
THIOPHANATE-METHYL
First draft prepared by
J. Taylor and M. Watson
Pesticides Safety Directorate, Ministry of Agriculture, Fisheries
and Food
Mallard House, Kings Pool, York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Effects on the thyroid and liver
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Thiophanate-methyl is a systemic fungicide of the benzimidazole
group. It was evaluated toxicologically by the Joint Meeting in 1973,
1975, and 1977 (Annex I, references 20, 24, and 28). The ADI of
0-0.08 mg/kg bw allocated in 1973 was confirmed by the 1975 and 1977
Joint Meetings. Since that time, additional data have become
available, and the results of these studies were reviewed at the
present Meeting. In order to facilitate review of the complete
database, information presented in the reports of the previous
monographs (Annex I, references 21, 25, and 29) are included in this
monograph. Thiophanate-methyl was reviewed by the present Meeting
within the periodic review programme of the CCPR.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
In a series of studies, an unspecified number of male dd-Y strain
mice were given a single dose of radiolabelled thiophanate-methyl by
gavage. Four radiolabelled thiophanate-methyl moieties were used,
14C-carbonyl thiophanate-methyl, 35S-thiophanate-methyl,
14C-methyl thiophanate-methyl, and 14C-phenyl thiophanate-methyl.
Faeces, urine, and expired gas were analysed 3, 6, 12, 24, 48, 60, 72,
84, and 96 h after administration, and blood samples were analysed
after 3, 6, 12, 24, 48, and 72 h. Conjugates were determined after
hydrolysis with hydrochloric acid or ß-glucuronidase. An unspecified
number of mice were killed 3, 6, 12, 24, 48, and 72 h after
administration, and tissue samples were analysed for radiolabel. The
contents of all moieties in blood and urine declined steadily from
peak levels within 3 h, and faecal excretion peaked at about 12 h in
all cases, declining significantly by 48 h. The rate of excretion
varied slightly between moieties, but total excretion had reached a
plateau at about 24 h. 14C-Methyl thiophanate-methyl showed a
different pattern of excretion, suggesting that the methyl group was
parted from the parent molecule and was rapidly absorbed and partially
converted to carbon dioxide. Total excretion is summarized in Table 1.
Table 1. Percentage of radiolabelled thiophanate-methyl excreted by male
dd-Y mice after 96 h
Compound Total radiolabel excreted (%) Total recovery (%)
of radiolabel by
day 4 or 5
Urine Faeces Expired gas
14C-Carbonyl thiophanate-methyl 78-83 18-20 ND 102 (day 5)
35S-Thiophanate-methyl 66-86 19-21 ND 105 (day 5)
14C-Methyl thiophanate-methyl 66-67 16-17 1 ND
14C-Phenyl thiophanate-methyl 78-89 27-29 ND 117 (day 4)
ND, not determined
None of the moieties accumulated in any organ or tissue, and
radiolabel disappeared relatively rapidly within the 96-h
investigation period. After three days, 14C-carbonyl thiophanate-
methyl radiolabel was detectable in liver and blood, 14C-methyl
thiophanate-methyl radiolabel primarily in liver, kidney, and blood,
and 14C-phenyl thiophanate-methyl radiolabel in liver, with low
levels in a number of tissues. 35S-Labelled thiophanate-methyl was
the only moiety found in bone after 96 h, suggesting that the labelled
sulfur might be cleaved and hence behave differently. Metabolites were
investigated in urine, faeces, organs, and tissues by thin-layer
chromatography. Untreated urine from 14C-phenyl thiophanate-methyl
produced eight possible metabolites. The parent molecule and three
metabolites were identified against known standards: carbendazim, an
intermediate (1-thioureido-2-(3-ethoxycarbonyl-2-thioureido)benzene),
and a minor metabolite (1,2-bis(3-ethoxycarbonyl-2-ureido)benzene).
Hydrolysis of the water-soluble fraction with hydrochloric acid or
ß-glucuronidase resulted in six spots, the majority of which were
identified as carbendazim and its hydroxy analogue 5-hydroxy-
carbendazim, which were found by co-chromatography with authentic
standards to be glucuronic acid conjugates. 1-Thioureido-2-
(3-ethoxycarbonyl-2-thioureido)benzene and 1,2-bis(3-ethoxy-
carbonyl-2-ureido)benzene were also found, but two further metabolites
were not identified. Faeces, liver, kidney, stomach, and intestine
were also investigated for metabolites. Thiophanate-methyl,
carbendazim, 5-hydroxy-carbendazim, and 1-thioureido-2-(3-ethoxy-
carbonyl-2-thioureido)benzene were identified in faeces; the parent
molecule, carbendazim, 5-hydroxycarbendazim, and 1,2-bis(3-ethoxy-
carbonyl-2-ureido)benzene were identified in liver; carbendazim,
5-hydroxycarbendazim, and 1,2-bis(3-ethoxycarbonyl-2-ureido)benzene
were identified in kidney; the parent molecule was the main compound
identified in stomach, with small amounts of carbendazim and
1,2-bis(3-ethoxycarbonyl-2-ureido)benzene; and the parent molecule and
carbendazim were identified in the intestine (Noguchi, 1970a, 1971,
1972; Fujino et al., 1973).
An unspecified number of male Wistar rats were given a single
dose of 14C-thiocarbonyl]-thiophanate-methyl by gavage. Most of the
radiolabel (83% of the total administered) was excreted within 24 h,
with 56% in faeces and 28% in urine. By 72 h, 89% of the label had
been excreted. Table 2 shows the 24-h faecal and urinary metabolites
that were characterized. Of the 56% radiolabel detected in the faeces,
1% in the water-soluble phase was uncharacterized and > 4% remained
in the residue; of the 28% radiolabel in urine, 14% was
uncharacterized because it was not extractable (Fujino et al.,
1973).
Table 2. Recovery of 14C-labelled compounds in rat urine and faeces 24 h after
administration of 14C-labelled thiophanate-methyl
Compound Recovery (as % of administered
14C-labelled thiophanate-methyl)
Faeces Urine
Thiophanate-methyl 38 1
4-Hydroxy-thiophanate-methyl 6 3
5-Hydroxy-carbendazim 2 6
4-Hydroxy-dimethyl-4,4'-O-phenylene bisallophanate 1 2
Dimethyl-4,4'-O-phenylene bisallophanate 1 2
Carbendazim 1 1
An unspecified number of male beagle dogs were given a single
dose of [14C-carbonyl]-thiophanate-methyl in a capsule. Blood, urine,
and faeces were collected 8, 15, and 30 min and 1, 2, 3, 6, 12, 24,
35, 48, 60, 72, and 96 h after administration. Urine contained 74% of
the total radiolabel and faeces, 14%. Maximal total excretion occurred
after about 24 h by both routes. The total recovery of radiolabel was
not specified. Carbendazim was identified by co-chromatography from
urine samples. Treatment of urine samples with ß-glucuronidase
released the 5-hydroxy analogue. No other metabolites were identified
(Noguchi, 1972; Fujino et al., 1973).
(b) Biotransformation
Male mice (strain unspecified) received 0.1 g/kg bw of an aqueous
thiophanate-methyl solution orally, and their urine and faeces were
collected at 24, 48, and 72 h. Metabolites were extracted into
ethylacetate; conjugated metabolites were extracted by enzymic
hydrolysis with ß-glucuronidase and arylsulfatase. As a proportion of
the total dose administered, 27% was identified as free metabolites in
urine, the majority (19%) being carbendazim; other metabolites,
2-aminobenzimidazole (2.5%), 5-hydroxy-2-aminobenzimidazole (0.4%),
and 5-hydroxycarbendazim (4.9%), accounted for the other 8% found free
in urine. Conjugates of glucuronide and/or sulfate were found in urine
and faeces, but the amounts of each type were not determined and only
two were identified (representing 8% of total dose), of which 6% was
5-hydroxycarbendazim and 2% aminobenzimidazole. In faeces, 16% of the
administered dose was identified as free metabolites, carbendazim
again being prevalent (11%); 2-amino-benzimidazole (3%), 5-hydroxy-
2-aminobenzimidazole (0.3%), and 5-hydroxy carbendazim (3%) accounted
for the other 6% found free in faeces. Of the total dose 8% was
identified as conjugated metabolites, with similar proportions of the
two metabolites found in urine. Thin-layer chromatography of faeces
and urine showed the presence of 10 conjugated metabolites.
Mouse liver preparations were incubated with 1 mmol/litre
thiophanate-methyl, and 11 metabolites were identified:
2-(3-methoxycarbonyl-2-thioureido)aniline,
1-(3-methoxycarbonyl-2-ureido)-2-(3-methoxycarbaryl-2-thioureido)
benzene,
1,2-bis(3-methoxycarbonyl-2-ureido)benzene,
2-(3-methoxycarbonyl-2-ureido)aniline,
1-thioureido-2-(3-methoxycarbonyl-2-thioureido)benzene,
1-(2-ureido)-2-(3-methoxy-2-thioureido)benzene,
1-(2-ureido)-2-(3-methoxycarbonyl-2-ureido)benzene,
2-aminobenzimidazole,
5-hydroxy-2-aminobenzimidazole,
methylbenzimidazole-2-ylcarbamate (carbendazim), and
methyl 5-hydroxybenzimidazol-2-ylcarbamate (5-hydroxy-
carbendazim).
Incubation with mouse kidney and brain preparations produced similar
metabolites, with thin-layer chromatography patterns that were
indistinguishable under fluorescence quenching. Incubation with
intestinal preparations did not produce cyclized benzimidazole
derivatives, but 2-(3-methoxycarbaryl-2-thioureido) aniline and
1-thioureido-2-(3-methoxycarbaryl-2-thioureido)benzene were detected.
Further experiments were carried out in vitro with non-cyclized
compounds (including the 11 thiophanate-methyl metabolites identified)
as the substrate in mouse liver preparations. The rate of carbendazim
formation from eight compounds, including thiophanate-methyl, was
measured. The 11 metabolites were relatively stable but 1-formamido-
2-(3-methoxy-2-thioureido)benzene and the 2-ureido analogue were
relatively unstable to nonenzymatic transformation. Formation of
carbendazim was rapid for substrates with an intact 3-ethoxy-carbonyl-
3-thioureido constituents, although the formamide and thioformamide
derivatives showed highest cyclization (the latter being cyclized at
about 80% of the former). The largest amounts of 2-aminobenzimidazole
were formed from substrates containing thioureido or ureido
side-chains. Formation of 2-aminobenzimidazole was 25-50% that of
carbendazim. Hydrolysis of carbendazim with subsequent cyclization was
proposed. Additional experiments to investigate the cofactor (NAD,
NADP, and glucose-6-phosphate) requirements for reactions leading to
carbendazim suggested that mixed-function oxidase enzymes were
responsible, as these reactions were inhibited by carbon monoxide and
SKF 525A (Douch, 1974).
14C-Thiocarbonyl-labelled thiophanate-methyl was added to liver
microsome preparations from homogenates obtained from male and female
Sprague-Dawley rats fed either a commercial diet or a diet containing
640 ppm thiophanate-methyl for three months. No sex difference and no
enzyme induction were seen; only one metabolite, carbendazim, was
identified, by thin-layer co-chromatography (Noguchi, 1970b).
Male and female Sprague-Dawley rats were fed either a commercial
diet or a diet containing 640 ppm of 14C-carbonyl thiophanate-
methyl, 35S-thiophanate-methyl, 14C-methyl thiophanate-methyl, or
14C-phenyl thiophanate-methyl for three months. No sex difference
and no enzyme induction was seen. Four metabolites of the 14C
moieties were identified by autoradiography: dimethyl-4,4'- O-
phenylenebisallophanate, an intermediate metabolite, carbendazim, and
5-hydroxycarbendazim; 35S-thiophanate-methyl did not result in
carbendazim (Noguchi, 1972). In a similar study, 4-hydroxythio-
phanate-methyl and dimethyl-4,4'- O-phenylene bisallophanate were
identified in vitro as metabolites of thiophanate-methyl in rat
liver microsome preparations (Fujino et al., 1973).
A proposed metabolic pathway for thiophanate-methyl is presented
in Figure 1.
(c) Effects on enzymes and other biochemical parameters
A number of experiments were performed on mice, rats, and rabbits
to determine the pharmacological properties of thiophanate-methyl
(purity, > 98%) (Singh & Garg, 1989). The results are summarized in
Table 3.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of thiophanate-methyl is summarized in Table
4. It has little acute toxicity. At high oral doses in older studies,
the active ingredient induced symptoms of toxicity which included
tremors and tonic and clonic convulsions. In a newer study with
thiophanate-methyl of 96.55% purity, there were no signs of toxicity
or deaths at 5000 mg/kg bw orally (Souma & Nishibe, 1990a). After
acute inhalation of 95.3% pure compound at concentrations close to the
LC50 (1.7-1.9 mg/litre), the symptoms of toxicity included ataxia,
decreased motor activity, tremor, and convulsions (Saika & Nishibe,
1987).
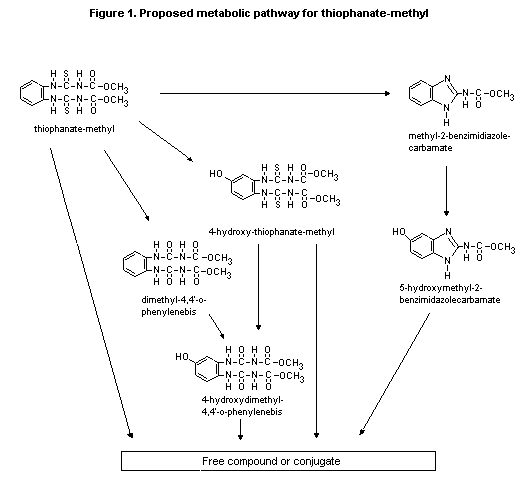 Table 3. Pharmacological effects of thiophanate-methyl
Parameter Species Dose and route Results
Body temperature Wistar rats 380-1500 mg/kg bw orally No effect
Analgesic activity Mice 500 mg/kg bw intraperitoneally No effect
Sedative or hypnotic activity Rats and mice 100-500 mg/kg bw subcutaneously No effect
Cardiovascular and respiratory Rabbits 20-100 mg/kg bw intravenously Reduced blood pressure
effects followed by bradycardia
Table 4. Acute toxicity of thiphanate-methyl
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male, female Orala 3400-3514 Noguchi & Hashimoto (1970a)
Mouse Male, female Intraperitoneal 792-1113 Noguchi & Hashimoto (1970a)
Mouse Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Mouse Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Oral 6640-7500 Noguchi & Hashimoto (1970a)
Rat Male, female Intraperitoneal 140-1640 Noguchi & Hashimoto (1970a)
Rat Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Inhalation 1.7-1.9 Nishibe (1987)
Rat Male, female Oralb > 5000 Souma & Nishibe (1990a)
Guinea-pig Male, female Oral 3640-6700 Noguchi & Hashimoto (1970a)
Guinea-pig Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Oral 2270-2500 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermalb > 2000 Souma & Nishibe (1990b)
a In gum arabic in 5% sodium chloride solution
b In distilled water
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 12 ICR mice of each sex were fed diets containing
thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or 8000 ppm for
six months. The homogeneity, stability, and concentrations of the diet
were not reported; clinical signs of toxicity were also not reported.
There were no treatment-related deaths. The body-weight gain of
animals of each sex at 8000 ppm was significantly reduced. Erythrocyte
counts were also reduced in these animals and in males at 1600 ppm,
and haematocrit values were reduced in animals of each sex at 1600 and
8000 ppm. No significant differences were seen in the weights of the
cerebrum, heart, lung, spleen, kidney, or testis, but in animals at
8000 ppm, liver weights were increased. Histopathological
investigation revealed a higher incidence of hepatic-cell irregularity
at the higher doses, with five cases in males and three in females at
8000 ppm and one case in an animal of each sex at 1600 ppm. Large
swollen hepatic cells, some with oedematous or granular protoplasm,
were found to a greater extent at 8000 ppm, especially in the central
lobules. Constriction of veins by the swollen cells was proposed to
explain the cloudy appearance of the liver. Sporadic formation of
acidiphilic bodies from hepatic-cell degeneration was recorded in two
females and one male at 8000 ppm and one male at 1600 ppm. The NOAEL
was 320 ppm, equivalent to approximately 48 mg/kg bw per day, on the
basis of hepatotoxic and haematological effects at 1600 and 8000 ppm
(Noguchi & Hashimoto, 1970b; Hashimoto et al., 1973).
Rats
Groups of 12 Sprague Dawley rats of each sex were fed diets
containing thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or
8000 ppm for six months. The homogeneity, stability, and
concentrations of the diet were not reported, and no clinical signs of
toxicity were reported. There were no treatment-related deaths. The
body-weight gain of animals of each sex at 8000 ppm was significantly
reduced, but food consumption did not differ significantly between
groups. Reduced erythrocyte counts were seen in animals of each sex at
8000 ppm. Urinalysis did not show significant treatment-related
differences. Blood cholesterol levels were raised significantly in
animals of each sex at 8000 ppm. The thyroid anti liver weights were
increased in these animals, and the weight of the thymus was increased
in females. Some histopathological effects on the thyroid were
reported in animals at this dose: four animals of each sex had small
thyroid follicles, four males and four females had thickened
follicular epithelium, and five animals of each sex had decreased
colloidal substance. There were no treatment-related effects on
protein-bound iodine levels. The NOAEL was 1600 ppm, equivalent to
approximately 80 mg/kg bw per day, mainly on the basis of effects on
thyroid, liver, and body weights (Noguchi & Hashimoto, 1970c;
Hashimoto et al., 1973).
Dogs
Groups of five beagle dogs of each sex (four at the highest dose)
were fed capsules containing thiophanate-methyl (purity unspecified)
at doses of 0, 2, 10, 50, or 250 mg/kg bw per day for two years. One
animal of each sex at each dose was killed and autopsied after 12
months. There were no deaths or adverse symptoms. Animals of each sex
at 250 mg/kg bw per day had reduced body weights and body-weight gain.
Gross pathological effects were not reported, and no effects on the
eyes or heart were found in periodic ophthalmoscopic and electro-
cardiographic investigations. There were no treatment-related changes
in clinical chemical, haematological, or urinary values. After 24
months, the weight of the thyroid gland was increased in animals of
each sex at 250 mg/kg bw per day and to a lesser extent at 50 mg/kg bw
per day. The effects on thyroid tissue at 12 months included altered
or decreased colloidal substance and slightly taller cuboid follicular
epithelial cells at 250 mg/kg bw per day. The NOAEL was 10 mg/kg bw
per day on the basis of effects on thyroid weight in animals of each
sex at 50 and 250 mg/kg bw per day (Taniguchi, 1972).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) for three months at
doses of 0, 50, 200, or 800 mg/kg bw per day, the last dose being
lowered to 400 mg/kg bw per day on test day 50 because of severe
toxicity. One male at the high dose was sacrificed on day 41 because
of severe toxicity; one male at 50 mg/kg bw per day died on day 36,
but this death did not appear to be related to treatment. Dose-related
clinical signs seen in animals at the high dose and to a lesser extent
at the middle dose included dehydration, thinness, and lethargy.
Dose-related decreases in body weights and marked decreases in food
consumption were seen at the middle and high doses. There were no
treatment-related ophthalmological findings. Slight anaemia, increased
platelet counts and cholesterol levels, and decreases in serum alanine
aminotransferase activity and albumin levels were seen at the middle
and high doses and increased activated partial thromboplastin time at
the high dose. Thyroid function tests revealed slightly decreased
triiodothyronine levels in males at the high dose and decreased
triiodothyronine and thyroxine levels in females at the middle and
high doses; no clear effects on thyroid-stimulating hormone values
were apparent. Urinalysis showed no treatment-related findings. At the
middle and high doses, the weights of the liver (in animals of each
sex) and thyroid (males only) were increased. Gross examination
post mortem revealed emaciation in one male at the middle dose and
three at the high dose. Dose-related histological alterations were
seen in a number of organs. In thyroids, hypertrophy of the follicular
epithelium was found in one, three, and four males and one, two, and
four females at the low, middle, and high doses, respectively, with
none in controls. The severity of the hypertrophy increased from
minimal to marked with dose. Hyperplasia of the follicular epithelium
was found in one male at the middle dose and in most animals at the
high dose. In two males at the high dose in which both hypertrophy and
hyperplasia were marked, a large decrease in the quantity of
intrafollicular colloid was seen. In animals at the middle and high
doses, dose-related changes were found in the liver (reduction in
vesiculation of the hepatocellular cytoplasm), gall-bladder
(intracytoplasmic vacuoles), pancreas (atrophy of acinar cells due to
a decreased quantity of zymogen granules), spleen (lymphoid-cell
depletion), thymus (involution), prostate (atrophy), uterus
(anoestrus), and ovaries (inactive). There was no NOAEL because of the
presence of follicular-cell hypertrophy in the thyroid of two dogs at
the low dose (Auletta, 1991).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) at doses of 0, 8, 40,
or 200 mg/kg bw per day for one year. There were no deaths. Tremors
were seen in all dogs at the high dose 2-4 h after treatment on one or
more occasions during the initial three weeks of the study but not
subsequently. The tremors were slight to moderate, except in one
animal which had severe tremors that progressed to apparent tonic
convulsions on three occasions. The body-weight gains of animals at
the high dose were reduced during the study, and body-weight losses
were noted in the first week of treatment; slight reductions in
body-weight gain were noted at 40 mg/kg bw per day. The food
consumption of dogs at the high dose was generally lower than that of
controls during the early months, but was close to the control value
thereafter. Ophthalmological examinations and urinalyses showed no
treatment-related effects. Haematological effects consisted of slight
decreases in total erythrocyte counts and haemoglobin and haematocrit
values in males at the high dose. These animals also had decreased
serum alanine aminotransferase activity, increased alkaline
phosphatase activity, increased cholesterol levels (also at the middle
dose), and decreased albumin:globulin ratios (also at the middle
dose), calcium (also at the middle dose), potassium, and phosphorus
levels. In females at 200 mg/kg bw per day, serum alanine
aminotransferase activity was decreased, and alkaline phosphatase
activity and cholesterol levels were increased. Thyroid function tests
revealed decreased thyroxine levels in males at the middle and high
doses but no clear effects on triiodothyronine or thyroid-stimulating
hormone. Abnormalities seen post mortem included increased liver
weights in dogs at the high dose and increased thyroid weights in
those at the middle and high doses. Microscopic alterations attributed
to treatment were limited to minimal to moderate hypertrophy and
slight hyperplasia of the follicular epithelium of the thyroid in the
dogs at the middle dose (hypertrophy in two females) and the high dose
(hypertrophy in four males and three females, and hyperplasia in one
male and one female). The NOAEL was 8 mg/kg bw per day (Auletta,
1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing 0,
10, 40, 160, or 640 ppm thiophanate-methyl (purity, > 94%) for two
years. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. The body-weight
gain of males at 640 ppm was reduced during the study. No
treatment-related effects on food consumption or on mortality or overt
signs of toxicity were seen. By 24 months, 74-94% of treated animals
had died, but the mean survival times were comparable. Organ weights
and clinical chemical and haematological parameters were not
investigated. No treatment-related gross macroscopic or histopatho-
logical alterations were seen, and there was no evidence of
carcinogenicity. The NOAEL was 160 ppm, equivalent to about 24 mg/kg
bw per day, on the basis of the reduction in body-weight gain in males
at 640 ppm (Kosaka, 1973).
Groups of 60 male and 60 female CD-1 mice were fed diets
containing 0, 150, 640, 3000, or 7000 ppm thiophanate-methyl (purity,
95.9-96.6%) for 18 months. Ten animals of each sex in each group were
necropsied after 39 weeks of treatment, and surviving animals were
necropsied after 78-79 weeks of treatment; all mice that died or were
killed in extremis were also autopsied. The homogeneity, stability,
and concentrations of thiophanate-methyl were acceptable. Haemato-
logical and thyroid function were assessed at the interim necropsy and
before the end of the study. No treatment-related clinical signs were
observed. The cumulative numbers of deaths up to 18 months of -
treatment were 10, 11, 14, 16, and 24 males and 12, 13, 15, 17, and 23
females at 0, 150, 640, 3000, and 7000 ppm, respectively; animals at
7000 ppm had a significantly higher mortality rate than controls. The
increased rates in females at 3000 ppm and males and females at
7000 ppm appeared to be related to amyloid deposition in tissues.
Body-weight gains were slightly reduced in animals of each sex at
3000 ppm and to a greater degree at 7000 ppm; food consumption was
slightly reduced in females at 3000 and 7000 ppm. There was no
treatment-related effect on ophthalmologic parameters or on the onset
or number of palpable masses. The only haematological parameter that
appeared to be altered by treatment was a slightly lower erythrocyte
count in males at 7000 ppm. At the nine-month interim necropsy,
enlarged thyroid glands were seen grossly in three of 10 males at
7000 ppm, the thyroid weights of males and females at 3000 and
7000 ppm were increased, and the thyroid-stimulating hormone levels
were higher and the thyroxine levels lower in males at 3000 ppm and
males and females at 7000 ppm than in controls. The differences in
thyroid weights and thyroid hormone levels were not seen at 18 months.
At the nine-month interim necropsy, the liver weights of males and
females at 3000 and 7000 ppm were significantly increased as a result
of an increased incidence and severity of hepatocellular centrilobular
hypertrophy at these two doses; the incidence of hepatocellular
centrilobular hypertrophy was also increased in females at 640 ppm,
five out of 10 having minimal hypertrophy. At 18 months, the mean
liver weights of animals at 7000 ppm were increased as a result of a
significantly increased number of liver masses, which was also
significantly increased at 3000 ppm. At the 18-month terminal
necropsy, an increased incidence of hepatocellular centrilobular
hypertrophy was seen in males at 3000 and 7000 ppm. Histopathological
examination revealed that the liver masses seen grossly were adenomas.
The incidence of hepatocellular adenomas was significantly increased
in males and females at 3000 and 7000 ppm. The slightly increased
incidence of hepatocellular adenomas in females at 640 ppm (8.6%) was
greater than the historical control range (0-2.7%). Two hepatocellular
carcinomas were seen, one in a male at 640 ppm and the other in a male
at 7000 ppm; this 2% incidence was similar to the mean incidence for
historical control males (1.4%; range, 0-6%). One male at 7000 ppm had
a hepatoblastoma, which is a relatively rare tumour (0.001% in
historical controls). The incidence of atrial thrombosis was increased
in females at 3000 ppm and in animals of each sex at 7000 ppm. The
NOAEL was 150 ppm, equivalent to 29 mg/kg bw per day, in view of the
possible treatment-related incidence of hepatocellular adenomas in
females at 640 ppm (Tompkins, 1992).
Rats
Groups of 35 Sprague-Dawley rats of each sex (50 of each sex for
controls) were fed diets containing thiophanate-methyl (purity, >
94%) at doses of 0, 10, 40, 160, or 640 ppm for two years. Five rats
of each sex at each dose were killed and autopsied at three and 12
months. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. There were no
treatment-related deaths, although survival was poor in some groups,
and there were no overt signs of toxicity. Body-weight gain was
reduced in animals of each sex at 640 ppm, but no significant
difference in food consumption was observed. No significant treatment-
related effects were seen on clinical chemical, haematological, or
urinary values. No significant adverse histopathological effects or
changes in organ weights were seen at any dose at three or 12 months.
Necropsy of animals killed after 24 months or which died during
treatment showed treatment-related histopathological findings in the
testes and thyroid gland. Males at 640 ppm had an increased incidence
of decreased colloidal substance and hypertrophy of follicular
epithelium in thyroid tissue (6/35 compared to 1/50 in controls). Five
animals at 640 ppm were found to be hypospermatogenic; one died at
week 91 with a pituitary tumour and was found to have atrophied
testes. Another male had diminished spermatogenesis at 24 months, and
another surviving male and one that died during treatment had
hyperplasia of the testicular interstitium. No other treatment-related
effects were seen. There was no evidence for carcinogenicity. The
NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per day, on the
basis of effects on the testes and thyroid gland and reduced growth at
640 ppm (Hashimoto, 1972).
Groups of 60 male and 60 female Fischer 344 rats were fed diets
containing 0, 75, 200, 1200, or 6000 ppm thiophanate-methyl (purity,
96.55%) for 24 months. The homogeneity, stability, and concentrations
of thiophanate-methyl in the diets were acceptable. After 12 months,
10 rats of each sex in each group (only five males at 6000 ppm) were
sacrificed. All survivors were sacrificed at the end of treatment. The
mortality rates are shown in Table 5. Only two males at 6000 ppm
survived to the end of the study; because of this high rate, no
statistical analysis was performed for this group at 24 months. The
main causes of death among these rats were nephropathy (22 rats),
thyroid follicular-cell tumour (10 rats), and leukaemia (six rats);
eight males at this dose died or were killed in extremis during
weeks 11-12 due to fractures of the nasal bone caused by the feeder
plate and subsequent dyspnoea (rhinorrhagia). The feeder plates were
removed.
No clinical signs attributable to treatment were noted in any
group during the first 52 weeks. After week 52, dose-related clinical
signs included pale skin and mucous membranes in males at 6000 ppm,
alopecia in females at 6000 ppm, and tissue masses on the skin and in
the subcutis including the lip in males at 1200 and 6000 ppm. These
changes attained statistical significance at week 77 or later.
Dose-related, statistically significant depressions in body-weight
gain were noted in both males and females at 1200 and 6000 ppm.
