
AMITROLE JMPR 1974
IDENTITY
Chemical name
3-amino-1,2,4-triazole; 3-amino-S-triazole.
Synonyms
Aminotriazole, ATA, AT, 3-AT, Weedazol(R)
Structural formula
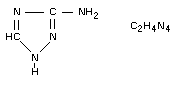 Other information on identity and properties
Amitrole is a white, crystalline solid with a molecular weight of
84 and a melting point of 150-153°C. It is soluble in water to the
extent of 28 g/100 g and in ethanol and methanol to an extent of
26 g/100 g. It is sparingly soluble in ethyl acetate and insoluble in
ether, acetone and most other organic solvents. Amitrole forms neutral
aqueous solutions but acts as a weak base. When exposed to UV light,
amitrole breaks down to form CO2, urea and cyanamide. Potts (1961)
has reviewed the chemistry of s-triazoles in considerable detail.
Amitrole behaves chemically as a typical aromatic amine.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
The metabolic fate of amitrole in animals has not yet been fully
elucidated. It is rapidly excreted from the body. Following
intraperitoneal administration, at least 90% of the injected dose was
observed in the urine unchanged within 24 hours (Bagdon et al., 1965).
When amitrole (5-14C) was fed to rats (Fang et al., 1964),
70-96% of the radioactivity was excreted in the urine during the first
24 hours as amitrole and two unidentified metabolites. There were
traces of 14C in the expired air and the faeces contained a small but
variable amount of activity. After absorption, amitrole was
distributed throughout most of the tissues. The maximum radioactivity
in all tissues was generally reached within one hour and started to
decrease 3-4 hours after dosing. Elimination of amitrole from all
tissues was rapid. The liver contained a metabolite but no free
amitrole. The rate of elimination of this metabolite from liver and
kidney was much slower than that of the parent compound.
Further studies on rats (Fang et al., 1966) have shown that
amitrole is not metabolically acetylated, and that the average
half-time for amitrole clearance in various tissues was 4.2 hours.
These studies were extended to include the metabolism of two
metabolites isolated from bean plants. One was readily excreated in
the urine, mainly unchanged but partly as two new metabolites. The
other bean metabolite was excreted much more slowly, apparently
unchanged.
Biotransformations in plants and soil are discussed in the
section "Fate of residues".
Effects on enzymes and other biochemical parameters
Acute administration of amitrole to rats results in depression of
catalase and peroxidase enzyme activity as well as the activity of
several other enzymes. Liver peroxidase was found to recover within 24
hours, while liver and kidney catalase depression was slower to
recover. Catalase returned to normal after seven days (Heim et al.,
1956). Insect-derived catalase was also shown to regenerate its
activity slowly following exposure to aminotriazole (Samio et al.,
1972).
Groups of rats (15 males per group) were administered radioactive
iodine alone or in combination with 0.15 or 0.78 mg amitrole
administered by intraperitoneal injection. Uptake of radioactivity by
the thyroid gland was significantly depressed at 0.78 mg/kg. The lower
dose reduced iodine uptake but the reduction was not statistically
significant. Amitrole significantly decreased fecal radioactivity,
while the urinary radioactivity was not significantly affected
(Fregly, 1968).
Amitrole inhibits peroxidase activity of rat thyroid and salivary
gland (Alexander, 1959a and b); liver and kidney catalase activity of
rats (Tephly et al., 1961; Heim et al., 1956); catalase activity of
human red blood cell (Margoliash and Novogrodsky, 1958; Magos et al.,
1974; Kudsk, 1969) catalase activity from other sources such as plant
tissue and commercial crystallized enzymes; the synthesis of
tryptophan peroxidase of rats (Auerbach et al., 1959);
delta-aminolevulinic acid dehydrase activity of mouse and rat liver
(Baron and Tephley, 1969; Tschudy and Collins, 1957); the inductive
effect of phenobarbital on hepatic microsomal cytochrome P-450 (Baron
and Tephly, 1969); the incorporation of iron (59FeCl3) into rat
hepatic microsomes (Tephly et al., 1971); microsomal heme synthesis
(Tephly et al., 1971); and hydroxylation of exogenous substrates by
the liver (Raisfeld et al., 1970). Amitrole caused no proliferation of
the microsomal endoplasmic reticulum (Raisfeld et al., 1970). Several
further studies have shown the effect of amitrole on certain aspects
of the drug metabolizing enzyme system in rat liver (Feytmans and
Leighton, 1973; Lotikar et al., 1973; Stenger and Johnson, 1972;
Levine, 1973; Matsushima and Weisburgh, 1972; Langhans and Shimassek,
1974). There is no clear picture of the effect of amitrole on
microsomal metabolizing enzyme systems in mammals although it does
serve to inhibit or reduce several specific biochemical reactions.
Kudsk (1969) observed that peroxide generating systems, such as
seen with methylene blue, accelerated the uptake of mercury in vitro
by human red blood cell preparations. Amitrole and methylene blue
which promoted the catalase-peroxide-complex I, had no effect on
mercury uptake. Magos (1974) suggested that the ability of red blood
cells to take up mercury from air saturated with mercury vapor was
reduced when cells were treated for three hours with amitrole and
methylene blue. In hemolysates mercury uptake was stimulated by
amitrole. In vivo administration of amitrole to rats resulted in
decreased lung concentration and increased liver concentrations of
mercury.
Tryptophan synthesis in plants was shown to be inhibited by
amitrole (Smith and Chang, 1973). Amitrole stimulated respiration in
Azotobacter and concurrently inhibited growth possibility through an
uncoupling of oxidative phosphorylation (Kretschmar and Günther,
1970). In algae, amino acid synthesis was inhibited and glucosamine
synthesis was stimulated (Schroeder, 1970).
In vitro, amitrole does not inhibit cholinesterase activity of
brain, submaxillary gland, serum, or ilium. Three hours after
intraperitoneal administration of 4 mg/kg to male rats cholinesterase
activity of brain, submaxillary gland, and serum was normal (Bagdon et
al., 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A testing procedure using groups of hybrid strains of mice (18
mice of each sex and each of two strains) evaluated amitrole for
carcinogenicity. The mice were administered amitrole orally from day 7
to day 28 of age at doses of 1000 mg/kg and thereafter in the diet at
a dose of 2192 ppm. None of the mice survived the 18 month test
interval. Hepatomas were evident in most animals, 67/72, and carcinoma
of the thyroid was reported in 67/71 of the animals examined (Innes et
al., 1969). A further study was designated as subcutaneous although
no details were given of the experimental design (Innes, 1966). The
majority of animals survived the 18 month test interval. There was no
evidence of heptoma or carcinoma of the thyroid.
Groups of mice (50 of each sex) were either treated with amitrole
by a single subcutaneous administration of 10 mg per mouse or by a
weekly dermal application of 0.1 mg/mouse. There was no abnormal
behaviour observed. When the animals were sacrificed and examined, no
signs of cancer were evident. No mention was made of examination of
the thyroid gland in this study (Hodge et al., 1966).
Rat
Amitrole was found to have an effect on the induction of liver
carcinogenesis by 4-dimethylaminoazobenzene. In an attempt to
determine the relationship between liver catalase activity and
carcinogenesis, Hoshino (1960) administered amitrole intraperitoneally
to albino rats at a dose of 1000 mg/kg every other day for 150 days
while feeding 4-dimethylaminoazobenzene at a dose that was known to
induce liver carcinoma. There was a reduction in the occurrence of
liver tumours in those animals administered amitrole (4/14) when
compared to the animals administered the carcinogen alone (12/16).
Napalkov (1962; 1969) administered amitrole to rats by
subcutaneous injection twice weekly (later reduced to a weekly
injection) at a dose of 125 mg/animal; in the water at a dose of 20-25
mg/day; in the diet at a dose of 250-500 mg/day; or by subcutaneous
implantation combined with subcutaneous administration twice weekly at
a dose of 125 mg/rat. Thyroid hyperplasia was observed after seven to
eight months. Liver tumours were observed initially in those animals
receiving amitrole in the diet and soon thereafter in those animals
receiving it by injection or via the drinking water. Sarcomas
developed where amitrole was implanted or injected. Concurrent daily
injections of 2.5 µg/100 g bw thyroxine and oral administration of 300
mg/day/rat amitrole in food for the length of the experiment resulted
in the development of only one thyroid adenoma but 9 liver tumors in
12 males. Amitrole alone induced thyroid tumors in 7/22 and liver
tumors in 12/23 male rats of the same age. No liver tumors but 2
thyroid cystic adenomas were observed in 51 control animals.
A comprehensive review of the carcinogenic risk of amitrole was
made by the International Agency for Research on Cancer commenting on
the animal and human data (IARC, 1974).
Special studies on mutagenicity
Mohandas and Grant (1972) observed that amitrole significantly
increased the frequency of chromosomal aberrations in root tips of
higher plants.
Mutagenic tests using Salmonella strains and yeast cells as
test indicators have been negative. A host mediated assay has been
reported to be negative although details of the test are not yet
available (Weir, 1974). A cytogenetic study, utilizing examination of
bone marrow cells arrested in C-metaphase from rats treated with
amitrole at levels of 0, 2.5, 25 and 250 mg/kg daily for 5 days, was
negative (Fabrizio, 1973).
Special studies on reproduction
Rat
Groups of rats (10 male and 10 female Sherman strain rats per
group) were fed amitrole in the diet at levels of 0, 25, 100, 500 and
1000 ppm for approximately two months and mated. In the first
generation at 500 and 1000 ppm the number of pups born and pup
survival was reduced. It was noted that almost all of the pups died
within one week after weaning and these dietary levels were
terminated. Dietary levels of 0, 25, and 100 ppm were fed through two
generations (two litters from the first generation and one litter from
the second generation). At 100 ppm in the diet there was no effect on
reproduction and all pups survived. At 100 ppm all animals were found
to have thyroid hyperplasia while the incidence of thyroid hyperplasia
was sporadic at 25 ppm (Gaines et al., 1973).
Oral administration of amitrole at doses of 400 and 1000 mg/kg to
rats on days 8 through 13 of gestation resulted in no signs of embryo
toxicity or of teratogenic effects. Groups of rats (5 males and 5
females per group) were administered amitrole orally at 100 mg/kg or
at 100 ppm in the drinking water for three months prior to mating and
in the females up to day 15 of gestation. There was no indication of
any effect on reproduction in either male or female rats (Hapke,
1967).
The effect of oral administration of amitrole on reproduction was
studied in three groups of eight pregnant Sherman strain rats
administered dosage levels of 0, 20 and 100 mg/kg bw/day from day 7
through 15 of gestation. There was no effect of amitrole on
reproduction and no abnormalities were observed in the offspring
through weaning (Gaines et al., 1973).
Special studies on teratogenicity
Amitrole injected into the yolk of chicken eggs at doses of 20-40
mg/egg, produced a dose dependent malformation of the beak and
abnormalities of the tibia shaft. This latter abnormality occurred
less frequently. At doses of 2 mg/egg, no effects ware noted. It was
observed that when amitrole was dissolved in DMSO it was more active
than when it was administered in water (Landauer et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of amitrole
LD50
Species Route (mg/kg bw) References
Rat oral >25 000 Bagdon et al., 1965
dermal > 2 500 Gaines et al., 1973
ip > 4 000 Bagdon et al., 1965
Mouse oral >14 700 Fogleman, 1954
iv > 1 600 Bagnon et al., 1965
Rabbit dermal >10 000 Elsea, 1954
oral > 2 150 Fogleman, 1954
Dog iv > 1 800 Fogleman, 1954
Cat iv > 1 750 Bagdon et al., 1965
Signs of poisoning include: depression, dyspnea, diarrhoea,
ataxia, altered respiration, coma and death. The G.I. tract was
severely irritated following acute doses. Acute doses administered to
dogs (50-1000 mg/kg) intravenously produced an immediate fall in blood
pressure followed by increased respiration. Following a dose of 1000
mg/kg, the pressor response to adrenalin was blocked suggestive of an
adrenalytic action with respect to blood pressure (Fogleman, 1954).
TABLE 2. Acute toxicity of glucose adduct
LD50
Species Sex Route (mg/kg bw) References
Rat M oral >10 000 Bagdon et al., 1965
Mouse M oral >10 000 Bagdon et al., 1965
M ip >10 000 Bagdon et al., 1965
M iv > 1 600 Bagdon et al,, 1965
Amitrole applied to unabraded skin as a paste at doses of
1-10 g/kg for 24 hours caused a mild dermal irritation at all dose
levels. The effect, a mild erythemia, was reduced within 24 hours. No
other effects were noted. Gross and microscopic examination of tissues
and organs was normal (Elsea, 1954).
Amitrole (3 mg) was applied to the conjuntival sac of rabbits.
Although mild irritation lasting 24-48 hours was observed, no
permanent damage was noted (Elsea, 1954).
Short term studies
Rat
Groups of rats (5 males and 15 females per group) were fed
amitrole in the diet at levels of 0, 0.01%, 0.1%, and 1.0% for 63
days. There was no mortality over the course of this study. Food
consumption and growth were depressed at 0.1% and above in both males
and females. Histological examination from selected tissues at the
conclusion of the study (thyroid was not examined) revealed
vacuolation of liver cells in those animals fed 0.1% and above. The
vacuoles were identified as fat globules indicative of fatty
metamorphosis associated with liver cell damage. No histological
effects were noted at 0.01% (Fogleman, 1954).
Groups of weanling rats were administered amitrole for up to 56
days by intraperitoneal injection three times/week for eight weeks at
1000 mg/kg. The administration of a recrystallized amitrole resulted
in no growth depression in two separate groups. The administration of
a "pure" material, not recrystallized, resulted in growth depression
(Heim et al., 1956).
Groups of rats (10 males and 10 females per group) were
administered amitrole at 1000 mg/kg by intraperitoneal administration
on alternate days for 42 days. Amitrole had to effect on bw gain or
food consumption but caused a 3-4 fold increase in thyroid weight
(Bagdon et al., 1965).
Groups of rats were administered amitrole daily, 5 days per week,
for four weeks at levels of 0, 100, 200 and 400 mg/kg. Growth rate was
reduced, relative thyroid weight increased, and iodine content of the
thyroid was reduced (Hapke, 1967).
Administration of dietary levels of 60 and 120 ppm for two weeks
resulted in an enlargement of the thyroid gland of rats and a
pronounced lowering of iodine uptake. Over this two week interval
there were no significant changes at levels of 15 and 30 ppm (Jukes
and Shaffer, 1960).
Administration of a high dose (0.04%) of amitrole in the drinking
water (approximately 60 mg/kg/day) has been shown to produce goitres
in rats within three days (Strum and Karnovsky, 1971; Tsuda et al.,
1973).
Groups of rats (10 male rats per group) were fed dietary
concentrations of amitrole for 32 days. One group was fed 500 ppm for
32 consecutive days, another group received 1000 ppm on alternate days
for the duration of the study and a group was fed the basal diet.
Behavior and mortality were not affected. Food intake and growth at
500 ppm were reduced. In the group receiving 1000 ppm on alternate
days food intake and growth data did not differ significantly from the
controls. At autopsy, gross examination of animals receiving 500 ppm
showed the thyroid gland to be hyperaemic and enlarged. The thyroid of
animals fed 1000 ppm appeared to be slightly hyperaemic but were
otherwise comparable to the controls. Mean thyroid weight/body weight
ratio for the 0, 1000 ppm, and 500 ppm groups was 58, 77, and 303
respectively (Shaffer et al., 1958).
A group of 30 male weanling rats was fed amitrole in the diet at
1000 ppm for two weeks. A comparable group received a similar dietary
level of propylthiouracil for two weeks. At the end of the feeding
interval several animals were sacrificed and the thyroid glands
weighed. The remaining animals were placed on control diets and
sacrificed at either the third or fourth week of feeding. Amitrole,
after two weeks produced a significant increase in the mean thyroid
weight (comparable to that found with propylthiouracil). Daring the
week following removal from the diet, the weight of the thyroid gland
diminished and by the end of the second week on a control diet
regression to normal size was almost complete (Bagdon et al., 1965).
Groups of rats (20 rats per group) were fed dietary l levels of 0
and 316 ppm for 100 days to examine the goitrogenic potential of
amitrole. Over the course of this experiment amitrole had a
significant effect on growth. Exophthalmia was not observed as in the
long term rat studies. Food intake was not drastically reduced. Gross
examination of the thyroid showed a laterally enlarged thyroid with
enlarged blood vessels. Microscopic examination showed hyperplasia of
the thyroid. There was no evidence of abnormalities observed in either
the liver or the kidney (Sanderson and Row, 1962).
Groups of rats (10 males per group) were administered amitrole in
the drinking water at concentrations of 0, 50, 250 and 1250 ppm for
106 days. The administration of amitrole resulted in a dose-dependent
depression of growth with a corresponding reduction of food and water
intake. Appearance, behaviour, and mortality were not affected by
amitrole. Gross and microscopic examination of tissues and organs
showed a marked increase in thyroid size at all dose levels. In rats
where reduced growth was noted, the kidneys, adrenals, liver and
spleen were proportionately smaller. Reproductive organs were not
affected. Microscopic examination showed general enlargement of the
thyroid at 50 ppm, with moderate stimulation of the thyroid epithelium
(no evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident (Bagdon et al., 1965).
Groups of rats (10 male rate per group) were fed dietary levels
of amitrole for 11-13 weeks at dosages of 0, 0.25, 0.50, 2, 10 and 50
ppm. At 0.5 ppm in the diet, no effect was observed in any of seven
separate measurements of thyroid function although iodine uptake was
slightly reduced and serum PBI concentrations were slightly increased.
Significant effects were noted at 2 ppm in the diet, especially with
regard to reduced PBI and reduced iodine uptake by thyroid. Gross and
microscopic examination of the thyroid confirmed the effects noted at
2 ppm (Fregly, 1968).
Dog
Groups of beagle dogs (3 males and 3 females were controls; 2
males and 4 females - 0.25 mg/kg; 3 males and 3 females - 1.25 mg/kg;
2 males and 4 females - 2.50 mg/kg; and 2 males and 2 females - 12.5
mg/kg) were administered amitrole orally by gelatin capsule six days a
week for 52 weeks. One male and one female per group were sacrificed
at 26 weeks. There was no mortality over the testing interval. Growth,
appearance, and behaviour were normal in all test animals. Results of
biochemical, haematological, and urological examinations were normal.
Gross and microscopic examination of tissues and organs showed no
evidence of abnormality associated with amitrole. There was no
apparent evidence of cytotoxic effects associated with the
administration of 12.5 mg amitrole kg bw/day for one year (Weir, 1958;
Dardin, 1958).
Long term studies
Dermal
Rat
Two groups of rats (25 males and 25 females per group) were
administered amitrole dermally at a level of 2.4 mg/kg weekly for 23
months. The application was allowed to contact the skin for thirty
minutes, after which the skin was rinsed and dried. Amitrole was found
to be non-irritating to the skin. There was no apparent effect of
amitrole on growth, behaviour, tumour formation or when tissues and
organs were observed on gross and microscopic examination. Statistical
studies with the liver and thyroid organ weight and organ-to-body
weight ratio did not reveal any differences in this experiment between
treated animals and controls (Rausina et al., 1972).
Inhalation
Rat
Groups of rats (25 males and 25 females per group) were exposed
to amitrole by inhalation for a one hour period every week for two
years. The animals were exposed to an aerosol of 0.2% (w/v) aqueous
solution by a head-only exposure to minimize oral ingestion. The
average analytical concentration of aerosol in the exposure chamber
was 2 mg/l air. There were no significant differences between the
treated and the control groups when examined with respect to
mortality, behaviour, growth, gross and microscopic examination of
thyroid and liver, or the incidence of tumor formation. No abnormal
effects were noted in this inhalation exposure (Grapenthien et al.,
1972).
Feeding
Rat
Groups of rats (35 males and 35 females per group) were fed
amitrole in the diet for two years at levels of 0, 10, 50, 100 and 500
ppm. Animals were sacrificed periodically over the course of the
experiment. The appearance of animals (especially females) fed 100 ppm
in the diet was altered. The incidence of protruding eyes
(exophthalmia) was considerably higher in the higher dose groups than
in the controls. Growth was reduced at 500 ppm. There was no apparent
effect of amitrole in the diet on survival, growth, or mortality in
the animals fed 50 ppm.
Gross examination of animals revealed a consistent finding of
thyroid enlargement. At 13 weeks, thyroid enlargement in males and
females was observed at 100 ppm. Microscopic examination suggested
hypofunction and a non-functional hyperplasia evident at 50 ppm and
above. The animals fed 500 ppm were removed from the diet at 19 weeks
and examined after two weeks on control diets. No evidence of
enlargement or hyperplasia was noted. At 26 weeks, thyroid enlargement
was evident at 50 ppm. Liver and kidney weight in both sexes was
decreased at 100 ppm. No effects were noted at 10 ppm. At 52 weeks,
growth of males was depressed at 100 ppm. At 68 weeks, growth of males
was again depressed at 100 ppm accompanied by enlargement of the
thyroid, pituitary, and liver. A cystic adenomatous structure was
observed at 100 ppm in the thyroid. At 50 ppm, thyroid hyperplasia and
hypofunction were noted. At 10 ppm, thyroid hypofunction was observed
in one male animal of the three males and three females examined. At
104 weeks, body weights were normal in all groups. At 100 ppm, liver
and kidney weights were normal while the thyroid was enlarged and
adenomas ranging from benign cysts to foetal malignant carcinoma were
observed. At 50 ppm, the thyroid was not enlarged. Adenomas were
observed in 3/16 rats. At 10 ppm, one rat showed an adenomatous nodule
with cellular hyperplasia (other sections suggested slight enlargement
while most control sections were normal). Thyroid enlargement was
observed only at 100 ppm. A no-effect level was not observed in this
study. Ten ppm was a minimal effect level (Keller, 1959; Dardin,
1959).
OBSERVATIONS IN MAN
A 39 year old woman ingested 20 mg/kg. This dose caused no signs
of intoxication and within a few hours of ingestion the compound
passed rapidly through the body and began to appear in concentrations
up to 100 mg/100 ml in the urine (Geldmacher-von Mallinckrodt, 1970).
Amitrole has been produced industrially since 1955 with no
evidence of ill effects other than mild contact dermatitis to the
occupationally exposed workers (Smagghe, 1974; Clyne, 1970). It was
suggested that from 1955-60, sixteen employees were exposed for five
to six months per year and from 1967 to 1970, nine employees were
exposed for approximately ten months per year with no ill effects. No
thyroid or liver tumors were observed "in excess of the general
population".
Oral administration of 100 mg amitrole to humans for treatment of
overactive thyroids inhibited the 131I-intake of the thyroid for 24
hours in normal persons and in hyperthyreotics. A dose of 10 mg had
only a very slight effect (Astwood, 1960).
A preliminary study of Swedish railway workers exposed to
amitrole showed two lung cancer cases. Although the number of subjects
was small, a combination of amitrole with smoking (or other
interacting substances) might have been responsible for the excess
lung cancer (Axelson et al., 1964).
COMMENTS
Amitrole is rapidly absorbed and eliminated primarily in urine.
It has an effect on a variety of biochemical systems including
catalase, peroxidase and certain enzymes associated with oxidative
metabolism. Amitrole is goitrogenic on continuous long-term exposure;
probably as a result of continuous inhibitions of peroxidase activity.
At high levels of exposure, antithyroid effects of amitrole have been
seen within three days. In adult female rats fed amitrole in the diet,
no effects on reproduction were noted below 500 ppm although goitre
was observed. At 500 ppm, reduced fecundity and a reduced lactation
index was observed but no malformation of pups was observed. Oral
administration to rats from days 7 to 15 of pregnancy resulted in no
effect on reproduction and no abnormal offspring. Amitrole, in the
presence of dimethyl sulfoxide, injected into chicken eggs produced
beak abnormalities and tibial malformations. Results of mutagenicity
tests using currently defined protocols were negative.
In two long-term studies, hepatomas have been produced in mice
and rats administered amitrole at exceptionally high levels. However,
a long-term feeding study at high dietary levels in rats did not
result in hepatomas. In a one-year dog study, no hepatic, goitrogenic
or other effects were noted at a dietary level of 12.5 mg/kg bw. The
no-effect level was based on a short-term study where normal PBI
values, a sensitive biochemical parameter of thyroid function, were
noted at 0.5 ppm. In addition, since no goitrogenic effect on
discontinuous exposure was observed, a conditional ADI was allocated.
The Meeting was reassured that in the use of amitrole, man has
only a remote, if any, chance of achieving the conditions where
continuous exposure is maintained. The Meeting emphasized that the ADI
was allocated with the condition that the uses of amitrole be
restricted to those where food residues would be unlikely to occur and
further to recommend that the use of materials in combination in the
same formulation be restricted, especially where effects on specific
target organs are expressed by both materials.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg bw.
Dog: 12.5 mg/kg bw.
ESTIMATE OF CONDITIONAL ACCEPTABLE DAILY INTAKE FOR MAN
0.00003 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Amitrole is a broad spectrum herbicide effective against a wide
range of grasses and broad-leafed weeds when applied as a foliar
spray. It was introduced as a herbicide in 1954. The major herbicidal
uses are on industrial land, roadsides, rights of way, railways,
forests, irrigation channels and other ditches, either used alone or
in admixture with other herbicides or as a combination with ammonium
thiocyanate.
There are numerous important uses on crop land and these are
summarized in Table 2. Amitrole is not selective and therefore all
applications in the vicinity of crop plants must be made in such a way
that growing parts of the plant are avoided.
Because of its quick action but relatively poor residual effect
amitrole is widely combined with triazine, substituted urea and uracil
herbicides to widen their spectrum of activity and to enhance the
knock down effect.
Amitrole is translocated within many plants and is thereby
effective against rhizomatous and stoloniferous grasses and bulbous
plants. Once translocated to the root systems of such plants amitrole
appears to remain effective until the next growing season or at least
its effect is observed in the regrowth, possibly owing to the
destruction of certain essential growth factors.
In order to evaluate properly the possibility of residues of
amitrole in raw agricultural commodities and foods it is important to
understand the mode of use. The following is an outline of the
application to crop land.
Apples and pears
In the spring, before fruit starts to form or after harvest,
amitrole is applied to the floor of the orchard to control broadleaf
weeds and grasses. The chemical treatment is a replacement for
mechanical cultivations designed to maintain the area at the base of
the trees free from all unwanted vegetation. Label directions state
that application should be made to weeds and that spray should be kept
off the trunk or foliage of trees. Actually, at the time applied,
there may be blossoms on the tree but very little foliage. Because of
this, very little transpiration is taking place and there is a minimum
of fluid transport within the tree. Thus, any amitrole that does come
into direct contact with the trunk or the roots would not be expected
to move around in the tree in detectable amounts. Extensive data were
available to the Meeting to judge the possible effect of accidental
misapplication or treatment in mid-summer when fruit is on the tree
and foliage is at a maximum.
Grapes
In many grape-growing areas where there is a winter rainfall,
winter annual weeds are a serious cultural problem. These weeds sprout
profusely with the first rains of the winter wet season and grow
strongly during winter and early spring while adequate moisture is
available. Several residual herbicides are effective for controlling
these weeds provided they are applied before the weeds emerge.
However, if application is made too late or if the amount of moisture
is inadequate these materials are often ineffective and must be
supplemented by a foliage-absorbed herbicide. During this period, the
vines are completely dormant with no leaves or berries. The spray is
directed to the weed foliage at the base of the grapevine with
instructions to keep all chemical off the grape plant itself. When
grapes are dormant, there is no transpiration of fluids within the
grapevine. Therefore, no moisture is going into the vine and thus
there is no vehicle for the amitrole. By the time the grape breaks
dormancy and transpiration and water uptake are significant, amitrole
apparently is degraded and not available for uptake.
The residual herbicides suffer the disadvantage of being
ineffective against some species. If these are left uncontrolled they
soon colonize the whole area and interfere with cultural practices.
Also there are numerous instances where the farmer has a perennial
weed problem in his cropped fields. This can either be an entire field
or a large patch within a field or merely scattered plants. The use of
amitrole alone or in combination with other herbicides as a spot
treatment is recognized as an important agricultural practice
essential to the maintenance of the productivity of perennial cultures
such as vineyards.
TABLE 3. Pattern of use of amitrol based herbicides
Rate
Use kg/ha When applied Remarks
Chemical fallow 1 Autumn/winter Apply after weeds
emerge.
Cropland 4-8 After harvest Do not plant crops
or cutting. or graze for 8
months.
Grapes 2 When vines are Directed spray.
dormant.
Orchards (apple 2 Before fruit Do not spray
and pear) forms/after foliage.
picking.
Corn 2 10-14 days Spray weeds 10-14
pre-planting. days before
ploughing.
Irrigation drains 2-8 When weeds Do not allow
and ditches 15 cm high. grazing.
Orchards/vineyards 2-8 When weeds are Spot treatment.
(perennial weeds) actively growing.
Non-crop areas 1-8 When weeds are Boom sprays-
actively growing. spot sprays.
Aerial Not to be made
application where spray or
drift might
contaminate crops
or potable water.
Maize
Selective herbicides have revolutionised the growing of maize and
many other crops but there still remains a serious problem of
perennial weeds such as thistles, Agropyron repens and
convolvulus. In the spring amitrole is applied to growing weeds.
After roughly 10-14 days, during which the chemical is allowed to
penetrate the weed foliage and translocate to the actively growing
meristematic tissue, the weedy field is ploughed in the normal
fashion. That is, the weed foliage is buried roughly 15.25 cm in the
ground and soil which was well under the surface is brought to the
top. Emphasis is placed on burying all treated weeds.
Chemical fallow
In areas where a fallow period is maintained because conservation
of all available moisture is essential to produce a satisfactory crop,
amitrole has been used alone or mixed with 2,4-D to control annual
weeds that sprout during the fallow period. If allowed to grow, these
weeds remove much of the moisture reserves in the soil.
Depending upon winter conditions, it may be desirable to spray in
the autumn or wait until weed growth starts in the spring. This
initial treatment is followed by one or more mechanical cultivations
to maintain the area fallow until the next crop is planted. It may be
necessary to repeat the herbicide application if fresh weeds emerge.
Practically speaking, there is no need to apply it less than 30 days
before planting cereal crops although the residue data show that
application could be as late as several days before planting without
producing detectable residues. There is no control whatsoever of weeds
that germinate after application.
Irrigation channels and ditch banks
Amitrole has been used for many years in some countries where
difficulty is found in maintaining irrigation channels and ditch banks
free from encroaching vegetation, particularly against those weeds
which grow prolifically in such situations and where a herbicide which
is rapidly translocated but rapidly disappears from soil or water is
required. In some countries there are restrictions on use near potable
water or catchments.
Pre-harvest treatments
There are no approved uses for direct application to crop plants.
Lack of selectivity precludes the possibility of such treatments.
Other uses
There are no known domestic or industrial uses which could
subject the general public to exposure to amitrole in any form.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable number of studies have been carried out to
determine whether, following the use of amitrole for the control of
weeds, residues could possibly occur in raw agricultural commodities
or food. Virtually without exception these studies indicate that the
parent compound is not found in any food commodity even when grossly
excessive rates are used or when the compound is applied at times and
under conditions not recommended as good agricultural practice.
Apples and pears
Otten (1970) reported before the Amitrole Advisory Committee of
the U.S. Environmental Protection Agency the results of many studies
on apple and pear trees. The approved treatment is 2-4 kg/ha under
trees in the spring before fruit forms or after harvest. In one series
of trials amitrole was applied for five successive years at the rate
of 4 kg/ha per year, or a total of 20 kg/ha during the 5 year period.
This is 10 times the normal use rate over the 5 year period. The
bottom 50 cm of the trunk was wet if necessary. No amitrole residues
were detected in the fruit.
Otten reported other tests in which amitrole was applied for 8
successive years using 4 or 20 kg/ha each year, a total of 160 kg of
amitrole per hectare during the period. Apple samples were taken from
trees with or without white shoots at the base (indicating amitrole in
the leaves) and analysed separately. No amitrole was detected in any
samples. The limit of determination of the method used is 0.01-0.02
mg/kg.
In both series of tests amitrole was applied during the summer
when the fruit was on the tree. Only when spray was applied directly
to the fruit was a residue detected.
Schubert (1965) carried out trials continuously over the period
1957-1964 in numerous orchards which received treatments of amitrole
over ground cover, fruit and leaf. Even in those trials where amitrole
was applied beneath trees right through the summer, mature fruit were
free from amitrole at harvest. In the early years apparent amitrole
residues were reported at levels in the range 0.05-0.09 ppm.
Subsequently Storherr and Burke (1961) developed improved methods to
overcome the extremely high absorbance backgrounds of most crops. Even
where chlorotic shoots were present on the trunk of trees, fruit at
harvest was found to have no residues (limit of determination 0.02
mg/kg). Substantial amitrole residues were found in mature apples
where foliage and/or fruit were directly treated with amitrole in
mid-summer. The residues from direct application to foliage were
similar to quantities found after dipping the fruit. When both leaves
and fruit were sprayed the residues were approximately doubled. The
residues resulting from these direct applications during the growing
season were in marked contrast to the results of a ground cover
application that avoided spray contact with foliage or fruit, where no
amitrole residues were found at harvest.
Maier-Bode and Bechtel (1968) reported trials with apple trees
carried out in Germany. When amitrole was applied at 2.5-4.2 times the
officially recognized dosage (6 kg/ha), no amitrole was detected in
the apples (residues of less than 0.01 ppm). Not until 50 or 100 kg/ha
of amitrole was used, i.e. 8.3-16.7 times the recognized dosage, were
small residues of amitrole, between 0.02 and 0.09 mg/kg found in the
apples. In practice such high doses are never used; the foliage of the
trees in these plots was distinctly chlorotic.
Maier-Bode and Bechtel (1968) also found that when grossly
excessive amounts of amitrole were applied to the soil, the amitrole
residues in apples were much higher when there was no weed cover
beneath the trees than when the herbicide was applied to densely
weed-covered plots. This was presumably because the lack of weed cover
to absorb and metabolize the amitrole allowed it to reach the root
zone of the trees.
Amchem (1965) reports show that apples, grown in orchards where
the floor of the orchard was cleared of poison ivy and general
broadleaf and grass weeds according to label instructions, show no
residues at harvest. These studies extended over 8 locations and
numerous varieties of apples. The methods of analysis used showed 96±
15% recovery at the 0.1 mg/kg level with a limit of determination of
0.01 mg/kg. Only when partially grown apples hanging on the trees were
sprayed to run-off with an amitrole spray mixture, could residues of
amitrole be found in the mature apples.
Moore (1968, 1969, 1970) reports three series of trials at
various locations in Australia in which amitrole was applied beneath
apple trees at varying rates between 2 and 4 kg/ha, at petal fall or
in mid-summer. To obtain the most severe conditions possible,
weed-free plots beneath trees were also sprayed in mid-summer. To
ensure the most complete uptake of herbicide by the tree an area of 5
metres × 5 metres was treated instead of the usual 3 metres × 3
metres. The author concludes that if any residues were present they
were below the analytical limit of determination of 0.01 ppm. There
was no build-up of residues in the second year of treatment.
Bayer (1973) reports studies carried out in Germany which showed
that approved uses of mixed herbicides containing amitrole applied for
the control of weeds in apple orchards did not produce detectable
residues of amitrole in mature apples (limit of determination
0.05 ppm).
The Netherlands authorities have submitted results of studies
carried out to determine amitrole residues in some fruits following
approved uses in that country. Pears and apples harvested in 1961 and
1962 following treatment of orchards for weed control 4-18 months
previously showed no indication of residues when analysed by the
method of Storherr and Burke (1961) having a limit of determination of
0.025 mg/kg (Wit and Van der Kamp, 1963).
Cherries
Maier-Bode and Bechtel (1968) report a number of experiments
which show that when amitrole is applied for weed control beneath
cherry trees 100 days before harvest no residues can be detected in
mature fruit by methods capable of determining 0.01 ppm.
Bayer reports experiments where amitrole was applied for weed
control in sour cherry orchards at the rate of 4 kg/ha. No residues
could be detected by methods sensitive to 0.05 ppm.
Citrus
An extensive study of the accumulation and depletion of amitrole
residues in citrus fruit and foliage was published by Day and
Hendrixson (1959) from California. Amitrole was usually applied to the
soil at rates of 2 and 4 kg/ha; in two instances a logarithmic series
of applications were made from 2-64 kg/ha. Treatments were replicated
4 times in each of 13 different orchards in various parts of the
citrus growing area. Treatments were applied during the winter months,
usually on dry soil surfaces, and orchards were irrigated during the
period of observation. The analytical method used was effective down
to 0.01 mg/kg. Several varieties of oranges and lemons were included
and samples were taken each month for the four months following
application. In spite of obvious symptoms of damage to the lower skirt
of the citrus trees most samples from the 2 and 4 kg/ha experiments
showed no detectable residues although a few were found to have
residues up to 0.05 mg/kg. In the logarithmic plots the level of
residues increased with increasing concentration of amitrole applied.
Those plots receiving 64 kg/ha showed 0.15 mg/kg in the whole oranges
4 months after application. Less was found after shorter intervals.
The authors concluded that citrus fruit from trees receiving
either 2 or 4 kg/ha contained residues only rarely. The residue level
found in citrus fruit does not appear to be affected by fruit variety,
soil type, climate, geographical area, cultural operations or rate of
application to the soil (except at grossly exaggerated rates).
Albinism appears only on the lower skirt area indicating foliar
contact rather than root uptake as the source of entry. Amitrole is
not re-distributed beyond the immediate area of contact when the
herbicide is applied at recommended rates. Field observations indicate
general systemic distribution at high rates of application.
Coffee beans
Information was available from only one trial carried out in
Hawaii.
Hylin (1962) reports results of analysis of coffee beans from
trees which had been treated twice within 6 weeks with amitrole,
applied at the rate of 2 and 4 kg/ha to substantially bare ground. A
total of 40 trees were involved in the trial. Great difficulty was
apparently encountered in developing a suitable analytical procedure
to deal with the coffee beans. Apparent amitrole residues were
reported to occur in the green beans at levels ranging from
0.02 - 0.48 mg/kg. In view of the analytical difficulties reported
and the experience of other workers, however, caution should be
exercised in interpreting the significance of these results.
Cranberries
Following the disclosure by Fleming (1959) that the practical use
of amitrole for the control of weeds in cranberry bogs led to
significant residues of amitrole in cranberries, the U.S. Food and
Drug Administration and the U.S. Department of Agriculture undertook
field and laboratory studies extending over 19 months (Onley et al.,
1963). Following the application of 4 and 8 kg of amitrole per ha of
cranberry bog the level of amitrole was determined in samples of
cranberry vines, soil, roots and fruits (when available). Results
indicated that though the residues in the soil declined steadily and
disappeared at the end of 12 months there was a distinct concentrating
effect in the roots and a pronounced effect in bushes where the
residues declined more slowly than in the soil. Cranberry fruit
harvested 3 and 12 months after application showed residues ranging up
to 0.4 mg/kg. The authors were unable to conclude whether the residue
represented the parent compound or metabolites.
Grapes
Studies using radio-labelled amitrole (Leonard and Weaver 1961)
showed that when amitrole was applied to the leaves, stem, clusters or
shoots of the grapevine a modest upward translocation occurred for
about 3 days. After this period the amitrole appears to be either
complexed or broken down. Amitrole was not recoverable, as such, from
the clusters except within 3 days after treatment.
Leonard and Lider (1961) studied the translocation of amitrole
and a number of other herbicides in the grapevine. Grape rootings were
allowed to absorb solutions of herbicide for 3 days before being
planted in pots. The distribution and fate of the herbicide was
studied by radio-autographs. There was very little indication of
translocation throughout the plant. Fruit from vines grown in
amitrole-treated soil under greenhouse conditions showed no positive
evidence of amitrole.
Trials with grapes in Germany involved the application of a
maximum of 4 kg/ha of amitrole weeds in vineyards, during both the
dormant and vegetative stages (Maier-Bode and Bechtel 1968). No trace
of amitrole was found in grapes when using a method capable of
detecting 0.01. Similar results are reported by Bayer (1973) from
trials involving the application of 4 kg of amitrole per ha of
vineyard.
Maize
Otten (1970) reports results obtained by both chemical analysis
and radio-labelling which show that no amitrole was detected in either
immature maize plants or at normal harvest time when amitrole was
applied to weeds 10 days before ploughing for the planting of maize
seed. When exaggerated rates were applied or maize was planted 1 or 2
days after spraying, amitrole was detected in the seedling plant but
had disappeared well before the crop would be used for silage or
mature grain.
In a statement before the Amitrole Committee, Amchem (1970)
summarized investigations carried out by a number of official research
workers investigating the possibility of amitrole residues finding
their way into corn plants and grain. Ercegovich (1957) analysed corn
from plots treated with amitrole at 0, 1, 2, 4 and 8 kg/ha and planted
1, 5, 9 and 13 days later. Harvests were made at 3, 4, 6, 10 and 16
weeks after planting. No residues of amitrole were found in any
samples.
Boyd applied radio-labelled amitrole directly to young corn
plants and followed the rapid decrease in residues over a 47-day
period. Initial residues of 4-10 mg/kg decreased to final levels of
0.1 - 0.2 mg/kg.
Nuts
Studies carried out in California involved the application of
amitrole to the orchard floor at rates of 1, 4 and 8 kg/ha.
Application was made during the winter months. Mature nuts collected
the following season were analysed for residues of amitrole. None of
the many samples examined was found to contain amitrole residues above
the limit of determination (0.02 mg/kg) (Hill 1962-63).
Peaches and plums
The Meeting had available numerous reports on residue trials
carried out in peach and plum orchards where amitrole was used for
weed control. None of the many samples analysed by methods capable of
determining as little as 0.02 mg/kg showed any indication of amitrole
residues (Hill 1962/63; Maier-Bode and Bechtel 1968; Bayer 1973).
Soybeans
The Meeting examined summaries of many separate trials carried
out in different areas of the USA and analysed by three separate
laboratories to determine the level and fate of amitrole in soybean
plants, pods and mature beans following different patterns of using
amitrole for weed control. In all cases samples were from treatments
at rates of 1´ - 2 times the maximum recommended rate. In no instances
were the residues, even in immature plants, found to be above the
limit of determination, 0.06 mg/kg (Amchem, 1969).
Montgomery and Freed (1963), wishing to know whether the proposed
Pre-plant use of amitrole could possible result in residues in
soybeans, studied various methods of extraction and analysis. They
reported that by each procedure samples from fields treated with up to
4 times the recommended rate of amitrole gave exactly the same results
as samples from untreated control fields. They applied a statistical
analysis to the extensive data and concluded that there was no reason
to suspect that any residues could occur following pre-plant
application. Further evidence of freedom from residues was provided by
failure to demonstrate any difference between the absorption spectra
of paired samples from control and treated plots when using a
double-beam spectrophotometer to compare them.
Sugar cane
Amitrole has been found to be useful for the control of certain
perennial grass and broad leaf weeds common in sugar cane fields and
on irrigation ditch banks. Application of directed sprays in sugar
cane produced a moderate amount of chlorosis which persisted for 3-4
weeks in the leaves without appearing to affect yields or subsequent
growth. Hilton et al. (1963) carried out a residue study designed to
determine the residual amitrole in sugar cane from several successive
applications made over the period of the crop cycle (2 years). Hilton
and Uyehara (1966) reporting on these studies noted that when sugar
cane was double treated with amitrole at 5, 10 or 20 kg/ha, 12 and 20
weeks after planting, the residues of amitrole diminished to less than
0.002 mg/kg by harvest time. When 10 times as much cane was taken for
analysis and treated in a manner to approximate the early raw sugar
processing, residues, if present, were less than 0.01 mg/kg even from
plots receiving the grossly exaggerated rate of 20 kg/ha.
Wheat and oats
Amitrole proved to be an effective means of controlling couch
grass (Agropyron repens) in cereal crops. Most proposed treatments
involved the application of amitrole to the grass 10-14 days before
ploughing in preparation for seeding with cereals. In Sweden the
procedure was to apply amitrole at the 3 leaf stage at a rate of 0.5
to 1 kg/ha. Svensson (1971) investigated the effect of such treatments
on oats and the fate of residues in the oat plants and grain. He
reports that when the treatment was carried out strictly in accordance
with approved directions no detectable residues could be found in the
grain. However when the rate was increased or application withheld for
2 weeks until the 5-leaf stage of the crop, the grain contained about
0.05 mg/kg. If the rate of application is increased to 2 kg/ha the
residue can exceed 0.1 ppm. Determinations were made on straw from a
number of trials but analytical difficulties rendered the results
somewhat unreliable. However it appears that application of amitrole
at any stage after the 3-leaf stage increases the likelihood that
residues will be found in the straw at harvest. Much higher residues
were found in the grain of oats treated with amitrole at the heading
and milk stages. 0.5 kg/ha applied at the milk stage gave rise to a
residue in the grain of 2.9 mg/kg.
