of twenty insecticides to
striped bass (Morone saxatilis). Calif. Fish Game, 60, 128-131.
Malcolm Pirnie Inc. (1987) The toxicity of Baytex technical, lot
no. 85RO1461 to Lemna gibba. Unpublished report No. 835 from
Malcolm Pirnie Inc., White Plains, New York, USA. Submitted to
WHO by Bayer AG.
Mayer, F.L. (1986) Acute toxicity handbook of chemicals to estuarine
organisms (Report no. EPA/600/X-86/231). Gulf Breeze, Texas, USA,
Environmental Research Laboratory, US Environmental Protection
Agency.
Mayer, F.L. & Ellersieck, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals (Resource publication 160). Washington DC, US
Department of the Interior, Fish and Wildlife Service.
McKenney, C.L. (1986) Influence of the organophosphate insecticide
fenthion on Mysidopsis bahia exposed during a complete life
cycle. I. Survival, reproduction and age-specific growth.
Dis. Aquat. Org., 1, 131-139.
Mobay Corp. (1978a) Soil adsorption and desorption of Baytex-
ring-1-14C. Unpublished report No. 66756 from Mobay Corp.
Agricultural Division, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1978b) The metabolism of Baytex-ring-1-13,14C on soil.
Unpublished report No. 66758 from Mobay Corp., Agricultural
Division, Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987a) Leaching characteristics of aged soil residues of
Baytex (Project No. BX-02-87). Unpublished report No. 94522 from
Mobay Corp., Agricultural Chemicals Division, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987b) Photodegradation of Baytex on soil (Project
No. BX-08-S). Unpublished report No. 94564 from Mobay Corp.,
Agricultural Chemicals Division, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
Mobay Corp. (1987c) Baytex Technical: Subacute dietary LC50 to
mallard ducks (Study No. 86-175-08). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987d) Baytex Technical: subacute dietary LC50 to
bobwhite quail (Study No. 86-175-09). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987e) Baytex (technical grade-acute LD50 to bobwhite
quail (Study No. 86-015-06). Unpublished report No. 831 from
Mobay Corp., Health, Environment and Safety Corporate Toxicology
Department, Stillwell, Kansas, USA. Submitted to WHO by Bayer AG.
O'Neill, E.J., Cripe, C.R., Mueller, L.H., Connolly, J.P. & Pritchard,
P.H. (1989) Fate of fenthion in salt-marsh environments: II.
Transport and biodegradation in microcosms. Environ. Toxicol.
Chem., 8, 759-768.
Patterson, R.S. & von Windeguth, D.L. (1964) The effects of Baytex on
some aquatic organisms. Mosq. News, 24, 46-49.
Powell, G.V.N. (1984) Reproduction by an altricial songbird, the
red-winged blackbird, in fields treated with the organophosphate
insecticide fenthion. J. Appl. Ecol., 21, 83-95.
Reissig, W.H., Heinrichs, E.A. & Valencia, S.L. (1982) Effects of
insecticides on Nilaparvata lugens and its predators: Spiders,
Microvelia atrolineata, and Cyrtorhinus lividipennis. Environ.
Entomol., 11, 193-199.
Saini, A. & Saxena, D.M. (1986) Effect of organophosphorus
insecticides on the growth of Tetrahymena pyriformis. Arch.
Protistenkd., 131, 143-152.
Schafer, W.E., Jr, Brunton, R.B., Lockyer, N.F & De Grazio, J.W.
(1973) Comparative toxicity of seventeen pesticides to the
quelea, house sparrow and red-winged blackbird. Toxicol. Appl.
Pharmacol., 26, 154-157.
Seabloom, R.W., Pearson, G.L., Oring, L.W. & Reilly, J.R. (1973) An
incident of fenthion mosquito control and subsequent arian
mortality. J. Wildl. Dis., 9, 18-20.
