
CHLORPYRIFOS JMPR 1972
IDENTITY
Chemical name
O,O-Diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
Synonyms
Dowco(R)179, ENT 27311, OMS 971, DURSBAN(R)
Structural formula
 Mol. Wt. 350.6
Empirical Formula C9H11Cl3NO3PS
Other relevant chemical and physical properties
State: white, granular, crystalline solid
Melting point: 42 - 43.5°C
Vapour pressure: 1.87 × 10-5mm Hg (at 25°C)
8.87 × 10-5mm Hg (at 35°C)
Solubility: readily soluble in acetone, benzene,
chloroform, methanol and iso-octane; slightly
soluble (0.4 ppm) in water.
When chlorpyrifos was exposed to UV light or to sunlight, it underwent
hydrolysis in the presence of water to liberate
3,5,6-trichloro-2-pyridinol, which underwent further decomposition to
diols and triols and ultimately cleavage of the ring to fragmentary
products (Smith, 1968). Hydrolysis in water occurs least readily at
about pH 6 and very readily above pH 8.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrates, dusts, wettable powders and
granules).
Purity: 99.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
As with other phosphorothioate esters chlorpyrifos is rapidly
absorbed, metabolized and excreted by mammals following oral
administration. When 36Cl chlorpyrifos was fed by intubation to rats
as a single sublethal dose (50 mg/kg), the radioactivity was
eliminated rapidly (90.1% in 26 h) via the urine (90%) and faeces
(10%). Products excreted were identified as 36Cl 3,
5,6-trichloro-2-pyridyl phosphate (75-80%),
3,5,6-trichloro-2-pyridinol (15-20%) and traces of chlorpyrifos. The
pyridyl phosphate was indicated to be a transient intermediary in the
hydrolysis of chlorpyrifos to the pyridinol moiety. Analyses showed
no appreciable residue in any tissue except fat, which showed
temporary retention of radioactivity (halflife of ca 62 h) identified
principally as chlorpyrifos (Smith et al., 1967b). This study
suggests that desethylation is a significant metabolic pathway in
rats. An additional study in the rat involving administration of 14C
chlorpyrifos by single dose intubation (19.1 mg/kg) essentially
verified the earlier result (Branson and Litchfield, 1971a,b). In this
study, as well as in an in vitro study involving breakdown of
chlorpyrifos by rat liver microsomes (Branson and Wass, 1970),
3,5,6-trichloro-2-pyridinol was identified as the only major
metabolite. A study in cows involving feeding of chlorpyrifos in the
diet at 5 ppm for four days indicated that the major pathway for
degradation was by hydrolysis to the pyridinol, as no desethyl
phosphate esters were found. In addition no chlorpyrifos or
metabolites were found in milk (Gutenmann et al., 1968).
Studies of 3,5,6-trichloro-2-pyridinol, the major enolic metabolite of
chlorpyrifos, have shown absorption, distribution and excretion in the
rat following single oral administration to be similar to that
reported for the parent compound. The metabolite is rapidly absorbed
and excreted primarily in the urine and faeces. Small tissue residues
(<0.3 ppm) were present and occurred mainly in the biological systems
involved with urinary excretion, i.e. liver, kidney and blood. Unlike
chlorpyrifos, residues of the pyridinol in fat were trivial, which is
consistent with its much greater polarity (Branson and Litchfield,
1971a,b; Smith et al., 1970).
Biotransformation
The metabolic fate of chlorpyrifos has been investigated in plants
(Smith et al., 1967a, 1967b) and data indicate an oxidative and/or
hydrolytic breakdown yielding des-mono-ethylchloropyrifos; desethyl
chlorpyrifos; 3,5,6-trichloro-2-pyridinol and further degradation
products. This is discussed further in the section on "Fate of
residues."
Effects on enzymes and other biochemical parameters
Chlorpyrifos is a cholinesterase inhibitor affecting this enzyme in
brain or blood, although differing in its response with different
animal species. In vitro studies have shown the oxygen analogue of
chlorpyrifos to be 106 times more active as an inhibitor of
cholinesterase than chlorpyrifos (I50 values: chlorpyrifos approx.
2.5 x 10-3M; oxygen analogue approx. 2.5 x 10-9M) (Smith, 1966).
Equimolar concentrations of chlorpyrifos administered to dogs and
monkeys affected RBC cholinesterase in monkeys while having almost no
effect on RBC cholinesterase in dogs. Plasma cholinesterase is the
most sensitive indicator of exposure to chlorpyrifos. In dogs and
monkeys administered subacute levels of chlorpyrifos, plasma
cholinesterase was maximally inhibited within 8 hours following
exposure. Recovery in dogs was complete within 24 hours while with
monkey, plasma cholinesterase in monkeys remained depressed at 48
hours (Coulston et al., 1971).
TOXICOLOGICAL STUDIES
Acute toxicity of the metabolite 3,5,6-trichloro-pyridinol has been
studied in rats and mice. Results are summarized in Table 1. Signs of
poisoning with this metabolite include facial paralysis with dyspnoea
and slight hypersalivation and exophthalmia (mice only) (Gerbig and
Emerson, 1970 a,b).
Special short-term studies on metabolites
Rat
Groups of rats (10 males and 10 females/group) were fed the metabolite
of chlorpyrifos, 3,5,6-trichloro-2-pyridinol, in the diet for 90 days
at dosage levels of 0, 100, 300, 1 000, 3 000 and 10 000 ppm. Diuresis
was observed at 3 000 ppm. At 10 000 ppm a reduction in body-weight
was observed which reflected a general retardation in growth in both
sexes. No effects were observed on mortality, terminal haematology or
gross and histological examination of tissues (Beatty and McCollister,
1964).
Dog
Groups of dogs (3 males and 3 females/group, 4 of each sex were
controls) were fed the metabolite of chlorpyrifos,
3,5,6-trichloro-2-pyridinol, in the diet for 91 days at dosage levels
of 0, 1, 3, 10 and 30 mg/kg/day (Emerson and Gerbig, 1970). Another
study utilized groups of dogs (2 males and 2 females at the higher
level and 4 male and 4 female at the lower level) which were fed the
metabolite at dietary levels of 26 and 80 mg/kg/day for 93 days
(Copeland, 1964). At 80 mg/kg the metabolite produced abnormalities
including growth depression in females, increased spleen/body-weight
ratios and histological changes in the liver. At 26 mg/kg an elevated
SAP accompanied by equivocal changes in the liver was observed. At 30
mg/kg increased SAP, SGOT and SGPT values were observed in the second
study. The increased biochemical parameters were noted in 1 of 3 dogs
examined at 10 mg/kg. Hepatic lesions noted in 1 dog at 10 mg/kg and
1 dog at 30 mg/kg did not appear to be chemically induced. No effects
were noted at 3 mg/kg/day on growth, behaviour, haematology, blood
biochemistry, urinalysis or gross and microscopic examination of
tissues.
TABLE 1 Acute toxicity of the metabolite 3,5,6-trichloropyridinol
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 794 Gerbig and Emerson, 1970a
F 870
Mouse M Oral 380 Gerbig and Emerson, 1970b
F 415
Duckling
Groups of Peking ducklings (10 per group, 20 used as controls) were
fed the metabolite at dosage levels of 0, 1, 3, 10, 30, 60, 100, 300
and 1 000 ppm for 3 weeks to determine cataractogenicity. Growth
retardation was evident at 1 000 ppm, and 3 of 10 ducklings had
elevated prothrombin values. Levels of 300 ppm and below produced no
effect on these parameters and no cataractous changes were observed at
any level (Molello and Sharp, 1968).
Special studies in neurotoxicity
Hens (3 DeKalb leghorns/group) were administered chlorpyrifos orally
at dosage levels of 40, 75, 100 and 150 mg/kg and observed for 27
days. Mortality occurred at the higher levels. Surviving birds showed
no signs of delayed ataxia or paralysis. Crufomate administered as a
positive control (1 000 mg/kg) produced clinical signs of paralysis
(Stevenson, 1966).
Special studies on potentiation
Rats administered subacute oral dosages (1/2 the LD50 value) of
chlorpyrifos in combination with malathion or dichlorvos showed slight
evidence for potentiation of the acute toxicity. A three-fold increase
in the acute toxicity was noted with malathion, but this was judged to
be of no practical significance (Dow Chemical Co., 1964; Norris,
1970).
Special studies on reproduction and teratogenicity
Groups of rats (10 males and 20 females/group with 20 males and 10
females/group as controls) were fed chlorpyrifos in the diet for one
generation (two litters) at dosage levels of 0, 0.03, 0.1 and 0.3
mg/kg and for two generations (two litters per generation) at dosage
levels of 0, 0.1, 0.3 and 1.0 mg/kg. The F3b litter was used in a
teratology study where the parents were fed dietary levels of
chlorpyrifos until day 6 - 15 of organogenesis when the dietary
supplement was removed and chlorpyrifos administered by gavage once
daily. After day 15 chlorpyrifos was again administered by dietary
supplement until day 20 when the females were sacrificed, foetuses
removed by caesarean section and viscera and skeletal structure
examined. Neonatal mortality was somewhat higher in F2a, F2b and F3a
litters at 1.0 mg/kg. No other significant effects were observed on
fertility, reproduction or lactation indices or on teratological
examination of F3b pups as a result of feeding chlorpyrifos at levels
up to 1.0 mg/kg/day. Cholinesterase depression was observed at levels
of 0.3 mg/kg and above, primarily in plasma (Thompson et al., 1971).
Acute toxicity
The acute toxicity of chlorpyrifos has been studied in several animal
species, and the results are summarized in Table 2.
TABLE 2 Acute toxicity of chlorpyrifos in different animal species
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 118 - 245 Taylor and Olson, 1963;
Gaines, 1969; Tucker and
Crabtree, 1970
F Oral 82 - 135 Taylor and Olson, 1963;
Gaines, 1969
Mouse M Oral 102 Coulston et al., 1971
- i.p. 31 USAEHA, 1968
White
footed - Oral 64 Kenaga, 1967
Mouse
Rabbit - Oral 1 000 - 2 000 Taylor and Olson, 1963
Cavy M Oral 504 Ibid.
Goat F Oral 500 - 1 000 Tucker and Crabtree, 1970
Chukar F Oral 61.1 Ibid.
M Oral 60.7 Ibid.
Bullfrog M Oral >400 Ibid.
Chick M Oral 25 - 35 Taylor and Olson, 1963;
Stevenson, 1966; Sherman
et al., 1967; Brust
Chicken - Oral 32 - 63 et al., 1971
The LD50 in a number of avian species has been determined by Tucker
and Crabtree (1970). The toxicity values range from 8 mg/kg in male
ring necked pheasant to 167 mg/kg in mallard ducklings.
Signs of poisoning with chlorpyrifos are typical of cholinesterase
inhibiting compounds. Mortality following acute oral dosing may be
delayed for as long as nine days in some species of animals (Tucker
and Crabtree, 1970).
Chlorpyrifos administered to the conjunctival sac of rabbits produced
transient redness of the conjunctiva. No corneal injury was observed
(Taylor and Olson, 1963). When applied to intact or abraded abdominal
skin of rabbits daily for up to 2 weeks no significant dermal damage
was observed. Prolonged contact produced burning and hardening of the
skin, slight swelling and mild hyperemia which returned to normal
within three weeks. Chlorpyrifos administered dermally as a technical
product to rabbits in an undiluted form produced no untoward
physiological effects. When administered as a 50% solution in
dipropylene glycol methyl ether, the same dose (2 000 mg/kg) was
rapidly absorbed and resulted in death in four out of six animals when
applied to either intact or abraded skin (Taylor and Olson, 1963).
Chlorpyrifos did not induce sensitization when examined on the guinea
pig (USAEHA, 1968).
Dogs and sheep were exposed for a 4-hour period to a thermal fog or
liquid aerosol at a concentration of 4 or 8 mg/cu ft. Mild plasma
cholinesterase depression in dogs at the higher dose was the only
effect noted (Dow Chemical Co., 1966a).
Five female rats were exposed for 7 hours/day to an atmosphere of
chlorpyrifos (calculated at 7 mg/l air) for 16 exposures over a 21-day
interval. There were no effects on growth, appearance, behaviour or
plasma and RBC cholinesterase activity (Dow Chemical Co., 1966b).
Short-term studies
Mallard duck
Groups of mallard ducklings (6 - 8 birds/group, 5 days old) were fed
chlorpyrifos for five days at dosage levels of 0, 100, 200, 300, 500,
700, 1 000 and 2 000 ppm in the diet. Mortality occurred at all dosage
levels (100-2/8; 200-4/7; 300-5/8; 500-6/8; 700 - 2000 - all dead)
except in controls (0/7 dead) (Stevenson, 1965).
Chickens
Groups of chicks (20 chicks/group) were fed chlorpyrifos in the diet
for two weeks at dosages of 0, 200, 400 and 800 ppm. Mortality was
observed at 400 ppm and plasma cholinesterase depression and reduced
weight gain was observed at all levels (Sherman et al., 1967).
Groups of chickens (30 per group) were administered chlorpyrifos in
the drinking water at dosage levels of 0, 1 ppb, 1 ppm and 100 ppm for
84 days. A higher level (1000 ppm) resulted in clinical signs of
cholinergic stimulation. Plasma cholinesterase was depressed at 100
ppm but not at 1 ppm. A 10 ppm group was tested for two weeks with no
signs of cholinesterase depression. Growth and behaviour were not
affected at 100 ppm (Stevenson and Daering, 1969). Chlorpyrifos added
to the drinking water of chicks at dosages of 0, 0.08, 0.32, 1.25, 5,
20, 80, 320 and 1 280 ppm for periods varying from 3 - 5 weeks induced
mortality and reduced growth at levels of 80 ppm and above. Whole
blood cholinesterase depression was evident only at 80 ppm and above
(Brust et al., 1971).
Chickens fed chlorpyrifos in the diet at levels of 0, 25, 50 and 100
ppm for four weeks exhibited reduced plasma cholinesterase activity
and reduced weight gain at all feeding levels. Calculations of food
conversion data show a reduced yield in production as a result of 25
ppm in the diet. No clinical signs of toxicity were observed
(Schlinke, 1970).
Rat
Groups of rats (20 males and 20 females/group) were fed chlorpyrifos
in the diet for six months at dosage levels of 0, 0.03, 0.15 and 0.75
mg/kg/day. Cholinesterase depression was noted at 0.75 mg/kg/day in
RBC and plasma. Brain cholinesterase activity was unaffected. No
effects were noted at any feeding level on mortality, behaviour,
growth, haematology or gross or histological examination of tissue
(Coulston et al., 1971).
Groups of rats (10 males and 10 females/group) were fed chlorpyrifos
in the diet for 90 days at levels of 0,10, 30, 100 and 300 ppm. Growth
reduction was observed in both males and females at 300 ppm. Increased
liver and kidney organ to body-weight ratios were also observed
(possibly as a result of reduced body-weight as organ weights were
comparable to the controls). Cholinesterase depression was observed at
30 ppm in blood and brain; at 10 ppm, brain cholinesterase was normal
although enzyme inhibition was observed in RBC and plasm. No adverse
effects were noted on growth, appearance, food consumption, mortality,
behaviour, haematology or gross and microscopic pathology at 100 ppm
(Beatty and McCollister, 1964).
Five groups of rats (10 males and 10 females/group) were fed
chlorpyrifos at levels varying from 0.03 - 10.0 mg/kg/day for 28 - 90
days to determine a "no-effect" level on cholinesterase depression.
Dosage levels of 0 and 0.3 mg/kg/day were fed for 90 days while
dosages of 1, 3 and 10 mg/kg/day were fed for 28 days, stopped for
three weeks (during which time the rats were fed control diets) and
the rats refed diets containing 0, 0.03 and 0.1 mg/kg/day for a full
90-day study. Behaviour and growth were affected at 3 and 10
mg/kg/day. Cholinesterase was depressed at 0.3 mg/kg/day and above in
both male and female rats. The rats in the three highest groups were
removed for three weeks during which time cholinesterase activity
returned to pretest intervals. On resumption of feeding chlorpyrifos
at 0.1 mg/kg/day, slight cholinesterase depression was observed in
plasma and erythrocytes. No cholinesterase depression was noted on
brain or blood enzymes at 0.03 mg/kg/day (Blackmore, 1968b).
Dog
Groups of dogs (2 males and 2 females/group; 4 of each sex were
controls) were fed chlorpyrifos for 93 days. The dosage levels of
feeding were initially 200, 600 and 2 000 ppm in the diet, but because
of the appearance of signs of cholinergic poisoning the dosages were
changed from 2 000 to 60 ppm after 5 days while the 200 ppm (5.8
mg/kg/day) level was maintained for 45 days. The dogs were then placed
on control diets for the remainder of the test. Another group of 2
male and 2 female dogs was added to the study at a dietary level of
200 ppm (3.4 mg/kg/day) for 27 days, removed from the test for 5 days
and returned to this dietary level for the final 2 weeks. A decrease
in food consumption at the 200 ppm level was accompanied by reduced
growth, especially in females. Growth of all dogs was slightly lower
than control levels. Increased SGPT values were seen in several dogs
at all feeding levels. RBC, plasma and brain cholinesterase values
were reduced in all animals fed chlorpyrifos in the diet at a level of
20 ppm (0.8 mg/kg/day) and above. No adverse effects were observed on
haematology, BUN, SAP, organ weights and gross or microscopic
examination of tissues (Copeland, 1964).
Groups of dogs were orally administered chlorpyrifos daily in a
gelatin capsule for periods of 32 to 90 days at doses of 0 (2 males
and 2 females - 90 days), 0.01 mg/kg/day (2 males and 2 females - 32
days), 0.03, 0.10, 0.30 and 1.00 mg/kg/day (2 males and 2
females/group - 90 days), and a final group (2 males and 2 females)
was given 1.00 mg/kg/day from day 0 to 18, control diet from days 19
to 42, 0.03 mg/kg/day from days 43 to 58, control diet from days 59 to
77, 0.01 mg/kg/day from days 78 to 94 and control diet until day 124.
Data obtained on appearance, body-weight and food consumption were
normal. A dose-dependent cholinesterase depression in plasma was
observed to begin at 0.03 mg/kg/day. At this level inhibition was
moderate and was more marked as the dietary level increased. Brain
cholinesterase was not affected at any level, and RBC cholinesterase
was affected at levels of 0.1 mg/kg/day and above. No effects were
noted at 0.01 mg/kg/day on any parameters tested over the 32-day
administration interval (Blackmore, 1968a).
Groups of dogs (7 males and 7 females/group) were fed chlorpyrifos in
the diet for up to two years at dose levels of 0, 0.01, 0.03, 0.1, 1.0
and 3.0 mg/kg/day. Inhibition of cholinesterase activity was the only
abnormality detected. Inhibition of RBC cholinesterase in males and
females was evident at 1.0 and 3.0 mg/kg. Marginal depression of brain
cholinesterase was shown at the highest level of feeding. Plasma
cholinesterase was depressed at 0.1 mg/kg/day in both males and
females. Occasional reduction at 0.03 mg/kg was noted. Studies on the
recovery of cholinesterase activity following one year feeding
indicate that plasma activity recovered to normal within two weeks
while RBC recovery was much slower. No significant effects were noted
on mortality, behaviour, food consumption, growth, haematology, blood
biochemistry, urinalysis, or gross and microscopic examination of
tissues. Gross examination showed an increased liver weight and ratio
at 3.0 mg/kg at two years but not at one year. All other tissues were
normal (McCollister et al., 1971a).
Monkey
Groups of Rhesus monkeys (2 males and 2 females - control; 2 males and
1 female - 0.08 or 0.40 mg/kg/day; 2 males and 2 females - 2.0
mg/kg/day) were administered chlorpyrifos orally by gavage daily for
six months. Plasma and RBC cholinesterase activity was depressed in
those animals that received 2.0 and 0.4 mg chlorpyrifos/kg/day. Plasma
cholinesterase activity was slightly depressed and RBC cholinesterase
activity was not depressed in animals that received 0.08 mg/kg/day.
Brain cholinesterase activity was not affected at any level. No
significant effects were noted on behaviour, growth, haematology,
clinical chemistry or gross or histological examination of tissues.
The histological examination included electron microscopy of liver and
kidneys and histochemical delineation of lysosomal distribution in
these two organs (Coulston et al., 1971).
Long-term studies
Rats
Groups of rats (57 males and 57 females/group) were fed dietary levels
of chlorpyrifos of 0, 0.01, 0.03, 0.1, 1.0 and 3.0 mg/kg/day for two
years. Blood cholinesterase activity, primarily in plasma, was
depressed in females at 0.1 mg/kg and above. Brain cholinesterase was
inhibited at 3.0 mg/kg and slightly depressed at 1.0 mg/kg.
Chlorpyrifos at all dosage levels had no significant effect on
behaviour, appearance, growth, mortality, haematology, urinalysis,
clinical biochemistry, gross or histopathology of tissues and organs
or the incidence of neoplasms (McCollister et al., 1971b).
OBSERVATIONS IN MAN
Groups of men (4 men/group) were administered chlorpyrifos by tablet
daily for 27 days (0.014 mg/kg/day), 20 days (0.03 mg/kg/day), 9 days
(0.10 mg/kg/day) and 48 days (control). No effects were noted on
behaviour, haematology, urinalysis or biochemical determinations in
blood. Blood plasma cholinesterase was depressed at the highest dosage
and this regimen was discontinued. Recovery was complete in four
weeks. At 0.03 mg/kg/day a statistically non-significant, although
suggestive, depression of plasma cholinesterase was noted. No effects
were noted at 0.014 mg/kg on plasma cholinesterase. RBC activity was
unaffected at any level of chlorpyrifos. The level of 0.03 mg/kg/day
appears to be the minimal threshold response level in humans to
chlorpyrifos based on plasma cholinesterase (Coulston et al., 1972).
COMMENT
The compound is rapidly absorbed and metabolized by mammals, the major
metabolite being a chlorinated pyridonal hydrolysis product of low
mammalian toxicity.
There is no evidence of neurotoxicity or cataractogenicity.
Potentiation has been demonstrated only with malathion.
Reproduction studies and examination of offspring for teratogenic
abnormalities did not reveal adverse effects other than an increase in
neonatal mortality at 1 mg/kg/day. Chlorpyrifos is an active
cholinesterase inhibitor, inhibiting plasma cholinesterase to a far
greater degree than other cholinesterases. One short-term study in
rats indicated an increase in sensitivity to plasma cholinesterase
depression following withdrawal after initial treatment. This study
needs confirmation.
No-effect levels have been demonstrated in dog, rat and man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.03 mg/kg/day
Dog: 0.01 mg/kg/day
Man: 0.014 mg/kg/day orally for 1 month
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In animals
Chlorpyrifos is used to control various species of fever ticks
(Boophilus sp.), ear ticks, lice and horn flies on beef cattle and
non-lactating dairy cattle, by use of emulsifiable liquid formulations
in water varying from 0.025 to 0.125% applied as a spray or dip.
Treatment for all but ear ticks is limited to six applications at
21-day intervals, and not within two weeks of slaughter.
Sheep dipped or sprayed with wettable powder or emulsifiable
formulations of chlorpyrifos are protected from blowfly, ticks, body
lice and sheep keds. A minimum interval of seven days is required
between treatment and slaughter.
Turkey chiggers are controlled through the use of wettable powder
formulations sprayed on soil in turkey pens at the rate of 4 lb/acre
chlorpyrifos, before the turkeys are introduced into the pens.
Treatment is limited to two applications, not within seven days of
slaughter.
In plants
Soil treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control soil pests such as wireworms,
white grubs, mole crickets, Maulwurf's Grill, garden symphylans,
seed corn maggots, cereal maggots, beet root weevils, the
Atomaria, Blaniulus, Tipula and cutworms. The chemical is
applied by means of broadcast row or furrow treatments and is
varied in concentrations from 300 to 4 000 g/ha a.i. as wettable
powder, emulsifiable or granular formulations before and after
planting, previous to harvest. The crops involved are maize,
spring cereals, beans, onions, tobacco, turnips, bulb flowers and
carrots.
2. Canada
Chlorpyrifos is used to control soil insect pests of lettuce,
onions and carrots before and after planting at dosages of 1 to 2
lb/acre applied as needed, but with the indicated 28- to 60-day
withdrawal period before harvest. Tobacco plants are treated in
soil water, preplant and by soil and foliar application.
Foliar treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control insect pests of rape, cereals,
potatoes, fodder crops, beans, peas and deciduous fruits such as
apples and pears.
2. Japan, Thailand, Philippines, India and Malaysia
Chlorpyrifos is used to control insect pests of apples, pears,
rice, tobacco, Chinese cabbage and kale.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
Considerable work has been done in U.S.A. on the analysis of cattle
tissues for residues of chlorpyrifos as well as its oxygen analogue
and pyridinol moiety. Typical distribution of residues in cattle
dipped in chlorpyrifos is shown in Table 3 (Claborn et al., 1970a).
In this study, 57 beef cattle were dipped one to six times in a 0.025%
emulsion of chlorpyrifos at intervals of 21 days. Animals were removed
from the dipping schedule after each dip, except the fifth, for
post-treatment slaughter at various time intervals. Fat muscle, kidney
and liver tissue samples were collected and analysed for residues of
chlorpyrifos, its oxygen analogue, and the 3,5,6-trichloro-2-pyridinol
moiety. Residues of chlorpyrifos were predominately in the fatty
tissues, and residues of 3,5,6-trichloro-2-pyridinol were in the
kidney and liver tissues. No residue of the oxygen analogue was
detected in any tissue.
Residues of the hydrolytic pyridinol were not detectable in muscle
tissue or in kidney and liver tissues of cattle, except at low levels
for the first 1 - 2 weeks after the last dip, even after six dippings.
It should be noted that this experiment began in February, so the
animals had their winter coat of hair through the first three dippings
and were shedding it at approximately the fourth dipping period. The
heavy winter hair coat carries more insecticide out of the vats than
the summer coat, as evidenced by the higher residues after the second
and third dippings and the drop in residues after the fourth dipping.
The effect of the winter coat is also reflected by the higher residues
after the first dip when compared to levels of residues after a single
dip (in the same concentration or higher concentration - 0.05% - of
chlorpyrifos) in the animals reported by Mann, 1966; Claborn et al.,
1968a,b; and Dishburger et al., 1966a,b.
Since the season for ticks extends from approximately April or May to
mid-September, animals would be expected to shed their winter coat
after the first or second dipping when treated according to label
instructions. Therefore, in practice residue levels as high as those
found in the test animals would not be expected after the third
dipping, since these animals would have been dipped three times before
shedding their winter coat of hair. In any case, it is believed that
this experiment represents the highest levels of chlorpyrifos that
would ever be encountered in the use of 0.025% chlorpyrifos as a dip.
In an early test, cattle were sprayed once with a 0.5% emulsion of
chlorpyrifos (20 times the label recommendation). Residues of the
compound in the fat were 1.3 ppm (maximum) at 20 days post-treatment
and had declined to 0.08 ppm (maximum) at 30 days post-treatment
(Dishburger and Rice, 1965).
In cattle treated with an emulsion containing 0.05% chlorpyrifos
(twice the label recommendation), the average amounts of residue in
the fat one week after a single-dip application were about twice those
resulting from a single-spray application. The maximum residues were
well below 1 ppm (0.209 for spray and 0.525 for dip application) at
the first sampling period at seven days post-treatment. Approximately
two weeks were required for residues to be reduced to 1 ppm in the fat
of animals given multiple treatments (3 dippings at 2-week intervals)
at this level (Mann, 1966; Claborn et al., 1968a,b).
TABLE 3 Chlorpyrifos residues in cattle in the United States1
Mean residues of
Weeks Mean residues of chlorpyrifos2 3,5,6-trichloro-2-pyridinol3
after last fat muscle kidney kidney liver
dipping (ppm) (ppm)
1 dipping
1 0.672 0.004 0.003 0.12 0.12
2 0.142 0.002 0 <0.05 <0.05
3 0.083 0.001 0 0 0
4 0.038 0 0 0 0
2 dippings4
1 1.245 0.011 0.008 0.30 0.26
3 dippings4
1 1.589 0.028 0.008 0.24 0.29
2 0.085 0.011 0.001 0.05 0.05
4 0.158 0 0 0 0
6 0.038 0 0 0 0
8 0.041 0 0 0 0
10 0.001 0 0 0 0
4 dippings4
1 0.693 0.011 0.001 0.10 0.12
6 dippings4
1 0.744 0.015 0.003 0.12 0.10
2 0.141 0.001 0 0 0
4 0.069 0 0 0 0
6 0.018 0 0 0 0
8 0.007 0 0 0 0
10 0 0 0 0 0
1 Residues of chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues
of beef cattle dipped in an emulsion containing 0.025% chlorpyrifos.
Residue levels corrected for % recovery from tissues.
2 No chlorpyrifos found in liver (0 - <0.002 ppm) or oxygen
analogue (0 - <0.005 ppm) in any tissue analysed (Claborn, 1970).
3 No 3,5,6-trichloro-2-pyridinol found in fat or muscle tissues
(0 - <0.05 ppm) (Dishburger and McKellar, 1971).
4 Dipping schedule was at 21 day intervals.
The oxygen analogue was found only in samples of fat containing the
higher residues of chlorpyrifos (approximately 2 ppm); it amounted to
about 1% of the residue. No increase occurred in the amount of the
oxygen analogue as the chlorpyrifos was eliminated, and no residues of
the analogue were detected in the muscle, brain or spleen tissues of
the animals that had the highest residues of chlorpyrifos in the fat
(Claborn et al., 1968a).
Data from Mann (1966), Claborn et al. (1968a) and Dishburger
et al. (1966a,b) indicated that the biological half-life of
chlorpyrifos in tissues of cattle following a single treatment with
DURSBAN insecticide containing 0.025% and 0.05% chlorpyrifos is
approximately 4 - 6 days. Data from Mann (1966) and Claborn et al.
(1968a,b) showing multiple dippings in DURSBAN insecticide containing
0.025% and 0.05% chlorpyrifos, further establish the consistency of
the degradation ratio following the last treatment. The data of
Claborn et al. (1968b) indicate that the biological half-life of
chlorpyrifos in tissues of cattle treated at three-week intervals for
as long as six weeks with DURSBAN 24E containing 0.025% chlorpyrifos
is approximately seven days. In summary, the experiments illustrate
the consistency of a 4 - 7 day biological half-life for chlorpyrifos
in fat.
Considerable work has been carried out in Australia on the
determination of residues from the vat dip and spray application of
chlorpyrifos to cattle (Hall, 1969; Snelson, 1969b). Results shown in
table 4 indicate that residues of chlorpyrifos in omental fat are in
the same order of magnitude as those for United States, considering
the fact that most tests were at double the concentration (0.05%) in
Australia.
Snelson (1969a) studied the disappearance rate of chlorpyrifos in
butterfat in milk after a single dip application of chlorpyrifos at
0.025% to milk cows. Residue declined from 1.1 ppm after 1 day to 0.01
ppm in 9 days, and a non-detectable level at 15 days (see Table 5).
Sheep
Sheep dipped in an 0.05% chlorpyrifos emulsion showed inconsistent
levels of residues of chlorpyrifos with no apparent change in residue
levels (0.081 - 0.179 ppm) from 3 - 28 days after application. No
detectable levels (<0.005 ppm) were seen in muscle, liver or kidney
tissues at any time (Dow Chemical Co., 1967).
TABLE 4 Chlorpyrifos residues in cattle in Australia
Chlorpyrifos
Days residues in
Chlorpyrifos Application between last No. of omental fat Reference
% type no. treatment and cattle (mean values
slaughter or range, ppm)
0.025 Vat dip 3 1 0.2 Watts, 1968
4 0.96
7 0.25
1 1
3 0.11 - 0.22 Ibid.
1 1,4,8,15 0.29 - 2.18 Ibid.
3,6 1,4,8,15 0.05 - 1.19 Ibid.
0.05 Vat dip 1 1,4,8 0.41 - 0.48 Ibid.
3 1 1.17 Ibid.
4 0.98
6 1 1.75 Ibid.
8 1.36
15 1.11
22 0.6
29 0.38
0.05 Vat dip 2 0 3 1.46 - 2.26 Hall, 1969
1 2 3 2.4 - 3.87
1 2 3 1.23 - 3.09
1 2 3 0.98 - 2.54
1 1 3 0.42 - 0.79
3 1 4 1.32 - 3.80
0.05 Dip 10 5? 12 0.70 - 7.131 Snelson, 1969b
0.05 Spray 30 1 3 1.6 - 4.2 Ibid.
5 3 0.34 - 1.1
9 2 0.1 - 0.15
13 1 0.06
28 1 0.01
1 Omental and/or perirenal fat.
TABLE 5 Chlorpyrifos residues in milk of cows in Australia
Days between Chlorpyrifos1
last treatment residue in butterfat
and sampling (ppm avg. 4 cows) Reference
1 1.1 Snelson, 1969a
2 0.96
3 0.57
4 0.14
5 0.08
6 0.05
9 0.01
15 N.D.2
1 One dip application containing 0.025% chlorpyrifos.
2 N.D. = not detectable
Turkeys and chickens
Claborn et al. (1970b) determined residues of chlorpyrifos in the
body tissues of turkeys confined in pens containing soil treated with
4 or 8 lb/acre of chlorpyrifos (in a 25% wettable powder formulation)
for control of the chigger, Neoschongastia americana. The higher
concentration caused maximum residues of 0.157 and 0.066 ppm of
chlorpyrifos in the skin and fat, respectively, one week
post-treatment, that decreased to less than 0.001 ppm after six weeks.
No significant residues were found in the other tissues (thigh,
breast, liver). The oxygen analogue of chlorpyrifos (diethyl
3,5,6-trichloro-2-pyridyl phosphate) was not detected (<0.005 ppm) in
any tissues.
In a study conducted by Mann and Ivey (1971), turkeys were maintained
on soils treated two times, at an interval of 28 days, with 4 lb
chlorpyrifos/acre each time. Two or three birds were necropsied at
weekly intervals for six weeks after the first application and for
eight weeks after the second application. Tissue samples of fat, skin,
muscle, liver and kidney were analysed for residues of chlorpyrifos,
the oxygen analogue and the pyridinol moiety
(3,5,6-trichloro-2-pyridinol). Lowlevel residues of chlorpyrifos were
found in fat (0.022 - 0.005 ppm), skin (0.033 - 0.152 ppm) and kidney
(<0.002 - 0.005 ppm) of birds after one week of exposure to the
first application of compound to the soil. Fat only was analysed for
the remainder of the test for each application. The residues in fat
were reduced to <0.002 ppm (the sensitivity of the method) six weeks
after the first application and eight weeks after the second
application. No oxygen analogue was detected in any of the tissues.
Small residues of the pyridinol moiety were found in the skin of one
hen (<0.003 - 0.007 ppm), the liver of one tom (<0.01 - 0.025 ppm)
and the kidney of one tom (<0.01 - 0.098 ppm) after one week of
exposure to the first application; and in the skin (<0.003 - 0.003
ppm), liver (<0.01 - 0.078 ppm) and kidney (<0.01 - 0.165 ppm) in
one hen after one week of exposure to the second application of
compound to the soil.
Hunt et al. (1969) developed a new rapid gas chromatographic method
for analyses of chlorpyrifos in turkey and chicken tissues. The
residues reported are from the same experiments as the toxicological
results reported by Schlinke (1970) in chickens and Schlinke et al.
(1969) in turkeys. The tests consisted of dietary and contact tests
with chlorpyrifos for four weeks. Residue analyses of muscle, liver,
heart, brain, gizzard, skin and fat tissues were taken after 28 days
of treatment. Only skin and fat tissues showed detectable amounts of
chlorpyrifos. In chickens fed 100, 50 and 25 ppm in the diet, the
highest tissue residue was 0.87 ppm chlorpyrifos in fat. In turkeys
fed 100 and 50 ppm in the diet, the highest residue was 0.34 in fat.
Dishburger et al. (1969b) determined the presence of chlorpyrifos
residues in skin, fat, muscle and liver tissues of turkeys which had
been confined to soil treated with 4.5 lb chlorpyrifos/acre as
emulsifiable, granular, or wettable powder formulations. Muscle and
liver tissues contained undetectable amounts of chlorpyrifos in all
tests. Skin and fat tissues contained <0.1 ppm at all times, but were
higher at seven days post-treatment than any of the other weekly
samplings up through 28 days post-treatment.
In plants
Residues found in experiments with plants in United States
Chlorpyrifos has been applied to various species of plants and
generally found to be lacking in residual insecticidal properties on
plant-feeding insects. The residue determinations reported here
actually never exceeded 28 ppm at the dosages tested (up to 1 lb/acre)
and soon diminished, exhibiting a half-life of 1 - 2 days, thus
confirming the reason for the observed lack of residual effectiveness
on plant-feeding insects (see Table 6).
There was no evidence of residue buildup on plants from repeated
application of chlorpyrifos.
TABLE 6 Chlorpyrifos residues found in experiments in plants in United States
Dosage Application Chlorpyrifos
chlorpyrifos (no.) (no. of days Crop residues Reference
(lb/A) since last) (ppm)
1.0 1 0 Bermuda grass 13.2 Leuck et al., 1968
14 0.37
1.0 1 0 Maize 5.60 Ibid.
14 0.42
0.05 8 0 Pasture grass 0.7 - 8.2 Winterlin et al.,
7 <0.1 - 0.2 1968
0.05 1 0 Grass 0.11 - 8.1 Ibid.
3 0.012 - 1.8
0.025 1 0 Rice 0.09 - 0.64 Ibid.
6-14 <0.005 - 0.07
1.0 1 0 Various wild 26.9 Hurlbert et al.,
1 plants 27.4 1970
7 1.1
14 0.1
0.025 4 0 Rice 0.07 - 0.21 Dishburger et al.,
9 <0.01 1969c.
1.0 1 0 Maize silage 15.1 Johnson et al., 1969
1 4.4
0.25 1 0 Maize silage 2.5 Ibid.
1 1.1
Leuck et al. (1968) studied the persistence of chlorpyrifos and its
oxygen analogue on field plots of coastal Bermuda grass and maize
forage using the method of Bowman and Beroza (1967a) with a minimum
sensitivity of 0.002 - 0.01 ppm. Chlorpyrifos was applied at the rate
of 1 lb/acre. The oxygen analogue constituted a very low residue and
was only a small proportion of the total insecticide. Chlorpyrifos
residues varied from about 6 - 13 ppm at the time of application to 4
- 8 ppm after 1 day, to around 1 ppm after 7 days and to nearly
negligible levels after 21 days. The residue half-life of chlorpyrifos
appeared to be in the order of 2 - 3 days on Bermuda grass and maize
plants.
Winterlin et al. (1968) studied the residues of chlorpyrifos on rice
and pasture grass and environment when applied experimentally for the
control of mosquitoes around Colusa, California. They used the method
of Bowman and Beroza (1967a) i.e. gas chromatography with a flame
ionization detector, which is sensitive to 0.005 ppm chlorpyrifos in
rice and 0.01 ppm in pasture grass, and found residues as high as 8.11
ppm chlorpyrifos immediately after application of 0.05 lb
chlorpyrifos/acre. Residues decreased rapidly after application to
below 1 ppm.
Myers et al. (1968) reported on residues resulting from application
of chlorpyrifos sprayed by aircraft at the rate of 0.1 lb/acre, eight
times, over a period of five months on irrigated pastures. Samples of
grass analysed for the insecticide 1 - 3 hours after each spray
application varied from 0.4 - 8.2 ppm, and after seven days varied
from <0.1 - 0.2 ppm, using an analytical method sensitive to 0.1 ppm
chlorpyrifos. No evidence of accumulated residues of chlorpyrifos were
detected as the result of multiple applications to grass with
chlorpyrifos.
Hurlbert et al. (1970) reported on the residues of chlorpyrifos on
watergrass, sprangletop and smartweed on the dikes surrounding ponds,
resulting from hand-sprayed applications of chlorpyrifos emulsifiable
insecticide. Dosages used were 1, 0.1, 0.05, and 0.01 lb/acre diluted
in a constant spray volume of 8 gal/acre. Under these conditions
residues over 1 ppm occurred only at 1 lb/acre after 4 h, 1 day, and 7
days, but not 14 days; and at 0.1 lb/acre after 4 h. Residues
determined at other dosages and post-application times were less than
1 ppm. The half-life of the foliar residues of chlorpyrifos was in the
order of 1 - 3 days.
Dosages of 0.025 lb chlorpyrifos were applied aerially four times, at
monthly intervals, to rice fields. Residues of chlorpyrifos in raw
(rough) rice grain ranged from 0.07 to 0.21 ppm at 0 day, following
the last of the four applications. No residues of the oxygen analogue
of chlorpyrifos were detected, nor did detectable chlorpyrifos
residues persist more than 9 days after application (Dishburger
et al., 1969c).
Johnson et al. (1969) treated maize plants in the field with 0.25,
0.5 and 1.0 lb chlorpyrifos/acre. The initial residues in maize and
silage were 2.5, 3.9 and 15.1 ppm, respectively (on an as-collected
basis). After one day the losses of residues were 56, 40 and 71%,
respectively. This represents a half-life of about one day. One day
after treatment the maize and silage were enclosed in a silage bin
where chlorpyrifos residues declined slowly over a period of seven
weeks.
Dishburger et al. (1969a) analysed samples of tobacco leaves from
soil and foliage treated with 0.5, 1.0 and 1.5 lb chlorpyrifos/acre.
No residues (<0.01 ppm) were detected on the samples harvested (2
months or more post-application).
Residues found in experiments with plants in Canada
Residue data from experiments with onions, lettuce, carrots, and
tobacco are shown in Tables 7 and 8 using the analytical method of
Braun (1971) for chlorpyrifos and McKellar (1971c) for the pyridinol.
