
BENOMYL JMPR 1973
IDENTITY
Chemical names
Methyl 1-(butylcarbamoyl)benzimidazol-2-ylcarbamate
Methyl 1-(butylcarbamoyl)-2-benzimidazole carbamate
1-(butylcarbamoyl)-2-benzimidazole carbamic acid methyl ester
Synonyms
Benlate (R), Du Pont Fungicide 1991
Structural formula
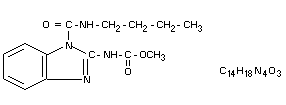 Other information on identity and properties
Molecular weight: 290.3
State: White crystalline solid
Melting point: Decomposition before melting
Solubility: Water: practically insoluble
Oil: practically insoluble
Chloroform: 9.4 g/100 ml
Dimethylformamide: 5.3 g/100 ml
Acetone: 1.8 g/100 ml
Xylene: 1.0 g/100 ml
Ethanol: 0.4 g/100 ml
Heptane: 0.04 g/100 ml
Purity: Technical benomyl better than
98% (w/w)
Chemical stability: Benomyl is easily hydrolyzed to
(see Fig. 1) methyl 2-benzimidazole carbamate
(MBC) in very dilute aqueous, and
in acidified methanolic solutions.
MBC in turn hydrolyzes under basic
conditions to give 2-aminobenzimidazole
(2-AB)
By direct alkaline treatment of benomyl
a triazine ring-closure takes place,
forming 3-butyl-S-triazino [1.2 a]
benzimidazole-2.4(1H, 3H)dione (STB)
which is not stable in hot alkali. It is
further converted to the stable
2-(3-butylureido)-benzimidazole (BUB)
(Ogawa et al., 1971; White et al.,
1973). By exclusion of moisture
formulated benomyl products are stable.
Use pattern
Benomyl is, since 1970, registered as a systemic fungicide in a
great number of countries, including the United States of America. It
is marketed mostly as a 50% wettable powder. Similar to other
benzimidazole fungicides it is active against a broad spectrum of
fungi, among which are Ascomycetes, Basidiomycetes and some
Deuteromycetes, while it is found completely inactive against the
Phycomycetes fungi (Edgington et al., 1971; Bollen, 1972). Among the
well-controlled fungal diseases are powdery mildew, apple scab
(Venturia inaequalis) and the grey mould fungus Botrytis cinerea
(Evans, 1971).
The major uses of benomyl for the control of fungal diseases on
fruits, vegetables and ornamenthals are preharvest applications which
usually involve one or more sprayings with from about 20 g to about 20
g a.i. per 100 litres (Table 1). Post-harvest treatments, by either
dipping or dusting, within the same concentration range are approved
for the protection of fruits, seeds and vegetables in storage. The
United States Environmental Protection Agency has registered
post-harvest uses of benomyl for apricots, cherries, citrus,
nectarines, peaches, plums and prunes.
Residues resulting from supervised trials
The levels of benomyl residues found in various crops in the
United States of America and other countries are summarized in Table 1
(Anonymous, 1972, 1973). The data are based on information supplied to
the United States Environmental Protection Agency to support the
establishment of United States tolerances, indicating the residues
resulting from approved use patterns, Residue levels are given as the
range of values observed in those crops showing benomyl residues
(incl. MBC).
Other information on identity and properties
Molecular weight: 290.3
State: White crystalline solid
Melting point: Decomposition before melting
Solubility: Water: practically insoluble
Oil: practically insoluble
Chloroform: 9.4 g/100 ml
Dimethylformamide: 5.3 g/100 ml
Acetone: 1.8 g/100 ml
Xylene: 1.0 g/100 ml
Ethanol: 0.4 g/100 ml
Heptane: 0.04 g/100 ml
Purity: Technical benomyl better than
98% (w/w)
Chemical stability: Benomyl is easily hydrolyzed to
(see Fig. 1) methyl 2-benzimidazole carbamate
(MBC) in very dilute aqueous, and
in acidified methanolic solutions.
MBC in turn hydrolyzes under basic
conditions to give 2-aminobenzimidazole
(2-AB)
By direct alkaline treatment of benomyl
a triazine ring-closure takes place,
forming 3-butyl-S-triazino [1.2 a]
benzimidazole-2.4(1H, 3H)dione (STB)
which is not stable in hot alkali. It is
further converted to the stable
2-(3-butylureido)-benzimidazole (BUB)
(Ogawa et al., 1971; White et al.,
1973). By exclusion of moisture
formulated benomyl products are stable.
Use pattern
Benomyl is, since 1970, registered as a systemic fungicide in a
great number of countries, including the United States of America. It
is marketed mostly as a 50% wettable powder. Similar to other
benzimidazole fungicides it is active against a broad spectrum of
fungi, among which are Ascomycetes, Basidiomycetes and some
Deuteromycetes, while it is found completely inactive against the
Phycomycetes fungi (Edgington et al., 1971; Bollen, 1972). Among the
well-controlled fungal diseases are powdery mildew, apple scab
(Venturia inaequalis) and the grey mould fungus Botrytis cinerea
(Evans, 1971).
The major uses of benomyl for the control of fungal diseases on
fruits, vegetables and ornamenthals are preharvest applications which
usually involve one or more sprayings with from about 20 g to about 20
g a.i. per 100 litres (Table 1). Post-harvest treatments, by either
dipping or dusting, within the same concentration range are approved
for the protection of fruits, seeds and vegetables in storage. The
United States Environmental Protection Agency has registered
post-harvest uses of benomyl for apricots, cherries, citrus,
nectarines, peaches, plums and prunes.
Residues resulting from supervised trials
The levels of benomyl residues found in various crops in the
United States of America and other countries are summarized in Table 1
(Anonymous, 1972, 1973). The data are based on information supplied to
the United States Environmental Protection Agency to support the
establishment of United States tolerances, indicating the residues
resulting from approved use patterns, Residue levels are given as the
range of values observed in those crops showing benomyl residues
(incl. MBC).
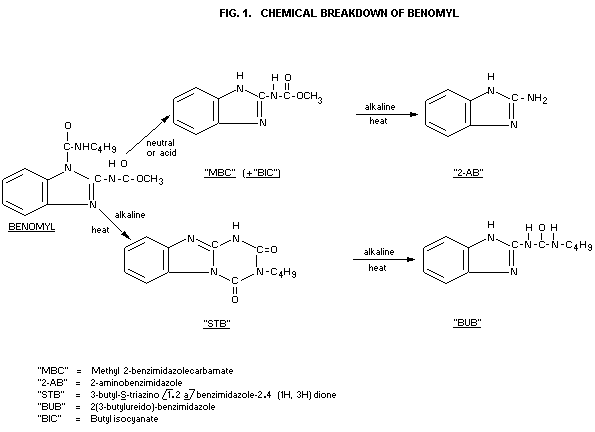 TABLE 1. RESIDUES OF BENOMYL IN VARIOUS CROPS
(Supervised trials)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Apricots 0.3-0.6 g/l 2-3 2.6-6.5
Dip
post-harvest 0.9-5.1
Almonds
nutmeats 1-2 kg/ha 1-3 <0.1
hulls 1-2 kg/ha 1-3 0.2-0.74
Apples 0.4-1.2 g/l 6-12 0.5-4.8
Dip
post - harvest 0.12-0.88
Avocados 1-2 kg/ha 3-6 0.11-0.18
Bananas 0.2-0.3 kg/ha 6-9 Pulp <0.1
0.3-0.6 g/l Dip 0.15-0.43
post-harvest
Barley 0.5 kg/ha 2 Straw 0.54
1.53 (53
days)
Grain 0.1
Beans dry 1.5-2 kg/ha 2-3 0.1-0.73
lim 1.5-2 kg/ha 2-3 0.5-1.8
snap 1.5-2 kg/ha 1-2 0.11-0.94
Bean vines 1.5-2 g/ha 2-3 0.2-4.6
Blackcurrant 0.45 kg/ha 1 4.0-5.1
(0 day)
1.7-2.4
(7 day)
1.3-2.4
(14 day)
Caneberries 0.5-2 kg/ha 2-5 0.75-6.0
Celery 0.2-0.5 kg/ha 4-11 0.1-2.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Cherries 0.3-0.6 g/l 2-3 0.2-12.6
0.3 g/l Dip 0.5-2.3
post-harvest
Citrus (whole 0.6-1.1 g/l 1-5 0.2-1.3
fruit) 500 ppm Dip 0.54-1.26
post-harvest
1000 ppm Dip 0.39-2.35
post-harvest
1250 ppm Dip 1.3-1.8
post-harvest
2500 ppm Dip 2.7-3.9
post-harvest
5000 ppm Dip 4.5-5.4
post-harvest
Cucumbers 0.2-0.5 kg/ha 2-6 0.13-0.55
Grapes 1-1.5 kg/ha 3-5 0.14-10.3
Macadamia nuts 2-2.5 kg/ha 4-10 <0.1
Mangoes 1-2 kg/ha 5-17 0.15-3.0
Melons 0.2-0.4 kg/ha 2-7 0.27-0.32
Mushroom 0.25 g/m2 1 0.31-0.57
0.5 g/m2 (peat
treatment) 1 0.23-0.51
1.0 g/m2 (peat
treatment) 1 0.88-1.5
Nectarines 0.5 kg/ha 1-3 1.6-2.2
Peaches 0.2-0.6 g/l 1-15 0.2-8.2
0.3 g/l Dip 3.9-4.7
post-harvest
Peanuts 0.2-0.5 kg/ha 2-13 <0.1
Peanuts hay 0.4-1 kg/ha 3-13 1.4-1.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Pears 0.6-1.2 g/l 3-5 1.7-3.2
Dip
post-harvest 0.1-0.55
Pecans 1-1.25 kg/ha 3-6 <0.1
Prunes, plums 0.3-0.6 g/l 2-4 0.4-1.4
0.3 g/l Dip 0.5-1.9
post-harvest
Potato 50 g/100 kg Tuber <0.1
dressing
Squash 0.4-0.8 kg/ha 2-5 0.10-0.50
Strawberries 0.3-0.6 g/l 3-7 0.43-2.6
0.3-0.6 g/l 6-7 2.7-15.2
(Under glass) (0.day)
0.3-0.6 g/l (Under glass) 1.5-2.6
(17.day)
Sugarbeet
roots 0.2-0.5 kg/ha 3-6 <0.1
tops 0.4-1 kg/ha 1-6 0.15-4.7
Tomato 0.3 g/plant 1 1.1-2.2
0.3-0.7 kg/ha 3 0.15-2.1
(Under glass)
Application Benomyl
a Total residue comprising benomyl, methyl benzimidazolecarbamate
and 2-aminobenzimidazole, expressed as benomyl.
(Nominal blank values either 0.1 or 0.2 ppm, apart from citrus,
strawberries, peanut hay, barley straw and lettuce (0.3-0.4 ppm).)
Fate of residues
General remarks on mode of action
As suggested by Clemons and Sisler (1969) (Kilgore and White,
1970; Helweg, 1973) and others, it is generally accepted that methyl
2-benzimidazole carbamate (MBC), which is the major metabolite of
benomyl, is primarily responsible for the fungitoxicity of the latter.
Comparisons between the effects of benomyl and MBC (Hammerschlag and
Sisler, 1973) indicated that both compounds caused an inhibition in
the synthesis of DNA, but that this was secondary to a mitotic arrest
of fungal cell division, followed by an inhibition of cytokinesis.
Benomyl partially inhibits respiration of subcellular particles
from fungal cells, while MBC had essentially no such effect. This
differential effect of benomyl and MBC on metabolism is attributed to
the formation, from benomyl, of volatile butyl isocyanate (BIC),
simultaneously to MBC production. Fungitoxicity of benomyl
preparations may thus be attributed to the combined effects of its two
first breakdown products, MBC and BIC.
Although the fungitoxic effects of benomyl are thus assumed to be
caused by its metabolites, MBC and BIC, much of its systemic functions
may be connected to benomyl per se. Controlled experiments of foliar
treatments on apples, cucumber, banana, orange and grape plants
indicate that from 48% to 77% of the 14C-benomyl remaining 21-23 days
after application under outdoor conditions was still in the form of
intact benomyl (Baude et al., 1973). Several workers have recently
found that plant penetration and movement through the cuticle after
foliar application is greater for benomyl than for MBC (Upham and
Delp, 1973; Hammerschlag and Sisler, 1972, 1973; Solel and Edgington,
1973). Systemic translocation within the plant, on the other hand, is
mostly in the form of MBC plus smaller amounts of 2-AB (Siegel and
Zabbia, 1972).
The two theoretical metabolites, STB and BUB (see Fig. 1), which
are formed chemically under alkaline conditions could not be detected
by Baude et al. (1973) as actual conversion products from benomyl
under greenhouse conditions or in field tests, even when subjected to
the influence of two alkaline pesticides, basic copper sulfate and
lime sulfur.
Plants
Under practical conditions a considerable part of the applied
benomyl is claimed to remain on the foliar surfaces, as relatively
stable residues, only gradually degrading to form MBC (Baude et al.,
1973).
Studies of the systemic uptake of benomyl after foliar treatments
indicate that while the total amount which penetrates the surface is
relatively small, residues can still be traced in untreated portions
of the plant (Lowen, 1973). The translocated residues consist mainly
of MBC together with smaller amounts of 2-AB. To the plants these
compounds move only passively, i.e. they are carried by the
intercellular transpiration stream towards the edges and tips of
leaves (Foldo, 1973), where they then accumulate.
Benomyl is readily absorbed through the root-system of plants.
The uptake in vegetable crops, such as lettuce and cucumber is clearly
demonstrated through greenhouse trials (see Table 2) in which normal
and excessive rates were applied through watering or through admixing
benomyl with the soil (Green-Lauridsen and Voldum-Clausen, 1973).
Citrus
The total 14C-benomyl residue (benomyl + MBC) was found to
decrease slowly in oranges during two months storage after
post-harvest dipping. While the major part of the residue remained in
the peel, a smaller, but significant amount gradually penetrated into
the edible portion (see Table 3). Two weeks after the dipping 61% of
the surface residue was present as unchanged benomyl, the remaining
39% being MBC (Lowen, 1973).
Grapes and wine
Lemperle et al. (1973) found that benomyl residues, as well as
other benzimidazole fungicides, are transferred almost quantitatively
into unclarified musts during wine production. During clarification
and fermentation a small amount may disappear but a considerable part
of the benomyl residue present in grapes may be found in wine.
Animals and animal products
Benomyl is readily hydrolyzed into MBC through cleavage of the
1-butyl carbamoyl side chain when ingested by mice, rabbits and sheep
(Douch, 1973). Further hydrolysis of the carbamate ester bond was
shown by these studies to yield 2-AB suggesting that benomyl may not
remain in the unchanged form more than momentarily when residues are
ingested into the alimentary tract of animals. Hydroxylated
derivatives of MBC and 2-AB were identified by these studies in urine
and faeces of all three species.
After feeding rats with benomyl Gardiner et al, (1968) identified
methyl 5-hydroxy-2-benzimidazole carbamate as the major metabolite
which was liberated in urine by enzyme hydrolysis of glucuronide
and/or sulfate conjugates. MBC is assumed to be the possible
intermediate metabolite for this conversion as well as for the
formation of a minor isomeric metabolite, methyl
4-hydroxy-2-benzimidazole carbamate, which was found in milk and meat
in later studies (Gardiner et al., 1972) when feeding cows with
rations containing 2, 10 and 50 ppm benomyl for 32 days (see Table 4).
Neither intact benomyl nor MBC was detected in these studies.