Seugé, J. & Bluzat, R. (1980) [Long-term toxicity of an
organophosphorus insecticide (fenthion) in the mollusc Lymnea
stagnalis.] Hydrobiology, 76, 241-248 (in French).
SLS Inc. (1988) The toxicity of technical grade fenthion (trade name
Baytex) to rainbow trout (Salmo gairdneri) embryos and larvae.
Unpublished report No. 95641 from Springborn Life Sciences Inc.,
Wareham, Massachusetts, USA. Submitted to WHO by Bayer AG.
TNO (1989) Reproduction test with Lebaycid EC 50 and Daphnia magna.
Unpublished report No. R 89/272 from TNO Division of Technology
for Society, Delft, Netherlands. Submitted to WHO by Bayer AG.
University of Southampton (1992) An evaluation of the side-effects of
Lebaycid 500 EC on larvae of the hoverfly Episyrphus balteatus
(Study No. BAY-91-1). Unpublished report from Agrochemical
Evaluation Unit, Department of Biology, University of
Southampton, Southampton, United Kingdom. Submitted to WHO by
Bayer AG.
US Department of Agriculture (1951) Soil Survey/Manual (Agriculture
Handbook No. 18). Washington DC, USA.
Van Meerendonk, J.H., Van Steenwijk, J.M., Phernambucq, A.J.W. &
Barreveld, H.L. (1994) [Tracking traces II. An inventory survey
for hazardous chemicals in the salt and freshwater systems in the
Netherlands] (RIKZ Report No. 94.007), The Hague, Netherlands,
National Institute for Coastal and Marine Management & National
Institute of Inland Water Management (in Dutch).
Wang, T.C., Lenaham, R.A., Tucker, J.W., Jr & Kadlac, T. (1987) Aerial
spray of mosquito adulticides in a salt marsh environment.
Int. Assoc. Water Qual., pp. 113-125.
WHO (1986) Organophosphorus Insecticides: A General Introduction
(Environmental Health Criteria 63). Geneva, International
Programme on Chemical Safety.
The following data are used in the calculations for situations
(a) and (b):
- water depth, 0.3 m
- 'worst-case' application rate on ornamental plants, three times 1
kg a.i./ha at seven-day interval, taking into account half-life
of seven days: 1.75 kg a.i./ha
- emission factor, 0.05 spray drift at 1 m
- tests periods of four days for algae, 21 days for crustaceans,
and 88 days for fish.
The half-life used is the longest value reported (see Table 3), which
can be considered a realistic 'worst-case' value. The lowest L(E)C50,
and NOEC values for algae, crustaceans, and fish were taken from
Tables 6 and 7.
It should be noted that limited information is available on
concentrations in the environment and toxicity. Only the water
compartment and a few species living in open water have been included.
Data on the toxicity of transformation products for aquatic organisms
were available only for 3-methyl-4-methylsulfinyl-phenol and
3-methylsulfonyl-phenol. The exposure concentrations are calculated
for the times equal to the duration of an experiment; e.g. the 21-day
NOEC for water fleas must be compared with the PEC21 days, calculated
as described above.
Table 9 gives a comparison of the estimated mean and maximal
exposure concentrations in the field with the lowest reported
L(E)C50 and NOEC values for acute and long-term exposure of
organisms in open water. The ratio between exposure and effect
concentration (TER) has been calculated. The Table is meant to serve
as a guide to classifying risk in the field and is not intended for
use in estimating the degree of effect likely to be seen. The
classification 'possible risk' is a simple one based on different
classification phrases for order-of-magnitude segments of the ratios.
Table 9 shows that crustaceans are at possible risk after either
acute or long-term exposure, but fish and algae appear not to be
affected. The few field observations available confirm the risk for
crustaceans (see above), but they are not altogether reliable, as some
relevant test conditions (e.g. physicochemical characteristics of the
water, temperature, actual concentrations of fenthion) are
incompletely described.
