
BENOMYL
First draft prepared by
M. Watson
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries and
Food, Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Neurotoxicity
Observations in humans
Exposure during field use
Medical surveillance of workers
Comments
Toxicological evaluation
References
Explanation
Benomyl was previously evaluated toxicologically by the Joint
Meeting in 1975 and 1983 (Annex I, references 24 and 40). In 1983, an
ADI of 0-0.02 mg/kg bw was established on the basis of a review of the
data on toxicity for carbendazim and benomyl and a safety factor that
is higher than normal in view of the paucity of data on individual
animals in many of the studies. The compound was reviewed at the
present Meeting as a result of the CCPR periodic review programme,
with particular attention to the recent WHO Environmental Health
Criteria monograph on benomyl (EHC 148). This monograph summarizes new
data on benomyl, data that were not reviewed previously, and relevant
data from the previous monographs on this pesticide.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Radiolabelled 14C-benomyl was administered by gavage to male
Sprague-Dawley rats either as a single dose of 1000 mg/kg bw to five
rats, which were sacrificed either 1, 2, 4, 7, or 24 h later, or as 10
repeated doses of 200 mg/kg bw per day to two rats, which were
sacrificed 1 or 24 h after the last dose. Blood and testes were then
analysed, as were those from rats fed 2500 ppm for one year. In rats
given 1000 mg/kg bw, the total amount of radiolabel (calculated as
benomyl) was 3-13 ppm in the blood and 2-4 ppm in the testes;
5-hydroxybenzimidazol-2-ylcarbamate (5-HBC) appeared in the blood and
testes as early as 1 h after dosing. The concentration of benomyl
and/or carbendazim decreased with time, with a corresponding increase
in the concentration of 5-HBC in blood and testes. In samples from
rats given repeated doses, no benomyl or carbendazim (< 0.1 ppm)
could be detected 1 h after the last dose, and only low levels of
5-HBC were found (1.5 ppm in blood and 0.3 ppm in testes); no benomyl,
carbendazim, or 5-HBC (< 0.1 ppm) was found 24 h after the last dose.
In blood and testes from rats fed 2500 ppm, no benomyl or carbendazim
(< 0.1 ppm) was detected, and only a minimal amount (0.2 ppm) of
5-HBC was found in blood and none in the testes (< 0.1 ppm) (Belasco
et al., 1969).
Benomyl and/or Benlate (a 50% benomyl formulation) were
administered by gavage or in the diet to pregnant Sprague-Dawley rats
on days 7-16 of gestation in order to determine the concentrations of
benomyl, carbendazim, and two carbendazim metabolites (4- and 5-HBC)
in maternal blood and embryonic tissue. The doses were 125 mg/kg bw
per day by gavage or 5000-10 000 ppm in the diet (about 400-800 mg/kg
bw). Blood samples from the dams and tissue samples from their embryos
were examined on days 1, 6, and 10 of dietary administration and on
days 12 and 16 of administration by gavage 1, 2, 4, 8, and 24 h after
gavage. The levels of benomyl and carbendazim in maternal blood and
embryonic tissues decreased markedly with the number of treatments
24 h after each dose. The level of benomyl 1 h after treatment was
0.98-8.4 mg/kg bw, with a mean of 5.0 mg/kg bw on the first day of
treatment. After 10 treatments, the levels of benomyl and carbendazim
1 h after the last treatment ranged from < 0.1 to 0.39 mg/kg bw.
Embryonic tissues contained 0.13 mg/kg benomyl and carbendazim after
the tenth treatment in comparison with a mean of 1.9 mg/kg after the
first treatment. The half-life of benomyl was about 45 min in maternal
blood and less in the embryos. The level of 5-HBC (0.84-2.9 mg/kg) 2 h
after the last gavage increased with the number of exposures, the
half-life in blood being 2-3 h in dams and 4-8 h in embryos. 4-HBC was
not detected. In the studies of dietary administration, the levels of
benomyl, carbendazim, and 4-HBC in embryonic tissue were too low to be
measured, and 4-HBC could not be detected in the dams. Irrespective of
the dose (5000 or 10 000 ppm) of benomyl or Benlate, the levels of
benomyl and carbendazim in maternal blood were very low. In three
separate groups of animals, the highest mean blood concentrations of
benomyl and carbendazim were 0.35, 0.61, and 0.23 mg/kg bw. The
highest mean value of 5-HBC, after administration of 5000 ppm benomyl
in the diet, was 0.44 mg/kg bw in the blood and 0.33 mg/kg bw in the
embryos. Animals fed benomyl or Benlate at 10 000 ppm had 5-HBC levels
an order of magnitude higher (Culik, 1981a,b).
Three groups of five rats of each sex were given [phenyl(U)-
14C]-carbendazim by gavage. One group received a single dose
of 50 mg/kg bw; the second received a single dose of 50 mg/kg bw
after 14 days of preconditioning with non-radiolabelled carbendazim at
50 mg/kg bw per day; and the third group received a single dose of
1000 mg/kg bw. In all groups, > 98% of the recovered radioactivity
had been excreted in the urine or faeces by the time of sacrifice
(72 h after treatment). Urinary excretion accounted for 62-66% of the
dose in males and for 54-62% of the dose in females given the low dose
or preconditioned; in animals given the high dose, this pathway
accounted for 41% of the dose. Elimination in the faeces accounted for
virtually all of the remaining radiolabel. There were no apparent
differences between male and female rats with respect to the extent of
absorption or the extent and rate of elimination of 14C-carbendazim
equivalents within a given treatment group. The amount of radiolabel
remaining in tissues represented < 1% of the applied dose (Monson,
1990).
Groups of four male Sprague-Dawley rats received dermal
applications of 0.1, 1, 10, or 100 mg benomyl (as [2-14C]-Benlate
50% wettable powder) at 0.5-, 1-, 2-, 4-, or 10-h intervals. Benomyl
was slowly absorbed across an area of skin representing 16% of the
total, appearing in the blood and urine within 30 min and reaching a
maximum at 2-4 h. The concentration of benomyl and its metabolites in
the blood peaked at 0.05 mg/litre (2-h sample) in the group given the
lowest dose and at 0.10 mg/litre (4-h sample) in that given the
highest dose, representing a 20-fold increase in blood concentration
for a 1000-fold increase in dose. Thus, absorption into the
bloodstream was non-linear with respect to dose (Belasco, 1979a).
Formulated benomyl (Benlate 50% wettable powder) poorly
penetrated human skin in vitro after application at the recommended
strength. Even less penetration was detected when dry concentrated
benomyl was applied (Ward & Scott, 1992).
After intravenous injection of 1 or 10 mg of radiolabelled
benomyl (as 14C-Benlate 50% wettable powder) to groups of 10 male
Sprague-Dawley rats, radiolabel was found in the urine as 5-HBC 6, 12,
and 24 h after dosing; little radiolabel was found in the blood or
faeces at these times. More than 80% of the dose was excreted,
predominantly in the urine, within 6 h after injection, and > 95% was
recovered within 24 h. No radiolabel (< 0.1%) was found in any tissue
after 24 h, although blood contained trace quantities of 14C
residues. Isolated cases of apparent faecal excretion of radiolabel
were reported probably to be due to wetting of faeces with urine (Han,
1979).
Blood levels of benomyl and its metabolites were measured 6 and
18 h after exposure of male rats to time-weighted average levels of
0.32 or 3.3 mg/litre of air for 0.5, 1, 2, and 6 h. The analytical
method did not allow distinction between benomyl and carbendazim. The
blood concentrations of benomyl and carbendazim were 0.39-2.3 mg/litre
after exposure to the low dose and 0.25-1.2 mg/litre after the high
dose; both were greater than that of 5-HBC 6 h after exposure. At 18 h
after exposure, only 5-HBC was detected in the blood (at 1.1 mg/litre)
and only at the highest dose. The urinary metabolites consisted
primarily of 5-HBC; small amounts of benomyl and carbendazim were also
detected (Turney, 1979)
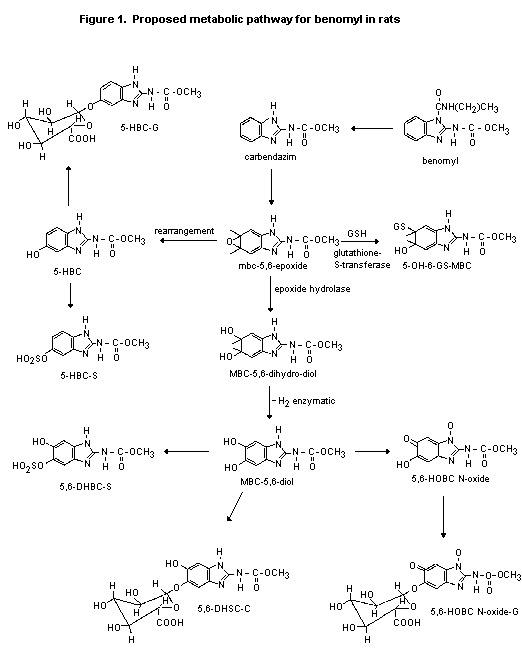
(b) Biotransformation
Benomyl is extensively metabolized by rats to carbendazim, which
is then further metabolized. Studies of rats administered benomyl
intravenously (Han, 1979), dermally (Belasco, 1979a), or by inhalation
(Turney, 1979) showed that 5-HBC is the main urinary metabolite,
although some carbendazim is also present.
The metabolism of benomyl was investigated in one male
Sprague-Dawley rat fed a diet containing 2500 ppm unlabelled benomyl
for 12 days and then a single oral dose of 7.7 mg methyl
1-(butylcarbamoyl)-2-14C-benzimidazole carbamate. Urine, faeces, and
carbon dioxide were collected for 72 h before the animal was killed
and its tissues analysed for radiolabel. Most (79%) of the radiolabel
was excreted in urine within 24 h; by 72 h, 85.8% of the label had
been excreted in the urine and 13.1% in the faeces. The tissue levels
were < 0.1% of the applied dose, except in the gastrointestinal tract
and liver where 0.2% of the applied dose was found; 0.02% of the dose
was found in the carcass. The 24-h urine sample was compared with
reference standards on silica gel thin-layer chromatography plates:
The main urinary metabolite was identified as methyl 5-HBC (Gardiner
et al., 1974).
Two male CD-1 mice were fed a diet containing 2500 ppm unlabelled
benomyl for 21 days and were then given a single oral dose of 2.5 mg
[2-14C]-benomyl. Urine and faeces were collected at 24-h intervals
for 72 h, and the mice were then killed and tissues were examined for
radiolabel. Most of the radiolabel was excreted within 24 h, with 64%
in urine and 11.7% in faeces. The tissue levels represented < 0.01%
of the applied dose except in the gastrointestinal tract (0.2%), liver
(0.12%), and skin (0.18%). Urine and faecal samples were analysed for
metabolites on silica gel thin-layer chromatography plates: The main
metabolite found in urine was methyl 5-HBC; only unchanged benomyl was
detected in faeces (Han, undated).
The metabolism of [2-14C]-benomyl was also investigated in
hamsters. The same procedure as described above was followed, except
that a dose of 7.5 mg radiolabelled benomyl was used. Excretion was
slower than in the mouse, 4.43% being excreted in the urine and 14.8%
in the faeces within 24 h. By 72 h, 67.7% of the applied dose had been
excreted in the urine and 22% in the faeces. The tissue levels were
again low: The highest levels were found in the gastrointestinal tract
(1.2% of the administered dose), liver (0.36%), and skin (0.94%). The
main metabolite detected in urine was methyl 5-HBC. In the faeces, the
main compound detected was unchanged benomyl, with traces of 5-HBC
(Han, undated).
Three Sprague-Dawley rats were given a single oral dose of 5-8 mg
14C benomyl-1-carbamoyl- n-[1-3H]butyl) in peanut oil. Within the
first 29 h, 55-69% of the 3H label and 76-89% of the 14C label had
been recovered, mainly in the urine and as exhaled carbon dioxide.
Chromatography of urine samples on an anion-exchange resin followed by
chromatography on silica gel allowed tentative identification of one
metabolite as butylamine (Axness & Fleeker, 1979).
In the study described above in which rats were given carbendazim
by gavage, 5-HBC- S(see Figure 1; 21-43% of dose) was identified as
the main metabolite, except in preconditioned female rats at the low
dose (5.5-10%); in all female rats, 5,6-HOBC-N-oxide-G was the
predominant metabolite (10-19%). 5,6-DHBC-S and 5,6-DHBC-G were
identified as minor metabolites. In faeces collected at the same times
as the urine, the total recovery was about 24% for males and 33-38%
for females at the low dose, with or without preconditioning, and
> 60% for males and females at the high dose. Unchanged carbendazim
represented 10-15% of the administered dose in the faeces of rats at
the high dose (Monson, 1990).
The proposed metabolic pathway of benomyl in rats is shown in
Figure 1.
(c) Effects on enzymes and other biochemical parameters
Acetylcholinesterase from bovine erythrocytes was not
inhibited by benomyl in vitro, the inhibition constant being
> 1 × 10-3 mol/litre (Belasco, 1979b). Krupka (1974) confirmed
that benomyl inhibits neither acetylcholinesterase nor butyryl-
cholinesterase in vitro.
 The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed diets containing benomyl or carbendazim at a concentration of 0,
10, 30, 100, 300, 1000 or 3000 ppm for 28 days. After sacrifice, liver
weights were recorded and microsomal epoxide hydrolase and cytosolic
glutathione- S-transferase were monitored in subcellular fractions
isolated from the liver. The mean absolute liver weights were elevated
in males and females fed 1000 or 3000 ppm carbendazim and in females
fed 300 ppm; however, the only significantly increase was found in
females fed 3000 ppm benomyl. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced epoxide
hydrolase in male and female rats and in mice fed 1000 or 3000 ppm,
and both induced glutathione- S-transferase at 3000 ppm. The level of
induction seemed to be slightly greater in females than males. There
was no substantial difference in enzyme induction between rats and
mice (Guengerich, 1981).
Administration of 1000 or 4000 ppm benomyl in the diet to groups
of eight female albino rats and female Swiss albino mice for 15 days
increased the activity of blood and liver gamma-glutamyl transpeptidase
in both species, and the degree of induction was dose related (Shukla
et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
1. The clinical signs of toxicity after treatment with benomyl were
generally nonspecific. Gross and histopathological changes were
evaluated in some studies; in male gonads, testicular degeneration,
necrosis of germinal epithelium, and aspermatogenesis were observed in
rats and dogs.
(b) Short-term toxicity
Rats
After intubation of benomyl into male Sprague-Dawley rats at 200
or 3400 mg/kg bw in peanut oil five times per week for two weeks, four
of six animals at the high dose died. At this dose, degeneration of
germinal epithelium, multinucleated giant cells, and reduction or
absence of sperm were seen. Very minor changes were observed in the
testes of the animals treated with the lower dose (Sherman & Krauss,
1966).
Male Sprague-Dawley rats treated orally for 10 days with
200 mg/kg bw per day of the metabolite 5-HBC (methyl ester) had no
toxic symptoms or evidence of effects on the testes (Snee, 1969).
Table 1. Acute toxicity of benomyl, benomyl formulations, and benomyl metabolites
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Benomyl Rat Oral > 10 000 > 95 Sherman (1969a)
Benomyl Dog Oral > 1 000 > 95 Sherman (1969b)
'50% wettable powder' Rat Oral > 1 000 > 95 Sherman (1969a)
'50% wettable powder' Rat Oral > 5 000 NR Sarver (1987)
'50% wettable powder' Rat Oral > 1 200 NR Hostetler (1977)
'50% wettable powder' Rabbit Oral > 3 400 NR Fritz (1969)
'50% wettable powder' Rabbit Dermal > 10 000 NR Busey (1968a)
'50% wettable powder' Rabbit Dermal > 2 000 NR Brock (1987)
'50% wettable powder' Rabbit Dermal > 2 000 NR Gargus & Zoetis (1983a)
'50% wettable powder' Rat Inhalation (4-h) > 0.82 NR Hornberger (1969)
'50% wettable powder' Rat Inhalation (4-h) > 4.01 NR Busey (1968b)
'50% wettable powder' Dog Inhalation (4-h) > 1.65 NR Littlefield & Busey (1969)
2-Benzimidazole carbamic Rat Intraperitoneal > 10 000 NR Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole Rat Oral > 7 500 > 95 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole Rat Oral > 3 400 > 95 Fritz & Sherman (1969)
Benzimidazole 2-(3-butylureido) Rat Oral > 17 000 NR Dashiell (1972)
S-Triazine, 3-butyl-benzimidazole Rat Oral > 17 000 NR Barbo & Carroll (1972)
(1,2a)-2,4(1H,3H)-dione
NR, Not reported
Groups of 16 male and 16 female Sprague Dawley rats were fed
benomyl (72% pure) in the diet at 0, 100, 500 and 2500 ppm (as
benomyl). After 96-103 days of continuous feeding, 10 male and 10
female rats in each group were killed, and selected organs were
weighed; additional organs were preserved for microscopic examination.
The remaining animals in each group were studied for reproductive
toxicity (see below). There were no gross toxic signs of poisoning and
no compound-related effects on weight gain, food consumption, food
efficiency, or haematological, biochemical, or urine parameters. The
liver body weight ratio was slightly increased in females at 2500 ppm
in comparison with control rats, Gross and microscopic examination of
tissues and organs showed no significant effect attributable to the
presence of benomyl in the diet at levels up to and including 2500 ppm
(Sherman et al., 1967).
Groups of 20 male and 20 female Sprague-Dawley rats were exposed
to benomyl by inhalation at 0, 10, 50, or 200 mg/m3 via the nose
only, for 6 h/day for 90 days. After 45 and 90 days, blood and urine
samples were collected from 10 rats of each sex per group for clinical
analysis; the animals were then killed for pathological examination.
After about 45 days of exposure, degeneration of the olfactory
epithelium attributable to benomyl was observed in all males and in
eight of the 10 females exposed to 200 mg/m3; two male rats exposed
to 50 mg/m3 had similar but less severe olfactory degeneration. After
about 90 days of exposure, all of the animals at 200 mg/m3 and three
males at 50 mg/m3 had olfactory degeneration. No other compound-
related pathological effects was observed. Male rats exposed to
200 mg/m3 had depressed mean body weights in comparison with
controls; the depression was correlated with a reduction in food
consumption (Warheit et al., 1989).
Rabbits
Groups of five male and five female New Zealand albino rabbits
were given 15 dermal applications for 6 h/day, five days per week for
three weeks of a 50% benomyl formulation (equivalent to 1000 mg/kg bw)
on both abraded and intact abdominal skin. After each application, the
abdomen was washed with tap water. Mortality and toxic effects were
monitored daily and body weight changes weekly; gross necropsy and
microscopic examinations were performed. Slight erythema, oedema, and
atonia were observed on both abraded and intact skin sites, and slight
to moderate desquamation was reported throughout the study. No
apparent compound-related changes in body weight or organ:body weight
ratios were reported. Treatment induced degeneration of the
spermatogenic elements of the seminiferous tubules of the testes,
including vacuolated and multinucleated spermatocytes. A slight
increase in haematogenic activity in the bone marrow and acanthosis
and hyperkeratosis of the skin were reported in treated animals
(Busey, 1968c).
Groups of five male and five female New Zealand albino rabbits
received benomyl at doses equivalent to 0, 50, 250, 500, 1000, and
5000 mg/kg bw on non-occluded, abraded dorsal skin for 6 h/day, five
days per week for three weeks. The material was removed by washing the
skin site and drying with a towel. The body weight gains of males and
females at > 1000 mg/kg bw were reduced, and mild-to-moderate skin
irritation was reported in all groups, especially at the higher doses.
Functional disturbances of the alimentary canal and kidney, including
diarrhoea, oliguria, and haematuria, were observed in males and
females at 1000 and 5000 mg/kg bw. Decreased testicular weights were
observed at 1000 mg/kg bw only, but these were considered not to be
directly related to treatment. No histopathological changes were
reported (Hood, 1969).
Dogs
Groups of four male and four female beagle dogs were given
benomyl (as a 50% wettable powder) in the diet at 0, 100, 500, or
2500 ppm (based on active ingredient) for three months. There was no
mortality or adverse clinical effect during the course of the study,
and growth and food consumption were normal. Urinary parameters were
unaffected by treatment. There were no dose-related effects on
haematological values, but alkaline phosphatase and alanine
aminotransferase activities were increased in males and females at the
highest dose, and the albumin:globulin ratio was significantly
decreased in both males and females fed 2500 ppm. In males and females
at the high dose, the weight of the thymus was decreased and that of
the thyroid was increased. One female fed 2500 ppm had an enlarged
spleen, which was consistent with the decreased erythrocyte count,
haemoglobin concentration, and haematocrit values. Histopathological
examination revealed myeloid hyperplasia of the spleen and bone marrow
and erythroid hyperplasia in the same animal. Three of four males fed
2500 ppm had reduced prostate weights in comparison with controls.
Microscopic examination of tissues and organs showed no consistent
effect. The NOAEL was 500 ppm (Sherman, 1968).
Groups of four male and four female beagle dogs were fed benomyl
(50% pure) in the diet at 0, 100, 500, or 2500 ppm (as benomyl) for
two years, with interim sacrifice after one year of one male and one
female from the control and high-dose groups. Organ weights, gross
necropsy, and histopathological evaluations were performed at the
conclusion of the study; only the livers and testes of animals fed 100
or 500 ppm were examined histologically. No deaths were attributable
to treatment. Body weight changes and food consumption were similar in
all groups, except for those at the high dose, which had decreased
food intake and body weight gain; one dog at the high dose lost its
appetite and was replaced. No other clinical signs of toxicity were
observed. The results of haematological evaluations and urinalysis
were similar to those of the controls. Males at 2500 ppm had
increased levels of cholesterol, alkaline phosphatase, and alanine
aminotransferase (initially) and decreased total protein and albumin:
globulin ratio. Similar, but less marked, effects were seen in
females at this dose. Biochemical analyses indicated adverse effects
on the liver, expressed as liver cirrhosis. Four of six dogs at this
dose had slight-to-marked bile duct proliferation. Haemosiderosis was
seen after specific staining for iron in one dog at the high dose
after one year but not in other dogs examined at two years.
Preparation of preserved wet tissue with oil red O and Sudan black for
hepatocyte vacuolation showed that benomyl is not hepatotoxic at 100
or 500 ppm in the diet. Focal testicular degeneration was seen in all
groups, including controls, with marked testicular degeneration
(reduced testicular weight, absence of spermatozoa, and spermatic
giant cells) in one of three dogs at 2500 ppm. The NOAEL was 500 ppm,
equivalent to 13 mg/kg bw per day (Sherman, 1970).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD-1 mice were given benomyl
(99% pure) in the diet at 0, 500, 1500, or 5000 ppm (reduced from
7500 ppm after 37 weeks) for two years. Survival was unaffected by
treatment. Male and female mice fed 1500 or 5000 ppm benomyl had
dose-related decreases in body weight. Food consumption was variable
throughout the study, although females at the high dose appeared to
consume less food. Clinical signs of toxicity were similar in all
groups; there were no apparent differences in palpable masses,
numbers of mice affected, or latency to detection. The results of
haematological examinations were unremarkable except for decreased
erythrocyte counts in males at 1500 ppm and in females at 5000 ppm.
Haemoglobin and haematocrit values were also depressed in males at the
intermediate dose. Several statistically significant changes in organ
weights were seen in treated groups, the most significant being
reduced absolute and relative liver weights in males at 1500 and
5000 ppm and females at 5000 ppm. Male mice at the high dose also had
decreased absolute testicular weights. The changes in liver and
testicular weights were accompanied by histomorphic changes in these
tissues. The incidence of hepatocellular carcinomas and benign hepatic
neoplasms in treated female mice was increased in a dose-dependent
fashion. In male mice, hepatocellular carcinomas and benign hepatic
neoplasms were significantly increased at 500 and 1500 ppm, but not at
the high dose. The incidences of lung tumours (alveologenic
carcinomas) were also significantly increased in males but not in
females at 500 and 1500 ppm. Non-neoplastic changes in males at
5000 ppm were confined to the liver (degeneration, pigment,
cytomegaly), thymus (atrophy), and the testis, epididymus, and
prostate (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini). The occurrence of splenic
haemosiderosis was significantly increased in female mice at 5000 ppm
and that of submucosal lymphocytic infiltration of the trachea at
1500 ppm. The latency for liver tumour induction (adenomas and
carcinomas), as determined from palpation, gross necropsy, and
histopathology performed on all animals throughout the study, was not
measurably different in treated and control animals. The authors
concluded that, under the conditions of the study, benomyl is
oncogenic in male and female animals. No NOAEL was identified for male
or female mice with respect to hepatocellular carcinomas or combined
hepatocellular neoplasms. The lowest dietary level, 500 ppm, was
equivalent to 64 mg/kg bw per day in males and to 103 mg/kg bw per day
in females (Wiechman, 1982).
Rats
Groups of 36 male and 36 female Charles River albino weanling
rats were fed benomyl (purity, 50-70%) in the diet for 104 weeks at
100, 500, or 2500 ppm (as benomyl); there were two control groups.
After one year, each group was reduced to 30 males and 30 females by
interim sacrifice for gross and microscopic evaluation. At the
conclusion of the study, all surviving animals were sacrificed and the
tissues and organs examined grossly. No deaths were attributable to
the presence of benomyl in the diet. Survival was decreased to about
50% during the second year but was comparable in all groups. Body
weight, food consumption, and food efficiency were unaffected by
treatment. There were no compound-related clinical manifestations of
toxicity, and the results of haematological, urinary, and liver
function examinations were unaffected by treatment. There were no
differences in organ weights, and histopathological examination
revealed no differences between treated and control groups. The most
frequently observed tumours were of the mammary, pituitary, and
adrenal glands, but these were equally distributed among the groups.
Hepatic toxicity was similar in all groups, including controls, and
there were no compound-related effects. As a high incidence of
testicular degeneration was observed in control males, no conclusion
could be drawn about compound-related effects on male gonads. Benomyl
had no adverse effects in this study at levels up to and including
2500 ppm, which was equivalent to 109 mg/kg bw per day in males and
128 mg/kg bw per day in females (Sherman, 1969c; Lee, 1977).
(d) Reproductive toxicity
In a short-term study of toxicity (see above), groups of six male
and six female Sprague-Dawley rats were fed diets containing benomyl
at 0, 100, 500, or 2500 ppm. Each female was caged separately and
exposed to each of three males at the same dose for five days. The
females were then allowed to deliver, and the offspring were observed
until weaning. Litters containing more than 10 pups were reduced to 10
by randomly removing pups on day 4. There was no treatment-related
effect on pregnancy rate, number of pups born live or dead, fertility,
gestation, viability, or lactation indices or on average body weight
(Sherman et al., 1975).
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal male Sprague-Dawley rats aged
33 days, which were gavaged daily for 10 days with 0 or 200 mg/kg bw.
Eight animals per group were killed 3, 17, 31, 45, and 59 days after
the last treatment. Selected tissues, including liver, kidneys,
testes, seminal vesicles, and epididymides, were removed, weighed, and
examined histologically; samples of seminal fluid from the vas
deferens were also examined. Observation intervals were preselected to
coincide with stages of spermatogenesis. No effects related to
treatment were seen on tissue weights, total epididymal sperm counts,
was deferens sperm concentrations, or testicular histology (Carter,
1982).
In a similar experiment, groups of five animals were dosed orally
on five consecutive days with 0, 125, 250, 500, or 1000 mg/kg bw per
day, beginning when animals were 33 (prepubertal), 54 (pubertal), or
75 (post-pubertal) days old. Animals were killed 31 days after
the last dose. There was no effect on body weight gain or on
haematological values. The weights of the testes and cauda
epididymides were reduced in animals given 500 or 1000 mg/kg bw per
day benomyl during the onset of puberty. Cauda epididymal and vas
deferens sperm counts were reduced in animals given > 250 mg/kg bw
per day benomyl during puberty. The incidence of diffuse hyposperma-
tocytogenesis was increased in pubertal and post-pubertal animals but
was most pronounced in the latter. The NOAEL for effects on
reproductive development was 12.5 mg/kg bw per day (Carter, 1982).
In a further experiment, adult (65-day-old) male Sprague-Dawley
rats received 10 daily doses of benomyl at 0, 200, or 400 mg/kg bw per
day by gavage. Fourteen days after the last treatment, body weights,
tissue weights, total epididymal sperm counts, and sperm concentration
in the vas deferens were measured, and the testis was examined
histologically. Production of testosterone by the Leydig cells was
artificially stimulated by subcutaneous injections of hypothalamic
chorionic gonadotrophin 2 h before sacrifice. There were no compound-
related effects on the weights of the body, liver, kidney, adrenal,
testis, or seminal vesicles, but caudate epididymal weights were
depressed by treatment and there were treatment-related reductions in
epididymal sperm count (caput and caudate) and in vas deferens sperm
concentration. In animals exposed to the highest dose, there was
histological evidence of hypospermatocytogenesis, with generalized
disruption of all stages of spermatogenesis (Carter & Laskey, 1982).
Benomyl was administered by gavage to Wistar rats at doses of 0,
15.6, or 31.2 mg/kg bw per day from day 7 of gestation to day 15 of
lactation (day 22 of gestation was considered day 0 of lactation). The
litters were each reduced to four male and four female pups on day 3
of lactation. The litters were weighed on days 8, 15, 22, 29, and 100,
and locomotor activity was evaluated periodically throughout the
study. When the animals were 100 days of age, several organs were
weighed, including adrenals, liver, kidney, ovary, testis, and ventral
prostate plus seminal vesicles. There were no compound-related effects
on litter size at birth or weaning or on body weights of fetuses.
Growth, survival, and locomotor activity were comparable to those of
controls throughout the study. Organ weights were comparable to t hose
of controls except for decreased weights of testes and ventral
prostate/seminal vesicles, which were significantly reduced at 31.2
but not at 15.6 mg/kg bw per day (Kavlock et at., 1982).
In a two-generation study, Sprague-Dawley rats were fed diets
containing 0, 100, 500, 3000, or 10 000 ppm benomyl. Parental rats
received the test diets for 71 days before being bred for production
of F1 parental rats with animals at the same dietary concentration.
F1 rats were mated for production of the F2a litter after
maintenance on their respective diets for at least 105 days after
weaning. F1 dams were mated again, to different, non-sibling males,
at least one week after weaning the F2a litter, to produce the
F2blitters. The following indices of reproductive Junction were
calculated for the F0 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive, and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption, and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental rats
were sacrificed lot gross examination and histopathological
examination of gross lesions and target organs; complete histopatho-
logical examination was conducted on controls and animals at the high
dose. Groups of 20 F2a and 20 F2bweanlings were also examined
grossly. There were no compound-related effects on parental mortality
at any concentration of benomyl. Mean body weights, body weight gains,
and overall food consumption of F0 and F1 male and female rats at
10 000 ppm were significantly lower than those in controls, and there
was a significant compound-related decrease in the number of F2a and
F2boffspring alive before culling (on day 4) at this dose. In
addition, male and female offspring of rats fed 10 000 ppm weighed
consistently less at birth than did the offspring of control rats.
With the exception of 14-day male pups of the F2bgeneration, F2a
and F2b offspring at 3000 ppm also had significantly depressed body
weights on days 14 and 21 of lactation. The testicular sperm counts of
F0 and F1 rats at 3000 and 10 000 ppm were decreased, and rats at
the highest dose had decreased testicular weight and histopathological
changes in the testes. Microscopic observation showed a trophy and
degeneration of the seminiferous tubules in the testes of rats at 3000
and 10 000 ppm and oligospermia in the epididymides of F0, rats at
the highest dose and in F1 rats at 3000 and 10 000 ppm. There were
no compound-related differences in mating indices, fertility indices,
or length of gestation that could be attributed to benomyl. The NOAEL
was 500 ppm, equivalent to about 37 mg/kg bw per day (Mebus, 1990).
In a study of adult male Wistar rats, groups of 27 animals were
fed 0, 1, 6.3, or 203 ppm for 70 days, and 14 animals per group were
allowed to recover for 70 days. Ejaculated sperm counts were
reportedly depressed during the feeding phase in the group fed
203 ppm. Relative testicular weights were also lowered, and a slightly
reduced male fertility index was seen in all treated groups. Benomyl
did not alter copulatory behaviour and did not induce dominant lethal
mutation. Plasma testosterone and gonadotropin levels remained
unchanged throughout the study. Identical studies conducted during the
recovery phase showed that the treatment-related effects were
completely reversible (Barnes et al., 1983).
Groups of 12 adult (102-day-old) Wistar male rats were given
benomyl at 0, 1, 5, 15, or 45 mg/kg bw per day by gavage daily for 62
days, were then bred to untreated females after 62 days of dosing, and
were killed after 76-79 days. Reproductive behaviour, seminal vesicle
weight, prostate weight, sperm motility, and the levels of serum
gonadotropin and gonadal hormones were no different from those in
controls. At necropsy, males exposed to 45 mg/kg bw per day had
decreased testicular and epididymal weights, reduced caudal sperm
reserves, decreased sperm production, increased numbers of decapitated
spermatozoa, and increased numbers of seminiferous tubules containing
multinucleated giant cells. There were no treatment-related effects on
the levels of serum luteinizing hormone, follicle stimulating hormone,
prolactin, or androgen binding protein (Linder et al., 1988).
Groups of 20 male Sprague-Dawley rats aged 100 days were given a
single dose of benomyl in corn oil at 0, 25, 50, 100, 200, 400, or
800 mg/kg bw. Eight animals per group were sacrificed after two days
of treatment and 12 animals per group after 70 days (except in the
group receiving 800 mg/kg bw) in order to examine the testis and
efferent ducts for effects on spermatogenesis or the epididymis. The
primary effects seen at day 2 were testicular swelling and occlusions
of the efferent ductules. Premature release of germ cells (sloughing)
was the most sensitive short-term response to benomyl. It was detected
in all treated groups but was statistically significant (P < 0.05)
only at doses > 100 mg/kg bw. Occlusion of the efferent ductules of
the testis was dose-dependent and was correlated with the increase in
testicular weight on day 2. The greatest increase in testicular weight
was observed at 400 mg/kg bw. Long-term effects (70 days) were seen
only in animals at > 100 mg/kg bw and included decreased testicular
weight at 400 mg/kg bw, dose-dependent increases in seminiferous
tubular atrophy, and increases in the number of reproductive tracts
containing occluded efferent ductules (Hess et al., 1991).
(e) Developmental toxicity
Mice
Groups of 20-25 pregnant CD-1 mice were given benomyl by gavage
at 0, 50, 100, or 200 mg/kg bw per day on days 7-17 of gestation. They
were killed on day 18, their pups were delivered by caesarean section,
the numbers of live, dead, and recorded fetuses were determined, and
the fetuses were examined for gross anomalies (one-half for visceral
abnormalities and the other half for skeletal abnormalities). Maternal
indexes were unaffected by treatment, but fetal mortality was
increased, fetal weight was decreased, and fetal development was
adversely affected by treatment. The high dose increased the
supraoccipital score, decreased the numbers of caudal and sternal
ossifications, and increased the incidences of enlarged lateral
ventricles and enlarged renal pelvises. The latter increase, while not
significant at the lowest dose, was dose-related at the other doses.
The occurrence of supernumerary ribs and subnormal vertebral centra
was significantly increased in a dose-related manner at all doses. The
numbers of abnormal litters and fetuses were significantly increased
over those among the controls at 100 and 200 mg/kg bw per day. Major
anomalies included exencephaly, hydrocephaly, cleft palate,
hydronephrosis, polydactyly, oligodactyly, umbilical hernia, fused
ribs, fused vertebrae, and short or kinky tail. Although benomyl
induced dose-related fetotoxicity at all levels, it was not
teratogenic at 50 mg/kg bw per day in mice (Kavlock et al., 1982).
In a study designed to investigate whether teratogens could be
detected by observing postnatal viability or impaired growth, groups
of 24 pregnant CD-1 mice were treated orally with benomyl at 0 or
200 mg/kg bw per day on days 8-12 of gestation. Postnatal viability
was reduced on days 1 and 3, and average pup weight was reduced on day
1 post partum (Chernoff & Kavlock, 1983).
