
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
OMETHOATE
Explanation
Omethoate was evaluated in 1971 and reviewed in 1975, 1978 and
1979 (FAO/WHO 1972b, 1976b, 1979b and 1980b).* A temporary ADI was
established in 1971 and the submission of a long-term study required.
In addition to the report of the long-term study in rats, the results
of a multi-generation study in rats, of an acute oral study, of a
mutagenicity study, degradation in soil and data on residues from
supervised trials have been received and are included in this
monograph addendum.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Acute toxicity
LD50 of omethoate metabolite in male rats, dosed orally with
aqueous solution, was 908 mg/kg (Flucke 1978).
Long-term studies
Rat
Four groups of 50 male and 50 female Wistar rats were maintained
for 24 months on a diet containing omethoate at concentrations of 0.3,
1, 0, 3 and 10 mg/kg. The control group consisted of 100 males and 100
females.
Omethoate did not clearly affect behaviour, body weight, survival
rate, food intake, haematological parameters, clinical chemistry and
urinalysis.
Plasma and erythrocyte cholinesterase activities, measured in 5
males and 5 females of each group at 1, 2, 4, 8, 13, 26, 52 and 78
weeks and at the end of the study, were statistically significantly
depressed in both sexes at 10 mg/kg diet. Erythrocyte cholinesterase
activity was also inhibited in both sexes in the 3 mg/kg diet group.
The suppression of brain cholinesterase, as measured in 10 males and
10 females per group, was dose-related in the 3 and 10 mg/kg diet
group in both sexes. In addition, brain cholinesterase was also
significantly affected in females in the 1 mg/kg group, which can be
considered as a marginal no-effect level.
* See Annex II for FAO and WHO documentation.
Gross and microscopic examination revealed no indication of a
diverse effect of omethoate. The tumour incidence was not clearly
affected by treatment (Bomhard et al 1979).
Special studies on reproduction
Groups of FB30 Long Evans rats (10 males and 20 females per
group) were fed omethoate (94%) in the diet at concentrations of 0, 1,
3 and 10 ppm for about 10 weeks, after which they were mated to
initiate a standard 3-generation, 2-litter-per-generation,
reproduction study. Four days after birth, the litters were reduced to
10. Immediately after birth, litters were examined for malformations.
At the age of 8 weeks, the offspring were sacrificed and subjected to
gross examination. Ten males and 10 female rats of the F3b generation
of all dose groups were examined histopathologically 4 weeks after
birth.
There were no clear effects either on mating performance and
pregnancy rate, mortality, or type and distribution of abnormalities.
In the second generation the litter size was reduced in the 3 and
10 ppm groups.
In the F2b litters, litter size was reduced at both 3 and 10 ppm
after 4 days and at 10 ppm only after 28 days. Since this effect was
observed only in a single progeny generation, 3 ppm could be
considered as a no-effect level with regard to reproduction (Löser
1981).
Special studies on mutagenicity
In a micronucleus test, male and female mice were given
2 × 6 mg/kg bw or 2 × 12 mg/ kg bw at 30 and 6h before necropsy.
Omethoate did not increase the frequency of micro-nucleated
erythrocytes in the bone marrow. The animals, especially those in the
high-dose group, showed clear clinical symptoms (Herbold 1981).
Omethoate was examined for mutagenic activity in a series of
in vitro microbiological assays, using the Salmonella typhimurium
strains TA 1535, TA 100, TA 1537 and TA 98. Each plate was run with
and without liver homogenate (S-9 mixture). Omethoate was mutagenic
in strain TA 1535 (2 experiments), TA 100 (2 experiments) and TA 98 (1
experiment). No mutagenic activity could be observed in strain TA 1537
(Herbold 1980).
RESIDUES IN FOOD
RESIDUES RESULTING FROM SUPERVISED TRIALS
Some information was provided (Bayer 1981) on residues of
omethoate occurring on potatoes and sugarbeet following repeated (3 or
4) treatments at recommended levels. In all cases, residues at harvest
(about 35 and 80 days after last treatment respectively) were below
the level of determination of 0.01 mg/kg. Steadily declining residues
were found on sugarbeet leaves (3, 0.9, 0.4, 0.2 and 0.1 mg/kg at 0,
14, 28 and 35 days respectively); at harvest (85 days) no detectable
residue remained.