TABLE 2. BENOMYL RESIDUES ABSORBED THROUGH THE ROOTS UNDER GREENHOUSE CONDITIONS
Sampling after Sampling after
Treatment Application 0-14 days 15-23 days Remarks
rate
Range Average Range Average
Summer lettuce
Watering 1.5-3.8 g/10 m2 5.21-17.8 11.3 n.d.-3.09 1.38 Practical use rate
Watering 7.5-15 g/10 m2 4.33-39.7 15.8 0.49-5.01 2.63 Excessive rates
Watering 22.5 g/10 m2 17.7-29.3 23.0
Winter lettuce
Watering 1.5-3.8 g/10 m2 11.7-18.6 15.3 7.08-14.7 10.5 Practical use rate
Watering 7.5-15 g/10 m2 20.4-60.6 36.8 6.74-27.1 15.9 Excessive rates
Watering 22.5 g/10 m2 56.5-97.1 74.5 28.2-32.1 30.2
Cucumber
Watering 1 g/plant n.d.-0.64 0.27 0.15-0.53 0.37
Soil treatmenta 1 g/plant 0.53-2.02 1.10b
a Composite Soil: 1/3 soil, 1/3 fertilizer, 1/3 peat mulch.
b Sampling after 34-55 days.
TABLE 3. 14C-BENOMYL RESIDUES ON ORANGES FOLLOWING POST-HARVEST
DIPPING (From Lowen, 1973)
ppm benomyl Days Whole
in dip post-treatment Peel Pulp fruit
(calculated)
Untreated - <0.05 <0.05 <0.05
100 0 0.80 <0.05 0.30
31 0.46 <0.05 0.16
62 0.54 <0.05 0.19
500 0 1.5 0.08 0.46
31 1.4 0.12 0.38
62 1.6 0.17 0.50
1 000 0 5.6 <0.05 1.3
31 4.4 0.11 0.95
62 3.0 0.40 0.93
After withdrawing the treated diet for one or two days the
residues of 5-hydroxy-MBC and 4-hydroxy-MBC in milk dropped to below
0.01 ppm.
Water
Benomyl rapidly breaks down on contact with water to give MBC,
conversion being complete within four days (Kilgore and White, 1970;
Peterson and Edgington, 1969).
Soil
Helweg (1973) isolated from soils four strains of bacteria and
two fungi which promoted the breakdown of benomyl to nonfungistatic
compounds. High humus content increased the rate of breakdown of
benomyl. Generally, however, it was concluded that benomyl is
relatively stable as a soil residue under temperate climatic
conditions.
TABLE 4. RESIDUES (PPM) OF METHYL 5-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(5-HYDROXY MBC) AND METHYL 4-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(4-HYDROXY MBC) FOUND IN MEAT AND MILK AFTER FEEDING COWS RATIONS
CONTAINING 2, 10, AND 50 PPM BENOMYL FOR 32 DAYS
(From Gardiner et al., 1972)
Feeding level
2 ppm 10 ppm 50 ppm
Sample
5- 4- 5- 4- 5- 4-
hydroxy hydroxy hydroxy hydroxy hydroxy hydroxy
MBC MBC MBC MBC MBC MBC
Milk <0.01 <0.01 0.01 <0.01 0.05 0.03
Meata <0.05 <0.05 <0.05 <0.05 <0.05
<0.05
a "Meat" represents liver, kidney, fat and muscle.
Studies over two years in experimental fields in Delaware, North
Carolina and Florida (Baude, 1972) showed that the amount of
non-volatile decomposition products of 14C-benomyl remaining in the
soil after one year were 57%, 49% and 57%, respectively, of the
quantity originally applied. After two years in soil in Florida, 27%
of the amount originally present remained. After one month only minor
amounts of unchanged benomyl were present, the principal metabolite
being MBC, with smaller amounts of 2-AB.
Uptake by crops grown in treated soils was studied in the same
experiments by Rhodes (1972) using 14C-benomyl. Trace amounts,
ranging from 0.02 to 0.08 ppm, could be found in tissues of carrots,
tomatoes, corn and beans, while edible snap beans, corn and tomatoes
contained less than the detection limit (i.e. <0.01 ppm). In this
study the benomyl content of the soil at the time of harvest was
0.9 ppm.
Methods of residue analysis
Pease and Gardiner (1969) describe colorimetric and fluorometric
procedures for determining benomyl in a variety of plant and animal
tissues and in soil. The residue is extracted, purified by partition,
converted to 2-aminobenzimidazole, and determined by direct
fluorometric measurement or by colorimetric analysis following
bromination. The procedure thus determines benomyl, its principal
degradation product methyl 2-benzimidazole carbamate, and the minor
residual component 2-aminobenzimidazole as a composite value. With a
sensitivity of 0.1 ppm for 50 g samples, recoveries were about 87% for
both methods. Pease and Holt (1971) improved this method to make it
less time-consuming and to include the analysis of citrus fruits. By
using fluorometric measurement from both acidic and basic media it was
possible to distinguish between benomyl residues and other
benzimidazole fungicides.
Peterson and Edgington (1969) report a method to determine
benomyl and the hydrolysis product, MBC, using a bioautograph
technique. A thin-layer plate is sprayed with a mixture of agar and
Penicillium spores, and the diameter of the zone of inhibited growth
above the fungitoxic spot is related to the amount of active material
in the spot. The sensitivity of the method is 0.1 ppm for a 50 g
sample. Somewhat similar agar plate methods have also been described
by Erwin et al. (1968), Baron (1971) and Holman and Fuchs (1970). A
two-dimensional TLC-technique for separation of benomyl, MBC, 2-AB and
thiophanate as well as thiophanate-methyl is described by von Stryk
(1972).
A UV-spectrophotometric method for determining residues in
grapes, must and wine has been described by Lemperle and Kerner
(1971). After addition of acetone the samples are extracted with
chloroform, the chloroform evaporated to dryness and the residues
measured in methanol at 301.5 nm. The limit of detection reported is
0.3 ppm for 100 g samples and recoveries of 89% for grapes, 75% for
must and 56% for wines. Mestres et al. (1971) used a
spectrophotometric method for fruits and vegetables, measuring the
hydrolysis product, MBC, at 282 nm. The limit of detection was 0.1 ppm
for 25 g samples.
Residues in meat and milk have been determined by the liquid
chromatographic procedure of Kirkland (1973). The method detects
4-hydroxymethyl-2-benzimidazole carbamate and
5-hydroxymethyl-2-benzimidazole carbamate, which are found in animal
systems, as well as benomyl and methyl-2-benzimidazole carbamate.
Kirkland et al. (1973) further adapted this method for the
determination of benomyl and/or MBC and 2-AB in soil and plant
material. Recoveries of the three compounds were 92, 88 and 72%,
respectively. The lower limit of sensitivity of the method is 0.05 ppm
for each of the components.
The methods based on spectrophotofluometry of 2-AB, on
UV-spectophotometry of MBC and the liquid chromatographic procedure
may all be adapted for regulatory purposes.
National tolerances (as notified to the meeting)
Country Commodity Tolerance, ppm
Australia Pome fruits, stone fruits 5
Vegetables, citrus 3
Grapes, mangoes, avocados
strawberries 2
Bananas 1
Peanuts 0.2
Belgium Apples, pears, cucumbers,
strawberries, celery,
small grain 1
Canada Peaches 15
Blackberries, boysenberries,
raspberries 6
Apples, apricots, cherries,
crabapples, grapes,
nectarines, pears, plums,
prunes, strawberries 5
Federal Citrus fruits 10
Republic Grapes 3.0
of Berries 2.0
Germany Vegetables (exc. cucumber), Total
stone fruit, bananas, citrus residue
fruits (without peel) 1.0 calculated
Cereals, cucumbers 0.5 as benomyl
Banana (without peel) 0.2
Other vegetable products 0.1
United Apricots, cherries, nectarines,
States of peaches and plums (including
America fresh prunes) (from post-
harvest and/or pre-harvest
applications), peanut hay,
peanut forage, sugar beet tops 15
Mushrooms 10
Apples, pears, blackberries,
boysenberries, dewberries,
loganberries, raspberries 7
Strawberries 5
Celery, mango 3
Snapbeans, peanut hulls 2
National Tolerances (cont'd)
Country Commodity Tolerance, ppm
Bananas (whole), cucumbers,
melons, summer squash, winter
squash, avocado, almond hulls 1
Banana (pulp), peanuts, sugar
beets (roots), nuts (almonds,
Brazil nuts, bush nuts,
butternuts, cashews, chestnuts,
filberts, hazelnuts, hickory nuts,
macadamia nuts, pecans, walnuts) 0.2
Milk and the meat, fat and meat
by-products of cattle, goats,
hogs, horses and sheep 0.1
Netherlands Fruit and vegetables 2 ) Determined
Raw cereals 0.5 ) and
Citrus fruits 3.5 ) expressed
) as
) MBC
South Fruits and vegetables 3
Africa
Appraisal
Benomyl is a relatively new systemic broad-spectrum fungicide
already established in most countries. It is marketed as wettable
powders and used in foliar applications, seed treatments and
post-harvest dipping procedures on a great number of food and forage
crops.
Benomyl is chemically easily hydrolysed into the relatively
stable methyl-2-benzimidazole carbamate (MBC) which is considered to
be the major fungitoxic principle of the compound. Formulated benomyl
may hydrolyze if not kept dry in storage.
A fungicidal effect additional to the MBC activity is claimed to
be connected with the simultaneous release of volatile butyl
isocyanate. Recent data has been presented suggesting that the
hydrolytic breakdown is of significance for the systemic properties of
benomyl facilitating the cuticular penetration. Evidence is given that
in the case of benomyl residues on foliar surfaces the formation of
MBC proceeds gradually. In a typical example a 50% cleavage of
residues remaining on apple leaves occurs three weeks after
application.
A further metabolic product found in plants is
2-aminobenzimidazole formed from MBC. The amount of this metabolite
found in plant material is, however, usually relatively small; so far
not being found in excess of 1% of total residues. For practical
purposes therefore, residues in plant material following application
of benomyl could be considered as the composite of benomyl, MBC and
2-AB or as the sum of benomyl and MBC alone.
Residue data justifying the establishment of several national
tolerances were available to the meeting, together with limited
information on residues on citrus fruits sampled from trade channels.
Included also is evidence of uptake into crops from treated soils.
Half-life of benomyl (metabolized to MBC) in soils varies from about
four months to one year indicating relatively long persistence with a
possibility of unintentional uptake by following crops.
Cow feeding trials show that hydroxylated methyl benzimidazole
carbamates are formed from benomyl with MBC as the probable
intermediate metabolite.
The most recent published report has confirmed this information
indicating that MBC (and possible 2-AB) are likely to be the primary
chemical entities to be absorbed from the alimentary tract following
the ingestion of benomyl residues.
The presence of hydroxylated MBC was demonstrated in milk and
meat residues of intact benomyl or MBC were detected.
Suitable methods of analysis for benomyl, MBC and 2-AB or for
benomyl and MBC as composite values have been published, both methods
being effective at the 0.1 ppm level. For one of these methods which
is based on spectrophotofluorometric measurement, modifications have
been introduced which allow the determination of benomyl and
metabolites and to differentiate these from other benzimidazole
fungicides such as thiabendazole. Although useful for regulatory
purposes neither of the two methods, however, permits the separate
determination of benomyl and metabolites when these are present
together.
RECOMMENDATIONS
As no acceptable daily intake was established it was not possible
to recommend tolerances. Following officially acceptable use in
various countries residues of benomyl and its metabolites can occur in
the following commodities up to levels indicated. Residues of benomyl
should be measured as the composite of benomyl and its main
metabolite, methyl benzimidazolecarbamate with or without minor
amounts of 2-aminobenzimidazole (usually less than 1% of total
residues), calculated as methyl benzimidazolecarbamate. The levels
indicated are not likely to be exceeded when benomyl is applied in
accordance with good agricultural practice, including either or both
pre-harvest and post-harvest treatments (when applicable). These
levels have been recommended as guideline levels.
For the underlined commodities adjustments have been made to
recommendations in order to accommodate good agricultural practices
for such alternative systemic fungicides of which MBC is also
recognized and identified as the major metabolite and/or chemical
entity (i.e. carbendazim and thiophanatemethyl).
Guideline levels
ppm
Citrus fruit (whole), cherries, grapes, peaches 10
Apples, pears, apricots, tomatoes, blackberries,
boysenberries, dewberries, loganberries, raspberries,
strawberries, blackcurrants 5
Celery, prunes, plums, beans (lima), mangoes,
beans (dry), beans (snap), nectarines 2
Mushroom, banana (whole) 1
cucumbers, melons, squash, brussels sprouts, avocados 0.5
Sugar beet, raw cereals, peanuts, almonds, macadamia 0.5
Nuts, pecans, potatoes 0.1a
Animal feedstuffs
Bean vines 30
Sugar beet leaves 5
Peanut hay, barley straw 2
Almond hulls 1
Meat of cattle and sheep 0.1a
Milk (whole)
a At or about limit of determination.
Residues to be measured as a composite of benomyl, MCB, 2-AB and
expressed as MBC.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Full toxicological data.
Desirable
1. Further development of analytical methods to adapt them
for regulatory purposes, especially to permit separate
determination of benomyl and metabolites when present together.
2. Information on residues in food in commerce.
3. Information on the nature and level of residues in poultry and
eggs following the feeding of benomyl residues in rations.
REFERENCES
Anon. Residues of benomyl in certain foods. E.I. du Pont de
1972, 1973 Nemours & Co., Delaware. Unpublished reports filed
with FAO
Baron, M. Determination, migration and distribution of a
1971 systemic fungicide (benomyl) in the leaves of the
banana plant. Fruits, 26: 643
Baude, F. J. Disappearance of benomyl-2-14C from field soil
1972 in Delaware, North Carolina, and Florida. Paper
submitted by E.I. de Pont de Nemours & Co.,
Delaware (Unpublished)
Baude, F. J., Gardiner, J. A. and Han, J. C.-Y. Characterization
1973 of residues on plants following spray applications
of benomyl. Prepublication of paper submitted to
the J. Agr. Food Chem.
Bollen, G, J. A comparison of the in vitro antifungal spectra
1972 of thiophanates and benomyl. Neth J. Plant. Path.
78: 55
CIVO-TNO Unpublished reports on benomyl residues from Central
1972, 1973 Institute of Food Research (CIVO-TNO) of 11
January 1972 and 9 August 1973
Clemons, G.P. and Sisler, H. D. Formation of a fungitoxic
1969 derivative from Benlate. Phytopathology, 59: 705
Douch, P. G. C. The metabolism of benomyl fungicide in animals.
1973 Xenobiotica, 3: 367
Edgington, L. V., Khew, K.L. and Barron, G.L. Fungitoxic spectrum
1971 of benzimidazole compounds. Phytopathology, 61:
42
Erwin, D. C., Mee, H. and Sims, J. J. The systemic effect of
1968 1-(butylcarbamoyl)-2-benzimidazole carbamic acid,
methyl ester, on verticillium wilt of cotton.
Phytopathology, 58: 528
Evans, E. Systemic fungicides in practice, Pesticide Science,
1971 2: 192
Foldo, N. E. Systemic fungicides. Effect, advantages and
1973 disadvantages (in Danish), Ugeskr. f. agronomer og
hortonomer, 40: 730
Gardiner, J. A., Brantley, R. K. and Sherman, H. Isolation
1968 and identification of a metabolite of methyl
1-(butylcarbamoyl)-2-benzimidazolecarbamate in rat
urine. J. Agr. Food Chem. 16: 1050
Gardiner, J. A., Kirkland, J. J., Pease, H. L., Wall, E. N.
