
AMITROLE JMPR 1977
Explanation
Amitrole was evaluated in 1974 (FAO/WHO, 1975). A conditional
acceptable daily-intake for humans of 0.00003 mg/kg b.w. was
established. Since then some new information has become available,
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Metabolism
3-Amino-1,2,4 triazole (5-14C) was administered orally to rats as a
single dose of 5 mg/kg body weight. The main part of the radioactivity
(about 79%) was excreted in the urine during the first 24 hrs. After
three days about 6% of the total dose was excreted as metabolites. The
two main metabolites were identified as 3-amino-5-mercapto-1,2,4
triazole (I) and 3-amino-1,2,4-traizo-5-yl-mercapturic acid (II). In
the faeces only 1.5% of the dose was excreted.
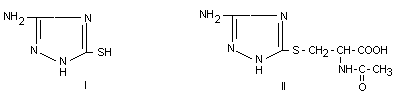 Metabolite I has in its tautomeric form the -NH-CS-NH-group, which was
thought to be partly responsible for the antithyroid action of
amitrole. However, according to the authors amitrole has much more
effect on the thyroid than 3-amino-5-mercapto-triazole (Grunow et al.,
1975).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
The LD50 of amitrole for Drosophila was 40 ppm in the medium, while
with 10 ppm a prolongation of development time was found. Therefore no
optimal concentrations for a mutagenicity test could be used. With 10
ppm in the medium no mutagenic effects were observed in the sex
chromosome non-disjunction test (females) or the sex-linked recessive
lethal test (males) (Laamanen et al., 1976).
Effects of amitrole on human leucocytes in culture were investigated.
The cell growth was inhibited in concentrations of 0.2%w/v and higher.
Selected metaphases were examined for the presence of chromosome and
chromatid aberrations. No breakage or other visible chromosome damage
has been found (Meretoja at al., 1976).
With bacteria two types of tests were carried out. A DNA repair test
was done with E. coli, B. subtilis and S. typhimurium strains.
No mutagenic effect was noted.
Negative results were also obtained in a revertant test with several
strains of Salmonella. In both types of test known mutagens were
used as positive controls (Bamford et al., 1976),
Special studies on carcinogenicity
40 female Wistar rats were given 2500 ppm amitrole in the drinking
water during their life-time. All animals surviving 30-70 weeks were
studied. In all animals goitre was observed. After 30 weeks the
follicles became small and stromal tissue became fibrotic. These small
follicles contained little or no colloid. The proliferating follicular
tissue gave invasive lesions in 19 of the 26 rats.
In 3 of the animals nodules of papillary adenoma were found. In the
liver two cases of cholangiofibrosis, clue to irregular proliferation
of the bile ducts and surrounding stroma, were found (Tsuda et al.,
1976).
Special studies on the antithyroid effect
Two dosages of amitrole (0.5 g/kg and 1.0 g/kg) were administered
intraperitoneally to chicks, daily from day 3-40 after hatching. The
animals showed growth inhibition and an increased thyroid/body weight
ratio, compared with the controls.
In another experiment chicks were injected up to day 20 post-hatching
at which time treatment with the drug was discontinued. The animals
were sacrificed at day 27, 34, and 41. When the amitrole treatment was
stopped, the thyroid/body weight ratio decreased markedly, but never
to the same levels which were obtained in the controls.
Histologically, the characteristic changes reported for other
goitrogens were observed in the thyroid (Wishe, 1976).
Short-term studies
Rat
Several short-term experiments were carried out in order establish a
no-effect level on thyroid function tests. In all experiments the
uptake of 131I by the thyroid was measured in an in vivo test, 6, 24
and 48 hours after administration of 0.6 Ác 131I per animal
intraperitoneally. In addition thyroid weight and PBI (protein bound
iodine) were measured and the thyroid was studied histopathologically.
In the first experiment 4 groups of 8 female Wistar rats received
respectively 0, 2, 20 and 200 ppm of amitrole in the diet during 6
weeks. After 5 days and 6 weeks the uptake of 131I was measured. At
both times a significantly increased uptake was found in the 200 ppm
group 6 hours after injection, which decreased rather rapidly after 24
and 48 hrs. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. In the two
lower dosages no significant effects were found.