Rats
Groups of 26-28 pregnant Sprague-Dawley rats were given benomyl
(purity, 53.5%) in the diet at 0, 100, 500, 2500, or 5000 ppm on days
6-15 of gestation to provide average doses equivalent to 0, 8.6, 43.5,
209.5, and 372.9 mg/kg bw per day. On day 20 of gestation, all
pregnant animals were sacrificed and their fetuses were delivered by
caesarean section. There were no deaths attributable to treatment, no
clinical signs of toxicity, and no adverse effects on the body weights
of dams. Dams at 5000 ppm had reduced food intake during treatment,
but consumption returned to control levels for the remainder of the
study. Indicators of reproductive health (numbers of implantation
sites, resorption sites, and live and dead fetuses) were not affected
by treatment up to and including the highest dietary level. The only
external or internal abnormalities reported were hydronephrosis and
retarded ossification of the interparietal and occipital bones in
three litters at the highest dose. None of these teratogenic effects
was associated with the administration of benomyl during the critical
period of organogenesis (Sherman et al., 1975).
Groups of 27-28 pregnant Wistar rats were fed benomyl in the diet
at 0, 1690, 3380, or 6760 ppm, equivalent to 0, 169, 298, or 505 mg/kg
bw per day, on days 7-16 of gestation. Fetuses were delivered by
caesarean section on day 21, weighed, examined grossly, and fixed for
evaluation of visceral and skeletal abnormalities. Food consumption
was decreased in dams at 3380 or 6760 ppm, with resultant decreases in
body weight gain, which were significant at the high dose. The weight
gain of fetuses at the two higher doses was significantly reduced, and
a significant decrease in the ossification of the supraoccipital bone
was seen at the high dose. The percentage of fetuses with an enlarged
renal pelvis was increased at the two higher doses. No dose-related
major malformations were seen at these dietary levels (Kavlock
et al., 1982)
Benomyl (99.2% pure) was administered by gavage to pregnant
Sprague-Dawley rats at doses of 0, 3, 10, 30, 62.5, or 125 mg/kg bw
per day on days 7-16 of gestation. A control group receiving corn oil
comprised 60 rats, and each test group comprised 27 rats. No clinical
signs of toxicity or mortality were observed at any dose. Body weight
gain, the incidences of pregnancy, corpora lutea, and implantation
sites, and the sex ratio were comparable to those of controls;
however, fetal body weight was significantly decreased at 62.5 and
125 mg/kg bw. There was also an increased incidence of embryofetal
mortality at 125 mg/kg bw. At doses > 10 mg/kg bw per day, some
fetuses had external and visceral malformations, which included
microphthalmia, anophthalmia, and hydrocephaly (distended lateral
ventricles), predominantly at the higher doses. Histological
examination of the eyes revealed pathological changes consisting of
irregular lenses, retrobulbar glandular adnexa, distorted or
compressed retinal layers, and thickened nerve fibres in the groups
receiving 10, 62.5, or 125 mg/kg bw per day; no alterations were seen
in controls or in animals at 30 mg/kg bw per day. The major skeletal
malformations observed at 125 mg/kg bw per day included fused ribs,
sternebrae, and thoracic arches. Additional skeletal variations seen
at 62.5 and 125 mg/kg bw per day included misaligned and unossified
sternebrae and bipartite vertebral centra. The authors concluded that
benomyl produced teratogenic responses in rats at doses > 10 mg/kg
bw per day. Microphthalmia seen at this dose was reported to be
related to treatment on the basis of the severity of the pathological
changes and the finding that the increased incidences at 62.5 and
125 mg/kg bw per day were not a direct reflection of reduced fetal
body weight. There was no evidence of maternal toxicity, even at the
highest dose (Staples, 1980).
Groups of pregnant Sprague-Dawley rats were given benomyl (99.1%
pure) by gavage at 0, 3, 6.25, 10, 20, 30, or 62.5 mg/kg bw per day on
days 7-16 of gestation. Each group consisted of 50 animals, except the
high-dose group, which comprised 20 rats. As the study was performed
to determine a no-effect level for microphthalmia and hydrocephaly,
only fetal heads were fixed and examined. Microphthalmia was
determined on the basis of the smallest eye in the control group
(< 1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two cases of malformation, both at
62.5 mg/kg bw per day: One fetus had internal hydrocephaly, and one in
another litter had unilateral microphthalmia. The only type of
variation reported was subcutaneous haematoma, which was not dose-
related. There was no teratogenic response at 30 mg/kg bw per day
(Staples, 1982).
On the basis of these two studies, the Meeting concluded that the
NOAEL for teratogenicity was 30 mg/kg bw per day, as the anophthalmia
at 10 mg/kg bw per day in the first study was not found in the second
study, which was specifically designed to look for this anomaly.
Furthermore, anophthalmia was not seen at 30 mg/kg bw per day in the
first study, and its occurrence at 10 mg/kg bw per day was not
accompanied by other evidence of teratogenic response.
The teratogenic potential of benomyl was examined in groups of
12-30 Wistar rats given oral doses of 0, 15.6, 31.2, 62.5, or
12.5 mg/kg bw per day on days 7-16 of gestation. Pups were delivered
by caesarean section on day 21 of gestation. The weight gain of the
dams at the highest dose was decreased on days 17-20 of gestation, and
the frequency of fetal resorption was increased, six litters being
completely resorbed. Fetal weight was also adversely affected, with
significant decreases at 62.5 and 125 mg/kg bw per day, and there was
a significant increase in fetal mortality at 125 mg/kg bw per day.
Several skeletal and visceral variants were observed among fetuses at
62.5 and 125 mg/kg bw per day, including increased supraoccipital
score, decreased numbers of sternal and caudal vertebrae, and
increased percentages of enlarged lateral ventricles and enlarged
renal pelvis. Major anomalies, observed primarily at > 62.5 mg/kg
bw per day, included encephaloceles, hydrocephaly, microphthalmia,
fused vertebrae, and fused ribs. The dose of 31.2 mg/kg bw per day
appeared to have no adverse effects on developing rat fetuses (Kavlock
et al., 1982).
Groups of pregnant Sprague-Dawley rats were treated orally with
benomyl at 0 or 62.5 mg/kg bw per day on days 7-16 (eight rats) or
7-20 of gestation (13 rats). There were no overt signs of toxicity.
Hydrocephaly was noted in 35% of fetuses examined on day 16 and in 76%
of fetuses examined on day 20 (Ellis et al., 1987, 1988).
In other studies, benomyl produced ocular and craniocerebral
malformations in Sprague-Dawley rats when administered by gavage at
doses of 31.2 or 62.4 mg/kg bw per day on days 7-21 of gestation.
Ocular anomalies (retinal dysplasia, cataracts, microphthalmia, and
anophthalmia) occurred in 43.3% of the fetuses of dams given the
higher dose. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose of
benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
consisting primarily of hydrocephaly occurred in the fetuses of dams
given 31.2 mg/kg bw per day in combination with the semipurified diet
(Zeman et al., 1986).
Rabbits
Groups of 15 artificially inseminated New Zealand albino rabbits
were fed a benomyl formulation (containing 50% benomyl) in the diet at
0, 100, or 500 ppm on days 8-16 of gestation. Mortality, clinical
parameters, and food consumption were determined daily, and body
weights were measured weekly. There were 12 pregnant does in the
control group, 13 in the low-dose group, and 9 in the high-dose group.
Of these, 6, 7 and 5, respectively, were sacrificed on day 29 or 30
and their fetuses were delivered by caesarean section; the remaining
does gave birth normally. No maternal toxicity was observed at any
dose. Except for a marginal increase in the frequency of rudimentary
ribs in animals at 500 ppm, no developmental toxicity was observed
(Busey, 1968d); however, the numbers of litters and of fetuses
examined were less than adequate to assess the fetotoxic or
teratogenic potential of benomyl.
Groups of 20 female New Zealand white female rabbits, presumed to
be pregnant, were given oral doses of 0, 15, 30, 90, or 180 mg/kg bw
per day benomyl (as a suspension in 0.5% methylcellulose) on days 7-28
of gestation. The animals were killed on day 29. The results of
analyses of the concentration, homogeneity, and stability of the
suspensions indicated acceptable conformity with nominal content. Only
one animal at 90 and one at 180 mg/kg bw per day did not become
pregnant. There were seven deaths before terminal sacrifice -- one
control, three treated with 30 mg/kg bw per day, one treated with
90 mg/kg bw per day, and two treated with 180 mg/kg bw per day -- all
of which were due to injuries associated with dosing. At 180 mg/kg bw
per day, an increased incidence of stained tails was seen, and food
consumption was significantly reduced, but there were no treatment-
related effects on maternal body weight. Five animals aborted during
the study: one in each of the groups treated with 0, 15, and 90 mg/kg
bw per day and two at 180 mg/kg bw per day. As the historical control
data showed no more than one abortion in a control group of 20 does,
the Meeting concluded that benomyl at 180 mg/kg bw per day had an
adverse effect. There were no other treatment-related effects on
reproductive parameters. Fetal mortality and fetal weights were not
affected by treatment, and there were no treatment-related external
malformations. At 180 mg/kg bw per day, two viable fetuses in two
litters had small renal papillae. The Meeting concluded that this
finding was treatment-related in view of the finding of a single
incidence in 11 studies in historical controls. The NOAEL for maternal
toxicity was 90 mg/kg bw per day, on the basis of reduced food
consumption, abortion, and clinical signs of toxicity at 180 mg/kg bw
per day. The NOAEL for developmental toxicity was also 90 mg/kg bw per
day, on the basis of the occurrence of small renal papillae at
180 mg/kg bw per day (Munley, 1995).
(f) Genotoxicity
Numerous studies have been reported in the open literature on the
mutagenic potential of benomyl, its metabolite carbendazim, and
several benomyl formulations. Many of the results are conflicting, and
few of the publications provide sufficient detail to evaluate the
reasons for the conflicts. Table 2 covers only those studies in which
sufficient experimental detail and data were reported.
Benomyl does not cause gene mutation or structural chromosomal
aberrations in somatic or germ cells, and it does not interact
directly with DNA in either mammalian or non-mammalian systems. Gene
mutations and structural chromosomal aberrations observed in mammalian
cells in vitro in several studies appear to have been the
consequence of the inherent sensitivity of some mammalian test systems
to cytotoxic agents. No gene mutations or structural chromosomal
aberrations were seen in mammals in vivo. Benomyl causes numerical
chromosomal aberrations (aneuploidy and/or polyploidy) both in vitro
and in vivo. Studies involving kinetochore staining indicated,
however, that benomyl is not clastogenic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Application for 24 h of doses > 0.5 g per animal of a 50%
wettable powder to the occluded, clipped, intact and abraded abdominal
skin of albino rabbits produced moderate to marked erythema, slight
oedema, and slight desquamation. Albino guinea-pigs similarly ex posed
to 10, 25, or 40% dilutions of technical-grade benomyl in dimethyl
phthalate had only mild irritation of intact and abraded skin (Majut,
1966; Busey, 1968a; Colburn, 1969; Frank, 1969). No dermal irritation
was observed 4 or 24 h after application of 0.5 g of Benlate 50 DF
(containing 50% benomyl) to six male New Zealand white rabbits. After
48 h, two rabbits had slight to mild erythema, which was still evident
after 72 h. The primary irritation scores ranged from 0 to 1 (not an
irritant) (Vick & Brock, 1987).
Draize scores were assessed 4 and 72 h after application of
benomyl (purity, 98%) to a shaved area of the back of four albino
rabbits at 5 mg/cm2. The lesions produced were classified as 'mild
irritation' (Desi, 1979).
The ocular irritating properties of technical-grade benomyl, a
50% wettable powder, and a suspension in mineral oil were examined in
albino rabbits in several tests. Mild conjunctival irritation and
minor transitory corneal opacity were reported after 48 to 96 h in all
tests (Reinke, 1966; Frank, 1972). Similar results were obtained with
Table 2. Results of tests for the genotoxicity of benomyl
End-point Test system Concentration Purity Results Reference
or dose (%)
Tests for chromosomal effects
Mitotic gene conversiona S. cerevisiae, A. nidulans < 3200 mg/ml Technical Negative DeBertoldi et al.
(1980)
Nondisjunction/crossing- A. nidulans < 2.8 mmol/litre Technical Negative, but DeBertoldi & Griselli
over spindle inhibition (1980)
observed
Sister chromatid exchangea Chinese hamster ovary > 150 mg/ml Technical Weakly positive Evans & Mitchell
cells (1980)
Chromosomal aberrationa Human lymphocytes < 2000 mg/ml 50% WP Negative Gupta & Legator
(1975)
Chromosomal aberrationa Human lymphocytes < 100 mg/ml Technical Negative Pilinskaya (1983)
Chromosomal aberrationa Chinese hamster lung cells < 90 mg/ml Technical Positive Sasaki (1988)
(98.7%)
Chromosomal effects Human/mouse monochromosomal < 15 mg/ml Technical Positive: aneuploidy Athwal & Sandhu (1985)
hybrid cells and polyploidy
(R3-5) (1.5 mg/ml threshold)
Chromosomal effects V79/AP4 Chinese hamster NR Technical Dose-related increase Rainaldi et al. (1989)
cells in numerical
chromosomal
aberrations
Chromosomal effects Chinese hamster ovary cells NR Technical Dose-related increase Eastmond & Tucker
in numerical (1989)
chromosomal
aberrations
Chromosomal effects Human lymphocytes NR Technical Dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations
(0.1 mg/ml threshold)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Chromosomal effects Chinese hamster/human NR Technical Dose-related increase Zelesco et al. (1990)
hybrid cells (EUBI) in numerical
chromosomal
aberrations
(2.0 mg/ml threshold)
Tests for gene mutation
Reverse mutationa S. typhimurium TA98, < 325 mg/plate Technical Doubtful mutagenic Rashid & Ercegovitch
TA 100, TA 1535, TA 1537, activity (1976)
TA 1538
Reverse mutationa S. typhimurium TA 1535, < 1 mg/ml 50% WP Positive Kappas et al. (1976)
TA1538, E. coil WP2,
WP2 uvra, CM 611
Reverse mutationa S. typhimurium TA98, < 1000 mg/ml Technical Negative Shirasu et al. (1978)
TA100, TA1535, TA1537,
TA1538, E. coil WP2 he;
Reverse mutationa S .typhimurium TA 1530, < 10 000 mg/ml Technical, Negative Fiscor et al. (1978)
TA1535, TA1950 50% WP
Reverse mutationa S. typhimurium TA 1530, < 10 000 mg/ml Technical, Negative DuPont de Nemours
TA1535, TA 1950 50% WP & Co. (1977)
Reverse mutationa S. typhimurium TA98, < 250 mg/plate Technical Negative Donovan & Krahn
TA 100, TA 1535, TA1537 (99.9%) (1981)
Reverse mutationa S. typhimurium TA98. < 10000 Technical Negative Rickard (1983a,b)
TA100, TA1535. TA1537 mg/plate (99%)
Reverse mutationa S. typhimurium TA98, < 500 mg/plate Technical Equivocal (positive Russell (1978a,b)
TA 100, TA 1535. TA 1537 in TA 1537 with
activation)
Reverse mutationa S. typhimurium TA 15~35, < 500 mg/disc Technical Negative Carere et al. (1978)
TA1536, TA 1537, TA1538,
Streptococcus coelicodor
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Reverse mutationa A. nidulans < 0.4 mg/ml Technical Positive Kappas et al. (1974);
Kappas & Bridges
(1981)
hprt mutationa Chinese hamster ovary < 172 mmol/litre Technical Negative Fitzpatrick (1980)
cells (99,9%)
tk mutation L5178Y mouse < 20 mmol/litre Technical Negative Amacher et al. (1979)
lymphoma cells
tk mutationa L5178Y mouse < 25 mmol/litre Technical Positive without McCooey et al.
lymphoma cells (991%) activation: negative (1983a, b)
with activation
Tests for DNA damage and repair
Unscheduled DNA Rat and mouse < 500 mg/ml Technical Negative Tong (1981)
synthesis hepatocytes
Rec assay B. subtilis 2000 mg/plate Technical Negative Shirasu et al. (1978)
Tests in vivo
Host-mediated mutation S. typhimurium 2000 mg/kg bw Technical Negative Shirasu et al. (1978)
in mice G46 his-
Host mediated mutation S. typhimurium 3 × 500 mg/kg bw 50% WP Negative Fiscor et al. (1978)
in mice G46 his-
Dominant lethal mutation Rat < 5000 ppm in 50% WP Negative Culik & Gibson
food for 7 days (1914)
Dominant lethal mutation Rat NR Technical Negative Sherman et al. (1975)
Dominant lethal mutation Rat < 203 ppm in Technical Negative Barnes et al. (1983)
food for 70 days
Dominant lethal mutation Rat < 50 mg/kg bw Technical Negative Georgieva et al.
per day for 70 days (1990)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Positive Seller (1976)
bw
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Equivocal Kirkhart (1980)
bw
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive Sasaki (1990)
bw (95%)
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive, KC+, Bentley (1992)
with KC+ staining bw (96.1-97.4%) benomyl classified
as aneugen
Chromosomal aberration Mouse bone marrow < 1 × 5000 mg/kg Technical Negative Stahl (1990)
bw (961%)
Chromosomal aberration Peripheral blood NR 50% WP Negative Ruzicska et al. (1976)
from workers
Chromosomal aberration Rat bone marrow < 500 mg/kg bw, 50% WP Bone marrow, Ruzicska et al. (1976)
and cultured embryo days 7-14 of negative; embryo
cells gestation cells, positive
Sex-linked recessive Drosophila melanogester 1.5 mg/ml Technical, Negative, but Lamb & Lilly (1980)
lethal mutation 50% WP sterility consistent
with observed
spindle effects
NR, not reported; 50% WP, wettable powder formulation; KC, kinetochore
a With and without metabolic activation
Benlate PNW, a 50% wettable powder (Gargus & Zoetis, 1983b,c). The
Draize score for ocular irritation induced by 5 mg of pure benomyl in
four albino rabbits indicates that it is a mild irritant (Desi, 1979).
Albino guinea-pigs exposed to technical-grade benomyl or a 50%
sucrose formulation had mild to moderate skin erythema during the
challenge phase after either intradermal injection or repeated
applications to abraded skin (Majut, 1966; Colburn, 1969; Frank,
1969).
Ten albino Hartley guinea-pigs received four weekly injections of
Benlate PNW (50% benomyl prepared as a 0.1% solution in saline), and
10 animals were injected with saline; 14 days after the final
injection, 8 or 80% Benlate PNW was used for challenge. An unequivocal
increase in sensitization was seen at two of 10 sites challenged with
the 8% suspension and at seven of 10 sites challenged with the 80%
suspension (Gargus & Zoetis, 1984).
Technical-grade benomyl sensitized all 10 guinea-pigs tested in a
maximization test (Matsushita & Aovama, 1981).
(ii) Neurotoxicity
Studies in hens given single oral doses of < 5000 mg/kg bw did
not indicate neurotoxic potential (Goldenthal, 1978; Jessup, 1979;
Jessup & Dean, 1979).
Studies in groups of 10 adult male CFY rats showed no alteration
in electroencephalographic potential or in behaviour (learning ability
assessed in a four-choice T-maze) in comparison with controls after
treatment with benomyl at 250 or 500 mg/kg bw per day for three months
(Desi, 1983).
Groups of 10 male and 10 female Sprague-Dawley rats received
single oral doses of 0, 500, 1000, or 2000 mg/kg bw benomyl (97.4%
pure). Functional and motor activity were assessed during the week
before the day of treatment and at 2 h, one day, seven days, and 14
days after dosing. Rats were then killed and subjected to a gross
post-mortem examination. Histopathological examination was restricted
to tissues from the central and peripheral nervous system of six rats
in the control and high-dose groups. There were no treatment-related
deaths during the study. Immediately after treatment, body weight gain
and food intake were decreased in all treated groups; however, overall
weight gain and food consumption were unaffected by treatment. In the
battery of functional tests, a small number of animals had soft or
liquid faeces and fur stained with urine or faeces on days 1 and 2,
but no other component of the battery, including assessments of other
autonomic functions, reactivity, and sensitivity, excitability, gait,
and sensorimotor coordination, forelimb and hindlimb grip strength,
abnormal clinical signs and body temperature, showed any reaction to
treatment. Motor activity was reduced, only on the day of treatment,
in females given 2000 mg/kg bw. Neuropathological observation revealed
no treatment-related abnormalities. It was concluded that benomyl
induced no lasting neurotoxicity at doses up to 2000 mg/kg bw (Foss
1993).
Groups of 11 male and 11 female Sprague-Dawley rats received
benomyl (purity, 97.4%) in the diet at 0, 100, 2500, or 7500 ppm for
90 days. Functional and motor activity were assessed during the week
before treatment and during weeks 4, 8, and 13. Rats were then killed
and subjected to gross post-mortem examination. Histopathological
examination was restricted to tissues from the central and peripheral
nervous system of six rats in the control and high-dose groups. There
were no treatment-related deaths during the study. Body weight gain
and food intake were reduced at the high dose throughout the treatment
period. The battery of functional tests showed no reaction to
treatment with benomyl. Motor activity was increased at 7500 ppm.
Neuropathological observation revealed no treatment-related
abnormalities. It was concluded that benomyl is not a specific
neurotoxicant, since the changes in motor activity occurred at the
same dietary level as other general toxicological changes (Foss,
1994).
3. Observations in humans
(a) Exposure during field use
Potential dermal and respiratory exposure to benomyl during
actual use was determined during mixing for aerial application,
re-entry into treated fields, and home use (garden, ornamental, and
greenhouse). Maximal exposure occurred during loading and mixing for
aerial application, with dermal exposure to 26 mg per mixing cycle,
primarily (90%) on the hands and forearms; the average respiratory
intake was 0.08 mg benomyl. During re-entry, the dermal exposure was
5.9 mg/h and the respiratory exposure < 0.002 mg/h. Home use resulted
in exposures of 1 mg per application cycle dermally and 0.003 mg by
the respiratory route. This study did not address reactions to
exposure (Everhart & Holt, 1982).
The potential dermal and respiratory exposure of workers mixing
and applying 20 and 100 gallons of Benlate per acre (224 and
1123 litres/ha) while spraying fruit orchards was examined. The
application cycle was about 70 min and resulted in a total dermal
exposure of 11 mg of benomyl per cycle and a total respiratory
exposure to 15 mg. Essentially all of the exposure was dermal,
resulting in 12.2 mg per cycle dermally and < 0.05 mg per cycle via
the respiratory route (DuPont de Nemours & Co., undated).
(b) Medical surveillance of workers
Benomyl caused contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et at., 1985).
In controlled patch tests of 200 agricultural, ex-agricultural, and
non-agricultural workers, only one agricultural worker showed contact
dermatitis to 0.1% benomyl (Lisi et al., 1986). In a survey of
cross-sensitization with benomyl and other pesticides in a group of
126 Japanese farmers who applied benomyl to their crops, 39 had
positive test results with benomyl, the highest incidence being found
among female farmers. There were cross-reactions between benomyl and
diazinon, saturn, daconil, and Z-bordeaux (Matsushita & Aovama, 1981).
Selected blood profiles of 50 factory workers involved in the
manufacture of Benlate were compared with those of a control group of
48 workers who were not exposed to Benlate. White blood count, red
blood count, haemoglobin, and haematocrit values were comparable in
the two groups. No quantitative estimates of exposure were given for
the factory workers (DuPont de Nemours & Co., 1979).
A survey was performed to determine whether potential exposure
to Benlate had an adverse effect on the fertility of 298 male
manufacturing workers exposed between 1970 and 1977. The ages of the
workers ranged from 19 to 64 years; 79% of the workers and 78% of
their spouses were aged 20-39. The duration of exposure ranged from
less than one month to 95 months, but more than 51% of the workers had
potentially been exposed for one to five months. The birth rates of
the spouses of the exposed workers were compared with those of four
populations from the same county, state, region, and country (United
States). There was no reduction in fertility, and the birth rates of
the study population were generally higher than those of the
comparison populations. Spermatogenesis was not examined (Gooch,
1978).
Comments
Benomyl is readily absorbed by animals after oral exposure and
rapidly metabolized. It is eliminated in the faeces and excreted in
the urine; 98% of the dose was excreted by 72 h after administration.
The tissue distribution showed no bioconcentration. In rats, the
metabolites carbendazim and methyl 5-hydroxybenzimidazol-2-ylcarbamate
(5-hydroxy-carbendazim) were found in the blood and in small amounts
in the testis and liver. The latter compound was the main metabolite
in urine. A 50% wettable powder formulation was poorly absorbed via
the dermal route by rats. After a 10-h exposure, less than 2% of a
single dose of 0.2 mg was excreted in the urine.
Benomyl has low acute toxicity, with an oral LD50 in rats of
> 10 000 mg/kg bw. The clinical signs of toxicity after high single
doses were generally nonspecific. Testicular degeneration, with
necrosis of germinal epithelium and aspermatogenesis, has been
observed after single doses in rats (> 100 mg/kg bw orally) and dogs
(1.65 mg/litre air by inhalation). Wettable powder formulations
containing benomyl have been shown to be mildly irritating to rabbit
skin and eyes and have induced skin sensitization reactions in
maximization tests. WHO has classified benomyl as unlikely to present
an acute hazard in normal use.
In a 90-day study, rats were given dietary doses of 0, 100, 500,
or 2500 ppm benomyl. Increased liver weight was seen at 2500 ppm; the
NOAEL was 500 ppm (50 mg/kg bw per day). Dogs were given dietary doses
of 0, 100, 500, or 2500 ppm benomyl for three months or two years and
rabbits were treated dermally, five days per week for three weeks,
with 0, 50, 250, 500, 1000, or 5000 mg/kg bw per day. Hepatotoxicity
was seen in the dogs but not in the rabbits; effects on male
reproductive organs were seen in both rabbits and dogs. The NOAEL was
500 ppm (equal to 13 mg/kg bw per day) in dogs and 500 mg/kg bw per
day in rabbits.
In a two-year study, benomyl was administered in the diet to rats
at 0, 100, 500, or 2500 ppm. Benomyl was not carcinogenic and showed
no compound-related effects at dietary levels up to and including
2500 ppm (equal to 109 mg/kg bw per day). In a two-year feeding study
in mice at dietary levels of 0, 500, 1500, or 5000 ppm, benomyl
caused liver tumours, and an NOAEL could not be established for
hepatocellular neoplasms. Male mice had decreased testicular weights
and thymic atrophy at 5000 ppm. The lowest dietary level was equal to
64 mg/kg bw per day.
In rats treated by gavage for 62 days with 45 mg/kg bw per day,
decreased testicular and epididymal weights, reduced caudal sperm
reserves, and decreased sperm production, with generalized disruption
of all stages of spermatogenesis were observed. After mating with
untreated females, no effect was seen on reproductive behaviour,
weight of the seminal vesicles, sperm mobility, or related
reproductive hormones. The NOAEL was 15 mg/kg bw per day. A lowering
of male fertility rates has been reported, but this effect was not
seen consistently. A single dose of 100 mg/kg bw or more administered
to rats by gavage had effects 70 days after exposure, which included
decreased testicular weight and atrophy of the seminiferous tubules.
The NOAEL was 50 mg/kg bw per day. In a recent study of reproductive
toxicity, rats received dietary doses of 0, 100, 500, 3000, or
10 000 ppm benomyl. The NOAEL was 500 ppm (equivalent to 37 mg/kg bw
per day), on the basis of effects on pup survival and pup growth and
on testicular changes. Fertility indices were not affected at dietary
levels up to 10 000 ppm.
In a study of developmental toxicity, mice were exposed by gavage
to benomyl at doses of 0, 50, 100, or 200 mg/kg bw per day on days
7-17 of gestation. There was no indication of maternal toxicity, but
benomyl was teratogenic at doses of 100 and 200 mg/kg bw per day and
fetotoxic at 50 mg/kg bw per day. The major abnormalities included
hydrocephaly, cleft palate, and limb defects. In studies of
teratogenicity, pregnant rats were exposed to benomyl at doses up to
and including 125 mg/kg bw per day on days 7-16 of gestation.
Benomyl was teratogenic, the major effects being microphthalmia and
hydrocephaly. The Meeting concluded that the NOAELs in rats were
30 mg/kg bw per day for teratogenicity and fetotoxicity and 125 mg/kg
bw per day for maternal toxicity. In rabbits, benomyl was not
teratogenic at doses up to 180 mg/kg bw per day (the highest dose
tested), and no effect was seen on maternal toxicity or fetotoxicity
at 90 mg/kg bw per day.
Benomyl has been adequately tested for genotoxicity in a range of
assays. The Meeting concluded that it does not directly damage genetic
material but does cause numerical chromosomal changes both in vitro
and in vivo as a result of its interference with mitotic spindle
proteins.
In a study of workers exposed to benomyl, there was no reduction
in fertility, as indicated by birth rates, among the study population.
Spermatogenesis in the workers was not examined. Cases of dermal
sensitization to benomyl have been reported.
An ADI of 0-0.1 mg/kg bw was established on the basis of the
NOAEL of 13 mg/kg bw per day in the two-year study in dogs and
applying a safety factor of 100. This ADI should be used when
assessing exposure to benomyl itself. Since the use of benomyl on
crops gives rise to residues of carbendazim and since the ADI for
carbendazim is lower than that which would be derived from the data on
benomyl, the Meeting concluded that the intake of residues in food
should be compared with the ADI of 0-0.03 mg/kg bw for carbendazim.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: < 500 ppm, equal to < 64 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
50 mg/kg bw per day (study of developmental toxicity)
< 50 mg/kg bw per day (fetotoxicity in a study of
teratogenicity)
Rat: 2500 ppm, equal to 109 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
500 ppm, equivalent to 37 mg/kg bw per day (study of
reproductive toxicity)
30 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
125 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 180 mg/kg bw per day (study of developmental toxicity)
90 mg/kg bw per day (maternal toxicity and fetotoxicity in
study of developmental toxicity)
Dog: 500 ppm, equal to 13 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw (benomyl)
0-0.03 mg/kg bw (carbendazim, with which residues of benomyl in
food should be compared)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to benomyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 >10 000 mg/kg bw
Dermal, toxicity, rabbit LD50 (50% wettable powder)
> 10 000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder; irritating
Ocular, irritation, rabbit 50% wettable powder; irritating
Dermal, sensitization, guinea-pig Positive in maximization test
Inhalation, toxicity, rat LC50 (50% wettable powder)
> 4.01 mg/litre air
Mid-term (1-26 weeks) Oral, 62 days, rat NOAEL = 15 mg/kg bw per day;
reduced spermatogenesis
Oral, developmental toxicity, rat NOAEL = 30 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary two years, toxicity, dog NOAEL = 13 mg/kg bw per day;
hepatotoxicity
See also toxicological criteria for carbendazim when considering dietary exposure to residues
References
Amacher, D.E., Paillet, S. & Ray, V.A. (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells:
Application to genetic toxicological testing. Mutat. Res., 64,
391-406.
Athwal, R.S. & Sandhu, S.S. (1985) Use of human x mouse hybrid cell
line to detect aneuploidy induced by environmental chemicals.
Mutat. Res., 149, 73-81.
Axness, M.E. & Fleeker, J.R. (1979) Metabolism of the butylcarbamoyl
moiety of benomyl in rat. Pestic. Biochem. Physiol., 11, 1-12.
Barbo, E.C. & Carroll, K.S. (1972) Oral ALD test ( S-triazine,
3-butylbenzimidazolo[1,2-a]-2,4[1 H,3 H]dione. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Barnes, T. B., Verlangieri, A.J. & Wilson, M.C. (1983) Reproductive
toxicity of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate
(benomyl) in male Wistar rats. Toxicology, 28, 103-115.
Belasco, I.J. (1979a) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied. Unpublished
report from DuPont de Nemours & Co., Biochemical Department,
Research Division, Experimental Station, Wilmington, Delaware,
USA.
Belasco I.J. (1979b) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Unpublished report from DuPont de Nemours & Co., Biochemical
Department, Research Division, Experimental Station, Wilmington,
Delaware, USA
Belasco, I.J., Kirkland, J.J., Pease, H.L. & Sherman, H (1969) Studies
with 2-14C-labelled methyl-1-(butylcarbamoyl)-2-
benzimidazolecarbamate (benomyl) in rats. Unpublished report from
DuPont de Nemours & Co., Biochemical Department, Research
Division, Experimental Station, Wilmington, Delaware, USA.
Bentley, K S. (1992) Classification of DPX-T1991-529 (Benomyl)-induced
micronuclei in mouse bone marrow erythrocytes using immuno-
fluorescence antikinetochore antibodies. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Brock, W.J. (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Unpublished report from DuPont de Nemours &
Co, Haskell Laboratory, Newark, Delaware, USA.
Busey, W.M. (1968a) Acute dermal LD50 test and dermal irritation
test on rabbits using a wettable powder formulation (50% benomyl)
with histological addendum. Unpublished report from Hazelton
Laboratories, Inc., Falls Church, Virginia, USA, prepared for
DuPont de Nemours & Co.
Busey, W.M. (1968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968c) Repeated dermal application test on rabbits using
a wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968d) Teratology study in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from
Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res., 57, 277-826.
Carter, S.D. (1982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Thesis, North Carolina State
University, Raleigh, North Carolina, USA.
Carter, S.D. & Laskey, J.W. (1982) Effect of benomyl on reproduction
in the male rat. Toxicol. Lett., 11, 87-94.
Chernoff, N. & Kavlock, R.J. (1983) A teratology test system which
utilises post natal growth and viability in the mouse. Environ.
Sci. Res., 27, 417-427.
Colburn, C.W. (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Culik, R. (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the
concepti of rats exposed to benomyl and Benlate by diet.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC), 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. and Gibson, J.R. (1974) 'Benlate' dominant lethal study in
male rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Dashiell, O.L. (1972) Acute oral test (benzimidazole, 2-(3-
butylureido)). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
De Bertoldi, M. & Griselli, M. (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat. Res., 74,
303-324.
De Bertoldi, M., Griselli. M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by metres of gene-conversion
in Saccharomyces cerevisiae and in Aspergillus nidulans.
Environ. Mutag., 2, 359-370.
Desi, I. (1979) Hygienic-toxicological evaluations of pesticides.
D.Sc. Thesis, Budapest, National Institute of Hygiene.
Desi, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5, 503-515.
Donovan, S.D. & Krahn, D.F. (1981) Mutagenic evaluation in
Salmonella typhimurium. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1977) Mutagenic activity of benomyl in the
Salmonella/microsome assay (50% benomyl as a wettable pow der
formulation). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1979) Benlate dust exposure survey -- blood
profile analysis. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (undated) Applicator exposure during filling
and spraying of Benlate benomyl fungicide -- orchard crops.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Eastmond, D.A. & Tucker, J.D. (1989) Kinetochore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat. Res., 224, 517-525.
Ellis W.G., Semple, J.L., Hoogenboom, E.R., Kavlock, R.J. & Zeman,
E.J. (1987) Benomyl-induced craniocerebral anomalies in fetuses
of adequately nourished and protein-deprived rats. Teratog.
Carcinog. Mutag., 7, 357-375.
Ellis W.G., De Roos, F., Kavlock, R.J. & Zeman, F.J. (1988)
Relationship of periventricular overgrowth to hydrocephalus in
brains of fetal rats exposed to benomyl. Teratog. Carcinog.
Mutag., 8, 377-391.
Evans, E.L. & Mitchell, A.D. (1980) An evaluation of the effect of
benomyl on sister chromatid exchange frequencies in cultured
Chinese hamster ovary cells. Unpublished report from SRI
International, Menlo Park, California, USA, prepared for DuPont
de Nemours & Co.
Everhart, L.P. & Holt, R.F. (1982) Potential Benlate fungicide
exposure during mixer/loader operations, crop harvest and home
use. J. Agric. Food Chem., 30, 222-227.
Fiscor, G., Bordas, S. & Stewart, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Fitzpatrick, K. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Foss J.A (1993) Acute neurotoxicity study of DPX-T1991-529 (Benomyl)
administered orally by gavage to Crl:CD BR VAF/Plus rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Foss, J.A. (1994) Subchronic neurotoxicity study of DPX-T1991-529
(Benomyl) administered orally via the diet to Crl:CD BR VAF/Plus
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. & Sherman, H. (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Gardiner, J.A., Kirkland, J.J., Klopping, H.L. & Sherman, H. (1974)
Fate of benomyl in animals. J. Agric. Chem., 22, 419-427.
Gargus, J.L. & Zoetis, T. (1983a) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines -- Benlate PNW).