Amchem (1970) provided a summary of a number of studies by
official workers in the USA who investigated the incidence, level and
fate of residues of amitrole in wheat grain and straw from crops grown
on soil treated according to label directions 14-28 days before
ploughing for planting. In some of these studies the application rate
was increased to 4 times the recommended rate and the time interval
was reduced to 1 day before planting. In no instance was any amitrole
residue found in cereal grains or straw at harvest time. Soil studies
on the same test plots showed detectable residues of amitrole on the
day of treatment but no detectable residues the day after treatment
even at the doubly exaggerated rate. The limits of detection were 0.02
ppm in grain and straw. Recoveries were 86-96% at levels of 0.2-0.4
ppm. On the basis of these data it was concluded that the use of up to
4 kg/ha of amitrole, one or more weeks before planting wheat or other
cereals, should cause no residues in the crop.
FATE OF RESIDUES
General
Kröller (1966) and Menzie (1969) have reviewed the metabolism of
amitrole in animals, plants, micro-organisms, etc. There is an
extensive literature on the mode of action of amitrole and on its
metabolism in plants. Many of these investigations were carried out
with the object of determining uptake, translocation, site of action,
reason for selectivity, and possible means of enhancing the activity.
Among the most useful publications are those of Boyd (1964/65), Carter
(1969), Herrett and Linck (1961a), Jukes (1961), Moser (1968), and
Rogers (1957a,b).
Many of these studies reveal that amitrole does not persist for
extended periods in plant tissues though there is considerable
disagreement as to whether it becomes conjugated, converted into a
more active material or destroyed and its fragments taken up into the
plant. There appears to be no clear-cut mode of action, but rather
multiple pathways by which it interferes with plant metabolism. Carter
(1969) reviewed the available literature on the effects of amitrole on
amino acid and protein metabolism, purine metabolism, flavin
synthesis, enzyme activity, chlorophyll synthesis and plastid
development. It is obvious that there is no single site of action but
the relative importance of the various effects has apparently not been
unravelled.
In animals
The fate of amitrole in rats is discussed in the section
"Biotransformation". There do not appear to be any grounds for
assuming that livestock grazing on plant materials growing on land
that had been treated with amitrole for the control of weeds would
absorb or retain significant amounts of amitrole or its metabolites.
In plants
The s-triazole nucleus is highly stable (Potts 1961); hence it
is not surprising that few workers have reported evidence of ring
cleavage under physiological conditions. Yost and Williams (1958)
reported disappearance of radio-labelled amitrole from corn plants in
approximately 6 weeks with a half-life of about 8 days. Disappearance
was also observed in soybeans but at a much slower rate.
Miller and Hall (1961) could not detect amitrole in cotton 4 days
after treatment. However, large quantities of metabolic products were
present. Fang et al. (1967) report the half-life of amitrole in
several plants as 18 to 28 hours. Little of the applied material was
recovered as CO2 from beet, corn or beans.
Freed et al. (1961) reported evolution of radio-active, CO2 from
treated oats and barley, indicating ring cleavage. Montgomery and
Freed (1963) observed some evolution of 14CO2 from treated soybeans,
with the remaining radioactivity apparently bound to components of
plant tissues. Resistant oats released CO2 more readily than
sensitive barley. Massini (1963) found no loss of CO2 from beans or
tomatoes. Muzik (1965) observed chlorosis in scions grafted onto
tomato plants 103 days after treatment with amitrole, indicating long
persistence of the toxic moiety.
Studies of amitrole degradation in plants have been complicated
by the fact that the material is available commercially only with the
5-carbon labelled. Apparently, the 5-carbon is quickly lost (as CO2
or formate) when ring rupture occurs and the remaining fragments are
thus unlabelled. However, if significant amounts of CO2 or
14C-formate were produced from 14C-amitrole in higher plants, one
would expect to find some incorporation of 14C into normal
metabolites. This is not the case. The vast majority of the literature
indicates that the extractable 14C from plants treated with
amitrole-5-14C remains in the intact ring as free amitrole or
conjugates. Considerable amounts of amitrole are attached to protein
(Brown and Carter 1968; Castelfranco and Brown 1963) or somehow bound
in an insoluble form (Racusen 1958). There is evidence of activation
to a free radical form which can react with amino acids (Carter and
Naylor 1960, 1961a, b; Herrett and Bagley 1964; Herrett and Linck
1961a; Miller and Hall 1961). Conclusive evidence of rapid and
extensive ring cleavage by higher plants has not been reported.
Most literature on metabolic alteration of amitrole in plants
deals with the formation and properties of conjugates between amitrole
and endogenous plant constituents. These "degradation" products
contain the intact triazole nucleus which often may be regenerated by
chemical treatment.
Rogers (1957a,b) reported a derivative of amitrole in several
plants which was "chromatographically identical" with an
amine-glucoside derivative of amitrole. The glucose derivative forms
quite readily in vitro (Fredrick and Gentile 1960) and its occurrence
in plant extracts is probably an artifact (Naylor 1964), since
numerous attempts by other workers to detect the compound in plant
extracts failed (Miller and Hall 1961; Massini 1963; Carter and Naylor
1959). However, Gentile and Fredrick (1959) and Frederick and Gentile
(1960, 1969, 1962 and 1965) have published a series of studies on the
properties and metabolism of the glucose derivative. These same
authors suggest that the triose derivative represents the true
structure of the amitrole derivatives reported by other workers.
Massini (1963) and Carter and Naylor (1960, 1961a) reported studies of
amitrole metabolism in which Massini identified one of the major
metabolites as 3-(3-amino-1,2,4-triazole-1-yl)-2-amino propionic acid
(3-ATAL), also referred to as
3-(3-amino-1,2,4-triazol-1-yl)-d-alanine. The formation of 3-ATAL
apparently represents a detoxication, since the derivative does not
appear to be nearly as toxic as amitrole, or as mobile. Furthermore
ammonium thiocyanate, which synergizes the action of amitrole,
inhibits the formation of 3-ATAL.
Smith and Chang (1973) studied the metabolism of amitrole in
Canada thistle (Cirsium arvense) and peas. They showed that excised
leaves of thistles metabolized amitrole into three major products one
of which was 3-ATAL. The other two were shown to be its metabolic
products, one being the precursor of the other. An enzyme preparation
from peas capable of synthesizing tryptophan was also able to
metabolize amitrole. Tryptophan synthesis with the enzyme preparation
was inhibited by amitrole and the authors deduced that amitrole
metabolism may follow a similar pathway to tryptophan synthesis.
A number of workers including Racusen (1958) reported another
metabolite which was stable to 6N HCI for 5 hours at 100°C.
Verification of the structure of this material and others awaited
further studies.
The most important outstanding question is whether the conjugates
and/or metabolites represent biologically active derivatives of
amitrole and if so whether these are available to react
physiologically and biochemically with animals receiving such
conjugates and metabolites in plant materials as part of their ration.
However, use patterns at present approved avoid the possibility that
significant residues are ingested by livestock or man.
In soil
Amitrole disappears rapidly from soils as shown by Sund (1956),
Bondarenko (1958), Ashton (1962), Riepma (1962), and Ercegovich and
Frear (1964). Disappearance has been attributed to absorption (Sund,
1956, Ercegovich and Frear, 1964) and microbial degradation, although
attempts to isolate organisms capable of degrading amitrole have not
been successful. Kretschmar (1970) showed that amitrole was not
degraded by Azotobacter.
Kaufmann (1965), Kaufman and co-workers (1968) and Plimmer et al.
(1967), have proposed that most of the amitrole degradation occurring
in soils proceeds by non-biological reactions. They showed that
approximately 69% of the radio-label from amitrole was released as
CO2 in 20 days by non-sterilized soil. Although autoclaved soil
released only 2% in a comparable period, soil treated with potassium
azide or ethylene oxide released 46 and 35% respectively, and
reinoculation of autoclaved soil did not restore the capacity to
metabolize amitrole. These authors propose that amitrole is degraded
in soil by an oxidative mechanism involving an attack on the triazole
nucleus. Microbial attack is not discounted however. Soil moisture,
temperature and pH markedly affected amitrole degradation (Riepma
1962), indicating possible microbial involvement. The studies by
Ercegovich and Frear (1964) show that amitrole degradation obeys
first-order kinetics, suggesting a chemical reaction. No-one has
reported an investigation of the possible involvement of
extra-cellular enzymes.
Whatever the mechanisms by which the triazole ring is opened,
there appears to be little doubt that ring opening does occur rapidly
in soils and the resulting products (urea, cyanamide and nitrogen)
should be readily metabolized by soil micro-organisms.
Norris (1970) showed that amitrole was rapidly lost from the
forest floor but that degradation was not completely biological, since
there was considerable loss in steam-sterilized material. He found
that ammonium thiocyanate applied with amitrole in the proportions
found in the commercial herbicide had no effect on the degradation of
amitrole.
Sund (1956) showed that amitrole becomes tightly adsorbed to soil
particles. He concluded that it takes part in the soil's base exchange
system, but also it has the tendency to complex metals. Studies of
amitrole toxicity to tomato seedlings were correlated with chemical
analysis and it was found that the biological response of plants is
proportional to the amount recoverable in any soil type.
MacRae and Alexander (1965) are in agreement with the results of
Ashton (1963) that microflora have an important function in the
degradation of amitrole. They confirmed the rapid biodegradation in
soil by plant bioassay.
Ercegovich and Frear obtained evidence of complex formation
between soil clay and amitrole by means of X-ray diffraction
measurements. Ashton (1963) noted that 13 compounds were formed in
addition to CO2 when amitrole was added to unsterile soil. One or
more metabolic compounds formed from amitrole are tenaciously bound to
the soil and appear to be resistant to degradation.
Day et al. (1960) studied the effect of soil type, temperature,
moisture and sterilization by steam on the fate of amitrole in 55
types of Californian citrus soil. Rates of decomposition in
steam-sterilized soils were much lower than in unsterilized soil,
apparently indicating that the decomposition is primarily due to the
action of soil microorganisms (but compare the results of Kaufman,
Plimmer and co-workers quoted above). Rates of decomposition of
amitrole were highly variable among the soils studied, apparently
because of differences in populations or levels of activity of the
soil micro-organisms concerned. A short residual life for amitrole was
more frequently found in the more highly evolved soils having finer
textures and more highly developed colloidal properties.
Freed and Furtick (1961) showed by simultaneous bioassay and
chemical analyses capable of detecting less than 0.05 mg/kg of
amitrole that it would be highly improbable that any residue of
amitrole would remain in the soil even a short while after
application.
It has generally been assumed that failure to recover amitrole
from soils following its use as a herbicide was due to elimination by
such processes as leaching, volatalization, or biological or chemical
destruction. Work by Groves and Chough (1971) demonstrated that new
solvent mixtures gave much better recoveries of amitrole from soil
than water extraction. Concentrated ammonium hydroxide/glycol mixture
(5/20) gave distinctly higher recoveries than water as indicated by
the following table.
TABLE 4. Extraction (%) of amitrole from soils with water and
ammonium hydroxide/glycol (A/G) mixture (5x20)
Time after Non-sterile soil Sterile soil
application (days) Water A/G Water A/G
% % % %
0 48.1
1 36.7 97.6 49.6 97.3
12 21.3 55.9 36.1 77.9
17 3.2 15.2 36.9 67.7
These results point to a need for caution in interpreting some of
the earlier results.
In water
Since amitrole is widely used for the control of ditch bank weeds
many studies have been carried out to determine the fate of any
amitrole reaching the water. Many of these studies (Segal 1960;
Marston et al. 1968; Frank 1969; Dunstar 1969; and Demint et al. 1970)
have largely involved the study of the rate of dissipation of amitrole
in irrigation water or flowing streams. Since only a small proportion
of the herbicide applied in such operations ever reaches the water,
dissipation and dilution is a recognized means of reducing any risk to
crops and municipal users. Where application has been made by aerial
spraying, measurable amounts were found in samples of water near the
downstream edge of the sprayed area for up to 5 days after spraying
(Marston et al. 1968).
Such studies are however of little value in determining whether
residues are lost by physical, chemical or biological means. Studies
were reported by Thoman (1963) from Florida where ponds with static
water levels were treated at the rate of 3 kg/ha of water surface,
giving a concentration of 0.45 mg/l in the water immediately after
treatment. Samples collected every 7 days thereafter were analysed.
The residue level declined slowly but 46 days elapsed before it
reached the limit of determination (0.02 mg/l). Samples taken
thereafter until the 74th day gave results comparable to pre-treatment
levels.
Nicholson (1963) reported experiments carried out in a fish
hatchery in South Carolina where ponds of 1 acre surface area were
treated with amitrole to yield a concentration of 1 mg/l in 80,000
cu.ft of pond water. Samples were taken each 7 days for 5 weeks and
thereafter at slightly greater intervals until the 201st day when the
pond became flooded. The concentration following treatment (1.16 mg/l)
gradually declined through 0.49 mg/l on the 68th day to 0.07 mg/l on
day 201. No information is given on the quality of the water in the
ponds but it is obvious that under these conditions amitrole remains
relatively stable for long periods.
In processing and cooking
No information was available on the effect of processing and
cooking on residues in plant materials. In view of the fact that none
of the recommended use patterns give rise to detectable residues in
raw agricultural commodities such studies are unlikely to have been
carried out.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Food moving in commerce
No data were available to the Meeting to indicate the level and
incidence of amitrole residues in food moving in commerce.
Food at the time of consumption
Results of several total diet studies in which an attempt was
made to find amitrole residues in many separate food groups were
available to the Meeting. Duggan et al. (1966) reported that amitrole
residues were not found in any food composites in the total diet study
carried out in the USA in 1964/65; the method used had a limit of
determination of 0.05 mg/kg. The same authors (1967), having repeated
the total diet study, reported that amitrole residues were not found
at or above the prescribed sensitivity limit. Corneliussen (1969,
1970), reporting the results of the 4th and 5th total diet studies
carried out in the USA, states "amitrole has never been found in any
total diet composites". The limit of determination was again
0.05 mg/kg.
METHODS OF RESIDUE ANALYSIS
One of the first methods for the determination of amitrole
residues was that of Sund (1956). This was a colorimetric method
applicable to soils, depending upon the formation of a green colour
with sodium nitroprusside reagent. It is sensitive to 1 mg/l of
amitrole in aqueous solutions and Sund reported that it conformed to
Beer's law. Bioassay with tomato seedlings correlated with chemical
analysis.
American Cyanamid (1958) published a colorimetric method for the
estimation of residues of amitrole in cranberry fruit. The extract is
purified by means of a cation exchange resin, treated with nitrous
acid and coupled with N-1-naphthyl-ethylenediamine dihydrochloride to
produce a pink-coloured solution. The intensity of the colour is
proportional to the amitrole concentration and is measured
spectrophotometrically at 512 nm. The limit of determination is
0.03 mg/kg.
Herrett and Linck (1961b) published what they describe as a
simple reproducible method for the separation and quantitative
determination of amitrole in biological systems. The method consists
of diazotization followed by coupling with
1-amino-8-naphthol-3,6-disulfonic acid (H-acid). The authors describe
the method as being sensitive in the range of 0.1-3.3 mg/kg.
Storherr and Burke (1961) in describing an improved method for
the determination of amitrole in crops refer to previous methods
published by the Food and Drug Administration which were applicable to
only a limited number of food crops. With their modifications the
method involves extraction of vegetable crops with ethanol, adsorption
on cation-exchange resin and desorption with aqueous ammonia. The
ammoniacal concentrate is subjected to a clean-up procedure using
acetonitrile and filter aid followed by acid digestion and clean-up
with activated carbon. The resulting solution is diazotized and
coupled with H-acid. The pink colour is measured at 455 nm. It is
necessary to carry out a blank determination. The apparent amitrole
found in control crops ranged from 0.003 to 0.02 mg/kg. The limit of
determination is considered to be 0.025 ppm using a 40 g sample.
Storherr and Onley (1962) later published a procedure for the
clean-up and separation of amitrole and its metabolites from vegetable
crops. This method utilizes a chromatrographic column packed with dry
cellulose for the removal of interfering substances from plant
extracts. It is reported to recover amitrole from some conjugates.
Hilton et al. (1963) modified the method of Storherr and Burke,
(1961) for use with sugar cane and sugar juice and were able to lower
the limit of determination to 0.002 mg/kg. Their modifications
involved an increase in the size of sample taken and a reduction in
the amount of activated carbon used in the acid digestion and cleanup
steps.
Segal (1960) and Hilton and Uyehara (1966) described a number of
minor modifications to the method of Storherr and Burke which lowered
the limit of determination of amitrole to 0.001 mg/kg in water and
sugar cane respectively.
Meissner (1971), on behalf of the Amitrole Advisory Committee to
the US Environmental Protection Agency, commenting on the claim that
the method of analysis developed by Storherr and Burke (1961)
determines not only amitrole but all known metabolites drew attention
to the lack of detailed information available on the metabolites of
amitrole in plants or on the influence of environmental and chemical
factors on plant metabolism. Groves and Chough (1971) report an
improved solvent mixture (concentrated ammonium hydroxide and ethylene
glycol) to extract amitrole from soil. Further details are given above
(see "Fate of residues in soil").
NATIONAL TOLERANCES
The information available to the Meeting indicated that most
countries with pesticide residue tolerance legislation either
registered amitrole on a "no-residue" basis or provided a zero
tolerance. It is assumed that zero would in effect mean "at or about
the limit of determination". In the Netherlands there is a general
tolerance of 0.02 mg/kg in fruit and vegetables. In Australia there is
a maximum residue limit for amitrole in water of 0.01 mg/l. This is at
or about the limit of determination. In the Federal Republic of
Germany, use of amitrole where potable water might be contaminated is
prohibited.
APPRAISAL
Amitrole is a non-selective, foliage-absorbed herbicide widely
used since 1954 for the control of unwanted vegetation on industrial
land, roads, railways, rights of way, ditch banks and similar
situations. It is also used for the destruction of weeds beneath trees
and vines in permanent horticultural crops, for spot treatment of
perennial weeds and for their destruction prior to the planting of
cereal crops. There are no known uses where application is made to
crop plants.
Extensive information indicates that amitrole is rapidly lost
from soil by a combination of chemical, biological and microbiological
attack. There is no indication that amitrole is taken up by the roots
of plants if currently approved practices are followed. Approved uses
involve the application of amitrole to weeds between the crop when
fruit trees, vines and similar horticultural crops are dormant or the
use of spot sprays and directed sprays which avoid contamination of
crop plants. In these cases there is no uptake of residues by crop
plants. Studies with radio-labelled amitrole confirm that it is not
taken up from soil following simulated normal practices.
Extensive residue trials have been carried out under many
conditions in many countries and all indicate that there is no residue
in fruit, vegetables or grain following the recommended use of
amitrole as a herbicide. Residues have been found experimentally only
when crop plants have been treated with excessive quantities of
amitrole applied directly over the crop when the fruiting parts are
already well formed.
There appears to be no single mode of action and the metabolic
pathways in plants appear most complex. There is evidence that when
amitrole is applied to the leaves of plants, most of the material
absorbed is metabolized. Some is complexed with various plant
materials, but the Meeting considered that the nature and biological
significance of conjugation products would only be of importance when
considering residues resulting from direct application over food-crop
plants. Where amitrole was administered to laboratory animals it was
shown that similar conjugates with sugars and proteins were formed in
the animal body.
There was some doubt whether the methods of analysis for residues
of the parent compound would adequately recover and determine all
conjugated materials or other biologically active metabolites. The
method of Storherr and Onley (1962) is the most sensitive and specific
method available, and is reported by several authors to recover
amitrole from some conjugates. Under the circumstances the Meeting
felt confident in recommending a maximum residue limit at or about the
limit of determination by the beet available method. The Meeting
however proposed certain precautions to avoid contamination of food
crops.
RECOMMENDATIONS
There is no reason to believe that significant residues occur in
any raw agricultural commodities when amitrole is used for the control
of weeds according to approved directions.
To reduce the possibility of contaminating food crops with
residues of amitrole, use-patterns should avoid the direct treatment
of food crops and should be limited to directed sprays, spot sprays
and, in the case of pre-planting and stubble treatments, application
to weeds at least 10 days before ploughing (see Table 2), the interval
to be conditioned by the temperature of the soil.
For regulatory purposes and as a means of determining whether
amitrole herbicides have been misused or incorrectly applied the
Meeting recommends a maximum residue limit at or about the limit of
determination by the best available analytical method.
CONDITIONAL TOLERANCE
mg/kg
Raw agricultural commodities of plant origin 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Long term feeding studies in a sufficient number of rats and mice
with low levels of amitrole of known composition and purity.
2. Studies to elucidate the possible relationship between the effects
of amitrole on the thyroid and on the liver.
3. Studies to show that the analytical methods determine not only the
parent compound but also biologically active metabolites.
4. Studies to develop a specific method sensitive to 0.005 mg/kg.
REFERENCES
Alexander, N.M. (1959a). Antithyroid action of 3-amino-1,2,4-triazole.
J. biol. Chem., 234:148-150.
Alexander, N.M. (1959b). Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by antithyroid compounds. J. biol. Chem.,
234:1530-1533.
Amchem (1965). Residues of amitrole in apples. Report Amchem Products
Inc. (Unpublished)
Amchem (1969). Analysis of soyabeans and soyabean plants for possible
residues of amitrole. Review of collaborative trials. Amchem Products
Inc. Report, Jan. 1969.
Amchem (1970a). Residues of aminotriazole in corn. Collection of
studies by various workers. Submission to Amitrole Committee, 16 Dec.
1970.
Amchem (1970b). Residues of amitrole in wheat and soil when used for
chemical fallow. Submission to Amitrole Committee, 16 Dec. 1970.
American Cyanamid (1958). Colorimetric method for the estimation of
residues of amitrole in cranberry fruit. American Cyanamid Co., 4 Feb.
1958.
Ashton, F.M. (1963). Fate of amitrole in soil. Weeds, 11:167-170.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. Am. med.
Ass., 172:1319-1320.
Auerbach, V.H., Piesinger, R.H. and Waisman, H.A. (1959). The effect
of 3-amino-1,2,4-triazole on the synthesis of tryptophan peroxidase.
Archs Biochem. Biophys., 82:370-379.
Axelson, O., Rehn, M. and Sundell, L. (1974). Herbicide exposure,
mortality and tumour incidence. An epidemiological investigation on
Swedish railroad workers. Envir. Hlth. (in press)
Bagdon, R., Vidone, L., Shaffer, C. and Golz, H. (1965).
Aminotriazole: acute and subacute toxicity. Report from American
Cyanamid Co. (Unpublished)
Baron, J. and Tephyly, T.R. (1969). Effect of 3-amino-1,2,4-triazole
on the stimulation of hepatic microsomal heme synthesis and induction
of hepatic microsomal oxidases produced by phenobarbital. Molec.
Pharmac., 5:10-20.
Bayer (1973). Amitrole residues in peaches, plums, grapes, cherries
and apples. Reports 434/72 - 443/72.
Bondarenko, D.D. (1958). Decomposition of amitrole in soil. The Ohio
Agricultural Experiment Station and The Ohio State University. Proc.
N. cent. Weed Control Conf.
Boyd, J.E. (1964, 1965). Some physiological and phytotoxicological
properties of amitrole. American Cyanamid Co.
Brown, J.C. and Carter, M.C. (1968). Influence of amitrole upon
protein metabolism in bean plants. Weed Sci., 16(2): 222-226.
Carter, M.C. (1969). Amitrole. In P.C. Kearney and D.D. Kaufman
(editors). Degradation of herbicides. Marcel Dekker, Inc., New York,
p. 187-206.
Carter, M.C. and Naylor, A.W. (1959). The formation, in vivo, of a
complex between 3-amino-1,2,4-triazole and a glycine-serine
derivative. Pl. Physiol., Lancaster, 34:(Suppl.) vi.
Carter, M.C. and Naylor, A.W. (1960, 1961). Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot. Gaz., 122:138-143.
Carter, M.C. and Naylor, A.W. (1961a). Studies on an unknown metabolic
product of 3-amino-1,2,4-triazole. Physiologia Pl., 14:20-27.
Carter, C. and Naylor, A.W. (1961b). The effect of
3-amino-1,2,4-triazole upon the metabolism of carbon labeled sodium
bicarbonate, glucose, succinate, glycine and serine by bean plants.
Physiologia Pl., 14:62-71.
Castelfranco, P. and Brown, M.S. (1963). A hypothesis of amitrole
action based on its behavior toward free radical generating systems.
Weeds, 11(2):116-124.
Clyne, R. (1970). Statement to Amitrole Advisory Committee.
Corneliussen, P.E. (1969). Residues in food and feed. Pesticide
residues in total diet samples (IV). Pestic. Monit. J., 2:140-152.
Corneliussen, P.E. (1970). Pesticide residues in total diet samples
(V). Pestic. Monit. J., 4(3):89-105.
Dardin, V. (1958). 3-amino-1,2,4-triazole - chronic oral
administration, beagle dogs, 52 weeks. Histology - supplement to
report. Report from Hazelton Laboratories, Inc. (Unpublished)
Dardin, V. (1959). 3-amino-1,2,4-triazole. Histology - supplement to
report of two year dietary feeding - rats. Report from Hazelton
Laboratories, Inc. (Unpublished)
Day, B.E. and Hendrixson, R.T. (1959). A study of the accumulation and
depletion of amitrole in citrus fruit and foliage. University of
California, Riverside, Research Report.
Day, B.E., Jordan, L.S., and Hendrixson, R.T. (1961). The
decomposition of amitrole in Californian soils. Weeds, 9:443-456.
Demint, R.J., Frank, P.A., Comes, R.D. (1970). Amitrole residues and
rate of dissipation in irrigation water. Weed Sci., 18(4):439-442.
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966). Pesticide residues
in total diet samples. Science, 151:101-104.
Duggan R.E., Barry, H.C. and Johnson, L.Y. (1967). Residues in food
and feed. Pesticide residues in total diet samples (II). Pestic.
Monit. J., 1(2):2.
Dunster, K.W. (1969). Amitrole persistence in irrigation canals.
Report of trials in Montana, Washington and Colorado. Amchem Report, 7
May 1969.
Elsea, J. (1954). Acute dermal application, acute eye application.
Report from Hazelton Laboratories, Inc. (Unpublished)
Ercegovich, C.D. (1957). Studies on the analysis and persistence of
3-amino-1,2,4-triazole in plant tissue and in soil. Ph.D. thesis,
Pennsylvania State University, University Park, Pa.
Ercegovich, C.D. and Frear, D.E.H. (1964). The fate of
3-amino-1,2,4-triazole in soils. J. agr. Food Chem., 12:26-29.
Fabrizio, D.P.A. (1973). Final report on cytogenicity study on
3-amino-1,2,4-triazole from Litton Bionetics, Inc. submitted to WHO by
Rohm and Haas Co. (Unpublished)
Fang, S.C., George, M. and Yu, T.C. (1964). Metabolism of 3-amino-1,2,
4-triazole-5-C14 by rats. J. agr. Food Chem., 12:219-223.
Fang, S.C., Khanna, S. and Rao, A.V. (1966). Further study on the
metabolism of labeled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. agr. Food Chem., 14:262-265.
Fang, S.C., Fallen, E. and Khanna, S. (1967). The metabolism of
labeled amitrole in plants. Weeds, 15:343-346.
Feytmans, E. and Leighton, F. (1973). Effects of pyrazole and
3-amino-1,2,4-triazole on methanol and ethanol metabolism by the rat.
Biochem. Pharmac., 22:349-360.
Fleming, A.S. (1959). Statement about amitrole residues in cranberries
- Press Release. Dept of Health, Education and Welfare, 9 Nov. 1959.
Fogleman, R. (1954). Amitrole - (3-amino-1,2,4-triazole) - Final
report: submitted by Hazelton Laboratories, Inc. (Unpublished)
Frank, P.A. (1969). Amitrole concentration in water of treated
irrigation channels. U.S. Dept of Agriculture A.R.S., Denver,
Colorado. Report, 14 Jan. 1969.
Fredrick, J.F. and Gentile, A.C. (1960). The formation of the glucose
derivative of 3-amino-1,2,4-triazole under physiological conditions.
Physiologia Pl., 13:761-765.
Fredrick, J.F. and Gentile, A.C. (1961). The structure of the
D-glucose adduct of 3-amino-1,2,4-triazole: Infrared absorption
studies. Arch. Biochem. Biophys., 92(2):356-359.
Fredrick, J.F. and Gentile, A.C. (1962). Studies of a phosphorylated
derivative of 3-amino-1,2,4-triazole formed by the action of yeast
hexokinase. Physiologia Pl., 15(1):186-193.
Fredrick, J.F. and Gentile, A.C. (1965). Physico-chemical studies of
the metabolic end product of 3-amino-1,2,4-triazole in yeast.
Phytochemistry, 4:851-856.
Freed, V.H., Furtick, W.R. (1961). The persistence of amitrole in
soil. Oregon Agricultural Experiment Station Technical Paper No. 1375.
Freed, V.H., Montgomery, M. and Kief, M. (1961). Proc. N East Weed
Control Conf., 1961:6-16.
Fregly, M.J. (1968). Effect of aminotriazole on thyroid function in
the rat. Toxic. appl. Pharmac., 13:271-286.
Gaines, T.B., Kimbrough, R.D. and Linder, R.E. (1973). Toxicity of
amitrole in the rat. Toxic. appl. Pharmac., 26:118-129.
Geldmacher-von Mallinckrodt, M. and Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Archs Toxik., 27:13-18. (English
summary)
Gentile, A.C. and Fredrick, J.F. (1959). Chemical activity of the
glucose adduct of 3-amino-1,2,4-triazole. Physiologia Pl., 12:862-867.
Grapenthien, J., Hathaway, D. and Keplinger, M. (1972). Two year
chronic aerosol inhalation toxicity study with amitrol 3AT in albino
rats. Report from Industrial Biotest Laboratories, Inc. (Unpublished)
Groves, K. and Chough, K.S. (1971). Extraction of 3-amino-1,2,
4-triazole (amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from
soils. J. agr. Food Chem., 19(5):840-841.
Hapke, H.J. (1967). The toxicity of aminotriazol for domestic animals.
Zbl. Vet. Med. A., 14:469-486. (English, French and Spanish summaries)
Heim, W.G., Appleman, D. and Pyfrom, H.T. (1956). Effects of
3-amino-1,2,4-triazole (AT) on catalase and other compounds. Am. J.
Physiol., 186:19-23.
Herrett, R.A. and Bagley, W.P. (1964). The metabolism and
translocation of 3-amino-1,2,4-triazole by Canada thistle. J. agr.
Food Chem., 12:17-20.
Herrett, R.A. and Linck, A.J. (1961a). Penetration of
3-amino-1,2,4-triazole in Canada thistle and field bindweed. Weeds,
9(2): 224-230.
Herrett, R.A. and Linck, A.J. (1961b). Quantitative determination of
3-amino-1,2,4-triazole. J. agr. Food Chem., 9(6):466-467.
Hill, R. (1962, 1963). Residues of amitrole in almonds, walnuts,
peaches and prunes. Diablo Laboratories, Berkley, Reports of Amchem
Products Inc.
Hilton, H.W., Uyehara, G. and Segal, H.S. (1963). Amitrole residues in
sugar cane. Rate of disappearance studies and improved methodology.
Hawaiian Sugar Planters Association Report.
Hilton, H.W. and Uyehara, G.K. (1966). Determination of
3-amino-1,2,4-triazole in sugarcane. J. agr. Food Chem., 14(1):90-94.
Hodge, H.C., Maynard, E.A., Downs, W.L., Ashton, J.K. and Salerno
L.L. (1966). Tests on mice for evaluating carcinogenicity. Toxic.
appl. Pharmac., 9:583-596.
Hoshino, M. (1960). Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature, 186:174-175.
Hylin, J.W. (1962). Determination of amitrole residues in coffee
beans. Report from University of Hawaii.
IARC (1974). Amitrole. In IARC Monographs on the Carcinogenic Risk
of Chemicals to Man. Vol. 7.
Innes, J. (1966). Amitrole - summary sheet - Carcinogenesis assay
studies - Subcutaneous. Data sheet. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969). Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Jukes, T.H. and Shaffer, C.B. (1960). Antithyroid effects of
amino-triazole. Science, 132:296-297.
Jukes, T.H. (1961). Antimetabolites and plant growth. Bull. Torrey
bot. Club, 88(5):321-327.
Kaufmann, D.D. (1966). Microbial degradation of herbicide
combinations: amitrole and dalapon. Weeds, 14:130-134.
Kaufmann, D.D., Plimmer, J.R., Kearney, P.C., Blake, J. and Guardia,
F.S. (1968). Chemical versus microbial decomposition of amitrole in
soil. Weed Sci., 16(2):266-272.
Keller, J. (1959). Aminotriazole (3-amino-1,2,4-triazole) two year
chronic feeding - rats. Report from Hazelton Laboratories, Inc.
(Unpublished).
Kretschmar, G. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) on the growth of Azotobacter. Z. allg. Mikrobiol.,
10:253-267.
Kretschmar, G. and Gunther, G. (1970). Der einflus von
3-amino-1,2,4-triazol (Amitrol) auf die Atmungvon Azobacter. A.
allg. Mikrobiol., 10:487-500.
Kröller, E. (1966). Anwendung und Eigenschaften des
3-amino-1,2,4-triazols im Hinblick auf seine Rückstände im
Lebensmitteln. Residue Reviews, 12:162-192.
Kudsk, F.N. (1969). Uptake of mercury vapor in blood in vivo and
in vitro from Hg-containing air. Acta pharmac. Tox., 27:149-160.
Landauer, W., Salam, N. and Sopher, D. (1971). The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Envir. Res.,
4:529-543.
Langhans, W. and Schimassek, H. (1974). Prevention of halothane
induced changes in rat liver enzymes by 3-amino-1,2,4-triazole.
Biochem. Pharmac., 23:403-409.
Leonard, O.A., and Lider, L.A. (1961). Toxicity and translocation of
herbicides supplied to grape rootings through solution culture. Am. J.
Enol. Vitic., 12(1):37-46.
Leonard, O.A., and Weaver, R.J. (1961). Absorption and translocation
of 2,4-D and amitrole in shoots of the Tokay grape. Hilgardia,
31(9):327-368.
Levine, W.G. (1973). Effect of phenobarbital and 3-amino-1,2,
4-triazole on the metabolism and biliary excretion of 3,4-benzypyrene
in the rat. Life Sci., 13:723-732.
Lotlikar, P., Wasserman, M. and Luha, L. (1973). Effect of
2-amino-1,2,4-triazole pretreatment on N and ring - hydroxylation of
2-acetylamino-fluorene by the rat. Proc. Soc. exp. Biol. Med.,
144:445-449.
Magos, L., Sugata, Y. and Clarkson, T.W. (1974). Effects of
3-amino-1,2,4-triazole on mercury uptake by in vitro human blood
samples and by whole rats. Toxic. appl. Pharmac., 28:367-373.
Maier-Bode, H. and Bechtel, W.D. (1968). Investigations of amitrole
residues in orchardry and viticulture. A. Pflanzenkrankheiten No. 4.
Proceedings of the 7th German Symposium on questions of weed biology
and control, 5 March 1968.
Margoliash, E. and Novogrodsky, A. A study of the inhibition of
catalase by 3-amino-1,2,4-triazole. Biochem. J., 68:468-475.
Marston, R.B., Schults, D.W., Shiroyama, T., and Snyder, L.V. (1968).
Pesticides in water. Amitrole concentrations in creek waters
downstream from an aerially sprayed watershed sub-basin. Pestic.
Monit. J., 2(3):123-128.
Massini, P. (1958). Uptake and translocation of 3-amino and
3-hydroxy-1,2,4-triazole in plants. Acta bot. neerl., 7:525-530.
Massini, P. (1959). Synthesis of (3-amino-1,2,4-triazolyl) alanine
(ATX) from 3-amino-1,2,4-triazole in plants. Biochem. Biophys. Acta,
36:548-549.
Massini, P. (1963). Aminotriazolylalanine; a metabolic product of
amitrole in plants. Acta bot. neerl., 12:64.
Matsushima, T. and Weisburger, J. (1972). Effect of carbon monoxide or
of 3-amino-triazole on C- and N- hydroxylation of the carcinogen
N-2-fluorenylacetamide by liver microsomes of hamsters pretreated with
3-methyl-cholanthrene. Xenobiotica, 2:423-430.
McRae, I.C. and Alexander, M. (1965). Microbial degradation of
selected herbicides in soil. J. agr. Food Chem., 13:72-76.
Meissner, W.A. (1971). Report of Amitrole Advisory Committee to
Environmental Protection Agency Washington, 12 March 1971.
Menzie, C.M. (1969). Metabolism of Pesticides. U.S. Dept. Interior,
Bureau of Sport Fisheries and Wildlife Special Scientific
Report - Wildlife No. 127:40-41.
Miller, C.S. and Hall, W.C. (1961). Absorption and metabolism of
aminotriazole in cotton. J. agr. Food Chem., 9:210-212.
Mohandas, T. and Grant, W.F. (1972). Cytogenetic effects of 2,4-D and
amitrole in relation to nuclear volume and DNA content in some higher
plants. Can. J. Genet. Cytol., 14:773-783.
Montgomery, M. and Freed, V.H. (1963). Residue studies of
amino-triazole in soyabeans. Report from Oregon State University, 25
April 1963.
Moore, B. (1968). Amitrole and simazine residue trials in Tasmanian
apples treated with D amatol 44. Geigy Australia Ltd., Report
68/8/290.
Moore, B. (1969). Amitrole and simazine residue trials at Orange, NSW,
1967-68. Geigy Australia Ltd., Technical Report 69/2/294.
Moore, B. (1970). Amitrole and simazine residue trials at Orange, NSW,
1968-69. Geigy Australia, Technical Report 70/1/309.
Moser, B. (1968). Summary statements on triazoles. Rutgers Univ. Plant
Physiology Seminar.
Muzik, T.J. (1965). Effect of temperature on the activity and
persistence of amitrole and 2,4-D. Weed Res., 5(3):207-212.
Napalkov, N. (1962). Tumor inducing action of 3-amino-1,2,4-triazole.
Truda prof. Zabol., 6:48-51.
Napalkov, N.P. (1969). On blastomogenic action of thyreostatic
substances. IECO USSR Acad. Med. Sci. Moscow, Monograph.
Naylor, A.W. (1964). Complexes of 3-amino-1,2,4-triazole in plant
metabolism. J. agr. Food Chem., 12:21-25.
Nicholson, H.P. (1963). Persistence of amitrole in pond water. U.S.
Public Health Service, Report 17 April 1963.
Norris, L.A. (1970). Degradation of herbicides in the forest floor. In
Tree Growth and Forest Soils, C.T. Youngberg and C.B. Daley (editors).
Onley, J.H., Storherr, R.W., Cluver, A.J. Jr. (1963). An Oregon
cranberry bog study for 3-amino-1,2,4-triazole residues, 1961-1962. J.
Ass. off. analyt. Chem., 46(6):996-1001.
Otten, R.S. (1970). Statement to Amitrole Advisory Committee, 16 Dec.
1970.
Plimmer, J.R., Kearney, P.C., Kaufman, D.D. and Guardia, F.S. (1967).
Amitrole decomposition by free radical-generating systems and by
soils. J. agr. Food Chem., 15: 996-999.
Potts, K.T. (1961). The chemistry of 1,2,4-triazoles. Chem. Rev.,
61:87-127.
Racusen, D. (1958). The metabolism and translocation of
3-amino-triazole. Archs Biochem. Biophys, 74(1):106-113.
Raisfeld, I., Bacchin, P., Hutterer, F. and Schaffner, F. (1970). The
effect of 3-amino-1,2,4-triazole on the phenobarbital induced
formation of hepatic micro-somal membranes. Molec. Pharmac., 6:231.
Rausina, B., Mastri, C., Keplinger, M. and Yost, D. (1972). Two year
chronic dermal toxicity study with amitrole 3-AT in albino rats.
Report from Industrial BioTest Laboratories, Inc. (Unpublished)
Riepma, P. (1962). Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in soil. Weed Res., 2:41-50.
Rogers, B.J. (1957a). Translocation and fate of amino triazole in
Plants. Weeds, 5:5-11.
Rogers, B.J. (1957b). The action of 3-amino-1,2,4-triazole in plants.
Hormolog 1:10.
Samio, H., Baird, M. and Massie, H. (1972). Renewal of catalase
activity in Drosophilia following treatment with 3-amino-1,2,
4-triazole. J. Insect. Physiol., 18:991-1000.
Sanderson, D. and Roe, F. (1962). Dietary toxicity of aminotriazole to
the female rat. Report from Fisons Ltd., Agrochemical Division and
Pathology Report from Chester Beatty Research Institute. (Unpublished)
Schroeder. I. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) in a non-herbicidal range on the protein biosynthesis of
the unicellular algae Poteriochromonas stipitata. A. Allg.
Mikrobiol., 10:295-298.
Schubert, O.E. (1965). Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Report from West Virginia
University.
Segal, H.S. (1960). Determination of amitrole in irrigation ditch
water. Amchem Laboratory Report 411, July 1960
Segal, H.S. (1963). Amitrole residues in Hawaiian irrigation ditch
water. Amchem Report, 27 Feb. 1963.
Shaffer, C., Vidone, L. and Golz, H. (1958). Aminotriazole: 32 day
repeated feeding to rats. Report from American Cyanamid Co.
(Unpublished)
Smagghe, G. (1974). Report from Chemical Products - Ugine - Kuhlmann.
(Unpublished)
Smith, L.W. and Chang, F.Y. (1973). Aminotriazole metabolism in
Cirsium arvense (L.) Scop. and Pisum sativum L. Weed Res.,
13:339-350.
Stenger, R. and Johnson, E. (1972). Modifying effect of
3-amino-1,2,4-triazole on phenobarbital induced changes in rat liver.
Expl. Molec. Pathol., 16:147-157.
Storherr, R.W. and Burke, J. (1961). Determination of 3-amino-1,2,
4-triazole in crops. J. Ass. off. analyt. Chem., 44(2):196-199.
Storherr, R.W. and Onley, J. (1962). Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J. Ass.
off. analyt. Chem., 45(2):382-387.
Strum, J. and Karnovsky, M. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity. Lab.
Invest., 24:1-12.
Sund, K.A. (1956). Residual activity of 3-amino-1,2,4-triazole in
soils. J. agr. Food Chem., 4(1):57-60.
Svensson, J.A. (1971). Residues of herbicides in cereals. Proceedings
of 12th Swedish Weed Conference, Jan. 1971.
Tephly, T.R., Mannering, G.J. and Parks, R.E., Jr. (1961). Studies on
the mechanism of inhibition of liver and erythrocyte catalase activity
by 3-amino-1,2,4-triazole (AT). J. Pharmac. exp. Ther., 134:77-82.
Tephly, T.R., Hasegawa, E. and Baron, J. (1971). Effect of drugs on
heme synthesis in the liver. Metabolism, 20:200.
Thoman, J.R. (1963). Persistence of amitrole in water (Borrow Pits)
Florida. U.S. Dept of Health, Education and Welfare, Report, 13 Nov.
1963.
Tschudy, D.P. and Collins, A. (1957). Effect of 3-amino-1,2,4-triazole
on delta-amino-levulinic acid dehydrase activity. Science, 126:168.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. and Hidosaka, Y.
(1973). Fine structure and localization of peroxidase activity in
aminotriazole goiter. Nagoya med. J., 18:183-190.
Weir, R., Jr. (1958). 3-amino-1,2,4-triazole chronic oral
administration - Beagle Dogs - 52 weeks - Final Report. Report from
Hazelton Laboratories, Inc. (Unpublished)
Weir, R. Jr. (1974). Personal communication to WHO.
Wit, S.L. and van der Kamp, C.G. (1963). Residues of amitrole in
apples. Report 26 February 1963, National Institute of Public Health,
Utrecht. Submitted by Netherlands Government to FAO (1974).
Yost, J.F. and Williams, E.F. (1958). Proc. N East Weed Control Conf.,
1958:9-15.
