
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
CHLORPYRIFOS
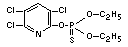 Explanation
Chlorpyrifos was reviewed by the Joint Meetings of 1972, 1974,
1975, 1977 and 1981 (FAO/WHO 1973, 1975, 1976, 1978, 1982)1/. An ADI
of 0-0.001 mg/kg bw was established, based on plasma cholinesterase
depression in humans. Additional reports on the toxicity of
chlorpyrifos have been submitted, including a two-year chronic
feeding/oncogenicity study in mice, a teratology study in mice, and a
delayed neurotoxicity study in hens. These studies have been reviewed
and summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 40-47 pregnant CF-1 mice were given 0, 1, 10 or
25 kg bw/day of chlorpyrifos by gavage on days 6 through 15 of
gestation. Administration of 25 mg/kg/day of chlorpyrifos resulted in
severe maternal toxicity. Additional groups of 35-41 pregnant mice
were gavaged with 0, 0.1, 1.0 and 10 mg chlorpyrifos/kg bw on days 6
through 15 of gestation, due to the maternal toxicity at 25 mg/kg body
weight. On day 18 of gestation all pregnant animals were sacrificed
and foetuses delivered by caesarean section. Maternal observations
included cageside observations; body, liver and gravid uterine
weights; food and water consumption; number and position of live,
dead, and resorbed foetuses and evidence of implantation sites. Foetal
body weights, crown-rump measurements, external alterations, together
with visceral and skeletal alterations, were similarly determined for
all foetuses. Separate groups of pregnant mice were also examined for
clinical evidence of cholinesterase depression during selected periods
of gestation, including ChE measurements of plasma and erythrocytes,
as well as the ChE of a foetal homogenate on gestation day 15.
1/ See Annex 2 for WHO and FAO documentation.
Evidence of maternal toxicity (including cholinesterase
inhibition, decreased body weight, decreased food and water
consumption and death) was observed among pregnant mice given 25 mg/kg
of chlorpyrifos. Plasma and erythrocyte cholinesterase levels were
significantly reduced among maternal mice at 1 mg/kg or greater and in
foetuses at dose levels of 10 mg/kg or greater.
Foetotoxicity, as evidenced by increased occurrence of minor
skeletal variants (i.e. delayed ossification of skull bones and
sternebrae) and decreased foetal body weight measurements, was noted
at the 25 mg/kg dose level.
Reproduction performance (as determined by implantation sites,
resorption sites and live young) was not affected by chlorpyrifos at
any dose level administered. Some malformations (exencephaly) were
observed among litters of mice given 1 mg/kg chlorpyrifos; however, a
dose-related teratogenic response was not observed nor repeated in the
additional mice given the same dose. No evidence of teratogenic
response was observed among mice given 0.1, 1, 10 or 25 mg/kg of
chlorpyrifos, during the critical period of organogenesis (Deacon
et al 1979).
Special Study on Neurotoxicity
Chicken
Groups of adult hens (av. 14.5 months) were administered
chlorpyrifos orally at dosage levels of 0, 25, 50, 100, 200 or
400 mg/kg bw, and the oral LD50 estimated to be 50 mg/kg bw. Separate
groups of non-fasted adult hens (av. 17 months) were given single oral
doses of 0, 50 and 100 mg chlorpyrifos/kg bw. A positive control
(TOTP, 232 mg/kg) was also used. Ten birds comprised each test group.
Atropine sulphate (30 mg/kg) was given to all birds prior to
administration of TOTP or chlorpyrifos. At the conclusion of the study
(21-24 days), all surviving hens were sacrificed and subjected to
gross and microscopic examinations. Axon and myelin degeneration were
assessed in the sciatic nerve and spinal cord.
There was no mortality in any group. Body weight was slightly,
but not statistically, depressed in the positive control (TOTP). There
were dose-dependent, acute cholinergic effects observed for the
chlorpyrifos treated groups. Symptoms included transient signs of
ataxia, CNS depression and paralysis, which recovered to normal within
2 to 4 days post-treatment. There were no signs of delayed
neurotoxicity observed with chlorpyrifos. The positive control animals
(TOTP) displayed delayed gross symptoms beginning on day 10 and
continued through to sacrifice. There were slight to severe signs of
ataxia in all birds, with partial paralysis in a few. Histologic
evaluation revealed no evidence of neurotoxic effects in either the
chlorpyrifos treated group or the negative control (atropine sulphate
only). Evidence of delayed neurotoxicity was apparent in the TOTP
group with the observation of swollen, degenerate, fragmented and
demyelinated axons in the spinal cord, plus swollen neurollemma
sheaths and vacuolation of the sciatic nerve. Based on the results of
this study, it was concluded that chlorpyrifos does not induce delayed
neurotoxic reactions in adult white leghorn hens when administered as
a single acute oral dose (Rowe et al 1978).
Special Study for Carcinogenicity
Mouse
Groups of CD-1 mice (56 male and 56 female/mice/group) were fed
chlorpyrifos in the diet at dosage levels of 0, 0.5, 5 and 15 ppm for
105 weeks. These dose levels were approximately equivalent to 0, 0.05,
0.5 and 1.5 mg/kg/day over the entire treatment period. The animals
were examined daily for mortality and adverse physical/behavioural
condition. Periodic evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All mice were killed by CO2 inhalation
and exsanguination via the axillary blood vessels. Peripheral blood
smears were prepared from tail blood from all survivors at terminal
sacrifice.
There were no unusual behavioural changes and no significant
effects on food consumption, body weight gain or mortality. Absolute
and relative organ weights of selected organs were varied and
unrelated to dose. Relative liver weights for both male and female
mice were significantly decreased (p <0.05) at the low- and mid-dose,
but not at the high dose. Histological examination revealed a
significant difference between control and mid-dose males for the
incidence of hyperplastic nodules of the liver. There was also a
significant increase in the incidence of spindle cell hyperplasia of
the adrenal gland for male mice at the low and mid-dose levels, and in
female mice at the low dose only. Lesions observed in the lung
included a significant increase of alveologenic adenomas in mid-dose
male mice only. The histologic changes identified appear to be
spontaneous in nature and without a demonstrated dose-response.
Furthermore, type A and B nodules of the liver parenchyma, as well as
the alveologenic carcinomas of the lung, occurred with equal frequency
between treatment and control group animals. There were no other
significant lesions or statistically increased hyperplastic and/or
nodular lesions. The results of this study demonstrate that
chlorpyrifos is not oncogenic in mice at dietary levels up to and
including 15 ppm for a period of 105 weeks (Warner et al 1980).
COMMENTS
The Meeting evaluated substantial new data submitted on
chlorpyrifos, including a mouse teratology study, a mouse
carcinogenicity study and a hen neurotoxicity study. Chlorpyrifos was
not teratogenic to mice at doses up to and including 25 mg/kg bw/day,
although severe maternal toxicity and foetotoxicity were evident at
that dose. It was also determined that 15 ppm of chlorpyrifos was not
oncogenic when administered in the diet of mice for 105 weeks. A
single acute oral dose of 100 mg/kg bw (twice the LD50) did not
induce a delayed neurotoxic response in hens. However, the Meeting
reiterated previously expressed JMPR opinions that a delayed
neurotoxicity study in hens requires multiple dosing, such that doses
are repeated 21 days apart for a total of two acute oral doses. In
this context, the present study may not fully address the delayed
neurotoxic potential of chlorpyrifos relative to residues in food.
Chlorpyrifos is recognized to be an active cholinesterase
inhibitor in several mammalian species, including humans. In all
species evaluated, plasma ChE or pseudo-cholinesterase was inhibited
at lower administered dose levels of chlorpyrifos than were RBC or
brain ChE (where determined). Chlorpyrifos was reviewed by the Joint
Meeting in 1972 (FAO/WHO 1973), which determined that chlorpyrifos
inhibited the plasma ChE in humans at 0.03 mg/kg bw, but not at
0.014 mg/kg bw (Coulston et al 1972). This served as a basis for the
ADI (0.0015 mg/kg), which was subsequently rounded off to 0.001 mg/kg
bw at the recommendation of the Joint Meeting of 1977 (FAO/WHO 1978).
However, appropriate statistical evaluation of the human
cholinesterase determinations in the Coulston et al study (1972)
(Park 1972) demonstrated 0.03 mg/kg to be a threshold level for
inhibition of plasma ChE, which was without statistical significance
when compared to current controls. Based upon repeated daily doses for
a period of 21 days, it was concluded by Coulston et al and Park
that a dose of 0.03 mg chlorpyrifos/kg bw has no significant
toxicological effect in humans. This conclusion is supported by the
evidence from studies in other mammalian species at comparable dose
levels (McCollister et al 1971a,b; Coulston et al 1971).
These same investigators determined that erythrocyte
cholinesterase activity was inhibited at comparable levels in
different species (e.g. 0.1 mg/kg for rat, dog and humans, and
0.08 mg/kg for monkey). Except for ChE depression, these levels were
without significant effects noted on mortality, behaviour, food
consumption growth, haematology, clinical chemistry, urinalysis, or
gross and microscopic examination of selected tissues (where
determined).
The Meeting accordingly increased the ADI from 0.001 to
0.01 mg/kg body weight, based on the level causing no inhibition of
RBC cholinesterase and not on the plasma cholinesterase.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0.1 mg/kg bw/day
Dog: 0.1 mg/kg bw/day
Man: 0.1 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Data on the inhibition, reactivation and ageing of cholinesterase
(in vitro).
REFERENCES
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
1971 and Norvell, M. Final report on safety evaluation and
metabolic studies on Dowco 179 (IN 151). Report from Insp.
Exp. Pathol. Toxicol., Albany Medical College, submitted to
the World Health Organization by Dow Chemical Company.
(Unpublished)
Coulston, F., Goldberg, L. and Griffin, T. Safety evaluation of
1972 Dowco 179 in human volunteers. Summary and tables 33 and 34,
reviewed by JMPR 1972. Report from Albany Medical College,
Albany, New York, submitted to the World Health Organization
by Dow Chemical Company. (Unpublished)
Deacon, M.M., Murray, J.S., Pilney, M.K., Dittenber, D.A., Hanley,
1979 T.R. Jr. and John, J.A. The effects of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Report from Dow Chemical Company submitted to the World
Health Organization by Dow Chemical Company. (Unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
1971a Results of two-year dietary feeding studies on DowcoR 179
in beagle dogs. Report T35.12.44793-18, from Dow Chemical
Company, submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
1971b Results of two-year dietary feeding studies on DowcoR179 in
rats. Report NBT35.12-44793-21, from Dow Chemical Company
submitted to the World Health Organization by Dow Chemical
Company (Unpublished)
Park, C. Analysis of plasma and red blood cell cholinesterase levels
1972 in humans ingesting DOWCO 179. Report from Dow Chemical
Company submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
Rowe, L.D., Warner, S.D. and Johnston, R.V. Acute delayed
1978 neurotoxicologic evaluation of chlorpyrifos in White Leghorn
hens. Report from Dow Chemical Company submitted to the
World Health Organization by Dow Chemical Company.
(Unpublished)
Warner, S.D., Gerbig, C.G., Strebing, R.J. and Molello, J.A. Results
1980 of a two-year toxicity and oncogenicity study of
chlorpyrifos administered to CD-1 mice in the diet. Report
from Dow Chemical Company, submitted to the World Health
Organization by Dow Chemical Company. (Unpublished)
CHLORPYRIFOS
Explanation
Chlorpyrifos was evaluated in 1972, 1974, 1975 and 1977 (FAO/WHO
1973, 1975, 1976, 1978)1/. Use patterns for chlorpyrifos have
undergone significant changes since 1972 with less emphasis on
previous animal applications, the development of "spot-on" animal
treatments and the emergence of more feed uses. At the 1982 meeting of
the CCPR, a question was raised concerning the recommended limit in
milk in view of these changing use patterns.
New information was made available to the Meeting on a survey of
international uses on dairy cattle, residues in meat from "spot-on"
treatment, residues in meat and milk from ingestion of treated feed,
metabolic fate in lactating goats, an improved method of analysis for
milk and cream and national maximum residue limits.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In Animals
A survey of uses of chlorpyrifos in animals was conducted by Dow
Chemical Co. in each geographic region in late 1981. This survey was
aimed specifically at clarifying whether dairy cattle are treated with
chlorpyrifos and, if so, whether such use is likely to lead to
residues greater than 0.1 mg/kg on a fat basis in milk or milk
products in international trade. Table 1 summarizes recommendations
for treatment of cattle with chlorpyrifos in each country where it has
been used.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Survey of Uses of Chlorpyrifos in Cattle: A. Developed Countries
Country Product Use Comment
Australia DURSBAN 1/24E For ticks and lice in cattle, Do not use on lactating cattle or in
all states 250 g/litre as dip at 1 litre/1 000 litres dairy cows later than 3 weeks before
1970-71 of water (0.025% a.i.), or as calving. Do not spray later than 3
spray at 200 ml/200 litres weeks before slaughter, or dip later
using at least 5 litres per than 3 days before slaughter of meat
animal. animals.
all states DURSBAN JS For louse control in cattle, Do not use in lactating dairy cattle
1977 50% a.i. w/v apply as a "one-shot" treatment or in dry dairy cows within 3 weeks
using 3 ml/100 kg bw (1.5 g before calving. Do not use later than
a.i./100 kg bw) as spot-on to 21 days before slaughter for meat
rump. animals.
New Zealand DURSBAN 10E For cattle lice control, use Do not use on dairy cows "in milk".
1971? License No. 1454 62.5 - 125 ml/100 litres of Treat dairy cows not later than 3
water weeks before calving. Treat beef
cattle not later than 3 weeks before
slaughtering.
1977? DURSBAN JS For control of cattle lice, use Do not use on dairy cows "in milk".
(400 g a.i./litre) 15-30 ml/100 litres, of water Spray no later than 3 weeks before
License No. 2941 applied as a saturating spray, calving for dry dairy cattle or
or 2 ml/50 kg live weight (1.6 before slaughter for meat animals.
g/100 kg bw) as a spot-on Apply spot-on no later than 5 weeks
treatment. before calving or slaughter.
1978 Ridlice For cattle, ectoparasites as Do not use on lactating dairy cattle
50% a.i. w/v one-shot application, use 3 ml/ or within 5 weeks before calving or
License No. 3485 100 kg bw (1.5 g a.i./100 kg bw) slaughter.
as spot-on to top of rump.
Table 1. (con't)
Country Product Use Comment
U.S.A. DURSBAN 24E For control of fever ticks in For use under supervision of qualified
1972 2 lb a.i./gal2/ beef cattle, use 1 ounce of state or federal personnel responsible
23.7% a.i., w/v product in 8 gallons of water for quarantine programmes. Withdrawal
EPA Reg No. (0.025% a.i.), and apply 3/4 to period of 2 weeks before slaughter.
464-450 1 gallon as spray treatment; or No more than 6 applications at 21-day
use 1/2 gallon of product per intervals per season.
500 gallons of water (0.025%
a.i.) for dip treatment.2/
U.S.A.