TABLE 7 Residues of chlorpyrifos from treatments of lettuce,
onions and carrots1
Application Residues on crop (ppm)
(lb/acre) (No.)2 lettuce onions carrots3
0.5 2 <0.001 0.001 <0.001
0.005 0.003 0.003
(54)4 (119) (138)
3 0.001 0.002 0.012
0.020 0.003 0.020
(31) (104) (123)
1.0 2 <0.001 0.002 0.012
0.008 0.005 0.034
(54) (118) (138)
3 0.002 0.002 0.006
0.009 0.005 0.018
(31) (104) (123)
2.0 2 0.003 0.008 0.015
0.029 0.013 0 046
(54) (119) (138)
2.0 3 0.008 0.003 0.019
0.034 0.005 0.033
(31) (104) (123)
1 McEwen and Frank, 1970.
2 First application was preplant soil treatment; second and third
soil and foliar treatments (Canada).
3 3,5,6-trichloro-2-pyridinol analysed for but not found at
detection limit of 0.005 ppm.
4 Days after chlorpyrifos withdrawal shown in ().
TABLE 8 Residues of chlorpyrifos from treatments of lettuce, onions,
carrots and celery
Withdrawal Applications1 Residues range
Crop (days) (no.) (ppm) References
Onions 57 1 0.001 - 0.003 McEwen, 1970
19 4 <0.0012 Harris, 1971
Celery 57 1 N.D.3 - 0.016 McEwen, 1970
Carrots 57 1 0.001 - 0.015 Ibid.
Lettuce 27 1 N.D. Ibid.
1 Treatments all post-planting, 2 lb/acre (Canada).
2 No oxygen analogue of chlorpyrifos detected at 0.01 ppm sensitivity
level.
3 N.D. = <0.001 ppm.
Residues found in experiments with plants in Europe and the Near
East
Residue data from experiments with cauliflower, red cabbage, eggplant,
beans, peppers, lettuce, mushrooms, carrots, potatoes, sugar beet,
corn, cotton, tomatoes, grapes, pears and apples are shown in Tables 9
and 10. These tests include soil and foliar applications.
Residues found in experiments with plants in Japan, Thailand,
Malaysia, and the Philippines
Residue data from experiments with Chinese cabbage and kale, rice and
apples are shown in Tables 11, 12 and 13.
TABLE 9 Residues of Chlorpyrifos in Food Crops, Europe/Near East Trials
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
FRANCE
Potatoes 1970 Granules Soil 1 1 500 g/ha 5 mo <0.005 Graf and Engeler,
5% active insecticide 1 3 500 g/ha 5 mo <0.005 1971a
W. GERMANY
Sugar beets 1971 Dursban EC Broadcast 1 1.15 kg/ha 112 d <0.13 Scheuerer & Hahn,
40.8% active Soil insect. 118 d 1972
Maize 1971 Dursban 4 EC Soil insect. 1 0.95 kg/ha 123 d <0.13 Ibid.
40.8% active Broadcast
HOLLAND
Cauliflower Strewpowder Soil insect. 1 120 mg 8 wk 0.002
Red cabbage (1) 1969 4% active 11 wk <0.002 Wit, 1970
Red cabbage (2) 85 wk <0.002
Lettuce (under 1970 3.2% active Soil insect. 1 1 900 g/ha 9 wk <0.01 Wit, 1971
glass) granule
Mushrooms 1971 25% wettable Spray 3 5 000 g/ha 3 wk 0.01 Olthof, 1971a
powder 3 wk 0.03
Carrots 1971 Dursban 25W Soil insect. 1 6 000 g/ha 3-4 mo 0.14 Olthof, 1971b
1 6 000 g/ha 3-4 mo 0.28
ISRAEL
Cotton 1970 Dursban E.C. Spray 6 730 g/ha 15 d <0.05 Graf, 1970a
40.8% active
Apples 1970 Dursban E.C. Spray 1 2 400 g/ha 7 d 0.54 Graf &
40% active 14 d 0.20 Engeler, 1970a
Tomatoes 1970 Dursban E.C. Spray 1 720 g/ha 1 d 0.24 Aharonson
40.8% active 4 d 0.16 et al., 1969
8 d 0.12
16 d 0.04
1 1 440 g/ha 1 d 0.45
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
4 d -
8 d 0.18
16 d 0.10
1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.27 Graf & Engeler,
40.8% active 5 d 0.25 1971b
Apples 1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.57 Graf & Engeler,
40.8% active 7 d 0.23 1971c
14 d 0.14
Spray 1 2 880 g/ha 1 d 0.52
7 d 0.28
14 d 0.24
Dursban Spray 1 1 440 g/ha 1 d 0.13
40.8% active 7 d 0.11
Spray 1 2 880 g/ha 1 d 0.29 Ibid.
7 d 0.24
ITALY
Sugar beets 1970 Dursban 5% Soil insect. 1 2 500 g/ha 3-6 mo <0.004 Foschi, 1971
Maize 1970 Granule Soil insect. 1 2 500 g/ha 3-6 mo <0.004
Lettuce 1971 Dursban E.C. Spray 1 500 g/ha 14 d 0.05 SIAPA, 1971
22.5% 1 1 000 g/ha 14 d 0.07
Apples 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.16 Graf, 1970b
40.8% active 29 d 0.10
1 1 224 g/ha 15 d 0.25
29 d 0.16
Pears 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.22 Graf, 1970c
40.8% active 29 d 0.14
1 1 224 g/ha 15 d 0.22
29 d 0.27
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
Turkey
Eggplant 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 0.26-0.40 Hollick &
E.C. 7 d 0.02-0.03 Collison, 1972
14 d 0.02-0.02
Beans 1972 Dursban 4 Spray 2 0.96 kg/ha 0 d 0.65-1.20 Ibid.
E.C. 7 d 0.08
14 d <0.04
Tomatoes 1970 Dursban E.C. Spray 1 347 g/ha 3 h 1.3 Graf & Engeler,
40.8% active 5 d 0.26 1970b
Peppers (under 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 2.00-5.20 Hollick &
glass) E.C. 7 d 0.04-0.10 Collison, 1972
14 d 0.13-0.25
UNITED KINGDOM
Raspberries 1971 Dursban E.C. Spray 1 0.1% 0 d 1.40 Burrows et al.,
40.8% active 5 d 0.32 1971d
2 0.5% 10 d 0.14
18 d 0.18
Tomatoes 1971 Dursban E.C. Spray 1 0 d 1.26 Burrows et al.,
40.8% active 3 d 0.10 1971e
7 d 0.08
14 d 0.01
28 d 0.01
1 All dosages given in a.i.
2 Last application to harvest
3 Limit of detection
TABLE 10 RESIDUES OF CELORPYRIPOS AND PYRIDINOL IN FOOD CROPS, EUROPE/NEAR EAST TRIALS
CROP, SAMPLE DOSAGE1 TIME BETWEEN RESIDUES RESIDUES REFERENCES
COUNTRY NO. LAST TREATMENT CHLORPYRIFOS 3,5,6-TRICHLORO-2-PYRIDINOL (ppm)
& YEAR AND HARVEST (ppm)
TURKEY
1971
Tomatoes 1 A 825 g/ha 3 hours 1.3* <0.05
2 A " 3 hours <0.05
4 A " 3 hours 0.05 Barrows & Mullin,
3 B " 5 days 0.05 1971b
5 B " 5 days 0.26 <0.05
6 B " 5 days <0.05
ENGLAND
1971
Tomatoes 720 g/ha 0 day 1.28 0.14
1.24 0.12
3 days 0.14 0.10
0.05 0.10 Burrows et al
7 days 0.08 0.08 1971e
0.08 0.08
14 days 0.01 0.12
0.01 0.10
28 days <0.01 0.10
<0.01 0.16
ITALY 1971
Apples 1 816 g/ha 29 days 0.10 <0.05 Burrows & Mullin,
4 1 224 g/ha 15 days 0.25 <0.05 1971b
Grapes 11 816 g/ha 29 days 0.10 <0.05
13 816 g/ha 15 days 1.10 0.06 Ibid.
12 1 224 g/ha 29 days 0.22 <0.05
14 1 224 g/ha 15 days 1.50 0.29
* Composite samples
1 All dosages given in a.i.
TABLE 11 CHLORPYRIPHOS RESIDUES ON CROPS IN JAPAN1
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
Chinese
cabbage
1967 40E 267 1 4 0.13-0.23
1100 l/ha
1969 40E 267 2 8 0.02-0.09
1000 l/ha 2 16 0.43-0.44
" 267 4 8 0.16-0.22
4 16 0.62-0.86
1969 40E 267 2 7 0.02-0.06
1500 l/ha 2 14 0.06-0.08
" 267 4 7 0.08-0.14
4 14 0.06-0.12
Apples
Rall's Janet
1967 25W 250 4 66 N.D.3
Johnathan
1967 25W 250 5 42 0.01-0.05
1969 25W 250 3 30 0.05-0.07
30 l/tree 45 0.05-0.06
1969 " 250 2 17 0.03-0.07
28 0.02-0.05
46 0.02-0.03
TABLE 11 (Cont'd.)
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
25W 250 3 7 0.03-0.09
30 l/tree
14 0.05-0.17
31 0.08-0.16
45 0.03-0.05
Aomori
1969 25W 250 2 7 0.10
14 0.12-0.14
21 0.08-0.03
1 Hamaguchi, 1970
2 Range of averages for two laboratories
3 N.D. = not detectable (<0.005 ppm)
TABLE 12 CHLORPYRIFOS RESIDUES ON CROPS IN THAILAND AND MALAYSIA
CROP FORMULATION APPLIC. DAYS BETWEEN TESTS RESIDUE1 REFERENCES
& DILUTION (no.) LAST SPRAY (no.) (ppm)
& HARVEST
Chinese 25W 6 5 6 N. D.2 - 0.023 Tucker & Zielinski,
cabbage 1972a
Chinese 187 ppm(a.i.) " " " 0.10 - 0.243 Ibid., 1972b
kale " " " " 0.09 - 0.763
Chinese 25W " " " N.D.3 Ibid., 1972a
cabbage
Chinese 375 ppm(a.i.) " " " 0.38 - 0.753 Ibid., 1972b
kale " " " " 0.35 - 0.763
Chinese 25W 16 2 3 0.07 - 0.104 Ibid., 1972,
kale
3 oz a.i./A " " " 0.01 - 0.35
25W " " " 0.13 - 0.244
4 oz a.i./A " " " 0.01 - 0.035
Tobacco 25W 10 7 4 0.03 - 0.053 Ibid., 1972d
187 ppm (a.i.)
25W " " " 0.21 - 0.383
375 ppm (a.i.)
1 Two analytical methods used in analyses
2 N.D. = not detectable (<0.01 ppm)
3 "Usable leaves" from Thailand
4 Leaf only from Malaysia
5 Stem only from Malaysia
TABLE 13 Chlorpyrifos residues on rice in the Philippines1
Chlorpyrifos Residue
applied (kg/ha, Tests (range of
Part analysed Formulation 3 applications) (no.) averages,
ppm)
Grain E.C. 0.10 4 N.D.3
0.15 4 N.D. - 0.008
0.20 3 N.D. - 0.005
0.25 2 0.008
Granule 0.10 3 N.D.
Grain and E.C. 0.10 4 N.D. - 0.007
straw2 0.15 4 N.D. - 0.006
0.20 4 0.005 - 0.008
0.25 2 0.008
Granule 0.10 3 <0.005
1 Tucker, 1971.
2 Very little straw, mostly grain.
3 N.D = not detectable (<0.005 ppm)
FATE OF RESIDUES
General comments
The fate of chlorpyrifos residues and its metabolites in different
species of animals are discussed in the section on "Biochemical
aspects".
In animals
Cow
Chlorpyrifos was not found in the urine and milk from a cow fed 5 ppm
(based on a daily ration of 50 lb) of the compound for four days. The
compound in absolute ethanol was mixed thoroughly with the grain. A
compound characterized by retention time as chlorpyrifos was found in
faecal samples taken on the last three days during which the
insecticide was fed and the first day after, and represented 1.7% of
the insecticide fed. Two metabolites were excreted in the urine which,
following methylation, had retention times identical to the methyl
esters of diethylthiophosphate and diethyl phosphate. They represented
35.9 and 26.8%, respectively, of the total insecticide fed on an
equivalent basis (Gutenmann et al., 1968).
Fish
Waters treated with 50 and 300 ppb of ring-labelled 14C chlorpyrifos
and containing goldfish were analysed for the types of metabolites
found in each. Metabolites I, III, IIIa and IIIb, shown in Figure 1,
as well as chlorpyrifos were found in fish, and metabolites I, IIIa
and IIIb were found in the water (Smith et al., 1966).
The major metabolic product in fish is 3,5,6-trichloro-2-pyridinol
which appears to be slowly broken down by dehalogenation and cleavage
of the ring (Smith et al., 1966).
Some of the derivatives of 3,5,6-trichloro-2-pyridinol may be those
shown in Smith (1968) which consist of dechlorination forming a series
of transient pyridine diols, triols and tetrols (Ia), followed by a
series of transient ring diketones (Ib), followed by a breakage of the
ring to elemental fragments (carbon dioxide and possibly aliphatic
amines).
Cholinesterase inhibition properties of metabolites
The house fly head cholinesterase activity of chlorpyrifos, and
metabolites I, II, III, IIIa and IIIb shown in Figure 1, were tested
for comparative purposes by Smith et al. 1966). Only the phosphate
derivative of chlorpyrifos (metabolite III) shows significant
inhibition and is the compound most likely to be responsible for the
cholinesterase inhibition found in mammals and other animal organisms
(Smith et al., 1966).
In plants
Studies on the absorption, translocation and metabolism of 14C and
36Cl labelled chlorpyrifos in plants show that for all practical
purposes uptake of the chemical or its degradation products into
plants does not occur, either through the foliage or through the roots
(Smith et al., 1967a, 1967c).
When radioactive chlorpyrifos was applied to single bean or corn
leaves, about 75 to 80% of the chemical was lost by vapourization from
the leaf surface within the first 48 h following treatment. The
chlorpyrifos remaining on the leaf was rapidly degraded (>95% in 5
days) and a trace of the resulting breakdown products (representing
<2% of the applied dose of chlorpyrifos) absorbed into and
translocated within the plant. The identity of the degradation
products was not totally elucidated; however, a variety of hydrolysis
products appeared to be formed, mainly the 3,5,6-trichloro-2-pyridinol
(I), ethyl-3,5,6-trichloro-2-pyridyl phoshate (IIIa) and
3,5,6-trichloro-2-pyridyl phosphate (IIIb) shown in figure 1 (Smith
et al., 1967a).
Mol. Wt. 350.6
Empirical Formula C9H11Cl3NO3PS
Other relevant chemical and physical properties
State: white, granular, crystalline solid
Melting point: 42 - 43.5°C
Vapour pressure: 1.87 × 10-5mm Hg (at 25°C)
8.87 × 10-5mm Hg (at 35°C)
Solubility: readily soluble in acetone, benzene,
chloroform, methanol and iso-octane; slightly
soluble (0.4 ppm) in water.
When chlorpyrifos was exposed to UV light or to sunlight, it underwent
hydrolysis in the presence of water to liberate
3,5,6-trichloro-2-pyridinol, which underwent further decomposition to
diols and triols and ultimately cleavage of the ring to fragmentary
products (Smith, 1968). Hydrolysis in water occurs least readily at
about pH 6 and very readily above pH 8.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrates, dusts, wettable powders and
granules).
Purity: 99.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
As with other phosphorothioate esters chlorpyrifos is rapidly
absorbed, metabolized and excreted by mammals following oral
administration. When 36Cl chlorpyrifos was fed by intubation to rats
as a single sublethal dose (50 mg/kg), the radioactivity was
eliminated rapidly (90.1% in 26 h) via the urine (90%) and faeces
(10%). Products excreted were identified as 36Cl 3,
5,6-trichloro-2-pyridyl phosphate (75-80%),
3,5,6-trichloro-2-pyridinol (15-20%) and traces of chlorpyrifos. The
pyridyl phosphate was indicated to be a transient intermediary in the
hydrolysis of chlorpyrifos to the pyridinol moiety. Analyses showed
no appreciable residue in any tissue except fat, which showed
temporary retention of radioactivity (halflife of ca 62 h) identified
principally as chlorpyrifos (Smith et al., 1967b). This study
suggests that desethylation is a significant metabolic pathway in
rats. An additional study in the rat involving administration of 14C
chlorpyrifos by single dose intubation (19.1 mg/kg) essentially
verified the earlier result (Branson and Litchfield, 1971a,b). In this
study, as well as in an in vitro study involving breakdown of
chlorpyrifos by rat liver microsomes (Branson and Wass, 1970),
3,5,6-trichloro-2-pyridinol was identified as the only major
metabolite. A study in cows involving feeding of chlorpyrifos in the
diet at 5 ppm for four days indicated that the major pathway for
degradation was by hydrolysis to the pyridinol, as no desethyl
phosphate esters were found. In addition no chlorpyrifos or
metabolites were found in milk (Gutenmann et al., 1968).
Studies of 3,5,6-trichloro-2-pyridinol, the major enolic metabolite of
chlorpyrifos, have shown absorption, distribution and excretion in the
rat following single oral administration to be similar to that
reported for the parent compound. The metabolite is rapidly absorbed
and excreted primarily in the urine and faeces. Small tissue residues
(<0.3 ppm) were present and occurred mainly in the biological systems
involved with urinary excretion, i.e. liver, kidney and blood. Unlike
chlorpyrifos, residues of the pyridinol in fat were trivial, which is
consistent with its much greater polarity (Branson and Litchfield,
1971a,b; Smith et al., 1970).
Biotransformation
The metabolic fate of chlorpyrifos has been investigated in plants
(Smith et al., 1967a, 1967b) and data indicate an oxidative and/or
hydrolytic breakdown yielding des-mono-ethylchloropyrifos; desethyl
chlorpyrifos; 3,5,6-trichloro-2-pyridinol and further degradation
products. This is discussed further in the section on "Fate of
residues."
Effects on enzymes and other biochemical parameters
Chlorpyrifos is a cholinesterase inhibitor affecting this enzyme in
brain or blood, although differing in its response with different
animal species. In vitro studies have shown the oxygen analogue of
chlorpyrifos to be 106 times more active as an inhibitor of
cholinesterase than chlorpyrifos (I50 values: chlorpyrifos approx.
2.5 x 10-3M; oxygen analogue approx. 2.5 x 10-9M) (Smith, 1966).
Equimolar concentrations of chlorpyrifos administered to dogs and
monkeys affected RBC cholinesterase in monkeys while having almost no
effect on RBC cholinesterase in dogs. Plasma cholinesterase is the
most sensitive indicator of exposure to chlorpyrifos. In dogs and
monkeys administered subacute levels of chlorpyrifos, plasma
cholinesterase was maximally inhibited within 8 hours following
exposure. Recovery in dogs was complete within 24 hours while with
monkey, plasma cholinesterase in monkeys remained depressed at 48
hours (Coulston et al., 1971).
TOXICOLOGICAL STUDIES
Acute toxicity of the metabolite 3,5,6-trichloro-pyridinol has been
studied in rats and mice. Results are summarized in Table 1. Signs of
poisoning with this metabolite include facial paralysis with dyspnoea
and slight hypersalivation and exophthalmia (mice only) (Gerbig and
Emerson, 1970 a,b).
Special short-term studies on metabolites
Rat
Groups of rats (10 males and 10 females/group) were fed the metabolite
of chlorpyrifos, 3,5,6-trichloro-2-pyridinol, in the diet for 90 days
at dosage levels of 0, 100, 300, 1 000, 3 000 and 10 000 ppm. Diuresis
was observed at 3 000 ppm. At 10 000 ppm a reduction in body-weight
was observed which reflected a general retardation in growth in both
sexes. No effects were observed on mortality, terminal haematology or
gross and histological examination of tissues (Beatty and McCollister,
1964).
Dog
Groups of dogs (3 males and 3 females/group, 4 of each sex were
controls) were fed the metabolite of chlorpyrifos,
3,5,6-trichloro-2-pyridinol, in the diet for 91 days at dosage levels
of 0, 1, 3, 10 and 30 mg/kg/day (Emerson and Gerbig, 1970). Another
study utilized groups of dogs (2 males and 2 females at the higher
level and 4 male and 4 female at the lower level) which were fed the
metabolite at dietary levels of 26 and 80 mg/kg/day for 93 days
(Copeland, 1964). At 80 mg/kg the metabolite produced abnormalities
including growth depression in females, increased spleen/body-weight
ratios and histological changes in the liver. At 26 mg/kg an elevated
SAP accompanied by equivocal changes in the liver was observed. At 30
mg/kg increased SAP, SGOT and SGPT values were observed in the second
study. The increased biochemical parameters were noted in 1 of 3 dogs
examined at 10 mg/kg. Hepatic lesions noted in 1 dog at 10 mg/kg and
1 dog at 30 mg/kg did not appear to be chemically induced. No effects
were noted at 3 mg/kg/day on growth, behaviour, haematology, blood
biochemistry, urinalysis or gross and microscopic examination of
tissues.
TABLE 1 Acute toxicity of the metabolite 3,5,6-trichloropyridinol
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 794 Gerbig and Emerson, 1970a
F 870
Mouse M Oral 380 Gerbig and Emerson, 1970b
F 415
Duckling
Groups of Peking ducklings (10 per group, 20 used as controls) were
fed the metabolite at dosage levels of 0, 1, 3, 10, 30, 60, 100, 300
and 1 000 ppm for 3 weeks to determine cataractogenicity. Growth
retardation was evident at 1 000 ppm, and 3 of 10 ducklings had
elevated prothrombin values. Levels of 300 ppm and below produced no
effect on these parameters and no cataractous changes were observed at
any level (Molello and Sharp, 1968).
Special studies in neurotoxicity
Hens (3 DeKalb leghorns/group) were administered chlorpyrifos orally
at dosage levels of 40, 75, 100 and 150 mg/kg and observed for 27
days. Mortality occurred at the higher levels. Surviving birds showed
no signs of delayed ataxia or paralysis. Crufomate administered as a
positive control (1 000 mg/kg) produced clinical signs of paralysis
(Stevenson, 1966).
Special studies on potentiation
Rats administered subacute oral dosages (1/2 the LD50 value) of
chlorpyrifos in combination with malathion or dichlorvos showed slight
evidence for potentiation of the acute toxicity. A three-fold increase
in the acute toxicity was noted with malathion, but this was judged to
be of no practical significance (Dow Chemical Co., 1964; Norris,
1970).
Special studies on reproduction and teratogenicity
Groups of rats (10 males and 20 females/group with 20 males and 10
females/group as controls) were fed chlorpyrifos in the diet for one
generation (two litters) at dosage levels of 0, 0.03, 0.1 and 0.3
mg/kg and for two generations (two litters per generation) at dosage
levels of 0, 0.1, 0.3 and 1.0 mg/kg. The F3b litter was used in a
teratology study where the parents were fed dietary levels of
chlorpyrifos until day 6 - 15 of organogenesis when the dietary
supplement was removed and chlorpyrifos administered by gavage once
daily. After day 15 chlorpyrifos was again administered by dietary
supplement until day 20 when the females were sacrificed, foetuses
removed by caesarean section and viscera and skeletal structure
examined. Neonatal mortality was somewhat higher in F2a, F2b and F3a
litters at 1.0 mg/kg. No other significant effects were observed on
fertility, reproduction or lactation indices or on teratological
examination of F3b pups as a result of feeding chlorpyrifos at levels
up to 1.0 mg/kg/day. Cholinesterase depression was observed at levels
of 0.3 mg/kg and above, primarily in plasma (Thompson et al., 1971).
Acute toxicity
The acute toxicity of chlorpyrifos has been studied in several animal
species, and the results are summarized in Table 2.
TABLE 2 Acute toxicity of chlorpyrifos in different animal species
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 118 - 245 Taylor and Olson, 1963;
Gaines, 1969; Tucker and
Crabtree, 1970
F Oral 82 - 135 Taylor and Olson, 1963;
Gaines, 1969
Mouse M Oral 102 Coulston et al., 1971
- i.p. 31 USAEHA, 1968
White
footed - Oral 64 Kenaga, 1967
Mouse
Rabbit - Oral 1 000 - 2 000 Taylor and Olson, 1963
Cavy M Oral 504 Ibid.
Goat F Oral 500 - 1 000 Tucker and Crabtree, 1970
Chukar F Oral 61.1 Ibid.
M Oral 60.7 Ibid.
Bullfrog M Oral >400 Ibid.
Chick M Oral 25 - 35 Taylor and Olson, 1963;
Stevenson, 1966; Sherman
et al., 1967; Brust
Chicken - Oral 32 - 63 et al., 1971
The LD50 in a number of avian species has been determined by Tucker
and Crabtree (1970). The toxicity values range from 8 mg/kg in male
ring necked pheasant to 167 mg/kg in mallard ducklings.
Signs of poisoning with chlorpyrifos are typical of cholinesterase
inhibiting compounds. Mortality following acute oral dosing may be
delayed for as long as nine days in some species of animals (Tucker
and Crabtree, 1970).
Chlorpyrifos administered to the conjunctival sac of rabbits produced
transient redness of the conjunctiva. No corneal injury was observed
(Taylor and Olson, 1963). When applied to intact or abraded abdominal
skin of rabbits daily for up to 2 weeks no significant dermal damage
was observed. Prolonged contact produced burning and hardening of the
skin, slight swelling and mild hyperemia which returned to normal
within three weeks. Chlorpyrifos administered dermally as a technical
product to rabbits in an undiluted form produced no untoward
physiological effects. When administered as a 50% solution in
dipropylene glycol methyl ether, the same dose (2 000 mg/kg) was
rapidly absorbed and resulted in death in four out of six animals when
applied to either intact or abraded skin (Taylor and Olson, 1963).
Chlorpyrifos did not induce sensitization when examined on the guinea
pig (USAEHA, 1968).
Dogs and sheep were exposed for a 4-hour period to a thermal fog or
liquid aerosol at a concentration of 4 or 8 mg/cu ft. Mild plasma
cholinesterase depression in dogs at the higher dose was the only
effect noted (Dow Chemical Co., 1966a).
Five female rats were exposed for 7 hours/day to an atmosphere of
chlorpyrifos (calculated at 7 mg/l air) for 16 exposures over a 21-day
interval. There were no effects on growth, appearance, behaviour or
plasma and RBC cholinesterase activity (Dow Chemical Co., 1966b).
Short-term studies
Mallard duck
Groups of mallard ducklings (6 - 8 birds/group, 5 days old) were fed
chlorpyrifos for five days at dosage levels of 0, 100, 200, 300, 500,
700, 1 000 and 2 000 ppm in the diet. Mortality occurred at all dosage
levels (100-2/8; 200-4/7; 300-5/8; 500-6/8; 700 - 2000 - all dead)
except in controls (0/7 dead) (Stevenson, 1965).
Chickens
Groups of chicks (20 chicks/group) were fed chlorpyrifos in the diet
for two weeks at dosages of 0, 200, 400 and 800 ppm. Mortality was
observed at 400 ppm and plasma cholinesterase depression and reduced
weight gain was observed at all levels (Sherman et al., 1967).
Groups of chickens (30 per group) were administered chlorpyrifos in
the drinking water at dosage levels of 0, 1 ppb, 1 ppm and 100 ppm for
84 days. A higher level (1000 ppm) resulted in clinical signs of
cholinergic stimulation. Plasma cholinesterase was depressed at 100
ppm but not at 1 ppm. A 10 ppm group was tested for two weeks with no
signs of cholinesterase depression. Growth and behaviour were not
affected at 100 ppm (Stevenson and Daering, 1969). Chlorpyrifos added
to the drinking water of chicks at dosages of 0, 0.08, 0.32, 1.25, 5,
20, 80, 320 and 1 280 ppm for periods varying from 3 - 5 weeks induced
mortality and reduced growth at levels of 80 ppm and above. Whole
blood cholinesterase depression was evident only at 80 ppm and above
(Brust et al., 1971).
Chickens fed chlorpyrifos in the diet at levels of 0, 25, 50 and 100
ppm for four weeks exhibited reduced plasma cholinesterase activity
and reduced weight gain at all feeding levels. Calculations of food
conversion data show a reduced yield in production as a result of 25
ppm in the diet. No clinical signs of toxicity were observed
(Schlinke, 1970).
Rat
Groups of rats (20 males and 20 females/group) were fed chlorpyrifos
in the diet for six months at dosage levels of 0, 0.03, 0.15 and 0.75
mg/kg/day. Cholinesterase depression was noted at 0.75 mg/kg/day in
RBC and plasma. Brain cholinesterase activity was unaffected. No
effects were noted at any feeding level on mortality, behaviour,
growth, haematology or gross or histological examination of tissue
(Coulston et al., 1971).
Groups of rats (10 males and 10 females/group) were fed chlorpyrifos
in the diet for 90 days at levels of 0,10, 30, 100 and 300 ppm. Growth
reduction was observed in both males and females at 300 ppm. Increased
liver and kidney organ to body-weight ratios were also observed
(possibly as a result of reduced body-weight as organ weights were
comparable to the controls). Cholinesterase depression was observed at
30 ppm in blood and brain; at 10 ppm, brain cholinesterase was normal
although enzyme inhibition was observed in RBC and plasm. No adverse
effects were noted on growth, appearance, food consumption, mortality,
behaviour, haematology or gross and microscopic pathology at 100 ppm
(Beatty and McCollister, 1964).
Five groups of rats (10 males and 10 females/group) were fed
chlorpyrifos at levels varying from 0.03 - 10.0 mg/kg/day for 28 - 90
days to determine a "no-effect" level on cholinesterase depression.
Dosage levels of 0 and 0.3 mg/kg/day were fed for 90 days while
dosages of 1, 3 and 10 mg/kg/day were fed for 28 days, stopped for
three weeks (during which time the rats were fed control diets) and
the rats refed diets containing 0, 0.03 and 0.1 mg/kg/day for a full
90-day study. Behaviour and growth were affected at 3 and 10
mg/kg/day. Cholinesterase was depressed at 0.3 mg/kg/day and above in
both male and female rats. The rats in the three highest groups were
removed for three weeks during which time cholinesterase activity
returned to pretest intervals. On resumption of feeding chlorpyrifos
at 0.1 mg/kg/day, slight cholinesterase depression was observed in
plasma and erythrocytes. No cholinesterase depression was noted on
brain or blood enzymes at 0.03 mg/kg/day (Blackmore, 1968b).
Dog
Groups of dogs (2 males and 2 females/group; 4 of each sex were
controls) were fed chlorpyrifos for 93 days. The dosage levels of
feeding were initially 200, 600 and 2 000 ppm in the diet, but because
of the appearance of signs of cholinergic poisoning the dosages were
changed from 2 000 to 60 ppm after 5 days while the 200 ppm (5.8
mg/kg/day) level was maintained for 45 days. The dogs were then placed
on control diets for the remainder of the test. Another group of 2
male and 2 female dogs was added to the study at a dietary level of
200 ppm (3.4 mg/kg/day) for 27 days, removed from the test for 5 days
and returned to this dietary level for the final 2 weeks. A decrease
in food consumption at the 200 ppm level was accompanied by reduced
growth, especially in females. Growth of all dogs was slightly lower
than control levels. Increased SGPT values were seen in several dogs
at all feeding levels. RBC, plasma and brain cholinesterase values
were reduced in all animals fed chlorpyrifos in the diet at a level of
20 ppm (0.8 mg/kg/day) and above. No adverse effects were observed on
haematology, BUN, SAP, organ weights and gross or microscopic
examination of tissues (Copeland, 1964).
Groups of dogs were orally administered chlorpyrifos daily in a
gelatin capsule for periods of 32 to 90 days at doses of 0 (2 males
and 2 females - 90 days), 0.01 mg/kg/day (2 males and 2 females - 32
days), 0.03, 0.10, 0.30 and 1.00 mg/kg/day (2 males and 2
females/group - 90 days), and a final group (2 males and 2 females)
was given 1.00 mg/kg/day from day 0 to 18, control diet from days 19
to 42, 0.03 mg/kg/day from days 43 to 58, control diet from days 59 to
77, 0.01 mg/kg/day from days 78 to 94 and control diet until day 124.
Data obtained on appearance, body-weight and food consumption were
normal. A dose-dependent cholinesterase depression in plasma was
observed to begin at 0.03 mg/kg/day. At this level inhibition was
moderate and was more marked as the dietary level increased. Brain
cholinesterase was not affected at any level, and RBC cholinesterase
was affected at levels of 0.1 mg/kg/day and above. No effects were
noted at 0.01 mg/kg/day on any parameters tested over the 32-day
administration interval (Blackmore, 1968a).
Groups of dogs (7 males and 7 females/group) were fed chlorpyrifos in
the diet for up to two years at dose levels of 0, 0.01, 0.03, 0.1, 1.0
and 3.0 mg/kg/day. Inhibition of cholinesterase activity was the only
abnormality detected. Inhibition of RBC cholinesterase in males and
females was evident at 1.0 and 3.0 mg/kg. Marginal depression of brain
cholinesterase was shown at the highest level of feeding. Plasma
cholinesterase was depressed at 0.1 mg/kg/day in both males and
females. Occasional reduction at 0.03 mg/kg was noted. Studies on the
recovery of cholinesterase activity following one year feeding
indicate that plasma activity recovered to normal within two weeks
while RBC recovery was much slower. No significant effects were noted
on mortality, behaviour, food consumption, growth, haematology, blood
biochemistry, urinalysis, or gross and microscopic examination of
tissues. Gross examination showed an increased liver weight and ratio
at 3.0 mg/kg at two years but not at one year. All other tissues were
normal (McCollister et al., 1971a).
Monkey
Groups of Rhesus monkeys (2 males and 2 females - control; 2 males and
1 female - 0.08 or 0.40 mg/kg/day; 2 males and 2 females - 2.0
mg/kg/day) were administered chlorpyrifos orally by gavage daily for
six months. Plasma and RBC cholinesterase activity was depressed in
those animals that received 2.0 and 0.4 mg chlorpyrifos/kg/day. Plasma
cholinesterase activity was slightly depressed and RBC cholinesterase
activity was not depressed in animals that received 0.08 mg/kg/day.
Brain cholinesterase activity was not affected at any level. No
significant effects were noted on behaviour, growth, haematology,
clinical chemistry or gross or histological examination of tissues.
The histological examination included electron microscopy of liver and
kidneys and histochemical delineation of lysosomal distribution in
these two organs (Coulston et al., 1971).
Long-term studies
Rats
Groups of rats (57 males and 57 females/group) were fed dietary levels
of chlorpyrifos of 0, 0.01, 0.03, 0.1, 1.0 and 3.0 mg/kg/day for two
years. Blood cholinesterase activity, primarily in plasma, was
depressed in females at 0.1 mg/kg and above. Brain cholinesterase was
inhibited at 3.0 mg/kg and slightly depressed at 1.0 mg/kg.
Chlorpyrifos at all dosage levels had no significant effect on
behaviour, appearance, growth, mortality, haematology, urinalysis,
clinical biochemistry, gross or histopathology of tissues and organs
or the incidence of neoplasms (McCollister et al., 1971b).
OBSERVATIONS IN MAN
Groups of men (4 men/group) were administered chlorpyrifos by tablet
daily for 27 days (0.014 mg/kg/day), 20 days (0.03 mg/kg/day), 9 days
(0.10 mg/kg/day) and 48 days (control). No effects were noted on
behaviour, haematology, urinalysis or biochemical determinations in
blood. Blood plasma cholinesterase was depressed at the highest dosage
and this regimen was discontinued. Recovery was complete in four
weeks. At 0.03 mg/kg/day a statistically non-significant, although
suggestive, depression of plasma cholinesterase was noted. No effects
were noted at 0.014 mg/kg on plasma cholinesterase. RBC activity was
unaffected at any level of chlorpyrifos. The level of 0.03 mg/kg/day
appears to be the minimal threshold response level in humans to
chlorpyrifos based on plasma cholinesterase (Coulston et al., 1972).
COMMENT
The compound is rapidly absorbed and metabolized by mammals, the major
metabolite being a chlorinated pyridonal hydrolysis product of low
mammalian toxicity.
There is no evidence of neurotoxicity or cataractogenicity.
Potentiation has been demonstrated only with malathion.
Reproduction studies and examination of offspring for teratogenic
abnormalities did not reveal adverse effects other than an increase in
neonatal mortality at 1 mg/kg/day. Chlorpyrifos is an active
cholinesterase inhibitor, inhibiting plasma cholinesterase to a far
greater degree than other cholinesterases. One short-term study in
rats indicated an increase in sensitivity to plasma cholinesterase
depression following withdrawal after initial treatment. This study
needs confirmation.
No-effect levels have been demonstrated in dog, rat and man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.03 mg/kg/day
Dog: 0.01 mg/kg/day
Man: 0.014 mg/kg/day orally for 1 month
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In animals
Chlorpyrifos is used to control various species of fever ticks
(Boophilus sp.), ear ticks, lice and horn flies on beef cattle and
non-lactating dairy cattle, by use of emulsifiable liquid formulations
in water varying from 0.025 to 0.125% applied as a spray or dip.
Treatment for all but ear ticks is limited to six applications at
21-day intervals, and not within two weeks of slaughter.
Sheep dipped or sprayed with wettable powder or emulsifiable
formulations of chlorpyrifos are protected from blowfly, ticks, body
lice and sheep keds. A minimum interval of seven days is required
between treatment and slaughter.
Turkey chiggers are controlled through the use of wettable powder
formulations sprayed on soil in turkey pens at the rate of 4 lb/acre
chlorpyrifos, before the turkeys are introduced into the pens.
Treatment is limited to two applications, not within seven days of
slaughter.
In plants
Soil treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control soil pests such as wireworms,
white grubs, mole crickets, Maulwurf's Grill, garden symphylans,
seed corn maggots, cereal maggots, beet root weevils, the
Atomaria, Blaniulus, Tipula and cutworms. The chemical is
applied by means of broadcast row or furrow treatments and is
varied in concentrations from 300 to 4 000 g/ha a.i. as wettable
powder, emulsifiable or granular formulations before and after
planting, previous to harvest. The crops involved are maize,
spring cereals, beans, onions, tobacco, turnips, bulb flowers and
carrots.
2. Canada
Chlorpyrifos is used to control soil insect pests of lettuce,
onions and carrots before and after planting at dosages of 1 to 2
lb/acre applied as needed, but with the indicated 28- to 60-day
withdrawal period before harvest. Tobacco plants are treated in
soil water, preplant and by soil and foliar application.
Foliar treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control insect pests of rape, cereals,
potatoes, fodder crops, beans, peas and deciduous fruits such as
apples and pears.
2. Japan, Thailand, Philippines, India and Malaysia
Chlorpyrifos is used to control insect pests of apples, pears,
rice, tobacco, Chinese cabbage and kale.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
Considerable work has been done in U.S.A. on the analysis of cattle
tissues for residues of chlorpyrifos as well as its oxygen analogue
and pyridinol moiety. Typical distribution of residues in cattle
dipped in chlorpyrifos is shown in Table 3 (Claborn et al., 1970a).
In this study, 57 beef cattle were dipped one to six times in a 0.025%
emulsion of chlorpyrifos at intervals of 21 days. Animals were removed
from the dipping schedule after each dip, except the fifth, for
post-treatment slaughter at various time intervals. Fat muscle, kidney
and liver tissue samples were collected and analysed for residues of
chlorpyrifos, its oxygen analogue, and the 3,5,6-trichloro-2-pyridinol
moiety. Residues of chlorpyrifos were predominately in the fatty
tissues, and residues of 3,5,6-trichloro-2-pyridinol were in the
kidney and liver tissues. No residue of the oxygen analogue was
detected in any tissue.
Residues of the hydrolytic pyridinol were not detectable in muscle
tissue or in kidney and liver tissues of cattle, except at low levels
for the first 1 - 2 weeks after the last dip, even after six dippings.
It should be noted that this experiment began in February, so the
animals had their winter coat of hair through the first three dippings
and were shedding it at approximately the fourth dipping period. The
heavy winter hair coat carries more insecticide out of the vats than
the summer coat, as evidenced by the higher residues after the second
and third dippings and the drop in residues after the fourth dipping.
The effect of the winter coat is also reflected by the higher residues
after the first dip when compared to levels of residues after a single
dip (in the same concentration or higher concentration - 0.05% - of
chlorpyrifos) in the animals reported by Mann, 1966; Claborn et al.,
1968a,b; and Dishburger et al., 1966a,b.
Since the season for ticks extends from approximately April or May to
mid-September, animals would be expected to shed their winter coat
after the first or second dipping when treated according to label
instructions. Therefore, in practice residue levels as high as those
found in the test animals would not be expected after the third
dipping, since these animals would have been dipped three times before
shedding their winter coat of hair. In any case, it is believed that
this experiment represents the highest levels of chlorpyrifos that
would ever be encountered in the use of 0.025% chlorpyrifos as a dip.
In an early test, cattle were sprayed once with a 0.5% emulsion of
chlorpyrifos (20 times the label recommendation). Residues of the
compound in the fat were 1.3 ppm (maximum) at 20 days post-treatment
and had declined to 0.08 ppm (maximum) at 30 days post-treatment
(Dishburger and Rice, 1965).
In cattle treated with an emulsion containing 0.05% chlorpyrifos
(twice the label recommendation), the average amounts of residue in
the fat one week after a single-dip application were about twice those
resulting from a single-spray application. The maximum residues were
well below 1 ppm (0.209 for spray and 0.525 for dip application) at
the first sampling period at seven days post-treatment. Approximately
two weeks were required for residues to be reduced to 1 ppm in the fat
of animals given multiple treatments (3 dippings at 2-week intervals)
at this level (Mann, 1966; Claborn et al., 1968a,b).
