
METHAMIDOPHOS JMPR 1976
IDENTITY
Chemical name
OS-dimethyl phosphoramidothioate
Synonyms
Tamaron R, Monitor R, SRA 5172, Bayer 71 628, RE 9006
Structural formula
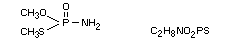 Other information on identity and properties
Molecular weight: 141.1
State: yellowish to colourless crystals
(technical)
colourless crystals (pure)
Melting point: 37 - 39°C (technical)
44.5°C (pure)
Vapour pressure: 3 x 10-4 mm Hg at 30°C (pure)
Density: 1.31
Solubility: Readily soluble in water, alcohols
ketones, aliphatic chlorinated
hydrocarbons; sparingly soluble
in ether; practically insoluble
in petroleum ether.
Stability (alkaline Half-life of 120 hours at pH 9
and acidic conditions and 37°C Half-life of 140 hours at
pH 2
and 40°C
Impurities in the technical product
Maximum level, %
Total impurities 27
OO-Dimethyl phosphoramidothioate 12
Other methyl esters of phosphoramido
and phosphoro-N-methylamidothioates 12
Methyl esters of phosphoric and
phosphorothioic acids 8
Phosphate, sulphate, sulphonium ion,
water 8
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male and female rats were orally administered methamidophos
(32p-labelled) following a two-week preconditioning period where
a daily dose of 0.5 mg/kg was orally administered. Male rats only
were administered 14C-methamidophos with no preconditioning at the
same dose level. In the 14C study (label = S-CH3) absorption and
a distribution was rapid. Elimination of 14C02 within the first
6 hours accounted for 32% of the recovered radioactivity with 9% of
the dose in the urine. Over a 5-day period only 50% of the
administered dose was recovered (39% as 14C02 and the rest
predominantly in urine). The remainder of the 14C was probably
present as residual radioactivity in the body, distributed
randomly. The major tissue residue was found in the polar lipid
fraction with lesser amounts as phospholipids and proteins
(probably incorporated as natural products). Studies with 32p
confirm the rapid absorption and distribution of radioactivity in
rats. The major amount of material recovered following 32p labelled
methamidophos dosing was observed in urine within 1 day. Feces
contained small quantities that were continually excreted (<20% of
the dose was excreted in feces over a 28-day test interval). Thus,
methamidophos is rapidly absorbed and distributed in the body.
Methamidophos and metabolites are excreted predominantly as urinary
products (Crossley and Tutass, 1969).
The metabolic fate of methamidophos in a lactating ruminant
(goat) was essentially the same as reported for the rat. Small
amounts of radioactivity were observed in milk, predominantly as
the parent molecule shortly after dosing. About 1% of the
radioactivity (<0.003 mg/kg) in the milk was methamidophos, which
disappeared rapidly. Distribution of the applied radioactivity in
the goat was rapid, predominantly as exhaled 14C02, in urine
and as radioactivity incorporated into some tissues (muscle, liver,
fat). 24% of the administered radioactivity was excreted within the
dosing period. In tissues, the predominant residue was not the
parent compound as evidenced by solvent partition characteristics
(Lee and Crossley, 1972).
Further details of the fate of metamidophos in the rat and
lactating goat are given in the section "Fate of residues".
Biotransformation
Methamidophos is rapidly degraded primarily by cleavage of the
P-N bond yielding dimethyl phosphoric acid derivatives. Microsomal
preparations in vitro accelerated the hydrolysis of the
phosphoramidate bond (Tutass, 1968a). A qualitative sequence of
metabolism is as follows:
Other information on identity and properties
Molecular weight: 141.1
State: yellowish to colourless crystals
(technical)
colourless crystals (pure)
Melting point: 37 - 39°C (technical)
44.5°C (pure)
Vapour pressure: 3 x 10-4 mm Hg at 30°C (pure)
Density: 1.31
Solubility: Readily soluble in water, alcohols
ketones, aliphatic chlorinated
hydrocarbons; sparingly soluble
in ether; practically insoluble
in petroleum ether.
Stability (alkaline Half-life of 120 hours at pH 9
and acidic conditions and 37°C Half-life of 140 hours at
pH 2
and 40°C
Impurities in the technical product
Maximum level, %
Total impurities 27
OO-Dimethyl phosphoramidothioate 12
Other methyl esters of phosphoramido
and phosphoro-N-methylamidothioates 12
Methyl esters of phosphoric and
phosphorothioic acids 8
Phosphate, sulphate, sulphonium ion,
water 8
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male and female rats were orally administered methamidophos
(32p-labelled) following a two-week preconditioning period where
a daily dose of 0.5 mg/kg was orally administered. Male rats only
were administered 14C-methamidophos with no preconditioning at the
same dose level. In the 14C study (label = S-CH3) absorption and
a distribution was rapid. Elimination of 14C02 within the first
6 hours accounted for 32% of the recovered radioactivity with 9% of
the dose in the urine. Over a 5-day period only 50% of the
administered dose was recovered (39% as 14C02 and the rest
predominantly in urine). The remainder of the 14C was probably
present as residual radioactivity in the body, distributed
randomly. The major tissue residue was found in the polar lipid
fraction with lesser amounts as phospholipids and proteins
(probably incorporated as natural products). Studies with 32p
confirm the rapid absorption and distribution of radioactivity in
rats. The major amount of material recovered following 32p labelled
methamidophos dosing was observed in urine within 1 day. Feces
contained small quantities that were continually excreted (<20% of
the dose was excreted in feces over a 28-day test interval). Thus,
methamidophos is rapidly absorbed and distributed in the body.
Methamidophos and metabolites are excreted predominantly as urinary
products (Crossley and Tutass, 1969).
The metabolic fate of methamidophos in a lactating ruminant
(goat) was essentially the same as reported for the rat. Small
amounts of radioactivity were observed in milk, predominantly as
the parent molecule shortly after dosing. About 1% of the
radioactivity (<0.003 mg/kg) in the milk was methamidophos, which
disappeared rapidly. Distribution of the applied radioactivity in
the goat was rapid, predominantly as exhaled 14C02, in urine
and as radioactivity incorporated into some tissues (muscle, liver,
fat). 24% of the administered radioactivity was excreted within the
dosing period. In tissues, the predominant residue was not the
parent compound as evidenced by solvent partition characteristics
(Lee and Crossley, 1972).
Further details of the fate of metamidophos in the rat and
lactating goat are given in the section "Fate of residues".
Biotransformation
Methamidophos is rapidly degraded primarily by cleavage of the
P-N bond yielding dimethyl phosphoric acid derivatives. Microsomal
preparations in vitro accelerated the hydrolysis of the
phosphoramidate bond (Tutass, 1968a). A qualitative sequence of
metabolism is as follows:
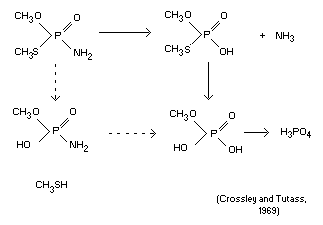 (Crossley and Tutass, 1969)
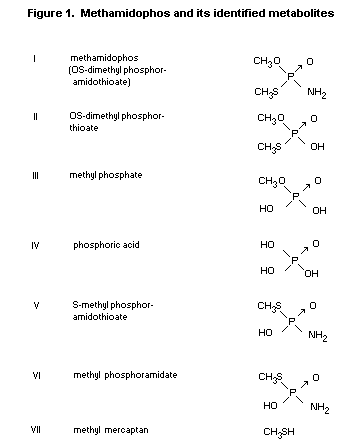
A list of the identified metabolites of methamidophos is given
in Figure 1.
Effects on enzymes and other biochemical parameters
Methamidophos is an active, direct cholinesterase inhibitor.
In blood, cholinesterase depression was observed to be maximum
within 3 hours following oral acute intoxication of male rats.
Cholinesterase activity was maintained at a low level for at least
24 hours following acute poisoning. Three days after treatment, the
enzyme activity of whole blood returned to a level that was almost
normal (Lorke and Kimmerle, 1967).
(Crossley and Tutass, 1969)
A list of the identified metabolites of methamidophos is given
in Figure 1.
Effects on enzymes and other biochemical parameters
Methamidophos is an active, direct cholinesterase inhibitor.
In blood, cholinesterase depression was observed to be maximum
within 3 hours following oral acute intoxication of male rats.
Cholinesterase activity was maintained at a low level for at least
24 hours following acute poisoning. Three days after treatment, the
enzyme activity of whole blood returned to a level that was almost
normal (Lorke and Kimmerle, 1967).
 RBC cholinesterase appears to be more sensitive than plasma
cholinesterase to inhibition as noted by the differing I50 values.
I50(M)
Bovine RBC 3.1 x 10-5
Human Plasma 1.6 x 10-4
(Tucker, 1972)
The sensitivity of RBC relative to plasma cholinesterase to
inhibition by methamidophos was noted in a 3-week dietary study
where methamidophos was fed at 0 and 10 ppm. No cumulative enzyme
depression was observed and RBC was affected slightly more than
plasma (Cavalli and Spence, 1970;) [see acephate - Cavalli and
Spence, 1970f, summary of the data in addendum].
TOXICOLOGICAL STUDIES
Special studies on delayed neurotoxicity
Groups of hens (10 mature laying hens - 1-2 years old/group)
were fed methamidophos in the diet (dosage levels were said to be
0, 0.3, and 3.0 mg/kg body weight) for four weeks. There was a
control of TOCP fed at a dose of 50 mg/kg body weight. No mortality
or severe weight loss was observed and clinical signs of ataxia
were not noted. The TOCP-treated hens developed clinical signs of
ataxia. Histological examination of nervous tissue showed no
abnormalities, even in the TOCP control group (Fletcher et al.,
1971).
In two trials, groups of adult hens (varying in number from 2
to 10/group) were administered methamidophos once orally or by
intraperitoneal injection in the presence or absence of 2-PAM and
atropine. The test protocol used doses equal to and exceeding the
LD50 values in an attempt to establish the neurotoxic potential for
methamidophos. Although mortality was observed, no clinical signs
of delayed neurotoxicity were evident. Histopathological
examinations were not performed (Lorke and Kimmerle, 1967).
A group of six hens each were administered methamidophos
orally at an LD50 dose level of 27.5 mg/kg, observed for 21 days,
re-treated with the same dose and maintained for an additional 21
days. Two separate control groups were used - an untreated control
and a TOCP (500 mg/kg, oral dose) positive control. While some of
the hens (2/6) died from acute toxicity, the survivors of the
methamidophos treatment did not lose weight or show signs of
delayed ataxia. The TOCP treatment group lost weight and showed
positive signs of ataxia within 14 days of poisoning.
Histopathology examination was not performed (Wolvin, et al.,
1968).
Special studies on reproduction
Rat
Groups of rats (8 male and 16 female/group) were fed
methamidophos in the diet at dose levels of 0, 3, 10, and 30 ppm
for 100 days and mated to begin a standard 3 generation, 2
litter/generation reproduction study. Body weights of parental
males (but not females) were reduced but the reduction was slight
and not dose-related. Cholinesterase activity values of the female
animals were measured after the mating trials were concluded. RBC
and plasma cholinesterase depression was evident at 30 ppm in males
and females (male plasma was also depressed at 10 ppm). There were
no significant effects noted on gross or microscopic examinations
of tissues and organs.
Reproductive indices were generally affected at the 30 ppm
dose level. The incidence of pregnancy (fertility index) incidence
of parturition (gestation index) and viability and lactation
indices were reduced at 30 ppm. Numbers of stillborn and
cannibalized pups were increased at the 30 ppm dose but no
suggestions of terata were presented. Histopathologic examination
of the progeny did not reveal any abnormalities. No effects on
reproduction were observed at 10 ppm (Arnold et al., 1970).
Special studies on teratogenesis
Groups of pregnant rabbits (from 17-23 does/group) were
administered methamidophos by gelatin capsule from day 6 through
day 18 of gestation at dosage levels of 0, 0.1, and 0.3 mg/kg. A
positive control (thalidomide, 37.5 mg/kg) was administered to a
group of 27 pregnant does. All animals were sacrificed on day 29 of
gestation. Positive teratogenic signs were observed as expected
with thalidomide. No effects were noted as a result of
administration of methamidophos on growth of does or on fetal
mortality. Fetus weight was normal and skeletal development was
unaffected. One of 63 pups at the 0.3 mg/kg dose level displayed
talipomanus, club hand. This malformation was not observed in 75-79
pups at the low dose or in the controls, but it occurred in 8 of 85
in the positive controls (Ladd et al., 1971). There were no other
obvious defects noted in the study with methamidophos. A larger
number of defects were noted with thalidomide (umbilical hernia,
harelip, cervical elongation, frontal fontanel) that were not seen
with methamidophos. Skeletal examinations of the pups from
methamidophos-treated does were normal.
Special studies on mutagenicity
Groups of mice (12 males/group) were administered
methamidophos by intraperitoneal injection at dosage levels of 0,
1, and 2 mg/kg and mated to virgin females for 6 consecutive weeks
in a dominant lethal test. A positive control group administered
ethylmethane sulfonate (EMS, 400 mg/kg) was included in the study.
Females sacrificed one week after breeding with the treated males
were examined for pregnancy and resorption of fetuses.
Mutagenicity, measured by comparing viable embryos in the test and
control groups, was not evident with methamidophos treatment. A
mutagenic response to EMS was indicated in the first two weeks of
the test (Arnold et al., 1971).
Results of in vitro tests using Salmonella and
Escherichia Spp tester strains were negative when methamidophos was
examined (Hanna and Dyer, 1975).
Special studies on antidotes
Antidotal effects were noted with atropine and atropine +
2-PAM combination administered following intoxication. BH6 and
2-PAM alone were not effective antidotes. Atropine + PAM was more
effective than atropine alone but less effective than atropine +
BH6 (Lorke and Kimmerle, 1967).