1973 and Morales, R. Unpublished studies. Cited from
Kirkland (1973)
Green-Lauridsen, M. and Voldum-Clausen, K. Reports on
1973 experiments on pesticide residues Nos 285-287,
290-293, 298-300, 302, 306, 308-310 from the
Danish National Food Institute and Government
Plant Pathology Institute, Copenhagen
(Unpublished)
Hammerschlag, R. S. and Sisler, H. D. Differential action
1972 of benomyl and methyl-2-benzimidazolecarbmate
(MBC) in Saccharomyces pastorianus. Pesticide
Biochem. Physiol. 2: 123
Hammerschlag, R. S. and Sisler, H. D. Benomyl and methyl-2
1973 benzimidazole (MBC): biochemical, cytological and
chemical aspects of toxicity to Ustilago
mavdis and Saccharomyces cerevesiae; Pesticide
Biochemistry and Physiology, 3: 42
Helweg, A. Persistence of benomyl in different soil types
1973a and microbial breakdown of the fungicide in soil
and agar culture. Tidskr. for Planteavl
(Copenhagen), 77: 232
Helweg, A. Influence of the fungicide benomyl on micro-organisms
1973b in soil. Tidsskrift for Planteavl (Copenhagen),
77: 375
Holman, A. L. and Fuchs, A. Direct bioautography on TLC as
1970 a method for detecting fungitoxic substances. J.
Chromatography, 51: 327
Kilgore, W. W. and White, E.R. Decomposition of the systemic
1970 fungicide 1991 (Benlate). Bull. Environm. Contam.
& Toxicol. 5: 67
Kirkland, J. Method for high-speed liquid chromatographic
1973 analysis of benomyl and/or metabolite residues in
cow milk, urine, feces and tissues. J. Agric. Food
Chem. 21: 171
Kirkland, J., Holt, R.F. and Pease, H.L. Determination of
1973 benomyl residues in soils and plant tissues by
high-speed cation exchange liquid chromatography.
J. Agr. Food Chem. 21: 368
Lemperle, E. and Kerner, E. UV-spektrophotometrische
1971 Bestimmung von Rücksttänden in Weintrauben,
Traubenmost und Wein nach Anwendung von Du Pont
Benomyl. Z. Anal. Chem. 254: 117
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A.
1973 Wirkstoffrückstände und Gärbeeinflussungen nach
Anwendung von Fungiziden im Weinbau. Deutsche
Leb.-Rdschau, 69: 313
Mestres, R., Tourte, T. and Campo, M. Dosage des residus de
1972 benomyl dans les fruits et les legumes. Ann. Fals.
Exp. Chim. 65: 239
Ogawa, J. A., Bose, E., Manji, B. T., White, E. R. and
1971 Kilgore, W. W. Phytopathology, 70: 905 (Cited
from Baude et al., 1973)
Pease, H. L. and Gardiner, J. A. Fluorometric and colorimetric
1969 procedures for determining residues of benomyl. J.
Agr. Fd. Chem. 17: 267
Pease, H. L. and Holt, R. F. Improved method for determining
1971 benomyl residues. J. Assoc. Off. Anal. Chem. 54:
1399
Peterson, C. A. and Edgington, L. V. Quantitative estimation
1969 of the fungicide benomyl using a bioautograph
technique. J. Agr. Food Chem. 77: 898
Rhodes, R. C. Uptake of 2-14 C-benomyl soil residues by
1972 crops. Paper submitted by du Pont de Nemours &
Co., Delaware (Unpublished)
Siegel, M. R. and Zabbia, A. J., jr Distribution and metabolic
1972 fate of the fungicide benomyl in dwarf pea.
Phytopathology, 62: 630
Solel, Z. and Edgington, L. V. Transcuticular movement of
1973 fungicides. Phytopathology, 63: 505
Upham, P. M. and Delp, C. J. Phytopathology (In press).
1973 (Cited from Baude et al., 1973)
von Stryk, F. G. Separation and determination of some
1972 systemic fungicides and their metabolites by thin-
layer chromatography. J. Chromatogr. 72: 410
White, E. R., Bose, E. A., Ogawa, J. M., Manji, B. T. and
1973 Kilgore, W. W. J. Agr. Food Chem. (in press)
(Cited from Baude et al., 1973)
TABLE 1. RESIDUES OF BENOMYL IN VARIOUS CROPS
(Supervised trials)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Apricots 0.3-0.6 g/l 2-3 2.6-6.5
Dip
post-harvest 0.9-5.1
Almonds
nutmeats 1-2 kg/ha 1-3 <0.1
hulls 1-2 kg/ha 1-3 0.2-0.74
Apples 0.4-1.2 g/l 6-12 0.5-4.8
Dip
post - harvest 0.12-0.88
Avocados 1-2 kg/ha 3-6 0.11-0.18
Bananas 0.2-0.3 kg/ha 6-9 Pulp <0.1
0.3-0.6 g/l Dip 0.15-0.43
post-harvest
Barley 0.5 kg/ha 2 Straw 0.54
1.53 (53
days)
Grain 0.1
Beans dry 1.5-2 kg/ha 2-3 0.1-0.73
lim 1.5-2 kg/ha 2-3 0.5-1.8
snap 1.5-2 kg/ha 1-2 0.11-0.94
Bean vines 1.5-2 g/ha 2-3 0.2-4.6
Blackcurrant 0.45 kg/ha 1 4.0-5.1
(0 day)
1.7-2.4
(7 day)
1.3-2.4
(14 day)
Caneberries 0.5-2 kg/ha 2-5 0.75-6.0
Celery 0.2-0.5 kg/ha 4-11 0.1-2.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Cherries 0.3-0.6 g/l 2-3 0.2-12.6
0.3 g/l Dip 0.5-2.3
post-harvest
Citrus (whole 0.6-1.1 g/l 1-5 0.2-1.3
fruit) 500 ppm Dip 0.54-1.26
post-harvest
1000 ppm Dip 0.39-2.35
post-harvest
1250 ppm Dip 1.3-1.8
post-harvest
2500 ppm Dip 2.7-3.9
post-harvest
5000 ppm Dip 4.5-5.4
post-harvest
Cucumbers 0.2-0.5 kg/ha 2-6 0.13-0.55
Grapes 1-1.5 kg/ha 3-5 0.14-10.3
Macadamia nuts 2-2.5 kg/ha 4-10 <0.1
Mangoes 1-2 kg/ha 5-17 0.15-3.0
Melons 0.2-0.4 kg/ha 2-7 0.27-0.32
Mushroom 0.25 g/m2 1 0.31-0.57
0.5 g/m2 (peat
treatment) 1 0.23-0.51
1.0 g/m2 (peat
treatment) 1 0.88-1.5
Nectarines 0.5 kg/ha 1-3 1.6-2.2
Peaches 0.2-0.6 g/l 1-15 0.2-8.2
0.3 g/l Dip 3.9-4.7
post-harvest
Peanuts 0.2-0.5 kg/ha 2-13 <0.1
Peanuts hay 0.4-1 kg/ha 3-13 1.4-1.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Pears 0.6-1.2 g/l 3-5 1.7-3.2
Dip
post-harvest 0.1-0.55
Pecans 1-1.25 kg/ha 3-6 <0.1
Prunes, plums 0.3-0.6 g/l 2-4 0.4-1.4
0.3 g/l Dip 0.5-1.9
post-harvest
Potato 50 g/100 kg Tuber <0.1
dressing
Squash 0.4-0.8 kg/ha 2-5 0.10-0.50
Strawberries 0.3-0.6 g/l 3-7 0.43-2.6
0.3-0.6 g/l 6-7 2.7-15.2
(Under glass) (0.day)
0.3-0.6 g/l (Under glass) 1.5-2.6
(17.day)
Sugarbeet
roots 0.2-0.5 kg/ha 3-6 <0.1
tops 0.4-1 kg/ha 1-6 0.15-4.7
Tomato 0.3 g/plant 1 1.1-2.2
0.3-0.7 kg/ha 3 0.15-2.1
(Under glass)
Application Benomyl
a Total residue comprising benomyl, methyl benzimidazolecarbamate
and 2-aminobenzimidazole, expressed as benomyl.
(Nominal blank values either 0.1 or 0.2 ppm, apart from citrus,
strawberries, peanut hay, barley straw and lettuce (0.3-0.4 ppm).)
Fate of residues
General remarks on mode of action
As suggested by Clemons and Sisler (1969) (Kilgore and White,
1970; Helweg, 1973) and others, it is generally accepted that methyl
2-benzimidazole carbamate (MBC), which is the major metabolite of
benomyl, is primarily responsible for the fungitoxicity of the latter.
Comparisons between the effects of benomyl and MBC (Hammerschlag and
Sisler, 1973) indicated that both compounds caused an inhibition in
the synthesis of DNA, but that this was secondary to a mitotic arrest
of fungal cell division, followed by an inhibition of cytokinesis.
Benomyl partially inhibits respiration of subcellular particles
from fungal cells, while MBC had essentially no such effect. This
differential effect of benomyl and MBC on metabolism is attributed to
the formation, from benomyl, of volatile butyl isocyanate (BIC),
simultaneously to MBC production. Fungitoxicity of benomyl
preparations may thus be attributed to the combined effects of its two
first breakdown products, MBC and BIC.
Although the fungitoxic effects of benomyl are thus assumed to be
caused by its metabolites, MBC and BIC, much of its systemic functions
may be connected to benomyl per se. Controlled experiments of foliar
treatments on apples, cucumber, banana, orange and grape plants
indicate that from 48% to 77% of the 14C-benomyl remaining 21-23 days
after application under outdoor conditions was still in the form of
intact benomyl (Baude et al., 1973). Several workers have recently
found that plant penetration and movement through the cuticle after
foliar application is greater for benomyl than for MBC (Upham and
Delp, 1973; Hammerschlag and Sisler, 1972, 1973; Solel and Edgington,
1973). Systemic translocation within the plant, on the other hand, is
mostly in the form of MBC plus smaller amounts of 2-AB (Siegel and
Zabbia, 1972).
The two theoretical metabolites, STB and BUB (see Fig. 1), which
are formed chemically under alkaline conditions could not be detected
by Baude et al. (1973) as actual conversion products from benomyl
under greenhouse conditions or in field tests, even when subjected to
the influence of two alkaline pesticides, basic copper sulfate and
lime sulfur.
Plants
Under practical conditions a considerable part of the applied
benomyl is claimed to remain on the foliar surfaces, as relatively
stable residues, only gradually degrading to form MBC (Baude et al.,
1973).
Studies of the systemic uptake of benomyl after foliar treatments
indicate that while the total amount which penetrates the surface is
relatively small, residues can still be traced in untreated portions
of the plant (Lowen, 1973). The translocated residues consist mainly
of MBC together with smaller amounts of 2-AB. To the plants these
compounds move only passively, i.e. they are carried by the
intercellular transpiration stream towards the edges and tips of
leaves (Foldo, 1973), where they then accumulate.
Benomyl is readily absorbed through the root-system of plants.
The uptake in vegetable crops, such as lettuce and cucumber is clearly
demonstrated through greenhouse trials (see Table 2) in which normal
and excessive rates were applied through watering or through admixing
benomyl with the soil (Green-Lauridsen and Voldum-Clausen, 1973).
Citrus
The total 14C-benomyl residue (benomyl + MBC) was found to
decrease slowly in oranges during two months storage after
post-harvest dipping. While the major part of the residue remained in
the peel, a smaller, but significant amount gradually penetrated into
the edible portion (see Table 3). Two weeks after the dipping 61% of
the surface residue was present as unchanged benomyl, the remaining
39% being MBC (Lowen, 1973).
Grapes and wine
Lemperle et al. (1973) found that benomyl residues, as well as
other benzimidazole fungicides, are transferred almost quantitatively
into unclarified musts during wine production. During clarification
and fermentation a small amount may disappear but a considerable part
of the benomyl residue present in grapes may be found in wine.
Animals and animal products
Benomyl is readily hydrolyzed into MBC through cleavage of the
1-butyl carbamoyl side chain when ingested by mice, rabbits and sheep
(Douch, 1973). Further hydrolysis of the carbamate ester bond was
shown by these studies to yield 2-AB suggesting that benomyl may not
remain in the unchanged form more than momentarily when residues are
ingested into the alimentary tract of animals. Hydroxylated
derivatives of MBC and 2-AB were identified by these studies in urine
and faeces of all three species.
After feeding rats with benomyl Gardiner et al, (1968) identified
methyl 5-hydroxy-2-benzimidazole carbamate as the major metabolite
which was liberated in urine by enzyme hydrolysis of glucuronide
and/or sulfate conjugates. MBC is assumed to be the possible
intermediate metabolite for this conversion as well as for the
formation of a minor isomeric metabolite, methyl
4-hydroxy-2-benzimidazole carbamate, which was found in milk and meat
in later studies (Gardiner et al., 1972) when feeding cows with
rations containing 2, 10 and 50 ppm benomyl for 32 days (see Table 4).
Neither intact benomyl nor MBC was detected in these studies.
TABLE 2. BENOMYL RESIDUES ABSORBED THROUGH THE ROOTS UNDER GREENHOUSE CONDITIONS
Sampling after Sampling after
Treatment Application 0-14 days 15-23 days Remarks
rate
Range Average Range Average
Summer lettuce
Watering 1.5-3.8 g/10 m2 5.21-17.8 11.3 n.d.-3.09 1.38 Practical use rate
Watering 7.5-15 g/10 m2 4.33-39.7 15.8 0.49-5.01 2.63 Excessive rates
Watering 22.5 g/10 m2 17.7-29.3 23.0
Winter lettuce
Watering 1.5-3.8 g/10 m2 11.7-18.6 15.3 7.08-14.7 10.5 Practical use rate
Watering 7.5-15 g/10 m2 20.4-60.6 36.8 6.74-27.1 15.9 Excessive rates
Watering 22.5 g/10 m2 56.5-97.1 74.5 28.2-32.1 30.2
Cucumber
Watering 1 g/plant n.d.-0.64 0.27 0.15-0.53 0.37
Soil treatmenta 1 g/plant 0.53-2.02 1.10b
a Composite Soil: 1/3 soil, 1/3 fertilizer, 1/3 peat mulch.
b Sampling after 34-55 days.
TABLE 3. 14C-BENOMYL RESIDUES ON ORANGES FOLLOWING POST-HARVEST
DIPPING (From Lowen, 1973)
ppm benomyl Days Whole
in dip post-treatment Peel Pulp fruit
(calculated)
Untreated - <0.05 <0.05 <0.05
100 0 0.80 <0.05 0.30
31 0.46 <0.05 0.16
62 0.54 <0.05 0.19
500 0 1.5 0.08 0.46
31 1.4 0.12 0.38
62 1.6 0.17 0.50
1 000 0 5.6 <0.05 1.3
31 4.4 0.11 0.95
62 3.0 0.40 0.93
After withdrawing the treated diet for one or two days the
residues of 5-hydroxy-MBC and 4-hydroxy-MBC in milk dropped to below
0.01 ppm.
Water
Benomyl rapidly breaks down on contact with water to give MBC,
conversion being complete within four days (Kilgore and White, 1970;
Peterson and Edgington, 1969).
Soil
Helweg (1973) isolated from soils four strains of bacteria and
two fungi which promoted the breakdown of benomyl to nonfungistatic
compounds. High humus content increased the rate of breakdown of
benomyl. Generally, however, it was concluded that benomyl is
relatively stable as a soil residue under temperate climatic
conditions.
TABLE 4. RESIDUES (PPM) OF METHYL 5-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(5-HYDROXY MBC) AND METHYL 4-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(4-HYDROXY MBC) FOUND IN MEAT AND MILK AFTER FEEDING COWS RATIONS
CONTAINING 2, 10, AND 50 PPM BENOMYL FOR 32 DAYS
(From Gardiner et al., 1972)
Feeding level
2 ppm 10 ppm 50 ppm
Sample
5- 4- 5- 4- 5- 4-
hydroxy hydroxy hydroxy hydroxy hydroxy hydroxy
MBC MBC MBC MBC MBC MBC
Milk <0.01 <0.01 0.01 <0.01 0.05 0.03
Meata <0.05 <0.05 <0.05 <0.05 <0.05
<0.05
a "Meat" represents liver, kidney, fat and muscle.