TABLE 3 Chlorpyrifos residues in cattle in the United States1
Mean residues of
Weeks Mean residues of chlorpyrifos2 3,5,6-trichloro-2-pyridinol3
after last fat muscle kidney kidney liver
dipping (ppm) (ppm)
1 dipping
1 0.672 0.004 0.003 0.12 0.12
2 0.142 0.002 0 <0.05 <0.05
3 0.083 0.001 0 0 0
4 0.038 0 0 0 0
2 dippings4
1 1.245 0.011 0.008 0.30 0.26
3 dippings4
1 1.589 0.028 0.008 0.24 0.29
2 0.085 0.011 0.001 0.05 0.05
4 0.158 0 0 0 0
6 0.038 0 0 0 0
8 0.041 0 0 0 0
10 0.001 0 0 0 0
4 dippings4
1 0.693 0.011 0.001 0.10 0.12
6 dippings4
1 0.744 0.015 0.003 0.12 0.10
2 0.141 0.001 0 0 0
4 0.069 0 0 0 0
6 0.018 0 0 0 0
8 0.007 0 0 0 0
10 0 0 0 0 0
1 Residues of chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues
of beef cattle dipped in an emulsion containing 0.025% chlorpyrifos.
Residue levels corrected for % recovery from tissues.
2 No chlorpyrifos found in liver (0 - <0.002 ppm) or oxygen
analogue (0 - <0.005 ppm) in any tissue analysed (Claborn, 1970).
3 No 3,5,6-trichloro-2-pyridinol found in fat or muscle tissues
(0 - <0.05 ppm) (Dishburger and McKellar, 1971).
4 Dipping schedule was at 21 day intervals.
The oxygen analogue was found only in samples of fat containing the
higher residues of chlorpyrifos (approximately 2 ppm); it amounted to
about 1% of the residue. No increase occurred in the amount of the
oxygen analogue as the chlorpyrifos was eliminated, and no residues of
the analogue were detected in the muscle, brain or spleen tissues of
the animals that had the highest residues of chlorpyrifos in the fat
(Claborn et al., 1968a).
Data from Mann (1966), Claborn et al. (1968a) and Dishburger
et al. (1966a,b) indicated that the biological half-life of
chlorpyrifos in tissues of cattle following a single treatment with
DURSBAN insecticide containing 0.025% and 0.05% chlorpyrifos is
approximately 4 - 6 days. Data from Mann (1966) and Claborn et al.
(1968a,b) showing multiple dippings in DURSBAN insecticide containing
0.025% and 0.05% chlorpyrifos, further establish the consistency of
the degradation ratio following the last treatment. The data of
Claborn et al. (1968b) indicate that the biological half-life of
chlorpyrifos in tissues of cattle treated at three-week intervals for
as long as six weeks with DURSBAN 24E containing 0.025% chlorpyrifos
is approximately seven days. In summary, the experiments illustrate
the consistency of a 4 - 7 day biological half-life for chlorpyrifos
in fat.
Considerable work has been carried out in Australia on the
determination of residues from the vat dip and spray application of
chlorpyrifos to cattle (Hall, 1969; Snelson, 1969b). Results shown in
table 4 indicate that residues of chlorpyrifos in omental fat are in
the same order of magnitude as those for United States, considering
the fact that most tests were at double the concentration (0.05%) in
Australia.
Snelson (1969a) studied the disappearance rate of chlorpyrifos in
butterfat in milk after a single dip application of chlorpyrifos at
0.025% to milk cows. Residue declined from 1.1 ppm after 1 day to 0.01
ppm in 9 days, and a non-detectable level at 15 days (see Table 5).
Sheep
Sheep dipped in an 0.05% chlorpyrifos emulsion showed inconsistent
levels of residues of chlorpyrifos with no apparent change in residue
levels (0.081 - 0.179 ppm) from 3 - 28 days after application. No
detectable levels (<0.005 ppm) were seen in muscle, liver or kidney
tissues at any time (Dow Chemical Co., 1967).
TABLE 4 Chlorpyrifos residues in cattle in Australia
Chlorpyrifos
Days residues in
Chlorpyrifos Application between last No. of omental fat Reference
% type no. treatment and cattle (mean values
slaughter or range, ppm)
0.025 Vat dip 3 1 0.2 Watts, 1968
4 0.96
7 0.25
1 1
3 0.11 - 0.22 Ibid.
1 1,4,8,15 0.29 - 2.18 Ibid.
3,6 1,4,8,15 0.05 - 1.19 Ibid.
0.05 Vat dip 1 1,4,8 0.41 - 0.48 Ibid.
3 1 1.17 Ibid.
4 0.98
6 1 1.75 Ibid.
8 1.36
15 1.11
22 0.6
29 0.38
0.05 Vat dip 2 0 3 1.46 - 2.26 Hall, 1969
1 2 3 2.4 - 3.87
1 2 3 1.23 - 3.09
1 2 3 0.98 - 2.54
1 1 3 0.42 - 0.79
3 1 4 1.32 - 3.80
0.05 Dip 10 5? 12 0.70 - 7.131 Snelson, 1969b
0.05 Spray 30 1 3 1.6 - 4.2 Ibid.
5 3 0.34 - 1.1
9 2 0.1 - 0.15
13 1 0.06
28 1 0.01
1 Omental and/or perirenal fat.
TABLE 5 Chlorpyrifos residues in milk of cows in Australia
Days between Chlorpyrifos1
last treatment residue in butterfat
and sampling (ppm avg. 4 cows) Reference
1 1.1 Snelson, 1969a
2 0.96
3 0.57
4 0.14
5 0.08
6 0.05
9 0.01
15 N.D.2
1 One dip application containing 0.025% chlorpyrifos.
2 N.D. = not detectable
Turkeys and chickens
Claborn et al. (1970b) determined residues of chlorpyrifos in the
body tissues of turkeys confined in pens containing soil treated with
4 or 8 lb/acre of chlorpyrifos (in a 25% wettable powder formulation)
for control of the chigger, Neoschongastia americana. The higher
concentration caused maximum residues of 0.157 and 0.066 ppm of
chlorpyrifos in the skin and fat, respectively, one week
post-treatment, that decreased to less than 0.001 ppm after six weeks.
No significant residues were found in the other tissues (thigh,
breast, liver). The oxygen analogue of chlorpyrifos (diethyl
3,5,6-trichloro-2-pyridyl phosphate) was not detected (<0.005 ppm) in
any tissues.
In a study conducted by Mann and Ivey (1971), turkeys were maintained
on soils treated two times, at an interval of 28 days, with 4 lb
chlorpyrifos/acre each time. Two or three birds were necropsied at
weekly intervals for six weeks after the first application and for
eight weeks after the second application. Tissue samples of fat, skin,
muscle, liver and kidney were analysed for residues of chlorpyrifos,
the oxygen analogue and the pyridinol moiety
(3,5,6-trichloro-2-pyridinol). Lowlevel residues of chlorpyrifos were
found in fat (0.022 - 0.005 ppm), skin (0.033 - 0.152 ppm) and kidney
(<0.002 - 0.005 ppm) of birds after one week of exposure to the
first application of compound to the soil. Fat only was analysed for
the remainder of the test for each application. The residues in fat
were reduced to <0.002 ppm (the sensitivity of the method) six weeks
after the first application and eight weeks after the second
application. No oxygen analogue was detected in any of the tissues.
Small residues of the pyridinol moiety were found in the skin of one
hen (<0.003 - 0.007 ppm), the liver of one tom (<0.01 - 0.025 ppm)
and the kidney of one tom (<0.01 - 0.098 ppm) after one week of
exposure to the first application; and in the skin (<0.003 - 0.003
ppm), liver (<0.01 - 0.078 ppm) and kidney (<0.01 - 0.165 ppm) in
one hen after one week of exposure to the second application of
compound to the soil.
Hunt et al. (1969) developed a new rapid gas chromatographic method
for analyses of chlorpyrifos in turkey and chicken tissues. The
residues reported are from the same experiments as the toxicological
results reported by Schlinke (1970) in chickens and Schlinke et al.
(1969) in turkeys. The tests consisted of dietary and contact tests
with chlorpyrifos for four weeks. Residue analyses of muscle, liver,
heart, brain, gizzard, skin and fat tissues were taken after 28 days
of treatment. Only skin and fat tissues showed detectable amounts of
chlorpyrifos. In chickens fed 100, 50 and 25 ppm in the diet, the
highest tissue residue was 0.87 ppm chlorpyrifos in fat. In turkeys
fed 100 and 50 ppm in the diet, the highest residue was 0.34 in fat.
Dishburger et al. (1969b) determined the presence of chlorpyrifos
residues in skin, fat, muscle and liver tissues of turkeys which had
been confined to soil treated with 4.5 lb chlorpyrifos/acre as
emulsifiable, granular, or wettable powder formulations. Muscle and
liver tissues contained undetectable amounts of chlorpyrifos in all
tests. Skin and fat tissues contained <0.1 ppm at all times, but were
higher at seven days post-treatment than any of the other weekly
samplings up through 28 days post-treatment.
In plants
Residues found in experiments with plants in United States
Chlorpyrifos has been applied to various species of plants and
generally found to be lacking in residual insecticidal properties on
plant-feeding insects. The residue determinations reported here
actually never exceeded 28 ppm at the dosages tested (up to 1 lb/acre)
and soon diminished, exhibiting a half-life of 1 - 2 days, thus
confirming the reason for the observed lack of residual effectiveness
on plant-feeding insects (see Table 6).
There was no evidence of residue buildup on plants from repeated
application of chlorpyrifos.
TABLE 6 Chlorpyrifos residues found in experiments in plants in United States
Dosage Application Chlorpyrifos
chlorpyrifos (no.) (no. of days Crop residues Reference
(lb/A) since last) (ppm)
1.0 1 0 Bermuda grass 13.2 Leuck et al., 1968
14 0.37
1.0 1 0 Maize 5.60 Ibid.
14 0.42
0.05 8 0 Pasture grass 0.7 - 8.2 Winterlin et al.,
7 <0.1 - 0.2 1968
0.05 1 0 Grass 0.11 - 8.1 Ibid.
3 0.012 - 1.8
0.025 1 0 Rice 0.09 - 0.64 Ibid.
6-14 <0.005 - 0.07
1.0 1 0 Various wild 26.9 Hurlbert et al.,
1 plants 27.4 1970
7 1.1
14 0.1
0.025 4 0 Rice 0.07 - 0.21 Dishburger et al.,
9 <0.01 1969c.
1.0 1 0 Maize silage 15.1 Johnson et al., 1969
1 4.4
0.25 1 0 Maize silage 2.5 Ibid.
1 1.1
Leuck et al. (1968) studied the persistence of chlorpyrifos and its
oxygen analogue on field plots of coastal Bermuda grass and maize
forage using the method of Bowman and Beroza (1967a) with a minimum
sensitivity of 0.002 - 0.01 ppm. Chlorpyrifos was applied at the rate
of 1 lb/acre. The oxygen analogue constituted a very low residue and
was only a small proportion of the total insecticide. Chlorpyrifos
residues varied from about 6 - 13 ppm at the time of application to 4
- 8 ppm after 1 day, to around 1 ppm after 7 days and to nearly
negligible levels after 21 days. The residue half-life of chlorpyrifos
appeared to be in the order of 2 - 3 days on Bermuda grass and maize
plants.
Winterlin et al. (1968) studied the residues of chlorpyrifos on rice
and pasture grass and environment when applied experimentally for the
control of mosquitoes around Colusa, California. They used the method
of Bowman and Beroza (1967a) i.e. gas chromatography with a flame
ionization detector, which is sensitive to 0.005 ppm chlorpyrifos in
rice and 0.01 ppm in pasture grass, and found residues as high as 8.11
ppm chlorpyrifos immediately after application of 0.05 lb
chlorpyrifos/acre. Residues decreased rapidly after application to
below 1 ppm.
Myers et al. (1968) reported on residues resulting from application
of chlorpyrifos sprayed by aircraft at the rate of 0.1 lb/acre, eight
times, over a period of five months on irrigated pastures. Samples of
grass analysed for the insecticide 1 - 3 hours after each spray
application varied from 0.4 - 8.2 ppm, and after seven days varied
from <0.1 - 0.2 ppm, using an analytical method sensitive to 0.1 ppm
chlorpyrifos. No evidence of accumulated residues of chlorpyrifos were
detected as the result of multiple applications to grass with
chlorpyrifos.
Hurlbert et al. (1970) reported on the residues of chlorpyrifos on
watergrass, sprangletop and smartweed on the dikes surrounding ponds,
resulting from hand-sprayed applications of chlorpyrifos emulsifiable
insecticide. Dosages used were 1, 0.1, 0.05, and 0.01 lb/acre diluted
in a constant spray volume of 8 gal/acre. Under these conditions
residues over 1 ppm occurred only at 1 lb/acre after 4 h, 1 day, and 7
days, but not 14 days; and at 0.1 lb/acre after 4 h. Residues
determined at other dosages and post-application times were less than
1 ppm. The half-life of the foliar residues of chlorpyrifos was in the
order of 1 - 3 days.
Dosages of 0.025 lb chlorpyrifos were applied aerially four times, at
monthly intervals, to rice fields. Residues of chlorpyrifos in raw
(rough) rice grain ranged from 0.07 to 0.21 ppm at 0 day, following
the last of the four applications. No residues of the oxygen analogue
of chlorpyrifos were detected, nor did detectable chlorpyrifos
residues persist more than 9 days after application (Dishburger
et al., 1969c).
Johnson et al. (1969) treated maize plants in the field with 0.25,
0.5 and 1.0 lb chlorpyrifos/acre. The initial residues in maize and
silage were 2.5, 3.9 and 15.1 ppm, respectively (on an as-collected
basis). After one day the losses of residues were 56, 40 and 71%,
respectively. This represents a half-life of about one day. One day
after treatment the maize and silage were enclosed in a silage bin
where chlorpyrifos residues declined slowly over a period of seven
weeks.
Dishburger et al. (1969a) analysed samples of tobacco leaves from
soil and foliage treated with 0.5, 1.0 and 1.5 lb chlorpyrifos/acre.
No residues (<0.01 ppm) were detected on the samples harvested (2
months or more post-application).
Residues found in experiments with plants in Canada
Residue data from experiments with onions, lettuce, carrots, and
tobacco are shown in Tables 7 and 8 using the analytical method of
Braun (1971) for chlorpyrifos and McKellar (1971c) for the pyridinol.
TABLE 7 Residues of chlorpyrifos from treatments of lettuce,
onions and carrots1
Application Residues on crop (ppm)
(lb/acre) (No.)2 lettuce onions carrots3
0.5 2 <0.001 0.001 <0.001
0.005 0.003 0.003
(54)4 (119) (138)
3 0.001 0.002 0.012
0.020 0.003 0.020
(31) (104) (123)
1.0 2 <0.001 0.002 0.012
0.008 0.005 0.034
(54) (118) (138)
3 0.002 0.002 0.006
0.009 0.005 0.018
(31) (104) (123)
2.0 2 0.003 0.008 0.015
0.029 0.013 0 046
(54) (119) (138)
2.0 3 0.008 0.003 0.019
0.034 0.005 0.033
(31) (104) (123)
1 McEwen and Frank, 1970.
2 First application was preplant soil treatment; second and third
soil and foliar treatments (Canada).
3 3,5,6-trichloro-2-pyridinol analysed for but not found at
detection limit of 0.005 ppm.
4 Days after chlorpyrifos withdrawal shown in ().
TABLE 8 Residues of chlorpyrifos from treatments of lettuce, onions,
carrots and celery
Withdrawal Applications1 Residues range
Crop (days) (no.) (ppm) References
Onions 57 1 0.001 - 0.003 McEwen, 1970
19 4 <0.0012 Harris, 1971
Celery 57 1 N.D.3 - 0.016 McEwen, 1970
Carrots 57 1 0.001 - 0.015 Ibid.
Lettuce 27 1 N.D. Ibid.
1 Treatments all post-planting, 2 lb/acre (Canada).
2 No oxygen analogue of chlorpyrifos detected at 0.01 ppm sensitivity
level.
3 N.D. = <0.001 ppm.
Residues found in experiments with plants in Europe and the Near
East
Residue data from experiments with cauliflower, red cabbage, eggplant,
beans, peppers, lettuce, mushrooms, carrots, potatoes, sugar beet,
corn, cotton, tomatoes, grapes, pears and apples are shown in Tables 9
and 10. These tests include soil and foliar applications.
Residues found in experiments with plants in Japan, Thailand,
Malaysia, and the Philippines
Residue data from experiments with Chinese cabbage and kale, rice and
apples are shown in Tables 11, 12 and 13.
TABLE 9 Residues of Chlorpyrifos in Food Crops, Europe/Near East Trials
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
FRANCE
Potatoes 1970 Granules Soil 1 1 500 g/ha 5 mo <0.005 Graf and Engeler,
5% active insecticide 1 3 500 g/ha 5 mo <0.005 1971a
W. GERMANY
Sugar beets 1971 Dursban EC Broadcast 1 1.15 kg/ha 112 d <0.13 Scheuerer & Hahn,
40.8% active Soil insect. 118 d 1972
Maize 1971 Dursban 4 EC Soil insect. 1 0.95 kg/ha 123 d <0.13 Ibid.
40.8% active Broadcast
HOLLAND
Cauliflower Strewpowder Soil insect. 1 120 mg 8 wk 0.002
Red cabbage (1) 1969 4% active 11 wk <0.002 Wit, 1970
Red cabbage (2) 85 wk <0.002
Lettuce (under 1970 3.2% active Soil insect. 1 1 900 g/ha 9 wk <0.01 Wit, 1971
glass) granule
Mushrooms 1971 25% wettable Spray 3 5 000 g/ha 3 wk 0.01 Olthof, 1971a
powder 3 wk 0.03
Carrots 1971 Dursban 25W Soil insect. 1 6 000 g/ha 3-4 mo 0.14 Olthof, 1971b
1 6 000 g/ha 3-4 mo 0.28
ISRAEL
Cotton 1970 Dursban E.C. Spray 6 730 g/ha 15 d <0.05 Graf, 1970a
40.8% active
Apples 1970 Dursban E.C. Spray 1 2 400 g/ha 7 d 0.54 Graf &
40% active 14 d 0.20 Engeler, 1970a
Tomatoes 1970 Dursban E.C. Spray 1 720 g/ha 1 d 0.24 Aharonson
40.8% active 4 d 0.16 et al., 1969
8 d 0.12
16 d 0.04
1 1 440 g/ha 1 d 0.45
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
4 d -
8 d 0.18
16 d 0.10
1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.27 Graf & Engeler,
40.8% active 5 d 0.25 1971b
Apples 1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.57 Graf & Engeler,
40.8% active 7 d 0.23 1971c
14 d 0.14
Spray 1 2 880 g/ha 1 d 0.52
7 d 0.28
14 d 0.24
Dursban Spray 1 1 440 g/ha 1 d 0.13
40.8% active 7 d 0.11
Spray 1 2 880 g/ha 1 d 0.29 Ibid.
7 d 0.24
ITALY
Sugar beets 1970 Dursban 5% Soil insect. 1 2 500 g/ha 3-6 mo <0.004 Foschi, 1971
Maize 1970 Granule Soil insect. 1 2 500 g/ha 3-6 mo <0.004
Lettuce 1971 Dursban E.C. Spray 1 500 g/ha 14 d 0.05 SIAPA, 1971
22.5% 1 1 000 g/ha 14 d 0.07
Apples 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.16 Graf, 1970b
40.8% active 29 d 0.10
1 1 224 g/ha 15 d 0.25
29 d 0.16
Pears 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.22 Graf, 1970c
40.8% active 29 d 0.14
1 1 224 g/ha 15 d 0.22
29 d 0.27
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
Turkey
Eggplant 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 0.26-0.40 Hollick &
E.C. 7 d 0.02-0.03 Collison, 1972
14 d 0.02-0.02
Beans 1972 Dursban 4 Spray 2 0.96 kg/ha 0 d 0.65-1.20 Ibid.
E.C. 7 d 0.08
14 d <0.04
Tomatoes 1970 Dursban E.C. Spray 1 347 g/ha 3 h 1.3 Graf & Engeler,
40.8% active 5 d 0.26 1970b
Peppers (under 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 2.00-5.20 Hollick &
glass) E.C. 7 d 0.04-0.10 Collison, 1972
14 d 0.13-0.25
UNITED KINGDOM
Raspberries 1971 Dursban E.C. Spray 1 0.1% 0 d 1.40 Burrows et al.,
40.8% active 5 d 0.32 1971d
2 0.5% 10 d 0.14
18 d 0.18
Tomatoes 1971 Dursban E.C. Spray 1 0 d 1.26 Burrows et al.,
40.8% active 3 d 0.10 1971e
7 d 0.08
14 d 0.01
28 d 0.01
1 All dosages given in a.i.
2 Last application to harvest
3 Limit of detection
TABLE 10 RESIDUES OF CELORPYRIPOS AND PYRIDINOL IN FOOD CROPS, EUROPE/NEAR EAST TRIALS
CROP, SAMPLE DOSAGE1 TIME BETWEEN RESIDUES RESIDUES REFERENCES
COUNTRY NO. LAST TREATMENT CHLORPYRIFOS 3,5,6-TRICHLORO-2-PYRIDINOL (ppm)
& YEAR AND HARVEST (ppm)
TURKEY
1971
Tomatoes 1 A 825 g/ha 3 hours 1.3* <0.05
2 A " 3 hours <0.05
4 A " 3 hours 0.05 Barrows & Mullin,
3 B " 5 days 0.05 1971b
5 B " 5 days 0.26 <0.05
6 B " 5 days <0.05
ENGLAND
1971
Tomatoes 720 g/ha 0 day 1.28 0.14
1.24 0.12
3 days 0.14 0.10
0.05 0.10 Burrows et al
7 days 0.08 0.08 1971e
0.08 0.08
14 days 0.01 0.12
0.01 0.10
28 days <0.01 0.10
<0.01 0.16
ITALY 1971
Apples 1 816 g/ha 29 days 0.10 <0.05 Burrows & Mullin,
4 1 224 g/ha 15 days 0.25 <0.05 1971b
Grapes 11 816 g/ha 29 days 0.10 <0.05
13 816 g/ha 15 days 1.10 0.06 Ibid.
12 1 224 g/ha 29 days 0.22 <0.05
14 1 224 g/ha 15 days 1.50 0.29
* Composite samples
1 All dosages given in a.i.
TABLE 11 CHLORPYRIPHOS RESIDUES ON CROPS IN JAPAN1
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
Chinese
cabbage
1967 40E 267 1 4 0.13-0.23
1100 l/ha
1969 40E 267 2 8 0.02-0.09
1000 l/ha 2 16 0.43-0.44
" 267 4 8 0.16-0.22
4 16 0.62-0.86
1969 40E 267 2 7 0.02-0.06
1500 l/ha 2 14 0.06-0.08
" 267 4 7 0.08-0.14
4 14 0.06-0.12
Apples
Rall's Janet
1967 25W 250 4 66 N.D.3
Johnathan
1967 25W 250 5 42 0.01-0.05
1969 25W 250 3 30 0.05-0.07
30 l/tree 45 0.05-0.06
1969 " 250 2 17 0.03-0.07
28 0.02-0.05
46 0.02-0.03
TABLE 11 (Cont'd.)
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
25W 250 3 7 0.03-0.09
30 l/tree
14 0.05-0.17
31 0.08-0.16
45 0.03-0.05
Aomori
1969 25W 250 2 7 0.10
14 0.12-0.14
21 0.08-0.03
1 Hamaguchi, 1970
2 Range of averages for two laboratories
3 N.D. = not detectable (<0.005 ppm)
TABLE 12 CHLORPYRIFOS RESIDUES ON CROPS IN THAILAND AND MALAYSIA
CROP FORMULATION APPLIC. DAYS BETWEEN TESTS RESIDUE1 REFERENCES
& DILUTION (no.) LAST SPRAY (no.) (ppm)
& HARVEST
Chinese 25W 6 5 6 N. D.2 - 0.023 Tucker & Zielinski,
cabbage 1972a
Chinese 187 ppm(a.i.) " " " 0.10 - 0.243 Ibid., 1972b
kale " " " " 0.09 - 0.763
Chinese 25W " " " N.D.3 Ibid., 1972a
cabbage
Chinese 375 ppm(a.i.) " " " 0.38 - 0.753 Ibid., 1972b
kale " " " " 0.35 - 0.763
Chinese 25W 16 2 3 0.07 - 0.104 Ibid., 1972,
kale
3 oz a.i./A " " " 0.01 - 0.35
25W " " " 0.13 - 0.244
4 oz a.i./A " " " 0.01 - 0.035
Tobacco 25W 10 7 4 0.03 - 0.053 Ibid., 1972d
187 ppm (a.i.)
25W " " " 0.21 - 0.383
375 ppm (a.i.)
1 Two analytical methods used in analyses
2 N.D. = not detectable (<0.01 ppm)
3 "Usable leaves" from Thailand
4 Leaf only from Malaysia
5 Stem only from Malaysia
TABLE 13 Chlorpyrifos residues on rice in the Philippines1
Chlorpyrifos Residue
applied (kg/ha, Tests (range of
Part analysed Formulation 3 applications) (no.) averages,
ppm)
Grain E.C. 0.10 4 N.D.3
0.15 4 N.D. - 0.008
0.20 3 N.D. - 0.005
0.25 2 0.008
Granule 0.10 3 N.D.
Grain and E.C. 0.10 4 N.D. - 0.007
straw2 0.15 4 N.D. - 0.006
0.20 4 0.005 - 0.008
0.25 2 0.008
Granule 0.10 3 <0.005
1 Tucker, 1971.
2 Very little straw, mostly grain.
3 N.D = not detectable (<0.005 ppm)
FATE OF RESIDUES
General comments
The fate of chlorpyrifos residues and its metabolites in different
species of animals are discussed in the section on "Biochemical
aspects".
In animals
Cow
Chlorpyrifos was not found in the urine and milk from a cow fed 5 ppm
(based on a daily ration of 50 lb) of the compound for four days. The
compound in absolute ethanol was mixed thoroughly with the grain. A
compound characterized by retention time as chlorpyrifos was found in
faecal samples taken on the last three days during which the
insecticide was fed and the first day after, and represented 1.7% of
the insecticide fed. Two metabolites were excreted in the urine which,
following methylation, had retention times identical to the methyl
esters of diethylthiophosphate and diethyl phosphate. They represented
35.9 and 26.8%, respectively, of the total insecticide fed on an
equivalent basis (Gutenmann et al., 1968).
Fish
Waters treated with 50 and 300 ppb of ring-labelled 14C chlorpyrifos
and containing goldfish were analysed for the types of metabolites
found in each. Metabolites I, III, IIIa and IIIb, shown in Figure 1,
as well as chlorpyrifos were found in fish, and metabolites I, IIIa
and IIIb were found in the water (Smith et al., 1966).
The major metabolic product in fish is 3,5,6-trichloro-2-pyridinol
which appears to be slowly broken down by dehalogenation and cleavage
of the ring (Smith et al., 1966).
Some of the derivatives of 3,5,6-trichloro-2-pyridinol may be those
shown in Smith (1968) which consist of dechlorination forming a series
of transient pyridine diols, triols and tetrols (Ia), followed by a
series of transient ring diketones (Ib), followed by a breakage of the
ring to elemental fragments (carbon dioxide and possibly aliphatic
amines).
Cholinesterase inhibition properties of metabolites
The house fly head cholinesterase activity of chlorpyrifos, and
metabolites I, II, III, IIIa and IIIb shown in Figure 1, were tested
for comparative purposes by Smith et al. 1966). Only the phosphate
derivative of chlorpyrifos (metabolite III) shows significant
inhibition and is the compound most likely to be responsible for the
cholinesterase inhibition found in mammals and other animal organisms
(Smith et al., 1966).
In plants
Studies on the absorption, translocation and metabolism of 14C and
36Cl labelled chlorpyrifos in plants show that for all practical
purposes uptake of the chemical or its degradation products into
plants does not occur, either through the foliage or through the roots
(Smith et al., 1967a, 1967c).
When radioactive chlorpyrifos was applied to single bean or corn
leaves, about 75 to 80% of the chemical was lost by vapourization from
the leaf surface within the first 48 h following treatment. The
chlorpyrifos remaining on the leaf was rapidly degraded (>95% in 5
days) and a trace of the resulting breakdown products (representing
<2% of the applied dose of chlorpyrifos) absorbed into and
translocated within the plant. The identity of the degradation
products was not totally elucidated; however, a variety of hydrolysis
products appeared to be formed, mainly the 3,5,6-trichloro-2-pyridinol
(I), ethyl-3,5,6-trichloro-2-pyridyl phoshate (IIIa) and
3,5,6-trichloro-2-pyridyl phosphate (IIIb) shown in figure 1 (Smith
et al., 1967a).
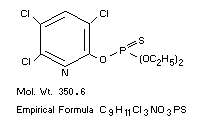 Nutrient culture experiments have shown uptake of chlorpyrifos by
plant roots to be essentially nil. Bean plants grown in nutrient
solution containing 10 mg (50 ppm) of 14C chlorpyrifos absorbed and
translocated to the plant tops less than 0.07% of the applied dose
during a 144 h exposure period. Based on the weight of plant tissue
analysed, this represented a residue <0.002 ppm of radioactive
compounds (expressed as chlorpyrifos). In this same experiment,
sizable amounts of chlorpyrifos were sorbed on the outside of the
plant roots, reaching 5 mg (50%) in 72 h. The negligible amounts of
radioactivity taken into the plants in this and other tests have made
it difficult to determine the nature of the compounds involved. Only
the 3,5,6-trichloro-2-pyridonol was identified. This compound, the
principal hydrolysis product of chlorpyrifos in soil, may or may not
be taken up by the plant depending on pH of the soil medium. The free
pyridinol, which is the predominant form of the compound below pH 6.0,
is essentially insoluble in water. Soil and nutrient culture
experiments have shown its uptake by plants to be insignificant. For
example, bean plants grown in nutrient solution (pH 5.5) containing 10
mg (50 ppm) of 36Cl labelled 3,5,6-trichloro-2-pyridinol showed about
0.007 ppm of radioactive compounds (expressed as the pyridinol) in the
plant tops after 72 h exposure. At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the Na salt) as well as rate of absorption by the plant
are enhanced relative to the free pyridinol. Regardless, soil and
nutrient culture tests have again shown only negligible amounts of the
compound to enter the plant and these undergo metabolism with the
liberation of chloride and the formation of trivial amounts of several
unidentified water soluble decomposition products (Smith et al.,
1967c).
Only through the use of specialized techniques has it been possible to
introduce sufficient chlorpyrifos into plants to determine the extent
to which the compound might be metabolized. Using the string
technique, 14C chlorpyrifos was introduced into the stems of
cranberry bean plants and the plants harvested at various time
intervals. Subsequent analysis of the plant tissues revealed the
presence principally of chlorpyrifos and its primary hydrolysis
products. Under these artificial conditions, chlorpyrifos once
introduced inside the stem, was distributed throughout the plant
(Smith et al., 1967c).
METHODS OF RESIDUE ANALYSIS
Chlorpyrifos, its oxygen analogue and hydrolytic pyridinol
Based on previously mentioned metabolite determinations, the main
residues from chlorpyrifos treatments searched for in various tissues
are chlorpyrifos, its oxygen analogue and the hydrolysed pyridinol.
Since the oxygen analogue is infrequently encountered in animal or
plant tissues, the principal analyses of residues are for chlorpyrifos
and 3,5,6-trichloro-2-pyridinol. The latter appears in small amounts,
mostly in non-fatty tissues. Analytical methods are available to
determine each of these two residues individually. However, for
regulatory purposes, chlorpyrifos is the most important residue to be
determined.
Claborn et al. (1968a,b) and Ivey and Claborn (1968) described the
basic extraction and cleanup procedures using an electron capture gas
chromatography method for detection of chlorpyrifos and its oxygen
analogue in milk and body tissues of cattle. Claborn et al. (1970b)
used a gas chromatography method and a flame photometric detector to
determine chlorpyrifos and its oxygen analogue in body tissues of
turkeys. Since the phosphate analogue is not searched for routinely, a
quicker method for determination of chlorpyrifos only is useful. In
order to do this a modification of the flame photometric gas
chromatography method of Claborn et al. (1970a,b) using a hexane
extraction for fat tissues was used instead of a
hexane-dichloromethane extraction. The method is sensitive to 0.01
ppm.
Further minor variations in the analytical method are related to
extraction and cleanup procedures, depending on the nature of the
substrate. Three analytical procedures are generally suitable for the
various substrates when the substrates are defined as (A) water; (B)
wet tissues and soil; and (C) dry or fatty tissues. The procedural
scheme for methods A, B and C is outlined in Figure 2.
More detailed procedures are specifically outlined as follows: Only
those asterisked methods accounted for the oxygen analogue. Procedure
for substrate (A), water (Rice and Dishburger, 1968; Wetters and
Dishburger, 1971c). Procedure for substrate (B), wet media, such as
sweet corn (Wetters, 1972c), corn forage (Wetters and Dishburger,
1971b), soil (Wetters, 1971a*,b), vegetables (Braun, 1971), liver,
kidney and muscle of pig (Wetters, 1972a; Bowman and Beroza, 1967b),
chicken muscle, liver, kidney and egg (Wetters, 1972b; Bowman and
Beroza, 1967b), peach (Wetters and Dishburger, 1971a*), milk (Craig,
1971), cattle tissues except fat (Claborn et al., 1968a), turkey
tissues except fat (Claborn et al., 1970b). Procedure for substrate
(C), dry or fatty media, such as corn grain and stover (Wetters and
Dishburger, 1971b; Wetters, 1972c), pig fat (Wetters, 1972a), chicken
fat (Wetters, 1972b; Bowman and Beroza, 1967a), cream (Graig, 1971),
cattle fat (Claborn et al., 1968a,b; Bowman and Beroza, 1967a),
turkey fat (Claborn et al., 1970b). Many other references on
chlorpyrifos in various media have been published (see References).
Nutrient culture experiments have shown uptake of chlorpyrifos by
plant roots to be essentially nil. Bean plants grown in nutrient
solution containing 10 mg (50 ppm) of 14C chlorpyrifos absorbed and
translocated to the plant tops less than 0.07% of the applied dose
during a 144 h exposure period. Based on the weight of plant tissue
analysed, this represented a residue <0.002 ppm of radioactive
compounds (expressed as chlorpyrifos). In this same experiment,
sizable amounts of chlorpyrifos were sorbed on the outside of the
plant roots, reaching 5 mg (50%) in 72 h. The negligible amounts of
radioactivity taken into the plants in this and other tests have made
it difficult to determine the nature of the compounds involved. Only
the 3,5,6-trichloro-2-pyridonol was identified. This compound, the
principal hydrolysis product of chlorpyrifos in soil, may or may not
be taken up by the plant depending on pH of the soil medium. The free
pyridinol, which is the predominant form of the compound below pH 6.0,
is essentially insoluble in water. Soil and nutrient culture
experiments have shown its uptake by plants to be insignificant. For
example, bean plants grown in nutrient solution (pH 5.5) containing 10
mg (50 ppm) of 36Cl labelled 3,5,6-trichloro-2-pyridinol showed about
0.007 ppm of radioactive compounds (expressed as the pyridinol) in the
plant tops after 72 h exposure. At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the Na salt) as well as rate of absorption by the plant
are enhanced relative to the free pyridinol. Regardless, soil and
nutrient culture tests have again shown only negligible amounts of the
compound to enter the plant and these undergo metabolism with the
liberation of chloride and the formation of trivial amounts of several
unidentified water soluble decomposition products (Smith et al.,
1967c).
Only through the use of specialized techniques has it been possible to
introduce sufficient chlorpyrifos into plants to determine the extent
to which the compound might be metabolized. Using the string
technique, 14C chlorpyrifos was introduced into the stems of
cranberry bean plants and the plants harvested at various time
intervals. Subsequent analysis of the plant tissues revealed the
presence principally of chlorpyrifos and its primary hydrolysis
products. Under these artificial conditions, chlorpyrifos once
introduced inside the stem, was distributed throughout the plant
(Smith et al., 1967c).
METHODS OF RESIDUE ANALYSIS
Chlorpyrifos, its oxygen analogue and hydrolytic pyridinol
Based on previously mentioned metabolite determinations, the main
residues from chlorpyrifos treatments searched for in various tissues
are chlorpyrifos, its oxygen analogue and the hydrolysed pyridinol.
Since the oxygen analogue is infrequently encountered in animal or
plant tissues, the principal analyses of residues are for chlorpyrifos
and 3,5,6-trichloro-2-pyridinol. The latter appears in small amounts,
mostly in non-fatty tissues. Analytical methods are available to
determine each of these two residues individually. However, for
regulatory purposes, chlorpyrifos is the most important residue to be
determined.
Claborn et al. (1968a,b) and Ivey and Claborn (1968) described the
basic extraction and cleanup procedures using an electron capture gas
chromatography method for detection of chlorpyrifos and its oxygen
analogue in milk and body tissues of cattle. Claborn et al. (1970b)
used a gas chromatography method and a flame photometric detector to
determine chlorpyrifos and its oxygen analogue in body tissues of
turkeys. Since the phosphate analogue is not searched for routinely, a
quicker method for determination of chlorpyrifos only is useful. In
order to do this a modification of the flame photometric gas
chromatography method of Claborn et al. (1970a,b) using a hexane
extraction for fat tissues was used instead of a
hexane-dichloromethane extraction. The method is sensitive to 0.01
ppm.
Further minor variations in the analytical method are related to
extraction and cleanup procedures, depending on the nature of the
substrate. Three analytical procedures are generally suitable for the
various substrates when the substrates are defined as (A) water; (B)
wet tissues and soil; and (C) dry or fatty tissues. The procedural
scheme for methods A, B and C is outlined in Figure 2.
More detailed procedures are specifically outlined as follows: Only
those asterisked methods accounted for the oxygen analogue. Procedure
for substrate (A), water (Rice and Dishburger, 1968; Wetters and
Dishburger, 1971c). Procedure for substrate (B), wet media, such as
sweet corn (Wetters, 1972c), corn forage (Wetters and Dishburger,
1971b), soil (Wetters, 1971a*,b), vegetables (Braun, 1971), liver,
kidney and muscle of pig (Wetters, 1972a; Bowman and Beroza, 1967b),
chicken muscle, liver, kidney and egg (Wetters, 1972b; Bowman and
Beroza, 1967b), peach (Wetters and Dishburger, 1971a*), milk (Craig,
1971), cattle tissues except fat (Claborn et al., 1968a), turkey
tissues except fat (Claborn et al., 1970b). Procedure for substrate
(C), dry or fatty media, such as corn grain and stover (Wetters and
Dishburger, 1971b; Wetters, 1972c), pig fat (Wetters, 1972a), chicken
fat (Wetters, 1972b; Bowman and Beroza, 1967a), cream (Graig, 1971),
cattle fat (Claborn et al., 1968a,b; Bowman and Beroza, 1967a),
turkey fat (Claborn et al., 1970b). Many other references on
chlorpyrifos in various media have been published (see References).
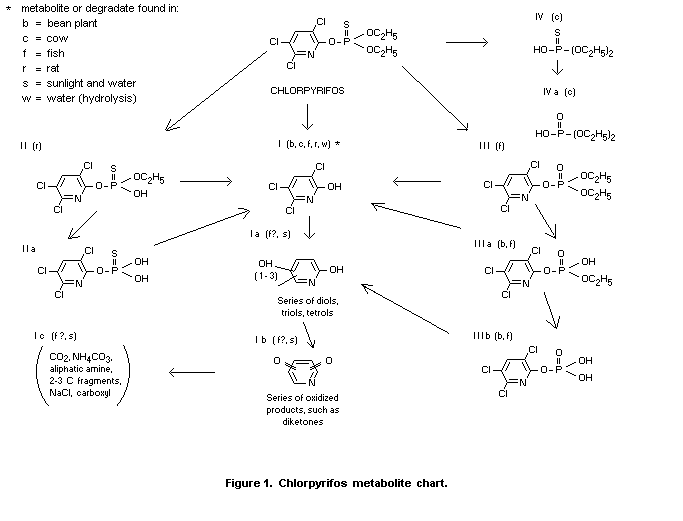 Residues of 3,5,6-trichloro-2-pyridinol may be determined by the
method of McKellar and Dishburger (1970) in bovine tissues; McKellar
(1971b) in corn grain, forage and stover; McKellar (1971c) in grass;
Dishburger et al. (1972) in chicken tissues and eggs and McKellar
(1971a) in milk and cream, at a detection level of 0.05 ppm.
The general cleanup procedures shown in Figure 2 followed by a gas
chromatographic determination utilizing one of the phosphorus specific
detectors would be suitable for most regulatory uses.
Examples of national tolerances as reported to meeting
Animals
Australia has a 2 ppm residue tolerance for chlorpyrifos in beef fat
when cattle are treated for ticks by dip or spray.
Plants
Canada has a tentative 0.1 ppm residue tolerance for head lettuce,
onions and carrots with 28, 60 and 60 days, respectively, required
between treatment and harvest.
APPRAISAL
Chlorpyrifos is a non-systemic organophosphorus insecticide used to
control ectoparasites of cattle, sheep and poultry; as a soil
insecticide for vegetables, cereals and tobacco; and as a foliar
insecticide for deciduous fruit, cereals, fodder crops, rape, cotton,
some vegetables, tobacco and rice.
Animal residues are found predominantly in the fat and are excreted
rapidly after cessation of treatment. When cattle were given multiple
dippings at the recommended dosage rates of 0.025%, the maximum total
residue in fat one week after the last dipping was 1.6 ppm. When milk
cows were dipped once in 0.025% chlorpyrifos, the residue in butterfat
fell to 0.01 ppm in nine days and was not detectable (<0.005 ppm)
after 15 days. When turkeys were confined over soil treated with
chlorpyrifos (4 or 8 lb/acre) for chigger control, maximum residues of
0.16 and 0.07 ppm were found in skin and fat, respectively, one week
post-treatment; the oxygen analogue was not detected in any tissues.
Small amounts of the pyridinol were found in the skin, livers and
kidneys of two birds with a maximum residue of 0.17 ppm in kidney
after one week exposure to the second application.
Extensive supervised experiments with foliar treatments on plants have
shown chlorpyrifos to have little persistence and virtually no uptake
occurs from either soil or foliar application. Up to 80% of applied
radioactive chlorpyrifos was lost by vapourization from single leaf
surfaces within the first 48 hours following treatment. There was no
evidence of residue buildup on plants from repeated applications.