1979 DURSBAN 44 For lice control in beef cattle Do not treat dairy cows during
43.2% a.i. w/v and non-lactating dairy cattle, lactation. Do not treat dry dairy cows
EPA Reg. No. apply to back as a "spot-on" or replacement heifers within 60 days
464-542 treatment using 2 ml/100 lb bw before freshening. Do not slaughter
(2 g a.i. 100 kg bw) or 16 ml meat animals for 14 days after initial
maximum per animal. treatment, or after a second treatment
applied 45 days or more after the first
treatment. Do not slaughter for 21
days after second treatment applied
30 days after first treatment.
Canada
Temp. 1979 DURSBAN 44 Temporary registration for louse Do not treat within 14 days before
Expired 1980 43.2% a.i. control in beef cattle slaughter.
Argentina DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1969 240 g a.i./litre spraying and dipping using 336 weeks of treatment.
Reg. No. 1307 g a.i./1 000 litres of water.
1975 DURSBAN 18A Dip or spray treatment for Preslaughter interval of 2 days for
180 g a.i./litre cattle. meat animals (previously 2 weeks)
Table 1. (con't)
Country Product Use Comment
Brazil DURSBAN 1E Tickicide for cattle as spray Withdrawal period of 14 days after
1969, 1973 13% a.i. w/v or dip at 1:500 - 1:300 treatment.
Reg. No. 973 dilution
1975 DURSBAN 24E Control of ticks on cattle by Preslaughter limitation of 14 days
240 g a.i./litre spraying and dipping at after treatment. Also 48 hours
NBR 96/75 1:1 000 - 1:600 dilution limitation on use of milk after
treatment.
Colombia DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1972 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of water.
Costa Rica DURSBAN 24E " "
Expired 240 g a.i./litre
1976 Reg. No 255
Dominican DURSBAN 24E " "
Republic 240 g a.i./litre
1975
Ecuador DURSBAN 24E " "
1975 240 g a.i./litre
El Salvador DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1975 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of
water.
Table 1. (con't)
Country Product Use Comment
Mexico DURSBAN 24E " "
1969 240 g a.i./litre
Reg.No. 0-0480-015
Nicaragua DURSBAN 24E " "
1975 240 g a.i./litre
Panama DURSBAN 24E " "
1975 240 g a.i./litre
Reg.No. 15130
Trinidad DURSBAN 24E " "
1972 240 g a.i./litre
Venezuela DURSBAN 24E " Not to be used for 20 hours before
1972 240 g a.i./litre milking or 15 days before slaughter
of meat animals.
1/ Trademark of The Dow Chemical Company;
2/ 1 gallon = 3.8 litres;
3/ 1 lb (16 oz) = 0.45 kg.
Labels approved recently in Australia, New Zealand and the U.S.
recommends a one-shot "spot-on" treatment rather than dipping or
spraying. Limitations specify withdrawal intervals prior to slaughter
of meat animals, and state that the product is not to be used on dairy
cattle during lactation, nor within a stated interval prior to
freshening or calving.
In Canada, temporary approval expired in 1980 for use of
chlorpyrifos to control lice in cattle and pigs. In Europe, only one
recommendation was noted, as a dip for sheep using a mixture of
chlorpyrifos and gamma HCH (Rycovet 365 Dip, Glasgow, June 1978).
Recommendations for chlorpyrifos in Latin America generally do
not differentiate between beef animals and dairy animals. However, the
use of these products has been declining steadily, and the total sold
in 1981 amounted to less than 40 000 kg active ingredient for the
entire region. Records of Dow Chemical Co. indicate that less than
1 000 kg was sold in Brazil in each of 1979, 1980 and 1981, and none
was sold in Venezuela in 1981.
Although it is difficult to estimate how much chlorpyrifos is
actually used in lactating animals, the rate of milk production under
present agricultural practice in developing countries is inadequate
even for local consumption. Thus, it is highly unlikely that minor use
of chlorpyrifos in cows in certain Latin American countries would
result in residues in milk products in international trade.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In Meat
Cattle were treated once with DURSBAN 44 Insecticide at the rate
of 2 ml/100 lb of body weight (equivalent to 20 mg chlorpyrifos per
kg bw) applied as a "spot-on" to the top midline just posterior to the
withers. Groups of three cattle each were sacrificed the day after
treatment and at 7-day intervals through 35 days after application.
The maximum residue of chlorpyrifos was 1.4 mg/kg in fat at 7 days
after treatment (Table 2). Residues in muscle were much lower than in
fat, and did not exceed 0.6 mg/kg chlorpyrifos at any sampling. Thus
residues from "spot-on" treatment are not likely to exceed the
recommended limit of 2 mg/kg for chlorpyrifos in carcase meat on a fat
basis (McKellar and Dishburger 1976).
Table 2. Summary of Average Residues of Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol in Tissues of Cattle1/
Days from Average Residue Found, mg/kg (range, mg/kg)
Treatment Chlorpyrifos 3,5,6-Trichloro-2-Pyridinol
to Sampling Muscle Liver Kidney Fat Muscle Liver Kidney Fat
1 0.02 0.01 0.07 0.28 ND 0.74 0.52 0.10
(0.01-0.03) (0.01) (0.06-0.08) (0.03-0.46) (0.52-0.97) (0.45-0.56) (<0.05-0.18)
7 0.04 0.04 0.12 1.1 0.09 1.0 0.86 0.19
(0.01-0.06) (0.01-0.08) (0.09-0.18) (0.95-1.4) (<0.05-0.15) (0.67-1.5) (0.46-1.4) (0.08-0.24)
14 0.01 0.02 0.11 0.80 ND 0.59 0.52 0.07
(ND-0.01) (0.02-0.03) (0.08-0.16) (0.61-0.99) (0.47-0.69) (0.48-0.58) (0.06-0.08)
21 0.01 0.01 0.08 0.05 ND 0.42 0.29 0.14
(0.01) (<0.01-0.01) (0.06-0.10) (ND-0.14) (0.31-0.53) (0.14-0.57) (0.09-0.24)
24 0.01 ND 0.07 0.47 ND 0.20 0.10 <0.05
(0.01) (0.03-0.09) (0.34-0.71) (0.11-0.27) (0.06-0.13) (ND-0.05)
35 ND ND 0.03 0.20 ND 0.13 0.05 ND
(0.01-0.05) (0.02-0.34) (0.05-0.23) (0.04-0.07)
1/ All animals received a single treatment of Dursban spot-on (M-4110) at a rate of 2 ml/45 kg body weight.
Values corrected for control and recovery. ND = No detectable differences between treated and control samples.
Groups of three cattle were fed chlorpyrifos for 30 days at
levels of 3, 10, 30 and 100 ppm on a daily, dry matter intake basis.
Residues of chlorpyrifos were mainly in the fat, reaching maxima of
4.70 mg/kg in renal fat at 100 fed and 1.09 mg/kg in renal fat at
30 ppm fed. The residues of chlorpyrifos declined to less than 2 mg/kg
in fat within 7 days after discontinuation of feeding 100 ppm in the
total diet of the cattle for 30 days (Table 2).
The oxygen analogue of chlorpyrifos was shown to be unstable in
liver and kidney, and no residue was found in any tissue at any
feeding level. The pyridinol metabolite was not detected in muscle at
feeding levels of 3 and 10 ppm chlorpyrifos, but was present in liver
and kidney at all feeding levels, reaching maxima of 2.61 mg/kg in
liver at 100 ppm fed and 1.67 mg/kg in liver at 30 ppm fed (Dishburger
et al 1977).
In Milk
Lactating cows were fed a complete ration containing chlorpyrifos
at levels from 0.3 to 30 ppm for 2 weeks at each level. At 30 ppm in
the total diet, maximum residues of chlorpyrifos were 0.01 mg/kg in
milk and 0.10 mg/kg in cream. The oxygen analogue was not detected in
any sample of milk or cream at any dietary level of chlorpyrifos. The
pyridinol metabolite was detected at 0.01 mg/kg in milk of cows fed
30 ppm chlorpyrifos, but does not concentrate in fat. Thus, residues
resulting from ingestion of feedstuffs containing chlorpyrifos
residues amounting to no more than 10 - 15 mg/kg in the total diet
should not exceed the current recommendation of 0.1 mg/kg for
chlorpyrifos in milk on a fat basis (McKellar et al 1976).
FATE OF RESIDUES
In Animals
An additional metabolic study has been conducted using 14C-ring
labelled chlorpyrifos in lactating goats. The material was
administered via capsule twice daily at doses equivalent to 16 and
25 ppm in the daily feed for 10 days. The majority of the total 14C
activity was recovered in the urine (80.3%), with small amounts in the
faeces (3.6%), gut (0.9%) tissues (0.8%) and milk (0.1%).
Most of the radioactivity in the urine was excreted as the
ß-glucuronide conjugate of 3,5,6-trichloro-2-pyridinol with small
amounts of free pyridinol metabolite. About 75% of the 14C activity
in the fat was chlorpyrifos (0.1 mg/kg) with most of the remaining
14C activity hydrolysable to the pyridinol metabolite. The results of
this study validate the methodology used in earlier animal residue
studies (Glas 1981).
METHODS OF RESIDUE ANALYSIS
The analytical method used for analysis of beef tissues from the
"spot-on" treatment, was the procedure developed in 1972 for
determination of chlorpyrifos residues in chicken tissues and eggs.
Analysis is by gas chromatography using flame photometric detection.
The validated lower level of sensitivity is 0.01 mg/kg chlorpyrifos in
muscle, liver, kidney and fat of beef animals, as well as the chicken
muscle, liver, kidney and eggs (McKellar and Dishburger 1976; Wetters
1972).
An improved analytical method for the determination of
chlorpyrifos in milk and cream has lower limits of detection at 0.01
and 0.05 mg/kg in these matrices, respectively. The samples are
extracted with hexane, the extract partitioned into acetonitrile,
cleaned up on deactivated silica gel and analysed by gas
chromatography with flame photometric detection (Jeffries 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
Maximum residue limits reported to the Meeting for chlorpyrifos
in foods of animal origin are summarized in Table 3. The Codex MRLs
included for comparison are in terms of chlorpyrifos alone, since the
pyridinol hydrolysis product is of low mammalian toxicity and is
excreted rapidly in the urine and faeces of animals (FAO/WHO 1973b,
p. 162 and 194).
U.S. tolerances include its pyridinol metabolite, expressed as
chlorpyrifos, and were increased recently to include the estimated
contribution from ingestion of feedstuffs treated with chlorpyrifos.
The tolerances in feedstuffs, which do not exceed 15 mg/kg in any
case, also represent maximum total residues of chlorpyrifos and its
pyridinol hydrolysis product, expressed as chlorpyrifos, without
differentiation as to the contribution due to each component (U.S.
Code of Federal Regulations 1982).
APPRAISAL
New information on current uses of chlorpyrifos on animals and on
residues in milk from ingestion of treated feed indicate that such
uses should not result in residues in excess of the proposed CCPR MRLs
of 2 mg/kg in carcase meat of cattle (for fat-soluble pesticides, a
portion of carcase fat is analysed and the MRLs apply to carcase fat)
and of 0.1 mg/kg in milk and milk products (both on a fat basis).
Because chlorpyrifos residues in whole milk from cows fed up to
Table 3. Maximum Residue Limits for Chlorpyrifos1/ in
Food Products of Animal Origin
Cattle
Codex 2.0 mg/kg in carcase meat (carcase fat).
Australia 2.0 mg/kg in fat of meat of cattle.
Canada 1.0 mg/kg (temporary) in meat and meat by-products
(fat basis) and in fat, liver and kidney.
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 2.0 mg/kg in fat, meat and meat by-products.
Eggs
Codex 0.01 mg/kg (on shell-free basis).
U.S. 0.1 mg/kg.
Goats
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Pigs
Australia 0.1 mg/kg in fat of meat of pigs.
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Horses
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Milk
Codex 0.1 mg/kg (on a fat basis) including milk products.
Switzerland 0.005 mg/kg in whole milk.
U.S. 0.5 mg/kg in fat (reflecting 0.02 mg/kg in whole
milk).
Poultry
Codex 0.1 mg/kg in carcase meat (carcase fat).
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Sheep
Codex 0.2 mg/kg in carcase meat (carcase fat).
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Table 3. (con't)
Turkeys
Codex 0.2 mg/kg in carcase meat (skin and carcase fat).
U.S. 0.2 mg/kg in fat, meat and meat by-products.
1/ Tolerances in Canada and the U.S. include residues of
3,5,6-trichloro-2-pyridinol expressed as chlorpyrifos.
30 mg/kg in the total diet reached the measurable level of 0.01 mg/kg
and, in consideration of the current CCPR approach to the
establishment of limits for fat-soluble pesticides in milk, the MRL
for milk should be changed to 0.01 mg/kg for chlorpyrifos (the limit
of determination) in whole milk, from which the MRL for milk products
containing more than 2% fat would be calculated on a fat basis,
assuming 4% fat in raw milk.
RECOMMENDATIONS
The recommended maximum residue limit for milk and milk products
on a fat basis is deleted and replaced with a new maximum residue
limit for milk. The limit applies to chlorpyrifos only.
Commodity Limit (mg/kg)
Milk 0.01*
* at or about the limit of determination.
REFERENCES
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. and Rice, J.R.
1977 Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of
cattle fed chlorpyrifos. Journal of Agricultural Food Chem.
25(6): 1325-1329.
Glas, R.D. The metabolic fate of 14C-chlorpyrifos fed to lactating
1981 goats. Report GH-C 1408R, Dow Chemical Co, (Unpublished)
Jeffries, T.K. Determination of residues of chlorpyrifos in milk and
1980 cream. Method ACR 80.5. Dow Chemical Co. (Unpublished)
McKellar, R.L. and Dishburger, H.J. Determination of residues of
1976 chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues of
cattle receiving a single treatment of DURSBAN spot-on.
Report GH-C-930, Dow Chemical Co. (Unpublished)
McKellar, R.L., Dishburger, H.J., Rice, J.R., Craig, L.F. and
Pennington, J. Residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in milk and cream
from cows fed chlorpyrifos. Journal of Agricultural Food
Chem. 24(2): 283-286.
U.S. Code of Federal Regulations. Title 40: Section 180.342 for
1982 chlorpyrifos tolerances in raw agricultural commodities;
Section 193.85 for chlorpyrifos tolerances in processed
foods; Section 561.98 for chlorpyrifos residues in processed
feeds.
Wetters, J.H. Determination of residues of 0,0-diethyl 0-(3,5,6-
1972 trichloro-2-pyridyl) phosphorothioate in chicken tissues and
eggs by gas chromatography with flame photometric detection.