Decreased food consumption was seen in animals of each sex at 6000 ppm
starting at week 76. No dose-related abnormalities were observed in
ophthalmoscopic parameters at 6, 12, 18, or 24 months. Treatment-
related signs of anaemia (decreased erythrocyte count, haemoglobin,
haematocrit, mean corpuscular volume, mean corpuscular haemoglobin,
and mean corpuscular haemoglobin concentration) were seen in animals
of each sex (mainly males) at 6000 ppm at 3, 6, 12, and 18 months.
Increased platelet and leukocyte counts were seen frequently in males
at 6000 ppm. Clinical chemical investigations revealed increased total
cholesterol and total protein and decreased albumin:globulin ratios in
animals of each sex at 1200 and/or 6000 ppm. At 24 months, treated
males had increased blood urea nitrogen (at 75, 200, and 1200 ppm) and
creatinine levels (at 1200 ppm), reduced thyroxine and triiod-
othyronine values, and increased thyroid-stimulating hormone values
(at 1200 and 6000 ppm, also in females at 6000 ppm). Dose-related
increases in urinary protein (semi-quantitative analysis) were noted
in animals of each sex at 6000 ppm at six and/or 12 months.
Table 5. Mortality rates in Fischer rats treated with thiophanate-methyl
Dose Male Female
(ppm)
Week 52 Week 80 Week 104 Week 52 Week 80 Week 104
No. % No. % No. % No. % No. % No. %
0 0/60 0 2/50 4 13/50 26 0/60 0 3/50 6 13/50 26
75 0/60 0 2/50 4 15/50 30 0/60 0 1/50 2 12/50 24
200 0/60 0 8/50 16 24/50 48* 0/60 0 1/50 2 8/50 16
1200 0/60 0 3/50 6 21/50 42 0/60 0 0/50 0 12/50 24
6000 8/60 13* 18/55 33** 53/55 96** 1/60 2 3/50 6 11/50 22
Significantly different from control at *P < 0.05; ** P < 0.001 (chi-square test)
Nephelometry indicated an increase in urinary protein in males at 1200
and 6000 ppm at various times. Although males at 200 ppm had a
statistically significant increase in urinary protein at 24 months,
histopathological examination revealed no treatment-related effect. In
males at 6000 ppm, ketone bodies, urinary volume, and water
consumption were increased, and pH and specific gravity were decreased
significantly at various times. Dose-related increases in the weights
of the liver, kidney, and thyroid and their body-weight ratios were
seen in animals of each sex at 1200 and/or 6000 ppm at both interim
and final sacrifice.
At interim sacrifice (week 53), treatment-related changes were
found macroscopically in the liver (brownish-black) and kidneys
(granular surface, brownish-black) of males and females at 6000 ppm.
At final sacrifice, males at 1200 ppm had granular kidneys. Females at
6000 ppm also had enlarged thyroids. In animals at 6000 ppm that died
during the study, swollen thyroids were seen in animals of each sex
and white areas in the liver and granular kidney in males. Dose-
related histopathological changes were found in the thyroid, liver,
kidney, and adrenal: Thyroid follicular-cell hyperplasia and
hypertrophy were noted in animals of each sex at 1200 and/or 6000 ppm
at 12 and 24 months, and the incidence of focal follicular-cell
hyperplasia was increased. Males at 6000 ppm also had a statistically
significant increased incidence of thyroid follicular-cell adenomas
(12/60), and one of two males at 6000 ppm that were killed at 24
months and 2/53 that were dead or were killed in extremis had
thyroid follicular-cell adenocarcinomas, with none in the other groups
of treated males. The incidence of retinal atrophy was increased in
females at 6000 ppm at 24 months, and those of parathyroid hypertrophy
and hyperplasia were increased in males at this dose. Centrilobular
hepatocellular hypertrophy and lipofuscin deposition were noted in
animals of each sex at 1200 and/or 6000 ppm at 12 and 24 months and in
most males and females at 6000 ppm which died during the study.
Microgranuloma and focal fatty degeneration in the liver were
increased in incidence in males treated at 6000 ppm. Lipidosis of the
adrenal cortex was noted in females at 1200 ppm and in animals of each
sex at 6000 ppm at 12 months. The severity of nephropathy was
increased in animals of each sex at 6000 ppm at 12 months and in those
at 1200 and 6000 ppm at 24 months. Most males at 6000 ppm that died or
were killed in extremis had severe nephropathy associated with
hyperplasia of the parathyroid, demineralization of the bone, and
metastatic calcification in various organs. The NOAEL was 200 ppm,
equivalent to 8.8 mg/kg bw per day in males and 10.2 mg/kg bw per day
in females. The increased blood urea nitrogen levels observed in males
at 75 and 200 ppm and the increased urinary protein levels in males at
200 ppm at 24 months were not considered to be adverse effects in view
of the absence of treatment-related pathological findings at these
doses (Takaori, 1993).
(d) Reproductive toxicity
Mice
Groups of eight male Swiss-Webster mice were given thiophanate-
methyl (purity unspecified) as an oral dose of 192 mg/kg bw per day
for five consecutive days. Twenty-four hours after the final dose
[1,2-3H]-testosterone was administered intraperitoneally at
10 µCi/kg bw in order to assess its assimilation by the anterior
prostate coagulating glands. Animals were killed after 5 min, and the
anterior prostate glands were removed. Thiophanate-methyl did not
significantly affect body weights, absolute testicular weights,
absolute or relative seminal vesicular weights, or total radiolabel
assimilation by the anterior prostate gland. The absolute prostate and
adrenal gland weights were significantly increased, and these changes
were considered by the authors to be due to stress induced by
thiophanate-methyl. The relative weights of the testes, prostate, and
adrenal were also increased. Spermatogenesis was not affected, no
sterile tubules were seen, and the interstitial (Leydig) cells
appeared to be normal (Thomas, 1974; Thomas & Schein, 1974).
Rats
In a three-generation study, groups of 10 male and 20 female
weaning Sprague-Dawley rats fed diets containing 0, 40 160, or 640 ppm
thiophanate-methyl (purity unspecified) were mated twice to produce
the F1a and F1b litters. Ten male and 20 female offspring were
selected from the F1b litters and mated to produce the F2a and
F2b litters; 10 male and 20 female F2b offspring were then
selected and mated to produce the F3a and F3b litters. The test
diets were fed throughout the study, animals in the F0, F1b, and
F2b generations being fed for 60 days before mating. In each case,
mating lasted for 20 days. Animals were re-mated to produce the second
litters about 10 days after weaning of the first litters. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were not reported. In F0 animals, no treatment-related
effects on mortality, food consumption, or body-weight gain or
clinical signs of toxicity were seen. No treatment-related effects
were observed on mating performance, length of gestation, or pregnancy
rate or at terminal autopsy in F0, F1b, or F2b rats. The total
litter sizes and weights at birth were slightly reduced in all animals
at 640 ppm except the F3a litter. Viable litter size and weight at
weaning were reduced in all generations at 640 ppm; mean pup weight
was not affected. Viable litter size and weight between birth and
weaning were slightly reduced in the second F2b litter at all doses,
the percentage losses increasing with dose; however, average pup
weights were not affected. The total litter loss and the incidence of
gross malformations of pups were not treatment-related. No
dose-related effects were seen on macroscopic appearance, organ
weight, histopathological parameters, or the skeleton in F3b
offspring. The NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per
day, on the basis of effects on litters at 640 ppm (Palmer et al.,
1972).
In a two-generation study, groups of 25 male and 25 female
Sprague Dawley rats were given diets containing thiophanate-methyl
(purity, 95.93%) at doses of 0, 200, 630, or 2000 ppm. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were acceptable. After 14 weeks of treatment, the parental
(F0) animals were mated for up to 21 days. The F0 females were
allowed to litter and to rear their offspring (F1a generation) to
weaning. After a 14-week maturation period after weaning, the parental
F1 animals, selected from the F1a offspring, were mated for up to
21 days and the females were allowed to litter and rear their
offspring (F2a generation) to weaning. After weaning of the F2a
pups and review of the results of rearing and weaning, including high
pup mortality in all groups, the F1 generation was mated again, six
weeks after weaning of the surviving F2a pups. The F1 animals were
allowed to mate for a maximum of 21 days with the same partner, as
during the first mating, and to produce a second litter (F2b). The
body-weight gain of males of the F0 generation and males and females
of the F1 generation at 2000 ppm was reduced, but only the
body-weight gain of male F1 animals at 630 ppm was affected. Food
consumption was affected by treatment before the first mating and
during the first and second gestations and first lactation only in
F1 females at 2000 ppm. At this dose, the males and females of the
F0 and F1 generations had elevated levels of thyroid-stimulating
hormone; slightly reduced triiodothyronine levels were noted in F0
females at necropsy; and males and females of the F0 generation had
lower thyroxine values at weeks 1 and 8 but not at necropsy. These
findings are consistent with the histological findings and changes in
organ weights found at this dose. Thyroid and liver weights were
increased in male and female animals of the F0 and F1 generations
at the high dose, and histopathological examinations of these animals
showed treatment-related centrilobular hepatocyte hypertrophy in the
liver and follicular-cell hypertrophy and hyperplasia (except in F1
females) in the thyroid. The weights of male and female F1a, male
F2a, and male and female F2b pups were reduced at 2000 ppm; at
630 ppm, only the weights of male and female F2b pups were reduced.
Very high pup losses, mainly after day 4 of lactation, occurred in all
animals of the F2a generation, including the controls. No clear
explanation was found for the pup losses. Those pups that did not die
had normal postnatal development, except for reduced body-weight gain
in male pups at the high dose. Such losses were not seen in the F2b
groups. Functional tests of F1a, F2a, and F2b animals showed no
treatment-related findings. The NOAEL was 630 ppm for male and female
F0 animals, 200 ppm for the F1 generation, 630 ppm for the F1a
and F2a offspring, and 200 ppm for the F2b offspring. The NOAEL
was 2000 ppm for fertility and general reproductive performance
(Muller, 1993).
Since treatment-related histopathological effects were found in
the thyroid and liver in rats at the high dose in the preceding study,
organs from all F0 and F1 animals at 200 and 630 ppm were further
evaluated. The incidence and severity of hepatocyte hypertrophy were
increased in a dose-related fashion at all doses in F0 males, and
the incidence and to a lesser extent the severity of thyroid
follicular-cell hypertrophy and hyperplasia were increased in the same
animals. F1 males also had increased (but to a lesser extent)
hepatocyte hypertrophy and thyroid follicular cell hyperplasia at all
doses. The minimal effect level was thus 200 ppm, equivalent to
9.7-14.7 mg/kg bw per day (Muller & Singer, 1995).
(e) Developmental toxicity
Mice
Groups of 20 female ICR mice were given thiophanate-methyl
(purity, 94%) at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day by
gavage on days 1-15 of gestation. Animals were killed on day 18 or 19
(discrepancy between the study report and publication). No deaths,
abnormalities, or significant differences in body weights were
recorded. The number of living fetuses per litter was reduced in
animals at 1000 mg/kg bw per day, partly as a result of increased
resorptions. No treatment-related effects were observed on
implantation numbers, litter sizes, fetal body weights, sex ratio, or
numbers of immature or malformed fetuses. The NOAEL was 1000 mg/kg bw
per day for maternal toxicity and 500 mg/kg bw per day for embryo- and
fetotoxicity, on the basis of the reduced number of live fetuses at
1000 mg/kg bw per day. There was no evidence of teratogenicity
(Noguchi, 1970c; Makita et al., 1973).
Rats
Groups of 25 female Sprague-Dawley rats were given
thiophanate-methyl (purity, 97.2%) at doses of 0, 100, 300, or
1000 mg/kg bw per day by gavage on days 6-19 of gestation. Animals
were killed on day 20 of gestation. There were five to nine nongravid
females per group, including the control group. One rat at 100 mg/kg
bw per day and one at 300 mg/kg bw per day delivered on days 13 and
11, respectively, and were killed on the day of delivery. The weight
and development of the offspring were comparable to those of animals
delivered later, suggesting incorrect assessment of copulation; no
malformations or maternal abnormalities were seen in these animals. No
deaths, treatment-related clinical symptoms, or gross pathological
abnormalities were reported in any dams. Body-weight gain was reduced
between days 6 and 9 in animals at 1000 mg/kg bw per day. There were
no treatment-related effects on total implantations, post-implantation
loss, fetal body weight, fetal sex ratio, or viable fetuses. There
were no dose-related fetal malformations or developmental
abnormalities, and the incidences of these parameters were within
those of historical controls. A positive control study in the same
strain with a single high dose of vitamin A produced the appropriate
results. The NOAEL was 300 mg/kg bw per day for maternal toxicity, on
the basis of the reductions in body-weight gain of dams at 1000 mg/kg
bw per day during treatment. There was no evidence of teratogenicity
or embryo- or fetotoxicity (Rodwell et al., 1981).
Rabbits
Groups of 15 female New Zealand white rabbits received
thiophanate-methyl (purity, 96.2%) at doses of 0, 2, 6, or 20 mg/kg bw
per day by gavage on days 6-19 of gestation. Samples of test solutions
were taken during the first and last weeks of treatment, and the
concentrations of thiophanate-methyl were found to be acceptable. The
animals were killed on day 29 of gestation. No treatment-related
maternal mortality or clinical or subsequent maternal effects were
seen. Dose-related losses in maternal body weight were observed mainly
at the beginning of treatment (days 6-8 at 6 mg/kg bw per day and days
6-14 at 20 mg/kg bw per day). The food consumption of animals at
20 mg/kg bw per day was reduced from the start of treatment, and large
reductions during days 6-12 were generally associated with subsequent
abortion or sacrifice in extremis. Water consumption was unaffected.
One of 12 animals at 2 mg/kg bw per day, one of 14 at 6 mg/kg bw per
day, and two of 13 at 0 mg/kg bw per day aborted. One control animal
and one at 20 mg/kg bw per day suffered total litter loss and
resorption; the control animal suffered early resorption of its only
implantation, while the treated animal lost five of five implantations.
No treatment-related trends were observed in resorptions, pre-
implantation losses, or fetal or placental weights. The numbers of
viable young were slightly decreased in animals at 20 mg/kg bw per day
due to abortions and total litter losses. Gross examination of the
fetuses showed that in animals at 6 and 20 mg/kg bw per day, there was
an apparent treatment-related trend in skeletal abnormalities in ribs,
vertebrae, and pelvis that was generally close to or slightly greater
than the upper limit of historical controls. Treatment-related effects
included increased incidences of 13 pairs of ribs, incomplete or
asymmetric ossification of costal elements of the sacral vertebrae, 27
presacral vertebrae, and asymmetric pelvises associated with different
sacral vertebrae. At 20 mg/kg bw per day, the incidences of one or
more ribs thickened at the costal cartilage were significantly
increased. The frequencies of these skeletal abnormalities are
summarized in Table 6. The NOAEL was 2 mg/kg bw per day for maternal
toxicity, on the basis of the effect on maternal growth rate, and
2 mg/kg bw per day for developmental toxicity, on the basis of the
increased incidence of skeletal abnormalities at 6 and 20 mg/kg bw per
day (Ross et al., 1986).
Table 6. Incidence of selected skeletal abnormalities in fetuses of rabbits
treated with thiophanate-methyl
Observation Dose (mg/kg bw per day) Historical controls
0 2 6 20 Mean Range
13 pairs of ribs 41 46 63 61 36 12-61
Thickened ribs 1 4 2 14 2 0-13
Incomplete or assymetrical 3 6 7 12 4 0-15
ossification
27 presacral vertebrae 16 18 38 43 18 7-44
Assymetrical pelvis 3 5 7 10 4 0-9
(f) Genotoxicity
The results of studies of the genotoxicity and mutagenicity of
thiophanate-methyl in vitro and in vivo are summarized in Table 7.
There was no evidence of genotoxicity or mutagenicity. Thiophanate-
methyl is partially metabolized in mammalian systems to carbendazim,
which has been shown to act as an aneugen.
The aneugenic potential of thiophanate-methyl (purity, 95%;
dispersed in 70% kaolin) was tested in male Swiss albino mice given
oral doses of 1000 mg/kg bw. Bone-marrow cells were analysed 16, 24,
36, and 48 h after treatment for micronuclei, chromosomal aberrations,
hyperdiploidy, and polyploidy. Large micronuclei were significantly
induced, but the response was relatively weak, and thiophanate-methyl
was less effective than benomyl or carbendazim. There was no increase
in the frequency of chromosomal aberrations. At 24 and 36 h, a
possibly treatment-related increase in the frequency of polyploid and
hyperdiploid cells was observed, which was of borderline significance
in view of the very low frequency of changes in ploidy (Barale
et al., 1993).
Table 7. Results of tests for the genttoxicity of thiophanate-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 10, 50, 100, 97.3 Negativea Shirasu et al. (1976)
TA100, TA1535, TA1537, 500, 1000 µg/plate
TA1538, E. coli WP2 hcr dissolved in DMSO
Reverse mutation S. typhimurium TA98, 39.1, 78.1, 156.3, 96.55 Negativea Kanaguchi & Nishibe
TA100, TA1535, TA1537, 312.5, 625, 1250, (1990)
E. coil WP2 uvrA 2500, 5000 µg/plate
in DMSO
Host-mediated S. typhimurium G46 1000, 3000 mg/kg 97.3 Negative Shirasu et al. (1976)
mutation bw twice
Gene mutation B. subtills H17, M45 20, 100, 200, 500, 97.3 Negative Shirasu et al. (1976)
1000, 2000 µg,/disk
Gene mutation Chinese hamster V79 cells, 6.25, 12.5, 25.0, 50, 96.36 Negativea Tippins et al. (1984)
hprt locus 100 µg/ml in DMSOa
Chromosomal aberration Chinese hamster ovary cells 100, 200, 300, 400 95.0 Negative Murli (1988)
µg/ml in DMSOa
250, 500, 750,
1000 µg/ml in DMSOb
Unscheduled DNA Primary hepatocytes 5, 10, 25, 50, 100, 100 Negative Myhr (1981)
synthesis 250, 500, 1000 µg/ml
In vivo
Dominant lethal mutation ICR mice 8-500 mg/kg bw 94 Negative Makita et al. (1973)
intraperitoneally
Cytogenicity Wistar rat bone marrow 62.5-1000 mg/kg bw 94 Negative Makita et al. (1973)
and spermatocytes intraperitoneally
DMSO, dimethyl sulfoxide
a Without metabolic activation
b With metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Thiophanate-methyl (0.5 g; purity, 96.2%) moistened with water
was applied to the backs of six male New Zealand white rabbits for 4 h
under an occlusive patch. There were no signs of irritation over a
72-h observation period (Souma & Nishibe, 1986a).
Nine male New Zealand white rabbits received 0.1 g
thiophanate-methyl (purity, 96.2%) in the left eye. The treated eyes
of three rabbits were washed with water, and those of the others
remained unwashed. Conjunctival redness was observed in four of the
unirrigated eyes and in all three of the irrigated eyes after 1 h.
These symptoms had resolved by 24 h in all animals except one with an
unwashed eye, in which irritation resolved by 48 h. Thiophanate-methyl
was considered to be a very mild eye irritant (Souma & Nishibe,
1986b).
The skin sensitizing potential of thiophanate-methyl (purity,
96.2%) was tested in 20 female Hartley guinea-pigs by the
Magnusson-Kligman technique. Thiophanate-methyl was sensitizing under
the conditions of the study (Souma & Nishibe, 1989). In a Buehler
test, thiophanate-methyl (purity, 96.55%) was not sensitizing in 10
female Hartley guinea-pigs (Mochizuki, 1993).
(ii) Effects on the thyroid and liver
A number of studies were carried out to elucidate the effects of
thiophanate-methyl on the thyroid and liver (Nishibe & Takaori, 1990).
Thiophanate-methyl (purity, 96.55%) was administered in the diets
of six-week-old male Fischer 344 rats for two or eight days at a
concentration of 6000 ppm. Propylthiouracil, an inhibitor of thyroid
hormone synthesis, was administered at 1000 ppm for two or eight days,
and phenobarbital, an inducer of drug-metabolizing enzymes, was
administered at 500 ppm for eight days, as positive controls.
Thiophanate-methyl reduced triiodothyronine and thyroxine levels,
increased thyroid-stimulating hormone activity (at day 8 only),
increased thyroid weights (at day 8 only), and increased liver
weights. Propylthiouracil caused similar but more marked changes in
thyroid hormone levels and weights. Phenobarbital increased liver
weights. All three chemicals increased serum cholesterol levels by day
8. Thiophanate-methyl and phenobarbital increased microsomal
cytochrome P450, cytochrome b5, total protein, and UDP-glucu-
ronosyltransferase activities.
Seven-week-old female Fischer 344 rats were given 6000 ppm
thiophanate-methyl or 500 ppm phenobarbital for eight days. Some were
killed at eight days, and the others were killed after an eight-day
recovery. Thyroid weights were increased at day 8.
Groups of eight-week-old rats (sex not specified) were fed diets
containing 0 or 6000 ppm thiophanate-methyl, 30 µg/kg bw thyroxine, or
6000 ppm thiophanate-methyl and daily injections of 30 µg/kg thyroxine
subcutaneously, for eight days. Thiophanate-methyl caused hypertrophy
of the thyroid and liver and increased the level of thyroid-
stimulating hormone; these changes were suppressed by concomitant
administration of thyroxine. Thiophanate with and without thyroxine
also increased serum cholesterol. Thyroxine alone caused none of these
changes.
Thiophanate-methyl was less effective than propylthiouracil in
inhibiting porcine thyroid microsomal peroxidase. The ED50 and ED0
were 6 × 10-4 mol/litre and 8 × 10-5 mol/litre for thiophanate-
methyl and 2 × 10-5 mol/litre and 4 × 10-7 mol/litre for
propylthiouracil.
Eleven-week-old male ICR mice and Fischer 344 rats were given
diets containing 6000 ppm thiophanate-methyl or 500 ppm phenobarbital
for two or eight days. Liver weights were increased in all treated
animals. The titre of hepatocyte proliferating cell nuclear antigen,
the polymerase delta accessory protein, was increased in rats at day 2
and mice at days 2 and 8 (Nishibe & Takaori, 1990).
3. Observations in humans
In 1969-72, 16 production workers continuously exposed to
Topsin-E containing thiophanate or Topsin-M containing thiophanate-
methyl were routinely tested for effects on health. Blood and urine
samples were taken for analysis every six months: blood was analysed
for erythrocyte and leukocyte counts, haemoglobin content, and
specific gravity, and urine for protein, sugar, and urobilinogen.
Serum alanine and aspartate aminotransferases activities were
determined twice during the first six months of 1972 only. The initial
tests were conducted in January 1969, prior to production of Topsin.
No significant effects were seen in any of the workers for any of the
parameters investigated (Mori, 1972).
The same tests reported by Mori (1972) were conducted twice a
year on 28 production workers exposed to Topsin M and/or Topsin E in
1973-77, except that leukocyte and erythrocyte counts and haemoglobin
levels were not tested during 1976 for undescribed 'personal reasons'.
Serum alanine and aspartate aminotransferase activities were
determined in all employees in October 1977. More than 13 employees
were tested each year, but the specific individuals tested changed
during the study so that only seven individuals were monitored
continuously. No adverse behavioural, haematological, clinical
chemical, or urinary findings were made (Ikeda, 1978).
Between 1985 and 1991, no adverse behavioural symptoms (including
skin or eye effects) or clinical findings (haematology, clinical
chemistry, or urinalysis) were observed in workers exposed to
thiophanate-methyl and thiophanate during production. Health
examinations were performed twice a year. The workers were involved
mainly in the production of thiophanate-methyl, usually for 11 months
of the year (Aizawa, 1991).
Comments
Thiophanate-methyl was rapidly absorbed after oral exposure of
mice, rats, and dogs. Most of the administered radiolabel was
recovered within 24 h of dosing, predominantly in the urine.
Carbendazim and 5-hydroxycarbendazim were among the metabolites of
thiophanate-methyl identified in vivo and in vitro.
Thiophanate-methyl has low acute toxicity in rats, with an oral
LD50 of about 7000 mg/kg bw. Clinical signs of toxicity after acute
dosing with thiophanate-methyl were generally non-specific. WHO has
classified thiophanate-methyl as unlikely to present an acute hazard
in normal use.
In a six-month study of toxicity, mice were given dietary doses
of 0, 13, 64, 320, 1600, or 8000 ppm thiophanate-methyl. The NOAEL was
320 ppm, equivalent to 48 mg/kg bw per day, on the basis of
haematological effects and hepatotoxicity. In a six-month study, rats
were exposed to thiophanate-methyl in the diet at levels of 0, 13, 64,
320, 1600, or 8000 ppm. Reduced erythrocyte counts, reduced
body-weight gain, and evidence of hepatotoxicity and thyroid
stimulation were seen at 8000 ppm. The NOAEL was 1600 ppm, equivalent
to 80 mg/kg bw per day. Dogs were exposed to thiophanate-methyl by
administration in capsules at doses of 0, 50, 200, or 400 mg/kg bw per
day for 13 weeks, 0, 8, 40 or 200 mg/kg bw per day for one year, or 0,
2, 10, 50, or 250 mg/kg bw per day for two years. Treatment-related
thyroid hyperplasia was seen in all three studies; the overall NOAEL
was 10 mg/kg bw per day.
In a two-year study of carcinogenicity in mice fed dietary levels
of 0, 10, 40, 160, or 640 ppm, there was no evidence of any
carcinogenic response; the NOAEL was 160 ppm (equal to 24 mg/kg bw per
day) on the basis of reduced body-weight gain. In a subsequent
18-month study in mice fed dietary levels of 0, 150, 640, 3000, or
7000 ppm, the NOAEL was 150 ppm (equal to 29 mg/kg bw per day) on the
basis of treatment-related hepatotoxicity and hepatocellular tumours.
In a two-year study of carcinogenicity in rats fed dietary levels of
0, 10, 40,160, or 640 ppm, effects were seen on the testes (mainly
hypospermatogenesis), thyroid gland, and growth rate at 640 ppm; the
NOAEL was 160 ppm (equivalent to 8 mg/kg bw per day). In a two-year
study in rats fed dietary levels of 0, 75, 200, 1200, or 6000 ppm, the
NOAEL was 200 ppm (equal to 9 mg/kg bw per day). Thyroid hyperplasia
and thyroid tumours were seen at higher doses, together with
parathyroid hyperplasia, nephrotoxicity, hepatotoxicity, and lipidosis
of the adrenal cortex.
In a three-generation study of reproductive toxicity in which
rats received dietary doses of 0, 40, 160, or 640 ppm, reductions in
total litter size and weight at birth and at weaning were seen at
640 ppm; the NOAEL was 160 ppm, equivalent to 8 mg/kg bw per day. In a
two-generation study in which rats received dietary doses of 0, 200,
630 or 2000 ppm, there was no evidence of a treatment-related effect
on reproduction. The lowest dose tested in the study, equivalent to
10 mg/kg bw per day, was a minimal-effect level on the basis of
hepatocyte hypertrophy, thyroid follicular-cell hypertrophy, and
hyperplasia at all treatment levels.
The teratogenic potential of thiophanate-methyl was investigated
in mice, rats, and rabbits. In mice exposed by gavage to thiophanate-
methyl at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day on days
1-15 of gestation, there was no evidence of teratogenicity or maternal
toxicity at the highest dose, although embryo- and fetotoxicity were
seen at this dose. In rats exposed by gavage to thiophanate-methyl at
doses of 0, 100, 300, or 1000 mg/kg bw per day on days 6-19 of
gestation, there was no evidence of fetotoxicity or teratogenicity,
but maternal toxicity, expressed as reduced body-weight gain, was seen
at the highest dose. In rabbits exposed by gavage to thiophanate-
methyl at doses of 0, 2, 6, or 20 mg/kg bw per day on days 6-19 of
gestation, maternal toxicity (reduced growth rate) was seen at 6 mg/kg
bw per day and above. A dose-related trend of increased fetal skeletal
abnormalities (ribs, vertebrae, and pelvis) at 6 and 20 mg/kg bw per
day indicated an NOAEL for developmental toxicity of 2 mg/kg bw per
day.
Thiophanate-methyl was adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The only significant
response was a small increase in the frequency of micronuclei
characterized by their large size in mouse bone-marrow cells, which
was not associated with the induction of structural chromosomal
aberrations. This is indicative of a weak aneugenic effect. The
Meeting concluded that thiophanate-methyl is not genotoxic.
Studies on the effects of thiophanate-methyl on the liver and
thyroid of rats showed liver enzyme induction, reductions in thyroid
hormones (triiodothyronine and thyroxine), and increases in the level
of thyroid-stimulating hormone and in thyroid and liver weights.
Thyroid hypertrophy and increases in thyroid-stimulating hormone
levels associated with treatment with thiophanate-methyl were
suppressed by concomitant treatment with thyroxine. Thiophanate-methyl
was shown to inhibit porcine thyroid microsomal peroxidase, an enzyme
involved in thyroid hormone synthesis. The thyroidal and hepatic
changes, including tumours, observed in the studies of toxicity may be
due to increased hepatocyte turnover, reductions in the thyroid
hormones triiodothyronine and thyroxine as a result of liver enzyme
induction, and inhibition of thyroid microsomal peroxidase.
Workers involved in manufacturing products containing
thiophanate-methyl showed no treatment-related adverse local (skin or
eyes) or systemic effects.
