
WHO Pesticide Residues Series, No. 1
1971 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
The evaluations contained in these monographs were prepared by the
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues that met
in Geneva from 22 to 29 November 1971.1
World Health Organization
Geneva
1972
1 Pesticide Residues in Food: Report of the 1971 Joint Meeting of
the FAO Working Party of Experts on Pesticide Residues and the WHO
Expert Committee on Pesticide Residues, Wld Hlth Org. techn. Rep.
Ser., No. 502; FAO Agricultural Studies, 1972, No. 88.
These monographs are also issued by the Food and Agriculture
Organization of the United Nations, Rome, as document AGP-1971/M/9/1.
FAO and WHO 1972
OMETHOATE
IDENTITY
Chemical names
dimethyl S-methylcarbamoylmethyl phosphorothiolate.
O,O-dimethyl S-(N-methylcarbamoylmethyl) phosphorothioate.
O,O-dimethyl S-(2-oxo-3-aza-butyl)-monothiophosphate.
Synonyms
Folimat (R), Bayer 45, 432, S 6876, P-O-dimethoate.
Empirical formula
C5H12NO4PS (213.1)
Structural formula
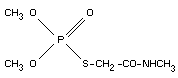 Physical and chemical properties
Colourless to slightly yellowish oil, b.p. ca. 135°C (decomposes when
distilled); v.p. 2.5 × 10-5 mm Hg at 20°C; volatility at 20°C, 0.29
mg/m3; d20 1.32; nD20 1.4987; readily soluble in water,
alcohol and acetone; slightly soluble in ethyl ether; almost insoluble
in petroleum ether. Stability to hydrolysis: half-life period, 611
hours at pH 7 and 24°C. The velocity of decomposition of the active
ingredient is essentially greater at higher temperatures or at pH
values 8-10.
Purity
Composition of a typical technical omethoate:
content of active ingredient 94.0 - 96.0%
O-methyl S-methylcarbamoylmethyl
phosphorothiolate 1.0 - 2.0%
dimethylphosphate 1.0 - 2.0%
1,2-dichloroethane max. 3.0%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biotransformation
Dauterman et al. (1959) treated rats orally with radioactive
omethoate. The urine from two male rats treated with a dose of 50
mg/kg was collected at 12, 24 and 48 hours. The cumulative percentages
of the administered radioactivity excreted over the indicated times
were 16, 19 and 30. Utilizing ion exchange chromatography the
metabolites found in a 24 hr/urine composite were:
O,O - dimethyl phosphoric acid 34%
Unknown A 52%
O,O - dimethyl phosphorothioic acid 9.5%
Unknown B 4.5%
Following treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine in 24 hours, while
following treatment with omethoate, only 19% was excreted in 24 hours
(Dauterman et al., 1959).
Apart from this preliminary study the biotransformation of omethoate
has not been examined although the fate of dimethoate, the
phosphorodithioate which is oxidized in vivo to omethoate, is well
documented (FAO/WHO, 1968). It is probable that the metabolic route of
omethoate will follow that observed for dimethoate in plants and
animals although the rate of the individual reactions may differ as
indicated in the study on male rats. In animals metabolism is
oxidative and hydrolytic and should yield compounds as follows:
Physical and chemical properties
Colourless to slightly yellowish oil, b.p. ca. 135°C (decomposes when
distilled); v.p. 2.5 × 10-5 mm Hg at 20°C; volatility at 20°C, 0.29
mg/m3; d20 1.32; nD20 1.4987; readily soluble in water,
alcohol and acetone; slightly soluble in ethyl ether; almost insoluble
in petroleum ether. Stability to hydrolysis: half-life period, 611
hours at pH 7 and 24°C. The velocity of decomposition of the active
ingredient is essentially greater at higher temperatures or at pH
values 8-10.
Purity
Composition of a typical technical omethoate:
content of active ingredient 94.0 - 96.0%
O-methyl S-methylcarbamoylmethyl
phosphorothiolate 1.0 - 2.0%
dimethylphosphate 1.0 - 2.0%
1,2-dichloroethane max. 3.0%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biotransformation
Dauterman et al. (1959) treated rats orally with radioactive
omethoate. The urine from two male rats treated with a dose of 50
mg/kg was collected at 12, 24 and 48 hours. The cumulative percentages
of the administered radioactivity excreted over the indicated times
were 16, 19 and 30. Utilizing ion exchange chromatography the
metabolites found in a 24 hr/urine composite were:
O,O - dimethyl phosphoric acid 34%
Unknown A 52%
O,O - dimethyl phosphorothioic acid 9.5%
Unknown B 4.5%
Following treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine in 24 hours, while
following treatment with omethoate, only 19% was excreted in 24 hours
(Dauterman et al., 1959).
Apart from this preliminary study the biotransformation of omethoate
has not been examined although the fate of dimethoate, the
phosphorodithioate which is oxidized in vivo to omethoate, is well
documented (FAO/WHO, 1968). It is probable that the metabolic route of
omethoate will follow that observed for dimethoate in plants and
animals although the rate of the individual reactions may differ as
indicated in the study on male rats. In animals metabolism is
oxidative and hydrolytic and should yield compounds as follows:
 (For reference to dimethoate metabolism in animals see: Hassan et al.
(1969), Lucier and Menzer (1970), FAO/WHO (1968), Brady and Arthur
(1963), and Dauterman et al. (1959); and in plants see: Morikawa and
Saito (1966) and Lucier and Menzer (1968; 1970).) Apparently all
metabolites of dimethoate observed in mammals are found in plants. In
plants a major reaction pathway includes demothoxylation which yields
a product that is not a major metabolite in mammals. (Morikawa and
Saito, 1966). However, in vitro studies with dimethoate using liver
and insect homogenates and in vivo studies with insects have shown
the presence of the dimethoxyl derivative. Dealkylation of omethoate
is a significant detoxication mechanism as evidence by fly head
cholinesterase bimolecular rate constant K1 values for omethoate of
9.2 × 10-5 L mole-1min-1 and 1.39 L mole-1min-1 for
demethoxylomethoate (Aharoni and O'Brien, 1968). Oxidative metabolism
of omethoate results in the de-N-methyl derivative which is as toxic
as the parent compound although less active as a cholinesterase
inhibitor (Lucier and Menzer, 1970). Hassan et al. (1969) whilst
investigating the metabolic fate of dimethoate in the rat concluded
that oxidation to omethoate occurred in vivo. They suggested two
major metabolic pathways for both dimethoate and omethoate. The first
involved cleavage of the C-N bond by a carboxy amidase, while the
second proceeded through esterase action on the S-C bond. Kinetic data
indicated that reaction between acetycholinesterase and omethoate was
irreversible and bimolecular. Omethoate was found to be 75-100 times
more potent than dimethoate in inhibiting rat brain
acetylcholinesterase.
Effects on enzymes and other biochemical parameters
Omethoate is a direct inhibitor of acetyl cholinesterase from various
sources. Sensitivity to inhibition is greater with invertebrate than
with mammalian sources. This is reflected in the omethoate bimolecular
rate constant (Ki) for rat brain of 1.65 × 10-3 L mole-1min-1
(Hassan et al., 1969) and house fly head of 9.2 × 10-5
L mole-1min-1 (Abaroni and O'Brien, 1968) as well as the 150 valve
(molar concentration of omethoate producing 50% inhibition of enzyme
activity) as seen in the following table:
Enzyme source I50 (M) Reference
Rat brain 1.2 × 10-5 Hassan et al., 1969
1.1 × 10-5 Sanderson and Edson, 1964
Bovine (RBC) 3.9 × 10-5 Santi and Pietri-Tonelli, 1969
Bovine liver 5.7 × 10-5 Villeneuve and McKinley, 1968
Human plasma 6.3 × 10-5 Lucier and Menzer, 1970
1.2 × 10-4 Sutherland, 1962
Housefly 1.7 × 10-7 Santi and Pietri-Tonelli, 1969
Following oral administrations of dimethoate to a cow at 10 mg/kg, red
blood cell cholinesterase was depressed in two stages; for six days
inhibition was constant at less than 20% and then slowly declined to
40% at 12 days (Dauterman et al., 1959), This biphasic inhibition was
also noted in sheep by Beck et al. (1968) who found that
cholinesterase activity continued to decrease for periods of time
after dimethoate treatment stopped. This is apparently due to slow
release of dimethoate and/or conversion rate of dimethoate to
omethoate.
Following acute oral administration to rabbits (20 mg/kg) various
enzymatic tests for liver function were not affected. These include:
serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic
transaminase (SGPT), serum sorbitol dehydrogenase (SDH) and
bromophthalein test (Kimmerle, 1962).
TOXICOLOGICAL STUDIES
Special studies
(a) Toxicity of the metabolites
Compound Species Route LD50 150 (M) pI50
(mg/kg) (human plasma)
Dimethoate Mouse ip 151 >0.1 <1
N-hydroxymethyl P(S) n.d. >0.1 <1
Des-N-methyl P(S) Mouse ip 190 >0.1 <1
Omethoate Mouse ip 13 6.5 × 10-5 4.2
N-hydroxymethyl P(O) n.d. 2.0 × 10-4 3.7
Des-N-methyl P(O) Mouse ip 10 4.0 × 10-4 3.4
n.d. = not determined.
Data from Lucier and Menzer (1970).
Deamination of omethoate results in a significantly less toxic
compound than omethoate. Carboxyomethoate ((CH3O)2 P(O)
(SCH2C(O)OH)), the deamination product of omethoate, was fed to rats
for 33 days at levels of 0, 330, 1000 and 3000 ppm in the diet. Plasma
cholinesterase activity was not affected. Red blood cell
cholinesterase was depressed at 1000 and 3000 ppm in both sexes and to
a slight degree in female rats at 330 ppm. Brain cholinesterase was
slightly, but significantly decreased in female rats at 3000 ppm,
Weight gain and food intake was depressed at 3000 ppm. No effects were
noted on survival, appearance, organ weights or histological
examination of organs and tissues (Levinskas and Shaffer, 1965a).
(b) Potentiation
Omethoate was observed to influence the acute oral toxicity of
malathion in rats. When administered at 1/2 LD50 levels the
mortality observed was slightly greater than theoretically anticipated
(Kimmerle and Lorke, 1967). Pretreatment of rats with phenobarbital
resulted in a threefold increase in the acute toxicity of omethoate
(Menzor and Best, 1968).
(c) Reproduction
Eighty male and 80 female rats were administered omethoate orally at a
daily dose of 0.5 mg/kg for six weeks and at the conclusion of
treatment they were mated. The pups were raised on normal diets for
four weeks after weaning and subsequently treated in a similar manner
for six weeks as the Fo generation. Daily oral treatment for six
weeks to the Fo generation had no effect on reproduction and the
same treatment did not grossly affect the F1 generation. Red blood
cell cholinesterase levels during the administration of omethoate
remained constant at 40-50% of normal while the plasma cholinesterase
was unaffected. This treatment did not affect growth of Fo or F1.
(Kimmerle, 1969).
(d) Neurotoxicity
Hens were dosed with 50-200 mg/kg of omethoate by intraperitoneal
injection under the protection of 2-PAM and atropine. Of 17 hens
treated, 14 were given 150 mg/kg and five died of acute intoxication.
The hens which survived were kept under observation for six weeks and
no signs of neurotoxic effect was observed (Kimmerle, 1962).
Hens (six hens/group) were fed omethoate at 0, 60, 120 and 240 ppm for
four weeks. At the end of the study three hens were sacrificed and
examined histologically (brain, thoracic spinal cord and sciatic
nerve) for myelin degeneration. The remainder were maintained on
normal diets for four weeks. No evidence was found, clinically or
histologically, for delayed neurotoxicity or demyelination (Levinskas
and Shaffer, 1965b).
(e) Antidotes
Administration of atropine alone and in combination with 2-PAM or BH-6
was effective in reducing the oral LD50 values for rats (Kimmerle,
1962; Kimmerle and Lorke, 1967). Administration of 2-PAM or BH-6 alone
was not effective although TMB-4 alone reduced the acute toxicity. The
optimum antidotal effects were noted with combinations of atropine and
oxime reactivators. At the first signs of poisoning with omethoate,
2-PAM or toxogonin and/or atropine were effective therapeutic agents
increasing the LD50 of omethoate from 50 to 200% (Lorke and
Kimmerle, 1969).
Acute toxicity
Animal Sex Route LD50 Reference
(mg/kg/bw)
Mouse M Oral 36 Kimmerle, 1968
27 Santi and Pietri-Tonelli, 1969
Ip 13 Lucier and Menzer, 1970
Iv 23 Kimmerle, 1962
Rat M and F Oral 28-65 Kimmerle and Lorke, 1967
Dauterman, 1959
Kimmerle, 1966; 1968
Kimmerle, 1962
50 Ben-Dyke, 1970
M Ip 14 Kimmerle, 1968
Ip 38 Kimmerle, 1962
Rabbit Oral 50 Kimmerle, 1962
Cat Oral 50 Kimmerle, 1962
Guinea-pig Oral 100 Kimmerle, 1962
Hen Oral 125 Kimmerle, 1962
100 Levinskas and Shaffer, 1965b
Signs of poisoning are typical of cholinergic stimulation as elicited
by other organophosphorus esters. The signs appear in from 5-60
minutes following poisoning and include salivation, lacrymation,
tremors, etc. The signs of poisoning may persist for one to three days
following intoxication (Kimmerle, 1968).
Rats (groups of three males per dose) were orally administered
omethoate at 2.5, 5 and 10 mg/kg. cholinesterase assay indicated
maximal depression at three hours (first interval examined after
exposure) with recovery in one to three days (Kimmerle, 1962),
Groups of rats (five male and five female/group) were orally
administered omethoate at a single dose of 0, 0.1, 0.5, 2.5, 5.0, 10.0
and 25 mg/kg and examined one, three, and seven days after treatment
for cholinesterase inhibition. Red blood cell cholinesterase was
depressed to a greater extent and duration than plasma. The response
of the RBC cholinesterase was the same in both sexes. With plasma,
females were more susceptible than males. Plasma was completely
recovered at all dose levels by day seven from a maximum of 67%
inhibition at day one at 25 mg/kg (female). Red blood cell
cholinesterase was still depressed at the conclusion of the test
(seven days) at doses of 10 mg/kg and above. Maximum inhibition
observed at 10 mg/kg on day one was 63%. The higher dose did not give
a greater reduction of activity (Kimmerle, 1969).
Short-term studies
Rat
Rats (20 males) were administered omethoate orally for five days/week
for eight weeks (42 doses) at 5 mg/kg/day. Tremors were transient
after each application. Cholinesterase was depressed 50-70% of normal
and quickly recovered after treatment ended (Kimmerle, 1962).
Rats (10 males per group) were orally administered omethoate at doses
of 0, 1, 2, 4, 8 and 16 mg/kg, five days/week for eight weeks. The
survivors were sacrificed four weeks after treatment ended. Mortality
occurred at 8 and 16 mg/kg. Cholinergic symptoms evident at 4 mg/kg,
decreased as time progressed. Slight trembling was observed at the
lowest doses. Organ weights, growth and gross and microscopic
examination of tissues were not affected (Kimmerle, 1962).
Groups of rats (10 male and 10 female per group) were administered
omethoate orally for 14 days at doses of 0.1 and 0.5 mg/kg/ day.
Plasma cholinesterase was not depressed during the 14-day interval.
Red blood cell cholinesterase was slightly depressed in males and
females at 0.5 mg/kg and this was maintained for the 14-day period. No
differences in sex were observed with regard to red blood cell enzyme
inhibition (Kimmerle, 1969).
Rats (10 male and 10 female per group) were fed omethoate (which was
mixed with the feed every day) for four weeks at 0, 2.5 and 15 ppm.
Growth was temporarily depressed at 15 ppm and cholinesterase was
depressed at both feeding levels. Red blood cell cholinesterase
activity was inhibited at 2.5 ppm and plasma at 15 ppm with the female
rats showing the higher sensitivity of plasma cholinesterase (Loser,
1968b).
Rats (15 males and 15 females per group, 30 of each sex were controls)
were fed omethoate at 0, 2.5, 5, 15, 50 and 150 ppm for four months.
Cholinergic stimulation was evident at 15 ppm and above.
Cholinesterase was depressed at 50 ppm and 150 ppm in females and 5
ppm and above in males. No effects were noted on growth, organ
weights, blood parameters or urinalysis at the feeding levels
including 50 ppm. At 150 ppm some animals died, the bodyweight and
food consumption was depressed and the relative liver weight in males
was increased (Loser and Lorke, 1967).
Groups of rats (80 male and 80 female per group) were administered
omethoate daily at an oral dose of 0.5 mg/kg for six weeks. RBC
cholinesterase was inhibited similarly in males and females at 40-50%
of normal. When placed on a normal diet for two weeks the enzyme
recovered to normal values. No significant adverse effects were
observed on growth or plasma cholinesterase activity (Kimmerle, 1969).
Rats (15 male and 15 female per group) were fed omethoate (mixed with
feed daily) at levels of 0, 0.5, 1.0, 2.0 and 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident at 4
ppm. Cholinesterase (red blood cell) was depressed at 2 ppm though in
females only slightly. At 4 ppm the inhibition was 30-50%. No effects
were noted on growth, food consumption, blood parameters, liver and
kidney function tests, organ weights (Loser, 1968a) and histological
examination of tissues (Vince and Spicer, 1971).
Dog
Dogs (four male and four female per group) were fed omethoate for 14
weeks at dietary concentrations of 0.4 ppm, 0.8 ppm and 1.6 ppm. No
adverse effects were observed on red blood cell and plasma
cholinesterase activity or other parameters measured including:
growth, survival, food consumption, blood analyses (haematocrit,
haemoglobin and total and differential leukocyte counts) and clinical
chemistry (alkaline phosphatase, glucose, urea nitrogen and
bromsulphthalein (BSP) retention) (Hutchison et al,, 1968).
Long-term studies
No data available.
Comments
Omethoate is an organophosphorus insecticide with a high acute
toxicity. Its toxicity is considerably higher than is phosphorothioate
analogue dimethoate, which was evaluated in 1967 and for which an
acceptable daily intake has been established. Although dimethoate is
converted to omethoate, quantitative data on the rate of conversion
following administration have not been reported.
Adequate short-term studies are available in both rats and dogs. The
lack of an acceptable reproduction study is partly offset by the
existence of an acceptable study of this kind in the case of
dimethoate. No long-term studies have been reported and there are no
observations in man comparable with those for dimethoate.
In view of the information available from dimethoate the Meeting
agreed that a temporary ADI for omethoate could be established based
on the 90-day study on omethoate in the rat. It was stressed however,
that long-term studies are required.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1.0 ppm in the dry diet equivalent to 0.05 mg/kg bodyweight per
day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg body-weight
per day
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
It is a systemic, organophosphorus insecticide and acaricide with a
broad sphere of action and good plant compatibility. It acts against
both sucking and biting insect pests and is active against spider
mites which are resistant to some other organophosphorus pesticides;
in this respect it differs from dimethoate, of which it is the oxygen
analogue. Its chief uses are for pre-harvest treatments of:
fruit (pome, stone and citrus) 65%
field crops (pasture, cotton, sugar cane, etc.) 25%
vegetables 5%
miscellaneous (e.g. ornamentals) 5%
It is available as 50% and 80% w/v emulsifiable concentrates and is
applied at about 0.025 to 0.075% w/v of active ingredient, equivalent
to up to 1500 g/ha a.i.
Residues resulting from supervised trials
The behaviour of omethoate on and in plants is characterized by a rate
of degradation which is relatively slow for an organophosphorus
pesticide. It seems that the surface condition of the crop, perhaps in
association with weather factors, can have a bearing on this matter.
Information on available residue data is given in Table 1 (Bayer,
1971). The application rates are expressed in percentage concentration
of active ingredient; the normal volume applied being about 2000 l/ha
for fruit crops and between 600-1000 l/ha for vegetable crops. Data on
the behaviour of residues in storage and processing and on the level
of residues in food moving in commerce are not available. Residues of
omethoate were not observed during a total diet study for
organophosphorus pesticide residues (Abbot et al, 1970).
TABLE I. OMETHOATE RESIDUES IN CROPS FROM SUPERVISED TRIALS
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Apples 0.03 1 0 0.66-1.0 0.8
1 0.16-0.4 0.3
2 0.04-0.2 0.1
3 0.01-0.14 0.07
Apples 0.05 3 0 1.55-2.07 1.75
1 0.32-0.6 0.45
2 0.11-0.17 0.15
3 - 0.10
4 0.07-0.09 0.08
5 - <0.05
Apples 0.064 1 0 - 3.3
1 - 1.9
2 - 1.6
3 - 1.2
4 - 0.4
Apples 0.075 2 0 2.3-5.2 3.75
2 1.1-2.4 1.62
3 0.75-1.3 1.02
4 0.55-1.4 0.95
5 0.5-1.1 0.70
6 0.25-0.85 0.51
Pears 0.064 1 0 - 2.15
1 - 2.0
2 - 1.45
3 - 1.05
4 - 0.9
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Pears 0.075 2 0 4.05-6.4 5.5
2 1.7-3.05 2.2
3 0.5-2.25 1.1
4 0.35-1.3 0.9
5 0.17-1.1 0.55
6 0.16-1.1 0.4
Pears 0.075 2-3 0 2.4-6.7 4.05
1 2.2-3.8 3.0
2 1.4-1.5 1.45
3 1.3-1.6 1.5
4 0.8-1.3 1.05
Apricots 0.075 1 0 - 3.1
1 - 1.8
2 - 1.8
3 - 0.4
Cherries 0.05 1 0 - 1.1
2 - 0.2
Grapes 0.05 1 0 - 5.7
1 - 3.9
2 - 2.35
3 - 1.8
4 - 1.25
Peaches 0.075 1 0 3.3-7.3 5.3
1 2.6-6.2 4.4
2 1.8-3.8 2.8
3 1.4-1.7 1.55
Plums 0.075 1 0 0.7-0.85 0.8
1 0.7-1.05 0.85
2 0.6-0.9 0.75
3 0.6-0.75 0.68
4 - 0.5
Lettuce* 0.03 1 0 3.3-3.7 3.5
1 0.3-0.9 0.6
2 0.11-0.17 0.15
3 - 0.03
Potatoes* 0.03 2 2 n.d.-0.21 0.11
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Sugar beets* 0.03 1 2 - 1.4
3 - 0.1
7 - 0.1
Sugar beets* 0.03 1 0 - 2.7
leaves 2 - 0.3
3 - 0.23
7 - 0.02
Hops (dried) 0.05 1 0 - 35
2 - 6.8
3 0.5-6.5 3.5
0.05 5-6 2“ 3.8-11.7 7.8
6 - 0.07
0.1 1 2 - 9.4
Note: slight underdosages used on these crops.