Method ARC 72.3. Dow Chemical Co. (Unpublished)
Explanation
Chlorpyrifos was reviewed by the Joint Meetings of 1972, 1974,
1975, 1977 and 1981 (FAO/WHO 1973, 1975, 1976, 1978, 1982)1/. An ADI
of 0-0.001 mg/kg bw was established, based on plasma cholinesterase
depression in humans. Additional reports on the toxicity of
chlorpyrifos have been submitted, including a two-year chronic
feeding/oncogenicity study in mice, a teratology study in mice, and a
delayed neurotoxicity study in hens. These studies have been reviewed
and summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 40-47 pregnant CF-1 mice were given 0, 1, 10 or
25 kg bw/day of chlorpyrifos by gavage on days 6 through 15 of
gestation. Administration of 25 mg/kg/day of chlorpyrifos resulted in
severe maternal toxicity. Additional groups of 35-41 pregnant mice
were gavaged with 0, 0.1, 1.0 and 10 mg chlorpyrifos/kg bw on days 6
through 15 of gestation, due to the maternal toxicity at 25 mg/kg body
weight. On day 18 of gestation all pregnant animals were sacrificed
and foetuses delivered by caesarean section. Maternal observations
included cageside observations; body, liver and gravid uterine
weights; food and water consumption; number and position of live,
dead, and resorbed foetuses and evidence of implantation sites. Foetal
body weights, crown-rump measurements, external alterations, together
with visceral and skeletal alterations, were similarly determined for
all foetuses. Separate groups of pregnant mice were also examined for
clinical evidence of cholinesterase depression during selected periods
of gestation, including ChE measurements of plasma and erythrocytes,
as well as the ChE of a foetal homogenate on gestation day 15.
1/ See Annex 2 for WHO and FAO documentation.
Evidence of maternal toxicity (including cholinesterase
inhibition, decreased body weight, decreased food and water
consumption and death) was observed among pregnant mice given 25 mg/kg
of chlorpyrifos. Plasma and erythrocyte cholinesterase levels were
significantly reduced among maternal mice at 1 mg/kg or greater and in
foetuses at dose levels of 10 mg/kg or greater.
Foetotoxicity, as evidenced by increased occurrence of minor
skeletal variants (i.e. delayed ossification of skull bones and
sternebrae) and decreased foetal body weight measurements, was noted
at the 25 mg/kg dose level.
Reproduction performance (as determined by implantation sites,
resorption sites and live young) was not affected by chlorpyrifos at
any dose level administered. Some malformations (exencephaly) were
observed among litters of mice given 1 mg/kg chlorpyrifos; however, a
dose-related teratogenic response was not observed nor repeated in the
additional mice given the same dose. No evidence of teratogenic
response was observed among mice given 0.1, 1, 10 or 25 mg/kg of
chlorpyrifos, during the critical period of organogenesis (Deacon
et al 1979).
Special Study on Neurotoxicity
Chicken
Groups of adult hens (av. 14.5 months) were administered
chlorpyrifos orally at dosage levels of 0, 25, 50, 100, 200 or
400 mg/kg bw, and the oral LD50 estimated to be 50 mg/kg bw. Separate
groups of non-fasted adult hens (av. 17 months) were given single oral
doses of 0, 50 and 100 mg chlorpyrifos/kg bw. A positive control
(TOTP, 232 mg/kg) was also used. Ten birds comprised each test group.
Atropine sulphate (30 mg/kg) was given to all birds prior to
administration of TOTP or chlorpyrifos. At the conclusion of the study
(21-24 days), all surviving hens were sacrificed and subjected to
gross and microscopic examinations. Axon and myelin degeneration were
assessed in the sciatic nerve and spinal cord.
There was no mortality in any group. Body weight was slightly,
but not statistically, depressed in the positive control (TOTP). There
were dose-dependent, acute cholinergic effects observed for the
chlorpyrifos treated groups. Symptoms included transient signs of
ataxia, CNS depression and paralysis, which recovered to normal within
2 to 4 days post-treatment. There were no signs of delayed
neurotoxicity observed with chlorpyrifos. The positive control animals
(TOTP) displayed delayed gross symptoms beginning on day 10 and
continued through to sacrifice. There were slight to severe signs of
ataxia in all birds, with partial paralysis in a few. Histologic
evaluation revealed no evidence of neurotoxic effects in either the
chlorpyrifos treated group or the negative control (atropine sulphate
only). Evidence of delayed neurotoxicity was apparent in the TOTP
group with the observation of swollen, degenerate, fragmented and
demyelinated axons in the spinal cord, plus swollen neurollemma
sheaths and vacuolation of the sciatic nerve. Based on the results of
this study, it was concluded that chlorpyrifos does not induce delayed
neurotoxic reactions in adult white leghorn hens when administered as
a single acute oral dose (Rowe et al 1978).
Special Study for Carcinogenicity
Mouse
Groups of CD-1 mice (56 male and 56 female/mice/group) were fed
chlorpyrifos in the diet at dosage levels of 0, 0.5, 5 and 15 ppm for
105 weeks. These dose levels were approximately equivalent to 0, 0.05,
0.5 and 1.5 mg/kg/day over the entire treatment period. The animals
were examined daily for mortality and adverse physical/behavioural
condition. Periodic evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All mice were killed by CO2 inhalation
and exsanguination via the axillary blood vessels. Peripheral blood
smears were prepared from tail blood from all survivors at terminal
sacrifice.
There were no unusual behavioural changes and no significant
effects on food consumption, body weight gain or mortality. Absolute
and relative organ weights of selected organs were varied and
unrelated to dose. Relative liver weights for both male and female
mice were significantly decreased (p <0.05) at the low- and mid-dose,
but not at the high dose. Histological examination revealed a
significant difference between control and mid-dose males for the
incidence of hyperplastic nodules of the liver. There was also a
significant increase in the incidence of spindle cell hyperplasia of
the adrenal gland for male mice at the low and mid-dose levels, and in
female mice at the low dose only. Lesions observed in the lung
included a significant increase of alveologenic adenomas in mid-dose
male mice only. The histologic changes identified appear to be
spontaneous in nature and without a demonstrated dose-response.
Furthermore, type A and B nodules of the liver parenchyma, as well as
the alveologenic carcinomas of the lung, occurred with equal frequency
between treatment and control group animals. There were no other
significant lesions or statistically increased hyperplastic and/or
nodular lesions. The results of this study demonstrate that
chlorpyrifos is not oncogenic in mice at dietary levels up to and
including 15 ppm for a period of 105 weeks (Warner et al 1980).
COMMENTS
The Meeting evaluated substantial new data submitted on
chlorpyrifos, including a mouse teratology study, a mouse
carcinogenicity study and a hen neurotoxicity study. Chlorpyrifos was
not teratogenic to mice at doses up to and including 25 mg/kg bw/day,
although severe maternal toxicity and foetotoxicity were evident at
that dose. It was also determined that 15 ppm of chlorpyrifos was not
oncogenic when administered in the diet of mice for 105 weeks. A
single acute oral dose of 100 mg/kg bw (twice the LD50) did not
induce a delayed neurotoxic response in hens. However, the Meeting
reiterated previously expressed JMPR opinions that a delayed
neurotoxicity study in hens requires multiple dosing, such that doses
are repeated 21 days apart for a total of two acute oral doses. In
this context, the present study may not fully address the delayed
neurotoxic potential of chlorpyrifos relative to residues in food.
Chlorpyrifos is recognized to be an active cholinesterase
inhibitor in several mammalian species, including humans. In all
species evaluated, plasma ChE or pseudo-cholinesterase was inhibited
at lower administered dose levels of chlorpyrifos than were RBC or
brain ChE (where determined). Chlorpyrifos was reviewed by the Joint
Meeting in 1972 (FAO/WHO 1973), which determined that chlorpyrifos
inhibited the plasma ChE in humans at 0.03 mg/kg bw, but not at
0.014 mg/kg bw (Coulston et al 1972). This served as a basis for the
ADI (0.0015 mg/kg), which was subsequently rounded off to 0.001 mg/kg
bw at the recommendation of the Joint Meeting of 1977 (FAO/WHO 1978).
However, appropriate statistical evaluation of the human
cholinesterase determinations in the Coulston et al study (1972)
(Park 1972) demonstrated 0.03 mg/kg to be a threshold level for
inhibition of plasma ChE, which was without statistical significance
when compared to current controls. Based upon repeated daily doses for
a period of 21 days, it was concluded by Coulston et al and Park
that a dose of 0.03 mg chlorpyrifos/kg bw has no significant
toxicological effect in humans. This conclusion is supported by the
evidence from studies in other mammalian species at comparable dose
levels (McCollister et al 1971a,b; Coulston et al 1971).
These same investigators determined that erythrocyte
cholinesterase activity was inhibited at comparable levels in
different species (e.g. 0.1 mg/kg for rat, dog and humans, and
0.08 mg/kg for monkey). Except for ChE depression, these levels were
without significant effects noted on mortality, behaviour, food
consumption growth, haematology, clinical chemistry, urinalysis, or
gross and microscopic examination of selected tissues (where
determined).
The Meeting accordingly increased the ADI from 0.001 to
0.01 mg/kg body weight, based on the level causing no inhibition of
RBC cholinesterase and not on the plasma cholinesterase.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0.1 mg/kg bw/day
Dog: 0.1 mg/kg bw/day
Man: 0.1 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Data on the inhibition, reactivation and ageing of cholinesterase
(in vitro).
REFERENCES
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
1971 and Norvell, M. Final report on safety evaluation and
metabolic studies on Dowco 179 (IN 151). Report from Insp.
Exp. Pathol. Toxicol., Albany Medical College, submitted to
the World Health Organization by Dow Chemical Company.
(Unpublished)
Coulston, F., Goldberg, L. and Griffin, T. Safety evaluation of
1972 Dowco 179 in human volunteers. Summary and tables 33 and 34,
reviewed by JMPR 1972. Report from Albany Medical College,
Albany, New York, submitted to the World Health Organization
by Dow Chemical Company. (Unpublished)
Deacon, M.M., Murray, J.S., Pilney, M.K., Dittenber, D.A., Hanley,
1979 T.R. Jr. and John, J.A. The effects of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Report from Dow Chemical Company submitted to the World
Health Organization by Dow Chemical Company. (Unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
1971a Results of two-year dietary feeding studies on DowcoR 179
in beagle dogs. Report T35.12.44793-18, from Dow Chemical
Company, submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
1971b Results of two-year dietary feeding studies on DowcoR179 in
rats. Report NBT35.12-44793-21, from Dow Chemical Company
submitted to the World Health Organization by Dow Chemical
Company (Unpublished)
Park, C. Analysis of plasma and red blood cell cholinesterase levels
1972 in humans ingesting DOWCO 179. Report from Dow Chemical
Company submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
Rowe, L.D., Warner, S.D. and Johnston, R.V. Acute delayed
1978 neurotoxicologic evaluation of chlorpyrifos in White Leghorn
hens. Report from Dow Chemical Company submitted to the
World Health Organization by Dow Chemical Company.
(Unpublished)
Warner, S.D., Gerbig, C.G., Strebing, R.J. and Molello, J.A. Results
1980 of a two-year toxicity and oncogenicity study of
chlorpyrifos administered to CD-1 mice in the diet. Report
from Dow Chemical Company, submitted to the World Health
Organization by Dow Chemical Company. (Unpublished)
CHLORPYRIFOS
Explanation
Chlorpyrifos was evaluated in 1972, 1974, 1975 and 1977 (FAO/WHO
1973, 1975, 1976, 1978)1/. Use patterns for chlorpyrifos have
undergone significant changes since 1972 with less emphasis on
previous animal applications, the development of "spot-on" animal
treatments and the emergence of more feed uses. At the 1982 meeting of
the CCPR, a question was raised concerning the recommended limit in
milk in view of these changing use patterns.
New information was made available to the Meeting on a survey of
international uses on dairy cattle, residues in meat from "spot-on"
treatment, residues in meat and milk from ingestion of treated feed,
metabolic fate in lactating goats, an improved method of analysis for
milk and cream and national maximum residue limits.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In Animals
A survey of uses of chlorpyrifos in animals was conducted by Dow
Chemical Co. in each geographic region in late 1981. This survey was
aimed specifically at clarifying whether dairy cattle are treated with
chlorpyrifos and, if so, whether such use is likely to lead to
residues greater than 0.1 mg/kg on a fat basis in milk or milk
products in international trade. Table 1 summarizes recommendations
for treatment of cattle with chlorpyrifos in each country where it has
been used.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Survey of Uses of Chlorpyrifos in Cattle: A. Developed Countries
Country Product Use Comment
Australia DURSBAN 1/24E For ticks and lice in cattle, Do not use on lactating cattle or in
all states 250 g/litre as dip at 1 litre/1 000 litres dairy cows later than 3 weeks before
1970-71 of water (0.025% a.i.), or as calving. Do not spray later than 3
spray at 200 ml/200 litres weeks before slaughter, or dip later
using at least 5 litres per than 3 days before slaughter of meat
animal. animals.
all states DURSBAN JS For louse control in cattle, Do not use in lactating dairy cattle
1977 50% a.i. w/v apply as a "one-shot" treatment or in dry dairy cows within 3 weeks
using 3 ml/100 kg bw (1.5 g before calving. Do not use later than
a.i./100 kg bw) as spot-on to 21 days before slaughter for meat
rump. animals.
New Zealand DURSBAN 10E For cattle lice control, use Do not use on dairy cows "in milk".
1971? License No. 1454 62.5 - 125 ml/100 litres of Treat dairy cows not later than 3
water weeks before calving. Treat beef
cattle not later than 3 weeks before
slaughtering.
1977? DURSBAN JS For control of cattle lice, use Do not use on dairy cows "in milk".
(400 g a.i./litre) 15-30 ml/100 litres, of water Spray no later than 3 weeks before
License No. 2941 applied as a saturating spray, calving for dry dairy cattle or
or 2 ml/50 kg live weight (1.6 before slaughter for meat animals.
g/100 kg bw) as a spot-on Apply spot-on no later than 5 weeks
treatment. before calving or slaughter.
1978 Ridlice For cattle, ectoparasites as Do not use on lactating dairy cattle
50% a.i. w/v one-shot application, use 3 ml/ or within 5 weeks before calving or
License No. 3485 100 kg bw (1.5 g a.i./100 kg bw) slaughter.
as spot-on to top of rump.
Table 1. (con't)
Country Product Use Comment
U.S.A. DURSBAN 24E For control of fever ticks in For use under supervision of qualified
1972 2 lb a.i./gal2/ beef cattle, use 1 ounce of state or federal personnel responsible
23.7% a.i., w/v product in 8 gallons of water for quarantine programmes. Withdrawal
EPA Reg No. (0.025% a.i.), and apply 3/4 to period of 2 weeks before slaughter.
464-450 1 gallon as spray treatment; or No more than 6 applications at 21-day
use 1/2 gallon of product per intervals per season.
500 gallons of water (0.025%
a.i.) for dip treatment.2/
U.S.A.
1979 DURSBAN 44 For lice control in beef cattle Do not treat dairy cows during
43.2% a.i. w/v and non-lactating dairy cattle, lactation. Do not treat dry dairy cows
EPA Reg. No. apply to back as a "spot-on" or replacement heifers within 60 days
464-542 treatment using 2 ml/100 lb bw before freshening. Do not slaughter
(2 g a.i. 100 kg bw) or 16 ml meat animals for 14 days after initial
maximum per animal. treatment, or after a second treatment
applied 45 days or more after the first
treatment. Do not slaughter for 21
days after second treatment applied
30 days after first treatment.