An ADI of 0-0.02 mg/kg bw was established on the basis of the
NOAEL of 2 mg/kg bw per day for developmental toxicity in rabbits and
a safety factor of 100.
The Meeting noted that the use of thiophanate-methyl on crops
gives rise to residues of carbendazim, although thiophanate-methyl can
also be detected as part of the residue to which consumers of treated
produce are exposed. Since the toxicity of thiophanate-methyl is
qualitatively and quantitatively (when corrected for relative
molecular mass) different from that of carbendazim, the Meeting
concluded that the intake of residues in food should initially be
compared with the ADI for thiophanate-methyl. If further refinement of
the risk assessment is necessary, the different components of the
residue (carbendazim and thiophanate-methyl) may be characterized.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 29 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
1000 mg/kg bw per day (maternal toxicity and teratogenicity
in study of reproductive toxicity)
500 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
160 ppm, equivalent to 8 mg/kg bw per day (study of
reproductive toxicity)
1000 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
300 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 2 mg/kg bw per day (maternal toxicity and teratogenicity in
study of developmental toxicity)
Dog: 10 mg/kg bw per day (studies of toxicity up to two years)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Comparison of the metabolism of thiophanate-methyl and
carbendazim in different species, including humans
2. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to thiophanate-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 7000 mg/kg bw
Dermal, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test; not sensitizing
in Buehler test
Inhalation, toxicity, rat LC50 = 1.8 mg/litre air
Mid-term (1-26 weeks) Oral, reproductive toxicity, rabbit NOAEL = 2 mg/kg bw per day; maternal and
developmental toxicity
Long-term (> one year) Dietary, toxicity and carcinogenicity, NOAEL = 9 mg/kg bw per day; thyroid rumours
two years, rat and hepatotoxicity
Oral, toxicity, two years, dog NOAEL = 10 mg/kg bw per day; hepatotoxicity and
thyroid effects
References
Aizawa, T. (1991) Human handling experiences from plant employees
manufacturing Topsins (3). Unpublished report No. RD-9153 from
Nippon Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Auletta, CS. (1991) A subchronic (3-month) oral toxicity study in the
dog via capsule administration with thiophanate-methyl.
Unpublished report Project No. 89-3525 (RE-9119) from
Bio/dynamics Inc. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Auletta, C.S. (1992) A chronic (1-year) oral toxicity study in the dog
via capsule administration with thiophanate-methyl. Unpublished
report Project No. 89-3526 (RD-9207) from Bio/dynamics Inc.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Barale, R., Scapoli, C., Meli, C., Casini, D., Minunni, M.,
Marrazzini, A., Loprieno, N. & Barrai, I. (1993) Cytogenetic
effects of benzimidazoles in mouse bone marrow. Mutat. Res.,
300, 15-28.
Douch, P.G.C. (1974) The metabolism of some thioureidobenzene
fungicides in mice and sheep. Xenobiotica, 4, 457-475.
Fujino, A., Ohnuma, N., Mori, T., Tanoue, T. & Kamimura, H. (1973) The
balance and metabolism studies of thiophanate-methyl in animals.
Unpublished report from the Nisso Institute for Life Science,
Nippon Soda Co. Ltd, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Hashimoto, Y. (1972) Final report on chronic oral toxicity studies on
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis 3-thioal-
lophanate in Sprague-Dawley strain rats. Unpublished report from
the Nisso Institute for Life Science, Nippon Soda Co. Ltd,
Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Hashimoto, Y., Makita, T., Nishibe, T., Mori, T., Ohnuma, N., Noguchi,
T. & Ohta, G. (1973) Subacute toxicity of thiophanate-methyl in
mice and rats. Pharmacometrics, 7, 929-945.
Ikeda, K. (1978) Human handling experiences from plant employees
manufacturing Topsin (2). Unpublished report from Nippon Soda Co.
Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Kanaguchi, Y. & Nishibe, T. (1990) Thiophanate-methyl reverse mutation
study on bacteria. Unpublished report No. RD-9065 from Nippon
Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Kosaka, S. (1973) The report on the carcinogenesis studies of
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis-(3-thioal-
lophanate) in mice of ICR-SLC strain for 24 months. Unpublished
report from the Nisso Institute for Life Science, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co., Ltd,
Tokyo, Japan.
Makita, T., Hashimoto, Y. & Noguchi, T. (1973) Mutagenic, cytogenetic
and teratogenic studies on thiophanate-methyl. Toxicol. Appl.
Pharmacol., 24, 206-215.
Mochizuki, N. (1993) Topsin M skin sensitization study in guinea pigs.
Unpublished report No. RD-9347 from Nippon Soda Co. Ltd.
Submitted to WHO by Nippon Soda Co., Ltd, Tokyo, Japan.
Mori, H. (1972) Human handling experiences from plant employees
manufacturing Topsin (1). Unpublished report from the Fine
Chemical Division, Nippon Soda Co. Ltd. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Muller, W. (1993) Topsin M two generation oral (dietary
administration) reproduction toxicity study in the rat.
Unpublished report HLA Study No. 683-004 (RD-9329) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Muller, W. & Singer, A. (1995) Topsin M final addendum histopathology
report and peer review pathology report to MRID 42899101. Two
generation oral (dietary administration) reproduction toxicity
study in the rat. Unpublished report HLA Study No. 683-004
addendum histopathology report (RD-9525) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Murli, H. (1988) Mutagenicity test on Topsin M technical (thiophanate-
methyl) in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished report HLA Study No 10345-0-437 (RD-9120) from
Hazleton Laboratories America, Inc. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Myhr B.C. (1981) Primary rat hepatocyte unscheduled DNA synthesis
assay of thiophanate-methyl. Unpublished report No. LBI Project
No 21191 (RD-8195) from Litton Bionetics. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation toxicity study
in rats. Unpublished report No. RD-8711 from the Toxicology
Institute, Environmental Toxicology Laboratory, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Nishibe, T. & Takaori, H. (1990) Summary of mechanistic investigation
of the effect of thiophanate-methyl on thyroid and liver.
Unpublished report from the Toxicology Institute, Environmental
Toxicology Laboratory, Nippon Soda Co. Ltd, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970a) Toxicological evaluation of thiophanate-methyl
(V). Some pharmacological properties of a new fungicide,
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970b) Studies on the biotransformation of thiophanate-
methyl in animal and plant (Part 1). Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970c) Toxicological evaluation of thiophanate-methyl
(IV). Studies on the teratogenic effect of thiophanate-methyl
upon the fetus of ICR strain of mice. Unpublished report from the
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1971) Studies on the biotransformation of
thiophanate-methyl in animal, plant and soil (Part II).
Unpublished report from the Chemical Research Laboratories, Nisso
Institute for Life Sciences, Kanagawa-ken, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1972) Studies on the biotransformation of
thiophanate-methyl in animal and plant. Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970a) Toxicological evaluation of
thiophanate-methyl (I). Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa-ken, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate-methyl (II). Studies on the subchronic oral toxicity
of thiophanate-methyl in mice. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity
of thiophanate-methyl in rats. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Palmer, A., Lovell., M. & Newman, A. (1972) Effect of
thiophanate-methyl on reproductive function of multiple
generations in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Rodwell, D.E. et al (1981) Teratology study in thiophanate-methyl in
rats. Unpublished report No. 449-006 (RD-8126) from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Ross, F.W. et al. (1986) Thiophanate-methyl: Teratology study in the
rabbit. Unpublished report No. 86/NIS010/11 (RD-8642) from Life
Science Research. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Saike, O. & Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation
toxicity study in rats. Unpublished report No. 0219 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Shirasu, Y. et al. (1976) Mutagenicity testing on thiophanate-methyl
in microbial systems. Unpublished report No. RD-8084 from the
Institute of Environmental Toxicology. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Singh, T. & Garg, B. (1989) Effect of thiophanate-methyl on
cardiovascular system. Indian Vet. J., 66, 1116-1119.
Souma, S. & Nishibe, T. (1986a) Thiophanate-methyl primary dermal
irritation study in rabbits. Unpublished report No. RD-8692 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1986b) Thiophanate-methyl primary eye
irritation study in rabbits. Unpublished report No. RD-8691 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1989) Thiophanate-methyl: Delayed contact
hypersensitivity study in guinea pigs. Unpublished report No.
RD-8924 from Nisso Institute for Life Sciences, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990a) Thiophanate-methyl acute oral toxicity
study in rats. Unpublished report No. RD-9083 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990b) Thiophanate-methyl acute dermal
toxicity study in rabbits. Unpublished report No. RD-9084 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Takaori, H. (1993) Thiophanate-methyl combined chronic
toxicity/oncogenicity study in rats. Unpublished report
No. RD-9327 from Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Taniguchi, T. (1972) Final report on the long-term oral toxicity
studies of thiophanate-methyl, dimethyl 4,4'-O-phenylene-
bis(3-thioallophanate) in beagle-dogs for 24 months. Unpublished
report from the Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Thomas, J. (1974) Actions of pesticides and other drugs on the male
reproductive system (EPA-650/1-74-011), Washington DC, US
Environmental Protection Agency. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Thomas, J. & Schein, L. (1974) Effects of thiophanate and thiophanate-
methyl on the male reproductive system of the mouse. Toxicol.
Appl. Pharmacol., 30, 129-133.
Tippins, R.S. et al. (1984) Gene mutation in Chinese hamster V79
cells with thiophanate-methyl. Unpublished LSR-RTC Report
No. 063013-M-05184 (RD-84109) from Life Science Research.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Tompkins, E.C. (1992) Topsin-M (thiophanate-methyl) 18-month dietary
oncogenicity study in mice. Unpublished report No. WIL-75024
(RD-9328) from WIL Research Laboratory, Inc. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Table 3. Pharmacological effects of thiophanate-methyl
Parameter Species Dose and route Results
Body temperature Wistar rats 380-1500 mg/kg bw orally No effect
Analgesic activity Mice 500 mg/kg bw intraperitoneally No effect
Sedative or hypnotic activity Rats and mice 100-500 mg/kg bw subcutaneously No effect
Cardiovascular and respiratory Rabbits 20-100 mg/kg bw intravenously Reduced blood pressure
effects followed by bradycardia
Table 4. Acute toxicity of thiphanate-methyl
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male, female Orala 3400-3514 Noguchi & Hashimoto (1970a)
Mouse Male, female Intraperitoneal 792-1113 Noguchi & Hashimoto (1970a)
Mouse Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Mouse Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Oral 6640-7500 Noguchi & Hashimoto (1970a)
Rat Male, female Intraperitoneal 140-1640 Noguchi & Hashimoto (1970a)
Rat Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Inhalation 1.7-1.9 Nishibe (1987)
Rat Male, female Oralb > 5000 Souma & Nishibe (1990a)
Guinea-pig Male, female Oral 3640-6700 Noguchi & Hashimoto (1970a)
Guinea-pig Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Oral 2270-2500 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermalb > 2000 Souma & Nishibe (1990b)
a In gum arabic in 5% sodium chloride solution
b In distilled water
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 12 ICR mice of each sex were fed diets containing
thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or 8000 ppm for
six months. The homogeneity, stability, and concentrations of the diet
were not reported; clinical signs of toxicity were also not reported.
There were no treatment-related deaths. The body-weight gain of
animals of each sex at 8000 ppm was significantly reduced. Erythrocyte
counts were also reduced in these animals and in males at 1600 ppm,
and haematocrit values were reduced in animals of each sex at 1600 and
8000 ppm. No significant differences were seen in the weights of the
cerebrum, heart, lung, spleen, kidney, or testis, but in animals at
8000 ppm, liver weights were increased. Histopathological
investigation revealed a higher incidence of hepatic-cell irregularity
at the higher doses, with five cases in males and three in females at
8000 ppm and one case in an animal of each sex at 1600 ppm. Large
swollen hepatic cells, some with oedematous or granular protoplasm,
were found to a greater extent at 8000 ppm, especially in the central
lobules. Constriction of veins by the swollen cells was proposed to
explain the cloudy appearance of the liver. Sporadic formation of
acidiphilic bodies from hepatic-cell degeneration was recorded in two
females and one male at 8000 ppm and one male at 1600 ppm. The NOAEL
was 320 ppm, equivalent to approximately 48 mg/kg bw per day, on the
basis of hepatotoxic and haematological effects at 1600 and 8000 ppm
(Noguchi & Hashimoto, 1970b; Hashimoto et al., 1973).
Rats
Groups of 12 Sprague Dawley rats of each sex were fed diets
containing thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or
8000 ppm for six months. The homogeneity, stability, and
concentrations of the diet were not reported, and no clinical signs of
toxicity were reported. There were no treatment-related deaths. The
body-weight gain of animals of each sex at 8000 ppm was significantly
reduced, but food consumption did not differ significantly between
groups. Reduced erythrocyte counts were seen in animals of each sex at
8000 ppm. Urinalysis did not show significant treatment-related
differences. Blood cholesterol levels were raised significantly in
animals of each sex at 8000 ppm. The thyroid anti liver weights were
increased in these animals, and the weight of the thymus was increased
in females. Some histopathological effects on the thyroid were
reported in animals at this dose: four animals of each sex had small
thyroid follicles, four males and four females had thickened
follicular epithelium, and five animals of each sex had decreased
colloidal substance. There were no treatment-related effects on
protein-bound iodine levels. The NOAEL was 1600 ppm, equivalent to
approximately 80 mg/kg bw per day, mainly on the basis of effects on
thyroid, liver, and body weights (Noguchi & Hashimoto, 1970c;
Hashimoto et al., 1973).
Dogs
Groups of five beagle dogs of each sex (four at the highest dose)
were fed capsules containing thiophanate-methyl (purity unspecified)
at doses of 0, 2, 10, 50, or 250 mg/kg bw per day for two years. One
animal of each sex at each dose was killed and autopsied after 12
months. There were no deaths or adverse symptoms. Animals of each sex
at 250 mg/kg bw per day had reduced body weights and body-weight gain.
Gross pathological effects were not reported, and no effects on the
eyes or heart were found in periodic ophthalmoscopic and electro-
cardiographic investigations. There were no treatment-related changes
in clinical chemical, haematological, or urinary values. After 24
months, the weight of the thyroid gland was increased in animals of
each sex at 250 mg/kg bw per day and to a lesser extent at 50 mg/kg bw
per day. The effects on thyroid tissue at 12 months included altered
or decreased colloidal substance and slightly taller cuboid follicular
epithelial cells at 250 mg/kg bw per day. The NOAEL was 10 mg/kg bw
per day on the basis of effects on thyroid weight in animals of each
sex at 50 and 250 mg/kg bw per day (Taniguchi, 1972).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) for three months at
doses of 0, 50, 200, or 800 mg/kg bw per day, the last dose being
lowered to 400 mg/kg bw per day on test day 50 because of severe
toxicity. One male at the high dose was sacrificed on day 41 because
of severe toxicity; one male at 50 mg/kg bw per day died on day 36,
but this death did not appear to be related to treatment. Dose-related
clinical signs seen in animals at the high dose and to a lesser extent
at the middle dose included dehydration, thinness, and lethargy.
Dose-related decreases in body weights and marked decreases in food
consumption were seen at the middle and high doses. There were no
treatment-related ophthalmological findings. Slight anaemia, increased
platelet counts and cholesterol levels, and decreases in serum alanine
aminotransferase activity and albumin levels were seen at the middle
and high doses and increased activated partial thromboplastin time at
the high dose. Thyroid function tests revealed slightly decreased
triiodothyronine levels in males at the high dose and decreased
triiodothyronine and thyroxine levels in females at the middle and
high doses; no clear effects on thyroid-stimulating hormone values
were apparent. Urinalysis showed no treatment-related findings. At the
middle and high doses, the weights of the liver (in animals of each
sex) and thyroid (males only) were increased. Gross examination
post mortem revealed emaciation in one male at the middle dose and
three at the high dose. Dose-related histological alterations were
seen in a number of organs. In thyroids, hypertrophy of the follicular
epithelium was found in one, three, and four males and one, two, and
four females at the low, middle, and high doses, respectively, with
none in controls. The severity of the hypertrophy increased from
minimal to marked with dose. Hyperplasia of the follicular epithelium
was found in one male at the middle dose and in most animals at the
high dose. In two males at the high dose in which both hypertrophy and
hyperplasia were marked, a large decrease in the quantity of
intrafollicular colloid was seen. In animals at the middle and high
doses, dose-related changes were found in the liver (reduction in
vesiculation of the hepatocellular cytoplasm), gall-bladder
(intracytoplasmic vacuoles), pancreas (atrophy of acinar cells due to
a decreased quantity of zymogen granules), spleen (lymphoid-cell
depletion), thymus (involution), prostate (atrophy), uterus
(anoestrus), and ovaries (inactive). There was no NOAEL because of the
presence of follicular-cell hypertrophy in the thyroid of two dogs at
the low dose (Auletta, 1991).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) at doses of 0, 8, 40,
or 200 mg/kg bw per day for one year. There were no deaths. Tremors
were seen in all dogs at the high dose 2-4 h after treatment on one or
more occasions during the initial three weeks of the study but not
subsequently. The tremors were slight to moderate, except in one
animal which had severe tremors that progressed to apparent tonic
convulsions on three occasions. The body-weight gains of animals at
the high dose were reduced during the study, and body-weight losses
were noted in the first week of treatment; slight reductions in
body-weight gain were noted at 40 mg/kg bw per day. The food
consumption of dogs at the high dose was generally lower than that of
controls during the early months, but was close to the control value
thereafter. Ophthalmological examinations and urinalyses showed no
treatment-related effects. Haematological effects consisted of slight
decreases in total erythrocyte counts and haemoglobin and haematocrit
values in males at the high dose. These animals also had decreased
serum alanine aminotransferase activity, increased alkaline
phosphatase activity, increased cholesterol levels (also at the middle
dose), and decreased albumin:globulin ratios (also at the middle
dose), calcium (also at the middle dose), potassium, and phosphorus
levels. In females at 200 mg/kg bw per day, serum alanine
aminotransferase activity was decreased, and alkaline phosphatase
activity and cholesterol levels were increased. Thyroid function tests
revealed decreased thyroxine levels in males at the middle and high
doses but no clear effects on triiodothyronine or thyroid-stimulating
hormone. Abnormalities seen post mortem included increased liver
weights in dogs at the high dose and increased thyroid weights in
those at the middle and high doses. Microscopic alterations attributed
to treatment were limited to minimal to moderate hypertrophy and
slight hyperplasia of the follicular epithelium of the thyroid in the
dogs at the middle dose (hypertrophy in two females) and the high dose
(hypertrophy in four males and three females, and hyperplasia in one
male and one female). The NOAEL was 8 mg/kg bw per day (Auletta,
1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing 0,
10, 40, 160, or 640 ppm thiophanate-methyl (purity, > 94%) for two
years. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. The body-weight
gain of males at 640 ppm was reduced during the study. No
treatment-related effects on food consumption or on mortality or overt
signs of toxicity were seen. By 24 months, 74-94% of treated animals
had died, but the mean survival times were comparable. Organ weights
and clinical chemical and haematological parameters were not
investigated. No treatment-related gross macroscopic or histopatho-
logical alterations were seen, and there was no evidence of
carcinogenicity. The NOAEL was 160 ppm, equivalent to about 24 mg/kg
bw per day, on the basis of the reduction in body-weight gain in males
at 640 ppm (Kosaka, 1973).
Groups of 60 male and 60 female CD-1 mice were fed diets
containing 0, 150, 640, 3000, or 7000 ppm thiophanate-methyl (purity,
95.9-96.6%) for 18 months. Ten animals of each sex in each group were
necropsied after 39 weeks of treatment, and surviving animals were
necropsied after 78-79 weeks of treatment; all mice that died or were
killed in extremis were also autopsied. The homogeneity, stability,
and concentrations of thiophanate-methyl were acceptable. Haemato-
logical and thyroid function were assessed at the interim necropsy and
before the end of the study. No treatment-related clinical signs were
observed. The cumulative numbers of deaths up to 18 months of -
treatment were 10, 11, 14, 16, and 24 males and 12, 13, 15, 17, and 23
females at 0, 150, 640, 3000, and 7000 ppm, respectively; animals at
7000 ppm had a significantly higher mortality rate than controls. The
increased rates in females at 3000 ppm and males and females at
7000 ppm appeared to be related to amyloid deposition in tissues.
Body-weight gains were slightly reduced in animals of each sex at
3000 ppm and to a greater degree at 7000 ppm; food consumption was
slightly reduced in females at 3000 and 7000 ppm. There was no
treatment-related effect on ophthalmologic parameters or on the onset
or number of palpable masses. The only haematological parameter that
appeared to be altered by treatment was a slightly lower erythrocyte
count in males at 7000 ppm. At the nine-month interim necropsy,
enlarged thyroid glands were seen grossly in three of 10 males at
7000 ppm, the thyroid weights of males and females at 3000 and
7000 ppm were increased, and the thyroid-stimulating hormone levels
were higher and the thyroxine levels lower in males at 3000 ppm and
males and females at 7000 ppm than in controls. The differences in
thyroid weights and thyroid hormone levels were not seen at 18 months.
At the nine-month interim necropsy, the liver weights of males and
females at 3000 and 7000 ppm were significantly increased as a result
of an increased incidence and severity of hepatocellular centrilobular
hypertrophy at these two doses; the incidence of hepatocellular
centrilobular hypertrophy was also increased in females at 640 ppm,
five out of 10 having minimal hypertrophy. At 18 months, the mean
liver weights of animals at 7000 ppm were increased as a result of a
significantly increased number of liver masses, which was also
significantly increased at 3000 ppm. At the 18-month terminal
necropsy, an increased incidence of hepatocellular centrilobular
hypertrophy was seen in males at 3000 and 7000 ppm. Histopathological
examination revealed that the liver masses seen grossly were adenomas.
The incidence of hepatocellular adenomas was significantly increased
in males and females at 3000 and 7000 ppm. The slightly increased
incidence of hepatocellular adenomas in females at 640 ppm (8.6%) was
greater than the historical control range (0-2.7%). Two hepatocellular
carcinomas were seen, one in a male at 640 ppm and the other in a male
at 7000 ppm; this 2% incidence was similar to the mean incidence for
historical control males (1.4%; range, 0-6%). One male at 7000 ppm had
a hepatoblastoma, which is a relatively rare tumour (0.001% in
historical controls). The incidence of atrial thrombosis was increased
in females at 3000 ppm and in animals of each sex at 7000 ppm. The
NOAEL was 150 ppm, equivalent to 29 mg/kg bw per day, in view of the
possible treatment-related incidence of hepatocellular adenomas in
females at 640 ppm (Tompkins, 1992).
Rats
Groups of 35 Sprague-Dawley rats of each sex (50 of each sex for
controls) were fed diets containing thiophanate-methyl (purity, >
94%) at doses of 0, 10, 40, 160, or 640 ppm for two years. Five rats
of each sex at each dose were killed and autopsied at three and 12
months. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. There were no
treatment-related deaths, although survival was poor in some groups,
and there were no overt signs of toxicity. Body-weight gain was
reduced in animals of each sex at 640 ppm, but no significant
difference in food consumption was observed. No significant treatment-
related effects were seen on clinical chemical, haematological, or
urinary values. No significant adverse histopathological effects or
changes in organ weights were seen at any dose at three or 12 months.
Necropsy of animals killed after 24 months or which died during
treatment showed treatment-related histopathological findings in the
testes and thyroid gland. Males at 640 ppm had an increased incidence
of decreased colloidal substance and hypertrophy of follicular
epithelium in thyroid tissue (6/35 compared to 1/50 in controls). Five
animals at 640 ppm were found to be hypospermatogenic; one died at
week 91 with a pituitary tumour and was found to have atrophied
testes. Another male had diminished spermatogenesis at 24 months, and
another surviving male and one that died during treatment had
hyperplasia of the testicular interstitium. No other treatment-related
effects were seen. There was no evidence for carcinogenicity. The
NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per day, on the
basis of effects on the testes and thyroid gland and reduced growth at
640 ppm (Hashimoto, 1972).
Groups of 60 male and 60 female Fischer 344 rats were fed diets
containing 0, 75, 200, 1200, or 6000 ppm thiophanate-methyl (purity,
96.55%) for 24 months. The homogeneity, stability, and concentrations
of thiophanate-methyl in the diets were acceptable. After 12 months,
10 rats of each sex in each group (only five males at 6000 ppm) were
sacrificed. All survivors were sacrificed at the end of treatment. The
mortality rates are shown in Table 5. Only two males at 6000 ppm
survived to the end of the study; because of this high rate, no
statistical analysis was performed for this group at 24 months. The
main causes of death among these rats were nephropathy (22 rats),
thyroid follicular-cell tumour (10 rats), and leukaemia (six rats);
eight males at this dose died or were killed in extremis during
weeks 11-12 due to fractures of the nasal bone caused by the feeder
plate and subsequent dyspnoea (rhinorrhagia). The feeder plates were
removed.
No clinical signs attributable to treatment were noted in any
group during the first 52 weeks. After week 52, dose-related clinical
signs included pale skin and mucous membranes in males at 6000 ppm,
alopecia in females at 6000 ppm, and tissue masses on the skin and in
the subcutis including the lip in males at 1200 and 6000 ppm. These
changes attained statistical significance at week 77 or later.
Dose-related, statistically significant depressions in body-weight
gain were noted in both males and females at 1200 and 6000 ppm.
Decreased food consumption was seen in animals of each sex at 6000 ppm
starting at week 76. No dose-related abnormalities were observed in
ophthalmoscopic parameters at 6, 12, 18, or 24 months. Treatment-
related signs of anaemia (decreased erythrocyte count, haemoglobin,
haematocrit, mean corpuscular volume, mean corpuscular haemoglobin,
and mean corpuscular haemoglobin concentration) were seen in animals
of each sex (mainly males) at 6000 ppm at 3, 6, 12, and 18 months.
Increased platelet and leukocyte counts were seen frequently in males
at 6000 ppm. Clinical chemical investigations revealed increased total
cholesterol and total protein and decreased albumin:globulin ratios in
animals of each sex at 1200 and/or 6000 ppm. At 24 months, treated
males had increased blood urea nitrogen (at 75, 200, and 1200 ppm) and
creatinine levels (at 1200 ppm), reduced thyroxine and triiod-
othyronine values, and increased thyroid-stimulating hormone values
(at 1200 and 6000 ppm, also in females at 6000 ppm). Dose-related
increases in urinary protein (semi-quantitative analysis) were noted
in animals of each sex at 6000 ppm at six and/or 12 months.
Table 5. Mortality rates in Fischer rats treated with thiophanate-methyl
Dose Male Female
(ppm)
Week 52 Week 80 Week 104 Week 52 Week 80 Week 104
No. % No. % No. % No. % No. % No. %
0 0/60 0 2/50 4 13/50 26 0/60 0 3/50 6 13/50 26
75 0/60 0 2/50 4 15/50 30 0/60 0 1/50 2 12/50 24
200 0/60 0 8/50 16 24/50 48* 0/60 0 1/50 2 8/50 16
1200 0/60 0 3/50 6 21/50 42 0/60 0 0/50 0 12/50 24
6000 8/60 13* 18/55 33** 53/55 96** 1/60 2 3/50 6 11/50 22
Significantly different from control at *P < 0.05; ** P < 0.001 (chi-square test)
Nephelometry indicated an increase in urinary protein in males at 1200
and 6000 ppm at various times. Although males at 200 ppm had a
statistically significant increase in urinary protein at 24 months,
histopathological examination revealed no treatment-related effect. In
males at 6000 ppm, ketone bodies, urinary volume, and water
consumption were increased, and pH and specific gravity were decreased
significantly at various times. Dose-related increases in the weights
of the liver, kidney, and thyroid and their body-weight ratios were
seen in animals of each sex at 1200 and/or 6000 ppm at both interim
and final sacrifice.
At interim sacrifice (week 53), treatment-related changes were
found macroscopically in the liver (brownish-black) and kidneys
(granular surface, brownish-black) of males and females at 6000 ppm.
At final sacrifice, males at 1200 ppm had granular kidneys. Females at
6000 ppm also had enlarged thyroids. In animals at 6000 ppm that died
during the study, swollen thyroids were seen in animals of each sex
and white areas in the liver and granular kidney in males. Dose-
related histopathological changes were found in the thyroid, liver,
kidney, and adrenal: Thyroid follicular-cell hyperplasia and
hypertrophy were noted in animals of each sex at 1200 and/or 6000 ppm
at 12 and 24 months, and the incidence of focal follicular-cell
hyperplasia was increased. Males at 6000 ppm also had a statistically
significant increased incidence of thyroid follicular-cell adenomas
(12/60), and one of two males at 6000 ppm that were killed at 24
months and 2/53 that were dead or were killed in extremis had
thyroid follicular-cell adenocarcinomas, with none in the other groups
of treated males. The incidence of retinal atrophy was increased in
females at 6000 ppm at 24 months, and those of parathyroid hypertrophy
and hyperplasia were increased in males at this dose. Centrilobular
hepatocellular hypertrophy and lipofuscin deposition were noted in
animals of each sex at 1200 and/or 6000 ppm at 12 and 24 months and in
most males and females at 6000 ppm which died during the study.
