
THIOPHANATE-METHYL JMPR 1973
IDENTITY
Chemical name
1.2-alpha-(3-methoxycarbonyl-2-thioureido)benzene.
Synonyms
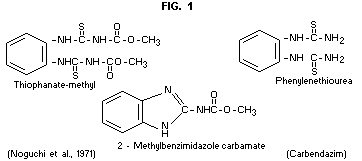
Dimethyl 4.4'-o-phenylene bis(3-thioallophanate)
Topsin-methylR, Cercobin-MethyR, NF 44.
Topsin - MR, Cercobin MR, MildothaneR, Pelt 44R,
Enovit MR, NeotopsinR
Structural formula
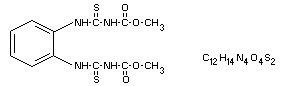 Other information on identity and properties
Molecular weight: 342.40
State: Crystalline solid, colourless
Melting point: 17°C decomposed
Solubility: Chloroform 2.62% (w/w)
(at 21°C) Methanol 2.92% "
Acetone 5.81% "
Ethyl acetate 1.19% "
Acetonitrile 2.44% "
Cyclohexane 4.30% "
Slightly soluble in n-Hexane and water.
Stability: Stable in acidic solutions. At pH = 7 slight,
but measurable formation of methyl
benzimidazol-2-ylcarbamate (MBC). Unstable in
alkaline solution.
Purity of
technical material: Purity 96.1%
Sulfur 1.0%
Sodium chloride 1.5%
Loss on drying 0.5%
Other components 0.9%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
The potential metabolites of thiophanate-methyl described on the
basis of chemical reactivity are as follows:
Other information on identity and properties
Molecular weight: 342.40
State: Crystalline solid, colourless
Melting point: 17°C decomposed
Solubility: Chloroform 2.62% (w/w)
(at 21°C) Methanol 2.92% "
Acetone 5.81% "
Ethyl acetate 1.19% "
Acetonitrile 2.44% "
Cyclohexane 4.30% "
Slightly soluble in n-Hexane and water.
Stability: Stable in acidic solutions. At pH = 7 slight,
but measurable formation of methyl
benzimidazol-2-ylcarbamate (MBC). Unstable in
alkaline solution.
Purity of
technical material: Purity 96.1%
Sulfur 1.0%
Sodium chloride 1.5%
Loss on drying 0.5%
Other components 0.9%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
The potential metabolites of thiophanate-methyl described on the
basis of chemical reactivity are as follows:
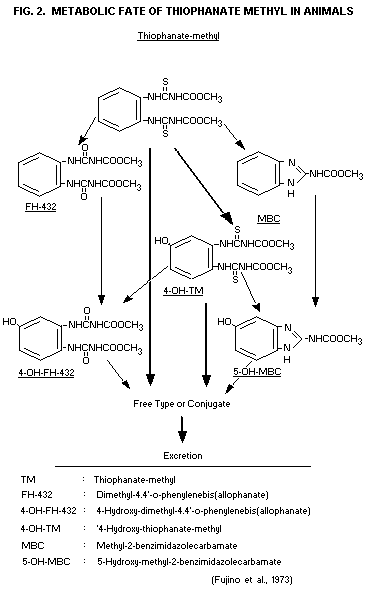 In vitro transformation of thiophanate methyl to methyl
benzimidazole carbamate, was dependent on pH and temperature (Fuchs et
al., 1972).
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973) 14C- or 35S-labelled thiophanate-methyl were fed to rats, mice
and dogs. 80-100% of the administered amounts were recovered in faeces
and urine within 96 hours after administration of the compound. With
faeces 60%, 16-27% and 14% respectively were excreted for the three
species, while urinary excretion accounted for 30%, 66-78% and 74%,
respectively, of the administered thiophanate-methyl. Fujino et al.
(1973) have suggested the metabolism scheme shown in Fig. 2. They
report that the major part of faecal excretion was in the form of
unmetabolized thiophanate-methyl, while the minor parts consisted of
4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-O-phenylenebisallophanate (FH-432). Methyl
2-benzimidazol carbamate (carbendazim) and 5-hydroxy-MBC (5-OH-MBC)
were also observed during TLC identifications of metabolites of faecal
extracts. It was, however, questioned whether these two were actual
metabolites in faeces or if they were compounds produced during the
analytical procedures from thiophanate-methyl and 4-OH-TM,
respectively.
Thiophanate-methyl and a number of metabolites could be liberated
enzymatically or by acid treatment from water soluble conjugates in
rat urine in the same studies by Fujino et al. (1973). Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (Fig. 2). As was the case in faeces, the two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical procedures.
In the earlier study by Noguchi et al. (1971) of the conversion
of thiophanate methyl by rat liver microsomes, MBC and 5-OH-MBC were
also found to be present. Evidence for enzyme induction could not be
attained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Although the presence of phenylene-thiourea has been suggested
there is no evidence from the studies in rats, mice and dogs that this
metabolite actually is formed.
Thiophanate-methyl has been reported to be reasonably unstable on
leaf surfaces with the majority of material degrading to MBC and the
oxygen analogue of thiophanate. In contrast to animal studies where
these two metabolites appear to be of minor significance they appear
to be significant metabolites in plants (Soeda et al., 1972a and b).
In aqueous solution following irradiation by UV or sunlight MBC was
the only metabolite found (Buchenauer et al., 1973).
The hazard to the environment associated with the use of
thiophanate-methyl appears to be minimal. It is unstable in soil
degrading within one week and appears to be relatively selective in
that levels of up to 1000 ppm had no effect on a soil population of
bacteria, fungi and actinomycetides (Noguchi, 1972).
Effects on enzymes and other biochemical parameters
Male rats administered 1000 mg/kg orally were examined for
cholinesterase activity in blood and brain four hours following
dosing. No significant change in erythrocyte, serum or brain
cholinesterase was observed over the period (Hashimoto et al., 1972b).
TOXICOLOGICAL STUDIES
Acute toxicity
Signs of poisoning include tremors 1-2 hours after high level
exposure which lead to tonic or clonic convulsions. Nose bleeding and
lacrimation were observed in rats. A slight decrease in respiratory
rate, lethargy, disappearance of tonus of abdominal muscle, discharge
from the eye and mydriasis were observed in rabbits and dogs
(Hashimoto of al., 1972a).
Species Route LD50 (mg/kg) References
Rat M oral 7 500 Hashimoto et al.1972a;
M ip 1 640 Noguchi of al., 1970a
F oral 6 640 "
F ip 1 140 "
M & F dermal >10 000 "
Mice M oral 3 510 "
M ip 790 "
F oral 3 400 "
F ip 1 110 "
M & F dermal >10 000 "
Guinea-pig M oral 3 640 "
F oral 6 700 "
(cont'd)
Species Route LD50 (mg/kg) References
Rabbit M 2 270 "
F 2 500 "
Dog M 4 000 "
F 4 000 "
Japanese
Quail M & F >5 000 "
Contact phototoxicity and photosensitivity
Thiophanate-methyl applied to the shaved skin of rabbits and
guinea-pigs was exposed to erythrogenic and nonerythrogenic light.
There was no evidence of phototoxicity in either species.
Thiophanate-methyl was topically applied to the shaved skin of
guinea-pigs four times over a nine-day period and three weeks later.
After exposure the area was exposed to erythrogenic and
nonerythrogenic radiation. No positive photosensitivity reactions were
noted (Noguchi and Hashimoto, 1971; Hashimoto et al., 1972).
Subacute dermal toxicity
Groups of guinea-pigs (five per group) were administered
thiophanate-methyl to the abraded dorsum daily for 30 days at a dosage
level of 0, 2, 20 and 200 mg/kg. No evidence of dermal toxicity or
irritation was noted (Noguchi and Hashimoto, 1972).
Inhalation toxicity
Groups of 10 male mice were exposed to formulations of
thiophanate-methyl for periods of time of 30, 60, 120 minutes at a
concentration of 100 mg/litre through an aerosol generator. There were
no deaths in any group including the controls although such findings
as lacrimation, salivation and nasal discharge were observed soon
after exposure. All animals appeared normal within 24 hours following
exposure (Hashimoto et al., 1972a).
Dermal irritation
Rabbits were dermally administered thiophanate-methyl at levels
of 1, 0.1, and 0.01 g daily for 21 days. A slight reversible erythema
was observed in the highest dose group. No skin irritation was evident
in the two lower dose groups (Noguchi and Hashimoto, 1970a; Hashimoto
et al., 1972a).
Cutaneous sensitization
Guinea-pigs were examined for cutaneous sensitization by
intradermal injection of 50 µl and 100 µl at a 1% aqueous solution on
alternate days for 20 days. Two weeks later the guinea-pigs were
challenged with 50 µl injected into the same area. Animals treated
with thiophanate-methyl exhibited no primary irritation and only a
slightly sensitive state (Noguchi and Hashimoto, 1970a; Hashimoto et
al., 1972a).
Pharmacological properties
Thiophanate-methyl administered to rats at dosages up to 1500
mg/kg caused a slight increase in body temperature. Mice administered
500 mg/kg subcutaneously appeared to have a slight transient
insensitivity to heat. Studies with mice and rats administered
thiophanate-methyl at levels of 100 or 500 mg/kg subcutaneously showed
neither a sedative nor hypnotic effect. Oral administration of 1000
mg/kg to mice resulted in no significant mydriatic effect. No surface
anaesthetic action or irritation of the mucous membrane of the eye of
rabbit was observed following the administration of up to 10%
thiophanate-methyl in saline instilled into the conjunctival sac of
rabbits. Studies on excised mouse intestine at levels of up to 1 X
10-3 mg/ml, resulted in no atropine-like or papaverine-like activity.
The same concentrations did not inhibit the contraction of excised
guinea-pig intestine by histamine hydrochloride or of an excised strip
of guinea-pig aorta by epinephrine. After a single oral dose of
thiophanate-methyl of 100 mg/kg, the blood pressure of rat did not
change. Following high oral administration of thiophanate-methyl to
rabbits, there was a slight transitory decrease in white blood cell
count. In a similar manner, the white cell count of rabbits was also
depressed within a short period of time following acute oral
administration of 1000 mg/kg.
A temporary fall in blood pressure in the rabbit, followed by a
persistent rise and a persistent bradycardia was observed following
i.v. or oral administration of 1000 mg/kg. There was no change in
respiration or ECG following acute oral administration. Lethal doses
of thiophanate-methyl showed an immediate blood pressure drop to zero,
gradual disturbance of the ECG followed by cessation of respiration.
Except at very high levels there was no unusual pharmacological
properties attributed to thiophanate-methyl. All adverse
pharmacological properties were completely reversible within a short
period of time (Hashimoto of al., 1972b; Noguchi and Hashimoto,
1970e).
Special studies on carcinogenicity
Mouse. A carcinogenicity study was performed on mice of the ICR-SCL
strain.
Groups of mice (50 males and 50 females per group) were fed
thiophanate-methyl in the diet at levels of 0, 10, 40, 160 and 640 ppm
for 24 months. A sample of thiophanate-methyl used in this study was
approximately 94% active ingredient with impurities of 2% sulfur and
2% inorganic chloride, 2% unknown volatile substances and less than
0.5% aromatic amines. Animals in this study were SPF-derived and were
maintained under barrier-conditions to minimize infection. Diets were
prepared at an institute outside of the laboratory performing the
study and food was allotted bi-weekly during which time food
consumption data were recorded. Body weights were recorded initially
and monthly throughout the study with daily mortality and behavioural
examinations. Pathological examinations, using H&E stains primarily,
were performed on all animals which died or were sacrificed prior to
the conclusion of the study. After 105 weeks all animals were
sacrificed and histological examinations were performed on a variety
of tissues.
There was a slight retardation of growth in males at 640 ppm in
the diet although this was not reflected in females. Food consumption
was comparable to that of controls. Mortality and average survival
time over 105 weeks was the same in controls and at all dietary
levels. A variety of tumours was found in all groups including the
controls. All hepatomas observed in the experiment were benign liver
cell adenomas except for three liver cell derived adenocarcinomas (1
female at 40 ppm and one male and one female at 160 ppm). The
incidence of hepatoma was similar in all of the experimental groups
including the controls. Pulmonary benign adenomas were also
significantly observed in all cases with no differences in the control
or any feeding group. Leukaemia was also observed frequently with a 20
to 30% incidence in each group. In consequence, there was essentially
no difference in occurrence of tumours between experimental and
control groups. The results of this study indicate that the ingestion
of thiophanate-methyl in the diet in levels up to 640 ppm to a mouse
strain which appears to be significantly susceptible to various
tumours results in no carcinogenic activity related to
thiophanate-methyl feeding (Kosaka and Tsubura, 1973).
Special studies on cytogenicity
Male rats were administered thiophanate-methyl daily for five
days by intraperitoneal injection at levels of 0, 62.5, 125, 250, 500
and 1000 mg/kg/day. Bone marrow and spermatogonial cells examined
showed no abnormal chromogomal configurations (Makita et al., 1973).
Special studies on mutagenicity
Groups of 10 males ICR-strain mice were administered
thiophanate-methyl intraperitoneally at a single dose level of 0, 8,
40, 200, 400 or 500 mg/kg and mated with virgin females which were
replaced weekly for a period of eight weeks. At a dosage of 400 mg/kg
and above there appeared to be a reduction in the incidence of
pregnancies. However, there was no systematic variation indicative of
a mutagenic potential over the entire eight-week mating period (Makita
et al., 1973).
Special studies on reproduction
Groups of Charles-River rats (10 males and 20 females per group)
were fed levels of thiophanate-methyl in the diet at 0, 40, 160, 640
ppm in a three-generation, two-litters per generation reproduction
study. There were no apparent effects of thiophanate-methyl at levels
up to and including 640 ppm on any of the reproduction parameters
measured in this experiment. In addition, gross and histological
examinations of the F3b generation were performed on several tissues
and organs of three-week-old rats and a further group of animals was
examined for skeletal abnormalities. In no instance was there any
effect of feeding thiophanate-methyl on reproduction. There was a
definitive effect on the growth of animals fed dietary levels of 640
ppm (Palmer et al., 1972).
Special studies on teratogenicity
Thiophanate-methyl was administered to pregnant ICR mice from day
1 to day 15 of gestation at levels of 0, 40, 200, 500 and 1000
mg/kg/day. At 1000 mg/kg/day there was a significantly reduced number
of living fetuses. No (significant) differences in the number of
implantation sites, body weight of fetuses or fetal mortality or body
weight were observed at the lower dosage levels. The administration of
thiophanate-methyl did not produce gross internal or external
abnormalities and the study did not reveal any teratogenic properties
under these experimental conditions (Noguchi and Hashimoto, 1970a;
Makita et al., 1973).
Short-term studies
Mouse. Groups of six-week-old SPFICR-strain mice (12 males and 12
females per group) were fed thiophanate-methyl at levels of 0, 12.8,
64, 390, 1600 and 8000 ppm in the diet for six months. Daily
observations were made on their behaviour and mortality and body
weight and food consumption data were recorded weekly. Haematological
and gross and microscopic examination of tissues and organs were
performed at the end of the feeding period.
There were no unusual behavioural patterns and incidence of
mortality was not related to the feeding of thiophanate. Growth of all
mice at levels of 1600 ppm and below was not affected by
thiophanate-methyl with a retardation of growth observed at 8000 ppm.
Food intake and haematological examinations were normal. Gross
pathology at the termination of the experiment showed that there was
an increase in the mean weight of liver in animals fed 8000 ppm with
an increase in liver to body weight ratio. This was noted in both
males and females. There were no effects of this kind noted at a dose
level of 1600 ppm. Pathological abnormalities were noted in liver at
8000 ppm in both males and females. There was swelling of large
hepatic cells with concurrent swelling. It was observed that the
protoplasm of these cells were oedema-like or granule-like. Although
such pathological abnormalities were found in some mice of other
groups as well as the control, it appeared to be more prevalent in the
8000 ppm animals. In the present experiment the no-effect level of
thiophanate-methyl is considered to be 1600 ppm (Noguchi and
Hashimoto, 1970b).
Groups of SPF-Sprague Dawley rats (12 males and 12 females per
group) were fed thiophanate-methyl in the diet at levels of 0, 12.8,
64, 320, 1600, 8000 ppm for six months under SPF conditions. Daily
checks of mortality and abnormal behaviour were made throughout the
experiment with body weight and food intake data recorded weekly. At
the conclusion of the study, haematology, clinical chemistry and
urinalyses were performed. Gross and microscopic examination of
tissues and organs was also performed at the conclusion of the feeding
period.
There were no behavioural abnormalities observed during the
six-month feeding study and mortality was not related to food intake
of thiophanate-methyl, Examination of growth curves indicated a severe
retardation of both males and females fed 8000 ppm in the diet. There
did not appear to be any retardation in growth of animals fed 1600 ppm
or below. Food intake of all groups was comparable with control
values. All haematological values were comparable with controls as
were the values obtained from urine analysis. At 8000 ppm there was a
slight decrease in glucose and GOT levels and an increase in total
cholesterol. Gross and histological examination of tissues showed only
the liver and thyroid glands to be slightly affected by
thiophanate-methyl in the diet. There was an increase in liver weight
and liver to body weight ratio although there were no pathological
changes such as cloudy swelling and irregularity in the size of the
hepatic cells which was noted in the studies in mice. In the thyroid
gland some abnormalities such as small follicles, cubic epithelium
cells and a decrease in colloidal substance were observed in those
animals fed 8000 ppm. These changes were not observed in animals fed
1600 ppm and were not accompanied by any change in the PBI values.
Abnormal findings were not noted in any other tissues.
From these studies it appears that a no-effect level of 1600 ppm
in the diet of rats for six months would result in no unusual effect
(Noguchi and Hashimoto, 1970c).
Dog. Groups of pure bred beagle dogs (five males and five females
per group - four males and four females per group at the highest dose
level) were administered thiophanate-methyl at levels of 0, 2, 10, 30
and 250 mg/kg/day for two years. The animals were approximately six
months old at the time of the inception of the testing and were housed
singly in indoor kennels. In all groups one male and one female were
sacrificed at 12 months with the remaining animals sacrificed after 24
months on test. Dosing was performed by oral administration of
thiophanate-methyl by capsule once a day seven days a week for two
years. Doses were given to individual animals related to the body
weight and were adjusted weekly. Clinical signs of any abnormalities
were recorded daily. Food consumption and body weight changes were
recorded weekly. Throughout the study the eyes were examined for any
changes and electro cardiographs were taken periodically.
Haematological examinations, blood chemistry analyses and urinalyses
were performed periodically. At the conclusion of the study gross and
microscopic examination of tissues and organs was performed on all
animals.
There was no mortality or adverse behavioural changes observed in
these animals during the course of the study. Growth of the animals
fed 250 mg/kg/day was slightly retarded in both males and females.
There did not appear to be a significant decrease in any other group.
There did not appear to be any significant differences in the
haematological studies, blood chemistry, urinalysis, ophthalmological
examinations, or the ECG during the course of the 24-month study.
Pathological (gross and histological) examination of the animals
sacrificed at 12 months did not show any significant changes in the
tissues examined with regard to gross pathology and organ weight
ratios. Slight changes observed at 250 mg/kg in the thyroid gland were
difficult to determine from the one year data on dogs.. Animals
sacrificed at 24 months showed an elevated thyroid weight in both
males and females at 50 and 250 mg/kg although there were no changes
in the PHI values. Although the average weight of the thyroid of the
two highest dose groups was heavier than that of the controls,
histological examination showed no differences in the thyroid of the
control and the experimental groups. Histological findings in all
other tissues were also normal.
The no-effect level in this two-year study in dogs is considered
to be 50 mg/kg/day based upon the marginal effect noted in thyroid
weight at the conclusion of the study. Although this increased thyroid
weight was not reflected in the histological examination or PBI
values, it has been noted in other species (rat). A more noticeable
effect was observed at 250 mg/kg relating to retarded growth in both
males and females (Hashimoto and Fukuda, 1972).
Long-term studies
Rat (See also under the section: "Special studies on
carcinogenicity"). Groups of SPF-Sprague-Dawley strain rats (35 males
and 35 females per group, 50 males and 50 females were used as
controls) were fed thiophanate-methyl in the diet at levels of 0, 10,
40, 160, 640 ppm for two years.
The animals were maintained under SPF conditions. The sample of
thiophanate-methyl fed in this experiment was approximately 94% active
ingredient with impurities of 2% sulfur, 2% inorganic chlorides, 2%
unknown volatile substances and less than 0.5% aromatic amines. The
animals were fed dietary levels of thiophanate methyl for a period of
10 days during which behaviour and mortality were recorded daily and
after which food consumption was measured on all animals. Body weight
and growth were recorded weekly. Haematology, blood chemistry and
urinalyses were performed periodically over the two-year study. At the
conclusion of the study animals were sacrificed and gross and
microscopic examination of tissues and organs was performed.
There was no effect of feeding levels of up to 640 ppm of
thiophanate-methyl in the diet of rats on behaviour and mortality over
a two-year period. Growth curves indicate a slight retardation of
growth in both male and female rats in fed diets containing 640 ppm.
Food consumption at all dietary levels was comparable with the control
groups. Haematological data, urinalysis data and clinical blood
chemistry data were not abnormal in any of the groups examined. Gross
pathological examination of animals showed no changes in organ weight
in any group with the exception of slight increases in kidney weight
and kidney to body weight ratio, primarily in the males at the highest
level of feeding. There was no indication of increased thyroid weight
or an increase in liver size as was observed in shorter duration test
with rats. In addition there were frequent observations of
inflammation of the lung, fibroadenoma of the breast and the
occurrences of pituitary tumours. The administration of
thiophanate-methyl was not believed to be responsible for the
occurrence of these abnormalities. At the end of two years there was a
dose response in a degenerative change in the testis which underwent a
degeneration and atrophy including spermatogenesis. In this particular
dose-response change several cases were noted in other groups
including the control. This effect was probably not related to
ingestion of thiophanate-methyl. At the end of two years there was
also some change in the thyroid where colloidal epithelial cells were
enlarged. This enlargement took place primarily in the males of the
highest dose group. There was no apparent effect on the occurrence of
tumours and the only significant effects appear to be in the decreased
growth observed at 640 ppm in the diet.
Based upon retardation of growth a no-effect level would be
considered to be 160 ppm in the rat fed thiophanate-methyl for two
years (Hashimoto and Tsubura, 1972).
Observations in man
Sixteen workers concerned with the production of
thiophanate-methyl were examined periodically for 3.5 years. Blood and
urine analysis were carried out every six months. No adverse effects
were found with these workers with regard to blood chemistry and urine
analysis (Mori, 1972).
Comments
Thiophanate-methyl is degraded in plants to carbendazim and
hydroxylated derivatives. A question was raised if the apparent
occurrence of carbendazim in mammals may be an analytical artefact. A
theoretical metabolite of potential toxicological significance,
phenylene thiourea, has not been reported in plant or animal systems.
Thiophanate-methyl has a low acute toxicity in various species of
animal and shows pharmacological activity only at very high levels.
Adequate short-term and long-term studies are available in both
rats and dogs. The results of a three-generation reproduction study,
mutagenesis studies and a cytogenicity study were negative.
Thiophanate-methyl did not appear to be a carcinogen in a susceptible
strain of mice and two-year studies in rats and dogs gave rise to
retardation of growth only at high dietary levels (640 ppm). There was
a marginal increase in thyroid weight at higher dose levels which was
not accompanied by adverse biochemical or histological effects. The
long-term study in the rat was used as a basis for estimating an ADI
for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 160 ppm in the diet equivalent to 23 mg/kg bw
Rat: 160 ppm in the diet equivalent to 8 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of an acceptable daily intake for man
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Thiophanate-methyl was first registered for use as a systemic
fungicide in Japan in May 1971. By 1973 it has obtained provisional
clearance for safe use in Great Britain for a number of specified
preharvest treatments on food crops and ornamentals and for
post-harvest dipping of apples.
Thiophanate-methyl is formulated into wettable powders containing
50 and 70% active ingredient.
Recommended use levels are from 0.02-0.07% a.i. usually applied
at the rate of 1-5 kg a.i. per ha for pears, peaches and citrus and of
up to 2 kg a.i. per ha for other fruits and vegetables. Repeated
applications are claimed to be advantageous.
Thiophanate-methyl is reported to be very similar to benomyl
(BenlateR) as regards both its systemic functions (Aelbers, 1970)
and the rather broad spectrum of fungal diseases against which it is
effective (Bollen, 1971). It is claimed effective against apple and
pear scab, powdery mildew and different moulds, rots and blight.
Pre-harvest application on citrus trees should effectively reduce
Penicillium decay on citrus fruits under post-harvest storage.