Canada
Temp. 1979 DURSBAN 44 Temporary registration for louse Do not treat within 14 days before
Expired 1980 43.2% a.i. control in beef cattle slaughter.
Argentina DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1969 240 g a.i./litre spraying and dipping using 336 weeks of treatment.
Reg. No. 1307 g a.i./1 000 litres of water.
1975 DURSBAN 18A Dip or spray treatment for Preslaughter interval of 2 days for
180 g a.i./litre cattle. meat animals (previously 2 weeks)
Table 1. (con't)
Country Product Use Comment
Brazil DURSBAN 1E Tickicide for cattle as spray Withdrawal period of 14 days after
1969, 1973 13% a.i. w/v or dip at 1:500 - 1:300 treatment.
Reg. No. 973 dilution
1975 DURSBAN 24E Control of ticks on cattle by Preslaughter limitation of 14 days
240 g a.i./litre spraying and dipping at after treatment. Also 48 hours
NBR 96/75 1:1 000 - 1:600 dilution limitation on use of milk after
treatment.
Colombia DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1972 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of water.
Costa Rica DURSBAN 24E " "
Expired 240 g a.i./litre
1976 Reg. No 255
Dominican DURSBAN 24E " "
Republic 240 g a.i./litre
1975
Ecuador DURSBAN 24E " "
1975 240 g a.i./litre
El Salvador DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1975 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of
water.
Table 1. (con't)
Country Product Use Comment
Mexico DURSBAN 24E " "
1969 240 g a.i./litre
Reg.No. 0-0480-015
Nicaragua DURSBAN 24E " "
1975 240 g a.i./litre
Panama DURSBAN 24E " "
1975 240 g a.i./litre
Reg.No. 15130
Trinidad DURSBAN 24E " "
1972 240 g a.i./litre
Venezuela DURSBAN 24E " Not to be used for 20 hours before
1972 240 g a.i./litre milking or 15 days before slaughter
of meat animals.
1/ Trademark of The Dow Chemical Company;
2/ 1 gallon = 3.8 litres;
3/ 1 lb (16 oz) = 0.45 kg.
Labels approved recently in Australia, New Zealand and the U.S.
recommends a one-shot "spot-on" treatment rather than dipping or
spraying. Limitations specify withdrawal intervals prior to slaughter
of meat animals, and state that the product is not to be used on dairy
cattle during lactation, nor within a stated interval prior to
freshening or calving.
In Canada, temporary approval expired in 1980 for use of
chlorpyrifos to control lice in cattle and pigs. In Europe, only one
recommendation was noted, as a dip for sheep using a mixture of
chlorpyrifos and gamma HCH (Rycovet 365 Dip, Glasgow, June 1978).
Recommendations for chlorpyrifos in Latin America generally do
not differentiate between beef animals and dairy animals. However, the
use of these products has been declining steadily, and the total sold
in 1981 amounted to less than 40 000 kg active ingredient for the
entire region. Records of Dow Chemical Co. indicate that less than
1 000 kg was sold in Brazil in each of 1979, 1980 and 1981, and none
was sold in Venezuela in 1981.
Although it is difficult to estimate how much chlorpyrifos is
actually used in lactating animals, the rate of milk production under
present agricultural practice in developing countries is inadequate
even for local consumption. Thus, it is highly unlikely that minor use
of chlorpyrifos in cows in certain Latin American countries would
result in residues in milk products in international trade.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In Meat
Cattle were treated once with DURSBAN 44 Insecticide at the rate
of 2 ml/100 lb of body weight (equivalent to 20 mg chlorpyrifos per
kg bw) applied as a "spot-on" to the top midline just posterior to the
withers. Groups of three cattle each were sacrificed the day after
treatment and at 7-day intervals through 35 days after application.
The maximum residue of chlorpyrifos was 1.4 mg/kg in fat at 7 days
after treatment (Table 2). Residues in muscle were much lower than in
fat, and did not exceed 0.6 mg/kg chlorpyrifos at any sampling. Thus
residues from "spot-on" treatment are not likely to exceed the
recommended limit of 2 mg/kg for chlorpyrifos in carcase meat on a fat
basis (McKellar and Dishburger 1976).
Table 2. Summary of Average Residues of Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol in Tissues of Cattle1/
Days from Average Residue Found, mg/kg (range, mg/kg)
Treatment Chlorpyrifos 3,5,6-Trichloro-2-Pyridinol
to Sampling Muscle Liver Kidney Fat Muscle Liver Kidney Fat
1 0.02 0.01 0.07 0.28 ND 0.74 0.52 0.10
(0.01-0.03) (0.01) (0.06-0.08) (0.03-0.46) (0.52-0.97) (0.45-0.56) (<0.05-0.18)
7 0.04 0.04 0.12 1.1 0.09 1.0 0.86 0.19
(0.01-0.06) (0.01-0.08) (0.09-0.18) (0.95-1.4) (<0.05-0.15) (0.67-1.5) (0.46-1.4) (0.08-0.24)
14 0.01 0.02 0.11 0.80 ND 0.59 0.52 0.07
(ND-0.01) (0.02-0.03) (0.08-0.16) (0.61-0.99) (0.47-0.69) (0.48-0.58) (0.06-0.08)
21 0.01 0.01 0.08 0.05 ND 0.42 0.29 0.14
(0.01) (<0.01-0.01) (0.06-0.10) (ND-0.14) (0.31-0.53) (0.14-0.57) (0.09-0.24)
24 0.01 ND 0.07 0.47 ND 0.20 0.10 <0.05
(0.01) (0.03-0.09) (0.34-0.71) (0.11-0.27) (0.06-0.13) (ND-0.05)
35 ND ND 0.03 0.20 ND 0.13 0.05 ND
(0.01-0.05) (0.02-0.34) (0.05-0.23) (0.04-0.07)
1/ All animals received a single treatment of Dursban spot-on (M-4110) at a rate of 2 ml/45 kg body weight.
Values corrected for control and recovery. ND = No detectable differences between treated and control samples.
Groups of three cattle were fed chlorpyrifos for 30 days at
levels of 3, 10, 30 and 100 ppm on a daily, dry matter intake basis.
Residues of chlorpyrifos were mainly in the fat, reaching maxima of
4.70 mg/kg in renal fat at 100 fed and 1.09 mg/kg in renal fat at
30 ppm fed. The residues of chlorpyrifos declined to less than 2 mg/kg
in fat within 7 days after discontinuation of feeding 100 ppm in the
total diet of the cattle for 30 days (Table 2).
The oxygen analogue of chlorpyrifos was shown to be unstable in
liver and kidney, and no residue was found in any tissue at any
feeding level. The pyridinol metabolite was not detected in muscle at
feeding levels of 3 and 10 ppm chlorpyrifos, but was present in liver
and kidney at all feeding levels, reaching maxima of 2.61 mg/kg in
liver at 100 ppm fed and 1.67 mg/kg in liver at 30 ppm fed (Dishburger
et al 1977).
In Milk
Lactating cows were fed a complete ration containing chlorpyrifos
at levels from 0.3 to 30 ppm for 2 weeks at each level. At 30 ppm in
the total diet, maximum residues of chlorpyrifos were 0.01 mg/kg in
milk and 0.10 mg/kg in cream. The oxygen analogue was not detected in
any sample of milk or cream at any dietary level of chlorpyrifos. The
pyridinol metabolite was detected at 0.01 mg/kg in milk of cows fed
30 ppm chlorpyrifos, but does not concentrate in fat. Thus, residues
resulting from ingestion of feedstuffs containing chlorpyrifos
residues amounting to no more than 10 - 15 mg/kg in the total diet
should not exceed the current recommendation of 0.1 mg/kg for
chlorpyrifos in milk on a fat basis (McKellar et al 1976).
FATE OF RESIDUES
In Animals
An additional metabolic study has been conducted using 14C-ring
labelled chlorpyrifos in lactating goats. The material was
administered via capsule twice daily at doses equivalent to 16 and
25 ppm in the daily feed for 10 days. The majority of the total 14C
activity was recovered in the urine (80.3%), with small amounts in the
faeces (3.6%), gut (0.9%) tissues (0.8%) and milk (0.1%).
Most of the radioactivity in the urine was excreted as the
ß-glucuronide conjugate of 3,5,6-trichloro-2-pyridinol with small
amounts of free pyridinol metabolite. About 75% of the 14C activity
in the fat was chlorpyrifos (0.1 mg/kg) with most of the remaining
14C activity hydrolysable to the pyridinol metabolite. The results of
this study validate the methodology used in earlier animal residue
studies (Glas 1981).
METHODS OF RESIDUE ANALYSIS
The analytical method used for analysis of beef tissues from the
"spot-on" treatment, was the procedure developed in 1972 for
determination of chlorpyrifos residues in chicken tissues and eggs.
Analysis is by gas chromatography using flame photometric detection.
The validated lower level of sensitivity is 0.01 mg/kg chlorpyrifos in
muscle, liver, kidney and fat of beef animals, as well as the chicken
muscle, liver, kidney and eggs (McKellar and Dishburger 1976; Wetters
1972).
An improved analytical method for the determination of
chlorpyrifos in milk and cream has lower limits of detection at 0.01
and 0.05 mg/kg in these matrices, respectively. The samples are
extracted with hexane, the extract partitioned into acetonitrile,
cleaned up on deactivated silica gel and analysed by gas
chromatography with flame photometric detection (Jeffries 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
Maximum residue limits reported to the Meeting for chlorpyrifos
in foods of animal origin are summarized in Table 3. The Codex MRLs
included for comparison are in terms of chlorpyrifos alone, since the
pyridinol hydrolysis product is of low mammalian toxicity and is
excreted rapidly in the urine and faeces of animals (FAO/WHO 1973b,
p. 162 and 194).
U.S. tolerances include its pyridinol metabolite, expressed as
chlorpyrifos, and were increased recently to include the estimated
contribution from ingestion of feedstuffs treated with chlorpyrifos.
The tolerances in feedstuffs, which do not exceed 15 mg/kg in any
case, also represent maximum total residues of chlorpyrifos and its
pyridinol hydrolysis product, expressed as chlorpyrifos, without
differentiation as to the contribution due to each component (U.S.
Code of Federal Regulations 1982).
APPRAISAL
New information on current uses of chlorpyrifos on animals and on
residues in milk from ingestion of treated feed indicate that such
uses should not result in residues in excess of the proposed CCPR MRLs
of 2 mg/kg in carcase meat of cattle (for fat-soluble pesticides, a
portion of carcase fat is analysed and the MRLs apply to carcase fat)
and of 0.1 mg/kg in milk and milk products (both on a fat basis).
Because chlorpyrifos residues in whole milk from cows fed up to
Table 3. Maximum Residue Limits for Chlorpyrifos1/ in
Food Products of Animal Origin
Cattle
Codex 2.0 mg/kg in carcase meat (carcase fat).
Australia 2.0 mg/kg in fat of meat of cattle.
Canada 1.0 mg/kg (temporary) in meat and meat by-products
(fat basis) and in fat, liver and kidney.
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 2.0 mg/kg in fat, meat and meat by-products.
Eggs
Codex 0.01 mg/kg (on shell-free basis).
U.S. 0.1 mg/kg.
Goats
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Pigs
Australia 0.1 mg/kg in fat of meat of pigs.
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Horses
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Milk
Codex 0.1 mg/kg (on a fat basis) including milk products.
Switzerland 0.005 mg/kg in whole milk.
U.S. 0.5 mg/kg in fat (reflecting 0.02 mg/kg in whole
milk).
Poultry
Codex 0.1 mg/kg in carcase meat (carcase fat).
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Sheep
Codex 0.2 mg/kg in carcase meat (carcase fat).
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Table 3. (con't)
Turkeys
Codex 0.2 mg/kg in carcase meat (skin and carcase fat).
U.S. 0.2 mg/kg in fat, meat and meat by-products.
1/ Tolerances in Canada and the U.S. include residues of
3,5,6-trichloro-2-pyridinol expressed as chlorpyrifos.
30 mg/kg in the total diet reached the measurable level of 0.01 mg/kg
and, in consideration of the current CCPR approach to the
establishment of limits for fat-soluble pesticides in milk, the MRL
for milk should be changed to 0.01 mg/kg for chlorpyrifos (the limit
of determination) in whole milk, from which the MRL for milk products
containing more than 2% fat would be calculated on a fat basis,
assuming 4% fat in raw milk.
RECOMMENDATIONS
The recommended maximum residue limit for milk and milk products
on a fat basis is deleted and replaced with a new maximum residue
limit for milk. The limit applies to chlorpyrifos only.
Commodity Limit (mg/kg)
Milk 0.01*
* at or about the limit of determination.
REFERENCES
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. and Rice, J.R.
1977 Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of
cattle fed chlorpyrifos. Journal of Agricultural Food Chem.
25(6): 1325-1329.
Glas, R.D. The metabolic fate of 14C-chlorpyrifos fed to lactating
1981 goats. Report GH-C 1408R, Dow Chemical Co, (Unpublished)
Jeffries, T.K. Determination of residues of chlorpyrifos in milk and
1980 cream. Method ACR 80.5. Dow Chemical Co. (Unpublished)
McKellar, R.L. and Dishburger, H.J. Determination of residues of
1976 chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues of
cattle receiving a single treatment of DURSBAN spot-on.
Report GH-C-930, Dow Chemical Co. (Unpublished)
McKellar, R.L., Dishburger, H.J., Rice, J.R., Craig, L.F. and
Pennington, J. Residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in milk and cream
from cows fed chlorpyrifos. Journal of Agricultural Food
Chem. 24(2): 283-286.
U.S. Code of Federal Regulations. Title 40: Section 180.342 for
1982 chlorpyrifos tolerances in raw agricultural commodities;
Section 193.85 for chlorpyrifos tolerances in processed
foods; Section 561.98 for chlorpyrifos residues in processed
feeds.
Wetters, J.H. Determination of residues of 0,0-diethyl 0-(3,5,6-
1972 trichloro-2-pyridyl) phosphorothioate in chicken tissues and
eggs by gas chromatography with flame photometric detection.
