
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
CHLORPYRIFOS
First draft prepared by
D. Wagner
Therapeutic Goods Administration,Department of Health and Aged Care,
Canberra, ACT, Australia
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies: Neurotoxicity
Studies on metabolites
Acute toxicity
Short-term studies of toxicity
Genotoxicity
Developmental toxicity
Observations in humans
Experimental studies
Case reports
Monitored field trials
Studies of morbidity
Comments
Toxicological evaluation
References
Explanation
Chlorpyrifos [ O,O-diethyl O-(3,5,6-trichloro-2-pyridinyl)
phosphorothioate] is a broad-spectrum organophosphorus pesticide. The
toxicology of chlorpyrifos was first evaluated by the 1972 Joint
Meeting (Annex 1, reference 18), when an ADI of 0-0.0015 mg/kg bw
was established on the basis of a NOAEL of 0.014 mg/kg bw per day in a
1-month study in humans. Further biochemical and toxicological
information was considered by the 1977 JMPR (Annex 1, reference
28), when the ADI was changed to 0-0.001 mg/kg bw. Additional
reports on the toxicology of chlorpyrifos were reviewed by the 1982
Joint Meeting (Annex 1, reference 38), which increased the ADI to
0-0.01 mg/kg bw on the basis of a NOAEL of 0.1 mg/kg bw per day in
humans exposed to chlorpyrifos for 9 days, with a 10-fold safety
factor. This ADI was supported by findings in rats and dogs.
Chlorpyrifos was reviewed at the present meeting within the periodic
review pro-gramme of the Codex Committee on Pesticide Residues.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Rats
A dose of 50 mg/kg bw [36Cl]chlorpyrifos given orally to male
Wistar rats by intubation was eliminated rapidly in the urine (about
90% of the dose) and faeces (about 10% of the dose). The compounds
excreted in the urine were identified as 3,5,6-trichloro-2-pyridyl
phosphated (5-80%), 3,5,6-trichloro-2-pyridinol (TCP; 15-20%), and
traces of chlorpyrifos. The concentrations of residue were highest in
liver and kidney 4 h after dosing, but the half-time in these tissues
was < 20 h. The longest half-time, 62 h, was recorded in fat (Smith
et al., 1967).
By 72 h after a single oral dose of 19 mg/kg bw
[14C]ring-labelled chlorpyrifos was given by intubation to male
Sprague-Dawley rats, 83-87% had been eliminated, mainly in the urine
(68-70%), faeces (14-15%), and expired air (0.15-0.39%). The residues
found at this time represented about 1.7% of the total dose, and the
concentration, while highest in fat, was < 1 ppm in any tissue
(Branson & Litchfield, 1971a). In a study to investigate the breakdown
of chlorpyrifos by microsomal enzymes in vitro (Branson & Wass,
1970), TCP was again identified as the major metabolite of
chlorpyrifos. In cows fed chlorpyrifos at a dose of 5 ppm in the diet
for four days, hydrolysis to the pyridinol was the major metabolic
pathway (Gutenmann et al., 1968).
Female Fischer 344 rats were given chlorpyrifos by oral gavage or
by inhalation in nose-only or whole-body chambers, while another group
was given [14C]chlorpyrifos by oral gavage to estimate the
efficiency of recovery. Oral dosing led to 30-80% recovery of the
administered dose in urine, while nose-only or whole-body inhalational
exposure led to an average urinary excretion of 0.28-0.58 µg of TCP
per ppb of chlorpyrifos in air, after adjustment for the route of
exposure. Rats with whole-body exposure absorbed more chlorpyrifos
than would have been expected to occur by inhalation alone, probably
due to grooming activity. The total combined recovery of radiolabel
from urine, faeces, and cage wash was 99.8% of the administered dose
(Nolan et al., 1986).
Single doses of [14C]ring-labelled chlorpyrifos (19 mg/kg bw)
or TCP (7 mg/kg bw) were given by oral gavage to Sprague-Dawley rats,
and the radiolabel was analysed in blood samples, expired air, and
excreta. The main compound found in urine was TCP. The biological
half-time of each chemical was estimated to be 8-17 h, and by 72 h
> 98% of each compound had been eliminated, mainly in the urine
(68-70% of chlorpyrifos, 73-76% of TCP) and faeces (14-15% of
chlorpyrifos, 6-7% of TCP). Elimination in the form of radiolabelled
carbon dioxide was a minor (< 1%) pathway. The residue concentrations
were consistently low in the brain, and the highest were found in bone
and intestine after administration of TCP and in fat and intestine
after administration of chlorpyrifos. Administration of single oral
doses of [14C]TCP or [14C]chlorpyrifos did not lead to
accumulation of these compounds in rat tissues (Branson & Litchfield,
1970, 1971b).
Groups of male and female Fischer 344 rats were given
[14C]chlorpyrifos as a single oral dose of 0.5 or 25 mg/kg bw or
unlabelled chlorpyrifos as 15 consecutive doses of 0.5 mg/kg bw per
day followed on day 16 by 0.5 mg/kg bw of [14C]chlorpyrifos. By 3
days after exposure, essentially all of the radiolabel had been
recovered (> 97%), mainly in the urine (84-92% of the administered
dose), with 6-12% in the faeces. Repeated dosing induced a slight
increase (6-7%) in urinary excretion as compared with the single dose
of 0.5 mg/kg bw per day. At sacrifice, the residues in the carcass
accounted for 0.2% of the administered dose of 25 mg/kg bw. The
residues were found mainly in perirenal fat and livers of males and in
the ovaries and fat of females. The half-time for excretion was 12 h
for males and 23 h for females at 25 mg/kg bw. No unchanged
chlorpyrifos was found in urine. The major metabolites were TCP (12%),
its glucuronide conjugate (80%), and, tentatively, a sulfate conjugate
(Nolan et al., 1987).
Hens
Groups of 36 laying hens were fed diets containing chlorpyrifos
at 0 (two groups), 0.3, 1, 3, or 10 (three groups) ppm for 30-45 days
before sacrifice. All control birds and 24 birds at each dose were
killed after 30 days, and the remaining 12 hens at each dose were fed
treated diet for a further 15 days; eggs were collected from these
birds throughout treatment. The additional two groups of birds at 10
ppm were allowed to recover from treatment for 7 and 21 days,
respectively, after the 30-day treatment period and were then killed.
Chlorpyrifos residues were detected only in fat, whereas TCP was found
in liver (0.15 ppm), kidney (0.33 ppm), and eggs (< 0.05 ppm) of hens
given 10 ppm chlorpyrifos. No residues were detected after the 7-day
withdrawal period (Dishburger et al., 1972a).
Goats
Two goats were fed [14C]ring labelled (positions 2 and 6)
chlorpyrifos twice daily in capsules for 10 days at concentrations
equivalent to 15-19 ppm in the feed. The majority (80%) of the
radiolabel was recovered in urine, with smaller amounts in faeces
(3.6%), gut (0.9%), tissues (0.8%), and milk (0.1%). The major urinary
metabolite (> 75% of the residual radiolabel) was the ß-glucuronide
conjugate of TCP, with smaller amounts of unconjugated TCP. The major
residue in fat was chlorpyrifos (0.12 ppm), while TCP was the major
residue in liver and kidney (Glas, 1981a). A similar pattern of
elimination was seen in a study in which lactating goats were fed
[14C]ring labelled chlorpyrifos twice daily by capsule; little
radiolabel (0.05-0.14%), mainly associated with chlorpyrifos, was
recovered in milk (Glas, 1981b).
Pigs
Groups of two boars and one sow were fed basal rations containing
0, 1, 3, or 10 ppm chlorpyrifos for 30 days, and tissue samples were
collected after withdrawal periods of 0, 7, and 21 days. Residues were
initially found predominantly in fat tissues, with lower
concentrations in muscle, liver, and kidney, but no residues were
detectable after 7 days of withdrawal (McKellar et al., 1972).
Weanling piglets of each sex were given daily oral doses of[14C]TCP
equivalent to 75 ppm in the diet for 7 days, and urine and tissue
samples were collected after withdrawal periods of up to 7 days. The
principal urinary excretion product was unchanged TCP. The liver and
kidney initially contained the highest residues; while the
concentrations decreased rapidly after withdrawal, the liver had the
slowest rate of clearance (Bauriedel & Miller, 1981).
Cattle
Calves were fed TCP in the feed at concentrations < 100 ppm
for 28 days, and tissue samples were taken on day 28 or after a 3-,
7-, or 21-day withdrawal period. The concentrations of residues were
highest in liver and kidney, with lower concentrations in fat and
muscle. These concentrations declined rapidly after the return to
untreated feed (Glas, 1977a). One calf fed
2-methoxy-3,5,6-trichloropyridine for 28 days showed significant
concentrations (> 2 ppm) of TCP but no
2-methoxy-3,5,6-trichloropyridine in liver, kidney, and fat
(Glas,1977b).
Female calves were fed one capsule of chlorpyrifos per day for 30
days at doses equivalent to daily concentrations of dry matter in the
diet of < 100 ppm. Tissue samples were taken on day 30 or after a
1-5-week withdrawal period. A dose-related increase in the
concentration of residues was seen in all tissues but especially in
fat, whereas TCP was found predominantly in the liver and kidney.
After the dose of 100 ppm, the residues of chlorpyrifos in fat were
0.93 ppm at 7 days and 0.02 ppm at 35 days after withdrawal of the
test material (Dishburger et al., 1972b, 1977).
Humans
In persons poisoned with chlorpyrifos formulations, chlorpyrifos
was detected in serum samples only and at lower concentration than the
diethylphosphorus metabolites, which were excreted mainly in urine.
The urinary diethylphosphorus metabolites were excreted by first-order
kinetics, with an average elimination half-time of 6.1 ± 2.2 h in the
fastest phase and 80 ± 26 h in the slowest (Drevenkar et al., 1993).
Male volunteers received chlorpyrifos as an oral dose of 0.5
mg/kg bw and 1 month later a dermal dose of 5 mg/kg bw. The time to
the maximal concentration of TCP in blood was 0.5 h after oral dosing
and 22 h after dermal treatment. The elimination half-time,
irrespective of the route of administration, was 27 h. The percentage
of the administered dose recovered from the urine was 70% after oral
dosing and 1.3% after dermal administration (Nolan et al., 1982,
1984).
(b) Biotransformation
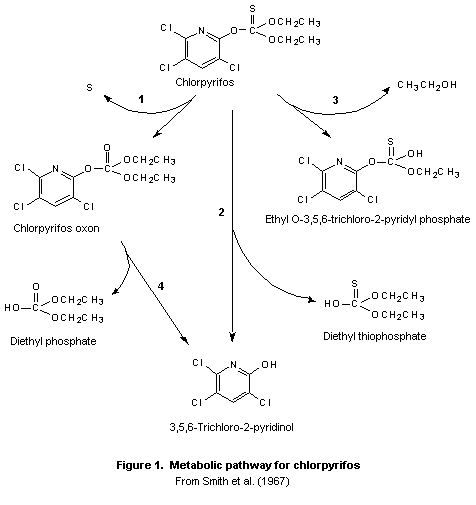
A generalized metabolic pathway for chlorpyrifos is shown in
Figure 1. Chlorpyrifos, like many of the most commonly used
organophosphorus insecticides, is a phosphorothionate.
Phosphorothionate insecticides are bioactivated by the microsomal
cytochrome P450 system in the bodies of vertebrates and insects to
their active oxon (phosphate ester) metabolites, which are about three
orders of magnitude more potent as anticholinesterases than the parent
compounds. Most bioactivation takes place in the liver, while
detoxification takes place in the liver and plasma. Chlorpyrifos is
rapidly metabolized by mixed-function oxidases to the highly reactive
chlorpyrifos oxon by oxidative desulfuration (step 1). Oxidative
metabolism involving concurrent activation and degradation via a
common intermediate appears to be the general pattern for P=S esters
(Yang et al., 1971; Wolcott et al., 1972; Nakatsugawa, 1992). The
oxidative desulfuration is believed to proceed via an electrophilic
phosphooxathiiran intermediate (Kamataki & Neil, 1976). The
degradation step occurs by conversion directly to TCP and diethyl
thiophosphate (step 2). The oxon can be deactivated by hydrolysis to
diethylphosphate and TCP (step 4) (Ma & Chambers, 1994; Sultatos &
Murphy, 1983). A minor reaction pathway is hydrolysis to monethyl
3,5,6-trichloro-2-pyridinyl phosphorothioate (step 3).
The moderate toxicity of chlorpyrifos, when compared with some
other phosphorothionates, may be due to hydrolytic detoxification of
the oxon by A-esterases, such as paraoxonase, and high serum
concentrations of paraoxonase may protect against poisoning by
organophosphorus insecticides with paraoxonase substrates as active
metabolites (Omenn, 1987; Geldmacher-von Mallinckrodt & Diepgen,
1988). This effect has been demonstrated in rats directly, in which
injection of purified paraoxonase before dosing with chlorpyrifos oxon
reduced the inhibition of brain acetylcholinesterase in these animals
by 2.5 times when compared with controls (Costa et al., 1990).
Twelve rats given chlorpyrifos at 5 mg/kg bw (3 mCi per rat)
orally by gavage excreted 88% of the administered radiolabel in urine
within 48 h. At least six metabolites were present, three of which
accounted for 97% of the excreted radiolabel. These were identified as
the glucuronide of TCP (80%), a glucoside of TCP (4%), and TCP itself
(12%) (Bakke et al., 1976).
 (c) Effects on enzymes and other biochemical parameters
Hydrolysis is the most important route of detoxification of
organophosphorus esters. Hydrolytic esterases are distributed
ubiquitously in the blood and tissues of virtually all animal
organisms and catalyse the hydrolysis of a variety of esters,
including organophosphorus esters, but have little activity on
organophosphorus itself. Esterases that interact with
organophosphoruses have been characterized as A- and B-esterases
according to their sensitivity to inhibition by organophosphorus
compounds. Plasma and tissues of mammals have significant
concentrations of the calcium-activated A-esterases, arylesterase (EC
3.1.1.2), and the high-density lipoprotein-associated paroxonase (EC
3.1.8.1). The B-esterases, including aliesterases (EC 3.1.1.1; also
called carboxylesterases) and cholinesterases (e.g. butyryl
cholinesterase, EC 3.1.1.8), are inhibited by organophosphorus
compounds such as chlorpyrifos oxon. While they bind them, they do not
hydrolyse them (Derelanko & Hollinger, 1995).
Butyrylcholinesterase is the predominant cholinesterase in human
serum (> 99%), while in rats there is an approximately equal
distribution of acetylcholinesterase and butyryl cholinesterase
(Nolan, 1997). These enzymes detoxify organophosphorus compounds by
sequestering them through binding to or phosphorylation by blood
proteins, such as albumin, which stoichiometrically degrades them
(Aldridge, 1953). These protein-bound organophosphorus esters are
secreted into the bile. Erythrocyte acetylcholinesterase (EC 3.1.1.7)
is similar to the acetylcholinesterase in nervous systems and can bind
to and sequester or hydrolyse organophosphorus compounds. The enzyme
activities attributable to A-esterases, B-esterases, and
cholinesterases vary widely within populations and are influenced by
genetic and environmental factors and disease states (Derelanko &
Hollinger, 1995). The serum activity of paraoxonase in rabbits is some
40 times greater than that in rats. Most avians, including hens, have
relatively low activity of A-esterases, and the mean value of serum
chlorpyrifos oxonase activity in humans is 10 times that of rat serum
(Furlong et al., 1989).
In humans, a substrate-dependent polymorphism of serum
paraoxonase is observed, in which one isoform of paraoxonase has a
high turnover for paraoxon and the other a low turnover (Furlong et
al., 1989; Smolen et al., 1991). The polymorphism is also observed
with the oxons of methyl parathion, chlothion, and
phenylphosphonothioic acid O-ethyl O-para-nitrophenyl ester. The
two isoforms appear, however, to hydrolyse chlorpyrifos oxon and
phenylacetate at the same rate. Cloning and sequencing of the human
paraoxonase cDNA has revealed the molecular basis of the polymorphism:
arginine at position 192 determines high paraoxonase activity, and
glutamine at this position encodes low paraoxonase activity (Humbert
et al., 1993). In addition to this polymorphism, a 13-fold variation
in serum enzyme activity of a given genetic class is seen (Furlong et
al., 1989). While chlorpyrifos oxon was hydrolysed by the same plasma
fraction that hydrolysed paraoxon in a study of plasma samples from
320 white blood donors (Furlong et al., 1988), the population
distribution of chlorpyrifos oxon hydrolysis was unimodal. This
contrasted with a bimodal population distribution of the activity for
paraoxon hydrolysis, and the variation in paraoxonase activity was
about 11-fold, while that in chlorpyrifos oxonase activity showed a
four- to fivefold variation.
Correlation of the LD50 values for orally administered
chlorpyrifos in rats with the activation rates measured in brain
rather than liver (Chambers, 1992) suggested that local
biotransformation in target tissues is an important determinant of its
toxicity. In a more recent review, however, Chambers & Carr (1995)
compared published LD50 or LC50 values for a variety of
insecticides in several vertebrate species. Studies in rats indicated
that the sensitivity of brain acetylcholinesterase activity to
inhibition by various phosphorothionate oxons did not correlate with
their acute toxicity. Chlorpyrifos oxon has a greater affinity for rat
brain acetylcholinesterase than does paraoxon, with median inhibitory
concentrations (IC50 values) of 4.0 and 22 nmol/L, respectively, but
a lower LD50. This finding is consistent with the greater affinity
(IC50, 0.75 nmol/L) of chlorpyrifos oxon for plasma aliesterases
than for rat brain acetylcholinesterase, indicating that aliesterases
provide protection against chlorpyrifos oxon.
Further work by Chambers & Carr (1995) indicated longer
inhibition of esterases after exposure chlorpyrifos than to parathion.
This was attributed to the greater lipophilicity of chlorpyrifos than
parathion (hexane:acetonitrile partition coefficients, 0.28 and 0.062,
respectively) and the assumption that a substantial fraction of the
dose of chlorpyrifos would have been sequestered by fat and released
gradually for later bioactivation. Studies of hepatic microsomal
metabolism of parathion and chlorpyrifos indicated that desulfuration
of parathion was favoured over dearylation (activation vs
deactivation), whereas the reverse was seen for chlorpyrifos (Ma &
Chambers, 1994). In channel catfish, however, the sensitivity of
acetylcholinesterase to inhibition by oxons reflects the acute
toxicity of these insecticides, and may be largely responsible for
their toxicity in this species. Thus, the metabolism of insecticides
appears to be more influential in some species than in others in
determining their toxicity.
Chlorpyrifos oxonase is a calcium-dependent A-esterase that
hydrolyses chlorpyrifos oxon, the active metabolite of chlorpyrifos.
As this activity may be related to that of paraoxonase, it was
determined spectrophotometrically by measuring the generation of TCP
(Furlong et al., 1989). Mortensen et al. (1996) hypothesized that
young rats have less chlorpyrifos oxonase activity than adults, and
they measured the activity of this enzyme in the brain, plasma, and
liver of male Long-Evans rats (CRL:(LE)BR) at postnatal day 4 and in
adults. No activity was detected in brain at either age, but the
activities in plasma and liver were markedly lower in younger than in
adult animals, the activities in plasma and liver being 1/11 and 1/2
of those in adults, respectively.
As the Michaelis-Menten constant for chlorpyrifos oxonase
activity was high (210-380 µmol/L), experiments were performed to
determine whether this enzyme could hydrolyse physiologically relevant
concentrations (nanomolar to low micromolar) of chlorpyrifos oxon. On
the assumption that tissue acetylcholinesterase inhibition provides a
sensitive bioassay for the concentration of chlorpyrifos oxon, changes
in the tissue acetylcholinesterase IC50 for chlorpyrifos oxon were
measured in the presence and absence of chlorpyrifos oxonase. Brain
acetylcholinesterase activity was determined spectrophotometrically,
while plasma and liver cholinesterase activities were determined by a
radiometric method with a [3H]acetylcholine iodide substrate. An
increase in the 'apparent' IC50 would indicate that chlorpyrifos
oxonase had hydrolysed substantial amounts of chlorpyrifos oxon during
the 30-min preincubation with the chlorpyrifos oxon. In the adult
rats, the apparent IC50 values for both plasma and liver
cholinesterase were higher in the presence of chlorpyrifos oxonase,
suggesting that the enzyme in those tissues could hydrolyse
physiologically relevant concentrations of chlorpyrifos oxon within
30 min. Young animals, however, showed less of a shift in the IC50
curves than adults, confirming that they have a lower capacity to
detoxify physiologically relevant concentrations of chlorpyrifos oxon
with chlorpyrifos oxonase.
2. Toxicological studies
(a) Acute toxicity
(i) Lethal doses
Numerous studies have been carried out with technical-grade
chlorpyrifos to establish LD50 and LC50 values (Table 1). The
lowest value after oral administration was 96 mg/kg bw (range,
96-480 mg/kg bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in
mice. The signs of acute intoxication were consistent with
cholinesterase inhibition and included inactivity, salivation,
dyspnoea, flaccid paralysis, vomiting, piloerection, exophthalmia, and
diarrhoea; female animals were generally more sensitive to the acute
effects of chlorpyrifos than males. The LD50 for dermally applied
chlorpyrifos was consistently lower than that for oral or inhalational
exposure and indicated the lower dermal absorption of chlorpyrifos
(about 2% in humans) when compared with the 70-90% absorption after
inhalational or oral exposure.
(ii) Dermal and ocular irritation and dermal sensitization
Ocular irritation: In a paper in which few procedural details
were provided (Taylor & Olson, 1963), slight conjunctival redness was
seen in rabbits treated with chlorpyrifos, regardless of whether the
eyes were washed after administration of the test material. The
redness persisted for more than 7 days in some animals. Transient
conjunctival and iridial irritation, possibly due to a direct physical
irritating effect of cholorpyrifos powder, was seen in three young
adult female New Zealand white rabbits after each had received 100 mg
of a finely ground commercial preparation in the left conjunctival
sac. The eyes of all animals were normal after 24 h (Jones, 1985a).
The ocular irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested (in accordance with GLP requirements) in six
young male New Zealand white rabbits, 100 mg of test material being
placed into the right eye and the untreated left eye serving as a
control. There were no signs of corneal or iridial irritation. Slight
to moderate conjunctival redness (mean Draize score, 1 at 1 h, 0.83 at
24 h), slight chemosis (mean Draize score, 0.83 at 1 h, 0.16 at 24 h),
and discharge (mean Draize score, 1.6 at 1 h, 0.83 at 24 h) were seen
up to 24 h after instillation, but no signs of irritation were
reported at 48 h (Jackson & Ogilvie, 1994b).
The ocular irritation potential of 0.1 g of chlorpyrifos (purity,
99.3%) was assessed in three male and three female New Zealand white
rabbits by instilling the test substance into the conjunctival sac of
the right eye, the left eye remaining untreated and serving as
control. This study was conducted according to GLP requirements.
Diffuse corneal opacities (score 1 for degree and area of opacity)
were observed in two animals at 24 h, but these effects had
disappeared by 48 h and no other corneal effects were seen. Iridial
inflammation (score 1) was noted in four animals at 1 h, and this
effect persisted for 24 h in one of the animals and for 48 h in
another. No iridial effects were observed after 72 h. Conjunctival
irritation was observed in all animals, with redness (score 2:
diffuse, deeper crimson red at 1-24 h; score 1: vessels definitely
injected above normal at 48 h), chemosis (score 2: obvious swelling
with partial eversion of lids up to 48 h), and discharge (scores 2-3:
moistening of the lids and hairs just adjacent to lids or a
considerable area around the eye only at 1 h). No signs of
conjunctival irritation was observed at 72 h. The maximum individual
irritation scores were seen at 1 h and ranged from 12 to 19, with a
mean score of 16. The mean scores at 24, 48, and 72 h were 9.3, 2.5,
and 0, respectively. The overall scores according to EEC Council
Directive 67/548/EEC were 2 (mean, 0.11) for corneal opacity, 3 (0.17)
for iridial inflammation, 11 (0.61) for conjunctival redness, and 8
(0.44) for conjunctival chemosis (Dreher, 1994d)
Technical-grade chlorpyrifos (100 mg; purity, 96.2%) was
instilled into the conjunctival sac of the left eye of Himalayan
albino rabbits, and the right eyes of the animals served as controls.
A group of control animals was similarly treated with distilled water
only. All animals were observed for signs of ocular irritation at 1,
4, 24, 48, and 72 h, and then daily up to day 7. At the 4-h
observation, very slight iridial irritation (score 1) and conjunctival
irritation (score 1) were reported. It was not clear from the report
whether the conjunctival irritation was redness, discharge, or
chemosis. No signs of irritation were reported at 24 or 72 h. A
'quality assurance statement' was issued for this study (Frederick
Institute of Plant Protection and Toxicology, 1995d).
Table 1. Studies of the acute toxicity of technical-grade chlorpyrifos
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Oral administration
Mouse SmithWebster M 1% aqueous gum 102 (94-110) Coulston et al. (1971)
tragacanth
Mouse NAMRU F Soya bean oil 152 (143-162) Berteau & Deen (1978)
Mouse Swiss albino M&F Vegetable oil 109 (93-127) QA Fredrick Institute (1995a)
Rat NR M 5% corn oil 163 (97-276) Taylor & Olson (1963)
F 135 (97-188)
Rat Sprague-Dawley F Soya bean oil 169 (146-196) Berteau & Deen (1978)
Rat Sprague-Dawley M Arachis oil 276 (167-455) GLP Dreher (1994a)
F 350 (285-429)
M&F 320 (260-393)
Rat Wistar M&F Vegetable oil 134 (102-163) QA Frederick Institute (1995b)
Rat Sprague-Dawley M Maize oil 264 GLP Wilson & MacBeth (1994)
F 141
M&F 192
Rat Sprague-Dawley M Maize oil 475 (311-727) Buch & Gardner (1981)
F 337 (220-515)
Rat Sprague-Dawley M Corn oil 221 (181-69) Nissimov & Nyska (1984a)
F 144 (105-200)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M Not reported 205 (134-299) Henck & Kociba (1980)
F 96 (72-140)
M 248 (170-379)
F 97 (80-112)
M 270 (188-426)
F 174 (130-244)
Guinea-pig NR M Corn oil 504 (300-850) GLP Lackenby (1985a)
Rabbit NR M Corn oil 1000-2000 GLP Lackenby (1985a)
Chicken NR M Capsule 32 (14-72) GLP Lackenby (1985a)
Chicken Leghorn M Diet 25 (21-31) Sherman et al. (1967)
Chicken Leghorn M Capsule 32 Stevenson (1963)
Chicken White Rock M&F Capsule 50-63 Stevenson (1966a)
Chicken NR M&F Capsule 20-50 Ross & Roberts (1974)
Chicken NR M&F Corn oil 102 (64-169) Ross & Roberts (1974)
Chicken NR M Capsule, 32 (14-72) Taylor & Olson (1963)
undiluted
Turkey Beltsville NR NR 32-63 Stevenson (1967)
small white
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Inhalation
Mouse NAMRU F 65% xylene 94 (83-106) Berteau & Deen (1978)
(27-50 min,
whole body,
aerosol)
Rat Albino M&F Vapour (4 h, > 200a GLP Hardy & Jackson (1984)
(HC/CFHB) whole body)
Rat Sprague-Dawley F 65% xylene 78 (57-108) Berteau & Deen (1978)
(60-180 min,
whole body,
aerosol)
Rat Sprague-Dawley M&F Undiluted (4 h, > 230b (max. GLP Anderson et al. (1995)
nose only, attainable
powder) concentration)
Rat Sprague-Dawley M 40% xylene > 4070a Buch (1980)
F (4 h, nose only, 2890b (2010-4160)
aerosol)
Rat Wistar M&F Undiluted (4 h, > 1020a GLP Kenny et al. (1987)
whole body,
aerosol)
Rat Fischer 344 M&F 1% aqueous > 3200a GLP Phillips & Lomax (1989)
solution (4 h,
whole body,
aerosol)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M&F Vapour (4 h, > 36a (max. GLP Blagden (1994)
nose only) attainable
concentration)
Rat Wistar M&F Acetone (4 h, 560b (360-950) Fredrick Institute (1996a)
whole body,
nebulized particles
< 5 µm)
Dermal application
Rat Sprague-Dawley M&F PEG > 2000 Lackenby (1985b)
Rat Sprague-Dawley M&F Undiluted > 2000 GLP Jackson & Ogilvie (1994a)
Rat NR M&F Undiluted > 2000 QA Nissimov & Nyska (1984b)
Rat Fischer M&F Undiluted > 2000 GLP Jeffrey et al. (1986)
Rat Sprague-Dawley M&F Arachis oil > 2000 GLP Dreher (1994b)
Rat Sprague-Dawley M&F Saline > 5000 (intact Buch et al. (1980)
and abraded)
Rabbit Himalayan M&F Water 1233 (993-1531) QA Fredrick Institute (1995c)
Rabbit New Zealand M&F Undiluted 1580 (828-2606) Henck & Kociba (1980)
white M&F 1598 (1243-1919)
M&F 1801 (1023-3152)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat CFY M&F Tween 20/ 147 (120-179) Davies & Kynoch (1970)
DMSO/water
2:3:5 (subcutaneous)
M, male; F, female; NR, not reported; GLP, good laboratory practice; QA, quality assurance; PEG, polyethylene glycol;
DMSO, dimethyl sulfoxide
Technical-grade chlorpyrifos (100 mg; fine powder) was placed
into the right eye of nine young adult albino rabbits. The eyes of
three animals were irrigated with saline solution 30 s after
treatment, while the eyes of the remaining animals were not irrigated.
Ocular examinations were conducted after 24, 48, and 72 h and at 4 and
7 days. Most of the animals displayed slight initial pain after
instillation of the test material. No signs of irritation were
observed in the cornea or the iris. Slight conjunctival redness was
observed in all animals with unwashed eyes, and in two animals whose
eyes were irrigated after 30 s. The irritation disappeared in most
animals by days 3-4 but persisted for 7 days in a single animal with
unirrigated eyes. The mean irritation scores in rabbits with unwashed
eyes were 2.3 at 24 h and 0.3 at 7 days, while in the rabbits with
irrigated eyes the scores were 1.3 and 0, respectively (Buch &
Gardner, 1980a).
Dermal irritation: A finely ground commercial preparation of
chlorpyrifos (500 mg) moistened with distilled water was applied under
an occlusive dressing to about 6 cm2 of clipped skin on the back of
three young adult female New Zealand white rabbits as a single 4-h
application. No primary irritation was observed (Jones, 1985b).
The dermal irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested in a study conducted according to GLP in six
young male New Zealand white rabbits, to which 500 mg of the material
(moistened with water) were applied under a gauze patch on clipped
intact skin on the trunk and then covered with an occlusive dressing.
The dressings were removed after 4 h and the skin wiped to remove
residual material. The skin was examined for signs of irritation 1,
24, 48, and 72 h after removal of the patch and scored for irritation
on the basis of a system recommended by the US Environmental
Protection Agency. Further assessments were conducted after 4, 5, and
6 days to determine the reversibility of the effects. Slight erythema
(score 1) was noted on three of six test sites at 1 h, and this effect
persisted until 72 h at one site and until day 5 in another animal. No
skin irritation was seen on day 6 (Jackson & Ogilvie, 1994dc).
A technical-grade, fine-powder commercial preparation (purity not
stated) was impregnated at a dose of 0.5 g onto unmedicated lint
patches and tested in six young adult male New Zealand white rabbits
on shaved abraded and intact sites on the back. The patches were held
in place under impermeable dressings, which were removed after 23 h.
The reactions at the test sites were assessed by the method of Draize
at 24 and 72 h and 7 days after administration of the test material.
Well-defined or very slight erythema was seen in all animals at 24 h,
on both intact and abraded sites. Very slight oedema was also seen at
the intact and abraded test sites of one animal at 24 h, another at 72
h, and a third at 7 days. No skin reactions were seen in other animals
at these intervals. The primary irritation index was calculated to be
0.67 (Buch & Gardner, 1980b).
Technical-grade chlorpyrifos (purity, 96.2%) was applied at a
dose of 500 g to clipped intact and abraded skin on the trunks of
three male and three female Himalayan albino rabbits, and the trunks
were wrapped with plastic sheets held in place with tapes for 24 h. A
control group was treated with distilled water. After exposure, the
coverings were removed, but the report did not indicate whether the
test sites were washed or cleaned. The skin was observed 24 and 72 h
after application for signs of irritation. No sign of erythema or
oedema was reported. A 'quality assurance statement' was issued for
this study (Frederick Institute of Plant Protection and Toxicology,
1995e).
Technical-grade chlorpyrifos (purity, 99.3%), moistened with
0.5 ml distilled water, was applied at a dose of 0.5 g to clipped
areas on the backs of New Zealand white rabbits in a study carried out
in accordance with GLP. Surgical gauze was applied over the sites and
held in place with a strip of surgical adhesive tape, and the trunk of
each rabbit was wrapped in an elasticized corset. After the 4-h
contact period, the corset and patches were removed, and the treated
skin was wiped with cotton-wool soaked in distilled water. One hour
and approximately 24, 48, and 72 h after removal of the patches, the
test sites were examined for evidence of dermal irritation and scored
according to the method of Draize. Very slight (score 1) to
well-defined (score 2) erythema and very slight (score 1) to slight
(score 2) oedema were observed in all animals 1-24 h after removal of
the patches. At 48 h, very slight erythema was observed in three
animals and very slight oedema in one. No sign of skin irritation was
seen at 72 h. The sum of the readings at 24 and 72 h was 10, and the
primary irritation index (the sum of the readings/12) was 0.8. This
score indicates mild irritation according to Draize (Dreher, 1994d).
Dermal sensitization: The skin sensitizing potential of
technical-grade chlorpyrifos (purity, 99.3%) was assessed in female
albino Dunkin-Hartley guinea-pigs in a study conducted according to
GLP. Very slight (score 1; 10 animals) to well-defined (score 2; 2
animals) erythema and very slight (score 1; 1 animal) oedema were
observed 1 h after topical induction, but no signs of irritation were
observed at 24 h. No skin reaction was observed in control animals,
and no skin reactions were observed at the test or vehicle control
sites after topical challenge 21 days after induction. One animal was
found dead at this stage of the study, but the cause of death was not
determined. The test material was not a skin sensitizer in this study
(Dreher, 1994e).
Technical-grade chlorpyrifos (purity, 96.2%) was prepared as a 1%
solution in 0.5% carboxymethyl cellulose and tested at a dose of
100 mg in male Hartley guinea-pigs. Test and positive control groups
received induction by exposure of the shaven skin of the left flank,
and challenge 14 days after the third induction dose. Control animals
received only a challenge with 0.5 ml of a 0.1% solution. The test
animals had barely perceptible (score 1; 4/10 animals) erythema or
slight oedema (score 1; 2/10 animals) 24 h after the challenge, but no
signs of irritation were observed at 48 h. A single control animal had
barely perceptible erythema at 24 h, but no other signs of skin
irritation were seen in this group. Positive control animals had
barely perceptible to deep-red erythema (score 1-3) and slight to
marked oedema (score 1-3) 24 h after the challenge and barely
susceptible erythema and slight to marked oedema at 48 h. This study
was issued a 'quality assurance statement' (Frederick Institute of
Plant Protection and Toxicology, 1996b).
After a study to determine a suitable dosing regimen, six male
and six female young albino Dunkin-Hartley guinea-pigs were treated
with a commercial preparation of chlorpyrifos in a standard Beuhler
skin sensitization assay. The test substance (0.3 ml, 100% w/v in
polyethylene glycol on a lint pad) was applied for 6 h under an
occlusive bandage to the freshly clipped left flank of the animals on
days 1, 8, and 15. Challenge doses of chlorpyrifos and solvent were
applied for 6 h on day 29 to the right and left flanks, respectively.
The negative controls were treated only on day 29, when they received
the same treatment as the test animals. The challenge sites of both
groups were evaluated 24 and 48 h after removal of the patch. No
adverse skin reactions were seen after exposure to chlorpyrifos or
vehicle alone (Jones, 1985c).
Technical-grade chlorpyrifos (purity, 99%) was tested in young
female Dunkin-Hartley guinea-pigs by the Magnusson-Kligman
maximization test in a study conducted according to GLP. The test
consists of an induction with intradermal injections of the test
material followed after 1 week by topical application, and a challenge
3 weeks after induction. The test sites were assessed for irritation
24, 48, and 72 h after injection and 24 and 48 h after removal of the
patch (48-h exposure). A concentration of 25% of the test material in
maize oil was selected for injection in the induction phase and 75% in
maize oil for the topical induction phase; a concentration of 75% in
maize oil was also selected for the challenge. During the induction
phase, slight or discrete erythema was observed in all test and
control animals 1 and 24 h after injection and 1 h after topical
application. A number of test and control animals also showed slight
or discrete erythema 24 h after topical application. During
application, two test animals became ataxic, thin, and subdued and
were removed from the study, and one test animal was removed from the
study before the challenge application because it had lesions at the
test site. A single test animal showed a positive response to
challenge, with slight to discrete erythema. None of the animals given
the vehicle alone reacted to the challenge application (Jackson &
Ogilvie, 1994a).
Technical-grade chlorpyrifos (purity, 96.8%) dissolved in 100%
dimethylsulfoxide and diluted in the same solvent to achieve the
desired concentrations was tested in a study that conformed to GLP in
albino Dunkin-Hartley guinea-pigs. In animals treated with a single
dose, irritation ranging from scattered mild redness to moderate and
diffuse redness was seen at 24 h in 5/10 animals; only mild redness
was seen at 48 h in 4/10 animals. In 20 animals induced and then
challenged with chlorpyrifos, only one had scattered mild redness
after the challenge dose at 24 h, and no skin reaction was seen at 48
h. In a positive control group, 9/10 animals had mild redness at 24 h
and 4/10 at 48 h (Berman, 1987).
(b) Short-term studies of toxicity
Mice
In a 2-week study conducted according to GLP requirements, young
adult CD-1 mice, were given a commercial preparation of
technical-grade chrlopyrifos (purity, 96.3%) at dietary concentrations
of 0 (control), 75, 150, 300, 600, or 1200 ppm, equaivalent to 0,
14-18, 30-32, 56-67, 89-100, and 130-180 mg/kg bw per day,
respectively. Deaths and treatment-related clinical signs, including
tremor, ocular opacity, ocular staining, hunching, and lachrymation,
were seen mainly in animals at the highest dose. Marked reductions in
body weight and feed consumption were observed in animals at doses
> 600 ppm. In males, cholinesterase activity was reduced at all
doses. At 75 ppm, plasma cholinesterase activity was reduced by
> 95%, erythrocyte acetylcholinesterase activity by almost 40%, and
brain acetylcholinesterase activity by about 50%. Inhibition of
erythrocyte acetylcholinesterase activity was not dependent on dose,
and approximately 35% inhibition being seen at all doses, but plasma
and brain cholinesterase activities were reduced in a dose-related
manner, with an inhibition of 99% in plasma and about 89% in brain at
the high dose. Similar patterns of cholinesterase inhibition were seen
in females. On the basis of the results of this study, doses of 5, 50,
200, 400, and 800 ppm were selected for a 13-week dietary study in
mice (Crown et al., 1984a).
Groups of 40 male and 40 female CD-1 mice were given chlorpyrifos
(purity, 95.7%) in the diet at 0 or 15 ppm, equal to 2.7 mg/kg bw per
day for males and 3.4 mg/kg bw per day for females; 20 animals of each
sex per group were killed after 1 week, and 20 were treated for a
total of 4 weeks. There were no significant clinical signs or
unscheduled deaths. Food consumption was unaffected by treatment, but
the body-weight gain of male mice was decreased by 25% at the end of
the study. Organ weights were not affected by treatment, and there
were no significant differences between groups. The findings at
necropsy were within normal limits for all groups. After 1 week,
plasma cholinesterase activity was depressed by 88-91% and erythrocyte
acetylcholinesterase activity was depressed by 40-53%; after 4 weeks,
plasma cholinesterase activity was depressed by 91% and erythrocyte
acetylcholinesterase activity by 53%. Brain acetylcholinesterase
activity was unaffected by treatment at either time. There was no
difference between the sexes in the alterations in plasma and
erythrocyte cholinesterase activity (Davies et al., 1985).
Groups of 12 Crl CD-1 mice of each sex were given chlorpyrifos
(purity, 93.5%) in the diet at concentrations of 0, 5, 50, 200, 400,
or 800 ppm (equivalent to 0, 0.7-1.3, 7.1-14, 32-53, 41-140, and
110-300 mg/kg bw per day, respectively) for 13 weeks. This study was
conducted to the requirements of GLP. Dose-related increases in the
mortality rate and the frequency of ocular opacities were seen at 400
and 800 ppm. Body weights were reduced by 10-15% in mice at 800 ppm
throughout the study. Plasma cholinesterase activity was markedly
decreased at all doses, and decreased erythrocyte acetylcholinesterase
activity was seen, but with no clear dose-dependency; brain
acetylcholinesterase activity was inhibited in a dose-dependent manner
in males at doses > 200 ppm and in females at doses > 50 ppm
(Table 2).
Table 2. Group mean values for inhibition of cholinesterase activity in
Crl CD-1 mice given chlorpyrifos in the diet (expressed as a
percentage of control values)
Cholinesterase Sex Dose (ppm)
5 50 200 400 800
Plasma Male 45*** 95*** 98*** 99*** 99***
Female 36*** 97*** 98*** 99 *** 99 ***
Erythrocyte Male 34a 21 16 12 26
Female 11 57*** 44** 48** 44**
Brain Male 43a 14* 80*** 85*** 87**
Female 3a 28*** 58*** 84*** 88***
Plasma, butyryl cholinesterase; erythrocyte and brain,
acetylcholinesterase
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased in comparison with controls
A single malignant lymphoma was reported in a female at the high
dose at terminal sacrifice, but no other neoplastic lesions were
reported. Given the isolated nature of this finding, it was considered
not to be related to treatment. The absolute and relative organ
weights were generally unaffected by treatment, although the relative
liver weights were increased in females at 200, 400, and 800 ppm. The
clinical signs included urogenital staining in males at doses
> 200 ppm and in females at 800 ppm. Ocular opacities occurred in
some animals at 400 and 800 ppm. Dose-related histopathological
findings were seen in the adrenal glands (including lipogenic
pigmentation at doses > 200 ppm in females and at > 400 ppm in
males) and in the eyes (acute or subchronic keratitis in two males and
four females at 800 ppm and in a single female at 400 ppm).
Erythrocyte acetylcholinesterase activity was variable, and the effect
of treatment on this parameter was equivocal. The NOAEL was 5 ppm,
equal to 0.7 mg/kg bw per day, on the basis of inhibition of brain
acetylcholinesterase activity at higher doses (Crown et al., 1987).
Rats
In a 2-week study to establish the doses to be used in a 13-week
study, technical-grade chlorpyrifos (purity, 95.5%) was administered
to groups of five rats of each sex at dietary concentrations of 0
(controls), 10, 30, 84, 240, or 694 ppm for 14 days, equivalent to 0,
1.4-1.9, 4.1-5.2, 12-14, 34-39, and 66-95 mg/kg bw per day,
respectively. At the high dose, clinical signs of intoxication were
reported, consisting of irritability, hunching, tremor, ataxia,
urogenital staining, pigmented orbital secretion, failure to groom,
and proneness. No treatment-related clinical signs were observed at
other doses, and treatment-related deaths were confined to animals at
the high dose. Body weights were reduced in males and females at
694 ppm and in females at 240 ppm, and food consumption was also
reduced in animals at 694 ppm. Dose-related decreases in blood
cholinesterase activity were seen at all doses, the inhibition ranging
from 42% at 10 ppm to 93% at 694 ppm. On the basis of these findings,
the doses selected for the 13-week study were 0.5, 10, and 100 ppm
(Crown et al., 1984b).
Plasma, erythrocyte, and brain cholinesterase activities were
markedly reduced in rats after dietary administration of chlorpyrifos
for 14 days at doses of 5 or 10 mg/kg bw per day in a study carried
out according to GLP. Inhibition of cholinesterase activity occurred
in the absence of clinical signs of intoxication. Feed consumption,
body weights, and gross pathological appearance were similar in
control and treated groups. Statistically significant increases in
absolute and relative adrenal weights (about 20% compared with
controls) were observed in females only at 10 mg/kg bw per day
(Liberacki et al., 1990).
Fischer 344 rats received technical-grade chlorpyrifos (purity,
95%) by nose-only inhalation for 6 h/day for 5 days at a target
concentration of 20 ppb in a study that conformed to GLP. No deaths or
treatment-related clinical signs were reported. Exposure of female
rats to a concentration of 0.34 mg/m3 (23 ppb) resulted in a
significant decrease in plasma cholinesterase activity. No effect on
erythrocyte or brain acetylcholinesterase activity was seen in females
or on plasma, erythrocyte, or brain cholinesterase activity in males
(Newton, 1988a).
Chlorpyrifos was administered by nose-only inhalation to female
Fischer 344 rats at a time-weighted average concentration of 12 ppb
(equivalent to 172 µg/m3) for 6 h/day on 5 days per week for 2 weeks
in a study that met GLP requirements. No clinical signs of
intoxication or treatment-related changes in body weights were
reported, and all rats survived until the scheduled termination.
Clinical chemistry showed no treatment-related changes in
haematological, clinical chemical, or urinary parameters, including
plasma, brain, and erythrocyte cholinesterase activity (Landry et al.,
1986).
In a study to determine a dose range, conducted to GLP
requirements, technical-grade chlorpyrifos was administered by
whole-body exposure of groups of Wistar rats for 6 h/day, 5 days per
week for 2 weeks at target concentrations of 0 (air control), 10, 100,
and 400 mg/m3 (actual concentrations, 0, 10, 94, and 388 mg/m3).
Exposure of animals to the high dose was terminated after five
exposures because they became moribund, with rapid weight loss and
deterioration of their general condition, and all surviving animals
were killed on day 9. No deaths was observed at other doses. There was
no NOAEL in this study, as significant inhibition of plasma,
erythrocyte, and brain cholinesterase activity was seen at all doses
in males and females. Plasma cholinesterase activity was inhibited by
78, 86, and 88% at the three doses, respectively, in males and by 91,
93, and 95%, respectively, in females when compared with controls.
Erythrocyte cholinesterase activity was inhibited by 78, 58, and 75%
in males and by 60, 80, and 73% in females, respectively. Brain
cholinesterase activity was inhibited by 50% at 10 mg/m3, about 70%
at 94 mg/m3, and about 72% at 388 mg/m3 in males and females.
Clinical signs of toxicity (tremors, salivation, and lachrymation),
decreased food consumption, and decreased body weights were observed
at the two higher doses. Increased adrenal weights were seen at 94 and
388 mg/m3, and effects in the forestomach including thickening and
congestion were observed at 388 mg/m3 (Kenny et al., 1988).
Fischer 344 rats were exposed to time-weighted average
concentrations (nominal) of 0, 1, or 5 ppb (0, 0.014, and
0.072 mg/m3) of chlorpyrifos vapour for 6 h/day, 5 days per week for
2 weeks in a study conducted according to GLP. The mean daily
time-weighted average concentrations found by analysis were 0.7 and
5 ppb, respectively. Although two control groups were used, their
cholinesterase activity did not differ statistically significantly,
and the activity in test groups was compared with that of the combined
control group. Statistically significant decreases in plasma,
erythrocyte, and brain cholinesterase activity were observed at 5 ppb,
but as the reductions in brain and erythrocyte acetylcholinesterase
activity did not reach 20% when compared with the control group, the
effect was considered not to be of toxicological significance. Plasma
cholinesterase activity was inhibited by > 20% in females at 5 ppb.
A statistically significant decrease in plasma cholinesterase activity
in females at 0.7 ppb was considered to be incidental to treatment.
There were no treatment-related deaths, clinical signs of toxicity, or
changes in body weight during the study. No treatment-related effects
were seen on body or organ weights, and no lesions were found at gross
examination. No significant adverse treatment-related effects were
observed at 5 ppb (approximately 0.07 mg/m3) (Landry et al., 1985;
Streeter et al., 1987).
Groups of 10 CDF Fischer 344 rats of each sex were fed diets
containing chlorpyrifos (purity, 95.7%) at concentrations that
provided doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day for 13 weeks in
a study that conformed to GLP. The treatment-related effects seen at
the highest dose consisted of decreased body weight and body-weight
gain in males, increased fatty vacuolation of the adrenal zone
fasciculata in males, and changes in haematological and clinical
chemical parameters consisting of decreased erythrocyte counts in both
sexes; increased platelet counts, reduced serum total protein,
albumin, and globulin concentrations, and decreased alanine
aminotransferase and alkaline phosphatase activity in males; and
decreased serum glucose concentration and increased urinary specific
gravity in females. Plasma and erythrocyte cholinesterase activities
were depressed at doses > 1 mg/kg bw per day, and the activity of
brain acetylcholinesterase was depressed at 5 and 15 mg/kg bw per day.
The NOAEL was 0.1 mg/kg bw per day on the basis of depression of
erythrocyte and brain acetylcholinesterase activity (Szabo et al.,
1988).
Groups of 10 rats of each sex were given diets containing
chlorpyrifos at 0, 10, 30, 300, or 1000 ppm, but treatment at the
highest concentration was discontinued after 4 weeks because of severe
clinical signs. Plasma cholinesterase activity was reduced in a
dose-related manner and was significantly lower than that of controls
at all doses. Erythrocyte cholinesterase activity was similarly
significantly reduced at all doses, but no dose-dependency was
demonstrated. Brain cholinesterase activity showed a clear
dose-related depression in animals of each sex at doses > 30 ppm.
At 30 ppm, the activity was inhibited by 22% in males and 24% in
females 41 days after administration. No other significant effects
were found on haematological or clinical chemical parameters, but few
were examined. No histopathological lesions were found that could be
linked to treatment. The NOAEL for inhibition of brain cholinesterase
activity was 0.5 mg/kg bw per day, and the LOAEL was 1.5 mg/kg bw per
day. There was no NOAEL for inhibition of erythrocyte or plasma
cholinesterase activity, as effects were seen at concentrations
> 10 ppm (0.5 mg/kg bw per day) (JMPR 1972; modified by reference
to the original reports of Beatty & McCollister, 1964; Beatty &
McCollister, 1971).
Five groups of 10 rats (strain not specified) of each sex
received chlorpyrifos (purity not specified) in the diet at
concentrations that provided doses of 0.03-10 mg/kg bw per day. The
doses of 0 and 0.3 mg/kg bw per day were fed for 90 days; the doses of
1, 3, and 10 mg/kg bw per day were fed for 28 days, when these animals
were fed control diets for 3 weeks and then diets containing 0, 0.03,
or 0.1 mg/kg bw per day. Behaviour and growth were affected at 3 and
10 mg/kg bw per day. Cholinesterase activity was depressed at 0.3
mg/kg bw per day. During the period of removal from treated diet, the
cholinesterase activity returned to pre-test levels; on resumption of
feeding at 0.1 mg/kg bw per day, only a slight depression was observed
in plasma and erythrocyte cholinesterase activity. In view of of the
unusual design of this study, the toxicological relevance of this
effect is considerede equivocal. No depression of brain or blood
enzymes was seen at 0.03 mg/kg bw per day (Blackmore, 1968).
Groups of 10 Crl rats of each sex were fed diets containing
chlorpyrifos (purity, 97.5%) at concentrations that provided a dose of
0.3 mg/kg bw per day for 13 weeks or 1, 3, or 10 mg/kg bw per day for
4 weeks. No deaths occurred during the study. After 4 weeks, there was
a clear dose-response relationship for inhibition of erythrocyte and
plasma cholinesterase activity at all doses, and animals at higher
doses showed clinical signs of toxicity. These animals were therefore
removed from exposure and allowed to recover for 3 weeks, during which
time the plasma and erythrocyte cholinesterase activities returned to
control values. These groups were then fed chlorpyrifos at doses of 0,
0.03, or 0.1 mg/kg bw per day for 13 weeks. The dose of 0.03 mg/kg bw
per day had no unequivocal effect on cholinesterase activity in
erythrocytes, plasma, or brain in either sex, but the dose of
0.1 mg/kg bw per day significantly reduced the erythrocyte
cholinesterase activity. The NOAEL was 0.03 mg/kg bw per day on the
basis of inhibition of erythrocyte cholinesterase activity after 13
weeks at 0.1 mg/kg bw per day (Hazleton Laboratories, 1968).
Groups of 20 Sprague-Dawley rats of each sex were given
chlorpyrifos (purity not stated) in the diet at 0, 0.03, 0.15, or
0.75 mg/kg bw per day for 6 months; an interim sacrifice of five
animals of each sex per dose was conducted after 3 months. A total of
16 animals across dose groups died from chronic murine pneumonia.
Cholinesterase activity was inhibited at the high dose in both
erythrocytes (50%) and plasma (65%), but the activity in brain was not
inhibited at any dose. Treatment did not change body-weight gain, food
consumption, or haematological or other clinical chemical parameters.
The NOAEL was 0.15 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholinesterase activity at 0.75 mg/kg bw per day
(JMPR, 1972; modified by reference to the original report of Coulston
et al., 1971).
Groups of 20 Crl CD Sprague-Dawley-derived rats of each sex were
given chlorpyrifos (purity, 95.5%) at dietary concentrations of 0
(control), 0.5, 10, or 200 ppm for 13 weeks in a study conducted
according to GLP. The minimum and maximum achieved doses of the test
material were calculated to be 0, 0.03 and 0.078, 0.6 and 1.5, and 13
and 31 mg/kg bw per day, respectively. All animals were inspected once
or twice daily for signs of ill-health and other treatment-related
signs and all were examined closely each week. No deaths or
significant clinical signs were observed at any dose. Clinical
chemistry, urinary analysis, and ophthalmoscopy revealed no findings
that were considered to be related to treatment. At 200 ppm
(approximately 13 mg/kg bw per day), reduced body weights, erythrocyte
count, erythrocyte volume fraction, and haemoglobin and increased food
consumption were observed. Statistically significant reductions
(> 20% inhibition compared with controls) in plasma cholinesterase
activity were observed in males at doses > 0.5 ppm (approximately
0.03 mg/kg bw per day) at 12 weeks and in females at doses > 10 ppm
(approximately 0.6 mg/kg bw per day). The inhibition was dose-related
in males but not in females. Erythrocyte and brain cholinesterase
activities were not measured in this study. The NOAEL was 10 ppm,
equal to 0.6 mg/kg bw per day, on the basis of reduced body weights
and statistically significant reductions in packed cell volume,
haemoglobin, and erythrocyte volume fraction at 200 ppm (Crown et al.,
1985).
Groups of 10 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 100%) by nose only at concentrations of 0, 5.2,
10.3, or 20.6 ppb (0, 75, 150, or 300 µg/m3) for 6 h/day, 5 days per
week for 13 weeks in a study that conformed to GLP. No
treatment-related deaths and only minimal clinical signs were observed
during the study. Body weights and urinary, haematological, and
clinical chemical parameters were unaffected by treatment. No effects
on plasma, erythrocyte, or brain cholinesterase activity were seen at
any dose. Gross and histopathological examination revealed no effects
associated with administration of chlorpyrifos (Corley et al., 1986).
Groups of 15 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 95%) by nose only for 6 h/day, 5 days per week,
for 13 weeks at target concentrations of 0 (control), 5, 10, and
20 ppb (0, 0.07, 0.14, and 0.28 mg/m3, respectively). The study was
conducted according to GLP requirements. There were no
treatment-related deaths or ophthalmic, haematological, or clinical
chemical changes. Plasma cholinesterase activity was inhibited by 23%
in males at the high dose ( p < 0.01), but other changes in plasma
cholinesterase activity were considered not to be related to
treatment. Erythrocyte and brain cholinesterase activities were
unaffected. Pathological and histopathological examination showed no
effect of treatment. The NOAEL was 20 ppb (0.28 mg/m3), on the basis
of inhibition of plasma cholinesterase activity. The absence of frank
toxicity at any dose in this study makes it difficult to draw
conclusions about the toxic potential of inhaled chlorpyrifos at doses
> 0.28 mg/m3 (Newton, 1988b).
In a study in Fischer 344 rats treated dermally, conducted
according to GLP requirements, the animals received chlorpyrifos
(purity, 100%) at 0, 0.1, 0.5, or 5 mg/kg bw per day in corn oil for a
total of 15 days over a 21-day period. No treatment-related effects
were observed at any dose, and plasma, erythrocyte, and brain
cholinesterase activities were not inhibited. Gross and microscopic
examination did not reveal any treatment-related changes, and body
weights and organ weights were unaffected by treatment. In the 4-day
range-finding component of this study, decreased plasma cholinesterase
activity (45%) and erythrocyte cholinesterase activity (16%) were
observed at 10 mg/kg bw per day, in the absence of clinical signs of
intoxication (Calhoun & Johnson, 1988, 1989).
Rabbits
No skin irritation was found when a formulation containing 61.5%
chlorpyrifos and 35% xylene was applied to the skin of the back and
abdomen of groups of two rabbits at doses of 5, 10, 25, and 50 mg/kg
bw 20, 4, 3, and 1 times, respectively. Both plasma and erythrocyte
cholinesterase activities were significantly inhibited in each dose
regimen. The values for plasma recovered more quickly than those for
erythrocytes, which were still significantly inhibited at day 40 after
the 3-, 4-, and 20-day exposure patterns (Pennington & Edwards, 1971).
Three rabbits were exposed for 5 min to a formulation containing
61.5% chlorpyrifos and 34.5% xylene from an ultra-light vapour cold
aerosol fog generator delivering 3.8 L/h. Two rabbits were exposed at
a distance of 8 m at a height of 1.3 m and one at a height of 0.8 m.
Exposure was terminated after 5 min because of ocular and pulmonary
irritation. The recorded concentration of chlorpyrifos in
breathing-space air was about 108 mg/L (range, 83-133 mg/L). By 24 h
after treatment, the rabbits had decreased activities of plasma
cholinesterase (< 33%) and erythrocyte cholinesterase (< 12%),
but these values had recovered almost to the control level by 72 h
after treatment (Pennington & Edwards, 1971).
Chickens
White Leghorn chickens were given chlorpyrifos in their
drinking-water at 1 ppb, 1 ppm, or 100 ppm for up to 84 days. Only
plasma cholinesterase activity was reported because of technical
difficulties with blood sampling. Significant inhibition (54%) of
plasma cholinesterase activity was recorded on day 84 in birds at 100
ppm (Stevenson, 1965).
Dogs
Technical-grade chlorpyrifos (purity, 95.8%) was administered to
groups of two pure-bred beagles of each sex orally in gelatine capsule
at doses of 0 (control; lactose only), 0.01, 0.03, 0.5, or 5 mg/kg bw
per day for 4 weeks, although only one animal of each sex was exposed
at 0 and 0.5 mg/kg bw per day. This study met GLP requirements. No
deaths occurred during the study, and no clinical signs associated
with treatment were observed. All animals gained weight steadily
during treatment, and food consumption was similar in all groups.
Rapid inhibition of plasma cholinesterase activity was observed at 0.5
and 5 mg/kg bw per day at all intervals, and the inhibition was both
dose- and time-dependent. Plasma cholinesterase activity was reduced
at 0.03 mg/kg bw per day, but the inhibition was considered not to be
significant owing to variation between groups and the small group
sizes. In animals at 5 mg/kg bw per day, erythrocyte cholinesterase
activity was 47% that of controls on day 7 and was still inhibited by
> 70% at the end of the study; brain cholinesterase activity was
inhibited by 32% in comparison with concurrent controls at the end of
the study. In animals at 0.5 mg/kg bw per day, erythrocyte
cholinesterase activity was inhibited by 30% on days 27-28 only, and
the effect was considered not to be toxicologically significant owing
to its transient nature. Macroscopic post-mortem examination showed no
changes that were considered to be of toxicological significance. The
NOAEL was 0.5 mg/kg bw per day, on the basis of inhibition of
erythrocyte and brain cholinesterase activity at 5 mg/kg bw per day
(Harling et al., 1989a).
Five dogs received 10 applications of a 0.087% commercial aerosol
formulation of chlorpyrifos in dichlorophene on their backs (with the
fur brushed against the grain) for 2 weeks, each application lasting
30 s. No deaths occurred, and no abnormalities were seen in clinical
signs, body-weight gain, or opthalmoscopic, haematological or clinical
chemical parameters. Erythrocyte cholinesterase activity was not
decreased, but plasma cholinesterase activity was inhibited from the
beginning of treatment, returning to normal 72 days after treatment.
The inhibition of plasma cholinesterase activity was not accompanied
by clinical signs (Sharp & Warner, 1968).
Groups of two young adult beagles of each sex were given daily
doses of chlorpyrifos orally by capsule at doses of 0, 0.03, 0.1, 0.3,
or 1 mg/kg bw per day for 90 days. Plasma cholinesterase activity was
inhibited at all doses. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day, and that for
inhibition of brain cholinesterase activity was 1 mg/kg bw per day
(Blackmore, 1968).
Technical-grade chlorpyrifos (purity, 95.8%) was administered
orally by capsule to groups of four pure-bred beagles of each sex at
doses of 0 (control), 0.01, 0.22, or 5 mg/kg bw per day for 13 weeks.
There were no unscheduled deaths or unequivocal clinical signs during
the study. The group mean body weights of animals at the high dose
were reduced. Haematology, clinical chemistry, urinary analysis, and
ophthalmoscopy revealed no findings that were considered to be related
to treatment. No treatment-related pathological macroscopic or
microscopic change was found. Statistically significant, dose-related
reductions in plasma cholinesterase activity were seen at 0.01 mg/kg
bw per day (bitches only; week 6 only; < 25%) and above (dogs and
bitches; all sample intervals; < 87%). Erythrocyte cholinesterase
activity was significantly inhibited in a dose-dependent manner at
0.22 mg/kg bw per day (< 46%) and at 5 mg/kg bw per day (< 85%).
At 0.01 mg/kg bw per day, erythrocyte cholinesterase was inhibited by
17-18% at week 12, but the reduction was not statistically significant
at any sample interval. Brain cholinesterase activity was inhibited in
animals of each sex (by 46%) at 5 mg/kg bw per day, and the NOAEL for
this effect was 0.22 mg/kg bw per day. The NOAEL for the study, on the
basis of inhibition of erythrocyte acetylcholinesterase activity, was
0.01 mg/kg bw per day (Harling et al., 1989b).
Beagles aged 10 and 11 months were given diets containing
chlorpyrifos (purity, 97.2%) at concentrations that provided doses of
0, 0.01, 0.03, 0.1, 1, or 3 mg/kg bw per day for 1 year (one animal of
each sex per dose), 1 year with a 3-month recovery period (two animals
of each sex per dose), or 2 years (four animals of each sex per dose).
Clinical signs were recorded daily, and body weights were recorded
weekly for the first 6 months and every 2 weeks thereafter. Food
intake was recorded weekly during the first 3 months and monthly
thereafter. The clinical investigations in the groups receiving 0, 1,
or 3 mg/kg bw per day included haematology (packed cell volume,
haemoglobin, erythrocyte and leukocyte counts, and prothrombin time)
and urinary analyses (specific gravity, occult blood, ketones, solids,
pH, albumin, and sugar). The serum concentration of blood urea
nitrogen and the activities of alkaline phosphatase and alanine and
aspartate aminotransferases were measured in all dogs before treatment
and at various intervals thereafter. Bromsulphalein retention was
measured before treatment and at the 1-year sacrifice of animals at 0,
1, or 3 mg/kg bw per day. Cholinesterase activity was determined in
plasma and erythrocytes in most groups before treatment and at various
intervals during the study, and the activity in brain was measured at
necropsy. Fasted dogs were necropsied and the brain, heart, liver,
kidney, spleen, and testes were weighed. Portions of these organs and
a standard set of tissues were preserved from the controls and animals
at 1 (after 1 year only) and 3 mg/kg bw per day for histopathological
examination. The animals fed chlorpyrifos for 2 years were given
complete physical examinations before the end of the study, including
routine neurological and opthalmoscopic evaluations. The data were
evaluated only with Student's t test.
The body weights were not significantly affected by treatment,
and food consumption was comparable in groups of the same sex.
Consumption of the test compound was monitored regularly and reported
to approximate the nominal concentrations closely. The organ weights
were not affected by treatment, except for an increase in the mean
relative liver weight in males at 3 mg/kg bw per day (3.5 g/100 g vs
2.5 g/100 g in controls) in the 2-year study. Details of the recorded
clinical signs were not presented, and the authors simply stated that
"No clinical signs of toxicity were observed in any of the dogs in
either phase of the experiment." The results of clinical pathology
were presented as individual and summary data. There were no
unequivocal treatment-related differences between control and treated
groups in the limited haematological parameters measured nor in
urinary analyses. No effect of treatment was found on blood urea
nitrogen, alkaline phosphatase or alanine and aspartate
aminotransferase activities or Bromsulphalein retention. The results
of gross pathological examination were reported in the form of a
general summary. None of the findings indicated an effect of
treatment. The results of the limited histopathological examinations
(control and high dose only) were reported in a summary table with no
indication of the severity of lesions or abnormalities; individual
data were not presented. The available data did not indicate any
treatment-related effects.
Cholinesterase activity was presented for individual animals and
for groups (Table 3). Inhibition was evident within nine days (the
first assay time) of the start of the study, and the values recorded
at termination were similar to those recorded at interim assays.
Plasma cholinesterase activity was decreased in a dose-related manner
at both times, and erythrocyte cholinesterase activity was similarly
depressed, females being slightly more severely affected than males.
Brain cholinesterase activity was the least affected by treatment. The
cholinesterase activity of animals that were allowed to recover,
assayed after 2, 6, and 13 weeks on normal diet, showed a return to
control levels after 2 weeks for plasma activity and after 13 weeks
for erythrocyte activity.
Table 3. Percentage inhibition of cholinesterase activity in beagles treated with
chlorpyrifos in the diet in comparison with concurrent controls
Dose Inhibition of cholinesterase activity (%)
(mg/kg bw per day)
Plasma Erythrocytes Brain
Male Female Male Female
One year (males and females)
3 72 70 79 82 8
1 62 62 63 61 3
0.1 39 36 18 30 0
0.03 18 17 2 33 0
0.01 1 1 16 13 0
Two years (males)
3 77 67 83 70 20
1 69 50 68 66 7
0.1 52 38 27 41 8
0.03 23 22 0 6 7
0.01 2 0 14 6 1
Average values at terminal sacrifice
Inadequacies in data collection and recording were noted.
Although the long-term effects of dietary intake of chlorpyrifos could
not be evaluated in the absence of complete pathological and
histopathological examination, the data were adequate for analysis of
inhibition of cholinesterase. Plasma cholinesterase activity was
inhibited in animals of each sex at 0.03 mg/kg bw per day. Erythrocyte
cholinesterase activity was inhibited by > 20% at 0.1 mg/kg bw per
day at both the 1- and 2-year intervals, but the inhibition did not
consistently reach statistical significance when compared with the
values in concurrent controls or with pre-treatment values. This was
due in part to the small group sizes and the variation in this
parameter between groups, including the control group, the activity
differing by as much as twofold between individual animals. Under
these conditions, the erythrocyte cholinesterase activity would have
had to be inhibited by as much as 50% in comparison with controls
before statistical significance was achieved. The NOAEL was 0.1 mg/kg
bw per day on the basis of inhibition of erythrocyte cholinesterase
activity at higher doses. Inhibition of brain cholinesterase activity
was seen only at 3 mg/kg bw per day after 2 years (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971a).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not
consistently include basic observations. Individual body weights,
ophthalmological data, the results of pre-treatment and terminal
physical examinations, and an inventory of histopathological findings
were included. The conclusions of the original study were unchanged,
with the additional observation that no effect of treatment was
detected by ophthalmology (Kociba et al., 1985).
Rhesus monkeys
Groups of one or two rhesus monkeys of each sex were given
technical-grade chlorpyrifos in 1% aqueous gum tragacanth by stomach
tube at doses of 0, 0.08, 0.4, or 2 mg/kg bw per day for 6 months.
There were no treatment-related changes in body-weight gain, food
consumption, clinical findings, or haematological, clinical chemical,
or histopathological parameters. Plasma cholinesterase activity was
depressed at all doses at 16 weeks and at > 0.4 mg/kg bw per day at
24 weeks, and erythrocyte cholinesterase activity was depressed at 0.4
and 2 mg/kg bw per day (Table 4). The NOAEL for erythrocyte
cholinesterase inhibition was 0.08 mg/kg bw per day and that for brain
cholinesterase inhibition was 2 mg/kg bw per day. The usefulness of
this study was limited by the small number of animals tested (JMPR
1972; modified by reference to the original paper of Coulston et al.,
1971).
Table 4. Mean cholinesterase activity (and percent inhibition in
comparison with controls) in rhesus monkeys given chlorpyrifos
by stomach tube
Cholinesterase Time Dose (mg/kg bw per day)
activity (weeks)
0 0.08 0.4 2
Plasma 16 6.0 4.4 (26%) 1.6 (72%) 1.8 (70%)
24 4.4 3.8 (14%) 1.7 (61%) 2.0 (55%)
Erythrocytes 16 9.5 8.2 (13%) 6.5 (32%) 2.6 (72%)
24 8.6 7.6 (13%) 6.3 (27%) 5.4 (38%)
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity conducted in accordance with test
guidelines 83-2 of the US Environmental Protection Agency and OECD 451
and in compliance with GLP standards, technical-grade chlorpyrifos
(stated purity, 95.9%) was mixed in maize oil and incorporated into a
commercially available powdered animal diet to produce dietary
concentrations of 0 (control), 5, 50, and 250 ppm, calculated to be
equal to 0.7-1.1, 6.1-12, and 32-55 mg/kg bw per day in males and
0.7-1.2, 6.6-12, and 34-62 mg/kg bw per day in females. The test diets
were administered to groups of 64 CD-1 of each sex, 3 weeks old, for
at least 79 weeks, terminal sacrifice being undertaken during weeks
80-82. The animals continued to receive the treated diets until
scheduled necropsy. An additional 12 males per group were treated
identically to the mice in the main groups and were used to replace
animals for reasons unrelated to treatment (such as excessive
aggression). All of the unused spares were discarded after 10 weeks of
treatment. Each animal was weighed weekly for the first 13 weeks of
the study and every 2 weeks thereafter, while food consumption was
measured weekly for 13 weeks and monthly thereafter. The animals were
examined daily for clinical signs of intoxication, and a careful
examination, including palpation, was conducted weekly. Blood smears
were prepared from all mice after 12 and 18 months for differential
leukocyte counts, but only those from controls and animals at the high
dose were examined. Plasma, erythrocyte, and brain cholinesterase
activity was determined in five animals of each sex at each dose at 9
and 18 months. All animals were examined grossly, the examination
including all external surfaces, the cranial, thoracic, abdominal, and
pelvic cavities, and the carcass. A large number of organs and tissues
from all animals were fixed for histopathological examination; the
eyes, kidneys, livers, lungs, and thyroids/parathyroids from animals
at all doses were examined, as were other tissues from control and
animals at the high dose and those from animals that died during the
study.
There was no adverse effect on survival, and no treatment-related
increase in the incidence of neoplastic lesions. Treatment-related
clinical signs (ocular opacities, excess lachrymation, and cranial
hair loss) and reductions in body weights and food consumption were
observed at 250 ppm (approximately 32 mg/kg bw per day). The effects
on cholinestrase activity are summarized in Table 5. Plasma
cholinesterase activity was inhibited at all doses. Determinations of
erythrocyte cholinesterase activity revealed > 20% inhibition at 42
weeks and about 30% inhibition at week 78 in animals at 250 ppm but
considerable intra-group variation at other doses. In addition, the
erythrocytes were washed with saline before the cholinesterase
determinations at 78 weeks but not at 42 weeks. The NOAEL for
inhibition of erythrocyte cholinesterase activity was 5 ppm, with a
statistically significant inhibition of 29% in males at 78 weeks and
41% in females at 42 weeks in animals at 50 ppm. Significant
inhibition of brain cholinesterase activity ( p < 0.001 or
p < 0.01) was observed in males and females at 250 ppm at 42 and 78
weeks, the activity being reduced by 80-86% when compared with
controls. At 50 ppm, decreases in activity of 43-47% were seen in
males at 42 and 78 weeks and in females at 42 weeks, but this finding
reached statistical significance ( p < 0.05) in females only at 42
weeks and in males only at 78 weeks. At 5 ppm, brain cholinesterase
activity was reduced in males at 78 weeks (27% reduction), but this
Table 5. Percent inhibition of cholinesterase activity in groups of five mice
fed diets containing chlorpyrifos
Cholinesterase Interval Dose (ppm)
(weeks)
5 50 250
Male Female Male Female Male Female
Plasma 42 4*** 45*** 95*** 97*** 98*** 99***
78 49* 50*** 95*** 96*** 98*** 98***
Erythrocyte 42 1 15 11 41** 22 23
78 - +10a 29* 4 31* 29**
Brain 42 8 1 43 46* 80** 85***
78 27 +13a 47 9 86** 84***
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased activity in comparison with controls
finding was not statistically significant and was considered to be
incidental to treatment. The NOAEL for inhibition of brain
cholinesterase activity was 5 ppm.
Non-neoplastic effects were observed at the high dose, in the
livers (slight subchronic pericholangitis, histiocytic proliferation,
and centrilobular hepatocytic fatty vacuolation) of males and in the
eyes of males and females. No treatment-related lesions were seen at
lower doses. In males at the high dose, an increased incidence of lung
nodules was seen macroscopically, with rates of 5/31 in controls, 4/26
at 5 ppm, 2/37 at 50 ppm, and 14/49 at 250 ppm. The nodules were
diagnosed histologically as bronchio-alveolar adenomas or carcinomas,
but the incidences were stated to be within the range in historical
controls. Nevertheless, two sets of data were reported for the
incidence of neoplasms in historical controls: values obtained from
24-month studies and a published report on spontaneous neoplasms in
CD-1 mice in a number of testing laboratories. Those used in the
report were less than ideal. The use of background incidence from
24-month studies would be expected to increase the range of
spontaneous tumours over that in the 78-week protocol used in this
study. In addition, the tumour incidence was reported in a published
paper (Maita et al., 1988) as a percentage of the animals examined,
while the historical data from the 24-month study and the tumour
incidence in this study were given as percentages of the number of
organs examined. Histopathological examination did not reveal any
statistically significant increase in the incidence of neoplasms in
treated animals when compared with controls. The NOAEL was 5 ppm
(0.7 mg/kg bw per day) on the basis of inhibition of erythrocyte and
brain acetylcholinesterase activity (Gur et al., 1991).
Groups of 56 CD-1 mice of each sex were maintained on diets
containing 0, 0.5, 5, or 15 ppm of chlorpyrifos (purity, 99.6%) for
105 weeks. They were observed daily for clinical signs, body weights
were recorded monthly, and daily food consumption was calculated from
a 3- or 4-day measurement made once a month. Gross pathological
examination was performed on all animals that died, when the weights
of the brain, testes, heart, kidneys, and liver were recorded and a
range of other tissues were collected. Sections were prepared of all
the these tissues for histopathology. Haematology was limited to
examination of smears of blood collected from the tail vein at
terminal sacrifice.
The body weights and absolute and relative organ weights were not
significantly affected by treatment over the course of the study, and
the mean body weight of all treated female mice was greater (< 13%)
than that of controls from day 84 onwards, although food consumption
was comparable in all groups. The mortality rate was also generally
unaffected by treatment, and treated males had slightly higher
survival rates than controls: 39% in controls, 46% at 0.5 ppm, 50% at
5 ppm, and 48% at 15 ppm; the survival rates of females were lower
than those of controls only at the highest dose: 46% in controls, 59%
at 0.5 ppm, 46% at 5 ppm, and 38% at 15 ppm. There were no clinical
signs or pathological findings that indicated an effect related to
treatment. A significant increase was observed in the incidence of
spindle-cell hyperplasia of the adrenal gland in animals of each sex
given 0.5 ppm chlorpyrifos and in males given 5 ppm. This finding was
not considered to be related to treatment as there was a high
background incidence in ageing mice and no dose-response relationship.
Vacuolation was found in sciatic nerve preparations from 2/56 male and
3/54 female controls, 5/54 males and 8/55 females at 0.5 ppm, 2/56
males and 2/52 females at 5 ppm, and 6/52 males and 8/53 females at 15
ppm. The incidences of alveologenic adenomas and hyperplastic nodules
in the liver were significantly increased only in males at 5 ppm.
These findings were considered to be incidental.
This study was barely adequate for assessing the carcinogenicity
and long-term toxicity of chlorpyrifos, as it had serious shortcomings
in design, method, and data collection. No clinical pathology was
performed, and in particular cholinesterase activity was not measured;
no clinical signs were reported; individual data were not presented
for several parameters, including histopathology; and intestinal
parasites (nematodes) were present in all groups (JMPR, 1982; modified
by reference to the original report of Warner et al., 1980).
Rats
In a 2-year study, groups of 25 male and 25 female Sherman rats
received chlorpyrifos (purity, 97.2%) in the diet at doses of 0, 0.01,
0.03, 0.1, 1, or 3 mg/kg bw per day (dietary concentrations not
provided). Supplementary animals were included at each dose to allow
interim sacrifices. The design of the study is shown in Table 6.
Clinical signs were recorded 'frequently', body weights were recorded
twice weekly for the first 4 weeks, weekly for 2-6 months, and every 2
weeks for the remainder of the study. The food intake of the main
group was recorded continuously during the first 3 months and once a
month thereafter. The clinical investigations included haematology
(packed cell volume, haemoglobin, and erythrocyte and leukocyte
counts) and urinary analysis (solids, pH, albumin, sugar, occult
blood, ketones) for five rats of each sex at 0, 1, and 3 mg/kg bw per
day; the serum concentration of urea nitrogen and the activities of
alkaline phosphatase and alanine aminotransferase were measured in all
rats at sacrifice at 12 and 18 months, in all male survivors and in
four to five females at 24 months. Cholinesterase activity was
determined in plasma, erythrocytes, and brain samples, as shown in
Table 6. Rats in groups A, H, I, and K were necropsied in a standard
manner, and the weights of the brain, heart, liver, kidney, spleen,
and testes were recorded. Portions of these organs and a standard set
of tissues were preserved for histopathology, but only the slides for
the controls, animals at the high dose, and animals at 1 mg/kg bw per
day at the 12-month necropsy were examined. Some tissues from animals
that died during the study and from those animals killed when moribund
were also examined histologically. Statistical evaluation of the data
was limited to Student's t test.
Body weights (data presented only as graphs) were generally not
significantly affected by treatment. No details of the recorded
clinical signs were presented; rather, the authors stated that "No
changes in appearance or demeanour or signs of toxicity were observed
grossly in any of the rats. No evidence of a cholinergic response was
noted at any time." The results of clinical pathology were presented
as individual and summary data, and no unequivocal treatment-related
differences between control and treated groups were found for the
limited haematological parameters measured or for the results of
urinary analyses. Treatment had no effect on serum concentrations of
urea nitrogen or on alkaline phosphatase and alanine aminotransferase
activity.
Plasma cholinesterase activity was significantly decreased at 3
mg/kg bw per day at all assay times, the inhibition ranging from 20 to
40% in males and 55 to 74% in females; the activity was less severely
inhibited at 1 mg/kg bw per day, but from 6 months onwards the
inhibition was 18-38% in males and 50-69% in females when compared
with controls, and these differences were statistically significant at
most assay times. Erythrocyte cholinesterase activity was severely
inhibited in animals of each sex at the higher doses at all assay
times, with rates of 60-100% at 3 mg/kg bw per day, 13-90% at 1 mg/kg
bw per day, and little effect at the other doses. Animals that had
Table 6. Design of the long-term study of toxicity and carcinogenicity in groups of
56 mice fed diets containing chlorpyrifos
Group No. of rats of Sacrifice interval End-point examined
each sex per dose
A 25 2 years Necropsy, blood and brain
cholinesterase
B 5 1 week Blood cholinesterase
C 5 1 month Blood cholinesterase
D 5 3 months Blood cholinesterase
E 5 6 months Blood and brain cholinesterase
F 5 9 months Blood cholinesterase
G 6 12 months Blood and brain cholinesterase
H 5 12 months Necropsy
I 7 12 months, Necropsy, blood and brain
7-8-week recovery cholinesterase
J 7 18 months Blood and brain cholinesterase
K 7 18 months Necropsy
been allowed to recover showed full restoration of cholinesterase
activity. Brain cholinesterase activity was significantly inhibited by
treatment at 3 mg/kg bw per day (30-53% inhibition) at all assay
times, to a lesser degree at 1 mg/kg bw per day (3-16%), and was
comparable to that of controls at other doses. Animals that were
allowed to recover showed restoration of brain cholinesterase
activity.
This study was considered to be inadequate for assessing the
carcinogenicity and long-term effects of chlorpyrifos, other than
clinical chemical findings including cholinesterase inhibition, as
there were serious shortcomings in data collection, and the reports of
gross and histopathology were inadequate and no clinical findings were
reported. The NOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day on the basis of
significant inhibition at 1 mg/kg bw per day. The NOAEL for inhibition
of brain acetylcholinesterase activity was 1 mg/kg bw per day on the
basis of significant inhibition at 3 mg/kg bw per day (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971b).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not include
basic observations such as abnormal behaviour or gait. Individual body
weights and gross and histopathological findings were included in the
supplement, but the histopathology was limited and variable, only a
small number of tissues from each rat being examined consistently. The
results were not presented as summary tables of incidence and
severity. The conclusions of the original study remain unchanged by
this supplement (McCollister et al., 1985).
In a 2-year study conducted according to accepted test guidelines
(OECD 451, USEPA 83-5) and GLP, groups of 60 Fischer 344 rats of each
sex were fed diets containing technical-grade chlorpyrifos (purity,
96.1%) at concentrations of 0, 0 (vehicle control), 0.2, 5, or
100 ppm. The vehicle used was 0.04% w/w maize oil. The diets were
prepared weekly and assayed regularly for stability and accuracy of
dosing. Body weights were generally recorded weekly; food consumption
was recorded weekly for 13 weeks and monthly thereafter. Clinical
signs were recorded daily and palpation performed weekly.
Cholinesterase activity was measured before treatment and at weeks 14,
32, 45, 50 (interim sacrifice of five animals of each sex per group
for measurement of brain acetylcholinesterase activity), 78, and 104
weeks (terminal sacrifice with measurement of brain
acetylcholinesterase activity in 10 animals of each sex per group),
and generally on 10 rats of each sex per dose. Haematological
parameters were measured in blood smears from 10 rats of each sex in
the vehicle control group and at 100 ppm at months 12 and 18. Gross
pathological findings were recorded for all animals at necropsy,
including those that died during the study or were killed at early
sacrifices. Necropsy included removal of the eyes with the optic nerve
and adnexa. The weights of the brain, testes, thyroids, adrenals,
kidneys, and liver were recorded, and a range of tissues was
collected. All gross lesions were sectioned, and sections from all
control animal and those at 100 ppm were examined. Appropriate
statistical tests were used to analyse the data.
The mean consumption of chlorpyrifos was estimated to be 0.012,
0.3, and 6 mg/kg bw per day for animals of each sex. Mortality rates
and the incidences of clinical signs or palpable masses were not
affected by treatment. A 5% reduction in the mean body weights of
males at 100 ppm was significant in comparison with controls at most
times during weeks 3-94, and the weights of females at this dose were
significantly lower (about 4%) than those of controls at most times
during weeks 2-64. A variety of non-neoplastic and neoplastic lesions
were recorded, and the incidence of these lesions occasionally showed
a positive trend with dose; however, the incidence was generally
within the range of historical controls in the laboratory or within
the range of published values from the National Toxicology Program in
the USA. The incidences of neoplastic lesions did not show unequivocal
or statistically significant dose-response relationships. The only
observation that was considered to be related to treatment was an
increased incidence of cataracts and diffuse retinal atrophy in
females (Table 7).
Table 7. Incidences of ocular abnormalities found microscopically in rats given
diets containing chlorpyrifos
Abnormality Sex Dose (ppm)
0 0 (vehicle) 0.2 5 100
Diffuse retinal atrophy Male 4/60 0/60 3/23 1/28 3/60
Female 5/60 15/59* 9/60 5/58 24/60*
Cataract Male 31/60 31/60 7/23 6/28 30/60
Female 38/60 38/59 33/60 32/58 51/60*
* Significantly different from controls at p < 0.01 or p < 0.001
Significant (> 20%) inhibition of plasma cholinesterase activity
relative to the control values was observed at doses > 0.3 mg/kg bw
per day (Table 8). While erythrocyte cholinesterase activity was
inhibited in animals at 100 ppm, the variation in the data and the
lack of statistical significance and/or dose-response relationships
mitigated against an unequivocal finding. The NOAEL for inhibition of
brain cholinesterase activity was 0.3 mg/kg bw per day (Crown et al.,
1988).
In a study conducted in compliance with GLP standards, groups of
60 Fischer 344 rats of each sex received diets containing chlorpyrifos
(purity, 98.5%) at concentrations that provided doses of 0 (control),
0.05, 0.1, 1, or 10 mg/kg bw per day for 24 months. Ten rats of each
sex at each dose were randomly designated at the start of the study
for interim sacrifice at 12 months. Blood was collected for
haematology, clinical chemistry, and measurement of plasma and
erythrocyte cholinesterase activity at 6, 12, and 18 months as well as
at the 24-month terminal sacrifice. The groups were observed daily for
deaths and signs of toxicity. All rats were examined clinically at
least once a week from after the sixth month. All animals were
palpated for externally detectable masses before treatment, before the
12-month kill, and monthly thereafter. Body weights and feed
consumption were determined weekly for the first 3 months and monthly
thereafter. All rats were weighed, but feed consumption was determined
for only 20 rats of each sex per group.
All clinical laboratory procedures scheduled for 6 and 12 months
were performed on rats designated for the 12-month interim kill,
whereas tests scheduled for 18 and 24 months were performed on rats
designated for terminal sacrifice. Blood samples were obtained for
haematology and clinical chemistry by orbital sinus puncture under
Table 8. Group mean percent inhibition of cholinesterase activity in comparison
with vehicle controls in rats given diets containing chlorpyrifos
Assay time Dose Cholinesterase inhibition (%)
(weeks) (ppm)
Plasma Erythrocytes Brain
Male Female Male Female Male Female
50 0 0 7 0* 31 0 35
0.2 1 4 0 42 0 14
5 15 51 0* 39 9 10
100 93 98 13 45 57* 80*
78 0 0 3 27 0
0.2 4 3 11 0
5 28* 47* 0 0
100 93* 97* 10 0
104 0 8 9 0 0 18 4
0.2 13 0 0 0 0 0
5 36 37* 17 11 0 0
100 95* 96* 34 18 58* 61*
Values the same as or higher than those of the vehicle control are recorded as
0 inhibition.
* Significantly different from control at p < 0.01 or p < 0.001
light anaesthesia. The haematological determinations consisted of
packed cell volume, haemoglobin concentration, erythrocyte count,
total and differential leukocyte counts, and platelet count. The
clinical biochemical determinations included blood urea nitrogen,
alkaline phosphatase and alanine and aspartate aminotransferase
activities, glucose, total protein, albumin, globulin (calculated),
creatine phosphokinase, total bilirubin, cholesterol, calcium,
phosphorus, sodium, potassium, and chloride. Urine samples were
obtained 1-2 weeks before the scheduled sacrifices at 12 and 24 months
and at 6 and 18 months from 10 rats of each sex per group. The urinary
parameters measured included specific gravity and semi-quantitative
estimates of bilirubin, glucose, ketones, occult blood, pH, protein,
and urobilinogen. Microscopy of a pooled sample was also conducted.
Plasma and erythrocyte cholinesterase activities were assayed in 10
rats of each sex per group at 6, 12, 18, and 24 months, and
acetylcholinesterase activity was measured in half-brain samples
obtained at the 12-month and 24-month scheduled necropsies. All
animals killed at the interim and terminal sacrifices or which died or
were killed when moribund were necropsied and subjected to a complete
gross examination. The brain, liver, kidneys, testes, ovaries, and
adrenal glands were weighed, and many organs and tissues were removed
and preserved in neutral, phosphate-buffered 10% formalin for
subsequent histopathological evaluation. Histological sections of the
formalin-fixed tissues from all controls and those at the highest dose
were prepared, stained with haematoxylin and eosin and examined
microscopically. Histopathological examination of tissues from animals
at the three lower doses was limited to the liver, kidneys, adrenals,
and tissues with gross lesions at both sacrifices. At terminal
sacrifice, the lungs, spleen, testes, pituitary, and
thyroid/parathyroid were also examined microscopically. Appropriate
statistical tests were applied to the data.
Males at the high dose showed a consistent decrease in
body-weight gain relative to controls in the absence of reduced food
consumption, depression of plasma (56-87%), erythrocyte (20-40%) and
brain (56-58%) cholinesterase activities, and an increase in the
weight of their adrenal glands which was characterized microscopically
by exacerbated fatty vacuolation of the zona fasciculata. Similar
effects were observed in females at this dose, but were generally less
pronounced than in males: for example, a transient decrease in
body-weight gain relative to controls with no reduction in food
consumption, depression of plasma (82-95%), erythrocyte (generally
> 20%), and brain (57-61%) cholinesterase activities, and an
increase in adrenal weight at the terminal sacrifice with no
associated histopathological lesions. At 1 mg/kg bw per day, the only
effects attributable to treatment were inhibition of plasma
cholinesterase activity (39-71% in males and 60-86% in females) and
erythrocyte cholinesterase activity (20-40% in males and < 22% in
females); brain cholinesterase activity was not affected. These
results are summarized in Table 9. No treatment-related effects were
observed at the two lower doses. There was no increase in the
incidence of any type of tumour. The NOAEL for inhibition of
erythrocyte cholinesterase activity was 0.1 mg/kg bw per day, on the
basis of toxicologically (> 20%) or statistically significant
inhibition at 1 mg/kg bw per day, and the NOAEL for inhibition of
brain cholinesterase activity was 1 mg/kg bw per day on the basis of
toxicologically and statistically significant inhibition at 10 mg/kg
bw per day (Young & Grandjean, 1988).
Chickens
Hens were fed diets containing chlorpyrifos at concentrations of
0, 25, 50, or 200 ppm (approximately 0, 2.5, 5, and 20 mg/kg bw per
day) for 52 weeks. Mortality was unaffected by treatment, with rates
of 13% in controls, 3% at 25 ppm, 7% at 50 ppm, and 10% at 200 ppm.
Plasma cholinesterase activity was assayed in three hens at each dose
1, 3, 7, 14, 22, and 29 days after the start of treatment, monthly
thereafter, and 1, 2, and 3 weeks after the end of treatment. The
onset of inhibition of cholinesterase activity was rapid and
dose-related, with 22% inhibition in week 1 at 25 ppm, 45% inhibition
at 50 ppm, and 76% inhibition at 200 ppm. It persisted at similar
levels throughout the study but returned to control levels during the
Table 9. Group mean cholinesterase activities in rats given diets containing chlorpyrifos,
expressed as percent of the respective control values for each sampling period
Cholinesterase Sex Sampling period Dose (mg/kg bw per day)
(months)
0 0.05 0.1 1 10
Plasma Male 6 100 96.5 95.1 60.9* 44.2*
12 100 94.5 97.8 28.6* 13.3*
18 100 93.4 80.1 36.6* 22.5*
24 100 92.4 85.5 40.0* 19.7*
Female 6 100 97.9 91.0 34.6* 16.9*
12 100 104 87.0* 13.8* 4.8*
18 100 99.1 85.8* 30.4* 12.4*
24 100 103 94.4 39.7* 17.9*
Erythrocytes Male 6 100 107 88.8 76.1* 75.6*
12 100 107 93.2 67.4 63.1
18 100 109 95.3 66.2* 71.0*
24 100 105 108 86.5 73.5*
Female 6 100 93.3 106 104 87.5
12 100 88.2 109 82.2 59.5*
18 100 114 99.7 78.0 81.9
24 100 107 87.2 83.6 79.9
Brain Male 12 100 93.8* 93.1* 91.0* 42.1*
24 100 102 100 103 44.3*
Female 12 100 97.9 102 95.3* 38.9*
24 100 101 100 96.2 42.9*
* Statistically significantly different from control by Dunnett's or Wilcoxon's test,
alpha = 0.05, one-sided
recovery period. Overall feed consumption, body weight, egg
production, feed efficiency, egg weight, and shell thickness were not
affected by treatment. There was no NOEL for inhibition of plasma
cholinesterase activity in this study, as significant inhibition was
seen at the lowest dose tested (Sherman & Herrick, 1973).
(d) Genotoxicity
Chlorpyrifos was not genotoxic in vitro or in vivo in a range
of studies (Table 10).
Table 10. Results of studies for the genotoxicity of chlorpyrifos
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
In vitro
Gene mutation S. cerevisiae D3 NR NR Negative Poole et al. (1977;
rec locus abstract only)
Gene mutation B. subtilus H17, 20 (100, 200, 500, 1000 95.83 Negative Shirasu et al. (1980)
M45 2000 µg/plate in DMSO
Reverse mutation S. typhimurium TA98, 10, 50, 100, 500, 1000, 95.83 Negativea Shirasu et al. (1980)
TA100, TA1535, 5000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 1, 3.162, 10, 31.62, 95.7 Negativea,b QA Bruce & Zempel (1986)
TA100, TA1535, 100 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 0.01, 0.1, 1, 5, 10, 95 Negativea,c Jai Research Foundation
TA100, TA1535, 100 µg/plate in DMSO (1996a)
TA1537
Reverse mutation S. typhimurium TA98, 30, 100, 300, 3000, 96.8 Negativea GLP Loveday et al. (1987)
TA100, TA1535, 10 000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative Poole et al. (1977;
TA100, TA1535, abstract only)
TA1537
Reverse mutation E. coli WP2 NR NR Negative Poole et al. (1977;
abstract only)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Relative toxicity E. coli and NR NR Negative Poole et al. (1977;
B. subtilis abstract only)
Reverse mutation S. typhimurium TA98, 5, 500, 5000 µg/plate 96.2 Negativea,d QA Fredrick Institute
TA100, TA1535, in DMSO (1996c)
TA1537, TA1538
Forward mutation Chinese hamster ovary 10, 20, 25, 30, 40, 95.7 Negativea Mendrala (1985)
cells, hprt locus 50 µmol/L
Forward mutation Chinese hamster ovary 5, 10, 25, 50, 75 µg/ml, 96.8 Negativea,e GLP Tu (1987)
cells, hprt locus 16 h, - S9
5, 10, 20, 30, 40, 50 µg/ml,
16 h, - S9
30, 50, 100, 300, 1000 µg/ml,
+S9, in DMSO
Sister chromatid Human lymphocytes 0.02, 0.2, 2 or 20 µg/ml NR Negativea,f Sobti et al. (1982)
exchange (Laz-007) in ethanol
Chromosomal Chinese hamster ovary 0.975, 1.47, 2.93, 4.89, 96.8 Negativea,g GLP Loveday (1987)
aberration cells 9.75, 14.7, 29.3, 48.9, 97.5,
147 µg/ml, 19 h, - S9
1.56, 3.12, 5.2, 10.4, 15.6,
31.2, 52, 104, 156 µg/ml,
10 h, - S9
9.75, 14.7, 29.3, 48.9, 97.5,
147, 293 µg/ml, 19 h, +S9
1, 1.5, 3, 5, 10, 15, 30, 50,
100 µg/ml, 10 h, + S9
2.95, 4.95, 9.85, 14.8, 29.6,
49.4, 98.5, 296 µg/ml, 10 h,
+S9
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Rat hepatocytes 16.7, 50, 167, 500, 1667, 98.6 Negativea,h GLP Linscombe et al. (1992)
aberration 5000 µg/ml harvested after
24 h and 5, 16.7, 50, 167
µg/ml harvested after
24 and 48 h, in DMSO
Sister chromatid Chinese hamster ovary 1, 10, 100 µg/ml in acetone NR Negative Muscarella et al. (1984)
exchange cells
Unscheduled DNA Rat hepatocytes 1, 3.16, 10, 31.6, 95.7 Negative Mendrala & Dryzaga
synthesis 100 µmol/L in DMSO (1986)
Reverse mutation S. typhimurium TA98, 0, 1, 3.16, 10, 31.6, 95.7- Negativea Gollapudi et al. (1995)
TA100, TA1535, 100 µg/plate in DMSO 98.6
TA1537, TA1538
Forward mutation Chinese hamster ovary 0, 3.5, 7, 8.8, 10.5, 14, 95.7-98.6 Negativea,i Gollapudi et al. (1995)
cells, hprt locus 17.5 µg/ml in DMSO
Chromosomal Rat lymphocytes 16.7-5000 µg/ml in 95.7-95.6 Negativea,j Gollapudi et al. (1995)
aberration DMSO
Unscheduled Rat hepatocytes 1, 3.16, 10, 31.6, 95.7-95.6 Negative Gollapudi et al. (1995)
DNA synthesis 100 µmol/L in DMSO
In vivo
Micronucleus Mouse (CD-ICR BR) 0, 7, 22, 70 mg/kg bw 95.7 Negative QA Gollapudi et al. (1985)
formation marrow cells orally in corn oil
Micronucleus Mouse (Swiss albino) 0, 15, 30, 60 mg/kg bw 96.2 Negative QA Fredrick Institute
formation marrow cells orally in vegetable oil (1996d)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Mouse (Swiss albino) 0.6, 3, 15 mg/kg bw per 96.2 Negative QA Fredrick Institute
aberration marrow cells day orally in vegetable oil (1996e)
Chromosomal Chick embryo (Cornell 1.11, 11.1, 111, 1110, NR Negativek Muscarella et al. (1984)
aberration K strain eggs) 2220 µg/embryo
Chromosomal Bovine blastocysts NR NR Negative Muscarella et al. (1984)
aberration
Micronucleus Mouse (CD-1 (ICR) BR) 90 mg/kg bw orally in 97.9 Negative GLP McClintock & Gollapudi
formation marrow cells corn oil (1989a)
QA, quality assurance; GLP, good laboratory practice; NR, not reported; S9, exogenous metabolic activation system from 9000 × g
fraction of rat liver; DMSO, dimethyl sulfoxide
Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Cytotoxicity observed at 100 µg/plate in TA100, 1535, 1537, 1538 with precipitation of test material; in TA98, precipitation in the
absence of toxicity
c Cytotoxicity at > 10 µg/plate; dose-related increase in revertant frequency (> twofold than controls) in all strains ± S9, but
increases lower than in positive controls by 2-50-fold and not statistically significant
d Increase in revertants at all dose, - S9 in TA1537 only; no dose-response relationship
e Cytotoxicity at 50 µg/ml in one assay - S9
f At 2 and 20 µg/ml, frequency was statistically significantly different from controls but not double the control frequency
g - S9: cytotoxicity at highest doses; increase in gaps only at 52 µg/ml and 10-h exposure; no increases in other aberrations
+S9: cytotoxicity at 15 µg/ml and 10-h incubation; in one 10-h assay, a significant increase in number of cells with aberrations
(including gaps) at 3 and 10 µg/ml; incidence of aberrations (excluding gaps) not statistically significantly increased and not
dose-dependent In repeat 10-h assay, no increase in incidence of aberrations.
h Cytotoxicity at > 500 µg/ml -S9 and > 167 µg/ml +S9 in first assay; no mitotic index measurable at 50 µg/ml - S9 or
167 µg/ml ±S9
i Test material precipitated at 10.5, 14, and 17.5 µg/ml ± S9
j Test material cytotoxic at > 50 µg/ml ± S9
k Increased mortality at 1110 and 2220 µg/embryo
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a three-generation study, Sprague-Dawley rats (two litters per
generation) were given diets containing chlorpyrifos (purity not
specified) at concentrations that provided doses of 0, 0.03, 0.1, or
0.3 mg/kg bw per day for the first generation and 0, 0.1, 0.3, or
1 mg/kg bw per day for the second and third generations. The sizes of
the groups were consistent in all generations, the controls comprising
20 males and 40 females and the treated groups comprising 10 males and
20 females. Treatment was started at 58 days of age, and F0 parents
were mated at 118 days of age at a 1:2 male:female ratio. Evidence of
conception (vaginal plug or sperm) was considered to be day 0 of
gestation. Pairing for the second litter in each generation was
started approximately 10 days after the first litter was weaned at 21
days, and the treated diets were fed to the litters from weaning. When
the progeny of the F1b or F2b numbered more than 10 per litter,
each litter was reduced to 10 after 5 days of age. The F3b fetuses
were examined for teratogenic changes. F2b parent animals were
continued on the test diet throughout the breeding period, except that
females were placed on normal diet during organogenesis and the test
substance was administered by gavage in acetone or corn oil. Dams were
killed on day 20 of gestation and the fetuses removed surgically and
examined for external abnormalities. Onethird were fixed for
examination of soft tissues, and two-thirds were stained with alizarin
red for skeletal examination; however, only the controls and rats at 1
mg/kg bw per day were examined. Clinical observations were stated to
have been performed 'frequently'. Body weights and food consumption
were recorded weekly until breeding commenced. During gestation and
lactation, the diets were adjusted on the basis of an average feed
consumption of 25 and 40 g/day; rats were weighed before breeding to
allow dietary adjustment. In the investigation of teratogenic effects,
the body weights of the dams were recorded on days 0, 6, 15, and 20 of
gestation, while food consumption was measured during the gestational
intervals 0-6, 6-16, and 16-20 days. Various indices of reproductive
performance were calculated: fertility index as pregnancies per
mating; gestation index as live litters born per number of
pregnancies; viability as rats alive at day 5 per rats born alive; and
lactation index as rats alive at day 21 per rats alive at day 5. All
animals that died and five pups of each sex per dose from the F1a,
F2a, and F3a litters were examined grossly. An extensive set of
tissues was prepared and fixed from these pups, but in the absence of
gross abnormalities only tissues from the F3a pups were examined
histopathologically. Clinical chemistry was limited to the F2
animals, and consisted of measurements of plasma and erythrocyte
cholinesterase activity in 11 control and five or six treated dams at
the time of removal of their litters and in five males per group.
Statistical analysis was limited.
No clinical signs of toxicity were seen in the parents or
offspring. The parental body weights were not significantly affected
by treatment, and food consumption was variable but unaffected. The
fertility, gestation, and lactation indices were comparable between
groups and generations. The viability index was decreased at 1 mg/kg
bw per day, but this effect was considered not to be clearly related
to treatment in view of the isolated nature of the finding and the
study design. The mean body-weight gain of the dams that produced the
F3b pups showed a dose-related increase, with gains of 114 g in
controls, 120 g at 0.1 mg/kg bw per day, 123 g at 0.3 mg/kg bw per
day, and 126 g at 1 mg/kg bw per day. This generation showed no
treatment-related effects on fertility (per cent pregnant), the mean
number of corpora lutea or implantations, viable litter size, or pup
weight after surgical removal. The skeletons of the pups showed common
minor variants such as incomplete ossification of sternebrae and the
occurrence of extra ribs. Visceral examination of the pups showed that
minor variants of the urogenital system (hydronephrosis or left,
right, or bilateral hydroureter) were more common in the treated
group. A single fetus with multiple abnormalities was found at 1 mg/kg
bw per day, but this isolated finding was considered not to be related
to treatment. The group average activity of plasma and erythrocyte
cholinesterase was decreased by > 20% at 1 mg/kg bw per day in males
and females of the F2 generation. The NOAEL was 0.1 mg/kg bw per day
on the basis of decreased plasma and erythrocyte cholinesterase
activity at 1 mg/kg bw per day and decreased erythrocyte
cholinesterase activity at 0.3 mg/kg bw per day (JMPR, 1972; modified
by reference to the original report of Thompson et al., 1971).
To supplement the finding of an equivocal decrease in neonatal
survival at 1 mg/kg bw per day, groups of 30 Sprague-Dawley rats of
each sex were fed diets containing chlorpyrifos (purity, 96.6-99%) at
concentrations providing doses of 0, 0.5, 0.8, or 1.2 mg/kg bw per day
for 135 days and then bred to produce F1 litters. On day 21 of
lactation, 30 pups of each sex were selected randomly, dosed in the
same manner as the F0 animals for 120 days, and then bred to produce
the F2 litters. The F2 pups were weaned on day 21 of lactation.
The F0 and F1 matings were in a 1:1 ratio, with pairing for 5
days, a 7-day rest, and a pairing with a different male for a further
5 days. The litters were culled on day 4 post partum, and the litter
weights and pup survival were recorded on days 1, 4, 7, 14, and 21;
individual pups were weighed on day 21. The parental animals were
examined daily for signs of toxicity, and body weight and food intake
were recorded weekly for animals of each sex until pairing;
thereafter, males were weighed after pairing until sacrifice, and
females were weighed on days 1, 4, 7, 14, and 21 of lactation. The
date of parturition, litter size at birth, and the numbers of live and
dead pups at birth were recorded. The dietary concentrations were
adjusted weekly during the pre-mating period according to the food
consumption and body weights of the rats; the concentrations were then
maintained at the last adjusted concentration from commencement of
cohabitation until sacrifice. Appropriate statistical tests were
applied.
The parental animals showed no significant clinical signs in
either generation. Body weight and food intake were unaffected by
treatment. The mean fertility index (number of females delivering a
litter expressed as the percentage of the total number of females
placed with a male) was reduced at 0.5 and 0.8 mg/kg bw per day but
not at the highest dose, and a similar inverted dose-response
relationship was seen for length of gestation; data on individual
animals were not presented. The litter sizes (10-11) were comparable
at all doses, as were the survival indices (> 91% in all groups on
days 1, 4, 7, 14, and 21) during lactation. The pup weights
(presumably litter weight per number of pups) were also comparable. In
F1 adults, the body weight of males was reduced at 1.2 mg/kg bw per
day on days 160-182 after cohabitation, and those of females showed
slight, sporadic increases when compared with controls. Food intake
was sporadically decreased in males but not in females. No effects
were seen on female body weight during lactation. The fertility index
in treated groups exceeded the control values. The litter sizes were
comparable in all groups, as were the pup weights and pup survival
during lactation. The adequacy of this study for regulatory purposes
was limited by the doses selected. As no adverse effects were found at
the highest dose, it was unclear whether the study clearly
demonstrated the reproductive toxicity of the test material in rats.
The lack of data for individual animals and the inverted dose-response
relationships for some parameters rendered the findings equivocal. In
the absence of clear adverse effects on reproductive parameters at any
dose, the NOAEL was 1.2 mg/kg bw per day (Dietz et al., 1983).
In a study conducted in compliance with GLP standards and the
requirements of the US Environmental Protection Agency 83-4,
technical-grade chlorpyrifos (stated purity, 95.8%) was given in the
diet to groups of male and female CrL: COBS CD (SD) BR rats at
concentrations of 0, 2, 10, or 50 ppm over two generations. These
concentrations corresponded to achieved intakes of 0.1-0.2, 0.5-0.9,
and 2.5-4.5 mg/kg bw per day, respectively, in F0 males and 0.1-0.2,
0.6-0.9, and 2.9-4.6 mg/kg bw per day, respectively, in F0 females.
The corresponding intakes in the F1 generation were 0.1-0.3,
0.7-1.6, and 3.3-8.1 mg/kg bw per day in males and 0.2-0.3, 0.8-1.6,
and 4-8.1 mg/kg bw per day in females. The animals of the F0
generation (28 of each sex per dose) were approximately 7 weeks of age
at the beginning of treatment and were maintained on their respective
diets for at least 56 days before mating. They were mated at a 1:1
ratio for 20 days, and then the dams were allowed to rear their young
to day 21 post partum. Before weaning, all F1 offspring in all
litters were examined for developmental indices (surface righting
reflex, startle reflex, air righting reflex, and pupil reflex). At day
21 post partum, 24 pups from each group were selected for mating;
excess pups were sacrificed on day 22 post partum and examined
macroscopically, and specified organs were weighed and tissues
preserved for histopathological examination from one animal of each
sex per litter. Shortly after the F1 pups were weaned, the F0
adults were killed and examined macroscopically, and a wide range of
organs and tissues were preserved, the ovaries, pituitary, prostate,
testes with epididymides, and uterus with cervix and vagina being
examined histopathologically.The animals in the F1 generation that
were selected for mating were reared on their respective diets for at
least 56 days (i.e. until they were approximately 12 weeks of age) and
were then mated 1:1 for 20 days; the dams were allowed to rear their
young until day 21 post partum. Pathological examination was
conducted as for the F0 generation. All animals were handled
regularly and examined for signs associated with treatment. All
animals that died or were killed were examined. The weight of each
animal was determined at the start of each generation and subsequently
at weekly intervals. All pups were weighed at birth and at 4, 8, 12,
and 21 days post partum. Cholinesterase activity was not determined
in the study. The doses were selected on the basis of those that
decreased cholinesterase activity in a preliminary study in which
chlorpyrifos was given in the diet for a single generation at doses up
to 250 ppm. In this preliminary study, plasma cholinesterase activity
was inhibited when compared with that in controls by 86% in adult
females and by about 60% in weanling males and females at 50 ppm. At
50 ppm, erythrocyte cholinesterase activity was also inhibited in
adult females (70% inhibition) and weanlings (about 50% inhibition),
and brain cholinesterase was inhibited by 38% in adult females
Food consumption was similar in control and treated groups and no
adverse, treatment-related effects on group mean body weights were
observed at any dose in either generation. Mating performance and the
duration of gestation were similar in control and treated groups.
Slight inter-group variation was observed in the incidence of total
pre-birth loss in both the F0 and F1 generations, but these
findings were considered not to be related to treatment in the absence
of a consistent relationship between dose and effect. The F0
generation showed statistically significant increases in litter size
and litter weight and decreased mean pup weight at a number of doses
at most sample intervals (Table 11), but these values were generally
only slightly different from those in controls and showed no
consistent relationship with dose. The decrease in mean pup weights
was probably related to the increased litter size. The data for the
litters of the F1 generation were similar for control and treated
groups, with the exception of a single, statistically significant
decrease in litter weight at 50 ppm on day 21. This isolated finding
was considered to be incidental to treatment. No consistent,
dose-related effect on organ weights was reported during the study.
Pathological examination revealed no macro- or microscopic effects
that were related to treatment. Under the conditions of this study, no
adverse treatment-related effects were observed at any dose. The
slight increase in mean body weights of F0 males at the high dose
was the only effect attributed to treatment. The NOAEL was 50 ppm,
equal to 2.5 mg/kg bw per day (James et al., 1988).
Four groups of Sprague-Dawley rats were used in a two-generation
(one litter per generation) study of reproductive toxicity in which
diets containing chlorpyrifos (purity, 97.8-98.5%) at doses of 0, 0.1,
1, or 5 mg/kg bw per day were administered. The study was conducted in
accordance with FIFRA guideline 83-4, OECD guideline 416, and GLP
principles. The F0 parental animals (30 of each sex per dose) were
Table 11. Statistically significant differences in group data for
litters of the F0 generation of rats given diets containing
chlorpyrifos
Time Litter parameter Dose (ppm)
0 2 10 50
At birth Litter size, total 12 13 14** 13
Litter size, live 12 13 14** 13
Litter weight (g) 70 72 79* 71
Pup weight (g) 6.1 5.6*** 5.7** 5.7*
Day 4 Litter size, live 11 13* 14*** 12
Litter weight (g) 100 110 120* 110
Mean pup weight (g) 9.3 8.7 8.6* 9.2
Day 8 Litter size, live 11 13** 13*** 12
Mean pup weight (g) 15 14* 14* 15
Day 12 Litter size, live 11 13** 13*** 12
Litter weight (g) 240 270 280** 250
Mean pup weight (g) 22 21 21* 22
Day 21 Litter size, live 11 13* 13*** 12
Litter weight (g) 460 520 530* 480
Mean pup weight (g) 44 42 40* 42
* p < 0.05; ** p < 0.01; *** p < 0.001
6 weeks of age at the beginning of the study and were mated after 10
weeks of exposure to produce the F1 litters. Groups of 30 rats of
each sex per dose were selected from the F1 weanlings and were
treated for 12 weeks before breeding to produce the F2 litters.
Pairing in both generations allowed for three periods of 7 days'
cohabitation in a 1:1 ratio, the males being changed weekly. Care was
taken to avoid sibling pairings. Females were removed from pairing
when vaginal lavage showed sperm, and this was considered to be day 0
of gestation. The F1 and F2 litters were culled if appropriate to
a total of eight pups on day 4. Clinical observations were performed
daily on all animals; litter size at birth, the numbers of live and
dead pups on days 0, 1, 4, 7, 14, and 21 post partum, and the sex
and weight of each pup were recorded on days 1, 4, 7, 14, and 21 of
lactation. Body weights and food consumption were recorded weekly
before and after breeding. The body weights of dams were recorded on
days 0, 7, 14, and 21 of gestation and on days 1, 4, 7, 14, and 21 of
lactation, whereas food consumption was recorded once during the first
week of lactation, twice during the second week, and every 2-3 days
during the third week. Inhibition of cholinesterase activity in
plasma, erythrocytes, and brain was measured in 10 F0 and F1
parental animals of each sex at necropsy after 19 and 21 weeks of
exposure. Complete necropsies were conducted on all F0 and F1
adults, and included ocular examinations and collection ofmany
tissues. Histopathological examinations were made of the adrenals,
brain, gross lesions, and reproductive tissues (cervix, coagulating
glands, epididymides, ovaries, oviducts, pituitary, prostate, seminal
vesicles, testes, uterus, and vagina) of controls and animals at the
high dose. The livers of 10 F1 parental males in the control and
high-dose groups were also examined microscopically. Only the adrenals
from animals at the intermediate and low doses were examined. Ten F1
and F2 pups of each sex per dose were autopsied grossly. Appropriate
statistical tests were applied to all data.
No significant effects of treatment were seen on clinical signs,
food intake, or body weight. A slight but not statistically
significant decrease in body weight was seen in F1 males at 5 mg/kg
bw per day, and F1 females at this dose showed reduced body-weight
gain during lactation. Plasma cholinesterase activity was inhibited in
parental F0 and F1 rats of each sex at 1 mg/kg bw per day (40-57%)
and at 5 mg/kg bw per day (< 72%). Plasma cholinesterase activity
was also inhibited in animals of both generations at 0.1 mg/kg bw per
day as part of a dose-related trend, although the inhibition was
generally not statistically significant at this dose. Erythrocyte
cholinesterase activity was strongly inhibited at 1 and 5 mg/kg bw per
day, but that of brain was inhibited only at 5 mg/kg bw per day (Table
12).
Gross observation of F0 and F1 parents revealed no
significant alterations, and the only significant histopathological
change in the parental animals was vacuolation consistent with fatty
changes in the adrenal zone fasciculata. Treatment had no effect on
fertility, length of gestation, survival dring gestation, time to
mating, sex ratio, or litter size in either generation. F1 pups at
1 mg/kg bw per day showed slightly decreased body-weight gain during
lactation and statistically significantly decreased body-weight gain
and survival at 5 mg/kg bw per day. No effect of treatment was seen
during gross or daily observation of the F1 weanlings. F2 pups did
not show dose-related decreases in body-weight gain during lactation,
but the survival of pups at 0 and 5 mg/kg bw per day was decreased,
with total loss of three and five litters, respectively; this effect
was stated to be due to maternal neglect, since the stomachs of pups
in these litters contained no milk. No effect of treatment was seen
during gross or daily observation of the F2 weanlings. The NOAEL for
inhibition of erythrocyte cholinesterase in adults was 0.1 mg/kg bw
per day and that for inhibition of brain cholinesterase activity and
materal toxicity was 1 mg/kg bw per day. The NOAEL for developmental
effects was 1 mg/kg bw per day, and the NOAEL for effects on fertility
and reproductive effects was 5 mg/kg bw per day (Breslin et al.,
1991).
Table 12. Mean cholinesterase activity in F0 and F1 parent rats at necropsy (percentage inhibition compared
with controls)
Generation Dose (mg/kg Cholinesterase activity (% inhibition)
bw per day)
Plasma (IU/ml) Erythrocytes (IU/ml) Brain (IU/g)
Male Female Male Female Male Female
F0 0 0.54 2.0 1.1 1.0 9.3 9.0
0.1 0.46 (15%) 1.6 (20%) 1.0 (5%) 1.0 9.2 (1%) 9.1
1 0.30a (44%) 0.8a (59%) 0.3b (92%) 0.36b (65%) 8.8 (6%) 8.7 (3%)
5 0.21a (61%) 0.6a (67%) 0.3b (92%) 0.31b (70%) 4.9b (48%) 4.6a (49%)
F1 0 0.53 1.8 1.1 0.96 9.7 9.4
0.1 0.43 (19%) 1.6 (15%) 0.98a (13%) 0.97 9.7 9.2 (2.5%)
1 0.30a (43%) 0.93a (52%) 0.37a (67%) 0.32b (67%) 9.4 (3%) 9.0 (4.5%)
5 0.19a (64%) 0.52a (72%) 0.33a (70%) 0.24b (75%) 4.6a (52%) 4.0b (58%)
a Statistically significantly different from control mean by Wilcoxon's test
b Statistically significantly different from control mean by Dunnett's test
(ii) Developmental toxicity
Mice
In the range-finding portion of a study of developmental
toxicity, pregnant mice were given chlorpyrifos at doses of up to 60
mg/kg bw per day by gavage. No toxic effects were seen in the dams or
offspring at 3, 10, and 20 mg/kg bw per day. In the main study, groups
of 40-47 pregnant CF-1 mice were initially given chlorpyrifos (purity,
96.8%) at 0, 1, 10, or 25 mg/kg bw per day by gavage in cottonseed oil
on days 6-15 of gestation. The day on which a vaginal plug was
observed was considered to be day 0 of gestation. Owing to severe
maternal toxicity at 25 mg/kg bw per day, additional groups of 35-41
mice were given 0, 0.1, 1, or 10 mg/kg bw per day by gavage on days
6-15 of gestation. The mice were observed for signs of toxicity and
were weighed daily on day 6-16 of gestation and then sacrificed on day
18. At the time of surgical removal of their fetuses, the weights of
the liver and gravid uterus with ovaries were recorded. The uteri of
non-pregnant mice were stained with sodium sulfide to ensure that no
implantations had occurred. The fetuses were weighed, measured
(crown-rump length), sexed, and examined externally. One-third of the
fetuses were dissected to examine the soft tissues. All of the heads
were preserved in Bouin's solution and subsequently sectioned. All
fetuses were stained with Alizarin red for examination of the
skeletons.
Additional mice were added to the study to assess clinical
changes. Cholinesterase (erythrocyte and plasma) activity was measured
in groups of 4-10 mice given 1, 10, or 25 mg/kg bw per day on day 6,
days 6-10, or days 6-15 of gestation, and these measurements were also
made in a group treated with 0, 0.1, 1, or 10 mg/kg bw per day. Blood
was collected for assay of cholinesterase 5 h after dosing on days 6,
10, and 15 of gestation, respectively. A homogenate of fetal tissue
was prepared from litters of mice killed on day 15 of gestation. Data
were not provided on the body weights or food and water consumption of
the animals in these groups.
Appropriate statistical analysis was applied to the experimental
data: fetal body weights and body measurements, maternal body weights,
weights of maternal livers and uteri, and food and water consumption
were analysed by one-way analysis of variance and Dunnett's test. The
incidences of fetal resorptions and alterations were analysed by a
modified Wilcoxon test. The Fisher exact test was applied to other
data.
In the dose-ranging study, severe cholinergic signs and
consequent reproductive failure were found at doses > 30 mg/kg bw
per day. In the first main study, dose-related increases in the
incidence of clinical signs including mortality, excessive salivation,
tremors, urine-soaked coat, ataxia and lethargy were found at 10 and
25 mg/kg bw per day. A single death and isolated clinical signs at
1 mg/kg bw per day were considered not to be related to treatment.
Body-weight gain was reduced at 25 mg/kg bw per day, but no effects
were observed on litter size, resorption incidence, or sex ratio.
Fetal body weight and crown-rump length were significantly reduced at
25 mg/kg bw per day. Plasma cholinesterase activity was depressed in a
dose-related manner, while the activities in erythrocytes and fetal
tissue homogenate were inhibited at 10 and 25 mg/kg bw per day (Table
13). Exencephaly was reported in all groups, but with no dose-response
relationship, and a statistically significant incidence of delayed
ossification was seen at 25 mg/kg bw per day (Table 14).
The second main study showed no cholinergic signs in dams at any
dose up to 10 mg/kg bw per day. Body weight, food intake, maternal
liver weights, gravid uterine weight, and adjusted body weights were
also unaffected. Cholinesterase activity was rapidly and significantly
inhibited in plasma and erythrocytes at 1 and 10 mg/kg bw per day but
was depressed in fetal tissue homogenates only at the high dose. No
effects were observed on materna1 parameters or on litter size, the
incidence of resorptions, the incidence of dead fetuses, the sex
ratio, fetal body weight, or crown-rump length (Table 15). Significant
fetal alterations are shown in Table 16.
Neither study provided evidence of teratogenic activity at any
dose, but fetal toxicity was seen at 25 mg/kg bw per day. The NOAEL
for developmental toxicity was therefore 10 mg/kg bw per day. The
NOAEL for inhibition of erythrocyte cholinesterase activity was 0.1
mg/kg bw per day, and the NOAEL for maternal toxicity was 1 mg/kg bw
per day (JMPR, 1982; modified by reference to the original reports of
Deacon et al., 1979, 1980).
Rats
In a range-finding study of developmental toxicity, which was
given a statement of quality assurance, Fischer 344 rats were fed
chlorpyrifos (purity, 96.6%) by gavage at doses of 0, 3, 10, 15, or
30 mg/kg bw per day during organogenesis. Slight maternal toxicity was
observed at 15 mg/kg bw per day, and severe toxicity was observed at
30 mg/kg bw per day, with typical cholinergic signs of excessive
salivation, lachrymation, urination, defaecation, and body tremors and
general observations of unkempt appearance, decreased body size, and
matting of perineal and facial hair. Deaths were also seen at 30 mg/kg
bw per day, and animals in this group had decreased body-weight gain,
decreased food consumption, enlarged adrenal glands, decreased liver
weight, and shrunken thymus glands. Both plasma and erythrocyte
cholinesterase activities were depressed at all doses. Maternal
toxicity was thus observed at all doses, but fetal toxicity was seen
only at 30 mg/kg bw per day (Ouelette et al., 1983a).
In the main study, which also had a statement of quality
assurance, groups of 31-33 pregnant Fischer 344 rats were given
chlorpyrifos (purity, 96.6%) by gavage in corn oil at doses of 0, 0.1,
3, or 15 mg/kg bw per day on days 6-15 of gestation. They were mated
1:1, and the day sperm were observed in a vaginal smear was considered
to be day 0 of gestation. The rats were observed daily for signs of
toxicity, were weighed daily on days 6-16 and day 21 of gestation, and
Table 13. Significant findings in mice and offspring given chlorpyrifos by gavage on days 6-15 of
gestation
Finding Dose (mg/kg bw per day)
0 1 10 25
No. of females 51 40 44 47
No. of animals with cholinergic signs 0 2 9 32
Deaths 0 1 1 4
Total pregnanta 40 (4) 31 (1) 32 (1) 34 (1)
No. with litters 36 29 30 29
Maternal body weight gain (g)b 21 ± 5 22 ± 3 22 ± 4 18 ± 4*
No. of implantation sites per dam 13 ± 3 13 ± 2 13 ± 1 12 ± 3
No. of fetuses per litter 11 ± 3 12 ± 2 12 ± 2 11 ± 3
Fetal body weight (g) 1.14 ± 0.10 1.13 ± 0.07 1.15 ± 0.08 1.03 ± 0.17*
Fetal crown-rump length (mm) 25.0 ± 1.2 25.2 ± 0.9 25.3 ± 0.9 24.4 ± 1.6*
Plasma cholinesterase activityc 100 (10) 15 (7) 4 (8) 2 (9)
Erythrocyte cholinesterase activityc 100 (10) 86 (6) 57 (9)* 43 (9)*
Fetal homogenate cholinesterase activity 100 81 65 35
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining (not resulting in
litters).
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals
Table 14. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 1 10 25
No. of fetuses examined 408 347 359 326
Exencephalya 1/1 5/5* 1/1 4/3
Ablephariaa 1/1 1/1 1/1 2/2
Cleft palatea 0 2/2 1/1 2/1
Small cerebral hemispherea 1/1 1/1 1/1 0
Multiple skeletal malformationsa 0 0 1/1 0
Fused ribsa 1/1 0 1/1 0
Skull: delayed ossification 5/5 9/5 10/6 34/10*
Sternebrae: delayed ossification 37/17 24/11 19/11 78/22*
Sternebrae: unfused 2/2 7/6* 3/3 8/7*
Sternebrae: fused 24/16 9/16* 23/12 6/5*
* Significantly different from control group
a Considered to be a major malformations
were sacrificed on day 21. At the time their fetuses were surgically
removed, the body and liver weights were recorded. The number of
corpora lutea and the number and position of live and resorbed fetuses
were recorded at necropsy. Fetuses were sexed, examined, and measured.
The uteri of non-pregnant rats were stained with sodium sulfide to
determine whether implantations had occurred in the absence of
litters. Separate groups of 10 rats were dosed on days 6-15 of
gestation and killed, when samples of maternal blood were collected by
heart puncture to measure erythrocyte and plasma cholinesterase
activity. Appropriate statistical analysis of the data was reported.
There were no unscheduled deaths. Clinical signs of maternal
toxicity were found, including excessive salivation, urine staining in
the perineal region, porphyrin deposits around the eyes, and vaginal
bleeding. Tremors were observed only at 15 mg/kg bw per day, and
animals at this dose also had decreased body-weight gain; no other
maternal findings were reported at necropsy. Both plasma and
erythrocyte cholinesterase activities were depressed at doses of 3 and
15 mg/kg bw per day (Table 17). No adverse effects were observed on
reproductive parameters, and no teratogenicity was observed.
Variations and malformations were found which were distributed
randomly by dose, and no effect of treatment on skeletal development
was seen. The NOAEL for maternal toxicity was 0.1 mg/kg bw per day, on
the basis of inhibition of erythrocyte cholinesterase activity in all
adult animals at higher doses. No fetal toxicity was observed
(Ouelette et al., 1983b).
Table 15. Significant findings in mice and offspring given chlorpyrifos by gavage on days
6-15 of gestation
Finding Dose (mg/kg bw per day)
0 0.1 1 10
No. of females bred 41 35 36 35
Total no. pregnanta 30 (0) 25 (1) 23 (0) 28 (3)
No. with litters 30 24 23 25
Maternal body weight gain (g)b 12 ± 6 14 ± 5 14 ± 5 14 ± 5
No. of implantation sites per dam 10 ± 3 10 ± 3 10 ± 3 9 ± 4
No. of fetuses per litter 8 ± 3 7 ± 3 7 ± 3 7 ± 3
Fetal body weight (g) 1.04 ± 1.11 1.07 ± 0.15 1.08 ± 0.18 1.09 ± 0.12*
Fetal crown-rump length (mm) 24.4 ± 1.1 24.8 ± 1.6 24.9 ± 2.1 24.8 ± 1.2*
Plasma cholinesterase activityc 100 (8) 86 (6) 25 (7)* 3 (6)*
Erythrocyte cholinesterase activityc 100 (8) 88 (6) 75 (7)* 50 (6)*
Fetal homogenate cholinesterase activityc 100 84 95 77
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals.
Table 16. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 0.1 1 10
Exencephalya 1/1 1/1 0 1/1
Ablephariaa 1/1 0 0 0
Cleft palatea 0 0 1/1 0
Omphalocelea 0 0 0 1/1
Skull: delayed ossification 18/10 11/6 3/2 3/3
Sternebrae: delayed ossification 50/20 25/13 25/13 17/11
Sternebrae: unfused 3/3 6/5 0 1/1
Sternebrae: fused 13/7 9/7 3/3 12/8
a Considered to be major malformations
Female CD Sprague-Dawley rats were mated 1:1 with stock males
from the same source and strain. Technical-grade chlorpyrifos (stated
purity, 96.1%) was formulated daily as solutions in maize oil and
given to groups of 32 mated females in a volume of 5 ml/kg bw by oral
gavage at doses of 0.5, 2.5, or 15 mg/kg bw per day on days 6-15
post coitum. A further group received maize oil only. This study was
conducted in accordance with US Food and Drug Administration and
Environmental Protection Agency guidelines for GLP and testing
guidelines of the OECD (No. 414) and US Environmental Protection
Agency (section 83-3). All females were examined daily for signs
associated with treatment. Animals were weighed on days 0, 3, 6-15,
17, and 20 of gestation, and food consumption was measured twice
weekly. Ten mated females were bled on day 15 for measurement of
plasma cholinesterase activity, blood samples being obtained from the
retro-orbital sinus under ether anaesthesia. The same procedure was
repeated for an additional 10 animals per dose immediately before
necropsy on day 20. The animals used for cholinesterase determinations
on day 15 were discarded without necropsy after being bled. All
remaining animals were killed on day 20, and the following were
recorded for each animal: any macroscopic abnormality of the
reproductive tract; number of corpora lutea in each ovary; weight of
gravid uterus; distribution of live and dead fetuses and distribution
of resorption sites in each uterine horn; individual placental
weights; individual fetal weight, length, and sex; and external
anomalies of individual fetuses. The thoracic and abdominal contents
of approximately one-half of each litter were dissected and examined,
and these fetuses were used for skeletal staining and evaluation; the
remaining fetuses were used for preparation of free-hand sections and
examination of visceral organs.
Table 17. Adverse effects in rats given chlorpyrifos by gavage
Finding Dose (mg/kg bw per day)
0 0.1 3 15
No. of females 31 32 33 31
No. of animals with cholinergic signs 0 0 0 12
Total no. pregnanta 30 (0) 28 (0) 26 (0)b 29 (1)
No. of litters 29 26 24 26
Maternal body weight gain (g)c 65 ± 11 64 ± 14 71 ± 12 58 ± 11
No. of implantation sites per dam 11 ± 2 10 ± 2 10 ± 2 10 ± 3
No. of fetuses per litter 10 ± 3 9 ± 2 10 ± 3 9 ± 3
Mean fetal body weight (g) 4.28 4.37 4.52* 4.46*
Mean fetal crown-rump length (mm) 43.91 43.93 43.96 43.64
Plasma cholinesterase activity (IU/ml)d 46.0 42.8 (93%) 4.9 (11%) 1.5 (3%)
Erythrocyte cholinesterase activity (IU/ml)d 12.1 11.9 (98%) 3.1 (26%) 2.5 (21%)
* Significantly different from control
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Two dams removed during dosing for reasons not related to treatment
c Days 6-20
d Mean of 6-8 dams on day 15, IU/ml (percentage of control)
No treatment-related deaths occurred during the study, and
clinical signs of intoxication were confined to tremors late in the
study in three animals at 15 mg/kg bw per day. Slight but
statistically significant decreases in mean food consumption (8%
reduction during day 79 only) and body-weight gain were seen at
15 mg/kg bw per day. Statistically significant, dose-related decreases
in plasma cholinesterase activity were observed at all doses;
erythrocyte and brain cholinesterase activities were not determined.
The effects on litters are summarized in Table 18. The total live
litter sizes were unaffected by treatment, but at 15 mg/kg bw per day
the mean number of live male fetuses was statistically significantly
decreased and post-implantation loss was slightly elevated.
Statistically significant increases in pre-implantation loss were seen
at all doses, but there was no consistent dose-response relationship
and the incidence was within historical control values for this
laboratory; the finding was considered not to be related to treatment.
At 15 mg/kg bw per day, slight but statistically significant increases
were seen in mean fetal weight and mean crown-rump length, but as the
increase in fetal weight was not accompanied by a decrease in litter
size, this finding may have been due to advanced fetal development and
was considered to be of no toxicological significance. Similarly, the
statistically significant decrease in the incidence of small fetuses
observed in all test groups was considered not to be toxicologically
significant. A number of minor structural variations were reported,
including a single incidence of anophthalmia at 0.5 mg/kg bw per day
and instances of microphthalmia at 0.5 and 2.5 mg/kg bw per day, but
the incidence of such findings was low and/or were not related to
dose. These findings were therefore considered to be incidental to
treatment. Skeletal examination showed no adverse treatment-related
findings. The NOAEL for frank maternal toxicity was 2.5 mg/kg bw per
day, on the basis of reduced body weight, tremors, and transient
reductions in food consumption at 15 mg/kg bw per day. The NOAEL for
fetal toxicity was 2.5 mg/kg bw per day, on the basis of a slight
increase in post-implantation loss at 15 mg/kg bw per day, probably
associated with maternal toxicity (Rubin et al., 1987a).