Other information on identity and properties
Amitrole is a white, crystalline solid with a molecular weight of
84 and a melting point of 150-153°C. It is soluble in water to the
extent of 28 g/100 g and in ethanol and methanol to an extent of
26 g/100 g. It is sparingly soluble in ethyl acetate and insoluble in
ether, acetone and most other organic solvents. Amitrole forms neutral
aqueous solutions but acts as a weak base. When exposed to UV light,
amitrole breaks down to form CO2, urea and cyanamide. Potts (1961)
has reviewed the chemistry of s-triazoles in considerable detail.
Amitrole behaves chemically as a typical aromatic amine.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
The metabolic fate of amitrole in animals has not yet been fully
elucidated. It is rapidly excreted from the body. Following
intraperitoneal administration, at least 90% of the injected dose was
observed in the urine unchanged within 24 hours (Bagdon et al., 1965).
When amitrole (5-14C) was fed to rats (Fang et al., 1964),
70-96% of the radioactivity was excreted in the urine during the first
24 hours as amitrole and two unidentified metabolites. There were
traces of 14C in the expired air and the faeces contained a small but
variable amount of activity. After absorption, amitrole was
distributed throughout most of the tissues. The maximum radioactivity
in all tissues was generally reached within one hour and started to
decrease 3-4 hours after dosing. Elimination of amitrole from all
tissues was rapid. The liver contained a metabolite but no free
amitrole. The rate of elimination of this metabolite from liver and
kidney was much slower than that of the parent compound.
Further studies on rats (Fang et al., 1966) have shown that
amitrole is not metabolically acetylated, and that the average
half-time for amitrole clearance in various tissues was 4.2 hours.
These studies were extended to include the metabolism of two
metabolites isolated from bean plants. One was readily excreated in
the urine, mainly unchanged but partly as two new metabolites. The
other bean metabolite was excreted much more slowly, apparently
unchanged.
Biotransformations in plants and soil are discussed in the
section "Fate of residues".
Effects on enzymes and other biochemical parameters
Acute administration of amitrole to rats results in depression of
catalase and peroxidase enzyme activity as well as the activity of
several other enzymes. Liver peroxidase was found to recover within 24
hours, while liver and kidney catalase depression was slower to
recover. Catalase returned to normal after seven days (Heim et al.,
1956). Insect-derived catalase was also shown to regenerate its
activity slowly following exposure to aminotriazole (Samio et al.,
1972).
Groups of rats (15 males per group) were administered radioactive
iodine alone or in combination with 0.15 or 0.78 mg amitrole
administered by intraperitoneal injection. Uptake of radioactivity by
the thyroid gland was significantly depressed at 0.78 mg/kg. The lower
dose reduced iodine uptake but the reduction was not statistically
significant. Amitrole significantly decreased fecal radioactivity,
while the urinary radioactivity was not significantly affected
(Fregly, 1968).
Amitrole inhibits peroxidase activity of rat thyroid and salivary
gland (Alexander, 1959a and b); liver and kidney catalase activity of
rats (Tephly et al., 1961; Heim et al., 1956); catalase activity of
human red blood cell (Margoliash and Novogrodsky, 1958; Magos et al.,
1974; Kudsk, 1969) catalase activity from other sources such as plant
tissue and commercial crystallized enzymes; the synthesis of
tryptophan peroxidase of rats (Auerbach et al., 1959);
delta-aminolevulinic acid dehydrase activity of mouse and rat liver
(Baron and Tephley, 1969; Tschudy and Collins, 1957); the inductive
effect of phenobarbital on hepatic microsomal cytochrome P-450 (Baron
and Tephly, 1969); the incorporation of iron (59FeCl3) into rat
hepatic microsomes (Tephly et al., 1971); microsomal heme synthesis
(Tephly et al., 1971); and hydroxylation of exogenous substrates by
the liver (Raisfeld et al., 1970). Amitrole caused no proliferation of
the microsomal endoplasmic reticulum (Raisfeld et al., 1970). Several
further studies have shown the effect of amitrole on certain aspects
of the drug metabolizing enzyme system in rat liver (Feytmans and
Leighton, 1973; Lotikar et al., 1973; Stenger and Johnson, 1972;
Levine, 1973; Matsushima and Weisburgh, 1972; Langhans and Shimassek,
1974). There is no clear picture of the effect of amitrole on
microsomal metabolizing enzyme systems in mammals although it does
serve to inhibit or reduce several specific biochemical reactions.
Kudsk (1969) observed that peroxide generating systems, such as
seen with methylene blue, accelerated the uptake of mercury in vitro
by human red blood cell preparations. Amitrole and methylene blue
which promoted the catalase-peroxide-complex I, had no effect on
mercury uptake. Magos (1974) suggested that the ability of red blood
cells to take up mercury from air saturated with mercury vapor was
reduced when cells were treated for three hours with amitrole and
methylene blue. In hemolysates mercury uptake was stimulated by
amitrole. In vivo administration of amitrole to rats resulted in
decreased lung concentration and increased liver concentrations of
mercury.
Tryptophan synthesis in plants was shown to be inhibited by
amitrole (Smith and Chang, 1973). Amitrole stimulated respiration in
Azotobacter and concurrently inhibited growth possibility through an
uncoupling of oxidative phosphorylation (Kretschmar and Günther,
1970). In algae, amino acid synthesis was inhibited and glucosamine
synthesis was stimulated (Schroeder, 1970).
In vitro, amitrole does not inhibit cholinesterase activity of
brain, submaxillary gland, serum, or ilium. Three hours after
intraperitoneal administration of 4 mg/kg to male rats cholinesterase
activity of brain, submaxillary gland, and serum was normal (Bagdon et
al., 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A testing procedure using groups of hybrid strains of mice (18
mice of each sex and each of two strains) evaluated amitrole for
carcinogenicity. The mice were administered amitrole orally from day 7
to day 28 of age at doses of 1000 mg/kg and thereafter in the diet at
a dose of 2192 ppm. None of the mice survived the 18 month test
interval. Hepatomas were evident in most animals, 67/72, and carcinoma
of the thyroid was reported in 67/71 of the animals examined (Innes et
al., 1969). A further study was designated as subcutaneous although
no details were given of the experimental design (Innes, 1966). The
majority of animals survived the 18 month test interval. There was no
evidence of heptoma or carcinoma of the thyroid.
Groups of mice (50 of each sex) were either treated with amitrole
by a single subcutaneous administration of 10 mg per mouse or by a
weekly dermal application of 0.1 mg/mouse. There was no abnormal
behaviour observed. When the animals were sacrificed and examined, no
signs of cancer were evident. No mention was made of examination of
the thyroid gland in this study (Hodge et al., 1966).
Rat
Amitrole was found to have an effect on the induction of liver
carcinogenesis by 4-dimethylaminoazobenzene. In an attempt to
determine the relationship between liver catalase activity and
carcinogenesis, Hoshino (1960) administered amitrole intraperitoneally
to albino rats at a dose of 1000 mg/kg every other day for 150 days
while feeding 4-dimethylaminoazobenzene at a dose that was known to
induce liver carcinoma. There was a reduction in the occurrence of
liver tumours in those animals administered amitrole (4/14) when
compared to the animals administered the carcinogen alone (12/16).
Napalkov (1962; 1969) administered amitrole to rats by
subcutaneous injection twice weekly (later reduced to a weekly
injection) at a dose of 125 mg/animal; in the water at a dose of 20-25
mg/day; in the diet at a dose of 250-500 mg/day; or by subcutaneous
implantation combined with subcutaneous administration twice weekly at
a dose of 125 mg/rat. Thyroid hyperplasia was observed after seven to
eight months. Liver tumours were observed initially in those animals
receiving amitrole in the diet and soon thereafter in those animals
receiving it by injection or via the drinking water. Sarcomas
developed where amitrole was implanted or injected. Concurrent daily
injections of 2.5 µg/100 g bw thyroxine and oral administration of 300
mg/day/rat amitrole in food for the length of the experiment resulted
in the development of only one thyroid adenoma but 9 liver tumors in
12 males. Amitrole alone induced thyroid tumors in 7/22 and liver
tumors in 12/23 male rats of the same age. No liver tumors but 2
thyroid cystic adenomas were observed in 51 control animals.
A comprehensive review of the carcinogenic risk of amitrole was
made by the International Agency for Research on Cancer commenting on
the animal and human data (IARC, 1974).
Special studies on mutagenicity
Mohandas and Grant (1972) observed that amitrole significantly
increased the frequency of chromosomal aberrations in root tips of
higher plants.
Mutagenic tests using Salmonella strains and yeast cells as
test indicators have been negative. A host mediated assay has been
reported to be negative although details of the test are not yet
available (Weir, 1974). A cytogenetic study, utilizing examination of
bone marrow cells arrested in C-metaphase from rats treated with
amitrole at levels of 0, 2.5, 25 and 250 mg/kg daily for 5 days, was
negative (Fabrizio, 1973).
Special studies on reproduction
Rat
Groups of rats (10 male and 10 female Sherman strain rats per
group) were fed amitrole in the diet at levels of 0, 25, 100, 500 and
1000 ppm for approximately two months and mated. In the first
generation at 500 and 1000 ppm the number of pups born and pup
survival was reduced. It was noted that almost all of the pups died
within one week after weaning and these dietary levels were
terminated. Dietary levels of 0, 25, and 100 ppm were fed through two
generations (two litters from the first generation and one litter from
the second generation). At 100 ppm in the diet there was no effect on
reproduction and all pups survived. At 100 ppm all animals were found
to have thyroid hyperplasia while the incidence of thyroid hyperplasia
was sporadic at 25 ppm (Gaines et al., 1973).
Oral administration of amitrole at doses of 400 and 1000 mg/kg to
rats on days 8 through 13 of gestation resulted in no signs of embryo
toxicity or of teratogenic effects. Groups of rats (5 males and 5
females per group) were administered amitrole orally at 100 mg/kg or
at 100 ppm in the drinking water for three months prior to mating and
in the females up to day 15 of gestation. There was no indication of
any effect on reproduction in either male or female rats (Hapke,
1967).
The effect of oral administration of amitrole on reproduction was
studied in three groups of eight pregnant Sherman strain rats
administered dosage levels of 0, 20 and 100 mg/kg bw/day from day 7
through 15 of gestation. There was no effect of amitrole on
reproduction and no abnormalities were observed in the offspring
through weaning (Gaines et al., 1973).
Special studies on teratogenicity
Amitrole injected into the yolk of chicken eggs at doses of 20-40
mg/egg, produced a dose dependent malformation of the beak and
abnormalities of the tibia shaft. This latter abnormality occurred
less frequently. At doses of 2 mg/egg, no effects ware noted. It was
observed that when amitrole was dissolved in DMSO it was more active
than when it was administered in water (Landauer et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of amitrole
LD50
Species Route (mg/kg bw) References
Rat oral >25 000 Bagdon et al., 1965
dermal > 2 500 Gaines et al., 1973
ip > 4 000 Bagdon et al., 1965
Mouse oral >14 700 Fogleman, 1954
iv > 1 600 Bagnon et al., 1965
Rabbit dermal >10 000 Elsea, 1954
oral > 2 150 Fogleman, 1954
Dog iv > 1 800 Fogleman, 1954
Cat iv > 1 750 Bagdon et al., 1965
Signs of poisoning include: depression, dyspnea, diarrhoea,
ataxia, altered respiration, coma and death. The G.I. tract was
severely irritated following acute doses. Acute doses administered to
dogs (50-1000 mg/kg) intravenously produced an immediate fall in blood
pressure followed by increased respiration. Following a dose of 1000
mg/kg, the pressor response to adrenalin was blocked suggestive of an
adrenalytic action with respect to blood pressure (Fogleman, 1954).
TABLE 2. Acute toxicity of glucose adduct
LD50
Species Sex Route (mg/kg bw) References
Rat M oral >10 000 Bagdon et al., 1965
Mouse M oral >10 000 Bagdon et al., 1965
M ip >10 000 Bagdon et al., 1965
M iv > 1 600 Bagdon et al,, 1965
Amitrole applied to unabraded skin as a paste at doses of
1-10 g/kg for 24 hours caused a mild dermal irritation at all dose
levels. The effect, a mild erythemia, was reduced within 24 hours. No
other effects were noted. Gross and microscopic examination of tissues
and organs was normal (Elsea, 1954).
Amitrole (3 mg) was applied to the conjuntival sac of rabbits.
Although mild irritation lasting 24-48 hours was observed, no
permanent damage was noted (Elsea, 1954).
Short term studies
Rat
Groups of rats (5 males and 15 females per group) were fed
amitrole in the diet at levels of 0, 0.01%, 0.1%, and 1.0% for 63
days. There was no mortality over the course of this study. Food
consumption and growth were depressed at 0.1% and above in both males
and females. Histological examination from selected tissues at the
conclusion of the study (thyroid was not examined) revealed
vacuolation of liver cells in those animals fed 0.1% and above. The
vacuoles were identified as fat globules indicative of fatty
metamorphosis associated with liver cell damage. No histological
effects were noted at 0.01% (Fogleman, 1954).
Groups of weanling rats were administered amitrole for up to 56
days by intraperitoneal injection three times/week for eight weeks at
1000 mg/kg. The administration of a recrystallized amitrole resulted
in no growth depression in two separate groups. The administration of
a "pure" material, not recrystallized, resulted in growth depression
(Heim et al., 1956).
Groups of rats (10 males and 10 females per group) were
administered amitrole at 1000 mg/kg by intraperitoneal administration
on alternate days for 42 days. Amitrole had to effect on bw gain or
food consumption but caused a 3-4 fold increase in thyroid weight
(Bagdon et al., 1965).
Groups of rats were administered amitrole daily, 5 days per week,
for four weeks at levels of 0, 100, 200 and 400 mg/kg. Growth rate was
reduced, relative thyroid weight increased, and iodine content of the
thyroid was reduced (Hapke, 1967).
Administration of dietary levels of 60 and 120 ppm for two weeks
resulted in an enlargement of the thyroid gland of rats and a
pronounced lowering of iodine uptake. Over this two week interval
there were no significant changes at levels of 15 and 30 ppm (Jukes
and Shaffer, 1960).
Administration of a high dose (0.04%) of amitrole in the drinking
water (approximately 60 mg/kg/day) has been shown to produce goitres
in rats within three days (Strum and Karnovsky, 1971; Tsuda et al.,
1973).
Groups of rats (10 male rats per group) were fed dietary
concentrations of amitrole for 32 days. One group was fed 500 ppm for
32 consecutive days, another group received 1000 ppm on alternate days
for the duration of the study and a group was fed the basal diet.
Behavior and mortality were not affected. Food intake and growth at
500 ppm were reduced. In the group receiving 1000 ppm on alternate
days food intake and growth data did not differ significantly from the
controls. At autopsy, gross examination of animals receiving 500 ppm
showed the thyroid gland to be hyperaemic and enlarged. The thyroid of
animals fed 1000 ppm appeared to be slightly hyperaemic but were
otherwise comparable to the controls. Mean thyroid weight/body weight
ratio for the 0, 1000 ppm, and 500 ppm groups was 58, 77, and 303
respectively (Shaffer et al., 1958).
A group of 30 male weanling rats was fed amitrole in the diet at
1000 ppm for two weeks. A comparable group received a similar dietary
level of propylthiouracil for two weeks. At the end of the feeding
interval several animals were sacrificed and the thyroid glands
weighed. The remaining animals were placed on control diets and
sacrificed at either the third or fourth week of feeding. Amitrole,
after two weeks produced a significant increase in the mean thyroid
weight (comparable to that found with propylthiouracil). Daring the
week following removal from the diet, the weight of the thyroid gland
diminished and by the end of the second week on a control diet
regression to normal size was almost complete (Bagdon et al., 1965).
Groups of rats (20 rats per group) were fed dietary l levels of 0
and 316 ppm for 100 days to examine the goitrogenic potential of
amitrole. Over the course of this experiment amitrole had a
significant effect on growth. Exophthalmia was not observed as in the
long term rat studies. Food intake was not drastically reduced. Gross
examination of the thyroid showed a laterally enlarged thyroid with
enlarged blood vessels. Microscopic examination showed hyperplasia of
the thyroid. There was no evidence of abnormalities observed in either
the liver or the kidney (Sanderson and Row, 1962).
Groups of rats (10 males per group) were administered amitrole in
the drinking water at concentrations of 0, 50, 250 and 1250 ppm for
106 days. The administration of amitrole resulted in a dose-dependent
depression of growth with a corresponding reduction of food and water
intake. Appearance, behaviour, and mortality were not affected by
amitrole. Gross and microscopic examination of tissues and organs
showed a marked increase in thyroid size at all dose levels. In rats
where reduced growth was noted, the kidneys, adrenals, liver and
spleen were proportionately smaller. Reproductive organs were not
affected. Microscopic examination showed general enlargement of the
thyroid at 50 ppm, with moderate stimulation of the thyroid epithelium
(no evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident (Bagdon et al., 1965).
Groups of rats (10 male rate per group) were fed dietary levels
of amitrole for 11-13 weeks at dosages of 0, 0.25, 0.50, 2, 10 and 50
ppm. At 0.5 ppm in the diet, no effect was observed in any of seven
separate measurements of thyroid function although iodine uptake was
slightly reduced and serum PBI concentrations were slightly increased.
Significant effects were noted at 2 ppm in the diet, especially with
regard to reduced PBI and reduced iodine uptake by thyroid. Gross and
microscopic examination of the thyroid confirmed the effects noted at
2 ppm (Fregly, 1968).
Dog
Groups of beagle dogs (3 males and 3 females were controls; 2
males and 4 females - 0.25 mg/kg; 3 males and 3 females - 1.25 mg/kg;
2 males and 4 females - 2.50 mg/kg; and 2 males and 2 females - 12.5
mg/kg) were administered amitrole orally by gelatin capsule six days a
week for 52 weeks. One male and one female per group were sacrificed
at 26 weeks. There was no mortality over the testing interval. Growth,
appearance, and behaviour were normal in all test animals. Results of
biochemical, haematological, and urological examinations were normal.
Gross and microscopic examination of tissues and organs showed no
evidence of abnormality associated with amitrole. There was no
apparent evidence of cytotoxic effects associated with the
administration of 12.5 mg amitrole kg bw/day for one year (Weir, 1958;
Dardin, 1958).
Long term studies
Dermal
Rat
Two groups of rats (25 males and 25 females per group) were
administered amitrole dermally at a level of 2.4 mg/kg weekly for 23
months. The application was allowed to contact the skin for thirty
minutes, after which the skin was rinsed and dried. Amitrole was found
to be non-irritating to the skin. There was no apparent effect of
amitrole on growth, behaviour, tumour formation or when tissues and
organs were observed on gross and microscopic examination. Statistical
studies with the liver and thyroid organ weight and organ-to-body
weight ratio did not reveal any differences in this experiment between
treated animals and controls (Rausina et al., 1972).
Inhalation
Rat
Groups of rats (25 males and 25 females per group) were exposed
to amitrole by inhalation for a one hour period every week for two
years. The animals were exposed to an aerosol of 0.2% (w/v) aqueous
solution by a head-only exposure to minimize oral ingestion. The
average analytical concentration of aerosol in the exposure chamber
was 2 mg/l air. There were no significant differences between the
treated and the control groups when examined with respect to
mortality, behaviour, growth, gross and microscopic examination of
thyroid and liver, or the incidence of tumor formation. No abnormal
effects were noted in this inhalation exposure (Grapenthien et al.,
1972).
Feeding
Rat
Groups of rats (35 males and 35 females per group) were fed
amitrole in the diet for two years at levels of 0, 10, 50, 100 and 500
ppm. Animals were sacrificed periodically over the course of the
experiment. The appearance of animals (especially females) fed 100 ppm
in the diet was altered. The incidence of protruding eyes
(exophthalmia) was considerably higher in the higher dose groups than
in the controls. Growth was reduced at 500 ppm. There was no apparent
effect of amitrole in the diet on survival, growth, or mortality in
the animals fed 50 ppm.
Gross examination of animals revealed a consistent finding of
thyroid enlargement. At 13 weeks, thyroid enlargement in males and
females was observed at 100 ppm. Microscopic examination suggested
hypofunction and a non-functional hyperplasia evident at 50 ppm and
above. The animals fed 500 ppm were removed from the diet at 19 weeks
and examined after two weeks on control diets. No evidence of
enlargement or hyperplasia was noted. At 26 weeks, thyroid enlargement
was evident at 50 ppm. Liver and kidney weight in both sexes was
decreased at 100 ppm. No effects were noted at 10 ppm. At 52 weeks,
growth of males was depressed at 100 ppm. At 68 weeks, growth of males
was again depressed at 100 ppm accompanied by enlargement of the
thyroid, pituitary, and liver. A cystic adenomatous structure was
observed at 100 ppm in the thyroid. At 50 ppm, thyroid hyperplasia and
hypofunction were noted. At 10 ppm, thyroid hypofunction was observed
in one male animal of the three males and three females examined. At
104 weeks, body weights were normal in all groups. At 100 ppm, liver
and kidney weights were normal while the thyroid was enlarged and
adenomas ranging from benign cysts to foetal malignant carcinoma were
observed. At 50 ppm, the thyroid was not enlarged. Adenomas were
observed in 3/16 rats. At 10 ppm, one rat showed an adenomatous nodule
with cellular hyperplasia (other sections suggested slight enlargement
while most control sections were normal). Thyroid enlargement was
observed only at 100 ppm. A no-effect level was not observed in this
study. Ten ppm was a minimal effect level (Keller, 1959; Dardin,
1959).
OBSERVATIONS IN MAN
A 39 year old woman ingested 20 mg/kg. This dose caused no signs
of intoxication and within a few hours of ingestion the compound
passed rapidly through the body and began to appear in concentrations
up to 100 mg/100 ml in the urine (Geldmacher-von Mallinckrodt, 1970).
Amitrole has been produced industrially since 1955 with no
evidence of ill effects other than mild contact dermatitis to the
occupationally exposed workers (Smagghe, 1974; Clyne, 1970). It was
suggested that from 1955-60, sixteen employees were exposed for five
to six months per year and from 1967 to 1970, nine employees were
exposed for approximately ten months per year with no ill effects. No
thyroid or liver tumors were observed "in excess of the general
population".
Oral administration of 100 mg amitrole to humans for treatment of
overactive thyroids inhibited the 131I-intake of the thyroid for 24
hours in normal persons and in hyperthyreotics. A dose of 10 mg had
only a very slight effect (Astwood, 1960).
A preliminary study of Swedish railway workers exposed to
amitrole showed two lung cancer cases. Although the number of subjects
was small, a combination of amitrole with smoking (or other
interacting substances) might have been responsible for the excess
lung cancer (Axelson et al., 1964).
COMMENTS
Amitrole is rapidly absorbed and eliminated primarily in urine.
It has an effect on a variety of biochemical systems including
catalase, peroxidase and certain enzymes associated with oxidative
metabolism. Amitrole is goitrogenic on continuous long-term exposure;
probably as a result of continuous inhibitions of peroxidase activity.
At high levels of exposure, antithyroid effects of amitrole have been
seen within three days. In adult female rats fed amitrole in the diet,
no effects on reproduction were noted below 500 ppm although goitre
was observed. At 500 ppm, reduced fecundity and a reduced lactation
index was observed but no malformation of pups was observed. Oral
administration to rats from days 7 to 15 of pregnancy resulted in no
effect on reproduction and no abnormal offspring. Amitrole, in the
presence of dimethyl sulfoxide, injected into chicken eggs produced
beak abnormalities and tibial malformations. Results of mutagenicity
tests using currently defined protocols were negative.
In two long-term studies, hepatomas have been produced in mice
and rats administered amitrole at exceptionally high levels. However,
a long-term feeding study at high dietary levels in rats did not
result in hepatomas. In a one-year dog study, no hepatic, goitrogenic
or other effects were noted at a dietary level of 12.5 mg/kg bw. The
no-effect level was based on a short-term study where normal PBI
values, a sensitive biochemical parameter of thyroid function, were
noted at 0.5 ppm. In addition, since no goitrogenic effect on
discontinuous exposure was observed, a conditional ADI was allocated.
The Meeting was reassured that in the use of amitrole, man has
only a remote, if any, chance of achieving the conditions where
continuous exposure is maintained. The Meeting emphasized that the ADI
was allocated with the condition that the uses of amitrole be
restricted to those where food residues would be unlikely to occur and
further to recommend that the use of materials in combination in the
same formulation be restricted, especially where effects on specific
target organs are expressed by both materials.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg bw.
Dog: 12.5 mg/kg bw.
ESTIMATE OF CONDITIONAL ACCEPTABLE DAILY INTAKE FOR MAN
0.00003 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Amitrole is a broad spectrum herbicide effective against a wide
range of grasses and broad-leafed weeds when applied as a foliar
spray. It was introduced as a herbicide in 1954. The major herbicidal
uses are on industrial land, roadsides, rights of way, railways,
forests, irrigation channels and other ditches, either used alone or
in admixture with other herbicides or as a combination with ammonium
thiocyanate.
There are numerous important uses on crop land and these are
summarized in Table 2. Amitrole is not selective and therefore all
applications in the vicinity of crop plants must be made in such a way
that growing parts of the plant are avoided.
Because of its quick action but relatively poor residual effect
amitrole is widely combined with triazine, substituted urea and uracil
herbicides to widen their spectrum of activity and to enhance the
knock down effect.
Amitrole is translocated within many plants and is thereby
effective against rhizomatous and stoloniferous grasses and bulbous
plants. Once translocated to the root systems of such plants amitrole
appears to remain effective until the next growing season or at least
its effect is observed in the regrowth, possibly owing to the
destruction of certain essential growth factors.
In order to evaluate properly the possibility of residues of
amitrole in raw agricultural commodities and foods it is important to
understand the mode of use. The following is an outline of the
application to crop land.
Apples and pears
In the spring, before fruit starts to form or after harvest,
amitrole is applied to the floor of the orchard to control broadleaf
weeds and grasses. The chemical treatment is a replacement for
mechanical cultivations designed to maintain the area at the base of
the trees free from all unwanted vegetation. Label directions state
that application should be made to weeds and that spray should be kept
off the trunk or foliage of trees. Actually, at the time applied,
there may be blossoms on the tree but very little foliage. Because of
this, very little transpiration is taking place and there is a minimum
of fluid transport within the tree. Thus, any amitrole that does come
into direct contact with the trunk or the roots would not be expected
to move around in the tree in detectable amounts. Extensive data were
available to the Meeting to judge the possible effect of accidental
misapplication or treatment in mid-summer when fruit is on the tree
and foliage is at a maximum.
Grapes
In many grape-growing areas where there is a winter rainfall,
winter annual weeds are a serious cultural problem. These weeds sprout
profusely with the first rains of the winter wet season and grow
strongly during winter and early spring while adequate moisture is
available. Several residual herbicides are effective for controlling
these weeds provided they are applied before the weeds emerge.
However, if application is made too late or if the amount of moisture
is inadequate these materials are often ineffective and must be
supplemented by a foliage-absorbed herbicide. During this period, the
vines are completely dormant with no leaves or berries. The spray is
directed to the weed foliage at the base of the grapevine with
instructions to keep all chemical off the grape plant itself. When
grapes are dormant, there is no transpiration of fluids within the
grapevine. Therefore, no moisture is going into the vine and thus
there is no vehicle for the amitrole. By the time the grape breaks
dormancy and transpiration and water uptake are significant, amitrole
apparently is degraded and not available for uptake.
The residual herbicides suffer the disadvantage of being
ineffective against some species. If these are left uncontrolled they
soon colonize the whole area and interfere with cultural practices.
Also there are numerous instances where the farmer has a perennial
weed problem in his cropped fields. This can either be an entire field
or a large patch within a field or merely scattered plants. The use of
amitrole alone or in combination with other herbicides as a spot
treatment is recognized as an important agricultural practice
essential to the maintenance of the productivity of perennial cultures
such as vineyards.
TABLE 3. Pattern of use of amitrol based herbicides
Rate
Use kg/ha When applied Remarks
Chemical fallow 1 Autumn/winter Apply after weeds
emerge.
Cropland 4-8 After harvest Do not plant crops
or cutting. or graze for 8
months.
Grapes 2 When vines are Directed spray.
dormant.
Orchards (apple 2 Before fruit Do not spray
and pear) forms/after foliage.
picking.
Corn 2 10-14 days Spray weeds 10-14
pre-planting. days before
ploughing.
Irrigation drains 2-8 When weeds Do not allow
and ditches 15 cm high. grazing.
Orchards/vineyards 2-8 When weeds are Spot treatment.
(perennial weeds) actively growing.
Non-crop areas 1-8 When weeds are Boom sprays-
actively growing. spot sprays.
Aerial Not to be made
application where spray or
drift might
contaminate crops
or potable water.
Maize
Selective herbicides have revolutionised the growing of maize and
many other crops but there still remains a serious problem of
perennial weeds such as thistles, Agropyron repens and
convolvulus. In the spring amitrole is applied to growing weeds.
After roughly 10-14 days, during which the chemical is allowed to
penetrate the weed foliage and translocate to the actively growing
meristematic tissue, the weedy field is ploughed in the normal
fashion. That is, the weed foliage is buried roughly 15.25 cm in the
ground and soil which was well under the surface is brought to the
top. Emphasis is placed on burying all treated weeds.
Chemical fallow
In areas where a fallow period is maintained because conservation
of all available moisture is essential to produce a satisfactory crop,
amitrole has been used alone or mixed with 2,4-D to control annual
weeds that sprout during the fallow period. If allowed to grow, these
weeds remove much of the moisture reserves in the soil.
Depending upon winter conditions, it may be desirable to spray in
the autumn or wait until weed growth starts in the spring. This
initial treatment is followed by one or more mechanical cultivations
to maintain the area fallow until the next crop is planted. It may be
necessary to repeat the herbicide application if fresh weeds emerge.
Practically speaking, there is no need to apply it less than 30 days
before planting cereal crops although the residue data show that
application could be as late as several days before planting without
producing detectable residues. There is no control whatsoever of weeds
that germinate after application.
Irrigation channels and ditch banks
Amitrole has been used for many years in some countries where
difficulty is found in maintaining irrigation channels and ditch banks
free from encroaching vegetation, particularly against those weeds
which grow prolifically in such situations and where a herbicide which
is rapidly translocated but rapidly disappears from soil or water is
required. In some countries there are restrictions on use near potable
water or catchments.
Pre-harvest treatments
There are no approved uses for direct application to crop plants.
Lack of selectivity precludes the possibility of such treatments.
Other uses
There are no known domestic or industrial uses which could
subject the general public to exposure to amitrole in any form.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable number of studies have been carried out to
determine whether, following the use of amitrole for the control of
weeds, residues could possibly occur in raw agricultural commodities
or food. Virtually without exception these studies indicate that the
parent compound is not found in any food commodity even when grossly
excessive rates are used or when the compound is applied at times and
under conditions not recommended as good agricultural practice.
Apples and pears
Otten (1970) reported before the Amitrole Advisory Committee of
the U.S. Environmental Protection Agency the results of many studies
on apple and pear trees. The approved treatment is 2-4 kg/ha under
trees in the spring before fruit forms or after harvest. In one series
of trials amitrole was applied for five successive years at the rate
of 4 kg/ha per year, or a total of 20 kg/ha during the 5 year period.
This is 10 times the normal use rate over the 5 year period. The
bottom 50 cm of the trunk was wet if necessary. No amitrole residues
were detected in the fruit.
Otten reported other tests in which amitrole was applied for 8
successive years using 4 or 20 kg/ha each year, a total of 160 kg of
amitrole per hectare during the period. Apple samples were taken from
trees with or without white shoots at the base (indicating amitrole in
the leaves) and analysed separately. No amitrole was detected in any
samples. The limit of determination of the method used is 0.01-0.02
mg/kg.
In both series of tests amitrole was applied during the summer
when the fruit was on the tree. Only when spray was applied directly
to the fruit was a residue detected.
Schubert (1965) carried out trials continuously over the period
1957-1964 in numerous orchards which received treatments of amitrole
over ground cover, fruit and leaf. Even in those trials where amitrole
was applied beneath trees right through the summer, mature fruit were
free from amitrole at harvest. In the early years apparent amitrole
residues were reported at levels in the range 0.05-0.09 ppm.
Subsequently Storherr and Burke (1961) developed improved methods to
overcome the extremely high absorbance backgrounds of most crops. Even
where chlorotic shoots were present on the trunk of trees, fruit at
harvest was found to have no residues (limit of determination 0.02
mg/kg). Substantial amitrole residues were found in mature apples
where foliage and/or fruit were directly treated with amitrole in
mid-summer. The residues from direct application to foliage were
similar to quantities found after dipping the fruit. When both leaves
and fruit were sprayed the residues were approximately doubled. The
residues resulting from these direct applications during the growing
season were in marked contrast to the results of a ground cover
application that avoided spray contact with foliage or fruit, where no
amitrole residues were found at harvest.
Maier-Bode and Bechtel (1968) reported trials with apple trees
carried out in Germany. When amitrole was applied at 2.5-4.2 times the
officially recognized dosage (6 kg/ha), no amitrole was detected in
the apples (residues of less than 0.01 ppm). Not until 50 or 100 kg/ha
of amitrole was used, i.e. 8.3-16.7 times the recognized dosage, were
small residues of amitrole, between 0.02 and 0.09 mg/kg found in the
apples. In practice such high doses are never used; the foliage of the
trees in these plots was distinctly chlorotic.
Maier-Bode and Bechtel (1968) also found that when grossly
excessive amounts of amitrole were applied to the soil, the amitrole
residues in apples were much higher when there was no weed cover
beneath the trees than when the herbicide was applied to densely
weed-covered plots. This was presumably because the lack of weed cover
to absorb and metabolize the amitrole allowed it to reach the root
zone of the trees.
Amchem (1965) reports show that apples, grown in orchards where
the floor of the orchard was cleared of poison ivy and general
broadleaf and grass weeds according to label instructions, show no
residues at harvest. These studies extended over 8 locations and
numerous varieties of apples. The methods of analysis used showed 96±
15% recovery at the 0.1 mg/kg level with a limit of determination of
0.01 mg/kg. Only when partially grown apples hanging on the trees were
sprayed to run-off with an amitrole spray mixture, could residues of
amitrole be found in the mature apples.
Moore (1968, 1969, 1970) reports three series of trials at
various locations in Australia in which amitrole was applied beneath
apple trees at varying rates between 2 and 4 kg/ha, at petal fall or
in mid-summer. To obtain the most severe conditions possible,
weed-free plots beneath trees were also sprayed in mid-summer. To
ensure the most complete uptake of herbicide by the tree an area of 5
metres × 5 metres was treated instead of the usual 3 metres × 3
metres. The author concludes that if any residues were present they
were below the analytical limit of determination of 0.01 ppm. There
was no build-up of residues in the second year of treatment.
Bayer (1973) reports studies carried out in Germany which showed
that approved uses of mixed herbicides containing amitrole applied for
the control of weeds in apple orchards did not produce detectable
residues of amitrole in mature apples (limit of determination
0.05 ppm).
The Netherlands authorities have submitted results of studies
carried out to determine amitrole residues in some fruits following
approved uses in that country. Pears and apples harvested in 1961 and
1962 following treatment of orchards for weed control 4-18 months
previously showed no indication of residues when analysed by the
method of Storherr and Burke (1961) having a limit of determination of
0.025 mg/kg (Wit and Van der Kamp, 1963).
Cherries
Maier-Bode and Bechtel (1968) report a number of experiments
which show that when amitrole is applied for weed control beneath
cherry trees 100 days before harvest no residues can be detected in
mature fruit by methods capable of determining 0.01 ppm.
Bayer reports experiments where amitrole was applied for weed
control in sour cherry orchards at the rate of 4 kg/ha. No residues
could be detected by methods sensitive to 0.05 ppm.
Citrus
An extensive study of the accumulation and depletion of amitrole
residues in citrus fruit and foliage was published by Day and
Hendrixson (1959) from California. Amitrole was usually applied to the
soil at rates of 2 and 4 kg/ha; in two instances a logarithmic series
of applications were made from 2-64 kg/ha. Treatments were replicated
4 times in each of 13 different orchards in various parts of the
citrus growing area. Treatments were applied during the winter months,
usually on dry soil surfaces, and orchards were irrigated during the
period of observation. The analytical method used was effective down
to 0.01 mg/kg. Several varieties of oranges and lemons were included
and samples were taken each month for the four months following
application. In spite of obvious symptoms of damage to the lower skirt
of the citrus trees most samples from the 2 and 4 kg/ha experiments
showed no detectable residues although a few were found to have
residues up to 0.05 mg/kg. In the logarithmic plots the level of
residues increased with increasing concentration of amitrole applied.
Those plots receiving 64 kg/ha showed 0.15 mg/kg in the whole oranges
4 months after application. Less was found after shorter intervals.
The authors concluded that citrus fruit from trees receiving
either 2 or 4 kg/ha contained residues only rarely. The residue level
found in citrus fruit does not appear to be affected by fruit variety,
soil type, climate, geographical area, cultural operations or rate of
application to the soil (except at grossly exaggerated rates).
Albinism appears only on the lower skirt area indicating foliar
contact rather than root uptake as the source of entry. Amitrole is
not re-distributed beyond the immediate area of contact when the
herbicide is applied at recommended rates. Field observations indicate
general systemic distribution at high rates of application.
Coffee beans
Information was available from only one trial carried out in
Hawaii.
Hylin (1962) reports results of analysis of coffee beans from
trees which had been treated twice within 6 weeks with amitrole,
applied at the rate of 2 and 4 kg/ha to substantially bare ground. A
total of 40 trees were involved in the trial. Great difficulty was
apparently encountered in developing a suitable analytical procedure
to deal with the coffee beans. Apparent amitrole residues were
reported to occur in the green beans at levels ranging from
0.02 - 0.48 mg/kg. In view of the analytical difficulties reported
and the experience of other workers, however, caution should be
exercised in interpreting the significance of these results.
Cranberries
Following the disclosure by Fleming (1959) that the practical use
of amitrole for the control of weeds in cranberry bogs led to
significant residues of amitrole in cranberries, the U.S. Food and
Drug Administration and the U.S. Department of Agriculture undertook
field and laboratory studies extending over 19 months (Onley et al.,
1963). Following the application of 4 and 8 kg of amitrole per ha of
cranberry bog the level of amitrole was determined in samples of
cranberry vines, soil, roots and fruits (when available). Results
indicated that though the residues in the soil declined steadily and
disappeared at the end of 12 months there was a distinct concentrating
effect in the roots and a pronounced effect in bushes where the
residues declined more slowly than in the soil. Cranberry fruit
harvested 3 and 12 months after application showed residues ranging up
to 0.4 mg/kg. The authors were unable to conclude whether the residue
represented the parent compound or metabolites.
Grapes
Studies using radio-labelled amitrole (Leonard and Weaver 1961)
showed that when amitrole was applied to the leaves, stem, clusters or
shoots of the grapevine a modest upward translocation occurred for
about 3 days. After this period the amitrole appears to be either
complexed or broken down. Amitrole was not recoverable, as such, from
the clusters except within 3 days after treatment.
Leonard and Lider (1961) studied the translocation of amitrole
and a number of other herbicides in the grapevine. Grape rootings were
allowed to absorb solutions of herbicide for 3 days before being
planted in pots. The distribution and fate of the herbicide was
studied by radio-autographs. There was very little indication of
translocation throughout the plant. Fruit from vines grown in
amitrole-treated soil under greenhouse conditions showed no positive
evidence of amitrole.
Trials with grapes in Germany involved the application of a
maximum of 4 kg/ha of amitrole weeds in vineyards, during both the
dormant and vegetative stages (Maier-Bode and Bechtel 1968). No trace
of amitrole was found in grapes when using a method capable of
detecting 0.01. Similar results are reported by Bayer (1973) from
trials involving the application of 4 kg of amitrole per ha of
vineyard.
Maize
Otten (1970) reports results obtained by both chemical analysis
and radio-labelling which show that no amitrole was detected in either
immature maize plants or at normal harvest time when amitrole was
applied to weeds 10 days before ploughing for the planting of maize
seed. When exaggerated rates were applied or maize was planted 1 or 2
days after spraying, amitrole was detected in the seedling plant but
had disappeared well before the crop would be used for silage or
mature grain.
In a statement before the Amitrole Committee, Amchem (1970)
summarized investigations carried out by a number of official research
workers investigating the possibility of amitrole residues finding
their way into corn plants and grain. Ercegovich (1957) analysed corn
from plots treated with amitrole at 0, 1, 2, 4 and 8 kg/ha and planted
1, 5, 9 and 13 days later. Harvests were made at 3, 4, 6, 10 and 16
weeks after planting. No residues of amitrole were found in any
samples.
Boyd applied radio-labelled amitrole directly to young corn
plants and followed the rapid decrease in residues over a 47-day
period. Initial residues of 4-10 mg/kg decreased to final levels of
0.1 - 0.2 mg/kg.
Nuts
Studies carried out in California involved the application of
amitrole to the orchard floor at rates of 1, 4 and 8 kg/ha.
Application was made during the winter months. Mature nuts collected
the following season were analysed for residues of amitrole. None of
the many samples examined was found to contain amitrole residues above
the limit of determination (0.02 mg/kg) (Hill 1962-63).
Peaches and plums
The Meeting had available numerous reports on residue trials
carried out in peach and plum orchards where amitrole was used for
weed control. None of the many samples analysed by methods capable of
determining as little as 0.02 mg/kg showed any indication of amitrole
residues (Hill 1962/63; Maier-Bode and Bechtel 1968; Bayer 1973).
Soybeans
The Meeting examined summaries of many separate trials carried
out in different areas of the USA and analysed by three separate
laboratories to determine the level and fate of amitrole in soybean
plants, pods and mature beans following different patterns of using
amitrole for weed control. In all cases samples were from treatments
at rates of 1´ - 2 times the maximum recommended rate. In no instances
were the residues, even in immature plants, found to be above the
limit of determination, 0.06 mg/kg (Amchem, 1969).
Montgomery and Freed (1963), wishing to know whether the proposed
Pre-plant use of amitrole could possible result in residues in
soybeans, studied various methods of extraction and analysis. They
reported that by each procedure samples from fields treated with up to
4 times the recommended rate of amitrole gave exactly the same results
as samples from untreated control fields. They applied a statistical
analysis to the extensive data and concluded that there was no reason
to suspect that any residues could occur following pre-plant
application. Further evidence of freedom from residues was provided by
failure to demonstrate any difference between the absorption spectra
of paired samples from control and treated plots when using a
double-beam spectrophotometer to compare them.
Sugar cane
Amitrole has been found to be useful for the control of certain
perennial grass and broad leaf weeds common in sugar cane fields and
on irrigation ditch banks. Application of directed sprays in sugar
cane produced a moderate amount of chlorosis which persisted for 3-4
weeks in the leaves without appearing to affect yields or subsequent
growth. Hilton et al. (1963) carried out a residue study designed to
determine the residual amitrole in sugar cane from several successive
applications made over the period of the crop cycle (2 years). Hilton
and Uyehara (1966) reporting on these studies noted that when sugar
cane was double treated with amitrole at 5, 10 or 20 kg/ha, 12 and 20
weeks after planting, the residues of amitrole diminished to less than
0.002 mg/kg by harvest time. When 10 times as much cane was taken for
analysis and treated in a manner to approximate the early raw sugar
processing, residues, if present, were less than 0.01 mg/kg even from
plots receiving the grossly exaggerated rate of 20 kg/ha.
Wheat and oats
Amitrole proved to be an effective means of controlling couch
grass (Agropyron repens) in cereal crops. Most proposed treatments
involved the application of amitrole to the grass 10-14 days before
ploughing in preparation for seeding with cereals. In Sweden the
procedure was to apply amitrole at the 3 leaf stage at a rate of 0.5
to 1 kg/ha. Svensson (1971) investigated the effect of such treatments
on oats and the fate of residues in the oat plants and grain. He
reports that when the treatment was carried out strictly in accordance
with approved directions no detectable residues could be found in the
grain. However when the rate was increased or application withheld for
2 weeks until the 5-leaf stage of the crop, the grain contained about
0.05 mg/kg. If the rate of application is increased to 2 kg/ha the
residue can exceed 0.1 ppm. Determinations were made on straw from a
number of trials but analytical difficulties rendered the results
somewhat unreliable. However it appears that application of amitrole
at any stage after the 3-leaf stage increases the likelihood that
residues will be found in the straw at harvest. Much higher residues
were found in the grain of oats treated with amitrole at the heading
and milk stages. 0.5 kg/ha applied at the milk stage gave rise to a
residue in the grain of 2.9 mg/kg.