FATE OF RESIDUES
In soil
Omethoate is rapidly degraded in soil. By 16 days after
application of 14C-labelled omethoate to a standard soil, 48% of the
14C activity had been eliminated as 14CO2 (Wagner et al 1978). As
intermediates in this degradation, the following conversion products,
shown in Figure 1, were isolated and identified by NMR and MS:
I Omethoate
II (Phosphonothio)acetic acid
III O-Methyl S-methylcarbamoylmethyl hydrogen phosphorothioate
IV 2-Mercapto-N-methylacetamide
V N, N'-Dimethyl-2,2'-dithiodi(acetamide)
VI 2-(Methylcarbamoylmethyldithio)acetic acid
VII N-Methyl-2-methylsulphinylacetamide
VIII N-Methyl-2-methylsulphonylacetamide
IX N,N' -Dimethyl(thio-oxamide)
X N,N'Dimethyloxamide
XI N-Methyloxamic acid
XII Dimethyl hydrogen phosphate
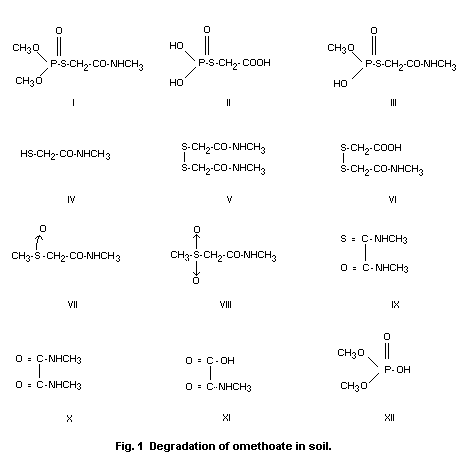 Of the compounds identified, amounts of I, II, III, V, VI and
VIII were measured over a period of 112 days as shown in Table 1. The
other materials listed were shown to be labile intermediates in the
degradation of omethoate in soil. Similar information is being
obtained on the degradation of omethoate in plant tissue.
TABLE 1. Degradation pattern of 14C-omethoate in soil
% of 14C applied
Days I II III+ VI1 V VIII Total
9 37 3.4 4.2 2.6 <0.1 49
21 29 5.2 4.0 2.3 0.8 45
49 17 6.3 4.9 1.3 1.8 32
85 2.4 0.7 3.9 0.2 2.4 11
112 <1 <0.3 3.5 <0.1 2.7 7
1 III and VI were not separated in these determinations.
EVALUATION
COMMENTS AND APPRAISAL
In a long-term study in rats, at levels of 3 and 10 ppm in the
diet, a clear indication of cholinesterase inhibition was evident in
brain, plasma and red blood cell cholinesterase. At the 1 ppm level,
no clear effect was observed on cholinesterase activity in plasma
or red cells in either sex. However, in females receiving 1 ppm
omethoate in the diet a slight depression (ca. 12%) of brain
acetylcholinesterase was observed. A no-effect level of 3 ppm in the
diet could be estimated from a rat 3-generation reproduction study.
Omethoate was mutagenic in strains TA 1535, 100 and 98 of
Salmonella typhimurium used in the Ames test. However, no activity
was observed in TA 1537. No mutagenic activity was detected in a
micronucleus test and a dominant lethal test previously evaluated. A
long-term rat study did not indicate carcinogenic potential. In this
study, the highest dose level (10 ppm) failed to elicit any effect
other than cholinesterase depression. The relatively low dose levels
used in this study preclude adequate carcinogenicity evaluation.
Therefore, the temporary ADI has been extended. Submission of results
of carcinogenicity studies at higher levels in a rodent species is
required by 1985.
Information regarding the degradation of omethoate in soil was
reviewed, together with a limited amount of additional data on
residues from supervised trials on potatoes and sugarbeet.
Levels causing no toxicological effect
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
No change is suggested in the maximum residue limits previously
recommended.
FURTHER WORK OR INFORMATION
Required (by 1985)
Carcinogenicity studies in a rodent species at higher dose
levels.
Desirable
Clarification of the mutagenic potential in micro-organisms, in
respect to the "marginal effect" of omethoate in some strains of
Salmonella.
REFERENCES
Bayer Reports on omethoate residue trials. Data from Bayer AG,
1981 Leverkusen. (Unpublished)
Bomhard, E. S-6876 - Chronische Toxizität an Ratten 22 Oktober,
1980 Stellungnahme zu Bericht Nr. 8607. Report of the Bayer AG
Institut für Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Bomhard, E., Löser, E. and Kaliner, G. S-6876 - chronic toxicity study
1979 on rats (two-year feeding experiment). Report of the Bayer
AG Institut f. Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Flucke, W. Akute orale Toxizität "Omethoat-Metabolit" 23 Oktober.