The residues most likely to be found in either plants or animals are
chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol;
the oxygen analogue or other possible metabolites are very rarely
found.
Both specific and general gas chromatographic methods of analysis are
available which are suitable for regulatory purposes. The generalized
cleanup schemes shown in Figure 2 followed by gas chromatographic
determination using a phosphorus specific detector are recommended.
Separate analytical procedures for determining
3,5,6-trichloro-2-pyridinol in animal tissues (liver, kidney, poultry
skin), fruits and vegetables have been developed. The pyridinol is
excreted rapidly in the urine and faeces of animals and there would
appear to be no need to include this compound in regulatory analysis.
In field experiments with plants the pyridinol is rarely found and
when present constitutes only a fraction of the total residue.
Although chlorpyrifos is recommended in some countries for use on
rape, cereal grains and certain vegetables, there were no data
available on these commodities from supervised experiments and no
recommendations for tolerances could be made for these commodities.
RECOMMENDATIONS
TOLERANCES
ppm
Fat of meat of cattle 2
Chinese cabbage, grapes, kale, apples 1
Carrots, pears, tomatoes 0.5
Fat of meat of sheep, poultry, beans,
eggplants, peppers, raspberries 0.2
Lettuce, rice (in husk), sugarbeets 0.1
Celery, cottonseed, cottonseed oil
(crude), mushrooms, onions 0.05
Cauliflower, milk (fat basis),
potatoes, red cabbage 0.01*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials for rape seed, cereal grains and
any vegetables not listed.
DESIRABLE
1. Elucidation of possible increased sensitivity to plasma
cholinesterase depression after withdrawal from an initial dose
regime.
2. Further information on residues in milk and milk products arising
from dipping of dairy cattle.
REFERENCES
Aharonson, N., Grünberg, R. and Resnick, C. (1969) Dursban residue
level in tomatoes and cotton. Ministry of Agriculture, Jaffa, Israel.
Beatty, S.C. and McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Report The Dow Chemical Co., revision.
(unpublished)
Blackmore, R.H. (1968a) Oral administration-dogs-Dursban final report.
Hazleton Lab. Inc. (unpublished)
Blackmore, R.H. (1968b) Short-term (subacute) dietary
administration-rats-organophosphate insecticide Dursban - final
report. Hazleton Lab. Inc. (unpublished)
Bowman, M.C. and Beroza, M. (1967a) Determination of Dursban and its
oxygen analogue in corn and grass by gas chromatography with flame
photometric detection. J. Agr. Fd. Chem., 15(4): 651-653.
Bowman, M.C. and Beroza, M. (1967b) Temperature-programmed gas
chromatography of 20 phosphorus-containing insecticides on 4 different
columns and its application to the analysis of milk and corn silage.
J. Ass. off. analyt. Chem., 50(6): 1228-1236.
Branson, D.R. and Litchfield, N.H. (1971a) Absorption, excretion and
distribution of 3,5,6-trichloro-2,6-14C-2-pyridinol in rats. Report
NBA-10 Dow Chemical Company. (unpublished)
Branson, D.R. and Litchfield, N.H. (1971b) Absorption, excretion and
distribution of O,O-diethyl O-3,5,6-trichloro-2,6-14C-2-pyridyl
phosphorothioate (14C Dowco 179) in rats. Report NBA-9 Dow Chemical
Company. (unpublished)
Branson, D.R. and Wass, M.N. (1970) Comparative metabolism of
insecticides. I. Preliminary studies of ring labelled O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate breakdown with rat
liver microsomes. Report R-582 Dow Chemical Company. (unpublished)
Braun, H.E. (1971) Dursban residues in vegetables. Ontario Department
of Food and Agriculture, Guelph, Ontario. Personal communications to
T. Haagsma.
Brust, R.A., Miyazaki, S. and Hodgson, G.C. (1971) Effect of Dursban
in the drinking water of chicks. J. Econ. Entomol., 64(5): 1179-1183.
Burrows, I.E., Fraser, W.D., Joyce, C.A., Jenkins, G. and Eden, A.
(1971a) Determination of decay curves for Dursban and its metabolite
in stored tomatoes. 4211/71/369. Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971b) Determination of residues of
Dursban metabolite in tomatoes, grapes and apples. 4109/71/267.
Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971c) Determination of residues of
Dursban in lettuce. 4140/71/298. Huntingdon Research Centre.
Burrows, I.E., Mullin, L.W. and Eden, A. (1971d) Determination of
residues of Dursban and its metabolites in raspberries. 4371/71/527.
Huntingdon Research Centre.
Burrows, I.E., Orme, J.P.R. and Ashton, J. (1971e) Determination of
residues of Dursban and its metabolite in tomatoes. 4496/71/652.
Huntingdon Research Centre.
Claborn, H.V., Hoffman, R.A., Mann, H.D. and Oehler, D.D. (1968a)
Residues of Dursban and its oxygen analogue in the body tissues of
treated cattle. J. Econ. Entomol., 61(4): 983-986.
Claborn, H.V., Mann, H.D. and Oehler, D.D. (1968b) Dursban(R)
determination in milk and body tissues of cattle. J. Ass. off. analyt.
Chem., 51(6): 1243-1245.
Claborn, H.V., Ivey, M.C., Mann, H.D. and Oehler, D.D. (1970a) Report
of residue analysis - Omental fat, muscle, kidney and liver. Report
PCK-70-1. USDA-ARS-Entomol. Res. Div.
Claborn, H.V., Kunz, S.E. and Mann, H.D. (1970b) Residues of Dursban
in the body tissues of turkeys confined in pens containing treated
soil. J. Econ. Entomol., 63(2): 422-424.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
0,0-diethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate in Beagle
hounds. Biochem. Res. Lab. Report T35.12-44793-3 Dow Chemical Co.
(unpublished)
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
and Norvell, M. (1971) Final report on safety evaluation and metabolic
studies on Dowco 179 (IN 151). Inst. Exp. Pathol. Toxicol., Albany
Medical College. (unpublished)
Coulston, F., Goldberg, L. and Griffin, T. (1972) Safety evaluation of
Dowco 179 in humans. Inst. Exp. Pathol. Toxicol., Albany Medical
College. (unpublished)
Craig, L.F. (1971) Determination of residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in milk and cream by
gas chromatography. Method ACR 71.1. Dow Chemical Co.
Czech, F.P. (1967) Analysis of Dursban in livestock dips and sprays.
J. Ass. off. analyt. Chem., 50(4): 861-868.
Dishburger, H.J. and Rice, J.R. (1965) Residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in animal fat when
applied as a spray on cattle. Report TA-301 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966a) The effect of
formulation on Dursban residues in omental fat of cattle following
single spray application. Report TA-336 Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966b) Dursban residues
in the omental fat of cattle following dip applications. Report TA-349
Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1967) Dursban residues
in the omental fat of cattle following two dip applications. Report
TA-372 Dow Chemical Co. (unpublished)
Dishburger, H.J., Pennington, J. and Rice, J.R. (1969a) Determination
of O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
tobacco by gas chromatography. Report TA-430 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R., McGregor, W.S. and Pennington, J.
(1969b) Residues of Dursban(R) insecticide in tissues from turkeys
confined on soil treated for chigger control. J. Econ. Entomol.,
62(1): 181-183.
Dishburger, H.J., Rice, J.R and Pennington, J. (1969c) Determination
of residues of Dursban insecticide and its oxygen analogue in soil by
gas chromatography. Report TA-439 Dow Chemical Co. (unpublished)
Dow Chemical Co. (1964) Potentiation studies with Dursban in
combination with Ruelene and malathion in rats. Report Dow Chemical
Co. (unpublished)
Dow Chemical Co. (1966a) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (1966b) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (U.K.) Ltd. (1967) Data sheets containing summarized
data supporting notification of Dursban(R) insecticide for
commercial use in the control of sheep ectoparasites. Submitted to
the Technical Secretary, Scientific Sub-Committee, Veterinary
Products Safety Precautions Scheme.
Düsch, M.E., Westlake, W.E. and Gunther, F.A. (1970) Determination of
Dursban insecticide in water, mud, vegetation, fish, ducks, insects
and crustacea. J. Agr. Fd. Chem., 18(1): 178-179.
Emerson, J.L. and Gerbig, C.G. (1970) 91 day-toxicology study in
Beagle dogs treated with 3,5,6-trichloro-2-pyridinol. Report HH-263
Dow Chemical Co. (unpublished)
Foschi, S. (1971) Determination del Dursban. Istituto di Patologia
Vegetale, Bologna, Italy. Personal communication to K.N. Komblas.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14: 515-534.
Gerbig, C.G and Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat. Report HH-239
Dow Chemical Co. (unpublished)
Gerbig, C.G and Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Report HH-240
Dow Chemical Co. (unpublished)
Graf, W.C. (1970a) Analyses of Dursban residues on cotton seeds. Dow
Chemical Europe S.A. Personal communication to K. Komblas.
Graf, W.C. (1970b) Dursban residue analyses on apples from SIAPA,
Italy. Report DC 53. Dow Chemical Europe S.A.
Graf, W.C. (1970c) Dursban residue analyses on pears from SIAPA,
Italy. Report DC 54 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970a) Dursban residue analyses on apples
from Israel. Report DEC 55 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970b) Dursban residue on tomatoes from
Turkey. Report DEC 56 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971a) Dowco 179 residue analysis in
potatoes from France. Report DEH 2 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971b) Dowco 179 residue analysis on
tomatoes from Israel. Dow Chemical Europe S.A.
Graf. W.C. and Engeler, V. (1971c) Dowco 179 residue analysis in
apples from Israel. Report DEH 36 Dow Chemical Europe S.A.
Gutenmann, W.H. St. John, Jr., L.E. and Lisk, D.L. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow. J. Agr. Fd.
Chem., 16(1): 45-47.
Hall, R.A. (1969) Dursban residues. Department of Agriculture,
Australia. Personal communication to J. Tollett.
Hamaguchi, H. (1970) Dowco 179 - residue analysis. Translation of
analytical data furnished by Japan Plant Protection Association.
Nissan Chemical Laboratory and Japan Analytical Chemical Research
Laboratory. Personal communication to R.H. Ferguson.
Harris, C.R. (1971) Dursban residue in onions. Canada Department of
Agriculture. Personal Communication to T. Haagsma.
Hollick, C.B. and Collison, R.J. (1972) Determination of residues of
O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
(Dowco-179) in eggplant, tomatoes, beans and peppers treated with
Dursban 4 insecticide in Turkey. Report Dow Chemical Company Ltd.
(unpublished)
Hunt, L.M., Gilbert, B.N. and Schlinke, J.C. (1969) Rapid gas
chromatographic method for analysis of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate (Dursban) in turkey and
chicken tissues. J. Agr. Fd. Chem., 17(6): 1166-1167.
Hurlbert, S.H., Mulla, M.S., Keith, J.O., Westlake, W.E. and Düsch,
M.E. (1970) Biological effects and persistence of Dursban(R) in
freshwater ponds. J. Econ. Entomol., 63(1): 43-52.
Ivey, M.C. and Claborn, H.V. (1968) Dursban oxygen analogue:
Determination in fortified milk and body tissues of cattle. J. Assoc.
Offic. Anal. Chem., 51(6): 1245-1246.
Johnson, J.C. Jr., Bowman, M.C. and Leuck, D.B. (1969) Responses from
cows fed silages containing Dursban residues. J. Dairy Sci., 52(8):
1253-1258.
Kenaga, E.E. (1967) Toxicity and repellency of Dursban to the
white-footed mouse (Peromyscus maniculatus). Report Dow Chemical Co.
(unpublished).
Leuck, D.B., Bowman, M.C. and Beck, E.W. (1968) Dursban(R) insecticide
persistence in grass and corn forage. J. Econ. Entomol., 61(3):
689-690.
Mann, H.D. (1966) Report of Dursban(R) insecticide residue analysis in
treated cattle. Report PCK-66-2. USDA-ARS-Entomol. Res. Div.
Mann, H.D. and Ivey, M.C. (1971) Report of residues analysis - Fat,
skin, muscle, liver and kidney tissues of turkeys. Report PCK-71-1.
USDA-ARS-Entomol. Res. Div.
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco(R) 179 in
Beagle dogs. Report T35.12-44793-18 Dow Chemical Co. (unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco(R) 179 in
rats. Report, NBT35.12-44793-21 Dow Chemical Co. (unpublished)
McCray, C.W. (1969) Field trials using Dursban. Letter report Animal
Res. Inst., Queensland.
McEwen, F.L. (1970) Tests with Dursban on onions, carrots and lettuce.
University of Guelph. Personal communication to T. Haagsma.
McEwen, F.L. and Frank, R. (1970) Tests with Dursban on onions,
carrots and lettuce. University of Guelph. Personal communication to
T. Haagsma.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-pyridinol in milk and cream by gas chromatography.
Method ACR 71.2. Dow Chemical Co.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-pyridinol in corn grain, forage and stover by gas
chromatography. Method ACR 71.19. Dow Chemical Co.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-pyridinol in grass by gas chromatography. Method ACR
71.5. Dow Chemical Co.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography. Method ACR 70.19. Dow Chemical Co.
Molello, J.A. and Sharp, L.D. (1968) Three week study on
cataractogenicity of 3,5,6-trichloro-2-pyridinol as part of the
dietary intake of Pekin ducklings. Report HH-134 Dow Chemical Co.
(unpublished)
Myers, C.M., Lewallen, L.L. and Nobe, B. (1968) Residue studies of
fenthion (Baytex(R)) and Dursban(R) in central California pastures.
California Vector Views, 15(5): 51-54.
Norris, J.M. (1970) Potentiation Study on Dowco(R) 179 and Vapona(R)
insecticide. Report Dow Chemical Co. (unpublished)
Olthof, P.D.A. (1971a) Residuen van Dursban in champignons. Report R
3563. Centraal Instituut voor Voedingsonderzoek, The Netherlands.
Olthof, P.D.A. (1971b) Residues of Dursban in carrots. Report R 3633.
English translation from report by Centraal Instituut voor
Voedingsonderzoek, The Netherlands.
Rice, J.R. and Dishburger, H.J. (1968) Determination of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate in
water and silt by gas chromatography. J. Agr. Fd. Chem., 16(5):
867-869.
Scheuerer, W. and Hahn, I. (1972) Dursban residues on vegetables.
Badische Anilin and Soda-Fabrik AG, West Germany. Communication to Dow
Chemical (Nederland) N.V.
Schlinke, J.C. (1970) Chronic toxicity of Dursban in chickens, 1969.
J. Econ. Entomol., 63(1): 319.
Schlinke, J.C., Palmer, J.S. and Hunt, L. (1969) Preliminary
toxicological study of a phosphorothioate compound in turkeys. Am. J.
Vet. Res., 30(9): 1705-1709.
Sherman, M., Herrick, R.B., Ross, E. and Chang, M.T.Y. (1967) Further
studies on the acute and subacute toxicity of insecticides to chicks.
Tox. Appl. Pharmacol., 11: 49-67.
SIAPA. (1971) Residue determinations on lettuce after treatment with
Dursban insecticide. Centro Esperienze e Ricerche, Italy.
Smith, G.N. (1966) Basic studies of Dursban insecticide. Down to
Earth, 22(2): 3-7.
Smith, G.N. (1968) Ultraviolet light decomposition studies with
Dursban and 3,5,6-trichloro-2-pyridinol. J. Econ. Entomol., 61(3):
793-799.
Smith, G.N. and Taylor, Y.S. (1970) An analytical method for the
determination of 3,5,6-trichloro-2-pyridinol in animal tissues and the
metabolism of the pyridinol in rats. Report OL 3132 Dow Chemical
(unpublished)
Smith, G.N., Watson, B.S. and Fischer, F.S. (1966) The metabolism of
(14C) O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) in fish. J. Econ. Entomol., 59(6):
1464-1475.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967a) Investigations on
Dursban insecticide. Uptake and translocation of (36Cl)
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate by
beans and corn. J. Agr. Fd. Chem., 15(1): 127-131.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967b) Investigations on
Dursban insecticide. Metabolism of (36Cl) O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in rats. J. Agr. Fd.
Chem., 15(1): 132-138.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967c) Investigations on
Dursban insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agr. Fd. Chem., 15(5):
870-877.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Report OL-3132 Dow
Chemical Co. (unpublished)
Snelson, J.T. (1969a) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Snelson, J.T. (1969b) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Stevenson, G.T. (1965) A gamebird toxicology study - acute dietary
feeding of Dursban to wild type mallard ducklings. Report GH-A-122 Dow
Chemical Co. (unpublished)
Stevenson, G.T. (1966) A neurotoxicity study of Dursban in laying
hens. Report GH-A-195 Dow Chemical Co. (unpublished)
Stevenson, G.T. and Daering, K.L. (1969) The effects of Dursban
insecticide (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) on blood plasma cholinesterase in chickens. Report
GH-A-410 Dow Chemical Co. (unpublished)
Taylor, M.L. and Olson, K.J. (1963) Toxicological properties of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report
T35.12-44793-4 Dow Chemical Co. (unpublished)
Thompson, D.J., Gerbig, C.G. and Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report HH-382 Dow
Chemical Co. (unpublished)
Tucker, K.E.B. (1971) Dursban/Dowco 214 residues in rice and straw.
Report 160 Dow Chemical (Australia) Ltd.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of the Interior, Fish and Wildlife
Service Resource Publication #84, p. 56-57.
Tucker, K.E.B. and Zielinski, W. (1972a) Chlorpyrifos and Dowco 214
residues in Chinese cabbage. Report 175 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972b) Chlorpyrifos and Dowco 214
residues in Chinese kale. Report 174 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972c) Residues of chlorpyrifos in
Chinese kale. Report 179 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972d) Residues of chlorpyrifos and
Dowco 214 in tobacco. Report 180 Dow Chemical (Australia) Ltd.
USAEHA. (1968) Report U.S. Army Environmental Hygiene Agency.
Watts, R.M. (1968) Residues of chlorpyrifos in cattle. Dept. of
Agriculture, Australia. Communication to M.K. Astill.
Wetters, J.H. (1971a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate and O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphate in soils by gas
chromatography with flame photometric detection. Method ACR 71.3. Dow
Chemical Co.
Wetters, J.H. (1971b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in soils by gas
chromatography using flame photometric detection and direct injection.
Method ACR 71.20. Dow Chemical Co.
Wetters, J.H. (1972a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in pig tissues by gas
chromatography with flame photometric detection. Method 72.1. Dow
Chemical Co.
Wetters, J.H. (1972b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in chicken tissues
and eggs by gas chromatography with flame photometric detection.
Method 72.3. Dow Chemical Co.
Wetters, J.H. (1972c) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sweet corn by gas
chromatography with flame photometric detection. Method ACR 72.9. Dow
Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971a) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
and O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphate in
peaches by gas chromatography with flame photometric detection. Method
ACR 71.14. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971b) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
corn by gas chromatography with flame photometric detection. Method
ACR 71.18. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971c) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
water by gas chromatography with flame photometric detection. Method
ACR 71.21. Dow Chemical Co.
Winterlin. W., Moilanen, K. and Burgoyne, W.E. (1968) Residues of
Dursban insecticide following mosquito control applications. Down to
Earth, 24(2): 34-37.
Wit, S.L. (1970) Residues of Dursban in cabbage. Report 49/70 Tox RoB.
English translation from report by State Institute for the Public
Health, The Netherlands.
Wit, S.L. (1971) Residues of O,O-diethyl
O-(3,5,6-trichloro-pyridine-(2)-yl) phosphorothioate (Dursban) for
the control of cutworms in lettuce under glass. Report 63/71 Tox-RoB.
English translation from report by Rijks Instituut voor de
Volksgezondheid, The Netherlands.
Residues of 3,5,6-trichloro-2-pyridinol may be determined by the
method of McKellar and Dishburger (1970) in bovine tissues; McKellar
(1971b) in corn grain, forage and stover; McKellar (1971c) in grass;
Dishburger et al. (1972) in chicken tissues and eggs and McKellar
(1971a) in milk and cream, at a detection level of 0.05 ppm.
The general cleanup procedures shown in Figure 2 followed by a gas
chromatographic determination utilizing one of the phosphorus specific
detectors would be suitable for most regulatory uses.
Examples of national tolerances as reported to meeting
Animals
Australia has a 2 ppm residue tolerance for chlorpyrifos in beef fat
when cattle are treated for ticks by dip or spray.
Plants
Canada has a tentative 0.1 ppm residue tolerance for head lettuce,
onions and carrots with 28, 60 and 60 days, respectively, required
between treatment and harvest.
APPRAISAL
Chlorpyrifos is a non-systemic organophosphorus insecticide used to
control ectoparasites of cattle, sheep and poultry; as a soil
insecticide for vegetables, cereals and tobacco; and as a foliar
insecticide for deciduous fruit, cereals, fodder crops, rape, cotton,
some vegetables, tobacco and rice.
Animal residues are found predominantly in the fat and are excreted
rapidly after cessation of treatment. When cattle were given multiple
dippings at the recommended dosage rates of 0.025%, the maximum total
residue in fat one week after the last dipping was 1.6 ppm. When milk
cows were dipped once in 0.025% chlorpyrifos, the residue in butterfat
fell to 0.01 ppm in nine days and was not detectable (<0.005 ppm)
after 15 days. When turkeys were confined over soil treated with
chlorpyrifos (4 or 8 lb/acre) for chigger control, maximum residues of
0.16 and 0.07 ppm were found in skin and fat, respectively, one week
post-treatment; the oxygen analogue was not detected in any tissues.
Small amounts of the pyridinol were found in the skin, livers and
kidneys of two birds with a maximum residue of 0.17 ppm in kidney
after one week exposure to the second application.
Extensive supervised experiments with foliar treatments on plants have
shown chlorpyrifos to have little persistence and virtually no uptake
occurs from either soil or foliar application. Up to 80% of applied
radioactive chlorpyrifos was lost by vapourization from single leaf
surfaces within the first 48 hours following treatment. There was no
evidence of residue buildup on plants from repeated applications.
The residues most likely to be found in either plants or animals are
chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol;
the oxygen analogue or other possible metabolites are very rarely
found.
Both specific and general gas chromatographic methods of analysis are
available which are suitable for regulatory purposes. The generalized
cleanup schemes shown in Figure 2 followed by gas chromatographic
determination using a phosphorus specific detector are recommended.
Separate analytical procedures for determining
3,5,6-trichloro-2-pyridinol in animal tissues (liver, kidney, poultry
skin), fruits and vegetables have been developed. The pyridinol is
excreted rapidly in the urine and faeces of animals and there would
appear to be no need to include this compound in regulatory analysis.
In field experiments with plants the pyridinol is rarely found and
when present constitutes only a fraction of the total residue.
Although chlorpyrifos is recommended in some countries for use on
rape, cereal grains and certain vegetables, there were no data
available on these commodities from supervised experiments and no
recommendations for tolerances could be made for these commodities.
RECOMMENDATIONS
TOLERANCES
ppm
Fat of meat of cattle 2
Chinese cabbage, grapes, kale, apples 1
Carrots, pears, tomatoes 0.5
Fat of meat of sheep, poultry, beans,
eggplants, peppers, raspberries 0.2
Lettuce, rice (in husk), sugarbeets 0.1
Celery, cottonseed, cottonseed oil
(crude), mushrooms, onions 0.05
Cauliflower, milk (fat basis),
potatoes, red cabbage 0.01*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials for rape seed, cereal grains and
any vegetables not listed.
DESIRABLE
1. Elucidation of possible increased sensitivity to plasma
cholinesterase depression after withdrawal from an initial dose
regime.
2. Further information on residues in milk and milk products arising
from dipping of dairy cattle.
REFERENCES
Aharonson, N., Grünberg, R. and Resnick, C. (1969) Dursban residue
level in tomatoes and cotton. Ministry of Agriculture, Jaffa, Israel.
Beatty, S.C. and McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Report The Dow Chemical Co., revision.
(unpublished)
Blackmore, R.H. (1968a) Oral administration-dogs-Dursban final report.
Hazleton Lab. Inc. (unpublished)
Blackmore, R.H. (1968b) Short-term (subacute) dietary
administration-rats-organophosphate insecticide Dursban - final
report. Hazleton Lab. Inc. (unpublished)
Bowman, M.C. and Beroza, M. (1967a) Determination of Dursban and its
oxygen analogue in corn and grass by gas chromatography with flame
photometric detection. J. Agr. Fd. Chem., 15(4): 651-653.
Bowman, M.C. and Beroza, M. (1967b) Temperature-programmed gas
chromatography of 20 phosphorus-containing insecticides on 4 different
columns and its application to the analysis of milk and corn silage.
J. Ass. off. analyt. Chem., 50(6): 1228-1236.
Branson, D.R. and Litchfield, N.H. (1971a) Absorption, excretion and
distribution of 3,5,6-trichloro-2,6-14C-2-pyridinol in rats. Report
NBA-10 Dow Chemical Company. (unpublished)
Branson, D.R. and Litchfield, N.H. (1971b) Absorption, excretion and
distribution of O,O-diethyl O-3,5,6-trichloro-2,6-14C-2-pyridyl
phosphorothioate (14C Dowco 179) in rats. Report NBA-9 Dow Chemical
Company. (unpublished)
Branson, D.R. and Wass, M.N. (1970) Comparative metabolism of
insecticides. I. Preliminary studies of ring labelled O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate breakdown with rat
liver microsomes. Report R-582 Dow Chemical Company. (unpublished)
Braun, H.E. (1971) Dursban residues in vegetables. Ontario Department
of Food and Agriculture, Guelph, Ontario. Personal communications to
T. Haagsma.
Brust, R.A., Miyazaki, S. and Hodgson, G.C. (1971) Effect of Dursban
in the drinking water of chicks. J. Econ. Entomol., 64(5): 1179-1183.
Burrows, I.E., Fraser, W.D., Joyce, C.A., Jenkins, G. and Eden, A.
(1971a) Determination of decay curves for Dursban and its metabolite
in stored tomatoes. 4211/71/369. Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971b) Determination of residues of
Dursban metabolite in tomatoes, grapes and apples. 4109/71/267.
Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971c) Determination of residues of
Dursban in lettuce. 4140/71/298. Huntingdon Research Centre.
Burrows, I.E., Mullin, L.W. and Eden, A. (1971d) Determination of
residues of Dursban and its metabolites in raspberries. 4371/71/527.
Huntingdon Research Centre.
Burrows, I.E., Orme, J.P.R. and Ashton, J. (1971e) Determination of
residues of Dursban and its metabolite in tomatoes. 4496/71/652.
Huntingdon Research Centre.
Claborn, H.V., Hoffman, R.A., Mann, H.D. and Oehler, D.D. (1968a)
Residues of Dursban and its oxygen analogue in the body tissues of
treated cattle. J. Econ. Entomol., 61(4): 983-986.
Claborn, H.V., Mann, H.D. and Oehler, D.D. (1968b) Dursban(R)
determination in milk and body tissues of cattle. J. Ass. off. analyt.
Chem., 51(6): 1243-1245.
Claborn, H.V., Ivey, M.C., Mann, H.D. and Oehler, D.D. (1970a) Report
of residue analysis - Omental fat, muscle, kidney and liver. Report
PCK-70-1. USDA-ARS-Entomol. Res. Div.
Claborn, H.V., Kunz, S.E. and Mann, H.D. (1970b) Residues of Dursban
in the body tissues of turkeys confined in pens containing treated
soil. J. Econ. Entomol., 63(2): 422-424.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
0,0-diethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate in Beagle
hounds. Biochem. Res. Lab. Report T35.12-44793-3 Dow Chemical Co.
(unpublished)
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
and Norvell, M. (1971) Final report on safety evaluation and metabolic
studies on Dowco 179 (IN 151). Inst. Exp. Pathol. Toxicol., Albany
Medical College. (unpublished)
Coulston, F., Goldberg, L. and Griffin, T. (1972) Safety evaluation of
Dowco 179 in humans. Inst. Exp. Pathol. Toxicol., Albany Medical
College. (unpublished)
Craig, L.F. (1971) Determination of residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in milk and cream by
gas chromatography. Method ACR 71.1. Dow Chemical Co.
Czech, F.P. (1967) Analysis of Dursban in livestock dips and sprays.
J. Ass. off. analyt. Chem., 50(4): 861-868.
Dishburger, H.J. and Rice, J.R. (1965) Residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in animal fat when
applied as a spray on cattle. Report TA-301 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966a) The effect of
formulation on Dursban residues in omental fat of cattle following
single spray application. Report TA-336 Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966b) Dursban residues
in the omental fat of cattle following dip applications. Report TA-349
Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1967) Dursban residues
in the omental fat of cattle following two dip applications. Report
TA-372 Dow Chemical Co. (unpublished)
Dishburger, H.J., Pennington, J. and Rice, J.R. (1969a) Determination
of O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
tobacco by gas chromatography. Report TA-430 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R., McGregor, W.S. and Pennington, J.
(1969b) Residues of Dursban(R) insecticide in tissues from turkeys
confined on soil treated for chigger control. J. Econ. Entomol.,
62(1): 181-183.
Dishburger, H.J., Rice, J.R and Pennington, J. (1969c) Determination
of residues of Dursban insecticide and its oxygen analogue in soil by
gas chromatography. Report TA-439 Dow Chemical Co. (unpublished)
Dow Chemical Co. (1964) Potentiation studies with Dursban in
combination with Ruelene and malathion in rats. Report Dow Chemical
Co. (unpublished)
Dow Chemical Co. (1966a) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (1966b) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (U.K.) Ltd. (1967) Data sheets containing summarized
data supporting notification of Dursban(R) insecticide for
commercial use in the control of sheep ectoparasites. Submitted to
the Technical Secretary, Scientific Sub-Committee, Veterinary
Products Safety Precautions Scheme.
Düsch, M.E., Westlake, W.E. and Gunther, F.A. (1970) Determination of
Dursban insecticide in water, mud, vegetation, fish, ducks, insects
and crustacea. J. Agr. Fd. Chem., 18(1): 178-179.
Emerson, J.L. and Gerbig, C.G. (1970) 91 day-toxicology study in
Beagle dogs treated with 3,5,6-trichloro-2-pyridinol. Report HH-263
Dow Chemical Co. (unpublished)
Foschi, S. (1971) Determination del Dursban. Istituto di Patologia
Vegetale, Bologna, Italy. Personal communication to K.N. Komblas.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14: 515-534.
Gerbig, C.G and Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat. Report HH-239
Dow Chemical Co. (unpublished)
Gerbig, C.G and Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Report HH-240
Dow Chemical Co. (unpublished)
Graf, W.C. (1970a) Analyses of Dursban residues on cotton seeds. Dow
Chemical Europe S.A. Personal communication to K. Komblas.
Graf, W.C. (1970b) Dursban residue analyses on apples from SIAPA,
Italy. Report DC 53. Dow Chemical Europe S.A.
Graf, W.C. (1970c) Dursban residue analyses on pears from SIAPA,
Italy. Report DC 54 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970a) Dursban residue analyses on apples
from Israel. Report DEC 55 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970b) Dursban residue on tomatoes from
Turkey. Report DEC 56 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971a) Dowco 179 residue analysis in
potatoes from France. Report DEH 2 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971b) Dowco 179 residue analysis on
tomatoes from Israel. Dow Chemical Europe S.A.
Graf. W.C. and Engeler, V. (1971c) Dowco 179 residue analysis in
apples from Israel. Report DEH 36 Dow Chemical Europe S.A.
Gutenmann, W.H. St. John, Jr., L.E. and Lisk, D.L. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow. J. Agr. Fd.
Chem., 16(1): 45-47.
Hall, R.A. (1969) Dursban residues. Department of Agriculture,
Australia. Personal communication to J. Tollett.
Hamaguchi, H. (1970) Dowco 179 - residue analysis. Translation of
analytical data furnished by Japan Plant Protection Association.
Nissan Chemical Laboratory and Japan Analytical Chemical Research
Laboratory. Personal communication to R.H. Ferguson.
Harris, C.R. (1971) Dursban residue in onions. Canada Department of
Agriculture. Personal Communication to T. Haagsma.
Hollick, C.B. and Collison, R.J. (1972) Determination of residues of
O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
(Dowco-179) in eggplant, tomatoes, beans and peppers treated with
Dursban 4 insecticide in Turkey. Report Dow Chemical Company Ltd.
(unpublished)
Hunt, L.M., Gilbert, B.N. and Schlinke, J.C. (1969) Rapid gas
chromatographic method for analysis of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate (Dursban) in turkey and
chicken tissues. J. Agr. Fd. Chem., 17(6): 1166-1167.
Hurlbert, S.H., Mulla, M.S., Keith, J.O., Westlake, W.E. and Düsch,
M.E. (1970) Biological effects and persistence of Dursban(R) in
freshwater ponds. J. Econ. Entomol., 63(1): 43-52.
Ivey, M.C. and Claborn, H.V. (1968) Dursban oxygen analogue:
Determination in fortified milk and body tissues of cattle. J. Assoc.
Offic. Anal. Chem., 51(6): 1245-1246.
Johnson, J.C. Jr., Bowman, M.C. and Leuck, D.B. (1969) Responses from
cows fed silages containing Dursban residues. J. Dairy Sci., 52(8):
1253-1258.
Kenaga, E.E. (1967) Toxicity and repellency of Dursban to the
white-footed mouse (Peromyscus maniculatus). Report Dow Chemical Co.
(unpublished).
Leuck, D.B., Bowman, M.C. and Beck, E.W. (1968) Dursban(R) insecticide
persistence in grass and corn forage. J. Econ. Entomol., 61(3):
689-690.
Mann, H.D. (1966) Report of Dursban(R) insecticide residue analysis in
treated cattle. Report PCK-66-2. USDA-ARS-Entomol. Res. Div.
Mann, H.D. and Ivey, M.C. (1971) Report of residues analysis - Fat,
skin, muscle, liver and kidney tissues of turkeys. Report PCK-71-1.
USDA-ARS-Entomol. Res. Div.
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco(R) 179 in
Beagle dogs. Report T35.12-44793-18 Dow Chemical Co. (unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco(R) 179 in
rats. Report, NBT35.12-44793-21 Dow Chemical Co. (unpublished)
McCray, C.W. (1969) Field trials using Dursban. Letter report Animal
Res. Inst., Queensland.
McEwen, F.L. (1970) Tests with Dursban on onions, carrots and lettuce.
University of Guelph. Personal communication to T. Haagsma.
McEwen, F.L. and Frank, R. (1970) Tests with Dursban on onions,
carrots and lettuce. University of Guelph. Personal communication to
T. Haagsma.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-pyridinol in milk and cream by gas chromatography.
Method ACR 71.2. Dow Chemical Co.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-pyridinol in corn grain, forage and stover by gas
chromatography. Method ACR 71.19. Dow Chemical Co.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-pyridinol in grass by gas chromatography. Method ACR
71.5. Dow Chemical Co.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography. Method ACR 70.19. Dow Chemical Co.
Molello, J.A. and Sharp, L.D. (1968) Three week study on
cataractogenicity of 3,5,6-trichloro-2-pyridinol as part of the
dietary intake of Pekin ducklings. Report HH-134 Dow Chemical Co.
(unpublished)
Myers, C.M., Lewallen, L.L. and Nobe, B. (1968) Residue studies of
fenthion (Baytex(R)) and Dursban(R) in central California pastures.
California Vector Views, 15(5): 51-54.
Norris, J.M. (1970) Potentiation Study on Dowco(R) 179 and Vapona(R)
insecticide. Report Dow Chemical Co. (unpublished)
Olthof, P.D.A. (1971a) Residuen van Dursban in champignons. Report R
3563. Centraal Instituut voor Voedingsonderzoek, The Netherlands.
Olthof, P.D.A. (1971b) Residues of Dursban in carrots. Report R 3633.
English translation from report by Centraal Instituut voor
Voedingsonderzoek, The Netherlands.
Rice, J.R. and Dishburger, H.J. (1968) Determination of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate in
water and silt by gas chromatography. J. Agr. Fd. Chem., 16(5):
867-869.
Scheuerer, W. and Hahn, I. (1972) Dursban residues on vegetables.
Badische Anilin and Soda-Fabrik AG, West Germany. Communication to Dow
Chemical (Nederland) N.V.
Schlinke, J.C. (1970) Chronic toxicity of Dursban in chickens, 1969.
J. Econ. Entomol., 63(1): 319.
Schlinke, J.C., Palmer, J.S. and Hunt, L. (1969) Preliminary
toxicological study of a phosphorothioate compound in turkeys. Am. J.
Vet. Res., 30(9): 1705-1709.
Sherman, M., Herrick, R.B., Ross, E. and Chang, M.T.Y. (1967) Further
studies on the acute and subacute toxicity of insecticides to chicks.
Tox. Appl. Pharmacol., 11: 49-67.
SIAPA. (1971) Residue determinations on lettuce after treatment with
Dursban insecticide. Centro Esperienze e Ricerche, Italy.
Smith, G.N. (1966) Basic studies of Dursban insecticide. Down to
Earth, 22(2): 3-7.
Smith, G.N. (1968) Ultraviolet light decomposition studies with
Dursban and 3,5,6-trichloro-2-pyridinol. J. Econ. Entomol., 61(3):
793-799.
Smith, G.N. and Taylor, Y.S. (1970) An analytical method for the
determination of 3,5,6-trichloro-2-pyridinol in animal tissues and the
metabolism of the pyridinol in rats. Report OL 3132 Dow Chemical
(unpublished)
Smith, G.N., Watson, B.S. and Fischer, F.S. (1966) The metabolism of
(14C) O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) in fish. J. Econ. Entomol., 59(6):
1464-1475.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967a) Investigations on
Dursban insecticide. Uptake and translocation of (36Cl)
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate by
beans and corn. J. Agr. Fd. Chem., 15(1): 127-131.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967b) Investigations on
Dursban insecticide. Metabolism of (36Cl) O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in rats. J. Agr. Fd.
Chem., 15(1): 132-138.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967c) Investigations on
Dursban insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agr. Fd. Chem., 15(5):
870-877.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Report OL-3132 Dow
Chemical Co. (unpublished)
Snelson, J.T. (1969a) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Snelson, J.T. (1969b) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Stevenson, G.T. (1965) A gamebird toxicology study - acute dietary
feeding of Dursban to wild type mallard ducklings. Report GH-A-122 Dow
Chemical Co. (unpublished)
Stevenson, G.T. (1966) A neurotoxicity study of Dursban in laying
hens. Report GH-A-195 Dow Chemical Co. (unpublished)
Stevenson, G.T. and Daering, K.L. (1969) The effects of Dursban
insecticide (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) on blood plasma cholinesterase in chickens. Report
GH-A-410 Dow Chemical Co. (unpublished)
Taylor, M.L. and Olson, K.J. (1963) Toxicological properties of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report
T35.12-44793-4 Dow Chemical Co. (unpublished)
Thompson, D.J., Gerbig, C.G. and Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report HH-382 Dow
Chemical Co. (unpublished)
Tucker, K.E.B. (1971) Dursban/Dowco 214 residues in rice and straw.
Report 160 Dow Chemical (Australia) Ltd.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of the Interior, Fish and Wildlife
Service Resource Publication #84, p. 56-57.
Tucker, K.E.B. and Zielinski, W. (1972a) Chlorpyrifos and Dowco 214
residues in Chinese cabbage. Report 175 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972b) Chlorpyrifos and Dowco 214
residues in Chinese kale. Report 174 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972c) Residues of chlorpyrifos in
Chinese kale. Report 179 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972d) Residues of chlorpyrifos and
Dowco 214 in tobacco. Report 180 Dow Chemical (Australia) Ltd.
USAEHA. (1968) Report U.S. Army Environmental Hygiene Agency.
Watts, R.M. (1968) Residues of chlorpyrifos in cattle. Dept. of
Agriculture, Australia. Communication to M.K. Astill.
Wetters, J.H. (1971a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate and O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphate in soils by gas
chromatography with flame photometric detection. Method ACR 71.3. Dow
Chemical Co.
Wetters, J.H. (1971b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in soils by gas
chromatography using flame photometric detection and direct injection.
Method ACR 71.20. Dow Chemical Co.
Wetters, J.H. (1972a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in pig tissues by gas
chromatography with flame photometric detection. Method 72.1. Dow
Chemical Co.
Wetters, J.H. (1972b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in chicken tissues
and eggs by gas chromatography with flame photometric detection.
Method 72.3. Dow Chemical Co.
Wetters, J.H. (1972c) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sweet corn by gas
chromatography with flame photometric detection. Method ACR 72.9. Dow
Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971a) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
and O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphate in
peaches by gas chromatography with flame photometric detection. Method
ACR 71.14. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971b) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
corn by gas chromatography with flame photometric detection. Method
ACR 71.18. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971c) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
water by gas chromatography with flame photometric detection. Method
ACR 71.21. Dow Chemical Co.
Winterlin. W., Moilanen, K. and Burgoyne, W.E. (1968) Residues of
Dursban insecticide following mosquito control applications. Down to
Earth, 24(2): 34-37.
Wit, S.L. (1970) Residues of Dursban in cabbage. Report 49/70 Tox RoB.
English translation from report by State Institute for the Public
Health, The Netherlands.
Wit, S.L. (1971) Residues of O,O-diethyl
O-(3,5,6-trichloro-pyridine-(2)-yl) phosphorothioate (Dursban) for
the control of cutworms in lettuce under glass. Report 63/71 Tox-RoB.
English translation from report by Rijks Instituut voor de
Volksgezondheid, The Netherlands.
 Mol. Wt. 350.6
Empirical Formula C9H11Cl3NO3PS
Other relevant chemical and physical properties
State: white, granular, crystalline solid
Melting point: 42 - 43.5°C
Vapour pressure: 1.87 × 10-5mm Hg (at 25°C)
8.87 × 10-5mm Hg (at 35°C)
Solubility: readily soluble in acetone, benzene,
chloroform, methanol and iso-octane; slightly
soluble (0.4 ppm) in water.