The similarity between thiophanate-methyl and benomyl seems well
explained by the fact that both compounds are converted into methyl
2-benzimidazolecarbamate (MBC), which is reported to be the principal
fungitoxic compound to which both chemicals owe their major activity
(Vonk and Sijpesteijn, 1971; Selling et al., 1970).
Residues resulting from supervised trials
Data derived from extensive field trials in several countries on
thiophanate-methyl residues have been presented by the manufacturer
(Nippon Soda Company Ltd, 1973a). A summarized list of a selection of
the maximum residues found in these trials is presented in Table 1
(Nippon Soda Company Ltd, 1973b). The majority of information in
Table 1 shows residue levels at 0 or 1 day after last application.
Generally, the major part of residues is in the form of unchanged
thiophanate-methyl, while methyl 2-benzimidazolecarbamate (MBC) is
usually detectable in smaller amounts, averaging 10% of the total.
Apples, pears, peaches
Generally, higher levels of thiophanate-methyl residues are found
in these fruits after post-harvest treatments than after pre-harvest
sprayings. This is partly due to higher initial deposits from dipping
and partly connected to volatility losses to which the pre-harvest
treated fruits are subjected. During storage experiments performed in
the United Kingdom by the manufacturer, pears treated after harvest
TABLE 1. RESIDUES OF THIOPHAMATE-METHYL AND MBC IN VARIOUS CROPSa,b
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Apple 1.4 kg/ha 10× 1.4 - Fed. Rep. Ger.
0.6 g/l 1× 0.83 0.14 Canada
1-2 kg/ha 17×+
+1000 pp. Dipping 3.8 0.8 UK
2100 ppm Dipping 3.4 Italy
Banana 1000 pp. Dipping Peel: 0.3 0.4 Australia
Pulp: 0.1 <0.2
Beans, broad 2 kg/ha 1× 0.1 0.5 UK
" dwarf 1 kg/ha 1× 2.3 0.1 UK
" french 1.5 kg/ha 1× 0.2 0.2 US
" kidney 0.75 kg/ha 2× 0.2 Japan
" runner 1 kg/h. 1× 1.8 0.1 UK
Blackcurrant 5.4-7.5 kg/ha 4× 0.day:19.4 4.9 UK
5.4-7.5 kg/ha 4× 7.day:7.0 3.3 UK
1 kg/ha 4.5× 3.0 2.3 UK
Carrots 1000 ppm Dipping 5.0 0.2 UK
Celery low pp. Dipping 28.0 0.8 UK
Cherries 1.4 g/l 3× 2.0 0.2 Australia
2 kg/ha 5× 0.46 0.64 USA
Citrus 3.5 kg/ha 1× Peel: 12.2 2.1 Japan
Pulp: 0.3 <0.2
Cucumber 1 kg/ha 6× 0.45 0.09 Japan
Gherkins 1 g/l 1.87 0.33 Netherlands
Gooseberries 1 kg/ha 4× 3.0 0.4 UK
Grapes 1.4 kg/ha 5× 3.3 - Fed. Rep. Ger.
1.4 kg/ha 19× 2.8 Japan
2.4 g/l 6× 0.6 <0.2 Australia
Lettuce 1 kg/ha 3× 0.day:10.0 0.6 UK
7.day:0.1 <0.2
2.8 kg/ha 6× 0.day:24.5 5.0 Japan
7.day:3.8 1.5
2.2 kg/ha 1× 0.day:4.1 New Zealand
TABLE 1. (Cont'd.)
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Mushroom about 5 g/m2 1× <0.1 0.7 UK
Onion 2.5 kg/ha 10× 0.05 0.02 Japan
Orange 1000 ppm Dipping Peel: 1.2 <0.3 Australia
Pulp: 0.2 <0.3 Australia
Juice: 0.4 <0.2 Australia
1000 ppm Dipping Peel: 2.3 0.23 USA
2100 ppm Dipping 8.8 Italy
Peach 2.4 g/l 4× 5.0 0.4 Australia
Pear 1000 ppm Dipping 2.2 <0.1 UK
2100 ppm Dipping 3.9 Italy
Plum 1 kg/ha 6× 0.86 0.37 USA
Raspberries 1 kg/ha 8× 9.6 2.3 UK
Strawberries 1.5 kg/ha 1× 0.day:14.6 UK
7.day:3.0
1 kg/ha 4-5× 0.day:3.8 1.2 Netherlands
(under glass) 7.day:3.6 0.9
14.day:0.9 0.6
Sugarbeet 0.35 kg/ha 2× Leaf:3.6 Fed. Rep. Oar.
Root: <0.2
Wheat, winter 0.5 kg/ha 1× Straw: <0.2 (50 days)
Grain: <0.2
Tomato 2 kg/ha 5× 9.7 0.4 UK
a Residues reported are maximum residues, i.e. levels found on 0. or 1. day after application,
except when otherwise stated.
b Analytical methods used were as follows (see monograph text):
TLC-method: Australia, Japan, New Zealand
Colorimetry: Canada, UK, USA
UV-spectrophotometry: Japan, Federal Republic of Germany
Oscillopolarography: Italy, Netherlands (TLC-bioassay for MBC).
with thiophanate-methyl showed no pronounced decrease of residues
(about 2 ppm) during 61 days, while on the other hand, residues of
about 1 ppm on apples decreased well below 0.1 ppm during four weeks
on the trees after spraying.
Grapes and wine
Lemperle et al. (1973) found that thiophanate-methyl residues, as
well as those of other benzimidazole fungicides, are transferred
nearly quantitatively into unclarified musts during wine production.
During clarification and fermentation small amounts may disappear. A
considerable part of the thiophanate-methyl residues which are present
in grapes may therefore be found in wines.
Leafy vegetables
The residue data supplied by the manufacturer indicate that even
relatively high residues are subject to pronounced losses, which may
be explained as usual growth dilution effect to which volatility
losses from the foliar surfaces may be added. From field trials with
lettuce performed in Japan, half-life values of the order of 1-2 days
for thiophanate-methyl have been deducted, while the MBC-metabolite
present in smaller amounts disappeared more slowly, corresponding to
half-life values of 5-6 days.
For comparison, the half-life of thiophanate-methyl on apple and
grape leaves under controlled conditions was found to be in the order
of 15 and 12 days, respectively (Soeda et al., 1972).
Carrots, celery, sugarbeets
When subjected to post-harvest treatments with thiophanate-methyl
these root-crops take up considerable amounts of residues. Dipping of
carrots and celery performed in the United Kingdom with 0.1% a.i. gave
total residues of thiophanate-methyl + MBC of 5.2 and 28.8 ppm,
respectively. Pre-harvest sprays gave high, though rapidly
disappearing, residues on the green tops above soil, but no detectable
residues in the edible roots.
Fate of residues
Substantial evidence has been presented by the manufacturer that
methyl 2-benzimidazolecarbamate (MBC) is produced as the major
metabolite (Noguchi et al., 1971) although only gradually released
from thiophanate-methyl when applied on foliar surfaces (Kikuchi,
1973). Vonk and Sijpesteijn (1971) found that the fungitoxic activity
of thiophanate-methyl is increased by aging in aqueous media. They
concluded that MBC is responsible for the fungitoxic effect of the
fungicide and indicated that the rate of conversion from
thiophanate-methyl may be increased by the fungal metabolic activity.
Selling et al. (1970) also observed the conversion of
thiophanate-methyl to MBC in tap water. A 4% transformation was noted
when kept at pH = 7.0 in water for seven days, while
thiophanate-methyl remained stable in methanol or chloroform for 50
days at 24°C (Noguchi et al., 1971).
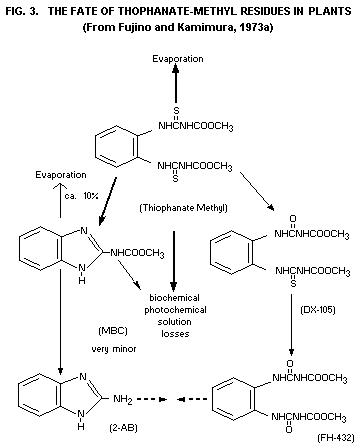
Plants
Conversion of thiophanate-methyl to MBC has been followed after
uptake through the roots and after foliar treatment of French bean
seedlings (Phaseolus vulgaris) by Noguchi et al. (1971). After 21 days
34.3% of the original amount had been converted on the leaves, while
87.5% was metabolized into MBC when absorbed through the roots in a
water culture. In a most recent publication, the formation of MBC from
thiophanate-methyl is further described by Buchenauer et al. (1973).
They found that the transformation to a great extent is
light-catalyzed (requiring at the same time the presence of water). A
42% transformation of thiophanate-methyl residues was found in cotton
leaves, when subjected to four days of sunlight as opposed to only
about 6%, when the plants were kept under dimlight conditions.
A minor metabolite, 2-aminobenzimidazole (2-AB), also formed from
MBC in benomyl treated plants is detected as a result of
thiophanate-methyl applications as well (Fujino, A. and Kamimura,
1973a). It is further reported by the same authors that two
oxygen-analogues (DX-105 = 1-(3-methyoxy-carbonyl-2
thioureido)-2-(3-methoxycarbonylureido) benzene and FH-132 =
dimethyl-4.4-O-phenylenbis(allophanate)) has recently been identified
by a reverse isotopedilution method (see Fig. 3).
Soil
Thiophanate-methyl is degraded almost entirely within seven days
in sandy loam and silty loam soils at temperatures from 23°C to 33°C
(Pujino and Kamumura, 1973b). MBC is identified as the major
metabolite, which is subsequently degraded at a more moderate rate.
Sixty days after treatment residual MBC was present at the level of
20% at 23°C and 2-15% at 33°C in both soil types, calculated on the
basis of the amount of thiophanate-methyl applied. No influence from
thiophanate-methyl was indicated in these experiments on soil
micro-organisms, when measured by bacterial counts (total bacteria,
gram-negative and actinomycetes). With increased levels of
thiophanate-methyl (up to 5000 ppm) increased oxygen uptake increased
significantly, suggesting that thiophanate-methyl was used as a source
of nitrogen by bacteriae.
The conversion of thiophanate-methyl to MBC is significantly more
rapid in unsterilized than in sterile soils. Similarly, the MBC formed
is less persistent in the unsterilized soil, which suggests that
micro-organisms may be involved in the degradation of both compounds.
TLC-identification patterns further indicate that
dimethyl-4.4'-o-phenyleneallophanate (or 111-432, see Fig. 3) may be
an intermediate metabolic step in the microflora-induced degradation,
as this compound is not found as a metabolite in sterilized soils
(Kosaka et al., 1972).
In vitro transformation of thiophanate methyl to methyl
benzimidazole carbamate, was dependent on pH and temperature (Fuchs et
al., 1972).
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973) 14C- or 35S-labelled thiophanate-methyl were fed to rats, mice
and dogs. 80-100% of the administered amounts were recovered in faeces
and urine within 96 hours after administration of the compound. With
faeces 60%, 16-27% and 14% respectively were excreted for the three
species, while urinary excretion accounted for 30%, 66-78% and 74%,
respectively, of the administered thiophanate-methyl. Fujino et al.
(1973) have suggested the metabolism scheme shown in Fig. 2. They
report that the major part of faecal excretion was in the form of
unmetabolized thiophanate-methyl, while the minor parts consisted of
4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-O-phenylenebisallophanate (FH-432). Methyl
2-benzimidazol carbamate (carbendazim) and 5-hydroxy-MBC (5-OH-MBC)
were also observed during TLC identifications of metabolites of faecal
extracts. It was, however, questioned whether these two were actual
metabolites in faeces or if they were compounds produced during the
analytical procedures from thiophanate-methyl and 4-OH-TM,
respectively.
Thiophanate-methyl and a number of metabolites could be liberated
enzymatically or by acid treatment from water soluble conjugates in
rat urine in the same studies by Fujino et al. (1973). Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (Fig. 2). As was the case in faeces, the two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical procedures.
In the earlier study by Noguchi et al. (1971) of the conversion
of thiophanate methyl by rat liver microsomes, MBC and 5-OH-MBC were
also found to be present. Evidence for enzyme induction could not be
attained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Although the presence of phenylene-thiourea has been suggested
there is no evidence from the studies in rats, mice and dogs that this
metabolite actually is formed.
Thiophanate-methyl has been reported to be reasonably unstable on
leaf surfaces with the majority of material degrading to MBC and the
oxygen analogue of thiophanate. In contrast to animal studies where
these two metabolites appear to be of minor significance they appear
to be significant metabolites in plants (Soeda et al., 1972a and b).
In aqueous solution following irradiation by UV or sunlight MBC was
the only metabolite found (Buchenauer et al., 1973).
The hazard to the environment associated with the use of
thiophanate-methyl appears to be minimal. It is unstable in soil
degrading within one week and appears to be relatively selective in
that levels of up to 1000 ppm had no effect on a soil population of
bacteria, fungi and actinomycetides (Noguchi, 1972).
Effects on enzymes and other biochemical parameters
Male rats administered 1000 mg/kg orally were examined for
cholinesterase activity in blood and brain four hours following
dosing. No significant change in erythrocyte, serum or brain
cholinesterase was observed over the period (Hashimoto et al., 1972b).
TOXICOLOGICAL STUDIES
Acute toxicity
Signs of poisoning include tremors 1-2 hours after high level
exposure which lead to tonic or clonic convulsions. Nose bleeding and
lacrimation were observed in rats. A slight decrease in respiratory
rate, lethargy, disappearance of tonus of abdominal muscle, discharge
from the eye and mydriasis were observed in rabbits and dogs
(Hashimoto of al., 1972a).
Species Route LD50 (mg/kg) References
Rat M oral 7 500 Hashimoto et al.1972a;
M ip 1 640 Noguchi of al., 1970a
F oral 6 640 "
F ip 1 140 "
M & F dermal >10 000 "
Mice M oral 3 510 "
M ip 790 "
F oral 3 400 "
F ip 1 110 "
M & F dermal >10 000 "
Guinea-pig M oral 3 640 "
F oral 6 700 "
(cont'd)
Species Route LD50 (mg/kg) References
Rabbit M 2 270 "
F 2 500 "
Dog M 4 000 "
F 4 000 "
Japanese
Quail M & F >5 000 "
Contact phototoxicity and photosensitivity
Thiophanate-methyl applied to the shaved skin of rabbits and
guinea-pigs was exposed to erythrogenic and nonerythrogenic light.
There was no evidence of phototoxicity in either species.
Thiophanate-methyl was topically applied to the shaved skin of
guinea-pigs four times over a nine-day period and three weeks later.
After exposure the area was exposed to erythrogenic and
nonerythrogenic radiation. No positive photosensitivity reactions were
noted (Noguchi and Hashimoto, 1971; Hashimoto et al., 1972).
Subacute dermal toxicity
Groups of guinea-pigs (five per group) were administered
thiophanate-methyl to the abraded dorsum daily for 30 days at a dosage
level of 0, 2, 20 and 200 mg/kg. No evidence of dermal toxicity or
irritation was noted (Noguchi and Hashimoto, 1972).
Inhalation toxicity
Groups of 10 male mice were exposed to formulations of
thiophanate-methyl for periods of time of 30, 60, 120 minutes at a
concentration of 100 mg/litre through an aerosol generator. There were
no deaths in any group including the controls although such findings
as lacrimation, salivation and nasal discharge were observed soon
after exposure. All animals appeared normal within 24 hours following
exposure (Hashimoto et al., 1972a).
Dermal irritation
Rabbits were dermally administered thiophanate-methyl at levels
of 1, 0.1, and 0.01 g daily for 21 days. A slight reversible erythema
was observed in the highest dose group. No skin irritation was evident
in the two lower dose groups (Noguchi and Hashimoto, 1970a; Hashimoto
et al., 1972a).
Cutaneous sensitization
Guinea-pigs were examined for cutaneous sensitization by
intradermal injection of 50 µl and 100 µl at a 1% aqueous solution on
alternate days for 20 days. Two weeks later the guinea-pigs were
challenged with 50 µl injected into the same area. Animals treated
with thiophanate-methyl exhibited no primary irritation and only a
slightly sensitive state (Noguchi and Hashimoto, 1970a; Hashimoto et
al., 1972a).
Pharmacological properties
Thiophanate-methyl administered to rats at dosages up to 1500
mg/kg caused a slight increase in body temperature. Mice administered
500 mg/kg subcutaneously appeared to have a slight transient
insensitivity to heat. Studies with mice and rats administered
thiophanate-methyl at levels of 100 or 500 mg/kg subcutaneously showed
neither a sedative nor hypnotic effect. Oral administration of 1000
mg/kg to mice resulted in no significant mydriatic effect. No surface
anaesthetic action or irritation of the mucous membrane of the eye of
rabbit was observed following the administration of up to 10%
thiophanate-methyl in saline instilled into the conjunctival sac of
rabbits. Studies on excised mouse intestine at levels of up to 1 X
10-3 mg/ml, resulted in no atropine-like or papaverine-like activity.
The same concentrations did not inhibit the contraction of excised
guinea-pig intestine by histamine hydrochloride or of an excised strip
of guinea-pig aorta by epinephrine. After a single oral dose of
thiophanate-methyl of 100 mg/kg, the blood pressure of rat did not
change. Following high oral administration of thiophanate-methyl to
rabbits, there was a slight transitory decrease in white blood cell
count. In a similar manner, the white cell count of rabbits was also
depressed within a short period of time following acute oral
administration of 1000 mg/kg.
A temporary fall in blood pressure in the rabbit, followed by a
persistent rise and a persistent bradycardia was observed following
i.v. or oral administration of 1000 mg/kg. There was no change in
respiration or ECG following acute oral administration. Lethal doses
of thiophanate-methyl showed an immediate blood pressure drop to zero,
gradual disturbance of the ECG followed by cessation of respiration.
Except at very high levels there was no unusual pharmacological
properties attributed to thiophanate-methyl. All adverse
pharmacological properties were completely reversible within a short
period of time (Hashimoto of al., 1972b; Noguchi and Hashimoto,
1970e).
Special studies on carcinogenicity
Mouse. A carcinogenicity study was performed on mice of the ICR-SCL
strain.
Groups of mice (50 males and 50 females per group) were fed
thiophanate-methyl in the diet at levels of 0, 10, 40, 160 and 640 ppm
for 24 months. A sample of thiophanate-methyl used in this study was
approximately 94% active ingredient with impurities of 2% sulfur and
2% inorganic chloride, 2% unknown volatile substances and less than
0.5% aromatic amines. Animals in this study were SPF-derived and were
maintained under barrier-conditions to minimize infection. Diets were
prepared at an institute outside of the laboratory performing the
study and food was allotted bi-weekly during which time food
consumption data were recorded. Body weights were recorded initially
and monthly throughout the study with daily mortality and behavioural
examinations. Pathological examinations, using H&E stains primarily,
were performed on all animals which died or were sacrificed prior to
the conclusion of the study. After 105 weeks all animals were
sacrificed and histological examinations were performed on a variety
of tissues.
There was a slight retardation of growth in males at 640 ppm in
the diet although this was not reflected in females. Food consumption
was comparable to that of controls. Mortality and average survival
time over 105 weeks was the same in controls and at all dietary
levels. A variety of tumours was found in all groups including the
controls. All hepatomas observed in the experiment were benign liver
cell adenomas except for three liver cell derived adenocarcinomas (1
female at 40 ppm and one male and one female at 160 ppm). The
incidence of hepatoma was similar in all of the experimental groups
including the controls. Pulmonary benign adenomas were also
significantly observed in all cases with no differences in the control
or any feeding group. Leukaemia was also observed frequently with a 20
to 30% incidence in each group. In consequence, there was essentially
no difference in occurrence of tumours between experimental and
control groups. The results of this study indicate that the ingestion
of thiophanate-methyl in the diet in levels up to 640 ppm to a mouse
strain which appears to be significantly susceptible to various
tumours results in no carcinogenic activity related to
thiophanate-methyl feeding (Kosaka and Tsubura, 1973).
Special studies on cytogenicity
Male rats were administered thiophanate-methyl daily for five
days by intraperitoneal injection at levels of 0, 62.5, 125, 250, 500
and 1000 mg/kg/day. Bone marrow and spermatogonial cells examined
showed no abnormal chromogomal configurations (Makita et al., 1973).
Special studies on mutagenicity
Groups of 10 males ICR-strain mice were administered
thiophanate-methyl intraperitoneally at a single dose level of 0, 8,
40, 200, 400 or 500 mg/kg and mated with virgin females which were
replaced weekly for a period of eight weeks. At a dosage of 400 mg/kg
and above there appeared to be a reduction in the incidence of
pregnancies. However, there was no systematic variation indicative of
a mutagenic potential over the entire eight-week mating period (Makita
et al., 1973).
Special studies on reproduction
Groups of Charles-River rats (10 males and 20 females per group)
were fed levels of thiophanate-methyl in the diet at 0, 40, 160, 640
ppm in a three-generation, two-litters per generation reproduction
study. There were no apparent effects of thiophanate-methyl at levels
up to and including 640 ppm on any of the reproduction parameters
measured in this experiment. In addition, gross and histological
examinations of the F3b generation were performed on several tissues
and organs of three-week-old rats and a further group of animals was
examined for skeletal abnormalities. In no instance was there any
effect of feeding thiophanate-methyl on reproduction. There was a
definitive effect on the growth of animals fed dietary levels of 640
ppm (Palmer et al., 1972).
Special studies on teratogenicity
Thiophanate-methyl was administered to pregnant ICR mice from day
1 to day 15 of gestation at levels of 0, 40, 200, 500 and 1000
mg/kg/day. At 1000 mg/kg/day there was a significantly reduced number
of living fetuses. No (significant) differences in the number of
implantation sites, body weight of fetuses or fetal mortality or body
weight were observed at the lower dosage levels. The administration of
thiophanate-methyl did not produce gross internal or external
abnormalities and the study did not reveal any teratogenic properties
under these experimental conditions (Noguchi and Hashimoto, 1970a;
Makita et al., 1973).
Short-term studies
Mouse. Groups of six-week-old SPFICR-strain mice (12 males and 12
females per group) were fed thiophanate-methyl at levels of 0, 12.8,
64, 390, 1600 and 8000 ppm in the diet for six months. Daily
observations were made on their behaviour and mortality and body
weight and food consumption data were recorded weekly. Haematological
and gross and microscopic examination of tissues and organs were
performed at the end of the feeding period.
There were no unusual behavioural patterns and incidence of
mortality was not related to the feeding of thiophanate. Growth of all
mice at levels of 1600 ppm and below was not affected by
thiophanate-methyl with a retardation of growth observed at 8000 ppm.
Food intake and haematological examinations were normal. Gross
pathology at the termination of the experiment showed that there was
an increase in the mean weight of liver in animals fed 8000 ppm with
an increase in liver to body weight ratio. This was noted in both
males and females. There were no effects of this kind noted at a dose
level of 1600 ppm. Pathological abnormalities were noted in liver at
8000 ppm in both males and females. There was swelling of large
hepatic cells with concurrent swelling. It was observed that the
protoplasm of these cells were oedema-like or granule-like. Although
such pathological abnormalities were found in some mice of other
groups as well as the control, it appeared to be more prevalent in the
8000 ppm animals. In the present experiment the no-effect level of
thiophanate-methyl is considered to be 1600 ppm (Noguchi and
Hashimoto, 1970b).
Groups of SPF-Sprague Dawley rats (12 males and 12 females per
group) were fed thiophanate-methyl in the diet at levels of 0, 12.8,
64, 320, 1600, 8000 ppm for six months under SPF conditions. Daily
checks of mortality and abnormal behaviour were made throughout the
experiment with body weight and food intake data recorded weekly. At
the conclusion of the study, haematology, clinical chemistry and
urinalyses were performed. Gross and microscopic examination of
tissues and organs was also performed at the conclusion of the feeding
period.
There were no behavioural abnormalities observed during the
six-month feeding study and mortality was not related to food intake
of thiophanate-methyl, Examination of growth curves indicated a severe
retardation of both males and females fed 8000 ppm in the diet. There
did not appear to be any retardation in growth of animals fed 1600 ppm
or below. Food intake of all groups was comparable with control
values. All haematological values were comparable with controls as
were the values obtained from urine analysis. At 8000 ppm there was a
slight decrease in glucose and GOT levels and an increase in total
cholesterol. Gross and histological examination of tissues showed only
the liver and thyroid glands to be slightly affected by
thiophanate-methyl in the diet. There was an increase in liver weight
and liver to body weight ratio although there were no pathological
changes such as cloudy swelling and irregularity in the size of the
hepatic cells which was noted in the studies in mice. In the thyroid
gland some abnormalities such as small follicles, cubic epithelium
cells and a decrease in colloidal substance were observed in those
animals fed 8000 ppm. These changes were not observed in animals fed
1600 ppm and were not accompanied by any change in the PBI values.
