
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
OMETHOATE
Explanation
Omethoate was evaluated in 1971 and reviewed in 1975, 1978, 1979
and 1981 1/.
The CCPR at its 12th (1980) Session (ALINORM 81/24 para 64)
concerned over the higher toxicity of omethoate than dimethoate,
requested the meeting to review the two compounds with a view to
recommending separate MRLs for each.
Dimethoate is reviewed elsewhere in these evaluations. Some
additional information concerning omethoate, received from Australia,
The Netherlands, New Zealand and the manufacturers of the compound, is
reviewed below.
IDENTITY
Purity
The only manufacturer of omethoate confirmed that technical
omethoate has the following composition.
Omethoate min 94.5%
O-desmethyl-omethoate max 2.0%
Dimethyl phosphite max 2.0%
Solvents max 2.0%
This is substantially identical with the purity of omethoate
referred to in 1971.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
The manufacturer provided an up-to-date list of registered uses
in many countries with recognised registration systems which has been
summarized in Table 1. This should be compared with the use pattern
for dimethoate. Dimethoate, having been introduced world-wide nine
years before the introduction of omethoate in 1965, was adopted for
many uses for which omethoate is suitable but which have not been
promoted by the manufacturer.
1/ See Annex II for FAO and WHO documentation.
Table 1. Registered Used, pre-harvest intervals and maximum residue limits (MRLs) for Omethoate
Omethoate
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Algeria Olives 60
Pome fruit 21
Argentina Cereals 21
Citrus fruit 21
Cotton 21
Foddercrops 21
Pastures 21
Pome fruit 21
Potatoes 21
Stone fruit 80 1.2 21
Vegetables 21
Australia Bananas 60 0.60 4 -
Cereals, grain 7 0.05
Cereals, green 7(grazing)
Citrus 60 1.2 7 2.0
Cotton 0.64 7 0.5
Forage crops 0.22 7(grazing)
Fruit 60 0.60 7 2.0
Oilseed 0.05
Oilseed crops (as food stuff) 7
Onions 60 0.60 7 2.0
Pastures 7(grazing)
Peppers 60 0.60 7 1.0
Tomatoes 60 0.60 7 1.0
Vegetables (excluding
tomatoes and peppers 60 0.60 7 2.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Austria Fruit 50 0.75 35 0.4
Potatoes 0.35-0.5 35 0.05
Sugar beets 0.2 35 0.4
Vegetables 0.35-0.5 35 0.4
Belgium Cereals 0.05
Chicory 0.4
Fruit(excl.citrus) 50-75 0.75 21 0.1
Hops 50-75 0.75 21
Sugar beets 21
Vegetables (excl. chicory) 50-75 0.75 0.1
Brazil (1) Coffee 14 0.01
Cottonseed 14 0.1
Soybeans 14 0.1
Wheat 14 0.1
Canada Apples 2.0
Beans 1.0
Beet leaves 2.0
Broccoli 2.0
Cabbage 2.0
Cauliflowers 2.0
Celery 1.0
Cherries 1.0
Citrus fruit 1.5
Kale 2.0
Lettuce 2.0
Pears 2.0
Peas 0.5
Peppers 0.5
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Spinach 2.0
Strawberries 1.0
Swiss chard 2.0
Tomatoes 0.5
Turnip leaves 2.0
Chile Field crops 0.3-0.7
Fruit 30-83 0.5-2.7
Cyprus Fruit 15-20
Grapes 15-20
Potatoes 15-20
Vegetables 15-20
Czechoslovakia Fodder beets 7
Sugar beets 7
Denmark Berry fruit 0.5
Grapes 0.5
Leafy vegetables 0.5
Pomefruit 0.5
Potatoes 0.05
Stone fruit 0.5
Other vegetables 0.5
European Artichokes 0.4
Community Berry frqit 0.1
Cherries 0.4
Chicory 0.4
Leek 0.1
Onions - 0.1
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Root vegetables 0.1
Spinach 0.4
Other food crops 0.2
FAO/WHO 1) Apples 2
Apricots 2
Citrus fruit 2
Cherries 2
Currants (black) 2
Grapes 2
Hops (dried) 3
Olives 2
Peaches 2
Pears 2
Peppers 1
Plums 2
Potatoes 0.05
Strawberries 1
Sugar beet leaves 1
Sugar beets 0.05
Tomatoes 1
Vegetables (not otherwise listed) 2
France Artichokes 62.5 15 0.4
Fruit 62.5-87.5 0.75-1.3 15 1.0
Grapes 62.5 0.75-0.93 7 1.0
Olives 21 0.2
Vegetables (excl.artichokes) 15 1.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
German Hops 28
Democratic Crops treated by drift:
Republic Food crops 21
Fodder crops 10
Germany, Artichokes 0.4
Federal Beets 35-42 3) 0.05
Republic Berry fruit 0.1
Cherries 0.4
Chicory 0.4
Hops 21
Leek 0.1
Onions 0.1
Potatoes 35 0.05
Root vegetables 0.1
Spinach 0.4
Other fruit 0.2
Other vegetables 0.2
Other food crops 0.05
Greece Citrus fruit 21
Cotton 21
Pome fruit 21
Vegetables 21
Great Britain Cereals 21
Grass crops 42
Hops 21
Pastures 7
Plums 35
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Israel Alfalfa 7
Citrus fruit 30 0.4
Common vetch 30
Grapes 15
Italy Cereals 0.375-0.75 30 0.05
Fruit 25-50 0.375-2.0 30 0.4
Grapes 25-50 0.375-1.0 30
Olives 25-50 0.375-0.75 30 0.4
Potatoes 25-50 0.5-1.0 30
Sugar beets 30 0.4
Vegetables 30 0.4
Kenya Apples 21 2.0
Beans 21 2.0
Broccoli 21 2.0
Cabbage 21 2.0
Cauliflower 21 2.0
Endive 21 2.0
Kale 21 2.0
Lemons 21 2.0
Lettuce 21 2.0
Melons 21 1.0
Oranges 21 2.0
Pears 21 2.0
Peas 21 2.0
Pecans 21 0.1
Peppers 21 2.0
Potatoes 21 0.2
Rutabagas 21 2.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Swiss chard 21 2.0
Spinach 21 2.0
Tomatoes 21 2.0
Wheat 21 0.04
Meat, fat and meat by-
products of cattle, 0.02
goats, hogs, horses
and sheep
Korea Apples 50-62.5 0.75-0.95
Citrus 50-62.5 2.0 -2.5
Luxemburg Cereals 0.05
Fruit 0.5
Vegetables 0.5
Other food crops 0.05
Mexico Alfalfa 10/28 2) 2.0
Apples 28 2.0
Barley 0.3-0.45 60 3) 0.04
Beans, dried 0.3-0.45 0.2
Beans, green 0.3-0.45 2.0
Broccoli 7 2.0
Brussels sprouts 3 2.0
Cabbage 3 2.0
Cauliflowers 7 2.0
Celery 7 2.0
Chick peas 0.3-0.45 21 2.0
Citrus fruit 1.0-1.25 28 2.0
Cotton 0.25-0.8 14 0.1
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Grapes 28 1.0
Lettuce 14 2.0
Maize 0.3-0.45 14 0.1
Melons 3 1.0
Oranges 15 2.0
Pears 28 2.0
Pecans 21 0.1
Peppers 2.0
Potatoes 0.2
Safflower 14 0.1
Snap beans 0.3-0.45 2.0
Sorghum 0.3-0.45 28 0.1
Soybeans 21 0.05
Stone fruit 0.85-1.0
Tomatoes 7 2.0
Tobacco 0.45-0.65 21
Wheat 0.3-0.45 60 3) 0.04
Morocco Fruit 0.25-0.75
Vegetables 0.25-0.75
Netherlands Artichokes 0.4
Berry fruit 0.1
Cherries 0.4
Chicory 0.4
Leak 0.1
Onions 0.1
Vegetables 0.1
Spinach 0.4
Strawberries 0.1
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Other fruit 0.2
Other vegetables 0.2
Other 0 4)
New Zealand Cereals 21
Citrus fruit 21
Fodder crops 14
Grapes 42
Lucerne 14
Maize (fodder) 14
Pome fruit 21
General 0.5
Portugal Apples 21
Citrus fruit 28
Cotton 21
Hops 21
Pears 21
Plums 21
South Africa Alfalfa 7 2.0
Apples 28 1.5
Citrus fruit 35 2.0
Clover 7 2.0
Cotton 50
Grapes 28 1.5
Pears 28 1.5
Peas 7 1.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Spain Citrus fruit 25-75 1.0-3.0 30 0.4
Cotton 0.25-1.0 30
Fruit 25-75 0.25-0.75 0.4
Grapes 50-75 0.75-1.5 30 0.4
Hops 25-75 0.25-0.75 30
Pome fruit 25-75 0.25-0.75 30 0.4
Potatoes
Stone fruit 25-75 0.25-0.75 30 0.4
Sugar beet
Vegetables 25-75 0.375-0.75 0.4
Sweden Fruit 0.1
Potatoes 0.1
Vegetables 0.1
Taiwan Apples 21
Guavas 21
Soybeans 60
Thailand Rice 0.75
Cotton 60 0.9
Tobacco 60 0.9
Citrus 60-80 1.5-2.5
Fruit 60-80 0.9-1.2
Turkey Fruit 50-200
Grapes 50-75 0.5-0.75
Cotton 0.5-0.75
Vegetables 50-75
Sugar beet 50
Citrus 50-75 1.0-1.5
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
USA 5) Alfalfa 2.0
Apples 2.0
Beans (dry, 2.0
lima, snap)
Broccoli 2.0
Cabbage 2.0
Cauliflowers 2.0
Celery 2.0
Cherries 2.0
Citrus pulp, dried 5.0
(as feed stuff)
Collards 2.0
Cottonseed 0.1
Endive 2.0
Grapefruit 2.0
Grapes 1.0
Kale 2.0
Lemons 2.0
Lettuce 2.0
Melons 1.0
Mustard greens 2.0
Oranges 2.0
Pears 2.0
Peas 2.0
Pecans 0.1
Peppers 2.0
Potatoes 0.2
Safflower seed 0.1
Sorghum forage 0.2
Sorghum grain 0.1
Soybean forage 2.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Soybean hay 2.0
Soybeans 0.05 6)
Spinach 2.0
Swiss chard 2.0
Tangerines 2.0
Tomatoes 2.0
Turnips (roots and tops) 2.0
Wheat grain 0.04 6)
Wheat, green fodder 2.0
Wheat straw 2.0
Eggs 0.02 6)
Meat, fat and meat 0.02 6)
by-products of cattle,
goats, hogs, horses,
poultry and sheep
Milk 0.002 6)
Venezuela Carrots 21 1.0
Cotton 21 1.0
Fruit 21 1.0
Grapes 21 1.0
Rice 21 1.0
Potatoes 21 1.0
Tobacco 21 1.0
Tomatoes 21 1.0
Vegetables 21 1.0
Table 1. (continued)
Country Crop Concentration Application Rate Pre-harvest MRL
(g/1001) (kg/ha) Interval (mg/kg)
(days)
Yugoslavia Fodder beets 42
Fruit 28 0.5
Hops 21
Onions 42
Sugar beets 42 0.5
Tobacco 21
Vegetables (excl. onions) 28
Other field crops 42
Other food crops 0.05
1) = Temporary tolerances
2) = Depending on formulation
3) = Application at time of sowing
4) = Under the limit of determination (0.02 mg/kg)
5) = Tolerances of dimethoate
6) = At or about the limit of determination
The nine major applications of omethoate represent approximately
70% of the world demand as indicated in Table 2.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Results of trials carried out with apples, peaches and hops in
Chile, Italy and the Federal Republic of Germany (Bayer, 1984) are
summarised in Table 3. These results are generally in agreement with
those considered previously and upon which recommendations for MRLs
were made. With the exception of one trial on apples from Italy, the
results are within the recommended MRLs. This Italian trial yielded a
residue of 2.3 mg/kg 37 days after the last of 3 treatments at the
rate of 1 kg/ha. The proposed Codex temporary MRL of 2 mg/kg is based
on a PHI of 21 days after a similar treatment.