The acute oral toxicity of methamidophos in male rats was
reduced 3-fold when animals were administered atropine alone or in
combination with 2-PAM. While 2-PAM alone was not as therapeutic as
atropine it reduced the toxicity by more than two-fold (Schoenig et
al., 1968).
Special studies on potentiation
Simultaneous administration of methamidophos to rats either by
oral gavage or intraperitoneal injection in combination with
malathion showed a significant potentiation (acute interaction in
the toxicity as measured by reduction of the LD50 value). Graduated
doses of malathion and methamidophos by oral administration
resulted in a greater than two-fold potentiation with malathion.
(Gröning, 1975). A slightly less than two-fold increase was
observed when ip injection was the route of administration used
(Crawford and Anderson, 1973). Oral administration of methamidophos
in combination with parathion or EPN did not result in potentiation
of the acute toxicity (Gröning, 1975).
Acute toxicity
TABLE 1. Acute toxicity of methamidophos
Species Sex Route LD50 Reference
(mg/kg)
Rat M Oral 15.6-32.3 Lorke & Kimmerle,
1967; Cavalli
et al., 1968a;
1968b; 1968g.
F Oral 13.0-29.6
M ip 21.3 Lorke & Kimmerle.
1967
F ip 26.4 Lorke & Kimmerle,
1967
TABLE 1. (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
Rat M Dermal
4-hr exp. 110 Lorke & Kimmerle,
7-day exp. 50 1967
Inhalation
1-hr exp. 0.525 mg/L Lorke & Kimmerle,
4-hr exp. 0.162 mg/L 1967
Guinea Pig Oral 30-50 Lorke & Kimmerle,
1967
Mice Oral 16.2 Cavalli et al.,
(Technical) 1968c
Oral (75% 18.0 Cavalli et al.,
Technical) 1968d
Rabbit Oral 10-30 Lorke & Kimmerle,
1967
Dermal 118-125 Cavalli et al.,
1968e; 1968f
Cat Oral 10-30 Lorke & Kimmerle,
1967
Dog Oral 10-30 Lorke & Kimmerle,
1967
Chicken F Oral 25 Lorke & Kimmerle,
1967
F ip 10 Lorke & Kimmerle,
1967
The observed signs of poisoning were typical of
anticholinesterase agents. The signs appeared rapidly following
poisoning (5-20 minutes) and disappeared in 4-6 days. Typical
parasympathomimetic signs of poisoning would be expected.
TABLE 2. Acute toxicity of impurities
LD50
Compound Species Sex Route (mg/kg) Reference
(CH3O)2P(S) Rat M Oral 633 Cavalli et al., 1968h
NH2 (RE9169) F Oral 549 Cavalli et al., 1968h
Signs of poisoning were typical of CNS depression
- paresis, hyporeflexia and anesthesia.
Rabbit Dermal 2,500- Cavalli et al., 1969
5,000
(intact
skin)
1,570 Cavalli et al., 1969
(abraded
skin)
See the monograph on acephate for the toxicity of other products.
Short-term studies
Rat
Groups of rats (15 females/group) were administered methamidophos
orally or by intraperitoneal injection, 5 days/week for 60 days to
examine possible cumulative effects. Oral application consisted of
daily doses of 0, 1.5, 3.0, 6.0, 10.0, and 15.0 mg/kg while ip
application consisted of doses of 0, 1.3, 2.6, 5.3, 8.8, and 13.2
mg/kg. While slight mortality was observed (oral = 3/15, ip = 6/15)
there was no evidence of cumulative action. The cumulative LD50 by
either oral or ip administration was estimated to be > 15 mg/kg body
weight (Lorke & Kimmerle, 1967).
Groups of rats (30 females/treatment group - 90 females were used
as controls) were fed methamidophos in the diet at dosage levels of 0,
0.25, 0.50, 1.00, 2.00 and 4.00 ppm for 13 weeks (a further 4-week
recovery period using a control diet followed this test interval).
There were no deaths or adverse behavioral reactions. Growth, as
evidenced by body weight gain, was normal at all treatment levels.
Cholinesterase depression of plasma, RBC and brain was reported only
in the high dose group.
At 4.0 ppm depression of enzyme activity was obvious in plasma
and RBC, less so in brain. A comparison of the total depression of
cholinesterase activity and recovery during the 4-week control diet
period suggests a no-effect level of 2.0 ppm (Reyna et al., 1973).
Groups of rats (35 males and 35 females/group) were fed
methamidophos in the diet for 13 weeks at dietary levels of 0, 0.3,
1.0, 3.0 and 10.0 ppm. Cholinesterase activity was examined at
periodic intervals using plasma, RBC, and brain tissue. Significant
dose-related depression of plasma cholinesterase activity was noted in
male rats at 3 and 10 ppm and in females at 10 ppm. Decreases in
cholinesterase activity of RBC and brain were noted at 3 and 10 ppm in
both sexes. RBC activity was depressed in both sexes at 10 ppm and in
males only, marginally depressed at 1 and 3 ppm. Recovery of enzyme
activity was noted after one week of control diets following 3 ppm and
at 4 weeks of control diets following 10 ppm (Plank et al., 1969).
Groups of rats (15 males and 15 females/group; 30 of each sex
were used as controls) were fed methamidophos in the diet at dosage
levels of 0, 2, 6, 20, and 60 ppm for 3 months. No mortality or
behavior changes were observed although growth was depressed
significantly at 60 ppm in both sexes. Blood chemistry, hematology,
and urine analysis parameters were normal (bilirubin content of males
only at 20 and 60 ppm was depressed at 90 days, and OCT
(Ornithinecarbamoyl transferase) activity was depressed in females fed
60 ppm at 90 days. These unusual effects were reported to be within
physiological limits). Cholinesterase activity was depressed at 6 ppm
and above in both plasma and RBC (RBC was slightly more sensitive). No
effects were noted at 2 ppm over the 13 week feeding interval. Gross,
macroscopic examination of tissues and organs showed a reduction in
both males and females in the size of several organs; thymus, liver,
spleen, adrenals, and gonads only at 60 ppm. There were no effects
noted at 20 ppm. Microscopic examination of the tissues did not show
any adverse effects related to the presence of methamidophos in the
diet. A no-effect level based on cholinesterase depression is 2 ppm in
the diet (Löser and Lorke, 1970b; Gröning, 1976).
Rabbit
Groups of rabbits (5 males and 5 females/group) were administered
methamidophos dermally to intact or abraded skin at dose levels of 0,
5, or 10 mg/kg (a 75% technical product). The application was made 6-7
hours/day, 5 days/ week for 3 weeks. Application was to a new site
each day. Mortality occurred in the 10 mg/kg abraded skin group
accompanied by signs of cholinergic stimulation. Hematologic studies,
blood chemistry and urinalysis parameters were unaffected by dermal
application of methamidophos. Gross and microscopic analyses of
tissues and organs were uneventful. Dermatologic reactions, such as
erythema, were minor. The affected skin returned to normal within 1-2
days. Growth was slightly retarded at the 10 mg/kg dose group but was
not accompanied by other adverse signs of poisoning (Mastri et al.,
1968).
Dog
Groups of dogs (3 male and 3 female/group) were administered
methamidophos orally by capsule daily, seven days per week, for 90
days at dosage levels of 0, 0.025, 0.075, and 0.25 mg/kg body weight.
There was no mortality. Growth and food consumption were unaffected.
Red blood cell and plasma cholinesterase activity, examined at several
intervals both before and during treatment, was normal. There was no
depression of cholinesterase noted in this study (Carlson et al.,
1969).
Groups of dogs (3 male and 3 female/group) were used in a repeat
of the above test to examine the 90-day subacute anticholinesterase
effects of methamidophos. Growth was reduced in males at the highest
dose level. In females, a reduced weight gain was observed at all dose
levels. Food consumption was reduced at the highest dose level in both
sexes. Depressed erythrocyte cholinesterase was observed at 0.25 mg/kg
in both males and females. No effects were noted on plasma enzymes. A
no-effect level based on RBC cholinesterase is 0.075 mg/kg (Lindberg
et al., 1970).
Groups of dogs (2 male and 2 female/group) were administered
methamidophos by capsule for periods varying from 21-28 days, at doses
ranging from 0 to 0.6 mg/kg/day. In this study each dog was examined
for 34 weeks to determine a pre-treatment baseline for cholinesterase
activity. Animals were then administered a dose of 0.025 mg/kg for 28
days after which the dose was increased to 0.05 mg/kg. The total
treatment sequence was as follows: 0.025, 0.05, 0.075, and 0.10 mg/kg
each administered for 28 days, followed by 0.125, 0.20, 0.30, 0.40,
0.50, and 0.60 mg/kg each administered for 21 days, after which a
control diet was administered for 28 days. Cholinesterase activity was
measured several times (generally at weekly intervals) during the
course of treatment. In both males and females a dose level was
reached where plasma and RBC cholinesterase activity was depressed. In
males, plasma and RBC cholinesterase was depressed when the dose
regimen reached 0.125 mg/kg. In females, plasma was depressed at a
dose of 0.3 mg/kg while RBC was affected at approximately 0.2 mg/kg.
Based on these data, no effects on cholinesterase would be expected to
occur at levels around 0.1 mg/kg/day. In this study, depression was
considered positive when the average value for cholinesterase activity
declined below the lowest range of the pre-treatment mean (Greco et
al., 1971).
Groups of dogs (2 male and 2 female beagles/group), 3 of each sex
were used as controls) were fed diets containing methamidophos at
dosage levels of 0, 1.5, 5, and 15 ppm for 90 days. There was no
mortality or behavioral change noted over the course of treatment.
Growth and food consumption were normal. There were no effects noted
in blood chemistry, hematology or urinalysis parameters.
Cholinesterase depression (RBC and plasma) was noted at 5 ppm and
above in both sexes (within the first week of dietary administration
at the high dose and after 2 months at the intermediate dose). Gross
macroscopic examination of tissues and calculation of relative organ
weights did not indicate any effects at the highest treatment level. A
dietary no-effect level in this study is 1.5 ppm (Löser and Lorke,
1970a)
Histopathological examination of tissues and organs showed no
effects of methamidophos (Gröning and Lorke, 1976).
Groups of dogs (3 male and 3 female beagles/group) were
administered methamidophos by capsule, orally, for two years at dosage
levels of 0, 0.075, 0.25 and 0.75 mg/kg. There were no deaths or
observable differences in behavior over the two-year study. Growth was
unaffected by methamidophos. There were no effects noted on clinical
chemistry, hematology, or urinalysis parameters recorded.
Cholinesterase activity was not reported. Gross and microscopic
examination of tissues and organs showed no abnormalities (Lindberg et
al., 1969).
Long-term studies
Rat
Groups of rats (45 males and 45 females/group) were fed
methamidophos in the diet at dosage levels of 0, 3, 10, and 30 ppm for
two years. Growth was reduced at the high dose level in males. Growth
in females was unaffected in this study. A separate paired feeding
study (21-day) was conducted where it was noted that growth was
unaffected at 30 ppm. Examination of food consumption data suggested
this effect to be due to diet rejection rather than a direct effect of
methamidophos in the diet. (Plank et al., 1968). In the long-term
study, there were no effects reported on behavior, hematologic blood
chemistry or urinalysis parameters. Gross and microscopic examination
of tissues and organs at 12 or 24 months showed no abnormalities
attributed to methamidophos in the diet. The tumor incidence was
unaffected and a definitive, no-effect level based on somatic changes
would be greater than 30 ppm. Cholinesterase activity was not measured
in the study (Plank et al., 1970).
OBSERVATIONS IN MAN
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. The groups received either a
combination mixture of 1:4 (methamidophos:acephate), 1:9 or a control
dose, daily (divided into 3 equal doses), for periods of 21 days. Each
group was administered the combination at a dose of 0.1 mg/kg/day for
21 days after which the dose was increased (to 0.2 mg/kg/day) and
continued for another 21 days. The protocol called for a total of 4
dose levels (up to 0.4 mg/kg/day) each to be administered for 21 days.
Blood cholinesterase (plasma and RBC) was examined periodically. No
effects were noted on RBC cholinesterase at any treatment level. There
was no substantial depression noted in the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The male
cohort was substantially affected at the 0.3 mg/kg dose and was not
retested at the higher dose level. The group (both males and females)
receiving the higher methamidophos:acephate ratio (1:4) showed
reduction of plasma cholinesterase after two weeks of receiving a dose
of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not used in the 1:4 dose
group. This study indicated the minimal dose necessary to decrease
plasma cholinesterase for the combination of acephate and
methamidophos was 0.2 mg/kg/day of a 1:4 combination of the two
toxicants. No effects with this combination were noted at 0.1 mg/kg
(Garofalo et al., 1973). See the monograph on acephate for a further
amplification of these data.
COMMENTS
Methamidophos, an anticholinesterase organophosphorus ester, is
also a metabolite of acephate. Methamidophos is rapidly absorbed,
distributed, metabolised and excreted in mammals. Bioaccumulation,
based on data on solubility properties, would not be expected to
occur. Biotransformation in mammals results in metabolites of
insignificant toxicological properties. Methamidophos is an acutely
toxic pesticide. The acute signs of poisoning can be relieved with the
aid of atropine (and reactivators, such as 2-PAM). In contrast to the
innocuous metabolites, methamidophos is an active cholinesterase
inhibitor, primarily affecting RBC (and presumably brain)
cholinesterase in animal models. In humans, plasma cholinesterase
appears to be the more sensitive enzyme parameter.
Methamidophos does not induce delayed neurotoxicity although
potentiation of the acute toxicity of malathion has been noted. In
reproduction studies, several parameters were affected at relatively
low levels (the levels were however sufficient to impart
cholinesterase depression in the parents). No terata were induced in
the reproduction study or in special teratological studies in rabbits,
although the dose levels used in this latter study were extremely low.
Mutagenicity tests were negative.