Abnormal findings were not noted in any other tissues.
From these studies it appears that a no-effect level of 1600 ppm
in the diet of rats for six months would result in no unusual effect
(Noguchi and Hashimoto, 1970c).
Dog. Groups of pure bred beagle dogs (five males and five females
per group - four males and four females per group at the highest dose
level) were administered thiophanate-methyl at levels of 0, 2, 10, 30
and 250 mg/kg/day for two years. The animals were approximately six
months old at the time of the inception of the testing and were housed
singly in indoor kennels. In all groups one male and one female were
sacrificed at 12 months with the remaining animals sacrificed after 24
months on test. Dosing was performed by oral administration of
thiophanate-methyl by capsule once a day seven days a week for two
years. Doses were given to individual animals related to the body
weight and were adjusted weekly. Clinical signs of any abnormalities
were recorded daily. Food consumption and body weight changes were
recorded weekly. Throughout the study the eyes were examined for any
changes and electro cardiographs were taken periodically.
Haematological examinations, blood chemistry analyses and urinalyses
were performed periodically. At the conclusion of the study gross and
microscopic examination of tissues and organs was performed on all
animals.
There was no mortality or adverse behavioural changes observed in
these animals during the course of the study. Growth of the animals
fed 250 mg/kg/day was slightly retarded in both males and females.
There did not appear to be a significant decrease in any other group.
There did not appear to be any significant differences in the
haematological studies, blood chemistry, urinalysis, ophthalmological
examinations, or the ECG during the course of the 24-month study.
Pathological (gross and histological) examination of the animals
sacrificed at 12 months did not show any significant changes in the
tissues examined with regard to gross pathology and organ weight
ratios. Slight changes observed at 250 mg/kg in the thyroid gland were
difficult to determine from the one year data on dogs.. Animals
sacrificed at 24 months showed an elevated thyroid weight in both
males and females at 50 and 250 mg/kg although there were no changes
in the PHI values. Although the average weight of the thyroid of the
two highest dose groups was heavier than that of the controls,
histological examination showed no differences in the thyroid of the
control and the experimental groups. Histological findings in all
other tissues were also normal.
The no-effect level in this two-year study in dogs is considered
to be 50 mg/kg/day based upon the marginal effect noted in thyroid
weight at the conclusion of the study. Although this increased thyroid
weight was not reflected in the histological examination or PBI
values, it has been noted in other species (rat). A more noticeable
effect was observed at 250 mg/kg relating to retarded growth in both
males and females (Hashimoto and Fukuda, 1972).
Long-term studies
Rat (See also under the section: "Special studies on
carcinogenicity"). Groups of SPF-Sprague-Dawley strain rats (35 males
and 35 females per group, 50 males and 50 females were used as
controls) were fed thiophanate-methyl in the diet at levels of 0, 10,
40, 160, 640 ppm for two years.
The animals were maintained under SPF conditions. The sample of
thiophanate-methyl fed in this experiment was approximately 94% active
ingredient with impurities of 2% sulfur, 2% inorganic chlorides, 2%
unknown volatile substances and less than 0.5% aromatic amines. The
animals were fed dietary levels of thiophanate methyl for a period of
10 days during which behaviour and mortality were recorded daily and
after which food consumption was measured on all animals. Body weight
and growth were recorded weekly. Haematology, blood chemistry and
urinalyses were performed periodically over the two-year study. At the
conclusion of the study animals were sacrificed and gross and
microscopic examination of tissues and organs was performed.
There was no effect of feeding levels of up to 640 ppm of
thiophanate-methyl in the diet of rats on behaviour and mortality over
a two-year period. Growth curves indicate a slight retardation of
growth in both male and female rats in fed diets containing 640 ppm.
Food consumption at all dietary levels was comparable with the control
groups. Haematological data, urinalysis data and clinical blood
chemistry data were not abnormal in any of the groups examined. Gross
pathological examination of animals showed no changes in organ weight
in any group with the exception of slight increases in kidney weight
and kidney to body weight ratio, primarily in the males at the highest
level of feeding. There was no indication of increased thyroid weight
or an increase in liver size as was observed in shorter duration test
with rats. In addition there were frequent observations of
inflammation of the lung, fibroadenoma of the breast and the
occurrences of pituitary tumours. The administration of
thiophanate-methyl was not believed to be responsible for the
occurrence of these abnormalities. At the end of two years there was a
dose response in a degenerative change in the testis which underwent a
degeneration and atrophy including spermatogenesis. In this particular
dose-response change several cases were noted in other groups
including the control. This effect was probably not related to
ingestion of thiophanate-methyl. At the end of two years there was
also some change in the thyroid where colloidal epithelial cells were
enlarged. This enlargement took place primarily in the males of the
highest dose group. There was no apparent effect on the occurrence of
tumours and the only significant effects appear to be in the decreased
growth observed at 640 ppm in the diet.
Based upon retardation of growth a no-effect level would be
considered to be 160 ppm in the rat fed thiophanate-methyl for two
years (Hashimoto and Tsubura, 1972).
Observations in man
Sixteen workers concerned with the production of
thiophanate-methyl were examined periodically for 3.5 years. Blood and
urine analysis were carried out every six months. No adverse effects
were found with these workers with regard to blood chemistry and urine
analysis (Mori, 1972).
Comments
Thiophanate-methyl is degraded in plants to carbendazim and
hydroxylated derivatives. A question was raised if the apparent
occurrence of carbendazim in mammals may be an analytical artefact. A
theoretical metabolite of potential toxicological significance,
phenylene thiourea, has not been reported in plant or animal systems.
Thiophanate-methyl has a low acute toxicity in various species of
animal and shows pharmacological activity only at very high levels.
Adequate short-term and long-term studies are available in both
rats and dogs. The results of a three-generation reproduction study,
mutagenesis studies and a cytogenicity study were negative.
Thiophanate-methyl did not appear to be a carcinogen in a susceptible
strain of mice and two-year studies in rats and dogs gave rise to
retardation of growth only at high dietary levels (640 ppm). There was
a marginal increase in thyroid weight at higher dose levels which was
not accompanied by adverse biochemical or histological effects. The
long-term study in the rat was used as a basis for estimating an ADI
for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 160 ppm in the diet equivalent to 23 mg/kg bw
Rat: 160 ppm in the diet equivalent to 8 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of an acceptable daily intake for man
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Thiophanate-methyl was first registered for use as a systemic
fungicide in Japan in May 1971. By 1973 it has obtained provisional
clearance for safe use in Great Britain for a number of specified
preharvest treatments on food crops and ornamentals and for
post-harvest dipping of apples.
Thiophanate-methyl is formulated into wettable powders containing
50 and 70% active ingredient.
Recommended use levels are from 0.02-0.07% a.i. usually applied
at the rate of 1-5 kg a.i. per ha for pears, peaches and citrus and of
up to 2 kg a.i. per ha for other fruits and vegetables. Repeated
applications are claimed to be advantageous.
Thiophanate-methyl is reported to be very similar to benomyl
(BenlateR) as regards both its systemic functions (Aelbers, 1970)
and the rather broad spectrum of fungal diseases against which it is
effective (Bollen, 1971). It is claimed effective against apple and
pear scab, powdery mildew and different moulds, rots and blight.
Pre-harvest application on citrus trees should effectively reduce
Penicillium decay on citrus fruits under post-harvest storage.
The similarity between thiophanate-methyl and benomyl seems well
explained by the fact that both compounds are converted into methyl
2-benzimidazolecarbamate (MBC), which is reported to be the principal
fungitoxic compound to which both chemicals owe their major activity
(Vonk and Sijpesteijn, 1971; Selling et al., 1970).
Residues resulting from supervised trials
Data derived from extensive field trials in several countries on
thiophanate-methyl residues have been presented by the manufacturer
(Nippon Soda Company Ltd, 1973a). A summarized list of a selection of
the maximum residues found in these trials is presented in Table 1
(Nippon Soda Company Ltd, 1973b). The majority of information in
Table 1 shows residue levels at 0 or 1 day after last application.
Generally, the major part of residues is in the form of unchanged
thiophanate-methyl, while methyl 2-benzimidazolecarbamate (MBC) is
usually detectable in smaller amounts, averaging 10% of the total.
Apples, pears, peaches
Generally, higher levels of thiophanate-methyl residues are found
in these fruits after post-harvest treatments than after pre-harvest
sprayings. This is partly due to higher initial deposits from dipping
and partly connected to volatility losses to which the pre-harvest
treated fruits are subjected. During storage experiments performed in
the United Kingdom by the manufacturer, pears treated after harvest
TABLE 1. RESIDUES OF THIOPHAMATE-METHYL AND MBC IN VARIOUS CROPSa,b
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Apple 1.4 kg/ha 10× 1.4 - Fed. Rep. Ger.
0.6 g/l 1× 0.83 0.14 Canada
1-2 kg/ha 17×+
+1000 pp. Dipping 3.8 0.8 UK
2100 ppm Dipping 3.4 Italy
Banana 1000 pp. Dipping Peel: 0.3 0.4 Australia
Pulp: 0.1 <0.2
Beans, broad 2 kg/ha 1× 0.1 0.5 UK
" dwarf 1 kg/ha 1× 2.3 0.1 UK
" french 1.5 kg/ha 1× 0.2 0.2 US
" kidney 0.75 kg/ha 2× 0.2 Japan
" runner 1 kg/h. 1× 1.8 0.1 UK
Blackcurrant 5.4-7.5 kg/ha 4× 0.day:19.4 4.9 UK
5.4-7.5 kg/ha 4× 7.day:7.0 3.3 UK
1 kg/ha 4.5× 3.0 2.3 UK
Carrots 1000 ppm Dipping 5.0 0.2 UK
Celery low pp. Dipping 28.0 0.8 UK
Cherries 1.4 g/l 3× 2.0 0.2 Australia
2 kg/ha 5× 0.46 0.64 USA
Citrus 3.5 kg/ha 1× Peel: 12.2 2.1 Japan
Pulp: 0.3 <0.2
Cucumber 1 kg/ha 6× 0.45 0.09 Japan
Gherkins 1 g/l 1.87 0.33 Netherlands
Gooseberries 1 kg/ha 4× 3.0 0.4 UK
Grapes 1.4 kg/ha 5× 3.3 - Fed. Rep. Ger.
1.4 kg/ha 19× 2.8 Japan
2.4 g/l 6× 0.6 <0.2 Australia
Lettuce 1 kg/ha 3× 0.day:10.0 0.6 UK
7.day:0.1 <0.2
2.8 kg/ha 6× 0.day:24.5 5.0 Japan
7.day:3.8 1.5
2.2 kg/ha 1× 0.day:4.1 New Zealand
TABLE 1. (Cont'd.)
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Mushroom about 5 g/m2 1× <0.1 0.7 UK
Onion 2.5 kg/ha 10× 0.05 0.02 Japan
Orange 1000 ppm Dipping Peel: 1.2 <0.3 Australia
Pulp: 0.2 <0.3 Australia
Juice: 0.4 <0.2 Australia
1000 ppm Dipping Peel: 2.3 0.23 USA
2100 ppm Dipping 8.8 Italy
Peach 2.4 g/l 4× 5.0 0.4 Australia
Pear 1000 ppm Dipping 2.2 <0.1 UK
2100 ppm Dipping 3.9 Italy
Plum 1 kg/ha 6× 0.86 0.37 USA
Raspberries 1 kg/ha 8× 9.6 2.3 UK
Strawberries 1.5 kg/ha 1× 0.day:14.6 UK
7.day:3.0
1 kg/ha 4-5× 0.day:3.8 1.2 Netherlands
(under glass) 7.day:3.6 0.9
14.day:0.9 0.6
Sugarbeet 0.35 kg/ha 2× Leaf:3.6 Fed. Rep. Oar.
Root: <0.2
Wheat, winter 0.5 kg/ha 1× Straw: <0.2 (50 days)
Grain: <0.2
Tomato 2 kg/ha 5× 9.7 0.4 UK
a Residues reported are maximum residues, i.e. levels found on 0. or 1. day after application,
except when otherwise stated.
b Analytical methods used were as follows (see monograph text):
TLC-method: Australia, Japan, New Zealand
Colorimetry: Canada, UK, USA
UV-spectrophotometry: Japan, Federal Republic of Germany
Oscillopolarography: Italy, Netherlands (TLC-bioassay for MBC).
with thiophanate-methyl showed no pronounced decrease of residues
(about 2 ppm) during 61 days, while on the other hand, residues of
about 1 ppm on apples decreased well below 0.1 ppm during four weeks
on the trees after spraying.
Grapes and wine
Lemperle et al. (1973) found that thiophanate-methyl residues, as
well as those of other benzimidazole fungicides, are transferred
nearly quantitatively into unclarified musts during wine production.
During clarification and fermentation small amounts may disappear. A
considerable part of the thiophanate-methyl residues which are present
in grapes may therefore be found in wines.
Leafy vegetables
The residue data supplied by the manufacturer indicate that even
relatively high residues are subject to pronounced losses, which may
be explained as usual growth dilution effect to which volatility
losses from the foliar surfaces may be added. From field trials with
lettuce performed in Japan, half-life values of the order of 1-2 days
for thiophanate-methyl have been deducted, while the MBC-metabolite
present in smaller amounts disappeared more slowly, corresponding to
half-life values of 5-6 days.
For comparison, the half-life of thiophanate-methyl on apple and
grape leaves under controlled conditions was found to be in the order
of 15 and 12 days, respectively (Soeda et al., 1972).
Carrots, celery, sugarbeets
When subjected to post-harvest treatments with thiophanate-methyl
these root-crops take up considerable amounts of residues. Dipping of
carrots and celery performed in the United Kingdom with 0.1% a.i. gave
total residues of thiophanate-methyl + MBC of 5.2 and 28.8 ppm,
respectively. Pre-harvest sprays gave high, though rapidly
disappearing, residues on the green tops above soil, but no detectable
residues in the edible roots.
Fate of residues
Substantial evidence has been presented by the manufacturer that
methyl 2-benzimidazolecarbamate (MBC) is produced as the major
metabolite (Noguchi et al., 1971) although only gradually released
from thiophanate-methyl when applied on foliar surfaces (Kikuchi,
1973). Vonk and Sijpesteijn (1971) found that the fungitoxic activity
of thiophanate-methyl is increased by aging in aqueous media. They
concluded that MBC is responsible for the fungitoxic effect of the
fungicide and indicated that the rate of conversion from
thiophanate-methyl may be increased by the fungal metabolic activity.
Selling et al. (1970) also observed the conversion of
thiophanate-methyl to MBC in tap water. A 4% transformation was noted
when kept at pH = 7.0 in water for seven days, while
thiophanate-methyl remained stable in methanol or chloroform for 50
days at 24°C (Noguchi et al., 1971).
Plants
Conversion of thiophanate-methyl to MBC has been followed after
uptake through the roots and after foliar treatment of French bean
seedlings (Phaseolus vulgaris) by Noguchi et al. (1971). After 21 days
34.3% of the original amount had been converted on the leaves, while
87.5% was metabolized into MBC when absorbed through the roots in a
water culture. In a most recent publication, the formation of MBC from
thiophanate-methyl is further described by Buchenauer et al. (1973).
They found that the transformation to a great extent is
light-catalyzed (requiring at the same time the presence of water). A
42% transformation of thiophanate-methyl residues was found in cotton
leaves, when subjected to four days of sunlight as opposed to only
about 6%, when the plants were kept under dimlight conditions.
A minor metabolite, 2-aminobenzimidazole (2-AB), also formed from
MBC in benomyl treated plants is detected as a result of
thiophanate-methyl applications as well (Fujino, A. and Kamimura,
1973a). It is further reported by the same authors that two
oxygen-analogues (DX-105 = 1-(3-methyoxy-carbonyl-2
thioureido)-2-(3-methoxycarbonylureido) benzene and FH-132 =
dimethyl-4.4-O-phenylenbis(allophanate)) has recently been identified
by a reverse isotopedilution method (see Fig. 3).
Soil
Thiophanate-methyl is degraded almost entirely within seven days
in sandy loam and silty loam soils at temperatures from 23°C to 33°C
(Pujino and Kamumura, 1973b). MBC is identified as the major
metabolite, which is subsequently degraded at a more moderate rate.
Sixty days after treatment residual MBC was present at the level of
20% at 23°C and 2-15% at 33°C in both soil types, calculated on the
basis of the amount of thiophanate-methyl applied. No influence from
thiophanate-methyl was indicated in these experiments on soil
micro-organisms, when measured by bacterial counts (total bacteria,
gram-negative and actinomycetes). With increased levels of
thiophanate-methyl (up to 5000 ppm) increased oxygen uptake increased
significantly, suggesting that thiophanate-methyl was used as a source
of nitrogen by bacteriae.
The conversion of thiophanate-methyl to MBC is significantly more
rapid in unsterilized than in sterile soils. Similarly, the MBC formed
is less persistent in the unsterilized soil, which suggests that
micro-organisms may be involved in the degradation of both compounds.
TLC-identification patterns further indicate that
dimethyl-4.4'-o-phenyleneallophanate (or 111-432, see Fig. 3) may be
an intermediate metabolic step in the microflora-induced degradation,
as this compound is not found as a metabolite in sterilized soils
(Kosaka et al., 1972).
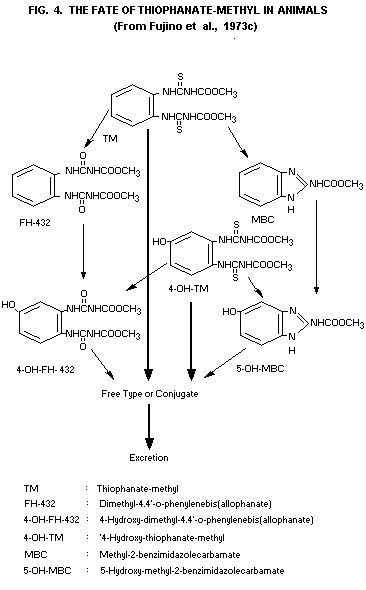 Radio-activity recovery experiments in which water was percolated
through treated loams (sandy and silty) did not give evidence of any
migration of thiophanate-methyl or of degradation products deeper than
4 cm from the treated surface layer.
Animals
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973c) 14C or 35S-labelled thiophanate-methyl was fed to rats, mice
and dogs. From 80 to 100% of the administered amounts were recovered
in faeces and urine within 96 hours after feeding. Sixty per cent.,
16-27% and 14% were excreted with the faeces by the three species
respectively, while urinary excretion accounted for 30%, 66-78% and
74% of the administered thiophanate-methyl respectively.
Fujino et al. (1973c) report that the major part of faecal
excretion was in the form of unmetabolized thiophanate-methyl, while
the minor part consisted of 4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-o-phenyleneallophanate (FH-432). MBC and 5-hydroxy-MBC
(5-OH-MBC) were also observed during TLC identifications of
metabolites from faecal extracts. It was, however, questioned whether
these two were actual metabolites in faeces or if they were compounds
produced during the analytical procedures from thiophanate-methyl and
4-OH-TM, respectively.
In the same studies by Fujino et al. (1973c) thiophanatemethyl
and a number of metabolites could be liberated enzymatically or by
acid treatment from water soluble conjugates in rat urine. Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (see Fig. 4). As was the case in faeces, two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical steps.
In the earlier study by Noguchi et al. (1971) of the effect of
thiophanate-methyl in rat liver microsomes, MBC and 5-OH-MBC were also
found to be present. Evidence for enzyme induction could not be
obtained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Methods of residue analysis
The methods of Pease and Gardiner (1969) and Pease and Holt
(1971) for benomyl could be applied to thiophanate-methyl after
conversion to MBC. It is expected that this method should be able to
differentiate thiophanate-methyl from other benzimidazole fungicides.
The manufacturer has presented detailed descriptions of methods
for thiophanate-methyl residue analyses by means of thin-layer
chromatography (Tannue et al., 1973), colorimetric determination based
on MBC reaction with bromcresol purple in chloroform (Sugioka et al.,
1973) and by UV-spectrophotometric measurement of MBC at 282 nm (Ono,
1973a).
The UV-spectrophotometric method which is applicable to various
crops is generally recommended. Thiophanate-methyl and MBC are
conveniently extracted together from the sample with methyl alcohol,
and then separated during the clean-up stages through liquid
partitioning between petroleum ether-iso-octane solvent, and methylene
chloride and aqueous methanol solutions. Thiophanate-methyl is
converted into MBC by reflux treatment with cupric acetate in aqueous
acetic acid solution and thiophanate-methyl and MBC are determined
separately as MBC by a corrected absorbance measurement technique at
292 nm from the spectrum recorded between 250 nm and 310 nm.
The limit of determination with this method is given to 0.02 ppm
for each of the compounds, thiophanate-methyl and MBC, when analysing
100 g samples. Recoveries are reported for a number of fruits and
vegetables from 72 to 81% for thiophanate-methyl and 77-84% for MBC.
The initial steps of the method (until the conversion of
thiophanate-methyl into MBC) should be carried out without prolonged
keeping and possibly under protected light conditions (Ono, 1973b).
An oscillopolarographic method for the separate determination of
unchanged thiophanate-methyl has been published (Martens and Chs,
1972). It has been adapted for residue determinations in a number of
fruits and vegetables with a limit of determination of 0.02 ppm in
combination with TLC-Bioassay Technique for the MBC (Civo TNO 1973) as
this compound gives no oscillopolarographic response.
National tolerances
The Meeting was aware of the following national tolerances:
Australia Apples, pears, stone fruit 3 ppm
citrus, bananas, grapes 1 ppm
Netherlands Fruit and vegetables 2 ppm
(thiophanate-methyl
without MBC)
Raw cereals 0.5 ppm
(thiophanate-methyl
+ MBC, under revision)
Switzerland Stone fruit, grapes,
strawberries 3 ppm (calculated
as MBC)
Celeriac 0.1 ppm
Appraisal
Thiophanate-methyl is a new systemic fungicide which is
characterized by broad-spectrum fungicidal effects as other chemicals
of the benzimidazole group. Thus it is claimed to be effective in the
control of different moulds rots and blights although ineffective
towards some specific plant-pathogenic fungi e.g. Pythium spp.,
Phytophora sop. and Alternaria spp.
In countries in which thiopbanate-methyl has obtained
registration, it is marketed as wettable powders for pre-harvest as
well as post-harvest treatments. Recommended use levels are from 0.02
to 0.07%, often with repeated applications.
Residue data from supervised trials performed with
thiophanate-methyl in several countries have been provided by the
manufacturer in support of registrations, including the establishment
of tolerances. It is noted that in a few cases the data was derived
from trials involving excessive application rates so that residue
levels were higher than would be likely following actual good
agricultural practice. In addition to information on numerous food
crops, the data includes experiments on soil residues, although direct
soil treatments are not indicated as recommended practice.
In plant material and in soils the major metabolite is found to
be methyl benzimidazolecarbamate, formed by hydrolysis and ring
closure from thiophanate-methyl. Methyl benzimidazolecarbamate is a
chemically stable compound which leaves relatively persistent
residues. Although somewhat conflicting evidence is presented as to
which degree of transformation takes place in and on plants (possibly
due to analytical difficulties) it is found reasonably well
established that methyl benzimidazolecarbamate is formed gradually
from thiophanate-methyl and that both compounds should be accounted
for in the evaluation of residue data.
A minor metabolite, 2-aminobenzimidazole, may be formed from
methyl benzimidazolecarbamate and be detectable in plant tissues
following thiophanate-methyl applications. Two oxygen analogues of
thiophanate-methyl,
1-(3-methoxy-carbonyl-2-thioureido)-2-(3-methoxycarbonyl ureido)
benzene and dimethyl-4, 4-0-phenylenbis (allophanate) have, in
addition, been described as possible intermediate metabolites. These
have recently been identified in trace amounts from plant material
by reverse isotope-dilution technique.
In soils the degradation of thiophanate-methyl to methyl
benzimidazolecarbamate is found to be complete within seven days in
both sandy and silty loams, whereafter the metabolite behaves as a
more persistent compound which disappears at a more moderate rate. An
uptake through the roots of plants has been clearly demonstrated.
Information, however, on the fate of thiophanate-methyl after
feeding to ruminants is not given, neither is data available on
residues in animal products, milk, meat and eggs.
Analytical methods which allow the determination of
thiophanate-methyl and its main metabolite (MBC) separately are
available. They are based on UV-spectrometric measurements of MBC
after separation of the two compounds and they may be adapted for
regulatory purposes, although a definite multi-residue technique for
distinguishing the different benzimidazole fungicide seems to be
lacking.