When chlorpyrifos was exposed to UV light or to sunlight, it underwent
hydrolysis in the presence of water to liberate
3,5,6-trichloro-2-pyridinol, which underwent further decomposition to
diols and triols and ultimately cleavage of the ring to fragmentary
products (Smith, 1968). Hydrolysis in water occurs least readily at
about pH 6 and very readily above pH 8.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrates, dusts, wettable powders and
granules).
Purity: 99.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
As with other phosphorothioate esters chlorpyrifos is rapidly
absorbed, metabolized and excreted by mammals following oral
administration. When 36Cl chlorpyrifos was fed by intubation to rats
as a single sublethal dose (50 mg/kg), the radioactivity was
eliminated rapidly (90.1% in 26 h) via the urine (90%) and faeces
(10%). Products excreted were identified as 36Cl 3,
5,6-trichloro-2-pyridyl phosphate (75-80%),
3,5,6-trichloro-2-pyridinol (15-20%) and traces of chlorpyrifos. The
pyridyl phosphate was indicated to be a transient intermediary in the
hydrolysis of chlorpyrifos to the pyridinol moiety. Analyses showed
no appreciable residue in any tissue except fat, which showed
temporary retention of radioactivity (halflife of ca 62 h) identified
principally as chlorpyrifos (Smith et al., 1967b). This study
suggests that desethylation is a significant metabolic pathway in
rats. An additional study in the rat involving administration of 14C
chlorpyrifos by single dose intubation (19.1 mg/kg) essentially
verified the earlier result (Branson and Litchfield, 1971a,b). In this
study, as well as in an in vitro study involving breakdown of
chlorpyrifos by rat liver microsomes (Branson and Wass, 1970),
3,5,6-trichloro-2-pyridinol was identified as the only major
metabolite. A study in cows involving feeding of chlorpyrifos in the
diet at 5 ppm for four days indicated that the major pathway for
degradation was by hydrolysis to the pyridinol, as no desethyl
phosphate esters were found. In addition no chlorpyrifos or
metabolites were found in milk (Gutenmann et al., 1968).
Studies of 3,5,6-trichloro-2-pyridinol, the major enolic metabolite of
chlorpyrifos, have shown absorption, distribution and excretion in the
rat following single oral administration to be similar to that
reported for the parent compound. The metabolite is rapidly absorbed
and excreted primarily in the urine and faeces. Small tissue residues
(<0.3 ppm) were present and occurred mainly in the biological systems
involved with urinary excretion, i.e. liver, kidney and blood. Unlike
chlorpyrifos, residues of the pyridinol in fat were trivial, which is
consistent with its much greater polarity (Branson and Litchfield,
1971a,b; Smith et al., 1970).
Biotransformation
The metabolic fate of chlorpyrifos has been investigated in plants
(Smith et al., 1967a, 1967b) and data indicate an oxidative and/or
hydrolytic breakdown yielding des-mono-ethylchloropyrifos; desethyl
chlorpyrifos; 3,5,6-trichloro-2-pyridinol and further degradation
products. This is discussed further in the section on "Fate of
residues."
Effects on enzymes and other biochemical parameters
Chlorpyrifos is a cholinesterase inhibitor affecting this enzyme in
brain or blood, although differing in its response with different
animal species. In vitro studies have shown the oxygen analogue of
chlorpyrifos to be 106 times more active as an inhibitor of
cholinesterase than chlorpyrifos (I50 values: chlorpyrifos approx.
2.5 x 10-3M; oxygen analogue approx. 2.5 x 10-9M) (Smith, 1966).
Equimolar concentrations of chlorpyrifos administered to dogs and
monkeys affected RBC cholinesterase in monkeys while having almost no
effect on RBC cholinesterase in dogs. Plasma cholinesterase is the
most sensitive indicator of exposure to chlorpyrifos. In dogs and
monkeys administered subacute levels of chlorpyrifos, plasma
cholinesterase was maximally inhibited within 8 hours following
exposure. Recovery in dogs was complete within 24 hours while with
monkey, plasma cholinesterase in monkeys remained depressed at 48
hours (Coulston et al., 1971).
TOXICOLOGICAL STUDIES
Acute toxicity of the metabolite 3,5,6-trichloro-pyridinol has been
studied in rats and mice. Results are summarized in Table 1. Signs of
poisoning with this metabolite include facial paralysis with dyspnoea
and slight hypersalivation and exophthalmia (mice only) (Gerbig and
Emerson, 1970 a,b).
Special short-term studies on metabolites
Rat
Groups of rats (10 males and 10 females/group) were fed the metabolite
of chlorpyrifos, 3,5,6-trichloro-2-pyridinol, in the diet for 90 days
at dosage levels of 0, 100, 300, 1 000, 3 000 and 10 000 ppm. Diuresis
was observed at 3 000 ppm. At 10 000 ppm a reduction in body-weight
was observed which reflected a general retardation in growth in both
sexes. No effects were observed on mortality, terminal haematology or
gross and histological examination of tissues (Beatty and McCollister,
1964).
Dog
Groups of dogs (3 males and 3 females/group, 4 of each sex were
controls) were fed the metabolite of chlorpyrifos,
3,5,6-trichloro-2-pyridinol, in the diet for 91 days at dosage levels
of 0, 1, 3, 10 and 30 mg/kg/day (Emerson and Gerbig, 1970). Another
study utilized groups of dogs (2 males and 2 females at the higher
level and 4 male and 4 female at the lower level) which were fed the
metabolite at dietary levels of 26 and 80 mg/kg/day for 93 days
(Copeland, 1964). At 80 mg/kg the metabolite produced abnormalities
including growth depression in females, increased spleen/body-weight
ratios and histological changes in the liver. At 26 mg/kg an elevated
SAP accompanied by equivocal changes in the liver was observed. At 30
mg/kg increased SAP, SGOT and SGPT values were observed in the second
study. The increased biochemical parameters were noted in 1 of 3 dogs
examined at 10 mg/kg. Hepatic lesions noted in 1 dog at 10 mg/kg and
1 dog at 30 mg/kg did not appear to be chemically induced. No effects
were noted at 3 mg/kg/day on growth, behaviour, haematology, blood
biochemistry, urinalysis or gross and microscopic examination of
tissues.
TABLE 1 Acute toxicity of the metabolite 3,5,6-trichloropyridinol
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 794 Gerbig and Emerson, 1970a
F 870
Mouse M Oral 380 Gerbig and Emerson, 1970b
F 415
Duckling
Groups of Peking ducklings (10 per group, 20 used as controls) were
fed the metabolite at dosage levels of 0, 1, 3, 10, 30, 60, 100, 300
and 1 000 ppm for 3 weeks to determine cataractogenicity. Growth
retardation was evident at 1 000 ppm, and 3 of 10 ducklings had
elevated prothrombin values. Levels of 300 ppm and below produced no
effect on these parameters and no cataractous changes were observed at
any level (Molello and Sharp, 1968).
Special studies in neurotoxicity
Hens (3 DeKalb leghorns/group) were administered chlorpyrifos orally
at dosage levels of 40, 75, 100 and 150 mg/kg and observed for 27
days. Mortality occurred at the higher levels. Surviving birds showed
no signs of delayed ataxia or paralysis. Crufomate administered as a
positive control (1 000 mg/kg) produced clinical signs of paralysis
(Stevenson, 1966).
Special studies on potentiation
Rats administered subacute oral dosages (1/2 the LD50 value) of
chlorpyrifos in combination with malathion or dichlorvos showed slight
evidence for potentiation of the acute toxicity. A three-fold increase
in the acute toxicity was noted with malathion, but this was judged to
be of no practical significance (Dow Chemical Co., 1964; Norris,
1970).
Special studies on reproduction and teratogenicity
Groups of rats (10 males and 20 females/group with 20 males and 10
females/group as controls) were fed chlorpyrifos in the diet for one
generation (two litters) at dosage levels of 0, 0.03, 0.1 and 0.3
mg/kg and for two generations (two litters per generation) at dosage
levels of 0, 0.1, 0.3 and 1.0 mg/kg. The F3b litter was used in a
teratology study where the parents were fed dietary levels of
chlorpyrifos until day 6 - 15 of organogenesis when the dietary
supplement was removed and chlorpyrifos administered by gavage once
daily. After day 15 chlorpyrifos was again administered by dietary
supplement until day 20 when the females were sacrificed, foetuses
removed by caesarean section and viscera and skeletal structure
examined. Neonatal mortality was somewhat higher in F2a, F2b and F3a
litters at 1.0 mg/kg. No other significant effects were observed on
fertility, reproduction or lactation indices or on teratological
examination of F3b pups as a result of feeding chlorpyrifos at levels
up to 1.0 mg/kg/day. Cholinesterase depression was observed at levels
of 0.3 mg/kg and above, primarily in plasma (Thompson et al., 1971).
Acute toxicity
The acute toxicity of chlorpyrifos has been studied in several animal
species, and the results are summarized in Table 2.
TABLE 2 Acute toxicity of chlorpyrifos in different animal species
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 118 - 245 Taylor and Olson, 1963;
Gaines, 1969; Tucker and
Crabtree, 1970
F Oral 82 - 135 Taylor and Olson, 1963;
Gaines, 1969
Mouse M Oral 102 Coulston et al., 1971
- i.p. 31 USAEHA, 1968
White
footed - Oral 64 Kenaga, 1967
Mouse
Rabbit - Oral 1 000 - 2 000 Taylor and Olson, 1963
Cavy M Oral 504 Ibid.
Goat F Oral 500 - 1 000 Tucker and Crabtree, 1970
Chukar F Oral 61.1 Ibid.
M Oral 60.7 Ibid.
Bullfrog M Oral >400 Ibid.
Chick M Oral 25 - 35 Taylor and Olson, 1963;
Stevenson, 1966; Sherman
et al., 1967; Brust
Chicken - Oral 32 - 63 et al., 1971
The LD50 in a number of avian species has been determined by Tucker
and Crabtree (1970). The toxicity values range from 8 mg/kg in male
ring necked pheasant to 167 mg/kg in mallard ducklings.
Signs of poisoning with chlorpyrifos are typical of cholinesterase
inhibiting compounds. Mortality following acute oral dosing may be
delayed for as long as nine days in some species of animals (Tucker
and Crabtree, 1970).
Chlorpyrifos administered to the conjunctival sac of rabbits produced
transient redness of the conjunctiva. No corneal injury was observed
(Taylor and Olson, 1963). When applied to intact or abraded abdominal
skin of rabbits daily for up to 2 weeks no significant dermal damage
was observed. Prolonged contact produced burning and hardening of the
skin, slight swelling and mild hyperemia which returned to normal
within three weeks. Chlorpyrifos administered dermally as a technical
product to rabbits in an undiluted form produced no untoward
physiological effects. When administered as a 50% solution in
dipropylene glycol methyl ether, the same dose (2 000 mg/kg) was
rapidly absorbed and resulted in death in four out of six animals when
applied to either intact or abraded skin (Taylor and Olson, 1963).
Chlorpyrifos did not induce sensitization when examined on the guinea
pig (USAEHA, 1968).
Dogs and sheep were exposed for a 4-hour period to a thermal fog or
liquid aerosol at a concentration of 4 or 8 mg/cu ft. Mild plasma
cholinesterase depression in dogs at the higher dose was the only
effect noted (Dow Chemical Co., 1966a).
Five female rats were exposed for 7 hours/day to an atmosphere of
chlorpyrifos (calculated at 7 mg/l air) for 16 exposures over a 21-day
interval. There were no effects on growth, appearance, behaviour or
plasma and RBC cholinesterase activity (Dow Chemical Co., 1966b).
Short-term studies
Mallard duck
Groups of mallard ducklings (6 - 8 birds/group, 5 days old) were fed
chlorpyrifos for five days at dosage levels of 0, 100, 200, 300, 500,
700, 1 000 and 2 000 ppm in the diet. Mortality occurred at all dosage
levels (100-2/8; 200-4/7; 300-5/8; 500-6/8; 700 - 2000 - all dead)
except in controls (0/7 dead) (Stevenson, 1965).
Chickens
Groups of chicks (20 chicks/group) were fed chlorpyrifos in the diet
for two weeks at dosages of 0, 200, 400 and 800 ppm. Mortality was
observed at 400 ppm and plasma cholinesterase depression and reduced
weight gain was observed at all levels (Sherman et al., 1967).
Groups of chickens (30 per group) were administered chlorpyrifos in
the drinking water at dosage levels of 0, 1 ppb, 1 ppm and 100 ppm for
84 days. A higher level (1000 ppm) resulted in clinical signs of
cholinergic stimulation. Plasma cholinesterase was depressed at 100
ppm but not at 1 ppm. A 10 ppm group was tested for two weeks with no
signs of cholinesterase depression. Growth and behaviour were not
affected at 100 ppm (Stevenson and Daering, 1969). Chlorpyrifos added
to the drinking water of chicks at dosages of 0, 0.08, 0.32, 1.25, 5,
20, 80, 320 and 1 280 ppm for periods varying from 3 - 5 weeks induced
mortality and reduced growth at levels of 80 ppm and above. Whole
blood cholinesterase depression was evident only at 80 ppm and above
(Brust et al., 1971).
Chickens fed chlorpyrifos in the diet at levels of 0, 25, 50 and 100
ppm for four weeks exhibited reduced plasma cholinesterase activity
and reduced weight gain at all feeding levels. Calculations of food
conversion data show a reduced yield in production as a result of 25
ppm in the diet. No clinical signs of toxicity were observed
(Schlinke, 1970).
Rat
Groups of rats (20 males and 20 females/group) were fed chlorpyrifos
in the diet for six months at dosage levels of 0, 0.03, 0.15 and 0.75
mg/kg/day. Cholinesterase depression was noted at 0.75 mg/kg/day in
RBC and plasma. Brain cholinesterase activity was unaffected. No
effects were noted at any feeding level on mortality, behaviour,
growth, haematology or gross or histological examination of tissue
(Coulston et al., 1971).
Groups of rats (10 males and 10 females/group) were fed chlorpyrifos
in the diet for 90 days at levels of 0,10, 30, 100 and 300 ppm. Growth
reduction was observed in both males and females at 300 ppm. Increased
liver and kidney organ to body-weight ratios were also observed
(possibly as a result of reduced body-weight as organ weights were
comparable to the controls). Cholinesterase depression was observed at
30 ppm in blood and brain; at 10 ppm, brain cholinesterase was normal
although enzyme inhibition was observed in RBC and plasm. No adverse
effects were noted on growth, appearance, food consumption, mortality,
behaviour, haematology or gross and microscopic pathology at 100 ppm
(Beatty and McCollister, 1964).
Five groups of rats (10 males and 10 females/group) were fed
chlorpyrifos at levels varying from 0.03 - 10.0 mg/kg/day for 28 - 90
days to determine a "no-effect" level on cholinesterase depression.
Dosage levels of 0 and 0.3 mg/kg/day were fed for 90 days while
dosages of 1, 3 and 10 mg/kg/day were fed for 28 days, stopped for
three weeks (during which time the rats were fed control diets) and
the rats refed diets containing 0, 0.03 and 0.1 mg/kg/day for a full
90-day study. Behaviour and growth were affected at 3 and 10
mg/kg/day. Cholinesterase was depressed at 0.3 mg/kg/day and above in
both male and female rats. The rats in the three highest groups were
removed for three weeks during which time cholinesterase activity
returned to pretest intervals. On resumption of feeding chlorpyrifos
at 0.1 mg/kg/day, slight cholinesterase depression was observed in
plasma and erythrocytes. No cholinesterase depression was noted on
brain or blood enzymes at 0.03 mg/kg/day (Blackmore, 1968b).
Dog
Groups of dogs (2 males and 2 females/group; 4 of each sex were
controls) were fed chlorpyrifos for 93 days. The dosage levels of
feeding were initially 200, 600 and 2 000 ppm in the diet, but because
of the appearance of signs of cholinergic poisoning the dosages were
changed from 2 000 to 60 ppm after 5 days while the 200 ppm (5.8
mg/kg/day) level was maintained for 45 days. The dogs were then placed
on control diets for the remainder of the test. Another group of 2
male and 2 female dogs was added to the study at a dietary level of
200 ppm (3.4 mg/kg/day) for 27 days, removed from the test for 5 days
and returned to this dietary level for the final 2 weeks. A decrease
in food consumption at the 200 ppm level was accompanied by reduced
growth, especially in females. Growth of all dogs was slightly lower
than control levels. Increased SGPT values were seen in several dogs
at all feeding levels. RBC, plasma and brain cholinesterase values
were reduced in all animals fed chlorpyrifos in the diet at a level of
20 ppm (0.8 mg/kg/day) and above. No adverse effects were observed on
haematology, BUN, SAP, organ weights and gross or microscopic
examination of tissues (Copeland, 1964).
Groups of dogs were orally administered chlorpyrifos daily in a
gelatin capsule for periods of 32 to 90 days at doses of 0 (2 males
and 2 females - 90 days), 0.01 mg/kg/day (2 males and 2 females - 32
days), 0.03, 0.10, 0.30 and 1.00 mg/kg/day (2 males and 2
females/group - 90 days), and a final group (2 males and 2 females)
was given 1.00 mg/kg/day from day 0 to 18, control diet from days 19
to 42, 0.03 mg/kg/day from days 43 to 58, control diet from days 59 to
77, 0.01 mg/kg/day from days 78 to 94 and control diet until day 124.
Data obtained on appearance, body-weight and food consumption were
normal. A dose-dependent cholinesterase depression in plasma was
observed to begin at 0.03 mg/kg/day. At this level inhibition was
moderate and was more marked as the dietary level increased. Brain
cholinesterase was not affected at any level, and RBC cholinesterase
was affected at levels of 0.1 mg/kg/day and above. No effects were
noted at 0.01 mg/kg/day on any parameters tested over the 32-day
administration interval (Blackmore, 1968a).
Groups of dogs (7 males and 7 females/group) were fed chlorpyrifos in
the diet for up to two years at dose levels of 0, 0.01, 0.03, 0.1, 1.0
and 3.0 mg/kg/day. Inhibition of cholinesterase activity was the only
abnormality detected. Inhibition of RBC cholinesterase in males and
females was evident at 1.0 and 3.0 mg/kg. Marginal depression of brain
cholinesterase was shown at the highest level of feeding. Plasma
cholinesterase was depressed at 0.1 mg/kg/day in both males and
females. Occasional reduction at 0.03 mg/kg was noted. Studies on the
recovery of cholinesterase activity following one year feeding
indicate that plasma activity recovered to normal within two weeks
while RBC recovery was much slower. No significant effects were noted
on mortality, behaviour, food consumption, growth, haematology, blood
biochemistry, urinalysis, or gross and microscopic examination of
tissues. Gross examination showed an increased liver weight and ratio
at 3.0 mg/kg at two years but not at one year. All other tissues were
normal (McCollister et al., 1971a).
Monkey
Groups of Rhesus monkeys (2 males and 2 females - control; 2 males and
1 female - 0.08 or 0.40 mg/kg/day; 2 males and 2 females - 2.0
mg/kg/day) were administered chlorpyrifos orally by gavage daily for
six months. Plasma and RBC cholinesterase activity was depressed in
those animals that received 2.0 and 0.4 mg chlorpyrifos/kg/day. Plasma
cholinesterase activity was slightly depressed and RBC cholinesterase
activity was not depressed in animals that received 0.08 mg/kg/day.
Brain cholinesterase activity was not affected at any level. No
significant effects were noted on behaviour, growth, haematology,
clinical chemistry or gross or histological examination of tissues.
The histological examination included electron microscopy of liver and
kidneys and histochemical delineation of lysosomal distribution in
these two organs (Coulston et al., 1971).
Long-term studies
Rats
Groups of rats (57 males and 57 females/group) were fed dietary levels
of chlorpyrifos of 0, 0.01, 0.03, 0.1, 1.0 and 3.0 mg/kg/day for two
years. Blood cholinesterase activity, primarily in plasma, was
depressed in females at 0.1 mg/kg and above. Brain cholinesterase was
inhibited at 3.0 mg/kg and slightly depressed at 1.0 mg/kg.
Chlorpyrifos at all dosage levels had no significant effect on
behaviour, appearance, growth, mortality, haematology, urinalysis,
clinical biochemistry, gross or histopathology of tissues and organs
or the incidence of neoplasms (McCollister et al., 1971b).
OBSERVATIONS IN MAN
Groups of men (4 men/group) were administered chlorpyrifos by tablet
daily for 27 days (0.014 mg/kg/day), 20 days (0.03 mg/kg/day), 9 days
(0.10 mg/kg/day) and 48 days (control). No effects were noted on
behaviour, haematology, urinalysis or biochemical determinations in
blood. Blood plasma cholinesterase was depressed at the highest dosage
and this regimen was discontinued. Recovery was complete in four
weeks. At 0.03 mg/kg/day a statistically non-significant, although
suggestive, depression of plasma cholinesterase was noted. No effects
were noted at 0.014 mg/kg on plasma cholinesterase. RBC activity was
unaffected at any level of chlorpyrifos. The level of 0.03 mg/kg/day
appears to be the minimal threshold response level in humans to
chlorpyrifos based on plasma cholinesterase (Coulston et al., 1972).
COMMENT
The compound is rapidly absorbed and metabolized by mammals, the major
metabolite being a chlorinated pyridonal hydrolysis product of low
mammalian toxicity.
There is no evidence of neurotoxicity or cataractogenicity.
Potentiation has been demonstrated only with malathion.
Reproduction studies and examination of offspring for teratogenic
abnormalities did not reveal adverse effects other than an increase in
neonatal mortality at 1 mg/kg/day. Chlorpyrifos is an active
cholinesterase inhibitor, inhibiting plasma cholinesterase to a far
greater degree than other cholinesterases. One short-term study in
rats indicated an increase in sensitivity to plasma cholinesterase
depression following withdrawal after initial treatment. This study
needs confirmation.
No-effect levels have been demonstrated in dog, rat and man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.03 mg/kg/day
Dog: 0.01 mg/kg/day
Man: 0.014 mg/kg/day orally for 1 month
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In animals
Chlorpyrifos is used to control various species of fever ticks
(Boophilus sp.), ear ticks, lice and horn flies on beef cattle and
non-lactating dairy cattle, by use of emulsifiable liquid formulations
in water varying from 0.025 to 0.125% applied as a spray or dip.
Treatment for all but ear ticks is limited to six applications at
21-day intervals, and not within two weeks of slaughter.
Sheep dipped or sprayed with wettable powder or emulsifiable
formulations of chlorpyrifos are protected from blowfly, ticks, body
lice and sheep keds. A minimum interval of seven days is required
between treatment and slaughter.
Turkey chiggers are controlled through the use of wettable powder
formulations sprayed on soil in turkey pens at the rate of 4 lb/acre
chlorpyrifos, before the turkeys are introduced into the pens.
Treatment is limited to two applications, not within seven days of
slaughter.
In plants
Soil treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control soil pests such as wireworms,
white grubs, mole crickets, Maulwurf's Grill, garden symphylans,
seed corn maggots, cereal maggots, beet root weevils, the
Atomaria, Blaniulus, Tipula and cutworms. The chemical is
applied by means of broadcast row or furrow treatments and is
varied in concentrations from 300 to 4 000 g/ha a.i. as wettable
powder, emulsifiable or granular formulations before and after
planting, previous to harvest. The crops involved are maize,
spring cereals, beans, onions, tobacco, turnips, bulb flowers and
carrots.
2. Canada
Chlorpyrifos is used to control soil insect pests of lettuce,
onions and carrots before and after planting at dosages of 1 to 2
lb/acre applied as needed, but with the indicated 28- to 60-day
withdrawal period before harvest. Tobacco plants are treated in
soil water, preplant and by soil and foliar application.
Foliar treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control insect pests of rape, cereals,
potatoes, fodder crops, beans, peas and deciduous fruits such as
apples and pears.
2. Japan, Thailand, Philippines, India and Malaysia
Chlorpyrifos is used to control insect pests of apples, pears,
rice, tobacco, Chinese cabbage and kale.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
Considerable work has been done in U.S.A. on the analysis of cattle
tissues for residues of chlorpyrifos as well as its oxygen analogue
and pyridinol moiety. Typical distribution of residues in cattle
dipped in chlorpyrifos is shown in Table 3 (Claborn et al., 1970a).
In this study, 57 beef cattle were dipped one to six times in a 0.025%
emulsion of chlorpyrifos at intervals of 21 days. Animals were removed
from the dipping schedule after each dip, except the fifth, for
post-treatment slaughter at various time intervals. Fat muscle, kidney
and liver tissue samples were collected and analysed for residues of
chlorpyrifos, its oxygen analogue, and the 3,5,6-trichloro-2-pyridinol
moiety. Residues of chlorpyrifos were predominately in the fatty
tissues, and residues of 3,5,6-trichloro-2-pyridinol were in the
kidney and liver tissues. No residue of the oxygen analogue was
detected in any tissue.
Residues of the hydrolytic pyridinol were not detectable in muscle
tissue or in kidney and liver tissues of cattle, except at low levels
for the first 1 - 2 weeks after the last dip, even after six dippings.
It should be noted that this experiment began in February, so the
animals had their winter coat of hair through the first three dippings
and were shedding it at approximately the fourth dipping period. The
heavy winter hair coat carries more insecticide out of the vats than
the summer coat, as evidenced by the higher residues after the second
and third dippings and the drop in residues after the fourth dipping.
The effect of the winter coat is also reflected by the higher residues
after the first dip when compared to levels of residues after a single
dip (in the same concentration or higher concentration - 0.05% - of
chlorpyrifos) in the animals reported by Mann, 1966; Claborn et al.,
1968a,b; and Dishburger et al., 1966a,b.
Since the season for ticks extends from approximately April or May to
mid-September, animals would be expected to shed their winter coat
after the first or second dipping when treated according to label
instructions. Therefore, in practice residue levels as high as those
found in the test animals would not be expected after the third
dipping, since these animals would have been dipped three times before
shedding their winter coat of hair. In any case, it is believed that
this experiment represents the highest levels of chlorpyrifos that
would ever be encountered in the use of 0.025% chlorpyrifos as a dip.
In an early test, cattle were sprayed once with a 0.5% emulsion of
chlorpyrifos (20 times the label recommendation). Residues of the
compound in the fat were 1.3 ppm (maximum) at 20 days post-treatment
and had declined to 0.08 ppm (maximum) at 30 days post-treatment
(Dishburger and Rice, 1965).
In cattle treated with an emulsion containing 0.05% chlorpyrifos
(twice the label recommendation), the average amounts of residue in
the fat one week after a single-dip application were about twice those
resulting from a single-spray application. The maximum residues were
well below 1 ppm (0.209 for spray and 0.525 for dip application) at
the first sampling period at seven days post-treatment. Approximately
two weeks were required for residues to be reduced to 1 ppm in the fat
of animals given multiple treatments (3 dippings at 2-week intervals)
at this level (Mann, 1966; Claborn et al., 1968a,b).
TABLE 3 Chlorpyrifos residues in cattle in the United States1
Mean residues of
Weeks Mean residues of chlorpyrifos2 3,5,6-trichloro-2-pyridinol3
after last fat muscle kidney kidney liver
dipping (ppm) (ppm)
1 dipping
1 0.672 0.004 0.003 0.12 0.12
2 0.142 0.002 0 <0.05 <0.05
3 0.083 0.001 0 0 0
4 0.038 0 0 0 0
2 dippings4
1 1.245 0.011 0.008 0.30 0.26
3 dippings4
1 1.589 0.028 0.008 0.24 0.29
2 0.085 0.011 0.001 0.05 0.05
4 0.158 0 0 0 0
6 0.038 0 0 0 0
8 0.041 0 0 0 0
10 0.001 0 0 0 0
4 dippings4
1 0.693 0.011 0.001 0.10 0.12
6 dippings4
1 0.744 0.015 0.003 0.12 0.10
2 0.141 0.001 0 0 0
4 0.069 0 0 0 0
6 0.018 0 0 0 0
8 0.007 0 0 0 0
10 0 0 0 0 0
1 Residues of chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues
of beef cattle dipped in an emulsion containing 0.025% chlorpyrifos.
Residue levels corrected for % recovery from tissues.
2 No chlorpyrifos found in liver (0 - <0.002 ppm) or oxygen
analogue (0 - <0.005 ppm) in any tissue analysed (Claborn, 1970).
3 No 3,5,6-trichloro-2-pyridinol found in fat or muscle tissues
(0 - <0.05 ppm) (Dishburger and McKellar, 1971).
4 Dipping schedule was at 21 day intervals.
The oxygen analogue was found only in samples of fat containing the
higher residues of chlorpyrifos (approximately 2 ppm); it amounted to
about 1% of the residue. No increase occurred in the amount of the
oxygen analogue as the chlorpyrifos was eliminated, and no residues of
the analogue were detected in the muscle, brain or spleen tissues of
the animals that had the highest residues of chlorpyrifos in the fat
(Claborn et al., 1968a).
Data from Mann (1966), Claborn et al. (1968a) and Dishburger
et al. (1966a,b) indicated that the biological half-life of
chlorpyrifos in tissues of cattle following a single treatment with
DURSBAN insecticide containing 0.025% and 0.05% chlorpyrifos is
approximately 4 - 6 days. Data from Mann (1966) and Claborn et al.
(1968a,b) showing multiple dippings in DURSBAN insecticide containing
0.025% and 0.05% chlorpyrifos, further establish the consistency of
the degradation ratio following the last treatment. The data of
Claborn et al. (1968b) indicate that the biological half-life of
chlorpyrifos in tissues of cattle treated at three-week intervals for
as long as six weeks with DURSBAN 24E containing 0.025% chlorpyrifos
is approximately seven days. In summary, the experiments illustrate
the consistency of a 4 - 7 day biological half-life for chlorpyrifos
in fat.
Considerable work has been carried out in Australia on the
determination of residues from the vat dip and spray application of
chlorpyrifos to cattle (Hall, 1969; Snelson, 1969b). Results shown in
table 4 indicate that residues of chlorpyrifos in omental fat are in
the same order of magnitude as those for United States, considering
the fact that most tests were at double the concentration (0.05%) in
Australia.
Snelson (1969a) studied the disappearance rate of chlorpyrifos in
butterfat in milk after a single dip application of chlorpyrifos at
0.025% to milk cows. Residue declined from 1.1 ppm after 1 day to 0.01
ppm in 9 days, and a non-detectable level at 15 days (see Table 5).
Sheep
Sheep dipped in an 0.05% chlorpyrifos emulsion showed inconsistent
levels of residues of chlorpyrifos with no apparent change in residue
levels (0.081 - 0.179 ppm) from 3 - 28 days after application. No
detectable levels (<0.005 ppm) were seen in muscle, liver or kidney
tissues at any time (Dow Chemical Co., 1967).
TABLE 4 Chlorpyrifos residues in cattle in Australia
Chlorpyrifos
Days residues in
Chlorpyrifos Application between last No. of omental fat Reference
% type no. treatment and cattle (mean values
slaughter or range, ppm)
0.025 Vat dip 3 1 0.2 Watts, 1968
4 0.96
7 0.25
1 1
3 0.11 - 0.22 Ibid.
1 1,4,8,15 0.29 - 2.18 Ibid.
3,6 1,4,8,15 0.05 - 1.19 Ibid.
0.05 Vat dip 1 1,4,8 0.41 - 0.48 Ibid.
3 1 1.17 Ibid.
4 0.98
6 1 1.75 Ibid.
8 1.36
15 1.11
22 0.6
29 0.38
0.05 Vat dip 2 0 3 1.46 - 2.26 Hall, 1969
1 2 3 2.4 - 3.87
1 2 3 1.23 - 3.09
1 2 3 0.98 - 2.54
1 1 3 0.42 - 0.79
3 1 4 1.32 - 3.80
0.05 Dip 10 5? 12 0.70 - 7.131 Snelson, 1969b
0.05 Spray 30 1 3 1.6 - 4.2 Ibid.
5 3 0.34 - 1.1
9 2 0.1 - 0.15
13 1 0.06
28 1 0.01
1 Omental and/or perirenal fat.
TABLE 5 Chlorpyrifos residues in milk of cows in Australia
Days between Chlorpyrifos1
last treatment residue in butterfat
and sampling (ppm avg. 4 cows) Reference
1 1.1 Snelson, 1969a
2 0.96
3 0.57
4 0.14
5 0.08
6 0.05
9 0.01
15 N.D.2
1 One dip application containing 0.025% chlorpyrifos.
2 N.D. = not detectable
Turkeys and chickens
Claborn et al. (1970b) determined residues of chlorpyrifos in the
body tissues of turkeys confined in pens containing soil treated with
4 or 8 lb/acre of chlorpyrifos (in a 25% wettable powder formulation)
for control of the chigger, Neoschongastia americana. The higher
concentration caused maximum residues of 0.157 and 0.066 ppm of
chlorpyrifos in the skin and fat, respectively, one week
post-treatment, that decreased to less than 0.001 ppm after six weeks.
No significant residues were found in the other tissues (thigh,
breast, liver). The oxygen analogue of chlorpyrifos (diethyl
3,5,6-trichloro-2-pyridyl phosphate) was not detected (<0.005 ppm) in
any tissues.
In a study conducted by Mann and Ivey (1971), turkeys were maintained
on soils treated two times, at an interval of 28 days, with 4 lb
chlorpyrifos/acre each time. Two or three birds were necropsied at
weekly intervals for six weeks after the first application and for
eight weeks after the second application. Tissue samples of fat, skin,
muscle, liver and kidney were analysed for residues of chlorpyrifos,
the oxygen analogue and the pyridinol moiety
(3,5,6-trichloro-2-pyridinol). Lowlevel residues of chlorpyrifos were
found in fat (0.022 - 0.005 ppm), skin (0.033 - 0.152 ppm) and kidney
(<0.002 - 0.005 ppm) of birds after one week of exposure to the
first application of compound to the soil. Fat only was analysed for
the remainder of the test for each application. The residues in fat
were reduced to <0.002 ppm (the sensitivity of the method) six weeks
after the first application and eight weeks after the second
application. No oxygen analogue was detected in any of the tissues.
Small residues of the pyridinol moiety were found in the skin of one
hen (<0.003 - 0.007 ppm), the liver of one tom (<0.01 - 0.025 ppm)
and the kidney of one tom (<0.01 - 0.098 ppm) after one week of
exposure to the first application; and in the skin (<0.003 - 0.003
ppm), liver (<0.01 - 0.078 ppm) and kidney (<0.01 - 0.165 ppm) in
one hen after one week of exposure to the second application of
compound to the soil.
Hunt et al. (1969) developed a new rapid gas chromatographic method
for analyses of chlorpyrifos in turkey and chicken tissues. The
residues reported are from the same experiments as the toxicological
results reported by Schlinke (1970) in chickens and Schlinke et al.
(1969) in turkeys. The tests consisted of dietary and contact tests
with chlorpyrifos for four weeks. Residue analyses of muscle, liver,
heart, brain, gizzard, skin and fat tissues were taken after 28 days
of treatment. Only skin and fat tissues showed detectable amounts of
chlorpyrifos. In chickens fed 100, 50 and 25 ppm in the diet, the
highest tissue residue was 0.87 ppm chlorpyrifos in fat. In turkeys
fed 100 and 50 ppm in the diet, the highest residue was 0.34 in fat.
Dishburger et al. (1969b) determined the presence of chlorpyrifos
residues in skin, fat, muscle and liver tissues of turkeys which had
been confined to soil treated with 4.5 lb chlorpyrifos/acre as
emulsifiable, granular, or wettable powder formulations. Muscle and
liver tissues contained undetectable amounts of chlorpyrifos in all
tests. Skin and fat tissues contained <0.1 ppm at all times, but were
higher at seven days post-treatment than any of the other weekly
samplings up through 28 days post-treatment.
In plants
Residues found in experiments with plants in United States
Chlorpyrifos has been applied to various species of plants and
generally found to be lacking in residual insecticidal properties on
plant-feeding insects. The residue determinations reported here
actually never exceeded 28 ppm at the dosages tested (up to 1 lb/acre)
and soon diminished, exhibiting a half-life of 1 - 2 days, thus
confirming the reason for the observed lack of residual effectiveness
on plant-feeding insects (see Table 6).
There was no evidence of residue buildup on plants from repeated
application of chlorpyrifos.
TABLE 6 Chlorpyrifos residues found in experiments in plants in United States
Dosage Application Chlorpyrifos
chlorpyrifos (no.) (no. of days Crop residues Reference
(lb/A) since last) (ppm)
1.0 1 0 Bermuda grass 13.2 Leuck et al., 1968
14 0.37
1.0 1 0 Maize 5.60 Ibid.
14 0.42
0.05 8 0 Pasture grass 0.7 - 8.2 Winterlin et al.,
7 <0.1 - 0.2 1968
0.05 1 0 Grass 0.11 - 8.1 Ibid.
3 0.012 - 1.8
0.025 1 0 Rice 0.09 - 0.64 Ibid.
6-14 <0.005 - 0.07
1.0 1 0 Various wild 26.9 Hurlbert et al.,
1 plants 27.4 1970
7 1.1
14 0.1
0.025 4 0 Rice 0.07 - 0.21 Dishburger et al.,
9 <0.01 1969c.
1.0 1 0 Maize silage 15.1 Johnson et al., 1969
1 4.4
0.25 1 0 Maize silage 2.5 Ibid.
1 1.1
Leuck et al. (1968) studied the persistence of chlorpyrifos and its
oxygen analogue on field plots of coastal Bermuda grass and maize
forage using the method of Bowman and Beroza (1967a) with a minimum
sensitivity of 0.002 - 0.01 ppm. Chlorpyrifos was applied at the rate
of 1 lb/acre. The oxygen analogue constituted a very low residue and
was only a small proportion of the total insecticide. Chlorpyrifos
residues varied from about 6 - 13 ppm at the time of application to 4
- 8 ppm after 1 day, to around 1 ppm after 7 days and to nearly
negligible levels after 21 days. The residue half-life of chlorpyrifos
appeared to be in the order of 2 - 3 days on Bermuda grass and maize
plants.
Winterlin et al. (1968) studied the residues of chlorpyrifos on rice
and pasture grass and environment when applied experimentally for the
control of mosquitoes around Colusa, California. They used the method
of Bowman and Beroza (1967a) i.e. gas chromatography with a flame
ionization detector, which is sensitive to 0.005 ppm chlorpyrifos in
rice and 0.01 ppm in pasture grass, and found residues as high as 8.11
ppm chlorpyrifos immediately after application of 0.05 lb
chlorpyrifos/acre. Residues decreased rapidly after application to
below 1 ppm.
Myers et al. (1968) reported on residues resulting from application
of chlorpyrifos sprayed by aircraft at the rate of 0.1 lb/acre, eight
times, over a period of five months on irrigated pastures. Samples of
grass analysed for the insecticide 1 - 3 hours after each spray
application varied from 0.4 - 8.2 ppm, and after seven days varied
from <0.1 - 0.2 ppm, using an analytical method sensitive to 0.1 ppm
chlorpyrifos. No evidence of accumulated residues of chlorpyrifos were
detected as the result of multiple applications to grass with
chlorpyrifos.
Hurlbert et al. (1970) reported on the residues of chlorpyrifos on
watergrass, sprangletop and smartweed on the dikes surrounding ponds,
resulting from hand-sprayed applications of chlorpyrifos emulsifiable
insecticide. Dosages used were 1, 0.1, 0.05, and 0.01 lb/acre diluted
in a constant spray volume of 8 gal/acre. Under these conditions
residues over 1 ppm occurred only at 1 lb/acre after 4 h, 1 day, and 7
days, but not 14 days; and at 0.1 lb/acre after 4 h. Residues
determined at other dosages and post-application times were less than
1 ppm. The half-life of the foliar residues of chlorpyrifos was in the
order of 1 - 3 days.
Dosages of 0.025 lb chlorpyrifos were applied aerially four times, at
monthly intervals, to rice fields. Residues of chlorpyrifos in raw
(rough) rice grain ranged from 0.07 to 0.21 ppm at 0 day, following
the last of the four applications. No residues of the oxygen analogue
of chlorpyrifos were detected, nor did detectable chlorpyrifos
residues persist more than 9 days after application (Dishburger
et al., 1969c).
Johnson et al. (1969) treated maize plants in the field with 0.25,
0.5 and 1.0 lb chlorpyrifos/acre. The initial residues in maize and
silage were 2.5, 3.9 and 15.1 ppm, respectively (on an as-collected
basis). After one day the losses of residues were 56, 40 and 71%,
respectively. This represents a half-life of about one day. One day
after treatment the maize and silage were enclosed in a silage bin
where chlorpyrifos residues declined slowly over a period of seven
weeks.
Dishburger et al. (1969a) analysed samples of tobacco leaves from
soil and foliage treated with 0.5, 1.0 and 1.5 lb chlorpyrifos/acre.
No residues (<0.01 ppm) were detected on the samples harvested (2
months or more post-application).
Residues found in experiments with plants in Canada
Residue data from experiments with onions, lettuce, carrots, and
tobacco are shown in Tables 7 and 8 using the analytical method of
Braun (1971) for chlorpyrifos and McKellar (1971c) for the pyridinol.
TABLE 7 Residues of chlorpyrifos from treatments of lettuce,
onions and carrots1
Application Residues on crop (ppm)
(lb/acre) (No.)2 lettuce onions carrots3
0.5 2 <0.001 0.001 <0.001
0.005 0.003 0.003
(54)4 (119) (138)
3 0.001 0.002 0.012
0.020 0.003 0.020
(31) (104) (123)
1.0 2 <0.001 0.002 0.012
0.008 0.005 0.034
(54) (118) (138)
3 0.002 0.002 0.006
0.009 0.005 0.018
(31) (104) (123)
2.0 2 0.003 0.008 0.015
0.029 0.013 0 046
(54) (119) (138)
2.0 3 0.008 0.003 0.019
0.034 0.005 0.033
(31) (104) (123)
1 McEwen and Frank, 1970.
2 First application was preplant soil treatment; second and third
soil and foliar treatments (Canada).
3 3,5,6-trichloro-2-pyridinol analysed for but not found at
detection limit of 0.005 ppm.