In short and long term studies no significant somatic effects
were noted. Cholinesterase depression was manifested in short term
studies. In 90 day dog studies, cholinesterase depression was observed
at 5 ppm while a dietary level of 1.5 ppm (equivalent to 0.04
mg/kg/day) caused no observable effects. In a 2 year dog study,
somatic effects were not noted at levels of 0.75 mg/kg/day but
cholinesterase activity was not determined. Depression of
cholinesterase activity in rat feeding studies was observed at dietary
levels of 3 ppm and above while 2 ppm was found to induce no
depression of cholinesterase activity. In a controlled study where a
combination dose of methamidophos and acephate was administered to a
group of male and female volunteers, cholinesterase depression was
observed predominantly when a higher methamidophos:acephate ratio was
used (1:4 rather than 1:9). Based on the results of this study a
no-effect level of 0.02 mg/kg was suggested. A higher total
concentration (0.2 mg/kg), administered in a lower ratio (1:9) also
gave no depression of enzyme activity. Based on the no-effect level of
0.04 mg/kg bw in dog an ADI was allocated. The no-effect level in
human studies was further assurance of the adequacy of the numerical
value of the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet equivalent to 0.1 mg/kg bw
Dog: 1.5 ppm in the diet equivalent to 0.04 mg/kg bw
Man: 0.02 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methamidophos is an organophosphorus insecticide with systemic
properties. It is a good stomach poison with further contact poison
action and has good residual activity. It is claimed to be effective
for the control of sucking pests such as aphids, whitefly and spider
mites as well as biting pests such as Laphygma, Prodenia, Trichoplusia
and others.
Methamidophos is used world-wide, chiefly in regions with a
subtropical climate. Formulations based on methamidophos are
registered and sold in more than 40 different countries of the world,
including Egypt, many countries of Latin America, Turkey, U.S.A.,
Canada and several countries of Europe. Methamidophos is marketed in
different emulsifiable concentrate formulations.
The major uses of methamidophos are pre-harvest treatments on
cotton, vegetables, potatoes, maize, sugar beet, stone fruit, tobacco,
hops and ornamentals. Further details on its uses and recommendations
are given in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In vegetables
Residue data are presented in Table 4 on a number of vegetable
crops, namely broccoli, Brussels sprouts, cabbage, cauliflower,
celery, cucumber, eggplant, lettuce, peaches, pepper, potatoes and
tomatoes. Most of the data have been received in detailed as well as
in summarised form from the manufacturers (Chevron Chemagro and
Bayer-reports in file), although with some supplementary material from
other sources (Davis et al., 1974).
Generally vegetable crops are treated with 0.5 - 1 kg/ha of
methamidophos, which leaves residues of a non-persistent nature and
with a well-known distribution pattern, viz. the outer leaves,
especially of leafy vegetables, carry considerably higher residues
than the internal parts of the plants. Some of these outer leaves may
or may not be discarded as "trimmings" when marketed.
With only occasional high values, the data in Table 4 indicate
that residues of methamidophos will rapidly dissipate to low levels.
With appropriate pre-harvest intervals, from 1-3 weeks, the average
residues in all vegetables are usually well below 1 mg/kg.
TABLE 3. Recommended use patterns of methamidophos
Crop Dose, Number Recommended
kg a.i./ha of pre-harvest
treatments interval, days
Cotton 0.05-1.5 3-5 21
Cucumbers 0.4 -0.75 3-4 3
Tomatoes 0.4 -0.75 3-4 3
Cauliflower 0.4 -1.12 3-5 7
(U.S.A. 14)
Brussels sprouts 0.56-1.12 3-5 7
Broccoli 0.56-1.12 3-5 7
Other brassicas 0.4 -1.12 3-5 7
Celery 0.56-1.12 5 21
Lettuce 0.56-1.12 3-4 21
Potatoes 0.5 -1.12 3 7
Maize 0.9 -1.5 3 21
Sugar beet 0.5 -0.6 3-5 28
Stone fruit 0.5 -1.0 2-3 21
(peach)
Tobacco 0.3 -0.6 3-4 14
Hops 0.3 -2.0 4 21
Rape 0.28-0.56 2 10
TABLE 4. Residues of methamidophos in vegetable crops (supervised trials)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Broccoli
heads 0.56-1.12 3-6 2 w n.d. - 2.84 0.45 21 U.S.A.
3 w n.d. - 0.14 0.03 9
4 w n.d. - 0.09 0.02 7
leaves 1.12 3-6 2 w 0.22 - 7.62 2.50 7 U.S.A.
3 w n.d. - 0.88 0.28 7
4 w n.d. - 0.54 0.16 7
total 1.12 3-6 2 w 0.16 - 3.74 1.41 7 U.S.A.
3 w n.d. - 0.57 0.18 7
4 w n.d. - 0.44 0.13 6
Brussels sprouts
heads 1.12 3-5 2 w 0.07 - 0.47 0.26 6 U.S.A.
3 w 0.08 - 0.64 0.27 6
4 w 0.05 - 0.22 0.12 6
leaves 1.12 3-5 2 w 0.16 - 0.17 0.17 2 U.S.A.
3 w 0.06 - 0.21 0.14 2
4 w n.d. - 0.10 0.05 2
total 1.12 3.5 2 w 0.10 - 0.11 0.11 2 U.S.A.
3 w 0.12 0.12 2
4 w 0.05 - 0.08 0.07 2
Cabbage
heads 0.56 - 1.12 2-9 1 w n.d. - 0.7 0.11 11 U.S.A.
2 w n.d. - 0.35 0.04 11 GFR
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Cabbage
savoy 0.3 3 1 w 0.09 1 GFR
2 w 0.07 1
Cauliflower
heads 0.56 - 1.12 3-12 2 w n.d. - 0.45 0.11 16 U.S.A.
3 w n.d. - 0.23 0.06 7 GFR
4 w n.d. - 0.12 0.03 7
leaves 0.56 - 1.12 3-12 2 w 0.1 - 6.28 1.85 8
3 w n.d. - 1.11 0.46 7
4 w n.d. - 0.62 0.24 7
Celery
stalks 1.12 5-7 3 w 0 - 1.55 0.33 6 U.S.A.
tops 1.12 5-7 3 w 0.08 - 6.0 2.59 6
whole plant 1.12 5-7 3 w 0.02 - 2.0 0.74 6
Cucumber 0.6 4-6 0 d 0.19 - 0.45 0.35 3 Mexico
(dust treatment) 1 d 0.13 - 0.44 0.32 3
3 d 0.05 . 0.41 0.24 3
5 d 0.06 - 0.2 0.15 3
7 d 0.03 - 0.31 0.16 3
Eggplant 0.45 - 0.6 7-9 0 d 0.03 - 0.10 0.07 2 Mexico
1 d 0.02 - 0.05 0.04 2
3 d 0.05 - 0.06 0.06 2
5 d 0.03 - 0.04 0.04 2
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Lettuce 7 d 0.05 - 0.06 0.06 2
head (1) 0.56 - 1.12 3-8 2 w 0.01 - 0.68 0.24 12 U.S.A.
3 w n.d. - 0.02 n.d. 5
wrapper
leaves (2) 1.12 3-4 3 w 0.1 - 0.77 0.19 5 U.S.A.
(1) + (2) 1.12 3-4 3 w n.d. - 0.25 0.07 5 U.S.A.
head 1.12 5 2 w n.d. - 1.40 0.21 9 U.S.A.
wrapper 3 w n.d. n.d. 6
leaves (2) 1.12 5 2 w 0.01 - 2.42 0.78 5 U.S.A.
3 w 0.02 - 0.15 0.07 6
(1) + (2) 1.12 5 2 w 0.05 - 0.93 0.31 5 U.S.A.
3 w n.d. - 0.39 0.09 6
Peppers 0.34 - 1.12 4-20 1 w 0.04 - 2.3 1.00 7 U.S.A.
2 w 0.08 - 1.09 0.38 8 Mexico
3 w n.d. - 0.58 0.16 8 ) Australia
4 w n.d. n.d. 4 )
Potatoes 1.12 6-8 1 w n.d. - 0.11 0.04 12 U.S.A.
2 w n.d. n.d. 5 U.S.A.
Canada
Tomatoes 0.5 4 1 d - 0.50 1 Venezuela
3 d - 0.28 1
7 d - 0.22 1
10 d - 0.23 1
14 d - 0.20 1
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
18 d - 0.19 1
Tomatoes 0.6 - 1.33 4-10 0 w 0.10 - 5.07 1.05 17 Australia
1 W 0.08 - 1.83 0.55 18 U.S.A.
2 w 0.03 - 1.62 0.30 18 Venezuela
3 w 0.03 - 0.18 0.09 6
In oil seeds, hops and sugar beets
Rape and cotton plants have been treated with varying amounts of
methamidophos, ranging from 0.28 kg/ha up to an overdose of 2.24 kg/ha
and with up to 4 applications. In these experiments residues in the
harvested seeds ranged from undetectable to 0.06 mg/kg 21-26 days
after the last treatment, and no residues could be detected at 32-72
days after application.
Limited residue data for methamidophos in hops are available.
After six applications with 0.66 kg/ha dried hops contained 3.0 mg/kg
at 17 days. After 3 spray applications with 1.8 kg/ha, the residue
levels of methamidophos amounted to 1.4-2.8 mg/kg at 20 days.
Sugar beets were sprayed on the foliage 4 times with 0.48 kg/ha.
The residue levels 23 days after the last application were 0.01 mg/kg
of methamidophos in the roots and 0.6 mg/kg in the leaves. After 28
days the roots were free of residues, while the tops contained 0.9
mg/kg methamidophos.
In peaches
Peaches were sprayed with 1 kg/ha of methamidophos under German
climatic conditions. 1 week after the second of 2 applications
residues varied from 0.50-1.05 mg/kg (average, 0.73 mg/kg), and 1 week
later they were 0.28-0.75 mg/kg (average 0.45 mg/kg).
FATE OF RESIDUES
The identified metabolites formed from methamidophos are shown in
Figure 1.
In animals
Studies of the absorption, distribution and excretion of
methamidophos in the rat (Crossley and Tutass, 1969), and lactating
goat (Lee and Crossley, 1972) are described in the section
"Biochemical aspects," p. ). Some features of these studies,
particularly the distribution in tissues, are amplified below.
Female and male rats each received a single dose of radiolabelled
methamidophos, administered intragastrically as an aqueous solution
(Crossley and Tutass, 1969). The doses received by each rat were
0.16-0.19 mg of 14C methylthio-labelled methamidophos and 0.21 mg of
32P-labelled methamidophos. In both experiments, the majority of the
applied dose was excreted within 24 hours. This period of fast
elimination in which over 50% of the activity was excreted was
followed by a second phase during which only 1-2% of the applied dose
was eliminated daily.
In the experiments with 14C-labelled methamidophos, the major
excretion route was via the breath (approx. 39% of the applied dose);
urine and faeces contained 11.1% and 1.5% respectively. In the
experiments with 32P-labelled methamidophos, on the other hand, the
majority of radioactivity was eliminated in the urine (until day 14),
and up to about 20% was eliminated in the faeces. There was no
accumulation in tissues or organs (see Table 5).
TABLE 5. Distribution of radioactivity in tissues and organs of
rats after dosing with labelled methamidophos (Crossley
and Tutass, 1969)
Radioactivity, % of applied dose
Organ/ 14C 32P
tissue female rats male rats female rats
Day 5 - 9 Day 1 Day 28 Day 1 Day 28
Carcass 21.9 9.9 6.7 11.3 4.4
Liver 0.4 6.9 0.1 5.6 0.1
Kidney 0.1 0.5 0.0 0.4 0.0
Heart 0.3 0.1 0.0 0.1 0.0
Lung 0.1 - - - -
Fat - 0.1 0.0 0.2 0.0
Muscle - 0.2 0.1 0.3 0.1
Total 22.6 17.4 6.9 17.4 4.6
The degradation of methamidophos in the animal body is hydrolytic
in nature (Crossley & Tutass, 1969; Tutass, 1968). The degradation
sequence (Crossley & Tutass, 1969), is shown above
("Biotransformation"). Data on the quantitative distribution of
residues 32P residues in the urine are presented in Table 6.
In the faeces, the majority of radioactivity was not extractable
with either water or acetone. That which was extractable showed the
same degradation profile as in the urine. The non-extractable
radioactivity was in all probability incorporated into natural
biochemical constituents.
TABLE 6. Distribution of 32P in urine of rats dosed with
32P-methamidophos (Crossley and Tutass, 1969)
32P, % of total in sample
Metabolite* Male rats Female rats
Day 1 Day 2 Day 1 Day 2
methamidophos (I) 40 10 40 trace
OS-dimethyl
phosphorothioate (II) 5 - 5 -
methylphosphoric
acid (III) 40 25 40 30
phosphoric acid (IV) 15 65 15 70
* Numbers in parenthesis refer to Figure 1.
S-methyl-14C-methamidophos was given orally to a goat in order
to assess its fate in a lactating ruminant (Lee and Crossley, 1972).
The goat received 3.75 mg. of 14C methamidophos daily after the
morning milking for 7 consecutive days. Methamidophos was rapidly
metabolized and about half of the radioactivity rapidly excreted. This
excreted radioactivity was distributed mainly between the breath and
urine and, to a lesser extent, the faeces and milk. The activity that
remained in the animal after the initial rapid excretion was
eliminated at a slower but constant rate. None of the radioactivity
remaining in the body was concentrated in any one organ, although
slightly higher levels were found in the liver than elsewhere. The
distribution of radioactivity in the goat is summarized in Table 7.
The results are essentially the same as those found in the rat study
(Table 5), although a somewhat higher proportion was excreted in the
breath of the rats.