RECOMMENDATIONS
Due to the fact that a certain, not definitely established
proportion of residues from thiophanate-ethyl applications consist of
MBC, for which an ADI has not been established, only temporary
tolerances can be recommended.
Tolerances given below are measured as the sum of thiophanate-
ethyl and its major metabolite, methyl benzimidazolecarbamate (MSC)
and expressed in terms of the latter. Levels indicated are such that
they are not likely to be exceeded when following good agricultural
practice, including pre-harvest and post-harvest treatments when
applicable.
For the underlined commodities individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides, of
which MBC is also recognized and identified as the major metabolic
and/or chemical entity (i.e. benomyl and carbendazim).
Temporary tolerances ppm
expressed as MBC
Celery 20
Citrus (whole), peaches, grapes, cherries 10
Apples, tomato*, pears, lettuce*, strawberries*,
raspberries, gooseberries, blackcurrants*,
carrots, sugar beet tops : 5
Beans (broad, dwarf, french, runner, kidney)
gherkins, plums 2
Mushrooms, bananas (whole) 1
Cucumbers 0.5
Sugar beet, raw cereals, onions 0.1
* At or about limit of determination.
FURTHER WORK OR INFORMATION
Required (before temporary tolerances can be confirmed)
1. Information on the nature and fate of residues of thiophanate-
ethyl in meat, milk, and eggs following the feeding of
thiophanate-methyl at levels likely to be found on forage and
foodstuffs.
Desirable
1. Further studies on the metabolism of thiophanatemethyl in
animals, with special reference to the occurrence of carbendazim.
2. Further studies on the effect of thiophanate-methyl on the
thyroid and the testes in the rat and other species of animals.
3. Further data on residues on raspberries following good
agricultural practice.
4. Further information on the need for post-harvest treatment of
carrots and celery.
REFERENCES
Aelbers, E., Mededel, Pak. (1970) Landbouw Wet. Gent., 36, 126.
Cited from Vonk & Sijpesteijn (1971)
Bollen, G.J. (1972) A comparison of the in vitro antifungal spectra
of thiophanates and benomyl. Neth. J. Pl. Path. 78, 55
Buchenauer, H., Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci., 4: 343-348
CIVO-INO Report No. R.4023 (1973) on residues of thiophanate-methyl
and its metabolite MBC in strawberries. From Central Institute for
Nutrition and Food Research, TNO, Zeist (Unpublished)
Fuchs, A. Van Den Berg, G.A. and Davidse, L.C.A (1972) comparison of
benomyl and thiophanates with respect to some chemical and systemic
fungitoxic characteristics. Pest. Biochem. Physiol., 2(2): 191-205
Fujino, A. and Kamimura, H. 1973a The fate of thiophanate-methyl
residue on plants under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A. and Kamimura, H. 1973b The fate of thiophanate-methyl
residue in soil under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A., Ohnuma, N. Mori, T., Tanoue, T. and Kamimura, H. (1973c)
The balance and metabolism studies of thiophanate methyl in animals.
Paper submitted by Nisso institute Per Life Sciences. Nippon Soda Co.
Ltd. (Unpublished)
Hashimoto, Y., Makita, T., Mori, T., Nishibe, T., Noguchi, T., Tsuboi,
S. and Ohta, G. (1970) Toxicological Evaluations of Thiophanate (I).
Acute and Subacute toxicity of a new fungicide, thiophanate (Acute
Ingredient of NF-35), 1,2 hrs. (ethoxy-carbonyl-thioureido)
- benzene. Pharmacometrics, 4(1): 5-21
Hashimoto, Y. Makita, T. Ohnuma, M. and Noguchi, T. (1972a) Acute
toxicity on Demethyl 4, 4'-O-phenylene bis (3-thio allophanate),
thiophanate-methyl fungicide. Toxicol. Appl. Pharmacol., 23: 606-615
Hashimoto, Y., Mori, T., Ohnuma, N. and Noguchi, T. (1972b) Some
Pharmacologic Properties of a new fungicide, thiephanate-methyl. Tox,
Appl. Pharmacol., 23: 616-622
Hashimoto, Y. and Fukuda, Y. (1972) Final Report on the long term oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4 -O-phenylenebis
(3-thioallophanate) in Beagle dogs for 24 months. Unpublished report
from Nisso Institute for Life Sciences submitted by Nippon Soda Co.
Ltd.
Hashimoto, Y. and Tsubura, Y. (1972) Final Report on the Chronic Oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4' -O-phenylenebis
(3-thioallophanate) in rats of Sprague-Dawley Strain for 24 months.
Unpublished report from Nisso Institute for Life Sciences submitted by
Nippon Soda Co. Ltd.
Kikuchi, Masayoshi. (1973) Actual state of thiophanate-methyl residues
on crops. Paper submitted by Fine Chemicals Division, Nippon Soda Co.
Ltd. (Unpublished)
Kosaka, S., Soeda, Y., Hagiwara, S., Hashimoto, S. and Naohara, T.
(1972) Influences of thiophanate-methyl to soil microorganisms add
fate of the chemical in soil. Paper submitted by Nisso Institute for
Life Science, Nippon Soda Co. Ltd. (Unpublished)
Lemperle, E., Kerner, B., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstünde und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Deutsche Leb.-Rdschau, 69, 313
Makita, T., Hashimoto, Y., and Noguchi, T. (1973) Mutagenic,
Cytogenetic and teratogenic Studies on thiophanate methyl. Toxicol
Appl. Pharmacol, 24(2): 206-215
Martens, P. H. and Chs, M. Détermination oscillopolarographique des
residue do thiophanate sur légumes divers. Model. Faknld.
Landbonetensch., Gent, 37, 891
Mori, H. 1972 Human Handling experiences from Plant Employees
manufacturing Topsin (1). Unpublished report from Nisso Takaoka
Hospital submitted by Nippon Soda Co. Ltd.
Nippon Soda Co. Ltd. (1973a) Information on usage and the occurrence
of residues of thiophanate-methyl. Report submitted in August 1973 by
Nippon Soda Co. Ltd. (Unpublished)
Nippon Soda Co. Ltd. (1973b) List of maximum residue of
thiophanate-methyl and its metabolite, MBC in/on edible crops in
the world. Compiled by the Nippon Soda Co. Ltd. October 1973
(Unpublished)
Noguchi, T. and Hashimoto, Y. (1970a) Toxicological evaluation of
Thiophanate Methyl (I) Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from Nisso Institute for Life
Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate methyl (II) studies on the subchronic oral toxicity of
thiophanate-methyl in mice. Unpublished report from Nisso Institute
for, Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity of
thiophanate-methyl in rats. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970d) Toxicological evaluation of
thiophanate-methyl (IV). Studies on the teratogenic effect of
thiophanate-methyl upon the fetus of ICR strain mice. Unpublished
report from Nisso Institute for Life Sciences submitted by Nippon Soda
Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970e) Toxicological evaluation of
thiophanate-methyl (V). Some pharmacologic properties of a new
fungicide thiophanate-methyl. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1971) Study on contact phototoxicity
and sensitivity to new fungicide, thiophates. Unpublished report from
Nisso Institute for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T., Ohkuma and Kosaka, S. (1971) Chemistry and Metabolism of
thiophanate fungicides. Proc. Int. Symp. Pesticide Terminal Residues,
Tot Aviv, pp. 257-270
Noguchi, T. (1972) Environmental Evaluation of Systemic Fungicides.
Environmental Toxicology of Pesticides, Academic Press N.Y., pp.
607-632
Ono, S. (1973a) Analytical method for residues of thiophanate-methyl
and 2-methyl benzimidazolecarbamate in crops by U.V.-spectrometry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Ono, S. (1973b) Stability of thiophanate-methyl under analytical
procedures. Paper submitted by Nippon Soda Co. Ltd. (Unpublished)
Palmer, A.K., Lowell, M.R, and Newman, A.J. (1972) Effect of
thiophanate-methyl on reproductive function of multiple generation in
the rat. Unpublished report from Huntingdon Research Center submitted
by Nippon Soda Co. Ltd.
Pease, H.L. and Gardiner, J.A. (1969) Fluorometric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.P. Improved method for determining benomyl
residues. J. Assoc. Off. Analyt. Chem., 54, 1399
Selling, H.A., Vonk, J.W. and Sijpesteijn, A.K. (1970) Transformation
of the systemic fungicide methyl thiophanate into 2-benzimidazole
carbamic acid methyl ester. Chem. Ind., pp. 1625-1626
Soeda, Y., Kosaka, S. and Noguchi, T. (1972a) The fate of thiophanate.
methyl fungicide and its metabolites on plant leaves and glass plates.
Agr. Biol. Chem., 36(6): 931-936
Soeda, Y., Kosaka, S. & Noguchi, T. (1972b) Identification of Alkyl
2-benzimidazole carbamates as a major metabolite of thiophanates
fungicide in/on the bean plant. Agr. Biol. Chem., 36(6): 817-823
Sugioka, K., Toyama, N. and Kamimura, H. (1973) Residue analytical
method of thiophanate-methyl and a transformed product, methyl
2-benzimidazole (MBC) in soils and plant tissues by colorimetry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Tanoue, T., Toyama, N. & Karimura, H. (1973) Residue analytical method
of thiophanate-methyl in plant tissues by thin layer chromatography.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Vonk, J.W. & Sijpesteijn, A.K. (1971) Methyl benzimidazol-2-
ylcarbamate, the fungitoxic principle of thiophanatemethyl. Pest.
Sci., 2, 160
Radio-activity recovery experiments in which water was percolated
through treated loams (sandy and silty) did not give evidence of any
migration of thiophanate-methyl or of degradation products deeper than
4 cm from the treated surface layer.
Animals
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973c) 14C or 35S-labelled thiophanate-methyl was fed to rats, mice
and dogs. From 80 to 100% of the administered amounts were recovered
in faeces and urine within 96 hours after feeding. Sixty per cent.,
16-27% and 14% were excreted with the faeces by the three species
respectively, while urinary excretion accounted for 30%, 66-78% and
74% of the administered thiophanate-methyl respectively.
Fujino et al. (1973c) report that the major part of faecal
excretion was in the form of unmetabolized thiophanate-methyl, while
the minor part consisted of 4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-o-phenyleneallophanate (FH-432). MBC and 5-hydroxy-MBC
(5-OH-MBC) were also observed during TLC identifications of
metabolites from faecal extracts. It was, however, questioned whether
these two were actual metabolites in faeces or if they were compounds
produced during the analytical procedures from thiophanate-methyl and
4-OH-TM, respectively.
In the same studies by Fujino et al. (1973c) thiophanatemethyl
and a number of metabolites could be liberated enzymatically or by
acid treatment from water soluble conjugates in rat urine. Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (see Fig. 4). As was the case in faeces, two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical steps.
In the earlier study by Noguchi et al. (1971) of the effect of
thiophanate-methyl in rat liver microsomes, MBC and 5-OH-MBC were also
found to be present. Evidence for enzyme induction could not be
obtained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Methods of residue analysis
The methods of Pease and Gardiner (1969) and Pease and Holt
(1971) for benomyl could be applied to thiophanate-methyl after
conversion to MBC. It is expected that this method should be able to
differentiate thiophanate-methyl from other benzimidazole fungicides.
The manufacturer has presented detailed descriptions of methods
for thiophanate-methyl residue analyses by means of thin-layer
chromatography (Tannue et al., 1973), colorimetric determination based
on MBC reaction with bromcresol purple in chloroform (Sugioka et al.,
1973) and by UV-spectrophotometric measurement of MBC at 282 nm (Ono,
1973a).
The UV-spectrophotometric method which is applicable to various
crops is generally recommended. Thiophanate-methyl and MBC are
conveniently extracted together from the sample with methyl alcohol,
and then separated during the clean-up stages through liquid
partitioning between petroleum ether-iso-octane solvent, and methylene
chloride and aqueous methanol solutions. Thiophanate-methyl is
converted into MBC by reflux treatment with cupric acetate in aqueous
acetic acid solution and thiophanate-methyl and MBC are determined
separately as MBC by a corrected absorbance measurement technique at
292 nm from the spectrum recorded between 250 nm and 310 nm.
The limit of determination with this method is given to 0.02 ppm
for each of the compounds, thiophanate-methyl and MBC, when analysing
100 g samples. Recoveries are reported for a number of fruits and
vegetables from 72 to 81% for thiophanate-methyl and 77-84% for MBC.
The initial steps of the method (until the conversion of
thiophanate-methyl into MBC) should be carried out without prolonged
keeping and possibly under protected light conditions (Ono, 1973b).
An oscillopolarographic method for the separate determination of
unchanged thiophanate-methyl has been published (Martens and Chs,
1972). It has been adapted for residue determinations in a number of
fruits and vegetables with a limit of determination of 0.02 ppm in
combination with TLC-Bioassay Technique for the MBC (Civo TNO 1973) as
this compound gives no oscillopolarographic response.
National tolerances
The Meeting was aware of the following national tolerances:
Australia Apples, pears, stone fruit 3 ppm
citrus, bananas, grapes 1 ppm
Netherlands Fruit and vegetables 2 ppm
(thiophanate-methyl
without MBC)
Raw cereals 0.5 ppm
(thiophanate-methyl
+ MBC, under revision)
Switzerland Stone fruit, grapes,
strawberries 3 ppm (calculated
as MBC)
Celeriac 0.1 ppm
Appraisal
Thiophanate-methyl is a new systemic fungicide which is
characterized by broad-spectrum fungicidal effects as other chemicals
of the benzimidazole group. Thus it is claimed to be effective in the
control of different moulds rots and blights although ineffective
towards some specific plant-pathogenic fungi e.g. Pythium spp.,
Phytophora sop. and Alternaria spp.
In countries in which thiopbanate-methyl has obtained
registration, it is marketed as wettable powders for pre-harvest as
well as post-harvest treatments. Recommended use levels are from 0.02
to 0.07%, often with repeated applications.
Residue data from supervised trials performed with
thiophanate-methyl in several countries have been provided by the
manufacturer in support of registrations, including the establishment
of tolerances. It is noted that in a few cases the data was derived
from trials involving excessive application rates so that residue
levels were higher than would be likely following actual good
agricultural practice. In addition to information on numerous food
crops, the data includes experiments on soil residues, although direct
soil treatments are not indicated as recommended practice.
In plant material and in soils the major metabolite is found to
be methyl benzimidazolecarbamate, formed by hydrolysis and ring
closure from thiophanate-methyl. Methyl benzimidazolecarbamate is a
chemically stable compound which leaves relatively persistent
residues. Although somewhat conflicting evidence is presented as to
which degree of transformation takes place in and on plants (possibly
due to analytical difficulties) it is found reasonably well
established that methyl benzimidazolecarbamate is formed gradually
from thiophanate-methyl and that both compounds should be accounted
for in the evaluation of residue data.
A minor metabolite, 2-aminobenzimidazole, may be formed from
methyl benzimidazolecarbamate and be detectable in plant tissues
following thiophanate-methyl applications. Two oxygen analogues of
thiophanate-methyl,
1-(3-methoxy-carbonyl-2-thioureido)-2-(3-methoxycarbonyl ureido)
benzene and dimethyl-4, 4-0-phenylenbis (allophanate) have, in
addition, been described as possible intermediate metabolites. These
have recently been identified in trace amounts from plant material
by reverse isotope-dilution technique.
In soils the degradation of thiophanate-methyl to methyl
benzimidazolecarbamate is found to be complete within seven days in
both sandy and silty loams, whereafter the metabolite behaves as a
more persistent compound which disappears at a more moderate rate. An
uptake through the roots of plants has been clearly demonstrated.
Information, however, on the fate of thiophanate-methyl after
feeding to ruminants is not given, neither is data available on
residues in animal products, milk, meat and eggs.
Analytical methods which allow the determination of
thiophanate-methyl and its main metabolite (MBC) separately are
available. They are based on UV-spectrometric measurements of MBC
after separation of the two compounds and they may be adapted for
regulatory purposes, although a definite multi-residue technique for
distinguishing the different benzimidazole fungicide seems to be
lacking.
RECOMMENDATIONS
Due to the fact that a certain, not definitely established
proportion of residues from thiophanate-ethyl applications consist of
MBC, for which an ADI has not been established, only temporary
tolerances can be recommended.
Tolerances given below are measured as the sum of thiophanate-
ethyl and its major metabolite, methyl benzimidazolecarbamate (MSC)
and expressed in terms of the latter. Levels indicated are such that
they are not likely to be exceeded when following good agricultural
practice, including pre-harvest and post-harvest treatments when
applicable.
For the underlined commodities individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides, of
which MBC is also recognized and identified as the major metabolic
and/or chemical entity (i.e. benomyl and carbendazim).
Temporary tolerances ppm
expressed as MBC
Celery 20
Citrus (whole), peaches, grapes, cherries 10
Apples, tomato*, pears, lettuce*, strawberries*,
raspberries, gooseberries, blackcurrants*,
carrots, sugar beet tops : 5
Beans (broad, dwarf, french, runner, kidney)
gherkins, plums 2
Mushrooms, bananas (whole) 1
Cucumbers 0.5
Sugar beet, raw cereals, onions 0.1
* At or about limit of determination.
FURTHER WORK OR INFORMATION
Required (before temporary tolerances can be confirmed)
1. Information on the nature and fate of residues of thiophanate-
ethyl in meat, milk, and eggs following the feeding of
thiophanate-methyl at levels likely to be found on forage and
foodstuffs.
Desirable
1. Further studies on the metabolism of thiophanatemethyl in
animals, with special reference to the occurrence of carbendazim.
2. Further studies on the effect of thiophanate-methyl on the
thyroid and the testes in the rat and other species of animals.
3. Further data on residues on raspberries following good
agricultural practice.
4. Further information on the need for post-harvest treatment of
carrots and celery.
REFERENCES
Aelbers, E., Mededel, Pak. (1970) Landbouw Wet. Gent., 36, 126.
Cited from Vonk & Sijpesteijn (1971)
Bollen, G.J. (1972) A comparison of the in vitro antifungal spectra
of thiophanates and benomyl. Neth. J. Pl. Path. 78, 55
Buchenauer, H., Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci., 4: 343-348
CIVO-INO Report No. R.4023 (1973) on residues of thiophanate-methyl
and its metabolite MBC in strawberries. From Central Institute for
Nutrition and Food Research, TNO, Zeist (Unpublished)
Fuchs, A. Van Den Berg, G.A. and Davidse, L.C.A (1972) comparison of
benomyl and thiophanates with respect to some chemical and systemic
fungitoxic characteristics. Pest. Biochem. Physiol., 2(2): 191-205
Fujino, A. and Kamimura, H. 1973a The fate of thiophanate-methyl
residue on plants under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A. and Kamimura, H. 1973b The fate of thiophanate-methyl
residue in soil under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A., Ohnuma, N. Mori, T., Tanoue, T. and Kamimura, H. (1973c)
The balance and metabolism studies of thiophanate methyl in animals.
Paper submitted by Nisso institute Per Life Sciences. Nippon Soda Co.
Ltd. (Unpublished)
Hashimoto, Y., Makita, T., Mori, T., Nishibe, T., Noguchi, T., Tsuboi,
S. and Ohta, G. (1970) Toxicological Evaluations of Thiophanate (I).
Acute and Subacute toxicity of a new fungicide, thiophanate (Acute
Ingredient of NF-35), 1,2 hrs. (ethoxy-carbonyl-thioureido)
- benzene. Pharmacometrics, 4(1): 5-21
Hashimoto, Y. Makita, T. Ohnuma, M. and Noguchi, T. (1972a) Acute
toxicity on Demethyl 4, 4'-O-phenylene bis (3-thio allophanate),
thiophanate-methyl fungicide. Toxicol. Appl. Pharmacol., 23: 606-615
Hashimoto, Y., Mori, T., Ohnuma, N. and Noguchi, T. (1972b) Some
Pharmacologic Properties of a new fungicide, thiephanate-methyl. Tox,
Appl. Pharmacol., 23: 616-622
Hashimoto, Y. and Fukuda, Y. (1972) Final Report on the long term oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4 -O-phenylenebis
(3-thioallophanate) in Beagle dogs for 24 months. Unpublished report
from Nisso Institute for Life Sciences submitted by Nippon Soda Co.
Ltd.
Hashimoto, Y. and Tsubura, Y. (1972) Final Report on the Chronic Oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4' -O-phenylenebis
(3-thioallophanate) in rats of Sprague-Dawley Strain for 24 months.
Unpublished report from Nisso Institute for Life Sciences submitted by
Nippon Soda Co. Ltd.
Kikuchi, Masayoshi. (1973) Actual state of thiophanate-methyl residues
on crops. Paper submitted by Fine Chemicals Division, Nippon Soda Co.
Ltd. (Unpublished)
Kosaka, S., Soeda, Y., Hagiwara, S., Hashimoto, S. and Naohara, T.
(1972) Influences of thiophanate-methyl to soil microorganisms add
fate of the chemical in soil. Paper submitted by Nisso Institute for
Life Science, Nippon Soda Co. Ltd. (Unpublished)
Lemperle, E., Kerner, B., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstünde und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Deutsche Leb.-Rdschau, 69, 313
Makita, T., Hashimoto, Y., and Noguchi, T. (1973) Mutagenic,
Cytogenetic and teratogenic Studies on thiophanate methyl. Toxicol
Appl. Pharmacol, 24(2): 206-215
Martens, P. H. and Chs, M. Détermination oscillopolarographique des
residue do thiophanate sur légumes divers. Model. Faknld.
Landbonetensch., Gent, 37, 891
Mori, H. 1972 Human Handling experiences from Plant Employees
manufacturing Topsin (1). Unpublished report from Nisso Takaoka
Hospital submitted by Nippon Soda Co. Ltd.
Nippon Soda Co. Ltd. (1973a) Information on usage and the occurrence
of residues of thiophanate-methyl. Report submitted in August 1973 by
Nippon Soda Co. Ltd. (Unpublished)
Nippon Soda Co. Ltd. (1973b) List of maximum residue of
thiophanate-methyl and its metabolite, MBC in/on edible crops in
the world. Compiled by the Nippon Soda Co. Ltd. October 1973
(Unpublished)
Noguchi, T. and Hashimoto, Y. (1970a) Toxicological evaluation of
Thiophanate Methyl (I) Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from Nisso Institute for Life
Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate methyl (II) studies on the subchronic oral toxicity of
thiophanate-methyl in mice. Unpublished report from Nisso Institute
for, Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity of
thiophanate-methyl in rats. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970d) Toxicological evaluation of
thiophanate-methyl (IV). Studies on the teratogenic effect of
thiophanate-methyl upon the fetus of ICR strain mice. Unpublished
report from Nisso Institute for Life Sciences submitted by Nippon Soda
Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970e) Toxicological evaluation of
thiophanate-methyl (V). Some pharmacologic properties of a new
fungicide thiophanate-methyl. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1971) Study on contact phototoxicity
and sensitivity to new fungicide, thiophates. Unpublished report from
Nisso Institute for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T., Ohkuma and Kosaka, S. (1971) Chemistry and Metabolism of
thiophanate fungicides. Proc. Int. Symp. Pesticide Terminal Residues,
Tot Aviv, pp. 257-270
Noguchi, T. (1972) Environmental Evaluation of Systemic Fungicides.
Environmental Toxicology of Pesticides, Academic Press N.Y., pp.
607-632
Ono, S. (1973a) Analytical method for residues of thiophanate-methyl
and 2-methyl benzimidazolecarbamate in crops by U.V.-spectrometry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Ono, S. (1973b) Stability of thiophanate-methyl under analytical
procedures. Paper submitted by Nippon Soda Co. Ltd. (Unpublished)
Palmer, A.K., Lowell, M.R, and Newman, A.J. (1972) Effect of
thiophanate-methyl on reproductive function of multiple generation in
the rat. Unpublished report from Huntingdon Research Center submitted
by Nippon Soda Co. Ltd.
Pease, H.L. and Gardiner, J.A. (1969) Fluorometric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.P. Improved method for determining benomyl
residues. J. Assoc. Off. Analyt. Chem., 54, 1399
Selling, H.A., Vonk, J.W. and Sijpesteijn, A.K. (1970) Transformation
of the systemic fungicide methyl thiophanate into 2-benzimidazole
carbamic acid methyl ester. Chem. Ind., pp. 1625-1626
Soeda, Y., Kosaka, S. and Noguchi, T. (1972a) The fate of thiophanate.
methyl fungicide and its metabolites on plant leaves and glass plates.