Method ARC 72.3. Dow Chemical Co. (Unpublished)
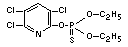 Explanation
Chlorpyrifos was reviewed by the Joint Meetings of 1972, 1974,
1975, 1977 and 1981 (FAO/WHO 1973, 1975, 1976, 1978, 1982)1/. An ADI
of 0-0.001 mg/kg bw was established, based on plasma cholinesterase
depression in humans. Additional reports on the toxicity of
chlorpyrifos have been submitted, including a two-year chronic
feeding/oncogenicity study in mice, a teratology study in mice, and a
delayed neurotoxicity study in hens. These studies have been reviewed
and summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 40-47 pregnant CF-1 mice were given 0, 1, 10 or
25 kg bw/day of chlorpyrifos by gavage on days 6 through 15 of
gestation. Administration of 25 mg/kg/day of chlorpyrifos resulted in
severe maternal toxicity. Additional groups of 35-41 pregnant mice
were gavaged with 0, 0.1, 1.0 and 10 mg chlorpyrifos/kg bw on days 6
through 15 of gestation, due to the maternal toxicity at 25 mg/kg body
weight. On day 18 of gestation all pregnant animals were sacrificed
and foetuses delivered by caesarean section. Maternal observations
included cageside observations; body, liver and gravid uterine
weights; food and water consumption; number and position of live,
dead, and resorbed foetuses and evidence of implantation sites. Foetal
body weights, crown-rump measurements, external alterations, together
with visceral and skeletal alterations, were similarly determined for
all foetuses. Separate groups of pregnant mice were also examined for
clinical evidence of cholinesterase depression during selected periods
of gestation, including ChE measurements of plasma and erythrocytes,
as well as the ChE of a foetal homogenate on gestation day 15.
1/ See Annex 2 for WHO and FAO documentation.
Evidence of maternal toxicity (including cholinesterase
inhibition, decreased body weight, decreased food and water
consumption and death) was observed among pregnant mice given 25 mg/kg
of chlorpyrifos. Plasma and erythrocyte cholinesterase levels were
significantly reduced among maternal mice at 1 mg/kg or greater and in
foetuses at dose levels of 10 mg/kg or greater.
Foetotoxicity, as evidenced by increased occurrence of minor
skeletal variants (i.e. delayed ossification of skull bones and
sternebrae) and decreased foetal body weight measurements, was noted
at the 25 mg/kg dose level.
Reproduction performance (as determined by implantation sites,
resorption sites and live young) was not affected by chlorpyrifos at
any dose level administered. Some malformations (exencephaly) were
observed among litters of mice given 1 mg/kg chlorpyrifos; however, a
dose-related teratogenic response was not observed nor repeated in the
additional mice given the same dose. No evidence of teratogenic
response was observed among mice given 0.1, 1, 10 or 25 mg/kg of
chlorpyrifos, during the critical period of organogenesis (Deacon
et al 1979).
Special Study on Neurotoxicity
Chicken
Groups of adult hens (av. 14.5 months) were administered
chlorpyrifos orally at dosage levels of 0, 25, 50, 100, 200 or
400 mg/kg bw, and the oral LD50 estimated to be 50 mg/kg bw. Separate
groups of non-fasted adult hens (av. 17 months) were given single oral
doses of 0, 50 and 100 mg chlorpyrifos/kg bw. A positive control
(TOTP, 232 mg/kg) was also used. Ten birds comprised each test group.
Atropine sulphate (30 mg/kg) was given to all birds prior to
administration of TOTP or chlorpyrifos. At the conclusion of the study
(21-24 days), all surviving hens were sacrificed and subjected to
gross and microscopic examinations. Axon and myelin degeneration were
assessed in the sciatic nerve and spinal cord.
There was no mortality in any group. Body weight was slightly,
but not statistically, depressed in the positive control (TOTP). There
were dose-dependent, acute cholinergic effects observed for the
chlorpyrifos treated groups. Symptoms included transient signs of
ataxia, CNS depression and paralysis, which recovered to normal within
2 to 4 days post-treatment. There were no signs of delayed
neurotoxicity observed with chlorpyrifos. The positive control animals
(TOTP) displayed delayed gross symptoms beginning on day 10 and
continued through to sacrifice. There were slight to severe signs of
ataxia in all birds, with partial paralysis in a few. Histologic
evaluation revealed no evidence of neurotoxic effects in either the
chlorpyrifos treated group or the negative control (atropine sulphate
only). Evidence of delayed neurotoxicity was apparent in the TOTP
group with the observation of swollen, degenerate, fragmented and
demyelinated axons in the spinal cord, plus swollen neurollemma
sheaths and vacuolation of the sciatic nerve. Based on the results of
this study, it was concluded that chlorpyrifos does not induce delayed
neurotoxic reactions in adult white leghorn hens when administered as
a single acute oral dose (Rowe et al 1978).
Special Study for Carcinogenicity
Mouse
Groups of CD-1 mice (56 male and 56 female/mice/group) were fed
chlorpyrifos in the diet at dosage levels of 0, 0.5, 5 and 15 ppm for
105 weeks. These dose levels were approximately equivalent to 0, 0.05,
0.5 and 1.5 mg/kg/day over the entire treatment period. The animals
were examined daily for mortality and adverse physical/behavioural
condition. Periodic evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All mice were killed by CO2 inhalation
and exsanguination via the axillary blood vessels. Peripheral blood
smears were prepared from tail blood from all survivors at terminal
sacrifice.
There were no unusual behavioural changes and no significant
effects on food consumption, body weight gain or mortality. Absolute
and relative organ weights of selected organs were varied and
unrelated to dose. Relative liver weights for both male and female
mice were significantly decreased (p <0.05) at the low- and mid-dose,
but not at the high dose. Histological examination revealed a
significant difference between control and mid-dose males for the
incidence of hyperplastic nodules of the liver. There was also a
significant increase in the incidence of spindle cell hyperplasia of
the adrenal gland for male mice at the low and mid-dose levels, and in
female mice at the low dose only. Lesions observed in the lung
included a significant increase of alveologenic adenomas in mid-dose
male mice only. The histologic changes identified appear to be
spontaneous in nature and without a demonstrated dose-response.
Furthermore, type A and B nodules of the liver parenchyma, as well as
the alveologenic carcinomas of the lung, occurred with equal frequency
between treatment and control group animals. There were no other
significant lesions or statistically increased hyperplastic and/or
nodular lesions. The results of this study demonstrate that
chlorpyrifos is not oncogenic in mice at dietary levels up to and
including 15 ppm for a period of 105 weeks (Warner et al 1980).
COMMENTS
The Meeting evaluated substantial new data submitted on
chlorpyrifos, including a mouse teratology study, a mouse
carcinogenicity study and a hen neurotoxicity study. Chlorpyrifos was
not teratogenic to mice at doses up to and including 25 mg/kg bw/day,
although severe maternal toxicity and foetotoxicity were evident at
that dose. It was also determined that 15 ppm of chlorpyrifos was not
oncogenic when administered in the diet of mice for 105 weeks. A
single acute oral dose of 100 mg/kg bw (twice the LD50) did not
induce a delayed neurotoxic response in hens. However, the Meeting
reiterated previously expressed JMPR opinions that a delayed
neurotoxicity study in hens requires multiple dosing, such that doses
are repeated 21 days apart for a total of two acute oral doses. In
this context, the present study may not fully address the delayed
neurotoxic potential of chlorpyrifos relative to residues in food.
Chlorpyrifos is recognized to be an active cholinesterase
inhibitor in several mammalian species, including humans. In all
species evaluated, plasma ChE or pseudo-cholinesterase was inhibited
at lower administered dose levels of chlorpyrifos than were RBC or
brain ChE (where determined). Chlorpyrifos was reviewed by the Joint
Meeting in 1972 (FAO/WHO 1973), which determined that chlorpyrifos
inhibited the plasma ChE in humans at 0.03 mg/kg bw, but not at
0.014 mg/kg bw (Coulston et al 1972). This served as a basis for the
ADI (0.0015 mg/kg), which was subsequently rounded off to 0.001 mg/kg
bw at the recommendation of the Joint Meeting of 1977 (FAO/WHO 1978).
However, appropriate statistical evaluation of the human
cholinesterase determinations in the Coulston et al study (1972)
(Park 1972) demonstrated 0.03 mg/kg to be a threshold level for
inhibition of plasma ChE, which was without statistical significance
when compared to current controls. Based upon repeated daily doses for
a period of 21 days, it was concluded by Coulston et al and Park
that a dose of 0.03 mg chlorpyrifos/kg bw has no significant
toxicological effect in humans. This conclusion is supported by the
evidence from studies in other mammalian species at comparable dose
levels (McCollister et al 1971a,b; Coulston et al 1971).
These same investigators determined that erythrocyte
cholinesterase activity was inhibited at comparable levels in
different species (e.g. 0.1 mg/kg for rat, dog and humans, and
0.08 mg/kg for monkey). Except for ChE depression, these levels were
without significant effects noted on mortality, behaviour, food
consumption growth, haematology, clinical chemistry, urinalysis, or
gross and microscopic examination of selected tissues (where
determined).
The Meeting accordingly increased the ADI from 0.001 to
0.01 mg/kg body weight, based on the level causing no inhibition of
RBC cholinesterase and not on the plasma cholinesterase.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0.1 mg/kg bw/day
Dog: 0.1 mg/kg bw/day
Man: 0.1 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Data on the inhibition, reactivation and ageing of cholinesterase
(in vitro).
REFERENCES
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
1971 and Norvell, M. Final report on safety evaluation and
metabolic studies on Dowco 179 (IN 151). Report from Insp.
Exp. Pathol. Toxicol., Albany Medical College, submitted to
the World Health Organization by Dow Chemical Company.
(Unpublished)
Coulston, F., Goldberg, L. and Griffin, T. Safety evaluation of
1972 Dowco 179 in human volunteers. Summary and tables 33 and 34,
reviewed by JMPR 1972. Report from Albany Medical College,
Albany, New York, submitted to the World Health Organization
by Dow Chemical Company. (Unpublished)
Deacon, M.M., Murray, J.S., Pilney, M.K., Dittenber, D.A., Hanley,
1979 T.R. Jr. and John, J.A. The effects of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Report from Dow Chemical Company submitted to the World
Health Organization by Dow Chemical Company. (Unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
1971a Results of two-year dietary feeding studies on DowcoR 179
in beagle dogs. Report T35.12.44793-18, from Dow Chemical
Company, submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
1971b Results of two-year dietary feeding studies on DowcoR179 in
rats. Report NBT35.12-44793-21, from Dow Chemical Company
submitted to the World Health Organization by Dow Chemical
Company (Unpublished)
Park, C. Analysis of plasma and red blood cell cholinesterase levels
1972 in humans ingesting DOWCO 179. Report from Dow Chemical
Company submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
Rowe, L.D., Warner, S.D. and Johnston, R.V. Acute delayed
1978 neurotoxicologic evaluation of chlorpyrifos in White Leghorn
hens. Report from Dow Chemical Company submitted to the
World Health Organization by Dow Chemical Company.
(Unpublished)
Warner, S.D., Gerbig, C.G., Strebing, R.J. and Molello, J.A. Results
1980 of a two-year toxicity and oncogenicity study of
chlorpyrifos administered to CD-1 mice in the diet. Report
from Dow Chemical Company, submitted to the World Health
Organization by Dow Chemical Company. (Unpublished)
CHLORPYRIFOS
Explanation
Chlorpyrifos was evaluated in 1972, 1974, 1975 and 1977 (FAO/WHO
1973, 1975, 1976, 1978)1/. Use patterns for chlorpyrifos have
undergone significant changes since 1972 with less emphasis on
previous animal applications, the development of "spot-on" animal
treatments and the emergence of more feed uses. At the 1982 meeting of
the CCPR, a question was raised concerning the recommended limit in
milk in view of these changing use patterns.
New information was made available to the Meeting on a survey of
international uses on dairy cattle, residues in meat from "spot-on"
treatment, residues in meat and milk from ingestion of treated feed,
metabolic fate in lactating goats, an improved method of analysis for
milk and cream and national maximum residue limits.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In Animals
A survey of uses of chlorpyrifos in animals was conducted by Dow
Chemical Co. in each geographic region in late 1981. This survey was
aimed specifically at clarifying whether dairy cattle are treated with
chlorpyrifos and, if so, whether such use is likely to lead to
residues greater than 0.1 mg/kg on a fat basis in milk or milk
products in international trade. Table 1 summarizes recommendations
for treatment of cattle with chlorpyrifos in each country where it has
been used.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Survey of Uses of Chlorpyrifos in Cattle: A. Developed Countries
Country Product Use Comment
Australia DURSBAN 1/24E For ticks and lice in cattle, Do not use on lactating cattle or in
all states 250 g/litre as dip at 1 litre/1 000 litres dairy cows later than 3 weeks before
1970-71 of water (0.025% a.i.), or as calving. Do not spray later than 3
spray at 200 ml/200 litres weeks before slaughter, or dip later
using at least 5 litres per than 3 days before slaughter of meat
animal. animals.
all states DURSBAN JS For louse control in cattle, Do not use in lactating dairy cattle
1977 50% a.i. w/v apply as a "one-shot" treatment or in dry dairy cows within 3 weeks
using 3 ml/100 kg bw (1.5 g before calving. Do not use later than
a.i./100 kg bw) as spot-on to 21 days before slaughter for meat
rump. animals.
New Zealand DURSBAN 10E For cattle lice control, use Do not use on dairy cows "in milk".
1971? License No. 1454 62.5 - 125 ml/100 litres of Treat dairy cows not later than 3
water weeks before calving. Treat beef
cattle not later than 3 weeks before
slaughtering.
1977? DURSBAN JS For control of cattle lice, use Do not use on dairy cows "in milk".
(400 g a.i./litre) 15-30 ml/100 litres, of water Spray no later than 3 weeks before
License No. 2941 applied as a saturating spray, calving for dry dairy cattle or
or 2 ml/50 kg live weight (1.6 before slaughter for meat animals.
g/100 kg bw) as a spot-on Apply spot-on no later than 5 weeks
treatment. before calving or slaughter.
1978 Ridlice For cattle, ectoparasites as Do not use on lactating dairy cattle
50% a.i. w/v one-shot application, use 3 ml/ or within 5 weeks before calving or
License No. 3485 100 kg bw (1.5 g a.i./100 kg bw) slaughter.
as spot-on to top of rump.
Table 1. (con't)
Country Product Use Comment
U.S.A. DURSBAN 24E For control of fever ticks in For use under supervision of qualified
1972 2 lb a.i./gal2/ beef cattle, use 1 ounce of state or federal personnel responsible
23.7% a.i., w/v product in 8 gallons of water for quarantine programmes. Withdrawal
EPA Reg No. (0.025% a.i.), and apply 3/4 to period of 2 weeks before slaughter.
464-450 1 gallon as spray treatment; or No more than 6 applications at 21-day
use 1/2 gallon of product per intervals per season.
500 gallons of water (0.025%
a.i.) for dip treatment.2/
U.S.A.
1979 DURSBAN 44 For lice control in beef cattle Do not treat dairy cows during
43.2% a.i. w/v and non-lactating dairy cattle, lactation. Do not treat dry dairy cows
EPA Reg. No. apply to back as a "spot-on" or replacement heifers within 60 days
464-542 treatment using 2 ml/100 lb bw before freshening. Do not slaughter
(2 g a.i. 100 kg bw) or 16 ml meat animals for 14 days after initial
maximum per animal. treatment, or after a second treatment
applied 45 days or more after the first
treatment. Do not slaughter for 21
days after second treatment applied
30 days after first treatment.