The residue value at 64 days after the last treatment
(0.46 mg/kg) from the same trial would tend to confirm that the
finding at 37 days was probably valid.
Results of official trials on Kiwi fruit received from New
Zealand are summarized in Table 4. The approved rate of application is
50 g a.i./100 l equivalent to 2.8 kg/ha with a withholding period of
21 days.
FATE OF RESIDUES
Attention is directed to the references to the fate of residues
in the accompanying monograph on dimethoate, the 1976 and 1970
evaluations of dimethoate, and the 1971 evaluation of omethoate.
In Animals
The work of the following investigators mentioned in the present
or earlier dimethoate evaluations should especially be noted:
Dauterman et al. (1959); Kaplanis et al (1959); Chamberlain
et al. (1961); Beck et al. (1968).
Ecker and Coelln (1981) studied the biotransformation of
omethoate in rats. Within 8 hours after oral administration of 5 mg/kg
of [carbonyl-14C] omethoate to male rats, about 88% of the
radioactivity was eliminated with the urine. Of this, 30-50% was
unchanged parent compound, about 11% was O-demethyl-omethoate and a
further 15-22% was identified as N-methyl-2-(methyldithio)acetamide.
It is fairly certain that omethoate is not stored in animal
tissues or fat nor excreted in milk unless the intake is at least one
to two orders of magnitude higher than livestock could encounter from
the direct or indirect use of dimethoate or omethoate in controlling
insect pests of crops, pasture or forage.
Table 2. Main uses of dimethoate (Bayer, 1984)
Crop % of world demand
Pome fruit 23
Citrus 16
Cotton 6
Tomatoes 6
Cereals other than rice and maize 5
Rice 5
Vegetables 3
Grapes 3
Maize 1.5
68.5%
Table 3. Residues of omethoate in crops resulting from supervised trials
Crops Formulation/Dose No. Country Days a. Residue (mg/kg) Bayer Report
(kg a.i./ha) Appl. 1.Appl. from-to average* No.
Apples Folimat 1000 EC 3 Chile 14 0.38 0.38 1) 4411/81
1.0 - 1.5 2 28 0.15 0.15 1) 4410/81
Folimat 500 SL 3 Italy 37 2.3 2.3 1) 4403/81
1.0 3 64 0.46 0.46 1) 4401/81
2 81 0.36 0.36 1) 4402/81
2 108 0.07 0.07 1) 4400/81
Peaches Folimat 500 SL 3 Italy 20 0.32-1.06 0.69 2) 4404-05/81
0.5 - 1.0 2 28 0.36 0.36 1) 4406/81
81 0.03 0.03 1) 4407/81
Hops Folimat spezial 5 FRG 0 14.5-16.8 15.7 2) 4500-01/81
0.54-2.16 7 6.3- 7.2 6.8 2)
14 2.4 2.4 2)
21 1.7 - 2.9 2.3 2)
28 <0.07- 2.1 1.1 2)
35 <0.07- 0.98 0.5 2)
* Values in parenthesis indicate number of individual results.
Table 4. Omethoate residues in Kiwi fruit - New Zealand
Omethoate residues, mg/kg
Trial No. g a.i./ kg/ha No. of Days after treatment
100 l Treatments
1 3 5 8 11 15 21 28 35
1 50 2.8 1 2.6 1.6 1.5 1.0 0.8 0.6 0.3 0.6 -
2 75 4.2 1 8.6 5.1 4.6 4.4 3.5 3.8 2-6 1.9 0.8
3 100 5.6 1 9.4 11.0 7.8 5.5 4.1 3.8 4.3 2.9 1.5
One trial in New Zealand when omethoate was applied to apricots at the rate of 60 g/100 l resulted in less than
0.1 mg/kg omethoate 89 days after application.
In Plants
Wagner et al. (1981) examined the metabolism of omethoate in
sugar beets in greenhouse studies.
The formation of degradation products was observed shortly after
the foliar application of [carbonyl-14C]omethoate. Only about 8% of
unaltered parent compound was found in the plants 21 days after the
beginning of the study.
The following metabolites were isolated and identified as
intermediates of omethoate degradation in sugar beets using nuclear
magnetic resonance (NMR) and mass spectrometry. The metabolic pathways
are shown in Figure 1.
a) Phosphorus-containing alteration products
O-Methyl S-methylcarbamoylmethyl phosphorothioate (II in Fig. 1)
S-Hydroxymethylcarbamoylmethyl O,O-dimethyl phosphorothioate
(III).
(Dimethoxyphosphinoylthio)acetic acid (IV)
b. Hydrolytic and oxidative alteration products
Methylcarbamoylmethyl(dithio) acet acid (V)
2,2'-Dithiobis(N-methylacetamide) (VI)
N-Methyl-2-(methyldithio)acetamide (VII)
methylcarbamoylmethyl-thio) acetic acid (VIII)
c. Biotransformation products
3-Hydroxy-3-(methylcarbamoylmethyl-thiol)propionic acid (IX)
3-Hydroxy-3-methylcarbamoylmethylsulphinyl)propionic acid (X)
2-[2-Carboxymethyl)sulphinyl]-2-hydroxyacetic acid (XI)
N-Methyl-3-methylcarbamoylmethyldithio)propionamide (XII)
3-hydroxy-3-(methylcarbamoylmethyldithio)propionic acid (XIII)
2-Hydroxy-N,N'-dimethylbutandiamide (XIV)
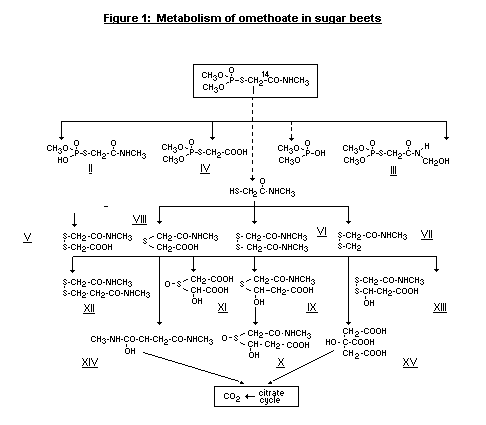 Citric acid (XV)
The mono-, di- and tricarboxylic acids were isolated and
identified after derivatization with diazomethane.
Approximately 30% of the initial radioactivity was found as
3-hydroxy-3(methylcarbamoylmethylthio)propionic acid (IX), which is
the major alteration product of omethoate in sugar beets. The
oxidation product X of IX was found at a concentration of 10% of the
initial radioactivity 21 days after application of the parent
compound. Both compounds were found predominantly in the polar
methanol and aqueous leaf percolates.
The water-soluble, highly polar demethyl-omethoate (II) reached a
concentration of 4.2% of the initial radioactivity 21 days after
application of the parent compound.
Up to 1.4% of the initial radioactivity was found as
2,2'-dithiobis (N-methylacetamide) (VI), which is also water-soluble
but distinctly less polar.
The authors were able to isolate "N-hydroxymethyl-omethoate"
(III), as a short-lived intermediate in the chloroform percolate of
the sugar beet leaves at a maximum concentration of 0.6% of the
initial radioactivity.
The occurrence of genuine intracellular, biochemical degradation
processes was confirmed by isolation and identification of
2-hydroxy-N,N'-dimethylbutandiamide (XIV) and citric acid, XV -
both at a maximum of 2.5% of the initial radioactivity, using
radioactively labelled material.
Vacuum infiltration studies (Maximov, 1951) using unlabelled
N-methyl thioglycolic acid amide, which must represent a key
intermediate product in omethoate metabolism, showed that
(methylcarbamoylmethyldithio)acetic acid (V) was the major alteration
product of isolated sugar beet leaves after only 30 minutes.
100% recovery of the initial radioactivity was achieved in a
"closed system" 14 days after parent compound application. An "open
system" resulted in 81, 80 and 73% recovery 7, 14 and 21 days after
the beginning of the study, respectively.
Components IV, VII and XIII were obtained from unlabelled
experimental batches with higher omethoate concentrations.
Oehlmann and Wagner (1983) reported that some of the highly polar
metabolites of omethoate formed in sugarbeets and soil did not give
interpretable electron impact mass spectra and also that the molecular
ion for the main metabolite could not be detected. In the field
desorption mass spectra the molecular ions of all 3 reported compounds
were found. They reported that the spectroscopic data confirmed the
structures of the metabolites.
In Soil
Attention is directed to the publications by Harris and Hitchon
(1970) and Duff and Menzer (1973) reviewed in the dimethoate
monograph, and to the 1967 evaluation of dimethoate.
The high solubility of omethoate in water (miscible in any ratio:
Wagner and Frehse, 1976) greatly increases the likelihood of leaching
but this is offset by the readiness with which the compound is
biodegraded in biologically active soil.