Fate of residues
In animals
Beck et al. (1968) fed 0.50 mg/kg dimethoate and 0.05 mg/kg of
omethoate for 14 days to cattle but found no residues in the milk.
When double these amounts were fed for an additional 14 days, residues
of omethoate ranging from 0.004 to 0.125 ppm were detected but
dimethoate was still absent; three days after ceasing the treatment no
omethoate was detected in the milk. See also "Biochemical aspects".
In plants
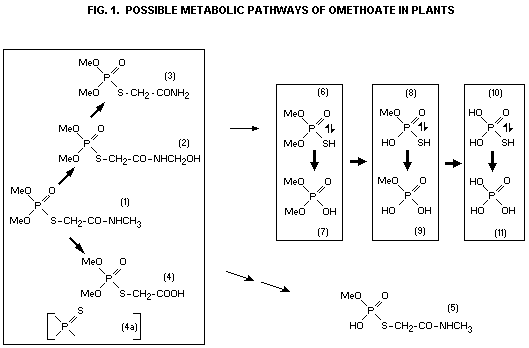
Dauterman et al. (1960) studied the metabolism of 32P-dimethoate
(surface and absorbed residues) after foliar treatment of corn,
cotton, pea, and potato plants. The amount of dimethoate, omethoate
and water-soluble metabolites on the surface of the plants was
investigated two and 12 days after treatment. With the longer time
interval there was a decrease in the total amount of omethoate on the
surface but an increase in the proportionate amount with respect to
dimethoate. The compound (4) (see Fig. 1) accounted for the largest
proportion of the water-soluble metabolites (up to 94%). Inside the
leaf, there was also a decrease with time in the total amount of
omethoate but a proportionate increase with respect to dimethoate.
Here, compound (4) accounted for no more than 10% of the water-soluble
metabolites.
Hydrolisis at the alkoxy group occurred mainly inside the leaves,
indicating an enzymatic action at this site, which also held for
omethoate. As none of the thio-carboxy derivative (4a) was found in or
on plants, formation of (4) probably proceeded by oxidation of
dimethoate to omethoate followed by hydrolysis of the carbamoyl group.
As (4) was found in the largest amount on the surface, this cleavage
was probably non-enzymatic. This is an important indication of the
mode of transformation of omethoate on the plant. However, these
considerations are not supported by the observations of Hacskaylo and
Bull (1963) who, using the excised-leaf technique, found large amounts
of (4a) as a metabolite whilst the presence of even slight amounts of
(4) could not be established with any certainty.
Lucier and Menzer (1968, 1970) studied the metabolism of dimethoate on
bean plants. The most important neutral metabolites which they found
were compound (3) and an unidentified compound (X) although only in
small amounts as compared with omethoate, as is shown below
(proportions expressed in relation to omethoate = 1X%).
Days after treatment
1 3 6
(2) 1.0% 0.4% 0.4%
(3) 7.7% 7.2% 5.7%
(X) 29.0% 5.0% 2.2%
The ratio of phosphorodithioates to phosphorothioates in the
N-demethylation scheme was approximately 1:1, possibly indicating a
reduced rate of hydrolytic metabolism for the desulfurated compounds.
However, the small proportions of the compounds (X), (2), and (3), in
relation to omethoate, suggests that these compounds had merely a
transitory character. It is interesting to note that compound (4) was
not found in these studies.
The simple phosphates and thiophosphates (6) to (11) have also been
identified as metabolites in plants, or presumed to be present, after
application of dimethoate:
(6), (7), (11); Dautermann et al. (1960); Hacskaylo and Bull (1963)
(6); Rowlands (1966); Lucier and Menzer (1968)
(8), not significant; Hacskaylo and Bull (1963)
(8), (9), (10), (11) not significant; Looter and Menzer (1968)
In vitro hydrolysis of omethoate by grain extracts (Rowlands, 1966)
provided clear evidence of the formation of (4) in addition to (6);
compound (5) was also found.
(For reference to dimethoate metabolism in animals see: Hassan et al.
(1969), Lucier and Menzer (1970), FAO/WHO (1968), Brady and Arthur
(1963), and Dauterman et al. (1959); and in plants see: Morikawa and
Saito (1966) and Lucier and Menzer (1968; 1970).) Apparently all
metabolites of dimethoate observed in mammals are found in plants. In
plants a major reaction pathway includes demothoxylation which yields
a product that is not a major metabolite in mammals. (Morikawa and
Saito, 1966). However, in vitro studies with dimethoate using liver
and insect homogenates and in vivo studies with insects have shown
the presence of the dimethoxyl derivative. Dealkylation of omethoate
is a significant detoxication mechanism as evidence by fly head
cholinesterase bimolecular rate constant K1 values for omethoate of
9.2 × 10-5 L mole-1min-1 and 1.39 L mole-1min-1 for
demethoxylomethoate (Aharoni and O'Brien, 1968). Oxidative metabolism
of omethoate results in the de-N-methyl derivative which is as toxic
as the parent compound although less active as a cholinesterase
inhibitor (Lucier and Menzer, 1970). Hassan et al. (1969) whilst
investigating the metabolic fate of dimethoate in the rat concluded
that oxidation to omethoate occurred in vivo. They suggested two
major metabolic pathways for both dimethoate and omethoate. The first
involved cleavage of the C-N bond by a carboxy amidase, while the
second proceeded through esterase action on the S-C bond. Kinetic data
indicated that reaction between acetycholinesterase and omethoate was
irreversible and bimolecular. Omethoate was found to be 75-100 times
more potent than dimethoate in inhibiting rat brain
acetylcholinesterase.
Effects on enzymes and other biochemical parameters
Omethoate is a direct inhibitor of acetyl cholinesterase from various
sources. Sensitivity to inhibition is greater with invertebrate than
with mammalian sources. This is reflected in the omethoate bimolecular
rate constant (Ki) for rat brain of 1.65 × 10-3 L mole-1min-1
(Hassan et al., 1969) and house fly head of 9.2 × 10-5
L mole-1min-1 (Abaroni and O'Brien, 1968) as well as the 150 valve
(molar concentration of omethoate producing 50% inhibition of enzyme
activity) as seen in the following table:
Enzyme source I50 (M) Reference
Rat brain 1.2 × 10-5 Hassan et al., 1969
1.1 × 10-5 Sanderson and Edson, 1964
Bovine (RBC) 3.9 × 10-5 Santi and Pietri-Tonelli, 1969
Bovine liver 5.7 × 10-5 Villeneuve and McKinley, 1968
Human plasma 6.3 × 10-5 Lucier and Menzer, 1970
1.2 × 10-4 Sutherland, 1962
Housefly 1.7 × 10-7 Santi and Pietri-Tonelli, 1969
Following oral administrations of dimethoate to a cow at 10 mg/kg, red
blood cell cholinesterase was depressed in two stages; for six days
inhibition was constant at less than 20% and then slowly declined to
40% at 12 days (Dauterman et al., 1959), This biphasic inhibition was
also noted in sheep by Beck et al. (1968) who found that
cholinesterase activity continued to decrease for periods of time
after dimethoate treatment stopped. This is apparently due to slow
release of dimethoate and/or conversion rate of dimethoate to
omethoate.
Following acute oral administration to rabbits (20 mg/kg) various
enzymatic tests for liver function were not affected. These include:
serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic
transaminase (SGPT), serum sorbitol dehydrogenase (SDH) and
bromophthalein test (Kimmerle, 1962).
TOXICOLOGICAL STUDIES
Special studies
(a) Toxicity of the metabolites
Compound Species Route LD50 150 (M) pI50
(mg/kg) (human plasma)
Dimethoate Mouse ip 151 >0.1 <1
N-hydroxymethyl P(S) n.d. >0.1 <1
Des-N-methyl P(S) Mouse ip 190 >0.1 <1
Omethoate Mouse ip 13 6.5 × 10-5 4.2
N-hydroxymethyl P(O) n.d. 2.0 × 10-4 3.7
Des-N-methyl P(O) Mouse ip 10 4.0 × 10-4 3.4
n.d. = not determined.
Data from Lucier and Menzer (1970).
Deamination of omethoate results in a significantly less toxic
compound than omethoate. Carboxyomethoate ((CH3O)2 P(O)
(SCH2C(O)OH)), the deamination product of omethoate, was fed to rats
for 33 days at levels of 0, 330, 1000 and 3000 ppm in the diet. Plasma
cholinesterase activity was not affected. Red blood cell
cholinesterase was depressed at 1000 and 3000 ppm in both sexes and to
a slight degree in female rats at 330 ppm. Brain cholinesterase was
slightly, but significantly decreased in female rats at 3000 ppm,
Weight gain and food intake was depressed at 3000 ppm. No effects were
noted on survival, appearance, organ weights or histological
examination of organs and tissues (Levinskas and Shaffer, 1965a).
(b) Potentiation
Omethoate was observed to influence the acute oral toxicity of
malathion in rats. When administered at 1/2 LD50 levels the
mortality observed was slightly greater than theoretically anticipated
(Kimmerle and Lorke, 1967). Pretreatment of rats with phenobarbital
resulted in a threefold increase in the acute toxicity of omethoate
(Menzor and Best, 1968).
(c) Reproduction
Eighty male and 80 female rats were administered omethoate orally at a
daily dose of 0.5 mg/kg for six weeks and at the conclusion of
treatment they were mated. The pups were raised on normal diets for
four weeks after weaning and subsequently treated in a similar manner
for six weeks as the Fo generation. Daily oral treatment for six
weeks to the Fo generation had no effect on reproduction and the
same treatment did not grossly affect the F1 generation. Red blood
cell cholinesterase levels during the administration of omethoate
remained constant at 40-50% of normal while the plasma cholinesterase
was unaffected. This treatment did not affect growth of Fo or F1.
(Kimmerle, 1969).
(d) Neurotoxicity
Hens were dosed with 50-200 mg/kg of omethoate by intraperitoneal
injection under the protection of 2-PAM and atropine. Of 17 hens
treated, 14 were given 150 mg/kg and five died of acute intoxication.
The hens which survived were kept under observation for six weeks and
no signs of neurotoxic effect was observed (Kimmerle, 1962).
Hens (six hens/group) were fed omethoate at 0, 60, 120 and 240 ppm for
four weeks. At the end of the study three hens were sacrificed and
examined histologically (brain, thoracic spinal cord and sciatic
nerve) for myelin degeneration. The remainder were maintained on
normal diets for four weeks. No evidence was found, clinically or
histologically, for delayed neurotoxicity or demyelination (Levinskas
and Shaffer, 1965b).
(e) Antidotes
Administration of atropine alone and in combination with 2-PAM or BH-6
was effective in reducing the oral LD50 values for rats (Kimmerle,
1962; Kimmerle and Lorke, 1967). Administration of 2-PAM or BH-6 alone
was not effective although TMB-4 alone reduced the acute toxicity. The
optimum antidotal effects were noted with combinations of atropine and
oxime reactivators. At the first signs of poisoning with omethoate,
2-PAM or toxogonin and/or atropine were effective therapeutic agents
increasing the LD50 of omethoate from 50 to 200% (Lorke and
Kimmerle, 1969).
Acute toxicity
Animal Sex Route LD50 Reference
(mg/kg/bw)
Mouse M Oral 36 Kimmerle, 1968
27 Santi and Pietri-Tonelli, 1969
Ip 13 Lucier and Menzer, 1970
Iv 23 Kimmerle, 1962
Rat M and F Oral 28-65 Kimmerle and Lorke, 1967
Dauterman, 1959
Kimmerle, 1966; 1968
Kimmerle, 1962
50 Ben-Dyke, 1970
M Ip 14 Kimmerle, 1968
Ip 38 Kimmerle, 1962
Rabbit Oral 50 Kimmerle, 1962
Cat Oral 50 Kimmerle, 1962
Guinea-pig Oral 100 Kimmerle, 1962
Hen Oral 125 Kimmerle, 1962
100 Levinskas and Shaffer, 1965b
Signs of poisoning are typical of cholinergic stimulation as elicited
by other organophosphorus esters. The signs appear in from 5-60
minutes following poisoning and include salivation, lacrymation,
tremors, etc. The signs of poisoning may persist for one to three days
following intoxication (Kimmerle, 1968).
Rats (groups of three males per dose) were orally administered
omethoate at 2.5, 5 and 10 mg/kg. cholinesterase assay indicated
maximal depression at three hours (first interval examined after
exposure) with recovery in one to three days (Kimmerle, 1962),
Groups of rats (five male and five female/group) were orally
administered omethoate at a single dose of 0, 0.1, 0.5, 2.5, 5.0, 10.0
and 25 mg/kg and examined one, three, and seven days after treatment
for cholinesterase inhibition. Red blood cell cholinesterase was
depressed to a greater extent and duration than plasma. The response
of the RBC cholinesterase was the same in both sexes. With plasma,
females were more susceptible than males. Plasma was completely
recovered at all dose levels by day seven from a maximum of 67%
inhibition at day one at 25 mg/kg (female). Red blood cell
cholinesterase was still depressed at the conclusion of the test
(seven days) at doses of 10 mg/kg and above. Maximum inhibition
observed at 10 mg/kg on day one was 63%. The higher dose did not give
a greater reduction of activity (Kimmerle, 1969).
Short-term studies
Rat
Rats (20 males) were administered omethoate orally for five days/week
for eight weeks (42 doses) at 5 mg/kg/day. Tremors were transient
after each application. Cholinesterase was depressed 50-70% of normal
and quickly recovered after treatment ended (Kimmerle, 1962).
Rats (10 males per group) were orally administered omethoate at doses
of 0, 1, 2, 4, 8 and 16 mg/kg, five days/week for eight weeks. The
survivors were sacrificed four weeks after treatment ended. Mortality
occurred at 8 and 16 mg/kg. Cholinergic symptoms evident at 4 mg/kg,
decreased as time progressed. Slight trembling was observed at the
lowest doses. Organ weights, growth and gross and microscopic
examination of tissues were not affected (Kimmerle, 1962).
Groups of rats (10 male and 10 female per group) were administered
omethoate orally for 14 days at doses of 0.1 and 0.5 mg/kg/ day.
Plasma cholinesterase was not depressed during the 14-day interval.
Red blood cell cholinesterase was slightly depressed in males and
females at 0.5 mg/kg and this was maintained for the 14-day period. No
differences in sex were observed with regard to red blood cell enzyme
inhibition (Kimmerle, 1969).
Rats (10 male and 10 female per group) were fed omethoate (which was
mixed with the feed every day) for four weeks at 0, 2.5 and 15 ppm.
Growth was temporarily depressed at 15 ppm and cholinesterase was
depressed at both feeding levels. Red blood cell cholinesterase
activity was inhibited at 2.5 ppm and plasma at 15 ppm with the female
rats showing the higher sensitivity of plasma cholinesterase (Loser,
1968b).
Rats (15 males and 15 females per group, 30 of each sex were controls)
were fed omethoate at 0, 2.5, 5, 15, 50 and 150 ppm for four months.
Cholinergic stimulation was evident at 15 ppm and above.
Cholinesterase was depressed at 50 ppm and 150 ppm in females and 5
ppm and above in males. No effects were noted on growth, organ
weights, blood parameters or urinalysis at the feeding levels
including 50 ppm. At 150 ppm some animals died, the bodyweight and
food consumption was depressed and the relative liver weight in males
was increased (Loser and Lorke, 1967).
Groups of rats (80 male and 80 female per group) were administered
omethoate daily at an oral dose of 0.5 mg/kg for six weeks. RBC
cholinesterase was inhibited similarly in males and females at 40-50%
of normal. When placed on a normal diet for two weeks the enzyme
recovered to normal values. No significant adverse effects were
observed on growth or plasma cholinesterase activity (Kimmerle, 1969).
Rats (15 male and 15 female per group) were fed omethoate (mixed with
feed daily) at levels of 0, 0.5, 1.0, 2.0 and 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident at 4
ppm. Cholinesterase (red blood cell) was depressed at 2 ppm though in
females only slightly. At 4 ppm the inhibition was 30-50%. No effects
were noted on growth, food consumption, blood parameters, liver and
kidney function tests, organ weights (Loser, 1968a) and histological
examination of tissues (Vince and Spicer, 1971).
Dog
Dogs (four male and four female per group) were fed omethoate for 14
weeks at dietary concentrations of 0.4 ppm, 0.8 ppm and 1.6 ppm. No
adverse effects were observed on red blood cell and plasma
cholinesterase activity or other parameters measured including:
growth, survival, food consumption, blood analyses (haematocrit,
haemoglobin and total and differential leukocyte counts) and clinical
chemistry (alkaline phosphatase, glucose, urea nitrogen and
bromsulphthalein (BSP) retention) (Hutchison et al,, 1968).
Long-term studies
No data available.
Comments
Omethoate is an organophosphorus insecticide with a high acute
toxicity. Its toxicity is considerably higher than is phosphorothioate
analogue dimethoate, which was evaluated in 1967 and for which an
acceptable daily intake has been established. Although dimethoate is
converted to omethoate, quantitative data on the rate of conversion
following administration have not been reported.
Adequate short-term studies are available in both rats and dogs. The
lack of an acceptable reproduction study is partly offset by the
existence of an acceptable study of this kind in the case of
dimethoate. No long-term studies have been reported and there are no
observations in man comparable with those for dimethoate.
In view of the information available from dimethoate the Meeting
agreed that a temporary ADI for omethoate could be established based
on the 90-day study on omethoate in the rat. It was stressed however,
that long-term studies are required.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1.0 ppm in the dry diet equivalent to 0.05 mg/kg bodyweight per
day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg body-weight
per day
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
It is a systemic, organophosphorus insecticide and acaricide with a
broad sphere of action and good plant compatibility. It acts against
both sucking and biting insect pests and is active against spider
mites which are resistant to some other organophosphorus pesticides;
in this respect it differs from dimethoate, of which it is the oxygen
analogue. Its chief uses are for pre-harvest treatments of:
fruit (pome, stone and citrus) 65%
field crops (pasture, cotton, sugar cane, etc.) 25%
vegetables 5%
miscellaneous (e.g. ornamentals) 5%
It is available as 50% and 80% w/v emulsifiable concentrates and is
applied at about 0.025 to 0.075% w/v of active ingredient, equivalent
to up to 1500 g/ha a.i.
Residues resulting from supervised trials
The behaviour of omethoate on and in plants is characterized by a rate
of degradation which is relatively slow for an organophosphorus
pesticide. It seems that the surface condition of the crop, perhaps in
association with weather factors, can have a bearing on this matter.
Information on available residue data is given in Table 1 (Bayer,
1971). The application rates are expressed in percentage concentration
of active ingredient; the normal volume applied being about 2000 l/ha
for fruit crops and between 600-1000 l/ha for vegetable crops. Data on
the behaviour of residues in storage and processing and on the level
of residues in food moving in commerce are not available. Residues of
omethoate were not observed during a total diet study for
organophosphorus pesticide residues (Abbot et al, 1970).
TABLE I. OMETHOATE RESIDUES IN CROPS FROM SUPERVISED TRIALS
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Apples 0.03 1 0 0.66-1.0 0.8
1 0.16-0.4 0.3
2 0.04-0.2 0.1
3 0.01-0.14 0.07
Apples 0.05 3 0 1.55-2.07 1.75
1 0.32-0.6 0.45
2 0.11-0.17 0.15
3 - 0.10
4 0.07-0.09 0.08
5 - <0.05
Apples 0.064 1 0 - 3.3
1 - 1.9
2 - 1.6
3 - 1.2
4 - 0.4
Apples 0.075 2 0 2.3-5.2 3.75
2 1.1-2.4 1.62
3 0.75-1.3 1.02
4 0.55-1.4 0.95
5 0.5-1.1 0.70
6 0.25-0.85 0.51
Pears 0.064 1 0 - 2.15
1 - 2.0
2 - 1.45
3 - 1.05
4 - 0.9
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Pears 0.075 2 0 4.05-6.4 5.5
2 1.7-3.05 2.2
3 0.5-2.25 1.1
4 0.35-1.3 0.9
5 0.17-1.1 0.55
6 0.16-1.1 0.4
Pears 0.075 2-3 0 2.4-6.7 4.05
1 2.2-3.8 3.0
2 1.4-1.5 1.45
3 1.3-1.6 1.5
4 0.8-1.3 1.05
Apricots 0.075 1 0 - 3.1
1 - 1.8
2 - 1.8
3 - 0.4
Cherries 0.05 1 0 - 1.1
2 - 0.2
Grapes 0.05 1 0 - 5.7
1 - 3.9
2 - 2.35
3 - 1.8
4 - 1.25
Peaches 0.075 1 0 3.3-7.3 5.3
1 2.6-6.2 4.4
2 1.8-3.8 2.8
3 1.4-1.7 1.55
Plums 0.075 1 0 0.7-0.85 0.8
1 0.7-1.05 0.85
2 0.6-0.9 0.75
3 0.6-0.75 0.68
4 - 0.5
Lettuce* 0.03 1 0 3.3-3.7 3.5
1 0.3-0.9 0.6
2 0.11-0.17 0.15
3 - 0.03
Potatoes* 0.03 2 2 n.d.-0.21 0.11
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Sugar beets* 0.03 1 2 - 1.4
3 - 0.1
7 - 0.1
Sugar beets* 0.03 1 0 - 2.7
leaves 2 - 0.3
3 - 0.23
7 - 0.02
Hops (dried) 0.05 1 0 - 35
2 - 6.8
3 0.5-6.5 3.5
0.05 5-6 2“ 3.8-11.7 7.8
6 - 0.07
0.1 1 2 - 9.4
Note: slight underdosages used on these crops.
Fate of residues
In animals
Beck et al. (1968) fed 0.50 mg/kg dimethoate and 0.05 mg/kg of
omethoate for 14 days to cattle but found no residues in the milk.
When double these amounts were fed for an additional 14 days, residues
of omethoate ranging from 0.004 to 0.125 ppm were detected but
dimethoate was still absent; three days after ceasing the treatment no
omethoate was detected in the milk. See also "Biochemical aspects".
