
THIOPHANATE - METHYL JMPR 1977
Explanation
Thiophante-methyl was previously evaluated in 1973 and 1975 (FAO/WHO,
1974, 1976). An acceptable daily intake of 0-0.08 mg/kg was allocated.
Further data on the degradation of thiophante-methyl in vivo and
in vitro have been made available and are summarized in the
following monograph addendum.
EVALUATION FOR THE ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Male mice (8 weeks old, 20-25 g b.w.) and rams (ca 26 weeks old,
ca 35 kg b.w.) received a single oral dose of 0.1 mg/kg
thiophanate-methyl (as aqueous suspension) and excreta were collected
for 72 h p.a. The excretion of metabolites in the sheep was similar to
that in the mouse:
urine faeces
carbendazim 16-19% 9-11%
5-hyclroxyoarbendazim 4-11% 5-9%
2-aminobenzimidazole 3% 3%
5-hydroxy-2-aminobenzimidazole 0.5-2% 1-2%
The metabolites carbendazim and 2-aminobenzimidazole appeared as free
compounds; the hydroxylated products were found both free and as
glucuronide and sulphate conjugates (Douch, 1974).
Data from quantitative and qualitative metabolism studies with enzyme
preparations from mouse and sheep liver give the following scheme for
the degradation of thiophanate methyl (Figure 1).
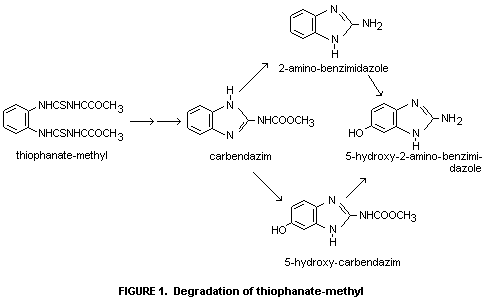 In vitro also some other metabolites have been isolated and
identified:
2-bis(3-methoxycarbonyl-2-thioureido)aniline
1- (3-methoxycarbonyl-2-ureido) -2- (3-methoxycarbonyl-2-thioureido)
benzene
1,2-bis(3-methoxycarbonyl-2-ureido)benzene
2-(3-methoxycarbonyl-2-ureido)aniline
1-thioureido-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-ureido)benzene
When incubated separately with liver enzymes, these intermediates gave
rise to the formation of the same carbendazim and imidazole compounds
as in vivo studies. Mouse kidney and brain enzymes produced the came
compounds, while enzymes from mouse intestine and sheep rumen fluid
did not produce the benzimidazole derivatives (Douch, 1974).
COMMENTS
New data on biochemical aspects were made available. The Meeting
reconfirmed the previously established ADI for humane of
0-0.08 mg/kg bw.
REFERENCES
Douch, P.G.C. (1974) The metabolism of some thicuraidobenzene
fungicides in mice and sheep. Xenobiotica 4, 457-475.
FAO/WHO (1974) 1971 evaluations of some pesticide residues in food.
AGP:1973/M/9/1; WHO Pesticide Residue Series No. 3.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/M/13; WHO Pesticide Residue Series No. 5.
In vitro also some other metabolites have been isolated and
identified:
2-bis(3-methoxycarbonyl-2-thioureido)aniline
1- (3-methoxycarbonyl-2-ureido) -2- (3-methoxycarbonyl-2-thioureido)
benzene
1,2-bis(3-methoxycarbonyl-2-ureido)benzene
2-(3-methoxycarbonyl-2-ureido)aniline
1-thioureido-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-ureido)benzene
When incubated separately with liver enzymes, these intermediates gave
rise to the formation of the same carbendazim and imidazole compounds
as in vivo studies. Mouse kidney and brain enzymes produced the came
compounds, while enzymes from mouse intestine and sheep rumen fluid
did not produce the benzimidazole derivatives (Douch, 1974).
COMMENTS
New data on biochemical aspects were made available. The Meeting
reconfirmed the previously established ADI for humane of
0-0.08 mg/kg bw.
REFERENCES
Douch, P.G.C. (1974) The metabolism of some thicuraidobenzene
fungicides in mice and sheep. Xenobiotica 4, 457-475.
FAO/WHO (1974) 1971 evaluations of some pesticide residues in food.
AGP:1973/M/9/1; WHO Pesticide Residue Series No. 3.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/M/13; WHO Pesticide Residue Series No. 5.
 In vitro also some other metabolites have been isolated and
identified:
2-bis(3-methoxycarbonyl-2-thioureido)aniline
1- (3-methoxycarbonyl-2-ureido) -2- (3-methoxycarbonyl-2-thioureido)
benzene
1,2-bis(3-methoxycarbonyl-2-ureido)benzene
2-(3-methoxycarbonyl-2-ureido)aniline
1-thioureido-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-ureido)benzene
When incubated separately with liver enzymes, these intermediates gave
rise to the formation of the same carbendazim and imidazole compounds
as in vivo studies. Mouse kidney and brain enzymes produced the came
compounds, while enzymes from mouse intestine and sheep rumen fluid
did not produce the benzimidazole derivatives (Douch, 1974).
COMMENTS
New data on biochemical aspects were made available. The Meeting
reconfirmed the previously established ADI for humane of
0-0.08 mg/kg bw.
REFERENCES
Douch, P.G.C. (1974) The metabolism of some thicuraidobenzene
fungicides in mice and sheep. Xenobiotica 4, 457-475.
FAO/WHO (1974) 1971 evaluations of some pesticide residues in food.
AGP:1973/M/9/1; WHO Pesticide Residue Series No. 3.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/M/13; WHO Pesticide Residue Series No. 5.
In vitro also some other metabolites have been isolated and
identified:
2-bis(3-methoxycarbonyl-2-thioureido)aniline
1- (3-methoxycarbonyl-2-ureido) -2- (3-methoxycarbonyl-2-thioureido)
benzene
1,2-bis(3-methoxycarbonyl-2-ureido)benzene
2-(3-methoxycarbonyl-2-ureido)aniline
1-thioureido-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-thioureido)benzene
1-(2-ureido)-2-(3-methoxycarbonyl-2-ureido)benzene
When incubated separately with liver enzymes, these intermediates gave
rise to the formation of the same carbendazim and imidazole compounds
as in vivo studies. Mouse kidney and brain enzymes produced the came
compounds, while enzymes from mouse intestine and sheep rumen fluid
did not produce the benzimidazole derivatives (Douch, 1974).
COMMENTS
New data on biochemical aspects were made available. The Meeting
reconfirmed the previously established ADI for humane of
0-0.08 mg/kg bw.
REFERENCES
Douch, P.G.C. (1974) The metabolism of some thicuraidobenzene
fungicides in mice and sheep. Xenobiotica 4, 457-475.
FAO/WHO (1974) 1971 evaluations of some pesticide residues in food.
AGP:1973/M/9/1; WHO Pesticide Residue Series No. 3.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/M/13; WHO Pesticide Residue Series No. 5.