In Water
Wagner and Frehse (1976) report the stability of dimethoate and
omethoate in water as follows:
Stability (half-life in days)
Temperature°C pH omethoate dimethoate
(1) 25° 2 62 176
(1) " 4 74 160
(1) " 6 32.5 125
(1) " 8 2.8 38
(2) 21° 9 0.3 5.8
(1) Taken from Grimmer et al. 1968
(2) Taken from Santi and de Pietri-Tonelli (1959)
Grimmer et al. (1968) also determined the following
distribution coefficients:
omethoate dimethoate
chloroform:water 1.15 8.4
ethyl acetate:water 0.5 5.1
benzene:water 0.25 2.2
In Processing and Cooking
Steller and Pasarella (1972) showed that omethoate was not
significantly affected by the fermentation process in the production
of wine by Kawar et al. (1979) showed that residues of dimethoate in
wine stored at 24°C were degraded by hydrolysis with a half-life of 30
days. Residues in wine were unchanged during 1 year in frozen storage.
The dimethoate monograph describes this work and gives information
concerning the fate of omethoate in the processing of olives into oil
and pickled olives.
Ten Broeke and Dornseiffen (1973) showed that the washing and
cooking of Witloof chicory, as practiced in domestic kitchens,
destroyed more than half the omethoate residues present in fresh
chicory. (See dimethoate monograph for details).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Dick et al. (1978) published the results of a survey of
pesticide residues in the New Zealand diet. This included
organophosphorus insecticides. The study involved the analysis of food
composites from a simulated New Zealand total diet which was collected
at quarterly intervals during 1974/75 from four major cities. The
foods were grouped into 8 classes. The methods used for analysis were
tested to determine the recovery of a range of pesticide residues at a
concentration of 0.01 mg/kg. The recovery of omethoate from 4 major
categories was reported to be 100%. Omethoate was found in the fruit
group from each of the 4 cities during 2 or 3 periods of the survey.
The concentration in the composites ranged from 0.03 mg/kg to
0.04 mg/kg.
The investigators reported daily intakes ranging from 6 to 59
micrograms for individuals in the separate cities during different
periods of the year. The temporary ADI is equivalent to 35 mg for an
individual weighing 70 kg. Only one of the 16 separate composites
represented an intake greater than 35 mg per day. The authors comment
that omethoate was detected in 60% of the fruit composites whereas a
similar survey carried out in 1974 revealed omethoate in only 1% of
the samples. It seems highly likely that the omethoate residues were
derived from the direct use of omethoate insecticides rather than the
metabolism of dimethoate.
The Netherlands Government supplied comprehensive information from
surveys conducted by the Food Inspection Service, on food commodities
produced in The Netherlands and imported. The results of monitoring
during 1982 are presented in Table 5 (Netherlands 1984). Since this
period the national MRLs have been amended. Additional monitoring data
on omethoate from The Netherlands are tabulated in the evaluation of
dimethoate.
METHODS OF RESIDUE ANALYSIS
Some analytical methods for the determination of omethoate
residues are included in the present evaluation of dimethoate. Earlier
methods are reviewed in the 1967 evaluation of dimethoate and the 1971
evaluation of omethoate.
Table 5 Omethoate residues found in monitoring in the Netherlands
Food Inspection Services of the Netherlands
omethoate 1982
Other
witloof french grape citrus
endive Parsley chicory apple clementine beans grape fruit orange fruit radish
source N N N I I I I I I I I
mg/kg
0.000
0.001 - 0.100 1 1 14 2 5 1 1 1 4 1 1
0.101 - 0.200 8 1 1 1
0.201 - 0.400 11
0.401 - 0.600 6
0.601 - 0.800 1
0.800 - 1.000 1 1
> 1.000 1
(2.1)
Total 1 1 42 3 5 1 1 1 6 2 1
N = Netherlands
I = imported
U = unknown
--- = national tolerance
Multi-residue methods recommended by the Ad Hoc Working Group
on Methods of Analysis of the CCPR which apply to both dimethoate and
omethoate are noted in the accompanying evaluation of dimethoate. Of
these, the methods of Steller and Pasarella (1972) and Wagner and
Frehse (1976) are described in more detail below.
Steller and Pasarella (1972) reported a gas-liquid
chromatographic method for the determination of omethoate and
dimethoate residues in plant and animal tissues, milk and eggs, using
a 60 cm. × 4 mm. i.d. glass column packed with 11% DC-200 on
60-80 mesh Gas-Chrom Q previously treated with 0.01% Versamid 900. The
GLC column was operated at 160°C using a flame photometric detector
with a phosphorus filter or at 165°C using an alkali flame ionization
detector. The column should be conditioned by injecting omethoate,
dimethoate and polyethylene glycol 400. Prior to gas chromatography,
it is essential that extracts from cattle and poultry tissues, milk,
eggs, cottonseed and oil, safflower seeds, wheat and sorghum grain and
alfalfa are cleaned up by silica gel chromatography, primarily to
remove oily or waxy extractives which, if not removed, would greatly
shorten the effective GLC column life.
Steller and Brand (1974) modified these conditions for the
determination of omethoate and dimethoate and the potential
metabolites de-N-methyl-omethoate, de-N-methyl-dimethoate,
N-hydroxymethyl-omethoate, N-hydroxymethyl-dimethoate and
N-hydroxymethyl-dimethoate-O-glucoside in grapes. A 120 cm. × 3 mm.
i.d. glass GLC column was packed with 1% EGSS-X on Gas-Chrom 0,
60-80 mesh, and operated at 180°C. The retention times were 1.2
minutes for omethoate; 1.5 for dimethoate; 2.3 minutes for
des-N-methyl- and N-hydroxymethyl-omethoate; 3.2 minutes for
des-N-methyl- and N-hydroxymethyl-dimethoate. The column must be
conditioned by firstly injecting Silyl 8 followed by injections of
des-N-methyl-dimethoate and grape extracts. The N-hydroxymethyl
derivatives are converted to their corresponding des-N-methyl
derivatives by heat on the GLC column. Of all the mentioned compounds,
only omethoate was present as a metabolite 28 days after the
application of dimethoate (sensitivity limit of 0.05 mg/kg).
Woodham et al. (1974a,b) determined both omethoate and
dimethoate on and in citrus fruits and leaves by a gas
chromatographic-flame photometric detection procedure (using
phosphorus and sulphur interference filters), although on separate
columns. They used 15 cm. × 6 mm. o.d. glass columns packed with 3%
DC-200 on 100-200 mesh Gas-Chrom 0 for dimethoate and 10% DC-200 on
100-200 mesh Gas-Chrom for omethoate. The temperature of each column
was 200°C. Retention times were not given. The recoveries ranged from
60 to 70%.
The method of Steller and Pasarella (1972) has superseded
an older method described by Bazzi et al. (1970) for the
gas-chromatographic determination of dimethoate residues in cattle
meat (using an electron-capture detector).
Wagner and Frehse (1976) described a method for the
gas-chromatographic determination of omethoate residues in plant
material, soil and water. It is intended chiefly for the determination
of residues after the application of omethoate, but also permits
determination of both omethoate and dimethoate or of dimethoate alone.
The prepared samples are extracted by macerating with acetone or
aqueous methanol, and the macerate is extracted with chloroform or
dichloromethane. The extracts are evaporated to dryness, dissolved in
acetone and, without further clean-up, analyzed by gas chromatography
using an AFID. Columns packed with 10% DC-200 + 1.5% 0F1 or with 10%
OV17, on 80-100 mesh Gas-Chrom 0 are the most suitable for the
separation of omethoate and dimethoate.
APPRAISAL
Omethoate was re-evaluated in conjunction with dimethoate in order
to determine whether it would be possible to recommend separate MRLs
for the two compounds for residues arising both as a consequence of
the metabolism of applied dimethoate and from the use of omethoate
directly as an insecticide.
Extensive information from the open scientific literature on
dimethoate was evaluated and recommendations were made. Additional
information on omethoate was received from Australia, The Netherlands,
New Zealand and the manufacturers. The similarity in the use patterns
of dimethoate and omethoate was noted.
Results from 22 residue trials on apples, peaches and hops were
reviewed. These served to confirm the validity of the MRLs previously
recommended.
Results of official trials on Kiwi fruit were received from New
Zealand. These enabled the meeting to estimate a maximum residue level
which was recommended as a temporary MRL.
An extensive study of the metabolism of omethoate in sugar beet
plants provided a further inside into the fate of omethoate in plants
and an indication that residues are converted into natural plant
products through a number of well-defined steps.
Results from several national monitoring programmes indicate that
the incidence and level of omethoate residues in the diet is low and
that the intake by consumers does not exceed the ADI.
RECOMMENDATIONS
The meeting recommends the following temporary MRL for omethoate
on the basis of residues found at harvest.
Commodity MRL PHI
(mg/kg)
Kiwi fruit 2 21
The nine major applications of omethoate represent approximately
70% of the world demand as indicated in Table 2.
REFERENCES
Bayer Omethoate - Information submitted to FAO. Bayer A.G.,
1984 Leverhusen.
Bazzi, G., Galluzzi, G., and Nesti, V. Gas-Chromatographic
1970 determination of dimethoate residues in cattle meat.
Contributi 1968-69, 1st Ric. Agr. Soc. Montecatini X, 25-30
(Oct. 1970)
Beck, E.W., Johnson, J.C., Getz, M.E., Skinner, F.B., Dawsey, L.H.,
1968 Woodham, D.W. and Derbyshire, J.C. Effects of feeding
dimethoate, its oxygen analog and dimethoate-treated silage
to cattle. J. Econ. Entom., 61 (3):605-610.
Chamberlain, W.F.P.E., Gatterdam and Hopkins, D.E. The metabolism of
1961 labeled dimethoate in sheep. J. Econ. Entomol., 54 (4): 733-
740.
Dauterman, W.C., Casida, J.E., Kraake, J.B., and Kowalczyk, T. Bovine
1959 metabolism of organophosphorus insecticides. Metabolism and
residues associated with the oral administration of
dimethoate to rats and three lactating cows. J. Agr., Food
Chem., 7:188.
Dick, G.L., Heenan, M.P., Love, J.L., Udy, P.B. and Davidson, F.
1978 Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus
pesticide residue content. N.Z.J. Sci., 21 (1):71-78.
Duff, W.G. and Menzer, R.E. Persistence, mobility, and degradation of
1973 14C-dimethoate in soils. Environ. Entomol. 2 (3):309-318.
Ecker, W., and Coelln, R. Biotransformation of omethoate. PF-Report:
1981 1574 Aug.7, 1981 from PH-E Isotope Laboratory, at the
Institute of Pharmacokinetics, Wuppertal. Mobay Chemical
Corporation (Unpublished).
Grimmer, F., Dedek, W. and Liebnitz, E. Zur Kenntris der
1968 N-Butylchomalogen des Dimethoate. I. Hydrolysegesch-
Wurdigkeit und-mechanismus. Z. Naturforsch. 23. 13-17.
Harris, C.R. and Hitchon, J.L. Laboratory evaluation of candidate
1970 materials as potential soil insecticides. II. J. Econ.