In plants
Dauterman et al. (1960) studied the metabolism of 32P-dimethoate
(surface and absorbed residues) after foliar treatment of corn,
cotton, pea, and potato plants. The amount of dimethoate, omethoate
and water-soluble metabolites on the surface of the plants was
investigated two and 12 days after treatment. With the longer time
interval there was a decrease in the total amount of omethoate on the
surface but an increase in the proportionate amount with respect to
dimethoate. The compound (4) (see Fig. 1) accounted for the largest
proportion of the water-soluble metabolites (up to 94%). Inside the
leaf, there was also a decrease with time in the total amount of
omethoate but a proportionate increase with respect to dimethoate.
Here, compound (4) accounted for no more than 10% of the water-soluble
metabolites.
Hydrolisis at the alkoxy group occurred mainly inside the leaves,
indicating an enzymatic action at this site, which also held for
omethoate. As none of the thio-carboxy derivative (4a) was found in or
on plants, formation of (4) probably proceeded by oxidation of
dimethoate to omethoate followed by hydrolysis of the carbamoyl group.
As (4) was found in the largest amount on the surface, this cleavage
was probably non-enzymatic. This is an important indication of the
mode of transformation of omethoate on the plant. However, these
considerations are not supported by the observations of Hacskaylo and
Bull (1963) who, using the excised-leaf technique, found large amounts
of (4a) as a metabolite whilst the presence of even slight amounts of
(4) could not be established with any certainty.
Lucier and Menzer (1968, 1970) studied the metabolism of dimethoate on
bean plants. The most important neutral metabolites which they found
were compound (3) and an unidentified compound (X) although only in
small amounts as compared with omethoate, as is shown below
(proportions expressed in relation to omethoate = 1X%).
Days after treatment
1 3 6
(2) 1.0% 0.4% 0.4%
(3) 7.7% 7.2% 5.7%
(X) 29.0% 5.0% 2.2%
The ratio of phosphorodithioates to phosphorothioates in the
N-demethylation scheme was approximately 1:1, possibly indicating a
reduced rate of hydrolytic metabolism for the desulfurated compounds.
However, the small proportions of the compounds (X), (2), and (3), in
relation to omethoate, suggests that these compounds had merely a
transitory character. It is interesting to note that compound (4) was
not found in these studies.
The simple phosphates and thiophosphates (6) to (11) have also been
identified as metabolites in plants, or presumed to be present, after
application of dimethoate:
(6), (7), (11); Dautermann et al. (1960); Hacskaylo and Bull (1963)
(6); Rowlands (1966); Lucier and Menzer (1968)
(8), not significant; Hacskaylo and Bull (1963)
(8), (9), (10), (11) not significant; Looter and Menzer (1968)
In vitro hydrolysis of omethoate by grain extracts (Rowlands, 1966)
provided clear evidence of the formation of (4) in addition to (6);
compound (5) was also found.
 The behaviour of omethoate in the plant is characterized by a
relatively high degree of persistence, which at first seems surprising
in view of the fact that excuse usually hydrolyze more readily than
thionates. In actual fact, omethoate hydrolyzes at pH 6 and 25°C about
four times faster than dimethoate (Table II). Omethoate was found to
have a biological half-life of 9.3 days as against 3.2 days for
dimethoate in tomato plants which were placed in a solution of
32P-labelled compound for 24 hours and then held in distilled water
for 14 days (Grimmer et al., 1968b). Confirmation of this relatively
slow breakdown of omethoate on and in plants has been obtained
following field application of the compound.
TABLE II. RATES OF HYDROLYSIS (HALF-LIFE IN DAYS) AT 25°C
(GRIMMER ET AL., 1968a)
pH Dimethoate Omethoate (4a)+ (4)+
2 176 62 2 0.02
4 160 74 14 0.33
6 122 32.5 124 10
8 38 2.8 20 2.25
9++ 5.8 0.3 - -
+ The acids (4a) and (4) are stabilized by formation of
salt in neutral and weakly alkaline media.
++ At 21°C; Santi and de Pietri-Tonelli (1960).
Methods of residue analysis
Most of the methods proposed for the determination of omethoate
residues have been developed in association with methods for
dimethoate, of which it is the oxygen-analogue; for reviews of these
see de Pietri-Tonelli et al. (1965); Smart (1966); Bazzi (1966); Joint
Dimethoate Residues Panel (1968); FAO/WHO (1968).
All methods for the extraction of omethoate from plant material must
make allowance for the extremely high solubility of the compound in
water. The distribution coefficients for omethoate between water and
some organic solvents are as follows (Grimmer et al., 1968a):
chloroform 1.15 <(0.7*)
n-butanol 1.55
ethyl acetate 0.5
benzene 0.25 <(0.02*)
* Santi and de Pietri-Tonelli (1960)
Ethyl acetate in a strongly mineral-acid solution is suitable for the
extraction of compound (4) (Grimmer at al., 1968a).
The total phosphorus method is suitable for samples from supervised
trials which have not been treated with other phosphoric esters. A
simple method was recommended by the Joint Dimethoate Residues Panel
(maceration with acetone; extraction with chloroform; Al2O3 column
(grade V), elution with chloroform/carbon tetrachloride (1 + 1); wet
ashing; colorimetric phosphorus determination). After an additional
clean-up step (acetonitrile/n-hexane partitioning), the residues can
be identified by two-dimensional thin-layer chromatography. This
method of analysis has since been modified (Frehse, 1969) in order to
obtain better recoveries. The aqueous extract from the plant material
is extracted four times with chloroform; the Al2O3 column is
eluted with 150 ml of a mixture of chloroform/methanol (1 + 1) which
contains 1% glacial acetic acid to stabilize the residues. In most
cases, plant material poor in chlorophyll can be analysed without
using the Al2O3 column.
Steller and Curry (1964) describe a total phosphorus method
incorporating thin-layer chromatographic isolation for the
determination of dimethoate and omethoate. The method includes an
extraction of hexane, which is done before shaking out with
chloroform; extracts with a very high content of chlorophyll must be
further cleaned-up with diatomaceous earth and charcoal. A
column-chromatographic clean-up need not be done before the thin-layer
chromatography. The compound-containing spots are scraped, eluted and
analysed for phosphorus. The recoveries are between 55 and 72% for
apples (0.4-0.8 ppm), 81-94% for green tomatoes (0.16-0.32 ppm), and
72-87% for alfalfa (0.16-0.4 ppm). Details of the thin-layer
chromatography of omethoate are by Ackermann (1966) and Mendoza et al.
(1969).
Sissons and Telling (1970) describe a total phosphorus procedure which
is similar to the above-mentioned method recommended by the panel. The
extraction is done without adding acetone from the acidified aqueous
phase. The authors point out that the reason for the recoveries for
omethoate being lower than for other organophosphorus compounds was
due to significant volatilization losses which occurred during
Kaderna-Danish evaporation of the extracts. The recoveries obtained by
the proposed method are between 70 and 80% for an addition of 0.1-0.2
ppm.
With the increasing use of gas chromatography, some methods had been
proposed for determination of dimethoate and omethoate residues but
they were not sufficiently developed at the time the Joint Dimethoate
Residues Panol (1968) concluded its deliberations; however, several
promising stationary phases were described.
Bache and Lisk (1966) used the emission detector for determining
omethoate residues in soil. The soil is blended with chloroform and
the filtered solution, concentrated to a small volume, is directly
injected into the gas chromatograph: 6 ft × 3/16 in i.d. glass column
packed with 5% FFAP on 80/100 mesh Gas Chrom Q, 130°C, retention time
9.8 min. Recovery at the 5 ppm level was 86%; the limit of detection
was reported to be about 0.02 ppm. Beck et al. (1968) developed a
method for corn silage and milk. Silage is extracted with ethyl
acetate, and filtered. The solvent is removed and the residue
partitioned twice between 10 ml volumes of iso-octane and 1:4 (v/v)
water/acetonitrile. The iso-octane layers are discarded, and 5 ml of
methylene chloride is added to the acetonitrile layer; the mixture is
shaken. The lower layer (methylene chloride acetonitrile) is cleaned
up with Norite SG-extra, filtered through gelite and the filter cake
worked with ethyl acetate. The filtrate is evaporated just to dryness
and then diluted with ethyl acetate to a suitable volume for gas
chromatography: 4 ft glass column, 1/4 in o.d.; 5% Carbowax 20 M on
80/90 mesh Chromosorb W, acid washed and silanized, 120°C, flame
photometric detector (526 mµ), retention time six minutes, limit of
determination, 0.01 ppm. For milk analysis, 100 g aliquots are heated
in a 60°C water bath for 20 minutes to facilitate protein
precipitation. The samples are homogenized at room temperature with
200 ml of acetonitrile and are then subjected to a similar clean-up as
for the silage extracts. The limit of determination is stated to be
0.001 ppm.
Ruzicka et al. (1967) were able to determine omethoate with the aid of
a thermionic detector on a glass column (150 cm × 5 mm o.d.) packed
with 3% Apiezon L + 0.2% Epikote 1001 on
dimethyl-dichlorosilane-treated Chromosorb G, 70/80 mesh, at 165°C.
Bache and Lisk (1968), using the micro-wave powered helium emission
detector and an EC detector, obtained for omethoate, at 160°C, a
retention time of 3.6 minutes on a 2 ft × 3/16 in i.d. column packed
with 10% OV-17 on 80/100 mesh Gas Chrom Q; a slight but perceptible
tailing was observed. Sissons and Telling (1970) used a 5 ft × 1/8 in
glass column packed with 3% OV-17 on AWDMCS Chromosorb G, 80/100 mesh,
and obtained a retention time of 2.5 minutes at 225°C.
After comparing the behaviour of over 60 organophosphorus compounds on
three GDC columns, using a KCI thermionic detector, Watts and Storherr
(1969) found the following conditions to be the most suitable for
omethoate:
6 ft × 4 mm i.d. glass column, 2% diethylene glycol succinate
(C6 stabilized) on 80/100 mesh Gas Chrom Q, 210°C (retention
time of 3.7 minutes).
Watts et al. (1969) recommended an extraction with ethyl acetate
followed by charcoal clean-up for many organophosphorus pesticide
residues in crop extracts, especially in kale. Omethoate was included
in the studies and a recovery of 98% was obtained at the 0.5 ppm level
by gas chromatography using the above-mentioned method of Watts and
Storherr (1969).
Abbott et al. (1970) included omethoate, too, in their method for the
determination of organophosphorus pesticide residues in total diet
samples. Extracts from six groups of foods and milk are cleaned up and
examined using four different types of column and thermionic detectors
with caesium bromide tips. The authors chiefly report on their results
and unfortunately do not state which of the columns used is the most
suitable for omethoate. The AOAC multi-residue method is not suitable
for determination of omethoate because the compound is not eluted from
the column with the elution solvents used in this method (Pardue,
1971).
A simple method (Frehse, 1970) for fruits (including citrus fruits)
and vegetables operates as follows: 200 g of the sample is macerated
twice (500, 250 ml) with acetone, the cleaned-up filtrates are freed
from acetone on the rotary evaporator, the aqueous remainder is
rapidly cooled (at first under running tap water, then for one hour in
a refrigerator), filtered and extracted four times with 200 ml
portions of chloroform. The combined chloroform extracts are
evaporated to dryness and made up to a volume of 10 ml with acetone.
The gas-chromatographic determination is done on a 100 × 1.5 mm i.d.
glass column packed with 1.6% DC-200 + 0.6% Reoplex on 60/80 mesh
Chromosorb G, 185°C, using a thermionic detector giving a retention
time of 2.2 minutes. The recoveries are between 80 and 90% at the
0.05 - 1.0 ppm level. With some cole crops an interference peak may
appear at the position of omethoate which must be removed by
additional clean-up steps (see e.g. Watts et al. (1969)).
Fechner at al. (1969) published a method for determining dimethoate
and omethoate in milk. The milk sample is freed from fat by
centrifuging and extracted with methylene chloride at 5°C. The extract
is filtered and evaporated almost to dryness (0.25 ml), Aliquots of
the concentrate are chromatographed on Kieselgel G plates with
benzene/acetone (2+1); the compound spots are visualized
enzymatically, and evaluated semiquantitatively by comparison with
standards. In this way, omethoate residues can be determined down to a
level of 0.002 - 0.004 ppm. Fischer (1969) has described a thin-layer
chromatographic procedure for the identification and quantitative
determination of omethoate in biological material. Omethoate can be
semiquantitatively determined by thin-layer chromatography in
cucumbers, cherries and apples (Anonymous, 1970). After extraction
with ethanol and clean-up, the concentrated extracts are
chromatographed on Kieselgel G (pre-development with
chloroform/acetone 3 + 1), then chromatographed twice with
toluene/n-heptane/acetone (1 + 2 + 4). The compound spots are
visualized with thymol (bright spots on brownish background); 2 µg of
omethoate can be determined.
For regulatory purposes, a gas chromatographic procedure using either
flame photometric or phosphorus-sensitive thermionic detection would
be the method of choice. Thin-layer chromatography can be used to
obtain additional evidence of identity of the residue found.
EXAMPLES OF NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Country Crop Tolerance Safety
in ppm interval
in days
Australia Apples, citrus fruits, peaches 2.0 21
(provisional)
Austria General - 35
Belgium General - 21
Apples, pears 0.5 -
Brazila Deciduous fruits 2.0 -
Tomatoes, peppers 1.0 -
Vegetables, potatoes, beets 2.0 -
Bulgaria Tobacco - 0
Pome and stone fruit - 0
France General - 21
Italy General - 20
Netherlands General, field-grown 0.1 21
New Zealand General 0.5 21
South Africa General 1.5
Apples, pears - 28
Vines - 28
Spain General - 28
United Apples, pears 2 28
States of Beans (green, lima, snap, dry) 2 -
Americaa Broccoli, cauliflower 2 7
Cabbage 2 3
Lemons, oranges 2 15-45
Collards, endive, kale, lettuce )
(leaf), mustard greens, )
spinach, Swiss chard, turnips ) 2 14
(greens and roots) )
Lettuce (head), tomatoes 2 7
Peas, peppers 2 -
Melons 1 3
Potatoes 0.2 -
Pecans 0.1 21
Milk 0.002 -
Meat, fat and meat by-products
of cattle 0.02 -
Wheat (grain) 0.04 60
Wheat (green fodder and straw) 2 14
(Alfalfa, cotton, tobacco) - 14-28
a "....dimethoate and its oxygen analogue".
Appraisal
Omethoate, which is the oxygen analogue of dimethoate, is a systemic,
organophosphorus insecticide and acaricide, active against both
sucking and biting pests. Its chief uses are for pre-harvest
treatments of tree fruits, especially apples and pears, field crops
and vegetables. Omethoate is more persistent than dimethoate in plant
tissue but it degrades similarly to give esters of phosphoric and
thiophosphoric acid; metabolism in animals is also similar to that of
dimethoate. Residues are therefore determined only in terms of the
parent omethoate. Information was available regarding residues
resulting from supervised trials but no residue data from foodstuffs
moving in commerce or total diet studies was presented. Gas
chromatographic procedures are available for the determination of
residues of omethoate which can be adapted for regulatory purposes as
required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
Temporary tolerances
The following temporary tolerances are recommended for omethoate
residues. The pre-harvest interval shown is an indication of the basis
on which the recommendation is made:
Tolerance Pre-harvest
Fruit (ppm) interval (days)
Apples, grapes, peaches, pears 2 21
Apricots, cherries, plums 2 7
Insufficient information was available to support recommendations for
tolerances for omethoate residues on lettuce, potatoes, sugar beet or
hops.
Further work or information
Required before 30 June 1975
A long-term feeding study in at least one species of animal.
Desirable
1. Relevant observations in man.
2. More information on the quantitative aspect of the metabolism of
omethoate as compared with that of dimethoate.
3. Reproduction study in a non-rodent species.
4. Studies on the fate of the compound during storage, processing
and preparation of food for consumption.
5. Further data on residues occurring in use on lettuce, potatoes,
sugar beet and hops.
6. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R., and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pesticide Sci.,
1: 10-13
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschichtchromatographischer Trennung. Nahrung,
10: 273-274
Aharoni, A. H., and O'Brien, R. D. (1968) The inhibition of
acetylcholinesterases by anionic organophosphorus compounds. Biochem.,
7: 1538-1544
Anonymous. (1970) Methods zur Bestimmung von Dimethoate- und
PO-Dimethoat-rückständen in pflanzlichem Material. Nahrung, 14:
689-693
Bache, C. A. and Lisk, D. J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
gas chromatography, J. Assoc. Offic. Anal. Chem., 49: 647-650
Bache, C. A. and Lisk, D. J. (1968) Note on the versatility of OV-17
substrate for gas chromatography of pesticides. J. Assoc. Offic. Anal.
Chem., 51: 1270-1271
Bayer. (1971) Omethoate residues data. Unpublished report from
Farbenfabriken Bayer A. G.
Bazzi, B. (1966) Problemes relatifs au metabolisme et la determination
dans les produits vegetaux d'un ester phosphorique amide: le
dimethoate, Mededel. Rijksfaculteit Landbouwwetenschappen Gent, 31:
489-505
Beck, E. W., Johnson, jr J. C., Getz, M. E., Skinner, F. B., Dawsey,
L. H., Woodham, D. W., and Derbyshire, J. C. (1968) Effects of feeding
dimethoate, its oxygen analogue and dimethoate-treated silage to
cattle. J. Econ. Entomol., 61: 605-610
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. (1970) Acute toxicity
data for pesticides. World Rev. Pest Control, 9: 119-271
Brady, U. E. jr, and Arthur, B. W. (1963) Biological and chemical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56: 477-482
Chamberlain, W. F., Gatterdam, P. E., and Hopkins, D. E. (1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54: 733-740
Dauterman, W. C., Casida, J. E., Knaak, J. B. and Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agr. Food Chem., 7: 188-193
Dautermann, W. C., Viado, G. B., Casida, J. E. and O'Brien, R. D.
(1960) Persistence of dimethoate and metabolites following foliar
application to plants. J. Agr. Food Chem., 8: 115-119
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. Rogor (1965)
(dimethoate) residues in food crops. Residue Rev., 11: 60-99
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30: pp. 117-132
Fechner, G., Ackermann, H. and Lexow, B. (1969)
Dünnschichtchromatographische Bestimmung von Dimethcat und
PO-Dimethoat in Milch. Z. Lebensm.-Untersuch. -Forsch., 140: 145-149
Fischer, R. (1969) Identification of organophosphate insecticides in
biological material. IV. Arch. Toxikol., 25: 216-222
Frehse, H. (1969) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Frehse, H. (1970) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Grimmer, F., Dedek, W. and Leibnitz, E. (1968a) Zur Kenntnis der
N-Butylhomologen des Dimethoats Hydrolysegeschwindigkeit
und-15-metabolismes. Z. Naturforsch., 23B: 10-17
Grimmer, F., Dedek, W. and Leibnitz, E. (1968b) Zur Kenntnis der
N-Butylhomologen des Dimethoats. Verhalten in Tomatenpflangen. Z.
Naturforsch., 23B: 834-838
Hacskaylo, J. and Bull, D. L. (1963) Metabolism of dimethoate in
cotton leaves. J. Agr. Food Chem., 11: 464-466
Hassan, A., Zayed, S. M. A. D. and Bahig, M. R. E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18: 2429-2438
Hutchinson, E. B., McNerney, J. M. and Levinskas, G. J. (1968) Report
on oxygen analogue of Cygon-dimethoate: 90-day repeated feeding to
dogs (CL-28580). Unpublished report submitted by American Cyanamid
Company.
Joint Dimethoate Residues Panel. (1968) The determination of
dimethoate residues in fruits and vegetables. Analyst, 93: 756-766
Kimmerle, G. (1962) Toxicological studies on active ingredient
Dr Schrader S6876. Unpublished report submitted by Farbenfabriken
Bayer A. G.
Kimmerle, G. (1966) S6876 P-O-dimethoate (Bayer 45432; LO-NR 113a-1),
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. Bayer (1968) 45432-Toxikologische untersuchhungen.
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. (1969) Omethoate and dimethoate. Vergleichende
toxikologische untersuchungen bei rattengenerationsversuch bei ratten
mit omethoate. Unpublished report submitted by Farbenfabriken Bayer
A. G.
Kimmerle, G. and Lorke, D. (1967) S6876 (Lo-No.274). Antidotal
effect and potentiation. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Levinskas, G. J. and Shaffer, C. B. (1965a) Oxy-carboxy dimethoate:
thirty-three day repeated feeding to rats. Unpublished report
submitted by American Cyanamid Company.
Levinskas, G. J. and Shaffer, C. B. (1965b) Oxygen analogue of
dimethoate: demyelination studies in white leghorn hens. Unpublished
report submitted by American Cyanamid Company.
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmacol., 263: 237-238
Loser, E. and Lorke, D. (1967) Bayer 45432 - Subchronische
toxikologische untersuchungen an ratten. Unpublished report submitted
by Farbenfabriken Bayer, A. G.
Loser, E. (1968b) Bayer 45432 - Subakute toxikologische untersuchungen
an ratten. Unpublished report submitted by Farbenfabriken Bayer A. G.
Lucier, G. W. and Menzer, R. E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem., 16: 936-945
Lucier, G. W. and Menzer, R. E. (1970) Nature of oxidative metabolites
of dimethoate formed in rats, liver microsomes, and bean plants. J.
Agr. Food Chem., 18: 698-704
Mendoza, C. E., Grant, D. L., Braceland, B. and McCully, K. A. (1969)
Evaluation of esterases from livers of beef, pig, sheep, monkey, and
chicken for detection of some pesticides by thin-layer
chromatographic-enzyme inhibition technique. Analyst, 94: 805-810
Menzer, R. E. and Best, N. H. (1968) Effect of phenobarbital on the
toxicity of several organophosphorus insecticides. Toxicol. appl.
Pharmacol., 13: 37-42
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects, and mammals. Botyu-Kagaku,
31: 130-136
Pardue, J. R. (1971) Recovery of organophosphorus compounds using the
AOAC multiresidue method. J. Assoc. Offic. Anal. Chem., 54: 359-360
Rowlands, D. G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Food Agr.,
17: 90-93
Ruzicka, J., Thomson, J. and Wheals, B. B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatog., 30: 92-99
Sanderson, D. M. and Edson, E. F. (1964) Toxicological properties of
the organophosphorus insecticide dimethoate. Brit. J. industr. Med.,
21: 52-64
Santi, R. and de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido
O,O-dimetilditiofosforilacetico. Contrib. 1959, Ist Ric. Agr. Soc.
Montecatini, 3: 3-29
Sissons, J. D. and Telling, G. M. (1970) A screening procedure for
water soluble insecticides. J. Chromatog., 48: 468-477
Smart, N. A. (1966) An assessment of some methods of analysis for
dimethoate residues in fruit and vegetables. Analyst, 91: 621-624
Steller, W. A. and Curry, A. N. (1964) Measurement of residues of
Cygon insecticide and its oxygen analogue by total phosphorus
determination after isolation by thin-layer chromatography. J. Assoc.