Amchem (1970) provided a summary of a number of studies by
official workers in the USA who investigated the incidence, level and
fate of residues of amitrole in wheat grain and straw from crops grown
on soil treated according to label directions 14-28 days before
ploughing for planting. In some of these studies the application rate
was increased to 4 times the recommended rate and the time interval
was reduced to 1 day before planting. In no instance was any amitrole
residue found in cereal grains or straw at harvest time. Soil studies
on the same test plots showed detectable residues of amitrole on the
day of treatment but no detectable residues the day after treatment
even at the doubly exaggerated rate. The limits of detection were 0.02
ppm in grain and straw. Recoveries were 86-96% at levels of 0.2-0.4
ppm. On the basis of these data it was concluded that the use of up to
4 kg/ha of amitrole, one or more weeks before planting wheat or other
cereals, should cause no residues in the crop.
FATE OF RESIDUES
General
Kröller (1966) and Menzie (1969) have reviewed the metabolism of
amitrole in animals, plants, micro-organisms, etc. There is an
extensive literature on the mode of action of amitrole and on its
metabolism in plants. Many of these investigations were carried out
with the object of determining uptake, translocation, site of action,
reason for selectivity, and possible means of enhancing the activity.
Among the most useful publications are those of Boyd (1964/65), Carter
(1969), Herrett and Linck (1961a), Jukes (1961), Moser (1968), and
Rogers (1957a,b).
Many of these studies reveal that amitrole does not persist for
extended periods in plant tissues though there is considerable
disagreement as to whether it becomes conjugated, converted into a
more active material or destroyed and its fragments taken up into the
plant. There appears to be no clear-cut mode of action, but rather
multiple pathways by which it interferes with plant metabolism. Carter
(1969) reviewed the available literature on the effects of amitrole on
amino acid and protein metabolism, purine metabolism, flavin
synthesis, enzyme activity, chlorophyll synthesis and plastid
development. It is obvious that there is no single site of action but
the relative importance of the various effects has apparently not been
unravelled.
In animals
The fate of amitrole in rats is discussed in the section
"Biotransformation". There do not appear to be any grounds for
assuming that livestock grazing on plant materials growing on land
that had been treated with amitrole for the control of weeds would
absorb or retain significant amounts of amitrole or its metabolites.
In plants
The s-triazole nucleus is highly stable (Potts 1961); hence it
is not surprising that few workers have reported evidence of ring
cleavage under physiological conditions. Yost and Williams (1958)
reported disappearance of radio-labelled amitrole from corn plants in
approximately 6 weeks with a half-life of about 8 days. Disappearance
was also observed in soybeans but at a much slower rate.
Miller and Hall (1961) could not detect amitrole in cotton 4 days
after treatment. However, large quantities of metabolic products were
present. Fang et al. (1967) report the half-life of amitrole in
several plants as 18 to 28 hours. Little of the applied material was
recovered as CO2 from beet, corn or beans.
Freed et al. (1961) reported evolution of radio-active, CO2 from
treated oats and barley, indicating ring cleavage. Montgomery and
Freed (1963) observed some evolution of 14CO2 from treated soybeans,
with the remaining radioactivity apparently bound to components of
plant tissues. Resistant oats released CO2 more readily than
sensitive barley. Massini (1963) found no loss of CO2 from beans or
tomatoes. Muzik (1965) observed chlorosis in scions grafted onto
tomato plants 103 days after treatment with amitrole, indicating long
persistence of the toxic moiety.
Studies of amitrole degradation in plants have been complicated
by the fact that the material is available commercially only with the
5-carbon labelled. Apparently, the 5-carbon is quickly lost (as CO2
or formate) when ring rupture occurs and the remaining fragments are
thus unlabelled. However, if significant amounts of CO2 or
14C-formate were produced from 14C-amitrole in higher plants, one
would expect to find some incorporation of 14C into normal
metabolites. This is not the case. The vast majority of the literature
indicates that the extractable 14C from plants treated with
amitrole-5-14C remains in the intact ring as free amitrole or
conjugates. Considerable amounts of amitrole are attached to protein
(Brown and Carter 1968; Castelfranco and Brown 1963) or somehow bound
in an insoluble form (Racusen 1958). There is evidence of activation
to a free radical form which can react with amino acids (Carter and
Naylor 1960, 1961a, b; Herrett and Bagley 1964; Herrett and Linck
1961a; Miller and Hall 1961). Conclusive evidence of rapid and
extensive ring cleavage by higher plants has not been reported.
Most literature on metabolic alteration of amitrole in plants
deals with the formation and properties of conjugates between amitrole
and endogenous plant constituents. These "degradation" products
contain the intact triazole nucleus which often may be regenerated by
chemical treatment.
Rogers (1957a,b) reported a derivative of amitrole in several
plants which was "chromatographically identical" with an
amine-glucoside derivative of amitrole. The glucose derivative forms
quite readily in vitro (Fredrick and Gentile 1960) and its occurrence
in plant extracts is probably an artifact (Naylor 1964), since
numerous attempts by other workers to detect the compound in plant
extracts failed (Miller and Hall 1961; Massini 1963; Carter and Naylor
1959). However, Gentile and Fredrick (1959) and Frederick and Gentile
(1960, 1969, 1962 and 1965) have published a series of studies on the
properties and metabolism of the glucose derivative. These same
authors suggest that the triose derivative represents the true
structure of the amitrole derivatives reported by other workers.
Massini (1963) and Carter and Naylor (1960, 1961a) reported studies of
amitrole metabolism in which Massini identified one of the major
metabolites as 3-(3-amino-1,2,4-triazole-1-yl)-2-amino propionic acid
(3-ATAL), also referred to as
3-(3-amino-1,2,4-triazol-1-yl)-d-alanine. The formation of 3-ATAL
apparently represents a detoxication, since the derivative does not
appear to be nearly as toxic as amitrole, or as mobile. Furthermore
ammonium thiocyanate, which synergizes the action of amitrole,
inhibits the formation of 3-ATAL.
Smith and Chang (1973) studied the metabolism of amitrole in
Canada thistle (Cirsium arvense) and peas. They showed that excised
leaves of thistles metabolized amitrole into three major products one
of which was 3-ATAL. The other two were shown to be its metabolic
products, one being the precursor of the other. An enzyme preparation
from peas capable of synthesizing tryptophan was also able to
metabolize amitrole. Tryptophan synthesis with the enzyme preparation
was inhibited by amitrole and the authors deduced that amitrole
metabolism may follow a similar pathway to tryptophan synthesis.
A number of workers including Racusen (1958) reported another
metabolite which was stable to 6N HCI for 5 hours at 100°C.
Verification of the structure of this material and others awaited
further studies.
The most important outstanding question is whether the conjugates
and/or metabolites represent biologically active derivatives of
amitrole and if so whether these are available to react
physiologically and biochemically with animals receiving such
conjugates and metabolites in plant materials as part of their ration.
However, use patterns at present approved avoid the possibility that
significant residues are ingested by livestock or man.
In soil
Amitrole disappears rapidly from soils as shown by Sund (1956),
Bondarenko (1958), Ashton (1962), Riepma (1962), and Ercegovich and
Frear (1964). Disappearance has been attributed to absorption (Sund,
1956, Ercegovich and Frear, 1964) and microbial degradation, although
attempts to isolate organisms capable of degrading amitrole have not
been successful. Kretschmar (1970) showed that amitrole was not
degraded by Azotobacter.
Kaufmann (1965), Kaufman and co-workers (1968) and Plimmer et al.
(1967), have proposed that most of the amitrole degradation occurring
in soils proceeds by non-biological reactions. They showed that
approximately 69% of the radio-label from amitrole was released as
CO2 in 20 days by non-sterilized soil. Although autoclaved soil
released only 2% in a comparable period, soil treated with potassium
azide or ethylene oxide released 46 and 35% respectively, and
reinoculation of autoclaved soil did not restore the capacity to
metabolize amitrole. These authors propose that amitrole is degraded
in soil by an oxidative mechanism involving an attack on the triazole
nucleus. Microbial attack is not discounted however. Soil moisture,
temperature and pH markedly affected amitrole degradation (Riepma
1962), indicating possible microbial involvement. The studies by
Ercegovich and Frear (1964) show that amitrole degradation obeys
first-order kinetics, suggesting a chemical reaction. No-one has
reported an investigation of the possible involvement of
extra-cellular enzymes.
Whatever the mechanisms by which the triazole ring is opened,
there appears to be little doubt that ring opening does occur rapidly
in soils and the resulting products (urea, cyanamide and nitrogen)
should be readily metabolized by soil micro-organisms.
Norris (1970) showed that amitrole was rapidly lost from the
forest floor but that degradation was not completely biological, since
there was considerable loss in steam-sterilized material. He found
that ammonium thiocyanate applied with amitrole in the proportions
found in the commercial herbicide had no effect on the degradation of
amitrole.
Sund (1956) showed that amitrole becomes tightly adsorbed to soil
particles. He concluded that it takes part in the soil's base exchange
system, but also it has the tendency to complex metals. Studies of
amitrole toxicity to tomato seedlings were correlated with chemical
analysis and it was found that the biological response of plants is
proportional to the amount recoverable in any soil type.
MacRae and Alexander (1965) are in agreement with the results of
Ashton (1963) that microflora have an important function in the
degradation of amitrole. They confirmed the rapid biodegradation in
soil by plant bioassay.
Ercegovich and Frear obtained evidence of complex formation
between soil clay and amitrole by means of X-ray diffraction
measurements. Ashton (1963) noted that 13 compounds were formed in
addition to CO2 when amitrole was added to unsterile soil. One or
more metabolic compounds formed from amitrole are tenaciously bound to
the soil and appear to be resistant to degradation.
Day et al. (1960) studied the effect of soil type, temperature,
moisture and sterilization by steam on the fate of amitrole in 55
types of Californian citrus soil. Rates of decomposition in
steam-sterilized soils were much lower than in unsterilized soil,
apparently indicating that the decomposition is primarily due to the
action of soil microorganisms (but compare the results of Kaufman,
Plimmer and co-workers quoted above). Rates of decomposition of
amitrole were highly variable among the soils studied, apparently
because of differences in populations or levels of activity of the
soil micro-organisms concerned. A short residual life for amitrole was
more frequently found in the more highly evolved soils having finer
textures and more highly developed colloidal properties.
Freed and Furtick (1961) showed by simultaneous bioassay and
chemical analyses capable of detecting less than 0.05 mg/kg of
amitrole that it would be highly improbable that any residue of
amitrole would remain in the soil even a short while after
application.
It has generally been assumed that failure to recover amitrole
from soils following its use as a herbicide was due to elimination by
such processes as leaching, volatalization, or biological or chemical
destruction. Work by Groves and Chough (1971) demonstrated that new
solvent mixtures gave much better recoveries of amitrole from soil
than water extraction. Concentrated ammonium hydroxide/glycol mixture
(5/20) gave distinctly higher recoveries than water as indicated by
the following table.
TABLE 4. Extraction (%) of amitrole from soils with water and
ammonium hydroxide/glycol (A/G) mixture (5x20)
Time after Non-sterile soil Sterile soil
application (days) Water A/G Water A/G
% % % %
0 48.1
1 36.7 97.6 49.6 97.3
12 21.3 55.9 36.1 77.9
17 3.2 15.2 36.9 67.7
These results point to a need for caution in interpreting some of
the earlier results.
In water
Since amitrole is widely used for the control of ditch bank weeds
many studies have been carried out to determine the fate of any
amitrole reaching the water. Many of these studies (Segal 1960;
Marston et al. 1968; Frank 1969; Dunstar 1969; and Demint et al. 1970)
have largely involved the study of the rate of dissipation of amitrole
in irrigation water or flowing streams. Since only a small proportion
of the herbicide applied in such operations ever reaches the water,
dissipation and dilution is a recognized means of reducing any risk to
crops and municipal users. Where application has been made by aerial
spraying, measurable amounts were found in samples of water near the
downstream edge of the sprayed area for up to 5 days after spraying
(Marston et al. 1968).
Such studies are however of little value in determining whether
residues are lost by physical, chemical or biological means. Studies
were reported by Thoman (1963) from Florida where ponds with static
water levels were treated at the rate of 3 kg/ha of water surface,
giving a concentration of 0.45 mg/l in the water immediately after
treatment. Samples collected every 7 days thereafter were analysed.
The residue level declined slowly but 46 days elapsed before it
reached the limit of determination (0.02 mg/l). Samples taken
thereafter until the 74th day gave results comparable to pre-treatment
levels.
Nicholson (1963) reported experiments carried out in a fish
hatchery in South Carolina where ponds of 1 acre surface area were
treated with amitrole to yield a concentration of 1 mg/l in 80,000
cu.ft of pond water. Samples were taken each 7 days for 5 weeks and
thereafter at slightly greater intervals until the 201st day when the
pond became flooded. The concentration following treatment (1.16 mg/l)
gradually declined through 0.49 mg/l on the 68th day to 0.07 mg/l on
day 201. No information is given on the quality of the water in the
ponds but it is obvious that under these conditions amitrole remains
relatively stable for long periods.
In processing and cooking
No information was available on the effect of processing and
cooking on residues in plant materials. In view of the fact that none
of the recommended use patterns give rise to detectable residues in
raw agricultural commodities such studies are unlikely to have been
carried out.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Food moving in commerce
No data were available to the Meeting to indicate the level and
incidence of amitrole residues in food moving in commerce.
Food at the time of consumption
Results of several total diet studies in which an attempt was
made to find amitrole residues in many separate food groups were
available to the Meeting. Duggan et al. (1966) reported that amitrole
residues were not found in any food composites in the total diet study
carried out in the USA in 1964/65; the method used had a limit of
determination of 0.05 mg/kg. The same authors (1967), having repeated
the total diet study, reported that amitrole residues were not found
at or above the prescribed sensitivity limit. Corneliussen (1969,
1970), reporting the results of the 4th and 5th total diet studies
carried out in the USA, states "amitrole has never been found in any
total diet composites". The limit of determination was again
0.05 mg/kg.
METHODS OF RESIDUE ANALYSIS
One of the first methods for the determination of amitrole
residues was that of Sund (1956). This was a colorimetric method
applicable to soils, depending upon the formation of a green colour
with sodium nitroprusside reagent. It is sensitive to 1 mg/l of
amitrole in aqueous solutions and Sund reported that it conformed to
Beer's law. Bioassay with tomato seedlings correlated with chemical
analysis.
American Cyanamid (1958) published a colorimetric method for the
estimation of residues of amitrole in cranberry fruit. The extract is
purified by means of a cation exchange resin, treated with nitrous
acid and coupled with N-1-naphthyl-ethylenediamine dihydrochloride to
produce a pink-coloured solution. The intensity of the colour is
proportional to the amitrole concentration and is measured
spectrophotometrically at 512 nm. The limit of determination is
0.03 mg/kg.
Herrett and Linck (1961b) published what they describe as a
simple reproducible method for the separation and quantitative
determination of amitrole in biological systems. The method consists
of diazotization followed by coupling with
1-amino-8-naphthol-3,6-disulfonic acid (H-acid). The authors describe
the method as being sensitive in the range of 0.1-3.3 mg/kg.
Storherr and Burke (1961) in describing an improved method for
the determination of amitrole in crops refer to previous methods
published by the Food and Drug Administration which were applicable to
only a limited number of food crops. With their modifications the
method involves extraction of vegetable crops with ethanol, adsorption
on cation-exchange resin and desorption with aqueous ammonia. The
ammoniacal concentrate is subjected to a clean-up procedure using
acetonitrile and filter aid followed by acid digestion and clean-up
with activated carbon. The resulting solution is diazotized and
coupled with H-acid. The pink colour is measured at 455 nm. It is
necessary to carry out a blank determination. The apparent amitrole
found in control crops ranged from 0.003 to 0.02 mg/kg. The limit of
determination is considered to be 0.025 ppm using a 40 g sample.
Storherr and Onley (1962) later published a procedure for the
clean-up and separation of amitrole and its metabolites from vegetable
crops. This method utilizes a chromatrographic column packed with dry
cellulose for the removal of interfering substances from plant
extracts. It is reported to recover amitrole from some conjugates.
Hilton et al. (1963) modified the method of Storherr and Burke,
(1961) for use with sugar cane and sugar juice and were able to lower
the limit of determination to 0.002 mg/kg. Their modifications
involved an increase in the size of sample taken and a reduction in
the amount of activated carbon used in the acid digestion and cleanup
steps.
Segal (1960) and Hilton and Uyehara (1966) described a number of
minor modifications to the method of Storherr and Burke which lowered
the limit of determination of amitrole to 0.001 mg/kg in water and
sugar cane respectively.
Meissner (1971), on behalf of the Amitrole Advisory Committee to
the US Environmental Protection Agency, commenting on the claim that
the method of analysis developed by Storherr and Burke (1961)
determines not only amitrole but all known metabolites drew attention
to the lack of detailed information available on the metabolites of
amitrole in plants or on the influence of environmental and chemical
factors on plant metabolism. Groves and Chough (1971) report an
improved solvent mixture (concentrated ammonium hydroxide and ethylene
glycol) to extract amitrole from soil. Further details are given above
(see "Fate of residues in soil").
NATIONAL TOLERANCES
The information available to the Meeting indicated that most
countries with pesticide residue tolerance legislation either
registered amitrole on a "no-residue" basis or provided a zero
tolerance. It is assumed that zero would in effect mean "at or about
the limit of determination". In the Netherlands there is a general
tolerance of 0.02 mg/kg in fruit and vegetables. In Australia there is
a maximum residue limit for amitrole in water of 0.01 mg/l. This is at
or about the limit of determination. In the Federal Republic of
Germany, use of amitrole where potable water might be contaminated is
prohibited.
APPRAISAL
Amitrole is a non-selective, foliage-absorbed herbicide widely
used since 1954 for the control of unwanted vegetation on industrial
land, roads, railways, rights of way, ditch banks and similar
situations. It is also used for the destruction of weeds beneath trees
and vines in permanent horticultural crops, for spot treatment of
perennial weeds and for their destruction prior to the planting of
cereal crops. There are no known uses where application is made to
crop plants.
Extensive information indicates that amitrole is rapidly lost
from soil by a combination of chemical, biological and microbiological
attack. There is no indication that amitrole is taken up by the roots
of plants if currently approved practices are followed. Approved uses
involve the application of amitrole to weeds between the crop when
fruit trees, vines and similar horticultural crops are dormant or the
use of spot sprays and directed sprays which avoid contamination of
crop plants. In these cases there is no uptake of residues by crop
plants. Studies with radio-labelled amitrole confirm that it is not
taken up from soil following simulated normal practices.
Extensive residue trials have been carried out under many
conditions in many countries and all indicate that there is no residue
in fruit, vegetables or grain following the recommended use of
amitrole as a herbicide. Residues have been found experimentally only
when crop plants have been treated with excessive quantities of
amitrole applied directly over the crop when the fruiting parts are
already well formed.
There appears to be no single mode of action and the metabolic
pathways in plants appear most complex. There is evidence that when
amitrole is applied to the leaves of plants, most of the material
absorbed is metabolized. Some is complexed with various plant
materials, but the Meeting considered that the nature and biological
significance of conjugation products would only be of importance when
considering residues resulting from direct application over food-crop
plants. Where amitrole was administered to laboratory animals it was
shown that similar conjugates with sugars and proteins were formed in
the animal body.
There was some doubt whether the methods of analysis for residues
of the parent compound would adequately recover and determine all
conjugated materials or other biologically active metabolites. The
method of Storherr and Onley (1962) is the most sensitive and specific
method available, and is reported by several authors to recover
amitrole from some conjugates. Under the circumstances the Meeting
felt confident in recommending a maximum residue limit at or about the
limit of determination by the beet available method. The Meeting
however proposed certain precautions to avoid contamination of food
crops.
RECOMMENDATIONS
There is no reason to believe that significant residues occur in
any raw agricultural commodities when amitrole is used for the control
of weeds according to approved directions.
To reduce the possibility of contaminating food crops with
residues of amitrole, use-patterns should avoid the direct treatment
of food crops and should be limited to directed sprays, spot sprays
and, in the case of pre-planting and stubble treatments, application
to weeds at least 10 days before ploughing (see Table 2), the interval
to be conditioned by the temperature of the soil.
For regulatory purposes and as a means of determining whether
amitrole herbicides have been misused or incorrectly applied the
Meeting recommends a maximum residue limit at or about the limit of
determination by the best available analytical method.
CONDITIONAL TOLERANCE
mg/kg
Raw agricultural commodities of plant origin 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Long term feeding studies in a sufficient number of rats and mice
with low levels of amitrole of known composition and purity.
2. Studies to elucidate the possible relationship between the effects
of amitrole on the thyroid and on the liver.
3. Studies to show that the analytical methods determine not only the
parent compound but also biologically active metabolites.
4. Studies to develop a specific method sensitive to 0.005 mg/kg.
REFERENCES
Alexander, N.M. (1959a). Antithyroid action of 3-amino-1,2,4-triazole.
J. biol. Chem., 234:148-150.
Alexander, N.M. (1959b). Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by antithyroid compounds. J. biol. Chem.,
234:1530-1533.
Amchem (1965). Residues of amitrole in apples. Report Amchem Products
Inc. (Unpublished)
Amchem (1969). Analysis of soyabeans and soyabean plants for possible
residues of amitrole. Review of collaborative trials. Amchem Products
Inc. Report, Jan. 1969.
Amchem (1970a). Residues of aminotriazole in corn. Collection of
studies by various workers. Submission to Amitrole Committee, 16 Dec.
1970.
Amchem (1970b). Residues of amitrole in wheat and soil when used for
chemical fallow. Submission to Amitrole Committee, 16 Dec. 1970.
American Cyanamid (1958). Colorimetric method for the estimation of
residues of amitrole in cranberry fruit. American Cyanamid Co., 4 Feb.
1958.
Ashton, F.M. (1963). Fate of amitrole in soil. Weeds, 11:167-170.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. Am. med.
Ass., 172:1319-1320.
Auerbach, V.H., Piesinger, R.H. and Waisman, H.A. (1959). The effect
of 3-amino-1,2,4-triazole on the synthesis of tryptophan peroxidase.
Archs Biochem. Biophys., 82:370-379.
Axelson, O., Rehn, M. and Sundell, L. (1974). Herbicide exposure,
mortality and tumour incidence. An epidemiological investigation on
Swedish railroad workers. Envir. Hlth. (in press)
Bagdon, R., Vidone, L., Shaffer, C. and Golz, H. (1965).
Aminotriazole: acute and subacute toxicity. Report from American
Cyanamid Co. (Unpublished)
Baron, J. and Tephyly, T.R. (1969). Effect of 3-amino-1,2,4-triazole
on the stimulation of hepatic microsomal heme synthesis and induction
of hepatic microsomal oxidases produced by phenobarbital. Molec.
Pharmac., 5:10-20.
Bayer (1973). Amitrole residues in peaches, plums, grapes, cherries
and apples. Reports 434/72 - 443/72.
Bondarenko, D.D. (1958). Decomposition of amitrole in soil. The Ohio
Agricultural Experiment Station and The Ohio State University. Proc.
N. cent. Weed Control Conf.
Boyd, J.E. (1964, 1965). Some physiological and phytotoxicological
properties of amitrole. American Cyanamid Co.
Brown, J.C. and Carter, M.C. (1968). Influence of amitrole upon
protein metabolism in bean plants. Weed Sci., 16(2): 222-226.
Carter, M.C. (1969). Amitrole. In P.C. Kearney and D.D. Kaufman
(editors). Degradation of herbicides. Marcel Dekker, Inc., New York,
p. 187-206.
Carter, M.C. and Naylor, A.W. (1959). The formation, in vivo, of a
complex between 3-amino-1,2,4-triazole and a glycine-serine
derivative. Pl. Physiol., Lancaster, 34:(Suppl.) vi.
Carter, M.C. and Naylor, A.W. (1960, 1961). Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot. Gaz., 122:138-143.
Carter, M.C. and Naylor, A.W. (1961a). Studies on an unknown metabolic
product of 3-amino-1,2,4-triazole. Physiologia Pl., 14:20-27.
Carter, C. and Naylor, A.W. (1961b). The effect of
3-amino-1,2,4-triazole upon the metabolism of carbon labeled sodium
bicarbonate, glucose, succinate, glycine and serine by bean plants.
Physiologia Pl., 14:62-71.
Castelfranco, P. and Brown, M.S. (1963). A hypothesis of amitrole
action based on its behavior toward free radical generating systems.
Weeds, 11(2):116-124.
Clyne, R. (1970). Statement to Amitrole Advisory Committee.
Corneliussen, P.E. (1969). Residues in food and feed. Pesticide
residues in total diet samples (IV). Pestic. Monit. J., 2:140-152.
Corneliussen, P.E. (1970). Pesticide residues in total diet samples
(V). Pestic. Monit. J., 4(3):89-105.
Dardin, V. (1958). 3-amino-1,2,4-triazole - chronic oral
administration, beagle dogs, 52 weeks. Histology - supplement to
report. Report from Hazelton Laboratories, Inc. (Unpublished)
Dardin, V. (1959). 3-amino-1,2,4-triazole. Histology - supplement to
report of two year dietary feeding - rats. Report from Hazelton
Laboratories, Inc. (Unpublished)
Day, B.E. and Hendrixson, R.T. (1959). A study of the accumulation and
depletion of amitrole in citrus fruit and foliage. University of
California, Riverside, Research Report.
Day, B.E., Jordan, L.S., and Hendrixson, R.T. (1961). The
decomposition of amitrole in Californian soils. Weeds, 9:443-456.
Demint, R.J., Frank, P.A., Comes, R.D. (1970). Amitrole residues and
rate of dissipation in irrigation water. Weed Sci., 18(4):439-442.
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966). Pesticide residues
in total diet samples. Science, 151:101-104.
Duggan R.E., Barry, H.C. and Johnson, L.Y. (1967). Residues in food
and feed. Pesticide residues in total diet samples (II). Pestic.
Monit. J., 1(2):2.
Dunster, K.W. (1969). Amitrole persistence in irrigation canals.
Report of trials in Montana, Washington and Colorado. Amchem Report, 7
May 1969.
Elsea, J. (1954). Acute dermal application, acute eye application.
Report from Hazelton Laboratories, Inc. (Unpublished)
Ercegovich, C.D. (1957). Studies on the analysis and persistence of
3-amino-1,2,4-triazole in plant tissue and in soil. Ph.D. thesis,
Pennsylvania State University, University Park, Pa.
Ercegovich, C.D. and Frear, D.E.H. (1964). The fate of
3-amino-1,2,4-triazole in soils. J. agr. Food Chem., 12:26-29.
Fabrizio, D.P.A. (1973). Final report on cytogenicity study on
3-amino-1,2,4-triazole from Litton Bionetics, Inc. submitted to WHO by
Rohm and Haas Co. (Unpublished)
Fang, S.C., George, M. and Yu, T.C. (1964). Metabolism of 3-amino-1,2,
4-triazole-5-C14 by rats. J. agr. Food Chem., 12:219-223.
Fang, S.C., Khanna, S. and Rao, A.V. (1966). Further study on the
metabolism of labeled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. agr. Food Chem., 14:262-265.
Fang, S.C., Fallen, E. and Khanna, S. (1967). The metabolism of
labeled amitrole in plants. Weeds, 15:343-346.
Feytmans, E. and Leighton, F. (1973). Effects of pyrazole and
3-amino-1,2,4-triazole on methanol and ethanol metabolism by the rat.
Biochem. Pharmac., 22:349-360.
Fleming, A.S. (1959). Statement about amitrole residues in cranberries
- Press Release. Dept of Health, Education and Welfare, 9 Nov. 1959.
Fogleman, R. (1954). Amitrole - (3-amino-1,2,4-triazole) - Final
report: submitted by Hazelton Laboratories, Inc. (Unpublished)
Frank, P.A. (1969). Amitrole concentration in water of treated
irrigation channels. U.S. Dept of Agriculture A.R.S., Denver,
Colorado. Report, 14 Jan. 1969.
Fredrick, J.F. and Gentile, A.C. (1960). The formation of the glucose
derivative of 3-amino-1,2,4-triazole under physiological conditions.
Physiologia Pl., 13:761-765.
Fredrick, J.F. and Gentile, A.C. (1961). The structure of the
D-glucose adduct of 3-amino-1,2,4-triazole: Infrared absorption
studies. Arch. Biochem. Biophys., 92(2):356-359.
Fredrick, J.F. and Gentile, A.C. (1962). Studies of a phosphorylated
derivative of 3-amino-1,2,4-triazole formed by the action of yeast
hexokinase. Physiologia Pl., 15(1):186-193.
Fredrick, J.F. and Gentile, A.C. (1965). Physico-chemical studies of
the metabolic end product of 3-amino-1,2,4-triazole in yeast.
Phytochemistry, 4:851-856.
Freed, V.H., Furtick, W.R. (1961). The persistence of amitrole in
soil. Oregon Agricultural Experiment Station Technical Paper No. 1375.
Freed, V.H., Montgomery, M. and Kief, M. (1961). Proc. N East Weed
Control Conf., 1961:6-16.
Fregly, M.J. (1968). Effect of aminotriazole on thyroid function in
the rat. Toxic. appl. Pharmac., 13:271-286.
Gaines, T.B., Kimbrough, R.D. and Linder, R.E. (1973). Toxicity of
amitrole in the rat. Toxic. appl. Pharmac., 26:118-129.
Geldmacher-von Mallinckrodt, M. and Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Archs Toxik., 27:13-18. (English
summary)
Gentile, A.C. and Fredrick, J.F. (1959). Chemical activity of the
glucose adduct of 3-amino-1,2,4-triazole. Physiologia Pl., 12:862-867.
Grapenthien, J., Hathaway, D. and Keplinger, M. (1972). Two year
chronic aerosol inhalation toxicity study with amitrol 3AT in albino
rats. Report from Industrial Biotest Laboratories, Inc. (Unpublished)
Groves, K. and Chough, K.S. (1971). Extraction of 3-amino-1,2,
4-triazole (amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from
soils. J. agr. Food Chem., 19(5):840-841.
Hapke, H.J. (1967). The toxicity of aminotriazol for domestic animals.
Zbl. Vet. Med. A., 14:469-486. (English, French and Spanish summaries)
Heim, W.G., Appleman, D. and Pyfrom, H.T. (1956). Effects of
3-amino-1,2,4-triazole (AT) on catalase and other compounds. Am. J.
Physiol., 186:19-23.
Herrett, R.A. and Bagley, W.P. (1964). The metabolism and
translocation of 3-amino-1,2,4-triazole by Canada thistle. J. agr.
Food Chem., 12:17-20.
Herrett, R.A. and Linck, A.J. (1961a). Penetration of
3-amino-1,2,4-triazole in Canada thistle and field bindweed. Weeds,
9(2): 224-230.
Herrett, R.A. and Linck, A.J. (1961b). Quantitative determination of
3-amino-1,2,4-triazole. J. agr. Food Chem., 9(6):466-467.
Hill, R. (1962, 1963). Residues of amitrole in almonds, walnuts,
peaches and prunes. Diablo Laboratories, Berkley, Reports of Amchem
Products Inc.
Hilton, H.W., Uyehara, G. and Segal, H.S. (1963). Amitrole residues in
sugar cane. Rate of disappearance studies and improved methodology.
Hawaiian Sugar Planters Association Report.
Hilton, H.W. and Uyehara, G.K. (1966). Determination of
3-amino-1,2,4-triazole in sugarcane. J. agr. Food Chem., 14(1):90-94.
Hodge, H.C., Maynard, E.A., Downs, W.L., Ashton, J.K. and Salerno
L.L. (1966). Tests on mice for evaluating carcinogenicity. Toxic.
appl. Pharmac., 9:583-596.
Hoshino, M. (1960). Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature, 186:174-175.
Hylin, J.W. (1962). Determination of amitrole residues in coffee
beans. Report from University of Hawaii.
IARC (1974). Amitrole. In IARC Monographs on the Carcinogenic Risk
of Chemicals to Man. Vol. 7.
Innes, J. (1966). Amitrole - summary sheet - Carcinogenesis assay
studies - Subcutaneous. Data sheet. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969). Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Jukes, T.H. and Shaffer, C.B. (1960). Antithyroid effects of
amino-triazole. Science, 132:296-297.
Jukes, T.H. (1961). Antimetabolites and plant growth. Bull. Torrey
bot. Club, 88(5):321-327.
Kaufmann, D.D. (1966). Microbial degradation of herbicide
combinations: amitrole and dalapon. Weeds, 14:130-134.
Kaufmann, D.D., Plimmer, J.R., Kearney, P.C., Blake, J. and Guardia,
F.S. (1968). Chemical versus microbial decomposition of amitrole in
soil. Weed Sci., 16(2):266-272.
Keller, J. (1959). Aminotriazole (3-amino-1,2,4-triazole) two year
chronic feeding - rats. Report from Hazelton Laboratories, Inc.
(Unpublished).
Kretschmar, G. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) on the growth of Azotobacter. Z. allg. Mikrobiol.,
10:253-267.
Kretschmar, G. and Gunther, G. (1970). Der einflus von
3-amino-1,2,4-triazol (Amitrol) auf die Atmungvon Azobacter. A.
allg. Mikrobiol., 10:487-500.
Kröller, E. (1966). Anwendung und Eigenschaften des
3-amino-1,2,4-triazols im Hinblick auf seine Rückstände im
Lebensmitteln. Residue Reviews, 12:162-192.
Kudsk, F.N. (1969). Uptake of mercury vapor in blood in vivo and
in vitro from Hg-containing air. Acta pharmac. Tox., 27:149-160.
Landauer, W., Salam, N. and Sopher, D. (1971). The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Envir. Res.,
4:529-543.
Langhans, W. and Schimassek, H. (1974). Prevention of halothane
induced changes in rat liver enzymes by 3-amino-1,2,4-triazole.
Biochem. Pharmac., 23:403-409.
Leonard, O.A., and Lider, L.A. (1961). Toxicity and translocation of
herbicides supplied to grape rootings through solution culture. Am. J.
Enol. Vitic., 12(1):37-46.
Leonard, O.A., and Weaver, R.J. (1961). Absorption and translocation
of 2,4-D and amitrole in shoots of the Tokay grape. Hilgardia,
31(9):327-368.
Levine, W.G. (1973). Effect of phenobarbital and 3-amino-1,2,
4-triazole on the metabolism and biliary excretion of 3,4-benzypyrene
in the rat. Life Sci., 13:723-732.
Lotlikar, P., Wasserman, M. and Luha, L. (1973). Effect of
2-amino-1,2,4-triazole pretreatment on N and ring - hydroxylation of
2-acetylamino-fluorene by the rat. Proc. Soc. exp. Biol. Med.,
144:445-449.
Magos, L., Sugata, Y. and Clarkson, T.W. (1974). Effects of
3-amino-1,2,4-triazole on mercury uptake by in vitro human blood
samples and by whole rats. Toxic. appl. Pharmac., 28:367-373.
Maier-Bode, H. and Bechtel, W.D. (1968). Investigations of amitrole
residues in orchardry and viticulture. A. Pflanzenkrankheiten No. 4.
Proceedings of the 7th German Symposium on questions of weed biology
and control, 5 March 1968.
Margoliash, E. and Novogrodsky, A. A study of the inhibition of
catalase by 3-amino-1,2,4-triazole. Biochem. J., 68:468-475.
Marston, R.B., Schults, D.W., Shiroyama, T., and Snyder, L.V. (1968).
Pesticides in water. Amitrole concentrations in creek waters
downstream from an aerially sprayed watershed sub-basin. Pestic.
Monit. J., 2(3):123-128.
Massini, P. (1958). Uptake and translocation of 3-amino and
3-hydroxy-1,2,4-triazole in plants. Acta bot. neerl., 7:525-530.
Massini, P. (1959). Synthesis of (3-amino-1,2,4-triazolyl) alanine
(ATX) from 3-amino-1,2,4-triazole in plants. Biochem. Biophys. Acta,
36:548-549.
Massini, P. (1963). Aminotriazolylalanine; a metabolic product of
amitrole in plants. Acta bot. neerl., 12:64.
Matsushima, T. and Weisburger, J. (1972). Effect of carbon monoxide or
of 3-amino-triazole on C- and N- hydroxylation of the carcinogen
N-2-fluorenylacetamide by liver microsomes of hamsters pretreated with
3-methyl-cholanthrene. Xenobiotica, 2:423-430.
McRae, I.C. and Alexander, M. (1965). Microbial degradation of
selected herbicides in soil. J. agr. Food Chem., 13:72-76.
Meissner, W.A. (1971). Report of Amitrole Advisory Committee to
Environmental Protection Agency Washington, 12 March 1971.
Menzie, C.M. (1969). Metabolism of Pesticides. U.S. Dept. Interior,
Bureau of Sport Fisheries and Wildlife Special Scientific
Report - Wildlife No. 127:40-41.
Miller, C.S. and Hall, W.C. (1961). Absorption and metabolism of
aminotriazole in cotton. J. agr. Food Chem., 9:210-212.
Mohandas, T. and Grant, W.F. (1972). Cytogenetic effects of 2,4-D and
amitrole in relation to nuclear volume and DNA content in some higher
plants. Can. J. Genet. Cytol., 14:773-783.
Montgomery, M. and Freed, V.H. (1963). Residue studies of
amino-triazole in soyabeans. Report from Oregon State University, 25
April 1963.
Moore, B. (1968). Amitrole and simazine residue trials in Tasmanian
apples treated with D amatol 44. Geigy Australia Ltd., Report
68/8/290.
Moore, B. (1969). Amitrole and simazine residue trials at Orange, NSW,
1967-68. Geigy Australia Ltd., Technical Report 69/2/294.
Moore, B. (1970). Amitrole and simazine residue trials at Orange, NSW,
1968-69. Geigy Australia, Technical Report 70/1/309.
Moser, B. (1968). Summary statements on triazoles. Rutgers Univ. Plant
Physiology Seminar.
Muzik, T.J. (1965). Effect of temperature on the activity and
persistence of amitrole and 2,4-D. Weed Res., 5(3):207-212.
Napalkov, N. (1962). Tumor inducing action of 3-amino-1,2,4-triazole.
Truda prof. Zabol., 6:48-51.
Napalkov, N.P. (1969). On blastomogenic action of thyreostatic
substances. IECO USSR Acad. Med. Sci. Moscow, Monograph.
Naylor, A.W. (1964). Complexes of 3-amino-1,2,4-triazole in plant
metabolism. J. agr. Food Chem., 12:21-25.
Nicholson, H.P. (1963). Persistence of amitrole in pond water. U.S.
Public Health Service, Report 17 April 1963.
Norris, L.A. (1970). Degradation of herbicides in the forest floor. In
Tree Growth and Forest Soils, C.T. Youngberg and C.B. Daley (editors).
Onley, J.H., Storherr, R.W., Cluver, A.J. Jr. (1963). An Oregon
cranberry bog study for 3-amino-1,2,4-triazole residues, 1961-1962. J.
Ass. off. analyt. Chem., 46(6):996-1001.
Otten, R.S. (1970). Statement to Amitrole Advisory Committee, 16 Dec.
1970.
Plimmer, J.R., Kearney, P.C., Kaufman, D.D. and Guardia, F.S. (1967).
Amitrole decomposition by free radical-generating systems and by
soils. J. agr. Food Chem., 15: 996-999.
Potts, K.T. (1961). The chemistry of 1,2,4-triazoles. Chem. Rev.,
61:87-127.
Racusen, D. (1958). The metabolism and translocation of
3-amino-triazole. Archs Biochem. Biophys, 74(1):106-113.
Raisfeld, I., Bacchin, P., Hutterer, F. and Schaffner, F. (1970). The
effect of 3-amino-1,2,4-triazole on the phenobarbital induced
formation of hepatic micro-somal membranes. Molec. Pharmac., 6:231.
Rausina, B., Mastri, C., Keplinger, M. and Yost, D. (1972). Two year
chronic dermal toxicity study with amitrole 3-AT in albino rats.
Report from Industrial BioTest Laboratories, Inc. (Unpublished)
Riepma, P. (1962). Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in soil. Weed Res., 2:41-50.
Rogers, B.J. (1957a). Translocation and fate of amino triazole in
Plants. Weeds, 5:5-11.
Rogers, B.J. (1957b). The action of 3-amino-1,2,4-triazole in plants.
Hormolog 1:10.
Samio, H., Baird, M. and Massie, H. (1972). Renewal of catalase
activity in Drosophilia following treatment with 3-amino-1,2,
4-triazole. J. Insect. Physiol., 18:991-1000.
Sanderson, D. and Roe, F. (1962). Dietary toxicity of aminotriazole to
the female rat. Report from Fisons Ltd., Agrochemical Division and
Pathology Report from Chester Beatty Research Institute. (Unpublished)
Schroeder. I. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) in a non-herbicidal range on the protein biosynthesis of
the unicellular algae Poteriochromonas stipitata. A. Allg.
Mikrobiol., 10:295-298.
Schubert, O.E. (1965). Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Report from West Virginia
University.
Segal, H.S. (1960). Determination of amitrole in irrigation ditch
water. Amchem Laboratory Report 411, July 1960
Segal, H.S. (1963). Amitrole residues in Hawaiian irrigation ditch
water. Amchem Report, 27 Feb. 1963.
Shaffer, C., Vidone, L. and Golz, H. (1958). Aminotriazole: 32 day
repeated feeding to rats. Report from American Cyanamid Co.
(Unpublished)
Smagghe, G. (1974). Report from Chemical Products - Ugine - Kuhlmann.
(Unpublished)
Smith, L.W. and Chang, F.Y. (1973). Aminotriazole metabolism in
Cirsium arvense (L.) Scop. and Pisum sativum L. Weed Res.,
13:339-350.
Stenger, R. and Johnson, E. (1972). Modifying effect of
3-amino-1,2,4-triazole on phenobarbital induced changes in rat liver.
Expl. Molec. Pathol., 16:147-157.
Storherr, R.W. and Burke, J. (1961). Determination of 3-amino-1,2,
4-triazole in crops. J. Ass. off. analyt. Chem., 44(2):196-199.
Storherr, R.W. and Onley, J. (1962). Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J. Ass.
off. analyt. Chem., 45(2):382-387.
Strum, J. and Karnovsky, M. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity. Lab.
Invest., 24:1-12.
Sund, K.A. (1956). Residual activity of 3-amino-1,2,4-triazole in
soils. J. agr. Food Chem., 4(1):57-60.
Svensson, J.A. (1971). Residues of herbicides in cereals. Proceedings
of 12th Swedish Weed Conference, Jan. 1971.
Tephly, T.R., Mannering, G.J. and Parks, R.E., Jr. (1961). Studies on
the mechanism of inhibition of liver and erythrocyte catalase activity
by 3-amino-1,2,4-triazole (AT). J. Pharmac. exp. Ther., 134:77-82.
Tephly, T.R., Hasegawa, E. and Baron, J. (1971). Effect of drugs on
heme synthesis in the liver. Metabolism, 20:200.
Thoman, J.R. (1963). Persistence of amitrole in water (Borrow Pits)
Florida. U.S. Dept of Health, Education and Welfare, Report, 13 Nov.
1963.
Tschudy, D.P. and Collins, A. (1957). Effect of 3-amino-1,2,4-triazole
on delta-amino-levulinic acid dehydrase activity. Science, 126:168.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. and Hidosaka, Y.
(1973). Fine structure and localization of peroxidase activity in
aminotriazole goiter. Nagoya med. J., 18:183-190.
Weir, R., Jr. (1958). 3-amino-1,2,4-triazole chronic oral
administration - Beagle Dogs - 52 weeks - Final Report. Report from
Hazelton Laboratories, Inc. (Unpublished)
Weir, R. Jr. (1974). Personal communication to WHO.
Wit, S.L. and van der Kamp, C.G. (1963). Residues of amitrole in
apples. Report 26 February 1963, National Institute of Public Health,
Utrecht. Submitted by Netherlands Government to FAO (1974).
Yost, J.F. and Williams, E.F. (1958). Proc. N East Weed Control Conf.,
1958:9-15.
 Other information on identity and properties
Amitrole is a white, crystalline solid with a molecular weight of
84 and a melting point of 150-153°C. It is soluble in water to the
extent of 28 g/100 g and in ethanol and methanol to an extent of
26 g/100 g. It is sparingly soluble in ethyl acetate and insoluble in
ether, acetone and most other organic solvents. Amitrole forms neutral
aqueous solutions but acts as a weak base. When exposed to UV light,
amitrole breaks down to form CO2, urea and cyanamide. Potts (1961)
has reviewed the chemistry of s-triazoles in considerable detail.