Entomol., 63 (1):2-7.
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
1959 R.E., and Treiber, G. The metabolism of dimethoate in
cattle. J. Econ. Entomol. 52 (6):1190-1194.
Kawar, N.S., Iwata, Y., Dusch, M.E. and Gunther, F.A. Behaviour of
1979 dialifor, dimethoate and methidathion in artificially
fortified grape juice processed into wine. J. Environ. Sci.
Health, Part B, 14 (5):505-513.
Maximow, N.A., Kurzes Lehrbuch der Pflanzenphysiologie, S. 354-361.
1951 Verlag Kultur and Fortschritt, Berlin.
Netherlands Information on pesticides included in the JMPR priority
1984 list. Submission to FAO dated 4 May 1984.
Oehlmann, L. and Wagner, K. Metabolism of omethoate in soil and sugar
1983 beet. Anal. Chem. Symp. Ser., Chromatogr. Mass Spectrom.
Biomed, 14, 2:53-6.
Santi, R. and de Pietri-Tonelli, P. Mode of action and biological
1959 properties of dimethoate. Nature 183:398 (quoted by
Dauterman et al., 1960).
Steller, W.A. and Pasarella, N.R. Gas-liquid chromatographic method
1972 for the determination of dimethoate and dimethoxon residues
in plant and animal tissues, milk and eggs. J. Assoc. Offic.
Anal. Chem., 55 (6):1280-1287.
Steller, W.A. and Brand, W.W. Analysis of dimethoate-treated grapes
1974 for the N-hydroxymethyl and de-N-methyl metabolites and for
their sugar adducts. Agr. Food Chem., 22 (3): 445-449.
Ten Broeke, R. and Dornseiffen, J.W., Residuen van dimethoate en
1973 omethoate in witlof in diverse stadia de hueshoudelijke
bereiding. Report KvW 173/WRV (73) 34.6.2.1 Keuringsdienst
van Waren - Amsterdam November 1973.
Wagner, K. and Frehse, H. Method for the determination of residues of
1976 Folimat insecticide/acaricide. Pflanzenschutz-Nachrichten
Bayer, 29 (1):54-66.
Wagner, K., Neitzel, H., Oehlmann, L. and Pahlke, H. Metabolism of
1981 omethoate in sugar beets. Bayer AG Report RA-6/OEL,2 Bayer
AG Leverkusen, Federal Republic of Germany, Jan. 23, 1981.
Woodham, D.W., Reeves, R.G., Williams, C.B., Richardson, H. and
1974a Bond,C.A. Residues of dimethoate and its oxygen analogue on
and in citrus leaves following helicopter treatment of the
trees with dimethoate ultra-low volume concentrate and high
volume spray. J. Agr. Food Chem., 22 (4):731-733.
Woodham, D.W., Hatchett, J.C. and Bond, C.A. Comparison of dimethoate
1974b and dimethoxon residues in citrus leaves and grapefruit
following foliar treatment with dimethoate wettable powder
with and without surfactant. J. Agr. Food Chem.,
22 (2):239-242.
Citric acid (XV)
The mono-, di- and tricarboxylic acids were isolated and
identified after derivatization with diazomethane.
Approximately 30% of the initial radioactivity was found as
3-hydroxy-3(methylcarbamoylmethylthio)propionic acid (IX), which is
the major alteration product of omethoate in sugar beets. The
oxidation product X of IX was found at a concentration of 10% of the
initial radioactivity 21 days after application of the parent
compound. Both compounds were found predominantly in the polar
methanol and aqueous leaf percolates.
The water-soluble, highly polar demethyl-omethoate (II) reached a
concentration of 4.2% of the initial radioactivity 21 days after
application of the parent compound.
Up to 1.4% of the initial radioactivity was found as
2,2'-dithiobis (N-methylacetamide) (VI), which is also water-soluble
but distinctly less polar.
The authors were able to isolate "N-hydroxymethyl-omethoate"
(III), as a short-lived intermediate in the chloroform percolate of
the sugar beet leaves at a maximum concentration of 0.6% of the
initial radioactivity.
The occurrence of genuine intracellular, biochemical degradation
processes was confirmed by isolation and identification of
2-hydroxy-N,N'-dimethylbutandiamide (XIV) and citric acid, XV -
both at a maximum of 2.5% of the initial radioactivity, using
radioactively labelled material.
Vacuum infiltration studies (Maximov, 1951) using unlabelled
N-methyl thioglycolic acid amide, which must represent a key
intermediate product in omethoate metabolism, showed that
(methylcarbamoylmethyldithio)acetic acid (V) was the major alteration
product of isolated sugar beet leaves after only 30 minutes.
100% recovery of the initial radioactivity was achieved in a
"closed system" 14 days after parent compound application. An "open
system" resulted in 81, 80 and 73% recovery 7, 14 and 21 days after
the beginning of the study, respectively.
Components IV, VII and XIII were obtained from unlabelled
experimental batches with higher omethoate concentrations.
Oehlmann and Wagner (1983) reported that some of the highly polar
metabolites of omethoate formed in sugarbeets and soil did not give
interpretable electron impact mass spectra and also that the molecular
ion for the main metabolite could not be detected. In the field
desorption mass spectra the molecular ions of all 3 reported compounds
were found. They reported that the spectroscopic data confirmed the
structures of the metabolites.
In Soil
Attention is directed to the publications by Harris and Hitchon
(1970) and Duff and Menzer (1973) reviewed in the dimethoate
monograph, and to the 1967 evaluation of dimethoate.
The high solubility of omethoate in water (miscible in any ratio:
Wagner and Frehse, 1976) greatly increases the likelihood of leaching
but this is offset by the readiness with which the compound is
biodegraded in biologically active soil.
In Water
Wagner and Frehse (1976) report the stability of dimethoate and
omethoate in water as follows:
Stability (half-life in days)
Temperature°C pH omethoate dimethoate
(1) 25° 2 62 176
(1) " 4 74 160
(1) " 6 32.5 125
(1) " 8 2.8 38
(2) 21° 9 0.3 5.8
(1) Taken from Grimmer et al. 1968
(2) Taken from Santi and de Pietri-Tonelli (1959)
Grimmer et al. (1968) also determined the following
distribution coefficients:
omethoate dimethoate
chloroform:water 1.15 8.4
ethyl acetate:water 0.5 5.1
benzene:water 0.25 2.2
In Processing and Cooking
Steller and Pasarella (1972) showed that omethoate was not
significantly affected by the fermentation process in the production
of wine by Kawar et al. (1979) showed that residues of dimethoate in
wine stored at 24°C were degraded by hydrolysis with a half-life of 30
days. Residues in wine were unchanged during 1 year in frozen storage.
The dimethoate monograph describes this work and gives information
concerning the fate of omethoate in the processing of olives into oil
and pickled olives.
Ten Broeke and Dornseiffen (1973) showed that the washing and
cooking of Witloof chicory, as practiced in domestic kitchens,
destroyed more than half the omethoate residues present in fresh
chicory. (See dimethoate monograph for details).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Dick et al. (1978) published the results of a survey of
pesticide residues in the New Zealand diet. This included
organophosphorus insecticides. The study involved the analysis of food
composites from a simulated New Zealand total diet which was collected
at quarterly intervals during 1974/75 from four major cities. The
foods were grouped into 8 classes. The methods used for analysis were
tested to determine the recovery of a range of pesticide residues at a
concentration of 0.01 mg/kg. The recovery of omethoate from 4 major
categories was reported to be 100%. Omethoate was found in the fruit
group from each of the 4 cities during 2 or 3 periods of the survey.
The concentration in the composites ranged from 0.03 mg/kg to
0.04 mg/kg.
The investigators reported daily intakes ranging from 6 to 59
micrograms for individuals in the separate cities during different
periods of the year. The temporary ADI is equivalent to 35 mg for an
individual weighing 70 kg. Only one of the 16 separate composites
represented an intake greater than 35 mg per day. The authors comment
that omethoate was detected in 60% of the fruit composites whereas a
similar survey carried out in 1974 revealed omethoate in only 1% of
the samples. It seems highly likely that the omethoate residues were
derived from the direct use of omethoate insecticides rather than the
metabolism of dimethoate.
The Netherlands Government supplied comprehensive information from
surveys conducted by the Food Inspection Service, on food commodities
produced in The Netherlands and imported. The results of monitoring
during 1982 are presented in Table 5 (Netherlands 1984). Since this
period the national MRLs have been amended. Additional monitoring data
on omethoate from The Netherlands are tabulated in the evaluation of
dimethoate.
METHODS OF RESIDUE ANALYSIS
Some analytical methods for the determination of omethoate
residues are included in the present evaluation of dimethoate. Earlier
methods are reviewed in the 1967 evaluation of dimethoate and the 1971
evaluation of omethoate.
Table 5 Omethoate residues found in monitoring in the Netherlands
Food Inspection Services of the Netherlands
omethoate 1982
Other
witloof french grape citrus
endive Parsley chicory apple clementine beans grape fruit orange fruit radish
source N N N I I I I I I I I
mg/kg
0.000
0.001 - 0.100 1 1 14 2 5 1 1 1 4 1 1
0.101 - 0.200 8 1 1 1
0.201 - 0.400 11
0.401 - 0.600 6
0.601 - 0.800 1
0.800 - 1.000 1 1
> 1.000 1
(2.1)
Total 1 1 42 3 5 1 1 1 6 2 1
N = Netherlands
I = imported
U = unknown
--- = national tolerance
Multi-residue methods recommended by the Ad Hoc Working Group
on Methods of Analysis of the CCPR which apply to both dimethoate and
omethoate are noted in the accompanying evaluation of dimethoate. Of
these, the methods of Steller and Pasarella (1972) and Wagner and
Frehse (1976) are described in more detail below.
Steller and Pasarella (1972) reported a gas-liquid
chromatographic method for the determination of omethoate and
dimethoate residues in plant and animal tissues, milk and eggs, using
a 60 cm. × 4 mm. i.d. glass column packed with 11% DC-200 on
60-80 mesh Gas-Chrom Q previously treated with 0.01% Versamid 900. The
GLC column was operated at 160°C using a flame photometric detector
with a phosphorus filter or at 165°C using an alkali flame ionization
detector. The column should be conditioned by injecting omethoate,
dimethoate and polyethylene glycol 400. Prior to gas chromatography,
it is essential that extracts from cattle and poultry tissues, milk,
eggs, cottonseed and oil, safflower seeds, wheat and sorghum grain and
alfalfa are cleaned up by silica gel chromatography, primarily to
remove oily or waxy extractives which, if not removed, would greatly
shorten the effective GLC column life.