Agr. Biol. Chem., 36(6): 931-936
Soeda, Y., Kosaka, S. & Noguchi, T. (1972b) Identification of Alkyl
2-benzimidazole carbamates as a major metabolite of thiophanates
fungicide in/on the bean plant. Agr. Biol. Chem., 36(6): 817-823
Sugioka, K., Toyama, N. and Kamimura, H. (1973) Residue analytical
method of thiophanate-methyl and a transformed product, methyl
2-benzimidazole (MBC) in soils and plant tissues by colorimetry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Tanoue, T., Toyama, N. & Karimura, H. (1973) Residue analytical method
of thiophanate-methyl in plant tissues by thin layer chromatography.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Vonk, J.W. & Sijpesteijn, A.K. (1971) Methyl benzimidazol-2-
ylcarbamate, the fungitoxic principle of thiophanatemethyl. Pest.
Sci., 2, 160
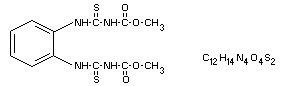 Other information on identity and properties
Molecular weight: 342.40
State: Crystalline solid, colourless
Melting point: 17°C decomposed
Solubility: Chloroform 2.62% (w/w)
(at 21°C) Methanol 2.92% "
Acetone 5.81% "
Ethyl acetate 1.19% "
Acetonitrile 2.44% "
Cyclohexane 4.30% "
Slightly soluble in n-Hexane and water.
Stability: Stable in acidic solutions. At pH = 7 slight,
but measurable formation of methyl
benzimidazol-2-ylcarbamate (MBC). Unstable in
alkaline solution.
Purity of
technical material: Purity 96.1%
Sulfur 1.0%
Sodium chloride 1.5%
Loss on drying 0.5%
Other components 0.9%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
The potential metabolites of thiophanate-methyl described on the
basis of chemical reactivity are as follows:
Other information on identity and properties
Molecular weight: 342.40
State: Crystalline solid, colourless
Melting point: 17°C decomposed
Solubility: Chloroform 2.62% (w/w)
(at 21°C) Methanol 2.92% "
Acetone 5.81% "
Ethyl acetate 1.19% "
Acetonitrile 2.44% "
Cyclohexane 4.30% "
Slightly soluble in n-Hexane and water.
Stability: Stable in acidic solutions. At pH = 7 slight,
but measurable formation of methyl
benzimidazol-2-ylcarbamate (MBC). Unstable in
alkaline solution.
Purity of
technical material: Purity 96.1%
Sulfur 1.0%
Sodium chloride 1.5%
Loss on drying 0.5%
Other components 0.9%
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biochemical aspects
The potential metabolites of thiophanate-methyl described on the
basis of chemical reactivity are as follows:

 In vitro transformation of thiophanate methyl to methyl
benzimidazole carbamate, was dependent on pH and temperature (Fuchs et
al., 1972).
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973) 14C- or 35S-labelled thiophanate-methyl were fed to rats, mice
and dogs. 80-100% of the administered amounts were recovered in faeces
and urine within 96 hours after administration of the compound. With
faeces 60%, 16-27% and 14% respectively were excreted for the three
species, while urinary excretion accounted for 30%, 66-78% and 74%,
respectively, of the administered thiophanate-methyl. Fujino et al.
(1973) have suggested the metabolism scheme shown in Fig. 2. They
report that the major part of faecal excretion was in the form of
unmetabolized thiophanate-methyl, while the minor parts consisted of
4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-O-phenylenebisallophanate (FH-432). Methyl
2-benzimidazol carbamate (carbendazim) and 5-hydroxy-MBC (5-OH-MBC)
were also observed during TLC identifications of metabolites of faecal
extracts. It was, however, questioned whether these two were actual
metabolites in faeces or if they were compounds produced during the
analytical procedures from thiophanate-methyl and 4-OH-TM,
respectively.
Thiophanate-methyl and a number of metabolites could be liberated
enzymatically or by acid treatment from water soluble conjugates in
rat urine in the same studies by Fujino et al. (1973). Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (Fig. 2). As was the case in faeces, the two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical procedures.
In the earlier study by Noguchi et al. (1971) of the conversion
of thiophanate methyl by rat liver microsomes, MBC and 5-OH-MBC were
also found to be present. Evidence for enzyme induction could not be
attained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Although the presence of phenylene-thiourea has been suggested
there is no evidence from the studies in rats, mice and dogs that this
metabolite actually is formed.
Thiophanate-methyl has been reported to be reasonably unstable on
leaf surfaces with the majority of material degrading to MBC and the
oxygen analogue of thiophanate. In contrast to animal studies where
these two metabolites appear to be of minor significance they appear
to be significant metabolites in plants (Soeda et al., 1972a and b).
In aqueous solution following irradiation by UV or sunlight MBC was
the only metabolite found (Buchenauer et al., 1973).
The hazard to the environment associated with the use of
thiophanate-methyl appears to be minimal. It is unstable in soil
degrading within one week and appears to be relatively selective in
that levels of up to 1000 ppm had no effect on a soil population of
bacteria, fungi and actinomycetides (Noguchi, 1972).
Effects on enzymes and other biochemical parameters
Male rats administered 1000 mg/kg orally were examined for
cholinesterase activity in blood and brain four hours following
dosing. No significant change in erythrocyte, serum or brain
cholinesterase was observed over the period (Hashimoto et al., 1972b).
TOXICOLOGICAL STUDIES
Acute toxicity
Signs of poisoning include tremors 1-2 hours after high level
exposure which lead to tonic or clonic convulsions. Nose bleeding and
lacrimation were observed in rats. A slight decrease in respiratory
rate, lethargy, disappearance of tonus of abdominal muscle, discharge
from the eye and mydriasis were observed in rabbits and dogs
(Hashimoto of al., 1972a).
Species Route LD50 (mg/kg) References
Rat M oral 7 500 Hashimoto et al.1972a;
M ip 1 640 Noguchi of al., 1970a
F oral 6 640 "
F ip 1 140 "
M & F dermal >10 000 "
Mice M oral 3 510 "
M ip 790 "
F oral 3 400 "
F ip 1 110 "
M & F dermal >10 000 "
Guinea-pig M oral 3 640 "
F oral 6 700 "
(cont'd)
Species Route LD50 (mg/kg) References
Rabbit M 2 270 "
F 2 500 "
Dog M 4 000 "
F 4 000 "
Japanese
Quail M & F >5 000 "
Contact phototoxicity and photosensitivity
Thiophanate-methyl applied to the shaved skin of rabbits and
guinea-pigs was exposed to erythrogenic and nonerythrogenic light.
There was no evidence of phototoxicity in either species.
Thiophanate-methyl was topically applied to the shaved skin of
guinea-pigs four times over a nine-day period and three weeks later.
After exposure the area was exposed to erythrogenic and
nonerythrogenic radiation. No positive photosensitivity reactions were
noted (Noguchi and Hashimoto, 1971; Hashimoto et al., 1972).
Subacute dermal toxicity
Groups of guinea-pigs (five per group) were administered
thiophanate-methyl to the abraded dorsum daily for 30 days at a dosage
level of 0, 2, 20 and 200 mg/kg. No evidence of dermal toxicity or
irritation was noted (Noguchi and Hashimoto, 1972).
Inhalation toxicity
Groups of 10 male mice were exposed to formulations of
thiophanate-methyl for periods of time of 30, 60, 120 minutes at a
concentration of 100 mg/litre through an aerosol generator. There were
no deaths in any group including the controls although such findings
as lacrimation, salivation and nasal discharge were observed soon
after exposure. All animals appeared normal within 24 hours following
exposure (Hashimoto et al., 1972a).
Dermal irritation
Rabbits were dermally administered thiophanate-methyl at levels
of 1, 0.1, and 0.01 g daily for 21 days. A slight reversible erythema
was observed in the highest dose group. No skin irritation was evident
in the two lower dose groups (Noguchi and Hashimoto, 1970a; Hashimoto
et al., 1972a).
Cutaneous sensitization
Guinea-pigs were examined for cutaneous sensitization by
intradermal injection of 50 µl and 100 µl at a 1% aqueous solution on
alternate days for 20 days. Two weeks later the guinea-pigs were
challenged with 50 µl injected into the same area. Animals treated
with thiophanate-methyl exhibited no primary irritation and only a
slightly sensitive state (Noguchi and Hashimoto, 1970a; Hashimoto et
al., 1972a).
Pharmacological properties
Thiophanate-methyl administered to rats at dosages up to 1500
mg/kg caused a slight increase in body temperature. Mice administered
500 mg/kg subcutaneously appeared to have a slight transient
insensitivity to heat. Studies with mice and rats administered
thiophanate-methyl at levels of 100 or 500 mg/kg subcutaneously showed
neither a sedative nor hypnotic effect. Oral administration of 1000
mg/kg to mice resulted in no significant mydriatic effect. No surface
anaesthetic action or irritation of the mucous membrane of the eye of
rabbit was observed following the administration of up to 10%
thiophanate-methyl in saline instilled into the conjunctival sac of
rabbits. Studies on excised mouse intestine at levels of up to 1 X
10-3 mg/ml, resulted in no atropine-like or papaverine-like activity.
The same concentrations did not inhibit the contraction of excised
guinea-pig intestine by histamine hydrochloride or of an excised strip
of guinea-pig aorta by epinephrine. After a single oral dose of
thiophanate-methyl of 100 mg/kg, the blood pressure of rat did not
change. Following high oral administration of thiophanate-methyl to
rabbits, there was a slight transitory decrease in white blood cell
count. In a similar manner, the white cell count of rabbits was also
depressed within a short period of time following acute oral
administration of 1000 mg/kg.
A temporary fall in blood pressure in the rabbit, followed by a
persistent rise and a persistent bradycardia was observed following
i.v. or oral administration of 1000 mg/kg. There was no change in
respiration or ECG following acute oral administration. Lethal doses
of thiophanate-methyl showed an immediate blood pressure drop to zero,
gradual disturbance of the ECG followed by cessation of respiration.
Except at very high levels there was no unusual pharmacological
properties attributed to thiophanate-methyl. All adverse
pharmacological properties were completely reversible within a short
period of time (Hashimoto of al., 1972b; Noguchi and Hashimoto,
1970e).
Special studies on carcinogenicity
Mouse. A carcinogenicity study was performed on mice of the ICR-SCL
strain.
Groups of mice (50 males and 50 females per group) were fed
thiophanate-methyl in the diet at levels of 0, 10, 40, 160 and 640 ppm
for 24 months. A sample of thiophanate-methyl used in this study was
approximately 94% active ingredient with impurities of 2% sulfur and
2% inorganic chloride, 2% unknown volatile substances and less than
0.5% aromatic amines. Animals in this study were SPF-derived and were
maintained under barrier-conditions to minimize infection. Diets were
prepared at an institute outside of the laboratory performing the
study and food was allotted bi-weekly during which time food
consumption data were recorded. Body weights were recorded initially
and monthly throughout the study with daily mortality and behavioural
examinations. Pathological examinations, using H&E stains primarily,
were performed on all animals which died or were sacrificed prior to
the conclusion of the study. After 105 weeks all animals were
sacrificed and histological examinations were performed on a variety
of tissues.
There was a slight retardation of growth in males at 640 ppm in
the diet although this was not reflected in females. Food consumption
was comparable to that of controls. Mortality and average survival
time over 105 weeks was the same in controls and at all dietary
levels. A variety of tumours was found in all groups including the
controls. All hepatomas observed in the experiment were benign liver
cell adenomas except for three liver cell derived adenocarcinomas (1
female at 40 ppm and one male and one female at 160 ppm). The
incidence of hepatoma was similar in all of the experimental groups
including the controls. Pulmonary benign adenomas were also
significantly observed in all cases with no differences in the control
or any feeding group. Leukaemia was also observed frequently with a 20
to 30% incidence in each group. In consequence, there was essentially
no difference in occurrence of tumours between experimental and
control groups. The results of this study indicate that the ingestion
of thiophanate-methyl in the diet in levels up to 640 ppm to a mouse
strain which appears to be significantly susceptible to various
tumours results in no carcinogenic activity related to
thiophanate-methyl feeding (Kosaka and Tsubura, 1973).
Special studies on cytogenicity
Male rats were administered thiophanate-methyl daily for five
days by intraperitoneal injection at levels of 0, 62.5, 125, 250, 500
and 1000 mg/kg/day. Bone marrow and spermatogonial cells examined
showed no abnormal chromogomal configurations (Makita et al., 1973).
Special studies on mutagenicity
Groups of 10 males ICR-strain mice were administered
thiophanate-methyl intraperitoneally at a single dose level of 0, 8,
40, 200, 400 or 500 mg/kg and mated with virgin females which were
replaced weekly for a period of eight weeks. At a dosage of 400 mg/kg
and above there appeared to be a reduction in the incidence of
pregnancies. However, there was no systematic variation indicative of
a mutagenic potential over the entire eight-week mating period (Makita
et al., 1973).
Special studies on reproduction
Groups of Charles-River rats (10 males and 20 females per group)
were fed levels of thiophanate-methyl in the diet at 0, 40, 160, 640
ppm in a three-generation, two-litters per generation reproduction
study. There were no apparent effects of thiophanate-methyl at levels
up to and including 640 ppm on any of the reproduction parameters
measured in this experiment. In addition, gross and histological
examinations of the F3b generation were performed on several tissues
and organs of three-week-old rats and a further group of animals was
examined for skeletal abnormalities. In no instance was there any
effect of feeding thiophanate-methyl on reproduction. There was a
definitive effect on the growth of animals fed dietary levels of 640
ppm (Palmer et al., 1972).
Special studies on teratogenicity
Thiophanate-methyl was administered to pregnant ICR mice from day
1 to day 15 of gestation at levels of 0, 40, 200, 500 and 1000
mg/kg/day. At 1000 mg/kg/day there was a significantly reduced number
of living fetuses. No (significant) differences in the number of
implantation sites, body weight of fetuses or fetal mortality or body
weight were observed at the lower dosage levels. The administration of
thiophanate-methyl did not produce gross internal or external
abnormalities and the study did not reveal any teratogenic properties
under these experimental conditions (Noguchi and Hashimoto, 1970a;
Makita et al., 1973).
Short-term studies
Mouse. Groups of six-week-old SPFICR-strain mice (12 males and 12
females per group) were fed thiophanate-methyl at levels of 0, 12.8,
64, 390, 1600 and 8000 ppm in the diet for six months. Daily
observations were made on their behaviour and mortality and body
weight and food consumption data were recorded weekly. Haematological
and gross and microscopic examination of tissues and organs were
performed at the end of the feeding period.
There were no unusual behavioural patterns and incidence of
mortality was not related to the feeding of thiophanate. Growth of all
mice at levels of 1600 ppm and below was not affected by
thiophanate-methyl with a retardation of growth observed at 8000 ppm.
Food intake and haematological examinations were normal. Gross
pathology at the termination of the experiment showed that there was
an increase in the mean weight of liver in animals fed 8000 ppm with
an increase in liver to body weight ratio. This was noted in both
males and females. There were no effects of this kind noted at a dose
level of 1600 ppm. Pathological abnormalities were noted in liver at
8000 ppm in both males and females. There was swelling of large
hepatic cells with concurrent swelling. It was observed that the
protoplasm of these cells were oedema-like or granule-like. Although
such pathological abnormalities were found in some mice of other
groups as well as the control, it appeared to be more prevalent in the
8000 ppm animals. In the present experiment the no-effect level of
thiophanate-methyl is considered to be 1600 ppm (Noguchi and
Hashimoto, 1970b).
Groups of SPF-Sprague Dawley rats (12 males and 12 females per
group) were fed thiophanate-methyl in the diet at levels of 0, 12.8,
64, 320, 1600, 8000 ppm for six months under SPF conditions. Daily
checks of mortality and abnormal behaviour were made throughout the
experiment with body weight and food intake data recorded weekly. At
the conclusion of the study, haematology, clinical chemistry and
urinalyses were performed. Gross and microscopic examination of
tissues and organs was also performed at the conclusion of the feeding
period.
There were no behavioural abnormalities observed during the
six-month feeding study and mortality was not related to food intake
of thiophanate-methyl, Examination of growth curves indicated a severe
retardation of both males and females fed 8000 ppm in the diet. There
did not appear to be any retardation in growth of animals fed 1600 ppm
or below. Food intake of all groups was comparable with control
values. All haematological values were comparable with controls as
were the values obtained from urine analysis. At 8000 ppm there was a
slight decrease in glucose and GOT levels and an increase in total
cholesterol. Gross and histological examination of tissues showed only
the liver and thyroid glands to be slightly affected by
thiophanate-methyl in the diet. There was an increase in liver weight
and liver to body weight ratio although there were no pathological
changes such as cloudy swelling and irregularity in the size of the
hepatic cells which was noted in the studies in mice. In the thyroid
gland some abnormalities such as small follicles, cubic epithelium
cells and a decrease in colloidal substance were observed in those
animals fed 8000 ppm. These changes were not observed in animals fed
1600 ppm and were not accompanied by any change in the PBI values.
Abnormal findings were not noted in any other tissues.
From these studies it appears that a no-effect level of 1600 ppm
in the diet of rats for six months would result in no unusual effect
(Noguchi and Hashimoto, 1970c).
Dog. Groups of pure bred beagle dogs (five males and five females
per group - four males and four females per group at the highest dose
level) were administered thiophanate-methyl at levels of 0, 2, 10, 30
and 250 mg/kg/day for two years. The animals were approximately six
months old at the time of the inception of the testing and were housed
singly in indoor kennels. In all groups one male and one female were
sacrificed at 12 months with the remaining animals sacrificed after 24
months on test. Dosing was performed by oral administration of
thiophanate-methyl by capsule once a day seven days a week for two
years. Doses were given to individual animals related to the body
weight and were adjusted weekly. Clinical signs of any abnormalities
were recorded daily. Food consumption and body weight changes were
recorded weekly. Throughout the study the eyes were examined for any
changes and electro cardiographs were taken periodically.
Haematological examinations, blood chemistry analyses and urinalyses
were performed periodically. At the conclusion of the study gross and
microscopic examination of tissues and organs was performed on all
animals.
There was no mortality or adverse behavioural changes observed in
these animals during the course of the study. Growth of the animals
fed 250 mg/kg/day was slightly retarded in both males and females.
There did not appear to be a significant decrease in any other group.
There did not appear to be any significant differences in the
haematological studies, blood chemistry, urinalysis, ophthalmological
examinations, or the ECG during the course of the 24-month study.
Pathological (gross and histological) examination of the animals
sacrificed at 12 months did not show any significant changes in the
tissues examined with regard to gross pathology and organ weight
ratios. Slight changes observed at 250 mg/kg in the thyroid gland were
difficult to determine from the one year data on dogs.. Animals
sacrificed at 24 months showed an elevated thyroid weight in both
males and females at 50 and 250 mg/kg although there were no changes
in the PHI values. Although the average weight of the thyroid of the
two highest dose groups was heavier than that of the controls,
histological examination showed no differences in the thyroid of the
control and the experimental groups. Histological findings in all
other tissues were also normal.
The no-effect level in this two-year study in dogs is considered
to be 50 mg/kg/day based upon the marginal effect noted in thyroid
weight at the conclusion of the study. Although this increased thyroid
weight was not reflected in the histological examination or PBI
values, it has been noted in other species (rat). A more noticeable
effect was observed at 250 mg/kg relating to retarded growth in both
males and females (Hashimoto and Fukuda, 1972).
Long-term studies
Rat (See also under the section: "Special studies on
carcinogenicity"). Groups of SPF-Sprague-Dawley strain rats (35 males
and 35 females per group, 50 males and 50 females were used as
controls) were fed thiophanate-methyl in the diet at levels of 0, 10,
40, 160, 640 ppm for two years.
The animals were maintained under SPF conditions. The sample of
thiophanate-methyl fed in this experiment was approximately 94% active
ingredient with impurities of 2% sulfur, 2% inorganic chlorides, 2%
unknown volatile substances and less than 0.5% aromatic amines. The
animals were fed dietary levels of thiophanate methyl for a period of
10 days during which behaviour and mortality were recorded daily and
after which food consumption was measured on all animals. Body weight
and growth were recorded weekly. Haematology, blood chemistry and
urinalyses were performed periodically over the two-year study. At the
conclusion of the study animals were sacrificed and gross and
microscopic examination of tissues and organs was performed.
There was no effect of feeding levels of up to 640 ppm of
thiophanate-methyl in the diet of rats on behaviour and mortality over
a two-year period. Growth curves indicate a slight retardation of
growth in both male and female rats in fed diets containing 640 ppm.
Food consumption at all dietary levels was comparable with the control
groups. Haematological data, urinalysis data and clinical blood
chemistry data were not abnormal in any of the groups examined. Gross
pathological examination of animals showed no changes in organ weight
in any group with the exception of slight increases in kidney weight
and kidney to body weight ratio, primarily in the males at the highest
level of feeding. There was no indication of increased thyroid weight
or an increase in liver size as was observed in shorter duration test
with rats. In addition there were frequent observations of
inflammation of the lung, fibroadenoma of the breast and the
occurrences of pituitary tumours. The administration of
thiophanate-methyl was not believed to be responsible for the
occurrence of these abnormalities. At the end of two years there was a
dose response in a degenerative change in the testis which underwent a
degeneration and atrophy including spermatogenesis. In this particular
dose-response change several cases were noted in other groups
including the control. This effect was probably not related to
ingestion of thiophanate-methyl. At the end of two years there was
also some change in the thyroid where colloidal epithelial cells were
enlarged. This enlargement took place primarily in the males of the
highest dose group. There was no apparent effect on the occurrence of
tumours and the only significant effects appear to be in the decreased
growth observed at 640 ppm in the diet.
Based upon retardation of growth a no-effect level would be
considered to be 160 ppm in the rat fed thiophanate-methyl for two
years (Hashimoto and Tsubura, 1972).
Observations in man
Sixteen workers concerned with the production of
thiophanate-methyl were examined periodically for 3.5 years. Blood and
urine analysis were carried out every six months. No adverse effects
were found with these workers with regard to blood chemistry and urine
analysis (Mori, 1972).
Comments
Thiophanate-methyl is degraded in plants to carbendazim and
hydroxylated derivatives. A question was raised if the apparent
occurrence of carbendazim in mammals may be an analytical artefact. A
theoretical metabolite of potential toxicological significance,
phenylene thiourea, has not been reported in plant or animal systems.
Thiophanate-methyl has a low acute toxicity in various species of
animal and shows pharmacological activity only at very high levels.
Adequate short-term and long-term studies are available in both
rats and dogs. The results of a three-generation reproduction study,
mutagenesis studies and a cytogenicity study were negative.
Thiophanate-methyl did not appear to be a carcinogen in a susceptible
strain of mice and two-year studies in rats and dogs gave rise to
retardation of growth only at high dietary levels (640 ppm). There was
a marginal increase in thyroid weight at higher dose levels which was
not accompanied by adverse biochemical or histological effects. The
long-term study in the rat was used as a basis for estimating an ADI
for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 160 ppm in the diet equivalent to 23 mg/kg bw
Rat: 160 ppm in the diet equivalent to 8 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of an acceptable daily intake for man
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Thiophanate-methyl was first registered for use as a systemic
fungicide in Japan in May 1971. By 1973 it has obtained provisional
clearance for safe use in Great Britain for a number of specified
preharvest treatments on food crops and ornamentals and for
post-harvest dipping of apples.
Thiophanate-methyl is formulated into wettable powders containing
50 and 70% active ingredient.
Recommended use levels are from 0.02-0.07% a.i. usually applied
at the rate of 1-5 kg a.i. per ha for pears, peaches and citrus and of
up to 2 kg a.i. per ha for other fruits and vegetables. Repeated
applications are claimed to be advantageous.
Thiophanate-methyl is reported to be very similar to benomyl
(BenlateR) as regards both its systemic functions (Aelbers, 1970)
and the rather broad spectrum of fungal diseases against which it is
effective (Bollen, 1971). It is claimed effective against apple and
pear scab, powdery mildew and different moulds, rots and blight.
Pre-harvest application on citrus trees should effectively reduce
Penicillium decay on citrus fruits under post-harvest storage.
The similarity between thiophanate-methyl and benomyl seems well
explained by the fact that both compounds are converted into methyl
2-benzimidazolecarbamate (MBC), which is reported to be the principal
fungitoxic compound to which both chemicals owe their major activity
(Vonk and Sijpesteijn, 1971; Selling et al., 1970).
Residues resulting from supervised trials
Data derived from extensive field trials in several countries on
thiophanate-methyl residues have been presented by the manufacturer
(Nippon Soda Company Ltd, 1973a). A summarized list of a selection of
the maximum residues found in these trials is presented in Table 1
(Nippon Soda Company Ltd, 1973b). The majority of information in
Table 1 shows residue levels at 0 or 1 day after last application.
Generally, the major part of residues is in the form of unchanged
thiophanate-methyl, while methyl 2-benzimidazolecarbamate (MBC) is
usually detectable in smaller amounts, averaging 10% of the total.