In the second experiment in which 8 female animals per group received
respectively 0, 20, 50 and 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition a significantly decreased PBI
was observed at the end of the experiment. With 50 ppm in the diet for
6 weeks a statistically increased uptake was found 6 hours after
injection of 131I. In this case the radioactivity in the thyroid
remained higher than the controls after 24 and 48 hrs.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment 0, 20, 50 and 200 ppm were given to 10 animals
per group during 13 weeks. The uptake of 131I by the thyroid was
significantly increased at 200 and 50 ppm after 6 and 12 weeks. The
difference between the groups was that with 50 ppm the radioactivity
in the thyroid remained high after 24 and 48 hrs., whereas with 200
ppm a very high uptake was found 6 hrs. after injection of 131I, but a
rapid decrease still lower than the controls after 48 hrs. With 200
ppm the PBI was decreased and the thyroid/body weight ration increased
by a factor of 6. With 50 ppm only a slightly increased relative
thyroid weight was found. Histologically a strong activation and
goitre were found with 200 ppm a slight activation with 50 ppm. In
this experiment a tendency to a higher uptake of 131I was found in the
20 ppm group.
The above mentioned experiments were carried out with a relatively low
iodine content in the diet (about 0.2-0.3 ppm). In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment 8 female rats per group received respectively 0, 20, 50,
200 and 500 ppm in the diet for 6 weeks, to see whether iodine could
protect against the anti-hyroid action of amitrole. With 500 ppm a
small increase in iodine uptake was found 5 hrs. after 131I injection,
but thereafter a very rapid decrease. With 200 ppm the uptake was much
higher and the same type of decrease was found as in the other
experiments, whereas with 50 ppm a significantly increased thyroid
radioactivity was found at all times. The 131I uptake of all animals
was of course much lower than in the increased and PBI only decreased
at 200 and 500 ppm. Histopathologically goitre and strongly activated
thyroids were found only at the two highest dosed levels. Some
activation was found in the 50 ppm group and a very slight activation
was also found in the 20 ppm group. (Den Tonkelaar and Kroest 1974)
COMMENTS
Several new studies on mutagenicity became available. No mutagenic
action could be demonstrated. In a recent carcinogenicity study with a
very high dose level, which had an influence on survival, a
carcinogenic action on the thyroid was found. In addition, two cases
of cholangiofibrosis in the liver have been observed. Two metabolites
of amitrole have been identified after oral administration to rats.
In four short-term studies the main effect was an increased uptake of
radioactive iodine by the thyroid at dose levels of 50, 200 and 500
ppm. This uptake correlated well with the histologically observed
activation. With 20 ppm only a very marginal effect was found while 2
ppm was without effect.
This Meeting confirmed the existing conditional ADI for humans pending
consideration of the whole concept of conditional ADIs for humans at a
future meeting.
FURTHER WORK OR INFORMATION
Desirable
See FAO/WHO Pesticide residues in food. Report of the 1974 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. p. 31
REFERENCES
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole III. Microbial tests
Mutation Res 401, 197-202.
Den Tonkelaar, E.M. and Kroes, R. (1974) Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol. Unpublished
report National Institute of Public Health, Bilthoven, the
Netherlands, 164/74 Tox.
Grunow, W., Altmann, H.J. and B÷hme, CHR. (1975) Uber Den Stoffwechsel
von 3-Amino-1,2,3-triazol in Ratten. Arch. Toxicol. 34, 315-324.
Laamanen, J., Sorsa, M., Banford, D., Gripenberg, U. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole I. Drosophila tests.
Mutation Res. 40, 185-190.
Meretoja, T., Gripenberg, U., Bamford, B., Laamanen, I. and Sorsa, M.