Table 10 gives a summary of results obtained for the example of
spraying ornamental plants. Crustaceans are again the organisms at
risk after either acute or long-term exposure. Algae are not affected,
and the effect on fish can be considered low.
It should be noted on the one hand that the risk classifications
shown in Tables 9 and 10 are for realistic 'worst-case' conditions:
the lowest reported values for toxicity were included; the slowest
reported dissipation rate was used; the concentrations used to
estimate mean exposure are only those from sites where fenthion was
actually detected; and the maximal estimated exposure concentration is
that used in aerial application for mosquito control. On the other
hand, only four groups of aquatic organisms are considered (e.g. no
sediment-dwelling organisms were included), adjuvants and transform-
ation products were not taken in to account, and the risks are not
relevant for water temperatures exceeding 22°C.
Table 9. Indications of environmental risk for aquatic organisms due to technical-grade fenthion
Effect Organism Estimated exposure Toxicity End-point Toxicity: Risk
concentration (µg/litre) exposure classification
(µg/litre) ratio
Mean estimated exposure concentration (monitoring programme)
Acute Crustaceans 0.020 EC50 = 5.7 Immobilization 285 Negligible
Acute Fish 0.020 LC50 = 830 Mortality > 1000 Negligible
Acute Amphibia 0.020 LC50 = 0.84 Mortality 42 Low
Chronic Algae 0.017 EC50 = 550 Biomass decrease > 1000 Negligible
Chronic Crustaceans 0.0084 NOEC = 0.018 Reproduction 2.1 Present
Chronic Fish 0.0023 MATC = 20a Reproduction > 1000 Negligible
Maximum estimated exposure concentration (salt-marsh)
Acute Crustaceans 1.7 EC50 = 5.7 Immobilization 3.4 Present
Acute Fish 1.7 LC50 = 830 Mortality 488 Negligible
Acute Amphibia 1.7 LC50 = 0.84 Mortality 0.5 High
Chronic Algae 1.4 EC50 = 550 Biomass decrease 393 Negligible
Chronic Crustaceans 0.71 NOEC = 0.018 Reproduction 0.03 Very high
Chronic Fish 0.2 MATC = 20a Reproduction 100 Negligible
a Mean of 13 and 27 µg/litre (see Table 7)
Table 10. Risk assessment for highest recommended rate of agricultural application based on
predicted environmental concentration
Effect Organism Preicted Toxicity End-point Toxicity: Risk
environmental (µg/litre) exposure classification
concentration ratio
(µg/litre)
Acute Crustaceans 29 EC50 = 5.7 Immobilization 0.2 High
Acute Fish 29 LC50 = 830 Mortality 28.6 Negligible
Acute Amphibia 29 LC50 = 0.84 Mortality 0.03 Very high
Chronic Algae 23.9 EC50 = 550 Biomass decrease 23.0 Negligible
Chronic Crustaceans 12.2 NOEC = 0.018 Reproduction 0.0015 Very high
Chronic Fish 3.3 MATC = 20 Reproduction 6.1 Present
Fenthion kills some beneficial insect predators, although the
results are variable; less effect was seen in the field than in the
laboratory. It is considered not to be hazardous to earthworms at
recommended rates of application. The risk to honey bees is considered
to be negligible provided they are not foraging. Fenthion is highly
acutely toxic to birds and is used as an avicide at high application
rates. Risk was assessed for both avicidal and general agricultural
use.