Rabbits
Groups of 14 female New Zealand white rabbits were mated 1:1 with
stock males from the same source and strain and given technical-grade
chlorpyrifos (stated purity, 96.1%), formulated daily as solutions in
maize oil, by oral gavage at doses of 1, 9, 81, or 140 mg/kg bw per
day (in a volume of 2 ml/kg bw) on days 7-19 post coitum. The doses
for animals at 81 and 140 mg/kg bw per day were prepared directly, and
those for the animals at 1 and 9 mg/kg bw per day were prepared by
diluting the dose of 81 mg/kg bw per day with maize oil. A further
group received maize oil only. This study was conducted in accordance
with US Food and Drug Administration and Environmental Protection
Agency guidelines for GLP and testing guidelines of the OECD (No. 414)
and US Environmental Protection Agency (section 83-3). The doses were
selected after a preliminary range-finding study. On day 15, three
does at 81 mg/kg bw per day were accidentally given a dose of
140 mg/kg bw, and the group was expanded to 21 animals so that the
overdosed animals could be excluded from the study if that was found
to be warranted. Two of the three animals were pregnant, but no
apparent difference was found from the females treated at 81 mg/kg bw
per day, and the maternal and fetal data for these animals were
included in the report. All animals were examined daily for clinical
signs associated with treatment. Any animals found dead or killed
in extremis were autopsied to determine the cause of death. Animals
were weighed on days 0, 3, 7-19, 22, 25, and 29 of gestation, and food
consumption was determined twice weekly. Plasma cholinesterase
activity was measured before mating and again after at least 10 days
of dosing. The does were killed on day 29 and the following were
recorded: any macroscopic abnormality of the reproductive tract; the
number of corpora lutea in each ovary; the weight of gravid uterus;
the distribution of live and dead fetuses and of resorption sites in
each uterine horn; individual placental weights; individual fetal
weight and crown-rump length; sex; and external anomalies of
individual fetuses. The thoracic and abdominal contents were dissected
and examined, and the skull of each fetus was sectioned transversely
through the frontal-parietal suture, and the brain examined. Each
fetus was used for skeletal staining and evaluation.
Table 18. Effects on fetuses of rats given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 0.5 2.5 15
Pre-implantation loss (%) 8.6 12.2*** 11.5*** 11.4***
Post-implantation loss (%) 7.0 6.0 5.8 9.0**
Mean fetal weight (g) 3.33 3.40 3.44 3.51**
Mean crown-rump length (mm) 36.0 36.2 36.3 36.7**
Live fetuses:
Male 7.6 8.0 6.8 6.0*
Female 6.8 7.0 7.3 7.5
Total 14.4 15.0 14.1 13.4
Small fetuses (no. observed) 48 (317) 25 (315)** 20 (311)*** 13 (282)***
Ablepheron (no. observed) 0 (317) 0 (315) 1 (311) 0 (282)
Major cranio-facial malformations: 0 (317) 1 (315) 0 (311) 0 (282)
proboscis, anophthalmia (no. observed)
Hydronephrosis (no. observed) 0 (161) 0 (158) 1 (158) 0 (146)
Hydroureter 0 (161) 0 (158) 1 (158) 0 (146)
Anophthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
Microthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
No treatment-related deaths were observed during the study, and
no treatment-related clinical signs were reported. Maternal group mean
food consumption was unaffected by treatment, and group mean body
weights and body-weight gains were not affected by treatment at doses
up to 81 mg/kg bw per day. At 140 mg/kg bw per day, body-weight gain
was inhibited during most of the treatment period, although it was
greater than normal after cessation of treatment, and by day 29 the
animals in this group had attained body weights similar to those in
the control group. Statistically significant, dose-related decreases
in plasma cholinesterase activity were seen at all doses after 10 days
of treatment, with inhibition of 56% at 1 mg/kg bw per day, 68% at 9
mg/kg bw per day, 70% at 81 mg/kg bw per day, and 72% at 140 mg/kg bw
per day compared with controls. Erythrocyte and brain cholinesterase
activities were not determined.
Statistically significant decreases in pre-implantation loss were
observed at most doses, but this finding was considered not to be
toxicologically significant. Statistically significant increases in
post-implantation loss were observed at 9 and 140 mg/kg bw per day but
not at 81 mg/kg bw per day. In the absence of a consistent
dose-response relationship, the toxicological significance of this
finding was unclear; no historical control data were supplied for this
effect from the laboratory. A slight but statistically significant
decrease in mean fetal crown-rump length and a decrease in mean fetal
weight were observed in animals at 140 mg/kg bw per day, and these
effects were considered to be related to treatment. A number of fetal
anomalies were observed but were considered not to be
treatment-related as the incidence was generally low and unrelated to
dose. An increased incidence of fetuses with fifth sternebra and/or
unossified xiphisternum seen at 140 mg/kg bw per day was considered to
be related to slightly delayed development at this dose, as indicated
by reduced fetal weight and length in this group. Given the variation
in the incidence of other skeletal observations in treated groups,
however, this finding may have been due to chance. The NOAEL for frank
maternal toxicity was 81 mg/kg bw per day, on the bais of decreased
body-weight gain at the higher dose. The NOAEL for fetal toxicity was
81 mg/kg bw per day, on the basis of a slight decrease in mean fetal
crown-rump length, decreased mean fetal weight, and an increased
incidence of fetuses with fifth sternebra and/or unossified
xiphisternum at the higher dose (see Table 19) (Rubin et al., 1987b).
(f) Special studies: Neurotoxicity
Chickens
No signs of delayed ataxia or paralysis were reported in groups
of three hens treated with single oral doses of chlorpyrifos at 40,
75, 100, or 150 mg/kg bw. The two higher doses resulted in deaths and
other clinical signs of toxicity (Stevenson, 1966a). Similarly, groups
of 10 hens given single oral doses of chlorpyrifos at up to 100 mg/kg
bw did not display delayed neurotoxicity, and no neuropathological
findings associated with treatment were detected (Rowe et al., 1978).
In another study, single oral doses of chlorpyrifos at up to five
Table 19. Effects on litters and fetuses of rabbits given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 1 9 81 140
Number of fetuses examined 108 117 126 142 99
Pre-implantation loss (%) 15.2 13.1 8.9*** 11.9* 11.5*
Post-implantation loss (%) 6.5 8.6 13.8*** 8.7 13.9***
Mean fetal weight (g) 45.4 44.3 43.3 41.8 40.7
Mean crown-rump length (mm) 97.4 95.9 95.0 94.2 93.2*
Teeth absent 0 0 1 0 0
Arthrogryposis, forelimb 0 0 2 0 0
Irregularly shaped thorax 0 0 0 0 1
Hydrocephalus 0 0 1 0 0
Enlarged aortic arch 0 0 1a 0 0
Rudimentary pulmonary artery 0 0 1a 0 0
Agenesis of interventricular cardiac septum 0 0 1a 0 0
Hydronephrosis (bilateral) 2 0 0 4 1
Hydronephrosis (unilateral) 2 2 1 2 1
Diffuse opacity of eye 0 0 0 2 0
No. of fetuses examined for skeletal defects 108 117 126 142 99
Reduced or incomplete ossification of interparietal bone 3 4 3 5 2
Unossified interparietal bone 0 1 0 0 3
Reduced or incomplete ossification of hyoid bone 2 10* 17*** 16** 8*
Irregular ossification of one or more of sternebrae 1-4 1 2 7* 8* 1
Bony plaque adjacent to sternebra 10 6 2** 3* 1**
Unossified fifth sternebra and/or xiphisternum 1 1 2 3 6*
Multiple malformations of ribs and lumbar spine 0 0 0 0 1
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
times the oral LD50 in hens (> 150 mg/kg) resulted in significant
inhibition of neuropathy target esterase and cholinesterase activity
(Capodicasa et al., 1991), with signs consistent with
organophosphate-induced delayed neuropathy. Extensive, aggressive
antidotal treatment, both before and throughout the treatment and
recovery periods, was required for the birds' survival.
Technical-grade chlorpyrifos (purity, 96.8%) was tested for acute
toxicity and delayed neurotoxicity in adult domestic hens in a study
carried out according to GLP. In the assessment of neurotoxicity,
groups of 10 birds were given corn oil, tri- ortho-cresyl phosphate
(positive control; 20% in corn oil; 500 mg/kg), chlorpyrifos
(110 mg/kg bw) plus atropine sulfate (intramuscular injection;
10 mg/kg bw), chlorpyrifos (110 mg/kg bw; 5.5% in corn oil) plus
pyridine 2-aldoxime methane sulfonate (intramuscular injection;
50 mg/kg bw; 5% aqueous solution), or were not treated and were
maintained as a source of additional birds if necessary. The hens were
observed for 21 days, at which time all those given the corn oil were
given a second dose, and all treated birds were given chlorpyrifos,
followed by another 21-day observation period.
No clinical signs of toxicity were observed in those given the
vehicle. In birds given tri- ortho-cresyl phosphate, no signs of
toxicity were observed immediately after dosing, but all birds
developed signs of delayed locomotor ataxia as early as day 11 after
treatment. The birds given chlorpyrifos showed signs of toxicity after
either dose, including subdued appearance, unsteadiness, inability to
stand, and weakness. Deaths were observed in both chlorpyrifos-treated
groups, four birds that died after the first dose with atropine
sulfate (no deaths after the second dose) and three after the first
dose of chlorpyrifos with pyridine 2-aldoxime methane sulfonate (one
death after the second dose). Surviving birds recovered completely
within 5-8 days of treatment. Additional doses of atropine sulfate
were given to several birds in both groups treated with chlorpyrifos
owing to the severity of the clinical signs observed.
Histopathological examination revealed a low incidence of minor
neurological changes (grade II on a scale of I-V of increasing
severity) in several birds given the vehicle, which was considered to
show a normal background incidence of axonal degeneration. Most of the
birds given tri- ortho-cresyl phosphate had grade III changes at at
least one level of spinal cord and grade III or IV changes in at least
one level of peripheral nerve, indicating significant
treatment-related axonal degeneration. Three birds treated with
chlorpyrifos showed grade II changes at one level of peripheral nerve
only, and these findings were considered to be similar to the
incidence and severity of changes seen in the vehicle controls. Thus,
oral administration of a single dose of chlorpyrifos at 110 mg/kg bw
to hens and another dose after 21 days did not induce clinical signs
of delayed neurotoxicity, as confirmed by histopathological
examination of nervous tissues (Roberts et al., 1987)
When chlorpyrifos was given to chickens for 91 days in the diet
at doses of < 10 mg/kg bw per day in a study conducted according to
GLP, the only compound-related clinical signs were transient, slight
gait alterations in a number of birds at the high dose. No
histopathological lesions of nerve tissues characteristic of
organophosphate-induced delayed neuropathy were seen. Concurrent
positive controls showed both toxic signs and histopathological
lesions of nerve tissues typical of those induced by a delayed
neurotoxicant (Barna-Lloyd et al., 1986).
To determine whether repeated doses of chlorpyrifos at the
maximum tolerated dose caused cumulative inhibition of neuropathy
target esterase and organophosphate-induced delayed neuropathy,
chlorpyrifos (purity, 100%) was given orally to 15 white Leghorn hens,
18 months of age, for 20 days at a dose of 10 mg/kg bw per day. Corn
oil was given to a control group of 18 birds. Physical observations
(including posture and gait) were made daily. Three hens per group
were killed on days 0 (controls only), 4, 10, 15, 20, and 48, and
brain acetylcholinesterase, plasma butyryl cholinesterase, and
lymphocyte and brain neuropathy target esterase were assayed. Body
weights were decreased during treatment, by 25% when compared with
controls on day 20, but had recovered to about 87% of that of controls
by the end of the study. Some birds showed acute effects (staggering
gait and diarrhoea) during week 1 of treatment. Diarrhoea was also
seen in controls and may have been associated with the corn oil. No
behavioural or perching defects were seen during the observation
period. During days 4-20, brain cholinesterase activity was depressed
by 58-70% and plasma cholinesterase activity by 49-80% when compared
with control values, but after the 4-week observation period brain
cholinesterase activity had recovered to 86% and plasma cholinesterase
to 134% of the controls values; neuropathy target esterase activity in
brain and lymphocytes throughout the study was 82-99% and 85-128% of
that in controls, respectively. This finding was not statistically
significant for lymphocytes, but the 18% inhibition of brain
neuropathy target esterase activity on days 10 and 20 was
statistically significant. There were no indications of delayed
neurotoxicity after repeated administration of a dose that caused
significant inhibition of brain cholinesterase activity and loss of
body weight (Richardson et al., 1993a).
In order to clarify the magnitude of the neuropathic risk
associated with exposure to chlorpyrifos, groups of three 18-month-old
white Leghorn hens were given single oral doses of chlorpyrifos
(purity, 100%) at 0, 75, 150, or 300 mg/kg bw with atropine (20 mg/kg
bw subcutaneously at the time of the first dose of chlorpyrifos and
additional doses as needed to maintain the hens for 4 days). Four days
after treament, when the hens were killed, brain acetylcholinesterase
activity was depressed by 0, 58, 75, and 86% and neuropathy target
esterase activity by 0, 21, 40, and 77% at the three doses,
respectively. In a further study, 18 hens were given corn oil alone
and 15 atropine-protected hens were given chlorpyrifos at a single
oral dose of 150 mg/kg bw. Three controls were killed on day 0, and
three treated and three control hens were killed on days 1, 2, 4, 8,
and 16. Brain acetylcholinesterase activity was inhibited by 86, 82,
72, 43, and 29% on days 1, 2, 4, 8, and 16 after treatment, and brain
neuropathy target esterase activity was inhibited by 30, 28, 38, 29,
and 6%. No signs of organophosphate-induced delayed neuropathy were
observed.
In studies in vitro in which chlorpyrifos-oxon was added to
whole brain homogenate, the rate constant for the enzyme-inhibitor
complex was 15.5 per mm per min for acetylcholinesterase and 0.145 per
mm per min for neuropathy target esterase. The calculated 20-min
fixed-time IC50 values were 2.2 nmol/L for acetylcholinesterase and
240 nmol/L for neuropathy target esterase. The measured IC50 values
based on fixed-time (20 min) preincubation were 2.2 nmol/L for
acetylcholinesterase and 210 nmol/L for neuropathy target esterase.
The ratio of the calculated values for neuropathy target
esterase:acetylcholinesterase is 110. As a ratio of > 1 generally
indicates that the dose that would be required to cause
organophosphate-induced delayed neuropathy would be in excess of the
LD50, acute exposure to chlorpyrifos, except at doses well in excess
of the LD50, would not be anticipated to induce this condition
(Richardson et al., 1993b).
The potential of chlorpyrifos to cause acute delayed
neurotoxicity was tested in white Leghorn hens given doses < 12
mg/kg bw per day for 21 days by oral gavage in peanut oil. Dose- and
time-related increases in the incidence and severity of ataxia were
observed. Plasma and whole blood cholinesterase activities were
reduced at 5 and 12 mg/kg bw per day. Ataxia was observed at 2, 5, and
12 mg/kg bw per day. Necropsy did not reveal any findings associated
with treatment. Histopathological examination of the brain and spinal
cord revealed a low incidence of several lesions, with no relation to
dose, and these findings were considered to be incidental to
treatment. Since continued administration of chlorpyrifos resulted in
ataxia, it would have been difficult to observe any delayed
neurological effects had they occurred (Jai Research Foundation,
1996b).
Rats
In rats, single oral doses of chlorpyrifos at 50-100 mg/kg bw
resulted in treatment-related effects and clinical signs for several
days after dosing. No deaths occurred at any dose. At 10 mg/kg bw,
effects were confined to isolated observations of perineal staining
and/or decreased activity. No neuropathological lesions were reported
(Wilmer, 1992; Mattsson et al., 1996).
In a study in adult Long-Evans rats carried out in accordance
with GLP, oral administration of chlorpyrifos at doses < 10 mg/kg
bw per day for 4 weeks resulted in dose-related inhibition of plasma
and erythrocyte cholinesterase activity at > 1 mg/kg bw per day and
of brain cholinesterase activity at > 3 mg/kg bw per day. These
effects were accompanied by clinical signs of intoxication at 10 mg/kg
bw per day. Cognitive behavioural tests indicated some non-cognitive
changes associated with impaired motor activity at 10 mg/kg bw per
day, but no clear treatment-related effects on cognitive function were
observed (Maurissen et al., 1996).
After oral administration of chlorpyrifos to rats at single doses
< 100 mg/kg bw in a study that conformed to GLP, no
treatment-related effects on neuropathy target esterase activity were
observed at any dose. Plasma, erythrocyte, and heart cholinesterase
activity was statistically significantly lower than that in controls
at doses > 5 mg/kg bw, and brain cholinesterase activity was
decreased at > 50 mg/kg bw. No significant inhibition was observed
at 0.5 or 1 mg/kg bw (Dittenber, 1997).
The neurotoxic potential of chlorpyrifos (purity, 99.5%) was
assessed in groups of 20 male Long-Evans hooded rats, approximately 70
days old with a mean body weight of 350 g. The test material was
dissolved in corn oil and given to the rats by oral gavage in a volume
of 1 ml/kg bw at doses of 0, 10, 30, 60, or 100 mg/kg bw. All rats
were evaluated for behavioural signs 3.5 h after dosing with a
functional observational battery of tests consisting of evaluations of
home cage, open-field, and manipulative neurobehaviour. The measures
were autonomic activity (lachrymation, salivation, miosis,
defaecation), motor activity (rearing, home cage posture), convulsive
activity (tremors), neuromuscular activity (gait score, landing foot
splay, righting reaction), general reactivity (arousal, removal
reactivity), sensorimotor activity (responses to tail pinch, click,
approach), and physiological reactions (body temperature, body
weight). Data on motor activity were recorded during a 1-h session in
a maze shortly after testing. Tissue samples were obtained after the
motor activity tests, 4.5 and 25 h after dosing. Half of the animals
in each group were killed at 3.5 h, and the remaining 10 animals were
examined again at 24 h and then killed. Immediately after sacrifice,
the brain, retina, liver, heart, diaphragm, and quadriceps femoris
muscle were collected from all animals. The brain was sectioned
mid-sagitally, and the remaining half-brain was further sectioned to
obtain the frontal cortex, hippocampus, striatum, hypothalamus,
cerebellum, and pons/medulla. Blood was collected from the trunk.
Muscarinic receptor density was determined in all brain regions and in
the heart and retina. Cholinesterase activity was determined in brain,
muscle, retina, plasma, erythrocytes, whole blood, and liver.
Cholinesterase activity was significantly inhibited in a range of
tissues, including brain, plasma, and erythrocytes, at all doses at
both 4.5 and 25 h (Table 20). As the data were represented only as
graphs, the percentages are approximations. Erythrocyte cholinesterase
activity was slightly more sensitive than that of plasma at the doses
used in this study, and plasma cholinesterase activity recovered more
quickly than that of erythrocytes. The degree of inhibition of
whole-blood cholinesterase activity lay between that of plasma and
erythrocyte activity at both 4.5 and 25 h. Whole-brain cholinesterase
activity can be considered representative of that in various regions
of the brain, although a number of regions showed less inhibition than
was measured in the half-brain in this study. In peripheral tissues,
Table 20. Percentage inhibition of cholinesterase activity compared with controls in rats given chlorpyrifos by gavage
Time (h) Dose Inhibition of cholinesterase activity (%)
(mg/kg bw)
Plasma Erythrocytes Blood Half-brain Retina Heart Diaphragm Quadriceps Liver
4.5 10 80 90 85 40 40 55 40 5 70
30 85 95 90 70 65 65 55 20 70
60 85 100 90 85 80 70 70 70 80
100 90 100 95 90 90 75 85 85 85
2.5 10 35 75 55 30 30 40 5 + 10 25
30 75 90 85 60 60 75 35 20 70
60 95 100 95 80 75 90 65 35 85
100 95 100 95 85 85 90 75 55 85
When inhibition was > 20% in comparison with corresponding controls, the change in cholinesterase activity was
statistically significant ( p < 0.05).
the inhibition was much lower in the quadriceps than in other tissues
at 4.5 h, and inhibition of heart, liver, and retina cholinesterase
activity was similar at 25 h.
Clinical and behavioural signs were observed at doses
> 30 mg/kg bw at 4.5 h. The doses selected for this study resulted
in a skew of some correlations, especially for cholinesterase
inhibition in blood and blood components, with behavioural and
clinical findings. In order to examine the relationship between
clinical and behavioural findings and cholinesterase inhibition, the
authors grouped rats according to the extent of cholinesterase
inhibition (instead of by dose) and indicated the incidence of
cholinergic signs related to individual cholinesterase inhibition. The
10 'cardinal' cholinergic signs that were used for this analysis were
temperature, gait, motor activity, smacking, foot splay, tail pinch,
tremors, lachrymation, pupil response, and salivation (Table 21). None
of these signs was observed in the 18 animals with < 60% inhibition
of brain cholinesterase activity. The incidence of these findings
increased in relation to brain cholinesterase inhibition. In animals
with < 80% inhibition of whole-blood cholinesterase activity, no
signs were observed, while all of the signs were seen at varying
incidences in animals with 90-100% inhibition. The correlation between
these end-points and inhibition of cholinesterase in other tissues or
in erythrocytes or plasma was not provided. These results were
interpreted by the authors as indicating a threshold effect, since no
clinical signs were observed until a certain percentage inhibition of
cholinesterase activity was reached. Because of the doses selected for
this study, marked inhibition was observed at the lowest dose in the
absence of clinical findings. The use of a single dose also limited
the usefulness of the findings. No clinical signs were seen when brain
cholinesterase activity was inhibited by < 60% or when whole blood
cholinesterase activity was inhibited by < 80% after a single dose of
chlorpyrifos. The data do not suggest that these values could be
extrapolated to repeated doses (Nostrandt et al., 1997).
Technical-grade chlorpyrifos (purity, 98.1%) was given to groups
of 10 male and 10 female Fischer 344 rats in the diet for 13 weeks at
doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day. The dietary
concentrations were adjusted on the basis of measured weight and food
consumption, but the range of dietary concentrations used to achieve
these target doses was not provided. The study was conducted in
accordance with GLP requirements and to meet US Environmental
Protection Agency FIFRA guideline No. 82-7. Body weights and feed
consumption were measured before treatment and weekly during
treatment. Ophthalmic examinations were conducted before treatment and
at necropsy. Cage-side observations were made twice daily and clinical
examinations weekly. A functional observational battery of tests was
applied to all rats before treatment and during weeks 4, 8, and 13 of
treatment. Five rats of each sex per dose were examined for gross
pathological alterations. Tissues for histopathological evaluation
were prepared only from rats in the control and high-dose groups. Nine
transverse sections of the brain included the olfactory bulb, cerebral
cortex (frontal, parietal, temporal, and occipital lobes),
Table 21. Incidence (%) of cardinal cholinergic signs in rats given chlorpyrifos by gavage and grouped according to
extent of inhibition of cholinesterase activity (numbers of rats in parentheses)
End-point Inhibition of brain cholinesterase activity (%) Inhibition of blood cholinesterase activity (%)
90-100 (10) 80-90 (8) 70-80 (7) 60-70 (7) < 60 (18) 90-100 (25) 80-90 (14) < 80 (11)
Temperature 100 100 86 57 0 92 36 0
Gait 100 100 57 71 0 84 43 0
Motor activity 90 88 86 57 0 84 36 0
Smacking 90 63 57 0 0 64 14 0
Foot splay 40 38 29 0 0 32 14 0
Tail pinch 70 38 0 0 0 40 0 0
Tremors 90 25 0 0 0 44 0 0
Lachrymation 80 13 0 0 0 32 7 0
Pupil response 60 0 0 0 0 24 0 0
Salivation 30 0 0 0 0 12 0 0
thalamus/hypothalamus, midbrain, pons, cerebellum, medulla oblongata,
and nucleus gracilis/cuneatus. Other tissues examined were the
trigeminal ganglion and nerve, pituitary, eyes (retina and optic
nerve), spinal cord (cervical and lumbar), olfactory epithelium, and
skeletal muscles (gastrocnemius and anterior tibial).
A low incidence of perineal staining was seen in females at 5 and
15 mg/kg bw per day. No decrease in body weight was recorded at any
dose. Gross pathological examination did not reveal any
treatment-related effects. Histopathological examination revealed a
small number of isolated findings that were similar in control and
treated groups and which were not considered to be related to
treatment. These included slight degeneration of individual nerve
fibres in the trapezoid body of the medulla oblongata in four control
males and three control females and in one male and two females at the
high dose; slight degeneration of individual nerve fibres in the
cervical spinal cord of a single male at the high dose; and myelin
ellipsoids in the lumbar spinal cord of one male at the high dose and
one female control. A slight reduction in motor activity was observed
at 15 mg/kg bw per day during week 4 only, but the toxicological
significance of this finding was unclear. Cholinesterase activity was
not measured in this study. No neuropathological findings related to
treatment were observed at any dose. The NOAEL for cholinergic effects
was 1 mg/kg bw per day, and that for neuropathy was 15 mg/kg bw per
day (Shankar et al., 1993).
In a study designed to investigate the effects of age on the
toxicity of chlorpyrifos, rats received corn oil or chlorpyrifos
orally at doses of 80 mg/kg bw for adults and 15 mg/kg bw for young
rats (at postnatal day 17). These doses were equally effective in
inhibiting cholinesterase activity. In adult rats, peak behavioural
changes and cholinesterase inhibition occurred in males 3.5 h after
dosing, while in females the onset of functional changes occurred
sooner, the time course was more protracted, and recovery was slower.
In young rats, the maximal behavioural effects and cholinesterase
inhibition occurred 6.5 h after dosing, with no sex-related
difference. Partial to full recovery from the behavioural changes was
seen at 24-72 h, whereas the cholinesterase activity recovered more
slowly. The blood and brain cholinesterase activity in young rats had
almost recovered by 1 week after dosing, whereas the brain
cholinesterase activity in adults had not recovered by 2 weeks. Assays
for binding to muscarinic receptors showed apparent down-regulation in
some areas of the brain, mostly at 24 and 72 h. Rats at postnatal day
17 generally showed more receptor down-regulation than adults, whereas
only adult females showed receptor changes in striatal tissue that
persisted for 2 weeks. Thus, young rats showed similar behavioural
changes and cholinesterase inhibition to adults, although at a
fivefold lower dose; the onset of maximal effects was somewhat delayed
in the young rats; cholinesterase activity tended to recover more
quickly in the young rats; young rats appeared to have more extensive
muscarinic receptor down-regulation; and young rats showed no
sex-related difference in functional changes (Moser & Padilla, 1998).
Chlorpyrifos was administered subcutaneously to neonatal rats at
1 or 5 mg/kg bw per day on postnatal days 1-4 or daily at 5 or 25
mg/kg bw per day on days 11-14, and the two groups of pups were
examined at postnatal days 5 and 10 or 15 and 20, respectively. Pups
treated at postnatal days 1-4 had a significant increased mortality
rate (> 50%) at 5 but not at 1 mg/kg bw per day; the body weights of
the affected pups were decreased by 10-15% and the brain was
significantly smaller than that of controls. The survivors showed
severe cell loss in the brainstem; brainstem growth was maintained by
enlargement of the remaining cells. The lower dose did not compromise
survival or growth. Neither dose had an adverse effect on the
forebrain, despite the fact that the brainstem and forebrain have
comparable cholinergic projections. Pups treated on postnatal days
11-14 showed no increase in mortality rate at 5 mg/kg bw per day but a
significant increase (> 80%) at 25 mg/kg bw per day at day 15. The
body weights of the affected pups were significantly decreased
(> 40%), and the major target for cell loss shifted from the
brainstem to the forebrain. The pups at 5 mg/kg bw per day had a
small, transient loss in body weight; there was no significant effect
on whole brain weight, but these pups showed loss of forebrain cells
between 15 and 20 days of age, and the total amount of DNA in the
forebrain declined whereas an increase was seen in the forebrains of
control pups. The brainstem showed a smaller but not significant cell
loss during this period. The cerebellum differed from the other
regions in that it showed short-term increases in DNA after exposure
to chlorpyrifos in either the early or the late postnatal period;
nevertheless, the values then regressed to subnormal, in parallel with
loss of cells in other regions.
Although regions rich in cholinergic projections, such as the
brainstem and forebrain, may be more severely affected than
non-cholinergic regions such as the cerebellum, the time of maturation
of each region (brainstem earliest, forebrain intermediate, cerebellum
last) appears to be more important in determining vulnerability. The
weights of the various regions remained within normal limits despite
severe reductions in cell numbers, probably because reactive
hypertrophy of the remaining cells masked the cell loss. These results
suggest that chlorpyrifos induces cellular deficits in the developing
brain even when growth or survival is unaffected; however, the route
of administration and the doses used in this study limit the relevance
of these findings to humans (Campbell et al., 1997).
Chlorpyrifos was administered subcutaneously to neonatal rats at
doses of 1 or 5 mg/kg bw per day on postnatal days 1-4, or at 5 mg/kg
bw per day on days 11-14, and the two groups of pups were examined on
postnatal days 5 and 10 or 15 and 20, respectively. Signs of
interference with the adenyl cyclase signalling cascade were
determined in the heart and developing brainstem and cerebellum. Pups
treated on postnatal days 1-4 at 1 mg/kg bw per day showed no signs of
toxicity, while there was significant mortality (> 50%) at 5 mg/kg bw
per day. Pups treated on postnatal days 11-14 at 5 mg/kg bw per day
showed no deaths or clinical signs. No significant changes in the
weights of the brain region were found at these apparently non-toxic
doses. Cholinesterase activity in the brainstem of the pups given
1 mg/kg bw per day was inhibited by 25% when measured 24 h after the
last dose, but recovery was substantial (< 10% inhibition) by day 10.
Cholinesterase activity in the brainstem of the pups given 5 mg/kg bw
per day on days 11-14 was inhibited by > 60% when measured 24 h after
the last dos, but recovery was again substantial (< 30% inhibition)
by postnatal day 20. Separate examinations of the forebrain,
cerebellum, and heart showed that chlorpyrifos had evoked deficits in
multiple components of the adenyl cyclase cascade: the expression and
activity of adenyl cyclase itself, functioning of the G-proteins that
link neurotransmitter and hormone receptors to cyclase activity, and
expression of neurotransmitter receptors that act through this
cascade. Disruption of signalling function was not restricted to
transduction of cholinergic signals but extended to adrenergic signals
as well. In most cases, the adverse effects were not evident during
administration of chlorpyrifos but appeared after several days. Most
of the results of this study were presented in summary form only (Song
et al., 1997).
The acute neurobehavioural effects (as determined by
observational tests of function and motor activity) of two carbamates,
carbaryl and aldicarb, and five organophosphates, chlorpyrifos,
diazinon, parathion, fenthion, and diisopropyl fluorophosphate, were
evaluated in 10-week-old male Long-Evans rats on the day of dosing at
the time of the peak effect, at 1 and 3 days, and 1 week after dosing.
All doses were administered by oral gavage in corn oil in a volume of
1 ml/kg bw, except carbaryl, which was given in 2 ml/kg bw. A weight
loss of about 10% was recorded with each substance only at the high
dose. Generally, all of the cholinesterase inhibitors induced
autonomic signs of cholinergic over-stimulation (salivation,
lachrymation, and miosis), hypothermia, mild tremors, mouth-smacking
(chewing motions), decreased motor activity, decreased tail-pinch
response, and altered neuromuscular function (gait changes and
increased foot splay). The measures found to be most sensitive on the
day of dosing were body temperature, motor activity, gait, and the
presence of mouth-smacking and fine tremors, although no single
measure was the most sensitive for all compounds. Differences in the
slopes of the dose-response curves were evident for some measures.
Many effects were still present at 24 h, but the rats recovered from
the effects of all compounds. Interestingly, residual effects were
found at 72 h with the carbamates and with the organophosphate
fenthion, but not with the other compounds; this may be due, at least
in the case of the carbamates, to slow absorption from the gut. Thus,
the overall clinical toxicity profile of these cholinesterase
inhibitors was similar, but compound-specific differences emerged in
terms of individual measures, dose-response relationships, and time
course; the behavioural effects induced by these compounds may be due
to both cholinergic and non-cholinergic mechanisms. Only summary data
were available for evaluation (Moser, 1995).
Chlorpyrifos was administered subcutaneously to neonatal rats at
2 mg/kg bw on postnatal day 1 or at 11 mg/kg bw per day on postnatal
days 6-9. The developing brain regions (brainstem, forebrain, and
cerebellum) were examined for signs of interference with cell
development by using markers of cell division. The 1-day-old rats
showed significant inhibition (about 15%) of DNA synthesis in all
brain regions within 4 h of treatment. Equivalent results were
obtained when a small dose (0.6 or 2 µg/g brain) was introduced
directly into the brain by intracisternal injection, indicating that
the effect was not secondary to systemic toxicity. Comparable
inhibition (> 10%) of DNA synthesis was seen in these animals at 8
days of age, but at this point there was regional selectivity, with
sparing of the cerebellum (about 1% inhibition). In another phase of
this study, 6-8-day-old animals were pretreated with mecamylamine, a
nicotinic receptor antagonist, and then with chlorpyrifos at 11 mg/kg
bw. The pretreatment caused a decrease in DNA synthesis and also
prevented any further decrement in DNA synthesis due to chlorpyrifos.
Administration of chlorpyrifos at 1 day of age caused severe (> 30%)
inhibition of protein synthesis throughout the brain; the effect was
distinct from that on DNA synthesis, as it had diminished
substantially by 8 days of age (< 10%) and did not show regional
selectivity. Assays of ornithine decarboxylase activity 4 and 48 h
after treatment with chlorpyrifos in 1- and 8-9-day-old rat pups
showed no significant alteration of activity in any brain region in
comparison with appropriate controls. The authors concluded that the
effects of chlorpyrifos on DNA and protein synthesis are not secondary
to generalized cell damage or suppression of cell metabolism, as
evidenced by the maintenance of normal ornithine decarboxylase
activity. The results of this study were presented only in summary
form (Whitney et al., 1995)
Pregnant Sprague-Dawley rats were given chlorpyrifos at doses of
0, 0.3, 1, or 5 mg/kg bw per day by gavage in corn oil from day 6 of
gestation through day 11 of lactation, and their neurobehavioural
performance was evaluated. A satellite group of mated females was
dosed similarly every day on days 6-20 of gestation and used to obtain
blood and brain samples for analysis of cholinesterase. This study was
conducted in accordance with GLP standards. There were no deaths and
only a few clinical signs (fasciculations, hyperpnoea,
hyper-reactivity, and decreased body-weight gain) in the dams at the
high dose. Between days 1 and 5 of lactation, three dams at 5 mg/kg bw
per day had total litter loss and an additional 57 pups died or were
cannibalized; these losses led to a decreased viability index.
Necropsy of many of the dead pups indicated treatment-related lack of
maternal care (no milk in the stomach). No similar effects of
treatment were seen at the other doses. In dams, cholinesterase
activity in plasma and erythrocytes was inhibited at all three doses,
with > 40% inhibition at 0.3 mg/kg bw per day. Brain cholinesterase
activity was inhibited at the two higher doses, but the inhibition at
1 mg/kg bw per day was not > 20% that of controls. Pups that were
exposed to chlorpyrifos in utero and for a period post partum
showed signs of toxicity at the high dose: the weights of male and
female pups were significantly lower than those at the other doses,
consistent with the decreased food consumption and lack of body-weight
gain by dams at this dose. Morphometric measurements of the brain and
brain sections of six pups of eachsex per dose showed a pattern of an
increase at the low dose and a decrease at the high dose, which was
more pronounced in male pups, paralleling the differences in brain
weight between the groups. Hence, the NOAEL for maternal toxicity was
1 mg/kg bw per day. The LOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.3 mg/kg bw per day, and the NOAEL for
inhibition of brain acetylcholinesterase activity was 1 mg/kg bw per
day. The NOAEL for toxic effects in the pups was 1 mg/kg bw per day on
the basis of the decreased viability index, relative brain weight, and
delayed sexual maturity, possibly associated with maternal toxicity
and subsequent diminished maternal care at the high dose. Cognitive
function (learning, memory, and habituation) in the pups were not
affected by treatment (Hoberman, 1998).
Adult male Long-Evans rats were trained to perform a test of
memory and motor function and were then injected subcutaneously with
single doses of 0, 60, 125, or 250 mg/kg bw of chlorpyrifos in peanut
oil (2 ml/kg bw) and tested on 5 days/week for 7 weeks. Unconditioned
behaviour was also rated for signs of cholinergic toxicity.
Cholinesterase activity was measured in whole blood and in several
brain regions, and muscarinic receptor density and the hypothermic
effect of oxotremorine challenge were determined. Clinical signs of
toxicity were seen in one rat at 250 mg/kg bw. Whole-blood
cholinesterase activity was inhibited by 60-75% in a dose-dependent
fashion on day 4 but had recovered to near control values by day 53 in
animals given 60 mg/kg bw and by day 74 in those given 125 mg/kg bw.
Brain cholinesterase activity was strongly inhibited in a
dose-dependent manner and was inhibited by < 95% on day 7 in
animals given 250 mg/kg bw. By day 21, partial recovery of
cholinesterase activity was seen in some brain regions after the doses
of 60 and 125 mg/kg bw, but no significant recovery was seen in
animals given 250 mg/kg bw. Muscarinic receptor density decreased with
dose and time. At 250 mg/kg bw, the receptor density in the
hippocampus, frontal cortex, and striatum had fallen to 70-75% of the
control values after 7 days and to 60% 21 days after dosing; the
hypothalamic receptors were less severely affected. In comparison with
controls, the hypothermia induced by oxotremorine challenge was
significantly less pronounced in each group receiving chlorpyrifos on
days 8 and 32, but was comparable to that of controls by day 52.
Unconditioned behaviour was relatively unaffected by treatment, with
fine tremor in the head and limbs, the only sign of cholinergic
overstimulation, peaking on day 9 and no longer observed by 14 days
after dosing. Functional deficits in working memory and motor function
appeared within 2 days of injection of a dose of chlorpyrifos that
caused severe cholinergic effects, but the animals recovered within
3 weeks. The definition of working memory in this paper was
non-specific, and the short-term memory of the animals was unaffected
by treatment (Bushnell et al., 1993).
The authors contrasted these findings to those seen in rats dosed
with the organophosphate diisopropyl fluorophosphate (Bushnell et al.,
1991). These rats showed progressive, persistent impairment of
cognitive and motor function over a 3-week period of daily exposure,
despite neurochemical and pharmacological evidence of tolerance to
diisopropyl fluorophosphate-induced inhibition of cholinesterase. This
difference suggests that the diisopropyl fluorophosphate-induced
behavioural changes cannot be attributed entirely to its effects on
cholinesterase activity and changes in muscarinic receptor binding.
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 200 mg/kg bw as a
single dose on day 12 of gestation and then killed on day 16 or 20 of
gestation or on postnatal day 3 for measurement of maternal and
developmental indicators of toxicity. While most treated dams showed
no overt cholinergic signs, 4/28 showed moderate-to-severe signs 2-3
days after treatment, and these rats were omitted from further
studies. Maternal body weight was decreased by 15% 3 days after
treatment, after which the body-weight increase was comparable to that
of controls. Chlorpyrifos did not significantly affect fetal body
weights or brain weights when assayed on day 16 or 20 of gestation.
Inhibition of acetylcholinesterase activity (by 82-88%) was seen in
maternal brain at all three times after exposures. At days 16 and 20
of gestation, fetal brain acetylcholinesterase activity was inhibited
by 42-44%. While some degree of recovery in acetylcholinesterase
activity was seen in pup brain by postnatal day 3, it was still
inhibited (by 30%) in treated pups cross-fostered by control dams.
Maternal brain muscarinic receptor binding at day 20 of gestation and
postnatal day 3 was more extensively reduced (30-32%) than in the
developing brain (16 and 11%, respectively). The response to a simple
postnatal reflex test of righting was transiently altered (present at
on day 1 but not on day 3) by exposure to chlorpyrifos. The results
suggested that acute exposure of dams during gestation induced more
extensive neurotoxicological effects in the dams than in their
developing fetuses, but the dose and the route of administration make
the relevance of this finding unclear (Chanda et al., 1995).
The maximum tolerated dose of chlorpyrifos in Sprague-Dawley rats
injected subcutaneously in peanut oil was 45 mg/kg bw in 7-day-old
pups and 280 mg/kg bw in adults (80-100 days old) (Pope et al., 1991).
At this dose, the neonates showed an initial transient decrease in
body weight when compared with controls. Adult rats also had an
initial decrease in body weight, but this had recovered by the end of
the 7-day observation period. Brain cholinesterase activity increased
by about 42% in neonates on days 7-14, whereas it remained constant in
adults. Neonatal brain cholinesterase activity was inhibited by 80% on
day 1 and by 45% on day 7, whereas 90% inhibition was seen in adults
on day 4, with little or no recovery. In plasma, inhibition of
cholinesterase activity was rapid and marked (95%) in pups on day 1
but had recovered to 50% of control by day 7. In adults, such
inhibition persisted through day 7, with no recovery of activity.
In a study to determine whether a measure of inhibitory potency
in vivo, the ED50, could be correlated with a measure of acute
toxicity, the maximum tolerated dose, in neonatal and adult rats,
chlorpyrifos was given by subcutaneous injection in peanut oil.
Cholinesterase inhibition was measured at the time of peak inhibition
(24 h in neonates and 4 days in adults). Depression of cholinesterase
activity versus log dose gave a straight line, and the slope of the
curve of inhibition of plasma cholinesterase was slightly steeper than
that of brain cholinesterase. The ED50 values for brain
cholinesterase activity were 20 (range, 17-23) in neonates and 44
(31-63) in adults, and those for plasma cholinesterase activity were
20 (13-31) in neonates and 70 (40-120) in adults. The correlation
between the two values was good for neonates but not for adult rats
(Pope & Chakraborti, 1992).
Groups of five 3-month old Sprague-Dawley rats were given
chlorpyrifos (purity, 98%) in peanut oil by subcutaneous injection at
a dose of 280 mg/kg bw, and trunk blood, cerebral cortex, and corpus
striatum were collected 2, 4, 6, and 12 weeks after dosing.
Acetylcholinesterase activity was assayed, and the binding of
[3H]quinuclidinyl benzilate to muscarinic receptors was measured in
membranes of the cortex and striatum. Locomotor activity was measured
for 30 min on 5 days/week. A challenge of scopolamine (1 mg/kg bw
intraperitoneally) was given 2 weeks after chlorpyrifos treatment and
then every two weeks for 12 weeks. Extensive inhibition of cortical
and striatal cholinesterase activity was seen 2 (94-96%), 4 (about
80%), 6 (about 60%), and 12 (15-20%) weeks after treatment. Plasma
cholinesterase activity was inhibited to a lesser extent, and recovery
was more rapid, with 85% inhibition at 2 weeks, 55% at 4 weeks, and
30% at 6 weeks; the activity was normal at 12 weeks. The changes in
cholinesterase activity were accompanied by reductions in muscarinic
receptor binding sites in the cortex (34, 33, and 18% reductions in
maximal binding) and striatum (48, 40, and 23% reductions) 2, 4, and 6
weeks after exposure. Tests of locomotor activity showed hypoactivity
in treated rats for 2-3 days after treatment. After the scopolamine
challenge, chlorpyrifos-treated rats showed increased activity at all
times except week 10 (Pope et al., 1992).
Chlorpyrifos was given to groups of six adult male Sprague-Dawley
rats by subcutaneous injection at a dose of 280 mg/kg bw, and the
animals were killed 2, 7, and 14 days after treatment. A significant
( p < 0.05), approximately 6% decrease in body weight was seen after
2-3 days, but the body weights recovered during the remainder of the
study. Chlorpyrifos caused marked inhibition of cholinesterase
activity in brain regions and plasma at all times, with about 90%
inhibition in the cortex, striatum, and plasma at day 2, and there was
virtually no recovery of this activity within the 14-day recovery
period. Reductions of < 55% in atropine-sensitive quinuclidinyl
benzilate binding (total muscarinic receptors) and binding in the
cortex and striatum were also observed (Chaudhuri et al., 1993).
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 25 mg/kg bw per day
on days 12-19 of gestation and killed on day 20 of gestation or
postnatal day 3. In a separate study to determine the dose-response
relationship, rats were similarly exposed to chlorpyrifos at 6.25 or
12.5 mg/kg bw per day on days 12-19 of gestation and killed on day 20
for analysis of various neurochemical markers. No maternal toxicity
was seen at any dose. Treatment with 25 mg/kg bw per day induced only
an initial, slight, transient decrease in maternal body weight.
Treatment from day 12 of gestation did not affect fetal weight at day
16 or 20, but pup weight on postnatal day 1 was significantly reduced
by treatment at this dose. Maternal brain cholinesterase activity
measured on day 20 of gestation was significantly inhibited in a
dose-related manner, with 75 and 90% inhibition at 6.25 and 25 mg/kg
bw per day, respectively. Fetal brain cholinesterase activity was less
severely inhibited, with 40 and 60% inhibition at the two doses,
respectively. Extensive inhibition of acetylcholinesterase activity,
to 10-17% of the control value, was seen in dams treated with 25 mg/kg
bw per day. Significant, dose-related downregulation of muscarinic
receptors in maternal and fetal brain was noted on day 20 of gestation
after exposure at all doses. The responses to righting reflex and
cliff avoidance tests were markedly altered after repeated exposures.
Generally, the neurochemical effects were more severe in dams than in
the developing fetuses, despite the greater sensitivity of fetal brain
cholinesterase to inhibition by chlorpyrifos. Comparison of these
results with those of an earlier study of acute exposure to
chlorpyrifos (Chanda et al., 1995) indicated that repeated exposure
causes greater down-regulation of muscarinic receptors than the
equivalent acute dose (Chanda & Pope, 1996).
Cats
Technical-grade chlorpyrifos dissolved in methylene chloride and
mixed in olive oil was given to adult male, colony-raised, short-hair
cats at doses of 0, 0.1, 0.5, 1, 5, 10, or 50 mg/kg bw to establish a
dose that would induce clinical signs of toxicosis but not death. Two
cats were used in this segment of the study, and each received
increasing doses of chlorpyrifos. On the basis of deaths at 50 mg/kg
bw, a dose of 40 mg/kg bw was selected for the next phase of the
study, in which three groups of six cats received corn oil,
chlorpyrifos, or chlorpyrifos followed by injections of atropine
sulfate every 12 h for 2 days. Two cats receiving the antidote also
received pyridine 2-aldoxime methane sulfonate every 12 h until the
clinical signs had abated. All cats given chlorpyrifos showed clinical
signs of toxicity, and one cat died 4 days later. Whole-blood and
plasma cholinesterase activity was reduced in all treated animals, but
the levels had returned to pre-test levels after 7-28 days. Brain
cholinesterase activity was normal in all animals that survived until
the end of the study, 28 days after treatment. Gross pathology
revealed no treatment-related lesions, and haematological parameters
were unaffected (Hooser et al., 1988).
Groups of five adult male cats received a single intramuscular
injection of corn oil (vehicle), diisopropyl fluorophosphate (positive
control; 5 mg/kg bw), or chlorpyrifos (purity, 99%; 300 mg/kg bw) and
were observed for 60 days. Atropine and pyridine 2-aldoxime methane
sulfonate were given to chlorpyrifos-treated animals once or twice
daily for 14-24 days. Ataxia was observed in positive controls (mean
onset, 16 days; range, 14-19 days) and in those given chlorpyrifos
(mean onset, 19 days; range, 17-21 days) . Functional deficits were
seen, characterized by a crouched-waddling gait, hypermetria, and
proprioceptive deficits, and these effects were still present in both
groups 56 days after exposure; the effects in chlorpyrifos-treated
animals began to subside after day 35. Lymphocyte neuropathy target
esterase activity was inhibited by < 96% in positive control
animals (24 h after dosing) and by 46% in chlorpyrifos-treated cats
(7 days after dosing) but had reached control levels by day 28 in the
latter group. Whole-blood cholinesterase activity was reduced by
< 86% by day 4 and slowly recovered to control levels by day 56.
Diisopropyl fluorophosphate had inhibited cholinesterase activity by
< 63% 24 h after dosing, but the recovery of activity was similar
to that in chlorpyrifos-treated animals. Histological examination
(Table 22) revealed degeneration of axons in animals treated with
chlorpyrifos and with diisopropyl fluorophosphate, and, while the
lesions were similar, they were more severe in chlorpyrifos-treated
animals. The effects were characterized by axonal degeneration with
vacuolar (spongy) changes in the white matter tracts of the cerebrum,
cerebellum, and spinal cord and occasionally in the peripheral nerves.
Vacuolization was seen, resulting from distension of axonal spaces
associated with focal axonal swelling and degeneration of myelin and
myelin-forming cells, with infiltration of snall numbers of
macrophages (Fikes et al., 1992). The route of administration and
magnitude of the dose in this study limit its usefulness.
(g) Studies on metabolites
(i) Acute toxicity
The main metabolite of chlorpyrifos, TCP, was generally of lower
acute toxicity than chlorpyrifos (Table 23).
(ii) Short-term studies of toxicity
Groups of 10 rats of each sex were fed diets containing 0, 0.01,
0.03, 0.1, 0.3, or 1% TCP for 90 days. The three lower doses had no
treatment-related effects on body-weight gain, mortality, food
consumption, haematological values, blood urea nitrogen, alkaline
phosphatase, or average body and organ weights. The NOAEL was 0.1% in
the diet, approximately 50 mg/kg bw per day, on the basis of reduced
body-weight gain at 1% (Beatty, 1964).
Groups of 15 rats of each sex were fed diets containing sodium
TCP at concentrations providing doses of 0, 10, 30, or 100 mg/kg bw
per day for 91 days. The dietary concentration was varied to allow for
increasing body weight and ranged from about 85, 250, and 825 ppm at
day 0 to about 160, 460, and 1540 ppm at day 84 at the three doses,
respectively. Significantly increased body-weight gain was observed in
males at 30 mg/kg bw per day from day 48 onwards. There were no
treatment-related changes in haematology, urinary analysis, or blood
chemistry values. The absolute weight of the heart was increased in
males at 100 mg/kg bw per day, and increased absolute and relative
liver and kidney weights were seen in animals of each sex at this
dose. There were no gross or histopathological alterations due to
treatment. The NOAEL was 30 mg/kg bw per day on the basis of increased
relative liver and kidney weights (Barna-Lloyd & Szabo, 1985).
Table 22. Occurrence and severity (score ± SD of histological changes in the nervous system of cats
given a single intramuscular injection of chlorpyrifos, diisopropyl fluorophosphate
(positive control), or corn oil
Location Treatment
Corn oil Diisopropyl fluorophosphate Chlorpyrifos
No. affected Score No. affected Score No. affected Score
Brain
Cerebruma 2/5 0.40 ± 0.49 4/5 0.80 ± 0.40 4/5 2.00 ± 0.63
Cerebellumb 1/5 0.20 ± 0.40 2/5 0.40 ± 0.49 3/5 1.00 ± 0.89
Spinal cord
C2 2/5 0.20 ± 0.49 4/5 0.80 ± 0.40 5/5 1.40 ± 0.49
C7 1/5 0.20 ± 0.40 3/5 0.60 ± 0.49 5/5 1.40 ± 0.49
T-L 4/5 0.80 ± 0.40 5/5 1.00 ± 0.0 4/5 0.80 ± 0.40
L4 0/5 0.00 ± 0.0 5/5 1.60 ± 0.49 5/5 1.80 ± 0.40
Sciatic nerve 0/5 0.00 ± 0.0 3/5 0.60 ± 0.49 1/5 0.20 ± 0.40
Severity of lesions (axonal swelling, vacuolar change, myelin degeneration) in each section was given the
score of the most severely affected area. The lesions were scored as 0 = normal or equivocal, 1 = mild
(1-5 nerve fibres affected/high power field), 2 = moderate (6-10 nerve fibres affected), 3 = marked
(11-15 nerve fibres affected), and 4 = severe change (> 20 nerve fibres affected).
a Anterior parietal cerebrum sectioned transversely immediately posterior to the optic chiasm and
anterior to the infundibulum
b Transverse section through the cerebellum and brain stem at the level of the trigeminal nerves
Table 23. Acute toxicity of 3,5,6-trichloro-2-pyridinol (TCP) administered orally
Species Strain Sex Vehicle LD50 (mg/kg bw Reference
and 95% CI or range)
Mouse Swiss M 0.5% hydroxypropylmethyl 380 (333-433) Gerbig & Emerson (1970a)
F cellulose (Methocel) 414 (367-469)
Rat Sprague-Dawley M 0.5% hydroxypropylmethyl 794 (709-889) Gerbig & Emerson (1970b)
F 870 (758-1009)
Doga Beagle NR Gelatine capsule > 1000 Gerbig & Emerson (1970c)
NR, not reported
a One dog received two doses of 1000 mg/kg bw TCP, a second received four doses of 500 mg/kg bw, and a third
received eight doses of 50 mg/kg bw. Emesis was seen in all animals within 1-3 h of dosing. There were no deaths.
Groups of two beagles of each sex were given diets containing TCP
at concentrations providing doses of 0, 26, or 80 mg/kg bw per day for
93 days. At termination, haematological parameters were normal. There
was no NOAEL as elevated serum alkaline phosphatase activity and
histopathological evidence of liver damage and increased testicular
weights were seen in the treated animals (JMPR, 1972; modified by
reference to the original report of Copeland, 1964).
Groups of three dogs of each sex were given diets containing TCP
at concentrations providing doses of 1, 3, 10, or 30 mg/kg bw per day
for 91 days. No toxic effects were seen at the three lower doses, but
at 30 mg/kg tbw per day evidence of liver toxicity was seen, with
increased activity of alkaline phosphatase and alanine and aspartate
aminotransferases although there was no unequivocal sign of liver
damage on histopathological examination (JMPR, 1972; modified by
reference to the original report of Emerson & Gerbig, 1970).
Groups of four beagles of each sex were fed diets containing TCP
at concentrations providing doses of 0, 3, 12, or 48 mg/kg bw per day
for 1 year in a study conducted according to GLP. No details of the
clinical observations were reported. The body weights of males were
not statistically significantly different between groups, but females
at the high dose had consistently lower body weights than controls and
statistically significantly lower body-weight gains. No changes in
food consumption were found in treated groups, and no alterations in
haematological or urinary parameters. The dose-related changes in
clinical chemistry values that were attributed to treatment were
increased serum alkaline phosphatase and alanine aminotransferase
activities in males and females at 3, 6, and 12 months. The values
were comparable to those of controls at 3 mg/kg bw per day,
consistently elevated but generally not to a statistically significant
degree at 12 mg/kg bw per day, and were statistically significantly
increased at 48 mg/kg bw per day at most times. There were no gross or
histopathological lesions that were associated with treatment and no
changes in absolute or relative organ weights. The NOAEL for systemic
effects was 12 mg/kg bw per day, on the basis of a lack of a
consistent, statistically significant increase in enzyme activities at
that dose (Zempel et al., 1987).
(iii) Genotoxicity
TCP was not genotoxic in a range of assays in vitro and
in vivo (Table 24).
(iv) Developmental toxicity
In a range-finding study, Fischer 344 rats were given TCP
(purity, 99.7%) at doses of 0, 43, 75, or 127.5 mg/kg bw per day on
days 6-15 of gestation by oral gavage. Clinical signs (perineal
soiling) were seen sporadically in all dams at the highest dose and in
some at 75 mg/kg bw per day. Food and water consumption were
unaffected by treatment. The signs of maternal toxicity were limited
to reduced body-weight gain in dams at the high dose. The relative and
Table 24. Results of tests for the genotoxicity of 3,5,6-trichloro-2-pyridinol
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 3.16, 10, 31.6, 99.7 Negativea,b Zempel & Bruce (1986)
TA98, TA100, 100, 316 µg/plate
TA1535, TA1537, in DMSO
TA1538
Unscheduled Rat hepatocytes 1-100 µg/ml in 99.7 Negativec Gollapudi (1987)
DNA synthesis (Fischer CDF 344) DMSO
Forward mutation Chinese hamster 62.5, 125, 250, 99.7 Negativea Linscombe & Gollapudi
ovary cells, hprt locus 500, 750 µg/ml (1986)
In vivo
Micronucleus Mouse (CD-1) 250, 500, 750, 99.9 Negative McClintock & Gollapudi
formation marrow cells 1000, 1500, 2000, (1989b; GLP)
3000 mg/kg bw
orally in corn oil
Micronucleus Mouse (CD-1) 0, 24, 76, 240 mg/kg 99.7 Negative Bruce et al. (1985)
formation marrow cells bw orally in
corn oil
GLP, good laboratory practice; DMSO, dimethyl sulfoxide
a With and without an exogenous metabolic system
b Toxic at 100 (some strains) and 316 (all strains) µg/plate
c Toxic at 50 and 100 µg/ml
absolute weights of the liver and kidney were unaffected by treatment,
and there was no observable effect on reproductive parameters
(Scortichini et al., 1986a).
Groups of Fischer 344 rats were given 0, 50, 100, or 150 mg/kg bw
per day of TCP (purity, 99.7%) on days 6-15 of gestation by oral
gavage in a study that conformed to GLP. No clinical signs of toxicity
were seen in any group. Slight vaginal bleeding occurred in one animal
at 50 mg/kg bw per day and two at 150 mg/kg bw per day on day 13. Food
consumption was slightly decreased in animals at 100 mg/kg bw per day
and decreased by about 10% in those at the high dose. Significant,
dose-related decreases in body-weight gain were recorded throughout
treatment at 100 and 150 mg/kg bw per day. Changes in the relative and
absolute liver and kidney weights were restricted to a significant
increase (4%) in the relative liver weight at 150 mg/kg bw per day,
consequent to a significant decrease (5%) in body weight at this dose.
There was a dose-related trend in the number of implantation sites per
dam, which was reflected in the number of fetuses per litter and
probably in gravid uterine weight; the latter trend may also reflect
the trend to lower body weight with higher dose. Fetal examination
revealed a small number of findings in each group, including controls.
Overall, treatment had no significant effect on reproductive
parameters or fetuses. The NOAEL for maternal effects was 50 mg/kg bw
per day on the basis of decreased body-weight gain, and that for fetal
toxicity and teratogenic effects was 150 mg/kg bw per day, the highest
dose tested (Hanley et al., 1987a).
In the first phase of a study in inseminated New Zealand white
rabbits, the animals were given TCP (purity, 99.7%) on days 6-18 of
gestation by oral gavage at doses of 0, 48.5, 80, or 130 mg/kg bw per
day. As there was uncertainty that the maximum tolerated dose had been
reached in this phase, additional groups of animals were given 130 or
210 mg/kg bw per day (phase 2). Maternal body-weight gain during
gestation was depressed but not statistically significantly and was
highly variable at the highest dose in both phases. Gross examination
of all animals showed no significant findings or any differences
between groups. At 210 mg/kg bw per day, resorptions were seen in all
litters, and the percent implantations resorbed and the percent
litters with resorptions were statistically significantly different
from those in the comparable control group in phase 2. There was no
observable effect of treatment on reproductive parameters at doses up
to 130 mg/kg bw per day (Scortichini et al., 1986b).
Groups of New Zealand white rabbits were given TCP (purity,
99.7%) by oral gavage on days 7-19 of gestation at doses of 0, 25,
100, or 250 mg/kg bw per day in a study that met GLP requirements.
Female animals were given injections of human chorionic gonadotropin
to synchronize estrus and were inseminated 3 weeks later, which was
presumed to be day 0 of gestation. No clinical signs of toxicity were
seen in any group. Water and food consumption were not reported. A
dose-related decrease in body-weight gain was seen during treatment,
which reached statistical significance at the high dose. There were no
treatment-related changes in the relative or absolute weights of the
liver or kidney. Fetal examination revealed a small number of
malformations in each group, including controls. Overall, there was no
statistically significant effect of treatment on reproductive
parameters or fetuses, but an increased incidence of central nervous
system malformations at the intermediate and high doses suggested a
possible teratogenic effect, and the authors concluded that these
findings provided evidence of teratogenic potential, despite a lack of
statistical significance. The malformations included severe dilatation
of the cerebral ventricles and hydrocephaly. The incidences of minor
external, visceral, or skeletal alterations were unchanged. The NOAEL
for maternal effects was 100 mg/kg bw per day on the basis of
decreased body-weight gain during treatment. The NOAEL for fetal
toxicity and teratogenic effects was 25 mg/kg bw per day, on the basis
of central nervous system malformations at higher doses (Hanley et
al., 1987b).
3. Observations in humans
3.1 Experimental studies
A commercial preparation of chlorpyrifos containing 35% xylene
was applied to the skin of the back and abdomen of four volunteers
(skin area not stated) at a dose of 5, 10, 25, or 50 mg/kg bw 20 (5
days/week), four, three, and two times, respectively. The material was
covered with an occlusive patch for 12 h, and after removal of the
patch each site was scored for irritation before washing. Each site
was used only once. Plasma and erythrocyte cholinesterase activity was
determined before and several times after treatment (exact times not
stated; for results, see Table 25). In another study, five volunteers
received dermal doses of 94-620 mg/kg bw for 12 h and showed similar
inhibition of erythrocyte and plasma cholinesterase activity 24, 48,
and 72 h after exposure, even at the highest dose. No skin irritation
was recorded. Plasma cholinesterase activity was significantly
inhibited with two of the dose regimens, the activity reaching a
minimum around 3 days after the last dose and then recovering to
control values within 4-10 days; erythrocyte cholinesterase activity
were not inhibited with any regimen (Kilian et al., 1970).
Four volunteers were exposed for 5 min to a formulation
containing 61.5% chlorpyrifos and 34.5% xylene from an ultra-low
volume cold aerosol fog generator delivering 3.8 L/h. Air sampling
showed a concentration of chlorpyrifos in the breathing space of about
108 mg/L (range, 83-133 mg/L). The subjects were exposed at a distance
of 8 m and wore plastic coveralls allowing exposure of the heads and
hands of two subjects and the heads, hands, and arms of the other two.
Exposure was terminated after 5 min because of the ocular and
pulmonary irritation induced. Plasma and erythrocyte cholinesterase
activity was not depressed 24 h after exposure (Pennington & Edwards,
1971).
Table 25. Cholinesterase activity after dermal dosing of humans (% of pre-treatment value)
Dose regimen End of dosing Day 1 Day 3
Plasma Erythrocytes Plasma Erythrocytes Plasma Erythrocytes
10 mg/kg bw for 4 × 12 h 99 102 97 103 97 100
50 mg/kg bw for 2 × 12 h 100 100 100 100 102 100
25 mg/kg bw for 3 × 12 h 67 98 48 98
5 mg/kg bw for 20 × 12 h 67 103 64 104 74 104
One subject per dose regimen
Groups of four healthy, adult, male volunteers received
chlorpyrifos in tablet form at doses of 0, 0.014, 0.03, or 0.1 mg/kg
bw per day for 27, 20, and 9 days, respectively, with breakfast.
Control subjects received a placebo for 48 days. Although not
specifically stated, the high dose appears to have been withdrawn
because of the severity of plasma cholinesterase inhibition on day 9.
No rationale was given for termination of treatment at the lower
doses. Heparinized blood samples were obtained from the subjects twice
weekly for determination of plasma and erythrocyte cholinesterase
activity until all values had returned to pre-treatment levels. As it
was not stated whether the blood samples were taken at the same time
on each sampling day, the interval between administration and sampling
is unknown. No indication was given of the frequency of observation
for clinical signs after treatment or how such observations were made.
A single subject at 0.1 mg/kg bw per day reported having a runny
nose, blurred vision, and a feeling of faintness on the final day of
dosing, and his plasma cholinesterase activity was found to be
inhibited by 70% when compared with pre-treatment levels. No decrease
in erythrocyte cholinesterase activity was seen in any subject during
the study, and no other treatment-related effects were noted. Rapid,
marked depression of plasma cholinesterase activity was observed in
subjects at the high dose, and the activity did not return to control
levels until about day 34. Similar results were obtained when the
plasma cholinesterase activity in these subjects was compared with the
baseline activity of the group before treatment. The mean plasma
cholinesterase activity of men at 0.03 mg/kg bw per day was inhibited
by > 20% on days 16-20 of treatment when compared with those given
placebo, and the activity did not return to control levels for several
weeks after cessation of treatment. The plasma cholinesterase activity
of men at 0.014 mg/kg bw per day was inhibited by 20% only on day 13
and was similar to the baseline activity from day 20 to day 27, even
with continued administration of chlorpyrifos. Two-way analysis of
variance indicated that the inhibition of mean plasma cholinesterase
activity was significant only at 0.1 mg/kg bw per day ( p < 0.05)
when compared with controls. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day, the highest dose
tested (JMPR, 1972; modified by reference to the original report of
Coulston et al., 1972).
Six white male volunteers aged 27-50 years were used in another
study. One volunteer was given a single oral dose of 0.5 mg/kg bw
chlorpyrifos in a lactose capsule, about 30 min after food, and about
1 month later the other volunteers received the same treatment and the
first volunteer was given a dermal dose of 0.5 mg/kg bw chlorpyrifos
dissolved in methylene chloride. Two weeks after the first dermal
dose, the first volunteer was given a further dermal dose of 0.5 mg/kg
bw in dipropylene glycol methyl ether. The other volunteers were given
a dermal dose of 5 mg/kg bw of chlorpyrifos in dipropylene glycol
methyl ether 4 weeks after their oral dose. All urine was collected
from volunteers from 24-48 h before dosing until 120 h after dosing.
No signs or symptoms of toxicity were reported in any of the men
during the study. After oral administration of 0.5 mg/kg bw, the
plasma cholinesterase activity of the first volunteer was inhibited by
71% from that before treatment and that of the other men by a mean of
85% within 12-24 h of treatment. The range of individual values was
not provided. Plasma cholinesterase activity had returned to > 80% of
the mean baseline value by 30 days after oral treatment. After dermal
application on day 30, the plasma cholinesterase activity had
decreased to approximately 70% of baseline, and returned to about
80-90% of those levels by day 40 of the study. The intra-group
variation was stated to be considerable, but the figures were not
provided. Erythrocyte cholinesterase activity was not significantly
inhibited by either oral or dermal doses of chlorpyrifos (Nolan et
al., 1982, 1984).
In a double-blind, randomized, placebo controlled study of the
effects of single oral doses of chlorpyrifos (purity, 99.8%), groups
of six fasted men and women aged 18-55 received doses of 0, 0.5, 1, or
2 mg/kg bw in lactose powder. The study was conducted in two phases
separated by 14 days. The volunteers were dosed with 0, 0.5, or 1
mg/kg bw in the first phase and the results were assessed before
administration of 0 or 2 mg/kg bw in the second phase. Blood samples
were collected 10 and 0 h before treatment and 2, 4, 8, 12, 24, 36,
48, 72, 96, 120, 144, and 168 h after treatment and analysed for
erythrocyte cholinesterase activity and chlorpyrifos and its
metabolites. In addition, all urine voided from 48 h before dosing to
168 h after dosing was collected at 12- or 6-h intervals and analysed
for chlorpyifos and metabolites. Haematology, clinical chemistry,
urinary analysis and a brief physical examination were performed at
completion of the study. Blood and urine were collected to determine
each volunteer's paraoxonase status and the concentrations of
chlorpyrifos and metabolites, but these data have not yet been
reported. The volunteers were screened for general health according to
set criteria and instructed to refrain from alcohol, strenuous
exercise, and prescription medications before and during the study.
The doses were taken by capsule after an overnight fast. The health
status of subjects was monitored closely; vital signs (blood pressure,
pulse, respiration, and temperature) were assessed before dosing and
1, 2, 4, 8, 12, 24, 48, and 168 h after treatment. The subjects were
questioned about their well-being at each sampling time, and the
symptoms were evaluated clinically. The subjects were aware of the
signs and symptoms of cholinergic toxicity and were instructed to
inform the study physician of any adverse effects. The subjects were
unaware of their treatment, and the signs and symptoms were assessed
and treated by a physician who was also unaware of the treatment
status of the subject.
There were no significant deviations from the study protocol. One
male control in phase 1 and one woman receiving 2 mg/kg bw did not
provide a complete series of blood and urine samples. Treatment had no
effect on general health or on clinical chemical parameters measured 7
days after dosing. The only treatment-related effect was found in the
woman who withdrew from the study, who had decreased erythrocyte
cholinesterase activity when compared with her pre-treatment values at
most sampling times, with 98.4% of the pretreatment value at 4 h after
dosing, 77% at 8 h, 72% at 12 h, 74% at 24 h, 81% at 36 h, and 80% at
48 h (Table 26). When the data for this subject are removed from the
analysis, the mean for women receiving 2 mg/kg bw is indistinguishable
from the value for concurrent controls (Table 26). The NOAEL for
clinical signs or symptoms was thus the highest dose tested, and the
NOAEL for inhibition of erythrocyte cholinesterase activity was 1
mg/kg bw on the basis of significant inhibition in one of 12 subjects
(Kisicki et al., 1999).
3.2 Case reports
Blondell & Dobozy (1997) summarized the case reports, case
series, statistical surveys, and epidemiological studies of acute and
chronic health effects reported to be related to exposure to
chlorpyrifos. The authors noted the limitations of their review,
including inadequate documentation of exposure and effects, reporting
biases, and absence of information on the denominator population at
risk. They concluded, largely on the basis of an examination of
reports from poison control centres, that chlorpyrifos is one of the
leading causes of acute insecticide poisoning in the USA. Certain uses
were considered to pose greater risks than others, the main concern
being use of liquid formulations in households or by pest control
operators indoors or outdoors, termite treatment, and application of
liquid sprays and dips to domestic animals. Most of the more serious
cases were associated with misuse or inappropriate use such as spills
and inadvertent contamination by a pest control operator.
Shurdut et al. (1997) commented that deficiencies in the report
of Blondell & Dobozy (1997) invalidated its use for reaching a
conclusion about the safety of chlorpyrifos. They concluded that the
data from poison control centres had been misinterpreted and in fact
indicated the relative safety of products containing chlorpyrifos.
They also claimed that the report failed to respect the limitations of
anecdotal information, failed to consider the extensive testing with
regard to neurological damage and the long history of use of products
containing chlorpyrifos, made inappropriate use of anecdotes and
studies of organophosphates to characterize the safety of
chlorpyrifos, failed to consider the extensive database on exposure
after use of chlorpyrifos products, and presented the data
selectively.
In a case report, a previously well 3-year-old boy was found
playing near an open, spilled bottle of insecticide containing
chlorpyrifos (concentration not stated). The authors noted that
clinical manifestations of organophosphate-induced polyneuropathy
usually begin 1-3 weeks after an acute cholinergic crisis and that the
results of examination of the child were consistent with distal,
symmetric, predominantly motor polyneuropathy. In this patient, the
symptoms of cholinesterase inhibition and manifestations of weakness
and areflexia 11 days after ingestion and electromyographic findings
confirmed the presence of acute transient polyneuropathy with a more
Table 26. Mean erythrocyte cholinesterase activity as percent of mean baseline values in volunteers given single oral
doses of chlorpyrifos
Dose Time in relation to dosing (h)
(mg/kg bw)
- 10 0 4 8 12 24 36 48
Women
0.0a 99.5 (2.1) 100.5 (2.1) 98.3 (2.0) 102.1 (2.4) 100.7 (1.8) 99.7 (3.9) 98.4 (1.7) 100.2 (2.3)
0.0b 99.2 (4.4) 100.8 (4.4) 102.5 (5.9) 103.1 (5.4) 98.4 (3.2) 99.4 (3.7) 94.6 (5.3) 97.7 (5.1)
0.5 101.2 (6.7) 98.8 (6.7) 96.3 (6.5) 98.1 (2.1) 95.8 (3.9) 97.0 (3.6) 94.7 (4.7) 97.1 (4.1)
1 98.2 (0.9) 101.8 (0.9) 104.6 (4.2) 100.8 (3.5) 98.9 (3.7) 100.9 (5.6) 96.0 (4.1) 98.9 (2.1)
2 99.8 (1.8) 100.2 (1.8) 100.3 (3.2) 96.6 (6.81) 91.1 (9.7) 95.2 (10.6) 95.9 (7.4) 94.5 (7.7)
Men
0.0a 100.6 (1.8) 99.4 (1.8) 99.1 (2.2) 99.6 (4.6) 99.8 (4.3) 98.4 (4.3) 97.6 (2.8) 98.2 (3.1)
0.0b 96.1 (5.1) 103.9 (5.1) 101.6 (4.0) 102.5 (5.2) 102.9 (5.8) 102.6 (2.3) 98.2 (3.8) 95.9 (6.8)
0.5 98.7 (1.1) 101.3 (1.1) 102.0 (3.0) 103.4 (4.9) 99.7 (3.0) 101.9 (3.3) 98.1 (3.0) 92.8 (4.7)
1 98.6 (1.9) 101.4 (1.9) 104.5 (7.8) 101.1 (4.2) 101.1 (4.7) 101.7 (4.7) 98.5 (4.6) 92.1 (2.0)
2 101.2 (2.3) 98.8 (2.3) 99.2 (3.1) 98.9 (3.5) 98.7 (4.0) 99.2 (2.6) 99.5 (5.1) 98.4 (3.1)
a Phase 2 control
b Phase 1 control
proximal distribution than is usually seen in adults. The acute,
reversible bilateral vocal cord paralysis reported in this patient had
not been seen previously in organophosphate-induced delayed
neuropathy, but the authors noted that the onset of stridor and vocal
cord paralysis coincided with the onset of muscle weakness and
resolved at the same time as normalization of the peripheral
neuropathy (Aiuto et al., 1993a).
Gutmann & Bodensteiner (1993), responding to the report of Aiuto
et al. (1993), suggested that this case may not be a clear example of
organophosphate-induced delayed neuropathy. They noted that
chlorpyrifos has been associated with such symptoms only after severe
intoxication characterized by extensive cholinesterase inhibition. The
inhibition of plasma cholinesterase activity in the reported case was
instead suggestive of mild chlorpyrifos intoxication. They also noted
that the clinical findings associated with organophosphate-induced
delayed neuropathy are predominantly motor polyneuropathy, flaccid
weakness and atrophy primarily in the distal limb muscles, and often
incomplete recovery usually after months or years. The case report
implied that the evoked compound muscle action potentials and sensory
nerve action potentials were of normal amplitude, which would run
counter to a diagnosis of organophosphate-induced delayed neuropathy.
In response to these comments, Aiuto et al. (1993b) noted that
the patient's symptoms were consistent with > 90% inhibition of
cholinesterase activity and that the plasma cholinesterase activity
did not necessarily correlate with the severity of poisoning. The
authors concluded that the patient had developed delayed,
life-threatening neuropathy, although they were unable to determine
the mechanism of the condition.
In another case report, a 38-year-old man drank an undefined
quantity of a solution of 25% chlorpyrifos. He was treated with 3 mg
of atropine intravenously every 2 h for 6 days (total, 400 mg), but
any attempt to stop the atropine treatment resulted in reappearance of
respiratory distress. After 6 days, the patient was stuporous,
catatonic, and suffering from respiratory distress with white mucous
secretions, and no cholinesterase activity could be detected in his
serum. When ipratropium was administered endotracheally at a dose of
0.5 mg as an aerosol mist over 10 min, the patient's clinical
condition improved, although the salivary secretion continued. The
patient continued to receive ipratropium at 1-2 mg/day, and
respiratory support was discontinued on the fifth day. Severe
catatonia and coarse tremor were relieved by administration of
dantrolene at 10 mg intravenously, followed by 25 mg orally, three
times daily. Cholinesterase activity was still undetectable in serum
1 month after the man was discharged (Shemesh et al., 1988).
In a case in which a 26-year-old man intentionally ingested
approximately 360 ml of a 6.7% chlorpyrifos formulation containing
2,4-D (10.8%) and mecoprop (11.6%) and a few granules of a warfarin
(0.025%) concentrate, the main clinical findings on admission were
coma, myoclonus, miosis, cardiac arrythmia with progression to
hypotension, oliguria, and death after several episodes of asystole.
The authors stated that such symptoms are consistent with those seen
in patients who have ingested chlorophenoxy acetic acids such as 2,4-D
and mecoprop. The patient did not show many of the usual signs of
organophosphate poisoning, such as lachrymation, salivation,
respiratory paralysis, or muscle fasciculation, but his erythrocyte
cholinesterase activity was inhibited by about 70% at 13 h and by
< 90% by 26 h. Plasma cholinesterase activity was inhibited
completely at all times. Lymphocyte neurotoxic esterase activity was
inhibited by about 50% after 13 h but was within normal limits after
19 h. The activity of neurotoxic esterase in the cerebral cortex was
within normal limits but that in peripheral nerves was inhibited by
about 70% when compared with normal levels. The inhibition of
neurotoxic esterase activity was found in the absence of significant
signs of organophosphate intoxication (Osterloh et al., 1983).
A 21-year-old man who ingested an unknown quantity of a
chlorpyrifos formulation required large amounts of atropine and
pyridine 2-aldoxime methane sulfonate, endotracheal intubation, and
ventilation to overcome the cholinergic effects, which consisted of
pupillary constriction, excess secretions, tachycardia, and impaired
consciousness. Bilateral vocal cord paralysis developed 16 days after
extubation (28 days after exposure), and this finding was considered
to be indicative of organophosphate-induced delayed neuropathy. The
patient was asymptomatic within 4 weeks of the onset of symptoms (de
Silva et al., 1994).
A 42-year-old man who attempted suicide by drinking a commercial
formulation of 41% chlorpyrifos (estimated dose, 300 mg/kg bw) went
into coma and showed respiratory insufficiency, lachrymation,
salivation, sweating, miosis, fasciculations, and bronchorrhoea on
admission to hospital 18 h after exposure. A chest X-ray showed right
basal bronchopneumonitis, electroencephalographic signs of diffuse
irritation were recorded, and chlorpyrifos was detected in gastric
lavage fluid. Plasma cholinesterase activity was inhibited by almost
100% by 36 h. The coma and cholinergic signs persisted for 17 days,
during which time atropine, pyridine 2-aldoxime methane sulfonate,
mechanical respiration, and antibiotics were used. The patient was
asymptomatic on day 24 after exposure, and drug administration was
stopped; no signs of peripheral or central nervous system involvement
were observed. On day 30, lymphocytic neurotoxic esterase, blood
acetylcholinesterase, and plasma butyryl cholinesterase activities
were markedly below the normal range but recovered slowly thereafter.
On day 43, weakness and paraesthesia in the legs with reduced tendon
reflexes and sensory conduction velocity were noted. By day 62, the
leg weakness was more severe, gait was impaired, and tendon reflexes
were absent. Electromyography of the leg muscles showed symmetrical
signs of denervation with spontaneous activity, and motor conduction
velocity was reduced. Electrophysiological studies of the arms
confirmed a symmetrical reduction of sensory conduction velocity of
the ulnar nerves. Sural nerve biopsy on day 63 revealed a few altered
myelinated fibres. Aspects of axon and myelin degeneration were also
found occasionally. The changes were consistent with mild distal
axonopathy. Chlorpyrifos was still detectable 10 days after exposure,
and neurotoxic esterase activity was still inhibited by 60% 4 weeks
after exposure (Lotti et al., 1986).
In a study in which toxicological and/or electrophysiological
examinations were conducted in 11 patients who had acute
organophosphate poisoning, three of the patients, one of whom had been
poisoned with chlorpyrifos, developed organophosphate-induced delayed
neuropathy (Moretto & Lotti, 1998). The findings in this individual
were reported by Lotti et al. (1986). The electro-physiological data
indicated organophosphate-induced delayed neuropathy with sensory and
motor components which persisted for 3 months, at which time the
patient was lost to follow-up.
Four incidents of birth defects allegedly associated with
exposure to chlorpyrifos were reported. The children were found to
have a range of birth defects, including ventricular, ocular, and
palate defects and growth retardation (all children), hydrocephaly,
microcephaly, mental retardation, blindness, hypotonia, widely spread
nipples, and deformities of the teeth, ears, and external genitalia.
The mothers of the affected children had reportedly been exposed to
chlorpyrifos either at the workplace or at home during the pregnancy.
Two of the children were born to the same mother. The exposure of the
mothers to chlorpyrifos was poorly characterized, and it was not
indicated whether it was severe or sustained during organogenesis. The
range of birth defects reported was not consistent with the results of
studies in laboratory animals (Sherman, 1996).
In a comment on this paper, Gibson (1996) noted that the medical
records of the cases indicated that the same effects were not seen in
all children and that the effects in some of the children were
consistent with a diagnosis of an autosomal recessive birth defect
syndrome of the brain and eye known as cerebro-oculo-facio-skeletal
syndrome or a closely related one known as the 'MICRO' syndrome.
Eight cases were reported in which exposure to chlorpyrifos was
claimed to have caused sensory neuropathy. The patients each presented
with a range of symptoms, and characterization of exposure was poor.
One patient who reported signs of cholinesterase inhibition, including
lachrymation, muscle twitching, and diarrhoea, was a pesticide
applicator who had reportedly been exposed to chlorpyrifos in a closed
environment for 6 months. The patient's erythrocyte cholinesterase
activity was reportedly to be 'low' initially but returned to normal
levels within 2 months. Neurological evaluations were conducted only 6
weeks after the other symptoms were reported; at this time, sensory
loss of all modalities was found in a 'stocking-glove distribution'
test, with mild distal weakness and areflexia in the lower
extremities. Studies of nerve conduction and quantitative sensory
threshold revealed changes consistent with peripheral neuropathy of
the distal anonopathy type. The decreased reflexes and mild distal
weakness were consistent with polyneuropathy. Follow-up examination at
1 year revealed remission of all symptoms. In the other seven patients
(four from the same family), the symptoms were nonspecific, and the
absence of symptoms of cholinesterase inhibition suggested that these
patients had not been as exposed to chlorpyrifos as extensively as the
first patient. The lack of immediate electrodiagnostic testing, the
poor exposure characterization, and the variation in the reported
clinical findings make interpretation of the case studies difficult.
Additionally, subjective questioning was used to obtain information
from the patients. Follow-up examinations for a range of parameters
were not conducted on several patients, and the patients did not
undergo the same initial testing regimen. Thus, one patient was
exposed to chlorpyrifos occupationally at a level sufficient to
decrease cholinesterase activity and cause cholinergic symptoms; he
also showed evidence of mild, reversible polyneuropathy, possibly in
the presence of decreased erythrocyte cholinesterase activity. For two
other patients, there was limited evidence of mild polyneuropathy
(Kaplan et al., 1993).
3.3 Monitored field trials
A monitored field test was used to determine the effect of
chlorpyrifos on spraymen applying treatment at the rates used in
programmes for the eradication of mosquitoes. Three studies were
conducted in 1966, 1967, and 1968. Cholinesterase activity was
measured, and exposure was terminated if the inhibition reached 50% of
the baseline values. In the first study, three spraymen showed marked
depression of plasma cholinesterase activity, with reductions of
68-82% from baseline values. The two remaining spraymen showed
reductions of 52-56% over 9 days, but the baseline values were not
available. The study was discontinued after 2 weeks because of the
significant decrease in cholinesterase activity. In the second study,
no treatment-related effect on cholinesterase activity was seen during
a short period. In the third study, erythrocyte cholinesterase
activity was not affected by treatment, but plasma cholinesterase
activity was reduced by 50-80% in spraymen using a suspension
formulation. The activity had generally returned to pre-exposure
levels within 2 months of cessation of use. Four spraymen using an
emulsion formulation did not show reduced plasma cholinesterase
activity during the exposure period (Kenaga, 1967; Eliason et al.,
1969).
3.4 Studies of morbidity
In a study in which the sample size was small and the statistical
power was limited, comparisons were made between 175 employees
potentially exposed to chlorpyrifos at a manufacturing plant where
they had held jobs between 1 January 1977 and 31 July 1985 and 335
unexposed controls matched on age (within 5 years), date of hire
(within 5 years), sex, race, and pay status. The source of information
for the observations (self, nurse, company physician, private
physician, laboratory) was recorded, as were use of tobacco, alcohol,
and cholinergic drugs. Exposure was grouped into high, moderate, and
low on the basis of industrial hygiene surveys and consultation with
veteran manufacturing personnel. The plasma cholinesterase activities
of the potentially exposed employees before exposure were available,
and follow-up was conducted at roughly monthly intervals. The mean
inhibition of plasma cholinesterase activity was 19 ± 2.9% in the
group with low exposure, 32 ± 2.8% in that with moderate exposure, and
32 ± 5.3% in that with high exposure. No cases of peripheral
neuropathy were recorded in the study group. Inhibition of plasma
cholinesterase activity was observed but was not associated with
illness. The exposed groups reported symptoms of dizziness, malaise,
and fatigue more frequently than the unexposed group, but the analysis
did not indicate any correlation with increasing exposure (Brenner et
al., 1989).
In a follow-up to this study (Burns et al., 1998), the data were
updated to include the period 1987-94, and additional medical
disorders were considered. Data on self-reported paraesthesia were
collected from 1982 to 1994. The prevalence of peripheral neuropathy
was not significantly increased in the group of workers exposed to
chlorpyrifos.
Comments
After oral administration to rats, radiolabelled chlorpyrifos was
rapidly and extensively absorbed (up to about 90% of the dose) and
eliminated, predominantly in the urine (68-93%) and faeces (6-15% of
the dose), within about 72 h of administration. The urinary
metabolites included the glucuronide (about 80%) and sulfate (about
5%) conjugates of chlorpyrifos, and 3,5,6-trichloro-2-pyridyl
phosphate (TCP; about 12%). The tissue concentrations of residues of
[14C]chlorpyrifos were very low (generally < 1 ppm) within 72 h of
dosing. The longest half-time of residues in rats was 62 h in fat, and
low levels were also detected in the fat of several other species and
in the milk of goats.
In humans who were poisoned with chlorpyrifos formulations,
diethylphosphorus metabolites were excreted in the urine by
first-order kinetics, with an average elimination half-time of 6.1 ±
2.2 h in the fast phase and of 80 ± 26 h in the slow phase. In
volunteers, the time to maximal concentration of 3,5,6-TCP in the
blood was 0.5 h after oral dosing and 22 h after dermal treatment, but
the elimination half-time by both routes was 27 h, and the percentage
of the administered dose recovered from the urine was 70% after oral
dosing and 1.3% after dermal administration.
Chlorpyrifos is rapidly metabolized by mixed-function oxidases to
the highly reactive chlorpyrifos oxon by oxidative desulfuration. The
oxon can be deactivated by hydrolysis to diethylphosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is hydrolysis
to monoethyl 3,5,6-trichloro-2-pyridinyl phosphorothioate.
The lowest oral LD50 value was 96 mg/kg bw (range, 96-475 mg/kg
bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in mice. Female
rats were generally more sensitive to the acute effects of
chlorpyrifos than males. The signs of acute intoxication with
chlorpyrifos were consistent with cholinesterase inhibition. The acute
dermal LD50 of chlorpyrifos was > 2000 mg/kg bw in rats and > 1200
mg/kg bw in rabbits.
WHO (1999) has classified chlorpyrifos as 'moderately hazardous'.
Chlorpyrifos was irritating to the eye and skin of rabbits, but
it did not sensitize the skin of guinea-pigs in Magnusson-Kligman
maximization or Buehler tests.
In short-term studies, the NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day in dogs and 0.1
mg/kg bw per day in rats. The NOAEL for inhibition of brain
cholinesterase activity was 1 mg/kg bw per day in dogs and rats. The
signs of toxicity were largely limited to cholinergic signs and
decreased body weights and/or food consumption. The NOAEL for these
effects in short-term studies was 1 mg/kg bw per day in rats, and the
NOAEL for clinical signs was 3 mg/kg bw per day in dogs. In mice,
ocular effects and histopathological alterations (including adrenal
lipogenic pigmentation and ocular keratitis) were observed (NOAEL, 50
ppm; equal to 7 mg/kg bw per day). In rats, the NOAEL for increased
fatty vacuolation of the adrenal zonal fasciculata and changes in
haematological and clinical chemical parameters was 5 mg/kg bw per
day. When rats received chlorpyrifos dermally for 21 days, the NOAEL
for inhibition of cholinesterase activity in erythrocytes and brain
was 5 mg/kg bw per day.
In long-term studies, inhibition of cholinesterase activity was
again the main toxicological finding in all species. In rats, the
NOAEL was 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity and 1 mg/kg bw per day for inhibition of
brain acetylcholinesterase activity, but clinical signs were not seen
at doses up to 10 mg/kg bw per day, and the NOAEL for reduction in
body weight was 1 mg/kg bw per day. In mice, erythrocyte and brain
acetylcholinesterase activities were inhibited at 50 ppm, equal to 6.1
mg/kg bw per day, and the NOAEL was 5 ppm, equal to 0.7 mg/kg bw per
day. Cholinergic signs and reductions in body weight were reported
only at the highest dietary concentration of 250 ppm (equal to 32
mg/kg bw per day). Other treatment-related findings included effects
on the liver in mice, with a NOAEL of 50 ppm (equal to 6.6 mg/kg bw
per day), and increased adrenal weight in rats with a NOAEL of 1 mg/kg
bw per day. There was no treatment-related increase in the incidence
of neoplastic lesions in any of the long-term studies. The Meeting
concluded that chlorpyrifos is unlikely to pose a carcinogenic risk to
humans.
Chlorpyrifos was not genotoxic in an adequate range of studies
in vitro and in vivo. The Meeting concluded that chlorpyrifos is
not genotoxic.
In multigeneration studies of reproductive toxicity in rats, the
treatment-related effects of chlorpyrifos were limited to inhibition
of cholinesterase activity, consistent with that seen in other short-
and long-term studies, and fetotoxicity characterized by reduced pup
viability, body weights and survival. No significant,
treatment-related clinical signs were reported. The NOAEL for
inhibition of maternal acetylcholinesterase activity was 0.1 mg/kg bw
per day for erythrocytes and 1 mg/kg bw per day for brain. The NOAEL
for developmental toxicity was 1 mg/kg bw per day. No effects on
reproductive parameters were observed at the highest dose tested,
5 mg/kg bw per day.
In studies of developmental toxicity in mice, rats, and rabbits,
the maternal effects included inhibition of erythrocyte and/or brain
acetylcholinesterase activity and cholinergic signs (lowest NOAEL,
1 mg/kg bw per day in rats and mice) and reductions in body weight and
food consumption (lowest NOAEL, 2.5 mg/kg bw per day in rats). The
observed fetal toxicity (lowest NOAEL, 2.5 mg/kg bw per day in rats)
and developmental toxicity (NOAEL, 1 mg/kg bw per day in rats) were
consistent with treatment-related maternal toxicity; there was no
evidence of treatment-related malformations in any of the studies.
There was no effect on cognitive function (learning, memory, and
habituation) in pups exposed to chlorpyrifos in utero and for a
period post partum at doses up to and including the highest dose of
5 mg/kg bw per day, while inhibition of cholinesterase activity,
decreased brain weight, and delayed development were seen at lower
doses, consistent with findings in other studies.
In studies of delayed neurotoxicity, chlorpyrifos was given to
chickens as either single or repeated doses. Significant inhibition of
both cholinesterase and neuropathy target esterase activity was
observed, and mild delayed neuropathy was seen in a number of studies;
aggressive antidotal therapy was always necessary to allow at least
some of the treated birds to survive. Despite the marked cholinergic
toxicity of chlorpyrifos, there was no evidence that it caused delayed
neurotoxicity, and there was no increase in the incidence of
histopathological lesions in the nerve tissues of birds treated at
doses up to 10 mg/kg bw per day for up to 91 days. In a number of
studies in rats given single doses of up to 100 mg/kg bw, repeated
doses of up to 10 mg/kg bw per day for 4 weeks, or repeated doses of
up to 15 mg/kg bw per day for 13 weeks, there were no
treatment-related neurological lesions or effects on cognition and no
inhibition of neuropathy target esterase activity, although
significant inhibition of erythrocyte, brain, and peripheral tissue
cholinesterase activity was seen at some doses. In a study that
included a functional observational battery of tests, clinical signs
of intoxication were observed after a single dose only when brain
acetylcholinesterase activity was inhibited by more than 60% or when
whole-blood cholinesterase activity was inhibited by more than 80%.
When chlorpyrifos was applied as a single dose of up to 5 mg/kg
bw to the skin of volunteers for 12 h, erythrocyte cholinesterase
activity was not significantly inhibited. Plasma cholinesterase
activity was inhibited after 20 12-h dermal exposures to 5 mg/kg bw
per day over 4 weeks or after three daily 12-h exposures to 25 mg/kg
bw per day on consecutive days, but erythrocyte cholinesterase
activity was not inhibited under any treatment regimen.
A single oral dose of up to 1 mg/kg bw or repeated doses of up to
0.1 mg/kg bw per day for 9 days did not significantly inhibit
erythrocyte acetylcholinesterase activity in volunteers. No clinical
signs were observed in these studies. Inhibition of erythrocyte
acetylcholinesterase activity was observed in a single female
volunteer (of a group of six men and six women) given a single oral
dose of 2 mg/kg bw.
In a case of human poisoning with chlorpyrifos at an estimated
dose of 300-400 mg/kg bw, significant inhibition of neuropathy target
esterase in lymphocytes and of plasma and erythrocyte
acetylcholinesterase activity was reported, with severe cholinergic
signs which required aggressive, extensive antidotal therapy and
artificial ventilation. Mild distal axonopathy consistent with
organophosphate-induced delayed polyneuropathy was reported some weeks
after the poisoning incident.
The ADI of 0-0.01 mg/kg bw established by the 1982 Meeting was
based on a NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in humans. The present Meeting affirmed
this ADI on the basis of the NOAEL of 1 mg/kg bw per day for
inhibition of brain acetylcholinesterase activity in studies in mice,
rats, and dogs, using a 100-fold safety factor, and on the basis of
the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in the study of human subjects exposed
for 9 days, using a 10-fold safety factor.
The Meeting allocated an acute reference dose of 0.1 mg/kg bw on
the basis of the NOAEL of 1 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity in a study in which volunteers received
a single oral dose of chlorpyrifos, with a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 0.7 mg/kg bw per day (toxicity in a 79-week
study of toxicity and carcinogenicity)
1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
10 mg/kg bw/day (fetal toxicity in a study of developmental
toxicity)
Rat: 1 mg/kg bw per day (toxicity in 2-year studies of toxicity
and carcinogenicity)
1 mg/kg bw per day (developmental and parental toxicity in a
two-generation study of reproductive toxicity)
1 mg/kg bw per day (maternal and developmental toxicity in a
study of developmental toxicity)
2.5 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Rabbit: 81 mg/kg bw per day (maternal and fetal toxicity in a study
of developmental toxicity)
Dog: 1 mg/kg bw per day (toxicity in a 2-year study of toxicity)
Human: 0.1 mg/kg bw per day (no inhibition of erythrocyte
cholinesterase activity at highest dose tested in men dosed
orally for 9 days)
1 mg/kg bw (inhibition of erythrocyte cholinesterase
activity in adult volunteers after a single oral dose)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to chlorpyrifos
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Up to 90% in rats within 72 h; about 70% in humans within 96 h
Dermal absorption Less than 2% in humans within 180 h; not determined in animals
Distribution Initially widely distributed; highest residues in liver, kidneys
and fat at 72 h in rats
Potential for accumulation Elimination half-times of < 24 h and low tissue residues after
72 h in rats. No evidence of potential for accumulation
Rate and extent of excretion > 95% within 72 h in rats, mainly in urine (68-93%) and faeces (6-15%)
Metabolism in animals Rapidly metabolized by mixed-function oxidases to chlorpyrifos oxon
via oxidative desulfuration and an electrophilic phosphooxathiiran
intermediate. Degradation by conversion directly to
3,5,6-trichloro-2-pyridyl phosphate and diethyl thiophosphate.
The oxon is hydrolysed to diethyl phosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is by
hydrolysis to monoethyl-3,5,6-trichloro-2-pyridinol phosphorothioate.
Toxicologically significant compounds Parent compound and oxon
(animals, plants and environment)
Acute toxicity
Rat,, LD50, oral 96 mg/kg bw
Rat LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 36 mg/m3 (4 h, vapour, nose-only exposure)
560 mg/m3 (4 h, nebulized particles < 5 mm, whole-body exposure)
Rabbit, dermal irritation Slightly irritating
Rabbit, ocular irritation Slightly irritating
Guinea-pig, dermal sensitization Not sensitizing
Short-term toxicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest critical oral NOAEL 1 mg/kg bw per day, dog, 2 years
1 mg/kg bw per day, rat, 13 weeks
Lowest relevant dermal NOAEL 5 mg/kg bw per day, rat; 21 days
Lowest relevant inhalation NOAEL 20.6 ppb (296 mg/m3), rat; 13 weeks
Long-term toxicity and carcinogenicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest relevant NOAEL 2 years, rat; 1 mg/kg bw per day
78 weeks, mouse; 0.7 mg/kg bw per day
Carcinogenicity Not carcinogenic in rats or mice
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Neonatal toxicity (reduced pup body weight and survival)
Lowest relevant reproductive NOAEL Two-generation, rat; 1 mg/kg bw per day
Developmental target/critical effect Fetal and perinatal toxicity at maternally toxic doses (including
an increase in delayed ossification, reduced crown-rump length,
reduced pup weight, increased postimplantation loss, delayed sexual
maturity)
Lowest relevant developmental NOAEL Rats; 1 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Reversible neurotoxicity consistent with cholinesterase
inhibition. No evidence of delayed neurotoxicity or
histopathological changes in nerves of hens (10 mg/kg bw per day)
and rats (15 mg/kg bw per day) for up to 13 weeks. At high acute
doses (up to 150 mg/kg bw), significant inhibition of neuropathy
target esterase and mild delayed neuropathy in hens, but at this
dose, extensive and aggressive antidote treatment were required
for birds' survival.
Other toxicological studies No effect on cognitive function in rat pups in a study of
developmental toxicity at doses up to 5 mg/kg bw per day
Medical data No inhibition of erythrocyte acetylcholinesterase activity in
volunteers after repeated oral doses of up to 0.1 mg/kg bw per day
(for 9 days), single oral doses of up to 1 mg/kg bw, or a single
dermal doses of 5 mg/kg bw. Poisoning case presented with severe
cholinergic effects, with evidence of delayed polyneuropathy and/or
distal axonopathy at a dose that required antidotal treatment and
artificial ventilation.
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw Rat, 2-year dietary 100
Rat, reproduction
Mouse, developmental toxicity
Dog, 2-year dietary
Human, 9-day oral 10
Acute reference dose 0.1 mg/kg bw Human, single dose 10
References
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993a) Life-threatening
organophosphate-induced delayed polyneuropathy in a child after
accidental chlorpyrifos ingestion. J. Pediatr., 122, 658-660.
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993b)
Organophosphate-induced delayed polyneuropathy (comment).
J. Pediatr., 123, 837.
Aldridge, W.N. (1953) Serum esterases I. J. Biochem., 53, 110-117.
Anderson, B.T., Walker, S.A. & Punler, M.J. (1995) Chlorpyrifos tech:
Acute inhalational toxicity study in rats. Unpublished report No.
10425 from Inveresk Research International, Tranent, Scotland.
Submitted to WHO by Luxembourg Industries, Tel Aviv, Israel.
Bakke, J.E., Feil, V.J. & Price, C.E. (1976). Rat urinary metabolites
from O,O-diethyl-O(3,5,6-trichloro-2-pyridyl) phosphorthioate.
J. Environ. Sci. Health Bull., 3, 225-230.
Barna-Lloyd, T. & Szabo, J.R. (1985) Sodium
3,5,6-trichloro-2-pyridinol: 3-month rat dietary toxicity study.
Unpublished report No. TexasT:K-065999-099 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Barna-Lloyd, T., Szabo, J.R. & Young, J.T. (1986) Chlorpyrifos:
Subchronic organophosphate-induced delayed-neurotoxicity (OPIDN)
study in laying chickens hens. Unpublished report No.
TexasT:K-044793-064 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Bauriedel, W.R. & Miller, J.H. (1981) The metabolic fate of the sodium
salt of C14-labelled 3,5,6-trichloro-2-pyridinol orally
administered to swine. Unpublished report No. GH-C 1427 from Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. (1964) Results of 90-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in rats. Unpublished report from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,Odiethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1971) Results of 90-day dietary
feeding studies of O,Odiethyl-O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Berman, C.L. (1987) Evaluation of chlorpyrifos (Pyrinex) for dermal
sensitization of guinea pig. Unpublished report No. 59487-01 from
Arthur D. Little Inc., Cambridge, Massachusetts, USA. Submitted
to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Berteau, P.E. & Deen, W.A. (1978) A comparison of oral and inhalation
toxicities of four insecticides to mice and rats. Bull.
Environ. Contam. Toxicol., 19, 113-120.
Blackmore, R.H. (1968) Oral administration -- dogs Dursban. Final
report. Unpublished report No. 174-115 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Blagden, S.M. (1994) Chlorpyrifos technical: Acute inhalation toxicity
study four-hour exposure (nose only) in the rat. Unpublished
report No. CHA 10-CYF from Safepharm Laboratories Ltd, Derby,
United Kingdom. Submitted to WHO by Cheminova Agro A/S, Lemvig,
Denmark.
Blondell, J. & Dobozy, V.A. (1997) Review of chlorpyrifos poisoning
data. Washington DC, Environmental Protection Agency.
Branson, D.R. & Litchfield, N.H. (1970) Comparative absorption,
elimination and distribution of DOWCO 179, its methyl analog
DOWCO 214 and their major metabolite,
3,5,6-trichloro-2-pyridinol. Unpublished report No. 70 4479 from
Dow Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971a) Absorption, excretion and
distribution of O,Odiethyl O-3,5,6-trichloro-2, 6-C14-2-pyridyl
phosphorothioate in rats. Unpublished report No. NBA-9 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971b) Absorption, excretion and
distribution of 3,5,6trichloro-2, 6-C14-2-pyridinol in rats.
Unpublished report No. NBA-10 from Dow Chemical Co., USA
Submitted to WHO by Dow Agrosciences, Indianapolis, IN, USA.
Brenner, F.E., Bond, G.G., McLaren, E.A., Greene, S. & Cook, R.R.
(1989) Morbidity among employees engaged in the manufacture or
formulation of chlorpyrifos. Br. J. Ind. Med., 46, 133-137.
Breslin, W.J., Liberacki, A.B., Dittenber, D.A., Brzak, K.A. & Quast,
J.F. (1991) Chlorpyrifos: Two-generation dietary reproduction
study in Sprague-Dawley rats. Unpublished report No. K-044793-088
from the Toxicology Research Laboratory, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Bruce, R.J. & Zempel, J.A. (1986) Chlorpyrifos: Evaluation in the Ames
Salmonella/mammalian-microsome mutagenicity assay. Unpublished
report No. TexasT:K044793-075 from Lake Jackson Research Center,
Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Bruce, R.J., Gollapudi, B.B. & Hinze, C.A. (1985) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008 from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Buch SA (1980) Pyrinex tech: Acute inhalation toxicity in rats.
Unpublished report No. 80/MA/025/362 from Life Science Research,
Stock, Essex, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980a) Pyrinex tech: Irritance to rabbit
eye. Unpublished report No. 80/MA/023/143 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980b) Pyrinex tech: Irritance to rabbit
skin. Unpublished report No. 80/MAK/024/144 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1981) Pyrinex technical: Acute oral
toxicity in the rat. Unpublished report No. 81/MAK035/104 from
Life Science Research, Stock, Essex, United Kingdom. Submitted to
WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A., Gardner, J.R. & Birnie, J.V. (1980) Pyrinex: Acute
percutaneous toxicity study in rats. Unpublished report No.
80/MAK022/300 from Life Science Research, Stock, Essex, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Burns, C.J., Cartmill, J.B., Powers, B.S. & Lee, M.K. (1998) Update of
the morbidity experienced of employees potentially exposed to
chlorpyrifos. Occup. Environ. Med., 55, 65-70.
Bushnell, P.J., Padilla, S.S., Ward, T., Pope, C.N. & Olszyk, V.B.
(1991) Behavioral and neurochemical changes in rats dosed
repeatedly with diisopropylfluorophosphate. J. Pharmacol.
Exp. Ther., 256, 741-750.
Bushnell, P.J., Pope, C.N. & Padilla, S. (1993) Behavioral and
neurochemical effects of acute chlorpyrifos in rats: Tolerance to
prolonged inhibition of cholinesterase. J. Pharmacol. Exp.
Ther., 266, 1007-1017.
Calhoun, L.L. & Johnson, K.A. (1988) Chlorpyrifos: 4-day dermal probe
and 21-day dermal toxicity studies in Fischer 344 rats.
Unpublished report No. HET K 044793-085,086 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Calhoun, L.L. & Johnson, K.A. (1989) Supplemental information to the
report entitled: Chlorpyrifos: 4-day dermal probe and 21 day
dermal toxicity studies in Fischer 344 rats. Unpublished report
No. K-044793-085(S) from Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Campbell, C.G., Seidler, F.J. & Slotkin, T.A. (1997) Chlorpyrifos
interferes with cell development in rat brain regions. Brain
Res. Bull., 43, 179-189.
Capodicasa, E., Scapellato, M.L., Moretto, A., Caroldi, S. & Lotti, M.
(1991) Chlorpyrifosinduced delayed polyneuropathy. Arch.
Toxicol., 65, 150-155.
Chambers, J.E. (1992) The role of target-site activation of
phosphorothionates in acute toxicity. In: Chambers, J.E., Levi,
P.E. & Levi, A.P., eds, Organophosphates, San Diego, Academic
Press.
Chambers, J.E. & Carr, R.L. (1995) Biochemical mechanisms contributing
to species differences in insecticidal toxicity. Toxicology,
105, 291-304.
Chanda, S.M. & Pope, C.N. (1996) Neurochemical and neurobehavioural
effects of repeated gestational exposure to chlorpyrifos in
maternal and developing rats. Pharmacol. Biochem. Behav., 53,
771-776.
Chanda, S.M., Harp, P., Liu, J. & Pope, C.N. (1995) Comparative
developmental and maternal neurotoxicity following acute
gestational exposure to chlorpyrifos in rats. J. Toxicol.
Environ. Health, 44, 189-202.
Chaudhuri, J., Chakraborti, T.K., Chanda, S. & Pope, C.N. (1993)
Differential modulation of organophosphate-sensitivity muscarinic
receptors in rat brain by parathion and chlorpyrifos.
J. Biochem. Toxicol., 8, 207-216.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in beagle hounds. Unpublished report
No. T35.12-38278-3 from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Corley, R.A., Landry, T.D., Calhoun, L.L., Dittenber, D.A. & Lomax,
L.G. (1986) Chlorpyrifos: 13-week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET K
044793-077 from Dow Chemical Company Laboratory, Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Costa, L.G., McDonald, B.E., Murphy, S.D., Omenn, G.S., Richter, R.J.,
Motulsky, A.G. & Furlong, C.E. (1990) Serum paraoxonase and its
influence on paraoxon and chlorpyrifosoxon toxicity in rats.
Toxicol. Appl. Pharmacol., 103, 66-76.
Coulston, F., Golberg, L., Abraham, R. & Benitz, F.K. (1971) Final
report on safety evaluations and metabolic studies on Dowco 179
(Indiana 151). Unpublished report No. K44793-(48)A from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Coulston, F., Golberg, L. & Griffin, T. (1972). Safety evaluation of
Dowco 179 in human volunteers. Unpublished report from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Crown, S., Weiss, A., Nyska, A. & Waner, T. (1984a) Pyrinex. Toxicity
in dietary administration to mice for two weeks: A range-finding
study. Unpublished report No. MAK/109/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A. & Waner, T. (1984b) Pyrinex technical. Two-week
range-finding study in dietary administration to rats.
Unpublished report No. MAK/057/a/PYR from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Gur, E., Nyska, A. & Waner, T. (1985) Pyrinex technical
toxicity in dietary administration to rats for 13 weeks.
Unpublished report No. MAK/058/PYRA from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Weiss, A., Nyska, A., Waner, T. & Pirak, M. (1987) Pyrinex
technical. Toxicity in dietary administration to mice for 13
weeks. A preliminary study. Unpublished report No. MAK/105/PYR
from Life Science Research Israel Inc., Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A., Pirak, M. & Waner, T. (1988) Pyrinex technical.
Oncogenicity study in the rat. Unpublished report No. MAK/095/PYR
from Life Science Research Israel Ltd, Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Davies, R.E. & Kynoch, S.R. (1970) Acute subcutaneous toxicity of
Dursban to rats and the effect of treatment with 1. Atropine
sulphate 2. Atropine sulphate and PAM. Unpublished report No.
A1A-275 or 3641/70/453 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Davies, D.B., Tollett, J.T. & Lomax, L.G. (1985) Chlorpyrifos: A
four-week dietary study in the CD-1 mice. Unpublished report No.
HET-K-044793-068 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Dittenber, D.A., Hanley,
T.R., Jr & John, J.A. (1979) The effect of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Unpublished report No. HET-K-44793-32 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Rao, K.S., Dittenber, D.A.,
Hanley, T.R., Jr & John, J.A. (1980) Embryotoxicity and
fetotoxicity of orally administered chlorpyrifos in mice.
Toxicol. Appl. Pharmacol., 54, 31-40.
Derelanko, M.J. & Hollinger, M.A., eds (1995) CRC Handbook of
Toxicology, Boca Raton, FL, CRC Press Inc.
Dietz, F.K., Mensik, D.C., Hinze, C.A., Rachunek, B.K. & Taylor, H.W.
(1983) Dursban insecticide: Assessment of neonatal survival in a
two-generation reproduction study in rats. Unpublished report No.
TexasT-K-44793-(45) from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L. & Wetters, J.H. (1972a) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues and eggs
from chickens fed chlorpyrifos. Unpublished report No. GH-C 555
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., Rice, J.R., McKellar, R.L. & Pennington, J.Y.
(1972b) Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of cattle
fed chlorpyrifos. Unpublished report No. GH-C 566 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. & Rice, J.R. (1977)
Determination of residues of chlorpyrifos, its oxygen analogue,
and 3,5,6-trichloro-2-pyridinol in tissues of cattle fed
chlorpyrifos. J. Agric. Food Chem., 25, 1325-1329.
Dittenber, D.A. (1997) Chlorpyrifos: Evaluation of single oral doses
on cholinesterase and neurotoxic esterase inhibition in F344
rats. Unpublished report No. 960036 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Dreher DM (1994a) Chlorpyrifos technical: Acute oral toxicity test in
the rat. Unpublished report No. CHA 8-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994b) Chlorpyrifos technical: Acute dermal toxicity (limit
test) in the rat. Unpublished report No. CHA 9-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994c) Chlorpyrifos technical: Acute eye irritation test in
the rabbit. Unpublished report No. CHA 12-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994d) Chlorpyrifos technical: Acute dermal irritation test
in the rabbit. Unpublished report No. CHA 11-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994e) Chlorpyrifos technical: Magnusson & Kligman
maximisation study in the guinea pig. Unpublished report No. CHA
12-CYF from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to Who by Cheminova Agro A/S, Lemvig, Denmark.
Drevenkar, V., Vasilic, Z., Stengl, B., Frobe, Z. & Rumenjak, V.
(1993) Chlorpyrifos metabolites in serum and urine of poisoned
persons. Chem.-Biol. Interactions, 87, 315-322.
Eliason, D.A., Crammer, M.F., von Windeguth, D.L., Kilpatrick, J.W.,
Suggs, J.E. & Schoof, H.F. (1969) Dursban premise applications
and their effect on the cholinesterase levels of spraymen.
Mosquito News, 29, 591-595.
Emerson, J.L. & Gerbig, C.L. (1970) 91 day toxicology study in beagle
dogs treated with 3,5,6-trichloro-2-pyridinol. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Fikes, J.D., Zacchary, J.F., Parker, A.J. & Beasley, V.R. (1992)
Clinical, biochemical, electrophysiologic and histologic
assessment of chlorpyrifos induced delayed neuropathy in the cat.
Neurotoxicology, 13, 663-678.
Fredrick Institute of Plant Protection and Toxicology (1995a) Acute
oral toxicity study of chlorpyrifos technical in Swiss albino
mice. Unpublished report No. 2739 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995b) Acute
oral toxicity study of chlorpyrifos technical in Wistar rats.
Unpublished report No. 2738 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995c) Acute
dermal toxicity study of chlorpyrifos technical in rabbits.
Unpublished report No. 2748 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995d)
Irritation of chlorpyrifos technical to mucous membrane in
rabbits. Unpublished report No. 2746 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995e) Primary
skin irritation of chlorpyrifos technical in rabbits. Unpublished
report No. 2747 from Fredrick Institute of Plant Protection and
Toxicology, Tamil Nadu, India. Submitted to WHO by Ficom
Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996a) Acute
inhalation study with chlorpyrifos technical in Wistar rats.
Unpublished report No. 2968 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996b) Allergy
and skin sensitization potential of chlorpyrifos technical in
guinea pigs. Unpublished report No. 2928 from Fredrick Institute
of Plant Protection and Toxicology, Tamil Nadu, India. Submitted
to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996c)
Mutagenicity evaluation of chlorpyrifos technical in the
point-mutation Ames Salmonella typhimurium test system.
Unpublished report No. 3049 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996d)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
micronucleus assay. Unpublished report No. 3054 from Fredrick
Institute of Plant Protection and Toxicology, Tamil Nadu, India.
Submitted to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996e)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
bone marrow cytogenic assay. Unpublished report No. 3076 from
Fredrick Institute of Plant Protection and Toxicology, Tamil
Nadu, India. Submitted to WHO by Ficom Organics, Bombay, India.
Furlong, C.E., Richter, R.J., Seidel, S.L. & Motulski, A.G. (1988)
Role of genetic polymorphism of human plasma
paraoxonase/arylesterase in hydrolysis of the insecticide
metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum.
Genet., 43, 230-238.
Furlong, C.E., Richter, R.J., Seidel, S.L., Costa, L.G. & Motulsky,
A.G. (1989) Spectrophotometric assays for the enzymatic
hydrolysis of the active metabolites of chlorpyrifos and
parathion by plasma paraoxonase/arylesterase. Anal. Biochem.,
180, 242-247.
Geldmacher-von Mallinckrodt, M. & Diepgen, T.L. (1988) The human serum
paraoxonase polymorphism and specificity. Toxicol. Environ.
Chem., 18, 79-196.
Gerbig,, C.G. & Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Unpublished
report No. HH-240 from Dow Chemical Co., Zionsville, Indianna,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gerbig,, C.G. & Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat.
Unpublished report No. HH-239 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gerbig,, C.G. & Emerson, J.L. (1970c) Determination of oral minimum
lethal dose of 3,5,6trichloro-2-pyridinol in beagle dogs.
Unpublished report No. HH-253 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gibson, J.E. (1996) Critical review of allegations associating Dursban
with human teratogenicity. Unpublished report No. JEG122396, from
Dow Elanco, USA. Submitted by Dow Agrosciences, Indianapolis,
Indiana, USA.
Glas, R.D. (1977a) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from calves fed
3,5,6-trichloro-2-pyridinol. Unpublished report No. GH-C 1053
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977b) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from a calf fed
2-methoxy-3,5,6-trichloro-2-pyridine. Unpublished report No. GH-C
1052 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977c) Residues of triclopyr, 3,5,6-trichloro-2-pyridinol
and 2-methoxy-3,5,6trichloropyridine in bovine tissues from
calves fed trichlopyr. Unpublished report No. GH-C 1047 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981a) The metabolic fate of 14C-chlorpyrifos fed to
lactating goats. Unpublished report No. GH-C 1408 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981b) Identification of 14C-labelled residues in milk
from goats fed 14Cchlorpyrifos. Unpublished report No. GH-C
1470 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Gollapudi, B.B. (1987) Evaluation of 3,5,6-trichloro-2-pyridinol (TCP)
in the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. TexasT-K-38278-012 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Linscombe, V.A. & Wilkerson, J.E. (1985) Evaluation
of chlorpyrifos in the mouse bone marrow micronucleus test.
Unpublished report No. TexasT-K-044793-067 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Mendrala, A.L. & Linscombe, V.A. (1995) Evaluation of
the genetic toxicity of the organophosphate insecticide
chlorpyrifos. Mutat. Res., 342, 25-36.
Gur, E., Nyska, A. & Waner, T. (1991) Pyrinex technical oncogenicity
study in the mouse. Unpublished report from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Isreal.
Gutenmann, W.H., St John, L.E., Jr & Lisk, D.J. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow.
Unpublished report from Cornell University, Ithaca, New York,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gutmann, L. & Bodensteiner, J.B. (1993) Organophosphate-induced
delayed polyneuropathy (comment). J. Pediatr., 123, 837.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987a)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in Fischer 344
rats. Unpublished report No. K-038278-011 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987b)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in New Zealand
white Rrabbits. Unpublished report No. K-038278-015 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hardy, C.J. & Jackson, G.C. (1984) Dursban technical: Acute inhalation
toxicity in rats. Unpublished report No. DET 368 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Dawe, I.S. &
Anderson, A. (1989a) Chlorpyrifos oral dose range finding
toxicity study in beagle dogs (final report -- repeated daily
dosage for 4 weeks). Unpublished report No. MBS 30/88675 from
Huntingdon Research Centre Inc, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Majeed, S.,
Gopinath, C., Dawe, I.S. & Anderson, A. (1989b) Chlorpyrifos oral
toxicity study in beagle dogs (repeated daily dosage for 13
weeks). Unpublished report No. MBS 31/88999 from Huntingdon
Research Centre Inc, Huntingdon, United Kingdom. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Hazleton Laboratories (1968) Short term (subacute) dietary
administration -- Rats organophosphate insecticide Dursban. Final
report. Unpublished report No. 174-114 from Hazleton
Laboratories, Falls Church, Virginia, USA. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Henck, J.W. & Kociba, R.J. (1980) Three samples of Dursban
insecticide: Acute oral toxicity and acute percutaneous
absorption potential. Unpublished report No. A1A-130 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hoberman, A.M. (1998) Developmental neurotoxicity study of
chlorpyrifos administered orally via gavage to Crl:CD.BR VAF/Plus
presumed pregnant rats. Unpublished report No. K-044793109 from
Argus Research Laboratories, Horsham, PA, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Hooser, S.B., Beasley, V.R., Sundberg, J.P. & Harlin, K. (1988)
Toxicologic evaluation of chlorpyrifos in cats. Am. J. Vet.
Res., 49, 1371-1375.
Humbert, R., Adler, D.A., Distechhe, C.M., Hassett, C., Omiecinski,
C.J. & Furlong, C.E. (1993) The molecular basis of the human
serum paraoxonase activity polymorphism. Nature Genet., 3,
73-76
Jackson, D. & Ogilvie, S.W. (1994a) Chlorpyrifos tech: Acute dermal
toxicity (LD50) test in rats. Unpublished report No. 10439 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994b) Chlorpyrifos tech: Primary eye
irritation test in rabbits. Unpublished report No. 10441 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994c) Chlorpyrifos tech: Primary skin
irritation test in rabbits. Unpublished report No. 10440 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994d) Chlorpyrifos tech:
Magnusson-Kligman maximisation test in guinea pigs. Unpublished
report No. 10442 from Inveresk Research International, Tranent,
Scotland. Submitted to WHO by Luxembourg Industries, Tel Aviv,
Israel.
Jai Research Foundation (1996a) Reverse gene mutation study of
chlorpyrifos tech (95%) in bacteria by Ames Salmonella/microsome
test. Unpublished report No. 5/JRF/MichiganC/96 from Department
of Microbiology, Jai Research Foundation, Gujarat, India.
Submitted to WHO by Mitsu Industries, Gujarat, India.
Jai Research Foundation (1996b) Neurotoxicity study of chlorpyrifos
technical to hen. Unpublished report No. 1539/JRF/TOX/96 from
Department of Toxicology, Jai Research Foundation, Gujarat,
India. Submitted to WHO by Mitsu Industries, Gujarat, India.
James, P., Stubbs, A., Parker, C.A., Offer, J.M. & Anderson, A. (1988)
The effect of Pyrinex (chlorpyrifos) on reproductive function of
two generations in the rat. Unpublished report No. MBS 29/881452
from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Jeffrey, M.M., Battjes, J.E. & Eisenbrandt, D.L. (1986) Dursban F
(tech): Acute dermal toxicity study in Fischer 344 rats.
Unpublished report No. HET-K 044793-083 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Jones, J.R. (1985a) Dursban F: Eye irritation study in the rabbit.
Unpublished report No. DET 424 from Hazleton Laboratories Europe,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985b) Dursban F: Primary skin irritation and corrosivity
study in the rabbit. Unpublished report No. DET 421 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985c) Dursban F: Delayed contact hypersensitivity study
in the guinea pig (Buehler Test). Unpublished report No. DET 475
from Hazleton Laboratories Europe, Harrogate, North Yorkshire,
United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kamataki, T. & Neil, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothienate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfur
atom. Mol. Pharmacol., 12, 933-944.
Kaplan, J.G., Kessler, J., Rosenberg, N., Pack, D. & Schaumburg, H.H.
(1993) Sensory neuropathy associated with Dursban (chlorpyrifos)
exposure [published erratum appears in Neurology 1994 44, 367].
Neurology, 43, 2193-2196.
Kenaga, E.E. (1967) Comments on data concerning cholinesterase effects
of Dursban in man following US Public Health Service experimental
yellow fever mosquito eradication tests in Florida during 1966
and 1967. Unpublished report No.A1A-394, TK 2-1684 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kenny, T.J., Coombs, D.W. & Hardy, C.J. (1987) Chlorpyrifos technical
acute inhalation toxicity in rats 4-hour exposure. Unpublished
report No. MBS 20/87575 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Kenny, T.J., Coombs, D.W., Hardy, C.J. & Crook, D. (1988) Chlorpyrifos
inhalation toxicity in the rat. 14-Day preliminary dose range
finding study. Unpublished report No. MBS 21/871329 from
Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted by Makteshim Chemical Works, Beer-Sheva, Israel.
Kilian, D.J., Edwards, H.N., Benge, M.C. & Tabatabai, Z. (1970)
Results of human skin exposure to Dowco 179. Unpublished report
No. TMD-70-2 from Dow Chemical Co., Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Kisicki, J.C., Seip, C.W. & Combs, M.L. (1999) A rising dose
toxicology study to determine the no-observable-effect-levels
(NOEL) for erythrocyte acetylcholinesterase (AChE) inhibition and
cholinergic signs and symptoms of chlorpyrifos at three dose
levels. Unpublished report No. LLC Study ID: DR#K-044793-284
dated 19 April 1999, from MDS Harris, Lincoln, NE, USA. Submitted
to WHO by Dow Agrosciences, Indianapolis, Indiana, USA.
Kociba, R.J., McCollister, S.B., Keyes, D.G. & Dittenber, D.A. (1985)
Supplement to original report entitled 'Results of two-year
dietary feeding studies on Dowco 179 in beagle dogs'. Unpublished
report No. NBT 3512-44793-18 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lackenby, F. (1985a) Dursban F and Dursban F (OP1): Acute oral
toxicity studies in the rat. Unpublished report No. 4703-50/390 &
591 from Hazleton Laboratories Europe, North Yorkshire, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Lackenby, F. (1985b) Dursban F: Acute dermal median lethal dose in the
rat. Unpublished report No. 4206-50/391 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Landry, T.D., Streeter, C.M. & Lomax, L.G. (1985) Chlorpyrifos: 2-week
vapor inhalation toxicity study in Fischer 344 rats. Protocol.
Unpublished report No. A1A-714 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Landry, T.D., Dittenber, D.A., Lomax, L.G., Calhoun, L.L. & Morabito,
P. (1986) Chlorpyrifos: 2 week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET
K-44793-81 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Liberacki, A.B., Breslin, W.J., Dittenber, D.A. & Quast, J.F. (1990)
Chlorpyrifos: Two week dietary probe study in Sprague-Dawley
rats. Unpublished report No. HET K 044793090 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A. & Gollapudi, B. (1986) Evaluation of
3,5,6-trtichloro-2-pyridinol (TCP) in the Chinese hamster ovary
cell/hypoxanthine-guanine-phosphoribosyl transferase (CHO/HGPRT)
forward mutation assay. Unpublished report No.
TexasT:K-038278-013 from Dow Chemical Co., HES, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A., Mensik, D.C. & Clem, B.A. (1992) Evaluation of
chlorpyrifos in an in vitro chromosomal aberration assay
utilizing rat lymphocytes. Unpublished report No. TexasT:K
044793-092 from Dow Chemical Co., Freeport, Texas, USA. Submitted
to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lotti, M., Moretto, A., Zoppellari, R., Dainese, R., Rizzuto, N. &
Barusco, G. (1986) Inhibition of lymphocytic neuropathy target
esterase predicts the development of organophosphate-induced
delayed polyneuropathy. Arch. Toxicol., 59, 176-179.
Loveday, K.S. (1987) In vitro chromosomal aberration assay on Pyrinex
(chlorpyrifos). Unpublished report No. R4660 from Arthur D.
Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Loveday, K.S., Findlen, K.M.B. & Yadlon, S. (1987) Evaluation of
Pyrinex in the Ames mutagenesis assay. Unpublished report No.
59487-00 from Arthur D. Little Inc., USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Ma, T. & Chambers, J.E. (1994) Kinetic parameters of desulfuration and
dearylation of parathion and chlorpyrifos by rat liver
microsomes. Food Chem. Toxicol., 32, 763-767.
Maita, K., Hirano, M., Harada, T., Mitsumori, K., Yoshida, A.,
Takahashi, K., Nakashima, N., Kitazawa, T., Enomoto, A. & Inui,
K. (1988) Mortality, major cause of moribundity aqnd spontaneous
tumours in CD-1 mice. Toxicol. Pathol., 16, 340-349.
Mattsson, J.L., Wilmer, J.W., Shankar, M.R., Berdasco, N.M., Crissman,
J.W., Maurissen, J.P. & Bond, D.M. (1996) Single-dose and 13-week
repeated-dose neurotoxicity screening studies of chlorpyrifos
insecticide. Food Chem. Toxicol., 34, 393-405.
Maurissen, J.P., Shankar, M.R. & Mattsson, J.L. (1996) Chlorpyrifos:
Cognitive study in adult Long-Evans rats. Unpublished report No.
K-044793-096 from Dow Chemical Co., USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989a) Evaluation of chlorpyrifos
in the mouse bone marrow micronucleus test. Unpublished report
No. TexasT:K 044793-067A from Lake Jackson Research Center, Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989b) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008A from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco 179
in beagle dogs. Unpublished report No. T35.1244793-18 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco 179
in rats. Unpublished report No. NBT35.12-4479321 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J. & Keyes, D.G. (1985) Supplement to
original report entitled 'Results of two-year dietary feeding
studies on Dowco 179 in rats'. Unpublished report No.
NBT35.12-44793-21 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McKellar, R.L., Wetters, J.H. & Dishburger, H.J. (1972) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues of swine
fed chlorpyrifos. Unpublished report No. GHC549 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Mendrala, A.L. (1985) Evaluation of chlorpyrifos in the Chinese
hamster ovary cellhypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Unpublished
report No. HET-K-044793-072 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Mendrala, A.L. & Dryzaga, M.D. (1986) Evaluation of chlorpyrifos in
the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. HET K 044793-073 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Moretto, A. & Lotti, M. (1998) Poisoning by
organophosphorusinsecticides and sensory neuropathy.
J. Neurol. Neurosurg. Psych., 64, 463-468.
Mortensen, S.R., Chanda, S.M., Hooper, M.J. & Padilla, S. (1996)
Maturational differences in chlorpyrifos-oxonase activity may
contribute to age-related sensitivity to chlorpyrifos.
J. Biochem. Toxicol., 11, 279-287.
Moser, V.C. (1995) Comparisons of the acute effects of cholinesterase
inhibitors using a neurobehavioral screening battery in rats.
Neurotoxicol. Teratol., 17, 617-625.
Moser, V.C. & Padilla, S. (1998) Age- and gender-related differences
in the time course of behavioral and biochemical effects produced
by oral chlorpyrifos in rats. Toxicol. Appl. Pharmacol., 149,
107-119.
Muscarella, D.E., Keown, J.F. & Bloom, S.E. (1984) Evaluation of the
genotoxic and embryotoxic potential of chlorpyrifos and its
metabolites in vivo and in vitro. Environ. Mutag., 6, 13-23.
Nakatsugawa, T. (1992) Hepatic disposition of organophosphorus
insecticides: A synthesis of in vitro, in situ and in vivo data.
In: Chambers, J.E., Levi, P.E. & Levi, A.P., eds,
Organophosphates, San Diego, Academic Press, Chapter 10.
Newton, P.E. (1988a) A 5-day nose-only inhalation toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
from Bio/Dynamics Inc., East Millstone, NJ, USA. Submitted by
Makteshim Chemical Works, Beer-Sheva, Israel.
Newton, P.E. (1988b) A thirteen week nose-only toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
No. R-4750 from Bio/dynamics Inc., East Millstone, NJ, USA.
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984a) Pyrinex tech: Acute oral toxicity in
the rat. Unpublished report No. MAK/056/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984b) Pyrinex tech: Acute dermal toxicity
in rabbits. Unpublished report No. MAK/059/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nolan, R.J. (1997) Proposed reference dose for acute and chronic
exposure to chlorpyrifos based on the criteria described by the
Acute Cholinesterase Risk Assessment Task Force and the available
animal and human data. Unpublished report No. K-044793-110 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1982)
Chlorpyrifos: pharmacokinetics in human volunteers following
single oral and dermal doses. Unpublished report No.
HEB-DR-0043-4946-4 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1984)
Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol.
Appl. Pharmacol., 73, 8-15.
Nolan, R.J., Streeter, C.M. & Kastl, P.E. (1986) Chlorpyrifos:
Absorption by female Fischer 344 rats exposed for 6 hours to
vapours of chlorpyrifos in a nose-only or a whole-body inhalation
chamber. Unpublished report No. HET K-044793-82 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Dryzga, M.D., Landenberger, B.D. & Kastl, P.E. (1987)
Chlorpyrifos: Tissue distribution and metabolism of orally
administered 14C labelled chlorpyrifos in Fischer 344.
Unpublished report No. HET K-044793-(76) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Nostrandt, A.C., Padilla, S. & Moser, V.C. (1997) The relationship of
oral chlorpyrifos effects on behaviour, cholinesterase
inhibition, and muscarinic receptor density in rat.
Pharmacol. Biochem. Behav., 58, 15-23.
Omenn, G.S. (1987) The role of genetic differences in human
susceptibility to pesticides. In: Costa, L.G., Galli, C.L. &
Murphy, S.D., eds, Toxicology of Pesticides: Experimental,
Clinical and Regulatory Perspectives, Berlin, Springer-Verlag.
Osterloh, J., Lotti, M. & Pond, S.M. (1983) Toxicologic studies in a
fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. Anal.
Toxicol., 7, 125-129.
Ouelette, J.H., Dittenber, D.A., Kociba, R.J. & John, J.A. (1983a)
Chlorpyrifos: Oral teratology probe study in rats. Unpublished
report No. HET K-44793-(46) from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Ouelette, J.H., Dittenber, D.A., Kloes, P.M. & John, J.A. (1983b)
Chlorpyrifos: Oral teratology study in Fischer 344 rats.
Unpublished report No. HET K-44793-(47) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Pennington, J.Y. & Edwards, N.H. (1971) Comparison of cholinesterase
depression in humans and rabbits following exposure to
chlorpyrifos. Unpublished report no. TA-477 from Dow Chemical
Company USA. Submitted to WHO by Dow Agrosciences, Indianapolis,
Indiana, USA.
Phillips, J.E. & Lomax, L.G. (1989) 1% aqueous dilution of Dursban
acute aerosol inhalation toxicity study in Fischer 344 rats.
Unpublished report No. M-004803-004A from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Poole, D.C., Simmon, V.F. & Newell, C.W. (1977). In vitro mutagenic
activity of fourteen pesticides. Toxicol. Appl. Pharmacol., 41,
196.
Pope, C.N. & Chakraborti, T.K. (1992) Dose related inhibition of brain
and plasma cholinesterase in neonatal and adult rats following
sub-lethal organophosphate exposure. Toxicology, 73, 35-43.
Pope, C.N., Chakraborti, T.K., Chapman, M.L., Farrar, J.D. & Arthun,
D. (1991) Comparison of in vivo cholinesterase inhibition in
neonatal and adult rats by three organophosphorothioate
insecticides. Toxicology, 68, 51-61.
Pope, C.N., Chakraborti, T.K., Chapman, M.L. & Farrar, J.D. (1992)
Long-term neurochemical and behavioral effects induced by acute
chlorpyrifos treatment. Pharmacol. Biochem. Behav., 42,
251-256.
Richardson, R.J., Moore, T.B., Kayyali, U.S. & Randall, J.C. (1993a)
Chlorpyrifos: Assessment of potential for delayed neurotoxicity
by repeated dosing in adult hens with monitoring of brain
acetylcholinesterase, brain and lymphocyte neurotoxic esterase
and plasma butyrylcholinesterase activities. Fundam. Appl.
Toxicol., 21, 89-96.
Richardson, R.J., Moore, T.B., Kayyali, U.S., Fowke, J.H. & Randall,
J.C. (1993b) Inhibition of hen brain acetylcholinesterase and
neurotoxic esterase by chlorpyrifos in vivo and kinetics of
inhibition by chlorpyrifos oxon in vitro: Application to
assessment of neuropathic risk. Fundam. Appl. Toxicol., 20,
273-279.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Begg, S., Anderson, A.
& Dawe, I.S. (1987) Acute delayed neurotoxicity study with
chlorpyrifos in the domestic hen. Unpublished report No. MBS
16/87764 from Huntingdon Research Centre Ltd, Huntingdon, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Ross, D.B. & Roberts, N.L. (1974) The acute oral toxicity (LD50) of
Dursban (DOWCO 179) to the chicken (DWC/234/74877). Unpublished
report from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Rowe, L.D., Warner, S.D. & Johnston, R.V. (1978) Acute delayed
neurotoxicologic evaluation of chlorpyrifos in white Leghorn
hens. Unpublished report No. A1A-715 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Rubin, Y.,. Gal, N., Waner, T. & Nyska, A. (1987a) Pyrinex.