Offic. Anal. Chem., 47: 645-651
Sutherland, G. L. (1962) Cholinesterase inhibitory activity of
dimethoate. Unpublished report submitted by American Cyanamid Company.
Villeneuve, D. C. and McKinley, W. P. (1968) Inhibition of beef liver
hydrolytic enzymes by organophosphorus pesticides. J. Agr. Food Chem.,
16: 290-294
Vince, A. A. and Spicer, E. J. F. (1971) Pathology report of Bay
45432-Subchronic toxicity in rats. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Watts, R. R. and Storherr, R. W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Offic. Anal. Chem., 52; 513-521
Watts, R. R., Storherr, R. W., Pardue, J. R. and Osgood, T. (1969)
Charcoal column cleanup method for many organophosphorus pesticide
residues in crop extracts. J. Assoc. Offic. Anal. Chem., 52: 522-526
The behaviour of omethoate in the plant is characterized by a
relatively high degree of persistence, which at first seems surprising
in view of the fact that excuse usually hydrolyze more readily than
thionates. In actual fact, omethoate hydrolyzes at pH 6 and 25°C about
four times faster than dimethoate (Table II). Omethoate was found to
have a biological half-life of 9.3 days as against 3.2 days for
dimethoate in tomato plants which were placed in a solution of
32P-labelled compound for 24 hours and then held in distilled water
for 14 days (Grimmer et al., 1968b). Confirmation of this relatively
slow breakdown of omethoate on and in plants has been obtained
following field application of the compound.
TABLE II. RATES OF HYDROLYSIS (HALF-LIFE IN DAYS) AT 25°C
(GRIMMER ET AL., 1968a)
pH Dimethoate Omethoate (4a)+ (4)+
2 176 62 2 0.02
4 160 74 14 0.33
6 122 32.5 124 10
8 38 2.8 20 2.25
9++ 5.8 0.3 - -
+ The acids (4a) and (4) are stabilized by formation of
salt in neutral and weakly alkaline media.
++ At 21°C; Santi and de Pietri-Tonelli (1960).
Methods of residue analysis
Most of the methods proposed for the determination of omethoate
residues have been developed in association with methods for
dimethoate, of which it is the oxygen-analogue; for reviews of these
see de Pietri-Tonelli et al. (1965); Smart (1966); Bazzi (1966); Joint
Dimethoate Residues Panel (1968); FAO/WHO (1968).
All methods for the extraction of omethoate from plant material must
make allowance for the extremely high solubility of the compound in
water. The distribution coefficients for omethoate between water and
some organic solvents are as follows (Grimmer et al., 1968a):
chloroform 1.15 <(0.7*)
n-butanol 1.55
ethyl acetate 0.5
benzene 0.25 <(0.02*)
* Santi and de Pietri-Tonelli (1960)
Ethyl acetate in a strongly mineral-acid solution is suitable for the
extraction of compound (4) (Grimmer at al., 1968a).
The total phosphorus method is suitable for samples from supervised
trials which have not been treated with other phosphoric esters. A
simple method was recommended by the Joint Dimethoate Residues Panel
(maceration with acetone; extraction with chloroform; Al2O3 column
(grade V), elution with chloroform/carbon tetrachloride (1 + 1); wet
ashing; colorimetric phosphorus determination). After an additional
clean-up step (acetonitrile/n-hexane partitioning), the residues can
be identified by two-dimensional thin-layer chromatography. This
method of analysis has since been modified (Frehse, 1969) in order to
obtain better recoveries. The aqueous extract from the plant material
is extracted four times with chloroform; the Al2O3 column is
eluted with 150 ml of a mixture of chloroform/methanol (1 + 1) which
contains 1% glacial acetic acid to stabilize the residues. In most
cases, plant material poor in chlorophyll can be analysed without
using the Al2O3 column.
Steller and Curry (1964) describe a total phosphorus method
incorporating thin-layer chromatographic isolation for the
determination of dimethoate and omethoate. The method includes an
extraction of hexane, which is done before shaking out with
chloroform; extracts with a very high content of chlorophyll must be
further cleaned-up with diatomaceous earth and charcoal. A
column-chromatographic clean-up need not be done before the thin-layer
chromatography. The compound-containing spots are scraped, eluted and
analysed for phosphorus. The recoveries are between 55 and 72% for
apples (0.4-0.8 ppm), 81-94% for green tomatoes (0.16-0.32 ppm), and
72-87% for alfalfa (0.16-0.4 ppm). Details of the thin-layer
chromatography of omethoate are by Ackermann (1966) and Mendoza et al.
(1969).
Sissons and Telling (1970) describe a total phosphorus procedure which
is similar to the above-mentioned method recommended by the panel. The
extraction is done without adding acetone from the acidified aqueous
phase. The authors point out that the reason for the recoveries for
omethoate being lower than for other organophosphorus compounds was
due to significant volatilization losses which occurred during
Kaderna-Danish evaporation of the extracts. The recoveries obtained by
the proposed method are between 70 and 80% for an addition of 0.1-0.2
ppm.
With the increasing use of gas chromatography, some methods had been
proposed for determination of dimethoate and omethoate residues but
they were not sufficiently developed at the time the Joint Dimethoate
Residues Panol (1968) concluded its deliberations; however, several
promising stationary phases were described.
Bache and Lisk (1966) used the emission detector for determining
omethoate residues in soil. The soil is blended with chloroform and
the filtered solution, concentrated to a small volume, is directly
injected into the gas chromatograph: 6 ft × 3/16 in i.d. glass column
packed with 5% FFAP on 80/100 mesh Gas Chrom Q, 130°C, retention time
9.8 min. Recovery at the 5 ppm level was 86%; the limit of detection
was reported to be about 0.02 ppm. Beck et al. (1968) developed a
method for corn silage and milk. Silage is extracted with ethyl
acetate, and filtered. The solvent is removed and the residue
partitioned twice between 10 ml volumes of iso-octane and 1:4 (v/v)
water/acetonitrile. The iso-octane layers are discarded, and 5 ml of
methylene chloride is added to the acetonitrile layer; the mixture is
shaken. The lower layer (methylene chloride acetonitrile) is cleaned
up with Norite SG-extra, filtered through gelite and the filter cake
worked with ethyl acetate. The filtrate is evaporated just to dryness
and then diluted with ethyl acetate to a suitable volume for gas
chromatography: 4 ft glass column, 1/4 in o.d.; 5% Carbowax 20 M on
80/90 mesh Chromosorb W, acid washed and silanized, 120°C, flame
photometric detector (526 mµ), retention time six minutes, limit of
determination, 0.01 ppm. For milk analysis, 100 g aliquots are heated
in a 60°C water bath for 20 minutes to facilitate protein
precipitation. The samples are homogenized at room temperature with
200 ml of acetonitrile and are then subjected to a similar clean-up as
for the silage extracts. The limit of determination is stated to be
0.001 ppm.
Ruzicka et al. (1967) were able to determine omethoate with the aid of
a thermionic detector on a glass column (150 cm × 5 mm o.d.) packed
with 3% Apiezon L + 0.2% Epikote 1001 on
dimethyl-dichlorosilane-treated Chromosorb G, 70/80 mesh, at 165°C.
Bache and Lisk (1968), using the micro-wave powered helium emission
detector and an EC detector, obtained for omethoate, at 160°C, a
retention time of 3.6 minutes on a 2 ft × 3/16 in i.d. column packed
with 10% OV-17 on 80/100 mesh Gas Chrom Q; a slight but perceptible
tailing was observed. Sissons and Telling (1970) used a 5 ft × 1/8 in
glass column packed with 3% OV-17 on AWDMCS Chromosorb G, 80/100 mesh,
and obtained a retention time of 2.5 minutes at 225°C.
After comparing the behaviour of over 60 organophosphorus compounds on
three GDC columns, using a KCI thermionic detector, Watts and Storherr
(1969) found the following conditions to be the most suitable for
omethoate:
6 ft × 4 mm i.d. glass column, 2% diethylene glycol succinate
(C6 stabilized) on 80/100 mesh Gas Chrom Q, 210°C (retention
time of 3.7 minutes).
Watts et al. (1969) recommended an extraction with ethyl acetate
followed by charcoal clean-up for many organophosphorus pesticide
residues in crop extracts, especially in kale. Omethoate was included
in the studies and a recovery of 98% was obtained at the 0.5 ppm level
by gas chromatography using the above-mentioned method of Watts and
Storherr (1969).
Abbott et al. (1970) included omethoate, too, in their method for the
determination of organophosphorus pesticide residues in total diet
samples. Extracts from six groups of foods and milk are cleaned up and
examined using four different types of column and thermionic detectors
with caesium bromide tips. The authors chiefly report on their results
and unfortunately do not state which of the columns used is the most
suitable for omethoate. The AOAC multi-residue method is not suitable
for determination of omethoate because the compound is not eluted from
the column with the elution solvents used in this method (Pardue,
1971).
A simple method (Frehse, 1970) for fruits (including citrus fruits)
and vegetables operates as follows: 200 g of the sample is macerated
twice (500, 250 ml) with acetone, the cleaned-up filtrates are freed
from acetone on the rotary evaporator, the aqueous remainder is
rapidly cooled (at first under running tap water, then for one hour in
a refrigerator), filtered and extracted four times with 200 ml
portions of chloroform. The combined chloroform extracts are
evaporated to dryness and made up to a volume of 10 ml with acetone.
The gas-chromatographic determination is done on a 100 × 1.5 mm i.d.
glass column packed with 1.6% DC-200 + 0.6% Reoplex on 60/80 mesh
Chromosorb G, 185°C, using a thermionic detector giving a retention
time of 2.2 minutes. The recoveries are between 80 and 90% at the
0.05 - 1.0 ppm level. With some cole crops an interference peak may
appear at the position of omethoate which must be removed by
additional clean-up steps (see e.g. Watts et al. (1969)).
Fechner at al. (1969) published a method for determining dimethoate
and omethoate in milk. The milk sample is freed from fat by
centrifuging and extracted with methylene chloride at 5°C. The extract
is filtered and evaporated almost to dryness (0.25 ml), Aliquots of
the concentrate are chromatographed on Kieselgel G plates with
benzene/acetone (2+1); the compound spots are visualized
enzymatically, and evaluated semiquantitatively by comparison with
standards. In this way, omethoate residues can be determined down to a
level of 0.002 - 0.004 ppm. Fischer (1969) has described a thin-layer
chromatographic procedure for the identification and quantitative
determination of omethoate in biological material. Omethoate can be
semiquantitatively determined by thin-layer chromatography in
cucumbers, cherries and apples (Anonymous, 1970). After extraction
with ethanol and clean-up, the concentrated extracts are
chromatographed on Kieselgel G (pre-development with
chloroform/acetone 3 + 1), then chromatographed twice with
toluene/n-heptane/acetone (1 + 2 + 4). The compound spots are
visualized with thymol (bright spots on brownish background); 2 µg of
omethoate can be determined.
For regulatory purposes, a gas chromatographic procedure using either
flame photometric or phosphorus-sensitive thermionic detection would
be the method of choice. Thin-layer chromatography can be used to
obtain additional evidence of identity of the residue found.
EXAMPLES OF NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Country Crop Tolerance Safety
in ppm interval
in days
Australia Apples, citrus fruits, peaches 2.0 21
(provisional)
Austria General - 35
Belgium General - 21
Apples, pears 0.5 -
Brazila Deciduous fruits 2.0 -
Tomatoes, peppers 1.0 -
Vegetables, potatoes, beets 2.0 -
Bulgaria Tobacco - 0
Pome and stone fruit - 0
France General - 21
Italy General - 20
Netherlands General, field-grown 0.1 21
New Zealand General 0.5 21
South Africa General 1.5
Apples, pears - 28
Vines - 28
Spain General - 28
United Apples, pears 2 28
States of Beans (green, lima, snap, dry) 2 -
Americaa Broccoli, cauliflower 2 7
Cabbage 2 3
Lemons, oranges 2 15-45
Collards, endive, kale, lettuce )
(leaf), mustard greens, )
spinach, Swiss chard, turnips ) 2 14
(greens and roots) )
Lettuce (head), tomatoes 2 7
Peas, peppers 2 -
Melons 1 3
Potatoes 0.2 -
Pecans 0.1 21
Milk 0.002 -
Meat, fat and meat by-products
of cattle 0.02 -
Wheat (grain) 0.04 60
Wheat (green fodder and straw) 2 14
(Alfalfa, cotton, tobacco) - 14-28
a "....dimethoate and its oxygen analogue".
Appraisal
Omethoate, which is the oxygen analogue of dimethoate, is a systemic,
organophosphorus insecticide and acaricide, active against both
sucking and biting pests. Its chief uses are for pre-harvest
treatments of tree fruits, especially apples and pears, field crops
and vegetables. Omethoate is more persistent than dimethoate in plant
tissue but it degrades similarly to give esters of phosphoric and
thiophosphoric acid; metabolism in animals is also similar to that of
dimethoate. Residues are therefore determined only in terms of the
parent omethoate. Information was available regarding residues
resulting from supervised trials but no residue data from foodstuffs
moving in commerce or total diet studies was presented. Gas
chromatographic procedures are available for the determination of
residues of omethoate which can be adapted for regulatory purposes as
required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
Temporary tolerances
The following temporary tolerances are recommended for omethoate
residues. The pre-harvest interval shown is an indication of the basis
on which the recommendation is made:
Tolerance Pre-harvest
Fruit (ppm) interval (days)
Apples, grapes, peaches, pears 2 21
Apricots, cherries, plums 2 7
Insufficient information was available to support recommendations for
tolerances for omethoate residues on lettuce, potatoes, sugar beet or
hops.
Further work or information
Required before 30 June 1975
A long-term feeding study in at least one species of animal.
Desirable
1. Relevant observations in man.
2. More information on the quantitative aspect of the metabolism of
omethoate as compared with that of dimethoate.
3. Reproduction study in a non-rodent species.
4. Studies on the fate of the compound during storage, processing
and preparation of food for consumption.
5. Further data on residues occurring in use on lettuce, potatoes,
sugar beet and hops.
6. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R., and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pesticide Sci.,
1: 10-13
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschichtchromatographischer Trennung. Nahrung,
10: 273-274
Aharoni, A. H., and O'Brien, R. D. (1968) The inhibition of
acetylcholinesterases by anionic organophosphorus compounds. Biochem.,
7: 1538-1544
Anonymous. (1970) Methods zur Bestimmung von Dimethoate- und
PO-Dimethoat-rückständen in pflanzlichem Material. Nahrung, 14:
689-693
Bache, C. A. and Lisk, D. J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
gas chromatography, J. Assoc. Offic. Anal. Chem., 49: 647-650
Bache, C. A. and Lisk, D. J. (1968) Note on the versatility of OV-17
substrate for gas chromatography of pesticides. J. Assoc. Offic. Anal.
Chem., 51: 1270-1271
Bayer. (1971) Omethoate residues data. Unpublished report from
Farbenfabriken Bayer A. G.
Bazzi, B. (1966) Problemes relatifs au metabolisme et la determination
dans les produits vegetaux d'un ester phosphorique amide: le
dimethoate, Mededel. Rijksfaculteit Landbouwwetenschappen Gent, 31:
489-505
Beck, E. W., Johnson, jr J. C., Getz, M. E., Skinner, F. B., Dawsey,
L. H., Woodham, D. W., and Derbyshire, J. C. (1968) Effects of feeding
dimethoate, its oxygen analogue and dimethoate-treated silage to
cattle. J. Econ. Entomol., 61: 605-610
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. (1970) Acute toxicity
data for pesticides. World Rev. Pest Control, 9: 119-271
Brady, U. E. jr, and Arthur, B. W. (1963) Biological and chemical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56: 477-482
Chamberlain, W. F., Gatterdam, P. E., and Hopkins, D. E. (1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54: 733-740
Dauterman, W. C., Casida, J. E., Knaak, J. B. and Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agr. Food Chem., 7: 188-193
Dautermann, W. C., Viado, G. B., Casida, J. E. and O'Brien, R. D.
(1960) Persistence of dimethoate and metabolites following foliar
application to plants. J. Agr. Food Chem., 8: 115-119
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. Rogor (1965)
(dimethoate) residues in food crops. Residue Rev., 11: 60-99
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30: pp. 117-132
Fechner, G., Ackermann, H. and Lexow, B. (1969)
Dünnschichtchromatographische Bestimmung von Dimethcat und
PO-Dimethoat in Milch. Z. Lebensm.-Untersuch. -Forsch., 140: 145-149
Fischer, R. (1969) Identification of organophosphate insecticides in
biological material. IV. Arch. Toxikol., 25: 216-222
Frehse, H. (1969) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Frehse, H. (1970) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Grimmer, F., Dedek, W. and Leibnitz, E. (1968a) Zur Kenntnis der
N-Butylhomologen des Dimethoats Hydrolysegeschwindigkeit
und-15-metabolismes. Z. Naturforsch., 23B: 10-17
Grimmer, F., Dedek, W. and Leibnitz, E. (1968b) Zur Kenntnis der
N-Butylhomologen des Dimethoats. Verhalten in Tomatenpflangen. Z.
Naturforsch., 23B: 834-838
Hacskaylo, J. and Bull, D. L. (1963) Metabolism of dimethoate in
cotton leaves. J. Agr. Food Chem., 11: 464-466
Hassan, A., Zayed, S. M. A. D. and Bahig, M. R. E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18: 2429-2438
Hutchinson, E. B., McNerney, J. M. and Levinskas, G. J. (1968) Report
on oxygen analogue of Cygon-dimethoate: 90-day repeated feeding to
dogs (CL-28580). Unpublished report submitted by American Cyanamid
Company.
Joint Dimethoate Residues Panel. (1968) The determination of
dimethoate residues in fruits and vegetables. Analyst, 93: 756-766
Kimmerle, G. (1962) Toxicological studies on active ingredient
Dr Schrader S6876. Unpublished report submitted by Farbenfabriken
Bayer A. G.
Kimmerle, G. (1966) S6876 P-O-dimethoate (Bayer 45432; LO-NR 113a-1),
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. Bayer (1968) 45432-Toxikologische untersuchhungen.
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. (1969) Omethoate and dimethoate. Vergleichende
toxikologische untersuchungen bei rattengenerationsversuch bei ratten
mit omethoate. Unpublished report submitted by Farbenfabriken Bayer
A. G.
Kimmerle, G. and Lorke, D. (1967) S6876 (Lo-No.274). Antidotal
effect and potentiation. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Levinskas, G. J. and Shaffer, C. B. (1965a) Oxy-carboxy dimethoate:
thirty-three day repeated feeding to rats. Unpublished report
submitted by American Cyanamid Company.
Levinskas, G. J. and Shaffer, C. B. (1965b) Oxygen analogue of
dimethoate: demyelination studies in white leghorn hens. Unpublished
report submitted by American Cyanamid Company.
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmacol., 263: 237-238
Loser, E. and Lorke, D. (1967) Bayer 45432 - Subchronische
toxikologische untersuchungen an ratten. Unpublished report submitted
by Farbenfabriken Bayer, A. G.
Loser, E. (1968b) Bayer 45432 - Subakute toxikologische untersuchungen
an ratten. Unpublished report submitted by Farbenfabriken Bayer A. G.
Lucier, G. W. and Menzer, R. E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem., 16: 936-945
Lucier, G. W. and Menzer, R. E. (1970) Nature of oxidative metabolites
of dimethoate formed in rats, liver microsomes, and bean plants. J.
Agr. Food Chem., 18: 698-704
Mendoza, C. E., Grant, D. L., Braceland, B. and McCully, K. A. (1969)
Evaluation of esterases from livers of beef, pig, sheep, monkey, and
chicken for detection of some pesticides by thin-layer
chromatographic-enzyme inhibition technique. Analyst, 94: 805-810
Menzer, R. E. and Best, N. H. (1968) Effect of phenobarbital on the
toxicity of several organophosphorus insecticides. Toxicol. appl.
Pharmacol., 13: 37-42
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects, and mammals. Botyu-Kagaku,
31: 130-136
Pardue, J. R. (1971) Recovery of organophosphorus compounds using the
AOAC multiresidue method. J. Assoc. Offic. Anal. Chem., 54: 359-360
Rowlands, D. G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Food Agr.,
17: 90-93
Ruzicka, J., Thomson, J. and Wheals, B. B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatog., 30: 92-99
Sanderson, D. M. and Edson, E. F. (1964) Toxicological properties of
the organophosphorus insecticide dimethoate. Brit. J. industr. Med.,
21: 52-64
Santi, R. and de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido
O,O-dimetilditiofosforilacetico. Contrib. 1959, Ist Ric. Agr. Soc.
Montecatini, 3: 3-29
Sissons, J. D. and Telling, G. M. (1970) A screening procedure for
water soluble insecticides. J. Chromatog., 48: 468-477
Smart, N. A. (1966) An assessment of some methods of analysis for
dimethoate residues in fruit and vegetables. Analyst, 91: 621-624
Steller, W. A. and Curry, A. N. (1964) Measurement of residues of
Cygon insecticide and its oxygen analogue by total phosphorus
determination after isolation by thin-layer chromatography. J. Assoc.
Offic. Anal. Chem., 47: 645-651
Sutherland, G. L. (1962) Cholinesterase inhibitory activity of
dimethoate. Unpublished report submitted by American Cyanamid Company.
Villeneuve, D. C. and McKinley, W. P. (1968) Inhibition of beef liver
hydrolytic enzymes by organophosphorus pesticides. J. Agr. Food Chem.,
16: 290-294
Vince, A. A. and Spicer, E. J. F. (1971) Pathology report of Bay
45432-Subchronic toxicity in rats. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Watts, R. R. and Storherr, R. W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Offic. Anal. Chem., 52; 513-521
Watts, R. R., Storherr, R. W., Pardue, J. R. and Osgood, T. (1969)
Charcoal column cleanup method for many organophosphorus pesticide
residues in crop extracts. J. Assoc. Offic. Anal. Chem., 52: 522-526
 Physical and chemical properties
Colourless to slightly yellowish oil, b.p. ca. 135°C (decomposes when
distilled); v.p. 2.5 × 10-5 mm Hg at 20°C; volatility at 20°C, 0.29
mg/m3; d20 1.32; nD20 1.4987; readily soluble in water,
alcohol and acetone; slightly soluble in ethyl ether; almost insoluble
in petroleum ether. Stability to hydrolysis: half-life period, 611
hours at pH 7 and 24°C. The velocity of decomposition of the active
ingredient is essentially greater at higher temperatures or at pH
values 8-10.