Steller and Brand (1974) modified these conditions for the
determination of omethoate and dimethoate and the potential
metabolites de-N-methyl-omethoate, de-N-methyl-dimethoate,
N-hydroxymethyl-omethoate, N-hydroxymethyl-dimethoate and
N-hydroxymethyl-dimethoate-O-glucoside in grapes. A 120 cm. × 3 mm.
i.d. glass GLC column was packed with 1% EGSS-X on Gas-Chrom 0,
60-80 mesh, and operated at 180°C. The retention times were 1.2
minutes for omethoate; 1.5 for dimethoate; 2.3 minutes for
des-N-methyl- and N-hydroxymethyl-omethoate; 3.2 minutes for
des-N-methyl- and N-hydroxymethyl-dimethoate. The column must be
conditioned by firstly injecting Silyl 8 followed by injections of
des-N-methyl-dimethoate and grape extracts. The N-hydroxymethyl
derivatives are converted to their corresponding des-N-methyl
derivatives by heat on the GLC column. Of all the mentioned compounds,
only omethoate was present as a metabolite 28 days after the
application of dimethoate (sensitivity limit of 0.05 mg/kg).
Woodham et al. (1974a,b) determined both omethoate and
dimethoate on and in citrus fruits and leaves by a gas
chromatographic-flame photometric detection procedure (using
phosphorus and sulphur interference filters), although on separate
columns. They used 15 cm. × 6 mm. o.d. glass columns packed with 3%
DC-200 on 100-200 mesh Gas-Chrom 0 for dimethoate and 10% DC-200 on
100-200 mesh Gas-Chrom for omethoate. The temperature of each column
was 200°C. Retention times were not given. The recoveries ranged from
60 to 70%.
The method of Steller and Pasarella (1972) has superseded
an older method described by Bazzi et al. (1970) for the
gas-chromatographic determination of dimethoate residues in cattle
meat (using an electron-capture detector).
Wagner and Frehse (1976) described a method for the
gas-chromatographic determination of omethoate residues in plant
material, soil and water. It is intended chiefly for the determination
of residues after the application of omethoate, but also permits
determination of both omethoate and dimethoate or of dimethoate alone.
The prepared samples are extracted by macerating with acetone or
aqueous methanol, and the macerate is extracted with chloroform or
dichloromethane. The extracts are evaporated to dryness, dissolved in
acetone and, without further clean-up, analyzed by gas chromatography
using an AFID. Columns packed with 10% DC-200 + 1.5% 0F1 or with 10%
OV17, on 80-100 mesh Gas-Chrom 0 are the most suitable for the
separation of omethoate and dimethoate.
APPRAISAL
Omethoate was re-evaluated in conjunction with dimethoate in order
to determine whether it would be possible to recommend separate MRLs
for the two compounds for residues arising both as a consequence of
the metabolism of applied dimethoate and from the use of omethoate
directly as an insecticide.
Extensive information from the open scientific literature on
dimethoate was evaluated and recommendations were made. Additional
information on omethoate was received from Australia, The Netherlands,
New Zealand and the manufacturers. The similarity in the use patterns
of dimethoate and omethoate was noted.
Results from 22 residue trials on apples, peaches and hops were
reviewed. These served to confirm the validity of the MRLs previously
recommended.
Results of official trials on Kiwi fruit were received from New
Zealand. These enabled the meeting to estimate a maximum residue level
which was recommended as a temporary MRL.
An extensive study of the metabolism of omethoate in sugar beet
plants provided a further inside into the fate of omethoate in plants
and an indication that residues are converted into natural plant
products through a number of well-defined steps.
Results from several national monitoring programmes indicate that
the incidence and level of omethoate residues in the diet is low and
that the intake by consumers does not exceed the ADI.
RECOMMENDATIONS
The meeting recommends the following temporary MRL for omethoate
on the basis of residues found at harvest.
Commodity MRL PHI
(mg/kg)
Kiwi fruit 2 21
The nine major applications of omethoate represent approximately
70% of the world demand as indicated in Table 2.
REFERENCES
Bayer Omethoate - Information submitted to FAO. Bayer A.G.,
1984 Leverhusen.
Bazzi, G., Galluzzi, G., and Nesti, V. Gas-Chromatographic
1970 determination of dimethoate residues in cattle meat.
Contributi 1968-69, 1st Ric. Agr. Soc. Montecatini X, 25-30
(Oct. 1970)
Beck, E.W., Johnson, J.C., Getz, M.E., Skinner, F.B., Dawsey, L.H.,
1968 Woodham, D.W. and Derbyshire, J.C. Effects of feeding
dimethoate, its oxygen analog and dimethoate-treated silage
to cattle. J. Econ. Entom., 61 (3):605-610.
Chamberlain, W.F.P.E., Gatterdam and Hopkins, D.E. The metabolism of
1961 labeled dimethoate in sheep. J. Econ. Entomol., 54 (4): 733-
740.
Dauterman, W.C., Casida, J.E., Kraake, J.B., and Kowalczyk, T. Bovine
1959 metabolism of organophosphorus insecticides. Metabolism and
residues associated with the oral administration of
dimethoate to rats and three lactating cows. J. Agr., Food
Chem., 7:188.
Dick, G.L., Heenan, M.P., Love, J.L., Udy, P.B. and Davidson, F.
1978 Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus
pesticide residue content. N.Z.J. Sci., 21 (1):71-78.
Duff, W.G. and Menzer, R.E. Persistence, mobility, and degradation of
1973 14C-dimethoate in soils. Environ. Entomol. 2 (3):309-318.
Ecker, W., and Coelln, R. Biotransformation of omethoate. PF-Report:
1981 1574 Aug.7, 1981 from PH-E Isotope Laboratory, at the
Institute of Pharmacokinetics, Wuppertal. Mobay Chemical
Corporation (Unpublished).
Grimmer, F., Dedek, W. and Liebnitz, E. Zur Kenntris der
1968 N-Butylchomalogen des Dimethoate. I. Hydrolysegesch-
Wurdigkeit und-mechanismus. Z. Naturforsch. 23. 13-17.
Harris, C.R. and Hitchon, J.L. Laboratory evaluation of candidate
1970 materials as potential soil insecticides. II. J. Econ.
Entomol., 63 (1):2-7.
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
1959 R.E., and Treiber, G. The metabolism of dimethoate in
cattle. J. Econ. Entomol. 52 (6):1190-1194.
Kawar, N.S., Iwata, Y., Dusch, M.E. and Gunther, F.A. Behaviour of
1979 dialifor, dimethoate and methidathion in artificially
fortified grape juice processed into wine. J. Environ. Sci.
Health, Part B, 14 (5):505-513.
Maximow, N.A., Kurzes Lehrbuch der Pflanzenphysiologie, S. 354-361.
1951 Verlag Kultur and Fortschritt, Berlin.
Netherlands Information on pesticides included in the JMPR priority
1984 list. Submission to FAO dated 4 May 1984.
Oehlmann, L. and Wagner, K. Metabolism of omethoate in soil and sugar
1983 beet. Anal. Chem. Symp. Ser., Chromatogr. Mass Spectrom.
Biomed, 14, 2:53-6.
Santi, R. and de Pietri-Tonelli, P. Mode of action and biological
1959 properties of dimethoate. Nature 183:398 (quoted by
Dauterman et al., 1960).
Steller, W.A. and Pasarella, N.R. Gas-liquid chromatographic method
1972 for the determination of dimethoate and dimethoxon residues
in plant and animal tissues, milk and eggs. J. Assoc. Offic.
Anal. Chem., 55 (6):1280-1287.
Steller, W.A. and Brand, W.W. Analysis of dimethoate-treated grapes
1974 for the N-hydroxymethyl and de-N-methyl metabolites and for
their sugar adducts. Agr. Food Chem., 22 (3): 445-449.
Ten Broeke, R. and Dornseiffen, J.W., Residuen van dimethoate en
1973 omethoate in witlof in diverse stadia de hueshoudelijke
bereiding. Report KvW 173/WRV (73) 34.6.2.1 Keuringsdienst
van Waren - Amsterdam November 1973.
Wagner, K. and Frehse, H. Method for the determination of residues of
1976 Folimat insecticide/acaricide. Pflanzenschutz-Nachrichten
Bayer, 29 (1):54-66.
Wagner, K., Neitzel, H., Oehlmann, L. and Pahlke, H. Metabolism of
1981 omethoate in sugar beets. Bayer AG Report RA-6/OEL,2 Bayer
AG Leverkusen, Federal Republic of Germany, Jan. 23, 1981.
Woodham, D.W., Reeves, R.G., Williams, C.B., Richardson, H. and
1974a Bond,C.A. Residues of dimethoate and its oxygen analogue on
and in citrus leaves following helicopter treatment of the
trees with dimethoate ultra-low volume concentrate and high
volume spray. J. Agr. Food Chem., 22 (4):731-733.
Woodham, D.W., Hatchett, J.C. and Bond, C.A. Comparison of dimethoate
1974b and dimethoxon residues in citrus leaves and grapefruit
following foliar treatment with dimethoate wettable powder
with and without surfactant. J. Agr. Food Chem.,
22 (2):239-242.
 Citric acid (XV)
The mono-, di- and tricarboxylic acids were isolated and
identified after derivatization with diazomethane.
Approximately 30% of the initial radioactivity was found as
3-hydroxy-3(methylcarbamoylmethylthio)propionic acid (IX), which is
the major alteration product of omethoate in sugar beets. The
oxidation product X of IX was found at a concentration of 10% of the
initial radioactivity 21 days after application of the parent
compound. Both compounds were found predominantly in the polar
methanol and aqueous leaf percolates.
The water-soluble, highly polar demethyl-omethoate (II) reached a
concentration of 4.2% of the initial radioactivity 21 days after
application of the parent compound.
Up to 1.4% of the initial radioactivity was found as
2,2'-dithiobis (N-methylacetamide) (VI), which is also water-soluble
but distinctly less polar.
The authors were able to isolate "N-hydroxymethyl-omethoate"
(III), as a short-lived intermediate in the chloroform percolate of
the sugar beet leaves at a maximum concentration of 0.6% of the
initial radioactivity.
The occurrence of genuine intracellular, biochemical degradation
processes was confirmed by isolation and identification of
2-hydroxy-N,N'-dimethylbutandiamide (XIV) and citric acid, XV -
both at a maximum of 2.5% of the initial radioactivity, using
radioactively labelled material.
Vacuum infiltration studies (Maximov, 1951) using unlabelled
N-methyl thioglycolic acid amide, which must represent a key
intermediate product in omethoate metabolism, showed that
(methylcarbamoylmethyldithio)acetic acid (V) was the major alteration
product of isolated sugar beet leaves after only 30 minutes.