Teratogenicity study in the rat. Unpublished report No.
MAK/101/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Rubin, Y., Nyska, A. & Waner, T. (1987b) Pyrinex. Pyrinex
teratogenicity study in the rabbit. Unpublished report No.
MAK/103/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Scortichini, B.H., Lomax, L.G., Wolfe, E.L. & Hanley, T.R., Jr (1986a)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in
Fischer 344 rats. Unpublished report No. HETK-038278-006 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Scortichini, B.H., Lomax, L.G., Wolf, E.L. & Hanley, T.R., Jr (1986b)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in New
Zealand white rabbits. Unpublished report No. HET-K-038278-007
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Shankar, M.R., Bond, D.M. & Crissman, J.W. (1993) Chlorpyrifos:
13-week neurotoxicity study in Fischer 344 rats. Unpublished
report No. K-044793-094 from Dow Chemical Co., USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Sharp, L.D. & Warner, S.D. (1968) The clinical toxicity of Dursban in
the dog after multiple applications of an aerosol formulation.
Unpublished report from Dow Chemical Co., USA. Submitted to WHO
by Dow Agrosciences, Indianapolis, Indiana, USA.
Shemesh, I., Bourvin, A., Gold, D. & Kutscherowsky, M. (1988)
Chlorpyrifos poisoning treated with ipratropium and dantrolene: a
case report. J. Toxicol. Clin. Toxicol., 26, 495-498.
Sherman, J.D. (1996) Chlorpyrifos (Dursban)-associated birth defects:
Report of four cases. Arch. Environ. Health, 51, 5-8.
Sherman, J.D. & Herrick, R.B. (1973) Fly control and chronic toxicity
from feeding Dursban (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) to laying hens. Poultry Sci., 52, 741-747.
Sherman, J.D., Herrick, R.B., Ross, E. & Chang, M.T.Y. (1967) Further
studies on the acute and sub-acute toxicity of insecticides to
chicks. Toxicol. Appl. Pharmacol., 11, 49-67.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) Mutagenicity test of
chlorpyrifos in bacteria. Unpublished report No. GHF-R-47 from
Dow Chemical Pacific, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Shurdut, B.A., Chen, W.L., Burns, C.J., McCormick, R.A., Nolan, R.J. &
Racke, K.D. (1997) Critical assessment of report entitled 'Review
of chlorpyrifos poisoning data' (by J. Blondell and V. Dobozy,
January 14, 1997). Unpublished report No. GH-C 4359, by Dow
Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, Indiana, USA.
de Silva, H.J., Sanmuganathan, P.S. & Senanayake, N. (1994) Isolated
bilateral recurrent laryngeal nerve paralysis: A delayed
complication of organophosphorus poisoning. Hum. Exp.
Toxicol., 13, 171-173.
Smith, G.N., Watson, B.S. & Fischer, F.S. (1967) Investigations on
Dursban insecticide. Metabolism of [36Cl] O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothiate in rats. Agric.
Food Chem., 15, 132-138.
Smolen, A., Eckerson, H.W., Gan, K.N., Hailat, N. & LaDu, B.N. (1991)
Characteristics of the genetically determined allozymic forms of
human paraoxonase/artylesterase. Drug Metab. Disposition, 19,
107-112
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: Organophosphates. Mutat. Res., 102,
89-102.
Song, X., Seidler, F.J., Saleh, J.L., Zhang, J., Padilla, S. &
Slotkin, T.A. (1997) Cellular mechanisms for developmental
toxicity of chlorpyrifos: Targeting the adenylyl cyclase
signaling cascade. Toxicol. Appl. Pharmacol., 145, 158-174.
Stevenson, G.T. (1963) An LD50 toxicity study of Dursban in Leghorn
chickens. Unpublished report from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1966a) A single oral dose LD50 toxicity study of
Dursban in broiler type chickens. Unpublished report No. A1A-364
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Stevenson, G.T. (1966b) A neurotoxicity study of Dursban in laying
hens. Unpublished report No. A1A-390 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1967) A single oral dose LD50 toxicity study of
Dursban in turkey/poultry. Unpublished report No. A1A-362 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Streeter, C.M., Lomax, L.G., Landry, T.D. & Dittenber, D.A. (1987)
Chlorpyrifos: 2-week whole-body vapor inhalation toxicity study
in Fischer 344 rats. Unpublished report No. HET K 044793-078 from
Dow Chemical Co., Midland, Michigan, USA Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Sultatos, L.G. & Murphy, S.D. (1983) Hepatic microsomal detoxification
of the organophosphates paraoxon and chlorpyrifos oxon in the
mouse. Drug Metab. Disposition, 11, 232-238.
Szabo, J.R., Young, J.T. & Granjean, M. (1988) Chlorpyrifos: 13-week
dietary toxicity study in Fisher 344 rats. Unpublished report No.
TexasT: K-044793-071 from Lake Jackson Research Center, Health
and Environmental Sciences, Freeport, Texas, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Taylor, M.L. & Olson, K.J. (1963) Toxicological properties of
O,O-diethyl-O-3,5,6trichloro-2-pyridyl phosphorothioate.
Unpublished report. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Thompson, D.J., Gerbig, C.C. & Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Tu, A.S. (1987) CHO/HGPRT in vitro mammalian cell mutation assay on
Pyrinex (chlorpyrifos). Unpublished report No. 59487 from Arthur
D. Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Warner, S.D., Gerbig, C.G., Strebing, R.J. & Molello, J.A. (1980)
Results of a two-year toxicity and oncogenic study of
chlorpyrifos administered to CD-1 mice in the diet. Unpublished
report from Dow Chemical Co., Indianapolis, Indiana, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Whitney, K.D., Seidler, F.J. & Slotkin, T.A. (1995) Developmental
neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicol.
Appl. Pharmacol., 134, 53-62.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Wilmer, J.W. (1992) Acute neurotoxicity study in Fischer 344 rats.
Unpublished report No. K-044793-093B from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Wilson, J.A. & MacBeth, D. (1994) Chlorpyrifos tech: Acute oral
toxicity (LD50) test in rats. Unpublished report No. 10521 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Wolcott, R.M., Vaughn, W.K. & Neal, R.A. (1972) Comparison of the
mixed function oxidase-catalyzed metabolism of a series of
dialkyl p-nitrophenyl phosphorothionates. Toxicol. Appl.
Pharmacol., 22, 213-220.
Yang, R.S.H., Hodgson, E. & Dauterman, W.C. (1971) Metabolism in vitro
of diazinon and diazoxon in rat liver. J. Agric. Food Chem.,
19, 10-13.
Young, J.T. & Grandjean, M. (1988) Chlorpyrifos: 2 year dietary
chronic toxicity-oncogenicity study in Fischer 344 rats.
Unpublished report No. K-044793-079 from Lake Jackson Research
Center, Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Zempel, J.A. & Bruce, R.J. (1986) 3,5,6-Trichloro-2-pyridinol:
Evaluation in the Ames Salmonella/mammalian-microsome
mutagenicity assay. Unpublished report No. TexasT:K038278-010
from Lake Jackson Research Center, Dow Chemical Co., Freeport,
Texas, USA. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Zempel, J.A., Rachunek, B.L. & Szabo, J.R. (1987)
3,5,6-Trichloro-2-pyridinol: Results of a one-year dietary study
in male and female beagle dogs. Unpublished report No.
TexasT:K038278-009 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
(c) Effects on enzymes and other biochemical parameters
Hydrolysis is the most important route of detoxification of
organophosphorus esters. Hydrolytic esterases are distributed
ubiquitously in the blood and tissues of virtually all animal
organisms and catalyse the hydrolysis of a variety of esters,
including organophosphorus esters, but have little activity on
organophosphorus itself. Esterases that interact with
organophosphoruses have been characterized as A- and B-esterases
according to their sensitivity to inhibition by organophosphorus
compounds. Plasma and tissues of mammals have significant
concentrations of the calcium-activated A-esterases, arylesterase (EC
3.1.1.2), and the high-density lipoprotein-associated paroxonase (EC
3.1.8.1). The B-esterases, including aliesterases (EC 3.1.1.1; also
called carboxylesterases) and cholinesterases (e.g. butyryl
cholinesterase, EC 3.1.1.8), are inhibited by organophosphorus
compounds such as chlorpyrifos oxon. While they bind them, they do not
hydrolyse them (Derelanko & Hollinger, 1995).
Butyrylcholinesterase is the predominant cholinesterase in human
serum (> 99%), while in rats there is an approximately equal
distribution of acetylcholinesterase and butyryl cholinesterase
(Nolan, 1997). These enzymes detoxify organophosphorus compounds by
sequestering them through binding to or phosphorylation by blood
proteins, such as albumin, which stoichiometrically degrades them
(Aldridge, 1953). These protein-bound organophosphorus esters are
secreted into the bile. Erythrocyte acetylcholinesterase (EC 3.1.1.7)
is similar to the acetylcholinesterase in nervous systems and can bind
to and sequester or hydrolyse organophosphorus compounds. The enzyme
activities attributable to A-esterases, B-esterases, and
cholinesterases vary widely within populations and are influenced by
genetic and environmental factors and disease states (Derelanko &
Hollinger, 1995). The serum activity of paraoxonase in rabbits is some
40 times greater than that in rats. Most avians, including hens, have
relatively low activity of A-esterases, and the mean value of serum
chlorpyrifos oxonase activity in humans is 10 times that of rat serum
(Furlong et al., 1989).
In humans, a substrate-dependent polymorphism of serum
paraoxonase is observed, in which one isoform of paraoxonase has a
high turnover for paraoxon and the other a low turnover (Furlong et
al., 1989; Smolen et al., 1991). The polymorphism is also observed
with the oxons of methyl parathion, chlothion, and
phenylphosphonothioic acid O-ethyl O-para-nitrophenyl ester. The
two isoforms appear, however, to hydrolyse chlorpyrifos oxon and
phenylacetate at the same rate. Cloning and sequencing of the human
paraoxonase cDNA has revealed the molecular basis of the polymorphism:
arginine at position 192 determines high paraoxonase activity, and
glutamine at this position encodes low paraoxonase activity (Humbert
et al., 1993). In addition to this polymorphism, a 13-fold variation
in serum enzyme activity of a given genetic class is seen (Furlong et
al., 1989). While chlorpyrifos oxon was hydrolysed by the same plasma
fraction that hydrolysed paraoxon in a study of plasma samples from
320 white blood donors (Furlong et al., 1988), the population
distribution of chlorpyrifos oxon hydrolysis was unimodal. This
contrasted with a bimodal population distribution of the activity for
paraoxon hydrolysis, and the variation in paraoxonase activity was
about 11-fold, while that in chlorpyrifos oxonase activity showed a
four- to fivefold variation.
Correlation of the LD50 values for orally administered
chlorpyrifos in rats with the activation rates measured in brain
rather than liver (Chambers, 1992) suggested that local
biotransformation in target tissues is an important determinant of its
toxicity. In a more recent review, however, Chambers & Carr (1995)
compared published LD50 or LC50 values for a variety of
insecticides in several vertebrate species. Studies in rats indicated
that the sensitivity of brain acetylcholinesterase activity to
inhibition by various phosphorothionate oxons did not correlate with
their acute toxicity. Chlorpyrifos oxon has a greater affinity for rat
brain acetylcholinesterase than does paraoxon, with median inhibitory
concentrations (IC50 values) of 4.0 and 22 nmol/L, respectively, but
a lower LD50. This finding is consistent with the greater affinity
(IC50, 0.75 nmol/L) of chlorpyrifos oxon for plasma aliesterases
than for rat brain acetylcholinesterase, indicating that aliesterases
provide protection against chlorpyrifos oxon.
Further work by Chambers & Carr (1995) indicated longer
inhibition of esterases after exposure chlorpyrifos than to parathion.
This was attributed to the greater lipophilicity of chlorpyrifos than
parathion (hexane:acetonitrile partition coefficients, 0.28 and 0.062,
respectively) and the assumption that a substantial fraction of the
dose of chlorpyrifos would have been sequestered by fat and released
gradually for later bioactivation. Studies of hepatic microsomal
metabolism of parathion and chlorpyrifos indicated that desulfuration
of parathion was favoured over dearylation (activation vs
deactivation), whereas the reverse was seen for chlorpyrifos (Ma &
Chambers, 1994). In channel catfish, however, the sensitivity of
acetylcholinesterase to inhibition by oxons reflects the acute
toxicity of these insecticides, and may be largely responsible for
their toxicity in this species. Thus, the metabolism of insecticides
appears to be more influential in some species than in others in
determining their toxicity.
Chlorpyrifos oxonase is a calcium-dependent A-esterase that
hydrolyses chlorpyrifos oxon, the active metabolite of chlorpyrifos.
As this activity may be related to that of paraoxonase, it was
determined spectrophotometrically by measuring the generation of TCP
(Furlong et al., 1989). Mortensen et al. (1996) hypothesized that
young rats have less chlorpyrifos oxonase activity than adults, and
they measured the activity of this enzyme in the brain, plasma, and
liver of male Long-Evans rats (CRL:(LE)BR) at postnatal day 4 and in
adults. No activity was detected in brain at either age, but the
activities in plasma and liver were markedly lower in younger than in
adult animals, the activities in plasma and liver being 1/11 and 1/2
of those in adults, respectively.
As the Michaelis-Menten constant for chlorpyrifos oxonase
activity was high (210-380 µmol/L), experiments were performed to
determine whether this enzyme could hydrolyse physiologically relevant
concentrations (nanomolar to low micromolar) of chlorpyrifos oxon. On
the assumption that tissue acetylcholinesterase inhibition provides a
sensitive bioassay for the concentration of chlorpyrifos oxon, changes
in the tissue acetylcholinesterase IC50 for chlorpyrifos oxon were
measured in the presence and absence of chlorpyrifos oxonase. Brain
acetylcholinesterase activity was determined spectrophotometrically,
while plasma and liver cholinesterase activities were determined by a
radiometric method with a [3H]acetylcholine iodide substrate. An
increase in the 'apparent' IC50 would indicate that chlorpyrifos
oxonase had hydrolysed substantial amounts of chlorpyrifos oxon during
the 30-min preincubation with the chlorpyrifos oxon. In the adult
rats, the apparent IC50 values for both plasma and liver
cholinesterase were higher in the presence of chlorpyrifos oxonase,
suggesting that the enzyme in those tissues could hydrolyse
physiologically relevant concentrations of chlorpyrifos oxon within
30 min. Young animals, however, showed less of a shift in the IC50
curves than adults, confirming that they have a lower capacity to
detoxify physiologically relevant concentrations of chlorpyrifos oxon
with chlorpyrifos oxonase.
2. Toxicological studies
(a) Acute toxicity
(i) Lethal doses
Numerous studies have been carried out with technical-grade
chlorpyrifos to establish LD50 and LC50 values (Table 1). The
lowest value after oral administration was 96 mg/kg bw (range,
96-480 mg/kg bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in
mice. The signs of acute intoxication were consistent with
cholinesterase inhibition and included inactivity, salivation,
dyspnoea, flaccid paralysis, vomiting, piloerection, exophthalmia, and
diarrhoea; female animals were generally more sensitive to the acute
effects of chlorpyrifos than males. The LD50 for dermally applied
chlorpyrifos was consistently lower than that for oral or inhalational
exposure and indicated the lower dermal absorption of chlorpyrifos
(about 2% in humans) when compared with the 70-90% absorption after
inhalational or oral exposure.
(ii) Dermal and ocular irritation and dermal sensitization
Ocular irritation: In a paper in which few procedural details
were provided (Taylor & Olson, 1963), slight conjunctival redness was
seen in rabbits treated with chlorpyrifos, regardless of whether the
eyes were washed after administration of the test material. The
redness persisted for more than 7 days in some animals. Transient
conjunctival and iridial irritation, possibly due to a direct physical
irritating effect of cholorpyrifos powder, was seen in three young
adult female New Zealand white rabbits after each had received 100 mg
of a finely ground commercial preparation in the left conjunctival
sac. The eyes of all animals were normal after 24 h (Jones, 1985a).
The ocular irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested (in accordance with GLP requirements) in six
young male New Zealand white rabbits, 100 mg of test material being
placed into the right eye and the untreated left eye serving as a
control. There were no signs of corneal or iridial irritation. Slight
to moderate conjunctival redness (mean Draize score, 1 at 1 h, 0.83 at
24 h), slight chemosis (mean Draize score, 0.83 at 1 h, 0.16 at 24 h),
and discharge (mean Draize score, 1.6 at 1 h, 0.83 at 24 h) were seen
up to 24 h after instillation, but no signs of irritation were
reported at 48 h (Jackson & Ogilvie, 1994b).
The ocular irritation potential of 0.1 g of chlorpyrifos (purity,
99.3%) was assessed in three male and three female New Zealand white
rabbits by instilling the test substance into the conjunctival sac of
the right eye, the left eye remaining untreated and serving as
control. This study was conducted according to GLP requirements.
Diffuse corneal opacities (score 1 for degree and area of opacity)
were observed in two animals at 24 h, but these effects had
disappeared by 48 h and no other corneal effects were seen. Iridial
inflammation (score 1) was noted in four animals at 1 h, and this
effect persisted for 24 h in one of the animals and for 48 h in
another. No iridial effects were observed after 72 h. Conjunctival
irritation was observed in all animals, with redness (score 2:
diffuse, deeper crimson red at 1-24 h; score 1: vessels definitely
injected above normal at 48 h), chemosis (score 2: obvious swelling
with partial eversion of lids up to 48 h), and discharge (scores 2-3:
moistening of the lids and hairs just adjacent to lids or a
considerable area around the eye only at 1 h). No signs of
conjunctival irritation was observed at 72 h. The maximum individual
irritation scores were seen at 1 h and ranged from 12 to 19, with a
mean score of 16. The mean scores at 24, 48, and 72 h were 9.3, 2.5,
and 0, respectively. The overall scores according to EEC Council
Directive 67/548/EEC were 2 (mean, 0.11) for corneal opacity, 3 (0.17)
for iridial inflammation, 11 (0.61) for conjunctival redness, and 8
(0.44) for conjunctival chemosis (Dreher, 1994d)
Technical-grade chlorpyrifos (100 mg; purity, 96.2%) was
instilled into the conjunctival sac of the left eye of Himalayan
albino rabbits, and the right eyes of the animals served as controls.
A group of control animals was similarly treated with distilled water
only. All animals were observed for signs of ocular irritation at 1,
4, 24, 48, and 72 h, and then daily up to day 7. At the 4-h
observation, very slight iridial irritation (score 1) and conjunctival
irritation (score 1) were reported. It was not clear from the report
whether the conjunctival irritation was redness, discharge, or
chemosis. No signs of irritation were reported at 24 or 72 h. A
'quality assurance statement' was issued for this study (Frederick
Institute of Plant Protection and Toxicology, 1995d).
Table 1. Studies of the acute toxicity of technical-grade chlorpyrifos
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Oral administration
Mouse SmithWebster M 1% aqueous gum 102 (94-110) Coulston et al. (1971)
tragacanth
Mouse NAMRU F Soya bean oil 152 (143-162) Berteau & Deen (1978)
Mouse Swiss albino M&F Vegetable oil 109 (93-127) QA Fredrick Institute (1995a)
Rat NR M 5% corn oil 163 (97-276) Taylor & Olson (1963)
F 135 (97-188)
Rat Sprague-Dawley F Soya bean oil 169 (146-196) Berteau & Deen (1978)
Rat Sprague-Dawley M Arachis oil 276 (167-455) GLP Dreher (1994a)
F 350 (285-429)
M&F 320 (260-393)
Rat Wistar M&F Vegetable oil 134 (102-163) QA Frederick Institute (1995b)
Rat Sprague-Dawley M Maize oil 264 GLP Wilson & MacBeth (1994)
F 141
M&F 192
Rat Sprague-Dawley M Maize oil 475 (311-727) Buch & Gardner (1981)
F 337 (220-515)
Rat Sprague-Dawley M Corn oil 221 (181-69) Nissimov & Nyska (1984a)
F 144 (105-200)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M Not reported 205 (134-299) Henck & Kociba (1980)
F 96 (72-140)
M 248 (170-379)
F 97 (80-112)
M 270 (188-426)
F 174 (130-244)
Guinea-pig NR M Corn oil 504 (300-850) GLP Lackenby (1985a)
Rabbit NR M Corn oil 1000-2000 GLP Lackenby (1985a)
Chicken NR M Capsule 32 (14-72) GLP Lackenby (1985a)
Chicken Leghorn M Diet 25 (21-31) Sherman et al. (1967)
Chicken Leghorn M Capsule 32 Stevenson (1963)
Chicken White Rock M&F Capsule 50-63 Stevenson (1966a)
Chicken NR M&F Capsule 20-50 Ross & Roberts (1974)
Chicken NR M&F Corn oil 102 (64-169) Ross & Roberts (1974)
Chicken NR M Capsule, 32 (14-72) Taylor & Olson (1963)
undiluted
Turkey Beltsville NR NR 32-63 Stevenson (1967)
small white
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Inhalation
Mouse NAMRU F 65% xylene 94 (83-106) Berteau & Deen (1978)
(27-50 min,
whole body,
aerosol)
Rat Albino M&F Vapour (4 h, > 200a GLP Hardy & Jackson (1984)
(HC/CFHB) whole body)
Rat Sprague-Dawley F 65% xylene 78 (57-108) Berteau & Deen (1978)
(60-180 min,
whole body,
aerosol)
Rat Sprague-Dawley M&F Undiluted (4 h, > 230b (max. GLP Anderson et al. (1995)
nose only, attainable
powder) concentration)
Rat Sprague-Dawley M 40% xylene > 4070a Buch (1980)
F (4 h, nose only, 2890b (2010-4160)
aerosol)
Rat Wistar M&F Undiluted (4 h, > 1020a GLP Kenny et al. (1987)
whole body,
aerosol)
Rat Fischer 344 M&F 1% aqueous > 3200a GLP Phillips & Lomax (1989)
solution (4 h,
whole body,
aerosol)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M&F Vapour (4 h, > 36a (max. GLP Blagden (1994)
nose only) attainable
concentration)
Rat Wistar M&F Acetone (4 h, 560b (360-950) Fredrick Institute (1996a)
whole body,
nebulized particles
< 5 µm)
Dermal application
Rat Sprague-Dawley M&F PEG > 2000 Lackenby (1985b)
Rat Sprague-Dawley M&F Undiluted > 2000 GLP Jackson & Ogilvie (1994a)
Rat NR M&F Undiluted > 2000 QA Nissimov & Nyska (1984b)
Rat Fischer M&F Undiluted > 2000 GLP Jeffrey et al. (1986)
Rat Sprague-Dawley M&F Arachis oil > 2000 GLP Dreher (1994b)
Rat Sprague-Dawley M&F Saline > 5000 (intact Buch et al. (1980)
and abraded)
Rabbit Himalayan M&F Water 1233 (993-1531) QA Fredrick Institute (1995c)
Rabbit New Zealand M&F Undiluted 1580 (828-2606) Henck & Kociba (1980)
white M&F 1598 (1243-1919)
M&F 1801 (1023-3152)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat CFY M&F Tween 20/ 147 (120-179) Davies & Kynoch (1970)
DMSO/water
2:3:5 (subcutaneous)
M, male; F, female; NR, not reported; GLP, good laboratory practice; QA, quality assurance; PEG, polyethylene glycol;
DMSO, dimethyl sulfoxide
Technical-grade chlorpyrifos (100 mg; fine powder) was placed
into the right eye of nine young adult albino rabbits. The eyes of
three animals were irrigated with saline solution 30 s after
treatment, while the eyes of the remaining animals were not irrigated.
Ocular examinations were conducted after 24, 48, and 72 h and at 4 and
7 days. Most of the animals displayed slight initial pain after
instillation of the test material. No signs of irritation were
observed in the cornea or the iris. Slight conjunctival redness was
observed in all animals with unwashed eyes, and in two animals whose
eyes were irrigated after 30 s. The irritation disappeared in most
animals by days 3-4 but persisted for 7 days in a single animal with
unirrigated eyes. The mean irritation scores in rabbits with unwashed
eyes were 2.3 at 24 h and 0.3 at 7 days, while in the rabbits with
irrigated eyes the scores were 1.3 and 0, respectively (Buch &
Gardner, 1980a).
Dermal irritation: A finely ground commercial preparation of
chlorpyrifos (500 mg) moistened with distilled water was applied under
an occlusive dressing to about 6 cm2 of clipped skin on the back of
three young adult female New Zealand white rabbits as a single 4-h
application. No primary irritation was observed (Jones, 1985b).
The dermal irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested in a study conducted according to GLP in six
young male New Zealand white rabbits, to which 500 mg of the material
(moistened with water) were applied under a gauze patch on clipped
intact skin on the trunk and then covered with an occlusive dressing.
The dressings were removed after 4 h and the skin wiped to remove
residual material. The skin was examined for signs of irritation 1,
24, 48, and 72 h after removal of the patch and scored for irritation
on the basis of a system recommended by the US Environmental
Protection Agency. Further assessments were conducted after 4, 5, and
6 days to determine the reversibility of the effects. Slight erythema
(score 1) was noted on three of six test sites at 1 h, and this effect
persisted until 72 h at one site and until day 5 in another animal. No
skin irritation was seen on day 6 (Jackson & Ogilvie, 1994dc).
A technical-grade, fine-powder commercial preparation (purity not
stated) was impregnated at a dose of 0.5 g onto unmedicated lint
patches and tested in six young adult male New Zealand white rabbits
on shaved abraded and intact sites on the back. The patches were held
in place under impermeable dressings, which were removed after 23 h.
The reactions at the test sites were assessed by the method of Draize
at 24 and 72 h and 7 days after administration of the test material.
Well-defined or very slight erythema was seen in all animals at 24 h,
on both intact and abraded sites. Very slight oedema was also seen at
the intact and abraded test sites of one animal at 24 h, another at 72
h, and a third at 7 days. No skin reactions were seen in other animals
at these intervals. The primary irritation index was calculated to be
0.67 (Buch & Gardner, 1980b).
Technical-grade chlorpyrifos (purity, 96.2%) was applied at a
dose of 500 g to clipped intact and abraded skin on the trunks of
three male and three female Himalayan albino rabbits, and the trunks
were wrapped with plastic sheets held in place with tapes for 24 h. A
control group was treated with distilled water. After exposure, the
coverings were removed, but the report did not indicate whether the
test sites were washed or cleaned. The skin was observed 24 and 72 h
after application for signs of irritation. No sign of erythema or
oedema was reported. A 'quality assurance statement' was issued for
this study (Frederick Institute of Plant Protection and Toxicology,
1995e).
Technical-grade chlorpyrifos (purity, 99.3%), moistened with
0.5 ml distilled water, was applied at a dose of 0.5 g to clipped
areas on the backs of New Zealand white rabbits in a study carried out
in accordance with GLP. Surgical gauze was applied over the sites and
held in place with a strip of surgical adhesive tape, and the trunk of
each rabbit was wrapped in an elasticized corset. After the 4-h
contact period, the corset and patches were removed, and the treated
skin was wiped with cotton-wool soaked in distilled water. One hour
and approximately 24, 48, and 72 h after removal of the patches, the
test sites were examined for evidence of dermal irritation and scored
according to the method of Draize. Very slight (score 1) to
well-defined (score 2) erythema and very slight (score 1) to slight
(score 2) oedema were observed in all animals 1-24 h after removal of
the patches. At 48 h, very slight erythema was observed in three
animals and very slight oedema in one. No sign of skin irritation was
seen at 72 h. The sum of the readings at 24 and 72 h was 10, and the
primary irritation index (the sum of the readings/12) was 0.8. This
score indicates mild irritation according to Draize (Dreher, 1994d).
Dermal sensitization: The skin sensitizing potential of
technical-grade chlorpyrifos (purity, 99.3%) was assessed in female
albino Dunkin-Hartley guinea-pigs in a study conducted according to
GLP. Very slight (score 1; 10 animals) to well-defined (score 2; 2
animals) erythema and very slight (score 1; 1 animal) oedema were
observed 1 h after topical induction, but no signs of irritation were
observed at 24 h. No skin reaction was observed in control animals,
and no skin reactions were observed at the test or vehicle control
sites after topical challenge 21 days after induction. One animal was
found dead at this stage of the study, but the cause of death was not
determined. The test material was not a skin sensitizer in this study
(Dreher, 1994e).
Technical-grade chlorpyrifos (purity, 96.2%) was prepared as a 1%
solution in 0.5% carboxymethyl cellulose and tested at a dose of
100 mg in male Hartley guinea-pigs. Test and positive control groups
received induction by exposure of the shaven skin of the left flank,
and challenge 14 days after the third induction dose. Control animals
received only a challenge with 0.5 ml of a 0.1% solution. The test
animals had barely perceptible (score 1; 4/10 animals) erythema or
slight oedema (score 1; 2/10 animals) 24 h after the challenge, but no
signs of irritation were observed at 48 h. A single control animal had
barely perceptible erythema at 24 h, but no other signs of skin
irritation were seen in this group. Positive control animals had
barely perceptible to deep-red erythema (score 1-3) and slight to
marked oedema (score 1-3) 24 h after the challenge and barely
susceptible erythema and slight to marked oedema at 48 h. This study
was issued a 'quality assurance statement' (Frederick Institute of
Plant Protection and Toxicology, 1996b).
After a study to determine a suitable dosing regimen, six male
and six female young albino Dunkin-Hartley guinea-pigs were treated
with a commercial preparation of chlorpyrifos in a standard Beuhler
skin sensitization assay. The test substance (0.3 ml, 100% w/v in
polyethylene glycol on a lint pad) was applied for 6 h under an
occlusive bandage to the freshly clipped left flank of the animals on
days 1, 8, and 15. Challenge doses of chlorpyrifos and solvent were
applied for 6 h on day 29 to the right and left flanks, respectively.
The negative controls were treated only on day 29, when they received
the same treatment as the test animals. The challenge sites of both
groups were evaluated 24 and 48 h after removal of the patch. No
adverse skin reactions were seen after exposure to chlorpyrifos or
vehicle alone (Jones, 1985c).
Technical-grade chlorpyrifos (purity, 99%) was tested in young
female Dunkin-Hartley guinea-pigs by the Magnusson-Kligman
maximization test in a study conducted according to GLP. The test
consists of an induction with intradermal injections of the test
material followed after 1 week by topical application, and a challenge
3 weeks after induction. The test sites were assessed for irritation
24, 48, and 72 h after injection and 24 and 48 h after removal of the
patch (48-h exposure). A concentration of 25% of the test material in
maize oil was selected for injection in the induction phase and 75% in
maize oil for the topical induction phase; a concentration of 75% in
maize oil was also selected for the challenge. During the induction
phase, slight or discrete erythema was observed in all test and
control animals 1 and 24 h after injection and 1 h after topical
application. A number of test and control animals also showed slight
or discrete erythema 24 h after topical application. During
application, two test animals became ataxic, thin, and subdued and
were removed from the study, and one test animal was removed from the
study before the challenge application because it had lesions at the
test site. A single test animal showed a positive response to
challenge, with slight to discrete erythema. None of the animals given
the vehicle alone reacted to the challenge application (Jackson &
Ogilvie, 1994a).
Technical-grade chlorpyrifos (purity, 96.8%) dissolved in 100%
dimethylsulfoxide and diluted in the same solvent to achieve the
desired concentrations was tested in a study that conformed to GLP in
albino Dunkin-Hartley guinea-pigs. In animals treated with a single
dose, irritation ranging from scattered mild redness to moderate and
diffuse redness was seen at 24 h in 5/10 animals; only mild redness
was seen at 48 h in 4/10 animals. In 20 animals induced and then
challenged with chlorpyrifos, only one had scattered mild redness
after the challenge dose at 24 h, and no skin reaction was seen at 48
h. In a positive control group, 9/10 animals had mild redness at 24 h
and 4/10 at 48 h (Berman, 1987).
(b) Short-term studies of toxicity
Mice
In a 2-week study conducted according to GLP requirements, young
adult CD-1 mice, were given a commercial preparation of
technical-grade chrlopyrifos (purity, 96.3%) at dietary concentrations
of 0 (control), 75, 150, 300, 600, or 1200 ppm, equaivalent to 0,
14-18, 30-32, 56-67, 89-100, and 130-180 mg/kg bw per day,
respectively. Deaths and treatment-related clinical signs, including
tremor, ocular opacity, ocular staining, hunching, and lachrymation,
were seen mainly in animals at the highest dose. Marked reductions in
body weight and feed consumption were observed in animals at doses
> 600 ppm. In males, cholinesterase activity was reduced at all
doses. At 75 ppm, plasma cholinesterase activity was reduced by
> 95%, erythrocyte acetylcholinesterase activity by almost 40%, and
brain acetylcholinesterase activity by about 50%. Inhibition of
erythrocyte acetylcholinesterase activity was not dependent on dose,
and approximately 35% inhibition being seen at all doses, but plasma
and brain cholinesterase activities were reduced in a dose-related
manner, with an inhibition of 99% in plasma and about 89% in brain at
the high dose. Similar patterns of cholinesterase inhibition were seen
in females. On the basis of the results of this study, doses of 5, 50,
200, 400, and 800 ppm were selected for a 13-week dietary study in
mice (Crown et al., 1984a).
Groups of 40 male and 40 female CD-1 mice were given chlorpyrifos
(purity, 95.7%) in the diet at 0 or 15 ppm, equal to 2.7 mg/kg bw per
day for males and 3.4 mg/kg bw per day for females; 20 animals of each
sex per group were killed after 1 week, and 20 were treated for a
total of 4 weeks. There were no significant clinical signs or
unscheduled deaths. Food consumption was unaffected by treatment, but
the body-weight gain of male mice was decreased by 25% at the end of
the study. Organ weights were not affected by treatment, and there
were no significant differences between groups. The findings at
necropsy were within normal limits for all groups. After 1 week,
plasma cholinesterase activity was depressed by 88-91% and erythrocyte
acetylcholinesterase activity was depressed by 40-53%; after 4 weeks,
plasma cholinesterase activity was depressed by 91% and erythrocyte
acetylcholinesterase activity by 53%. Brain acetylcholinesterase
activity was unaffected by treatment at either time. There was no
difference between the sexes in the alterations in plasma and
erythrocyte cholinesterase activity (Davies et al., 1985).
Groups of 12 Crl CD-1 mice of each sex were given chlorpyrifos
(purity, 93.5%) in the diet at concentrations of 0, 5, 50, 200, 400,
or 800 ppm (equivalent to 0, 0.7-1.3, 7.1-14, 32-53, 41-140, and
110-300 mg/kg bw per day, respectively) for 13 weeks. This study was
conducted to the requirements of GLP. Dose-related increases in the
mortality rate and the frequency of ocular opacities were seen at 400
and 800 ppm. Body weights were reduced by 10-15% in mice at 800 ppm
throughout the study. Plasma cholinesterase activity was markedly
decreased at all doses, and decreased erythrocyte acetylcholinesterase
activity was seen, but with no clear dose-dependency; brain
acetylcholinesterase activity was inhibited in a dose-dependent manner
in males at doses > 200 ppm and in females at doses > 50 ppm
(Table 2).
Table 2. Group mean values for inhibition of cholinesterase activity in
Crl CD-1 mice given chlorpyrifos in the diet (expressed as a
percentage of control values)
Cholinesterase Sex Dose (ppm)
5 50 200 400 800
Plasma Male 45*** 95*** 98*** 99*** 99***
Female 36*** 97*** 98*** 99 *** 99 ***
Erythrocyte Male 34a 21 16 12 26
Female 11 57*** 44** 48** 44**
Brain Male 43a 14* 80*** 85*** 87**
Female 3a 28*** 58*** 84*** 88***
Plasma, butyryl cholinesterase; erythrocyte and brain,
acetylcholinesterase
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased in comparison with controls
A single malignant lymphoma was reported in a female at the high
dose at terminal sacrifice, but no other neoplastic lesions were
reported. Given the isolated nature of this finding, it was considered
not to be related to treatment. The absolute and relative organ
weights were generally unaffected by treatment, although the relative
liver weights were increased in females at 200, 400, and 800 ppm. The
clinical signs included urogenital staining in males at doses
> 200 ppm and in females at 800 ppm. Ocular opacities occurred in
some animals at 400 and 800 ppm. Dose-related histopathological
findings were seen in the adrenal glands (including lipogenic
pigmentation at doses > 200 ppm in females and at > 400 ppm in
males) and in the eyes (acute or subchronic keratitis in two males and
four females at 800 ppm and in a single female at 400 ppm).
Erythrocyte acetylcholinesterase activity was variable, and the effect
of treatment on this parameter was equivocal. The NOAEL was 5 ppm,
equal to 0.7 mg/kg bw per day, on the basis of inhibition of brain
acetylcholinesterase activity at higher doses (Crown et al., 1987).
Rats
In a 2-week study to establish the doses to be used in a 13-week
study, technical-grade chlorpyrifos (purity, 95.5%) was administered
to groups of five rats of each sex at dietary concentrations of 0
(controls), 10, 30, 84, 240, or 694 ppm for 14 days, equivalent to 0,
1.4-1.9, 4.1-5.2, 12-14, 34-39, and 66-95 mg/kg bw per day,
respectively. At the high dose, clinical signs of intoxication were
reported, consisting of irritability, hunching, tremor, ataxia,
urogenital staining, pigmented orbital secretion, failure to groom,
and proneness. No treatment-related clinical signs were observed at
other doses, and treatment-related deaths were confined to animals at
the high dose. Body weights were reduced in males and females at
694 ppm and in females at 240 ppm, and food consumption was also
reduced in animals at 694 ppm. Dose-related decreases in blood
cholinesterase activity were seen at all doses, the inhibition ranging
from 42% at 10 ppm to 93% at 694 ppm. On the basis of these findings,
the doses selected for the 13-week study were 0.5, 10, and 100 ppm
(Crown et al., 1984b).
Plasma, erythrocyte, and brain cholinesterase activities were
markedly reduced in rats after dietary administration of chlorpyrifos
for 14 days at doses of 5 or 10 mg/kg bw per day in a study carried
out according to GLP. Inhibition of cholinesterase activity occurred
in the absence of clinical signs of intoxication. Feed consumption,
body weights, and gross pathological appearance were similar in
control and treated groups. Statistically significant increases in
absolute and relative adrenal weights (about 20% compared with
controls) were observed in females only at 10 mg/kg bw per day
(Liberacki et al., 1990).
Fischer 344 rats received technical-grade chlorpyrifos (purity,
95%) by nose-only inhalation for 6 h/day for 5 days at a target
concentration of 20 ppb in a study that conformed to GLP. No deaths or
treatment-related clinical signs were reported. Exposure of female
rats to a concentration of 0.34 mg/m3 (23 ppb) resulted in a
significant decrease in plasma cholinesterase activity. No effect on
erythrocyte or brain acetylcholinesterase activity was seen in females
or on plasma, erythrocyte, or brain cholinesterase activity in males
(Newton, 1988a).
Chlorpyrifos was administered by nose-only inhalation to female
Fischer 344 rats at a time-weighted average concentration of 12 ppb
(equivalent to 172 µg/m3) for 6 h/day on 5 days per week for 2 weeks
in a study that met GLP requirements. No clinical signs of
intoxication or treatment-related changes in body weights were
reported, and all rats survived until the scheduled termination.
Clinical chemistry showed no treatment-related changes in
haematological, clinical chemical, or urinary parameters, including
plasma, brain, and erythrocyte cholinesterase activity (Landry et al.,
1986).
In a study to determine a dose range, conducted to GLP
requirements, technical-grade chlorpyrifos was administered by
whole-body exposure of groups of Wistar rats for 6 h/day, 5 days per
week for 2 weeks at target concentrations of 0 (air control), 10, 100,
and 400 mg/m3 (actual concentrations, 0, 10, 94, and 388 mg/m3).
Exposure of animals to the high dose was terminated after five
exposures because they became moribund, with rapid weight loss and
deterioration of their general condition, and all surviving animals
were killed on day 9. No deaths was observed at other doses. There was
no NOAEL in this study, as significant inhibition of plasma,
erythrocyte, and brain cholinesterase activity was seen at all doses
in males and females. Plasma cholinesterase activity was inhibited by
78, 86, and 88% at the three doses, respectively, in males and by 91,
93, and 95%, respectively, in females when compared with controls.
Erythrocyte cholinesterase activity was inhibited by 78, 58, and 75%
in males and by 60, 80, and 73% in females, respectively. Brain
cholinesterase activity was inhibited by 50% at 10 mg/m3, about 70%
at 94 mg/m3, and about 72% at 388 mg/m3 in males and females.
Clinical signs of toxicity (tremors, salivation, and lachrymation),
decreased food consumption, and decreased body weights were observed
at the two higher doses. Increased adrenal weights were seen at 94 and
388 mg/m3, and effects in the forestomach including thickening and
congestion were observed at 388 mg/m3 (Kenny et al., 1988).
Fischer 344 rats were exposed to time-weighted average
concentrations (nominal) of 0, 1, or 5 ppb (0, 0.014, and
0.072 mg/m3) of chlorpyrifos vapour for 6 h/day, 5 days per week for
2 weeks in a study conducted according to GLP. The mean daily
time-weighted average concentrations found by analysis were 0.7 and
5 ppb, respectively. Although two control groups were used, their
cholinesterase activity did not differ statistically significantly,
and the activity in test groups was compared with that of the combined
control group. Statistically significant decreases in plasma,
erythrocyte, and brain cholinesterase activity were observed at 5 ppb,
but as the reductions in brain and erythrocyte acetylcholinesterase
activity did not reach 20% when compared with the control group, the
effect was considered not to be of toxicological significance. Plasma
cholinesterase activity was inhibited by > 20% in females at 5 ppb.
A statistically significant decrease in plasma cholinesterase activity
in females at 0.7 ppb was considered to be incidental to treatment.
There were no treatment-related deaths, clinical signs of toxicity, or
changes in body weight during the study. No treatment-related effects
were seen on body or organ weights, and no lesions were found at gross
examination. No significant adverse treatment-related effects were
observed at 5 ppb (approximately 0.07 mg/m3) (Landry et al., 1985;
Streeter et al., 1987).
Groups of 10 CDF Fischer 344 rats of each sex were fed diets
containing chlorpyrifos (purity, 95.7%) at concentrations that
provided doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day for 13 weeks in
a study that conformed to GLP. The treatment-related effects seen at
the highest dose consisted of decreased body weight and body-weight
gain in males, increased fatty vacuolation of the adrenal zone
fasciculata in males, and changes in haematological and clinical
chemical parameters consisting of decreased erythrocyte counts in both
sexes; increased platelet counts, reduced serum total protein,
albumin, and globulin concentrations, and decreased alanine
aminotransferase and alkaline phosphatase activity in males; and
decreased serum glucose concentration and increased urinary specific
gravity in females. Plasma and erythrocyte cholinesterase activities
were depressed at doses > 1 mg/kg bw per day, and the activity of
brain acetylcholinesterase was depressed at 5 and 15 mg/kg bw per day.
The NOAEL was 0.1 mg/kg bw per day on the basis of depression of
erythrocyte and brain acetylcholinesterase activity (Szabo et al.,
1988).
Groups of 10 rats of each sex were given diets containing
chlorpyrifos at 0, 10, 30, 300, or 1000 ppm, but treatment at the
highest concentration was discontinued after 4 weeks because of severe
clinical signs. Plasma cholinesterase activity was reduced in a
dose-related manner and was significantly lower than that of controls
at all doses. Erythrocyte cholinesterase activity was similarly
significantly reduced at all doses, but no dose-dependency was
demonstrated. Brain cholinesterase activity showed a clear
dose-related depression in animals of each sex at doses > 30 ppm.
At 30 ppm, the activity was inhibited by 22% in males and 24% in
females 41 days after administration. No other significant effects
were found on haematological or clinical chemical parameters, but few
were examined. No histopathological lesions were found that could be
linked to treatment. The NOAEL for inhibition of brain cholinesterase
activity was 0.5 mg/kg bw per day, and the LOAEL was 1.5 mg/kg bw per
day. There was no NOAEL for inhibition of erythrocyte or plasma
cholinesterase activity, as effects were seen at concentrations
> 10 ppm (0.5 mg/kg bw per day) (JMPR 1972; modified by reference
to the original reports of Beatty & McCollister, 1964; Beatty &
McCollister, 1971).
Five groups of 10 rats (strain not specified) of each sex
received chlorpyrifos (purity not specified) in the diet at
concentrations that provided doses of 0.03-10 mg/kg bw per day. The
doses of 0 and 0.3 mg/kg bw per day were fed for 90 days; the doses of
1, 3, and 10 mg/kg bw per day were fed for 28 days, when these animals
were fed control diets for 3 weeks and then diets containing 0, 0.03,
or 0.1 mg/kg bw per day. Behaviour and growth were affected at 3 and
10 mg/kg bw per day. Cholinesterase activity was depressed at 0.3
mg/kg bw per day. During the period of removal from treated diet, the
cholinesterase activity returned to pre-test levels; on resumption of
feeding at 0.1 mg/kg bw per day, only a slight depression was observed
in plasma and erythrocyte cholinesterase activity. In view of of the
unusual design of this study, the toxicological relevance of this
effect is considerede equivocal. No depression of brain or blood
enzymes was seen at 0.03 mg/kg bw per day (Blackmore, 1968).
Groups of 10 Crl rats of each sex were fed diets containing
chlorpyrifos (purity, 97.5%) at concentrations that provided a dose of
0.3 mg/kg bw per day for 13 weeks or 1, 3, or 10 mg/kg bw per day for
4 weeks. No deaths occurred during the study. After 4 weeks, there was
a clear dose-response relationship for inhibition of erythrocyte and
plasma cholinesterase activity at all doses, and animals at higher
doses showed clinical signs of toxicity. These animals were therefore
removed from exposure and allowed to recover for 3 weeks, during which
time the plasma and erythrocyte cholinesterase activities returned to
control values. These groups were then fed chlorpyrifos at doses of 0,
0.03, or 0.1 mg/kg bw per day for 13 weeks. The dose of 0.03 mg/kg bw
per day had no unequivocal effect on cholinesterase activity in
erythrocytes, plasma, or brain in either sex, but the dose of
0.1 mg/kg bw per day significantly reduced the erythrocyte
cholinesterase activity. The NOAEL was 0.03 mg/kg bw per day on the
basis of inhibition of erythrocyte cholinesterase activity after 13
weeks at 0.1 mg/kg bw per day (Hazleton Laboratories, 1968).
Groups of 20 Sprague-Dawley rats of each sex were given
chlorpyrifos (purity not stated) in the diet at 0, 0.03, 0.15, or
0.75 mg/kg bw per day for 6 months; an interim sacrifice of five
animals of each sex per dose was conducted after 3 months. A total of
16 animals across dose groups died from chronic murine pneumonia.
Cholinesterase activity was inhibited at the high dose in both
erythrocytes (50%) and plasma (65%), but the activity in brain was not
inhibited at any dose. Treatment did not change body-weight gain, food
consumption, or haematological or other clinical chemical parameters.
The NOAEL was 0.15 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholinesterase activity at 0.75 mg/kg bw per day
(JMPR, 1972; modified by reference to the original report of Coulston
et al., 1971).
Groups of 20 Crl CD Sprague-Dawley-derived rats of each sex were
given chlorpyrifos (purity, 95.5%) at dietary concentrations of 0
(control), 0.5, 10, or 200 ppm for 13 weeks in a study conducted
according to GLP. The minimum and maximum achieved doses of the test
material were calculated to be 0, 0.03 and 0.078, 0.6 and 1.5, and 13
and 31 mg/kg bw per day, respectively. All animals were inspected once
or twice daily for signs of ill-health and other treatment-related
signs and all were examined closely each week. No deaths or
significant clinical signs were observed at any dose. Clinical
chemistry, urinary analysis, and ophthalmoscopy revealed no findings
that were considered to be related to treatment. At 200 ppm
(approximately 13 mg/kg bw per day), reduced body weights, erythrocyte
count, erythrocyte volume fraction, and haemoglobin and increased food
consumption were observed. Statistically significant reductions
(> 20% inhibition compared with controls) in plasma cholinesterase
activity were observed in males at doses > 0.5 ppm (approximately
0.03 mg/kg bw per day) at 12 weeks and in females at doses > 10 ppm
(approximately 0.6 mg/kg bw per day). The inhibition was dose-related
in males but not in females. Erythrocyte and brain cholinesterase
activities were not measured in this study. The NOAEL was 10 ppm,
equal to 0.6 mg/kg bw per day, on the basis of reduced body weights
and statistically significant reductions in packed cell volume,
haemoglobin, and erythrocyte volume fraction at 200 ppm (Crown et al.,
1985).
Groups of 10 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 100%) by nose only at concentrations of 0, 5.2,
10.3, or 20.6 ppb (0, 75, 150, or 300 µg/m3) for 6 h/day, 5 days per
week for 13 weeks in a study that conformed to GLP. No
treatment-related deaths and only minimal clinical signs were observed
during the study. Body weights and urinary, haematological, and
clinical chemical parameters were unaffected by treatment. No effects
on plasma, erythrocyte, or brain cholinesterase activity were seen at
any dose. Gross and histopathological examination revealed no effects
associated with administration of chlorpyrifos (Corley et al., 1986).
Groups of 15 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 95%) by nose only for 6 h/day, 5 days per week,
for 13 weeks at target concentrations of 0 (control), 5, 10, and
20 ppb (0, 0.07, 0.14, and 0.28 mg/m3, respectively). The study was
conducted according to GLP requirements. There were no
treatment-related deaths or ophthalmic, haematological, or clinical
chemical changes. Plasma cholinesterase activity was inhibited by 23%
in males at the high dose ( p < 0.01), but other changes in plasma
cholinesterase activity were considered not to be related to
treatment. Erythrocyte and brain cholinesterase activities were
unaffected. Pathological and histopathological examination showed no
effect of treatment. The NOAEL was 20 ppb (0.28 mg/m3), on the basis
of inhibition of plasma cholinesterase activity. The absence of frank
toxicity at any dose in this study makes it difficult to draw
conclusions about the toxic potential of inhaled chlorpyrifos at doses
> 0.28 mg/m3 (Newton, 1988b).
In a study in Fischer 344 rats treated dermally, conducted
according to GLP requirements, the animals received chlorpyrifos
(purity, 100%) at 0, 0.1, 0.5, or 5 mg/kg bw per day in corn oil for a
total of 15 days over a 21-day period. No treatment-related effects
were observed at any dose, and plasma, erythrocyte, and brain
cholinesterase activities were not inhibited. Gross and microscopic
examination did not reveal any treatment-related changes, and body
weights and organ weights were unaffected by treatment. In the 4-day
range-finding component of this study, decreased plasma cholinesterase
activity (45%) and erythrocyte cholinesterase activity (16%) were
observed at 10 mg/kg bw per day, in the absence of clinical signs of
intoxication (Calhoun & Johnson, 1988, 1989).
Rabbits
No skin irritation was found when a formulation containing 61.5%
chlorpyrifos and 35% xylene was applied to the skin of the back and
abdomen of groups of two rabbits at doses of 5, 10, 25, and 50 mg/kg
bw 20, 4, 3, and 1 times, respectively. Both plasma and erythrocyte
cholinesterase activities were significantly inhibited in each dose
regimen. The values for plasma recovered more quickly than those for
erythrocytes, which were still significantly inhibited at day 40 after
the 3-, 4-, and 20-day exposure patterns (Pennington & Edwards, 1971).
Three rabbits were exposed for 5 min to a formulation containing
61.5% chlorpyrifos and 34.5% xylene from an ultra-light vapour cold
aerosol fog generator delivering 3.8 L/h. Two rabbits were exposed at
a distance of 8 m at a height of 1.3 m and one at a height of 0.8 m.
Exposure was terminated after 5 min because of ocular and pulmonary
irritation. The recorded concentration of chlorpyrifos in
breathing-space air was about 108 mg/L (range, 83-133 mg/L). By 24 h
after treatment, the rabbits had decreased activities of plasma
cholinesterase (< 33%) and erythrocyte cholinesterase (< 12%),
but these values had recovered almost to the control level by 72 h
after treatment (Pennington & Edwards, 1971).
Chickens
White Leghorn chickens were given chlorpyrifos in their
drinking-water at 1 ppb, 1 ppm, or 100 ppm for up to 84 days. Only
plasma cholinesterase activity was reported because of technical
difficulties with blood sampling. Significant inhibition (54%) of
plasma cholinesterase activity was recorded on day 84 in birds at 100
ppm (Stevenson, 1965).
Dogs
Technical-grade chlorpyrifos (purity, 95.8%) was administered to
groups of two pure-bred beagles of each sex orally in gelatine capsule
at doses of 0 (control; lactose only), 0.01, 0.03, 0.5, or 5 mg/kg bw
per day for 4 weeks, although only one animal of each sex was exposed
at 0 and 0.5 mg/kg bw per day. This study met GLP requirements. No
deaths occurred during the study, and no clinical signs associated
with treatment were observed. All animals gained weight steadily
during treatment, and food consumption was similar in all groups.
Rapid inhibition of plasma cholinesterase activity was observed at 0.5
and 5 mg/kg bw per day at all intervals, and the inhibition was both
dose- and time-dependent. Plasma cholinesterase activity was reduced
at 0.03 mg/kg bw per day, but the inhibition was considered not to be
significant owing to variation between groups and the small group
sizes. In animals at 5 mg/kg bw per day, erythrocyte cholinesterase
activity was 47% that of controls on day 7 and was still inhibited by
> 70% at the end of the study; brain cholinesterase activity was
inhibited by 32% in comparison with concurrent controls at the end of
the study. In animals at 0.5 mg/kg bw per day, erythrocyte
cholinesterase activity was inhibited by 30% on days 27-28 only, and
the effect was considered not to be toxicologically significant owing
to its transient nature. Macroscopic post-mortem examination showed no
changes that were considered to be of toxicological significance. The
NOAEL was 0.5 mg/kg bw per day, on the basis of inhibition of
erythrocyte and brain cholinesterase activity at 5 mg/kg bw per day
(Harling et al., 1989a).
Five dogs received 10 applications of a 0.087% commercial aerosol
formulation of chlorpyrifos in dichlorophene on their backs (with the
fur brushed against the grain) for 2 weeks, each application lasting
30 s. No deaths occurred, and no abnormalities were seen in clinical
signs, body-weight gain, or opthalmoscopic, haematological or clinical
chemical parameters. Erythrocyte cholinesterase activity was not
decreased, but plasma cholinesterase activity was inhibited from the
beginning of treatment, returning to normal 72 days after treatment.
The inhibition of plasma cholinesterase activity was not accompanied
by clinical signs (Sharp & Warner, 1968).
Groups of two young adult beagles of each sex were given daily
doses of chlorpyrifos orally by capsule at doses of 0, 0.03, 0.1, 0.3,
or 1 mg/kg bw per day for 90 days. Plasma cholinesterase activity was
inhibited at all doses. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day, and that for
inhibition of brain cholinesterase activity was 1 mg/kg bw per day
(Blackmore, 1968).
Technical-grade chlorpyrifos (purity, 95.8%) was administered
orally by capsule to groups of four pure-bred beagles of each sex at
doses of 0 (control), 0.01, 0.22, or 5 mg/kg bw per day for 13 weeks.
There were no unscheduled deaths or unequivocal clinical signs during
the study. The group mean body weights of animals at the high dose
were reduced. Haematology, clinical chemistry, urinary analysis, and
ophthalmoscopy revealed no findings that were considered to be related
to treatment. No treatment-related pathological macroscopic or
microscopic change was found. Statistically significant, dose-related
reductions in plasma cholinesterase activity were seen at 0.01 mg/kg
bw per day (bitches only; week 6 only; < 25%) and above (dogs and
bitches; all sample intervals; < 87%). Erythrocyte cholinesterase
activity was significantly inhibited in a dose-dependent manner at
0.22 mg/kg bw per day (< 46%) and at 5 mg/kg bw per day (< 85%).
At 0.01 mg/kg bw per day, erythrocyte cholinesterase was inhibited by
17-18% at week 12, but the reduction was not statistically significant
at any sample interval. Brain cholinesterase activity was inhibited in
animals of each sex (by 46%) at 5 mg/kg bw per day, and the NOAEL for
this effect was 0.22 mg/kg bw per day. The NOAEL for the study, on the
basis of inhibition of erythrocyte acetylcholinesterase activity, was
0.01 mg/kg bw per day (Harling et al., 1989b).
Beagles aged 10 and 11 months were given diets containing
chlorpyrifos (purity, 97.2%) at concentrations that provided doses of
0, 0.01, 0.03, 0.1, 1, or 3 mg/kg bw per day for 1 year (one animal of
each sex per dose), 1 year with a 3-month recovery period (two animals
of each sex per dose), or 2 years (four animals of each sex per dose).
Clinical signs were recorded daily, and body weights were recorded
weekly for the first 6 months and every 2 weeks thereafter. Food
intake was recorded weekly during the first 3 months and monthly
thereafter. The clinical investigations in the groups receiving 0, 1,
or 3 mg/kg bw per day included haematology (packed cell volume,
haemoglobin, erythrocyte and leukocyte counts, and prothrombin time)
and urinary analyses (specific gravity, occult blood, ketones, solids,
pH, albumin, and sugar). The serum concentration of blood urea
nitrogen and the activities of alkaline phosphatase and alanine and
aspartate aminotransferases were measured in all dogs before treatment
and at various intervals thereafter. Bromsulphalein retention was
measured before treatment and at the 1-year sacrifice of animals at 0,
1, or 3 mg/kg bw per day. Cholinesterase activity was determined in
plasma and erythrocytes in most groups before treatment and at various
intervals during the study, and the activity in brain was measured at
necropsy. Fasted dogs were necropsied and the brain, heart, liver,
kidney, spleen, and testes were weighed. Portions of these organs and
a standard set of tissues were preserved from the controls and animals
at 1 (after 1 year only) and 3 mg/kg bw per day for histopathological
examination. The animals fed chlorpyrifos for 2 years were given
complete physical examinations before the end of the study, including
routine neurological and opthalmoscopic evaluations. The data were
evaluated only with Student's t test.
The body weights were not significantly affected by treatment,
and food consumption was comparable in groups of the same sex.
Consumption of the test compound was monitored regularly and reported
to approximate the nominal concentrations closely. The organ weights
were not affected by treatment, except for an increase in the mean
relative liver weight in males at 3 mg/kg bw per day (3.5 g/100 g vs
2.5 g/100 g in controls) in the 2-year study. Details of the recorded
clinical signs were not presented, and the authors simply stated that
"No clinical signs of toxicity were observed in any of the dogs in
either phase of the experiment." The results of clinical pathology
were presented as individual and summary data. There were no
unequivocal treatment-related differences between control and treated
groups in the limited haematological parameters measured nor in
urinary analyses. No effect of treatment was found on blood urea
nitrogen, alkaline phosphatase or alanine and aspartate
aminotransferase activities or Bromsulphalein retention. The results
of gross pathological examination were reported in the form of a
general summary. None of the findings indicated an effect of
treatment. The results of the limited histopathological examinations
(control and high dose only) were reported in a summary table with no
indication of the severity of lesions or abnormalities; individual
data were not presented. The available data did not indicate any
treatment-related effects.
Cholinesterase activity was presented for individual animals and
for groups (Table 3). Inhibition was evident within nine days (the
first assay time) of the start of the study, and the values recorded
at termination were similar to those recorded at interim assays.
Plasma cholinesterase activity was decreased in a dose-related manner
at both times, and erythrocyte cholinesterase activity was similarly
depressed, females being slightly more severely affected than males.
Brain cholinesterase activity was the least affected by treatment. The
cholinesterase activity of animals that were allowed to recover,
assayed after 2, 6, and 13 weeks on normal diet, showed a return to
control levels after 2 weeks for plasma activity and after 13 weeks
for erythrocyte activity.
Table 3. Percentage inhibition of cholinesterase activity in beagles treated with
chlorpyrifos in the diet in comparison with concurrent controls
Dose Inhibition of cholinesterase activity (%)
(mg/kg bw per day)
Plasma Erythrocytes Brain
Male Female Male Female
One year (males and females)
3 72 70 79 82 8
1 62 62 63 61 3
0.1 39 36 18 30 0
0.03 18 17 2 33 0
0.01 1 1 16 13 0
Two years (males)
3 77 67 83 70 20
1 69 50 68 66 7
0.1 52 38 27 41 8
0.03 23 22 0 6 7
0.01 2 0 14 6 1
Average values at terminal sacrifice
Inadequacies in data collection and recording were noted.
Although the long-term effects of dietary intake of chlorpyrifos could
not be evaluated in the absence of complete pathological and
histopathological examination, the data were adequate for analysis of
inhibition of cholinesterase. Plasma cholinesterase activity was
inhibited in animals of each sex at 0.03 mg/kg bw per day. Erythrocyte
cholinesterase activity was inhibited by > 20% at 0.1 mg/kg bw per
day at both the 1- and 2-year intervals, but the inhibition did not
consistently reach statistical significance when compared with the
values in concurrent controls or with pre-treatment values. This was
due in part to the small group sizes and the variation in this
parameter between groups, including the control group, the activity
differing by as much as twofold between individual animals. Under
these conditions, the erythrocyte cholinesterase activity would have
had to be inhibited by as much as 50% in comparison with controls
before statistical significance was achieved. The NOAEL was 0.1 mg/kg
bw per day on the basis of inhibition of erythrocyte cholinesterase
activity at higher doses. Inhibition of brain cholinesterase activity
was seen only at 3 mg/kg bw per day after 2 years (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971a).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not
consistently include basic observations. Individual body weights,
ophthalmological data, the results of pre-treatment and terminal
physical examinations, and an inventory of histopathological findings
were included. The conclusions of the original study were unchanged,
with the additional observation that no effect of treatment was
detected by ophthalmology (Kociba et al., 1985).
Rhesus monkeys
Groups of one or two rhesus monkeys of each sex were given
technical-grade chlorpyrifos in 1% aqueous gum tragacanth by stomach
tube at doses of 0, 0.08, 0.4, or 2 mg/kg bw per day for 6 months.
There were no treatment-related changes in body-weight gain, food
consumption, clinical findings, or haematological, clinical chemical,
or histopathological parameters. Plasma cholinesterase activity was
depressed at all doses at 16 weeks and at > 0.4 mg/kg bw per day at
24 weeks, and erythrocyte cholinesterase activity was depressed at 0.4
and 2 mg/kg bw per day (Table 4). The NOAEL for erythrocyte
cholinesterase inhibition was 0.08 mg/kg bw per day and that for brain
cholinesterase inhibition was 2 mg/kg bw per day. The usefulness of
this study was limited by the small number of animals tested (JMPR
1972; modified by reference to the original paper of Coulston et al.,
1971).
Table 4. Mean cholinesterase activity (and percent inhibition in
comparison with controls) in rhesus monkeys given chlorpyrifos
by stomach tube
Cholinesterase Time Dose (mg/kg bw per day)
activity (weeks)
0 0.08 0.4 2
Plasma 16 6.0 4.4 (26%) 1.6 (72%) 1.8 (70%)
24 4.4 3.8 (14%) 1.7 (61%) 2.0 (55%)
Erythrocytes 16 9.5 8.2 (13%) 6.5 (32%) 2.6 (72%)
24 8.6 7.6 (13%) 6.3 (27%) 5.4 (38%)
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity conducted in accordance with test
guidelines 83-2 of the US Environmental Protection Agency and OECD 451
and in compliance with GLP standards, technical-grade chlorpyrifos
(stated purity, 95.9%) was mixed in maize oil and incorporated into a
commercially available powdered animal diet to produce dietary
concentrations of 0 (control), 5, 50, and 250 ppm, calculated to be
equal to 0.7-1.1, 6.1-12, and 32-55 mg/kg bw per day in males and
0.7-1.2, 6.6-12, and 34-62 mg/kg bw per day in females. The test diets
were administered to groups of 64 CD-1 of each sex, 3 weeks old, for
at least 79 weeks, terminal sacrifice being undertaken during weeks
80-82. The animals continued to receive the treated diets until
scheduled necropsy. An additional 12 males per group were treated
identically to the mice in the main groups and were used to replace
animals for reasons unrelated to treatment (such as excessive
aggression). All of the unused spares were discarded after 10 weeks of
treatment. Each animal was weighed weekly for the first 13 weeks of
the study and every 2 weeks thereafter, while food consumption was
measured weekly for 13 weeks and monthly thereafter. The animals were
examined daily for clinical signs of intoxication, and a careful
examination, including palpation, was conducted weekly. Blood smears
were prepared from all mice after 12 and 18 months for differential
leukocyte counts, but only those from controls and animals at the high
dose were examined. Plasma, erythrocyte, and brain cholinesterase
activity was determined in five animals of each sex at each dose at 9
and 18 months. All animals were examined grossly, the examination
including all external surfaces, the cranial, thoracic, abdominal, and
pelvic cavities, and the carcass. A large number of organs and tissues
from all animals were fixed for histopathological examination; the
eyes, kidneys, livers, lungs, and thyroids/parathyroids from animals
at all doses were examined, as were other tissues from control and
animals at the high dose and those from animals that died during the
study.
There was no adverse effect on survival, and no treatment-related
increase in the incidence of neoplastic lesions. Treatment-related
clinical signs (ocular opacities, excess lachrymation, and cranial
hair loss) and reductions in body weights and food consumption were
observed at 250 ppm (approximately 32 mg/kg bw per day). The effects
on cholinestrase activity are summarized in Table 5. Plasma
cholinesterase activity was inhibited at all doses. Determinations of
erythrocyte cholinesterase activity revealed > 20% inhibition at 42
weeks and about 30% inhibition at week 78 in animals at 250 ppm but
considerable intra-group variation at other doses. In addition, the
erythrocytes were washed with saline before the cholinesterase
determinations at 78 weeks but not at 42 weeks. The NOAEL for
inhibition of erythrocyte cholinesterase activity was 5 ppm, with a
statistically significant inhibition of 29% in males at 78 weeks and
41% in females at 42 weeks in animals at 50 ppm. Significant
inhibition of brain cholinesterase activity ( p < 0.001 or
p < 0.01) was observed in males and females at 250 ppm at 42 and 78
weeks, the activity being reduced by 80-86% when compared with
controls. At 50 ppm, decreases in activity of 43-47% were seen in
males at 42 and 78 weeks and in females at 42 weeks, but this finding
reached statistical significance ( p < 0.05) in females only at 42
weeks and in males only at 78 weeks. At 5 ppm, brain cholinesterase
activity was reduced in males at 78 weeks (27% reduction), but this
Table 5. Percent inhibition of cholinesterase activity in groups of five mice
fed diets containing chlorpyrifos
Cholinesterase Interval Dose (ppm)
(weeks)
5 50 250
Male Female Male Female Male Female
Plasma 42 4*** 45*** 95*** 97*** 98*** 99***
78 49* 50*** 95*** 96*** 98*** 98***
Erythrocyte 42 1 15 11 41** 22 23
78 - +10a 29* 4 31* 29**
Brain 42 8 1 43 46* 80** 85***
78 27 +13a 47 9 86** 84***
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased activity in comparison with controls
finding was not statistically significant and was considered to be
incidental to treatment. The NOAEL for inhibition of brain
cholinesterase activity was 5 ppm.
Non-neoplastic effects were observed at the high dose, in the
livers (slight subchronic pericholangitis, histiocytic proliferation,
and centrilobular hepatocytic fatty vacuolation) of males and in the
eyes of males and females. No treatment-related lesions were seen at
lower doses. In males at the high dose, an increased incidence of lung
nodules was seen macroscopically, with rates of 5/31 in controls, 4/26
at 5 ppm, 2/37 at 50 ppm, and 14/49 at 250 ppm. The nodules were
diagnosed histologically as bronchio-alveolar adenomas or carcinomas,
but the incidences were stated to be within the range in historical
controls. Nevertheless, two sets of data were reported for the
incidence of neoplasms in historical controls: values obtained from
24-month studies and a published report on spontaneous neoplasms in
CD-1 mice in a number of testing laboratories. Those used in the
report were less than ideal. The use of background incidence from
24-month studies would be expected to increase the range of
spontaneous tumours over that in the 78-week protocol used in this
study. In addition, the tumour incidence was reported in a published
paper (Maita et al., 1988) as a percentage of the animals examined,
while the historical data from the 24-month study and the tumour
incidence in this study were given as percentages of the number of
organs examined. Histopathological examination did not reveal any
statistically significant increase in the incidence of neoplasms in
treated animals when compared with controls. The NOAEL was 5 ppm
(0.7 mg/kg bw per day) on the basis of inhibition of erythrocyte and
brain acetylcholinesterase activity (Gur et al., 1991).
Groups of 56 CD-1 mice of each sex were maintained on diets
containing 0, 0.5, 5, or 15 ppm of chlorpyrifos (purity, 99.6%) for
105 weeks. They were observed daily for clinical signs, body weights
were recorded monthly, and daily food consumption was calculated from
a 3- or 4-day measurement made once a month. Gross pathological
examination was performed on all animals that died, when the weights
of the brain, testes, heart, kidneys, and liver were recorded and a
range of other tissues were collected. Sections were prepared of all
the these tissues for histopathology. Haematology was limited to
examination of smears of blood collected from the tail vein at
terminal sacrifice.
The body weights and absolute and relative organ weights were not
significantly affected by treatment over the course of the study, and
the mean body weight of all treated female mice was greater (< 13%)
than that of controls from day 84 onwards, although food consumption
was comparable in all groups. The mortality rate was also generally
unaffected by treatment, and treated males had slightly higher
survival rates than controls: 39% in controls, 46% at 0.5 ppm, 50% at
5 ppm, and 48% at 15 ppm; the survival rates of females were lower
than those of controls only at the highest dose: 46% in controls, 59%
at 0.5 ppm, 46% at 5 ppm, and 38% at 15 ppm. There were no clinical
signs or pathological findings that indicated an effect related to
treatment. A significant increase was observed in the incidence of
spindle-cell hyperplasia of the adrenal gland in animals of each sex
given 0.5 ppm chlorpyrifos and in males given 5 ppm. This finding was
not considered to be related to treatment as there was a high
background incidence in ageing mice and no dose-response relationship.
Vacuolation was found in sciatic nerve preparations from 2/56 male and
3/54 female controls, 5/54 males and 8/55 females at 0.5 ppm, 2/56
males and 2/52 females at 5 ppm, and 6/52 males and 8/53 females at 15
ppm. The incidences of alveologenic adenomas and hyperplastic nodules
in the liver were significantly increased only in males at 5 ppm.
These findings were considered to be incidental.
This study was barely adequate for assessing the carcinogenicity
and long-term toxicity of chlorpyrifos, as it had serious shortcomings
in design, method, and data collection. No clinical pathology was
performed, and in particular cholinesterase activity was not measured;
no clinical signs were reported; individual data were not presented
for several parameters, including histopathology; and intestinal
parasites (nematodes) were present in all groups (JMPR, 1982; modified
by reference to the original report of Warner et al., 1980).
Rats
In a 2-year study, groups of 25 male and 25 female Sherman rats
received chlorpyrifos (purity, 97.2%) in the diet at doses of 0, 0.01,
0.03, 0.1, 1, or 3 mg/kg bw per day (dietary concentrations not
provided). Supplementary animals were included at each dose to allow
interim sacrifices. The design of the study is shown in Table 6.
Clinical signs were recorded 'frequently', body weights were recorded
twice weekly for the first 4 weeks, weekly for 2-6 months, and every 2
weeks for the remainder of the study. The food intake of the main
group was recorded continuously during the first 3 months and once a
month thereafter. The clinical investigations included haematology
(packed cell volume, haemoglobin, and erythrocyte and leukocyte
counts) and urinary analysis (solids, pH, albumin, sugar, occult
blood, ketones) for five rats of each sex at 0, 1, and 3 mg/kg bw per
day; the serum concentration of urea nitrogen and the activities of
alkaline phosphatase and alanine aminotransferase were measured in all
rats at sacrifice at 12 and 18 months, in all male survivors and in
four to five females at 24 months. Cholinesterase activity was
determined in plasma, erythrocytes, and brain samples, as shown in
Table 6. Rats in groups A, H, I, and K were necropsied in a standard
manner, and the weights of the brain, heart, liver, kidney, spleen,
and testes were recorded. Portions of these organs and a standard set
of tissues were preserved for histopathology, but only the slides for
the controls, animals at the high dose, and animals at 1 mg/kg bw per
day at the 12-month necropsy were examined. Some tissues from animals
that died during the study and from those animals killed when moribund
were also examined histologically. Statistical evaluation of the data
was limited to Student's t test.
Body weights (data presented only as graphs) were generally not
significantly affected by treatment. No details of the recorded
clinical signs were presented; rather, the authors stated that "No
changes in appearance or demeanour or signs of toxicity were observed
grossly in any of the rats. No evidence of a cholinergic response was
noted at any time." The results of clinical pathology were presented
as individual and summary data, and no unequivocal treatment-related
differences between control and treated groups were found for the
limited haematological parameters measured or for the results of
urinary analyses. Treatment had no effect on serum concentrations of
urea nitrogen or on alkaline phosphatase and alanine aminotransferase
activity.
Plasma cholinesterase activity was significantly decreased at 3
mg/kg bw per day at all assay times, the inhibition ranging from 20 to
40% in males and 55 to 74% in females; the activity was less severely
inhibited at 1 mg/kg bw per day, but from 6 months onwards the
inhibition was 18-38% in males and 50-69% in females when compared
with controls, and these differences were statistically significant at
most assay times. Erythrocyte cholinesterase activity was severely
inhibited in animals of each sex at the higher doses at all assay
times, with rates of 60-100% at 3 mg/kg bw per day, 13-90% at 1 mg/kg
bw per day, and little effect at the other doses. Animals that had
Table 6. Design of the long-term study of toxicity and carcinogenicity in groups of
56 mice fed diets containing chlorpyrifos
Group No. of rats of Sacrifice interval End-point examined
each sex per dose
A 25 2 years Necropsy, blood and brain
cholinesterase
B 5 1 week Blood cholinesterase
C 5 1 month Blood cholinesterase
D 5 3 months Blood cholinesterase
E 5 6 months Blood and brain cholinesterase
F 5 9 months Blood cholinesterase
G 6 12 months Blood and brain cholinesterase
H 5 12 months Necropsy
I 7 12 months, Necropsy, blood and brain
7-8-week recovery cholinesterase
J 7 18 months Blood and brain cholinesterase
K 7 18 months Necropsy
been allowed to recover showed full restoration of cholinesterase
activity. Brain cholinesterase activity was significantly inhibited by
treatment at 3 mg/kg bw per day (30-53% inhibition) at all assay
times, to a lesser degree at 1 mg/kg bw per day (3-16%), and was
comparable to that of controls at other doses. Animals that were
allowed to recover showed restoration of brain cholinesterase
activity.
This study was considered to be inadequate for assessing the
carcinogenicity and long-term effects of chlorpyrifos, other than
clinical chemical findings including cholinesterase inhibition, as
there were serious shortcomings in data collection, and the reports of
gross and histopathology were inadequate and no clinical findings were
reported. The NOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day on the basis of
significant inhibition at 1 mg/kg bw per day. The NOAEL for inhibition
of brain acetylcholinesterase activity was 1 mg/kg bw per day on the
basis of significant inhibition at 3 mg/kg bw per day (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971b).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not include
basic observations such as abnormal behaviour or gait. Individual body
weights and gross and histopathological findings were included in the
supplement, but the histopathology was limited and variable, only a
small number of tissues from each rat being examined consistently. The
results were not presented as summary tables of incidence and
severity. The conclusions of the original study remain unchanged by
this supplement (McCollister et al., 1985).
In a 2-year study conducted according to accepted test guidelines
(OECD 451, USEPA 83-5) and GLP, groups of 60 Fischer 344 rats of each
sex were fed diets containing technical-grade chlorpyrifos (purity,
96.1%) at concentrations of 0, 0 (vehicle control), 0.2, 5, or
100 ppm. The vehicle used was 0.04% w/w maize oil. The diets were
prepared weekly and assayed regularly for stability and accuracy of
dosing. Body weights were generally recorded weekly; food consumption
was recorded weekly for 13 weeks and monthly thereafter. Clinical
signs were recorded daily and palpation performed weekly.
Cholinesterase activity was measured before treatment and at weeks 14,
32, 45, 50 (interim sacrifice of five animals of each sex per group
for measurement of brain acetylcholinesterase activity), 78, and 104
weeks (terminal sacrifice with measurement of brain
acetylcholinesterase activity in 10 animals of each sex per group),
and generally on 10 rats of each sex per dose. Haematological
parameters were measured in blood smears from 10 rats of each sex in
the vehicle control group and at 100 ppm at months 12 and 18. Gross
pathological findings were recorded for all animals at necropsy,
including those that died during the study or were killed at early
sacrifices. Necropsy included removal of the eyes with the optic nerve
and adnexa. The weights of the brain, testes, thyroids, adrenals,
kidneys, and liver were recorded, and a range of tissues was
collected. All gross lesions were sectioned, and sections from all
control animal and those at 100 ppm were examined. Appropriate
statistical tests were used to analyse the data.
The mean consumption of chlorpyrifos was estimated to be 0.012,
0.3, and 6 mg/kg bw per day for animals of each sex. Mortality rates
and the incidences of clinical signs or palpable masses were not
affected by treatment. A 5% reduction in the mean body weights of
males at 100 ppm was significant in comparison with controls at most
times during weeks 3-94, and the weights of females at this dose were
significantly lower (about 4%) than those of controls at most times
during weeks 2-64. A variety of non-neoplastic and neoplastic lesions
were recorded, and the incidence of these lesions occasionally showed
a positive trend with dose; however, the incidence was generally
within the range of historical controls in the laboratory or within
the range of published values from the National Toxicology Program in
the USA. The incidences of neoplastic lesions did not show unequivocal
or statistically significant dose-response relationships. The only
observation that was considered to be related to treatment was an
increased incidence of cataracts and diffuse retinal atrophy in
females (Table 7).
Table 7. Incidences of ocular abnormalities found microscopically in rats given
diets containing chlorpyrifos
Abnormality Sex Dose (ppm)
0 0 (vehicle) 0.2 5 100
Diffuse retinal atrophy Male 4/60 0/60 3/23 1/28 3/60
Female 5/60 15/59* 9/60 5/58 24/60*
Cataract Male 31/60 31/60 7/23 6/28 30/60
Female 38/60 38/59 33/60 32/58 51/60*
* Significantly different from controls at p < 0.01 or p < 0.001
Significant (> 20%) inhibition of plasma cholinesterase activity
relative to the control values was observed at doses > 0.3 mg/kg bw
per day (Table 8). While erythrocyte cholinesterase activity was
inhibited in animals at 100 ppm, the variation in the data and the
lack of statistical significance and/or dose-response relationships
mitigated against an unequivocal finding. The NOAEL for inhibition of
brain cholinesterase activity was 0.3 mg/kg bw per day (Crown et al.,
1988).
In a study conducted in compliance with GLP standards, groups of
60 Fischer 344 rats of each sex received diets containing chlorpyrifos
(purity, 98.5%) at concentrations that provided doses of 0 (control),
0.05, 0.1, 1, or 10 mg/kg bw per day for 24 months. Ten rats of each
sex at each dose were randomly designated at the start of the study
for interim sacrifice at 12 months. Blood was collected for
haematology, clinical chemistry, and measurement of plasma and
erythrocyte cholinesterase activity at 6, 12, and 18 months as well as
at the 24-month terminal sacrifice. The groups were observed daily for
deaths and signs of toxicity. All rats were examined clinically at
least once a week from after the sixth month. All animals were
palpated for externally detectable masses before treatment, before the
12-month kill, and monthly thereafter. Body weights and feed
consumption were determined weekly for the first 3 months and monthly
thereafter. All rats were weighed, but feed consumption was determined
for only 20 rats of each sex per group.
All clinical laboratory procedures scheduled for 6 and 12 months
were performed on rats designated for the 12-month interim kill,
whereas tests scheduled for 18 and 24 months were performed on rats
designated for terminal sacrifice. Blood samples were obtained for
haematology and clinical chemistry by orbital sinus puncture under
Table 8. Group mean percent inhibition of cholinesterase activity in comparison
with vehicle controls in rats given diets containing chlorpyrifos
Assay time Dose Cholinesterase inhibition (%)
(weeks) (ppm)
Plasma Erythrocytes Brain
Male Female Male Female Male Female
50 0 0 7 0* 31 0 35
0.2 1 4 0 42 0 14
5 15 51 0* 39 9 10
100 93 98 13 45 57* 80*
78 0 0 3 27 0
0.2 4 3 11 0
5 28* 47* 0 0
100 93* 97* 10 0
104 0 8 9 0 0 18 4
0.2 13 0 0 0 0 0
5 36 37* 17 11 0 0
100 95* 96* 34 18 58* 61*
Values the same as or higher than those of the vehicle control are recorded as
0 inhibition.
* Significantly different from control at p < 0.01 or p < 0.001
light anaesthesia. The haematological determinations consisted of
packed cell volume, haemoglobin concentration, erythrocyte count,
total and differential leukocyte counts, and platelet count. The
clinical biochemical determinations included blood urea nitrogen,
alkaline phosphatase and alanine and aspartate aminotransferase
activities, glucose, total protein, albumin, globulin (calculated),
creatine phosphokinase, total bilirubin, cholesterol, calcium,
phosphorus, sodium, potassium, and chloride. Urine samples were
obtained 1-2 weeks before the scheduled sacrifices at 12 and 24 months
and at 6 and 18 months from 10 rats of each sex per group. The urinary
parameters measured included specific gravity and semi-quantitative
estimates of bilirubin, glucose, ketones, occult blood, pH, protein,
and urobilinogen. Microscopy of a pooled sample was also conducted.
Plasma and erythrocyte cholinesterase activities were assayed in 10
rats of each sex per group at 6, 12, 18, and 24 months, and
acetylcholinesterase activity was measured in half-brain samples
obtained at the 12-month and 24-month scheduled necropsies. All
animals killed at the interim and terminal sacrifices or which died or
were killed when moribund were necropsied and subjected to a complete
gross examination. The brain, liver, kidneys, testes, ovaries, and
adrenal glands were weighed, and many organs and tissues were removed
and preserved in neutral, phosphate-buffered 10% formalin for
subsequent histopathological evaluation. Histological sections of the
formalin-fixed tissues from all controls and those at the highest dose
were prepared, stained with haematoxylin and eosin and examined
microscopically. Histopathological examination of tissues from animals
at the three lower doses was limited to the liver, kidneys, adrenals,
and tissues with gross lesions at both sacrifices. At terminal
sacrifice, the lungs, spleen, testes, pituitary, and
thyroid/parathyroid were also examined microscopically. Appropriate
statistical tests were applied to the data.
Males at the high dose showed a consistent decrease in
body-weight gain relative to controls in the absence of reduced food
consumption, depression of plasma (56-87%), erythrocyte (20-40%) and
brain (56-58%) cholinesterase activities, and an increase in the
weight of their adrenal glands which was characterized microscopically
by exacerbated fatty vacuolation of the zona fasciculata. Similar
effects were observed in females at this dose, but were generally less
pronounced than in males: for example, a transient decrease in
body-weight gain relative to controls with no reduction in food
consumption, depression of plasma (82-95%), erythrocyte (generally
> 20%), and brain (57-61%) cholinesterase activities, and an
increase in adrenal weight at the terminal sacrifice with no
associated histopathological lesions. At 1 mg/kg bw per day, the only
effects attributable to treatment were inhibition of plasma
cholinesterase activity (39-71% in males and 60-86% in females) and
erythrocyte cholinesterase activity (20-40% in males and < 22% in
females); brain cholinesterase activity was not affected. These
results are summarized in Table 9. No treatment-related effects were
observed at the two lower doses. There was no increase in the
incidence of any type of tumour. The NOAEL for inhibition of
erythrocyte cholinesterase activity was 0.1 mg/kg bw per day, on the
basis of toxicologically (> 20%) or statistically significant
inhibition at 1 mg/kg bw per day, and the NOAEL for inhibition of
brain cholinesterase activity was 1 mg/kg bw per day on the basis of
toxicologically and statistically significant inhibition at 10 mg/kg
bw per day (Young & Grandjean, 1988).
Chickens
Hens were fed diets containing chlorpyrifos at concentrations of
0, 25, 50, or 200 ppm (approximately 0, 2.5, 5, and 20 mg/kg bw per
day) for 52 weeks. Mortality was unaffected by treatment, with rates
of 13% in controls, 3% at 25 ppm, 7% at 50 ppm, and 10% at 200 ppm.
Plasma cholinesterase activity was assayed in three hens at each dose
1, 3, 7, 14, 22, and 29 days after the start of treatment, monthly
thereafter, and 1, 2, and 3 weeks after the end of treatment. The
onset of inhibition of cholinesterase activity was rapid and
dose-related, with 22% inhibition in week 1 at 25 ppm, 45% inhibition
at 50 ppm, and 76% inhibition at 200 ppm. It persisted at similar
levels throughout the study but returned to control levels during the
Table 9. Group mean cholinesterase activities in rats given diets containing chlorpyrifos,
expressed as percent of the respective control values for each sampling period
Cholinesterase Sex Sampling period Dose (mg/kg bw per day)
(months)
0 0.05 0.1 1 10
Plasma Male 6 100 96.5 95.1 60.9* 44.2*
12 100 94.5 97.8 28.6* 13.3*
18 100 93.4 80.1 36.6* 22.5*
24 100 92.4 85.5 40.0* 19.7*
Female 6 100 97.9 91.0 34.6* 16.9*
12 100 104 87.0* 13.8* 4.8*
18 100 99.1 85.8* 30.4* 12.4*
24 100 103 94.4 39.7* 17.9*
Erythrocytes Male 6 100 107 88.8 76.1* 75.6*
12 100 107 93.2 67.4 63.1
18 100 109 95.3 66.2* 71.0*
24 100 105 108 86.5 73.5*
Female 6 100 93.3 106 104 87.5
12 100 88.2 109 82.2 59.5*
18 100 114 99.7 78.0 81.9
24 100 107 87.2 83.6 79.9
Brain Male 12 100 93.8* 93.1* 91.0* 42.1*
24 100 102 100 103 44.3*
Female 12 100 97.9 102 95.3* 38.9*
24 100 101 100 96.2 42.9*
* Statistically significantly different from control by Dunnett's or Wilcoxon's test,
alpha = 0.05, one-sided
recovery period. Overall feed consumption, body weight, egg
production, feed efficiency, egg weight, and shell thickness were not
affected by treatment. There was no NOEL for inhibition of plasma
cholinesterase activity in this study, as significant inhibition was
seen at the lowest dose tested (Sherman & Herrick, 1973).
(d) Genotoxicity
Chlorpyrifos was not genotoxic in vitro or in vivo in a range
of studies (Table 10).
Table 10. Results of studies for the genotoxicity of chlorpyrifos
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
In vitro
Gene mutation S. cerevisiae D3 NR NR Negative Poole et al. (1977;
rec locus abstract only)
Gene mutation B. subtilus H17, 20 (100, 200, 500, 1000 95.83 Negative Shirasu et al. (1980)
M45 2000 µg/plate in DMSO
Reverse mutation S. typhimurium TA98, 10, 50, 100, 500, 1000, 95.83 Negativea Shirasu et al. (1980)
TA100, TA1535, 5000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 1, 3.162, 10, 31.62, 95.7 Negativea,b QA Bruce & Zempel (1986)
TA100, TA1535, 100 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 0.01, 0.1, 1, 5, 10, 95 Negativea,c Jai Research Foundation
TA100, TA1535, 100 µg/plate in DMSO (1996a)
TA1537
Reverse mutation S. typhimurium TA98, 30, 100, 300, 3000, 96.8 Negativea GLP Loveday et al. (1987)
TA100, TA1535, 10 000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative Poole et al. (1977;
TA100, TA1535, abstract only)
TA1537
Reverse mutation E. coli WP2 NR NR Negative Poole et al. (1977;
abstract only)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Relative toxicity E. coli and NR NR Negative Poole et al. (1977;
B. subtilis abstract only)
Reverse mutation S. typhimurium TA98, 5, 500, 5000 µg/plate 96.2 Negativea,d QA Fredrick Institute
TA100, TA1535, in DMSO (1996c)
TA1537, TA1538
Forward mutation Chinese hamster ovary 10, 20, 25, 30, 40, 95.7 Negativea Mendrala (1985)
cells, hprt locus 50 µmol/L
Forward mutation Chinese hamster ovary 5, 10, 25, 50, 75 µg/ml, 96.8 Negativea,e GLP Tu (1987)
cells, hprt locus 16 h, - S9
5, 10, 20, 30, 40, 50 µg/ml,
16 h, - S9
30, 50, 100, 300, 1000 µg/ml,
+S9, in DMSO
Sister chromatid Human lymphocytes 0.02, 0.2, 2 or 20 µg/ml NR Negativea,f Sobti et al. (1982)
exchange (Laz-007) in ethanol
Chromosomal Chinese hamster ovary 0.975, 1.47, 2.93, 4.89, 96.8 Negativea,g GLP Loveday (1987)
aberration cells 9.75, 14.7, 29.3, 48.9, 97.5,
147 µg/ml, 19 h, - S9
1.56, 3.12, 5.2, 10.4, 15.6,
31.2, 52, 104, 156 µg/ml,
10 h, - S9
9.75, 14.7, 29.3, 48.9, 97.5,
147, 293 µg/ml, 19 h, +S9
1, 1.5, 3, 5, 10, 15, 30, 50,
100 µg/ml, 10 h, + S9
2.95, 4.95, 9.85, 14.8, 29.6,
49.4, 98.5, 296 µg/ml, 10 h,
+S9
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Rat hepatocytes 16.7, 50, 167, 500, 1667, 98.6 Negativea,h GLP Linscombe et al. (1992)
aberration 5000 µg/ml harvested after
24 h and 5, 16.7, 50, 167
µg/ml harvested after
24 and 48 h, in DMSO
Sister chromatid Chinese hamster ovary 1, 10, 100 µg/ml in acetone NR Negative Muscarella et al. (1984)
exchange cells
Unscheduled DNA Rat hepatocytes 1, 3.16, 10, 31.6, 95.7 Negative Mendrala & Dryzaga
synthesis 100 µmol/L in DMSO (1986)
Reverse mutation S. typhimurium TA98, 0, 1, 3.16, 10, 31.6, 95.7- Negativea Gollapudi et al. (1995)
TA100, TA1535, 100 µg/plate in DMSO 98.6
TA1537, TA1538
Forward mutation Chinese hamster ovary 0, 3.5, 7, 8.8, 10.5, 14, 95.7-98.6 Negativea,i Gollapudi et al. (1995)
cells, hprt locus 17.5 µg/ml in DMSO
Chromosomal Rat lymphocytes 16.7-5000 µg/ml in 95.7-95.6 Negativea,j Gollapudi et al. (1995)
aberration DMSO
Unscheduled Rat hepatocytes 1, 3.16, 10, 31.6, 95.7-95.6 Negative Gollapudi et al. (1995)
DNA synthesis 100 µmol/L in DMSO
In vivo
Micronucleus Mouse (CD-ICR BR) 0, 7, 22, 70 mg/kg bw 95.7 Negative QA Gollapudi et al. (1985)
formation marrow cells orally in corn oil
Micronucleus Mouse (Swiss albino) 0, 15, 30, 60 mg/kg bw 96.2 Negative QA Fredrick Institute
formation marrow cells orally in vegetable oil (1996d)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Mouse (Swiss albino) 0.6, 3, 15 mg/kg bw per 96.2 Negative QA Fredrick Institute
aberration marrow cells day orally in vegetable oil (1996e)
Chromosomal Chick embryo (Cornell 1.11, 11.1, 111, 1110, NR Negativek Muscarella et al. (1984)
aberration K strain eggs) 2220 µg/embryo
Chromosomal Bovine blastocysts NR NR Negative Muscarella et al. (1984)
aberration
Micronucleus Mouse (CD-1 (ICR) BR) 90 mg/kg bw orally in 97.9 Negative GLP McClintock & Gollapudi
formation marrow cells corn oil (1989a)
QA, quality assurance; GLP, good laboratory practice; NR, not reported; S9, exogenous metabolic activation system from 9000 × g
fraction of rat liver; DMSO, dimethyl sulfoxide
Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Cytotoxicity observed at 100 µg/plate in TA100, 1535, 1537, 1538 with precipitation of test material; in TA98, precipitation in the
absence of toxicity
c Cytotoxicity at > 10 µg/plate; dose-related increase in revertant frequency (> twofold than controls) in all strains ± S9, but
increases lower than in positive controls by 2-50-fold and not statistically significant
d Increase in revertants at all dose, - S9 in TA1537 only; no dose-response relationship
e Cytotoxicity at 50 µg/ml in one assay - S9
f At 2 and 20 µg/ml, frequency was statistically significantly different from controls but not double the control frequency
g - S9: cytotoxicity at highest doses; increase in gaps only at 52 µg/ml and 10-h exposure; no increases in other aberrations
+S9: cytotoxicity at 15 µg/ml and 10-h incubation; in one 10-h assay, a significant increase in number of cells with aberrations
(including gaps) at 3 and 10 µg/ml; incidence of aberrations (excluding gaps) not statistically significantly increased and not
dose-dependent In repeat 10-h assay, no increase in incidence of aberrations.
h Cytotoxicity at > 500 µg/ml -S9 and > 167 µg/ml +S9 in first assay; no mitotic index measurable at 50 µg/ml - S9 or
167 µg/ml ±S9
i Test material precipitated at 10.5, 14, and 17.5 µg/ml ± S9
j Test material cytotoxic at > 50 µg/ml ± S9
k Increased mortality at 1110 and 2220 µg/embryo
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a three-generation study, Sprague-Dawley rats (two litters per
generation) were given diets containing chlorpyrifos (purity not
specified) at concentrations that provided doses of 0, 0.03, 0.1, or
0.3 mg/kg bw per day for the first generation and 0, 0.1, 0.3, or
1 mg/kg bw per day for the second and third generations. The sizes of
the groups were consistent in all generations, the controls comprising
20 males and 40 females and the treated groups comprising 10 males and
20 females. Treatment was started at 58 days of age, and F0 parents
were mated at 118 days of age at a 1:2 male:female ratio. Evidence of
conception (vaginal plug or sperm) was considered to be day 0 of
gestation. Pairing for the second litter in each generation was
started approximately 10 days after the first litter was weaned at 21
days, and the treated diets were fed to the litters from weaning. When
the progeny of the F1b or F2b numbered more than 10 per litter,
each litter was reduced to 10 after 5 days of age. The F3b fetuses
were examined for teratogenic changes. F2b parent animals were
continued on the test diet throughout the breeding period, except that
females were placed on normal diet during organogenesis and the test
substance was administered by gavage in acetone or corn oil. Dams were
killed on day 20 of gestation and the fetuses removed surgically and
examined for external abnormalities. Onethird were fixed for
examination of soft tissues, and two-thirds were stained with alizarin
red for skeletal examination; however, only the controls and rats at 1
mg/kg bw per day were examined. Clinical observations were stated to
have been performed 'frequently'. Body weights and food consumption
were recorded weekly until breeding commenced. During gestation and
lactation, the diets were adjusted on the basis of an average feed
consumption of 25 and 40 g/day; rats were weighed before breeding to
allow dietary adjustment. In the investigation of teratogenic effects,
the body weights of the dams were recorded on days 0, 6, 15, and 20 of
gestation, while food consumption was measured during the gestational
intervals 0-6, 6-16, and 16-20 days. Various indices of reproductive
performance were calculated: fertility index as pregnancies per
mating; gestation index as live litters born per number of
pregnancies; viability as rats alive at day 5 per rats born alive; and
lactation index as rats alive at day 21 per rats alive at day 5. All
animals that died and five pups of each sex per dose from the F1a,
F2a, and F3a litters were examined grossly. An extensive set of
tissues was prepared and fixed from these pups, but in the absence of
gross abnormalities only tissues from the F3a pups were examined
histopathologically. Clinical chemistry was limited to the F2
animals, and consisted of measurements of plasma and erythrocyte
cholinesterase activity in 11 control and five or six treated dams at
the time of removal of their litters and in five males per group.
Statistical analysis was limited.
No clinical signs of toxicity were seen in the parents or
offspring. The parental body weights were not significantly affected
by treatment, and food consumption was variable but unaffected. The
fertility, gestation, and lactation indices were comparable between
groups and generations. The viability index was decreased at 1 mg/kg
bw per day, but this effect was considered not to be clearly related
to treatment in view of the isolated nature of the finding and the
study design. The mean body-weight gain of the dams that produced the
F3b pups showed a dose-related increase, with gains of 114 g in
controls, 120 g at 0.1 mg/kg bw per day, 123 g at 0.3 mg/kg bw per
day, and 126 g at 1 mg/kg bw per day. This generation showed no
treatment-related effects on fertility (per cent pregnant), the mean
number of corpora lutea or implantations, viable litter size, or pup
weight after surgical removal. The skeletons of the pups showed common
minor variants such as incomplete ossification of sternebrae and the
occurrence of extra ribs. Visceral examination of the pups showed that
minor variants of the urogenital system (hydronephrosis or left,
right, or bilateral hydroureter) were more common in the treated
group. A single fetus with multiple abnormalities was found at 1 mg/kg
bw per day, but this isolated finding was considered not to be related
to treatment. The group average activity of plasma and erythrocyte
cholinesterase was decreased by > 20% at 1 mg/kg bw per day in males
and females of the F2 generation. The NOAEL was 0.1 mg/kg bw per day
on the basis of decreased plasma and erythrocyte cholinesterase
activity at 1 mg/kg bw per day and decreased erythrocyte
cholinesterase activity at 0.3 mg/kg bw per day (JMPR, 1972; modified
by reference to the original report of Thompson et al., 1971).
To supplement the finding of an equivocal decrease in neonatal
survival at 1 mg/kg bw per day, groups of 30 Sprague-Dawley rats of
each sex were fed diets containing chlorpyrifos (purity, 96.6-99%) at
concentrations providing doses of 0, 0.5, 0.8, or 1.2 mg/kg bw per day
for 135 days and then bred to produce F1 litters. On day 21 of
lactation, 30 pups of each sex were selected randomly, dosed in the
same manner as the F0 animals for 120 days, and then bred to produce
the F2 litters. The F2 pups were weaned on day 21 of lactation.
The F0 and F1 matings were in a 1:1 ratio, with pairing for 5
days, a 7-day rest, and a pairing with a different male for a further
5 days. The litters were culled on day 4 post partum, and the litter
weights and pup survival were recorded on days 1, 4, 7, 14, and 21;
individual pups were weighed on day 21. The parental animals were
examined daily for signs of toxicity, and body weight and food intake
were recorded weekly for animals of each sex until pairing;
thereafter, males were weighed after pairing until sacrifice, and
females were weighed on days 1, 4, 7, 14, and 21 of lactation. The
date of parturition, litter size at birth, and the numbers of live and
dead pups at birth were recorded. The dietary concentrations were
adjusted weekly during the pre-mating period according to the food
consumption and body weights of the rats; the concentrations were then
maintained at the last adjusted concentration from commencement of
cohabitation until sacrifice. Appropriate statistical tests were
applied.
The parental animals showed no significant clinical signs in
either generation. Body weight and food intake were unaffected by
treatment. The mean fertility index (number of females delivering a
litter expressed as the percentage of the total number of females
placed with a male) was reduced at 0.5 and 0.8 mg/kg bw per day but
not at the highest dose, and a similar inverted dose-response
relationship was seen for length of gestation; data on individual
animals were not presented. The litter sizes (10-11) were comparable
at all doses, as were the survival indices (> 91% in all groups on
days 1, 4, 7, 14, and 21) during lactation. The pup weights
(presumably litter weight per number of pups) were also comparable. In
F1 adults, the body weight of males was reduced at 1.2 mg/kg bw per
day on days 160-182 after cohabitation, and those of females showed
slight, sporadic increases when compared with controls. Food intake
was sporadically decreased in males but not in females. No effects
were seen on female body weight during lactation. The fertility index
in treated groups exceeded the control values. The litter sizes were
comparable in all groups, as were the pup weights and pup survival
during lactation. The adequacy of this study for regulatory purposes
was limited by the doses selected. As no adverse effects were found at
the highest dose, it was unclear whether the study clearly
demonstrated the reproductive toxicity of the test material in rats.
The lack of data for individual animals and the inverted dose-response
relationships for some parameters rendered the findings equivocal. In
the absence of clear adverse effects on reproductive parameters at any
dose, the NOAEL was 1.2 mg/kg bw per day (Dietz et al., 1983).
In a study conducted in compliance with GLP standards and the
requirements of the US Environmental Protection Agency 83-4,
technical-grade chlorpyrifos (stated purity, 95.8%) was given in the
diet to groups of male and female CrL: COBS CD (SD) BR rats at
concentrations of 0, 2, 10, or 50 ppm over two generations. These
concentrations corresponded to achieved intakes of 0.1-0.2, 0.5-0.9,
and 2.5-4.5 mg/kg bw per day, respectively, in F0 males and 0.1-0.2,
0.6-0.9, and 2.9-4.6 mg/kg bw per day, respectively, in F0 females.
The corresponding intakes in the F1 generation were 0.1-0.3,
0.7-1.6, and 3.3-8.1 mg/kg bw per day in males and 0.2-0.3, 0.8-1.6,
and 4-8.1 mg/kg bw per day in females. The animals of the F0
generation (28 of each sex per dose) were approximately 7 weeks of age
at the beginning of treatment and were maintained on their respective
diets for at least 56 days before mating. They were mated at a 1:1
ratio for 20 days, and then the dams were allowed to rear their young
to day 21 post partum. Before weaning, all F1 offspring in all
litters were examined for developmental indices (surface righting
reflex, startle reflex, air righting reflex, and pupil reflex). At day
21 post partum, 24 pups from each group were selected for mating;
excess pups were sacrificed on day 22 post partum and examined
macroscopically, and specified organs were weighed and tissues
preserved for histopathological examination from one animal of each
sex per litter. Shortly after the F1 pups were weaned, the F0
adults were killed and examined macroscopically, and a wide range of
organs and tissues were preserved, the ovaries, pituitary, prostate,
testes with epididymides, and uterus with cervix and vagina being
examined histopathologically.The animals in the F1 generation that
were selected for mating were reared on their respective diets for at
least 56 days (i.e. until they were approximately 12 weeks of age) and
were then mated 1:1 for 20 days; the dams were allowed to rear their
young until day 21 post partum. Pathological examination was
conducted as for the F0 generation. All animals were handled
regularly and examined for signs associated with treatment. All
animals that died or were killed were examined. The weight of each
animal was determined at the start of each generation and subsequently
at weekly intervals. All pups were weighed at birth and at 4, 8, 12,
and 21 days post partum. Cholinesterase activity was not determined
in the study. The doses were selected on the basis of those that
decreased cholinesterase activity in a preliminary study in which
chlorpyrifos was given in the diet for a single generation at doses up
to 250 ppm. In this preliminary study, plasma cholinesterase activity
was inhibited when compared with that in controls by 86% in adult
females and by about 60% in weanling males and females at 50 ppm. At
50 ppm, erythrocyte cholinesterase activity was also inhibited in
adult females (70% inhibition) and weanlings (about 50% inhibition),
and brain cholinesterase was inhibited by 38% in adult females
Food consumption was similar in control and treated groups and no
adverse, treatment-related effects on group mean body weights were
observed at any dose in either generation. Mating performance and the
duration of gestation were similar in control and treated groups.
Slight inter-group variation was observed in the incidence of total
pre-birth loss in both the F0 and F1 generations, but these
findings were considered not to be related to treatment in the absence
of a consistent relationship between dose and effect. The F0
generation showed statistically significant increases in litter size
and litter weight and decreased mean pup weight at a number of doses
at most sample intervals (Table 11), but these values were generally
only slightly different from those in controls and showed no
consistent relationship with dose. The decrease in mean pup weights
was probably related to the increased litter size. The data for the
litters of the F1 generation were similar for control and treated
groups, with the exception of a single, statistically significant
decrease in litter weight at 50 ppm on day 21. This isolated finding
was considered to be incidental to treatment. No consistent,
dose-related effect on organ weights was reported during the study.
Pathological examination revealed no macro- or microscopic effects
that were related to treatment. Under the conditions of this study, no
adverse treatment-related effects were observed at any dose. The
slight increase in mean body weights of F0 males at the high dose
was the only effect attributed to treatment. The NOAEL was 50 ppm,
equal to 2.5 mg/kg bw per day (James et al., 1988).
Four groups of Sprague-Dawley rats were used in a two-generation
(one litter per generation) study of reproductive toxicity in which
diets containing chlorpyrifos (purity, 97.8-98.5%) at doses of 0, 0.1,
1, or 5 mg/kg bw per day were administered. The study was conducted in
accordance with FIFRA guideline 83-4, OECD guideline 416, and GLP
principles. The F0 parental animals (30 of each sex per dose) were
Table 11. Statistically significant differences in group data for
litters of the F0 generation of rats given diets containing
chlorpyrifos
Time Litter parameter Dose (ppm)
0 2 10 50
At birth Litter size, total 12 13 14** 13
Litter size, live 12 13 14** 13
Litter weight (g) 70 72 79* 71
Pup weight (g) 6.1 5.6*** 5.7** 5.7*
Day 4 Litter size, live 11 13* 14*** 12
Litter weight (g) 100 110 120* 110
Mean pup weight (g) 9.3 8.7 8.6* 9.2
Day 8 Litter size, live 11 13** 13*** 12
Mean pup weight (g) 15 14* 14* 15
Day 12 Litter size, live 11 13** 13*** 12
Litter weight (g) 240 270 280** 250
Mean pup weight (g) 22 21 21* 22
Day 21 Litter size, live 11 13* 13*** 12
Litter weight (g) 460 520 530* 480
Mean pup weight (g) 44 42 40* 42
* p < 0.05; ** p < 0.01; *** p < 0.001
6 weeks of age at the beginning of the study and were mated after 10
weeks of exposure to produce the F1 litters. Groups of 30 rats of
each sex per dose were selected from the F1 weanlings and were
treated for 12 weeks before breeding to produce the F2 litters.
Pairing in both generations allowed for three periods of 7 days'
cohabitation in a 1:1 ratio, the males being changed weekly. Care was
taken to avoid sibling pairings. Females were removed from pairing
when vaginal lavage showed sperm, and this was considered to be day 0
of gestation. The F1 and F2 litters were culled if appropriate to
a total of eight pups on day 4. Clinical observations were performed
daily on all animals; litter size at birth, the numbers of live and
dead pups on days 0, 1, 4, 7, 14, and 21 post partum, and the sex
and weight of each pup were recorded on days 1, 4, 7, 14, and 21 of
lactation. Body weights and food consumption were recorded weekly
before and after breeding. The body weights of dams were recorded on
days 0, 7, 14, and 21 of gestation and on days 1, 4, 7, 14, and 21 of
lactation, whereas food consumption was recorded once during the first
week of lactation, twice during the second week, and every 2-3 days
during the third week. Inhibition of cholinesterase activity in
plasma, erythrocytes, and brain was measured in 10 F0 and F1
parental animals of each sex at necropsy after 19 and 21 weeks of
exposure. Complete necropsies were conducted on all F0 and F1
adults, and included ocular examinations and collection ofmany
tissues. Histopathological examinations were made of the adrenals,
brain, gross lesions, and reproductive tissues (cervix, coagulating
glands, epididymides, ovaries, oviducts, pituitary, prostate, seminal
vesicles, testes, uterus, and vagina) of controls and animals at the
high dose. The livers of 10 F1 parental males in the control and
high-dose groups were also examined microscopically. Only the adrenals
from animals at the intermediate and low doses were examined. Ten F1
and F2 pups of each sex per dose were autopsied grossly. Appropriate
statistical tests were applied to all data.
No significant effects of treatment were seen on clinical signs,
food intake, or body weight. A slight but not statistically
significant decrease in body weight was seen in F1 males at 5 mg/kg
bw per day, and F1 females at this dose showed reduced body-weight
gain during lactation. Plasma cholinesterase activity was inhibited in
parental F0 and F1 rats of each sex at 1 mg/kg bw per day (40-57%)
and at 5 mg/kg bw per day (< 72%). Plasma cholinesterase activity
was also inhibited in animals of both generations at 0.1 mg/kg bw per
day as part of a dose-related trend, although the inhibition was
generally not statistically significant at this dose. Erythrocyte
cholinesterase activity was strongly inhibited at 1 and 5 mg/kg bw per
day, but that of brain was inhibited only at 5 mg/kg bw per day (Table
12).
Gross observation of F0 and F1 parents revealed no
significant alterations, and the only significant histopathological
change in the parental animals was vacuolation consistent with fatty
changes in the adrenal zone fasciculata. Treatment had no effect on
fertility, length of gestation, survival dring gestation, time to
mating, sex ratio, or litter size in either generation. F1 pups at
1 mg/kg bw per day showed slightly decreased body-weight gain during
lactation and statistically significantly decreased body-weight gain
and survival at 5 mg/kg bw per day. No effect of treatment was seen
during gross or daily observation of the F1 weanlings. F2 pups did
not show dose-related decreases in body-weight gain during lactation,
but the survival of pups at 0 and 5 mg/kg bw per day was decreased,
with total loss of three and five litters, respectively; this effect
was stated to be due to maternal neglect, since the stomachs of pups
in these litters contained no milk. No effect of treatment was seen
during gross or daily observation of the F2 weanlings. The NOAEL for
inhibition of erythrocyte cholinesterase in adults was 0.1 mg/kg bw
per day and that for inhibition of brain cholinesterase activity and
materal toxicity was 1 mg/kg bw per day. The NOAEL for developmental
effects was 1 mg/kg bw per day, and the NOAEL for effects on fertility
and reproductive effects was 5 mg/kg bw per day (Breslin et al.,
1991).
Table 12. Mean cholinesterase activity in F0 and F1 parent rats at necropsy (percentage inhibition compared
with controls)
Generation Dose (mg/kg Cholinesterase activity (% inhibition)
bw per day)
Plasma (IU/ml) Erythrocytes (IU/ml) Brain (IU/g)
Male Female Male Female Male Female
F0 0 0.54 2.0 1.1 1.0 9.3 9.0
0.1 0.46 (15%) 1.6 (20%) 1.0 (5%) 1.0 9.2 (1%) 9.1
1 0.30a (44%) 0.8a (59%) 0.3b (92%) 0.36b (65%) 8.8 (6%) 8.7 (3%)
5 0.21a (61%) 0.6a (67%) 0.3b (92%) 0.31b (70%) 4.9b (48%) 4.6a (49%)
F1 0 0.53 1.8 1.1 0.96 9.7 9.4
0.1 0.43 (19%) 1.6 (15%) 0.98a (13%) 0.97 9.7 9.2 (2.5%)
1 0.30a (43%) 0.93a (52%) 0.37a (67%) 0.32b (67%) 9.4 (3%) 9.0 (4.5%)
5 0.19a (64%) 0.52a (72%) 0.33a (70%) 0.24b (75%) 4.6a (52%) 4.0b (58%)
a Statistically significantly different from control mean by Wilcoxon's test
b Statistically significantly different from control mean by Dunnett's test
(ii) Developmental toxicity
Mice
In the range-finding portion of a study of developmental
toxicity, pregnant mice were given chlorpyrifos at doses of up to 60
mg/kg bw per day by gavage. No toxic effects were seen in the dams or
offspring at 3, 10, and 20 mg/kg bw per day. In the main study, groups
of 40-47 pregnant CF-1 mice were initially given chlorpyrifos (purity,
96.8%) at 0, 1, 10, or 25 mg/kg bw per day by gavage in cottonseed oil
on days 6-15 of gestation. The day on which a vaginal plug was
observed was considered to be day 0 of gestation. Owing to severe
maternal toxicity at 25 mg/kg bw per day, additional groups of 35-41
mice were given 0, 0.1, 1, or 10 mg/kg bw per day by gavage on days
6-15 of gestation. The mice were observed for signs of toxicity and
were weighed daily on day 6-16 of gestation and then sacrificed on day
18. At the time of surgical removal of their fetuses, the weights of
the liver and gravid uterus with ovaries were recorded. The uteri of
non-pregnant mice were stained with sodium sulfide to ensure that no
implantations had occurred. The fetuses were weighed, measured
(crown-rump length), sexed, and examined externally. One-third of the
fetuses were dissected to examine the soft tissues. All of the heads
were preserved in Bouin's solution and subsequently sectioned. All
fetuses were stained with Alizarin red for examination of the
skeletons.
Additional mice were added to the study to assess clinical
changes. Cholinesterase (erythrocyte and plasma) activity was measured
in groups of 4-10 mice given 1, 10, or 25 mg/kg bw per day on day 6,
days 6-10, or days 6-15 of gestation, and these measurements were also
made in a group treated with 0, 0.1, 1, or 10 mg/kg bw per day. Blood
was collected for assay of cholinesterase 5 h after dosing on days 6,
10, and 15 of gestation, respectively. A homogenate of fetal tissue
was prepared from litters of mice killed on day 15 of gestation. Data
were not provided on the body weights or food and water consumption of
the animals in these groups.
Appropriate statistical analysis was applied to the experimental
data: fetal body weights and body measurements, maternal body weights,
weights of maternal livers and uteri, and food and water consumption
were analysed by one-way analysis of variance and Dunnett's test. The
incidences of fetal resorptions and alterations were analysed by a
modified Wilcoxon test. The Fisher exact test was applied to other
data.
In the dose-ranging study, severe cholinergic signs and
consequent reproductive failure were found at doses > 30 mg/kg bw
per day. In the first main study, dose-related increases in the
incidence of clinical signs including mortality, excessive salivation,
tremors, urine-soaked coat, ataxia and lethargy were found at 10 and
25 mg/kg bw per day. A single death and isolated clinical signs at
1 mg/kg bw per day were considered not to be related to treatment.
Body-weight gain was reduced at 25 mg/kg bw per day, but no effects
were observed on litter size, resorption incidence, or sex ratio.
Fetal body weight and crown-rump length were significantly reduced at
25 mg/kg bw per day. Plasma cholinesterase activity was depressed in a
dose-related manner, while the activities in erythrocytes and fetal
tissue homogenate were inhibited at 10 and 25 mg/kg bw per day (Table
13). Exencephaly was reported in all groups, but with no dose-response
relationship, and a statistically significant incidence of delayed
ossification was seen at 25 mg/kg bw per day (Table 14).
The second main study showed no cholinergic signs in dams at any
dose up to 10 mg/kg bw per day. Body weight, food intake, maternal
liver weights, gravid uterine weight, and adjusted body weights were
also unaffected. Cholinesterase activity was rapidly and significantly
inhibited in plasma and erythrocytes at 1 and 10 mg/kg bw per day but
was depressed in fetal tissue homogenates only at the high dose. No
effects were observed on materna1 parameters or on litter size, the
incidence of resorptions, the incidence of dead fetuses, the sex
ratio, fetal body weight, or crown-rump length (Table 15). Significant
fetal alterations are shown in Table 16.
Neither study provided evidence of teratogenic activity at any
dose, but fetal toxicity was seen at 25 mg/kg bw per day. The NOAEL
for developmental toxicity was therefore 10 mg/kg bw per day. The
NOAEL for inhibition of erythrocyte cholinesterase activity was 0.1
mg/kg bw per day, and the NOAEL for maternal toxicity was 1 mg/kg bw
per day (JMPR, 1982; modified by reference to the original reports of
Deacon et al., 1979, 1980).
Rats
In a range-finding study of developmental toxicity, which was
given a statement of quality assurance, Fischer 344 rats were fed
chlorpyrifos (purity, 96.6%) by gavage at doses of 0, 3, 10, 15, or
30 mg/kg bw per day during organogenesis. Slight maternal toxicity was
observed at 15 mg/kg bw per day, and severe toxicity was observed at
30 mg/kg bw per day, with typical cholinergic signs of excessive
salivation, lachrymation, urination, defaecation, and body tremors and
general observations of unkempt appearance, decreased body size, and
matting of perineal and facial hair. Deaths were also seen at 30 mg/kg
bw per day, and animals in this group had decreased body-weight gain,
decreased food consumption, enlarged adrenal glands, decreased liver
weight, and shrunken thymus glands. Both plasma and erythrocyte
cholinesterase activities were depressed at all doses. Maternal
toxicity was thus observed at all doses, but fetal toxicity was seen
only at 30 mg/kg bw per day (Ouelette et al., 1983a).
In the main study, which also had a statement of quality
assurance, groups of 31-33 pregnant Fischer 344 rats were given
chlorpyrifos (purity, 96.6%) by gavage in corn oil at doses of 0, 0.1,
3, or 15 mg/kg bw per day on days 6-15 of gestation. They were mated
1:1, and the day sperm were observed in a vaginal smear was considered
to be day 0 of gestation. The rats were observed daily for signs of
toxicity, were weighed daily on days 6-16 and day 21 of gestation, and
Table 13. Significant findings in mice and offspring given chlorpyrifos by gavage on days 6-15 of
gestation
Finding Dose (mg/kg bw per day)
0 1 10 25
No. of females 51 40 44 47
No. of animals with cholinergic signs 0 2 9 32
Deaths 0 1 1 4
Total pregnanta 40 (4) 31 (1) 32 (1) 34 (1)
No. with litters 36 29 30 29
Maternal body weight gain (g)b 21 ± 5 22 ± 3 22 ± 4 18 ± 4*
No. of implantation sites per dam 13 ± 3 13 ± 2 13 ± 1 12 ± 3
No. of fetuses per litter 11 ± 3 12 ± 2 12 ± 2 11 ± 3
Fetal body weight (g) 1.14 ± 0.10 1.13 ± 0.07 1.15 ± 0.08 1.03 ± 0.17*
Fetal crown-rump length (mm) 25.0 ± 1.2 25.2 ± 0.9 25.3 ± 0.9 24.4 ± 1.6*
Plasma cholinesterase activityc 100 (10) 15 (7) 4 (8) 2 (9)
Erythrocyte cholinesterase activityc 100 (10) 86 (6) 57 (9)* 43 (9)*
Fetal homogenate cholinesterase activity 100 81 65 35
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining (not resulting in
litters).
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals
Table 14. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 1 10 25
No. of fetuses examined 408 347 359 326
Exencephalya 1/1 5/5* 1/1 4/3
Ablephariaa 1/1 1/1 1/1 2/2
Cleft palatea 0 2/2 1/1 2/1
Small cerebral hemispherea 1/1 1/1 1/1 0
Multiple skeletal malformationsa 0 0 1/1 0
Fused ribsa 1/1 0 1/1 0
Skull: delayed ossification 5/5 9/5 10/6 34/10*
Sternebrae: delayed ossification 37/17 24/11 19/11 78/22*
Sternebrae: unfused 2/2 7/6* 3/3 8/7*
Sternebrae: fused 24/16 9/16* 23/12 6/5*
* Significantly different from control group
a Considered to be a major malformations
were sacrificed on day 21. At the time their fetuses were surgically
removed, the body and liver weights were recorded. The number of
corpora lutea and the number and position of live and resorbed fetuses
were recorded at necropsy. Fetuses were sexed, examined, and measured.
The uteri of non-pregnant rats were stained with sodium sulfide to
determine whether implantations had occurred in the absence of
litters. Separate groups of 10 rats were dosed on days 6-15 of
gestation and killed, when samples of maternal blood were collected by
heart puncture to measure erythrocyte and plasma cholinesterase
activity. Appropriate statistical analysis of the data was reported.
There were no unscheduled deaths. Clinical signs of maternal
toxicity were found, including excessive salivation, urine staining in
the perineal region, porphyrin deposits around the eyes, and vaginal
bleeding. Tremors were observed only at 15 mg/kg bw per day, and
animals at this dose also had decreased body-weight gain; no other
maternal findings were reported at necropsy. Both plasma and
erythrocyte cholinesterase activities were depressed at doses of 3 and
15 mg/kg bw per day (Table 17). No adverse effects were observed on
reproductive parameters, and no teratogenicity was observed.
Variations and malformations were found which were distributed
randomly by dose, and no effect of treatment on skeletal development
was seen. The NOAEL for maternal toxicity was 0.1 mg/kg bw per day, on
the basis of inhibition of erythrocyte cholinesterase activity in all
adult animals at higher doses. No fetal toxicity was observed
(Ouelette et al., 1983b).
Table 15. Significant findings in mice and offspring given chlorpyrifos by gavage on days
6-15 of gestation
Finding Dose (mg/kg bw per day)
0 0.1 1 10
No. of females bred 41 35 36 35
Total no. pregnanta 30 (0) 25 (1) 23 (0) 28 (3)
No. with litters 30 24 23 25
Maternal body weight gain (g)b 12 ± 6 14 ± 5 14 ± 5 14 ± 5
No. of implantation sites per dam 10 ± 3 10 ± 3 10 ± 3 9 ± 4
No. of fetuses per litter 8 ± 3 7 ± 3 7 ± 3 7 ± 3
Fetal body weight (g) 1.04 ± 1.11 1.07 ± 0.15 1.08 ± 0.18 1.09 ± 0.12*
Fetal crown-rump length (mm) 24.4 ± 1.1 24.8 ± 1.6 24.9 ± 2.1 24.8 ± 1.2*
Plasma cholinesterase activityc 100 (8) 86 (6) 25 (7)* 3 (6)*
Erythrocyte cholinesterase activityc 100 (8) 88 (6) 75 (7)* 50 (6)*
Fetal homogenate cholinesterase activityc 100 84 95 77
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals.
Table 16. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 0.1 1 10
Exencephalya 1/1 1/1 0 1/1
Ablephariaa 1/1 0 0 0
Cleft palatea 0 0 1/1 0
Omphalocelea 0 0 0 1/1
Skull: delayed ossification 18/10 11/6 3/2 3/3
Sternebrae: delayed ossification 50/20 25/13 25/13 17/11
Sternebrae: unfused 3/3 6/5 0 1/1
Sternebrae: fused 13/7 9/7 3/3 12/8
a Considered to be major malformations
Female CD Sprague-Dawley rats were mated 1:1 with stock males
from the same source and strain. Technical-grade chlorpyrifos (stated
purity, 96.1%) was formulated daily as solutions in maize oil and
given to groups of 32 mated females in a volume of 5 ml/kg bw by oral
gavage at doses of 0.5, 2.5, or 15 mg/kg bw per day on days 6-15
post coitum. A further group received maize oil only. This study was
conducted in accordance with US Food and Drug Administration and
Environmental Protection Agency guidelines for GLP and testing
guidelines of the OECD (No. 414) and US Environmental Protection
Agency (section 83-3). All females were examined daily for signs
associated with treatment. Animals were weighed on days 0, 3, 6-15,
17, and 20 of gestation, and food consumption was measured twice
weekly. Ten mated females were bled on day 15 for measurement of
plasma cholinesterase activity, blood samples being obtained from the
retro-orbital sinus under ether anaesthesia. The same procedure was
repeated for an additional 10 animals per dose immediately before
necropsy on day 20. The animals used for cholinesterase determinations
on day 15 were discarded without necropsy after being bled. All
remaining animals were killed on day 20, and the following were
recorded for each animal: any macroscopic abnormality of the
reproductive tract; number of corpora lutea in each ovary; weight of
gravid uterus; distribution of live and dead fetuses and distribution
of resorption sites in each uterine horn; individual placental
weights; individual fetal weight, length, and sex; and external
anomalies of individual fetuses. The thoracic and abdominal contents
of approximately one-half of each litter were dissected and examined,
and these fetuses were used for skeletal staining and evaluation; the
remaining fetuses were used for preparation of free-hand sections and
examination of visceral organs.
Table 17. Adverse effects in rats given chlorpyrifos by gavage
Finding Dose (mg/kg bw per day)
0 0.1 3 15
No. of females 31 32 33 31
No. of animals with cholinergic signs 0 0 0 12
Total no. pregnanta 30 (0) 28 (0) 26 (0)b 29 (1)
No. of litters 29 26 24 26
Maternal body weight gain (g)c 65 ± 11 64 ± 14 71 ± 12 58 ± 11
No. of implantation sites per dam 11 ± 2 10 ± 2 10 ± 2 10 ± 3
No. of fetuses per litter 10 ± 3 9 ± 2 10 ± 3 9 ± 3
Mean fetal body weight (g) 4.28 4.37 4.52* 4.46*
Mean fetal crown-rump length (mm) 43.91 43.93 43.96 43.64
Plasma cholinesterase activity (IU/ml)d 46.0 42.8 (93%) 4.9 (11%) 1.5 (3%)
Erythrocyte cholinesterase activity (IU/ml)d 12.1 11.9 (98%) 3.1 (26%) 2.5 (21%)
* Significantly different from control
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Two dams removed during dosing for reasons not related to treatment
c Days 6-20
d Mean of 6-8 dams on day 15, IU/ml (percentage of control)
No treatment-related deaths occurred during the study, and
clinical signs of intoxication were confined to tremors late in the
study in three animals at 15 mg/kg bw per day. Slight but
statistically significant decreases in mean food consumption (8%
reduction during day 79 only) and body-weight gain were seen at
15 mg/kg bw per day. Statistically significant, dose-related decreases
in plasma cholinesterase activity were observed at all doses;
erythrocyte and brain cholinesterase activities were not determined.
The effects on litters are summarized in Table 18. The total live
litter sizes were unaffected by treatment, but at 15 mg/kg bw per day
the mean number of live male fetuses was statistically significantly
decreased and post-implantation loss was slightly elevated.
Statistically significant increases in pre-implantation loss were seen
at all doses, but there was no consistent dose-response relationship
and the incidence was within historical control values for this
laboratory; the finding was considered not to be related to treatment.
At 15 mg/kg bw per day, slight but statistically significant increases
were seen in mean fetal weight and mean crown-rump length, but as the
increase in fetal weight was not accompanied by a decrease in litter
size, this finding may have been due to advanced fetal development and
was considered to be of no toxicological significance. Similarly, the
statistically significant decrease in the incidence of small fetuses
observed in all test groups was considered not to be toxicologically
significant. A number of minor structural variations were reported,
including a single incidence of anophthalmia at 0.5 mg/kg bw per day
and instances of microphthalmia at 0.5 and 2.5 mg/kg bw per day, but
the incidence of such findings was low and/or were not related to
dose. These findings were therefore considered to be incidental to
treatment. Skeletal examination showed no adverse treatment-related
findings. The NOAEL for frank maternal toxicity was 2.5 mg/kg bw per
day, on the basis of reduced body weight, tremors, and transient
reductions in food consumption at 15 mg/kg bw per day. The NOAEL for
fetal toxicity was 2.5 mg/kg bw per day, on the basis of a slight
increase in post-implantation loss at 15 mg/kg bw per day, probably
associated with maternal toxicity (Rubin et al., 1987a).
Rabbits
Groups of 14 female New Zealand white rabbits were mated 1:1 with
stock males from the same source and strain and given technical-grade
chlorpyrifos (stated purity, 96.1%), formulated daily as solutions in
maize oil, by oral gavage at doses of 1, 9, 81, or 140 mg/kg bw per
day (in a volume of 2 ml/kg bw) on days 7-19 post coitum. The doses
for animals at 81 and 140 mg/kg bw per day were prepared directly, and
those for the animals at 1 and 9 mg/kg bw per day were prepared by
diluting the dose of 81 mg/kg bw per day with maize oil. A further
group received maize oil only. This study was conducted in accordance
with US Food and Drug Administration and Environmental Protection
Agency guidelines for GLP and testing guidelines of the OECD (No. 414)
and US Environmental Protection Agency (section 83-3). The doses were
selected after a preliminary range-finding study. On day 15, three
does at 81 mg/kg bw per day were accidentally given a dose of
140 mg/kg bw, and the group was expanded to 21 animals so that the
overdosed animals could be excluded from the study if that was found
to be warranted. Two of the three animals were pregnant, but no
apparent difference was found from the females treated at 81 mg/kg bw
per day, and the maternal and fetal data for these animals were
included in the report. All animals were examined daily for clinical
signs associated with treatment. Any animals found dead or killed
in extremis were autopsied to determine the cause of death. Animals
were weighed on days 0, 3, 7-19, 22, 25, and 29 of gestation, and food
consumption was determined twice weekly. Plasma cholinesterase
activity was measured before mating and again after at least 10 days
of dosing. The does were killed on day 29 and the following were
recorded: any macroscopic abnormality of the reproductive tract; the
number of corpora lutea in each ovary; the weight of gravid uterus;
the distribution of live and dead fetuses and of resorption sites in
each uterine horn; individual placental weights; individual fetal
weight and crown-rump length; sex; and external anomalies of
individual fetuses. The thoracic and abdominal contents were dissected
and examined, and the skull of each fetus was sectioned transversely
through the frontal-parietal suture, and the brain examined. Each
fetus was used for skeletal staining and evaluation.
Table 18. Effects on fetuses of rats given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 0.5 2.5 15
Pre-implantation loss (%) 8.6 12.2*** 11.5*** 11.4***
Post-implantation loss (%) 7.0 6.0 5.8 9.0**
Mean fetal weight (g) 3.33 3.40 3.44 3.51**
Mean crown-rump length (mm) 36.0 36.2 36.3 36.7**
Live fetuses:
Male 7.6 8.0 6.8 6.0*
Female 6.8 7.0 7.3 7.5
Total 14.4 15.0 14.1 13.4
Small fetuses (no. observed) 48 (317) 25 (315)** 20 (311)*** 13 (282)***
Ablepheron (no. observed) 0 (317) 0 (315) 1 (311) 0 (282)
Major cranio-facial malformations: 0 (317) 1 (315) 0 (311) 0 (282)
proboscis, anophthalmia (no. observed)
Hydronephrosis (no. observed) 0 (161) 0 (158) 1 (158) 0 (146)
Hydroureter 0 (161) 0 (158) 1 (158) 0 (146)
Anophthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
Microthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
No treatment-related deaths were observed during the study, and
no treatment-related clinical signs were reported. Maternal group mean
food consumption was unaffected by treatment, and group mean body
weights and body-weight gains were not affected by treatment at doses
up to 81 mg/kg bw per day. At 140 mg/kg bw per day, body-weight gain
was inhibited during most of the treatment period, although it was
greater than normal after cessation of treatment, and by day 29 the
animals in this group had attained body weights similar to those in
the control group. Statistically significant, dose-related decreases
in plasma cholinesterase activity were seen at all doses after 10 days
of treatment, with inhibition of 56% at 1 mg/kg bw per day, 68% at 9
mg/kg bw per day, 70% at 81 mg/kg bw per day, and 72% at 140 mg/kg bw
per day compared with controls. Erythrocyte and brain cholinesterase
activities were not determined.
Statistically significant decreases in pre-implantation loss were
observed at most doses, but this finding was considered not to be
toxicologically significant. Statistically significant increases in
post-implantation loss were observed at 9 and 140 mg/kg bw per day but
not at 81 mg/kg bw per day. In the absence of a consistent
dose-response relationship, the toxicological significance of this
finding was unclear; no historical control data were supplied for this
effect from the laboratory. A slight but statistically significant
decrease in mean fetal crown-rump length and a decrease in mean fetal
weight were observed in animals at 140 mg/kg bw per day, and these
effects were considered to be related to treatment. A number of fetal
anomalies were observed but were considered not to be
treatment-related as the incidence was generally low and unrelated to
dose. An increased incidence of fetuses with fifth sternebra and/or
unossified xiphisternum seen at 140 mg/kg bw per day was considered to
be related to slightly delayed development at this dose, as indicated
by reduced fetal weight and length in this group. Given the variation
in the incidence of other skeletal observations in treated groups,
however, this finding may have been due to chance. The NOAEL for frank
maternal toxicity was 81 mg/kg bw per day, on the bais of decreased
body-weight gain at the higher dose. The NOAEL for fetal toxicity was
81 mg/kg bw per day, on the basis of a slight decrease in mean fetal
crown-rump length, decreased mean fetal weight, and an increased
incidence of fetuses with fifth sternebra and/or unossified
xiphisternum at the higher dose (see Table 19) (Rubin et al., 1987b).
(f) Special studies: Neurotoxicity
Chickens
No signs of delayed ataxia or paralysis were reported in groups
of three hens treated with single oral doses of chlorpyrifos at 40,
75, 100, or 150 mg/kg bw. The two higher doses resulted in deaths and
other clinical signs of toxicity (Stevenson, 1966a). Similarly, groups
of 10 hens given single oral doses of chlorpyrifos at up to 100 mg/kg
bw did not display delayed neurotoxicity, and no neuropathological
findings associated with treatment were detected (Rowe et al., 1978).