Purity
Composition of a typical technical omethoate:
content of active ingredient 94.0 - 96.0%
O-methyl S-methylcarbamoylmethyl
phosphorothiolate 1.0 - 2.0%
dimethylphosphate 1.0 - 2.0%
1,2-dichloroethane max. 3.0%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biotransformation
Dauterman et al. (1959) treated rats orally with radioactive
omethoate. The urine from two male rats treated with a dose of 50
mg/kg was collected at 12, 24 and 48 hours. The cumulative percentages
of the administered radioactivity excreted over the indicated times
were 16, 19 and 30. Utilizing ion exchange chromatography the
metabolites found in a 24 hr/urine composite were:
O,O - dimethyl phosphoric acid 34%
Unknown A 52%
O,O - dimethyl phosphorothioic acid 9.5%
Unknown B 4.5%
Following treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine in 24 hours, while
following treatment with omethoate, only 19% was excreted in 24 hours
(Dauterman et al., 1959).
Apart from this preliminary study the biotransformation of omethoate
has not been examined although the fate of dimethoate, the
phosphorodithioate which is oxidized in vivo to omethoate, is well
documented (FAO/WHO, 1968). It is probable that the metabolic route of
omethoate will follow that observed for dimethoate in plants and
animals although the rate of the individual reactions may differ as
indicated in the study on male rats. In animals metabolism is
oxidative and hydrolytic and should yield compounds as follows:
Physical and chemical properties
Colourless to slightly yellowish oil, b.p. ca. 135°C (decomposes when
distilled); v.p. 2.5 × 10-5 mm Hg at 20°C; volatility at 20°C, 0.29
mg/m3; d20 1.32; nD20 1.4987; readily soluble in water,
alcohol and acetone; slightly soluble in ethyl ether; almost insoluble
in petroleum ether. Stability to hydrolysis: half-life period, 611
hours at pH 7 and 24°C. The velocity of decomposition of the active
ingredient is essentially greater at higher temperatures or at pH
values 8-10.
Purity
Composition of a typical technical omethoate:
content of active ingredient 94.0 - 96.0%
O-methyl S-methylcarbamoylmethyl
phosphorothiolate 1.0 - 2.0%
dimethylphosphate 1.0 - 2.0%
1,2-dichloroethane max. 3.0%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
Biotransformation
Dauterman et al. (1959) treated rats orally with radioactive
omethoate. The urine from two male rats treated with a dose of 50
mg/kg was collected at 12, 24 and 48 hours. The cumulative percentages
of the administered radioactivity excreted over the indicated times
were 16, 19 and 30. Utilizing ion exchange chromatography the
metabolites found in a 24 hr/urine composite were:
O,O - dimethyl phosphoric acid 34%
Unknown A 52%
O,O - dimethyl phosphorothioic acid 9.5%
Unknown B 4.5%
Following treatment of male rats with dimethoate, 81% of the
administered dose was excreted in the urine in 24 hours, while
following treatment with omethoate, only 19% was excreted in 24 hours
(Dauterman et al., 1959).
Apart from this preliminary study the biotransformation of omethoate
has not been examined although the fate of dimethoate, the
phosphorodithioate which is oxidized in vivo to omethoate, is well
documented (FAO/WHO, 1968). It is probable that the metabolic route of
omethoate will follow that observed for dimethoate in plants and
animals although the rate of the individual reactions may differ as
indicated in the study on male rats. In animals metabolism is
oxidative and hydrolytic and should yield compounds as follows:
 (For reference to dimethoate metabolism in animals see: Hassan et al.
(1969), Lucier and Menzer (1970), FAO/WHO (1968), Brady and Arthur
(1963), and Dauterman et al. (1959); and in plants see: Morikawa and
Saito (1966) and Lucier and Menzer (1968; 1970).) Apparently all
metabolites of dimethoate observed in mammals are found in plants. In
plants a major reaction pathway includes demothoxylation which yields
a product that is not a major metabolite in mammals. (Morikawa and
Saito, 1966). However, in vitro studies with dimethoate using liver
and insect homogenates and in vivo studies with insects have shown
the presence of the dimethoxyl derivative. Dealkylation of omethoate
is a significant detoxication mechanism as evidence by fly head
cholinesterase bimolecular rate constant K1 values for omethoate of
9.2 × 10-5 L mole-1min-1 and 1.39 L mole-1min-1 for
demethoxylomethoate (Aharoni and O'Brien, 1968). Oxidative metabolism
of omethoate results in the de-N-methyl derivative which is as toxic
as the parent compound although less active as a cholinesterase
inhibitor (Lucier and Menzer, 1970). Hassan et al. (1969) whilst
investigating the metabolic fate of dimethoate in the rat concluded
that oxidation to omethoate occurred in vivo. They suggested two
major metabolic pathways for both dimethoate and omethoate. The first
involved cleavage of the C-N bond by a carboxy amidase, while the
second proceeded through esterase action on the S-C bond. Kinetic data
indicated that reaction between acetycholinesterase and omethoate was
irreversible and bimolecular. Omethoate was found to be 75-100 times
more potent than dimethoate in inhibiting rat brain
acetylcholinesterase.
Effects on enzymes and other biochemical parameters
Omethoate is a direct inhibitor of acetyl cholinesterase from various
sources. Sensitivity to inhibition is greater with invertebrate than
with mammalian sources. This is reflected in the omethoate bimolecular
rate constant (Ki) for rat brain of 1.65 × 10-3 L mole-1min-1
(Hassan et al., 1969) and house fly head of 9.2 × 10-5
L mole-1min-1 (Abaroni and O'Brien, 1968) as well as the 150 valve
(molar concentration of omethoate producing 50% inhibition of enzyme
activity) as seen in the following table:
Enzyme source I50 (M) Reference
Rat brain 1.2 × 10-5 Hassan et al., 1969
1.1 × 10-5 Sanderson and Edson, 1964
Bovine (RBC) 3.9 × 10-5 Santi and Pietri-Tonelli, 1969
Bovine liver 5.7 × 10-5 Villeneuve and McKinley, 1968
Human plasma 6.3 × 10-5 Lucier and Menzer, 1970
1.2 × 10-4 Sutherland, 1962
Housefly 1.7 × 10-7 Santi and Pietri-Tonelli, 1969
Following oral administrations of dimethoate to a cow at 10 mg/kg, red
blood cell cholinesterase was depressed in two stages; for six days
inhibition was constant at less than 20% and then slowly declined to
40% at 12 days (Dauterman et al., 1959), This biphasic inhibition was
also noted in sheep by Beck et al. (1968) who found that
cholinesterase activity continued to decrease for periods of time
after dimethoate treatment stopped. This is apparently due to slow
release of dimethoate and/or conversion rate of dimethoate to
omethoate.
Following acute oral administration to rabbits (20 mg/kg) various
enzymatic tests for liver function were not affected. These include:
serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic
transaminase (SGPT), serum sorbitol dehydrogenase (SDH) and
bromophthalein test (Kimmerle, 1962).
TOXICOLOGICAL STUDIES
Special studies
(a) Toxicity of the metabolites
Compound Species Route LD50 150 (M) pI50
(mg/kg) (human plasma)
Dimethoate Mouse ip 151 >0.1 <1
N-hydroxymethyl P(S) n.d. >0.1 <1
Des-N-methyl P(S) Mouse ip 190 >0.1 <1
Omethoate Mouse ip 13 6.5 × 10-5 4.2
N-hydroxymethyl P(O) n.d. 2.0 × 10-4 3.7
Des-N-methyl P(O) Mouse ip 10 4.0 × 10-4 3.4
n.d. = not determined.
Data from Lucier and Menzer (1970).
Deamination of omethoate results in a significantly less toxic
compound than omethoate. Carboxyomethoate ((CH3O)2 P(O)
(SCH2C(O)OH)), the deamination product of omethoate, was fed to rats
for 33 days at levels of 0, 330, 1000 and 3000 ppm in the diet. Plasma
cholinesterase activity was not affected. Red blood cell
cholinesterase was depressed at 1000 and 3000 ppm in both sexes and to
a slight degree in female rats at 330 ppm. Brain cholinesterase was
slightly, but significantly decreased in female rats at 3000 ppm,
Weight gain and food intake was depressed at 3000 ppm. No effects were
noted on survival, appearance, organ weights or histological
examination of organs and tissues (Levinskas and Shaffer, 1965a).
(b) Potentiation
Omethoate was observed to influence the acute oral toxicity of
malathion in rats. When administered at 1/2 LD50 levels the
mortality observed was slightly greater than theoretically anticipated
(Kimmerle and Lorke, 1967). Pretreatment of rats with phenobarbital
resulted in a threefold increase in the acute toxicity of omethoate
(Menzor and Best, 1968).
(c) Reproduction
Eighty male and 80 female rats were administered omethoate orally at a
daily dose of 0.5 mg/kg for six weeks and at the conclusion of
treatment they were mated. The pups were raised on normal diets for
four weeks after weaning and subsequently treated in a similar manner
for six weeks as the Fo generation. Daily oral treatment for six
weeks to the Fo generation had no effect on reproduction and the
same treatment did not grossly affect the F1 generation. Red blood
cell cholinesterase levels during the administration of omethoate
remained constant at 40-50% of normal while the plasma cholinesterase
was unaffected. This treatment did not affect growth of Fo or F1.
(Kimmerle, 1969).
(d) Neurotoxicity
Hens were dosed with 50-200 mg/kg of omethoate by intraperitoneal
injection under the protection of 2-PAM and atropine. Of 17 hens
treated, 14 were given 150 mg/kg and five died of acute intoxication.
The hens which survived were kept under observation for six weeks and
no signs of neurotoxic effect was observed (Kimmerle, 1962).
Hens (six hens/group) were fed omethoate at 0, 60, 120 and 240 ppm for
four weeks. At the end of the study three hens were sacrificed and
examined histologically (brain, thoracic spinal cord and sciatic
nerve) for myelin degeneration. The remainder were maintained on
normal diets for four weeks. No evidence was found, clinically or
histologically, for delayed neurotoxicity or demyelination (Levinskas
and Shaffer, 1965b).
(e) Antidotes
Administration of atropine alone and in combination with 2-PAM or BH-6
was effective in reducing the oral LD50 values for rats (Kimmerle,
1962; Kimmerle and Lorke, 1967). Administration of 2-PAM or BH-6 alone
was not effective although TMB-4 alone reduced the acute toxicity. The
optimum antidotal effects were noted with combinations of atropine and
oxime reactivators. At the first signs of poisoning with omethoate,
2-PAM or toxogonin and/or atropine were effective therapeutic agents
increasing the LD50 of omethoate from 50 to 200% (Lorke and
Kimmerle, 1969).
Acute toxicity
Animal Sex Route LD50 Reference
(mg/kg/bw)
Mouse M Oral 36 Kimmerle, 1968
27 Santi and Pietri-Tonelli, 1969
Ip 13 Lucier and Menzer, 1970
Iv 23 Kimmerle, 1962
Rat M and F Oral 28-65 Kimmerle and Lorke, 1967
Dauterman, 1959
Kimmerle, 1966; 1968
Kimmerle, 1962
50 Ben-Dyke, 1970
M Ip 14 Kimmerle, 1968
Ip 38 Kimmerle, 1962
Rabbit Oral 50 Kimmerle, 1962
Cat Oral 50 Kimmerle, 1962
Guinea-pig Oral 100 Kimmerle, 1962
Hen Oral 125 Kimmerle, 1962
100 Levinskas and Shaffer, 1965b
Signs of poisoning are typical of cholinergic stimulation as elicited
by other organophosphorus esters. The signs appear in from 5-60
minutes following poisoning and include salivation, lacrymation,
tremors, etc. The signs of poisoning may persist for one to three days
following intoxication (Kimmerle, 1968).
Rats (groups of three males per dose) were orally administered
omethoate at 2.5, 5 and 10 mg/kg. cholinesterase assay indicated
maximal depression at three hours (first interval examined after
exposure) with recovery in one to three days (Kimmerle, 1962),
Groups of rats (five male and five female/group) were orally
administered omethoate at a single dose of 0, 0.1, 0.5, 2.5, 5.0, 10.0
and 25 mg/kg and examined one, three, and seven days after treatment
for cholinesterase inhibition. Red blood cell cholinesterase was
depressed to a greater extent and duration than plasma. The response
of the RBC cholinesterase was the same in both sexes. With plasma,
females were more susceptible than males. Plasma was completely
recovered at all dose levels by day seven from a maximum of 67%
inhibition at day one at 25 mg/kg (female). Red blood cell
cholinesterase was still depressed at the conclusion of the test
(seven days) at doses of 10 mg/kg and above. Maximum inhibition
observed at 10 mg/kg on day one was 63%. The higher dose did not give
a greater reduction of activity (Kimmerle, 1969).
Short-term studies
Rat
Rats (20 males) were administered omethoate orally for five days/week
for eight weeks (42 doses) at 5 mg/kg/day. Tremors were transient
after each application. Cholinesterase was depressed 50-70% of normal
and quickly recovered after treatment ended (Kimmerle, 1962).
Rats (10 males per group) were orally administered omethoate at doses
of 0, 1, 2, 4, 8 and 16 mg/kg, five days/week for eight weeks. The
survivors were sacrificed four weeks after treatment ended. Mortality
occurred at 8 and 16 mg/kg. Cholinergic symptoms evident at 4 mg/kg,
decreased as time progressed. Slight trembling was observed at the
lowest doses. Organ weights, growth and gross and microscopic
examination of tissues were not affected (Kimmerle, 1962).
Groups of rats (10 male and 10 female per group) were administered
omethoate orally for 14 days at doses of 0.1 and 0.5 mg/kg/ day.
Plasma cholinesterase was not depressed during the 14-day interval.
Red blood cell cholinesterase was slightly depressed in males and
females at 0.5 mg/kg and this was maintained for the 14-day period. No
differences in sex were observed with regard to red blood cell enzyme
inhibition (Kimmerle, 1969).
Rats (10 male and 10 female per group) were fed omethoate (which was
mixed with the feed every day) for four weeks at 0, 2.5 and 15 ppm.
Growth was temporarily depressed at 15 ppm and cholinesterase was
depressed at both feeding levels. Red blood cell cholinesterase
activity was inhibited at 2.5 ppm and plasma at 15 ppm with the female
rats showing the higher sensitivity of plasma cholinesterase (Loser,
1968b).
Rats (15 males and 15 females per group, 30 of each sex were controls)
were fed omethoate at 0, 2.5, 5, 15, 50 and 150 ppm for four months.
Cholinergic stimulation was evident at 15 ppm and above.
Cholinesterase was depressed at 50 ppm and 150 ppm in females and 5
ppm and above in males. No effects were noted on growth, organ
weights, blood parameters or urinalysis at the feeding levels
including 50 ppm. At 150 ppm some animals died, the bodyweight and
food consumption was depressed and the relative liver weight in males
was increased (Loser and Lorke, 1967).
Groups of rats (80 male and 80 female per group) were administered
omethoate daily at an oral dose of 0.5 mg/kg for six weeks. RBC
cholinesterase was inhibited similarly in males and females at 40-50%
of normal. When placed on a normal diet for two weeks the enzyme
recovered to normal values. No significant adverse effects were
observed on growth or plasma cholinesterase activity (Kimmerle, 1969).
Rats (15 male and 15 female per group) were fed omethoate (mixed with
feed daily) at levels of 0, 0.5, 1.0, 2.0 and 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident at 4
ppm. Cholinesterase (red blood cell) was depressed at 2 ppm though in
females only slightly. At 4 ppm the inhibition was 30-50%. No effects
were noted on growth, food consumption, blood parameters, liver and
kidney function tests, organ weights (Loser, 1968a) and histological
examination of tissues (Vince and Spicer, 1971).
Dog
Dogs (four male and four female per group) were fed omethoate for 14
weeks at dietary concentrations of 0.4 ppm, 0.8 ppm and 1.6 ppm. No
adverse effects were observed on red blood cell and plasma
cholinesterase activity or other parameters measured including:
growth, survival, food consumption, blood analyses (haematocrit,
haemoglobin and total and differential leukocyte counts) and clinical
chemistry (alkaline phosphatase, glucose, urea nitrogen and
bromsulphthalein (BSP) retention) (Hutchison et al,, 1968).
Long-term studies
No data available.
Comments
Omethoate is an organophosphorus insecticide with a high acute
toxicity. Its toxicity is considerably higher than is phosphorothioate
analogue dimethoate, which was evaluated in 1967 and for which an
acceptable daily intake has been established. Although dimethoate is
converted to omethoate, quantitative data on the rate of conversion
following administration have not been reported.
Adequate short-term studies are available in both rats and dogs. The
lack of an acceptable reproduction study is partly offset by the
existence of an acceptable study of this kind in the case of
dimethoate. No long-term studies have been reported and there are no
observations in man comparable with those for dimethoate.
In view of the information available from dimethoate the Meeting
agreed that a temporary ADI for omethoate could be established based
on the 90-day study on omethoate in the rat. It was stressed however,
that long-term studies are required.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1.0 ppm in the dry diet equivalent to 0.05 mg/kg bodyweight per
day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg body-weight
per day
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
It is a systemic, organophosphorus insecticide and acaricide with a
broad sphere of action and good plant compatibility. It acts against
both sucking and biting insect pests and is active against spider
mites which are resistant to some other organophosphorus pesticides;
in this respect it differs from dimethoate, of which it is the oxygen
analogue. Its chief uses are for pre-harvest treatments of:
fruit (pome, stone and citrus) 65%
field crops (pasture, cotton, sugar cane, etc.) 25%
vegetables 5%
miscellaneous (e.g. ornamentals) 5%
It is available as 50% and 80% w/v emulsifiable concentrates and is
applied at about 0.025 to 0.075% w/v of active ingredient, equivalent
to up to 1500 g/ha a.i.
Residues resulting from supervised trials
The behaviour of omethoate on and in plants is characterized by a rate
of degradation which is relatively slow for an organophosphorus
pesticide. It seems that the surface condition of the crop, perhaps in
association with weather factors, can have a bearing on this matter.
Information on available residue data is given in Table 1 (Bayer,
1971). The application rates are expressed in percentage concentration
of active ingredient; the normal volume applied being about 2000 l/ha
for fruit crops and between 600-1000 l/ha for vegetable crops. Data on
the behaviour of residues in storage and processing and on the level
of residues in food moving in commerce are not available. Residues of
omethoate were not observed during a total diet study for
organophosphorus pesticide residues (Abbot et al, 1970).
TABLE I. OMETHOATE RESIDUES IN CROPS FROM SUPERVISED TRIALS
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Apples 0.03 1 0 0.66-1.0 0.8
1 0.16-0.4 0.3
2 0.04-0.2 0.1
3 0.01-0.14 0.07
Apples 0.05 3 0 1.55-2.07 1.75
1 0.32-0.6 0.45
2 0.11-0.17 0.15
3 - 0.10
4 0.07-0.09 0.08
5 - <0.05
Apples 0.064 1 0 - 3.3
1 - 1.9
2 - 1.6
3 - 1.2
4 - 0.4
Apples 0.075 2 0 2.3-5.2 3.75
2 1.1-2.4 1.62
3 0.75-1.3 1.02
4 0.55-1.4 0.95
5 0.5-1.1 0.70
6 0.25-0.85 0.51
Pears 0.064 1 0 - 2.15
1 - 2.0
2 - 1.45
3 - 1.05
4 - 0.9
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Pears 0.075 2 0 4.05-6.4 5.5
2 1.7-3.05 2.2
3 0.5-2.25 1.1
4 0.35-1.3 0.9
5 0.17-1.1 0.55
6 0.16-1.1 0.4
Pears 0.075 2-3 0 2.4-6.7 4.05
1 2.2-3.8 3.0
2 1.4-1.5 1.45
3 1.3-1.6 1.5
4 0.8-1.3 1.05
Apricots 0.075 1 0 - 3.1
1 - 1.8
2 - 1.8
3 - 0.4
Cherries 0.05 1 0 - 1.1
2 - 0.2
Grapes 0.05 1 0 - 5.7
1 - 3.9
2 - 2.35
3 - 1.8
4 - 1.25
Peaches 0.075 1 0 3.3-7.3 5.3
1 2.6-6.2 4.4
2 1.8-3.8 2.8
3 1.4-1.7 1.55
Plums 0.075 1 0 0.7-0.85 0.8
1 0.7-1.05 0.85
2 0.6-0.9 0.75
3 0.6-0.75 0.68
4 - 0.5
Lettuce* 0.03 1 0 3.3-3.7 3.5
1 0.3-0.9 0.6
2 0.11-0.17 0.15
3 - 0.03
Potatoes* 0.03 2 2 n.d.-0.21 0.11
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Sugar beets* 0.03 1 2 - 1.4
3 - 0.1
7 - 0.1
Sugar beets* 0.03 1 0 - 2.7
leaves 2 - 0.3
3 - 0.23
7 - 0.02
Hops (dried) 0.05 1 0 - 35
2 - 6.8
3 0.5-6.5 3.5
0.05 5-6 2“ 3.8-11.7 7.8
6 - 0.07
0.1 1 2 - 9.4
Note: slight underdosages used on these crops.
Fate of residues
In animals
Beck et al. (1968) fed 0.50 mg/kg dimethoate and 0.05 mg/kg of
omethoate for 14 days to cattle but found no residues in the milk.
When double these amounts were fed for an additional 14 days, residues
of omethoate ranging from 0.004 to 0.125 ppm were detected but
dimethoate was still absent; three days after ceasing the treatment no
omethoate was detected in the milk. See also "Biochemical aspects".
In plants
Dauterman et al. (1960) studied the metabolism of 32P-dimethoate
(surface and absorbed residues) after foliar treatment of corn,
cotton, pea, and potato plants. The amount of dimethoate, omethoate
and water-soluble metabolites on the surface of the plants was
investigated two and 12 days after treatment. With the longer time
interval there was a decrease in the total amount of omethoate on the
surface but an increase in the proportionate amount with respect to
dimethoate. The compound (4) (see Fig. 1) accounted for the largest
proportion of the water-soluble metabolites (up to 94%). Inside the
leaf, there was also a decrease with time in the total amount of
omethoate but a proportionate increase with respect to dimethoate.
Here, compound (4) accounted for no more than 10% of the water-soluble
metabolites.