(1976) Mutagenicity and toxicity of amitrole II. Human lymphocyte
culture tests. Mutation Res. 40, 191-196
Tsuda, H., Hananouchi, M., Tatematsu, M., Hirose, M., Hirao, K.,
Takahashi, M. and Ito, N. (1976) Tumorigenic effect of
3-amino-1-H-1,2,3 triazole on rat thyroid. J. Natl. Cancer Inst. 57,
861-864.
Wishe, H.J. (1976) The effect of aminotriazole on the thyroid gland
and development of the white leghorn chicks. Diss. Abstr. Int. B.,
37, 1066-1067. Cited in Rest. Abstr. 9, (1976).
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP: 1974/M/11; WHO Pesticide Residue Series, No. 4.
Metabolite I has in its tautomeric form the -NH-CS-NH-group, which was
thought to be partly responsible for the antithyroid action of
amitrole. However, according to the authors amitrole has much more
effect on the thyroid than 3-amino-5-mercapto-triazole (Grunow et al.,
1975).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
The LD50 of amitrole for Drosophila was 40 ppm in the medium, while
with 10 ppm a prolongation of development time was found. Therefore no
optimal concentrations for a mutagenicity test could be used. With 10
ppm in the medium no mutagenic effects were observed in the sex
chromosome non-disjunction test (females) or the sex-linked recessive
lethal test (males) (Laamanen et al., 1976).
Effects of amitrole on human leucocytes in culture were investigated.
The cell growth was inhibited in concentrations of 0.2%w/v and higher.
Selected metaphases were examined for the presence of chromosome and
chromatid aberrations. No breakage or other visible chromosome damage
has been found (Meretoja at al., 1976).
With bacteria two types of tests were carried out. A DNA repair test
was done with E. coli, B. subtilis and S. typhimurium strains.
No mutagenic effect was noted.
Negative results were also obtained in a revertant test with several
strains of Salmonella. In both types of test known mutagens were
used as positive controls (Bamford et al., 1976),
Special studies on carcinogenicity
40 female Wistar rats were given 2500 ppm amitrole in the drinking
water during their life-time. All animals surviving 30-70 weeks were
studied. In all animals goitre was observed. After 30 weeks the
follicles became small and stromal tissue became fibrotic. These small
follicles contained little or no colloid. The proliferating follicular
tissue gave invasive lesions in 19 of the 26 rats.
In 3 of the animals nodules of papillary adenoma were found. In the
liver two cases of cholangiofibrosis, clue to irregular proliferation
of the bile ducts and surrounding stroma, were found (Tsuda et al.,
1976).
Special studies on the antithyroid effect
Two dosages of amitrole (0.5 g/kg and 1.0 g/kg) were administered
intraperitoneally to chicks, daily from day 3-40 after hatching. The
animals showed growth inhibition and an increased thyroid/body weight
ratio, compared with the controls.
In another experiment chicks were injected up to day 20 post-hatching
at which time treatment with the drug was discontinued. The animals
were sacrificed at day 27, 34, and 41. When the amitrole treatment was
stopped, the thyroid/body weight ratio decreased markedly, but never
to the same levels which were obtained in the controls.
Histologically, the characteristic changes reported for other
goitrogens were observed in the thyroid (Wishe, 1976).
Short-term studies
Rat
Several short-term experiments were carried out in order establish a
no-effect level on thyroid function tests. In all experiments the
uptake of 131I by the thyroid was measured in an in vivo test, 6, 24
and 48 hours after administration of 0.6 Ác 131I per animal
intraperitoneally. In addition thyroid weight and PBI (protein bound
iodine) were measured and the thyroid was studied histopathologically.
In the first experiment 4 groups of 8 female Wistar rats received
respectively 0, 2, 20 and 200 ppm of amitrole in the diet during 6
weeks. After 5 days and 6 weeks the uptake of 131I was measured. At
both times a significantly increased uptake was found in the 200 ppm
group 6 hours after injection, which decreased rather rapidly after 24
and 48 hrs. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. In the two
lower dosages no significant effects were found.