Studies over two years in experimental fields in Delaware, North
Carolina and Florida (Baude, 1972) showed that the amount of
non-volatile decomposition products of 14C-benomyl remaining in the
soil after one year were 57%, 49% and 57%, respectively, of the
quantity originally applied. After two years in soil in Florida, 27%
of the amount originally present remained. After one month only minor
amounts of unchanged benomyl were present, the principal metabolite
being MBC, with smaller amounts of 2-AB.
Uptake by crops grown in treated soils was studied in the same
experiments by Rhodes (1972) using 14C-benomyl. Trace amounts,
ranging from 0.02 to 0.08 ppm, could be found in tissues of carrots,
tomatoes, corn and beans, while edible snap beans, corn and tomatoes
contained less than the detection limit (i.e. <0.01 ppm). In this
study the benomyl content of the soil at the time of harvest was
0.9 ppm.
Methods of residue analysis
Pease and Gardiner (1969) describe colorimetric and fluorometric
procedures for determining benomyl in a variety of plant and animal
tissues and in soil. The residue is extracted, purified by partition,
converted to 2-aminobenzimidazole, and determined by direct
fluorometric measurement or by colorimetric analysis following
bromination. The procedure thus determines benomyl, its principal
degradation product methyl 2-benzimidazole carbamate, and the minor
residual component 2-aminobenzimidazole as a composite value. With a
sensitivity of 0.1 ppm for 50 g samples, recoveries were about 87% for
both methods. Pease and Holt (1971) improved this method to make it
less time-consuming and to include the analysis of citrus fruits. By
using fluorometric measurement from both acidic and basic media it was
possible to distinguish between benomyl residues and other
benzimidazole fungicides.
Peterson and Edgington (1969) report a method to determine
benomyl and the hydrolysis product, MBC, using a bioautograph
technique. A thin-layer plate is sprayed with a mixture of agar and
Penicillium spores, and the diameter of the zone of inhibited growth
above the fungitoxic spot is related to the amount of active material
in the spot. The sensitivity of the method is 0.1 ppm for a 50 g
sample. Somewhat similar agar plate methods have also been described
by Erwin et al. (1968), Baron (1971) and Holman and Fuchs (1970). A
two-dimensional TLC-technique for separation of benomyl, MBC, 2-AB and
thiophanate as well as thiophanate-methyl is described by von Stryk
(1972).
A UV-spectrophotometric method for determining residues in
grapes, must and wine has been described by Lemperle and Kerner
(1971). After addition of acetone the samples are extracted with
chloroform, the chloroform evaporated to dryness and the residues
measured in methanol at 301.5 nm. The limit of detection reported is
0.3 ppm for 100 g samples and recoveries of 89% for grapes, 75% for
must and 56% for wines. Mestres et al. (1971) used a
spectrophotometric method for fruits and vegetables, measuring the
hydrolysis product, MBC, at 282 nm. The limit of detection was 0.1 ppm
for 25 g samples.
Residues in meat and milk have been determined by the liquid
chromatographic procedure of Kirkland (1973). The method detects
4-hydroxymethyl-2-benzimidazole carbamate and
5-hydroxymethyl-2-benzimidazole carbamate, which are found in animal
systems, as well as benomyl and methyl-2-benzimidazole carbamate.
Kirkland et al. (1973) further adapted this method for the
determination of benomyl and/or MBC and 2-AB in soil and plant
material. Recoveries of the three compounds were 92, 88 and 72%,
respectively. The lower limit of sensitivity of the method is 0.05 ppm
for each of the components.
The methods based on spectrophotofluometry of 2-AB, on
UV-spectophotometry of MBC and the liquid chromatographic procedure
may all be adapted for regulatory purposes.
National tolerances (as notified to the meeting)
Country Commodity Tolerance, ppm
Australia Pome fruits, stone fruits 5
Vegetables, citrus 3
Grapes, mangoes, avocados
strawberries 2
Bananas 1
Peanuts 0.2
Belgium Apples, pears, cucumbers,
strawberries, celery,
small grain 1
Canada Peaches 15
Blackberries, boysenberries,
raspberries 6
Apples, apricots, cherries,
crabapples, grapes,
nectarines, pears, plums,
prunes, strawberries 5
Federal Citrus fruits 10
Republic Grapes 3.0
of Berries 2.0
Germany Vegetables (exc. cucumber), Total
stone fruit, bananas, citrus residue
fruits (without peel) 1.0 calculated
Cereals, cucumbers 0.5 as benomyl
Banana (without peel) 0.2
Other vegetable products 0.1
United Apricots, cherries, nectarines,
States of peaches and plums (including
America fresh prunes) (from post-
harvest and/or pre-harvest
applications), peanut hay,
peanut forage, sugar beet tops 15
Mushrooms 10
Apples, pears, blackberries,
boysenberries, dewberries,
loganberries, raspberries 7
Strawberries 5
Celery, mango 3
Snapbeans, peanut hulls 2
National Tolerances (cont'd)
Country Commodity Tolerance, ppm
Bananas (whole), cucumbers,
melons, summer squash, winter
squash, avocado, almond hulls 1
Banana (pulp), peanuts, sugar
beets (roots), nuts (almonds,
Brazil nuts, bush nuts,
butternuts, cashews, chestnuts,
filberts, hazelnuts, hickory nuts,
macadamia nuts, pecans, walnuts) 0.2
Milk and the meat, fat and meat
by-products of cattle, goats,
hogs, horses and sheep 0.1
Netherlands Fruit and vegetables 2 ) Determined
Raw cereals 0.5 ) and
Citrus fruits 3.5 ) expressed
) as
) MBC
South Fruits and vegetables 3
Africa
Appraisal
Benomyl is a relatively new systemic broad-spectrum fungicide
already established in most countries. It is marketed as wettable
powders and used in foliar applications, seed treatments and
post-harvest dipping procedures on a great number of food and forage
crops.
Benomyl is chemically easily hydrolysed into the relatively
stable methyl-2-benzimidazole carbamate (MBC) which is considered to
be the major fungitoxic principle of the compound. Formulated benomyl
may hydrolyze if not kept dry in storage.
A fungicidal effect additional to the MBC activity is claimed to
be connected with the simultaneous release of volatile butyl
isocyanate. Recent data has been presented suggesting that the
hydrolytic breakdown is of significance for the systemic properties of
benomyl facilitating the cuticular penetration. Evidence is given that
in the case of benomyl residues on foliar surfaces the formation of
MBC proceeds gradually. In a typical example a 50% cleavage of
residues remaining on apple leaves occurs three weeks after
application.
A further metabolic product found in plants is
2-aminobenzimidazole formed from MBC. The amount of this metabolite
found in plant material is, however, usually relatively small; so far
not being found in excess of 1% of total residues. For practical
purposes therefore, residues in plant material following application
of benomyl could be considered as the composite of benomyl, MBC and
2-AB or as the sum of benomyl and MBC alone.
Residue data justifying the establishment of several national
tolerances were available to the meeting, together with limited
information on residues on citrus fruits sampled from trade channels.
Included also is evidence of uptake into crops from treated soils.
Half-life of benomyl (metabolized to MBC) in soils varies from about
four months to one year indicating relatively long persistence with a
possibility of unintentional uptake by following crops.
Cow feeding trials show that hydroxylated methyl benzimidazole
carbamates are formed from benomyl with MBC as the probable
intermediate metabolite.
The most recent published report has confirmed this information
indicating that MBC (and possible 2-AB) are likely to be the primary
chemical entities to be absorbed from the alimentary tract following
the ingestion of benomyl residues.
The presence of hydroxylated MBC was demonstrated in milk and
meat residues of intact benomyl or MBC were detected.
Suitable methods of analysis for benomyl, MBC and 2-AB or for
benomyl and MBC as composite values have been published, both methods
being effective at the 0.1 ppm level. For one of these methods which
is based on spectrophotofluorometric measurement, modifications have
been introduced which allow the determination of benomyl and
metabolites and to differentiate these from other benzimidazole
fungicides such as thiabendazole. Although useful for regulatory
purposes neither of the two methods, however, permits the separate
determination of benomyl and metabolites when these are present
together.
RECOMMENDATIONS
As no acceptable daily intake was established it was not possible
to recommend tolerances. Following officially acceptable use in
various countries residues of benomyl and its metabolites can occur in
the following commodities up to levels indicated. Residues of benomyl
should be measured as the composite of benomyl and its main
metabolite, methyl benzimidazolecarbamate with or without minor
amounts of 2-aminobenzimidazole (usually less than 1% of total
residues), calculated as methyl benzimidazolecarbamate. The levels
indicated are not likely to be exceeded when benomyl is applied in
accordance with good agricultural practice, including either or both
pre-harvest and post-harvest treatments (when applicable). These
levels have been recommended as guideline levels.
For the underlined commodities adjustments have been made to
recommendations in order to accommodate good agricultural practices
for such alternative systemic fungicides of which MBC is also
recognized and identified as the major metabolite and/or chemical
entity (i.e. carbendazim and thiophanatemethyl).
Guideline levels
ppm
Citrus fruit (whole), cherries, grapes, peaches 10
Apples, pears, apricots, tomatoes, blackberries,
boysenberries, dewberries, loganberries, raspberries,
strawberries, blackcurrants 5
Celery, prunes, plums, beans (lima), mangoes,
beans (dry), beans (snap), nectarines 2
Mushroom, banana (whole) 1
cucumbers, melons, squash, brussels sprouts, avocados 0.5
Sugar beet, raw cereals, peanuts, almonds, macadamia 0.5
Nuts, pecans, potatoes 0.1a
Animal feedstuffs
Bean vines 30
Sugar beet leaves 5
Peanut hay, barley straw 2
Almond hulls 1
Meat of cattle and sheep 0.1a
Milk (whole)
a At or about limit of determination.
Residues to be measured as a composite of benomyl, MCB, 2-AB and
expressed as MBC.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Full toxicological data.
Desirable
1. Further development of analytical methods to adapt them
for regulatory purposes, especially to permit separate
determination of benomyl and metabolites when present together.
2. Information on residues in food in commerce.
3. Information on the nature and level of residues in poultry and
eggs following the feeding of benomyl residues in rations.
REFERENCES
Anon. Residues of benomyl in certain foods. E.I. du Pont de
1972, 1973 Nemours & Co., Delaware. Unpublished reports filed
with FAO
Baron, M. Determination, migration and distribution of a
1971 systemic fungicide (benomyl) in the leaves of the
banana plant. Fruits, 26: 643
Baude, F. J. Disappearance of benomyl-2-14C from field soil
1972 in Delaware, North Carolina, and Florida. Paper
submitted by E.I. de Pont de Nemours & Co.,
Delaware (Unpublished)
Baude, F. J., Gardiner, J. A. and Han, J. C.-Y. Characterization
1973 of residues on plants following spray applications
of benomyl. Prepublication of paper submitted to
the J. Agr. Food Chem.
Bollen, G, J. A comparison of the in vitro antifungal spectra
1972 of thiophanates and benomyl. Neth J. Plant. Path.
78: 55
CIVO-TNO Unpublished reports on benomyl residues from Central
1972, 1973 Institute of Food Research (CIVO-TNO) of 11
January 1972 and 9 August 1973
Clemons, G.P. and Sisler, H. D. Formation of a fungitoxic
1969 derivative from Benlate. Phytopathology, 59: 705
Douch, P. G. C. The metabolism of benomyl fungicide in animals.
1973 Xenobiotica, 3: 367
Edgington, L. V., Khew, K.L. and Barron, G.L. Fungitoxic spectrum
1971 of benzimidazole compounds. Phytopathology, 61:
42
Erwin, D. C., Mee, H. and Sims, J. J. The systemic effect of
1968 1-(butylcarbamoyl)-2-benzimidazole carbamic acid,
methyl ester, on verticillium wilt of cotton.
Phytopathology, 58: 528
Evans, E. Systemic fungicides in practice, Pesticide Science,
1971 2: 192
Foldo, N. E. Systemic fungicides. Effect, advantages and
1973 disadvantages (in Danish), Ugeskr. f. agronomer og
hortonomer, 40: 730
Gardiner, J. A., Brantley, R. K. and Sherman, H. Isolation
1968 and identification of a metabolite of methyl
1-(butylcarbamoyl)-2-benzimidazolecarbamate in rat
urine. J. Agr. Food Chem. 16: 1050
Gardiner, J. A., Kirkland, J. J., Pease, H. L., Wall, E. N.
1973 and Morales, R. Unpublished studies. Cited from
Kirkland (1973)
Green-Lauridsen, M. and Voldum-Clausen, K. Reports on
1973 experiments on pesticide residues Nos 285-287,
290-293, 298-300, 302, 306, 308-310 from the
Danish National Food Institute and Government
Plant Pathology Institute, Copenhagen
(Unpublished)
Hammerschlag, R. S. and Sisler, H. D. Differential action
1972 of benomyl and methyl-2-benzimidazolecarbmate
(MBC) in Saccharomyces pastorianus. Pesticide
Biochem. Physiol. 2: 123
Hammerschlag, R. S. and Sisler, H. D. Benomyl and methyl-2
1973 benzimidazole (MBC): biochemical, cytological and
chemical aspects of toxicity to Ustilago
mavdis and Saccharomyces cerevesiae; Pesticide
Biochemistry and Physiology, 3: 42
Helweg, A. Persistence of benomyl in different soil types
1973a and microbial breakdown of the fungicide in soil
and agar culture. Tidskr. for Planteavl
(Copenhagen), 77: 232
Helweg, A. Influence of the fungicide benomyl on micro-organisms
1973b in soil. Tidsskrift for Planteavl (Copenhagen),
77: 375
Holman, A. L. and Fuchs, A. Direct bioautography on TLC as
1970 a method for detecting fungitoxic substances. J.
Chromatography, 51: 327
Kilgore, W. W. and White, E.R. Decomposition of the systemic
1970 fungicide 1991 (Benlate). Bull. Environm. Contam.
& Toxicol. 5: 67
Kirkland, J. Method for high-speed liquid chromatographic
1973 analysis of benomyl and/or metabolite residues in
cow milk, urine, feces and tissues. J. Agric. Food
Chem. 21: 171
Kirkland, J., Holt, R.F. and Pease, H.L. Determination of
1973 benomyl residues in soils and plant tissues by
high-speed cation exchange liquid chromatography.
J. Agr. Food Chem. 21: 368
Lemperle, E. and Kerner, E. UV-spektrophotometrische
1971 Bestimmung von Rücksttänden in Weintrauben,
Traubenmost und Wein nach Anwendung von Du Pont
Benomyl. Z. Anal. Chem. 254: 117
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A.
1973 Wirkstoffrückstände und Gärbeeinflussungen nach
Anwendung von Fungiziden im Weinbau. Deutsche
Leb.-Rdschau, 69: 313
Mestres, R., Tourte, T. and Campo, M. Dosage des residus de
1972 benomyl dans les fruits et les legumes. Ann. Fals.
Exp. Chim. 65: 239
Ogawa, J. A., Bose, E., Manji, B. T., White, E. R. and
1971 Kilgore, W. W. Phytopathology, 70: 905 (Cited
from Baude et al., 1973)
Pease, H. L. and Gardiner, J. A. Fluorometric and colorimetric
1969 procedures for determining residues of benomyl. J.
Agr. Fd. Chem. 17: 267
Pease, H. L. and Holt, R. F. Improved method for determining
1971 benomyl residues. J. Assoc. Off. Anal. Chem. 54:
1399
Peterson, C. A. and Edgington, L. V. Quantitative estimation
1969 of the fungicide benomyl using a bioautograph
technique. J. Agr. Food Chem. 77: 898
Rhodes, R. C. Uptake of 2-14 C-benomyl soil residues by
1972 crops. Paper submitted by du Pont de Nemours &
Co., Delaware (Unpublished)
Siegel, M. R. and Zabbia, A. J., jr Distribution and metabolic
1972 fate of the fungicide benomyl in dwarf pea.
Phytopathology, 62: 630
Solel, Z. and Edgington, L. V. Transcuticular movement of
1973 fungicides. Phytopathology, 63: 505
Upham, P. M. and Delp, C. J. Phytopathology (In press).