TABLE 7. Distribution of radioactivity in goat dosed with 14C-methamidophos expressed as
percentage of applied dose or mg/kg methamidophos equivalents (Lee and Crossley, 1972)
Sample Dosing Period Recovery Period Total Blood and Tissues
(days 0 - 8) (days 9 - 17) Excreted
% % % % mg/kg
Urine 17.24 0.63 17.87 Bloodx 5.87 -
Faeces 4.00 0.98 4.98 Liver 3.93 0.22
Milk 2.49 0.74 3.23 Kidney 0.61 0.16
Total 23.73 2.35 26.08 Heart 0.61 0.16
Mammary 0.82 0.10
Brain 0.21 0.08
Fatxx 4.13 0.16
Musclexxx 33.10 0.16
Total recovered = 75% Total 49.28 1.04
x Assuming blood to be 10% of the body weight
xx Assuming fat to be 5% of the body weight
xxx Assuming muscle to be 40% of the body weight
TLC of urinary samples revealed the presence of methamidophos and
the first metabolite OS-dimethyl phosphorothioate (II in Figure 1). In
the sample taken 12 hours after the third dose, 7% of the activity in
the urine was found to be parent compound and 16% metabolite II.
Extraction procedures applied to the samples of tissues, blood, milk,
urine and faeces extracted only small proportions of their activity,
indicating that the main source of activity was not methamidophos. The
maximum possible level of methamidophos in milk estimated on this
basis was 0.008 mg/kg. The maximum content in the milk found by direct
analysis was 0.003 mg/kg (about 1% of the total activity in the milk).
Since the data obtained from the goat are similar to those from the
rats; it is believed that the non-extractable activity in the goat
tissues, milk, etc. is due to the incorporation of 14C into the
natural constituents of the body, as in rats.
In plants
Methamidophos is an insecticide with systemic activity (Werner,
1972). The physical and chemical basis of systemic movement in the
cotton plant was investigated by Hussain et al. (1974).
Root and leaf treatment of tomato, cabbage and bean plants
demonstrated that 14C-methamidophos is readily taken up by roots and
leaves and transported with the transpiration water towards the
margins of leaves, while little is retained in the stem and petioles.
The labelled compound accumulating at the margins of leaves is
essentially only methamidophos, while in the centre of the leaf some
more polar compounds are present, apparently as the result of
metabolic degradation. The uptake of methamidophos by roots appears to
be independent of pH within the physiological range of 3.5-7.5
(Tutass, 1968b).
Horler et al., (1975) showed that in tomato plants there is only
slight movement of the intact insecticide from treated leaves to the
fruit. After rapid penetration of 14C-methamidophos or/and
32P-methamidophos into tomato leaves, the residue in leaves and fruit
in the initial phase has a biological half-life of 7-10 days. However,
especially in the fruit, after this initial period the rate slows to
give a half-life of about 6 weeks (Horler et al., 1975). The authors
assume that intact insecticide is lost from fruits and leaves by a
mechanism associated with transpiration, rather than by metabolism and
translocation.
Werner (1973) also reports rapid absorption of
methylthio-14C-methamidophos by the roots of Loblolly pine seedlings.
After 96 hours exposure, an accumulation of radioactivity was evident
in the needle tips. Besides unchanged methamidophos, 3 compounds were
isolated from the seedlings. One was identified as the major
metabolite, OS-dimethyl phosphorothioate (II, Figure 1), and in
addition two unidentified compounds were detected, of which one
(Unknown 1) was probably phosphoric acid (VI, Figure 1).
Methamidophos, II and Unknown 1 were detected in the roots, stems and
needles, but Unknown 2 only in the needles, of seedlings treated for
72 and 96 hours (see Table 8).
TABLE 8. Percentage distribution of radioactive methamidophos
and 14C-compounds from TLC and GLC of pine seedling
extracts (Werner, 1973)
Seedling exposure period (h)
Compound* Plant part 6 12 24 48 72 96
I (methamidophos) Roots 80.0 46.0 40.0 35.7 3.3 0.9
Stem 0 0 1.6 1.0 1.1 1.7
Needles 0 31.0 27.9 26.3 22.0 30.5
II (OS-dimethyl Roots 20.0 3.8 1.6 1.0 3.3 3.5
phosphorothioate)
Stem 0 3.8 13.1 6.1 11.0 10.4
Needles 0 46.0 26.2 18.4 16.5 17.4
Unknown 1 Roots 0 0 1.6 1.0 2.2 1.7
Stem 0 3.8 4.9 3.1 4.4 2.6
Needles 0 19.2 18.1 17.4 19.7 10.4
Unknown 2 Roots 0 0 0 0 0 0
Stem 0 0 0 0 0 0
Needles 0 0 0 0 16.5 20.9
* Roman numerals refer to Figure 1.
Experiments with methylthio-14C-methamidophos on cabbages and
tomatoes as well as on plant tissue cultures of sweet potatoes and
tobacco have shown that degradation occurs chiefly by hydrolysis
(Chevron, 1968). Hydrolysis first occurs at the P-N bond to yield
OS-dimethyl phosphorothioate (II, Figure 1), which was consistently
detected as a metabolite in all tissues investigated. Continued,
though somewhat slower, hydrolysis of II apparently leads to cleavage
of CH3S-P and CH3O-P bonds yielding VII and III as major products.
Quantitative data on the residues of these metabolites are not given.
The final fate of the radiolabel is its incorporation into plant
constituents such as pigments, amino acids, carbohydrates and
structural material (up to 19% of the activity in the sample of
cabbage plants).
In soil
Methamidophos is degraded at a relatively fast rate in soils. In
laboratory experiments, Leary and Tutass (1968) found half-lives of
1.9 days in silt soil, 4.8 days in loam soil and 6.1 days in sandy
soil. Sterilization of silt soil by autoclaving resulted in a
considerable increase in the half-life, to about 6 weeks.
In laboratory experiments conducted by Horler et al. (1975),
methamidophos was also found to undergo rapid degradation. In basic
soils (pH 7.40 and 7.75), only 3-7% of the original activity was found
after 4 days; in an acid soil (pH 4.30), 25% was present after 9 days.
In the basic soils, there were differences in the degradation of the
metabolites. In clay-loam soil (pH 7.40), the metabolite degraded at a
slower rate than in loam soil (pH 7.75). Since both soils had a
similar pH, such differences may be connected with the fact that
microorganisms play a role in the decomposition.
In degradation experiments with field soils sampled from depths
of 0-10 cm, and from depths of 0-15 cm and 15-30 cm, it was noted that
at sampling on the day of application to the sail, only a fraction of
the applied methamidophos was still present (Bayer, 1972; Chemagro,
1974). Within one week, the residue dropped further to 10% of the
level measured on the day of application. At depths of 15-30 cm, only
very small amounts of residue (maximum of 0.04 mg/kg) were found in
the soil, and no accumulation was noted after several applications.
Leary and Tutass (1968) found that metabolic degradation of
S-methyl-14-methamidophos in silt soil under aerobic conditions
resulted after 3 days in the evolution of 70% of the applied dose as
14CO2. On the other hand, only 7.6% was evolved as 14CO2 under
aerobic conditions, with 84.6% of the applied activity remaining in
the soil. Up to about 30% of the activity that remained in the soil
was not acetone-extractable. Most of the extractable radioactivity was
in the form of OS-dimethyl phosphorothioate (II, Figure 1), while
minor amounts were in the form of methamidophos, amino acids and
carbohydrates. Quantitative data on these constituents were not given.
Horler et al (1975) also identified OS-dimethylphosphorothioate
(II) as the major metabolite in soil. However, they also found very
small amounts of V, VI and III in clay loam soil. In loam soil, VI was
not found. It is stated that the loss of the methoxy group results in
the evolution of CO2. Quantitative data are not given.
In water
The hydrolysis of methamidophos was examined at elevated
temperatures in aqueous solutions as a function of pH (Leary, 1968;
Magee, 1966). In solutions with a pH below 2, the half-life was a
matter of hours. At pH 9, the half-life was 2.6 days at 25°C and 1.5
days at 37°C. In mildly acidic or neutral solutions, methamidophos
showed remarkable stability at temperatures up to 80°C (less than 5%
hydrolysis in 25 hours). The P-NH2 bond is broken first, followed by
hydrolysis of the CH3SP grouping.
Leaching experiments with methamidophos and three soil types
(muck, sandy loam and loam) (Tutass, 1968c), showed little retention
of the compound. Soil type apparently had only a minor effect upon
retention. The compound was least retained in loam.
Experiments to study the leaching behaviour of methamidophos with
a standardized soil column technique revealed that about two-thirds of
the compound was leached out of sandy and sandy loam soils while only
about one-fifth was leached out of a soil with an increased content of
organic matter (Bayer, 1972).
Flint and Shaw (1972) also studied the absorption of
methamidophos by and its leaching from three different soil types. The
absorption by soil was very slight, and it was further decreased by
increasing temperature and decreasing pH. Methamidophos was not
retarded by the soil when passed through soil columns in water. The
chemical was found in soil run-off water only in the first two of five
irrigations. It was degraded in a natural water system outdoors with
half-lives of 15.9 days in water and 7.5 days in silt. It was
concluded that although it was leached, methamidophos was degraded
rapidly in natural water systems.
In fish
Bass fingerlings which had been in water containing 0.01 mg/1 of
methamidophos for eight days, were analyzed. Less than 0.02 mg/kg of
methamidophos was found in all samples (Stanley, 1971 b).
In processing and storage
The effect of simulated commercial processing on methamidophos
residues in tomatoes was investigated by Morris and Olson (1974). The
tomatoes were processed to juice (pressed, pasteurized), to canned
tomatoes (sterilized), to ketchup. (sterilized, pasteurized), to dry
tomato pomace and to dry tomato pulp. The residues in juice, canned
tomatoes and ketchup were smaller than those found in whole tomatoes,
amounting to 89.5, 73.0 and 68.4% respectively, of the residue levels
prior to processing. On the other hand, the residues in dry tomato
pomace and dry tomato pulp were 2.0 and 2.5 times the original levels
respectively.
The effect on residues of washing in a manner simulating
commercial packing practice depended upon the treated crop. After
treatment, the fruits were allowed to stand outdoors for 24 hours.
Whereas washing green peppers and eggplants did not remove any
significant residue, washing tomatoes removed approximately 15% and
washing cucumbers approximately 20% of the methamidophos residue
(Thornton, 1973).
Dehydrated celery flakes were prepared commercially by placing
celery leaves on trays and drying at temperatures of up to 60°C
(Morris, 1975). Dehydration removed 80% of the weight as water, and it
was concluded that the dehydration process did not destroy any of the
methamidophos residue present in fresh celery leaves.
Cottonseed was processed into delinted seed, meal, hulls. and
oil. No residue was found in any of the seed samples or in any of the
fractions from the processed seed. The residue found in the trash
ranged from 0.1 to 2.7 mg/kg (Chevron, 1976).
Rapeseed was processed into oil and meal (Chemagro, 1976). The
following residue levels were measured: rapeseed, 0.02-0.25 mg/kg,
rapeseed oil, n.d.-0.02 mg/kg and rapeseed meal 0.01-0.30 mg/kg.
Leary (1968) conducted a study to determine the stability of
weathered residues of methamidophos on chopped crop samples (broccoli,
lettuce, cabbage, cauliflower, Brussels sprouts) stored deep frozen
for several months. No significant evidence of decomposition was
obtained and there was no indication of any trend towards
decomposition with increased time of storage.
Möllhoff (1971), however, noted that methamidophos residues
decreased by 10-40% in cauliflower samples stored for three months at
-20°C, and by up to 10% in tomatoes stored under the same conditions.
In further studies conducted by Chemagro (1976), to investigate
the effect of frozen storage at about -20°C on methamidophos residues
in peanut meat, peanut forage, sorghum grain, rapeseed, celery and
peppers, decomposition ranged from 0 to 22%.
The effect of frozen storage at about -20°C on methamidophos
residues in cattle tissues and milk and in poultry tissues and eggs
has also been studied (Chemagro, 1976). It was found that
decomposition in poultry liver amounted to 33% after 60 days and in
cattle liver to 85% after 150 days. On the other hand, the residues
remained completely stable in cattle steak, fat and milk, and
decomposition amounted to a maximum of only 13% in eggs, heart and
gizzard of poultry.
METHODS OF RESIDUE ANALYSIS
Methods for the gas-chromatographic determination of
methamidophos have been developed and published in several
modifications, but in practically all cases are based on clean-up
procedures with silica gel columns and GLC with thermionic detection.
Möllhoff (1971) developed a method for determination in plants,
soil and water. Maceration with acetone or an acetone/water mixture is
used for extraction. The limit of determination is generally about
0.01 mg/kg, but for oil seeds only 0.05 mg/kg and for hops and tobacco
0.1 mg/ kg. Recoveries in the 0.01-1.0 mg/kg range vary between 80%
and 100%.
Leary (1971) also determined methamidophos in crops, but with the
use of ethyl acetate for extraction. The GLC instrument in this case
was equipped with a caesium bromide thermionic detector and the method
is claimed to have a limit of determination of 0.003-0.005 mg/kg. At
levels of 0.5-0.1 mg/kg the majority of recovery values were from
90-100%.
Both these methods have been tested for specificity in
conjunction with other organophosphorus insecticides. They were found
to be adequately specific and without interferences from extractives
with a number of different crops (Olson, 1973).
Stanley and co-workers have developed methods for residues in
different forage crops such as hay, peanut meal, sorghum grain and
rape seed (McNamara & Stanley, 1975) and in animal tissues and milk
(Stanley, 1971c). Extractions were with either methanol/chloroform or
acetonitrile and Skellysolve B.
With minor amendments these methods have been found suitable also
for the determination of methamidophos in fish and water with limits
of determination down to 0.02 mg/kg and 0.004 mg/kg respectively
(Stanley 1971 a).
These methods for the determination of methamidophos follow
closely the general pattern for organophosphorus insecticides in
current multi-residue procedures. Undoubtedly, therefore, they will be
suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
TABLE 9. National tolerances reported to the meeting
Country Crop Tolerance, Pre-harvest
mg/kg interval
Australia Capsicums 0.25 14 days
Peaches 0.25 21 days
Tomatoes 0.25 21 days
TABLE 9. (Cont'd.)