Apples, pears, peaches
Generally, higher levels of thiophanate-methyl residues are found
in these fruits after post-harvest treatments than after pre-harvest
sprayings. This is partly due to higher initial deposits from dipping
and partly connected to volatility losses to which the pre-harvest
treated fruits are subjected. During storage experiments performed in
the United Kingdom by the manufacturer, pears treated after harvest
TABLE 1. RESIDUES OF THIOPHAMATE-METHYL AND MBC IN VARIOUS CROPSa,b
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Apple 1.4 kg/ha 10× 1.4 - Fed. Rep. Ger.
0.6 g/l 1× 0.83 0.14 Canada
1-2 kg/ha 17×+
+1000 pp. Dipping 3.8 0.8 UK
2100 ppm Dipping 3.4 Italy
Banana 1000 pp. Dipping Peel: 0.3 0.4 Australia
Pulp: 0.1 <0.2
Beans, broad 2 kg/ha 1× 0.1 0.5 UK
" dwarf 1 kg/ha 1× 2.3 0.1 UK
" french 1.5 kg/ha 1× 0.2 0.2 US
" kidney 0.75 kg/ha 2× 0.2 Japan
" runner 1 kg/h. 1× 1.8 0.1 UK
Blackcurrant 5.4-7.5 kg/ha 4× 0.day:19.4 4.9 UK
5.4-7.5 kg/ha 4× 7.day:7.0 3.3 UK
1 kg/ha 4.5× 3.0 2.3 UK
Carrots 1000 ppm Dipping 5.0 0.2 UK
Celery low pp. Dipping 28.0 0.8 UK
Cherries 1.4 g/l 3× 2.0 0.2 Australia
2 kg/ha 5× 0.46 0.64 USA
Citrus 3.5 kg/ha 1× Peel: 12.2 2.1 Japan
Pulp: 0.3 <0.2
Cucumber 1 kg/ha 6× 0.45 0.09 Japan
Gherkins 1 g/l 1.87 0.33 Netherlands
Gooseberries 1 kg/ha 4× 3.0 0.4 UK
Grapes 1.4 kg/ha 5× 3.3 - Fed. Rep. Ger.
1.4 kg/ha 19× 2.8 Japan
2.4 g/l 6× 0.6 <0.2 Australia
Lettuce 1 kg/ha 3× 0.day:10.0 0.6 UK
7.day:0.1 <0.2
2.8 kg/ha 6× 0.day:24.5 5.0 Japan
7.day:3.8 1.5
2.2 kg/ha 1× 0.day:4.1 New Zealand
TABLE 1. (Cont'd.)
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Mushroom about 5 g/m2 1× <0.1 0.7 UK
Onion 2.5 kg/ha 10× 0.05 0.02 Japan
Orange 1000 ppm Dipping Peel: 1.2 <0.3 Australia
Pulp: 0.2 <0.3 Australia
Juice: 0.4 <0.2 Australia
1000 ppm Dipping Peel: 2.3 0.23 USA
2100 ppm Dipping 8.8 Italy
Peach 2.4 g/l 4× 5.0 0.4 Australia
Pear 1000 ppm Dipping 2.2 <0.1 UK
2100 ppm Dipping 3.9 Italy
Plum 1 kg/ha 6× 0.86 0.37 USA
Raspberries 1 kg/ha 8× 9.6 2.3 UK
Strawberries 1.5 kg/ha 1× 0.day:14.6 UK
7.day:3.0
1 kg/ha 4-5× 0.day:3.8 1.2 Netherlands
(under glass) 7.day:3.6 0.9
14.day:0.9 0.6
Sugarbeet 0.35 kg/ha 2× Leaf:3.6 Fed. Rep. Oar.
Root: <0.2
Wheat, winter 0.5 kg/ha 1× Straw: <0.2 (50 days)
Grain: <0.2
Tomato 2 kg/ha 5× 9.7 0.4 UK
a Residues reported are maximum residues, i.e. levels found on 0. or 1. day after application,
except when otherwise stated.
b Analytical methods used were as follows (see monograph text):
TLC-method: Australia, Japan, New Zealand
Colorimetry: Canada, UK, USA
UV-spectrophotometry: Japan, Federal Republic of Germany
Oscillopolarography: Italy, Netherlands (TLC-bioassay for MBC).
with thiophanate-methyl showed no pronounced decrease of residues
(about 2 ppm) during 61 days, while on the other hand, residues of
about 1 ppm on apples decreased well below 0.1 ppm during four weeks
on the trees after spraying.
Grapes and wine
Lemperle et al. (1973) found that thiophanate-methyl residues, as
well as those of other benzimidazole fungicides, are transferred
nearly quantitatively into unclarified musts during wine production.
During clarification and fermentation small amounts may disappear. A
considerable part of the thiophanate-methyl residues which are present
in grapes may therefore be found in wines.
Leafy vegetables
The residue data supplied by the manufacturer indicate that even
relatively high residues are subject to pronounced losses, which may
be explained as usual growth dilution effect to which volatility
losses from the foliar surfaces may be added. From field trials with
lettuce performed in Japan, half-life values of the order of 1-2 days
for thiophanate-methyl have been deducted, while the MBC-metabolite
present in smaller amounts disappeared more slowly, corresponding to
half-life values of 5-6 days.
For comparison, the half-life of thiophanate-methyl on apple and
grape leaves under controlled conditions was found to be in the order
of 15 and 12 days, respectively (Soeda et al., 1972).
Carrots, celery, sugarbeets
When subjected to post-harvest treatments with thiophanate-methyl
these root-crops take up considerable amounts of residues. Dipping of
carrots and celery performed in the United Kingdom with 0.1% a.i. gave
total residues of thiophanate-methyl + MBC of 5.2 and 28.8 ppm,
respectively. Pre-harvest sprays gave high, though rapidly
disappearing, residues on the green tops above soil, but no detectable
residues in the edible roots.
Fate of residues
Substantial evidence has been presented by the manufacturer that
methyl 2-benzimidazolecarbamate (MBC) is produced as the major
metabolite (Noguchi et al., 1971) although only gradually released
from thiophanate-methyl when applied on foliar surfaces (Kikuchi,
1973). Vonk and Sijpesteijn (1971) found that the fungitoxic activity
of thiophanate-methyl is increased by aging in aqueous media. They
concluded that MBC is responsible for the fungitoxic effect of the
fungicide and indicated that the rate of conversion from
thiophanate-methyl may be increased by the fungal metabolic activity.
Selling et al. (1970) also observed the conversion of
thiophanate-methyl to MBC in tap water. A 4% transformation was noted
when kept at pH = 7.0 in water for seven days, while
thiophanate-methyl remained stable in methanol or chloroform for 50
days at 24°C (Noguchi et al., 1971).
Plants
Conversion of thiophanate-methyl to MBC has been followed after
uptake through the roots and after foliar treatment of French bean
seedlings (Phaseolus vulgaris) by Noguchi et al. (1971). After 21 days
34.3% of the original amount had been converted on the leaves, while
87.5% was metabolized into MBC when absorbed through the roots in a
water culture. In a most recent publication, the formation of MBC from
thiophanate-methyl is further described by Buchenauer et al. (1973).
They found that the transformation to a great extent is
light-catalyzed (requiring at the same time the presence of water). A
42% transformation of thiophanate-methyl residues was found in cotton
leaves, when subjected to four days of sunlight as opposed to only
about 6%, when the plants were kept under dimlight conditions.
A minor metabolite, 2-aminobenzimidazole (2-AB), also formed from
MBC in benomyl treated plants is detected as a result of
thiophanate-methyl applications as well (Fujino, A. and Kamimura,
1973a). It is further reported by the same authors that two
oxygen-analogues (DX-105 = 1-(3-methyoxy-carbonyl-2
thioureido)-2-(3-methoxycarbonylureido) benzene and FH-132 =
dimethyl-4.4-O-phenylenbis(allophanate)) has recently been identified
by a reverse isotopedilution method (see Fig. 3).
Soil
Thiophanate-methyl is degraded almost entirely within seven days
in sandy loam and silty loam soils at temperatures from 23°C to 33°C
(Pujino and Kamumura, 1973b). MBC is identified as the major
metabolite, which is subsequently degraded at a more moderate rate.
Sixty days after treatment residual MBC was present at the level of
20% at 23°C and 2-15% at 33°C in both soil types, calculated on the
basis of the amount of thiophanate-methyl applied. No influence from
thiophanate-methyl was indicated in these experiments on soil
micro-organisms, when measured by bacterial counts (total bacteria,
gram-negative and actinomycetes). With increased levels of
thiophanate-methyl (up to 5000 ppm) increased oxygen uptake increased
significantly, suggesting that thiophanate-methyl was used as a source
of nitrogen by bacteriae.
The conversion of thiophanate-methyl to MBC is significantly more
rapid in unsterilized than in sterile soils. Similarly, the MBC formed
is less persistent in the unsterilized soil, which suggests that
micro-organisms may be involved in the degradation of both compounds.
TLC-identification patterns further indicate that
dimethyl-4.4'-o-phenyleneallophanate (or 111-432, see Fig. 3) may be
an intermediate metabolic step in the microflora-induced degradation,
as this compound is not found as a metabolite in sterilized soils
(Kosaka et al., 1972).
In vitro transformation of thiophanate methyl to methyl
benzimidazole carbamate, was dependent on pH and temperature (Fuchs et
al., 1972).
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973) 14C- or 35S-labelled thiophanate-methyl were fed to rats, mice
and dogs. 80-100% of the administered amounts were recovered in faeces
and urine within 96 hours after administration of the compound. With
faeces 60%, 16-27% and 14% respectively were excreted for the three
species, while urinary excretion accounted for 30%, 66-78% and 74%,
respectively, of the administered thiophanate-methyl. Fujino et al.
(1973) have suggested the metabolism scheme shown in Fig. 2. They
report that the major part of faecal excretion was in the form of
unmetabolized thiophanate-methyl, while the minor parts consisted of
4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-O-phenylenebisallophanate (FH-432). Methyl
2-benzimidazol carbamate (carbendazim) and 5-hydroxy-MBC (5-OH-MBC)
were also observed during TLC identifications of metabolites of faecal
extracts. It was, however, questioned whether these two were actual
metabolites in faeces or if they were compounds produced during the
analytical procedures from thiophanate-methyl and 4-OH-TM,
respectively.
Thiophanate-methyl and a number of metabolites could be liberated
enzymatically or by acid treatment from water soluble conjugates in
rat urine in the same studies by Fujino et al. (1973). Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (Fig. 2). As was the case in faeces, the two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical procedures.
In the earlier study by Noguchi et al. (1971) of the conversion
of thiophanate methyl by rat liver microsomes, MBC and 5-OH-MBC were
also found to be present. Evidence for enzyme induction could not be
attained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Although the presence of phenylene-thiourea has been suggested
there is no evidence from the studies in rats, mice and dogs that this
metabolite actually is formed.
Thiophanate-methyl has been reported to be reasonably unstable on
leaf surfaces with the majority of material degrading to MBC and the
oxygen analogue of thiophanate. In contrast to animal studies where
these two metabolites appear to be of minor significance they appear
to be significant metabolites in plants (Soeda et al., 1972a and b).
In aqueous solution following irradiation by UV or sunlight MBC was
the only metabolite found (Buchenauer et al., 1973).
The hazard to the environment associated with the use of
thiophanate-methyl appears to be minimal. It is unstable in soil
degrading within one week and appears to be relatively selective in
that levels of up to 1000 ppm had no effect on a soil population of
bacteria, fungi and actinomycetides (Noguchi, 1972).
Effects on enzymes and other biochemical parameters
Male rats administered 1000 mg/kg orally were examined for
cholinesterase activity in blood and brain four hours following
dosing. No significant change in erythrocyte, serum or brain
cholinesterase was observed over the period (Hashimoto et al., 1972b).
TOXICOLOGICAL STUDIES
Acute toxicity
Signs of poisoning include tremors 1-2 hours after high level
exposure which lead to tonic or clonic convulsions. Nose bleeding and
lacrimation were observed in rats. A slight decrease in respiratory
rate, lethargy, disappearance of tonus of abdominal muscle, discharge
from the eye and mydriasis were observed in rabbits and dogs
(Hashimoto of al., 1972a).
Species Route LD50 (mg/kg) References
Rat M oral 7 500 Hashimoto et al.1972a;
M ip 1 640 Noguchi of al., 1970a
F oral 6 640 "
F ip 1 140 "
M & F dermal >10 000 "
Mice M oral 3 510 "
M ip 790 "
F oral 3 400 "
F ip 1 110 "
M & F dermal >10 000 "
Guinea-pig M oral 3 640 "
F oral 6 700 "
(cont'd)
Species Route LD50 (mg/kg) References
Rabbit M 2 270 "
F 2 500 "
Dog M 4 000 "
F 4 000 "
Japanese
Quail M & F >5 000 "
Contact phototoxicity and photosensitivity
Thiophanate-methyl applied to the shaved skin of rabbits and
guinea-pigs was exposed to erythrogenic and nonerythrogenic light.
There was no evidence of phototoxicity in either species.
Thiophanate-methyl was topically applied to the shaved skin of
guinea-pigs four times over a nine-day period and three weeks later.
After exposure the area was exposed to erythrogenic and
nonerythrogenic radiation. No positive photosensitivity reactions were
noted (Noguchi and Hashimoto, 1971; Hashimoto et al., 1972).
Subacute dermal toxicity
Groups of guinea-pigs (five per group) were administered
thiophanate-methyl to the abraded dorsum daily for 30 days at a dosage
level of 0, 2, 20 and 200 mg/kg. No evidence of dermal toxicity or
irritation was noted (Noguchi and Hashimoto, 1972).
Inhalation toxicity
Groups of 10 male mice were exposed to formulations of
thiophanate-methyl for periods of time of 30, 60, 120 minutes at a
concentration of 100 mg/litre through an aerosol generator. There were
no deaths in any group including the controls although such findings
as lacrimation, salivation and nasal discharge were observed soon
after exposure. All animals appeared normal within 24 hours following
exposure (Hashimoto et al., 1972a).
Dermal irritation
Rabbits were dermally administered thiophanate-methyl at levels
of 1, 0.1, and 0.01 g daily for 21 days. A slight reversible erythema
was observed in the highest dose group. No skin irritation was evident
in the two lower dose groups (Noguchi and Hashimoto, 1970a; Hashimoto
et al., 1972a).
Cutaneous sensitization
Guinea-pigs were examined for cutaneous sensitization by
intradermal injection of 50 µl and 100 µl at a 1% aqueous solution on
alternate days for 20 days. Two weeks later the guinea-pigs were
challenged with 50 µl injected into the same area. Animals treated
with thiophanate-methyl exhibited no primary irritation and only a
slightly sensitive state (Noguchi and Hashimoto, 1970a; Hashimoto et
al., 1972a).
Pharmacological properties
Thiophanate-methyl administered to rats at dosages up to 1500
mg/kg caused a slight increase in body temperature. Mice administered
500 mg/kg subcutaneously appeared to have a slight transient
insensitivity to heat. Studies with mice and rats administered
thiophanate-methyl at levels of 100 or 500 mg/kg subcutaneously showed
neither a sedative nor hypnotic effect. Oral administration of 1000
mg/kg to mice resulted in no significant mydriatic effect. No surface
anaesthetic action or irritation of the mucous membrane of the eye of
rabbit was observed following the administration of up to 10%
thiophanate-methyl in saline instilled into the conjunctival sac of
rabbits. Studies on excised mouse intestine at levels of up to 1 X
10-3 mg/ml, resulted in no atropine-like or papaverine-like activity.
The same concentrations did not inhibit the contraction of excised
guinea-pig intestine by histamine hydrochloride or of an excised strip
of guinea-pig aorta by epinephrine. After a single oral dose of
thiophanate-methyl of 100 mg/kg, the blood pressure of rat did not
change. Following high oral administration of thiophanate-methyl to
rabbits, there was a slight transitory decrease in white blood cell
count. In a similar manner, the white cell count of rabbits was also
depressed within a short period of time following acute oral
administration of 1000 mg/kg.
A temporary fall in blood pressure in the rabbit, followed by a
persistent rise and a persistent bradycardia was observed following
i.v. or oral administration of 1000 mg/kg. There was no change in
respiration or ECG following acute oral administration. Lethal doses
of thiophanate-methyl showed an immediate blood pressure drop to zero,
gradual disturbance of the ECG followed by cessation of respiration.
Except at very high levels there was no unusual pharmacological
properties attributed to thiophanate-methyl. All adverse
pharmacological properties were completely reversible within a short
period of time (Hashimoto of al., 1972b; Noguchi and Hashimoto,
1970e).
Special studies on carcinogenicity
Mouse. A carcinogenicity study was performed on mice of the ICR-SCL
strain.
Groups of mice (50 males and 50 females per group) were fed
thiophanate-methyl in the diet at levels of 0, 10, 40, 160 and 640 ppm
for 24 months. A sample of thiophanate-methyl used in this study was
approximately 94% active ingredient with impurities of 2% sulfur and
2% inorganic chloride, 2% unknown volatile substances and less than
0.5% aromatic amines. Animals in this study were SPF-derived and were
maintained under barrier-conditions to minimize infection. Diets were
prepared at an institute outside of the laboratory performing the
study and food was allotted bi-weekly during which time food
consumption data were recorded. Body weights were recorded initially
and monthly throughout the study with daily mortality and behavioural
examinations. Pathological examinations, using H&E stains primarily,
were performed on all animals which died or were sacrificed prior to
the conclusion of the study. After 105 weeks all animals were
sacrificed and histological examinations were performed on a variety
of tissues.
There was a slight retardation of growth in males at 640 ppm in
the diet although this was not reflected in females. Food consumption
was comparable to that of controls. Mortality and average survival
time over 105 weeks was the same in controls and at all dietary
levels. A variety of tumours was found in all groups including the
controls. All hepatomas observed in the experiment were benign liver
cell adenomas except for three liver cell derived adenocarcinomas (1
female at 40 ppm and one male and one female at 160 ppm). The
incidence of hepatoma was similar in all of the experimental groups
including the controls. Pulmonary benign adenomas were also
significantly observed in all cases with no differences in the control
or any feeding group. Leukaemia was also observed frequently with a 20
to 30% incidence in each group. In consequence, there was essentially
no difference in occurrence of tumours between experimental and
control groups. The results of this study indicate that the ingestion
of thiophanate-methyl in the diet in levels up to 640 ppm to a mouse
strain which appears to be significantly susceptible to various
tumours results in no carcinogenic activity related to
thiophanate-methyl feeding (Kosaka and Tsubura, 1973).
Special studies on cytogenicity
Male rats were administered thiophanate-methyl daily for five
days by intraperitoneal injection at levels of 0, 62.5, 125, 250, 500
and 1000 mg/kg/day. Bone marrow and spermatogonial cells examined
showed no abnormal chromogomal configurations (Makita et al., 1973).
Special studies on mutagenicity
Groups of 10 males ICR-strain mice were administered
thiophanate-methyl intraperitoneally at a single dose level of 0, 8,
40, 200, 400 or 500 mg/kg and mated with virgin females which were
replaced weekly for a period of eight weeks. At a dosage of 400 mg/kg
and above there appeared to be a reduction in the incidence of
pregnancies. However, there was no systematic variation indicative of
a mutagenic potential over the entire eight-week mating period (Makita
et al., 1973).
Special studies on reproduction
Groups of Charles-River rats (10 males and 20 females per group)
were fed levels of thiophanate-methyl in the diet at 0, 40, 160, 640
ppm in a three-generation, two-litters per generation reproduction
study. There were no apparent effects of thiophanate-methyl at levels
up to and including 640 ppm on any of the reproduction parameters
measured in this experiment. In addition, gross and histological
examinations of the F3b generation were performed on several tissues
and organs of three-week-old rats and a further group of animals was
examined for skeletal abnormalities. In no instance was there any
effect of feeding thiophanate-methyl on reproduction. There was a
definitive effect on the growth of animals fed dietary levels of 640
ppm (Palmer et al., 1972).
Special studies on teratogenicity
Thiophanate-methyl was administered to pregnant ICR mice from day
1 to day 15 of gestation at levels of 0, 40, 200, 500 and 1000
mg/kg/day. At 1000 mg/kg/day there was a significantly reduced number
of living fetuses. No (significant) differences in the number of
implantation sites, body weight of fetuses or fetal mortality or body
weight were observed at the lower dosage levels. The administration of
thiophanate-methyl did not produce gross internal or external
abnormalities and the study did not reveal any teratogenic properties
under these experimental conditions (Noguchi and Hashimoto, 1970a;
Makita et al., 1973).
Short-term studies
Mouse. Groups of six-week-old SPFICR-strain mice (12 males and 12
females per group) were fed thiophanate-methyl at levels of 0, 12.8,
64, 390, 1600 and 8000 ppm in the diet for six months. Daily
observations were made on their behaviour and mortality and body
weight and food consumption data were recorded weekly. Haematological
and gross and microscopic examination of tissues and organs were
performed at the end of the feeding period.
There were no unusual behavioural patterns and incidence of
mortality was not related to the feeding of thiophanate. Growth of all
mice at levels of 1600 ppm and below was not affected by
thiophanate-methyl with a retardation of growth observed at 8000 ppm.
Food intake and haematological examinations were normal. Gross
pathology at the termination of the experiment showed that there was
an increase in the mean weight of liver in animals fed 8000 ppm with
an increase in liver to body weight ratio. This was noted in both
males and females. There were no effects of this kind noted at a dose
level of 1600 ppm. Pathological abnormalities were noted in liver at
8000 ppm in both males and females. There was swelling of large
hepatic cells with concurrent swelling. It was observed that the
protoplasm of these cells were oedema-like or granule-like. Although
such pathological abnormalities were found in some mice of other
groups as well as the control, it appeared to be more prevalent in the
8000 ppm animals. In the present experiment the no-effect level of
thiophanate-methyl is considered to be 1600 ppm (Noguchi and
Hashimoto, 1970b).
Groups of SPF-Sprague Dawley rats (12 males and 12 females per
group) were fed thiophanate-methyl in the diet at levels of 0, 12.8,
64, 320, 1600, 8000 ppm for six months under SPF conditions. Daily
checks of mortality and abnormal behaviour were made throughout the
experiment with body weight and food intake data recorded weekly. At
the conclusion of the study, haematology, clinical chemistry and
urinalyses were performed. Gross and microscopic examination of
tissues and organs was also performed at the conclusion of the feeding
period.
There were no behavioural abnormalities observed during the
six-month feeding study and mortality was not related to food intake
of thiophanate-methyl, Examination of growth curves indicated a severe
retardation of both males and females fed 8000 ppm in the diet. There
did not appear to be any retardation in growth of animals fed 1600 ppm
or below. Food intake of all groups was comparable with control
values. All haematological values were comparable with controls as
were the values obtained from urine analysis. At 8000 ppm there was a
slight decrease in glucose and GOT levels and an increase in total
cholesterol. Gross and histological examination of tissues showed only
the liver and thyroid glands to be slightly affected by
thiophanate-methyl in the diet. There was an increase in liver weight
and liver to body weight ratio although there were no pathological
changes such as cloudy swelling and irregularity in the size of the
hepatic cells which was noted in the studies in mice. In the thyroid
gland some abnormalities such as small follicles, cubic epithelium
cells and a decrease in colloidal substance were observed in those
animals fed 8000 ppm. These changes were not observed in animals fed
1600 ppm and were not accompanied by any change in the PBI values.
Abnormal findings were not noted in any other tissues.
From these studies it appears that a no-effect level of 1600 ppm
in the diet of rats for six months would result in no unusual effect
(Noguchi and Hashimoto, 1970c).
Dog. Groups of pure bred beagle dogs (five males and five females
per group - four males and four females per group at the highest dose
level) were administered thiophanate-methyl at levels of 0, 2, 10, 30
and 250 mg/kg/day for two years. The animals were approximately six
months old at the time of the inception of the testing and were housed
singly in indoor kennels. In all groups one male and one female were
sacrificed at 12 months with the remaining animals sacrificed after 24
months on test. Dosing was performed by oral administration of
thiophanate-methyl by capsule once a day seven days a week for two
years. Doses were given to individual animals related to the body
weight and were adjusted weekly. Clinical signs of any abnormalities
were recorded daily. Food consumption and body weight changes were
recorded weekly. Throughout the study the eyes were examined for any
changes and electro cardiographs were taken periodically.
Haematological examinations, blood chemistry analyses and urinalyses
were performed periodically. At the conclusion of the study gross and
microscopic examination of tissues and organs was performed on all
animals.
There was no mortality or adverse behavioural changes observed in
these animals during the course of the study. Growth of the animals
fed 250 mg/kg/day was slightly retarded in both males and females.