Unpublished report from Hazelton Laboratories Inc., Vienna,
Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983b) Primary irritation study in rabbits
(Benlate PNW). Unpublished report from Hazelton Laboratories
Inc., Vienna, Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983c) Eye irritation test in rabbits (EPA
pesticide registration Benlate PNW). Unpublished report from
Hazelton Laboratories Inc., Vienna, Virginia, USA, prepared for
DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Unpublished
report from Hazelton Laboratories Inc., Vienna, Virginia, USA,
prepared for DuPont de Nemours & Co.
Georgieva, V., Vachkova, R., Tzoneva, M. & Kappas, A. (1990) Genotoxic
activity of benomyl in different test systems. Environ. Mol.
Mutag., 16, 32-36.
Goldenthal, E.I. (1978) Neurotoxicity study in hens using technical
benomyl (< 95% benomyl). Unpublished report from International
Research and Development Corporation, Mattawan, Michigan, USA,
prepared for DuPont de Nemours & Co.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from DuPont de Nemours & Co.,
Wilmington, Delaware, USA.
Goodman, N.C. (1975) Intraperitoneal LD50 test in rats. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Guengerich, F.P. (1981) Enzyme induction with DuPont compounds H11,
202-02 and H10, 962-02. Unpublished report from Vanderbilt
University, School of Medicine, Nashville, Tennessee, USA,
prepared for DuPont de Nemours & Co.
Gupta, A.K. & Legator, M.S. (1975) Chromosome aberrations in cultured
human lymphocytes after treatment with fungicide 'Benlate'. In:
Proceedings of the Symposium on Mutagenicity, Carcinogenicity
and Teratogenicity of Chemicals, New Delhi, Department of
Atomic Energy, pp. 95-103
Han, J.C.Y. (1979) 2-14C-Benomyl (50% WP) rat study -- intravenous
injection. Unpublished report from DuPont de Nemours & Co.,
Biochemical Department, Research Division, Experimental Station,
Wilmington, Delaware, USA.
Han, J.C.Y. (undated) Metabolism of 14C-labelled benomyl in the
mouse and hamster. Unpublished report from DuPont de Nemours &
Co., Biochemical Department, Research Division, Experimental
Station, Wilmington, Delaware, USA
Hess, R.A., Moore, B.J., Forrer, J., Linder, R.E. & Abuel-Atta, A.A.
(1991) The fungicide benomyl [(methyl)1-(butylcarbomyl)-2-
benzimidazole carbamate] causes testicular dysfunction by
inducing the sloughing of germ cells and occlusion of efferent
ductules. Fundam. Appl. Toxicol., 17, 733-745.
Hood, D.B. (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Hoogenboom, E.R., Ransdell, J.F., Ellis, W.G., Kavlock, R.J. & Zeman,
E.J. (1991) Effects on the fetal rat eye of maternal benomyl
exposure and protein malnutrition. Curr. Eye Res., 10, 601-612.
Hornberger, C.S. (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Hostetler, K.H. (1977) Oral LD50 test (Benlate OD). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Jessup, D.C. (1979) Acute delayed neurotoxicity study in chickens
using technical benomyl (> 95% benomyl). Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA, prepared for DuPont de Nemours & Co.
Jessup, D.C. & Dean, W. (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl).
Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, prepared for DuPont de
Nemours & Co.
van Joost, T.H., Naafs, B. & van Ketel, W G. (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9, 153-154.
Kappas, A. & Bridges, B.A. (1981) Induction of point mutations by
benomyl in DNA-repair deficient Aspergillus nidulans. Mutat.
Res., 91, 115-118.
Kappas, A., Georgopoulos, S G. & Hastie, A.C. (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat. Res., 26, 17-27.
Kappas, A., Green, M.H.L., Bridges, B.A., Rogers, A.M. & Muriel, W.J.
(1976) Benomyl -- a novel type of base analogue mutagen? Mutat.
Res., 40, 379-382.
Kavlock, R.J., Chernoff, N., Gray, L.E., Gray, J A & Whitehouse, D.
(1982) Teratogenic effects of benomyl in the Wistar rat and CD-1
mouse, with emphasis on the route of administration. Toxicol.
Appl. Pharmacol., 62, 44-54.
Kirkhart, B. (1980) Micronucleus test on benomyl (> 95% benomyl).
Unpublished report from SRI International, Menlo Park,
California, USA. prepared for DuPont de Nemours & Co.
Krupka R.M. (1974) On the anti-cholinesterase activity of benomyl.
Pestic. Sci., 5, 211-216.
Kuelme, G., Heise, H., Plottke, B. & Puskeller, T (1985) Dermatitis
after Benlate contact. Z. Ges. Hyg. Grenzgeb., 31, 710-711.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Lee K.P. (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Linder, R.E., Rehnberg, G.L., Strader, L.F. & Diggs, J.P. (1988)
Evaluation of reproductive parameters in adult male Wistar rats
after subchronic exposure. J. Toxicol. Environ. Health, 25,
285-298.
Lisi, P., Caraffini, S. & Assalve, D. (1986) A test series for
pesticide dermatitis. Contact Derm., 15, 266-269.
Littlefield, N.A. & Busey, W.M. (1969) Four-hour acute inhalation
exposure test in dogs using a wettable powder formulation (50%
benomyl). Unpublished report from Hazleton Laboratories, Inc.,
Falls Church, Virginia, USA, prepared for DuPont de Nemours & Co.
Majut, J.C. (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Matsushita, T. & Aovama, K. (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind.
Health, 19, 77-83.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983a) L5178Y
mouse lymphoma cell assay for mutagenicity (benomyl). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983b) L5178Y
mouse lymphoma cell assay for mutagenicity. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Mebus, C.A. (1990) Reproductive and fertility effects with
DPX-1991-529 (benomyl). Multigeneration reproduction study in
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Monson, K.D. (1990) Metabolism of [phenyl(U)14C]carbendazim in rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Munley, S.M. (1995) Developmental toxicity study of DPX-T1991-529
(Benomyl) in rabbits. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Pilinskaya, M.A. (1.983) Investigation of the cytogenetic action of
the pesticides captan and benomyl in a culture of human
peripheral blood lymphocytes in the absence and presence of a
system of metabolic activation. Cytol. Genet., 17, 29-33
Rainaldi, G., Flori, L., Colella, C.M, Mariani, T., Piras, A., Simi,
S. & Simili, M. (1989) Analysis by BrUdR-labelling technique of
induced aneuploidy in mammalian cells in culture. Mutat. Res.,
177, 255-260.
Rashid, K.A. & Ercegovitch, C.D. (1976) New laboratory tests evaluate
chemicals for cancer or gene damage. Sci. Agric., 23, 7.
Reinke, RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Ruzicska, P., Peter, S., Laczi, J. & Czeizel, E. (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP Egeszegtudomany, 20,
74-83.
Sarver, J.W. (1987) Acute oral toxicity study with IN-T1991 in male
and female rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sasaki, Y.F.X. (1988) Benomyl: In vitro cytogenetics test. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Sasaki, Y.F.X. (1990) Benomyl: Micronucleus test in mice. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives, VI: Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and the
Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. & Krauss, W.C. (1966) Acute oral test [benomyl].
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Sherman, H., Barnes, J.R. & Krauss, W.C. (1967) Ninety-day feeding
study with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl
ester (INT-1991). Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H., Culik, R. & Jackson, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. Appl.
Pharmacol., 32, 305-315.
Shirasu, Y., Moriva, M. & Kato, K. (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan, prepared for DuPont de Nemours & Co.
Shukla, Y., Antony, M. & Mehrota, N.K. (1989) Studies on
gamma-glutamyl transpeptidase in rodents exposed to benomyl.
Bull. Environ. Contam. Toxicol., 42, 301-306.
Snee, D.A. (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test). Unpublished. report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Stahl, R.G., Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Staples, R.E. (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Staples, R.E. (1982) Teratogenicity study in the rat using technical
benomyl (> 95% benomyl) administered by gavage. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Tong, C. (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA, prepared for DuPont de Nemours & Co.
Turney, R.T. (1979) Rat inhalation study -- Benlate. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Vick, D.A. & Brock, W.J. (1987) Primary dermal irritation study with
Benlate 50 DF fungicide in rabbits. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Ward, R.S. & Scott, R.C. (1992) Benomyl: in vitro absorption of a
500 g.kg-1 WP formulation through human epidermis. Unpublished
report from Imperial Chemical Industries (ICI), Fernhurst,
Haslemere, Surrey, United Kingdom.
Warheit, D.B., Kelly, D.P., Carakostas M.C. & Singer, A.W. (1989) A
90-day inhalation toxicity study with benomyl in rats. Fundam.
Appl. Toxicol., 12, 333-345.
Wiechman, B.E. (1982) Long term feeding study with methyl
1-(butylcarbamoyl)-2-benzimidazole carbamate in mice (INT-1991;
> 95% benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Zelesco, P.A., Barbieri, I. & Graves, J.A.M. (1990) Use of a cell
hybrid test system to demonstrate that benomyl induces aneuploidy
and polyploidy. Mutat. Res., 242, 329-335.
Zeman, E.J., Hoogenboom, E.R., Kavlock, R.I. & Semple, J.L. (1986)
Effects on the fetus of maternal benomyl exposure in the
protein-deprived rat. J. Toxicol. Environ. Health, 17, 405-417.
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed diets containing benomyl or carbendazim at a concentration of 0,
10, 30, 100, 300, 1000 or 3000 ppm for 28 days. After sacrifice, liver
weights were recorded and microsomal epoxide hydrolase and cytosolic
glutathione- S-transferase were monitored in subcellular fractions
isolated from the liver. The mean absolute liver weights were elevated
in males and females fed 1000 or 3000 ppm carbendazim and in females
fed 300 ppm; however, the only significantly increase was found in
females fed 3000 ppm benomyl. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced epoxide
hydrolase in male and female rats and in mice fed 1000 or 3000 ppm,
and both induced glutathione- S-transferase at 3000 ppm. The level of
induction seemed to be slightly greater in females than males. There
was no substantial difference in enzyme induction between rats and
mice (Guengerich, 1981).
Administration of 1000 or 4000 ppm benomyl in the diet to groups
of eight female albino rats and female Swiss albino mice for 15 days
increased the activity of blood and liver gamma-glutamyl transpeptidase
in both species, and the degree of induction was dose related (Shukla
et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
1. The clinical signs of toxicity after treatment with benomyl were
generally nonspecific. Gross and histopathological changes were
evaluated in some studies; in male gonads, testicular degeneration,
necrosis of germinal epithelium, and aspermatogenesis were observed in
rats and dogs.
(b) Short-term toxicity
Rats
After intubation of benomyl into male Sprague-Dawley rats at 200
or 3400 mg/kg bw in peanut oil five times per week for two weeks, four
of six animals at the high dose died. At this dose, degeneration of
germinal epithelium, multinucleated giant cells, and reduction or
absence of sperm were seen. Very minor changes were observed in the
testes of the animals treated with the lower dose (Sherman & Krauss,
1966).
Male Sprague-Dawley rats treated orally for 10 days with
200 mg/kg bw per day of the metabolite 5-HBC (methyl ester) had no
toxic symptoms or evidence of effects on the testes (Snee, 1969).
Table 1. Acute toxicity of benomyl, benomyl formulations, and benomyl metabolites
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Benomyl Rat Oral > 10 000 > 95 Sherman (1969a)
Benomyl Dog Oral > 1 000 > 95 Sherman (1969b)
'50% wettable powder' Rat Oral > 1 000 > 95 Sherman (1969a)
'50% wettable powder' Rat Oral > 5 000 NR Sarver (1987)
'50% wettable powder' Rat Oral > 1 200 NR Hostetler (1977)
'50% wettable powder' Rabbit Oral > 3 400 NR Fritz (1969)
'50% wettable powder' Rabbit Dermal > 10 000 NR Busey (1968a)
'50% wettable powder' Rabbit Dermal > 2 000 NR Brock (1987)
'50% wettable powder' Rabbit Dermal > 2 000 NR Gargus & Zoetis (1983a)
'50% wettable powder' Rat Inhalation (4-h) > 0.82 NR Hornberger (1969)
'50% wettable powder' Rat Inhalation (4-h) > 4.01 NR Busey (1968b)
'50% wettable powder' Dog Inhalation (4-h) > 1.65 NR Littlefield & Busey (1969)
2-Benzimidazole carbamic Rat Intraperitoneal > 10 000 NR Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole Rat Oral > 7 500 > 95 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole Rat Oral > 3 400 > 95 Fritz & Sherman (1969)
Benzimidazole 2-(3-butylureido) Rat Oral > 17 000 NR Dashiell (1972)
S-Triazine, 3-butyl-benzimidazole Rat Oral > 17 000 NR Barbo & Carroll (1972)
(1,2a)-2,4(1H,3H)-dione
NR, Not reported
Groups of 16 male and 16 female Sprague Dawley rats were fed
benomyl (72% pure) in the diet at 0, 100, 500 and 2500 ppm (as
benomyl). After 96-103 days of continuous feeding, 10 male and 10
female rats in each group were killed, and selected organs were
weighed; additional organs were preserved for microscopic examination.
The remaining animals in each group were studied for reproductive
toxicity (see below). There were no gross toxic signs of poisoning and
no compound-related effects on weight gain, food consumption, food
efficiency, or haematological, biochemical, or urine parameters. The
liver body weight ratio was slightly increased in females at 2500 ppm
in comparison with control rats, Gross and microscopic examination of
tissues and organs showed no significant effect attributable to the
presence of benomyl in the diet at levels up to and including 2500 ppm
(Sherman et al., 1967).
Groups of 20 male and 20 female Sprague-Dawley rats were exposed
to benomyl by inhalation at 0, 10, 50, or 200 mg/m3 via the nose
only, for 6 h/day for 90 days. After 45 and 90 days, blood and urine
samples were collected from 10 rats of each sex per group for clinical
analysis; the animals were then killed for pathological examination.
After about 45 days of exposure, degeneration of the olfactory
epithelium attributable to benomyl was observed in all males and in
eight of the 10 females exposed to 200 mg/m3; two male rats exposed
to 50 mg/m3 had similar but less severe olfactory degeneration. After
about 90 days of exposure, all of the animals at 200 mg/m3 and three
males at 50 mg/m3 had olfactory degeneration. No other compound-
related pathological effects was observed. Male rats exposed to
200 mg/m3 had depressed mean body weights in comparison with
controls; the depression was correlated with a reduction in food
consumption (Warheit et al., 1989).
Rabbits
Groups of five male and five female New Zealand albino rabbits
were given 15 dermal applications for 6 h/day, five days per week for
three weeks of a 50% benomyl formulation (equivalent to 1000 mg/kg bw)
on both abraded and intact abdominal skin. After each application, the
abdomen was washed with tap water. Mortality and toxic effects were
monitored daily and body weight changes weekly; gross necropsy and
microscopic examinations were performed. Slight erythema, oedema, and
atonia were observed on both abraded and intact skin sites, and slight
to moderate desquamation was reported throughout the study. No
apparent compound-related changes in body weight or organ:body weight
ratios were reported. Treatment induced degeneration of the
spermatogenic elements of the seminiferous tubules of the testes,
including vacuolated and multinucleated spermatocytes. A slight
increase in haematogenic activity in the bone marrow and acanthosis
and hyperkeratosis of the skin were reported in treated animals
(Busey, 1968c).
Groups of five male and five female New Zealand albino rabbits
received benomyl at doses equivalent to 0, 50, 250, 500, 1000, and
5000 mg/kg bw on non-occluded, abraded dorsal skin for 6 h/day, five
days per week for three weeks. The material was removed by washing the
skin site and drying with a towel. The body weight gains of males and
females at > 1000 mg/kg bw were reduced, and mild-to-moderate skin
irritation was reported in all groups, especially at the higher doses.
Functional disturbances of the alimentary canal and kidney, including
diarrhoea, oliguria, and haematuria, were observed in males and
females at 1000 and 5000 mg/kg bw. Decreased testicular weights were
observed at 1000 mg/kg bw only, but these were considered not to be
directly related to treatment. No histopathological changes were
reported (Hood, 1969).
Dogs
Groups of four male and four female beagle dogs were given
benomyl (as a 50% wettable powder) in the diet at 0, 100, 500, or
2500 ppm (based on active ingredient) for three months. There was no
mortality or adverse clinical effect during the course of the study,
and growth and food consumption were normal. Urinary parameters were
unaffected by treatment. There were no dose-related effects on
haematological values, but alkaline phosphatase and alanine
aminotransferase activities were increased in males and females at the
highest dose, and the albumin:globulin ratio was significantly
decreased in both males and females fed 2500 ppm. In males and females
at the high dose, the weight of the thymus was decreased and that of
the thyroid was increased. One female fed 2500 ppm had an enlarged
spleen, which was consistent with the decreased erythrocyte count,
haemoglobin concentration, and haematocrit values. Histopathological
examination revealed myeloid hyperplasia of the spleen and bone marrow
and erythroid hyperplasia in the same animal. Three of four males fed
2500 ppm had reduced prostate weights in comparison with controls.
Microscopic examination of tissues and organs showed no consistent
effect. The NOAEL was 500 ppm (Sherman, 1968).
Groups of four male and four female beagle dogs were fed benomyl
(50% pure) in the diet at 0, 100, 500, or 2500 ppm (as benomyl) for
two years, with interim sacrifice after one year of one male and one
female from the control and high-dose groups. Organ weights, gross
necropsy, and histopathological evaluations were performed at the
conclusion of the study; only the livers and testes of animals fed 100
or 500 ppm were examined histologically. No deaths were attributable
to treatment. Body weight changes and food consumption were similar in
all groups, except for those at the high dose, which had decreased
food intake and body weight gain; one dog at the high dose lost its
appetite and was replaced. No other clinical signs of toxicity were
observed. The results of haematological evaluations and urinalysis
were similar to those of the controls. Males at 2500 ppm had
increased levels of cholesterol, alkaline phosphatase, and alanine
aminotransferase (initially) and decreased total protein and albumin:
globulin ratio. Similar, but less marked, effects were seen in
females at this dose. Biochemical analyses indicated adverse effects
on the liver, expressed as liver cirrhosis. Four of six dogs at this
dose had slight-to-marked bile duct proliferation. Haemosiderosis was
seen after specific staining for iron in one dog at the high dose
after one year but not in other dogs examined at two years.
Preparation of preserved wet tissue with oil red O and Sudan black for
hepatocyte vacuolation showed that benomyl is not hepatotoxic at 100
or 500 ppm in the diet. Focal testicular degeneration was seen in all
groups, including controls, with marked testicular degeneration
(reduced testicular weight, absence of spermatozoa, and spermatic
giant cells) in one of three dogs at 2500 ppm. The NOAEL was 500 ppm,
equivalent to 13 mg/kg bw per day (Sherman, 1970).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD-1 mice were given benomyl
(99% pure) in the diet at 0, 500, 1500, or 5000 ppm (reduced from
7500 ppm after 37 weeks) for two years. Survival was unaffected by
treatment. Male and female mice fed 1500 or 5000 ppm benomyl had
dose-related decreases in body weight. Food consumption was variable
throughout the study, although females at the high dose appeared to
consume less food. Clinical signs of toxicity were similar in all
groups; there were no apparent differences in palpable masses,
numbers of mice affected, or latency to detection. The results of
haematological examinations were unremarkable except for decreased
erythrocyte counts in males at 1500 ppm and in females at 5000 ppm.
Haemoglobin and haematocrit values were also depressed in males at the
intermediate dose. Several statistically significant changes in organ
weights were seen in treated groups, the most significant being
reduced absolute and relative liver weights in males at 1500 and
5000 ppm and females at 5000 ppm. Male mice at the high dose also had
decreased absolute testicular weights. The changes in liver and
testicular weights were accompanied by histomorphic changes in these
tissues. The incidence of hepatocellular carcinomas and benign hepatic
neoplasms in treated female mice was increased in a dose-dependent
fashion. In male mice, hepatocellular carcinomas and benign hepatic
neoplasms were significantly increased at 500 and 1500 ppm, but not at
the high dose. The incidences of lung tumours (alveologenic
carcinomas) were also significantly increased in males but not in
females at 500 and 1500 ppm. Non-neoplastic changes in males at
5000 ppm were confined to the liver (degeneration, pigment,
cytomegaly), thymus (atrophy), and the testis, epididymus, and
prostate (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini). The occurrence of splenic
haemosiderosis was significantly increased in female mice at 5000 ppm
and that of submucosal lymphocytic infiltration of the trachea at
1500 ppm. The latency for liver tumour induction (adenomas and
carcinomas), as determined from palpation, gross necropsy, and
histopathology performed on all animals throughout the study, was not
measurably different in treated and control animals. The authors
concluded that, under the conditions of the study, benomyl is
oncogenic in male and female animals. No NOAEL was identified for male
or female mice with respect to hepatocellular carcinomas or combined
hepatocellular neoplasms. The lowest dietary level, 500 ppm, was
equivalent to 64 mg/kg bw per day in males and to 103 mg/kg bw per day
in females (Wiechman, 1982).
Rats
Groups of 36 male and 36 female Charles River albino weanling
rats were fed benomyl (purity, 50-70%) in the diet for 104 weeks at
100, 500, or 2500 ppm (as benomyl); there were two control groups.
After one year, each group was reduced to 30 males and 30 females by
interim sacrifice for gross and microscopic evaluation. At the
conclusion of the study, all surviving animals were sacrificed and the
tissues and organs examined grossly. No deaths were attributable to
the presence of benomyl in the diet. Survival was decreased to about
50% during the second year but was comparable in all groups. Body
weight, food consumption, and food efficiency were unaffected by
treatment. There were no compound-related clinical manifestations of
toxicity, and the results of haematological, urinary, and liver
function examinations were unaffected by treatment. There were no
differences in organ weights, and histopathological examination
revealed no differences between treated and control groups. The most
frequently observed tumours were of the mammary, pituitary, and
adrenal glands, but these were equally distributed among the groups.
Hepatic toxicity was similar in all groups, including controls, and
there were no compound-related effects. As a high incidence of
testicular degeneration was observed in control males, no conclusion
could be drawn about compound-related effects on male gonads. Benomyl
had no adverse effects in this study at levels up to and including
2500 ppm, which was equivalent to 109 mg/kg bw per day in males and
128 mg/kg bw per day in females (Sherman, 1969c; Lee, 1977).
(d) Reproductive toxicity
In a short-term study of toxicity (see above), groups of six male
and six female Sprague-Dawley rats were fed diets containing benomyl
at 0, 100, 500, or 2500 ppm. Each female was caged separately and
exposed to each of three males at the same dose for five days. The
females were then allowed to deliver, and the offspring were observed
until weaning. Litters containing more than 10 pups were reduced to 10
by randomly removing pups on day 4. There was no treatment-related
effect on pregnancy rate, number of pups born live or dead, fertility,
gestation, viability, or lactation indices or on average body weight
(Sherman et al., 1975).
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal male Sprague-Dawley rats aged
33 days, which were gavaged daily for 10 days with 0 or 200 mg/kg bw.
Eight animals per group were killed 3, 17, 31, 45, and 59 days after
the last treatment. Selected tissues, including liver, kidneys,
testes, seminal vesicles, and epididymides, were removed, weighed, and
examined histologically; samples of seminal fluid from the vas
deferens were also examined. Observation intervals were preselected to
coincide with stages of spermatogenesis. No effects related to
treatment were seen on tissue weights, total epididymal sperm counts,
was deferens sperm concentrations, or testicular histology (Carter,
1982).
In a similar experiment, groups of five animals were dosed orally
on five consecutive days with 0, 125, 250, 500, or 1000 mg/kg bw per
day, beginning when animals were 33 (prepubertal), 54 (pubertal), or
75 (post-pubertal) days old. Animals were killed 31 days after
the last dose. There was no effect on body weight gain or on
haematological values. The weights of the testes and cauda
epididymides were reduced in animals given 500 or 1000 mg/kg bw per
day benomyl during the onset of puberty. Cauda epididymal and vas
deferens sperm counts were reduced in animals given > 250 mg/kg bw
per day benomyl during puberty. The incidence of diffuse hyposperma-
tocytogenesis was increased in pubertal and post-pubertal animals but
was most pronounced in the latter. The NOAEL for effects on
reproductive development was 12.5 mg/kg bw per day (Carter, 1982).
In a further experiment, adult (65-day-old) male Sprague-Dawley
rats received 10 daily doses of benomyl at 0, 200, or 400 mg/kg bw per
day by gavage. Fourteen days after the last treatment, body weights,
tissue weights, total epididymal sperm counts, and sperm concentration
in the vas deferens were measured, and the testis was examined
histologically. Production of testosterone by the Leydig cells was
artificially stimulated by subcutaneous injections of hypothalamic
chorionic gonadotrophin 2 h before sacrifice. There were no compound-
related effects on the weights of the body, liver, kidney, adrenal,
testis, or seminal vesicles, but caudate epididymal weights were
depressed by treatment and there were treatment-related reductions in
epididymal sperm count (caput and caudate) and in vas deferens sperm
concentration. In animals exposed to the highest dose, there was
histological evidence of hypospermatocytogenesis, with generalized
disruption of all stages of spermatogenesis (Carter & Laskey, 1982).
Benomyl was administered by gavage to Wistar rats at doses of 0,
15.6, or 31.2 mg/kg bw per day from day 7 of gestation to day 15 of
lactation (day 22 of gestation was considered day 0 of lactation). The
litters were each reduced to four male and four female pups on day 3
of lactation. The litters were weighed on days 8, 15, 22, 29, and 100,
and locomotor activity was evaluated periodically throughout the
study. When the animals were 100 days of age, several organs were
weighed, including adrenals, liver, kidney, ovary, testis, and ventral
prostate plus seminal vesicles. There were no compound-related effects
on litter size at birth or weaning or on body weights of fetuses.
Growth, survival, and locomotor activity were comparable to those of
controls throughout the study. Organ weights were comparable to t hose
of controls except for decreased weights of testes and ventral
prostate/seminal vesicles, which were significantly reduced at 31.2
but not at 15.6 mg/kg bw per day (Kavlock et at., 1982).
In a two-generation study, Sprague-Dawley rats were fed diets
containing 0, 100, 500, 3000, or 10 000 ppm benomyl. Parental rats
received the test diets for 71 days before being bred for production
of F1 parental rats with animals at the same dietary concentration.
F1 rats were mated for production of the F2a litter after
maintenance on their respective diets for at least 105 days after
weaning. F1 dams were mated again, to different, non-sibling males,
at least one week after weaning the F2a litter, to produce the
F2blitters. The following indices of reproductive Junction were
calculated for the F0 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive, and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption, and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental rats
were sacrificed lot gross examination and histopathological
examination of gross lesions and target organs; complete histopatho-
logical examination was conducted on controls and animals at the high
dose. Groups of 20 F2a and 20 F2bweanlings were also examined
grossly. There were no compound-related effects on parental mortality
at any concentration of benomyl. Mean body weights, body weight gains,
and overall food consumption of F0 and F1 male and female rats at
10 000 ppm were significantly lower than those in controls, and there
was a significant compound-related decrease in the number of F2a and
F2boffspring alive before culling (on day 4) at this dose. In
addition, male and female offspring of rats fed 10 000 ppm weighed
consistently less at birth than did the offspring of control rats.
With the exception of 14-day male pups of the F2bgeneration, F2a
and F2b offspring at 3000 ppm also had significantly depressed body
weights on days 14 and 21 of lactation. The testicular sperm counts of
F0 and F1 rats at 3000 and 10 000 ppm were decreased, and rats at
the highest dose had decreased testicular weight and histopathological
changes in the testes. Microscopic observation showed a trophy and
degeneration of the seminiferous tubules in the testes of rats at 3000
and 10 000 ppm and oligospermia in the epididymides of F0, rats at
the highest dose and in F1 rats at 3000 and 10 000 ppm. There were
no compound-related differences in mating indices, fertility indices,
or length of gestation that could be attributed to benomyl. The NOAEL
was 500 ppm, equivalent to about 37 mg/kg bw per day (Mebus, 1990).
In a study of adult male Wistar rats, groups of 27 animals were
fed 0, 1, 6.3, or 203 ppm for 70 days, and 14 animals per group were
allowed to recover for 70 days. Ejaculated sperm counts were
reportedly depressed during the feeding phase in the group fed
203 ppm. Relative testicular weights were also lowered, and a slightly
reduced male fertility index was seen in all treated groups. Benomyl
did not alter copulatory behaviour and did not induce dominant lethal
mutation. Plasma testosterone and gonadotropin levels remained
unchanged throughout the study. Identical studies conducted during the
recovery phase showed that the treatment-related effects were
completely reversible (Barnes et al., 1983).
Groups of 12 adult (102-day-old) Wistar male rats were given
benomyl at 0, 1, 5, 15, or 45 mg/kg bw per day by gavage daily for 62
days, were then bred to untreated females after 62 days of dosing, and
were killed after 76-79 days. Reproductive behaviour, seminal vesicle
weight, prostate weight, sperm motility, and the levels of serum
gonadotropin and gonadal hormones were no different from those in
controls. At necropsy, males exposed to 45 mg/kg bw per day had
decreased testicular and epididymal weights, reduced caudal sperm
reserves, decreased sperm production, increased numbers of decapitated
spermatozoa, and increased numbers of seminiferous tubules containing
multinucleated giant cells. There were no treatment-related effects on
the levels of serum luteinizing hormone, follicle stimulating hormone,
prolactin, or androgen binding protein (Linder et al., 1988).
Groups of 20 male Sprague-Dawley rats aged 100 days were given a
single dose of benomyl in corn oil at 0, 25, 50, 100, 200, 400, or
800 mg/kg bw. Eight animals per group were sacrificed after two days
of treatment and 12 animals per group after 70 days (except in the
group receiving 800 mg/kg bw) in order to examine the testis and
efferent ducts for effects on spermatogenesis or the epididymis. The
primary effects seen at day 2 were testicular swelling and occlusions
of the efferent ductules. Premature release of germ cells (sloughing)
was the most sensitive short-term response to benomyl. It was detected
in all treated groups but was statistically significant (P < 0.05)
only at doses > 100 mg/kg bw. Occlusion of the efferent ductules of
the testis was dose-dependent and was correlated with the increase in
testicular weight on day 2. The greatest increase in testicular weight
was observed at 400 mg/kg bw. Long-term effects (70 days) were seen
only in animals at > 100 mg/kg bw and included decreased testicular
weight at 400 mg/kg bw, dose-dependent increases in seminiferous
tubular atrophy, and increases in the number of reproductive tracts
containing occluded efferent ductules (Hess et al., 1991).
(e) Developmental toxicity
Mice
Groups of 20-25 pregnant CD-1 mice were given benomyl by gavage
at 0, 50, 100, or 200 mg/kg bw per day on days 7-17 of gestation. They
were killed on day 18, their pups were delivered by caesarean section,
the numbers of live, dead, and recorded fetuses were determined, and
the fetuses were examined for gross anomalies (one-half for visceral
abnormalities and the other half for skeletal abnormalities). Maternal
indexes were unaffected by treatment, but fetal mortality was
increased, fetal weight was decreased, and fetal development was
adversely affected by treatment. The high dose increased the
supraoccipital score, decreased the numbers of caudal and sternal
ossifications, and increased the incidences of enlarged lateral
ventricles and enlarged renal pelvises. The latter increase, while not
significant at the lowest dose, was dose-related at the other doses.
The occurrence of supernumerary ribs and subnormal vertebral centra
was significantly increased in a dose-related manner at all doses. The
numbers of abnormal litters and fetuses were significantly increased
over those among the controls at 100 and 200 mg/kg bw per day. Major
anomalies included exencephaly, hydrocephaly, cleft palate,
hydronephrosis, polydactyly, oligodactyly, umbilical hernia, fused
ribs, fused vertebrae, and short or kinky tail. Although benomyl
induced dose-related fetotoxicity at all levels, it was not
teratogenic at 50 mg/kg bw per day in mice (Kavlock et al., 1982).
In a study designed to investigate whether teratogens could be
detected by observing postnatal viability or impaired growth, groups
of 24 pregnant CD-1 mice were treated orally with benomyl at 0 or
200 mg/kg bw per day on days 8-12 of gestation. Postnatal viability
was reduced on days 1 and 3, and average pup weight was reduced on day
1 post partum (Chernoff & Kavlock, 1983).
Rats
Groups of 26-28 pregnant Sprague-Dawley rats were given benomyl
(purity, 53.5%) in the diet at 0, 100, 500, 2500, or 5000 ppm on days
6-15 of gestation to provide average doses equivalent to 0, 8.6, 43.5,
209.5, and 372.9 mg/kg bw per day. On day 20 of gestation, all
pregnant animals were sacrificed and their fetuses were delivered by
caesarean section. There were no deaths attributable to treatment, no
clinical signs of toxicity, and no adverse effects on the body weights
of dams. Dams at 5000 ppm had reduced food intake during treatment,
but consumption returned to control levels for the remainder of the
study. Indicators of reproductive health (numbers of implantation
sites, resorption sites, and live and dead fetuses) were not affected
by treatment up to and including the highest dietary level. The only
external or internal abnormalities reported were hydronephrosis and
retarded ossification of the interparietal and occipital bones in
three litters at the highest dose. None of these teratogenic effects
was associated with the administration of benomyl during the critical
period of organogenesis (Sherman et al., 1975).
Groups of 27-28 pregnant Wistar rats were fed benomyl in the diet
at 0, 1690, 3380, or 6760 ppm, equivalent to 0, 169, 298, or 505 mg/kg
bw per day, on days 7-16 of gestation. Fetuses were delivered by
caesarean section on day 21, weighed, examined grossly, and fixed for
evaluation of visceral and skeletal abnormalities. Food consumption
was decreased in dams at 3380 or 6760 ppm, with resultant decreases in
body weight gain, which were significant at the high dose. The weight
gain of fetuses at the two higher doses was significantly reduced, and
a significant decrease in the ossification of the supraoccipital bone
was seen at the high dose. The percentage of fetuses with an enlarged
renal pelvis was increased at the two higher doses. No dose-related
major malformations were seen at these dietary levels (Kavlock
et al., 1982)
Benomyl (99.2% pure) was administered by gavage to pregnant
Sprague-Dawley rats at doses of 0, 3, 10, 30, 62.5, or 125 mg/kg bw
per day on days 7-16 of gestation. A control group receiving corn oil
comprised 60 rats, and each test group comprised 27 rats. No clinical
signs of toxicity or mortality were observed at any dose. Body weight
gain, the incidences of pregnancy, corpora lutea, and implantation
sites, and the sex ratio were comparable to those of controls;
however, fetal body weight was significantly decreased at 62.5 and
125 mg/kg bw. There was also an increased incidence of embryofetal
mortality at 125 mg/kg bw. At doses > 10 mg/kg bw per day, some
fetuses had external and visceral malformations, which included
microphthalmia, anophthalmia, and hydrocephaly (distended lateral
ventricles), predominantly at the higher doses. Histological
examination of the eyes revealed pathological changes consisting of
irregular lenses, retrobulbar glandular adnexa, distorted or
compressed retinal layers, and thickened nerve fibres in the groups
receiving 10, 62.5, or 125 mg/kg bw per day; no alterations were seen
in controls or in animals at 30 mg/kg bw per day. The major skeletal
malformations observed at 125 mg/kg bw per day included fused ribs,
sternebrae, and thoracic arches. Additional skeletal variations seen
at 62.5 and 125 mg/kg bw per day included misaligned and unossified
sternebrae and bipartite vertebral centra. The authors concluded that
benomyl produced teratogenic responses in rats at doses > 10 mg/kg
bw per day. Microphthalmia seen at this dose was reported to be
related to treatment on the basis of the severity of the pathological
changes and the finding that the increased incidences at 62.5 and
125 mg/kg bw per day were not a direct reflection of reduced fetal
body weight. There was no evidence of maternal toxicity, even at the
highest dose (Staples, 1980).
Groups of pregnant Sprague-Dawley rats were given benomyl (99.1%
pure) by gavage at 0, 3, 6.25, 10, 20, 30, or 62.5 mg/kg bw per day on
days 7-16 of gestation. Each group consisted of 50 animals, except the
high-dose group, which comprised 20 rats. As the study was performed
to determine a no-effect level for microphthalmia and hydrocephaly,
only fetal heads were fixed and examined. Microphthalmia was
determined on the basis of the smallest eye in the control group
(< 1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two cases of malformation, both at
62.5 mg/kg bw per day: One fetus had internal hydrocephaly, and one in
another litter had unilateral microphthalmia. The only type of
variation reported was subcutaneous haematoma, which was not dose-
related. There was no teratogenic response at 30 mg/kg bw per day
(Staples, 1982).