4 Days after chlorpyrifos withdrawal shown in ().
TABLE 8 Residues of chlorpyrifos from treatments of lettuce, onions,
carrots and celery
Withdrawal Applications1 Residues range
Crop (days) (no.) (ppm) References
Onions 57 1 0.001 - 0.003 McEwen, 1970
19 4 <0.0012 Harris, 1971
Celery 57 1 N.D.3 - 0.016 McEwen, 1970
Carrots 57 1 0.001 - 0.015 Ibid.
Lettuce 27 1 N.D. Ibid.
1 Treatments all post-planting, 2 lb/acre (Canada).
2 No oxygen analogue of chlorpyrifos detected at 0.01 ppm sensitivity
level.
3 N.D. = <0.001 ppm.
Residues found in experiments with plants in Europe and the Near
East
Residue data from experiments with cauliflower, red cabbage, eggplant,
beans, peppers, lettuce, mushrooms, carrots, potatoes, sugar beet,
corn, cotton, tomatoes, grapes, pears and apples are shown in Tables 9
and 10. These tests include soil and foliar applications.
Residues found in experiments with plants in Japan, Thailand,
Malaysia, and the Philippines
Residue data from experiments with Chinese cabbage and kale, rice and
apples are shown in Tables 11, 12 and 13.
TABLE 9 Residues of Chlorpyrifos in Food Crops, Europe/Near East Trials
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
FRANCE
Potatoes 1970 Granules Soil 1 1 500 g/ha 5 mo <0.005 Graf and Engeler,
5% active insecticide 1 3 500 g/ha 5 mo <0.005 1971a
W. GERMANY
Sugar beets 1971 Dursban EC Broadcast 1 1.15 kg/ha 112 d <0.13 Scheuerer & Hahn,
40.8% active Soil insect. 118 d 1972
Maize 1971 Dursban 4 EC Soil insect. 1 0.95 kg/ha 123 d <0.13 Ibid.
40.8% active Broadcast
HOLLAND
Cauliflower Strewpowder Soil insect. 1 120 mg 8 wk 0.002
Red cabbage (1) 1969 4% active 11 wk <0.002 Wit, 1970
Red cabbage (2) 85 wk <0.002
Lettuce (under 1970 3.2% active Soil insect. 1 1 900 g/ha 9 wk <0.01 Wit, 1971
glass) granule
Mushrooms 1971 25% wettable Spray 3 5 000 g/ha 3 wk 0.01 Olthof, 1971a
powder 3 wk 0.03
Carrots 1971 Dursban 25W Soil insect. 1 6 000 g/ha 3-4 mo 0.14 Olthof, 1971b
1 6 000 g/ha 3-4 mo 0.28
ISRAEL
Cotton 1970 Dursban E.C. Spray 6 730 g/ha 15 d <0.05 Graf, 1970a
40.8% active
Apples 1970 Dursban E.C. Spray 1 2 400 g/ha 7 d 0.54 Graf &
40% active 14 d 0.20 Engeler, 1970a
Tomatoes 1970 Dursban E.C. Spray 1 720 g/ha 1 d 0.24 Aharonson
40.8% active 4 d 0.16 et al., 1969
8 d 0.12
16 d 0.04
1 1 440 g/ha 1 d 0.45
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
4 d -
8 d 0.18
16 d 0.10
1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.27 Graf & Engeler,
40.8% active 5 d 0.25 1971b
Apples 1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.57 Graf & Engeler,
40.8% active 7 d 0.23 1971c
14 d 0.14
Spray 1 2 880 g/ha 1 d 0.52
7 d 0.28
14 d 0.24
Dursban Spray 1 1 440 g/ha 1 d 0.13
40.8% active 7 d 0.11
Spray 1 2 880 g/ha 1 d 0.29 Ibid.
7 d 0.24
ITALY
Sugar beets 1970 Dursban 5% Soil insect. 1 2 500 g/ha 3-6 mo <0.004 Foschi, 1971
Maize 1970 Granule Soil insect. 1 2 500 g/ha 3-6 mo <0.004
Lettuce 1971 Dursban E.C. Spray 1 500 g/ha 14 d 0.05 SIAPA, 1971
22.5% 1 1 000 g/ha 14 d 0.07
Apples 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.16 Graf, 1970b
40.8% active 29 d 0.10
1 1 224 g/ha 15 d 0.25
29 d 0.16
Pears 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.22 Graf, 1970c
40.8% active 29 d 0.14
1 1 224 g/ha 15 d 0.22
29 d 0.27
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
Turkey
Eggplant 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 0.26-0.40 Hollick &
E.C. 7 d 0.02-0.03 Collison, 1972
14 d 0.02-0.02
Beans 1972 Dursban 4 Spray 2 0.96 kg/ha 0 d 0.65-1.20 Ibid.
E.C. 7 d 0.08
14 d <0.04
Tomatoes 1970 Dursban E.C. Spray 1 347 g/ha 3 h 1.3 Graf & Engeler,
40.8% active 5 d 0.26 1970b
Peppers (under 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 2.00-5.20 Hollick &
glass) E.C. 7 d 0.04-0.10 Collison, 1972
14 d 0.13-0.25
UNITED KINGDOM
Raspberries 1971 Dursban E.C. Spray 1 0.1% 0 d 1.40 Burrows et al.,
40.8% active 5 d 0.32 1971d
2 0.5% 10 d 0.14
18 d 0.18
Tomatoes 1971 Dursban E.C. Spray 1 0 d 1.26 Burrows et al.,
40.8% active 3 d 0.10 1971e
7 d 0.08
14 d 0.01
28 d 0.01
1 All dosages given in a.i.
2 Last application to harvest
3 Limit of detection
TABLE 10 RESIDUES OF CELORPYRIPOS AND PYRIDINOL IN FOOD CROPS, EUROPE/NEAR EAST TRIALS
CROP, SAMPLE DOSAGE1 TIME BETWEEN RESIDUES RESIDUES REFERENCES
COUNTRY NO. LAST TREATMENT CHLORPYRIFOS 3,5,6-TRICHLORO-2-PYRIDINOL (ppm)
& YEAR AND HARVEST (ppm)
TURKEY
1971
Tomatoes 1 A 825 g/ha 3 hours 1.3* <0.05
2 A " 3 hours <0.05
4 A " 3 hours 0.05 Barrows & Mullin,
3 B " 5 days 0.05 1971b
5 B " 5 days 0.26 <0.05
6 B " 5 days <0.05
ENGLAND
1971
Tomatoes 720 g/ha 0 day 1.28 0.14
1.24 0.12
3 days 0.14 0.10
0.05 0.10 Burrows et al
7 days 0.08 0.08 1971e
0.08 0.08
14 days 0.01 0.12
0.01 0.10
28 days <0.01 0.10
<0.01 0.16
ITALY 1971
Apples 1 816 g/ha 29 days 0.10 <0.05 Burrows & Mullin,
4 1 224 g/ha 15 days 0.25 <0.05 1971b
Grapes 11 816 g/ha 29 days 0.10 <0.05
13 816 g/ha 15 days 1.10 0.06 Ibid.
12 1 224 g/ha 29 days 0.22 <0.05
14 1 224 g/ha 15 days 1.50 0.29
* Composite samples
1 All dosages given in a.i.
TABLE 11 CHLORPYRIPHOS RESIDUES ON CROPS IN JAPAN1
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
Chinese
cabbage
1967 40E 267 1 4 0.13-0.23
1100 l/ha
1969 40E 267 2 8 0.02-0.09
1000 l/ha 2 16 0.43-0.44
" 267 4 8 0.16-0.22
4 16 0.62-0.86
1969 40E 267 2 7 0.02-0.06
1500 l/ha 2 14 0.06-0.08
" 267 4 7 0.08-0.14
4 14 0.06-0.12
Apples
Rall's Janet
1967 25W 250 4 66 N.D.3
Johnathan
1967 25W 250 5 42 0.01-0.05
1969 25W 250 3 30 0.05-0.07
30 l/tree 45 0.05-0.06
1969 " 250 2 17 0.03-0.07
28 0.02-0.05
46 0.02-0.03
TABLE 11 (Cont'd.)
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
25W 250 3 7 0.03-0.09
30 l/tree
14 0.05-0.17
31 0.08-0.16
45 0.03-0.05
Aomori
1969 25W 250 2 7 0.10
14 0.12-0.14
21 0.08-0.03
1 Hamaguchi, 1970
2 Range of averages for two laboratories
3 N.D. = not detectable (<0.005 ppm)
TABLE 12 CHLORPYRIFOS RESIDUES ON CROPS IN THAILAND AND MALAYSIA
CROP FORMULATION APPLIC. DAYS BETWEEN TESTS RESIDUE1 REFERENCES
& DILUTION (no.) LAST SPRAY (no.) (ppm)
& HARVEST
Chinese 25W 6 5 6 N. D.2 - 0.023 Tucker & Zielinski,
cabbage 1972a
Chinese 187 ppm(a.i.) " " " 0.10 - 0.243 Ibid., 1972b
kale " " " " 0.09 - 0.763
Chinese 25W " " " N.D.3 Ibid., 1972a
cabbage
Chinese 375 ppm(a.i.) " " " 0.38 - 0.753 Ibid., 1972b
kale " " " " 0.35 - 0.763
Chinese 25W 16 2 3 0.07 - 0.104 Ibid., 1972,
kale
3 oz a.i./A " " " 0.01 - 0.35
25W " " " 0.13 - 0.244
4 oz a.i./A " " " 0.01 - 0.035
Tobacco 25W 10 7 4 0.03 - 0.053 Ibid., 1972d
187 ppm (a.i.)
25W " " " 0.21 - 0.383
375 ppm (a.i.)
1 Two analytical methods used in analyses
2 N.D. = not detectable (<0.01 ppm)
3 "Usable leaves" from Thailand
4 Leaf only from Malaysia
5 Stem only from Malaysia
TABLE 13 Chlorpyrifos residues on rice in the Philippines1
Chlorpyrifos Residue
applied (kg/ha, Tests (range of
Part analysed Formulation 3 applications) (no.) averages,
ppm)
Grain E.C. 0.10 4 N.D.3
0.15 4 N.D. - 0.008
0.20 3 N.D. - 0.005
0.25 2 0.008
Granule 0.10 3 N.D.
Grain and E.C. 0.10 4 N.D. - 0.007
straw2 0.15 4 N.D. - 0.006
0.20 4 0.005 - 0.008
0.25 2 0.008
Granule 0.10 3 <0.005
1 Tucker, 1971.
2 Very little straw, mostly grain.
3 N.D = not detectable (<0.005 ppm)
FATE OF RESIDUES
General comments
The fate of chlorpyrifos residues and its metabolites in different
species of animals are discussed in the section on "Biochemical
aspects".
In animals
Cow
Chlorpyrifos was not found in the urine and milk from a cow fed 5 ppm
(based on a daily ration of 50 lb) of the compound for four days. The
compound in absolute ethanol was mixed thoroughly with the grain. A
compound characterized by retention time as chlorpyrifos was found in
faecal samples taken on the last three days during which the
insecticide was fed and the first day after, and represented 1.7% of
the insecticide fed. Two metabolites were excreted in the urine which,
following methylation, had retention times identical to the methyl
esters of diethylthiophosphate and diethyl phosphate. They represented
35.9 and 26.8%, respectively, of the total insecticide fed on an
equivalent basis (Gutenmann et al., 1968).
Fish
Waters treated with 50 and 300 ppb of ring-labelled 14C chlorpyrifos
and containing goldfish were analysed for the types of metabolites
found in each. Metabolites I, III, IIIa and IIIb, shown in Figure 1,
as well as chlorpyrifos were found in fish, and metabolites I, IIIa
and IIIb were found in the water (Smith et al., 1966).
The major metabolic product in fish is 3,5,6-trichloro-2-pyridinol
which appears to be slowly broken down by dehalogenation and cleavage
of the ring (Smith et al., 1966).
Some of the derivatives of 3,5,6-trichloro-2-pyridinol may be those
shown in Smith (1968) which consist of dechlorination forming a series
of transient pyridine diols, triols and tetrols (Ia), followed by a
series of transient ring diketones (Ib), followed by a breakage of the
ring to elemental fragments (carbon dioxide and possibly aliphatic
amines).
Cholinesterase inhibition properties of metabolites
The house fly head cholinesterase activity of chlorpyrifos, and
metabolites I, II, III, IIIa and IIIb shown in Figure 1, were tested
for comparative purposes by Smith et al. 1966). Only the phosphate
derivative of chlorpyrifos (metabolite III) shows significant
inhibition and is the compound most likely to be responsible for the
cholinesterase inhibition found in mammals and other animal organisms
(Smith et al., 1966).
In plants
Studies on the absorption, translocation and metabolism of 14C and
36Cl labelled chlorpyrifos in plants show that for all practical
purposes uptake of the chemical or its degradation products into
plants does not occur, either through the foliage or through the roots
(Smith et al., 1967a, 1967c).
When radioactive chlorpyrifos was applied to single bean or corn
leaves, about 75 to 80% of the chemical was lost by vapourization from
the leaf surface within the first 48 h following treatment. The
chlorpyrifos remaining on the leaf was rapidly degraded (>95% in 5
days) and a trace of the resulting breakdown products (representing
<2% of the applied dose of chlorpyrifos) absorbed into and
translocated within the plant. The identity of the degradation
products was not totally elucidated; however, a variety of hydrolysis
products appeared to be formed, mainly the 3,5,6-trichloro-2-pyridinol
(I), ethyl-3,5,6-trichloro-2-pyridyl phoshate (IIIa) and
3,5,6-trichloro-2-pyridyl phosphate (IIIb) shown in figure 1 (Smith
et al., 1967a).
Mol. Wt. 350.6
Empirical Formula C9H11Cl3NO3PS
Other relevant chemical and physical properties
State: white, granular, crystalline solid
Melting point: 42 - 43.5°C
Vapour pressure: 1.87 × 10-5mm Hg (at 25°C)
8.87 × 10-5mm Hg (at 35°C)
Solubility: readily soluble in acetone, benzene,
chloroform, methanol and iso-octane; slightly
soluble (0.4 ppm) in water.
When chlorpyrifos was exposed to UV light or to sunlight, it underwent
hydrolysis in the presence of water to liberate
3,5,6-trichloro-2-pyridinol, which underwent further decomposition to
diols and triols and ultimately cleavage of the ring to fragmentary
products (Smith, 1968). Hydrolysis in water occurs least readily at
about pH 6 and very readily above pH 8.
The technical product is supplied in a variety of concentrations and
formulations (emulsifiable concentrates, dusts, wettable powders and
granules).
Purity: 99.5%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
As with other phosphorothioate esters chlorpyrifos is rapidly
absorbed, metabolized and excreted by mammals following oral
administration. When 36Cl chlorpyrifos was fed by intubation to rats
as a single sublethal dose (50 mg/kg), the radioactivity was
eliminated rapidly (90.1% in 26 h) via the urine (90%) and faeces
(10%). Products excreted were identified as 36Cl 3,
5,6-trichloro-2-pyridyl phosphate (75-80%),
3,5,6-trichloro-2-pyridinol (15-20%) and traces of chlorpyrifos. The
pyridyl phosphate was indicated to be a transient intermediary in the
hydrolysis of chlorpyrifos to the pyridinol moiety. Analyses showed
no appreciable residue in any tissue except fat, which showed
temporary retention of radioactivity (halflife of ca 62 h) identified
principally as chlorpyrifos (Smith et al., 1967b). This study
suggests that desethylation is a significant metabolic pathway in
rats. An additional study in the rat involving administration of 14C
chlorpyrifos by single dose intubation (19.1 mg/kg) essentially
verified the earlier result (Branson and Litchfield, 1971a,b). In this
study, as well as in an in vitro study involving breakdown of
chlorpyrifos by rat liver microsomes (Branson and Wass, 1970),
3,5,6-trichloro-2-pyridinol was identified as the only major
metabolite. A study in cows involving feeding of chlorpyrifos in the
diet at 5 ppm for four days indicated that the major pathway for
degradation was by hydrolysis to the pyridinol, as no desethyl
phosphate esters were found. In addition no chlorpyrifos or
metabolites were found in milk (Gutenmann et al., 1968).
Studies of 3,5,6-trichloro-2-pyridinol, the major enolic metabolite of
chlorpyrifos, have shown absorption, distribution and excretion in the
rat following single oral administration to be similar to that
reported for the parent compound. The metabolite is rapidly absorbed
and excreted primarily in the urine and faeces. Small tissue residues
(<0.3 ppm) were present and occurred mainly in the biological systems
involved with urinary excretion, i.e. liver, kidney and blood. Unlike
chlorpyrifos, residues of the pyridinol in fat were trivial, which is
consistent with its much greater polarity (Branson and Litchfield,
1971a,b; Smith et al., 1970).
Biotransformation
The metabolic fate of chlorpyrifos has been investigated in plants
(Smith et al., 1967a, 1967b) and data indicate an oxidative and/or
hydrolytic breakdown yielding des-mono-ethylchloropyrifos; desethyl
chlorpyrifos; 3,5,6-trichloro-2-pyridinol and further degradation
products. This is discussed further in the section on "Fate of
residues."
Effects on enzymes and other biochemical parameters
Chlorpyrifos is a cholinesterase inhibitor affecting this enzyme in
brain or blood, although differing in its response with different
animal species. In vitro studies have shown the oxygen analogue of
chlorpyrifos to be 106 times more active as an inhibitor of
cholinesterase than chlorpyrifos (I50 values: chlorpyrifos approx.
2.5 x 10-3M; oxygen analogue approx. 2.5 x 10-9M) (Smith, 1966).
Equimolar concentrations of chlorpyrifos administered to dogs and
monkeys affected RBC cholinesterase in monkeys while having almost no
effect on RBC cholinesterase in dogs. Plasma cholinesterase is the
most sensitive indicator of exposure to chlorpyrifos. In dogs and
monkeys administered subacute levels of chlorpyrifos, plasma
cholinesterase was maximally inhibited within 8 hours following
exposure. Recovery in dogs was complete within 24 hours while with
monkey, plasma cholinesterase in monkeys remained depressed at 48
hours (Coulston et al., 1971).
TOXICOLOGICAL STUDIES
Acute toxicity of the metabolite 3,5,6-trichloro-pyridinol has been
studied in rats and mice. Results are summarized in Table 1. Signs of
poisoning with this metabolite include facial paralysis with dyspnoea
and slight hypersalivation and exophthalmia (mice only) (Gerbig and
Emerson, 1970 a,b).
Special short-term studies on metabolites
Rat
Groups of rats (10 males and 10 females/group) were fed the metabolite
of chlorpyrifos, 3,5,6-trichloro-2-pyridinol, in the diet for 90 days
at dosage levels of 0, 100, 300, 1 000, 3 000 and 10 000 ppm. Diuresis
was observed at 3 000 ppm. At 10 000 ppm a reduction in body-weight
was observed which reflected a general retardation in growth in both
sexes. No effects were observed on mortality, terminal haematology or
gross and histological examination of tissues (Beatty and McCollister,
1964).
Dog
Groups of dogs (3 males and 3 females/group, 4 of each sex were
controls) were fed the metabolite of chlorpyrifos,
3,5,6-trichloro-2-pyridinol, in the diet for 91 days at dosage levels
of 0, 1, 3, 10 and 30 mg/kg/day (Emerson and Gerbig, 1970). Another
study utilized groups of dogs (2 males and 2 females at the higher
level and 4 male and 4 female at the lower level) which were fed the
metabolite at dietary levels of 26 and 80 mg/kg/day for 93 days
(Copeland, 1964). At 80 mg/kg the metabolite produced abnormalities
including growth depression in females, increased spleen/body-weight
ratios and histological changes in the liver. At 26 mg/kg an elevated
SAP accompanied by equivocal changes in the liver was observed. At 30
mg/kg increased SAP, SGOT and SGPT values were observed in the second
study. The increased biochemical parameters were noted in 1 of 3 dogs
examined at 10 mg/kg. Hepatic lesions noted in 1 dog at 10 mg/kg and
1 dog at 30 mg/kg did not appear to be chemically induced. No effects
were noted at 3 mg/kg/day on growth, behaviour, haematology, blood
biochemistry, urinalysis or gross and microscopic examination of
tissues.
TABLE 1 Acute toxicity of the metabolite 3,5,6-trichloropyridinol
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 794 Gerbig and Emerson, 1970a
F 870
Mouse M Oral 380 Gerbig and Emerson, 1970b
F 415
Duckling
Groups of Peking ducklings (10 per group, 20 used as controls) were
fed the metabolite at dosage levels of 0, 1, 3, 10, 30, 60, 100, 300
and 1 000 ppm for 3 weeks to determine cataractogenicity. Growth
retardation was evident at 1 000 ppm, and 3 of 10 ducklings had
elevated prothrombin values. Levels of 300 ppm and below produced no
effect on these parameters and no cataractous changes were observed at
any level (Molello and Sharp, 1968).
Special studies in neurotoxicity
Hens (3 DeKalb leghorns/group) were administered chlorpyrifos orally
at dosage levels of 40, 75, 100 and 150 mg/kg and observed for 27
days. Mortality occurred at the higher levels. Surviving birds showed
no signs of delayed ataxia or paralysis. Crufomate administered as a
positive control (1 000 mg/kg) produced clinical signs of paralysis
(Stevenson, 1966).
Special studies on potentiation
Rats administered subacute oral dosages (1/2 the LD50 value) of
chlorpyrifos in combination with malathion or dichlorvos showed slight
evidence for potentiation of the acute toxicity. A three-fold increase
in the acute toxicity was noted with malathion, but this was judged to
be of no practical significance (Dow Chemical Co., 1964; Norris,
1970).
Special studies on reproduction and teratogenicity
Groups of rats (10 males and 20 females/group with 20 males and 10
females/group as controls) were fed chlorpyrifos in the diet for one
generation (two litters) at dosage levels of 0, 0.03, 0.1 and 0.3
mg/kg and for two generations (two litters per generation) at dosage
levels of 0, 0.1, 0.3 and 1.0 mg/kg. The F3b litter was used in a
teratology study where the parents were fed dietary levels of
chlorpyrifos until day 6 - 15 of organogenesis when the dietary
supplement was removed and chlorpyrifos administered by gavage once
daily. After day 15 chlorpyrifos was again administered by dietary
supplement until day 20 when the females were sacrificed, foetuses
removed by caesarean section and viscera and skeletal structure
examined. Neonatal mortality was somewhat higher in F2a, F2b and F3a
litters at 1.0 mg/kg. No other significant effects were observed on
fertility, reproduction or lactation indices or on teratological
examination of F3b pups as a result of feeding chlorpyrifos at levels
up to 1.0 mg/kg/day. Cholinesterase depression was observed at levels
of 0.3 mg/kg and above, primarily in plasma (Thompson et al., 1971).
Acute toxicity
The acute toxicity of chlorpyrifos has been studied in several animal
species, and the results are summarized in Table 2.
TABLE 2 Acute toxicity of chlorpyrifos in different animal species
Species Sex Route LD50 (mg/kg) Reference
Rat M Oral 118 - 245 Taylor and Olson, 1963;
Gaines, 1969; Tucker and
Crabtree, 1970
F Oral 82 - 135 Taylor and Olson, 1963;
Gaines, 1969
Mouse M Oral 102 Coulston et al., 1971
- i.p. 31 USAEHA, 1968
White
footed - Oral 64 Kenaga, 1967
Mouse
Rabbit - Oral 1 000 - 2 000 Taylor and Olson, 1963
Cavy M Oral 504 Ibid.
Goat F Oral 500 - 1 000 Tucker and Crabtree, 1970
Chukar F Oral 61.1 Ibid.
M Oral 60.7 Ibid.
Bullfrog M Oral >400 Ibid.
Chick M Oral 25 - 35 Taylor and Olson, 1963;
Stevenson, 1966; Sherman
et al., 1967; Brust
Chicken - Oral 32 - 63 et al., 1971
The LD50 in a number of avian species has been determined by Tucker
and Crabtree (1970). The toxicity values range from 8 mg/kg in male
ring necked pheasant to 167 mg/kg in mallard ducklings.
Signs of poisoning with chlorpyrifos are typical of cholinesterase
inhibiting compounds. Mortality following acute oral dosing may be
delayed for as long as nine days in some species of animals (Tucker
and Crabtree, 1970).
Chlorpyrifos administered to the conjunctival sac of rabbits produced
transient redness of the conjunctiva. No corneal injury was observed
(Taylor and Olson, 1963). When applied to intact or abraded abdominal
skin of rabbits daily for up to 2 weeks no significant dermal damage
was observed. Prolonged contact produced burning and hardening of the
skin, slight swelling and mild hyperemia which returned to normal
within three weeks. Chlorpyrifos administered dermally as a technical
product to rabbits in an undiluted form produced no untoward
physiological effects. When administered as a 50% solution in
dipropylene glycol methyl ether, the same dose (2 000 mg/kg) was
rapidly absorbed and resulted in death in four out of six animals when
applied to either intact or abraded skin (Taylor and Olson, 1963).
Chlorpyrifos did not induce sensitization when examined on the guinea
pig (USAEHA, 1968).
Dogs and sheep were exposed for a 4-hour period to a thermal fog or
liquid aerosol at a concentration of 4 or 8 mg/cu ft. Mild plasma
cholinesterase depression in dogs at the higher dose was the only
effect noted (Dow Chemical Co., 1966a).
Five female rats were exposed for 7 hours/day to an atmosphere of
chlorpyrifos (calculated at 7 mg/l air) for 16 exposures over a 21-day
interval. There were no effects on growth, appearance, behaviour or
plasma and RBC cholinesterase activity (Dow Chemical Co., 1966b).
Short-term studies
Mallard duck
Groups of mallard ducklings (6 - 8 birds/group, 5 days old) were fed
chlorpyrifos for five days at dosage levels of 0, 100, 200, 300, 500,
700, 1 000 and 2 000 ppm in the diet. Mortality occurred at all dosage
levels (100-2/8; 200-4/7; 300-5/8; 500-6/8; 700 - 2000 - all dead)
except in controls (0/7 dead) (Stevenson, 1965).
Chickens
Groups of chicks (20 chicks/group) were fed chlorpyrifos in the diet
for two weeks at dosages of 0, 200, 400 and 800 ppm. Mortality was
observed at 400 ppm and plasma cholinesterase depression and reduced
weight gain was observed at all levels (Sherman et al., 1967).
Groups of chickens (30 per group) were administered chlorpyrifos in
the drinking water at dosage levels of 0, 1 ppb, 1 ppm and 100 ppm for
84 days. A higher level (1000 ppm) resulted in clinical signs of
cholinergic stimulation. Plasma cholinesterase was depressed at 100
ppm but not at 1 ppm. A 10 ppm group was tested for two weeks with no
signs of cholinesterase depression. Growth and behaviour were not
affected at 100 ppm (Stevenson and Daering, 1969). Chlorpyrifos added
to the drinking water of chicks at dosages of 0, 0.08, 0.32, 1.25, 5,
20, 80, 320 and 1 280 ppm for periods varying from 3 - 5 weeks induced
mortality and reduced growth at levels of 80 ppm and above. Whole
blood cholinesterase depression was evident only at 80 ppm and above
(Brust et al., 1971).
Chickens fed chlorpyrifos in the diet at levels of 0, 25, 50 and 100
ppm for four weeks exhibited reduced plasma cholinesterase activity
and reduced weight gain at all feeding levels. Calculations of food
conversion data show a reduced yield in production as a result of 25
ppm in the diet. No clinical signs of toxicity were observed
(Schlinke, 1970).
Rat
Groups of rats (20 males and 20 females/group) were fed chlorpyrifos
in the diet for six months at dosage levels of 0, 0.03, 0.15 and 0.75
mg/kg/day. Cholinesterase depression was noted at 0.75 mg/kg/day in
RBC and plasma. Brain cholinesterase activity was unaffected. No
effects were noted at any feeding level on mortality, behaviour,
growth, haematology or gross or histological examination of tissue
(Coulston et al., 1971).
Groups of rats (10 males and 10 females/group) were fed chlorpyrifos
in the diet for 90 days at levels of 0,10, 30, 100 and 300 ppm. Growth
reduction was observed in both males and females at 300 ppm. Increased
liver and kidney organ to body-weight ratios were also observed
(possibly as a result of reduced body-weight as organ weights were
comparable to the controls). Cholinesterase depression was observed at
30 ppm in blood and brain; at 10 ppm, brain cholinesterase was normal
although enzyme inhibition was observed in RBC and plasm. No adverse
effects were noted on growth, appearance, food consumption, mortality,
behaviour, haematology or gross and microscopic pathology at 100 ppm
(Beatty and McCollister, 1964).
Five groups of rats (10 males and 10 females/group) were fed
chlorpyrifos at levels varying from 0.03 - 10.0 mg/kg/day for 28 - 90
days to determine a "no-effect" level on cholinesterase depression.
Dosage levels of 0 and 0.3 mg/kg/day were fed for 90 days while
dosages of 1, 3 and 10 mg/kg/day were fed for 28 days, stopped for
three weeks (during which time the rats were fed control diets) and
the rats refed diets containing 0, 0.03 and 0.1 mg/kg/day for a full
90-day study. Behaviour and growth were affected at 3 and 10
mg/kg/day. Cholinesterase was depressed at 0.3 mg/kg/day and above in
both male and female rats. The rats in the three highest groups were
removed for three weeks during which time cholinesterase activity
returned to pretest intervals. On resumption of feeding chlorpyrifos
at 0.1 mg/kg/day, slight cholinesterase depression was observed in
plasma and erythrocytes. No cholinesterase depression was noted on
brain or blood enzymes at 0.03 mg/kg/day (Blackmore, 1968b).
Dog
Groups of dogs (2 males and 2 females/group; 4 of each sex were
controls) were fed chlorpyrifos for 93 days. The dosage levels of
feeding were initially 200, 600 and 2 000 ppm in the diet, but because
of the appearance of signs of cholinergic poisoning the dosages were
changed from 2 000 to 60 ppm after 5 days while the 200 ppm (5.8
mg/kg/day) level was maintained for 45 days. The dogs were then placed
on control diets for the remainder of the test. Another group of 2
male and 2 female dogs was added to the study at a dietary level of
200 ppm (3.4 mg/kg/day) for 27 days, removed from the test for 5 days
and returned to this dietary level for the final 2 weeks. A decrease
in food consumption at the 200 ppm level was accompanied by reduced
growth, especially in females. Growth of all dogs was slightly lower
than control levels. Increased SGPT values were seen in several dogs
at all feeding levels. RBC, plasma and brain cholinesterase values
were reduced in all animals fed chlorpyrifos in the diet at a level of
20 ppm (0.8 mg/kg/day) and above. No adverse effects were observed on
haematology, BUN, SAP, organ weights and gross or microscopic
examination of tissues (Copeland, 1964).
Groups of dogs were orally administered chlorpyrifos daily in a
gelatin capsule for periods of 32 to 90 days at doses of 0 (2 males
and 2 females - 90 days), 0.01 mg/kg/day (2 males and 2 females - 32
days), 0.03, 0.10, 0.30 and 1.00 mg/kg/day (2 males and 2
females/group - 90 days), and a final group (2 males and 2 females)
was given 1.00 mg/kg/day from day 0 to 18, control diet from days 19
to 42, 0.03 mg/kg/day from days 43 to 58, control diet from days 59 to
77, 0.01 mg/kg/day from days 78 to 94 and control diet until day 124.
Data obtained on appearance, body-weight and food consumption were
normal. A dose-dependent cholinesterase depression in plasma was
observed to begin at 0.03 mg/kg/day. At this level inhibition was
moderate and was more marked as the dietary level increased. Brain
cholinesterase was not affected at any level, and RBC cholinesterase
was affected at levels of 0.1 mg/kg/day and above. No effects were
noted at 0.01 mg/kg/day on any parameters tested over the 32-day
administration interval (Blackmore, 1968a).
Groups of dogs (7 males and 7 females/group) were fed chlorpyrifos in
the diet for up to two years at dose levels of 0, 0.01, 0.03, 0.1, 1.0
and 3.0 mg/kg/day. Inhibition of cholinesterase activity was the only
abnormality detected. Inhibition of RBC cholinesterase in males and
females was evident at 1.0 and 3.0 mg/kg. Marginal depression of brain
cholinesterase was shown at the highest level of feeding. Plasma
cholinesterase was depressed at 0.1 mg/kg/day in both males and
females. Occasional reduction at 0.03 mg/kg was noted. Studies on the
recovery of cholinesterase activity following one year feeding
indicate that plasma activity recovered to normal within two weeks
while RBC recovery was much slower. No significant effects were noted
on mortality, behaviour, food consumption, growth, haematology, blood
biochemistry, urinalysis, or gross and microscopic examination of
tissues. Gross examination showed an increased liver weight and ratio
at 3.0 mg/kg at two years but not at one year. All other tissues were
normal (McCollister et al., 1971a).
Monkey
Groups of Rhesus monkeys (2 males and 2 females - control; 2 males and
1 female - 0.08 or 0.40 mg/kg/day; 2 males and 2 females - 2.0
mg/kg/day) were administered chlorpyrifos orally by gavage daily for
six months. Plasma and RBC cholinesterase activity was depressed in
those animals that received 2.0 and 0.4 mg chlorpyrifos/kg/day. Plasma
cholinesterase activity was slightly depressed and RBC cholinesterase
activity was not depressed in animals that received 0.08 mg/kg/day.
Brain cholinesterase activity was not affected at any level. No
significant effects were noted on behaviour, growth, haematology,
clinical chemistry or gross or histological examination of tissues.
The histological examination included electron microscopy of liver and
kidneys and histochemical delineation of lysosomal distribution in
these two organs (Coulston et al., 1971).
Long-term studies
Rats
Groups of rats (57 males and 57 females/group) were fed dietary levels
of chlorpyrifos of 0, 0.01, 0.03, 0.1, 1.0 and 3.0 mg/kg/day for two
years. Blood cholinesterase activity, primarily in plasma, was
depressed in females at 0.1 mg/kg and above. Brain cholinesterase was
inhibited at 3.0 mg/kg and slightly depressed at 1.0 mg/kg.
Chlorpyrifos at all dosage levels had no significant effect on
behaviour, appearance, growth, mortality, haematology, urinalysis,
clinical biochemistry, gross or histopathology of tissues and organs
or the incidence of neoplasms (McCollister et al., 1971b).
OBSERVATIONS IN MAN
Groups of men (4 men/group) were administered chlorpyrifos by tablet
daily for 27 days (0.014 mg/kg/day), 20 days (0.03 mg/kg/day), 9 days
(0.10 mg/kg/day) and 48 days (control). No effects were noted on
behaviour, haematology, urinalysis or biochemical determinations in
blood. Blood plasma cholinesterase was depressed at the highest dosage
and this regimen was discontinued. Recovery was complete in four
weeks. At 0.03 mg/kg/day a statistically non-significant, although
suggestive, depression of plasma cholinesterase was noted. No effects
were noted at 0.014 mg/kg on plasma cholinesterase. RBC activity was
unaffected at any level of chlorpyrifos. The level of 0.03 mg/kg/day
appears to be the minimal threshold response level in humans to
chlorpyrifos based on plasma cholinesterase (Coulston et al., 1972).
COMMENT
The compound is rapidly absorbed and metabolized by mammals, the major
metabolite being a chlorinated pyridonal hydrolysis product of low
mammalian toxicity.
There is no evidence of neurotoxicity or cataractogenicity.
Potentiation has been demonstrated only with malathion.
Reproduction studies and examination of offspring for teratogenic
abnormalities did not reveal adverse effects other than an increase in
neonatal mortality at 1 mg/kg/day. Chlorpyrifos is an active
cholinesterase inhibitor, inhibiting plasma cholinesterase to a far
greater degree than other cholinesterases. One short-term study in
rats indicated an increase in sensitivity to plasma cholinesterase
depression following withdrawal after initial treatment. This study
needs confirmation.
No-effect levels have been demonstrated in dog, rat and man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.03 mg/kg/day
Dog: 0.01 mg/kg/day
Man: 0.014 mg/kg/day orally for 1 month
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.0015 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In animals
Chlorpyrifos is used to control various species of fever ticks
(Boophilus sp.), ear ticks, lice and horn flies on beef cattle and
non-lactating dairy cattle, by use of emulsifiable liquid formulations
in water varying from 0.025 to 0.125% applied as a spray or dip.
Treatment for all but ear ticks is limited to six applications at
21-day intervals, and not within two weeks of slaughter.
Sheep dipped or sprayed with wettable powder or emulsifiable
formulations of chlorpyrifos are protected from blowfly, ticks, body
lice and sheep keds. A minimum interval of seven days is required
between treatment and slaughter.
Turkey chiggers are controlled through the use of wettable powder
formulations sprayed on soil in turkey pens at the rate of 4 lb/acre
chlorpyrifos, before the turkeys are introduced into the pens.
Treatment is limited to two applications, not within seven days of
slaughter.
In plants
Soil treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control soil pests such as wireworms,
white grubs, mole crickets, Maulwurf's Grill, garden symphylans,
seed corn maggots, cereal maggots, beet root weevils, the
Atomaria, Blaniulus, Tipula and cutworms. The chemical is
applied by means of broadcast row or furrow treatments and is
varied in concentrations from 300 to 4 000 g/ha a.i. as wettable
powder, emulsifiable or granular formulations before and after
planting, previous to harvest. The crops involved are maize,
spring cereals, beans, onions, tobacco, turnips, bulb flowers and
carrots.
2. Canada
Chlorpyrifos is used to control soil insect pests of lettuce,
onions and carrots before and after planting at dosages of 1 to 2
lb/acre applied as needed, but with the indicated 28- to 60-day
withdrawal period before harvest. Tobacco plants are treated in
soil water, preplant and by soil and foliar application.
Foliar treatments
1. Europe, Near East and South Africa
Chlorpyrifos is used to control insect pests of rape, cereals,
potatoes, fodder crops, beans, peas and deciduous fruits such as
apples and pears.
2. Japan, Thailand, Philippines, India and Malaysia
Chlorpyrifos is used to control insect pests of apples, pears,
rice, tobacco, Chinese cabbage and kale.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In animals
Cattle
Considerable work has been done in U.S.A. on the analysis of cattle
tissues for residues of chlorpyrifos as well as its oxygen analogue
and pyridinol moiety. Typical distribution of residues in cattle
dipped in chlorpyrifos is shown in Table 3 (Claborn et al., 1970a).
In this study, 57 beef cattle were dipped one to six times in a 0.025%
emulsion of chlorpyrifos at intervals of 21 days. Animals were removed
from the dipping schedule after each dip, except the fifth, for
post-treatment slaughter at various time intervals. Fat muscle, kidney
and liver tissue samples were collected and analysed for residues of
chlorpyrifos, its oxygen analogue, and the 3,5,6-trichloro-2-pyridinol
moiety. Residues of chlorpyrifos were predominately in the fatty
tissues, and residues of 3,5,6-trichloro-2-pyridinol were in the
kidney and liver tissues. No residue of the oxygen analogue was
detected in any tissue.
Residues of the hydrolytic pyridinol were not detectable in muscle
tissue or in kidney and liver tissues of cattle, except at low levels
for the first 1 - 2 weeks after the last dip, even after six dippings.
It should be noted that this experiment began in February, so the
animals had their winter coat of hair through the first three dippings
and were shedding it at approximately the fourth dipping period. The
heavy winter hair coat carries more insecticide out of the vats than
the summer coat, as evidenced by the higher residues after the second
and third dippings and the drop in residues after the fourth dipping.
The effect of the winter coat is also reflected by the higher residues
after the first dip when compared to levels of residues after a single
dip (in the same concentration or higher concentration - 0.05% - of
chlorpyrifos) in the animals reported by Mann, 1966; Claborn et al.,
1968a,b; and Dishburger et al., 1966a,b.
Since the season for ticks extends from approximately April or May to
mid-September, animals would be expected to shed their winter coat
after the first or second dipping when treated according to label
instructions. Therefore, in practice residue levels as high as those
found in the test animals would not be expected after the third
dipping, since these animals would have been dipped three times before
shedding their winter coat of hair. In any case, it is believed that
this experiment represents the highest levels of chlorpyrifos that
would ever be encountered in the use of 0.025% chlorpyrifos as a dip.
In an early test, cattle were sprayed once with a 0.5% emulsion of
chlorpyrifos (20 times the label recommendation). Residues of the
compound in the fat were 1.3 ppm (maximum) at 20 days post-treatment
and had declined to 0.08 ppm (maximum) at 30 days post-treatment
(Dishburger and Rice, 1965).
In cattle treated with an emulsion containing 0.05% chlorpyrifos
(twice the label recommendation), the average amounts of residue in
the fat one week after a single-dip application were about twice those
resulting from a single-spray application. The maximum residues were
well below 1 ppm (0.209 for spray and 0.525 for dip application) at
the first sampling period at seven days post-treatment. Approximately
two weeks were required for residues to be reduced to 1 ppm in the fat
of animals given multiple treatments (3 dippings at 2-week intervals)
at this level (Mann, 1966; Claborn et al., 1968a,b).
TABLE 3 Chlorpyrifos residues in cattle in the United States1
Mean residues of
Weeks Mean residues of chlorpyrifos2 3,5,6-trichloro-2-pyridinol3
after last fat muscle kidney kidney liver
dipping (ppm) (ppm)
1 dipping
1 0.672 0.004 0.003 0.12 0.12
2 0.142 0.002 0 <0.05 <0.05
3 0.083 0.001 0 0 0
4 0.038 0 0 0 0
2 dippings4
1 1.245 0.011 0.008 0.30 0.26
3 dippings4
1 1.589 0.028 0.008 0.24 0.29
2 0.085 0.011 0.001 0.05 0.05
4 0.158 0 0 0 0
6 0.038 0 0 0 0
8 0.041 0 0 0 0
10 0.001 0 0 0 0
4 dippings4
1 0.693 0.011 0.001 0.10 0.12
6 dippings4
1 0.744 0.015 0.003 0.12 0.10
2 0.141 0.001 0 0 0
4 0.069 0 0 0 0
6 0.018 0 0 0 0
8 0.007 0 0 0 0
10 0 0 0 0 0
1 Residues of chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues
of beef cattle dipped in an emulsion containing 0.025% chlorpyrifos.