Amitrole behaves chemically as a typical aromatic amine.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
The metabolic fate of amitrole in animals has not yet been fully
elucidated. It is rapidly excreted from the body. Following
intraperitoneal administration, at least 90% of the injected dose was
observed in the urine unchanged within 24 hours (Bagdon et al., 1965).
When amitrole (5-14C) was fed to rats (Fang et al., 1964),
70-96% of the radioactivity was excreted in the urine during the first
24 hours as amitrole and two unidentified metabolites. There were
traces of 14C in the expired air and the faeces contained a small but
variable amount of activity. After absorption, amitrole was
distributed throughout most of the tissues. The maximum radioactivity
in all tissues was generally reached within one hour and started to
decrease 3-4 hours after dosing. Elimination of amitrole from all
tissues was rapid. The liver contained a metabolite but no free
amitrole. The rate of elimination of this metabolite from liver and
kidney was much slower than that of the parent compound.
Further studies on rats (Fang et al., 1966) have shown that
amitrole is not metabolically acetylated, and that the average
half-time for amitrole clearance in various tissues was 4.2 hours.
These studies were extended to include the metabolism of two
metabolites isolated from bean plants. One was readily excreated in
the urine, mainly unchanged but partly as two new metabolites. The
other bean metabolite was excreted much more slowly, apparently
unchanged.
Biotransformations in plants and soil are discussed in the
section "Fate of residues".
Effects on enzymes and other biochemical parameters
Acute administration of amitrole to rats results in depression of
catalase and peroxidase enzyme activity as well as the activity of
several other enzymes. Liver peroxidase was found to recover within 24
hours, while liver and kidney catalase depression was slower to
recover. Catalase returned to normal after seven days (Heim et al.,
1956). Insect-derived catalase was also shown to regenerate its
activity slowly following exposure to aminotriazole (Samio et al.,
1972).
Groups of rats (15 males per group) were administered radioactive
iodine alone or in combination with 0.15 or 0.78 mg amitrole
administered by intraperitoneal injection. Uptake of radioactivity by
the thyroid gland was significantly depressed at 0.78 mg/kg. The lower
dose reduced iodine uptake but the reduction was not statistically
significant. Amitrole significantly decreased fecal radioactivity,
while the urinary radioactivity was not significantly affected
(Fregly, 1968).
Amitrole inhibits peroxidase activity of rat thyroid and salivary
gland (Alexander, 1959a and b); liver and kidney catalase activity of
rats (Tephly et al., 1961; Heim et al., 1956); catalase activity of
human red blood cell (Margoliash and Novogrodsky, 1958; Magos et al.,
1974; Kudsk, 1969) catalase activity from other sources such as plant
tissue and commercial crystallized enzymes; the synthesis of
tryptophan peroxidase of rats (Auerbach et al., 1959);
delta-aminolevulinic acid dehydrase activity of mouse and rat liver
(Baron and Tephley, 1969; Tschudy and Collins, 1957); the inductive
effect of phenobarbital on hepatic microsomal cytochrome P-450 (Baron
and Tephly, 1969); the incorporation of iron (59FeCl3) into rat
hepatic microsomes (Tephly et al., 1971); microsomal heme synthesis
(Tephly et al., 1971); and hydroxylation of exogenous substrates by
the liver (Raisfeld et al., 1970). Amitrole caused no proliferation of
the microsomal endoplasmic reticulum (Raisfeld et al., 1970). Several
further studies have shown the effect of amitrole on certain aspects
of the drug metabolizing enzyme system in rat liver (Feytmans and
Leighton, 1973; Lotikar et al., 1973; Stenger and Johnson, 1972;
Levine, 1973; Matsushima and Weisburgh, 1972; Langhans and Shimassek,
1974). There is no clear picture of the effect of amitrole on
microsomal metabolizing enzyme systems in mammals although it does
serve to inhibit or reduce several specific biochemical reactions.
Kudsk (1969) observed that peroxide generating systems, such as
seen with methylene blue, accelerated the uptake of mercury in vitro
by human red blood cell preparations. Amitrole and methylene blue
which promoted the catalase-peroxide-complex I, had no effect on
mercury uptake. Magos (1974) suggested that the ability of red blood
cells to take up mercury from air saturated with mercury vapor was
reduced when cells were treated for three hours with amitrole and
methylene blue. In hemolysates mercury uptake was stimulated by
amitrole. In vivo administration of amitrole to rats resulted in
decreased lung concentration and increased liver concentrations of
mercury.
Tryptophan synthesis in plants was shown to be inhibited by
amitrole (Smith and Chang, 1973). Amitrole stimulated respiration in
Azotobacter and concurrently inhibited growth possibility through an
uncoupling of oxidative phosphorylation (Kretschmar and Günther,
1970). In algae, amino acid synthesis was inhibited and glucosamine
synthesis was stimulated (Schroeder, 1970).
In vitro, amitrole does not inhibit cholinesterase activity of
brain, submaxillary gland, serum, or ilium. Three hours after
intraperitoneal administration of 4 mg/kg to male rats cholinesterase
activity of brain, submaxillary gland, and serum was normal (Bagdon et
al., 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A testing procedure using groups of hybrid strains of mice (18
mice of each sex and each of two strains) evaluated amitrole for
carcinogenicity. The mice were administered amitrole orally from day 7
to day 28 of age at doses of 1000 mg/kg and thereafter in the diet at
a dose of 2192 ppm. None of the mice survived the 18 month test
interval. Hepatomas were evident in most animals, 67/72, and carcinoma
of the thyroid was reported in 67/71 of the animals examined (Innes et
al., 1969). A further study was designated as subcutaneous although
no details were given of the experimental design (Innes, 1966). The
majority of animals survived the 18 month test interval. There was no
evidence of heptoma or carcinoma of the thyroid.
Groups of mice (50 of each sex) were either treated with amitrole
by a single subcutaneous administration of 10 mg per mouse or by a
weekly dermal application of 0.1 mg/mouse. There was no abnormal
behaviour observed. When the animals were sacrificed and examined, no
signs of cancer were evident. No mention was made of examination of
the thyroid gland in this study (Hodge et al., 1966).
Rat
Amitrole was found to have an effect on the induction of liver
carcinogenesis by 4-dimethylaminoazobenzene. In an attempt to
determine the relationship between liver catalase activity and
carcinogenesis, Hoshino (1960) administered amitrole intraperitoneally
to albino rats at a dose of 1000 mg/kg every other day for 150 days
while feeding 4-dimethylaminoazobenzene at a dose that was known to
induce liver carcinoma. There was a reduction in the occurrence of
liver tumours in those animals administered amitrole (4/14) when
compared to the animals administered the carcinogen alone (12/16).
Napalkov (1962; 1969) administered amitrole to rats by
subcutaneous injection twice weekly (later reduced to a weekly
injection) at a dose of 125 mg/animal; in the water at a dose of 20-25
mg/day; in the diet at a dose of 250-500 mg/day; or by subcutaneous
implantation combined with subcutaneous administration twice weekly at
a dose of 125 mg/rat. Thyroid hyperplasia was observed after seven to
eight months. Liver tumours were observed initially in those animals
receiving amitrole in the diet and soon thereafter in those animals
receiving it by injection or via the drinking water. Sarcomas
developed where amitrole was implanted or injected. Concurrent daily
injections of 2.5 µg/100 g bw thyroxine and oral administration of 300
mg/day/rat amitrole in food for the length of the experiment resulted
in the development of only one thyroid adenoma but 9 liver tumors in
12 males. Amitrole alone induced thyroid tumors in 7/22 and liver
tumors in 12/23 male rats of the same age. No liver tumors but 2
thyroid cystic adenomas were observed in 51 control animals.
A comprehensive review of the carcinogenic risk of amitrole was
made by the International Agency for Research on Cancer commenting on
the animal and human data (IARC, 1974).
Special studies on mutagenicity
Mohandas and Grant (1972) observed that amitrole significantly
increased the frequency of chromosomal aberrations in root tips of
higher plants.
Mutagenic tests using Salmonella strains and yeast cells as
test indicators have been negative. A host mediated assay has been
reported to be negative although details of the test are not yet
available (Weir, 1974). A cytogenetic study, utilizing examination of
bone marrow cells arrested in C-metaphase from rats treated with
amitrole at levels of 0, 2.5, 25 and 250 mg/kg daily for 5 days, was
negative (Fabrizio, 1973).
Special studies on reproduction
Rat
Groups of rats (10 male and 10 female Sherman strain rats per
group) were fed amitrole in the diet at levels of 0, 25, 100, 500 and
1000 ppm for approximately two months and mated. In the first
generation at 500 and 1000 ppm the number of pups born and pup
survival was reduced. It was noted that almost all of the pups died
within one week after weaning and these dietary levels were
terminated. Dietary levels of 0, 25, and 100 ppm were fed through two
generations (two litters from the first generation and one litter from
the second generation). At 100 ppm in the diet there was no effect on
reproduction and all pups survived. At 100 ppm all animals were found
to have thyroid hyperplasia while the incidence of thyroid hyperplasia
was sporadic at 25 ppm (Gaines et al., 1973).
Oral administration of amitrole at doses of 400 and 1000 mg/kg to
rats on days 8 through 13 of gestation resulted in no signs of embryo
toxicity or of teratogenic effects. Groups of rats (5 males and 5
females per group) were administered amitrole orally at 100 mg/kg or
at 100 ppm in the drinking water for three months prior to mating and
in the females up to day 15 of gestation. There was no indication of
any effect on reproduction in either male or female rats (Hapke,
1967).
The effect of oral administration of amitrole on reproduction was
studied in three groups of eight pregnant Sherman strain rats
administered dosage levels of 0, 20 and 100 mg/kg bw/day from day 7
through 15 of gestation. There was no effect of amitrole on
reproduction and no abnormalities were observed in the offspring
through weaning (Gaines et al., 1973).
Special studies on teratogenicity
Amitrole injected into the yolk of chicken eggs at doses of 20-40
mg/egg, produced a dose dependent malformation of the beak and
abnormalities of the tibia shaft. This latter abnormality occurred
less frequently. At doses of 2 mg/egg, no effects ware noted. It was
observed that when amitrole was dissolved in DMSO it was more active
than when it was administered in water (Landauer et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of amitrole
LD50
Species Route (mg/kg bw) References
Rat oral >25 000 Bagdon et al., 1965
dermal > 2 500 Gaines et al., 1973
ip > 4 000 Bagdon et al., 1965
Mouse oral >14 700 Fogleman, 1954
iv > 1 600 Bagnon et al., 1965
Rabbit dermal >10 000 Elsea, 1954
oral > 2 150 Fogleman, 1954
Dog iv > 1 800 Fogleman, 1954
Cat iv > 1 750 Bagdon et al., 1965
Signs of poisoning include: depression, dyspnea, diarrhoea,
ataxia, altered respiration, coma and death. The G.I. tract was
severely irritated following acute doses. Acute doses administered to
dogs (50-1000 mg/kg) intravenously produced an immediate fall in blood
pressure followed by increased respiration. Following a dose of 1000
mg/kg, the pressor response to adrenalin was blocked suggestive of an
adrenalytic action with respect to blood pressure (Fogleman, 1954).
TABLE 2. Acute toxicity of glucose adduct
LD50
Species Sex Route (mg/kg bw) References
Rat M oral >10 000 Bagdon et al., 1965
Mouse M oral >10 000 Bagdon et al., 1965
M ip >10 000 Bagdon et al., 1965
M iv > 1 600 Bagdon et al,, 1965
Amitrole applied to unabraded skin as a paste at doses of
1-10 g/kg for 24 hours caused a mild dermal irritation at all dose
levels. The effect, a mild erythemia, was reduced within 24 hours. No
other effects were noted. Gross and microscopic examination of tissues
and organs was normal (Elsea, 1954).
Amitrole (3 mg) was applied to the conjuntival sac of rabbits.
Although mild irritation lasting 24-48 hours was observed, no
permanent damage was noted (Elsea, 1954).
Short term studies
Rat
Groups of rats (5 males and 15 females per group) were fed
amitrole in the diet at levels of 0, 0.01%, 0.1%, and 1.0% for 63
days. There was no mortality over the course of this study. Food
consumption and growth were depressed at 0.1% and above in both males
and females. Histological examination from selected tissues at the
conclusion of the study (thyroid was not examined) revealed
vacuolation of liver cells in those animals fed 0.1% and above. The
vacuoles were identified as fat globules indicative of fatty
metamorphosis associated with liver cell damage. No histological
effects were noted at 0.01% (Fogleman, 1954).
Groups of weanling rats were administered amitrole for up to 56
days by intraperitoneal injection three times/week for eight weeks at
1000 mg/kg. The administration of a recrystallized amitrole resulted
in no growth depression in two separate groups. The administration of
a "pure" material, not recrystallized, resulted in growth depression
(Heim et al., 1956).
Groups of rats (10 males and 10 females per group) were
administered amitrole at 1000 mg/kg by intraperitoneal administration
on alternate days for 42 days. Amitrole had to effect on bw gain or
food consumption but caused a 3-4 fold increase in thyroid weight
(Bagdon et al., 1965).
Groups of rats were administered amitrole daily, 5 days per week,
for four weeks at levels of 0, 100, 200 and 400 mg/kg. Growth rate was
reduced, relative thyroid weight increased, and iodine content of the
thyroid was reduced (Hapke, 1967).
Administration of dietary levels of 60 and 120 ppm for two weeks
resulted in an enlargement of the thyroid gland of rats and a
pronounced lowering of iodine uptake. Over this two week interval
there were no significant changes at levels of 15 and 30 ppm (Jukes
and Shaffer, 1960).
Administration of a high dose (0.04%) of amitrole in the drinking
water (approximately 60 mg/kg/day) has been shown to produce goitres
in rats within three days (Strum and Karnovsky, 1971; Tsuda et al.,
1973).
Groups of rats (10 male rats per group) were fed dietary
concentrations of amitrole for 32 days. One group was fed 500 ppm for
32 consecutive days, another group received 1000 ppm on alternate days
for the duration of the study and a group was fed the basal diet.
Behavior and mortality were not affected. Food intake and growth at
500 ppm were reduced. In the group receiving 1000 ppm on alternate
days food intake and growth data did not differ significantly from the
controls. At autopsy, gross examination of animals receiving 500 ppm
showed the thyroid gland to be hyperaemic and enlarged. The thyroid of
animals fed 1000 ppm appeared to be slightly hyperaemic but were
otherwise comparable to the controls. Mean thyroid weight/body weight
ratio for the 0, 1000 ppm, and 500 ppm groups was 58, 77, and 303
respectively (Shaffer et al., 1958).
A group of 30 male weanling rats was fed amitrole in the diet at
1000 ppm for two weeks. A comparable group received a similar dietary
level of propylthiouracil for two weeks. At the end of the feeding
interval several animals were sacrificed and the thyroid glands
weighed. The remaining animals were placed on control diets and
sacrificed at either the third or fourth week of feeding. Amitrole,
after two weeks produced a significant increase in the mean thyroid
weight (comparable to that found with propylthiouracil). Daring the
week following removal from the diet, the weight of the thyroid gland
diminished and by the end of the second week on a control diet
regression to normal size was almost complete (Bagdon et al., 1965).
Groups of rats (20 rats per group) were fed dietary l levels of 0
and 316 ppm for 100 days to examine the goitrogenic potential of
amitrole. Over the course of this experiment amitrole had a
significant effect on growth. Exophthalmia was not observed as in the
long term rat studies. Food intake was not drastically reduced. Gross
examination of the thyroid showed a laterally enlarged thyroid with
enlarged blood vessels. Microscopic examination showed hyperplasia of
the thyroid. There was no evidence of abnormalities observed in either
the liver or the kidney (Sanderson and Row, 1962).
Groups of rats (10 males per group) were administered amitrole in
the drinking water at concentrations of 0, 50, 250 and 1250 ppm for
106 days. The administration of amitrole resulted in a dose-dependent
depression of growth with a corresponding reduction of food and water
intake. Appearance, behaviour, and mortality were not affected by
amitrole. Gross and microscopic examination of tissues and organs
showed a marked increase in thyroid size at all dose levels. In rats
where reduced growth was noted, the kidneys, adrenals, liver and
spleen were proportionately smaller. Reproductive organs were not
affected. Microscopic examination showed general enlargement of the
thyroid at 50 ppm, with moderate stimulation of the thyroid epithelium
(no evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident (Bagdon et al., 1965).
Groups of rats (10 male rate per group) were fed dietary levels
of amitrole for 11-13 weeks at dosages of 0, 0.25, 0.50, 2, 10 and 50
ppm. At 0.5 ppm in the diet, no effect was observed in any of seven
separate measurements of thyroid function although iodine uptake was
slightly reduced and serum PBI concentrations were slightly increased.
Significant effects were noted at 2 ppm in the diet, especially with
regard to reduced PBI and reduced iodine uptake by thyroid. Gross and
microscopic examination of the thyroid confirmed the effects noted at
2 ppm (Fregly, 1968).
Dog
Groups of beagle dogs (3 males and 3 females were controls; 2
males and 4 females - 0.25 mg/kg; 3 males and 3 females - 1.25 mg/kg;
2 males and 4 females - 2.50 mg/kg; and 2 males and 2 females - 12.5
mg/kg) were administered amitrole orally by gelatin capsule six days a
week for 52 weeks. One male and one female per group were sacrificed
at 26 weeks. There was no mortality over the testing interval. Growth,
appearance, and behaviour were normal in all test animals. Results of
biochemical, haematological, and urological examinations were normal.
Gross and microscopic examination of tissues and organs showed no
evidence of abnormality associated with amitrole. There was no
apparent evidence of cytotoxic effects associated with the
administration of 12.5 mg amitrole kg bw/day for one year (Weir, 1958;
Dardin, 1958).
Long term studies
Dermal
Rat
Two groups of rats (25 males and 25 females per group) were
administered amitrole dermally at a level of 2.4 mg/kg weekly for 23
months. The application was allowed to contact the skin for thirty
minutes, after which the skin was rinsed and dried. Amitrole was found
to be non-irritating to the skin. There was no apparent effect of
amitrole on growth, behaviour, tumour formation or when tissues and
organs were observed on gross and microscopic examination. Statistical
studies with the liver and thyroid organ weight and organ-to-body
weight ratio did not reveal any differences in this experiment between
treated animals and controls (Rausina et al., 1972).
Inhalation
Rat
Groups of rats (25 males and 25 females per group) were exposed
to amitrole by inhalation for a one hour period every week for two
years. The animals were exposed to an aerosol of 0.2% (w/v) aqueous
solution by a head-only exposure to minimize oral ingestion. The
average analytical concentration of aerosol in the exposure chamber
was 2 mg/l air. There were no significant differences between the
treated and the control groups when examined with respect to
mortality, behaviour, growth, gross and microscopic examination of
thyroid and liver, or the incidence of tumor formation. No abnormal
effects were noted in this inhalation exposure (Grapenthien et al.,
1972).
Feeding
Rat
Groups of rats (35 males and 35 females per group) were fed
amitrole in the diet for two years at levels of 0, 10, 50, 100 and 500
ppm. Animals were sacrificed periodically over the course of the
experiment. The appearance of animals (especially females) fed 100 ppm
in the diet was altered. The incidence of protruding eyes
(exophthalmia) was considerably higher in the higher dose groups than
in the controls. Growth was reduced at 500 ppm. There was no apparent
effect of amitrole in the diet on survival, growth, or mortality in
the animals fed 50 ppm.
Gross examination of animals revealed a consistent finding of
thyroid enlargement. At 13 weeks, thyroid enlargement in males and
females was observed at 100 ppm. Microscopic examination suggested
hypofunction and a non-functional hyperplasia evident at 50 ppm and
above. The animals fed 500 ppm were removed from the diet at 19 weeks
and examined after two weeks on control diets. No evidence of
enlargement or hyperplasia was noted. At 26 weeks, thyroid enlargement
was evident at 50 ppm. Liver and kidney weight in both sexes was
decreased at 100 ppm. No effects were noted at 10 ppm. At 52 weeks,
growth of males was depressed at 100 ppm. At 68 weeks, growth of males
was again depressed at 100 ppm accompanied by enlargement of the
thyroid, pituitary, and liver. A cystic adenomatous structure was
observed at 100 ppm in the thyroid. At 50 ppm, thyroid hyperplasia and
hypofunction were noted. At 10 ppm, thyroid hypofunction was observed
in one male animal of the three males and three females examined. At
104 weeks, body weights were normal in all groups. At 100 ppm, liver
and kidney weights were normal while the thyroid was enlarged and
adenomas ranging from benign cysts to foetal malignant carcinoma were
observed. At 50 ppm, the thyroid was not enlarged. Adenomas were
observed in 3/16 rats. At 10 ppm, one rat showed an adenomatous nodule
with cellular hyperplasia (other sections suggested slight enlargement
while most control sections were normal). Thyroid enlargement was
observed only at 100 ppm. A no-effect level was not observed in this
study. Ten ppm was a minimal effect level (Keller, 1959; Dardin,
1959).
OBSERVATIONS IN MAN
A 39 year old woman ingested 20 mg/kg. This dose caused no signs
of intoxication and within a few hours of ingestion the compound
passed rapidly through the body and began to appear in concentrations
up to 100 mg/100 ml in the urine (Geldmacher-von Mallinckrodt, 1970).
Amitrole has been produced industrially since 1955 with no
evidence of ill effects other than mild contact dermatitis to the
occupationally exposed workers (Smagghe, 1974; Clyne, 1970). It was
suggested that from 1955-60, sixteen employees were exposed for five
to six months per year and from 1967 to 1970, nine employees were
exposed for approximately ten months per year with no ill effects. No
thyroid or liver tumors were observed "in excess of the general
population".
Oral administration of 100 mg amitrole to humans for treatment of
overactive thyroids inhibited the 131I-intake of the thyroid for 24
hours in normal persons and in hyperthyreotics. A dose of 10 mg had
only a very slight effect (Astwood, 1960).
A preliminary study of Swedish railway workers exposed to
amitrole showed two lung cancer cases. Although the number of subjects
was small, a combination of amitrole with smoking (or other
interacting substances) might have been responsible for the excess
lung cancer (Axelson et al., 1964).
COMMENTS
Amitrole is rapidly absorbed and eliminated primarily in urine.
It has an effect on a variety of biochemical systems including
catalase, peroxidase and certain enzymes associated with oxidative
metabolism. Amitrole is goitrogenic on continuous long-term exposure;
probably as a result of continuous inhibitions of peroxidase activity.
At high levels of exposure, antithyroid effects of amitrole have been
seen within three days. In adult female rats fed amitrole in the diet,
no effects on reproduction were noted below 500 ppm although goitre
was observed. At 500 ppm, reduced fecundity and a reduced lactation
index was observed but no malformation of pups was observed. Oral
administration to rats from days 7 to 15 of pregnancy resulted in no
effect on reproduction and no abnormal offspring. Amitrole, in the
presence of dimethyl sulfoxide, injected into chicken eggs produced
beak abnormalities and tibial malformations. Results of mutagenicity
tests using currently defined protocols were negative.
In two long-term studies, hepatomas have been produced in mice
and rats administered amitrole at exceptionally high levels. However,
a long-term feeding study at high dietary levels in rats did not
result in hepatomas. In a one-year dog study, no hepatic, goitrogenic
or other effects were noted at a dietary level of 12.5 mg/kg bw. The
no-effect level was based on a short-term study where normal PBI
values, a sensitive biochemical parameter of thyroid function, were
noted at 0.5 ppm. In addition, since no goitrogenic effect on
discontinuous exposure was observed, a conditional ADI was allocated.
The Meeting was reassured that in the use of amitrole, man has
only a remote, if any, chance of achieving the conditions where
continuous exposure is maintained. The Meeting emphasized that the ADI
was allocated with the condition that the uses of amitrole be
restricted to those where food residues would be unlikely to occur and
further to recommend that the use of materials in combination in the
same formulation be restricted, especially where effects on specific
target organs are expressed by both materials.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg bw.
Dog: 12.5 mg/kg bw.
ESTIMATE OF CONDITIONAL ACCEPTABLE DAILY INTAKE FOR MAN
0.00003 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Amitrole is a broad spectrum herbicide effective against a wide
range of grasses and broad-leafed weeds when applied as a foliar
spray. It was introduced as a herbicide in 1954. The major herbicidal
uses are on industrial land, roadsides, rights of way, railways,
forests, irrigation channels and other ditches, either used alone or
in admixture with other herbicides or as a combination with ammonium
thiocyanate.
There are numerous important uses on crop land and these are
summarized in Table 2. Amitrole is not selective and therefore all
applications in the vicinity of crop plants must be made in such a way
that growing parts of the plant are avoided.
Because of its quick action but relatively poor residual effect
amitrole is widely combined with triazine, substituted urea and uracil
herbicides to widen their spectrum of activity and to enhance the
knock down effect.
Amitrole is translocated within many plants and is thereby
effective against rhizomatous and stoloniferous grasses and bulbous
plants. Once translocated to the root systems of such plants amitrole
appears to remain effective until the next growing season or at least
its effect is observed in the regrowth, possibly owing to the
destruction of certain essential growth factors.
In order to evaluate properly the possibility of residues of
amitrole in raw agricultural commodities and foods it is important to
understand the mode of use. The following is an outline of the
application to crop land.
Apples and pears
In the spring, before fruit starts to form or after harvest,
amitrole is applied to the floor of the orchard to control broadleaf
weeds and grasses. The chemical treatment is a replacement for
mechanical cultivations designed to maintain the area at the base of
the trees free from all unwanted vegetation. Label directions state
that application should be made to weeds and that spray should be kept
off the trunk or foliage of trees. Actually, at the time applied,
there may be blossoms on the tree but very little foliage. Because of
this, very little transpiration is taking place and there is a minimum
of fluid transport within the tree. Thus, any amitrole that does come
into direct contact with the trunk or the roots would not be expected
to move around in the tree in detectable amounts. Extensive data were
available to the Meeting to judge the possible effect of accidental
misapplication or treatment in mid-summer when fruit is on the tree
and foliage is at a maximum.
Grapes
In many grape-growing areas where there is a winter rainfall,
winter annual weeds are a serious cultural problem. These weeds sprout
profusely with the first rains of the winter wet season and grow
strongly during winter and early spring while adequate moisture is
available. Several residual herbicides are effective for controlling
these weeds provided they are applied before the weeds emerge.
However, if application is made too late or if the amount of moisture
is inadequate these materials are often ineffective and must be
supplemented by a foliage-absorbed herbicide. During this period, the
vines are completely dormant with no leaves or berries. The spray is
directed to the weed foliage at the base of the grapevine with
instructions to keep all chemical off the grape plant itself. When
grapes are dormant, there is no transpiration of fluids within the
grapevine. Therefore, no moisture is going into the vine and thus
there is no vehicle for the amitrole. By the time the grape breaks
dormancy and transpiration and water uptake are significant, amitrole
apparently is degraded and not available for uptake.
The residual herbicides suffer the disadvantage of being
ineffective against some species. If these are left uncontrolled they
soon colonize the whole area and interfere with cultural practices.
Also there are numerous instances where the farmer has a perennial
weed problem in his cropped fields. This can either be an entire field
or a large patch within a field or merely scattered plants. The use of
amitrole alone or in combination with other herbicides as a spot
treatment is recognized as an important agricultural practice
essential to the maintenance of the productivity of perennial cultures
such as vineyards.
TABLE 3. Pattern of use of amitrol based herbicides
Rate
Use kg/ha When applied Remarks
Chemical fallow 1 Autumn/winter Apply after weeds
emerge.
Cropland 4-8 After harvest Do not plant crops
or cutting. or graze for 8
months.
Grapes 2 When vines are Directed spray.
dormant.
Orchards (apple 2 Before fruit Do not spray
and pear) forms/after foliage.
picking.
Corn 2 10-14 days Spray weeds 10-14
pre-planting. days before
ploughing.
Irrigation drains 2-8 When weeds Do not allow
and ditches 15 cm high. grazing.
Orchards/vineyards 2-8 When weeds are Spot treatment.
(perennial weeds) actively growing.
Non-crop areas 1-8 When weeds are Boom sprays-
actively growing. spot sprays.
Aerial Not to be made
application where spray or
drift might
contaminate crops
or potable water.
Maize
Selective herbicides have revolutionised the growing of maize and
many other crops but there still remains a serious problem of
perennial weeds such as thistles, Agropyron repens and
convolvulus. In the spring amitrole is applied to growing weeds.
After roughly 10-14 days, during which the chemical is allowed to
penetrate the weed foliage and translocate to the actively growing
meristematic tissue, the weedy field is ploughed in the normal
fashion. That is, the weed foliage is buried roughly 15.25 cm in the
ground and soil which was well under the surface is brought to the
top. Emphasis is placed on burying all treated weeds.
Chemical fallow
In areas where a fallow period is maintained because conservation
of all available moisture is essential to produce a satisfactory crop,
amitrole has been used alone or mixed with 2,4-D to control annual
weeds that sprout during the fallow period. If allowed to grow, these
weeds remove much of the moisture reserves in the soil.
Depending upon winter conditions, it may be desirable to spray in
the autumn or wait until weed growth starts in the spring. This
initial treatment is followed by one or more mechanical cultivations
to maintain the area fallow until the next crop is planted. It may be
necessary to repeat the herbicide application if fresh weeds emerge.
Practically speaking, there is no need to apply it less than 30 days
before planting cereal crops although the residue data show that
application could be as late as several days before planting without
producing detectable residues. There is no control whatsoever of weeds
that germinate after application.
Irrigation channels and ditch banks
Amitrole has been used for many years in some countries where
difficulty is found in maintaining irrigation channels and ditch banks
free from encroaching vegetation, particularly against those weeds
which grow prolifically in such situations and where a herbicide which
is rapidly translocated but rapidly disappears from soil or water is
required. In some countries there are restrictions on use near potable
water or catchments.
Pre-harvest treatments
There are no approved uses for direct application to crop plants.
Lack of selectivity precludes the possibility of such treatments.
Other uses
There are no known domestic or industrial uses which could
subject the general public to exposure to amitrole in any form.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable number of studies have been carried out to
determine whether, following the use of amitrole for the control of
weeds, residues could possibly occur in raw agricultural commodities
or food. Virtually without exception these studies indicate that the
parent compound is not found in any food commodity even when grossly
excessive rates are used or when the compound is applied at times and
under conditions not recommended as good agricultural practice.
Apples and pears
Otten (1970) reported before the Amitrole Advisory Committee of
the U.S. Environmental Protection Agency the results of many studies
on apple and pear trees. The approved treatment is 2-4 kg/ha under
trees in the spring before fruit forms or after harvest. In one series
of trials amitrole was applied for five successive years at the rate
of 4 kg/ha per year, or a total of 20 kg/ha during the 5 year period.
This is 10 times the normal use rate over the 5 year period. The
bottom 50 cm of the trunk was wet if necessary. No amitrole residues
were detected in the fruit.
Otten reported other tests in which amitrole was applied for 8
successive years using 4 or 20 kg/ha each year, a total of 160 kg of
amitrole per hectare during the period. Apple samples were taken from
trees with or without white shoots at the base (indicating amitrole in
the leaves) and analysed separately. No amitrole was detected in any
samples. The limit of determination of the method used is 0.01-0.02
mg/kg.
In both series of tests amitrole was applied during the summer
when the fruit was on the tree. Only when spray was applied directly
to the fruit was a residue detected.
Schubert (1965) carried out trials continuously over the period
1957-1964 in numerous orchards which received treatments of amitrole
over ground cover, fruit and leaf. Even in those trials where amitrole
was applied beneath trees right through the summer, mature fruit were
free from amitrole at harvest. In the early years apparent amitrole
residues were reported at levels in the range 0.05-0.09 ppm.
Subsequently Storherr and Burke (1961) developed improved methods to
overcome the extremely high absorbance backgrounds of most crops. Even
where chlorotic shoots were present on the trunk of trees, fruit at
harvest was found to have no residues (limit of determination 0.02
mg/kg). Substantial amitrole residues were found in mature apples
where foliage and/or fruit were directly treated with amitrole in
mid-summer. The residues from direct application to foliage were
similar to quantities found after dipping the fruit. When both leaves
and fruit were sprayed the residues were approximately doubled. The
residues resulting from these direct applications during the growing
season were in marked contrast to the results of a ground cover
application that avoided spray contact with foliage or fruit, where no
amitrole residues were found at harvest.
Maier-Bode and Bechtel (1968) reported trials with apple trees
carried out in Germany. When amitrole was applied at 2.5-4.2 times the
officially recognized dosage (6 kg/ha), no amitrole was detected in
the apples (residues of less than 0.01 ppm). Not until 50 or 100 kg/ha
of amitrole was used, i.e. 8.3-16.7 times the recognized dosage, were
small residues of amitrole, between 0.02 and 0.09 mg/kg found in the
apples. In practice such high doses are never used; the foliage of the
trees in these plots was distinctly chlorotic.
Maier-Bode and Bechtel (1968) also found that when grossly
excessive amounts of amitrole were applied to the soil, the amitrole
residues in apples were much higher when there was no weed cover
beneath the trees than when the herbicide was applied to densely
weed-covered plots. This was presumably because the lack of weed cover
to absorb and metabolize the amitrole allowed it to reach the root
zone of the trees.
Amchem (1965) reports show that apples, grown in orchards where
the floor of the orchard was cleared of poison ivy and general
broadleaf and grass weeds according to label instructions, show no
residues at harvest. These studies extended over 8 locations and
numerous varieties of apples. The methods of analysis used showed 96±
15% recovery at the 0.1 mg/kg level with a limit of determination of
0.01 mg/kg. Only when partially grown apples hanging on the trees were
sprayed to run-off with an amitrole spray mixture, could residues of
amitrole be found in the mature apples.
Moore (1968, 1969, 1970) reports three series of trials at
various locations in Australia in which amitrole was applied beneath
apple trees at varying rates between 2 and 4 kg/ha, at petal fall or
in mid-summer. To obtain the most severe conditions possible,
weed-free plots beneath trees were also sprayed in mid-summer. To
ensure the most complete uptake of herbicide by the tree an area of 5
metres × 5 metres was treated instead of the usual 3 metres × 3
metres. The author concludes that if any residues were present they
were below the analytical limit of determination of 0.01 ppm. There
was no build-up of residues in the second year of treatment.
Bayer (1973) reports studies carried out in Germany which showed
that approved uses of mixed herbicides containing amitrole applied for
the control of weeds in apple orchards did not produce detectable
residues of amitrole in mature apples (limit of determination
0.05 ppm).
The Netherlands authorities have submitted results of studies
carried out to determine amitrole residues in some fruits following
approved uses in that country. Pears and apples harvested in 1961 and
1962 following treatment of orchards for weed control 4-18 months
previously showed no indication of residues when analysed by the
method of Storherr and Burke (1961) having a limit of determination of
0.025 mg/kg (Wit and Van der Kamp, 1963).
Cherries
Maier-Bode and Bechtel (1968) report a number of experiments
which show that when amitrole is applied for weed control beneath
cherry trees 100 days before harvest no residues can be detected in
mature fruit by methods capable of determining 0.01 ppm.
Bayer reports experiments where amitrole was applied for weed
control in sour cherry orchards at the rate of 4 kg/ha. No residues
could be detected by methods sensitive to 0.05 ppm.
Citrus
An extensive study of the accumulation and depletion of amitrole
residues in citrus fruit and foliage was published by Day and
Hendrixson (1959) from California. Amitrole was usually applied to the
soil at rates of 2 and 4 kg/ha; in two instances a logarithmic series
of applications were made from 2-64 kg/ha. Treatments were replicated
4 times in each of 13 different orchards in various parts of the
citrus growing area. Treatments were applied during the winter months,
usually on dry soil surfaces, and orchards were irrigated during the
period of observation. The analytical method used was effective down
to 0.01 mg/kg. Several varieties of oranges and lemons were included
and samples were taken each month for the four months following
application. In spite of obvious symptoms of damage to the lower skirt
of the citrus trees most samples from the 2 and 4 kg/ha experiments
showed no detectable residues although a few were found to have
residues up to 0.05 mg/kg. In the logarithmic plots the level of
residues increased with increasing concentration of amitrole applied.
Those plots receiving 64 kg/ha showed 0.15 mg/kg in the whole oranges
4 months after application. Less was found after shorter intervals.
The authors concluded that citrus fruit from trees receiving
either 2 or 4 kg/ha contained residues only rarely. The residue level
found in citrus fruit does not appear to be affected by fruit variety,
soil type, climate, geographical area, cultural operations or rate of
application to the soil (except at grossly exaggerated rates).
Albinism appears only on the lower skirt area indicating foliar
contact rather than root uptake as the source of entry. Amitrole is
not re-distributed beyond the immediate area of contact when the
herbicide is applied at recommended rates. Field observations indicate
general systemic distribution at high rates of application.
Coffee beans
Information was available from only one trial carried out in
Hawaii.
Hylin (1962) reports results of analysis of coffee beans from
trees which had been treated twice within 6 weeks with amitrole,
applied at the rate of 2 and 4 kg/ha to substantially bare ground. A
total of 40 trees were involved in the trial. Great difficulty was
apparently encountered in developing a suitable analytical procedure
to deal with the coffee beans. Apparent amitrole residues were
reported to occur in the green beans at levels ranging from
0.02 - 0.48 mg/kg. In view of the analytical difficulties reported
and the experience of other workers, however, caution should be
exercised in interpreting the significance of these results.
Cranberries
Following the disclosure by Fleming (1959) that the practical use
of amitrole for the control of weeds in cranberry bogs led to
significant residues of amitrole in cranberries, the U.S. Food and
Drug Administration and the U.S. Department of Agriculture undertook
field and laboratory studies extending over 19 months (Onley et al.,
1963). Following the application of 4 and 8 kg of amitrole per ha of
cranberry bog the level of amitrole was determined in samples of
cranberry vines, soil, roots and fruits (when available). Results
indicated that though the residues in the soil declined steadily and
disappeared at the end of 12 months there was a distinct concentrating
effect in the roots and a pronounced effect in bushes where the
residues declined more slowly than in the soil. Cranberry fruit
harvested 3 and 12 months after application showed residues ranging up
to 0.4 mg/kg. The authors were unable to conclude whether the residue
represented the parent compound or metabolites.
Grapes
Studies using radio-labelled amitrole (Leonard and Weaver 1961)
showed that when amitrole was applied to the leaves, stem, clusters or
shoots of the grapevine a modest upward translocation occurred for
about 3 days. After this period the amitrole appears to be either
complexed or broken down. Amitrole was not recoverable, as such, from
the clusters except within 3 days after treatment.
Leonard and Lider (1961) studied the translocation of amitrole
and a number of other herbicides in the grapevine. Grape rootings were
allowed to absorb solutions of herbicide for 3 days before being
planted in pots. The distribution and fate of the herbicide was
studied by radio-autographs. There was very little indication of
translocation throughout the plant. Fruit from vines grown in
amitrole-treated soil under greenhouse conditions showed no positive
evidence of amitrole.
Trials with grapes in Germany involved the application of a
maximum of 4 kg/ha of amitrole weeds in vineyards, during both the
dormant and vegetative stages (Maier-Bode and Bechtel 1968). No trace
of amitrole was found in grapes when using a method capable of
detecting 0.01. Similar results are reported by Bayer (1973) from
trials involving the application of 4 kg of amitrole per ha of
vineyard.
Maize
Otten (1970) reports results obtained by both chemical analysis
and radio-labelling which show that no amitrole was detected in either
immature maize plants or at normal harvest time when amitrole was
applied to weeds 10 days before ploughing for the planting of maize
seed. When exaggerated rates were applied or maize was planted 1 or 2
days after spraying, amitrole was detected in the seedling plant but
had disappeared well before the crop would be used for silage or
mature grain.
In a statement before the Amitrole Committee, Amchem (1970)
summarized investigations carried out by a number of official research
workers investigating the possibility of amitrole residues finding
their way into corn plants and grain. Ercegovich (1957) analysed corn
from plots treated with amitrole at 0, 1, 2, 4 and 8 kg/ha and planted
1, 5, 9 and 13 days later. Harvests were made at 3, 4, 6, 10 and 16
weeks after planting. No residues of amitrole were found in any
samples.
Boyd applied radio-labelled amitrole directly to young corn
plants and followed the rapid decrease in residues over a 47-day
period. Initial residues of 4-10 mg/kg decreased to final levels of
0.1 - 0.2 mg/kg.
Nuts
Studies carried out in California involved the application of
amitrole to the orchard floor at rates of 1, 4 and 8 kg/ha.
Application was made during the winter months. Mature nuts collected
the following season were analysed for residues of amitrole. None of
the many samples examined was found to contain amitrole residues above
the limit of determination (0.02 mg/kg) (Hill 1962-63).
Peaches and plums
The Meeting had available numerous reports on residue trials
carried out in peach and plum orchards where amitrole was used for
weed control. None of the many samples analysed by methods capable of
determining as little as 0.02 mg/kg showed any indication of amitrole
residues (Hill 1962/63; Maier-Bode and Bechtel 1968; Bayer 1973).
Soybeans
The Meeting examined summaries of many separate trials carried
out in different areas of the USA and analysed by three separate
laboratories to determine the level and fate of amitrole in soybean
plants, pods and mature beans following different patterns of using
amitrole for weed control. In all cases samples were from treatments
at rates of 1´ - 2 times the maximum recommended rate. In no instances
were the residues, even in immature plants, found to be above the
limit of determination, 0.06 mg/kg (Amchem, 1969).
Montgomery and Freed (1963), wishing to know whether the proposed
Pre-plant use of amitrole could possible result in residues in
soybeans, studied various methods of extraction and analysis. They
reported that by each procedure samples from fields treated with up to
4 times the recommended rate of amitrole gave exactly the same results
as samples from untreated control fields. They applied a statistical
analysis to the extensive data and concluded that there was no reason
to suspect that any residues could occur following pre-plant
application. Further evidence of freedom from residues was provided by
failure to demonstrate any difference between the absorption spectra
of paired samples from control and treated plots when using a
double-beam spectrophotometer to compare them.
Sugar cane
Amitrole has been found to be useful for the control of certain
perennial grass and broad leaf weeds common in sugar cane fields and
on irrigation ditch banks. Application of directed sprays in sugar
cane produced a moderate amount of chlorosis which persisted for 3-4
weeks in the leaves without appearing to affect yields or subsequent
growth. Hilton et al. (1963) carried out a residue study designed to
determine the residual amitrole in sugar cane from several successive
applications made over the period of the crop cycle (2 years). Hilton
and Uyehara (1966) reporting on these studies noted that when sugar
cane was double treated with amitrole at 5, 10 or 20 kg/ha, 12 and 20
weeks after planting, the residues of amitrole diminished to less than
0.002 mg/kg by harvest time. When 10 times as much cane was taken for
analysis and treated in a manner to approximate the early raw sugar
processing, residues, if present, were less than 0.01 mg/kg even from
plots receiving the grossly exaggerated rate of 20 kg/ha.
Wheat and oats
Amitrole proved to be an effective means of controlling couch
grass (Agropyron repens) in cereal crops. Most proposed treatments
involved the application of amitrole to the grass 10-14 days before
ploughing in preparation for seeding with cereals. In Sweden the
procedure was to apply amitrole at the 3 leaf stage at a rate of 0.5
to 1 kg/ha. Svensson (1971) investigated the effect of such treatments
on oats and the fate of residues in the oat plants and grain. He
reports that when the treatment was carried out strictly in accordance
with approved directions no detectable residues could be found in the
grain. However when the rate was increased or application withheld for
2 weeks until the 5-leaf stage of the crop, the grain contained about
0.05 mg/kg. If the rate of application is increased to 2 kg/ha the
residue can exceed 0.1 ppm. Determinations were made on straw from a
number of trials but analytical difficulties rendered the results
somewhat unreliable. However it appears that application of amitrole
at any stage after the 3-leaf stage increases the likelihood that
residues will be found in the straw at harvest. Much higher residues
were found in the grain of oats treated with amitrole at the heading
and milk stages. 0.5 kg/ha applied at the milk stage gave rise to a
residue in the grain of 2.9 mg/kg.
Amchem (1970) provided a summary of a number of studies by
official workers in the USA who investigated the incidence, level and
fate of residues of amitrole in wheat grain and straw from crops grown
on soil treated according to label directions 14-28 days before
ploughing for planting. In some of these studies the application rate
was increased to 4 times the recommended rate and the time interval
was reduced to 1 day before planting. In no instance was any amitrole
residue found in cereal grains or straw at harvest time. Soil studies
on the same test plots showed detectable residues of amitrole on the
day of treatment but no detectable residues the day after treatment
even at the doubly exaggerated rate. The limits of detection were 0.02
ppm in grain and straw. Recoveries were 86-96% at levels of 0.2-0.4
ppm. On the basis of these data it was concluded that the use of up to
4 kg/ha of amitrole, one or more weeks before planting wheat or other
cereals, should cause no residues in the crop.