100% recovery of the initial radioactivity was achieved in a
"closed system" 14 days after parent compound application. An "open
system" resulted in 81, 80 and 73% recovery 7, 14 and 21 days after
the beginning of the study, respectively.
Components IV, VII and XIII were obtained from unlabelled
experimental batches with higher omethoate concentrations.
Oehlmann and Wagner (1983) reported that some of the highly polar
metabolites of omethoate formed in sugarbeets and soil did not give
interpretable electron impact mass spectra and also that the molecular
ion for the main metabolite could not be detected. In the field
desorption mass spectra the molecular ions of all 3 reported compounds
were found. They reported that the spectroscopic data confirmed the
structures of the metabolites.
In Soil
Attention is directed to the publications by Harris and Hitchon
(1970) and Duff and Menzer (1973) reviewed in the dimethoate
monograph, and to the 1967 evaluation of dimethoate.
The high solubility of omethoate in water (miscible in any ratio:
Wagner and Frehse, 1976) greatly increases the likelihood of leaching
but this is offset by the readiness with which the compound is
biodegraded in biologically active soil.
In Water
Wagner and Frehse (1976) report the stability of dimethoate and
omethoate in water as follows:
Stability (half-life in days)
Temperature°C pH omethoate dimethoate
(1) 25° 2 62 176
(1) " 4 74 160
(1) " 6 32.5 125
(1) " 8 2.8 38
(2) 21° 9 0.3 5.8
(1) Taken from Grimmer et al. 1968
(2) Taken from Santi and de Pietri-Tonelli (1959)
Grimmer et al. (1968) also determined the following
distribution coefficients:
omethoate dimethoate
chloroform:water 1.15 8.4
ethyl acetate:water 0.5 5.1
benzene:water 0.25 2.2
In Processing and Cooking
Steller and Pasarella (1972) showed that omethoate was not
significantly affected by the fermentation process in the production
of wine by Kawar et al. (1979) showed that residues of dimethoate in
wine stored at 24°C were degraded by hydrolysis with a half-life of 30
days. Residues in wine were unchanged during 1 year in frozen storage.
The dimethoate monograph describes this work and gives information
concerning the fate of omethoate in the processing of olives into oil
and pickled olives.
Ten Broeke and Dornseiffen (1973) showed that the washing and
cooking of Witloof chicory, as practiced in domestic kitchens,
destroyed more than half the omethoate residues present in fresh
chicory. (See dimethoate monograph for details).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Dick et al. (1978) published the results of a survey of
pesticide residues in the New Zealand diet. This included
organophosphorus insecticides. The study involved the analysis of food
composites from a simulated New Zealand total diet which was collected
at quarterly intervals during 1974/75 from four major cities. The
foods were grouped into 8 classes. The methods used for analysis were
tested to determine the recovery of a range of pesticide residues at a
concentration of 0.01 mg/kg. The recovery of omethoate from 4 major
categories was reported to be 100%. Omethoate was found in the fruit
group from each of the 4 cities during 2 or 3 periods of the survey.
The concentration in the composites ranged from 0.03 mg/kg to
0.04 mg/kg.
The investigators reported daily intakes ranging from 6 to 59
micrograms for individuals in the separate cities during different
periods of the year. The temporary ADI is equivalent to 35 mg for an
individual weighing 70 kg. Only one of the 16 separate composites
represented an intake greater than 35 mg per day. The authors comment
that omethoate was detected in 60% of the fruit composites whereas a
similar survey carried out in 1974 revealed omethoate in only 1% of
the samples. It seems highly likely that the omethoate residues were
derived from the direct use of omethoate insecticides rather than the
metabolism of dimethoate.
The Netherlands Government supplied comprehensive information from
surveys conducted by the Food Inspection Service, on food commodities
produced in The Netherlands and imported. The results of monitoring
during 1982 are presented in Table 5 (Netherlands 1984). Since this
period the national MRLs have been amended. Additional monitoring data
on omethoate from The Netherlands are tabulated in the evaluation of
dimethoate.
METHODS OF RESIDUE ANALYSIS
Some analytical methods for the determination of omethoate
residues are included in the present evaluation of dimethoate. Earlier
methods are reviewed in the 1967 evaluation of dimethoate and the 1971
evaluation of omethoate.
Table 5 Omethoate residues found in monitoring in the Netherlands
Food Inspection Services of the Netherlands
omethoate 1982
Other
witloof french grape citrus
endive Parsley chicory apple clementine beans grape fruit orange fruit radish
source N N N I I I I I I I I
mg/kg
0.000
0.001 - 0.100 1 1 14 2 5 1 1 1 4 1 1
0.101 - 0.200 8 1 1 1
0.201 - 0.400 11
0.401 - 0.600 6
0.601 - 0.800 1
0.800 - 1.000 1 1
> 1.000 1
(2.1)
Total 1 1 42 3 5 1 1 1 6 2 1
N = Netherlands
I = imported
U = unknown
--- = national tolerance
Multi-residue methods recommended by the Ad Hoc Working Group
on Methods of Analysis of the CCPR which apply to both dimethoate and
omethoate are noted in the accompanying evaluation of dimethoate. Of
these, the methods of Steller and Pasarella (1972) and Wagner and
Frehse (1976) are described in more detail below.
Steller and Pasarella (1972) reported a gas-liquid
chromatographic method for the determination of omethoate and
dimethoate residues in plant and animal tissues, milk and eggs, using
a 60 cm. × 4 mm. i.d. glass column packed with 11% DC-200 on
60-80 mesh Gas-Chrom Q previously treated with 0.01% Versamid 900. The
GLC column was operated at 160°C using a flame photometric detector
with a phosphorus filter or at 165°C using an alkali flame ionization
detector. The column should be conditioned by injecting omethoate,
dimethoate and polyethylene glycol 400. Prior to gas chromatography,
it is essential that extracts from cattle and poultry tissues, milk,
eggs, cottonseed and oil, safflower seeds, wheat and sorghum grain and
alfalfa are cleaned up by silica gel chromatography, primarily to
remove oily or waxy extractives which, if not removed, would greatly
shorten the effective GLC column life.
Steller and Brand (1974) modified these conditions for the
determination of omethoate and dimethoate and the potential
metabolites de-N-methyl-omethoate, de-N-methyl-dimethoate,
N-hydroxymethyl-omethoate, N-hydroxymethyl-dimethoate and
N-hydroxymethyl-dimethoate-O-glucoside in grapes. A 120 cm. × 3 mm.
i.d. glass GLC column was packed with 1% EGSS-X on Gas-Chrom 0,
60-80 mesh, and operated at 180°C. The retention times were 1.2
minutes for omethoate; 1.5 for dimethoate; 2.3 minutes for
des-N-methyl- and N-hydroxymethyl-omethoate; 3.2 minutes for
des-N-methyl- and N-hydroxymethyl-dimethoate. The column must be
conditioned by firstly injecting Silyl 8 followed by injections of
des-N-methyl-dimethoate and grape extracts. The N-hydroxymethyl
derivatives are converted to their corresponding des-N-methyl
derivatives by heat on the GLC column. Of all the mentioned compounds,
only omethoate was present as a metabolite 28 days after the
application of dimethoate (sensitivity limit of 0.05 mg/kg).
Woodham et al. (1974a,b) determined both omethoate and
dimethoate on and in citrus fruits and leaves by a gas
chromatographic-flame photometric detection procedure (using
phosphorus and sulphur interference filters), although on separate
columns. They used 15 cm. × 6 mm. o.d. glass columns packed with 3%
DC-200 on 100-200 mesh Gas-Chrom 0 for dimethoate and 10% DC-200 on
100-200 mesh Gas-Chrom for omethoate. The temperature of each column
was 200°C. Retention times were not given. The recoveries ranged from
60 to 70%.
The method of Steller and Pasarella (1972) has superseded
an older method described by Bazzi et al. (1970) for the
gas-chromatographic determination of dimethoate residues in cattle
meat (using an electron-capture detector).
Wagner and Frehse (1976) described a method for the
gas-chromatographic determination of omethoate residues in plant
material, soil and water. It is intended chiefly for the determination
of residues after the application of omethoate, but also permits
determination of both omethoate and dimethoate or of dimethoate alone.
The prepared samples are extracted by macerating with acetone or
aqueous methanol, and the macerate is extracted with chloroform or
dichloromethane. The extracts are evaporated to dryness, dissolved in
acetone and, without further clean-up, analyzed by gas chromatography
using an AFID. Columns packed with 10% DC-200 + 1.5% 0F1 or with 10%
OV17, on 80-100 mesh Gas-Chrom 0 are the most suitable for the
separation of omethoate and dimethoate.
APPRAISAL
Omethoate was re-evaluated in conjunction with dimethoate in order
to determine whether it would be possible to recommend separate MRLs
for the two compounds for residues arising both as a consequence of
the metabolism of applied dimethoate and from the use of omethoate
directly as an insecticide.
Extensive information from the open scientific literature on
dimethoate was evaluated and recommendations were made. Additional
information on omethoate was received from Australia, The Netherlands,
New Zealand and the manufacturers. The similarity in the use patterns
of dimethoate and omethoate was noted.
Results from 22 residue trials on apples, peaches and hops were
reviewed. These served to confirm the validity of the MRLs previously
recommended.
Results of official trials on Kiwi fruit were received from New
Zealand. These enabled the meeting to estimate a maximum residue level
which was recommended as a temporary MRL.
An extensive study of the metabolism of omethoate in sugar beet
plants provided a further inside into the fate of omethoate in plants
and an indication that residues are converted into natural plant
products through a number of well-defined steps.
Results from several national monitoring programmes indicate that
the incidence and level of omethoate residues in the diet is low and
that the intake by consumers does not exceed the ADI.
RECOMMENDATIONS
The meeting recommends the following temporary MRL for omethoate
on the basis of residues found at harvest.
Commodity MRL PHI
(mg/kg)
Kiwi fruit 2 21
The nine major applications of omethoate represent approximately
70% of the world demand as indicated in Table 2.
REFERENCES
Bayer Omethoate - Information submitted to FAO. Bayer A.G.,
1984 Leverhusen.
Bazzi, G., Galluzzi, G., and Nesti, V. Gas-Chromatographic
1970 determination of dimethoate residues in cattle meat.
Contributi 1968-69, 1st Ric. Agr. Soc. Montecatini X, 25-30
(Oct. 1970)
Beck, E.W., Johnson, J.C., Getz, M.E., Skinner, F.B., Dawsey, L.H.,
1968 Woodham, D.W. and Derbyshire, J.C. Effects of feeding
dimethoate, its oxygen analog and dimethoate-treated silage
to cattle. J. Econ. Entom., 61 (3):605-610.