There did not appear to be a significant decrease in any other group.
There did not appear to be any significant differences in the
haematological studies, blood chemistry, urinalysis, ophthalmological
examinations, or the ECG during the course of the 24-month study.
Pathological (gross and histological) examination of the animals
sacrificed at 12 months did not show any significant changes in the
tissues examined with regard to gross pathology and organ weight
ratios. Slight changes observed at 250 mg/kg in the thyroid gland were
difficult to determine from the one year data on dogs.. Animals
sacrificed at 24 months showed an elevated thyroid weight in both
males and females at 50 and 250 mg/kg although there were no changes
in the PHI values. Although the average weight of the thyroid of the
two highest dose groups was heavier than that of the controls,
histological examination showed no differences in the thyroid of the
control and the experimental groups. Histological findings in all
other tissues were also normal.
The no-effect level in this two-year study in dogs is considered
to be 50 mg/kg/day based upon the marginal effect noted in thyroid
weight at the conclusion of the study. Although this increased thyroid
weight was not reflected in the histological examination or PBI
values, it has been noted in other species (rat). A more noticeable
effect was observed at 250 mg/kg relating to retarded growth in both
males and females (Hashimoto and Fukuda, 1972).
Long-term studies
Rat (See also under the section: "Special studies on
carcinogenicity"). Groups of SPF-Sprague-Dawley strain rats (35 males
and 35 females per group, 50 males and 50 females were used as
controls) were fed thiophanate-methyl in the diet at levels of 0, 10,
40, 160, 640 ppm for two years.
The animals were maintained under SPF conditions. The sample of
thiophanate-methyl fed in this experiment was approximately 94% active
ingredient with impurities of 2% sulfur, 2% inorganic chlorides, 2%
unknown volatile substances and less than 0.5% aromatic amines. The
animals were fed dietary levels of thiophanate methyl for a period of
10 days during which behaviour and mortality were recorded daily and
after which food consumption was measured on all animals. Body weight
and growth were recorded weekly. Haematology, blood chemistry and
urinalyses were performed periodically over the two-year study. At the
conclusion of the study animals were sacrificed and gross and
microscopic examination of tissues and organs was performed.
There was no effect of feeding levels of up to 640 ppm of
thiophanate-methyl in the diet of rats on behaviour and mortality over
a two-year period. Growth curves indicate a slight retardation of
growth in both male and female rats in fed diets containing 640 ppm.
Food consumption at all dietary levels was comparable with the control
groups. Haematological data, urinalysis data and clinical blood
chemistry data were not abnormal in any of the groups examined. Gross
pathological examination of animals showed no changes in organ weight
in any group with the exception of slight increases in kidney weight
and kidney to body weight ratio, primarily in the males at the highest
level of feeding. There was no indication of increased thyroid weight
or an increase in liver size as was observed in shorter duration test
with rats. In addition there were frequent observations of
inflammation of the lung, fibroadenoma of the breast and the
occurrences of pituitary tumours. The administration of
thiophanate-methyl was not believed to be responsible for the
occurrence of these abnormalities. At the end of two years there was a
dose response in a degenerative change in the testis which underwent a
degeneration and atrophy including spermatogenesis. In this particular
dose-response change several cases were noted in other groups
including the control. This effect was probably not related to
ingestion of thiophanate-methyl. At the end of two years there was
also some change in the thyroid where colloidal epithelial cells were
enlarged. This enlargement took place primarily in the males of the
highest dose group. There was no apparent effect on the occurrence of
tumours and the only significant effects appear to be in the decreased
growth observed at 640 ppm in the diet.
Based upon retardation of growth a no-effect level would be
considered to be 160 ppm in the rat fed thiophanate-methyl for two
years (Hashimoto and Tsubura, 1972).
Observations in man
Sixteen workers concerned with the production of
thiophanate-methyl were examined periodically for 3.5 years. Blood and
urine analysis were carried out every six months. No adverse effects
were found with these workers with regard to blood chemistry and urine
analysis (Mori, 1972).
Comments
Thiophanate-methyl is degraded in plants to carbendazim and
hydroxylated derivatives. A question was raised if the apparent
occurrence of carbendazim in mammals may be an analytical artefact. A
theoretical metabolite of potential toxicological significance,
phenylene thiourea, has not been reported in plant or animal systems.
Thiophanate-methyl has a low acute toxicity in various species of
animal and shows pharmacological activity only at very high levels.
Adequate short-term and long-term studies are available in both
rats and dogs. The results of a three-generation reproduction study,
mutagenesis studies and a cytogenicity study were negative.
Thiophanate-methyl did not appear to be a carcinogen in a susceptible
strain of mice and two-year studies in rats and dogs gave rise to
retardation of growth only at high dietary levels (640 ppm). There was
a marginal increase in thyroid weight at higher dose levels which was
not accompanied by adverse biochemical or histological effects. The
long-term study in the rat was used as a basis for estimating an ADI
for man.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 160 ppm in the diet equivalent to 23 mg/kg bw
Rat: 160 ppm in the diet equivalent to 8 mg/kg bw
Dog: 50 mg/kg bw/day
Estimate of an acceptable daily intake for man
0-0.08 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
Thiophanate-methyl was first registered for use as a systemic
fungicide in Japan in May 1971. By 1973 it has obtained provisional
clearance for safe use in Great Britain for a number of specified
preharvest treatments on food crops and ornamentals and for
post-harvest dipping of apples.
Thiophanate-methyl is formulated into wettable powders containing
50 and 70% active ingredient.
Recommended use levels are from 0.02-0.07% a.i. usually applied
at the rate of 1-5 kg a.i. per ha for pears, peaches and citrus and of
up to 2 kg a.i. per ha for other fruits and vegetables. Repeated
applications are claimed to be advantageous.
Thiophanate-methyl is reported to be very similar to benomyl
(BenlateR) as regards both its systemic functions (Aelbers, 1970)
and the rather broad spectrum of fungal diseases against which it is
effective (Bollen, 1971). It is claimed effective against apple and
pear scab, powdery mildew and different moulds, rots and blight.
Pre-harvest application on citrus trees should effectively reduce
Penicillium decay on citrus fruits under post-harvest storage.
The similarity between thiophanate-methyl and benomyl seems well
explained by the fact that both compounds are converted into methyl
2-benzimidazolecarbamate (MBC), which is reported to be the principal
fungitoxic compound to which both chemicals owe their major activity
(Vonk and Sijpesteijn, 1971; Selling et al., 1970).
Residues resulting from supervised trials
Data derived from extensive field trials in several countries on
thiophanate-methyl residues have been presented by the manufacturer
(Nippon Soda Company Ltd, 1973a). A summarized list of a selection of
the maximum residues found in these trials is presented in Table 1
(Nippon Soda Company Ltd, 1973b). The majority of information in
Table 1 shows residue levels at 0 or 1 day after last application.
Generally, the major part of residues is in the form of unchanged
thiophanate-methyl, while methyl 2-benzimidazolecarbamate (MBC) is
usually detectable in smaller amounts, averaging 10% of the total.
Apples, pears, peaches
Generally, higher levels of thiophanate-methyl residues are found
in these fruits after post-harvest treatments than after pre-harvest
sprayings. This is partly due to higher initial deposits from dipping
and partly connected to volatility losses to which the pre-harvest
treated fruits are subjected. During storage experiments performed in
the United Kingdom by the manufacturer, pears treated after harvest
TABLE 1. RESIDUES OF THIOPHAMATE-METHYL AND MBC IN VARIOUS CROPSa,b
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Apple 1.4 kg/ha 10× 1.4 - Fed. Rep. Ger.
0.6 g/l 1× 0.83 0.14 Canada
1-2 kg/ha 17×+
+1000 pp. Dipping 3.8 0.8 UK
2100 ppm Dipping 3.4 Italy
Banana 1000 pp. Dipping Peel: 0.3 0.4 Australia
Pulp: 0.1 <0.2
Beans, broad 2 kg/ha 1× 0.1 0.5 UK
" dwarf 1 kg/ha 1× 2.3 0.1 UK
" french 1.5 kg/ha 1× 0.2 0.2 US
" kidney 0.75 kg/ha 2× 0.2 Japan
" runner 1 kg/h. 1× 1.8 0.1 UK
Blackcurrant 5.4-7.5 kg/ha 4× 0.day:19.4 4.9 UK
5.4-7.5 kg/ha 4× 7.day:7.0 3.3 UK
1 kg/ha 4.5× 3.0 2.3 UK
Carrots 1000 ppm Dipping 5.0 0.2 UK
Celery low pp. Dipping 28.0 0.8 UK
Cherries 1.4 g/l 3× 2.0 0.2 Australia
2 kg/ha 5× 0.46 0.64 USA
Citrus 3.5 kg/ha 1× Peel: 12.2 2.1 Japan
Pulp: 0.3 <0.2
Cucumber 1 kg/ha 6× 0.45 0.09 Japan
Gherkins 1 g/l 1.87 0.33 Netherlands
Gooseberries 1 kg/ha 4× 3.0 0.4 UK
Grapes 1.4 kg/ha 5× 3.3 - Fed. Rep. Ger.
1.4 kg/ha 19× 2.8 Japan
2.4 g/l 6× 0.6 <0.2 Australia
Lettuce 1 kg/ha 3× 0.day:10.0 0.6 UK
7.day:0.1 <0.2
2.8 kg/ha 6× 0.day:24.5 5.0 Japan
7.day:3.8 1.5
2.2 kg/ha 1× 0.day:4.1 New Zealand
TABLE 1. (Cont'd.)
Crop Application Residues Found (ppm)
Rate, approx. Frequency Thioph.-methyl MBC Country
Mushroom about 5 g/m2 1× <0.1 0.7 UK
Onion 2.5 kg/ha 10× 0.05 0.02 Japan
Orange 1000 ppm Dipping Peel: 1.2 <0.3 Australia
Pulp: 0.2 <0.3 Australia
Juice: 0.4 <0.2 Australia
1000 ppm Dipping Peel: 2.3 0.23 USA
2100 ppm Dipping 8.8 Italy
Peach 2.4 g/l 4× 5.0 0.4 Australia
Pear 1000 ppm Dipping 2.2 <0.1 UK
2100 ppm Dipping 3.9 Italy
Plum 1 kg/ha 6× 0.86 0.37 USA
Raspberries 1 kg/ha 8× 9.6 2.3 UK
Strawberries 1.5 kg/ha 1× 0.day:14.6 UK
7.day:3.0
1 kg/ha 4-5× 0.day:3.8 1.2 Netherlands
(under glass) 7.day:3.6 0.9
14.day:0.9 0.6
Sugarbeet 0.35 kg/ha 2× Leaf:3.6 Fed. Rep. Oar.
Root: <0.2
Wheat, winter 0.5 kg/ha 1× Straw: <0.2 (50 days)
Grain: <0.2
Tomato 2 kg/ha 5× 9.7 0.4 UK
a Residues reported are maximum residues, i.e. levels found on 0. or 1. day after application,
except when otherwise stated.
b Analytical methods used were as follows (see monograph text):
TLC-method: Australia, Japan, New Zealand
Colorimetry: Canada, UK, USA
UV-spectrophotometry: Japan, Federal Republic of Germany
Oscillopolarography: Italy, Netherlands (TLC-bioassay for MBC).
with thiophanate-methyl showed no pronounced decrease of residues
(about 2 ppm) during 61 days, while on the other hand, residues of
about 1 ppm on apples decreased well below 0.1 ppm during four weeks
on the trees after spraying.
Grapes and wine
Lemperle et al. (1973) found that thiophanate-methyl residues, as
well as those of other benzimidazole fungicides, are transferred
nearly quantitatively into unclarified musts during wine production.
During clarification and fermentation small amounts may disappear. A
considerable part of the thiophanate-methyl residues which are present
in grapes may therefore be found in wines.
Leafy vegetables
The residue data supplied by the manufacturer indicate that even
relatively high residues are subject to pronounced losses, which may
be explained as usual growth dilution effect to which volatility
losses from the foliar surfaces may be added. From field trials with
lettuce performed in Japan, half-life values of the order of 1-2 days
for thiophanate-methyl have been deducted, while the MBC-metabolite
present in smaller amounts disappeared more slowly, corresponding to
half-life values of 5-6 days.
For comparison, the half-life of thiophanate-methyl on apple and
grape leaves under controlled conditions was found to be in the order
of 15 and 12 days, respectively (Soeda et al., 1972).
Carrots, celery, sugarbeets
When subjected to post-harvest treatments with thiophanate-methyl
these root-crops take up considerable amounts of residues. Dipping of
carrots and celery performed in the United Kingdom with 0.1% a.i. gave
total residues of thiophanate-methyl + MBC of 5.2 and 28.8 ppm,
respectively. Pre-harvest sprays gave high, though rapidly
disappearing, residues on the green tops above soil, but no detectable
residues in the edible roots.
Fate of residues
Substantial evidence has been presented by the manufacturer that
methyl 2-benzimidazolecarbamate (MBC) is produced as the major
metabolite (Noguchi et al., 1971) although only gradually released
from thiophanate-methyl when applied on foliar surfaces (Kikuchi,
1973). Vonk and Sijpesteijn (1971) found that the fungitoxic activity
of thiophanate-methyl is increased by aging in aqueous media. They
concluded that MBC is responsible for the fungitoxic effect of the
fungicide and indicated that the rate of conversion from
thiophanate-methyl may be increased by the fungal metabolic activity.
Selling et al. (1970) also observed the conversion of
thiophanate-methyl to MBC in tap water. A 4% transformation was noted
when kept at pH = 7.0 in water for seven days, while
thiophanate-methyl remained stable in methanol or chloroform for 50
days at 24°C (Noguchi et al., 1971).
Plants
Conversion of thiophanate-methyl to MBC has been followed after
uptake through the roots and after foliar treatment of French bean
seedlings (Phaseolus vulgaris) by Noguchi et al. (1971). After 21 days
34.3% of the original amount had been converted on the leaves, while
87.5% was metabolized into MBC when absorbed through the roots in a
water culture. In a most recent publication, the formation of MBC from
thiophanate-methyl is further described by Buchenauer et al. (1973).
They found that the transformation to a great extent is
light-catalyzed (requiring at the same time the presence of water). A
42% transformation of thiophanate-methyl residues was found in cotton
leaves, when subjected to four days of sunlight as opposed to only
about 6%, when the plants were kept under dimlight conditions.
A minor metabolite, 2-aminobenzimidazole (2-AB), also formed from
MBC in benomyl treated plants is detected as a result of
thiophanate-methyl applications as well (Fujino, A. and Kamimura,
1973a). It is further reported by the same authors that two
oxygen-analogues (DX-105 = 1-(3-methyoxy-carbonyl-2
thioureido)-2-(3-methoxycarbonylureido) benzene and FH-132 =
dimethyl-4.4-O-phenylenbis(allophanate)) has recently been identified
by a reverse isotopedilution method (see Fig. 3).
Soil
Thiophanate-methyl is degraded almost entirely within seven days
in sandy loam and silty loam soils at temperatures from 23°C to 33°C
(Pujino and Kamumura, 1973b). MBC is identified as the major
metabolite, which is subsequently degraded at a more moderate rate.
Sixty days after treatment residual MBC was present at the level of
20% at 23°C and 2-15% at 33°C in both soil types, calculated on the
basis of the amount of thiophanate-methyl applied. No influence from
thiophanate-methyl was indicated in these experiments on soil
micro-organisms, when measured by bacterial counts (total bacteria,
gram-negative and actinomycetes). With increased levels of
thiophanate-methyl (up to 5000 ppm) increased oxygen uptake increased
significantly, suggesting that thiophanate-methyl was used as a source
of nitrogen by bacteriae.
The conversion of thiophanate-methyl to MBC is significantly more
rapid in unsterilized than in sterile soils. Similarly, the MBC formed
is less persistent in the unsterilized soil, which suggests that
micro-organisms may be involved in the degradation of both compounds.
TLC-identification patterns further indicate that
dimethyl-4.4'-o-phenyleneallophanate (or 111-432, see Fig. 3) may be
an intermediate metabolic step in the microflora-induced degradation,
as this compound is not found as a metabolite in sterilized soils
(Kosaka et al., 1972).

 Radio-activity recovery experiments in which water was percolated
through treated loams (sandy and silty) did not give evidence of any
migration of thiophanate-methyl or of degradation products deeper than
4 cm from the treated surface layer.
Animals
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973c) 14C or 35S-labelled thiophanate-methyl was fed to rats, mice
and dogs. From 80 to 100% of the administered amounts were recovered
in faeces and urine within 96 hours after feeding. Sixty per cent.,
16-27% and 14% were excreted with the faeces by the three species
respectively, while urinary excretion accounted for 30%, 66-78% and
74% of the administered thiophanate-methyl respectively.
Fujino et al. (1973c) report that the major part of faecal
excretion was in the form of unmetabolized thiophanate-methyl, while
the minor part consisted of 4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-o-phenyleneallophanate (FH-432). MBC and 5-hydroxy-MBC
(5-OH-MBC) were also observed during TLC identifications of
metabolites from faecal extracts. It was, however, questioned whether
these two were actual metabolites in faeces or if they were compounds
produced during the analytical procedures from thiophanate-methyl and
4-OH-TM, respectively.
In the same studies by Fujino et al. (1973c) thiophanatemethyl
and a number of metabolites could be liberated enzymatically or by
acid treatment from water soluble conjugates in rat urine. Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (see Fig. 4). As was the case in faeces, two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical steps.
In the earlier study by Noguchi et al. (1971) of the effect of
thiophanate-methyl in rat liver microsomes, MBC and 5-OH-MBC were also
found to be present. Evidence for enzyme induction could not be
obtained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Methods of residue analysis
The methods of Pease and Gardiner (1969) and Pease and Holt
(1971) for benomyl could be applied to thiophanate-methyl after
conversion to MBC. It is expected that this method should be able to
differentiate thiophanate-methyl from other benzimidazole fungicides.
The manufacturer has presented detailed descriptions of methods
for thiophanate-methyl residue analyses by means of thin-layer
chromatography (Tannue et al., 1973), colorimetric determination based
on MBC reaction with bromcresol purple in chloroform (Sugioka et al.,
1973) and by UV-spectrophotometric measurement of MBC at 282 nm (Ono,
1973a).
The UV-spectrophotometric method which is applicable to various
crops is generally recommended. Thiophanate-methyl and MBC are
conveniently extracted together from the sample with methyl alcohol,
and then separated during the clean-up stages through liquid
partitioning between petroleum ether-iso-octane solvent, and methylene
chloride and aqueous methanol solutions. Thiophanate-methyl is
converted into MBC by reflux treatment with cupric acetate in aqueous
acetic acid solution and thiophanate-methyl and MBC are determined
separately as MBC by a corrected absorbance measurement technique at
292 nm from the spectrum recorded between 250 nm and 310 nm.
The limit of determination with this method is given to 0.02 ppm
for each of the compounds, thiophanate-methyl and MBC, when analysing
100 g samples. Recoveries are reported for a number of fruits and
vegetables from 72 to 81% for thiophanate-methyl and 77-84% for MBC.
The initial steps of the method (until the conversion of
thiophanate-methyl into MBC) should be carried out without prolonged
keeping and possibly under protected light conditions (Ono, 1973b).
An oscillopolarographic method for the separate determination of
unchanged thiophanate-methyl has been published (Martens and Chs,
1972). It has been adapted for residue determinations in a number of
fruits and vegetables with a limit of determination of 0.02 ppm in
combination with TLC-Bioassay Technique for the MBC (Civo TNO 1973) as
this compound gives no oscillopolarographic response.
National tolerances
The Meeting was aware of the following national tolerances:
Australia Apples, pears, stone fruit 3 ppm
citrus, bananas, grapes 1 ppm
Netherlands Fruit and vegetables 2 ppm
(thiophanate-methyl
without MBC)
Raw cereals 0.5 ppm
(thiophanate-methyl
+ MBC, under revision)
Switzerland Stone fruit, grapes,
strawberries 3 ppm (calculated
as MBC)
Celeriac 0.1 ppm
Appraisal
Thiophanate-methyl is a new systemic fungicide which is
characterized by broad-spectrum fungicidal effects as other chemicals
of the benzimidazole group. Thus it is claimed to be effective in the
control of different moulds rots and blights although ineffective
towards some specific plant-pathogenic fungi e.g. Pythium spp.,
Phytophora sop. and Alternaria spp.
In countries in which thiopbanate-methyl has obtained
registration, it is marketed as wettable powders for pre-harvest as
well as post-harvest treatments. Recommended use levels are from 0.02
to 0.07%, often with repeated applications.
Residue data from supervised trials performed with
thiophanate-methyl in several countries have been provided by the
manufacturer in support of registrations, including the establishment
of tolerances. It is noted that in a few cases the data was derived
from trials involving excessive application rates so that residue
levels were higher than would be likely following actual good
agricultural practice. In addition to information on numerous food
crops, the data includes experiments on soil residues, although direct
soil treatments are not indicated as recommended practice.
In plant material and in soils the major metabolite is found to
be methyl benzimidazolecarbamate, formed by hydrolysis and ring
closure from thiophanate-methyl. Methyl benzimidazolecarbamate is a
chemically stable compound which leaves relatively persistent
residues. Although somewhat conflicting evidence is presented as to
which degree of transformation takes place in and on plants (possibly
due to analytical difficulties) it is found reasonably well
established that methyl benzimidazolecarbamate is formed gradually
from thiophanate-methyl and that both compounds should be accounted
for in the evaluation of residue data.
A minor metabolite, 2-aminobenzimidazole, may be formed from
methyl benzimidazolecarbamate and be detectable in plant tissues
following thiophanate-methyl applications. Two oxygen analogues of
thiophanate-methyl,
1-(3-methoxy-carbonyl-2-thioureido)-2-(3-methoxycarbonyl ureido)
benzene and dimethyl-4, 4-0-phenylenbis (allophanate) have, in
addition, been described as possible intermediate metabolites. These
have recently been identified in trace amounts from plant material
by reverse isotope-dilution technique.
In soils the degradation of thiophanate-methyl to methyl
benzimidazolecarbamate is found to be complete within seven days in
both sandy and silty loams, whereafter the metabolite behaves as a
more persistent compound which disappears at a more moderate rate. An
uptake through the roots of plants has been clearly demonstrated.
Information, however, on the fate of thiophanate-methyl after
feeding to ruminants is not given, neither is data available on
residues in animal products, milk, meat and eggs.
Analytical methods which allow the determination of
thiophanate-methyl and its main metabolite (MBC) separately are
available. They are based on UV-spectrometric measurements of MBC
after separation of the two compounds and they may be adapted for
regulatory purposes, although a definite multi-residue technique for
distinguishing the different benzimidazole fungicide seems to be
lacking.
RECOMMENDATIONS
Due to the fact that a certain, not definitely established
proportion of residues from thiophanate-ethyl applications consist of
MBC, for which an ADI has not been established, only temporary
tolerances can be recommended.
Tolerances given below are measured as the sum of thiophanate-
ethyl and its major metabolite, methyl benzimidazolecarbamate (MSC)
and expressed in terms of the latter. Levels indicated are such that
they are not likely to be exceeded when following good agricultural
practice, including pre-harvest and post-harvest treatments when
applicable.
For the underlined commodities individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides, of
which MBC is also recognized and identified as the major metabolic
and/or chemical entity (i.e. benomyl and carbendazim).
Temporary tolerances ppm
expressed as MBC
Celery 20
Citrus (whole), peaches, grapes, cherries 10
Apples, tomato*, pears, lettuce*, strawberries*,
raspberries, gooseberries, blackcurrants*,
carrots, sugar beet tops : 5
Beans (broad, dwarf, french, runner, kidney)
gherkins, plums 2
Mushrooms, bananas (whole) 1
Cucumbers 0.5
Sugar beet, raw cereals, onions 0.1
* At or about limit of determination.
FURTHER WORK OR INFORMATION
Required (before temporary tolerances can be confirmed)
1. Information on the nature and fate of residues of thiophanate-
ethyl in meat, milk, and eggs following the feeding of
thiophanate-methyl at levels likely to be found on forage and
foodstuffs.
Desirable
1. Further studies on the metabolism of thiophanatemethyl in
animals, with special reference to the occurrence of carbendazim.
2. Further studies on the effect of thiophanate-methyl on the
thyroid and the testes in the rat and other species of animals.
3. Further data on residues on raspberries following good
agricultural practice.
4. Further information on the need for post-harvest treatment of
carrots and celery.
REFERENCES
Aelbers, E., Mededel, Pak. (1970) Landbouw Wet. Gent., 36, 126.