FATE OF RESIDUES
General
Kröller (1966) and Menzie (1969) have reviewed the metabolism of
amitrole in animals, plants, micro-organisms, etc. There is an
extensive literature on the mode of action of amitrole and on its
metabolism in plants. Many of these investigations were carried out
with the object of determining uptake, translocation, site of action,
reason for selectivity, and possible means of enhancing the activity.
Among the most useful publications are those of Boyd (1964/65), Carter
(1969), Herrett and Linck (1961a), Jukes (1961), Moser (1968), and
Rogers (1957a,b).
Many of these studies reveal that amitrole does not persist for
extended periods in plant tissues though there is considerable
disagreement as to whether it becomes conjugated, converted into a
more active material or destroyed and its fragments taken up into the
plant. There appears to be no clear-cut mode of action, but rather
multiple pathways by which it interferes with plant metabolism. Carter
(1969) reviewed the available literature on the effects of amitrole on
amino acid and protein metabolism, purine metabolism, flavin
synthesis, enzyme activity, chlorophyll synthesis and plastid
development. It is obvious that there is no single site of action but
the relative importance of the various effects has apparently not been
unravelled.
In animals
The fate of amitrole in rats is discussed in the section
"Biotransformation". There do not appear to be any grounds for
assuming that livestock grazing on plant materials growing on land
that had been treated with amitrole for the control of weeds would
absorb or retain significant amounts of amitrole or its metabolites.
In plants
The s-triazole nucleus is highly stable (Potts 1961); hence it
is not surprising that few workers have reported evidence of ring
cleavage under physiological conditions. Yost and Williams (1958)
reported disappearance of radio-labelled amitrole from corn plants in
approximately 6 weeks with a half-life of about 8 days. Disappearance
was also observed in soybeans but at a much slower rate.
Miller and Hall (1961) could not detect amitrole in cotton 4 days
after treatment. However, large quantities of metabolic products were
present. Fang et al. (1967) report the half-life of amitrole in
several plants as 18 to 28 hours. Little of the applied material was
recovered as CO2 from beet, corn or beans.
Freed et al. (1961) reported evolution of radio-active, CO2 from
treated oats and barley, indicating ring cleavage. Montgomery and
Freed (1963) observed some evolution of 14CO2 from treated soybeans,
with the remaining radioactivity apparently bound to components of
plant tissues. Resistant oats released CO2 more readily than
sensitive barley. Massini (1963) found no loss of CO2 from beans or
tomatoes. Muzik (1965) observed chlorosis in scions grafted onto
tomato plants 103 days after treatment with amitrole, indicating long
persistence of the toxic moiety.
Studies of amitrole degradation in plants have been complicated
by the fact that the material is available commercially only with the
5-carbon labelled. Apparently, the 5-carbon is quickly lost (as CO2
or formate) when ring rupture occurs and the remaining fragments are
thus unlabelled. However, if significant amounts of CO2 or
14C-formate were produced from 14C-amitrole in higher plants, one
would expect to find some incorporation of 14C into normal
metabolites. This is not the case. The vast majority of the literature
indicates that the extractable 14C from plants treated with
amitrole-5-14C remains in the intact ring as free amitrole or
conjugates. Considerable amounts of amitrole are attached to protein
(Brown and Carter 1968; Castelfranco and Brown 1963) or somehow bound
in an insoluble form (Racusen 1958). There is evidence of activation
to a free radical form which can react with amino acids (Carter and
Naylor 1960, 1961a, b; Herrett and Bagley 1964; Herrett and Linck
1961a; Miller and Hall 1961). Conclusive evidence of rapid and
extensive ring cleavage by higher plants has not been reported.
Most literature on metabolic alteration of amitrole in plants
deals with the formation and properties of conjugates between amitrole
and endogenous plant constituents. These "degradation" products
contain the intact triazole nucleus which often may be regenerated by
chemical treatment.
Rogers (1957a,b) reported a derivative of amitrole in several
plants which was "chromatographically identical" with an
amine-glucoside derivative of amitrole. The glucose derivative forms
quite readily in vitro (Fredrick and Gentile 1960) and its occurrence
in plant extracts is probably an artifact (Naylor 1964), since
numerous attempts by other workers to detect the compound in plant
extracts failed (Miller and Hall 1961; Massini 1963; Carter and Naylor
1959). However, Gentile and Fredrick (1959) and Frederick and Gentile
(1960, 1969, 1962 and 1965) have published a series of studies on the
properties and metabolism of the glucose derivative. These same
authors suggest that the triose derivative represents the true
structure of the amitrole derivatives reported by other workers.
Massini (1963) and Carter and Naylor (1960, 1961a) reported studies of
amitrole metabolism in which Massini identified one of the major
metabolites as 3-(3-amino-1,2,4-triazole-1-yl)-2-amino propionic acid
(3-ATAL), also referred to as
3-(3-amino-1,2,4-triazol-1-yl)-d-alanine. The formation of 3-ATAL
apparently represents a detoxication, since the derivative does not
appear to be nearly as toxic as amitrole, or as mobile. Furthermore
ammonium thiocyanate, which synergizes the action of amitrole,
inhibits the formation of 3-ATAL.
Smith and Chang (1973) studied the metabolism of amitrole in
Canada thistle (Cirsium arvense) and peas. They showed that excised
leaves of thistles metabolized amitrole into three major products one
of which was 3-ATAL. The other two were shown to be its metabolic
products, one being the precursor of the other. An enzyme preparation
from peas capable of synthesizing tryptophan was also able to
metabolize amitrole. Tryptophan synthesis with the enzyme preparation
was inhibited by amitrole and the authors deduced that amitrole
metabolism may follow a similar pathway to tryptophan synthesis.
A number of workers including Racusen (1958) reported another
metabolite which was stable to 6N HCI for 5 hours at 100°C.
Verification of the structure of this material and others awaited
further studies.
The most important outstanding question is whether the conjugates
and/or metabolites represent biologically active derivatives of
amitrole and if so whether these are available to react
physiologically and biochemically with animals receiving such
conjugates and metabolites in plant materials as part of their ration.
However, use patterns at present approved avoid the possibility that
significant residues are ingested by livestock or man.
In soil
Amitrole disappears rapidly from soils as shown by Sund (1956),
Bondarenko (1958), Ashton (1962), Riepma (1962), and Ercegovich and
Frear (1964). Disappearance has been attributed to absorption (Sund,
1956, Ercegovich and Frear, 1964) and microbial degradation, although
attempts to isolate organisms capable of degrading amitrole have not
been successful. Kretschmar (1970) showed that amitrole was not
degraded by Azotobacter.
Kaufmann (1965), Kaufman and co-workers (1968) and Plimmer et al.
(1967), have proposed that most of the amitrole degradation occurring
in soils proceeds by non-biological reactions. They showed that
approximately 69% of the radio-label from amitrole was released as
CO2 in 20 days by non-sterilized soil. Although autoclaved soil
released only 2% in a comparable period, soil treated with potassium
azide or ethylene oxide released 46 and 35% respectively, and
reinoculation of autoclaved soil did not restore the capacity to
metabolize amitrole. These authors propose that amitrole is degraded
in soil by an oxidative mechanism involving an attack on the triazole
nucleus. Microbial attack is not discounted however. Soil moisture,
temperature and pH markedly affected amitrole degradation (Riepma
1962), indicating possible microbial involvement. The studies by
Ercegovich and Frear (1964) show that amitrole degradation obeys
first-order kinetics, suggesting a chemical reaction. No-one has
reported an investigation of the possible involvement of
extra-cellular enzymes.
Whatever the mechanisms by which the triazole ring is opened,
there appears to be little doubt that ring opening does occur rapidly
in soils and the resulting products (urea, cyanamide and nitrogen)
should be readily metabolized by soil micro-organisms.
Norris (1970) showed that amitrole was rapidly lost from the
forest floor but that degradation was not completely biological, since
there was considerable loss in steam-sterilized material. He found
that ammonium thiocyanate applied with amitrole in the proportions
found in the commercial herbicide had no effect on the degradation of
amitrole.
Sund (1956) showed that amitrole becomes tightly adsorbed to soil
particles. He concluded that it takes part in the soil's base exchange
system, but also it has the tendency to complex metals. Studies of
amitrole toxicity to tomato seedlings were correlated with chemical
analysis and it was found that the biological response of plants is
proportional to the amount recoverable in any soil type.
MacRae and Alexander (1965) are in agreement with the results of
Ashton (1963) that microflora have an important function in the
degradation of amitrole. They confirmed the rapid biodegradation in
soil by plant bioassay.
Ercegovich and Frear obtained evidence of complex formation
between soil clay and amitrole by means of X-ray diffraction
measurements. Ashton (1963) noted that 13 compounds were formed in
addition to CO2 when amitrole was added to unsterile soil. One or
more metabolic compounds formed from amitrole are tenaciously bound to
the soil and appear to be resistant to degradation.
Day et al. (1960) studied the effect of soil type, temperature,
moisture and sterilization by steam on the fate of amitrole in 55
types of Californian citrus soil. Rates of decomposition in
steam-sterilized soils were much lower than in unsterilized soil,
apparently indicating that the decomposition is primarily due to the
action of soil microorganisms (but compare the results of Kaufman,
Plimmer and co-workers quoted above). Rates of decomposition of
amitrole were highly variable among the soils studied, apparently
because of differences in populations or levels of activity of the
soil micro-organisms concerned. A short residual life for amitrole was
more frequently found in the more highly evolved soils having finer
textures and more highly developed colloidal properties.
Freed and Furtick (1961) showed by simultaneous bioassay and
chemical analyses capable of detecting less than 0.05 mg/kg of
amitrole that it would be highly improbable that any residue of
amitrole would remain in the soil even a short while after
application.
It has generally been assumed that failure to recover amitrole
from soils following its use as a herbicide was due to elimination by
such processes as leaching, volatalization, or biological or chemical
destruction. Work by Groves and Chough (1971) demonstrated that new
solvent mixtures gave much better recoveries of amitrole from soil
than water extraction. Concentrated ammonium hydroxide/glycol mixture
(5/20) gave distinctly higher recoveries than water as indicated by
the following table.
TABLE 4. Extraction (%) of amitrole from soils with water and
ammonium hydroxide/glycol (A/G) mixture (5x20)
Time after Non-sterile soil Sterile soil
application (days) Water A/G Water A/G
% % % %
0 48.1
1 36.7 97.6 49.6 97.3
12 21.3 55.9 36.1 77.9
17 3.2 15.2 36.9 67.7
These results point to a need for caution in interpreting some of
the earlier results.
In water
Since amitrole is widely used for the control of ditch bank weeds
many studies have been carried out to determine the fate of any
amitrole reaching the water. Many of these studies (Segal 1960;
Marston et al. 1968; Frank 1969; Dunstar 1969; and Demint et al. 1970)
have largely involved the study of the rate of dissipation of amitrole
in irrigation water or flowing streams. Since only a small proportion
of the herbicide applied in such operations ever reaches the water,
dissipation and dilution is a recognized means of reducing any risk to
crops and municipal users. Where application has been made by aerial
spraying, measurable amounts were found in samples of water near the
downstream edge of the sprayed area for up to 5 days after spraying
(Marston et al. 1968).
Such studies are however of little value in determining whether
residues are lost by physical, chemical or biological means. Studies
were reported by Thoman (1963) from Florida where ponds with static
water levels were treated at the rate of 3 kg/ha of water surface,
giving a concentration of 0.45 mg/l in the water immediately after
treatment. Samples collected every 7 days thereafter were analysed.
The residue level declined slowly but 46 days elapsed before it
reached the limit of determination (0.02 mg/l). Samples taken
thereafter until the 74th day gave results comparable to pre-treatment
levels.
Nicholson (1963) reported experiments carried out in a fish
hatchery in South Carolina where ponds of 1 acre surface area were
treated with amitrole to yield a concentration of 1 mg/l in 80,000
cu.ft of pond water. Samples were taken each 7 days for 5 weeks and
thereafter at slightly greater intervals until the 201st day when the
pond became flooded. The concentration following treatment (1.16 mg/l)
gradually declined through 0.49 mg/l on the 68th day to 0.07 mg/l on
day 201. No information is given on the quality of the water in the
ponds but it is obvious that under these conditions amitrole remains
relatively stable for long periods.
In processing and cooking
No information was available on the effect of processing and
cooking on residues in plant materials. In view of the fact that none
of the recommended use patterns give rise to detectable residues in
raw agricultural commodities such studies are unlikely to have been
carried out.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Food moving in commerce
No data were available to the Meeting to indicate the level and
incidence of amitrole residues in food moving in commerce.
Food at the time of consumption
Results of several total diet studies in which an attempt was
made to find amitrole residues in many separate food groups were
available to the Meeting. Duggan et al. (1966) reported that amitrole
residues were not found in any food composites in the total diet study
carried out in the USA in 1964/65; the method used had a limit of
determination of 0.05 mg/kg. The same authors (1967), having repeated
the total diet study, reported that amitrole residues were not found
at or above the prescribed sensitivity limit. Corneliussen (1969,
1970), reporting the results of the 4th and 5th total diet studies
carried out in the USA, states "amitrole has never been found in any
total diet composites". The limit of determination was again
0.05 mg/kg.
METHODS OF RESIDUE ANALYSIS
One of the first methods for the determination of amitrole
residues was that of Sund (1956). This was a colorimetric method
applicable to soils, depending upon the formation of a green colour
with sodium nitroprusside reagent. It is sensitive to 1 mg/l of
amitrole in aqueous solutions and Sund reported that it conformed to
Beer's law. Bioassay with tomato seedlings correlated with chemical
analysis.
American Cyanamid (1958) published a colorimetric method for the
estimation of residues of amitrole in cranberry fruit. The extract is
purified by means of a cation exchange resin, treated with nitrous
acid and coupled with N-1-naphthyl-ethylenediamine dihydrochloride to
produce a pink-coloured solution. The intensity of the colour is
proportional to the amitrole concentration and is measured
spectrophotometrically at 512 nm. The limit of determination is
0.03 mg/kg.
Herrett and Linck (1961b) published what they describe as a
simple reproducible method for the separation and quantitative
determination of amitrole in biological systems. The method consists
of diazotization followed by coupling with
1-amino-8-naphthol-3,6-disulfonic acid (H-acid). The authors describe
the method as being sensitive in the range of 0.1-3.3 mg/kg.
Storherr and Burke (1961) in describing an improved method for
the determination of amitrole in crops refer to previous methods
published by the Food and Drug Administration which were applicable to
only a limited number of food crops. With their modifications the
method involves extraction of vegetable crops with ethanol, adsorption
on cation-exchange resin and desorption with aqueous ammonia. The
ammoniacal concentrate is subjected to a clean-up procedure using
acetonitrile and filter aid followed by acid digestion and clean-up
with activated carbon. The resulting solution is diazotized and
coupled with H-acid. The pink colour is measured at 455 nm. It is
necessary to carry out a blank determination. The apparent amitrole
found in control crops ranged from 0.003 to 0.02 mg/kg. The limit of
determination is considered to be 0.025 ppm using a 40 g sample.
Storherr and Onley (1962) later published a procedure for the
clean-up and separation of amitrole and its metabolites from vegetable
crops. This method utilizes a chromatrographic column packed with dry
cellulose for the removal of interfering substances from plant
extracts. It is reported to recover amitrole from some conjugates.
Hilton et al. (1963) modified the method of Storherr and Burke,
(1961) for use with sugar cane and sugar juice and were able to lower
the limit of determination to 0.002 mg/kg. Their modifications
involved an increase in the size of sample taken and a reduction in
the amount of activated carbon used in the acid digestion and cleanup
steps.
Segal (1960) and Hilton and Uyehara (1966) described a number of
minor modifications to the method of Storherr and Burke which lowered
the limit of determination of amitrole to 0.001 mg/kg in water and
sugar cane respectively.
Meissner (1971), on behalf of the Amitrole Advisory Committee to
the US Environmental Protection Agency, commenting on the claim that
the method of analysis developed by Storherr and Burke (1961)
determines not only amitrole but all known metabolites drew attention
to the lack of detailed information available on the metabolites of
amitrole in plants or on the influence of environmental and chemical
factors on plant metabolism. Groves and Chough (1971) report an
improved solvent mixture (concentrated ammonium hydroxide and ethylene
glycol) to extract amitrole from soil. Further details are given above
(see "Fate of residues in soil").
NATIONAL TOLERANCES
The information available to the Meeting indicated that most
countries with pesticide residue tolerance legislation either
registered amitrole on a "no-residue" basis or provided a zero
tolerance. It is assumed that zero would in effect mean "at or about
the limit of determination". In the Netherlands there is a general
tolerance of 0.02 mg/kg in fruit and vegetables. In Australia there is
a maximum residue limit for amitrole in water of 0.01 mg/l. This is at
or about the limit of determination. In the Federal Republic of
Germany, use of amitrole where potable water might be contaminated is
prohibited.
APPRAISAL
Amitrole is a non-selective, foliage-absorbed herbicide widely
used since 1954 for the control of unwanted vegetation on industrial
land, roads, railways, rights of way, ditch banks and similar
situations. It is also used for the destruction of weeds beneath trees
and vines in permanent horticultural crops, for spot treatment of
perennial weeds and for their destruction prior to the planting of
cereal crops. There are no known uses where application is made to
crop plants.
Extensive information indicates that amitrole is rapidly lost
from soil by a combination of chemical, biological and microbiological
attack. There is no indication that amitrole is taken up by the roots
of plants if currently approved practices are followed. Approved uses
involve the application of amitrole to weeds between the crop when
fruit trees, vines and similar horticultural crops are dormant or the
use of spot sprays and directed sprays which avoid contamination of
crop plants. In these cases there is no uptake of residues by crop
plants. Studies with radio-labelled amitrole confirm that it is not
taken up from soil following simulated normal practices.
Extensive residue trials have been carried out under many
conditions in many countries and all indicate that there is no residue
in fruit, vegetables or grain following the recommended use of
amitrole as a herbicide. Residues have been found experimentally only
when crop plants have been treated with excessive quantities of
amitrole applied directly over the crop when the fruiting parts are
already well formed.
There appears to be no single mode of action and the metabolic
pathways in plants appear most complex. There is evidence that when
amitrole is applied to the leaves of plants, most of the material
absorbed is metabolized. Some is complexed with various plant
materials, but the Meeting considered that the nature and biological
significance of conjugation products would only be of importance when
considering residues resulting from direct application over food-crop
plants. Where amitrole was administered to laboratory animals it was
shown that similar conjugates with sugars and proteins were formed in
the animal body.
There was some doubt whether the methods of analysis for residues
of the parent compound would adequately recover and determine all
conjugated materials or other biologically active metabolites. The
method of Storherr and Onley (1962) is the most sensitive and specific
method available, and is reported by several authors to recover
amitrole from some conjugates. Under the circumstances the Meeting
felt confident in recommending a maximum residue limit at or about the
limit of determination by the beet available method. The Meeting
however proposed certain precautions to avoid contamination of food
crops.
RECOMMENDATIONS
There is no reason to believe that significant residues occur in
any raw agricultural commodities when amitrole is used for the control
of weeds according to approved directions.
To reduce the possibility of contaminating food crops with
residues of amitrole, use-patterns should avoid the direct treatment
of food crops and should be limited to directed sprays, spot sprays
and, in the case of pre-planting and stubble treatments, application
to weeds at least 10 days before ploughing (see Table 2), the interval
to be conditioned by the temperature of the soil.
For regulatory purposes and as a means of determining whether
amitrole herbicides have been misused or incorrectly applied the
Meeting recommends a maximum residue limit at or about the limit of
determination by the best available analytical method.
CONDITIONAL TOLERANCE
mg/kg
Raw agricultural commodities of plant origin 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Long term feeding studies in a sufficient number of rats and mice
with low levels of amitrole of known composition and purity.
2. Studies to elucidate the possible relationship between the effects
of amitrole on the thyroid and on the liver.
3. Studies to show that the analytical methods determine not only the
parent compound but also biologically active metabolites.
4. Studies to develop a specific method sensitive to 0.005 mg/kg.
REFERENCES
Alexander, N.M. (1959a). Antithyroid action of 3-amino-1,2,4-triazole.
J. biol. Chem., 234:148-150.
Alexander, N.M. (1959b). Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by antithyroid compounds. J. biol. Chem.,
234:1530-1533.
Amchem (1965). Residues of amitrole in apples. Report Amchem Products
Inc. (Unpublished)
Amchem (1969). Analysis of soyabeans and soyabean plants for possible
residues of amitrole. Review of collaborative trials. Amchem Products
Inc. Report, Jan. 1969.
Amchem (1970a). Residues of aminotriazole in corn. Collection of
studies by various workers. Submission to Amitrole Committee, 16 Dec.
1970.
Amchem (1970b). Residues of amitrole in wheat and soil when used for
chemical fallow. Submission to Amitrole Committee, 16 Dec. 1970.
American Cyanamid (1958). Colorimetric method for the estimation of
residues of amitrole in cranberry fruit. American Cyanamid Co., 4 Feb.
1958.
Ashton, F.M. (1963). Fate of amitrole in soil. Weeds, 11:167-170.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. Am. med.
Ass., 172:1319-1320.
Auerbach, V.H., Piesinger, R.H. and Waisman, H.A. (1959). The effect
of 3-amino-1,2,4-triazole on the synthesis of tryptophan peroxidase.
Archs Biochem. Biophys., 82:370-379.
Axelson, O., Rehn, M. and Sundell, L. (1974). Herbicide exposure,
mortality and tumour incidence. An epidemiological investigation on
Swedish railroad workers. Envir. Hlth. (in press)
Bagdon, R., Vidone, L., Shaffer, C. and Golz, H. (1965).
Aminotriazole: acute and subacute toxicity. Report from American
Cyanamid Co. (Unpublished)
Baron, J. and Tephyly, T.R. (1969). Effect of 3-amino-1,2,4-triazole
on the stimulation of hepatic microsomal heme synthesis and induction
of hepatic microsomal oxidases produced by phenobarbital. Molec.
Pharmac., 5:10-20.
Bayer (1973). Amitrole residues in peaches, plums, grapes, cherries
and apples. Reports 434/72 - 443/72.
Bondarenko, D.D. (1958). Decomposition of amitrole in soil. The Ohio
Agricultural Experiment Station and The Ohio State University. Proc.
N. cent. Weed Control Conf.
Boyd, J.E. (1964, 1965). Some physiological and phytotoxicological
properties of amitrole. American Cyanamid Co.
Brown, J.C. and Carter, M.C. (1968). Influence of amitrole upon
protein metabolism in bean plants. Weed Sci., 16(2): 222-226.
Carter, M.C. (1969). Amitrole. In P.C. Kearney and D.D. Kaufman
(editors). Degradation of herbicides. Marcel Dekker, Inc., New York,
p. 187-206.
Carter, M.C. and Naylor, A.W. (1959). The formation, in vivo, of a
complex between 3-amino-1,2,4-triazole and a glycine-serine
derivative. Pl. Physiol., Lancaster, 34:(Suppl.) vi.
Carter, M.C. and Naylor, A.W. (1960, 1961). Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot. Gaz., 122:138-143.
Carter, M.C. and Naylor, A.W. (1961a). Studies on an unknown metabolic
product of 3-amino-1,2,4-triazole. Physiologia Pl., 14:20-27.
Carter, C. and Naylor, A.W. (1961b). The effect of
3-amino-1,2,4-triazole upon the metabolism of carbon labeled sodium
bicarbonate, glucose, succinate, glycine and serine by bean plants.
Physiologia Pl., 14:62-71.
Castelfranco, P. and Brown, M.S. (1963). A hypothesis of amitrole
action based on its behavior toward free radical generating systems.
Weeds, 11(2):116-124.
Clyne, R. (1970). Statement to Amitrole Advisory Committee.
Corneliussen, P.E. (1969). Residues in food and feed. Pesticide
residues in total diet samples (IV). Pestic. Monit. J., 2:140-152.
Corneliussen, P.E. (1970). Pesticide residues in total diet samples
(V). Pestic. Monit. J., 4(3):89-105.
Dardin, V. (1958). 3-amino-1,2,4-triazole - chronic oral
administration, beagle dogs, 52 weeks. Histology - supplement to
report. Report from Hazelton Laboratories, Inc. (Unpublished)
Dardin, V. (1959). 3-amino-1,2,4-triazole. Histology - supplement to
report of two year dietary feeding - rats. Report from Hazelton
Laboratories, Inc. (Unpublished)
Day, B.E. and Hendrixson, R.T. (1959). A study of the accumulation and
depletion of amitrole in citrus fruit and foliage. University of
California, Riverside, Research Report.
Day, B.E., Jordan, L.S., and Hendrixson, R.T. (1961). The
decomposition of amitrole in Californian soils. Weeds, 9:443-456.
Demint, R.J., Frank, P.A., Comes, R.D. (1970). Amitrole residues and
rate of dissipation in irrigation water. Weed Sci., 18(4):439-442.
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966). Pesticide residues
in total diet samples. Science, 151:101-104.
Duggan R.E., Barry, H.C. and Johnson, L.Y. (1967). Residues in food
and feed. Pesticide residues in total diet samples (II). Pestic.
Monit. J., 1(2):2.
Dunster, K.W. (1969). Amitrole persistence in irrigation canals.
Report of trials in Montana, Washington and Colorado. Amchem Report, 7
May 1969.
Elsea, J. (1954). Acute dermal application, acute eye application.
Report from Hazelton Laboratories, Inc. (Unpublished)
Ercegovich, C.D. (1957). Studies on the analysis and persistence of
3-amino-1,2,4-triazole in plant tissue and in soil. Ph.D. thesis,
Pennsylvania State University, University Park, Pa.
Ercegovich, C.D. and Frear, D.E.H. (1964). The fate of
3-amino-1,2,4-triazole in soils. J. agr. Food Chem., 12:26-29.
Fabrizio, D.P.A. (1973). Final report on cytogenicity study on
3-amino-1,2,4-triazole from Litton Bionetics, Inc. submitted to WHO by
Rohm and Haas Co. (Unpublished)
Fang, S.C., George, M. and Yu, T.C. (1964). Metabolism of 3-amino-1,2,
4-triazole-5-C14 by rats. J. agr. Food Chem., 12:219-223.
Fang, S.C., Khanna, S. and Rao, A.V. (1966). Further study on the
metabolism of labeled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. agr. Food Chem., 14:262-265.
Fang, S.C., Fallen, E. and Khanna, S. (1967). The metabolism of
labeled amitrole in plants. Weeds, 15:343-346.
Feytmans, E. and Leighton, F. (1973). Effects of pyrazole and
3-amino-1,2,4-triazole on methanol and ethanol metabolism by the rat.
Biochem. Pharmac., 22:349-360.
Fleming, A.S. (1959). Statement about amitrole residues in cranberries
- Press Release. Dept of Health, Education and Welfare, 9 Nov. 1959.
Fogleman, R. (1954). Amitrole - (3-amino-1,2,4-triazole) - Final
report: submitted by Hazelton Laboratories, Inc. (Unpublished)
Frank, P.A. (1969). Amitrole concentration in water of treated
irrigation channels. U.S. Dept of Agriculture A.R.S., Denver,
Colorado. Report, 14 Jan. 1969.
Fredrick, J.F. and Gentile, A.C. (1960). The formation of the glucose
derivative of 3-amino-1,2,4-triazole under physiological conditions.
Physiologia Pl., 13:761-765.
Fredrick, J.F. and Gentile, A.C. (1961). The structure of the
D-glucose adduct of 3-amino-1,2,4-triazole: Infrared absorption
studies. Arch. Biochem. Biophys., 92(2):356-359.
Fredrick, J.F. and Gentile, A.C. (1962). Studies of a phosphorylated
derivative of 3-amino-1,2,4-triazole formed by the action of yeast
hexokinase. Physiologia Pl., 15(1):186-193.
Fredrick, J.F. and Gentile, A.C. (1965). Physico-chemical studies of
the metabolic end product of 3-amino-1,2,4-triazole in yeast.
Phytochemistry, 4:851-856.
Freed, V.H., Furtick, W.R. (1961). The persistence of amitrole in
soil. Oregon Agricultural Experiment Station Technical Paper No. 1375.
Freed, V.H., Montgomery, M. and Kief, M. (1961). Proc. N East Weed
Control Conf., 1961:6-16.
Fregly, M.J. (1968). Effect of aminotriazole on thyroid function in
the rat. Toxic. appl. Pharmac., 13:271-286.
Gaines, T.B., Kimbrough, R.D. and Linder, R.E. (1973). Toxicity of
amitrole in the rat. Toxic. appl. Pharmac., 26:118-129.
Geldmacher-von Mallinckrodt, M. and Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Archs Toxik., 27:13-18. (English
summary)
Gentile, A.C. and Fredrick, J.F. (1959). Chemical activity of the
glucose adduct of 3-amino-1,2,4-triazole. Physiologia Pl., 12:862-867.
Grapenthien, J., Hathaway, D. and Keplinger, M. (1972). Two year
chronic aerosol inhalation toxicity study with amitrol 3AT in albino
rats. Report from Industrial Biotest Laboratories, Inc. (Unpublished)
Groves, K. and Chough, K.S. (1971). Extraction of 3-amino-1,2,
4-triazole (amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from
soils. J. agr. Food Chem., 19(5):840-841.
Hapke, H.J. (1967). The toxicity of aminotriazol for domestic animals.
Zbl. Vet. Med. A., 14:469-486. (English, French and Spanish summaries)
Heim, W.G., Appleman, D. and Pyfrom, H.T. (1956). Effects of
3-amino-1,2,4-triazole (AT) on catalase and other compounds. Am. J.
Physiol., 186:19-23.
Herrett, R.A. and Bagley, W.P. (1964). The metabolism and
translocation of 3-amino-1,2,4-triazole by Canada thistle. J. agr.
Food Chem., 12:17-20.
Herrett, R.A. and Linck, A.J. (1961a). Penetration of
3-amino-1,2,4-triazole in Canada thistle and field bindweed. Weeds,
9(2): 224-230.
Herrett, R.A. and Linck, A.J. (1961b). Quantitative determination of
3-amino-1,2,4-triazole. J. agr. Food Chem., 9(6):466-467.
Hill, R. (1962, 1963). Residues of amitrole in almonds, walnuts,
peaches and prunes. Diablo Laboratories, Berkley, Reports of Amchem
Products Inc.
Hilton, H.W., Uyehara, G. and Segal, H.S. (1963). Amitrole residues in
sugar cane. Rate of disappearance studies and improved methodology.
Hawaiian Sugar Planters Association Report.
Hilton, H.W. and Uyehara, G.K. (1966). Determination of
3-amino-1,2,4-triazole in sugarcane. J. agr. Food Chem., 14(1):90-94.
Hodge, H.C., Maynard, E.A., Downs, W.L., Ashton, J.K. and Salerno
L.L. (1966). Tests on mice for evaluating carcinogenicity. Toxic.
appl. Pharmac., 9:583-596.
Hoshino, M. (1960). Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature, 186:174-175.
Hylin, J.W. (1962). Determination of amitrole residues in coffee
beans. Report from University of Hawaii.
IARC (1974). Amitrole. In IARC Monographs on the Carcinogenic Risk
of Chemicals to Man. Vol. 7.
Innes, J. (1966). Amitrole - summary sheet - Carcinogenesis assay
studies - Subcutaneous. Data sheet. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969). Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Jukes, T.H. and Shaffer, C.B. (1960). Antithyroid effects of
amino-triazole. Science, 132:296-297.
Jukes, T.H. (1961). Antimetabolites and plant growth. Bull. Torrey
bot. Club, 88(5):321-327.
Kaufmann, D.D. (1966). Microbial degradation of herbicide
combinations: amitrole and dalapon. Weeds, 14:130-134.
Kaufmann, D.D., Plimmer, J.R., Kearney, P.C., Blake, J. and Guardia,
F.S. (1968). Chemical versus microbial decomposition of amitrole in
soil. Weed Sci., 16(2):266-272.
Keller, J. (1959). Aminotriazole (3-amino-1,2,4-triazole) two year
chronic feeding - rats. Report from Hazelton Laboratories, Inc.
(Unpublished).
Kretschmar, G. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) on the growth of Azotobacter. Z. allg. Mikrobiol.,
10:253-267.
Kretschmar, G. and Gunther, G. (1970). Der einflus von
3-amino-1,2,4-triazol (Amitrol) auf die Atmungvon Azobacter. A.
allg. Mikrobiol., 10:487-500.
Kröller, E. (1966). Anwendung und Eigenschaften des
3-amino-1,2,4-triazols im Hinblick auf seine Rückstände im
Lebensmitteln. Residue Reviews, 12:162-192.
Kudsk, F.N. (1969). Uptake of mercury vapor in blood in vivo and
in vitro from Hg-containing air. Acta pharmac. Tox., 27:149-160.
Landauer, W., Salam, N. and Sopher, D. (1971). The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Envir. Res.,
4:529-543.
Langhans, W. and Schimassek, H. (1974). Prevention of halothane
induced changes in rat liver enzymes by 3-amino-1,2,4-triazole.
Biochem. Pharmac., 23:403-409.
Leonard, O.A., and Lider, L.A. (1961). Toxicity and translocation of
herbicides supplied to grape rootings through solution culture. Am. J.
Enol. Vitic., 12(1):37-46.
Leonard, O.A., and Weaver, R.J. (1961). Absorption and translocation
of 2,4-D and amitrole in shoots of the Tokay grape. Hilgardia,
31(9):327-368.
Levine, W.G. (1973). Effect of phenobarbital and 3-amino-1,2,
4-triazole on the metabolism and biliary excretion of 3,4-benzypyrene
in the rat. Life Sci., 13:723-732.
Lotlikar, P., Wasserman, M. and Luha, L. (1973). Effect of
2-amino-1,2,4-triazole pretreatment on N and ring - hydroxylation of
2-acetylamino-fluorene by the rat. Proc. Soc. exp. Biol. Med.,
144:445-449.
Magos, L., Sugata, Y. and Clarkson, T.W. (1974). Effects of
3-amino-1,2,4-triazole on mercury uptake by in vitro human blood
samples and by whole rats. Toxic. appl. Pharmac., 28:367-373.
Maier-Bode, H. and Bechtel, W.D. (1968). Investigations of amitrole
residues in orchardry and viticulture. A. Pflanzenkrankheiten No. 4.
Proceedings of the 7th German Symposium on questions of weed biology
and control, 5 March 1968.
Margoliash, E. and Novogrodsky, A. A study of the inhibition of
catalase by 3-amino-1,2,4-triazole. Biochem. J., 68:468-475.
Marston, R.B., Schults, D.W., Shiroyama, T., and Snyder, L.V. (1968).
Pesticides in water. Amitrole concentrations in creek waters
downstream from an aerially sprayed watershed sub-basin. Pestic.
Monit. J., 2(3):123-128.
Massini, P. (1958). Uptake and translocation of 3-amino and
3-hydroxy-1,2,4-triazole in plants. Acta bot. neerl., 7:525-530.
Massini, P. (1959). Synthesis of (3-amino-1,2,4-triazolyl) alanine
(ATX) from 3-amino-1,2,4-triazole in plants. Biochem. Biophys. Acta,
36:548-549.
Massini, P. (1963). Aminotriazolylalanine; a metabolic product of
amitrole in plants. Acta bot. neerl., 12:64.
Matsushima, T. and Weisburger, J. (1972). Effect of carbon monoxide or
of 3-amino-triazole on C- and N- hydroxylation of the carcinogen
N-2-fluorenylacetamide by liver microsomes of hamsters pretreated with
3-methyl-cholanthrene. Xenobiotica, 2:423-430.
McRae, I.C. and Alexander, M. (1965). Microbial degradation of
selected herbicides in soil. J. agr. Food Chem., 13:72-76.
Meissner, W.A. (1971). Report of Amitrole Advisory Committee to
Environmental Protection Agency Washington, 12 March 1971.
Menzie, C.M. (1969). Metabolism of Pesticides. U.S. Dept. Interior,
Bureau of Sport Fisheries and Wildlife Special Scientific
Report - Wildlife No. 127:40-41.
Miller, C.S. and Hall, W.C. (1961). Absorption and metabolism of
aminotriazole in cotton. J. agr. Food Chem., 9:210-212.
Mohandas, T. and Grant, W.F. (1972). Cytogenetic effects of 2,4-D and
amitrole in relation to nuclear volume and DNA content in some higher
plants. Can. J. Genet. Cytol., 14:773-783.
Montgomery, M. and Freed, V.H. (1963). Residue studies of
amino-triazole in soyabeans. Report from Oregon State University, 25
April 1963.
Moore, B. (1968). Amitrole and simazine residue trials in Tasmanian
apples treated with D amatol 44. Geigy Australia Ltd., Report
68/8/290.
Moore, B. (1969). Amitrole and simazine residue trials at Orange, NSW,
1967-68. Geigy Australia Ltd., Technical Report 69/2/294.
Moore, B. (1970). Amitrole and simazine residue trials at Orange, NSW,
1968-69. Geigy Australia, Technical Report 70/1/309.
Moser, B. (1968). Summary statements on triazoles. Rutgers Univ. Plant
Physiology Seminar.
Muzik, T.J. (1965). Effect of temperature on the activity and
persistence of amitrole and 2,4-D. Weed Res., 5(3):207-212.
Napalkov, N. (1962). Tumor inducing action of 3-amino-1,2,4-triazole.
Truda prof. Zabol., 6:48-51.
Napalkov, N.P. (1969). On blastomogenic action of thyreostatic
substances. IECO USSR Acad. Med. Sci. Moscow, Monograph.
Naylor, A.W. (1964). Complexes of 3-amino-1,2,4-triazole in plant
metabolism. J. agr. Food Chem., 12:21-25.
Nicholson, H.P. (1963). Persistence of amitrole in pond water. U.S.
Public Health Service, Report 17 April 1963.
Norris, L.A. (1970). Degradation of herbicides in the forest floor. In
Tree Growth and Forest Soils, C.T. Youngberg and C.B. Daley (editors).
Onley, J.H., Storherr, R.W., Cluver, A.J. Jr. (1963). An Oregon
cranberry bog study for 3-amino-1,2,4-triazole residues, 1961-1962. J.
Ass. off. analyt. Chem., 46(6):996-1001.
Otten, R.S. (1970). Statement to Amitrole Advisory Committee, 16 Dec.
1970.
Plimmer, J.R., Kearney, P.C., Kaufman, D.D. and Guardia, F.S. (1967).
Amitrole decomposition by free radical-generating systems and by
soils. J. agr. Food Chem., 15: 996-999.
Potts, K.T. (1961). The chemistry of 1,2,4-triazoles. Chem. Rev.,
61:87-127.
Racusen, D. (1958). The metabolism and translocation of
3-amino-triazole. Archs Biochem. Biophys, 74(1):106-113.
Raisfeld, I., Bacchin, P., Hutterer, F. and Schaffner, F. (1970). The
effect of 3-amino-1,2,4-triazole on the phenobarbital induced
formation of hepatic micro-somal membranes. Molec. Pharmac., 6:231.
Rausina, B., Mastri, C., Keplinger, M. and Yost, D. (1972). Two year
chronic dermal toxicity study with amitrole 3-AT in albino rats.
Report from Industrial BioTest Laboratories, Inc. (Unpublished)
Riepma, P. (1962). Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in soil. Weed Res., 2:41-50.
Rogers, B.J. (1957a). Translocation and fate of amino triazole in
Plants. Weeds, 5:5-11.
Rogers, B.J. (1957b). The action of 3-amino-1,2,4-triazole in plants.
Hormolog 1:10.
Samio, H., Baird, M. and Massie, H. (1972). Renewal of catalase
activity in Drosophilia following treatment with 3-amino-1,2,
4-triazole. J. Insect. Physiol., 18:991-1000.
Sanderson, D. and Roe, F. (1962). Dietary toxicity of aminotriazole to
the female rat. Report from Fisons Ltd., Agrochemical Division and
Pathology Report from Chester Beatty Research Institute. (Unpublished)
Schroeder. I. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) in a non-herbicidal range on the protein biosynthesis of
the unicellular algae Poteriochromonas stipitata. A. Allg.
Mikrobiol., 10:295-298.
Schubert, O.E. (1965). Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Report from West Virginia
University.
Segal, H.S. (1960). Determination of amitrole in irrigation ditch
water. Amchem Laboratory Report 411, July 1960
Segal, H.S. (1963). Amitrole residues in Hawaiian irrigation ditch
water. Amchem Report, 27 Feb. 1963.
Shaffer, C., Vidone, L. and Golz, H. (1958). Aminotriazole: 32 day
repeated feeding to rats. Report from American Cyanamid Co.
(Unpublished)
Smagghe, G. (1974). Report from Chemical Products - Ugine - Kuhlmann.
(Unpublished)
Smith, L.W. and Chang, F.Y. (1973). Aminotriazole metabolism in
Cirsium arvense (L.) Scop. and Pisum sativum L. Weed Res.,
13:339-350.
Stenger, R. and Johnson, E. (1972). Modifying effect of
3-amino-1,2,4-triazole on phenobarbital induced changes in rat liver.
Expl. Molec. Pathol., 16:147-157.
Storherr, R.W. and Burke, J. (1961). Determination of 3-amino-1,2,
4-triazole in crops. J. Ass. off. analyt. Chem., 44(2):196-199.
Storherr, R.W. and Onley, J. (1962). Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J. Ass.
off. analyt. Chem., 45(2):382-387.
Strum, J. and Karnovsky, M. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity. Lab.
Invest., 24:1-12.
Sund, K.A. (1956). Residual activity of 3-amino-1,2,4-triazole in
soils. J. agr. Food Chem., 4(1):57-60.
Svensson, J.A. (1971). Residues of herbicides in cereals. Proceedings
of 12th Swedish Weed Conference, Jan. 1971.
Tephly, T.R., Mannering, G.J. and Parks, R.E., Jr. (1961). Studies on
the mechanism of inhibition of liver and erythrocyte catalase activity
by 3-amino-1,2,4-triazole (AT). J. Pharmac. exp. Ther., 134:77-82.
Tephly, T.R., Hasegawa, E. and Baron, J. (1971). Effect of drugs on
heme synthesis in the liver. Metabolism, 20:200.
Thoman, J.R. (1963). Persistence of amitrole in water (Borrow Pits)
Florida. U.S. Dept of Health, Education and Welfare, Report, 13 Nov.
1963.
Tschudy, D.P. and Collins, A. (1957). Effect of 3-amino-1,2,4-triazole
on delta-amino-levulinic acid dehydrase activity. Science, 126:168.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. and Hidosaka, Y.
(1973). Fine structure and localization of peroxidase activity in
aminotriazole goiter. Nagoya med. J., 18:183-190.
Weir, R., Jr. (1958). 3-amino-1,2,4-triazole chronic oral
administration - Beagle Dogs - 52 weeks - Final Report. Report from
Hazelton Laboratories, Inc. (Unpublished)
Weir, R. Jr. (1974). Personal communication to WHO.
Wit, S.L. and van der Kamp, C.G. (1963). Residues of amitrole in
apples. Report 26 February 1963, National Institute of Public Health,
Utrecht. Submitted by Netherlands Government to FAO (1974).
Yost, J.F. and Williams, E.F. (1958). Proc. N East Weed Control Conf.,
1958:9-15.
Other information on identity and properties
Amitrole is a white, crystalline solid with a molecular weight of
84 and a melting point of 150-153°C. It is soluble in water to the
extent of 28 g/100 g and in ethanol and methanol to an extent of
26 g/100 g. It is sparingly soluble in ethyl acetate and insoluble in
ether, acetone and most other organic solvents. Amitrole forms neutral
aqueous solutions but acts as a weak base. When exposed to UV light,
amitrole breaks down to form CO2, urea and cyanamide. Potts (1961)
has reviewed the chemistry of s-triazoles in considerable detail.
Amitrole behaves chemically as a typical aromatic amine.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Biotransformation
The metabolic fate of amitrole in animals has not yet been fully
elucidated. It is rapidly excreted from the body. Following
intraperitoneal administration, at least 90% of the injected dose was
observed in the urine unchanged within 24 hours (Bagdon et al., 1965).