In another study, single oral doses of chlorpyrifos at up to five
Table 19. Effects on litters and fetuses of rabbits given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 1 9 81 140
Number of fetuses examined 108 117 126 142 99
Pre-implantation loss (%) 15.2 13.1 8.9*** 11.9* 11.5*
Post-implantation loss (%) 6.5 8.6 13.8*** 8.7 13.9***
Mean fetal weight (g) 45.4 44.3 43.3 41.8 40.7
Mean crown-rump length (mm) 97.4 95.9 95.0 94.2 93.2*
Teeth absent 0 0 1 0 0
Arthrogryposis, forelimb 0 0 2 0 0
Irregularly shaped thorax 0 0 0 0 1
Hydrocephalus 0 0 1 0 0
Enlarged aortic arch 0 0 1a 0 0
Rudimentary pulmonary artery 0 0 1a 0 0
Agenesis of interventricular cardiac septum 0 0 1a 0 0
Hydronephrosis (bilateral) 2 0 0 4 1
Hydronephrosis (unilateral) 2 2 1 2 1
Diffuse opacity of eye 0 0 0 2 0
No. of fetuses examined for skeletal defects 108 117 126 142 99
Reduced or incomplete ossification of interparietal bone 3 4 3 5 2
Unossified interparietal bone 0 1 0 0 3
Reduced or incomplete ossification of hyoid bone 2 10* 17*** 16** 8*
Irregular ossification of one or more of sternebrae 1-4 1 2 7* 8* 1
Bony plaque adjacent to sternebra 10 6 2** 3* 1**
Unossified fifth sternebra and/or xiphisternum 1 1 2 3 6*
Multiple malformations of ribs and lumbar spine 0 0 0 0 1
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
times the oral LD50 in hens (> 150 mg/kg) resulted in significant
inhibition of neuropathy target esterase and cholinesterase activity
(Capodicasa et al., 1991), with signs consistent with
organophosphate-induced delayed neuropathy. Extensive, aggressive
antidotal treatment, both before and throughout the treatment and
recovery periods, was required for the birds' survival.
Technical-grade chlorpyrifos (purity, 96.8%) was tested for acute
toxicity and delayed neurotoxicity in adult domestic hens in a study
carried out according to GLP. In the assessment of neurotoxicity,
groups of 10 birds were given corn oil, tri- ortho-cresyl phosphate
(positive control; 20% in corn oil; 500 mg/kg), chlorpyrifos
(110 mg/kg bw) plus atropine sulfate (intramuscular injection;
10 mg/kg bw), chlorpyrifos (110 mg/kg bw; 5.5% in corn oil) plus
pyridine 2-aldoxime methane sulfonate (intramuscular injection;
50 mg/kg bw; 5% aqueous solution), or were not treated and were
maintained as a source of additional birds if necessary. The hens were
observed for 21 days, at which time all those given the corn oil were
given a second dose, and all treated birds were given chlorpyrifos,
followed by another 21-day observation period.
No clinical signs of toxicity were observed in those given the
vehicle. In birds given tri- ortho-cresyl phosphate, no signs of
toxicity were observed immediately after dosing, but all birds
developed signs of delayed locomotor ataxia as early as day 11 after
treatment. The birds given chlorpyrifos showed signs of toxicity after
either dose, including subdued appearance, unsteadiness, inability to
stand, and weakness. Deaths were observed in both chlorpyrifos-treated
groups, four birds that died after the first dose with atropine
sulfate (no deaths after the second dose) and three after the first
dose of chlorpyrifos with pyridine 2-aldoxime methane sulfonate (one
death after the second dose). Surviving birds recovered completely
within 5-8 days of treatment. Additional doses of atropine sulfate
were given to several birds in both groups treated with chlorpyrifos
owing to the severity of the clinical signs observed.
Histopathological examination revealed a low incidence of minor
neurological changes (grade II on a scale of I-V of increasing
severity) in several birds given the vehicle, which was considered to
show a normal background incidence of axonal degeneration. Most of the
birds given tri- ortho-cresyl phosphate had grade III changes at at
least one level of spinal cord and grade III or IV changes in at least
one level of peripheral nerve, indicating significant
treatment-related axonal degeneration. Three birds treated with
chlorpyrifos showed grade II changes at one level of peripheral nerve
only, and these findings were considered to be similar to the
incidence and severity of changes seen in the vehicle controls. Thus,
oral administration of a single dose of chlorpyrifos at 110 mg/kg bw
to hens and another dose after 21 days did not induce clinical signs
of delayed neurotoxicity, as confirmed by histopathological
examination of nervous tissues (Roberts et al., 1987)
When chlorpyrifos was given to chickens for 91 days in the diet
at doses of < 10 mg/kg bw per day in a study conducted according to
GLP, the only compound-related clinical signs were transient, slight
gait alterations in a number of birds at the high dose. No
histopathological lesions of nerve tissues characteristic of
organophosphate-induced delayed neuropathy were seen. Concurrent
positive controls showed both toxic signs and histopathological
lesions of nerve tissues typical of those induced by a delayed
neurotoxicant (Barna-Lloyd et al., 1986).
To determine whether repeated doses of chlorpyrifos at the
maximum tolerated dose caused cumulative inhibition of neuropathy
target esterase and organophosphate-induced delayed neuropathy,
chlorpyrifos (purity, 100%) was given orally to 15 white Leghorn hens,
18 months of age, for 20 days at a dose of 10 mg/kg bw per day. Corn
oil was given to a control group of 18 birds. Physical observations
(including posture and gait) were made daily. Three hens per group
were killed on days 0 (controls only), 4, 10, 15, 20, and 48, and
brain acetylcholinesterase, plasma butyryl cholinesterase, and
lymphocyte and brain neuropathy target esterase were assayed. Body
weights were decreased during treatment, by 25% when compared with
controls on day 20, but had recovered to about 87% of that of controls
by the end of the study. Some birds showed acute effects (staggering
gait and diarrhoea) during week 1 of treatment. Diarrhoea was also
seen in controls and may have been associated with the corn oil. No
behavioural or perching defects were seen during the observation
period. During days 4-20, brain cholinesterase activity was depressed
by 58-70% and plasma cholinesterase activity by 49-80% when compared
with control values, but after the 4-week observation period brain
cholinesterase activity had recovered to 86% and plasma cholinesterase
to 134% of the controls values; neuropathy target esterase activity in
brain and lymphocytes throughout the study was 82-99% and 85-128% of
that in controls, respectively. This finding was not statistically
significant for lymphocytes, but the 18% inhibition of brain
neuropathy target esterase activity on days 10 and 20 was
statistically significant. There were no indications of delayed
neurotoxicity after repeated administration of a dose that caused
significant inhibition of brain cholinesterase activity and loss of
body weight (Richardson et al., 1993a).
In order to clarify the magnitude of the neuropathic risk
associated with exposure to chlorpyrifos, groups of three 18-month-old
white Leghorn hens were given single oral doses of chlorpyrifos
(purity, 100%) at 0, 75, 150, or 300 mg/kg bw with atropine (20 mg/kg
bw subcutaneously at the time of the first dose of chlorpyrifos and
additional doses as needed to maintain the hens for 4 days). Four days
after treament, when the hens were killed, brain acetylcholinesterase
activity was depressed by 0, 58, 75, and 86% and neuropathy target
esterase activity by 0, 21, 40, and 77% at the three doses,
respectively. In a further study, 18 hens were given corn oil alone
and 15 atropine-protected hens were given chlorpyrifos at a single
oral dose of 150 mg/kg bw. Three controls were killed on day 0, and
three treated and three control hens were killed on days 1, 2, 4, 8,
and 16. Brain acetylcholinesterase activity was inhibited by 86, 82,
72, 43, and 29% on days 1, 2, 4, 8, and 16 after treatment, and brain
neuropathy target esterase activity was inhibited by 30, 28, 38, 29,
and 6%. No signs of organophosphate-induced delayed neuropathy were
observed.
In studies in vitro in which chlorpyrifos-oxon was added to
whole brain homogenate, the rate constant for the enzyme-inhibitor
complex was 15.5 per mm per min for acetylcholinesterase and 0.145 per
mm per min for neuropathy target esterase. The calculated 20-min
fixed-time IC50 values were 2.2 nmol/L for acetylcholinesterase and
240 nmol/L for neuropathy target esterase. The measured IC50 values
based on fixed-time (20 min) preincubation were 2.2 nmol/L for
acetylcholinesterase and 210 nmol/L for neuropathy target esterase.
The ratio of the calculated values for neuropathy target
esterase:acetylcholinesterase is 110. As a ratio of > 1 generally
indicates that the dose that would be required to cause
organophosphate-induced delayed neuropathy would be in excess of the
LD50, acute exposure to chlorpyrifos, except at doses well in excess
of the LD50, would not be anticipated to induce this condition
(Richardson et al., 1993b).
The potential of chlorpyrifos to cause acute delayed
neurotoxicity was tested in white Leghorn hens given doses < 12
mg/kg bw per day for 21 days by oral gavage in peanut oil. Dose- and
time-related increases in the incidence and severity of ataxia were
observed. Plasma and whole blood cholinesterase activities were
reduced at 5 and 12 mg/kg bw per day. Ataxia was observed at 2, 5, and
12 mg/kg bw per day. Necropsy did not reveal any findings associated
with treatment. Histopathological examination of the brain and spinal
cord revealed a low incidence of several lesions, with no relation to
dose, and these findings were considered to be incidental to
treatment. Since continued administration of chlorpyrifos resulted in
ataxia, it would have been difficult to observe any delayed
neurological effects had they occurred (Jai Research Foundation,
1996b).
Rats
In rats, single oral doses of chlorpyrifos at 50-100 mg/kg bw
resulted in treatment-related effects and clinical signs for several
days after dosing. No deaths occurred at any dose. At 10 mg/kg bw,
effects were confined to isolated observations of perineal staining
and/or decreased activity. No neuropathological lesions were reported
(Wilmer, 1992; Mattsson et al., 1996).
In a study in adult Long-Evans rats carried out in accordance
with GLP, oral administration of chlorpyrifos at doses < 10 mg/kg
bw per day for 4 weeks resulted in dose-related inhibition of plasma
and erythrocyte cholinesterase activity at > 1 mg/kg bw per day and
of brain cholinesterase activity at > 3 mg/kg bw per day. These
effects were accompanied by clinical signs of intoxication at 10 mg/kg
bw per day. Cognitive behavioural tests indicated some non-cognitive
changes associated with impaired motor activity at 10 mg/kg bw per
day, but no clear treatment-related effects on cognitive function were
observed (Maurissen et al., 1996).
After oral administration of chlorpyrifos to rats at single doses
< 100 mg/kg bw in a study that conformed to GLP, no
treatment-related effects on neuropathy target esterase activity were
observed at any dose. Plasma, erythrocyte, and heart cholinesterase
activity was statistically significantly lower than that in controls
at doses > 5 mg/kg bw, and brain cholinesterase activity was
decreased at > 50 mg/kg bw. No significant inhibition was observed
at 0.5 or 1 mg/kg bw (Dittenber, 1997).
The neurotoxic potential of chlorpyrifos (purity, 99.5%) was
assessed in groups of 20 male Long-Evans hooded rats, approximately 70
days old with a mean body weight of 350 g. The test material was
dissolved in corn oil and given to the rats by oral gavage in a volume
of 1 ml/kg bw at doses of 0, 10, 30, 60, or 100 mg/kg bw. All rats
were evaluated for behavioural signs 3.5 h after dosing with a
functional observational battery of tests consisting of evaluations of
home cage, open-field, and manipulative neurobehaviour. The measures
were autonomic activity (lachrymation, salivation, miosis,
defaecation), motor activity (rearing, home cage posture), convulsive
activity (tremors), neuromuscular activity (gait score, landing foot
splay, righting reaction), general reactivity (arousal, removal
reactivity), sensorimotor activity (responses to tail pinch, click,
approach), and physiological reactions (body temperature, body
weight). Data on motor activity were recorded during a 1-h session in
a maze shortly after testing. Tissue samples were obtained after the
motor activity tests, 4.5 and 25 h after dosing. Half of the animals
in each group were killed at 3.5 h, and the remaining 10 animals were
examined again at 24 h and then killed. Immediately after sacrifice,
the brain, retina, liver, heart, diaphragm, and quadriceps femoris
muscle were collected from all animals. The brain was sectioned
mid-sagitally, and the remaining half-brain was further sectioned to
obtain the frontal cortex, hippocampus, striatum, hypothalamus,
cerebellum, and pons/medulla. Blood was collected from the trunk.
Muscarinic receptor density was determined in all brain regions and in
the heart and retina. Cholinesterase activity was determined in brain,
muscle, retina, plasma, erythrocytes, whole blood, and liver.
Cholinesterase activity was significantly inhibited in a range of
tissues, including brain, plasma, and erythrocytes, at all doses at
both 4.5 and 25 h (Table 20). As the data were represented only as
graphs, the percentages are approximations. Erythrocyte cholinesterase
activity was slightly more sensitive than that of plasma at the doses
used in this study, and plasma cholinesterase activity recovered more
quickly than that of erythrocytes. The degree of inhibition of
whole-blood cholinesterase activity lay between that of plasma and
erythrocyte activity at both 4.5 and 25 h. Whole-brain cholinesterase
activity can be considered representative of that in various regions
of the brain, although a number of regions showed less inhibition than
was measured in the half-brain in this study. In peripheral tissues,
Table 20. Percentage inhibition of cholinesterase activity compared with controls in rats given chlorpyrifos by gavage
Time (h) Dose Inhibition of cholinesterase activity (%)
(mg/kg bw)
Plasma Erythrocytes Blood Half-brain Retina Heart Diaphragm Quadriceps Liver
4.5 10 80 90 85 40 40 55 40 5 70
30 85 95 90 70 65 65 55 20 70
60 85 100 90 85 80 70 70 70 80
100 90 100 95 90 90 75 85 85 85
2.5 10 35 75 55 30 30 40 5 + 10 25
30 75 90 85 60 60 75 35 20 70
60 95 100 95 80 75 90 65 35 85
100 95 100 95 85 85 90 75 55 85
When inhibition was > 20% in comparison with corresponding controls, the change in cholinesterase activity was
statistically significant ( p < 0.05).
the inhibition was much lower in the quadriceps than in other tissues
at 4.5 h, and inhibition of heart, liver, and retina cholinesterase
activity was similar at 25 h.
Clinical and behavioural signs were observed at doses
> 30 mg/kg bw at 4.5 h. The doses selected for this study resulted
in a skew of some correlations, especially for cholinesterase
inhibition in blood and blood components, with behavioural and
clinical findings. In order to examine the relationship between
clinical and behavioural findings and cholinesterase inhibition, the
authors grouped rats according to the extent of cholinesterase
inhibition (instead of by dose) and indicated the incidence of
cholinergic signs related to individual cholinesterase inhibition. The
10 'cardinal' cholinergic signs that were used for this analysis were
temperature, gait, motor activity, smacking, foot splay, tail pinch,
tremors, lachrymation, pupil response, and salivation (Table 21). None
of these signs was observed in the 18 animals with < 60% inhibition
of brain cholinesterase activity. The incidence of these findings
increased in relation to brain cholinesterase inhibition. In animals
with < 80% inhibition of whole-blood cholinesterase activity, no
signs were observed, while all of the signs were seen at varying
incidences in animals with 90-100% inhibition. The correlation between
these end-points and inhibition of cholinesterase in other tissues or
in erythrocytes or plasma was not provided. These results were
interpreted by the authors as indicating a threshold effect, since no
clinical signs were observed until a certain percentage inhibition of
cholinesterase activity was reached. Because of the doses selected for
this study, marked inhibition was observed at the lowest dose in the
absence of clinical findings. The use of a single dose also limited
the usefulness of the findings. No clinical signs were seen when brain
cholinesterase activity was inhibited by < 60% or when whole blood
cholinesterase activity was inhibited by < 80% after a single dose of
chlorpyrifos. The data do not suggest that these values could be
extrapolated to repeated doses (Nostrandt et al., 1997).
Technical-grade chlorpyrifos (purity, 98.1%) was given to groups
of 10 male and 10 female Fischer 344 rats in the diet for 13 weeks at
doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day. The dietary
concentrations were adjusted on the basis of measured weight and food
consumption, but the range of dietary concentrations used to achieve
these target doses was not provided. The study was conducted in
accordance with GLP requirements and to meet US Environmental
Protection Agency FIFRA guideline No. 82-7. Body weights and feed
consumption were measured before treatment and weekly during
treatment. Ophthalmic examinations were conducted before treatment and
at necropsy. Cage-side observations were made twice daily and clinical
examinations weekly. A functional observational battery of tests was
applied to all rats before treatment and during weeks 4, 8, and 13 of
treatment. Five rats of each sex per dose were examined for gross
pathological alterations. Tissues for histopathological evaluation
were prepared only from rats in the control and high-dose groups. Nine
transverse sections of the brain included the olfactory bulb, cerebral
cortex (frontal, parietal, temporal, and occipital lobes),
Table 21. Incidence (%) of cardinal cholinergic signs in rats given chlorpyrifos by gavage and grouped according to
extent of inhibition of cholinesterase activity (numbers of rats in parentheses)
End-point Inhibition of brain cholinesterase activity (%) Inhibition of blood cholinesterase activity (%)
90-100 (10) 80-90 (8) 70-80 (7) 60-70 (7) < 60 (18) 90-100 (25) 80-90 (14) < 80 (11)
Temperature 100 100 86 57 0 92 36 0
Gait 100 100 57 71 0 84 43 0
Motor activity 90 88 86 57 0 84 36 0
Smacking 90 63 57 0 0 64 14 0
Foot splay 40 38 29 0 0 32 14 0
Tail pinch 70 38 0 0 0 40 0 0
Tremors 90 25 0 0 0 44 0 0
Lachrymation 80 13 0 0 0 32 7 0
Pupil response 60 0 0 0 0 24 0 0
Salivation 30 0 0 0 0 12 0 0
thalamus/hypothalamus, midbrain, pons, cerebellum, medulla oblongata,
and nucleus gracilis/cuneatus. Other tissues examined were the
trigeminal ganglion and nerve, pituitary, eyes (retina and optic
nerve), spinal cord (cervical and lumbar), olfactory epithelium, and
skeletal muscles (gastrocnemius and anterior tibial).
A low incidence of perineal staining was seen in females at 5 and
15 mg/kg bw per day. No decrease in body weight was recorded at any
dose. Gross pathological examination did not reveal any
treatment-related effects. Histopathological examination revealed a
small number of isolated findings that were similar in control and
treated groups and which were not considered to be related to
treatment. These included slight degeneration of individual nerve
fibres in the trapezoid body of the medulla oblongata in four control
males and three control females and in one male and two females at the
high dose; slight degeneration of individual nerve fibres in the
cervical spinal cord of a single male at the high dose; and myelin
ellipsoids in the lumbar spinal cord of one male at the high dose and
one female control. A slight reduction in motor activity was observed
at 15 mg/kg bw per day during week 4 only, but the toxicological
significance of this finding was unclear. Cholinesterase activity was
not measured in this study. No neuropathological findings related to
treatment were observed at any dose. The NOAEL for cholinergic effects
was 1 mg/kg bw per day, and that for neuropathy was 15 mg/kg bw per
day (Shankar et al., 1993).
In a study designed to investigate the effects of age on the
toxicity of chlorpyrifos, rats received corn oil or chlorpyrifos
orally at doses of 80 mg/kg bw for adults and 15 mg/kg bw for young
rats (at postnatal day 17). These doses were equally effective in
inhibiting cholinesterase activity. In adult rats, peak behavioural
changes and cholinesterase inhibition occurred in males 3.5 h after
dosing, while in females the onset of functional changes occurred
sooner, the time course was more protracted, and recovery was slower.
In young rats, the maximal behavioural effects and cholinesterase
inhibition occurred 6.5 h after dosing, with no sex-related
difference. Partial to full recovery from the behavioural changes was
seen at 24-72 h, whereas the cholinesterase activity recovered more
slowly. The blood and brain cholinesterase activity in young rats had
almost recovered by 1 week after dosing, whereas the brain
cholinesterase activity in adults had not recovered by 2 weeks. Assays
for binding to muscarinic receptors showed apparent down-regulation in
some areas of the brain, mostly at 24 and 72 h. Rats at postnatal day
17 generally showed more receptor down-regulation than adults, whereas
only adult females showed receptor changes in striatal tissue that
persisted for 2 weeks. Thus, young rats showed similar behavioural
changes and cholinesterase inhibition to adults, although at a
fivefold lower dose; the onset of maximal effects was somewhat delayed
in the young rats; cholinesterase activity tended to recover more
quickly in the young rats; young rats appeared to have more extensive
muscarinic receptor down-regulation; and young rats showed no
sex-related difference in functional changes (Moser & Padilla, 1998).
Chlorpyrifos was administered subcutaneously to neonatal rats at
1 or 5 mg/kg bw per day on postnatal days 1-4 or daily at 5 or 25
mg/kg bw per day on days 11-14, and the two groups of pups were
examined at postnatal days 5 and 10 or 15 and 20, respectively. Pups
treated at postnatal days 1-4 had a significant increased mortality
rate (> 50%) at 5 but not at 1 mg/kg bw per day; the body weights of
the affected pups were decreased by 10-15% and the brain was
significantly smaller than that of controls. The survivors showed
severe cell loss in the brainstem; brainstem growth was maintained by
enlargement of the remaining cells. The lower dose did not compromise
survival or growth. Neither dose had an adverse effect on the
forebrain, despite the fact that the brainstem and forebrain have
comparable cholinergic projections. Pups treated on postnatal days
11-14 showed no increase in mortality rate at 5 mg/kg bw per day but a
significant increase (> 80%) at 25 mg/kg bw per day at day 15. The
body weights of the affected pups were significantly decreased
(> 40%), and the major target for cell loss shifted from the
brainstem to the forebrain. The pups at 5 mg/kg bw per day had a
small, transient loss in body weight; there was no significant effect
on whole brain weight, but these pups showed loss of forebrain cells
between 15 and 20 days of age, and the total amount of DNA in the
forebrain declined whereas an increase was seen in the forebrains of
control pups. The brainstem showed a smaller but not significant cell
loss during this period. The cerebellum differed from the other
regions in that it showed short-term increases in DNA after exposure
to chlorpyrifos in either the early or the late postnatal period;
nevertheless, the values then regressed to subnormal, in parallel with
loss of cells in other regions.
Although regions rich in cholinergic projections, such as the
brainstem and forebrain, may be more severely affected than
non-cholinergic regions such as the cerebellum, the time of maturation
of each region (brainstem earliest, forebrain intermediate, cerebellum
last) appears to be more important in determining vulnerability. The
weights of the various regions remained within normal limits despite
severe reductions in cell numbers, probably because reactive
hypertrophy of the remaining cells masked the cell loss. These results
suggest that chlorpyrifos induces cellular deficits in the developing
brain even when growth or survival is unaffected; however, the route
of administration and the doses used in this study limit the relevance
of these findings to humans (Campbell et al., 1997).
Chlorpyrifos was administered subcutaneously to neonatal rats at
doses of 1 or 5 mg/kg bw per day on postnatal days 1-4, or at 5 mg/kg
bw per day on days 11-14, and the two groups of pups were examined on
postnatal days 5 and 10 or 15 and 20, respectively. Signs of
interference with the adenyl cyclase signalling cascade were
determined in the heart and developing brainstem and cerebellum. Pups
treated on postnatal days 1-4 at 1 mg/kg bw per day showed no signs of
toxicity, while there was significant mortality (> 50%) at 5 mg/kg bw
per day. Pups treated on postnatal days 11-14 at 5 mg/kg bw per day
showed no deaths or clinical signs. No significant changes in the
weights of the brain region were found at these apparently non-toxic
doses. Cholinesterase activity in the brainstem of the pups given
1 mg/kg bw per day was inhibited by 25% when measured 24 h after the
last dose, but recovery was substantial (< 10% inhibition) by day 10.
Cholinesterase activity in the brainstem of the pups given 5 mg/kg bw
per day on days 11-14 was inhibited by > 60% when measured 24 h after
the last dos, but recovery was again substantial (< 30% inhibition)
by postnatal day 20. Separate examinations of the forebrain,
cerebellum, and heart showed that chlorpyrifos had evoked deficits in
multiple components of the adenyl cyclase cascade: the expression and
activity of adenyl cyclase itself, functioning of the G-proteins that
link neurotransmitter and hormone receptors to cyclase activity, and
expression of neurotransmitter receptors that act through this
cascade. Disruption of signalling function was not restricted to
transduction of cholinergic signals but extended to adrenergic signals
as well. In most cases, the adverse effects were not evident during
administration of chlorpyrifos but appeared after several days. Most
of the results of this study were presented in summary form only (Song
et al., 1997).
The acute neurobehavioural effects (as determined by
observational tests of function and motor activity) of two carbamates,
carbaryl and aldicarb, and five organophosphates, chlorpyrifos,
diazinon, parathion, fenthion, and diisopropyl fluorophosphate, were
evaluated in 10-week-old male Long-Evans rats on the day of dosing at
the time of the peak effect, at 1 and 3 days, and 1 week after dosing.
All doses were administered by oral gavage in corn oil in a volume of
1 ml/kg bw, except carbaryl, which was given in 2 ml/kg bw. A weight
loss of about 10% was recorded with each substance only at the high
dose. Generally, all of the cholinesterase inhibitors induced
autonomic signs of cholinergic over-stimulation (salivation,
lachrymation, and miosis), hypothermia, mild tremors, mouth-smacking
(chewing motions), decreased motor activity, decreased tail-pinch
response, and altered neuromuscular function (gait changes and
increased foot splay). The measures found to be most sensitive on the
day of dosing were body temperature, motor activity, gait, and the
presence of mouth-smacking and fine tremors, although no single
measure was the most sensitive for all compounds. Differences in the
slopes of the dose-response curves were evident for some measures.
Many effects were still present at 24 h, but the rats recovered from
the effects of all compounds. Interestingly, residual effects were
found at 72 h with the carbamates and with the organophosphate
fenthion, but not with the other compounds; this may be due, at least
in the case of the carbamates, to slow absorption from the gut. Thus,
the overall clinical toxicity profile of these cholinesterase
inhibitors was similar, but compound-specific differences emerged in
terms of individual measures, dose-response relationships, and time
course; the behavioural effects induced by these compounds may be due
to both cholinergic and non-cholinergic mechanisms. Only summary data
were available for evaluation (Moser, 1995).
Chlorpyrifos was administered subcutaneously to neonatal rats at
2 mg/kg bw on postnatal day 1 or at 11 mg/kg bw per day on postnatal
days 6-9. The developing brain regions (brainstem, forebrain, and
cerebellum) were examined for signs of interference with cell
development by using markers of cell division. The 1-day-old rats
showed significant inhibition (about 15%) of DNA synthesis in all
brain regions within 4 h of treatment. Equivalent results were
obtained when a small dose (0.6 or 2 µg/g brain) was introduced
directly into the brain by intracisternal injection, indicating that
the effect was not secondary to systemic toxicity. Comparable
inhibition (> 10%) of DNA synthesis was seen in these animals at 8
days of age, but at this point there was regional selectivity, with
sparing of the cerebellum (about 1% inhibition). In another phase of
this study, 6-8-day-old animals were pretreated with mecamylamine, a
nicotinic receptor antagonist, and then with chlorpyrifos at 11 mg/kg
bw. The pretreatment caused a decrease in DNA synthesis and also
prevented any further decrement in DNA synthesis due to chlorpyrifos.
Administration of chlorpyrifos at 1 day of age caused severe (> 30%)
inhibition of protein synthesis throughout the brain; the effect was
distinct from that on DNA synthesis, as it had diminished
substantially by 8 days of age (< 10%) and did not show regional
selectivity. Assays of ornithine decarboxylase activity 4 and 48 h
after treatment with chlorpyrifos in 1- and 8-9-day-old rat pups
showed no significant alteration of activity in any brain region in
comparison with appropriate controls. The authors concluded that the
effects of chlorpyrifos on DNA and protein synthesis are not secondary
to generalized cell damage or suppression of cell metabolism, as
evidenced by the maintenance of normal ornithine decarboxylase
activity. The results of this study were presented only in summary
form (Whitney et al., 1995)
Pregnant Sprague-Dawley rats were given chlorpyrifos at doses of
0, 0.3, 1, or 5 mg/kg bw per day by gavage in corn oil from day 6 of
gestation through day 11 of lactation, and their neurobehavioural
performance was evaluated. A satellite group of mated females was
dosed similarly every day on days 6-20 of gestation and used to obtain
blood and brain samples for analysis of cholinesterase. This study was
conducted in accordance with GLP standards. There were no deaths and
only a few clinical signs (fasciculations, hyperpnoea,
hyper-reactivity, and decreased body-weight gain) in the dams at the
high dose. Between days 1 and 5 of lactation, three dams at 5 mg/kg bw
per day had total litter loss and an additional 57 pups died or were
cannibalized; these losses led to a decreased viability index.
Necropsy of many of the dead pups indicated treatment-related lack of
maternal care (no milk in the stomach). No similar effects of
treatment were seen at the other doses. In dams, cholinesterase
activity in plasma and erythrocytes was inhibited at all three doses,
with > 40% inhibition at 0.3 mg/kg bw per day. Brain cholinesterase
activity was inhibited at the two higher doses, but the inhibition at
1 mg/kg bw per day was not > 20% that of controls. Pups that were
exposed to chlorpyrifos in utero and for a period post partum
showed signs of toxicity at the high dose: the weights of male and
female pups were significantly lower than those at the other doses,
consistent with the decreased food consumption and lack of body-weight
gain by dams at this dose. Morphometric measurements of the brain and
brain sections of six pups of eachsex per dose showed a pattern of an
increase at the low dose and a decrease at the high dose, which was
more pronounced in male pups, paralleling the differences in brain
weight between the groups. Hence, the NOAEL for maternal toxicity was
1 mg/kg bw per day. The LOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.3 mg/kg bw per day, and the NOAEL for
inhibition of brain acetylcholinesterase activity was 1 mg/kg bw per
day. The NOAEL for toxic effects in the pups was 1 mg/kg bw per day on
the basis of the decreased viability index, relative brain weight, and
delayed sexual maturity, possibly associated with maternal toxicity
and subsequent diminished maternal care at the high dose. Cognitive
function (learning, memory, and habituation) in the pups were not
affected by treatment (Hoberman, 1998).
Adult male Long-Evans rats were trained to perform a test of
memory and motor function and were then injected subcutaneously with
single doses of 0, 60, 125, or 250 mg/kg bw of chlorpyrifos in peanut
oil (2 ml/kg bw) and tested on 5 days/week for 7 weeks. Unconditioned
behaviour was also rated for signs of cholinergic toxicity.
Cholinesterase activity was measured in whole blood and in several
brain regions, and muscarinic receptor density and the hypothermic
effect of oxotremorine challenge were determined. Clinical signs of
toxicity were seen in one rat at 250 mg/kg bw. Whole-blood
cholinesterase activity was inhibited by 60-75% in a dose-dependent
fashion on day 4 but had recovered to near control values by day 53 in
animals given 60 mg/kg bw and by day 74 in those given 125 mg/kg bw.
Brain cholinesterase activity was strongly inhibited in a
dose-dependent manner and was inhibited by < 95% on day 7 in
animals given 250 mg/kg bw. By day 21, partial recovery of
cholinesterase activity was seen in some brain regions after the doses
of 60 and 125 mg/kg bw, but no significant recovery was seen in
animals given 250 mg/kg bw. Muscarinic receptor density decreased with
dose and time. At 250 mg/kg bw, the receptor density in the
hippocampus, frontal cortex, and striatum had fallen to 70-75% of the
control values after 7 days and to 60% 21 days after dosing; the
hypothalamic receptors were less severely affected. In comparison with
controls, the hypothermia induced by oxotremorine challenge was
significantly less pronounced in each group receiving chlorpyrifos on
days 8 and 32, but was comparable to that of controls by day 52.
Unconditioned behaviour was relatively unaffected by treatment, with
fine tremor in the head and limbs, the only sign of cholinergic
overstimulation, peaking on day 9 and no longer observed by 14 days
after dosing. Functional deficits in working memory and motor function
appeared within 2 days of injection of a dose of chlorpyrifos that
caused severe cholinergic effects, but the animals recovered within
3 weeks. The definition of working memory in this paper was
non-specific, and the short-term memory of the animals was unaffected
by treatment (Bushnell et al., 1993).
The authors contrasted these findings to those seen in rats dosed
with the organophosphate diisopropyl fluorophosphate (Bushnell et al.,
1991). These rats showed progressive, persistent impairment of
cognitive and motor function over a 3-week period of daily exposure,
despite neurochemical and pharmacological evidence of tolerance to
diisopropyl fluorophosphate-induced inhibition of cholinesterase. This
difference suggests that the diisopropyl fluorophosphate-induced
behavioural changes cannot be attributed entirely to its effects on
cholinesterase activity and changes in muscarinic receptor binding.
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 200 mg/kg bw as a
single dose on day 12 of gestation and then killed on day 16 or 20 of
gestation or on postnatal day 3 for measurement of maternal and
developmental indicators of toxicity. While most treated dams showed
no overt cholinergic signs, 4/28 showed moderate-to-severe signs 2-3
days after treatment, and these rats were omitted from further
studies. Maternal body weight was decreased by 15% 3 days after
treatment, after which the body-weight increase was comparable to that
of controls. Chlorpyrifos did not significantly affect fetal body
weights or brain weights when assayed on day 16 or 20 of gestation.
Inhibition of acetylcholinesterase activity (by 82-88%) was seen in
maternal brain at all three times after exposures. At days 16 and 20
of gestation, fetal brain acetylcholinesterase activity was inhibited
by 42-44%. While some degree of recovery in acetylcholinesterase
activity was seen in pup brain by postnatal day 3, it was still
inhibited (by 30%) in treated pups cross-fostered by control dams.
Maternal brain muscarinic receptor binding at day 20 of gestation and
postnatal day 3 was more extensively reduced (30-32%) than in the
developing brain (16 and 11%, respectively). The response to a simple
postnatal reflex test of righting was transiently altered (present at
on day 1 but not on day 3) by exposure to chlorpyrifos. The results
suggested that acute exposure of dams during gestation induced more
extensive neurotoxicological effects in the dams than in their
developing fetuses, but the dose and the route of administration make
the relevance of this finding unclear (Chanda et al., 1995).
The maximum tolerated dose of chlorpyrifos in Sprague-Dawley rats
injected subcutaneously in peanut oil was 45 mg/kg bw in 7-day-old
pups and 280 mg/kg bw in adults (80-100 days old) (Pope et al., 1991).
At this dose, the neonates showed an initial transient decrease in
body weight when compared with controls. Adult rats also had an
initial decrease in body weight, but this had recovered by the end of
the 7-day observation period. Brain cholinesterase activity increased
by about 42% in neonates on days 7-14, whereas it remained constant in
adults. Neonatal brain cholinesterase activity was inhibited by 80% on
day 1 and by 45% on day 7, whereas 90% inhibition was seen in adults
on day 4, with little or no recovery. In plasma, inhibition of
cholinesterase activity was rapid and marked (95%) in pups on day 1
but had recovered to 50% of control by day 7. In adults, such
inhibition persisted through day 7, with no recovery of activity.
In a study to determine whether a measure of inhibitory potency
in vivo, the ED50, could be correlated with a measure of acute
toxicity, the maximum tolerated dose, in neonatal and adult rats,
chlorpyrifos was given by subcutaneous injection in peanut oil.
Cholinesterase inhibition was measured at the time of peak inhibition
(24 h in neonates and 4 days in adults). Depression of cholinesterase
activity versus log dose gave a straight line, and the slope of the
curve of inhibition of plasma cholinesterase was slightly steeper than
that of brain cholinesterase. The ED50 values for brain
cholinesterase activity were 20 (range, 17-23) in neonates and 44
(31-63) in adults, and those for plasma cholinesterase activity were
20 (13-31) in neonates and 70 (40-120) in adults. The correlation
between the two values was good for neonates but not for adult rats
(Pope & Chakraborti, 1992).
Groups of five 3-month old Sprague-Dawley rats were given
chlorpyrifos (purity, 98%) in peanut oil by subcutaneous injection at
a dose of 280 mg/kg bw, and trunk blood, cerebral cortex, and corpus
striatum were collected 2, 4, 6, and 12 weeks after dosing.
Acetylcholinesterase activity was assayed, and the binding of
[3H]quinuclidinyl benzilate to muscarinic receptors was measured in
membranes of the cortex and striatum. Locomotor activity was measured
for 30 min on 5 days/week. A challenge of scopolamine (1 mg/kg bw
intraperitoneally) was given 2 weeks after chlorpyrifos treatment and
then every two weeks for 12 weeks. Extensive inhibition of cortical
and striatal cholinesterase activity was seen 2 (94-96%), 4 (about
80%), 6 (about 60%), and 12 (15-20%) weeks after treatment. Plasma
cholinesterase activity was inhibited to a lesser extent, and recovery
was more rapid, with 85% inhibition at 2 weeks, 55% at 4 weeks, and
30% at 6 weeks; the activity was normal at 12 weeks. The changes in
cholinesterase activity were accompanied by reductions in muscarinic
receptor binding sites in the cortex (34, 33, and 18% reductions in
maximal binding) and striatum (48, 40, and 23% reductions) 2, 4, and 6
weeks after exposure. Tests of locomotor activity showed hypoactivity
in treated rats for 2-3 days after treatment. After the scopolamine
challenge, chlorpyrifos-treated rats showed increased activity at all
times except week 10 (Pope et al., 1992).
Chlorpyrifos was given to groups of six adult male Sprague-Dawley
rats by subcutaneous injection at a dose of 280 mg/kg bw, and the
animals were killed 2, 7, and 14 days after treatment. A significant
( p < 0.05), approximately 6% decrease in body weight was seen after
2-3 days, but the body weights recovered during the remainder of the
study. Chlorpyrifos caused marked inhibition of cholinesterase
activity in brain regions and plasma at all times, with about 90%
inhibition in the cortex, striatum, and plasma at day 2, and there was
virtually no recovery of this activity within the 14-day recovery
period. Reductions of < 55% in atropine-sensitive quinuclidinyl
benzilate binding (total muscarinic receptors) and binding in the
cortex and striatum were also observed (Chaudhuri et al., 1993).
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 25 mg/kg bw per day
on days 12-19 of gestation and killed on day 20 of gestation or
postnatal day 3. In a separate study to determine the dose-response
relationship, rats were similarly exposed to chlorpyrifos at 6.25 or
12.5 mg/kg bw per day on days 12-19 of gestation and killed on day 20
for analysis of various neurochemical markers. No maternal toxicity
was seen at any dose. Treatment with 25 mg/kg bw per day induced only
an initial, slight, transient decrease in maternal body weight.
Treatment from day 12 of gestation did not affect fetal weight at day
16 or 20, but pup weight on postnatal day 1 was significantly reduced
by treatment at this dose. Maternal brain cholinesterase activity
measured on day 20 of gestation was significantly inhibited in a
dose-related manner, with 75 and 90% inhibition at 6.25 and 25 mg/kg
bw per day, respectively. Fetal brain cholinesterase activity was less
severely inhibited, with 40 and 60% inhibition at the two doses,
respectively. Extensive inhibition of acetylcholinesterase activity,
to 10-17% of the control value, was seen in dams treated with 25 mg/kg
bw per day. Significant, dose-related downregulation of muscarinic
receptors in maternal and fetal brain was noted on day 20 of gestation
after exposure at all doses. The responses to righting reflex and
cliff avoidance tests were markedly altered after repeated exposures.
Generally, the neurochemical effects were more severe in dams than in
the developing fetuses, despite the greater sensitivity of fetal brain
cholinesterase to inhibition by chlorpyrifos. Comparison of these
results with those of an earlier study of acute exposure to
chlorpyrifos (Chanda et al., 1995) indicated that repeated exposure
causes greater down-regulation of muscarinic receptors than the
equivalent acute dose (Chanda & Pope, 1996).
Cats
Technical-grade chlorpyrifos dissolved in methylene chloride and
mixed in olive oil was given to adult male, colony-raised, short-hair
cats at doses of 0, 0.1, 0.5, 1, 5, 10, or 50 mg/kg bw to establish a
dose that would induce clinical signs of toxicosis but not death. Two
cats were used in this segment of the study, and each received
increasing doses of chlorpyrifos. On the basis of deaths at 50 mg/kg
bw, a dose of 40 mg/kg bw was selected for the next phase of the
study, in which three groups of six cats received corn oil,
chlorpyrifos, or chlorpyrifos followed by injections of atropine
sulfate every 12 h for 2 days. Two cats receiving the antidote also
received pyridine 2-aldoxime methane sulfonate every 12 h until the
clinical signs had abated. All cats given chlorpyrifos showed clinical
signs of toxicity, and one cat died 4 days later. Whole-blood and
plasma cholinesterase activity was reduced in all treated animals, but
the levels had returned to pre-test levels after 7-28 days. Brain
cholinesterase activity was normal in all animals that survived until
the end of the study, 28 days after treatment. Gross pathology
revealed no treatment-related lesions, and haematological parameters
were unaffected (Hooser et al., 1988).
Groups of five adult male cats received a single intramuscular
injection of corn oil (vehicle), diisopropyl fluorophosphate (positive
control; 5 mg/kg bw), or chlorpyrifos (purity, 99%; 300 mg/kg bw) and
were observed for 60 days. Atropine and pyridine 2-aldoxime methane
sulfonate were given to chlorpyrifos-treated animals once or twice
daily for 14-24 days. Ataxia was observed in positive controls (mean
onset, 16 days; range, 14-19 days) and in those given chlorpyrifos
(mean onset, 19 days; range, 17-21 days) . Functional deficits were
seen, characterized by a crouched-waddling gait, hypermetria, and
proprioceptive deficits, and these effects were still present in both
groups 56 days after exposure; the effects in chlorpyrifos-treated
animals began to subside after day 35. Lymphocyte neuropathy target
esterase activity was inhibited by < 96% in positive control
animals (24 h after dosing) and by 46% in chlorpyrifos-treated cats
(7 days after dosing) but had reached control levels by day 28 in the
latter group. Whole-blood cholinesterase activity was reduced by
< 86% by day 4 and slowly recovered to control levels by day 56.
Diisopropyl fluorophosphate had inhibited cholinesterase activity by
< 63% 24 h after dosing, but the recovery of activity was similar
to that in chlorpyrifos-treated animals. Histological examination
(Table 22) revealed degeneration of axons in animals treated with
chlorpyrifos and with diisopropyl fluorophosphate, and, while the
lesions were similar, they were more severe in chlorpyrifos-treated
animals. The effects were characterized by axonal degeneration with
vacuolar (spongy) changes in the white matter tracts of the cerebrum,
cerebellum, and spinal cord and occasionally in the peripheral nerves.
Vacuolization was seen, resulting from distension of axonal spaces
associated with focal axonal swelling and degeneration of myelin and
myelin-forming cells, with infiltration of snall numbers of
macrophages (Fikes et al., 1992). The route of administration and
magnitude of the dose in this study limit its usefulness.
(g) Studies on metabolites
(i) Acute toxicity
The main metabolite of chlorpyrifos, TCP, was generally of lower
acute toxicity than chlorpyrifos (Table 23).
(ii) Short-term studies of toxicity
Groups of 10 rats of each sex were fed diets containing 0, 0.01,
0.03, 0.1, 0.3, or 1% TCP for 90 days. The three lower doses had no
treatment-related effects on body-weight gain, mortality, food
consumption, haematological values, blood urea nitrogen, alkaline
phosphatase, or average body and organ weights. The NOAEL was 0.1% in
the diet, approximately 50 mg/kg bw per day, on the basis of reduced
body-weight gain at 1% (Beatty, 1964).
Groups of 15 rats of each sex were fed diets containing sodium
TCP at concentrations providing doses of 0, 10, 30, or 100 mg/kg bw
per day for 91 days. The dietary concentration was varied to allow for
increasing body weight and ranged from about 85, 250, and 825 ppm at
day 0 to about 160, 460, and 1540 ppm at day 84 at the three doses,
respectively. Significantly increased body-weight gain was observed in
males at 30 mg/kg bw per day from day 48 onwards. There were no
treatment-related changes in haematology, urinary analysis, or blood
chemistry values. The absolute weight of the heart was increased in
males at 100 mg/kg bw per day, and increased absolute and relative
liver and kidney weights were seen in animals of each sex at this
dose. There were no gross or histopathological alterations due to
treatment. The NOAEL was 30 mg/kg bw per day on the basis of increased
relative liver and kidney weights (Barna-Lloyd & Szabo, 1985).
Table 22. Occurrence and severity (score ± SD of histological changes in the nervous system of cats
given a single intramuscular injection of chlorpyrifos, diisopropyl fluorophosphate
(positive control), or corn oil
Location Treatment
Corn oil Diisopropyl fluorophosphate Chlorpyrifos
No. affected Score No. affected Score No. affected Score
Brain
Cerebruma 2/5 0.40 ± 0.49 4/5 0.80 ± 0.40 4/5 2.00 ± 0.63
Cerebellumb 1/5 0.20 ± 0.40 2/5 0.40 ± 0.49 3/5 1.00 ± 0.89
Spinal cord
C2 2/5 0.20 ± 0.49 4/5 0.80 ± 0.40 5/5 1.40 ± 0.49
C7 1/5 0.20 ± 0.40 3/5 0.60 ± 0.49 5/5 1.40 ± 0.49
T-L 4/5 0.80 ± 0.40 5/5 1.00 ± 0.0 4/5 0.80 ± 0.40
L4 0/5 0.00 ± 0.0 5/5 1.60 ± 0.49 5/5 1.80 ± 0.40
Sciatic nerve 0/5 0.00 ± 0.0 3/5 0.60 ± 0.49 1/5 0.20 ± 0.40
Severity of lesions (axonal swelling, vacuolar change, myelin degeneration) in each section was given the
score of the most severely affected area. The lesions were scored as 0 = normal or equivocal, 1 = mild
(1-5 nerve fibres affected/high power field), 2 = moderate (6-10 nerve fibres affected), 3 = marked
(11-15 nerve fibres affected), and 4 = severe change (> 20 nerve fibres affected).
a Anterior parietal cerebrum sectioned transversely immediately posterior to the optic chiasm and
anterior to the infundibulum
b Transverse section through the cerebellum and brain stem at the level of the trigeminal nerves
Table 23. Acute toxicity of 3,5,6-trichloro-2-pyridinol (TCP) administered orally
Species Strain Sex Vehicle LD50 (mg/kg bw Reference
and 95% CI or range)
Mouse Swiss M 0.5% hydroxypropylmethyl 380 (333-433) Gerbig & Emerson (1970a)
F cellulose (Methocel) 414 (367-469)
Rat Sprague-Dawley M 0.5% hydroxypropylmethyl 794 (709-889) Gerbig & Emerson (1970b)
F 870 (758-1009)
Doga Beagle NR Gelatine capsule > 1000 Gerbig & Emerson (1970c)
NR, not reported
a One dog received two doses of 1000 mg/kg bw TCP, a second received four doses of 500 mg/kg bw, and a third
received eight doses of 50 mg/kg bw. Emesis was seen in all animals within 1-3 h of dosing. There were no deaths.
Groups of two beagles of each sex were given diets containing TCP
at concentrations providing doses of 0, 26, or 80 mg/kg bw per day for
93 days. At termination, haematological parameters were normal. There
was no NOAEL as elevated serum alkaline phosphatase activity and
histopathological evidence of liver damage and increased testicular
weights were seen in the treated animals (JMPR, 1972; modified by
reference to the original report of Copeland, 1964).
Groups of three dogs of each sex were given diets containing TCP
at concentrations providing doses of 1, 3, 10, or 30 mg/kg bw per day
for 91 days. No toxic effects were seen at the three lower doses, but
at 30 mg/kg tbw per day evidence of liver toxicity was seen, with
increased activity of alkaline phosphatase and alanine and aspartate
aminotransferases although there was no unequivocal sign of liver
damage on histopathological examination (JMPR, 1972; modified by
reference to the original report of Emerson & Gerbig, 1970).
Groups of four beagles of each sex were fed diets containing TCP
at concentrations providing doses of 0, 3, 12, or 48 mg/kg bw per day
for 1 year in a study conducted according to GLP. No details of the
clinical observations were reported. The body weights of males were
not statistically significantly different between groups, but females
at the high dose had consistently lower body weights than controls and
statistically significantly lower body-weight gains. No changes in
food consumption were found in treated groups, and no alterations in
haematological or urinary parameters. The dose-related changes in
clinical chemistry values that were attributed to treatment were
increased serum alkaline phosphatase and alanine aminotransferase
activities in males and females at 3, 6, and 12 months. The values
were comparable to those of controls at 3 mg/kg bw per day,
consistently elevated but generally not to a statistically significant
degree at 12 mg/kg bw per day, and were statistically significantly
increased at 48 mg/kg bw per day at most times. There were no gross or
histopathological lesions that were associated with treatment and no
changes in absolute or relative organ weights. The NOAEL for systemic
effects was 12 mg/kg bw per day, on the basis of a lack of a
consistent, statistically significant increase in enzyme activities at
that dose (Zempel et al., 1987).
(iii) Genotoxicity
TCP was not genotoxic in a range of assays in vitro and
in vivo (Table 24).
(iv) Developmental toxicity
In a range-finding study, Fischer 344 rats were given TCP
(purity, 99.7%) at doses of 0, 43, 75, or 127.5 mg/kg bw per day on
days 6-15 of gestation by oral gavage. Clinical signs (perineal
soiling) were seen sporadically in all dams at the highest dose and in
some at 75 mg/kg bw per day. Food and water consumption were
unaffected by treatment. The signs of maternal toxicity were limited
to reduced body-weight gain in dams at the high dose. The relative and
Table 24. Results of tests for the genotoxicity of 3,5,6-trichloro-2-pyridinol
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 3.16, 10, 31.6, 99.7 Negativea,b Zempel & Bruce (1986)
TA98, TA100, 100, 316 µg/plate
TA1535, TA1537, in DMSO
TA1538
Unscheduled Rat hepatocytes 1-100 µg/ml in 99.7 Negativec Gollapudi (1987)
DNA synthesis (Fischer CDF 344) DMSO
Forward mutation Chinese hamster 62.5, 125, 250, 99.7 Negativea Linscombe & Gollapudi
ovary cells, hprt locus 500, 750 µg/ml (1986)
In vivo
Micronucleus Mouse (CD-1) 250, 500, 750, 99.9 Negative McClintock & Gollapudi
formation marrow cells 1000, 1500, 2000, (1989b; GLP)
3000 mg/kg bw
orally in corn oil
Micronucleus Mouse (CD-1) 0, 24, 76, 240 mg/kg 99.7 Negative Bruce et al. (1985)
formation marrow cells bw orally in
corn oil
GLP, good laboratory practice; DMSO, dimethyl sulfoxide
a With and without an exogenous metabolic system
b Toxic at 100 (some strains) and 316 (all strains) µg/plate
c Toxic at 50 and 100 µg/ml
absolute weights of the liver and kidney were unaffected by treatment,
and there was no observable effect on reproductive parameters
(Scortichini et al., 1986a).
Groups of Fischer 344 rats were given 0, 50, 100, or 150 mg/kg bw
per day of TCP (purity, 99.7%) on days 6-15 of gestation by oral
gavage in a study that conformed to GLP. No clinical signs of toxicity
were seen in any group. Slight vaginal bleeding occurred in one animal
at 50 mg/kg bw per day and two at 150 mg/kg bw per day on day 13. Food
consumption was slightly decreased in animals at 100 mg/kg bw per day
and decreased by about 10% in those at the high dose. Significant,
dose-related decreases in body-weight gain were recorded throughout
treatment at 100 and 150 mg/kg bw per day. Changes in the relative and
absolute liver and kidney weights were restricted to a significant
increase (4%) in the relative liver weight at 150 mg/kg bw per day,
consequent to a significant decrease (5%) in body weight at this dose.
There was a dose-related trend in the number of implantation sites per
dam, which was reflected in the number of fetuses per litter and
probably in gravid uterine weight; the latter trend may also reflect
the trend to lower body weight with higher dose. Fetal examination
revealed a small number of findings in each group, including controls.
Overall, treatment had no significant effect on reproductive
parameters or fetuses. The NOAEL for maternal effects was 50 mg/kg bw
per day on the basis of decreased body-weight gain, and that for fetal
toxicity and teratogenic effects was 150 mg/kg bw per day, the highest
dose tested (Hanley et al., 1987a).
In the first phase of a study in inseminated New Zealand white
rabbits, the animals were given TCP (purity, 99.7%) on days 6-18 of
gestation by oral gavage at doses of 0, 48.5, 80, or 130 mg/kg bw per
day. As there was uncertainty that the maximum tolerated dose had been
reached in this phase, additional groups of animals were given 130 or
210 mg/kg bw per day (phase 2). Maternal body-weight gain during
gestation was depressed but not statistically significantly and was
highly variable at the highest dose in both phases. Gross examination
of all animals showed no significant findings or any differences
between groups. At 210 mg/kg bw per day, resorptions were seen in all
litters, and the percent implantations resorbed and the percent
litters with resorptions were statistically significantly different
from those in the comparable control group in phase 2. There was no
observable effect of treatment on reproductive parameters at doses up
to 130 mg/kg bw per day (Scortichini et al., 1986b).
Groups of New Zealand white rabbits were given TCP (purity,
99.7%) by oral gavage on days 7-19 of gestation at doses of 0, 25,
100, or 250 mg/kg bw per day in a study that met GLP requirements.
Female animals were given injections of human chorionic gonadotropin
to synchronize estrus and were inseminated 3 weeks later, which was
presumed to be day 0 of gestation. No clinical signs of toxicity were
seen in any group. Water and food consumption were not reported. A
dose-related decrease in body-weight gain was seen during treatment,
which reached statistical significance at the high dose. There were no
treatment-related changes in the relative or absolute weights of the
liver or kidney. Fetal examination revealed a small number of
malformations in each group, including controls. Overall, there was no
statistically significant effect of treatment on reproductive
parameters or fetuses, but an increased incidence of central nervous
system malformations at the intermediate and high doses suggested a
possible teratogenic effect, and the authors concluded that these
findings provided evidence of teratogenic potential, despite a lack of
statistical significance. The malformations included severe dilatation
of the cerebral ventricles and hydrocephaly. The incidences of minor
external, visceral, or skeletal alterations were unchanged. The NOAEL
for maternal effects was 100 mg/kg bw per day on the basis of
decreased body-weight gain during treatment. The NOAEL for fetal
toxicity and teratogenic effects was 25 mg/kg bw per day, on the basis
of central nervous system malformations at higher doses (Hanley et
al., 1987b).
3. Observations in humans
3.1 Experimental studies
A commercial preparation of chlorpyrifos containing 35% xylene
was applied to the skin of the back and abdomen of four volunteers
(skin area not stated) at a dose of 5, 10, 25, or 50 mg/kg bw 20 (5
days/week), four, three, and two times, respectively. The material was
covered with an occlusive patch for 12 h, and after removal of the
patch each site was scored for irritation before washing. Each site
was used only once. Plasma and erythrocyte cholinesterase activity was
determined before and several times after treatment (exact times not
stated; for results, see Table 25). In another study, five volunteers
received dermal doses of 94-620 mg/kg bw for 12 h and showed similar
inhibition of erythrocyte and plasma cholinesterase activity 24, 48,
and 72 h after exposure, even at the highest dose. No skin irritation
was recorded. Plasma cholinesterase activity was significantly
inhibited with two of the dose regimens, the activity reaching a
minimum around 3 days after the last dose and then recovering to
control values within 4-10 days; erythrocyte cholinesterase activity
were not inhibited with any regimen (Kilian et al., 1970).
Four volunteers were exposed for 5 min to a formulation
containing 61.5% chlorpyrifos and 34.5% xylene from an ultra-low
volume cold aerosol fog generator delivering 3.8 L/h. Air sampling
showed a concentration of chlorpyrifos in the breathing space of about
108 mg/L (range, 83-133 mg/L). The subjects were exposed at a distance
of 8 m and wore plastic coveralls allowing exposure of the heads and
hands of two subjects and the heads, hands, and arms of the other two.
Exposure was terminated after 5 min because of the ocular and
pulmonary irritation induced. Plasma and erythrocyte cholinesterase
activity was not depressed 24 h after exposure (Pennington & Edwards,
1971).
Table 25. Cholinesterase activity after dermal dosing of humans (% of pre-treatment value)
Dose regimen End of dosing Day 1 Day 3
Plasma Erythrocytes Plasma Erythrocytes Plasma Erythrocytes
10 mg/kg bw for 4 × 12 h 99 102 97 103 97 100
50 mg/kg bw for 2 × 12 h 100 100 100 100 102 100
25 mg/kg bw for 3 × 12 h 67 98 48 98
5 mg/kg bw for 20 × 12 h 67 103 64 104 74 104
One subject per dose regimen
Groups of four healthy, adult, male volunteers received
chlorpyrifos in tablet form at doses of 0, 0.014, 0.03, or 0.1 mg/kg
bw per day for 27, 20, and 9 days, respectively, with breakfast.
Control subjects received a placebo for 48 days. Although not
specifically stated, the high dose appears to have been withdrawn
because of the severity of plasma cholinesterase inhibition on day 9.
No rationale was given for termination of treatment at the lower
doses. Heparinized blood samples were obtained from the subjects twice
weekly for determination of plasma and erythrocyte cholinesterase
activity until all values had returned to pre-treatment levels. As it
was not stated whether the blood samples were taken at the same time
on each sampling day, the interval between administration and sampling
is unknown. No indication was given of the frequency of observation
for clinical signs after treatment or how such observations were made.
A single subject at 0.1 mg/kg bw per day reported having a runny
nose, blurred vision, and a feeling of faintness on the final day of
dosing, and his plasma cholinesterase activity was found to be
inhibited by 70% when compared with pre-treatment levels. No decrease
in erythrocyte cholinesterase activity was seen in any subject during
the study, and no other treatment-related effects were noted. Rapid,
marked depression of plasma cholinesterase activity was observed in
subjects at the high dose, and the activity did not return to control
levels until about day 34. Similar results were obtained when the
plasma cholinesterase activity in these subjects was compared with the
baseline activity of the group before treatment. The mean plasma
cholinesterase activity of men at 0.03 mg/kg bw per day was inhibited
by > 20% on days 16-20 of treatment when compared with those given
placebo, and the activity did not return to control levels for several
weeks after cessation of treatment. The plasma cholinesterase activity
of men at 0.014 mg/kg bw per day was inhibited by 20% only on day 13
and was similar to the baseline activity from day 20 to day 27, even
with continued administration of chlorpyrifos. Two-way analysis of
variance indicated that the inhibition of mean plasma cholinesterase
activity was significant only at 0.1 mg/kg bw per day ( p < 0.05)
when compared with controls. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day, the highest dose
tested (JMPR, 1972; modified by reference to the original report of
Coulston et al., 1972).
Six white male volunteers aged 27-50 years were used in another
study. One volunteer was given a single oral dose of 0.5 mg/kg bw
chlorpyrifos in a lactose capsule, about 30 min after food, and about
1 month later the other volunteers received the same treatment and the
first volunteer was given a dermal dose of 0.5 mg/kg bw chlorpyrifos
dissolved in methylene chloride. Two weeks after the first dermal
dose, the first volunteer was given a further dermal dose of 0.5 mg/kg
bw in dipropylene glycol methyl ether. The other volunteers were given
a dermal dose of 5 mg/kg bw of chlorpyrifos in dipropylene glycol
methyl ether 4 weeks after their oral dose. All urine was collected
from volunteers from 24-48 h before dosing until 120 h after dosing.
No signs or symptoms of toxicity were reported in any of the men
during the study. After oral administration of 0.5 mg/kg bw, the
plasma cholinesterase activity of the first volunteer was inhibited by
71% from that before treatment and that of the other men by a mean of
85% within 12-24 h of treatment. The range of individual values was
not provided. Plasma cholinesterase activity had returned to > 80% of
the mean baseline value by 30 days after oral treatment. After dermal
application on day 30, the plasma cholinesterase activity had
decreased to approximately 70% of baseline, and returned to about
80-90% of those levels by day 40 of the study. The intra-group
variation was stated to be considerable, but the figures were not
provided. Erythrocyte cholinesterase activity was not significantly
inhibited by either oral or dermal doses of chlorpyrifos (Nolan et
al., 1982, 1984).
In a double-blind, randomized, placebo controlled study of the
effects of single oral doses of chlorpyrifos (purity, 99.8%), groups
of six fasted men and women aged 18-55 received doses of 0, 0.5, 1, or
2 mg/kg bw in lactose powder. The study was conducted in two phases
separated by 14 days. The volunteers were dosed with 0, 0.5, or 1
mg/kg bw in the first phase and the results were assessed before
administration of 0 or 2 mg/kg bw in the second phase. Blood samples
were collected 10 and 0 h before treatment and 2, 4, 8, 12, 24, 36,
48, 72, 96, 120, 144, and 168 h after treatment and analysed for
erythrocyte cholinesterase activity and chlorpyrifos and its
metabolites. In addition, all urine voided from 48 h before dosing to
168 h after dosing was collected at 12- or 6-h intervals and analysed
for chlorpyifos and metabolites. Haematology, clinical chemistry,
urinary analysis and a brief physical examination were performed at
completion of the study. Blood and urine were collected to determine
each volunteer's paraoxonase status and the concentrations of
chlorpyrifos and metabolites, but these data have not yet been
reported. The volunteers were screened for general health according to
set criteria and instructed to refrain from alcohol, strenuous
exercise, and prescription medications before and during the study.
The doses were taken by capsule after an overnight fast. The health
status of subjects was monitored closely; vital signs (blood pressure,
pulse, respiration, and temperature) were assessed before dosing and
1, 2, 4, 8, 12, 24, 48, and 168 h after treatment. The subjects were
questioned about their well-being at each sampling time, and the
symptoms were evaluated clinically. The subjects were aware of the
signs and symptoms of cholinergic toxicity and were instructed to
inform the study physician of any adverse effects. The subjects were
unaware of their treatment, and the signs and symptoms were assessed
and treated by a physician who was also unaware of the treatment
status of the subject.
There were no significant deviations from the study protocol. One
male control in phase 1 and one woman receiving 2 mg/kg bw did not
provide a complete series of blood and urine samples. Treatment had no
effect on general health or on clinical chemical parameters measured 7
days after dosing. The only treatment-related effect was found in the
woman who withdrew from the study, who had decreased erythrocyte
cholinesterase activity when compared with her pre-treatment values at
most sampling times, with 98.4% of the pretreatment value at 4 h after
dosing, 77% at 8 h, 72% at 12 h, 74% at 24 h, 81% at 36 h, and 80% at
48 h (Table 26). When the data for this subject are removed from the
analysis, the mean for women receiving 2 mg/kg bw is indistinguishable
from the value for concurrent controls (Table 26). The NOAEL for
clinical signs or symptoms was thus the highest dose tested, and the
NOAEL for inhibition of erythrocyte cholinesterase activity was 1
mg/kg bw on the basis of significant inhibition in one of 12 subjects
(Kisicki et al., 1999).
3.2 Case reports
Blondell & Dobozy (1997) summarized the case reports, case
series, statistical surveys, and epidemiological studies of acute and
chronic health effects reported to be related to exposure to
chlorpyrifos. The authors noted the limitations of their review,
including inadequate documentation of exposure and effects, reporting
biases, and absence of information on the denominator population at
risk. They concluded, largely on the basis of an examination of
reports from poison control centres, that chlorpyrifos is one of the
leading causes of acute insecticide poisoning in the USA. Certain uses
were considered to pose greater risks than others, the main concern
being use of liquid formulations in households or by pest control
operators indoors or outdoors, termite treatment, and application of
liquid sprays and dips to domestic animals. Most of the more serious
cases were associated with misuse or inappropriate use such as spills
and inadvertent contamination by a pest control operator.
Shurdut et al. (1997) commented that deficiencies in the report
of Blondell & Dobozy (1997) invalidated its use for reaching a
conclusion about the safety of chlorpyrifos. They concluded that the
data from poison control centres had been misinterpreted and in fact
indicated the relative safety of products containing chlorpyrifos.
They also claimed that the report failed to respect the limitations of
anecdotal information, failed to consider the extensive testing with
regard to neurological damage and the long history of use of products
containing chlorpyrifos, made inappropriate use of anecdotes and
studies of organophosphates to characterize the safety of
chlorpyrifos, failed to consider the extensive database on exposure
after use of chlorpyrifos products, and presented the data
selectively.
In a case report, a previously well 3-year-old boy was found
playing near an open, spilled bottle of insecticide containing
chlorpyrifos (concentration not stated). The authors noted that
clinical manifestations of organophosphate-induced polyneuropathy
usually begin 1-3 weeks after an acute cholinergic crisis and that the
results of examination of the child were consistent with distal,
symmetric, predominantly motor polyneuropathy. In this patient, the
symptoms of cholinesterase inhibition and manifestations of weakness
and areflexia 11 days after ingestion and electromyographic findings
confirmed the presence of acute transient polyneuropathy with a more
Table 26. Mean erythrocyte cholinesterase activity as percent of mean baseline values in volunteers given single oral
doses of chlorpyrifos
Dose Time in relation to dosing (h)
(mg/kg bw)
- 10 0 4 8 12 24 36 48
Women
0.0a 99.5 (2.1) 100.5 (2.1) 98.3 (2.0) 102.1 (2.4) 100.7 (1.8) 99.7 (3.9) 98.4 (1.7) 100.2 (2.3)
0.0b 99.2 (4.4) 100.8 (4.4) 102.5 (5.9) 103.1 (5.4) 98.4 (3.2) 99.4 (3.7) 94.6 (5.3) 97.7 (5.1)
0.5 101.2 (6.7) 98.8 (6.7) 96.3 (6.5) 98.1 (2.1) 95.8 (3.9) 97.0 (3.6) 94.7 (4.7) 97.1 (4.1)
1 98.2 (0.9) 101.8 (0.9) 104.6 (4.2) 100.8 (3.5) 98.9 (3.7) 100.9 (5.6) 96.0 (4.1) 98.9 (2.1)
2 99.8 (1.8) 100.2 (1.8) 100.3 (3.2) 96.6 (6.81) 91.1 (9.7) 95.2 (10.6) 95.9 (7.4) 94.5 (7.7)
Men
0.0a 100.6 (1.8) 99.4 (1.8) 99.1 (2.2) 99.6 (4.6) 99.8 (4.3) 98.4 (4.3) 97.6 (2.8) 98.2 (3.1)
0.0b 96.1 (5.1) 103.9 (5.1) 101.6 (4.0) 102.5 (5.2) 102.9 (5.8) 102.6 (2.3) 98.2 (3.8) 95.9 (6.8)
0.5 98.7 (1.1) 101.3 (1.1) 102.0 (3.0) 103.4 (4.9) 99.7 (3.0) 101.9 (3.3) 98.1 (3.0) 92.8 (4.7)
1 98.6 (1.9) 101.4 (1.9) 104.5 (7.8) 101.1 (4.2) 101.1 (4.7) 101.7 (4.7) 98.5 (4.6) 92.1 (2.0)
2 101.2 (2.3) 98.8 (2.3) 99.2 (3.1) 98.9 (3.5) 98.7 (4.0) 99.2 (2.6) 99.5 (5.1) 98.4 (3.1)
a Phase 2 control
b Phase 1 control
proximal distribution than is usually seen in adults. The acute,
reversible bilateral vocal cord paralysis reported in this patient had
not been seen previously in organophosphate-induced delayed
neuropathy, but the authors noted that the onset of stridor and vocal
cord paralysis coincided with the onset of muscle weakness and
resolved at the same time as normalization of the peripheral
neuropathy (Aiuto et al., 1993a).
Gutmann & Bodensteiner (1993), responding to the report of Aiuto
et al. (1993), suggested that this case may not be a clear example of
organophosphate-induced delayed neuropathy. They noted that
chlorpyrifos has been associated with such symptoms only after severe
intoxication characterized by extensive cholinesterase inhibition. The
inhibition of plasma cholinesterase activity in the reported case was
instead suggestive of mild chlorpyrifos intoxication. They also noted
that the clinical findings associated with organophosphate-induced
delayed neuropathy are predominantly motor polyneuropathy, flaccid
weakness and atrophy primarily in the distal limb muscles, and often
incomplete recovery usually after months or years. The case report
implied that the evoked compound muscle action potentials and sensory
nerve action potentials were of normal amplitude, which would run
counter to a diagnosis of organophosphate-induced delayed neuropathy.
In response to these comments, Aiuto et al. (1993b) noted that
the patient's symptoms were consistent with > 90% inhibition of
cholinesterase activity and that the plasma cholinesterase activity
did not necessarily correlate with the severity of poisoning. The
authors concluded that the patient had developed delayed,
life-threatening neuropathy, although they were unable to determine
the mechanism of the condition.
In another case report, a 38-year-old man drank an undefined
quantity of a solution of 25% chlorpyrifos. He was treated with 3 mg
of atropine intravenously every 2 h for 6 days (total, 400 mg), but
any attempt to stop the atropine treatment resulted in reappearance of
respiratory distress. After 6 days, the patient was stuporous,
catatonic, and suffering from respiratory distress with white mucous
secretions, and no cholinesterase activity could be detected in his
serum. When ipratropium was administered endotracheally at a dose of
0.5 mg as an aerosol mist over 10 min, the patient's clinical
condition improved, although the salivary secretion continued. The
patient continued to receive ipratropium at 1-2 mg/day, and
respiratory support was discontinued on the fifth day. Severe
catatonia and coarse tremor were relieved by administration of
dantrolene at 10 mg intravenously, followed by 25 mg orally, three
times daily. Cholinesterase activity was still undetectable in serum
1 month after the man was discharged (Shemesh et al., 1988).
In a case in which a 26-year-old man intentionally ingested
approximately 360 ml of a 6.7% chlorpyrifos formulation containing
2,4-D (10.8%) and mecoprop (11.6%) and a few granules of a warfarin
(0.025%) concentrate, the main clinical findings on admission were
coma, myoclonus, miosis, cardiac arrythmia with progression to
hypotension, oliguria, and death after several episodes of asystole.
The authors stated that such symptoms are consistent with those seen
in patients who have ingested chlorophenoxy acetic acids such as 2,4-D
and mecoprop. The patient did not show many of the usual signs of
organophosphate poisoning, such as lachrymation, salivation,
respiratory paralysis, or muscle fasciculation, but his erythrocyte
cholinesterase activity was inhibited by about 70% at 13 h and by
< 90% by 26 h. Plasma cholinesterase activity was inhibited
completely at all times. Lymphocyte neurotoxic esterase activity was
inhibited by about 50% after 13 h but was within normal limits after
19 h. The activity of neurotoxic esterase in the cerebral cortex was
within normal limits but that in peripheral nerves was inhibited by
about 70% when compared with normal levels. The inhibition of
neurotoxic esterase activity was found in the absence of significant
signs of organophosphate intoxication (Osterloh et al., 1983).
A 21-year-old man who ingested an unknown quantity of a
chlorpyrifos formulation required large amounts of atropine and
pyridine 2-aldoxime methane sulfonate, endotracheal intubation, and
ventilation to overcome the cholinergic effects, which consisted of
pupillary constriction, excess secretions, tachycardia, and impaired
consciousness. Bilateral vocal cord paralysis developed 16 days after
extubation (28 days after exposure), and this finding was considered
to be indicative of organophosphate-induced delayed neuropathy. The
patient was asymptomatic within 4 weeks of the onset of symptoms (de
Silva et al., 1994).
A 42-year-old man who attempted suicide by drinking a commercial
formulation of 41% chlorpyrifos (estimated dose, 300 mg/kg bw) went
into coma and showed respiratory insufficiency, lachrymation,
salivation, sweating, miosis, fasciculations, and bronchorrhoea on
admission to hospital 18 h after exposure. A chest X-ray showed right
basal bronchopneumonitis, electroencephalographic signs of diffuse
irritation were recorded, and chlorpyrifos was detected in gastric
lavage fluid. Plasma cholinesterase activity was inhibited by almost
100% by 36 h. The coma and cholinergic signs persisted for 17 days,
during which time atropine, pyridine 2-aldoxime methane sulfonate,
mechanical respiration, and antibiotics were used. The patient was
asymptomatic on day 24 after exposure, and drug administration was
stopped; no signs of peripheral or central nervous system involvement
were observed. On day 30, lymphocytic neurotoxic esterase, blood
acetylcholinesterase, and plasma butyryl cholinesterase activities
were markedly below the normal range but recovered slowly thereafter.
On day 43, weakness and paraesthesia in the legs with reduced tendon
reflexes and sensory conduction velocity were noted. By day 62, the
leg weakness was more severe, gait was impaired, and tendon reflexes
were absent. Electromyography of the leg muscles showed symmetrical
signs of denervation with spontaneous activity, and motor conduction
velocity was reduced. Electrophysiological studies of the arms
confirmed a symmetrical reduction of sensory conduction velocity of
the ulnar nerves. Sural nerve biopsy on day 63 revealed a few altered
myelinated fibres. Aspects of axon and myelin degeneration were also
found occasionally. The changes were consistent with mild distal
axonopathy. Chlorpyrifos was still detectable 10 days after exposure,
and neurotoxic esterase activity was still inhibited by 60% 4 weeks
after exposure (Lotti et al., 1986).
In a study in which toxicological and/or electrophysiological
examinations were conducted in 11 patients who had acute
organophosphate poisoning, three of the patients, one of whom had been
poisoned with chlorpyrifos, developed organophosphate-induced delayed
neuropathy (Moretto & Lotti, 1998). The findings in this individual
were reported by Lotti et al. (1986). The electro-physiological data
indicated organophosphate-induced delayed neuropathy with sensory and
motor components which persisted for 3 months, at which time the
patient was lost to follow-up.
Four incidents of birth defects allegedly associated with
exposure to chlorpyrifos were reported. The children were found to
have a range of birth defects, including ventricular, ocular, and
palate defects and growth retardation (all children), hydrocephaly,
microcephaly, mental retardation, blindness, hypotonia, widely spread
nipples, and deformities of the teeth, ears, and external genitalia.
The mothers of the affected children had reportedly been exposed to
chlorpyrifos either at the workplace or at home during the pregnancy.
Two of the children were born to the same mother. The exposure of the
mothers to chlorpyrifos was poorly characterized, and it was not
indicated whether it was severe or sustained during organogenesis. The
range of birth defects reported was not consistent with the results of
studies in laboratory animals (Sherman, 1996).
In a comment on this paper, Gibson (1996) noted that the medical
records of the cases indicated that the same effects were not seen in
all children and that the effects in some of the children were
consistent with a diagnosis of an autosomal recessive birth defect
syndrome of the brain and eye known as cerebro-oculo-facio-skeletal
syndrome or a closely related one known as the 'MICRO' syndrome.
Eight cases were reported in which exposure to chlorpyrifos was
claimed to have caused sensory neuropathy. The patients each presented
with a range of symptoms, and characterization of exposure was poor.
One patient who reported signs of cholinesterase inhibition, including
lachrymation, muscle twitching, and diarrhoea, was a pesticide
applicator who had reportedly been exposed to chlorpyrifos in a closed
environment for 6 months. The patient's erythrocyte cholinesterase
activity was reportedly to be 'low' initially but returned to normal
levels within 2 months. Neurological evaluations were conducted only 6
weeks after the other symptoms were reported; at this time, sensory
loss of all modalities was found in a 'stocking-glove distribution'
test, with mild distal weakness and areflexia in the lower
extremities. Studies of nerve conduction and quantitative sensory
threshold revealed changes consistent with peripheral neuropathy of
the distal anonopathy type. The decreased reflexes and mild distal
weakness were consistent with polyneuropathy. Follow-up examination at
1 year revealed remission of all symptoms. In the other seven patients
(four from the same family), the symptoms were nonspecific, and the
absence of symptoms of cholinesterase inhibition suggested that these
patients had not been as exposed to chlorpyrifos as extensively as the
first patient. The lack of immediate electrodiagnostic testing, the
poor exposure characterization, and the variation in the reported
clinical findings make interpretation of the case studies difficult.
Additionally, subjective questioning was used to obtain information
from the patients. Follow-up examinations for a range of parameters
were not conducted on several patients, and the patients did not
undergo the same initial testing regimen. Thus, one patient was
exposed to chlorpyrifos occupationally at a level sufficient to
decrease cholinesterase activity and cause cholinergic symptoms; he
also showed evidence of mild, reversible polyneuropathy, possibly in
the presence of decreased erythrocyte cholinesterase activity. For two
other patients, there was limited evidence of mild polyneuropathy
(Kaplan et al., 1993).
3.3 Monitored field trials
A monitored field test was used to determine the effect of
chlorpyrifos on spraymen applying treatment at the rates used in
programmes for the eradication of mosquitoes. Three studies were
conducted in 1966, 1967, and 1968. Cholinesterase activity was
measured, and exposure was terminated if the inhibition reached 50% of
the baseline values. In the first study, three spraymen showed marked
depression of plasma cholinesterase activity, with reductions of
68-82% from baseline values. The two remaining spraymen showed
reductions of 52-56% over 9 days, but the baseline values were not
available. The study was discontinued after 2 weeks because of the
significant decrease in cholinesterase activity. In the second study,
no treatment-related effect on cholinesterase activity was seen during
a short period. In the third study, erythrocyte cholinesterase
activity was not affected by treatment, but plasma cholinesterase
activity was reduced by 50-80% in spraymen using a suspension
formulation. The activity had generally returned to pre-exposure
levels within 2 months of cessation of use. Four spraymen using an
emulsion formulation did not show reduced plasma cholinesterase
activity during the exposure period (Kenaga, 1967; Eliason et al.,
1969).
3.4 Studies of morbidity
In a study in which the sample size was small and the statistical
power was limited, comparisons were made between 175 employees
potentially exposed to chlorpyrifos at a manufacturing plant where
they had held jobs between 1 January 1977 and 31 July 1985 and 335
unexposed controls matched on age (within 5 years), date of hire
(within 5 years), sex, race, and pay status. The source of information
for the observations (self, nurse, company physician, private
physician, laboratory) was recorded, as were use of tobacco, alcohol,
and cholinergic drugs. Exposure was grouped into high, moderate, and
low on the basis of industrial hygiene surveys and consultation with
veteran manufacturing personnel. The plasma cholinesterase activities
of the potentially exposed employees before exposure were available,
and follow-up was conducted at roughly monthly intervals. The mean
inhibition of plasma cholinesterase activity was 19 ± 2.9% in the
group with low exposure, 32 ± 2.8% in that with moderate exposure, and
32 ± 5.3% in that with high exposure. No cases of peripheral
neuropathy were recorded in the study group. Inhibition of plasma
cholinesterase activity was observed but was not associated with
illness. The exposed groups reported symptoms of dizziness, malaise,
and fatigue more frequently than the unexposed group, but the analysis
did not indicate any correlation with increasing exposure (Brenner et
al., 1989).
In a follow-up to this study (Burns et al., 1998), the data were
updated to include the period 1987-94, and additional medical
disorders were considered. Data on self-reported paraesthesia were
collected from 1982 to 1994. The prevalence of peripheral neuropathy
was not significantly increased in the group of workers exposed to
chlorpyrifos.
Comments
After oral administration to rats, radiolabelled chlorpyrifos was
rapidly and extensively absorbed (up to about 90% of the dose) and
eliminated, predominantly in the urine (68-93%) and faeces (6-15% of
the dose), within about 72 h of administration. The urinary
metabolites included the glucuronide (about 80%) and sulfate (about
5%) conjugates of chlorpyrifos, and 3,5,6-trichloro-2-pyridyl
phosphate (TCP; about 12%). The tissue concentrations of residues of
[14C]chlorpyrifos were very low (generally < 1 ppm) within 72 h of
dosing. The longest half-time of residues in rats was 62 h in fat, and
low levels were also detected in the fat of several other species and
in the milk of goats.
In humans who were poisoned with chlorpyrifos formulations,
diethylphosphorus metabolites were excreted in the urine by
first-order kinetics, with an average elimination half-time of 6.1 ±
2.2 h in the fast phase and of 80 ± 26 h in the slow phase. In
volunteers, the time to maximal concentration of 3,5,6-TCP in the
blood was 0.5 h after oral dosing and 22 h after dermal treatment, but
the elimination half-time by both routes was 27 h, and the percentage
of the administered dose recovered from the urine was 70% after oral
dosing and 1.3% after dermal administration.
Chlorpyrifos is rapidly metabolized by mixed-function oxidases to
the highly reactive chlorpyrifos oxon by oxidative desulfuration. The
oxon can be deactivated by hydrolysis to diethylphosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is hydrolysis
to monoethyl 3,5,6-trichloro-2-pyridinyl phosphorothioate.
The lowest oral LD50 value was 96 mg/kg bw (range, 96-475 mg/kg
bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in mice. Female
rats were generally more sensitive to the acute effects of
chlorpyrifos than males. The signs of acute intoxication with
chlorpyrifos were consistent with cholinesterase inhibition. The acute
dermal LD50 of chlorpyrifos was > 2000 mg/kg bw in rats and > 1200
mg/kg bw in rabbits.
WHO (1999) has classified chlorpyrifos as 'moderately hazardous'.
Chlorpyrifos was irritating to the eye and skin of rabbits, but
it did not sensitize the skin of guinea-pigs in Magnusson-Kligman
maximization or Buehler tests.
In short-term studies, the NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day in dogs and 0.1
mg/kg bw per day in rats. The NOAEL for inhibition of brain
cholinesterase activity was 1 mg/kg bw per day in dogs and rats. The
signs of toxicity were largely limited to cholinergic signs and
decreased body weights and/or food consumption. The NOAEL for these
effects in short-term studies was 1 mg/kg bw per day in rats, and the
NOAEL for clinical signs was 3 mg/kg bw per day in dogs. In mice,
ocular effects and histopathological alterations (including adrenal
lipogenic pigmentation and ocular keratitis) were observed (NOAEL, 50
ppm; equal to 7 mg/kg bw per day). In rats, the NOAEL for increased
fatty vacuolation of the adrenal zonal fasciculata and changes in
haematological and clinical chemical parameters was 5 mg/kg bw per
day. When rats received chlorpyrifos dermally for 21 days, the NOAEL
for inhibition of cholinesterase activity in erythrocytes and brain
was 5 mg/kg bw per day.
In long-term studies, inhibition of cholinesterase activity was
again the main toxicological finding in all species. In rats, the
NOAEL was 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity and 1 mg/kg bw per day for inhibition of
brain acetylcholinesterase activity, but clinical signs were not seen
at doses up to 10 mg/kg bw per day, and the NOAEL for reduction in
body weight was 1 mg/kg bw per day. In mice, erythrocyte and brain
acetylcholinesterase activities were inhibited at 50 ppm, equal to 6.1
mg/kg bw per day, and the NOAEL was 5 ppm, equal to 0.7 mg/kg bw per
day. Cholinergic signs and reductions in body weight were reported
only at the highest dietary concentration of 250 ppm (equal to 32
mg/kg bw per day). Other treatment-related findings included effects
on the liver in mice, with a NOAEL of 50 ppm (equal to 6.6 mg/kg bw
per day), and increased adrenal weight in rats with a NOAEL of 1 mg/kg
bw per day. There was no treatment-related increase in the incidence
of neoplastic lesions in any of the long-term studies. The Meeting
concluded that chlorpyrifos is unlikely to pose a carcinogenic risk to
humans.
Chlorpyrifos was not genotoxic in an adequate range of studies
in vitro and in vivo. The Meeting concluded that chlorpyrifos is
not genotoxic.
In multigeneration studies of reproductive toxicity in rats, the
treatment-related effects of chlorpyrifos were limited to inhibition
of cholinesterase activity, consistent with that seen in other short-
and long-term studies, and fetotoxicity characterized by reduced pup
viability, body weights and survival. No significant,
treatment-related clinical signs were reported. The NOAEL for
inhibition of maternal acetylcholinesterase activity was 0.1 mg/kg bw
per day for erythrocytes and 1 mg/kg bw per day for brain. The NOAEL
for developmental toxicity was 1 mg/kg bw per day. No effects on
reproductive parameters were observed at the highest dose tested,
5 mg/kg bw per day.
In studies of developmental toxicity in mice, rats, and rabbits,
the maternal effects included inhibition of erythrocyte and/or brain
acetylcholinesterase activity and cholinergic signs (lowest NOAEL,
1 mg/kg bw per day in rats and mice) and reductions in body weight and
food consumption (lowest NOAEL, 2.5 mg/kg bw per day in rats). The
observed fetal toxicity (lowest NOAEL, 2.5 mg/kg bw per day in rats)
and developmental toxicity (NOAEL, 1 mg/kg bw per day in rats) were
consistent with treatment-related maternal toxicity; there was no
evidence of treatment-related malformations in any of the studies.
There was no effect on cognitive function (learning, memory, and
habituation) in pups exposed to chlorpyrifos in utero and for a
period post partum at doses up to and including the highest dose of
5 mg/kg bw per day, while inhibition of cholinesterase activity,
decreased brain weight, and delayed development were seen at lower
doses, consistent with findings in other studies.
In studies of delayed neurotoxicity, chlorpyrifos was given to
chickens as either single or repeated doses. Significant inhibition of
both cholinesterase and neuropathy target esterase activity was
observed, and mild delayed neuropathy was seen in a number of studies;
aggressive antidotal therapy was always necessary to allow at least
some of the treated birds to survive. Despite the marked cholinergic
toxicity of chlorpyrifos, there was no evidence that it caused delayed
neurotoxicity, and there was no increase in the incidence of
histopathological lesions in the nerve tissues of birds treated at
doses up to 10 mg/kg bw per day for up to 91 days. In a number of
studies in rats given single doses of up to 100 mg/kg bw, repeated
doses of up to 10 mg/kg bw per day for 4 weeks, or repeated doses of
up to 15 mg/kg bw per day for 13 weeks, there were no
treatment-related neurological lesions or effects on cognition and no
inhibition of neuropathy target esterase activity, although
significant inhibition of erythrocyte, brain, and peripheral tissue
cholinesterase activity was seen at some doses. In a study that
included a functional observational battery of tests, clinical signs
of intoxication were observed after a single dose only when brain
acetylcholinesterase activity was inhibited by more than 60% or when
whole-blood cholinesterase activity was inhibited by more than 80%.
When chlorpyrifos was applied as a single dose of up to 5 mg/kg
bw to the skin of volunteers for 12 h, erythrocyte cholinesterase
activity was not significantly inhibited. Plasma cholinesterase
activity was inhibited after 20 12-h dermal exposures to 5 mg/kg bw
per day over 4 weeks or after three daily 12-h exposures to 25 mg/kg
bw per day on consecutive days, but erythrocyte cholinesterase
activity was not inhibited under any treatment regimen.
A single oral dose of up to 1 mg/kg bw or repeated doses of up to
0.1 mg/kg bw per day for 9 days did not significantly inhibit
erythrocyte acetylcholinesterase activity in volunteers. No clinical
signs were observed in these studies. Inhibition of erythrocyte
acetylcholinesterase activity was observed in a single female
volunteer (of a group of six men and six women) given a single oral
dose of 2 mg/kg bw.
In a case of human poisoning with chlorpyrifos at an estimated
dose of 300-400 mg/kg bw, significant inhibition of neuropathy target
esterase in lymphocytes and of plasma and erythrocyte
acetylcholinesterase activity was reported, with severe cholinergic
signs which required aggressive, extensive antidotal therapy and
artificial ventilation. Mild distal axonopathy consistent with
organophosphate-induced delayed polyneuropathy was reported some weeks
after the poisoning incident.
The ADI of 0-0.01 mg/kg bw established by the 1982 Meeting was
based on a NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in humans. The present Meeting affirmed
this ADI on the basis of the NOAEL of 1 mg/kg bw per day for
inhibition of brain acetylcholinesterase activity in studies in mice,
rats, and dogs, using a 100-fold safety factor, and on the basis of
the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in the study of human subjects exposed
for 9 days, using a 10-fold safety factor.
The Meeting allocated an acute reference dose of 0.1 mg/kg bw on
the basis of the NOAEL of 1 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity in a study in which volunteers received
a single oral dose of chlorpyrifos, with a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 0.7 mg/kg bw per day (toxicity in a 79-week
study of toxicity and carcinogenicity)
1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
10 mg/kg bw/day (fetal toxicity in a study of developmental
toxicity)
Rat: 1 mg/kg bw per day (toxicity in 2-year studies of toxicity
and carcinogenicity)
1 mg/kg bw per day (developmental and parental toxicity in a
two-generation study of reproductive toxicity)
1 mg/kg bw per day (maternal and developmental toxicity in a
study of developmental toxicity)
2.5 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Rabbit: 81 mg/kg bw per day (maternal and fetal toxicity in a study
of developmental toxicity)
Dog: 1 mg/kg bw per day (toxicity in a 2-year study of toxicity)
Human: 0.1 mg/kg bw per day (no inhibition of erythrocyte
cholinesterase activity at highest dose tested in men dosed
orally for 9 days)
1 mg/kg bw (inhibition of erythrocyte cholinesterase
activity in adult volunteers after a single oral dose)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to chlorpyrifos
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Up to 90% in rats within 72 h; about 70% in humans within 96 h
Dermal absorption Less than 2% in humans within 180 h; not determined in animals
Distribution Initially widely distributed; highest residues in liver, kidneys
and fat at 72 h in rats
Potential for accumulation Elimination half-times of < 24 h and low tissue residues after
72 h in rats. No evidence of potential for accumulation
Rate and extent of excretion > 95% within 72 h in rats, mainly in urine (68-93%) and faeces (6-15%)
Metabolism in animals Rapidly metabolized by mixed-function oxidases to chlorpyrifos oxon
via oxidative desulfuration and an electrophilic phosphooxathiiran
intermediate. Degradation by conversion directly to
3,5,6-trichloro-2-pyridyl phosphate and diethyl thiophosphate.
The oxon is hydrolysed to diethyl phosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is by
hydrolysis to monoethyl-3,5,6-trichloro-2-pyridinol phosphorothioate.
Toxicologically significant compounds Parent compound and oxon
(animals, plants and environment)
Acute toxicity
Rat,, LD50, oral 96 mg/kg bw
Rat LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 36 mg/m3 (4 h, vapour, nose-only exposure)
560 mg/m3 (4 h, nebulized particles < 5 mm, whole-body exposure)
Rabbit, dermal irritation Slightly irritating
Rabbit, ocular irritation Slightly irritating
Guinea-pig, dermal sensitization Not sensitizing
Short-term toxicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest critical oral NOAEL 1 mg/kg bw per day, dog, 2 years
1 mg/kg bw per day, rat, 13 weeks
Lowest relevant dermal NOAEL 5 mg/kg bw per day, rat; 21 days
Lowest relevant inhalation NOAEL 20.6 ppb (296 mg/m3), rat; 13 weeks
Long-term toxicity and carcinogenicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest relevant NOAEL 2 years, rat; 1 mg/kg bw per day
78 weeks, mouse; 0.7 mg/kg bw per day
Carcinogenicity Not carcinogenic in rats or mice
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Neonatal toxicity (reduced pup body weight and survival)
Lowest relevant reproductive NOAEL Two-generation, rat; 1 mg/kg bw per day
Developmental target/critical effect Fetal and perinatal toxicity at maternally toxic doses (including
an increase in delayed ossification, reduced crown-rump length,
reduced pup weight, increased postimplantation loss, delayed sexual
maturity)
Lowest relevant developmental NOAEL Rats; 1 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Reversible neurotoxicity consistent with cholinesterase
inhibition. No evidence of delayed neurotoxicity or
histopathological changes in nerves of hens (10 mg/kg bw per day)
and rats (15 mg/kg bw per day) for up to 13 weeks. At high acute
doses (up to 150 mg/kg bw), significant inhibition of neuropathy
target esterase and mild delayed neuropathy in hens, but at this
dose, extensive and aggressive antidote treatment were required
for birds' survival.
Other toxicological studies No effect on cognitive function in rat pups in a study of
developmental toxicity at doses up to 5 mg/kg bw per day
Medical data No inhibition of erythrocyte acetylcholinesterase activity in
volunteers after repeated oral doses of up to 0.1 mg/kg bw per day
(for 9 days), single oral doses of up to 1 mg/kg bw, or a single
dermal doses of 5 mg/kg bw. Poisoning case presented with severe
cholinergic effects, with evidence of delayed polyneuropathy and/or
distal axonopathy at a dose that required antidotal treatment and
artificial ventilation.
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw Rat, 2-year dietary 100
Rat, reproduction
Mouse, developmental toxicity
Dog, 2-year dietary
Human, 9-day oral 10
Acute reference dose 0.1 mg/kg bw Human, single dose 10
References
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993a) Life-threatening
organophosphate-induced delayed polyneuropathy in a child after
accidental chlorpyrifos ingestion. J. Pediatr., 122, 658-660.
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993b)
Organophosphate-induced delayed polyneuropathy (comment).
J. Pediatr., 123, 837.
Aldridge, W.N. (1953) Serum esterases I. J. Biochem., 53, 110-117.
Anderson, B.T., Walker, S.A. & Punler, M.J. (1995) Chlorpyrifos tech:
Acute inhalational toxicity study in rats. Unpublished report No.
10425 from Inveresk Research International, Tranent, Scotland.
Submitted to WHO by Luxembourg Industries, Tel Aviv, Israel.
Bakke, J.E., Feil, V.J. & Price, C.E. (1976). Rat urinary metabolites
from O,O-diethyl-O(3,5,6-trichloro-2-pyridyl) phosphorthioate.
J. Environ. Sci. Health Bull., 3, 225-230.
Barna-Lloyd, T. & Szabo, J.R. (1985) Sodium
3,5,6-trichloro-2-pyridinol: 3-month rat dietary toxicity study.
Unpublished report No. TexasT:K-065999-099 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Barna-Lloyd, T., Szabo, J.R. & Young, J.T. (1986) Chlorpyrifos:
Subchronic organophosphate-induced delayed-neurotoxicity (OPIDN)
study in laying chickens hens. Unpublished report No.
TexasT:K-044793-064 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Bauriedel, W.R. & Miller, J.H. (1981) The metabolic fate of the sodium
salt of C14-labelled 3,5,6-trichloro-2-pyridinol orally
administered to swine. Unpublished report No. GH-C 1427 from Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. (1964) Results of 90-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in rats. Unpublished report from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,Odiethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1971) Results of 90-day dietary
feeding studies of O,Odiethyl-O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Berman, C.L. (1987) Evaluation of chlorpyrifos (Pyrinex) for dermal
sensitization of guinea pig. Unpublished report No. 59487-01 from
Arthur D. Little Inc., Cambridge, Massachusetts, USA. Submitted
to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Berteau, P.E. & Deen, W.A. (1978) A comparison of oral and inhalation
toxicities of four insecticides to mice and rats. Bull.
Environ. Contam. Toxicol., 19, 113-120.
Blackmore, R.H. (1968) Oral administration -- dogs Dursban. Final
report. Unpublished report No. 174-115 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Blagden, S.M. (1994) Chlorpyrifos technical: Acute inhalation toxicity
study four-hour exposure (nose only) in the rat. Unpublished
report No. CHA 10-CYF from Safepharm Laboratories Ltd, Derby,
United Kingdom. Submitted to WHO by Cheminova Agro A/S, Lemvig,
Denmark.
Blondell, J. & Dobozy, V.A. (1997) Review of chlorpyrifos poisoning
data. Washington DC, Environmental Protection Agency.
Branson, D.R. & Litchfield, N.H. (1970) Comparative absorption,
elimination and distribution of DOWCO 179, its methyl analog
DOWCO 214 and their major metabolite,
3,5,6-trichloro-2-pyridinol. Unpublished report No. 70 4479 from
Dow Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971a) Absorption, excretion and
distribution of O,Odiethyl O-3,5,6-trichloro-2, 6-C14-2-pyridyl
phosphorothioate in rats. Unpublished report No. NBA-9 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971b) Absorption, excretion and
distribution of 3,5,6trichloro-2, 6-C14-2-pyridinol in rats.
Unpublished report No. NBA-10 from Dow Chemical Co., USA
Submitted to WHO by Dow Agrosciences, Indianapolis, IN, USA.
Brenner, F.E., Bond, G.G., McLaren, E.A., Greene, S. & Cook, R.R.
(1989) Morbidity among employees engaged in the manufacture or
formulation of chlorpyrifos. Br. J. Ind. Med., 46, 133-137.
Breslin, W.J., Liberacki, A.B., Dittenber, D.A., Brzak, K.A. & Quast,
J.F. (1991) Chlorpyrifos: Two-generation dietary reproduction
study in Sprague-Dawley rats. Unpublished report No. K-044793-088
from the Toxicology Research Laboratory, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Bruce, R.J. & Zempel, J.A. (1986) Chlorpyrifos: Evaluation in the Ames
Salmonella/mammalian-microsome mutagenicity assay. Unpublished
report No. TexasT:K044793-075 from Lake Jackson Research Center,
Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Bruce, R.J., Gollapudi, B.B. & Hinze, C.A. (1985) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008 from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Buch SA (1980) Pyrinex tech: Acute inhalation toxicity in rats.
Unpublished report No. 80/MA/025/362 from Life Science Research,
Stock, Essex, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980a) Pyrinex tech: Irritance to rabbit
eye. Unpublished report No. 80/MA/023/143 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980b) Pyrinex tech: Irritance to rabbit
skin. Unpublished report No. 80/MAK/024/144 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1981) Pyrinex technical: Acute oral
toxicity in the rat. Unpublished report No. 81/MAK035/104 from
Life Science Research, Stock, Essex, United Kingdom. Submitted to
WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A., Gardner, J.R. & Birnie, J.V. (1980) Pyrinex: Acute
percutaneous toxicity study in rats. Unpublished report No.
80/MAK022/300 from Life Science Research, Stock, Essex, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Burns, C.J., Cartmill, J.B., Powers, B.S. & Lee, M.K. (1998) Update of
the morbidity experienced of employees potentially exposed to
chlorpyrifos. Occup. Environ. Med., 55, 65-70.
Bushnell, P.J., Padilla, S.S., Ward, T., Pope, C.N. & Olszyk, V.B.
(1991) Behavioral and neurochemical changes in rats dosed
repeatedly with diisopropylfluorophosphate. J. Pharmacol.
Exp. Ther., 256, 741-750.
Bushnell, P.J., Pope, C.N. & Padilla, S. (1993) Behavioral and
neurochemical effects of acute chlorpyrifos in rats: Tolerance to
prolonged inhibition of cholinesterase. J. Pharmacol. Exp.
Ther., 266, 1007-1017.
Calhoun, L.L. & Johnson, K.A. (1988) Chlorpyrifos: 4-day dermal probe
and 21-day dermal toxicity studies in Fischer 344 rats.
Unpublished report No. HET K 044793-085,086 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Calhoun, L.L. & Johnson, K.A. (1989) Supplemental information to the
report entitled: Chlorpyrifos: 4-day dermal probe and 21 day
dermal toxicity studies in Fischer 344 rats. Unpublished report
No. K-044793-085(S) from Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Campbell, C.G., Seidler, F.J. & Slotkin, T.A. (1997) Chlorpyrifos
interferes with cell development in rat brain regions. Brain
Res. Bull., 43, 179-189.
Capodicasa, E., Scapellato, M.L., Moretto, A., Caroldi, S. & Lotti, M.
(1991) Chlorpyrifosinduced delayed polyneuropathy. Arch.
Toxicol., 65, 150-155.
Chambers, J.E. (1992) The role of target-site activation of
phosphorothionates in acute toxicity. In: Chambers, J.E., Levi,
P.E. & Levi, A.P., eds, Organophosphates, San Diego, Academic
Press.
Chambers, J.E. & Carr, R.L. (1995) Biochemical mechanisms contributing
to species differences in insecticidal toxicity. Toxicology,
105, 291-304.
Chanda, S.M. & Pope, C.N. (1996) Neurochemical and neurobehavioural
effects of repeated gestational exposure to chlorpyrifos in
maternal and developing rats. Pharmacol. Biochem. Behav., 53,
771-776.
Chanda, S.M., Harp, P., Liu, J. & Pope, C.N. (1995) Comparative
developmental and maternal neurotoxicity following acute
gestational exposure to chlorpyrifos in rats. J. Toxicol.
Environ. Health, 44, 189-202.
Chaudhuri, J., Chakraborti, T.K., Chanda, S. & Pope, C.N. (1993)
Differential modulation of organophosphate-sensitivity muscarinic
receptors in rat brain by parathion and chlorpyrifos.
J. Biochem. Toxicol., 8, 207-216.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in beagle hounds. Unpublished report
No. T35.12-38278-3 from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Corley, R.A., Landry, T.D., Calhoun, L.L., Dittenber, D.A. & Lomax,
L.G. (1986) Chlorpyrifos: 13-week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET K
044793-077 from Dow Chemical Company Laboratory, Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Costa, L.G., McDonald, B.E., Murphy, S.D., Omenn, G.S., Richter, R.J.,
Motulsky, A.G. & Furlong, C.E. (1990) Serum paraoxonase and its
influence on paraoxon and chlorpyrifosoxon toxicity in rats.
Toxicol. Appl. Pharmacol., 103, 66-76.
Coulston, F., Golberg, L., Abraham, R. & Benitz, F.K. (1971) Final
report on safety evaluations and metabolic studies on Dowco 179
(Indiana 151). Unpublished report No. K44793-(48)A from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Coulston, F., Golberg, L. & Griffin, T. (1972). Safety evaluation of
Dowco 179 in human volunteers. Unpublished report from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Crown, S., Weiss, A., Nyska, A. & Waner, T. (1984a) Pyrinex. Toxicity
in dietary administration to mice for two weeks: A range-finding
study. Unpublished report No. MAK/109/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A. & Waner, T. (1984b) Pyrinex technical. Two-week
range-finding study in dietary administration to rats.
Unpublished report No. MAK/057/a/PYR from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Gur, E., Nyska, A. & Waner, T. (1985) Pyrinex technical
toxicity in dietary administration to rats for 13 weeks.
Unpublished report No. MAK/058/PYRA from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Weiss, A., Nyska, A., Waner, T. & Pirak, M. (1987) Pyrinex
technical. Toxicity in dietary administration to mice for 13
weeks. A preliminary study. Unpublished report No. MAK/105/PYR
from Life Science Research Israel Inc., Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A., Pirak, M. & Waner, T. (1988) Pyrinex technical.
Oncogenicity study in the rat. Unpublished report No. MAK/095/PYR
from Life Science Research Israel Ltd, Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Davies, R.E. & Kynoch, S.R. (1970) Acute subcutaneous toxicity of
Dursban to rats and the effect of treatment with 1. Atropine
sulphate 2. Atropine sulphate and PAM. Unpublished report No.
A1A-275 or 3641/70/453 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Davies, D.B., Tollett, J.T. & Lomax, L.G. (1985) Chlorpyrifos: A
four-week dietary study in the CD-1 mice. Unpublished report No.
HET-K-044793-068 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Dittenber, D.A., Hanley,
T.R., Jr & John, J.A. (1979) The effect of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Unpublished report No. HET-K-44793-32 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Rao, K.S., Dittenber, D.A.,
Hanley, T.R., Jr & John, J.A. (1980) Embryotoxicity and
fetotoxicity of orally administered chlorpyrifos in mice.
Toxicol. Appl. Pharmacol., 54, 31-40.
Derelanko, M.J. & Hollinger, M.A., eds (1995) CRC Handbook of
Toxicology, Boca Raton, FL, CRC Press Inc.
Dietz, F.K., Mensik, D.C., Hinze, C.A., Rachunek, B.K. & Taylor, H.W.
(1983) Dursban insecticide: Assessment of neonatal survival in a
two-generation reproduction study in rats. Unpublished report No.
TexasT-K-44793-(45) from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L. & Wetters, J.H. (1972a) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues and eggs
from chickens fed chlorpyrifos. Unpublished report No. GH-C 555
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., Rice, J.R., McKellar, R.L. & Pennington, J.Y.
(1972b) Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of cattle
fed chlorpyrifos. Unpublished report No. GH-C 566 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. & Rice, J.R. (1977)
Determination of residues of chlorpyrifos, its oxygen analogue,
and 3,5,6-trichloro-2-pyridinol in tissues of cattle fed
chlorpyrifos. J. Agric. Food Chem., 25, 1325-1329.
Dittenber, D.A. (1997) Chlorpyrifos: Evaluation of single oral doses
on cholinesterase and neurotoxic esterase inhibition in F344
rats. Unpublished report No. 960036 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Dreher DM (1994a) Chlorpyrifos technical: Acute oral toxicity test in
the rat. Unpublished report No. CHA 8-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994b) Chlorpyrifos technical: Acute dermal toxicity (limit
test) in the rat. Unpublished report No. CHA 9-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994c) Chlorpyrifos technical: Acute eye irritation test in
the rabbit. Unpublished report No. CHA 12-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994d) Chlorpyrifos technical: Acute dermal irritation test
in the rabbit. Unpublished report No. CHA 11-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994e) Chlorpyrifos technical: Magnusson & Kligman
maximisation study in the guinea pig. Unpublished report No. CHA
12-CYF from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to Who by Cheminova Agro A/S, Lemvig, Denmark.
Drevenkar, V., Vasilic, Z., Stengl, B., Frobe, Z. & Rumenjak, V.
(1993) Chlorpyrifos metabolites in serum and urine of poisoned
persons. Chem.-Biol. Interactions, 87, 315-322.
Eliason, D.A., Crammer, M.F., von Windeguth, D.L., Kilpatrick, J.W.,
Suggs, J.E. & Schoof, H.F. (1969) Dursban premise applications
and their effect on the cholinesterase levels of spraymen.
Mosquito News, 29, 591-595.
Emerson, J.L. & Gerbig, C.L. (1970) 91 day toxicology study in beagle
dogs treated with 3,5,6-trichloro-2-pyridinol. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Fikes, J.D., Zacchary, J.F., Parker, A.J. & Beasley, V.R. (1992)
Clinical, biochemical, electrophysiologic and histologic
assessment of chlorpyrifos induced delayed neuropathy in the cat.
Neurotoxicology, 13, 663-678.
Fredrick Institute of Plant Protection and Toxicology (1995a) Acute
oral toxicity study of chlorpyrifos technical in Swiss albino
mice. Unpublished report No. 2739 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995b) Acute
oral toxicity study of chlorpyrifos technical in Wistar rats.
Unpublished report No. 2738 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995c) Acute
dermal toxicity study of chlorpyrifos technical in rabbits.
Unpublished report No. 2748 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995d)
Irritation of chlorpyrifos technical to mucous membrane in
rabbits. Unpublished report No. 2746 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995e) Primary
skin irritation of chlorpyrifos technical in rabbits. Unpublished
report No. 2747 from Fredrick Institute of Plant Protection and
Toxicology, Tamil Nadu, India. Submitted to WHO by Ficom
Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996a) Acute
inhalation study with chlorpyrifos technical in Wistar rats.
Unpublished report No. 2968 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996b) Allergy
and skin sensitization potential of chlorpyrifos technical in
guinea pigs. Unpublished report No. 2928 from Fredrick Institute
of Plant Protection and Toxicology, Tamil Nadu, India. Submitted
to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996c)
Mutagenicity evaluation of chlorpyrifos technical in the
point-mutation Ames Salmonella typhimurium test system.
Unpublished report No. 3049 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996d)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
micronucleus assay. Unpublished report No. 3054 from Fredrick
Institute of Plant Protection and Toxicology, Tamil Nadu, India.
Submitted to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996e)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
bone marrow cytogenic assay. Unpublished report No. 3076 from
Fredrick Institute of Plant Protection and Toxicology, Tamil
Nadu, India. Submitted to WHO by Ficom Organics, Bombay, India.
Furlong, C.E., Richter, R.J., Seidel, S.L. & Motulski, A.G. (1988)
Role of genetic polymorphism of human plasma
paraoxonase/arylesterase in hydrolysis of the insecticide
metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum.
Genet., 43, 230-238.
Furlong, C.E., Richter, R.J., Seidel, S.L., Costa, L.G. & Motulsky,
A.G. (1989) Spectrophotometric assays for the enzymatic
hydrolysis of the active metabolites of chlorpyrifos and
parathion by plasma paraoxonase/arylesterase. Anal. Biochem.,
180, 242-247.
Geldmacher-von Mallinckrodt, M. & Diepgen, T.L. (1988) The human serum
paraoxonase polymorphism and specificity. Toxicol. Environ.
Chem., 18, 79-196.
Gerbig,, C.G. & Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Unpublished
report No. HH-240 from Dow Chemical Co., Zionsville, Indianna,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gerbig,, C.G. & Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat.
Unpublished report No. HH-239 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gerbig,, C.G. & Emerson, J.L. (1970c) Determination of oral minimum
lethal dose of 3,5,6trichloro-2-pyridinol in beagle dogs.
Unpublished report No. HH-253 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gibson, J.E. (1996) Critical review of allegations associating Dursban
with human teratogenicity. Unpublished report No. JEG122396, from
Dow Elanco, USA. Submitted by Dow Agrosciences, Indianapolis,
Indiana, USA.
Glas, R.D. (1977a) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from calves fed
3,5,6-trichloro-2-pyridinol. Unpublished report No. GH-C 1053
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977b) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from a calf fed
2-methoxy-3,5,6-trichloro-2-pyridine. Unpublished report No. GH-C
1052 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977c) Residues of triclopyr, 3,5,6-trichloro-2-pyridinol
and 2-methoxy-3,5,6trichloropyridine in bovine tissues from
calves fed trichlopyr. Unpublished report No. GH-C 1047 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981a) The metabolic fate of 14C-chlorpyrifos fed to
lactating goats. Unpublished report No. GH-C 1408 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981b) Identification of 14C-labelled residues in milk
from goats fed 14Cchlorpyrifos. Unpublished report No. GH-C
1470 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Gollapudi, B.B. (1987) Evaluation of 3,5,6-trichloro-2-pyridinol (TCP)
in the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. TexasT-K-38278-012 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Linscombe, V.A. & Wilkerson, J.E. (1985) Evaluation
of chlorpyrifos in the mouse bone marrow micronucleus test.
Unpublished report No. TexasT-K-044793-067 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Mendrala, A.L. & Linscombe, V.A. (1995) Evaluation of
the genetic toxicity of the organophosphate insecticide
chlorpyrifos. Mutat. Res., 342, 25-36.
Gur, E., Nyska, A. & Waner, T. (1991) Pyrinex technical oncogenicity
study in the mouse. Unpublished report from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Isreal.
Gutenmann, W.H., St John, L.E., Jr & Lisk, D.J. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow.
Unpublished report from Cornell University, Ithaca, New York,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gutmann, L. & Bodensteiner, J.B. (1993) Organophosphate-induced
delayed polyneuropathy (comment). J. Pediatr., 123, 837.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987a)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in Fischer 344
rats. Unpublished report No. K-038278-011 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987b)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in New Zealand
white Rrabbits. Unpublished report No. K-038278-015 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hardy, C.J. & Jackson, G.C. (1984) Dursban technical: Acute inhalation
toxicity in rats. Unpublished report No. DET 368 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Dawe, I.S. &
Anderson, A. (1989a) Chlorpyrifos oral dose range finding
toxicity study in beagle dogs (final report -- repeated daily
dosage for 4 weeks). Unpublished report No. MBS 30/88675 from
Huntingdon Research Centre Inc, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Majeed, S.,
Gopinath, C., Dawe, I.S. & Anderson, A. (1989b) Chlorpyrifos oral
toxicity study in beagle dogs (repeated daily dosage for 13
weeks). Unpublished report No. MBS 31/88999 from Huntingdon
Research Centre Inc, Huntingdon, United Kingdom. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Hazleton Laboratories (1968) Short term (subacute) dietary
administration -- Rats organophosphate insecticide Dursban. Final
report. Unpublished report No. 174-114 from Hazleton
Laboratories, Falls Church, Virginia, USA. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Henck, J.W. & Kociba, R.J. (1980) Three samples of Dursban
insecticide: Acute oral toxicity and acute percutaneous
absorption potential. Unpublished report No. A1A-130 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hoberman, A.M. (1998) Developmental neurotoxicity study of
chlorpyrifos administered orally via gavage to Crl:CD.BR VAF/Plus
presumed pregnant rats. Unpublished report No. K-044793109 from
Argus Research Laboratories, Horsham, PA, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Hooser, S.B., Beasley, V.R., Sundberg, J.P. & Harlin, K. (1988)
Toxicologic evaluation of chlorpyrifos in cats. Am. J. Vet.
Res., 49, 1371-1375.
Humbert, R., Adler, D.A., Distechhe, C.M., Hassett, C., Omiecinski,
C.J. & Furlong, C.E. (1993) The molecular basis of the human
serum paraoxonase activity polymorphism. Nature Genet., 3,
73-76
Jackson, D. & Ogilvie, S.W. (1994a) Chlorpyrifos tech: Acute dermal
toxicity (LD50) test in rats. Unpublished report No. 10439 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994b) Chlorpyrifos tech: Primary eye
irritation test in rabbits. Unpublished report No. 10441 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994c) Chlorpyrifos tech: Primary skin
irritation test in rabbits. Unpublished report No. 10440 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994d) Chlorpyrifos tech:
Magnusson-Kligman maximisation test in guinea pigs. Unpublished
report No. 10442 from Inveresk Research International, Tranent,
Scotland. Submitted to WHO by Luxembourg Industries, Tel Aviv,
Israel.
Jai Research Foundation (1996a) Reverse gene mutation study of
chlorpyrifos tech (95%) in bacteria by Ames Salmonella/microsome
test. Unpublished report No. 5/JRF/MichiganC/96 from Department
of Microbiology, Jai Research Foundation, Gujarat, India.
Submitted to WHO by Mitsu Industries, Gujarat, India.
Jai Research Foundation (1996b) Neurotoxicity study of chlorpyrifos
technical to hen. Unpublished report No. 1539/JRF/TOX/96 from
Department of Toxicology, Jai Research Foundation, Gujarat,
India. Submitted to WHO by Mitsu Industries, Gujarat, India.
James, P., Stubbs, A., Parker, C.A., Offer, J.M. & Anderson, A. (1988)
The effect of Pyrinex (chlorpyrifos) on reproductive function of
two generations in the rat. Unpublished report No. MBS 29/881452
from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Jeffrey, M.M., Battjes, J.E. & Eisenbrandt, D.L. (1986) Dursban F
(tech): Acute dermal toxicity study in Fischer 344 rats.
Unpublished report No. HET-K 044793-083 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Jones, J.R. (1985a) Dursban F: Eye irritation study in the rabbit.
Unpublished report No. DET 424 from Hazleton Laboratories Europe,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985b) Dursban F: Primary skin irritation and corrosivity
study in the rabbit. Unpublished report No. DET 421 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985c) Dursban F: Delayed contact hypersensitivity study
in the guinea pig (Buehler Test). Unpublished report No. DET 475
from Hazleton Laboratories Europe, Harrogate, North Yorkshire,
United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kamataki, T. & Neil, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothienate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfur
atom. Mol. Pharmacol., 12, 933-944.
Kaplan, J.G., Kessler, J., Rosenberg, N., Pack, D. & Schaumburg, H.H.
(1993) Sensory neuropathy associated with Dursban (chlorpyrifos)
exposure [published erratum appears in Neurology 1994 44, 367].
Neurology, 43, 2193-2196.
Kenaga, E.E. (1967) Comments on data concerning cholinesterase effects
of Dursban in man following US Public Health Service experimental
yellow fever mosquito eradication tests in Florida during 1966
and 1967. Unpublished report No.A1A-394, TK 2-1684 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kenny, T.J., Coombs, D.W. & Hardy, C.J. (1987) Chlorpyrifos technical
acute inhalation toxicity in rats 4-hour exposure. Unpublished
report No. MBS 20/87575 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Kenny, T.J., Coombs, D.W., Hardy, C.J. & Crook, D. (1988) Chlorpyrifos
inhalation toxicity in the rat. 14-Day preliminary dose range
finding study. Unpublished report No. MBS 21/871329 from
Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted by Makteshim Chemical Works, Beer-Sheva, Israel.
Kilian, D.J., Edwards, H.N., Benge, M.C. & Tabatabai, Z. (1970)
Results of human skin exposure to Dowco 179. Unpublished report
No. TMD-70-2 from Dow Chemical Co., Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Kisicki, J.C., Seip, C.W. & Combs, M.L. (1999) A rising dose
toxicology study to determine the no-observable-effect-levels
(NOEL) for erythrocyte acetylcholinesterase (AChE) inhibition and
cholinergic signs and symptoms of chlorpyrifos at three dose
levels. Unpublished report No. LLC Study ID: DR#K-044793-284
dated 19 April 1999, from MDS Harris, Lincoln, NE, USA. Submitted
to WHO by Dow Agrosciences, Indianapolis, Indiana, USA.
Kociba, R.J., McCollister, S.B., Keyes, D.G. & Dittenber, D.A. (1985)
Supplement to original report entitled 'Results of two-year
dietary feeding studies on Dowco 179 in beagle dogs'. Unpublished
report No. NBT 3512-44793-18 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lackenby, F. (1985a) Dursban F and Dursban F (OP1): Acute oral
toxicity studies in the rat. Unpublished report No. 4703-50/390 &
591 from Hazleton Laboratories Europe, North Yorkshire, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Lackenby, F. (1985b) Dursban F: Acute dermal median lethal dose in the
rat. Unpublished report No. 4206-50/391 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Landry, T.D., Streeter, C.M. & Lomax, L.G. (1985) Chlorpyrifos: 2-week
vapor inhalation toxicity study in Fischer 344 rats. Protocol.
Unpublished report No. A1A-714 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Landry, T.D., Dittenber, D.A., Lomax, L.G., Calhoun, L.L. & Morabito,
P. (1986) Chlorpyrifos: 2 week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET
K-44793-81 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Liberacki, A.B., Breslin, W.J., Dittenber, D.A. & Quast, J.F. (1990)
Chlorpyrifos: Two week dietary probe study in Sprague-Dawley
rats. Unpublished report No. HET K 044793090 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A. & Gollapudi, B. (1986) Evaluation of
3,5,6-trtichloro-2-pyridinol (TCP) in the Chinese hamster ovary
cell/hypoxanthine-guanine-phosphoribosyl transferase (CHO/HGPRT)
forward mutation assay. Unpublished report No.
TexasT:K-038278-013 from Dow Chemical Co., HES, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A., Mensik, D.C. & Clem, B.A. (1992) Evaluation of
chlorpyrifos in an in vitro chromosomal aberration assay
utilizing rat lymphocytes. Unpublished report No. TexasT:K
044793-092 from Dow Chemical Co., Freeport, Texas, USA. Submitted
to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lotti, M., Moretto, A., Zoppellari, R., Dainese, R., Rizzuto, N. &
Barusco, G. (1986) Inhibition of lymphocytic neuropathy target
esterase predicts the development of organophosphate-induced
delayed polyneuropathy. Arch. Toxicol., 59, 176-179.
Loveday, K.S. (1987) In vitro chromosomal aberration assay on Pyrinex
(chlorpyrifos). Unpublished report No. R4660 from Arthur D.
Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Loveday, K.S., Findlen, K.M.B. & Yadlon, S. (1987) Evaluation of
Pyrinex in the Ames mutagenesis assay. Unpublished report No.
59487-00 from Arthur D. Little Inc., USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Ma, T. & Chambers, J.E. (1994) Kinetic parameters of desulfuration and
dearylation of parathion and chlorpyrifos by rat liver
microsomes. Food Chem. Toxicol., 32, 763-767.
Maita, K., Hirano, M., Harada, T., Mitsumori, K., Yoshida, A.,
Takahashi, K., Nakashima, N., Kitazawa, T., Enomoto, A. & Inui,
K. (1988) Mortality, major cause of moribundity aqnd spontaneous
tumours in CD-1 mice. Toxicol. Pathol., 16, 340-349.
Mattsson, J.L., Wilmer, J.W., Shankar, M.R., Berdasco, N.M., Crissman,
J.W., Maurissen, J.P. & Bond, D.M. (1996) Single-dose and 13-week
repeated-dose neurotoxicity screening studies of chlorpyrifos
insecticide. Food Chem. Toxicol., 34, 393-405.
Maurissen, J.P., Shankar, M.R. & Mattsson, J.L. (1996) Chlorpyrifos:
Cognitive study in adult Long-Evans rats. Unpublished report No.
K-044793-096 from Dow Chemical Co., USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989a) Evaluation of chlorpyrifos
in the mouse bone marrow micronucleus test. Unpublished report
No. TexasT:K 044793-067A from Lake Jackson Research Center, Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989b) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008A from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco 179
in beagle dogs. Unpublished report No. T35.1244793-18 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco 179
in rats. Unpublished report No. NBT35.12-4479321 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J. & Keyes, D.G. (1985) Supplement to
original report entitled 'Results of two-year dietary feeding
studies on Dowco 179 in rats'. Unpublished report No.
NBT35.12-44793-21 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McKellar, R.L., Wetters, J.H. & Dishburger, H.J. (1972) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues of swine
fed chlorpyrifos. Unpublished report No. GHC549 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Mendrala, A.L. (1985) Evaluation of chlorpyrifos in the Chinese
hamster ovary cellhypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Unpublished
report No. HET-K-044793-072 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Mendrala, A.L. & Dryzaga, M.D. (1986) Evaluation of chlorpyrifos in
the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. HET K 044793-073 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Moretto, A. & Lotti, M. (1998) Poisoning by
organophosphorusinsecticides and sensory neuropathy.
J. Neurol. Neurosurg. Psych., 64, 463-468.
Mortensen, S.R., Chanda, S.M., Hooper, M.J. & Padilla, S. (1996)
Maturational differences in chlorpyrifos-oxonase activity may
contribute to age-related sensitivity to chlorpyrifos.
J. Biochem. Toxicol., 11, 279-287.
Moser, V.C. (1995) Comparisons of the acute effects of cholinesterase
inhibitors using a neurobehavioral screening battery in rats.
Neurotoxicol. Teratol., 17, 617-625.
Moser, V.C. & Padilla, S. (1998) Age- and gender-related differences
in the time course of behavioral and biochemical effects produced
by oral chlorpyrifos in rats. Toxicol. Appl. Pharmacol., 149,
107-119.
Muscarella, D.E., Keown, J.F. & Bloom, S.E. (1984) Evaluation of the
genotoxic and embryotoxic potential of chlorpyrifos and its
metabolites in vivo and in vitro. Environ. Mutag., 6, 13-23.
Nakatsugawa, T. (1992) Hepatic disposition of organophosphorus
insecticides: A synthesis of in vitro, in situ and in vivo data.
In: Chambers, J.E., Levi, P.E. & Levi, A.P., eds,
Organophosphates, San Diego, Academic Press, Chapter 10.
Newton, P.E. (1988a) A 5-day nose-only inhalation toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
from Bio/Dynamics Inc., East Millstone, NJ, USA. Submitted by
Makteshim Chemical Works, Beer-Sheva, Israel.
Newton, P.E. (1988b) A thirteen week nose-only toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
No. R-4750 from Bio/dynamics Inc., East Millstone, NJ, USA.
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984a) Pyrinex tech: Acute oral toxicity in
the rat. Unpublished report No. MAK/056/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984b) Pyrinex tech: Acute dermal toxicity
in rabbits. Unpublished report No. MAK/059/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nolan, R.J. (1997) Proposed reference dose for acute and chronic
exposure to chlorpyrifos based on the criteria described by the
Acute Cholinesterase Risk Assessment Task Force and the available
animal and human data. Unpublished report No. K-044793-110 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1982)
Chlorpyrifos: pharmacokinetics in human volunteers following
single oral and dermal doses. Unpublished report No.
HEB-DR-0043-4946-4 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1984)
Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol.
Appl. Pharmacol., 73, 8-15.
Nolan, R.J., Streeter, C.M. & Kastl, P.E. (1986) Chlorpyrifos:
Absorption by female Fischer 344 rats exposed for 6 hours to
vapours of chlorpyrifos in a nose-only or a whole-body inhalation
chamber. Unpublished report No. HET K-044793-82 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Dryzga, M.D., Landenberger, B.D. & Kastl, P.E. (1987)
Chlorpyrifos: Tissue distribution and metabolism of orally
administered 14C labelled chlorpyrifos in Fischer 344.
Unpublished report No. HET K-044793-(76) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Nostrandt, A.C., Padilla, S. & Moser, V.C. (1997) The relationship of
oral chlorpyrifos effects on behaviour, cholinesterase
inhibition, and muscarinic receptor density in rat.
Pharmacol. Biochem. Behav., 58, 15-23.
Omenn, G.S. (1987) The role of genetic differences in human
susceptibility to pesticides. In: Costa, L.G., Galli, C.L. &
Murphy, S.D., eds, Toxicology of Pesticides: Experimental,
Clinical and Regulatory Perspectives, Berlin, Springer-Verlag.
Osterloh, J., Lotti, M. & Pond, S.M. (1983) Toxicologic studies in a
fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. Anal.
Toxicol., 7, 125-129.
Ouelette, J.H., Dittenber, D.A., Kociba, R.J. & John, J.A. (1983a)
Chlorpyrifos: Oral teratology probe study in rats. Unpublished
report No. HET K-44793-(46) from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Ouelette, J.H., Dittenber, D.A., Kloes, P.M. & John, J.A. (1983b)
Chlorpyrifos: Oral teratology study in Fischer 344 rats.
Unpublished report No. HET K-44793-(47) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Pennington, J.Y. & Edwards, N.H. (1971) Comparison of cholinesterase
depression in humans and rabbits following exposure to
chlorpyrifos. Unpublished report no. TA-477 from Dow Chemical
Company USA. Submitted to WHO by Dow Agrosciences, Indianapolis,
Indiana, USA.
Phillips, J.E. & Lomax, L.G. (1989) 1% aqueous dilution of Dursban
acute aerosol inhalation toxicity study in Fischer 344 rats.
Unpublished report No. M-004803-004A from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Poole, D.C., Simmon, V.F. & Newell, C.W. (1977). In vitro mutagenic
activity of fourteen pesticides. Toxicol. Appl. Pharmacol., 41,
196.
Pope, C.N. & Chakraborti, T.K. (1992) Dose related inhibition of brain
and plasma cholinesterase in neonatal and adult rats following
sub-lethal organophosphate exposure. Toxicology, 73, 35-43.
Pope, C.N., Chakraborti, T.K., Chapman, M.L., Farrar, J.D. & Arthun,
D. (1991) Comparison of in vivo cholinesterase inhibition in
neonatal and adult rats by three organophosphorothioate
insecticides. Toxicology, 68, 51-61.
Pope, C.N., Chakraborti, T.K., Chapman, M.L. & Farrar, J.D. (1992)
Long-term neurochemical and behavioral effects induced by acute
chlorpyrifos treatment. Pharmacol. Biochem. Behav., 42,
251-256.
Richardson, R.J., Moore, T.B., Kayyali, U.S. & Randall, J.C. (1993a)
Chlorpyrifos: Assessment of potential for delayed neurotoxicity
by repeated dosing in adult hens with monitoring of brain
acetylcholinesterase, brain and lymphocyte neurotoxic esterase
and plasma butyrylcholinesterase activities. Fundam. Appl.
Toxicol., 21, 89-96.
Richardson, R.J., Moore, T.B., Kayyali, U.S., Fowke, J.H. & Randall,
J.C. (1993b) Inhibition of hen brain acetylcholinesterase and
neurotoxic esterase by chlorpyrifos in vivo and kinetics of
inhibition by chlorpyrifos oxon in vitro: Application to
assessment of neuropathic risk. Fundam. Appl. Toxicol., 20,
273-279.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Begg, S., Anderson, A.
& Dawe, I.S. (1987) Acute delayed neurotoxicity study with
chlorpyrifos in the domestic hen. Unpublished report No. MBS
16/87764 from Huntingdon Research Centre Ltd, Huntingdon, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Ross, D.B. & Roberts, N.L. (1974) The acute oral toxicity (LD50) of
Dursban (DOWCO 179) to the chicken (DWC/234/74877). Unpublished
report from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Rowe, L.D., Warner, S.D. & Johnston, R.V. (1978) Acute delayed
neurotoxicologic evaluation of chlorpyrifos in white Leghorn
hens. Unpublished report No. A1A-715 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Rubin, Y.,. Gal, N., Waner, T. & Nyska, A. (1987a) Pyrinex.
Teratogenicity study in the rat. Unpublished report No.
MAK/101/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Rubin, Y., Nyska, A. & Waner, T. (1987b) Pyrinex. Pyrinex
teratogenicity study in the rabbit. Unpublished report No.
MAK/103/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Scortichini, B.H., Lomax, L.G., Wolfe, E.L. & Hanley, T.R., Jr (1986a)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in
Fischer 344 rats. Unpublished report No. HETK-038278-006 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Scortichini, B.H., Lomax, L.G., Wolf, E.L. & Hanley, T.R., Jr (1986b)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in New
Zealand white rabbits. Unpublished report No. HET-K-038278-007
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Shankar, M.R., Bond, D.M. & Crissman, J.W. (1993) Chlorpyrifos:
13-week neurotoxicity study in Fischer 344 rats. Unpublished
report No. K-044793-094 from Dow Chemical Co., USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Sharp, L.D. & Warner, S.D. (1968) The clinical toxicity of Dursban in
the dog after multiple applications of an aerosol formulation.
Unpublished report from Dow Chemical Co., USA. Submitted to WHO
by Dow Agrosciences, Indianapolis, Indiana, USA.
Shemesh, I., Bourvin, A., Gold, D. & Kutscherowsky, M. (1988)
Chlorpyrifos poisoning treated with ipratropium and dantrolene: a
case report. J. Toxicol. Clin. Toxicol., 26, 495-498.
Sherman, J.D. (1996) Chlorpyrifos (Dursban)-associated birth defects:
Report of four cases. Arch. Environ. Health, 51, 5-8.
Sherman, J.D. & Herrick, R.B. (1973) Fly control and chronic toxicity
from feeding Dursban (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) to laying hens. Poultry Sci., 52, 741-747.
Sherman, J.D., Herrick, R.B., Ross, E. & Chang, M.T.Y. (1967) Further
studies on the acute and sub-acute toxicity of insecticides to
chicks. Toxicol. Appl. Pharmacol., 11, 49-67.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) Mutagenicity test of
chlorpyrifos in bacteria. Unpublished report No. GHF-R-47 from
Dow Chemical Pacific, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Shurdut, B.A., Chen, W.L., Burns, C.J., McCormick, R.A., Nolan, R.J. &
Racke, K.D. (1997) Critical assessment of report entitled 'Review
of chlorpyrifos poisoning data' (by J. Blondell and V. Dobozy,
January 14, 1997). Unpublished report No. GH-C 4359, by Dow
Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, Indiana, USA.
de Silva, H.J., Sanmuganathan, P.S. & Senanayake, N. (1994) Isolated
bilateral recurrent laryngeal nerve paralysis: A delayed
complication of organophosphorus poisoning. Hum. Exp.
Toxicol., 13, 171-173.
Smith, G.N., Watson, B.S. & Fischer, F.S. (1967) Investigations on
Dursban insecticide. Metabolism of [36Cl] O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothiate in rats. Agric.
Food Chem., 15, 132-138.
Smolen, A., Eckerson, H.W., Gan, K.N., Hailat, N. & LaDu, B.N. (1991)
Characteristics of the genetically determined allozymic forms of
human paraoxonase/artylesterase. Drug Metab. Disposition, 19,
107-112
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: Organophosphates. Mutat. Res., 102,
89-102.
Song, X., Seidler, F.J., Saleh, J.L., Zhang, J., Padilla, S. &
Slotkin, T.A. (1997) Cellular mechanisms for developmental
toxicity of chlorpyrifos: Targeting the adenylyl cyclase
signaling cascade. Toxicol. Appl. Pharmacol., 145, 158-174.
Stevenson, G.T. (1963) An LD50 toxicity study of Dursban in Leghorn
chickens. Unpublished report from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1966a) A single oral dose LD50 toxicity study of
Dursban in broiler type chickens. Unpublished report No. A1A-364
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Stevenson, G.T. (1966b) A neurotoxicity study of Dursban in laying
hens. Unpublished report No. A1A-390 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1967) A single oral dose LD50 toxicity study of
Dursban in turkey/poultry. Unpublished report No. A1A-362 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Streeter, C.M., Lomax, L.G., Landry, T.D. & Dittenber, D.A. (1987)
Chlorpyrifos: 2-week whole-body vapor inhalation toxicity study
in Fischer 344 rats. Unpublished report No. HET K 044793-078 from
Dow Chemical Co., Midland, Michigan, USA Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Sultatos, L.G. & Murphy, S.D. (1983) Hepatic microsomal detoxification
of the organophosphates paraoxon and chlorpyrifos oxon in the
mouse. Drug Metab. Disposition, 11, 232-238.
Szabo, J.R., Young, J.T. & Granjean, M. (1988) Chlorpyrifos: 13-week
dietary toxicity study in Fisher 344 rats. Unpublished report No.
TexasT: K-044793-071 from Lake Jackson Research Center, Health
and Environmental Sciences, Freeport, Texas, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Taylor, M.L. & Olson, K.J. (1963) Toxicological properties of
O,O-diethyl-O-3,5,6trichloro-2-pyridyl phosphorothioate.
Unpublished report. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Thompson, D.J., Gerbig, C.C. & Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Tu, A.S. (1987) CHO/HGPRT in vitro mammalian cell mutation assay on
Pyrinex (chlorpyrifos). Unpublished report No. 59487 from Arthur
D. Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Warner, S.D., Gerbig, C.G., Strebing, R.J. & Molello, J.A. (1980)
Results of a two-year toxicity and oncogenic study of
chlorpyrifos administered to CD-1 mice in the diet. Unpublished
report from Dow Chemical Co., Indianapolis, Indiana, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Whitney, K.D., Seidler, F.J. & Slotkin, T.A. (1995) Developmental
neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicol.