1973 (Cited from Baude et al., 1973)
von Stryk, F. G. Separation and determination of some
1972 systemic fungicides and their metabolites by thin-
layer chromatography. J. Chromatogr. 72: 410
White, E. R., Bose, E. A., Ogawa, J. M., Manji, B. T. and
1973 Kilgore, W. W. J. Agr. Food Chem. (in press)
(Cited from Baude et al., 1973)
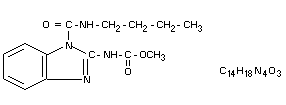 Other information on identity and properties
Molecular weight: 290.3
State: White crystalline solid
Melting point: Decomposition before melting
Solubility: Water: practically insoluble
Oil: practically insoluble
Chloroform: 9.4 g/100 ml
Dimethylformamide: 5.3 g/100 ml
Acetone: 1.8 g/100 ml
Xylene: 1.0 g/100 ml
Ethanol: 0.4 g/100 ml
Heptane: 0.04 g/100 ml
Purity: Technical benomyl better than
98% (w/w)
Chemical stability: Benomyl is easily hydrolyzed to
(see Fig. 1) methyl 2-benzimidazole carbamate
(MBC) in very dilute aqueous, and
in acidified methanolic solutions.
MBC in turn hydrolyzes under basic
conditions to give 2-aminobenzimidazole
(2-AB)
By direct alkaline treatment of benomyl
a triazine ring-closure takes place,
forming 3-butyl-S-triazino [1.2 a]
benzimidazole-2.4(1H, 3H)dione (STB)
which is not stable in hot alkali. It is
further converted to the stable
2-(3-butylureido)-benzimidazole (BUB)
(Ogawa et al., 1971; White et al.,
1973). By exclusion of moisture
formulated benomyl products are stable.
Use pattern
Benomyl is, since 1970, registered as a systemic fungicide in a
great number of countries, including the United States of America. It
is marketed mostly as a 50% wettable powder. Similar to other
benzimidazole fungicides it is active against a broad spectrum of
fungi, among which are Ascomycetes, Basidiomycetes and some
Deuteromycetes, while it is found completely inactive against the
Phycomycetes fungi (Edgington et al., 1971; Bollen, 1972). Among the
well-controlled fungal diseases are powdery mildew, apple scab
(Venturia inaequalis) and the grey mould fungus Botrytis cinerea
(Evans, 1971).
The major uses of benomyl for the control of fungal diseases on
fruits, vegetables and ornamenthals are preharvest applications which
usually involve one or more sprayings with from about 20 g to about 20
g a.i. per 100 litres (Table 1). Post-harvest treatments, by either
dipping or dusting, within the same concentration range are approved
for the protection of fruits, seeds and vegetables in storage. The
United States Environmental Protection Agency has registered
post-harvest uses of benomyl for apricots, cherries, citrus,
nectarines, peaches, plums and prunes.
Residues resulting from supervised trials
The levels of benomyl residues found in various crops in the
United States of America and other countries are summarized in Table 1
(Anonymous, 1972, 1973). The data are based on information supplied to
the United States Environmental Protection Agency to support the
establishment of United States tolerances, indicating the residues
resulting from approved use patterns, Residue levels are given as the
range of values observed in those crops showing benomyl residues
(incl. MBC).
Other information on identity and properties
Molecular weight: 290.3
State: White crystalline solid
Melting point: Decomposition before melting
Solubility: Water: practically insoluble
Oil: practically insoluble
Chloroform: 9.4 g/100 ml
Dimethylformamide: 5.3 g/100 ml
Acetone: 1.8 g/100 ml
Xylene: 1.0 g/100 ml
Ethanol: 0.4 g/100 ml
Heptane: 0.04 g/100 ml
Purity: Technical benomyl better than
98% (w/w)
Chemical stability: Benomyl is easily hydrolyzed to
(see Fig. 1) methyl 2-benzimidazole carbamate
(MBC) in very dilute aqueous, and
in acidified methanolic solutions.
MBC in turn hydrolyzes under basic
conditions to give 2-aminobenzimidazole
(2-AB)
By direct alkaline treatment of benomyl
a triazine ring-closure takes place,
forming 3-butyl-S-triazino [1.2 a]
benzimidazole-2.4(1H, 3H)dione (STB)
which is not stable in hot alkali. It is
further converted to the stable
2-(3-butylureido)-benzimidazole (BUB)
(Ogawa et al., 1971; White et al.,
1973). By exclusion of moisture
formulated benomyl products are stable.
Use pattern
Benomyl is, since 1970, registered as a systemic fungicide in a
great number of countries, including the United States of America. It
is marketed mostly as a 50% wettable powder. Similar to other
benzimidazole fungicides it is active against a broad spectrum of
fungi, among which are Ascomycetes, Basidiomycetes and some
Deuteromycetes, while it is found completely inactive against the
Phycomycetes fungi (Edgington et al., 1971; Bollen, 1972). Among the
well-controlled fungal diseases are powdery mildew, apple scab
(Venturia inaequalis) and the grey mould fungus Botrytis cinerea
(Evans, 1971).
The major uses of benomyl for the control of fungal diseases on
fruits, vegetables and ornamenthals are preharvest applications which
usually involve one or more sprayings with from about 20 g to about 20
g a.i. per 100 litres (Table 1). Post-harvest treatments, by either
dipping or dusting, within the same concentration range are approved
for the protection of fruits, seeds and vegetables in storage. The
United States Environmental Protection Agency has registered
post-harvest uses of benomyl for apricots, cherries, citrus,
nectarines, peaches, plums and prunes.
Residues resulting from supervised trials
The levels of benomyl residues found in various crops in the
United States of America and other countries are summarized in Table 1
(Anonymous, 1972, 1973). The data are based on information supplied to
the United States Environmental Protection Agency to support the
establishment of United States tolerances, indicating the residues
resulting from approved use patterns, Residue levels are given as the
range of values observed in those crops showing benomyl residues
(incl. MBC).
 TABLE 1. RESIDUES OF BENOMYL IN VARIOUS CROPS
(Supervised trials)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Apricots 0.3-0.6 g/l 2-3 2.6-6.5
Dip
post-harvest 0.9-5.1
Almonds
nutmeats 1-2 kg/ha 1-3 <0.1
hulls 1-2 kg/ha 1-3 0.2-0.74
Apples 0.4-1.2 g/l 6-12 0.5-4.8
Dip
post - harvest 0.12-0.88
Avocados 1-2 kg/ha 3-6 0.11-0.18
Bananas 0.2-0.3 kg/ha 6-9 Pulp <0.1
0.3-0.6 g/l Dip 0.15-0.43
post-harvest
Barley 0.5 kg/ha 2 Straw 0.54
1.53 (53
days)
Grain 0.1
Beans dry 1.5-2 kg/ha 2-3 0.1-0.73
lim 1.5-2 kg/ha 2-3 0.5-1.8
snap 1.5-2 kg/ha 1-2 0.11-0.94
Bean vines 1.5-2 g/ha 2-3 0.2-4.6
Blackcurrant 0.45 kg/ha 1 4.0-5.1
(0 day)
1.7-2.4
(7 day)
1.3-2.4
(14 day)
Caneberries 0.5-2 kg/ha 2-5 0.75-6.0
Celery 0.2-0.5 kg/ha 4-11 0.1-2.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Cherries 0.3-0.6 g/l 2-3 0.2-12.6
0.3 g/l Dip 0.5-2.3
post-harvest
Citrus (whole 0.6-1.1 g/l 1-5 0.2-1.3
fruit) 500 ppm Dip 0.54-1.26
post-harvest
1000 ppm Dip 0.39-2.35
post-harvest
1250 ppm Dip 1.3-1.8
post-harvest
2500 ppm Dip 2.7-3.9
post-harvest
5000 ppm Dip 4.5-5.4
post-harvest
Cucumbers 0.2-0.5 kg/ha 2-6 0.13-0.55
Grapes 1-1.5 kg/ha 3-5 0.14-10.3
Macadamia nuts 2-2.5 kg/ha 4-10 <0.1
Mangoes 1-2 kg/ha 5-17 0.15-3.0
Melons 0.2-0.4 kg/ha 2-7 0.27-0.32
Mushroom 0.25 g/m2 1 0.31-0.57
0.5 g/m2 (peat
treatment) 1 0.23-0.51
1.0 g/m2 (peat
treatment) 1 0.88-1.5
Nectarines 0.5 kg/ha 1-3 1.6-2.2
Peaches 0.2-0.6 g/l 1-15 0.2-8.2
0.3 g/l Dip 3.9-4.7
post-harvest
Peanuts 0.2-0.5 kg/ha 2-13 <0.1
Peanuts hay 0.4-1 kg/ha 3-13 1.4-1.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Pears 0.6-1.2 g/l 3-5 1.7-3.2
Dip
post-harvest 0.1-0.55
Pecans 1-1.25 kg/ha 3-6 <0.1
Prunes, plums 0.3-0.6 g/l 2-4 0.4-1.4
0.3 g/l Dip 0.5-1.9
post-harvest
Potato 50 g/100 kg Tuber <0.1
dressing
Squash 0.4-0.8 kg/ha 2-5 0.10-0.50
Strawberries 0.3-0.6 g/l 3-7 0.43-2.6
0.3-0.6 g/l 6-7 2.7-15.2
(Under glass) (0.day)
0.3-0.6 g/l (Under glass) 1.5-2.6
(17.day)
Sugarbeet
roots 0.2-0.5 kg/ha 3-6 <0.1
tops 0.4-1 kg/ha 1-6 0.15-4.7
Tomato 0.3 g/plant 1 1.1-2.2
0.3-0.7 kg/ha 3 0.15-2.1
(Under glass)
Application Benomyl
a Total residue comprising benomyl, methyl benzimidazolecarbamate
and 2-aminobenzimidazole, expressed as benomyl.
(Nominal blank values either 0.1 or 0.2 ppm, apart from citrus,
strawberries, peanut hay, barley straw and lettuce (0.3-0.4 ppm).)
Fate of residues
General remarks on mode of action
As suggested by Clemons and Sisler (1969) (Kilgore and White,
1970; Helweg, 1973) and others, it is generally accepted that methyl
2-benzimidazole carbamate (MBC), which is the major metabolite of
benomyl, is primarily responsible for the fungitoxicity of the latter.
Comparisons between the effects of benomyl and MBC (Hammerschlag and
Sisler, 1973) indicated that both compounds caused an inhibition in
the synthesis of DNA, but that this was secondary to a mitotic arrest
of fungal cell division, followed by an inhibition of cytokinesis.
Benomyl partially inhibits respiration of subcellular particles
from fungal cells, while MBC had essentially no such effect. This
differential effect of benomyl and MBC on metabolism is attributed to
the formation, from benomyl, of volatile butyl isocyanate (BIC),
simultaneously to MBC production. Fungitoxicity of benomyl
preparations may thus be attributed to the combined effects of its two
first breakdown products, MBC and BIC.
Although the fungitoxic effects of benomyl are thus assumed to be
caused by its metabolites, MBC and BIC, much of its systemic functions
may be connected to benomyl per se. Controlled experiments of foliar
treatments on apples, cucumber, banana, orange and grape plants
indicate that from 48% to 77% of the 14C-benomyl remaining 21-23 days
after application under outdoor conditions was still in the form of
intact benomyl (Baude et al., 1973). Several workers have recently
found that plant penetration and movement through the cuticle after
foliar application is greater for benomyl than for MBC (Upham and
Delp, 1973; Hammerschlag and Sisler, 1972, 1973; Solel and Edgington,
1973). Systemic translocation within the plant, on the other hand, is
mostly in the form of MBC plus smaller amounts of 2-AB (Siegel and
Zabbia, 1972).
The two theoretical metabolites, STB and BUB (see Fig. 1), which
are formed chemically under alkaline conditions could not be detected
by Baude et al. (1973) as actual conversion products from benomyl
under greenhouse conditions or in field tests, even when subjected to
the influence of two alkaline pesticides, basic copper sulfate and
lime sulfur.
Plants
Under practical conditions a considerable part of the applied
benomyl is claimed to remain on the foliar surfaces, as relatively
stable residues, only gradually degrading to form MBC (Baude et al.,
1973).
Studies of the systemic uptake of benomyl after foliar treatments
indicate that while the total amount which penetrates the surface is
relatively small, residues can still be traced in untreated portions
of the plant (Lowen, 1973). The translocated residues consist mainly
of MBC together with smaller amounts of 2-AB. To the plants these
compounds move only passively, i.e. they are carried by the
intercellular transpiration stream towards the edges and tips of
leaves (Foldo, 1973), where they then accumulate.
Benomyl is readily absorbed through the root-system of plants.
The uptake in vegetable crops, such as lettuce and cucumber is clearly
demonstrated through greenhouse trials (see Table 2) in which normal
and excessive rates were applied through watering or through admixing
benomyl with the soil (Green-Lauridsen and Voldum-Clausen, 1973).
Citrus
The total 14C-benomyl residue (benomyl + MBC) was found to
decrease slowly in oranges during two months storage after
post-harvest dipping. While the major part of the residue remained in
the peel, a smaller, but significant amount gradually penetrated into
the edible portion (see Table 3). Two weeks after the dipping 61% of
the surface residue was present as unchanged benomyl, the remaining
39% being MBC (Lowen, 1973).
Grapes and wine
Lemperle et al. (1973) found that benomyl residues, as well as
other benzimidazole fungicides, are transferred almost quantitatively
into unclarified musts during wine production. During clarification
and fermentation a small amount may disappear but a considerable part
of the benomyl residue present in grapes may be found in wine.
Animals and animal products
Benomyl is readily hydrolyzed into MBC through cleavage of the
1-butyl carbamoyl side chain when ingested by mice, rabbits and sheep
(Douch, 1973). Further hydrolysis of the carbamate ester bond was
shown by these studies to yield 2-AB suggesting that benomyl may not
remain in the unchanged form more than momentarily when residues are
ingested into the alimentary tract of animals. Hydroxylated
derivatives of MBC and 2-AB were identified by these studies in urine
and faeces of all three species.
After feeding rats with benomyl Gardiner et al, (1968) identified
methyl 5-hydroxy-2-benzimidazole carbamate as the major metabolite
which was liberated in urine by enzyme hydrolysis of glucuronide
and/or sulfate conjugates. MBC is assumed to be the possible
intermediate metabolite for this conversion as well as for the
formation of a minor isomeric metabolite, methyl
4-hydroxy-2-benzimidazole carbamate, which was found in milk and meat
in later studies (Gardiner et al., 1972) when feeding cows with
rations containing 2, 10 and 50 ppm benomyl for 32 days (see Table 4).
Neither intact benomyl nor MBC was detected in these studies.
TABLE 2. BENOMYL RESIDUES ABSORBED THROUGH THE ROOTS UNDER GREENHOUSE CONDITIONS
Sampling after Sampling after
Treatment Application 0-14 days 15-23 days Remarks
rate
Range Average Range Average
Summer lettuce
Watering 1.5-3.8 g/10 m2 5.21-17.8 11.3 n.d.-3.09 1.38 Practical use rate
Watering 7.5-15 g/10 m2 4.33-39.7 15.8 0.49-5.01 2.63 Excessive rates
Watering 22.5 g/10 m2 17.7-29.3 23.0
Winter lettuce
Watering 1.5-3.8 g/10 m2 11.7-18.6 15.3 7.08-14.7 10.5 Practical use rate
Watering 7.5-15 g/10 m2 20.4-60.6 36.8 6.74-27.1 15.9 Excessive rates
Watering 22.5 g/10 m2 56.5-97.1 74.5 28.2-32.1 30.2
Cucumber
Watering 1 g/plant n.d.-0.64 0.27 0.15-0.53 0.37
Soil treatmenta 1 g/plant 0.53-2.02 1.10b
a Composite Soil: 1/3 soil, 1/3 fertilizer, 1/3 peat mulch.
b Sampling after 34-55 days.