1978 Report of the Bayer AG Institut für Toxikologie, submitted
to WHO by Bayer AG; (Unpublished)
Herbold, B. S-6876-Salmonella/Mikrosomen-Test zur Untersuchung auf
1980 punktmutagene Wirking. Report of the Bayer AG Institut für
Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Löser, E. S-6876-Folimat-Wirkstoff Multigenerationeversuche an Ratten
1981 21 Januar, Bericht Nr. 9731. Report of the Bayer AG Institut
für Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Addendum: Histopathology - Consultox Laboratories Ltd.,
London.
Wagner, K., Neitzel, H. and Oehlmann, L. Metabolismus von Omethoat im
1978 Boden. Report submitted by Bayer AG, Leverkusen.
(Unpublished)
Of the compounds identified, amounts of I, II, III, V, VI and
VIII were measured over a period of 112 days as shown in Table 1. The
other materials listed were shown to be labile intermediates in the
degradation of omethoate in soil. Similar information is being
obtained on the degradation of omethoate in plant tissue.
TABLE 1. Degradation pattern of 14C-omethoate in soil
% of 14C applied
Days I II III+ VI1 V VIII Total
9 37 3.4 4.2 2.6 <0.1 49
21 29 5.2 4.0 2.3 0.8 45
49 17 6.3 4.9 1.3 1.8 32
85 2.4 0.7 3.9 0.2 2.4 11
112 <1 <0.3 3.5 <0.1 2.7 7
1 III and VI were not separated in these determinations.
EVALUATION
COMMENTS AND APPRAISAL
In a long-term study in rats, at levels of 3 and 10 ppm in the
diet, a clear indication of cholinesterase inhibition was evident in
brain, plasma and red blood cell cholinesterase. At the 1 ppm level,
no clear effect was observed on cholinesterase activity in plasma
or red cells in either sex. However, in females receiving 1 ppm
omethoate in the diet a slight depression (ca. 12%) of brain
acetylcholinesterase was observed. A no-effect level of 3 ppm in the
diet could be estimated from a rat 3-generation reproduction study.
Omethoate was mutagenic in strains TA 1535, 100 and 98 of
Salmonella typhimurium used in the Ames test. However, no activity
was observed in TA 1537. No mutagenic activity was detected in a
micronucleus test and a dominant lethal test previously evaluated. A
long-term rat study did not indicate carcinogenic potential. In this
study, the highest dose level (10 ppm) failed to elicit any effect
other than cholinesterase depression. The relatively low dose levels
used in this study preclude adequate carcinogenicity evaluation.
Therefore, the temporary ADI has been extended. Submission of results
of carcinogenicity studies at higher levels in a rodent species is
required by 1985.
Information regarding the degradation of omethoate in soil was
reviewed, together with a limited amount of additional data on
residues from supervised trials on potatoes and sugarbeet.
Levels causing no toxicological effect
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
No change is suggested in the maximum residue limits previously
recommended.
FURTHER WORK OR INFORMATION
Required (by 1985)
Carcinogenicity studies in a rodent species at higher dose
levels.
Desirable
Clarification of the mutagenic potential in micro-organisms, in
respect to the "marginal effect" of omethoate in some strains of
Salmonella.
REFERENCES
Bayer Reports on omethoate residue trials. Data from Bayer AG,
1981 Leverkusen. (Unpublished)
Bomhard, E. S-6876 - Chronische Toxizität an Ratten 22 Oktober,
1980 Stellungnahme zu Bericht Nr. 8607. Report of the Bayer AG
Institut für Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Bomhard, E., Löser, E. and Kaliner, G. S-6876 - chronic toxicity study
1979 on rats (two-year feeding experiment). Report of the Bayer
AG Institut f. Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Flucke, W. Akute orale Toxizität "Omethoat-Metabolit" 23 Oktober.
1978 Report of the Bayer AG Institut für Toxikologie, submitted
to WHO by Bayer AG; (Unpublished)
Herbold, B. S-6876-Salmonella/Mikrosomen-Test zur Untersuchung auf
1980 punktmutagene Wirking. Report of the Bayer AG Institut für
Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Löser, E. S-6876-Folimat-Wirkstoff Multigenerationeversuche an Ratten
1981 21 Januar, Bericht Nr. 9731. Report of the Bayer AG Institut
für Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Addendum: Histopathology - Consultox Laboratories Ltd.,
London.