Microgranuloma and focal fatty degeneration in the liver were
increased in incidence in males treated at 6000 ppm. Lipidosis of the
adrenal cortex was noted in females at 1200 ppm and in animals of each
sex at 6000 ppm at 12 months. The severity of nephropathy was
increased in animals of each sex at 6000 ppm at 12 months and in those
at 1200 and 6000 ppm at 24 months. Most males at 6000 ppm that died or
were killed in extremis had severe nephropathy associated with
hyperplasia of the parathyroid, demineralization of the bone, and
metastatic calcification in various organs. The NOAEL was 200 ppm,
equivalent to 8.8 mg/kg bw per day in males and 10.2 mg/kg bw per day
in females. The increased blood urea nitrogen levels observed in males
at 75 and 200 ppm and the increased urinary protein levels in males at
200 ppm at 24 months were not considered to be adverse effects in view
of the absence of treatment-related pathological findings at these
doses (Takaori, 1993).
(d) Reproductive toxicity
Mice
Groups of eight male Swiss-Webster mice were given thiophanate-
methyl (purity unspecified) as an oral dose of 192 mg/kg bw per day
for five consecutive days. Twenty-four hours after the final dose
[1,2-3H]-testosterone was administered intraperitoneally at
10 µCi/kg bw in order to assess its assimilation by the anterior
prostate coagulating glands. Animals were killed after 5 min, and the
anterior prostate glands were removed. Thiophanate-methyl did not
significantly affect body weights, absolute testicular weights,
absolute or relative seminal vesicular weights, or total radiolabel
assimilation by the anterior prostate gland. The absolute prostate and
adrenal gland weights were significantly increased, and these changes
were considered by the authors to be due to stress induced by
thiophanate-methyl. The relative weights of the testes, prostate, and
adrenal were also increased. Spermatogenesis was not affected, no
sterile tubules were seen, and the interstitial (Leydig) cells
appeared to be normal (Thomas, 1974; Thomas & Schein, 1974).
Rats
In a three-generation study, groups of 10 male and 20 female
weaning Sprague-Dawley rats fed diets containing 0, 40 160, or 640 ppm
thiophanate-methyl (purity unspecified) were mated twice to produce
the F1a and F1b litters. Ten male and 20 female offspring were
selected from the F1b litters and mated to produce the F2a and
F2b litters; 10 male and 20 female F2b offspring were then
selected and mated to produce the F3a and F3b litters. The test
diets were fed throughout the study, animals in the F0, F1b, and
F2b generations being fed for 60 days before mating. In each case,
mating lasted for 20 days. Animals were re-mated to produce the second
litters about 10 days after weaning of the first litters. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were not reported. In F0 animals, no treatment-related
effects on mortality, food consumption, or body-weight gain or
clinical signs of toxicity were seen. No treatment-related effects
were observed on mating performance, length of gestation, or pregnancy
rate or at terminal autopsy in F0, F1b, or F2b rats. The total
litter sizes and weights at birth were slightly reduced in all animals
at 640 ppm except the F3a litter. Viable litter size and weight at
weaning were reduced in all generations at 640 ppm; mean pup weight
was not affected. Viable litter size and weight between birth and
weaning were slightly reduced in the second F2b litter at all doses,
the percentage losses increasing with dose; however, average pup
weights were not affected. The total litter loss and the incidence of
gross malformations of pups were not treatment-related. No
dose-related effects were seen on macroscopic appearance, organ
weight, histopathological parameters, or the skeleton in F3b
offspring. The NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per
day, on the basis of effects on litters at 640 ppm (Palmer et al.,
1972).
In a two-generation study, groups of 25 male and 25 female
Sprague Dawley rats were given diets containing thiophanate-methyl
(purity, 95.93%) at doses of 0, 200, 630, or 2000 ppm. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were acceptable. After 14 weeks of treatment, the parental
(F0) animals were mated for up to 21 days. The F0 females were
allowed to litter and to rear their offspring (F1a generation) to
weaning. After a 14-week maturation period after weaning, the parental
F1 animals, selected from the F1a offspring, were mated for up to
21 days and the females were allowed to litter and rear their
offspring (F2a generation) to weaning. After weaning of the F2a
pups and review of the results of rearing and weaning, including high
pup mortality in all groups, the F1 generation was mated again, six
weeks after weaning of the surviving F2a pups. The F1 animals were
allowed to mate for a maximum of 21 days with the same partner, as
during the first mating, and to produce a second litter (F2b). The
body-weight gain of males of the F0 generation and males and females
of the F1 generation at 2000 ppm was reduced, but only the
body-weight gain of male F1 animals at 630 ppm was affected. Food
consumption was affected by treatment before the first mating and
during the first and second gestations and first lactation only in
F1 females at 2000 ppm. At this dose, the males and females of the
F0 and F1 generations had elevated levels of thyroid-stimulating
hormone; slightly reduced triiodothyronine levels were noted in F0
females at necropsy; and males and females of the F0 generation had
lower thyroxine values at weeks 1 and 8 but not at necropsy. These
findings are consistent with the histological findings and changes in
organ weights found at this dose. Thyroid and liver weights were
increased in male and female animals of the F0 and F1 generations
at the high dose, and histopathological examinations of these animals
showed treatment-related centrilobular hepatocyte hypertrophy in the
liver and follicular-cell hypertrophy and hyperplasia (except in F1
females) in the thyroid. The weights of male and female F1a, male
F2a, and male and female F2b pups were reduced at 2000 ppm; at
630 ppm, only the weights of male and female F2b pups were reduced.
Very high pup losses, mainly after day 4 of lactation, occurred in all
animals of the F2a generation, including the controls. No clear
explanation was found for the pup losses. Those pups that did not die
had normal postnatal development, except for reduced body-weight gain
in male pups at the high dose. Such losses were not seen in the F2b
groups. Functional tests of F1a, F2a, and F2b animals showed no
treatment-related findings. The NOAEL was 630 ppm for male and female
F0 animals, 200 ppm for the F1 generation, 630 ppm for the F1a
and F2a offspring, and 200 ppm for the F2b offspring. The NOAEL
was 2000 ppm for fertility and general reproductive performance
(Muller, 1993).
Since treatment-related histopathological effects were found in
the thyroid and liver in rats at the high dose in the preceding study,
organs from all F0 and F1 animals at 200 and 630 ppm were further
evaluated. The incidence and severity of hepatocyte hypertrophy were
increased in a dose-related fashion at all doses in F0 males, and
the incidence and to a lesser extent the severity of thyroid
follicular-cell hypertrophy and hyperplasia were increased in the same
animals. F1 males also had increased (but to a lesser extent)
hepatocyte hypertrophy and thyroid follicular cell hyperplasia at all
doses. The minimal effect level was thus 200 ppm, equivalent to
9.7-14.7 mg/kg bw per day (Muller & Singer, 1995).
(e) Developmental toxicity
Mice
Groups of 20 female ICR mice were given thiophanate-methyl
(purity, 94%) at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day by
gavage on days 1-15 of gestation. Animals were killed on day 18 or 19
(discrepancy between the study report and publication). No deaths,
abnormalities, or significant differences in body weights were
recorded. The number of living fetuses per litter was reduced in
animals at 1000 mg/kg bw per day, partly as a result of increased
resorptions. No treatment-related effects were observed on
implantation numbers, litter sizes, fetal body weights, sex ratio, or
numbers of immature or malformed fetuses. The NOAEL was 1000 mg/kg bw
per day for maternal toxicity and 500 mg/kg bw per day for embryo- and
fetotoxicity, on the basis of the reduced number of live fetuses at
1000 mg/kg bw per day. There was no evidence of teratogenicity
(Noguchi, 1970c; Makita et al., 1973).
Rats
Groups of 25 female Sprague-Dawley rats were given
thiophanate-methyl (purity, 97.2%) at doses of 0, 100, 300, or
1000 mg/kg bw per day by gavage on days 6-19 of gestation. Animals
were killed on day 20 of gestation. There were five to nine nongravid
females per group, including the control group. One rat at 100 mg/kg
bw per day and one at 300 mg/kg bw per day delivered on days 13 and
11, respectively, and were killed on the day of delivery. The weight
and development of the offspring were comparable to those of animals
delivered later, suggesting incorrect assessment of copulation; no
malformations or maternal abnormalities were seen in these animals. No
deaths, treatment-related clinical symptoms, or gross pathological
abnormalities were reported in any dams. Body-weight gain was reduced
between days 6 and 9 in animals at 1000 mg/kg bw per day. There were
no treatment-related effects on total implantations, post-implantation
loss, fetal body weight, fetal sex ratio, or viable fetuses. There
were no dose-related fetal malformations or developmental
abnormalities, and the incidences of these parameters were within
those of historical controls. A positive control study in the same
strain with a single high dose of vitamin A produced the appropriate
results. The NOAEL was 300 mg/kg bw per day for maternal toxicity, on
the basis of the reductions in body-weight gain of dams at 1000 mg/kg
bw per day during treatment. There was no evidence of teratogenicity
or embryo- or fetotoxicity (Rodwell et al., 1981).
Rabbits
Groups of 15 female New Zealand white rabbits received
thiophanate-methyl (purity, 96.2%) at doses of 0, 2, 6, or 20 mg/kg bw
per day by gavage on days 6-19 of gestation. Samples of test solutions
were taken during the first and last weeks of treatment, and the
concentrations of thiophanate-methyl were found to be acceptable. The
animals were killed on day 29 of gestation. No treatment-related
maternal mortality or clinical or subsequent maternal effects were
seen. Dose-related losses in maternal body weight were observed mainly
at the beginning of treatment (days 6-8 at 6 mg/kg bw per day and days
6-14 at 20 mg/kg bw per day). The food consumption of animals at
20 mg/kg bw per day was reduced from the start of treatment, and large
reductions during days 6-12 were generally associated with subsequent
abortion or sacrifice in extremis. Water consumption was unaffected.
One of 12 animals at 2 mg/kg bw per day, one of 14 at 6 mg/kg bw per
day, and two of 13 at 0 mg/kg bw per day aborted. One control animal
and one at 20 mg/kg bw per day suffered total litter loss and
resorption; the control animal suffered early resorption of its only
implantation, while the treated animal lost five of five implantations.
No treatment-related trends were observed in resorptions, pre-
implantation losses, or fetal or placental weights. The numbers of
viable young were slightly decreased in animals at 20 mg/kg bw per day
due to abortions and total litter losses. Gross examination of the
fetuses showed that in animals at 6 and 20 mg/kg bw per day, there was
an apparent treatment-related trend in skeletal abnormalities in ribs,
vertebrae, and pelvis that was generally close to or slightly greater
than the upper limit of historical controls. Treatment-related effects
included increased incidences of 13 pairs of ribs, incomplete or
asymmetric ossification of costal elements of the sacral vertebrae, 27
presacral vertebrae, and asymmetric pelvises associated with different
sacral vertebrae. At 20 mg/kg bw per day, the incidences of one or
more ribs thickened at the costal cartilage were significantly
increased. The frequencies of these skeletal abnormalities are
summarized in Table 6. The NOAEL was 2 mg/kg bw per day for maternal
toxicity, on the basis of the effect on maternal growth rate, and
2 mg/kg bw per day for developmental toxicity, on the basis of the
increased incidence of skeletal abnormalities at 6 and 20 mg/kg bw per
day (Ross et al., 1986).
Table 6. Incidence of selected skeletal abnormalities in fetuses of rabbits
treated with thiophanate-methyl
Observation Dose (mg/kg bw per day) Historical controls
0 2 6 20 Mean Range
13 pairs of ribs 41 46 63 61 36 12-61
Thickened ribs 1 4 2 14 2 0-13
Incomplete or assymetrical 3 6 7 12 4 0-15
ossification
27 presacral vertebrae 16 18 38 43 18 7-44
Assymetrical pelvis 3 5 7 10 4 0-9
(f) Genotoxicity
The results of studies of the genotoxicity and mutagenicity of
thiophanate-methyl in vitro and in vivo are summarized in Table 7.
There was no evidence of genotoxicity or mutagenicity. Thiophanate-
methyl is partially metabolized in mammalian systems to carbendazim,
which has been shown to act as an aneugen.
The aneugenic potential of thiophanate-methyl (purity, 95%;
dispersed in 70% kaolin) was tested in male Swiss albino mice given
oral doses of 1000 mg/kg bw. Bone-marrow cells were analysed 16, 24,
36, and 48 h after treatment for micronuclei, chromosomal aberrations,
hyperdiploidy, and polyploidy. Large micronuclei were significantly
induced, but the response was relatively weak, and thiophanate-methyl
was less effective than benomyl or carbendazim. There was no increase
in the frequency of chromosomal aberrations. At 24 and 36 h, a
possibly treatment-related increase in the frequency of polyploid and
hyperdiploid cells was observed, which was of borderline significance
in view of the very low frequency of changes in ploidy (Barale
et al., 1993).
Table 7. Results of tests for the genttoxicity of thiophanate-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 10, 50, 100, 97.3 Negativea Shirasu et al. (1976)
TA100, TA1535, TA1537, 500, 1000 µg/plate
TA1538, E. coli WP2 hcr dissolved in DMSO
Reverse mutation S. typhimurium TA98, 39.1, 78.1, 156.3, 96.55 Negativea Kanaguchi & Nishibe
TA100, TA1535, TA1537, 312.5, 625, 1250, (1990)
E. coil WP2 uvrA 2500, 5000 µg/plate
in DMSO
Host-mediated S. typhimurium G46 1000, 3000 mg/kg 97.3 Negative Shirasu et al. (1976)
mutation bw twice
Gene mutation B. subtills H17, M45 20, 100, 200, 500, 97.3 Negative Shirasu et al. (1976)
1000, 2000 µg,/disk
Gene mutation Chinese hamster V79 cells, 6.25, 12.5, 25.0, 50, 96.36 Negativea Tippins et al. (1984)
hprt locus 100 µg/ml in DMSOa
Chromosomal aberration Chinese hamster ovary cells 100, 200, 300, 400 95.0 Negative Murli (1988)
µg/ml in DMSOa
250, 500, 750,
1000 µg/ml in DMSOb
Unscheduled DNA Primary hepatocytes 5, 10, 25, 50, 100, 100 Negative Myhr (1981)
synthesis 250, 500, 1000 µg/ml
In vivo
Dominant lethal mutation ICR mice 8-500 mg/kg bw 94 Negative Makita et al. (1973)
intraperitoneally
Cytogenicity Wistar rat bone marrow 62.5-1000 mg/kg bw 94 Negative Makita et al. (1973)
and spermatocytes intraperitoneally
DMSO, dimethyl sulfoxide
a Without metabolic activation
b With metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Thiophanate-methyl (0.5 g; purity, 96.2%) moistened with water
was applied to the backs of six male New Zealand white rabbits for 4 h
under an occlusive patch. There were no signs of irritation over a
72-h observation period (Souma & Nishibe, 1986a).
Nine male New Zealand white rabbits received 0.1 g
thiophanate-methyl (purity, 96.2%) in the left eye. The treated eyes
of three rabbits were washed with water, and those of the others
remained unwashed. Conjunctival redness was observed in four of the
unirrigated eyes and in all three of the irrigated eyes after 1 h.
These symptoms had resolved by 24 h in all animals except one with an
unwashed eye, in which irritation resolved by 48 h. Thiophanate-methyl
was considered to be a very mild eye irritant (Souma & Nishibe,
1986b).
The skin sensitizing potential of thiophanate-methyl (purity,
96.2%) was tested in 20 female Hartley guinea-pigs by the
Magnusson-Kligman technique. Thiophanate-methyl was sensitizing under
the conditions of the study (Souma & Nishibe, 1989). In a Buehler
test, thiophanate-methyl (purity, 96.55%) was not sensitizing in 10
female Hartley guinea-pigs (Mochizuki, 1993).
(ii) Effects on the thyroid and liver
A number of studies were carried out to elucidate the effects of
thiophanate-methyl on the thyroid and liver (Nishibe & Takaori, 1990).
Thiophanate-methyl (purity, 96.55%) was administered in the diets
of six-week-old male Fischer 344 rats for two or eight days at a
concentration of 6000 ppm. Propylthiouracil, an inhibitor of thyroid
hormone synthesis, was administered at 1000 ppm for two or eight days,
and phenobarbital, an inducer of drug-metabolizing enzymes, was
administered at 500 ppm for eight days, as positive controls.
Thiophanate-methyl reduced triiodothyronine and thyroxine levels,
increased thyroid-stimulating hormone activity (at day 8 only),
increased thyroid weights (at day 8 only), and increased liver
weights. Propylthiouracil caused similar but more marked changes in
thyroid hormone levels and weights. Phenobarbital increased liver
weights. All three chemicals increased serum cholesterol levels by day
8. Thiophanate-methyl and phenobarbital increased microsomal
cytochrome P450, cytochrome b5, total protein, and UDP-glucu-
ronosyltransferase activities.
Seven-week-old female Fischer 344 rats were given 6000 ppm
thiophanate-methyl or 500 ppm phenobarbital for eight days. Some were
killed at eight days, and the others were killed after an eight-day
recovery. Thyroid weights were increased at day 8.
Groups of eight-week-old rats (sex not specified) were fed diets
containing 0 or 6000 ppm thiophanate-methyl, 30 µg/kg bw thyroxine, or
6000 ppm thiophanate-methyl and daily injections of 30 µg/kg thyroxine
subcutaneously, for eight days. Thiophanate-methyl caused hypertrophy
of the thyroid and liver and increased the level of thyroid-
stimulating hormone; these changes were suppressed by concomitant
administration of thyroxine. Thiophanate with and without thyroxine
also increased serum cholesterol. Thyroxine alone caused none of these
changes.
Thiophanate-methyl was less effective than propylthiouracil in
inhibiting porcine thyroid microsomal peroxidase. The ED50 and ED0
were 6 × 10-4 mol/litre and 8 × 10-5 mol/litre for thiophanate-
methyl and 2 × 10-5 mol/litre and 4 × 10-7 mol/litre for
propylthiouracil.
Eleven-week-old male ICR mice and Fischer 344 rats were given
diets containing 6000 ppm thiophanate-methyl or 500 ppm phenobarbital
for two or eight days. Liver weights were increased in all treated
animals. The titre of hepatocyte proliferating cell nuclear antigen,
the polymerase delta accessory protein, was increased in rats at day 2
and mice at days 2 and 8 (Nishibe & Takaori, 1990).
3. Observations in humans
In 1969-72, 16 production workers continuously exposed to
Topsin-E containing thiophanate or Topsin-M containing thiophanate-
methyl were routinely tested for effects on health. Blood and urine
samples were taken for analysis every six months: blood was analysed
for erythrocyte and leukocyte counts, haemoglobin content, and
specific gravity, and urine for protein, sugar, and urobilinogen.
Serum alanine and aspartate aminotransferases activities were
determined twice during the first six months of 1972 only. The initial
tests were conducted in January 1969, prior to production of Topsin.
No significant effects were seen in any of the workers for any of the
parameters investigated (Mori, 1972).
The same tests reported by Mori (1972) were conducted twice a
year on 28 production workers exposed to Topsin M and/or Topsin E in
1973-77, except that leukocyte and erythrocyte counts and haemoglobin
levels were not tested during 1976 for undescribed 'personal reasons'.
Serum alanine and aspartate aminotransferase activities were
determined in all employees in October 1977. More than 13 employees
were tested each year, but the specific individuals tested changed
during the study so that only seven individuals were monitored
continuously. No adverse behavioural, haematological, clinical
chemical, or urinary findings were made (Ikeda, 1978).
Between 1985 and 1991, no adverse behavioural symptoms (including
skin or eye effects) or clinical findings (haematology, clinical
chemistry, or urinalysis) were observed in workers exposed to
thiophanate-methyl and thiophanate during production. Health
examinations were performed twice a year. The workers were involved
mainly in the production of thiophanate-methyl, usually for 11 months
of the year (Aizawa, 1991).
Comments
Thiophanate-methyl was rapidly absorbed after oral exposure of
mice, rats, and dogs. Most of the administered radiolabel was
recovered within 24 h of dosing, predominantly in the urine.
Carbendazim and 5-hydroxycarbendazim were among the metabolites of
thiophanate-methyl identified in vivo and in vitro.
Thiophanate-methyl has low acute toxicity in rats, with an oral
LD50 of about 7000 mg/kg bw. Clinical signs of toxicity after acute
dosing with thiophanate-methyl were generally non-specific. WHO has
classified thiophanate-methyl as unlikely to present an acute hazard
in normal use.
In a six-month study of toxicity, mice were given dietary doses
of 0, 13, 64, 320, 1600, or 8000 ppm thiophanate-methyl. The NOAEL was
320 ppm, equivalent to 48 mg/kg bw per day, on the basis of
haematological effects and hepatotoxicity. In a six-month study, rats
were exposed to thiophanate-methyl in the diet at levels of 0, 13, 64,
320, 1600, or 8000 ppm. Reduced erythrocyte counts, reduced
body-weight gain, and evidence of hepatotoxicity and thyroid
stimulation were seen at 8000 ppm. The NOAEL was 1600 ppm, equivalent
to 80 mg/kg bw per day. Dogs were exposed to thiophanate-methyl by
administration in capsules at doses of 0, 50, 200, or 400 mg/kg bw per
day for 13 weeks, 0, 8, 40 or 200 mg/kg bw per day for one year, or 0,
2, 10, 50, or 250 mg/kg bw per day for two years. Treatment-related
thyroid hyperplasia was seen in all three studies; the overall NOAEL
was 10 mg/kg bw per day.
In a two-year study of carcinogenicity in mice fed dietary levels
of 0, 10, 40, 160, or 640 ppm, there was no evidence of any
carcinogenic response; the NOAEL was 160 ppm (equal to 24 mg/kg bw per
day) on the basis of reduced body-weight gain. In a subsequent
18-month study in mice fed dietary levels of 0, 150, 640, 3000, or
7000 ppm, the NOAEL was 150 ppm (equal to 29 mg/kg bw per day) on the
basis of treatment-related hepatotoxicity and hepatocellular tumours.
In a two-year study of carcinogenicity in rats fed dietary levels of
0, 10, 40,160, or 640 ppm, effects were seen on the testes (mainly
hypospermatogenesis), thyroid gland, and growth rate at 640 ppm; the
NOAEL was 160 ppm (equivalent to 8 mg/kg bw per day). In a two-year
study in rats fed dietary levels of 0, 75, 200, 1200, or 6000 ppm, the
NOAEL was 200 ppm (equal to 9 mg/kg bw per day). Thyroid hyperplasia
and thyroid tumours were seen at higher doses, together with
parathyroid hyperplasia, nephrotoxicity, hepatotoxicity, and lipidosis
of the adrenal cortex.
In a three-generation study of reproductive toxicity in which
rats received dietary doses of 0, 40, 160, or 640 ppm, reductions in
total litter size and weight at birth and at weaning were seen at
640 ppm; the NOAEL was 160 ppm, equivalent to 8 mg/kg bw per day. In a
two-generation study in which rats received dietary doses of 0, 200,
630 or 2000 ppm, there was no evidence of a treatment-related effect
on reproduction. The lowest dose tested in the study, equivalent to
10 mg/kg bw per day, was a minimal-effect level on the basis of
hepatocyte hypertrophy, thyroid follicular-cell hypertrophy, and
hyperplasia at all treatment levels.
The teratogenic potential of thiophanate-methyl was investigated
in mice, rats, and rabbits. In mice exposed by gavage to thiophanate-
methyl at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day on days
1-15 of gestation, there was no evidence of teratogenicity or maternal
toxicity at the highest dose, although embryo- and fetotoxicity were
seen at this dose. In rats exposed by gavage to thiophanate-methyl at
doses of 0, 100, 300, or 1000 mg/kg bw per day on days 6-19 of
gestation, there was no evidence of fetotoxicity or teratogenicity,
but maternal toxicity, expressed as reduced body-weight gain, was seen
at the highest dose. In rabbits exposed by gavage to thiophanate-
methyl at doses of 0, 2, 6, or 20 mg/kg bw per day on days 6-19 of
gestation, maternal toxicity (reduced growth rate) was seen at 6 mg/kg
bw per day and above. A dose-related trend of increased fetal skeletal
abnormalities (ribs, vertebrae, and pelvis) at 6 and 20 mg/kg bw per
day indicated an NOAEL for developmental toxicity of 2 mg/kg bw per
day.
Thiophanate-methyl was adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The only significant
response was a small increase in the frequency of micronuclei
characterized by their large size in mouse bone-marrow cells, which
was not associated with the induction of structural chromosomal
aberrations. This is indicative of a weak aneugenic effect. The
Meeting concluded that thiophanate-methyl is not genotoxic.
Studies on the effects of thiophanate-methyl on the liver and
thyroid of rats showed liver enzyme induction, reductions in thyroid
hormones (triiodothyronine and thyroxine), and increases in the level
of thyroid-stimulating hormone and in thyroid and liver weights.
Thyroid hypertrophy and increases in thyroid-stimulating hormone
levels associated with treatment with thiophanate-methyl were
suppressed by concomitant treatment with thyroxine. Thiophanate-methyl
was shown to inhibit porcine thyroid microsomal peroxidase, an enzyme
involved in thyroid hormone synthesis. The thyroidal and hepatic
changes, including tumours, observed in the studies of toxicity may be
due to increased hepatocyte turnover, reductions in the thyroid
hormones triiodothyronine and thyroxine as a result of liver enzyme
induction, and inhibition of thyroid microsomal peroxidase.
Workers involved in manufacturing products containing
thiophanate-methyl showed no treatment-related adverse local (skin or
eyes) or systemic effects.
An ADI of 0-0.02 mg/kg bw was established on the basis of the
NOAEL of 2 mg/kg bw per day for developmental toxicity in rabbits and
a safety factor of 100.
The Meeting noted that the use of thiophanate-methyl on crops
gives rise to residues of carbendazim, although thiophanate-methyl can
also be detected as part of the residue to which consumers of treated
produce are exposed. Since the toxicity of thiophanate-methyl is
qualitatively and quantitatively (when corrected for relative
molecular mass) different from that of carbendazim, the Meeting
concluded that the intake of residues in food should initially be
compared with the ADI for thiophanate-methyl. If further refinement of
the risk assessment is necessary, the different components of the
residue (carbendazim and thiophanate-methyl) may be characterized.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 29 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
1000 mg/kg bw per day (maternal toxicity and teratogenicity
in study of reproductive toxicity)
500 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
160 ppm, equivalent to 8 mg/kg bw per day (study of
reproductive toxicity)
1000 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
300 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 2 mg/kg bw per day (maternal toxicity and teratogenicity in
study of developmental toxicity)
Dog: 10 mg/kg bw per day (studies of toxicity up to two years)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Comparison of the metabolism of thiophanate-methyl and
carbendazim in different species, including humans
2. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to thiophanate-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 7000 mg/kg bw
Dermal, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test; not sensitizing
in Buehler test
Inhalation, toxicity, rat LC50 = 1.8 mg/litre air
Mid-term (1-26 weeks) Oral, reproductive toxicity, rabbit NOAEL = 2 mg/kg bw per day; maternal and
developmental toxicity
Long-term (> one year) Dietary, toxicity and carcinogenicity, NOAEL = 9 mg/kg bw per day; thyroid rumours
two years, rat and hepatotoxicity
Oral, toxicity, two years, dog NOAEL = 10 mg/kg bw per day; hepatotoxicity and
thyroid effects
References
Aizawa, T. (1991) Human handling experiences from plant employees
manufacturing Topsins (3). Unpublished report No. RD-9153 from
Nippon Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Auletta, CS. (1991) A subchronic (3-month) oral toxicity study in the
dog via capsule administration with thiophanate-methyl.
Unpublished report Project No. 89-3525 (RE-9119) from
Bio/dynamics Inc. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Auletta, C.S. (1992) A chronic (1-year) oral toxicity study in the dog
via capsule administration with thiophanate-methyl. Unpublished
report Project No. 89-3526 (RD-9207) from Bio/dynamics Inc.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Barale, R., Scapoli, C., Meli, C., Casini, D., Minunni, M.,
Marrazzini, A., Loprieno, N. & Barrai, I. (1993) Cytogenetic
effects of benzimidazoles in mouse bone marrow. Mutat. Res.,
300, 15-28.
Douch, P.G.C. (1974) The metabolism of some thioureidobenzene
fungicides in mice and sheep. Xenobiotica, 4, 457-475.
Fujino, A., Ohnuma, N., Mori, T., Tanoue, T. & Kamimura, H. (1973) The
balance and metabolism studies of thiophanate-methyl in animals.
Unpublished report from the Nisso Institute for Life Science,
Nippon Soda Co. Ltd, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Hashimoto, Y. (1972) Final report on chronic oral toxicity studies on
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis 3-thioal-
lophanate in Sprague-Dawley strain rats. Unpublished report from
the Nisso Institute for Life Science, Nippon Soda Co. Ltd,
Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Hashimoto, Y., Makita, T., Nishibe, T., Mori, T., Ohnuma, N., Noguchi,
T. & Ohta, G. (1973) Subacute toxicity of thiophanate-methyl in
mice and rats. Pharmacometrics, 7, 929-945.
Ikeda, K. (1978) Human handling experiences from plant employees
manufacturing Topsin (2). Unpublished report from Nippon Soda Co.
Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Kanaguchi, Y. & Nishibe, T. (1990) Thiophanate-methyl reverse mutation
study on bacteria. Unpublished report No. RD-9065 from Nippon
Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Kosaka, S. (1973) The report on the carcinogenesis studies of
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis-(3-thioal-
lophanate) in mice of ICR-SLC strain for 24 months. Unpublished
report from the Nisso Institute for Life Science, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co., Ltd,
Tokyo, Japan.
Makita, T., Hashimoto, Y. & Noguchi, T. (1973) Mutagenic, cytogenetic
and teratogenic studies on thiophanate-methyl. Toxicol. Appl.