Canada
Temp. 1979 DURSBAN 44 Temporary registration for louse Do not treat within 14 days before
Expired 1980 43.2% a.i. control in beef cattle slaughter.
Argentina DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1969 240 g a.i./litre spraying and dipping using 336 weeks of treatment.
Reg. No. 1307 g a.i./1 000 litres of water.
1975 DURSBAN 18A Dip or spray treatment for Preslaughter interval of 2 days for
180 g a.i./litre cattle. meat animals (previously 2 weeks)
Table 1. (con't)
Country Product Use Comment
Brazil DURSBAN 1E Tickicide for cattle as spray Withdrawal period of 14 days after
1969, 1973 13% a.i. w/v or dip at 1:500 - 1:300 treatment.
Reg. No. 973 dilution
1975 DURSBAN 24E Control of ticks on cattle by Preslaughter limitation of 14 days
240 g a.i./litre spraying and dipping at after treatment. Also 48 hours
NBR 96/75 1:1 000 - 1:600 dilution limitation on use of milk after
treatment.
Colombia DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1972 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of water.
Costa Rica DURSBAN 24E " "
Expired 240 g a.i./litre
1976 Reg. No 255
Dominican DURSBAN 24E " "
Republic 240 g a.i./litre
1975
Ecuador DURSBAN 24E " "
1975 240 g a.i./litre
El Salvador DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1975 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of
water.
Table 1. (con't)
Country Product Use Comment
Mexico DURSBAN 24E " "
1969 240 g a.i./litre
Reg.No. 0-0480-015
Nicaragua DURSBAN 24E " "
1975 240 g a.i./litre
Panama DURSBAN 24E " "
1975 240 g a.i./litre
Reg.No. 15130
Trinidad DURSBAN 24E " "
1972 240 g a.i./litre
Venezuela DURSBAN 24E " Not to be used for 20 hours before
1972 240 g a.i./litre milking or 15 days before slaughter
of meat animals.
1/ Trademark of The Dow Chemical Company;
2/ 1 gallon = 3.8 litres;
3/ 1 lb (16 oz) = 0.45 kg.
Labels approved recently in Australia, New Zealand and the U.S.
recommends a one-shot "spot-on" treatment rather than dipping or
spraying. Limitations specify withdrawal intervals prior to slaughter
of meat animals, and state that the product is not to be used on dairy
cattle during lactation, nor within a stated interval prior to
freshening or calving.
In Canada, temporary approval expired in 1980 for use of
chlorpyrifos to control lice in cattle and pigs. In Europe, only one
recommendation was noted, as a dip for sheep using a mixture of
chlorpyrifos and gamma HCH (Rycovet 365 Dip, Glasgow, June 1978).
Recommendations for chlorpyrifos in Latin America generally do
not differentiate between beef animals and dairy animals. However, the
use of these products has been declining steadily, and the total sold
in 1981 amounted to less than 40 000 kg active ingredient for the
entire region. Records of Dow Chemical Co. indicate that less than
1 000 kg was sold in Brazil in each of 1979, 1980 and 1981, and none
was sold in Venezuela in 1981.
Although it is difficult to estimate how much chlorpyrifos is
actually used in lactating animals, the rate of milk production under
present agricultural practice in developing countries is inadequate
even for local consumption. Thus, it is highly unlikely that minor use
of chlorpyrifos in cows in certain Latin American countries would
result in residues in milk products in international trade.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In Meat
Cattle were treated once with DURSBAN 44 Insecticide at the rate
of 2 ml/100 lb of body weight (equivalent to 20 mg chlorpyrifos per
kg bw) applied as a "spot-on" to the top midline just posterior to the
withers. Groups of three cattle each were sacrificed the day after
treatment and at 7-day intervals through 35 days after application.
The maximum residue of chlorpyrifos was 1.4 mg/kg in fat at 7 days
after treatment (Table 2). Residues in muscle were much lower than in
fat, and did not exceed 0.6 mg/kg chlorpyrifos at any sampling. Thus
residues from "spot-on" treatment are not likely to exceed the
recommended limit of 2 mg/kg for chlorpyrifos in carcase meat on a fat
basis (McKellar and Dishburger 1976).
Table 2. Summary of Average Residues of Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol in Tissues of Cattle1/
Days from Average Residue Found, mg/kg (range, mg/kg)
Treatment Chlorpyrifos 3,5,6-Trichloro-2-Pyridinol
to Sampling Muscle Liver Kidney Fat Muscle Liver Kidney Fat
1 0.02 0.01 0.07 0.28 ND 0.74 0.52 0.10
(0.01-0.03) (0.01) (0.06-0.08) (0.03-0.46) (0.52-0.97) (0.45-0.56) (<0.05-0.18)
7 0.04 0.04 0.12 1.1 0.09 1.0 0.86 0.19
(0.01-0.06) (0.01-0.08) (0.09-0.18) (0.95-1.4) (<0.05-0.15) (0.67-1.5) (0.46-1.4) (0.08-0.24)
14 0.01 0.02 0.11 0.80 ND 0.59 0.52 0.07
(ND-0.01) (0.02-0.03) (0.08-0.16) (0.61-0.99) (0.47-0.69) (0.48-0.58) (0.06-0.08)
21 0.01 0.01 0.08 0.05 ND 0.42 0.29 0.14
(0.01) (<0.01-0.01) (0.06-0.10) (ND-0.14) (0.31-0.53) (0.14-0.57) (0.09-0.24)
24 0.01 ND 0.07 0.47 ND 0.20 0.10 <0.05
(0.01) (0.03-0.09) (0.34-0.71) (0.11-0.27) (0.06-0.13) (ND-0.05)
35 ND ND 0.03 0.20 ND 0.13 0.05 ND
(0.01-0.05) (0.02-0.34) (0.05-0.23) (0.04-0.07)
1/ All animals received a single treatment of Dursban spot-on (M-4110) at a rate of 2 ml/45 kg body weight.
Values corrected for control and recovery. ND = No detectable differences between treated and control samples.
Groups of three cattle were fed chlorpyrifos for 30 days at
levels of 3, 10, 30 and 100 ppm on a daily, dry matter intake basis.
Residues of chlorpyrifos were mainly in the fat, reaching maxima of
4.70 mg/kg in renal fat at 100 fed and 1.09 mg/kg in renal fat at
30 ppm fed. The residues of chlorpyrifos declined to less than 2 mg/kg
in fat within 7 days after discontinuation of feeding 100 ppm in the
total diet of the cattle for 30 days (Table 2).
The oxygen analogue of chlorpyrifos was shown to be unstable in
liver and kidney, and no residue was found in any tissue at any
feeding level. The pyridinol metabolite was not detected in muscle at
feeding levels of 3 and 10 ppm chlorpyrifos, but was present in liver
and kidney at all feeding levels, reaching maxima of 2.61 mg/kg in
liver at 100 ppm fed and 1.67 mg/kg in liver at 30 ppm fed (Dishburger
et al 1977).
In Milk
Lactating cows were fed a complete ration containing chlorpyrifos
at levels from 0.3 to 30 ppm for 2 weeks at each level. At 30 ppm in
the total diet, maximum residues of chlorpyrifos were 0.01 mg/kg in
milk and 0.10 mg/kg in cream. The oxygen analogue was not detected in
any sample of milk or cream at any dietary level of chlorpyrifos. The
pyridinol metabolite was detected at 0.01 mg/kg in milk of cows fed
30 ppm chlorpyrifos, but does not concentrate in fat. Thus, residues
resulting from ingestion of feedstuffs containing chlorpyrifos
residues amounting to no more than 10 - 15 mg/kg in the total diet
should not exceed the current recommendation of 0.1 mg/kg for
chlorpyrifos in milk on a fat basis (McKellar et al 1976).
FATE OF RESIDUES
In Animals
An additional metabolic study has been conducted using 14C-ring
labelled chlorpyrifos in lactating goats. The material was
administered via capsule twice daily at doses equivalent to 16 and
25 ppm in the daily feed for 10 days. The majority of the total 14C
activity was recovered in the urine (80.3%), with small amounts in the
faeces (3.6%), gut (0.9%) tissues (0.8%) and milk (0.1%).
Most of the radioactivity in the urine was excreted as the
ß-glucuronide conjugate of 3,5,6-trichloro-2-pyridinol with small
amounts of free pyridinol metabolite. About 75% of the 14C activity
in the fat was chlorpyrifos (0.1 mg/kg) with most of the remaining
14C activity hydrolysable to the pyridinol metabolite. The results of
this study validate the methodology used in earlier animal residue
studies (Glas 1981).
METHODS OF RESIDUE ANALYSIS
The analytical method used for analysis of beef tissues from the
"spot-on" treatment, was the procedure developed in 1972 for
determination of chlorpyrifos residues in chicken tissues and eggs.
Analysis is by gas chromatography using flame photometric detection.
The validated lower level of sensitivity is 0.01 mg/kg chlorpyrifos in
muscle, liver, kidney and fat of beef animals, as well as the chicken
muscle, liver, kidney and eggs (McKellar and Dishburger 1976; Wetters
1972).
An improved analytical method for the determination of
chlorpyrifos in milk and cream has lower limits of detection at 0.01
and 0.05 mg/kg in these matrices, respectively. The samples are
extracted with hexane, the extract partitioned into acetonitrile,
cleaned up on deactivated silica gel and analysed by gas
chromatography with flame photometric detection (Jeffries 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
Maximum residue limits reported to the Meeting for chlorpyrifos
in foods of animal origin are summarized in Table 3. The Codex MRLs
included for comparison are in terms of chlorpyrifos alone, since the
pyridinol hydrolysis product is of low mammalian toxicity and is
excreted rapidly in the urine and faeces of animals (FAO/WHO 1973b,
p. 162 and 194).
U.S. tolerances include its pyridinol metabolite, expressed as
chlorpyrifos, and were increased recently to include the estimated
contribution from ingestion of feedstuffs treated with chlorpyrifos.
The tolerances in feedstuffs, which do not exceed 15 mg/kg in any
case, also represent maximum total residues of chlorpyrifos and its
pyridinol hydrolysis product, expressed as chlorpyrifos, without
differentiation as to the contribution due to each component (U.S.
Code of Federal Regulations 1982).
APPRAISAL
New information on current uses of chlorpyrifos on animals and on
residues in milk from ingestion of treated feed indicate that such
uses should not result in residues in excess of the proposed CCPR MRLs
of 2 mg/kg in carcase meat of cattle (for fat-soluble pesticides, a
portion of carcase fat is analysed and the MRLs apply to carcase fat)
and of 0.1 mg/kg in milk and milk products (both on a fat basis).
Because chlorpyrifos residues in whole milk from cows fed up to
Table 3. Maximum Residue Limits for Chlorpyrifos1/ in
Food Products of Animal Origin
Cattle
Codex 2.0 mg/kg in carcase meat (carcase fat).
Australia 2.0 mg/kg in fat of meat of cattle.
Canada 1.0 mg/kg (temporary) in meat and meat by-products
(fat basis) and in fat, liver and kidney.
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 2.0 mg/kg in fat, meat and meat by-products.
Eggs
Codex 0.01 mg/kg (on shell-free basis).
U.S. 0.1 mg/kg.
Goats
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Pigs
Australia 0.1 mg/kg in fat of meat of pigs.
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Horses
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Milk
Codex 0.1 mg/kg (on a fat basis) including milk products.
Switzerland 0.005 mg/kg in whole milk.
U.S. 0.5 mg/kg in fat (reflecting 0.02 mg/kg in whole
milk).
Poultry
Codex 0.1 mg/kg in carcase meat (carcase fat).
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Sheep
Codex 0.2 mg/kg in carcase meat (carcase fat).
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Table 3. (con't)
Turkeys
Codex 0.2 mg/kg in carcase meat (skin and carcase fat).
U.S. 0.2 mg/kg in fat, meat and meat by-products.
1/ Tolerances in Canada and the U.S. include residues of
3,5,6-trichloro-2-pyridinol expressed as chlorpyrifos.
30 mg/kg in the total diet reached the measurable level of 0.01 mg/kg
and, in consideration of the current CCPR approach to the
establishment of limits for fat-soluble pesticides in milk, the MRL
for milk should be changed to 0.01 mg/kg for chlorpyrifos (the limit
of determination) in whole milk, from which the MRL for milk products
containing more than 2% fat would be calculated on a fat basis,
assuming 4% fat in raw milk.
RECOMMENDATIONS
The recommended maximum residue limit for milk and milk products
on a fat basis is deleted and replaced with a new maximum residue
limit for milk. The limit applies to chlorpyrifos only.
Commodity Limit (mg/kg)
Milk 0.01*
* at or about the limit of determination.
REFERENCES
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. and Rice, J.R.
1977 Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of
cattle fed chlorpyrifos. Journal of Agricultural Food Chem.
25(6): 1325-1329.
Glas, R.D. The metabolic fate of 14C-chlorpyrifos fed to lactating
1981 goats. Report GH-C 1408R, Dow Chemical Co, (Unpublished)
Jeffries, T.K. Determination of residues of chlorpyrifos in milk and
1980 cream. Method ACR 80.5. Dow Chemical Co. (Unpublished)
McKellar, R.L. and Dishburger, H.J. Determination of residues of
1976 chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues of
cattle receiving a single treatment of DURSBAN spot-on.
Report GH-C-930, Dow Chemical Co. (Unpublished)
McKellar, R.L., Dishburger, H.J., Rice, J.R., Craig, L.F. and
Pennington, J. Residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in milk and cream
from cows fed chlorpyrifos. Journal of Agricultural Food
Chem. 24(2): 283-286.
U.S. Code of Federal Regulations. Title 40: Section 180.342 for
1982 chlorpyrifos tolerances in raw agricultural commodities;
Section 193.85 for chlorpyrifos tolerances in processed
foods; Section 561.98 for chlorpyrifos residues in processed
feeds.
Wetters, J.H. Determination of residues of 0,0-diethyl 0-(3,5,6-
1972 trichloro-2-pyridyl) phosphorothioate in chicken tissues and
eggs by gas chromatography with flame photometric detection.
Method ARC 72.3. Dow Chemical Co. (Unpublished)
Explanation
Chlorpyrifos was reviewed by the Joint Meetings of 1972, 1974,
1975, 1977 and 1981 (FAO/WHO 1973, 1975, 1976, 1978, 1982)1/. An ADI
of 0-0.001 mg/kg bw was established, based on plasma cholinesterase
depression in humans. Additional reports on the toxicity of
chlorpyrifos have been submitted, including a two-year chronic
feeding/oncogenicity study in mice, a teratology study in mice, and a
delayed neurotoxicity study in hens. These studies have been reviewed
and summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
TOXICOLOGICAL STUDIES
Special Study on Teratogenicity
Groups of 40-47 pregnant CF-1 mice were given 0, 1, 10 or
25 kg bw/day of chlorpyrifos by gavage on days 6 through 15 of
gestation. Administration of 25 mg/kg/day of chlorpyrifos resulted in
severe maternal toxicity. Additional groups of 35-41 pregnant mice
were gavaged with 0, 0.1, 1.0 and 10 mg chlorpyrifos/kg bw on days 6
through 15 of gestation, due to the maternal toxicity at 25 mg/kg body
weight. On day 18 of gestation all pregnant animals were sacrificed
and foetuses delivered by caesarean section. Maternal observations
included cageside observations; body, liver and gravid uterine
weights; food and water consumption; number and position of live,
dead, and resorbed foetuses and evidence of implantation sites. Foetal
body weights, crown-rump measurements, external alterations, together
with visceral and skeletal alterations, were similarly determined for
all foetuses. Separate groups of pregnant mice were also examined for
clinical evidence of cholinesterase depression during selected periods
of gestation, including ChE measurements of plasma and erythrocytes,
as well as the ChE of a foetal homogenate on gestation day 15.