Cited from Vonk & Sijpesteijn (1971)
Bollen, G.J. (1972) A comparison of the in vitro antifungal spectra
of thiophanates and benomyl. Neth. J. Pl. Path. 78, 55
Buchenauer, H., Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci., 4: 343-348
CIVO-INO Report No. R.4023 (1973) on residues of thiophanate-methyl
and its metabolite MBC in strawberries. From Central Institute for
Nutrition and Food Research, TNO, Zeist (Unpublished)
Fuchs, A. Van Den Berg, G.A. and Davidse, L.C.A (1972) comparison of
benomyl and thiophanates with respect to some chemical and systemic
fungitoxic characteristics. Pest. Biochem. Physiol., 2(2): 191-205
Fujino, A. and Kamimura, H. 1973a The fate of thiophanate-methyl
residue on plants under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A. and Kamimura, H. 1973b The fate of thiophanate-methyl
residue in soil under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A., Ohnuma, N. Mori, T., Tanoue, T. and Kamimura, H. (1973c)
The balance and metabolism studies of thiophanate methyl in animals.
Paper submitted by Nisso institute Per Life Sciences. Nippon Soda Co.
Ltd. (Unpublished)
Hashimoto, Y., Makita, T., Mori, T., Nishibe, T., Noguchi, T., Tsuboi,
S. and Ohta, G. (1970) Toxicological Evaluations of Thiophanate (I).
Acute and Subacute toxicity of a new fungicide, thiophanate (Acute
Ingredient of NF-35), 1,2 hrs. (ethoxy-carbonyl-thioureido)
- benzene. Pharmacometrics, 4(1): 5-21
Hashimoto, Y. Makita, T. Ohnuma, M. and Noguchi, T. (1972a) Acute
toxicity on Demethyl 4, 4'-O-phenylene bis (3-thio allophanate),
thiophanate-methyl fungicide. Toxicol. Appl. Pharmacol., 23: 606-615
Hashimoto, Y., Mori, T., Ohnuma, N. and Noguchi, T. (1972b) Some
Pharmacologic Properties of a new fungicide, thiephanate-methyl. Tox,
Appl. Pharmacol., 23: 616-622
Hashimoto, Y. and Fukuda, Y. (1972) Final Report on the long term oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4 -O-phenylenebis
(3-thioallophanate) in Beagle dogs for 24 months. Unpublished report
from Nisso Institute for Life Sciences submitted by Nippon Soda Co.
Ltd.
Hashimoto, Y. and Tsubura, Y. (1972) Final Report on the Chronic Oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4' -O-phenylenebis
(3-thioallophanate) in rats of Sprague-Dawley Strain for 24 months.
Unpublished report from Nisso Institute for Life Sciences submitted by
Nippon Soda Co. Ltd.
Kikuchi, Masayoshi. (1973) Actual state of thiophanate-methyl residues
on crops. Paper submitted by Fine Chemicals Division, Nippon Soda Co.
Ltd. (Unpublished)
Kosaka, S., Soeda, Y., Hagiwara, S., Hashimoto, S. and Naohara, T.
(1972) Influences of thiophanate-methyl to soil microorganisms add
fate of the chemical in soil. Paper submitted by Nisso Institute for
Life Science, Nippon Soda Co. Ltd. (Unpublished)
Lemperle, E., Kerner, B., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstünde und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Deutsche Leb.-Rdschau, 69, 313
Makita, T., Hashimoto, Y., and Noguchi, T. (1973) Mutagenic,
Cytogenetic and teratogenic Studies on thiophanate methyl. Toxicol
Appl. Pharmacol, 24(2): 206-215
Martens, P. H. and Chs, M. Détermination oscillopolarographique des
residue do thiophanate sur légumes divers. Model. Faknld.
Landbonetensch., Gent, 37, 891
Mori, H. 1972 Human Handling experiences from Plant Employees
manufacturing Topsin (1). Unpublished report from Nisso Takaoka
Hospital submitted by Nippon Soda Co. Ltd.
Nippon Soda Co. Ltd. (1973a) Information on usage and the occurrence
of residues of thiophanate-methyl. Report submitted in August 1973 by
Nippon Soda Co. Ltd. (Unpublished)
Nippon Soda Co. Ltd. (1973b) List of maximum residue of
thiophanate-methyl and its metabolite, MBC in/on edible crops in
the world. Compiled by the Nippon Soda Co. Ltd. October 1973
(Unpublished)
Noguchi, T. and Hashimoto, Y. (1970a) Toxicological evaluation of
Thiophanate Methyl (I) Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from Nisso Institute for Life
Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate methyl (II) studies on the subchronic oral toxicity of
thiophanate-methyl in mice. Unpublished report from Nisso Institute
for, Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity of
thiophanate-methyl in rats. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970d) Toxicological evaluation of
thiophanate-methyl (IV). Studies on the teratogenic effect of
thiophanate-methyl upon the fetus of ICR strain mice. Unpublished
report from Nisso Institute for Life Sciences submitted by Nippon Soda
Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970e) Toxicological evaluation of
thiophanate-methyl (V). Some pharmacologic properties of a new
fungicide thiophanate-methyl. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1971) Study on contact phototoxicity
and sensitivity to new fungicide, thiophates. Unpublished report from
Nisso Institute for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T., Ohkuma and Kosaka, S. (1971) Chemistry and Metabolism of
thiophanate fungicides. Proc. Int. Symp. Pesticide Terminal Residues,
Tot Aviv, pp. 257-270
Noguchi, T. (1972) Environmental Evaluation of Systemic Fungicides.
Environmental Toxicology of Pesticides, Academic Press N.Y., pp.
607-632
Ono, S. (1973a) Analytical method for residues of thiophanate-methyl
and 2-methyl benzimidazolecarbamate in crops by U.V.-spectrometry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Ono, S. (1973b) Stability of thiophanate-methyl under analytical
procedures. Paper submitted by Nippon Soda Co. Ltd. (Unpublished)
Palmer, A.K., Lowell, M.R, and Newman, A.J. (1972) Effect of
thiophanate-methyl on reproductive function of multiple generation in
the rat. Unpublished report from Huntingdon Research Center submitted
by Nippon Soda Co. Ltd.
Pease, H.L. and Gardiner, J.A. (1969) Fluorometric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.P. Improved method for determining benomyl
residues. J. Assoc. Off. Analyt. Chem., 54, 1399
Selling, H.A., Vonk, J.W. and Sijpesteijn, A.K. (1970) Transformation
of the systemic fungicide methyl thiophanate into 2-benzimidazole
carbamic acid methyl ester. Chem. Ind., pp. 1625-1626
Soeda, Y., Kosaka, S. and Noguchi, T. (1972a) The fate of thiophanate.
methyl fungicide and its metabolites on plant leaves and glass plates.
Agr. Biol. Chem., 36(6): 931-936
Soeda, Y., Kosaka, S. & Noguchi, T. (1972b) Identification of Alkyl
2-benzimidazole carbamates as a major metabolite of thiophanates
fungicide in/on the bean plant. Agr. Biol. Chem., 36(6): 817-823
Sugioka, K., Toyama, N. and Kamimura, H. (1973) Residue analytical
method of thiophanate-methyl and a transformed product, methyl
2-benzimidazole (MBC) in soils and plant tissues by colorimetry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Tanoue, T., Toyama, N. & Karimura, H. (1973) Residue analytical method
of thiophanate-methyl in plant tissues by thin layer chromatography.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Vonk, J.W. & Sijpesteijn, A.K. (1971) Methyl benzimidazol-2-
ylcarbamate, the fungitoxic principle of thiophanatemethyl. Pest.
Sci., 2, 160
Radio-activity recovery experiments in which water was percolated
through treated loams (sandy and silty) did not give evidence of any
migration of thiophanate-methyl or of degradation products deeper than
4 cm from the treated surface layer.
Animals
In studies reported by Noguchi et al. (1971) and Fujino et al.
(1973c) 14C or 35S-labelled thiophanate-methyl was fed to rats, mice
and dogs. From 80 to 100% of the administered amounts were recovered
in faeces and urine within 96 hours after feeding. Sixty per cent.,
16-27% and 14% were excreted with the faeces by the three species
respectively, while urinary excretion accounted for 30%, 66-78% and
74% of the administered thiophanate-methyl respectively.
Fujino et al. (1973c) report that the major part of faecal
excretion was in the form of unmetabolized thiophanate-methyl, while
the minor part consisted of 4-hydroxy-thiophanate-methyl (4-OH-TM) and
dimethyl-4.4'-o-phenyleneallophanate (FH-432). MBC and 5-hydroxy-MBC
(5-OH-MBC) were also observed during TLC identifications of
metabolites from faecal extracts. It was, however, questioned whether
these two were actual metabolites in faeces or if they were compounds
produced during the analytical procedures from thiophanate-methyl and
4-OH-TM, respectively.
In the same studies by Fujino et al. (1973c) thiophanatemethyl
and a number of metabolites could be liberated enzymatically or by
acid treatment from water soluble conjugates in rat urine. Identified
compounds were thiophanate-methyl, 4-OH-TM, 4-OH-FH-432, FH-432,
5-OH-MBC and MBC (see Fig. 4). As was the case in faeces, two of these
compounds, namely MBC and 5-OH-MBC, may possibly have been formed
during the analytical steps.
In the earlier study by Noguchi et al. (1971) of the effect of
thiophanate-methyl in rat liver microsomes, MBC and 5-OH-MBC were also
found to be present. Evidence for enzyme induction could not be
obtained with liver microsomes prepared from rats fed daily with 600
ppm of thiophanate-methyl for three months.
Methods of residue analysis
The methods of Pease and Gardiner (1969) and Pease and Holt
(1971) for benomyl could be applied to thiophanate-methyl after
conversion to MBC. It is expected that this method should be able to
differentiate thiophanate-methyl from other benzimidazole fungicides.
The manufacturer has presented detailed descriptions of methods
for thiophanate-methyl residue analyses by means of thin-layer
chromatography (Tannue et al., 1973), colorimetric determination based
on MBC reaction with bromcresol purple in chloroform (Sugioka et al.,
1973) and by UV-spectrophotometric measurement of MBC at 282 nm (Ono,
1973a).
The UV-spectrophotometric method which is applicable to various
crops is generally recommended. Thiophanate-methyl and MBC are
conveniently extracted together from the sample with methyl alcohol,
and then separated during the clean-up stages through liquid
partitioning between petroleum ether-iso-octane solvent, and methylene
chloride and aqueous methanol solutions. Thiophanate-methyl is
converted into MBC by reflux treatment with cupric acetate in aqueous
acetic acid solution and thiophanate-methyl and MBC are determined
separately as MBC by a corrected absorbance measurement technique at
292 nm from the spectrum recorded between 250 nm and 310 nm.
The limit of determination with this method is given to 0.02 ppm
for each of the compounds, thiophanate-methyl and MBC, when analysing
100 g samples. Recoveries are reported for a number of fruits and
vegetables from 72 to 81% for thiophanate-methyl and 77-84% for MBC.
The initial steps of the method (until the conversion of
thiophanate-methyl into MBC) should be carried out without prolonged
keeping and possibly under protected light conditions (Ono, 1973b).
An oscillopolarographic method for the separate determination of
unchanged thiophanate-methyl has been published (Martens and Chs,
1972). It has been adapted for residue determinations in a number of
fruits and vegetables with a limit of determination of 0.02 ppm in
combination with TLC-Bioassay Technique for the MBC (Civo TNO 1973) as
this compound gives no oscillopolarographic response.
National tolerances
The Meeting was aware of the following national tolerances:
Australia Apples, pears, stone fruit 3 ppm
citrus, bananas, grapes 1 ppm
Netherlands Fruit and vegetables 2 ppm
(thiophanate-methyl
without MBC)
Raw cereals 0.5 ppm
(thiophanate-methyl
+ MBC, under revision)
Switzerland Stone fruit, grapes,
strawberries 3 ppm (calculated
as MBC)
Celeriac 0.1 ppm
Appraisal
Thiophanate-methyl is a new systemic fungicide which is
characterized by broad-spectrum fungicidal effects as other chemicals
of the benzimidazole group. Thus it is claimed to be effective in the
control of different moulds rots and blights although ineffective
towards some specific plant-pathogenic fungi e.g. Pythium spp.,
Phytophora sop. and Alternaria spp.
In countries in which thiopbanate-methyl has obtained
registration, it is marketed as wettable powders for pre-harvest as
well as post-harvest treatments. Recommended use levels are from 0.02
to 0.07%, often with repeated applications.
Residue data from supervised trials performed with
thiophanate-methyl in several countries have been provided by the
manufacturer in support of registrations, including the establishment
of tolerances. It is noted that in a few cases the data was derived
from trials involving excessive application rates so that residue
levels were higher than would be likely following actual good
agricultural practice. In addition to information on numerous food
crops, the data includes experiments on soil residues, although direct
soil treatments are not indicated as recommended practice.
In plant material and in soils the major metabolite is found to
be methyl benzimidazolecarbamate, formed by hydrolysis and ring
closure from thiophanate-methyl. Methyl benzimidazolecarbamate is a
chemically stable compound which leaves relatively persistent
residues. Although somewhat conflicting evidence is presented as to
which degree of transformation takes place in and on plants (possibly
due to analytical difficulties) it is found reasonably well
established that methyl benzimidazolecarbamate is formed gradually
from thiophanate-methyl and that both compounds should be accounted
for in the evaluation of residue data.
A minor metabolite, 2-aminobenzimidazole, may be formed from
methyl benzimidazolecarbamate and be detectable in plant tissues
following thiophanate-methyl applications. Two oxygen analogues of
thiophanate-methyl,
1-(3-methoxy-carbonyl-2-thioureido)-2-(3-methoxycarbonyl ureido)
benzene and dimethyl-4, 4-0-phenylenbis (allophanate) have, in
addition, been described as possible intermediate metabolites. These
have recently been identified in trace amounts from plant material
by reverse isotope-dilution technique.
In soils the degradation of thiophanate-methyl to methyl
benzimidazolecarbamate is found to be complete within seven days in
both sandy and silty loams, whereafter the metabolite behaves as a
more persistent compound which disappears at a more moderate rate. An
uptake through the roots of plants has been clearly demonstrated.
Information, however, on the fate of thiophanate-methyl after
feeding to ruminants is not given, neither is data available on
residues in animal products, milk, meat and eggs.
Analytical methods which allow the determination of
thiophanate-methyl and its main metabolite (MBC) separately are
available. They are based on UV-spectrometric measurements of MBC
after separation of the two compounds and they may be adapted for
regulatory purposes, although a definite multi-residue technique for
distinguishing the different benzimidazole fungicide seems to be
lacking.
RECOMMENDATIONS
Due to the fact that a certain, not definitely established
proportion of residues from thiophanate-ethyl applications consist of
MBC, for which an ADI has not been established, only temporary
tolerances can be recommended.
Tolerances given below are measured as the sum of thiophanate-
ethyl and its major metabolite, methyl benzimidazolecarbamate (MSC)
and expressed in terms of the latter. Levels indicated are such that
they are not likely to be exceeded when following good agricultural
practice, including pre-harvest and post-harvest treatments when
applicable.
For the underlined commodities individual adjustments of
recommendations have been made in order to accommodate good
agricultural practices for such alternative systemic fungicides, of
which MBC is also recognized and identified as the major metabolic
and/or chemical entity (i.e. benomyl and carbendazim).
Temporary tolerances ppm
expressed as MBC
Celery 20
Citrus (whole), peaches, grapes, cherries 10
Apples, tomato*, pears, lettuce*, strawberries*,
raspberries, gooseberries, blackcurrants*,
carrots, sugar beet tops : 5
Beans (broad, dwarf, french, runner, kidney)
gherkins, plums 2
Mushrooms, bananas (whole) 1
Cucumbers 0.5
Sugar beet, raw cereals, onions 0.1
* At or about limit of determination.
FURTHER WORK OR INFORMATION
Required (before temporary tolerances can be confirmed)
1. Information on the nature and fate of residues of thiophanate-
ethyl in meat, milk, and eggs following the feeding of
thiophanate-methyl at levels likely to be found on forage and
foodstuffs.
Desirable
1. Further studies on the metabolism of thiophanatemethyl in
animals, with special reference to the occurrence of carbendazim.
2. Further studies on the effect of thiophanate-methyl on the
thyroid and the testes in the rat and other species of animals.
3. Further data on residues on raspberries following good
agricultural practice.
4. Further information on the need for post-harvest treatment of
carrots and celery.
REFERENCES
Aelbers, E., Mededel, Pak. (1970) Landbouw Wet. Gent., 36, 126.
Cited from Vonk & Sijpesteijn (1971)
Bollen, G.J. (1972) A comparison of the in vitro antifungal spectra
of thiophanates and benomyl. Neth. J. Pl. Path. 78, 55
Buchenauer, H., Edgington, L.V. and Grossmann, F. (1973) Photochemical
transformation of thiophanate-methyl and thiophanate to alkyl
benzimidazole-2-yl carbamates. Pest. Sci., 4: 343-348
CIVO-INO Report No. R.4023 (1973) on residues of thiophanate-methyl
and its metabolite MBC in strawberries. From Central Institute for
Nutrition and Food Research, TNO, Zeist (Unpublished)
Fuchs, A. Van Den Berg, G.A. and Davidse, L.C.A (1972) comparison of
benomyl and thiophanates with respect to some chemical and systemic
fungitoxic characteristics. Pest. Biochem. Physiol., 2(2): 191-205
Fujino, A. and Kamimura, H. 1973a The fate of thiophanate-methyl
residue on plants under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A. and Kamimura, H. 1973b The fate of thiophanate-methyl
residue in soil under practical use. Paper submitted by Chemical
Research Division, Nippon Soda Co. Ltd. (Unpublished)
Fujino, A., Ohnuma, N. Mori, T., Tanoue, T. and Kamimura, H. (1973c)
The balance and metabolism studies of thiophanate methyl in animals.
Paper submitted by Nisso institute Per Life Sciences. Nippon Soda Co.
Ltd. (Unpublished)
Hashimoto, Y., Makita, T., Mori, T., Nishibe, T., Noguchi, T., Tsuboi,
S. and Ohta, G. (1970) Toxicological Evaluations of Thiophanate (I).
Acute and Subacute toxicity of a new fungicide, thiophanate (Acute
Ingredient of NF-35), 1,2 hrs. (ethoxy-carbonyl-thioureido)
- benzene. Pharmacometrics, 4(1): 5-21
Hashimoto, Y. Makita, T. Ohnuma, M. and Noguchi, T. (1972a) Acute
toxicity on Demethyl 4, 4'-O-phenylene bis (3-thio allophanate),
thiophanate-methyl fungicide. Toxicol. Appl. Pharmacol., 23: 606-615
Hashimoto, Y., Mori, T., Ohnuma, N. and Noguchi, T. (1972b) Some
Pharmacologic Properties of a new fungicide, thiephanate-methyl. Tox,
Appl. Pharmacol., 23: 616-622
Hashimoto, Y. and Fukuda, Y. (1972) Final Report on the long term oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4 -O-phenylenebis
(3-thioallophanate) in Beagle dogs for 24 months. Unpublished report
from Nisso Institute for Life Sciences submitted by Nippon Soda Co.
Ltd.
Hashimoto, Y. and Tsubura, Y. (1972) Final Report on the Chronic Oral
toxicity studies of thiophanate-methyl, Dimethyl 4, 4' -O-phenylenebis
(3-thioallophanate) in rats of Sprague-Dawley Strain for 24 months.
Unpublished report from Nisso Institute for Life Sciences submitted by
Nippon Soda Co. Ltd.
Kikuchi, Masayoshi. (1973) Actual state of thiophanate-methyl residues
on crops. Paper submitted by Fine Chemicals Division, Nippon Soda Co.
Ltd. (Unpublished)
Kosaka, S., Soeda, Y., Hagiwara, S., Hashimoto, S. and Naohara, T.
(1972) Influences of thiophanate-methyl to soil microorganisms add
fate of the chemical in soil. Paper submitted by Nisso Institute for
Life Science, Nippon Soda Co. Ltd. (Unpublished)
Lemperle, E., Kerner, B., Strecker, H. and Waibel, A. (1973)
Wirkstoffrückstünde und Gärbeeinflussungen nach Anwendung von
Fungiziden im Weinbau. Deutsche Leb.-Rdschau, 69, 313
Makita, T., Hashimoto, Y., and Noguchi, T. (1973) Mutagenic,
Cytogenetic and teratogenic Studies on thiophanate methyl. Toxicol
Appl. Pharmacol, 24(2): 206-215
Martens, P. H. and Chs, M. Détermination oscillopolarographique des
residue do thiophanate sur légumes divers. Model. Faknld.
Landbonetensch., Gent, 37, 891
Mori, H. 1972 Human Handling experiences from Plant Employees
manufacturing Topsin (1). Unpublished report from Nisso Takaoka
Hospital submitted by Nippon Soda Co. Ltd.
Nippon Soda Co. Ltd. (1973a) Information on usage and the occurrence
of residues of thiophanate-methyl. Report submitted in August 1973 by
Nippon Soda Co. Ltd. (Unpublished)
Nippon Soda Co. Ltd. (1973b) List of maximum residue of
thiophanate-methyl and its metabolite, MBC in/on edible crops in
the world. Compiled by the Nippon Soda Co. Ltd. October 1973
(Unpublished)
Noguchi, T. and Hashimoto, Y. (1970a) Toxicological evaluation of
Thiophanate Methyl (I) Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from Nisso Institute for Life
Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate methyl (II) studies on the subchronic oral toxicity of
thiophanate-methyl in mice. Unpublished report from Nisso Institute
for, Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity of
thiophanate-methyl in rats. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970d) Toxicological evaluation of
thiophanate-methyl (IV). Studies on the teratogenic effect of
thiophanate-methyl upon the fetus of ICR strain mice. Unpublished
report from Nisso Institute for Life Sciences submitted by Nippon Soda
Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1970e) Toxicological evaluation of
thiophanate-methyl (V). Some pharmacologic properties of a new
fungicide thiophanate-methyl. Unpublished report from Nisso Institute
for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T. and Hashimoto, Y. (1971) Study on contact phototoxicity
and sensitivity to new fungicide, thiophates. Unpublished report from
Nisso Institute for Life Sciences submitted by Nippon Soda Co. Ltd.
Noguchi, T., Ohkuma and Kosaka, S. (1971) Chemistry and Metabolism of
thiophanate fungicides. Proc. Int. Symp. Pesticide Terminal Residues,
Tot Aviv, pp. 257-270
Noguchi, T. (1972) Environmental Evaluation of Systemic Fungicides.
Environmental Toxicology of Pesticides, Academic Press N.Y., pp.
607-632
Ono, S. (1973a) Analytical method for residues of thiophanate-methyl
and 2-methyl benzimidazolecarbamate in crops by U.V.-spectrometry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Ono, S. (1973b) Stability of thiophanate-methyl under analytical
procedures. Paper submitted by Nippon Soda Co. Ltd. (Unpublished)
Palmer, A.K., Lowell, M.R, and Newman, A.J. (1972) Effect of
thiophanate-methyl on reproductive function of multiple generation in
the rat. Unpublished report from Huntingdon Research Center submitted
by Nippon Soda Co. Ltd.
Pease, H.L. and Gardiner, J.A. (1969) Fluorometric and colorimetric
procedures for determining residues of benomyl. J. Agr. Food Chem.
17, 267
Pease, H.L. and Holt, R.P. Improved method for determining benomyl
residues. J. Assoc. Off. Analyt. Chem., 54, 1399
Selling, H.A., Vonk, J.W. and Sijpesteijn, A.K. (1970) Transformation
of the systemic fungicide methyl thiophanate into 2-benzimidazole
carbamic acid methyl ester. Chem. Ind., pp. 1625-1626
Soeda, Y., Kosaka, S. and Noguchi, T. (1972a) The fate of thiophanate.
methyl fungicide and its metabolites on plant leaves and glass plates.
Agr. Biol. Chem., 36(6): 931-936
Soeda, Y., Kosaka, S. & Noguchi, T. (1972b) Identification of Alkyl
2-benzimidazole carbamates as a major metabolite of thiophanates
fungicide in/on the bean plant. Agr. Biol. Chem., 36(6): 817-823
Sugioka, K., Toyama, N. and Kamimura, H. (1973) Residue analytical
method of thiophanate-methyl and a transformed product, methyl
2-benzimidazole (MBC) in soils and plant tissues by colorimetry.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Tanoue, T., Toyama, N. & Karimura, H. (1973) Residue analytical method
of thiophanate-methyl in plant tissues by thin layer chromatography.
Submitted by Nippon Soda Co. Ltd. (Unpublished)
Vonk, J.W. & Sijpesteijn, A.K. (1971) Methyl benzimidazol-2-
ylcarbamate, the fungitoxic principle of thiophanatemethyl. Pest.
Sci., 2, 160