Residue levels corrected for % recovery from tissues.
2 No chlorpyrifos found in liver (0 - <0.002 ppm) or oxygen
analogue (0 - <0.005 ppm) in any tissue analysed (Claborn, 1970).
3 No 3,5,6-trichloro-2-pyridinol found in fat or muscle tissues
(0 - <0.05 ppm) (Dishburger and McKellar, 1971).
4 Dipping schedule was at 21 day intervals.
The oxygen analogue was found only in samples of fat containing the
higher residues of chlorpyrifos (approximately 2 ppm); it amounted to
about 1% of the residue. No increase occurred in the amount of the
oxygen analogue as the chlorpyrifos was eliminated, and no residues of
the analogue were detected in the muscle, brain or spleen tissues of
the animals that had the highest residues of chlorpyrifos in the fat
(Claborn et al., 1968a).
Data from Mann (1966), Claborn et al. (1968a) and Dishburger
et al. (1966a,b) indicated that the biological half-life of
chlorpyrifos in tissues of cattle following a single treatment with
DURSBAN insecticide containing 0.025% and 0.05% chlorpyrifos is
approximately 4 - 6 days. Data from Mann (1966) and Claborn et al.
(1968a,b) showing multiple dippings in DURSBAN insecticide containing
0.025% and 0.05% chlorpyrifos, further establish the consistency of
the degradation ratio following the last treatment. The data of
Claborn et al. (1968b) indicate that the biological half-life of
chlorpyrifos in tissues of cattle treated at three-week intervals for
as long as six weeks with DURSBAN 24E containing 0.025% chlorpyrifos
is approximately seven days. In summary, the experiments illustrate
the consistency of a 4 - 7 day biological half-life for chlorpyrifos
in fat.
Considerable work has been carried out in Australia on the
determination of residues from the vat dip and spray application of
chlorpyrifos to cattle (Hall, 1969; Snelson, 1969b). Results shown in
table 4 indicate that residues of chlorpyrifos in omental fat are in
the same order of magnitude as those for United States, considering
the fact that most tests were at double the concentration (0.05%) in
Australia.
Snelson (1969a) studied the disappearance rate of chlorpyrifos in
butterfat in milk after a single dip application of chlorpyrifos at
0.025% to milk cows. Residue declined from 1.1 ppm after 1 day to 0.01
ppm in 9 days, and a non-detectable level at 15 days (see Table 5).
Sheep
Sheep dipped in an 0.05% chlorpyrifos emulsion showed inconsistent
levels of residues of chlorpyrifos with no apparent change in residue
levels (0.081 - 0.179 ppm) from 3 - 28 days after application. No
detectable levels (<0.005 ppm) were seen in muscle, liver or kidney
tissues at any time (Dow Chemical Co., 1967).
TABLE 4 Chlorpyrifos residues in cattle in Australia
Chlorpyrifos
Days residues in
Chlorpyrifos Application between last No. of omental fat Reference
% type no. treatment and cattle (mean values
slaughter or range, ppm)
0.025 Vat dip 3 1 0.2 Watts, 1968
4 0.96
7 0.25
1 1
3 0.11 - 0.22 Ibid.
1 1,4,8,15 0.29 - 2.18 Ibid.
3,6 1,4,8,15 0.05 - 1.19 Ibid.
0.05 Vat dip 1 1,4,8 0.41 - 0.48 Ibid.
3 1 1.17 Ibid.
4 0.98
6 1 1.75 Ibid.
8 1.36
15 1.11
22 0.6
29 0.38
0.05 Vat dip 2 0 3 1.46 - 2.26 Hall, 1969
1 2 3 2.4 - 3.87
1 2 3 1.23 - 3.09
1 2 3 0.98 - 2.54
1 1 3 0.42 - 0.79
3 1 4 1.32 - 3.80
0.05 Dip 10 5? 12 0.70 - 7.131 Snelson, 1969b
0.05 Spray 30 1 3 1.6 - 4.2 Ibid.
5 3 0.34 - 1.1
9 2 0.1 - 0.15
13 1 0.06
28 1 0.01
1 Omental and/or perirenal fat.
TABLE 5 Chlorpyrifos residues in milk of cows in Australia
Days between Chlorpyrifos1
last treatment residue in butterfat
and sampling (ppm avg. 4 cows) Reference
1 1.1 Snelson, 1969a
2 0.96
3 0.57
4 0.14
5 0.08
6 0.05
9 0.01
15 N.D.2
1 One dip application containing 0.025% chlorpyrifos.
2 N.D. = not detectable
Turkeys and chickens
Claborn et al. (1970b) determined residues of chlorpyrifos in the
body tissues of turkeys confined in pens containing soil treated with
4 or 8 lb/acre of chlorpyrifos (in a 25% wettable powder formulation)
for control of the chigger, Neoschongastia americana. The higher
concentration caused maximum residues of 0.157 and 0.066 ppm of
chlorpyrifos in the skin and fat, respectively, one week
post-treatment, that decreased to less than 0.001 ppm after six weeks.
No significant residues were found in the other tissues (thigh,
breast, liver). The oxygen analogue of chlorpyrifos (diethyl
3,5,6-trichloro-2-pyridyl phosphate) was not detected (<0.005 ppm) in
any tissues.
In a study conducted by Mann and Ivey (1971), turkeys were maintained
on soils treated two times, at an interval of 28 days, with 4 lb
chlorpyrifos/acre each time. Two or three birds were necropsied at
weekly intervals for six weeks after the first application and for
eight weeks after the second application. Tissue samples of fat, skin,
muscle, liver and kidney were analysed for residues of chlorpyrifos,
the oxygen analogue and the pyridinol moiety
(3,5,6-trichloro-2-pyridinol). Lowlevel residues of chlorpyrifos were
found in fat (0.022 - 0.005 ppm), skin (0.033 - 0.152 ppm) and kidney
(<0.002 - 0.005 ppm) of birds after one week of exposure to the
first application of compound to the soil. Fat only was analysed for
the remainder of the test for each application. The residues in fat
were reduced to <0.002 ppm (the sensitivity of the method) six weeks
after the first application and eight weeks after the second
application. No oxygen analogue was detected in any of the tissues.
Small residues of the pyridinol moiety were found in the skin of one
hen (<0.003 - 0.007 ppm), the liver of one tom (<0.01 - 0.025 ppm)
and the kidney of one tom (<0.01 - 0.098 ppm) after one week of
exposure to the first application; and in the skin (<0.003 - 0.003
ppm), liver (<0.01 - 0.078 ppm) and kidney (<0.01 - 0.165 ppm) in
one hen after one week of exposure to the second application of
compound to the soil.
Hunt et al. (1969) developed a new rapid gas chromatographic method
for analyses of chlorpyrifos in turkey and chicken tissues. The
residues reported are from the same experiments as the toxicological
results reported by Schlinke (1970) in chickens and Schlinke et al.
(1969) in turkeys. The tests consisted of dietary and contact tests
with chlorpyrifos for four weeks. Residue analyses of muscle, liver,
heart, brain, gizzard, skin and fat tissues were taken after 28 days
of treatment. Only skin and fat tissues showed detectable amounts of
chlorpyrifos. In chickens fed 100, 50 and 25 ppm in the diet, the
highest tissue residue was 0.87 ppm chlorpyrifos in fat. In turkeys
fed 100 and 50 ppm in the diet, the highest residue was 0.34 in fat.
Dishburger et al. (1969b) determined the presence of chlorpyrifos
residues in skin, fat, muscle and liver tissues of turkeys which had
been confined to soil treated with 4.5 lb chlorpyrifos/acre as
emulsifiable, granular, or wettable powder formulations. Muscle and
liver tissues contained undetectable amounts of chlorpyrifos in all
tests. Skin and fat tissues contained <0.1 ppm at all times, but were
higher at seven days post-treatment than any of the other weekly
samplings up through 28 days post-treatment.
In plants
Residues found in experiments with plants in United States
Chlorpyrifos has been applied to various species of plants and
generally found to be lacking in residual insecticidal properties on
plant-feeding insects. The residue determinations reported here
actually never exceeded 28 ppm at the dosages tested (up to 1 lb/acre)
and soon diminished, exhibiting a half-life of 1 - 2 days, thus
confirming the reason for the observed lack of residual effectiveness
on plant-feeding insects (see Table 6).
There was no evidence of residue buildup on plants from repeated
application of chlorpyrifos.
TABLE 6 Chlorpyrifos residues found in experiments in plants in United States
Dosage Application Chlorpyrifos
chlorpyrifos (no.) (no. of days Crop residues Reference
(lb/A) since last) (ppm)
1.0 1 0 Bermuda grass 13.2 Leuck et al., 1968
14 0.37
1.0 1 0 Maize 5.60 Ibid.
14 0.42
0.05 8 0 Pasture grass 0.7 - 8.2 Winterlin et al.,
7 <0.1 - 0.2 1968
0.05 1 0 Grass 0.11 - 8.1 Ibid.
3 0.012 - 1.8
0.025 1 0 Rice 0.09 - 0.64 Ibid.
6-14 <0.005 - 0.07
1.0 1 0 Various wild 26.9 Hurlbert et al.,
1 plants 27.4 1970
7 1.1
14 0.1
0.025 4 0 Rice 0.07 - 0.21 Dishburger et al.,
9 <0.01 1969c.
1.0 1 0 Maize silage 15.1 Johnson et al., 1969
1 4.4
0.25 1 0 Maize silage 2.5 Ibid.
1 1.1
Leuck et al. (1968) studied the persistence of chlorpyrifos and its
oxygen analogue on field plots of coastal Bermuda grass and maize
forage using the method of Bowman and Beroza (1967a) with a minimum
sensitivity of 0.002 - 0.01 ppm. Chlorpyrifos was applied at the rate
of 1 lb/acre. The oxygen analogue constituted a very low residue and
was only a small proportion of the total insecticide. Chlorpyrifos
residues varied from about 6 - 13 ppm at the time of application to 4
- 8 ppm after 1 day, to around 1 ppm after 7 days and to nearly
negligible levels after 21 days. The residue half-life of chlorpyrifos
appeared to be in the order of 2 - 3 days on Bermuda grass and maize
plants.
Winterlin et al. (1968) studied the residues of chlorpyrifos on rice
and pasture grass and environment when applied experimentally for the
control of mosquitoes around Colusa, California. They used the method
of Bowman and Beroza (1967a) i.e. gas chromatography with a flame
ionization detector, which is sensitive to 0.005 ppm chlorpyrifos in
rice and 0.01 ppm in pasture grass, and found residues as high as 8.11
ppm chlorpyrifos immediately after application of 0.05 lb
chlorpyrifos/acre. Residues decreased rapidly after application to
below 1 ppm.
Myers et al. (1968) reported on residues resulting from application
of chlorpyrifos sprayed by aircraft at the rate of 0.1 lb/acre, eight
times, over a period of five months on irrigated pastures. Samples of
grass analysed for the insecticide 1 - 3 hours after each spray
application varied from 0.4 - 8.2 ppm, and after seven days varied
from <0.1 - 0.2 ppm, using an analytical method sensitive to 0.1 ppm
chlorpyrifos. No evidence of accumulated residues of chlorpyrifos were
detected as the result of multiple applications to grass with
chlorpyrifos.
Hurlbert et al. (1970) reported on the residues of chlorpyrifos on
watergrass, sprangletop and smartweed on the dikes surrounding ponds,
resulting from hand-sprayed applications of chlorpyrifos emulsifiable
insecticide. Dosages used were 1, 0.1, 0.05, and 0.01 lb/acre diluted
in a constant spray volume of 8 gal/acre. Under these conditions
residues over 1 ppm occurred only at 1 lb/acre after 4 h, 1 day, and 7
days, but not 14 days; and at 0.1 lb/acre after 4 h. Residues
determined at other dosages and post-application times were less than
1 ppm. The half-life of the foliar residues of chlorpyrifos was in the
order of 1 - 3 days.
Dosages of 0.025 lb chlorpyrifos were applied aerially four times, at
monthly intervals, to rice fields. Residues of chlorpyrifos in raw
(rough) rice grain ranged from 0.07 to 0.21 ppm at 0 day, following
the last of the four applications. No residues of the oxygen analogue
of chlorpyrifos were detected, nor did detectable chlorpyrifos
residues persist more than 9 days after application (Dishburger
et al., 1969c).
Johnson et al. (1969) treated maize plants in the field with 0.25,
0.5 and 1.0 lb chlorpyrifos/acre. The initial residues in maize and
silage were 2.5, 3.9 and 15.1 ppm, respectively (on an as-collected
basis). After one day the losses of residues were 56, 40 and 71%,
respectively. This represents a half-life of about one day. One day
after treatment the maize and silage were enclosed in a silage bin
where chlorpyrifos residues declined slowly over a period of seven
weeks.
Dishburger et al. (1969a) analysed samples of tobacco leaves from
soil and foliage treated with 0.5, 1.0 and 1.5 lb chlorpyrifos/acre.
No residues (<0.01 ppm) were detected on the samples harvested (2
months or more post-application).
Residues found in experiments with plants in Canada
Residue data from experiments with onions, lettuce, carrots, and
tobacco are shown in Tables 7 and 8 using the analytical method of
Braun (1971) for chlorpyrifos and McKellar (1971c) for the pyridinol.
TABLE 7 Residues of chlorpyrifos from treatments of lettuce,
onions and carrots1
Application Residues on crop (ppm)
(lb/acre) (No.)2 lettuce onions carrots3
0.5 2 <0.001 0.001 <0.001
0.005 0.003 0.003
(54)4 (119) (138)
3 0.001 0.002 0.012
0.020 0.003 0.020
(31) (104) (123)
1.0 2 <0.001 0.002 0.012
0.008 0.005 0.034
(54) (118) (138)
3 0.002 0.002 0.006
0.009 0.005 0.018
(31) (104) (123)
2.0 2 0.003 0.008 0.015
0.029 0.013 0 046
(54) (119) (138)
2.0 3 0.008 0.003 0.019
0.034 0.005 0.033
(31) (104) (123)
1 McEwen and Frank, 1970.
2 First application was preplant soil treatment; second and third
soil and foliar treatments (Canada).
3 3,5,6-trichloro-2-pyridinol analysed for but not found at
detection limit of 0.005 ppm.
4 Days after chlorpyrifos withdrawal shown in ().
TABLE 8 Residues of chlorpyrifos from treatments of lettuce, onions,
carrots and celery
Withdrawal Applications1 Residues range
Crop (days) (no.) (ppm) References
Onions 57 1 0.001 - 0.003 McEwen, 1970
19 4 <0.0012 Harris, 1971
Celery 57 1 N.D.3 - 0.016 McEwen, 1970
Carrots 57 1 0.001 - 0.015 Ibid.
Lettuce 27 1 N.D. Ibid.
1 Treatments all post-planting, 2 lb/acre (Canada).
2 No oxygen analogue of chlorpyrifos detected at 0.01 ppm sensitivity
level.
3 N.D. = <0.001 ppm.
Residues found in experiments with plants in Europe and the Near
East
Residue data from experiments with cauliflower, red cabbage, eggplant,
beans, peppers, lettuce, mushrooms, carrots, potatoes, sugar beet,
corn, cotton, tomatoes, grapes, pears and apples are shown in Tables 9
and 10. These tests include soil and foliar applications.
Residues found in experiments with plants in Japan, Thailand,
Malaysia, and the Philippines
Residue data from experiments with Chinese cabbage and kale, rice and
apples are shown in Tables 11, 12 and 13.
TABLE 9 Residues of Chlorpyrifos in Food Crops, Europe/Near East Trials
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
FRANCE
Potatoes 1970 Granules Soil 1 1 500 g/ha 5 mo <0.005 Graf and Engeler,
5% active insecticide 1 3 500 g/ha 5 mo <0.005 1971a
W. GERMANY
Sugar beets 1971 Dursban EC Broadcast 1 1.15 kg/ha 112 d <0.13 Scheuerer & Hahn,
40.8% active Soil insect. 118 d 1972
Maize 1971 Dursban 4 EC Soil insect. 1 0.95 kg/ha 123 d <0.13 Ibid.
40.8% active Broadcast
HOLLAND
Cauliflower Strewpowder Soil insect. 1 120 mg 8 wk 0.002
Red cabbage (1) 1969 4% active 11 wk <0.002 Wit, 1970
Red cabbage (2) 85 wk <0.002
Lettuce (under 1970 3.2% active Soil insect. 1 1 900 g/ha 9 wk <0.01 Wit, 1971
glass) granule
Mushrooms 1971 25% wettable Spray 3 5 000 g/ha 3 wk 0.01 Olthof, 1971a
powder 3 wk 0.03
Carrots 1971 Dursban 25W Soil insect. 1 6 000 g/ha 3-4 mo 0.14 Olthof, 1971b
1 6 000 g/ha 3-4 mo 0.28
ISRAEL
Cotton 1970 Dursban E.C. Spray 6 730 g/ha 15 d <0.05 Graf, 1970a
40.8% active
Apples 1970 Dursban E.C. Spray 1 2 400 g/ha 7 d 0.54 Graf &
40% active 14 d 0.20 Engeler, 1970a
Tomatoes 1970 Dursban E.C. Spray 1 720 g/ha 1 d 0.24 Aharonson
40.8% active 4 d 0.16 et al., 1969
8 d 0.12
16 d 0.04
1 1 440 g/ha 1 d 0.45
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
4 d -
8 d 0.18
16 d 0.10
1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.27 Graf & Engeler,
40.8% active 5 d 0.25 1971b
Apples 1971 Dursban E.C. Spray 1 1 440 g/ha 1 d 0.57 Graf & Engeler,
40.8% active 7 d 0.23 1971c
14 d 0.14
Spray 1 2 880 g/ha 1 d 0.52
7 d 0.28
14 d 0.24
Dursban Spray 1 1 440 g/ha 1 d 0.13
40.8% active 7 d 0.11
Spray 1 2 880 g/ha 1 d 0.29 Ibid.
7 d 0.24
ITALY
Sugar beets 1970 Dursban 5% Soil insect. 1 2 500 g/ha 3-6 mo <0.004 Foschi, 1971
Maize 1970 Granule Soil insect. 1 2 500 g/ha 3-6 mo <0.004
Lettuce 1971 Dursban E.C. Spray 1 500 g/ha 14 d 0.05 SIAPA, 1971
22.5% 1 1 000 g/ha 14 d 0.07
Apples 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.16 Graf, 1970b
40.8% active 29 d 0.10
1 1 224 g/ha 15 d 0.25
29 d 0.16
Pears 1970 Dursban E.C. Spray 1 816 g/ha 15 d 0.22 Graf, 1970c
40.8% active 29 d 0.14
1 1 224 g/ha 15 d 0.22
29 d 0.27
TABLE 9 (Cont'd.)
COUNTRY YEAR FORMULATION APPLICATION DOSAGE1 INTERVAL2 RESIDUES REFERENCES
and CROP TYPE NO. (ppm)
Turkey
Eggplant 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 0.26-0.40 Hollick &
E.C. 7 d 0.02-0.03 Collison, 1972
14 d 0.02-0.02
Beans 1972 Dursban 4 Spray 2 0.96 kg/ha 0 d 0.65-1.20 Ibid.
E.C. 7 d 0.08
14 d <0.04
Tomatoes 1970 Dursban E.C. Spray 1 347 g/ha 3 h 1.3 Graf & Engeler,
40.8% active 5 d 0.26 1970b
Peppers (under 1972 Dursban 4 Spray 3 0.96 kg/ha 0 d 2.00-5.20 Hollick &
glass) E.C. 7 d 0.04-0.10 Collison, 1972
14 d 0.13-0.25
UNITED KINGDOM
Raspberries 1971 Dursban E.C. Spray 1 0.1% 0 d 1.40 Burrows et al.,
40.8% active 5 d 0.32 1971d
2 0.5% 10 d 0.14
18 d 0.18
Tomatoes 1971 Dursban E.C. Spray 1 0 d 1.26 Burrows et al.,
40.8% active 3 d 0.10 1971e
7 d 0.08
14 d 0.01
28 d 0.01
1 All dosages given in a.i.
2 Last application to harvest
3 Limit of detection
TABLE 10 RESIDUES OF CELORPYRIPOS AND PYRIDINOL IN FOOD CROPS, EUROPE/NEAR EAST TRIALS
CROP, SAMPLE DOSAGE1 TIME BETWEEN RESIDUES RESIDUES REFERENCES
COUNTRY NO. LAST TREATMENT CHLORPYRIFOS 3,5,6-TRICHLORO-2-PYRIDINOL (ppm)
& YEAR AND HARVEST (ppm)
TURKEY
1971
Tomatoes 1 A 825 g/ha 3 hours 1.3* <0.05
2 A " 3 hours <0.05
4 A " 3 hours 0.05 Barrows & Mullin,
3 B " 5 days 0.05 1971b
5 B " 5 days 0.26 <0.05
6 B " 5 days <0.05
ENGLAND
1971
Tomatoes 720 g/ha 0 day 1.28 0.14
1.24 0.12
3 days 0.14 0.10
0.05 0.10 Burrows et al
7 days 0.08 0.08 1971e
0.08 0.08
14 days 0.01 0.12
0.01 0.10
28 days <0.01 0.10
<0.01 0.16
ITALY 1971
Apples 1 816 g/ha 29 days 0.10 <0.05 Burrows & Mullin,
4 1 224 g/ha 15 days 0.25 <0.05 1971b
Grapes 11 816 g/ha 29 days 0.10 <0.05
13 816 g/ha 15 days 1.10 0.06 Ibid.
12 1 224 g/ha 29 days 0.22 <0.05
14 1 224 g/ha 15 days 1.50 0.29
* Composite samples
1 All dosages given in a.i.
TABLE 11 CHLORPYRIPHOS RESIDUES ON CROPS IN JAPAN1
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
Chinese
cabbage
1967 40E 267 1 4 0.13-0.23
1100 l/ha
1969 40E 267 2 8 0.02-0.09
1000 l/ha 2 16 0.43-0.44
" 267 4 8 0.16-0.22
4 16 0.62-0.86
1969 40E 267 2 7 0.02-0.06
1500 l/ha 2 14 0.06-0.08
" 267 4 7 0.08-0.14
4 14 0.06-0.12
Apples
Rall's Janet
1967 25W 250 4 66 N.D.3
Johnathan
1967 25W 250 5 42 0.01-0.05
1969 25W 250 3 30 0.05-0.07
30 l/tree 45 0.05-0.06
1969 " 250 2 17 0.03-0.07
28 0.02-0.05
46 0.02-0.03
TABLE 11 (Cont'd.)
CROP FORMULATION CHLORPYRIFOS APPLIC. DAYS BETWEEN RESIDUE2
& and DILUTION IN SPRAY (no.) LAST SPRAY (ppm)
YEAR (ppm) AND HARVEST
25W 250 3 7 0.03-0.09
30 l/tree
14 0.05-0.17
31 0.08-0.16
45 0.03-0.05
Aomori
1969 25W 250 2 7 0.10
14 0.12-0.14
21 0.08-0.03
1 Hamaguchi, 1970
2 Range of averages for two laboratories
3 N.D. = not detectable (<0.005 ppm)
TABLE 12 CHLORPYRIFOS RESIDUES ON CROPS IN THAILAND AND MALAYSIA
CROP FORMULATION APPLIC. DAYS BETWEEN TESTS RESIDUE1 REFERENCES
& DILUTION (no.) LAST SPRAY (no.) (ppm)
& HARVEST
Chinese 25W 6 5 6 N. D.2 - 0.023 Tucker & Zielinski,
cabbage 1972a
Chinese 187 ppm(a.i.) " " " 0.10 - 0.243 Ibid., 1972b
kale " " " " 0.09 - 0.763
Chinese 25W " " " N.D.3 Ibid., 1972a
cabbage
Chinese 375 ppm(a.i.) " " " 0.38 - 0.753 Ibid., 1972b
kale " " " " 0.35 - 0.763
Chinese 25W 16 2 3 0.07 - 0.104 Ibid., 1972,
kale
3 oz a.i./A " " " 0.01 - 0.35
25W " " " 0.13 - 0.244
4 oz a.i./A " " " 0.01 - 0.035
Tobacco 25W 10 7 4 0.03 - 0.053 Ibid., 1972d
187 ppm (a.i.)
25W " " " 0.21 - 0.383
375 ppm (a.i.)
1 Two analytical methods used in analyses
2 N.D. = not detectable (<0.01 ppm)
3 "Usable leaves" from Thailand
4 Leaf only from Malaysia
5 Stem only from Malaysia
TABLE 13 Chlorpyrifos residues on rice in the Philippines1
Chlorpyrifos Residue
applied (kg/ha, Tests (range of
Part analysed Formulation 3 applications) (no.) averages,
ppm)
Grain E.C. 0.10 4 N.D.3
0.15 4 N.D. - 0.008
0.20 3 N.D. - 0.005
0.25 2 0.008
Granule 0.10 3 N.D.
Grain and E.C. 0.10 4 N.D. - 0.007
straw2 0.15 4 N.D. - 0.006
0.20 4 0.005 - 0.008
0.25 2 0.008
Granule 0.10 3 <0.005
1 Tucker, 1971.
2 Very little straw, mostly grain.
3 N.D = not detectable (<0.005 ppm)
FATE OF RESIDUES
General comments
The fate of chlorpyrifos residues and its metabolites in different
species of animals are discussed in the section on "Biochemical
aspects".
In animals
Cow
Chlorpyrifos was not found in the urine and milk from a cow fed 5 ppm
(based on a daily ration of 50 lb) of the compound for four days. The
compound in absolute ethanol was mixed thoroughly with the grain. A
compound characterized by retention time as chlorpyrifos was found in
faecal samples taken on the last three days during which the
insecticide was fed and the first day after, and represented 1.7% of
the insecticide fed. Two metabolites were excreted in the urine which,
following methylation, had retention times identical to the methyl
esters of diethylthiophosphate and diethyl phosphate. They represented
35.9 and 26.8%, respectively, of the total insecticide fed on an
equivalent basis (Gutenmann et al., 1968).
Fish
Waters treated with 50 and 300 ppb of ring-labelled 14C chlorpyrifos
and containing goldfish were analysed for the types of metabolites
found in each. Metabolites I, III, IIIa and IIIb, shown in Figure 1,
as well as chlorpyrifos were found in fish, and metabolites I, IIIa
and IIIb were found in the water (Smith et al., 1966).
The major metabolic product in fish is 3,5,6-trichloro-2-pyridinol
which appears to be slowly broken down by dehalogenation and cleavage
of the ring (Smith et al., 1966).
Some of the derivatives of 3,5,6-trichloro-2-pyridinol may be those
shown in Smith (1968) which consist of dechlorination forming a series
of transient pyridine diols, triols and tetrols (Ia), followed by a
series of transient ring diketones (Ib), followed by a breakage of the
ring to elemental fragments (carbon dioxide and possibly aliphatic
amines).
Cholinesterase inhibition properties of metabolites
The house fly head cholinesterase activity of chlorpyrifos, and
metabolites I, II, III, IIIa and IIIb shown in Figure 1, were tested
for comparative purposes by Smith et al. 1966). Only the phosphate
derivative of chlorpyrifos (metabolite III) shows significant
inhibition and is the compound most likely to be responsible for the
cholinesterase inhibition found in mammals and other animal organisms
(Smith et al., 1966).
In plants
Studies on the absorption, translocation and metabolism of 14C and
36Cl labelled chlorpyrifos in plants show that for all practical
purposes uptake of the chemical or its degradation products into
plants does not occur, either through the foliage or through the roots
(Smith et al., 1967a, 1967c).
When radioactive chlorpyrifos was applied to single bean or corn
leaves, about 75 to 80% of the chemical was lost by vapourization from
the leaf surface within the first 48 h following treatment. The
chlorpyrifos remaining on the leaf was rapidly degraded (>95% in 5
days) and a trace of the resulting breakdown products (representing
<2% of the applied dose of chlorpyrifos) absorbed into and
translocated within the plant. The identity of the degradation
products was not totally elucidated; however, a variety of hydrolysis
products appeared to be formed, mainly the 3,5,6-trichloro-2-pyridinol
(I), ethyl-3,5,6-trichloro-2-pyridyl phoshate (IIIa) and
3,5,6-trichloro-2-pyridyl phosphate (IIIb) shown in figure 1 (Smith
et al., 1967a).
 Nutrient culture experiments have shown uptake of chlorpyrifos by
plant roots to be essentially nil. Bean plants grown in nutrient
solution containing 10 mg (50 ppm) of 14C chlorpyrifos absorbed and
translocated to the plant tops less than 0.07% of the applied dose
during a 144 h exposure period. Based on the weight of plant tissue
analysed, this represented a residue <0.002 ppm of radioactive
compounds (expressed as chlorpyrifos). In this same experiment,
sizable amounts of chlorpyrifos were sorbed on the outside of the
plant roots, reaching 5 mg (50%) in 72 h. The negligible amounts of
radioactivity taken into the plants in this and other tests have made
it difficult to determine the nature of the compounds involved. Only
the 3,5,6-trichloro-2-pyridonol was identified. This compound, the
principal hydrolysis product of chlorpyrifos in soil, may or may not
be taken up by the plant depending on pH of the soil medium. The free
pyridinol, which is the predominant form of the compound below pH 6.0,
is essentially insoluble in water. Soil and nutrient culture
experiments have shown its uptake by plants to be insignificant. For
example, bean plants grown in nutrient solution (pH 5.5) containing 10
mg (50 ppm) of 36Cl labelled 3,5,6-trichloro-2-pyridinol showed about
0.007 ppm of radioactive compounds (expressed as the pyridinol) in the
plant tops after 72 h exposure. At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the Na salt) as well as rate of absorption by the plant
are enhanced relative to the free pyridinol. Regardless, soil and
nutrient culture tests have again shown only negligible amounts of the
compound to enter the plant and these undergo metabolism with the
liberation of chloride and the formation of trivial amounts of several
unidentified water soluble decomposition products (Smith et al.,
1967c).
Only through the use of specialized techniques has it been possible to
introduce sufficient chlorpyrifos into plants to determine the extent
to which the compound might be metabolized. Using the string
technique, 14C chlorpyrifos was introduced into the stems of
cranberry bean plants and the plants harvested at various time
intervals. Subsequent analysis of the plant tissues revealed the
presence principally of chlorpyrifos and its primary hydrolysis
products. Under these artificial conditions, chlorpyrifos once
introduced inside the stem, was distributed throughout the plant
(Smith et al., 1967c).
METHODS OF RESIDUE ANALYSIS
Chlorpyrifos, its oxygen analogue and hydrolytic pyridinol
Based on previously mentioned metabolite determinations, the main
residues from chlorpyrifos treatments searched for in various tissues
are chlorpyrifos, its oxygen analogue and the hydrolysed pyridinol.
Since the oxygen analogue is infrequently encountered in animal or
plant tissues, the principal analyses of residues are for chlorpyrifos
and 3,5,6-trichloro-2-pyridinol. The latter appears in small amounts,
mostly in non-fatty tissues. Analytical methods are available to
determine each of these two residues individually. However, for
regulatory purposes, chlorpyrifos is the most important residue to be
determined.
Claborn et al. (1968a,b) and Ivey and Claborn (1968) described the
basic extraction and cleanup procedures using an electron capture gas
chromatography method for detection of chlorpyrifos and its oxygen
analogue in milk and body tissues of cattle. Claborn et al. (1970b)
used a gas chromatography method and a flame photometric detector to
determine chlorpyrifos and its oxygen analogue in body tissues of
turkeys. Since the phosphate analogue is not searched for routinely, a
quicker method for determination of chlorpyrifos only is useful. In
order to do this a modification of the flame photometric gas
chromatography method of Claborn et al. (1970a,b) using a hexane
extraction for fat tissues was used instead of a
hexane-dichloromethane extraction. The method is sensitive to 0.01
ppm.
Further minor variations in the analytical method are related to
extraction and cleanup procedures, depending on the nature of the
substrate. Three analytical procedures are generally suitable for the
various substrates when the substrates are defined as (A) water; (B)
wet tissues and soil; and (C) dry or fatty tissues. The procedural
scheme for methods A, B and C is outlined in Figure 2.
More detailed procedures are specifically outlined as follows: Only
those asterisked methods accounted for the oxygen analogue. Procedure
for substrate (A), water (Rice and Dishburger, 1968; Wetters and
Dishburger, 1971c). Procedure for substrate (B), wet media, such as
sweet corn (Wetters, 1972c), corn forage (Wetters and Dishburger,
1971b), soil (Wetters, 1971a*,b), vegetables (Braun, 1971), liver,
kidney and muscle of pig (Wetters, 1972a; Bowman and Beroza, 1967b),
chicken muscle, liver, kidney and egg (Wetters, 1972b; Bowman and
Beroza, 1967b), peach (Wetters and Dishburger, 1971a*), milk (Craig,
1971), cattle tissues except fat (Claborn et al., 1968a), turkey
tissues except fat (Claborn et al., 1970b). Procedure for substrate
(C), dry or fatty media, such as corn grain and stover (Wetters and
Dishburger, 1971b; Wetters, 1972c), pig fat (Wetters, 1972a), chicken
fat (Wetters, 1972b; Bowman and Beroza, 1967a), cream (Graig, 1971),
cattle fat (Claborn et al., 1968a,b; Bowman and Beroza, 1967a),
turkey fat (Claborn et al., 1970b). Many other references on
chlorpyrifos in various media have been published (see References).
Nutrient culture experiments have shown uptake of chlorpyrifos by
plant roots to be essentially nil. Bean plants grown in nutrient
solution containing 10 mg (50 ppm) of 14C chlorpyrifos absorbed and
translocated to the plant tops less than 0.07% of the applied dose
during a 144 h exposure period. Based on the weight of plant tissue
analysed, this represented a residue <0.002 ppm of radioactive
compounds (expressed as chlorpyrifos). In this same experiment,
sizable amounts of chlorpyrifos were sorbed on the outside of the
plant roots, reaching 5 mg (50%) in 72 h. The negligible amounts of
radioactivity taken into the plants in this and other tests have made
it difficult to determine the nature of the compounds involved. Only
the 3,5,6-trichloro-2-pyridonol was identified. This compound, the
principal hydrolysis product of chlorpyrifos in soil, may or may not
be taken up by the plant depending on pH of the soil medium. The free
pyridinol, which is the predominant form of the compound below pH 6.0,
is essentially insoluble in water. Soil and nutrient culture
experiments have shown its uptake by plants to be insignificant. For
example, bean plants grown in nutrient solution (pH 5.5) containing 10
mg (50 ppm) of 36Cl labelled 3,5,6-trichloro-2-pyridinol showed about
0.007 ppm of radioactive compounds (expressed as the pyridinol) in the
plant tops after 72 h exposure. At or above pH 7, the pyridinol is
readily converted to a salt in which form water solubility (4.4 g/100
ml at 25°C for the Na salt) as well as rate of absorption by the plant
are enhanced relative to the free pyridinol. Regardless, soil and
nutrient culture tests have again shown only negligible amounts of the
compound to enter the plant and these undergo metabolism with the
liberation of chloride and the formation of trivial amounts of several
unidentified water soluble decomposition products (Smith et al.,
1967c).
Only through the use of specialized techniques has it been possible to
introduce sufficient chlorpyrifos into plants to determine the extent
to which the compound might be metabolized. Using the string
technique, 14C chlorpyrifos was introduced into the stems of
cranberry bean plants and the plants harvested at various time
intervals. Subsequent analysis of the plant tissues revealed the
presence principally of chlorpyrifos and its primary hydrolysis
products. Under these artificial conditions, chlorpyrifos once
introduced inside the stem, was distributed throughout the plant
(Smith et al., 1967c).
METHODS OF RESIDUE ANALYSIS
Chlorpyrifos, its oxygen analogue and hydrolytic pyridinol
Based on previously mentioned metabolite determinations, the main
residues from chlorpyrifos treatments searched for in various tissues
are chlorpyrifos, its oxygen analogue and the hydrolysed pyridinol.
Since the oxygen analogue is infrequently encountered in animal or
plant tissues, the principal analyses of residues are for chlorpyrifos
and 3,5,6-trichloro-2-pyridinol. The latter appears in small amounts,
mostly in non-fatty tissues. Analytical methods are available to
determine each of these two residues individually. However, for
regulatory purposes, chlorpyrifos is the most important residue to be
determined.
Claborn et al. (1968a,b) and Ivey and Claborn (1968) described the
basic extraction and cleanup procedures using an electron capture gas
chromatography method for detection of chlorpyrifos and its oxygen
analogue in milk and body tissues of cattle. Claborn et al. (1970b)
used a gas chromatography method and a flame photometric detector to
determine chlorpyrifos and its oxygen analogue in body tissues of
turkeys. Since the phosphate analogue is not searched for routinely, a
quicker method for determination of chlorpyrifos only is useful. In
order to do this a modification of the flame photometric gas
chromatography method of Claborn et al. (1970a,b) using a hexane
extraction for fat tissues was used instead of a
hexane-dichloromethane extraction. The method is sensitive to 0.01
ppm.
Further minor variations in the analytical method are related to
extraction and cleanup procedures, depending on the nature of the
substrate. Three analytical procedures are generally suitable for the
various substrates when the substrates are defined as (A) water; (B)
wet tissues and soil; and (C) dry or fatty tissues. The procedural
scheme for methods A, B and C is outlined in Figure 2.
More detailed procedures are specifically outlined as follows: Only
those asterisked methods accounted for the oxygen analogue. Procedure
for substrate (A), water (Rice and Dishburger, 1968; Wetters and
Dishburger, 1971c). Procedure for substrate (B), wet media, such as
sweet corn (Wetters, 1972c), corn forage (Wetters and Dishburger,
1971b), soil (Wetters, 1971a*,b), vegetables (Braun, 1971), liver,
kidney and muscle of pig (Wetters, 1972a; Bowman and Beroza, 1967b),
chicken muscle, liver, kidney and egg (Wetters, 1972b; Bowman and
Beroza, 1967b), peach (Wetters and Dishburger, 1971a*), milk (Craig,
1971), cattle tissues except fat (Claborn et al., 1968a), turkey
tissues except fat (Claborn et al., 1970b). Procedure for substrate
(C), dry or fatty media, such as corn grain and stover (Wetters and
Dishburger, 1971b; Wetters, 1972c), pig fat (Wetters, 1972a), chicken
fat (Wetters, 1972b; Bowman and Beroza, 1967a), cream (Graig, 1971),
cattle fat (Claborn et al., 1968a,b; Bowman and Beroza, 1967a),
turkey fat (Claborn et al., 1970b). Many other references on
chlorpyrifos in various media have been published (see References).
 Residues of 3,5,6-trichloro-2-pyridinol may be determined by the
method of McKellar and Dishburger (1970) in bovine tissues; McKellar
(1971b) in corn grain, forage and stover; McKellar (1971c) in grass;
Dishburger et al. (1972) in chicken tissues and eggs and McKellar
(1971a) in milk and cream, at a detection level of 0.05 ppm.
The general cleanup procedures shown in Figure 2 followed by a gas
chromatographic determination utilizing one of the phosphorus specific
detectors would be suitable for most regulatory uses.
Examples of national tolerances as reported to meeting
Animals
Australia has a 2 ppm residue tolerance for chlorpyrifos in beef fat
when cattle are treated for ticks by dip or spray.
Plants
Canada has a tentative 0.1 ppm residue tolerance for head lettuce,
onions and carrots with 28, 60 and 60 days, respectively, required
between treatment and harvest.
APPRAISAL
Chlorpyrifos is a non-systemic organophosphorus insecticide used to
control ectoparasites of cattle, sheep and poultry; as a soil
insecticide for vegetables, cereals and tobacco; and as a foliar
insecticide for deciduous fruit, cereals, fodder crops, rape, cotton,
some vegetables, tobacco and rice.
Animal residues are found predominantly in the fat and are excreted
rapidly after cessation of treatment. When cattle were given multiple
dippings at the recommended dosage rates of 0.025%, the maximum total
residue in fat one week after the last dipping was 1.6 ppm. When milk
cows were dipped once in 0.025% chlorpyrifos, the residue in butterfat
fell to 0.01 ppm in nine days and was not detectable (<0.005 ppm)
after 15 days. When turkeys were confined over soil treated with
chlorpyrifos (4 or 8 lb/acre) for chigger control, maximum residues of
0.16 and 0.07 ppm were found in skin and fat, respectively, one week
post-treatment; the oxygen analogue was not detected in any tissues.
Small amounts of the pyridinol were found in the skin, livers and
kidneys of two birds with a maximum residue of 0.17 ppm in kidney
after one week exposure to the second application.
Extensive supervised experiments with foliar treatments on plants have
shown chlorpyrifos to have little persistence and virtually no uptake
occurs from either soil or foliar application. Up to 80% of applied
radioactive chlorpyrifos was lost by vapourization from single leaf
surfaces within the first 48 hours following treatment. There was no
evidence of residue buildup on plants from repeated applications.
The residues most likely to be found in either plants or animals are
chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol;
the oxygen analogue or other possible metabolites are very rarely
found.
Both specific and general gas chromatographic methods of analysis are
available which are suitable for regulatory purposes. The generalized
cleanup schemes shown in Figure 2 followed by gas chromatographic
determination using a phosphorus specific detector are recommended.
Separate analytical procedures for determining
3,5,6-trichloro-2-pyridinol in animal tissues (liver, kidney, poultry
skin), fruits and vegetables have been developed. The pyridinol is
excreted rapidly in the urine and faeces of animals and there would
appear to be no need to include this compound in regulatory analysis.
In field experiments with plants the pyridinol is rarely found and
when present constitutes only a fraction of the total residue.
Although chlorpyrifos is recommended in some countries for use on
rape, cereal grains and certain vegetables, there were no data
available on these commodities from supervised experiments and no
recommendations for tolerances could be made for these commodities.
RECOMMENDATIONS
TOLERANCES
ppm
Fat of meat of cattle 2
Chinese cabbage, grapes, kale, apples 1
Carrots, pears, tomatoes 0.5
Fat of meat of sheep, poultry, beans,
eggplants, peppers, raspberries 0.2
Lettuce, rice (in husk), sugarbeets 0.1
Celery, cottonseed, cottonseed oil
(crude), mushrooms, onions 0.05
Cauliflower, milk (fat basis),
potatoes, red cabbage 0.01*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials for rape seed, cereal grains and
any vegetables not listed.