1/ See Annex 2 for WHO and FAO documentation.
Evidence of maternal toxicity (including cholinesterase
inhibition, decreased body weight, decreased food and water
consumption and death) was observed among pregnant mice given 25 mg/kg
of chlorpyrifos. Plasma and erythrocyte cholinesterase levels were
significantly reduced among maternal mice at 1 mg/kg or greater and in
foetuses at dose levels of 10 mg/kg or greater.
Foetotoxicity, as evidenced by increased occurrence of minor
skeletal variants (i.e. delayed ossification of skull bones and
sternebrae) and decreased foetal body weight measurements, was noted
at the 25 mg/kg dose level.
Reproduction performance (as determined by implantation sites,
resorption sites and live young) was not affected by chlorpyrifos at
any dose level administered. Some malformations (exencephaly) were
observed among litters of mice given 1 mg/kg chlorpyrifos; however, a
dose-related teratogenic response was not observed nor repeated in the
additional mice given the same dose. No evidence of teratogenic
response was observed among mice given 0.1, 1, 10 or 25 mg/kg of
chlorpyrifos, during the critical period of organogenesis (Deacon
et al 1979).
Special Study on Neurotoxicity
Chicken
Groups of adult hens (av. 14.5 months) were administered
chlorpyrifos orally at dosage levels of 0, 25, 50, 100, 200 or
400 mg/kg bw, and the oral LD50 estimated to be 50 mg/kg bw. Separate
groups of non-fasted adult hens (av. 17 months) were given single oral
doses of 0, 50 and 100 mg chlorpyrifos/kg bw. A positive control
(TOTP, 232 mg/kg) was also used. Ten birds comprised each test group.
Atropine sulphate (30 mg/kg) was given to all birds prior to
administration of TOTP or chlorpyrifos. At the conclusion of the study
(21-24 days), all surviving hens were sacrificed and subjected to
gross and microscopic examinations. Axon and myelin degeneration were
assessed in the sciatic nerve and spinal cord.
There was no mortality in any group. Body weight was slightly,
but not statistically, depressed in the positive control (TOTP). There
were dose-dependent, acute cholinergic effects observed for the
chlorpyrifos treated groups. Symptoms included transient signs of
ataxia, CNS depression and paralysis, which recovered to normal within
2 to 4 days post-treatment. There were no signs of delayed
neurotoxicity observed with chlorpyrifos. The positive control animals
(TOTP) displayed delayed gross symptoms beginning on day 10 and
continued through to sacrifice. There were slight to severe signs of
ataxia in all birds, with partial paralysis in a few. Histologic
evaluation revealed no evidence of neurotoxic effects in either the
chlorpyrifos treated group or the negative control (atropine sulphate
only). Evidence of delayed neurotoxicity was apparent in the TOTP
group with the observation of swollen, degenerate, fragmented and
demyelinated axons in the spinal cord, plus swollen neurollemma
sheaths and vacuolation of the sciatic nerve. Based on the results of
this study, it was concluded that chlorpyrifos does not induce delayed
neurotoxic reactions in adult white leghorn hens when administered as
a single acute oral dose (Rowe et al 1978).
Special Study for Carcinogenicity
Mouse
Groups of CD-1 mice (56 male and 56 female/mice/group) were fed
chlorpyrifos in the diet at dosage levels of 0, 0.5, 5 and 15 ppm for
105 weeks. These dose levels were approximately equivalent to 0, 0.05,
0.5 and 1.5 mg/kg/day over the entire treatment period. The animals
were examined daily for mortality and adverse physical/behavioural
condition. Periodic evaluation of food consumption and body weights
were performed. At the conclusion of the study, or at death, organ
weights and gross and microscopic examination of tissues and organs of
all animals were performed. All mice were killed by CO2 inhalation
and exsanguination via the axillary blood vessels. Peripheral blood
smears were prepared from tail blood from all survivors at terminal
sacrifice.
There were no unusual behavioural changes and no significant
effects on food consumption, body weight gain or mortality. Absolute
and relative organ weights of selected organs were varied and
unrelated to dose. Relative liver weights for both male and female
mice were significantly decreased (p <0.05) at the low- and mid-dose,
but not at the high dose. Histological examination revealed a
significant difference between control and mid-dose males for the
incidence of hyperplastic nodules of the liver. There was also a
significant increase in the incidence of spindle cell hyperplasia of
the adrenal gland for male mice at the low and mid-dose levels, and in
female mice at the low dose only. Lesions observed in the lung
included a significant increase of alveologenic adenomas in mid-dose
male mice only. The histologic changes identified appear to be
spontaneous in nature and without a demonstrated dose-response.
Furthermore, type A and B nodules of the liver parenchyma, as well as
the alveologenic carcinomas of the lung, occurred with equal frequency
between treatment and control group animals. There were no other
significant lesions or statistically increased hyperplastic and/or
nodular lesions. The results of this study demonstrate that
chlorpyrifos is not oncogenic in mice at dietary levels up to and
including 15 ppm for a period of 105 weeks (Warner et al 1980).
COMMENTS
The Meeting evaluated substantial new data submitted on
chlorpyrifos, including a mouse teratology study, a mouse
carcinogenicity study and a hen neurotoxicity study. Chlorpyrifos was
not teratogenic to mice at doses up to and including 25 mg/kg bw/day,
although severe maternal toxicity and foetotoxicity were evident at
that dose. It was also determined that 15 ppm of chlorpyrifos was not
oncogenic when administered in the diet of mice for 105 weeks. A
single acute oral dose of 100 mg/kg bw (twice the LD50) did not
induce a delayed neurotoxic response in hens. However, the Meeting
reiterated previously expressed JMPR opinions that a delayed
neurotoxicity study in hens requires multiple dosing, such that doses
are repeated 21 days apart for a total of two acute oral doses. In
this context, the present study may not fully address the delayed
neurotoxic potential of chlorpyrifos relative to residues in food.
Chlorpyrifos is recognized to be an active cholinesterase
inhibitor in several mammalian species, including humans. In all
species evaluated, plasma ChE or pseudo-cholinesterase was inhibited
at lower administered dose levels of chlorpyrifos than were RBC or
brain ChE (where determined). Chlorpyrifos was reviewed by the Joint
Meeting in 1972 (FAO/WHO 1973), which determined that chlorpyrifos
inhibited the plasma ChE in humans at 0.03 mg/kg bw, but not at
0.014 mg/kg bw (Coulston et al 1972). This served as a basis for the
ADI (0.0015 mg/kg), which was subsequently rounded off to 0.001 mg/kg
bw at the recommendation of the Joint Meeting of 1977 (FAO/WHO 1978).
However, appropriate statistical evaluation of the human
cholinesterase determinations in the Coulston et al study (1972)
(Park 1972) demonstrated 0.03 mg/kg to be a threshold level for
inhibition of plasma ChE, which was without statistical significance
when compared to current controls. Based upon repeated daily doses for
a period of 21 days, it was concluded by Coulston et al and Park
that a dose of 0.03 mg chlorpyrifos/kg bw has no significant
toxicological effect in humans. This conclusion is supported by the
evidence from studies in other mammalian species at comparable dose
levels (McCollister et al 1971a,b; Coulston et al 1971).
These same investigators determined that erythrocyte
cholinesterase activity was inhibited at comparable levels in
different species (e.g. 0.1 mg/kg for rat, dog and humans, and
0.08 mg/kg for monkey). Except for ChE depression, these levels were
without significant effects noted on mortality, behaviour, food
consumption growth, haematology, clinical chemistry, urinalysis, or
gross and microscopic examination of selected tissues (where
determined).
The Meeting accordingly increased the ADI from 0.001 to
0.01 mg/kg body weight, based on the level causing no inhibition of
RBC cholinesterase and not on the plasma cholinesterase.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 0.1 mg/kg bw/day
Dog: 0.1 mg/kg bw/day
Man: 0.1 mg/kg bw/day
Estimate of Acceptable Daily Intake for Man
0 - 0.01 mg/kg bw.
FURTHER WORK OR INFORMATION
Desirable
Data on the inhibition, reactivation and ageing of cholinesterase
(in vitro).
REFERENCES
Coulston, F., Goldberg, L., Abraham, R., Benitz, K.F., Griffin, T.B.
1971 and Norvell, M. Final report on safety evaluation and
metabolic studies on Dowco 179 (IN 151). Report from Insp.
Exp. Pathol. Toxicol., Albany Medical College, submitted to
the World Health Organization by Dow Chemical Company.
(Unpublished)
Coulston, F., Goldberg, L. and Griffin, T. Safety evaluation of
1972 Dowco 179 in human volunteers. Summary and tables 33 and 34,
reviewed by JMPR 1972. Report from Albany Medical College,
Albany, New York, submitted to the World Health Organization
by Dow Chemical Company. (Unpublished)
Deacon, M.M., Murray, J.S., Pilney, M.K., Dittenber, D.A., Hanley,
1979 T.R. Jr. and John, J.A. The effects of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Report from Dow Chemical Company submitted to the World
Health Organization by Dow Chemical Company. (Unpublished)
McCollister, S.B., Kociba, R.J., Gehring, P.J. and Humiston, C.G.
1971a Results of two-year dietary feeding studies on DowcoR 179
in beagle dogs. Report T35.12.44793-18, from Dow Chemical
Company, submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
1971b Results of two-year dietary feeding studies on DowcoR179 in
rats. Report NBT35.12-44793-21, from Dow Chemical Company
submitted to the World Health Organization by Dow Chemical
Company (Unpublished)
Park, C. Analysis of plasma and red blood cell cholinesterase levels
1972 in humans ingesting DOWCO 179. Report from Dow Chemical
Company submitted to the World Health Organization by Dow
Chemical Company. (Unpublished)
Rowe, L.D., Warner, S.D. and Johnston, R.V. Acute delayed
1978 neurotoxicologic evaluation of chlorpyrifos in White Leghorn
hens. Report from Dow Chemical Company submitted to the
World Health Organization by Dow Chemical Company.
(Unpublished)
Warner, S.D., Gerbig, C.G., Strebing, R.J. and Molello, J.A. Results
1980 of a two-year toxicity and oncogenicity study of
chlorpyrifos administered to CD-1 mice in the diet. Report
from Dow Chemical Company, submitted to the World Health
Organization by Dow Chemical Company. (Unpublished)
CHLORPYRIFOS
Explanation
Chlorpyrifos was evaluated in 1972, 1974, 1975 and 1977 (FAO/WHO
1973, 1975, 1976, 1978)1/. Use patterns for chlorpyrifos have
undergone significant changes since 1972 with less emphasis on
previous animal applications, the development of "spot-on" animal
treatments and the emergence of more feed uses. At the 1982 meeting of
the CCPR, a question was raised concerning the recommended limit in
milk in view of these changing use patterns.
New information was made available to the Meeting on a survey of
international uses on dairy cattle, residues in meat from "spot-on"
treatment, residues in meat and milk from ingestion of treated feed,
metabolic fate in lactating goats, an improved method of analysis for
milk and cream and national maximum residue limits.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
In Animals
A survey of uses of chlorpyrifos in animals was conducted by Dow
Chemical Co. in each geographic region in late 1981. This survey was
aimed specifically at clarifying whether dairy cattle are treated with
chlorpyrifos and, if so, whether such use is likely to lead to
residues greater than 0.1 mg/kg on a fat basis in milk or milk
products in international trade. Table 1 summarizes recommendations
for treatment of cattle with chlorpyrifos in each country where it has
been used.
1/ See Annex 2 for FAO and WHO documentation.
Table 1. Survey of Uses of Chlorpyrifos in Cattle: A. Developed Countries
Country Product Use Comment
Australia DURSBAN 1/24E For ticks and lice in cattle, Do not use on lactating cattle or in
all states 250 g/litre as dip at 1 litre/1 000 litres dairy cows later than 3 weeks before
1970-71 of water (0.025% a.i.), or as calving. Do not spray later than 3
spray at 200 ml/200 litres weeks before slaughter, or dip later
using at least 5 litres per than 3 days before slaughter of meat
animal. animals.
all states DURSBAN JS For louse control in cattle, Do not use in lactating dairy cattle
1977 50% a.i. w/v apply as a "one-shot" treatment or in dry dairy cows within 3 weeks
using 3 ml/100 kg bw (1.5 g before calving. Do not use later than
a.i./100 kg bw) as spot-on to 21 days before slaughter for meat
rump. animals.
New Zealand DURSBAN 10E For cattle lice control, use Do not use on dairy cows "in milk".
1971? License No. 1454 62.5 - 125 ml/100 litres of Treat dairy cows not later than 3
water weeks before calving. Treat beef
cattle not later than 3 weeks before
slaughtering.
1977? DURSBAN JS For control of cattle lice, use Do not use on dairy cows "in milk".
(400 g a.i./litre) 15-30 ml/100 litres, of water Spray no later than 3 weeks before
License No. 2941 applied as a saturating spray, calving for dry dairy cattle or
or 2 ml/50 kg live weight (1.6 before slaughter for meat animals.
g/100 kg bw) as a spot-on Apply spot-on no later than 5 weeks
treatment. before calving or slaughter.
1978 Ridlice For cattle, ectoparasites as Do not use on lactating dairy cattle
50% a.i. w/v one-shot application, use 3 ml/ or within 5 weeks before calving or
License No. 3485 100 kg bw (1.5 g a.i./100 kg bw) slaughter.
as spot-on to top of rump.
Table 1. (con't)
Country Product Use Comment
U.S.A. DURSBAN 24E For control of fever ticks in For use under supervision of qualified
1972 2 lb a.i./gal2/ beef cattle, use 1 ounce of state or federal personnel responsible
23.7% a.i., w/v product in 8 gallons of water for quarantine programmes. Withdrawal
EPA Reg No. (0.025% a.i.), and apply 3/4 to period of 2 weeks before slaughter.
464-450 1 gallon as spray treatment; or No more than 6 applications at 21-day
use 1/2 gallon of product per intervals per season.
500 gallons of water (0.025%
a.i.) for dip treatment.2/
U.S.A.