Appl. Pharmacol., 134, 53-62.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Wilmer, J.W. (1992) Acute neurotoxicity study in Fischer 344 rats.
Unpublished report No. K-044793-093B from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Wilson, J.A. & MacBeth, D. (1994) Chlorpyrifos tech: Acute oral
toxicity (LD50) test in rats. Unpublished report No. 10521 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Wolcott, R.M., Vaughn, W.K. & Neal, R.A. (1972) Comparison of the
mixed function oxidase-catalyzed metabolism of a series of
dialkyl p-nitrophenyl phosphorothionates. Toxicol. Appl.
Pharmacol., 22, 213-220.
Yang, R.S.H., Hodgson, E. & Dauterman, W.C. (1971) Metabolism in vitro
of diazinon and diazoxon in rat liver. J. Agric. Food Chem.,
19, 10-13.
Young, J.T. & Grandjean, M. (1988) Chlorpyrifos: 2 year dietary
chronic toxicity-oncogenicity study in Fischer 344 rats.
Unpublished report No. K-044793-079 from Lake Jackson Research
Center, Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Zempel, J.A. & Bruce, R.J. (1986) 3,5,6-Trichloro-2-pyridinol:
Evaluation in the Ames Salmonella/mammalian-microsome
mutagenicity assay. Unpublished report No. TexasT:K038278-010
from Lake Jackson Research Center, Dow Chemical Co., Freeport,
Texas, USA. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Zempel, J.A., Rachunek, B.L. & Szabo, J.R. (1987)
3,5,6-Trichloro-2-pyridinol: Results of a one-year dietary study
in male and female beagle dogs. Unpublished report No.
TexasT:K038278-009 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
 (c) Effects on enzymes and other biochemical parameters
Hydrolysis is the most important route of detoxification of
organophosphorus esters. Hydrolytic esterases are distributed
ubiquitously in the blood and tissues of virtually all animal
organisms and catalyse the hydrolysis of a variety of esters,
including organophosphorus esters, but have little activity on
organophosphorus itself. Esterases that interact with
organophosphoruses have been characterized as A- and B-esterases
according to their sensitivity to inhibition by organophosphorus
compounds. Plasma and tissues of mammals have significant
concentrations of the calcium-activated A-esterases, arylesterase (EC
3.1.1.2), and the high-density lipoprotein-associated paroxonase (EC
3.1.8.1). The B-esterases, including aliesterases (EC 3.1.1.1; also
called carboxylesterases) and cholinesterases (e.g. butyryl
cholinesterase, EC 3.1.1.8), are inhibited by organophosphorus
compounds such as chlorpyrifos oxon. While they bind them, they do not
hydrolyse them (Derelanko & Hollinger, 1995).
Butyrylcholinesterase is the predominant cholinesterase in human
serum (> 99%), while in rats there is an approximately equal
distribution of acetylcholinesterase and butyryl cholinesterase
(Nolan, 1997). These enzymes detoxify organophosphorus compounds by
sequestering them through binding to or phosphorylation by blood
proteins, such as albumin, which stoichiometrically degrades them
(Aldridge, 1953). These protein-bound organophosphorus esters are
secreted into the bile. Erythrocyte acetylcholinesterase (EC 3.1.1.7)
is similar to the acetylcholinesterase in nervous systems and can bind
to and sequester or hydrolyse organophosphorus compounds. The enzyme
activities attributable to A-esterases, B-esterases, and
cholinesterases vary widely within populations and are influenced by
genetic and environmental factors and disease states (Derelanko &
Hollinger, 1995). The serum activity of paraoxonase in rabbits is some
40 times greater than that in rats. Most avians, including hens, have
relatively low activity of A-esterases, and the mean value of serum
chlorpyrifos oxonase activity in humans is 10 times that of rat serum
(Furlong et al., 1989).
In humans, a substrate-dependent polymorphism of serum
paraoxonase is observed, in which one isoform of paraoxonase has a
high turnover for paraoxon and the other a low turnover (Furlong et
al., 1989; Smolen et al., 1991). The polymorphism is also observed
with the oxons of methyl parathion, chlothion, and
phenylphosphonothioic acid O-ethyl O-para-nitrophenyl ester. The
two isoforms appear, however, to hydrolyse chlorpyrifos oxon and
phenylacetate at the same rate. Cloning and sequencing of the human
paraoxonase cDNA has revealed the molecular basis of the polymorphism:
arginine at position 192 determines high paraoxonase activity, and
glutamine at this position encodes low paraoxonase activity (Humbert
et al., 1993). In addition to this polymorphism, a 13-fold variation
in serum enzyme activity of a given genetic class is seen (Furlong et
al., 1989). While chlorpyrifos oxon was hydrolysed by the same plasma
fraction that hydrolysed paraoxon in a study of plasma samples from
320 white blood donors (Furlong et al., 1988), the population
distribution of chlorpyrifos oxon hydrolysis was unimodal. This
contrasted with a bimodal population distribution of the activity for
paraoxon hydrolysis, and the variation in paraoxonase activity was
about 11-fold, while that in chlorpyrifos oxonase activity showed a
four- to fivefold variation.
Correlation of the LD50 values for orally administered
chlorpyrifos in rats with the activation rates measured in brain
rather than liver (Chambers, 1992) suggested that local
biotransformation in target tissues is an important determinant of its
toxicity. In a more recent review, however, Chambers & Carr (1995)
compared published LD50 or LC50 values for a variety of
insecticides in several vertebrate species. Studies in rats indicated
that the sensitivity of brain acetylcholinesterase activity to
inhibition by various phosphorothionate oxons did not correlate with
their acute toxicity. Chlorpyrifos oxon has a greater affinity for rat
brain acetylcholinesterase than does paraoxon, with median inhibitory
concentrations (IC50 values) of 4.0 and 22 nmol/L, respectively, but
a lower LD50. This finding is consistent with the greater affinity
(IC50, 0.75 nmol/L) of chlorpyrifos oxon for plasma aliesterases
than for rat brain acetylcholinesterase, indicating that aliesterases
provide protection against chlorpyrifos oxon.
Further work by Chambers & Carr (1995) indicated longer
inhibition of esterases after exposure chlorpyrifos than to parathion.
This was attributed to the greater lipophilicity of chlorpyrifos than
parathion (hexane:acetonitrile partition coefficients, 0.28 and 0.062,
respectively) and the assumption that a substantial fraction of the
dose of chlorpyrifos would have been sequestered by fat and released
gradually for later bioactivation. Studies of hepatic microsomal
metabolism of parathion and chlorpyrifos indicated that desulfuration
of parathion was favoured over dearylation (activation vs
deactivation), whereas the reverse was seen for chlorpyrifos (Ma &
Chambers, 1994). In channel catfish, however, the sensitivity of
acetylcholinesterase to inhibition by oxons reflects the acute
toxicity of these insecticides, and may be largely responsible for
their toxicity in this species. Thus, the metabolism of insecticides
appears to be more influential in some species than in others in
determining their toxicity.
Chlorpyrifos oxonase is a calcium-dependent A-esterase that
hydrolyses chlorpyrifos oxon, the active metabolite of chlorpyrifos.
As this activity may be related to that of paraoxonase, it was
determined spectrophotometrically by measuring the generation of TCP
(Furlong et al., 1989). Mortensen et al. (1996) hypothesized that
young rats have less chlorpyrifos oxonase activity than adults, and
they measured the activity of this enzyme in the brain, plasma, and
liver of male Long-Evans rats (CRL:(LE)BR) at postnatal day 4 and in
adults. No activity was detected in brain at either age, but the
activities in plasma and liver were markedly lower in younger than in
adult animals, the activities in plasma and liver being 1/11 and 1/2
of those in adults, respectively.
As the Michaelis-Menten constant for chlorpyrifos oxonase
activity was high (210-380 µmol/L), experiments were performed to
determine whether this enzyme could hydrolyse physiologically relevant
concentrations (nanomolar to low micromolar) of chlorpyrifos oxon. On
the assumption that tissue acetylcholinesterase inhibition provides a
sensitive bioassay for the concentration of chlorpyrifos oxon, changes
in the tissue acetylcholinesterase IC50 for chlorpyrifos oxon were
measured in the presence and absence of chlorpyrifos oxonase. Brain
acetylcholinesterase activity was determined spectrophotometrically,
while plasma and liver cholinesterase activities were determined by a
radiometric method with a [3H]acetylcholine iodide substrate. An
increase in the 'apparent' IC50 would indicate that chlorpyrifos
oxonase had hydrolysed substantial amounts of chlorpyrifos oxon during
the 30-min preincubation with the chlorpyrifos oxon. In the adult
rats, the apparent IC50 values for both plasma and liver
cholinesterase were higher in the presence of chlorpyrifos oxonase,
suggesting that the enzyme in those tissues could hydrolyse
physiologically relevant concentrations of chlorpyrifos oxon within
30 min. Young animals, however, showed less of a shift in the IC50
curves than adults, confirming that they have a lower capacity to
detoxify physiologically relevant concentrations of chlorpyrifos oxon
with chlorpyrifos oxonase.
2. Toxicological studies
(a) Acute toxicity
(i) Lethal doses
Numerous studies have been carried out with technical-grade
chlorpyrifos to establish LD50 and LC50 values (Table 1). The
lowest value after oral administration was 96 mg/kg bw (range,
96-480 mg/kg bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in
mice. The signs of acute intoxication were consistent with
cholinesterase inhibition and included inactivity, salivation,
dyspnoea, flaccid paralysis, vomiting, piloerection, exophthalmia, and
diarrhoea; female animals were generally more sensitive to the acute
effects of chlorpyrifos than males. The LD50 for dermally applied
chlorpyrifos was consistently lower than that for oral or inhalational
exposure and indicated the lower dermal absorption of chlorpyrifos
(about 2% in humans) when compared with the 70-90% absorption after
inhalational or oral exposure.
(ii) Dermal and ocular irritation and dermal sensitization
Ocular irritation: In a paper in which few procedural details
were provided (Taylor & Olson, 1963), slight conjunctival redness was
seen in rabbits treated with chlorpyrifos, regardless of whether the
eyes were washed after administration of the test material. The
redness persisted for more than 7 days in some animals. Transient
conjunctival and iridial irritation, possibly due to a direct physical
irritating effect of cholorpyrifos powder, was seen in three young
adult female New Zealand white rabbits after each had received 100 mg
of a finely ground commercial preparation in the left conjunctival
sac. The eyes of all animals were normal after 24 h (Jones, 1985a).
The ocular irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested (in accordance with GLP requirements) in six
young male New Zealand white rabbits, 100 mg of test material being
placed into the right eye and the untreated left eye serving as a
control. There were no signs of corneal or iridial irritation. Slight
to moderate conjunctival redness (mean Draize score, 1 at 1 h, 0.83 at
24 h), slight chemosis (mean Draize score, 0.83 at 1 h, 0.16 at 24 h),
and discharge (mean Draize score, 1.6 at 1 h, 0.83 at 24 h) were seen
up to 24 h after instillation, but no signs of irritation were
reported at 48 h (Jackson & Ogilvie, 1994b).
The ocular irritation potential of 0.1 g of chlorpyrifos (purity,
99.3%) was assessed in three male and three female New Zealand white
rabbits by instilling the test substance into the conjunctival sac of
the right eye, the left eye remaining untreated and serving as
control. This study was conducted according to GLP requirements.
Diffuse corneal opacities (score 1 for degree and area of opacity)
were observed in two animals at 24 h, but these effects had
disappeared by 48 h and no other corneal effects were seen. Iridial
inflammation (score 1) was noted in four animals at 1 h, and this
effect persisted for 24 h in one of the animals and for 48 h in
another. No iridial effects were observed after 72 h. Conjunctival
irritation was observed in all animals, with redness (score 2:
diffuse, deeper crimson red at 1-24 h; score 1: vessels definitely
injected above normal at 48 h), chemosis (score 2: obvious swelling
with partial eversion of lids up to 48 h), and discharge (scores 2-3:
moistening of the lids and hairs just adjacent to lids or a
considerable area around the eye only at 1 h). No signs of
conjunctival irritation was observed at 72 h. The maximum individual
irritation scores were seen at 1 h and ranged from 12 to 19, with a
mean score of 16. The mean scores at 24, 48, and 72 h were 9.3, 2.5,
and 0, respectively. The overall scores according to EEC Council
Directive 67/548/EEC were 2 (mean, 0.11) for corneal opacity, 3 (0.17)
for iridial inflammation, 11 (0.61) for conjunctival redness, and 8
(0.44) for conjunctival chemosis (Dreher, 1994d)
Technical-grade chlorpyrifos (100 mg; purity, 96.2%) was
instilled into the conjunctival sac of the left eye of Himalayan
albino rabbits, and the right eyes of the animals served as controls.
A group of control animals was similarly treated with distilled water
only. All animals were observed for signs of ocular irritation at 1,
4, 24, 48, and 72 h, and then daily up to day 7. At the 4-h
observation, very slight iridial irritation (score 1) and conjunctival
irritation (score 1) were reported. It was not clear from the report
whether the conjunctival irritation was redness, discharge, or
chemosis. No signs of irritation were reported at 24 or 72 h. A
'quality assurance statement' was issued for this study (Frederick
Institute of Plant Protection and Toxicology, 1995d).
Table 1. Studies of the acute toxicity of technical-grade chlorpyrifos
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Oral administration
Mouse SmithWebster M 1% aqueous gum 102 (94-110) Coulston et al. (1971)
tragacanth
Mouse NAMRU F Soya bean oil 152 (143-162) Berteau & Deen (1978)
Mouse Swiss albino M&F Vegetable oil 109 (93-127) QA Fredrick Institute (1995a)
Rat NR M 5% corn oil 163 (97-276) Taylor & Olson (1963)
F 135 (97-188)
Rat Sprague-Dawley F Soya bean oil 169 (146-196) Berteau & Deen (1978)
Rat Sprague-Dawley M Arachis oil 276 (167-455) GLP Dreher (1994a)
F 350 (285-429)
M&F 320 (260-393)
Rat Wistar M&F Vegetable oil 134 (102-163) QA Frederick Institute (1995b)
Rat Sprague-Dawley M Maize oil 264 GLP Wilson & MacBeth (1994)
F 141
M&F 192
Rat Sprague-Dawley M Maize oil 475 (311-727) Buch & Gardner (1981)
F 337 (220-515)
Rat Sprague-Dawley M Corn oil 221 (181-69) Nissimov & Nyska (1984a)
F 144 (105-200)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M Not reported 205 (134-299) Henck & Kociba (1980)
F 96 (72-140)
M 248 (170-379)
F 97 (80-112)
M 270 (188-426)
F 174 (130-244)
Guinea-pig NR M Corn oil 504 (300-850) GLP Lackenby (1985a)
Rabbit NR M Corn oil 1000-2000 GLP Lackenby (1985a)
Chicken NR M Capsule 32 (14-72) GLP Lackenby (1985a)
Chicken Leghorn M Diet 25 (21-31) Sherman et al. (1967)
Chicken Leghorn M Capsule 32 Stevenson (1963)
Chicken White Rock M&F Capsule 50-63 Stevenson (1966a)
Chicken NR M&F Capsule 20-50 Ross & Roberts (1974)
Chicken NR M&F Corn oil 102 (64-169) Ross & Roberts (1974)
Chicken NR M Capsule, 32 (14-72) Taylor & Olson (1963)
undiluted
Turkey Beltsville NR NR 32-63 Stevenson (1967)
small white
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Inhalation
Mouse NAMRU F 65% xylene 94 (83-106) Berteau & Deen (1978)
(27-50 min,
whole body,
aerosol)
Rat Albino M&F Vapour (4 h, > 200a GLP Hardy & Jackson (1984)
(HC/CFHB) whole body)
Rat Sprague-Dawley F 65% xylene 78 (57-108) Berteau & Deen (1978)
(60-180 min,
whole body,
aerosol)
Rat Sprague-Dawley M&F Undiluted (4 h, > 230b (max. GLP Anderson et al. (1995)
nose only, attainable
powder) concentration)
Rat Sprague-Dawley M 40% xylene > 4070a Buch (1980)
F (4 h, nose only, 2890b (2010-4160)
aerosol)
Rat Wistar M&F Undiluted (4 h, > 1020a GLP Kenny et al. (1987)
whole body,
aerosol)
Rat Fischer 344 M&F 1% aqueous > 3200a GLP Phillips & Lomax (1989)
solution (4 h,
whole body,
aerosol)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M&F Vapour (4 h, > 36a (max. GLP Blagden (1994)
nose only) attainable
concentration)
Rat Wistar M&F Acetone (4 h, 560b (360-950) Fredrick Institute (1996a)
whole body,
nebulized particles
< 5 µm)
Dermal application
Rat Sprague-Dawley M&F PEG > 2000 Lackenby (1985b)
Rat Sprague-Dawley M&F Undiluted > 2000 GLP Jackson & Ogilvie (1994a)
Rat NR M&F Undiluted > 2000 QA Nissimov & Nyska (1984b)
Rat Fischer M&F Undiluted > 2000 GLP Jeffrey et al. (1986)
Rat Sprague-Dawley M&F Arachis oil > 2000 GLP Dreher (1994b)
Rat Sprague-Dawley M&F Saline > 5000 (intact Buch et al. (1980)
and abraded)
Rabbit Himalayan M&F Water 1233 (993-1531) QA Fredrick Institute (1995c)
Rabbit New Zealand M&F Undiluted 1580 (828-2606) Henck & Kociba (1980)
white M&F 1598 (1243-1919)
M&F 1801 (1023-3152)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat CFY M&F Tween 20/ 147 (120-179) Davies & Kynoch (1970)
DMSO/water
2:3:5 (subcutaneous)
M, male; F, female; NR, not reported; GLP, good laboratory practice; QA, quality assurance; PEG, polyethylene glycol;
DMSO, dimethyl sulfoxide
Technical-grade chlorpyrifos (100 mg; fine powder) was placed
into the right eye of nine young adult albino rabbits. The eyes of
three animals were irrigated with saline solution 30 s after
treatment, while the eyes of the remaining animals were not irrigated.
Ocular examinations were conducted after 24, 48, and 72 h and at 4 and
7 days. Most of the animals displayed slight initial pain after
instillation of the test material. No signs of irritation were
observed in the cornea or the iris. Slight conjunctival redness was
observed in all animals with unwashed eyes, and in two animals whose
eyes were irrigated after 30 s. The irritation disappeared in most
animals by days 3-4 but persisted for 7 days in a single animal with
unirrigated eyes. The mean irritation scores in rabbits with unwashed
eyes were 2.3 at 24 h and 0.3 at 7 days, while in the rabbits with
irrigated eyes the scores were 1.3 and 0, respectively (Buch &
Gardner, 1980a).
Dermal irritation: A finely ground commercial preparation of
chlorpyrifos (500 mg) moistened with distilled water was applied under
an occlusive dressing to about 6 cm2 of clipped skin on the back of
three young adult female New Zealand white rabbits as a single 4-h
application. No primary irritation was observed (Jones, 1985b).
The dermal irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested in a study conducted according to GLP in six
young male New Zealand white rabbits, to which 500 mg of the material
(moistened with water) were applied under a gauze patch on clipped
intact skin on the trunk and then covered with an occlusive dressing.
The dressings were removed after 4 h and the skin wiped to remove
residual material. The skin was examined for signs of irritation 1,
24, 48, and 72 h after removal of the patch and scored for irritation
on the basis of a system recommended by the US Environmental
Protection Agency. Further assessments were conducted after 4, 5, and
6 days to determine the reversibility of the effects. Slight erythema
(score 1) was noted on three of six test sites at 1 h, and this effect
persisted until 72 h at one site and until day 5 in another animal. No
skin irritation was seen on day 6 (Jackson & Ogilvie, 1994dc).
A technical-grade, fine-powder commercial preparation (purity not
stated) was impregnated at a dose of 0.5 g onto unmedicated lint
patches and tested in six young adult male New Zealand white rabbits
on shaved abraded and intact sites on the back. The patches were held
in place under impermeable dressings, which were removed after 23 h.
The reactions at the test sites were assessed by the method of Draize
at 24 and 72 h and 7 days after administration of the test material.
Well-defined or very slight erythema was seen in all animals at 24 h,
on both intact and abraded sites. Very slight oedema was also seen at
the intact and abraded test sites of one animal at 24 h, another at 72
h, and a third at 7 days. No skin reactions were seen in other animals
at these intervals. The primary irritation index was calculated to be
0.67 (Buch & Gardner, 1980b).
Technical-grade chlorpyrifos (purity, 96.2%) was applied at a
dose of 500 g to clipped intact and abraded skin on the trunks of
three male and three female Himalayan albino rabbits, and the trunks
were wrapped with plastic sheets held in place with tapes for 24 h. A
control group was treated with distilled water. After exposure, the
coverings were removed, but the report did not indicate whether the
test sites were washed or cleaned. The skin was observed 24 and 72 h
after application for signs of irritation. No sign of erythema or
oedema was reported. A 'quality assurance statement' was issued for
this study (Frederick Institute of Plant Protection and Toxicology,
1995e).
Technical-grade chlorpyrifos (purity, 99.3%), moistened with
0.5 ml distilled water, was applied at a dose of 0.5 g to clipped
areas on the backs of New Zealand white rabbits in a study carried out
in accordance with GLP. Surgical gauze was applied over the sites and
held in place with a strip of surgical adhesive tape, and the trunk of
each rabbit was wrapped in an elasticized corset. After the 4-h
contact period, the corset and patches were removed, and the treated
skin was wiped with cotton-wool soaked in distilled water. One hour
and approximately 24, 48, and 72 h after removal of the patches, the
test sites were examined for evidence of dermal irritation and scored
according to the method of Draize. Very slight (score 1) to
well-defined (score 2) erythema and very slight (score 1) to slight
(score 2) oedema were observed in all animals 1-24 h after removal of
the patches. At 48 h, very slight erythema was observed in three
animals and very slight oedema in one. No sign of skin irritation was
seen at 72 h. The sum of the readings at 24 and 72 h was 10, and the
primary irritation index (the sum of the readings/12) was 0.8. This
score indicates mild irritation according to Draize (Dreher, 1994d).
Dermal sensitization: The skin sensitizing potential of
technical-grade chlorpyrifos (purity, 99.3%) was assessed in female
albino Dunkin-Hartley guinea-pigs in a study conducted according to
GLP. Very slight (score 1; 10 animals) to well-defined (score 2; 2
animals) erythema and very slight (score 1; 1 animal) oedema were
observed 1 h after topical induction, but no signs of irritation were
observed at 24 h. No skin reaction was observed in control animals,
and no skin reactions were observed at the test or vehicle control
sites after topical challenge 21 days after induction. One animal was
found dead at this stage of the study, but the cause of death was not
determined. The test material was not a skin sensitizer in this study
(Dreher, 1994e).
Technical-grade chlorpyrifos (purity, 96.2%) was prepared as a 1%
solution in 0.5% carboxymethyl cellulose and tested at a dose of
100 mg in male Hartley guinea-pigs. Test and positive control groups
received induction by exposure of the shaven skin of the left flank,
and challenge 14 days after the third induction dose. Control animals
received only a challenge with 0.5 ml of a 0.1% solution. The test
animals had barely perceptible (score 1; 4/10 animals) erythema or
slight oedema (score 1; 2/10 animals) 24 h after the challenge, but no
signs of irritation were observed at 48 h. A single control animal had
barely perceptible erythema at 24 h, but no other signs of skin
irritation were seen in this group. Positive control animals had
barely perceptible to deep-red erythema (score 1-3) and slight to
marked oedema (score 1-3) 24 h after the challenge and barely
susceptible erythema and slight to marked oedema at 48 h. This study
was issued a 'quality assurance statement' (Frederick Institute of
Plant Protection and Toxicology, 1996b).
After a study to determine a suitable dosing regimen, six male
and six female young albino Dunkin-Hartley guinea-pigs were treated
with a commercial preparation of chlorpyrifos in a standard Beuhler
skin sensitization assay. The test substance (0.3 ml, 100% w/v in
polyethylene glycol on a lint pad) was applied for 6 h under an
occlusive bandage to the freshly clipped left flank of the animals on
days 1, 8, and 15. Challenge doses of chlorpyrifos and solvent were
applied for 6 h on day 29 to the right and left flanks, respectively.
The negative controls were treated only on day 29, when they received
the same treatment as the test animals. The challenge sites of both
groups were evaluated 24 and 48 h after removal of the patch. No
adverse skin reactions were seen after exposure to chlorpyrifos or
vehicle alone (Jones, 1985c).
Technical-grade chlorpyrifos (purity, 99%) was tested in young
female Dunkin-Hartley guinea-pigs by the Magnusson-Kligman
maximization test in a study conducted according to GLP. The test
consists of an induction with intradermal injections of the test
material followed after 1 week by topical application, and a challenge
3 weeks after induction. The test sites were assessed for irritation
24, 48, and 72 h after injection and 24 and 48 h after removal of the
patch (48-h exposure). A concentration of 25% of the test material in
maize oil was selected for injection in the induction phase and 75% in
maize oil for the topical induction phase; a concentration of 75% in
maize oil was also selected for the challenge. During the induction
phase, slight or discrete erythema was observed in all test and
control animals 1 and 24 h after injection and 1 h after topical
application. A number of test and control animals also showed slight
or discrete erythema 24 h after topical application. During
application, two test animals became ataxic, thin, and subdued and
were removed from the study, and one test animal was removed from the
study before the challenge application because it had lesions at the
test site. A single test animal showed a positive response to
challenge, with slight to discrete erythema. None of the animals given
the vehicle alone reacted to the challenge application (Jackson &
Ogilvie, 1994a).
Technical-grade chlorpyrifos (purity, 96.8%) dissolved in 100%
dimethylsulfoxide and diluted in the same solvent to achieve the
desired concentrations was tested in a study that conformed to GLP in
albino Dunkin-Hartley guinea-pigs. In animals treated with a single
dose, irritation ranging from scattered mild redness to moderate and
diffuse redness was seen at 24 h in 5/10 animals; only mild redness
was seen at 48 h in 4/10 animals. In 20 animals induced and then
challenged with chlorpyrifos, only one had scattered mild redness
after the challenge dose at 24 h, and no skin reaction was seen at 48
h. In a positive control group, 9/10 animals had mild redness at 24 h
and 4/10 at 48 h (Berman, 1987).
(b) Short-term studies of toxicity
Mice
In a 2-week study conducted according to GLP requirements, young
adult CD-1 mice, were given a commercial preparation of
technical-grade chrlopyrifos (purity, 96.3%) at dietary concentrations
of 0 (control), 75, 150, 300, 600, or 1200 ppm, equaivalent to 0,
14-18, 30-32, 56-67, 89-100, and 130-180 mg/kg bw per day,
respectively. Deaths and treatment-related clinical signs, including
tremor, ocular opacity, ocular staining, hunching, and lachrymation,
were seen mainly in animals at the highest dose. Marked reductions in
body weight and feed consumption were observed in animals at doses
> 600 ppm. In males, cholinesterase activity was reduced at all
doses. At 75 ppm, plasma cholinesterase activity was reduced by
> 95%, erythrocyte acetylcholinesterase activity by almost 40%, and
brain acetylcholinesterase activity by about 50%. Inhibition of
erythrocyte acetylcholinesterase activity was not dependent on dose,
and approximately 35% inhibition being seen at all doses, but plasma
and brain cholinesterase activities were reduced in a dose-related
manner, with an inhibition of 99% in plasma and about 89% in brain at
the high dose. Similar patterns of cholinesterase inhibition were seen
in females. On the basis of the results of this study, doses of 5, 50,
200, 400, and 800 ppm were selected for a 13-week dietary study in
mice (Crown et al., 1984a).
Groups of 40 male and 40 female CD-1 mice were given chlorpyrifos
(purity, 95.7%) in the diet at 0 or 15 ppm, equal to 2.7 mg/kg bw per
day for males and 3.4 mg/kg bw per day for females; 20 animals of each
sex per group were killed after 1 week, and 20 were treated for a
total of 4 weeks. There were no significant clinical signs or
unscheduled deaths. Food consumption was unaffected by treatment, but
the body-weight gain of male mice was decreased by 25% at the end of
the study. Organ weights were not affected by treatment, and there
were no significant differences between groups. The findings at
necropsy were within normal limits for all groups. After 1 week,
plasma cholinesterase activity was depressed by 88-91% and erythrocyte
acetylcholinesterase activity was depressed by 40-53%; after 4 weeks,
plasma cholinesterase activity was depressed by 91% and erythrocyte
acetylcholinesterase activity by 53%. Brain acetylcholinesterase
activity was unaffected by treatment at either time. There was no
difference between the sexes in the alterations in plasma and
erythrocyte cholinesterase activity (Davies et al., 1985).
Groups of 12 Crl CD-1 mice of each sex were given chlorpyrifos
(purity, 93.5%) in the diet at concentrations of 0, 5, 50, 200, 400,
or 800 ppm (equivalent to 0, 0.7-1.3, 7.1-14, 32-53, 41-140, and
110-300 mg/kg bw per day, respectively) for 13 weeks. This study was
conducted to the requirements of GLP. Dose-related increases in the
mortality rate and the frequency of ocular opacities were seen at 400
and 800 ppm. Body weights were reduced by 10-15% in mice at 800 ppm
throughout the study. Plasma cholinesterase activity was markedly
decreased at all doses, and decreased erythrocyte acetylcholinesterase
activity was seen, but with no clear dose-dependency; brain
acetylcholinesterase activity was inhibited in a dose-dependent manner
in males at doses > 200 ppm and in females at doses > 50 ppm
(Table 2).
Table 2. Group mean values for inhibition of cholinesterase activity in
Crl CD-1 mice given chlorpyrifos in the diet (expressed as a
percentage of control values)
Cholinesterase Sex Dose (ppm)
5 50 200 400 800
Plasma Male 45*** 95*** 98*** 99*** 99***
Female 36*** 97*** 98*** 99 *** 99 ***
Erythrocyte Male 34a 21 16 12 26
Female 11 57*** 44** 48** 44**
Brain Male 43a 14* 80*** 85*** 87**
Female 3a 28*** 58*** 84*** 88***
Plasma, butyryl cholinesterase; erythrocyte and brain,
acetylcholinesterase
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased in comparison with controls
A single malignant lymphoma was reported in a female at the high
dose at terminal sacrifice, but no other neoplastic lesions were
reported. Given the isolated nature of this finding, it was considered
not to be related to treatment. The absolute and relative organ
weights were generally unaffected by treatment, although the relative
liver weights were increased in females at 200, 400, and 800 ppm. The
clinical signs included urogenital staining in males at doses
> 200 ppm and in females at 800 ppm. Ocular opacities occurred in
some animals at 400 and 800 ppm. Dose-related histopathological
findings were seen in the adrenal glands (including lipogenic
pigmentation at doses > 200 ppm in females and at > 400 ppm in
males) and in the eyes (acute or subchronic keratitis in two males and
four females at 800 ppm and in a single female at 400 ppm).
Erythrocyte acetylcholinesterase activity was variable, and the effect
of treatment on this parameter was equivocal. The NOAEL was 5 ppm,
equal to 0.7 mg/kg bw per day, on the basis of inhibition of brain
acetylcholinesterase activity at higher doses (Crown et al., 1987).
Rats
In a 2-week study to establish the doses to be used in a 13-week
study, technical-grade chlorpyrifos (purity, 95.5%) was administered
to groups of five rats of each sex at dietary concentrations of 0
(controls), 10, 30, 84, 240, or 694 ppm for 14 days, equivalent to 0,
1.4-1.9, 4.1-5.2, 12-14, 34-39, and 66-95 mg/kg bw per day,
respectively. At the high dose, clinical signs of intoxication were
reported, consisting of irritability, hunching, tremor, ataxia,
urogenital staining, pigmented orbital secretion, failure to groom,
and proneness. No treatment-related clinical signs were observed at
other doses, and treatment-related deaths were confined to animals at
the high dose. Body weights were reduced in males and females at
694 ppm and in females at 240 ppm, and food consumption was also
reduced in animals at 694 ppm. Dose-related decreases in blood
cholinesterase activity were seen at all doses, the inhibition ranging
from 42% at 10 ppm to 93% at 694 ppm. On the basis of these findings,
the doses selected for the 13-week study were 0.5, 10, and 100 ppm
(Crown et al., 1984b).
Plasma, erythrocyte, and brain cholinesterase activities were
markedly reduced in rats after dietary administration of chlorpyrifos
for 14 days at doses of 5 or 10 mg/kg bw per day in a study carried
out according to GLP. Inhibition of cholinesterase activity occurred
in the absence of clinical signs of intoxication. Feed consumption,
body weights, and gross pathological appearance were similar in
control and treated groups. Statistically significant increases in
absolute and relative adrenal weights (about 20% compared with
controls) were observed in females only at 10 mg/kg bw per day
(Liberacki et al., 1990).
Fischer 344 rats received technical-grade chlorpyrifos (purity,
95%) by nose-only inhalation for 6 h/day for 5 days at a target
concentration of 20 ppb in a study that conformed to GLP. No deaths or
treatment-related clinical signs were reported. Exposure of female
rats to a concentration of 0.34 mg/m3 (23 ppb) resulted in a
significant decrease in plasma cholinesterase activity. No effect on
erythrocyte or brain acetylcholinesterase activity was seen in females
or on plasma, erythrocyte, or brain cholinesterase activity in males
(Newton, 1988a).
Chlorpyrifos was administered by nose-only inhalation to female
Fischer 344 rats at a time-weighted average concentration of 12 ppb
(equivalent to 172 µg/m3) for 6 h/day on 5 days per week for 2 weeks
in a study that met GLP requirements. No clinical signs of
intoxication or treatment-related changes in body weights were
reported, and all rats survived until the scheduled termination.
Clinical chemistry showed no treatment-related changes in
haematological, clinical chemical, or urinary parameters, including
plasma, brain, and erythrocyte cholinesterase activity (Landry et al.,
1986).
In a study to determine a dose range, conducted to GLP
requirements, technical-grade chlorpyrifos was administered by
whole-body exposure of groups of Wistar rats for 6 h/day, 5 days per
week for 2 weeks at target concentrations of 0 (air control), 10, 100,
and 400 mg/m3 (actual concentrations, 0, 10, 94, and 388 mg/m3).
Exposure of animals to the high dose was terminated after five
exposures because they became moribund, with rapid weight loss and
deterioration of their general condition, and all surviving animals
were killed on day 9. No deaths was observed at other doses. There was
no NOAEL in this study, as significant inhibition of plasma,
erythrocyte, and brain cholinesterase activity was seen at all doses
in males and females. Plasma cholinesterase activity was inhibited by
78, 86, and 88% at the three doses, respectively, in males and by 91,
93, and 95%, respectively, in females when compared with controls.
Erythrocyte cholinesterase activity was inhibited by 78, 58, and 75%
in males and by 60, 80, and 73% in females, respectively. Brain
cholinesterase activity was inhibited by 50% at 10 mg/m3, about 70%
at 94 mg/m3, and about 72% at 388 mg/m3 in males and females.
Clinical signs of toxicity (tremors, salivation, and lachrymation),
decreased food consumption, and decreased body weights were observed
at the two higher doses. Increased adrenal weights were seen at 94 and
388 mg/m3, and effects in the forestomach including thickening and
congestion were observed at 388 mg/m3 (Kenny et al., 1988).
Fischer 344 rats were exposed to time-weighted average
concentrations (nominal) of 0, 1, or 5 ppb (0, 0.014, and
0.072 mg/m3) of chlorpyrifos vapour for 6 h/day, 5 days per week for
2 weeks in a study conducted according to GLP. The mean daily
time-weighted average concentrations found by analysis were 0.7 and
5 ppb, respectively. Although two control groups were used, their
cholinesterase activity did not differ statistically significantly,
and the activity in test groups was compared with that of the combined
control group. Statistically significant decreases in plasma,
erythrocyte, and brain cholinesterase activity were observed at 5 ppb,
but as the reductions in brain and erythrocyte acetylcholinesterase
activity did not reach 20% when compared with the control group, the
effect was considered not to be of toxicological significance. Plasma
cholinesterase activity was inhibited by > 20% in females at 5 ppb.
A statistically significant decrease in plasma cholinesterase activity
in females at 0.7 ppb was considered to be incidental to treatment.
There were no treatment-related deaths, clinical signs of toxicity, or
changes in body weight during the study. No treatment-related effects
were seen on body or organ weights, and no lesions were found at gross
examination. No significant adverse treatment-related effects were
observed at 5 ppb (approximately 0.07 mg/m3) (Landry et al., 1985;
Streeter et al., 1987).
Groups of 10 CDF Fischer 344 rats of each sex were fed diets
containing chlorpyrifos (purity, 95.7%) at concentrations that
provided doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day for 13 weeks in
a study that conformed to GLP. The treatment-related effects seen at
the highest dose consisted of decreased body weight and body-weight
gain in males, increased fatty vacuolation of the adrenal zone
fasciculata in males, and changes in haematological and clinical
chemical parameters consisting of decreased erythrocyte counts in both
sexes; increased platelet counts, reduced serum total protein,
albumin, and globulin concentrations, and decreased alanine
aminotransferase and alkaline phosphatase activity in males; and
decreased serum glucose concentration and increased urinary specific
gravity in females. Plasma and erythrocyte cholinesterase activities
were depressed at doses > 1 mg/kg bw per day, and the activity of
brain acetylcholinesterase was depressed at 5 and 15 mg/kg bw per day.
The NOAEL was 0.1 mg/kg bw per day on the basis of depression of
erythrocyte and brain acetylcholinesterase activity (Szabo et al.,
1988).
Groups of 10 rats of each sex were given diets containing
chlorpyrifos at 0, 10, 30, 300, or 1000 ppm, but treatment at the
highest concentration was discontinued after 4 weeks because of severe
clinical signs. Plasma cholinesterase activity was reduced in a
dose-related manner and was significantly lower than that of controls
at all doses. Erythrocyte cholinesterase activity was similarly
significantly reduced at all doses, but no dose-dependency was
demonstrated. Brain cholinesterase activity showed a clear
dose-related depression in animals of each sex at doses > 30 ppm.
At 30 ppm, the activity was inhibited by 22% in males and 24% in
females 41 days after administration. No other significant effects
were found on haematological or clinical chemical parameters, but few
were examined. No histopathological lesions were found that could be
linked to treatment. The NOAEL for inhibition of brain cholinesterase
activity was 0.5 mg/kg bw per day, and the LOAEL was 1.5 mg/kg bw per
day. There was no NOAEL for inhibition of erythrocyte or plasma
cholinesterase activity, as effects were seen at concentrations
> 10 ppm (0.5 mg/kg bw per day) (JMPR 1972; modified by reference
to the original reports of Beatty & McCollister, 1964; Beatty &
McCollister, 1971).
Five groups of 10 rats (strain not specified) of each sex
received chlorpyrifos (purity not specified) in the diet at
concentrations that provided doses of 0.03-10 mg/kg bw per day. The
doses of 0 and 0.3 mg/kg bw per day were fed for 90 days; the doses of
1, 3, and 10 mg/kg bw per day were fed for 28 days, when these animals
were fed control diets for 3 weeks and then diets containing 0, 0.03,
or 0.1 mg/kg bw per day. Behaviour and growth were affected at 3 and
10 mg/kg bw per day. Cholinesterase activity was depressed at 0.3
mg/kg bw per day. During the period of removal from treated diet, the
cholinesterase activity returned to pre-test levels; on resumption of
feeding at 0.1 mg/kg bw per day, only a slight depression was observed
in plasma and erythrocyte cholinesterase activity. In view of of the
unusual design of this study, the toxicological relevance of this
effect is considerede equivocal. No depression of brain or blood
enzymes was seen at 0.03 mg/kg bw per day (Blackmore, 1968).
Groups of 10 Crl rats of each sex were fed diets containing
chlorpyrifos (purity, 97.5%) at concentrations that provided a dose of
0.3 mg/kg bw per day for 13 weeks or 1, 3, or 10 mg/kg bw per day for
4 weeks. No deaths occurred during the study. After 4 weeks, there was
a clear dose-response relationship for inhibition of erythrocyte and
plasma cholinesterase activity at all doses, and animals at higher
doses showed clinical signs of toxicity. These animals were therefore
removed from exposure and allowed to recover for 3 weeks, during which
time the plasma and erythrocyte cholinesterase activities returned to
control values. These groups were then fed chlorpyrifos at doses of 0,
0.03, or 0.1 mg/kg bw per day for 13 weeks. The dose of 0.03 mg/kg bw
per day had no unequivocal effect on cholinesterase activity in
erythrocytes, plasma, or brain in either sex, but the dose of
0.1 mg/kg bw per day significantly reduced the erythrocyte
cholinesterase activity. The NOAEL was 0.03 mg/kg bw per day on the
basis of inhibition of erythrocyte cholinesterase activity after 13
weeks at 0.1 mg/kg bw per day (Hazleton Laboratories, 1968).
Groups of 20 Sprague-Dawley rats of each sex were given
chlorpyrifos (purity not stated) in the diet at 0, 0.03, 0.15, or
0.75 mg/kg bw per day for 6 months; an interim sacrifice of five
animals of each sex per dose was conducted after 3 months. A total of
16 animals across dose groups died from chronic murine pneumonia.
Cholinesterase activity was inhibited at the high dose in both
erythrocytes (50%) and plasma (65%), but the activity in brain was not
inhibited at any dose. Treatment did not change body-weight gain, food
consumption, or haematological or other clinical chemical parameters.
The NOAEL was 0.15 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholinesterase activity at 0.75 mg/kg bw per day
(JMPR, 1972; modified by reference to the original report of Coulston
et al., 1971).
Groups of 20 Crl CD Sprague-Dawley-derived rats of each sex were
given chlorpyrifos (purity, 95.5%) at dietary concentrations of 0
(control), 0.5, 10, or 200 ppm for 13 weeks in a study conducted
according to GLP. The minimum and maximum achieved doses of the test
material were calculated to be 0, 0.03 and 0.078, 0.6 and 1.5, and 13
and 31 mg/kg bw per day, respectively. All animals were inspected once
or twice daily for signs of ill-health and other treatment-related
signs and all were examined closely each week. No deaths or
significant clinical signs were observed at any dose. Clinical
chemistry, urinary analysis, and ophthalmoscopy revealed no findings
that were considered to be related to treatment. At 200 ppm
(approximately 13 mg/kg bw per day), reduced body weights, erythrocyte
count, erythrocyte volume fraction, and haemoglobin and increased food
consumption were observed. Statistically significant reductions
(> 20% inhibition compared with controls) in plasma cholinesterase
activity were observed in males at doses > 0.5 ppm (approximately
0.03 mg/kg bw per day) at 12 weeks and in females at doses > 10 ppm
(approximately 0.6 mg/kg bw per day). The inhibition was dose-related
in males but not in females. Erythrocyte and brain cholinesterase
activities were not measured in this study. The NOAEL was 10 ppm,
equal to 0.6 mg/kg bw per day, on the basis of reduced body weights
and statistically significant reductions in packed cell volume,
haemoglobin, and erythrocyte volume fraction at 200 ppm (Crown et al.,
1985).
Groups of 10 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 100%) by nose only at concentrations of 0, 5.2,
10.3, or 20.6 ppb (0, 75, 150, or 300 µg/m3) for 6 h/day, 5 days per
week for 13 weeks in a study that conformed to GLP. No
treatment-related deaths and only minimal clinical signs were observed
during the study. Body weights and urinary, haematological, and
clinical chemical parameters were unaffected by treatment. No effects
on plasma, erythrocyte, or brain cholinesterase activity were seen at
any dose. Gross and histopathological examination revealed no effects
associated with administration of chlorpyrifos (Corley et al., 1986).
Groups of 15 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 95%) by nose only for 6 h/day, 5 days per week,
for 13 weeks at target concentrations of 0 (control), 5, 10, and
20 ppb (0, 0.07, 0.14, and 0.28 mg/m3, respectively). The study was
conducted according to GLP requirements. There were no
treatment-related deaths or ophthalmic, haematological, or clinical
chemical changes. Plasma cholinesterase activity was inhibited by 23%
in males at the high dose ( p < 0.01), but other changes in plasma
cholinesterase activity were considered not to be related to
treatment. Erythrocyte and brain cholinesterase activities were
unaffected. Pathological and histopathological examination showed no
effect of treatment. The NOAEL was 20 ppb (0.28 mg/m3), on the basis
of inhibition of plasma cholinesterase activity. The absence of frank
toxicity at any dose in this study makes it difficult to draw
conclusions about the toxic potential of inhaled chlorpyrifos at doses
> 0.28 mg/m3 (Newton, 1988b).
In a study in Fischer 344 rats treated dermally, conducted
according to GLP requirements, the animals received chlorpyrifos
(purity, 100%) at 0, 0.1, 0.5, or 5 mg/kg bw per day in corn oil for a
total of 15 days over a 21-day period. No treatment-related effects
were observed at any dose, and plasma, erythrocyte, and brain
cholinesterase activities were not inhibited. Gross and microscopic
examination did not reveal any treatment-related changes, and body
weights and organ weights were unaffected by treatment. In the 4-day
range-finding component of this study, decreased plasma cholinesterase
activity (45%) and erythrocyte cholinesterase activity (16%) were
observed at 10 mg/kg bw per day, in the absence of clinical signs of
intoxication (Calhoun & Johnson, 1988, 1989).
Rabbits
No skin irritation was found when a formulation containing 61.5%
chlorpyrifos and 35% xylene was applied to the skin of the back and
abdomen of groups of two rabbits at doses of 5, 10, 25, and 50 mg/kg
bw 20, 4, 3, and 1 times, respectively. Both plasma and erythrocyte
cholinesterase activities were significantly inhibited in each dose
regimen. The values for plasma recovered more quickly than those for
erythrocytes, which were still significantly inhibited at day 40 after
the 3-, 4-, and 20-day exposure patterns (Pennington & Edwards, 1971).
Three rabbits were exposed for 5 min to a formulation containing
61.5% chlorpyrifos and 34.5% xylene from an ultra-light vapour cold
aerosol fog generator delivering 3.8 L/h. Two rabbits were exposed at
a distance of 8 m at a height of 1.3 m and one at a height of 0.8 m.
Exposure was terminated after 5 min because of ocular and pulmonary
irritation. The recorded concentration of chlorpyrifos in
breathing-space air was about 108 mg/L (range, 83-133 mg/L). By 24 h
after treatment, the rabbits had decreased activities of plasma
cholinesterase (< 33%) and erythrocyte cholinesterase (< 12%),
but these values had recovered almost to the control level by 72 h
after treatment (Pennington & Edwards, 1971).
Chickens
White Leghorn chickens were given chlorpyrifos in their
drinking-water at 1 ppb, 1 ppm, or 100 ppm for up to 84 days. Only
plasma cholinesterase activity was reported because of technical
difficulties with blood sampling. Significant inhibition (54%) of
plasma cholinesterase activity was recorded on day 84 in birds at 100
ppm (Stevenson, 1965).
Dogs
Technical-grade chlorpyrifos (purity, 95.8%) was administered to
groups of two pure-bred beagles of each sex orally in gelatine capsule
at doses of 0 (control; lactose only), 0.01, 0.03, 0.5, or 5 mg/kg bw
per day for 4 weeks, although only one animal of each sex was exposed
at 0 and 0.5 mg/kg bw per day. This study met GLP requirements. No
deaths occurred during the study, and no clinical signs associated
with treatment were observed. All animals gained weight steadily
during treatment, and food consumption was similar in all groups.
Rapid inhibition of plasma cholinesterase activity was observed at 0.5
and 5 mg/kg bw per day at all intervals, and the inhibition was both
dose- and time-dependent. Plasma cholinesterase activity was reduced
at 0.03 mg/kg bw per day, but the inhibition was considered not to be
significant owing to variation between groups and the small group
sizes. In animals at 5 mg/kg bw per day, erythrocyte cholinesterase
activity was 47% that of controls on day 7 and was still inhibited by
> 70% at the end of the study; brain cholinesterase activity was
inhibited by 32% in comparison with concurrent controls at the end of
the study. In animals at 0.5 mg/kg bw per day, erythrocyte
cholinesterase activity was inhibited by 30% on days 27-28 only, and
the effect was considered not to be toxicologically significant owing
to its transient nature. Macroscopic post-mortem examination showed no
changes that were considered to be of toxicological significance. The
NOAEL was 0.5 mg/kg bw per day, on the basis of inhibition of
erythrocyte and brain cholinesterase activity at 5 mg/kg bw per day
(Harling et al., 1989a).
Five dogs received 10 applications of a 0.087% commercial aerosol
formulation of chlorpyrifos in dichlorophene on their backs (with the
fur brushed against the grain) for 2 weeks, each application lasting
30 s. No deaths occurred, and no abnormalities were seen in clinical
signs, body-weight gain, or opthalmoscopic, haematological or clinical
chemical parameters. Erythrocyte cholinesterase activity was not
decreased, but plasma cholinesterase activity was inhibited from the
beginning of treatment, returning to normal 72 days after treatment.
The inhibition of plasma cholinesterase activity was not accompanied
by clinical signs (Sharp & Warner, 1968).
Groups of two young adult beagles of each sex were given daily
doses of chlorpyrifos orally by capsule at doses of 0, 0.03, 0.1, 0.3,
or 1 mg/kg bw per day for 90 days. Plasma cholinesterase activity was
inhibited at all doses. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day, and that for
inhibition of brain cholinesterase activity was 1 mg/kg bw per day
(Blackmore, 1968).
Technical-grade chlorpyrifos (purity, 95.8%) was administered
orally by capsule to groups of four pure-bred beagles of each sex at
doses of 0 (control), 0.01, 0.22, or 5 mg/kg bw per day for 13 weeks.
There were no unscheduled deaths or unequivocal clinical signs during
the study. The group mean body weights of animals at the high dose
were reduced. Haematology, clinical chemistry, urinary analysis, and
ophthalmoscopy revealed no findings that were considered to be related
to treatment. No treatment-related pathological macroscopic or
microscopic change was found. Statistically significant, dose-related
reductions in plasma cholinesterase activity were seen at 0.01 mg/kg
bw per day (bitches only; week 6 only; < 25%) and above (dogs and
bitches; all sample intervals; < 87%). Erythrocyte cholinesterase
activity was significantly inhibited in a dose-dependent manner at
0.22 mg/kg bw per day (< 46%) and at 5 mg/kg bw per day (< 85%).
At 0.01 mg/kg bw per day, erythrocyte cholinesterase was inhibited by
17-18% at week 12, but the reduction was not statistically significant
at any sample interval. Brain cholinesterase activity was inhibited in
animals of each sex (by 46%) at 5 mg/kg bw per day, and the NOAEL for
this effect was 0.22 mg/kg bw per day. The NOAEL for the study, on the
basis of inhibition of erythrocyte acetylcholinesterase activity, was
0.01 mg/kg bw per day (Harling et al., 1989b).
Beagles aged 10 and 11 months were given diets containing
chlorpyrifos (purity, 97.2%) at concentrations that provided doses of
0, 0.01, 0.03, 0.1, 1, or 3 mg/kg bw per day for 1 year (one animal of
each sex per dose), 1 year with a 3-month recovery period (two animals
of each sex per dose), or 2 years (four animals of each sex per dose).
Clinical signs were recorded daily, and body weights were recorded
weekly for the first 6 months and every 2 weeks thereafter. Food
intake was recorded weekly during the first 3 months and monthly
thereafter. The clinical investigations in the groups receiving 0, 1,
or 3 mg/kg bw per day included haematology (packed cell volume,
haemoglobin, erythrocyte and leukocyte counts, and prothrombin time)
and urinary analyses (specific gravity, occult blood, ketones, solids,
pH, albumin, and sugar). The serum concentration of blood urea
nitrogen and the activities of alkaline phosphatase and alanine and
aspartate aminotransferases were measured in all dogs before treatment
and at various intervals thereafter. Bromsulphalein retention was
measured before treatment and at the 1-year sacrifice of animals at 0,
1, or 3 mg/kg bw per day. Cholinesterase activity was determined in
plasma and erythrocytes in most groups before treatment and at various
intervals during the study, and the activity in brain was measured at
necropsy. Fasted dogs were necropsied and the brain, heart, liver,
kidney, spleen, and testes were weighed. Portions of these organs and
a standard set of tissues were preserved from the controls and animals
at 1 (after 1 year only) and 3 mg/kg bw per day for histopathological
examination. The animals fed chlorpyrifos for 2 years were given
complete physical examinations before the end of the study, including
routine neurological and opthalmoscopic evaluations. The data were
evaluated only with Student's t test.
The body weights were not significantly affected by treatment,
and food consumption was comparable in groups of the same sex.
Consumption of the test compound was monitored regularly and reported
to approximate the nominal concentrations closely. The organ weights
were not affected by treatment, except for an increase in the mean
relative liver weight in males at 3 mg/kg bw per day (3.5 g/100 g vs
2.5 g/100 g in controls) in the 2-year study. Details of the recorded
clinical signs were not presented, and the authors simply stated that
"No clinical signs of toxicity were observed in any of the dogs in
either phase of the experiment." The results of clinical pathology
were presented as individual and summary data. There were no
unequivocal treatment-related differences between control and treated
groups in the limited haematological parameters measured nor in
urinary analyses. No effect of treatment was found on blood urea
nitrogen, alkaline phosphatase or alanine and aspartate
aminotransferase activities or Bromsulphalein retention. The results
of gross pathological examination were reported in the form of a
general summary. None of the findings indicated an effect of
treatment. The results of the limited histopathological examinations
(control and high dose only) were reported in a summary table with no
indication of the severity of lesions or abnormalities; individual
data were not presented. The available data did not indicate any
treatment-related effects.
Cholinesterase activity was presented for individual animals and
for groups (Table 3). Inhibition was evident within nine days (the
first assay time) of the start of the study, and the values recorded
at termination were similar to those recorded at interim assays.
Plasma cholinesterase activity was decreased in a dose-related manner
at both times, and erythrocyte cholinesterase activity was similarly
depressed, females being slightly more severely affected than males.
Brain cholinesterase activity was the least affected by treatment. The
cholinesterase activity of animals that were allowed to recover,
assayed after 2, 6, and 13 weeks on normal diet, showed a return to
control levels after 2 weeks for plasma activity and after 13 weeks
for erythrocyte activity.
Table 3. Percentage inhibition of cholinesterase activity in beagles treated with
chlorpyrifos in the diet in comparison with concurrent controls
Dose Inhibition of cholinesterase activity (%)
(mg/kg bw per day)
Plasma Erythrocytes Brain
Male Female Male Female
One year (males and females)
3 72 70 79 82 8
1 62 62 63 61 3
0.1 39 36 18 30 0
0.03 18 17 2 33 0
0.01 1 1 16 13 0
Two years (males)
3 77 67 83 70 20
1 69 50 68 66 7
0.1 52 38 27 41 8
0.03 23 22 0 6 7
0.01 2 0 14 6 1
Average values at terminal sacrifice
Inadequacies in data collection and recording were noted.
Although the long-term effects of dietary intake of chlorpyrifos could
not be evaluated in the absence of complete pathological and
histopathological examination, the data were adequate for analysis of
inhibition of cholinesterase. Plasma cholinesterase activity was
inhibited in animals of each sex at 0.03 mg/kg bw per day. Erythrocyte
cholinesterase activity was inhibited by > 20% at 0.1 mg/kg bw per
day at both the 1- and 2-year intervals, but the inhibition did not
consistently reach statistical significance when compared with the
values in concurrent controls or with pre-treatment values. This was
due in part to the small group sizes and the variation in this
parameter between groups, including the control group, the activity
differing by as much as twofold between individual animals. Under
these conditions, the erythrocyte cholinesterase activity would have
had to be inhibited by as much as 50% in comparison with controls
before statistical significance was achieved. The NOAEL was 0.1 mg/kg
bw per day on the basis of inhibition of erythrocyte cholinesterase
activity at higher doses. Inhibition of brain cholinesterase activity
was seen only at 3 mg/kg bw per day after 2 years (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971a).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not
consistently include basic observations. Individual body weights,
ophthalmological data, the results of pre-treatment and terminal
physical examinations, and an inventory of histopathological findings
were included. The conclusions of the original study were unchanged,
with the additional observation that no effect of treatment was
detected by ophthalmology (Kociba et al., 1985).
Rhesus monkeys
Groups of one or two rhesus monkeys of each sex were given
technical-grade chlorpyrifos in 1% aqueous gum tragacanth by stomach
tube at doses of 0, 0.08, 0.4, or 2 mg/kg bw per day for 6 months.
There were no treatment-related changes in body-weight gain, food
consumption, clinical findings, or haematological, clinical chemical,
or histopathological parameters. Plasma cholinesterase activity was
depressed at all doses at 16 weeks and at > 0.4 mg/kg bw per day at
24 weeks, and erythrocyte cholinesterase activity was depressed at 0.4
and 2 mg/kg bw per day (Table 4). The NOAEL for erythrocyte
cholinesterase inhibition was 0.08 mg/kg bw per day and that for brain
cholinesterase inhibition was 2 mg/kg bw per day. The usefulness of
this study was limited by the small number of animals tested (JMPR
1972; modified by reference to the original paper of Coulston et al.,
1971).
Table 4. Mean cholinesterase activity (and percent inhibition in
comparison with controls) in rhesus monkeys given chlorpyrifos
by stomach tube
Cholinesterase Time Dose (mg/kg bw per day)
activity (weeks)
0 0.08 0.4 2
Plasma 16 6.0 4.4 (26%) 1.6 (72%) 1.8 (70%)
24 4.4 3.8 (14%) 1.7 (61%) 2.0 (55%)
Erythrocytes 16 9.5 8.2 (13%) 6.5 (32%) 2.6 (72%)
24 8.6 7.6 (13%) 6.3 (27%) 5.4 (38%)
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity conducted in accordance with test
guidelines 83-2 of the US Environmental Protection Agency and OECD 451
and in compliance with GLP standards, technical-grade chlorpyrifos
(stated purity, 95.9%) was mixed in maize oil and incorporated into a
commercially available powdered animal diet to produce dietary
concentrations of 0 (control), 5, 50, and 250 ppm, calculated to be
equal to 0.7-1.1, 6.1-12, and 32-55 mg/kg bw per day in males and
0.7-1.2, 6.6-12, and 34-62 mg/kg bw per day in females. The test diets
were administered to groups of 64 CD-1 of each sex, 3 weeks old, for
at least 79 weeks, terminal sacrifice being undertaken during weeks
80-82. The animals continued to receive the treated diets until
scheduled necropsy. An additional 12 males per group were treated
identically to the mice in the main groups and were used to replace
animals for reasons unrelated to treatment (such as excessive
aggression). All of the unused spares were discarded after 10 weeks of
treatment. Each animal was weighed weekly for the first 13 weeks of
the study and every 2 weeks thereafter, while food consumption was
measured weekly for 13 weeks and monthly thereafter. The animals were
examined daily for clinical signs of intoxication, and a careful
examination, including palpation, was conducted weekly. Blood smears
were prepared from all mice after 12 and 18 months for differential
leukocyte counts, but only those from controls and animals at the high
dose were examined. Plasma, erythrocyte, and brain cholinesterase
activity was determined in five animals of each sex at each dose at 9
and 18 months. All animals were examined grossly, the examination
including all external surfaces, the cranial, thoracic, abdominal, and
pelvic cavities, and the carcass. A large number of organs and tissues
from all animals were fixed for histopathological examination; the
eyes, kidneys, livers, lungs, and thyroids/parathyroids from animals
at all doses were examined, as were other tissues from control and
animals at the high dose and those from animals that died during the
study.
There was no adverse effect on survival, and no treatment-related
increase in the incidence of neoplastic lesions. Treatment-related
clinical signs (ocular opacities, excess lachrymation, and cranial
hair loss) and reductions in body weights and food consumption were
observed at 250 ppm (approximately 32 mg/kg bw per day). The effects
on cholinestrase activity are summarized in Table 5. Plasma
cholinesterase activity was inhibited at all doses. Determinations of
erythrocyte cholinesterase activity revealed > 20% inhibition at 42
weeks and about 30% inhibition at week 78 in animals at 250 ppm but
considerable intra-group variation at other doses. In addition, the
erythrocytes were washed with saline before the cholinesterase
determinations at 78 weeks but not at 42 weeks. The NOAEL for
inhibition of erythrocyte cholinesterase activity was 5 ppm, with a
statistically significant inhibition of 29% in males at 78 weeks and
41% in females at 42 weeks in animals at 50 ppm. Significant
inhibition of brain cholinesterase activity ( p < 0.001 or
p < 0.01) was observed in males and females at 250 ppm at 42 and 78
weeks, the activity being reduced by 80-86% when compared with
controls. At 50 ppm, decreases in activity of 43-47% were seen in
males at 42 and 78 weeks and in females at 42 weeks, but this finding
reached statistical significance ( p < 0.05) in females only at 42
weeks and in males only at 78 weeks. At 5 ppm, brain cholinesterase
activity was reduced in males at 78 weeks (27% reduction), but this
Table 5. Percent inhibition of cholinesterase activity in groups of five mice
fed diets containing chlorpyrifos
Cholinesterase Interval Dose (ppm)
(weeks)
5 50 250
Male Female Male Female Male Female
Plasma 42 4*** 45*** 95*** 97*** 98*** 99***
78 49* 50*** 95*** 96*** 98*** 98***
Erythrocyte 42 1 15 11 41** 22 23
78 - +10a 29* 4 31* 29**
Brain 42 8 1 43 46* 80** 85***
78 27 +13a 47 9 86** 84***
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased activity in comparison with controls
finding was not statistically significant and was considered to be
incidental to treatment. The NOAEL for inhibition of brain
cholinesterase activity was 5 ppm.
Non-neoplastic effects were observed at the high dose, in the
livers (slight subchronic pericholangitis, histiocytic proliferation,
and centrilobular hepatocytic fatty vacuolation) of males and in the
eyes of males and females. No treatment-related lesions were seen at
lower doses. In males at the high dose, an increased incidence of lung
nodules was seen macroscopically, with rates of 5/31 in controls, 4/26
at 5 ppm, 2/37 at 50 ppm, and 14/49 at 250 ppm. The nodules were
diagnosed histologically as bronchio-alveolar adenomas or carcinomas,
but the incidences were stated to be within the range in historical
controls. Nevertheless, two sets of data were reported for the
incidence of neoplasms in historical controls: values obtained from
24-month studies and a published report on spontaneous neoplasms in
CD-1 mice in a number of testing laboratories. Those used in the
report were less than ideal. The use of background incidence from
24-month studies would be expected to increase the range of
spontaneous tumours over that in the 78-week protocol used in this
study. In addition, the tumour incidence was reported in a published
paper (Maita et al., 1988) as a percentage of the animals examined,
while the historical data from the 24-month study and the tumour
incidence in this study were given as percentages of the number of
organs examined. Histopathological examination did not reveal any
statistically significant increase in the incidence of neoplasms in
treated animals when compared with controls. The NOAEL was 5 ppm
(0.7 mg/kg bw per day) on the basis of inhibition of erythrocyte and
brain acetylcholinesterase activity (Gur et al., 1991).
Groups of 56 CD-1 mice of each sex were maintained on diets
containing 0, 0.5, 5, or 15 ppm of chlorpyrifos (purity, 99.6%) for
105 weeks. They were observed daily for clinical signs, body weights
were recorded monthly, and daily food consumption was calculated from
a 3- or 4-day measurement made once a month. Gross pathological
examination was performed on all animals that died, when the weights
of the brain, testes, heart, kidneys, and liver were recorded and a
range of other tissues were collected. Sections were prepared of all
the these tissues for histopathology. Haematology was limited to
examination of smears of blood collected from the tail vein at
terminal sacrifice.
The body weights and absolute and relative organ weights were not
significantly affected by treatment over the course of the study, and
the mean body weight of all treated female mice was greater (< 13%)
than that of controls from day 84 onwards, although food consumption
was comparable in all groups. The mortality rate was also generally
unaffected by treatment, and treated males had slightly higher
survival rates than controls: 39% in controls, 46% at 0.5 ppm, 50% at
5 ppm, and 48% at 15 ppm; the survival rates of females were lower
than those of controls only at the highest dose: 46% in controls, 59%
at 0.5 ppm, 46% at 5 ppm, and 38% at 15 ppm. There were no clinical
signs or pathological findings that indicated an effect related to
treatment. A significant increase was observed in the incidence of
spindle-cell hyperplasia of the adrenal gland in animals of each sex
given 0.5 ppm chlorpyrifos and in males given 5 ppm. This finding was
not considered to be related to treatment as there was a high
background incidence in ageing mice and no dose-response relationship.
Vacuolation was found in sciatic nerve preparations from 2/56 male and
3/54 female controls, 5/54 males and 8/55 females at 0.5 ppm, 2/56
males and 2/52 females at 5 ppm, and 6/52 males and 8/53 females at 15
ppm. The incidences of alveologenic adenomas and hyperplastic nodules
in the liver were significantly increased only in males at 5 ppm.
These findings were considered to be incidental.
This study was barely adequate for assessing the carcinogenicity
and long-term toxicity of chlorpyrifos, as it had serious shortcomings
in design, method, and data collection. No clinical pathology was
performed, and in particular cholinesterase activity was not measured;
no clinical signs were reported; individual data were not presented
for several parameters, including histopathology; and intestinal
parasites (nematodes) were present in all groups (JMPR, 1982; modified
by reference to the original report of Warner et al., 1980).
Rats
In a 2-year study, groups of 25 male and 25 female Sherman rats
received chlorpyrifos (purity, 97.2%) in the diet at doses of 0, 0.01,
0.03, 0.1, 1, or 3 mg/kg bw per day (dietary concentrations not
provided). Supplementary animals were included at each dose to allow
interim sacrifices. The design of the study is shown in Table 6.
Clinical signs were recorded 'frequently', body weights were recorded
twice weekly for the first 4 weeks, weekly for 2-6 months, and every 2
weeks for the remainder of the study. The food intake of the main
group was recorded continuously during the first 3 months and once a
month thereafter. The clinical investigations included haematology
(packed cell volume, haemoglobin, and erythrocyte and leukocyte
counts) and urinary analysis (solids, pH, albumin, sugar, occult
blood, ketones) for five rats of each sex at 0, 1, and 3 mg/kg bw per
day; the serum concentration of urea nitrogen and the activities of
alkaline phosphatase and alanine aminotransferase were measured in all
rats at sacrifice at 12 and 18 months, in all male survivors and in
four to five females at 24 months. Cholinesterase activity was
determined in plasma, erythrocytes, and brain samples, as shown in
Table 6. Rats in groups A, H, I, and K were necropsied in a standard
manner, and the weights of the brain, heart, liver, kidney, spleen,
and testes were recorded. Portions of these organs and a standard set
of tissues were preserved for histopathology, but only the slides for
the controls, animals at the high dose, and animals at 1 mg/kg bw per
day at the 12-month necropsy were examined. Some tissues from animals
that died during the study and from those animals killed when moribund
were also examined histologically. Statistical evaluation of the data
was limited to Student's t test.
Body weights (data presented only as graphs) were generally not
significantly affected by treatment. No details of the recorded
clinical signs were presented; rather, the authors stated that "No
changes in appearance or demeanour or signs of toxicity were observed
grossly in any of the rats. No evidence of a cholinergic response was
noted at any time." The results of clinical pathology were presented
as individual and summary data, and no unequivocal treatment-related
differences between control and treated groups were found for the
limited haematological parameters measured or for the results of
urinary analyses. Treatment had no effect on serum concentrations of
urea nitrogen or on alkaline phosphatase and alanine aminotransferase
activity.
Plasma cholinesterase activity was significantly decreased at 3
mg/kg bw per day at all assay times, the inhibition ranging from 20 to
40% in males and 55 to 74% in females; the activity was less severely
inhibited at 1 mg/kg bw per day, but from 6 months onwards the
inhibition was 18-38% in males and 50-69% in females when compared
with controls, and these differences were statistically significant at
most assay times. Erythrocyte cholinesterase activity was severely
inhibited in animals of each sex at the higher doses at all assay
times, with rates of 60-100% at 3 mg/kg bw per day, 13-90% at 1 mg/kg
bw per day, and little effect at the other doses. Animals that had
Table 6. Design of the long-term study of toxicity and carcinogenicity in groups of
56 mice fed diets containing chlorpyrifos
Group No. of rats of Sacrifice interval End-point examined
each sex per dose
A 25 2 years Necropsy, blood and brain
cholinesterase
B 5 1 week Blood cholinesterase
C 5 1 month Blood cholinesterase
D 5 3 months Blood cholinesterase
E 5 6 months Blood and brain cholinesterase
F 5 9 months Blood cholinesterase
G 6 12 months Blood and brain cholinesterase
H 5 12 months Necropsy
I 7 12 months, Necropsy, blood and brain
7-8-week recovery cholinesterase
J 7 18 months Blood and brain cholinesterase
K 7 18 months Necropsy
been allowed to recover showed full restoration of cholinesterase
activity. Brain cholinesterase activity was significantly inhibited by
treatment at 3 mg/kg bw per day (30-53% inhibition) at all assay
times, to a lesser degree at 1 mg/kg bw per day (3-16%), and was
comparable to that of controls at other doses. Animals that were
allowed to recover showed restoration of brain cholinesterase
activity.
This study was considered to be inadequate for assessing the
carcinogenicity and long-term effects of chlorpyrifos, other than
clinical chemical findings including cholinesterase inhibition, as
there were serious shortcomings in data collection, and the reports of
gross and histopathology were inadequate and no clinical findings were
reported. The NOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day on the basis of
significant inhibition at 1 mg/kg bw per day. The NOAEL for inhibition
of brain acetylcholinesterase activity was 1 mg/kg bw per day on the
basis of significant inhibition at 3 mg/kg bw per day (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971b).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not include
basic observations such as abnormal behaviour or gait. Individual body
weights and gross and histopathological findings were included in the
supplement, but the histopathology was limited and variable, only a
small number of tissues from each rat being examined consistently. The
results were not presented as summary tables of incidence and
severity. The conclusions of the original study remain unchanged by
this supplement (McCollister et al., 1985).
In a 2-year study conducted according to accepted test guidelines
(OECD 451, USEPA 83-5) and GLP, groups of 60 Fischer 344 rats of each
sex were fed diets containing technical-grade chlorpyrifos (purity,
96.1%) at concentrations of 0, 0 (vehicle control), 0.2, 5, or
100 ppm. The vehicle used was 0.04% w/w maize oil. The diets were
prepared weekly and assayed regularly for stability and accuracy of
dosing. Body weights were generally recorded weekly; food consumption
was recorded weekly for 13 weeks and monthly thereafter. Clinical
signs were recorded daily and palpation performed weekly.
Cholinesterase activity was measured before treatment and at weeks 14,
32, 45, 50 (interim sacrifice of five animals of each sex per group
for measurement of brain acetylcholinesterase activity), 78, and 104
weeks (terminal sacrifice with measurement of brain
acetylcholinesterase activity in 10 animals of each sex per group),
and generally on 10 rats of each sex per dose. Haematological
parameters were measured in blood smears from 10 rats of each sex in
the vehicle control group and at 100 ppm at months 12 and 18. Gross
pathological findings were recorded for all animals at necropsy,
including those that died during the study or were killed at early
sacrifices. Necropsy included removal of the eyes with the optic nerve
and adnexa. The weights of the brain, testes, thyroids, adrenals,
kidneys, and liver were recorded, and a range of tissues was
collected. All gross lesions were sectioned, and sections from all
control animal and those at 100 ppm were examined. Appropriate
statistical tests were used to analyse the data.
The mean consumption of chlorpyrifos was estimated to be 0.012,
0.3, and 6 mg/kg bw per day for animals of each sex. Mortality rates
and the incidences of clinical signs or palpable masses were not
affected by treatment. A 5% reduction in the mean body weights of
males at 100 ppm was significant in comparison with controls at most
times during weeks 3-94, and the weights of females at this dose were
significantly lower (about 4%) than those of controls at most times
during weeks 2-64. A variety of non-neoplastic and neoplastic lesions
were recorded, and the incidence of these lesions occasionally showed
a positive trend with dose; however, the incidence was generally
within the range of historical controls in the laboratory or within
the range of published values from the National Toxicology Program in
the USA. The incidences of neoplastic lesions did not show unequivocal
or statistically significant dose-response relationships. The only
observation that was considered to be related to treatment was an
increased incidence of cataracts and diffuse retinal atrophy in
females (Table 7).
Table 7. Incidences of ocular abnormalities found microscopically in rats given
diets containing chlorpyrifos
Abnormality Sex Dose (ppm)
0 0 (vehicle) 0.2 5 100
Diffuse retinal atrophy Male 4/60 0/60 3/23 1/28 3/60
Female 5/60 15/59* 9/60 5/58 24/60*
Cataract Male 31/60 31/60 7/23 6/28 30/60
Female 38/60 38/59 33/60 32/58 51/60*
* Significantly different from controls at p < 0.01 or p < 0.001
Significant (> 20%) inhibition of plasma cholinesterase activity
relative to the control values was observed at doses > 0.3 mg/kg bw
per day (Table 8). While erythrocyte cholinesterase activity was
inhibited in animals at 100 ppm, the variation in the data and the
lack of statistical significance and/or dose-response relationships
mitigated against an unequivocal finding. The NOAEL for inhibition of
brain cholinesterase activity was 0.3 mg/kg bw per day (Crown et al.,
1988).
In a study conducted in compliance with GLP standards, groups of
60 Fischer 344 rats of each sex received diets containing chlorpyrifos
(purity, 98.5%) at concentrations that provided doses of 0 (control),
0.05, 0.1, 1, or 10 mg/kg bw per day for 24 months. Ten rats of each
sex at each dose were randomly designated at the start of the study
for interim sacrifice at 12 months. Blood was collected for
haematology, clinical chemistry, and measurement of plasma and
erythrocyte cholinesterase activity at 6, 12, and 18 months as well as
at the 24-month terminal sacrifice. The groups were observed daily for
deaths and signs of toxicity. All rats were examined clinically at
least once a week from after the sixth month. All animals were
palpated for externally detectable masses before treatment, before the
12-month kill, and monthly thereafter. Body weights and feed
consumption were determined weekly for the first 3 months and monthly
thereafter. All rats were weighed, but feed consumption was determined
for only 20 rats of each sex per group.
All clinical laboratory procedures scheduled for 6 and 12 months
were performed on rats designated for the 12-month interim kill,
whereas tests scheduled for 18 and 24 months were performed on rats
designated for terminal sacrifice. Blood samples were obtained for
haematology and clinical chemistry by orbital sinus puncture under
Table 8. Group mean percent inhibition of cholinesterase activity in comparison
with vehicle controls in rats given diets containing chlorpyrifos
Assay time Dose Cholinesterase inhibition (%)
(weeks) (ppm)
Plasma Erythrocytes Brain
Male Female Male Female Male Female
50 0 0 7 0* 31 0 35
0.2 1 4 0 42 0 14
5 15 51 0* 39 9 10
100 93 98 13 45 57* 80*
78 0 0 3 27 0
0.2 4 3 11 0
5 28* 47* 0 0
100 93* 97* 10 0
104 0 8 9 0 0 18 4
0.2 13 0 0 0 0 0
5 36 37* 17 11 0 0
100 95* 96* 34 18 58* 61*
Values the same as or higher than those of the vehicle control are recorded as
0 inhibition.
* Significantly different from control at p < 0.01 or p < 0.001
light anaesthesia. The haematological determinations consisted of
packed cell volume, haemoglobin concentration, erythrocyte count,
total and differential leukocyte counts, and platelet count. The
clinical biochemical determinations included blood urea nitrogen,
alkaline phosphatase and alanine and aspartate aminotransferase
activities, glucose, total protein, albumin, globulin (calculated),
creatine phosphokinase, total bilirubin, cholesterol, calcium,
phosphorus, sodium, potassium, and chloride. Urine samples were
obtained 1-2 weeks before the scheduled sacrifices at 12 and 24 months
and at 6 and 18 months from 10 rats of each sex per group. The urinary
parameters measured included specific gravity and semi-quantitative
estimates of bilirubin, glucose, ketones, occult blood, pH, protein,
and urobilinogen. Microscopy of a pooled sample was also conducted.
Plasma and erythrocyte cholinesterase activities were assayed in 10
rats of each sex per group at 6, 12, 18, and 24 months, and
acetylcholinesterase activity was measured in half-brain samples
obtained at the 12-month and 24-month scheduled necropsies. All
animals killed at the interim and terminal sacrifices or which died or
were killed when moribund were necropsied and subjected to a complete
gross examination. The brain, liver, kidneys, testes, ovaries, and
adrenal glands were weighed, and many organs and tissues were removed
and preserved in neutral, phosphate-buffered 10% formalin for
subsequent histopathological evaluation. Histological sections of the
formalin-fixed tissues from all controls and those at the highest dose
were prepared, stained with haematoxylin and eosin and examined
microscopically. Histopathological examination of tissues from animals
at the three lower doses was limited to the liver, kidneys, adrenals,
and tissues with gross lesions at both sacrifices. At terminal
sacrifice, the lungs, spleen, testes, pituitary, and
thyroid/parathyroid were also examined microscopically. Appropriate
statistical tests were applied to the data.
Males at the high dose showed a consistent decrease in
body-weight gain relative to controls in the absence of reduced food
consumption, depression of plasma (56-87%), erythrocyte (20-40%) and
brain (56-58%) cholinesterase activities, and an increase in the
weight of their adrenal glands which was characterized microscopically
by exacerbated fatty vacuolation of the zona fasciculata. Similar
effects were observed in females at this dose, but were generally less
pronounced than in males: for example, a transient decrease in
body-weight gain relative to controls with no reduction in food
consumption, depression of plasma (82-95%), erythrocyte (generally
> 20%), and brain (57-61%) cholinesterase activities, and an
increase in adrenal weight at the terminal sacrifice with no
associated histopathological lesions. At 1 mg/kg bw per day, the only
effects attributable to treatment were inhibition of plasma
cholinesterase activity (39-71% in males and 60-86% in females) and
erythrocyte cholinesterase activity (20-40% in males and < 22% in
females); brain cholinesterase activity was not affected. These
results are summarized in Table 9. No treatment-related effects were
observed at the two lower doses. There was no increase in the
incidence of any type of tumour. The NOAEL for inhibition of
erythrocyte cholinesterase activity was 0.1 mg/kg bw per day, on the
basis of toxicologically (> 20%) or statistically significant
inhibition at 1 mg/kg bw per day, and the NOAEL for inhibition of
brain cholinesterase activity was 1 mg/kg bw per day on the basis of
toxicologically and statistically significant inhibition at 10 mg/kg
bw per day (Young & Grandjean, 1988).
Chickens
Hens were fed diets containing chlorpyrifos at concentrations of
0, 25, 50, or 200 ppm (approximately 0, 2.5, 5, and 20 mg/kg bw per
day) for 52 weeks. Mortality was unaffected by treatment, with rates
of 13% in controls, 3% at 25 ppm, 7% at 50 ppm, and 10% at 200 ppm.
Plasma cholinesterase activity was assayed in three hens at each dose
1, 3, 7, 14, 22, and 29 days after the start of treatment, monthly
thereafter, and 1, 2, and 3 weeks after the end of treatment. The
onset of inhibition of cholinesterase activity was rapid and
dose-related, with 22% inhibition in week 1 at 25 ppm, 45% inhibition
at 50 ppm, and 76% inhibition at 200 ppm. It persisted at similar
levels throughout the study but returned to control levels during the
Table 9. Group mean cholinesterase activities in rats given diets containing chlorpyrifos,
expressed as percent of the respective control values for each sampling period
Cholinesterase Sex Sampling period Dose (mg/kg bw per day)
(months)
0 0.05 0.1 1 10
Plasma Male 6 100 96.5 95.1 60.9* 44.2*
12 100 94.5 97.8 28.6* 13.3*
18 100 93.4 80.1 36.6* 22.5*
24 100 92.4 85.5 40.0* 19.7*
Female 6 100 97.9 91.0 34.6* 16.9*
12 100 104 87.0* 13.8* 4.8*
18 100 99.1 85.8* 30.4* 12.4*
24 100 103 94.4 39.7* 17.9*
Erythrocytes Male 6 100 107 88.8 76.1* 75.6*
12 100 107 93.2 67.4 63.1
18 100 109 95.3 66.2* 71.0*
24 100 105 108 86.5 73.5*
Female 6 100 93.3 106 104 87.5
12 100 88.2 109 82.2 59.5*
18 100 114 99.7 78.0 81.9
24 100 107 87.2 83.6 79.9
Brain Male 12 100 93.8* 93.1* 91.0* 42.1*
24 100 102 100 103 44.3*
Female 12 100 97.9 102 95.3* 38.9*
24 100 101 100 96.2 42.9*
* Statistically significantly different from control by Dunnett's or Wilcoxon's test,
alpha = 0.05, one-sided
recovery period. Overall feed consumption, body weight, egg
production, feed efficiency, egg weight, and shell thickness were not
affected by treatment. There was no NOEL for inhibition of plasma
cholinesterase activity in this study, as significant inhibition was
seen at the lowest dose tested (Sherman & Herrick, 1973).
(d) Genotoxicity
Chlorpyrifos was not genotoxic in vitro or in vivo in a range
of studies (Table 10).
Table 10. Results of studies for the genotoxicity of chlorpyrifos
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
In vitro
Gene mutation S. cerevisiae D3 NR NR Negative Poole et al. (1977;
rec locus abstract only)
Gene mutation B. subtilus H17, 20 (100, 200, 500, 1000 95.83 Negative Shirasu et al. (1980)
M45 2000 µg/plate in DMSO
Reverse mutation S. typhimurium TA98, 10, 50, 100, 500, 1000, 95.83 Negativea Shirasu et al. (1980)
TA100, TA1535, 5000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 1, 3.162, 10, 31.62, 95.7 Negativea,b QA Bruce & Zempel (1986)
TA100, TA1535, 100 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 0.01, 0.1, 1, 5, 10, 95 Negativea,c Jai Research Foundation
TA100, TA1535, 100 µg/plate in DMSO (1996a)
TA1537
Reverse mutation S. typhimurium TA98, 30, 100, 300, 3000, 96.8 Negativea GLP Loveday et al. (1987)
TA100, TA1535, 10 000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative Poole et al. (1977;
TA100, TA1535, abstract only)
TA1537
Reverse mutation E. coli WP2 NR NR Negative Poole et al. (1977;
abstract only)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Relative toxicity E. coli and NR NR Negative Poole et al. (1977;
B. subtilis abstract only)
Reverse mutation S. typhimurium TA98, 5, 500, 5000 µg/plate 96.2 Negativea,d QA Fredrick Institute
TA100, TA1535, in DMSO (1996c)
TA1537, TA1538
Forward mutation Chinese hamster ovary 10, 20, 25, 30, 40, 95.7 Negativea Mendrala (1985)
cells, hprt locus 50 µmol/L
Forward mutation Chinese hamster ovary 5, 10, 25, 50, 75 µg/ml, 96.8 Negativea,e GLP Tu (1987)
cells, hprt locus 16 h, - S9
5, 10, 20, 30, 40, 50 µg/ml,
16 h, - S9
30, 50, 100, 300, 1000 µg/ml,
+S9, in DMSO
Sister chromatid Human lymphocytes 0.02, 0.2, 2 or 20 µg/ml NR Negativea,f Sobti et al. (1982)
exchange (Laz-007) in ethanol
Chromosomal Chinese hamster ovary 0.975, 1.47, 2.93, 4.89, 96.8 Negativea,g GLP Loveday (1987)
aberration cells 9.75, 14.7, 29.3, 48.9, 97.5,
147 µg/ml, 19 h, - S9
1.56, 3.12, 5.2, 10.4, 15.6,
31.2, 52, 104, 156 µg/ml,
10 h, - S9
9.75, 14.7, 29.3, 48.9, 97.5,
147, 293 µg/ml, 19 h, +S9
1, 1.5, 3, 5, 10, 15, 30, 50,
100 µg/ml, 10 h, + S9
2.95, 4.95, 9.85, 14.8, 29.6,
49.4, 98.5, 296 µg/ml, 10 h,
+S9
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Rat hepatocytes 16.7, 50, 167, 500, 1667, 98.6 Negativea,h GLP Linscombe et al. (1992)
aberration 5000 µg/ml harvested after
24 h and 5, 16.7, 50, 167
µg/ml harvested after
24 and 48 h, in DMSO
Sister chromatid Chinese hamster ovary 1, 10, 100 µg/ml in acetone NR Negative Muscarella et al. (1984)
exchange cells
Unscheduled DNA Rat hepatocytes 1, 3.16, 10, 31.6, 95.7 Negative Mendrala & Dryzaga
synthesis 100 µmol/L in DMSO (1986)
Reverse mutation S. typhimurium TA98, 0, 1, 3.16, 10, 31.6, 95.7- Negativea Gollapudi et al. (1995)
TA100, TA1535, 100 µg/plate in DMSO 98.6
TA1537, TA1538
Forward mutation Chinese hamster ovary 0, 3.5, 7, 8.8, 10.5, 14, 95.7-98.6 Negativea,i Gollapudi et al. (1995)
cells, hprt locus 17.5 µg/ml in DMSO
Chromosomal Rat lymphocytes 16.7-5000 µg/ml in 95.7-95.6 Negativea,j Gollapudi et al. (1995)
aberration DMSO
Unscheduled Rat hepatocytes 1, 3.16, 10, 31.6, 95.7-95.6 Negative Gollapudi et al. (1995)
DNA synthesis 100 µmol/L in DMSO
In vivo
Micronucleus Mouse (CD-ICR BR) 0, 7, 22, 70 mg/kg bw 95.7 Negative QA Gollapudi et al. (1985)
formation marrow cells orally in corn oil
Micronucleus Mouse (Swiss albino) 0, 15, 30, 60 mg/kg bw 96.2 Negative QA Fredrick Institute
formation marrow cells orally in vegetable oil (1996d)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Mouse (Swiss albino) 0.6, 3, 15 mg/kg bw per 96.2 Negative QA Fredrick Institute
aberration marrow cells day orally in vegetable oil (1996e)
Chromosomal Chick embryo (Cornell 1.11, 11.1, 111, 1110, NR Negativek Muscarella et al. (1984)
aberration K strain eggs) 2220 µg/embryo
Chromosomal Bovine blastocysts NR NR Negative Muscarella et al. (1984)
aberration
Micronucleus Mouse (CD-1 (ICR) BR) 90 mg/kg bw orally in 97.9 Negative GLP McClintock & Gollapudi
formation marrow cells corn oil (1989a)
QA, quality assurance; GLP, good laboratory practice; NR, not reported; S9, exogenous metabolic activation system from 9000 × g
fraction of rat liver; DMSO, dimethyl sulfoxide
Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Cytotoxicity observed at 100 µg/plate in TA100, 1535, 1537, 1538 with precipitation of test material; in TA98, precipitation in the
absence of toxicity
c Cytotoxicity at > 10 µg/plate; dose-related increase in revertant frequency (> twofold than controls) in all strains ± S9, but
increases lower than in positive controls by 2-50-fold and not statistically significant
d Increase in revertants at all dose, - S9 in TA1537 only; no dose-response relationship
e Cytotoxicity at 50 µg/ml in one assay - S9
f At 2 and 20 µg/ml, frequency was statistically significantly different from controls but not double the control frequency
g - S9: cytotoxicity at highest doses; increase in gaps only at 52 µg/ml and 10-h exposure; no increases in other aberrations
+S9: cytotoxicity at 15 µg/ml and 10-h incubation; in one 10-h assay, a significant increase in number of cells with aberrations
(including gaps) at 3 and 10 µg/ml; incidence of aberrations (excluding gaps) not statistically significantly increased and not
dose-dependent In repeat 10-h assay, no increase in incidence of aberrations.
h Cytotoxicity at > 500 µg/ml -S9 and > 167 µg/ml +S9 in first assay; no mitotic index measurable at 50 µg/ml - S9 or
167 µg/ml ±S9
i Test material precipitated at 10.5, 14, and 17.5 µg/ml ± S9
j Test material cytotoxic at > 50 µg/ml ± S9
k Increased mortality at 1110 and 2220 µg/embryo
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a three-generation study, Sprague-Dawley rats (two litters per
generation) were given diets containing chlorpyrifos (purity not
specified) at concentrations that provided doses of 0, 0.03, 0.1, or
0.3 mg/kg bw per day for the first generation and 0, 0.1, 0.3, or
1 mg/kg bw per day for the second and third generations. The sizes of
the groups were consistent in all generations, the controls comprising
20 males and 40 females and the treated groups comprising 10 males and
20 females. Treatment was started at 58 days of age, and F0 parents
were mated at 118 days of age at a 1:2 male:female ratio. Evidence of
conception (vaginal plug or sperm) was considered to be day 0 of
gestation. Pairing for the second litter in each generation was
started approximately 10 days after the first litter was weaned at 21
days, and the treated diets were fed to the litters from weaning. When
the progeny of the F1b or F2b numbered more than 10 per litter,
each litter was reduced to 10 after 5 days of age. The F3b fetuses
were examined for teratogenic changes. F2b parent animals were
continued on the test diet throughout the breeding period, except that
females were placed on normal diet during organogenesis and the test
substance was administered by gavage in acetone or corn oil. Dams were
killed on day 20 of gestation and the fetuses removed surgically and
examined for external abnormalities. Onethird were fixed for
examination of soft tissues, and two-thirds were stained with alizarin
red for skeletal examination; however, only the controls and rats at 1
mg/kg bw per day were examined. Clinical observations were stated to
have been performed 'frequently'. Body weights and food consumption
were recorded weekly until breeding commenced. During gestation and
lactation, the diets were adjusted on the basis of an average feed
consumption of 25 and 40 g/day; rats were weighed before breeding to
allow dietary adjustment. In the investigation of teratogenic effects,
the body weights of the dams were recorded on days 0, 6, 15, and 20 of
gestation, while food consumption was measured during the gestational
intervals 0-6, 6-16, and 16-20 days. Various indices of reproductive
performance were calculated: fertility index as pregnancies per
mating; gestation index as live litters born per number of
pregnancies; viability as rats alive at day 5 per rats born alive; and
lactation index as rats alive at day 21 per rats alive at day 5. All
animals that died and five pups of each sex per dose from the F1a,
F2a, and F3a litters were examined grossly. An extensive set of
tissues was prepared and fixed from these pups, but in the absence of
gross abnormalities only tissues from the F3a pups were examined
histopathologically. Clinical chemistry was limited to the F2
animals, and consisted of measurements of plasma and erythrocyte
cholinesterase activity in 11 control and five or six treated dams at
the time of removal of their litters and in five males per group.
Statistical analysis was limited.
No clinical signs of toxicity were seen in the parents or
offspring. The parental body weights were not significantly affected
by treatment, and food consumption was variable but unaffected. The
fertility, gestation, and lactation indices were comparable between
groups and generations. The viability index was decreased at 1 mg/kg
bw per day, but this effect was considered not to be clearly related
to treatment in view of the isolated nature of the finding and the
study design. The mean body-weight gain of the dams that produced the
F3b pups showed a dose-related increase, with gains of 114 g in
controls, 120 g at 0.1 mg/kg bw per day, 123 g at 0.3 mg/kg bw per
day, and 126 g at 1 mg/kg bw per day. This generation showed no
treatment-related effects on fertility (per cent pregnant), the mean
number of corpora lutea or implantations, viable litter size, or pup
weight after surgical removal. The skeletons of the pups showed common
minor variants such as incomplete ossification of sternebrae and the
occurrence of extra ribs. Visceral examination of the pups showed that
minor variants of the urogenital system (hydronephrosis or left,
right, or bilateral hydroureter) were more common in the treated
group. A single fetus with multiple abnormalities was found at 1 mg/kg
bw per day, but this isolated finding was considered not to be related
to treatment. The group average activity of plasma and erythrocyte
cholinesterase was decreased by > 20% at 1 mg/kg bw per day in males
and females of the F2 generation. The NOAEL was 0.1 mg/kg bw per day
on the basis of decreased plasma and erythrocyte cholinesterase
activity at 1 mg/kg bw per day and decreased erythrocyte
cholinesterase activity at 0.3 mg/kg bw per day (JMPR, 1972; modified
by reference to the original report of Thompson et al., 1971).
To supplement the finding of an equivocal decrease in neonatal
survival at 1 mg/kg bw per day, groups of 30 Sprague-Dawley rats of
each sex were fed diets containing chlorpyrifos (purity, 96.6-99%) at
concentrations providing doses of 0, 0.5, 0.8, or 1.2 mg/kg bw per day
for 135 days and then bred to produce F1 litters. On day 21 of
lactation, 30 pups of each sex were selected randomly, dosed in the
same manner as the F0 animals for 120 days, and then bred to produce
the F2 litters. The F2 pups were weaned on day 21 of lactation.
The F0 and F1 matings were in a 1:1 ratio, with pairing for 5
days, a 7-day rest, and a pairing with a different male for a further
5 days. The litters were culled on day 4 post partum, and the litter
weights and pup survival were recorded on days 1, 4, 7, 14, and 21;
individual pups were weighed on day 21. The parental animals were
examined daily for signs of toxicity, and body weight and food intake
were recorded weekly for animals of each sex until pairing;
thereafter, males were weighed after pairing until sacrifice, and
females were weighed on days 1, 4, 7, 14, and 21 of lactation. The
date of parturition, litter size at birth, and the numbers of live and
dead pups at birth were recorded. The dietary concentrations were
adjusted weekly during the pre-mating period according to the food
consumption and body weights of the rats; the concentrations were then
maintained at the last adjusted concentration from commencement of
cohabitation until sacrifice. Appropriate statistical tests were
applied.
The parental animals showed no significant clinical signs in
either generation. Body weight and food intake were unaffected by
treatment. The mean fertility index (number of females delivering a
litter expressed as the percentage of the total number of females
placed with a male) was reduced at 0.5 and 0.8 mg/kg bw per day but
not at the highest dose, and a similar inverted dose-response
relationship was seen for length of gestation; data on individual
animals were not presented. The litter sizes (10-11) were comparable
at all doses, as were the survival indices (> 91% in all groups on
days 1, 4, 7, 14, and 21) during lactation. The pup weights
(presumably litter weight per number of pups) were also comparable. In
F1 adults, the body weight of males was reduced at 1.2 mg/kg bw per
day on days 160-182 after cohabitation, and those of females showed
slight, sporadic increases when compared with controls. Food intake
was sporadically decreased in males but not in females. No effects
were seen on female body weight during lactation. The fertility index
in treated groups exceeded the control values. The litter sizes were
comparable in all groups, as were the pup weights and pup survival
during lactation. The adequacy of this study for regulatory purposes
was limited by the doses selected. As no adverse effects were found at
the highest dose, it was unclear whether the study clearly
demonstrated the reproductive toxicity of the test material in rats.
The lack of data for individual animals and the inverted dose-response
relationships for some parameters rendered the findings equivocal. In
the absence of clear adverse effects on reproductive parameters at any
dose, the NOAEL was 1.2 mg/kg bw per day (Dietz et al., 1983).
In a study conducted in compliance with GLP standards and the
requirements of the US Environmental Protection Agency 83-4,
technical-grade chlorpyrifos (stated purity, 95.8%) was given in the
diet to groups of male and female CrL: COBS CD (SD) BR rats at
concentrations of 0, 2, 10, or 50 ppm over two generations. These
concentrations corresponded to achieved intakes of 0.1-0.2, 0.5-0.9,
and 2.5-4.5 mg/kg bw per day, respectively, in F0 males and 0.1-0.2,
0.6-0.9, and 2.9-4.6 mg/kg bw per day, respectively, in F0 females.
The corresponding intakes in the F1 generation were 0.1-0.3,
0.7-1.6, and 3.3-8.1 mg/kg bw per day in males and 0.2-0.3, 0.8-1.6,
and 4-8.1 mg/kg bw per day in females. The animals of the F0
generation (28 of each sex per dose) were approximately 7 weeks of age
at the beginning of treatment and were maintained on their respective
diets for at least 56 days before mating. They were mated at a 1:1
ratio for 20 days, and then the dams were allowed to rear their young
to day 21 post partum. Before weaning, all F1 offspring in all
litters were examined for developmental indices (surface righting
reflex, startle reflex, air righting reflex, and pupil reflex). At day
21 post partum, 24 pups from each group were selected for mating;
excess pups were sacrificed on day 22 post partum and examined
macroscopically, and specified organs were weighed and tissues
preserved for histopathological examination from one animal of each
sex per litter. Shortly after the F1 pups were weaned, the F0
adults were killed and examined macroscopically, and a wide range of
organs and tissues were preserved, the ovaries, pituitary, prostate,
testes with epididymides, and uterus with cervix and vagina being
examined histopathologically.The animals in the F1 generation that
were selected for mating were reared on their respective diets for at
least 56 days (i.e. until they were approximately 12 weeks of age) and
were then mated 1:1 for 20 days; the dams were allowed to rear their
young until day 21 post partum. Pathological examination was
conducted as for the F0 generation. All animals were handled
regularly and examined for signs associated with treatment. All
animals that died or were killed were examined. The weight of each
animal was determined at the start of each generation and subsequently
at weekly intervals. All pups were weighed at birth and at 4, 8, 12,
and 21 days post partum. Cholinesterase activity was not determined
in the study. The doses were selected on the basis of those that
decreased cholinesterase activity in a preliminary study in which
chlorpyrifos was given in the diet for a single generation at doses up
to 250 ppm. In this preliminary study, plasma cholinesterase activity
was inhibited when compared with that in controls by 86% in adult
females and by about 60% in weanling males and females at 50 ppm. At
50 ppm, erythrocyte cholinesterase activity was also inhibited in
adult females (70% inhibition) and weanlings (about 50% inhibition),
and brain cholinesterase was inhibited by 38% in adult females
Food consumption was similar in control and treated groups and no
adverse, treatment-related effects on group mean body weights were
observed at any dose in either generation. Mating performance and the
duration of gestation were similar in control and treated groups.
Slight inter-group variation was observed in the incidence of total
pre-birth loss in both the F0 and F1 generations, but these
findings were considered not to be related to treatment in the absence
of a consistent relationship between dose and effect. The F0
generation showed statistically significant increases in litter size
and litter weight and decreased mean pup weight at a number of doses
at most sample intervals (Table 11), but these values were generally
only slightly different from those in controls and showed no
consistent relationship with dose. The decrease in mean pup weights
was probably related to the increased litter size. The data for the
litters of the F1 generation were similar for control and treated
groups, with the exception of a single, statistically significant
decrease in litter weight at 50 ppm on day 21. This isolated finding
was considered to be incidental to treatment. No consistent,
dose-related effect on organ weights was reported during the study.
Pathological examination revealed no macro- or microscopic effects
that were related to treatment. Under the conditions of this study, no
adverse treatment-related effects were observed at any dose. The
slight increase in mean body weights of F0 males at the high dose
was the only effect attributed to treatment. The NOAEL was 50 ppm,
equal to 2.5 mg/kg bw per day (James et al., 1988).
Four groups of Sprague-Dawley rats were used in a two-generation
(one litter per generation) study of reproductive toxicity in which
diets containing chlorpyrifos (purity, 97.8-98.5%) at doses of 0, 0.1,
1, or 5 mg/kg bw per day were administered. The study was conducted in
accordance with FIFRA guideline 83-4, OECD guideline 416, and GLP
principles. The F0 parental animals (30 of each sex per dose) were
Table 11. Statistically significant differences in group data for
litters of the F0 generation of rats given diets containing
chlorpyrifos
Time Litter parameter Dose (ppm)
0 2 10 50
At birth Litter size, total 12 13 14** 13
Litter size, live 12 13 14** 13
Litter weight (g) 70 72 79* 71
Pup weight (g) 6.1 5.6*** 5.7** 5.7*
Day 4 Litter size, live 11 13* 14*** 12
Litter weight (g) 100 110 120* 110
Mean pup weight (g) 9.3 8.7 8.6* 9.2
Day 8 Litter size, live 11 13** 13*** 12
Mean pup weight (g) 15 14* 14* 15
Day 12 Litter size, live 11 13** 13*** 12
Litter weight (g) 240 270 280** 250
Mean pup weight (g) 22 21 21* 22
Day 21 Litter size, live 11 13* 13*** 12
Litter weight (g) 460 520 530* 480
Mean pup weight (g) 44 42 40* 42
* p < 0.05; ** p < 0.01; *** p < 0.001
6 weeks of age at the beginning of the study and were mated after 10
weeks of exposure to produce the F1 litters. Groups of 30 rats of
each sex per dose were selected from the F1 weanlings and were
treated for 12 weeks before breeding to produce the F2 litters.
Pairing in both generations allowed for three periods of 7 days'
cohabitation in a 1:1 ratio, the males being changed weekly. Care was
taken to avoid sibling pairings. Females were removed from pairing
when vaginal lavage showed sperm, and this was considered to be day 0
of gestation. The F1 and F2 litters were culled if appropriate to
a total of eight pups on day 4. Clinical observations were performed
daily on all animals; litter size at birth, the numbers of live and
dead pups on days 0, 1, 4, 7, 14, and 21 post partum, and the sex
and weight of each pup were recorded on days 1, 4, 7, 14, and 21 of
lactation. Body weights and food consumption were recorded weekly
before and after breeding. The body weights of dams were recorded on
days 0, 7, 14, and 21 of gestation and on days 1, 4, 7, 14, and 21 of
lactation, whereas food consumption was recorded once during the first
week of lactation, twice during the second week, and every 2-3 days
during the third week. Inhibition of cholinesterase activity in
plasma, erythrocytes, and brain was measured in 10 F0 and F1
parental animals of each sex at necropsy after 19 and 21 weeks of
exposure. Complete necropsies were conducted on all F0 and F1
adults, and included ocular examinations and collection ofmany
tissues. Histopathological examinations were made of the adrenals,
brain, gross lesions, and reproductive tissues (cervix, coagulating
glands, epididymides, ovaries, oviducts, pituitary, prostate, seminal
vesicles, testes, uterus, and vagina) of controls and animals at the
high dose. The livers of 10 F1 parental males in the control and
high-dose groups were also examined microscopically. Only the adrenals
from animals at the intermediate and low doses were examined. Ten F1
and F2 pups of each sex per dose were autopsied grossly. Appropriate
statistical tests were applied to all data.
No significant effects of treatment were seen on clinical signs,
food intake, or body weight. A slight but not statistically
significant decrease in body weight was seen in F1 males at 5 mg/kg
bw per day, and F1 females at this dose showed reduced body-weight
gain during lactation. Plasma cholinesterase activity was inhibited in
parental F0 and F1 rats of each sex at 1 mg/kg bw per day (40-57%)
and at 5 mg/kg bw per day (< 72%). Plasma cholinesterase activity
was also inhibited in animals of both generations at 0.1 mg/kg bw per
day as part of a dose-related trend, although the inhibition was
generally not statistically significant at this dose. Erythrocyte
cholinesterase activity was strongly inhibited at 1 and 5 mg/kg bw per
day, but that of brain was inhibited only at 5 mg/kg bw per day (Table
12).
Gross observation of F0 and F1 parents revealed no
significant alterations, and the only significant histopathological
change in the parental animals was vacuolation consistent with fatty
changes in the adrenal zone fasciculata. Treatment had no effect on
fertility, length of gestation, survival dring gestation, time to
mating, sex ratio, or litter size in either generation. F1 pups at
1 mg/kg bw per day showed slightly decreased body-weight gain during
lactation and statistically significantly decreased body-weight gain
and survival at 5 mg/kg bw per day. No effect of treatment was seen
during gross or daily observation of the F1 weanlings. F2 pups did
not show dose-related decreases in body-weight gain during lactation,
but the survival of pups at 0 and 5 mg/kg bw per day was decreased,
with total loss of three and five litters, respectively; this effect
was stated to be due to maternal neglect, since the stomachs of pups
in these litters contained no milk. No effect of treatment was seen
during gross or daily observation of the F2 weanlings. The NOAEL for
inhibition of erythrocyte cholinesterase in adults was 0.1 mg/kg bw
per day and that for inhibition of brain cholinesterase activity and
materal toxicity was 1 mg/kg bw per day. The NOAEL for developmental
effects was 1 mg/kg bw per day, and the NOAEL for effects on fertility
and reproductive effects was 5 mg/kg bw per day (Breslin et al.,
1991).
Table 12. Mean cholinesterase activity in F0 and F1 parent rats at necropsy (percentage inhibition compared
with controls)
Generation Dose (mg/kg Cholinesterase activity (% inhibition)
bw per day)
Plasma (IU/ml) Erythrocytes (IU/ml) Brain (IU/g)
Male Female Male Female Male Female
F0 0 0.54 2.0 1.1 1.0 9.3 9.0
0.1 0.46 (15%) 1.6 (20%) 1.0 (5%) 1.0 9.2 (1%) 9.1
1 0.30a (44%) 0.8a (59%) 0.3b (92%) 0.36b (65%) 8.8 (6%) 8.7 (3%)
5 0.21a (61%) 0.6a (67%) 0.3b (92%) 0.31b (70%) 4.9b (48%) 4.6a (49%)
F1 0 0.53 1.8 1.1 0.96 9.7 9.4
0.1 0.43 (19%) 1.6 (15%) 0.98a (13%) 0.97 9.7 9.2 (2.5%)
1 0.30a (43%) 0.93a (52%) 0.37a (67%) 0.32b (67%) 9.4 (3%) 9.0 (4.5%)
5 0.19a (64%) 0.52a (72%) 0.33a (70%) 0.24b (75%) 4.6a (52%) 4.0b (58%)
a Statistically significantly different from control mean by Wilcoxon's test
b Statistically significantly different from control mean by Dunnett's test
(ii) Developmental toxicity
Mice
In the range-finding portion of a study of developmental
toxicity, pregnant mice were given chlorpyrifos at doses of up to 60
mg/kg bw per day by gavage. No toxic effects were seen in the dams or
offspring at 3, 10, and 20 mg/kg bw per day. In the main study, groups
of 40-47 pregnant CF-1 mice were initially given chlorpyrifos (purity,
96.8%) at 0, 1, 10, or 25 mg/kg bw per day by gavage in cottonseed oil
on days 6-15 of gestation. The day on which a vaginal plug was
observed was considered to be day 0 of gestation. Owing to severe
maternal toxicity at 25 mg/kg bw per day, additional groups of 35-41
mice were given 0, 0.1, 1, or 10 mg/kg bw per day by gavage on days
6-15 of gestation. The mice were observed for signs of toxicity and
were weighed daily on day 6-16 of gestation and then sacrificed on day
18. At the time of surgical removal of their fetuses, the weights of
the liver and gravid uterus with ovaries were recorded. The uteri of
non-pregnant mice were stained with sodium sulfide to ensure that no
implantations had occurred. The fetuses were weighed, measured
(crown-rump length), sexed, and examined externally. One-third of the
fetuses were dissected to examine the soft tissues. All of the heads
were preserved in Bouin's solution and subsequently sectioned. All
fetuses were stained with Alizarin red for examination of the
skeletons.
Additional mice were added to the study to assess clinical
changes. Cholinesterase (erythrocyte and plasma) activity was measured
in groups of 4-10 mice given 1, 10, or 25 mg/kg bw per day on day 6,
days 6-10, or days 6-15 of gestation, and these measurements were also
made in a group treated with 0, 0.1, 1, or 10 mg/kg bw per day. Blood
was collected for assay of cholinesterase 5 h after dosing on days 6,
10, and 15 of gestation, respectively. A homogenate of fetal tissue
was prepared from litters of mice killed on day 15 of gestation. Data
were not provided on the body weights or food and water consumption of
the animals in these groups.
Appropriate statistical analysis was applied to the experimental
data: fetal body weights and body measurements, maternal body weights,
weights of maternal livers and uteri, and food and water consumption
were analysed by one-way analysis of variance and Dunnett's test. The
incidences of fetal resorptions and alterations were analysed by a
modified Wilcoxon test. The Fisher exact test was applied to other
data.
In the dose-ranging study, severe cholinergic signs and
consequent reproductive failure were found at doses > 30 mg/kg bw
per day. In the first main study, dose-related increases in the
incidence of clinical signs including mortality, excessive salivation,
tremors, urine-soaked coat, ataxia and lethargy were found at 10 and
25 mg/kg bw per day. A single death and isolated clinical signs at
1 mg/kg bw per day were considered not to be related to treatment.
Body-weight gain was reduced at 25 mg/kg bw per day, but no effects
were observed on litter size, resorption incidence, or sex ratio.
Fetal body weight and crown-rump length were significantly reduced at
25 mg/kg bw per day. Plasma cholinesterase activity was depressed in a
dose-related manner, while the activities in erythrocytes and fetal
tissue homogenate were inhibited at 10 and 25 mg/kg bw per day (Table
13). Exencephaly was reported in all groups, but with no dose-response
relationship, and a statistically significant incidence of delayed
ossification was seen at 25 mg/kg bw per day (Table 14).
The second main study showed no cholinergic signs in dams at any
dose up to 10 mg/kg bw per day. Body weight, food intake, maternal
liver weights, gravid uterine weight, and adjusted body weights were
also unaffected. Cholinesterase activity was rapidly and significantly
inhibited in plasma and erythrocytes at 1 and 10 mg/kg bw per day but
was depressed in fetal tissue homogenates only at the high dose. No
effects were observed on materna1 parameters or on litter size, the
incidence of resorptions, the incidence of dead fetuses, the sex
ratio, fetal body weight, or crown-rump length (Table 15). Significant
fetal alterations are shown in Table 16.
Neither study provided evidence of teratogenic activity at any
dose, but fetal toxicity was seen at 25 mg/kg bw per day. The NOAEL
for developmental toxicity was therefore 10 mg/kg bw per day. The
NOAEL for inhibition of erythrocyte cholinesterase activity was 0.1
mg/kg bw per day, and the NOAEL for maternal toxicity was 1 mg/kg bw
per day (JMPR, 1982; modified by reference to the original reports of
Deacon et al., 1979, 1980).
Rats
In a range-finding study of developmental toxicity, which was
given a statement of quality assurance, Fischer 344 rats were fed
chlorpyrifos (purity, 96.6%) by gavage at doses of 0, 3, 10, 15, or
30 mg/kg bw per day during organogenesis. Slight maternal toxicity was
observed at 15 mg/kg bw per day, and severe toxicity was observed at
30 mg/kg bw per day, with typical cholinergic signs of excessive
salivation, lachrymation, urination, defaecation, and body tremors and
general observations of unkempt appearance, decreased body size, and
matting of perineal and facial hair. Deaths were also seen at 30 mg/kg
bw per day, and animals in this group had decreased body-weight gain,
decreased food consumption, enlarged adrenal glands, decreased liver
weight, and shrunken thymus glands. Both plasma and erythrocyte
cholinesterase activities were depressed at all doses. Maternal
toxicity was thus observed at all doses, but fetal toxicity was seen
only at 30 mg/kg bw per day (Ouelette et al., 1983a).
In the main study, which also had a statement of quality
assurance, groups of 31-33 pregnant Fischer 344 rats were given
chlorpyrifos (purity, 96.6%) by gavage in corn oil at doses of 0, 0.1,
3, or 15 mg/kg bw per day on days 6-15 of gestation. They were mated
1:1, and the day sperm were observed in a vaginal smear was considered
to be day 0 of gestation. The rats were observed daily for signs of
toxicity, were weighed daily on days 6-16 and day 21 of gestation, and
Table 13. Significant findings in mice and offspring given chlorpyrifos by gavage on days 6-15 of
gestation
Finding Dose (mg/kg bw per day)
0 1 10 25
No. of females 51 40 44 47
No. of animals with cholinergic signs 0 2 9 32
Deaths 0 1 1 4
Total pregnanta 40 (4) 31 (1) 32 (1) 34 (1)
No. with litters 36 29 30 29
Maternal body weight gain (g)b 21 ± 5 22 ± 3 22 ± 4 18 ± 4*
No. of implantation sites per dam 13 ± 3 13 ± 2 13 ± 1 12 ± 3
No. of fetuses per litter 11 ± 3 12 ± 2 12 ± 2 11 ± 3
Fetal body weight (g) 1.14 ± 0.10 1.13 ± 0.07 1.15 ± 0.08 1.03 ± 0.17*
Fetal crown-rump length (mm) 25.0 ± 1.2 25.2 ± 0.9 25.3 ± 0.9 24.4 ± 1.6*
Plasma cholinesterase activityc 100 (10) 15 (7) 4 (8) 2 (9)
Erythrocyte cholinesterase activityc 100 (10) 86 (6) 57 (9)* 43 (9)*
Fetal homogenate cholinesterase activity 100 81 65 35
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining (not resulting in
litters).
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals
Table 14. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 1 10 25
No. of fetuses examined 408 347 359 326
Exencephalya 1/1 5/5* 1/1 4/3
Ablephariaa 1/1 1/1 1/1 2/2
Cleft palatea 0 2/2 1/1 2/1
Small cerebral hemispherea 1/1 1/1 1/1 0
Multiple skeletal malformationsa 0 0 1/1 0
Fused ribsa 1/1 0 1/1 0
Skull: delayed ossification 5/5 9/5 10/6 34/10*
Sternebrae: delayed ossification 37/17 24/11 19/11 78/22*
Sternebrae: unfused 2/2 7/6* 3/3 8/7*
Sternebrae: fused 24/16 9/16* 23/12 6/5*
* Significantly different from control group
a Considered to be a major malformations
were sacrificed on day 21. At the time their fetuses were surgically
removed, the body and liver weights were recorded. The number of
corpora lutea and the number and position of live and resorbed fetuses
were recorded at necropsy. Fetuses were sexed, examined, and measured.
The uteri of non-pregnant rats were stained with sodium sulfide to
determine whether implantations had occurred in the absence of
litters. Separate groups of 10 rats were dosed on days 6-15 of
gestation and killed, when samples of maternal blood were collected by
heart puncture to measure erythrocyte and plasma cholinesterase
activity. Appropriate statistical analysis of the data was reported.
There were no unscheduled deaths. Clinical signs of maternal
toxicity were found, including excessive salivation, urine staining in
the perineal region, porphyrin deposits around the eyes, and vaginal
bleeding. Tremors were observed only at 15 mg/kg bw per day, and
animals at this dose also had decreased body-weight gain; no other
maternal findings were reported at necropsy. Both plasma and
erythrocyte cholinesterase activities were depressed at doses of 3 and
15 mg/kg bw per day (Table 17). No adverse effects were observed on
reproductive parameters, and no teratogenicity was observed.
Variations and malformations were found which were distributed
randomly by dose, and no effect of treatment on skeletal development
was seen. The NOAEL for maternal toxicity was 0.1 mg/kg bw per day, on
the basis of inhibition of erythrocyte cholinesterase activity in all
adult animals at higher doses. No fetal toxicity was observed
(Ouelette et al., 1983b).
Table 15. Significant findings in mice and offspring given chlorpyrifos by gavage on days
6-15 of gestation
Finding Dose (mg/kg bw per day)
0 0.1 1 10
No. of females bred 41 35 36 35
Total no. pregnanta 30 (0) 25 (1) 23 (0) 28 (3)
No. with litters 30 24 23 25
Maternal body weight gain (g)b 12 ± 6 14 ± 5 14 ± 5 14 ± 5
No. of implantation sites per dam 10 ± 3 10 ± 3 10 ± 3 9 ± 4
No. of fetuses per litter 8 ± 3 7 ± 3 7 ± 3 7 ± 3
Fetal body weight (g) 1.04 ± 1.11 1.07 ± 0.15 1.08 ± 0.18 1.09 ± 0.12*
Fetal crown-rump length (mm) 24.4 ± 1.1 24.8 ± 1.6 24.9 ± 2.1 24.8 ± 1.2*
Plasma cholinesterase activityc 100 (8) 86 (6) 25 (7)* 3 (6)*
Erythrocyte cholinesterase activityc 100 (8) 88 (6) 75 (7)* 50 (6)*
Fetal homogenate cholinesterase activityc 100 84 95 77
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals.
Table 16. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 0.1 1 10
Exencephalya 1/1 1/1 0 1/1
Ablephariaa 1/1 0 0 0
Cleft palatea 0 0 1/1 0
Omphalocelea 0 0 0 1/1
Skull: delayed ossification 18/10 11/6 3/2 3/3
Sternebrae: delayed ossification 50/20 25/13 25/13 17/11
Sternebrae: unfused 3/3 6/5 0 1/1
Sternebrae: fused 13/7 9/7 3/3 12/8
a Considered to be major malformations
Female CD Sprague-Dawley rats were mated 1:1 with stock males
from the same source and strain. Technical-grade chlorpyrifos (stated
purity, 96.1%) was formulated daily as solutions in maize oil and
given to groups of 32 mated females in a volume of 5 ml/kg bw by oral
gavage at doses of 0.5, 2.5, or 15 mg/kg bw per day on days 6-15
post coitum. A further group received maize oil only. This study was
conducted in accordance with US Food and Drug Administration and
Environmental Protection Agency guidelines for GLP and testing
guidelines of the OECD (No. 414) and US Environmental Protection
Agency (section 83-3). All females were examined daily for signs
associated with treatment. Animals were weighed on days 0, 3, 6-15,
17, and 20 of gestation, and food consumption was measured twice
weekly. Ten mated females were bled on day 15 for measurement of
plasma cholinesterase activity, blood samples being obtained from the
retro-orbital sinus under ether anaesthesia. The same procedure was
repeated for an additional 10 animals per dose immediately before
necropsy on day 20. The animals used for cholinesterase determinations
on day 15 were discarded without necropsy after being bled. All
remaining animals were killed on day 20, and the following were
recorded for each animal: any macroscopic abnormality of the
reproductive tract; number of corpora lutea in each ovary; weight of
gravid uterus; distribution of live and dead fetuses and distribution
of resorption sites in each uterine horn; individual placental
weights; individual fetal weight, length, and sex; and external
anomalies of individual fetuses. The thoracic and abdominal contents
of approximately one-half of each litter were dissected and examined,
and these fetuses were used for skeletal staining and evaluation; the
remaining fetuses were used for preparation of free-hand sections and
examination of visceral organs.
Table 17. Adverse effects in rats given chlorpyrifos by gavage
Finding Dose (mg/kg bw per day)
0 0.1 3 15
No. of females 31 32 33 31
No. of animals with cholinergic signs 0 0 0 12
Total no. pregnanta 30 (0) 28 (0) 26 (0)b 29 (1)
No. of litters 29 26 24 26
Maternal body weight gain (g)c 65 ± 11 64 ± 14 71 ± 12 58 ± 11
No. of implantation sites per dam 11 ± 2 10 ± 2 10 ± 2 10 ± 3
No. of fetuses per litter 10 ± 3 9 ± 2 10 ± 3 9 ± 3
Mean fetal body weight (g) 4.28 4.37 4.52* 4.46*
Mean fetal crown-rump length (mm) 43.91 43.93 43.96 43.64
Plasma cholinesterase activity (IU/ml)d 46.0 42.8 (93%) 4.9 (11%) 1.5 (3%)
Erythrocyte cholinesterase activity (IU/ml)d 12.1 11.9 (98%) 3.1 (26%) 2.5 (21%)
* Significantly different from control
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Two dams removed during dosing for reasons not related to treatment
c Days 6-20
d Mean of 6-8 dams on day 15, IU/ml (percentage of control)
No treatment-related deaths occurred during the study, and
clinical signs of intoxication were confined to tremors late in the
study in three animals at 15 mg/kg bw per day. Slight but
statistically significant decreases in mean food consumption (8%
reduction during day 79 only) and body-weight gain were seen at
15 mg/kg bw per day. Statistically significant, dose-related decreases
in plasma cholinesterase activity were observed at all doses;
erythrocyte and brain cholinesterase activities were not determined.
The effects on litters are summarized in Table 18. The total live
litter sizes were unaffected by treatment, but at 15 mg/kg bw per day
the mean number of live male fetuses was statistically significantly
decreased and post-implantation loss was slightly elevated.
Statistically significant increases in pre-implantation loss were seen
at all doses, but there was no consistent dose-response relationship
and the incidence was within historical control values for this
laboratory; the finding was considered not to be related to treatment.
At 15 mg/kg bw per day, slight but statistically significant increases
were seen in mean fetal weight and mean crown-rump length, but as the
increase in fetal weight was not accompanied by a decrease in litter
size, this finding may have been due to advanced fetal development and
was considered to be of no toxicological significance. Similarly, the
statistically significant decrease in the incidence of small fetuses
observed in all test groups was considered not to be toxicologically
significant. A number of minor structural variations were reported,
including a single incidence of anophthalmia at 0.5 mg/kg bw per day
and instances of microphthalmia at 0.5 and 2.5 mg/kg bw per day, but
the incidence of such findings was low and/or were not related to
dose. These findings were therefore considered to be incidental to
treatment. Skeletal examination showed no adverse treatment-related
findings. The NOAEL for frank maternal toxicity was 2.5 mg/kg bw per
day, on the basis of reduced body weight, tremors, and transient
reductions in food consumption at 15 mg/kg bw per day. The NOAEL for
fetal toxicity was 2.5 mg/kg bw per day, on the basis of a slight
increase in post-implantation loss at 15 mg/kg bw per day, probably
associated with maternal toxicity (Rubin et al., 1987a).
Rabbits
Groups of 14 female New Zealand white rabbits were mated 1:1 with
stock males from the same source and strain and given technical-grade
chlorpyrifos (stated purity, 96.1%), formulated daily as solutions in
maize oil, by oral gavage at doses of 1, 9, 81, or 140 mg/kg bw per
day (in a volume of 2 ml/kg bw) on days 7-19 post coitum. The doses
for animals at 81 and 140 mg/kg bw per day were prepared directly, and
those for the animals at 1 and 9 mg/kg bw per day were prepared by
diluting the dose of 81 mg/kg bw per day with maize oil. A further
group received maize oil only. This study was conducted in accordance
with US Food and Drug Administration and Environmental Protection
Agency guidelines for GLP and testing guidelines of the OECD (No. 414)
and US Environmental Protection Agency (section 83-3). The doses were
selected after a preliminary range-finding study. On day 15, three
does at 81 mg/kg bw per day were accidentally given a dose of
140 mg/kg bw, and the group was expanded to 21 animals so that the
overdosed animals could be excluded from the study if that was found
to be warranted. Two of the three animals were pregnant, but no
apparent difference was found from the females treated at 81 mg/kg bw
per day, and the maternal and fetal data for these animals were
included in the report. All animals were examined daily for clinical
signs associated with treatment. Any animals found dead or killed
in extremis were autopsied to determine the cause of death. Animals
were weighed on days 0, 3, 7-19, 22, 25, and 29 of gestation, and food
consumption was determined twice weekly. Plasma cholinesterase
activity was measured before mating and again after at least 10 days
of dosing. The does were killed on day 29 and the following were
recorded: any macroscopic abnormality of the reproductive tract; the
number of corpora lutea in each ovary; the weight of gravid uterus;
the distribution of live and dead fetuses and of resorption sites in
each uterine horn; individual placental weights; individual fetal
weight and crown-rump length; sex; and external anomalies of
individual fetuses. The thoracic and abdominal contents were dissected
and examined, and the skull of each fetus was sectioned transversely
through the frontal-parietal suture, and the brain examined. Each
fetus was used for skeletal staining and evaluation.
Table 18. Effects on fetuses of rats given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 0.5 2.5 15
Pre-implantation loss (%) 8.6 12.2*** 11.5*** 11.4***
Post-implantation loss (%) 7.0 6.0 5.8 9.0**
Mean fetal weight (g) 3.33 3.40 3.44 3.51**
Mean crown-rump length (mm) 36.0 36.2 36.3 36.7**
Live fetuses:
Male 7.6 8.0 6.8 6.0*
Female 6.8 7.0 7.3 7.5
Total 14.4 15.0 14.1 13.4
Small fetuses (no. observed) 48 (317) 25 (315)** 20 (311)*** 13 (282)***
Ablepheron (no. observed) 0 (317) 0 (315) 1 (311) 0 (282)
Major cranio-facial malformations: 0 (317) 1 (315) 0 (311) 0 (282)
proboscis, anophthalmia (no. observed)
Hydronephrosis (no. observed) 0 (161) 0 (158) 1 (158) 0 (146)
Hydroureter 0 (161) 0 (158) 1 (158) 0 (146)
Anophthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
Microthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
No treatment-related deaths were observed during the study, and
no treatment-related clinical signs were reported. Maternal group mean
food consumption was unaffected by treatment, and group mean body
weights and body-weight gains were not affected by treatment at doses
up to 81 mg/kg bw per day. At 140 mg/kg bw per day, body-weight gain
was inhibited during most of the treatment period, although it was
greater than normal after cessation of treatment, and by day 29 the
animals in this group had attained body weights similar to those in
the control group. Statistically significant, dose-related decreases
in plasma cholinesterase activity were seen at all doses after 10 days
of treatment, with inhibition of 56% at 1 mg/kg bw per day, 68% at 9
mg/kg bw per day, 70% at 81 mg/kg bw per day, and 72% at 140 mg/kg bw
per day compared with controls. Erythrocyte and brain cholinesterase
activities were not determined.
Statistically significant decreases in pre-implantation loss were
observed at most doses, but this finding was considered not to be
toxicologically significant. Statistically significant increases in
post-implantation loss were observed at 9 and 140 mg/kg bw per day but
not at 81 mg/kg bw per day. In the absence of a consistent
dose-response relationship, the toxicological significance of this
finding was unclear; no historical control data were supplied for this
effect from the laboratory. A slight but statistically significant
decrease in mean fetal crown-rump length and a decrease in mean fetal
weight were observed in animals at 140 mg/kg bw per day, and these
effects were considered to be related to treatment. A number of fetal
anomalies were observed but were considered not to be
treatment-related as the incidence was generally low and unrelated to
dose. An increased incidence of fetuses with fifth sternebra and/or
unossified xiphisternum seen at 140 mg/kg bw per day was considered to
be related to slightly delayed development at this dose, as indicated
by reduced fetal weight and length in this group. Given the variation
in the incidence of other skeletal observations in treated groups,
however, this finding may have been due to chance. The NOAEL for frank
maternal toxicity was 81 mg/kg bw per day, on the bais of decreased
body-weight gain at the higher dose. The NOAEL for fetal toxicity was
81 mg/kg bw per day, on the basis of a slight decrease in mean fetal
crown-rump length, decreased mean fetal weight, and an increased
incidence of fetuses with fifth sternebra and/or unossified
xiphisternum at the higher dose (see Table 19) (Rubin et al., 1987b).
(f) Special studies: Neurotoxicity
Chickens
No signs of delayed ataxia or paralysis were reported in groups
of three hens treated with single oral doses of chlorpyrifos at 40,
75, 100, or 150 mg/kg bw. The two higher doses resulted in deaths and
other clinical signs of toxicity (Stevenson, 1966a). Similarly, groups
of 10 hens given single oral doses of chlorpyrifos at up to 100 mg/kg
bw did not display delayed neurotoxicity, and no neuropathological
findings associated with treatment were detected (Rowe et al., 1978).
In another study, single oral doses of chlorpyrifos at up to five
Table 19. Effects on litters and fetuses of rabbits given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 1 9 81 140
Number of fetuses examined 108 117 126 142 99
Pre-implantation loss (%) 15.2 13.1 8.9*** 11.9* 11.5*
Post-implantation loss (%) 6.5 8.6 13.8*** 8.7 13.9***
Mean fetal weight (g) 45.4 44.3 43.3 41.8 40.7
Mean crown-rump length (mm) 97.4 95.9 95.0 94.2 93.2*
Teeth absent 0 0 1 0 0
Arthrogryposis, forelimb 0 0 2 0 0
Irregularly shaped thorax 0 0 0 0 1
Hydrocephalus 0 0 1 0 0
Enlarged aortic arch 0 0 1a 0 0
Rudimentary pulmonary artery 0 0 1a 0 0
Agenesis of interventricular cardiac septum 0 0 1a 0 0
Hydronephrosis (bilateral) 2 0 0 4 1
Hydronephrosis (unilateral) 2 2 1 2 1
Diffuse opacity of eye 0 0 0 2 0
No. of fetuses examined for skeletal defects 108 117 126 142 99
Reduced or incomplete ossification of interparietal bone 3 4 3 5 2
Unossified interparietal bone 0 1 0 0 3
Reduced or incomplete ossification of hyoid bone 2 10* 17*** 16** 8*
Irregular ossification of one or more of sternebrae 1-4 1 2 7* 8* 1
Bony plaque adjacent to sternebra 10 6 2** 3* 1**
Unossified fifth sternebra and/or xiphisternum 1 1 2 3 6*
Multiple malformations of ribs and lumbar spine 0 0 0 0 1
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
times the oral LD50 in hens (> 150 mg/kg) resulted in significant
inhibition of neuropathy target esterase and cholinesterase activity
(Capodicasa et al., 1991), with signs consistent with
organophosphate-induced delayed neuropathy. Extensive, aggressive
antidotal treatment, both before and throughout the treatment and
recovery periods, was required for the birds' survival.
Technical-grade chlorpyrifos (purity, 96.8%) was tested for acute
toxicity and delayed neurotoxicity in adult domestic hens in a study
carried out according to GLP. In the assessment of neurotoxicity,
groups of 10 birds were given corn oil, tri- ortho-cresyl phosphate
(positive control; 20% in corn oil; 500 mg/kg), chlorpyrifos
(110 mg/kg bw) plus atropine sulfate (intramuscular injection;
10 mg/kg bw), chlorpyrifos (110 mg/kg bw; 5.5% in corn oil) plus
pyridine 2-aldoxime methane sulfonate (intramuscular injection;
50 mg/kg bw; 5% aqueous solution), or were not treated and were
maintained as a source of additional birds if necessary. The hens were
observed for 21 days, at which time all those given the corn oil were
given a second dose, and all treated birds were given chlorpyrifos,
followed by another 21-day observation period.
No clinical signs of toxicity were observed in those given the
vehicle. In birds given tri- ortho-cresyl phosphate, no signs of
toxicity were observed immediately after dosing, but all birds
developed signs of delayed locomotor ataxia as early as day 11 after
treatment. The birds given chlorpyrifos showed signs of toxicity after
either dose, including subdued appearance, unsteadiness, inability to
stand, and weakness. Deaths were observed in both chlorpyrifos-treated
groups, four birds that died after the first dose with atropine
sulfate (no deaths after the second dose) and three after the first
dose of chlorpyrifos with pyridine 2-aldoxime methane sulfonate (one
death after the second dose). Surviving birds recovered completely
within 5-8 days of treatment. Additional doses of atropine sulfate
were given to several birds in both groups treated with chlorpyrifos
owing to the severity of the clinical signs observed.
Histopathological examination revealed a low incidence of minor
neurological changes (grade II on a scale of I-V of increasing
severity) in several birds given the vehicle, which was considered to
show a normal background incidence of axonal degeneration. Most of the
birds given tri- ortho-cresyl phosphate had grade III changes at at
least one level of spinal cord and grade III or IV changes in at least
one level of peripheral nerve, indicating significant
treatment-related axonal degeneration. Three birds treated with
chlorpyrifos showed grade II changes at one level of peripheral nerve
only, and these findings were considered to be similar to the
incidence and severity of changes seen in the vehicle controls. Thus,
oral administration of a single dose of chlorpyrifos at 110 mg/kg bw
to hens and another dose after 21 days did not induce clinical signs
of delayed neurotoxicity, as confirmed by histopathological
examination of nervous tissues (Roberts et al., 1987)
When chlorpyrifos was given to chickens for 91 days in the diet
at doses of < 10 mg/kg bw per day in a study conducted according to
GLP, the only compound-related clinical signs were transient, slight
gait alterations in a number of birds at the high dose. No
histopathological lesions of nerve tissues characteristic of
organophosphate-induced delayed neuropathy were seen. Concurrent
positive controls showed both toxic signs and histopathological
lesions of nerve tissues typical of those induced by a delayed
neurotoxicant (Barna-Lloyd et al., 1986).
To determine whether repeated doses of chlorpyrifos at the
maximum tolerated dose caused cumulative inhibition of neuropathy
target esterase and organophosphate-induced delayed neuropathy,
chlorpyrifos (purity, 100%) was given orally to 15 white Leghorn hens,
18 months of age, for 20 days at a dose of 10 mg/kg bw per day. Corn
oil was given to a control group of 18 birds. Physical observations
(including posture and gait) were made daily. Three hens per group
were killed on days 0 (controls only), 4, 10, 15, 20, and 48, and
brain acetylcholinesterase, plasma butyryl cholinesterase, and
lymphocyte and brain neuropathy target esterase were assayed. Body
weights were decreased during treatment, by 25% when compared with
controls on day 20, but had recovered to about 87% of that of controls
by the end of the study. Some birds showed acute effects (staggering
gait and diarrhoea) during week 1 of treatment. Diarrhoea was also
seen in controls and may have been associated with the corn oil. No
behavioural or perching defects were seen during the observation
period. During days 4-20, brain cholinesterase activity was depressed
by 58-70% and plasma cholinesterase activity by 49-80% when compared
with control values, but after the 4-week observation period brain
cholinesterase activity had recovered to 86% and plasma cholinesterase
to 134% of the controls values; neuropathy target esterase activity in
brain and lymphocytes throughout the study was 82-99% and 85-128% of
that in controls, respectively. This finding was not statistically
significant for lymphocytes, but the 18% inhibition of brain
neuropathy target esterase activity on days 10 and 20 was
statistically significant. There were no indications of delayed
neurotoxicity after repeated administration of a dose that caused
significant inhibition of brain cholinesterase activity and loss of
body weight (Richardson et al., 1993a).