DESIRABLE
1. Elucidation of possible increased sensitivity to plasma
cholinesterase depression after withdrawal from an initial dose
regime.
2. Further information on residues in milk and milk products arising
from dipping of dairy cattle.
REFERENCES
Aharonson, N., Grünberg, R. and Resnick, C. (1969) Dursban residue
level in tomatoes and cotton. Ministry of Agriculture, Jaffa, Israel.
Beatty, S.C. and McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Report The Dow Chemical Co., revision.
(unpublished)
Blackmore, R.H. (1968a) Oral administration-dogs-Dursban final report.
Hazleton Lab. Inc. (unpublished)
Blackmore, R.H. (1968b) Short-term (subacute) dietary
administration-rats-organophosphate insecticide Dursban - final
report. Hazleton Lab. Inc. (unpublished)
Bowman, M.C. and Beroza, M. (1967a) Determination of Dursban and its
oxygen analogue in corn and grass by gas chromatography with flame
photometric detection. J. Agr. Fd. Chem., 15(4): 651-653.
Bowman, M.C. and Beroza, M. (1967b) Temperature-programmed gas
chromatography of 20 phosphorus-containing insecticides on 4 different
columns and its application to the analysis of milk and corn silage.
J. Ass. off. analyt. Chem., 50(6): 1228-1236.
Branson, D.R. and Litchfield, N.H. (1971a) Absorption, excretion and
distribution of 3,5,6-trichloro-2,6-14C-2-pyridinol in rats. Report
NBA-10 Dow Chemical Company. (unpublished)
Branson, D.R. and Litchfield, N.H. (1971b) Absorption, excretion and
distribution of O,O-diethyl O-3,5,6-trichloro-2,6-14C-2-pyridyl
phosphorothioate (14C Dowco 179) in rats. Report NBA-9 Dow Chemical
Company. (unpublished)
Branson, D.R. and Wass, M.N. (1970) Comparative metabolism of
insecticides. I. Preliminary studies of ring labelled O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate breakdown with rat
liver microsomes. Report R-582 Dow Chemical Company. (unpublished)
Braun, H.E. (1971) Dursban residues in vegetables. Ontario Department
of Food and Agriculture, Guelph, Ontario. Personal communications to
T. Haagsma.
Brust, R.A., Miyazaki, S. and Hodgson, G.C. (1971) Effect of Dursban
in the drinking water of chicks. J. Econ. Entomol., 64(5): 1179-1183.
Burrows, I.E., Fraser, W.D., Joyce, C.A., Jenkins, G. and Eden, A.
(1971a) Determination of decay curves for Dursban and its metabolite
in stored tomatoes. 4211/71/369. Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971b) Determination of residues of
Dursban metabolite in tomatoes, grapes and apples. 4109/71/267.
Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971c) Determination of residues of
Dursban in lettuce. 4140/71/298. Huntingdon Research Centre.
Burrows, I.E., Mullin, L.W. and Eden, A. (1971d) Determination of
residues of Dursban and its metabolites in raspberries. 4371/71/527.
Huntingdon Research Centre.
Burrows, I.E., Orme, J.P.R. and Ashton, J. (1971e) Determination of
residues of Dursban and its metabolite in tomatoes. 4496/71/652.
Huntingdon Research Centre.
Claborn, H.V., Hoffman, R.A., Mann, H.D. and Oehler, D.D. (1968a)
Residues of Dursban and its oxygen analogue in the body tissues of
treated cattle. J. Econ. Entomol., 61(4): 983-986.
Claborn, H.V., Mann, H.D. and Oehler, D.D. (1968b) Dursban(R)
determination in milk and body tissues of cattle. J. Ass. off. analyt.
Chem., 51(6): 1243-1245.
Claborn, H.V., Ivey, M.C., Mann, H.D. and Oehler, D.D. (1970a) Report
of residue analysis - Omental fat, muscle, kidney and liver. Report
PCK-70-1. USDA-ARS-Entomol. Res. Div.
Claborn, H.V., Kunz, S.E. and Mann, H.D. (1970b) Residues of Dursban
in the body tissues of turkeys confined in pens containing treated
soil. J. Econ. Entomol., 63(2): 422-424.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
0,0-diethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate in Beagle
hounds. Biochem. Res. Lab. Report T35.12-44793-3 Dow Chemical Co.
(unpublished)
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
and Norvell, M. (1971) Final report on safety evaluation and metabolic
studies on Dowco 179 (IN 151). Inst. Exp. Pathol. Toxicol., Albany
Medical College. (unpublished)
Coulston, F., Goldberg, L. and Griffin, T. (1972) Safety evaluation of
Dowco 179 in humans. Inst. Exp. Pathol. Toxicol., Albany Medical
College. (unpublished)
Craig, L.F. (1971) Determination of residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in milk and cream by
gas chromatography. Method ACR 71.1. Dow Chemical Co.
Czech, F.P. (1967) Analysis of Dursban in livestock dips and sprays.
J. Ass. off. analyt. Chem., 50(4): 861-868.
Dishburger, H.J. and Rice, J.R. (1965) Residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in animal fat when
applied as a spray on cattle. Report TA-301 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966a) The effect of
formulation on Dursban residues in omental fat of cattle following
single spray application. Report TA-336 Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966b) Dursban residues
in the omental fat of cattle following dip applications. Report TA-349
Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1967) Dursban residues
in the omental fat of cattle following two dip applications. Report
TA-372 Dow Chemical Co. (unpublished)
Dishburger, H.J., Pennington, J. and Rice, J.R. (1969a) Determination
of O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
tobacco by gas chromatography. Report TA-430 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R., McGregor, W.S. and Pennington, J.
(1969b) Residues of Dursban(R) insecticide in tissues from turkeys
confined on soil treated for chigger control. J. Econ. Entomol.,
62(1): 181-183.
Dishburger, H.J., Rice, J.R and Pennington, J. (1969c) Determination
of residues of Dursban insecticide and its oxygen analogue in soil by
gas chromatography. Report TA-439 Dow Chemical Co. (unpublished)
Dow Chemical Co. (1964) Potentiation studies with Dursban in
combination with Ruelene and malathion in rats. Report Dow Chemical
Co. (unpublished)
Dow Chemical Co. (1966a) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (1966b) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (U.K.) Ltd. (1967) Data sheets containing summarized
data supporting notification of Dursban(R) insecticide for
commercial use in the control of sheep ectoparasites. Submitted to
the Technical Secretary, Scientific Sub-Committee, Veterinary
Products Safety Precautions Scheme.
Düsch, M.E., Westlake, W.E. and Gunther, F.A. (1970) Determination of
Dursban insecticide in water, mud, vegetation, fish, ducks, insects
and crustacea. J. Agr. Fd. Chem., 18(1): 178-179.
Emerson, J.L. and Gerbig, C.G. (1970) 91 day-toxicology study in
Beagle dogs treated with 3,5,6-trichloro-2-pyridinol. Report HH-263
Dow Chemical Co. (unpublished)
Foschi, S. (1971) Determination del Dursban. Istituto di Patologia
Vegetale, Bologna, Italy. Personal communication to K.N. Komblas.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14: 515-534.
Gerbig, C.G and Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat. Report HH-239
Dow Chemical Co. (unpublished)
Gerbig, C.G and Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Report HH-240
Dow Chemical Co. (unpublished)
Graf, W.C. (1970a) Analyses of Dursban residues on cotton seeds. Dow
Chemical Europe S.A. Personal communication to K. Komblas.
Graf, W.C. (1970b) Dursban residue analyses on apples from SIAPA,
Italy. Report DC 53. Dow Chemical Europe S.A.
Graf, W.C. (1970c) Dursban residue analyses on pears from SIAPA,
Italy. Report DC 54 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970a) Dursban residue analyses on apples
from Israel. Report DEC 55 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970b) Dursban residue on tomatoes from
Turkey. Report DEC 56 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971a) Dowco 179 residue analysis in
potatoes from France. Report DEH 2 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971b) Dowco 179 residue analysis on
tomatoes from Israel. Dow Chemical Europe S.A.
Graf. W.C. and Engeler, V. (1971c) Dowco 179 residue analysis in
apples from Israel. Report DEH 36 Dow Chemical Europe S.A.
Gutenmann, W.H. St. John, Jr., L.E. and Lisk, D.L. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow. J. Agr. Fd.
Chem., 16(1): 45-47.
Hall, R.A. (1969) Dursban residues. Department of Agriculture,
Australia. Personal communication to J. Tollett.
Hamaguchi, H. (1970) Dowco 179 - residue analysis. Translation of
analytical data furnished by Japan Plant Protection Association.
Nissan Chemical Laboratory and Japan Analytical Chemical Research
Laboratory. Personal communication to R.H. Ferguson.
Harris, C.R. (1971) Dursban residue in onions. Canada Department of
Agriculture. Personal Communication to T. Haagsma.
Hollick, C.B. and Collison, R.J. (1972) Determination of residues of
O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
(Dowco-179) in eggplant, tomatoes, beans and peppers treated with
Dursban 4 insecticide in Turkey. Report Dow Chemical Company Ltd.
(unpublished)
Hunt, L.M., Gilbert, B.N. and Schlinke, J.C. (1969) Rapid gas
chromatographic method for analysis of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate (Dursban) in turkey and
chicken tissues. J. Agr. Fd. Chem., 17(6): 1166-1167.
Hurlbert, S.H., Mulla, M.S., Keith, J.O., Westlake, W.E. and Düsch,
M.E. (1970) Biological effects and persistence of Dursban(R) in
freshwater ponds. J. Econ. Entomol., 63(1): 43-52.
Ivey, M.C. and Claborn, H.V. (1968) Dursban oxygen analogue:
Determination in fortified milk and body tissues of cattle. J. Assoc.
Offic. Anal. Chem., 51(6): 1245-1246.
Johnson, J.C. Jr., Bowman, M.C. and Leuck, D.B. (1969) Responses from
cows fed silages containing Dursban residues. J. Dairy Sci., 52(8):
1253-1258.
Kenaga, E.E. (1967) Toxicity and repellency of Dursban to the
white-footed mouse (Peromyscus maniculatus). Report Dow Chemical Co.
(unpublished).
Leuck, D.B., Bowman, M.C. and Beck, E.W. (1968) Dursban(R) insecticide
persistence in grass and corn forage. J. Econ. Entomol., 61(3):
689-690.
Mann, H.D. (1966) Report of Dursban(R) insecticide residue analysis in
treated cattle. Report PCK-66-2. USDA-ARS-Entomol. Res. Div.
Mann, H.D. and Ivey, M.C. (1971) Report of residues analysis - Fat,
skin, muscle, liver and kidney tissues of turkeys. Report PCK-71-1.
USDA-ARS-Entomol. Res. Div.
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco(R) 179 in
Beagle dogs. Report T35.12-44793-18 Dow Chemical Co. (unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco(R) 179 in
rats. Report, NBT35.12-44793-21 Dow Chemical Co. (unpublished)
McCray, C.W. (1969) Field trials using Dursban. Letter report Animal
Res. Inst., Queensland.
McEwen, F.L. (1970) Tests with Dursban on onions, carrots and lettuce.
University of Guelph. Personal communication to T. Haagsma.
McEwen, F.L. and Frank, R. (1970) Tests with Dursban on onions,
carrots and lettuce. University of Guelph. Personal communication to
T. Haagsma.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-pyridinol in milk and cream by gas chromatography.
Method ACR 71.2. Dow Chemical Co.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-pyridinol in corn grain, forage and stover by gas
chromatography. Method ACR 71.19. Dow Chemical Co.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-pyridinol in grass by gas chromatography. Method ACR
71.5. Dow Chemical Co.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography. Method ACR 70.19. Dow Chemical Co.
Molello, J.A. and Sharp, L.D. (1968) Three week study on
cataractogenicity of 3,5,6-trichloro-2-pyridinol as part of the
dietary intake of Pekin ducklings. Report HH-134 Dow Chemical Co.
(unpublished)
Myers, C.M., Lewallen, L.L. and Nobe, B. (1968) Residue studies of
fenthion (Baytex(R)) and Dursban(R) in central California pastures.
California Vector Views, 15(5): 51-54.
Norris, J.M. (1970) Potentiation Study on Dowco(R) 179 and Vapona(R)
insecticide. Report Dow Chemical Co. (unpublished)
Olthof, P.D.A. (1971a) Residuen van Dursban in champignons. Report R
3563. Centraal Instituut voor Voedingsonderzoek, The Netherlands.
Olthof, P.D.A. (1971b) Residues of Dursban in carrots. Report R 3633.
English translation from report by Centraal Instituut voor
Voedingsonderzoek, The Netherlands.
Rice, J.R. and Dishburger, H.J. (1968) Determination of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate in
water and silt by gas chromatography. J. Agr. Fd. Chem., 16(5):
867-869.
Scheuerer, W. and Hahn, I. (1972) Dursban residues on vegetables.
Badische Anilin and Soda-Fabrik AG, West Germany. Communication to Dow
Chemical (Nederland) N.V.
Schlinke, J.C. (1970) Chronic toxicity of Dursban in chickens, 1969.
J. Econ. Entomol., 63(1): 319.
Schlinke, J.C., Palmer, J.S. and Hunt, L. (1969) Preliminary
toxicological study of a phosphorothioate compound in turkeys. Am. J.
Vet. Res., 30(9): 1705-1709.
Sherman, M., Herrick, R.B., Ross, E. and Chang, M.T.Y. (1967) Further
studies on the acute and subacute toxicity of insecticides to chicks.
Tox. Appl. Pharmacol., 11: 49-67.
SIAPA. (1971) Residue determinations on lettuce after treatment with
Dursban insecticide. Centro Esperienze e Ricerche, Italy.
Smith, G.N. (1966) Basic studies of Dursban insecticide. Down to
Earth, 22(2): 3-7.
Smith, G.N. (1968) Ultraviolet light decomposition studies with
Dursban and 3,5,6-trichloro-2-pyridinol. J. Econ. Entomol., 61(3):
793-799.
Smith, G.N. and Taylor, Y.S. (1970) An analytical method for the
determination of 3,5,6-trichloro-2-pyridinol in animal tissues and the
metabolism of the pyridinol in rats. Report OL 3132 Dow Chemical
(unpublished)
Smith, G.N., Watson, B.S. and Fischer, F.S. (1966) The metabolism of
(14C) O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) in fish. J. Econ. Entomol., 59(6):
1464-1475.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967a) Investigations on
Dursban insecticide. Uptake and translocation of (36Cl)
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate by
beans and corn. J. Agr. Fd. Chem., 15(1): 127-131.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967b) Investigations on
Dursban insecticide. Metabolism of (36Cl) O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in rats. J. Agr. Fd.
Chem., 15(1): 132-138.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967c) Investigations on
Dursban insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agr. Fd. Chem., 15(5):
870-877.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Report OL-3132 Dow
Chemical Co. (unpublished)
Snelson, J.T. (1969a) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Snelson, J.T. (1969b) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Stevenson, G.T. (1965) A gamebird toxicology study - acute dietary
feeding of Dursban to wild type mallard ducklings. Report GH-A-122 Dow
Chemical Co. (unpublished)
Stevenson, G.T. (1966) A neurotoxicity study of Dursban in laying
hens. Report GH-A-195 Dow Chemical Co. (unpublished)
Stevenson, G.T. and Daering, K.L. (1969) The effects of Dursban
insecticide (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) on blood plasma cholinesterase in chickens. Report
GH-A-410 Dow Chemical Co. (unpublished)
Taylor, M.L. and Olson, K.J. (1963) Toxicological properties of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report
T35.12-44793-4 Dow Chemical Co. (unpublished)
Thompson, D.J., Gerbig, C.G. and Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report HH-382 Dow
Chemical Co. (unpublished)
Tucker, K.E.B. (1971) Dursban/Dowco 214 residues in rice and straw.
Report 160 Dow Chemical (Australia) Ltd.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of the Interior, Fish and Wildlife
Service Resource Publication #84, p. 56-57.
Tucker, K.E.B. and Zielinski, W. (1972a) Chlorpyrifos and Dowco 214
residues in Chinese cabbage. Report 175 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972b) Chlorpyrifos and Dowco 214
residues in Chinese kale. Report 174 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972c) Residues of chlorpyrifos in
Chinese kale. Report 179 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972d) Residues of chlorpyrifos and
Dowco 214 in tobacco. Report 180 Dow Chemical (Australia) Ltd.
USAEHA. (1968) Report U.S. Army Environmental Hygiene Agency.
Watts, R.M. (1968) Residues of chlorpyrifos in cattle. Dept. of
Agriculture, Australia. Communication to M.K. Astill.
Wetters, J.H. (1971a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate and O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphate in soils by gas
chromatography with flame photometric detection. Method ACR 71.3. Dow
Chemical Co.
Wetters, J.H. (1971b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in soils by gas
chromatography using flame photometric detection and direct injection.
Method ACR 71.20. Dow Chemical Co.
Wetters, J.H. (1972a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in pig tissues by gas
chromatography with flame photometric detection. Method 72.1. Dow
Chemical Co.
Wetters, J.H. (1972b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in chicken tissues
and eggs by gas chromatography with flame photometric detection.
Method 72.3. Dow Chemical Co.
Wetters, J.H. (1972c) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sweet corn by gas
chromatography with flame photometric detection. Method ACR 72.9. Dow
Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971a) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
and O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphate in
peaches by gas chromatography with flame photometric detection. Method
ACR 71.14. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971b) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
corn by gas chromatography with flame photometric detection. Method
ACR 71.18. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971c) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
water by gas chromatography with flame photometric detection. Method
ACR 71.21. Dow Chemical Co.
Winterlin. W., Moilanen, K. and Burgoyne, W.E. (1968) Residues of
Dursban insecticide following mosquito control applications. Down to
Earth, 24(2): 34-37.
Wit, S.L. (1970) Residues of Dursban in cabbage. Report 49/70 Tox RoB.
English translation from report by State Institute for the Public
Health, The Netherlands.
Wit, S.L. (1971) Residues of O,O-diethyl
O-(3,5,6-trichloro-pyridine-(2)-yl) phosphorothioate (Dursban) for
the control of cutworms in lettuce under glass. Report 63/71 Tox-RoB.
English translation from report by Rijks Instituut voor de
Volksgezondheid, The Netherlands.
Residues of 3,5,6-trichloro-2-pyridinol may be determined by the
method of McKellar and Dishburger (1970) in bovine tissues; McKellar
(1971b) in corn grain, forage and stover; McKellar (1971c) in grass;
Dishburger et al. (1972) in chicken tissues and eggs and McKellar
(1971a) in milk and cream, at a detection level of 0.05 ppm.
The general cleanup procedures shown in Figure 2 followed by a gas
chromatographic determination utilizing one of the phosphorus specific
detectors would be suitable for most regulatory uses.
Examples of national tolerances as reported to meeting
Animals
Australia has a 2 ppm residue tolerance for chlorpyrifos in beef fat
when cattle are treated for ticks by dip or spray.
Plants
Canada has a tentative 0.1 ppm residue tolerance for head lettuce,
onions and carrots with 28, 60 and 60 days, respectively, required
between treatment and harvest.
APPRAISAL
Chlorpyrifos is a non-systemic organophosphorus insecticide used to
control ectoparasites of cattle, sheep and poultry; as a soil
insecticide for vegetables, cereals and tobacco; and as a foliar
insecticide for deciduous fruit, cereals, fodder crops, rape, cotton,
some vegetables, tobacco and rice.
Animal residues are found predominantly in the fat and are excreted
rapidly after cessation of treatment. When cattle were given multiple
dippings at the recommended dosage rates of 0.025%, the maximum total
residue in fat one week after the last dipping was 1.6 ppm. When milk
cows were dipped once in 0.025% chlorpyrifos, the residue in butterfat
fell to 0.01 ppm in nine days and was not detectable (<0.005 ppm)
after 15 days. When turkeys were confined over soil treated with
chlorpyrifos (4 or 8 lb/acre) for chigger control, maximum residues of
0.16 and 0.07 ppm were found in skin and fat, respectively, one week
post-treatment; the oxygen analogue was not detected in any tissues.
Small amounts of the pyridinol were found in the skin, livers and
kidneys of two birds with a maximum residue of 0.17 ppm in kidney
after one week exposure to the second application.
Extensive supervised experiments with foliar treatments on plants have
shown chlorpyrifos to have little persistence and virtually no uptake
occurs from either soil or foliar application. Up to 80% of applied
radioactive chlorpyrifos was lost by vapourization from single leaf
surfaces within the first 48 hours following treatment. There was no
evidence of residue buildup on plants from repeated applications.
The residues most likely to be found in either plants or animals are
chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol;
the oxygen analogue or other possible metabolites are very rarely
found.
Both specific and general gas chromatographic methods of analysis are
available which are suitable for regulatory purposes. The generalized
cleanup schemes shown in Figure 2 followed by gas chromatographic
determination using a phosphorus specific detector are recommended.
Separate analytical procedures for determining
3,5,6-trichloro-2-pyridinol in animal tissues (liver, kidney, poultry
skin), fruits and vegetables have been developed. The pyridinol is
excreted rapidly in the urine and faeces of animals and there would
appear to be no need to include this compound in regulatory analysis.
In field experiments with plants the pyridinol is rarely found and
when present constitutes only a fraction of the total residue.
Although chlorpyrifos is recommended in some countries for use on
rape, cereal grains and certain vegetables, there were no data
available on these commodities from supervised experiments and no
recommendations for tolerances could be made for these commodities.
RECOMMENDATIONS
TOLERANCES
ppm
Fat of meat of cattle 2
Chinese cabbage, grapes, kale, apples 1
Carrots, pears, tomatoes 0.5
Fat of meat of sheep, poultry, beans,
eggplants, peppers, raspberries 0.2
Lettuce, rice (in husk), sugarbeets 0.1
Celery, cottonseed, cottonseed oil
(crude), mushrooms, onions 0.05
Cauliflower, milk (fat basis),
potatoes, red cabbage 0.01*
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before tolerances can be recommended)
Residue data from supervised trials for rape seed, cereal grains and
any vegetables not listed.
DESIRABLE
1. Elucidation of possible increased sensitivity to plasma
cholinesterase depression after withdrawal from an initial dose
regime.
2. Further information on residues in milk and milk products arising
from dipping of dairy cattle.
REFERENCES
Aharonson, N., Grünberg, R. and Resnick, C. (1969) Dursban residue
level in tomatoes and cotton. Ministry of Agriculture, Jaffa, Israel.
Beatty, S.C. and McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Report The Dow Chemical Co., revision.
(unpublished)
Blackmore, R.H. (1968a) Oral administration-dogs-Dursban final report.
Hazleton Lab. Inc. (unpublished)
Blackmore, R.H. (1968b) Short-term (subacute) dietary
administration-rats-organophosphate insecticide Dursban - final
report. Hazleton Lab. Inc. (unpublished)
Bowman, M.C. and Beroza, M. (1967a) Determination of Dursban and its
oxygen analogue in corn and grass by gas chromatography with flame
photometric detection. J. Agr. Fd. Chem., 15(4): 651-653.
Bowman, M.C. and Beroza, M. (1967b) Temperature-programmed gas
chromatography of 20 phosphorus-containing insecticides on 4 different
columns and its application to the analysis of milk and corn silage.
J. Ass. off. analyt. Chem., 50(6): 1228-1236.
Branson, D.R. and Litchfield, N.H. (1971a) Absorption, excretion and
distribution of 3,5,6-trichloro-2,6-14C-2-pyridinol in rats. Report
NBA-10 Dow Chemical Company. (unpublished)
Branson, D.R. and Litchfield, N.H. (1971b) Absorption, excretion and
distribution of O,O-diethyl O-3,5,6-trichloro-2,6-14C-2-pyridyl
phosphorothioate (14C Dowco 179) in rats. Report NBA-9 Dow Chemical
Company. (unpublished)
Branson, D.R. and Wass, M.N. (1970) Comparative metabolism of
insecticides. I. Preliminary studies of ring labelled O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate breakdown with rat
liver microsomes. Report R-582 Dow Chemical Company. (unpublished)
Braun, H.E. (1971) Dursban residues in vegetables. Ontario Department
of Food and Agriculture, Guelph, Ontario. Personal communications to
T. Haagsma.
Brust, R.A., Miyazaki, S. and Hodgson, G.C. (1971) Effect of Dursban
in the drinking water of chicks. J. Econ. Entomol., 64(5): 1179-1183.
Burrows, I.E., Fraser, W.D., Joyce, C.A., Jenkins, G. and Eden, A.
(1971a) Determination of decay curves for Dursban and its metabolite
in stored tomatoes. 4211/71/369. Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971b) Determination of residues of
Dursban metabolite in tomatoes, grapes and apples. 4109/71/267.
Huntingdon Research Centre.
Burrows, I.E. and Mullin, L.W. (1971c) Determination of residues of
Dursban in lettuce. 4140/71/298. Huntingdon Research Centre.
Burrows, I.E., Mullin, L.W. and Eden, A. (1971d) Determination of
residues of Dursban and its metabolites in raspberries. 4371/71/527.
Huntingdon Research Centre.
Burrows, I.E., Orme, J.P.R. and Ashton, J. (1971e) Determination of
residues of Dursban and its metabolite in tomatoes. 4496/71/652.
Huntingdon Research Centre.
Claborn, H.V., Hoffman, R.A., Mann, H.D. and Oehler, D.D. (1968a)
Residues of Dursban and its oxygen analogue in the body tissues of
treated cattle. J. Econ. Entomol., 61(4): 983-986.
Claborn, H.V., Mann, H.D. and Oehler, D.D. (1968b) Dursban(R)
determination in milk and body tissues of cattle. J. Ass. off. analyt.
Chem., 51(6): 1243-1245.
Claborn, H.V., Ivey, M.C., Mann, H.D. and Oehler, D.D. (1970a) Report
of residue analysis - Omental fat, muscle, kidney and liver. Report
PCK-70-1. USDA-ARS-Entomol. Res. Div.
Claborn, H.V., Kunz, S.E. and Mann, H.D. (1970b) Residues of Dursban
in the body tissues of turkeys confined in pens containing treated
soil. J. Econ. Entomol., 63(2): 422-424.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
0,0-diethyl 0-3,5,6-trichloro-2-pyridyl phosphorothioate in Beagle
hounds. Biochem. Res. Lab. Report T35.12-44793-3 Dow Chemical Co.
(unpublished)
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
and Norvell, M. (1971) Final report on safety evaluation and metabolic
studies on Dowco 179 (IN 151). Inst. Exp. Pathol. Toxicol., Albany
Medical College. (unpublished)
Coulston, F., Goldberg, L. and Griffin, T. (1972) Safety evaluation of
Dowco 179 in humans. Inst. Exp. Pathol. Toxicol., Albany Medical
College. (unpublished)
Craig, L.F. (1971) Determination of residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in milk and cream by
gas chromatography. Method ACR 71.1. Dow Chemical Co.
Czech, F.P. (1967) Analysis of Dursban in livestock dips and sprays.
J. Ass. off. analyt. Chem., 50(4): 861-868.
Dishburger, H.J. and Rice, J.R. (1965) Residues of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in animal fat when
applied as a spray on cattle. Report TA-301 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966a) The effect of
formulation on Dursban residues in omental fat of cattle following
single spray application. Report TA-336 Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1966b) Dursban residues
in the omental fat of cattle following dip applications. Report TA-349
Dow Chemical Co. (unpublished)
Dishburger, H.J., Rice, J.R. and Muniza, R.A. (1967) Dursban residues
in the omental fat of cattle following two dip applications. Report
TA-372 Dow Chemical Co. (unpublished)
Dishburger, H.J., Pennington, J. and Rice, J.R. (1969a) Determination
of O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
tobacco by gas chromatography. Report TA-430 Dow Chemical Co.
(unpublished)
Dishburger, H.J., Rice, J.R., McGregor, W.S. and Pennington, J.
(1969b) Residues of Dursban(R) insecticide in tissues from turkeys
confined on soil treated for chigger control. J. Econ. Entomol.,
62(1): 181-183.
Dishburger, H.J., Rice, J.R and Pennington, J. (1969c) Determination
of residues of Dursban insecticide and its oxygen analogue in soil by
gas chromatography. Report TA-439 Dow Chemical Co. (unpublished)
Dow Chemical Co. (1964) Potentiation studies with Dursban in
combination with Ruelene and malathion in rats. Report Dow Chemical
Co. (unpublished)
Dow Chemical Co. (1966a) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (1966b) Report submitted by Dow Chemical Co.
(unpublished)
Dow Chemical Co. (U.K.) Ltd. (1967) Data sheets containing summarized
data supporting notification of Dursban(R) insecticide for
commercial use in the control of sheep ectoparasites. Submitted to
the Technical Secretary, Scientific Sub-Committee, Veterinary
Products Safety Precautions Scheme.
Düsch, M.E., Westlake, W.E. and Gunther, F.A. (1970) Determination of
Dursban insecticide in water, mud, vegetation, fish, ducks, insects
and crustacea. J. Agr. Fd. Chem., 18(1): 178-179.
Emerson, J.L. and Gerbig, C.G. (1970) 91 day-toxicology study in
Beagle dogs treated with 3,5,6-trichloro-2-pyridinol. Report HH-263
Dow Chemical Co. (unpublished)
Foschi, S. (1971) Determination del Dursban. Istituto di Patologia
Vegetale, Bologna, Italy. Personal communication to K.N. Komblas.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14: 515-534.
Gerbig, C.G and Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat. Report HH-239
Dow Chemical Co. (unpublished)
Gerbig, C.G and Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Report HH-240
Dow Chemical Co. (unpublished)
Graf, W.C. (1970a) Analyses of Dursban residues on cotton seeds. Dow
Chemical Europe S.A. Personal communication to K. Komblas.
Graf, W.C. (1970b) Dursban residue analyses on apples from SIAPA,
Italy. Report DC 53. Dow Chemical Europe S.A.
Graf, W.C. (1970c) Dursban residue analyses on pears from SIAPA,
Italy. Report DC 54 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970a) Dursban residue analyses on apples
from Israel. Report DEC 55 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1970b) Dursban residue on tomatoes from
Turkey. Report DEC 56 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971a) Dowco 179 residue analysis in
potatoes from France. Report DEH 2 Dow Chemical Europe S.A.
Graf, W.C. and Engeler, V. (1971b) Dowco 179 residue analysis on
tomatoes from Israel. Dow Chemical Europe S.A.
Graf. W.C. and Engeler, V. (1971c) Dowco 179 residue analysis in
apples from Israel. Report DEH 36 Dow Chemical Europe S.A.
Gutenmann, W.H. St. John, Jr., L.E. and Lisk, D.L. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow. J. Agr. Fd.
Chem., 16(1): 45-47.
Hall, R.A. (1969) Dursban residues. Department of Agriculture,
Australia. Personal communication to J. Tollett.
Hamaguchi, H. (1970) Dowco 179 - residue analysis. Translation of
analytical data furnished by Japan Plant Protection Association.
Nissan Chemical Laboratory and Japan Analytical Chemical Research
Laboratory. Personal communication to R.H. Ferguson.
Harris, C.R. (1971) Dursban residue in onions. Canada Department of
Agriculture. Personal Communication to T. Haagsma.
Hollick, C.B. and Collison, R.J. (1972) Determination of residues of
O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
(Dowco-179) in eggplant, tomatoes, beans and peppers treated with
Dursban 4 insecticide in Turkey. Report Dow Chemical Company Ltd.
(unpublished)
Hunt, L.M., Gilbert, B.N. and Schlinke, J.C. (1969) Rapid gas
chromatographic method for analysis of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate (Dursban) in turkey and
chicken tissues. J. Agr. Fd. Chem., 17(6): 1166-1167.
Hurlbert, S.H., Mulla, M.S., Keith, J.O., Westlake, W.E. and Düsch,
M.E. (1970) Biological effects and persistence of Dursban(R) in
freshwater ponds. J. Econ. Entomol., 63(1): 43-52.
Ivey, M.C. and Claborn, H.V. (1968) Dursban oxygen analogue:
Determination in fortified milk and body tissues of cattle. J. Assoc.
Offic. Anal. Chem., 51(6): 1245-1246.
Johnson, J.C. Jr., Bowman, M.C. and Leuck, D.B. (1969) Responses from
cows fed silages containing Dursban residues. J. Dairy Sci., 52(8):
1253-1258.
Kenaga, E.E. (1967) Toxicity and repellency of Dursban to the
white-footed mouse (Peromyscus maniculatus). Report Dow Chemical Co.
(unpublished).
Leuck, D.B., Bowman, M.C. and Beck, E.W. (1968) Dursban(R) insecticide
persistence in grass and corn forage. J. Econ. Entomol., 61(3):
689-690.
Mann, H.D. (1966) Report of Dursban(R) insecticide residue analysis in
treated cattle. Report PCK-66-2. USDA-ARS-Entomol. Res. Div.
Mann, H.D. and Ivey, M.C. (1971) Report of residues analysis - Fat,
skin, muscle, liver and kidney tissues of turkeys. Report PCK-71-1.
USDA-ARS-Entomol. Res. Div.
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco(R) 179 in
Beagle dogs. Report T35.12-44793-18 Dow Chemical Co. (unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco(R) 179 in
rats. Report, NBT35.12-44793-21 Dow Chemical Co. (unpublished)
McCray, C.W. (1969) Field trials using Dursban. Letter report Animal
Res. Inst., Queensland.
McEwen, F.L. (1970) Tests with Dursban on onions, carrots and lettuce.
University of Guelph. Personal communication to T. Haagsma.
McEwen, F.L. and Frank, R. (1970) Tests with Dursban on onions,
carrots and lettuce. University of Guelph. Personal communication to
T. Haagsma.
McKellar, R.L. (1971a) Determination of residues of
3,5,6-trichloro-pyridinol in milk and cream by gas chromatography.
Method ACR 71.2. Dow Chemical Co.
McKellar, R.L. (1971b) Determination of residues of
3,5,6-trichloro-pyridinol in corn grain, forage and stover by gas
chromatography. Method ACR 71.19. Dow Chemical Co.
McKellar, R.L. (1971c) Determination of residues of
3,5,6-trichloro-pyridinol in grass by gas chromatography. Method ACR
71.5. Dow Chemical Co.
McKellar, R.L. and Dishburger, H.J. (1970) Determination of residues
of 3,5,6-trichloro-2-pyridinol in bovine tissues by gas
chromatography. Method ACR 70.19. Dow Chemical Co.
Molello, J.A. and Sharp, L.D. (1968) Three week study on
cataractogenicity of 3,5,6-trichloro-2-pyridinol as part of the
dietary intake of Pekin ducklings. Report HH-134 Dow Chemical Co.
(unpublished)
Myers, C.M., Lewallen, L.L. and Nobe, B. (1968) Residue studies of
fenthion (Baytex(R)) and Dursban(R) in central California pastures.
California Vector Views, 15(5): 51-54.
Norris, J.M. (1970) Potentiation Study on Dowco(R) 179 and Vapona(R)
insecticide. Report Dow Chemical Co. (unpublished)
Olthof, P.D.A. (1971a) Residuen van Dursban in champignons. Report R
3563. Centraal Instituut voor Voedingsonderzoek, The Netherlands.
Olthof, P.D.A. (1971b) Residues of Dursban in carrots. Report R 3633.
English translation from report by Centraal Instituut voor
Voedingsonderzoek, The Netherlands.
Rice, J.R. and Dishburger, H.J. (1968) Determination of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate in
water and silt by gas chromatography. J. Agr. Fd. Chem., 16(5):
867-869.
Scheuerer, W. and Hahn, I. (1972) Dursban residues on vegetables.
Badische Anilin and Soda-Fabrik AG, West Germany. Communication to Dow
Chemical (Nederland) N.V.
Schlinke, J.C. (1970) Chronic toxicity of Dursban in chickens, 1969.
J. Econ. Entomol., 63(1): 319.
Schlinke, J.C., Palmer, J.S. and Hunt, L. (1969) Preliminary
toxicological study of a phosphorothioate compound in turkeys. Am. J.
Vet. Res., 30(9): 1705-1709.
Sherman, M., Herrick, R.B., Ross, E. and Chang, M.T.Y. (1967) Further
studies on the acute and subacute toxicity of insecticides to chicks.
Tox. Appl. Pharmacol., 11: 49-67.
SIAPA. (1971) Residue determinations on lettuce after treatment with
Dursban insecticide. Centro Esperienze e Ricerche, Italy.
Smith, G.N. (1966) Basic studies of Dursban insecticide. Down to
Earth, 22(2): 3-7.
Smith, G.N. (1968) Ultraviolet light decomposition studies with
Dursban and 3,5,6-trichloro-2-pyridinol. J. Econ. Entomol., 61(3):
793-799.
Smith, G.N. and Taylor, Y.S. (1970) An analytical method for the
determination of 3,5,6-trichloro-2-pyridinol in animal tissues and the
metabolism of the pyridinol in rats. Report OL 3132 Dow Chemical
(unpublished)
Smith, G.N., Watson, B.S. and Fischer, F.S. (1966) The metabolism of
(14C) O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) in fish. J. Econ. Entomol., 59(6):
1464-1475.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967a) Investigations on
Dursban insecticide. Uptake and translocation of (36Cl)
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate by
beans and corn. J. Agr. Fd. Chem., 15(1): 127-131.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967b) Investigations on
Dursban insecticide. Metabolism of (36Cl) O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate in rats. J. Agr. Fd.
Chem., 15(1): 132-138.
Smith, G.N., Watson, B.S. and Fischer, F.S. (1967c) Investigations on
Dursban insecticide. Metabolism of O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate and
3,5,6-trichloro-2-pyridinol in plants. J. Agr. Fd. Chem., 15(5):
870-877.
Smith, G.N., Taylor, Y.S. and Watson, B.S. (1970) An analytical method
for the determination of 3,5,6-trichloro-2-pyridinol in animal tissues
and the metabolism of the pyridinol in rats. Report OL-3132 Dow
Chemical Co. (unpublished)
Snelson, J.T. (1969a) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Snelson, J.T. (1969b) Dursban residues. Dept. of Primary Ind.,
Australia. Personal communication.
Stevenson, G.T. (1965) A gamebird toxicology study - acute dietary
feeding of Dursban to wild type mallard ducklings. Report GH-A-122 Dow
Chemical Co. (unpublished)
Stevenson, G.T. (1966) A neurotoxicity study of Dursban in laying
hens. Report GH-A-195 Dow Chemical Co. (unpublished)
Stevenson, G.T. and Daering, K.L. (1969) The effects of Dursban
insecticide (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) on blood plasma cholinesterase in chickens. Report
GH-A-410 Dow Chemical Co. (unpublished)
Taylor, M.L. and Olson, K.J. (1963) Toxicological properties of
O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report
T35.12-44793-4 Dow Chemical Co. (unpublished)
Thompson, D.J., Gerbig, C.G. and Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Report HH-382 Dow
Chemical Co. (unpublished)
Tucker, K.E.B. (1971) Dursban/Dowco 214 residues in rice and straw.
Report 160 Dow Chemical (Australia) Ltd.
Tucker, R.K. and Crabtree, D.G. (1970) Handbook of toxicity of
pesticides to wildlife. U.S. Dept. of the Interior, Fish and Wildlife
Service Resource Publication #84, p. 56-57.
Tucker, K.E.B. and Zielinski, W. (1972a) Chlorpyrifos and Dowco 214
residues in Chinese cabbage. Report 175 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972b) Chlorpyrifos and Dowco 214
residues in Chinese kale. Report 174 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972c) Residues of chlorpyrifos in
Chinese kale. Report 179 Dow Chemical (Australia) Ltd.
Tucker, K.E.B. and Zielinski, W. (1972d) Residues of chlorpyrifos and
Dowco 214 in tobacco. Report 180 Dow Chemical (Australia) Ltd.
USAEHA. (1968) Report U.S. Army Environmental Hygiene Agency.
Watts, R.M. (1968) Residues of chlorpyrifos in cattle. Dept. of
Agriculture, Australia. Communication to M.K. Astill.
Wetters, J.H. (1971a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate and O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphate in soils by gas
chromatography with flame photometric detection. Method ACR 71.3. Dow
Chemical Co.
Wetters, J.H. (1971b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in soils by gas
chromatography using flame photometric detection and direct injection.
Method ACR 71.20. Dow Chemical Co.
Wetters, J.H. (1972a) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in pig tissues by gas
chromatography with flame photometric detection. Method 72.1. Dow
Chemical Co.
Wetters, J.H. (1972b) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in chicken tissues
and eggs by gas chromatography with flame photometric detection.
Method 72.3. Dow Chemical Co.
Wetters, J.H. (1972c) Determination of residues of O,O-diethyl
O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in sweet corn by gas
chromatography with flame photometric detection. Method ACR 72.9. Dow
Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971a) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate
and O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphate in
peaches by gas chromatography with flame photometric detection. Method
ACR 71.14. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971b) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
corn by gas chromatography with flame photometric detection. Method
ACR 71.18. Dow Chemical Co.
Wetters, J.H. and Dishburger, H.J. (1971c) Determination of residues
of O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate in
water by gas chromatography with flame photometric detection. Method
ACR 71.21. Dow Chemical Co.
Winterlin. W., Moilanen, K. and Burgoyne, W.E. (1968) Residues of
Dursban insecticide following mosquito control applications. Down to
Earth, 24(2): 34-37.
Wit, S.L. (1970) Residues of Dursban in cabbage. Report 49/70 Tox RoB.
English translation from report by State Institute for the Public
Health, The Netherlands.
Wit, S.L. (1971) Residues of O,O-diethyl
O-(3,5,6-trichloro-pyridine-(2)-yl) phosphorothioate (Dursban) for
the control of cutworms in lettuce under glass. Report 63/71 Tox-RoB.
English translation from report by Rijks Instituut voor de
Volksgezondheid, The Netherlands.