When amitrole (5-14C) was fed to rats (Fang et al., 1964),
70-96% of the radioactivity was excreted in the urine during the first
24 hours as amitrole and two unidentified metabolites. There were
traces of 14C in the expired air and the faeces contained a small but
variable amount of activity. After absorption, amitrole was
distributed throughout most of the tissues. The maximum radioactivity
in all tissues was generally reached within one hour and started to
decrease 3-4 hours after dosing. Elimination of amitrole from all
tissues was rapid. The liver contained a metabolite but no free
amitrole. The rate of elimination of this metabolite from liver and
kidney was much slower than that of the parent compound.
Further studies on rats (Fang et al., 1966) have shown that
amitrole is not metabolically acetylated, and that the average
half-time for amitrole clearance in various tissues was 4.2 hours.
These studies were extended to include the metabolism of two
metabolites isolated from bean plants. One was readily excreated in
the urine, mainly unchanged but partly as two new metabolites. The
other bean metabolite was excreted much more slowly, apparently
unchanged.
Biotransformations in plants and soil are discussed in the
section "Fate of residues".
Effects on enzymes and other biochemical parameters
Acute administration of amitrole to rats results in depression of
catalase and peroxidase enzyme activity as well as the activity of
several other enzymes. Liver peroxidase was found to recover within 24
hours, while liver and kidney catalase depression was slower to
recover. Catalase returned to normal after seven days (Heim et al.,
1956). Insect-derived catalase was also shown to regenerate its
activity slowly following exposure to aminotriazole (Samio et al.,
1972).
Groups of rats (15 males per group) were administered radioactive
iodine alone or in combination with 0.15 or 0.78 mg amitrole
administered by intraperitoneal injection. Uptake of radioactivity by
the thyroid gland was significantly depressed at 0.78 mg/kg. The lower
dose reduced iodine uptake but the reduction was not statistically
significant. Amitrole significantly decreased fecal radioactivity,
while the urinary radioactivity was not significantly affected
(Fregly, 1968).
Amitrole inhibits peroxidase activity of rat thyroid and salivary
gland (Alexander, 1959a and b); liver and kidney catalase activity of
rats (Tephly et al., 1961; Heim et al., 1956); catalase activity of
human red blood cell (Margoliash and Novogrodsky, 1958; Magos et al.,
1974; Kudsk, 1969) catalase activity from other sources such as plant
tissue and commercial crystallized enzymes; the synthesis of
tryptophan peroxidase of rats (Auerbach et al., 1959);
delta-aminolevulinic acid dehydrase activity of mouse and rat liver
(Baron and Tephley, 1969; Tschudy and Collins, 1957); the inductive
effect of phenobarbital on hepatic microsomal cytochrome P-450 (Baron
and Tephly, 1969); the incorporation of iron (59FeCl3) into rat
hepatic microsomes (Tephly et al., 1971); microsomal heme synthesis
(Tephly et al., 1971); and hydroxylation of exogenous substrates by
the liver (Raisfeld et al., 1970). Amitrole caused no proliferation of
the microsomal endoplasmic reticulum (Raisfeld et al., 1970). Several
further studies have shown the effect of amitrole on certain aspects
of the drug metabolizing enzyme system in rat liver (Feytmans and
Leighton, 1973; Lotikar et al., 1973; Stenger and Johnson, 1972;
Levine, 1973; Matsushima and Weisburgh, 1972; Langhans and Shimassek,
1974). There is no clear picture of the effect of amitrole on
microsomal metabolizing enzyme systems in mammals although it does
serve to inhibit or reduce several specific biochemical reactions.
Kudsk (1969) observed that peroxide generating systems, such as
seen with methylene blue, accelerated the uptake of mercury in vitro
by human red blood cell preparations. Amitrole and methylene blue
which promoted the catalase-peroxide-complex I, had no effect on
mercury uptake. Magos (1974) suggested that the ability of red blood
cells to take up mercury from air saturated with mercury vapor was
reduced when cells were treated for three hours with amitrole and
methylene blue. In hemolysates mercury uptake was stimulated by
amitrole. In vivo administration of amitrole to rats resulted in
decreased lung concentration and increased liver concentrations of
mercury.
Tryptophan synthesis in plants was shown to be inhibited by
amitrole (Smith and Chang, 1973). Amitrole stimulated respiration in
Azotobacter and concurrently inhibited growth possibility through an
uncoupling of oxidative phosphorylation (Kretschmar and Günther,
1970). In algae, amino acid synthesis was inhibited and glucosamine
synthesis was stimulated (Schroeder, 1970).
In vitro, amitrole does not inhibit cholinesterase activity of
brain, submaxillary gland, serum, or ilium. Three hours after
intraperitoneal administration of 4 mg/kg to male rats cholinesterase
activity of brain, submaxillary gland, and serum was normal (Bagdon et
al., 1965).
TOXICOLOGICAL STUDIES
Special studies on carcinogenicity
Mouse
A testing procedure using groups of hybrid strains of mice (18
mice of each sex and each of two strains) evaluated amitrole for
carcinogenicity. The mice were administered amitrole orally from day 7
to day 28 of age at doses of 1000 mg/kg and thereafter in the diet at
a dose of 2192 ppm. None of the mice survived the 18 month test
interval. Hepatomas were evident in most animals, 67/72, and carcinoma
of the thyroid was reported in 67/71 of the animals examined (Innes et
al., 1969). A further study was designated as subcutaneous although
no details were given of the experimental design (Innes, 1966). The
majority of animals survived the 18 month test interval. There was no
evidence of heptoma or carcinoma of the thyroid.
Groups of mice (50 of each sex) were either treated with amitrole
by a single subcutaneous administration of 10 mg per mouse or by a
weekly dermal application of 0.1 mg/mouse. There was no abnormal
behaviour observed. When the animals were sacrificed and examined, no
signs of cancer were evident. No mention was made of examination of
the thyroid gland in this study (Hodge et al., 1966).
Rat
Amitrole was found to have an effect on the induction of liver
carcinogenesis by 4-dimethylaminoazobenzene. In an attempt to
determine the relationship between liver catalase activity and
carcinogenesis, Hoshino (1960) administered amitrole intraperitoneally
to albino rats at a dose of 1000 mg/kg every other day for 150 days
while feeding 4-dimethylaminoazobenzene at a dose that was known to
induce liver carcinoma. There was a reduction in the occurrence of
liver tumours in those animals administered amitrole (4/14) when
compared to the animals administered the carcinogen alone (12/16).
Napalkov (1962; 1969) administered amitrole to rats by
subcutaneous injection twice weekly (later reduced to a weekly
injection) at a dose of 125 mg/animal; in the water at a dose of 20-25
mg/day; in the diet at a dose of 250-500 mg/day; or by subcutaneous
implantation combined with subcutaneous administration twice weekly at
a dose of 125 mg/rat. Thyroid hyperplasia was observed after seven to
eight months. Liver tumours were observed initially in those animals
receiving amitrole in the diet and soon thereafter in those animals
receiving it by injection or via the drinking water. Sarcomas
developed where amitrole was implanted or injected. Concurrent daily
injections of 2.5 µg/100 g bw thyroxine and oral administration of 300
mg/day/rat amitrole in food for the length of the experiment resulted
in the development of only one thyroid adenoma but 9 liver tumors in
12 males. Amitrole alone induced thyroid tumors in 7/22 and liver
tumors in 12/23 male rats of the same age. No liver tumors but 2
thyroid cystic adenomas were observed in 51 control animals.
A comprehensive review of the carcinogenic risk of amitrole was
made by the International Agency for Research on Cancer commenting on
the animal and human data (IARC, 1974).
Special studies on mutagenicity
Mohandas and Grant (1972) observed that amitrole significantly
increased the frequency of chromosomal aberrations in root tips of
higher plants.
Mutagenic tests using Salmonella strains and yeast cells as
test indicators have been negative. A host mediated assay has been
reported to be negative although details of the test are not yet
available (Weir, 1974). A cytogenetic study, utilizing examination of
bone marrow cells arrested in C-metaphase from rats treated with
amitrole at levels of 0, 2.5, 25 and 250 mg/kg daily for 5 days, was
negative (Fabrizio, 1973).
Special studies on reproduction
Rat
Groups of rats (10 male and 10 female Sherman strain rats per
group) were fed amitrole in the diet at levels of 0, 25, 100, 500 and
1000 ppm for approximately two months and mated. In the first
generation at 500 and 1000 ppm the number of pups born and pup
survival was reduced. It was noted that almost all of the pups died
within one week after weaning and these dietary levels were
terminated. Dietary levels of 0, 25, and 100 ppm were fed through two
generations (two litters from the first generation and one litter from
the second generation). At 100 ppm in the diet there was no effect on
reproduction and all pups survived. At 100 ppm all animals were found
to have thyroid hyperplasia while the incidence of thyroid hyperplasia
was sporadic at 25 ppm (Gaines et al., 1973).
Oral administration of amitrole at doses of 400 and 1000 mg/kg to
rats on days 8 through 13 of gestation resulted in no signs of embryo
toxicity or of teratogenic effects. Groups of rats (5 males and 5
females per group) were administered amitrole orally at 100 mg/kg or
at 100 ppm in the drinking water for three months prior to mating and
in the females up to day 15 of gestation. There was no indication of
any effect on reproduction in either male or female rats (Hapke,
1967).
The effect of oral administration of amitrole on reproduction was
studied in three groups of eight pregnant Sherman strain rats
administered dosage levels of 0, 20 and 100 mg/kg bw/day from day 7
through 15 of gestation. There was no effect of amitrole on
reproduction and no abnormalities were observed in the offspring
through weaning (Gaines et al., 1973).
Special studies on teratogenicity
Amitrole injected into the yolk of chicken eggs at doses of 20-40
mg/egg, produced a dose dependent malformation of the beak and
abnormalities of the tibia shaft. This latter abnormality occurred
less frequently. At doses of 2 mg/egg, no effects ware noted. It was
observed that when amitrole was dissolved in DMSO it was more active
than when it was administered in water (Landauer et al., 1971).
Acute toxicity
TABLE 1. Acute toxicity of amitrole
LD50
Species Route (mg/kg bw) References
Rat oral >25 000 Bagdon et al., 1965
dermal > 2 500 Gaines et al., 1973
ip > 4 000 Bagdon et al., 1965
Mouse oral >14 700 Fogleman, 1954
iv > 1 600 Bagnon et al., 1965
Rabbit dermal >10 000 Elsea, 1954
oral > 2 150 Fogleman, 1954
Dog iv > 1 800 Fogleman, 1954
Cat iv > 1 750 Bagdon et al., 1965
Signs of poisoning include: depression, dyspnea, diarrhoea,
ataxia, altered respiration, coma and death. The G.I. tract was
severely irritated following acute doses. Acute doses administered to
dogs (50-1000 mg/kg) intravenously produced an immediate fall in blood
pressure followed by increased respiration. Following a dose of 1000
mg/kg, the pressor response to adrenalin was blocked suggestive of an
adrenalytic action with respect to blood pressure (Fogleman, 1954).
TABLE 2. Acute toxicity of glucose adduct
LD50
Species Sex Route (mg/kg bw) References
Rat M oral >10 000 Bagdon et al., 1965
Mouse M oral >10 000 Bagdon et al., 1965
M ip >10 000 Bagdon et al., 1965
M iv > 1 600 Bagdon et al,, 1965
Amitrole applied to unabraded skin as a paste at doses of
1-10 g/kg for 24 hours caused a mild dermal irritation at all dose
levels. The effect, a mild erythemia, was reduced within 24 hours. No
other effects were noted. Gross and microscopic examination of tissues
and organs was normal (Elsea, 1954).
Amitrole (3 mg) was applied to the conjuntival sac of rabbits.
Although mild irritation lasting 24-48 hours was observed, no
permanent damage was noted (Elsea, 1954).
Short term studies
Rat
Groups of rats (5 males and 15 females per group) were fed
amitrole in the diet at levels of 0, 0.01%, 0.1%, and 1.0% for 63
days. There was no mortality over the course of this study. Food
consumption and growth were depressed at 0.1% and above in both males
and females. Histological examination from selected tissues at the
conclusion of the study (thyroid was not examined) revealed
vacuolation of liver cells in those animals fed 0.1% and above. The
vacuoles were identified as fat globules indicative of fatty
metamorphosis associated with liver cell damage. No histological
effects were noted at 0.01% (Fogleman, 1954).
Groups of weanling rats were administered amitrole for up to 56
days by intraperitoneal injection three times/week for eight weeks at
1000 mg/kg. The administration of a recrystallized amitrole resulted
in no growth depression in two separate groups. The administration of
a "pure" material, not recrystallized, resulted in growth depression
(Heim et al., 1956).
Groups of rats (10 males and 10 females per group) were
administered amitrole at 1000 mg/kg by intraperitoneal administration
on alternate days for 42 days. Amitrole had to effect on bw gain or
food consumption but caused a 3-4 fold increase in thyroid weight
(Bagdon et al., 1965).
Groups of rats were administered amitrole daily, 5 days per week,
for four weeks at levels of 0, 100, 200 and 400 mg/kg. Growth rate was
reduced, relative thyroid weight increased, and iodine content of the
thyroid was reduced (Hapke, 1967).
Administration of dietary levels of 60 and 120 ppm for two weeks
resulted in an enlargement of the thyroid gland of rats and a
pronounced lowering of iodine uptake. Over this two week interval
there were no significant changes at levels of 15 and 30 ppm (Jukes
and Shaffer, 1960).
Administration of a high dose (0.04%) of amitrole in the drinking
water (approximately 60 mg/kg/day) has been shown to produce goitres
in rats within three days (Strum and Karnovsky, 1971; Tsuda et al.,
1973).
Groups of rats (10 male rats per group) were fed dietary
concentrations of amitrole for 32 days. One group was fed 500 ppm for
32 consecutive days, another group received 1000 ppm on alternate days
for the duration of the study and a group was fed the basal diet.
Behavior and mortality were not affected. Food intake and growth at
500 ppm were reduced. In the group receiving 1000 ppm on alternate
days food intake and growth data did not differ significantly from the
controls. At autopsy, gross examination of animals receiving 500 ppm
showed the thyroid gland to be hyperaemic and enlarged. The thyroid of
animals fed 1000 ppm appeared to be slightly hyperaemic but were
otherwise comparable to the controls. Mean thyroid weight/body weight
ratio for the 0, 1000 ppm, and 500 ppm groups was 58, 77, and 303
respectively (Shaffer et al., 1958).
A group of 30 male weanling rats was fed amitrole in the diet at
1000 ppm for two weeks. A comparable group received a similar dietary
level of propylthiouracil for two weeks. At the end of the feeding
interval several animals were sacrificed and the thyroid glands
weighed. The remaining animals were placed on control diets and
sacrificed at either the third or fourth week of feeding. Amitrole,
after two weeks produced a significant increase in the mean thyroid
weight (comparable to that found with propylthiouracil). Daring the
week following removal from the diet, the weight of the thyroid gland
diminished and by the end of the second week on a control diet
regression to normal size was almost complete (Bagdon et al., 1965).
Groups of rats (20 rats per group) were fed dietary l levels of 0
and 316 ppm for 100 days to examine the goitrogenic potential of
amitrole. Over the course of this experiment amitrole had a
significant effect on growth. Exophthalmia was not observed as in the
long term rat studies. Food intake was not drastically reduced. Gross
examination of the thyroid showed a laterally enlarged thyroid with
enlarged blood vessels. Microscopic examination showed hyperplasia of
the thyroid. There was no evidence of abnormalities observed in either
the liver or the kidney (Sanderson and Row, 1962).
Groups of rats (10 males per group) were administered amitrole in
the drinking water at concentrations of 0, 50, 250 and 1250 ppm for
106 days. The administration of amitrole resulted in a dose-dependent
depression of growth with a corresponding reduction of food and water
intake. Appearance, behaviour, and mortality were not affected by
amitrole. Gross and microscopic examination of tissues and organs
showed a marked increase in thyroid size at all dose levels. In rats
where reduced growth was noted, the kidneys, adrenals, liver and
spleen were proportionately smaller. Reproductive organs were not
affected. Microscopic examination showed general enlargement of the
thyroid at 50 ppm, with moderate stimulation of the thyroid epithelium
(no evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident (Bagdon et al., 1965).
Groups of rats (10 male rate per group) were fed dietary levels
of amitrole for 11-13 weeks at dosages of 0, 0.25, 0.50, 2, 10 and 50
ppm. At 0.5 ppm in the diet, no effect was observed in any of seven
separate measurements of thyroid function although iodine uptake was
slightly reduced and serum PBI concentrations were slightly increased.
Significant effects were noted at 2 ppm in the diet, especially with
regard to reduced PBI and reduced iodine uptake by thyroid. Gross and
microscopic examination of the thyroid confirmed the effects noted at
2 ppm (Fregly, 1968).
Dog
Groups of beagle dogs (3 males and 3 females were controls; 2
males and 4 females - 0.25 mg/kg; 3 males and 3 females - 1.25 mg/kg;
2 males and 4 females - 2.50 mg/kg; and 2 males and 2 females - 12.5
mg/kg) were administered amitrole orally by gelatin capsule six days a
week for 52 weeks. One male and one female per group were sacrificed
at 26 weeks. There was no mortality over the testing interval. Growth,
appearance, and behaviour were normal in all test animals. Results of
biochemical, haematological, and urological examinations were normal.
Gross and microscopic examination of tissues and organs showed no
evidence of abnormality associated with amitrole. There was no
apparent evidence of cytotoxic effects associated with the
administration of 12.5 mg amitrole kg bw/day for one year (Weir, 1958;
Dardin, 1958).
Long term studies
Dermal
Rat
Two groups of rats (25 males and 25 females per group) were
administered amitrole dermally at a level of 2.4 mg/kg weekly for 23
months. The application was allowed to contact the skin for thirty
minutes, after which the skin was rinsed and dried. Amitrole was found
to be non-irritating to the skin. There was no apparent effect of
amitrole on growth, behaviour, tumour formation or when tissues and
organs were observed on gross and microscopic examination. Statistical
studies with the liver and thyroid organ weight and organ-to-body
weight ratio did not reveal any differences in this experiment between
treated animals and controls (Rausina et al., 1972).
Inhalation
Rat
Groups of rats (25 males and 25 females per group) were exposed
to amitrole by inhalation for a one hour period every week for two
years. The animals were exposed to an aerosol of 0.2% (w/v) aqueous
solution by a head-only exposure to minimize oral ingestion. The
average analytical concentration of aerosol in the exposure chamber
was 2 mg/l air. There were no significant differences between the
treated and the control groups when examined with respect to
mortality, behaviour, growth, gross and microscopic examination of
thyroid and liver, or the incidence of tumor formation. No abnormal
effects were noted in this inhalation exposure (Grapenthien et al.,
1972).
Feeding
Rat
Groups of rats (35 males and 35 females per group) were fed
amitrole in the diet for two years at levels of 0, 10, 50, 100 and 500
ppm. Animals were sacrificed periodically over the course of the
experiment. The appearance of animals (especially females) fed 100 ppm
in the diet was altered. The incidence of protruding eyes
(exophthalmia) was considerably higher in the higher dose groups than
in the controls. Growth was reduced at 500 ppm. There was no apparent
effect of amitrole in the diet on survival, growth, or mortality in
the animals fed 50 ppm.
Gross examination of animals revealed a consistent finding of
thyroid enlargement. At 13 weeks, thyroid enlargement in males and
females was observed at 100 ppm. Microscopic examination suggested
hypofunction and a non-functional hyperplasia evident at 50 ppm and
above. The animals fed 500 ppm were removed from the diet at 19 weeks
and examined after two weeks on control diets. No evidence of
enlargement or hyperplasia was noted. At 26 weeks, thyroid enlargement
was evident at 50 ppm. Liver and kidney weight in both sexes was
decreased at 100 ppm. No effects were noted at 10 ppm. At 52 weeks,
growth of males was depressed at 100 ppm. At 68 weeks, growth of males
was again depressed at 100 ppm accompanied by enlargement of the
thyroid, pituitary, and liver. A cystic adenomatous structure was
observed at 100 ppm in the thyroid. At 50 ppm, thyroid hyperplasia and
hypofunction were noted. At 10 ppm, thyroid hypofunction was observed
in one male animal of the three males and three females examined. At
104 weeks, body weights were normal in all groups. At 100 ppm, liver
and kidney weights were normal while the thyroid was enlarged and
adenomas ranging from benign cysts to foetal malignant carcinoma were
observed. At 50 ppm, the thyroid was not enlarged. Adenomas were
observed in 3/16 rats. At 10 ppm, one rat showed an adenomatous nodule
with cellular hyperplasia (other sections suggested slight enlargement
while most control sections were normal). Thyroid enlargement was
observed only at 100 ppm. A no-effect level was not observed in this
study. Ten ppm was a minimal effect level (Keller, 1959; Dardin,
1959).
OBSERVATIONS IN MAN
A 39 year old woman ingested 20 mg/kg. This dose caused no signs
of intoxication and within a few hours of ingestion the compound
passed rapidly through the body and began to appear in concentrations
up to 100 mg/100 ml in the urine (Geldmacher-von Mallinckrodt, 1970).
Amitrole has been produced industrially since 1955 with no
evidence of ill effects other than mild contact dermatitis to the
occupationally exposed workers (Smagghe, 1974; Clyne, 1970). It was
suggested that from 1955-60, sixteen employees were exposed for five
to six months per year and from 1967 to 1970, nine employees were
exposed for approximately ten months per year with no ill effects. No
thyroid or liver tumors were observed "in excess of the general
population".
Oral administration of 100 mg amitrole to humans for treatment of
overactive thyroids inhibited the 131I-intake of the thyroid for 24
hours in normal persons and in hyperthyreotics. A dose of 10 mg had
only a very slight effect (Astwood, 1960).
A preliminary study of Swedish railway workers exposed to
amitrole showed two lung cancer cases. Although the number of subjects
was small, a combination of amitrole with smoking (or other
interacting substances) might have been responsible for the excess
lung cancer (Axelson et al., 1964).
COMMENTS
Amitrole is rapidly absorbed and eliminated primarily in urine.
It has an effect on a variety of biochemical systems including
catalase, peroxidase and certain enzymes associated with oxidative
metabolism. Amitrole is goitrogenic on continuous long-term exposure;
probably as a result of continuous inhibitions of peroxidase activity.
At high levels of exposure, antithyroid effects of amitrole have been
seen within three days. In adult female rats fed amitrole in the diet,
no effects on reproduction were noted below 500 ppm although goitre
was observed. At 500 ppm, reduced fecundity and a reduced lactation
index was observed but no malformation of pups was observed. Oral
administration to rats from days 7 to 15 of pregnancy resulted in no
effect on reproduction and no abnormal offspring. Amitrole, in the
presence of dimethyl sulfoxide, injected into chicken eggs produced
beak abnormalities and tibial malformations. Results of mutagenicity
tests using currently defined protocols were negative.
In two long-term studies, hepatomas have been produced in mice
and rats administered amitrole at exceptionally high levels. However,
a long-term feeding study at high dietary levels in rats did not
result in hepatomas. In a one-year dog study, no hepatic, goitrogenic
or other effects were noted at a dietary level of 12.5 mg/kg bw. The
no-effect level was based on a short-term study where normal PBI
values, a sensitive biochemical parameter of thyroid function, were
noted at 0.5 ppm. In addition, since no goitrogenic effect on
discontinuous exposure was observed, a conditional ADI was allocated.
The Meeting was reassured that in the use of amitrole, man has
only a remote, if any, chance of achieving the conditions where
continuous exposure is maintained. The Meeting emphasized that the ADI
was allocated with the condition that the uses of amitrole be
restricted to those where food residues would be unlikely to occur and
further to recommend that the use of materials in combination in the
same formulation be restricted, especially where effects on specific
target organs are expressed by both materials.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.5 ppm in the diet, equivalent to 0.025 mg/kg bw.
Dog: 12.5 mg/kg bw.
ESTIMATE OF CONDITIONAL ACCEPTABLE DAILY INTAKE FOR MAN
0.00003 mg/kg bw.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Amitrole is a broad spectrum herbicide effective against a wide
range of grasses and broad-leafed weeds when applied as a foliar
spray. It was introduced as a herbicide in 1954. The major herbicidal
uses are on industrial land, roadsides, rights of way, railways,
forests, irrigation channels and other ditches, either used alone or
in admixture with other herbicides or as a combination with ammonium
thiocyanate.
There are numerous important uses on crop land and these are
summarized in Table 2. Amitrole is not selective and therefore all
applications in the vicinity of crop plants must be made in such a way
that growing parts of the plant are avoided.
Because of its quick action but relatively poor residual effect
amitrole is widely combined with triazine, substituted urea and uracil
herbicides to widen their spectrum of activity and to enhance the
knock down effect.
Amitrole is translocated within many plants and is thereby
effective against rhizomatous and stoloniferous grasses and bulbous
plants. Once translocated to the root systems of such plants amitrole
appears to remain effective until the next growing season or at least
its effect is observed in the regrowth, possibly owing to the
destruction of certain essential growth factors.
In order to evaluate properly the possibility of residues of
amitrole in raw agricultural commodities and foods it is important to
understand the mode of use. The following is an outline of the
application to crop land.
Apples and pears
In the spring, before fruit starts to form or after harvest,
amitrole is applied to the floor of the orchard to control broadleaf
weeds and grasses. The chemical treatment is a replacement for
mechanical cultivations designed to maintain the area at the base of
the trees free from all unwanted vegetation. Label directions state
that application should be made to weeds and that spray should be kept
off the trunk or foliage of trees. Actually, at the time applied,
there may be blossoms on the tree but very little foliage. Because of
this, very little transpiration is taking place and there is a minimum
of fluid transport within the tree. Thus, any amitrole that does come
into direct contact with the trunk or the roots would not be expected
to move around in the tree in detectable amounts. Extensive data were
available to the Meeting to judge the possible effect of accidental
misapplication or treatment in mid-summer when fruit is on the tree
and foliage is at a maximum.
Grapes
In many grape-growing areas where there is a winter rainfall,
winter annual weeds are a serious cultural problem. These weeds sprout
profusely with the first rains of the winter wet season and grow
strongly during winter and early spring while adequate moisture is
available. Several residual herbicides are effective for controlling
these weeds provided they are applied before the weeds emerge.
However, if application is made too late or if the amount of moisture
is inadequate these materials are often ineffective and must be
supplemented by a foliage-absorbed herbicide. During this period, the
vines are completely dormant with no leaves or berries. The spray is
directed to the weed foliage at the base of the grapevine with
instructions to keep all chemical off the grape plant itself. When
grapes are dormant, there is no transpiration of fluids within the
grapevine. Therefore, no moisture is going into the vine and thus
there is no vehicle for the amitrole. By the time the grape breaks
dormancy and transpiration and water uptake are significant, amitrole
apparently is degraded and not available for uptake.
The residual herbicides suffer the disadvantage of being
ineffective against some species. If these are left uncontrolled they
soon colonize the whole area and interfere with cultural practices.
Also there are numerous instances where the farmer has a perennial
weed problem in his cropped fields. This can either be an entire field
or a large patch within a field or merely scattered plants. The use of
amitrole alone or in combination with other herbicides as a spot
treatment is recognized as an important agricultural practice
essential to the maintenance of the productivity of perennial cultures
such as vineyards.
TABLE 3. Pattern of use of amitrol based herbicides
Rate
Use kg/ha When applied Remarks
Chemical fallow 1 Autumn/winter Apply after weeds
emerge.
Cropland 4-8 After harvest Do not plant crops
or cutting. or graze for 8
months.
Grapes 2 When vines are Directed spray.
dormant.
Orchards (apple 2 Before fruit Do not spray
and pear) forms/after foliage.
picking.
Corn 2 10-14 days Spray weeds 10-14
pre-planting. days before
ploughing.
Irrigation drains 2-8 When weeds Do not allow
and ditches 15 cm high. grazing.
Orchards/vineyards 2-8 When weeds are Spot treatment.
(perennial weeds) actively growing.
Non-crop areas 1-8 When weeds are Boom sprays-
actively growing. spot sprays.
Aerial Not to be made
application where spray or
drift might
contaminate crops
or potable water.
Maize
Selective herbicides have revolutionised the growing of maize and
many other crops but there still remains a serious problem of
perennial weeds such as thistles, Agropyron repens and
convolvulus. In the spring amitrole is applied to growing weeds.
After roughly 10-14 days, during which the chemical is allowed to
penetrate the weed foliage and translocate to the actively growing
meristematic tissue, the weedy field is ploughed in the normal
fashion. That is, the weed foliage is buried roughly 15.25 cm in the
ground and soil which was well under the surface is brought to the
top. Emphasis is placed on burying all treated weeds.
Chemical fallow
In areas where a fallow period is maintained because conservation
of all available moisture is essential to produce a satisfactory crop,
amitrole has been used alone or mixed with 2,4-D to control annual
weeds that sprout during the fallow period. If allowed to grow, these
weeds remove much of the moisture reserves in the soil.
Depending upon winter conditions, it may be desirable to spray in
the autumn or wait until weed growth starts in the spring. This
initial treatment is followed by one or more mechanical cultivations
to maintain the area fallow until the next crop is planted. It may be
necessary to repeat the herbicide application if fresh weeds emerge.
Practically speaking, there is no need to apply it less than 30 days
before planting cereal crops although the residue data show that
application could be as late as several days before planting without
producing detectable residues. There is no control whatsoever of weeds
that germinate after application.
Irrigation channels and ditch banks
Amitrole has been used for many years in some countries where
difficulty is found in maintaining irrigation channels and ditch banks
free from encroaching vegetation, particularly against those weeds
which grow prolifically in such situations and where a herbicide which
is rapidly translocated but rapidly disappears from soil or water is
required. In some countries there are restrictions on use near potable
water or catchments.
Pre-harvest treatments
There are no approved uses for direct application to crop plants.
Lack of selectivity precludes the possibility of such treatments.
Other uses
There are no known domestic or industrial uses which could
subject the general public to exposure to amitrole in any form.
RESIDUES RESULTING FROM SUPERVISED TRIALS
A considerable number of studies have been carried out to
determine whether, following the use of amitrole for the control of
weeds, residues could possibly occur in raw agricultural commodities
or food. Virtually without exception these studies indicate that the
parent compound is not found in any food commodity even when grossly
excessive rates are used or when the compound is applied at times and
under conditions not recommended as good agricultural practice.
Apples and pears
Otten (1970) reported before the Amitrole Advisory Committee of
the U.S. Environmental Protection Agency the results of many studies
on apple and pear trees. The approved treatment is 2-4 kg/ha under
trees in the spring before fruit forms or after harvest. In one series
of trials amitrole was applied for five successive years at the rate
of 4 kg/ha per year, or a total of 20 kg/ha during the 5 year period.
This is 10 times the normal use rate over the 5 year period. The
bottom 50 cm of the trunk was wet if necessary. No amitrole residues
were detected in the fruit.
Otten reported other tests in which amitrole was applied for 8
successive years using 4 or 20 kg/ha each year, a total of 160 kg of
amitrole per hectare during the period. Apple samples were taken from
trees with or without white shoots at the base (indicating amitrole in
the leaves) and analysed separately. No amitrole was detected in any
samples. The limit of determination of the method used is 0.01-0.02
mg/kg.
In both series of tests amitrole was applied during the summer
when the fruit was on the tree. Only when spray was applied directly
to the fruit was a residue detected.
Schubert (1965) carried out trials continuously over the period
1957-1964 in numerous orchards which received treatments of amitrole
over ground cover, fruit and leaf. Even in those trials where amitrole
was applied beneath trees right through the summer, mature fruit were
free from amitrole at harvest. In the early years apparent amitrole
residues were reported at levels in the range 0.05-0.09 ppm.
Subsequently Storherr and Burke (1961) developed improved methods to
overcome the extremely high absorbance backgrounds of most crops. Even
where chlorotic shoots were present on the trunk of trees, fruit at
harvest was found to have no residues (limit of determination 0.02
mg/kg). Substantial amitrole residues were found in mature apples
where foliage and/or fruit were directly treated with amitrole in
mid-summer. The residues from direct application to foliage were
similar to quantities found after dipping the fruit. When both leaves
and fruit were sprayed the residues were approximately doubled. The
residues resulting from these direct applications during the growing
season were in marked contrast to the results of a ground cover
application that avoided spray contact with foliage or fruit, where no
amitrole residues were found at harvest.
Maier-Bode and Bechtel (1968) reported trials with apple trees
carried out in Germany. When amitrole was applied at 2.5-4.2 times the
officially recognized dosage (6 kg/ha), no amitrole was detected in
the apples (residues of less than 0.01 ppm). Not until 50 or 100 kg/ha
of amitrole was used, i.e. 8.3-16.7 times the recognized dosage, were
small residues of amitrole, between 0.02 and 0.09 mg/kg found in the
apples. In practice such high doses are never used; the foliage of the
trees in these plots was distinctly chlorotic.
Maier-Bode and Bechtel (1968) also found that when grossly
excessive amounts of amitrole were applied to the soil, the amitrole
residues in apples were much higher when there was no weed cover
beneath the trees than when the herbicide was applied to densely
weed-covered plots. This was presumably because the lack of weed cover
to absorb and metabolize the amitrole allowed it to reach the root
zone of the trees.
Amchem (1965) reports show that apples, grown in orchards where
the floor of the orchard was cleared of poison ivy and general
broadleaf and grass weeds according to label instructions, show no
residues at harvest. These studies extended over 8 locations and
numerous varieties of apples. The methods of analysis used showed 96±
15% recovery at the 0.1 mg/kg level with a limit of determination of
0.01 mg/kg. Only when partially grown apples hanging on the trees were
sprayed to run-off with an amitrole spray mixture, could residues of
amitrole be found in the mature apples.
Moore (1968, 1969, 1970) reports three series of trials at
various locations in Australia in which amitrole was applied beneath
apple trees at varying rates between 2 and 4 kg/ha, at petal fall or
in mid-summer. To obtain the most severe conditions possible,
weed-free plots beneath trees were also sprayed in mid-summer. To
ensure the most complete uptake of herbicide by the tree an area of 5
metres × 5 metres was treated instead of the usual 3 metres × 3
metres. The author concludes that if any residues were present they
were below the analytical limit of determination of 0.01 ppm. There
was no build-up of residues in the second year of treatment.
Bayer (1973) reports studies carried out in Germany which showed
that approved uses of mixed herbicides containing amitrole applied for
the control of weeds in apple orchards did not produce detectable
residues of amitrole in mature apples (limit of determination
0.05 ppm).
The Netherlands authorities have submitted results of studies
carried out to determine amitrole residues in some fruits following
approved uses in that country. Pears and apples harvested in 1961 and
1962 following treatment of orchards for weed control 4-18 months
previously showed no indication of residues when analysed by the
method of Storherr and Burke (1961) having a limit of determination of
0.025 mg/kg (Wit and Van der Kamp, 1963).
Cherries
Maier-Bode and Bechtel (1968) report a number of experiments
which show that when amitrole is applied for weed control beneath
cherry trees 100 days before harvest no residues can be detected in
mature fruit by methods capable of determining 0.01 ppm.
Bayer reports experiments where amitrole was applied for weed
control in sour cherry orchards at the rate of 4 kg/ha. No residues
could be detected by methods sensitive to 0.05 ppm.
Citrus
An extensive study of the accumulation and depletion of amitrole
residues in citrus fruit and foliage was published by Day and
Hendrixson (1959) from California. Amitrole was usually applied to the
soil at rates of 2 and 4 kg/ha; in two instances a logarithmic series
of applications were made from 2-64 kg/ha. Treatments were replicated
4 times in each of 13 different orchards in various parts of the
citrus growing area. Treatments were applied during the winter months,
usually on dry soil surfaces, and orchards were irrigated during the
period of observation. The analytical method used was effective down
to 0.01 mg/kg. Several varieties of oranges and lemons were included
and samples were taken each month for the four months following
application. In spite of obvious symptoms of damage to the lower skirt
of the citrus trees most samples from the 2 and 4 kg/ha experiments
showed no detectable residues although a few were found to have
residues up to 0.05 mg/kg. In the logarithmic plots the level of
residues increased with increasing concentration of amitrole applied.
Those plots receiving 64 kg/ha showed 0.15 mg/kg in the whole oranges
4 months after application. Less was found after shorter intervals.
The authors concluded that citrus fruit from trees receiving
either 2 or 4 kg/ha contained residues only rarely. The residue level
found in citrus fruit does not appear to be affected by fruit variety,
soil type, climate, geographical area, cultural operations or rate of
application to the soil (except at grossly exaggerated rates).
Albinism appears only on the lower skirt area indicating foliar
contact rather than root uptake as the source of entry. Amitrole is
not re-distributed beyond the immediate area of contact when the
herbicide is applied at recommended rates. Field observations indicate
general systemic distribution at high rates of application.
Coffee beans
Information was available from only one trial carried out in
Hawaii.
Hylin (1962) reports results of analysis of coffee beans from
trees which had been treated twice within 6 weeks with amitrole,
applied at the rate of 2 and 4 kg/ha to substantially bare ground. A
total of 40 trees were involved in the trial. Great difficulty was
apparently encountered in developing a suitable analytical procedure
to deal with the coffee beans. Apparent amitrole residues were
reported to occur in the green beans at levels ranging from
0.02 - 0.48 mg/kg. In view of the analytical difficulties reported
and the experience of other workers, however, caution should be
exercised in interpreting the significance of these results.
Cranberries
Following the disclosure by Fleming (1959) that the practical use
of amitrole for the control of weeds in cranberry bogs led to
significant residues of amitrole in cranberries, the U.S. Food and
Drug Administration and the U.S. Department of Agriculture undertook
field and laboratory studies extending over 19 months (Onley et al.,
1963). Following the application of 4 and 8 kg of amitrole per ha of
cranberry bog the level of amitrole was determined in samples of
cranberry vines, soil, roots and fruits (when available). Results
indicated that though the residues in the soil declined steadily and
disappeared at the end of 12 months there was a distinct concentrating
effect in the roots and a pronounced effect in bushes where the
residues declined more slowly than in the soil. Cranberry fruit
harvested 3 and 12 months after application showed residues ranging up
to 0.4 mg/kg. The authors were unable to conclude whether the residue
represented the parent compound or metabolites.
Grapes
Studies using radio-labelled amitrole (Leonard and Weaver 1961)
showed that when amitrole was applied to the leaves, stem, clusters or
shoots of the grapevine a modest upward translocation occurred for
about 3 days. After this period the amitrole appears to be either
complexed or broken down. Amitrole was not recoverable, as such, from
the clusters except within 3 days after treatment.
Leonard and Lider (1961) studied the translocation of amitrole
and a number of other herbicides in the grapevine. Grape rootings were
allowed to absorb solutions of herbicide for 3 days before being
planted in pots. The distribution and fate of the herbicide was
studied by radio-autographs. There was very little indication of
translocation throughout the plant. Fruit from vines grown in
amitrole-treated soil under greenhouse conditions showed no positive
evidence of amitrole.
Trials with grapes in Germany involved the application of a
maximum of 4 kg/ha of amitrole weeds in vineyards, during both the
dormant and vegetative stages (Maier-Bode and Bechtel 1968). No trace
of amitrole was found in grapes when using a method capable of
detecting 0.01. Similar results are reported by Bayer (1973) from
trials involving the application of 4 kg of amitrole per ha of
vineyard.
Maize
Otten (1970) reports results obtained by both chemical analysis
and radio-labelling which show that no amitrole was detected in either
immature maize plants or at normal harvest time when amitrole was
applied to weeds 10 days before ploughing for the planting of maize
seed. When exaggerated rates were applied or maize was planted 1 or 2
days after spraying, amitrole was detected in the seedling plant but
had disappeared well before the crop would be used for silage or
mature grain.
In a statement before the Amitrole Committee, Amchem (1970)
summarized investigations carried out by a number of official research
workers investigating the possibility of amitrole residues finding
their way into corn plants and grain. Ercegovich (1957) analysed corn
from plots treated with amitrole at 0, 1, 2, 4 and 8 kg/ha and planted
1, 5, 9 and 13 days later. Harvests were made at 3, 4, 6, 10 and 16
weeks after planting. No residues of amitrole were found in any
samples.
Boyd applied radio-labelled amitrole directly to young corn
plants and followed the rapid decrease in residues over a 47-day
period. Initial residues of 4-10 mg/kg decreased to final levels of
0.1 - 0.2 mg/kg.
Nuts
Studies carried out in California involved the application of
amitrole to the orchard floor at rates of 1, 4 and 8 kg/ha.
Application was made during the winter months. Mature nuts collected
the following season were analysed for residues of amitrole. None of
the many samples examined was found to contain amitrole residues above
the limit of determination (0.02 mg/kg) (Hill 1962-63).
Peaches and plums
The Meeting had available numerous reports on residue trials
carried out in peach and plum orchards where amitrole was used for
weed control. None of the many samples analysed by methods capable of
determining as little as 0.02 mg/kg showed any indication of amitrole
residues (Hill 1962/63; Maier-Bode and Bechtel 1968; Bayer 1973).
Soybeans
The Meeting examined summaries of many separate trials carried
out in different areas of the USA and analysed by three separate
laboratories to determine the level and fate of amitrole in soybean
plants, pods and mature beans following different patterns of using
amitrole for weed control. In all cases samples were from treatments
at rates of 1´ - 2 times the maximum recommended rate. In no instances
were the residues, even in immature plants, found to be above the
limit of determination, 0.06 mg/kg (Amchem, 1969).
Montgomery and Freed (1963), wishing to know whether the proposed
Pre-plant use of amitrole could possible result in residues in
soybeans, studied various methods of extraction and analysis. They
reported that by each procedure samples from fields treated with up to
4 times the recommended rate of amitrole gave exactly the same results
as samples from untreated control fields. They applied a statistical
analysis to the extensive data and concluded that there was no reason
to suspect that any residues could occur following pre-plant
application. Further evidence of freedom from residues was provided by
failure to demonstrate any difference between the absorption spectra
of paired samples from control and treated plots when using a
double-beam spectrophotometer to compare them.
Sugar cane
Amitrole has been found to be useful for the control of certain
perennial grass and broad leaf weeds common in sugar cane fields and
on irrigation ditch banks. Application of directed sprays in sugar
cane produced a moderate amount of chlorosis which persisted for 3-4
weeks in the leaves without appearing to affect yields or subsequent
growth. Hilton et al. (1963) carried out a residue study designed to
determine the residual amitrole in sugar cane from several successive
applications made over the period of the crop cycle (2 years). Hilton
and Uyehara (1966) reporting on these studies noted that when sugar
cane was double treated with amitrole at 5, 10 or 20 kg/ha, 12 and 20
weeks after planting, the residues of amitrole diminished to less than
0.002 mg/kg by harvest time. When 10 times as much cane was taken for
analysis and treated in a manner to approximate the early raw sugar
processing, residues, if present, were less than 0.01 mg/kg even from
plots receiving the grossly exaggerated rate of 20 kg/ha.
Wheat and oats
Amitrole proved to be an effective means of controlling couch
grass (Agropyron repens) in cereal crops. Most proposed treatments
involved the application of amitrole to the grass 10-14 days before
ploughing in preparation for seeding with cereals. In Sweden the
procedure was to apply amitrole at the 3 leaf stage at a rate of 0.5
to 1 kg/ha. Svensson (1971) investigated the effect of such treatments
on oats and the fate of residues in the oat plants and grain. He
reports that when the treatment was carried out strictly in accordance
with approved directions no detectable residues could be found in the
grain. However when the rate was increased or application withheld for
2 weeks until the 5-leaf stage of the crop, the grain contained about
0.05 mg/kg. If the rate of application is increased to 2 kg/ha the
residue can exceed 0.1 ppm. Determinations were made on straw from a
number of trials but analytical difficulties rendered the results
somewhat unreliable. However it appears that application of amitrole
at any stage after the 3-leaf stage increases the likelihood that
residues will be found in the straw at harvest. Much higher residues
were found in the grain of oats treated with amitrole at the heading
and milk stages. 0.5 kg/ha applied at the milk stage gave rise to a
residue in the grain of 2.9 mg/kg.
Amchem (1970) provided a summary of a number of studies by
official workers in the USA who investigated the incidence, level and
fate of residues of amitrole in wheat grain and straw from crops grown
on soil treated according to label directions 14-28 days before
ploughing for planting. In some of these studies the application rate
was increased to 4 times the recommended rate and the time interval
was reduced to 1 day before planting. In no instance was any amitrole
residue found in cereal grains or straw at harvest time. Soil studies
on the same test plots showed detectable residues of amitrole on the
day of treatment but no detectable residues the day after treatment
even at the doubly exaggerated rate. The limits of detection were 0.02
ppm in grain and straw. Recoveries were 86-96% at levels of 0.2-0.4
ppm. On the basis of these data it was concluded that the use of up to
4 kg/ha of amitrole, one or more weeks before planting wheat or other
cereals, should cause no residues in the crop.