Country Crop Tolerance, Pre-harvest
mg/kg interval
Potatoes 0.05 7 days
Canada Broccoli, Brussels
sprouts, lettuce,
peppers 1.0
Cabbage, cauliflower,
cucumbers, eggplant,
tomatoes 0.5
Potatoes, melons negligible
residue
Netherlands Brassica 0.1
Potatoes 0.01x
New Zealand Brassicas, potatoes 0.1
Tomatoes 0.1 3 days
South Africa Peaches 1.0 21 days
Cabbage 1.0 21 days
Potatoes 1.0 14 days
U.S.A. Cauliflower, lettuce
tomatoes 2.0
Broccoli, Brussels
sprouts, cabbage,
peppers, cucumbers,
eggplant 1.0
Melons 0.5
Cottonseed, potatoes 0.1xx
x = limit of determination
xx = negligible residues
APPRAISAL
Methamidophos is a chemically simple organophosphorus insecticide
with broad spectrum effects used to control caterpillars, aphids,
larvae etc. It is active by contact as well as systemically.
Since 1969 methamidophos has been registered in a number of
countries around the world, chiefly in regions with a sub-tropical
climate. Its major uses are pre-harvest treatments of industrial
crops, fruit and vegetables, mainly with spray solutions at rates from
0.5-1.5 kg/ha.
Detailed studies on the metabolic fate of methamidophos indicate
that the compound is readily taken up by plant roots and translocated
with the transpiration stream within the plants, i.e. towards the
edges of the foliage. In studies with the radio-labelled compound, the
further fate has been followed and a complete degradation pathway with
final incorporation into plant constituents is suggested, mainly
through steps involving hydrolytic breakdown.
The degradation of methamidophos in the animal body is also
hydrolytic and there are no signs of accumulation in tissues or
organs. Most of the phosphorus-containing moiety of methamidophos (up
to about 70%) is eliminated via the urine, while a major part of the
carbon (about 40% in the rat) is excreted as CO2 with the breath, the
remainder being found in the urine and faeces.
The results of extensive residue trials on a number of vegetable
and agricultural crops are available. The data confirm methamidophos
as a systemic compound of relatively low persistence. Most of the data
are from trials under practical conditions which are likely to
represent good agricultural practice.
Methamidophos penetrates into the flesh of the fruit in, e.g.,
tomatoes, peppers, eggplants, etc. Washing or peeling such products
therefore does not significantly remove residues. In the frozen
storage of crops treated with methamidophos, residues are found to be
stable for several months.
Methamidophos has been found to be degraded fairly rapidly in
soils and in natural water systems without any signs of accumulation
or concentration in living organisms.
Gas-chromatographic methods making use of thermionic detectors
are available for the determination of methamidopho in various crops
and animal products. They are combined with appropriate extraction and
clean-up procedures and are likely to be suitable for regulatory
purposes.
RECOMMENDATIONS
Maximum residue limits are recommended for methamidophos in the
following commodities at the levels indicated. They refer to the
parent compound.
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Hops 5 Peppers 1
Broccoli 2 Sugar beet leaves 1
Cauliflower 2 Cottonseed 0.1
Celery 2 Potatoes 0.1
Lettuce 2 Rapeseed 0.1
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Tomatoes 2 Sugar beets 0.1
Brussels Meat and fat of
sprouts 1 cattle, goats
Cabbage 1 and sheep 0.01*
Cucumber 1 Milk 0.01*
Eggplant 1
Peaches 1
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies an the teratogenic potential in a suitable
species.
2. Further studies to elucidate the contribution of acephate and
metamidophos alone or in combination in depressing cholinesterase
activity.
3. Further information on the level and fate of residues during
cooking and processing of fruit and vegetables.
REFERENCES
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E.
1971 Mutagenic Study with Monitor Technical (SX No.
171) in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Arnold, D., Keplinger, M.L. and Fancher, O. E. Three Generation
1970 Reproduction Study on SX-171 (Technical RE-9006 -
75%) in Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Bayer Berichte No. 12,13/1972; No. 11,12/1973. Submitted
1972 to the Joint Meeting.
Carlson, D., Vondruska, J. F. and Fancher, O. E. Effects of RE 9006
1969 - 75% SX-171 on the Cholinesterase Activity in the
Beagle Dog. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Chevron Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity of
1968a Monitor Technical in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1908b of Monitor 6S in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, D.H. Acute Oral Toxicity
1968c of RE 9006 (95%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968d of RE 9006 (75%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.D. Acute Dermal Toxicity
1968e of Monitor 6S. Unpublished report from Industrial
Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1968f of Monitor Technical. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968g of RE 9006 (95%) in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968h of RE 9169 (SX-196) in Rats. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
the Chevron Chemical Company.
Cavalli, R.E., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1969 RE 9169 (SX 198) in Rabbits. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
Chevron Chemical Company.
Cavalli, R.E. and Spence, J.A. Effect of Repeated Doses of RE 12420
1970 on Cholinesterase Activity in Rats. Unpublished
report from Standard Oil of California, submitted
to the WHO by Chevron Chemical Company.
Chemagro Reports nos. 44249, 44250 on methamidophos in
1974 soil. Submitted to the Joint Meeting.
Chevron Metabolism of Monitor insecticide by plants.
1968 Chevron report submitted to the Joint Meeting. In
file at FAO, Plant Protection Division.
Crawford, C.R. and Anderson, R.H. The Acute Toxicity of Monitor
1973 Technical in Combination with Marathion to Female
Rats. Unpublished report from Chemagro Division of
Baychem Corporation, submitted to the WHO by
Bayer, A.G.
Crossley, J. and Tutass, H.O. Metabolism of Monitor Insecticide
1969 by rats. Unpublished report no. 721.14 from the
Ortho Division, Chevron Chemical Company,
submitted to the WHO by Bayer, A.G.
Davis, A.C., Bourke, J.B. and Kuhr, R.J. Disappearance of Monitor
1974 Residues from Cole crops. J. Econ. Entomol. 67,
766-768.
Fletcher, D., Jenkins, D.H., Keplinger, M.L. and Fancher, O.E.
1971 Demyelination Study with Monitor (RE 9006)
Technical in Mature Laying Hens. Unpublished study
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Flint, D.R. and Shaw II, H.R. The Mobility and persistence of
1972 MONITOR in soil and water. Report No. 34 483
submitted by Chemagro.
Garofalo, M., Palazzolo, R. and Sanders, R. A Study on the Effect
1973 of Orthene and Monitor on Plasma and Erythrocyte
Cholinesterase Activity in Human Subjects During
Subacute Oral Administration. Unpublished report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Chevron Chemical Company.
Greco, R.A., Lindberg, D.C., Keplinger, M.L. and Fancher, O.E.
1971 Effect of RE 9006 on Plasma and Erythrocyte
Cholinesterase Activity in Beagle Dogs.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Gröning, P. Tamaron. Special Toxicity Studies (Potentiation).
1975 Unpublished report from Institut für Toxikologie,
submitted to the WHO by Bayer, A.G.
Gröning, P. Bayer 17628 (Tamaron) Untersuchung Der Subchronischen
Toxizitat an ratten (3-monate-futterungversuch).
Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Gröning, P. and Lorke, D. Untersuchung der subchronischen toxizitat
1976 an hunden. Unpublished report from the Institut
für Toxikologie, submitted to the WHO by Bayer,
A.G.
Hanna, P.J. and Dyer, K.F. Mutagenicity of Organophosphorus Compounds
1975 in Bacteria and Drosophila. Mut. Res. 28:
405-20.
Horler, D.F., Lubkowitz, J.A., Revilla, A.P., Baruel, J. and Cermeli,
1975 M.M. Uptake and Degradation of Monitor by Tomato
Plants. Pesticides, IUPAC 3rd Int. Congr. Pest.
Chem., Helsinki, 151-156.
Hussain, M., Fukuto, T.R., and Reynolds, H.T. Physical and Chemical
1974 Basis for Systemic Movement of Organophosphorus
Esters in the Cotton Plant. J. Agr. Fd Chem. 22
(2): 225-230.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L.
1971 Teratogenic Study with Monitor Technical in Albino
Rabbits. Unpublished report from Industrial Bio
Test Laboratories, Inc. submitted to the WHO by
Bayer, A.G.
Leary, J.B. Stability of Monitor insecticide residues in frozen
crops and in aqueous solutions. Chevron report
files No. 740.01 and No. 721.2.
Leary, J.B. and Tutass, H.O. Degradation of Monitor insecticide
1968 in soil. Chevron report file No. 721.2.
Leary, J.B. Gas-chromatographic Determination of Monitor
1971 (OS-Dimethyl Phosphoramidothioate) Residues in
Crops. J. Ass. Off. Analyt. Chem. 54 (6):
1396-98.
Lee, H. and Crossley, J. The Fate of Monitor in a Lactating
1972 Ruminant (Goat). Unpublished report from Ortho
Division, Chevron Chemical Company, submitted to
the WHO by Bayer, A.G.
Lindberg, D., Vondruska, J.F. and Fancher, O.E. Two Year Chronic
1969 Oral Toxicity of RE 9006-III, SX-116 in Beagle
Dogs. Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO, by
Bayer, A.G.
Lindberg, D., Keplinger, M.L. and Fancher, O.E. RE 9006 (70%)
1970 SX 171 on the Cholinesterase in the Beagle Dog.
Unpublished study from Industrial Bio-Test
Laboratories Inc., submitted to the WHO by Chevron
Chemical Company.
Lorke, D. and Kimmerle, G. Toxicological Studies on the Active
1967 Ingredient Bayer 71 628. Unpublished report from
the Institut für Toxikologie, submitted to the WHO
by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on Dogs
1970a (Three Month Feeding Experiment). Unpublished
report from Institut für Toxikologie, submitted to
the WHO by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on rats.
1970b Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Magee, P.S. Hydrolysis of Monitor insecticide. Chevron Report
1966 File No. 721.2.
Mastri, C., Keplinger, M.L. and Fancher, O.E. Twenty-one Day
1968 Subacute Dermal Toxicity of Monitor (RE 9006) 75%
Technical SX 171. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Chevron Chemical Company.
McNamara, F.T., and Stanley, C.W. Gas-Chromatographic Method for
1975 the Determination of Residues of MONITOR in Peanut
Meat and Hay, Sorghum Grain, and Rapeseed.
Chemagro Report No. 45.439.
Möllhoff, E. Metode zur gaschromatographischen Bestimmung
1971 von Tamaron-Rückständen in Pflanzen.
Pflanzenschutz-Nachr. 24 (2): 256-262.
Morris, R.A. Effect of Dehydration on Residues of MONITOR
1975 in Celery Leaves. Chemagro Report No. 45.586.
Morris, R.A., and Olson, T.J. The Effect of Processing on MONITOR
1974 Residues in Tomatoes. Chemagro Report No. 41.107.
Olson, T.J. Interference Study on MONITOR (TAMARON) in Various
1973 Crops. Chemagro Reports No. 37.380 and No. 37.392.
Plank, J., Keplinger, M.L. and Fancher, O.E. Paired Feeding Study
1968 of RE 9006 - III SX-116 Albino Rats. Unpublished
report from Industrial Bio-Test Laboratories.
Inc., submitted to the WHO by Chevron Chemical
Company.
Plank, J., Keplinger, M.L. and Fancher, O.E. Ninety-Day
1969 Cholinesterase Study of SX-171 Monitor (RE 9006) -
Albino Rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Bayer, A.G.
Plank, J., Keplinger, M.L. and Fancher, O.E. Two Year Chronic
1970 Oral Toxicity of RE 9006 - III, SX-116 in Albino
Rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Ninety-Day Subacute
1973 Oral Cholinesterase Study with Monitor Technical
in Female Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Schoenig, G., Keplinger, M.L. and Fancher, O.E. Study on the Efficacy
1968 of Atropine Sulfate and 2-PAM C1 as Antidotes for
Monitor (RE 9006) 75% Technical SX-171. Report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971a of MONITOR in Fish and Water. Chemagro Report No.
30.975.
Stanley, C.W. Analysis of Bass and Water for MONITOR. Chemagro
1971b Report No. 30.979.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971c of Residues of MONITOR in Animal Tissues and Milk.
Chemagro Report No. 31.093.
Thornton, J.S. Effect of Washing on Residues in Tomatoes, Cucumbers,
1973 Eggplants and Peppers. Chemagro Reports Nos.
37322, 37539, 37540 and 37541 submitted to the
Joint Meeting. In File at FAO, Plant Protection
Division.
Tucker, B. Acetylcholinesterase Inhibition of Orthene and Ortho
1972 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the WHO by
Chevron Chemical Company.
Tutass, H.O. Microsomal Oxidation of Monitor. Unpublished report
1968a no. 721.2 from the Ortho Division of Chevron
Chemical Company, submitted to the WHO by Bayer,
A.G.
Tutass, H.O. Uptake and Translocation of Monitor Insecticide
1968b by tomato, cabbage and bean plants. Chevron Report
File No. 721.2.
Tutass, H.O. Leaching of Monitor insecticide in soils. Chevron
1968c Report File No. 721.2
Werner, R.A. Systemic insecticidal action of disulfoton and
1972 Monitor in Loblolly pine seedlings. J. Georgia
Entomol. Soc. 7 (1): 64-67.
Werner, R.A. Absorption, Translocation and Metabolism of Root-
1973 Absorbed 14C Monitor in Loblolly Pine Seedlings.
J. Econ. Entomol. 66 (4): 867-872.
Wolvin, A.A., Palazzolo, R.J. and Fancher, O.E. Neurotoxicity
1968 Study - Chickens Monitor RE 9006, 75% Technical.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
RBC cholinesterase appears to be more sensitive than plasma
cholinesterase to inhibition as noted by the differing I50 values.