On the basis of these two studies, the Meeting concluded that the
NOAEL for teratogenicity was 30 mg/kg bw per day, as the anophthalmia
at 10 mg/kg bw per day in the first study was not found in the second
study, which was specifically designed to look for this anomaly.
Furthermore, anophthalmia was not seen at 30 mg/kg bw per day in the
first study, and its occurrence at 10 mg/kg bw per day was not
accompanied by other evidence of teratogenic response.
The teratogenic potential of benomyl was examined in groups of
12-30 Wistar rats given oral doses of 0, 15.6, 31.2, 62.5, or
12.5 mg/kg bw per day on days 7-16 of gestation. Pups were delivered
by caesarean section on day 21 of gestation. The weight gain of the
dams at the highest dose was decreased on days 17-20 of gestation, and
the frequency of fetal resorption was increased, six litters being
completely resorbed. Fetal weight was also adversely affected, with
significant decreases at 62.5 and 125 mg/kg bw per day, and there was
a significant increase in fetal mortality at 125 mg/kg bw per day.
Several skeletal and visceral variants were observed among fetuses at
62.5 and 125 mg/kg bw per day, including increased supraoccipital
score, decreased numbers of sternal and caudal vertebrae, and
increased percentages of enlarged lateral ventricles and enlarged
renal pelvis. Major anomalies, observed primarily at > 62.5 mg/kg
bw per day, included encephaloceles, hydrocephaly, microphthalmia,
fused vertebrae, and fused ribs. The dose of 31.2 mg/kg bw per day
appeared to have no adverse effects on developing rat fetuses (Kavlock
et al., 1982).
Groups of pregnant Sprague-Dawley rats were treated orally with
benomyl at 0 or 62.5 mg/kg bw per day on days 7-16 (eight rats) or
7-20 of gestation (13 rats). There were no overt signs of toxicity.
Hydrocephaly was noted in 35% of fetuses examined on day 16 and in 76%
of fetuses examined on day 20 (Ellis et al., 1987, 1988).
In other studies, benomyl produced ocular and craniocerebral
malformations in Sprague-Dawley rats when administered by gavage at
doses of 31.2 or 62.4 mg/kg bw per day on days 7-21 of gestation.
Ocular anomalies (retinal dysplasia, cataracts, microphthalmia, and
anophthalmia) occurred in 43.3% of the fetuses of dams given the
higher dose. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose of
benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
consisting primarily of hydrocephaly occurred in the fetuses of dams
given 31.2 mg/kg bw per day in combination with the semipurified diet
(Zeman et al., 1986).
Rabbits
Groups of 15 artificially inseminated New Zealand albino rabbits
were fed a benomyl formulation (containing 50% benomyl) in the diet at
0, 100, or 500 ppm on days 8-16 of gestation. Mortality, clinical
parameters, and food consumption were determined daily, and body
weights were measured weekly. There were 12 pregnant does in the
control group, 13 in the low-dose group, and 9 in the high-dose group.
Of these, 6, 7 and 5, respectively, were sacrificed on day 29 or 30
and their fetuses were delivered by caesarean section; the remaining
does gave birth normally. No maternal toxicity was observed at any
dose. Except for a marginal increase in the frequency of rudimentary
ribs in animals at 500 ppm, no developmental toxicity was observed
(Busey, 1968d); however, the numbers of litters and of fetuses
examined were less than adequate to assess the fetotoxic or
teratogenic potential of benomyl.
Groups of 20 female New Zealand white female rabbits, presumed to
be pregnant, were given oral doses of 0, 15, 30, 90, or 180 mg/kg bw
per day benomyl (as a suspension in 0.5% methylcellulose) on days 7-28
of gestation. The animals were killed on day 29. The results of
analyses of the concentration, homogeneity, and stability of the
suspensions indicated acceptable conformity with nominal content. Only
one animal at 90 and one at 180 mg/kg bw per day did not become
pregnant. There were seven deaths before terminal sacrifice -- one
control, three treated with 30 mg/kg bw per day, one treated with
90 mg/kg bw per day, and two treated with 180 mg/kg bw per day -- all
of which were due to injuries associated with dosing. At 180 mg/kg bw
per day, an increased incidence of stained tails was seen, and food
consumption was significantly reduced, but there were no treatment-
related effects on maternal body weight. Five animals aborted during
the study: one in each of the groups treated with 0, 15, and 90 mg/kg
bw per day and two at 180 mg/kg bw per day. As the historical control
data showed no more than one abortion in a control group of 20 does,
the Meeting concluded that benomyl at 180 mg/kg bw per day had an
adverse effect. There were no other treatment-related effects on
reproductive parameters. Fetal mortality and fetal weights were not
affected by treatment, and there were no treatment-related external
malformations. At 180 mg/kg bw per day, two viable fetuses in two
litters had small renal papillae. The Meeting concluded that this
finding was treatment-related in view of the finding of a single
incidence in 11 studies in historical controls. The NOAEL for maternal
toxicity was 90 mg/kg bw per day, on the basis of reduced food
consumption, abortion, and clinical signs of toxicity at 180 mg/kg bw
per day. The NOAEL for developmental toxicity was also 90 mg/kg bw per
day, on the basis of the occurrence of small renal papillae at
180 mg/kg bw per day (Munley, 1995).
(f) Genotoxicity
Numerous studies have been reported in the open literature on the
mutagenic potential of benomyl, its metabolite carbendazim, and
several benomyl formulations. Many of the results are conflicting, and
few of the publications provide sufficient detail to evaluate the
reasons for the conflicts. Table 2 covers only those studies in which
sufficient experimental detail and data were reported.
Benomyl does not cause gene mutation or structural chromosomal
aberrations in somatic or germ cells, and it does not interact
directly with DNA in either mammalian or non-mammalian systems. Gene
mutations and structural chromosomal aberrations observed in mammalian
cells in vitro in several studies appear to have been the
consequence of the inherent sensitivity of some mammalian test systems
to cytotoxic agents. No gene mutations or structural chromosomal
aberrations were seen in mammals in vivo. Benomyl causes numerical
chromosomal aberrations (aneuploidy and/or polyploidy) both in vitro
and in vivo. Studies involving kinetochore staining indicated,
however, that benomyl is not clastogenic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Application for 24 h of doses > 0.5 g per animal of a 50%
wettable powder to the occluded, clipped, intact and abraded abdominal
skin of albino rabbits produced moderate to marked erythema, slight
oedema, and slight desquamation. Albino guinea-pigs similarly ex posed
to 10, 25, or 40% dilutions of technical-grade benomyl in dimethyl
phthalate had only mild irritation of intact and abraded skin (Majut,
1966; Busey, 1968a; Colburn, 1969; Frank, 1969). No dermal irritation
was observed 4 or 24 h after application of 0.5 g of Benlate 50 DF
(containing 50% benomyl) to six male New Zealand white rabbits. After
48 h, two rabbits had slight to mild erythema, which was still evident
after 72 h. The primary irritation scores ranged from 0 to 1 (not an
irritant) (Vick & Brock, 1987).
Draize scores were assessed 4 and 72 h after application of
benomyl (purity, 98%) to a shaved area of the back of four albino
rabbits at 5 mg/cm2. The lesions produced were classified as 'mild
irritation' (Desi, 1979).
The ocular irritating properties of technical-grade benomyl, a
50% wettable powder, and a suspension in mineral oil were examined in
albino rabbits in several tests. Mild conjunctival irritation and
minor transitory corneal opacity were reported after 48 to 96 h in all
tests (Reinke, 1966; Frank, 1972). Similar results were obtained with
Table 2. Results of tests for the genotoxicity of benomyl
End-point Test system Concentration Purity Results Reference
or dose (%)
Tests for chromosomal effects
Mitotic gene conversiona S. cerevisiae, A. nidulans < 3200 mg/ml Technical Negative DeBertoldi et al.
(1980)
Nondisjunction/crossing- A. nidulans < 2.8 mmol/litre Technical Negative, but DeBertoldi & Griselli
over spindle inhibition (1980)
observed
Sister chromatid exchangea Chinese hamster ovary > 150 mg/ml Technical Weakly positive Evans & Mitchell
cells (1980)
Chromosomal aberrationa Human lymphocytes < 2000 mg/ml 50% WP Negative Gupta & Legator
(1975)
Chromosomal aberrationa Human lymphocytes < 100 mg/ml Technical Negative Pilinskaya (1983)
Chromosomal aberrationa Chinese hamster lung cells < 90 mg/ml Technical Positive Sasaki (1988)
(98.7%)
Chromosomal effects Human/mouse monochromosomal < 15 mg/ml Technical Positive: aneuploidy Athwal & Sandhu (1985)
hybrid cells and polyploidy
(R3-5) (1.5 mg/ml threshold)
Chromosomal effects V79/AP4 Chinese hamster NR Technical Dose-related increase Rainaldi et al. (1989)
cells in numerical
chromosomal
aberrations
Chromosomal effects Chinese hamster ovary cells NR Technical Dose-related increase Eastmond & Tucker
in numerical (1989)
chromosomal
aberrations
Chromosomal effects Human lymphocytes NR Technical Dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations
(0.1 mg/ml threshold)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Chromosomal effects Chinese hamster/human NR Technical Dose-related increase Zelesco et al. (1990)
hybrid cells (EUBI) in numerical
chromosomal
aberrations
(2.0 mg/ml threshold)
Tests for gene mutation
Reverse mutationa S. typhimurium TA98, < 325 mg/plate Technical Doubtful mutagenic Rashid & Ercegovitch
TA 100, TA 1535, TA 1537, activity (1976)
TA 1538
Reverse mutationa S. typhimurium TA 1535, < 1 mg/ml 50% WP Positive Kappas et al. (1976)
TA1538, E. coil WP2,
WP2 uvra, CM 611
Reverse mutationa S. typhimurium TA98, < 1000 mg/ml Technical Negative Shirasu et al. (1978)
TA100, TA1535, TA1537,
TA1538, E. coil WP2 he;
Reverse mutationa S .typhimurium TA 1530, < 10 000 mg/ml Technical, Negative Fiscor et al. (1978)
TA1535, TA1950 50% WP
Reverse mutationa S. typhimurium TA 1530, < 10 000 mg/ml Technical, Negative DuPont de Nemours
TA1535, TA 1950 50% WP & Co. (1977)
Reverse mutationa S. typhimurium TA98, < 250 mg/plate Technical Negative Donovan & Krahn
TA 100, TA 1535, TA1537 (99.9%) (1981)
Reverse mutationa S. typhimurium TA98. < 10000 Technical Negative Rickard (1983a,b)
TA100, TA1535. TA1537 mg/plate (99%)
Reverse mutationa S. typhimurium TA98, < 500 mg/plate Technical Equivocal (positive Russell (1978a,b)
TA 100, TA 1535. TA 1537 in TA 1537 with
activation)
Reverse mutationa S. typhimurium TA 15~35, < 500 mg/disc Technical Negative Carere et al. (1978)
TA1536, TA 1537, TA1538,
Streptococcus coelicodor
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Reverse mutationa A. nidulans < 0.4 mg/ml Technical Positive Kappas et al. (1974);
Kappas & Bridges
(1981)
hprt mutationa Chinese hamster ovary < 172 mmol/litre Technical Negative Fitzpatrick (1980)
cells (99,9%)
tk mutation L5178Y mouse < 20 mmol/litre Technical Negative Amacher et al. (1979)
lymphoma cells
tk mutationa L5178Y mouse < 25 mmol/litre Technical Positive without McCooey et al.
lymphoma cells (991%) activation: negative (1983a, b)
with activation
Tests for DNA damage and repair
Unscheduled DNA Rat and mouse < 500 mg/ml Technical Negative Tong (1981)
synthesis hepatocytes
Rec assay B. subtilis 2000 mg/plate Technical Negative Shirasu et al. (1978)
Tests in vivo
Host-mediated mutation S. typhimurium 2000 mg/kg bw Technical Negative Shirasu et al. (1978)
in mice G46 his-
Host mediated mutation S. typhimurium 3 × 500 mg/kg bw 50% WP Negative Fiscor et al. (1978)
in mice G46 his-
Dominant lethal mutation Rat < 5000 ppm in 50% WP Negative Culik & Gibson
food for 7 days (1914)
Dominant lethal mutation Rat NR Technical Negative Sherman et al. (1975)
Dominant lethal mutation Rat < 203 ppm in Technical Negative Barnes et al. (1983)
food for 70 days
Dominant lethal mutation Rat < 50 mg/kg bw Technical Negative Georgieva et al.
per day for 70 days (1990)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Positive Seller (1976)
bw
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Equivocal Kirkhart (1980)
bw
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive Sasaki (1990)
bw (95%)
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive, KC+, Bentley (1992)
with KC+ staining bw (96.1-97.4%) benomyl classified
as aneugen
Chromosomal aberration Mouse bone marrow < 1 × 5000 mg/kg Technical Negative Stahl (1990)
bw (961%)
Chromosomal aberration Peripheral blood NR 50% WP Negative Ruzicska et al. (1976)
from workers
Chromosomal aberration Rat bone marrow < 500 mg/kg bw, 50% WP Bone marrow, Ruzicska et al. (1976)
and cultured embryo days 7-14 of negative; embryo
cells gestation cells, positive
Sex-linked recessive Drosophila melanogester 1.5 mg/ml Technical, Negative, but Lamb & Lilly (1980)
lethal mutation 50% WP sterility consistent
with observed
spindle effects
NR, not reported; 50% WP, wettable powder formulation; KC, kinetochore
a With and without metabolic activation
Benlate PNW, a 50% wettable powder (Gargus & Zoetis, 1983b,c). The
Draize score for ocular irritation induced by 5 mg of pure benomyl in
four albino rabbits indicates that it is a mild irritant (Desi, 1979).
Albino guinea-pigs exposed to technical-grade benomyl or a 50%
sucrose formulation had mild to moderate skin erythema during the
challenge phase after either intradermal injection or repeated
applications to abraded skin (Majut, 1966; Colburn, 1969; Frank,
1969).
Ten albino Hartley guinea-pigs received four weekly injections of
Benlate PNW (50% benomyl prepared as a 0.1% solution in saline), and
10 animals were injected with saline; 14 days after the final
injection, 8 or 80% Benlate PNW was used for challenge. An unequivocal
increase in sensitization was seen at two of 10 sites challenged with
the 8% suspension and at seven of 10 sites challenged with the 80%
suspension (Gargus & Zoetis, 1984).
Technical-grade benomyl sensitized all 10 guinea-pigs tested in a
maximization test (Matsushita & Aovama, 1981).
(ii) Neurotoxicity
Studies in hens given single oral doses of < 5000 mg/kg bw did
not indicate neurotoxic potential (Goldenthal, 1978; Jessup, 1979;
Jessup & Dean, 1979).
Studies in groups of 10 adult male CFY rats showed no alteration
in electroencephalographic potential or in behaviour (learning ability
assessed in a four-choice T-maze) in comparison with controls after
treatment with benomyl at 250 or 500 mg/kg bw per day for three months
(Desi, 1983).
Groups of 10 male and 10 female Sprague-Dawley rats received
single oral doses of 0, 500, 1000, or 2000 mg/kg bw benomyl (97.4%
pure). Functional and motor activity were assessed during the week
before the day of treatment and at 2 h, one day, seven days, and 14
days after dosing. Rats were then killed and subjected to a gross
post-mortem examination. Histopathological examination was restricted
to tissues from the central and peripheral nervous system of six rats
in the control and high-dose groups. There were no treatment-related
deaths during the study. Immediately after treatment, body weight gain
and food intake were decreased in all treated groups; however, overall
weight gain and food consumption were unaffected by treatment. In the
battery of functional tests, a small number of animals had soft or
liquid faeces and fur stained with urine or faeces on days 1 and 2,
but no other component of the battery, including assessments of other
autonomic functions, reactivity, and sensitivity, excitability, gait,
and sensorimotor coordination, forelimb and hindlimb grip strength,
abnormal clinical signs and body temperature, showed any reaction to
treatment. Motor activity was reduced, only on the day of treatment,
in females given 2000 mg/kg bw. Neuropathological observation revealed
no treatment-related abnormalities. It was concluded that benomyl
induced no lasting neurotoxicity at doses up to 2000 mg/kg bw (Foss
1993).
Groups of 11 male and 11 female Sprague-Dawley rats received
benomyl (purity, 97.4%) in the diet at 0, 100, 2500, or 7500 ppm for
90 days. Functional and motor activity were assessed during the week
before treatment and during weeks 4, 8, and 13. Rats were then killed
and subjected to gross post-mortem examination. Histopathological
examination was restricted to tissues from the central and peripheral
nervous system of six rats in the control and high-dose groups. There
were no treatment-related deaths during the study. Body weight gain
and food intake were reduced at the high dose throughout the treatment
period. The battery of functional tests showed no reaction to
treatment with benomyl. Motor activity was increased at 7500 ppm.
Neuropathological observation revealed no treatment-related
abnormalities. It was concluded that benomyl is not a specific
neurotoxicant, since the changes in motor activity occurred at the
same dietary level as other general toxicological changes (Foss,
1994).
3. Observations in humans
(a) Exposure during field use
Potential dermal and respiratory exposure to benomyl during
actual use was determined during mixing for aerial application,
re-entry into treated fields, and home use (garden, ornamental, and
greenhouse). Maximal exposure occurred during loading and mixing for
aerial application, with dermal exposure to 26 mg per mixing cycle,
primarily (90%) on the hands and forearms; the average respiratory
intake was 0.08 mg benomyl. During re-entry, the dermal exposure was
5.9 mg/h and the respiratory exposure < 0.002 mg/h. Home use resulted
in exposures of 1 mg per application cycle dermally and 0.003 mg by
the respiratory route. This study did not address reactions to
exposure (Everhart & Holt, 1982).
The potential dermal and respiratory exposure of workers mixing
and applying 20 and 100 gallons of Benlate per acre (224 and
1123 litres/ha) while spraying fruit orchards was examined. The
application cycle was about 70 min and resulted in a total dermal
exposure of 11 mg of benomyl per cycle and a total respiratory
exposure to 15 mg. Essentially all of the exposure was dermal,
resulting in 12.2 mg per cycle dermally and < 0.05 mg per cycle via
the respiratory route (DuPont de Nemours & Co., undated).
(b) Medical surveillance of workers
Benomyl caused contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et at., 1985).
In controlled patch tests of 200 agricultural, ex-agricultural, and
non-agricultural workers, only one agricultural worker showed contact
dermatitis to 0.1% benomyl (Lisi et al., 1986). In a survey of
cross-sensitization with benomyl and other pesticides in a group of
126 Japanese farmers who applied benomyl to their crops, 39 had
positive test results with benomyl, the highest incidence being found
among female farmers. There were cross-reactions between benomyl and
diazinon, saturn, daconil, and Z-bordeaux (Matsushita & Aovama, 1981).
Selected blood profiles of 50 factory workers involved in the
manufacture of Benlate were compared with those of a control group of
48 workers who were not exposed to Benlate. White blood count, red
blood count, haemoglobin, and haematocrit values were comparable in
the two groups. No quantitative estimates of exposure were given for
the factory workers (DuPont de Nemours & Co., 1979).
A survey was performed to determine whether potential exposure
to Benlate had an adverse effect on the fertility of 298 male
manufacturing workers exposed between 1970 and 1977. The ages of the
workers ranged from 19 to 64 years; 79% of the workers and 78% of
their spouses were aged 20-39. The duration of exposure ranged from
less than one month to 95 months, but more than 51% of the workers had
potentially been exposed for one to five months. The birth rates of
the spouses of the exposed workers were compared with those of four
populations from the same county, state, region, and country (United
States). There was no reduction in fertility, and the birth rates of
the study population were generally higher than those of the
comparison populations. Spermatogenesis was not examined (Gooch,
1978).
Comments
Benomyl is readily absorbed by animals after oral exposure and
rapidly metabolized. It is eliminated in the faeces and excreted in
the urine; 98% of the dose was excreted by 72 h after administration.
The tissue distribution showed no bioconcentration. In rats, the
metabolites carbendazim and methyl 5-hydroxybenzimidazol-2-ylcarbamate
(5-hydroxy-carbendazim) were found in the blood and in small amounts
in the testis and liver. The latter compound was the main metabolite
in urine. A 50% wettable powder formulation was poorly absorbed via
the dermal route by rats. After a 10-h exposure, less than 2% of a
single dose of 0.2 mg was excreted in the urine.
Benomyl has low acute toxicity, with an oral LD50 in rats of
> 10 000 mg/kg bw. The clinical signs of toxicity after high single
doses were generally nonspecific. Testicular degeneration, with
necrosis of germinal epithelium and aspermatogenesis, has been
observed after single doses in rats (> 100 mg/kg bw orally) and dogs
(1.65 mg/litre air by inhalation). Wettable powder formulations
containing benomyl have been shown to be mildly irritating to rabbit
skin and eyes and have induced skin sensitization reactions in
maximization tests. WHO has classified benomyl as unlikely to present
an acute hazard in normal use.
In a 90-day study, rats were given dietary doses of 0, 100, 500,
or 2500 ppm benomyl. Increased liver weight was seen at 2500 ppm; the
NOAEL was 500 ppm (50 mg/kg bw per day). Dogs were given dietary doses
of 0, 100, 500, or 2500 ppm benomyl for three months or two years and
rabbits were treated dermally, five days per week for three weeks,
with 0, 50, 250, 500, 1000, or 5000 mg/kg bw per day. Hepatotoxicity
was seen in the dogs but not in the rabbits; effects on male
reproductive organs were seen in both rabbits and dogs. The NOAEL was
500 ppm (equal to 13 mg/kg bw per day) in dogs and 500 mg/kg bw per
day in rabbits.
In a two-year study, benomyl was administered in the diet to rats
at 0, 100, 500, or 2500 ppm. Benomyl was not carcinogenic and showed
no compound-related effects at dietary levels up to and including
2500 ppm (equal to 109 mg/kg bw per day). In a two-year feeding study
in mice at dietary levels of 0, 500, 1500, or 5000 ppm, benomyl
caused liver tumours, and an NOAEL could not be established for
hepatocellular neoplasms. Male mice had decreased testicular weights
and thymic atrophy at 5000 ppm. The lowest dietary level was equal to
64 mg/kg bw per day.
In rats treated by gavage for 62 days with 45 mg/kg bw per day,
decreased testicular and epididymal weights, reduced caudal sperm
reserves, and decreased sperm production, with generalized disruption
of all stages of spermatogenesis were observed. After mating with
untreated females, no effect was seen on reproductive behaviour,
weight of the seminal vesicles, sperm mobility, or related
reproductive hormones. The NOAEL was 15 mg/kg bw per day. A lowering
of male fertility rates has been reported, but this effect was not
seen consistently. A single dose of 100 mg/kg bw or more administered
to rats by gavage had effects 70 days after exposure, which included
decreased testicular weight and atrophy of the seminiferous tubules.
The NOAEL was 50 mg/kg bw per day. In a recent study of reproductive
toxicity, rats received dietary doses of 0, 100, 500, 3000, or
10 000 ppm benomyl. The NOAEL was 500 ppm (equivalent to 37 mg/kg bw
per day), on the basis of effects on pup survival and pup growth and
on testicular changes. Fertility indices were not affected at dietary
levels up to 10 000 ppm.
In a study of developmental toxicity, mice were exposed by gavage
to benomyl at doses of 0, 50, 100, or 200 mg/kg bw per day on days
7-17 of gestation. There was no indication of maternal toxicity, but
benomyl was teratogenic at doses of 100 and 200 mg/kg bw per day and
fetotoxic at 50 mg/kg bw per day. The major abnormalities included
hydrocephaly, cleft palate, and limb defects. In studies of
teratogenicity, pregnant rats were exposed to benomyl at doses up to
and including 125 mg/kg bw per day on days 7-16 of gestation.
Benomyl was teratogenic, the major effects being microphthalmia and
hydrocephaly. The Meeting concluded that the NOAELs in rats were
30 mg/kg bw per day for teratogenicity and fetotoxicity and 125 mg/kg
bw per day for maternal toxicity. In rabbits, benomyl was not
teratogenic at doses up to 180 mg/kg bw per day (the highest dose
tested), and no effect was seen on maternal toxicity or fetotoxicity
at 90 mg/kg bw per day.
Benomyl has been adequately tested for genotoxicity in a range of
assays. The Meeting concluded that it does not directly damage genetic
material but does cause numerical chromosomal changes both in vitro
and in vivo as a result of its interference with mitotic spindle
proteins.
In a study of workers exposed to benomyl, there was no reduction
in fertility, as indicated by birth rates, among the study population.
Spermatogenesis in the workers was not examined. Cases of dermal
sensitization to benomyl have been reported.
An ADI of 0-0.1 mg/kg bw was established on the basis of the
NOAEL of 13 mg/kg bw per day in the two-year study in dogs and
applying a safety factor of 100. This ADI should be used when
assessing exposure to benomyl itself. Since the use of benomyl on
crops gives rise to residues of carbendazim and since the ADI for
carbendazim is lower than that which would be derived from the data on
benomyl, the Meeting concluded that the intake of residues in food
should be compared with the ADI of 0-0.03 mg/kg bw for carbendazim.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: < 500 ppm, equal to < 64 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
50 mg/kg bw per day (study of developmental toxicity)
< 50 mg/kg bw per day (fetotoxicity in a study of
teratogenicity)
Rat: 2500 ppm, equal to 109 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
500 ppm, equivalent to 37 mg/kg bw per day (study of
reproductive toxicity)
30 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
125 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 180 mg/kg bw per day (study of developmental toxicity)
90 mg/kg bw per day (maternal toxicity and fetotoxicity in
study of developmental toxicity)
Dog: 500 ppm, equal to 13 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw (benomyl)
0-0.03 mg/kg bw (carbendazim, with which residues of benomyl in
food should be compared)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to benomyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 >10 000 mg/kg bw
Dermal, toxicity, rabbit LD50 (50% wettable powder)
> 10 000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder; irritating
Ocular, irritation, rabbit 50% wettable powder; irritating
Dermal, sensitization, guinea-pig Positive in maximization test
Inhalation, toxicity, rat LC50 (50% wettable powder)
> 4.01 mg/litre air
Mid-term (1-26 weeks) Oral, 62 days, rat NOAEL = 15 mg/kg bw per day;
reduced spermatogenesis
Oral, developmental toxicity, rat NOAEL = 30 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary two years, toxicity, dog NOAEL = 13 mg/kg bw per day;
hepatotoxicity
See also toxicological criteria for carbendazim when considering dietary exposure to residues
References
Amacher, D.E., Paillet, S. & Ray, V.A. (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells:
Application to genetic toxicological testing. Mutat. Res., 64,
391-406.
Athwal, R.S. & Sandhu, S.S. (1985) Use of human x mouse hybrid cell
line to detect aneuploidy induced by environmental chemicals.
Mutat. Res., 149, 73-81.
Axness, M.E. & Fleeker, J.R. (1979) Metabolism of the butylcarbamoyl
moiety of benomyl in rat. Pestic. Biochem. Physiol., 11, 1-12.
Barbo, E.C. & Carroll, K.S. (1972) Oral ALD test ( S-triazine,
3-butylbenzimidazolo[1,2-a]-2,4[1 H,3 H]dione. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Barnes, T. B., Verlangieri, A.J. & Wilson, M.C. (1983) Reproductive
toxicity of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate
(benomyl) in male Wistar rats. Toxicology, 28, 103-115.
Belasco, I.J. (1979a) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied. Unpublished
report from DuPont de Nemours & Co., Biochemical Department,
Research Division, Experimental Station, Wilmington, Delaware,
USA.
Belasco I.J. (1979b) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Unpublished report from DuPont de Nemours & Co., Biochemical
Department, Research Division, Experimental Station, Wilmington,
Delaware, USA
Belasco, I.J., Kirkland, J.J., Pease, H.L. & Sherman, H (1969) Studies
with 2-14C-labelled methyl-1-(butylcarbamoyl)-2-
benzimidazolecarbamate (benomyl) in rats. Unpublished report from
DuPont de Nemours & Co., Biochemical Department, Research
Division, Experimental Station, Wilmington, Delaware, USA.
Bentley, K S. (1992) Classification of DPX-T1991-529 (Benomyl)-induced
micronuclei in mouse bone marrow erythrocytes using immuno-
fluorescence antikinetochore antibodies. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Brock, W.J. (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Unpublished report from DuPont de Nemours &
Co, Haskell Laboratory, Newark, Delaware, USA.
Busey, W.M. (1968a) Acute dermal LD50 test and dermal irritation
test on rabbits using a wettable powder formulation (50% benomyl)
with histological addendum. Unpublished report from Hazelton
Laboratories, Inc., Falls Church, Virginia, USA, prepared for
DuPont de Nemours & Co.
Busey, W.M. (1968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968c) Repeated dermal application test on rabbits using
a wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968d) Teratology study in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from
Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res., 57, 277-826.
Carter, S.D. (1982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Thesis, North Carolina State
University, Raleigh, North Carolina, USA.
Carter, S.D. & Laskey, J.W. (1982) Effect of benomyl on reproduction
in the male rat. Toxicol. Lett., 11, 87-94.
Chernoff, N. & Kavlock, R.J. (1983) A teratology test system which
utilises post natal growth and viability in the mouse. Environ.
Sci. Res., 27, 417-427.
Colburn, C.W. (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Culik, R. (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the
concepti of rats exposed to benomyl and Benlate by diet.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC), 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. and Gibson, J.R. (1974) 'Benlate' dominant lethal study in
male rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Dashiell, O.L. (1972) Acute oral test (benzimidazole, 2-(3-
butylureido)). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
De Bertoldi, M. & Griselli, M. (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat. Res., 74,
303-324.
De Bertoldi, M., Griselli. M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by metres of gene-conversion
in Saccharomyces cerevisiae and in Aspergillus nidulans.
Environ. Mutag., 2, 359-370.
Desi, I. (1979) Hygienic-toxicological evaluations of pesticides.
D.Sc. Thesis, Budapest, National Institute of Hygiene.
Desi, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5, 503-515.
Donovan, S.D. & Krahn, D.F. (1981) Mutagenic evaluation in
Salmonella typhimurium. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1977) Mutagenic activity of benomyl in the
Salmonella/microsome assay (50% benomyl as a wettable pow der
formulation). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1979) Benlate dust exposure survey -- blood
profile analysis. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (undated) Applicator exposure during filling
and spraying of Benlate benomyl fungicide -- orchard crops.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Eastmond, D.A. & Tucker, J.D. (1989) Kinetochore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat. Res., 224, 517-525.
Ellis W.G., Semple, J.L., Hoogenboom, E.R., Kavlock, R.J. & Zeman,
E.J. (1987) Benomyl-induced craniocerebral anomalies in fetuses
of adequately nourished and protein-deprived rats. Teratog.
Carcinog. Mutag., 7, 357-375.
Ellis W.G., De Roos, F., Kavlock, R.J. & Zeman, F.J. (1988)
Relationship of periventricular overgrowth to hydrocephalus in
brains of fetal rats exposed to benomyl. Teratog. Carcinog.
Mutag., 8, 377-391.
Evans, E.L. & Mitchell, A.D. (1980) An evaluation of the effect of
benomyl on sister chromatid exchange frequencies in cultured
Chinese hamster ovary cells. Unpublished report from SRI
International, Menlo Park, California, USA, prepared for DuPont
de Nemours & Co.
Everhart, L.P. & Holt, R.F. (1982) Potential Benlate fungicide
exposure during mixer/loader operations, crop harvest and home
use. J. Agric. Food Chem., 30, 222-227.
Fiscor, G., Bordas, S. & Stewart, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Fitzpatrick, K. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Foss J.A (1993) Acute neurotoxicity study of DPX-T1991-529 (Benomyl)
administered orally by gavage to Crl:CD BR VAF/Plus rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Foss, J.A. (1994) Subchronic neurotoxicity study of DPX-T1991-529
(Benomyl) administered orally via the diet to Crl:CD BR VAF/Plus
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. & Sherman, H. (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Gardiner, J.A., Kirkland, J.J., Klopping, H.L. & Sherman, H. (1974)
Fate of benomyl in animals. J. Agric. Chem., 22, 419-427.
Gargus, J.L. & Zoetis, T. (1983a) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines -- Benlate PNW).
Unpublished report from Hazelton Laboratories Inc., Vienna,
Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983b) Primary irritation study in rabbits
(Benlate PNW). Unpublished report from Hazelton Laboratories
Inc., Vienna, Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983c) Eye irritation test in rabbits (EPA
pesticide registration Benlate PNW). Unpublished report from
Hazelton Laboratories Inc., Vienna, Virginia, USA, prepared for
DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Unpublished
report from Hazelton Laboratories Inc., Vienna, Virginia, USA,
prepared for DuPont de Nemours & Co.
Georgieva, V., Vachkova, R., Tzoneva, M. & Kappas, A. (1990) Genotoxic
activity of benomyl in different test systems. Environ. Mol.
Mutag., 16, 32-36.
Goldenthal, E.I. (1978) Neurotoxicity study in hens using technical
benomyl (< 95% benomyl). Unpublished report from International
Research and Development Corporation, Mattawan, Michigan, USA,
prepared for DuPont de Nemours & Co.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from DuPont de Nemours & Co.,
Wilmington, Delaware, USA.
Goodman, N.C. (1975) Intraperitoneal LD50 test in rats. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Guengerich, F.P. (1981) Enzyme induction with DuPont compounds H11,
202-02 and H10, 962-02. Unpublished report from Vanderbilt
University, School of Medicine, Nashville, Tennessee, USA,
prepared for DuPont de Nemours & Co.
Gupta, A.K. & Legator, M.S. (1975) Chromosome aberrations in cultured
human lymphocytes after treatment with fungicide 'Benlate'. In:
Proceedings of the Symposium on Mutagenicity, Carcinogenicity
and Teratogenicity of Chemicals, New Delhi, Department of
Atomic Energy, pp. 95-103
Han, J.C.Y. (1979) 2-14C-Benomyl (50% WP) rat study -- intravenous
injection. Unpublished report from DuPont de Nemours & Co.,
Biochemical Department, Research Division, Experimental Station,
Wilmington, Delaware, USA.
Han, J.C.Y. (undated) Metabolism of 14C-labelled benomyl in the
mouse and hamster. Unpublished report from DuPont de Nemours &
Co., Biochemical Department, Research Division, Experimental
Station, Wilmington, Delaware, USA
Hess, R.A., Moore, B.J., Forrer, J., Linder, R.E. & Abuel-Atta, A.A.
(1991) The fungicide benomyl [(methyl)1-(butylcarbomyl)-2-
benzimidazole carbamate] causes testicular dysfunction by
inducing the sloughing of germ cells and occlusion of efferent
ductules. Fundam. Appl. Toxicol., 17, 733-745.
Hood, D.B. (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Hoogenboom, E.R., Ransdell, J.F., Ellis, W.G., Kavlock, R.J. & Zeman,
E.J. (1991) Effects on the fetal rat eye of maternal benomyl
exposure and protein malnutrition. Curr. Eye Res., 10, 601-612.
Hornberger, C.S. (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Hostetler, K.H. (1977) Oral LD50 test (Benlate OD). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Jessup, D.C. (1979) Acute delayed neurotoxicity study in chickens
using technical benomyl (> 95% benomyl). Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA, prepared for DuPont de Nemours & Co.
Jessup, D.C. & Dean, W. (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl).
Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, prepared for DuPont de
Nemours & Co.
van Joost, T.H., Naafs, B. & van Ketel, W G. (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9, 153-154.
Kappas, A. & Bridges, B.A. (1981) Induction of point mutations by
benomyl in DNA-repair deficient Aspergillus nidulans. Mutat.
Res., 91, 115-118.
Kappas, A., Georgopoulos, S G. & Hastie, A.C. (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat. Res., 26, 17-27.
Kappas, A., Green, M.H.L., Bridges, B.A., Rogers, A.M. & Muriel, W.J.
(1976) Benomyl -- a novel type of base analogue mutagen? Mutat.
Res., 40, 379-382.
Kavlock, R.J., Chernoff, N., Gray, L.E., Gray, J A & Whitehouse, D.
(1982) Teratogenic effects of benomyl in the Wistar rat and CD-1
mouse, with emphasis on the route of administration. Toxicol.
Appl. Pharmacol., 62, 44-54.
Kirkhart, B. (1980) Micronucleus test on benomyl (> 95% benomyl).
Unpublished report from SRI International, Menlo Park,
California, USA. prepared for DuPont de Nemours & Co.