Hydrolisis at the alkoxy group occurred mainly inside the leaves,
indicating an enzymatic action at this site, which also held for
omethoate. As none of the thio-carboxy derivative (4a) was found in or
on plants, formation of (4) probably proceeded by oxidation of
dimethoate to omethoate followed by hydrolysis of the carbamoyl group.
As (4) was found in the largest amount on the surface, this cleavage
was probably non-enzymatic. This is an important indication of the
mode of transformation of omethoate on the plant. However, these
considerations are not supported by the observations of Hacskaylo and
Bull (1963) who, using the excised-leaf technique, found large amounts
of (4a) as a metabolite whilst the presence of even slight amounts of
(4) could not be established with any certainty.
Lucier and Menzer (1968, 1970) studied the metabolism of dimethoate on
bean plants. The most important neutral metabolites which they found
were compound (3) and an unidentified compound (X) although only in
small amounts as compared with omethoate, as is shown below
(proportions expressed in relation to omethoate = 1X%).
Days after treatment
1 3 6
(2) 1.0% 0.4% 0.4%
(3) 7.7% 7.2% 5.7%
(X) 29.0% 5.0% 2.2%
The ratio of phosphorodithioates to phosphorothioates in the
N-demethylation scheme was approximately 1:1, possibly indicating a
reduced rate of hydrolytic metabolism for the desulfurated compounds.
However, the small proportions of the compounds (X), (2), and (3), in
relation to omethoate, suggests that these compounds had merely a
transitory character. It is interesting to note that compound (4) was
not found in these studies.
The simple phosphates and thiophosphates (6) to (11) have also been
identified as metabolites in plants, or presumed to be present, after
application of dimethoate:
(6), (7), (11); Dautermann et al. (1960); Hacskaylo and Bull (1963)
(6); Rowlands (1966); Lucier and Menzer (1968)
(8), not significant; Hacskaylo and Bull (1963)
(8), (9), (10), (11) not significant; Looter and Menzer (1968)
In vitro hydrolysis of omethoate by grain extracts (Rowlands, 1966)
provided clear evidence of the formation of (4) in addition to (6);
compound (5) was also found.
(For reference to dimethoate metabolism in animals see: Hassan et al.
(1969), Lucier and Menzer (1970), FAO/WHO (1968), Brady and Arthur
(1963), and Dauterman et al. (1959); and in plants see: Morikawa and
Saito (1966) and Lucier and Menzer (1968; 1970).) Apparently all
metabolites of dimethoate observed in mammals are found in plants. In
plants a major reaction pathway includes demothoxylation which yields
a product that is not a major metabolite in mammals. (Morikawa and
Saito, 1966). However, in vitro studies with dimethoate using liver
and insect homogenates and in vivo studies with insects have shown
the presence of the dimethoxyl derivative. Dealkylation of omethoate
is a significant detoxication mechanism as evidence by fly head
cholinesterase bimolecular rate constant K1 values for omethoate of
9.2 × 10-5 L mole-1min-1 and 1.39 L mole-1min-1 for
demethoxylomethoate (Aharoni and O'Brien, 1968). Oxidative metabolism
of omethoate results in the de-N-methyl derivative which is as toxic
as the parent compound although less active as a cholinesterase
inhibitor (Lucier and Menzer, 1970). Hassan et al. (1969) whilst
investigating the metabolic fate of dimethoate in the rat concluded
that oxidation to omethoate occurred in vivo. They suggested two
major metabolic pathways for both dimethoate and omethoate. The first
involved cleavage of the C-N bond by a carboxy amidase, while the
second proceeded through esterase action on the S-C bond. Kinetic data
indicated that reaction between acetycholinesterase and omethoate was
irreversible and bimolecular. Omethoate was found to be 75-100 times
more potent than dimethoate in inhibiting rat brain
acetylcholinesterase.
Effects on enzymes and other biochemical parameters
Omethoate is a direct inhibitor of acetyl cholinesterase from various
sources. Sensitivity to inhibition is greater with invertebrate than
with mammalian sources. This is reflected in the omethoate bimolecular
rate constant (Ki) for rat brain of 1.65 × 10-3 L mole-1min-1
(Hassan et al., 1969) and house fly head of 9.2 × 10-5
L mole-1min-1 (Abaroni and O'Brien, 1968) as well as the 150 valve
(molar concentration of omethoate producing 50% inhibition of enzyme
activity) as seen in the following table:
Enzyme source I50 (M) Reference
Rat brain 1.2 × 10-5 Hassan et al., 1969
1.1 × 10-5 Sanderson and Edson, 1964
Bovine (RBC) 3.9 × 10-5 Santi and Pietri-Tonelli, 1969
Bovine liver 5.7 × 10-5 Villeneuve and McKinley, 1968
Human plasma 6.3 × 10-5 Lucier and Menzer, 1970
1.2 × 10-4 Sutherland, 1962
Housefly 1.7 × 10-7 Santi and Pietri-Tonelli, 1969
Following oral administrations of dimethoate to a cow at 10 mg/kg, red
blood cell cholinesterase was depressed in two stages; for six days
inhibition was constant at less than 20% and then slowly declined to
40% at 12 days (Dauterman et al., 1959), This biphasic inhibition was
also noted in sheep by Beck et al. (1968) who found that
cholinesterase activity continued to decrease for periods of time
after dimethoate treatment stopped. This is apparently due to slow
release of dimethoate and/or conversion rate of dimethoate to
omethoate.
Following acute oral administration to rabbits (20 mg/kg) various
enzymatic tests for liver function were not affected. These include:
serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic
transaminase (SGPT), serum sorbitol dehydrogenase (SDH) and
bromophthalein test (Kimmerle, 1962).
TOXICOLOGICAL STUDIES
Special studies
(a) Toxicity of the metabolites
Compound Species Route LD50 150 (M) pI50
(mg/kg) (human plasma)
Dimethoate Mouse ip 151 >0.1 <1
N-hydroxymethyl P(S) n.d. >0.1 <1
Des-N-methyl P(S) Mouse ip 190 >0.1 <1
Omethoate Mouse ip 13 6.5 × 10-5 4.2
N-hydroxymethyl P(O) n.d. 2.0 × 10-4 3.7
Des-N-methyl P(O) Mouse ip 10 4.0 × 10-4 3.4
n.d. = not determined.
Data from Lucier and Menzer (1970).
Deamination of omethoate results in a significantly less toxic
compound than omethoate. Carboxyomethoate ((CH3O)2 P(O)
(SCH2C(O)OH)), the deamination product of omethoate, was fed to rats
for 33 days at levels of 0, 330, 1000 and 3000 ppm in the diet. Plasma
cholinesterase activity was not affected. Red blood cell
cholinesterase was depressed at 1000 and 3000 ppm in both sexes and to
a slight degree in female rats at 330 ppm. Brain cholinesterase was
slightly, but significantly decreased in female rats at 3000 ppm,
Weight gain and food intake was depressed at 3000 ppm. No effects were
noted on survival, appearance, organ weights or histological
examination of organs and tissues (Levinskas and Shaffer, 1965a).
(b) Potentiation
Omethoate was observed to influence the acute oral toxicity of
malathion in rats. When administered at 1/2 LD50 levels the
mortality observed was slightly greater than theoretically anticipated
(Kimmerle and Lorke, 1967). Pretreatment of rats with phenobarbital
resulted in a threefold increase in the acute toxicity of omethoate
(Menzor and Best, 1968).
(c) Reproduction
Eighty male and 80 female rats were administered omethoate orally at a
daily dose of 0.5 mg/kg for six weeks and at the conclusion of
treatment they were mated. The pups were raised on normal diets for
four weeks after weaning and subsequently treated in a similar manner
for six weeks as the Fo generation. Daily oral treatment for six
weeks to the Fo generation had no effect on reproduction and the
same treatment did not grossly affect the F1 generation. Red blood
cell cholinesterase levels during the administration of omethoate
remained constant at 40-50% of normal while the plasma cholinesterase
was unaffected. This treatment did not affect growth of Fo or F1.
(Kimmerle, 1969).
(d) Neurotoxicity
Hens were dosed with 50-200 mg/kg of omethoate by intraperitoneal
injection under the protection of 2-PAM and atropine. Of 17 hens
treated, 14 were given 150 mg/kg and five died of acute intoxication.
The hens which survived were kept under observation for six weeks and
no signs of neurotoxic effect was observed (Kimmerle, 1962).
Hens (six hens/group) were fed omethoate at 0, 60, 120 and 240 ppm for
four weeks. At the end of the study three hens were sacrificed and
examined histologically (brain, thoracic spinal cord and sciatic
nerve) for myelin degeneration. The remainder were maintained on
normal diets for four weeks. No evidence was found, clinically or
histologically, for delayed neurotoxicity or demyelination (Levinskas
and Shaffer, 1965b).
(e) Antidotes
Administration of atropine alone and in combination with 2-PAM or BH-6
was effective in reducing the oral LD50 values for rats (Kimmerle,
1962; Kimmerle and Lorke, 1967). Administration of 2-PAM or BH-6 alone
was not effective although TMB-4 alone reduced the acute toxicity. The
optimum antidotal effects were noted with combinations of atropine and
oxime reactivators. At the first signs of poisoning with omethoate,
2-PAM or toxogonin and/or atropine were effective therapeutic agents
increasing the LD50 of omethoate from 50 to 200% (Lorke and
Kimmerle, 1969).
Acute toxicity
Animal Sex Route LD50 Reference
(mg/kg/bw)
Mouse M Oral 36 Kimmerle, 1968
27 Santi and Pietri-Tonelli, 1969
Ip 13 Lucier and Menzer, 1970
Iv 23 Kimmerle, 1962
Rat M and F Oral 28-65 Kimmerle and Lorke, 1967
Dauterman, 1959
Kimmerle, 1966; 1968
Kimmerle, 1962
50 Ben-Dyke, 1970
M Ip 14 Kimmerle, 1968
Ip 38 Kimmerle, 1962
Rabbit Oral 50 Kimmerle, 1962
Cat Oral 50 Kimmerle, 1962
Guinea-pig Oral 100 Kimmerle, 1962
Hen Oral 125 Kimmerle, 1962
100 Levinskas and Shaffer, 1965b
Signs of poisoning are typical of cholinergic stimulation as elicited
by other organophosphorus esters. The signs appear in from 5-60
minutes following poisoning and include salivation, lacrymation,
tremors, etc. The signs of poisoning may persist for one to three days
following intoxication (Kimmerle, 1968).
Rats (groups of three males per dose) were orally administered
omethoate at 2.5, 5 and 10 mg/kg. cholinesterase assay indicated
maximal depression at three hours (first interval examined after
exposure) with recovery in one to three days (Kimmerle, 1962),
Groups of rats (five male and five female/group) were orally
administered omethoate at a single dose of 0, 0.1, 0.5, 2.5, 5.0, 10.0
and 25 mg/kg and examined one, three, and seven days after treatment
for cholinesterase inhibition. Red blood cell cholinesterase was
depressed to a greater extent and duration than plasma. The response
of the RBC cholinesterase was the same in both sexes. With plasma,
females were more susceptible than males. Plasma was completely
recovered at all dose levels by day seven from a maximum of 67%
inhibition at day one at 25 mg/kg (female). Red blood cell
cholinesterase was still depressed at the conclusion of the test
(seven days) at doses of 10 mg/kg and above. Maximum inhibition
observed at 10 mg/kg on day one was 63%. The higher dose did not give
a greater reduction of activity (Kimmerle, 1969).
Short-term studies
Rat
Rats (20 males) were administered omethoate orally for five days/week
for eight weeks (42 doses) at 5 mg/kg/day. Tremors were transient
after each application. Cholinesterase was depressed 50-70% of normal
and quickly recovered after treatment ended (Kimmerle, 1962).
Rats (10 males per group) were orally administered omethoate at doses
of 0, 1, 2, 4, 8 and 16 mg/kg, five days/week for eight weeks. The
survivors were sacrificed four weeks after treatment ended. Mortality
occurred at 8 and 16 mg/kg. Cholinergic symptoms evident at 4 mg/kg,
decreased as time progressed. Slight trembling was observed at the
lowest doses. Organ weights, growth and gross and microscopic
examination of tissues were not affected (Kimmerle, 1962).
Groups of rats (10 male and 10 female per group) were administered
omethoate orally for 14 days at doses of 0.1 and 0.5 mg/kg/ day.
Plasma cholinesterase was not depressed during the 14-day interval.
Red blood cell cholinesterase was slightly depressed in males and
females at 0.5 mg/kg and this was maintained for the 14-day period. No
differences in sex were observed with regard to red blood cell enzyme
inhibition (Kimmerle, 1969).
Rats (10 male and 10 female per group) were fed omethoate (which was
mixed with the feed every day) for four weeks at 0, 2.5 and 15 ppm.
Growth was temporarily depressed at 15 ppm and cholinesterase was
depressed at both feeding levels. Red blood cell cholinesterase
activity was inhibited at 2.5 ppm and plasma at 15 ppm with the female
rats showing the higher sensitivity of plasma cholinesterase (Loser,
1968b).
Rats (15 males and 15 females per group, 30 of each sex were controls)
were fed omethoate at 0, 2.5, 5, 15, 50 and 150 ppm for four months.
Cholinergic stimulation was evident at 15 ppm and above.
Cholinesterase was depressed at 50 ppm and 150 ppm in females and 5
ppm and above in males. No effects were noted on growth, organ
weights, blood parameters or urinalysis at the feeding levels
including 50 ppm. At 150 ppm some animals died, the bodyweight and
food consumption was depressed and the relative liver weight in males
was increased (Loser and Lorke, 1967).
Groups of rats (80 male and 80 female per group) were administered
omethoate daily at an oral dose of 0.5 mg/kg for six weeks. RBC
cholinesterase was inhibited similarly in males and females at 40-50%
of normal. When placed on a normal diet for two weeks the enzyme
recovered to normal values. No significant adverse effects were
observed on growth or plasma cholinesterase activity (Kimmerle, 1969).
Rats (15 male and 15 female per group) were fed omethoate (mixed with
feed daily) at levels of 0, 0.5, 1.0, 2.0 and 4.0 ppm for three
months. Clinical signs of cholinergic stimulation were evident at 4
ppm. Cholinesterase (red blood cell) was depressed at 2 ppm though in
females only slightly. At 4 ppm the inhibition was 30-50%. No effects
were noted on growth, food consumption, blood parameters, liver and
kidney function tests, organ weights (Loser, 1968a) and histological
examination of tissues (Vince and Spicer, 1971).
Dog
Dogs (four male and four female per group) were fed omethoate for 14
weeks at dietary concentrations of 0.4 ppm, 0.8 ppm and 1.6 ppm. No
adverse effects were observed on red blood cell and plasma
cholinesterase activity or other parameters measured including:
growth, survival, food consumption, blood analyses (haematocrit,
haemoglobin and total and differential leukocyte counts) and clinical
chemistry (alkaline phosphatase, glucose, urea nitrogen and
bromsulphthalein (BSP) retention) (Hutchison et al,, 1968).
Long-term studies
No data available.
Comments
Omethoate is an organophosphorus insecticide with a high acute
toxicity. Its toxicity is considerably higher than is phosphorothioate
analogue dimethoate, which was evaluated in 1967 and for which an
acceptable daily intake has been established. Although dimethoate is
converted to omethoate, quantitative data on the rate of conversion
following administration have not been reported.
Adequate short-term studies are available in both rats and dogs. The
lack of an acceptable reproduction study is partly offset by the
existence of an acceptable study of this kind in the case of
dimethoate. No long-term studies have been reported and there are no
observations in man comparable with those for dimethoate.
In view of the information available from dimethoate the Meeting
agreed that a temporary ADI for omethoate could be established based
on the 90-day study on omethoate in the rat. It was stressed however,
that long-term studies are required.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 1.0 ppm in the dry diet equivalent to 0.05 mg/kg bodyweight per
day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg body-weight
per day
Estimate of temporary acceptable daily intake for man
0-0.0005 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
It is a systemic, organophosphorus insecticide and acaricide with a
broad sphere of action and good plant compatibility. It acts against
both sucking and biting insect pests and is active against spider
mites which are resistant to some other organophosphorus pesticides;
in this respect it differs from dimethoate, of which it is the oxygen
analogue. Its chief uses are for pre-harvest treatments of:
fruit (pome, stone and citrus) 65%
field crops (pasture, cotton, sugar cane, etc.) 25%
vegetables 5%
miscellaneous (e.g. ornamentals) 5%
It is available as 50% and 80% w/v emulsifiable concentrates and is
applied at about 0.025 to 0.075% w/v of active ingredient, equivalent
to up to 1500 g/ha a.i.
Residues resulting from supervised trials
The behaviour of omethoate on and in plants is characterized by a rate
of degradation which is relatively slow for an organophosphorus
pesticide. It seems that the surface condition of the crop, perhaps in
association with weather factors, can have a bearing on this matter.
Information on available residue data is given in Table 1 (Bayer,
1971). The application rates are expressed in percentage concentration
of active ingredient; the normal volume applied being about 2000 l/ha
for fruit crops and between 600-1000 l/ha for vegetable crops. Data on
the behaviour of residues in storage and processing and on the level
of residues in food moving in commerce are not available. Residues of
omethoate were not observed during a total diet study for
organophosphorus pesticide residues (Abbot et al, 1970).
TABLE I. OMETHOATE RESIDUES IN CROPS FROM SUPERVISED TRIALS
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Apples 0.03 1 0 0.66-1.0 0.8
1 0.16-0.4 0.3
2 0.04-0.2 0.1
3 0.01-0.14 0.07
Apples 0.05 3 0 1.55-2.07 1.75
1 0.32-0.6 0.45
2 0.11-0.17 0.15
3 - 0.10
4 0.07-0.09 0.08
5 - <0.05
Apples 0.064 1 0 - 3.3
1 - 1.9
2 - 1.6
3 - 1.2
4 - 0.4
Apples 0.075 2 0 2.3-5.2 3.75
2 1.1-2.4 1.62
3 0.75-1.3 1.02
4 0.55-1.4 0.95
5 0.5-1.1 0.70
6 0.25-0.85 0.51
Pears 0.064 1 0 - 2.15
1 - 2.0
2 - 1.45
3 - 1.05
4 - 0.9
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Pears 0.075 2 0 4.05-6.4 5.5
2 1.7-3.05 2.2
3 0.5-2.25 1.1
4 0.35-1.3 0.9
5 0.17-1.1 0.55
6 0.16-1.1 0.4
Pears 0.075 2-3 0 2.4-6.7 4.05
1 2.2-3.8 3.0
2 1.4-1.5 1.45
3 1.3-1.6 1.5
4 0.8-1.3 1.05
Apricots 0.075 1 0 - 3.1
1 - 1.8
2 - 1.8
3 - 0.4
Cherries 0.05 1 0 - 1.1
2 - 0.2
Grapes 0.05 1 0 - 5.7
1 - 3.9
2 - 2.35
3 - 1.8
4 - 1.25
Peaches 0.075 1 0 3.3-7.3 5.3
1 2.6-6.2 4.4
2 1.8-3.8 2.8
3 1.4-1.7 1.55
Plums 0.075 1 0 0.7-0.85 0.8
1 0.7-1.05 0.85
2 0.6-0.9 0.75
3 0.6-0.75 0.68
4 - 0.5
Lettuce* 0.03 1 0 3.3-3.7 3.5
1 0.3-0.9 0.6
2 0.11-0.17 0.15
3 - 0.03
Potatoes* 0.03 2 2 n.d.-0.21 0.11
TABLE I. (Continued)
Crop a.i.% No. of Weeks after Residues found
w/v applications last (ppm)
application Range Mean
Sugar beets* 0.03 1 2 - 1.4
3 - 0.1
7 - 0.1
Sugar beets* 0.03 1 0 - 2.7
leaves 2 - 0.3
3 - 0.23
7 - 0.02
Hops (dried) 0.05 1 0 - 35
2 - 6.8
3 0.5-6.5 3.5
0.05 5-6 2“ 3.8-11.7 7.8
6 - 0.07
0.1 1 2 - 9.4
Note: slight underdosages used on these crops.
Fate of residues
In animals
Beck et al. (1968) fed 0.50 mg/kg dimethoate and 0.05 mg/kg of
omethoate for 14 days to cattle but found no residues in the milk.
When double these amounts were fed for an additional 14 days, residues
of omethoate ranging from 0.004 to 0.125 ppm were detected but
dimethoate was still absent; three days after ceasing the treatment no
omethoate was detected in the milk. See also "Biochemical aspects".
In plants
Dauterman et al. (1960) studied the metabolism of 32P-dimethoate
(surface and absorbed residues) after foliar treatment of corn,
cotton, pea, and potato plants. The amount of dimethoate, omethoate
and water-soluble metabolites on the surface of the plants was
investigated two and 12 days after treatment. With the longer time
interval there was a decrease in the total amount of omethoate on the
surface but an increase in the proportionate amount with respect to
dimethoate. The compound (4) (see Fig. 1) accounted for the largest
proportion of the water-soluble metabolites (up to 94%). Inside the
leaf, there was also a decrease with time in the total amount of
omethoate but a proportionate increase with respect to dimethoate.
Here, compound (4) accounted for no more than 10% of the water-soluble
metabolites.
Hydrolisis at the alkoxy group occurred mainly inside the leaves,
indicating an enzymatic action at this site, which also held for
omethoate. As none of the thio-carboxy derivative (4a) was found in or
on plants, formation of (4) probably proceeded by oxidation of
dimethoate to omethoate followed by hydrolysis of the carbamoyl group.
As (4) was found in the largest amount on the surface, this cleavage
was probably non-enzymatic. This is an important indication of the
mode of transformation of omethoate on the plant. However, these
considerations are not supported by the observations of Hacskaylo and
Bull (1963) who, using the excised-leaf technique, found large amounts
of (4a) as a metabolite whilst the presence of even slight amounts of
(4) could not be established with any certainty.
Lucier and Menzer (1968, 1970) studied the metabolism of dimethoate on
bean plants. The most important neutral metabolites which they found
were compound (3) and an unidentified compound (X) although only in
small amounts as compared with omethoate, as is shown below
(proportions expressed in relation to omethoate = 1X%).
Days after treatment
1 3 6
(2) 1.0% 0.4% 0.4%
(3) 7.7% 7.2% 5.7%
(X) 29.0% 5.0% 2.2%
The ratio of phosphorodithioates to phosphorothioates in the
N-demethylation scheme was approximately 1:1, possibly indicating a
reduced rate of hydrolytic metabolism for the desulfurated compounds.