I50(M)
Bovine RBC 3.1 x 10-5
Human Plasma 1.6 x 10-4
(Tucker, 1972)
The sensitivity of RBC relative to plasma cholinesterase to
inhibition by methamidophos was noted in a 3-week dietary study
where methamidophos was fed at 0 and 10 ppm. No cumulative enzyme
depression was observed and RBC was affected slightly more than
plasma (Cavalli and Spence, 1970;) [see acephate - Cavalli and
Spence, 1970f, summary of the data in addendum].
TOXICOLOGICAL STUDIES
Special studies on delayed neurotoxicity
Groups of hens (10 mature laying hens - 1-2 years old/group)
were fed methamidophos in the diet (dosage levels were said to be
0, 0.3, and 3.0 mg/kg body weight) for four weeks. There was a
control of TOCP fed at a dose of 50 mg/kg body weight. No mortality
or severe weight loss was observed and clinical signs of ataxia
were not noted. The TOCP-treated hens developed clinical signs of
ataxia. Histological examination of nervous tissue showed no
abnormalities, even in the TOCP control group (Fletcher et al.,
1971).
In two trials, groups of adult hens (varying in number from 2
to 10/group) were administered methamidophos once orally or by
intraperitoneal injection in the presence or absence of 2-PAM and
atropine. The test protocol used doses equal to and exceeding the
LD50 values in an attempt to establish the neurotoxic potential for
methamidophos. Although mortality was observed, no clinical signs
of delayed neurotoxicity were evident. Histopathological
examinations were not performed (Lorke and Kimmerle, 1967).
A group of six hens each were administered methamidophos
orally at an LD50 dose level of 27.5 mg/kg, observed for 21 days,
re-treated with the same dose and maintained for an additional 21
days. Two separate control groups were used - an untreated control
and a TOCP (500 mg/kg, oral dose) positive control. While some of
the hens (2/6) died from acute toxicity, the survivors of the
methamidophos treatment did not lose weight or show signs of
delayed ataxia. The TOCP treatment group lost weight and showed
positive signs of ataxia within 14 days of poisoning.
Histopathology examination was not performed (Wolvin, et al.,
1968).
Special studies on reproduction
Rat
Groups of rats (8 male and 16 female/group) were fed
methamidophos in the diet at dose levels of 0, 3, 10, and 30 ppm
for 100 days and mated to begin a standard 3 generation, 2
litter/generation reproduction study. Body weights of parental
males (but not females) were reduced but the reduction was slight
and not dose-related. Cholinesterase activity values of the female
animals were measured after the mating trials were concluded. RBC
and plasma cholinesterase depression was evident at 30 ppm in males
and females (male plasma was also depressed at 10 ppm). There were
no significant effects noted on gross or microscopic examinations
of tissues and organs.
Reproductive indices were generally affected at the 30 ppm
dose level. The incidence of pregnancy (fertility index) incidence
of parturition (gestation index) and viability and lactation
indices were reduced at 30 ppm. Numbers of stillborn and
cannibalized pups were increased at the 30 ppm dose but no
suggestions of terata were presented. Histopathologic examination
of the progeny did not reveal any abnormalities. No effects on
reproduction were observed at 10 ppm (Arnold et al., 1970).
Special studies on teratogenesis
Groups of pregnant rabbits (from 17-23 does/group) were
administered methamidophos by gelatin capsule from day 6 through
day 18 of gestation at dosage levels of 0, 0.1, and 0.3 mg/kg. A
positive control (thalidomide, 37.5 mg/kg) was administered to a
group of 27 pregnant does. All animals were sacrificed on day 29 of
gestation. Positive teratogenic signs were observed as expected
with thalidomide. No effects were noted as a result of
administration of methamidophos on growth of does or on fetal
mortality. Fetus weight was normal and skeletal development was
unaffected. One of 63 pups at the 0.3 mg/kg dose level displayed
talipomanus, club hand. This malformation was not observed in 75-79
pups at the low dose or in the controls, but it occurred in 8 of 85
in the positive controls (Ladd et al., 1971). There were no other
obvious defects noted in the study with methamidophos. A larger
number of defects were noted with thalidomide (umbilical hernia,
harelip, cervical elongation, frontal fontanel) that were not seen
with methamidophos. Skeletal examinations of the pups from
methamidophos-treated does were normal.
Special studies on mutagenicity
Groups of mice (12 males/group) were administered
methamidophos by intraperitoneal injection at dosage levels of 0,
1, and 2 mg/kg and mated to virgin females for 6 consecutive weeks
in a dominant lethal test. A positive control group administered
ethylmethane sulfonate (EMS, 400 mg/kg) was included in the study.
Females sacrificed one week after breeding with the treated males
were examined for pregnancy and resorption of fetuses.
Mutagenicity, measured by comparing viable embryos in the test and
control groups, was not evident with methamidophos treatment. A
mutagenic response to EMS was indicated in the first two weeks of
the test (Arnold et al., 1971).
Results of in vitro tests using Salmonella and
Escherichia Spp tester strains were negative when methamidophos was
examined (Hanna and Dyer, 1975).
Special studies on antidotes
Antidotal effects were noted with atropine and atropine +
2-PAM combination administered following intoxication. BH6 and
2-PAM alone were not effective antidotes. Atropine + PAM was more
effective than atropine alone but less effective than atropine +
BH6 (Lorke and Kimmerle, 1967).
The acute oral toxicity of methamidophos in male rats was
reduced 3-fold when animals were administered atropine alone or in
combination with 2-PAM. While 2-PAM alone was not as therapeutic as
atropine it reduced the toxicity by more than two-fold (Schoenig et
al., 1968).
Special studies on potentiation
Simultaneous administration of methamidophos to rats either by
oral gavage or intraperitoneal injection in combination with
malathion showed a significant potentiation (acute interaction in
the toxicity as measured by reduction of the LD50 value). Graduated
doses of malathion and methamidophos by oral administration
resulted in a greater than two-fold potentiation with malathion.
(Gröning, 1975). A slightly less than two-fold increase was
observed when ip injection was the route of administration used
(Crawford and Anderson, 1973). Oral administration of methamidophos
in combination with parathion or EPN did not result in potentiation
of the acute toxicity (Gröning, 1975).
Acute toxicity
TABLE 1. Acute toxicity of methamidophos
Species Sex Route LD50 Reference
(mg/kg)
Rat M Oral 15.6-32.3 Lorke & Kimmerle,
1967; Cavalli
et al., 1968a;
1968b; 1968g.
F Oral 13.0-29.6
M ip 21.3 Lorke & Kimmerle.
1967
F ip 26.4 Lorke & Kimmerle,
1967
TABLE 1. (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
Rat M Dermal
4-hr exp. 110 Lorke & Kimmerle,
7-day exp. 50 1967
Inhalation
1-hr exp. 0.525 mg/L Lorke & Kimmerle,
4-hr exp. 0.162 mg/L 1967
Guinea Pig Oral 30-50 Lorke & Kimmerle,
1967
Mice Oral 16.2 Cavalli et al.,
(Technical) 1968c
Oral (75% 18.0 Cavalli et al.,
Technical) 1968d
Rabbit Oral 10-30 Lorke & Kimmerle,
1967
Dermal 118-125 Cavalli et al.,
1968e; 1968f
Cat Oral 10-30 Lorke & Kimmerle,
1967
Dog Oral 10-30 Lorke & Kimmerle,
1967
Chicken F Oral 25 Lorke & Kimmerle,
1967
F ip 10 Lorke & Kimmerle,
1967
The observed signs of poisoning were typical of
anticholinesterase agents. The signs appeared rapidly following
poisoning (5-20 minutes) and disappeared in 4-6 days. Typical
parasympathomimetic signs of poisoning would be expected.
TABLE 2. Acute toxicity of impurities
LD50
Compound Species Sex Route (mg/kg) Reference
(CH3O)2P(S) Rat M Oral 633 Cavalli et al., 1968h
NH2 (RE9169) F Oral 549 Cavalli et al., 1968h
Signs of poisoning were typical of CNS depression
- paresis, hyporeflexia and anesthesia.
Rabbit Dermal 2,500- Cavalli et al., 1969
5,000
(intact
skin)
1,570 Cavalli et al., 1969
(abraded
skin)
See the monograph on acephate for the toxicity of other products.
Short-term studies
Rat
Groups of rats (15 females/group) were administered methamidophos
orally or by intraperitoneal injection, 5 days/week for 60 days to
examine possible cumulative effects. Oral application consisted of
daily doses of 0, 1.5, 3.0, 6.0, 10.0, and 15.0 mg/kg while ip
application consisted of doses of 0, 1.3, 2.6, 5.3, 8.8, and 13.2
mg/kg. While slight mortality was observed (oral = 3/15, ip = 6/15)
there was no evidence of cumulative action. The cumulative LD50 by
either oral or ip administration was estimated to be > 15 mg/kg body
weight (Lorke & Kimmerle, 1967).
Groups of rats (30 females/treatment group - 90 females were used
as controls) were fed methamidophos in the diet at dosage levels of 0,
0.25, 0.50, 1.00, 2.00 and 4.00 ppm for 13 weeks (a further 4-week
recovery period using a control diet followed this test interval).
There were no deaths or adverse behavioral reactions. Growth, as
evidenced by body weight gain, was normal at all treatment levels.
Cholinesterase depression of plasma, RBC and brain was reported only
in the high dose group.
At 4.0 ppm depression of enzyme activity was obvious in plasma
and RBC, less so in brain. A comparison of the total depression of
cholinesterase activity and recovery during the 4-week control diet
period suggests a no-effect level of 2.0 ppm (Reyna et al., 1973).
Groups of rats (35 males and 35 females/group) were fed
methamidophos in the diet for 13 weeks at dietary levels of 0, 0.3,
1.0, 3.0 and 10.0 ppm. Cholinesterase activity was examined at
periodic intervals using plasma, RBC, and brain tissue. Significant
dose-related depression of plasma cholinesterase activity was noted in
male rats at 3 and 10 ppm and in females at 10 ppm. Decreases in
cholinesterase activity of RBC and brain were noted at 3 and 10 ppm in
both sexes. RBC activity was depressed in both sexes at 10 ppm and in
males only, marginally depressed at 1 and 3 ppm. Recovery of enzyme
activity was noted after one week of control diets following 3 ppm and
at 4 weeks of control diets following 10 ppm (Plank et al., 1969).
Groups of rats (15 males and 15 females/group; 30 of each sex
were used as controls) were fed methamidophos in the diet at dosage
levels of 0, 2, 6, 20, and 60 ppm for 3 months. No mortality or
behavior changes were observed although growth was depressed
significantly at 60 ppm in both sexes. Blood chemistry, hematology,
and urine analysis parameters were normal (bilirubin content of males
only at 20 and 60 ppm was depressed at 90 days, and OCT
(Ornithinecarbamoyl transferase) activity was depressed in females fed
60 ppm at 90 days. These unusual effects were reported to be within
physiological limits). Cholinesterase activity was depressed at 6 ppm
and above in both plasma and RBC (RBC was slightly more sensitive). No
effects were noted at 2 ppm over the 13 week feeding interval. Gross,
macroscopic examination of tissues and organs showed a reduction in
both males and females in the size of several organs; thymus, liver,
spleen, adrenals, and gonads only at 60 ppm. There were no effects
noted at 20 ppm. Microscopic examination of the tissues did not show
any adverse effects related to the presence of methamidophos in the
diet. A no-effect level based on cholinesterase depression is 2 ppm in
the diet (Löser and Lorke, 1970b; Gröning, 1976).
Rabbit
Groups of rabbits (5 males and 5 females/group) were administered
methamidophos dermally to intact or abraded skin at dose levels of 0,
5, or 10 mg/kg (a 75% technical product). The application was made 6-7
hours/day, 5 days/ week for 3 weeks. Application was to a new site
each day. Mortality occurred in the 10 mg/kg abraded skin group
accompanied by signs of cholinergic stimulation. Hematologic studies,
blood chemistry and urinalysis parameters were unaffected by dermal
application of methamidophos. Gross and microscopic analyses of
tissues and organs were uneventful. Dermatologic reactions, such as
erythema, were minor. The affected skin returned to normal within 1-2
days. Growth was slightly retarded at the 10 mg/kg dose group but was
not accompanied by other adverse signs of poisoning (Mastri et al.,
1968).
Dog
Groups of dogs (3 male and 3 female/group) were administered
methamidophos orally by capsule daily, seven days per week, for 90
days at dosage levels of 0, 0.025, 0.075, and 0.25 mg/kg body weight.
There was no mortality. Growth and food consumption were unaffected.
Red blood cell and plasma cholinesterase activity, examined at several
intervals both before and during treatment, was normal. There was no
depression of cholinesterase noted in this study (Carlson et al.,
1969).
Groups of dogs (3 male and 3 female/group) were used in a repeat
of the above test to examine the 90-day subacute anticholinesterase
effects of methamidophos. Growth was reduced in males at the highest
dose level. In females, a reduced weight gain was observed at all dose
levels. Food consumption was reduced at the highest dose level in both
sexes. Depressed erythrocyte cholinesterase was observed at 0.25 mg/kg
in both males and females. No effects were noted on plasma enzymes. A
no-effect level based on RBC cholinesterase is 0.075 mg/kg (Lindberg
et al., 1970).
Groups of dogs (2 male and 2 female/group) were administered
methamidophos by capsule for periods varying from 21-28 days, at doses
ranging from 0 to 0.6 mg/kg/day. In this study each dog was examined
for 34 weeks to determine a pre-treatment baseline for cholinesterase
activity. Animals were then administered a dose of 0.025 mg/kg for 28
days after which the dose was increased to 0.05 mg/kg. The total
treatment sequence was as follows: 0.025, 0.05, 0.075, and 0.10 mg/kg
each administered for 28 days, followed by 0.125, 0.20, 0.30, 0.40,
0.50, and 0.60 mg/kg each administered for 21 days, after which a
control diet was administered for 28 days. Cholinesterase activity was
measured several times (generally at weekly intervals) during the
course of treatment. In both males and females a dose level was
reached where plasma and RBC cholinesterase activity was depressed. In
males, plasma and RBC cholinesterase was depressed when the dose
regimen reached 0.125 mg/kg. In females, plasma was depressed at a
dose of 0.3 mg/kg while RBC was affected at approximately 0.2 mg/kg.