7.2 Use as an avicide
A single report has been made of secondary poisoning of a bird of
prey after ingestion of poisoned house sparrows. The dietary LC50
for the bobwhite quail is 60 mg/kg diet. On the basis of comparative
figures for body weight and food consumption, the LC50 values for
predatory birds that eat avian prey can be estimated from the
following equations to be 267 mg/kg diet for the European kestrel and
282 mg/kg diet for the sparrowhawk:
[Test species] LD50 [Test species] LD50 (mg/kg diet) × bw (kg)
(mg/kg per day) =
Food consumption (kg)
LC50 (mg/kg dry weight [Test species] LD50 (mg/kg diet) × bw (kg)
diet per day) =
Food consumption (kg)
The total daily intake that leads to death is 4.2 and 4.8 mg for the
two species, respectively. The residue concentrations reported in
house sparrows poisoned by fenthion used as an avicide were 6 µg/g in
carcass, 631 µg/g in feathers and skin, and 1152 µg/g in feet; the
total fenthion content of the carcass would be 166 µg on the basis of
a body weight of 27.7 g. Ingestion of the carcass of a sparrow should
not therefore result in a lethal dose, but ingestion of residues on
feathers and feet during plucking of the prey could result in a lethal
intake. A range of bird species may therefore have comparable
sensitivity to fenthion, and it can be assumed that any bird-eating
raptor would be killed by eating contaminated prey.
7.3 Agricultural use
Use of the vertebrate scheme of the European and Mediterranean
Plant Protection Organisation/Council of Europe and the highest
recommended agricultural application rate of 1.25 kg/ha gave predicted
environmental concentrations of 140 mg/kg for grass, 3.4 mg/kg for
insects, and 3.4 mg/kg for grains. The LC50 and TER values for
various bird species that use these items as food were estimated on
the basis of the data on toxicity for the bobwhite quail and are shown
in Table 11, which shows that use of fenthion at the highest
recommended application rate would be likely to cause deaths among
birds. The most susceptible species are those with a low body weight
that feed on vegetation.
Table 11. Toxicity:exposure ratios for birds after application of fenthion at 1.25 kg/ha
Species Estimated LC50 Predicted environmental Toxicity:exposure Risk
(mg/kg diet) concentration (mg/kg) ratio classification
Common quail (Coturnix coturnix) 211 140 1.5 Present
Greylag goose (Anser anser) 658 140 4.7 Present
Wren (Troglodytes troglodytes) 46 3.4 13.5 Low
Jackdaw (Corvus monedula) 285 3.4 84 Low
Reed bunting (Emberiza shoeniclus) 73 3.4 21.5 Low
Red-legged partridge (Alectroris nifa) 355 3.4 104 Very low
References
ABC Inc. (1986a) Acute flow-through toxicity of Baytex to rainbow
trout. Unpublished report No. 35313 by Analytical Biochemistry
Laboratories Inc., Columbia, Missouri, USA. Submitted to WHO by
Bayer AG.
ABC Inc. (1986b) Acute toxicity of Baytex to Selenastrum
capricornutum. Unpublished report No. 35314 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA.
Submitted to WHO by Bayer AG.
ABC Inc. (1987) Acute flow-through toxicity of Baytex to bluegill
sunfish (Lepomis macrochirus). Unpublished report No. 35597 by
Analytical Biochemistry Laboratories Inc., Columbia, Missouri,
USA. Submitted to WHO by Bayer AG.
ABC Inc. (1988) Soil adsorption/desorption with 14C-Baytex (Project
No. 36998). Unpublished report No. 98450 by Analytical
Biochemistry Laboratories Inc., Columbia, Missouri, USA Submitted
to WHO by Bayer AG.
von Afify, A.M., Farghaly, H.T., & Hassanein, M.H. (1970) Freiland-
Untersuchungen über die Wirkung verschiedener Insektizide auf das
Vorkommen von zwei entomophagen Coccinelliden an Baumwolle in
Oberägypten. Schaedlungskd Pflanzensch. Umweltsch., 43, 8-13.
Bayer AG (1974) Verhalten des Pflanzenschutzmittelwirkstoffes im
Boden. Unpublished report No. RR5000/74 from Bayer AG
Pflanzenschutz Anwendungstechnik, Biologische Forschung,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1983a) Note on hydrolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG.
Bayer AG (1983b) Note on photolytic stability of fenthion. Unpublished
report submitted to WHO by Bayer AG).