TABLE 3. 14C-BENOMYL RESIDUES ON ORANGES FOLLOWING POST-HARVEST
DIPPING (From Lowen, 1973)
ppm benomyl Days Whole
in dip post-treatment Peel Pulp fruit
(calculated)
Untreated - <0.05 <0.05 <0.05
100 0 0.80 <0.05 0.30
31 0.46 <0.05 0.16
62 0.54 <0.05 0.19
500 0 1.5 0.08 0.46
31 1.4 0.12 0.38
62 1.6 0.17 0.50
1 000 0 5.6 <0.05 1.3
31 4.4 0.11 0.95
62 3.0 0.40 0.93
After withdrawing the treated diet for one or two days the
residues of 5-hydroxy-MBC and 4-hydroxy-MBC in milk dropped to below
0.01 ppm.
Water
Benomyl rapidly breaks down on contact with water to give MBC,
conversion being complete within four days (Kilgore and White, 1970;
Peterson and Edgington, 1969).
Soil
Helweg (1973) isolated from soils four strains of bacteria and
two fungi which promoted the breakdown of benomyl to nonfungistatic
compounds. High humus content increased the rate of breakdown of
benomyl. Generally, however, it was concluded that benomyl is
relatively stable as a soil residue under temperate climatic
conditions.
TABLE 4. RESIDUES (PPM) OF METHYL 5-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(5-HYDROXY MBC) AND METHYL 4-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(4-HYDROXY MBC) FOUND IN MEAT AND MILK AFTER FEEDING COWS RATIONS
CONTAINING 2, 10, AND 50 PPM BENOMYL FOR 32 DAYS
(From Gardiner et al., 1972)
Feeding level
2 ppm 10 ppm 50 ppm
Sample
5- 4- 5- 4- 5- 4-
hydroxy hydroxy hydroxy hydroxy hydroxy hydroxy
MBC MBC MBC MBC MBC MBC
Milk <0.01 <0.01 0.01 <0.01 0.05 0.03
Meata <0.05 <0.05 <0.05 <0.05 <0.05
<0.05
a "Meat" represents liver, kidney, fat and muscle.
Studies over two years in experimental fields in Delaware, North
Carolina and Florida (Baude, 1972) showed that the amount of
non-volatile decomposition products of 14C-benomyl remaining in the
soil after one year were 57%, 49% and 57%, respectively, of the
quantity originally applied. After two years in soil in Florida, 27%
of the amount originally present remained. After one month only minor
amounts of unchanged benomyl were present, the principal metabolite
being MBC, with smaller amounts of 2-AB.
Uptake by crops grown in treated soils was studied in the same
experiments by Rhodes (1972) using 14C-benomyl. Trace amounts,
ranging from 0.02 to 0.08 ppm, could be found in tissues of carrots,
tomatoes, corn and beans, while edible snap beans, corn and tomatoes
contained less than the detection limit (i.e. <0.01 ppm). In this
study the benomyl content of the soil at the time of harvest was
0.9 ppm.
Methods of residue analysis
Pease and Gardiner (1969) describe colorimetric and fluorometric
procedures for determining benomyl in a variety of plant and animal
tissues and in soil. The residue is extracted, purified by partition,
converted to 2-aminobenzimidazole, and determined by direct
fluorometric measurement or by colorimetric analysis following
bromination. The procedure thus determines benomyl, its principal
degradation product methyl 2-benzimidazole carbamate, and the minor
residual component 2-aminobenzimidazole as a composite value. With a
sensitivity of 0.1 ppm for 50 g samples, recoveries were about 87% for
both methods. Pease and Holt (1971) improved this method to make it
less time-consuming and to include the analysis of citrus fruits. By
using fluorometric measurement from both acidic and basic media it was
possible to distinguish between benomyl residues and other
benzimidazole fungicides.
Peterson and Edgington (1969) report a method to determine
benomyl and the hydrolysis product, MBC, using a bioautograph
technique. A thin-layer plate is sprayed with a mixture of agar and
Penicillium spores, and the diameter of the zone of inhibited growth
above the fungitoxic spot is related to the amount of active material
in the spot. The sensitivity of the method is 0.1 ppm for a 50 g
sample. Somewhat similar agar plate methods have also been described
by Erwin et al. (1968), Baron (1971) and Holman and Fuchs (1970). A
two-dimensional TLC-technique for separation of benomyl, MBC, 2-AB and
thiophanate as well as thiophanate-methyl is described by von Stryk
(1972).
A UV-spectrophotometric method for determining residues in
grapes, must and wine has been described by Lemperle and Kerner
(1971). After addition of acetone the samples are extracted with
chloroform, the chloroform evaporated to dryness and the residues
measured in methanol at 301.5 nm. The limit of detection reported is
0.3 ppm for 100 g samples and recoveries of 89% for grapes, 75% for
must and 56% for wines. Mestres et al. (1971) used a
spectrophotometric method for fruits and vegetables, measuring the
hydrolysis product, MBC, at 282 nm. The limit of detection was 0.1 ppm
for 25 g samples.
Residues in meat and milk have been determined by the liquid
chromatographic procedure of Kirkland (1973). The method detects
4-hydroxymethyl-2-benzimidazole carbamate and
5-hydroxymethyl-2-benzimidazole carbamate, which are found in animal
systems, as well as benomyl and methyl-2-benzimidazole carbamate.
Kirkland et al. (1973) further adapted this method for the
determination of benomyl and/or MBC and 2-AB in soil and plant
material. Recoveries of the three compounds were 92, 88 and 72%,
respectively. The lower limit of sensitivity of the method is 0.05 ppm
for each of the components.
The methods based on spectrophotofluometry of 2-AB, on
UV-spectophotometry of MBC and the liquid chromatographic procedure
may all be adapted for regulatory purposes.
National tolerances (as notified to the meeting)
Country Commodity Tolerance, ppm
Australia Pome fruits, stone fruits 5
Vegetables, citrus 3
Grapes, mangoes, avocados
strawberries 2
Bananas 1
Peanuts 0.2
Belgium Apples, pears, cucumbers,
strawberries, celery,
small grain 1
Canada Peaches 15
Blackberries, boysenberries,
raspberries 6
Apples, apricots, cherries,
crabapples, grapes,
nectarines, pears, plums,
prunes, strawberries 5
Federal Citrus fruits 10
Republic Grapes 3.0
of Berries 2.0
Germany Vegetables (exc. cucumber), Total
stone fruit, bananas, citrus residue
fruits (without peel) 1.0 calculated
Cereals, cucumbers 0.5 as benomyl
Banana (without peel) 0.2
Other vegetable products 0.1
United Apricots, cherries, nectarines,
States of peaches and plums (including
America fresh prunes) (from post-
harvest and/or pre-harvest
applications), peanut hay,
peanut forage, sugar beet tops 15
Mushrooms 10
Apples, pears, blackberries,
boysenberries, dewberries,
loganberries, raspberries 7
Strawberries 5
Celery, mango 3
Snapbeans, peanut hulls 2
National Tolerances (cont'd)
Country Commodity Tolerance, ppm
Bananas (whole), cucumbers,
melons, summer squash, winter
squash, avocado, almond hulls 1
Banana (pulp), peanuts, sugar
beets (roots), nuts (almonds,
Brazil nuts, bush nuts,
butternuts, cashews, chestnuts,
filberts, hazelnuts, hickory nuts,
macadamia nuts, pecans, walnuts) 0.2
Milk and the meat, fat and meat
by-products of cattle, goats,
hogs, horses and sheep 0.1
Netherlands Fruit and vegetables 2 ) Determined
Raw cereals 0.5 ) and
Citrus fruits 3.5 ) expressed
) as
) MBC
South Fruits and vegetables 3
Africa
Appraisal
Benomyl is a relatively new systemic broad-spectrum fungicide
already established in most countries. It is marketed as wettable
powders and used in foliar applications, seed treatments and
post-harvest dipping procedures on a great number of food and forage
crops.
Benomyl is chemically easily hydrolysed into the relatively
stable methyl-2-benzimidazole carbamate (MBC) which is considered to
be the major fungitoxic principle of the compound. Formulated benomyl
may hydrolyze if not kept dry in storage.
A fungicidal effect additional to the MBC activity is claimed to
be connected with the simultaneous release of volatile butyl
isocyanate. Recent data has been presented suggesting that the
hydrolytic breakdown is of significance for the systemic properties of
benomyl facilitating the cuticular penetration. Evidence is given that
in the case of benomyl residues on foliar surfaces the formation of
MBC proceeds gradually. In a typical example a 50% cleavage of
residues remaining on apple leaves occurs three weeks after
application.
A further metabolic product found in plants is
2-aminobenzimidazole formed from MBC. The amount of this metabolite
found in plant material is, however, usually relatively small; so far
not being found in excess of 1% of total residues. For practical
purposes therefore, residues in plant material following application
of benomyl could be considered as the composite of benomyl, MBC and
2-AB or as the sum of benomyl and MBC alone.
Residue data justifying the establishment of several national
tolerances were available to the meeting, together with limited
information on residues on citrus fruits sampled from trade channels.
Included also is evidence of uptake into crops from treated soils.
Half-life of benomyl (metabolized to MBC) in soils varies from about
four months to one year indicating relatively long persistence with a
possibility of unintentional uptake by following crops.
Cow feeding trials show that hydroxylated methyl benzimidazole
carbamates are formed from benomyl with MBC as the probable
intermediate metabolite.
The most recent published report has confirmed this information
indicating that MBC (and possible 2-AB) are likely to be the primary
chemical entities to be absorbed from the alimentary tract following
the ingestion of benomyl residues.
The presence of hydroxylated MBC was demonstrated in milk and
meat residues of intact benomyl or MBC were detected.
Suitable methods of analysis for benomyl, MBC and 2-AB or for
benomyl and MBC as composite values have been published, both methods
being effective at the 0.1 ppm level. For one of these methods which
is based on spectrophotofluorometric measurement, modifications have
been introduced which allow the determination of benomyl and
metabolites and to differentiate these from other benzimidazole
fungicides such as thiabendazole. Although useful for regulatory
purposes neither of the two methods, however, permits the separate
determination of benomyl and metabolites when these are present
together.
RECOMMENDATIONS
As no acceptable daily intake was established it was not possible
to recommend tolerances. Following officially acceptable use in
various countries residues of benomyl and its metabolites can occur in
the following commodities up to levels indicated. Residues of benomyl
should be measured as the composite of benomyl and its main
metabolite, methyl benzimidazolecarbamate with or without minor
amounts of 2-aminobenzimidazole (usually less than 1% of total
residues), calculated as methyl benzimidazolecarbamate. The levels
indicated are not likely to be exceeded when benomyl is applied in
accordance with good agricultural practice, including either or both
pre-harvest and post-harvest treatments (when applicable). These
levels have been recommended as guideline levels.
For the underlined commodities adjustments have been made to
recommendations in order to accommodate good agricultural practices
for such alternative systemic fungicides of which MBC is also
recognized and identified as the major metabolite and/or chemical
entity (i.e. carbendazim and thiophanatemethyl).
Guideline levels
ppm
Citrus fruit (whole), cherries, grapes, peaches 10
Apples, pears, apricots, tomatoes, blackberries,
boysenberries, dewberries, loganberries, raspberries,
strawberries, blackcurrants 5
Celery, prunes, plums, beans (lima), mangoes,
beans (dry), beans (snap), nectarines 2
Mushroom, banana (whole) 1
cucumbers, melons, squash, brussels sprouts, avocados 0.5
Sugar beet, raw cereals, peanuts, almonds, macadamia 0.5
Nuts, pecans, potatoes 0.1a
Animal feedstuffs
Bean vines 30
Sugar beet leaves 5
Peanut hay, barley straw 2
Almond hulls 1
Meat of cattle and sheep 0.1a
Milk (whole)
a At or about limit of determination.
Residues to be measured as a composite of benomyl, MCB, 2-AB and
expressed as MBC.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Full toxicological data.
Desirable
1. Further development of analytical methods to adapt them
for regulatory purposes, especially to permit separate
determination of benomyl and metabolites when present together.
2. Information on residues in food in commerce.
3. Information on the nature and level of residues in poultry and
eggs following the feeding of benomyl residues in rations.
REFERENCES
Anon. Residues of benomyl in certain foods. E.I. du Pont de
1972, 1973 Nemours & Co., Delaware. Unpublished reports filed
with FAO
Baron, M. Determination, migration and distribution of a
1971 systemic fungicide (benomyl) in the leaves of the
banana plant. Fruits, 26: 643
Baude, F. J. Disappearance of benomyl-2-14C from field soil
1972 in Delaware, North Carolina, and Florida. Paper
submitted by E.I. de Pont de Nemours & Co.,
Delaware (Unpublished)
Baude, F. J., Gardiner, J. A. and Han, J. C.-Y. Characterization
1973 of residues on plants following spray applications
of benomyl. Prepublication of paper submitted to
the J. Agr. Food Chem.
Bollen, G, J. A comparison of the in vitro antifungal spectra
1972 of thiophanates and benomyl. Neth J. Plant. Path.
78: 55
CIVO-TNO Unpublished reports on benomyl residues from Central
1972, 1973 Institute of Food Research (CIVO-TNO) of 11
January 1972 and 9 August 1973
Clemons, G.P. and Sisler, H. D. Formation of a fungitoxic
1969 derivative from Benlate. Phytopathology, 59: 705
Douch, P. G. C. The metabolism of benomyl fungicide in animals.
1973 Xenobiotica, 3: 367
Edgington, L. V., Khew, K.L. and Barron, G.L. Fungitoxic spectrum
1971 of benzimidazole compounds. Phytopathology, 61:
42
Erwin, D. C., Mee, H. and Sims, J. J. The systemic effect of
1968 1-(butylcarbamoyl)-2-benzimidazole carbamic acid,
methyl ester, on verticillium wilt of cotton.
Phytopathology, 58: 528
Evans, E. Systemic fungicides in practice, Pesticide Science,
1971 2: 192
Foldo, N. E. Systemic fungicides. Effect, advantages and
1973 disadvantages (in Danish), Ugeskr. f. agronomer og
hortonomer, 40: 730
Gardiner, J. A., Brantley, R. K. and Sherman, H. Isolation
1968 and identification of a metabolite of methyl
1-(butylcarbamoyl)-2-benzimidazolecarbamate in rat
urine. J. Agr. Food Chem. 16: 1050
Gardiner, J. A., Kirkland, J. J., Pease, H. L., Wall, E. N.
1973 and Morales, R. Unpublished studies. Cited from
Kirkland (1973)
Green-Lauridsen, M. and Voldum-Clausen, K. Reports on
1973 experiments on pesticide residues Nos 285-287,
290-293, 298-300, 302, 306, 308-310 from the
Danish National Food Institute and Government
Plant Pathology Institute, Copenhagen
(Unpublished)
Hammerschlag, R. S. and Sisler, H. D. Differential action
1972 of benomyl and methyl-2-benzimidazolecarbmate
(MBC) in Saccharomyces pastorianus. Pesticide
Biochem. Physiol. 2: 123
Hammerschlag, R. S. and Sisler, H. D. Benomyl and methyl-2
1973 benzimidazole (MBC): biochemical, cytological and
chemical aspects of toxicity to Ustilago
mavdis and Saccharomyces cerevesiae; Pesticide
Biochemistry and Physiology, 3: 42
Helweg, A. Persistence of benomyl in different soil types
1973a and microbial breakdown of the fungicide in soil
and agar culture. Tidskr. for Planteavl
(Copenhagen), 77: 232
Helweg, A. Influence of the fungicide benomyl on micro-organisms
1973b in soil. Tidsskrift for Planteavl (Copenhagen),
77: 375
Holman, A. L. and Fuchs, A. Direct bioautography on TLC as
1970 a method for detecting fungitoxic substances. J.