However, the small proportions of the compounds (X), (2), and (3), in
relation to omethoate, suggests that these compounds had merely a
transitory character. It is interesting to note that compound (4) was
not found in these studies.
The simple phosphates and thiophosphates (6) to (11) have also been
identified as metabolites in plants, or presumed to be present, after
application of dimethoate:
(6), (7), (11); Dautermann et al. (1960); Hacskaylo and Bull (1963)
(6); Rowlands (1966); Lucier and Menzer (1968)
(8), not significant; Hacskaylo and Bull (1963)
(8), (9), (10), (11) not significant; Looter and Menzer (1968)
In vitro hydrolysis of omethoate by grain extracts (Rowlands, 1966)
provided clear evidence of the formation of (4) in addition to (6);
compound (5) was also found.
 The behaviour of omethoate in the plant is characterized by a
relatively high degree of persistence, which at first seems surprising
in view of the fact that excuse usually hydrolyze more readily than
thionates. In actual fact, omethoate hydrolyzes at pH 6 and 25°C about
four times faster than dimethoate (Table II). Omethoate was found to
have a biological half-life of 9.3 days as against 3.2 days for
dimethoate in tomato plants which were placed in a solution of
32P-labelled compound for 24 hours and then held in distilled water
for 14 days (Grimmer et al., 1968b). Confirmation of this relatively
slow breakdown of omethoate on and in plants has been obtained
following field application of the compound.
TABLE II. RATES OF HYDROLYSIS (HALF-LIFE IN DAYS) AT 25°C
(GRIMMER ET AL., 1968a)
pH Dimethoate Omethoate (4a)+ (4)+
2 176 62 2 0.02
4 160 74 14 0.33
6 122 32.5 124 10
8 38 2.8 20 2.25
9++ 5.8 0.3 - -
+ The acids (4a) and (4) are stabilized by formation of
salt in neutral and weakly alkaline media.
++ At 21°C; Santi and de Pietri-Tonelli (1960).
Methods of residue analysis
Most of the methods proposed for the determination of omethoate
residues have been developed in association with methods for
dimethoate, of which it is the oxygen-analogue; for reviews of these
see de Pietri-Tonelli et al. (1965); Smart (1966); Bazzi (1966); Joint
Dimethoate Residues Panel (1968); FAO/WHO (1968).
All methods for the extraction of omethoate from plant material must
make allowance for the extremely high solubility of the compound in
water. The distribution coefficients for omethoate between water and
some organic solvents are as follows (Grimmer et al., 1968a):
chloroform 1.15 <(0.7*)
n-butanol 1.55
ethyl acetate 0.5
benzene 0.25 <(0.02*)
* Santi and de Pietri-Tonelli (1960)
Ethyl acetate in a strongly mineral-acid solution is suitable for the
extraction of compound (4) (Grimmer at al., 1968a).
The total phosphorus method is suitable for samples from supervised
trials which have not been treated with other phosphoric esters. A
simple method was recommended by the Joint Dimethoate Residues Panel
(maceration with acetone; extraction with chloroform; Al2O3 column
(grade V), elution with chloroform/carbon tetrachloride (1 + 1); wet
ashing; colorimetric phosphorus determination). After an additional
clean-up step (acetonitrile/n-hexane partitioning), the residues can
be identified by two-dimensional thin-layer chromatography. This
method of analysis has since been modified (Frehse, 1969) in order to
obtain better recoveries. The aqueous extract from the plant material
is extracted four times with chloroform; the Al2O3 column is
eluted with 150 ml of a mixture of chloroform/methanol (1 + 1) which
contains 1% glacial acetic acid to stabilize the residues. In most
cases, plant material poor in chlorophyll can be analysed without
using the Al2O3 column.
Steller and Curry (1964) describe a total phosphorus method
incorporating thin-layer chromatographic isolation for the
determination of dimethoate and omethoate. The method includes an
extraction of hexane, which is done before shaking out with
chloroform; extracts with a very high content of chlorophyll must be
further cleaned-up with diatomaceous earth and charcoal. A
column-chromatographic clean-up need not be done before the thin-layer
chromatography. The compound-containing spots are scraped, eluted and
analysed for phosphorus. The recoveries are between 55 and 72% for
apples (0.4-0.8 ppm), 81-94% for green tomatoes (0.16-0.32 ppm), and
72-87% for alfalfa (0.16-0.4 ppm). Details of the thin-layer
chromatography of omethoate are by Ackermann (1966) and Mendoza et al.
(1969).
Sissons and Telling (1970) describe a total phosphorus procedure which
is similar to the above-mentioned method recommended by the panel. The
extraction is done without adding acetone from the acidified aqueous
phase. The authors point out that the reason for the recoveries for
omethoate being lower than for other organophosphorus compounds was
due to significant volatilization losses which occurred during
Kaderna-Danish evaporation of the extracts. The recoveries obtained by
the proposed method are between 70 and 80% for an addition of 0.1-0.2
ppm.
With the increasing use of gas chromatography, some methods had been
proposed for determination of dimethoate and omethoate residues but
they were not sufficiently developed at the time the Joint Dimethoate
Residues Panol (1968) concluded its deliberations; however, several
promising stationary phases were described.
Bache and Lisk (1966) used the emission detector for determining
omethoate residues in soil. The soil is blended with chloroform and
the filtered solution, concentrated to a small volume, is directly
injected into the gas chromatograph: 6 ft × 3/16 in i.d. glass column
packed with 5% FFAP on 80/100 mesh Gas Chrom Q, 130°C, retention time
9.8 min. Recovery at the 5 ppm level was 86%; the limit of detection
was reported to be about 0.02 ppm. Beck et al. (1968) developed a
method for corn silage and milk. Silage is extracted with ethyl
acetate, and filtered. The solvent is removed and the residue
partitioned twice between 10 ml volumes of iso-octane and 1:4 (v/v)
water/acetonitrile. The iso-octane layers are discarded, and 5 ml of
methylene chloride is added to the acetonitrile layer; the mixture is
shaken. The lower layer (methylene chloride acetonitrile) is cleaned
up with Norite SG-extra, filtered through gelite and the filter cake
worked with ethyl acetate. The filtrate is evaporated just to dryness
and then diluted with ethyl acetate to a suitable volume for gas
chromatography: 4 ft glass column, 1/4 in o.d.; 5% Carbowax 20 M on
80/90 mesh Chromosorb W, acid washed and silanized, 120°C, flame
photometric detector (526 mµ), retention time six minutes, limit of
determination, 0.01 ppm. For milk analysis, 100 g aliquots are heated
in a 60°C water bath for 20 minutes to facilitate protein
precipitation. The samples are homogenized at room temperature with
200 ml of acetonitrile and are then subjected to a similar clean-up as
for the silage extracts. The limit of determination is stated to be
0.001 ppm.
Ruzicka et al. (1967) were able to determine omethoate with the aid of
a thermionic detector on a glass column (150 cm × 5 mm o.d.) packed
with 3% Apiezon L + 0.2% Epikote 1001 on
dimethyl-dichlorosilane-treated Chromosorb G, 70/80 mesh, at 165°C.
Bache and Lisk (1968), using the micro-wave powered helium emission
detector and an EC detector, obtained for omethoate, at 160°C, a
retention time of 3.6 minutes on a 2 ft × 3/16 in i.d. column packed
with 10% OV-17 on 80/100 mesh Gas Chrom Q; a slight but perceptible
tailing was observed. Sissons and Telling (1970) used a 5 ft × 1/8 in
glass column packed with 3% OV-17 on AWDMCS Chromosorb G, 80/100 mesh,
and obtained a retention time of 2.5 minutes at 225°C.
After comparing the behaviour of over 60 organophosphorus compounds on
three GDC columns, using a KCI thermionic detector, Watts and Storherr
(1969) found the following conditions to be the most suitable for
omethoate:
6 ft × 4 mm i.d. glass column, 2% diethylene glycol succinate
(C6 stabilized) on 80/100 mesh Gas Chrom Q, 210°C (retention
time of 3.7 minutes).
Watts et al. (1969) recommended an extraction with ethyl acetate
followed by charcoal clean-up for many organophosphorus pesticide
residues in crop extracts, especially in kale. Omethoate was included
in the studies and a recovery of 98% was obtained at the 0.5 ppm level
by gas chromatography using the above-mentioned method of Watts and
Storherr (1969).
Abbott et al. (1970) included omethoate, too, in their method for the
determination of organophosphorus pesticide residues in total diet
samples. Extracts from six groups of foods and milk are cleaned up and
examined using four different types of column and thermionic detectors
with caesium bromide tips. The authors chiefly report on their results
and unfortunately do not state which of the columns used is the most
suitable for omethoate. The AOAC multi-residue method is not suitable
for determination of omethoate because the compound is not eluted from
the column with the elution solvents used in this method (Pardue,
1971).
A simple method (Frehse, 1970) for fruits (including citrus fruits)
and vegetables operates as follows: 200 g of the sample is macerated
twice (500, 250 ml) with acetone, the cleaned-up filtrates are freed
from acetone on the rotary evaporator, the aqueous remainder is
rapidly cooled (at first under running tap water, then for one hour in
a refrigerator), filtered and extracted four times with 200 ml
portions of chloroform. The combined chloroform extracts are
evaporated to dryness and made up to a volume of 10 ml with acetone.
The gas-chromatographic determination is done on a 100 × 1.5 mm i.d.
glass column packed with 1.6% DC-200 + 0.6% Reoplex on 60/80 mesh
Chromosorb G, 185°C, using a thermionic detector giving a retention
time of 2.2 minutes. The recoveries are between 80 and 90% at the
0.05 - 1.0 ppm level. With some cole crops an interference peak may
appear at the position of omethoate which must be removed by
additional clean-up steps (see e.g. Watts et al. (1969)).
Fechner at al. (1969) published a method for determining dimethoate
and omethoate in milk. The milk sample is freed from fat by
centrifuging and extracted with methylene chloride at 5°C. The extract
is filtered and evaporated almost to dryness (0.25 ml), Aliquots of
the concentrate are chromatographed on Kieselgel G plates with
benzene/acetone (2+1); the compound spots are visualized
enzymatically, and evaluated semiquantitatively by comparison with
standards. In this way, omethoate residues can be determined down to a
level of 0.002 - 0.004 ppm. Fischer (1969) has described a thin-layer
chromatographic procedure for the identification and quantitative
determination of omethoate in biological material. Omethoate can be
semiquantitatively determined by thin-layer chromatography in
cucumbers, cherries and apples (Anonymous, 1970). After extraction
with ethanol and clean-up, the concentrated extracts are
chromatographed on Kieselgel G (pre-development with
chloroform/acetone 3 + 1), then chromatographed twice with
toluene/n-heptane/acetone (1 + 2 + 4). The compound spots are
visualized with thymol (bright spots on brownish background); 2 µg of
omethoate can be determined.
For regulatory purposes, a gas chromatographic procedure using either
flame photometric or phosphorus-sensitive thermionic detection would
be the method of choice. Thin-layer chromatography can be used to
obtain additional evidence of identity of the residue found.
EXAMPLES OF NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Country Crop Tolerance Safety
in ppm interval
in days
Australia Apples, citrus fruits, peaches 2.0 21
(provisional)
Austria General - 35
Belgium General - 21
Apples, pears 0.5 -
Brazila Deciduous fruits 2.0 -
Tomatoes, peppers 1.0 -
Vegetables, potatoes, beets 2.0 -
Bulgaria Tobacco - 0
Pome and stone fruit - 0
France General - 21
Italy General - 20
Netherlands General, field-grown 0.1 21
New Zealand General 0.5 21
South Africa General 1.5
Apples, pears - 28
Vines - 28
Spain General - 28
United Apples, pears 2 28
States of Beans (green, lima, snap, dry) 2 -
Americaa Broccoli, cauliflower 2 7
Cabbage 2 3
Lemons, oranges 2 15-45
Collards, endive, kale, lettuce )
(leaf), mustard greens, )
spinach, Swiss chard, turnips ) 2 14
(greens and roots) )
Lettuce (head), tomatoes 2 7
Peas, peppers 2 -
Melons 1 3
Potatoes 0.2 -
Pecans 0.1 21
Milk 0.002 -
Meat, fat and meat by-products
of cattle 0.02 -
Wheat (grain) 0.04 60
Wheat (green fodder and straw) 2 14
(Alfalfa, cotton, tobacco) - 14-28
a "....dimethoate and its oxygen analogue".
Appraisal
Omethoate, which is the oxygen analogue of dimethoate, is a systemic,
organophosphorus insecticide and acaricide, active against both
sucking and biting pests. Its chief uses are for pre-harvest
treatments of tree fruits, especially apples and pears, field crops
and vegetables. Omethoate is more persistent than dimethoate in plant
tissue but it degrades similarly to give esters of phosphoric and
thiophosphoric acid; metabolism in animals is also similar to that of
dimethoate. Residues are therefore determined only in terms of the
parent omethoate. Information was available regarding residues
resulting from supervised trials but no residue data from foodstuffs
moving in commerce or total diet studies was presented. Gas
chromatographic procedures are available for the determination of
residues of omethoate which can be adapted for regulatory purposes as
required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
Temporary tolerances
The following temporary tolerances are recommended for omethoate
residues. The pre-harvest interval shown is an indication of the basis
on which the recommendation is made:
Tolerance Pre-harvest
Fruit (ppm) interval (days)
Apples, grapes, peaches, pears 2 21
Apricots, cherries, plums 2 7
Insufficient information was available to support recommendations for
tolerances for omethoate residues on lettuce, potatoes, sugar beet or
hops.
Further work or information
Required before 30 June 1975
A long-term feeding study in at least one species of animal.
Desirable
1. Relevant observations in man.
2. More information on the quantitative aspect of the metabolism of
omethoate as compared with that of dimethoate.
3. Reproduction study in a non-rodent species.
4. Studies on the fate of the compound during storage, processing
and preparation of food for consumption.
5. Further data on residues occurring in use on lettuce, potatoes,
sugar beet and hops.
6. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R., and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pesticide Sci.,
1: 10-13
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschichtchromatographischer Trennung. Nahrung,
10: 273-274
Aharoni, A. H., and O'Brien, R. D. (1968) The inhibition of
acetylcholinesterases by anionic organophosphorus compounds. Biochem.,
7: 1538-1544
Anonymous. (1970) Methods zur Bestimmung von Dimethoate- und
PO-Dimethoat-rückständen in pflanzlichem Material. Nahrung, 14:
689-693
Bache, C. A. and Lisk, D. J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
gas chromatography, J. Assoc. Offic. Anal. Chem., 49: 647-650
Bache, C. A. and Lisk, D. J. (1968) Note on the versatility of OV-17
substrate for gas chromatography of pesticides. J. Assoc. Offic. Anal.
Chem., 51: 1270-1271
Bayer. (1971) Omethoate residues data. Unpublished report from
Farbenfabriken Bayer A. G.
Bazzi, B. (1966) Problemes relatifs au metabolisme et la determination
dans les produits vegetaux d'un ester phosphorique amide: le
dimethoate, Mededel. Rijksfaculteit Landbouwwetenschappen Gent, 31:
489-505
Beck, E. W., Johnson, jr J. C., Getz, M. E., Skinner, F. B., Dawsey,
L. H., Woodham, D. W., and Derbyshire, J. C. (1968) Effects of feeding
dimethoate, its oxygen analogue and dimethoate-treated silage to
cattle. J. Econ. Entomol., 61: 605-610
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. (1970) Acute toxicity
data for pesticides. World Rev. Pest Control, 9: 119-271
Brady, U. E. jr, and Arthur, B. W. (1963) Biological and chemical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56: 477-482
Chamberlain, W. F., Gatterdam, P. E., and Hopkins, D. E. (1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54: 733-740
Dauterman, W. C., Casida, J. E., Knaak, J. B. and Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agr. Food Chem., 7: 188-193
Dautermann, W. C., Viado, G. B., Casida, J. E. and O'Brien, R. D.
(1960) Persistence of dimethoate and metabolites following foliar
application to plants. J. Agr. Food Chem., 8: 115-119
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. Rogor (1965)
(dimethoate) residues in food crops. Residue Rev., 11: 60-99
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30: pp. 117-132
Fechner, G., Ackermann, H. and Lexow, B. (1969)
Dünnschichtchromatographische Bestimmung von Dimethcat und
PO-Dimethoat in Milch. Z. Lebensm.-Untersuch. -Forsch., 140: 145-149
Fischer, R. (1969) Identification of organophosphate insecticides in
biological material. IV. Arch. Toxikol., 25: 216-222
Frehse, H. (1969) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Frehse, H. (1970) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Grimmer, F., Dedek, W. and Leibnitz, E. (1968a) Zur Kenntnis der
N-Butylhomologen des Dimethoats Hydrolysegeschwindigkeit
und-15-metabolismes. Z. Naturforsch., 23B: 10-17
Grimmer, F., Dedek, W. and Leibnitz, E. (1968b) Zur Kenntnis der
N-Butylhomologen des Dimethoats. Verhalten in Tomatenpflangen. Z.
Naturforsch., 23B: 834-838
Hacskaylo, J. and Bull, D. L. (1963) Metabolism of dimethoate in
cotton leaves. J. Agr. Food Chem., 11: 464-466
Hassan, A., Zayed, S. M. A. D. and Bahig, M. R. E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18: 2429-2438
Hutchinson, E. B., McNerney, J. M. and Levinskas, G. J. (1968) Report
on oxygen analogue of Cygon-dimethoate: 90-day repeated feeding to
dogs (CL-28580). Unpublished report submitted by American Cyanamid
Company.
Joint Dimethoate Residues Panel. (1968) The determination of
dimethoate residues in fruits and vegetables. Analyst, 93: 756-766
Kimmerle, G. (1962) Toxicological studies on active ingredient
Dr Schrader S6876. Unpublished report submitted by Farbenfabriken
Bayer A. G.
Kimmerle, G. (1966) S6876 P-O-dimethoate (Bayer 45432; LO-NR 113a-1),
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. Bayer (1968) 45432-Toxikologische untersuchhungen.
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. (1969) Omethoate and dimethoate. Vergleichende
toxikologische untersuchungen bei rattengenerationsversuch bei ratten
mit omethoate. Unpublished report submitted by Farbenfabriken Bayer
A. G.
Kimmerle, G. and Lorke, D. (1967) S6876 (Lo-No.274). Antidotal
effect and potentiation. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Levinskas, G. J. and Shaffer, C. B. (1965a) Oxy-carboxy dimethoate:
thirty-three day repeated feeding to rats. Unpublished report
submitted by American Cyanamid Company.
Levinskas, G. J. and Shaffer, C. B. (1965b) Oxygen analogue of
dimethoate: demyelination studies in white leghorn hens. Unpublished
report submitted by American Cyanamid Company.
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmacol., 263: 237-238
Loser, E. and Lorke, D. (1967) Bayer 45432 - Subchronische
toxikologische untersuchungen an ratten. Unpublished report submitted
by Farbenfabriken Bayer, A. G.
Loser, E. (1968b) Bayer 45432 - Subakute toxikologische untersuchungen
an ratten. Unpublished report submitted by Farbenfabriken Bayer A. G.
Lucier, G. W. and Menzer, R. E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem., 16: 936-945
Lucier, G. W. and Menzer, R. E. (1970) Nature of oxidative metabolites
of dimethoate formed in rats, liver microsomes, and bean plants. J.
Agr. Food Chem., 18: 698-704
Mendoza, C. E., Grant, D. L., Braceland, B. and McCully, K. A. (1969)
Evaluation of esterases from livers of beef, pig, sheep, monkey, and
chicken for detection of some pesticides by thin-layer
chromatographic-enzyme inhibition technique. Analyst, 94: 805-810
Menzer, R. E. and Best, N. H. (1968) Effect of phenobarbital on the
toxicity of several organophosphorus insecticides. Toxicol. appl.
Pharmacol., 13: 37-42
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects, and mammals. Botyu-Kagaku,
31: 130-136
Pardue, J. R. (1971) Recovery of organophosphorus compounds using the
AOAC multiresidue method. J. Assoc. Offic. Anal. Chem., 54: 359-360
Rowlands, D. G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Food Agr.,
17: 90-93
Ruzicka, J., Thomson, J. and Wheals, B. B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatog., 30: 92-99
Sanderson, D. M. and Edson, E. F. (1964) Toxicological properties of
the organophosphorus insecticide dimethoate. Brit. J. industr. Med.,
21: 52-64
Santi, R. and de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido
O,O-dimetilditiofosforilacetico. Contrib. 1959, Ist Ric. Agr. Soc.
Montecatini, 3: 3-29
Sissons, J. D. and Telling, G. M. (1970) A screening procedure for
water soluble insecticides. J. Chromatog., 48: 468-477
Smart, N. A. (1966) An assessment of some methods of analysis for
dimethoate residues in fruit and vegetables. Analyst, 91: 621-624
Steller, W. A. and Curry, A. N. (1964) Measurement of residues of
Cygon insecticide and its oxygen analogue by total phosphorus
determination after isolation by thin-layer chromatography. J. Assoc.
Offic. Anal. Chem., 47: 645-651
Sutherland, G. L. (1962) Cholinesterase inhibitory activity of
dimethoate. Unpublished report submitted by American Cyanamid Company.
Villeneuve, D. C. and McKinley, W. P. (1968) Inhibition of beef liver
hydrolytic enzymes by organophosphorus pesticides. J. Agr. Food Chem.,
16: 290-294
Vince, A. A. and Spicer, E. J. F. (1971) Pathology report of Bay
45432-Subchronic toxicity in rats. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Watts, R. R. and Storherr, R. W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Offic. Anal. Chem., 52; 513-521
Watts, R. R., Storherr, R. W., Pardue, J. R. and Osgood, T. (1969)
Charcoal column cleanup method for many organophosphorus pesticide
residues in crop extracts. J. Assoc. Offic. Anal. Chem., 52: 522-526
The behaviour of omethoate in the plant is characterized by a
relatively high degree of persistence, which at first seems surprising
in view of the fact that excuse usually hydrolyze more readily than
thionates. In actual fact, omethoate hydrolyzes at pH 6 and 25°C about
four times faster than dimethoate (Table II). Omethoate was found to
have a biological half-life of 9.3 days as against 3.2 days for
dimethoate in tomato plants which were placed in a solution of
32P-labelled compound for 24 hours and then held in distilled water
for 14 days (Grimmer et al., 1968b). Confirmation of this relatively
slow breakdown of omethoate on and in plants has been obtained
following field application of the compound.