1979 DURSBAN 44 For lice control in beef cattle Do not treat dairy cows during
43.2% a.i. w/v and non-lactating dairy cattle, lactation. Do not treat dry dairy cows
EPA Reg. No. apply to back as a "spot-on" or replacement heifers within 60 days
464-542 treatment using 2 ml/100 lb bw before freshening. Do not slaughter
(2 g a.i. 100 kg bw) or 16 ml meat animals for 14 days after initial
maximum per animal. treatment, or after a second treatment
applied 45 days or more after the first
treatment. Do not slaughter for 21
days after second treatment applied
30 days after first treatment.
Canada
Temp. 1979 DURSBAN 44 Temporary registration for louse Do not treat within 14 days before
Expired 1980 43.2% a.i. control in beef cattle slaughter.
Argentina DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1969 240 g a.i./litre spraying and dipping using 336 weeks of treatment.
Reg. No. 1307 g a.i./1 000 litres of water.
1975 DURSBAN 18A Dip or spray treatment for Preslaughter interval of 2 days for
180 g a.i./litre cattle. meat animals (previously 2 weeks)
Table 1. (con't)
Country Product Use Comment
Brazil DURSBAN 1E Tickicide for cattle as spray Withdrawal period of 14 days after
1969, 1973 13% a.i. w/v or dip at 1:500 - 1:300 treatment.
Reg. No. 973 dilution
1975 DURSBAN 24E Control of ticks on cattle by Preslaughter limitation of 14 days
240 g a.i./litre spraying and dipping at after treatment. Also 48 hours
NBR 96/75 1:1 000 - 1:600 dilution limitation on use of milk after
treatment.
Colombia DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1972 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of water.
Costa Rica DURSBAN 24E " "
Expired 240 g a.i./litre
1976 Reg. No 255
Dominican DURSBAN 24E " "
Republic 240 g a.i./litre
1975
Ecuador DURSBAN 24E " "
1975 240 g a.i./litre
El Salvador DURSBAN 24E Control of ticks on cattle by Do not slaughter cattle within 3
1975 240 g a.i./litre spraying and dipping using weeks of treatment.
336 g a.i./1 000 litres of
water.
Table 1. (con't)
Country Product Use Comment
Mexico DURSBAN 24E " "
1969 240 g a.i./litre
Reg.No. 0-0480-015
Nicaragua DURSBAN 24E " "
1975 240 g a.i./litre
Panama DURSBAN 24E " "
1975 240 g a.i./litre
Reg.No. 15130
Trinidad DURSBAN 24E " "
1972 240 g a.i./litre
Venezuela DURSBAN 24E " Not to be used for 20 hours before
1972 240 g a.i./litre milking or 15 days before slaughter
of meat animals.
1/ Trademark of The Dow Chemical Company;
2/ 1 gallon = 3.8 litres;
3/ 1 lb (16 oz) = 0.45 kg.
Labels approved recently in Australia, New Zealand and the U.S.
recommends a one-shot "spot-on" treatment rather than dipping or
spraying. Limitations specify withdrawal intervals prior to slaughter
of meat animals, and state that the product is not to be used on dairy
cattle during lactation, nor within a stated interval prior to
freshening or calving.
In Canada, temporary approval expired in 1980 for use of
chlorpyrifos to control lice in cattle and pigs. In Europe, only one
recommendation was noted, as a dip for sheep using a mixture of
chlorpyrifos and gamma HCH (Rycovet 365 Dip, Glasgow, June 1978).
Recommendations for chlorpyrifos in Latin America generally do
not differentiate between beef animals and dairy animals. However, the
use of these products has been declining steadily, and the total sold
in 1981 amounted to less than 40 000 kg active ingredient for the
entire region. Records of Dow Chemical Co. indicate that less than
1 000 kg was sold in Brazil in each of 1979, 1980 and 1981, and none
was sold in Venezuela in 1981.
Although it is difficult to estimate how much chlorpyrifos is
actually used in lactating animals, the rate of milk production under
present agricultural practice in developing countries is inadequate
even for local consumption. Thus, it is highly unlikely that minor use
of chlorpyrifos in cows in certain Latin American countries would
result in residues in milk products in international trade.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In Meat
Cattle were treated once with DURSBAN 44 Insecticide at the rate
of 2 ml/100 lb of body weight (equivalent to 20 mg chlorpyrifos per
kg bw) applied as a "spot-on" to the top midline just posterior to the
withers. Groups of three cattle each were sacrificed the day after
treatment and at 7-day intervals through 35 days after application.
The maximum residue of chlorpyrifos was 1.4 mg/kg in fat at 7 days
after treatment (Table 2). Residues in muscle were much lower than in
fat, and did not exceed 0.6 mg/kg chlorpyrifos at any sampling. Thus
residues from "spot-on" treatment are not likely to exceed the
recommended limit of 2 mg/kg for chlorpyrifos in carcase meat on a fat
basis (McKellar and Dishburger 1976).
Table 2. Summary of Average Residues of Chlorpyrifos and 3,5,6-Trichloro-2-Pyridinol in Tissues of Cattle1/
Days from Average Residue Found, mg/kg (range, mg/kg)
Treatment Chlorpyrifos 3,5,6-Trichloro-2-Pyridinol
to Sampling Muscle Liver Kidney Fat Muscle Liver Kidney Fat
1 0.02 0.01 0.07 0.28 ND 0.74 0.52 0.10
(0.01-0.03) (0.01) (0.06-0.08) (0.03-0.46) (0.52-0.97) (0.45-0.56) (<0.05-0.18)
7 0.04 0.04 0.12 1.1 0.09 1.0 0.86 0.19
(0.01-0.06) (0.01-0.08) (0.09-0.18) (0.95-1.4) (<0.05-0.15) (0.67-1.5) (0.46-1.4) (0.08-0.24)
14 0.01 0.02 0.11 0.80 ND 0.59 0.52 0.07
(ND-0.01) (0.02-0.03) (0.08-0.16) (0.61-0.99) (0.47-0.69) (0.48-0.58) (0.06-0.08)
21 0.01 0.01 0.08 0.05 ND 0.42 0.29 0.14
(0.01) (<0.01-0.01) (0.06-0.10) (ND-0.14) (0.31-0.53) (0.14-0.57) (0.09-0.24)
24 0.01 ND 0.07 0.47 ND 0.20 0.10 <0.05
(0.01) (0.03-0.09) (0.34-0.71) (0.11-0.27) (0.06-0.13) (ND-0.05)
35 ND ND 0.03 0.20 ND 0.13 0.05 ND
(0.01-0.05) (0.02-0.34) (0.05-0.23) (0.04-0.07)
1/ All animals received a single treatment of Dursban spot-on (M-4110) at a rate of 2 ml/45 kg body weight.
Values corrected for control and recovery. ND = No detectable differences between treated and control samples.
Groups of three cattle were fed chlorpyrifos for 30 days at
levels of 3, 10, 30 and 100 ppm on a daily, dry matter intake basis.
Residues of chlorpyrifos were mainly in the fat, reaching maxima of
4.70 mg/kg in renal fat at 100 fed and 1.09 mg/kg in renal fat at
30 ppm fed. The residues of chlorpyrifos declined to less than 2 mg/kg
in fat within 7 days after discontinuation of feeding 100 ppm in the
total diet of the cattle for 30 days (Table 2).
The oxygen analogue of chlorpyrifos was shown to be unstable in
liver and kidney, and no residue was found in any tissue at any
feeding level. The pyridinol metabolite was not detected in muscle at
feeding levels of 3 and 10 ppm chlorpyrifos, but was present in liver
and kidney at all feeding levels, reaching maxima of 2.61 mg/kg in
liver at 100 ppm fed and 1.67 mg/kg in liver at 30 ppm fed (Dishburger
et al 1977).
In Milk
Lactating cows were fed a complete ration containing chlorpyrifos
at levels from 0.3 to 30 ppm for 2 weeks at each level. At 30 ppm in
the total diet, maximum residues of chlorpyrifos were 0.01 mg/kg in
milk and 0.10 mg/kg in cream. The oxygen analogue was not detected in
any sample of milk or cream at any dietary level of chlorpyrifos. The
pyridinol metabolite was detected at 0.01 mg/kg in milk of cows fed
30 ppm chlorpyrifos, but does not concentrate in fat. Thus, residues
resulting from ingestion of feedstuffs containing chlorpyrifos
residues amounting to no more than 10 - 15 mg/kg in the total diet
should not exceed the current recommendation of 0.1 mg/kg for
chlorpyrifos in milk on a fat basis (McKellar et al 1976).
FATE OF RESIDUES
In Animals
An additional metabolic study has been conducted using 14C-ring
labelled chlorpyrifos in lactating goats. The material was
administered via capsule twice daily at doses equivalent to 16 and
25 ppm in the daily feed for 10 days. The majority of the total 14C
activity was recovered in the urine (80.3%), with small amounts in the
faeces (3.6%), gut (0.9%) tissues (0.8%) and milk (0.1%).
Most of the radioactivity in the urine was excreted as the
ß-glucuronide conjugate of 3,5,6-trichloro-2-pyridinol with small
amounts of free pyridinol metabolite. About 75% of the 14C activity
in the fat was chlorpyrifos (0.1 mg/kg) with most of the remaining
14C activity hydrolysable to the pyridinol metabolite. The results of
this study validate the methodology used in earlier animal residue
studies (Glas 1981).
METHODS OF RESIDUE ANALYSIS
The analytical method used for analysis of beef tissues from the
"spot-on" treatment, was the procedure developed in 1972 for
determination of chlorpyrifos residues in chicken tissues and eggs.
Analysis is by gas chromatography using flame photometric detection.
The validated lower level of sensitivity is 0.01 mg/kg chlorpyrifos in
muscle, liver, kidney and fat of beef animals, as well as the chicken
muscle, liver, kidney and eggs (McKellar and Dishburger 1976; Wetters
1972).
An improved analytical method for the determination of
chlorpyrifos in milk and cream has lower limits of detection at 0.01
and 0.05 mg/kg in these matrices, respectively. The samples are
extracted with hexane, the extract partitioned into acetonitrile,
cleaned up on deactivated silica gel and analysed by gas
chromatography with flame photometric detection (Jeffries 1980).
NATIONAL MAXIMUM RESIDUE LIMITS
Maximum residue limits reported to the Meeting for chlorpyrifos
in foods of animal origin are summarized in Table 3. The Codex MRLs
included for comparison are in terms of chlorpyrifos alone, since the
pyridinol hydrolysis product is of low mammalian toxicity and is
excreted rapidly in the urine and faeces of animals (FAO/WHO 1973b,
p. 162 and 194).
U.S. tolerances include its pyridinol metabolite, expressed as
chlorpyrifos, and were increased recently to include the estimated
contribution from ingestion of feedstuffs treated with chlorpyrifos.
The tolerances in feedstuffs, which do not exceed 15 mg/kg in any
case, also represent maximum total residues of chlorpyrifos and its
pyridinol hydrolysis product, expressed as chlorpyrifos, without
differentiation as to the contribution due to each component (U.S.
Code of Federal Regulations 1982).
APPRAISAL
New information on current uses of chlorpyrifos on animals and on
residues in milk from ingestion of treated feed indicate that such
uses should not result in residues in excess of the proposed CCPR MRLs
of 2 mg/kg in carcase meat of cattle (for fat-soluble pesticides, a
portion of carcase fat is analysed and the MRLs apply to carcase fat)
and of 0.1 mg/kg in milk and milk products (both on a fat basis).
Because chlorpyrifos residues in whole milk from cows fed up to
Table 3. Maximum Residue Limits for Chlorpyrifos1/ in
Food Products of Animal Origin
Cattle
Codex 2.0 mg/kg in carcase meat (carcase fat).
Australia 2.0 mg/kg in fat of meat of cattle.
Canada 1.0 mg/kg (temporary) in meat and meat by-products
(fat basis) and in fat, liver and kidney.
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 2.0 mg/kg in fat, meat and meat by-products.
Eggs
Codex 0.01 mg/kg (on shell-free basis).
U.S. 0.1 mg/kg.
Goats
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Pigs
Australia 0.1 mg/kg in fat of meat of pigs.
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Horses
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Milk
Codex 0.1 mg/kg (on a fat basis) including milk products.
Switzerland 0.005 mg/kg in whole milk.
U.S. 0.5 mg/kg in fat (reflecting 0.02 mg/kg in whole
milk).
Poultry
Codex 0.1 mg/kg in carcase meat (carcase fat).
U.S. 0.5 mg/kg in fat, meat and meat by-products.
Sheep
Codex 0.2 mg/kg in carcase meat (carcase fat).
New Zealand 1.5 mg/kg in meat fat in any food.
U.S. 1.0 mg/kg in fat, meat and meat by-products.
Table 3. (con't)
Turkeys
Codex 0.2 mg/kg in carcase meat (skin and carcase fat).
U.S. 0.2 mg/kg in fat, meat and meat by-products.
1/ Tolerances in Canada and the U.S. include residues of
3,5,6-trichloro-2-pyridinol expressed as chlorpyrifos.
30 mg/kg in the total diet reached the measurable level of 0.01 mg/kg
and, in consideration of the current CCPR approach to the
establishment of limits for fat-soluble pesticides in milk, the MRL
for milk should be changed to 0.01 mg/kg for chlorpyrifos (the limit
of determination) in whole milk, from which the MRL for milk products
containing more than 2% fat would be calculated on a fat basis,
assuming 4% fat in raw milk.
RECOMMENDATIONS
The recommended maximum residue limit for milk and milk products
on a fat basis is deleted and replaced with a new maximum residue
limit for milk. The limit applies to chlorpyrifos only.
Commodity Limit (mg/kg)
Milk 0.01*
* at or about the limit of determination.
REFERENCES
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. and Rice, J.R.
1977 Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of
cattle fed chlorpyrifos. Journal of Agricultural Food Chem.
25(6): 1325-1329.
Glas, R.D. The metabolic fate of 14C-chlorpyrifos fed to lactating
1981 goats. Report GH-C 1408R, Dow Chemical Co, (Unpublished)
Jeffries, T.K. Determination of residues of chlorpyrifos in milk and
1980 cream. Method ACR 80.5. Dow Chemical Co. (Unpublished)
McKellar, R.L. and Dishburger, H.J. Determination of residues of
1976 chlorpyrifos and 3,5,6-trichloro-2-pyridinol in tissues of
cattle receiving a single treatment of DURSBAN spot-on.
Report GH-C-930, Dow Chemical Co. (Unpublished)
McKellar, R.L., Dishburger, H.J., Rice, J.R., Craig, L.F. and
Pennington, J. Residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in milk and cream
from cows fed chlorpyrifos. Journal of Agricultural Food
Chem. 24(2): 283-286.
U.S. Code of Federal Regulations. Title 40: Section 180.342 for
1982 chlorpyrifos tolerances in raw agricultural commodities;
Section 193.85 for chlorpyrifos tolerances in processed
foods; Section 561.98 for chlorpyrifos residues in processed
feeds.
Wetters, J.H. Determination of residues of 0,0-diethyl 0-(3,5,6-
1972 trichloro-2-pyridyl) phosphorothioate in chicken tissues and
eggs by gas chromatography with flame photometric detection.
Method ARC 72.3. Dow Chemical Co. (Unpublished)