Chromatography, 51: 327
Kilgore, W. W. and White, E.R. Decomposition of the systemic
1970 fungicide 1991 (Benlate). Bull. Environm. Contam.
& Toxicol. 5: 67
Kirkland, J. Method for high-speed liquid chromatographic
1973 analysis of benomyl and/or metabolite residues in
cow milk, urine, feces and tissues. J. Agric. Food
Chem. 21: 171
Kirkland, J., Holt, R.F. and Pease, H.L. Determination of
1973 benomyl residues in soils and plant tissues by
high-speed cation exchange liquid chromatography.
J. Agr. Food Chem. 21: 368
Lemperle, E. and Kerner, E. UV-spektrophotometrische
1971 Bestimmung von Rücksttänden in Weintrauben,
Traubenmost und Wein nach Anwendung von Du Pont
Benomyl. Z. Anal. Chem. 254: 117
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A.
1973 Wirkstoffrückstände und Gärbeeinflussungen nach
Anwendung von Fungiziden im Weinbau. Deutsche
Leb.-Rdschau, 69: 313
Mestres, R., Tourte, T. and Campo, M. Dosage des residus de
1972 benomyl dans les fruits et les legumes. Ann. Fals.
Exp. Chim. 65: 239
Ogawa, J. A., Bose, E., Manji, B. T., White, E. R. and
1971 Kilgore, W. W. Phytopathology, 70: 905 (Cited
from Baude et al., 1973)
Pease, H. L. and Gardiner, J. A. Fluorometric and colorimetric
1969 procedures for determining residues of benomyl. J.
Agr. Fd. Chem. 17: 267
Pease, H. L. and Holt, R. F. Improved method for determining
1971 benomyl residues. J. Assoc. Off. Anal. Chem. 54:
1399
Peterson, C. A. and Edgington, L. V. Quantitative estimation
1969 of the fungicide benomyl using a bioautograph
technique. J. Agr. Food Chem. 77: 898
Rhodes, R. C. Uptake of 2-14 C-benomyl soil residues by
1972 crops. Paper submitted by du Pont de Nemours &
Co., Delaware (Unpublished)
Siegel, M. R. and Zabbia, A. J., jr Distribution and metabolic
1972 fate of the fungicide benomyl in dwarf pea.
Phytopathology, 62: 630
Solel, Z. and Edgington, L. V. Transcuticular movement of
1973 fungicides. Phytopathology, 63: 505
Upham, P. M. and Delp, C. J. Phytopathology (In press).
1973 (Cited from Baude et al., 1973)
von Stryk, F. G. Separation and determination of some
1972 systemic fungicides and their metabolites by thin-
layer chromatography. J. Chromatogr. 72: 410
White, E. R., Bose, E. A., Ogawa, J. M., Manji, B. T. and
1973 Kilgore, W. W. J. Agr. Food Chem. (in press)
(Cited from Baude et al., 1973)
TABLE 1. RESIDUES OF BENOMYL IN VARIOUS CROPS
(Supervised trials)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Apricots 0.3-0.6 g/l 2-3 2.6-6.5
Dip
post-harvest 0.9-5.1
Almonds
nutmeats 1-2 kg/ha 1-3 <0.1
hulls 1-2 kg/ha 1-3 0.2-0.74
Apples 0.4-1.2 g/l 6-12 0.5-4.8
Dip
post - harvest 0.12-0.88
Avocados 1-2 kg/ha 3-6 0.11-0.18
Bananas 0.2-0.3 kg/ha 6-9 Pulp <0.1
0.3-0.6 g/l Dip 0.15-0.43
post-harvest
Barley 0.5 kg/ha 2 Straw 0.54
1.53 (53
days)
Grain 0.1
Beans dry 1.5-2 kg/ha 2-3 0.1-0.73
lim 1.5-2 kg/ha 2-3 0.5-1.8
snap 1.5-2 kg/ha 1-2 0.11-0.94
Bean vines 1.5-2 g/ha 2-3 0.2-4.6
Blackcurrant 0.45 kg/ha 1 4.0-5.1
(0 day)
1.7-2.4
(7 day)
1.3-2.4
(14 day)
Caneberries 0.5-2 kg/ha 2-5 0.75-6.0
Celery 0.2-0.5 kg/ha 4-11 0.1-2.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Cherries 0.3-0.6 g/l 2-3 0.2-12.6
0.3 g/l Dip 0.5-2.3
post-harvest
Citrus (whole 0.6-1.1 g/l 1-5 0.2-1.3
fruit) 500 ppm Dip 0.54-1.26
post-harvest
1000 ppm Dip 0.39-2.35
post-harvest
1250 ppm Dip 1.3-1.8
post-harvest
2500 ppm Dip 2.7-3.9
post-harvest
5000 ppm Dip 4.5-5.4
post-harvest
Cucumbers 0.2-0.5 kg/ha 2-6 0.13-0.55
Grapes 1-1.5 kg/ha 3-5 0.14-10.3
Macadamia nuts 2-2.5 kg/ha 4-10 <0.1
Mangoes 1-2 kg/ha 5-17 0.15-3.0
Melons 0.2-0.4 kg/ha 2-7 0.27-0.32
Mushroom 0.25 g/m2 1 0.31-0.57
0.5 g/m2 (peat
treatment) 1 0.23-0.51
1.0 g/m2 (peat
treatment) 1 0.88-1.5
Nectarines 0.5 kg/ha 1-3 1.6-2.2
Peaches 0.2-0.6 g/l 1-15 0.2-8.2
0.3 g/l Dip 3.9-4.7
post-harvest
Peanuts 0.2-0.5 kg/ha 2-13 <0.1
Peanuts hay 0.4-1 kg/ha 3-13 1.4-1.6
TABLE 1. (Cont'd.)
Application Benomyl
Crop Rate No. per residue
(approx.) season founda
range (ppm)
Pears 0.6-1.2 g/l 3-5 1.7-3.2
Dip
post-harvest 0.1-0.55
Pecans 1-1.25 kg/ha 3-6 <0.1
Prunes, plums 0.3-0.6 g/l 2-4 0.4-1.4
0.3 g/l Dip 0.5-1.9
post-harvest
Potato 50 g/100 kg Tuber <0.1
dressing
Squash 0.4-0.8 kg/ha 2-5 0.10-0.50
Strawberries 0.3-0.6 g/l 3-7 0.43-2.6
0.3-0.6 g/l 6-7 2.7-15.2
(Under glass) (0.day)
0.3-0.6 g/l (Under glass) 1.5-2.6
(17.day)
Sugarbeet
roots 0.2-0.5 kg/ha 3-6 <0.1
tops 0.4-1 kg/ha 1-6 0.15-4.7
Tomato 0.3 g/plant 1 1.1-2.2
0.3-0.7 kg/ha 3 0.15-2.1
(Under glass)
Application Benomyl
a Total residue comprising benomyl, methyl benzimidazolecarbamate
and 2-aminobenzimidazole, expressed as benomyl.
(Nominal blank values either 0.1 or 0.2 ppm, apart from citrus,
strawberries, peanut hay, barley straw and lettuce (0.3-0.4 ppm).)
Fate of residues
General remarks on mode of action
As suggested by Clemons and Sisler (1969) (Kilgore and White,
1970; Helweg, 1973) and others, it is generally accepted that methyl
2-benzimidazole carbamate (MBC), which is the major metabolite of
benomyl, is primarily responsible for the fungitoxicity of the latter.
Comparisons between the effects of benomyl and MBC (Hammerschlag and
Sisler, 1973) indicated that both compounds caused an inhibition in
the synthesis of DNA, but that this was secondary to a mitotic arrest
of fungal cell division, followed by an inhibition of cytokinesis.
Benomyl partially inhibits respiration of subcellular particles
from fungal cells, while MBC had essentially no such effect. This
differential effect of benomyl and MBC on metabolism is attributed to
the formation, from benomyl, of volatile butyl isocyanate (BIC),
simultaneously to MBC production. Fungitoxicity of benomyl
preparations may thus be attributed to the combined effects of its two
first breakdown products, MBC and BIC.
Although the fungitoxic effects of benomyl are thus assumed to be
caused by its metabolites, MBC and BIC, much of its systemic functions
may be connected to benomyl per se. Controlled experiments of foliar
treatments on apples, cucumber, banana, orange and grape plants
indicate that from 48% to 77% of the 14C-benomyl remaining 21-23 days
after application under outdoor conditions was still in the form of
intact benomyl (Baude et al., 1973). Several workers have recently
found that plant penetration and movement through the cuticle after
foliar application is greater for benomyl than for MBC (Upham and
Delp, 1973; Hammerschlag and Sisler, 1972, 1973; Solel and Edgington,
1973). Systemic translocation within the plant, on the other hand, is
mostly in the form of MBC plus smaller amounts of 2-AB (Siegel and
Zabbia, 1972).
The two theoretical metabolites, STB and BUB (see Fig. 1), which
are formed chemically under alkaline conditions could not be detected
by Baude et al. (1973) as actual conversion products from benomyl
under greenhouse conditions or in field tests, even when subjected to
the influence of two alkaline pesticides, basic copper sulfate and
lime sulfur.
Plants
Under practical conditions a considerable part of the applied
benomyl is claimed to remain on the foliar surfaces, as relatively
stable residues, only gradually degrading to form MBC (Baude et al.,
1973).
Studies of the systemic uptake of benomyl after foliar treatments
indicate that while the total amount which penetrates the surface is
relatively small, residues can still be traced in untreated portions
of the plant (Lowen, 1973). The translocated residues consist mainly
of MBC together with smaller amounts of 2-AB. To the plants these
compounds move only passively, i.e. they are carried by the
intercellular transpiration stream towards the edges and tips of
leaves (Foldo, 1973), where they then accumulate.
Benomyl is readily absorbed through the root-system of plants.
The uptake in vegetable crops, such as lettuce and cucumber is clearly
demonstrated through greenhouse trials (see Table 2) in which normal
and excessive rates were applied through watering or through admixing
benomyl with the soil (Green-Lauridsen and Voldum-Clausen, 1973).
Citrus
The total 14C-benomyl residue (benomyl + MBC) was found to
decrease slowly in oranges during two months storage after
post-harvest dipping. While the major part of the residue remained in
the peel, a smaller, but significant amount gradually penetrated into
the edible portion (see Table 3). Two weeks after the dipping 61% of
the surface residue was present as unchanged benomyl, the remaining
39% being MBC (Lowen, 1973).
Grapes and wine
Lemperle et al. (1973) found that benomyl residues, as well as
other benzimidazole fungicides, are transferred almost quantitatively
into unclarified musts during wine production. During clarification
and fermentation a small amount may disappear but a considerable part
of the benomyl residue present in grapes may be found in wine.
Animals and animal products
Benomyl is readily hydrolyzed into MBC through cleavage of the
1-butyl carbamoyl side chain when ingested by mice, rabbits and sheep
(Douch, 1973). Further hydrolysis of the carbamate ester bond was
shown by these studies to yield 2-AB suggesting that benomyl may not
remain in the unchanged form more than momentarily when residues are
ingested into the alimentary tract of animals. Hydroxylated
derivatives of MBC and 2-AB were identified by these studies in urine
and faeces of all three species.
After feeding rats with benomyl Gardiner et al, (1968) identified
methyl 5-hydroxy-2-benzimidazole carbamate as the major metabolite
which was liberated in urine by enzyme hydrolysis of glucuronide
and/or sulfate conjugates. MBC is assumed to be the possible
intermediate metabolite for this conversion as well as for the
formation of a minor isomeric metabolite, methyl
4-hydroxy-2-benzimidazole carbamate, which was found in milk and meat
in later studies (Gardiner et al., 1972) when feeding cows with
rations containing 2, 10 and 50 ppm benomyl for 32 days (see Table 4).
Neither intact benomyl nor MBC was detected in these studies.
TABLE 2. BENOMYL RESIDUES ABSORBED THROUGH THE ROOTS UNDER GREENHOUSE CONDITIONS
Sampling after Sampling after
Treatment Application 0-14 days 15-23 days Remarks
rate
Range Average Range Average
Summer lettuce
Watering 1.5-3.8 g/10 m2 5.21-17.8 11.3 n.d.-3.09 1.38 Practical use rate
Watering 7.5-15 g/10 m2 4.33-39.7 15.8 0.49-5.01 2.63 Excessive rates
Watering 22.5 g/10 m2 17.7-29.3 23.0
Winter lettuce
Watering 1.5-3.8 g/10 m2 11.7-18.6 15.3 7.08-14.7 10.5 Practical use rate
Watering 7.5-15 g/10 m2 20.4-60.6 36.8 6.74-27.1 15.9 Excessive rates
Watering 22.5 g/10 m2 56.5-97.1 74.5 28.2-32.1 30.2
Cucumber
Watering 1 g/plant n.d.-0.64 0.27 0.15-0.53 0.37
Soil treatmenta 1 g/plant 0.53-2.02 1.10b
a Composite Soil: 1/3 soil, 1/3 fertilizer, 1/3 peat mulch.
b Sampling after 34-55 days.
TABLE 3. 14C-BENOMYL RESIDUES ON ORANGES FOLLOWING POST-HARVEST
DIPPING (From Lowen, 1973)
ppm benomyl Days Whole
in dip post-treatment Peel Pulp fruit
(calculated)
Untreated - <0.05 <0.05 <0.05
100 0 0.80 <0.05 0.30
31 0.46 <0.05 0.16
62 0.54 <0.05 0.19
500 0 1.5 0.08 0.46
31 1.4 0.12 0.38
62 1.6 0.17 0.50
1 000 0 5.6 <0.05 1.3
31 4.4 0.11 0.95
62 3.0 0.40 0.93
After withdrawing the treated diet for one or two days the
residues of 5-hydroxy-MBC and 4-hydroxy-MBC in milk dropped to below
0.01 ppm.
Water
Benomyl rapidly breaks down on contact with water to give MBC,
conversion being complete within four days (Kilgore and White, 1970;
Peterson and Edgington, 1969).
Soil
Helweg (1973) isolated from soils four strains of bacteria and
two fungi which promoted the breakdown of benomyl to nonfungistatic
compounds. High humus content increased the rate of breakdown of
benomyl. Generally, however, it was concluded that benomyl is
relatively stable as a soil residue under temperate climatic
conditions.
TABLE 4. RESIDUES (PPM) OF METHYL 5-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(5-HYDROXY MBC) AND METHYL 4-HYDROXY-2-BENZIMIDAZOLECARBAMATE
(4-HYDROXY MBC) FOUND IN MEAT AND MILK AFTER FEEDING COWS RATIONS
CONTAINING 2, 10, AND 50 PPM BENOMYL FOR 32 DAYS
(From Gardiner et al., 1972)
Feeding level
2 ppm 10 ppm 50 ppm
Sample
5- 4- 5- 4- 5- 4-
hydroxy hydroxy hydroxy hydroxy hydroxy hydroxy
MBC MBC MBC MBC MBC MBC
Milk <0.01 <0.01 0.01 <0.01 0.05 0.03
Meata <0.05 <0.05 <0.05 <0.05 <0.05
<0.05
a "Meat" represents liver, kidney, fat and muscle.
Studies over two years in experimental fields in Delaware, North
Carolina and Florida (Baude, 1972) showed that the amount of
non-volatile decomposition products of 14C-benomyl remaining in the
soil after one year were 57%, 49% and 57%, respectively, of the
quantity originally applied. After two years in soil in Florida, 27%
of the amount originally present remained. After one month only minor
amounts of unchanged benomyl were present, the principal metabolite
being MBC, with smaller amounts of 2-AB.
Uptake by crops grown in treated soils was studied in the same
experiments by Rhodes (1972) using 14C-benomyl. Trace amounts,
ranging from 0.02 to 0.08 ppm, could be found in tissues of carrots,
tomatoes, corn and beans, while edible snap beans, corn and tomatoes
contained less than the detection limit (i.e. <0.01 ppm). In this
study the benomyl content of the soil at the time of harvest was
0.9 ppm.