Krupka R.M. (1974) On the anti-cholinesterase activity of benomyl.
Pestic. Sci., 5, 211-216.
Kuelme, G., Heise, H., Plottke, B. & Puskeller, T (1985) Dermatitis
after Benlate contact. Z. Ges. Hyg. Grenzgeb., 31, 710-711.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Lee K.P. (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Linder, R.E., Rehnberg, G.L., Strader, L.F. & Diggs, J.P. (1988)
Evaluation of reproductive parameters in adult male Wistar rats
after subchronic exposure. J. Toxicol. Environ. Health, 25,
285-298.
Lisi, P., Caraffini, S. & Assalve, D. (1986) A test series for
pesticide dermatitis. Contact Derm., 15, 266-269.
Littlefield, N.A. & Busey, W.M. (1969) Four-hour acute inhalation
exposure test in dogs using a wettable powder formulation (50%
benomyl). Unpublished report from Hazleton Laboratories, Inc.,
Falls Church, Virginia, USA, prepared for DuPont de Nemours & Co.
Majut, J.C. (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Matsushita, T. & Aovama, K. (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind.
Health, 19, 77-83.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983a) L5178Y
mouse lymphoma cell assay for mutagenicity (benomyl). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983b) L5178Y
mouse lymphoma cell assay for mutagenicity. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Mebus, C.A. (1990) Reproductive and fertility effects with
DPX-1991-529 (benomyl). Multigeneration reproduction study in
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Monson, K.D. (1990) Metabolism of [phenyl(U)14C]carbendazim in rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Munley, S.M. (1995) Developmental toxicity study of DPX-T1991-529
(Benomyl) in rabbits. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Pilinskaya, M.A. (1.983) Investigation of the cytogenetic action of
the pesticides captan and benomyl in a culture of human
peripheral blood lymphocytes in the absence and presence of a
system of metabolic activation. Cytol. Genet., 17, 29-33
Rainaldi, G., Flori, L., Colella, C.M, Mariani, T., Piras, A., Simi,
S. & Simili, M. (1989) Analysis by BrUdR-labelling technique of
induced aneuploidy in mammalian cells in culture. Mutat. Res.,
177, 255-260.
Rashid, K.A. & Ercegovitch, C.D. (1976) New laboratory tests evaluate
chemicals for cancer or gene damage. Sci. Agric., 23, 7.
Reinke, RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Ruzicska, P., Peter, S., Laczi, J. & Czeizel, E. (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP Egeszegtudomany, 20,
74-83.
Sarver, J.W. (1987) Acute oral toxicity study with IN-T1991 in male
and female rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sasaki, Y.F.X. (1988) Benomyl: In vitro cytogenetics test. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Sasaki, Y.F.X. (1990) Benomyl: Micronucleus test in mice. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives, VI: Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and the
Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. & Krauss, W.C. (1966) Acute oral test [benomyl].
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Sherman, H., Barnes, J.R. & Krauss, W.C. (1967) Ninety-day feeding
study with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl
ester (INT-1991). Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H., Culik, R. & Jackson, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. Appl.
Pharmacol., 32, 305-315.
Shirasu, Y., Moriva, M. & Kato, K. (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan, prepared for DuPont de Nemours & Co.
Shukla, Y., Antony, M. & Mehrota, N.K. (1989) Studies on
gamma-glutamyl transpeptidase in rodents exposed to benomyl.
Bull. Environ. Contam. Toxicol., 42, 301-306.
Snee, D.A. (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test). Unpublished. report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Stahl, R.G., Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Staples, R.E. (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Staples, R.E. (1982) Teratogenicity study in the rat using technical
benomyl (> 95% benomyl) administered by gavage. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Tong, C. (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA, prepared for DuPont de Nemours & Co.
Turney, R.T. (1979) Rat inhalation study -- Benlate. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Vick, D.A. & Brock, W.J. (1987) Primary dermal irritation study with
Benlate 50 DF fungicide in rabbits. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Ward, R.S. & Scott, R.C. (1992) Benomyl: in vitro absorption of a
500 g.kg-1 WP formulation through human epidermis. Unpublished
report from Imperial Chemical Industries (ICI), Fernhurst,
Haslemere, Surrey, United Kingdom.
Warheit, D.B., Kelly, D.P., Carakostas M.C. & Singer, A.W. (1989) A
90-day inhalation toxicity study with benomyl in rats. Fundam.
Appl. Toxicol., 12, 333-345.
Wiechman, B.E. (1982) Long term feeding study with methyl
1-(butylcarbamoyl)-2-benzimidazole carbamate in mice (INT-1991;
> 95% benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Zelesco, P.A., Barbieri, I. & Graves, J.A.M. (1990) Use of a cell
hybrid test system to demonstrate that benomyl induces aneuploidy
and polyploidy. Mutat. Res., 242, 329-335.
Zeman, E.J., Hoogenboom, E.R., Kavlock, R.I. & Semple, J.L. (1986)
Effects on the fetus of maternal benomyl exposure in the
protein-deprived rat. J. Toxicol. Environ. Health, 17, 405-417.
 The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed diets containing benomyl or carbendazim at a concentration of 0,
10, 30, 100, 300, 1000 or 3000 ppm for 28 days. After sacrifice, liver
weights were recorded and microsomal epoxide hydrolase and cytosolic
glutathione- S-transferase were monitored in subcellular fractions
isolated from the liver. The mean absolute liver weights were elevated
in males and females fed 1000 or 3000 ppm carbendazim and in females
fed 300 ppm; however, the only significantly increase was found in
females fed 3000 ppm benomyl. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced epoxide
hydrolase in male and female rats and in mice fed 1000 or 3000 ppm,
and both induced glutathione- S-transferase at 3000 ppm. The level of
induction seemed to be slightly greater in females than males. There
was no substantial difference in enzyme induction between rats and
mice (Guengerich, 1981).
Administration of 1000 or 4000 ppm benomyl in the diet to groups
of eight female albino rats and female Swiss albino mice for 15 days
increased the activity of blood and liver gamma-glutamyl transpeptidase
in both species, and the degree of induction was dose related (Shukla
et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
1. The clinical signs of toxicity after treatment with benomyl were
generally nonspecific. Gross and histopathological changes were
evaluated in some studies; in male gonads, testicular degeneration,
necrosis of germinal epithelium, and aspermatogenesis were observed in
rats and dogs.
(b) Short-term toxicity
Rats
After intubation of benomyl into male Sprague-Dawley rats at 200
or 3400 mg/kg bw in peanut oil five times per week for two weeks, four
of six animals at the high dose died. At this dose, degeneration of
germinal epithelium, multinucleated giant cells, and reduction or
absence of sperm were seen. Very minor changes were observed in the
testes of the animals treated with the lower dose (Sherman & Krauss,
1966).
Male Sprague-Dawley rats treated orally for 10 days with
200 mg/kg bw per day of the metabolite 5-HBC (methyl ester) had no
toxic symptoms or evidence of effects on the testes (Snee, 1969).
Table 1. Acute toxicity of benomyl, benomyl formulations, and benomyl metabolites
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Benomyl Rat Oral > 10 000 > 95 Sherman (1969a)
Benomyl Dog Oral > 1 000 > 95 Sherman (1969b)
'50% wettable powder' Rat Oral > 1 000 > 95 Sherman (1969a)
'50% wettable powder' Rat Oral > 5 000 NR Sarver (1987)
'50% wettable powder' Rat Oral > 1 200 NR Hostetler (1977)
'50% wettable powder' Rabbit Oral > 3 400 NR Fritz (1969)
'50% wettable powder' Rabbit Dermal > 10 000 NR Busey (1968a)
'50% wettable powder' Rabbit Dermal > 2 000 NR Brock (1987)
'50% wettable powder' Rabbit Dermal > 2 000 NR Gargus & Zoetis (1983a)
'50% wettable powder' Rat Inhalation (4-h) > 0.82 NR Hornberger (1969)
'50% wettable powder' Rat Inhalation (4-h) > 4.01 NR Busey (1968b)
'50% wettable powder' Dog Inhalation (4-h) > 1.65 NR Littlefield & Busey (1969)
2-Benzimidazole carbamic Rat Intraperitoneal > 10 000 NR Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole Rat Oral > 7 500 > 95 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole Rat Oral > 3 400 > 95 Fritz & Sherman (1969)
Benzimidazole 2-(3-butylureido) Rat Oral > 17 000 NR Dashiell (1972)
S-Triazine, 3-butyl-benzimidazole Rat Oral > 17 000 NR Barbo & Carroll (1972)
(1,2a)-2,4(1H,3H)-dione
NR, Not reported
Groups of 16 male and 16 female Sprague Dawley rats were fed
benomyl (72% pure) in the diet at 0, 100, 500 and 2500 ppm (as
benomyl). After 96-103 days of continuous feeding, 10 male and 10
female rats in each group were killed, and selected organs were
weighed; additional organs were preserved for microscopic examination.
The remaining animals in each group were studied for reproductive
toxicity (see below). There were no gross toxic signs of poisoning and
no compound-related effects on weight gain, food consumption, food
efficiency, or haematological, biochemical, or urine parameters. The
liver body weight ratio was slightly increased in females at 2500 ppm
in comparison with control rats, Gross and microscopic examination of
tissues and organs showed no significant effect attributable to the
presence of benomyl in the diet at levels up to and including 2500 ppm
(Sherman et al., 1967).
Groups of 20 male and 20 female Sprague-Dawley rats were exposed
to benomyl by inhalation at 0, 10, 50, or 200 mg/m3 via the nose
only, for 6 h/day for 90 days. After 45 and 90 days, blood and urine
samples were collected from 10 rats of each sex per group for clinical
analysis; the animals were then killed for pathological examination.
After about 45 days of exposure, degeneration of the olfactory
epithelium attributable to benomyl was observed in all males and in
eight of the 10 females exposed to 200 mg/m3; two male rats exposed
to 50 mg/m3 had similar but less severe olfactory degeneration. After
about 90 days of exposure, all of the animals at 200 mg/m3 and three
males at 50 mg/m3 had olfactory degeneration. No other compound-
related pathological effects was observed. Male rats exposed to
200 mg/m3 had depressed mean body weights in comparison with
controls; the depression was correlated with a reduction in food
consumption (Warheit et al., 1989).
Rabbits
Groups of five male and five female New Zealand albino rabbits
were given 15 dermal applications for 6 h/day, five days per week for
three weeks of a 50% benomyl formulation (equivalent to 1000 mg/kg bw)
on both abraded and intact abdominal skin. After each application, the
abdomen was washed with tap water. Mortality and toxic effects were
monitored daily and body weight changes weekly; gross necropsy and
microscopic examinations were performed. Slight erythema, oedema, and
atonia were observed on both abraded and intact skin sites, and slight
to moderate desquamation was reported throughout the study. No
apparent compound-related changes in body weight or organ:body weight
ratios were reported. Treatment induced degeneration of the
spermatogenic elements of the seminiferous tubules of the testes,
including vacuolated and multinucleated spermatocytes. A slight
increase in haematogenic activity in the bone marrow and acanthosis
and hyperkeratosis of the skin were reported in treated animals
(Busey, 1968c).
Groups of five male and five female New Zealand albino rabbits
received benomyl at doses equivalent to 0, 50, 250, 500, 1000, and
5000 mg/kg bw on non-occluded, abraded dorsal skin for 6 h/day, five
days per week for three weeks. The material was removed by washing the
skin site and drying with a towel. The body weight gains of males and
females at > 1000 mg/kg bw were reduced, and mild-to-moderate skin
irritation was reported in all groups, especially at the higher doses.
Functional disturbances of the alimentary canal and kidney, including
diarrhoea, oliguria, and haematuria, were observed in males and
females at 1000 and 5000 mg/kg bw. Decreased testicular weights were
observed at 1000 mg/kg bw only, but these were considered not to be
directly related to treatment. No histopathological changes were
reported (Hood, 1969).
Dogs
Groups of four male and four female beagle dogs were given
benomyl (as a 50% wettable powder) in the diet at 0, 100, 500, or
2500 ppm (based on active ingredient) for three months. There was no
mortality or adverse clinical effect during the course of the study,
and growth and food consumption were normal. Urinary parameters were
unaffected by treatment. There were no dose-related effects on
haematological values, but alkaline phosphatase and alanine
aminotransferase activities were increased in males and females at the
highest dose, and the albumin:globulin ratio was significantly
decreased in both males and females fed 2500 ppm. In males and females
at the high dose, the weight of the thymus was decreased and that of
the thyroid was increased. One female fed 2500 ppm had an enlarged
spleen, which was consistent with the decreased erythrocyte count,
haemoglobin concentration, and haematocrit values. Histopathological
examination revealed myeloid hyperplasia of the spleen and bone marrow
and erythroid hyperplasia in the same animal. Three of four males fed
2500 ppm had reduced prostate weights in comparison with controls.
Microscopic examination of tissues and organs showed no consistent
effect. The NOAEL was 500 ppm (Sherman, 1968).
Groups of four male and four female beagle dogs were fed benomyl
(50% pure) in the diet at 0, 100, 500, or 2500 ppm (as benomyl) for
two years, with interim sacrifice after one year of one male and one
female from the control and high-dose groups. Organ weights, gross
necropsy, and histopathological evaluations were performed at the
conclusion of the study; only the livers and testes of animals fed 100
or 500 ppm were examined histologically. No deaths were attributable
to treatment. Body weight changes and food consumption were similar in
all groups, except for those at the high dose, which had decreased
food intake and body weight gain; one dog at the high dose lost its
appetite and was replaced. No other clinical signs of toxicity were
observed. The results of haematological evaluations and urinalysis
were similar to those of the controls. Males at 2500 ppm had
increased levels of cholesterol, alkaline phosphatase, and alanine
aminotransferase (initially) and decreased total protein and albumin:
globulin ratio. Similar, but less marked, effects were seen in
females at this dose. Biochemical analyses indicated adverse effects
on the liver, expressed as liver cirrhosis. Four of six dogs at this
dose had slight-to-marked bile duct proliferation. Haemosiderosis was
seen after specific staining for iron in one dog at the high dose
after one year but not in other dogs examined at two years.
Preparation of preserved wet tissue with oil red O and Sudan black for
hepatocyte vacuolation showed that benomyl is not hepatotoxic at 100
or 500 ppm in the diet. Focal testicular degeneration was seen in all
groups, including controls, with marked testicular degeneration
(reduced testicular weight, absence of spermatozoa, and spermatic
giant cells) in one of three dogs at 2500 ppm. The NOAEL was 500 ppm,
equivalent to 13 mg/kg bw per day (Sherman, 1970).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD-1 mice were given benomyl
(99% pure) in the diet at 0, 500, 1500, or 5000 ppm (reduced from
7500 ppm after 37 weeks) for two years. Survival was unaffected by
treatment. Male and female mice fed 1500 or 5000 ppm benomyl had
dose-related decreases in body weight. Food consumption was variable
throughout the study, although females at the high dose appeared to
consume less food. Clinical signs of toxicity were similar in all
groups; there were no apparent differences in palpable masses,
numbers of mice affected, or latency to detection. The results of
haematological examinations were unremarkable except for decreased
erythrocyte counts in males at 1500 ppm and in females at 5000 ppm.
Haemoglobin and haematocrit values were also depressed in males at the
intermediate dose. Several statistically significant changes in organ
weights were seen in treated groups, the most significant being
reduced absolute and relative liver weights in males at 1500 and
5000 ppm and females at 5000 ppm. Male mice at the high dose also had
decreased absolute testicular weights. The changes in liver and
testicular weights were accompanied by histomorphic changes in these
tissues. The incidence of hepatocellular carcinomas and benign hepatic
neoplasms in treated female mice was increased in a dose-dependent
fashion. In male mice, hepatocellular carcinomas and benign hepatic
neoplasms were significantly increased at 500 and 1500 ppm, but not at
the high dose. The incidences of lung tumours (alveologenic
carcinomas) were also significantly increased in males but not in
females at 500 and 1500 ppm. Non-neoplastic changes in males at
5000 ppm were confined to the liver (degeneration, pigment,
cytomegaly), thymus (atrophy), and the testis, epididymus, and
prostate (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini). The occurrence of splenic
haemosiderosis was significantly increased in female mice at 5000 ppm
and that of submucosal lymphocytic infiltration of the trachea at
1500 ppm. The latency for liver tumour induction (adenomas and
carcinomas), as determined from palpation, gross necropsy, and
histopathology performed on all animals throughout the study, was not
measurably different in treated and control animals. The authors
concluded that, under the conditions of the study, benomyl is
oncogenic in male and female animals. No NOAEL was identified for male
or female mice with respect to hepatocellular carcinomas or combined
hepatocellular neoplasms. The lowest dietary level, 500 ppm, was
equivalent to 64 mg/kg bw per day in males and to 103 mg/kg bw per day
in females (Wiechman, 1982).
Rats
Groups of 36 male and 36 female Charles River albino weanling
rats were fed benomyl (purity, 50-70%) in the diet for 104 weeks at
100, 500, or 2500 ppm (as benomyl); there were two control groups.
After one year, each group was reduced to 30 males and 30 females by
interim sacrifice for gross and microscopic evaluation. At the
conclusion of the study, all surviving animals were sacrificed and the
tissues and organs examined grossly. No deaths were attributable to
the presence of benomyl in the diet. Survival was decreased to about
50% during the second year but was comparable in all groups. Body
weight, food consumption, and food efficiency were unaffected by
treatment. There were no compound-related clinical manifestations of
toxicity, and the results of haematological, urinary, and liver
function examinations were unaffected by treatment. There were no
differences in organ weights, and histopathological examination
revealed no differences between treated and control groups. The most
frequently observed tumours were of the mammary, pituitary, and
adrenal glands, but these were equally distributed among the groups.
Hepatic toxicity was similar in all groups, including controls, and
there were no compound-related effects. As a high incidence of
testicular degeneration was observed in control males, no conclusion
could be drawn about compound-related effects on male gonads. Benomyl
had no adverse effects in this study at levels up to and including
2500 ppm, which was equivalent to 109 mg/kg bw per day in males and
128 mg/kg bw per day in females (Sherman, 1969c; Lee, 1977).
(d) Reproductive toxicity
In a short-term study of toxicity (see above), groups of six male
and six female Sprague-Dawley rats were fed diets containing benomyl
at 0, 100, 500, or 2500 ppm. Each female was caged separately and
exposed to each of three males at the same dose for five days. The
females were then allowed to deliver, and the offspring were observed
until weaning. Litters containing more than 10 pups were reduced to 10
by randomly removing pups on day 4. There was no treatment-related
effect on pregnancy rate, number of pups born live or dead, fertility,
gestation, viability, or lactation indices or on average body weight
(Sherman et al., 1975).
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal male Sprague-Dawley rats aged
33 days, which were gavaged daily for 10 days with 0 or 200 mg/kg bw.
Eight animals per group were killed 3, 17, 31, 45, and 59 days after
the last treatment. Selected tissues, including liver, kidneys,
testes, seminal vesicles, and epididymides, were removed, weighed, and
examined histologically; samples of seminal fluid from the vas
deferens were also examined. Observation intervals were preselected to
coincide with stages of spermatogenesis. No effects related to
treatment were seen on tissue weights, total epididymal sperm counts,
was deferens sperm concentrations, or testicular histology (Carter,
1982).
In a similar experiment, groups of five animals were dosed orally
on five consecutive days with 0, 125, 250, 500, or 1000 mg/kg bw per
day, beginning when animals were 33 (prepubertal), 54 (pubertal), or
75 (post-pubertal) days old. Animals were killed 31 days after
the last dose. There was no effect on body weight gain or on
haematological values. The weights of the testes and cauda
epididymides were reduced in animals given 500 or 1000 mg/kg bw per
day benomyl during the onset of puberty. Cauda epididymal and vas
deferens sperm counts were reduced in animals given > 250 mg/kg bw
per day benomyl during puberty. The incidence of diffuse hyposperma-
tocytogenesis was increased in pubertal and post-pubertal animals but
was most pronounced in the latter. The NOAEL for effects on
reproductive development was 12.5 mg/kg bw per day (Carter, 1982).
In a further experiment, adult (65-day-old) male Sprague-Dawley
rats received 10 daily doses of benomyl at 0, 200, or 400 mg/kg bw per
day by gavage. Fourteen days after the last treatment, body weights,
tissue weights, total epididymal sperm counts, and sperm concentration
in the vas deferens were measured, and the testis was examined
histologically. Production of testosterone by the Leydig cells was
artificially stimulated by subcutaneous injections of hypothalamic
chorionic gonadotrophin 2 h before sacrifice. There were no compound-
related effects on the weights of the body, liver, kidney, adrenal,
testis, or seminal vesicles, but caudate epididymal weights were
depressed by treatment and there were treatment-related reductions in
epididymal sperm count (caput and caudate) and in vas deferens sperm
concentration. In animals exposed to the highest dose, there was
histological evidence of hypospermatocytogenesis, with generalized
disruption of all stages of spermatogenesis (Carter & Laskey, 1982).
Benomyl was administered by gavage to Wistar rats at doses of 0,
15.6, or 31.2 mg/kg bw per day from day 7 of gestation to day 15 of
lactation (day 22 of gestation was considered day 0 of lactation). The
litters were each reduced to four male and four female pups on day 3
of lactation. The litters were weighed on days 8, 15, 22, 29, and 100,
and locomotor activity was evaluated periodically throughout the
study. When the animals were 100 days of age, several organs were
weighed, including adrenals, liver, kidney, ovary, testis, and ventral
prostate plus seminal vesicles. There were no compound-related effects
on litter size at birth or weaning or on body weights of fetuses.
Growth, survival, and locomotor activity were comparable to those of
controls throughout the study. Organ weights were comparable to t hose
of controls except for decreased weights of testes and ventral
prostate/seminal vesicles, which were significantly reduced at 31.2
but not at 15.6 mg/kg bw per day (Kavlock et at., 1982).
In a two-generation study, Sprague-Dawley rats were fed diets
containing 0, 100, 500, 3000, or 10 000 ppm benomyl. Parental rats
received the test diets for 71 days before being bred for production
of F1 parental rats with animals at the same dietary concentration.
F1 rats were mated for production of the F2a litter after
maintenance on their respective diets for at least 105 days after
weaning. F1 dams were mated again, to different, non-sibling males,
at least one week after weaning the F2a litter, to produce the
F2blitters. The following indices of reproductive Junction were
calculated for the F0 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive, and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption, and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental rats
were sacrificed lot gross examination and histopathological
examination of gross lesions and target organs; complete histopatho-
logical examination was conducted on controls and animals at the high
dose. Groups of 20 F2a and 20 F2bweanlings were also examined
grossly. There were no compound-related effects on parental mortality
at any concentration of benomyl. Mean body weights, body weight gains,
and overall food consumption of F0 and F1 male and female rats at
10 000 ppm were significantly lower than those in controls, and there
was a significant compound-related decrease in the number of F2a and
F2boffspring alive before culling (on day 4) at this dose. In
addition, male and female offspring of rats fed 10 000 ppm weighed
consistently less at birth than did the offspring of control rats.
With the exception of 14-day male pups of the F2bgeneration, F2a
and F2b offspring at 3000 ppm also had significantly depressed body
weights on days 14 and 21 of lactation. The testicular sperm counts of
F0 and F1 rats at 3000 and 10 000 ppm were decreased, and rats at
the highest dose had decreased testicular weight and histopathological
changes in the testes. Microscopic observation showed a trophy and
degeneration of the seminiferous tubules in the testes of rats at 3000
and 10 000 ppm and oligospermia in the epididymides of F0, rats at
the highest dose and in F1 rats at 3000 and 10 000 ppm. There were
no compound-related differences in mating indices, fertility indices,
or length of gestation that could be attributed to benomyl. The NOAEL
was 500 ppm, equivalent to about 37 mg/kg bw per day (Mebus, 1990).
In a study of adult male Wistar rats, groups of 27 animals were
fed 0, 1, 6.3, or 203 ppm for 70 days, and 14 animals per group were
allowed to recover for 70 days. Ejaculated sperm counts were
reportedly depressed during the feeding phase in the group fed
203 ppm. Relative testicular weights were also lowered, and a slightly
reduced male fertility index was seen in all treated groups. Benomyl
did not alter copulatory behaviour and did not induce dominant lethal
mutation. Plasma testosterone and gonadotropin levels remained
unchanged throughout the study. Identical studies conducted during the
recovery phase showed that the treatment-related effects were
completely reversible (Barnes et al., 1983).
Groups of 12 adult (102-day-old) Wistar male rats were given
benomyl at 0, 1, 5, 15, or 45 mg/kg bw per day by gavage daily for 62
days, were then bred to untreated females after 62 days of dosing, and
were killed after 76-79 days. Reproductive behaviour, seminal vesicle
weight, prostate weight, sperm motility, and the levels of serum
gonadotropin and gonadal hormones were no different from those in
controls. At necropsy, males exposed to 45 mg/kg bw per day had
decreased testicular and epididymal weights, reduced caudal sperm
reserves, decreased sperm production, increased numbers of decapitated
spermatozoa, and increased numbers of seminiferous tubules containing
multinucleated giant cells. There were no treatment-related effects on
the levels of serum luteinizing hormone, follicle stimulating hormone,
prolactin, or androgen binding protein (Linder et al., 1988).
Groups of 20 male Sprague-Dawley rats aged 100 days were given a
single dose of benomyl in corn oil at 0, 25, 50, 100, 200, 400, or
800 mg/kg bw. Eight animals per group were sacrificed after two days
of treatment and 12 animals per group after 70 days (except in the
group receiving 800 mg/kg bw) in order to examine the testis and
efferent ducts for effects on spermatogenesis or the epididymis. The
primary effects seen at day 2 were testicular swelling and occlusions
of the efferent ductules. Premature release of germ cells (sloughing)
was the most sensitive short-term response to benomyl. It was detected
in all treated groups but was statistically significant (P < 0.05)
only at doses > 100 mg/kg bw. Occlusion of the efferent ductules of
the testis was dose-dependent and was correlated with the increase in
testicular weight on day 2. The greatest increase in testicular weight
was observed at 400 mg/kg bw. Long-term effects (70 days) were seen
only in animals at > 100 mg/kg bw and included decreased testicular
weight at 400 mg/kg bw, dose-dependent increases in seminiferous
tubular atrophy, and increases in the number of reproductive tracts
containing occluded efferent ductules (Hess et al., 1991).
(e) Developmental toxicity
Mice
Groups of 20-25 pregnant CD-1 mice were given benomyl by gavage
at 0, 50, 100, or 200 mg/kg bw per day on days 7-17 of gestation. They
were killed on day 18, their pups were delivered by caesarean section,
the numbers of live, dead, and recorded fetuses were determined, and
the fetuses were examined for gross anomalies (one-half for visceral
abnormalities and the other half for skeletal abnormalities). Maternal
indexes were unaffected by treatment, but fetal mortality was
increased, fetal weight was decreased, and fetal development was
adversely affected by treatment. The high dose increased the
supraoccipital score, decreased the numbers of caudal and sternal
ossifications, and increased the incidences of enlarged lateral
ventricles and enlarged renal pelvises. The latter increase, while not
significant at the lowest dose, was dose-related at the other doses.
The occurrence of supernumerary ribs and subnormal vertebral centra
was significantly increased in a dose-related manner at all doses. The
numbers of abnormal litters and fetuses were significantly increased
over those among the controls at 100 and 200 mg/kg bw per day. Major
anomalies included exencephaly, hydrocephaly, cleft palate,
hydronephrosis, polydactyly, oligodactyly, umbilical hernia, fused
ribs, fused vertebrae, and short or kinky tail. Although benomyl
induced dose-related fetotoxicity at all levels, it was not
teratogenic at 50 mg/kg bw per day in mice (Kavlock et al., 1982).
In a study designed to investigate whether teratogens could be
detected by observing postnatal viability or impaired growth, groups
of 24 pregnant CD-1 mice were treated orally with benomyl at 0 or
200 mg/kg bw per day on days 8-12 of gestation. Postnatal viability
was reduced on days 1 and 3, and average pup weight was reduced on day
1 post partum (Chernoff & Kavlock, 1983).
Rats
Groups of 26-28 pregnant Sprague-Dawley rats were given benomyl
(purity, 53.5%) in the diet at 0, 100, 500, 2500, or 5000 ppm on days
6-15 of gestation to provide average doses equivalent to 0, 8.6, 43.5,
209.5, and 372.9 mg/kg bw per day. On day 20 of gestation, all
pregnant animals were sacrificed and their fetuses were delivered by
caesarean section. There were no deaths attributable to treatment, no
clinical signs of toxicity, and no adverse effects on the body weights
of dams. Dams at 5000 ppm had reduced food intake during treatment,
but consumption returned to control levels for the remainder of the
study. Indicators of reproductive health (numbers of implantation
sites, resorption sites, and live and dead fetuses) were not affected
by treatment up to and including the highest dietary level. The only
external or internal abnormalities reported were hydronephrosis and
retarded ossification of the interparietal and occipital bones in
three litters at the highest dose. None of these teratogenic effects
was associated with the administration of benomyl during the critical
period of organogenesis (Sherman et al., 1975).
Groups of 27-28 pregnant Wistar rats were fed benomyl in the diet
at 0, 1690, 3380, or 6760 ppm, equivalent to 0, 169, 298, or 505 mg/kg
bw per day, on days 7-16 of gestation. Fetuses were delivered by
caesarean section on day 21, weighed, examined grossly, and fixed for
evaluation of visceral and skeletal abnormalities. Food consumption
was decreased in dams at 3380 or 6760 ppm, with resultant decreases in
body weight gain, which were significant at the high dose. The weight
gain of fetuses at the two higher doses was significantly reduced, and
a significant decrease in the ossification of the supraoccipital bone
was seen at the high dose. The percentage of fetuses with an enlarged
renal pelvis was increased at the two higher doses. No dose-related
major malformations were seen at these dietary levels (Kavlock
et al., 1982)
Benomyl (99.2% pure) was administered by gavage to pregnant
Sprague-Dawley rats at doses of 0, 3, 10, 30, 62.5, or 125 mg/kg bw
per day on days 7-16 of gestation. A control group receiving corn oil
comprised 60 rats, and each test group comprised 27 rats. No clinical
signs of toxicity or mortality were observed at any dose. Body weight
gain, the incidences of pregnancy, corpora lutea, and implantation
sites, and the sex ratio were comparable to those of controls;
however, fetal body weight was significantly decreased at 62.5 and
125 mg/kg bw. There was also an increased incidence of embryofetal
mortality at 125 mg/kg bw. At doses > 10 mg/kg bw per day, some
fetuses had external and visceral malformations, which included
microphthalmia, anophthalmia, and hydrocephaly (distended lateral
ventricles), predominantly at the higher doses. Histological
examination of the eyes revealed pathological changes consisting of
irregular lenses, retrobulbar glandular adnexa, distorted or
compressed retinal layers, and thickened nerve fibres in the groups
receiving 10, 62.5, or 125 mg/kg bw per day; no alterations were seen
in controls or in animals at 30 mg/kg bw per day. The major skeletal
malformations observed at 125 mg/kg bw per day included fused ribs,
sternebrae, and thoracic arches. Additional skeletal variations seen
at 62.5 and 125 mg/kg bw per day included misaligned and unossified
sternebrae and bipartite vertebral centra. The authors concluded that
benomyl produced teratogenic responses in rats at doses > 10 mg/kg
bw per day. Microphthalmia seen at this dose was reported to be
related to treatment on the basis of the severity of the pathological
changes and the finding that the increased incidences at 62.5 and
125 mg/kg bw per day were not a direct reflection of reduced fetal
body weight. There was no evidence of maternal toxicity, even at the
highest dose (Staples, 1980).
Groups of pregnant Sprague-Dawley rats were given benomyl (99.1%
pure) by gavage at 0, 3, 6.25, 10, 20, 30, or 62.5 mg/kg bw per day on
days 7-16 of gestation. Each group consisted of 50 animals, except the
high-dose group, which comprised 20 rats. As the study was performed
to determine a no-effect level for microphthalmia and hydrocephaly,
only fetal heads were fixed and examined. Microphthalmia was
determined on the basis of the smallest eye in the control group
(< 1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two cases of malformation, both at
62.5 mg/kg bw per day: One fetus had internal hydrocephaly, and one in
another litter had unilateral microphthalmia. The only type of
variation reported was subcutaneous haematoma, which was not dose-
related. There was no teratogenic response at 30 mg/kg bw per day
(Staples, 1982).
On the basis of these two studies, the Meeting concluded that the
NOAEL for teratogenicity was 30 mg/kg bw per day, as the anophthalmia
at 10 mg/kg bw per day in the first study was not found in the second
study, which was specifically designed to look for this anomaly.
Furthermore, anophthalmia was not seen at 30 mg/kg bw per day in the
first study, and its occurrence at 10 mg/kg bw per day was not
accompanied by other evidence of teratogenic response.
The teratogenic potential of benomyl was examined in groups of
12-30 Wistar rats given oral doses of 0, 15.6, 31.2, 62.5, or
12.5 mg/kg bw per day on days 7-16 of gestation. Pups were delivered
by caesarean section on day 21 of gestation. The weight gain of the
dams at the highest dose was decreased on days 17-20 of gestation, and
the frequency of fetal resorption was increased, six litters being
completely resorbed. Fetal weight was also adversely affected, with
significant decreases at 62.5 and 125 mg/kg bw per day, and there was
a significant increase in fetal mortality at 125 mg/kg bw per day.
Several skeletal and visceral variants were observed among fetuses at
62.5 and 125 mg/kg bw per day, including increased supraoccipital
score, decreased numbers of sternal and caudal vertebrae, and
increased percentages of enlarged lateral ventricles and enlarged
renal pelvis. Major anomalies, observed primarily at > 62.5 mg/kg
bw per day, included encephaloceles, hydrocephaly, microphthalmia,
fused vertebrae, and fused ribs. The dose of 31.2 mg/kg bw per day
appeared to have no adverse effects on developing rat fetuses (Kavlock
et al., 1982).
Groups of pregnant Sprague-Dawley rats were treated orally with
benomyl at 0 or 62.5 mg/kg bw per day on days 7-16 (eight rats) or
7-20 of gestation (13 rats). There were no overt signs of toxicity.
Hydrocephaly was noted in 35% of fetuses examined on day 16 and in 76%
of fetuses examined on day 20 (Ellis et al., 1987, 1988).
In other studies, benomyl produced ocular and craniocerebral
malformations in Sprague-Dawley rats when administered by gavage at
doses of 31.2 or 62.4 mg/kg bw per day on days 7-21 of gestation.
Ocular anomalies (retinal dysplasia, cataracts, microphthalmia, and
anophthalmia) occurred in 43.3% of the fetuses of dams given the
higher dose. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose of
benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
consisting primarily of hydrocephaly occurred in the fetuses of dams
given 31.2 mg/kg bw per day in combination with the semipurified diet
(Zeman et al., 1986).
Rabbits
Groups of 15 artificially inseminated New Zealand albino rabbits
were fed a benomyl formulation (containing 50% benomyl) in the diet at
0, 100, or 500 ppm on days 8-16 of gestation. Mortality, clinical
parameters, and food consumption were determined daily, and body
weights were measured weekly. There were 12 pregnant does in the
control group, 13 in the low-dose group, and 9 in the high-dose group.
Of these, 6, 7 and 5, respectively, were sacrificed on day 29 or 30
and their fetuses were delivered by caesarean section; the remaining
does gave birth normally. No maternal toxicity was observed at any
dose. Except for a marginal increase in the frequency of rudimentary
ribs in animals at 500 ppm, no developmental toxicity was observed
(Busey, 1968d); however, the numbers of litters and of fetuses
examined were less than adequate to assess the fetotoxic or
teratogenic potential of benomyl.