Bayer AG (1985a) Growth inhibition of green algae (Scenedesmus
subspicatus) by fenthion (technical). Unpublished report
No. 89082 from Bayer Institute of Environmental Biology,
Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1985b) Acute toxicity of fenthion (technical) to water
fleas. Unpublished report No. HBF/Dm 52 from Bayer Institute of
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988a) Degradation of fenthion ((R)Baytex) in the system
water/sediment (Project No. M 151 0186-2). Unpublished report
No. 3026 from Bayer AG Geschäftsbereich Pflanzenschutz, Institut
für Metabolismusforschung, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1988b) Degradation of crop protectants under anaerobic
conditions in the system water/sediment: fenthion (Project
No. 1520194-2). Unpublished report No. PF-3028 from
Bayer AG Geschäftsbereich Pflanzenschutz, Institut für
Metabolismusforschung, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1988c) Method of estimating the direct photodegradation of
organic compounds in water under environmental conditions.
Unpublished report No. 2974 from Bayer AG Geschäftsbereich
Pflanzenschutz, Institut für Metabolismusforschung, Leverkusen,
Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989a) Influence of the commercial product (R)Lebaycid on
soil respiration after amendment with glucose (Study No. E 330
0279-4). Unpublished report No. AJO/71589 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989b) Influence of the commercial product (R)Lebaycid on
nitrification in soils (Study No. E 331 0278-4). Unpublished
report No. BSI/71689 from Bayer Crop Protection Research,
Chemical Product Development, Institute for Environmental
Biology, Leverkusen, Germany. Submitted to WHO by Bayer AG.
Bayer AG (1989c) Growth inhibition of green algae (Scenedesmus
subspicatus) by (R)Lebaycid EC 50 (Study No. E 323 0280-8).
Unpublished report No. HBF/A1 68 from Bayer Crop Protection
Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1989d) Toxicity of (R)Lebaycid to earthworms (Study
No. E 310 0293-8). Unpublished report No. HBF/Rg 105 from Bayer
Crop Protection Research, Chemical Product Development, Institute
for Environmental Biology, Leverkusen, Germany. Submitted to WHO
by Bayer AG.
Bayer AG (1994a) Calculation of the chemical lifetime of fenthion in
the troposphere. Unpublished report No. HPO-114 from Bayer Crop
Protection Research, Chemical Product Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994b) Studies on the ecological behaviour of Lebaycid N.
Unpublished report from Bayer AG, Institute for Environmental
Analysis and Assessments, Leverkusen, Germany. Submitted to WHO
by Bayer AG).
Bayer AG (1994c) Lebaycid 500 EC-acute toxicity (96 hours) to rainbow
trout in a static test. Unpublished revised study report No. DOM
94003 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1994d) Lebaycid 500 EC-acute toxicity (96 hours) to golden
orfe in a static test. Unpublished revised study report No. DOM
94002 from Bayer Crop Protection Development, Institute for
Environmental Biology, Leverkusen, Germany. Submitted to WHO by
Bayer AG.
Bayer AG (1995) JMPE monograph for fenthion. Unpublished report of
28-03-95 submitted to WHO by Bayer AG.
Bowman & Assoc. (1989) Fenthion-acute toxicity test for freshwater
fish (Project No. FEN89C,D,E & F). Unpublished report No. 73916
from MC Bowman & Assoc., Inc., Mount Ida, Arkansas, USA.
Submitted to WHO by Bayer AG.
Bruggers, R.L., Jaeger, M.M., Keith, J.O., Hegdal, P.L., Bourassa,
J.B., Latigo, A.A. & Gillis, J.N. (1989) Impact of fenthion on
nontarget birds during Quelea control in Kenya. Wildl. Soc.
Bull., 17, 149-160.
ChemAgro (1972) The mobility and persistence of Baytex in soil and
water. Unpublished report No. 31985 from ChemAgro, Research and
Development Department, Kansas City, Missouri, USA Submitted to
WHO by Bayer AG.