In the second experiment in which 8 female animals per group received
respectively 0, 20, 50 and 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition a significantly decreased PBI
was observed at the end of the experiment. With 50 ppm in the diet for
6 weeks a statistically increased uptake was found 6 hours after
injection of 131I. In this case the radioactivity in the thyroid
remained higher than the controls after 24 and 48 hrs.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment 0, 20, 50 and 200 ppm were given to 10 animals
per group during 13 weeks. The uptake of 131I by the thyroid was
significantly increased at 200 and 50 ppm after 6 and 12 weeks. The
difference between the groups was that with 50 ppm the radioactivity
in the thyroid remained high after 24 and 48 hrs., whereas with 200
ppm a very high uptake was found 6 hrs. after injection of 131I, but a
rapid decrease still lower than the controls after 48 hrs. With 200
ppm the PBI was decreased and the thyroid/body weight ration increased
by a factor of 6. With 50 ppm only a slightly increased relative
thyroid weight was found. Histologically a strong activation and
goitre were found with 200 ppm a slight activation with 50 ppm. In
this experiment a tendency to a higher uptake of 131I was found in the
20 ppm group.
The above mentioned experiments were carried out with a relatively low
iodine content in the diet (about 0.2-0.3 ppm). In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment 8 female rats per group received respectively 0, 20, 50,
200 and 500 ppm in the diet for 6 weeks, to see whether iodine could
protect against the anti-hyroid action of amitrole. With 500 ppm a
small increase in iodine uptake was found 5 hrs. after 131I injection,
but thereafter a very rapid decrease. With 200 ppm the uptake was much
higher and the same type of decrease was found as in the other
experiments, whereas with 50 ppm a significantly increased thyroid
radioactivity was found at all times. The 131I uptake of all animals
was of course much lower than in the increased and PBI only decreased
at 200 and 500 ppm. Histopathologically goitre and strongly activated
thyroids were found only at the two highest dosed levels. Some
activation was found in the 50 ppm group and a very slight activation
was also found in the 20 ppm group. (Den Tonkelaar and Kroest 1974)
COMMENTS
Several new studies on mutagenicity became available. No mutagenic
action could be demonstrated. In a recent carcinogenicity study with a
very high dose level, which had an influence on survival, a
carcinogenic action on the thyroid was found. In addition, two cases
of cholangiofibrosis in the liver have been observed. Two metabolites
of amitrole have been identified after oral administration to rats.
In four short-term studies the main effect was an increased uptake of
radioactive iodine by the thyroid at dose levels of 50, 200 and 500
ppm. This uptake correlated well with the histologically observed
activation. With 20 ppm only a very marginal effect was found while 2
ppm was without effect.
This Meeting confirmed the existing conditional ADI for humans pending
consideration of the whole concept of conditional ADIs for humans at a
future meeting.
FURTHER WORK OR INFORMATION
Desirable
See FAO/WHO Pesticide residues in food. Report of the 1974 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. p. 31
REFERENCES
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole III. Microbial tests
Mutation Res 401, 197-202.
Den Tonkelaar, E.M. and Kroes, R. (1974) Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol. Unpublished
report National Institute of Public Health, Bilthoven, the
Netherlands, 164/74 Tox.
Grunow, W., Altmann, H.J. and B÷hme, CHR. (1975) Uber Den Stoffwechsel
von 3-Amino-1,2,3-triazol in Ratten. Arch. Toxicol. 34, 315-324.
Laamanen, J., Sorsa, M., Banford, D., Gripenberg, U. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole I. Drosophila tests.
Mutation Res. 40, 185-190.
Meretoja, T., Gripenberg, U., Bamford, B., Laamanen, I. and Sorsa, M.
(1976) Mutagenicity and toxicity of amitrole II. Human lymphocyte
culture tests. Mutation Res. 40, 191-196
Tsuda, H., Hananouchi, M., Tatematsu, M., Hirose, M., Hirao, K.,
Takahashi, M. and Ito, N. (1976) Tumorigenic effect of
3-amino-1-H-1,2,3 triazole on rat thyroid. J. Natl. Cancer Inst. 57,
861-864.