Groups of 20 female New Zealand white female rabbits, presumed to
be pregnant, were given oral doses of 0, 15, 30, 90, or 180 mg/kg bw
per day benomyl (as a suspension in 0.5% methylcellulose) on days 7-28
of gestation. The animals were killed on day 29. The results of
analyses of the concentration, homogeneity, and stability of the
suspensions indicated acceptable conformity with nominal content. Only
one animal at 90 and one at 180 mg/kg bw per day did not become
pregnant. There were seven deaths before terminal sacrifice -- one
control, three treated with 30 mg/kg bw per day, one treated with
90 mg/kg bw per day, and two treated with 180 mg/kg bw per day -- all
of which were due to injuries associated with dosing. At 180 mg/kg bw
per day, an increased incidence of stained tails was seen, and food
consumption was significantly reduced, but there were no treatment-
related effects on maternal body weight. Five animals aborted during
the study: one in each of the groups treated with 0, 15, and 90 mg/kg
bw per day and two at 180 mg/kg bw per day. As the historical control
data showed no more than one abortion in a control group of 20 does,
the Meeting concluded that benomyl at 180 mg/kg bw per day had an
adverse effect. There were no other treatment-related effects on
reproductive parameters. Fetal mortality and fetal weights were not
affected by treatment, and there were no treatment-related external
malformations. At 180 mg/kg bw per day, two viable fetuses in two
litters had small renal papillae. The Meeting concluded that this
finding was treatment-related in view of the finding of a single
incidence in 11 studies in historical controls. The NOAEL for maternal
toxicity was 90 mg/kg bw per day, on the basis of reduced food
consumption, abortion, and clinical signs of toxicity at 180 mg/kg bw
per day. The NOAEL for developmental toxicity was also 90 mg/kg bw per
day, on the basis of the occurrence of small renal papillae at
180 mg/kg bw per day (Munley, 1995).
(f) Genotoxicity
Numerous studies have been reported in the open literature on the
mutagenic potential of benomyl, its metabolite carbendazim, and
several benomyl formulations. Many of the results are conflicting, and
few of the publications provide sufficient detail to evaluate the
reasons for the conflicts. Table 2 covers only those studies in which
sufficient experimental detail and data were reported.
Benomyl does not cause gene mutation or structural chromosomal
aberrations in somatic or germ cells, and it does not interact
directly with DNA in either mammalian or non-mammalian systems. Gene
mutations and structural chromosomal aberrations observed in mammalian
cells in vitro in several studies appear to have been the
consequence of the inherent sensitivity of some mammalian test systems
to cytotoxic agents. No gene mutations or structural chromosomal
aberrations were seen in mammals in vivo. Benomyl causes numerical
chromosomal aberrations (aneuploidy and/or polyploidy) both in vitro
and in vivo. Studies involving kinetochore staining indicated,
however, that benomyl is not clastogenic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Application for 24 h of doses > 0.5 g per animal of a 50%
wettable powder to the occluded, clipped, intact and abraded abdominal
skin of albino rabbits produced moderate to marked erythema, slight
oedema, and slight desquamation. Albino guinea-pigs similarly ex posed
to 10, 25, or 40% dilutions of technical-grade benomyl in dimethyl
phthalate had only mild irritation of intact and abraded skin (Majut,
1966; Busey, 1968a; Colburn, 1969; Frank, 1969). No dermal irritation
was observed 4 or 24 h after application of 0.5 g of Benlate 50 DF
(containing 50% benomyl) to six male New Zealand white rabbits. After
48 h, two rabbits had slight to mild erythema, which was still evident
after 72 h. The primary irritation scores ranged from 0 to 1 (not an
irritant) (Vick & Brock, 1987).
Draize scores were assessed 4 and 72 h after application of
benomyl (purity, 98%) to a shaved area of the back of four albino
rabbits at 5 mg/cm2. The lesions produced were classified as 'mild
irritation' (Desi, 1979).
The ocular irritating properties of technical-grade benomyl, a
50% wettable powder, and a suspension in mineral oil were examined in
albino rabbits in several tests. Mild conjunctival irritation and
minor transitory corneal opacity were reported after 48 to 96 h in all
tests (Reinke, 1966; Frank, 1972). Similar results were obtained with
Table 2. Results of tests for the genotoxicity of benomyl
End-point Test system Concentration Purity Results Reference
or dose (%)
Tests for chromosomal effects
Mitotic gene conversiona S. cerevisiae, A. nidulans < 3200 mg/ml Technical Negative DeBertoldi et al.
(1980)
Nondisjunction/crossing- A. nidulans < 2.8 mmol/litre Technical Negative, but DeBertoldi & Griselli
over spindle inhibition (1980)
observed
Sister chromatid exchangea Chinese hamster ovary > 150 mg/ml Technical Weakly positive Evans & Mitchell
cells (1980)
Chromosomal aberrationa Human lymphocytes < 2000 mg/ml 50% WP Negative Gupta & Legator
(1975)
Chromosomal aberrationa Human lymphocytes < 100 mg/ml Technical Negative Pilinskaya (1983)
Chromosomal aberrationa Chinese hamster lung cells < 90 mg/ml Technical Positive Sasaki (1988)
(98.7%)
Chromosomal effects Human/mouse monochromosomal < 15 mg/ml Technical Positive: aneuploidy Athwal & Sandhu (1985)
hybrid cells and polyploidy
(R3-5) (1.5 mg/ml threshold)
Chromosomal effects V79/AP4 Chinese hamster NR Technical Dose-related increase Rainaldi et al. (1989)
cells in numerical
chromosomal
aberrations
Chromosomal effects Chinese hamster ovary cells NR Technical Dose-related increase Eastmond & Tucker
in numerical (1989)
chromosomal
aberrations
Chromosomal effects Human lymphocytes NR Technical Dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations
(0.1 mg/ml threshold)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Chromosomal effects Chinese hamster/human NR Technical Dose-related increase Zelesco et al. (1990)
hybrid cells (EUBI) in numerical
chromosomal
aberrations
(2.0 mg/ml threshold)
Tests for gene mutation
Reverse mutationa S. typhimurium TA98, < 325 mg/plate Technical Doubtful mutagenic Rashid & Ercegovitch
TA 100, TA 1535, TA 1537, activity (1976)
TA 1538
Reverse mutationa S. typhimurium TA 1535, < 1 mg/ml 50% WP Positive Kappas et al. (1976)
TA1538, E. coil WP2,
WP2 uvra, CM 611
Reverse mutationa S. typhimurium TA98, < 1000 mg/ml Technical Negative Shirasu et al. (1978)
TA100, TA1535, TA1537,
TA1538, E. coil WP2 he;
Reverse mutationa S .typhimurium TA 1530, < 10 000 mg/ml Technical, Negative Fiscor et al. (1978)
TA1535, TA1950 50% WP
Reverse mutationa S. typhimurium TA 1530, < 10 000 mg/ml Technical, Negative DuPont de Nemours
TA1535, TA 1950 50% WP & Co. (1977)
Reverse mutationa S. typhimurium TA98, < 250 mg/plate Technical Negative Donovan & Krahn
TA 100, TA 1535, TA1537 (99.9%) (1981)
Reverse mutationa S. typhimurium TA98. < 10000 Technical Negative Rickard (1983a,b)
TA100, TA1535. TA1537 mg/plate (99%)
Reverse mutationa S. typhimurium TA98, < 500 mg/plate Technical Equivocal (positive Russell (1978a,b)
TA 100, TA 1535. TA 1537 in TA 1537 with
activation)
Reverse mutationa S. typhimurium TA 15~35, < 500 mg/disc Technical Negative Carere et al. (1978)
TA1536, TA 1537, TA1538,
Streptococcus coelicodor
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Reverse mutationa A. nidulans < 0.4 mg/ml Technical Positive Kappas et al. (1974);
Kappas & Bridges
(1981)
hprt mutationa Chinese hamster ovary < 172 mmol/litre Technical Negative Fitzpatrick (1980)
cells (99,9%)
tk mutation L5178Y mouse < 20 mmol/litre Technical Negative Amacher et al. (1979)
lymphoma cells
tk mutationa L5178Y mouse < 25 mmol/litre Technical Positive without McCooey et al.
lymphoma cells (991%) activation: negative (1983a, b)
with activation
Tests for DNA damage and repair
Unscheduled DNA Rat and mouse < 500 mg/ml Technical Negative Tong (1981)
synthesis hepatocytes
Rec assay B. subtilis 2000 mg/plate Technical Negative Shirasu et al. (1978)
Tests in vivo
Host-mediated mutation S. typhimurium 2000 mg/kg bw Technical Negative Shirasu et al. (1978)
in mice G46 his-
Host mediated mutation S. typhimurium 3 × 500 mg/kg bw 50% WP Negative Fiscor et al. (1978)
in mice G46 his-
Dominant lethal mutation Rat < 5000 ppm in 50% WP Negative Culik & Gibson
food for 7 days (1914)
Dominant lethal mutation Rat NR Technical Negative Sherman et al. (1975)
Dominant lethal mutation Rat < 203 ppm in Technical Negative Barnes et al. (1983)
food for 70 days
Dominant lethal mutation Rat < 50 mg/kg bw Technical Negative Georgieva et al.
per day for 70 days (1990)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Positive Seller (1976)
bw
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Equivocal Kirkhart (1980)
bw
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive Sasaki (1990)
bw (95%)
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive, KC+, Bentley (1992)
with KC+ staining bw (96.1-97.4%) benomyl classified
as aneugen
Chromosomal aberration Mouse bone marrow < 1 × 5000 mg/kg Technical Negative Stahl (1990)
bw (961%)
Chromosomal aberration Peripheral blood NR 50% WP Negative Ruzicska et al. (1976)
from workers
Chromosomal aberration Rat bone marrow < 500 mg/kg bw, 50% WP Bone marrow, Ruzicska et al. (1976)
and cultured embryo days 7-14 of negative; embryo
cells gestation cells, positive
Sex-linked recessive Drosophila melanogester 1.5 mg/ml Technical, Negative, but Lamb & Lilly (1980)
lethal mutation 50% WP sterility consistent
with observed
spindle effects
NR, not reported; 50% WP, wettable powder formulation; KC, kinetochore
a With and without metabolic activation
Benlate PNW, a 50% wettable powder (Gargus & Zoetis, 1983b,c). The
Draize score for ocular irritation induced by 5 mg of pure benomyl in
four albino rabbits indicates that it is a mild irritant (Desi, 1979).
Albino guinea-pigs exposed to technical-grade benomyl or a 50%
sucrose formulation had mild to moderate skin erythema during the
challenge phase after either intradermal injection or repeated
applications to abraded skin (Majut, 1966; Colburn, 1969; Frank,
1969).
Ten albino Hartley guinea-pigs received four weekly injections of
Benlate PNW (50% benomyl prepared as a 0.1% solution in saline), and
10 animals were injected with saline; 14 days after the final
injection, 8 or 80% Benlate PNW was used for challenge. An unequivocal
increase in sensitization was seen at two of 10 sites challenged with
the 8% suspension and at seven of 10 sites challenged with the 80%
suspension (Gargus & Zoetis, 1984).
Technical-grade benomyl sensitized all 10 guinea-pigs tested in a
maximization test (Matsushita & Aovama, 1981).
(ii) Neurotoxicity
Studies in hens given single oral doses of < 5000 mg/kg bw did
not indicate neurotoxic potential (Goldenthal, 1978; Jessup, 1979;
Jessup & Dean, 1979).
Studies in groups of 10 adult male CFY rats showed no alteration
in electroencephalographic potential or in behaviour (learning ability
assessed in a four-choice T-maze) in comparison with controls after
treatment with benomyl at 250 or 500 mg/kg bw per day for three months
(Desi, 1983).
Groups of 10 male and 10 female Sprague-Dawley rats received
single oral doses of 0, 500, 1000, or 2000 mg/kg bw benomyl (97.4%
pure). Functional and motor activity were assessed during the week
before the day of treatment and at 2 h, one day, seven days, and 14
days after dosing. Rats were then killed and subjected to a gross
post-mortem examination. Histopathological examination was restricted
to tissues from the central and peripheral nervous system of six rats
in the control and high-dose groups. There were no treatment-related
deaths during the study. Immediately after treatment, body weight gain
and food intake were decreased in all treated groups; however, overall
weight gain and food consumption were unaffected by treatment. In the
battery of functional tests, a small number of animals had soft or
liquid faeces and fur stained with urine or faeces on days 1 and 2,
but no other component of the battery, including assessments of other
autonomic functions, reactivity, and sensitivity, excitability, gait,
and sensorimotor coordination, forelimb and hindlimb grip strength,
abnormal clinical signs and body temperature, showed any reaction to
treatment. Motor activity was reduced, only on the day of treatment,
in females given 2000 mg/kg bw. Neuropathological observation revealed
no treatment-related abnormalities. It was concluded that benomyl
induced no lasting neurotoxicity at doses up to 2000 mg/kg bw (Foss
1993).
Groups of 11 male and 11 female Sprague-Dawley rats received
benomyl (purity, 97.4%) in the diet at 0, 100, 2500, or 7500 ppm for
90 days. Functional and motor activity were assessed during the week
before treatment and during weeks 4, 8, and 13. Rats were then killed
and subjected to gross post-mortem examination. Histopathological
examination was restricted to tissues from the central and peripheral
nervous system of six rats in the control and high-dose groups. There
were no treatment-related deaths during the study. Body weight gain
and food intake were reduced at the high dose throughout the treatment
period. The battery of functional tests showed no reaction to
treatment with benomyl. Motor activity was increased at 7500 ppm.
Neuropathological observation revealed no treatment-related
abnormalities. It was concluded that benomyl is not a specific
neurotoxicant, since the changes in motor activity occurred at the
same dietary level as other general toxicological changes (Foss,
1994).
3. Observations in humans
(a) Exposure during field use
Potential dermal and respiratory exposure to benomyl during
actual use was determined during mixing for aerial application,
re-entry into treated fields, and home use (garden, ornamental, and
greenhouse). Maximal exposure occurred during loading and mixing for
aerial application, with dermal exposure to 26 mg per mixing cycle,
primarily (90%) on the hands and forearms; the average respiratory
intake was 0.08 mg benomyl. During re-entry, the dermal exposure was
5.9 mg/h and the respiratory exposure < 0.002 mg/h. Home use resulted
in exposures of 1 mg per application cycle dermally and 0.003 mg by
the respiratory route. This study did not address reactions to
exposure (Everhart & Holt, 1982).
The potential dermal and respiratory exposure of workers mixing
and applying 20 and 100 gallons of Benlate per acre (224 and
1123 litres/ha) while spraying fruit orchards was examined. The
application cycle was about 70 min and resulted in a total dermal
exposure of 11 mg of benomyl per cycle and a total respiratory
exposure to 15 mg. Essentially all of the exposure was dermal,
resulting in 12.2 mg per cycle dermally and < 0.05 mg per cycle via
the respiratory route (DuPont de Nemours & Co., undated).
(b) Medical surveillance of workers
Benomyl caused contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et at., 1985).
In controlled patch tests of 200 agricultural, ex-agricultural, and
non-agricultural workers, only one agricultural worker showed contact
dermatitis to 0.1% benomyl (Lisi et al., 1986). In a survey of
cross-sensitization with benomyl and other pesticides in a group of
126 Japanese farmers who applied benomyl to their crops, 39 had
positive test results with benomyl, the highest incidence being found
among female farmers. There were cross-reactions between benomyl and
diazinon, saturn, daconil, and Z-bordeaux (Matsushita & Aovama, 1981).
Selected blood profiles of 50 factory workers involved in the
manufacture of Benlate were compared with those of a control group of
48 workers who were not exposed to Benlate. White blood count, red
blood count, haemoglobin, and haematocrit values were comparable in
the two groups. No quantitative estimates of exposure were given for
the factory workers (DuPont de Nemours & Co., 1979).
A survey was performed to determine whether potential exposure
to Benlate had an adverse effect on the fertility of 298 male
manufacturing workers exposed between 1970 and 1977. The ages of the
workers ranged from 19 to 64 years; 79% of the workers and 78% of
their spouses were aged 20-39. The duration of exposure ranged from
less than one month to 95 months, but more than 51% of the workers had
potentially been exposed for one to five months. The birth rates of
the spouses of the exposed workers were compared with those of four
populations from the same county, state, region, and country (United
States). There was no reduction in fertility, and the birth rates of
the study population were generally higher than those of the
comparison populations. Spermatogenesis was not examined (Gooch,
1978).
Comments
Benomyl is readily absorbed by animals after oral exposure and
rapidly metabolized. It is eliminated in the faeces and excreted in
the urine; 98% of the dose was excreted by 72 h after administration.
The tissue distribution showed no bioconcentration. In rats, the
metabolites carbendazim and methyl 5-hydroxybenzimidazol-2-ylcarbamate
(5-hydroxy-carbendazim) were found in the blood and in small amounts
in the testis and liver. The latter compound was the main metabolite
in urine. A 50% wettable powder formulation was poorly absorbed via
the dermal route by rats. After a 10-h exposure, less than 2% of a
single dose of 0.2 mg was excreted in the urine.
Benomyl has low acute toxicity, with an oral LD50 in rats of
> 10 000 mg/kg bw. The clinical signs of toxicity after high single
doses were generally nonspecific. Testicular degeneration, with
necrosis of germinal epithelium and aspermatogenesis, has been
observed after single doses in rats (> 100 mg/kg bw orally) and dogs
(1.65 mg/litre air by inhalation). Wettable powder formulations
containing benomyl have been shown to be mildly irritating to rabbit
skin and eyes and have induced skin sensitization reactions in
maximization tests. WHO has classified benomyl as unlikely to present
an acute hazard in normal use.
In a 90-day study, rats were given dietary doses of 0, 100, 500,
or 2500 ppm benomyl. Increased liver weight was seen at 2500 ppm; the
NOAEL was 500 ppm (50 mg/kg bw per day). Dogs were given dietary doses
of 0, 100, 500, or 2500 ppm benomyl for three months or two years and
rabbits were treated dermally, five days per week for three weeks,
with 0, 50, 250, 500, 1000, or 5000 mg/kg bw per day. Hepatotoxicity
was seen in the dogs but not in the rabbits; effects on male
reproductive organs were seen in both rabbits and dogs. The NOAEL was
500 ppm (equal to 13 mg/kg bw per day) in dogs and 500 mg/kg bw per
day in rabbits.
In a two-year study, benomyl was administered in the diet to rats
at 0, 100, 500, or 2500 ppm. Benomyl was not carcinogenic and showed
no compound-related effects at dietary levels up to and including
2500 ppm (equal to 109 mg/kg bw per day). In a two-year feeding study
in mice at dietary levels of 0, 500, 1500, or 5000 ppm, benomyl
caused liver tumours, and an NOAEL could not be established for
hepatocellular neoplasms. Male mice had decreased testicular weights
and thymic atrophy at 5000 ppm. The lowest dietary level was equal to
64 mg/kg bw per day.
In rats treated by gavage for 62 days with 45 mg/kg bw per day,
decreased testicular and epididymal weights, reduced caudal sperm
reserves, and decreased sperm production, with generalized disruption
of all stages of spermatogenesis were observed. After mating with
untreated females, no effect was seen on reproductive behaviour,
weight of the seminal vesicles, sperm mobility, or related
reproductive hormones. The NOAEL was 15 mg/kg bw per day. A lowering
of male fertility rates has been reported, but this effect was not
seen consistently. A single dose of 100 mg/kg bw or more administered
to rats by gavage had effects 70 days after exposure, which included
decreased testicular weight and atrophy of the seminiferous tubules.
The NOAEL was 50 mg/kg bw per day. In a recent study of reproductive
toxicity, rats received dietary doses of 0, 100, 500, 3000, or
10 000 ppm benomyl. The NOAEL was 500 ppm (equivalent to 37 mg/kg bw
per day), on the basis of effects on pup survival and pup growth and
on testicular changes. Fertility indices were not affected at dietary
levels up to 10 000 ppm.
In a study of developmental toxicity, mice were exposed by gavage
to benomyl at doses of 0, 50, 100, or 200 mg/kg bw per day on days
7-17 of gestation. There was no indication of maternal toxicity, but
benomyl was teratogenic at doses of 100 and 200 mg/kg bw per day and
fetotoxic at 50 mg/kg bw per day. The major abnormalities included
hydrocephaly, cleft palate, and limb defects. In studies of
teratogenicity, pregnant rats were exposed to benomyl at doses up to
and including 125 mg/kg bw per day on days 7-16 of gestation.
Benomyl was teratogenic, the major effects being microphthalmia and
hydrocephaly. The Meeting concluded that the NOAELs in rats were
30 mg/kg bw per day for teratogenicity and fetotoxicity and 125 mg/kg
bw per day for maternal toxicity. In rabbits, benomyl was not
teratogenic at doses up to 180 mg/kg bw per day (the highest dose
tested), and no effect was seen on maternal toxicity or fetotoxicity
at 90 mg/kg bw per day.
Benomyl has been adequately tested for genotoxicity in a range of
assays. The Meeting concluded that it does not directly damage genetic
material but does cause numerical chromosomal changes both in vitro
and in vivo as a result of its interference with mitotic spindle
proteins.
In a study of workers exposed to benomyl, there was no reduction
in fertility, as indicated by birth rates, among the study population.
Spermatogenesis in the workers was not examined. Cases of dermal
sensitization to benomyl have been reported.
An ADI of 0-0.1 mg/kg bw was established on the basis of the
NOAEL of 13 mg/kg bw per day in the two-year study in dogs and
applying a safety factor of 100. This ADI should be used when
assessing exposure to benomyl itself. Since the use of benomyl on
crops gives rise to residues of carbendazim and since the ADI for
carbendazim is lower than that which would be derived from the data on
benomyl, the Meeting concluded that the intake of residues in food
should be compared with the ADI of 0-0.03 mg/kg bw for carbendazim.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: < 500 ppm, equal to < 64 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
50 mg/kg bw per day (study of developmental toxicity)
< 50 mg/kg bw per day (fetotoxicity in a study of
teratogenicity)
Rat: 2500 ppm, equal to 109 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
500 ppm, equivalent to 37 mg/kg bw per day (study of
reproductive toxicity)
30 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
125 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 180 mg/kg bw per day (study of developmental toxicity)
90 mg/kg bw per day (maternal toxicity and fetotoxicity in
study of developmental toxicity)
Dog: 500 ppm, equal to 13 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw (benomyl)
0-0.03 mg/kg bw (carbendazim, with which residues of benomyl in
food should be compared)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to benomyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 >10 000 mg/kg bw
Dermal, toxicity, rabbit LD50 (50% wettable powder)
> 10 000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder; irritating
Ocular, irritation, rabbit 50% wettable powder; irritating
Dermal, sensitization, guinea-pig Positive in maximization test
Inhalation, toxicity, rat LC50 (50% wettable powder)
> 4.01 mg/litre air
Mid-term (1-26 weeks) Oral, 62 days, rat NOAEL = 15 mg/kg bw per day;
reduced spermatogenesis
Oral, developmental toxicity, rat NOAEL = 30 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary two years, toxicity, dog NOAEL = 13 mg/kg bw per day;
hepatotoxicity
See also toxicological criteria for carbendazim when considering dietary exposure to residues
References
Amacher, D.E., Paillet, S. & Ray, V.A. (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells:
Application to genetic toxicological testing. Mutat. Res., 64,
391-406.
Athwal, R.S. & Sandhu, S.S. (1985) Use of human x mouse hybrid cell
line to detect aneuploidy induced by environmental chemicals.
Mutat. Res., 149, 73-81.
Axness, M.E. & Fleeker, J.R. (1979) Metabolism of the butylcarbamoyl
moiety of benomyl in rat. Pestic. Biochem. Physiol., 11, 1-12.
Barbo, E.C. & Carroll, K.S. (1972) Oral ALD test ( S-triazine,
3-butylbenzimidazolo[1,2-a]-2,4[1 H,3 H]dione. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Barnes, T. B., Verlangieri, A.J. & Wilson, M.C. (1983) Reproductive
toxicity of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate
(benomyl) in male Wistar rats. Toxicology, 28, 103-115.
Belasco, I.J. (1979a) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied. Unpublished
report from DuPont de Nemours & Co., Biochemical Department,
Research Division, Experimental Station, Wilmington, Delaware,
USA.
Belasco I.J. (1979b) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Unpublished report from DuPont de Nemours & Co., Biochemical
Department, Research Division, Experimental Station, Wilmington,
Delaware, USA
Belasco, I.J., Kirkland, J.J., Pease, H.L. & Sherman, H (1969) Studies
with 2-14C-labelled methyl-1-(butylcarbamoyl)-2-
benzimidazolecarbamate (benomyl) in rats. Unpublished report from
DuPont de Nemours & Co., Biochemical Department, Research
Division, Experimental Station, Wilmington, Delaware, USA.
Bentley, K S. (1992) Classification of DPX-T1991-529 (Benomyl)-induced
micronuclei in mouse bone marrow erythrocytes using immuno-
fluorescence antikinetochore antibodies. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Brock, W.J. (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Unpublished report from DuPont de Nemours &
Co, Haskell Laboratory, Newark, Delaware, USA.
Busey, W.M. (1968a) Acute dermal LD50 test and dermal irritation
test on rabbits using a wettable powder formulation (50% benomyl)
with histological addendum. Unpublished report from Hazelton
Laboratories, Inc., Falls Church, Virginia, USA, prepared for
DuPont de Nemours & Co.
Busey, W.M. (1968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968c) Repeated dermal application test on rabbits using
a wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968d) Teratology study in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from
Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res., 57, 277-826.
Carter, S.D. (1982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Thesis, North Carolina State
University, Raleigh, North Carolina, USA.
Carter, S.D. & Laskey, J.W. (1982) Effect of benomyl on reproduction
in the male rat. Toxicol. Lett., 11, 87-94.
Chernoff, N. & Kavlock, R.J. (1983) A teratology test system which
utilises post natal growth and viability in the mouse. Environ.
Sci. Res., 27, 417-427.
Colburn, C.W. (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Culik, R. (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the
concepti of rats exposed to benomyl and Benlate by diet.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC), 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. and Gibson, J.R. (1974) 'Benlate' dominant lethal study in
male rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Dashiell, O.L. (1972) Acute oral test (benzimidazole, 2-(3-
butylureido)). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
De Bertoldi, M. & Griselli, M. (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat. Res., 74,
303-324.
De Bertoldi, M., Griselli. M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by metres of gene-conversion
in Saccharomyces cerevisiae and in Aspergillus nidulans.
Environ. Mutag., 2, 359-370.
Desi, I. (1979) Hygienic-toxicological evaluations of pesticides.
D.Sc. Thesis, Budapest, National Institute of Hygiene.
Desi, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5, 503-515.
Donovan, S.D. & Krahn, D.F. (1981) Mutagenic evaluation in
Salmonella typhimurium. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1977) Mutagenic activity of benomyl in the
Salmonella/microsome assay (50% benomyl as a wettable pow der
formulation). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1979) Benlate dust exposure survey -- blood
profile analysis. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (undated) Applicator exposure during filling
and spraying of Benlate benomyl fungicide -- orchard crops.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Eastmond, D.A. & Tucker, J.D. (1989) Kinetochore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat. Res., 224, 517-525.
Ellis W.G., Semple, J.L., Hoogenboom, E.R., Kavlock, R.J. & Zeman,
E.J. (1987) Benomyl-induced craniocerebral anomalies in fetuses
of adequately nourished and protein-deprived rats. Teratog.
Carcinog. Mutag., 7, 357-375.
Ellis W.G., De Roos, F., Kavlock, R.J. & Zeman, F.J. (1988)
Relationship of periventricular overgrowth to hydrocephalus in
brains of fetal rats exposed to benomyl. Teratog. Carcinog.
Mutag., 8, 377-391.
Evans, E.L. & Mitchell, A.D. (1980) An evaluation of the effect of
benomyl on sister chromatid exchange frequencies in cultured
Chinese hamster ovary cells. Unpublished report from SRI
International, Menlo Park, California, USA, prepared for DuPont
de Nemours & Co.
Everhart, L.P. & Holt, R.F. (1982) Potential Benlate fungicide
exposure during mixer/loader operations, crop harvest and home
use. J. Agric. Food Chem., 30, 222-227.
Fiscor, G., Bordas, S. & Stewart, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Fitzpatrick, K. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Foss J.A (1993) Acute neurotoxicity study of DPX-T1991-529 (Benomyl)
administered orally by gavage to Crl:CD BR VAF/Plus rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Foss, J.A. (1994) Subchronic neurotoxicity study of DPX-T1991-529
(Benomyl) administered orally via the diet to Crl:CD BR VAF/Plus
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. & Sherman, H. (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Gardiner, J.A., Kirkland, J.J., Klopping, H.L. & Sherman, H. (1974)
Fate of benomyl in animals. J. Agric. Chem., 22, 419-427.
Gargus, J.L. & Zoetis, T. (1983a) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines -- Benlate PNW).
Unpublished report from Hazelton Laboratories Inc., Vienna,
Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983b) Primary irritation study in rabbits
(Benlate PNW). Unpublished report from Hazelton Laboratories
Inc., Vienna, Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983c) Eye irritation test in rabbits (EPA
pesticide registration Benlate PNW). Unpublished report from
Hazelton Laboratories Inc., Vienna, Virginia, USA, prepared for
DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Unpublished
report from Hazelton Laboratories Inc., Vienna, Virginia, USA,
prepared for DuPont de Nemours & Co.
Georgieva, V., Vachkova, R., Tzoneva, M. & Kappas, A. (1990) Genotoxic
activity of benomyl in different test systems. Environ. Mol.
Mutag., 16, 32-36.
Goldenthal, E.I. (1978) Neurotoxicity study in hens using technical
benomyl (< 95% benomyl). Unpublished report from International
Research and Development Corporation, Mattawan, Michigan, USA,
prepared for DuPont de Nemours & Co.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from DuPont de Nemours & Co.,
Wilmington, Delaware, USA.
Goodman, N.C. (1975) Intraperitoneal LD50 test in rats. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Guengerich, F.P. (1981) Enzyme induction with DuPont compounds H11,
202-02 and H10, 962-02. Unpublished report from Vanderbilt
University, School of Medicine, Nashville, Tennessee, USA,
prepared for DuPont de Nemours & Co.
Gupta, A.K. & Legator, M.S. (1975) Chromosome aberrations in cultured
human lymphocytes after treatment with fungicide 'Benlate'. In:
Proceedings of the Symposium on Mutagenicity, Carcinogenicity
and Teratogenicity of Chemicals, New Delhi, Department of
Atomic Energy, pp. 95-103
Han, J.C.Y. (1979) 2-14C-Benomyl (50% WP) rat study -- intravenous
injection. Unpublished report from DuPont de Nemours & Co.,
Biochemical Department, Research Division, Experimental Station,
Wilmington, Delaware, USA.
Han, J.C.Y. (undated) Metabolism of 14C-labelled benomyl in the
mouse and hamster. Unpublished report from DuPont de Nemours &
Co., Biochemical Department, Research Division, Experimental
Station, Wilmington, Delaware, USA
Hess, R.A., Moore, B.J., Forrer, J., Linder, R.E. & Abuel-Atta, A.A.
(1991) The fungicide benomyl [(methyl)1-(butylcarbomyl)-2-
benzimidazole carbamate] causes testicular dysfunction by
inducing the sloughing of germ cells and occlusion of efferent
ductules. Fundam. Appl. Toxicol., 17, 733-745.
Hood, D.B. (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Hoogenboom, E.R., Ransdell, J.F., Ellis, W.G., Kavlock, R.J. & Zeman,
E.J. (1991) Effects on the fetal rat eye of maternal benomyl
exposure and protein malnutrition. Curr. Eye Res., 10, 601-612.
Hornberger, C.S. (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Hostetler, K.H. (1977) Oral LD50 test (Benlate OD). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Jessup, D.C. (1979) Acute delayed neurotoxicity study in chickens
using technical benomyl (> 95% benomyl). Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA, prepared for DuPont de Nemours & Co.
Jessup, D.C. & Dean, W. (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl).
Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, prepared for DuPont de
Nemours & Co.
van Joost, T.H., Naafs, B. & van Ketel, W G. (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9, 153-154.
Kappas, A. & Bridges, B.A. (1981) Induction of point mutations by
benomyl in DNA-repair deficient Aspergillus nidulans. Mutat.
Res., 91, 115-118.
Kappas, A., Georgopoulos, S G. & Hastie, A.C. (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat. Res., 26, 17-27.
Kappas, A., Green, M.H.L., Bridges, B.A., Rogers, A.M. & Muriel, W.J.
(1976) Benomyl -- a novel type of base analogue mutagen? Mutat.
Res., 40, 379-382.
Kavlock, R.J., Chernoff, N., Gray, L.E., Gray, J A & Whitehouse, D.
(1982) Teratogenic effects of benomyl in the Wistar rat and CD-1
mouse, with emphasis on the route of administration. Toxicol.
Appl. Pharmacol., 62, 44-54.
Kirkhart, B. (1980) Micronucleus test on benomyl (> 95% benomyl).
Unpublished report from SRI International, Menlo Park,
California, USA. prepared for DuPont de Nemours & Co.
Krupka R.M. (1974) On the anti-cholinesterase activity of benomyl.
Pestic. Sci., 5, 211-216.
Kuelme, G., Heise, H., Plottke, B. & Puskeller, T (1985) Dermatitis
after Benlate contact. Z. Ges. Hyg. Grenzgeb., 31, 710-711.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Lee K.P. (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Linder, R.E., Rehnberg, G.L., Strader, L.F. & Diggs, J.P. (1988)
Evaluation of reproductive parameters in adult male Wistar rats
after subchronic exposure. J. Toxicol. Environ. Health, 25,
285-298.
Lisi, P., Caraffini, S. & Assalve, D. (1986) A test series for
pesticide dermatitis. Contact Derm., 15, 266-269.
Littlefield, N.A. & Busey, W.M. (1969) Four-hour acute inhalation
exposure test in dogs using a wettable powder formulation (50%
benomyl). Unpublished report from Hazleton Laboratories, Inc.,
Falls Church, Virginia, USA, prepared for DuPont de Nemours & Co.
Majut, J.C. (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Matsushita, T. & Aovama, K. (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind.
Health, 19, 77-83.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983a) L5178Y
mouse lymphoma cell assay for mutagenicity (benomyl). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983b) L5178Y
mouse lymphoma cell assay for mutagenicity. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Mebus, C.A. (1990) Reproductive and fertility effects with
DPX-1991-529 (benomyl). Multigeneration reproduction study in
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Monson, K.D. (1990) Metabolism of [phenyl(U)14C]carbendazim in rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Munley, S.M. (1995) Developmental toxicity study of DPX-T1991-529
(Benomyl) in rabbits. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Pilinskaya, M.A. (1.983) Investigation of the cytogenetic action of
the pesticides captan and benomyl in a culture of human
peripheral blood lymphocytes in the absence and presence of a
system of metabolic activation. Cytol. Genet., 17, 29-33
Rainaldi, G., Flori, L., Colella, C.M, Mariani, T., Piras, A., Simi,
S. & Simili, M. (1989) Analysis by BrUdR-labelling technique of
induced aneuploidy in mammalian cells in culture. Mutat. Res.,
177, 255-260.
Rashid, K.A. & Ercegovitch, C.D. (1976) New laboratory tests evaluate
chemicals for cancer or gene damage. Sci. Agric., 23, 7.
Reinke, RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Ruzicska, P., Peter, S., Laczi, J. & Czeizel, E. (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP Egeszegtudomany, 20,
74-83.
Sarver, J.W. (1987) Acute oral toxicity study with IN-T1991 in male
and female rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sasaki, Y.F.X. (1988) Benomyl: In vitro cytogenetics test. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Sasaki, Y.F.X. (1990) Benomyl: Micronucleus test in mice. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives, VI: Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and the
Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. & Krauss, W.C. (1966) Acute oral test [benomyl].
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Sherman, H., Barnes, J.R. & Krauss, W.C. (1967) Ninety-day feeding
study with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl
ester (INT-1991). Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H., Culik, R. & Jackson, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. Appl.
Pharmacol., 32, 305-315.
Shirasu, Y., Moriva, M. & Kato, K. (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan, prepared for DuPont de Nemours & Co.
Shukla, Y., Antony, M. & Mehrota, N.K. (1989) Studies on
gamma-glutamyl transpeptidase in rodents exposed to benomyl.
Bull. Environ. Contam. Toxicol., 42, 301-306.
Snee, D.A. (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test). Unpublished. report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Stahl, R.G., Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Staples, R.E. (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Staples, R.E. (1982) Teratogenicity study in the rat using technical
benomyl (> 95% benomyl) administered by gavage. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Tong, C. (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA, prepared for DuPont de Nemours & Co.
Turney, R.T. (1979) Rat inhalation study -- Benlate. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Vick, D.A. & Brock, W.J. (1987) Primary dermal irritation study with
Benlate 50 DF fungicide in rabbits. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Ward, R.S. & Scott, R.C. (1992) Benomyl: in vitro absorption of a
500 g.kg-1 WP formulation through human epidermis. Unpublished
report from Imperial Chemical Industries (ICI), Fernhurst,
Haslemere, Surrey, United Kingdom.