In order to clarify the magnitude of the neuropathic risk
associated with exposure to chlorpyrifos, groups of three 18-month-old
white Leghorn hens were given single oral doses of chlorpyrifos
(purity, 100%) at 0, 75, 150, or 300 mg/kg bw with atropine (20 mg/kg
bw subcutaneously at the time of the first dose of chlorpyrifos and
additional doses as needed to maintain the hens for 4 days). Four days
after treament, when the hens were killed, brain acetylcholinesterase
activity was depressed by 0, 58, 75, and 86% and neuropathy target
esterase activity by 0, 21, 40, and 77% at the three doses,
respectively. In a further study, 18 hens were given corn oil alone
and 15 atropine-protected hens were given chlorpyrifos at a single
oral dose of 150 mg/kg bw. Three controls were killed on day 0, and
three treated and three control hens were killed on days 1, 2, 4, 8,
and 16. Brain acetylcholinesterase activity was inhibited by 86, 82,
72, 43, and 29% on days 1, 2, 4, 8, and 16 after treatment, and brain
neuropathy target esterase activity was inhibited by 30, 28, 38, 29,
and 6%. No signs of organophosphate-induced delayed neuropathy were
observed.
In studies in vitro in which chlorpyrifos-oxon was added to
whole brain homogenate, the rate constant for the enzyme-inhibitor
complex was 15.5 per mm per min for acetylcholinesterase and 0.145 per
mm per min for neuropathy target esterase. The calculated 20-min
fixed-time IC50 values were 2.2 nmol/L for acetylcholinesterase and
240 nmol/L for neuropathy target esterase. The measured IC50 values
based on fixed-time (20 min) preincubation were 2.2 nmol/L for
acetylcholinesterase and 210 nmol/L for neuropathy target esterase.
The ratio of the calculated values for neuropathy target
esterase:acetylcholinesterase is 110. As a ratio of > 1 generally
indicates that the dose that would be required to cause
organophosphate-induced delayed neuropathy would be in excess of the
LD50, acute exposure to chlorpyrifos, except at doses well in excess
of the LD50, would not be anticipated to induce this condition
(Richardson et al., 1993b).
The potential of chlorpyrifos to cause acute delayed
neurotoxicity was tested in white Leghorn hens given doses < 12
mg/kg bw per day for 21 days by oral gavage in peanut oil. Dose- and
time-related increases in the incidence and severity of ataxia were
observed. Plasma and whole blood cholinesterase activities were
reduced at 5 and 12 mg/kg bw per day. Ataxia was observed at 2, 5, and
12 mg/kg bw per day. Necropsy did not reveal any findings associated
with treatment. Histopathological examination of the brain and spinal
cord revealed a low incidence of several lesions, with no relation to
dose, and these findings were considered to be incidental to
treatment. Since continued administration of chlorpyrifos resulted in
ataxia, it would have been difficult to observe any delayed
neurological effects had they occurred (Jai Research Foundation,
1996b).
Rats
In rats, single oral doses of chlorpyrifos at 50-100 mg/kg bw
resulted in treatment-related effects and clinical signs for several
days after dosing. No deaths occurred at any dose. At 10 mg/kg bw,
effects were confined to isolated observations of perineal staining
and/or decreased activity. No neuropathological lesions were reported
(Wilmer, 1992; Mattsson et al., 1996).
In a study in adult Long-Evans rats carried out in accordance
with GLP, oral administration of chlorpyrifos at doses < 10 mg/kg
bw per day for 4 weeks resulted in dose-related inhibition of plasma
and erythrocyte cholinesterase activity at > 1 mg/kg bw per day and
of brain cholinesterase activity at > 3 mg/kg bw per day. These
effects were accompanied by clinical signs of intoxication at 10 mg/kg
bw per day. Cognitive behavioural tests indicated some non-cognitive
changes associated with impaired motor activity at 10 mg/kg bw per
day, but no clear treatment-related effects on cognitive function were
observed (Maurissen et al., 1996).
After oral administration of chlorpyrifos to rats at single doses
< 100 mg/kg bw in a study that conformed to GLP, no
treatment-related effects on neuropathy target esterase activity were
observed at any dose. Plasma, erythrocyte, and heart cholinesterase
activity was statistically significantly lower than that in controls
at doses > 5 mg/kg bw, and brain cholinesterase activity was
decreased at > 50 mg/kg bw. No significant inhibition was observed
at 0.5 or 1 mg/kg bw (Dittenber, 1997).
The neurotoxic potential of chlorpyrifos (purity, 99.5%) was
assessed in groups of 20 male Long-Evans hooded rats, approximately 70
days old with a mean body weight of 350 g. The test material was
dissolved in corn oil and given to the rats by oral gavage in a volume
of 1 ml/kg bw at doses of 0, 10, 30, 60, or 100 mg/kg bw. All rats
were evaluated for behavioural signs 3.5 h after dosing with a
functional observational battery of tests consisting of evaluations of
home cage, open-field, and manipulative neurobehaviour. The measures
were autonomic activity (lachrymation, salivation, miosis,
defaecation), motor activity (rearing, home cage posture), convulsive
activity (tremors), neuromuscular activity (gait score, landing foot
splay, righting reaction), general reactivity (arousal, removal
reactivity), sensorimotor activity (responses to tail pinch, click,
approach), and physiological reactions (body temperature, body
weight). Data on motor activity were recorded during a 1-h session in
a maze shortly after testing. Tissue samples were obtained after the
motor activity tests, 4.5 and 25 h after dosing. Half of the animals
in each group were killed at 3.5 h, and the remaining 10 animals were
examined again at 24 h and then killed. Immediately after sacrifice,
the brain, retina, liver, heart, diaphragm, and quadriceps femoris
muscle were collected from all animals. The brain was sectioned
mid-sagitally, and the remaining half-brain was further sectioned to
obtain the frontal cortex, hippocampus, striatum, hypothalamus,
cerebellum, and pons/medulla. Blood was collected from the trunk.
Muscarinic receptor density was determined in all brain regions and in
the heart and retina. Cholinesterase activity was determined in brain,
muscle, retina, plasma, erythrocytes, whole blood, and liver.
Cholinesterase activity was significantly inhibited in a range of
tissues, including brain, plasma, and erythrocytes, at all doses at
both 4.5 and 25 h (Table 20). As the data were represented only as
graphs, the percentages are approximations. Erythrocyte cholinesterase
activity was slightly more sensitive than that of plasma at the doses
used in this study, and plasma cholinesterase activity recovered more
quickly than that of erythrocytes. The degree of inhibition of
whole-blood cholinesterase activity lay between that of plasma and
erythrocyte activity at both 4.5 and 25 h. Whole-brain cholinesterase
activity can be considered representative of that in various regions
of the brain, although a number of regions showed less inhibition than
was measured in the half-brain in this study. In peripheral tissues,
Table 20. Percentage inhibition of cholinesterase activity compared with controls in rats given chlorpyrifos by gavage
Time (h) Dose Inhibition of cholinesterase activity (%)
(mg/kg bw)
Plasma Erythrocytes Blood Half-brain Retina Heart Diaphragm Quadriceps Liver
4.5 10 80 90 85 40 40 55 40 5 70
30 85 95 90 70 65 65 55 20 70
60 85 100 90 85 80 70 70 70 80
100 90 100 95 90 90 75 85 85 85
2.5 10 35 75 55 30 30 40 5 + 10 25
30 75 90 85 60 60 75 35 20 70
60 95 100 95 80 75 90 65 35 85
100 95 100 95 85 85 90 75 55 85
When inhibition was > 20% in comparison with corresponding controls, the change in cholinesterase activity was
statistically significant ( p < 0.05).
the inhibition was much lower in the quadriceps than in other tissues
at 4.5 h, and inhibition of heart, liver, and retina cholinesterase
activity was similar at 25 h.
Clinical and behavioural signs were observed at doses
> 30 mg/kg bw at 4.5 h. The doses selected for this study resulted
in a skew of some correlations, especially for cholinesterase
inhibition in blood and blood components, with behavioural and
clinical findings. In order to examine the relationship between
clinical and behavioural findings and cholinesterase inhibition, the
authors grouped rats according to the extent of cholinesterase
inhibition (instead of by dose) and indicated the incidence of
cholinergic signs related to individual cholinesterase inhibition. The
10 'cardinal' cholinergic signs that were used for this analysis were
temperature, gait, motor activity, smacking, foot splay, tail pinch,
tremors, lachrymation, pupil response, and salivation (Table 21). None
of these signs was observed in the 18 animals with < 60% inhibition
of brain cholinesterase activity. The incidence of these findings
increased in relation to brain cholinesterase inhibition. In animals
with < 80% inhibition of whole-blood cholinesterase activity, no
signs were observed, while all of the signs were seen at varying
incidences in animals with 90-100% inhibition. The correlation between
these end-points and inhibition of cholinesterase in other tissues or
in erythrocytes or plasma was not provided. These results were
interpreted by the authors as indicating a threshold effect, since no
clinical signs were observed until a certain percentage inhibition of
cholinesterase activity was reached. Because of the doses selected for
this study, marked inhibition was observed at the lowest dose in the
absence of clinical findings. The use of a single dose also limited
the usefulness of the findings. No clinical signs were seen when brain
cholinesterase activity was inhibited by < 60% or when whole blood
cholinesterase activity was inhibited by < 80% after a single dose of
chlorpyrifos. The data do not suggest that these values could be
extrapolated to repeated doses (Nostrandt et al., 1997).
Technical-grade chlorpyrifos (purity, 98.1%) was given to groups
of 10 male and 10 female Fischer 344 rats in the diet for 13 weeks at
doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day. The dietary
concentrations were adjusted on the basis of measured weight and food
consumption, but the range of dietary concentrations used to achieve
these target doses was not provided. The study was conducted in
accordance with GLP requirements and to meet US Environmental
Protection Agency FIFRA guideline No. 82-7. Body weights and feed
consumption were measured before treatment and weekly during
treatment. Ophthalmic examinations were conducted before treatment and
at necropsy. Cage-side observations were made twice daily and clinical
examinations weekly. A functional observational battery of tests was
applied to all rats before treatment and during weeks 4, 8, and 13 of
treatment. Five rats of each sex per dose were examined for gross
pathological alterations. Tissues for histopathological evaluation
were prepared only from rats in the control and high-dose groups. Nine
transverse sections of the brain included the olfactory bulb, cerebral
cortex (frontal, parietal, temporal, and occipital lobes),
Table 21. Incidence (%) of cardinal cholinergic signs in rats given chlorpyrifos by gavage and grouped according to
extent of inhibition of cholinesterase activity (numbers of rats in parentheses)
End-point Inhibition of brain cholinesterase activity (%) Inhibition of blood cholinesterase activity (%)
90-100 (10) 80-90 (8) 70-80 (7) 60-70 (7) < 60 (18) 90-100 (25) 80-90 (14) < 80 (11)
Temperature 100 100 86 57 0 92 36 0
Gait 100 100 57 71 0 84 43 0
Motor activity 90 88 86 57 0 84 36 0
Smacking 90 63 57 0 0 64 14 0
Foot splay 40 38 29 0 0 32 14 0
Tail pinch 70 38 0 0 0 40 0 0
Tremors 90 25 0 0 0 44 0 0
Lachrymation 80 13 0 0 0 32 7 0
Pupil response 60 0 0 0 0 24 0 0
Salivation 30 0 0 0 0 12 0 0
thalamus/hypothalamus, midbrain, pons, cerebellum, medulla oblongata,
and nucleus gracilis/cuneatus. Other tissues examined were the
trigeminal ganglion and nerve, pituitary, eyes (retina and optic
nerve), spinal cord (cervical and lumbar), olfactory epithelium, and
skeletal muscles (gastrocnemius and anterior tibial).
A low incidence of perineal staining was seen in females at 5 and
15 mg/kg bw per day. No decrease in body weight was recorded at any
dose. Gross pathological examination did not reveal any
treatment-related effects. Histopathological examination revealed a
small number of isolated findings that were similar in control and
treated groups and which were not considered to be related to
treatment. These included slight degeneration of individual nerve
fibres in the trapezoid body of the medulla oblongata in four control
males and three control females and in one male and two females at the
high dose; slight degeneration of individual nerve fibres in the
cervical spinal cord of a single male at the high dose; and myelin
ellipsoids in the lumbar spinal cord of one male at the high dose and
one female control. A slight reduction in motor activity was observed
at 15 mg/kg bw per day during week 4 only, but the toxicological
significance of this finding was unclear. Cholinesterase activity was
not measured in this study. No neuropathological findings related to
treatment were observed at any dose. The NOAEL for cholinergic effects
was 1 mg/kg bw per day, and that for neuropathy was 15 mg/kg bw per
day (Shankar et al., 1993).
In a study designed to investigate the effects of age on the
toxicity of chlorpyrifos, rats received corn oil or chlorpyrifos
orally at doses of 80 mg/kg bw for adults and 15 mg/kg bw for young
rats (at postnatal day 17). These doses were equally effective in
inhibiting cholinesterase activity. In adult rats, peak behavioural
changes and cholinesterase inhibition occurred in males 3.5 h after
dosing, while in females the onset of functional changes occurred
sooner, the time course was more protracted, and recovery was slower.
In young rats, the maximal behavioural effects and cholinesterase
inhibition occurred 6.5 h after dosing, with no sex-related
difference. Partial to full recovery from the behavioural changes was
seen at 24-72 h, whereas the cholinesterase activity recovered more
slowly. The blood and brain cholinesterase activity in young rats had
almost recovered by 1 week after dosing, whereas the brain
cholinesterase activity in adults had not recovered by 2 weeks. Assays
for binding to muscarinic receptors showed apparent down-regulation in
some areas of the brain, mostly at 24 and 72 h. Rats at postnatal day
17 generally showed more receptor down-regulation than adults, whereas
only adult females showed receptor changes in striatal tissue that
persisted for 2 weeks. Thus, young rats showed similar behavioural
changes and cholinesterase inhibition to adults, although at a
fivefold lower dose; the onset of maximal effects was somewhat delayed
in the young rats; cholinesterase activity tended to recover more
quickly in the young rats; young rats appeared to have more extensive
muscarinic receptor down-regulation; and young rats showed no
sex-related difference in functional changes (Moser & Padilla, 1998).
Chlorpyrifos was administered subcutaneously to neonatal rats at
1 or 5 mg/kg bw per day on postnatal days 1-4 or daily at 5 or 25
mg/kg bw per day on days 11-14, and the two groups of pups were
examined at postnatal days 5 and 10 or 15 and 20, respectively. Pups
treated at postnatal days 1-4 had a significant increased mortality
rate (> 50%) at 5 but not at 1 mg/kg bw per day; the body weights of
the affected pups were decreased by 10-15% and the brain was
significantly smaller than that of controls. The survivors showed
severe cell loss in the brainstem; brainstem growth was maintained by
enlargement of the remaining cells. The lower dose did not compromise
survival or growth. Neither dose had an adverse effect on the
forebrain, despite the fact that the brainstem and forebrain have
comparable cholinergic projections. Pups treated on postnatal days
11-14 showed no increase in mortality rate at 5 mg/kg bw per day but a
significant increase (> 80%) at 25 mg/kg bw per day at day 15. The
body weights of the affected pups were significantly decreased
(> 40%), and the major target for cell loss shifted from the
brainstem to the forebrain. The pups at 5 mg/kg bw per day had a
small, transient loss in body weight; there was no significant effect
on whole brain weight, but these pups showed loss of forebrain cells
between 15 and 20 days of age, and the total amount of DNA in the
forebrain declined whereas an increase was seen in the forebrains of
control pups. The brainstem showed a smaller but not significant cell
loss during this period. The cerebellum differed from the other
regions in that it showed short-term increases in DNA after exposure
to chlorpyrifos in either the early or the late postnatal period;
nevertheless, the values then regressed to subnormal, in parallel with
loss of cells in other regions.
Although regions rich in cholinergic projections, such as the
brainstem and forebrain, may be more severely affected than
non-cholinergic regions such as the cerebellum, the time of maturation
of each region (brainstem earliest, forebrain intermediate, cerebellum
last) appears to be more important in determining vulnerability. The
weights of the various regions remained within normal limits despite
severe reductions in cell numbers, probably because reactive
hypertrophy of the remaining cells masked the cell loss. These results
suggest that chlorpyrifos induces cellular deficits in the developing
brain even when growth or survival is unaffected; however, the route
of administration and the doses used in this study limit the relevance
of these findings to humans (Campbell et al., 1997).
Chlorpyrifos was administered subcutaneously to neonatal rats at
doses of 1 or 5 mg/kg bw per day on postnatal days 1-4, or at 5 mg/kg
bw per day on days 11-14, and the two groups of pups were examined on
postnatal days 5 and 10 or 15 and 20, respectively. Signs of
interference with the adenyl cyclase signalling cascade were
determined in the heart and developing brainstem and cerebellum. Pups
treated on postnatal days 1-4 at 1 mg/kg bw per day showed no signs of
toxicity, while there was significant mortality (> 50%) at 5 mg/kg bw
per day. Pups treated on postnatal days 11-14 at 5 mg/kg bw per day
showed no deaths or clinical signs. No significant changes in the
weights of the brain region were found at these apparently non-toxic
doses. Cholinesterase activity in the brainstem of the pups given
1 mg/kg bw per day was inhibited by 25% when measured 24 h after the
last dose, but recovery was substantial (< 10% inhibition) by day 10.
Cholinesterase activity in the brainstem of the pups given 5 mg/kg bw
per day on days 11-14 was inhibited by > 60% when measured 24 h after
the last dos, but recovery was again substantial (< 30% inhibition)
by postnatal day 20. Separate examinations of the forebrain,
cerebellum, and heart showed that chlorpyrifos had evoked deficits in
multiple components of the adenyl cyclase cascade: the expression and
activity of adenyl cyclase itself, functioning of the G-proteins that
link neurotransmitter and hormone receptors to cyclase activity, and
expression of neurotransmitter receptors that act through this
cascade. Disruption of signalling function was not restricted to
transduction of cholinergic signals but extended to adrenergic signals
as well. In most cases, the adverse effects were not evident during
administration of chlorpyrifos but appeared after several days. Most
of the results of this study were presented in summary form only (Song
et al., 1997).
The acute neurobehavioural effects (as determined by
observational tests of function and motor activity) of two carbamates,
carbaryl and aldicarb, and five organophosphates, chlorpyrifos,
diazinon, parathion, fenthion, and diisopropyl fluorophosphate, were
evaluated in 10-week-old male Long-Evans rats on the day of dosing at
the time of the peak effect, at 1 and 3 days, and 1 week after dosing.
All doses were administered by oral gavage in corn oil in a volume of
1 ml/kg bw, except carbaryl, which was given in 2 ml/kg bw. A weight
loss of about 10% was recorded with each substance only at the high
dose. Generally, all of the cholinesterase inhibitors induced
autonomic signs of cholinergic over-stimulation (salivation,
lachrymation, and miosis), hypothermia, mild tremors, mouth-smacking
(chewing motions), decreased motor activity, decreased tail-pinch
response, and altered neuromuscular function (gait changes and
increased foot splay). The measures found to be most sensitive on the
day of dosing were body temperature, motor activity, gait, and the
presence of mouth-smacking and fine tremors, although no single
measure was the most sensitive for all compounds. Differences in the
slopes of the dose-response curves were evident for some measures.
Many effects were still present at 24 h, but the rats recovered from
the effects of all compounds. Interestingly, residual effects were
found at 72 h with the carbamates and with the organophosphate
fenthion, but not with the other compounds; this may be due, at least
in the case of the carbamates, to slow absorption from the gut. Thus,
the overall clinical toxicity profile of these cholinesterase
inhibitors was similar, but compound-specific differences emerged in
terms of individual measures, dose-response relationships, and time
course; the behavioural effects induced by these compounds may be due
to both cholinergic and non-cholinergic mechanisms. Only summary data
were available for evaluation (Moser, 1995).
Chlorpyrifos was administered subcutaneously to neonatal rats at
2 mg/kg bw on postnatal day 1 or at 11 mg/kg bw per day on postnatal
days 6-9. The developing brain regions (brainstem, forebrain, and
cerebellum) were examined for signs of interference with cell
development by using markers of cell division. The 1-day-old rats
showed significant inhibition (about 15%) of DNA synthesis in all
brain regions within 4 h of treatment. Equivalent results were
obtained when a small dose (0.6 or 2 µg/g brain) was introduced
directly into the brain by intracisternal injection, indicating that
the effect was not secondary to systemic toxicity. Comparable
inhibition (> 10%) of DNA synthesis was seen in these animals at 8
days of age, but at this point there was regional selectivity, with
sparing of the cerebellum (about 1% inhibition). In another phase of
this study, 6-8-day-old animals were pretreated with mecamylamine, a
nicotinic receptor antagonist, and then with chlorpyrifos at 11 mg/kg
bw. The pretreatment caused a decrease in DNA synthesis and also
prevented any further decrement in DNA synthesis due to chlorpyrifos.
Administration of chlorpyrifos at 1 day of age caused severe (> 30%)
inhibition of protein synthesis throughout the brain; the effect was
distinct from that on DNA synthesis, as it had diminished
substantially by 8 days of age (< 10%) and did not show regional
selectivity. Assays of ornithine decarboxylase activity 4 and 48 h
after treatment with chlorpyrifos in 1- and 8-9-day-old rat pups
showed no significant alteration of activity in any brain region in
comparison with appropriate controls. The authors concluded that the
effects of chlorpyrifos on DNA and protein synthesis are not secondary
to generalized cell damage or suppression of cell metabolism, as
evidenced by the maintenance of normal ornithine decarboxylase
activity. The results of this study were presented only in summary
form (Whitney et al., 1995)
Pregnant Sprague-Dawley rats were given chlorpyrifos at doses of
0, 0.3, 1, or 5 mg/kg bw per day by gavage in corn oil from day 6 of
gestation through day 11 of lactation, and their neurobehavioural
performance was evaluated. A satellite group of mated females was
dosed similarly every day on days 6-20 of gestation and used to obtain
blood and brain samples for analysis of cholinesterase. This study was
conducted in accordance with GLP standards. There were no deaths and
only a few clinical signs (fasciculations, hyperpnoea,
hyper-reactivity, and decreased body-weight gain) in the dams at the
high dose. Between days 1 and 5 of lactation, three dams at 5 mg/kg bw
per day had total litter loss and an additional 57 pups died or were
cannibalized; these losses led to a decreased viability index.
Necropsy of many of the dead pups indicated treatment-related lack of
maternal care (no milk in the stomach). No similar effects of
treatment were seen at the other doses. In dams, cholinesterase
activity in plasma and erythrocytes was inhibited at all three doses,
with > 40% inhibition at 0.3 mg/kg bw per day. Brain cholinesterase
activity was inhibited at the two higher doses, but the inhibition at
1 mg/kg bw per day was not > 20% that of controls. Pups that were
exposed to chlorpyrifos in utero and for a period post partum
showed signs of toxicity at the high dose: the weights of male and
female pups were significantly lower than those at the other doses,
consistent with the decreased food consumption and lack of body-weight
gain by dams at this dose. Morphometric measurements of the brain and
brain sections of six pups of eachsex per dose showed a pattern of an
increase at the low dose and a decrease at the high dose, which was
more pronounced in male pups, paralleling the differences in brain
weight between the groups. Hence, the NOAEL for maternal toxicity was
1 mg/kg bw per day. The LOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.3 mg/kg bw per day, and the NOAEL for
inhibition of brain acetylcholinesterase activity was 1 mg/kg bw per
day. The NOAEL for toxic effects in the pups was 1 mg/kg bw per day on
the basis of the decreased viability index, relative brain weight, and
delayed sexual maturity, possibly associated with maternal toxicity
and subsequent diminished maternal care at the high dose. Cognitive
function (learning, memory, and habituation) in the pups were not
affected by treatment (Hoberman, 1998).
Adult male Long-Evans rats were trained to perform a test of
memory and motor function and were then injected subcutaneously with
single doses of 0, 60, 125, or 250 mg/kg bw of chlorpyrifos in peanut
oil (2 ml/kg bw) and tested on 5 days/week for 7 weeks. Unconditioned
behaviour was also rated for signs of cholinergic toxicity.
Cholinesterase activity was measured in whole blood and in several
brain regions, and muscarinic receptor density and the hypothermic
effect of oxotremorine challenge were determined. Clinical signs of
toxicity were seen in one rat at 250 mg/kg bw. Whole-blood
cholinesterase activity was inhibited by 60-75% in a dose-dependent
fashion on day 4 but had recovered to near control values by day 53 in
animals given 60 mg/kg bw and by day 74 in those given 125 mg/kg bw.
Brain cholinesterase activity was strongly inhibited in a
dose-dependent manner and was inhibited by < 95% on day 7 in
animals given 250 mg/kg bw. By day 21, partial recovery of
cholinesterase activity was seen in some brain regions after the doses
of 60 and 125 mg/kg bw, but no significant recovery was seen in
animals given 250 mg/kg bw. Muscarinic receptor density decreased with
dose and time. At 250 mg/kg bw, the receptor density in the
hippocampus, frontal cortex, and striatum had fallen to 70-75% of the
control values after 7 days and to 60% 21 days after dosing; the
hypothalamic receptors were less severely affected. In comparison with
controls, the hypothermia induced by oxotremorine challenge was
significantly less pronounced in each group receiving chlorpyrifos on
days 8 and 32, but was comparable to that of controls by day 52.
Unconditioned behaviour was relatively unaffected by treatment, with
fine tremor in the head and limbs, the only sign of cholinergic
overstimulation, peaking on day 9 and no longer observed by 14 days
after dosing. Functional deficits in working memory and motor function
appeared within 2 days of injection of a dose of chlorpyrifos that
caused severe cholinergic effects, but the animals recovered within
3 weeks. The definition of working memory in this paper was
non-specific, and the short-term memory of the animals was unaffected
by treatment (Bushnell et al., 1993).
The authors contrasted these findings to those seen in rats dosed
with the organophosphate diisopropyl fluorophosphate (Bushnell et al.,
1991). These rats showed progressive, persistent impairment of
cognitive and motor function over a 3-week period of daily exposure,
despite neurochemical and pharmacological evidence of tolerance to
diisopropyl fluorophosphate-induced inhibition of cholinesterase. This
difference suggests that the diisopropyl fluorophosphate-induced
behavioural changes cannot be attributed entirely to its effects on
cholinesterase activity and changes in muscarinic receptor binding.
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 200 mg/kg bw as a
single dose on day 12 of gestation and then killed on day 16 or 20 of
gestation or on postnatal day 3 for measurement of maternal and
developmental indicators of toxicity. While most treated dams showed
no overt cholinergic signs, 4/28 showed moderate-to-severe signs 2-3
days after treatment, and these rats were omitted from further
studies. Maternal body weight was decreased by 15% 3 days after
treatment, after which the body-weight increase was comparable to that
of controls. Chlorpyrifos did not significantly affect fetal body
weights or brain weights when assayed on day 16 or 20 of gestation.
Inhibition of acetylcholinesterase activity (by 82-88%) was seen in
maternal brain at all three times after exposures. At days 16 and 20
of gestation, fetal brain acetylcholinesterase activity was inhibited
by 42-44%. While some degree of recovery in acetylcholinesterase
activity was seen in pup brain by postnatal day 3, it was still
inhibited (by 30%) in treated pups cross-fostered by control dams.
Maternal brain muscarinic receptor binding at day 20 of gestation and
postnatal day 3 was more extensively reduced (30-32%) than in the
developing brain (16 and 11%, respectively). The response to a simple
postnatal reflex test of righting was transiently altered (present at
on day 1 but not on day 3) by exposure to chlorpyrifos. The results
suggested that acute exposure of dams during gestation induced more
extensive neurotoxicological effects in the dams than in their
developing fetuses, but the dose and the route of administration make
the relevance of this finding unclear (Chanda et al., 1995).
The maximum tolerated dose of chlorpyrifos in Sprague-Dawley rats
injected subcutaneously in peanut oil was 45 mg/kg bw in 7-day-old
pups and 280 mg/kg bw in adults (80-100 days old) (Pope et al., 1991).
At this dose, the neonates showed an initial transient decrease in
body weight when compared with controls. Adult rats also had an
initial decrease in body weight, but this had recovered by the end of
the 7-day observation period. Brain cholinesterase activity increased
by about 42% in neonates on days 7-14, whereas it remained constant in
adults. Neonatal brain cholinesterase activity was inhibited by 80% on
day 1 and by 45% on day 7, whereas 90% inhibition was seen in adults
on day 4, with little or no recovery. In plasma, inhibition of
cholinesterase activity was rapid and marked (95%) in pups on day 1
but had recovered to 50% of control by day 7. In adults, such
inhibition persisted through day 7, with no recovery of activity.
In a study to determine whether a measure of inhibitory potency
in vivo, the ED50, could be correlated with a measure of acute
toxicity, the maximum tolerated dose, in neonatal and adult rats,
chlorpyrifos was given by subcutaneous injection in peanut oil.
Cholinesterase inhibition was measured at the time of peak inhibition
(24 h in neonates and 4 days in adults). Depression of cholinesterase
activity versus log dose gave a straight line, and the slope of the
curve of inhibition of plasma cholinesterase was slightly steeper than
that of brain cholinesterase. The ED50 values for brain
cholinesterase activity were 20 (range, 17-23) in neonates and 44
(31-63) in adults, and those for plasma cholinesterase activity were
20 (13-31) in neonates and 70 (40-120) in adults. The correlation
between the two values was good for neonates but not for adult rats
(Pope & Chakraborti, 1992).
Groups of five 3-month old Sprague-Dawley rats were given
chlorpyrifos (purity, 98%) in peanut oil by subcutaneous injection at
a dose of 280 mg/kg bw, and trunk blood, cerebral cortex, and corpus
striatum were collected 2, 4, 6, and 12 weeks after dosing.
Acetylcholinesterase activity was assayed, and the binding of
[3H]quinuclidinyl benzilate to muscarinic receptors was measured in
membranes of the cortex and striatum. Locomotor activity was measured
for 30 min on 5 days/week. A challenge of scopolamine (1 mg/kg bw
intraperitoneally) was given 2 weeks after chlorpyrifos treatment and
then every two weeks for 12 weeks. Extensive inhibition of cortical
and striatal cholinesterase activity was seen 2 (94-96%), 4 (about
80%), 6 (about 60%), and 12 (15-20%) weeks after treatment. Plasma
cholinesterase activity was inhibited to a lesser extent, and recovery
was more rapid, with 85% inhibition at 2 weeks, 55% at 4 weeks, and
30% at 6 weeks; the activity was normal at 12 weeks. The changes in
cholinesterase activity were accompanied by reductions in muscarinic
receptor binding sites in the cortex (34, 33, and 18% reductions in
maximal binding) and striatum (48, 40, and 23% reductions) 2, 4, and 6
weeks after exposure. Tests of locomotor activity showed hypoactivity
in treated rats for 2-3 days after treatment. After the scopolamine
challenge, chlorpyrifos-treated rats showed increased activity at all
times except week 10 (Pope et al., 1992).
Chlorpyrifos was given to groups of six adult male Sprague-Dawley
rats by subcutaneous injection at a dose of 280 mg/kg bw, and the
animals were killed 2, 7, and 14 days after treatment. A significant
( p < 0.05), approximately 6% decrease in body weight was seen after
2-3 days, but the body weights recovered during the remainder of the
study. Chlorpyrifos caused marked inhibition of cholinesterase
activity in brain regions and plasma at all times, with about 90%
inhibition in the cortex, striatum, and plasma at day 2, and there was
virtually no recovery of this activity within the 14-day recovery
period. Reductions of < 55% in atropine-sensitive quinuclidinyl
benzilate binding (total muscarinic receptors) and binding in the
cortex and striatum were also observed (Chaudhuri et al., 1993).
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 25 mg/kg bw per day
on days 12-19 of gestation and killed on day 20 of gestation or
postnatal day 3. In a separate study to determine the dose-response
relationship, rats were similarly exposed to chlorpyrifos at 6.25 or
12.5 mg/kg bw per day on days 12-19 of gestation and killed on day 20
for analysis of various neurochemical markers. No maternal toxicity
was seen at any dose. Treatment with 25 mg/kg bw per day induced only
an initial, slight, transient decrease in maternal body weight.
Treatment from day 12 of gestation did not affect fetal weight at day
16 or 20, but pup weight on postnatal day 1 was significantly reduced
by treatment at this dose. Maternal brain cholinesterase activity
measured on day 20 of gestation was significantly inhibited in a
dose-related manner, with 75 and 90% inhibition at 6.25 and 25 mg/kg
bw per day, respectively. Fetal brain cholinesterase activity was less
severely inhibited, with 40 and 60% inhibition at the two doses,
respectively. Extensive inhibition of acetylcholinesterase activity,
to 10-17% of the control value, was seen in dams treated with 25 mg/kg
bw per day. Significant, dose-related downregulation of muscarinic
receptors in maternal and fetal brain was noted on day 20 of gestation
after exposure at all doses. The responses to righting reflex and
cliff avoidance tests were markedly altered after repeated exposures.
Generally, the neurochemical effects were more severe in dams than in
the developing fetuses, despite the greater sensitivity of fetal brain
cholinesterase to inhibition by chlorpyrifos. Comparison of these
results with those of an earlier study of acute exposure to
chlorpyrifos (Chanda et al., 1995) indicated that repeated exposure
causes greater down-regulation of muscarinic receptors than the
equivalent acute dose (Chanda & Pope, 1996).
Cats
Technical-grade chlorpyrifos dissolved in methylene chloride and
mixed in olive oil was given to adult male, colony-raised, short-hair
cats at doses of 0, 0.1, 0.5, 1, 5, 10, or 50 mg/kg bw to establish a
dose that would induce clinical signs of toxicosis but not death. Two
cats were used in this segment of the study, and each received
increasing doses of chlorpyrifos. On the basis of deaths at 50 mg/kg
bw, a dose of 40 mg/kg bw was selected for the next phase of the
study, in which three groups of six cats received corn oil,
chlorpyrifos, or chlorpyrifos followed by injections of atropine
sulfate every 12 h for 2 days. Two cats receiving the antidote also
received pyridine 2-aldoxime methane sulfonate every 12 h until the
clinical signs had abated. All cats given chlorpyrifos showed clinical
signs of toxicity, and one cat died 4 days later. Whole-blood and
plasma cholinesterase activity was reduced in all treated animals, but
the levels had returned to pre-test levels after 7-28 days. Brain
cholinesterase activity was normal in all animals that survived until
the end of the study, 28 days after treatment. Gross pathology
revealed no treatment-related lesions, and haematological parameters
were unaffected (Hooser et al., 1988).
Groups of five adult male cats received a single intramuscular
injection of corn oil (vehicle), diisopropyl fluorophosphate (positive
control; 5 mg/kg bw), or chlorpyrifos (purity, 99%; 300 mg/kg bw) and
were observed for 60 days. Atropine and pyridine 2-aldoxime methane
sulfonate were given to chlorpyrifos-treated animals once or twice
daily for 14-24 days. Ataxia was observed in positive controls (mean
onset, 16 days; range, 14-19 days) and in those given chlorpyrifos
(mean onset, 19 days; range, 17-21 days) . Functional deficits were
seen, characterized by a crouched-waddling gait, hypermetria, and
proprioceptive deficits, and these effects were still present in both
groups 56 days after exposure; the effects in chlorpyrifos-treated
animals began to subside after day 35. Lymphocyte neuropathy target
esterase activity was inhibited by < 96% in positive control
animals (24 h after dosing) and by 46% in chlorpyrifos-treated cats
(7 days after dosing) but had reached control levels by day 28 in the
latter group. Whole-blood cholinesterase activity was reduced by
< 86% by day 4 and slowly recovered to control levels by day 56.
Diisopropyl fluorophosphate had inhibited cholinesterase activity by
< 63% 24 h after dosing, but the recovery of activity was similar
to that in chlorpyrifos-treated animals. Histological examination
(Table 22) revealed degeneration of axons in animals treated with
chlorpyrifos and with diisopropyl fluorophosphate, and, while the
lesions were similar, they were more severe in chlorpyrifos-treated
animals. The effects were characterized by axonal degeneration with
vacuolar (spongy) changes in the white matter tracts of the cerebrum,
cerebellum, and spinal cord and occasionally in the peripheral nerves.
Vacuolization was seen, resulting from distension of axonal spaces
associated with focal axonal swelling and degeneration of myelin and
myelin-forming cells, with infiltration of snall numbers of
macrophages (Fikes et al., 1992). The route of administration and
magnitude of the dose in this study limit its usefulness.
(g) Studies on metabolites
(i) Acute toxicity
The main metabolite of chlorpyrifos, TCP, was generally of lower
acute toxicity than chlorpyrifos (Table 23).
(ii) Short-term studies of toxicity
Groups of 10 rats of each sex were fed diets containing 0, 0.01,
0.03, 0.1, 0.3, or 1% TCP for 90 days. The three lower doses had no
treatment-related effects on body-weight gain, mortality, food
consumption, haematological values, blood urea nitrogen, alkaline
phosphatase, or average body and organ weights. The NOAEL was 0.1% in
the diet, approximately 50 mg/kg bw per day, on the basis of reduced
body-weight gain at 1% (Beatty, 1964).
Groups of 15 rats of each sex were fed diets containing sodium
TCP at concentrations providing doses of 0, 10, 30, or 100 mg/kg bw
per day for 91 days. The dietary concentration was varied to allow for
increasing body weight and ranged from about 85, 250, and 825 ppm at
day 0 to about 160, 460, and 1540 ppm at day 84 at the three doses,
respectively. Significantly increased body-weight gain was observed in
males at 30 mg/kg bw per day from day 48 onwards. There were no
treatment-related changes in haematology, urinary analysis, or blood
chemistry values. The absolute weight of the heart was increased in
males at 100 mg/kg bw per day, and increased absolute and relative
liver and kidney weights were seen in animals of each sex at this
dose. There were no gross or histopathological alterations due to
treatment. The NOAEL was 30 mg/kg bw per day on the basis of increased
relative liver and kidney weights (Barna-Lloyd & Szabo, 1985).
Table 22. Occurrence and severity (score ± SD of histological changes in the nervous system of cats
given a single intramuscular injection of chlorpyrifos, diisopropyl fluorophosphate
(positive control), or corn oil
Location Treatment
Corn oil Diisopropyl fluorophosphate Chlorpyrifos
No. affected Score No. affected Score No. affected Score
Brain
Cerebruma 2/5 0.40 ± 0.49 4/5 0.80 ± 0.40 4/5 2.00 ± 0.63
Cerebellumb 1/5 0.20 ± 0.40 2/5 0.40 ± 0.49 3/5 1.00 ± 0.89
Spinal cord
C2 2/5 0.20 ± 0.49 4/5 0.80 ± 0.40 5/5 1.40 ± 0.49
C7 1/5 0.20 ± 0.40 3/5 0.60 ± 0.49 5/5 1.40 ± 0.49
T-L 4/5 0.80 ± 0.40 5/5 1.00 ± 0.0 4/5 0.80 ± 0.40
L4 0/5 0.00 ± 0.0 5/5 1.60 ± 0.49 5/5 1.80 ± 0.40
Sciatic nerve 0/5 0.00 ± 0.0 3/5 0.60 ± 0.49 1/5 0.20 ± 0.40
Severity of lesions (axonal swelling, vacuolar change, myelin degeneration) in each section was given the
score of the most severely affected area. The lesions were scored as 0 = normal or equivocal, 1 = mild
(1-5 nerve fibres affected/high power field), 2 = moderate (6-10 nerve fibres affected), 3 = marked
(11-15 nerve fibres affected), and 4 = severe change (> 20 nerve fibres affected).
a Anterior parietal cerebrum sectioned transversely immediately posterior to the optic chiasm and
anterior to the infundibulum
b Transverse section through the cerebellum and brain stem at the level of the trigeminal nerves
Table 23. Acute toxicity of 3,5,6-trichloro-2-pyridinol (TCP) administered orally
Species Strain Sex Vehicle LD50 (mg/kg bw Reference
and 95% CI or range)
Mouse Swiss M 0.5% hydroxypropylmethyl 380 (333-433) Gerbig & Emerson (1970a)
F cellulose (Methocel) 414 (367-469)
Rat Sprague-Dawley M 0.5% hydroxypropylmethyl 794 (709-889) Gerbig & Emerson (1970b)
F 870 (758-1009)
Doga Beagle NR Gelatine capsule > 1000 Gerbig & Emerson (1970c)
NR, not reported
a One dog received two doses of 1000 mg/kg bw TCP, a second received four doses of 500 mg/kg bw, and a third
received eight doses of 50 mg/kg bw. Emesis was seen in all animals within 1-3 h of dosing. There were no deaths.
Groups of two beagles of each sex were given diets containing TCP
at concentrations providing doses of 0, 26, or 80 mg/kg bw per day for
93 days. At termination, haematological parameters were normal. There
was no NOAEL as elevated serum alkaline phosphatase activity and
histopathological evidence of liver damage and increased testicular
weights were seen in the treated animals (JMPR, 1972; modified by
reference to the original report of Copeland, 1964).
Groups of three dogs of each sex were given diets containing TCP
at concentrations providing doses of 1, 3, 10, or 30 mg/kg bw per day
for 91 days. No toxic effects were seen at the three lower doses, but
at 30 mg/kg tbw per day evidence of liver toxicity was seen, with
increased activity of alkaline phosphatase and alanine and aspartate
aminotransferases although there was no unequivocal sign of liver
damage on histopathological examination (JMPR, 1972; modified by
reference to the original report of Emerson & Gerbig, 1970).
Groups of four beagles of each sex were fed diets containing TCP
at concentrations providing doses of 0, 3, 12, or 48 mg/kg bw per day
for 1 year in a study conducted according to GLP. No details of the
clinical observations were reported. The body weights of males were
not statistically significantly different between groups, but females
at the high dose had consistently lower body weights than controls and
statistically significantly lower body-weight gains. No changes in
food consumption were found in treated groups, and no alterations in
haematological or urinary parameters. The dose-related changes in
clinical chemistry values that were attributed to treatment were
increased serum alkaline phosphatase and alanine aminotransferase
activities in males and females at 3, 6, and 12 months. The values
were comparable to those of controls at 3 mg/kg bw per day,
consistently elevated but generally not to a statistically significant
degree at 12 mg/kg bw per day, and were statistically significantly
increased at 48 mg/kg bw per day at most times. There were no gross or
histopathological lesions that were associated with treatment and no
changes in absolute or relative organ weights. The NOAEL for systemic
effects was 12 mg/kg bw per day, on the basis of a lack of a
consistent, statistically significant increase in enzyme activities at
that dose (Zempel et al., 1987).
(iii) Genotoxicity
TCP was not genotoxic in a range of assays in vitro and
in vivo (Table 24).
(iv) Developmental toxicity
In a range-finding study, Fischer 344 rats were given TCP
(purity, 99.7%) at doses of 0, 43, 75, or 127.5 mg/kg bw per day on
days 6-15 of gestation by oral gavage. Clinical signs (perineal
soiling) were seen sporadically in all dams at the highest dose and in
some at 75 mg/kg bw per day. Food and water consumption were
unaffected by treatment. The signs of maternal toxicity were limited
to reduced body-weight gain in dams at the high dose. The relative and
Table 24. Results of tests for the genotoxicity of 3,5,6-trichloro-2-pyridinol
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 3.16, 10, 31.6, 99.7 Negativea,b Zempel & Bruce (1986)
TA98, TA100, 100, 316 µg/plate
TA1535, TA1537, in DMSO
TA1538
Unscheduled Rat hepatocytes 1-100 µg/ml in 99.7 Negativec Gollapudi (1987)
DNA synthesis (Fischer CDF 344) DMSO
Forward mutation Chinese hamster 62.5, 125, 250, 99.7 Negativea Linscombe & Gollapudi
ovary cells, hprt locus 500, 750 µg/ml (1986)
In vivo
Micronucleus Mouse (CD-1) 250, 500, 750, 99.9 Negative McClintock & Gollapudi
formation marrow cells 1000, 1500, 2000, (1989b; GLP)
3000 mg/kg bw
orally in corn oil
Micronucleus Mouse (CD-1) 0, 24, 76, 240 mg/kg 99.7 Negative Bruce et al. (1985)
formation marrow cells bw orally in
corn oil
GLP, good laboratory practice; DMSO, dimethyl sulfoxide
a With and without an exogenous metabolic system
b Toxic at 100 (some strains) and 316 (all strains) µg/plate
c Toxic at 50 and 100 µg/ml
absolute weights of the liver and kidney were unaffected by treatment,
and there was no observable effect on reproductive parameters
(Scortichini et al., 1986a).
Groups of Fischer 344 rats were given 0, 50, 100, or 150 mg/kg bw
per day of TCP (purity, 99.7%) on days 6-15 of gestation by oral
gavage in a study that conformed to GLP. No clinical signs of toxicity
were seen in any group. Slight vaginal bleeding occurred in one animal
at 50 mg/kg bw per day and two at 150 mg/kg bw per day on day 13. Food
consumption was slightly decreased in animals at 100 mg/kg bw per day
and decreased by about 10% in those at the high dose. Significant,
dose-related decreases in body-weight gain were recorded throughout
treatment at 100 and 150 mg/kg bw per day. Changes in the relative and
absolute liver and kidney weights were restricted to a significant
increase (4%) in the relative liver weight at 150 mg/kg bw per day,
consequent to a significant decrease (5%) in body weight at this dose.
There was a dose-related trend in the number of implantation sites per
dam, which was reflected in the number of fetuses per litter and
probably in gravid uterine weight; the latter trend may also reflect
the trend to lower body weight with higher dose. Fetal examination
revealed a small number of findings in each group, including controls.
Overall, treatment had no significant effect on reproductive
parameters or fetuses. The NOAEL for maternal effects was 50 mg/kg bw
per day on the basis of decreased body-weight gain, and that for fetal
toxicity and teratogenic effects was 150 mg/kg bw per day, the highest
dose tested (Hanley et al., 1987a).
In the first phase of a study in inseminated New Zealand white
rabbits, the animals were given TCP (purity, 99.7%) on days 6-18 of
gestation by oral gavage at doses of 0, 48.5, 80, or 130 mg/kg bw per
day. As there was uncertainty that the maximum tolerated dose had been
reached in this phase, additional groups of animals were given 130 or
210 mg/kg bw per day (phase 2). Maternal body-weight gain during
gestation was depressed but not statistically significantly and was
highly variable at the highest dose in both phases. Gross examination
of all animals showed no significant findings or any differences
between groups. At 210 mg/kg bw per day, resorptions were seen in all
litters, and the percent implantations resorbed and the percent
litters with resorptions were statistically significantly different
from those in the comparable control group in phase 2. There was no
observable effect of treatment on reproductive parameters at doses up
to 130 mg/kg bw per day (Scortichini et al., 1986b).
Groups of New Zealand white rabbits were given TCP (purity,
99.7%) by oral gavage on days 7-19 of gestation at doses of 0, 25,
100, or 250 mg/kg bw per day in a study that met GLP requirements.
Female animals were given injections of human chorionic gonadotropin
to synchronize estrus and were inseminated 3 weeks later, which was
presumed to be day 0 of gestation. No clinical signs of toxicity were
seen in any group. Water and food consumption were not reported. A
dose-related decrease in body-weight gain was seen during treatment,
which reached statistical significance at the high dose. There were no
treatment-related changes in the relative or absolute weights of the
liver or kidney. Fetal examination revealed a small number of
malformations in each group, including controls. Overall, there was no
statistically significant effect of treatment on reproductive
parameters or fetuses, but an increased incidence of central nervous
system malformations at the intermediate and high doses suggested a
possible teratogenic effect, and the authors concluded that these
findings provided evidence of teratogenic potential, despite a lack of
statistical significance. The malformations included severe dilatation
of the cerebral ventricles and hydrocephaly. The incidences of minor
external, visceral, or skeletal alterations were unchanged. The NOAEL
for maternal effects was 100 mg/kg bw per day on the basis of
decreased body-weight gain during treatment. The NOAEL for fetal
toxicity and teratogenic effects was 25 mg/kg bw per day, on the basis
of central nervous system malformations at higher doses (Hanley et
al., 1987b).
3. Observations in humans
3.1 Experimental studies
A commercial preparation of chlorpyrifos containing 35% xylene
was applied to the skin of the back and abdomen of four volunteers
(skin area not stated) at a dose of 5, 10, 25, or 50 mg/kg bw 20 (5
days/week), four, three, and two times, respectively. The material was
covered with an occlusive patch for 12 h, and after removal of the
patch each site was scored for irritation before washing. Each site
was used only once. Plasma and erythrocyte cholinesterase activity was
determined before and several times after treatment (exact times not
stated; for results, see Table 25). In another study, five volunteers
received dermal doses of 94-620 mg/kg bw for 12 h and showed similar
inhibition of erythrocyte and plasma cholinesterase activity 24, 48,
and 72 h after exposure, even at the highest dose. No skin irritation
was recorded. Plasma cholinesterase activity was significantly
inhibited with two of the dose regimens, the activity reaching a
minimum around 3 days after the last dose and then recovering to
control values within 4-10 days; erythrocyte cholinesterase activity
were not inhibited with any regimen (Kilian et al., 1970).
Four volunteers were exposed for 5 min to a formulation
containing 61.5% chlorpyrifos and 34.5% xylene from an ultra-low
volume cold aerosol fog generator delivering 3.8 L/h. Air sampling
showed a concentration of chlorpyrifos in the breathing space of about
108 mg/L (range, 83-133 mg/L). The subjects were exposed at a distance
of 8 m and wore plastic coveralls allowing exposure of the heads and
hands of two subjects and the heads, hands, and arms of the other two.
Exposure was terminated after 5 min because of the ocular and
pulmonary irritation induced. Plasma and erythrocyte cholinesterase
activity was not depressed 24 h after exposure (Pennington & Edwards,
1971).
Table 25. Cholinesterase activity after dermal dosing of humans (% of pre-treatment value)
Dose regimen End of dosing Day 1 Day 3
Plasma Erythrocytes Plasma Erythrocytes Plasma Erythrocytes
10 mg/kg bw for 4 × 12 h 99 102 97 103 97 100
50 mg/kg bw for 2 × 12 h 100 100 100 100 102 100
25 mg/kg bw for 3 × 12 h 67 98 48 98
5 mg/kg bw for 20 × 12 h 67 103 64 104 74 104
One subject per dose regimen
Groups of four healthy, adult, male volunteers received
chlorpyrifos in tablet form at doses of 0, 0.014, 0.03, or 0.1 mg/kg
bw per day for 27, 20, and 9 days, respectively, with breakfast.
Control subjects received a placebo for 48 days. Although not
specifically stated, the high dose appears to have been withdrawn
because of the severity of plasma cholinesterase inhibition on day 9.
No rationale was given for termination of treatment at the lower
doses. Heparinized blood samples were obtained from the subjects twice
weekly for determination of plasma and erythrocyte cholinesterase
activity until all values had returned to pre-treatment levels. As it
was not stated whether the blood samples were taken at the same time
on each sampling day, the interval between administration and sampling
is unknown. No indication was given of the frequency of observation
for clinical signs after treatment or how such observations were made.
A single subject at 0.1 mg/kg bw per day reported having a runny
nose, blurred vision, and a feeling of faintness on the final day of
dosing, and his plasma cholinesterase activity was found to be
inhibited by 70% when compared with pre-treatment levels. No decrease
in erythrocyte cholinesterase activity was seen in any subject during
the study, and no other treatment-related effects were noted. Rapid,
marked depression of plasma cholinesterase activity was observed in
subjects at the high dose, and the activity did not return to control
levels until about day 34. Similar results were obtained when the
plasma cholinesterase activity in these subjects was compared with the
baseline activity of the group before treatment. The mean plasma
cholinesterase activity of men at 0.03 mg/kg bw per day was inhibited
by > 20% on days 16-20 of treatment when compared with those given
placebo, and the activity did not return to control levels for several
weeks after cessation of treatment. The plasma cholinesterase activity
of men at 0.014 mg/kg bw per day was inhibited by 20% only on day 13
and was similar to the baseline activity from day 20 to day 27, even
with continued administration of chlorpyrifos. Two-way analysis of
variance indicated that the inhibition of mean plasma cholinesterase
activity was significant only at 0.1 mg/kg bw per day ( p < 0.05)
when compared with controls. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day, the highest dose
tested (JMPR, 1972; modified by reference to the original report of
Coulston et al., 1972).
Six white male volunteers aged 27-50 years were used in another
study. One volunteer was given a single oral dose of 0.5 mg/kg bw
chlorpyrifos in a lactose capsule, about 30 min after food, and about
1 month later the other volunteers received the same treatment and the
first volunteer was given a dermal dose of 0.5 mg/kg bw chlorpyrifos
dissolved in methylene chloride. Two weeks after the first dermal
dose, the first volunteer was given a further dermal dose of 0.5 mg/kg
bw in dipropylene glycol methyl ether. The other volunteers were given
a dermal dose of 5 mg/kg bw of chlorpyrifos in dipropylene glycol
methyl ether 4 weeks after their oral dose. All urine was collected
from volunteers from 24-48 h before dosing until 120 h after dosing.
No signs or symptoms of toxicity were reported in any of the men
during the study. After oral administration of 0.5 mg/kg bw, the
plasma cholinesterase activity of the first volunteer was inhibited by
71% from that before treatment and that of the other men by a mean of
85% within 12-24 h of treatment. The range of individual values was
not provided. Plasma cholinesterase activity had returned to > 80% of
the mean baseline value by 30 days after oral treatment. After dermal
application on day 30, the plasma cholinesterase activity had
decreased to approximately 70% of baseline, and returned to about
80-90% of those levels by day 40 of the study. The intra-group
variation was stated to be considerable, but the figures were not
provided. Erythrocyte cholinesterase activity was not significantly
inhibited by either oral or dermal doses of chlorpyrifos (Nolan et
al., 1982, 1984).
In a double-blind, randomized, placebo controlled study of the
effects of single oral doses of chlorpyrifos (purity, 99.8%), groups
of six fasted men and women aged 18-55 received doses of 0, 0.5, 1, or
2 mg/kg bw in lactose powder. The study was conducted in two phases
separated by 14 days. The volunteers were dosed with 0, 0.5, or 1
mg/kg bw in the first phase and the results were assessed before
administration of 0 or 2 mg/kg bw in the second phase. Blood samples
were collected 10 and 0 h before treatment and 2, 4, 8, 12, 24, 36,
48, 72, 96, 120, 144, and 168 h after treatment and analysed for
erythrocyte cholinesterase activity and chlorpyrifos and its
metabolites. In addition, all urine voided from 48 h before dosing to
168 h after dosing was collected at 12- or 6-h intervals and analysed
for chlorpyifos and metabolites. Haematology, clinical chemistry,
urinary analysis and a brief physical examination were performed at
completion of the study. Blood and urine were collected to determine
each volunteer's paraoxonase status and the concentrations of
chlorpyrifos and metabolites, but these data have not yet been
reported. The volunteers were screened for general health according to
set criteria and instructed to refrain from alcohol, strenuous
exercise, and prescription medications before and during the study.
The doses were taken by capsule after an overnight fast. The health
status of subjects was monitored closely; vital signs (blood pressure,
pulse, respiration, and temperature) were assessed before dosing and
1, 2, 4, 8, 12, 24, 48, and 168 h after treatment. The subjects were
questioned about their well-being at each sampling time, and the
symptoms were evaluated clinically. The subjects were aware of the
signs and symptoms of cholinergic toxicity and were instructed to
inform the study physician of any adverse effects. The subjects were
unaware of their treatment, and the signs and symptoms were assessed
and treated by a physician who was also unaware of the treatment
status of the subject.
There were no significant deviations from the study protocol. One
male control in phase 1 and one woman receiving 2 mg/kg bw did not
provide a complete series of blood and urine samples. Treatment had no
effect on general health or on clinical chemical parameters measured 7
days after dosing. The only treatment-related effect was found in the
woman who withdrew from the study, who had decreased erythrocyte
cholinesterase activity when compared with her pre-treatment values at
most sampling times, with 98.4% of the pretreatment value at 4 h after
dosing, 77% at 8 h, 72% at 12 h, 74% at 24 h, 81% at 36 h, and 80% at
48 h (Table 26). When the data for this subject are removed from the
analysis, the mean for women receiving 2 mg/kg bw is indistinguishable
from the value for concurrent controls (Table 26). The NOAEL for
clinical signs or symptoms was thus the highest dose tested, and the
NOAEL for inhibition of erythrocyte cholinesterase activity was 1
mg/kg bw on the basis of significant inhibition in one of 12 subjects
(Kisicki et al., 1999).
3.2 Case reports
Blondell & Dobozy (1997) summarized the case reports, case
series, statistical surveys, and epidemiological studies of acute and
chronic health effects reported to be related to exposure to
chlorpyrifos. The authors noted the limitations of their review,
including inadequate documentation of exposure and effects, reporting
biases, and absence of information on the denominator population at
risk. They concluded, largely on the basis of an examination of
reports from poison control centres, that chlorpyrifos is one of the
leading causes of acute insecticide poisoning in the USA. Certain uses
were considered to pose greater risks than others, the main concern
being use of liquid formulations in households or by pest control
operators indoors or outdoors, termite treatment, and application of
liquid sprays and dips to domestic animals. Most of the more serious
cases were associated with misuse or inappropriate use such as spills
and inadvertent contamination by a pest control operator.
Shurdut et al. (1997) commented that deficiencies in the report
of Blondell & Dobozy (1997) invalidated its use for reaching a
conclusion about the safety of chlorpyrifos. They concluded that the
data from poison control centres had been misinterpreted and in fact
indicated the relative safety of products containing chlorpyrifos.
They also claimed that the report failed to respect the limitations of
anecdotal information, failed to consider the extensive testing with
regard to neurological damage and the long history of use of products
containing chlorpyrifos, made inappropriate use of anecdotes and
studies of organophosphates to characterize the safety of
chlorpyrifos, failed to consider the extensive database on exposure
after use of chlorpyrifos products, and presented the data
selectively.
In a case report, a previously well 3-year-old boy was found
playing near an open, spilled bottle of insecticide containing
chlorpyrifos (concentration not stated). The authors noted that
clinical manifestations of organophosphate-induced polyneuropathy
usually begin 1-3 weeks after an acute cholinergic crisis and that the
results of examination of the child were consistent with distal,
symmetric, predominantly motor polyneuropathy. In this patient, the
symptoms of cholinesterase inhibition and manifestations of weakness
and areflexia 11 days after ingestion and electromyographic findings
confirmed the presence of acute transient polyneuropathy with a more
Table 26. Mean erythrocyte cholinesterase activity as percent of mean baseline values in volunteers given single oral
doses of chlorpyrifos
Dose Time in relation to dosing (h)
(mg/kg bw)
- 10 0 4 8 12 24 36 48
Women
0.0a 99.5 (2.1) 100.5 (2.1) 98.3 (2.0) 102.1 (2.4) 100.7 (1.8) 99.7 (3.9) 98.4 (1.7) 100.2 (2.3)
0.0b 99.2 (4.4) 100.8 (4.4) 102.5 (5.9) 103.1 (5.4) 98.4 (3.2) 99.4 (3.7) 94.6 (5.3) 97.7 (5.1)
0.5 101.2 (6.7) 98.8 (6.7) 96.3 (6.5) 98.1 (2.1) 95.8 (3.9) 97.0 (3.6) 94.7 (4.7) 97.1 (4.1)
1 98.2 (0.9) 101.8 (0.9) 104.6 (4.2) 100.8 (3.5) 98.9 (3.7) 100.9 (5.6) 96.0 (4.1) 98.9 (2.1)
2 99.8 (1.8) 100.2 (1.8) 100.3 (3.2) 96.6 (6.81) 91.1 (9.7) 95.2 (10.6) 95.9 (7.4) 94.5 (7.7)
Men
0.0a 100.6 (1.8) 99.4 (1.8) 99.1 (2.2) 99.6 (4.6) 99.8 (4.3) 98.4 (4.3) 97.6 (2.8) 98.2 (3.1)
0.0b 96.1 (5.1) 103.9 (5.1) 101.6 (4.0) 102.5 (5.2) 102.9 (5.8) 102.6 (2.3) 98.2 (3.8) 95.9 (6.8)
0.5 98.7 (1.1) 101.3 (1.1) 102.0 (3.0) 103.4 (4.9) 99.7 (3.0) 101.9 (3.3) 98.1 (3.0) 92.8 (4.7)
1 98.6 (1.9) 101.4 (1.9) 104.5 (7.8) 101.1 (4.2) 101.1 (4.7) 101.7 (4.7) 98.5 (4.6) 92.1 (2.0)
2 101.2 (2.3) 98.8 (2.3) 99.2 (3.1) 98.9 (3.5) 98.7 (4.0) 99.2 (2.6) 99.5 (5.1) 98.4 (3.1)
a Phase 2 control
b Phase 1 control
proximal distribution than is usually seen in adults. The acute,
reversible bilateral vocal cord paralysis reported in this patient had
not been seen previously in organophosphate-induced delayed
neuropathy, but the authors noted that the onset of stridor and vocal
cord paralysis coincided with the onset of muscle weakness and
resolved at the same time as normalization of the peripheral
neuropathy (Aiuto et al., 1993a).
Gutmann & Bodensteiner (1993), responding to the report of Aiuto
et al. (1993), suggested that this case may not be a clear example of
organophosphate-induced delayed neuropathy. They noted that
chlorpyrifos has been associated with such symptoms only after severe
intoxication characterized by extensive cholinesterase inhibition. The
inhibition of plasma cholinesterase activity in the reported case was
instead suggestive of mild chlorpyrifos intoxication. They also noted
that the clinical findings associated with organophosphate-induced
delayed neuropathy are predominantly motor polyneuropathy, flaccid
weakness and atrophy primarily in the distal limb muscles, and often
incomplete recovery usually after months or years. The case report
implied that the evoked compound muscle action potentials and sensory
nerve action potentials were of normal amplitude, which would run
counter to a diagnosis of organophosphate-induced delayed neuropathy.
In response to these comments, Aiuto et al. (1993b) noted that
the patient's symptoms were consistent with > 90% inhibition of
cholinesterase activity and that the plasma cholinesterase activity
did not necessarily correlate with the severity of poisoning. The
authors concluded that the patient had developed delayed,
life-threatening neuropathy, although they were unable to determine
the mechanism of the condition.
In another case report, a 38-year-old man drank an undefined
quantity of a solution of 25% chlorpyrifos. He was treated with 3 mg
of atropine intravenously every 2 h for 6 days (total, 400 mg), but
any attempt to stop the atropine treatment resulted in reappearance of
respiratory distress. After 6 days, the patient was stuporous,
catatonic, and suffering from respiratory distress with white mucous
secretions, and no cholinesterase activity could be detected in his
serum. When ipratropium was administered endotracheally at a dose of
0.5 mg as an aerosol mist over 10 min, the patient's clinical
condition improved, although the salivary secretion continued. The
patient continued to receive ipratropium at 1-2 mg/day, and
respiratory support was discontinued on the fifth day. Severe
catatonia and coarse tremor were relieved by administration of
dantrolene at 10 mg intravenously, followed by 25 mg orally, three
times daily. Cholinesterase activity was still undetectable in serum
1 month after the man was discharged (Shemesh et al., 1988).
In a case in which a 26-year-old man intentionally ingested
approximately 360 ml of a 6.7% chlorpyrifos formulation containing
2,4-D (10.8%) and mecoprop (11.6%) and a few granules of a warfarin
(0.025%) concentrate, the main clinical findings on admission were
coma, myoclonus, miosis, cardiac arrythmia with progression to
hypotension, oliguria, and death after several episodes of asystole.
The authors stated that such symptoms are consistent with those seen
in patients who have ingested chlorophenoxy acetic acids such as 2,4-D
and mecoprop. The patient did not show many of the usual signs of
organophosphate poisoning, such as lachrymation, salivation,
respiratory paralysis, or muscle fasciculation, but his erythrocyte
cholinesterase activity was inhibited by about 70% at 13 h and by
< 90% by 26 h. Plasma cholinesterase activity was inhibited
completely at all times. Lymphocyte neurotoxic esterase activity was
inhibited by about 50% after 13 h but was within normal limits after
19 h. The activity of neurotoxic esterase in the cerebral cortex was
within normal limits but that in peripheral nerves was inhibited by
about 70% when compared with normal levels. The inhibition of
neurotoxic esterase activity was found in the absence of significant
signs of organophosphate intoxication (Osterloh et al., 1983).
A 21-year-old man who ingested an unknown quantity of a
chlorpyrifos formulation required large amounts of atropine and
pyridine 2-aldoxime methane sulfonate, endotracheal intubation, and
ventilation to overcome the cholinergic effects, which consisted of
pupillary constriction, excess secretions, tachycardia, and impaired
consciousness. Bilateral vocal cord paralysis developed 16 days after
extubation (28 days after exposure), and this finding was considered
to be indicative of organophosphate-induced delayed neuropathy. The
patient was asymptomatic within 4 weeks of the onset of symptoms (de
Silva et al., 1994).
A 42-year-old man who attempted suicide by drinking a commercial
formulation of 41% chlorpyrifos (estimated dose, 300 mg/kg bw) went
into coma and showed respiratory insufficiency, lachrymation,
salivation, sweating, miosis, fasciculations, and bronchorrhoea on
admission to hospital 18 h after exposure. A chest X-ray showed right
basal bronchopneumonitis, electroencephalographic signs of diffuse
irritation were recorded, and chlorpyrifos was detected in gastric
lavage fluid. Plasma cholinesterase activity was inhibited by almost
100% by 36 h. The coma and cholinergic signs persisted for 17 days,
during which time atropine, pyridine 2-aldoxime methane sulfonate,
mechanical respiration, and antibiotics were used. The patient was
asymptomatic on day 24 after exposure, and drug administration was
stopped; no signs of peripheral or central nervous system involvement
were observed. On day 30, lymphocytic neurotoxic esterase, blood
acetylcholinesterase, and plasma butyryl cholinesterase activities
were markedly below the normal range but recovered slowly thereafter.
On day 43, weakness and paraesthesia in the legs with reduced tendon
reflexes and sensory conduction velocity were noted. By day 62, the
leg weakness was more severe, gait was impaired, and tendon reflexes
were absent. Electromyography of the leg muscles showed symmetrical
signs of denervation with spontaneous activity, and motor conduction
velocity was reduced. Electrophysiological studies of the arms
confirmed a symmetrical reduction of sensory conduction velocity of
the ulnar nerves. Sural nerve biopsy on day 63 revealed a few altered
myelinated fibres. Aspects of axon and myelin degeneration were also
found occasionally. The changes were consistent with mild distal
axonopathy. Chlorpyrifos was still detectable 10 days after exposure,
and neurotoxic esterase activity was still inhibited by 60% 4 weeks
after exposure (Lotti et al., 1986).
In a study in which toxicological and/or electrophysiological
examinations were conducted in 11 patients who had acute
organophosphate poisoning, three of the patients, one of whom had been
poisoned with chlorpyrifos, developed organophosphate-induced delayed
neuropathy (Moretto & Lotti, 1998). The findings in this individual
were reported by Lotti et al. (1986). The electro-physiological data
indicated organophosphate-induced delayed neuropathy with sensory and
motor components which persisted for 3 months, at which time the
patient was lost to follow-up.
Four incidents of birth defects allegedly associated with
exposure to chlorpyrifos were reported. The children were found to
have a range of birth defects, including ventricular, ocular, and
palate defects and growth retardation (all children), hydrocephaly,
microcephaly, mental retardation, blindness, hypotonia, widely spread
nipples, and deformities of the teeth, ears, and external genitalia.
The mothers of the affected children had reportedly been exposed to
chlorpyrifos either at the workplace or at home during the pregnancy.
Two of the children were born to the same mother. The exposure of the
mothers to chlorpyrifos was poorly characterized, and it was not
indicated whether it was severe or sustained during organogenesis. The
range of birth defects reported was not consistent with the results of
studies in laboratory animals (Sherman, 1996).
In a comment on this paper, Gibson (1996) noted that the medical
records of the cases indicated that the same effects were not seen in
all children and that the effects in some of the children were
consistent with a diagnosis of an autosomal recessive birth defect
syndrome of the brain and eye known as cerebro-oculo-facio-skeletal
syndrome or a closely related one known as the 'MICRO' syndrome.
Eight cases were reported in which exposure to chlorpyrifos was
claimed to have caused sensory neuropathy. The patients each presented
with a range of symptoms, and characterization of exposure was poor.
One patient who reported signs of cholinesterase inhibition, including
lachrymation, muscle twitching, and diarrhoea, was a pesticide
applicator who had reportedly been exposed to chlorpyrifos in a closed
environment for 6 months. The patient's erythrocyte cholinesterase
activity was reportedly to be 'low' initially but returned to normal
levels within 2 months. Neurological evaluations were conducted only 6
weeks after the other symptoms were reported; at this time, sensory
loss of all modalities was found in a 'stocking-glove distribution'
test, with mild distal weakness and areflexia in the lower
extremities. Studies of nerve conduction and quantitative sensory
threshold revealed changes consistent with peripheral neuropathy of
the distal anonopathy type. The decreased reflexes and mild distal
weakness were consistent with polyneuropathy. Follow-up examination at
1 year revealed remission of all symptoms. In the other seven patients
(four from the same family), the symptoms were nonspecific, and the
absence of symptoms of cholinesterase inhibition suggested that these
patients had not been as exposed to chlorpyrifos as extensively as the
first patient. The lack of immediate electrodiagnostic testing, the
poor exposure characterization, and the variation in the reported
clinical findings make interpretation of the case studies difficult.
Additionally, subjective questioning was used to obtain information
from the patients. Follow-up examinations for a range of parameters
were not conducted on several patients, and the patients did not
undergo the same initial testing regimen. Thus, one patient was
exposed to chlorpyrifos occupationally at a level sufficient to
decrease cholinesterase activity and cause cholinergic symptoms; he
also showed evidence of mild, reversible polyneuropathy, possibly in
the presence of decreased erythrocyte cholinesterase activity. For two
other patients, there was limited evidence of mild polyneuropathy
(Kaplan et al., 1993).
3.3 Monitored field trials
A monitored field test was used to determine the effect of
chlorpyrifos on spraymen applying treatment at the rates used in
programmes for the eradication of mosquitoes. Three studies were
conducted in 1966, 1967, and 1968. Cholinesterase activity was
measured, and exposure was terminated if the inhibition reached 50% of
the baseline values. In the first study, three spraymen showed marked
depression of plasma cholinesterase activity, with reductions of
68-82% from baseline values. The two remaining spraymen showed
reductions of 52-56% over 9 days, but the baseline values were not
available. The study was discontinued after 2 weeks because of the
significant decrease in cholinesterase activity. In the second study,
no treatment-related effect on cholinesterase activity was seen during
a short period. In the third study, erythrocyte cholinesterase
activity was not affected by treatment, but plasma cholinesterase
activity was reduced by 50-80% in spraymen using a suspension
formulation. The activity had generally returned to pre-exposure
levels within 2 months of cessation of use. Four spraymen using an
emulsion formulation did not show reduced plasma cholinesterase
activity during the exposure period (Kenaga, 1967; Eliason et al.,
1969).
3.4 Studies of morbidity
In a study in which the sample size was small and the statistical
power was limited, comparisons were made between 175 employees
potentially exposed to chlorpyrifos at a manufacturing plant where
they had held jobs between 1 January 1977 and 31 July 1985 and 335
unexposed controls matched on age (within 5 years), date of hire
(within 5 years), sex, race, and pay status. The source of information
for the observations (self, nurse, company physician, private
physician, laboratory) was recorded, as were use of tobacco, alcohol,
and cholinergic drugs. Exposure was grouped into high, moderate, and
low on the basis of industrial hygiene surveys and consultation with
veteran manufacturing personnel. The plasma cholinesterase activities
of the potentially exposed employees before exposure were available,
and follow-up was conducted at roughly monthly intervals. The mean
inhibition of plasma cholinesterase activity was 19 ± 2.9% in the
group with low exposure, 32 ± 2.8% in that with moderate exposure, and
32 ± 5.3% in that with high exposure. No cases of peripheral
neuropathy were recorded in the study group. Inhibition of plasma
cholinesterase activity was observed but was not associated with
illness. The exposed groups reported symptoms of dizziness, malaise,
and fatigue more frequently than the unexposed group, but the analysis
did not indicate any correlation with increasing exposure (Brenner et
al., 1989).
In a follow-up to this study (Burns et al., 1998), the data were
updated to include the period 1987-94, and additional medical
disorders were considered. Data on self-reported paraesthesia were
collected from 1982 to 1994. The prevalence of peripheral neuropathy
was not significantly increased in the group of workers exposed to
chlorpyrifos.
Comments
After oral administration to rats, radiolabelled chlorpyrifos was
rapidly and extensively absorbed (up to about 90% of the dose) and
eliminated, predominantly in the urine (68-93%) and faeces (6-15% of
the dose), within about 72 h of administration. The urinary
metabolites included the glucuronide (about 80%) and sulfate (about
5%) conjugates of chlorpyrifos, and 3,5,6-trichloro-2-pyridyl
phosphate (TCP; about 12%). The tissue concentrations of residues of
[14C]chlorpyrifos were very low (generally < 1 ppm) within 72 h of
dosing. The longest half-time of residues in rats was 62 h in fat, and
low levels were also detected in the fat of several other species and
in the milk of goats.
In humans who were poisoned with chlorpyrifos formulations,
diethylphosphorus metabolites were excreted in the urine by
first-order kinetics, with an average elimination half-time of 6.1 ±
2.2 h in the fast phase and of 80 ± 26 h in the slow phase. In
volunteers, the time to maximal concentration of 3,5,6-TCP in the
blood was 0.5 h after oral dosing and 22 h after dermal treatment, but
the elimination half-time by both routes was 27 h, and the percentage
of the administered dose recovered from the urine was 70% after oral
dosing and 1.3% after dermal administration.
Chlorpyrifos is rapidly metabolized by mixed-function oxidases to
the highly reactive chlorpyrifos oxon by oxidative desulfuration. The
oxon can be deactivated by hydrolysis to diethylphosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is hydrolysis
to monoethyl 3,5,6-trichloro-2-pyridinyl phosphorothioate.
The lowest oral LD50 value was 96 mg/kg bw (range, 96-475 mg/kg
bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in mice. Female
rats were generally more sensitive to the acute effects of
chlorpyrifos than males. The signs of acute intoxication with
chlorpyrifos were consistent with cholinesterase inhibition. The acute
dermal LD50 of chlorpyrifos was > 2000 mg/kg bw in rats and > 1200
mg/kg bw in rabbits.
WHO (1999) has classified chlorpyrifos as 'moderately hazardous'.
Chlorpyrifos was irritating to the eye and skin of rabbits, but
it did not sensitize the skin of guinea-pigs in Magnusson-Kligman
maximization or Buehler tests.
In short-term studies, the NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day in dogs and 0.1
mg/kg bw per day in rats. The NOAEL for inhibition of brain
cholinesterase activity was 1 mg/kg bw per day in dogs and rats. The
signs of toxicity were largely limited to cholinergic signs and
decreased body weights and/or food consumption. The NOAEL for these
effects in short-term studies was 1 mg/kg bw per day in rats, and the
NOAEL for clinical signs was 3 mg/kg bw per day in dogs. In mice,
ocular effects and histopathological alterations (including adrenal
lipogenic pigmentation and ocular keratitis) were observed (NOAEL, 50
ppm; equal to 7 mg/kg bw per day). In rats, the NOAEL for increased
fatty vacuolation of the adrenal zonal fasciculata and changes in
haematological and clinical chemical parameters was 5 mg/kg bw per
day. When rats received chlorpyrifos dermally for 21 days, the NOAEL
for inhibition of cholinesterase activity in erythrocytes and brain
was 5 mg/kg bw per day.
In long-term studies, inhibition of cholinesterase activity was
again the main toxicological finding in all species. In rats, the
NOAEL was 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity and 1 mg/kg bw per day for inhibition of
brain acetylcholinesterase activity, but clinical signs were not seen
at doses up to 10 mg/kg bw per day, and the NOAEL for reduction in
body weight was 1 mg/kg bw per day. In mice, erythrocyte and brain
acetylcholinesterase activities were inhibited at 50 ppm, equal to 6.1
mg/kg bw per day, and the NOAEL was 5 ppm, equal to 0.7 mg/kg bw per
day. Cholinergic signs and reductions in body weight were reported
only at the highest dietary concentration of 250 ppm (equal to 32
mg/kg bw per day). Other treatment-related findings included effects
on the liver in mice, with a NOAEL of 50 ppm (equal to 6.6 mg/kg bw
per day), and increased adrenal weight in rats with a NOAEL of 1 mg/kg
bw per day. There was no treatment-related increase in the incidence
of neoplastic lesions in any of the long-term studies. The Meeting
concluded that chlorpyrifos is unlikely to pose a carcinogenic risk to
humans.
Chlorpyrifos was not genotoxic in an adequate range of studies
in vitro and in vivo. The Meeting concluded that chlorpyrifos is
not genotoxic.
In multigeneration studies of reproductive toxicity in rats, the
treatment-related effects of chlorpyrifos were limited to inhibition
of cholinesterase activity, consistent with that seen in other short-
and long-term studies, and fetotoxicity characterized by reduced pup
viability, body weights and survival. No significant,
treatment-related clinical signs were reported. The NOAEL for
inhibition of maternal acetylcholinesterase activity was 0.1 mg/kg bw
per day for erythrocytes and 1 mg/kg bw per day for brain. The NOAEL
for developmental toxicity was 1 mg/kg bw per day. No effects on
reproductive parameters were observed at the highest dose tested,
5 mg/kg bw per day.
In studies of developmental toxicity in mice, rats, and rabbits,
the maternal effects included inhibition of erythrocyte and/or brain
acetylcholinesterase activity and cholinergic signs (lowest NOAEL,
1 mg/kg bw per day in rats and mice) and reductions in body weight and
food consumption (lowest NOAEL, 2.5 mg/kg bw per day in rats). The
observed fetal toxicity (lowest NOAEL, 2.5 mg/kg bw per day in rats)
and developmental toxicity (NOAEL, 1 mg/kg bw per day in rats) were
consistent with treatment-related maternal toxicity; there was no
evidence of treatment-related malformations in any of the studies.
There was no effect on cognitive function (learning, memory, and
habituation) in pups exposed to chlorpyrifos in utero and for a
period post partum at doses up to and including the highest dose of
5 mg/kg bw per day, while inhibition of cholinesterase activity,
decreased brain weight, and delayed development were seen at lower
doses, consistent with findings in other studies.
In studies of delayed neurotoxicity, chlorpyrifos was given to
chickens as either single or repeated doses. Significant inhibition of
both cholinesterase and neuropathy target esterase activity was
observed, and mild delayed neuropathy was seen in a number of studies;
aggressive antidotal therapy was always necessary to allow at least
some of the treated birds to survive. Despite the marked cholinergic
toxicity of chlorpyrifos, there was no evidence that it caused delayed
neurotoxicity, and there was no increase in the incidence of
histopathological lesions in the nerve tissues of birds treated at
doses up to 10 mg/kg bw per day for up to 91 days. In a number of
studies in rats given single doses of up to 100 mg/kg bw, repeated
doses of up to 10 mg/kg bw per day for 4 weeks, or repeated doses of
up to 15 mg/kg bw per day for 13 weeks, there were no
treatment-related neurological lesions or effects on cognition and no
inhibition of neuropathy target esterase activity, although
significant inhibition of erythrocyte, brain, and peripheral tissue
cholinesterase activity was seen at some doses. In a study that
included a functional observational battery of tests, clinical signs
of intoxication were observed after a single dose only when brain
acetylcholinesterase activity was inhibited by more than 60% or when
whole-blood cholinesterase activity was inhibited by more than 80%.
When chlorpyrifos was applied as a single dose of up to 5 mg/kg
bw to the skin of volunteers for 12 h, erythrocyte cholinesterase
activity was not significantly inhibited. Plasma cholinesterase
activity was inhibited after 20 12-h dermal exposures to 5 mg/kg bw
per day over 4 weeks or after three daily 12-h exposures to 25 mg/kg
bw per day on consecutive days, but erythrocyte cholinesterase
activity was not inhibited under any treatment regimen.
A single oral dose of up to 1 mg/kg bw or repeated doses of up to
0.1 mg/kg bw per day for 9 days did not significantly inhibit
erythrocyte acetylcholinesterase activity in volunteers. No clinical
signs were observed in these studies. Inhibition of erythrocyte
acetylcholinesterase activity was observed in a single female
volunteer (of a group of six men and six women) given a single oral
dose of 2 mg/kg bw.
In a case of human poisoning with chlorpyrifos at an estimated
dose of 300-400 mg/kg bw, significant inhibition of neuropathy target
esterase in lymphocytes and of plasma and erythrocyte
acetylcholinesterase activity was reported, with severe cholinergic
signs which required aggressive, extensive antidotal therapy and
artificial ventilation. Mild distal axonopathy consistent with
organophosphate-induced delayed polyneuropathy was reported some weeks
after the poisoning incident.
The ADI of 0-0.01 mg/kg bw established by the 1982 Meeting was
based on a NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in humans. The present Meeting affirmed
this ADI on the basis of the NOAEL of 1 mg/kg bw per day for
inhibition of brain acetylcholinesterase activity in studies in mice,
rats, and dogs, using a 100-fold safety factor, and on the basis of
the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in the study of human subjects exposed
for 9 days, using a 10-fold safety factor.
The Meeting allocated an acute reference dose of 0.1 mg/kg bw on
the basis of the NOAEL of 1 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity in a study in which volunteers received
a single oral dose of chlorpyrifos, with a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 0.7 mg/kg bw per day (toxicity in a 79-week
study of toxicity and carcinogenicity)
1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
10 mg/kg bw/day (fetal toxicity in a study of developmental
toxicity)
Rat: 1 mg/kg bw per day (toxicity in 2-year studies of toxicity
and carcinogenicity)
1 mg/kg bw per day (developmental and parental toxicity in a
two-generation study of reproductive toxicity)
1 mg/kg bw per day (maternal and developmental toxicity in a
study of developmental toxicity)
2.5 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Rabbit: 81 mg/kg bw per day (maternal and fetal toxicity in a study
of developmental toxicity)
Dog: 1 mg/kg bw per day (toxicity in a 2-year study of toxicity)
Human: 0.1 mg/kg bw per day (no inhibition of erythrocyte
cholinesterase activity at highest dose tested in men dosed
orally for 9 days)
1 mg/kg bw (inhibition of erythrocyte cholinesterase
activity in adult volunteers after a single oral dose)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to chlorpyrifos
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Up to 90% in rats within 72 h; about 70% in humans within 96 h
Dermal absorption Less than 2% in humans within 180 h; not determined in animals
Distribution Initially widely distributed; highest residues in liver, kidneys
and fat at 72 h in rats
Potential for accumulation Elimination half-times of < 24 h and low tissue residues after
72 h in rats. No evidence of potential for accumulation
Rate and extent of excretion > 95% within 72 h in rats, mainly in urine (68-93%) and faeces (6-15%)
Metabolism in animals Rapidly metabolized by mixed-function oxidases to chlorpyrifos oxon
via oxidative desulfuration and an electrophilic phosphooxathiiran
intermediate. Degradation by conversion directly to
3,5,6-trichloro-2-pyridyl phosphate and diethyl thiophosphate.
The oxon is hydrolysed to diethyl phosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is by
hydrolysis to monoethyl-3,5,6-trichloro-2-pyridinol phosphorothioate.
Toxicologically significant compounds Parent compound and oxon
(animals, plants and environment)
Acute toxicity
Rat,, LD50, oral 96 mg/kg bw
Rat LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 36 mg/m3 (4 h, vapour, nose-only exposure)
560 mg/m3 (4 h, nebulized particles < 5 mm, whole-body exposure)
Rabbit, dermal irritation Slightly irritating
Rabbit, ocular irritation Slightly irritating
Guinea-pig, dermal sensitization Not sensitizing
Short-term toxicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest critical oral NOAEL 1 mg/kg bw per day, dog, 2 years
1 mg/kg bw per day, rat, 13 weeks
Lowest relevant dermal NOAEL 5 mg/kg bw per day, rat; 21 days
Lowest relevant inhalation NOAEL 20.6 ppb (296 mg/m3), rat; 13 weeks
Long-term toxicity and carcinogenicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest relevant NOAEL 2 years, rat; 1 mg/kg bw per day
78 weeks, mouse; 0.7 mg/kg bw per day
Carcinogenicity Not carcinogenic in rats or mice
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Neonatal toxicity (reduced pup body weight and survival)
Lowest relevant reproductive NOAEL Two-generation, rat; 1 mg/kg bw per day
Developmental target/critical effect Fetal and perinatal toxicity at maternally toxic doses (including
an increase in delayed ossification, reduced crown-rump length,
reduced pup weight, increased postimplantation loss, delayed sexual
maturity)
Lowest relevant developmental NOAEL Rats; 1 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Reversible neurotoxicity consistent with cholinesterase
inhibition. No evidence of delayed neurotoxicity or
histopathological changes in nerves of hens (10 mg/kg bw per day)
and rats (15 mg/kg bw per day) for up to 13 weeks. At high acute
doses (up to 150 mg/kg bw), significant inhibition of neuropathy
target esterase and mild delayed neuropathy in hens, but at this
dose, extensive and aggressive antidote treatment were required
for birds' survival.
Other toxicological studies No effect on cognitive function in rat pups in a study of
developmental toxicity at doses up to 5 mg/kg bw per day
Medical data No inhibition of erythrocyte acetylcholinesterase activity in
volunteers after repeated oral doses of up to 0.1 mg/kg bw per day
(for 9 days), single oral doses of up to 1 mg/kg bw, or a single
dermal doses of 5 mg/kg bw. Poisoning case presented with severe
cholinergic effects, with evidence of delayed polyneuropathy and/or
distal axonopathy at a dose that required antidotal treatment and
artificial ventilation.
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw Rat, 2-year dietary 100
Rat, reproduction
Mouse, developmental toxicity
Dog, 2-year dietary
Human, 9-day oral 10
Acute reference dose 0.1 mg/kg bw Human, single dose 10
References
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993a) Life-threatening
organophosphate-induced delayed polyneuropathy in a child after
accidental chlorpyrifos ingestion. J. Pediatr., 122, 658-660.
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993b)
Organophosphate-induced delayed polyneuropathy (comment).
J. Pediatr., 123, 837.
Aldridge, W.N. (1953) Serum esterases I. J. Biochem., 53, 110-117.
Anderson, B.T., Walker, S.A. & Punler, M.J. (1995) Chlorpyrifos tech:
Acute inhalational toxicity study in rats. Unpublished report No.
10425 from Inveresk Research International, Tranent, Scotland.
Submitted to WHO by Luxembourg Industries, Tel Aviv, Israel.
Bakke, J.E., Feil, V.J. & Price, C.E. (1976). Rat urinary metabolites
from O,O-diethyl-O(3,5,6-trichloro-2-pyridyl) phosphorthioate.
J. Environ. Sci. Health Bull., 3, 225-230.
Barna-Lloyd, T. & Szabo, J.R. (1985) Sodium
3,5,6-trichloro-2-pyridinol: 3-month rat dietary toxicity study.
Unpublished report No. TexasT:K-065999-099 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Barna-Lloyd, T., Szabo, J.R. & Young, J.T. (1986) Chlorpyrifos:
Subchronic organophosphate-induced delayed-neurotoxicity (OPIDN)
study in laying chickens hens. Unpublished report No.
TexasT:K-044793-064 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Bauriedel, W.R. & Miller, J.H. (1981) The metabolic fate of the sodium
salt of C14-labelled 3,5,6-trichloro-2-pyridinol orally
administered to swine. Unpublished report No. GH-C 1427 from Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. (1964) Results of 90-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in rats. Unpublished report from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,Odiethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1971) Results of 90-day dietary
feeding studies of O,Odiethyl-O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Berman, C.L. (1987) Evaluation of chlorpyrifos (Pyrinex) for dermal
sensitization of guinea pig. Unpublished report No. 59487-01 from
Arthur D. Little Inc., Cambridge, Massachusetts, USA. Submitted
to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Berteau, P.E. & Deen, W.A. (1978) A comparison of oral and inhalation
toxicities of four insecticides to mice and rats. Bull.
Environ. Contam. Toxicol., 19, 113-120.
Blackmore, R.H. (1968) Oral administration -- dogs Dursban. Final
report. Unpublished report No. 174-115 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Blagden, S.M. (1994) Chlorpyrifos technical: Acute inhalation toxicity
study four-hour exposure (nose only) in the rat. Unpublished
report No. CHA 10-CYF from Safepharm Laboratories Ltd, Derby,
United Kingdom. Submitted to WHO by Cheminova Agro A/S, Lemvig,
Denmark.
Blondell, J. & Dobozy, V.A. (1997) Review of chlorpyrifos poisoning
data. Washington DC, Environmental Protection Agency.
Branson, D.R. & Litchfield, N.H. (1970) Comparative absorption,
elimination and distribution of DOWCO 179, its methyl analog
DOWCO 214 and their major metabolite,
3,5,6-trichloro-2-pyridinol. Unpublished report No. 70 4479 from
Dow Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971a) Absorption, excretion and
distribution of O,Odiethyl O-3,5,6-trichloro-2, 6-C14-2-pyridyl
phosphorothioate in rats. Unpublished report No. NBA-9 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971b) Absorption, excretion and
distribution of 3,5,6trichloro-2, 6-C14-2-pyridinol in rats.
Unpublished report No. NBA-10 from Dow Chemical Co., USA
Submitted to WHO by Dow Agrosciences, Indianapolis, IN, USA.
Brenner, F.E., Bond, G.G., McLaren, E.A., Greene, S. & Cook, R.R.
(1989) Morbidity among employees engaged in the manufacture or
formulation of chlorpyrifos. Br. J. Ind. Med., 46, 133-137.
Breslin, W.J., Liberacki, A.B., Dittenber, D.A., Brzak, K.A. & Quast,
J.F. (1991) Chlorpyrifos: Two-generation dietary reproduction
study in Sprague-Dawley rats. Unpublished report No. K-044793-088
from the Toxicology Research Laboratory, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Bruce, R.J. & Zempel, J.A. (1986) Chlorpyrifos: Evaluation in the Ames
Salmonella/mammalian-microsome mutagenicity assay. Unpublished
report No. TexasT:K044793-075 from Lake Jackson Research Center,
Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Bruce, R.J., Gollapudi, B.B. & Hinze, C.A. (1985) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008 from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Buch SA (1980) Pyrinex tech: Acute inhalation toxicity in rats.
Unpublished report No. 80/MA/025/362 from Life Science Research,
Stock, Essex, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980a) Pyrinex tech: Irritance to rabbit
eye. Unpublished report No. 80/MA/023/143 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980b) Pyrinex tech: Irritance to rabbit
skin. Unpublished report No. 80/MAK/024/144 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1981) Pyrinex technical: Acute oral
toxicity in the rat. Unpublished report No. 81/MAK035/104 from
Life Science Research, Stock, Essex, United Kingdom. Submitted to
WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A., Gardner, J.R. & Birnie, J.V. (1980) Pyrinex: Acute
percutaneous toxicity study in rats. Unpublished report No.
80/MAK022/300 from Life Science Research, Stock, Essex, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Burns, C.J., Cartmill, J.B., Powers, B.S. & Lee, M.K. (1998) Update of
the morbidity experienced of employees potentially exposed to
chlorpyrifos. Occup. Environ. Med., 55, 65-70.
Bushnell, P.J., Padilla, S.S., Ward, T., Pope, C.N. & Olszyk, V.B.
(1991) Behavioral and neurochemical changes in rats dosed
repeatedly with diisopropylfluorophosphate. J. Pharmacol.
Exp. Ther., 256, 741-750.
Bushnell, P.J., Pope, C.N. & Padilla, S. (1993) Behavioral and
neurochemical effects of acute chlorpyrifos in rats: Tolerance to
prolonged inhibition of cholinesterase. J. Pharmacol. Exp.
Ther., 266, 1007-1017.
Calhoun, L.L. & Johnson, K.A. (1988) Chlorpyrifos: 4-day dermal probe
and 21-day dermal toxicity studies in Fischer 344 rats.
Unpublished report No. HET K 044793-085,086 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Calhoun, L.L. & Johnson, K.A. (1989) Supplemental information to the
report entitled: Chlorpyrifos: 4-day dermal probe and 21 day
dermal toxicity studies in Fischer 344 rats. Unpublished report
No. K-044793-085(S) from Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Campbell, C.G., Seidler, F.J. & Slotkin, T.A. (1997) Chlorpyrifos
interferes with cell development in rat brain regions. Brain
Res. Bull., 43, 179-189.
Capodicasa, E., Scapellato, M.L., Moretto, A., Caroldi, S. & Lotti, M.
(1991) Chlorpyrifosinduced delayed polyneuropathy. Arch.
Toxicol., 65, 150-155.
Chambers, J.E. (1992) The role of target-site activation of
phosphorothionates in acute toxicity. In: Chambers, J.E., Levi,
P.E. & Levi, A.P., eds, Organophosphates, San Diego, Academic
Press.
Chambers, J.E. & Carr, R.L. (1995) Biochemical mechanisms contributing
to species differences in insecticidal toxicity. Toxicology,
105, 291-304.
Chanda, S.M. & Pope, C.N. (1996) Neurochemical and neurobehavioural
effects of repeated gestational exposure to chlorpyrifos in
maternal and developing rats. Pharmacol. Biochem. Behav., 53,
771-776.
Chanda, S.M., Harp, P., Liu, J. & Pope, C.N. (1995) Comparative
developmental and maternal neurotoxicity following acute
gestational exposure to chlorpyrifos in rats. J. Toxicol.
Environ. Health, 44, 189-202.
Chaudhuri, J., Chakraborti, T.K., Chanda, S. & Pope, C.N. (1993)
Differential modulation of organophosphate-sensitivity muscarinic
receptors in rat brain by parathion and chlorpyrifos.
J. Biochem. Toxicol., 8, 207-216.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in beagle hounds. Unpublished report
No. T35.12-38278-3 from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Corley, R.A., Landry, T.D., Calhoun, L.L., Dittenber, D.A. & Lomax,
L.G. (1986) Chlorpyrifos: 13-week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET K
044793-077 from Dow Chemical Company Laboratory, Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Costa, L.G., McDonald, B.E., Murphy, S.D., Omenn, G.S., Richter, R.J.,
Motulsky, A.G. & Furlong, C.E. (1990) Serum paraoxonase and its
influence on paraoxon and chlorpyrifosoxon toxicity in rats.
Toxicol. Appl. Pharmacol., 103, 66-76.
Coulston, F., Golberg, L., Abraham, R. & Benitz, F.K. (1971) Final
report on safety evaluations and metabolic studies on Dowco 179
(Indiana 151). Unpublished report No. K44793-(48)A from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Coulston, F., Golberg, L. & Griffin, T. (1972). Safety evaluation of
Dowco 179 in human volunteers. Unpublished report from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Crown, S., Weiss, A., Nyska, A. & Waner, T. (1984a) Pyrinex. Toxicity
in dietary administration to mice for two weeks: A range-finding
study. Unpublished report No. MAK/109/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A. & Waner, T. (1984b) Pyrinex technical. Two-week
range-finding study in dietary administration to rats.
Unpublished report No. MAK/057/a/PYR from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Gur, E., Nyska, A. & Waner, T. (1985) Pyrinex technical
toxicity in dietary administration to rats for 13 weeks.
Unpublished report No. MAK/058/PYRA from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Weiss, A., Nyska, A., Waner, T. & Pirak, M. (1987) Pyrinex
technical. Toxicity in dietary administration to mice for 13
weeks. A preliminary study. Unpublished report No. MAK/105/PYR
from Life Science Research Israel Inc., Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A., Pirak, M. & Waner, T. (1988) Pyrinex technical.
Oncogenicity study in the rat. Unpublished report No. MAK/095/PYR
from Life Science Research Israel Ltd, Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Davies, R.E. & Kynoch, S.R. (1970) Acute subcutaneous toxicity of
Dursban to rats and the effect of treatment with 1. Atropine
sulphate 2. Atropine sulphate and PAM. Unpublished report No.
A1A-275 or 3641/70/453 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Davies, D.B., Tollett, J.T. & Lomax, L.G. (1985) Chlorpyrifos: A
four-week dietary study in the CD-1 mice. Unpublished report No.
HET-K-044793-068 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Dittenber, D.A., Hanley,
T.R., Jr & John, J.A. (1979) The effect of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Unpublished report No. HET-K-44793-32 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Rao, K.S., Dittenber, D.A.,
Hanley, T.R., Jr & John, J.A. (1980) Embryotoxicity and
fetotoxicity of orally administered chlorpyrifos in mice.
Toxicol. Appl. Pharmacol., 54, 31-40.
Derelanko, M.J. & Hollinger, M.A., eds (1995) CRC Handbook of
Toxicology, Boca Raton, FL, CRC Press Inc.
Dietz, F.K., Mensik, D.C., Hinze, C.A., Rachunek, B.K. & Taylor, H.W.
(1983) Dursban insecticide: Assessment of neonatal survival in a
two-generation reproduction study in rats. Unpublished report No.
TexasT-K-44793-(45) from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L. & Wetters, J.H. (1972a) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues and eggs
from chickens fed chlorpyrifos. Unpublished report No. GH-C 555
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., Rice, J.R., McKellar, R.L. & Pennington, J.Y.
(1972b) Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of cattle
fed chlorpyrifos. Unpublished report No. GH-C 566 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. & Rice, J.R. (1977)
Determination of residues of chlorpyrifos, its oxygen analogue,
and 3,5,6-trichloro-2-pyridinol in tissues of cattle fed
chlorpyrifos. J. Agric. Food Chem., 25, 1325-1329.
Dittenber, D.A. (1997) Chlorpyrifos: Evaluation of single oral doses
on cholinesterase and neurotoxic esterase inhibition in F344
rats. Unpublished report No. 960036 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Dreher DM (1994a) Chlorpyrifos technical: Acute oral toxicity test in
the rat. Unpublished report No. CHA 8-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994b) Chlorpyrifos technical: Acute dermal toxicity (limit
test) in the rat. Unpublished report No. CHA 9-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994c) Chlorpyrifos technical: Acute eye irritation test in
the rabbit. Unpublished report No. CHA 12-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994d) Chlorpyrifos technical: Acute dermal irritation test
in the rabbit. Unpublished report No. CHA 11-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994e) Chlorpyrifos technical: Magnusson & Kligman
maximisation study in the guinea pig. Unpublished report No. CHA
12-CYF from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to Who by Cheminova Agro A/S, Lemvig, Denmark.
Drevenkar, V., Vasilic, Z., Stengl, B., Frobe, Z. & Rumenjak, V.
(1993) Chlorpyrifos metabolites in serum and urine of poisoned
persons. Chem.-Biol. Interactions, 87, 315-322.
Eliason, D.A., Crammer, M.F., von Windeguth, D.L., Kilpatrick, J.W.,
Suggs, J.E. & Schoof, H.F. (1969) Dursban premise applications
and their effect on the cholinesterase levels of spraymen.
Mosquito News, 29, 591-595.
Emerson, J.L. & Gerbig, C.L. (1970) 91 day toxicology study in beagle
dogs treated with 3,5,6-trichloro-2-pyridinol. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Fikes, J.D., Zacchary, J.F., Parker, A.J. & Beasley, V.R. (1992)
Clinical, biochemical, electrophysiologic and histologic
assessment of chlorpyrifos induced delayed neuropathy in the cat.
Neurotoxicology, 13, 663-678.
Fredrick Institute of Plant Protection and Toxicology (1995a) Acute
oral toxicity study of chlorpyrifos technical in Swiss albino
mice. Unpublished report No. 2739 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995b) Acute
oral toxicity study of chlorpyrifos technical in Wistar rats.
Unpublished report No. 2738 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995c) Acute
dermal toxicity study of chlorpyrifos technical in rabbits.
Unpublished report No. 2748 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995d)
Irritation of chlorpyrifos technical to mucous membrane in
rabbits. Unpublished report No. 2746 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995e) Primary
skin irritation of chlorpyrifos technical in rabbits. Unpublished
report No. 2747 from Fredrick Institute of Plant Protection and
Toxicology, Tamil Nadu, India. Submitted to WHO by Ficom
Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996a) Acute
inhalation study with chlorpyrifos technical in Wistar rats.
Unpublished report No. 2968 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996b) Allergy
and skin sensitization potential of chlorpyrifos technical in
guinea pigs. Unpublished report No. 2928 from Fredrick Institute
of Plant Protection and Toxicology, Tamil Nadu, India. Submitted
to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996c)
Mutagenicity evaluation of chlorpyrifos technical in the
point-mutation Ames Salmonella typhimurium test system.
Unpublished report No. 3049 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996d)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
micronucleus assay. Unpublished report No. 3054 from Fredrick
Institute of Plant Protection and Toxicology, Tamil Nadu, India.
Submitted to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996e)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
bone marrow cytogenic assay. Unpublished report No. 3076 from
Fredrick Institute of Plant Protection and Toxicology, Tamil
Nadu, India. Submitted to WHO by Ficom Organics, Bombay, India.
Furlong, C.E., Richter, R.J., Seidel, S.L. & Motulski, A.G. (1988)
Role of genetic polymorphism of human plasma
paraoxonase/arylesterase in hydrolysis of the insecticide
metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum.
Genet., 43, 230-238.
Furlong, C.E., Richter, R.J., Seidel, S.L., Costa, L.G. & Motulsky,
A.G. (1989) Spectrophotometric assays for the enzymatic
hydrolysis of the active metabolites of chlorpyrifos and
parathion by plasma paraoxonase/arylesterase. Anal. Biochem.,
180, 242-247.
Geldmacher-von Mallinckrodt, M. & Diepgen, T.L. (1988) The human serum
paraoxonase polymorphism and specificity. Toxicol. Environ.
Chem., 18, 79-196.
Gerbig,, C.G. & Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Unpublished
report No. HH-240 from Dow Chemical Co., Zionsville, Indianna,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gerbig,, C.G. & Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat.
Unpublished report No. HH-239 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gerbig,, C.G. & Emerson, J.L. (1970c) Determination of oral minimum
lethal dose of 3,5,6trichloro-2-pyridinol in beagle dogs.
Unpublished report No. HH-253 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gibson, J.E. (1996) Critical review of allegations associating Dursban
with human teratogenicity. Unpublished report No. JEG122396, from
Dow Elanco, USA. Submitted by Dow Agrosciences, Indianapolis,
Indiana, USA.
Glas, R.D. (1977a) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from calves fed
3,5,6-trichloro-2-pyridinol. Unpublished report No. GH-C 1053
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977b) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from a calf fed
2-methoxy-3,5,6-trichloro-2-pyridine. Unpublished report No. GH-C
1052 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977c) Residues of triclopyr, 3,5,6-trichloro-2-pyridinol
and 2-methoxy-3,5,6trichloropyridine in bovine tissues from
calves fed trichlopyr. Unpublished report No. GH-C 1047 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981a) The metabolic fate of 14C-chlorpyrifos fed to
lactating goats. Unpublished report No. GH-C 1408 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981b) Identification of 14C-labelled residues in milk
from goats fed 14Cchlorpyrifos. Unpublished report No. GH-C
1470 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Gollapudi, B.B. (1987) Evaluation of 3,5,6-trichloro-2-pyridinol (TCP)
in the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. TexasT-K-38278-012 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Linscombe, V.A. & Wilkerson, J.E. (1985) Evaluation
of chlorpyrifos in the mouse bone marrow micronucleus test.
Unpublished report No. TexasT-K-044793-067 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Mendrala, A.L. & Linscombe, V.A. (1995) Evaluation of
the genetic toxicity of the organophosphate insecticide
chlorpyrifos. Mutat. Res., 342, 25-36.
Gur, E., Nyska, A. & Waner, T. (1991) Pyrinex technical oncogenicity
study in the mouse. Unpublished report from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Isreal.
Gutenmann, W.H., St John, L.E., Jr & Lisk, D.J. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow.
Unpublished report from Cornell University, Ithaca, New York,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gutmann, L. & Bodensteiner, J.B. (1993) Organophosphate-induced
delayed polyneuropathy (comment). J. Pediatr., 123, 837.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987a)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in Fischer 344
rats. Unpublished report No. K-038278-011 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987b)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in New Zealand
white Rrabbits. Unpublished report No. K-038278-015 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hardy, C.J. & Jackson, G.C. (1984) Dursban technical: Acute inhalation
toxicity in rats. Unpublished report No. DET 368 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Dawe, I.S. &
Anderson, A. (1989a) Chlorpyrifos oral dose range finding
toxicity study in beagle dogs (final report -- repeated daily
dosage for 4 weeks). Unpublished report No. MBS 30/88675 from
Huntingdon Research Centre Inc, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Majeed, S.,
Gopinath, C., Dawe, I.S. & Anderson, A. (1989b) Chlorpyrifos oral
toxicity study in beagle dogs (repeated daily dosage for 13
weeks). Unpublished report No. MBS 31/88999 from Huntingdon
Research Centre Inc, Huntingdon, United Kingdom. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Hazleton Laboratories (1968) Short term (subacute) dietary
administration -- Rats organophosphate insecticide Dursban. Final
report. Unpublished report No. 174-114 from Hazleton
Laboratories, Falls Church, Virginia, USA. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Henck, J.W. & Kociba, R.J. (1980) Three samples of Dursban
insecticide: Acute oral toxicity and acute percutaneous
absorption potential. Unpublished report No. A1A-130 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hoberman, A.M. (1998) Developmental neurotoxicity study of
chlorpyrifos administered orally via gavage to Crl:CD.BR VAF/Plus
presumed pregnant rats. Unpublished report No. K-044793109 from
Argus Research Laboratories, Horsham, PA, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Hooser, S.B., Beasley, V.R., Sundberg, J.P. & Harlin, K. (1988)
Toxicologic evaluation of chlorpyrifos in cats. Am. J. Vet.
Res., 49, 1371-1375.
Humbert, R., Adler, D.A., Distechhe, C.M., Hassett, C., Omiecinski,
C.J. & Furlong, C.E. (1993) The molecular basis of the human
serum paraoxonase activity polymorphism. Nature Genet., 3,
73-76
Jackson, D. & Ogilvie, S.W. (1994a) Chlorpyrifos tech: Acute dermal
toxicity (LD50) test in rats. Unpublished report No. 10439 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994b) Chlorpyrifos tech: Primary eye
irritation test in rabbits. Unpublished report No. 10441 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994c) Chlorpyrifos tech: Primary skin
irritation test in rabbits. Unpublished report No. 10440 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994d) Chlorpyrifos tech:
Magnusson-Kligman maximisation test in guinea pigs. Unpublished
report No. 10442 from Inveresk Research International, Tranent,
Scotland. Submitted to WHO by Luxembourg Industries, Tel Aviv,
Israel.
Jai Research Foundation (1996a) Reverse gene mutation study of
chlorpyrifos tech (95%) in bacteria by Ames Salmonella/microsome
test. Unpublished report No. 5/JRF/MichiganC/96 from Department
of Microbiology, Jai Research Foundation, Gujarat, India.
Submitted to WHO by Mitsu Industries, Gujarat, India.
Jai Research Foundation (1996b) Neurotoxicity study of chlorpyrifos
technical to hen. Unpublished report No. 1539/JRF/TOX/96 from
Department of Toxicology, Jai Research Foundation, Gujarat,
India. Submitted to WHO by Mitsu Industries, Gujarat, India.
James, P., Stubbs, A., Parker, C.A., Offer, J.M. & Anderson, A. (1988)
The effect of Pyrinex (chlorpyrifos) on reproductive function of
two generations in the rat. Unpublished report No. MBS 29/881452
from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Jeffrey, M.M., Battjes, J.E. & Eisenbrandt, D.L. (1986) Dursban F
(tech): Acute dermal toxicity study in Fischer 344 rats.
Unpublished report No. HET-K 044793-083 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Jones, J.R. (1985a) Dursban F: Eye irritation study in the rabbit.
Unpublished report No. DET 424 from Hazleton Laboratories Europe,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985b) Dursban F: Primary skin irritation and corrosivity
study in the rabbit. Unpublished report No. DET 421 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985c) Dursban F: Delayed contact hypersensitivity study
in the guinea pig (Buehler Test). Unpublished report No. DET 475
from Hazleton Laboratories Europe, Harrogate, North Yorkshire,
United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kamataki, T. & Neil, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothienate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfur
atom. Mol. Pharmacol., 12, 933-944.
Kaplan, J.G., Kessler, J., Rosenberg, N., Pack, D. & Schaumburg, H.H.
(1993) Sensory neuropathy associated with Dursban (chlorpyrifos)
exposure [published erratum appears in Neurology 1994 44, 367].
Neurology, 43, 2193-2196.
Kenaga, E.E. (1967) Comments on data concerning cholinesterase effects
of Dursban in man following US Public Health Service experimental
yellow fever mosquito eradication tests in Florida during 1966
and 1967. Unpublished report No.A1A-394, TK 2-1684 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kenny, T.J., Coombs, D.W. & Hardy, C.J. (1987) Chlorpyrifos technical
acute inhalation toxicity in rats 4-hour exposure. Unpublished
report No. MBS 20/87575 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Kenny, T.J., Coombs, D.W., Hardy, C.J. & Crook, D. (1988) Chlorpyrifos
inhalation toxicity in the rat. 14-Day preliminary dose range
finding study. Unpublished report No. MBS 21/871329 from
Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted by Makteshim Chemical Works, Beer-Sheva, Israel.
Kilian, D.J., Edwards, H.N., Benge, M.C. & Tabatabai, Z. (1970)
Results of human skin exposure to Dowco 179. Unpublished report
No. TMD-70-2 from Dow Chemical Co., Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Kisicki, J.C., Seip, C.W. & Combs, M.L. (1999) A rising dose
toxicology study to determine the no-observable-effect-levels
(NOEL) for erythrocyte acetylcholinesterase (AChE) inhibition and
cholinergic signs and symptoms of chlorpyrifos at three dose
levels. Unpublished report No. LLC Study ID: DR#K-044793-284
dated 19 April 1999, from MDS Harris, Lincoln, NE, USA. Submitted
to WHO by Dow Agrosciences, Indianapolis, Indiana, USA.
Kociba, R.J., McCollister, S.B., Keyes, D.G. & Dittenber, D.A. (1985)
Supplement to original report entitled 'Results of two-year
dietary feeding studies on Dowco 179 in beagle dogs'. Unpublished
report No. NBT 3512-44793-18 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lackenby, F. (1985a) Dursban F and Dursban F (OP1): Acute oral
toxicity studies in the rat. Unpublished report No. 4703-50/390 &
591 from Hazleton Laboratories Europe, North Yorkshire, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Lackenby, F. (1985b) Dursban F: Acute dermal median lethal dose in the
rat. Unpublished report No. 4206-50/391 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Landry, T.D., Streeter, C.M. & Lomax, L.G. (1985) Chlorpyrifos: 2-week
vapor inhalation toxicity study in Fischer 344 rats. Protocol.
Unpublished report No. A1A-714 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Landry, T.D., Dittenber, D.A., Lomax, L.G., Calhoun, L.L. & Morabito,
P. (1986) Chlorpyrifos: 2 week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET
K-44793-81 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Liberacki, A.B., Breslin, W.J., Dittenber, D.A. & Quast, J.F. (1990)
Chlorpyrifos: Two week dietary probe study in Sprague-Dawley
rats. Unpublished report No. HET K 044793090 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A. & Gollapudi, B. (1986) Evaluation of
3,5,6-trtichloro-2-pyridinol (TCP) in the Chinese hamster ovary
cell/hypoxanthine-guanine-phosphoribosyl transferase (CHO/HGPRT)
forward mutation assay. Unpublished report No.
TexasT:K-038278-013 from Dow Chemical Co., HES, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A., Mensik, D.C. & Clem, B.A. (1992) Evaluation of
chlorpyrifos in an in vitro chromosomal aberration assay
utilizing rat lymphocytes. Unpublished report No. TexasT:K
044793-092 from Dow Chemical Co., Freeport, Texas, USA. Submitted
to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lotti, M., Moretto, A., Zoppellari, R., Dainese, R., Rizzuto, N. &
Barusco, G. (1986) Inhibition of lymphocytic neuropathy target
esterase predicts the development of organophosphate-induced
delayed polyneuropathy. Arch. Toxicol., 59, 176-179.
Loveday, K.S. (1987) In vitro chromosomal aberration assay on Pyrinex
(chlorpyrifos). Unpublished report No. R4660 from Arthur D.
Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Loveday, K.S., Findlen, K.M.B. & Yadlon, S. (1987) Evaluation of
Pyrinex in the Ames mutagenesis assay. Unpublished report No.
59487-00 from Arthur D. Little Inc., USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Ma, T. & Chambers, J.E. (1994) Kinetic parameters of desulfuration and
dearylation of parathion and chlorpyrifos by rat liver
microsomes. Food Chem. Toxicol., 32, 763-767.
Maita, K., Hirano, M., Harada, T., Mitsumori, K., Yoshida, A.,
Takahashi, K., Nakashima, N., Kitazawa, T., Enomoto, A. & Inui,
K. (1988) Mortality, major cause of moribundity aqnd spontaneous
tumours in CD-1 mice. Toxicol. Pathol., 16, 340-349.
Mattsson, J.L., Wilmer, J.W., Shankar, M.R., Berdasco, N.M., Crissman,
J.W., Maurissen, J.P. & Bond, D.M. (1996) Single-dose and 13-week
repeated-dose neurotoxicity screening studies of chlorpyrifos
insecticide. Food Chem. Toxicol., 34, 393-405.
Maurissen, J.P., Shankar, M.R. & Mattsson, J.L. (1996) Chlorpyrifos:
Cognitive study in adult Long-Evans rats. Unpublished report No.
K-044793-096 from Dow Chemical Co., USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989a) Evaluation of chlorpyrifos
in the mouse bone marrow micronucleus test. Unpublished report
No. TexasT:K 044793-067A from Lake Jackson Research Center, Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989b) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008A from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco 179
in beagle dogs. Unpublished report No. T35.1244793-18 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco 179
in rats. Unpublished report No. NBT35.12-4479321 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J. & Keyes, D.G. (1985) Supplement to
original report entitled 'Results of two-year dietary feeding
studies on Dowco 179 in rats'. Unpublished report No.
NBT35.12-44793-21 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McKellar, R.L., Wetters, J.H. & Dishburger, H.J. (1972) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues of swine
fed chlorpyrifos. Unpublished report No. GHC549 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Mendrala, A.L. (1985) Evaluation of chlorpyrifos in the Chinese
hamster ovary cellhypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Unpublished
report No. HET-K-044793-072 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Mendrala, A.L. & Dryzaga, M.D. (1986) Evaluation of chlorpyrifos in
the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. HET K 044793-073 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Moretto, A. & Lotti, M. (1998) Poisoning by
organophosphorusinsecticides and sensory neuropathy.
J. Neurol. Neurosurg. Psych., 64, 463-468.
Mortensen, S.R., Chanda, S.M., Hooper, M.J. & Padilla, S. (1996)
Maturational differences in chlorpyrifos-oxonase activity may
contribute to age-related sensitivity to chlorpyrifos.
J. Biochem. Toxicol., 11, 279-287.
Moser, V.C. (1995) Comparisons of the acute effects of cholinesterase
inhibitors using a neurobehavioral screening battery in rats.
Neurotoxicol. Teratol., 17, 617-625.
Moser, V.C. & Padilla, S. (1998) Age- and gender-related differences
in the time course of behavioral and biochemical effects produced
by oral chlorpyrifos in rats. Toxicol. Appl. Pharmacol., 149,
107-119.
Muscarella, D.E., Keown, J.F. & Bloom, S.E. (1984) Evaluation of the
genotoxic and embryotoxic potential of chlorpyrifos and its
metabolites in vivo and in vitro. Environ. Mutag., 6, 13-23.
Nakatsugawa, T. (1992) Hepatic disposition of organophosphorus
insecticides: A synthesis of in vitro, in situ and in vivo data.
In: Chambers, J.E., Levi, P.E. & Levi, A.P., eds,
Organophosphates, San Diego, Academic Press, Chapter 10.
Newton, P.E. (1988a) A 5-day nose-only inhalation toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
from Bio/Dynamics Inc., East Millstone, NJ, USA. Submitted by
Makteshim Chemical Works, Beer-Sheva, Israel.
Newton, P.E. (1988b) A thirteen week nose-only toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
No. R-4750 from Bio/dynamics Inc., East Millstone, NJ, USA.
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984a) Pyrinex tech: Acute oral toxicity in
the rat. Unpublished report No. MAK/056/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984b) Pyrinex tech: Acute dermal toxicity
in rabbits. Unpublished report No. MAK/059/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nolan, R.J. (1997) Proposed reference dose for acute and chronic
exposure to chlorpyrifos based on the criteria described by the
Acute Cholinesterase Risk Assessment Task Force and the available
animal and human data. Unpublished report No. K-044793-110 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1982)
Chlorpyrifos: pharmacokinetics in human volunteers following
single oral and dermal doses. Unpublished report No.
HEB-DR-0043-4946-4 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1984)
Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol.
Appl. Pharmacol., 73, 8-15.
Nolan, R.J., Streeter, C.M. & Kastl, P.E. (1986) Chlorpyrifos:
Absorption by female Fischer 344 rats exposed for 6 hours to
vapours of chlorpyrifos in a nose-only or a whole-body inhalation
chamber. Unpublished report No. HET K-044793-82 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Dryzga, M.D., Landenberger, B.D. & Kastl, P.E. (1987)
Chlorpyrifos: Tissue distribution and metabolism of orally
administered 14C labelled chlorpyrifos in Fischer 344.
Unpublished report No. HET K-044793-(76) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Nostrandt, A.C., Padilla, S. & Moser, V.C. (1997) The relationship of
oral chlorpyrifos effects on behaviour, cholinesterase
inhibition, and muscarinic receptor density in rat.
Pharmacol. Biochem. Behav., 58, 15-23.
Omenn, G.S. (1987) The role of genetic differences in human
susceptibility to pesticides. In: Costa, L.G., Galli, C.L. &
Murphy, S.D., eds, Toxicology of Pesticides: Experimental,
Clinical and Regulatory Perspectives, Berlin, Springer-Verlag.
Osterloh, J., Lotti, M. & Pond, S.M. (1983) Toxicologic studies in a
fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. Anal.
Toxicol., 7, 125-129.
Ouelette, J.H., Dittenber, D.A., Kociba, R.J. & John, J.A. (1983a)
Chlorpyrifos: Oral teratology probe study in rats. Unpublished
report No. HET K-44793-(46) from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Ouelette, J.H., Dittenber, D.A., Kloes, P.M. & John, J.A. (1983b)
Chlorpyrifos: Oral teratology study in Fischer 344 rats.
Unpublished report No. HET K-44793-(47) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Pennington, J.Y. & Edwards, N.H. (1971) Comparison of cholinesterase
depression in humans and rabbits following exposure to
chlorpyrifos. Unpublished report no. TA-477 from Dow Chemical
Company USA. Submitted to WHO by Dow Agrosciences, Indianapolis,
Indiana, USA.
Phillips, J.E. & Lomax, L.G. (1989) 1% aqueous dilution of Dursban
acute aerosol inhalation toxicity study in Fischer 344 rats.
Unpublished report No. M-004803-004A from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Poole, D.C., Simmon, V.F. & Newell, C.W. (1977). In vitro mutagenic
activity of fourteen pesticides. Toxicol. Appl. Pharmacol., 41,
196.
Pope, C.N. & Chakraborti, T.K. (1992) Dose related inhibition of brain
and plasma cholinesterase in neonatal and adult rats following
sub-lethal organophosphate exposure. Toxicology, 73, 35-43.
Pope, C.N., Chakraborti, T.K., Chapman, M.L., Farrar, J.D. & Arthun,
D. (1991) Comparison of in vivo cholinesterase inhibition in
neonatal and adult rats by three organophosphorothioate
insecticides. Toxicology, 68, 51-61.
Pope, C.N., Chakraborti, T.K., Chapman, M.L. & Farrar, J.D. (1992)
Long-term neurochemical and behavioral effects induced by acute
chlorpyrifos treatment. Pharmacol. Biochem. Behav., 42,
251-256.
Richardson, R.J., Moore, T.B., Kayyali, U.S. & Randall, J.C. (1993a)
Chlorpyrifos: Assessment of potential for delayed neurotoxicity
by repeated dosing in adult hens with monitoring of brain
acetylcholinesterase, brain and lymphocyte neurotoxic esterase
and plasma butyrylcholinesterase activities. Fundam. Appl.
Toxicol., 21, 89-96.
Richardson, R.J., Moore, T.B., Kayyali, U.S., Fowke, J.H. & Randall,
J.C. (1993b) Inhibition of hen brain acetylcholinesterase and
neurotoxic esterase by chlorpyrifos in vivo and kinetics of
inhibition by chlorpyrifos oxon in vitro: Application to
assessment of neuropathic risk. Fundam. Appl. Toxicol., 20,
273-279.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Begg, S., Anderson, A.
& Dawe, I.S. (1987) Acute delayed neurotoxicity study with
chlorpyrifos in the domestic hen. Unpublished report No. MBS
16/87764 from Huntingdon Research Centre Ltd, Huntingdon, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Ross, D.B. & Roberts, N.L. (1974) The acute oral toxicity (LD50) of
Dursban (DOWCO 179) to the chicken (DWC/234/74877). Unpublished
report from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Rowe, L.D., Warner, S.D. & Johnston, R.V. (1978) Acute delayed
neurotoxicologic evaluation of chlorpyrifos in white Leghorn
hens. Unpublished report No. A1A-715 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Rubin, Y.,. Gal, N., Waner, T. & Nyska, A. (1987a) Pyrinex.
Teratogenicity study in the rat. Unpublished report No.
MAK/101/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Rubin, Y., Nyska, A. & Waner, T. (1987b) Pyrinex. Pyrinex
teratogenicity study in the rabbit. Unpublished report No.
MAK/103/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Scortichini, B.H., Lomax, L.G., Wolfe, E.L. & Hanley, T.R., Jr (1986a)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in
Fischer 344 rats. Unpublished report No. HETK-038278-006 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Scortichini, B.H., Lomax, L.G., Wolf, E.L. & Hanley, T.R., Jr (1986b)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in New
Zealand white rabbits. Unpublished report No. HET-K-038278-007
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Shankar, M.R., Bond, D.M. & Crissman, J.W. (1993) Chlorpyrifos:
13-week neurotoxicity study in Fischer 344 rats. Unpublished
report No. K-044793-094 from Dow Chemical Co., USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Sharp, L.D. & Warner, S.D. (1968) The clinical toxicity of Dursban in
the dog after multiple applications of an aerosol formulation.
Unpublished report from Dow Chemical Co., USA. Submitted to WHO
by Dow Agrosciences, Indianapolis, Indiana, USA.
Shemesh, I., Bourvin, A., Gold, D. & Kutscherowsky, M. (1988)
Chlorpyrifos poisoning treated with ipratropium and dantrolene: a
case report. J. Toxicol. Clin. Toxicol., 26, 495-498.
Sherman, J.D. (1996) Chlorpyrifos (Dursban)-associated birth defects:
Report of four cases. Arch. Environ. Health, 51, 5-8.
Sherman, J.D. & Herrick, R.B. (1973) Fly control and chronic toxicity
from feeding Dursban (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) to laying hens. Poultry Sci., 52, 741-747.
Sherman, J.D., Herrick, R.B., Ross, E. & Chang, M.T.Y. (1967) Further
studies on the acute and sub-acute toxicity of insecticides to
chicks. Toxicol. Appl. Pharmacol., 11, 49-67.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) Mutagenicity test of
chlorpyrifos in bacteria. Unpublished report No. GHF-R-47 from
Dow Chemical Pacific, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Shurdut, B.A., Chen, W.L., Burns, C.J., McCormick, R.A., Nolan, R.J. &
Racke, K.D. (1997) Critical assessment of report entitled 'Review
of chlorpyrifos poisoning data' (by J. Blondell and V. Dobozy,
January 14, 1997). Unpublished report No. GH-C 4359, by Dow
Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, Indiana, USA.
de Silva, H.J., Sanmuganathan, P.S. & Senanayake, N. (1994) Isolated
bilateral recurrent laryngeal nerve paralysis: A delayed
complication of organophosphorus poisoning. Hum. Exp.
Toxicol., 13, 171-173.
Smith, G.N., Watson, B.S. & Fischer, F.S. (1967) Investigations on
Dursban insecticide. Metabolism of [36Cl] O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothiate in rats. Agric.
Food Chem., 15, 132-138.
Smolen, A., Eckerson, H.W., Gan, K.N., Hailat, N. & LaDu, B.N. (1991)
Characteristics of the genetically determined allozymic forms of
human paraoxonase/artylesterase. Drug Metab. Disposition, 19,
107-112
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: Organophosphates. Mutat. Res., 102,
89-102.
Song, X., Seidler, F.J., Saleh, J.L., Zhang, J., Padilla, S. &
Slotkin, T.A. (1997) Cellular mechanisms for developmental
toxicity of chlorpyrifos: Targeting the adenylyl cyclase
signaling cascade. Toxicol. Appl. Pharmacol., 145, 158-174.
Stevenson, G.T. (1963) An LD50 toxicity study of Dursban in Leghorn
chickens. Unpublished report from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1966a) A single oral dose LD50 toxicity study of
Dursban in broiler type chickens. Unpublished report No. A1A-364
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Stevenson, G.T. (1966b) A neurotoxicity study of Dursban in laying
hens. Unpublished report No. A1A-390 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1967) A single oral dose LD50 toxicity study of
Dursban in turkey/poultry. Unpublished report No. A1A-362 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Streeter, C.M., Lomax, L.G., Landry, T.D. & Dittenber, D.A. (1987)
Chlorpyrifos: 2-week whole-body vapor inhalation toxicity study
in Fischer 344 rats. Unpublished report No. HET K 044793-078 from
Dow Chemical Co., Midland, Michigan, USA Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Sultatos, L.G. & Murphy, S.D. (1983) Hepatic microsomal detoxification
of the organophosphates paraoxon and chlorpyrifos oxon in the
mouse. Drug Metab. Disposition, 11, 232-238.
Szabo, J.R., Young, J.T. & Granjean, M. (1988) Chlorpyrifos: 13-week
dietary toxicity study in Fisher 344 rats. Unpublished report No.
TexasT: K-044793-071 from Lake Jackson Research Center, Health
and Environmental Sciences, Freeport, Texas, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Taylor, M.L. & Olson, K.J. (1963) Toxicological properties of
O,O-diethyl-O-3,5,6trichloro-2-pyridyl phosphorothioate.
Unpublished report. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Thompson, D.J., Gerbig, C.C. & Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Tu, A.S. (1987) CHO/HGPRT in vitro mammalian cell mutation assay on
Pyrinex (chlorpyrifos). Unpublished report No. 59487 from Arthur
D. Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Warner, S.D., Gerbig, C.G., Strebing, R.J. & Molello, J.A. (1980)
Results of a two-year toxicity and oncogenic study of
chlorpyrifos administered to CD-1 mice in the diet. Unpublished
report from Dow Chemical Co., Indianapolis, Indiana, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Whitney, K.D., Seidler, F.J. & Slotkin, T.A. (1995) Developmental
neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicol.
Appl. Pharmacol., 134, 53-62.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Wilmer, J.W. (1992) Acute neurotoxicity study in Fischer 344 rats.
Unpublished report No. K-044793-093B from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Wilson, J.A. & MacBeth, D. (1994) Chlorpyrifos tech: Acute oral
toxicity (LD50) test in rats. Unpublished report No. 10521 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Wolcott, R.M., Vaughn, W.K. & Neal, R.A. (1972) Comparison of the
mixed function oxidase-catalyzed metabolism of a series of
dialkyl p-nitrophenyl phosphorothionates. Toxicol. Appl.
Pharmacol., 22, 213-220.
Yang, R.S.H., Hodgson, E. & Dauterman, W.C. (1971) Metabolism in vitro
of diazinon and diazoxon in rat liver. J. Agric. Food Chem.,
19, 10-13.
Young, J.T. & Grandjean, M. (1988) Chlorpyrifos: 2 year dietary
chronic toxicity-oncogenicity study in Fischer 344 rats.
Unpublished report No. K-044793-079 from Lake Jackson Research
Center, Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Zempel, J.A. & Bruce, R.J. (1986) 3,5,6-Trichloro-2-pyridinol:
Evaluation in the Ames Salmonella/mammalian-microsome
mutagenicity assay. Unpublished report No. TexasT:K038278-010
from Lake Jackson Research Center, Dow Chemical Co., Freeport,
Texas, USA. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Zempel, J.A., Rachunek, B.L. & Szabo, J.R. (1987)
3,5,6-Trichloro-2-pyridinol: Results of a one-year dietary study
in male and female beagle dogs. Unpublished report No.
TexasT:K038278-009 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
(c) Effects on enzymes and other biochemical parameters
Hydrolysis is the most important route of detoxification of
organophosphorus esters. Hydrolytic esterases are distributed
ubiquitously in the blood and tissues of virtually all animal
organisms and catalyse the hydrolysis of a variety of esters,
including organophosphorus esters, but have little activity on
organophosphorus itself. Esterases that interact with
organophosphoruses have been characterized as A- and B-esterases
according to their sensitivity to inhibition by organophosphorus
compounds. Plasma and tissues of mammals have significant
concentrations of the calcium-activated A-esterases, arylesterase (EC
3.1.1.2), and the high-density lipoprotein-associated paroxonase (EC
3.1.8.1). The B-esterases, including aliesterases (EC 3.1.1.1; also
called carboxylesterases) and cholinesterases (e.g. butyryl
cholinesterase, EC 3.1.1.8), are inhibited by organophosphorus
compounds such as chlorpyrifos oxon. While they bind them, they do not
hydrolyse them (Derelanko & Hollinger, 1995).
Butyrylcholinesterase is the predominant cholinesterase in human
serum (> 99%), while in rats there is an approximately equal
distribution of acetylcholinesterase and butyryl cholinesterase
(Nolan, 1997). These enzymes detoxify organophosphorus compounds by
sequestering them through binding to or phosphorylation by blood
proteins, such as albumin, which stoichiometrically degrades them
(Aldridge, 1953). These protein-bound organophosphorus esters are
secreted into the bile. Erythrocyte acetylcholinesterase (EC 3.1.1.7)
is similar to the acetylcholinesterase in nervous systems and can bind
to and sequester or hydrolyse organophosphorus compounds. The enzyme
activities attributable to A-esterases, B-esterases, and
cholinesterases vary widely within populations and are influenced by
genetic and environmental factors and disease states (Derelanko &
Hollinger, 1995). The serum activity of paraoxonase in rabbits is some
40 times greater than that in rats. Most avians, including hens, have
relatively low activity of A-esterases, and the mean value of serum
chlorpyrifos oxonase activity in humans is 10 times that of rat serum
(Furlong et al., 1989).
In humans, a substrate-dependent polymorphism of serum
paraoxonase is observed, in which one isoform of paraoxonase has a
high turnover for paraoxon and the other a low turnover (Furlong et
al., 1989; Smolen et al., 1991). The polymorphism is also observed
with the oxons of methyl parathion, chlothion, and
phenylphosphonothioic acid O-ethyl O-para-nitrophenyl ester. The
two isoforms appear, however, to hydrolyse chlorpyrifos oxon and
phenylacetate at the same rate. Cloning and sequencing of the human
paraoxonase cDNA has revealed the molecular basis of the polymorphism:
arginine at position 192 determines high paraoxonase activity, and
glutamine at this position encodes low paraoxonase activity (Humbert
et al., 1993). In addition to this polymorphism, a 13-fold variation
in serum enzyme activity of a given genetic class is seen (Furlong et
al., 1989). While chlorpyrifos oxon was hydrolysed by the same plasma
fraction that hydrolysed paraoxon in a study of plasma samples from
320 white blood donors (Furlong et al., 1988), the population
distribution of chlorpyrifos oxon hydrolysis was unimodal. This
contrasted with a bimodal population distribution of the activity for
paraoxon hydrolysis, and the variation in paraoxonase activity was
about 11-fold, while that in chlorpyrifos oxonase activity showed a
four- to fivefold variation.
Correlation of the LD50 values for orally administered
chlorpyrifos in rats with the activation rates measured in brain
rather than liver (Chambers, 1992) suggested that local
biotransformation in target tissues is an important determinant of its
toxicity. In a more recent review, however, Chambers & Carr (1995)
compared published LD50 or LC50 values for a variety of
insecticides in several vertebrate species. Studies in rats indicated
that the sensitivity of brain acetylcholinesterase activity to
inhibition by various phosphorothionate oxons did not correlate with
their acute toxicity. Chlorpyrifos oxon has a greater affinity for rat
brain acetylcholinesterase than does paraoxon, with median inhibitory
concentrations (IC50 values) of 4.0 and 22 nmol/L, respectively, but
a lower LD50. This finding is consistent with the greater affinity
(IC50, 0.75 nmol/L) of chlorpyrifos oxon for plasma aliesterases
than for rat brain acetylcholinesterase, indicating that aliesterases
provide protection against chlorpyrifos oxon.