Pharmacol., 24, 206-215.
Mochizuki, N. (1993) Topsin M skin sensitization study in guinea pigs.
Unpublished report No. RD-9347 from Nippon Soda Co. Ltd.
Submitted to WHO by Nippon Soda Co., Ltd, Tokyo, Japan.
Mori, H. (1972) Human handling experiences from plant employees
manufacturing Topsin (1). Unpublished report from the Fine
Chemical Division, Nippon Soda Co. Ltd. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Muller, W. (1993) Topsin M two generation oral (dietary
administration) reproduction toxicity study in the rat.
Unpublished report HLA Study No. 683-004 (RD-9329) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Muller, W. & Singer, A. (1995) Topsin M final addendum histopathology
report and peer review pathology report to MRID 42899101. Two
generation oral (dietary administration) reproduction toxicity
study in the rat. Unpublished report HLA Study No. 683-004
addendum histopathology report (RD-9525) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Murli, H. (1988) Mutagenicity test on Topsin M technical (thiophanate-
methyl) in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished report HLA Study No 10345-0-437 (RD-9120) from
Hazleton Laboratories America, Inc. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Myhr B.C. (1981) Primary rat hepatocyte unscheduled DNA synthesis
assay of thiophanate-methyl. Unpublished report No. LBI Project
No 21191 (RD-8195) from Litton Bionetics. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation toxicity study
in rats. Unpublished report No. RD-8711 from the Toxicology
Institute, Environmental Toxicology Laboratory, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Nishibe, T. & Takaori, H. (1990) Summary of mechanistic investigation
of the effect of thiophanate-methyl on thyroid and liver.
Unpublished report from the Toxicology Institute, Environmental
Toxicology Laboratory, Nippon Soda Co. Ltd, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970a) Toxicological evaluation of thiophanate-methyl
(V). Some pharmacological properties of a new fungicide,
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970b) Studies on the biotransformation of thiophanate-
methyl in animal and plant (Part 1). Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970c) Toxicological evaluation of thiophanate-methyl
(IV). Studies on the teratogenic effect of thiophanate-methyl
upon the fetus of ICR strain of mice. Unpublished report from the
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1971) Studies on the biotransformation of
thiophanate-methyl in animal, plant and soil (Part II).
Unpublished report from the Chemical Research Laboratories, Nisso
Institute for Life Sciences, Kanagawa-ken, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1972) Studies on the biotransformation of
thiophanate-methyl in animal and plant. Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970a) Toxicological evaluation of
thiophanate-methyl (I). Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa-ken, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate-methyl (II). Studies on the subchronic oral toxicity
of thiophanate-methyl in mice. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity
of thiophanate-methyl in rats. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Palmer, A., Lovell., M. & Newman, A. (1972) Effect of
thiophanate-methyl on reproductive function of multiple
generations in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Rodwell, D.E. et al (1981) Teratology study in thiophanate-methyl in
rats. Unpublished report No. 449-006 (RD-8126) from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Ross, F.W. et al. (1986) Thiophanate-methyl: Teratology study in the
rabbit. Unpublished report No. 86/NIS010/11 (RD-8642) from Life
Science Research. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Saike, O. & Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation
toxicity study in rats. Unpublished report No. 0219 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Shirasu, Y. et al. (1976) Mutagenicity testing on thiophanate-methyl
in microbial systems. Unpublished report No. RD-8084 from the
Institute of Environmental Toxicology. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Singh, T. & Garg, B. (1989) Effect of thiophanate-methyl on
cardiovascular system. Indian Vet. J., 66, 1116-1119.
Souma, S. & Nishibe, T. (1986a) Thiophanate-methyl primary dermal
irritation study in rabbits. Unpublished report No. RD-8692 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1986b) Thiophanate-methyl primary eye
irritation study in rabbits. Unpublished report No. RD-8691 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1989) Thiophanate-methyl: Delayed contact
hypersensitivity study in guinea pigs. Unpublished report No.
RD-8924 from Nisso Institute for Life Sciences, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990a) Thiophanate-methyl acute oral toxicity
study in rats. Unpublished report No. RD-9083 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990b) Thiophanate-methyl acute dermal
toxicity study in rabbits. Unpublished report No. RD-9084 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Takaori, H. (1993) Thiophanate-methyl combined chronic
toxicity/oncogenicity study in rats. Unpublished report
No. RD-9327 from Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Taniguchi, T. (1972) Final report on the long-term oral toxicity
studies of thiophanate-methyl, dimethyl 4,4'-O-phenylene-
bis(3-thioallophanate) in beagle-dogs for 24 months. Unpublished
report from the Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Thomas, J. (1974) Actions of pesticides and other drugs on the male
reproductive system (EPA-650/1-74-011), Washington DC, US
Environmental Protection Agency. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Thomas, J. & Schein, L. (1974) Effects of thiophanate and thiophanate-
methyl on the male reproductive system of the mouse. Toxicol.
Appl. Pharmacol., 30, 129-133.
Tippins, R.S. et al. (1984) Gene mutation in Chinese hamster V79
cells with thiophanate-methyl. Unpublished LSR-RTC Report
No. 063013-M-05184 (RD-84109) from Life Science Research.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Tompkins, E.C. (1992) Topsin-M (thiophanate-methyl) 18-month dietary
oncogenicity study in mice. Unpublished report No. WIL-75024
(RD-9328) from WIL Research Laboratory, Inc. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
 Table 3. Pharmacological effects of thiophanate-methyl
Parameter Species Dose and route Results
Body temperature Wistar rats 380-1500 mg/kg bw orally No effect
Analgesic activity Mice 500 mg/kg bw intraperitoneally No effect
Sedative or hypnotic activity Rats and mice 100-500 mg/kg bw subcutaneously No effect
Cardiovascular and respiratory Rabbits 20-100 mg/kg bw intravenously Reduced blood pressure
effects followed by bradycardia
Table 4. Acute toxicity of thiphanate-methyl
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male, female Orala 3400-3514 Noguchi & Hashimoto (1970a)
Mouse Male, female Intraperitoneal 792-1113 Noguchi & Hashimoto (1970a)
Mouse Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Mouse Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Oral 6640-7500 Noguchi & Hashimoto (1970a)
Rat Male, female Intraperitoneal 140-1640 Noguchi & Hashimoto (1970a)
Rat Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Inhalation 1.7-1.9 Nishibe (1987)
Rat Male, female Oralb > 5000 Souma & Nishibe (1990a)
Guinea-pig Male, female Oral 3640-6700 Noguchi & Hashimoto (1970a)
Guinea-pig Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Oral 2270-2500 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermalb > 2000 Souma & Nishibe (1990b)
a In gum arabic in 5% sodium chloride solution
b In distilled water
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 12 ICR mice of each sex were fed diets containing
thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or 8000 ppm for
six months. The homogeneity, stability, and concentrations of the diet
were not reported; clinical signs of toxicity were also not reported.
There were no treatment-related deaths. The body-weight gain of
animals of each sex at 8000 ppm was significantly reduced. Erythrocyte
counts were also reduced in these animals and in males at 1600 ppm,
and haematocrit values were reduced in animals of each sex at 1600 and
8000 ppm. No significant differences were seen in the weights of the
cerebrum, heart, lung, spleen, kidney, or testis, but in animals at
8000 ppm, liver weights were increased. Histopathological
investigation revealed a higher incidence of hepatic-cell irregularity
at the higher doses, with five cases in males and three in females at
8000 ppm and one case in an animal of each sex at 1600 ppm. Large
swollen hepatic cells, some with oedematous or granular protoplasm,
were found to a greater extent at 8000 ppm, especially in the central
lobules. Constriction of veins by the swollen cells was proposed to
explain the cloudy appearance of the liver. Sporadic formation of
acidiphilic bodies from hepatic-cell degeneration was recorded in two
females and one male at 8000 ppm and one male at 1600 ppm. The NOAEL
was 320 ppm, equivalent to approximately 48 mg/kg bw per day, on the
basis of hepatotoxic and haematological effects at 1600 and 8000 ppm
(Noguchi & Hashimoto, 1970b; Hashimoto et al., 1973).
Rats
Groups of 12 Sprague Dawley rats of each sex were fed diets
containing thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or
8000 ppm for six months. The homogeneity, stability, and
concentrations of the diet were not reported, and no clinical signs of
toxicity were reported. There were no treatment-related deaths. The
body-weight gain of animals of each sex at 8000 ppm was significantly
reduced, but food consumption did not differ significantly between
groups. Reduced erythrocyte counts were seen in animals of each sex at
8000 ppm. Urinalysis did not show significant treatment-related
differences. Blood cholesterol levels were raised significantly in
animals of each sex at 8000 ppm. The thyroid anti liver weights were
increased in these animals, and the weight of the thymus was increased
in females. Some histopathological effects on the thyroid were
reported in animals at this dose: four animals of each sex had small
thyroid follicles, four males and four females had thickened
follicular epithelium, and five animals of each sex had decreased
colloidal substance. There were no treatment-related effects on
protein-bound iodine levels. The NOAEL was 1600 ppm, equivalent to
approximately 80 mg/kg bw per day, mainly on the basis of effects on
thyroid, liver, and body weights (Noguchi & Hashimoto, 1970c;
Hashimoto et al., 1973).
Dogs
Groups of five beagle dogs of each sex (four at the highest dose)
were fed capsules containing thiophanate-methyl (purity unspecified)
at doses of 0, 2, 10, 50, or 250 mg/kg bw per day for two years. One
animal of each sex at each dose was killed and autopsied after 12
months. There were no deaths or adverse symptoms. Animals of each sex
at 250 mg/kg bw per day had reduced body weights and body-weight gain.
Gross pathological effects were not reported, and no effects on the
eyes or heart were found in periodic ophthalmoscopic and electro-
cardiographic investigations. There were no treatment-related changes
in clinical chemical, haematological, or urinary values. After 24
months, the weight of the thyroid gland was increased in animals of
each sex at 250 mg/kg bw per day and to a lesser extent at 50 mg/kg bw
per day. The effects on thyroid tissue at 12 months included altered
or decreased colloidal substance and slightly taller cuboid follicular
epithelial cells at 250 mg/kg bw per day. The NOAEL was 10 mg/kg bw
per day on the basis of effects on thyroid weight in animals of each
sex at 50 and 250 mg/kg bw per day (Taniguchi, 1972).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) for three months at
doses of 0, 50, 200, or 800 mg/kg bw per day, the last dose being
lowered to 400 mg/kg bw per day on test day 50 because of severe
toxicity. One male at the high dose was sacrificed on day 41 because
of severe toxicity; one male at 50 mg/kg bw per day died on day 36,
but this death did not appear to be related to treatment. Dose-related
clinical signs seen in animals at the high dose and to a lesser extent
at the middle dose included dehydration, thinness, and lethargy.
Dose-related decreases in body weights and marked decreases in food
consumption were seen at the middle and high doses. There were no
treatment-related ophthalmological findings. Slight anaemia, increased
platelet counts and cholesterol levels, and decreases in serum alanine
aminotransferase activity and albumin levels were seen at the middle
and high doses and increased activated partial thromboplastin time at
the high dose. Thyroid function tests revealed slightly decreased
triiodothyronine levels in males at the high dose and decreased
triiodothyronine and thyroxine levels in females at the middle and
high doses; no clear effects on thyroid-stimulating hormone values
were apparent. Urinalysis showed no treatment-related findings. At the
middle and high doses, the weights of the liver (in animals of each
sex) and thyroid (males only) were increased. Gross examination
post mortem revealed emaciation in one male at the middle dose and
three at the high dose. Dose-related histological alterations were
seen in a number of organs. In thyroids, hypertrophy of the follicular
epithelium was found in one, three, and four males and one, two, and
four females at the low, middle, and high doses, respectively, with
none in controls. The severity of the hypertrophy increased from
minimal to marked with dose. Hyperplasia of the follicular epithelium
was found in one male at the middle dose and in most animals at the
high dose. In two males at the high dose in which both hypertrophy and
hyperplasia were marked, a large decrease in the quantity of
intrafollicular colloid was seen. In animals at the middle and high
doses, dose-related changes were found in the liver (reduction in
vesiculation of the hepatocellular cytoplasm), gall-bladder
(intracytoplasmic vacuoles), pancreas (atrophy of acinar cells due to
a decreased quantity of zymogen granules), spleen (lymphoid-cell
depletion), thymus (involution), prostate (atrophy), uterus
(anoestrus), and ovaries (inactive). There was no NOAEL because of the
presence of follicular-cell hypertrophy in the thyroid of two dogs at
the low dose (Auletta, 1991).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) at doses of 0, 8, 40,
or 200 mg/kg bw per day for one year. There were no deaths. Tremors
were seen in all dogs at the high dose 2-4 h after treatment on one or
more occasions during the initial three weeks of the study but not
subsequently. The tremors were slight to moderate, except in one
animal which had severe tremors that progressed to apparent tonic
convulsions on three occasions. The body-weight gains of animals at
the high dose were reduced during the study, and body-weight losses
were noted in the first week of treatment; slight reductions in
body-weight gain were noted at 40 mg/kg bw per day. The food
consumption of dogs at the high dose was generally lower than that of
controls during the early months, but was close to the control value
thereafter. Ophthalmological examinations and urinalyses showed no
treatment-related effects. Haematological effects consisted of slight
decreases in total erythrocyte counts and haemoglobin and haematocrit
values in males at the high dose. These animals also had decreased
serum alanine aminotransferase activity, increased alkaline
phosphatase activity, increased cholesterol levels (also at the middle
dose), and decreased albumin:globulin ratios (also at the middle
dose), calcium (also at the middle dose), potassium, and phosphorus
levels. In females at 200 mg/kg bw per day, serum alanine
aminotransferase activity was decreased, and alkaline phosphatase
activity and cholesterol levels were increased. Thyroid function tests
revealed decreased thyroxine levels in males at the middle and high
doses but no clear effects on triiodothyronine or thyroid-stimulating
hormone. Abnormalities seen post mortem included increased liver
weights in dogs at the high dose and increased thyroid weights in
those at the middle and high doses. Microscopic alterations attributed
to treatment were limited to minimal to moderate hypertrophy and
slight hyperplasia of the follicular epithelium of the thyroid in the
dogs at the middle dose (hypertrophy in two females) and the high dose
(hypertrophy in four males and three females, and hyperplasia in one
male and one female). The NOAEL was 8 mg/kg bw per day (Auletta,
1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing 0,
10, 40, 160, or 640 ppm thiophanate-methyl (purity, > 94%) for two
years. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. The body-weight
gain of males at 640 ppm was reduced during the study. No
treatment-related effects on food consumption or on mortality or overt
signs of toxicity were seen. By 24 months, 74-94% of treated animals
had died, but the mean survival times were comparable. Organ weights
and clinical chemical and haematological parameters were not
investigated. No treatment-related gross macroscopic or histopatho-
logical alterations were seen, and there was no evidence of
carcinogenicity. The NOAEL was 160 ppm, equivalent to about 24 mg/kg
bw per day, on the basis of the reduction in body-weight gain in males
at 640 ppm (Kosaka, 1973).
Groups of 60 male and 60 female CD-1 mice were fed diets
containing 0, 150, 640, 3000, or 7000 ppm thiophanate-methyl (purity,
95.9-96.6%) for 18 months. Ten animals of each sex in each group were
necropsied after 39 weeks of treatment, and surviving animals were
necropsied after 78-79 weeks of treatment; all mice that died or were
killed in extremis were also autopsied. The homogeneity, stability,
and concentrations of thiophanate-methyl were acceptable. Haemato-
logical and thyroid function were assessed at the interim necropsy and
before the end of the study. No treatment-related clinical signs were
observed. The cumulative numbers of deaths up to 18 months of -
treatment were 10, 11, 14, 16, and 24 males and 12, 13, 15, 17, and 23
females at 0, 150, 640, 3000, and 7000 ppm, respectively; animals at
7000 ppm had a significantly higher mortality rate than controls. The
increased rates in females at 3000 ppm and males and females at
7000 ppm appeared to be related to amyloid deposition in tissues.
Body-weight gains were slightly reduced in animals of each sex at
3000 ppm and to a greater degree at 7000 ppm; food consumption was
slightly reduced in females at 3000 and 7000 ppm. There was no
treatment-related effect on ophthalmologic parameters or on the onset
or number of palpable masses. The only haematological parameter that
appeared to be altered by treatment was a slightly lower erythrocyte
count in males at 7000 ppm. At the nine-month interim necropsy,
enlarged thyroid glands were seen grossly in three of 10 males at
7000 ppm, the thyroid weights of males and females at 3000 and
7000 ppm were increased, and the thyroid-stimulating hormone levels
were higher and the thyroxine levels lower in males at 3000 ppm and
males and females at 7000 ppm than in controls. The differences in
thyroid weights and thyroid hormone levels were not seen at 18 months.
At the nine-month interim necropsy, the liver weights of males and
females at 3000 and 7000 ppm were significantly increased as a result
of an increased incidence and severity of hepatocellular centrilobular
hypertrophy at these two doses; the incidence of hepatocellular
centrilobular hypertrophy was also increased in females at 640 ppm,
five out of 10 having minimal hypertrophy. At 18 months, the mean
liver weights of animals at 7000 ppm were increased as a result of a
significantly increased number of liver masses, which was also
significantly increased at 3000 ppm. At the 18-month terminal
necropsy, an increased incidence of hepatocellular centrilobular
hypertrophy was seen in males at 3000 and 7000 ppm. Histopathological
examination revealed that the liver masses seen grossly were adenomas.
The incidence of hepatocellular adenomas was significantly increased
in males and females at 3000 and 7000 ppm. The slightly increased
incidence of hepatocellular adenomas in females at 640 ppm (8.6%) was
greater than the historical control range (0-2.7%). Two hepatocellular
carcinomas were seen, one in a male at 640 ppm and the other in a male
at 7000 ppm; this 2% incidence was similar to the mean incidence for
historical control males (1.4%; range, 0-6%). One male at 7000 ppm had
a hepatoblastoma, which is a relatively rare tumour (0.001% in
historical controls). The incidence of atrial thrombosis was increased
in females at 3000 ppm and in animals of each sex at 7000 ppm. The
NOAEL was 150 ppm, equivalent to 29 mg/kg bw per day, in view of the
possible treatment-related incidence of hepatocellular adenomas in
females at 640 ppm (Tompkins, 1992).
Rats
Groups of 35 Sprague-Dawley rats of each sex (50 of each sex for
controls) were fed diets containing thiophanate-methyl (purity, >
94%) at doses of 0, 10, 40, 160, or 640 ppm for two years. Five rats
of each sex at each dose were killed and autopsied at three and 12
months. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. There were no
treatment-related deaths, although survival was poor in some groups,
and there were no overt signs of toxicity. Body-weight gain was
reduced in animals of each sex at 640 ppm, but no significant
difference in food consumption was observed. No significant treatment-
related effects were seen on clinical chemical, haematological, or
urinary values. No significant adverse histopathological effects or
changes in organ weights were seen at any dose at three or 12 months.
Necropsy of animals killed after 24 months or which died during
treatment showed treatment-related histopathological findings in the
testes and thyroid gland. Males at 640 ppm had an increased incidence
of decreased colloidal substance and hypertrophy of follicular
epithelium in thyroid tissue (6/35 compared to 1/50 in controls). Five
animals at 640 ppm were found to be hypospermatogenic; one died at
week 91 with a pituitary tumour and was found to have atrophied
testes. Another male had diminished spermatogenesis at 24 months, and
another surviving male and one that died during treatment had
hyperplasia of the testicular interstitium. No other treatment-related
effects were seen. There was no evidence for carcinogenicity. The
NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per day, on the
basis of effects on the testes and thyroid gland and reduced growth at
640 ppm (Hashimoto, 1972).
Groups of 60 male and 60 female Fischer 344 rats were fed diets
containing 0, 75, 200, 1200, or 6000 ppm thiophanate-methyl (purity,
96.55%) for 24 months. The homogeneity, stability, and concentrations
of thiophanate-methyl in the diets were acceptable. After 12 months,
10 rats of each sex in each group (only five males at 6000 ppm) were
sacrificed. All survivors were sacrificed at the end of treatment. The
mortality rates are shown in Table 5. Only two males at 6000 ppm
survived to the end of the study; because of this high rate, no
statistical analysis was performed for this group at 24 months. The
main causes of death among these rats were nephropathy (22 rats),
thyroid follicular-cell tumour (10 rats), and leukaemia (six rats);
eight males at this dose died or were killed in extremis during
weeks 11-12 due to fractures of the nasal bone caused by the feeder
plate and subsequent dyspnoea (rhinorrhagia). The feeder plates were
removed.
No clinical signs attributable to treatment were noted in any
group during the first 52 weeks. After week 52, dose-related clinical
signs included pale skin and mucous membranes in males at 6000 ppm,
alopecia in females at 6000 ppm, and tissue masses on the skin and in
the subcutis including the lip in males at 1200 and 6000 ppm. These
changes attained statistical significance at week 77 or later.
Dose-related, statistically significant depressions in body-weight
gain were noted in both males and females at 1200 and 6000 ppm.
Decreased food consumption was seen in animals of each sex at 6000 ppm
starting at week 76. No dose-related abnormalities were observed in
ophthalmoscopic parameters at 6, 12, 18, or 24 months. Treatment-
related signs of anaemia (decreased erythrocyte count, haemoglobin,
haematocrit, mean corpuscular volume, mean corpuscular haemoglobin,
and mean corpuscular haemoglobin concentration) were seen in animals
of each sex (mainly males) at 6000 ppm at 3, 6, 12, and 18 months.
Increased platelet and leukocyte counts were seen frequently in males
at 6000 ppm. Clinical chemical investigations revealed increased total
cholesterol and total protein and decreased albumin:globulin ratios in
animals of each sex at 1200 and/or 6000 ppm. At 24 months, treated
males had increased blood urea nitrogen (at 75, 200, and 1200 ppm) and
creatinine levels (at 1200 ppm), reduced thyroxine and triiod-
othyronine values, and increased thyroid-stimulating hormone values
(at 1200 and 6000 ppm, also in females at 6000 ppm). Dose-related
increases in urinary protein (semi-quantitative analysis) were noted
in animals of each sex at 6000 ppm at six and/or 12 months.
Table 5. Mortality rates in Fischer rats treated with thiophanate-methyl
Dose Male Female
(ppm)
Week 52 Week 80 Week 104 Week 52 Week 80 Week 104
No. % No. % No. % No. % No. % No. %
0 0/60 0 2/50 4 13/50 26 0/60 0 3/50 6 13/50 26
75 0/60 0 2/50 4 15/50 30 0/60 0 1/50 2 12/50 24
200 0/60 0 8/50 16 24/50 48* 0/60 0 1/50 2 8/50 16
1200 0/60 0 3/50 6 21/50 42 0/60 0 0/50 0 12/50 24
6000 8/60 13* 18/55 33** 53/55 96** 1/60 2 3/50 6 11/50 22
Significantly different from control at *P < 0.05; ** P < 0.001 (chi-square test)
Nephelometry indicated an increase in urinary protein in males at 1200
and 6000 ppm at various times. Although males at 200 ppm had a
statistically significant increase in urinary protein at 24 months,
histopathological examination revealed no treatment-related effect. In
males at 6000 ppm, ketone bodies, urinary volume, and water
consumption were increased, and pH and specific gravity were decreased
significantly at various times. Dose-related increases in the weights
of the liver, kidney, and thyroid and their body-weight ratios were
seen in animals of each sex at 1200 and/or 6000 ppm at both interim
and final sacrifice.
At interim sacrifice (week 53), treatment-related changes were
found macroscopically in the liver (brownish-black) and kidneys
(granular surface, brownish-black) of males and females at 6000 ppm.
At final sacrifice, males at 1200 ppm had granular kidneys. Females at
6000 ppm also had enlarged thyroids. In animals at 6000 ppm that died
during the study, swollen thyroids were seen in animals of each sex
and white areas in the liver and granular kidney in males. Dose-
related histopathological changes were found in the thyroid, liver,
kidney, and adrenal: Thyroid follicular-cell hyperplasia and
hypertrophy were noted in animals of each sex at 1200 and/or 6000 ppm
at 12 and 24 months, and the incidence of focal follicular-cell
hyperplasia was increased. Males at 6000 ppm also had a statistically
significant increased incidence of thyroid follicular-cell adenomas
(12/60), and one of two males at 6000 ppm that were killed at 24
months and 2/53 that were dead or were killed in extremis had
thyroid follicular-cell adenocarcinomas, with none in the other groups
of treated males. The incidence of retinal atrophy was increased in
females at 6000 ppm at 24 months, and those of parathyroid hypertrophy
and hyperplasia were increased in males at this dose. Centrilobular
hepatocellular hypertrophy and lipofuscin deposition were noted in
animals of each sex at 1200 and/or 6000 ppm at 12 and 24 months and in
most males and females at 6000 ppm which died during the study.
Microgranuloma and focal fatty degeneration in the liver were
increased in incidence in males treated at 6000 ppm. Lipidosis of the
adrenal cortex was noted in females at 1200 ppm and in animals of each
sex at 6000 ppm at 12 months. The severity of nephropathy was
increased in animals of each sex at 6000 ppm at 12 months and in those
at 1200 and 6000 ppm at 24 months. Most males at 6000 ppm that died or
were killed in extremis had severe nephropathy associated with
hyperplasia of the parathyroid, demineralization of the bone, and
metastatic calcification in various organs. The NOAEL was 200 ppm,
equivalent to 8.8 mg/kg bw per day in males and 10.2 mg/kg bw per day
in females. The increased blood urea nitrogen levels observed in males
at 75 and 200 ppm and the increased urinary protein levels in males at
200 ppm at 24 months were not considered to be adverse effects in view
of the absence of treatment-related pathological findings at these
doses (Takaori, 1993).
(d) Reproductive toxicity
Mice
Groups of eight male Swiss-Webster mice were given thiophanate-
methyl (purity unspecified) as an oral dose of 192 mg/kg bw per day
for five consecutive days. Twenty-four hours after the final dose
[1,2-3H]-testosterone was administered intraperitoneally at
10 µCi/kg bw in order to assess its assimilation by the anterior
prostate coagulating glands. Animals were killed after 5 min, and the
anterior prostate glands were removed. Thiophanate-methyl did not
significantly affect body weights, absolute testicular weights,
absolute or relative seminal vesicular weights, or total radiolabel
assimilation by the anterior prostate gland. The absolute prostate and
adrenal gland weights were significantly increased, and these changes
were considered by the authors to be due to stress induced by
thiophanate-methyl. The relative weights of the testes, prostate, and
adrenal were also increased. Spermatogenesis was not affected, no
sterile tubules were seen, and the interstitial (Leydig) cells
appeared to be normal (Thomas, 1974; Thomas & Schein, 1974).
Rats
In a three-generation study, groups of 10 male and 20 female
weaning Sprague-Dawley rats fed diets containing 0, 40 160, or 640 ppm
thiophanate-methyl (purity unspecified) were mated twice to produce
the F1a and F1b litters. Ten male and 20 female offspring were
selected from the F1b litters and mated to produce the F2a and
F2b litters; 10 male and 20 female F2b offspring were then
selected and mated to produce the F3a and F3b litters. The test
diets were fed throughout the study, animals in the F0, F1b, and
F2b generations being fed for 60 days before mating. In each case,
mating lasted for 20 days. Animals were re-mated to produce the second
litters about 10 days after weaning of the first litters. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were not reported. In F0 animals, no treatment-related
effects on mortality, food consumption, or body-weight gain or
clinical signs of toxicity were seen. No treatment-related effects
were observed on mating performance, length of gestation, or pregnancy
rate or at terminal autopsy in F0, F1b, or F2b rats. The total
litter sizes and weights at birth were slightly reduced in all animals
at 640 ppm except the F3a litter. Viable litter size and weight at
weaning were reduced in all generations at 640 ppm; mean pup weight
was not affected. Viable litter size and weight between birth and
weaning were slightly reduced in the second F2b litter at all doses,
the percentage losses increasing with dose; however, average pup
weights were not affected. The total litter loss and the incidence of
gross malformations of pups were not treatment-related. No
dose-related effects were seen on macroscopic appearance, organ
weight, histopathological parameters, or the skeleton in F3b
offspring. The NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per
day, on the basis of effects on litters at 640 ppm (Palmer et al.,
1972).
In a two-generation study, groups of 25 male and 25 female
Sprague Dawley rats were given diets containing thiophanate-methyl
(purity, 95.93%) at doses of 0, 200, 630, or 2000 ppm. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were acceptable. After 14 weeks of treatment, the parental
(F0) animals were mated for up to 21 days. The F0 females were
allowed to litter and to rear their offspring (F1a generation) to
weaning. After a 14-week maturation period after weaning, the parental
F1 animals, selected from the F1a offspring, were mated for up to
21 days and the females were allowed to litter and rear their
offspring (F2a generation) to weaning. After weaning of the F2a
pups and review of the results of rearing and weaning, including high
pup mortality in all groups, the F1 generation was mated again, six
weeks after weaning of the surviving F2a pups. The F1 animals were
allowed to mate for a maximum of 21 days with the same partner, as
during the first mating, and to produce a second litter (F2b). The
body-weight gain of males of the F0 generation and males and females
of the F1 generation at 2000 ppm was reduced, but only the
body-weight gain of male F1 animals at 630 ppm was affected. Food
consumption was affected by treatment before the first mating and
during the first and second gestations and first lactation only in
F1 females at 2000 ppm. At this dose, the males and females of the
F0 and F1 generations had elevated levels of thyroid-stimulating
hormone; slightly reduced triiodothyronine levels were noted in F0
females at necropsy; and males and females of the F0 generation had
lower thyroxine values at weeks 1 and 8 but not at necropsy. These
findings are consistent with the histological findings and changes in
organ weights found at this dose. Thyroid and liver weights were
increased in male and female animals of the F0 and F1 generations
at the high dose, and histopathological examinations of these animals
showed treatment-related centrilobular hepatocyte hypertrophy in the
liver and follicular-cell hypertrophy and hyperplasia (except in F1
females) in the thyroid. The weights of male and female F1a, male
F2a, and male and female F2b pups were reduced at 2000 ppm; at
630 ppm, only the weights of male and female F2b pups were reduced.