FATE OF RESIDUES
General
Kröller (1966) and Menzie (1969) have reviewed the metabolism of
amitrole in animals, plants, micro-organisms, etc. There is an
extensive literature on the mode of action of amitrole and on its
metabolism in plants. Many of these investigations were carried out
with the object of determining uptake, translocation, site of action,
reason for selectivity, and possible means of enhancing the activity.
Among the most useful publications are those of Boyd (1964/65), Carter
(1969), Herrett and Linck (1961a), Jukes (1961), Moser (1968), and
Rogers (1957a,b).
Many of these studies reveal that amitrole does not persist for
extended periods in plant tissues though there is considerable
disagreement as to whether it becomes conjugated, converted into a
more active material or destroyed and its fragments taken up into the
plant. There appears to be no clear-cut mode of action, but rather
multiple pathways by which it interferes with plant metabolism. Carter
(1969) reviewed the available literature on the effects of amitrole on
amino acid and protein metabolism, purine metabolism, flavin
synthesis, enzyme activity, chlorophyll synthesis and plastid
development. It is obvious that there is no single site of action but
the relative importance of the various effects has apparently not been
unravelled.
In animals
The fate of amitrole in rats is discussed in the section
"Biotransformation". There do not appear to be any grounds for
assuming that livestock grazing on plant materials growing on land
that had been treated with amitrole for the control of weeds would
absorb or retain significant amounts of amitrole or its metabolites.
In plants
The s-triazole nucleus is highly stable (Potts 1961); hence it
is not surprising that few workers have reported evidence of ring
cleavage under physiological conditions. Yost and Williams (1958)
reported disappearance of radio-labelled amitrole from corn plants in
approximately 6 weeks with a half-life of about 8 days. Disappearance
was also observed in soybeans but at a much slower rate.
Miller and Hall (1961) could not detect amitrole in cotton 4 days
after treatment. However, large quantities of metabolic products were
present. Fang et al. (1967) report the half-life of amitrole in
several plants as 18 to 28 hours. Little of the applied material was
recovered as CO2 from beet, corn or beans.
Freed et al. (1961) reported evolution of radio-active, CO2 from
treated oats and barley, indicating ring cleavage. Montgomery and
Freed (1963) observed some evolution of 14CO2 from treated soybeans,
with the remaining radioactivity apparently bound to components of
plant tissues. Resistant oats released CO2 more readily than
sensitive barley. Massini (1963) found no loss of CO2 from beans or
tomatoes. Muzik (1965) observed chlorosis in scions grafted onto
tomato plants 103 days after treatment with amitrole, indicating long
persistence of the toxic moiety.
Studies of amitrole degradation in plants have been complicated
by the fact that the material is available commercially only with the
5-carbon labelled. Apparently, the 5-carbon is quickly lost (as CO2
or formate) when ring rupture occurs and the remaining fragments are
thus unlabelled. However, if significant amounts of CO2 or
14C-formate were produced from 14C-amitrole in higher plants, one
would expect to find some incorporation of 14C into normal
metabolites. This is not the case. The vast majority of the literature
indicates that the extractable 14C from plants treated with
amitrole-5-14C remains in the intact ring as free amitrole or
conjugates. Considerable amounts of amitrole are attached to protein
(Brown and Carter 1968; Castelfranco and Brown 1963) or somehow bound
in an insoluble form (Racusen 1958). There is evidence of activation
to a free radical form which can react with amino acids (Carter and
Naylor 1960, 1961a, b; Herrett and Bagley 1964; Herrett and Linck
1961a; Miller and Hall 1961). Conclusive evidence of rapid and
extensive ring cleavage by higher plants has not been reported.
Most literature on metabolic alteration of amitrole in plants
deals with the formation and properties of conjugates between amitrole
and endogenous plant constituents. These "degradation" products
contain the intact triazole nucleus which often may be regenerated by
chemical treatment.
Rogers (1957a,b) reported a derivative of amitrole in several
plants which was "chromatographically identical" with an
amine-glucoside derivative of amitrole. The glucose derivative forms
quite readily in vitro (Fredrick and Gentile 1960) and its occurrence
in plant extracts is probably an artifact (Naylor 1964), since
numerous attempts by other workers to detect the compound in plant
extracts failed (Miller and Hall 1961; Massini 1963; Carter and Naylor
1959). However, Gentile and Fredrick (1959) and Frederick and Gentile
(1960, 1969, 1962 and 1965) have published a series of studies on the
properties and metabolism of the glucose derivative. These same
authors suggest that the triose derivative represents the true
structure of the amitrole derivatives reported by other workers.
Massini (1963) and Carter and Naylor (1960, 1961a) reported studies of
amitrole metabolism in which Massini identified one of the major
metabolites as 3-(3-amino-1,2,4-triazole-1-yl)-2-amino propionic acid
(3-ATAL), also referred to as
3-(3-amino-1,2,4-triazol-1-yl)-d-alanine. The formation of 3-ATAL
apparently represents a detoxication, since the derivative does not
appear to be nearly as toxic as amitrole, or as mobile. Furthermore
ammonium thiocyanate, which synergizes the action of amitrole,
inhibits the formation of 3-ATAL.
Smith and Chang (1973) studied the metabolism of amitrole in
Canada thistle (Cirsium arvense) and peas. They showed that excised
leaves of thistles metabolized amitrole into three major products one
of which was 3-ATAL. The other two were shown to be its metabolic
products, one being the precursor of the other. An enzyme preparation
from peas capable of synthesizing tryptophan was also able to
metabolize amitrole. Tryptophan synthesis with the enzyme preparation
was inhibited by amitrole and the authors deduced that amitrole
metabolism may follow a similar pathway to tryptophan synthesis.
A number of workers including Racusen (1958) reported another
metabolite which was stable to 6N HCI for 5 hours at 100°C.
Verification of the structure of this material and others awaited
further studies.
The most important outstanding question is whether the conjugates
and/or metabolites represent biologically active derivatives of
amitrole and if so whether these are available to react
physiologically and biochemically with animals receiving such
conjugates and metabolites in plant materials as part of their ration.
However, use patterns at present approved avoid the possibility that
significant residues are ingested by livestock or man.
In soil
Amitrole disappears rapidly from soils as shown by Sund (1956),
Bondarenko (1958), Ashton (1962), Riepma (1962), and Ercegovich and
Frear (1964). Disappearance has been attributed to absorption (Sund,
1956, Ercegovich and Frear, 1964) and microbial degradation, although
attempts to isolate organisms capable of degrading amitrole have not
been successful. Kretschmar (1970) showed that amitrole was not
degraded by Azotobacter.
Kaufmann (1965), Kaufman and co-workers (1968) and Plimmer et al.
(1967), have proposed that most of the amitrole degradation occurring
in soils proceeds by non-biological reactions. They showed that
approximately 69% of the radio-label from amitrole was released as
CO2 in 20 days by non-sterilized soil. Although autoclaved soil
released only 2% in a comparable period, soil treated with potassium
azide or ethylene oxide released 46 and 35% respectively, and
reinoculation of autoclaved soil did not restore the capacity to
metabolize amitrole. These authors propose that amitrole is degraded
in soil by an oxidative mechanism involving an attack on the triazole
nucleus. Microbial attack is not discounted however. Soil moisture,
temperature and pH markedly affected amitrole degradation (Riepma
1962), indicating possible microbial involvement. The studies by
Ercegovich and Frear (1964) show that amitrole degradation obeys
first-order kinetics, suggesting a chemical reaction. No-one has
reported an investigation of the possible involvement of
extra-cellular enzymes.
Whatever the mechanisms by which the triazole ring is opened,
there appears to be little doubt that ring opening does occur rapidly
in soils and the resulting products (urea, cyanamide and nitrogen)
should be readily metabolized by soil micro-organisms.
Norris (1970) showed that amitrole was rapidly lost from the
forest floor but that degradation was not completely biological, since
there was considerable loss in steam-sterilized material. He found
that ammonium thiocyanate applied with amitrole in the proportions
found in the commercial herbicide had no effect on the degradation of
amitrole.
Sund (1956) showed that amitrole becomes tightly adsorbed to soil
particles. He concluded that it takes part in the soil's base exchange
system, but also it has the tendency to complex metals. Studies of
amitrole toxicity to tomato seedlings were correlated with chemical
analysis and it was found that the biological response of plants is
proportional to the amount recoverable in any soil type.
MacRae and Alexander (1965) are in agreement with the results of
Ashton (1963) that microflora have an important function in the
degradation of amitrole. They confirmed the rapid biodegradation in
soil by plant bioassay.
Ercegovich and Frear obtained evidence of complex formation
between soil clay and amitrole by means of X-ray diffraction
measurements. Ashton (1963) noted that 13 compounds were formed in
addition to CO2 when amitrole was added to unsterile soil. One or
more metabolic compounds formed from amitrole are tenaciously bound to
the soil and appear to be resistant to degradation.
Day et al. (1960) studied the effect of soil type, temperature,
moisture and sterilization by steam on the fate of amitrole in 55
types of Californian citrus soil. Rates of decomposition in
steam-sterilized soils were much lower than in unsterilized soil,
apparently indicating that the decomposition is primarily due to the
action of soil microorganisms (but compare the results of Kaufman,
Plimmer and co-workers quoted above). Rates of decomposition of
amitrole were highly variable among the soils studied, apparently
because of differences in populations or levels of activity of the
soil micro-organisms concerned. A short residual life for amitrole was
more frequently found in the more highly evolved soils having finer
textures and more highly developed colloidal properties.
Freed and Furtick (1961) showed by simultaneous bioassay and
chemical analyses capable of detecting less than 0.05 mg/kg of
amitrole that it would be highly improbable that any residue of
amitrole would remain in the soil even a short while after
application.
It has generally been assumed that failure to recover amitrole
from soils following its use as a herbicide was due to elimination by
such processes as leaching, volatalization, or biological or chemical
destruction. Work by Groves and Chough (1971) demonstrated that new
solvent mixtures gave much better recoveries of amitrole from soil
than water extraction. Concentrated ammonium hydroxide/glycol mixture
(5/20) gave distinctly higher recoveries than water as indicated by
the following table.
TABLE 4. Extraction (%) of amitrole from soils with water and
ammonium hydroxide/glycol (A/G) mixture (5x20)
Time after Non-sterile soil Sterile soil
application (days) Water A/G Water A/G
% % % %
0 48.1
1 36.7 97.6 49.6 97.3
12 21.3 55.9 36.1 77.9
17 3.2 15.2 36.9 67.7
These results point to a need for caution in interpreting some of
the earlier results.
In water
Since amitrole is widely used for the control of ditch bank weeds
many studies have been carried out to determine the fate of any
amitrole reaching the water. Many of these studies (Segal 1960;
Marston et al. 1968; Frank 1969; Dunstar 1969; and Demint et al. 1970)
have largely involved the study of the rate of dissipation of amitrole
in irrigation water or flowing streams. Since only a small proportion
of the herbicide applied in such operations ever reaches the water,
dissipation and dilution is a recognized means of reducing any risk to
crops and municipal users. Where application has been made by aerial
spraying, measurable amounts were found in samples of water near the
downstream edge of the sprayed area for up to 5 days after spraying
(Marston et al. 1968).
Such studies are however of little value in determining whether
residues are lost by physical, chemical or biological means. Studies
were reported by Thoman (1963) from Florida where ponds with static
water levels were treated at the rate of 3 kg/ha of water surface,
giving a concentration of 0.45 mg/l in the water immediately after
treatment. Samples collected every 7 days thereafter were analysed.
The residue level declined slowly but 46 days elapsed before it
reached the limit of determination (0.02 mg/l). Samples taken
thereafter until the 74th day gave results comparable to pre-treatment
levels.
Nicholson (1963) reported experiments carried out in a fish
hatchery in South Carolina where ponds of 1 acre surface area were
treated with amitrole to yield a concentration of 1 mg/l in 80,000
cu.ft of pond water. Samples were taken each 7 days for 5 weeks and
thereafter at slightly greater intervals until the 201st day when the
pond became flooded. The concentration following treatment (1.16 mg/l)
gradually declined through 0.49 mg/l on the 68th day to 0.07 mg/l on
day 201. No information is given on the quality of the water in the
ponds but it is obvious that under these conditions amitrole remains
relatively stable for long periods.
In processing and cooking
No information was available on the effect of processing and
cooking on residues in plant materials. In view of the fact that none
of the recommended use patterns give rise to detectable residues in
raw agricultural commodities such studies are unlikely to have been
carried out.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Food moving in commerce
No data were available to the Meeting to indicate the level and
incidence of amitrole residues in food moving in commerce.
Food at the time of consumption
Results of several total diet studies in which an attempt was
made to find amitrole residues in many separate food groups were
available to the Meeting. Duggan et al. (1966) reported that amitrole
residues were not found in any food composites in the total diet study
carried out in the USA in 1964/65; the method used had a limit of
determination of 0.05 mg/kg. The same authors (1967), having repeated
the total diet study, reported that amitrole residues were not found
at or above the prescribed sensitivity limit. Corneliussen (1969,
1970), reporting the results of the 4th and 5th total diet studies
carried out in the USA, states "amitrole has never been found in any
total diet composites". The limit of determination was again
0.05 mg/kg.
METHODS OF RESIDUE ANALYSIS
One of the first methods for the determination of amitrole
residues was that of Sund (1956). This was a colorimetric method
applicable to soils, depending upon the formation of a green colour
with sodium nitroprusside reagent. It is sensitive to 1 mg/l of
amitrole in aqueous solutions and Sund reported that it conformed to
Beer's law. Bioassay with tomato seedlings correlated with chemical
analysis.
American Cyanamid (1958) published a colorimetric method for the
estimation of residues of amitrole in cranberry fruit. The extract is
purified by means of a cation exchange resin, treated with nitrous
acid and coupled with N-1-naphthyl-ethylenediamine dihydrochloride to
produce a pink-coloured solution. The intensity of the colour is
proportional to the amitrole concentration and is measured
spectrophotometrically at 512 nm. The limit of determination is
0.03 mg/kg.
Herrett and Linck (1961b) published what they describe as a
simple reproducible method for the separation and quantitative
determination of amitrole in biological systems. The method consists
of diazotization followed by coupling with
1-amino-8-naphthol-3,6-disulfonic acid (H-acid). The authors describe
the method as being sensitive in the range of 0.1-3.3 mg/kg.
Storherr and Burke (1961) in describing an improved method for
the determination of amitrole in crops refer to previous methods
published by the Food and Drug Administration which were applicable to
only a limited number of food crops. With their modifications the
method involves extraction of vegetable crops with ethanol, adsorption
on cation-exchange resin and desorption with aqueous ammonia. The
ammoniacal concentrate is subjected to a clean-up procedure using
acetonitrile and filter aid followed by acid digestion and clean-up
with activated carbon. The resulting solution is diazotized and
coupled with H-acid. The pink colour is measured at 455 nm. It is
necessary to carry out a blank determination. The apparent amitrole
found in control crops ranged from 0.003 to 0.02 mg/kg. The limit of
determination is considered to be 0.025 ppm using a 40 g sample.
Storherr and Onley (1962) later published a procedure for the
clean-up and separation of amitrole and its metabolites from vegetable
crops. This method utilizes a chromatrographic column packed with dry
cellulose for the removal of interfering substances from plant
extracts. It is reported to recover amitrole from some conjugates.
Hilton et al. (1963) modified the method of Storherr and Burke,
(1961) for use with sugar cane and sugar juice and were able to lower
the limit of determination to 0.002 mg/kg. Their modifications
involved an increase in the size of sample taken and a reduction in
the amount of activated carbon used in the acid digestion and cleanup
steps.
Segal (1960) and Hilton and Uyehara (1966) described a number of
minor modifications to the method of Storherr and Burke which lowered
the limit of determination of amitrole to 0.001 mg/kg in water and
sugar cane respectively.
Meissner (1971), on behalf of the Amitrole Advisory Committee to
the US Environmental Protection Agency, commenting on the claim that
the method of analysis developed by Storherr and Burke (1961)
determines not only amitrole but all known metabolites drew attention
to the lack of detailed information available on the metabolites of
amitrole in plants or on the influence of environmental and chemical
factors on plant metabolism. Groves and Chough (1971) report an
improved solvent mixture (concentrated ammonium hydroxide and ethylene
glycol) to extract amitrole from soil. Further details are given above
(see "Fate of residues in soil").
NATIONAL TOLERANCES
The information available to the Meeting indicated that most
countries with pesticide residue tolerance legislation either
registered amitrole on a "no-residue" basis or provided a zero
tolerance. It is assumed that zero would in effect mean "at or about
the limit of determination". In the Netherlands there is a general
tolerance of 0.02 mg/kg in fruit and vegetables. In Australia there is
a maximum residue limit for amitrole in water of 0.01 mg/l. This is at
or about the limit of determination. In the Federal Republic of
Germany, use of amitrole where potable water might be contaminated is
prohibited.
APPRAISAL
Amitrole is a non-selective, foliage-absorbed herbicide widely
used since 1954 for the control of unwanted vegetation on industrial
land, roads, railways, rights of way, ditch banks and similar
situations. It is also used for the destruction of weeds beneath trees
and vines in permanent horticultural crops, for spot treatment of
perennial weeds and for their destruction prior to the planting of
cereal crops. There are no known uses where application is made to
crop plants.
Extensive information indicates that amitrole is rapidly lost
from soil by a combination of chemical, biological and microbiological
attack. There is no indication that amitrole is taken up by the roots
of plants if currently approved practices are followed. Approved uses
involve the application of amitrole to weeds between the crop when
fruit trees, vines and similar horticultural crops are dormant or the
use of spot sprays and directed sprays which avoid contamination of
crop plants. In these cases there is no uptake of residues by crop
plants. Studies with radio-labelled amitrole confirm that it is not
taken up from soil following simulated normal practices.
Extensive residue trials have been carried out under many
conditions in many countries and all indicate that there is no residue
in fruit, vegetables or grain following the recommended use of
amitrole as a herbicide. Residues have been found experimentally only
when crop plants have been treated with excessive quantities of
amitrole applied directly over the crop when the fruiting parts are
already well formed.
There appears to be no single mode of action and the metabolic
pathways in plants appear most complex. There is evidence that when
amitrole is applied to the leaves of plants, most of the material
absorbed is metabolized. Some is complexed with various plant
materials, but the Meeting considered that the nature and biological
significance of conjugation products would only be of importance when
considering residues resulting from direct application over food-crop
plants. Where amitrole was administered to laboratory animals it was
shown that similar conjugates with sugars and proteins were formed in
the animal body.
There was some doubt whether the methods of analysis for residues
of the parent compound would adequately recover and determine all
conjugated materials or other biologically active metabolites. The
method of Storherr and Onley (1962) is the most sensitive and specific
method available, and is reported by several authors to recover
amitrole from some conjugates. Under the circumstances the Meeting
felt confident in recommending a maximum residue limit at or about the
limit of determination by the beet available method. The Meeting
however proposed certain precautions to avoid contamination of food
crops.
RECOMMENDATIONS
There is no reason to believe that significant residues occur in
any raw agricultural commodities when amitrole is used for the control
of weeds according to approved directions.
To reduce the possibility of contaminating food crops with
residues of amitrole, use-patterns should avoid the direct treatment
of food crops and should be limited to directed sprays, spot sprays
and, in the case of pre-planting and stubble treatments, application
to weeds at least 10 days before ploughing (see Table 2), the interval
to be conditioned by the temperature of the soil.
For regulatory purposes and as a means of determining whether
amitrole herbicides have been misused or incorrectly applied the
Meeting recommends a maximum residue limit at or about the limit of
determination by the best available analytical method.
CONDITIONAL TOLERANCE
mg/kg
Raw agricultural commodities of plant origin 0.02*
* at or about the limit of determination.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Long term feeding studies in a sufficient number of rats and mice
with low levels of amitrole of known composition and purity.
2. Studies to elucidate the possible relationship between the effects
of amitrole on the thyroid and on the liver.
3. Studies to show that the analytical methods determine not only the
parent compound but also biologically active metabolites.
4. Studies to develop a specific method sensitive to 0.005 mg/kg.
REFERENCES
Alexander, N.M. (1959a). Antithyroid action of 3-amino-1,2,4-triazole.
J. biol. Chem., 234:148-150.
Alexander, N.M. (1959b). Iodide peroxidase in rat thyroid and salivary
glands and its inhibition by antithyroid compounds. J. biol. Chem.,
234:1530-1533.
Amchem (1965). Residues of amitrole in apples. Report Amchem Products
Inc. (Unpublished)
Amchem (1969). Analysis of soyabeans and soyabean plants for possible
residues of amitrole. Review of collaborative trials. Amchem Products
Inc. Report, Jan. 1969.
Amchem (1970a). Residues of aminotriazole in corn. Collection of
studies by various workers. Submission to Amitrole Committee, 16 Dec.
1970.
Amchem (1970b). Residues of amitrole in wheat and soil when used for
chemical fallow. Submission to Amitrole Committee, 16 Dec. 1970.
American Cyanamid (1958). Colorimetric method for the estimation of
residues of amitrole in cranberry fruit. American Cyanamid Co., 4 Feb.
1958.
Ashton, F.M. (1963). Fate of amitrole in soil. Weeds, 11:167-170.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. Am. med.
Ass., 172:1319-1320.
Auerbach, V.H., Piesinger, R.H. and Waisman, H.A. (1959). The effect
of 3-amino-1,2,4-triazole on the synthesis of tryptophan peroxidase.
Archs Biochem. Biophys., 82:370-379.
Axelson, O., Rehn, M. and Sundell, L. (1974). Herbicide exposure,
mortality and tumour incidence. An epidemiological investigation on
Swedish railroad workers. Envir. Hlth. (in press)
Bagdon, R., Vidone, L., Shaffer, C. and Golz, H. (1965).
Aminotriazole: acute and subacute toxicity. Report from American
Cyanamid Co. (Unpublished)
Baron, J. and Tephyly, T.R. (1969). Effect of 3-amino-1,2,4-triazole
on the stimulation of hepatic microsomal heme synthesis and induction
of hepatic microsomal oxidases produced by phenobarbital. Molec.
Pharmac., 5:10-20.
Bayer (1973). Amitrole residues in peaches, plums, grapes, cherries
and apples. Reports 434/72 - 443/72.
Bondarenko, D.D. (1958). Decomposition of amitrole in soil. The Ohio
Agricultural Experiment Station and The Ohio State University. Proc.
N. cent. Weed Control Conf.
Boyd, J.E. (1964, 1965). Some physiological and phytotoxicological
properties of amitrole. American Cyanamid Co.
Brown, J.C. and Carter, M.C. (1968). Influence of amitrole upon
protein metabolism in bean plants. Weed Sci., 16(2): 222-226.
Carter, M.C. (1969). Amitrole. In P.C. Kearney and D.D. Kaufman
(editors). Degradation of herbicides. Marcel Dekker, Inc., New York,
p. 187-206.
Carter, M.C. and Naylor, A.W. (1959). The formation, in vivo, of a
complex between 3-amino-1,2,4-triazole and a glycine-serine
derivative. Pl. Physiol., Lancaster, 34:(Suppl.) vi.
Carter, M.C. and Naylor, A.W. (1960, 1961). Metabolism of
3-amino-1,2,4-triazole-5-C14 in plants. Bot. Gaz., 122:138-143.
Carter, M.C. and Naylor, A.W. (1961a). Studies on an unknown metabolic
product of 3-amino-1,2,4-triazole. Physiologia Pl., 14:20-27.
Carter, C. and Naylor, A.W. (1961b). The effect of
3-amino-1,2,4-triazole upon the metabolism of carbon labeled sodium
bicarbonate, glucose, succinate, glycine and serine by bean plants.
Physiologia Pl., 14:62-71.
Castelfranco, P. and Brown, M.S. (1963). A hypothesis of amitrole
action based on its behavior toward free radical generating systems.
Weeds, 11(2):116-124.
Clyne, R. (1970). Statement to Amitrole Advisory Committee.
Corneliussen, P.E. (1969). Residues in food and feed. Pesticide
residues in total diet samples (IV). Pestic. Monit. J., 2:140-152.
Corneliussen, P.E. (1970). Pesticide residues in total diet samples
(V). Pestic. Monit. J., 4(3):89-105.
Dardin, V. (1958). 3-amino-1,2,4-triazole - chronic oral
administration, beagle dogs, 52 weeks. Histology - supplement to
report. Report from Hazelton Laboratories, Inc. (Unpublished)
Dardin, V. (1959). 3-amino-1,2,4-triazole. Histology - supplement to
report of two year dietary feeding - rats. Report from Hazelton
Laboratories, Inc. (Unpublished)
Day, B.E. and Hendrixson, R.T. (1959). A study of the accumulation and
depletion of amitrole in citrus fruit and foliage. University of
California, Riverside, Research Report.
Day, B.E., Jordan, L.S., and Hendrixson, R.T. (1961). The
decomposition of amitrole in Californian soils. Weeds, 9:443-456.
Demint, R.J., Frank, P.A., Comes, R.D. (1970). Amitrole residues and
rate of dissipation in irrigation water. Weed Sci., 18(4):439-442.
Duggan, R.E., Barry, H.C. and Johnson, L.Y. (1966). Pesticide residues
in total diet samples. Science, 151:101-104.
Duggan R.E., Barry, H.C. and Johnson, L.Y. (1967). Residues in food
and feed. Pesticide residues in total diet samples (II). Pestic.
Monit. J., 1(2):2.
Dunster, K.W. (1969). Amitrole persistence in irrigation canals.
Report of trials in Montana, Washington and Colorado. Amchem Report, 7
May 1969.
Elsea, J. (1954). Acute dermal application, acute eye application.
Report from Hazelton Laboratories, Inc. (Unpublished)
Ercegovich, C.D. (1957). Studies on the analysis and persistence of
3-amino-1,2,4-triazole in plant tissue and in soil. Ph.D. thesis,
Pennsylvania State University, University Park, Pa.
Ercegovich, C.D. and Frear, D.E.H. (1964). The fate of
3-amino-1,2,4-triazole in soils. J. agr. Food Chem., 12:26-29.
Fabrizio, D.P.A. (1973). Final report on cytogenicity study on
3-amino-1,2,4-triazole from Litton Bionetics, Inc. submitted to WHO by
Rohm and Haas Co. (Unpublished)
Fang, S.C., George, M. and Yu, T.C. (1964). Metabolism of 3-amino-1,2,
4-triazole-5-C14 by rats. J. agr. Food Chem., 12:219-223.
Fang, S.C., Khanna, S. and Rao, A.V. (1966). Further study on the
metabolism of labeled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. agr. Food Chem., 14:262-265.
Fang, S.C., Fallen, E. and Khanna, S. (1967). The metabolism of
labeled amitrole in plants. Weeds, 15:343-346.
Feytmans, E. and Leighton, F. (1973). Effects of pyrazole and
3-amino-1,2,4-triazole on methanol and ethanol metabolism by the rat.
Biochem. Pharmac., 22:349-360.
Fleming, A.S. (1959). Statement about amitrole residues in cranberries
- Press Release. Dept of Health, Education and Welfare, 9 Nov. 1959.
Fogleman, R. (1954). Amitrole - (3-amino-1,2,4-triazole) - Final
report: submitted by Hazelton Laboratories, Inc. (Unpublished)
Frank, P.A. (1969). Amitrole concentration in water of treated
irrigation channels. U.S. Dept of Agriculture A.R.S., Denver,
Colorado. Report, 14 Jan. 1969.
Fredrick, J.F. and Gentile, A.C. (1960). The formation of the glucose
derivative of 3-amino-1,2,4-triazole under physiological conditions.
Physiologia Pl., 13:761-765.
Fredrick, J.F. and Gentile, A.C. (1961). The structure of the
D-glucose adduct of 3-amino-1,2,4-triazole: Infrared absorption
studies. Arch. Biochem. Biophys., 92(2):356-359.
Fredrick, J.F. and Gentile, A.C. (1962). Studies of a phosphorylated
derivative of 3-amino-1,2,4-triazole formed by the action of yeast
hexokinase. Physiologia Pl., 15(1):186-193.
Fredrick, J.F. and Gentile, A.C. (1965). Physico-chemical studies of
the metabolic end product of 3-amino-1,2,4-triazole in yeast.
Phytochemistry, 4:851-856.
Freed, V.H., Furtick, W.R. (1961). The persistence of amitrole in
soil. Oregon Agricultural Experiment Station Technical Paper No. 1375.
Freed, V.H., Montgomery, M. and Kief, M. (1961). Proc. N East Weed
Control Conf., 1961:6-16.
Fregly, M.J. (1968). Effect of aminotriazole on thyroid function in
the rat. Toxic. appl. Pharmac., 13:271-286.
Gaines, T.B., Kimbrough, R.D. and Linder, R.E. (1973). Toxicity of
amitrole in the rat. Toxic. appl. Pharmac., 26:118-129.
Geldmacher-von Mallinckrodt, M. and Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Archs Toxik., 27:13-18. (English
summary)
Gentile, A.C. and Fredrick, J.F. (1959). Chemical activity of the
glucose adduct of 3-amino-1,2,4-triazole. Physiologia Pl., 12:862-867.
Grapenthien, J., Hathaway, D. and Keplinger, M. (1972). Two year
chronic aerosol inhalation toxicity study with amitrol 3AT in albino
rats. Report from Industrial Biotest Laboratories, Inc. (Unpublished)
Groves, K. and Chough, K.S. (1971). Extraction of 3-amino-1,2,
4-triazole (amitrole) and 2,6-dichloro-4-nitroaniline (DCNA) from
soils. J. agr. Food Chem., 19(5):840-841.
Hapke, H.J. (1967). The toxicity of aminotriazol for domestic animals.
Zbl. Vet. Med. A., 14:469-486. (English, French and Spanish summaries)
Heim, W.G., Appleman, D. and Pyfrom, H.T. (1956). Effects of
3-amino-1,2,4-triazole (AT) on catalase and other compounds. Am. J.
Physiol., 186:19-23.
Herrett, R.A. and Bagley, W.P. (1964). The metabolism and
translocation of 3-amino-1,2,4-triazole by Canada thistle. J. agr.
Food Chem., 12:17-20.
Herrett, R.A. and Linck, A.J. (1961a). Penetration of
3-amino-1,2,4-triazole in Canada thistle and field bindweed. Weeds,
9(2): 224-230.
Herrett, R.A. and Linck, A.J. (1961b). Quantitative determination of
3-amino-1,2,4-triazole. J. agr. Food Chem., 9(6):466-467.
Hill, R. (1962, 1963). Residues of amitrole in almonds, walnuts,
peaches and prunes. Diablo Laboratories, Berkley, Reports of Amchem
Products Inc.
Hilton, H.W., Uyehara, G. and Segal, H.S. (1963). Amitrole residues in
sugar cane. Rate of disappearance studies and improved methodology.
Hawaiian Sugar Planters Association Report.
Hilton, H.W. and Uyehara, G.K. (1966). Determination of
3-amino-1,2,4-triazole in sugarcane. J. agr. Food Chem., 14(1):90-94.
Hodge, H.C., Maynard, E.A., Downs, W.L., Ashton, J.K. and Salerno
L.L. (1966). Tests on mice for evaluating carcinogenicity. Toxic.
appl. Pharmac., 9:583-596.
Hoshino, M. (1960). Effect of 3-amino-1,2,4-triazole on the
experimental production of liver cancer. Nature, 186:174-175.
Hylin, J.W. (1962). Determination of amitrole residues in coffee
beans. Report from University of Hawaii.
IARC (1974). Amitrole. In IARC Monographs on the Carcinogenic Risk
of Chemicals to Man. Vol. 7.
Innes, J. (1966). Amitrole - summary sheet - Carcinogenesis assay
studies - Subcutaneous. Data sheet. (Unpublished)
Innes, J.R.M., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
I., Hart, E.R., Pallotta, A.J., Bates, R.R., Falk, H.L., Gart, J.J.,
Klein, M., Mitchell, I. and Peters, J. (1969). Bioassay of pesticides
and industrial chemicals for tumorigenicity in mice: a preliminary
note. J. natn. Cancer Inst., 42:1101-1114.
Jukes, T.H. and Shaffer, C.B. (1960). Antithyroid effects of
amino-triazole. Science, 132:296-297.
Jukes, T.H. (1961). Antimetabolites and plant growth. Bull. Torrey
bot. Club, 88(5):321-327.
Kaufmann, D.D. (1966). Microbial degradation of herbicide
combinations: amitrole and dalapon. Weeds, 14:130-134.
Kaufmann, D.D., Plimmer, J.R., Kearney, P.C., Blake, J. and Guardia,
F.S. (1968). Chemical versus microbial decomposition of amitrole in
soil. Weed Sci., 16(2):266-272.
Keller, J. (1959). Aminotriazole (3-amino-1,2,4-triazole) two year
chronic feeding - rats. Report from Hazelton Laboratories, Inc.
(Unpublished).
Kretschmar, G. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) on the growth of Azotobacter. Z. allg. Mikrobiol.,
10:253-267.
Kretschmar, G. and Gunther, G. (1970). Der einflus von
3-amino-1,2,4-triazol (Amitrol) auf die Atmungvon Azobacter. A.
allg. Mikrobiol., 10:487-500.
Kröller, E. (1966). Anwendung und Eigenschaften des
3-amino-1,2,4-triazols im Hinblick auf seine Rückstände im
Lebensmitteln. Residue Reviews, 12:162-192.
Kudsk, F.N. (1969). Uptake of mercury vapor in blood in vivo and
in vitro from Hg-containing air. Acta pharmac. Tox., 27:149-160.
Landauer, W., Salam, N. and Sopher, D. (1971). The herbicide
3-amino-1,2,4-triazole (amitrole) as teratogen. Envir. Res.,
4:529-543.
Langhans, W. and Schimassek, H. (1974). Prevention of halothane
induced changes in rat liver enzymes by 3-amino-1,2,4-triazole.
Biochem. Pharmac., 23:403-409.
Leonard, O.A., and Lider, L.A. (1961). Toxicity and translocation of
herbicides supplied to grape rootings through solution culture. Am. J.
Enol. Vitic., 12(1):37-46.
Leonard, O.A., and Weaver, R.J. (1961). Absorption and translocation
of 2,4-D and amitrole in shoots of the Tokay grape. Hilgardia,
31(9):327-368.
Levine, W.G. (1973). Effect of phenobarbital and 3-amino-1,2,
4-triazole on the metabolism and biliary excretion of 3,4-benzypyrene
in the rat. Life Sci., 13:723-732.
Lotlikar, P., Wasserman, M. and Luha, L. (1973). Effect of
2-amino-1,2,4-triazole pretreatment on N and ring - hydroxylation of
2-acetylamino-fluorene by the rat. Proc. Soc. exp. Biol. Med.,
144:445-449.
Magos, L., Sugata, Y. and Clarkson, T.W. (1974). Effects of
3-amino-1,2,4-triazole on mercury uptake by in vitro human blood
samples and by whole rats. Toxic. appl. Pharmac., 28:367-373.
Maier-Bode, H. and Bechtel, W.D. (1968). Investigations of amitrole
residues in orchardry and viticulture. A. Pflanzenkrankheiten No. 4.
Proceedings of the 7th German Symposium on questions of weed biology
and control, 5 March 1968.
Margoliash, E. and Novogrodsky, A. A study of the inhibition of
catalase by 3-amino-1,2,4-triazole. Biochem. J., 68:468-475.
Marston, R.B., Schults, D.W., Shiroyama, T., and Snyder, L.V. (1968).
Pesticides in water. Amitrole concentrations in creek waters
downstream from an aerially sprayed watershed sub-basin. Pestic.
Monit. J., 2(3):123-128.
Massini, P. (1958). Uptake and translocation of 3-amino and
3-hydroxy-1,2,4-triazole in plants. Acta bot. neerl., 7:525-530.
Massini, P. (1959). Synthesis of (3-amino-1,2,4-triazolyl) alanine
(ATX) from 3-amino-1,2,4-triazole in plants. Biochem. Biophys. Acta,
36:548-549.
Massini, P. (1963). Aminotriazolylalanine; a metabolic product of
amitrole in plants. Acta bot. neerl., 12:64.
Matsushima, T. and Weisburger, J. (1972). Effect of carbon monoxide or
of 3-amino-triazole on C- and N- hydroxylation of the carcinogen
N-2-fluorenylacetamide by liver microsomes of hamsters pretreated with
3-methyl-cholanthrene. Xenobiotica, 2:423-430.
McRae, I.C. and Alexander, M. (1965). Microbial degradation of
selected herbicides in soil. J. agr. Food Chem., 13:72-76.
Meissner, W.A. (1971). Report of Amitrole Advisory Committee to
Environmental Protection Agency Washington, 12 March 1971.
Menzie, C.M. (1969). Metabolism of Pesticides. U.S. Dept. Interior,
Bureau of Sport Fisheries and Wildlife Special Scientific
Report - Wildlife No. 127:40-41.
Miller, C.S. and Hall, W.C. (1961). Absorption and metabolism of
aminotriazole in cotton. J. agr. Food Chem., 9:210-212.
Mohandas, T. and Grant, W.F. (1972). Cytogenetic effects of 2,4-D and
amitrole in relation to nuclear volume and DNA content in some higher
plants. Can. J. Genet. Cytol., 14:773-783.
Montgomery, M. and Freed, V.H. (1963). Residue studies of
amino-triazole in soyabeans. Report from Oregon State University, 25
April 1963.
Moore, B. (1968). Amitrole and simazine residue trials in Tasmanian
apples treated with D amatol 44. Geigy Australia Ltd., Report
68/8/290.
Moore, B. (1969). Amitrole and simazine residue trials at Orange, NSW,
1967-68. Geigy Australia Ltd., Technical Report 69/2/294.
Moore, B. (1970). Amitrole and simazine residue trials at Orange, NSW,
1968-69. Geigy Australia, Technical Report 70/1/309.
Moser, B. (1968). Summary statements on triazoles. Rutgers Univ. Plant
Physiology Seminar.
Muzik, T.J. (1965). Effect of temperature on the activity and
persistence of amitrole and 2,4-D. Weed Res., 5(3):207-212.
Napalkov, N. (1962). Tumor inducing action of 3-amino-1,2,4-triazole.
Truda prof. Zabol., 6:48-51.
Napalkov, N.P. (1969). On blastomogenic action of thyreostatic
substances. IECO USSR Acad. Med. Sci. Moscow, Monograph.
Naylor, A.W. (1964). Complexes of 3-amino-1,2,4-triazole in plant
metabolism. J. agr. Food Chem., 12:21-25.
Nicholson, H.P. (1963). Persistence of amitrole in pond water. U.S.
Public Health Service, Report 17 April 1963.
Norris, L.A. (1970). Degradation of herbicides in the forest floor. In
Tree Growth and Forest Soils, C.T. Youngberg and C.B. Daley (editors).
Onley, J.H., Storherr, R.W., Cluver, A.J. Jr. (1963). An Oregon
cranberry bog study for 3-amino-1,2,4-triazole residues, 1961-1962. J.
Ass. off. analyt. Chem., 46(6):996-1001.
Otten, R.S. (1970). Statement to Amitrole Advisory Committee, 16 Dec.
1970.
Plimmer, J.R., Kearney, P.C., Kaufman, D.D. and Guardia, F.S. (1967).
Amitrole decomposition by free radical-generating systems and by
soils. J. agr. Food Chem., 15: 996-999.
Potts, K.T. (1961). The chemistry of 1,2,4-triazoles. Chem. Rev.,
61:87-127.
Racusen, D. (1958). The metabolism and translocation of
3-amino-triazole. Archs Biochem. Biophys, 74(1):106-113.
Raisfeld, I., Bacchin, P., Hutterer, F. and Schaffner, F. (1970). The
effect of 3-amino-1,2,4-triazole on the phenobarbital induced
formation of hepatic micro-somal membranes. Molec. Pharmac., 6:231.
Rausina, B., Mastri, C., Keplinger, M. and Yost, D. (1972). Two year
chronic dermal toxicity study with amitrole 3-AT in albino rats.
Report from Industrial BioTest Laboratories, Inc. (Unpublished)
Riepma, P. (1962). Preliminary observations on the breakdown of
3-amino-1,2,4-triazole in soil. Weed Res., 2:41-50.
Rogers, B.J. (1957a). Translocation and fate of amino triazole in
Plants. Weeds, 5:5-11.
Rogers, B.J. (1957b). The action of 3-amino-1,2,4-triazole in plants.
Hormolog 1:10.
Samio, H., Baird, M. and Massie, H. (1972). Renewal of catalase
activity in Drosophilia following treatment with 3-amino-1,2,
4-triazole. J. Insect. Physiol., 18:991-1000.
Sanderson, D. and Roe, F. (1962). Dietary toxicity of aminotriazole to
the female rat. Report from Fisons Ltd., Agrochemical Division and
Pathology Report from Chester Beatty Research Institute. (Unpublished)
Schroeder. I. (1970). The influence of 3-amino-1,2,4-triazole
(amitrole) in a non-herbicidal range on the protein biosynthesis of
the unicellular algae Poteriochromonas stipitata. A. Allg.
Mikrobiol., 10:295-298.
Schubert, O.E. (1965). Residues of amitrole in apple fruit following
ground cover, fruit and leaf applications. Report from West Virginia
University.
Segal, H.S. (1960). Determination of amitrole in irrigation ditch
water. Amchem Laboratory Report 411, July 1960
Segal, H.S. (1963). Amitrole residues in Hawaiian irrigation ditch
water. Amchem Report, 27 Feb. 1963.
Shaffer, C., Vidone, L. and Golz, H. (1958). Aminotriazole: 32 day
repeated feeding to rats. Report from American Cyanamid Co.
(Unpublished)
Smagghe, G. (1974). Report from Chemical Products - Ugine - Kuhlmann.
(Unpublished)
Smith, L.W. and Chang, F.Y. (1973). Aminotriazole metabolism in
Cirsium arvense (L.) Scop. and Pisum sativum L. Weed Res.,
13:339-350.
Stenger, R. and Johnson, E. (1972). Modifying effect of
3-amino-1,2,4-triazole on phenobarbital induced changes in rat liver.
Expl. Molec. Pathol., 16:147-157.
Storherr, R.W. and Burke, J. (1961). Determination of 3-amino-1,2,
4-triazole in crops. J. Ass. off. analyt. Chem., 44(2):196-199.
Storherr, R.W. and Onley, J. (1962). Cleanup and separation of
3-amino-1,2,4-triazole and metabolites from vegetable crops. J. Ass.
off. analyt. Chem., 45(2):382-387.
Strum, J. and Karnovsky, M. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity. Lab.
Invest., 24:1-12.
Sund, K.A. (1956). Residual activity of 3-amino-1,2,4-triazole in
soils. J. agr. Food Chem., 4(1):57-60.
Svensson, J.A. (1971). Residues of herbicides in cereals. Proceedings
of 12th Swedish Weed Conference, Jan. 1971.
Tephly, T.R., Mannering, G.J. and Parks, R.E., Jr. (1961). Studies on
the mechanism of inhibition of liver and erythrocyte catalase activity
by 3-amino-1,2,4-triazole (AT). J. Pharmac. exp. Ther., 134:77-82.
Tephly, T.R., Hasegawa, E. and Baron, J. (1971). Effect of drugs on
heme synthesis in the liver. Metabolism, 20:200.
Thoman, J.R. (1963). Persistence of amitrole in water (Borrow Pits)
Florida. U.S. Dept of Health, Education and Welfare, Report, 13 Nov.
1963.
Tschudy, D.P. and Collins, A. (1957). Effect of 3-amino-1,2,4-triazole
on delta-amino-levulinic acid dehydrase activity. Science, 126:168.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. and Hidosaka, Y.
(1973). Fine structure and localization of peroxidase activity in
aminotriazole goiter. Nagoya med. J., 18:183-190.
Weir, R., Jr. (1958). 3-amino-1,2,4-triazole chronic oral
administration - Beagle Dogs - 52 weeks - Final Report. Report from
Hazelton Laboratories, Inc. (Unpublished)
Weir, R. Jr. (1974). Personal communication to WHO.
Wit, S.L. and van der Kamp, C.G. (1963). Residues of amitrole in
apples. Report 26 February 1963, National Institute of Public Health,
Utrecht. Submitted by Netherlands Government to FAO (1974).
Yost, J.F. and Williams, E.F. (1958). Proc. N East Weed Control Conf.,
1958:9-15.