Warheit, D.B., Kelly, D.P., Carakostas M.C. & Singer, A.W. (1989) A
90-day inhalation toxicity study with benomyl in rats. Fundam.
Appl. Toxicol., 12, 333-345.
Wiechman, B.E. (1982) Long term feeding study with methyl
1-(butylcarbamoyl)-2-benzimidazole carbamate in mice (INT-1991;
> 95% benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Zelesco, P.A., Barbieri, I. & Graves, J.A.M. (1990) Use of a cell
hybrid test system to demonstrate that benomyl induces aneuploidy
and polyploidy. Mutat. Res., 242, 329-335.
Zeman, E.J., Hoogenboom, E.R., Kavlock, R.I. & Semple, J.L. (1986)
Effects on the fetus of maternal benomyl exposure in the
protein-deprived rat. J. Toxicol. Environ. Health, 17, 405-417.
The effects of benomyl and carbendazim on hepatic enzymes were
studied in male and female Sprague-Dawley rats and Swiss albino mice
fed diets containing benomyl or carbendazim at a concentration of 0,
10, 30, 100, 300, 1000 or 3000 ppm for 28 days. After sacrifice, liver
weights were recorded and microsomal epoxide hydrolase and cytosolic
glutathione- S-transferase were monitored in subcellular fractions
isolated from the liver. The mean absolute liver weights were elevated
in males and females fed 1000 or 3000 ppm carbendazim and in females
fed 300 ppm; however, the only significantly increase was found in
females fed 3000 ppm benomyl. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced epoxide
hydrolase in male and female rats and in mice fed 1000 or 3000 ppm,
and both induced glutathione- S-transferase at 3000 ppm. The level of
induction seemed to be slightly greater in females than males. There
was no substantial difference in enzyme induction between rats and
mice (Guengerich, 1981).
Administration of 1000 or 4000 ppm benomyl in the diet to groups
of eight female albino rats and female Swiss albino mice for 15 days
increased the activity of blood and liver gamma-glutamyl transpeptidase
in both species, and the degree of induction was dose related (Shukla
et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of acute toxicity are summarized in Table
1. The clinical signs of toxicity after treatment with benomyl were
generally nonspecific. Gross and histopathological changes were
evaluated in some studies; in male gonads, testicular degeneration,
necrosis of germinal epithelium, and aspermatogenesis were observed in
rats and dogs.
(b) Short-term toxicity
Rats
After intubation of benomyl into male Sprague-Dawley rats at 200
or 3400 mg/kg bw in peanut oil five times per week for two weeks, four
of six animals at the high dose died. At this dose, degeneration of
germinal epithelium, multinucleated giant cells, and reduction or
absence of sperm were seen. Very minor changes were observed in the
testes of the animals treated with the lower dose (Sherman & Krauss,
1966).
Male Sprague-Dawley rats treated orally for 10 days with
200 mg/kg bw per day of the metabolite 5-HBC (methyl ester) had no
toxic symptoms or evidence of effects on the testes (Snee, 1969).
Table 1. Acute toxicity of benomyl, benomyl formulations, and benomyl metabolites
Test material Species Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
Benomyl Rat Oral > 10 000 > 95 Sherman (1969a)
Benomyl Dog Oral > 1 000 > 95 Sherman (1969b)
'50% wettable powder' Rat Oral > 1 000 > 95 Sherman (1969a)
'50% wettable powder' Rat Oral > 5 000 NR Sarver (1987)
'50% wettable powder' Rat Oral > 1 200 NR Hostetler (1977)
'50% wettable powder' Rabbit Oral > 3 400 NR Fritz (1969)
'50% wettable powder' Rabbit Dermal > 10 000 NR Busey (1968a)
'50% wettable powder' Rabbit Dermal > 2 000 NR Brock (1987)
'50% wettable powder' Rabbit Dermal > 2 000 NR Gargus & Zoetis (1983a)
'50% wettable powder' Rat Inhalation (4-h) > 0.82 NR Hornberger (1969)
'50% wettable powder' Rat Inhalation (4-h) > 4.01 NR Busey (1968b)
'50% wettable powder' Dog Inhalation (4-h) > 1.65 NR Littlefield & Busey (1969)
2-Benzimidazole carbamic Rat Intraperitoneal > 10 000 NR Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole Rat Oral > 7 500 > 95 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole Rat Oral > 3 400 > 95 Fritz & Sherman (1969)
Benzimidazole 2-(3-butylureido) Rat Oral > 17 000 NR Dashiell (1972)
S-Triazine, 3-butyl-benzimidazole Rat Oral > 17 000 NR Barbo & Carroll (1972)
(1,2a)-2,4(1H,3H)-dione
NR, Not reported
Groups of 16 male and 16 female Sprague Dawley rats were fed
benomyl (72% pure) in the diet at 0, 100, 500 and 2500 ppm (as
benomyl). After 96-103 days of continuous feeding, 10 male and 10
female rats in each group were killed, and selected organs were
weighed; additional organs were preserved for microscopic examination.
The remaining animals in each group were studied for reproductive
toxicity (see below). There were no gross toxic signs of poisoning and
no compound-related effects on weight gain, food consumption, food
efficiency, or haematological, biochemical, or urine parameters. The
liver body weight ratio was slightly increased in females at 2500 ppm
in comparison with control rats, Gross and microscopic examination of
tissues and organs showed no significant effect attributable to the
presence of benomyl in the diet at levels up to and including 2500 ppm
(Sherman et al., 1967).
Groups of 20 male and 20 female Sprague-Dawley rats were exposed
to benomyl by inhalation at 0, 10, 50, or 200 mg/m3 via the nose
only, for 6 h/day for 90 days. After 45 and 90 days, blood and urine
samples were collected from 10 rats of each sex per group for clinical
analysis; the animals were then killed for pathological examination.
After about 45 days of exposure, degeneration of the olfactory
epithelium attributable to benomyl was observed in all males and in
eight of the 10 females exposed to 200 mg/m3; two male rats exposed
to 50 mg/m3 had similar but less severe olfactory degeneration. After
about 90 days of exposure, all of the animals at 200 mg/m3 and three
males at 50 mg/m3 had olfactory degeneration. No other compound-
related pathological effects was observed. Male rats exposed to
200 mg/m3 had depressed mean body weights in comparison with
controls; the depression was correlated with a reduction in food
consumption (Warheit et al., 1989).
Rabbits
Groups of five male and five female New Zealand albino rabbits
were given 15 dermal applications for 6 h/day, five days per week for
three weeks of a 50% benomyl formulation (equivalent to 1000 mg/kg bw)
on both abraded and intact abdominal skin. After each application, the
abdomen was washed with tap water. Mortality and toxic effects were
monitored daily and body weight changes weekly; gross necropsy and
microscopic examinations were performed. Slight erythema, oedema, and
atonia were observed on both abraded and intact skin sites, and slight
to moderate desquamation was reported throughout the study. No
apparent compound-related changes in body weight or organ:body weight
ratios were reported. Treatment induced degeneration of the
spermatogenic elements of the seminiferous tubules of the testes,
including vacuolated and multinucleated spermatocytes. A slight
increase in haematogenic activity in the bone marrow and acanthosis
and hyperkeratosis of the skin were reported in treated animals
(Busey, 1968c).
Groups of five male and five female New Zealand albino rabbits
received benomyl at doses equivalent to 0, 50, 250, 500, 1000, and
5000 mg/kg bw on non-occluded, abraded dorsal skin for 6 h/day, five
days per week for three weeks. The material was removed by washing the
skin site and drying with a towel. The body weight gains of males and
females at > 1000 mg/kg bw were reduced, and mild-to-moderate skin
irritation was reported in all groups, especially at the higher doses.
Functional disturbances of the alimentary canal and kidney, including
diarrhoea, oliguria, and haematuria, were observed in males and
females at 1000 and 5000 mg/kg bw. Decreased testicular weights were
observed at 1000 mg/kg bw only, but these were considered not to be
directly related to treatment. No histopathological changes were
reported (Hood, 1969).
Dogs
Groups of four male and four female beagle dogs were given
benomyl (as a 50% wettable powder) in the diet at 0, 100, 500, or
2500 ppm (based on active ingredient) for three months. There was no
mortality or adverse clinical effect during the course of the study,
and growth and food consumption were normal. Urinary parameters were
unaffected by treatment. There were no dose-related effects on
haematological values, but alkaline phosphatase and alanine
aminotransferase activities were increased in males and females at the
highest dose, and the albumin:globulin ratio was significantly
decreased in both males and females fed 2500 ppm. In males and females
at the high dose, the weight of the thymus was decreased and that of
the thyroid was increased. One female fed 2500 ppm had an enlarged
spleen, which was consistent with the decreased erythrocyte count,
haemoglobin concentration, and haematocrit values. Histopathological
examination revealed myeloid hyperplasia of the spleen and bone marrow
and erythroid hyperplasia in the same animal. Three of four males fed
2500 ppm had reduced prostate weights in comparison with controls.
Microscopic examination of tissues and organs showed no consistent
effect. The NOAEL was 500 ppm (Sherman, 1968).
Groups of four male and four female beagle dogs were fed benomyl
(50% pure) in the diet at 0, 100, 500, or 2500 ppm (as benomyl) for
two years, with interim sacrifice after one year of one male and one
female from the control and high-dose groups. Organ weights, gross
necropsy, and histopathological evaluations were performed at the
conclusion of the study; only the livers and testes of animals fed 100
or 500 ppm were examined histologically. No deaths were attributable
to treatment. Body weight changes and food consumption were similar in
all groups, except for those at the high dose, which had decreased
food intake and body weight gain; one dog at the high dose lost its
appetite and was replaced. No other clinical signs of toxicity were
observed. The results of haematological evaluations and urinalysis
were similar to those of the controls. Males at 2500 ppm had
increased levels of cholesterol, alkaline phosphatase, and alanine
aminotransferase (initially) and decreased total protein and albumin:
globulin ratio. Similar, but less marked, effects were seen in
females at this dose. Biochemical analyses indicated adverse effects
on the liver, expressed as liver cirrhosis. Four of six dogs at this
dose had slight-to-marked bile duct proliferation. Haemosiderosis was
seen after specific staining for iron in one dog at the high dose
after one year but not in other dogs examined at two years.
Preparation of preserved wet tissue with oil red O and Sudan black for
hepatocyte vacuolation showed that benomyl is not hepatotoxic at 100
or 500 ppm in the diet. Focal testicular degeneration was seen in all
groups, including controls, with marked testicular degeneration
(reduced testicular weight, absence of spermatozoa, and spermatic
giant cells) in one of three dogs at 2500 ppm. The NOAEL was 500 ppm,
equivalent to 13 mg/kg bw per day (Sherman, 1970).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female CD-1 mice were given benomyl
(99% pure) in the diet at 0, 500, 1500, or 5000 ppm (reduced from
7500 ppm after 37 weeks) for two years. Survival was unaffected by
treatment. Male and female mice fed 1500 or 5000 ppm benomyl had
dose-related decreases in body weight. Food consumption was variable
throughout the study, although females at the high dose appeared to
consume less food. Clinical signs of toxicity were similar in all
groups; there were no apparent differences in palpable masses,
numbers of mice affected, or latency to detection. The results of
haematological examinations were unremarkable except for decreased
erythrocyte counts in males at 1500 ppm and in females at 5000 ppm.
Haemoglobin and haematocrit values were also depressed in males at the
intermediate dose. Several statistically significant changes in organ
weights were seen in treated groups, the most significant being
reduced absolute and relative liver weights in males at 1500 and
5000 ppm and females at 5000 ppm. Male mice at the high dose also had
decreased absolute testicular weights. The changes in liver and
testicular weights were accompanied by histomorphic changes in these
tissues. The incidence of hepatocellular carcinomas and benign hepatic
neoplasms in treated female mice was increased in a dose-dependent
fashion. In male mice, hepatocellular carcinomas and benign hepatic
neoplasms were significantly increased at 500 and 1500 ppm, but not at
the high dose. The incidences of lung tumours (alveologenic
carcinomas) were also significantly increased in males but not in
females at 500 and 1500 ppm. Non-neoplastic changes in males at
5000 ppm were confined to the liver (degeneration, pigment,
cytomegaly), thymus (atrophy), and the testis, epididymus, and
prostate (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini). The occurrence of splenic
haemosiderosis was significantly increased in female mice at 5000 ppm
and that of submucosal lymphocytic infiltration of the trachea at
1500 ppm. The latency for liver tumour induction (adenomas and
carcinomas), as determined from palpation, gross necropsy, and
histopathology performed on all animals throughout the study, was not
measurably different in treated and control animals. The authors
concluded that, under the conditions of the study, benomyl is
oncogenic in male and female animals. No NOAEL was identified for male
or female mice with respect to hepatocellular carcinomas or combined
hepatocellular neoplasms. The lowest dietary level, 500 ppm, was
equivalent to 64 mg/kg bw per day in males and to 103 mg/kg bw per day
in females (Wiechman, 1982).
Rats
Groups of 36 male and 36 female Charles River albino weanling
rats were fed benomyl (purity, 50-70%) in the diet for 104 weeks at
100, 500, or 2500 ppm (as benomyl); there were two control groups.
After one year, each group was reduced to 30 males and 30 females by
interim sacrifice for gross and microscopic evaluation. At the
conclusion of the study, all surviving animals were sacrificed and the
tissues and organs examined grossly. No deaths were attributable to
the presence of benomyl in the diet. Survival was decreased to about
50% during the second year but was comparable in all groups. Body
weight, food consumption, and food efficiency were unaffected by
treatment. There were no compound-related clinical manifestations of
toxicity, and the results of haematological, urinary, and liver
function examinations were unaffected by treatment. There were no
differences in organ weights, and histopathological examination
revealed no differences between treated and control groups. The most
frequently observed tumours were of the mammary, pituitary, and
adrenal glands, but these were equally distributed among the groups.
Hepatic toxicity was similar in all groups, including controls, and
there were no compound-related effects. As a high incidence of
testicular degeneration was observed in control males, no conclusion
could be drawn about compound-related effects on male gonads. Benomyl
had no adverse effects in this study at levels up to and including
2500 ppm, which was equivalent to 109 mg/kg bw per day in males and
128 mg/kg bw per day in females (Sherman, 1969c; Lee, 1977).
(d) Reproductive toxicity
In a short-term study of toxicity (see above), groups of six male
and six female Sprague-Dawley rats were fed diets containing benomyl
at 0, 100, 500, or 2500 ppm. Each female was caged separately and
exposed to each of three males at the same dose for five days. The
females were then allowed to deliver, and the offspring were observed
until weaning. Litters containing more than 10 pups were reduced to 10
by randomly removing pups on day 4. There was no treatment-related
effect on pregnancy rate, number of pups born live or dead, fertility,
gestation, viability, or lactation indices or on average body weight
(Sherman et al., 1975).
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal male Sprague-Dawley rats aged
33 days, which were gavaged daily for 10 days with 0 or 200 mg/kg bw.
Eight animals per group were killed 3, 17, 31, 45, and 59 days after
the last treatment. Selected tissues, including liver, kidneys,
testes, seminal vesicles, and epididymides, were removed, weighed, and
examined histologically; samples of seminal fluid from the vas
deferens were also examined. Observation intervals were preselected to
coincide with stages of spermatogenesis. No effects related to
treatment were seen on tissue weights, total epididymal sperm counts,
was deferens sperm concentrations, or testicular histology (Carter,
1982).
In a similar experiment, groups of five animals were dosed orally
on five consecutive days with 0, 125, 250, 500, or 1000 mg/kg bw per
day, beginning when animals were 33 (prepubertal), 54 (pubertal), or
75 (post-pubertal) days old. Animals were killed 31 days after
the last dose. There was no effect on body weight gain or on
haematological values. The weights of the testes and cauda
epididymides were reduced in animals given 500 or 1000 mg/kg bw per
day benomyl during the onset of puberty. Cauda epididymal and vas
deferens sperm counts were reduced in animals given > 250 mg/kg bw
per day benomyl during puberty. The incidence of diffuse hyposperma-
tocytogenesis was increased in pubertal and post-pubertal animals but
was most pronounced in the latter. The NOAEL for effects on
reproductive development was 12.5 mg/kg bw per day (Carter, 1982).
In a further experiment, adult (65-day-old) male Sprague-Dawley
rats received 10 daily doses of benomyl at 0, 200, or 400 mg/kg bw per
day by gavage. Fourteen days after the last treatment, body weights,
tissue weights, total epididymal sperm counts, and sperm concentration
in the vas deferens were measured, and the testis was examined
histologically. Production of testosterone by the Leydig cells was
artificially stimulated by subcutaneous injections of hypothalamic
chorionic gonadotrophin 2 h before sacrifice. There were no compound-
related effects on the weights of the body, liver, kidney, adrenal,
testis, or seminal vesicles, but caudate epididymal weights were
depressed by treatment and there were treatment-related reductions in
epididymal sperm count (caput and caudate) and in vas deferens sperm
concentration. In animals exposed to the highest dose, there was
histological evidence of hypospermatocytogenesis, with generalized
disruption of all stages of spermatogenesis (Carter & Laskey, 1982).
Benomyl was administered by gavage to Wistar rats at doses of 0,
15.6, or 31.2 mg/kg bw per day from day 7 of gestation to day 15 of
lactation (day 22 of gestation was considered day 0 of lactation). The
litters were each reduced to four male and four female pups on day 3
of lactation. The litters were weighed on days 8, 15, 22, 29, and 100,
and locomotor activity was evaluated periodically throughout the
study. When the animals were 100 days of age, several organs were
weighed, including adrenals, liver, kidney, ovary, testis, and ventral
prostate plus seminal vesicles. There were no compound-related effects
on litter size at birth or weaning or on body weights of fetuses.
Growth, survival, and locomotor activity were comparable to those of
controls throughout the study. Organ weights were comparable to t hose
of controls except for decreased weights of testes and ventral
prostate/seminal vesicles, which were significantly reduced at 31.2
but not at 15.6 mg/kg bw per day (Kavlock et at., 1982).
In a two-generation study, Sprague-Dawley rats were fed diets
containing 0, 100, 500, 3000, or 10 000 ppm benomyl. Parental rats
received the test diets for 71 days before being bred for production
of F1 parental rats with animals at the same dietary concentration.
F1 rats were mated for production of the F2a litter after
maintenance on their respective diets for at least 105 days after
weaning. F1 dams were mated again, to different, non-sibling males,
at least one week after weaning the F2a litter, to produce the
F2blitters. The following indices of reproductive Junction were
calculated for the F0 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive, and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption, and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental rats
were sacrificed lot gross examination and histopathological
examination of gross lesions and target organs; complete histopatho-
logical examination was conducted on controls and animals at the high
dose. Groups of 20 F2a and 20 F2bweanlings were also examined
grossly. There were no compound-related effects on parental mortality
at any concentration of benomyl. Mean body weights, body weight gains,
and overall food consumption of F0 and F1 male and female rats at
10 000 ppm were significantly lower than those in controls, and there
was a significant compound-related decrease in the number of F2a and
F2boffspring alive before culling (on day 4) at this dose. In
addition, male and female offspring of rats fed 10 000 ppm weighed
consistently less at birth than did the offspring of control rats.
With the exception of 14-day male pups of the F2bgeneration, F2a
and F2b offspring at 3000 ppm also had significantly depressed body
weights on days 14 and 21 of lactation. The testicular sperm counts of
F0 and F1 rats at 3000 and 10 000 ppm were decreased, and rats at
the highest dose had decreased testicular weight and histopathological
changes in the testes. Microscopic observation showed a trophy and
degeneration of the seminiferous tubules in the testes of rats at 3000
and 10 000 ppm and oligospermia in the epididymides of F0, rats at
the highest dose and in F1 rats at 3000 and 10 000 ppm. There were
no compound-related differences in mating indices, fertility indices,
or length of gestation that could be attributed to benomyl. The NOAEL
was 500 ppm, equivalent to about 37 mg/kg bw per day (Mebus, 1990).
In a study of adult male Wistar rats, groups of 27 animals were
fed 0, 1, 6.3, or 203 ppm for 70 days, and 14 animals per group were
allowed to recover for 70 days. Ejaculated sperm counts were
reportedly depressed during the feeding phase in the group fed
203 ppm. Relative testicular weights were also lowered, and a slightly
reduced male fertility index was seen in all treated groups. Benomyl
did not alter copulatory behaviour and did not induce dominant lethal
mutation. Plasma testosterone and gonadotropin levels remained
unchanged throughout the study. Identical studies conducted during the
recovery phase showed that the treatment-related effects were
completely reversible (Barnes et al., 1983).
Groups of 12 adult (102-day-old) Wistar male rats were given
benomyl at 0, 1, 5, 15, or 45 mg/kg bw per day by gavage daily for 62
days, were then bred to untreated females after 62 days of dosing, and
were killed after 76-79 days. Reproductive behaviour, seminal vesicle
weight, prostate weight, sperm motility, and the levels of serum
gonadotropin and gonadal hormones were no different from those in
controls. At necropsy, males exposed to 45 mg/kg bw per day had
decreased testicular and epididymal weights, reduced caudal sperm
reserves, decreased sperm production, increased numbers of decapitated
spermatozoa, and increased numbers of seminiferous tubules containing
multinucleated giant cells. There were no treatment-related effects on
the levels of serum luteinizing hormone, follicle stimulating hormone,
prolactin, or androgen binding protein (Linder et al., 1988).
Groups of 20 male Sprague-Dawley rats aged 100 days were given a
single dose of benomyl in corn oil at 0, 25, 50, 100, 200, 400, or
800 mg/kg bw. Eight animals per group were sacrificed after two days
of treatment and 12 animals per group after 70 days (except in the
group receiving 800 mg/kg bw) in order to examine the testis and
efferent ducts for effects on spermatogenesis or the epididymis. The
primary effects seen at day 2 were testicular swelling and occlusions
of the efferent ductules. Premature release of germ cells (sloughing)
was the most sensitive short-term response to benomyl. It was detected
in all treated groups but was statistically significant (P < 0.05)
only at doses > 100 mg/kg bw. Occlusion of the efferent ductules of
the testis was dose-dependent and was correlated with the increase in
testicular weight on day 2. The greatest increase in testicular weight
was observed at 400 mg/kg bw. Long-term effects (70 days) were seen
only in animals at > 100 mg/kg bw and included decreased testicular
weight at 400 mg/kg bw, dose-dependent increases in seminiferous
tubular atrophy, and increases in the number of reproductive tracts
containing occluded efferent ductules (Hess et al., 1991).
(e) Developmental toxicity
Mice
Groups of 20-25 pregnant CD-1 mice were given benomyl by gavage
at 0, 50, 100, or 200 mg/kg bw per day on days 7-17 of gestation. They
were killed on day 18, their pups were delivered by caesarean section,
the numbers of live, dead, and recorded fetuses were determined, and
the fetuses were examined for gross anomalies (one-half for visceral
abnormalities and the other half for skeletal abnormalities). Maternal
indexes were unaffected by treatment, but fetal mortality was
increased, fetal weight was decreased, and fetal development was
adversely affected by treatment. The high dose increased the
supraoccipital score, decreased the numbers of caudal and sternal
ossifications, and increased the incidences of enlarged lateral
ventricles and enlarged renal pelvises. The latter increase, while not
significant at the lowest dose, was dose-related at the other doses.
The occurrence of supernumerary ribs and subnormal vertebral centra
was significantly increased in a dose-related manner at all doses. The
numbers of abnormal litters and fetuses were significantly increased
over those among the controls at 100 and 200 mg/kg bw per day. Major
anomalies included exencephaly, hydrocephaly, cleft palate,
hydronephrosis, polydactyly, oligodactyly, umbilical hernia, fused
ribs, fused vertebrae, and short or kinky tail. Although benomyl
induced dose-related fetotoxicity at all levels, it was not
teratogenic at 50 mg/kg bw per day in mice (Kavlock et al., 1982).
In a study designed to investigate whether teratogens could be
detected by observing postnatal viability or impaired growth, groups
of 24 pregnant CD-1 mice were treated orally with benomyl at 0 or
200 mg/kg bw per day on days 8-12 of gestation. Postnatal viability
was reduced on days 1 and 3, and average pup weight was reduced on day
1 post partum (Chernoff & Kavlock, 1983).
Rats
Groups of 26-28 pregnant Sprague-Dawley rats were given benomyl
(purity, 53.5%) in the diet at 0, 100, 500, 2500, or 5000 ppm on days
6-15 of gestation to provide average doses equivalent to 0, 8.6, 43.5,
209.5, and 372.9 mg/kg bw per day. On day 20 of gestation, all
pregnant animals were sacrificed and their fetuses were delivered by
caesarean section. There were no deaths attributable to treatment, no
clinical signs of toxicity, and no adverse effects on the body weights
of dams. Dams at 5000 ppm had reduced food intake during treatment,
but consumption returned to control levels for the remainder of the
study. Indicators of reproductive health (numbers of implantation
sites, resorption sites, and live and dead fetuses) were not affected
by treatment up to and including the highest dietary level. The only
external or internal abnormalities reported were hydronephrosis and
retarded ossification of the interparietal and occipital bones in
three litters at the highest dose. None of these teratogenic effects
was associated with the administration of benomyl during the critical
period of organogenesis (Sherman et al., 1975).
Groups of 27-28 pregnant Wistar rats were fed benomyl in the diet
at 0, 1690, 3380, or 6760 ppm, equivalent to 0, 169, 298, or 505 mg/kg
bw per day, on days 7-16 of gestation. Fetuses were delivered by
caesarean section on day 21, weighed, examined grossly, and fixed for
evaluation of visceral and skeletal abnormalities. Food consumption
was decreased in dams at 3380 or 6760 ppm, with resultant decreases in
body weight gain, which were significant at the high dose. The weight
gain of fetuses at the two higher doses was significantly reduced, and
a significant decrease in the ossification of the supraoccipital bone
was seen at the high dose. The percentage of fetuses with an enlarged
renal pelvis was increased at the two higher doses. No dose-related
major malformations were seen at these dietary levels (Kavlock
et al., 1982)
Benomyl (99.2% pure) was administered by gavage to pregnant
Sprague-Dawley rats at doses of 0, 3, 10, 30, 62.5, or 125 mg/kg bw
per day on days 7-16 of gestation. A control group receiving corn oil
comprised 60 rats, and each test group comprised 27 rats. No clinical
signs of toxicity or mortality were observed at any dose. Body weight
gain, the incidences of pregnancy, corpora lutea, and implantation
sites, and the sex ratio were comparable to those of controls;
however, fetal body weight was significantly decreased at 62.5 and
125 mg/kg bw. There was also an increased incidence of embryofetal
mortality at 125 mg/kg bw. At doses > 10 mg/kg bw per day, some
fetuses had external and visceral malformations, which included
microphthalmia, anophthalmia, and hydrocephaly (distended lateral
ventricles), predominantly at the higher doses. Histological
examination of the eyes revealed pathological changes consisting of
irregular lenses, retrobulbar glandular adnexa, distorted or
compressed retinal layers, and thickened nerve fibres in the groups
receiving 10, 62.5, or 125 mg/kg bw per day; no alterations were seen
in controls or in animals at 30 mg/kg bw per day. The major skeletal
malformations observed at 125 mg/kg bw per day included fused ribs,
sternebrae, and thoracic arches. Additional skeletal variations seen
at 62.5 and 125 mg/kg bw per day included misaligned and unossified
sternebrae and bipartite vertebral centra. The authors concluded that
benomyl produced teratogenic responses in rats at doses > 10 mg/kg
bw per day. Microphthalmia seen at this dose was reported to be
related to treatment on the basis of the severity of the pathological
changes and the finding that the increased incidences at 62.5 and
125 mg/kg bw per day were not a direct reflection of reduced fetal
body weight. There was no evidence of maternal toxicity, even at the
highest dose (Staples, 1980).
Groups of pregnant Sprague-Dawley rats were given benomyl (99.1%
pure) by gavage at 0, 3, 6.25, 10, 20, 30, or 62.5 mg/kg bw per day on
days 7-16 of gestation. Each group consisted of 50 animals, except the
high-dose group, which comprised 20 rats. As the study was performed
to determine a no-effect level for microphthalmia and hydrocephaly,
only fetal heads were fixed and examined. Microphthalmia was
determined on the basis of the smallest eye in the control group
(< 1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two cases of malformation, both at
62.5 mg/kg bw per day: One fetus had internal hydrocephaly, and one in
another litter had unilateral microphthalmia. The only type of
variation reported was subcutaneous haematoma, which was not dose-
related. There was no teratogenic response at 30 mg/kg bw per day
(Staples, 1982).
On the basis of these two studies, the Meeting concluded that the
NOAEL for teratogenicity was 30 mg/kg bw per day, as the anophthalmia
at 10 mg/kg bw per day in the first study was not found in the second
study, which was specifically designed to look for this anomaly.
Furthermore, anophthalmia was not seen at 30 mg/kg bw per day in the
first study, and its occurrence at 10 mg/kg bw per day was not
accompanied by other evidence of teratogenic response.
The teratogenic potential of benomyl was examined in groups of
12-30 Wistar rats given oral doses of 0, 15.6, 31.2, 62.5, or
12.5 mg/kg bw per day on days 7-16 of gestation. Pups were delivered
by caesarean section on day 21 of gestation. The weight gain of the
dams at the highest dose was decreased on days 17-20 of gestation, and
the frequency of fetal resorption was increased, six litters being
completely resorbed. Fetal weight was also adversely affected, with
significant decreases at 62.5 and 125 mg/kg bw per day, and there was
a significant increase in fetal mortality at 125 mg/kg bw per day.
Several skeletal and visceral variants were observed among fetuses at
62.5 and 125 mg/kg bw per day, including increased supraoccipital
score, decreased numbers of sternal and caudal vertebrae, and
increased percentages of enlarged lateral ventricles and enlarged
renal pelvis. Major anomalies, observed primarily at > 62.5 mg/kg
bw per day, included encephaloceles, hydrocephaly, microphthalmia,
fused vertebrae, and fused ribs. The dose of 31.2 mg/kg bw per day
appeared to have no adverse effects on developing rat fetuses (Kavlock
et al., 1982).
Groups of pregnant Sprague-Dawley rats were treated orally with
benomyl at 0 or 62.5 mg/kg bw per day on days 7-16 (eight rats) or
7-20 of gestation (13 rats). There were no overt signs of toxicity.
Hydrocephaly was noted in 35% of fetuses examined on day 16 and in 76%
of fetuses examined on day 20 (Ellis et al., 1987, 1988).
In other studies, benomyl produced ocular and craniocerebral
malformations in Sprague-Dawley rats when administered by gavage at
doses of 31.2 or 62.4 mg/kg bw per day on days 7-21 of gestation.
Ocular anomalies (retinal dysplasia, cataracts, microphthalmia, and
anophthalmia) occurred in 43.3% of the fetuses of dams given the
higher dose. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose of
benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
consisting primarily of hydrocephaly occurred in the fetuses of dams
given 31.2 mg/kg bw per day in combination with the semipurified diet
(Zeman et al., 1986).
Rabbits
Groups of 15 artificially inseminated New Zealand albino rabbits
were fed a benomyl formulation (containing 50% benomyl) in the diet at
0, 100, or 500 ppm on days 8-16 of gestation. Mortality, clinical
parameters, and food consumption were determined daily, and body
weights were measured weekly. There were 12 pregnant does in the
control group, 13 in the low-dose group, and 9 in the high-dose group.
Of these, 6, 7 and 5, respectively, were sacrificed on day 29 or 30
and their fetuses were delivered by caesarean section; the remaining
does gave birth normally. No maternal toxicity was observed at any
dose. Except for a marginal increase in the frequency of rudimentary
ribs in animals at 500 ppm, no developmental toxicity was observed
(Busey, 1968d); however, the numbers of litters and of fetuses
examined were less than adequate to assess the fetotoxic or
teratogenic potential of benomyl.
Groups of 20 female New Zealand white female rabbits, presumed to
be pregnant, were given oral doses of 0, 15, 30, 90, or 180 mg/kg bw
per day benomyl (as a suspension in 0.5% methylcellulose) on days 7-28
of gestation. The animals were killed on day 29. The results of
analyses of the concentration, homogeneity, and stability of the
suspensions indicated acceptable conformity with nominal content. Only
one animal at 90 and one at 180 mg/kg bw per day did not become
pregnant. There were seven deaths before terminal sacrifice -- one
control, three treated with 30 mg/kg bw per day, one treated with
90 mg/kg bw per day, and two treated with 180 mg/kg bw per day -- all
of which were due to injuries associated with dosing. At 180 mg/kg bw
per day, an increased incidence of stained tails was seen, and food
consumption was significantly reduced, but there were no treatment-
related effects on maternal body weight. Five animals aborted during
the study: one in each of the groups treated with 0, 15, and 90 mg/kg
bw per day and two at 180 mg/kg bw per day. As the historical control
data showed no more than one abortion in a control group of 20 does,
the Meeting concluded that benomyl at 180 mg/kg bw per day had an
adverse effect. There were no other treatment-related effects on
reproductive parameters. Fetal mortality and fetal weights were not
affected by treatment, and there were no treatment-related external
malformations. At 180 mg/kg bw per day, two viable fetuses in two
litters had small renal papillae. The Meeting concluded that this
finding was treatment-related in view of the finding of a single
incidence in 11 studies in historical controls. The NOAEL for maternal
toxicity was 90 mg/kg bw per day, on the basis of reduced food
consumption, abortion, and clinical signs of toxicity at 180 mg/kg bw
per day. The NOAEL for developmental toxicity was also 90 mg/kg bw per
day, on the basis of the occurrence of small renal papillae at
180 mg/kg bw per day (Munley, 1995).
(f) Genotoxicity
Numerous studies have been reported in the open literature on the
mutagenic potential of benomyl, its metabolite carbendazim, and
several benomyl formulations. Many of the results are conflicting, and
few of the publications provide sufficient detail to evaluate the
reasons for the conflicts. Table 2 covers only those studies in which
sufficient experimental detail and data were reported.
Benomyl does not cause gene mutation or structural chromosomal
aberrations in somatic or germ cells, and it does not interact
directly with DNA in either mammalian or non-mammalian systems. Gene
mutations and structural chromosomal aberrations observed in mammalian
cells in vitro in several studies appear to have been the
consequence of the inherent sensitivity of some mammalian test systems
to cytotoxic agents. No gene mutations or structural chromosomal
aberrations were seen in mammals in vivo. Benomyl causes numerical
chromosomal aberrations (aneuploidy and/or polyploidy) both in vitro
and in vivo. Studies involving kinetochore staining indicated,
however, that benomyl is not clastogenic.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Application for 24 h of doses > 0.5 g per animal of a 50%
wettable powder to the occluded, clipped, intact and abraded abdominal
skin of albino rabbits produced moderate to marked erythema, slight
oedema, and slight desquamation. Albino guinea-pigs similarly ex posed
to 10, 25, or 40% dilutions of technical-grade benomyl in dimethyl
phthalate had only mild irritation of intact and abraded skin (Majut,
1966; Busey, 1968a; Colburn, 1969; Frank, 1969). No dermal irritation
was observed 4 or 24 h after application of 0.5 g of Benlate 50 DF
(containing 50% benomyl) to six male New Zealand white rabbits. After
48 h, two rabbits had slight to mild erythema, which was still evident
after 72 h. The primary irritation scores ranged from 0 to 1 (not an
irritant) (Vick & Brock, 1987).
Draize scores were assessed 4 and 72 h after application of
benomyl (purity, 98%) to a shaved area of the back of four albino
rabbits at 5 mg/cm2. The lesions produced were classified as 'mild
irritation' (Desi, 1979).
The ocular irritating properties of technical-grade benomyl, a
50% wettable powder, and a suspension in mineral oil were examined in
albino rabbits in several tests. Mild conjunctival irritation and
minor transitory corneal opacity were reported after 48 to 96 h in all
tests (Reinke, 1966; Frank, 1972). Similar results were obtained with
Table 2. Results of tests for the genotoxicity of benomyl
End-point Test system Concentration Purity Results Reference
or dose (%)
Tests for chromosomal effects
Mitotic gene conversiona S. cerevisiae, A. nidulans < 3200 mg/ml Technical Negative DeBertoldi et al.