ChemAgro (1974) Leaching of aged residues of Baytex-ring-UL-14C in
sandy loam soil. Unpublished report No. 40566 from ChemAgro,
Research and Development Department, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975a) Accumulation and persistence of residues in catfish
and bluegill fish exposed to Baytex-14C. Unpublished report
No. 43455 from ChemAgro Agricultural Division, Mobay Chemical
Corp., Research and Development, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
ChemAgro (1975b) Leaching characteristics of Baytex. Unpublished
report No. 45653 from ChemAgro Agricultural Division, Mobay
Chemical Corp., Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
ChemAgro (1976a) Persistence and fate of Baytex in a natural aquatic
environment. Unpublished report No. 47404 from ChemAgro, Research
and Development Department, Kansas City, Missouri, USA. Submitted
to WHO by Bayer AG.
ChemAgro (1976b) Soil thin-layer mobility of twenty four pesticides
chemicals. Unpublished report No. 51016 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976c) Stability of Baytex in sterile aqueous buffer
solutions. Unpublished report No. 49130 from ChemAgro
Agricultural Division, Mobay Chemical Corp., Research and
Development, Kansas City, Missouri, USA. Submitted to WHO by
Bayer AG.
ChemAgro (1976d) Photodecomposition of Baytex. Unpublished report
No. 49347 from ChemAgro, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Conrad Appel GmbH (1989) Study on the effect of Lebaycid on
Trichogramma cacoeciae (Study No. 40/89 - 38/89). Unpublished
report from Conrad Appel GmbH, Department Trichogramma,
Darmstadt, Germany. Submitted to WHO by Bayer AG.
De Bruijn, J. & Hermens, J.L.M. (1991) Uptake and elimination kinetics
of organophosphorous pesticides in the guppy (Poecilia
reticulata): Correlations with the octanol/water partitioning
coefficient. Environ. Toxicol. Chem., 10, 791-804.
Derby, S.B. & Ruber, E. (1971) Primary production: Depression of
oxygen evolution in algal cultures by organophosphorus
insecticides. Bull. Environ. Contain. Toxicol., 5, 553-558.
DeWeese, L.R., McEwen, L.C., Settimi, L.A. & Deblinger, R.D. (1983)
Effects on birds of fenthion aerial application for mosquito
control. J. Econ. Entomol., 76, 906-911.
Eichelberger, J.W. & Lichtenberg, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5, 541-544.
FAO/WHO (1973) 1971 Evaluations of some pesticide residues in food.
Report of the 1971 Joint Meeting of the FAO Working Party of
Experts on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues (WHO Technical Report No. 502), Geneva.
GAB Biotechnologie GmbH (1991) Detection of side effects of Lebaycid
on the green lacewing Chrysoperla carnea Steph. in the
laboratory (Study No. 008/01-Cc). Unpublished report from GAB
Biotechnologie GmbH, Niefern-Öschelbronn, Germany. Submitted to
WHO by Bayer AG.
Gohre, K. & Miller, G.C. (1986) Photooxidation of thioether pesticides
on soil surfaces. J. Agric. Food Chem., 34, 709-713.
Grue, C.E. (1982) Response of common grackles to dietary concen-
trations of four organophosphate pesticides. Arch. Environ.
Contain. Toxicol., 11, 617-626.
Henny, C.J., Kolbe, E.J., Hill, E.F. & Blus, L.J. (1987) Case
histories of bald eagles and other raptors killed by organophos-
phorus insecticides topically applied to livestock. J. Wildl.
Dis., 23, 292-295.
Hill, E.F., Heath, R.G., Sparta, J.W. & Williams, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds (Special
scientific report-wildlife no. 191). Washington DC, US Fish and
Wildlife Service.
Hunt, K.A., Bird, D.M., Mineau, P. & Shutt, L. (1991) Secondary
poisoning hazard of fenthion to American kestrels. Arch.