Very high pup losses, mainly after day 4 of lactation, occurred in all
animals of the F2a generation, including the controls. No clear
explanation was found for the pup losses. Those pups that did not die
had normal postnatal development, except for reduced body-weight gain
in male pups at the high dose. Such losses were not seen in the F2b
groups. Functional tests of F1a, F2a, and F2b animals showed no
treatment-related findings. The NOAEL was 630 ppm for male and female
F0 animals, 200 ppm for the F1 generation, 630 ppm for the F1a
and F2a offspring, and 200 ppm for the F2b offspring. The NOAEL
was 2000 ppm for fertility and general reproductive performance
(Muller, 1993).
Since treatment-related histopathological effects were found in
the thyroid and liver in rats at the high dose in the preceding study,
organs from all F0 and F1 animals at 200 and 630 ppm were further
evaluated. The incidence and severity of hepatocyte hypertrophy were
increased in a dose-related fashion at all doses in F0 males, and
the incidence and to a lesser extent the severity of thyroid
follicular-cell hypertrophy and hyperplasia were increased in the same
animals. F1 males also had increased (but to a lesser extent)
hepatocyte hypertrophy and thyroid follicular cell hyperplasia at all
doses. The minimal effect level was thus 200 ppm, equivalent to
9.7-14.7 mg/kg bw per day (Muller & Singer, 1995).
(e) Developmental toxicity
Mice
Groups of 20 female ICR mice were given thiophanate-methyl
(purity, 94%) at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day by
gavage on days 1-15 of gestation. Animals were killed on day 18 or 19
(discrepancy between the study report and publication). No deaths,
abnormalities, or significant differences in body weights were
recorded. The number of living fetuses per litter was reduced in
animals at 1000 mg/kg bw per day, partly as a result of increased
resorptions. No treatment-related effects were observed on
implantation numbers, litter sizes, fetal body weights, sex ratio, or
numbers of immature or malformed fetuses. The NOAEL was 1000 mg/kg bw
per day for maternal toxicity and 500 mg/kg bw per day for embryo- and
fetotoxicity, on the basis of the reduced number of live fetuses at
1000 mg/kg bw per day. There was no evidence of teratogenicity
(Noguchi, 1970c; Makita et al., 1973).
Rats
Groups of 25 female Sprague-Dawley rats were given
thiophanate-methyl (purity, 97.2%) at doses of 0, 100, 300, or
1000 mg/kg bw per day by gavage on days 6-19 of gestation. Animals
were killed on day 20 of gestation. There were five to nine nongravid
females per group, including the control group. One rat at 100 mg/kg
bw per day and one at 300 mg/kg bw per day delivered on days 13 and
11, respectively, and were killed on the day of delivery. The weight
and development of the offspring were comparable to those of animals
delivered later, suggesting incorrect assessment of copulation; no
malformations or maternal abnormalities were seen in these animals. No
deaths, treatment-related clinical symptoms, or gross pathological
abnormalities were reported in any dams. Body-weight gain was reduced
between days 6 and 9 in animals at 1000 mg/kg bw per day. There were
no treatment-related effects on total implantations, post-implantation
loss, fetal body weight, fetal sex ratio, or viable fetuses. There
were no dose-related fetal malformations or developmental
abnormalities, and the incidences of these parameters were within
those of historical controls. A positive control study in the same
strain with a single high dose of vitamin A produced the appropriate
results. The NOAEL was 300 mg/kg bw per day for maternal toxicity, on
the basis of the reductions in body-weight gain of dams at 1000 mg/kg
bw per day during treatment. There was no evidence of teratogenicity
or embryo- or fetotoxicity (Rodwell et al., 1981).
Rabbits
Groups of 15 female New Zealand white rabbits received
thiophanate-methyl (purity, 96.2%) at doses of 0, 2, 6, or 20 mg/kg bw
per day by gavage on days 6-19 of gestation. Samples of test solutions
were taken during the first and last weeks of treatment, and the
concentrations of thiophanate-methyl were found to be acceptable. The
animals were killed on day 29 of gestation. No treatment-related
maternal mortality or clinical or subsequent maternal effects were
seen. Dose-related losses in maternal body weight were observed mainly
at the beginning of treatment (days 6-8 at 6 mg/kg bw per day and days
6-14 at 20 mg/kg bw per day). The food consumption of animals at
20 mg/kg bw per day was reduced from the start of treatment, and large
reductions during days 6-12 were generally associated with subsequent
abortion or sacrifice in extremis. Water consumption was unaffected.
One of 12 animals at 2 mg/kg bw per day, one of 14 at 6 mg/kg bw per
day, and two of 13 at 0 mg/kg bw per day aborted. One control animal
and one at 20 mg/kg bw per day suffered total litter loss and
resorption; the control animal suffered early resorption of its only
implantation, while the treated animal lost five of five implantations.
No treatment-related trends were observed in resorptions, pre-
implantation losses, or fetal or placental weights. The numbers of
viable young were slightly decreased in animals at 20 mg/kg bw per day
due to abortions and total litter losses. Gross examination of the
fetuses showed that in animals at 6 and 20 mg/kg bw per day, there was
an apparent treatment-related trend in skeletal abnormalities in ribs,
vertebrae, and pelvis that was generally close to or slightly greater
than the upper limit of historical controls. Treatment-related effects
included increased incidences of 13 pairs of ribs, incomplete or
asymmetric ossification of costal elements of the sacral vertebrae, 27
presacral vertebrae, and asymmetric pelvises associated with different
sacral vertebrae. At 20 mg/kg bw per day, the incidences of one or
more ribs thickened at the costal cartilage were significantly
increased. The frequencies of these skeletal abnormalities are
summarized in Table 6. The NOAEL was 2 mg/kg bw per day for maternal
toxicity, on the basis of the effect on maternal growth rate, and
2 mg/kg bw per day for developmental toxicity, on the basis of the
increased incidence of skeletal abnormalities at 6 and 20 mg/kg bw per
day (Ross et al., 1986).
Table 6. Incidence of selected skeletal abnormalities in fetuses of rabbits
treated with thiophanate-methyl
Observation Dose (mg/kg bw per day) Historical controls
0 2 6 20 Mean Range
13 pairs of ribs 41 46 63 61 36 12-61
Thickened ribs 1 4 2 14 2 0-13
Incomplete or assymetrical 3 6 7 12 4 0-15
ossification
27 presacral vertebrae 16 18 38 43 18 7-44
Assymetrical pelvis 3 5 7 10 4 0-9
(f) Genotoxicity
The results of studies of the genotoxicity and mutagenicity of
thiophanate-methyl in vitro and in vivo are summarized in Table 7.
There was no evidence of genotoxicity or mutagenicity. Thiophanate-
methyl is partially metabolized in mammalian systems to carbendazim,
which has been shown to act as an aneugen.
The aneugenic potential of thiophanate-methyl (purity, 95%;
dispersed in 70% kaolin) was tested in male Swiss albino mice given
oral doses of 1000 mg/kg bw. Bone-marrow cells were analysed 16, 24,
36, and 48 h after treatment for micronuclei, chromosomal aberrations,
hyperdiploidy, and polyploidy. Large micronuclei were significantly
induced, but the response was relatively weak, and thiophanate-methyl
was less effective than benomyl or carbendazim. There was no increase
in the frequency of chromosomal aberrations. At 24 and 36 h, a
possibly treatment-related increase in the frequency of polyploid and
hyperdiploid cells was observed, which was of borderline significance
in view of the very low frequency of changes in ploidy (Barale
et al., 1993).
Table 7. Results of tests for the genttoxicity of thiophanate-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 10, 50, 100, 97.3 Negativea Shirasu et al. (1976)
TA100, TA1535, TA1537, 500, 1000 µg/plate
TA1538, E. coli WP2 hcr dissolved in DMSO
Reverse mutation S. typhimurium TA98, 39.1, 78.1, 156.3, 96.55 Negativea Kanaguchi & Nishibe
TA100, TA1535, TA1537, 312.5, 625, 1250, (1990)
E. coil WP2 uvrA 2500, 5000 µg/plate
in DMSO
Host-mediated S. typhimurium G46 1000, 3000 mg/kg 97.3 Negative Shirasu et al. (1976)
mutation bw twice
Gene mutation B. subtills H17, M45 20, 100, 200, 500, 97.3 Negative Shirasu et al. (1976)
1000, 2000 µg,/disk
Gene mutation Chinese hamster V79 cells, 6.25, 12.5, 25.0, 50, 96.36 Negativea Tippins et al. (1984)
hprt locus 100 µg/ml in DMSOa
Chromosomal aberration Chinese hamster ovary cells 100, 200, 300, 400 95.0 Negative Murli (1988)
µg/ml in DMSOa
250, 500, 750,
1000 µg/ml in DMSOb
Unscheduled DNA Primary hepatocytes 5, 10, 25, 50, 100, 100 Negative Myhr (1981)
synthesis 250, 500, 1000 µg/ml
In vivo
Dominant lethal mutation ICR mice 8-500 mg/kg bw 94 Negative Makita et al. (1973)
intraperitoneally
Cytogenicity Wistar rat bone marrow 62.5-1000 mg/kg bw 94 Negative Makita et al. (1973)
and spermatocytes intraperitoneally
DMSO, dimethyl sulfoxide
a Without metabolic activation
b With metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Thiophanate-methyl (0.5 g; purity, 96.2%) moistened with water
was applied to the backs of six male New Zealand white rabbits for 4 h
under an occlusive patch. There were no signs of irritation over a
72-h observation period (Souma & Nishibe, 1986a).
Nine male New Zealand white rabbits received 0.1 g
thiophanate-methyl (purity, 96.2%) in the left eye. The treated eyes
of three rabbits were washed with water, and those of the others
remained unwashed. Conjunctival redness was observed in four of the
unirrigated eyes and in all three of the irrigated eyes after 1 h.
These symptoms had resolved by 24 h in all animals except one with an
unwashed eye, in which irritation resolved by 48 h. Thiophanate-methyl
was considered to be a very mild eye irritant (Souma & Nishibe,
1986b).
The skin sensitizing potential of thiophanate-methyl (purity,
96.2%) was tested in 20 female Hartley guinea-pigs by the
Magnusson-Kligman technique. Thiophanate-methyl was sensitizing under
the conditions of the study (Souma & Nishibe, 1989). In a Buehler
test, thiophanate-methyl (purity, 96.55%) was not sensitizing in 10
female Hartley guinea-pigs (Mochizuki, 1993).
(ii) Effects on the thyroid and liver
A number of studies were carried out to elucidate the effects of
thiophanate-methyl on the thyroid and liver (Nishibe & Takaori, 1990).
Thiophanate-methyl (purity, 96.55%) was administered in the diets
of six-week-old male Fischer 344 rats for two or eight days at a
concentration of 6000 ppm. Propylthiouracil, an inhibitor of thyroid
hormone synthesis, was administered at 1000 ppm for two or eight days,
and phenobarbital, an inducer of drug-metabolizing enzymes, was
administered at 500 ppm for eight days, as positive controls.
Thiophanate-methyl reduced triiodothyronine and thyroxine levels,
increased thyroid-stimulating hormone activity (at day 8 only),
increased thyroid weights (at day 8 only), and increased liver
weights. Propylthiouracil caused similar but more marked changes in
thyroid hormone levels and weights. Phenobarbital increased liver
weights. All three chemicals increased serum cholesterol levels by day
8. Thiophanate-methyl and phenobarbital increased microsomal
cytochrome P450, cytochrome b5, total protein, and UDP-glucu-
ronosyltransferase activities.
Seven-week-old female Fischer 344 rats were given 6000 ppm
thiophanate-methyl or 500 ppm phenobarbital for eight days. Some were
killed at eight days, and the others were killed after an eight-day
recovery. Thyroid weights were increased at day 8.
Groups of eight-week-old rats (sex not specified) were fed diets
containing 0 or 6000 ppm thiophanate-methyl, 30 µg/kg bw thyroxine, or
6000 ppm thiophanate-methyl and daily injections of 30 µg/kg thyroxine
subcutaneously, for eight days. Thiophanate-methyl caused hypertrophy
of the thyroid and liver and increased the level of thyroid-
stimulating hormone; these changes were suppressed by concomitant
administration of thyroxine. Thiophanate with and without thyroxine
also increased serum cholesterol. Thyroxine alone caused none of these
changes.
Thiophanate-methyl was less effective than propylthiouracil in
inhibiting porcine thyroid microsomal peroxidase. The ED50 and ED0
were 6 × 10-4 mol/litre and 8 × 10-5 mol/litre for thiophanate-
methyl and 2 × 10-5 mol/litre and 4 × 10-7 mol/litre for
propylthiouracil.
Eleven-week-old male ICR mice and Fischer 344 rats were given
diets containing 6000 ppm thiophanate-methyl or 500 ppm phenobarbital
for two or eight days. Liver weights were increased in all treated
animals. The titre of hepatocyte proliferating cell nuclear antigen,
the polymerase delta accessory protein, was increased in rats at day 2
and mice at days 2 and 8 (Nishibe & Takaori, 1990).
3. Observations in humans
In 1969-72, 16 production workers continuously exposed to
Topsin-E containing thiophanate or Topsin-M containing thiophanate-
methyl were routinely tested for effects on health. Blood and urine
samples were taken for analysis every six months: blood was analysed
for erythrocyte and leukocyte counts, haemoglobin content, and
specific gravity, and urine for protein, sugar, and urobilinogen.
Serum alanine and aspartate aminotransferases activities were
determined twice during the first six months of 1972 only. The initial
tests were conducted in January 1969, prior to production of Topsin.
No significant effects were seen in any of the workers for any of the
parameters investigated (Mori, 1972).
The same tests reported by Mori (1972) were conducted twice a
year on 28 production workers exposed to Topsin M and/or Topsin E in
1973-77, except that leukocyte and erythrocyte counts and haemoglobin
levels were not tested during 1976 for undescribed 'personal reasons'.
Serum alanine and aspartate aminotransferase activities were
determined in all employees in October 1977. More than 13 employees
were tested each year, but the specific individuals tested changed
during the study so that only seven individuals were monitored
continuously. No adverse behavioural, haematological, clinical
chemical, or urinary findings were made (Ikeda, 1978).
Between 1985 and 1991, no adverse behavioural symptoms (including
skin or eye effects) or clinical findings (haematology, clinical
chemistry, or urinalysis) were observed in workers exposed to
thiophanate-methyl and thiophanate during production. Health
examinations were performed twice a year. The workers were involved
mainly in the production of thiophanate-methyl, usually for 11 months
of the year (Aizawa, 1991).
Comments
Thiophanate-methyl was rapidly absorbed after oral exposure of
mice, rats, and dogs. Most of the administered radiolabel was
recovered within 24 h of dosing, predominantly in the urine.
Carbendazim and 5-hydroxycarbendazim were among the metabolites of
thiophanate-methyl identified in vivo and in vitro.
Thiophanate-methyl has low acute toxicity in rats, with an oral
LD50 of about 7000 mg/kg bw. Clinical signs of toxicity after acute
dosing with thiophanate-methyl were generally non-specific. WHO has
classified thiophanate-methyl as unlikely to present an acute hazard
in normal use.
In a six-month study of toxicity, mice were given dietary doses
of 0, 13, 64, 320, 1600, or 8000 ppm thiophanate-methyl. The NOAEL was
320 ppm, equivalent to 48 mg/kg bw per day, on the basis of
haematological effects and hepatotoxicity. In a six-month study, rats
were exposed to thiophanate-methyl in the diet at levels of 0, 13, 64,
320, 1600, or 8000 ppm. Reduced erythrocyte counts, reduced
body-weight gain, and evidence of hepatotoxicity and thyroid
stimulation were seen at 8000 ppm. The NOAEL was 1600 ppm, equivalent
to 80 mg/kg bw per day. Dogs were exposed to thiophanate-methyl by
administration in capsules at doses of 0, 50, 200, or 400 mg/kg bw per
day for 13 weeks, 0, 8, 40 or 200 mg/kg bw per day for one year, or 0,
2, 10, 50, or 250 mg/kg bw per day for two years. Treatment-related
thyroid hyperplasia was seen in all three studies; the overall NOAEL
was 10 mg/kg bw per day.
In a two-year study of carcinogenicity in mice fed dietary levels
of 0, 10, 40, 160, or 640 ppm, there was no evidence of any
carcinogenic response; the NOAEL was 160 ppm (equal to 24 mg/kg bw per
day) on the basis of reduced body-weight gain. In a subsequent
18-month study in mice fed dietary levels of 0, 150, 640, 3000, or
7000 ppm, the NOAEL was 150 ppm (equal to 29 mg/kg bw per day) on the
basis of treatment-related hepatotoxicity and hepatocellular tumours.
In a two-year study of carcinogenicity in rats fed dietary levels of
0, 10, 40,160, or 640 ppm, effects were seen on the testes (mainly
hypospermatogenesis), thyroid gland, and growth rate at 640 ppm; the
NOAEL was 160 ppm (equivalent to 8 mg/kg bw per day). In a two-year
study in rats fed dietary levels of 0, 75, 200, 1200, or 6000 ppm, the
NOAEL was 200 ppm (equal to 9 mg/kg bw per day). Thyroid hyperplasia
and thyroid tumours were seen at higher doses, together with
parathyroid hyperplasia, nephrotoxicity, hepatotoxicity, and lipidosis
of the adrenal cortex.
In a three-generation study of reproductive toxicity in which
rats received dietary doses of 0, 40, 160, or 640 ppm, reductions in
total litter size and weight at birth and at weaning were seen at
640 ppm; the NOAEL was 160 ppm, equivalent to 8 mg/kg bw per day. In a
two-generation study in which rats received dietary doses of 0, 200,
630 or 2000 ppm, there was no evidence of a treatment-related effect
on reproduction. The lowest dose tested in the study, equivalent to
10 mg/kg bw per day, was a minimal-effect level on the basis of
hepatocyte hypertrophy, thyroid follicular-cell hypertrophy, and
hyperplasia at all treatment levels.
The teratogenic potential of thiophanate-methyl was investigated
in mice, rats, and rabbits. In mice exposed by gavage to thiophanate-
methyl at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day on days
1-15 of gestation, there was no evidence of teratogenicity or maternal
toxicity at the highest dose, although embryo- and fetotoxicity were
seen at this dose. In rats exposed by gavage to thiophanate-methyl at
doses of 0, 100, 300, or 1000 mg/kg bw per day on days 6-19 of
gestation, there was no evidence of fetotoxicity or teratogenicity,
but maternal toxicity, expressed as reduced body-weight gain, was seen
at the highest dose. In rabbits exposed by gavage to thiophanate-
methyl at doses of 0, 2, 6, or 20 mg/kg bw per day on days 6-19 of
gestation, maternal toxicity (reduced growth rate) was seen at 6 mg/kg
bw per day and above. A dose-related trend of increased fetal skeletal
abnormalities (ribs, vertebrae, and pelvis) at 6 and 20 mg/kg bw per
day indicated an NOAEL for developmental toxicity of 2 mg/kg bw per
day.
Thiophanate-methyl was adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The only significant
response was a small increase in the frequency of micronuclei
characterized by their large size in mouse bone-marrow cells, which
was not associated with the induction of structural chromosomal
aberrations. This is indicative of a weak aneugenic effect. The
Meeting concluded that thiophanate-methyl is not genotoxic.
Studies on the effects of thiophanate-methyl on the liver and
thyroid of rats showed liver enzyme induction, reductions in thyroid
hormones (triiodothyronine and thyroxine), and increases in the level
of thyroid-stimulating hormone and in thyroid and liver weights.
Thyroid hypertrophy and increases in thyroid-stimulating hormone
levels associated with treatment with thiophanate-methyl were
suppressed by concomitant treatment with thyroxine. Thiophanate-methyl
was shown to inhibit porcine thyroid microsomal peroxidase, an enzyme
involved in thyroid hormone synthesis. The thyroidal and hepatic
changes, including tumours, observed in the studies of toxicity may be
due to increased hepatocyte turnover, reductions in the thyroid
hormones triiodothyronine and thyroxine as a result of liver enzyme
induction, and inhibition of thyroid microsomal peroxidase.
Workers involved in manufacturing products containing
thiophanate-methyl showed no treatment-related adverse local (skin or
eyes) or systemic effects.
An ADI of 0-0.02 mg/kg bw was established on the basis of the
NOAEL of 2 mg/kg bw per day for developmental toxicity in rabbits and
a safety factor of 100.
The Meeting noted that the use of thiophanate-methyl on crops
gives rise to residues of carbendazim, although thiophanate-methyl can
also be detected as part of the residue to which consumers of treated
produce are exposed. Since the toxicity of thiophanate-methyl is
qualitatively and quantitatively (when corrected for relative
molecular mass) different from that of carbendazim, the Meeting
concluded that the intake of residues in food should initially be
compared with the ADI for thiophanate-methyl. If further refinement of
the risk assessment is necessary, the different components of the
residue (carbendazim and thiophanate-methyl) may be characterized.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 29 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
1000 mg/kg bw per day (maternal toxicity and teratogenicity
in study of reproductive toxicity)
500 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
160 ppm, equivalent to 8 mg/kg bw per day (study of
reproductive toxicity)
1000 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
300 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 2 mg/kg bw per day (maternal toxicity and teratogenicity in
study of developmental toxicity)
Dog: 10 mg/kg bw per day (studies of toxicity up to two years)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Comparison of the metabolism of thiophanate-methyl and
carbendazim in different species, including humans
2. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to thiophanate-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 7000 mg/kg bw
Dermal, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test; not sensitizing
in Buehler test
Inhalation, toxicity, rat LC50 = 1.8 mg/litre air
Mid-term (1-26 weeks) Oral, reproductive toxicity, rabbit NOAEL = 2 mg/kg bw per day; maternal and
developmental toxicity
Long-term (> one year) Dietary, toxicity and carcinogenicity, NOAEL = 9 mg/kg bw per day; thyroid rumours
two years, rat and hepatotoxicity
Oral, toxicity, two years, dog NOAEL = 10 mg/kg bw per day; hepatotoxicity and
thyroid effects
References
Aizawa, T. (1991) Human handling experiences from plant employees
manufacturing Topsins (3). Unpublished report No. RD-9153 from
Nippon Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Auletta, CS. (1991) A subchronic (3-month) oral toxicity study in the
dog via capsule administration with thiophanate-methyl.
Unpublished report Project No. 89-3525 (RE-9119) from
Bio/dynamics Inc. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Auletta, C.S. (1992) A chronic (1-year) oral toxicity study in the dog
via capsule administration with thiophanate-methyl. Unpublished
report Project No. 89-3526 (RD-9207) from Bio/dynamics Inc.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Barale, R., Scapoli, C., Meli, C., Casini, D., Minunni, M.,
Marrazzini, A., Loprieno, N. & Barrai, I. (1993) Cytogenetic
effects of benzimidazoles in mouse bone marrow. Mutat. Res.,
300, 15-28.
Douch, P.G.C. (1974) The metabolism of some thioureidobenzene
fungicides in mice and sheep. Xenobiotica, 4, 457-475.
Fujino, A., Ohnuma, N., Mori, T., Tanoue, T. & Kamimura, H. (1973) The
balance and metabolism studies of thiophanate-methyl in animals.
Unpublished report from the Nisso Institute for Life Science,
Nippon Soda Co. Ltd, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Hashimoto, Y. (1972) Final report on chronic oral toxicity studies on
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis 3-thioal-
lophanate in Sprague-Dawley strain rats. Unpublished report from
the Nisso Institute for Life Science, Nippon Soda Co. Ltd,
Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Hashimoto, Y., Makita, T., Nishibe, T., Mori, T., Ohnuma, N., Noguchi,
T. & Ohta, G. (1973) Subacute toxicity of thiophanate-methyl in
mice and rats. Pharmacometrics, 7, 929-945.
Ikeda, K. (1978) Human handling experiences from plant employees
manufacturing Topsin (2). Unpublished report from Nippon Soda Co.
Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Kanaguchi, Y. & Nishibe, T. (1990) Thiophanate-methyl reverse mutation
study on bacteria. Unpublished report No. RD-9065 from Nippon
Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Kosaka, S. (1973) The report on the carcinogenesis studies of
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis-(3-thioal-
lophanate) in mice of ICR-SLC strain for 24 months. Unpublished
report from the Nisso Institute for Life Science, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co., Ltd,
Tokyo, Japan.
Makita, T., Hashimoto, Y. & Noguchi, T. (1973) Mutagenic, cytogenetic
and teratogenic studies on thiophanate-methyl. Toxicol. Appl.
Pharmacol., 24, 206-215.
Mochizuki, N. (1993) Topsin M skin sensitization study in guinea pigs.
Unpublished report No. RD-9347 from Nippon Soda Co. Ltd.
Submitted to WHO by Nippon Soda Co., Ltd, Tokyo, Japan.
Mori, H. (1972) Human handling experiences from plant employees
manufacturing Topsin (1). Unpublished report from the Fine
Chemical Division, Nippon Soda Co. Ltd. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Muller, W. (1993) Topsin M two generation oral (dietary
administration) reproduction toxicity study in the rat.
Unpublished report HLA Study No. 683-004 (RD-9329) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Muller, W. & Singer, A. (1995) Topsin M final addendum histopathology
report and peer review pathology report to MRID 42899101. Two
generation oral (dietary administration) reproduction toxicity
study in the rat. Unpublished report HLA Study No. 683-004
addendum histopathology report (RD-9525) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Murli, H. (1988) Mutagenicity test on Topsin M technical (thiophanate-
methyl) in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished report HLA Study No 10345-0-437 (RD-9120) from
Hazleton Laboratories America, Inc. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Myhr B.C. (1981) Primary rat hepatocyte unscheduled DNA synthesis
assay of thiophanate-methyl. Unpublished report No. LBI Project
No 21191 (RD-8195) from Litton Bionetics. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation toxicity study
in rats. Unpublished report No. RD-8711 from the Toxicology
Institute, Environmental Toxicology Laboratory, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Nishibe, T. & Takaori, H. (1990) Summary of mechanistic investigation
of the effect of thiophanate-methyl on thyroid and liver.
Unpublished report from the Toxicology Institute, Environmental
Toxicology Laboratory, Nippon Soda Co. Ltd, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970a) Toxicological evaluation of thiophanate-methyl
(V). Some pharmacological properties of a new fungicide,
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970b) Studies on the biotransformation of thiophanate-
methyl in animal and plant (Part 1). Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970c) Toxicological evaluation of thiophanate-methyl
(IV). Studies on the teratogenic effect of thiophanate-methyl
upon the fetus of ICR strain of mice. Unpublished report from the
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1971) Studies on the biotransformation of
thiophanate-methyl in animal, plant and soil (Part II).
Unpublished report from the Chemical Research Laboratories, Nisso
Institute for Life Sciences, Kanagawa-ken, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1972) Studies on the biotransformation of
thiophanate-methyl in animal and plant. Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970a) Toxicological evaluation of
thiophanate-methyl (I). Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa-ken, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate-methyl (II). Studies on the subchronic oral toxicity
of thiophanate-methyl in mice. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity
of thiophanate-methyl in rats. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Palmer, A., Lovell., M. & Newman, A. (1972) Effect of
thiophanate-methyl on reproductive function of multiple
generations in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Rodwell, D.E. et al (1981) Teratology study in thiophanate-methyl in
rats. Unpublished report No. 449-006 (RD-8126) from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Ross, F.W. et al. (1986) Thiophanate-methyl: Teratology study in the
rabbit. Unpublished report No. 86/NIS010/11 (RD-8642) from Life
Science Research. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Saike, O. & Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation
toxicity study in rats. Unpublished report No. 0219 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Shirasu, Y. et al. (1976) Mutagenicity testing on thiophanate-methyl
in microbial systems. Unpublished report No. RD-8084 from the
Institute of Environmental Toxicology. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Singh, T. & Garg, B. (1989) Effect of thiophanate-methyl on
cardiovascular system. Indian Vet. J., 66, 1116-1119.
Souma, S. & Nishibe, T. (1986a) Thiophanate-methyl primary dermal
irritation study in rabbits. Unpublished report No. RD-8692 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1986b) Thiophanate-methyl primary eye
irritation study in rabbits. Unpublished report No. RD-8691 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1989) Thiophanate-methyl: Delayed contact
hypersensitivity study in guinea pigs. Unpublished report No.