Wishe, H.J. (1976) The effect of aminotriazole on the thyroid gland
and development of the white leghorn chicks. Diss. Abstr. Int. B.,
37, 1066-1067. Cited in Rest. Abstr. 9, (1976).
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP: 1974/M/11; WHO Pesticide Residue Series, No. 4.
 Metabolite I has in its tautomeric form the -NH-CS-NH-group, which was
thought to be partly responsible for the antithyroid action of
amitrole. However, according to the authors amitrole has much more
effect on the thyroid than 3-amino-5-mercapto-triazole (Grunow et al.,
1975).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
The LD50 of amitrole for Drosophila was 40 ppm in the medium, while
with 10 ppm a prolongation of development time was found. Therefore no
optimal concentrations for a mutagenicity test could be used. With 10
ppm in the medium no mutagenic effects were observed in the sex
chromosome non-disjunction test (females) or the sex-linked recessive
lethal test (males) (Laamanen et al., 1976).
Effects of amitrole on human leucocytes in culture were investigated.
The cell growth was inhibited in concentrations of 0.2%w/v and higher.
Selected metaphases were examined for the presence of chromosome and
chromatid aberrations. No breakage or other visible chromosome damage
has been found (Meretoja at al., 1976).
With bacteria two types of tests were carried out. A DNA repair test
was done with E. coli, B. subtilis and S. typhimurium strains.
No mutagenic effect was noted.
Negative results were also obtained in a revertant test with several
strains of Salmonella. In both types of test known mutagens were
used as positive controls (Bamford et al., 1976),
Special studies on carcinogenicity
40 female Wistar rats were given 2500 ppm amitrole in the drinking
water during their life-time. All animals surviving 30-70 weeks were
studied. In all animals goitre was observed. After 30 weeks the
follicles became small and stromal tissue became fibrotic. These small
follicles contained little or no colloid. The proliferating follicular
tissue gave invasive lesions in 19 of the 26 rats.
In 3 of the animals nodules of papillary adenoma were found. In the
liver two cases of cholangiofibrosis, clue to irregular proliferation
of the bile ducts and surrounding stroma, were found (Tsuda et al.,
1976).
Special studies on the antithyroid effect
Two dosages of amitrole (0.5 g/kg and 1.0 g/kg) were administered
intraperitoneally to chicks, daily from day 3-40 after hatching. The
animals showed growth inhibition and an increased thyroid/body weight
ratio, compared with the controls.
In another experiment chicks were injected up to day 20 post-hatching
at which time treatment with the drug was discontinued. The animals
were sacrificed at day 27, 34, and 41. When the amitrole treatment was
stopped, the thyroid/body weight ratio decreased markedly, but never
to the same levels which were obtained in the controls.
Histologically, the characteristic changes reported for other
goitrogens were observed in the thyroid (Wishe, 1976).
Short-term studies
Rat
Several short-term experiments were carried out in order establish a
no-effect level on thyroid function tests. In all experiments the
uptake of 131I by the thyroid was measured in an in vivo test, 6, 24
and 48 hours after administration of 0.6 Ác 131I per animal
intraperitoneally. In addition thyroid weight and PBI (protein bound
iodine) were measured and the thyroid was studied histopathologically.
In the first experiment 4 groups of 8 female Wistar rats received
respectively 0, 2, 20 and 200 ppm of amitrole in the diet during 6
weeks. After 5 days and 6 weeks the uptake of 131I was measured. At
both times a significantly increased uptake was found in the 200 ppm
group 6 hours after injection, which decreased rather rapidly after 24
and 48 hrs. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. In the two
lower dosages no significant effects were found.