Environ. Contam. Toxicol., 21, 84-90.
Johansen, C.A., Mayer, D.F., Eves, J.D. & Kious, C.W. (1983)
Pesticides and bees. Environ. Entomol., 12, 1513-1518.
Kenaga, E.E. (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down to Earth, 35, 25-31.
Kendall, R.J. & Akerman, J. (1992) Terrestrial wildlife exposed to
agrochemicals: An ecological risk assessment perspective.
Environ. Toxicol. Chem., 11, 1727-1749.
Khangarot, B.S., Sehgal, A. & Bhasin, M.K. (1985) 'Man and
biosphere'-studies on the Sikkim Himalayas. Part 6: Toxicity of
selected pesticides to frog tadpole Rana hexadactyla (Lesson).
Acta Hydrochim. Hydrobiol., 13, 391-394.
Kira, M.T., Tawfik, M.F.S., & Metwally, S.M.I. (1972) Effect of DDT,
Gusathion and Lebaycid on corn pests and their predators. Bull.
Entomol. Soc. Egypt, 6, 221-230.
von Kniehase, U. & Zoebelein, G. (1990) [Testing the effects of
pesticides on the predator mite Phytoseiulus persimilis
Athias-Henriot by means of a new laboratory method approaching to
the practice.] Anz. Schaedlungskd. Pfianzensch. Umweltsch.,
63, 105-113 (in German).
Korn, S. & Earnest, R. (1974) Acute toxicity of twenty insecticides to
striped bass (Morone saxatilis). Calif. Fish Game, 60, 128-131.
Malcolm Pirnie Inc. (1987) The toxicity of Baytex technical, lot
no. 85RO1461 to Lemna gibba. Unpublished report No. 835 from
Malcolm Pirnie Inc., White Plains, New York, USA. Submitted to
WHO by Bayer AG.
Mayer, F.L. (1986) Acute toxicity handbook of chemicals to estuarine
organisms (Report no. EPA/600/X-86/231). Gulf Breeze, Texas, USA,
Environmental Research Laboratory, US Environmental Protection
Agency.
Mayer, F.L. & Ellersieck, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals (Resource publication 160). Washington DC, US
Department of the Interior, Fish and Wildlife Service.
McKenney, C.L. (1986) Influence of the organophosphate insecticide
fenthion on Mysidopsis bahia exposed during a complete life
cycle. I. Survival, reproduction and age-specific growth.
Dis. Aquat. Org., 1, 131-139.
Mobay Corp. (1978a) Soil adsorption and desorption of Baytex-
ring-1-14C. Unpublished report No. 66756 from Mobay Corp.
Agricultural Division, Research and Development Department,
Kansas City, Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1978b) The metabolism of Baytex-ring-1-13,14C on soil.
Unpublished report No. 66758 from Mobay Corp., Agricultural
Division, Research and Development Department, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987a) Leaching characteristics of aged soil residues of
Baytex (Project No. BX-02-87). Unpublished report No. 94522 from
Mobay Corp., Agricultural Chemicals Division, Kansas City,
Missouri, USA. Submitted to WHO by Bayer AG.
Mobay Corp. (1987b) Photodegradation of Baytex on soil (Project
No. BX-08-S). Unpublished report No. 94564 from Mobay Corp.,
Agricultural Chemicals Division, Kansas City, Missouri, USA.
Submitted to WHO by Bayer AG.
Mobay Corp. (1987c) Baytex Technical: Subacute dietary LC50 to
mallard ducks (Study No. 86-175-08). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987d) Baytex Technical: subacute dietary LC50 to
bobwhite quail (Study No. 86-175-09). Unpublished report No. 830
from Mobay Corp., Health, Environment and Safety Corporate
Toxicology Department, Stillwell, Kansas, USA. Submitted to WHO
by Bayer AG.