Based on these data, no effects on cholinesterase would be expected to
occur at levels around 0.1 mg/kg/day. In this study, depression was
considered positive when the average value for cholinesterase activity
declined below the lowest range of the pre-treatment mean (Greco et
al., 1971).
Groups of dogs (2 male and 2 female beagles/group), 3 of each sex
were used as controls) were fed diets containing methamidophos at
dosage levels of 0, 1.5, 5, and 15 ppm for 90 days. There was no
mortality or behavioral change noted over the course of treatment.
Growth and food consumption were normal. There were no effects noted
in blood chemistry, hematology or urinalysis parameters.
Cholinesterase depression (RBC and plasma) was noted at 5 ppm and
above in both sexes (within the first week of dietary administration
at the high dose and after 2 months at the intermediate dose). Gross
macroscopic examination of tissues and calculation of relative organ
weights did not indicate any effects at the highest treatment level. A
dietary no-effect level in this study is 1.5 ppm (Löser and Lorke,
1970a)
Histopathological examination of tissues and organs showed no
effects of methamidophos (Gröning and Lorke, 1976).
Groups of dogs (3 male and 3 female beagles/group) were
administered methamidophos by capsule, orally, for two years at dosage
levels of 0, 0.075, 0.25 and 0.75 mg/kg. There were no deaths or
observable differences in behavior over the two-year study. Growth was
unaffected by methamidophos. There were no effects noted on clinical
chemistry, hematology, or urinalysis parameters recorded.
Cholinesterase activity was not reported. Gross and microscopic
examination of tissues and organs showed no abnormalities (Lindberg et
al., 1969).
Long-term studies
Rat
Groups of rats (45 males and 45 females/group) were fed
methamidophos in the diet at dosage levels of 0, 3, 10, and 30 ppm for
two years. Growth was reduced at the high dose level in males. Growth
in females was unaffected in this study. A separate paired feeding
study (21-day) was conducted where it was noted that growth was
unaffected at 30 ppm. Examination of food consumption data suggested
this effect to be due to diet rejection rather than a direct effect of
methamidophos in the diet. (Plank et al., 1968). In the long-term
study, there were no effects reported on behavior, hematologic blood
chemistry or urinalysis parameters. Gross and microscopic examination
of tissues and organs at 12 or 24 months showed no abnormalities
attributed to methamidophos in the diet. The tumor incidence was
unaffected and a definitive, no-effect level based on somatic changes
would be greater than 30 ppm. Cholinesterase activity was not measured
in the study (Plank et al., 1970).
OBSERVATIONS IN MAN
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. The groups received either a
combination mixture of 1:4 (methamidophos:acephate), 1:9 or a control
dose, daily (divided into 3 equal doses), for periods of 21 days. Each
group was administered the combination at a dose of 0.1 mg/kg/day for
21 days after which the dose was increased (to 0.2 mg/kg/day) and
continued for another 21 days. The protocol called for a total of 4
dose levels (up to 0.4 mg/kg/day) each to be administered for 21 days.
Blood cholinesterase (plasma and RBC) was examined periodically. No
effects were noted on RBC cholinesterase at any treatment level. There
was no substantial depression noted in the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The male
cohort was substantially affected at the 0.3 mg/kg dose and was not
retested at the higher dose level. The group (both males and females)
receiving the higher methamidophos:acephate ratio (1:4) showed
reduction of plasma cholinesterase after two weeks of receiving a dose
of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not used in the 1:4 dose
group. This study indicated the minimal dose necessary to decrease
plasma cholinesterase for the combination of acephate and
methamidophos was 0.2 mg/kg/day of a 1:4 combination of the two
toxicants. No effects with this combination were noted at 0.1 mg/kg
(Garofalo et al., 1973). See the monograph on acephate for a further
amplification of these data.
COMMENTS
Methamidophos, an anticholinesterase organophosphorus ester, is
also a metabolite of acephate. Methamidophos is rapidly absorbed,
distributed, metabolised and excreted in mammals. Bioaccumulation,
based on data on solubility properties, would not be expected to
occur. Biotransformation in mammals results in metabolites of
insignificant toxicological properties. Methamidophos is an acutely
toxic pesticide. The acute signs of poisoning can be relieved with the
aid of atropine (and reactivators, such as 2-PAM). In contrast to the
innocuous metabolites, methamidophos is an active cholinesterase
inhibitor, primarily affecting RBC (and presumably brain)
cholinesterase in animal models. In humans, plasma cholinesterase
appears to be the more sensitive enzyme parameter.
Methamidophos does not induce delayed neurotoxicity although
potentiation of the acute toxicity of malathion has been noted. In
reproduction studies, several parameters were affected at relatively
low levels (the levels were however sufficient to impart
cholinesterase depression in the parents). No terata were induced in
the reproduction study or in special teratological studies in rabbits,
although the dose levels used in this latter study were extremely low.
Mutagenicity tests were negative.
In short and long term studies no significant somatic effects
were noted. Cholinesterase depression was manifested in short term
studies. In 90 day dog studies, cholinesterase depression was observed
at 5 ppm while a dietary level of 1.5 ppm (equivalent to 0.04
mg/kg/day) caused no observable effects. In a 2 year dog study,
somatic effects were not noted at levels of 0.75 mg/kg/day but
cholinesterase activity was not determined. Depression of
cholinesterase activity in rat feeding studies was observed at dietary
levels of 3 ppm and above while 2 ppm was found to induce no
depression of cholinesterase activity. In a controlled study where a
combination dose of methamidophos and acephate was administered to a
group of male and female volunteers, cholinesterase depression was
observed predominantly when a higher methamidophos:acephate ratio was
used (1:4 rather than 1:9). Based on the results of this study a
no-effect level of 0.02 mg/kg was suggested. A higher total
concentration (0.2 mg/kg), administered in a lower ratio (1:9) also
gave no depression of enzyme activity. Based on the no-effect level of
0.04 mg/kg bw in dog an ADI was allocated. The no-effect level in
human studies was further assurance of the adequacy of the numerical
value of the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet equivalent to 0.1 mg/kg bw
Dog: 1.5 ppm in the diet equivalent to 0.04 mg/kg bw
Man: 0.02 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methamidophos is an organophosphorus insecticide with systemic
properties. It is a good stomach poison with further contact poison
action and has good residual activity. It is claimed to be effective
for the control of sucking pests such as aphids, whitefly and spider
mites as well as biting pests such as Laphygma, Prodenia, Trichoplusia
and others.
Methamidophos is used world-wide, chiefly in regions with a
subtropical climate. Formulations based on methamidophos are
registered and sold in more than 40 different countries of the world,
including Egypt, many countries of Latin America, Turkey, U.S.A.,
Canada and several countries of Europe. Methamidophos is marketed in
different emulsifiable concentrate formulations.
The major uses of methamidophos are pre-harvest treatments on
cotton, vegetables, potatoes, maize, sugar beet, stone fruit, tobacco,
hops and ornamentals. Further details on its uses and recommendations
are given in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In vegetables
Residue data are presented in Table 4 on a number of vegetable
crops, namely broccoli, Brussels sprouts, cabbage, cauliflower,
celery, cucumber, eggplant, lettuce, peaches, pepper, potatoes and
tomatoes. Most of the data have been received in detailed as well as
in summarised form from the manufacturers (Chevron Chemagro and
Bayer-reports in file), although with some supplementary material from
other sources (Davis et al., 1974).
Generally vegetable crops are treated with 0.5 - 1 kg/ha of
methamidophos, which leaves residues of a non-persistent nature and
with a well-known distribution pattern, viz. the outer leaves,
especially of leafy vegetables, carry considerably higher residues
than the internal parts of the plants. Some of these outer leaves may
or may not be discarded as "trimmings" when marketed.
With only occasional high values, the data in Table 4 indicate
that residues of methamidophos will rapidly dissipate to low levels.
With appropriate pre-harvest intervals, from 1-3 weeks, the average
residues in all vegetables are usually well below 1 mg/kg.
TABLE 3. Recommended use patterns of methamidophos
Crop Dose, Number Recommended
kg a.i./ha of pre-harvest
treatments interval, days
Cotton 0.05-1.5 3-5 21
Cucumbers 0.4 -0.75 3-4 3
Tomatoes 0.4 -0.75 3-4 3
Cauliflower 0.4 -1.12 3-5 7
(U.S.A. 14)
Brussels sprouts 0.56-1.12 3-5 7
Broccoli 0.56-1.12 3-5 7
Other brassicas 0.4 -1.12 3-5 7
Celery 0.56-1.12 5 21
Lettuce 0.56-1.12 3-4 21
Potatoes 0.5 -1.12 3 7
Maize 0.9 -1.5 3 21
Sugar beet 0.5 -0.6 3-5 28
Stone fruit 0.5 -1.0 2-3 21
(peach)
Tobacco 0.3 -0.6 3-4 14
Hops 0.3 -2.0 4 21
Rape 0.28-0.56 2 10
TABLE 4. Residues of methamidophos in vegetable crops (supervised trials)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Broccoli
heads 0.56-1.12 3-6 2 w n.d. - 2.84 0.45 21 U.S.A.
3 w n.d. - 0.14 0.03 9
4 w n.d. - 0.09 0.02 7
leaves 1.12 3-6 2 w 0.22 - 7.62 2.50 7 U.S.A.
3 w n.d. - 0.88 0.28 7
4 w n.d. - 0.54 0.16 7
total 1.12 3-6 2 w 0.16 - 3.74 1.41 7 U.S.A.
3 w n.d. - 0.57 0.18 7
4 w n.d. - 0.44 0.13 6
Brussels sprouts
heads 1.12 3-5 2 w 0.07 - 0.47 0.26 6 U.S.A.
3 w 0.08 - 0.64 0.27 6
4 w 0.05 - 0.22 0.12 6
leaves 1.12 3-5 2 w 0.16 - 0.17 0.17 2 U.S.A.
3 w 0.06 - 0.21 0.14 2
4 w n.d. - 0.10 0.05 2
total 1.12 3.5 2 w 0.10 - 0.11 0.11 2 U.S.A.
3 w 0.12 0.12 2
4 w 0.05 - 0.08 0.07 2
Cabbage
heads 0.56 - 1.12 2-9 1 w n.d. - 0.7 0.11 11 U.S.A.
2 w n.d. - 0.35 0.04 11 GFR
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Cabbage
savoy 0.3 3 1 w 0.09 1 GFR
2 w 0.07 1
Cauliflower
heads 0.56 - 1.12 3-12 2 w n.d. - 0.45 0.11 16 U.S.A.
3 w n.d. - 0.23 0.06 7 GFR
4 w n.d. - 0.12 0.03 7
leaves 0.56 - 1.12 3-12 2 w 0.1 - 6.28 1.85 8
3 w n.d. - 1.11 0.46 7
4 w n.d. - 0.62 0.24 7
Celery
stalks 1.12 5-7 3 w 0 - 1.55 0.33 6 U.S.A.
tops 1.12 5-7 3 w 0.08 - 6.0 2.59 6
whole plant 1.12 5-7 3 w 0.02 - 2.0 0.74 6
Cucumber 0.6 4-6 0 d 0.19 - 0.45 0.35 3 Mexico
(dust treatment) 1 d 0.13 - 0.44 0.32 3
3 d 0.05 . 0.41 0.24 3
5 d 0.06 - 0.2 0.15 3
7 d 0.03 - 0.31 0.16 3
Eggplant 0.45 - 0.6 7-9 0 d 0.03 - 0.10 0.07 2 Mexico
1 d 0.02 - 0.05 0.04 2
3 d 0.05 - 0.06 0.06 2
5 d 0.03 - 0.04 0.04 2
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Lettuce 7 d 0.05 - 0.06 0.06 2
head (1) 0.56 - 1.12 3-8 2 w 0.01 - 0.68 0.24 12 U.S.A.
3 w n.d. - 0.02 n.d. 5
wrapper
leaves (2) 1.12 3-4 3 w 0.1 - 0.77 0.19 5 U.S.A.
(1) + (2) 1.12 3-4 3 w n.d. - 0.25 0.07 5 U.S.A.
head 1.12 5 2 w n.d. - 1.40 0.21 9 U.S.A.
wrapper 3 w n.d. n.d. 6
leaves (2) 1.12 5 2 w 0.01 - 2.42 0.78 5 U.S.A.
3 w 0.02 - 0.15 0.07 6
(1) + (2) 1.12 5 2 w 0.05 - 0.93 0.31 5 U.S.A.
3 w n.d. - 0.39 0.09 6
Peppers 0.34 - 1.12 4-20 1 w 0.04 - 2.3 1.00 7 U.S.A.
2 w 0.08 - 1.09 0.38 8 Mexico
3 w n.d. - 0.58 0.16 8 ) Australia
4 w n.d. n.d. 4 )
Potatoes 1.12 6-8 1 w n.d. - 0.11 0.04 12 U.S.A.
2 w n.d. n.d. 5 U.S.A.
Canada
Tomatoes 0.5 4 1 d - 0.50 1 Venezuela
3 d - 0.28 1
7 d - 0.22 1
10 d - 0.23 1
14 d - 0.20 1
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
18 d - 0.19 1
Tomatoes 0.6 - 1.33 4-10 0 w 0.10 - 5.07 1.05 17 Australia
1 W 0.08 - 1.83 0.55 18 U.S.A.
2 w 0.03 - 1.62 0.30 18 Venezuela
3 w 0.03 - 0.18 0.09 6
In oil seeds, hops and sugar beets
Rape and cotton plants have been treated with varying amounts of
methamidophos, ranging from 0.28 kg/ha up to an overdose of 2.24 kg/ha
and with up to 4 applications. In these experiments residues in the
harvested seeds ranged from undetectable to 0.06 mg/kg 21-26 days
after the last treatment, and no residues could be detected at 32-72
days after application.