Chamberlain, W.F.P.E., Gatterdam and Hopkins, D.E. The metabolism of
1961 labeled dimethoate in sheep. J. Econ. Entomol., 54 (4): 733-
740.
Dauterman, W.C., Casida, J.E., Kraake, J.B., and Kowalczyk, T. Bovine
1959 metabolism of organophosphorus insecticides. Metabolism and
residues associated with the oral administration of
dimethoate to rats and three lactating cows. J. Agr., Food
Chem., 7:188.
Dick, G.L., Heenan, M.P., Love, J.L., Udy, P.B. and Davidson, F.
1978 Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus
pesticide residue content. N.Z.J. Sci., 21 (1):71-78.
Duff, W.G. and Menzer, R.E. Persistence, mobility, and degradation of
1973 14C-dimethoate in soils. Environ. Entomol. 2 (3):309-318.
Ecker, W., and Coelln, R. Biotransformation of omethoate. PF-Report:
1981 1574 Aug.7, 1981 from PH-E Isotope Laboratory, at the
Institute of Pharmacokinetics, Wuppertal. Mobay Chemical
Corporation (Unpublished).
Grimmer, F., Dedek, W. and Liebnitz, E. Zur Kenntris der
1968 N-Butylchomalogen des Dimethoate. I. Hydrolysegesch-
Wurdigkeit und-mechanismus. Z. Naturforsch. 23. 13-17.
Harris, C.R. and Hitchon, J.L. Laboratory evaluation of candidate
1970 materials as potential soil insecticides. II. J. Econ.
Entomol., 63 (1):2-7.
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
1959 R.E., and Treiber, G. The metabolism of dimethoate in
cattle. J. Econ. Entomol. 52 (6):1190-1194.
Kawar, N.S., Iwata, Y., Dusch, M.E. and Gunther, F.A. Behaviour of
1979 dialifor, dimethoate and methidathion in artificially
fortified grape juice processed into wine. J. Environ. Sci.
Health, Part B, 14 (5):505-513.
Maximow, N.A., Kurzes Lehrbuch der Pflanzenphysiologie, S. 354-361.
1951 Verlag Kultur and Fortschritt, Berlin.
Netherlands Information on pesticides included in the JMPR priority
1984 list. Submission to FAO dated 4 May 1984.
Oehlmann, L. and Wagner, K. Metabolism of omethoate in soil and sugar
1983 beet. Anal. Chem. Symp. Ser., Chromatogr. Mass Spectrom.
Biomed, 14, 2:53-6.
Santi, R. and de Pietri-Tonelli, P. Mode of action and biological
1959 properties of dimethoate. Nature 183:398 (quoted by
Dauterman et al., 1960).
Steller, W.A. and Pasarella, N.R. Gas-liquid chromatographic method
1972 for the determination of dimethoate and dimethoxon residues
in plant and animal tissues, milk and eggs. J. Assoc. Offic.
Anal. Chem., 55 (6):1280-1287.
Steller, W.A. and Brand, W.W. Analysis of dimethoate-treated grapes
1974 for the N-hydroxymethyl and de-N-methyl metabolites and for
their sugar adducts. Agr. Food Chem., 22 (3): 445-449.
Ten Broeke, R. and Dornseiffen, J.W., Residuen van dimethoate en
1973 omethoate in witlof in diverse stadia de hueshoudelijke
bereiding. Report KvW 173/WRV (73) 34.6.2.1 Keuringsdienst
van Waren - Amsterdam November 1973.
Wagner, K. and Frehse, H. Method for the determination of residues of
1976 Folimat insecticide/acaricide. Pflanzenschutz-Nachrichten
Bayer, 29 (1):54-66.
Wagner, K., Neitzel, H., Oehlmann, L. and Pahlke, H. Metabolism of
1981 omethoate in sugar beets. Bayer AG Report RA-6/OEL,2 Bayer
AG Leverkusen, Federal Republic of Germany, Jan. 23, 1981.
Woodham, D.W., Reeves, R.G., Williams, C.B., Richardson, H. and
1974a Bond,C.A. Residues of dimethoate and its oxygen analogue on
and in citrus leaves following helicopter treatment of the
trees with dimethoate ultra-low volume concentrate and high
volume spray. J. Agr. Food Chem., 22 (4):731-733.
Woodham, D.W., Hatchett, J.C. and Bond, C.A. Comparison of dimethoate
1974b and dimethoxon residues in citrus leaves and grapefruit
following foliar treatment with dimethoate wettable powder
with and without surfactant. J. Agr. Food Chem.,
22 (2):239-242.
Citric acid (XV)
The mono-, di- and tricarboxylic acids were isolated and
identified after derivatization with diazomethane.
Approximately 30% of the initial radioactivity was found as
3-hydroxy-3(methylcarbamoylmethylthio)propionic acid (IX), which is
the major alteration product of omethoate in sugar beets. The
oxidation product X of IX was found at a concentration of 10% of the
initial radioactivity 21 days after application of the parent
compound. Both compounds were found predominantly in the polar
methanol and aqueous leaf percolates.
The water-soluble, highly polar demethyl-omethoate (II) reached a
concentration of 4.2% of the initial radioactivity 21 days after
application of the parent compound.
Up to 1.4% of the initial radioactivity was found as
2,2'-dithiobis (N-methylacetamide) (VI), which is also water-soluble
but distinctly less polar.
The authors were able to isolate "N-hydroxymethyl-omethoate"
(III), as a short-lived intermediate in the chloroform percolate of
the sugar beet leaves at a maximum concentration of 0.6% of the
initial radioactivity.
The occurrence of genuine intracellular, biochemical degradation
processes was confirmed by isolation and identification of
2-hydroxy-N,N'-dimethylbutandiamide (XIV) and citric acid, XV -
both at a maximum of 2.5% of the initial radioactivity, using
radioactively labelled material.
Vacuum infiltration studies (Maximov, 1951) using unlabelled
N-methyl thioglycolic acid amide, which must represent a key
intermediate product in omethoate metabolism, showed that
(methylcarbamoylmethyldithio)acetic acid (V) was the major alteration
product of isolated sugar beet leaves after only 30 minutes.
100% recovery of the initial radioactivity was achieved in a
"closed system" 14 days after parent compound application. An "open
system" resulted in 81, 80 and 73% recovery 7, 14 and 21 days after
the beginning of the study, respectively.
Components IV, VII and XIII were obtained from unlabelled
experimental batches with higher omethoate concentrations.
Oehlmann and Wagner (1983) reported that some of the highly polar
metabolites of omethoate formed in sugarbeets and soil did not give
interpretable electron impact mass spectra and also that the molecular
ion for the main metabolite could not be detected. In the field
desorption mass spectra the molecular ions of all 3 reported compounds
were found. They reported that the spectroscopic data confirmed the
structures of the metabolites.
In Soil
Attention is directed to the publications by Harris and Hitchon
(1970) and Duff and Menzer (1973) reviewed in the dimethoate
monograph, and to the 1967 evaluation of dimethoate.
The high solubility of omethoate in water (miscible in any ratio:
Wagner and Frehse, 1976) greatly increases the likelihood of leaching
but this is offset by the readiness with which the compound is
biodegraded in biologically active soil.
In Water
Wagner and Frehse (1976) report the stability of dimethoate and
omethoate in water as follows:
Stability (half-life in days)
Temperature°C pH omethoate dimethoate
(1) 25° 2 62 176
(1) " 4 74 160
(1) " 6 32.5 125
(1) " 8 2.8 38
(2) 21° 9 0.3 5.8
(1) Taken from Grimmer et al. 1968
(2) Taken from Santi and de Pietri-Tonelli (1959)
Grimmer et al. (1968) also determined the following
distribution coefficients:
omethoate dimethoate
chloroform:water 1.15 8.4
ethyl acetate:water 0.5 5.1
benzene:water 0.25 2.2
In Processing and Cooking
Steller and Pasarella (1972) showed that omethoate was not
significantly affected by the fermentation process in the production
of wine by Kawar et al. (1979) showed that residues of dimethoate in
wine stored at 24°C were degraded by hydrolysis with a half-life of 30
days. Residues in wine were unchanged during 1 year in frozen storage.
The dimethoate monograph describes this work and gives information
concerning the fate of omethoate in the processing of olives into oil
and pickled olives.
Ten Broeke and Dornseiffen (1973) showed that the washing and
cooking of Witloof chicory, as practiced in domestic kitchens,
destroyed more than half the omethoate residues present in fresh
chicory. (See dimethoate monograph for details).
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Dick et al. (1978) published the results of a survey of
pesticide residues in the New Zealand diet. This included
organophosphorus insecticides. The study involved the analysis of food
composites from a simulated New Zealand total diet which was collected
at quarterly intervals during 1974/75 from four major cities. The
foods were grouped into 8 classes. The methods used for analysis were
tested to determine the recovery of a range of pesticide residues at a
concentration of 0.01 mg/kg. The recovery of omethoate from 4 major
categories was reported to be 100%. Omethoate was found in the fruit
group from each of the 4 cities during 2 or 3 periods of the survey.
The concentration in the composites ranged from 0.03 mg/kg to
0.04 mg/kg.
The investigators reported daily intakes ranging from 6 to 59
micrograms for individuals in the separate cities during different
periods of the year. The temporary ADI is equivalent to 35 mg for an
individual weighing 70 kg. Only one of the 16 separate composites
represented an intake greater than 35 mg per day. The authors comment
that omethoate was detected in 60% of the fruit composites whereas a
similar survey carried out in 1974 revealed omethoate in only 1% of
the samples. It seems highly likely that the omethoate residues were
derived from the direct use of omethoate insecticides rather than the
metabolism of dimethoate.
The Netherlands Government supplied comprehensive information from
surveys conducted by the Food Inspection Service, on food commodities
produced in The Netherlands and imported. The results of monitoring
during 1982 are presented in Table 5 (Netherlands 1984). Since this
period the national MRLs have been amended. Additional monitoring data
on omethoate from The Netherlands are tabulated in the evaluation of
dimethoate.
METHODS OF RESIDUE ANALYSIS
Some analytical methods for the determination of omethoate
residues are included in the present evaluation of dimethoate. Earlier
methods are reviewed in the 1967 evaluation of dimethoate and the 1971
evaluation of omethoate.