RD-8924 from Nisso Institute for Life Sciences, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990a) Thiophanate-methyl acute oral toxicity
study in rats. Unpublished report No. RD-9083 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990b) Thiophanate-methyl acute dermal
toxicity study in rabbits. Unpublished report No. RD-9084 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Takaori, H. (1993) Thiophanate-methyl combined chronic
toxicity/oncogenicity study in rats. Unpublished report
No. RD-9327 from Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Taniguchi, T. (1972) Final report on the long-term oral toxicity
studies of thiophanate-methyl, dimethyl 4,4'-O-phenylene-
bis(3-thioallophanate) in beagle-dogs for 24 months. Unpublished
report from the Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Thomas, J. (1974) Actions of pesticides and other drugs on the male
reproductive system (EPA-650/1-74-011), Washington DC, US
Environmental Protection Agency. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Thomas, J. & Schein, L. (1974) Effects of thiophanate and thiophanate-
methyl on the male reproductive system of the mouse. Toxicol.
Appl. Pharmacol., 30, 129-133.
Tippins, R.S. et al. (1984) Gene mutation in Chinese hamster V79
cells with thiophanate-methyl. Unpublished LSR-RTC Report
No. 063013-M-05184 (RD-84109) from Life Science Research.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Tompkins, E.C. (1992) Topsin-M (thiophanate-methyl) 18-month dietary
oncogenicity study in mice. Unpublished report No. WIL-75024
(RD-9328) from WIL Research Laboratory, Inc. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Table 3. Pharmacological effects of thiophanate-methyl
Parameter Species Dose and route Results
Body temperature Wistar rats 380-1500 mg/kg bw orally No effect
Analgesic activity Mice 500 mg/kg bw intraperitoneally No effect
Sedative or hypnotic activity Rats and mice 100-500 mg/kg bw subcutaneously No effect
Cardiovascular and respiratory Rabbits 20-100 mg/kg bw intravenously Reduced blood pressure
effects followed by bradycardia
Table 4. Acute toxicity of thiphanate-methyl
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male, female Orala 3400-3514 Noguchi & Hashimoto (1970a)
Mouse Male, female Intraperitoneal 792-1113 Noguchi & Hashimoto (1970a)
Mouse Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Mouse Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Oral 6640-7500 Noguchi & Hashimoto (1970a)
Rat Male, female Intraperitoneal 140-1640 Noguchi & Hashimoto (1970a)
Rat Male, female Subcutaneous > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rat Male, female Inhalation 1.7-1.9 Nishibe (1987)
Rat Male, female Oralb > 5000 Souma & Nishibe (1990a)
Guinea-pig Male, female Oral 3640-6700 Noguchi & Hashimoto (1970a)
Guinea-pig Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Oral 2270-2500 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermal > 10 000 Noguchi & Hashimoto (1970a)
Rabbit Male, female Dermalb > 2000 Souma & Nishibe (1990b)
a In gum arabic in 5% sodium chloride solution
b In distilled water
c Mortality rates: 0 at 350 mg/kg bw per day, 2 at 500 mg/kg bw per day, 1 at 650 mg/kg bw per day,
0 at 800 mg/kg bw per day, 3 at 1100 mg/kg bw per day, and 3 at 1400 mg/kg bw per day
(b) Short-term toxicity
Mice
Groups of 12 ICR mice of each sex were fed diets containing
thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or 8000 ppm for
six months. The homogeneity, stability, and concentrations of the diet
were not reported; clinical signs of toxicity were also not reported.
There were no treatment-related deaths. The body-weight gain of
animals of each sex at 8000 ppm was significantly reduced. Erythrocyte
counts were also reduced in these animals and in males at 1600 ppm,
and haematocrit values were reduced in animals of each sex at 1600 and
8000 ppm. No significant differences were seen in the weights of the
cerebrum, heart, lung, spleen, kidney, or testis, but in animals at
8000 ppm, liver weights were increased. Histopathological
investigation revealed a higher incidence of hepatic-cell irregularity
at the higher doses, with five cases in males and three in females at
8000 ppm and one case in an animal of each sex at 1600 ppm. Large
swollen hepatic cells, some with oedematous or granular protoplasm,
were found to a greater extent at 8000 ppm, especially in the central
lobules. Constriction of veins by the swollen cells was proposed to
explain the cloudy appearance of the liver. Sporadic formation of
acidiphilic bodies from hepatic-cell degeneration was recorded in two
females and one male at 8000 ppm and one male at 1600 ppm. The NOAEL
was 320 ppm, equivalent to approximately 48 mg/kg bw per day, on the
basis of hepatotoxic and haematological effects at 1600 and 8000 ppm
(Noguchi & Hashimoto, 1970b; Hashimoto et al., 1973).
Rats
Groups of 12 Sprague Dawley rats of each sex were fed diets
containing thiophanate-methyl at doses of 0, 12.8, 64, 320, 1600, or
8000 ppm for six months. The homogeneity, stability, and
concentrations of the diet were not reported, and no clinical signs of
toxicity were reported. There were no treatment-related deaths. The
body-weight gain of animals of each sex at 8000 ppm was significantly
reduced, but food consumption did not differ significantly between
groups. Reduced erythrocyte counts were seen in animals of each sex at
8000 ppm. Urinalysis did not show significant treatment-related
differences. Blood cholesterol levels were raised significantly in
animals of each sex at 8000 ppm. The thyroid anti liver weights were
increased in these animals, and the weight of the thymus was increased
in females. Some histopathological effects on the thyroid were
reported in animals at this dose: four animals of each sex had small
thyroid follicles, four males and four females had thickened
follicular epithelium, and five animals of each sex had decreased
colloidal substance. There were no treatment-related effects on
protein-bound iodine levels. The NOAEL was 1600 ppm, equivalent to
approximately 80 mg/kg bw per day, mainly on the basis of effects on
thyroid, liver, and body weights (Noguchi & Hashimoto, 1970c;
Hashimoto et al., 1973).
Dogs
Groups of five beagle dogs of each sex (four at the highest dose)
were fed capsules containing thiophanate-methyl (purity unspecified)
at doses of 0, 2, 10, 50, or 250 mg/kg bw per day for two years. One
animal of each sex at each dose was killed and autopsied after 12
months. There were no deaths or adverse symptoms. Animals of each sex
at 250 mg/kg bw per day had reduced body weights and body-weight gain.
Gross pathological effects were not reported, and no effects on the
eyes or heart were found in periodic ophthalmoscopic and electro-
cardiographic investigations. There were no treatment-related changes
in clinical chemical, haematological, or urinary values. After 24
months, the weight of the thyroid gland was increased in animals of
each sex at 250 mg/kg bw per day and to a lesser extent at 50 mg/kg bw
per day. The effects on thyroid tissue at 12 months included altered
or decreased colloidal substance and slightly taller cuboid follicular
epithelial cells at 250 mg/kg bw per day. The NOAEL was 10 mg/kg bw
per day on the basis of effects on thyroid weight in animals of each
sex at 50 and 250 mg/kg bw per day (Taniguchi, 1972).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) for three months at
doses of 0, 50, 200, or 800 mg/kg bw per day, the last dose being
lowered to 400 mg/kg bw per day on test day 50 because of severe
toxicity. One male at the high dose was sacrificed on day 41 because
of severe toxicity; one male at 50 mg/kg bw per day died on day 36,
but this death did not appear to be related to treatment. Dose-related
clinical signs seen in animals at the high dose and to a lesser extent
at the middle dose included dehydration, thinness, and lethargy.
Dose-related decreases in body weights and marked decreases in food
consumption were seen at the middle and high doses. There were no
treatment-related ophthalmological findings. Slight anaemia, increased
platelet counts and cholesterol levels, and decreases in serum alanine
aminotransferase activity and albumin levels were seen at the middle
and high doses and increased activated partial thromboplastin time at
the high dose. Thyroid function tests revealed slightly decreased
triiodothyronine levels in males at the high dose and decreased
triiodothyronine and thyroxine levels in females at the middle and
high doses; no clear effects on thyroid-stimulating hormone values
were apparent. Urinalysis showed no treatment-related findings. At the
middle and high doses, the weights of the liver (in animals of each
sex) and thyroid (males only) were increased. Gross examination
post mortem revealed emaciation in one male at the middle dose and
three at the high dose. Dose-related histological alterations were
seen in a number of organs. In thyroids, hypertrophy of the follicular
epithelium was found in one, three, and four males and one, two, and
four females at the low, middle, and high doses, respectively, with
none in controls. The severity of the hypertrophy increased from
minimal to marked with dose. Hyperplasia of the follicular epithelium
was found in one male at the middle dose and in most animals at the
high dose. In two males at the high dose in which both hypertrophy and
hyperplasia were marked, a large decrease in the quantity of
intrafollicular colloid was seen. In animals at the middle and high
doses, dose-related changes were found in the liver (reduction in
vesiculation of the hepatocellular cytoplasm), gall-bladder
(intracytoplasmic vacuoles), pancreas (atrophy of acinar cells due to
a decreased quantity of zymogen granules), spleen (lymphoid-cell
depletion), thymus (involution), prostate (atrophy), uterus
(anoestrus), and ovaries (inactive). There was no NOAEL because of the
presence of follicular-cell hypertrophy in the thyroid of two dogs at
the low dose (Auletta, 1991).
Groups of four male and four female beagle dogs were fed capsules
containing thiophanate-methyl (purity, 96.55%) at doses of 0, 8, 40,
or 200 mg/kg bw per day for one year. There were no deaths. Tremors
were seen in all dogs at the high dose 2-4 h after treatment on one or
more occasions during the initial three weeks of the study but not
subsequently. The tremors were slight to moderate, except in one
animal which had severe tremors that progressed to apparent tonic
convulsions on three occasions. The body-weight gains of animals at
the high dose were reduced during the study, and body-weight losses
were noted in the first week of treatment; slight reductions in
body-weight gain were noted at 40 mg/kg bw per day. The food
consumption of dogs at the high dose was generally lower than that of
controls during the early months, but was close to the control value
thereafter. Ophthalmological examinations and urinalyses showed no
treatment-related effects. Haematological effects consisted of slight
decreases in total erythrocyte counts and haemoglobin and haematocrit
values in males at the high dose. These animals also had decreased
serum alanine aminotransferase activity, increased alkaline
phosphatase activity, increased cholesterol levels (also at the middle
dose), and decreased albumin:globulin ratios (also at the middle
dose), calcium (also at the middle dose), potassium, and phosphorus
levels. In females at 200 mg/kg bw per day, serum alanine
aminotransferase activity was decreased, and alkaline phosphatase
activity and cholesterol levels were increased. Thyroid function tests
revealed decreased thyroxine levels in males at the middle and high
doses but no clear effects on triiodothyronine or thyroid-stimulating
hormone. Abnormalities seen post mortem included increased liver
weights in dogs at the high dose and increased thyroid weights in
those at the middle and high doses. Microscopic alterations attributed
to treatment were limited to minimal to moderate hypertrophy and
slight hyperplasia of the follicular epithelium of the thyroid in the
dogs at the middle dose (hypertrophy in two females) and the high dose
(hypertrophy in four males and three females, and hyperplasia in one
male and one female). The NOAEL was 8 mg/kg bw per day (Auletta,
1992).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing 0,
10, 40, 160, or 640 ppm thiophanate-methyl (purity, > 94%) for two
years. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. The body-weight
gain of males at 640 ppm was reduced during the study. No
treatment-related effects on food consumption or on mortality or overt
signs of toxicity were seen. By 24 months, 74-94% of treated animals
had died, but the mean survival times were comparable. Organ weights
and clinical chemical and haematological parameters were not
investigated. No treatment-related gross macroscopic or histopatho-
logical alterations were seen, and there was no evidence of
carcinogenicity. The NOAEL was 160 ppm, equivalent to about 24 mg/kg
bw per day, on the basis of the reduction in body-weight gain in males
at 640 ppm (Kosaka, 1973).
Groups of 60 male and 60 female CD-1 mice were fed diets
containing 0, 150, 640, 3000, or 7000 ppm thiophanate-methyl (purity,
95.9-96.6%) for 18 months. Ten animals of each sex in each group were
necropsied after 39 weeks of treatment, and surviving animals were
necropsied after 78-79 weeks of treatment; all mice that died or were
killed in extremis were also autopsied. The homogeneity, stability,
and concentrations of thiophanate-methyl were acceptable. Haemato-
logical and thyroid function were assessed at the interim necropsy and
before the end of the study. No treatment-related clinical signs were
observed. The cumulative numbers of deaths up to 18 months of -
treatment were 10, 11, 14, 16, and 24 males and 12, 13, 15, 17, and 23
females at 0, 150, 640, 3000, and 7000 ppm, respectively; animals at
7000 ppm had a significantly higher mortality rate than controls. The
increased rates in females at 3000 ppm and males and females at
7000 ppm appeared to be related to amyloid deposition in tissues.
Body-weight gains were slightly reduced in animals of each sex at
3000 ppm and to a greater degree at 7000 ppm; food consumption was
slightly reduced in females at 3000 and 7000 ppm. There was no
treatment-related effect on ophthalmologic parameters or on the onset
or number of palpable masses. The only haematological parameter that
appeared to be altered by treatment was a slightly lower erythrocyte
count in males at 7000 ppm. At the nine-month interim necropsy,
enlarged thyroid glands were seen grossly in three of 10 males at
7000 ppm, the thyroid weights of males and females at 3000 and
7000 ppm were increased, and the thyroid-stimulating hormone levels
were higher and the thyroxine levels lower in males at 3000 ppm and
males and females at 7000 ppm than in controls. The differences in
thyroid weights and thyroid hormone levels were not seen at 18 months.
At the nine-month interim necropsy, the liver weights of males and
females at 3000 and 7000 ppm were significantly increased as a result
of an increased incidence and severity of hepatocellular centrilobular
hypertrophy at these two doses; the incidence of hepatocellular
centrilobular hypertrophy was also increased in females at 640 ppm,
five out of 10 having minimal hypertrophy. At 18 months, the mean
liver weights of animals at 7000 ppm were increased as a result of a
significantly increased number of liver masses, which was also
significantly increased at 3000 ppm. At the 18-month terminal
necropsy, an increased incidence of hepatocellular centrilobular
hypertrophy was seen in males at 3000 and 7000 ppm. Histopathological
examination revealed that the liver masses seen grossly were adenomas.
The incidence of hepatocellular adenomas was significantly increased
in males and females at 3000 and 7000 ppm. The slightly increased
incidence of hepatocellular adenomas in females at 640 ppm (8.6%) was
greater than the historical control range (0-2.7%). Two hepatocellular
carcinomas were seen, one in a male at 640 ppm and the other in a male
at 7000 ppm; this 2% incidence was similar to the mean incidence for
historical control males (1.4%; range, 0-6%). One male at 7000 ppm had
a hepatoblastoma, which is a relatively rare tumour (0.001% in
historical controls). The incidence of atrial thrombosis was increased
in females at 3000 ppm and in animals of each sex at 7000 ppm. The
NOAEL was 150 ppm, equivalent to 29 mg/kg bw per day, in view of the
possible treatment-related incidence of hepatocellular adenomas in
females at 640 ppm (Tompkins, 1992).
Rats
Groups of 35 Sprague-Dawley rats of each sex (50 of each sex for
controls) were fed diets containing thiophanate-methyl (purity, >
94%) at doses of 0, 10, 40, 160, or 640 ppm for two years. Five rats
of each sex at each dose were killed and autopsied at three and 12
months. The homogeneity, stability, and concentrations of
thiophanate-methyl in the diets were not reported. There were no
treatment-related deaths, although survival was poor in some groups,
and there were no overt signs of toxicity. Body-weight gain was
reduced in animals of each sex at 640 ppm, but no significant
difference in food consumption was observed. No significant treatment-
related effects were seen on clinical chemical, haematological, or
urinary values. No significant adverse histopathological effects or
changes in organ weights were seen at any dose at three or 12 months.
Necropsy of animals killed after 24 months or which died during
treatment showed treatment-related histopathological findings in the
testes and thyroid gland. Males at 640 ppm had an increased incidence
of decreased colloidal substance and hypertrophy of follicular
epithelium in thyroid tissue (6/35 compared to 1/50 in controls). Five
animals at 640 ppm were found to be hypospermatogenic; one died at
week 91 with a pituitary tumour and was found to have atrophied
testes. Another male had diminished spermatogenesis at 24 months, and
another surviving male and one that died during treatment had
hyperplasia of the testicular interstitium. No other treatment-related
effects were seen. There was no evidence for carcinogenicity. The
NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per day, on the
basis of effects on the testes and thyroid gland and reduced growth at
640 ppm (Hashimoto, 1972).
Groups of 60 male and 60 female Fischer 344 rats were fed diets
containing 0, 75, 200, 1200, or 6000 ppm thiophanate-methyl (purity,
96.55%) for 24 months. The homogeneity, stability, and concentrations
of thiophanate-methyl in the diets were acceptable. After 12 months,
10 rats of each sex in each group (only five males at 6000 ppm) were
sacrificed. All survivors were sacrificed at the end of treatment. The
mortality rates are shown in Table 5. Only two males at 6000 ppm
survived to the end of the study; because of this high rate, no
statistical analysis was performed for this group at 24 months. The
main causes of death among these rats were nephropathy (22 rats),
thyroid follicular-cell tumour (10 rats), and leukaemia (six rats);
eight males at this dose died or were killed in extremis during
weeks 11-12 due to fractures of the nasal bone caused by the feeder
plate and subsequent dyspnoea (rhinorrhagia). The feeder plates were
removed.
No clinical signs attributable to treatment were noted in any
group during the first 52 weeks. After week 52, dose-related clinical
signs included pale skin and mucous membranes in males at 6000 ppm,
alopecia in females at 6000 ppm, and tissue masses on the skin and in
the subcutis including the lip in males at 1200 and 6000 ppm. These
changes attained statistical significance at week 77 or later.
Dose-related, statistically significant depressions in body-weight
gain were noted in both males and females at 1200 and 6000 ppm.
Decreased food consumption was seen in animals of each sex at 6000 ppm
starting at week 76. No dose-related abnormalities were observed in
ophthalmoscopic parameters at 6, 12, 18, or 24 months. Treatment-
related signs of anaemia (decreased erythrocyte count, haemoglobin,
haematocrit, mean corpuscular volume, mean corpuscular haemoglobin,
and mean corpuscular haemoglobin concentration) were seen in animals
of each sex (mainly males) at 6000 ppm at 3, 6, 12, and 18 months.
Increased platelet and leukocyte counts were seen frequently in males
at 6000 ppm. Clinical chemical investigations revealed increased total
cholesterol and total protein and decreased albumin:globulin ratios in
animals of each sex at 1200 and/or 6000 ppm. At 24 months, treated
males had increased blood urea nitrogen (at 75, 200, and 1200 ppm) and
creatinine levels (at 1200 ppm), reduced thyroxine and triiod-
othyronine values, and increased thyroid-stimulating hormone values
(at 1200 and 6000 ppm, also in females at 6000 ppm). Dose-related
increases in urinary protein (semi-quantitative analysis) were noted
in animals of each sex at 6000 ppm at six and/or 12 months.
Table 5. Mortality rates in Fischer rats treated with thiophanate-methyl
Dose Male Female
(ppm)
Week 52 Week 80 Week 104 Week 52 Week 80 Week 104
No. % No. % No. % No. % No. % No. %
0 0/60 0 2/50 4 13/50 26 0/60 0 3/50 6 13/50 26
75 0/60 0 2/50 4 15/50 30 0/60 0 1/50 2 12/50 24
200 0/60 0 8/50 16 24/50 48* 0/60 0 1/50 2 8/50 16
1200 0/60 0 3/50 6 21/50 42 0/60 0 0/50 0 12/50 24
6000 8/60 13* 18/55 33** 53/55 96** 1/60 2 3/50 6 11/50 22
Significantly different from control at *P < 0.05; ** P < 0.001 (chi-square test)
Nephelometry indicated an increase in urinary protein in males at 1200
and 6000 ppm at various times. Although males at 200 ppm had a
statistically significant increase in urinary protein at 24 months,
histopathological examination revealed no treatment-related effect. In
males at 6000 ppm, ketone bodies, urinary volume, and water
consumption were increased, and pH and specific gravity were decreased
significantly at various times. Dose-related increases in the weights
of the liver, kidney, and thyroid and their body-weight ratios were
seen in animals of each sex at 1200 and/or 6000 ppm at both interim
and final sacrifice.
At interim sacrifice (week 53), treatment-related changes were
found macroscopically in the liver (brownish-black) and kidneys
(granular surface, brownish-black) of males and females at 6000 ppm.
At final sacrifice, males at 1200 ppm had granular kidneys. Females at
6000 ppm also had enlarged thyroids. In animals at 6000 ppm that died
during the study, swollen thyroids were seen in animals of each sex
and white areas in the liver and granular kidney in males. Dose-
related histopathological changes were found in the thyroid, liver,
kidney, and adrenal: Thyroid follicular-cell hyperplasia and
hypertrophy were noted in animals of each sex at 1200 and/or 6000 ppm
at 12 and 24 months, and the incidence of focal follicular-cell
hyperplasia was increased. Males at 6000 ppm also had a statistically
significant increased incidence of thyroid follicular-cell adenomas
(12/60), and one of two males at 6000 ppm that were killed at 24
months and 2/53 that were dead or were killed in extremis had
thyroid follicular-cell adenocarcinomas, with none in the other groups
of treated males. The incidence of retinal atrophy was increased in
females at 6000 ppm at 24 months, and those of parathyroid hypertrophy
and hyperplasia were increased in males at this dose. Centrilobular
hepatocellular hypertrophy and lipofuscin deposition were noted in
animals of each sex at 1200 and/or 6000 ppm at 12 and 24 months and in
most males and females at 6000 ppm which died during the study.
Microgranuloma and focal fatty degeneration in the liver were
increased in incidence in males treated at 6000 ppm. Lipidosis of the
adrenal cortex was noted in females at 1200 ppm and in animals of each
sex at 6000 ppm at 12 months. The severity of nephropathy was
increased in animals of each sex at 6000 ppm at 12 months and in those
at 1200 and 6000 ppm at 24 months. Most males at 6000 ppm that died or
were killed in extremis had severe nephropathy associated with
hyperplasia of the parathyroid, demineralization of the bone, and
metastatic calcification in various organs. The NOAEL was 200 ppm,
equivalent to 8.8 mg/kg bw per day in males and 10.2 mg/kg bw per day
in females. The increased blood urea nitrogen levels observed in males
at 75 and 200 ppm and the increased urinary protein levels in males at
200 ppm at 24 months were not considered to be adverse effects in view
of the absence of treatment-related pathological findings at these
doses (Takaori, 1993).
(d) Reproductive toxicity
Mice
Groups of eight male Swiss-Webster mice were given thiophanate-
methyl (purity unspecified) as an oral dose of 192 mg/kg bw per day
for five consecutive days. Twenty-four hours after the final dose
[1,2-3H]-testosterone was administered intraperitoneally at
10 µCi/kg bw in order to assess its assimilation by the anterior
prostate coagulating glands. Animals were killed after 5 min, and the
anterior prostate glands were removed. Thiophanate-methyl did not
significantly affect body weights, absolute testicular weights,
absolute or relative seminal vesicular weights, or total radiolabel
assimilation by the anterior prostate gland. The absolute prostate and
adrenal gland weights were significantly increased, and these changes
were considered by the authors to be due to stress induced by
thiophanate-methyl. The relative weights of the testes, prostate, and
adrenal were also increased. Spermatogenesis was not affected, no
sterile tubules were seen, and the interstitial (Leydig) cells
appeared to be normal (Thomas, 1974; Thomas & Schein, 1974).
Rats
In a three-generation study, groups of 10 male and 20 female
weaning Sprague-Dawley rats fed diets containing 0, 40 160, or 640 ppm
thiophanate-methyl (purity unspecified) were mated twice to produce
the F1a and F1b litters. Ten male and 20 female offspring were
selected from the F1b litters and mated to produce the F2a and
F2b litters; 10 male and 20 female F2b offspring were then
selected and mated to produce the F3a and F3b litters. The test
diets were fed throughout the study, animals in the F0, F1b, and
F2b generations being fed for 60 days before mating. In each case,
mating lasted for 20 days. Animals were re-mated to produce the second
litters about 10 days after weaning of the first litters. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were not reported. In F0 animals, no treatment-related
effects on mortality, food consumption, or body-weight gain or
clinical signs of toxicity were seen. No treatment-related effects
were observed on mating performance, length of gestation, or pregnancy
rate or at terminal autopsy in F0, F1b, or F2b rats. The total
litter sizes and weights at birth were slightly reduced in all animals
at 640 ppm except the F3a litter. Viable litter size and weight at
weaning were reduced in all generations at 640 ppm; mean pup weight
was not affected. Viable litter size and weight between birth and
weaning were slightly reduced in the second F2b litter at all doses,
the percentage losses increasing with dose; however, average pup
weights were not affected. The total litter loss and the incidence of
gross malformations of pups were not treatment-related. No
dose-related effects were seen on macroscopic appearance, organ
weight, histopathological parameters, or the skeleton in F3b
offspring. The NOAEL was 160 ppm, equivalent to about 8 mg/kg bw per
day, on the basis of effects on litters at 640 ppm (Palmer et al.,
1972).
In a two-generation study, groups of 25 male and 25 female
Sprague Dawley rats were given diets containing thiophanate-methyl
(purity, 95.93%) at doses of 0, 200, 630, or 2000 ppm. The
homogeneity, stability, and concentrations of thiophanate-methyl in
the diets were acceptable. After 14 weeks of treatment, the parental
(F0) animals were mated for up to 21 days. The F0 females were
allowed to litter and to rear their offspring (F1a generation) to
weaning. After a 14-week maturation period after weaning, the parental
F1 animals, selected from the F1a offspring, were mated for up to
21 days and the females were allowed to litter and rear their
offspring (F2a generation) to weaning. After weaning of the F2a
pups and review of the results of rearing and weaning, including high
pup mortality in all groups, the F1 generation was mated again, six
weeks after weaning of the surviving F2a pups. The F1 animals were
allowed to mate for a maximum of 21 days with the same partner, as
during the first mating, and to produce a second litter (F2b). The
body-weight gain of males of the F0 generation and males and females
of the F1 generation at 2000 ppm was reduced, but only the
body-weight gain of male F1 animals at 630 ppm was affected. Food
consumption was affected by treatment before the first mating and
during the first and second gestations and first lactation only in
F1 females at 2000 ppm. At this dose, the males and females of the
F0 and F1 generations had elevated levels of thyroid-stimulating
hormone; slightly reduced triiodothyronine levels were noted in F0
females at necropsy; and males and females of the F0 generation had
lower thyroxine values at weeks 1 and 8 but not at necropsy. These
findings are consistent with the histological findings and changes in
organ weights found at this dose. Thyroid and liver weights were
increased in male and female animals of the F0 and F1 generations
at the high dose, and histopathological examinations of these animals
showed treatment-related centrilobular hepatocyte hypertrophy in the
liver and follicular-cell hypertrophy and hyperplasia (except in F1
females) in the thyroid. The weights of male and female F1a, male
F2a, and male and female F2b pups were reduced at 2000 ppm; at
630 ppm, only the weights of male and female F2b pups were reduced.
Very high pup losses, mainly after day 4 of lactation, occurred in all
animals of the F2a generation, including the controls. No clear
explanation was found for the pup losses. Those pups that did not die
had normal postnatal development, except for reduced body-weight gain
in male pups at the high dose. Such losses were not seen in the F2b
groups. Functional tests of F1a, F2a, and F2b animals showed no
treatment-related findings. The NOAEL was 630 ppm for male and female
F0 animals, 200 ppm for the F1 generation, 630 ppm for the F1a
and F2a offspring, and 200 ppm for the F2b offspring. The NOAEL
was 2000 ppm for fertility and general reproductive performance
(Muller, 1993).
Since treatment-related histopathological effects were found in
the thyroid and liver in rats at the high dose in the preceding study,
organs from all F0 and F1 animals at 200 and 630 ppm were further
evaluated. The incidence and severity of hepatocyte hypertrophy were
increased in a dose-related fashion at all doses in F0 males, and
the incidence and to a lesser extent the severity of thyroid
follicular-cell hypertrophy and hyperplasia were increased in the same
animals. F1 males also had increased (but to a lesser extent)
hepatocyte hypertrophy and thyroid follicular cell hyperplasia at all
doses. The minimal effect level was thus 200 ppm, equivalent to
9.7-14.7 mg/kg bw per day (Muller & Singer, 1995).
(e) Developmental toxicity
Mice
Groups of 20 female ICR mice were given thiophanate-methyl
(purity, 94%) at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day by
gavage on days 1-15 of gestation. Animals were killed on day 18 or 19
(discrepancy between the study report and publication). No deaths,
abnormalities, or significant differences in body weights were
recorded. The number of living fetuses per litter was reduced in
animals at 1000 mg/kg bw per day, partly as a result of increased
resorptions. No treatment-related effects were observed on
implantation numbers, litter sizes, fetal body weights, sex ratio, or
numbers of immature or malformed fetuses. The NOAEL was 1000 mg/kg bw
per day for maternal toxicity and 500 mg/kg bw per day for embryo- and
fetotoxicity, on the basis of the reduced number of live fetuses at
1000 mg/kg bw per day. There was no evidence of teratogenicity
(Noguchi, 1970c; Makita et al., 1973).