In the second experiment in which 8 female animals per group received
respectively 0, 20, 50 and 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition a significantly decreased PBI
was observed at the end of the experiment. With 50 ppm in the diet for
6 weeks a statistically increased uptake was found 6 hours after
injection of 131I. In this case the radioactivity in the thyroid
remained higher than the controls after 24 and 48 hrs.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment 0, 20, 50 and 200 ppm were given to 10 animals
per group during 13 weeks. The uptake of 131I by the thyroid was
significantly increased at 200 and 50 ppm after 6 and 12 weeks. The
difference between the groups was that with 50 ppm the radioactivity
in the thyroid remained high after 24 and 48 hrs., whereas with 200
ppm a very high uptake was found 6 hrs. after injection of 131I, but a
rapid decrease still lower than the controls after 48 hrs. With 200
ppm the PBI was decreased and the thyroid/body weight ration increased
by a factor of 6. With 50 ppm only a slightly increased relative
thyroid weight was found. Histologically a strong activation and
goitre were found with 200 ppm a slight activation with 50 ppm. In
this experiment a tendency to a higher uptake of 131I was found in the
20 ppm group.
The above mentioned experiments were carried out with a relatively low
iodine content in the diet (about 0.2-0.3 ppm). In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment 8 female rats per group received respectively 0, 20, 50,
200 and 500 ppm in the diet for 6 weeks, to see whether iodine could
protect against the anti-hyroid action of amitrole. With 500 ppm a
small increase in iodine uptake was found 5 hrs. after 131I injection,
but thereafter a very rapid decrease. With 200 ppm the uptake was much
higher and the same type of decrease was found as in the other
experiments, whereas with 50 ppm a significantly increased thyroid
radioactivity was found at all times. The 131I uptake of all animals
was of course much lower than in the increased and PBI only decreased
at 200 and 500 ppm. Histopathologically goitre and strongly activated
thyroids were found only at the two highest dosed levels. Some
activation was found in the 50 ppm group and a very slight activation
was also found in the 20 ppm group. (Den Tonkelaar and Kroest 1974)
COMMENTS
Several new studies on mutagenicity became available. No mutagenic
action could be demonstrated. In a recent carcinogenicity study with a
very high dose level, which had an influence on survival, a
carcinogenic action on the thyroid was found. In addition, two cases
of cholangiofibrosis in the liver have been observed. Two metabolites
of amitrole have been identified after oral administration to rats.
In four short-term studies the main effect was an increased uptake of
radioactive iodine by the thyroid at dose levels of 50, 200 and 500
ppm. This uptake correlated well with the histologically observed
activation. With 20 ppm only a very marginal effect was found while 2
ppm was without effect.
This Meeting confirmed the existing conditional ADI for humans pending
consideration of the whole concept of conditional ADIs for humans at a
future meeting.
FURTHER WORK OR INFORMATION
Desirable
See FAO/WHO Pesticide residues in food. Report of the 1974 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. p. 31
REFERENCES
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole III. Microbial tests
Mutation Res 401, 197-202.
Den Tonkelaar, E.M. and Kroes, R. (1974) Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol. Unpublished
report National Institute of Public Health, Bilthoven, the
Netherlands, 164/74 Tox.
Grunow, W., Altmann, H.J. and B÷hme, CHR. (1975) Uber Den Stoffwechsel
von 3-Amino-1,2,3-triazol in Ratten. Arch. Toxicol. 34, 315-324.
Laamanen, J., Sorsa, M., Banford, D., Gripenberg, U. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole I. Drosophila tests.
Mutation Res. 40, 185-190.
Meretoja, T., Gripenberg, U., Bamford, B., Laamanen, I. and Sorsa, M.
(1976) Mutagenicity and toxicity of amitrole II. Human lymphocyte
culture tests. Mutation Res. 40, 191-196
Tsuda, H., Hananouchi, M., Tatematsu, M., Hirose, M., Hirao, K.,
Takahashi, M. and Ito, N. (1976) Tumorigenic effect of
3-amino-1-H-1,2,3 triazole on rat thyroid. J. Natl. Cancer Inst. 57,
861-864.
Wishe, H.J. (1976) The effect of aminotriazole on the thyroid gland
and development of the white leghorn chicks. Diss. Abstr. Int. B.,
37, 1066-1067. Cited in Rest. Abstr. 9, (1976).
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP: 1974/M/11; WHO Pesticide Residue Series, No. 4.