(1980)
Nondisjunction/crossing- A. nidulans < 2.8 mmol/litre Technical Negative, but DeBertoldi & Griselli
over spindle inhibition (1980)
observed
Sister chromatid exchangea Chinese hamster ovary > 150 mg/ml Technical Weakly positive Evans & Mitchell
cells (1980)
Chromosomal aberrationa Human lymphocytes < 2000 mg/ml 50% WP Negative Gupta & Legator
(1975)
Chromosomal aberrationa Human lymphocytes < 100 mg/ml Technical Negative Pilinskaya (1983)
Chromosomal aberrationa Chinese hamster lung cells < 90 mg/ml Technical Positive Sasaki (1988)
(98.7%)
Chromosomal effects Human/mouse monochromosomal < 15 mg/ml Technical Positive: aneuploidy Athwal & Sandhu (1985)
hybrid cells and polyploidy
(R3-5) (1.5 mg/ml threshold)
Chromosomal effects V79/AP4 Chinese hamster NR Technical Dose-related increase Rainaldi et al. (1989)
cells in numerical
chromosomal
aberrations
Chromosomal effects Chinese hamster ovary cells NR Technical Dose-related increase Eastmond & Tucker
in numerical (1989)
chromosomal
aberrations
Chromosomal effects Human lymphocytes NR Technical Dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations
(0.1 mg/ml threshold)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Chromosomal effects Chinese hamster/human NR Technical Dose-related increase Zelesco et al. (1990)
hybrid cells (EUBI) in numerical
chromosomal
aberrations
(2.0 mg/ml threshold)
Tests for gene mutation
Reverse mutationa S. typhimurium TA98, < 325 mg/plate Technical Doubtful mutagenic Rashid & Ercegovitch
TA 100, TA 1535, TA 1537, activity (1976)
TA 1538
Reverse mutationa S. typhimurium TA 1535, < 1 mg/ml 50% WP Positive Kappas et al. (1976)
TA1538, E. coil WP2,
WP2 uvra, CM 611
Reverse mutationa S. typhimurium TA98, < 1000 mg/ml Technical Negative Shirasu et al. (1978)
TA100, TA1535, TA1537,
TA1538, E. coil WP2 he;
Reverse mutationa S .typhimurium TA 1530, < 10 000 mg/ml Technical, Negative Fiscor et al. (1978)
TA1535, TA1950 50% WP
Reverse mutationa S. typhimurium TA 1530, < 10 000 mg/ml Technical, Negative DuPont de Nemours
TA1535, TA 1950 50% WP & Co. (1977)
Reverse mutationa S. typhimurium TA98, < 250 mg/plate Technical Negative Donovan & Krahn
TA 100, TA 1535, TA1537 (99.9%) (1981)
Reverse mutationa S. typhimurium TA98. < 10000 Technical Negative Rickard (1983a,b)
TA100, TA1535. TA1537 mg/plate (99%)
Reverse mutationa S. typhimurium TA98, < 500 mg/plate Technical Equivocal (positive Russell (1978a,b)
TA 100, TA 1535. TA 1537 in TA 1537 with
activation)
Reverse mutationa S. typhimurium TA 15~35, < 500 mg/disc Technical Negative Carere et al. (1978)
TA1536, TA 1537, TA1538,
Streptococcus coelicodor
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Reverse mutationa A. nidulans < 0.4 mg/ml Technical Positive Kappas et al. (1974);
Kappas & Bridges
(1981)
hprt mutationa Chinese hamster ovary < 172 mmol/litre Technical Negative Fitzpatrick (1980)
cells (99,9%)
tk mutation L5178Y mouse < 20 mmol/litre Technical Negative Amacher et al. (1979)
lymphoma cells
tk mutationa L5178Y mouse < 25 mmol/litre Technical Positive without McCooey et al.
lymphoma cells (991%) activation: negative (1983a, b)
with activation
Tests for DNA damage and repair
Unscheduled DNA Rat and mouse < 500 mg/ml Technical Negative Tong (1981)
synthesis hepatocytes
Rec assay B. subtilis 2000 mg/plate Technical Negative Shirasu et al. (1978)
Tests in vivo
Host-mediated mutation S. typhimurium 2000 mg/kg bw Technical Negative Shirasu et al. (1978)
in mice G46 his-
Host mediated mutation S. typhimurium 3 × 500 mg/kg bw 50% WP Negative Fiscor et al. (1978)
in mice G46 his-
Dominant lethal mutation Rat < 5000 ppm in 50% WP Negative Culik & Gibson
food for 7 days (1914)
Dominant lethal mutation Rat NR Technical Negative Sherman et al. (1975)
Dominant lethal mutation Rat < 203 ppm in Technical Negative Barnes et al. (1983)
food for 70 days
Dominant lethal mutation Rat < 50 mg/kg bw Technical Negative Georgieva et al.
per day for 70 days (1990)
Table 2. (Con't)
End-point Test system Concentration Purity Results Reference
or dose (%)
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Positive Seller (1976)
bw
Micronucleus formation Mouse < 2 × 1000 mg/kg Technical Equivocal Kirkhart (1980)
bw
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive Sasaki (1990)
bw (95%)
Micronucleus formation Mouse < 1 × 5000 mg/kg Technical Positive, KC+, Bentley (1992)
with KC+ staining bw (96.1-97.4%) benomyl classified
as aneugen
Chromosomal aberration Mouse bone marrow < 1 × 5000 mg/kg Technical Negative Stahl (1990)
bw (961%)
Chromosomal aberration Peripheral blood NR 50% WP Negative Ruzicska et al. (1976)
from workers
Chromosomal aberration Rat bone marrow < 500 mg/kg bw, 50% WP Bone marrow, Ruzicska et al. (1976)
and cultured embryo days 7-14 of negative; embryo
cells gestation cells, positive
Sex-linked recessive Drosophila melanogester 1.5 mg/ml Technical, Negative, but Lamb & Lilly (1980)
lethal mutation 50% WP sterility consistent
with observed
spindle effects
NR, not reported; 50% WP, wettable powder formulation; KC, kinetochore
a With and without metabolic activation
Benlate PNW, a 50% wettable powder (Gargus & Zoetis, 1983b,c). The
Draize score for ocular irritation induced by 5 mg of pure benomyl in
four albino rabbits indicates that it is a mild irritant (Desi, 1979).
Albino guinea-pigs exposed to technical-grade benomyl or a 50%
sucrose formulation had mild to moderate skin erythema during the
challenge phase after either intradermal injection or repeated
applications to abraded skin (Majut, 1966; Colburn, 1969; Frank,
1969).
Ten albino Hartley guinea-pigs received four weekly injections of
Benlate PNW (50% benomyl prepared as a 0.1% solution in saline), and
10 animals were injected with saline; 14 days after the final
injection, 8 or 80% Benlate PNW was used for challenge. An unequivocal
increase in sensitization was seen at two of 10 sites challenged with
the 8% suspension and at seven of 10 sites challenged with the 80%
suspension (Gargus & Zoetis, 1984).
Technical-grade benomyl sensitized all 10 guinea-pigs tested in a
maximization test (Matsushita & Aovama, 1981).
(ii) Neurotoxicity
Studies in hens given single oral doses of < 5000 mg/kg bw did
not indicate neurotoxic potential (Goldenthal, 1978; Jessup, 1979;
Jessup & Dean, 1979).
Studies in groups of 10 adult male CFY rats showed no alteration
in electroencephalographic potential or in behaviour (learning ability
assessed in a four-choice T-maze) in comparison with controls after
treatment with benomyl at 250 or 500 mg/kg bw per day for three months
(Desi, 1983).
Groups of 10 male and 10 female Sprague-Dawley rats received
single oral doses of 0, 500, 1000, or 2000 mg/kg bw benomyl (97.4%
pure). Functional and motor activity were assessed during the week
before the day of treatment and at 2 h, one day, seven days, and 14
days after dosing. Rats were then killed and subjected to a gross
post-mortem examination. Histopathological examination was restricted
to tissues from the central and peripheral nervous system of six rats
in the control and high-dose groups. There were no treatment-related
deaths during the study. Immediately after treatment, body weight gain
and food intake were decreased in all treated groups; however, overall
weight gain and food consumption were unaffected by treatment. In the
battery of functional tests, a small number of animals had soft or
liquid faeces and fur stained with urine or faeces on days 1 and 2,
but no other component of the battery, including assessments of other
autonomic functions, reactivity, and sensitivity, excitability, gait,
and sensorimotor coordination, forelimb and hindlimb grip strength,
abnormal clinical signs and body temperature, showed any reaction to
treatment. Motor activity was reduced, only on the day of treatment,
in females given 2000 mg/kg bw. Neuropathological observation revealed
no treatment-related abnormalities. It was concluded that benomyl
induced no lasting neurotoxicity at doses up to 2000 mg/kg bw (Foss
1993).
Groups of 11 male and 11 female Sprague-Dawley rats received
benomyl (purity, 97.4%) in the diet at 0, 100, 2500, or 7500 ppm for
90 days. Functional and motor activity were assessed during the week
before treatment and during weeks 4, 8, and 13. Rats were then killed
and subjected to gross post-mortem examination. Histopathological
examination was restricted to tissues from the central and peripheral
nervous system of six rats in the control and high-dose groups. There
were no treatment-related deaths during the study. Body weight gain
and food intake were reduced at the high dose throughout the treatment
period. The battery of functional tests showed no reaction to
treatment with benomyl. Motor activity was increased at 7500 ppm.
Neuropathological observation revealed no treatment-related
abnormalities. It was concluded that benomyl is not a specific
neurotoxicant, since the changes in motor activity occurred at the
same dietary level as other general toxicological changes (Foss,
1994).
3. Observations in humans
(a) Exposure during field use
Potential dermal and respiratory exposure to benomyl during
actual use was determined during mixing for aerial application,
re-entry into treated fields, and home use (garden, ornamental, and
greenhouse). Maximal exposure occurred during loading and mixing for
aerial application, with dermal exposure to 26 mg per mixing cycle,
primarily (90%) on the hands and forearms; the average respiratory
intake was 0.08 mg benomyl. During re-entry, the dermal exposure was
5.9 mg/h and the respiratory exposure < 0.002 mg/h. Home use resulted
in exposures of 1 mg per application cycle dermally and 0.003 mg by
the respiratory route. This study did not address reactions to
exposure (Everhart & Holt, 1982).
The potential dermal and respiratory exposure of workers mixing
and applying 20 and 100 gallons of Benlate per acre (224 and
1123 litres/ha) while spraying fruit orchards was examined. The
application cycle was about 70 min and resulted in a total dermal
exposure of 11 mg of benomyl per cycle and a total respiratory
exposure to 15 mg. Essentially all of the exposure was dermal,
resulting in 12.2 mg per cycle dermally and < 0.05 mg per cycle via
the respiratory route (DuPont de Nemours & Co., undated).
(b) Medical surveillance of workers
Benomyl caused contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et at., 1985).
In controlled patch tests of 200 agricultural, ex-agricultural, and
non-agricultural workers, only one agricultural worker showed contact
dermatitis to 0.1% benomyl (Lisi et al., 1986). In a survey of
cross-sensitization with benomyl and other pesticides in a group of
126 Japanese farmers who applied benomyl to their crops, 39 had
positive test results with benomyl, the highest incidence being found
among female farmers. There were cross-reactions between benomyl and
diazinon, saturn, daconil, and Z-bordeaux (Matsushita & Aovama, 1981).
Selected blood profiles of 50 factory workers involved in the
manufacture of Benlate were compared with those of a control group of
48 workers who were not exposed to Benlate. White blood count, red
blood count, haemoglobin, and haematocrit values were comparable in
the two groups. No quantitative estimates of exposure were given for
the factory workers (DuPont de Nemours & Co., 1979).
A survey was performed to determine whether potential exposure
to Benlate had an adverse effect on the fertility of 298 male
manufacturing workers exposed between 1970 and 1977. The ages of the
workers ranged from 19 to 64 years; 79% of the workers and 78% of
their spouses were aged 20-39. The duration of exposure ranged from
less than one month to 95 months, but more than 51% of the workers had
potentially been exposed for one to five months. The birth rates of
the spouses of the exposed workers were compared with those of four
populations from the same county, state, region, and country (United
States). There was no reduction in fertility, and the birth rates of
the study population were generally higher than those of the
comparison populations. Spermatogenesis was not examined (Gooch,
1978).
Comments
Benomyl is readily absorbed by animals after oral exposure and
rapidly metabolized. It is eliminated in the faeces and excreted in
the urine; 98% of the dose was excreted by 72 h after administration.
The tissue distribution showed no bioconcentration. In rats, the
metabolites carbendazim and methyl 5-hydroxybenzimidazol-2-ylcarbamate
(5-hydroxy-carbendazim) were found in the blood and in small amounts
in the testis and liver. The latter compound was the main metabolite
in urine. A 50% wettable powder formulation was poorly absorbed via
the dermal route by rats. After a 10-h exposure, less than 2% of a
single dose of 0.2 mg was excreted in the urine.
Benomyl has low acute toxicity, with an oral LD50 in rats of
> 10 000 mg/kg bw. The clinical signs of toxicity after high single
doses were generally nonspecific. Testicular degeneration, with
necrosis of germinal epithelium and aspermatogenesis, has been
observed after single doses in rats (> 100 mg/kg bw orally) and dogs
(1.65 mg/litre air by inhalation). Wettable powder formulations
containing benomyl have been shown to be mildly irritating to rabbit
skin and eyes and have induced skin sensitization reactions in
maximization tests. WHO has classified benomyl as unlikely to present
an acute hazard in normal use.
In a 90-day study, rats were given dietary doses of 0, 100, 500,
or 2500 ppm benomyl. Increased liver weight was seen at 2500 ppm; the
NOAEL was 500 ppm (50 mg/kg bw per day). Dogs were given dietary doses
of 0, 100, 500, or 2500 ppm benomyl for three months or two years and
rabbits were treated dermally, five days per week for three weeks,
with 0, 50, 250, 500, 1000, or 5000 mg/kg bw per day. Hepatotoxicity
was seen in the dogs but not in the rabbits; effects on male
reproductive organs were seen in both rabbits and dogs. The NOAEL was
500 ppm (equal to 13 mg/kg bw per day) in dogs and 500 mg/kg bw per
day in rabbits.
In a two-year study, benomyl was administered in the diet to rats
at 0, 100, 500, or 2500 ppm. Benomyl was not carcinogenic and showed
no compound-related effects at dietary levels up to and including
2500 ppm (equal to 109 mg/kg bw per day). In a two-year feeding study
in mice at dietary levels of 0, 500, 1500, or 5000 ppm, benomyl
caused liver tumours, and an NOAEL could not be established for
hepatocellular neoplasms. Male mice had decreased testicular weights
and thymic atrophy at 5000 ppm. The lowest dietary level was equal to
64 mg/kg bw per day.
In rats treated by gavage for 62 days with 45 mg/kg bw per day,
decreased testicular and epididymal weights, reduced caudal sperm
reserves, and decreased sperm production, with generalized disruption
of all stages of spermatogenesis were observed. After mating with
untreated females, no effect was seen on reproductive behaviour,
weight of the seminal vesicles, sperm mobility, or related
reproductive hormones. The NOAEL was 15 mg/kg bw per day. A lowering
of male fertility rates has been reported, but this effect was not
seen consistently. A single dose of 100 mg/kg bw or more administered
to rats by gavage had effects 70 days after exposure, which included
decreased testicular weight and atrophy of the seminiferous tubules.
The NOAEL was 50 mg/kg bw per day. In a recent study of reproductive
toxicity, rats received dietary doses of 0, 100, 500, 3000, or
10 000 ppm benomyl. The NOAEL was 500 ppm (equivalent to 37 mg/kg bw
per day), on the basis of effects on pup survival and pup growth and
on testicular changes. Fertility indices were not affected at dietary
levels up to 10 000 ppm.
In a study of developmental toxicity, mice were exposed by gavage
to benomyl at doses of 0, 50, 100, or 200 mg/kg bw per day on days
7-17 of gestation. There was no indication of maternal toxicity, but
benomyl was teratogenic at doses of 100 and 200 mg/kg bw per day and
fetotoxic at 50 mg/kg bw per day. The major abnormalities included
hydrocephaly, cleft palate, and limb defects. In studies of
teratogenicity, pregnant rats were exposed to benomyl at doses up to
and including 125 mg/kg bw per day on days 7-16 of gestation.
Benomyl was teratogenic, the major effects being microphthalmia and
hydrocephaly. The Meeting concluded that the NOAELs in rats were
30 mg/kg bw per day for teratogenicity and fetotoxicity and 125 mg/kg
bw per day for maternal toxicity. In rabbits, benomyl was not
teratogenic at doses up to 180 mg/kg bw per day (the highest dose
tested), and no effect was seen on maternal toxicity or fetotoxicity
at 90 mg/kg bw per day.
Benomyl has been adequately tested for genotoxicity in a range of
assays. The Meeting concluded that it does not directly damage genetic
material but does cause numerical chromosomal changes both in vitro
and in vivo as a result of its interference with mitotic spindle
proteins.
In a study of workers exposed to benomyl, there was no reduction
in fertility, as indicated by birth rates, among the study population.
Spermatogenesis in the workers was not examined. Cases of dermal
sensitization to benomyl have been reported.
An ADI of 0-0.1 mg/kg bw was established on the basis of the
NOAEL of 13 mg/kg bw per day in the two-year study in dogs and
applying a safety factor of 100. This ADI should be used when
assessing exposure to benomyl itself. Since the use of benomyl on
crops gives rise to residues of carbendazim and since the ADI for
carbendazim is lower than that which would be derived from the data on
benomyl, the Meeting concluded that the intake of residues in food
should be compared with the ADI of 0-0.03 mg/kg bw for carbendazim.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: < 500 ppm, equal to < 64 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
50 mg/kg bw per day (study of developmental toxicity)
< 50 mg/kg bw per day (fetotoxicity in a study of
teratogenicity)
Rat: 2500 ppm, equal to 109 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
500 ppm, equivalent to 37 mg/kg bw per day (study of
reproductive toxicity)
30 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
125 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Rabbit: 180 mg/kg bw per day (study of developmental toxicity)
90 mg/kg bw per day (maternal toxicity and fetotoxicity in
study of developmental toxicity)
Dog: 500 ppm, equal to 13 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw (benomyl)
0-0.03 mg/kg bw (carbendazim, with which residues of benomyl in
food should be compared)
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to benomyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 >10 000 mg/kg bw
Dermal, toxicity, rabbit LD50 (50% wettable powder)
> 10 000 mg/kg bw
Dermal, irritation, rabbit 50% wettable powder; irritating
Ocular, irritation, rabbit 50% wettable powder; irritating
Dermal, sensitization, guinea-pig Positive in maximization test
Inhalation, toxicity, rat LC50 (50% wettable powder)
> 4.01 mg/litre air
Mid-term (1-26 weeks) Oral, 62 days, rat NOAEL = 15 mg/kg bw per day;
reduced spermatogenesis
Oral, developmental toxicity, rat NOAEL = 30 mg/kg bw per day;
fetotoxicity and teratogenicity
Long-term (> one year) Dietary two years, toxicity, dog NOAEL = 13 mg/kg bw per day;
hepatotoxicity
See also toxicological criteria for carbendazim when considering dietary exposure to residues
References
Amacher, D.E., Paillet, S. & Ray, V.A. (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells:
Application to genetic toxicological testing. Mutat. Res., 64,
391-406.
Athwal, R.S. & Sandhu, S.S. (1985) Use of human x mouse hybrid cell
line to detect aneuploidy induced by environmental chemicals.
Mutat. Res., 149, 73-81.
Axness, M.E. & Fleeker, J.R. (1979) Metabolism of the butylcarbamoyl
moiety of benomyl in rat. Pestic. Biochem. Physiol., 11, 1-12.
Barbo, E.C. & Carroll, K.S. (1972) Oral ALD test ( S-triazine,
3-butylbenzimidazolo[1,2-a]-2,4[1 H,3 H]dione. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Barnes, T. B., Verlangieri, A.J. & Wilson, M.C. (1983) Reproductive
toxicity of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate
(benomyl) in male Wistar rats. Toxicology, 28, 103-115.
Belasco, I.J. (1979a) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied. Unpublished
report from DuPont de Nemours & Co., Biochemical Department,
Research Division, Experimental Station, Wilmington, Delaware,
USA.
Belasco I.J. (1979b) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Unpublished report from DuPont de Nemours & Co., Biochemical
Department, Research Division, Experimental Station, Wilmington,
Delaware, USA
Belasco, I.J., Kirkland, J.J., Pease, H.L. & Sherman, H (1969) Studies
with 2-14C-labelled methyl-1-(butylcarbamoyl)-2-
benzimidazolecarbamate (benomyl) in rats. Unpublished report from
DuPont de Nemours & Co., Biochemical Department, Research
Division, Experimental Station, Wilmington, Delaware, USA.
Bentley, K S. (1992) Classification of DPX-T1991-529 (Benomyl)-induced
micronuclei in mouse bone marrow erythrocytes using immuno-
fluorescence antikinetochore antibodies. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Brock, W.J. (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Unpublished report from DuPont de Nemours &
Co, Haskell Laboratory, Newark, Delaware, USA.
Busey, W.M. (1968a) Acute dermal LD50 test and dermal irritation
test on rabbits using a wettable powder formulation (50% benomyl)
with histological addendum. Unpublished report from Hazelton
Laboratories, Inc., Falls Church, Virginia, USA, prepared for
DuPont de Nemours & Co.
Busey, W.M. (1968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968c) Repeated dermal application test on rabbits using
a wettable powder formulation (50% benomyl). Unpublished report
from Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Busey, W.M. (1968d) Teratology study in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from
Hazelton Laboratories, Inc., Falls Church, Virginia, USA,
prepared for DuPont de Nemours & Co.
Carere, A., Ortali, V.A., Cardamone, G., Torracca, A.M. & Raschetti,
R. (1978) Microbiological mutagenicity studies of pesticides in
vitro. Mutat. Res., 57, 277-826.
Carter, S.D. (1982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Thesis, North Carolina State
University, Raleigh, North Carolina, USA.
Carter, S.D. & Laskey, J.W. (1982) Effect of benomyl on reproduction
in the male rat. Toxicol. Lett., 11, 87-94.
Chernoff, N. & Kavlock, R.J. (1983) A teratology test system which
utilises post natal growth and viability in the mouse. Environ.
Sci. Res., 27, 417-427.
Colburn, C.W. (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Culik, R. (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the
concepti of rats exposed to benomyl and Benlate by diet.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC), 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Culik, R. and Gibson, J.R. (1974) 'Benlate' dominant lethal study in
male rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Dashiell, O.L. (1972) Acute oral test (benzimidazole, 2-(3-
butylureido)). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
De Bertoldi, M. & Griselli, M. (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat. Res., 74,
303-324.
De Bertoldi, M., Griselli. M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by metres of gene-conversion
in Saccharomyces cerevisiae and in Aspergillus nidulans.
Environ. Mutag., 2, 359-370.
Desi, I. (1979) Hygienic-toxicological evaluations of pesticides.
D.Sc. Thesis, Budapest, National Institute of Hygiene.
Desi, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5, 503-515.
Donovan, S.D. & Krahn, D.F. (1981) Mutagenic evaluation in
Salmonella typhimurium. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1977) Mutagenic activity of benomyl in the
Salmonella/microsome assay (50% benomyl as a wettable pow der
formulation). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (1979) Benlate dust exposure survey -- blood
profile analysis. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
DuPont de Nemours & Co. (undated) Applicator exposure during filling
and spraying of Benlate benomyl fungicide -- orchard crops.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Eastmond, D.A. & Tucker, J.D. (1989) Kinetochore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat. Res., 224, 517-525.
Ellis W.G., Semple, J.L., Hoogenboom, E.R., Kavlock, R.J. & Zeman,
E.J. (1987) Benomyl-induced craniocerebral anomalies in fetuses
of adequately nourished and protein-deprived rats. Teratog.
Carcinog. Mutag., 7, 357-375.
Ellis W.G., De Roos, F., Kavlock, R.J. & Zeman, F.J. (1988)
Relationship of periventricular overgrowth to hydrocephalus in
brains of fetal rats exposed to benomyl. Teratog. Carcinog.
Mutag., 8, 377-391.
Evans, E.L. & Mitchell, A.D. (1980) An evaluation of the effect of
benomyl on sister chromatid exchange frequencies in cultured
Chinese hamster ovary cells. Unpublished report from SRI
International, Menlo Park, California, USA, prepared for DuPont
de Nemours & Co.
Everhart, L.P. & Holt, R.F. (1982) Potential Benlate fungicide
exposure during mixer/loader operations, crop harvest and home
use. J. Agric. Food Chem., 30, 222-227.
Fiscor, G., Bordas, S. & Stewart, S.J. (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl- N'-nitro- N-nitrosoguanine in Salmonella
typhimurium in vitro and in rodent host-mediated assays.
Mutat. Res., 51, 151-164.
Fitzpatrick, K. (1980) Chinese hamster ovary cell assay for
mutagenicity. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Foss J.A (1993) Acute neurotoxicity study of DPX-T1991-529 (Benomyl)
administered orally by gavage to Crl:CD BR VAF/Plus rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Foss, J.A. (1994) Subchronic neurotoxicity study of DPX-T1991-529
(Benomyl) administered orally via the diet to Crl:CD BR VAF/Plus
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Frank, K.M. (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Fritz, S.B. & Sherman, H. (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Gardiner, J.A., Kirkland, J.J., Klopping, H.L. & Sherman, H. (1974)
Fate of benomyl in animals. J. Agric. Chem., 22, 419-427.
Gargus, J.L. & Zoetis, T. (1983a) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines -- Benlate PNW).
Unpublished report from Hazelton Laboratories Inc., Vienna,
Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983b) Primary irritation study in rabbits
(Benlate PNW). Unpublished report from Hazelton Laboratories
Inc., Vienna, Virginia, USA, prepared for DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1983c) Eye irritation test in rabbits (EPA
pesticide registration Benlate PNW). Unpublished report from
Hazelton Laboratories Inc., Vienna, Virginia, USA, prepared for
DuPont de Nemours & Co.
Gargus, J.L. & Zoetis, T. (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Unpublished
report from Hazelton Laboratories Inc., Vienna, Virginia, USA,
prepared for DuPont de Nemours & Co.
Georgieva, V., Vachkova, R., Tzoneva, M. & Kappas, A. (1990) Genotoxic
activity of benomyl in different test systems. Environ. Mol.
Mutag., 16, 32-36.
Goldenthal, E.I. (1978) Neurotoxicity study in hens using technical
benomyl (< 95% benomyl). Unpublished report from International
Research and Development Corporation, Mattawan, Michigan, USA,
prepared for DuPont de Nemours & Co.
Gooch, J.J. (1978) Fertility of workers potentially exposed to
benomyl. Unpublished report from DuPont de Nemours & Co.,
Wilmington, Delaware, USA.
Goodman, N.C. (1975) Intraperitoneal LD50 test in rats. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Guengerich, F.P. (1981) Enzyme induction with DuPont compounds H11,
202-02 and H10, 962-02. Unpublished report from Vanderbilt
University, School of Medicine, Nashville, Tennessee, USA,
prepared for DuPont de Nemours & Co.
Gupta, A.K. & Legator, M.S. (1975) Chromosome aberrations in cultured
human lymphocytes after treatment with fungicide 'Benlate'. In:
Proceedings of the Symposium on Mutagenicity, Carcinogenicity
and Teratogenicity of Chemicals, New Delhi, Department of
Atomic Energy, pp. 95-103
Han, J.C.Y. (1979) 2-14C-Benomyl (50% WP) rat study -- intravenous
injection. Unpublished report from DuPont de Nemours & Co.,
Biochemical Department, Research Division, Experimental Station,
Wilmington, Delaware, USA.
Han, J.C.Y. (undated) Metabolism of 14C-labelled benomyl in the
mouse and hamster. Unpublished report from DuPont de Nemours &
Co., Biochemical Department, Research Division, Experimental
Station, Wilmington, Delaware, USA
Hess, R.A., Moore, B.J., Forrer, J., Linder, R.E. & Abuel-Atta, A.A.
(1991) The fungicide benomyl [(methyl)1-(butylcarbomyl)-2-
benzimidazole carbamate] causes testicular dysfunction by
inducing the sloughing of germ cells and occlusion of efferent
ductules. Fundam. Appl. Toxicol., 17, 733-745.
Hood, D.B. (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Hoogenboom, E.R., Ransdell, J.F., Ellis, W.G., Kavlock, R.J. & Zeman,
E.J. (1991) Effects on the fetal rat eye of maternal benomyl
exposure and protein malnutrition. Curr. Eye Res., 10, 601-612.
Hornberger, C.S. (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Hostetler, K.H. (1977) Oral LD50 test (Benlate OD). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Jessup, D.C. (1979) Acute delayed neurotoxicity study in chickens
using technical benomyl (> 95% benomyl). Unpublished report from
International Research and Development Corporation, Mattawan,
Michigan, USA, prepared for DuPont de Nemours & Co.
Jessup, D.C. & Dean, W. (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl).
Unpublished report from International Research and Development
Corporation, Mattawan, Michigan, USA, prepared for DuPont de
Nemours & Co.
van Joost, T.H., Naafs, B. & van Ketel, W G. (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9, 153-154.
Kappas, A. & Bridges, B.A. (1981) Induction of point mutations by
benomyl in DNA-repair deficient Aspergillus nidulans. Mutat.
Res., 91, 115-118.
Kappas, A., Georgopoulos, S G. & Hastie, A.C. (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat. Res., 26, 17-27.
Kappas, A., Green, M.H.L., Bridges, B.A., Rogers, A.M. & Muriel, W.J.
(1976) Benomyl -- a novel type of base analogue mutagen? Mutat.
Res., 40, 379-382.
Kavlock, R.J., Chernoff, N., Gray, L.E., Gray, J A & Whitehouse, D.
(1982) Teratogenic effects of benomyl in the Wistar rat and CD-1
mouse, with emphasis on the route of administration. Toxicol.
Appl. Pharmacol., 62, 44-54.
Kirkhart, B. (1980) Micronucleus test on benomyl (> 95% benomyl).
Unpublished report from SRI International, Menlo Park,
California, USA. prepared for DuPont de Nemours & Co.
Krupka R.M. (1974) On the anti-cholinesterase activity of benomyl.
Pestic. Sci., 5, 211-216.
Kuelme, G., Heise, H., Plottke, B. & Puskeller, T (1985) Dermatitis
after Benlate contact. Z. Ges. Hyg. Grenzgeb., 31, 710-711.
Lamb, M.J. & Lilly, L.J. (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17,
83-95.
Lee K.P. (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Linder, R.E., Rehnberg, G.L., Strader, L.F. & Diggs, J.P. (1988)
Evaluation of reproductive parameters in adult male Wistar rats
after subchronic exposure. J. Toxicol. Environ. Health, 25,
285-298.
Lisi, P., Caraffini, S. & Assalve, D. (1986) A test series for
pesticide dermatitis. Contact Derm., 15, 266-269.
Littlefield, N.A. & Busey, W.M. (1969) Four-hour acute inhalation
exposure test in dogs using a wettable powder formulation (50%
benomyl). Unpublished report from Hazleton Laboratories, Inc.,
Falls Church, Virginia, USA, prepared for DuPont de Nemours & Co.
Majut, J.C. (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Matsushita, T. & Aovama, K. (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind.
Health, 19, 77-83.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983a) L5178Y
mouse lymphoma cell assay for mutagenicity (benomyl). Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
McCooey, K.T., Arce, G.T., Sarrif, A.M. & Krahn, D.F. (1983b) L5178Y
mouse lymphoma cell assay for mutagenicity. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Mebus, C.A. (1990) Reproductive and fertility effects with
DPX-1991-529 (benomyl). Multigeneration reproduction study in
rats. Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Monson, K.D. (1990) Metabolism of [phenyl(U)14C]carbendazim in rats.
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Munley, S.M. (1995) Developmental toxicity study of DPX-T1991-529
(Benomyl) in rabbits. Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Pilinskaya, M.A. (1.983) Investigation of the cytogenetic action of
the pesticides captan and benomyl in a culture of human
peripheral blood lymphocytes in the absence and presence of a
system of metabolic activation. Cytol. Genet., 17, 29-33
Rainaldi, G., Flori, L., Colella, C.M, Mariani, T., Piras, A., Simi,
S. & Simili, M. (1989) Analysis by BrUdR-labelling technique of
induced aneuploidy in mammalian cells in culture. Mutat. Res.,
177, 255-260.
Rashid, K.A. & Ercegovitch, C.D. (1976) New laboratory tests evaluate
chemicals for cancer or gene damage. Sci. Agric., 23, 7.
Reinke, RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Rickard, L.B. (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Russell, J.F. (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/
microsome assay. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Ruzicska, P., Peter, S., Laczi, J. & Czeizel, E. (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP Egeszegtudomany, 20,
74-83.
Sarver, J.W. (1987) Acute oral toxicity study with IN-T1991 in male
and female rats. Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sasaki, Y.F.X. (1988) Benomyl: In vitro cytogenetics test. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Sasaki, Y.F.X. (1990) Benomyl: Micronucleus test in mice. Unpublished
report from Kodaira Laboratories, Institute of Environmental
Toxicology, Tokyo, Japan, prepared for DuPont de Nemours & Co.
Seiler, J.P. (1976) The mutagenicity of benzimidazole and
benzimidazole derivatives, VI: Cytogenetic effects of
benzimidazole derivatives in the bone marrow of the mouse and the
Chinese hamster. Mutat. Res., 40, 339-348.
Sherman, H. (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Unpublished report from DuPont
de Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Unpublished report from DuPont de
Nemours & Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Sherman, H. & Krauss, W.C. (1966) Acute oral test [benomyl].
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Sherman, H., Barnes, J.R. & Krauss, W.C. (1967) Ninety-day feeding
study with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl
ester (INT-1991). Unpublished report from DuPont de Nemours &
Co., Haskell Laboratory, Newark, Delaware, USA.
Sherman, H., Culik, R. & Jackson, R.A. (1975) Reproduction,
teratogenic and mutagenic studies with benomyl. Toxicol. Appl.
Pharmacol., 32, 305-315.
Shirasu, Y., Moriva, M. & Kato, K. (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Unpublished report from
Kodaira Laboratories, Institute of Environmental Toxicology,
Tokyo, Japan, prepared for DuPont de Nemours & Co.
Shukla, Y., Antony, M. & Mehrota, N.K. (1989) Studies on
gamma-glutamyl transpeptidase in rodents exposed to benomyl.
Bull. Environ. Contam. Toxicol., 42, 301-306.
Snee, D.A. (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test). Unpublished. report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Stahl, R.G., Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Unpublished report
from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Staples, R.E. (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl).
Unpublished report from DuPont de Nemours & Co., Haskell
Laboratory, Newark, Delaware, USA.
Staples, R.E. (1982) Teratogenicity study in the rat using technical
benomyl (> 95% benomyl) administered by gavage. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Tong, C. (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Unpublished report from Naylor Dana Institute, Valhalla, New
York, USA, prepared for DuPont de Nemours & Co.
Turney, R.T. (1979) Rat inhalation study -- Benlate. Unpublished
report from DuPont de Nemours & Co., Haskell Laboratory, Newark,
Delaware, USA.
Vick, D.A. & Brock, W.J. (1987) Primary dermal irritation study with
Benlate 50 DF fungicide in rabbits. Unpublished report from
DuPont de Nemours & Co., Haskell Laboratory, Newark, Delaware,
USA.
Ward, R.S. & Scott, R.C. (1992) Benomyl: in vitro absorption of a
500 g.kg-1 WP formulation through human epidermis. Unpublished
report from Imperial Chemical Industries (ICI), Fernhurst,
Haslemere, Surrey, United Kingdom.
Warheit, D.B., Kelly, D.P., Carakostas M.C. & Singer, A.W. (1989) A
90-day inhalation toxicity study with benomyl in rats. Fundam.
Appl. Toxicol., 12, 333-345.
Wiechman, B.E. (1982) Long term feeding study with methyl
1-(butylcarbamoyl)-2-benzimidazole carbamate in mice (INT-1991;
> 95% benomyl). Unpublished report from DuPont de Nemours & Co.,
Haskell Laboratory, Newark, Delaware, USA.
Zelesco, P.A., Barbieri, I. & Graves, J.A.M. (1990) Use of a cell
hybrid test system to demonstrate that benomyl induces aneuploidy
and polyploidy. Mutat. Res., 242, 329-335.
Zeman, E.J., Hoogenboom, E.R., Kavlock, R.I. & Semple, J.L. (1986)
Effects on the fetus of maternal benomyl exposure in the
protein-deprived rat. J. Toxicol. Environ. Health, 17, 405-417.