Wagner, K., Neitzel, H. and Oehlmann, L. Metabolismus von Omethoat im
1978 Boden. Report submitted by Bayer AG, Leverkusen.
(Unpublished)
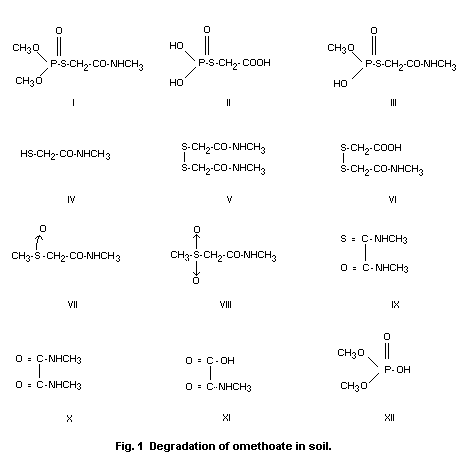 Of the compounds identified, amounts of I, II, III, V, VI and
VIII were measured over a period of 112 days as shown in Table 1. The
other materials listed were shown to be labile intermediates in the
degradation of omethoate in soil. Similar information is being
obtained on the degradation of omethoate in plant tissue.
TABLE 1. Degradation pattern of 14C-omethoate in soil
% of 14C applied
Days I II III+ VI1 V VIII Total
9 37 3.4 4.2 2.6 <0.1 49
21 29 5.2 4.0 2.3 0.8 45
49 17 6.3 4.9 1.3 1.8 32
85 2.4 0.7 3.9 0.2 2.4 11
112 <1 <0.3 3.5 <0.1 2.7 7
1 III and VI were not separated in these determinations.
EVALUATION
COMMENTS AND APPRAISAL
In a long-term study in rats, at levels of 3 and 10 ppm in the
diet, a clear indication of cholinesterase inhibition was evident in
brain, plasma and red blood cell cholinesterase. At the 1 ppm level,
no clear effect was observed on cholinesterase activity in plasma
or red cells in either sex. However, in females receiving 1 ppm
omethoate in the diet a slight depression (ca. 12%) of brain
acetylcholinesterase was observed. A no-effect level of 3 ppm in the
diet could be estimated from a rat 3-generation reproduction study.
Omethoate was mutagenic in strains TA 1535, 100 and 98 of
Salmonella typhimurium used in the Ames test. However, no activity
was observed in TA 1537. No mutagenic activity was detected in a
micronucleus test and a dominant lethal test previously evaluated. A
long-term rat study did not indicate carcinogenic potential. In this
study, the highest dose level (10 ppm) failed to elicit any effect
other than cholinesterase depression. The relatively low dose levels
used in this study preclude adequate carcinogenicity evaluation.
Therefore, the temporary ADI has been extended. Submission of results
of carcinogenicity studies at higher levels in a rodent species is
required by 1985.
Information regarding the degradation of omethoate in soil was
reviewed, together with a limited amount of additional data on
residues from supervised trials on potatoes and sugarbeet.
Levels causing no toxicological effect
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
No change is suggested in the maximum residue limits previously
recommended.
FURTHER WORK OR INFORMATION
Required (by 1985)
Carcinogenicity studies in a rodent species at higher dose
levels.
Desirable
Clarification of the mutagenic potential in micro-organisms, in
respect to the "marginal effect" of omethoate in some strains of
Salmonella.
REFERENCES
Bayer Reports on omethoate residue trials. Data from Bayer AG,
1981 Leverkusen. (Unpublished)
Bomhard, E. S-6876 - Chronische Toxizität an Ratten 22 Oktober,
1980 Stellungnahme zu Bericht Nr. 8607. Report of the Bayer AG
Institut für Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Bomhard, E., Löser, E. and Kaliner, G. S-6876 - chronic toxicity study
1979 on rats (two-year feeding experiment). Report of the Bayer
AG Institut f. Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Flucke, W. Akute orale Toxizität "Omethoat-Metabolit" 23 Oktober.
1978 Report of the Bayer AG Institut für Toxikologie, submitted
to WHO by Bayer AG; (Unpublished)
Herbold, B. S-6876-Salmonella/Mikrosomen-Test zur Untersuchung auf
1980 punktmutagene Wirking. Report of the Bayer AG Institut für
Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Löser, E. S-6876-Folimat-Wirkstoff Multigenerationeversuche an Ratten
1981 21 Januar, Bericht Nr. 9731. Report of the Bayer AG Institut
für Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Addendum: Histopathology - Consultox Laboratories Ltd.,
London.