Metabolite I has in its tautomeric form the -NH-CS-NH-group, which was
thought to be partly responsible for the antithyroid action of
amitrole. However, according to the authors amitrole has much more
effect on the thyroid than 3-amino-5-mercapto-triazole (Grunow et al.,
1975).
TOXICOLOGICAL STUDIES
Special studies on mutagenicity
The LD50 of amitrole for Drosophila was 40 ppm in the medium, while
with 10 ppm a prolongation of development time was found. Therefore no
optimal concentrations for a mutagenicity test could be used. With 10
ppm in the medium no mutagenic effects were observed in the sex
chromosome non-disjunction test (females) or the sex-linked recessive
lethal test (males) (Laamanen et al., 1976).
Effects of amitrole on human leucocytes in culture were investigated.
The cell growth was inhibited in concentrations of 0.2%w/v and higher.
Selected metaphases were examined for the presence of chromosome and
chromatid aberrations. No breakage or other visible chromosome damage
has been found (Meretoja at al., 1976).
With bacteria two types of tests were carried out. A DNA repair test
was done with E. coli, B. subtilis and S. typhimurium strains.
No mutagenic effect was noted.
Negative results were also obtained in a revertant test with several
strains of Salmonella. In both types of test known mutagens were
used as positive controls (Bamford et al., 1976),
Special studies on carcinogenicity
40 female Wistar rats were given 2500 ppm amitrole in the drinking
water during their life-time. All animals surviving 30-70 weeks were
studied. In all animals goitre was observed. After 30 weeks the
follicles became small and stromal tissue became fibrotic. These small
follicles contained little or no colloid. The proliferating follicular
tissue gave invasive lesions in 19 of the 26 rats.
In 3 of the animals nodules of papillary adenoma were found. In the
liver two cases of cholangiofibrosis, clue to irregular proliferation
of the bile ducts and surrounding stroma, were found (Tsuda et al.,
1976).
Special studies on the antithyroid effect
Two dosages of amitrole (0.5 g/kg and 1.0 g/kg) were administered
intraperitoneally to chicks, daily from day 3-40 after hatching. The
animals showed growth inhibition and an increased thyroid/body weight
ratio, compared with the controls.
In another experiment chicks were injected up to day 20 post-hatching
at which time treatment with the drug was discontinued. The animals
were sacrificed at day 27, 34, and 41. When the amitrole treatment was
stopped, the thyroid/body weight ratio decreased markedly, but never
to the same levels which were obtained in the controls.
Histologically, the characteristic changes reported for other
goitrogens were observed in the thyroid (Wishe, 1976).
Short-term studies
Rat
Several short-term experiments were carried out in order establish a
no-effect level on thyroid function tests. In all experiments the
uptake of 131I by the thyroid was measured in an in vivo test, 6, 24
and 48 hours after administration of 0.6 Ác 131I per animal
intraperitoneally. In addition thyroid weight and PBI (protein bound
iodine) were measured and the thyroid was studied histopathologically.
In the first experiment 4 groups of 8 female Wistar rats received
respectively 0, 2, 20 and 200 ppm of amitrole in the diet during 6
weeks. After 5 days and 6 weeks the uptake of 131I was measured. At
both times a significantly increased uptake was found in the 200 ppm
group 6 hours after injection, which decreased rather rapidly after 24
and 48 hrs. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. In the two
lower dosages no significant effects were found.
In the second experiment in which 8 female animals per group received
respectively 0, 20, 50 and 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition a significantly decreased PBI
was observed at the end of the experiment. With 50 ppm in the diet for
6 weeks a statistically increased uptake was found 6 hours after
injection of 131I. In this case the radioactivity in the thyroid
remained higher than the controls after 24 and 48 hrs.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment 0, 20, 50 and 200 ppm were given to 10 animals
per group during 13 weeks. The uptake of 131I by the thyroid was
significantly increased at 200 and 50 ppm after 6 and 12 weeks. The
difference between the groups was that with 50 ppm the radioactivity
in the thyroid remained high after 24 and 48 hrs., whereas with 200
ppm a very high uptake was found 6 hrs. after injection of 131I, but a
rapid decrease still lower than the controls after 48 hrs. With 200
ppm the PBI was decreased and the thyroid/body weight ration increased
by a factor of 6. With 50 ppm only a slightly increased relative
thyroid weight was found. Histologically a strong activation and
goitre were found with 200 ppm a slight activation with 50 ppm. In
this experiment a tendency to a higher uptake of 131I was found in the
20 ppm group.