Further work by Chambers & Carr (1995) indicated longer
inhibition of esterases after exposure chlorpyrifos than to parathion.
This was attributed to the greater lipophilicity of chlorpyrifos than
parathion (hexane:acetonitrile partition coefficients, 0.28 and 0.062,
respectively) and the assumption that a substantial fraction of the
dose of chlorpyrifos would have been sequestered by fat and released
gradually for later bioactivation. Studies of hepatic microsomal
metabolism of parathion and chlorpyrifos indicated that desulfuration
of parathion was favoured over dearylation (activation vs
deactivation), whereas the reverse was seen for chlorpyrifos (Ma &
Chambers, 1994). In channel catfish, however, the sensitivity of
acetylcholinesterase to inhibition by oxons reflects the acute
toxicity of these insecticides, and may be largely responsible for
their toxicity in this species. Thus, the metabolism of insecticides
appears to be more influential in some species than in others in
determining their toxicity.
Chlorpyrifos oxonase is a calcium-dependent A-esterase that
hydrolyses chlorpyrifos oxon, the active metabolite of chlorpyrifos.
As this activity may be related to that of paraoxonase, it was
determined spectrophotometrically by measuring the generation of TCP
(Furlong et al., 1989). Mortensen et al. (1996) hypothesized that
young rats have less chlorpyrifos oxonase activity than adults, and
they measured the activity of this enzyme in the brain, plasma, and
liver of male Long-Evans rats (CRL:(LE)BR) at postnatal day 4 and in
adults. No activity was detected in brain at either age, but the
activities in plasma and liver were markedly lower in younger than in
adult animals, the activities in plasma and liver being 1/11 and 1/2
of those in adults, respectively.
As the Michaelis-Menten constant for chlorpyrifos oxonase
activity was high (210-380 µmol/L), experiments were performed to
determine whether this enzyme could hydrolyse physiologically relevant
concentrations (nanomolar to low micromolar) of chlorpyrifos oxon. On
the assumption that tissue acetylcholinesterase inhibition provides a
sensitive bioassay for the concentration of chlorpyrifos oxon, changes
in the tissue acetylcholinesterase IC50 for chlorpyrifos oxon were
measured in the presence and absence of chlorpyrifos oxonase. Brain
acetylcholinesterase activity was determined spectrophotometrically,
while plasma and liver cholinesterase activities were determined by a
radiometric method with a [3H]acetylcholine iodide substrate. An
increase in the 'apparent' IC50 would indicate that chlorpyrifos
oxonase had hydrolysed substantial amounts of chlorpyrifos oxon during
the 30-min preincubation with the chlorpyrifos oxon. In the adult
rats, the apparent IC50 values for both plasma and liver
cholinesterase were higher in the presence of chlorpyrifos oxonase,
suggesting that the enzyme in those tissues could hydrolyse
physiologically relevant concentrations of chlorpyrifos oxon within
30 min. Young animals, however, showed less of a shift in the IC50
curves than adults, confirming that they have a lower capacity to
detoxify physiologically relevant concentrations of chlorpyrifos oxon
with chlorpyrifos oxonase.
2. Toxicological studies
(a) Acute toxicity
(i) Lethal doses
Numerous studies have been carried out with technical-grade
chlorpyrifos to establish LD50 and LC50 values (Table 1). The
lowest value after oral administration was 96 mg/kg bw (range,
96-480 mg/kg bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in
mice. The signs of acute intoxication were consistent with
cholinesterase inhibition and included inactivity, salivation,
dyspnoea, flaccid paralysis, vomiting, piloerection, exophthalmia, and
diarrhoea; female animals were generally more sensitive to the acute
effects of chlorpyrifos than males. The LD50 for dermally applied
chlorpyrifos was consistently lower than that for oral or inhalational
exposure and indicated the lower dermal absorption of chlorpyrifos
(about 2% in humans) when compared with the 70-90% absorption after
inhalational or oral exposure.
(ii) Dermal and ocular irritation and dermal sensitization
Ocular irritation: In a paper in which few procedural details
were provided (Taylor & Olson, 1963), slight conjunctival redness was
seen in rabbits treated with chlorpyrifos, regardless of whether the
eyes were washed after administration of the test material. The
redness persisted for more than 7 days in some animals. Transient
conjunctival and iridial irritation, possibly due to a direct physical
irritating effect of cholorpyrifos powder, was seen in three young
adult female New Zealand white rabbits after each had received 100 mg
of a finely ground commercial preparation in the left conjunctival
sac. The eyes of all animals were normal after 24 h (Jones, 1985a).
The ocular irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested (in accordance with GLP requirements) in six
young male New Zealand white rabbits, 100 mg of test material being
placed into the right eye and the untreated left eye serving as a
control. There were no signs of corneal or iridial irritation. Slight
to moderate conjunctival redness (mean Draize score, 1 at 1 h, 0.83 at
24 h), slight chemosis (mean Draize score, 0.83 at 1 h, 0.16 at 24 h),
and discharge (mean Draize score, 1.6 at 1 h, 0.83 at 24 h) were seen
up to 24 h after instillation, but no signs of irritation were
reported at 48 h (Jackson & Ogilvie, 1994b).
The ocular irritation potential of 0.1 g of chlorpyrifos (purity,
99.3%) was assessed in three male and three female New Zealand white
rabbits by instilling the test substance into the conjunctival sac of
the right eye, the left eye remaining untreated and serving as
control. This study was conducted according to GLP requirements.
Diffuse corneal opacities (score 1 for degree and area of opacity)
were observed in two animals at 24 h, but these effects had
disappeared by 48 h and no other corneal effects were seen. Iridial
inflammation (score 1) was noted in four animals at 1 h, and this
effect persisted for 24 h in one of the animals and for 48 h in
another. No iridial effects were observed after 72 h. Conjunctival
irritation was observed in all animals, with redness (score 2:
diffuse, deeper crimson red at 1-24 h; score 1: vessels definitely
injected above normal at 48 h), chemosis (score 2: obvious swelling
with partial eversion of lids up to 48 h), and discharge (scores 2-3:
moistening of the lids and hairs just adjacent to lids or a
considerable area around the eye only at 1 h). No signs of
conjunctival irritation was observed at 72 h. The maximum individual
irritation scores were seen at 1 h and ranged from 12 to 19, with a
mean score of 16. The mean scores at 24, 48, and 72 h were 9.3, 2.5,
and 0, respectively. The overall scores according to EEC Council
Directive 67/548/EEC were 2 (mean, 0.11) for corneal opacity, 3 (0.17)
for iridial inflammation, 11 (0.61) for conjunctival redness, and 8
(0.44) for conjunctival chemosis (Dreher, 1994d)
Technical-grade chlorpyrifos (100 mg; purity, 96.2%) was
instilled into the conjunctival sac of the left eye of Himalayan
albino rabbits, and the right eyes of the animals served as controls.
A group of control animals was similarly treated with distilled water
only. All animals were observed for signs of ocular irritation at 1,
4, 24, 48, and 72 h, and then daily up to day 7. At the 4-h
observation, very slight iridial irritation (score 1) and conjunctival
irritation (score 1) were reported. It was not clear from the report
whether the conjunctival irritation was redness, discharge, or
chemosis. No signs of irritation were reported at 24 or 72 h. A
'quality assurance statement' was issued for this study (Frederick
Institute of Plant Protection and Toxicology, 1995d).
Table 1. Studies of the acute toxicity of technical-grade chlorpyrifos
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Oral administration
Mouse SmithWebster M 1% aqueous gum 102 (94-110) Coulston et al. (1971)
tragacanth
Mouse NAMRU F Soya bean oil 152 (143-162) Berteau & Deen (1978)
Mouse Swiss albino M&F Vegetable oil 109 (93-127) QA Fredrick Institute (1995a)
Rat NR M 5% corn oil 163 (97-276) Taylor & Olson (1963)
F 135 (97-188)
Rat Sprague-Dawley F Soya bean oil 169 (146-196) Berteau & Deen (1978)
Rat Sprague-Dawley M Arachis oil 276 (167-455) GLP Dreher (1994a)
F 350 (285-429)
M&F 320 (260-393)
Rat Wistar M&F Vegetable oil 134 (102-163) QA Frederick Institute (1995b)
Rat Sprague-Dawley M Maize oil 264 GLP Wilson & MacBeth (1994)
F 141
M&F 192
Rat Sprague-Dawley M Maize oil 475 (311-727) Buch & Gardner (1981)
F 337 (220-515)
Rat Sprague-Dawley M Corn oil 221 (181-69) Nissimov & Nyska (1984a)
F 144 (105-200)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M Not reported 205 (134-299) Henck & Kociba (1980)
F 96 (72-140)
M 248 (170-379)
F 97 (80-112)
M 270 (188-426)
F 174 (130-244)
Guinea-pig NR M Corn oil 504 (300-850) GLP Lackenby (1985a)
Rabbit NR M Corn oil 1000-2000 GLP Lackenby (1985a)
Chicken NR M Capsule 32 (14-72) GLP Lackenby (1985a)
Chicken Leghorn M Diet 25 (21-31) Sherman et al. (1967)
Chicken Leghorn M Capsule 32 Stevenson (1963)
Chicken White Rock M&F Capsule 50-63 Stevenson (1966a)
Chicken NR M&F Capsule 20-50 Ross & Roberts (1974)
Chicken NR M&F Corn oil 102 (64-169) Ross & Roberts (1974)
Chicken NR M Capsule, 32 (14-72) Taylor & Olson (1963)
undiluted
Turkey Beltsville NR NR 32-63 Stevenson (1967)
small white
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Inhalation
Mouse NAMRU F 65% xylene 94 (83-106) Berteau & Deen (1978)
(27-50 min,
whole body,
aerosol)
Rat Albino M&F Vapour (4 h, > 200a GLP Hardy & Jackson (1984)
(HC/CFHB) whole body)
Rat Sprague-Dawley F 65% xylene 78 (57-108) Berteau & Deen (1978)
(60-180 min,
whole body,
aerosol)
Rat Sprague-Dawley M&F Undiluted (4 h, > 230b (max. GLP Anderson et al. (1995)
nose only, attainable
powder) concentration)
Rat Sprague-Dawley M 40% xylene > 4070a Buch (1980)
F (4 h, nose only, 2890b (2010-4160)
aerosol)
Rat Wistar M&F Undiluted (4 h, > 1020a GLP Kenny et al. (1987)
whole body,
aerosol)
Rat Fischer 344 M&F 1% aqueous > 3200a GLP Phillips & Lomax (1989)
solution (4 h,
whole body,
aerosol)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat Sprague-Dawley M&F Vapour (4 h, > 36a (max. GLP Blagden (1994)
nose only) attainable
concentration)
Rat Wistar M&F Acetone (4 h, 560b (360-950) Fredrick Institute (1996a)
whole body,
nebulized particles
< 5 µm)
Dermal application
Rat Sprague-Dawley M&F PEG > 2000 Lackenby (1985b)
Rat Sprague-Dawley M&F Undiluted > 2000 GLP Jackson & Ogilvie (1994a)
Rat NR M&F Undiluted > 2000 QA Nissimov & Nyska (1984b)
Rat Fischer M&F Undiluted > 2000 GLP Jeffrey et al. (1986)
Rat Sprague-Dawley M&F Arachis oil > 2000 GLP Dreher (1994b)
Rat Sprague-Dawley M&F Saline > 5000 (intact Buch et al. (1980)
and abraded)
Rabbit Himalayan M&F Water 1233 (993-1531) QA Fredrick Institute (1995c)
Rabbit New Zealand M&F Undiluted 1580 (828-2606) Henck & Kociba (1980)
white M&F 1598 (1243-1919)
M&F 1801 (1023-3152)
Table 1. (continued)
Species Strain Sex Vehicle LD50 (mg/kg bw; GLP Reference
95% CI or range) or QA
Rat CFY M&F Tween 20/ 147 (120-179) Davies & Kynoch (1970)
DMSO/water
2:3:5 (subcutaneous)
M, male; F, female; NR, not reported; GLP, good laboratory practice; QA, quality assurance; PEG, polyethylene glycol;
DMSO, dimethyl sulfoxide
Technical-grade chlorpyrifos (100 mg; fine powder) was placed
into the right eye of nine young adult albino rabbits. The eyes of
three animals were irrigated with saline solution 30 s after
treatment, while the eyes of the remaining animals were not irrigated.
Ocular examinations were conducted after 24, 48, and 72 h and at 4 and
7 days. Most of the animals displayed slight initial pain after
instillation of the test material. No signs of irritation were
observed in the cornea or the iris. Slight conjunctival redness was
observed in all animals with unwashed eyes, and in two animals whose
eyes were irrigated after 30 s. The irritation disappeared in most
animals by days 3-4 but persisted for 7 days in a single animal with
unirrigated eyes. The mean irritation scores in rabbits with unwashed
eyes were 2.3 at 24 h and 0.3 at 7 days, while in the rabbits with
irrigated eyes the scores were 1.3 and 0, respectively (Buch &
Gardner, 1980a).
Dermal irritation: A finely ground commercial preparation of
chlorpyrifos (500 mg) moistened with distilled water was applied under
an occlusive dressing to about 6 cm2 of clipped skin on the back of
three young adult female New Zealand white rabbits as a single 4-h
application. No primary irritation was observed (Jones, 1985b).
The dermal irritation potential of technical-grade chlorpyrifos
(purity, 99%) was tested in a study conducted according to GLP in six
young male New Zealand white rabbits, to which 500 mg of the material
(moistened with water) were applied under a gauze patch on clipped
intact skin on the trunk and then covered with an occlusive dressing.
The dressings were removed after 4 h and the skin wiped to remove
residual material. The skin was examined for signs of irritation 1,
24, 48, and 72 h after removal of the patch and scored for irritation
on the basis of a system recommended by the US Environmental
Protection Agency. Further assessments were conducted after 4, 5, and
6 days to determine the reversibility of the effects. Slight erythema
(score 1) was noted on three of six test sites at 1 h, and this effect
persisted until 72 h at one site and until day 5 in another animal. No
skin irritation was seen on day 6 (Jackson & Ogilvie, 1994dc).
A technical-grade, fine-powder commercial preparation (purity not
stated) was impregnated at a dose of 0.5 g onto unmedicated lint
patches and tested in six young adult male New Zealand white rabbits
on shaved abraded and intact sites on the back. The patches were held
in place under impermeable dressings, which were removed after 23 h.
The reactions at the test sites were assessed by the method of Draize
at 24 and 72 h and 7 days after administration of the test material.
Well-defined or very slight erythema was seen in all animals at 24 h,
on both intact and abraded sites. Very slight oedema was also seen at
the intact and abraded test sites of one animal at 24 h, another at 72
h, and a third at 7 days. No skin reactions were seen in other animals
at these intervals. The primary irritation index was calculated to be
0.67 (Buch & Gardner, 1980b).
Technical-grade chlorpyrifos (purity, 96.2%) was applied at a
dose of 500 g to clipped intact and abraded skin on the trunks of
three male and three female Himalayan albino rabbits, and the trunks
were wrapped with plastic sheets held in place with tapes for 24 h. A
control group was treated with distilled water. After exposure, the
coverings were removed, but the report did not indicate whether the
test sites were washed or cleaned. The skin was observed 24 and 72 h
after application for signs of irritation. No sign of erythema or
oedema was reported. A 'quality assurance statement' was issued for
this study (Frederick Institute of Plant Protection and Toxicology,
1995e).
Technical-grade chlorpyrifos (purity, 99.3%), moistened with
0.5 ml distilled water, was applied at a dose of 0.5 g to clipped
areas on the backs of New Zealand white rabbits in a study carried out
in accordance with GLP. Surgical gauze was applied over the sites and
held in place with a strip of surgical adhesive tape, and the trunk of
each rabbit was wrapped in an elasticized corset. After the 4-h
contact period, the corset and patches were removed, and the treated
skin was wiped with cotton-wool soaked in distilled water. One hour
and approximately 24, 48, and 72 h after removal of the patches, the
test sites were examined for evidence of dermal irritation and scored
according to the method of Draize. Very slight (score 1) to
well-defined (score 2) erythema and very slight (score 1) to slight
(score 2) oedema were observed in all animals 1-24 h after removal of
the patches. At 48 h, very slight erythema was observed in three
animals and very slight oedema in one. No sign of skin irritation was
seen at 72 h. The sum of the readings at 24 and 72 h was 10, and the
primary irritation index (the sum of the readings/12) was 0.8. This
score indicates mild irritation according to Draize (Dreher, 1994d).
Dermal sensitization: The skin sensitizing potential of
technical-grade chlorpyrifos (purity, 99.3%) was assessed in female
albino Dunkin-Hartley guinea-pigs in a study conducted according to
GLP. Very slight (score 1; 10 animals) to well-defined (score 2; 2
animals) erythema and very slight (score 1; 1 animal) oedema were
observed 1 h after topical induction, but no signs of irritation were
observed at 24 h. No skin reaction was observed in control animals,
and no skin reactions were observed at the test or vehicle control
sites after topical challenge 21 days after induction. One animal was
found dead at this stage of the study, but the cause of death was not
determined. The test material was not a skin sensitizer in this study
(Dreher, 1994e).
Technical-grade chlorpyrifos (purity, 96.2%) was prepared as a 1%
solution in 0.5% carboxymethyl cellulose and tested at a dose of
100 mg in male Hartley guinea-pigs. Test and positive control groups
received induction by exposure of the shaven skin of the left flank,
and challenge 14 days after the third induction dose. Control animals
received only a challenge with 0.5 ml of a 0.1% solution. The test
animals had barely perceptible (score 1; 4/10 animals) erythema or
slight oedema (score 1; 2/10 animals) 24 h after the challenge, but no
signs of irritation were observed at 48 h. A single control animal had
barely perceptible erythema at 24 h, but no other signs of skin
irritation were seen in this group. Positive control animals had
barely perceptible to deep-red erythema (score 1-3) and slight to
marked oedema (score 1-3) 24 h after the challenge and barely
susceptible erythema and slight to marked oedema at 48 h. This study
was issued a 'quality assurance statement' (Frederick Institute of
Plant Protection and Toxicology, 1996b).
After a study to determine a suitable dosing regimen, six male
and six female young albino Dunkin-Hartley guinea-pigs were treated
with a commercial preparation of chlorpyrifos in a standard Beuhler
skin sensitization assay. The test substance (0.3 ml, 100% w/v in
polyethylene glycol on a lint pad) was applied for 6 h under an
occlusive bandage to the freshly clipped left flank of the animals on
days 1, 8, and 15. Challenge doses of chlorpyrifos and solvent were
applied for 6 h on day 29 to the right and left flanks, respectively.
The negative controls were treated only on day 29, when they received
the same treatment as the test animals. The challenge sites of both
groups were evaluated 24 and 48 h after removal of the patch. No
adverse skin reactions were seen after exposure to chlorpyrifos or
vehicle alone (Jones, 1985c).
Technical-grade chlorpyrifos (purity, 99%) was tested in young
female Dunkin-Hartley guinea-pigs by the Magnusson-Kligman
maximization test in a study conducted according to GLP. The test
consists of an induction with intradermal injections of the test
material followed after 1 week by topical application, and a challenge
3 weeks after induction. The test sites were assessed for irritation
24, 48, and 72 h after injection and 24 and 48 h after removal of the
patch (48-h exposure). A concentration of 25% of the test material in
maize oil was selected for injection in the induction phase and 75% in
maize oil for the topical induction phase; a concentration of 75% in
maize oil was also selected for the challenge. During the induction
phase, slight or discrete erythema was observed in all test and
control animals 1 and 24 h after injection and 1 h after topical
application. A number of test and control animals also showed slight
or discrete erythema 24 h after topical application. During
application, two test animals became ataxic, thin, and subdued and
were removed from the study, and one test animal was removed from the
study before the challenge application because it had lesions at the
test site. A single test animal showed a positive response to
challenge, with slight to discrete erythema. None of the animals given
the vehicle alone reacted to the challenge application (Jackson &
Ogilvie, 1994a).
Technical-grade chlorpyrifos (purity, 96.8%) dissolved in 100%
dimethylsulfoxide and diluted in the same solvent to achieve the
desired concentrations was tested in a study that conformed to GLP in
albino Dunkin-Hartley guinea-pigs. In animals treated with a single
dose, irritation ranging from scattered mild redness to moderate and
diffuse redness was seen at 24 h in 5/10 animals; only mild redness
was seen at 48 h in 4/10 animals. In 20 animals induced and then
challenged with chlorpyrifos, only one had scattered mild redness
after the challenge dose at 24 h, and no skin reaction was seen at 48
h. In a positive control group, 9/10 animals had mild redness at 24 h
and 4/10 at 48 h (Berman, 1987).
(b) Short-term studies of toxicity
Mice
In a 2-week study conducted according to GLP requirements, young
adult CD-1 mice, were given a commercial preparation of
technical-grade chrlopyrifos (purity, 96.3%) at dietary concentrations
of 0 (control), 75, 150, 300, 600, or 1200 ppm, equaivalent to 0,
14-18, 30-32, 56-67, 89-100, and 130-180 mg/kg bw per day,
respectively. Deaths and treatment-related clinical signs, including
tremor, ocular opacity, ocular staining, hunching, and lachrymation,
were seen mainly in animals at the highest dose. Marked reductions in
body weight and feed consumption were observed in animals at doses
> 600 ppm. In males, cholinesterase activity was reduced at all
doses. At 75 ppm, plasma cholinesterase activity was reduced by
> 95%, erythrocyte acetylcholinesterase activity by almost 40%, and
brain acetylcholinesterase activity by about 50%. Inhibition of
erythrocyte acetylcholinesterase activity was not dependent on dose,
and approximately 35% inhibition being seen at all doses, but plasma
and brain cholinesterase activities were reduced in a dose-related
manner, with an inhibition of 99% in plasma and about 89% in brain at
the high dose. Similar patterns of cholinesterase inhibition were seen
in females. On the basis of the results of this study, doses of 5, 50,
200, 400, and 800 ppm were selected for a 13-week dietary study in
mice (Crown et al., 1984a).
Groups of 40 male and 40 female CD-1 mice were given chlorpyrifos
(purity, 95.7%) in the diet at 0 or 15 ppm, equal to 2.7 mg/kg bw per
day for males and 3.4 mg/kg bw per day for females; 20 animals of each
sex per group were killed after 1 week, and 20 were treated for a
total of 4 weeks. There were no significant clinical signs or
unscheduled deaths. Food consumption was unaffected by treatment, but
the body-weight gain of male mice was decreased by 25% at the end of
the study. Organ weights were not affected by treatment, and there
were no significant differences between groups. The findings at
necropsy were within normal limits for all groups. After 1 week,
plasma cholinesterase activity was depressed by 88-91% and erythrocyte
acetylcholinesterase activity was depressed by 40-53%; after 4 weeks,
plasma cholinesterase activity was depressed by 91% and erythrocyte
acetylcholinesterase activity by 53%. Brain acetylcholinesterase
activity was unaffected by treatment at either time. There was no
difference between the sexes in the alterations in plasma and
erythrocyte cholinesterase activity (Davies et al., 1985).
Groups of 12 Crl CD-1 mice of each sex were given chlorpyrifos
(purity, 93.5%) in the diet at concentrations of 0, 5, 50, 200, 400,
or 800 ppm (equivalent to 0, 0.7-1.3, 7.1-14, 32-53, 41-140, and
110-300 mg/kg bw per day, respectively) for 13 weeks. This study was
conducted to the requirements of GLP. Dose-related increases in the
mortality rate and the frequency of ocular opacities were seen at 400
and 800 ppm. Body weights were reduced by 10-15% in mice at 800 ppm
throughout the study. Plasma cholinesterase activity was markedly
decreased at all doses, and decreased erythrocyte acetylcholinesterase
activity was seen, but with no clear dose-dependency; brain
acetylcholinesterase activity was inhibited in a dose-dependent manner
in males at doses > 200 ppm and in females at doses > 50 ppm
(Table 2).
Table 2. Group mean values for inhibition of cholinesterase activity in
Crl CD-1 mice given chlorpyrifos in the diet (expressed as a
percentage of control values)
Cholinesterase Sex Dose (ppm)
5 50 200 400 800
Plasma Male 45*** 95*** 98*** 99*** 99***
Female 36*** 97*** 98*** 99 *** 99 ***
Erythrocyte Male 34a 21 16 12 26
Female 11 57*** 44** 48** 44**
Brain Male 43a 14* 80*** 85*** 87**
Female 3a 28*** 58*** 84*** 88***
Plasma, butyryl cholinesterase; erythrocyte and brain,
acetylcholinesterase
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased in comparison with controls
A single malignant lymphoma was reported in a female at the high
dose at terminal sacrifice, but no other neoplastic lesions were
reported. Given the isolated nature of this finding, it was considered
not to be related to treatment. The absolute and relative organ
weights were generally unaffected by treatment, although the relative
liver weights were increased in females at 200, 400, and 800 ppm. The
clinical signs included urogenital staining in males at doses
> 200 ppm and in females at 800 ppm. Ocular opacities occurred in
some animals at 400 and 800 ppm. Dose-related histopathological
findings were seen in the adrenal glands (including lipogenic
pigmentation at doses > 200 ppm in females and at > 400 ppm in
males) and in the eyes (acute or subchronic keratitis in two males and
four females at 800 ppm and in a single female at 400 ppm).
Erythrocyte acetylcholinesterase activity was variable, and the effect
of treatment on this parameter was equivocal. The NOAEL was 5 ppm,
equal to 0.7 mg/kg bw per day, on the basis of inhibition of brain
acetylcholinesterase activity at higher doses (Crown et al., 1987).
Rats
In a 2-week study to establish the doses to be used in a 13-week
study, technical-grade chlorpyrifos (purity, 95.5%) was administered
to groups of five rats of each sex at dietary concentrations of 0
(controls), 10, 30, 84, 240, or 694 ppm for 14 days, equivalent to 0,
1.4-1.9, 4.1-5.2, 12-14, 34-39, and 66-95 mg/kg bw per day,
respectively. At the high dose, clinical signs of intoxication were
reported, consisting of irritability, hunching, tremor, ataxia,
urogenital staining, pigmented orbital secretion, failure to groom,
and proneness. No treatment-related clinical signs were observed at
other doses, and treatment-related deaths were confined to animals at
the high dose. Body weights were reduced in males and females at
694 ppm and in females at 240 ppm, and food consumption was also
reduced in animals at 694 ppm. Dose-related decreases in blood
cholinesterase activity were seen at all doses, the inhibition ranging
from 42% at 10 ppm to 93% at 694 ppm. On the basis of these findings,
the doses selected for the 13-week study were 0.5, 10, and 100 ppm
(Crown et al., 1984b).
Plasma, erythrocyte, and brain cholinesterase activities were
markedly reduced in rats after dietary administration of chlorpyrifos
for 14 days at doses of 5 or 10 mg/kg bw per day in a study carried
out according to GLP. Inhibition of cholinesterase activity occurred
in the absence of clinical signs of intoxication. Feed consumption,
body weights, and gross pathological appearance were similar in
control and treated groups. Statistically significant increases in
absolute and relative adrenal weights (about 20% compared with
controls) were observed in females only at 10 mg/kg bw per day
(Liberacki et al., 1990).
Fischer 344 rats received technical-grade chlorpyrifos (purity,
95%) by nose-only inhalation for 6 h/day for 5 days at a target
concentration of 20 ppb in a study that conformed to GLP. No deaths or
treatment-related clinical signs were reported. Exposure of female
rats to a concentration of 0.34 mg/m3 (23 ppb) resulted in a
significant decrease in plasma cholinesterase activity. No effect on
erythrocyte or brain acetylcholinesterase activity was seen in females
or on plasma, erythrocyte, or brain cholinesterase activity in males
(Newton, 1988a).
Chlorpyrifos was administered by nose-only inhalation to female
Fischer 344 rats at a time-weighted average concentration of 12 ppb
(equivalent to 172 µg/m3) for 6 h/day on 5 days per week for 2 weeks
in a study that met GLP requirements. No clinical signs of
intoxication or treatment-related changes in body weights were
reported, and all rats survived until the scheduled termination.
Clinical chemistry showed no treatment-related changes in
haematological, clinical chemical, or urinary parameters, including
plasma, brain, and erythrocyte cholinesterase activity (Landry et al.,
1986).
In a study to determine a dose range, conducted to GLP
requirements, technical-grade chlorpyrifos was administered by
whole-body exposure of groups of Wistar rats for 6 h/day, 5 days per
week for 2 weeks at target concentrations of 0 (air control), 10, 100,
and 400 mg/m3 (actual concentrations, 0, 10, 94, and 388 mg/m3).
Exposure of animals to the high dose was terminated after five
exposures because they became moribund, with rapid weight loss and
deterioration of their general condition, and all surviving animals
were killed on day 9. No deaths was observed at other doses. There was
no NOAEL in this study, as significant inhibition of plasma,
erythrocyte, and brain cholinesterase activity was seen at all doses
in males and females. Plasma cholinesterase activity was inhibited by
78, 86, and 88% at the three doses, respectively, in males and by 91,
93, and 95%, respectively, in females when compared with controls.
Erythrocyte cholinesterase activity was inhibited by 78, 58, and 75%
in males and by 60, 80, and 73% in females, respectively. Brain
cholinesterase activity was inhibited by 50% at 10 mg/m3, about 70%
at 94 mg/m3, and about 72% at 388 mg/m3 in males and females.
Clinical signs of toxicity (tremors, salivation, and lachrymation),
decreased food consumption, and decreased body weights were observed
at the two higher doses. Increased adrenal weights were seen at 94 and
388 mg/m3, and effects in the forestomach including thickening and
congestion were observed at 388 mg/m3 (Kenny et al., 1988).
Fischer 344 rats were exposed to time-weighted average
concentrations (nominal) of 0, 1, or 5 ppb (0, 0.014, and
0.072 mg/m3) of chlorpyrifos vapour for 6 h/day, 5 days per week for
2 weeks in a study conducted according to GLP. The mean daily
time-weighted average concentrations found by analysis were 0.7 and
5 ppb, respectively. Although two control groups were used, their
cholinesterase activity did not differ statistically significantly,
and the activity in test groups was compared with that of the combined
control group. Statistically significant decreases in plasma,
erythrocyte, and brain cholinesterase activity were observed at 5 ppb,
but as the reductions in brain and erythrocyte acetylcholinesterase
activity did not reach 20% when compared with the control group, the
effect was considered not to be of toxicological significance. Plasma
cholinesterase activity was inhibited by > 20% in females at 5 ppb.
A statistically significant decrease in plasma cholinesterase activity
in females at 0.7 ppb was considered to be incidental to treatment.
There were no treatment-related deaths, clinical signs of toxicity, or
changes in body weight during the study. No treatment-related effects
were seen on body or organ weights, and no lesions were found at gross
examination. No significant adverse treatment-related effects were
observed at 5 ppb (approximately 0.07 mg/m3) (Landry et al., 1985;
Streeter et al., 1987).
Groups of 10 CDF Fischer 344 rats of each sex were fed diets
containing chlorpyrifos (purity, 95.7%) at concentrations that
provided doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day for 13 weeks in
a study that conformed to GLP. The treatment-related effects seen at
the highest dose consisted of decreased body weight and body-weight
gain in males, increased fatty vacuolation of the adrenal zone
fasciculata in males, and changes in haematological and clinical
chemical parameters consisting of decreased erythrocyte counts in both
sexes; increased platelet counts, reduced serum total protein,
albumin, and globulin concentrations, and decreased alanine
aminotransferase and alkaline phosphatase activity in males; and
decreased serum glucose concentration and increased urinary specific
gravity in females. Plasma and erythrocyte cholinesterase activities
were depressed at doses > 1 mg/kg bw per day, and the activity of
brain acetylcholinesterase was depressed at 5 and 15 mg/kg bw per day.
The NOAEL was 0.1 mg/kg bw per day on the basis of depression of
erythrocyte and brain acetylcholinesterase activity (Szabo et al.,
1988).
Groups of 10 rats of each sex were given diets containing
chlorpyrifos at 0, 10, 30, 300, or 1000 ppm, but treatment at the
highest concentration was discontinued after 4 weeks because of severe
clinical signs. Plasma cholinesterase activity was reduced in a
dose-related manner and was significantly lower than that of controls
at all doses. Erythrocyte cholinesterase activity was similarly
significantly reduced at all doses, but no dose-dependency was
demonstrated. Brain cholinesterase activity showed a clear
dose-related depression in animals of each sex at doses > 30 ppm.
At 30 ppm, the activity was inhibited by 22% in males and 24% in
females 41 days after administration. No other significant effects
were found on haematological or clinical chemical parameters, but few
were examined. No histopathological lesions were found that could be
linked to treatment. The NOAEL for inhibition of brain cholinesterase
activity was 0.5 mg/kg bw per day, and the LOAEL was 1.5 mg/kg bw per
day. There was no NOAEL for inhibition of erythrocyte or plasma
cholinesterase activity, as effects were seen at concentrations
> 10 ppm (0.5 mg/kg bw per day) (JMPR 1972; modified by reference
to the original reports of Beatty & McCollister, 1964; Beatty &
McCollister, 1971).
Five groups of 10 rats (strain not specified) of each sex
received chlorpyrifos (purity not specified) in the diet at
concentrations that provided doses of 0.03-10 mg/kg bw per day. The
doses of 0 and 0.3 mg/kg bw per day were fed for 90 days; the doses of
1, 3, and 10 mg/kg bw per day were fed for 28 days, when these animals
were fed control diets for 3 weeks and then diets containing 0, 0.03,
or 0.1 mg/kg bw per day. Behaviour and growth were affected at 3 and
10 mg/kg bw per day. Cholinesterase activity was depressed at 0.3
mg/kg bw per day. During the period of removal from treated diet, the
cholinesterase activity returned to pre-test levels; on resumption of
feeding at 0.1 mg/kg bw per day, only a slight depression was observed
in plasma and erythrocyte cholinesterase activity. In view of of the
unusual design of this study, the toxicological relevance of this
effect is considerede equivocal. No depression of brain or blood
enzymes was seen at 0.03 mg/kg bw per day (Blackmore, 1968).
Groups of 10 Crl rats of each sex were fed diets containing
chlorpyrifos (purity, 97.5%) at concentrations that provided a dose of
0.3 mg/kg bw per day for 13 weeks or 1, 3, or 10 mg/kg bw per day for
4 weeks. No deaths occurred during the study. After 4 weeks, there was
a clear dose-response relationship for inhibition of erythrocyte and
plasma cholinesterase activity at all doses, and animals at higher
doses showed clinical signs of toxicity. These animals were therefore
removed from exposure and allowed to recover for 3 weeks, during which
time the plasma and erythrocyte cholinesterase activities returned to
control values. These groups were then fed chlorpyrifos at doses of 0,
0.03, or 0.1 mg/kg bw per day for 13 weeks. The dose of 0.03 mg/kg bw
per day had no unequivocal effect on cholinesterase activity in
erythrocytes, plasma, or brain in either sex, but the dose of
0.1 mg/kg bw per day significantly reduced the erythrocyte
cholinesterase activity. The NOAEL was 0.03 mg/kg bw per day on the
basis of inhibition of erythrocyte cholinesterase activity after 13
weeks at 0.1 mg/kg bw per day (Hazleton Laboratories, 1968).
Groups of 20 Sprague-Dawley rats of each sex were given
chlorpyrifos (purity not stated) in the diet at 0, 0.03, 0.15, or
0.75 mg/kg bw per day for 6 months; an interim sacrifice of five
animals of each sex per dose was conducted after 3 months. A total of
16 animals across dose groups died from chronic murine pneumonia.
Cholinesterase activity was inhibited at the high dose in both
erythrocytes (50%) and plasma (65%), but the activity in brain was not
inhibited at any dose. Treatment did not change body-weight gain, food
consumption, or haematological or other clinical chemical parameters.
The NOAEL was 0.15 mg/kg bw per day, on the basis of inhibition of
erythrocyte acetylcholinesterase activity at 0.75 mg/kg bw per day
(JMPR, 1972; modified by reference to the original report of Coulston
et al., 1971).
Groups of 20 Crl CD Sprague-Dawley-derived rats of each sex were
given chlorpyrifos (purity, 95.5%) at dietary concentrations of 0
(control), 0.5, 10, or 200 ppm for 13 weeks in a study conducted
according to GLP. The minimum and maximum achieved doses of the test
material were calculated to be 0, 0.03 and 0.078, 0.6 and 1.5, and 13
and 31 mg/kg bw per day, respectively. All animals were inspected once
or twice daily for signs of ill-health and other treatment-related
signs and all were examined closely each week. No deaths or
significant clinical signs were observed at any dose. Clinical
chemistry, urinary analysis, and ophthalmoscopy revealed no findings
that were considered to be related to treatment. At 200 ppm
(approximately 13 mg/kg bw per day), reduced body weights, erythrocyte
count, erythrocyte volume fraction, and haemoglobin and increased food
consumption were observed. Statistically significant reductions
(> 20% inhibition compared with controls) in plasma cholinesterase
activity were observed in males at doses > 0.5 ppm (approximately
0.03 mg/kg bw per day) at 12 weeks and in females at doses > 10 ppm
(approximately 0.6 mg/kg bw per day). The inhibition was dose-related
in males but not in females. Erythrocyte and brain cholinesterase
activities were not measured in this study. The NOAEL was 10 ppm,
equal to 0.6 mg/kg bw per day, on the basis of reduced body weights
and statistically significant reductions in packed cell volume,
haemoglobin, and erythrocyte volume fraction at 200 ppm (Crown et al.,
1985).
Groups of 10 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 100%) by nose only at concentrations of 0, 5.2,
10.3, or 20.6 ppb (0, 75, 150, or 300 µg/m3) for 6 h/day, 5 days per
week for 13 weeks in a study that conformed to GLP. No
treatment-related deaths and only minimal clinical signs were observed
during the study. Body weights and urinary, haematological, and
clinical chemical parameters were unaffected by treatment. No effects
on plasma, erythrocyte, or brain cholinesterase activity were seen at
any dose. Gross and histopathological examination revealed no effects
associated with administration of chlorpyrifos (Corley et al., 1986).
Groups of 15 Fischer 344 rats of each sex were exposed to
chlorpyrifos (purity, 95%) by nose only for 6 h/day, 5 days per week,
for 13 weeks at target concentrations of 0 (control), 5, 10, and
20 ppb (0, 0.07, 0.14, and 0.28 mg/m3, respectively). The study was
conducted according to GLP requirements. There were no
treatment-related deaths or ophthalmic, haematological, or clinical
chemical changes. Plasma cholinesterase activity was inhibited by 23%
in males at the high dose ( p < 0.01), but other changes in plasma
cholinesterase activity were considered not to be related to
treatment. Erythrocyte and brain cholinesterase activities were
unaffected. Pathological and histopathological examination showed no
effect of treatment. The NOAEL was 20 ppb (0.28 mg/m3), on the basis
of inhibition of plasma cholinesterase activity. The absence of frank
toxicity at any dose in this study makes it difficult to draw
conclusions about the toxic potential of inhaled chlorpyrifos at doses
> 0.28 mg/m3 (Newton, 1988b).
In a study in Fischer 344 rats treated dermally, conducted
according to GLP requirements, the animals received chlorpyrifos
(purity, 100%) at 0, 0.1, 0.5, or 5 mg/kg bw per day in corn oil for a
total of 15 days over a 21-day period. No treatment-related effects
were observed at any dose, and plasma, erythrocyte, and brain
cholinesterase activities were not inhibited. Gross and microscopic
examination did not reveal any treatment-related changes, and body
weights and organ weights were unaffected by treatment. In the 4-day
range-finding component of this study, decreased plasma cholinesterase
activity (45%) and erythrocyte cholinesterase activity (16%) were
observed at 10 mg/kg bw per day, in the absence of clinical signs of
intoxication (Calhoun & Johnson, 1988, 1989).
Rabbits
No skin irritation was found when a formulation containing 61.5%
chlorpyrifos and 35% xylene was applied to the skin of the back and
abdomen of groups of two rabbits at doses of 5, 10, 25, and 50 mg/kg
bw 20, 4, 3, and 1 times, respectively. Both plasma and erythrocyte
cholinesterase activities were significantly inhibited in each dose
regimen. The values for plasma recovered more quickly than those for
erythrocytes, which were still significantly inhibited at day 40 after
the 3-, 4-, and 20-day exposure patterns (Pennington & Edwards, 1971).
Three rabbits were exposed for 5 min to a formulation containing
61.5% chlorpyrifos and 34.5% xylene from an ultra-light vapour cold
aerosol fog generator delivering 3.8 L/h. Two rabbits were exposed at
a distance of 8 m at a height of 1.3 m and one at a height of 0.8 m.
Exposure was terminated after 5 min because of ocular and pulmonary
irritation. The recorded concentration of chlorpyrifos in
breathing-space air was about 108 mg/L (range, 83-133 mg/L). By 24 h
after treatment, the rabbits had decreased activities of plasma
cholinesterase (< 33%) and erythrocyte cholinesterase (< 12%),
but these values had recovered almost to the control level by 72 h
after treatment (Pennington & Edwards, 1971).
Chickens
White Leghorn chickens were given chlorpyrifos in their
drinking-water at 1 ppb, 1 ppm, or 100 ppm for up to 84 days. Only
plasma cholinesterase activity was reported because of technical
difficulties with blood sampling. Significant inhibition (54%) of
plasma cholinesterase activity was recorded on day 84 in birds at 100
ppm (Stevenson, 1965).
Dogs
Technical-grade chlorpyrifos (purity, 95.8%) was administered to
groups of two pure-bred beagles of each sex orally in gelatine capsule
at doses of 0 (control; lactose only), 0.01, 0.03, 0.5, or 5 mg/kg bw
per day for 4 weeks, although only one animal of each sex was exposed
at 0 and 0.5 mg/kg bw per day. This study met GLP requirements. No
deaths occurred during the study, and no clinical signs associated
with treatment were observed. All animals gained weight steadily
during treatment, and food consumption was similar in all groups.
Rapid inhibition of plasma cholinesterase activity was observed at 0.5
and 5 mg/kg bw per day at all intervals, and the inhibition was both
dose- and time-dependent. Plasma cholinesterase activity was reduced
at 0.03 mg/kg bw per day, but the inhibition was considered not to be
significant owing to variation between groups and the small group
sizes. In animals at 5 mg/kg bw per day, erythrocyte cholinesterase
activity was 47% that of controls on day 7 and was still inhibited by
> 70% at the end of the study; brain cholinesterase activity was
inhibited by 32% in comparison with concurrent controls at the end of
the study. In animals at 0.5 mg/kg bw per day, erythrocyte
cholinesterase activity was inhibited by 30% on days 27-28 only, and
the effect was considered not to be toxicologically significant owing
to its transient nature. Macroscopic post-mortem examination showed no
changes that were considered to be of toxicological significance. The
NOAEL was 0.5 mg/kg bw per day, on the basis of inhibition of
erythrocyte and brain cholinesterase activity at 5 mg/kg bw per day
(Harling et al., 1989a).
Five dogs received 10 applications of a 0.087% commercial aerosol
formulation of chlorpyrifos in dichlorophene on their backs (with the
fur brushed against the grain) for 2 weeks, each application lasting
30 s. No deaths occurred, and no abnormalities were seen in clinical
signs, body-weight gain, or opthalmoscopic, haematological or clinical
chemical parameters. Erythrocyte cholinesterase activity was not
decreased, but plasma cholinesterase activity was inhibited from the
beginning of treatment, returning to normal 72 days after treatment.
The inhibition of plasma cholinesterase activity was not accompanied
by clinical signs (Sharp & Warner, 1968).
Groups of two young adult beagles of each sex were given daily
doses of chlorpyrifos orally by capsule at doses of 0, 0.03, 0.1, 0.3,
or 1 mg/kg bw per day for 90 days. Plasma cholinesterase activity was
inhibited at all doses. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day, and that for
inhibition of brain cholinesterase activity was 1 mg/kg bw per day
(Blackmore, 1968).
Technical-grade chlorpyrifos (purity, 95.8%) was administered
orally by capsule to groups of four pure-bred beagles of each sex at
doses of 0 (control), 0.01, 0.22, or 5 mg/kg bw per day for 13 weeks.
There were no unscheduled deaths or unequivocal clinical signs during
the study. The group mean body weights of animals at the high dose
were reduced. Haematology, clinical chemistry, urinary analysis, and
ophthalmoscopy revealed no findings that were considered to be related
to treatment. No treatment-related pathological macroscopic or
microscopic change was found. Statistically significant, dose-related
reductions in plasma cholinesterase activity were seen at 0.01 mg/kg
bw per day (bitches only; week 6 only; < 25%) and above (dogs and
bitches; all sample intervals; < 87%). Erythrocyte cholinesterase
activity was significantly inhibited in a dose-dependent manner at
0.22 mg/kg bw per day (< 46%) and at 5 mg/kg bw per day (< 85%).
At 0.01 mg/kg bw per day, erythrocyte cholinesterase was inhibited by
17-18% at week 12, but the reduction was not statistically significant
at any sample interval. Brain cholinesterase activity was inhibited in
animals of each sex (by 46%) at 5 mg/kg bw per day, and the NOAEL for
this effect was 0.22 mg/kg bw per day. The NOAEL for the study, on the
basis of inhibition of erythrocyte acetylcholinesterase activity, was
0.01 mg/kg bw per day (Harling et al., 1989b).
Beagles aged 10 and 11 months were given diets containing
chlorpyrifos (purity, 97.2%) at concentrations that provided doses of
0, 0.01, 0.03, 0.1, 1, or 3 mg/kg bw per day for 1 year (one animal of
each sex per dose), 1 year with a 3-month recovery period (two animals
of each sex per dose), or 2 years (four animals of each sex per dose).
Clinical signs were recorded daily, and body weights were recorded
weekly for the first 6 months and every 2 weeks thereafter. Food
intake was recorded weekly during the first 3 months and monthly
thereafter. The clinical investigations in the groups receiving 0, 1,
or 3 mg/kg bw per day included haematology (packed cell volume,
haemoglobin, erythrocyte and leukocyte counts, and prothrombin time)
and urinary analyses (specific gravity, occult blood, ketones, solids,
pH, albumin, and sugar). The serum concentration of blood urea
nitrogen and the activities of alkaline phosphatase and alanine and
aspartate aminotransferases were measured in all dogs before treatment
and at various intervals thereafter. Bromsulphalein retention was
measured before treatment and at the 1-year sacrifice of animals at 0,
1, or 3 mg/kg bw per day. Cholinesterase activity was determined in
plasma and erythrocytes in most groups before treatment and at various
intervals during the study, and the activity in brain was measured at
necropsy. Fasted dogs were necropsied and the brain, heart, liver,
kidney, spleen, and testes were weighed. Portions of these organs and
a standard set of tissues were preserved from the controls and animals
at 1 (after 1 year only) and 3 mg/kg bw per day for histopathological
examination. The animals fed chlorpyrifos for 2 years were given
complete physical examinations before the end of the study, including
routine neurological and opthalmoscopic evaluations. The data were
evaluated only with Student's t test.
The body weights were not significantly affected by treatment,
and food consumption was comparable in groups of the same sex.
Consumption of the test compound was monitored regularly and reported
to approximate the nominal concentrations closely. The organ weights
were not affected by treatment, except for an increase in the mean
relative liver weight in males at 3 mg/kg bw per day (3.5 g/100 g vs
2.5 g/100 g in controls) in the 2-year study. Details of the recorded
clinical signs were not presented, and the authors simply stated that
"No clinical signs of toxicity were observed in any of the dogs in
either phase of the experiment." The results of clinical pathology
were presented as individual and summary data. There were no
unequivocal treatment-related differences between control and treated
groups in the limited haematological parameters measured nor in
urinary analyses. No effect of treatment was found on blood urea
nitrogen, alkaline phosphatase or alanine and aspartate
aminotransferase activities or Bromsulphalein retention. The results
of gross pathological examination were reported in the form of a
general summary. None of the findings indicated an effect of
treatment. The results of the limited histopathological examinations
(control and high dose only) were reported in a summary table with no
indication of the severity of lesions or abnormalities; individual
data were not presented. The available data did not indicate any
treatment-related effects.
Cholinesterase activity was presented for individual animals and
for groups (Table 3). Inhibition was evident within nine days (the
first assay time) of the start of the study, and the values recorded
at termination were similar to those recorded at interim assays.
Plasma cholinesterase activity was decreased in a dose-related manner
at both times, and erythrocyte cholinesterase activity was similarly
depressed, females being slightly more severely affected than males.
Brain cholinesterase activity was the least affected by treatment. The
cholinesterase activity of animals that were allowed to recover,
assayed after 2, 6, and 13 weeks on normal diet, showed a return to
control levels after 2 weeks for plasma activity and after 13 weeks
for erythrocyte activity.
Table 3. Percentage inhibition of cholinesterase activity in beagles treated with
chlorpyrifos in the diet in comparison with concurrent controls
Dose Inhibition of cholinesterase activity (%)
(mg/kg bw per day)
Plasma Erythrocytes Brain
Male Female Male Female
One year (males and females)
3 72 70 79 82 8
1 62 62 63 61 3
0.1 39 36 18 30 0
0.03 18 17 2 33 0
0.01 1 1 16 13 0
Two years (males)
3 77 67 83 70 20
1 69 50 68 66 7
0.1 52 38 27 41 8
0.03 23 22 0 6 7
0.01 2 0 14 6 1
Average values at terminal sacrifice
Inadequacies in data collection and recording were noted.
Although the long-term effects of dietary intake of chlorpyrifos could
not be evaluated in the absence of complete pathological and
histopathological examination, the data were adequate for analysis of
inhibition of cholinesterase. Plasma cholinesterase activity was
inhibited in animals of each sex at 0.03 mg/kg bw per day. Erythrocyte
cholinesterase activity was inhibited by > 20% at 0.1 mg/kg bw per
day at both the 1- and 2-year intervals, but the inhibition did not
consistently reach statistical significance when compared with the
values in concurrent controls or with pre-treatment values. This was
due in part to the small group sizes and the variation in this
parameter between groups, including the control group, the activity
differing by as much as twofold between individual animals. Under
these conditions, the erythrocyte cholinesterase activity would have
had to be inhibited by as much as 50% in comparison with controls
before statistical significance was achieved. The NOAEL was 0.1 mg/kg
bw per day on the basis of inhibition of erythrocyte cholinesterase
activity at higher doses. Inhibition of brain cholinesterase activity
was seen only at 3 mg/kg bw per day after 2 years (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971a).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not
consistently include basic observations. Individual body weights,
ophthalmological data, the results of pre-treatment and terminal
physical examinations, and an inventory of histopathological findings
were included. The conclusions of the original study were unchanged,
with the additional observation that no effect of treatment was
detected by ophthalmology (Kociba et al., 1985).
Rhesus monkeys
Groups of one or two rhesus monkeys of each sex were given
technical-grade chlorpyrifos in 1% aqueous gum tragacanth by stomach
tube at doses of 0, 0.08, 0.4, or 2 mg/kg bw per day for 6 months.
There were no treatment-related changes in body-weight gain, food
consumption, clinical findings, or haematological, clinical chemical,
or histopathological parameters. Plasma cholinesterase activity was
depressed at all doses at 16 weeks and at > 0.4 mg/kg bw per day at
24 weeks, and erythrocyte cholinesterase activity was depressed at 0.4
and 2 mg/kg bw per day (Table 4). The NOAEL for erythrocyte
cholinesterase inhibition was 0.08 mg/kg bw per day and that for brain
cholinesterase inhibition was 2 mg/kg bw per day. The usefulness of
this study was limited by the small number of animals tested (JMPR
1972; modified by reference to the original paper of Coulston et al.,
1971).
Table 4. Mean cholinesterase activity (and percent inhibition in
comparison with controls) in rhesus monkeys given chlorpyrifos
by stomach tube
Cholinesterase Time Dose (mg/kg bw per day)
activity (weeks)
0 0.08 0.4 2
Plasma 16 6.0 4.4 (26%) 1.6 (72%) 1.8 (70%)
24 4.4 3.8 (14%) 1.7 (61%) 2.0 (55%)
Erythrocytes 16 9.5 8.2 (13%) 6.5 (32%) 2.6 (72%)
24 8.6 7.6 (13%) 6.3 (27%) 5.4 (38%)
(c) Long-term studies of toxicity and carcinogenicity
Mice
In a study of carcinogenicity conducted in accordance with test
guidelines 83-2 of the US Environmental Protection Agency and OECD 451
and in compliance with GLP standards, technical-grade chlorpyrifos
(stated purity, 95.9%) was mixed in maize oil and incorporated into a
commercially available powdered animal diet to produce dietary
concentrations of 0 (control), 5, 50, and 250 ppm, calculated to be
equal to 0.7-1.1, 6.1-12, and 32-55 mg/kg bw per day in males and
0.7-1.2, 6.6-12, and 34-62 mg/kg bw per day in females. The test diets
were administered to groups of 64 CD-1 of each sex, 3 weeks old, for
at least 79 weeks, terminal sacrifice being undertaken during weeks
80-82. The animals continued to receive the treated diets until
scheduled necropsy. An additional 12 males per group were treated
identically to the mice in the main groups and were used to replace
animals for reasons unrelated to treatment (such as excessive
aggression). All of the unused spares were discarded after 10 weeks of
treatment. Each animal was weighed weekly for the first 13 weeks of
the study and every 2 weeks thereafter, while food consumption was
measured weekly for 13 weeks and monthly thereafter. The animals were
examined daily for clinical signs of intoxication, and a careful
examination, including palpation, was conducted weekly. Blood smears
were prepared from all mice after 12 and 18 months for differential
leukocyte counts, but only those from controls and animals at the high
dose were examined. Plasma, erythrocyte, and brain cholinesterase
activity was determined in five animals of each sex at each dose at 9
and 18 months. All animals were examined grossly, the examination
including all external surfaces, the cranial, thoracic, abdominal, and
pelvic cavities, and the carcass. A large number of organs and tissues
from all animals were fixed for histopathological examination; the
eyes, kidneys, livers, lungs, and thyroids/parathyroids from animals
at all doses were examined, as were other tissues from control and
animals at the high dose and those from animals that died during the
study.
There was no adverse effect on survival, and no treatment-related
increase in the incidence of neoplastic lesions. Treatment-related
clinical signs (ocular opacities, excess lachrymation, and cranial
hair loss) and reductions in body weights and food consumption were
observed at 250 ppm (approximately 32 mg/kg bw per day). The effects
on cholinestrase activity are summarized in Table 5. Plasma
cholinesterase activity was inhibited at all doses. Determinations of
erythrocyte cholinesterase activity revealed > 20% inhibition at 42
weeks and about 30% inhibition at week 78 in animals at 250 ppm but
considerable intra-group variation at other doses. In addition, the
erythrocytes were washed with saline before the cholinesterase
determinations at 78 weeks but not at 42 weeks. The NOAEL for
inhibition of erythrocyte cholinesterase activity was 5 ppm, with a
statistically significant inhibition of 29% in males at 78 weeks and
41% in females at 42 weeks in animals at 50 ppm. Significant
inhibition of brain cholinesterase activity ( p < 0.001 or
p < 0.01) was observed in males and females at 250 ppm at 42 and 78
weeks, the activity being reduced by 80-86% when compared with
controls. At 50 ppm, decreases in activity of 43-47% were seen in
males at 42 and 78 weeks and in females at 42 weeks, but this finding
reached statistical significance ( p < 0.05) in females only at 42
weeks and in males only at 78 weeks. At 5 ppm, brain cholinesterase
activity was reduced in males at 78 weeks (27% reduction), but this
Table 5. Percent inhibition of cholinesterase activity in groups of five mice
fed diets containing chlorpyrifos
Cholinesterase Interval Dose (ppm)
(weeks)
5 50 250
Male Female Male Female Male Female
Plasma 42 4*** 45*** 95*** 97*** 98*** 99***
78 49* 50*** 95*** 96*** 98*** 98***
Erythrocyte 42 1 15 11 41** 22 23
78 - +10a 29* 4 31* 29**
Brain 42 8 1 43 46* 80** 85***
78 27 +13a 47 9 86** 84***
* p < 0.05; ** p < 0.01; *** p < 0.001
a Increased activity in comparison with controls
finding was not statistically significant and was considered to be
incidental to treatment. The NOAEL for inhibition of brain
cholinesterase activity was 5 ppm.
Non-neoplastic effects were observed at the high dose, in the
livers (slight subchronic pericholangitis, histiocytic proliferation,
and centrilobular hepatocytic fatty vacuolation) of males and in the
eyes of males and females. No treatment-related lesions were seen at
lower doses. In males at the high dose, an increased incidence of lung
nodules was seen macroscopically, with rates of 5/31 in controls, 4/26
at 5 ppm, 2/37 at 50 ppm, and 14/49 at 250 ppm. The nodules were
diagnosed histologically as bronchio-alveolar adenomas or carcinomas,
but the incidences were stated to be within the range in historical
controls. Nevertheless, two sets of data were reported for the
incidence of neoplasms in historical controls: values obtained from
24-month studies and a published report on spontaneous neoplasms in
CD-1 mice in a number of testing laboratories. Those used in the
report were less than ideal. The use of background incidence from
24-month studies would be expected to increase the range of
spontaneous tumours over that in the 78-week protocol used in this
study. In addition, the tumour incidence was reported in a published
paper (Maita et al., 1988) as a percentage of the animals examined,
while the historical data from the 24-month study and the tumour
incidence in this study were given as percentages of the number of
organs examined. Histopathological examination did not reveal any
statistically significant increase in the incidence of neoplasms in
treated animals when compared with controls. The NOAEL was 5 ppm
(0.7 mg/kg bw per day) on the basis of inhibition of erythrocyte and
brain acetylcholinesterase activity (Gur et al., 1991).
Groups of 56 CD-1 mice of each sex were maintained on diets
containing 0, 0.5, 5, or 15 ppm of chlorpyrifos (purity, 99.6%) for
105 weeks. They were observed daily for clinical signs, body weights
were recorded monthly, and daily food consumption was calculated from
a 3- or 4-day measurement made once a month. Gross pathological
examination was performed on all animals that died, when the weights
of the brain, testes, heart, kidneys, and liver were recorded and a
range of other tissues were collected. Sections were prepared of all
the these tissues for histopathology. Haematology was limited to
examination of smears of blood collected from the tail vein at
terminal sacrifice.
The body weights and absolute and relative organ weights were not
significantly affected by treatment over the course of the study, and
the mean body weight of all treated female mice was greater (< 13%)
than that of controls from day 84 onwards, although food consumption
was comparable in all groups. The mortality rate was also generally
unaffected by treatment, and treated males had slightly higher
survival rates than controls: 39% in controls, 46% at 0.5 ppm, 50% at
5 ppm, and 48% at 15 ppm; the survival rates of females were lower
than those of controls only at the highest dose: 46% in controls, 59%
at 0.5 ppm, 46% at 5 ppm, and 38% at 15 ppm. There were no clinical
signs or pathological findings that indicated an effect related to
treatment. A significant increase was observed in the incidence of
spindle-cell hyperplasia of the adrenal gland in animals of each sex
given 0.5 ppm chlorpyrifos and in males given 5 ppm. This finding was
not considered to be related to treatment as there was a high
background incidence in ageing mice and no dose-response relationship.
Vacuolation was found in sciatic nerve preparations from 2/56 male and
3/54 female controls, 5/54 males and 8/55 females at 0.5 ppm, 2/56
males and 2/52 females at 5 ppm, and 6/52 males and 8/53 females at 15
ppm. The incidences of alveologenic adenomas and hyperplastic nodules
in the liver were significantly increased only in males at 5 ppm.
These findings were considered to be incidental.
This study was barely adequate for assessing the carcinogenicity
and long-term toxicity of chlorpyrifos, as it had serious shortcomings
in design, method, and data collection. No clinical pathology was
performed, and in particular cholinesterase activity was not measured;
no clinical signs were reported; individual data were not presented
for several parameters, including histopathology; and intestinal
parasites (nematodes) were present in all groups (JMPR, 1982; modified
by reference to the original report of Warner et al., 1980).
Rats
In a 2-year study, groups of 25 male and 25 female Sherman rats
received chlorpyrifos (purity, 97.2%) in the diet at doses of 0, 0.01,
0.03, 0.1, 1, or 3 mg/kg bw per day (dietary concentrations not
provided). Supplementary animals were included at each dose to allow
interim sacrifices. The design of the study is shown in Table 6.
Clinical signs were recorded 'frequently', body weights were recorded
twice weekly for the first 4 weeks, weekly for 2-6 months, and every 2
weeks for the remainder of the study. The food intake of the main
group was recorded continuously during the first 3 months and once a
month thereafter. The clinical investigations included haematology
(packed cell volume, haemoglobin, and erythrocyte and leukocyte
counts) and urinary analysis (solids, pH, albumin, sugar, occult
blood, ketones) for five rats of each sex at 0, 1, and 3 mg/kg bw per
day; the serum concentration of urea nitrogen and the activities of
alkaline phosphatase and alanine aminotransferase were measured in all
rats at sacrifice at 12 and 18 months, in all male survivors and in
four to five females at 24 months. Cholinesterase activity was
determined in plasma, erythrocytes, and brain samples, as shown in
Table 6. Rats in groups A, H, I, and K were necropsied in a standard
manner, and the weights of the brain, heart, liver, kidney, spleen,
and testes were recorded. Portions of these organs and a standard set
of tissues were preserved for histopathology, but only the slides for
the controls, animals at the high dose, and animals at 1 mg/kg bw per
day at the 12-month necropsy were examined. Some tissues from animals
that died during the study and from those animals killed when moribund
were also examined histologically. Statistical evaluation of the data
was limited to Student's t test.
Body weights (data presented only as graphs) were generally not
significantly affected by treatment. No details of the recorded
clinical signs were presented; rather, the authors stated that "No
changes in appearance or demeanour or signs of toxicity were observed
grossly in any of the rats. No evidence of a cholinergic response was
noted at any time." The results of clinical pathology were presented
as individual and summary data, and no unequivocal treatment-related
differences between control and treated groups were found for the
limited haematological parameters measured or for the results of
urinary analyses. Treatment had no effect on serum concentrations of
urea nitrogen or on alkaline phosphatase and alanine aminotransferase
activity.
Plasma cholinesterase activity was significantly decreased at 3
mg/kg bw per day at all assay times, the inhibition ranging from 20 to
40% in males and 55 to 74% in females; the activity was less severely
inhibited at 1 mg/kg bw per day, but from 6 months onwards the
inhibition was 18-38% in males and 50-69% in females when compared
with controls, and these differences were statistically significant at
most assay times. Erythrocyte cholinesterase activity was severely
inhibited in animals of each sex at the higher doses at all assay
times, with rates of 60-100% at 3 mg/kg bw per day, 13-90% at 1 mg/kg
bw per day, and little effect at the other doses. Animals that had
Table 6. Design of the long-term study of toxicity and carcinogenicity in groups of
56 mice fed diets containing chlorpyrifos
Group No. of rats of Sacrifice interval End-point examined
each sex per dose
A 25 2 years Necropsy, blood and brain
cholinesterase
B 5 1 week Blood cholinesterase
C 5 1 month Blood cholinesterase
D 5 3 months Blood cholinesterase
E 5 6 months Blood and brain cholinesterase
F 5 9 months Blood cholinesterase
G 6 12 months Blood and brain cholinesterase
H 5 12 months Necropsy
I 7 12 months, Necropsy, blood and brain
7-8-week recovery cholinesterase
J 7 18 months Blood and brain cholinesterase
K 7 18 months Necropsy
been allowed to recover showed full restoration of cholinesterase
activity. Brain cholinesterase activity was significantly inhibited by
treatment at 3 mg/kg bw per day (30-53% inhibition) at all assay
times, to a lesser degree at 1 mg/kg bw per day (3-16%), and was
comparable to that of controls at other doses. Animals that were
allowed to recover showed restoration of brain cholinesterase
activity.
This study was considered to be inadequate for assessing the
carcinogenicity and long-term effects of chlorpyrifos, other than
clinical chemical findings including cholinesterase inhibition, as
there were serious shortcomings in data collection, and the reports of
gross and histopathology were inadequate and no clinical findings were
reported. The NOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day on the basis of
significant inhibition at 1 mg/kg bw per day. The NOAEL for inhibition
of brain acetylcholinesterase activity was 1 mg/kg bw per day on the
basis of significant inhibition at 3 mg/kg bw per day (JMPR, 1972;
modified by reference to the original report of McCollister et al.,
1971b).
A supplementary report provided some of the data that were
lacking or inadequate in the original report, but the observations
made in individual animal during life were limited and did not include
basic observations such as abnormal behaviour or gait. Individual body
weights and gross and histopathological findings were included in the
supplement, but the histopathology was limited and variable, only a
small number of tissues from each rat being examined consistently. The
results were not presented as summary tables of incidence and
severity. The conclusions of the original study remain unchanged by
this supplement (McCollister et al., 1985).
In a 2-year study conducted according to accepted test guidelines
(OECD 451, USEPA 83-5) and GLP, groups of 60 Fischer 344 rats of each
sex were fed diets containing technical-grade chlorpyrifos (purity,
96.1%) at concentrations of 0, 0 (vehicle control), 0.2, 5, or
100 ppm. The vehicle used was 0.04% w/w maize oil. The diets were
prepared weekly and assayed regularly for stability and accuracy of
dosing. Body weights were generally recorded weekly; food consumption
was recorded weekly for 13 weeks and monthly thereafter. Clinical
signs were recorded daily and palpation performed weekly.
Cholinesterase activity was measured before treatment and at weeks 14,
32, 45, 50 (interim sacrifice of five animals of each sex per group
for measurement of brain acetylcholinesterase activity), 78, and 104
weeks (terminal sacrifice with measurement of brain
acetylcholinesterase activity in 10 animals of each sex per group),
and generally on 10 rats of each sex per dose. Haematological
parameters were measured in blood smears from 10 rats of each sex in
the vehicle control group and at 100 ppm at months 12 and 18. Gross
pathological findings were recorded for all animals at necropsy,
including those that died during the study or were killed at early
sacrifices. Necropsy included removal of the eyes with the optic nerve
and adnexa. The weights of the brain, testes, thyroids, adrenals,
kidneys, and liver were recorded, and a range of tissues was
collected. All gross lesions were sectioned, and sections from all
control animal and those at 100 ppm were examined. Appropriate
statistical tests were used to analyse the data.
The mean consumption of chlorpyrifos was estimated to be 0.012,
0.3, and 6 mg/kg bw per day for animals of each sex. Mortality rates
and the incidences of clinical signs or palpable masses were not
affected by treatment. A 5% reduction in the mean body weights of
males at 100 ppm was significant in comparison with controls at most
times during weeks 3-94, and the weights of females at this dose were
significantly lower (about 4%) than those of controls at most times
during weeks 2-64. A variety of non-neoplastic and neoplastic lesions
were recorded, and the incidence of these lesions occasionally showed
a positive trend with dose; however, the incidence was generally
within the range of historical controls in the laboratory or within
the range of published values from the National Toxicology Program in
the USA. The incidences of neoplastic lesions did not show unequivocal
or statistically significant dose-response relationships. The only
observation that was considered to be related to treatment was an
increased incidence of cataracts and diffuse retinal atrophy in
females (Table 7).
Table 7. Incidences of ocular abnormalities found microscopically in rats given
diets containing chlorpyrifos
Abnormality Sex Dose (ppm)
0 0 (vehicle) 0.2 5 100
Diffuse retinal atrophy Male 4/60 0/60 3/23 1/28 3/60
Female 5/60 15/59* 9/60 5/58 24/60*
Cataract Male 31/60 31/60 7/23 6/28 30/60
Female 38/60 38/59 33/60 32/58 51/60*
* Significantly different from controls at p < 0.01 or p < 0.001
Significant (> 20%) inhibition of plasma cholinesterase activity
relative to the control values was observed at doses > 0.3 mg/kg bw
per day (Table 8). While erythrocyte cholinesterase activity was
inhibited in animals at 100 ppm, the variation in the data and the
lack of statistical significance and/or dose-response relationships
mitigated against an unequivocal finding. The NOAEL for inhibition of
brain cholinesterase activity was 0.3 mg/kg bw per day (Crown et al.,
1988).
In a study conducted in compliance with GLP standards, groups of
60 Fischer 344 rats of each sex received diets containing chlorpyrifos
(purity, 98.5%) at concentrations that provided doses of 0 (control),
0.05, 0.1, 1, or 10 mg/kg bw per day for 24 months. Ten rats of each
sex at each dose were randomly designated at the start of the study
for interim sacrifice at 12 months. Blood was collected for
haematology, clinical chemistry, and measurement of plasma and
erythrocyte cholinesterase activity at 6, 12, and 18 months as well as
at the 24-month terminal sacrifice. The groups were observed daily for
deaths and signs of toxicity. All rats were examined clinically at
least once a week from after the sixth month. All animals were
palpated for externally detectable masses before treatment, before the
12-month kill, and monthly thereafter. Body weights and feed
consumption were determined weekly for the first 3 months and monthly
thereafter. All rats were weighed, but feed consumption was determined
for only 20 rats of each sex per group.
All clinical laboratory procedures scheduled for 6 and 12 months
were performed on rats designated for the 12-month interim kill,
whereas tests scheduled for 18 and 24 months were performed on rats
designated for terminal sacrifice. Blood samples were obtained for
haematology and clinical chemistry by orbital sinus puncture under
Table 8. Group mean percent inhibition of cholinesterase activity in comparison
with vehicle controls in rats given diets containing chlorpyrifos
Assay time Dose Cholinesterase inhibition (%)
(weeks) (ppm)
Plasma Erythrocytes Brain
Male Female Male Female Male Female
50 0 0 7 0* 31 0 35
0.2 1 4 0 42 0 14
5 15 51 0* 39 9 10
100 93 98 13 45 57* 80*
78 0 0 3 27 0
0.2 4 3 11 0
5 28* 47* 0 0
100 93* 97* 10 0
104 0 8 9 0 0 18 4
0.2 13 0 0 0 0 0
5 36 37* 17 11 0 0
100 95* 96* 34 18 58* 61*
Values the same as or higher than those of the vehicle control are recorded as
0 inhibition.
* Significantly different from control at p < 0.01 or p < 0.001
light anaesthesia. The haematological determinations consisted of
packed cell volume, haemoglobin concentration, erythrocyte count,
total and differential leukocyte counts, and platelet count. The
clinical biochemical determinations included blood urea nitrogen,
alkaline phosphatase and alanine and aspartate aminotransferase
activities, glucose, total protein, albumin, globulin (calculated),
creatine phosphokinase, total bilirubin, cholesterol, calcium,
phosphorus, sodium, potassium, and chloride. Urine samples were
obtained 1-2 weeks before the scheduled sacrifices at 12 and 24 months
and at 6 and 18 months from 10 rats of each sex per group. The urinary
parameters measured included specific gravity and semi-quantitative
estimates of bilirubin, glucose, ketones, occult blood, pH, protein,
and urobilinogen. Microscopy of a pooled sample was also conducted.
Plasma and erythrocyte cholinesterase activities were assayed in 10
rats of each sex per group at 6, 12, 18, and 24 months, and
acetylcholinesterase activity was measured in half-brain samples
obtained at the 12-month and 24-month scheduled necropsies. All
animals killed at the interim and terminal sacrifices or which died or
were killed when moribund were necropsied and subjected to a complete
gross examination. The brain, liver, kidneys, testes, ovaries, and
adrenal glands were weighed, and many organs and tissues were removed
and preserved in neutral, phosphate-buffered 10% formalin for
subsequent histopathological evaluation. Histological sections of the
formalin-fixed tissues from all controls and those at the highest dose
were prepared, stained with haematoxylin and eosin and examined
microscopically. Histopathological examination of tissues from animals
at the three lower doses was limited to the liver, kidneys, adrenals,
and tissues with gross lesions at both sacrifices. At terminal
sacrifice, the lungs, spleen, testes, pituitary, and
thyroid/parathyroid were also examined microscopically. Appropriate
statistical tests were applied to the data.
Males at the high dose showed a consistent decrease in
body-weight gain relative to controls in the absence of reduced food
consumption, depression of plasma (56-87%), erythrocyte (20-40%) and
brain (56-58%) cholinesterase activities, and an increase in the
weight of their adrenal glands which was characterized microscopically
by exacerbated fatty vacuolation of the zona fasciculata. Similar
effects were observed in females at this dose, but were generally less
pronounced than in males: for example, a transient decrease in
body-weight gain relative to controls with no reduction in food
consumption, depression of plasma (82-95%), erythrocyte (generally
> 20%), and brain (57-61%) cholinesterase activities, and an
increase in adrenal weight at the terminal sacrifice with no
associated histopathological lesions. At 1 mg/kg bw per day, the only
effects attributable to treatment were inhibition of plasma
cholinesterase activity (39-71% in males and 60-86% in females) and
erythrocyte cholinesterase activity (20-40% in males and < 22% in
females); brain cholinesterase activity was not affected. These
results are summarized in Table 9. No treatment-related effects were
observed at the two lower doses. There was no increase in the
incidence of any type of tumour. The NOAEL for inhibition of
erythrocyte cholinesterase activity was 0.1 mg/kg bw per day, on the
basis of toxicologically (> 20%) or statistically significant
inhibition at 1 mg/kg bw per day, and the NOAEL for inhibition of
brain cholinesterase activity was 1 mg/kg bw per day on the basis of
toxicologically and statistically significant inhibition at 10 mg/kg
bw per day (Young & Grandjean, 1988).
Chickens
Hens were fed diets containing chlorpyrifos at concentrations of
0, 25, 50, or 200 ppm (approximately 0, 2.5, 5, and 20 mg/kg bw per
day) for 52 weeks. Mortality was unaffected by treatment, with rates
of 13% in controls, 3% at 25 ppm, 7% at 50 ppm, and 10% at 200 ppm.
Plasma cholinesterase activity was assayed in three hens at each dose
1, 3, 7, 14, 22, and 29 days after the start of treatment, monthly
thereafter, and 1, 2, and 3 weeks after the end of treatment. The
onset of inhibition of cholinesterase activity was rapid and
dose-related, with 22% inhibition in week 1 at 25 ppm, 45% inhibition
at 50 ppm, and 76% inhibition at 200 ppm. It persisted at similar
levels throughout the study but returned to control levels during the
Table 9. Group mean cholinesterase activities in rats given diets containing chlorpyrifos,
expressed as percent of the respective control values for each sampling period
Cholinesterase Sex Sampling period Dose (mg/kg bw per day)
(months)
0 0.05 0.1 1 10
Plasma Male 6 100 96.5 95.1 60.9* 44.2*
12 100 94.5 97.8 28.6* 13.3*
18 100 93.4 80.1 36.6* 22.5*
24 100 92.4 85.5 40.0* 19.7*
Female 6 100 97.9 91.0 34.6* 16.9*
12 100 104 87.0* 13.8* 4.8*
18 100 99.1 85.8* 30.4* 12.4*
24 100 103 94.4 39.7* 17.9*
Erythrocytes Male 6 100 107 88.8 76.1* 75.6*
12 100 107 93.2 67.4 63.1
18 100 109 95.3 66.2* 71.0*
24 100 105 108 86.5 73.5*
Female 6 100 93.3 106 104 87.5
12 100 88.2 109 82.2 59.5*
18 100 114 99.7 78.0 81.9
24 100 107 87.2 83.6 79.9
Brain Male 12 100 93.8* 93.1* 91.0* 42.1*
24 100 102 100 103 44.3*
Female 12 100 97.9 102 95.3* 38.9*
24 100 101 100 96.2 42.9*
* Statistically significantly different from control by Dunnett's or Wilcoxon's test,
alpha = 0.05, one-sided
recovery period. Overall feed consumption, body weight, egg
production, feed efficiency, egg weight, and shell thickness were not
affected by treatment. There was no NOEL for inhibition of plasma
cholinesterase activity in this study, as significant inhibition was
seen at the lowest dose tested (Sherman & Herrick, 1973).
(d) Genotoxicity
Chlorpyrifos was not genotoxic in vitro or in vivo in a range
of studies (Table 10).
Table 10. Results of studies for the genotoxicity of chlorpyrifos
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
In vitro
Gene mutation S. cerevisiae D3 NR NR Negative Poole et al. (1977;
rec locus abstract only)
Gene mutation B. subtilus H17, 20 (100, 200, 500, 1000 95.83 Negative Shirasu et al. (1980)
M45 2000 µg/plate in DMSO
Reverse mutation S. typhimurium TA98, 10, 50, 100, 500, 1000, 95.83 Negativea Shirasu et al. (1980)
TA100, TA1535, 5000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 1, 3.162, 10, 31.62, 95.7 Negativea,b QA Bruce & Zempel (1986)
TA100, TA1535, 100 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, 0.01, 0.1, 1, 5, 10, 95 Negativea,c Jai Research Foundation
TA100, TA1535, 100 µg/plate in DMSO (1996a)
TA1537
Reverse mutation S. typhimurium TA98, 30, 100, 300, 3000, 96.8 Negativea GLP Loveday et al. (1987)
TA100, TA1535, 10 000 µg/plate in DMSO
TA1537, TA1538
Reverse mutation S. typhimurium TA98, NR NR Negative Poole et al. (1977;
TA100, TA1535, abstract only)
TA1537
Reverse mutation E. coli WP2 NR NR Negative Poole et al. (1977;
abstract only)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Relative toxicity E. coli and NR NR Negative Poole et al. (1977;
B. subtilis abstract only)
Reverse mutation S. typhimurium TA98, 5, 500, 5000 µg/plate 96.2 Negativea,d QA Fredrick Institute
TA100, TA1535, in DMSO (1996c)
TA1537, TA1538
Forward mutation Chinese hamster ovary 10, 20, 25, 30, 40, 95.7 Negativea Mendrala (1985)
cells, hprt locus 50 µmol/L
Forward mutation Chinese hamster ovary 5, 10, 25, 50, 75 µg/ml, 96.8 Negativea,e GLP Tu (1987)
cells, hprt locus 16 h, - S9
5, 10, 20, 30, 40, 50 µg/ml,
16 h, - S9
30, 50, 100, 300, 1000 µg/ml,
+S9, in DMSO
Sister chromatid Human lymphocytes 0.02, 0.2, 2 or 20 µg/ml NR Negativea,f Sobti et al. (1982)
exchange (Laz-007) in ethanol
Chromosomal Chinese hamster ovary 0.975, 1.47, 2.93, 4.89, 96.8 Negativea,g GLP Loveday (1987)
aberration cells 9.75, 14.7, 29.3, 48.9, 97.5,
147 µg/ml, 19 h, - S9
1.56, 3.12, 5.2, 10.4, 15.6,
31.2, 52, 104, 156 µg/ml,
10 h, - S9
9.75, 14.7, 29.3, 48.9, 97.5,
147, 293 µg/ml, 19 h, +S9
1, 1.5, 3, 5, 10, 15, 30, 50,
100 µg/ml, 10 h, + S9
2.95, 4.95, 9.85, 14.8, 29.6,
49.4, 98.5, 296 µg/ml, 10 h,
+S9
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Rat hepatocytes 16.7, 50, 167, 500, 1667, 98.6 Negativea,h GLP Linscombe et al. (1992)
aberration 5000 µg/ml harvested after
24 h and 5, 16.7, 50, 167
µg/ml harvested after
24 and 48 h, in DMSO
Sister chromatid Chinese hamster ovary 1, 10, 100 µg/ml in acetone NR Negative Muscarella et al. (1984)
exchange cells
Unscheduled DNA Rat hepatocytes 1, 3.16, 10, 31.6, 95.7 Negative Mendrala & Dryzaga
synthesis 100 µmol/L in DMSO (1986)
Reverse mutation S. typhimurium TA98, 0, 1, 3.16, 10, 31.6, 95.7- Negativea Gollapudi et al. (1995)
TA100, TA1535, 100 µg/plate in DMSO 98.6
TA1537, TA1538
Forward mutation Chinese hamster ovary 0, 3.5, 7, 8.8, 10.5, 14, 95.7-98.6 Negativea,i Gollapudi et al. (1995)
cells, hprt locus 17.5 µg/ml in DMSO
Chromosomal Rat lymphocytes 16.7-5000 µg/ml in 95.7-95.6 Negativea,j Gollapudi et al. (1995)
aberration DMSO
Unscheduled Rat hepatocytes 1, 3.16, 10, 31.6, 95.7-95.6 Negative Gollapudi et al. (1995)
DNA synthesis 100 µmol/L in DMSO
In vivo
Micronucleus Mouse (CD-ICR BR) 0, 7, 22, 70 mg/kg bw 95.7 Negative QA Gollapudi et al. (1985)
formation marrow cells orally in corn oil
Micronucleus Mouse (Swiss albino) 0, 15, 30, 60 mg/kg bw 96.2 Negative QA Fredrick Institute
formation marrow cells orally in vegetable oil (1996d)
Table 10. (continued)
End-point Test object Concentration Purity Results GLP Reference
(%) or QA
Chromosomal Mouse (Swiss albino) 0.6, 3, 15 mg/kg bw per 96.2 Negative QA Fredrick Institute
aberration marrow cells day orally in vegetable oil (1996e)
Chromosomal Chick embryo (Cornell 1.11, 11.1, 111, 1110, NR Negativek Muscarella et al. (1984)
aberration K strain eggs) 2220 µg/embryo
Chromosomal Bovine blastocysts NR NR Negative Muscarella et al. (1984)
aberration
Micronucleus Mouse (CD-1 (ICR) BR) 90 mg/kg bw orally in 97.9 Negative GLP McClintock & Gollapudi
formation marrow cells corn oil (1989a)
QA, quality assurance; GLP, good laboratory practice; NR, not reported; S9, exogenous metabolic activation system from 9000 × g
fraction of rat liver; DMSO, dimethyl sulfoxide
Positive control substances were used in all assays and gave the expected results.
a With and without metabolic activation
b Cytotoxicity observed at 100 µg/plate in TA100, 1535, 1537, 1538 with precipitation of test material; in TA98, precipitation in the
absence of toxicity
c Cytotoxicity at > 10 µg/plate; dose-related increase in revertant frequency (> twofold than controls) in all strains ± S9, but
increases lower than in positive controls by 2-50-fold and not statistically significant
d Increase in revertants at all dose, - S9 in TA1537 only; no dose-response relationship
e Cytotoxicity at 50 µg/ml in one assay - S9
f At 2 and 20 µg/ml, frequency was statistically significantly different from controls but not double the control frequency
g - S9: cytotoxicity at highest doses; increase in gaps only at 52 µg/ml and 10-h exposure; no increases in other aberrations
+S9: cytotoxicity at 15 µg/ml and 10-h incubation; in one 10-h assay, a significant increase in number of cells with aberrations
(including gaps) at 3 and 10 µg/ml; incidence of aberrations (excluding gaps) not statistically significantly increased and not
dose-dependent In repeat 10-h assay, no increase in incidence of aberrations.
h Cytotoxicity at > 500 µg/ml -S9 and > 167 µg/ml +S9 in first assay; no mitotic index measurable at 50 µg/ml - S9 or
167 µg/ml ±S9
i Test material precipitated at 10.5, 14, and 17.5 µg/ml ± S9
j Test material cytotoxic at > 50 µg/ml ± S9
k Increased mortality at 1110 and 2220 µg/embryo
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
In a three-generation study, Sprague-Dawley rats (two litters per
generation) were given diets containing chlorpyrifos (purity not
specified) at concentrations that provided doses of 0, 0.03, 0.1, or
0.3 mg/kg bw per day for the first generation and 0, 0.1, 0.3, or
1 mg/kg bw per day for the second and third generations. The sizes of
the groups were consistent in all generations, the controls comprising
20 males and 40 females and the treated groups comprising 10 males and
20 females. Treatment was started at 58 days of age, and F0 parents
were mated at 118 days of age at a 1:2 male:female ratio. Evidence of
conception (vaginal plug or sperm) was considered to be day 0 of
gestation. Pairing for the second litter in each generation was
started approximately 10 days after the first litter was weaned at 21
days, and the treated diets were fed to the litters from weaning. When
the progeny of the F1b or F2b numbered more than 10 per litter,
each litter was reduced to 10 after 5 days of age. The F3b fetuses
were examined for teratogenic changes. F2b parent animals were
continued on the test diet throughout the breeding period, except that
females were placed on normal diet during organogenesis and the test
substance was administered by gavage in acetone or corn oil. Dams were
killed on day 20 of gestation and the fetuses removed surgically and
examined for external abnormalities. Onethird were fixed for
examination of soft tissues, and two-thirds were stained with alizarin
red for skeletal examination; however, only the controls and rats at 1
mg/kg bw per day were examined. Clinical observations were stated to
have been performed 'frequently'. Body weights and food consumption
were recorded weekly until breeding commenced. During gestation and
lactation, the diets were adjusted on the basis of an average feed
consumption of 25 and 40 g/day; rats were weighed before breeding to
allow dietary adjustment. In the investigation of teratogenic effects,
the body weights of the dams were recorded on days 0, 6, 15, and 20 of
gestation, while food consumption was measured during the gestational
intervals 0-6, 6-16, and 16-20 days. Various indices of reproductive
performance were calculated: fertility index as pregnancies per
mating; gestation index as live litters born per number of
pregnancies; viability as rats alive at day 5 per rats born alive; and
lactation index as rats alive at day 21 per rats alive at day 5. All
animals that died and five pups of each sex per dose from the F1a,
F2a, and F3a litters were examined grossly. An extensive set of
tissues was prepared and fixed from these pups, but in the absence of
gross abnormalities only tissues from the F3a pups were examined
histopathologically. Clinical chemistry was limited to the F2
animals, and consisted of measurements of plasma and erythrocyte
cholinesterase activity in 11 control and five or six treated dams at
the time of removal of their litters and in five males per group.
Statistical analysis was limited.
No clinical signs of toxicity were seen in the parents or
offspring. The parental body weights were not significantly affected
by treatment, and food consumption was variable but unaffected. The
fertility, gestation, and lactation indices were comparable between
groups and generations. The viability index was decreased at 1 mg/kg
bw per day, but this effect was considered not to be clearly related
to treatment in view of the isolated nature of the finding and the
study design. The mean body-weight gain of the dams that produced the
F3b pups showed a dose-related increase, with gains of 114 g in
controls, 120 g at 0.1 mg/kg bw per day, 123 g at 0.3 mg/kg bw per
day, and 126 g at 1 mg/kg bw per day. This generation showed no
treatment-related effects on fertility (per cent pregnant), the mean
number of corpora lutea or implantations, viable litter size, or pup
weight after surgical removal. The skeletons of the pups showed common
minor variants such as incomplete ossification of sternebrae and the
occurrence of extra ribs. Visceral examination of the pups showed that
minor variants of the urogenital system (hydronephrosis or left,
right, or bilateral hydroureter) were more common in the treated
group. A single fetus with multiple abnormalities was found at 1 mg/kg
bw per day, but this isolated finding was considered not to be related
to treatment. The group average activity of plasma and erythrocyte
cholinesterase was decreased by > 20% at 1 mg/kg bw per day in males
and females of the F2 generation. The NOAEL was 0.1 mg/kg bw per day
on the basis of decreased plasma and erythrocyte cholinesterase
activity at 1 mg/kg bw per day and decreased erythrocyte
cholinesterase activity at 0.3 mg/kg bw per day (JMPR, 1972; modified
by reference to the original report of Thompson et al., 1971).
To supplement the finding of an equivocal decrease in neonatal
survival at 1 mg/kg bw per day, groups of 30 Sprague-Dawley rats of
each sex were fed diets containing chlorpyrifos (purity, 96.6-99%) at
concentrations providing doses of 0, 0.5, 0.8, or 1.2 mg/kg bw per day
for 135 days and then bred to produce F1 litters. On day 21 of
lactation, 30 pups of each sex were selected randomly, dosed in the
same manner as the F0 animals for 120 days, and then bred to produce
the F2 litters. The F2 pups were weaned on day 21 of lactation.
The F0 and F1 matings were in a 1:1 ratio, with pairing for 5
days, a 7-day rest, and a pairing with a different male for a further
5 days. The litters were culled on day 4 post partum, and the litter
weights and pup survival were recorded on days 1, 4, 7, 14, and 21;
individual pups were weighed on day 21. The parental animals were
examined daily for signs of toxicity, and body weight and food intake
were recorded weekly for animals of each sex until pairing;
thereafter, males were weighed after pairing until sacrifice, and
females were weighed on days 1, 4, 7, 14, and 21 of lactation. The
date of parturition, litter size at birth, and the numbers of live and
dead pups at birth were recorded. The dietary concentrations were
adjusted weekly during the pre-mating period according to the food
consumption and body weights of the rats; the concentrations were then
maintained at the last adjusted concentration from commencement of
cohabitation until sacrifice. Appropriate statistical tests were
applied.
The parental animals showed no significant clinical signs in
either generation. Body weight and food intake were unaffected by
treatment. The mean fertility index (number of females delivering a
litter expressed as the percentage of the total number of females
placed with a male) was reduced at 0.5 and 0.8 mg/kg bw per day but
not at the highest dose, and a similar inverted dose-response
relationship was seen for length of gestation; data on individual
animals were not presented. The litter sizes (10-11) were comparable
at all doses, as were the survival indices (> 91% in all groups on
days 1, 4, 7, 14, and 21) during lactation. The pup weights
(presumably litter weight per number of pups) were also comparable. In
F1 adults, the body weight of males was reduced at 1.2 mg/kg bw per
day on days 160-182 after cohabitation, and those of females showed
slight, sporadic increases when compared with controls. Food intake
was sporadically decreased in males but not in females. No effects
were seen on female body weight during lactation. The fertility index
in treated groups exceeded the control values. The litter sizes were
comparable in all groups, as were the pup weights and pup survival
during lactation. The adequacy of this study for regulatory purposes
was limited by the doses selected. As no adverse effects were found at
the highest dose, it was unclear whether the study clearly
demonstrated the reproductive toxicity of the test material in rats.
The lack of data for individual animals and the inverted dose-response
relationships for some parameters rendered the findings equivocal. In
the absence of clear adverse effects on reproductive parameters at any
dose, the NOAEL was 1.2 mg/kg bw per day (Dietz et al., 1983).
In a study conducted in compliance with GLP standards and the
requirements of the US Environmental Protection Agency 83-4,
technical-grade chlorpyrifos (stated purity, 95.8%) was given in the
diet to groups of male and female CrL: COBS CD (SD) BR rats at
concentrations of 0, 2, 10, or 50 ppm over two generations. These
concentrations corresponded to achieved intakes of 0.1-0.2, 0.5-0.9,
and 2.5-4.5 mg/kg bw per day, respectively, in F0 males and 0.1-0.2,
0.6-0.9, and 2.9-4.6 mg/kg bw per day, respectively, in F0 females.
The corresponding intakes in the F1 generation were 0.1-0.3,
0.7-1.6, and 3.3-8.1 mg/kg bw per day in males and 0.2-0.3, 0.8-1.6,
and 4-8.1 mg/kg bw per day in females. The animals of the F0
generation (28 of each sex per dose) were approximately 7 weeks of age
at the beginning of treatment and were maintained on their respective
diets for at least 56 days before mating. They were mated at a 1:1
ratio for 20 days, and then the dams were allowed to rear their young
to day 21 post partum. Before weaning, all F1 offspring in all
litters were examined for developmental indices (surface righting
reflex, startle reflex, air righting reflex, and pupil reflex). At day
21 post partum, 24 pups from each group were selected for mating;
excess pups were sacrificed on day 22 post partum and examined
macroscopically, and specified organs were weighed and tissues
preserved for histopathological examination from one animal of each
sex per litter. Shortly after the F1 pups were weaned, the F0
adults were killed and examined macroscopically, and a wide range of
organs and tissues were preserved, the ovaries, pituitary, prostate,
testes with epididymides, and uterus with cervix and vagina being
examined histopathologically.The animals in the F1 generation that
were selected for mating were reared on their respective diets for at
least 56 days (i.e. until they were approximately 12 weeks of age) and
were then mated 1:1 for 20 days; the dams were allowed to rear their
young until day 21 post partum. Pathological examination was
conducted as for the F0 generation. All animals were handled
regularly and examined for signs associated with treatment. All
animals that died or were killed were examined. The weight of each
animal was determined at the start of each generation and subsequently
at weekly intervals. All pups were weighed at birth and at 4, 8, 12,
and 21 days post partum. Cholinesterase activity was not determined
in the study. The doses were selected on the basis of those that
decreased cholinesterase activity in a preliminary study in which
chlorpyrifos was given in the diet for a single generation at doses up
to 250 ppm. In this preliminary study, plasma cholinesterase activity
was inhibited when compared with that in controls by 86% in adult
females and by about 60% in weanling males and females at 50 ppm. At
50 ppm, erythrocyte cholinesterase activity was also inhibited in
adult females (70% inhibition) and weanlings (about 50% inhibition),
and brain cholinesterase was inhibited by 38% in adult females
Food consumption was similar in control and treated groups and no
adverse, treatment-related effects on group mean body weights were
observed at any dose in either generation. Mating performance and the
duration of gestation were similar in control and treated groups.
Slight inter-group variation was observed in the incidence of total
pre-birth loss in both the F0 and F1 generations, but these
findings were considered not to be related to treatment in the absence
of a consistent relationship between dose and effect. The F0
generation showed statistically significant increases in litter size
and litter weight and decreased mean pup weight at a number of doses
at most sample intervals (Table 11), but these values were generally
only slightly different from those in controls and showed no
consistent relationship with dose. The decrease in mean pup weights
was probably related to the increased litter size. The data for the
litters of the F1 generation were similar for control and treated
groups, with the exception of a single, statistically significant
decrease in litter weight at 50 ppm on day 21. This isolated finding
was considered to be incidental to treatment. No consistent,
dose-related effect on organ weights was reported during the study.
Pathological examination revealed no macro- or microscopic effects
that were related to treatment. Under the conditions of this study, no
adverse treatment-related effects were observed at any dose. The
slight increase in mean body weights of F0 males at the high dose
was the only effect attributed to treatment. The NOAEL was 50 ppm,
equal to 2.5 mg/kg bw per day (James et al., 1988).
Four groups of Sprague-Dawley rats were used in a two-generation
(one litter per generation) study of reproductive toxicity in which
diets containing chlorpyrifos (purity, 97.8-98.5%) at doses of 0, 0.1,
1, or 5 mg/kg bw per day were administered. The study was conducted in
accordance with FIFRA guideline 83-4, OECD guideline 416, and GLP
principles. The F0 parental animals (30 of each sex per dose) were
Table 11. Statistically significant differences in group data for
litters of the F0 generation of rats given diets containing
chlorpyrifos
Time Litter parameter Dose (ppm)
0 2 10 50
At birth Litter size, total 12 13 14** 13
Litter size, live 12 13 14** 13
Litter weight (g) 70 72 79* 71
Pup weight (g) 6.1 5.6*** 5.7** 5.7*
Day 4 Litter size, live 11 13* 14*** 12
Litter weight (g) 100 110 120* 110
Mean pup weight (g) 9.3 8.7 8.6* 9.2
Day 8 Litter size, live 11 13** 13*** 12
Mean pup weight (g) 15 14* 14* 15
Day 12 Litter size, live 11 13** 13*** 12
Litter weight (g) 240 270 280** 250
Mean pup weight (g) 22 21 21* 22
Day 21 Litter size, live 11 13* 13*** 12
Litter weight (g) 460 520 530* 480
Mean pup weight (g) 44 42 40* 42
* p < 0.05; ** p < 0.01; *** p < 0.001
6 weeks of age at the beginning of the study and were mated after 10
weeks of exposure to produce the F1 litters. Groups of 30 rats of
each sex per dose were selected from the F1 weanlings and were
treated for 12 weeks before breeding to produce the F2 litters.
Pairing in both generations allowed for three periods of 7 days'
cohabitation in a 1:1 ratio, the males being changed weekly. Care was
taken to avoid sibling pairings. Females were removed from pairing
when vaginal lavage showed sperm, and this was considered to be day 0
of gestation. The F1 and F2 litters were culled if appropriate to
a total of eight pups on day 4. Clinical observations were performed
daily on all animals; litter size at birth, the numbers of live and
dead pups on days 0, 1, 4, 7, 14, and 21 post partum, and the sex
and weight of each pup were recorded on days 1, 4, 7, 14, and 21 of
lactation. Body weights and food consumption were recorded weekly
before and after breeding. The body weights of dams were recorded on
days 0, 7, 14, and 21 of gestation and on days 1, 4, 7, 14, and 21 of
lactation, whereas food consumption was recorded once during the first
week of lactation, twice during the second week, and every 2-3 days
during the third week. Inhibition of cholinesterase activity in
plasma, erythrocytes, and brain was measured in 10 F0 and F1
parental animals of each sex at necropsy after 19 and 21 weeks of
exposure. Complete necropsies were conducted on all F0 and F1
adults, and included ocular examinations and collection ofmany
tissues. Histopathological examinations were made of the adrenals,
brain, gross lesions, and reproductive tissues (cervix, coagulating
glands, epididymides, ovaries, oviducts, pituitary, prostate, seminal
vesicles, testes, uterus, and vagina) of controls and animals at the
high dose. The livers of 10 F1 parental males in the control and
high-dose groups were also examined microscopically. Only the adrenals
from animals at the intermediate and low doses were examined. Ten F1
and F2 pups of each sex per dose were autopsied grossly. Appropriate
statistical tests were applied to all data.
No significant effects of treatment were seen on clinical signs,
food intake, or body weight. A slight but not statistically
significant decrease in body weight was seen in F1 males at 5 mg/kg
bw per day, and F1 females at this dose showed reduced body-weight
gain during lactation. Plasma cholinesterase activity was inhibited in
parental F0 and F1 rats of each sex at 1 mg/kg bw per day (40-57%)
and at 5 mg/kg bw per day (< 72%). Plasma cholinesterase activity
was also inhibited in animals of both generations at 0.1 mg/kg bw per
day as part of a dose-related trend, although the inhibition was
generally not statistically significant at this dose. Erythrocyte
cholinesterase activity was strongly inhibited at 1 and 5 mg/kg bw per
day, but that of brain was inhibited only at 5 mg/kg bw per day (Table
12).
Gross observation of F0 and F1 parents revealed no
significant alterations, and the only significant histopathological
change in the parental animals was vacuolation consistent with fatty
changes in the adrenal zone fasciculata. Treatment had no effect on
fertility, length of gestation, survival dring gestation, time to
mating, sex ratio, or litter size in either generation. F1 pups at
1 mg/kg bw per day showed slightly decreased body-weight gain during
lactation and statistically significantly decreased body-weight gain
and survival at 5 mg/kg bw per day. No effect of treatment was seen
during gross or daily observation of the F1 weanlings. F2 pups did
not show dose-related decreases in body-weight gain during lactation,
but the survival of pups at 0 and 5 mg/kg bw per day was decreased,
with total loss of three and five litters, respectively; this effect
was stated to be due to maternal neglect, since the stomachs of pups
in these litters contained no milk. No effect of treatment was seen
during gross or daily observation of the F2 weanlings. The NOAEL for
inhibition of erythrocyte cholinesterase in adults was 0.1 mg/kg bw
per day and that for inhibition of brain cholinesterase activity and
materal toxicity was 1 mg/kg bw per day. The NOAEL for developmental
effects was 1 mg/kg bw per day, and the NOAEL for effects on fertility
and reproductive effects was 5 mg/kg bw per day (Breslin et al.,
1991).
Table 12. Mean cholinesterase activity in F0 and F1 parent rats at necropsy (percentage inhibition compared
with controls)
Generation Dose (mg/kg Cholinesterase activity (% inhibition)
bw per day)
Plasma (IU/ml) Erythrocytes (IU/ml) Brain (IU/g)
Male Female Male Female Male Female
F0 0 0.54 2.0 1.1 1.0 9.3 9.0
0.1 0.46 (15%) 1.6 (20%) 1.0 (5%) 1.0 9.2 (1%) 9.1
1 0.30a (44%) 0.8a (59%) 0.3b (92%) 0.36b (65%) 8.8 (6%) 8.7 (3%)
5 0.21a (61%) 0.6a (67%) 0.3b (92%) 0.31b (70%) 4.9b (48%) 4.6a (49%)
F1 0 0.53 1.8 1.1 0.96 9.7 9.4
0.1 0.43 (19%) 1.6 (15%) 0.98a (13%) 0.97 9.7 9.2 (2.5%)
1 0.30a (43%) 0.93a (52%) 0.37a (67%) 0.32b (67%) 9.4 (3%) 9.0 (4.5%)
5 0.19a (64%) 0.52a (72%) 0.33a (70%) 0.24b (75%) 4.6a (52%) 4.0b (58%)
a Statistically significantly different from control mean by Wilcoxon's test
b Statistically significantly different from control mean by Dunnett's test
(ii) Developmental toxicity
Mice
In the range-finding portion of a study of developmental
toxicity, pregnant mice were given chlorpyrifos at doses of up to 60
mg/kg bw per day by gavage. No toxic effects were seen in the dams or
offspring at 3, 10, and 20 mg/kg bw per day. In the main study, groups
of 40-47 pregnant CF-1 mice were initially given chlorpyrifos (purity,
96.8%) at 0, 1, 10, or 25 mg/kg bw per day by gavage in cottonseed oil
on days 6-15 of gestation. The day on which a vaginal plug was
observed was considered to be day 0 of gestation. Owing to severe
maternal toxicity at 25 mg/kg bw per day, additional groups of 35-41
mice were given 0, 0.1, 1, or 10 mg/kg bw per day by gavage on days
6-15 of gestation. The mice were observed for signs of toxicity and
were weighed daily on day 6-16 of gestation and then sacrificed on day
18. At the time of surgical removal of their fetuses, the weights of
the liver and gravid uterus with ovaries were recorded. The uteri of
non-pregnant mice were stained with sodium sulfide to ensure that no
implantations had occurred. The fetuses were weighed, measured
(crown-rump length), sexed, and examined externally. One-third of the
fetuses were dissected to examine the soft tissues. All of the heads
were preserved in Bouin's solution and subsequently sectioned. All
fetuses were stained with Alizarin red for examination of the
skeletons.
Additional mice were added to the study to assess clinical
changes. Cholinesterase (erythrocyte and plasma) activity was measured
in groups of 4-10 mice given 1, 10, or 25 mg/kg bw per day on day 6,
days 6-10, or days 6-15 of gestation, and these measurements were also
made in a group treated with 0, 0.1, 1, or 10 mg/kg bw per day. Blood
was collected for assay of cholinesterase 5 h after dosing on days 6,
10, and 15 of gestation, respectively. A homogenate of fetal tissue
was prepared from litters of mice killed on day 15 of gestation. Data
were not provided on the body weights or food and water consumption of
the animals in these groups.
Appropriate statistical analysis was applied to the experimental
data: fetal body weights and body measurements, maternal body weights,
weights of maternal livers and uteri, and food and water consumption
were analysed by one-way analysis of variance and Dunnett's test. The
incidences of fetal resorptions and alterations were analysed by a
modified Wilcoxon test. The Fisher exact test was applied to other
data.
In the dose-ranging study, severe cholinergic signs and
consequent reproductive failure were found at doses > 30 mg/kg bw
per day. In the first main study, dose-related increases in the
incidence of clinical signs including mortality, excessive salivation,
tremors, urine-soaked coat, ataxia and lethargy were found at 10 and
25 mg/kg bw per day. A single death and isolated clinical signs at
1 mg/kg bw per day were considered not to be related to treatment.
Body-weight gain was reduced at 25 mg/kg bw per day, but no effects
were observed on litter size, resorption incidence, or sex ratio.
Fetal body weight and crown-rump length were significantly reduced at
25 mg/kg bw per day. Plasma cholinesterase activity was depressed in a
dose-related manner, while the activities in erythrocytes and fetal
tissue homogenate were inhibited at 10 and 25 mg/kg bw per day (Table
13). Exencephaly was reported in all groups, but with no dose-response
relationship, and a statistically significant incidence of delayed
ossification was seen at 25 mg/kg bw per day (Table 14).
The second main study showed no cholinergic signs in dams at any
dose up to 10 mg/kg bw per day. Body weight, food intake, maternal
liver weights, gravid uterine weight, and adjusted body weights were
also unaffected. Cholinesterase activity was rapidly and significantly
inhibited in plasma and erythrocytes at 1 and 10 mg/kg bw per day but
was depressed in fetal tissue homogenates only at the high dose. No
effects were observed on materna1 parameters or on litter size, the
incidence of resorptions, the incidence of dead fetuses, the sex
ratio, fetal body weight, or crown-rump length (Table 15). Significant
fetal alterations are shown in Table 16.
Neither study provided evidence of teratogenic activity at any
dose, but fetal toxicity was seen at 25 mg/kg bw per day. The NOAEL
for developmental toxicity was therefore 10 mg/kg bw per day. The
NOAEL for inhibition of erythrocyte cholinesterase activity was 0.1
mg/kg bw per day, and the NOAEL for maternal toxicity was 1 mg/kg bw
per day (JMPR, 1982; modified by reference to the original reports of
Deacon et al., 1979, 1980).
Rats
In a range-finding study of developmental toxicity, which was
given a statement of quality assurance, Fischer 344 rats were fed
chlorpyrifos (purity, 96.6%) by gavage at doses of 0, 3, 10, 15, or
30 mg/kg bw per day during organogenesis. Slight maternal toxicity was
observed at 15 mg/kg bw per day, and severe toxicity was observed at
30 mg/kg bw per day, with typical cholinergic signs of excessive
salivation, lachrymation, urination, defaecation, and body tremors and
general observations of unkempt appearance, decreased body size, and
matting of perineal and facial hair. Deaths were also seen at 30 mg/kg
bw per day, and animals in this group had decreased body-weight gain,
decreased food consumption, enlarged adrenal glands, decreased liver
weight, and shrunken thymus glands. Both plasma and erythrocyte
cholinesterase activities were depressed at all doses. Maternal
toxicity was thus observed at all doses, but fetal toxicity was seen
only at 30 mg/kg bw per day (Ouelette et al., 1983a).
In the main study, which also had a statement of quality
assurance, groups of 31-33 pregnant Fischer 344 rats were given
chlorpyrifos (purity, 96.6%) by gavage in corn oil at doses of 0, 0.1,
3, or 15 mg/kg bw per day on days 6-15 of gestation. They were mated
1:1, and the day sperm were observed in a vaginal smear was considered
to be day 0 of gestation. The rats were observed daily for signs of
toxicity, were weighed daily on days 6-16 and day 21 of gestation, and
Table 13. Significant findings in mice and offspring given chlorpyrifos by gavage on days 6-15 of
gestation
Finding Dose (mg/kg bw per day)
0 1 10 25
No. of females 51 40 44 47
No. of animals with cholinergic signs 0 2 9 32
Deaths 0 1 1 4
Total pregnanta 40 (4) 31 (1) 32 (1) 34 (1)
No. with litters 36 29 30 29
Maternal body weight gain (g)b 21 ± 5 22 ± 3 22 ± 4 18 ± 4*
No. of implantation sites per dam 13 ± 3 13 ± 2 13 ± 1 12 ± 3
No. of fetuses per litter 11 ± 3 12 ± 2 12 ± 2 11 ± 3
Fetal body weight (g) 1.14 ± 0.10 1.13 ± 0.07 1.15 ± 0.08 1.03 ± 0.17*
Fetal crown-rump length (mm) 25.0 ± 1.2 25.2 ± 0.9 25.3 ± 0.9 24.4 ± 1.6*
Plasma cholinesterase activityc 100 (10) 15 (7) 4 (8) 2 (9)
Erythrocyte cholinesterase activityc 100 (10) 86 (6) 57 (9)* 43 (9)*
Fetal homogenate cholinesterase activity 100 81 65 35
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining (not resulting in
litters).
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals
Table 14. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 1 10 25
No. of fetuses examined 408 347 359 326
Exencephalya 1/1 5/5* 1/1 4/3
Ablephariaa 1/1 1/1 1/1 2/2
Cleft palatea 0 2/2 1/1 2/1
Small cerebral hemispherea 1/1 1/1 1/1 0
Multiple skeletal malformationsa 0 0 1/1 0
Fused ribsa 1/1 0 1/1 0
Skull: delayed ossification 5/5 9/5 10/6 34/10*
Sternebrae: delayed ossification 37/17 24/11 19/11 78/22*
Sternebrae: unfused 2/2 7/6* 3/3 8/7*
Sternebrae: fused 24/16 9/16* 23/12 6/5*
* Significantly different from control group
a Considered to be a major malformations
were sacrificed on day 21. At the time their fetuses were surgically
removed, the body and liver weights were recorded. The number of
corpora lutea and the number and position of live and resorbed fetuses
were recorded at necropsy. Fetuses were sexed, examined, and measured.
The uteri of non-pregnant rats were stained with sodium sulfide to
determine whether implantations had occurred in the absence of
litters. Separate groups of 10 rats were dosed on days 6-15 of
gestation and killed, when samples of maternal blood were collected by
heart puncture to measure erythrocyte and plasma cholinesterase
activity. Appropriate statistical analysis of the data was reported.
There were no unscheduled deaths. Clinical signs of maternal
toxicity were found, including excessive salivation, urine staining in
the perineal region, porphyrin deposits around the eyes, and vaginal
bleeding. Tremors were observed only at 15 mg/kg bw per day, and
animals at this dose also had decreased body-weight gain; no other
maternal findings were reported at necropsy. Both plasma and
erythrocyte cholinesterase activities were depressed at doses of 3 and
15 mg/kg bw per day (Table 17). No adverse effects were observed on
reproductive parameters, and no teratogenicity was observed.
Variations and malformations were found which were distributed
randomly by dose, and no effect of treatment on skeletal development
was seen. The NOAEL for maternal toxicity was 0.1 mg/kg bw per day, on
the basis of inhibition of erythrocyte cholinesterase activity in all
adult animals at higher doses. No fetal toxicity was observed
(Ouelette et al., 1983b).
Table 15. Significant findings in mice and offspring given chlorpyrifos by gavage on days
6-15 of gestation
Finding Dose (mg/kg bw per day)
0 0.1 1 10
No. of females bred 41 35 36 35
Total no. pregnanta 30 (0) 25 (1) 23 (0) 28 (3)
No. with litters 30 24 23 25
Maternal body weight gain (g)b 12 ± 6 14 ± 5 14 ± 5 14 ± 5
No. of implantation sites per dam 10 ± 3 10 ± 3 10 ± 3 9 ± 4
No. of fetuses per litter 8 ± 3 7 ± 3 7 ± 3 7 ± 3
Fetal body weight (g) 1.04 ± 1.11 1.07 ± 0.15 1.08 ± 0.18 1.09 ± 0.12*
Fetal crown-rump length (mm) 24.4 ± 1.1 24.8 ± 1.6 24.9 ± 2.1 24.8 ± 1.2*
Plasma cholinesterase activityc 100 (8) 86 (6) 25 (7)* 3 (6)*
Erythrocyte cholinesterase activityc 100 (8) 88 (6) 75 (7)* 50 (6)*
Fetal homogenate cholinesterase activityc 100 84 95 77
* Significantly different from control at p < 0.05
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Days 6-17 of gestation
c Percent of control; numbers in parentheses are numbers of animals.
Table 16. Incidences (no. of fetuses/no. of litters) of significant
alterations in fetuses of mice given chlorpyrifos by gavage
on days 6-15 of gestation
Alteration Dose (mg/kg bw per day)
0 0.1 1 10
Exencephalya 1/1 1/1 0 1/1
Ablephariaa 1/1 0 0 0
Cleft palatea 0 0 1/1 0
Omphalocelea 0 0 0 1/1
Skull: delayed ossification 18/10 11/6 3/2 3/3
Sternebrae: delayed ossification 50/20 25/13 25/13 17/11
Sternebrae: unfused 3/3 6/5 0 1/1
Sternebrae: fused 13/7 9/7 3/3 12/8
a Considered to be major malformations
Female CD Sprague-Dawley rats were mated 1:1 with stock males
from the same source and strain. Technical-grade chlorpyrifos (stated
purity, 96.1%) was formulated daily as solutions in maize oil and
given to groups of 32 mated females in a volume of 5 ml/kg bw by oral
gavage at doses of 0.5, 2.5, or 15 mg/kg bw per day on days 6-15
post coitum. A further group received maize oil only. This study was
conducted in accordance with US Food and Drug Administration and
Environmental Protection Agency guidelines for GLP and testing
guidelines of the OECD (No. 414) and US Environmental Protection
Agency (section 83-3). All females were examined daily for signs
associated with treatment. Animals were weighed on days 0, 3, 6-15,
17, and 20 of gestation, and food consumption was measured twice
weekly. Ten mated females were bled on day 15 for measurement of
plasma cholinesterase activity, blood samples being obtained from the
retro-orbital sinus under ether anaesthesia. The same procedure was
repeated for an additional 10 animals per dose immediately before
necropsy on day 20. The animals used for cholinesterase determinations
on day 15 were discarded without necropsy after being bled. All
remaining animals were killed on day 20, and the following were
recorded for each animal: any macroscopic abnormality of the
reproductive tract; number of corpora lutea in each ovary; weight of
gravid uterus; distribution of live and dead fetuses and distribution
of resorption sites in each uterine horn; individual placental
weights; individual fetal weight, length, and sex; and external
anomalies of individual fetuses. The thoracic and abdominal contents
of approximately one-half of each litter were dissected and examined,
and these fetuses were used for skeletal staining and evaluation; the
remaining fetuses were used for preparation of free-hand sections and
examination of visceral organs.
Table 17. Adverse effects in rats given chlorpyrifos by gavage
Finding Dose (mg/kg bw per day)
0 0.1 3 15
No. of females 31 32 33 31
No. of animals with cholinergic signs 0 0 0 12
Total no. pregnanta 30 (0) 28 (0) 26 (0)b 29 (1)
No. of litters 29 26 24 26
Maternal body weight gain (g)c 65 ± 11 64 ± 14 71 ± 12 58 ± 11
No. of implantation sites per dam 11 ± 2 10 ± 2 10 ± 2 10 ± 3
No. of fetuses per litter 10 ± 3 9 ± 2 10 ± 3 9 ± 3
Mean fetal body weight (g) 4.28 4.37 4.52* 4.46*
Mean fetal crown-rump length (mm) 43.91 43.93 43.96 43.64
Plasma cholinesterase activity (IU/ml)d 46.0 42.8 (93%) 4.9 (11%) 1.5 (3%)
Erythrocyte cholinesterase activity (IU/ml)d 12.1 11.9 (98%) 3.1 (26%) 2.5 (21%)
* Significantly different from control
a Numbers in parentheses are pregnancies detected by sodium sulfide staining.
b Two dams removed during dosing for reasons not related to treatment
c Days 6-20
d Mean of 6-8 dams on day 15, IU/ml (percentage of control)
No treatment-related deaths occurred during the study, and
clinical signs of intoxication were confined to tremors late in the
study in three animals at 15 mg/kg bw per day. Slight but
statistically significant decreases in mean food consumption (8%
reduction during day 79 only) and body-weight gain were seen at
15 mg/kg bw per day. Statistically significant, dose-related decreases
in plasma cholinesterase activity were observed at all doses;
erythrocyte and brain cholinesterase activities were not determined.
The effects on litters are summarized in Table 18. The total live
litter sizes were unaffected by treatment, but at 15 mg/kg bw per day
the mean number of live male fetuses was statistically significantly
decreased and post-implantation loss was slightly elevated.
Statistically significant increases in pre-implantation loss were seen
at all doses, but there was no consistent dose-response relationship
and the incidence was within historical control values for this
laboratory; the finding was considered not to be related to treatment.
At 15 mg/kg bw per day, slight but statistically significant increases
were seen in mean fetal weight and mean crown-rump length, but as the
increase in fetal weight was not accompanied by a decrease in litter
size, this finding may have been due to advanced fetal development and
was considered to be of no toxicological significance. Similarly, the
statistically significant decrease in the incidence of small fetuses
observed in all test groups was considered not to be toxicologically
significant. A number of minor structural variations were reported,
including a single incidence of anophthalmia at 0.5 mg/kg bw per day
and instances of microphthalmia at 0.5 and 2.5 mg/kg bw per day, but
the incidence of such findings was low and/or were not related to
dose. These findings were therefore considered to be incidental to
treatment. Skeletal examination showed no adverse treatment-related
findings. The NOAEL for frank maternal toxicity was 2.5 mg/kg bw per
day, on the basis of reduced body weight, tremors, and transient
reductions in food consumption at 15 mg/kg bw per day. The NOAEL for
fetal toxicity was 2.5 mg/kg bw per day, on the basis of a slight
increase in post-implantation loss at 15 mg/kg bw per day, probably
associated with maternal toxicity (Rubin et al., 1987a).
Rabbits
Groups of 14 female New Zealand white rabbits were mated 1:1 with
stock males from the same source and strain and given technical-grade
chlorpyrifos (stated purity, 96.1%), formulated daily as solutions in
maize oil, by oral gavage at doses of 1, 9, 81, or 140 mg/kg bw per
day (in a volume of 2 ml/kg bw) on days 7-19 post coitum. The doses
for animals at 81 and 140 mg/kg bw per day were prepared directly, and
those for the animals at 1 and 9 mg/kg bw per day were prepared by
diluting the dose of 81 mg/kg bw per day with maize oil. A further
group received maize oil only. This study was conducted in accordance
with US Food and Drug Administration and Environmental Protection
Agency guidelines for GLP and testing guidelines of the OECD (No. 414)
and US Environmental Protection Agency (section 83-3). The doses were
selected after a preliminary range-finding study. On day 15, three
does at 81 mg/kg bw per day were accidentally given a dose of
140 mg/kg bw, and the group was expanded to 21 animals so that the
overdosed animals could be excluded from the study if that was found
to be warranted. Two of the three animals were pregnant, but no
apparent difference was found from the females treated at 81 mg/kg bw
per day, and the maternal and fetal data for these animals were
included in the report. All animals were examined daily for clinical
signs associated with treatment. Any animals found dead or killed
in extremis were autopsied to determine the cause of death. Animals
were weighed on days 0, 3, 7-19, 22, 25, and 29 of gestation, and food
consumption was determined twice weekly. Plasma cholinesterase
activity was measured before mating and again after at least 10 days
of dosing. The does were killed on day 29 and the following were
recorded: any macroscopic abnormality of the reproductive tract; the
number of corpora lutea in each ovary; the weight of gravid uterus;
the distribution of live and dead fetuses and of resorption sites in
each uterine horn; individual placental weights; individual fetal
weight and crown-rump length; sex; and external anomalies of
individual fetuses. The thoracic and abdominal contents were dissected
and examined, and the skull of each fetus was sectioned transversely
through the frontal-parietal suture, and the brain examined. Each
fetus was used for skeletal staining and evaluation.
Table 18. Effects on fetuses of rats given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 0.5 2.5 15
Pre-implantation loss (%) 8.6 12.2*** 11.5*** 11.4***
Post-implantation loss (%) 7.0 6.0 5.8 9.0**
Mean fetal weight (g) 3.33 3.40 3.44 3.51**
Mean crown-rump length (mm) 36.0 36.2 36.3 36.7**
Live fetuses:
Male 7.6 8.0 6.8 6.0*
Female 6.8 7.0 7.3 7.5
Total 14.4 15.0 14.1 13.4
Small fetuses (no. observed) 48 (317) 25 (315)** 20 (311)*** 13 (282)***
Ablepheron (no. observed) 0 (317) 0 (315) 1 (311) 0 (282)
Major cranio-facial malformations: 0 (317) 1 (315) 0 (311) 0 (282)
proboscis, anophthalmia (no. observed)
Hydronephrosis (no. observed) 0 (161) 0 (158) 1 (158) 0 (146)
Hydroureter 0 (161) 0 (158) 1 (158) 0 (146)
Anophthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
Microthalmia: free-hand section 0 (156) 1 (157)a 0 (153) 0 (136)
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
No treatment-related deaths were observed during the study, and
no treatment-related clinical signs were reported. Maternal group mean
food consumption was unaffected by treatment, and group mean body
weights and body-weight gains were not affected by treatment at doses
up to 81 mg/kg bw per day. At 140 mg/kg bw per day, body-weight gain
was inhibited during most of the treatment period, although it was
greater than normal after cessation of treatment, and by day 29 the
animals in this group had attained body weights similar to those in
the control group. Statistically significant, dose-related decreases
in plasma cholinesterase activity were seen at all doses after 10 days
of treatment, with inhibition of 56% at 1 mg/kg bw per day, 68% at 9
mg/kg bw per day, 70% at 81 mg/kg bw per day, and 72% at 140 mg/kg bw
per day compared with controls. Erythrocyte and brain cholinesterase
activities were not determined.
Statistically significant decreases in pre-implantation loss were
observed at most doses, but this finding was considered not to be
toxicologically significant. Statistically significant increases in
post-implantation loss were observed at 9 and 140 mg/kg bw per day but
not at 81 mg/kg bw per day. In the absence of a consistent
dose-response relationship, the toxicological significance of this
finding was unclear; no historical control data were supplied for this
effect from the laboratory. A slight but statistically significant
decrease in mean fetal crown-rump length and a decrease in mean fetal
weight were observed in animals at 140 mg/kg bw per day, and these
effects were considered to be related to treatment. A number of fetal
anomalies were observed but were considered not to be
treatment-related as the incidence was generally low and unrelated to
dose. An increased incidence of fetuses with fifth sternebra and/or
unossified xiphisternum seen at 140 mg/kg bw per day was considered to
be related to slightly delayed development at this dose, as indicated
by reduced fetal weight and length in this group. Given the variation
in the incidence of other skeletal observations in treated groups,
however, this finding may have been due to chance. The NOAEL for frank
maternal toxicity was 81 mg/kg bw per day, on the bais of decreased
body-weight gain at the higher dose. The NOAEL for fetal toxicity was
81 mg/kg bw per day, on the basis of a slight decrease in mean fetal
crown-rump length, decreased mean fetal weight, and an increased
incidence of fetuses with fifth sternebra and/or unossified
xiphisternum at the higher dose (see Table 19) (Rubin et al., 1987b).
(f) Special studies: Neurotoxicity
Chickens
No signs of delayed ataxia or paralysis were reported in groups
of three hens treated with single oral doses of chlorpyrifos at 40,
75, 100, or 150 mg/kg bw. The two higher doses resulted in deaths and
other clinical signs of toxicity (Stevenson, 1966a). Similarly, groups
of 10 hens given single oral doses of chlorpyrifos at up to 100 mg/kg
bw did not display delayed neurotoxicity, and no neuropathological
findings associated with treatment were detected (Rowe et al., 1978).
In another study, single oral doses of chlorpyrifos at up to five
Table 19. Effects on litters and fetuses of rabbits given chlorpyrifos by gavage
Effect Dose (mg/kg bw per day)
0 1 9 81 140
Number of fetuses examined 108 117 126 142 99
Pre-implantation loss (%) 15.2 13.1 8.9*** 11.9* 11.5*
Post-implantation loss (%) 6.5 8.6 13.8*** 8.7 13.9***
Mean fetal weight (g) 45.4 44.3 43.3 41.8 40.7
Mean crown-rump length (mm) 97.4 95.9 95.0 94.2 93.2*
Teeth absent 0 0 1 0 0
Arthrogryposis, forelimb 0 0 2 0 0
Irregularly shaped thorax 0 0 0 0 1
Hydrocephalus 0 0 1 0 0
Enlarged aortic arch 0 0 1a 0 0
Rudimentary pulmonary artery 0 0 1a 0 0
Agenesis of interventricular cardiac septum 0 0 1a 0 0
Hydronephrosis (bilateral) 2 0 0 4 1
Hydronephrosis (unilateral) 2 2 1 2 1
Diffuse opacity of eye 0 0 0 2 0
No. of fetuses examined for skeletal defects 108 117 126 142 99
Reduced or incomplete ossification of interparietal bone 3 4 3 5 2
Unossified interparietal bone 0 1 0 0 3
Reduced or incomplete ossification of hyoid bone 2 10* 17*** 16** 8*
Irregular ossification of one or more of sternebrae 1-4 1 2 7* 8* 1
Bony plaque adjacent to sternebra 10 6 2** 3* 1**
Unossified fifth sternebra and/or xiphisternum 1 1 2 3 6*
Multiple malformations of ribs and lumbar spine 0 0 0 0 1
*, p < 0.05; **, p < 0.01; ***, p < 0.001
a Same fetus
times the oral LD50 in hens (> 150 mg/kg) resulted in significant
inhibition of neuropathy target esterase and cholinesterase activity
(Capodicasa et al., 1991), with signs consistent with
organophosphate-induced delayed neuropathy. Extensive, aggressive
antidotal treatment, both before and throughout the treatment and
recovery periods, was required for the birds' survival.
Technical-grade chlorpyrifos (purity, 96.8%) was tested for acute
toxicity and delayed neurotoxicity in adult domestic hens in a study
carried out according to GLP. In the assessment of neurotoxicity,
groups of 10 birds were given corn oil, tri- ortho-cresyl phosphate
(positive control; 20% in corn oil; 500 mg/kg), chlorpyrifos
(110 mg/kg bw) plus atropine sulfate (intramuscular injection;
10 mg/kg bw), chlorpyrifos (110 mg/kg bw; 5.5% in corn oil) plus
pyridine 2-aldoxime methane sulfonate (intramuscular injection;
50 mg/kg bw; 5% aqueous solution), or were not treated and were
maintained as a source of additional birds if necessary. The hens were
observed for 21 days, at which time all those given the corn oil were
given a second dose, and all treated birds were given chlorpyrifos,
followed by another 21-day observation period.
No clinical signs of toxicity were observed in those given the
vehicle. In birds given tri- ortho-cresyl phosphate, no signs of
toxicity were observed immediately after dosing, but all birds
developed signs of delayed locomotor ataxia as early as day 11 after
treatment. The birds given chlorpyrifos showed signs of toxicity after
either dose, including subdued appearance, unsteadiness, inability to
stand, and weakness. Deaths were observed in both chlorpyrifos-treated
groups, four birds that died after the first dose with atropine
sulfate (no deaths after the second dose) and three after the first
dose of chlorpyrifos with pyridine 2-aldoxime methane sulfonate (one
death after the second dose). Surviving birds recovered completely
within 5-8 days of treatment. Additional doses of atropine sulfate
were given to several birds in both groups treated with chlorpyrifos
owing to the severity of the clinical signs observed.
Histopathological examination revealed a low incidence of minor
neurological changes (grade II on a scale of I-V of increasing
severity) in several birds given the vehicle, which was considered to
show a normal background incidence of axonal degeneration. Most of the
birds given tri- ortho-cresyl phosphate had grade III changes at at
least one level of spinal cord and grade III or IV changes in at least
one level of peripheral nerve, indicating significant
treatment-related axonal degeneration. Three birds treated with
chlorpyrifos showed grade II changes at one level of peripheral nerve
only, and these findings were considered to be similar to the
incidence and severity of changes seen in the vehicle controls. Thus,
oral administration of a single dose of chlorpyrifos at 110 mg/kg bw
to hens and another dose after 21 days did not induce clinical signs
of delayed neurotoxicity, as confirmed by histopathological
examination of nervous tissues (Roberts et al., 1987)
When chlorpyrifos was given to chickens for 91 days in the diet
at doses of < 10 mg/kg bw per day in a study conducted according to
GLP, the only compound-related clinical signs were transient, slight
gait alterations in a number of birds at the high dose. No
histopathological lesions of nerve tissues characteristic of
organophosphate-induced delayed neuropathy were seen. Concurrent
positive controls showed both toxic signs and histopathological
lesions of nerve tissues typical of those induced by a delayed
neurotoxicant (Barna-Lloyd et al., 1986).
To determine whether repeated doses of chlorpyrifos at the
maximum tolerated dose caused cumulative inhibition of neuropathy
target esterase and organophosphate-induced delayed neuropathy,
chlorpyrifos (purity, 100%) was given orally to 15 white Leghorn hens,
18 months of age, for 20 days at a dose of 10 mg/kg bw per day. Corn
oil was given to a control group of 18 birds. Physical observations
(including posture and gait) were made daily. Three hens per group
were killed on days 0 (controls only), 4, 10, 15, 20, and 48, and
brain acetylcholinesterase, plasma butyryl cholinesterase, and
lymphocyte and brain neuropathy target esterase were assayed. Body
weights were decreased during treatment, by 25% when compared with
controls on day 20, but had recovered to about 87% of that of controls
by the end of the study. Some birds showed acute effects (staggering
gait and diarrhoea) during week 1 of treatment. Diarrhoea was also
seen in controls and may have been associated with the corn oil. No
behavioural or perching defects were seen during the observation
period. During days 4-20, brain cholinesterase activity was depressed
by 58-70% and plasma cholinesterase activity by 49-80% when compared
with control values, but after the 4-week observation period brain
cholinesterase activity had recovered to 86% and plasma cholinesterase
to 134% of the controls values; neuropathy target esterase activity in
brain and lymphocytes throughout the study was 82-99% and 85-128% of
that in controls, respectively. This finding was not statistically
significant for lymphocytes, but the 18% inhibition of brain
neuropathy target esterase activity on days 10 and 20 was
statistically significant. There were no indications of delayed
neurotoxicity after repeated administration of a dose that caused
significant inhibition of brain cholinesterase activity and loss of
body weight (Richardson et al., 1993a).
In order to clarify the magnitude of the neuropathic risk
associated with exposure to chlorpyrifos, groups of three 18-month-old
white Leghorn hens were given single oral doses of chlorpyrifos
(purity, 100%) at 0, 75, 150, or 300 mg/kg bw with atropine (20 mg/kg
bw subcutaneously at the time of the first dose of chlorpyrifos and
additional doses as needed to maintain the hens for 4 days). Four days
after treament, when the hens were killed, brain acetylcholinesterase
activity was depressed by 0, 58, 75, and 86% and neuropathy target
esterase activity by 0, 21, 40, and 77% at the three doses,
respectively. In a further study, 18 hens were given corn oil alone
and 15 atropine-protected hens were given chlorpyrifos at a single
oral dose of 150 mg/kg bw. Three controls were killed on day 0, and
three treated and three control hens were killed on days 1, 2, 4, 8,
and 16. Brain acetylcholinesterase activity was inhibited by 86, 82,
72, 43, and 29% on days 1, 2, 4, 8, and 16 after treatment, and brain
neuropathy target esterase activity was inhibited by 30, 28, 38, 29,
and 6%. No signs of organophosphate-induced delayed neuropathy were
observed.
In studies in vitro in which chlorpyrifos-oxon was added to
whole brain homogenate, the rate constant for the enzyme-inhibitor
complex was 15.5 per mm per min for acetylcholinesterase and 0.145 per
mm per min for neuropathy target esterase. The calculated 20-min
fixed-time IC50 values were 2.2 nmol/L for acetylcholinesterase and
240 nmol/L for neuropathy target esterase. The measured IC50 values
based on fixed-time (20 min) preincubation were 2.2 nmol/L for
acetylcholinesterase and 210 nmol/L for neuropathy target esterase.
The ratio of the calculated values for neuropathy target
esterase:acetylcholinesterase is 110. As a ratio of > 1 generally
indicates that the dose that would be required to cause
organophosphate-induced delayed neuropathy would be in excess of the
LD50, acute exposure to chlorpyrifos, except at doses well in excess
of the LD50, would not be anticipated to induce this condition
(Richardson et al., 1993b).
The potential of chlorpyrifos to cause acute delayed
neurotoxicity was tested in white Leghorn hens given doses < 12
mg/kg bw per day for 21 days by oral gavage in peanut oil. Dose- and
time-related increases in the incidence and severity of ataxia were
observed. Plasma and whole blood cholinesterase activities were
reduced at 5 and 12 mg/kg bw per day. Ataxia was observed at 2, 5, and
12 mg/kg bw per day. Necropsy did not reveal any findings associated
with treatment. Histopathological examination of the brain and spinal
cord revealed a low incidence of several lesions, with no relation to
dose, and these findings were considered to be incidental to
treatment. Since continued administration of chlorpyrifos resulted in
ataxia, it would have been difficult to observe any delayed
neurological effects had they occurred (Jai Research Foundation,
1996b).
Rats
In rats, single oral doses of chlorpyrifos at 50-100 mg/kg bw
resulted in treatment-related effects and clinical signs for several
days after dosing. No deaths occurred at any dose. At 10 mg/kg bw,
effects were confined to isolated observations of perineal staining
and/or decreased activity. No neuropathological lesions were reported
(Wilmer, 1992; Mattsson et al., 1996).
In a study in adult Long-Evans rats carried out in accordance
with GLP, oral administration of chlorpyrifos at doses < 10 mg/kg
bw per day for 4 weeks resulted in dose-related inhibition of plasma
and erythrocyte cholinesterase activity at > 1 mg/kg bw per day and
of brain cholinesterase activity at > 3 mg/kg bw per day. These
effects were accompanied by clinical signs of intoxication at 10 mg/kg
bw per day. Cognitive behavioural tests indicated some non-cognitive
changes associated with impaired motor activity at 10 mg/kg bw per
day, but no clear treatment-related effects on cognitive function were
observed (Maurissen et al., 1996).