Wagner, K., Neitzel, H. and Oehlmann, L. Metabolismus von Omethoat im
1978 Boden. Report submitted by Bayer AG, Leverkusen.
(Unpublished)
Of the compounds identified, amounts of I, II, III, V, VI and
VIII were measured over a period of 112 days as shown in Table 1. The
other materials listed were shown to be labile intermediates in the
degradation of omethoate in soil. Similar information is being
obtained on the degradation of omethoate in plant tissue.
TABLE 1. Degradation pattern of 14C-omethoate in soil
% of 14C applied
Days I II III+ VI1 V VIII Total
9 37 3.4 4.2 2.6 <0.1 49
21 29 5.2 4.0 2.3 0.8 45
49 17 6.3 4.9 1.3 1.8 32
85 2.4 0.7 3.9 0.2 2.4 11
112 <1 <0.3 3.5 <0.1 2.7 7
1 III and VI were not separated in these determinations.
EVALUATION
COMMENTS AND APPRAISAL
In a long-term study in rats, at levels of 3 and 10 ppm in the
diet, a clear indication of cholinesterase inhibition was evident in
brain, plasma and red blood cell cholinesterase. At the 1 ppm level,
no clear effect was observed on cholinesterase activity in plasma
or red cells in either sex. However, in females receiving 1 ppm
omethoate in the diet a slight depression (ca. 12%) of brain
acetylcholinesterase was observed. A no-effect level of 3 ppm in the
diet could be estimated from a rat 3-generation reproduction study.
Omethoate was mutagenic in strains TA 1535, 100 and 98 of
Salmonella typhimurium used in the Ames test. However, no activity
was observed in TA 1537. No mutagenic activity was detected in a
micronucleus test and a dominant lethal test previously evaluated. A
long-term rat study did not indicate carcinogenic potential. In this
study, the highest dose level (10 ppm) failed to elicit any effect
other than cholinesterase depression. The relatively low dose levels
used in this study preclude adequate carcinogenicity evaluation.
Therefore, the temporary ADI has been extended. Submission of results
of carcinogenicity studies at higher levels in a rodent species is
required by 1985.
Information regarding the degradation of omethoate in soil was
reviewed, together with a limited amount of additional data on
residues from supervised trials on potatoes and sugarbeet.
Levels causing no toxicological effect
Rat: 1 ppm in the diet equivalent to 0.05 mg/kg bw/day
Dog: 1.6 ppm in the moist diet equivalent to 0.12 mg/kg bw/day.
Estimate of temporary acceptable daily intake for man
0 - 0.0005 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
No change is suggested in the maximum residue limits previously
recommended.
FURTHER WORK OR INFORMATION
Required (by 1985)
Carcinogenicity studies in a rodent species at higher dose
levels.
Desirable
Clarification of the mutagenic potential in micro-organisms, in
respect to the "marginal effect" of omethoate in some strains of
Salmonella.
REFERENCES
Bayer Reports on omethoate residue trials. Data from Bayer AG,
1981 Leverkusen. (Unpublished)
Bomhard, E. S-6876 - Chronische Toxizität an Ratten 22 Oktober,
1980 Stellungnahme zu Bericht Nr. 8607. Report of the Bayer AG
Institut für Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Bomhard, E., Löser, E. and Kaliner, G. S-6876 - chronic toxicity study
1979 on rats (two-year feeding experiment). Report of the Bayer
AG Institut f. Toxikologie, submitted to WHO by Bayer AG.
(Unpublished)
Flucke, W. Akute orale Toxizität "Omethoat-Metabolit" 23 Oktober.
1978 Report of the Bayer AG Institut für Toxikologie, submitted
to WHO by Bayer AG; (Unpublished)
Herbold, B. S-6876-Salmonella/Mikrosomen-Test zur Untersuchung auf
1980 punktmutagene Wirking. Report of the Bayer AG Institut für
Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Löser, E. S-6876-Folimat-Wirkstoff Multigenerationeversuche an Ratten
1981 21 Januar, Bericht Nr. 9731. Report of the Bayer AG Institut
für Toxikologie, submitted to WHO by Bayer AG. (Unpublished)
Addendum: Histopathology - Consultox Laboratories Ltd.,
London.
Wagner, K., Neitzel, H. and Oehlmann, L. Metabolismus von Omethoat im
1978 Boden. Report submitted by Bayer AG, Leverkusen.
(Unpublished)