Rats
Groups of 25 female Sprague-Dawley rats were given
thiophanate-methyl (purity, 97.2%) at doses of 0, 100, 300, or
1000 mg/kg bw per day by gavage on days 6-19 of gestation. Animals
were killed on day 20 of gestation. There were five to nine nongravid
females per group, including the control group. One rat at 100 mg/kg
bw per day and one at 300 mg/kg bw per day delivered on days 13 and
11, respectively, and were killed on the day of delivery. The weight
and development of the offspring were comparable to those of animals
delivered later, suggesting incorrect assessment of copulation; no
malformations or maternal abnormalities were seen in these animals. No
deaths, treatment-related clinical symptoms, or gross pathological
abnormalities were reported in any dams. Body-weight gain was reduced
between days 6 and 9 in animals at 1000 mg/kg bw per day. There were
no treatment-related effects on total implantations, post-implantation
loss, fetal body weight, fetal sex ratio, or viable fetuses. There
were no dose-related fetal malformations or developmental
abnormalities, and the incidences of these parameters were within
those of historical controls. A positive control study in the same
strain with a single high dose of vitamin A produced the appropriate
results. The NOAEL was 300 mg/kg bw per day for maternal toxicity, on
the basis of the reductions in body-weight gain of dams at 1000 mg/kg
bw per day during treatment. There was no evidence of teratogenicity
or embryo- or fetotoxicity (Rodwell et al., 1981).
Rabbits
Groups of 15 female New Zealand white rabbits received
thiophanate-methyl (purity, 96.2%) at doses of 0, 2, 6, or 20 mg/kg bw
per day by gavage on days 6-19 of gestation. Samples of test solutions
were taken during the first and last weeks of treatment, and the
concentrations of thiophanate-methyl were found to be acceptable. The
animals were killed on day 29 of gestation. No treatment-related
maternal mortality or clinical or subsequent maternal effects were
seen. Dose-related losses in maternal body weight were observed mainly
at the beginning of treatment (days 6-8 at 6 mg/kg bw per day and days
6-14 at 20 mg/kg bw per day). The food consumption of animals at
20 mg/kg bw per day was reduced from the start of treatment, and large
reductions during days 6-12 were generally associated with subsequent
abortion or sacrifice in extremis. Water consumption was unaffected.
One of 12 animals at 2 mg/kg bw per day, one of 14 at 6 mg/kg bw per
day, and two of 13 at 0 mg/kg bw per day aborted. One control animal
and one at 20 mg/kg bw per day suffered total litter loss and
resorption; the control animal suffered early resorption of its only
implantation, while the treated animal lost five of five implantations.
No treatment-related trends were observed in resorptions, pre-
implantation losses, or fetal or placental weights. The numbers of
viable young were slightly decreased in animals at 20 mg/kg bw per day
due to abortions and total litter losses. Gross examination of the
fetuses showed that in animals at 6 and 20 mg/kg bw per day, there was
an apparent treatment-related trend in skeletal abnormalities in ribs,
vertebrae, and pelvis that was generally close to or slightly greater
than the upper limit of historical controls. Treatment-related effects
included increased incidences of 13 pairs of ribs, incomplete or
asymmetric ossification of costal elements of the sacral vertebrae, 27
presacral vertebrae, and asymmetric pelvises associated with different
sacral vertebrae. At 20 mg/kg bw per day, the incidences of one or
more ribs thickened at the costal cartilage were significantly
increased. The frequencies of these skeletal abnormalities are
summarized in Table 6. The NOAEL was 2 mg/kg bw per day for maternal
toxicity, on the basis of the effect on maternal growth rate, and
2 mg/kg bw per day for developmental toxicity, on the basis of the
increased incidence of skeletal abnormalities at 6 and 20 mg/kg bw per
day (Ross et al., 1986).
Table 6. Incidence of selected skeletal abnormalities in fetuses of rabbits
treated with thiophanate-methyl
Observation Dose (mg/kg bw per day) Historical controls
0 2 6 20 Mean Range
13 pairs of ribs 41 46 63 61 36 12-61
Thickened ribs 1 4 2 14 2 0-13
Incomplete or assymetrical 3 6 7 12 4 0-15
ossification
27 presacral vertebrae 16 18 38 43 18 7-44
Assymetrical pelvis 3 5 7 10 4 0-9
(f) Genotoxicity
The results of studies of the genotoxicity and mutagenicity of
thiophanate-methyl in vitro and in vivo are summarized in Table 7.
There was no evidence of genotoxicity or mutagenicity. Thiophanate-
methyl is partially metabolized in mammalian systems to carbendazim,
which has been shown to act as an aneugen.
The aneugenic potential of thiophanate-methyl (purity, 95%;
dispersed in 70% kaolin) was tested in male Swiss albino mice given
oral doses of 1000 mg/kg bw. Bone-marrow cells were analysed 16, 24,
36, and 48 h after treatment for micronuclei, chromosomal aberrations,
hyperdiploidy, and polyploidy. Large micronuclei were significantly
induced, but the response was relatively weak, and thiophanate-methyl
was less effective than benomyl or carbendazim. There was no increase
in the frequency of chromosomal aberrations. At 24 and 36 h, a
possibly treatment-related increase in the frequency of polyploid and
hyperdiploid cells was observed, which was of borderline significance
in view of the very low frequency of changes in ploidy (Barale
et al., 1993).
Table 7. Results of tests for the genttoxicity of thiophanate-methyl
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation S. typhimurium TA98, 10, 50, 100, 97.3 Negativea Shirasu et al. (1976)
TA100, TA1535, TA1537, 500, 1000 µg/plate
TA1538, E. coli WP2 hcr dissolved in DMSO
Reverse mutation S. typhimurium TA98, 39.1, 78.1, 156.3, 96.55 Negativea Kanaguchi & Nishibe
TA100, TA1535, TA1537, 312.5, 625, 1250, (1990)
E. coil WP2 uvrA 2500, 5000 µg/plate
in DMSO
Host-mediated S. typhimurium G46 1000, 3000 mg/kg 97.3 Negative Shirasu et al. (1976)
mutation bw twice
Gene mutation B. subtills H17, M45 20, 100, 200, 500, 97.3 Negative Shirasu et al. (1976)
1000, 2000 µg,/disk
Gene mutation Chinese hamster V79 cells, 6.25, 12.5, 25.0, 50, 96.36 Negativea Tippins et al. (1984)
hprt locus 100 µg/ml in DMSOa
Chromosomal aberration Chinese hamster ovary cells 100, 200, 300, 400 95.0 Negative Murli (1988)
µg/ml in DMSOa
250, 500, 750,
1000 µg/ml in DMSOb
Unscheduled DNA Primary hepatocytes 5, 10, 25, 50, 100, 100 Negative Myhr (1981)
synthesis 250, 500, 1000 µg/ml
In vivo
Dominant lethal mutation ICR mice 8-500 mg/kg bw 94 Negative Makita et al. (1973)
intraperitoneally
Cytogenicity Wistar rat bone marrow 62.5-1000 mg/kg bw 94 Negative Makita et al. (1973)
and spermatocytes intraperitoneally
DMSO, dimethyl sulfoxide
a Without metabolic activation
b With metabolic activation
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Thiophanate-methyl (0.5 g; purity, 96.2%) moistened with water
was applied to the backs of six male New Zealand white rabbits for 4 h
under an occlusive patch. There were no signs of irritation over a
72-h observation period (Souma & Nishibe, 1986a).
Nine male New Zealand white rabbits received 0.1 g
thiophanate-methyl (purity, 96.2%) in the left eye. The treated eyes
of three rabbits were washed with water, and those of the others
remained unwashed. Conjunctival redness was observed in four of the
unirrigated eyes and in all three of the irrigated eyes after 1 h.
These symptoms had resolved by 24 h in all animals except one with an
unwashed eye, in which irritation resolved by 48 h. Thiophanate-methyl
was considered to be a very mild eye irritant (Souma & Nishibe,
1986b).
The skin sensitizing potential of thiophanate-methyl (purity,
96.2%) was tested in 20 female Hartley guinea-pigs by the
Magnusson-Kligman technique. Thiophanate-methyl was sensitizing under
the conditions of the study (Souma & Nishibe, 1989). In a Buehler
test, thiophanate-methyl (purity, 96.55%) was not sensitizing in 10
female Hartley guinea-pigs (Mochizuki, 1993).
(ii) Effects on the thyroid and liver
A number of studies were carried out to elucidate the effects of
thiophanate-methyl on the thyroid and liver (Nishibe & Takaori, 1990).
Thiophanate-methyl (purity, 96.55%) was administered in the diets
of six-week-old male Fischer 344 rats for two or eight days at a
concentration of 6000 ppm. Propylthiouracil, an inhibitor of thyroid
hormone synthesis, was administered at 1000 ppm for two or eight days,
and phenobarbital, an inducer of drug-metabolizing enzymes, was
administered at 500 ppm for eight days, as positive controls.
Thiophanate-methyl reduced triiodothyronine and thyroxine levels,
increased thyroid-stimulating hormone activity (at day 8 only),
increased thyroid weights (at day 8 only), and increased liver
weights. Propylthiouracil caused similar but more marked changes in
thyroid hormone levels and weights. Phenobarbital increased liver
weights. All three chemicals increased serum cholesterol levels by day
8. Thiophanate-methyl and phenobarbital increased microsomal
cytochrome P450, cytochrome b5, total protein, and UDP-glucu-
ronosyltransferase activities.
Seven-week-old female Fischer 344 rats were given 6000 ppm
thiophanate-methyl or 500 ppm phenobarbital for eight days. Some were
killed at eight days, and the others were killed after an eight-day
recovery. Thyroid weights were increased at day 8.
Groups of eight-week-old rats (sex not specified) were fed diets
containing 0 or 6000 ppm thiophanate-methyl, 30 µg/kg bw thyroxine, or
6000 ppm thiophanate-methyl and daily injections of 30 µg/kg thyroxine
subcutaneously, for eight days. Thiophanate-methyl caused hypertrophy
of the thyroid and liver and increased the level of thyroid-
stimulating hormone; these changes were suppressed by concomitant
administration of thyroxine. Thiophanate with and without thyroxine
also increased serum cholesterol. Thyroxine alone caused none of these
changes.
Thiophanate-methyl was less effective than propylthiouracil in
inhibiting porcine thyroid microsomal peroxidase. The ED50 and ED0
were 6 × 10-4 mol/litre and 8 × 10-5 mol/litre for thiophanate-
methyl and 2 × 10-5 mol/litre and 4 × 10-7 mol/litre for
propylthiouracil.
Eleven-week-old male ICR mice and Fischer 344 rats were given
diets containing 6000 ppm thiophanate-methyl or 500 ppm phenobarbital
for two or eight days. Liver weights were increased in all treated
animals. The titre of hepatocyte proliferating cell nuclear antigen,
the polymerase delta accessory protein, was increased in rats at day 2
and mice at days 2 and 8 (Nishibe & Takaori, 1990).
3. Observations in humans
In 1969-72, 16 production workers continuously exposed to
Topsin-E containing thiophanate or Topsin-M containing thiophanate-
methyl were routinely tested for effects on health. Blood and urine
samples were taken for analysis every six months: blood was analysed
for erythrocyte and leukocyte counts, haemoglobin content, and
specific gravity, and urine for protein, sugar, and urobilinogen.
Serum alanine and aspartate aminotransferases activities were
determined twice during the first six months of 1972 only. The initial
tests were conducted in January 1969, prior to production of Topsin.
No significant effects were seen in any of the workers for any of the
parameters investigated (Mori, 1972).
The same tests reported by Mori (1972) were conducted twice a
year on 28 production workers exposed to Topsin M and/or Topsin E in
1973-77, except that leukocyte and erythrocyte counts and haemoglobin
levels were not tested during 1976 for undescribed 'personal reasons'.
Serum alanine and aspartate aminotransferase activities were
determined in all employees in October 1977. More than 13 employees
were tested each year, but the specific individuals tested changed
during the study so that only seven individuals were monitored
continuously. No adverse behavioural, haematological, clinical
chemical, or urinary findings were made (Ikeda, 1978).
Between 1985 and 1991, no adverse behavioural symptoms (including
skin or eye effects) or clinical findings (haematology, clinical
chemistry, or urinalysis) were observed in workers exposed to
thiophanate-methyl and thiophanate during production. Health
examinations were performed twice a year. The workers were involved
mainly in the production of thiophanate-methyl, usually for 11 months
of the year (Aizawa, 1991).
Comments
Thiophanate-methyl was rapidly absorbed after oral exposure of
mice, rats, and dogs. Most of the administered radiolabel was
recovered within 24 h of dosing, predominantly in the urine.
Carbendazim and 5-hydroxycarbendazim were among the metabolites of
thiophanate-methyl identified in vivo and in vitro.
Thiophanate-methyl has low acute toxicity in rats, with an oral
LD50 of about 7000 mg/kg bw. Clinical signs of toxicity after acute
dosing with thiophanate-methyl were generally non-specific. WHO has
classified thiophanate-methyl as unlikely to present an acute hazard
in normal use.
In a six-month study of toxicity, mice were given dietary doses
of 0, 13, 64, 320, 1600, or 8000 ppm thiophanate-methyl. The NOAEL was
320 ppm, equivalent to 48 mg/kg bw per day, on the basis of
haematological effects and hepatotoxicity. In a six-month study, rats
were exposed to thiophanate-methyl in the diet at levels of 0, 13, 64,
320, 1600, or 8000 ppm. Reduced erythrocyte counts, reduced
body-weight gain, and evidence of hepatotoxicity and thyroid
stimulation were seen at 8000 ppm. The NOAEL was 1600 ppm, equivalent
to 80 mg/kg bw per day. Dogs were exposed to thiophanate-methyl by
administration in capsules at doses of 0, 50, 200, or 400 mg/kg bw per
day for 13 weeks, 0, 8, 40 or 200 mg/kg bw per day for one year, or 0,
2, 10, 50, or 250 mg/kg bw per day for two years. Treatment-related
thyroid hyperplasia was seen in all three studies; the overall NOAEL
was 10 mg/kg bw per day.
In a two-year study of carcinogenicity in mice fed dietary levels
of 0, 10, 40, 160, or 640 ppm, there was no evidence of any
carcinogenic response; the NOAEL was 160 ppm (equal to 24 mg/kg bw per
day) on the basis of reduced body-weight gain. In a subsequent
18-month study in mice fed dietary levels of 0, 150, 640, 3000, or
7000 ppm, the NOAEL was 150 ppm (equal to 29 mg/kg bw per day) on the
basis of treatment-related hepatotoxicity and hepatocellular tumours.
In a two-year study of carcinogenicity in rats fed dietary levels of
0, 10, 40,160, or 640 ppm, effects were seen on the testes (mainly
hypospermatogenesis), thyroid gland, and growth rate at 640 ppm; the
NOAEL was 160 ppm (equivalent to 8 mg/kg bw per day). In a two-year
study in rats fed dietary levels of 0, 75, 200, 1200, or 6000 ppm, the
NOAEL was 200 ppm (equal to 9 mg/kg bw per day). Thyroid hyperplasia
and thyroid tumours were seen at higher doses, together with
parathyroid hyperplasia, nephrotoxicity, hepatotoxicity, and lipidosis
of the adrenal cortex.
In a three-generation study of reproductive toxicity in which
rats received dietary doses of 0, 40, 160, or 640 ppm, reductions in
total litter size and weight at birth and at weaning were seen at
640 ppm; the NOAEL was 160 ppm, equivalent to 8 mg/kg bw per day. In a
two-generation study in which rats received dietary doses of 0, 200,
630 or 2000 ppm, there was no evidence of a treatment-related effect
on reproduction. The lowest dose tested in the study, equivalent to
10 mg/kg bw per day, was a minimal-effect level on the basis of
hepatocyte hypertrophy, thyroid follicular-cell hypertrophy, and
hyperplasia at all treatment levels.
The teratogenic potential of thiophanate-methyl was investigated
in mice, rats, and rabbits. In mice exposed by gavage to thiophanate-
methyl at doses of 0, 40, 200, 500, or 1000 mg/kg bw per day on days
1-15 of gestation, there was no evidence of teratogenicity or maternal
toxicity at the highest dose, although embryo- and fetotoxicity were
seen at this dose. In rats exposed by gavage to thiophanate-methyl at
doses of 0, 100, 300, or 1000 mg/kg bw per day on days 6-19 of
gestation, there was no evidence of fetotoxicity or teratogenicity,
but maternal toxicity, expressed as reduced body-weight gain, was seen
at the highest dose. In rabbits exposed by gavage to thiophanate-
methyl at doses of 0, 2, 6, or 20 mg/kg bw per day on days 6-19 of
gestation, maternal toxicity (reduced growth rate) was seen at 6 mg/kg
bw per day and above. A dose-related trend of increased fetal skeletal
abnormalities (ribs, vertebrae, and pelvis) at 6 and 20 mg/kg bw per
day indicated an NOAEL for developmental toxicity of 2 mg/kg bw per
day.
Thiophanate-methyl was adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The only significant
response was a small increase in the frequency of micronuclei
characterized by their large size in mouse bone-marrow cells, which
was not associated with the induction of structural chromosomal
aberrations. This is indicative of a weak aneugenic effect. The
Meeting concluded that thiophanate-methyl is not genotoxic.
Studies on the effects of thiophanate-methyl on the liver and
thyroid of rats showed liver enzyme induction, reductions in thyroid
hormones (triiodothyronine and thyroxine), and increases in the level
of thyroid-stimulating hormone and in thyroid and liver weights.
Thyroid hypertrophy and increases in thyroid-stimulating hormone
levels associated with treatment with thiophanate-methyl were
suppressed by concomitant treatment with thyroxine. Thiophanate-methyl
was shown to inhibit porcine thyroid microsomal peroxidase, an enzyme
involved in thyroid hormone synthesis. The thyroidal and hepatic
changes, including tumours, observed in the studies of toxicity may be
due to increased hepatocyte turnover, reductions in the thyroid
hormones triiodothyronine and thyroxine as a result of liver enzyme
induction, and inhibition of thyroid microsomal peroxidase.
Workers involved in manufacturing products containing
thiophanate-methyl showed no treatment-related adverse local (skin or
eyes) or systemic effects.
An ADI of 0-0.02 mg/kg bw was established on the basis of the
NOAEL of 2 mg/kg bw per day for developmental toxicity in rabbits and
a safety factor of 100.
The Meeting noted that the use of thiophanate-methyl on crops
gives rise to residues of carbendazim, although thiophanate-methyl can
also be detected as part of the residue to which consumers of treated
produce are exposed. Since the toxicity of thiophanate-methyl is
qualitatively and quantitatively (when corrected for relative
molecular mass) different from that of carbendazim, the Meeting
concluded that the intake of residues in food should initially be
compared with the ADI for thiophanate-methyl. If further refinement of
the risk assessment is necessary, the different components of the
residue (carbendazim and thiophanate-methyl) may be characterized.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 150 ppm, equal to 29 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
1000 mg/kg bw per day (maternal toxicity and teratogenicity
in study of reproductive toxicity)
500 mg/kg bw per day (fetotoxicity in study of developmental
toxicity)
Rat: 200 ppm, equal to 9 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
160 ppm, equivalent to 8 mg/kg bw per day (study of
reproductive toxicity)
1000 mg/kg bw per day (teratogenicity and fetotoxicity in
study of developmental toxicity)
300 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Rabbit: 2 mg/kg bw per day (maternal toxicity and teratogenicity in
study of developmental toxicity)
Dog: 10 mg/kg bw per day (studies of toxicity up to two years)
Estimate of acceptable daily intake for humans
0-0.02 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Comparison of the metabolism of thiophanate-methyl and
carbendazim in different species, including humans
2. Further observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to thiophanate-methyl
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, toxicity, rat LD50 = 7000 mg/kg bw
Dermal, toxicity, rat LD50 > 10 000 mg/kg bw
Dermal, irritation, rabbit Not irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Sensitizing in maximization test; not sensitizing
in Buehler test
Inhalation, toxicity, rat LC50 = 1.8 mg/litre air
Mid-term (1-26 weeks) Oral, reproductive toxicity, rabbit NOAEL = 2 mg/kg bw per day; maternal and
developmental toxicity
Long-term (> one year) Dietary, toxicity and carcinogenicity, NOAEL = 9 mg/kg bw per day; thyroid rumours
two years, rat and hepatotoxicity
Oral, toxicity, two years, dog NOAEL = 10 mg/kg bw per day; hepatotoxicity and
thyroid effects
References
Aizawa, T. (1991) Human handling experiences from plant employees
manufacturing Topsins (3). Unpublished report No. RD-9153 from
Nippon Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Auletta, CS. (1991) A subchronic (3-month) oral toxicity study in the
dog via capsule administration with thiophanate-methyl.
Unpublished report Project No. 89-3525 (RE-9119) from
Bio/dynamics Inc. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Auletta, C.S. (1992) A chronic (1-year) oral toxicity study in the dog
via capsule administration with thiophanate-methyl. Unpublished
report Project No. 89-3526 (RD-9207) from Bio/dynamics Inc.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Barale, R., Scapoli, C., Meli, C., Casini, D., Minunni, M.,
Marrazzini, A., Loprieno, N. & Barrai, I. (1993) Cytogenetic
effects of benzimidazoles in mouse bone marrow. Mutat. Res.,
300, 15-28.
Douch, P.G.C. (1974) The metabolism of some thioureidobenzene
fungicides in mice and sheep. Xenobiotica, 4, 457-475.
Fujino, A., Ohnuma, N., Mori, T., Tanoue, T. & Kamimura, H. (1973) The
balance and metabolism studies of thiophanate-methyl in animals.
Unpublished report from the Nisso Institute for Life Science,
Nippon Soda Co. Ltd, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Hashimoto, Y. (1972) Final report on chronic oral toxicity studies on
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis 3-thioal-
lophanate in Sprague-Dawley strain rats. Unpublished report from
the Nisso Institute for Life Science, Nippon Soda Co. Ltd,
Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Hashimoto, Y., Makita, T., Nishibe, T., Mori, T., Ohnuma, N., Noguchi,
T. & Ohta, G. (1973) Subacute toxicity of thiophanate-methyl in
mice and rats. Pharmacometrics, 7, 929-945.
Ikeda, K. (1978) Human handling experiences from plant employees
manufacturing Topsin (2). Unpublished report from Nippon Soda Co.
Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Kanaguchi, Y. & Nishibe, T. (1990) Thiophanate-methyl reverse mutation
study on bacteria. Unpublished report No. RD-9065 from Nippon
Soda Co. Ltd. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Kosaka, S. (1973) The report on the carcinogenesis studies of
thiophanate-methyl, dimethyl 4,4'-O-phenylene bis-(3-thioal-
lophanate) in mice of ICR-SLC strain for 24 months. Unpublished
report from the Nisso Institute for Life Science, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co., Ltd,
Tokyo, Japan.
Makita, T., Hashimoto, Y. & Noguchi, T. (1973) Mutagenic, cytogenetic
and teratogenic studies on thiophanate-methyl. Toxicol. Appl.
Pharmacol., 24, 206-215.
Mochizuki, N. (1993) Topsin M skin sensitization study in guinea pigs.
Unpublished report No. RD-9347 from Nippon Soda Co. Ltd.
Submitted to WHO by Nippon Soda Co., Ltd, Tokyo, Japan.
Mori, H. (1972) Human handling experiences from plant employees
manufacturing Topsin (1). Unpublished report from the Fine
Chemical Division, Nippon Soda Co. Ltd. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Muller, W. (1993) Topsin M two generation oral (dietary
administration) reproduction toxicity study in the rat.
Unpublished report HLA Study No. 683-004 (RD-9329) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Muller, W. & Singer, A. (1995) Topsin M final addendum histopathology
report and peer review pathology report to MRID 42899101. Two
generation oral (dietary administration) reproduction toxicity
study in the rat. Unpublished report HLA Study No. 683-004
addendum histopathology report (RD-9525) from Hazleton
Deutschland GmbH. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Murli, H. (1988) Mutagenicity test on Topsin M technical (thiophanate-
methyl) in an in-vitro cytogenetic assay measuring chromosomal
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished report HLA Study No 10345-0-437 (RD-9120) from
Hazleton Laboratories America, Inc. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Myhr B.C. (1981) Primary rat hepatocyte unscheduled DNA synthesis
assay of thiophanate-methyl. Unpublished report No. LBI Project
No 21191 (RD-8195) from Litton Bionetics. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation toxicity study
in rats. Unpublished report No. RD-8711 from the Toxicology
Institute, Environmental Toxicology Laboratory, Nippon Soda Co.
Ltd, Kanagawa, Japan. Submitted to WHO by Nippon Soda Co. Ltd,
Tokyo, Japan.
Nishibe, T. & Takaori, H. (1990) Summary of mechanistic investigation
of the effect of thiophanate-methyl on thyroid and liver.
Unpublished report from the Toxicology Institute, Environmental
Toxicology Laboratory, Nippon Soda Co. Ltd, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970a) Toxicological evaluation of thiophanate-methyl
(V). Some pharmacological properties of a new fungicide,
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa, Japan. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970b) Studies on the biotransformation of thiophanate-
methyl in animal and plant (Part 1). Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. (1970c) Toxicological evaluation of thiophanate-methyl
(IV). Studies on the teratogenic effect of thiophanate-methyl
upon the fetus of ICR strain of mice. Unpublished report from the
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1971) Studies on the biotransformation of
thiophanate-methyl in animal, plant and soil (Part II).
Unpublished report from the Chemical Research Laboratories, Nisso
Institute for Life Sciences, Kanagawa-ken, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. (1972) Studies on the biotransformation of
thiophanate-methyl in animal and plant. Unpublished report from
the Chemical Research Laboratories, Nisso Institute for Life
Sciences, Kanagawa-ken, Japan. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970a) Toxicological evaluation of
thiophanate-methyl (I). Acute and subacute toxicity of
thiophanate-methyl. Unpublished report from the Nisso Institute
for Life Sciences, Kanagawa-ken, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970b) Toxicological evaluation of
thiophanate-methyl (II). Studies on the subchronic oral toxicity
of thiophanate-methyl in mice. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Noguchi, T. & Hashimoto, Y. (1970c) Toxicological evaluation of
thiophanate-methyl (III). Studies on the subchronic oral toxicity
of thiophanate-methyl in rats. Unpublished report from the Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Palmer, A., Lovell., M. & Newman, A. (1972) Effect of
thiophanate-methyl on reproductive function of multiple
generations in the rat. Unpublished report from Huntingdon
Research Centre, Huntingdon, Cambs, United Kingdom. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Rodwell, D.E. et al (1981) Teratology study in thiophanate-methyl in
rats. Unpublished report No. 449-006 (RD-8126) from International
Research and Development Corporation, Mattawan, Michigan, USA.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Ross, F.W. et al. (1986) Thiophanate-methyl: Teratology study in the
rabbit. Unpublished report No. 86/NIS010/11 (RD-8642) from Life
Science Research. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo,
Japan.
Saike, O. & Nishibe, T. (1987) Thiophanate-methyl: Acute inhalation
toxicity study in rats. Unpublished report No. 0219 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Shirasu, Y. et al. (1976) Mutagenicity testing on thiophanate-methyl
in microbial systems. Unpublished report No. RD-8084 from the
Institute of Environmental Toxicology. Submitted to WHO by Nippon
Soda Co. Ltd, Tokyo, Japan.
Singh, T. & Garg, B. (1989) Effect of thiophanate-methyl on
cardiovascular system. Indian Vet. J., 66, 1116-1119.
Souma, S. & Nishibe, T. (1986a) Thiophanate-methyl primary dermal
irritation study in rabbits. Unpublished report No. RD-8692 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1986b) Thiophanate-methyl primary eye
irritation study in rabbits. Unpublished report No. RD-8691 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1989) Thiophanate-methyl: Delayed contact
hypersensitivity study in guinea pigs. Unpublished report No.
RD-8924 from Nisso Institute for Life Sciences, Kanagawa, Japan.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990a) Thiophanate-methyl acute oral toxicity
study in rats. Unpublished report No. RD-9083 from Nisso
Institute for Life Sciences, Kanagawa, Japan. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
Souma, S. & Nishibe, T. (1990b) Thiophanate-methyl acute dermal
toxicity study in rabbits. Unpublished report No. RD-9084 from
Nisso Institute for Life Sciences, Kanagawa, Japan. Submitted to
WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Takaori, H. (1993) Thiophanate-methyl combined chronic
toxicity/oncogenicity study in rats. Unpublished report
No. RD-9327 from Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Taniguchi, T. (1972) Final report on the long-term oral toxicity
studies of thiophanate-methyl, dimethyl 4,4'-O-phenylene-
bis(3-thioallophanate) in beagle-dogs for 24 months. Unpublished
report from the Nisso Institute for Life Sciences, Kanagawa,
Japan. Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Thomas, J. (1974) Actions of pesticides and other drugs on the male
reproductive system (EPA-650/1-74-011), Washington DC, US
Environmental Protection Agency. Submitted to WHO by Nippon Soda
Co. Ltd, Tokyo, Japan.
Thomas, J. & Schein, L. (1974) Effects of thiophanate and thiophanate-
methyl on the male reproductive system of the mouse. Toxicol.
Appl. Pharmacol., 30, 129-133.
Tippins, R.S. et al. (1984) Gene mutation in Chinese hamster V79
cells with thiophanate-methyl. Unpublished LSR-RTC Report
No. 063013-M-05184 (RD-84109) from Life Science Research.
Submitted to WHO by Nippon Soda Co. Ltd, Tokyo, Japan.
Tompkins, E.C. (1992) Topsin-M (thiophanate-methyl) 18-month dietary
oncogenicity study in mice. Unpublished report No. WIL-75024
(RD-9328) from WIL Research Laboratory, Inc. Submitted to WHO by
Nippon Soda Co. Ltd, Tokyo, Japan.