Table 5 Omethoate residues found in monitoring in the Netherlands
Food Inspection Services of the Netherlands
omethoate 1982
Other
witloof french grape citrus
endive Parsley chicory apple clementine beans grape fruit orange fruit radish
source N N N I I I I I I I I
mg/kg
0.000
0.001 - 0.100 1 1 14 2 5 1 1 1 4 1 1
0.101 - 0.200 8 1 1 1
0.201 - 0.400 11
0.401 - 0.600 6
0.601 - 0.800 1
0.800 - 1.000 1 1
> 1.000 1
(2.1)
Total 1 1 42 3 5 1 1 1 6 2 1
N = Netherlands
I = imported
U = unknown
--- = national tolerance
Multi-residue methods recommended by the Ad Hoc Working Group
on Methods of Analysis of the CCPR which apply to both dimethoate and
omethoate are noted in the accompanying evaluation of dimethoate. Of
these, the methods of Steller and Pasarella (1972) and Wagner and
Frehse (1976) are described in more detail below.
Steller and Pasarella (1972) reported a gas-liquid
chromatographic method for the determination of omethoate and
dimethoate residues in plant and animal tissues, milk and eggs, using
a 60 cm. × 4 mm. i.d. glass column packed with 11% DC-200 on
60-80 mesh Gas-Chrom Q previously treated with 0.01% Versamid 900. The
GLC column was operated at 160°C using a flame photometric detector
with a phosphorus filter or at 165°C using an alkali flame ionization
detector. The column should be conditioned by injecting omethoate,
dimethoate and polyethylene glycol 400. Prior to gas chromatography,
it is essential that extracts from cattle and poultry tissues, milk,
eggs, cottonseed and oil, safflower seeds, wheat and sorghum grain and
alfalfa are cleaned up by silica gel chromatography, primarily to
remove oily or waxy extractives which, if not removed, would greatly
shorten the effective GLC column life.
Steller and Brand (1974) modified these conditions for the
determination of omethoate and dimethoate and the potential
metabolites de-N-methyl-omethoate, de-N-methyl-dimethoate,
N-hydroxymethyl-omethoate, N-hydroxymethyl-dimethoate and
N-hydroxymethyl-dimethoate-O-glucoside in grapes. A 120 cm. × 3 mm.
i.d. glass GLC column was packed with 1% EGSS-X on Gas-Chrom 0,
60-80 mesh, and operated at 180°C. The retention times were 1.2
minutes for omethoate; 1.5 for dimethoate; 2.3 minutes for
des-N-methyl- and N-hydroxymethyl-omethoate; 3.2 minutes for
des-N-methyl- and N-hydroxymethyl-dimethoate. The column must be
conditioned by firstly injecting Silyl 8 followed by injections of
des-N-methyl-dimethoate and grape extracts. The N-hydroxymethyl
derivatives are converted to their corresponding des-N-methyl
derivatives by heat on the GLC column. Of all the mentioned compounds,
only omethoate was present as a metabolite 28 days after the
application of dimethoate (sensitivity limit of 0.05 mg/kg).
Woodham et al. (1974a,b) determined both omethoate and
dimethoate on and in citrus fruits and leaves by a gas
chromatographic-flame photometric detection procedure (using
phosphorus and sulphur interference filters), although on separate
columns. They used 15 cm. × 6 mm. o.d. glass columns packed with 3%
DC-200 on 100-200 mesh Gas-Chrom 0 for dimethoate and 10% DC-200 on
100-200 mesh Gas-Chrom for omethoate. The temperature of each column
was 200°C. Retention times were not given. The recoveries ranged from
60 to 70%.
The method of Steller and Pasarella (1972) has superseded
an older method described by Bazzi et al. (1970) for the
gas-chromatographic determination of dimethoate residues in cattle
meat (using an electron-capture detector).
Wagner and Frehse (1976) described a method for the
gas-chromatographic determination of omethoate residues in plant
material, soil and water. It is intended chiefly for the determination
of residues after the application of omethoate, but also permits
determination of both omethoate and dimethoate or of dimethoate alone.
The prepared samples are extracted by macerating with acetone or
aqueous methanol, and the macerate is extracted with chloroform or
dichloromethane. The extracts are evaporated to dryness, dissolved in
acetone and, without further clean-up, analyzed by gas chromatography
using an AFID. Columns packed with 10% DC-200 + 1.5% 0F1 or with 10%
OV17, on 80-100 mesh Gas-Chrom 0 are the most suitable for the
separation of omethoate and dimethoate.
APPRAISAL
Omethoate was re-evaluated in conjunction with dimethoate in order
to determine whether it would be possible to recommend separate MRLs
for the two compounds for residues arising both as a consequence of
the metabolism of applied dimethoate and from the use of omethoate
directly as an insecticide.
Extensive information from the open scientific literature on
dimethoate was evaluated and recommendations were made. Additional
information on omethoate was received from Australia, The Netherlands,
New Zealand and the manufacturers. The similarity in the use patterns
of dimethoate and omethoate was noted.
Results from 22 residue trials on apples, peaches and hops were
reviewed. These served to confirm the validity of the MRLs previously
recommended.
Results of official trials on Kiwi fruit were received from New
Zealand. These enabled the meeting to estimate a maximum residue level
which was recommended as a temporary MRL.
An extensive study of the metabolism of omethoate in sugar beet
plants provided a further inside into the fate of omethoate in plants
and an indication that residues are converted into natural plant
products through a number of well-defined steps.
Results from several national monitoring programmes indicate that
the incidence and level of omethoate residues in the diet is low and
that the intake by consumers does not exceed the ADI.
RECOMMENDATIONS
The meeting recommends the following temporary MRL for omethoate
on the basis of residues found at harvest.
Commodity MRL PHI
(mg/kg)
Kiwi fruit 2 21
The nine major applications of omethoate represent approximately
70% of the world demand as indicated in Table 2.
REFERENCES
Bayer Omethoate - Information submitted to FAO. Bayer A.G.,
1984 Leverhusen.
Bazzi, G., Galluzzi, G., and Nesti, V. Gas-Chromatographic
1970 determination of dimethoate residues in cattle meat.
Contributi 1968-69, 1st Ric. Agr. Soc. Montecatini X, 25-30
(Oct. 1970)
Beck, E.W., Johnson, J.C., Getz, M.E., Skinner, F.B., Dawsey, L.H.,
1968 Woodham, D.W. and Derbyshire, J.C. Effects of feeding
dimethoate, its oxygen analog and dimethoate-treated silage
to cattle. J. Econ. Entom., 61 (3):605-610.
Chamberlain, W.F.P.E., Gatterdam and Hopkins, D.E. The metabolism of
1961 labeled dimethoate in sheep. J. Econ. Entomol., 54 (4): 733-
740.
Dauterman, W.C., Casida, J.E., Kraake, J.B., and Kowalczyk, T. Bovine
1959 metabolism of organophosphorus insecticides. Metabolism and
residues associated with the oral administration of
dimethoate to rats and three lactating cows. J. Agr., Food
Chem., 7:188.
Dick, G.L., Heenan, M.P., Love, J.L., Udy, P.B. and Davidson, F.
1978 Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus
pesticide residue content. N.Z.J. Sci., 21 (1):71-78.
Duff, W.G. and Menzer, R.E. Persistence, mobility, and degradation of
1973 14C-dimethoate in soils. Environ. Entomol. 2 (3):309-318.
Ecker, W., and Coelln, R. Biotransformation of omethoate. PF-Report:
1981 1574 Aug.7, 1981 from PH-E Isotope Laboratory, at the
Institute of Pharmacokinetics, Wuppertal. Mobay Chemical
Corporation (Unpublished).
Grimmer, F., Dedek, W. and Liebnitz, E. Zur Kenntris der
1968 N-Butylchomalogen des Dimethoate. I. Hydrolysegesch-
Wurdigkeit und-mechanismus. Z. Naturforsch. 23. 13-17.
Harris, C.R. and Hitchon, J.L. Laboratory evaluation of candidate
1970 materials as potential soil insecticides. II. J. Econ.
Entomol., 63 (1):2-7.
Kaplanis, J.N., Robbins, W.E., Darrow, D.I., Hopkins, D.E., Monroe,
1959 R.E., and Treiber, G. The metabolism of dimethoate in
cattle. J. Econ. Entomol. 52 (6):1190-1194.
Kawar, N.S., Iwata, Y., Dusch, M.E. and Gunther, F.A. Behaviour of
1979 dialifor, dimethoate and methidathion in artificially
fortified grape juice processed into wine. J. Environ. Sci.
Health, Part B, 14 (5):505-513.
Maximow, N.A., Kurzes Lehrbuch der Pflanzenphysiologie, S. 354-361.
1951 Verlag Kultur and Fortschritt, Berlin.
Netherlands Information on pesticides included in the JMPR priority
1984 list. Submission to FAO dated 4 May 1984.
Oehlmann, L. and Wagner, K. Metabolism of omethoate in soil and sugar
1983 beet. Anal. Chem. Symp. Ser., Chromatogr. Mass Spectrom.
Biomed, 14, 2:53-6.
Santi, R. and de Pietri-Tonelli, P. Mode of action and biological
1959 properties of dimethoate. Nature 183:398 (quoted by
Dauterman et al., 1960).
Steller, W.A. and Pasarella, N.R. Gas-liquid chromatographic method
1972 for the determination of dimethoate and dimethoxon residues
in plant and animal tissues, milk and eggs. J. Assoc. Offic.
Anal. Chem., 55 (6):1280-1287.
Steller, W.A. and Brand, W.W. Analysis of dimethoate-treated grapes
1974 for the N-hydroxymethyl and de-N-methyl metabolites and for
their sugar adducts. Agr. Food Chem., 22 (3): 445-449.
Ten Broeke, R. and Dornseiffen, J.W., Residuen van dimethoate en
1973 omethoate in witlof in diverse stadia de hueshoudelijke
bereiding. Report KvW 173/WRV (73) 34.6.2.1 Keuringsdienst
van Waren - Amsterdam November 1973.
Wagner, K. and Frehse, H. Method for the determination of residues of
1976 Folimat insecticide/acaricide. Pflanzenschutz-Nachrichten
Bayer, 29 (1):54-66.
Wagner, K., Neitzel, H., Oehlmann, L. and Pahlke, H. Metabolism of
1981 omethoate in sugar beets. Bayer AG Report RA-6/OEL,2 Bayer
AG Leverkusen, Federal Republic of Germany, Jan. 23, 1981.
Woodham, D.W., Reeves, R.G., Williams, C.B., Richardson, H. and
1974a Bond,C.A. Residues of dimethoate and its oxygen analogue on
and in citrus leaves following helicopter treatment of the
trees with dimethoate ultra-low volume concentrate and high
volume spray. J. Agr. Food Chem., 22 (4):731-733.
Woodham, D.W., Hatchett, J.C. and Bond, C.A. Comparison of dimethoate
1974b and dimethoxon residues in citrus leaves and grapefruit
following foliar treatment with dimethoate wettable powder
with and without surfactant. J. Agr. Food Chem.,
22 (2):239-242.