After oral administration of chlorpyrifos to rats at single doses
< 100 mg/kg bw in a study that conformed to GLP, no
treatment-related effects on neuropathy target esterase activity were
observed at any dose. Plasma, erythrocyte, and heart cholinesterase
activity was statistically significantly lower than that in controls
at doses > 5 mg/kg bw, and brain cholinesterase activity was
decreased at > 50 mg/kg bw. No significant inhibition was observed
at 0.5 or 1 mg/kg bw (Dittenber, 1997).
The neurotoxic potential of chlorpyrifos (purity, 99.5%) was
assessed in groups of 20 male Long-Evans hooded rats, approximately 70
days old with a mean body weight of 350 g. The test material was
dissolved in corn oil and given to the rats by oral gavage in a volume
of 1 ml/kg bw at doses of 0, 10, 30, 60, or 100 mg/kg bw. All rats
were evaluated for behavioural signs 3.5 h after dosing with a
functional observational battery of tests consisting of evaluations of
home cage, open-field, and manipulative neurobehaviour. The measures
were autonomic activity (lachrymation, salivation, miosis,
defaecation), motor activity (rearing, home cage posture), convulsive
activity (tremors), neuromuscular activity (gait score, landing foot
splay, righting reaction), general reactivity (arousal, removal
reactivity), sensorimotor activity (responses to tail pinch, click,
approach), and physiological reactions (body temperature, body
weight). Data on motor activity were recorded during a 1-h session in
a maze shortly after testing. Tissue samples were obtained after the
motor activity tests, 4.5 and 25 h after dosing. Half of the animals
in each group were killed at 3.5 h, and the remaining 10 animals were
examined again at 24 h and then killed. Immediately after sacrifice,
the brain, retina, liver, heart, diaphragm, and quadriceps femoris
muscle were collected from all animals. The brain was sectioned
mid-sagitally, and the remaining half-brain was further sectioned to
obtain the frontal cortex, hippocampus, striatum, hypothalamus,
cerebellum, and pons/medulla. Blood was collected from the trunk.
Muscarinic receptor density was determined in all brain regions and in
the heart and retina. Cholinesterase activity was determined in brain,
muscle, retina, plasma, erythrocytes, whole blood, and liver.
Cholinesterase activity was significantly inhibited in a range of
tissues, including brain, plasma, and erythrocytes, at all doses at
both 4.5 and 25 h (Table 20). As the data were represented only as
graphs, the percentages are approximations. Erythrocyte cholinesterase
activity was slightly more sensitive than that of plasma at the doses
used in this study, and plasma cholinesterase activity recovered more
quickly than that of erythrocytes. The degree of inhibition of
whole-blood cholinesterase activity lay between that of plasma and
erythrocyte activity at both 4.5 and 25 h. Whole-brain cholinesterase
activity can be considered representative of that in various regions
of the brain, although a number of regions showed less inhibition than
was measured in the half-brain in this study. In peripheral tissues,
Table 20. Percentage inhibition of cholinesterase activity compared with controls in rats given chlorpyrifos by gavage
Time (h) Dose Inhibition of cholinesterase activity (%)
(mg/kg bw)
Plasma Erythrocytes Blood Half-brain Retina Heart Diaphragm Quadriceps Liver
4.5 10 80 90 85 40 40 55 40 5 70
30 85 95 90 70 65 65 55 20 70
60 85 100 90 85 80 70 70 70 80
100 90 100 95 90 90 75 85 85 85
2.5 10 35 75 55 30 30 40 5 + 10 25
30 75 90 85 60 60 75 35 20 70
60 95 100 95 80 75 90 65 35 85
100 95 100 95 85 85 90 75 55 85
When inhibition was > 20% in comparison with corresponding controls, the change in cholinesterase activity was
statistically significant ( p < 0.05).
the inhibition was much lower in the quadriceps than in other tissues
at 4.5 h, and inhibition of heart, liver, and retina cholinesterase
activity was similar at 25 h.
Clinical and behavioural signs were observed at doses
> 30 mg/kg bw at 4.5 h. The doses selected for this study resulted
in a skew of some correlations, especially for cholinesterase
inhibition in blood and blood components, with behavioural and
clinical findings. In order to examine the relationship between
clinical and behavioural findings and cholinesterase inhibition, the
authors grouped rats according to the extent of cholinesterase
inhibition (instead of by dose) and indicated the incidence of
cholinergic signs related to individual cholinesterase inhibition. The
10 'cardinal' cholinergic signs that were used for this analysis were
temperature, gait, motor activity, smacking, foot splay, tail pinch,
tremors, lachrymation, pupil response, and salivation (Table 21). None
of these signs was observed in the 18 animals with < 60% inhibition
of brain cholinesterase activity. The incidence of these findings
increased in relation to brain cholinesterase inhibition. In animals
with < 80% inhibition of whole-blood cholinesterase activity, no
signs were observed, while all of the signs were seen at varying
incidences in animals with 90-100% inhibition. The correlation between
these end-points and inhibition of cholinesterase in other tissues or
in erythrocytes or plasma was not provided. These results were
interpreted by the authors as indicating a threshold effect, since no
clinical signs were observed until a certain percentage inhibition of
cholinesterase activity was reached. Because of the doses selected for
this study, marked inhibition was observed at the lowest dose in the
absence of clinical findings. The use of a single dose also limited
the usefulness of the findings. No clinical signs were seen when brain
cholinesterase activity was inhibited by < 60% or when whole blood
cholinesterase activity was inhibited by < 80% after a single dose of
chlorpyrifos. The data do not suggest that these values could be
extrapolated to repeated doses (Nostrandt et al., 1997).
Technical-grade chlorpyrifos (purity, 98.1%) was given to groups
of 10 male and 10 female Fischer 344 rats in the diet for 13 weeks at
doses of 0, 0.1, 1, 5, or 15 mg/kg bw per day. The dietary
concentrations were adjusted on the basis of measured weight and food
consumption, but the range of dietary concentrations used to achieve
these target doses was not provided. The study was conducted in
accordance with GLP requirements and to meet US Environmental
Protection Agency FIFRA guideline No. 82-7. Body weights and feed
consumption were measured before treatment and weekly during
treatment. Ophthalmic examinations were conducted before treatment and
at necropsy. Cage-side observations were made twice daily and clinical
examinations weekly. A functional observational battery of tests was
applied to all rats before treatment and during weeks 4, 8, and 13 of
treatment. Five rats of each sex per dose were examined for gross
pathological alterations. Tissues for histopathological evaluation
were prepared only from rats in the control and high-dose groups. Nine
transverse sections of the brain included the olfactory bulb, cerebral
cortex (frontal, parietal, temporal, and occipital lobes),
Table 21. Incidence (%) of cardinal cholinergic signs in rats given chlorpyrifos by gavage and grouped according to
extent of inhibition of cholinesterase activity (numbers of rats in parentheses)
End-point Inhibition of brain cholinesterase activity (%) Inhibition of blood cholinesterase activity (%)
90-100 (10) 80-90 (8) 70-80 (7) 60-70 (7) < 60 (18) 90-100 (25) 80-90 (14) < 80 (11)
Temperature 100 100 86 57 0 92 36 0
Gait 100 100 57 71 0 84 43 0
Motor activity 90 88 86 57 0 84 36 0
Smacking 90 63 57 0 0 64 14 0
Foot splay 40 38 29 0 0 32 14 0
Tail pinch 70 38 0 0 0 40 0 0
Tremors 90 25 0 0 0 44 0 0
Lachrymation 80 13 0 0 0 32 7 0
Pupil response 60 0 0 0 0 24 0 0
Salivation 30 0 0 0 0 12 0 0
thalamus/hypothalamus, midbrain, pons, cerebellum, medulla oblongata,
and nucleus gracilis/cuneatus. Other tissues examined were the
trigeminal ganglion and nerve, pituitary, eyes (retina and optic
nerve), spinal cord (cervical and lumbar), olfactory epithelium, and
skeletal muscles (gastrocnemius and anterior tibial).
A low incidence of perineal staining was seen in females at 5 and
15 mg/kg bw per day. No decrease in body weight was recorded at any
dose. Gross pathological examination did not reveal any
treatment-related effects. Histopathological examination revealed a
small number of isolated findings that were similar in control and
treated groups and which were not considered to be related to
treatment. These included slight degeneration of individual nerve
fibres in the trapezoid body of the medulla oblongata in four control
males and three control females and in one male and two females at the
high dose; slight degeneration of individual nerve fibres in the
cervical spinal cord of a single male at the high dose; and myelin
ellipsoids in the lumbar spinal cord of one male at the high dose and
one female control. A slight reduction in motor activity was observed
at 15 mg/kg bw per day during week 4 only, but the toxicological
significance of this finding was unclear. Cholinesterase activity was
not measured in this study. No neuropathological findings related to
treatment were observed at any dose. The NOAEL for cholinergic effects
was 1 mg/kg bw per day, and that for neuropathy was 15 mg/kg bw per
day (Shankar et al., 1993).
In a study designed to investigate the effects of age on the
toxicity of chlorpyrifos, rats received corn oil or chlorpyrifos
orally at doses of 80 mg/kg bw for adults and 15 mg/kg bw for young
rats (at postnatal day 17). These doses were equally effective in
inhibiting cholinesterase activity. In adult rats, peak behavioural
changes and cholinesterase inhibition occurred in males 3.5 h after
dosing, while in females the onset of functional changes occurred
sooner, the time course was more protracted, and recovery was slower.
In young rats, the maximal behavioural effects and cholinesterase
inhibition occurred 6.5 h after dosing, with no sex-related
difference. Partial to full recovery from the behavioural changes was
seen at 24-72 h, whereas the cholinesterase activity recovered more
slowly. The blood and brain cholinesterase activity in young rats had
almost recovered by 1 week after dosing, whereas the brain
cholinesterase activity in adults had not recovered by 2 weeks. Assays
for binding to muscarinic receptors showed apparent down-regulation in
some areas of the brain, mostly at 24 and 72 h. Rats at postnatal day
17 generally showed more receptor down-regulation than adults, whereas
only adult females showed receptor changes in striatal tissue that
persisted for 2 weeks. Thus, young rats showed similar behavioural
changes and cholinesterase inhibition to adults, although at a
fivefold lower dose; the onset of maximal effects was somewhat delayed
in the young rats; cholinesterase activity tended to recover more
quickly in the young rats; young rats appeared to have more extensive
muscarinic receptor down-regulation; and young rats showed no
sex-related difference in functional changes (Moser & Padilla, 1998).
Chlorpyrifos was administered subcutaneously to neonatal rats at
1 or 5 mg/kg bw per day on postnatal days 1-4 or daily at 5 or 25
mg/kg bw per day on days 11-14, and the two groups of pups were
examined at postnatal days 5 and 10 or 15 and 20, respectively. Pups
treated at postnatal days 1-4 had a significant increased mortality
rate (> 50%) at 5 but not at 1 mg/kg bw per day; the body weights of
the affected pups were decreased by 10-15% and the brain was
significantly smaller than that of controls. The survivors showed
severe cell loss in the brainstem; brainstem growth was maintained by
enlargement of the remaining cells. The lower dose did not compromise
survival or growth. Neither dose had an adverse effect on the
forebrain, despite the fact that the brainstem and forebrain have
comparable cholinergic projections. Pups treated on postnatal days
11-14 showed no increase in mortality rate at 5 mg/kg bw per day but a
significant increase (> 80%) at 25 mg/kg bw per day at day 15. The
body weights of the affected pups were significantly decreased
(> 40%), and the major target for cell loss shifted from the
brainstem to the forebrain. The pups at 5 mg/kg bw per day had a
small, transient loss in body weight; there was no significant effect
on whole brain weight, but these pups showed loss of forebrain cells
between 15 and 20 days of age, and the total amount of DNA in the
forebrain declined whereas an increase was seen in the forebrains of
control pups. The brainstem showed a smaller but not significant cell
loss during this period. The cerebellum differed from the other
regions in that it showed short-term increases in DNA after exposure
to chlorpyrifos in either the early or the late postnatal period;
nevertheless, the values then regressed to subnormal, in parallel with
loss of cells in other regions.
Although regions rich in cholinergic projections, such as the
brainstem and forebrain, may be more severely affected than
non-cholinergic regions such as the cerebellum, the time of maturation
of each region (brainstem earliest, forebrain intermediate, cerebellum
last) appears to be more important in determining vulnerability. The
weights of the various regions remained within normal limits despite
severe reductions in cell numbers, probably because reactive
hypertrophy of the remaining cells masked the cell loss. These results
suggest that chlorpyrifos induces cellular deficits in the developing
brain even when growth or survival is unaffected; however, the route
of administration and the doses used in this study limit the relevance
of these findings to humans (Campbell et al., 1997).
Chlorpyrifos was administered subcutaneously to neonatal rats at
doses of 1 or 5 mg/kg bw per day on postnatal days 1-4, or at 5 mg/kg
bw per day on days 11-14, and the two groups of pups were examined on
postnatal days 5 and 10 or 15 and 20, respectively. Signs of
interference with the adenyl cyclase signalling cascade were
determined in the heart and developing brainstem and cerebellum. Pups
treated on postnatal days 1-4 at 1 mg/kg bw per day showed no signs of
toxicity, while there was significant mortality (> 50%) at 5 mg/kg bw
per day. Pups treated on postnatal days 11-14 at 5 mg/kg bw per day
showed no deaths or clinical signs. No significant changes in the
weights of the brain region were found at these apparently non-toxic
doses. Cholinesterase activity in the brainstem of the pups given
1 mg/kg bw per day was inhibited by 25% when measured 24 h after the
last dose, but recovery was substantial (< 10% inhibition) by day 10.
Cholinesterase activity in the brainstem of the pups given 5 mg/kg bw
per day on days 11-14 was inhibited by > 60% when measured 24 h after
the last dos, but recovery was again substantial (< 30% inhibition)
by postnatal day 20. Separate examinations of the forebrain,
cerebellum, and heart showed that chlorpyrifos had evoked deficits in
multiple components of the adenyl cyclase cascade: the expression and
activity of adenyl cyclase itself, functioning of the G-proteins that
link neurotransmitter and hormone receptors to cyclase activity, and
expression of neurotransmitter receptors that act through this
cascade. Disruption of signalling function was not restricted to
transduction of cholinergic signals but extended to adrenergic signals
as well. In most cases, the adverse effects were not evident during
administration of chlorpyrifos but appeared after several days. Most
of the results of this study were presented in summary form only (Song
et al., 1997).
The acute neurobehavioural effects (as determined by
observational tests of function and motor activity) of two carbamates,
carbaryl and aldicarb, and five organophosphates, chlorpyrifos,
diazinon, parathion, fenthion, and diisopropyl fluorophosphate, were
evaluated in 10-week-old male Long-Evans rats on the day of dosing at
the time of the peak effect, at 1 and 3 days, and 1 week after dosing.
All doses were administered by oral gavage in corn oil in a volume of
1 ml/kg bw, except carbaryl, which was given in 2 ml/kg bw. A weight
loss of about 10% was recorded with each substance only at the high
dose. Generally, all of the cholinesterase inhibitors induced
autonomic signs of cholinergic over-stimulation (salivation,
lachrymation, and miosis), hypothermia, mild tremors, mouth-smacking
(chewing motions), decreased motor activity, decreased tail-pinch
response, and altered neuromuscular function (gait changes and
increased foot splay). The measures found to be most sensitive on the
day of dosing were body temperature, motor activity, gait, and the
presence of mouth-smacking and fine tremors, although no single
measure was the most sensitive for all compounds. Differences in the
slopes of the dose-response curves were evident for some measures.
Many effects were still present at 24 h, but the rats recovered from
the effects of all compounds. Interestingly, residual effects were
found at 72 h with the carbamates and with the organophosphate
fenthion, but not with the other compounds; this may be due, at least
in the case of the carbamates, to slow absorption from the gut. Thus,
the overall clinical toxicity profile of these cholinesterase
inhibitors was similar, but compound-specific differences emerged in
terms of individual measures, dose-response relationships, and time
course; the behavioural effects induced by these compounds may be due
to both cholinergic and non-cholinergic mechanisms. Only summary data
were available for evaluation (Moser, 1995).
Chlorpyrifos was administered subcutaneously to neonatal rats at
2 mg/kg bw on postnatal day 1 or at 11 mg/kg bw per day on postnatal
days 6-9. The developing brain regions (brainstem, forebrain, and
cerebellum) were examined for signs of interference with cell
development by using markers of cell division. The 1-day-old rats
showed significant inhibition (about 15%) of DNA synthesis in all
brain regions within 4 h of treatment. Equivalent results were
obtained when a small dose (0.6 or 2 µg/g brain) was introduced
directly into the brain by intracisternal injection, indicating that
the effect was not secondary to systemic toxicity. Comparable
inhibition (> 10%) of DNA synthesis was seen in these animals at 8
days of age, but at this point there was regional selectivity, with
sparing of the cerebellum (about 1% inhibition). In another phase of
this study, 6-8-day-old animals were pretreated with mecamylamine, a
nicotinic receptor antagonist, and then with chlorpyrifos at 11 mg/kg
bw. The pretreatment caused a decrease in DNA synthesis and also
prevented any further decrement in DNA synthesis due to chlorpyrifos.
Administration of chlorpyrifos at 1 day of age caused severe (> 30%)
inhibition of protein synthesis throughout the brain; the effect was
distinct from that on DNA synthesis, as it had diminished
substantially by 8 days of age (< 10%) and did not show regional
selectivity. Assays of ornithine decarboxylase activity 4 and 48 h
after treatment with chlorpyrifos in 1- and 8-9-day-old rat pups
showed no significant alteration of activity in any brain region in
comparison with appropriate controls. The authors concluded that the
effects of chlorpyrifos on DNA and protein synthesis are not secondary
to generalized cell damage or suppression of cell metabolism, as
evidenced by the maintenance of normal ornithine decarboxylase
activity. The results of this study were presented only in summary
form (Whitney et al., 1995)
Pregnant Sprague-Dawley rats were given chlorpyrifos at doses of
0, 0.3, 1, or 5 mg/kg bw per day by gavage in corn oil from day 6 of
gestation through day 11 of lactation, and their neurobehavioural
performance was evaluated. A satellite group of mated females was
dosed similarly every day on days 6-20 of gestation and used to obtain
blood and brain samples for analysis of cholinesterase. This study was
conducted in accordance with GLP standards. There were no deaths and
only a few clinical signs (fasciculations, hyperpnoea,
hyper-reactivity, and decreased body-weight gain) in the dams at the
high dose. Between days 1 and 5 of lactation, three dams at 5 mg/kg bw
per day had total litter loss and an additional 57 pups died or were
cannibalized; these losses led to a decreased viability index.
Necropsy of many of the dead pups indicated treatment-related lack of
maternal care (no milk in the stomach). No similar effects of
treatment were seen at the other doses. In dams, cholinesterase
activity in plasma and erythrocytes was inhibited at all three doses,
with > 40% inhibition at 0.3 mg/kg bw per day. Brain cholinesterase
activity was inhibited at the two higher doses, but the inhibition at
1 mg/kg bw per day was not > 20% that of controls. Pups that were
exposed to chlorpyrifos in utero and for a period post partum
showed signs of toxicity at the high dose: the weights of male and
female pups were significantly lower than those at the other doses,
consistent with the decreased food consumption and lack of body-weight
gain by dams at this dose. Morphometric measurements of the brain and
brain sections of six pups of eachsex per dose showed a pattern of an
increase at the low dose and a decrease at the high dose, which was
more pronounced in male pups, paralleling the differences in brain
weight between the groups. Hence, the NOAEL for maternal toxicity was
1 mg/kg bw per day. The LOAEL for inhibition of plasma and erythrocyte
cholinesterase activity was 0.3 mg/kg bw per day, and the NOAEL for
inhibition of brain acetylcholinesterase activity was 1 mg/kg bw per
day. The NOAEL for toxic effects in the pups was 1 mg/kg bw per day on
the basis of the decreased viability index, relative brain weight, and
delayed sexual maturity, possibly associated with maternal toxicity
and subsequent diminished maternal care at the high dose. Cognitive
function (learning, memory, and habituation) in the pups were not
affected by treatment (Hoberman, 1998).
Adult male Long-Evans rats were trained to perform a test of
memory and motor function and were then injected subcutaneously with
single doses of 0, 60, 125, or 250 mg/kg bw of chlorpyrifos in peanut
oil (2 ml/kg bw) and tested on 5 days/week for 7 weeks. Unconditioned
behaviour was also rated for signs of cholinergic toxicity.
Cholinesterase activity was measured in whole blood and in several
brain regions, and muscarinic receptor density and the hypothermic
effect of oxotremorine challenge were determined. Clinical signs of
toxicity were seen in one rat at 250 mg/kg bw. Whole-blood
cholinesterase activity was inhibited by 60-75% in a dose-dependent
fashion on day 4 but had recovered to near control values by day 53 in
animals given 60 mg/kg bw and by day 74 in those given 125 mg/kg bw.
Brain cholinesterase activity was strongly inhibited in a
dose-dependent manner and was inhibited by < 95% on day 7 in
animals given 250 mg/kg bw. By day 21, partial recovery of
cholinesterase activity was seen in some brain regions after the doses
of 60 and 125 mg/kg bw, but no significant recovery was seen in
animals given 250 mg/kg bw. Muscarinic receptor density decreased with
dose and time. At 250 mg/kg bw, the receptor density in the
hippocampus, frontal cortex, and striatum had fallen to 70-75% of the
control values after 7 days and to 60% 21 days after dosing; the
hypothalamic receptors were less severely affected. In comparison with
controls, the hypothermia induced by oxotremorine challenge was
significantly less pronounced in each group receiving chlorpyrifos on
days 8 and 32, but was comparable to that of controls by day 52.
Unconditioned behaviour was relatively unaffected by treatment, with
fine tremor in the head and limbs, the only sign of cholinergic
overstimulation, peaking on day 9 and no longer observed by 14 days
after dosing. Functional deficits in working memory and motor function
appeared within 2 days of injection of a dose of chlorpyrifos that
caused severe cholinergic effects, but the animals recovered within
3 weeks. The definition of working memory in this paper was
non-specific, and the short-term memory of the animals was unaffected
by treatment (Bushnell et al., 1993).
The authors contrasted these findings to those seen in rats dosed
with the organophosphate diisopropyl fluorophosphate (Bushnell et al.,
1991). These rats showed progressive, persistent impairment of
cognitive and motor function over a 3-week period of daily exposure,
despite neurochemical and pharmacological evidence of tolerance to
diisopropyl fluorophosphate-induced inhibition of cholinesterase. This
difference suggests that the diisopropyl fluorophosphate-induced
behavioural changes cannot be attributed entirely to its effects on
cholinesterase activity and changes in muscarinic receptor binding.
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 200 mg/kg bw as a
single dose on day 12 of gestation and then killed on day 16 or 20 of
gestation or on postnatal day 3 for measurement of maternal and
developmental indicators of toxicity. While most treated dams showed
no overt cholinergic signs, 4/28 showed moderate-to-severe signs 2-3
days after treatment, and these rats were omitted from further
studies. Maternal body weight was decreased by 15% 3 days after
treatment, after which the body-weight increase was comparable to that
of controls. Chlorpyrifos did not significantly affect fetal body
weights or brain weights when assayed on day 16 or 20 of gestation.
Inhibition of acetylcholinesterase activity (by 82-88%) was seen in
maternal brain at all three times after exposures. At days 16 and 20
of gestation, fetal brain acetylcholinesterase activity was inhibited
by 42-44%. While some degree of recovery in acetylcholinesterase
activity was seen in pup brain by postnatal day 3, it was still
inhibited (by 30%) in treated pups cross-fostered by control dams.
Maternal brain muscarinic receptor binding at day 20 of gestation and
postnatal day 3 was more extensively reduced (30-32%) than in the
developing brain (16 and 11%, respectively). The response to a simple
postnatal reflex test of righting was transiently altered (present at
on day 1 but not on day 3) by exposure to chlorpyrifos. The results
suggested that acute exposure of dams during gestation induced more
extensive neurotoxicological effects in the dams than in their
developing fetuses, but the dose and the route of administration make
the relevance of this finding unclear (Chanda et al., 1995).
The maximum tolerated dose of chlorpyrifos in Sprague-Dawley rats
injected subcutaneously in peanut oil was 45 mg/kg bw in 7-day-old
pups and 280 mg/kg bw in adults (80-100 days old) (Pope et al., 1991).
At this dose, the neonates showed an initial transient decrease in
body weight when compared with controls. Adult rats also had an
initial decrease in body weight, but this had recovered by the end of
the 7-day observation period. Brain cholinesterase activity increased
by about 42% in neonates on days 7-14, whereas it remained constant in
adults. Neonatal brain cholinesterase activity was inhibited by 80% on
day 1 and by 45% on day 7, whereas 90% inhibition was seen in adults
on day 4, with little or no recovery. In plasma, inhibition of
cholinesterase activity was rapid and marked (95%) in pups on day 1
but had recovered to 50% of control by day 7. In adults, such
inhibition persisted through day 7, with no recovery of activity.
In a study to determine whether a measure of inhibitory potency
in vivo, the ED50, could be correlated with a measure of acute
toxicity, the maximum tolerated dose, in neonatal and adult rats,
chlorpyrifos was given by subcutaneous injection in peanut oil.
Cholinesterase inhibition was measured at the time of peak inhibition
(24 h in neonates and 4 days in adults). Depression of cholinesterase
activity versus log dose gave a straight line, and the slope of the
curve of inhibition of plasma cholinesterase was slightly steeper than
that of brain cholinesterase. The ED50 values for brain
cholinesterase activity were 20 (range, 17-23) in neonates and 44
(31-63) in adults, and those for plasma cholinesterase activity were
20 (13-31) in neonates and 70 (40-120) in adults. The correlation
between the two values was good for neonates but not for adult rats
(Pope & Chakraborti, 1992).
Groups of five 3-month old Sprague-Dawley rats were given
chlorpyrifos (purity, 98%) in peanut oil by subcutaneous injection at
a dose of 280 mg/kg bw, and trunk blood, cerebral cortex, and corpus
striatum were collected 2, 4, 6, and 12 weeks after dosing.
Acetylcholinesterase activity was assayed, and the binding of
[3H]quinuclidinyl benzilate to muscarinic receptors was measured in
membranes of the cortex and striatum. Locomotor activity was measured
for 30 min on 5 days/week. A challenge of scopolamine (1 mg/kg bw
intraperitoneally) was given 2 weeks after chlorpyrifos treatment and
then every two weeks for 12 weeks. Extensive inhibition of cortical
and striatal cholinesterase activity was seen 2 (94-96%), 4 (about
80%), 6 (about 60%), and 12 (15-20%) weeks after treatment. Plasma
cholinesterase activity was inhibited to a lesser extent, and recovery
was more rapid, with 85% inhibition at 2 weeks, 55% at 4 weeks, and
30% at 6 weeks; the activity was normal at 12 weeks. The changes in
cholinesterase activity were accompanied by reductions in muscarinic
receptor binding sites in the cortex (34, 33, and 18% reductions in
maximal binding) and striatum (48, 40, and 23% reductions) 2, 4, and 6
weeks after exposure. Tests of locomotor activity showed hypoactivity
in treated rats for 2-3 days after treatment. After the scopolamine
challenge, chlorpyrifos-treated rats showed increased activity at all
times except week 10 (Pope et al., 1992).
Chlorpyrifos was given to groups of six adult male Sprague-Dawley
rats by subcutaneous injection at a dose of 280 mg/kg bw, and the
animals were killed 2, 7, and 14 days after treatment. A significant
( p < 0.05), approximately 6% decrease in body weight was seen after
2-3 days, but the body weights recovered during the remainder of the
study. Chlorpyrifos caused marked inhibition of cholinesterase
activity in brain regions and plasma at all times, with about 90%
inhibition in the cortex, striatum, and plasma at day 2, and there was
virtually no recovery of this activity within the 14-day recovery
period. Reductions of < 55% in atropine-sensitive quinuclidinyl
benzilate binding (total muscarinic receptors) and binding in the
cortex and striatum were also observed (Chaudhuri et al., 1993).
Pregnant Sprague-Dawley rats were injected subcutaneously with
either peanut oil or chlorpyrifos (purity, 98%) at 25 mg/kg bw per day
on days 12-19 of gestation and killed on day 20 of gestation or
postnatal day 3. In a separate study to determine the dose-response
relationship, rats were similarly exposed to chlorpyrifos at 6.25 or
12.5 mg/kg bw per day on days 12-19 of gestation and killed on day 20
for analysis of various neurochemical markers. No maternal toxicity
was seen at any dose. Treatment with 25 mg/kg bw per day induced only
an initial, slight, transient decrease in maternal body weight.
Treatment from day 12 of gestation did not affect fetal weight at day
16 or 20, but pup weight on postnatal day 1 was significantly reduced
by treatment at this dose. Maternal brain cholinesterase activity
measured on day 20 of gestation was significantly inhibited in a
dose-related manner, with 75 and 90% inhibition at 6.25 and 25 mg/kg
bw per day, respectively. Fetal brain cholinesterase activity was less
severely inhibited, with 40 and 60% inhibition at the two doses,
respectively. Extensive inhibition of acetylcholinesterase activity,
to 10-17% of the control value, was seen in dams treated with 25 mg/kg
bw per day. Significant, dose-related downregulation of muscarinic
receptors in maternal and fetal brain was noted on day 20 of gestation
after exposure at all doses. The responses to righting reflex and
cliff avoidance tests were markedly altered after repeated exposures.
Generally, the neurochemical effects were more severe in dams than in
the developing fetuses, despite the greater sensitivity of fetal brain
cholinesterase to inhibition by chlorpyrifos. Comparison of these
results with those of an earlier study of acute exposure to
chlorpyrifos (Chanda et al., 1995) indicated that repeated exposure
causes greater down-regulation of muscarinic receptors than the
equivalent acute dose (Chanda & Pope, 1996).
Cats
Technical-grade chlorpyrifos dissolved in methylene chloride and
mixed in olive oil was given to adult male, colony-raised, short-hair
cats at doses of 0, 0.1, 0.5, 1, 5, 10, or 50 mg/kg bw to establish a
dose that would induce clinical signs of toxicosis but not death. Two
cats were used in this segment of the study, and each received
increasing doses of chlorpyrifos. On the basis of deaths at 50 mg/kg
bw, a dose of 40 mg/kg bw was selected for the next phase of the
study, in which three groups of six cats received corn oil,
chlorpyrifos, or chlorpyrifos followed by injections of atropine
sulfate every 12 h for 2 days. Two cats receiving the antidote also
received pyridine 2-aldoxime methane sulfonate every 12 h until the
clinical signs had abated. All cats given chlorpyrifos showed clinical
signs of toxicity, and one cat died 4 days later. Whole-blood and
plasma cholinesterase activity was reduced in all treated animals, but
the levels had returned to pre-test levels after 7-28 days. Brain
cholinesterase activity was normal in all animals that survived until
the end of the study, 28 days after treatment. Gross pathology
revealed no treatment-related lesions, and haematological parameters
were unaffected (Hooser et al., 1988).
Groups of five adult male cats received a single intramuscular
injection of corn oil (vehicle), diisopropyl fluorophosphate (positive
control; 5 mg/kg bw), or chlorpyrifos (purity, 99%; 300 mg/kg bw) and
were observed for 60 days. Atropine and pyridine 2-aldoxime methane
sulfonate were given to chlorpyrifos-treated animals once or twice
daily for 14-24 days. Ataxia was observed in positive controls (mean
onset, 16 days; range, 14-19 days) and in those given chlorpyrifos
(mean onset, 19 days; range, 17-21 days) . Functional deficits were
seen, characterized by a crouched-waddling gait, hypermetria, and
proprioceptive deficits, and these effects were still present in both
groups 56 days after exposure; the effects in chlorpyrifos-treated
animals began to subside after day 35. Lymphocyte neuropathy target
esterase activity was inhibited by < 96% in positive control
animals (24 h after dosing) and by 46% in chlorpyrifos-treated cats
(7 days after dosing) but had reached control levels by day 28 in the
latter group. Whole-blood cholinesterase activity was reduced by
< 86% by day 4 and slowly recovered to control levels by day 56.
Diisopropyl fluorophosphate had inhibited cholinesterase activity by
< 63% 24 h after dosing, but the recovery of activity was similar
to that in chlorpyrifos-treated animals. Histological examination
(Table 22) revealed degeneration of axons in animals treated with
chlorpyrifos and with diisopropyl fluorophosphate, and, while the
lesions were similar, they were more severe in chlorpyrifos-treated
animals. The effects were characterized by axonal degeneration with
vacuolar (spongy) changes in the white matter tracts of the cerebrum,
cerebellum, and spinal cord and occasionally in the peripheral nerves.
Vacuolization was seen, resulting from distension of axonal spaces
associated with focal axonal swelling and degeneration of myelin and
myelin-forming cells, with infiltration of snall numbers of
macrophages (Fikes et al., 1992). The route of administration and
magnitude of the dose in this study limit its usefulness.
(g) Studies on metabolites
(i) Acute toxicity
The main metabolite of chlorpyrifos, TCP, was generally of lower
acute toxicity than chlorpyrifos (Table 23).
(ii) Short-term studies of toxicity
Groups of 10 rats of each sex were fed diets containing 0, 0.01,
0.03, 0.1, 0.3, or 1% TCP for 90 days. The three lower doses had no
treatment-related effects on body-weight gain, mortality, food
consumption, haematological values, blood urea nitrogen, alkaline
phosphatase, or average body and organ weights. The NOAEL was 0.1% in
the diet, approximately 50 mg/kg bw per day, on the basis of reduced
body-weight gain at 1% (Beatty, 1964).
Groups of 15 rats of each sex were fed diets containing sodium
TCP at concentrations providing doses of 0, 10, 30, or 100 mg/kg bw
per day for 91 days. The dietary concentration was varied to allow for
increasing body weight and ranged from about 85, 250, and 825 ppm at
day 0 to about 160, 460, and 1540 ppm at day 84 at the three doses,
respectively. Significantly increased body-weight gain was observed in
males at 30 mg/kg bw per day from day 48 onwards. There were no
treatment-related changes in haematology, urinary analysis, or blood
chemistry values. The absolute weight of the heart was increased in
males at 100 mg/kg bw per day, and increased absolute and relative
liver and kidney weights were seen in animals of each sex at this
dose. There were no gross or histopathological alterations due to
treatment. The NOAEL was 30 mg/kg bw per day on the basis of increased
relative liver and kidney weights (Barna-Lloyd & Szabo, 1985).
Table 22. Occurrence and severity (score ± SD of histological changes in the nervous system of cats
given a single intramuscular injection of chlorpyrifos, diisopropyl fluorophosphate
(positive control), or corn oil
Location Treatment
Corn oil Diisopropyl fluorophosphate Chlorpyrifos
No. affected Score No. affected Score No. affected Score
Brain
Cerebruma 2/5 0.40 ± 0.49 4/5 0.80 ± 0.40 4/5 2.00 ± 0.63
Cerebellumb 1/5 0.20 ± 0.40 2/5 0.40 ± 0.49 3/5 1.00 ± 0.89
Spinal cord
C2 2/5 0.20 ± 0.49 4/5 0.80 ± 0.40 5/5 1.40 ± 0.49
C7 1/5 0.20 ± 0.40 3/5 0.60 ± 0.49 5/5 1.40 ± 0.49
T-L 4/5 0.80 ± 0.40 5/5 1.00 ± 0.0 4/5 0.80 ± 0.40
L4 0/5 0.00 ± 0.0 5/5 1.60 ± 0.49 5/5 1.80 ± 0.40
Sciatic nerve 0/5 0.00 ± 0.0 3/5 0.60 ± 0.49 1/5 0.20 ± 0.40
Severity of lesions (axonal swelling, vacuolar change, myelin degeneration) in each section was given the
score of the most severely affected area. The lesions were scored as 0 = normal or equivocal, 1 = mild
(1-5 nerve fibres affected/high power field), 2 = moderate (6-10 nerve fibres affected), 3 = marked
(11-15 nerve fibres affected), and 4 = severe change (> 20 nerve fibres affected).
a Anterior parietal cerebrum sectioned transversely immediately posterior to the optic chiasm and
anterior to the infundibulum
b Transverse section through the cerebellum and brain stem at the level of the trigeminal nerves
Table 23. Acute toxicity of 3,5,6-trichloro-2-pyridinol (TCP) administered orally
Species Strain Sex Vehicle LD50 (mg/kg bw Reference
and 95% CI or range)
Mouse Swiss M 0.5% hydroxypropylmethyl 380 (333-433) Gerbig & Emerson (1970a)
F cellulose (Methocel) 414 (367-469)
Rat Sprague-Dawley M 0.5% hydroxypropylmethyl 794 (709-889) Gerbig & Emerson (1970b)
F 870 (758-1009)
Doga Beagle NR Gelatine capsule > 1000 Gerbig & Emerson (1970c)
NR, not reported
a One dog received two doses of 1000 mg/kg bw TCP, a second received four doses of 500 mg/kg bw, and a third
received eight doses of 50 mg/kg bw. Emesis was seen in all animals within 1-3 h of dosing. There were no deaths.
Groups of two beagles of each sex were given diets containing TCP
at concentrations providing doses of 0, 26, or 80 mg/kg bw per day for
93 days. At termination, haematological parameters were normal. There
was no NOAEL as elevated serum alkaline phosphatase activity and
histopathological evidence of liver damage and increased testicular
weights were seen in the treated animals (JMPR, 1972; modified by
reference to the original report of Copeland, 1964).
Groups of three dogs of each sex were given diets containing TCP
at concentrations providing doses of 1, 3, 10, or 30 mg/kg bw per day
for 91 days. No toxic effects were seen at the three lower doses, but
at 30 mg/kg tbw per day evidence of liver toxicity was seen, with
increased activity of alkaline phosphatase and alanine and aspartate
aminotransferases although there was no unequivocal sign of liver
damage on histopathological examination (JMPR, 1972; modified by
reference to the original report of Emerson & Gerbig, 1970).
Groups of four beagles of each sex were fed diets containing TCP
at concentrations providing doses of 0, 3, 12, or 48 mg/kg bw per day
for 1 year in a study conducted according to GLP. No details of the
clinical observations were reported. The body weights of males were
not statistically significantly different between groups, but females
at the high dose had consistently lower body weights than controls and
statistically significantly lower body-weight gains. No changes in
food consumption were found in treated groups, and no alterations in
haematological or urinary parameters. The dose-related changes in
clinical chemistry values that were attributed to treatment were
increased serum alkaline phosphatase and alanine aminotransferase
activities in males and females at 3, 6, and 12 months. The values
were comparable to those of controls at 3 mg/kg bw per day,
consistently elevated but generally not to a statistically significant
degree at 12 mg/kg bw per day, and were statistically significantly
increased at 48 mg/kg bw per day at most times. There were no gross or
histopathological lesions that were associated with treatment and no
changes in absolute or relative organ weights. The NOAEL for systemic
effects was 12 mg/kg bw per day, on the basis of a lack of a
consistent, statistically significant increase in enzyme activities at
that dose (Zempel et al., 1987).
(iii) Genotoxicity
TCP was not genotoxic in a range of assays in vitro and
in vivo (Table 24).
(iv) Developmental toxicity
In a range-finding study, Fischer 344 rats were given TCP
(purity, 99.7%) at doses of 0, 43, 75, or 127.5 mg/kg bw per day on
days 6-15 of gestation by oral gavage. Clinical signs (perineal
soiling) were seen sporadically in all dams at the highest dose and in
some at 75 mg/kg bw per day. Food and water consumption were
unaffected by treatment. The signs of maternal toxicity were limited
to reduced body-weight gain in dams at the high dose. The relative and
Table 24. Results of tests for the genotoxicity of 3,5,6-trichloro-2-pyridinol
End-point Test object Concentration Purity Results Reference
(%)
In vitro
Reverse mutation S. typhimurium 3.16, 10, 31.6, 99.7 Negativea,b Zempel & Bruce (1986)
TA98, TA100, 100, 316 µg/plate
TA1535, TA1537, in DMSO
TA1538
Unscheduled Rat hepatocytes 1-100 µg/ml in 99.7 Negativec Gollapudi (1987)
DNA synthesis (Fischer CDF 344) DMSO
Forward mutation Chinese hamster 62.5, 125, 250, 99.7 Negativea Linscombe & Gollapudi
ovary cells, hprt locus 500, 750 µg/ml (1986)
In vivo
Micronucleus Mouse (CD-1) 250, 500, 750, 99.9 Negative McClintock & Gollapudi
formation marrow cells 1000, 1500, 2000, (1989b; GLP)
3000 mg/kg bw
orally in corn oil
Micronucleus Mouse (CD-1) 0, 24, 76, 240 mg/kg 99.7 Negative Bruce et al. (1985)
formation marrow cells bw orally in
corn oil
GLP, good laboratory practice; DMSO, dimethyl sulfoxide
a With and without an exogenous metabolic system
b Toxic at 100 (some strains) and 316 (all strains) µg/plate
c Toxic at 50 and 100 µg/ml
absolute weights of the liver and kidney were unaffected by treatment,
and there was no observable effect on reproductive parameters
(Scortichini et al., 1986a).
Groups of Fischer 344 rats were given 0, 50, 100, or 150 mg/kg bw
per day of TCP (purity, 99.7%) on days 6-15 of gestation by oral
gavage in a study that conformed to GLP. No clinical signs of toxicity
were seen in any group. Slight vaginal bleeding occurred in one animal
at 50 mg/kg bw per day and two at 150 mg/kg bw per day on day 13. Food
consumption was slightly decreased in animals at 100 mg/kg bw per day
and decreased by about 10% in those at the high dose. Significant,
dose-related decreases in body-weight gain were recorded throughout
treatment at 100 and 150 mg/kg bw per day. Changes in the relative and
absolute liver and kidney weights were restricted to a significant
increase (4%) in the relative liver weight at 150 mg/kg bw per day,
consequent to a significant decrease (5%) in body weight at this dose.
There was a dose-related trend in the number of implantation sites per
dam, which was reflected in the number of fetuses per litter and
probably in gravid uterine weight; the latter trend may also reflect
the trend to lower body weight with higher dose. Fetal examination
revealed a small number of findings in each group, including controls.
Overall, treatment had no significant effect on reproductive
parameters or fetuses. The NOAEL for maternal effects was 50 mg/kg bw
per day on the basis of decreased body-weight gain, and that for fetal
toxicity and teratogenic effects was 150 mg/kg bw per day, the highest
dose tested (Hanley et al., 1987a).
In the first phase of a study in inseminated New Zealand white
rabbits, the animals were given TCP (purity, 99.7%) on days 6-18 of
gestation by oral gavage at doses of 0, 48.5, 80, or 130 mg/kg bw per
day. As there was uncertainty that the maximum tolerated dose had been
reached in this phase, additional groups of animals were given 130 or
210 mg/kg bw per day (phase 2). Maternal body-weight gain during
gestation was depressed but not statistically significantly and was
highly variable at the highest dose in both phases. Gross examination
of all animals showed no significant findings or any differences
between groups. At 210 mg/kg bw per day, resorptions were seen in all
litters, and the percent implantations resorbed and the percent
litters with resorptions were statistically significantly different
from those in the comparable control group in phase 2. There was no
observable effect of treatment on reproductive parameters at doses up
to 130 mg/kg bw per day (Scortichini et al., 1986b).
Groups of New Zealand white rabbits were given TCP (purity,
99.7%) by oral gavage on days 7-19 of gestation at doses of 0, 25,
100, or 250 mg/kg bw per day in a study that met GLP requirements.
Female animals were given injections of human chorionic gonadotropin
to synchronize estrus and were inseminated 3 weeks later, which was
presumed to be day 0 of gestation. No clinical signs of toxicity were
seen in any group. Water and food consumption were not reported. A
dose-related decrease in body-weight gain was seen during treatment,
which reached statistical significance at the high dose. There were no
treatment-related changes in the relative or absolute weights of the
liver or kidney. Fetal examination revealed a small number of
malformations in each group, including controls. Overall, there was no
statistically significant effect of treatment on reproductive
parameters or fetuses, but an increased incidence of central nervous
system malformations at the intermediate and high doses suggested a
possible teratogenic effect, and the authors concluded that these
findings provided evidence of teratogenic potential, despite a lack of
statistical significance. The malformations included severe dilatation
of the cerebral ventricles and hydrocephaly. The incidences of minor
external, visceral, or skeletal alterations were unchanged. The NOAEL
for maternal effects was 100 mg/kg bw per day on the basis of
decreased body-weight gain during treatment. The NOAEL for fetal
toxicity and teratogenic effects was 25 mg/kg bw per day, on the basis
of central nervous system malformations at higher doses (Hanley et
al., 1987b).
3. Observations in humans
3.1 Experimental studies
A commercial preparation of chlorpyrifos containing 35% xylene
was applied to the skin of the back and abdomen of four volunteers
(skin area not stated) at a dose of 5, 10, 25, or 50 mg/kg bw 20 (5
days/week), four, three, and two times, respectively. The material was
covered with an occlusive patch for 12 h, and after removal of the
patch each site was scored for irritation before washing. Each site
was used only once. Plasma and erythrocyte cholinesterase activity was
determined before and several times after treatment (exact times not
stated; for results, see Table 25). In another study, five volunteers
received dermal doses of 94-620 mg/kg bw for 12 h and showed similar
inhibition of erythrocyte and plasma cholinesterase activity 24, 48,
and 72 h after exposure, even at the highest dose. No skin irritation
was recorded. Plasma cholinesterase activity was significantly
inhibited with two of the dose regimens, the activity reaching a
minimum around 3 days after the last dose and then recovering to
control values within 4-10 days; erythrocyte cholinesterase activity
were not inhibited with any regimen (Kilian et al., 1970).
Four volunteers were exposed for 5 min to a formulation
containing 61.5% chlorpyrifos and 34.5% xylene from an ultra-low
volume cold aerosol fog generator delivering 3.8 L/h. Air sampling
showed a concentration of chlorpyrifos in the breathing space of about
108 mg/L (range, 83-133 mg/L). The subjects were exposed at a distance
of 8 m and wore plastic coveralls allowing exposure of the heads and
hands of two subjects and the heads, hands, and arms of the other two.
Exposure was terminated after 5 min because of the ocular and
pulmonary irritation induced. Plasma and erythrocyte cholinesterase
activity was not depressed 24 h after exposure (Pennington & Edwards,
1971).
Table 25. Cholinesterase activity after dermal dosing of humans (% of pre-treatment value)
Dose regimen End of dosing Day 1 Day 3
Plasma Erythrocytes Plasma Erythrocytes Plasma Erythrocytes
10 mg/kg bw for 4 × 12 h 99 102 97 103 97 100
50 mg/kg bw for 2 × 12 h 100 100 100 100 102 100
25 mg/kg bw for 3 × 12 h 67 98 48 98
5 mg/kg bw for 20 × 12 h 67 103 64 104 74 104
One subject per dose regimen
Groups of four healthy, adult, male volunteers received
chlorpyrifos in tablet form at doses of 0, 0.014, 0.03, or 0.1 mg/kg
bw per day for 27, 20, and 9 days, respectively, with breakfast.
Control subjects received a placebo for 48 days. Although not
specifically stated, the high dose appears to have been withdrawn
because of the severity of plasma cholinesterase inhibition on day 9.
No rationale was given for termination of treatment at the lower
doses. Heparinized blood samples were obtained from the subjects twice
weekly for determination of plasma and erythrocyte cholinesterase
activity until all values had returned to pre-treatment levels. As it
was not stated whether the blood samples were taken at the same time
on each sampling day, the interval between administration and sampling
is unknown. No indication was given of the frequency of observation
for clinical signs after treatment or how such observations were made.
A single subject at 0.1 mg/kg bw per day reported having a runny
nose, blurred vision, and a feeling of faintness on the final day of
dosing, and his plasma cholinesterase activity was found to be
inhibited by 70% when compared with pre-treatment levels. No decrease
in erythrocyte cholinesterase activity was seen in any subject during
the study, and no other treatment-related effects were noted. Rapid,
marked depression of plasma cholinesterase activity was observed in
subjects at the high dose, and the activity did not return to control
levels until about day 34. Similar results were obtained when the
plasma cholinesterase activity in these subjects was compared with the
baseline activity of the group before treatment. The mean plasma
cholinesterase activity of men at 0.03 mg/kg bw per day was inhibited
by > 20% on days 16-20 of treatment when compared with those given
placebo, and the activity did not return to control levels for several
weeks after cessation of treatment. The plasma cholinesterase activity
of men at 0.014 mg/kg bw per day was inhibited by 20% only on day 13
and was similar to the baseline activity from day 20 to day 27, even
with continued administration of chlorpyrifos. Two-way analysis of
variance indicated that the inhibition of mean plasma cholinesterase
activity was significant only at 0.1 mg/kg bw per day ( p < 0.05)
when compared with controls. The NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.1 mg/kg bw per day, the highest dose
tested (JMPR, 1972; modified by reference to the original report of
Coulston et al., 1972).
Six white male volunteers aged 27-50 years were used in another
study. One volunteer was given a single oral dose of 0.5 mg/kg bw
chlorpyrifos in a lactose capsule, about 30 min after food, and about
1 month later the other volunteers received the same treatment and the
first volunteer was given a dermal dose of 0.5 mg/kg bw chlorpyrifos
dissolved in methylene chloride. Two weeks after the first dermal
dose, the first volunteer was given a further dermal dose of 0.5 mg/kg
bw in dipropylene glycol methyl ether. The other volunteers were given
a dermal dose of 5 mg/kg bw of chlorpyrifos in dipropylene glycol
methyl ether 4 weeks after their oral dose. All urine was collected
from volunteers from 24-48 h before dosing until 120 h after dosing.
No signs or symptoms of toxicity were reported in any of the men
during the study. After oral administration of 0.5 mg/kg bw, the
plasma cholinesterase activity of the first volunteer was inhibited by
71% from that before treatment and that of the other men by a mean of
85% within 12-24 h of treatment. The range of individual values was
not provided. Plasma cholinesterase activity had returned to > 80% of
the mean baseline value by 30 days after oral treatment. After dermal
application on day 30, the plasma cholinesterase activity had
decreased to approximately 70% of baseline, and returned to about
80-90% of those levels by day 40 of the study. The intra-group
variation was stated to be considerable, but the figures were not
provided. Erythrocyte cholinesterase activity was not significantly
inhibited by either oral or dermal doses of chlorpyrifos (Nolan et
al., 1982, 1984).
In a double-blind, randomized, placebo controlled study of the
effects of single oral doses of chlorpyrifos (purity, 99.8%), groups
of six fasted men and women aged 18-55 received doses of 0, 0.5, 1, or
2 mg/kg bw in lactose powder. The study was conducted in two phases
separated by 14 days. The volunteers were dosed with 0, 0.5, or 1
mg/kg bw in the first phase and the results were assessed before
administration of 0 or 2 mg/kg bw in the second phase. Blood samples
were collected 10 and 0 h before treatment and 2, 4, 8, 12, 24, 36,
48, 72, 96, 120, 144, and 168 h after treatment and analysed for
erythrocyte cholinesterase activity and chlorpyrifos and its
metabolites. In addition, all urine voided from 48 h before dosing to
168 h after dosing was collected at 12- or 6-h intervals and analysed
for chlorpyifos and metabolites. Haematology, clinical chemistry,
urinary analysis and a brief physical examination were performed at
completion of the study. Blood and urine were collected to determine
each volunteer's paraoxonase status and the concentrations of
chlorpyrifos and metabolites, but these data have not yet been
reported. The volunteers were screened for general health according to
set criteria and instructed to refrain from alcohol, strenuous
exercise, and prescription medications before and during the study.
The doses were taken by capsule after an overnight fast. The health
status of subjects was monitored closely; vital signs (blood pressure,
pulse, respiration, and temperature) were assessed before dosing and
1, 2, 4, 8, 12, 24, 48, and 168 h after treatment. The subjects were
questioned about their well-being at each sampling time, and the
symptoms were evaluated clinically. The subjects were aware of the
signs and symptoms of cholinergic toxicity and were instructed to
inform the study physician of any adverse effects. The subjects were
unaware of their treatment, and the signs and symptoms were assessed
and treated by a physician who was also unaware of the treatment
status of the subject.
There were no significant deviations from the study protocol. One
male control in phase 1 and one woman receiving 2 mg/kg bw did not
provide a complete series of blood and urine samples. Treatment had no
effect on general health or on clinical chemical parameters measured 7
days after dosing. The only treatment-related effect was found in the
woman who withdrew from the study, who had decreased erythrocyte
cholinesterase activity when compared with her pre-treatment values at
most sampling times, with 98.4% of the pretreatment value at 4 h after
dosing, 77% at 8 h, 72% at 12 h, 74% at 24 h, 81% at 36 h, and 80% at
48 h (Table 26). When the data for this subject are removed from the
analysis, the mean for women receiving 2 mg/kg bw is indistinguishable
from the value for concurrent controls (Table 26). The NOAEL for
clinical signs or symptoms was thus the highest dose tested, and the
NOAEL for inhibition of erythrocyte cholinesterase activity was 1
mg/kg bw on the basis of significant inhibition in one of 12 subjects
(Kisicki et al., 1999).
3.2 Case reports
Blondell & Dobozy (1997) summarized the case reports, case
series, statistical surveys, and epidemiological studies of acute and
chronic health effects reported to be related to exposure to
chlorpyrifos. The authors noted the limitations of their review,
including inadequate documentation of exposure and effects, reporting
biases, and absence of information on the denominator population at
risk. They concluded, largely on the basis of an examination of
reports from poison control centres, that chlorpyrifos is one of the
leading causes of acute insecticide poisoning in the USA. Certain uses
were considered to pose greater risks than others, the main concern
being use of liquid formulations in households or by pest control
operators indoors or outdoors, termite treatment, and application of
liquid sprays and dips to domestic animals. Most of the more serious
cases were associated with misuse or inappropriate use such as spills
and inadvertent contamination by a pest control operator.
Shurdut et al. (1997) commented that deficiencies in the report
of Blondell & Dobozy (1997) invalidated its use for reaching a
conclusion about the safety of chlorpyrifos. They concluded that the
data from poison control centres had been misinterpreted and in fact
indicated the relative safety of products containing chlorpyrifos.
They also claimed that the report failed to respect the limitations of
anecdotal information, failed to consider the extensive testing with
regard to neurological damage and the long history of use of products
containing chlorpyrifos, made inappropriate use of anecdotes and
studies of organophosphates to characterize the safety of
chlorpyrifos, failed to consider the extensive database on exposure
after use of chlorpyrifos products, and presented the data
selectively.
In a case report, a previously well 3-year-old boy was found
playing near an open, spilled bottle of insecticide containing
chlorpyrifos (concentration not stated). The authors noted that
clinical manifestations of organophosphate-induced polyneuropathy
usually begin 1-3 weeks after an acute cholinergic crisis and that the
results of examination of the child were consistent with distal,
symmetric, predominantly motor polyneuropathy. In this patient, the
symptoms of cholinesterase inhibition and manifestations of weakness
and areflexia 11 days after ingestion and electromyographic findings
confirmed the presence of acute transient polyneuropathy with a more
Table 26. Mean erythrocyte cholinesterase activity as percent of mean baseline values in volunteers given single oral
doses of chlorpyrifos
Dose Time in relation to dosing (h)
(mg/kg bw)
- 10 0 4 8 12 24 36 48
Women
0.0a 99.5 (2.1) 100.5 (2.1) 98.3 (2.0) 102.1 (2.4) 100.7 (1.8) 99.7 (3.9) 98.4 (1.7) 100.2 (2.3)
0.0b 99.2 (4.4) 100.8 (4.4) 102.5 (5.9) 103.1 (5.4) 98.4 (3.2) 99.4 (3.7) 94.6 (5.3) 97.7 (5.1)
0.5 101.2 (6.7) 98.8 (6.7) 96.3 (6.5) 98.1 (2.1) 95.8 (3.9) 97.0 (3.6) 94.7 (4.7) 97.1 (4.1)
1 98.2 (0.9) 101.8 (0.9) 104.6 (4.2) 100.8 (3.5) 98.9 (3.7) 100.9 (5.6) 96.0 (4.1) 98.9 (2.1)
2 99.8 (1.8) 100.2 (1.8) 100.3 (3.2) 96.6 (6.81) 91.1 (9.7) 95.2 (10.6) 95.9 (7.4) 94.5 (7.7)
Men
0.0a 100.6 (1.8) 99.4 (1.8) 99.1 (2.2) 99.6 (4.6) 99.8 (4.3) 98.4 (4.3) 97.6 (2.8) 98.2 (3.1)
0.0b 96.1 (5.1) 103.9 (5.1) 101.6 (4.0) 102.5 (5.2) 102.9 (5.8) 102.6 (2.3) 98.2 (3.8) 95.9 (6.8)
0.5 98.7 (1.1) 101.3 (1.1) 102.0 (3.0) 103.4 (4.9) 99.7 (3.0) 101.9 (3.3) 98.1 (3.0) 92.8 (4.7)
1 98.6 (1.9) 101.4 (1.9) 104.5 (7.8) 101.1 (4.2) 101.1 (4.7) 101.7 (4.7) 98.5 (4.6) 92.1 (2.0)
2 101.2 (2.3) 98.8 (2.3) 99.2 (3.1) 98.9 (3.5) 98.7 (4.0) 99.2 (2.6) 99.5 (5.1) 98.4 (3.1)
a Phase 2 control
b Phase 1 control
proximal distribution than is usually seen in adults. The acute,
reversible bilateral vocal cord paralysis reported in this patient had
not been seen previously in organophosphate-induced delayed
neuropathy, but the authors noted that the onset of stridor and vocal
cord paralysis coincided with the onset of muscle weakness and
resolved at the same time as normalization of the peripheral
neuropathy (Aiuto et al., 1993a).
Gutmann & Bodensteiner (1993), responding to the report of Aiuto
et al. (1993), suggested that this case may not be a clear example of
organophosphate-induced delayed neuropathy. They noted that
chlorpyrifos has been associated with such symptoms only after severe
intoxication characterized by extensive cholinesterase inhibition. The
inhibition of plasma cholinesterase activity in the reported case was
instead suggestive of mild chlorpyrifos intoxication. They also noted
that the clinical findings associated with organophosphate-induced
delayed neuropathy are predominantly motor polyneuropathy, flaccid
weakness and atrophy primarily in the distal limb muscles, and often
incomplete recovery usually after months or years. The case report
implied that the evoked compound muscle action potentials and sensory
nerve action potentials were of normal amplitude, which would run
counter to a diagnosis of organophosphate-induced delayed neuropathy.
In response to these comments, Aiuto et al. (1993b) noted that
the patient's symptoms were consistent with > 90% inhibition of
cholinesterase activity and that the plasma cholinesterase activity
did not necessarily correlate with the severity of poisoning. The
authors concluded that the patient had developed delayed,
life-threatening neuropathy, although they were unable to determine
the mechanism of the condition.
In another case report, a 38-year-old man drank an undefined
quantity of a solution of 25% chlorpyrifos. He was treated with 3 mg
of atropine intravenously every 2 h for 6 days (total, 400 mg), but
any attempt to stop the atropine treatment resulted in reappearance of
respiratory distress. After 6 days, the patient was stuporous,
catatonic, and suffering from respiratory distress with white mucous
secretions, and no cholinesterase activity could be detected in his
serum. When ipratropium was administered endotracheally at a dose of
0.5 mg as an aerosol mist over 10 min, the patient's clinical
condition improved, although the salivary secretion continued. The
patient continued to receive ipratropium at 1-2 mg/day, and
respiratory support was discontinued on the fifth day. Severe
catatonia and coarse tremor were relieved by administration of
dantrolene at 10 mg intravenously, followed by 25 mg orally, three
times daily. Cholinesterase activity was still undetectable in serum
1 month after the man was discharged (Shemesh et al., 1988).
In a case in which a 26-year-old man intentionally ingested
approximately 360 ml of a 6.7% chlorpyrifos formulation containing
2,4-D (10.8%) and mecoprop (11.6%) and a few granules of a warfarin
(0.025%) concentrate, the main clinical findings on admission were
coma, myoclonus, miosis, cardiac arrythmia with progression to
hypotension, oliguria, and death after several episodes of asystole.
The authors stated that such symptoms are consistent with those seen
in patients who have ingested chlorophenoxy acetic acids such as 2,4-D
and mecoprop. The patient did not show many of the usual signs of
organophosphate poisoning, such as lachrymation, salivation,
respiratory paralysis, or muscle fasciculation, but his erythrocyte
cholinesterase activity was inhibited by about 70% at 13 h and by
< 90% by 26 h. Plasma cholinesterase activity was inhibited
completely at all times. Lymphocyte neurotoxic esterase activity was
inhibited by about 50% after 13 h but was within normal limits after
19 h. The activity of neurotoxic esterase in the cerebral cortex was
within normal limits but that in peripheral nerves was inhibited by
about 70% when compared with normal levels. The inhibition of
neurotoxic esterase activity was found in the absence of significant
signs of organophosphate intoxication (Osterloh et al., 1983).
A 21-year-old man who ingested an unknown quantity of a
chlorpyrifos formulation required large amounts of atropine and
pyridine 2-aldoxime methane sulfonate, endotracheal intubation, and
ventilation to overcome the cholinergic effects, which consisted of
pupillary constriction, excess secretions, tachycardia, and impaired
consciousness. Bilateral vocal cord paralysis developed 16 days after
extubation (28 days after exposure), and this finding was considered
to be indicative of organophosphate-induced delayed neuropathy. The
patient was asymptomatic within 4 weeks of the onset of symptoms (de
Silva et al., 1994).
A 42-year-old man who attempted suicide by drinking a commercial
formulation of 41% chlorpyrifos (estimated dose, 300 mg/kg bw) went
into coma and showed respiratory insufficiency, lachrymation,
salivation, sweating, miosis, fasciculations, and bronchorrhoea on
admission to hospital 18 h after exposure. A chest X-ray showed right
basal bronchopneumonitis, electroencephalographic signs of diffuse
irritation were recorded, and chlorpyrifos was detected in gastric
lavage fluid. Plasma cholinesterase activity was inhibited by almost
100% by 36 h. The coma and cholinergic signs persisted for 17 days,
during which time atropine, pyridine 2-aldoxime methane sulfonate,
mechanical respiration, and antibiotics were used. The patient was
asymptomatic on day 24 after exposure, and drug administration was
stopped; no signs of peripheral or central nervous system involvement
were observed. On day 30, lymphocytic neurotoxic esterase, blood
acetylcholinesterase, and plasma butyryl cholinesterase activities
were markedly below the normal range but recovered slowly thereafter.
On day 43, weakness and paraesthesia in the legs with reduced tendon
reflexes and sensory conduction velocity were noted. By day 62, the
leg weakness was more severe, gait was impaired, and tendon reflexes
were absent. Electromyography of the leg muscles showed symmetrical
signs of denervation with spontaneous activity, and motor conduction
velocity was reduced. Electrophysiological studies of the arms
confirmed a symmetrical reduction of sensory conduction velocity of
the ulnar nerves. Sural nerve biopsy on day 63 revealed a few altered
myelinated fibres. Aspects of axon and myelin degeneration were also
found occasionally. The changes were consistent with mild distal
axonopathy. Chlorpyrifos was still detectable 10 days after exposure,
and neurotoxic esterase activity was still inhibited by 60% 4 weeks
after exposure (Lotti et al., 1986).
In a study in which toxicological and/or electrophysiological
examinations were conducted in 11 patients who had acute
organophosphate poisoning, three of the patients, one of whom had been
poisoned with chlorpyrifos, developed organophosphate-induced delayed
neuropathy (Moretto & Lotti, 1998). The findings in this individual
were reported by Lotti et al. (1986). The electro-physiological data
indicated organophosphate-induced delayed neuropathy with sensory and
motor components which persisted for 3 months, at which time the
patient was lost to follow-up.
Four incidents of birth defects allegedly associated with
exposure to chlorpyrifos were reported. The children were found to
have a range of birth defects, including ventricular, ocular, and
palate defects and growth retardation (all children), hydrocephaly,
microcephaly, mental retardation, blindness, hypotonia, widely spread
nipples, and deformities of the teeth, ears, and external genitalia.
The mothers of the affected children had reportedly been exposed to
chlorpyrifos either at the workplace or at home during the pregnancy.
Two of the children were born to the same mother. The exposure of the
mothers to chlorpyrifos was poorly characterized, and it was not
indicated whether it was severe or sustained during organogenesis. The
range of birth defects reported was not consistent with the results of
studies in laboratory animals (Sherman, 1996).
In a comment on this paper, Gibson (1996) noted that the medical
records of the cases indicated that the same effects were not seen in
all children and that the effects in some of the children were
consistent with a diagnosis of an autosomal recessive birth defect
syndrome of the brain and eye known as cerebro-oculo-facio-skeletal
syndrome or a closely related one known as the 'MICRO' syndrome.
Eight cases were reported in which exposure to chlorpyrifos was
claimed to have caused sensory neuropathy. The patients each presented
with a range of symptoms, and characterization of exposure was poor.
One patient who reported signs of cholinesterase inhibition, including
lachrymation, muscle twitching, and diarrhoea, was a pesticide
applicator who had reportedly been exposed to chlorpyrifos in a closed
environment for 6 months. The patient's erythrocyte cholinesterase
activity was reportedly to be 'low' initially but returned to normal
levels within 2 months. Neurological evaluations were conducted only 6
weeks after the other symptoms were reported; at this time, sensory
loss of all modalities was found in a 'stocking-glove distribution'
test, with mild distal weakness and areflexia in the lower
extremities. Studies of nerve conduction and quantitative sensory
threshold revealed changes consistent with peripheral neuropathy of
the distal anonopathy type. The decreased reflexes and mild distal
weakness were consistent with polyneuropathy. Follow-up examination at
1 year revealed remission of all symptoms. In the other seven patients
(four from the same family), the symptoms were nonspecific, and the
absence of symptoms of cholinesterase inhibition suggested that these
patients had not been as exposed to chlorpyrifos as extensively as the
first patient. The lack of immediate electrodiagnostic testing, the
poor exposure characterization, and the variation in the reported
clinical findings make interpretation of the case studies difficult.
Additionally, subjective questioning was used to obtain information
from the patients. Follow-up examinations for a range of parameters
were not conducted on several patients, and the patients did not
undergo the same initial testing regimen. Thus, one patient was
exposed to chlorpyrifos occupationally at a level sufficient to
decrease cholinesterase activity and cause cholinergic symptoms; he
also showed evidence of mild, reversible polyneuropathy, possibly in
the presence of decreased erythrocyte cholinesterase activity. For two
other patients, there was limited evidence of mild polyneuropathy
(Kaplan et al., 1993).
3.3 Monitored field trials
A monitored field test was used to determine the effect of
chlorpyrifos on spraymen applying treatment at the rates used in
programmes for the eradication of mosquitoes. Three studies were
conducted in 1966, 1967, and 1968. Cholinesterase activity was
measured, and exposure was terminated if the inhibition reached 50% of
the baseline values. In the first study, three spraymen showed marked
depression of plasma cholinesterase activity, with reductions of
68-82% from baseline values. The two remaining spraymen showed
reductions of 52-56% over 9 days, but the baseline values were not
available. The study was discontinued after 2 weeks because of the
significant decrease in cholinesterase activity. In the second study,
no treatment-related effect on cholinesterase activity was seen during
a short period. In the third study, erythrocyte cholinesterase
activity was not affected by treatment, but plasma cholinesterase
activity was reduced by 50-80% in spraymen using a suspension
formulation. The activity had generally returned to pre-exposure
levels within 2 months of cessation of use. Four spraymen using an
emulsion formulation did not show reduced plasma cholinesterase
activity during the exposure period (Kenaga, 1967; Eliason et al.,
1969).
3.4 Studies of morbidity
In a study in which the sample size was small and the statistical
power was limited, comparisons were made between 175 employees
potentially exposed to chlorpyrifos at a manufacturing plant where
they had held jobs between 1 January 1977 and 31 July 1985 and 335
unexposed controls matched on age (within 5 years), date of hire
(within 5 years), sex, race, and pay status. The source of information
for the observations (self, nurse, company physician, private
physician, laboratory) was recorded, as were use of tobacco, alcohol,
and cholinergic drugs. Exposure was grouped into high, moderate, and
low on the basis of industrial hygiene surveys and consultation with
veteran manufacturing personnel. The plasma cholinesterase activities
of the potentially exposed employees before exposure were available,
and follow-up was conducted at roughly monthly intervals. The mean
inhibition of plasma cholinesterase activity was 19 ± 2.9% in the
group with low exposure, 32 ± 2.8% in that with moderate exposure, and
32 ± 5.3% in that with high exposure. No cases of peripheral
neuropathy were recorded in the study group. Inhibition of plasma
cholinesterase activity was observed but was not associated with
illness. The exposed groups reported symptoms of dizziness, malaise,
and fatigue more frequently than the unexposed group, but the analysis
did not indicate any correlation with increasing exposure (Brenner et
al., 1989).
In a follow-up to this study (Burns et al., 1998), the data were
updated to include the period 1987-94, and additional medical
disorders were considered. Data on self-reported paraesthesia were
collected from 1982 to 1994. The prevalence of peripheral neuropathy
was not significantly increased in the group of workers exposed to
chlorpyrifos.
Comments
After oral administration to rats, radiolabelled chlorpyrifos was
rapidly and extensively absorbed (up to about 90% of the dose) and
eliminated, predominantly in the urine (68-93%) and faeces (6-15% of
the dose), within about 72 h of administration. The urinary
metabolites included the glucuronide (about 80%) and sulfate (about
5%) conjugates of chlorpyrifos, and 3,5,6-trichloro-2-pyridyl
phosphate (TCP; about 12%). The tissue concentrations of residues of
[14C]chlorpyrifos were very low (generally < 1 ppm) within 72 h of
dosing. The longest half-time of residues in rats was 62 h in fat, and
low levels were also detected in the fat of several other species and
in the milk of goats.
In humans who were poisoned with chlorpyrifos formulations,
diethylphosphorus metabolites were excreted in the urine by
first-order kinetics, with an average elimination half-time of 6.1 ±
2.2 h in the fast phase and of 80 ± 26 h in the slow phase. In
volunteers, the time to maximal concentration of 3,5,6-TCP in the
blood was 0.5 h after oral dosing and 22 h after dermal treatment, but
the elimination half-time by both routes was 27 h, and the percentage
of the administered dose recovered from the urine was 70% after oral
dosing and 1.3% after dermal administration.
Chlorpyrifos is rapidly metabolized by mixed-function oxidases to
the highly reactive chlorpyrifos oxon by oxidative desulfuration. The
oxon can be deactivated by hydrolysis to diethylphosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is hydrolysis
to monoethyl 3,5,6-trichloro-2-pyridinyl phosphorothioate.
The lowest oral LD50 value was 96 mg/kg bw (range, 96-475 mg/kg
bw) in rats and 100 mg/kg bw (range, 100-150 mg/kg bw) in mice. Female
rats were generally more sensitive to the acute effects of
chlorpyrifos than males. The signs of acute intoxication with
chlorpyrifos were consistent with cholinesterase inhibition. The acute
dermal LD50 of chlorpyrifos was > 2000 mg/kg bw in rats and > 1200
mg/kg bw in rabbits.
WHO (1999) has classified chlorpyrifos as 'moderately hazardous'.
Chlorpyrifos was irritating to the eye and skin of rabbits, but
it did not sensitize the skin of guinea-pigs in Magnusson-Kligman
maximization or Buehler tests.
In short-term studies, the NOAEL for inhibition of erythrocyte
cholinesterase activity was 0.03 mg/kg bw per day in dogs and 0.1
mg/kg bw per day in rats. The NOAEL for inhibition of brain
cholinesterase activity was 1 mg/kg bw per day in dogs and rats. The
signs of toxicity were largely limited to cholinergic signs and
decreased body weights and/or food consumption. The NOAEL for these
effects in short-term studies was 1 mg/kg bw per day in rats, and the
NOAEL for clinical signs was 3 mg/kg bw per day in dogs. In mice,
ocular effects and histopathological alterations (including adrenal
lipogenic pigmentation and ocular keratitis) were observed (NOAEL, 50
ppm; equal to 7 mg/kg bw per day). In rats, the NOAEL for increased
fatty vacuolation of the adrenal zonal fasciculata and changes in
haematological and clinical chemical parameters was 5 mg/kg bw per
day. When rats received chlorpyrifos dermally for 21 days, the NOAEL
for inhibition of cholinesterase activity in erythrocytes and brain
was 5 mg/kg bw per day.
In long-term studies, inhibition of cholinesterase activity was
again the main toxicological finding in all species. In rats, the
NOAEL was 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity and 1 mg/kg bw per day for inhibition of
brain acetylcholinesterase activity, but clinical signs were not seen
at doses up to 10 mg/kg bw per day, and the NOAEL for reduction in
body weight was 1 mg/kg bw per day. In mice, erythrocyte and brain
acetylcholinesterase activities were inhibited at 50 ppm, equal to 6.1
mg/kg bw per day, and the NOAEL was 5 ppm, equal to 0.7 mg/kg bw per
day. Cholinergic signs and reductions in body weight were reported
only at the highest dietary concentration of 250 ppm (equal to 32
mg/kg bw per day). Other treatment-related findings included effects
on the liver in mice, with a NOAEL of 50 ppm (equal to 6.6 mg/kg bw
per day), and increased adrenal weight in rats with a NOAEL of 1 mg/kg
bw per day. There was no treatment-related increase in the incidence
of neoplastic lesions in any of the long-term studies. The Meeting
concluded that chlorpyrifos is unlikely to pose a carcinogenic risk to
humans.
Chlorpyrifos was not genotoxic in an adequate range of studies
in vitro and in vivo. The Meeting concluded that chlorpyrifos is
not genotoxic.
In multigeneration studies of reproductive toxicity in rats, the
treatment-related effects of chlorpyrifos were limited to inhibition
of cholinesterase activity, consistent with that seen in other short-
and long-term studies, and fetotoxicity characterized by reduced pup
viability, body weights and survival. No significant,
treatment-related clinical signs were reported. The NOAEL for
inhibition of maternal acetylcholinesterase activity was 0.1 mg/kg bw
per day for erythrocytes and 1 mg/kg bw per day for brain. The NOAEL
for developmental toxicity was 1 mg/kg bw per day. No effects on
reproductive parameters were observed at the highest dose tested,
5 mg/kg bw per day.
In studies of developmental toxicity in mice, rats, and rabbits,
the maternal effects included inhibition of erythrocyte and/or brain
acetylcholinesterase activity and cholinergic signs (lowest NOAEL,
1 mg/kg bw per day in rats and mice) and reductions in body weight and
food consumption (lowest NOAEL, 2.5 mg/kg bw per day in rats). The
observed fetal toxicity (lowest NOAEL, 2.5 mg/kg bw per day in rats)
and developmental toxicity (NOAEL, 1 mg/kg bw per day in rats) were
consistent with treatment-related maternal toxicity; there was no
evidence of treatment-related malformations in any of the studies.
There was no effect on cognitive function (learning, memory, and
habituation) in pups exposed to chlorpyrifos in utero and for a
period post partum at doses up to and including the highest dose of
5 mg/kg bw per day, while inhibition of cholinesterase activity,
decreased brain weight, and delayed development were seen at lower
doses, consistent with findings in other studies.
In studies of delayed neurotoxicity, chlorpyrifos was given to
chickens as either single or repeated doses. Significant inhibition of
both cholinesterase and neuropathy target esterase activity was
observed, and mild delayed neuropathy was seen in a number of studies;
aggressive antidotal therapy was always necessary to allow at least
some of the treated birds to survive. Despite the marked cholinergic
toxicity of chlorpyrifos, there was no evidence that it caused delayed
neurotoxicity, and there was no increase in the incidence of
histopathological lesions in the nerve tissues of birds treated at
doses up to 10 mg/kg bw per day for up to 91 days. In a number of
studies in rats given single doses of up to 100 mg/kg bw, repeated
doses of up to 10 mg/kg bw per day for 4 weeks, or repeated doses of
up to 15 mg/kg bw per day for 13 weeks, there were no
treatment-related neurological lesions or effects on cognition and no
inhibition of neuropathy target esterase activity, although
significant inhibition of erythrocyte, brain, and peripheral tissue
cholinesterase activity was seen at some doses. In a study that
included a functional observational battery of tests, clinical signs
of intoxication were observed after a single dose only when brain
acetylcholinesterase activity was inhibited by more than 60% or when
whole-blood cholinesterase activity was inhibited by more than 80%.
When chlorpyrifos was applied as a single dose of up to 5 mg/kg
bw to the skin of volunteers for 12 h, erythrocyte cholinesterase
activity was not significantly inhibited. Plasma cholinesterase
activity was inhibited after 20 12-h dermal exposures to 5 mg/kg bw
per day over 4 weeks or after three daily 12-h exposures to 25 mg/kg
bw per day on consecutive days, but erythrocyte cholinesterase
activity was not inhibited under any treatment regimen.
A single oral dose of up to 1 mg/kg bw or repeated doses of up to
0.1 mg/kg bw per day for 9 days did not significantly inhibit
erythrocyte acetylcholinesterase activity in volunteers. No clinical
signs were observed in these studies. Inhibition of erythrocyte
acetylcholinesterase activity was observed in a single female
volunteer (of a group of six men and six women) given a single oral
dose of 2 mg/kg bw.
In a case of human poisoning with chlorpyrifos at an estimated
dose of 300-400 mg/kg bw, significant inhibition of neuropathy target
esterase in lymphocytes and of plasma and erythrocyte
acetylcholinesterase activity was reported, with severe cholinergic
signs which required aggressive, extensive antidotal therapy and
artificial ventilation. Mild distal axonopathy consistent with
organophosphate-induced delayed polyneuropathy was reported some weeks
after the poisoning incident.
The ADI of 0-0.01 mg/kg bw established by the 1982 Meeting was
based on a NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in humans. The present Meeting affirmed
this ADI on the basis of the NOAEL of 1 mg/kg bw per day for
inhibition of brain acetylcholinesterase activity in studies in mice,
rats, and dogs, using a 100-fold safety factor, and on the basis of
the NOAEL of 0.1 mg/kg bw per day for inhibition of erythrocyte
acetylcholinesterase activity in the study of human subjects exposed
for 9 days, using a 10-fold safety factor.
The Meeting allocated an acute reference dose of 0.1 mg/kg bw on
the basis of the NOAEL of 1 mg/kg bw for inhibition of erythrocyte
acetylcholinesterase activity in a study in which volunteers received
a single oral dose of chlorpyrifos, with a safety factor of 10.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 5 ppm, equal to 0.7 mg/kg bw per day (toxicity in a 79-week
study of toxicity and carcinogenicity)
1 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
10 mg/kg bw/day (fetal toxicity in a study of developmental
toxicity)
Rat: 1 mg/kg bw per day (toxicity in 2-year studies of toxicity
and carcinogenicity)
1 mg/kg bw per day (developmental and parental toxicity in a
two-generation study of reproductive toxicity)
1 mg/kg bw per day (maternal and developmental toxicity in a
study of developmental toxicity)
2.5 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Rabbit: 81 mg/kg bw per day (maternal and fetal toxicity in a study
of developmental toxicity)
Dog: 1 mg/kg bw per day (toxicity in a 2-year study of toxicity)
Human: 0.1 mg/kg bw per day (no inhibition of erythrocyte
cholinesterase activity at highest dose tested in men dosed
orally for 9 days)
1 mg/kg bw (inhibition of erythrocyte cholinesterase
activity in adult volunteers after a single oral dose)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Estimate of acute reference dose
0.1 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological end-points relevant for setting guidance values for dietary and non-dietary exposure to chlorpyrifos
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of oral absorption Up to 90% in rats within 72 h; about 70% in humans within 96 h
Dermal absorption Less than 2% in humans within 180 h; not determined in animals
Distribution Initially widely distributed; highest residues in liver, kidneys
and fat at 72 h in rats
Potential for accumulation Elimination half-times of < 24 h and low tissue residues after
72 h in rats. No evidence of potential for accumulation
Rate and extent of excretion > 95% within 72 h in rats, mainly in urine (68-93%) and faeces (6-15%)
Metabolism in animals Rapidly metabolized by mixed-function oxidases to chlorpyrifos oxon
via oxidative desulfuration and an electrophilic phosphooxathiiran
intermediate. Degradation by conversion directly to
3,5,6-trichloro-2-pyridyl phosphate and diethyl thiophosphate.
The oxon is hydrolysed to diethyl phosphate and
3,5,6-trichloropyridinol, while a minor reaction pathway is by
hydrolysis to monoethyl-3,5,6-trichloro-2-pyridinol phosphorothioate.
Toxicologically significant compounds Parent compound and oxon
(animals, plants and environment)
Acute toxicity
Rat,, LD50, oral 96 mg/kg bw
Rat LD50, dermal > 2000 mg/kg bw
Rat, LC50, inhalation > 36 mg/m3 (4 h, vapour, nose-only exposure)
560 mg/m3 (4 h, nebulized particles < 5 mm, whole-body exposure)
Rabbit, dermal irritation Slightly irritating
Rabbit, ocular irritation Slightly irritating
Guinea-pig, dermal sensitization Not sensitizing
Short-term toxicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest critical oral NOAEL 1 mg/kg bw per day, dog, 2 years
1 mg/kg bw per day, rat, 13 weeks
Lowest relevant dermal NOAEL 5 mg/kg bw per day, rat; 21 days
Lowest relevant inhalation NOAEL 20.6 ppb (296 mg/m3), rat; 13 weeks
Long-term toxicity and carcinogenicity
Target/critical effect Inhibition of brain cholinesterase activity
Lowest relevant NOAEL 2 years, rat; 1 mg/kg bw per day
78 weeks, mouse; 0.7 mg/kg bw per day
Carcinogenicity Not carcinogenic in rats or mice
Genotoxicity Not genotoxic
Reproductive toxicity
Reproductive target/critical effect Neonatal toxicity (reduced pup body weight and survival)
Lowest relevant reproductive NOAEL Two-generation, rat; 1 mg/kg bw per day
Developmental target/critical effect Fetal and perinatal toxicity at maternally toxic doses (including
an increase in delayed ossification, reduced crown-rump length,
reduced pup weight, increased postimplantation loss, delayed sexual
maturity)
Lowest relevant developmental NOAEL Rats; 1 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Reversible neurotoxicity consistent with cholinesterase
inhibition. No evidence of delayed neurotoxicity or
histopathological changes in nerves of hens (10 mg/kg bw per day)
and rats (15 mg/kg bw per day) for up to 13 weeks. At high acute
doses (up to 150 mg/kg bw), significant inhibition of neuropathy
target esterase and mild delayed neuropathy in hens, but at this
dose, extensive and aggressive antidote treatment were required
for birds' survival.
Other toxicological studies No effect on cognitive function in rat pups in a study of
developmental toxicity at doses up to 5 mg/kg bw per day
Medical data No inhibition of erythrocyte acetylcholinesterase activity in
volunteers after repeated oral doses of up to 0.1 mg/kg bw per day
(for 9 days), single oral doses of up to 1 mg/kg bw, or a single
dermal doses of 5 mg/kg bw. Poisoning case presented with severe
cholinergic effects, with evidence of delayed polyneuropathy and/or
distal axonopathy at a dose that required antidotal treatment and
artificial ventilation.
Summary Value Study Safety factor
ADI 0-0.01 mg/kg bw Rat, 2-year dietary 100
Rat, reproduction
Mouse, developmental toxicity
Dog, 2-year dietary
Human, 9-day oral 10
Acute reference dose 0.1 mg/kg bw Human, single dose 10
References
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993a) Life-threatening
organophosphate-induced delayed polyneuropathy in a child after
accidental chlorpyrifos ingestion. J. Pediatr., 122, 658-660.
Aiuto, L.A., Pavlakis, S.G. & Boxer, R.A. (1993b)
Organophosphate-induced delayed polyneuropathy (comment).
J. Pediatr., 123, 837.
Aldridge, W.N. (1953) Serum esterases I. J. Biochem., 53, 110-117.
Anderson, B.T., Walker, S.A. & Punler, M.J. (1995) Chlorpyrifos tech:
Acute inhalational toxicity study in rats. Unpublished report No.
10425 from Inveresk Research International, Tranent, Scotland.
Submitted to WHO by Luxembourg Industries, Tel Aviv, Israel.
Bakke, J.E., Feil, V.J. & Price, C.E. (1976). Rat urinary metabolites
from O,O-diethyl-O(3,5,6-trichloro-2-pyridyl) phosphorthioate.
J. Environ. Sci. Health Bull., 3, 225-230.
Barna-Lloyd, T. & Szabo, J.R. (1985) Sodium
3,5,6-trichloro-2-pyridinol: 3-month rat dietary toxicity study.
Unpublished report No. TexasT:K-065999-099 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Barna-Lloyd, T., Szabo, J.R. & Young, J.T. (1986) Chlorpyrifos:
Subchronic organophosphate-induced delayed-neurotoxicity (OPIDN)
study in laying chickens hens. Unpublished report No.
TexasT:K-044793-064 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Bauriedel, W.R. & Miller, J.H. (1981) The metabolic fate of the sodium
salt of C14-labelled 3,5,6-trichloro-2-pyridinol orally
administered to swine. Unpublished report No. GH-C 1427 from Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. (1964) Results of 90-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in rats. Unpublished report from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1964) Results of 90-day dietary
feeding studies of O,Odiethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Beatty, S.C. & McCollister, D.D. (1971) Results of 90-day dietary
feeding studies of O,Odiethyl-O-3,5,6-trichloro-2-pyridyl
phosphorothioate in rats. Unpublished report from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, IN, USA.
Berman, C.L. (1987) Evaluation of chlorpyrifos (Pyrinex) for dermal
sensitization of guinea pig. Unpublished report No. 59487-01 from
Arthur D. Little Inc., Cambridge, Massachusetts, USA. Submitted
to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Berteau, P.E. & Deen, W.A. (1978) A comparison of oral and inhalation
toxicities of four insecticides to mice and rats. Bull.
Environ. Contam. Toxicol., 19, 113-120.
Blackmore, R.H. (1968) Oral administration -- dogs Dursban. Final
report. Unpublished report No. 174-115 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, IN, USA.
Blagden, S.M. (1994) Chlorpyrifos technical: Acute inhalation toxicity
study four-hour exposure (nose only) in the rat. Unpublished
report No. CHA 10-CYF from Safepharm Laboratories Ltd, Derby,
United Kingdom. Submitted to WHO by Cheminova Agro A/S, Lemvig,
Denmark.
Blondell, J. & Dobozy, V.A. (1997) Review of chlorpyrifos poisoning
data. Washington DC, Environmental Protection Agency.
Branson, D.R. & Litchfield, N.H. (1970) Comparative absorption,
elimination and distribution of DOWCO 179, its methyl analog
DOWCO 214 and their major metabolite,
3,5,6-trichloro-2-pyridinol. Unpublished report No. 70 4479 from
Dow Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971a) Absorption, excretion and
distribution of O,Odiethyl O-3,5,6-trichloro-2, 6-C14-2-pyridyl
phosphorothioate in rats. Unpublished report No. NBA-9 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, IN, USA.
Branson, D.R. & Litchfield, N.H. (1971b) Absorption, excretion and
distribution of 3,5,6trichloro-2, 6-C14-2-pyridinol in rats.
Unpublished report No. NBA-10 from Dow Chemical Co., USA
Submitted to WHO by Dow Agrosciences, Indianapolis, IN, USA.
Brenner, F.E., Bond, G.G., McLaren, E.A., Greene, S. & Cook, R.R.
(1989) Morbidity among employees engaged in the manufacture or
formulation of chlorpyrifos. Br. J. Ind. Med., 46, 133-137.
Breslin, W.J., Liberacki, A.B., Dittenber, D.A., Brzak, K.A. & Quast,
J.F. (1991) Chlorpyrifos: Two-generation dietary reproduction
study in Sprague-Dawley rats. Unpublished report No. K-044793-088
from the Toxicology Research Laboratory, Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Bruce, R.J. & Zempel, J.A. (1986) Chlorpyrifos: Evaluation in the Ames
Salmonella/mammalian-microsome mutagenicity assay. Unpublished
report No. TexasT:K044793-075 from Lake Jackson Research Center,
Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Bruce, R.J., Gollapudi, B.B. & Hinze, C.A. (1985) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008 from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Buch SA (1980) Pyrinex tech: Acute inhalation toxicity in rats.
Unpublished report No. 80/MA/025/362 from Life Science Research,
Stock, Essex, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980a) Pyrinex tech: Irritance to rabbit
eye. Unpublished report No. 80/MA/023/143 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1980b) Pyrinex tech: Irritance to rabbit
skin. Unpublished report No. 80/MAK/024/144 from Life Science
Research, Stock, Essex, United Kingdom. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A. & Gardner, J.R. (1981) Pyrinex technical: Acute oral
toxicity in the rat. Unpublished report No. 81/MAK035/104 from
Life Science Research, Stock, Essex, United Kingdom. Submitted to
WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Buch, S.A., Gardner, J.R. & Birnie, J.V. (1980) Pyrinex: Acute
percutaneous toxicity study in rats. Unpublished report No.
80/MAK022/300 from Life Science Research, Stock, Essex, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Burns, C.J., Cartmill, J.B., Powers, B.S. & Lee, M.K. (1998) Update of
the morbidity experienced of employees potentially exposed to
chlorpyrifos. Occup. Environ. Med., 55, 65-70.
Bushnell, P.J., Padilla, S.S., Ward, T., Pope, C.N. & Olszyk, V.B.
(1991) Behavioral and neurochemical changes in rats dosed
repeatedly with diisopropylfluorophosphate. J. Pharmacol.
Exp. Ther., 256, 741-750.
Bushnell, P.J., Pope, C.N. & Padilla, S. (1993) Behavioral and
neurochemical effects of acute chlorpyrifos in rats: Tolerance to
prolonged inhibition of cholinesterase. J. Pharmacol. Exp.
Ther., 266, 1007-1017.
Calhoun, L.L. & Johnson, K.A. (1988) Chlorpyrifos: 4-day dermal probe
and 21-day dermal toxicity studies in Fischer 344 rats.
Unpublished report No. HET K 044793-085,086 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Calhoun, L.L. & Johnson, K.A. (1989) Supplemental information to the
report entitled: Chlorpyrifos: 4-day dermal probe and 21 day
dermal toxicity studies in Fischer 344 rats. Unpublished report
No. K-044793-085(S) from Dow Chemical Co., Midland, Michigan,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Campbell, C.G., Seidler, F.J. & Slotkin, T.A. (1997) Chlorpyrifos
interferes with cell development in rat brain regions. Brain
Res. Bull., 43, 179-189.
Capodicasa, E., Scapellato, M.L., Moretto, A., Caroldi, S. & Lotti, M.
(1991) Chlorpyrifosinduced delayed polyneuropathy. Arch.
Toxicol., 65, 150-155.
Chambers, J.E. (1992) The role of target-site activation of
phosphorothionates in acute toxicity. In: Chambers, J.E., Levi,
P.E. & Levi, A.P., eds, Organophosphates, San Diego, Academic
Press.
Chambers, J.E. & Carr, R.L. (1995) Biochemical mechanisms contributing
to species differences in insecticidal toxicity. Toxicology,
105, 291-304.
Chanda, S.M. & Pope, C.N. (1996) Neurochemical and neurobehavioural
effects of repeated gestational exposure to chlorpyrifos in
maternal and developing rats. Pharmacol. Biochem. Behav., 53,
771-776.
Chanda, S.M., Harp, P., Liu, J. & Pope, C.N. (1995) Comparative
developmental and maternal neurotoxicity following acute
gestational exposure to chlorpyrifos in rats. J. Toxicol.
Environ. Health, 44, 189-202.
Chaudhuri, J., Chakraborti, T.K., Chanda, S. & Pope, C.N. (1993)
Differential modulation of organophosphate-sensitivity muscarinic
receptors in rat brain by parathion and chlorpyrifos.
J. Biochem. Toxicol., 8, 207-216.
Copeland, J.R. (1964) Results of 93-day dietary feeding studies of
3,5,6-trichloro-2-pyridinol in beagle hounds. Unpublished report
No. T35.12-38278-3 from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Corley, R.A., Landry, T.D., Calhoun, L.L., Dittenber, D.A. & Lomax,
L.G. (1986) Chlorpyrifos: 13-week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET K
044793-077 from Dow Chemical Company Laboratory, Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Costa, L.G., McDonald, B.E., Murphy, S.D., Omenn, G.S., Richter, R.J.,
Motulsky, A.G. & Furlong, C.E. (1990) Serum paraoxonase and its
influence on paraoxon and chlorpyrifosoxon toxicity in rats.
Toxicol. Appl. Pharmacol., 103, 66-76.
Coulston, F., Golberg, L., Abraham, R. & Benitz, F.K. (1971) Final
report on safety evaluations and metabolic studies on Dowco 179
(Indiana 151). Unpublished report No. K44793-(48)A from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Coulston, F., Golberg, L. & Griffin, T. (1972). Safety evaluation of
Dowco 179 in human volunteers. Unpublished report from Institute
of Experimental Pathology and Toxicology, Albany Medical College,
Albany, New York, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Crown, S., Weiss, A., Nyska, A. & Waner, T. (1984a) Pyrinex. Toxicity
in dietary administration to mice for two weeks: A range-finding
study. Unpublished report No. MAK/109/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A. & Waner, T. (1984b) Pyrinex technical. Two-week
range-finding study in dietary administration to rats.
Unpublished report No. MAK/057/a/PYR from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Gur, E., Nyska, A. & Waner, T. (1985) Pyrinex technical
toxicity in dietary administration to rats for 13 weeks.
Unpublished report No. MAK/058/PYRA from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Crown, S., Weiss, A., Nyska, A., Waner, T. & Pirak, M. (1987) Pyrinex
technical. Toxicity in dietary administration to mice for 13
weeks. A preliminary study. Unpublished report No. MAK/105/PYR
from Life Science Research Israel Inc., Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Crown, S., Nyska, A., Pirak, M. & Waner, T. (1988) Pyrinex technical.
Oncogenicity study in the rat. Unpublished report No. MAK/095/PYR
from Life Science Research Israel Ltd, Ness Ziona, Israel.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Davies, R.E. & Kynoch, S.R. (1970) Acute subcutaneous toxicity of
Dursban to rats and the effect of treatment with 1. Atropine
sulphate 2. Atropine sulphate and PAM. Unpublished report No.
A1A-275 or 3641/70/453 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Davies, D.B., Tollett, J.T. & Lomax, L.G. (1985) Chlorpyrifos: A
four-week dietary study in the CD-1 mice. Unpublished report No.
HET-K-044793-068 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Dittenber, D.A., Hanley,
T.R., Jr & John, J.A. (1979) The effect of orally administered
chlorpyrifos on embryonal and foetal development in mice.
Unpublished report No. HET-K-44793-32 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Deacon, M.M., Murray, J.S., Pilny, M.K., Rao, K.S., Dittenber, D.A.,
Hanley, T.R., Jr & John, J.A. (1980) Embryotoxicity and
fetotoxicity of orally administered chlorpyrifos in mice.
Toxicol. Appl. Pharmacol., 54, 31-40.
Derelanko, M.J. & Hollinger, M.A., eds (1995) CRC Handbook of
Toxicology, Boca Raton, FL, CRC Press Inc.
Dietz, F.K., Mensik, D.C., Hinze, C.A., Rachunek, B.K. & Taylor, H.W.
(1983) Dursban insecticide: Assessment of neonatal survival in a
two-generation reproduction study in rats. Unpublished report No.
TexasT-K-44793-(45) from Dow Chemical Co., USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L. & Wetters, J.H. (1972a) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues and eggs
from chickens fed chlorpyrifos. Unpublished report No. GH-C 555
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., Rice, J.R., McKellar, R.L. & Pennington, J.Y.
(1972b) Determination of residues of chlorpyrifos, its oxygen
analogue, and 3,5,6-trichloro-2-pyridinol in tissues of cattle
fed chlorpyrifos. Unpublished report No. GH-C 566 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Dishburger, H.J., McKellar, R.L., Pennington, J.Y. & Rice, J.R. (1977)
Determination of residues of chlorpyrifos, its oxygen analogue,
and 3,5,6-trichloro-2-pyridinol in tissues of cattle fed
chlorpyrifos. J. Agric. Food Chem., 25, 1325-1329.
Dittenber, D.A. (1997) Chlorpyrifos: Evaluation of single oral doses
on cholinesterase and neurotoxic esterase inhibition in F344
rats. Unpublished report No. 960036 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Dreher DM (1994a) Chlorpyrifos technical: Acute oral toxicity test in
the rat. Unpublished report No. CHA 8-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994b) Chlorpyrifos technical: Acute dermal toxicity (limit
test) in the rat. Unpublished report No. CHA 9-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994c) Chlorpyrifos technical: Acute eye irritation test in
the rabbit. Unpublished report No. CHA 12-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994d) Chlorpyrifos technical: Acute dermal irritation test
in the rabbit. Unpublished report No. CHA 11-CYF from Safepharm
Laboratories Ltd, Derby, United Kingdom. Submitted to WHO by
Cheminova Agro A/S, Lemvig, Denmark.
Dreher DM (1994e) Chlorpyrifos technical: Magnusson & Kligman
maximisation study in the guinea pig. Unpublished report No. CHA
12-CYF from Safepharm Laboratories Ltd, Derby, United Kingdom.
Submitted to Who by Cheminova Agro A/S, Lemvig, Denmark.
Drevenkar, V., Vasilic, Z., Stengl, B., Frobe, Z. & Rumenjak, V.
(1993) Chlorpyrifos metabolites in serum and urine of poisoned
persons. Chem.-Biol. Interactions, 87, 315-322.
Eliason, D.A., Crammer, M.F., von Windeguth, D.L., Kilpatrick, J.W.,
Suggs, J.E. & Schoof, H.F. (1969) Dursban premise applications
and their effect on the cholinesterase levels of spraymen.
Mosquito News, 29, 591-595.
Emerson, J.L. & Gerbig, C.L. (1970) 91 day toxicology study in beagle
dogs treated with 3,5,6-trichloro-2-pyridinol. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Fikes, J.D., Zacchary, J.F., Parker, A.J. & Beasley, V.R. (1992)
Clinical, biochemical, electrophysiologic and histologic
assessment of chlorpyrifos induced delayed neuropathy in the cat.
Neurotoxicology, 13, 663-678.
Fredrick Institute of Plant Protection and Toxicology (1995a) Acute
oral toxicity study of chlorpyrifos technical in Swiss albino
mice. Unpublished report No. 2739 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995b) Acute
oral toxicity study of chlorpyrifos technical in Wistar rats.
Unpublished report No. 2738 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995c) Acute
dermal toxicity study of chlorpyrifos technical in rabbits.
Unpublished report No. 2748 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995d)
Irritation of chlorpyrifos technical to mucous membrane in
rabbits. Unpublished report No. 2746 from Fredrick Institute of
Plant Protection and Toxicology, Tamil Nadu, India. Submitted to
WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1995e) Primary
skin irritation of chlorpyrifos technical in rabbits. Unpublished
report No. 2747 from Fredrick Institute of Plant Protection and
Toxicology, Tamil Nadu, India. Submitted to WHO by Ficom
Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996a) Acute
inhalation study with chlorpyrifos technical in Wistar rats.
Unpublished report No. 2968 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996b) Allergy
and skin sensitization potential of chlorpyrifos technical in
guinea pigs. Unpublished report No. 2928 from Fredrick Institute
of Plant Protection and Toxicology, Tamil Nadu, India. Submitted
to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996c)
Mutagenicity evaluation of chlorpyrifos technical in the
point-mutation Ames Salmonella typhimurium test system.
Unpublished report No. 3049 from Fredrick Institute of Plant
Protection and Toxicology, Tamil Nadu, India. Submitted to WHO by
Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996d)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
micronucleus assay. Unpublished report No. 3054 from Fredrick
Institute of Plant Protection and Toxicology, Tamil Nadu, India.
Submitted to WHO by Ficom Organics, Bombay, India.
Fredrick Institute of Plant Protection and Toxicology (1996e)
Mutagenicity evaluation of chlorpyrifos technical in the mouse
bone marrow cytogenic assay. Unpublished report No. 3076 from
Fredrick Institute of Plant Protection and Toxicology, Tamil
Nadu, India. Submitted to WHO by Ficom Organics, Bombay, India.
Furlong, C.E., Richter, R.J., Seidel, S.L. & Motulski, A.G. (1988)
Role of genetic polymorphism of human plasma
paraoxonase/arylesterase in hydrolysis of the insecticide
metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum.
Genet., 43, 230-238.
Furlong, C.E., Richter, R.J., Seidel, S.L., Costa, L.G. & Motulsky,
A.G. (1989) Spectrophotometric assays for the enzymatic
hydrolysis of the active metabolites of chlorpyrifos and
parathion by plasma paraoxonase/arylesterase. Anal. Biochem.,
180, 242-247.
Geldmacher-von Mallinckrodt, M. & Diepgen, T.L. (1988) The human serum
paraoxonase polymorphism and specificity. Toxicol. Environ.
Chem., 18, 79-196.
Gerbig,, C.G. & Emerson, J.L. (1970a) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in mice. Unpublished
report No. HH-240 from Dow Chemical Co., Zionsville, Indianna,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gerbig,, C.G. & Emerson, J.L. (1970b) Oral median lethal dose (LD50)
determination of 3,5,6-trichloro-2-pyridinol in the rat.
Unpublished report No. HH-239 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gerbig,, C.G. & Emerson, J.L. (1970c) Determination of oral minimum
lethal dose of 3,5,6trichloro-2-pyridinol in beagle dogs.
Unpublished report No. HH-253 from Dow Chemical Co., Zionsville,
Indianna, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Gibson, J.E. (1996) Critical review of allegations associating Dursban
with human teratogenicity. Unpublished report No. JEG122396, from
Dow Elanco, USA. Submitted by Dow Agrosciences, Indianapolis,
Indiana, USA.
Glas, R.D. (1977a) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from calves fed
3,5,6-trichloro-2-pyridinol. Unpublished report No. GH-C 1053
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977b) Residues of 3,5,6-trichloro-2-pyridinol and
2-methoxy-3,5,6trichloropyridine in tissues from a calf fed
2-methoxy-3,5,6-trichloro-2-pyridine. Unpublished report No. GH-C
1052 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1977c) Residues of triclopyr, 3,5,6-trichloro-2-pyridinol
and 2-methoxy-3,5,6trichloropyridine in bovine tissues from
calves fed trichlopyr. Unpublished report No. GH-C 1047 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981a) The metabolic fate of 14C-chlorpyrifos fed to
lactating goats. Unpublished report No. GH-C 1408 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Glas, R.D. (1981b) Identification of 14C-labelled residues in milk
from goats fed 14Cchlorpyrifos. Unpublished report No. GH-C
1470 from Dow Chemical Co., Midland, Michigan, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Gollapudi, B.B. (1987) Evaluation of 3,5,6-trichloro-2-pyridinol (TCP)
in the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. TexasT-K-38278-012 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Linscombe, V.A. & Wilkerson, J.E. (1985) Evaluation
of chlorpyrifos in the mouse bone marrow micronucleus test.
Unpublished report No. TexasT-K-044793-067 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gollapudi, B.B., Mendrala, A.L. & Linscombe, V.A. (1995) Evaluation of
the genetic toxicity of the organophosphate insecticide
chlorpyrifos. Mutat. Res., 342, 25-36.
Gur, E., Nyska, A. & Waner, T. (1991) Pyrinex technical oncogenicity
study in the mouse. Unpublished report from Life Science Research
Israel Inc., Ness Ziona, Israel. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Isreal.
Gutenmann, W.H., St John, L.E., Jr & Lisk, D.J. (1968) Metabolic
studies with O,O-diethyl O-(3,5,6-trichloro-2-pyridyl)
phosphorothioate (Dursban) insecticide in a lactating cow.
Unpublished report from Cornell University, Ithaca, New York,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Gutmann, L. & Bodensteiner, J.B. (1993) Organophosphate-induced
delayed polyneuropathy (comment). J. Pediatr., 123, 837.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987a)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in Fischer 344
rats. Unpublished report No. K-038278-011 from Dow Chemical Co.,
USA. Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana,
USA.
Hanley, T.R., Jr, Zielke, G.J. & Lomax, L.G. (1987b)
3,5,6-Trichloro-2-pyridinol: Oral teratology study in New Zealand
white Rrabbits. Unpublished report No. K-038278-015 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hardy, C.J. & Jackson, G.C. (1984) Dursban technical: Acute inhalation
toxicity in rats. Unpublished report No. DET 368 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Dawe, I.S. &
Anderson, A. (1989a) Chlorpyrifos oral dose range finding
toxicity study in beagle dogs (final report -- repeated daily
dosage for 4 weeks). Unpublished report No. MBS 30/88675 from
Huntingdon Research Centre Inc, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Harling, R.J., Barker, M.H., Buist, D.P., Crook, D., Majeed, S.,
Gopinath, C., Dawe, I.S. & Anderson, A. (1989b) Chlorpyrifos oral
toxicity study in beagle dogs (repeated daily dosage for 13
weeks). Unpublished report No. MBS 31/88999 from Huntingdon
Research Centre Inc, Huntingdon, United Kingdom. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Hazleton Laboratories (1968) Short term (subacute) dietary
administration -- Rats organophosphate insecticide Dursban. Final
report. Unpublished report No. 174-114 from Hazleton
Laboratories, Falls Church, Virginia, USA. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Henck, J.W. & Kociba, R.J. (1980) Three samples of Dursban
insecticide: Acute oral toxicity and acute percutaneous
absorption potential. Unpublished report No. A1A-130 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Hoberman, A.M. (1998) Developmental neurotoxicity study of
chlorpyrifos administered orally via gavage to Crl:CD.BR VAF/Plus
presumed pregnant rats. Unpublished report No. K-044793109 from
Argus Research Laboratories, Horsham, PA, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Hooser, S.B., Beasley, V.R., Sundberg, J.P. & Harlin, K. (1988)
Toxicologic evaluation of chlorpyrifos in cats. Am. J. Vet.
Res., 49, 1371-1375.
Humbert, R., Adler, D.A., Distechhe, C.M., Hassett, C., Omiecinski,
C.J. & Furlong, C.E. (1993) The molecular basis of the human
serum paraoxonase activity polymorphism. Nature Genet., 3,
73-76
Jackson, D. & Ogilvie, S.W. (1994a) Chlorpyrifos tech: Acute dermal
toxicity (LD50) test in rats. Unpublished report No. 10439 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994b) Chlorpyrifos tech: Primary eye
irritation test in rabbits. Unpublished report No. 10441 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994c) Chlorpyrifos tech: Primary skin
irritation test in rabbits. Unpublished report No. 10440 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Jackson, D. & Ogilvie, S.W. (1994d) Chlorpyrifos tech:
Magnusson-Kligman maximisation test in guinea pigs. Unpublished
report No. 10442 from Inveresk Research International, Tranent,
Scotland. Submitted to WHO by Luxembourg Industries, Tel Aviv,
Israel.
Jai Research Foundation (1996a) Reverse gene mutation study of
chlorpyrifos tech (95%) in bacteria by Ames Salmonella/microsome
test. Unpublished report No. 5/JRF/MichiganC/96 from Department
of Microbiology, Jai Research Foundation, Gujarat, India.
Submitted to WHO by Mitsu Industries, Gujarat, India.
Jai Research Foundation (1996b) Neurotoxicity study of chlorpyrifos
technical to hen. Unpublished report No. 1539/JRF/TOX/96 from
Department of Toxicology, Jai Research Foundation, Gujarat,
India. Submitted to WHO by Mitsu Industries, Gujarat, India.
James, P., Stubbs, A., Parker, C.A., Offer, J.M. & Anderson, A. (1988)
The effect of Pyrinex (chlorpyrifos) on reproductive function of
two generations in the rat. Unpublished report No. MBS 29/881452
from Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted to WHO by Makteshim Chemical Works, Beer-Sheva, Israel.
Jeffrey, M.M., Battjes, J.E. & Eisenbrandt, D.L. (1986) Dursban F
(tech): Acute dermal toxicity study in Fischer 344 rats.
Unpublished report No. HET-K 044793-083 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Jones, J.R. (1985a) Dursban F: Eye irritation study in the rabbit.
Unpublished report No. DET 424 from Hazleton Laboratories Europe,
Harrogate, North Yorkshire, United Kingdom. Submitted to WHO by
Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985b) Dursban F: Primary skin irritation and corrosivity
study in the rabbit. Unpublished report No. DET 421 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Jones, J.R. (1985c) Dursban F: Delayed contact hypersensitivity study
in the guinea pig (Buehler Test). Unpublished report No. DET 475
from Hazleton Laboratories Europe, Harrogate, North Yorkshire,
United Kingdom. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kamataki, T. & Neil, R.A. (1976) Metabolism of diethyl A-nitrophenyl
phosphorothienate (parathion) by a reconstituted mixed-function
oxidase enzyme system: Studies of covalent binding of the sulfur
atom. Mol. Pharmacol., 12, 933-944.
Kaplan, J.G., Kessler, J., Rosenberg, N., Pack, D. & Schaumburg, H.H.
(1993) Sensory neuropathy associated with Dursban (chlorpyrifos)
exposure [published erratum appears in Neurology 1994 44, 367].
Neurology, 43, 2193-2196.
Kenaga, E.E. (1967) Comments on data concerning cholinesterase effects
of Dursban in man following US Public Health Service experimental
yellow fever mosquito eradication tests in Florida during 1966
and 1967. Unpublished report No.A1A-394, TK 2-1684 from Dow
Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Kenny, T.J., Coombs, D.W. & Hardy, C.J. (1987) Chlorpyrifos technical
acute inhalation toxicity in rats 4-hour exposure. Unpublished
report No. MBS 20/87575 from Huntingdon Research Centre,
Huntingdon, United Kingdom. Submitted to WHO by Makteshim
Chemical Works, Beer-Sheva, Israel.
Kenny, T.J., Coombs, D.W., Hardy, C.J. & Crook, D. (1988) Chlorpyrifos
inhalation toxicity in the rat. 14-Day preliminary dose range
finding study. Unpublished report No. MBS 21/871329 from
Huntingdon Research Centre Ltd, Huntingdon, United Kingdom.
Submitted by Makteshim Chemical Works, Beer-Sheva, Israel.
Kilian, D.J., Edwards, H.N., Benge, M.C. & Tabatabai, Z. (1970)
Results of human skin exposure to Dowco 179. Unpublished report
No. TMD-70-2 from Dow Chemical Co., Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Kisicki, J.C., Seip, C.W. & Combs, M.L. (1999) A rising dose
toxicology study to determine the no-observable-effect-levels
(NOEL) for erythrocyte acetylcholinesterase (AChE) inhibition and
cholinergic signs and symptoms of chlorpyrifos at three dose
levels. Unpublished report No. LLC Study ID: DR#K-044793-284
dated 19 April 1999, from MDS Harris, Lincoln, NE, USA. Submitted
to WHO by Dow Agrosciences, Indianapolis, Indiana, USA.
Kociba, R.J., McCollister, S.B., Keyes, D.G. & Dittenber, D.A. (1985)
Supplement to original report entitled 'Results of two-year
dietary feeding studies on Dowco 179 in beagle dogs'. Unpublished
report No. NBT 3512-44793-18 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lackenby, F. (1985a) Dursban F and Dursban F (OP1): Acute oral
toxicity studies in the rat. Unpublished report No. 4703-50/390 &
591 from Hazleton Laboratories Europe, North Yorkshire, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Lackenby, F. (1985b) Dursban F: Acute dermal median lethal dose in the
rat. Unpublished report No. 4206-50/391 from Hazleton
Laboratories Europe, Harrogate, North Yorkshire, United Kingdom.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Landry, T.D., Streeter, C.M. & Lomax, L.G. (1985) Chlorpyrifos: 2-week
vapor inhalation toxicity study in Fischer 344 rats. Protocol.
Unpublished report No. A1A-714 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Landry, T.D., Dittenber, D.A., Lomax, L.G., Calhoun, L.L. & Morabito,
P. (1986) Chlorpyrifos: 2 week nose-only vapor inhalation
exposure study in Fischer 344 rats. Unpublished report No. HET
K-44793-81 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Liberacki, A.B., Breslin, W.J., Dittenber, D.A. & Quast, J.F. (1990)
Chlorpyrifos: Two week dietary probe study in Sprague-Dawley
rats. Unpublished report No. HET K 044793090 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A. & Gollapudi, B. (1986) Evaluation of
3,5,6-trtichloro-2-pyridinol (TCP) in the Chinese hamster ovary
cell/hypoxanthine-guanine-phosphoribosyl transferase (CHO/HGPRT)
forward mutation assay. Unpublished report No.
TexasT:K-038278-013 from Dow Chemical Co., HES, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Linscombe, V.A., Mensik, D.C. & Clem, B.A. (1992) Evaluation of
chlorpyrifos in an in vitro chromosomal aberration assay
utilizing rat lymphocytes. Unpublished report No. TexasT:K
044793-092 from Dow Chemical Co., Freeport, Texas, USA. Submitted
to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Lotti, M., Moretto, A., Zoppellari, R., Dainese, R., Rizzuto, N. &
Barusco, G. (1986) Inhibition of lymphocytic neuropathy target
esterase predicts the development of organophosphate-induced
delayed polyneuropathy. Arch. Toxicol., 59, 176-179.
Loveday, K.S. (1987) In vitro chromosomal aberration assay on Pyrinex
(chlorpyrifos). Unpublished report No. R4660 from Arthur D.
Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Loveday, K.S., Findlen, K.M.B. & Yadlon, S. (1987) Evaluation of
Pyrinex in the Ames mutagenesis assay. Unpublished report No.
59487-00 from Arthur D. Little Inc., USA. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Ma, T. & Chambers, J.E. (1994) Kinetic parameters of desulfuration and
dearylation of parathion and chlorpyrifos by rat liver
microsomes. Food Chem. Toxicol., 32, 763-767.
Maita, K., Hirano, M., Harada, T., Mitsumori, K., Yoshida, A.,
Takahashi, K., Nakashima, N., Kitazawa, T., Enomoto, A. & Inui,
K. (1988) Mortality, major cause of moribundity aqnd spontaneous
tumours in CD-1 mice. Toxicol. Pathol., 16, 340-349.
Mattsson, J.L., Wilmer, J.W., Shankar, M.R., Berdasco, N.M., Crissman,
J.W., Maurissen, J.P. & Bond, D.M. (1996) Single-dose and 13-week
repeated-dose neurotoxicity screening studies of chlorpyrifos
insecticide. Food Chem. Toxicol., 34, 393-405.
Maurissen, J.P., Shankar, M.R. & Mattsson, J.L. (1996) Chlorpyrifos:
Cognitive study in adult Long-Evans rats. Unpublished report No.
K-044793-096 from Dow Chemical Co., USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989a) Evaluation of chlorpyrifos
in the mouse bone marrow micronucleus test. Unpublished report
No. TexasT:K 044793-067A from Lake Jackson Research Center, Dow
Chemical Co., Freeport, Texas, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McClintock, M.L. & Gollapudi, B.B. (1989b) Evaluation of
3,5,6-trichloro-2-pyridinol in the mouse bone marrow micronucleus
test. Unpublished report No. K-038278-008A from Lake Jackson
Research Center, Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971a) Results of two-year dietary feeding studies on Dowco 179
in beagle dogs. Unpublished report No. T35.1244793-18 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J., Gehring, P.J. & Humiston, C.G.
(1971b) Results of two-year dietary feeding studies on Dowco 179
in rats. Unpublished report No. NBT35.12-4479321 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
McCollister, S.B., Kociba, R.J. & Keyes, D.G. (1985) Supplement to
original report entitled 'Results of two-year dietary feeding
studies on Dowco 179 in rats'. Unpublished report No.
NBT35.12-44793-21 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
McKellar, R.L., Wetters, J.H. & Dishburger, H.J. (1972) Residues of
chlorpyrifos and 3,5,6trichloro-2-pyridinol in tissues of swine
fed chlorpyrifos. Unpublished report No. GHC549 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Mendrala, A.L. (1985) Evaluation of chlorpyrifos in the Chinese
hamster ovary cellhypoxanthine (guanine) phosphoribosyl
transferase (CHO/HGPRT) forward mutation assay. Unpublished
report No. HET-K-044793-072 from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Mendrala, A.L. & Dryzaga, M.D. (1986) Evaluation of chlorpyrifos in
the rat hepatocyte unscheduled DNA synthesis (UDS) assay.
Unpublished report No. HET K 044793-073 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Moretto, A. & Lotti, M. (1998) Poisoning by
organophosphorusinsecticides and sensory neuropathy.
J. Neurol. Neurosurg. Psych., 64, 463-468.
Mortensen, S.R., Chanda, S.M., Hooper, M.J. & Padilla, S. (1996)
Maturational differences in chlorpyrifos-oxonase activity may
contribute to age-related sensitivity to chlorpyrifos.
J. Biochem. Toxicol., 11, 279-287.
Moser, V.C. (1995) Comparisons of the acute effects of cholinesterase
inhibitors using a neurobehavioral screening battery in rats.
Neurotoxicol. Teratol., 17, 617-625.
Moser, V.C. & Padilla, S. (1998) Age- and gender-related differences
in the time course of behavioral and biochemical effects produced
by oral chlorpyrifos in rats. Toxicol. Appl. Pharmacol., 149,
107-119.
Muscarella, D.E., Keown, J.F. & Bloom, S.E. (1984) Evaluation of the
genotoxic and embryotoxic potential of chlorpyrifos and its
metabolites in vivo and in vitro. Environ. Mutag., 6, 13-23.
Nakatsugawa, T. (1992) Hepatic disposition of organophosphorus
insecticides: A synthesis of in vitro, in situ and in vivo data.
In: Chambers, J.E., Levi, P.E. & Levi, A.P., eds,
Organophosphates, San Diego, Academic Press, Chapter 10.
Newton, P.E. (1988a) A 5-day nose-only inhalation toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
from Bio/Dynamics Inc., East Millstone, NJ, USA. Submitted by
Makteshim Chemical Works, Beer-Sheva, Israel.
Newton, P.E. (1988b) A thirteen week nose-only toxicity study of
chlorpyrifos technical (Pyrinex) in the rat. Unpublished report
No. R-4750 from Bio/dynamics Inc., East Millstone, NJ, USA.
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984a) Pyrinex tech: Acute oral toxicity in
the rat. Unpublished report No. MAK/056/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nissimov, S. & Nyska, A. (1984b) Pyrinex tech: Acute dermal toxicity
in rabbits. Unpublished report No. MAK/059/PYR from Life Science
Research Israel Inc., Ness Ziona, Israel. Submitted to WHO by
Makteshim Chemical Works, Beer-Sheva, Israel.
Nolan, R.J. (1997) Proposed reference dose for acute and chronic
exposure to chlorpyrifos based on the criteria described by the
Acute Cholinesterase Risk Assessment Task Force and the available
animal and human data. Unpublished report No. K-044793-110 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1982)
Chlorpyrifos: pharmacokinetics in human volunteers following
single oral and dermal doses. Unpublished report No.
HEB-DR-0043-4946-4 from Dow Chemical Co., Midland, Michigan, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Rick, D.L., Freshour, N.L. & Saunders, J.H. (1984)
Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol.
Appl. Pharmacol., 73, 8-15.
Nolan, R.J., Streeter, C.M. & Kastl, P.E. (1986) Chlorpyrifos:
Absorption by female Fischer 344 rats exposed for 6 hours to
vapours of chlorpyrifos in a nose-only or a whole-body inhalation
chamber. Unpublished report No. HET K-044793-82 from Dow Chemical
Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Nolan, R.J., Dryzga, M.D., Landenberger, B.D. & Kastl, P.E. (1987)
Chlorpyrifos: Tissue distribution and metabolism of orally
administered 14C labelled chlorpyrifos in Fischer 344.
Unpublished report No. HET K-044793-(76) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Nostrandt, A.C., Padilla, S. & Moser, V.C. (1997) The relationship of
oral chlorpyrifos effects on behaviour, cholinesterase
inhibition, and muscarinic receptor density in rat.
Pharmacol. Biochem. Behav., 58, 15-23.
Omenn, G.S. (1987) The role of genetic differences in human
susceptibility to pesticides. In: Costa, L.G., Galli, C.L. &
Murphy, S.D., eds, Toxicology of Pesticides: Experimental,
Clinical and Regulatory Perspectives, Berlin, Springer-Verlag.
Osterloh, J., Lotti, M. & Pond, S.M. (1983) Toxicologic studies in a
fatal overdose of 2,4-D, MCPP, and chlorpyrifos. J. Anal.
Toxicol., 7, 125-129.
Ouelette, J.H., Dittenber, D.A., Kociba, R.J. & John, J.A. (1983a)
Chlorpyrifos: Oral teratology probe study in rats. Unpublished
report No. HET K-44793-(46) from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Ouelette, J.H., Dittenber, D.A., Kloes, P.M. & John, J.A. (1983b)
Chlorpyrifos: Oral teratology study in Fischer 344 rats.
Unpublished report No. HET K-44793-(47) from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Pennington, J.Y. & Edwards, N.H. (1971) Comparison of cholinesterase
depression in humans and rabbits following exposure to
chlorpyrifos. Unpublished report no. TA-477 from Dow Chemical
Company USA. Submitted to WHO by Dow Agrosciences, Indianapolis,
Indiana, USA.
Phillips, J.E. & Lomax, L.G. (1989) 1% aqueous dilution of Dursban
acute aerosol inhalation toxicity study in Fischer 344 rats.
Unpublished report No. M-004803-004A from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Poole, D.C., Simmon, V.F. & Newell, C.W. (1977). In vitro mutagenic
activity of fourteen pesticides. Toxicol. Appl. Pharmacol., 41,
196.
Pope, C.N. & Chakraborti, T.K. (1992) Dose related inhibition of brain
and plasma cholinesterase in neonatal and adult rats following
sub-lethal organophosphate exposure. Toxicology, 73, 35-43.
Pope, C.N., Chakraborti, T.K., Chapman, M.L., Farrar, J.D. & Arthun,
D. (1991) Comparison of in vivo cholinesterase inhibition in
neonatal and adult rats by three organophosphorothioate
insecticides. Toxicology, 68, 51-61.
Pope, C.N., Chakraborti, T.K., Chapman, M.L. & Farrar, J.D. (1992)
Long-term neurochemical and behavioral effects induced by acute
chlorpyrifos treatment. Pharmacol. Biochem. Behav., 42,
251-256.
Richardson, R.J., Moore, T.B., Kayyali, U.S. & Randall, J.C. (1993a)
Chlorpyrifos: Assessment of potential for delayed neurotoxicity
by repeated dosing in adult hens with monitoring of brain
acetylcholinesterase, brain and lymphocyte neurotoxic esterase
and plasma butyrylcholinesterase activities. Fundam. Appl.
Toxicol., 21, 89-96.
Richardson, R.J., Moore, T.B., Kayyali, U.S., Fowke, J.H. & Randall,
J.C. (1993b) Inhibition of hen brain acetylcholinesterase and
neurotoxic esterase by chlorpyrifos in vivo and kinetics of
inhibition by chlorpyrifos oxon in vitro: Application to
assessment of neuropathic risk. Fundam. Appl. Toxicol., 20,
273-279.
Roberts, N.L., Phillips, C.N.K., Gopinath, C., Begg, S., Anderson, A.
& Dawe, I.S. (1987) Acute delayed neurotoxicity study with
chlorpyrifos in the domestic hen. Unpublished report No. MBS
16/87764 from Huntingdon Research Centre Ltd, Huntingdon, United
Kingdom. Submitted to WHO by Makteshim Chemical Works,
Beer-Sheva, Israel.
Ross, D.B. & Roberts, N.L. (1974) The acute oral toxicity (LD50) of
Dursban (DOWCO 179) to the chicken (DWC/234/74877). Unpublished
report from Huntingdon Research Centre, Huntingdon, United
Kingdom. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Rowe, L.D., Warner, S.D. & Johnston, R.V. (1978) Acute delayed
neurotoxicologic evaluation of chlorpyrifos in white Leghorn
hens. Unpublished report No. A1A-715 from Dow Chemical Co., USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Rubin, Y.,. Gal, N., Waner, T. & Nyska, A. (1987a) Pyrinex.
Teratogenicity study in the rat. Unpublished report No.
MAK/101/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Rubin, Y., Nyska, A. & Waner, T. (1987b) Pyrinex. Pyrinex
teratogenicity study in the rabbit. Unpublished report No.
MAK/103/PYR from Life Science Research Israel Ltd, Ness Ziona,
Israel. Submitted to WHO by Makteshim Chemical Works, Beer-Sheva,
Israel.
Scortichini, B.H., Lomax, L.G., Wolfe, E.L. & Hanley, T.R., Jr (1986a)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in
Fischer 344 rats. Unpublished report No. HETK-038278-006 from Dow
Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Scortichini, B.H., Lomax, L.G., Wolf, E.L. & Hanley, T.R., Jr (1986b)
3,5,6-Trichloro-2pyridinol: Oral teratology probe study in New
Zealand white rabbits. Unpublished report No. HET-K-038278-007
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Shankar, M.R., Bond, D.M. & Crissman, J.W. (1993) Chlorpyrifos:
13-week neurotoxicity study in Fischer 344 rats. Unpublished
report No. K-044793-094 from Dow Chemical Co., USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Sharp, L.D. & Warner, S.D. (1968) The clinical toxicity of Dursban in
the dog after multiple applications of an aerosol formulation.
Unpublished report from Dow Chemical Co., USA. Submitted to WHO
by Dow Agrosciences, Indianapolis, Indiana, USA.
Shemesh, I., Bourvin, A., Gold, D. & Kutscherowsky, M. (1988)
Chlorpyrifos poisoning treated with ipratropium and dantrolene: a
case report. J. Toxicol. Clin. Toxicol., 26, 495-498.
Sherman, J.D. (1996) Chlorpyrifos (Dursban)-associated birth defects:
Report of four cases. Arch. Environ. Health, 51, 5-8.
Sherman, J.D. & Herrick, R.B. (1973) Fly control and chronic toxicity
from feeding Dursban (O,O-diethyl O-3,5,6-trichloro-2-pyridyl
phosphorothioate) to laying hens. Poultry Sci., 52, 741-747.
Sherman, J.D., Herrick, R.B., Ross, E. & Chang, M.T.Y. (1967) Further
studies on the acute and sub-acute toxicity of insecticides to
chicks. Toxicol. Appl. Pharmacol., 11, 49-67.
Shirasu, Y., Moriya, M. & Ohta, T. (1980) Mutagenicity test of
chlorpyrifos in bacteria. Unpublished report No. GHF-R-47 from
Dow Chemical Pacific, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Shurdut, B.A., Chen, W.L., Burns, C.J., McCormick, R.A., Nolan, R.J. &
Racke, K.D. (1997) Critical assessment of report entitled 'Review
of chlorpyrifos poisoning data' (by J. Blondell and V. Dobozy,
January 14, 1997). Unpublished report No. GH-C 4359, by Dow
Chemical Co., USA. Submitted to WHO by Dow Agrosciences,
Indianapolis, Indiana, USA.
de Silva, H.J., Sanmuganathan, P.S. & Senanayake, N. (1994) Isolated
bilateral recurrent laryngeal nerve paralysis: A delayed
complication of organophosphorus poisoning. Hum. Exp.
Toxicol., 13, 171-173.
Smith, G.N., Watson, B.S. & Fischer, F.S. (1967) Investigations on
Dursban insecticide. Metabolism of [36Cl] O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothiate in rats. Agric.
Food Chem., 15, 132-138.
Smolen, A., Eckerson, H.W., Gan, K.N., Hailat, N. & LaDu, B.N. (1991)
Characteristics of the genetically determined allozymic forms of
human paraoxonase/artylesterase. Drug Metab. Disposition, 19,
107-112
Sobti, R.C., Krishan, A. & Pfaffenberger, C.D. (1982) Cytokinetic and
cytogenetic effects of some agricultural chemicals on human
lymphoid cells in vitro: Organophosphates. Mutat. Res., 102,
89-102.
Song, X., Seidler, F.J., Saleh, J.L., Zhang, J., Padilla, S. &
Slotkin, T.A. (1997) Cellular mechanisms for developmental
toxicity of chlorpyrifos: Targeting the adenylyl cyclase
signaling cascade. Toxicol. Appl. Pharmacol., 145, 158-174.
Stevenson, G.T. (1963) An LD50 toxicity study of Dursban in Leghorn
chickens. Unpublished report from Dow Chemical Co., Midland,
Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1966a) A single oral dose LD50 toxicity study of
Dursban in broiler type chickens. Unpublished report No. A1A-364
from Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Stevenson, G.T. (1966b) A neurotoxicity study of Dursban in laying
hens. Unpublished report No. A1A-390 from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Stevenson, G.T. (1967) A single oral dose LD50 toxicity study of
Dursban in turkey/poultry. Unpublished report No. A1A-362 from
Dow Chemical Co., Midland, Michigan, USA. Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Streeter, C.M., Lomax, L.G., Landry, T.D. & Dittenber, D.A. (1987)
Chlorpyrifos: 2-week whole-body vapor inhalation toxicity study
in Fischer 344 rats. Unpublished report No. HET K 044793-078 from
Dow Chemical Co., Midland, Michigan, USA Submitted to WHO by Dow
AgroSciences, Indianapolis, Indiana, USA.
Sultatos, L.G. & Murphy, S.D. (1983) Hepatic microsomal detoxification
of the organophosphates paraoxon and chlorpyrifos oxon in the
mouse. Drug Metab. Disposition, 11, 232-238.
Szabo, J.R., Young, J.T. & Granjean, M. (1988) Chlorpyrifos: 13-week
dietary toxicity study in Fisher 344 rats. Unpublished report No.
TexasT: K-044793-071 from Lake Jackson Research Center, Health
and Environmental Sciences, Freeport, Texas, USA. Submitted to
WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Taylor, M.L. & Olson, K.J. (1963) Toxicological properties of
O,O-diethyl-O-3,5,6trichloro-2-pyridyl phosphorothioate.
Unpublished report. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Thompson, D.J., Gerbig, C.C. & Warner, S.D. (1971) Three generation
reproduction and teratology study in the rat following prolonged
dietary exposure to Dursban O,O-diethyl
O-3,5,6-trichloro-2-pyridyl phosphorothioate. Unpublished report
from Dow Chemical Co., USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Tu, A.S. (1987) CHO/HGPRT in vitro mammalian cell mutation assay on
Pyrinex (chlorpyrifos). Unpublished report No. 59487 from Arthur
D. Little Inc., Cambridge, Massachusetts, USA. Submitted to WHO
by Makteshim Chemical Works, Beer-Sheva, Israel.
Warner, S.D., Gerbig, C.G., Strebing, R.J. & Molello, J.A. (1980)
Results of a two-year toxicity and oncogenic study of
chlorpyrifos administered to CD-1 mice in the diet. Unpublished
report from Dow Chemical Co., Indianapolis, Indiana, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
Whitney, K.D., Seidler, F.J. & Slotkin, T.A. (1995) Developmental
neurotoxicity of chlorpyrifos: Cellular mechanisms. Toxicol.
Appl. Pharmacol., 134, 53-62.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Wilmer, J.W. (1992) Acute neurotoxicity study in Fischer 344 rats.
Unpublished report No. K-044793-093B from Dow Chemical Co.,
Midland, Michigan, USA. Submitted to WHO by Dow AgroSciences,
Indianapolis, Indiana, USA.
Wilson, J.A. & MacBeth, D. (1994) Chlorpyrifos tech: Acute oral
toxicity (LD50) test in rats. Unpublished report No. 10521 from
Inveresk Research International, Tranent, Scotland. Submitted to
WHO by Luxembourg Industries, Tel Aviv, Israel.
Wolcott, R.M., Vaughn, W.K. & Neal, R.A. (1972) Comparison of the
mixed function oxidase-catalyzed metabolism of a series of
dialkyl p-nitrophenyl phosphorothionates. Toxicol. Appl.
Pharmacol., 22, 213-220.
Yang, R.S.H., Hodgson, E. & Dauterman, W.C. (1971) Metabolism in vitro
of diazinon and diazoxon in rat liver. J. Agric. Food Chem.,
19, 10-13.
Young, J.T. & Grandjean, M. (1988) Chlorpyrifos: 2 year dietary
chronic toxicity-oncogenicity study in Fischer 344 rats.
Unpublished report No. K-044793-079 from Lake Jackson Research
Center, Dow Chemical Co., Freeport, Texas, USA. Submitted to WHO
by Dow AgroSciences, Indianapolis, Indiana, USA.
Zempel, J.A. & Bruce, R.J. (1986) 3,5,6-Trichloro-2-pyridinol:
Evaluation in the Ames Salmonella/mammalian-microsome
mutagenicity assay. Unpublished report No. TexasT:K038278-010
from Lake Jackson Research Center, Dow Chemical Co., Freeport,
Texas, USA. Submitted to WHO by Dow AgroSciences, Indianapolis,
Indiana, USA.
Zempel, J.A., Rachunek, B.L. & Szabo, J.R. (1987)
3,5,6-Trichloro-2-pyridinol: Results of a one-year dietary study
in male and female beagle dogs. Unpublished report No.
TexasT:K038278-009 from Dow Chemical Co., Freeport, Texas, USA.
Submitted to WHO by Dow AgroSciences, Indianapolis, Indiana, USA.