TABLE II. RATES OF HYDROLYSIS (HALF-LIFE IN DAYS) AT 25°C
(GRIMMER ET AL., 1968a)
pH Dimethoate Omethoate (4a)+ (4)+
2 176 62 2 0.02
4 160 74 14 0.33
6 122 32.5 124 10
8 38 2.8 20 2.25
9++ 5.8 0.3 - -
+ The acids (4a) and (4) are stabilized by formation of
salt in neutral and weakly alkaline media.
++ At 21°C; Santi and de Pietri-Tonelli (1960).
Methods of residue analysis
Most of the methods proposed for the determination of omethoate
residues have been developed in association with methods for
dimethoate, of which it is the oxygen-analogue; for reviews of these
see de Pietri-Tonelli et al. (1965); Smart (1966); Bazzi (1966); Joint
Dimethoate Residues Panel (1968); FAO/WHO (1968).
All methods for the extraction of omethoate from plant material must
make allowance for the extremely high solubility of the compound in
water. The distribution coefficients for omethoate between water and
some organic solvents are as follows (Grimmer et al., 1968a):
chloroform 1.15 <(0.7*)
n-butanol 1.55
ethyl acetate 0.5
benzene 0.25 <(0.02*)
* Santi and de Pietri-Tonelli (1960)
Ethyl acetate in a strongly mineral-acid solution is suitable for the
extraction of compound (4) (Grimmer at al., 1968a).
The total phosphorus method is suitable for samples from supervised
trials which have not been treated with other phosphoric esters. A
simple method was recommended by the Joint Dimethoate Residues Panel
(maceration with acetone; extraction with chloroform; Al2O3 column
(grade V), elution with chloroform/carbon tetrachloride (1 + 1); wet
ashing; colorimetric phosphorus determination). After an additional
clean-up step (acetonitrile/n-hexane partitioning), the residues can
be identified by two-dimensional thin-layer chromatography. This
method of analysis has since been modified (Frehse, 1969) in order to
obtain better recoveries. The aqueous extract from the plant material
is extracted four times with chloroform; the Al2O3 column is
eluted with 150 ml of a mixture of chloroform/methanol (1 + 1) which
contains 1% glacial acetic acid to stabilize the residues. In most
cases, plant material poor in chlorophyll can be analysed without
using the Al2O3 column.
Steller and Curry (1964) describe a total phosphorus method
incorporating thin-layer chromatographic isolation for the
determination of dimethoate and omethoate. The method includes an
extraction of hexane, which is done before shaking out with
chloroform; extracts with a very high content of chlorophyll must be
further cleaned-up with diatomaceous earth and charcoal. A
column-chromatographic clean-up need not be done before the thin-layer
chromatography. The compound-containing spots are scraped, eluted and
analysed for phosphorus. The recoveries are between 55 and 72% for
apples (0.4-0.8 ppm), 81-94% for green tomatoes (0.16-0.32 ppm), and
72-87% for alfalfa (0.16-0.4 ppm). Details of the thin-layer
chromatography of omethoate are by Ackermann (1966) and Mendoza et al.
(1969).
Sissons and Telling (1970) describe a total phosphorus procedure which
is similar to the above-mentioned method recommended by the panel. The
extraction is done without adding acetone from the acidified aqueous
phase. The authors point out that the reason for the recoveries for
omethoate being lower than for other organophosphorus compounds was
due to significant volatilization losses which occurred during
Kaderna-Danish evaporation of the extracts. The recoveries obtained by
the proposed method are between 70 and 80% for an addition of 0.1-0.2
ppm.
With the increasing use of gas chromatography, some methods had been
proposed for determination of dimethoate and omethoate residues but
they were not sufficiently developed at the time the Joint Dimethoate
Residues Panol (1968) concluded its deliberations; however, several
promising stationary phases were described.
Bache and Lisk (1966) used the emission detector for determining
omethoate residues in soil. The soil is blended with chloroform and
the filtered solution, concentrated to a small volume, is directly
injected into the gas chromatograph: 6 ft × 3/16 in i.d. glass column
packed with 5% FFAP on 80/100 mesh Gas Chrom Q, 130°C, retention time
9.8 min. Recovery at the 5 ppm level was 86%; the limit of detection
was reported to be about 0.02 ppm. Beck et al. (1968) developed a
method for corn silage and milk. Silage is extracted with ethyl
acetate, and filtered. The solvent is removed and the residue
partitioned twice between 10 ml volumes of iso-octane and 1:4 (v/v)
water/acetonitrile. The iso-octane layers are discarded, and 5 ml of
methylene chloride is added to the acetonitrile layer; the mixture is
shaken. The lower layer (methylene chloride acetonitrile) is cleaned
up with Norite SG-extra, filtered through gelite and the filter cake
worked with ethyl acetate. The filtrate is evaporated just to dryness
and then diluted with ethyl acetate to a suitable volume for gas
chromatography: 4 ft glass column, 1/4 in o.d.; 5% Carbowax 20 M on
80/90 mesh Chromosorb W, acid washed and silanized, 120°C, flame
photometric detector (526 mµ), retention time six minutes, limit of
determination, 0.01 ppm. For milk analysis, 100 g aliquots are heated
in a 60°C water bath for 20 minutes to facilitate protein
precipitation. The samples are homogenized at room temperature with
200 ml of acetonitrile and are then subjected to a similar clean-up as
for the silage extracts. The limit of determination is stated to be
0.001 ppm.
Ruzicka et al. (1967) were able to determine omethoate with the aid of
a thermionic detector on a glass column (150 cm × 5 mm o.d.) packed
with 3% Apiezon L + 0.2% Epikote 1001 on
dimethyl-dichlorosilane-treated Chromosorb G, 70/80 mesh, at 165°C.
Bache and Lisk (1968), using the micro-wave powered helium emission
detector and an EC detector, obtained for omethoate, at 160°C, a
retention time of 3.6 minutes on a 2 ft × 3/16 in i.d. column packed
with 10% OV-17 on 80/100 mesh Gas Chrom Q; a slight but perceptible
tailing was observed. Sissons and Telling (1970) used a 5 ft × 1/8 in
glass column packed with 3% OV-17 on AWDMCS Chromosorb G, 80/100 mesh,
and obtained a retention time of 2.5 minutes at 225°C.
After comparing the behaviour of over 60 organophosphorus compounds on
three GDC columns, using a KCI thermionic detector, Watts and Storherr
(1969) found the following conditions to be the most suitable for
omethoate:
6 ft × 4 mm i.d. glass column, 2% diethylene glycol succinate
(C6 stabilized) on 80/100 mesh Gas Chrom Q, 210°C (retention
time of 3.7 minutes).
Watts et al. (1969) recommended an extraction with ethyl acetate
followed by charcoal clean-up for many organophosphorus pesticide
residues in crop extracts, especially in kale. Omethoate was included
in the studies and a recovery of 98% was obtained at the 0.5 ppm level
by gas chromatography using the above-mentioned method of Watts and
Storherr (1969).
Abbott et al. (1970) included omethoate, too, in their method for the
determination of organophosphorus pesticide residues in total diet
samples. Extracts from six groups of foods and milk are cleaned up and
examined using four different types of column and thermionic detectors
with caesium bromide tips. The authors chiefly report on their results
and unfortunately do not state which of the columns used is the most
suitable for omethoate. The AOAC multi-residue method is not suitable
for determination of omethoate because the compound is not eluted from
the column with the elution solvents used in this method (Pardue,
1971).
A simple method (Frehse, 1970) for fruits (including citrus fruits)
and vegetables operates as follows: 200 g of the sample is macerated
twice (500, 250 ml) with acetone, the cleaned-up filtrates are freed
from acetone on the rotary evaporator, the aqueous remainder is
rapidly cooled (at first under running tap water, then for one hour in
a refrigerator), filtered and extracted four times with 200 ml
portions of chloroform. The combined chloroform extracts are
evaporated to dryness and made up to a volume of 10 ml with acetone.
The gas-chromatographic determination is done on a 100 × 1.5 mm i.d.
glass column packed with 1.6% DC-200 + 0.6% Reoplex on 60/80 mesh
Chromosorb G, 185°C, using a thermionic detector giving a retention
time of 2.2 minutes. The recoveries are between 80 and 90% at the
0.05 - 1.0 ppm level. With some cole crops an interference peak may
appear at the position of omethoate which must be removed by
additional clean-up steps (see e.g. Watts et al. (1969)).
Fechner at al. (1969) published a method for determining dimethoate
and omethoate in milk. The milk sample is freed from fat by
centrifuging and extracted with methylene chloride at 5°C. The extract
is filtered and evaporated almost to dryness (0.25 ml), Aliquots of
the concentrate are chromatographed on Kieselgel G plates with
benzene/acetone (2+1); the compound spots are visualized
enzymatically, and evaluated semiquantitatively by comparison with
standards. In this way, omethoate residues can be determined down to a
level of 0.002 - 0.004 ppm. Fischer (1969) has described a thin-layer
chromatographic procedure for the identification and quantitative
determination of omethoate in biological material. Omethoate can be
semiquantitatively determined by thin-layer chromatography in
cucumbers, cherries and apples (Anonymous, 1970). After extraction
with ethanol and clean-up, the concentrated extracts are
chromatographed on Kieselgel G (pre-development with
chloroform/acetone 3 + 1), then chromatographed twice with
toluene/n-heptane/acetone (1 + 2 + 4). The compound spots are
visualized with thymol (bright spots on brownish background); 2 µg of
omethoate can be determined.
For regulatory purposes, a gas chromatographic procedure using either
flame photometric or phosphorus-sensitive thermionic detection would
be the method of choice. Thin-layer chromatography can be used to
obtain additional evidence of identity of the residue found.
EXAMPLES OF NATIONAL TOLERANCES AND WITHHOLDING PERIODS
Country Crop Tolerance Safety
in ppm interval
in days
Australia Apples, citrus fruits, peaches 2.0 21
(provisional)
Austria General - 35
Belgium General - 21
Apples, pears 0.5 -
Brazila Deciduous fruits 2.0 -
Tomatoes, peppers 1.0 -
Vegetables, potatoes, beets 2.0 -
Bulgaria Tobacco - 0
Pome and stone fruit - 0
France General - 21
Italy General - 20
Netherlands General, field-grown 0.1 21
New Zealand General 0.5 21
South Africa General 1.5
Apples, pears - 28
Vines - 28
Spain General - 28
United Apples, pears 2 28
States of Beans (green, lima, snap, dry) 2 -
Americaa Broccoli, cauliflower 2 7
Cabbage 2 3
Lemons, oranges 2 15-45
Collards, endive, kale, lettuce )
(leaf), mustard greens, )
spinach, Swiss chard, turnips ) 2 14
(greens and roots) )
Lettuce (head), tomatoes 2 7
Peas, peppers 2 -
Melons 1 3
Potatoes 0.2 -
Pecans 0.1 21
Milk 0.002 -
Meat, fat and meat by-products
of cattle 0.02 -
Wheat (grain) 0.04 60
Wheat (green fodder and straw) 2 14
(Alfalfa, cotton, tobacco) - 14-28
a "....dimethoate and its oxygen analogue".
Appraisal
Omethoate, which is the oxygen analogue of dimethoate, is a systemic,
organophosphorus insecticide and acaricide, active against both
sucking and biting pests. Its chief uses are for pre-harvest
treatments of tree fruits, especially apples and pears, field crops
and vegetables. Omethoate is more persistent than dimethoate in plant
tissue but it degrades similarly to give esters of phosphoric and
thiophosphoric acid; metabolism in animals is also similar to that of
dimethoate. Residues are therefore determined only in terms of the
parent omethoate. Information was available regarding residues
resulting from supervised trials but no residue data from foodstuffs
moving in commerce or total diet studies was presented. Gas
chromatographic procedures are available for the determination of
residues of omethoate which can be adapted for regulatory purposes as
required.
RECOMMENDATIONS FOR TOLERANCES, TEMPORARY TOLERANCES
OR PRACTICAL RESIDUE LIMITS
Temporary tolerances
The following temporary tolerances are recommended for omethoate
residues. The pre-harvest interval shown is an indication of the basis
on which the recommendation is made:
Tolerance Pre-harvest
Fruit (ppm) interval (days)
Apples, grapes, peaches, pears 2 21
Apricots, cherries, plums 2 7
Insufficient information was available to support recommendations for
tolerances for omethoate residues on lettuce, potatoes, sugar beet or
hops.
Further work or information
Required before 30 June 1975
A long-term feeding study in at least one species of animal.
Desirable
1. Relevant observations in man.
2. More information on the quantitative aspect of the metabolism of
omethoate as compared with that of dimethoate.
3. Reproduction study in a non-rodent species.
4. Studies on the fate of the compound during storage, processing
and preparation of food for consumption.
5. Further data on residues occurring in use on lettuce, potatoes,
sugar beet and hops.
6. Information on residues occurring in food in commerce and in
total diet studies.
REFERENCES
Abbott, D. C., Crisp, S., Tarrant, K. R., and Tatton, J. O'G. (1970)
Organophosphorus pesticide residues in the total diet. Pesticide Sci.,
1: 10-13
Ackermann, H. (1966) Enzymatischer Nachweis phosphororganischer
Insektizide nach dünnschichtchromatographischer Trennung. Nahrung,
10: 273-274
Aharoni, A. H., and O'Brien, R. D. (1968) The inhibition of
acetylcholinesterases by anionic organophosphorus compounds. Biochem.,
7: 1538-1544
Anonymous. (1970) Methods zur Bestimmung von Dimethoate- und
PO-Dimethoat-rückständen in pflanzlichem Material. Nahrung, 14:
689-693
Bache, C. A. and Lisk, D. J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
gas chromatography, J. Assoc. Offic. Anal. Chem., 49: 647-650
Bache, C. A. and Lisk, D. J. (1968) Note on the versatility of OV-17
substrate for gas chromatography of pesticides. J. Assoc. Offic. Anal.
Chem., 51: 1270-1271
Bayer. (1971) Omethoate residues data. Unpublished report from
Farbenfabriken Bayer A. G.
Bazzi, B. (1966) Problemes relatifs au metabolisme et la determination
dans les produits vegetaux d'un ester phosphorique amide: le
dimethoate, Mededel. Rijksfaculteit Landbouwwetenschappen Gent, 31:
489-505
Beck, E. W., Johnson, jr J. C., Getz, M. E., Skinner, F. B., Dawsey,
L. H., Woodham, D. W., and Derbyshire, J. C. (1968) Effects of feeding
dimethoate, its oxygen analogue and dimethoate-treated silage to
cattle. J. Econ. Entomol., 61: 605-610
Ben-Dyke, R., Sanderson, D. M. and Noakes, D. N. (1970) Acute toxicity
data for pesticides. World Rev. Pest Control, 9: 119-271
Brady, U. E. jr, and Arthur, B. W. (1963) Biological and chemical
properties of dimethoate and related derivatives. J. Econ. Entomol.,
56: 477-482
Chamberlain, W. F., Gatterdam, P. E., and Hopkins, D. E. (1961) The
metabolism of P32 labelled dimethoate in sheep. J. Econ. Entomol.,
54: 733-740
Dauterman, W. C., Casida, J. E., Knaak, J. B. and Kowalczyk, T. (1959)
Bovine metabolism of organophosphorus insecticides. Metabolism and
residues associated with oral administration of dimethoate to rats and
three lactating cows. J. Agr. Food Chem., 7: 188-193
Dautermann, W. C., Viado, G. B., Casida, J. E. and O'Brien, R. D.
(1960) Persistence of dimethoate and metabolites following foliar
application to plants. J. Agr. Food Chem., 8: 115-119
de Pietri-Tonelli, P., Bazzi, B. and Santi, R. Rogor (1965)
(dimethoate) residues in food crops. Residue Rev., 11: 60-99
FAO/WHO. (1968) 1967 evaluations of some pesticide residues in food.
FAO/PL: 1967/M/11/1; WHO/Food Add./68.30: pp. 117-132
Fechner, G., Ackermann, H. and Lexow, B. (1969)
Dünnschichtchromatographische Bestimmung von Dimethcat und
PO-Dimethoat in Milch. Z. Lebensm.-Untersuch. -Forsch., 140: 145-149
Fischer, R. (1969) Identification of organophosphate insecticides in
biological material. IV. Arch. Toxikol., 25: 216-222
Frehse, H. (1969) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Frehse, H. (1970) Bayer, Pflanzenschutz ATEA, Biologische Forschung,
(unpublished)
Grimmer, F., Dedek, W. and Leibnitz, E. (1968a) Zur Kenntnis der
N-Butylhomologen des Dimethoats Hydrolysegeschwindigkeit
und-15-metabolismes. Z. Naturforsch., 23B: 10-17
Grimmer, F., Dedek, W. and Leibnitz, E. (1968b) Zur Kenntnis der
N-Butylhomologen des Dimethoats. Verhalten in Tomatenpflangen. Z.
Naturforsch., 23B: 834-838
Hacskaylo, J. and Bull, D. L. (1963) Metabolism of dimethoate in
cotton leaves. J. Agr. Food Chem., 11: 464-466
Hassan, A., Zayed, S. M. A. D. and Bahig, M. R. E. (1969) Metabolism
of organophosphorus insecticides. XI. Metabolic fate of dimethoate in
the rat. Biochem. Pharmacol., 18: 2429-2438
Hutchinson, E. B., McNerney, J. M. and Levinskas, G. J. (1968) Report
on oxygen analogue of Cygon-dimethoate: 90-day repeated feeding to
dogs (CL-28580). Unpublished report submitted by American Cyanamid
Company.
Joint Dimethoate Residues Panel. (1968) The determination of
dimethoate residues in fruits and vegetables. Analyst, 93: 756-766
Kimmerle, G. (1962) Toxicological studies on active ingredient
Dr Schrader S6876. Unpublished report submitted by Farbenfabriken
Bayer A. G.
Kimmerle, G. (1966) S6876 P-O-dimethoate (Bayer 45432; LO-NR 113a-1),
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. Bayer (1968) 45432-Toxikologische untersuchhungen.
Unpublished report submitted by Farbenfabriken Bayer A. G.
Kimmerle, G. (1969) Omethoate and dimethoate. Vergleichende
toxikologische untersuchungen bei rattengenerationsversuch bei ratten
mit omethoate. Unpublished report submitted by Farbenfabriken Bayer
A. G.
Kimmerle, G. and Lorke, D. (1967) S6876 (Lo-No.274). Antidotal
effect and potentiation. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Levinskas, G. J. and Shaffer, C. B. (1965a) Oxy-carboxy dimethoate:
thirty-three day repeated feeding to rats. Unpublished report
submitted by American Cyanamid Company.
Levinskas, G. J. and Shaffer, C. B. (1965b) Oxygen analogue of
dimethoate: demyelination studies in white leghorn hens. Unpublished
report submitted by American Cyanamid Company.
Lorke, D. and Kimmerle, G. (1969) The action of reactivators in
phosphoric acid ester poisoning. Arch. Pharmacol., 263: 237-238
Loser, E. and Lorke, D. (1967) Bayer 45432 - Subchronische
toxikologische untersuchungen an ratten. Unpublished report submitted
by Farbenfabriken Bayer, A. G.
Loser, E. (1968b) Bayer 45432 - Subakute toxikologische untersuchungen
an ratten. Unpublished report submitted by Farbenfabriken Bayer A. G.
Lucier, G. W. and Menzer, R. E. (1968) Metabolism of dimethoate in
bean plants in relation to its mode of application. J. Agr. Food
Chem., 16: 936-945
Lucier, G. W. and Menzer, R. E. (1970) Nature of oxidative metabolites
of dimethoate formed in rats, liver microsomes, and bean plants. J.
Agr. Food Chem., 18: 698-704
Mendoza, C. E., Grant, D. L., Braceland, B. and McCully, K. A. (1969)
Evaluation of esterases from livers of beef, pig, sheep, monkey, and
chicken for detection of some pesticides by thin-layer
chromatographic-enzyme inhibition technique. Analyst, 94: 805-810
Menzer, R. E. and Best, N. H. (1968) Effect of phenobarbital on the
toxicity of several organophosphorus insecticides. Toxicol. appl.
Pharmacol., 13: 37-42
Morikawa, O. and Saito, T. (1966) Degradations of vamidothion and
dimethoate in plants, insects, and mammals. Botyu-Kagaku,
31: 130-136
Pardue, J. R. (1971) Recovery of organophosphorus compounds using the
AOAC multiresidue method. J. Assoc. Offic. Anal. Chem., 54: 359-360
Rowlands, D. G. (1966) The in vitro and in vivo metabolism of
dimethoate by stored wheat and sorghum grains. J. Sci. Food Agr.,
17: 90-93
Ruzicka, J., Thomson, J. and Wheals, B. B. (1967) The
gas-chromatographic examination of organophosphorus pesticides and
their oxidation products. J. Chromatog., 30: 92-99
Sanderson, D. M. and Edson, E. F. (1964) Toxicological properties of
the organophosphorus insecticide dimethoate. Brit. J. industr. Med.,
21: 52-64
Santi, R. and de Pietri-Tonelli, P. (1960) Ricerche sul meccanismo
d'azione della N-monometilammide dell'acido
O,O-dimetilditiofosforilacetico. Contrib. 1959, Ist Ric. Agr. Soc.
Montecatini, 3: 3-29
Sissons, J. D. and Telling, G. M. (1970) A screening procedure for
water soluble insecticides. J. Chromatog., 48: 468-477
Smart, N. A. (1966) An assessment of some methods of analysis for
dimethoate residues in fruit and vegetables. Analyst, 91: 621-624
Steller, W. A. and Curry, A. N. (1964) Measurement of residues of
Cygon insecticide and its oxygen analogue by total phosphorus
determination after isolation by thin-layer chromatography. J. Assoc.
Offic. Anal. Chem., 47: 645-651
Sutherland, G. L. (1962) Cholinesterase inhibitory activity of
dimethoate. Unpublished report submitted by American Cyanamid Company.
Villeneuve, D. C. and McKinley, W. P. (1968) Inhibition of beef liver
hydrolytic enzymes by organophosphorus pesticides. J. Agr. Food Chem.,
16: 290-294
Vince, A. A. and Spicer, E. J. F. (1971) Pathology report of Bay
45432-Subchronic toxicity in rats. Unpublished report submitted by
Farbenfabriken Bayer A. G.
Watts, R. R. and Storherr, R. W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Offic. Anal. Chem., 52; 513-521
Watts, R. R., Storherr, R. W., Pardue, J. R. and Osgood, T. (1969)
Charcoal column cleanup method for many organophosphorus pesticide
residues in crop extracts. J. Assoc. Offic. Anal. Chem., 52: 522-526