Methods of residue analysis
Pease and Gardiner (1969) describe colorimetric and fluorometric
procedures for determining benomyl in a variety of plant and animal
tissues and in soil. The residue is extracted, purified by partition,
converted to 2-aminobenzimidazole, and determined by direct
fluorometric measurement or by colorimetric analysis following
bromination. The procedure thus determines benomyl, its principal
degradation product methyl 2-benzimidazole carbamate, and the minor
residual component 2-aminobenzimidazole as a composite value. With a
sensitivity of 0.1 ppm for 50 g samples, recoveries were about 87% for
both methods. Pease and Holt (1971) improved this method to make it
less time-consuming and to include the analysis of citrus fruits. By
using fluorometric measurement from both acidic and basic media it was
possible to distinguish between benomyl residues and other
benzimidazole fungicides.
Peterson and Edgington (1969) report a method to determine
benomyl and the hydrolysis product, MBC, using a bioautograph
technique. A thin-layer plate is sprayed with a mixture of agar and
Penicillium spores, and the diameter of the zone of inhibited growth
above the fungitoxic spot is related to the amount of active material
in the spot. The sensitivity of the method is 0.1 ppm for a 50 g
sample. Somewhat similar agar plate methods have also been described
by Erwin et al. (1968), Baron (1971) and Holman and Fuchs (1970). A
two-dimensional TLC-technique for separation of benomyl, MBC, 2-AB and
thiophanate as well as thiophanate-methyl is described by von Stryk
(1972).
A UV-spectrophotometric method for determining residues in
grapes, must and wine has been described by Lemperle and Kerner
(1971). After addition of acetone the samples are extracted with
chloroform, the chloroform evaporated to dryness and the residues
measured in methanol at 301.5 nm. The limit of detection reported is
0.3 ppm for 100 g samples and recoveries of 89% for grapes, 75% for
must and 56% for wines. Mestres et al. (1971) used a
spectrophotometric method for fruits and vegetables, measuring the
hydrolysis product, MBC, at 282 nm. The limit of detection was 0.1 ppm
for 25 g samples.
Residues in meat and milk have been determined by the liquid
chromatographic procedure of Kirkland (1973). The method detects
4-hydroxymethyl-2-benzimidazole carbamate and
5-hydroxymethyl-2-benzimidazole carbamate, which are found in animal
systems, as well as benomyl and methyl-2-benzimidazole carbamate.
Kirkland et al. (1973) further adapted this method for the
determination of benomyl and/or MBC and 2-AB in soil and plant
material. Recoveries of the three compounds were 92, 88 and 72%,
respectively. The lower limit of sensitivity of the method is 0.05 ppm
for each of the components.
The methods based on spectrophotofluometry of 2-AB, on
UV-spectophotometry of MBC and the liquid chromatographic procedure
may all be adapted for regulatory purposes.
National tolerances (as notified to the meeting)
Country Commodity Tolerance, ppm
Australia Pome fruits, stone fruits 5
Vegetables, citrus 3
Grapes, mangoes, avocados
strawberries 2
Bananas 1
Peanuts 0.2
Belgium Apples, pears, cucumbers,
strawberries, celery,
small grain 1
Canada Peaches 15
Blackberries, boysenberries,
raspberries 6
Apples, apricots, cherries,
crabapples, grapes,
nectarines, pears, plums,
prunes, strawberries 5
Federal Citrus fruits 10
Republic Grapes 3.0
of Berries 2.0
Germany Vegetables (exc. cucumber), Total
stone fruit, bananas, citrus residue
fruits (without peel) 1.0 calculated
Cereals, cucumbers 0.5 as benomyl
Banana (without peel) 0.2
Other vegetable products 0.1
United Apricots, cherries, nectarines,
States of peaches and plums (including
America fresh prunes) (from post-
harvest and/or pre-harvest
applications), peanut hay,
peanut forage, sugar beet tops 15
Mushrooms 10
Apples, pears, blackberries,
boysenberries, dewberries,
loganberries, raspberries 7
Strawberries 5
Celery, mango 3
Snapbeans, peanut hulls 2
National Tolerances (cont'd)
Country Commodity Tolerance, ppm
Bananas (whole), cucumbers,
melons, summer squash, winter
squash, avocado, almond hulls 1
Banana (pulp), peanuts, sugar
beets (roots), nuts (almonds,
Brazil nuts, bush nuts,
butternuts, cashews, chestnuts,
filberts, hazelnuts, hickory nuts,
macadamia nuts, pecans, walnuts) 0.2
Milk and the meat, fat and meat
by-products of cattle, goats,
hogs, horses and sheep 0.1
Netherlands Fruit and vegetables 2 ) Determined
Raw cereals 0.5 ) and
Citrus fruits 3.5 ) expressed
) as
) MBC
South Fruits and vegetables 3
Africa
Appraisal
Benomyl is a relatively new systemic broad-spectrum fungicide
already established in most countries. It is marketed as wettable
powders and used in foliar applications, seed treatments and
post-harvest dipping procedures on a great number of food and forage
crops.
Benomyl is chemically easily hydrolysed into the relatively
stable methyl-2-benzimidazole carbamate (MBC) which is considered to
be the major fungitoxic principle of the compound. Formulated benomyl
may hydrolyze if not kept dry in storage.
A fungicidal effect additional to the MBC activity is claimed to
be connected with the simultaneous release of volatile butyl
isocyanate. Recent data has been presented suggesting that the
hydrolytic breakdown is of significance for the systemic properties of
benomyl facilitating the cuticular penetration. Evidence is given that
in the case of benomyl residues on foliar surfaces the formation of
MBC proceeds gradually. In a typical example a 50% cleavage of
residues remaining on apple leaves occurs three weeks after
application.
A further metabolic product found in plants is
2-aminobenzimidazole formed from MBC. The amount of this metabolite
found in plant material is, however, usually relatively small; so far
not being found in excess of 1% of total residues. For practical
purposes therefore, residues in plant material following application
of benomyl could be considered as the composite of benomyl, MBC and
2-AB or as the sum of benomyl and MBC alone.
Residue data justifying the establishment of several national
tolerances were available to the meeting, together with limited
information on residues on citrus fruits sampled from trade channels.
Included also is evidence of uptake into crops from treated soils.
Half-life of benomyl (metabolized to MBC) in soils varies from about
four months to one year indicating relatively long persistence with a
possibility of unintentional uptake by following crops.
Cow feeding trials show that hydroxylated methyl benzimidazole
carbamates are formed from benomyl with MBC as the probable
intermediate metabolite.
The most recent published report has confirmed this information
indicating that MBC (and possible 2-AB) are likely to be the primary
chemical entities to be absorbed from the alimentary tract following
the ingestion of benomyl residues.
The presence of hydroxylated MBC was demonstrated in milk and
meat residues of intact benomyl or MBC were detected.
Suitable methods of analysis for benomyl, MBC and 2-AB or for
benomyl and MBC as composite values have been published, both methods
being effective at the 0.1 ppm level. For one of these methods which
is based on spectrophotofluorometric measurement, modifications have
been introduced which allow the determination of benomyl and
metabolites and to differentiate these from other benzimidazole
fungicides such as thiabendazole. Although useful for regulatory
purposes neither of the two methods, however, permits the separate
determination of benomyl and metabolites when these are present
together.
RECOMMENDATIONS
As no acceptable daily intake was established it was not possible
to recommend tolerances. Following officially acceptable use in
various countries residues of benomyl and its metabolites can occur in
the following commodities up to levels indicated. Residues of benomyl
should be measured as the composite of benomyl and its main
metabolite, methyl benzimidazolecarbamate with or without minor
amounts of 2-aminobenzimidazole (usually less than 1% of total
residues), calculated as methyl benzimidazolecarbamate. The levels
indicated are not likely to be exceeded when benomyl is applied in
accordance with good agricultural practice, including either or both
pre-harvest and post-harvest treatments (when applicable). These
levels have been recommended as guideline levels.
For the underlined commodities adjustments have been made to
recommendations in order to accommodate good agricultural practices
for such alternative systemic fungicides of which MBC is also
recognized and identified as the major metabolite and/or chemical
entity (i.e. carbendazim and thiophanatemethyl).
Guideline levels
ppm
Citrus fruit (whole), cherries, grapes, peaches 10
Apples, pears, apricots, tomatoes, blackberries,
boysenberries, dewberries, loganberries, raspberries,
strawberries, blackcurrants 5
Celery, prunes, plums, beans (lima), mangoes,
beans (dry), beans (snap), nectarines 2
Mushroom, banana (whole) 1
cucumbers, melons, squash, brussels sprouts, avocados 0.5
Sugar beet, raw cereals, peanuts, almonds, macadamia 0.5
Nuts, pecans, potatoes 0.1a
Animal feedstuffs
Bean vines 30
Sugar beet leaves 5
Peanut hay, barley straw 2
Almond hulls 1
Meat of cattle and sheep 0.1a
Milk (whole)
a At or about limit of determination.
Residues to be measured as a composite of benomyl, MCB, 2-AB and
expressed as MBC.
FURTHER WORK OR INFORMATION
Required (before an acceptable daily intake can be estimated)
1. Full toxicological data.
Desirable
1. Further development of analytical methods to adapt them
for regulatory purposes, especially to permit separate
determination of benomyl and metabolites when present together.
2. Information on residues in food in commerce.
3. Information on the nature and level of residues in poultry and
eggs following the feeding of benomyl residues in rations.
REFERENCES
Anon. Residues of benomyl in certain foods. E.I. du Pont de
1972, 1973 Nemours & Co., Delaware. Unpublished reports filed
with FAO
Baron, M. Determination, migration and distribution of a
1971 systemic fungicide (benomyl) in the leaves of the
banana plant. Fruits, 26: 643
Baude, F. J. Disappearance of benomyl-2-14C from field soil
1972 in Delaware, North Carolina, and Florida. Paper
submitted by E.I. de Pont de Nemours & Co.,
Delaware (Unpublished)
Baude, F. J., Gardiner, J. A. and Han, J. C.-Y. Characterization
1973 of residues on plants following spray applications
of benomyl. Prepublication of paper submitted to
the J. Agr. Food Chem.
Bollen, G, J. A comparison of the in vitro antifungal spectra
1972 of thiophanates and benomyl. Neth J. Plant. Path.
78: 55
CIVO-TNO Unpublished reports on benomyl residues from Central
1972, 1973 Institute of Food Research (CIVO-TNO) of 11
January 1972 and 9 August 1973
Clemons, G.P. and Sisler, H. D. Formation of a fungitoxic
1969 derivative from Benlate. Phytopathology, 59: 705
Douch, P. G. C. The metabolism of benomyl fungicide in animals.
1973 Xenobiotica, 3: 367
Edgington, L. V., Khew, K.L. and Barron, G.L. Fungitoxic spectrum
1971 of benzimidazole compounds. Phytopathology, 61:
42
Erwin, D. C., Mee, H. and Sims, J. J. The systemic effect of
1968 1-(butylcarbamoyl)-2-benzimidazole carbamic acid,
methyl ester, on verticillium wilt of cotton.
Phytopathology, 58: 528
Evans, E. Systemic fungicides in practice, Pesticide Science,
1971 2: 192
Foldo, N. E. Systemic fungicides. Effect, advantages and
1973 disadvantages (in Danish), Ugeskr. f. agronomer og
hortonomer, 40: 730
Gardiner, J. A., Brantley, R. K. and Sherman, H. Isolation
1968 and identification of a metabolite of methyl
1-(butylcarbamoyl)-2-benzimidazolecarbamate in rat
urine. J. Agr. Food Chem. 16: 1050
Gardiner, J. A., Kirkland, J. J., Pease, H. L., Wall, E. N.
1973 and Morales, R. Unpublished studies. Cited from
Kirkland (1973)
Green-Lauridsen, M. and Voldum-Clausen, K. Reports on
1973 experiments on pesticide residues Nos 285-287,
290-293, 298-300, 302, 306, 308-310 from the
Danish National Food Institute and Government
Plant Pathology Institute, Copenhagen
(Unpublished)
Hammerschlag, R. S. and Sisler, H. D. Differential action
1972 of benomyl and methyl-2-benzimidazolecarbmate
(MBC) in Saccharomyces pastorianus. Pesticide
Biochem. Physiol. 2: 123
Hammerschlag, R. S. and Sisler, H. D. Benomyl and methyl-2
1973 benzimidazole (MBC): biochemical, cytological and
chemical aspects of toxicity to Ustilago
mavdis and Saccharomyces cerevesiae; Pesticide
Biochemistry and Physiology, 3: 42
Helweg, A. Persistence of benomyl in different soil types
1973a and microbial breakdown of the fungicide in soil
and agar culture. Tidskr. for Planteavl
(Copenhagen), 77: 232
Helweg, A. Influence of the fungicide benomyl on micro-organisms
1973b in soil. Tidsskrift for Planteavl (Copenhagen),
77: 375
Holman, A. L. and Fuchs, A. Direct bioautography on TLC as
1970 a method for detecting fungitoxic substances. J.
Chromatography, 51: 327
Kilgore, W. W. and White, E.R. Decomposition of the systemic
1970 fungicide 1991 (Benlate). Bull. Environm. Contam.
& Toxicol. 5: 67
Kirkland, J. Method for high-speed liquid chromatographic
1973 analysis of benomyl and/or metabolite residues in
cow milk, urine, feces and tissues. J. Agric. Food
Chem. 21: 171
Kirkland, J., Holt, R.F. and Pease, H.L. Determination of
1973 benomyl residues in soils and plant tissues by
high-speed cation exchange liquid chromatography.
J. Agr. Food Chem. 21: 368
Lemperle, E. and Kerner, E. UV-spektrophotometrische
1971 Bestimmung von Rücksttänden in Weintrauben,
Traubenmost und Wein nach Anwendung von Du Pont
Benomyl. Z. Anal. Chem. 254: 117
Lemperle, E., Kerner, E., Strecker, H. and Waibel, A.
1973 Wirkstoffrückstände und Gärbeeinflussungen nach
Anwendung von Fungiziden im Weinbau. Deutsche
Leb.-Rdschau, 69: 313
Mestres, R., Tourte, T. and Campo, M. Dosage des residus de
1972 benomyl dans les fruits et les legumes. Ann. Fals.
Exp. Chim. 65: 239
Ogawa, J. A., Bose, E., Manji, B. T., White, E. R. and
1971 Kilgore, W. W. Phytopathology, 70: 905 (Cited
from Baude et al., 1973)
Pease, H. L. and Gardiner, J. A. Fluorometric and colorimetric
1969 procedures for determining residues of benomyl. J.
Agr. Fd. Chem. 17: 267
Pease, H. L. and Holt, R. F. Improved method for determining
1971 benomyl residues. J. Assoc. Off. Anal. Chem. 54:
1399
Peterson, C. A. and Edgington, L. V. Quantitative estimation
1969 of the fungicide benomyl using a bioautograph
technique. J. Agr. Food Chem. 77: 898
Rhodes, R. C. Uptake of 2-14 C-benomyl soil residues by
1972 crops. Paper submitted by du Pont de Nemours &
Co., Delaware (Unpublished)
Siegel, M. R. and Zabbia, A. J., jr Distribution and metabolic
1972 fate of the fungicide benomyl in dwarf pea.
Phytopathology, 62: 630
Solel, Z. and Edgington, L. V. Transcuticular movement of
1973 fungicides. Phytopathology, 63: 505
Upham, P. M. and Delp, C. J. Phytopathology (In press).
1973 (Cited from Baude et al., 1973)
von Stryk, F. G. Separation and determination of some
1972 systemic fungicides and their metabolites by thin-
layer chromatography. J. Chromatogr. 72: 410
White, E. R., Bose, E. A., Ogawa, J. M., Manji, B. T. and
1973 Kilgore, W. W. J. Agr. Food Chem. (in press)
(Cited from Baude et al., 1973)