The above mentioned experiments were carried out with a relatively low
iodine content in the diet (about 0.2-0.3 ppm). In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment 8 female rats per group received respectively 0, 20, 50,
200 and 500 ppm in the diet for 6 weeks, to see whether iodine could
protect against the anti-hyroid action of amitrole. With 500 ppm a
small increase in iodine uptake was found 5 hrs. after 131I injection,
but thereafter a very rapid decrease. With 200 ppm the uptake was much
higher and the same type of decrease was found as in the other
experiments, whereas with 50 ppm a significantly increased thyroid
radioactivity was found at all times. The 131I uptake of all animals
was of course much lower than in the increased and PBI only decreased
at 200 and 500 ppm. Histopathologically goitre and strongly activated
thyroids were found only at the two highest dosed levels. Some
activation was found in the 50 ppm group and a very slight activation
was also found in the 20 ppm group. (Den Tonkelaar and Kroest 1974)
COMMENTS
Several new studies on mutagenicity became available. No mutagenic
action could be demonstrated. In a recent carcinogenicity study with a
very high dose level, which had an influence on survival, a
carcinogenic action on the thyroid was found. In addition, two cases
of cholangiofibrosis in the liver have been observed. Two metabolites
of amitrole have been identified after oral administration to rats.
In four short-term studies the main effect was an increased uptake of
radioactive iodine by the thyroid at dose levels of 50, 200 and 500
ppm. This uptake correlated well with the histologically observed
activation. With 20 ppm only a very marginal effect was found while 2
ppm was without effect.
This Meeting confirmed the existing conditional ADI for humans pending
consideration of the whole concept of conditional ADIs for humans at a
future meeting.
FURTHER WORK OR INFORMATION
Desirable
See FAO/WHO Pesticide residues in food. Report of the 1974 Joint
Meeting of the FAO Working Party of Experts on Pesticide Residues and
the WHO Expert Committee on Pesticide Residues. p. 31
REFERENCES
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole III. Microbial tests
Mutation Res 401, 197-202.
Den Tonkelaar, E.M. and Kroes, R. (1974) Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol. Unpublished
report National Institute of Public Health, Bilthoven, the
Netherlands, 164/74 Tox.
Grunow, W., Altmann, H.J. and B÷hme, CHR. (1975) Uber Den Stoffwechsel
von 3-Amino-1,2,3-triazol in Ratten. Arch. Toxicol. 34, 315-324.
Laamanen, J., Sorsa, M., Banford, D., Gripenberg, U. and Meretoja, T.
(1976) Mutagenicity and toxicity of amitrole I. Drosophila tests.
Mutation Res. 40, 185-190.
Meretoja, T., Gripenberg, U., Bamford, B., Laamanen, I. and Sorsa, M.
(1976) Mutagenicity and toxicity of amitrole II. Human lymphocyte
culture tests. Mutation Res. 40, 191-196
Tsuda, H., Hananouchi, M., Tatematsu, M., Hirose, M., Hirao, K.,
Takahashi, M. and Ito, N. (1976) Tumorigenic effect of
3-amino-1-H-1,2,3 triazole on rat thyroid. J. Natl. Cancer Inst. 57,
861-864.
Wishe, H.J. (1976) The effect of aminotriazole on the thyroid gland
and development of the white leghorn chicks. Diss. Abstr. Int. B.,
37, 1066-1067. Cited in Rest. Abstr. 9, (1976).
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP: 1974/M/11; WHO Pesticide Residue Series, No. 4.