Mobay Corp. (1987e) Baytex (technical grade-acute LD50 to bobwhite
quail (Study No. 86-015-06). Unpublished report No. 831 from
Mobay Corp., Health, Environment and Safety Corporate Toxicology
Department, Stillwell, Kansas, USA. Submitted to WHO by Bayer AG.
O'Neill, E.J., Cripe, C.R., Mueller, L.H., Connolly, J.P. & Pritchard,
P.H. (1989) Fate of fenthion in salt-marsh environments: II.
Transport and biodegradation in microcosms. Environ. Toxicol.
Chem., 8, 759-768.
Patterson, R.S. & von Windeguth, D.L. (1964) The effects of Baytex on
some aquatic organisms. Mosq. News, 24, 46-49.
Powell, G.V.N. (1984) Reproduction by an altricial songbird, the
red-winged blackbird, in fields treated with the organophosphate
insecticide fenthion. J. Appl. Ecol., 21, 83-95.
Reissig, W.H., Heinrichs, E.A. & Valencia, S.L. (1982) Effects of
insecticides on Nilaparvata lugens and its predators: Spiders,
Microvelia atrolineata, and Cyrtorhinus lividipennis. Environ.
Entomol., 11, 193-199.
Saini, A. & Saxena, D.M. (1986) Effect of organophosphorus
insecticides on the growth of Tetrahymena pyriformis. Arch.
Protistenkd., 131, 143-152.
Schafer, W.E., Jr, Brunton, R.B., Lockyer, N.F & De Grazio, J.W.
(1973) Comparative toxicity of seventeen pesticides to the
quelea, house sparrow and red-winged blackbird. Toxicol. Appl.
Pharmacol., 26, 154-157.
Seabloom, R.W., Pearson, G.L., Oring, L.W. & Reilly, J.R. (1973) An
incident of fenthion mosquito control and subsequent arian
mortality. J. Wildl. Dis., 9, 18-20.
Seugé, J. & Bluzat, R. (1980) [Long-term toxicity of an
organophosphorus insecticide (fenthion) in the mollusc Lymnea
stagnalis.] Hydrobiology, 76, 241-248 (in French).
SLS Inc. (1988) The toxicity of technical grade fenthion (trade name
Baytex) to rainbow trout (Salmo gairdneri) embryos and larvae.
Unpublished report No. 95641 from Springborn Life Sciences Inc.,
Wareham, Massachusetts, USA. Submitted to WHO by Bayer AG.
TNO (1989) Reproduction test with Lebaycid EC 50 and Daphnia magna.
Unpublished report No. R 89/272 from TNO Division of Technology
for Society, Delft, Netherlands. Submitted to WHO by Bayer AG.
University of Southampton (1992) An evaluation of the side-effects of
Lebaycid 500 EC on larvae of the hoverfly Episyrphus balteatus
(Study No. BAY-91-1). Unpublished report from Agrochemical
Evaluation Unit, Department of Biology, University of
Southampton, Southampton, United Kingdom. Submitted to WHO by
Bayer AG.
US Department of Agriculture (1951) Soil Survey/Manual (Agriculture
Handbook No. 18). Washington DC, USA.
Van Meerendonk, J.H., Van Steenwijk, J.M., Phernambucq, A.J.W. &
Barreveld, H.L. (1994) [Tracking traces II. An inventory survey
for hazardous chemicals in the salt and freshwater systems in the
Netherlands] (RIKZ Report No. 94.007), The Hague, Netherlands,
National Institute for Coastal and Marine Management & National
Institute of Inland Water Management (in Dutch).
Wang, T.C., Lenaham, R.A., Tucker, J.W., Jr & Kadlac, T. (1987) Aerial
spray of mosquito adulticides in a salt marsh environment.
Int. Assoc. Water Qual., pp. 113-125.
WHO (1986) Organophosphorus Insecticides: A General Introduction
(Environmental Health Criteria 63). Geneva, International
Programme on Chemical Safety.