Limited residue data for methamidophos in hops are available.
After six applications with 0.66 kg/ha dried hops contained 3.0 mg/kg
at 17 days. After 3 spray applications with 1.8 kg/ha, the residue
levels of methamidophos amounted to 1.4-2.8 mg/kg at 20 days.
Sugar beets were sprayed on the foliage 4 times with 0.48 kg/ha.
The residue levels 23 days after the last application were 0.01 mg/kg
of methamidophos in the roots and 0.6 mg/kg in the leaves. After 28
days the roots were free of residues, while the tops contained 0.9
mg/kg methamidophos.
In peaches
Peaches were sprayed with 1 kg/ha of methamidophos under German
climatic conditions. 1 week after the second of 2 applications
residues varied from 0.50-1.05 mg/kg (average, 0.73 mg/kg), and 1 week
later they were 0.28-0.75 mg/kg (average 0.45 mg/kg).
FATE OF RESIDUES
The identified metabolites formed from methamidophos are shown in
Figure 1.
In animals
Studies of the absorption, distribution and excretion of
methamidophos in the rat (Crossley and Tutass, 1969), and lactating
goat (Lee and Crossley, 1972) are described in the section
"Biochemical aspects," p. ). Some features of these studies,
particularly the distribution in tissues, are amplified below.
Female and male rats each received a single dose of radiolabelled
methamidophos, administered intragastrically as an aqueous solution
(Crossley and Tutass, 1969). The doses received by each rat were
0.16-0.19 mg of 14C methylthio-labelled methamidophos and 0.21 mg of
32P-labelled methamidophos. In both experiments, the majority of the
applied dose was excreted within 24 hours. This period of fast
elimination in which over 50% of the activity was excreted was
followed by a second phase during which only 1-2% of the applied dose
was eliminated daily.
In the experiments with 14C-labelled methamidophos, the major
excretion route was via the breath (approx. 39% of the applied dose);
urine and faeces contained 11.1% and 1.5% respectively. In the
experiments with 32P-labelled methamidophos, on the other hand, the
majority of radioactivity was eliminated in the urine (until day 14),
and up to about 20% was eliminated in the faeces. There was no
accumulation in tissues or organs (see Table 5).
TABLE 5. Distribution of radioactivity in tissues and organs of
rats after dosing with labelled methamidophos (Crossley
and Tutass, 1969)
Radioactivity, % of applied dose
Organ/ 14C 32P
tissue female rats male rats female rats
Day 5 - 9 Day 1 Day 28 Day 1 Day 28
Carcass 21.9 9.9 6.7 11.3 4.4
Liver 0.4 6.9 0.1 5.6 0.1
Kidney 0.1 0.5 0.0 0.4 0.0
Heart 0.3 0.1 0.0 0.1 0.0
Lung 0.1 - - - -
Fat - 0.1 0.0 0.2 0.0
Muscle - 0.2 0.1 0.3 0.1
Total 22.6 17.4 6.9 17.4 4.6
The degradation of methamidophos in the animal body is hydrolytic
in nature (Crossley & Tutass, 1969; Tutass, 1968). The degradation
sequence (Crossley & Tutass, 1969), is shown above
("Biotransformation"). Data on the quantitative distribution of
residues 32P residues in the urine are presented in Table 6.
In the faeces, the majority of radioactivity was not extractable
with either water or acetone. That which was extractable showed the
same degradation profile as in the urine. The non-extractable
radioactivity was in all probability incorporated into natural
biochemical constituents.
TABLE 6. Distribution of 32P in urine of rats dosed with
32P-methamidophos (Crossley and Tutass, 1969)
32P, % of total in sample
Metabolite* Male rats Female rats
Day 1 Day 2 Day 1 Day 2
methamidophos (I) 40 10 40 trace
OS-dimethyl
phosphorothioate (II) 5 - 5 -
methylphosphoric
acid (III) 40 25 40 30
phosphoric acid (IV) 15 65 15 70
* Numbers in parenthesis refer to Figure 1.
S-methyl-14C-methamidophos was given orally to a goat in order
to assess its fate in a lactating ruminant (Lee and Crossley, 1972).
The goat received 3.75 mg. of 14C methamidophos daily after the
morning milking for 7 consecutive days. Methamidophos was rapidly
metabolized and about half of the radioactivity rapidly excreted. This
excreted radioactivity was distributed mainly between the breath and
urine and, to a lesser extent, the faeces and milk. The activity that
remained in the animal after the initial rapid excretion was
eliminated at a slower but constant rate. None of the radioactivity
remaining in the body was concentrated in any one organ, although
slightly higher levels were found in the liver than elsewhere. The
distribution of radioactivity in the goat is summarized in Table 7.
The results are essentially the same as those found in the rat study
(Table 5), although a somewhat higher proportion was excreted in the
breath of the rats.
TABLE 7. Distribution of radioactivity in goat dosed with 14C-methamidophos expressed as
percentage of applied dose or mg/kg methamidophos equivalents (Lee and Crossley, 1972)
Sample Dosing Period Recovery Period Total Blood and Tissues
(days 0 - 8) (days 9 - 17) Excreted
% % % % mg/kg
Urine 17.24 0.63 17.87 Bloodx 5.87 -
Faeces 4.00 0.98 4.98 Liver 3.93 0.22
Milk 2.49 0.74 3.23 Kidney 0.61 0.16
Total 23.73 2.35 26.08 Heart 0.61 0.16
Mammary 0.82 0.10
Brain 0.21 0.08
Fatxx 4.13 0.16
Musclexxx 33.10 0.16
Total recovered = 75% Total 49.28 1.04
x Assuming blood to be 10% of the body weight
xx Assuming fat to be 5% of the body weight
xxx Assuming muscle to be 40% of the body weight
TLC of urinary samples revealed the presence of methamidophos and
the first metabolite OS-dimethyl phosphorothioate (II in Figure 1). In
the sample taken 12 hours after the third dose, 7% of the activity in
the urine was found to be parent compound and 16% metabolite II.
Extraction procedures applied to the samples of tissues, blood, milk,
urine and faeces extracted only small proportions of their activity,
indicating that the main source of activity was not methamidophos. The
maximum possible level of methamidophos in milk estimated on this
basis was 0.008 mg/kg. The maximum content in the milk found by direct
analysis was 0.003 mg/kg (about 1% of the total activity in the milk).
Since the data obtained from the goat are similar to those from the
rats; it is believed that the non-extractable activity in the goat
tissues, milk, etc. is due to the incorporation of 14C into the
natural constituents of the body, as in rats.
In plants
Methamidophos is an insecticide with systemic activity (Werner,
1972). The physical and chemical basis of systemic movement in the
cotton plant was investigated by Hussain et al. (1974).
Root and leaf treatment of tomato, cabbage and bean plants
demonstrated that 14C-methamidophos is readily taken up by roots and
leaves and transported with the transpiration water towards the
margins of leaves, while little is retained in the stem and petioles.
The labelled compound accumulating at the margins of leaves is
essentially only methamidophos, while in the centre of the leaf some
more polar compounds are present, apparently as the result of
metabolic degradation. The uptake of methamidophos by roots appears to
be independent of pH within the physiological range of 3.5-7.5
(Tutass, 1968b).
Horler et al., (1975) showed that in tomato plants there is only
slight movement of the intact insecticide from treated leaves to the
fruit. After rapid penetration of 14C-methamidophos or/and
32P-methamidophos into tomato leaves, the residue in leaves and fruit
in the initial phase has a biological half-life of 7-10 days. However,
especially in the fruit, after this initial period the rate slows to
give a half-life of about 6 weeks (Horler et al., 1975). The authors
assume that intact insecticide is lost from fruits and leaves by a
mechanism associated with transpiration, rather than by metabolism and
translocation.
Werner (1973) also reports rapid absorption of
methylthio-14C-methamidophos by the roots of Loblolly pine seedlings.
After 96 hours exposure, an accumulation of radioactivity was evident
in the needle tips. Besides unchanged methamidophos, 3 compounds were
isolated from the seedlings. One was identified as the major
metabolite, OS-dimethyl phosphorothioate (II, Figure 1), and in
addition two unidentified compounds were detected, of which one
(Unknown 1) was probably phosphoric acid (VI, Figure 1).
Methamidophos, II and Unknown 1 were detected in the roots, stems and
needles, but Unknown 2 only in the needles, of seedlings treated for
72 and 96 hours (see Table 8).
TABLE 8. Percentage distribution of radioactive methamidophos
and 14C-compounds from TLC and GLC of pine seedling
extracts (Werner, 1973)
Seedling exposure period (h)
Compound* Plant part 6 12 24 48 72 96
I (methamidophos) Roots 80.0 46.0 40.0 35.7 3.3 0.9
Stem 0 0 1.6 1.0 1.1 1.7
Needles 0 31.0 27.9 26.3 22.0 30.5
II (OS-dimethyl Roots 20.0 3.8 1.6 1.0 3.3 3.5
phosphorothioate)
Stem 0 3.8 13.1 6.1 11.0 10.4
Needles 0 46.0 26.2 18.4 16.5 17.4
Unknown 1 Roots 0 0 1.6 1.0 2.2 1.7
Stem 0 3.8 4.9 3.1 4.4 2.6
Needles 0 19.2 18.1 17.4 19.7 10.4
Unknown 2 Roots 0 0 0 0 0 0
Stem 0 0 0 0 0 0
Needles 0 0 0 0 16.5 20.9
* Roman numerals refer to Figure 1.
Experiments with methylthio-14C-methamidophos on cabbages and
tomatoes as well as on plant tissue cultures of sweet potatoes and
tobacco have shown that degradation occurs chiefly by hydrolysis
(Chevron, 1968). Hydrolysis first occurs at the P-N bond to yield
OS-dimethyl phosphorothioate (II, Figure 1), which was consistently
detected as a metabolite in all tissues investigated. Continued,
though somewhat slower, hydrolysis of II apparently leads to cleavage
of CH3S-P and CH3O-P bonds yielding VII and III as major products.
Quantitative data on the residues of these metabolites are not given.
The final fate of the radiolabel is its incorporation into plant
constituents such as pigments, amino acids, carbohydrates and
structural material (up to 19% of the activity in the sample of
cabbage plants).
In soil
Methamidophos is degraded at a relatively fast rate in soils. In
laboratory experiments, Leary and Tutass (1968) found half-lives of
1.9 days in silt soil, 4.8 days in loam soil and 6.1 days in sandy
soil. Sterilization of silt soil by autoclaving resulted in a
considerable increase in the half-life, to about 6 weeks.
In laboratory experiments conducted by Horler et al. (1975),
methamidophos was also found to undergo rapid degradation. In basic
soils (pH 7.40 and 7.75), only 3-7% of the original activity was found
after 4 days; in an acid soil (pH 4.30), 25% was present after 9 days.
In the basic soils, there were differences in the degradation of the
metabolites. In clay-loam soil (pH 7.40), the metabolite degraded at a
slower rate than in loam soil (pH 7.75). Since both soils had a
similar pH, such differences may be connected with the fact that
microorganisms play a role in the decomposition.
In degradation experiments with field soils sampled from depths
of 0-10 cm, and from depths of 0-15 cm and 15-30 cm, it was noted that
at sampling on the day of application to the sail, only a fraction of
the applied methamidophos was still present (Bayer, 1972; Chemagro,
1974). Within one week, the residue dropped further to 10% of the
level measured on the day of application. At depths of 15-30 cm, only
very small amounts of residue (maximum of 0.04 mg/kg) were found in
the soil, and no accumulation was noted after several applications.
Leary and Tutass (1968) found that metabolic degradation of
S-methyl-14-methamidophos in silt soil under aerobic conditions
resulted after 3 days in the evolution of 70% of the applied dose as
14CO2. On the other hand, only 7.6% was evolved as 14CO2 under
aerobic conditions, with 84.6% of the applied activity remaining in
the soil. Up to about 30% of the activity that remained in the soil
was not acetone-extractable. Most of the extractable radioactivity was
in the form of OS-dimethyl phosphorothioate (II, Figure 1), while
minor amounts were in the form of methamidophos, amino acids and
carbohydrates. Quantitative data on these constituents were not given.
Horler et al (1975) also identified OS-dimethylphosphorothioate
(II) as the major metabolite in soil. However, they also found very
small amounts of V, VI and III in clay loam soil. In loam soil, VI was
not found. It is stated that the loss of the methoxy group results in
the evolution of CO2. Quantitative data are not given.
In water
The hydrolysis of methamidophos was examined at elevated
temperatures in aqueous solutions as a function of pH (Leary, 1968;
Magee, 1966). In solutions with a pH below 2, the half-life was a
matter of hours. At pH 9, the half-life was 2.6 days at 25°C and 1.5
days at 37°C. In mildly acidic or neutral solutions, methamidophos
showed remarkable stability at temperatures up to 80°C (less than 5%
hydrolysis in 25 hours). The P-NH2 bond is broken first, followed by
hydrolysis of the CH3SP grouping.
Leaching experiments with methamidophos and three soil types
(muck, sandy loam and loam) (Tutass, 1968c), showed little retention
of the compound. Soil type apparently had only a minor effect upon
retention. The compound was least retained in loam.
Experiments to study the leaching behaviour of methamidophos with
a standardized soil column technique revealed that about two-thirds of
the compound was leached out of sandy and sandy loam soils while only
about one-fifth was leached out of a soil with an increased content of
organic matter (Bayer, 1972).
Flint and Shaw (1972) also studied the absorption of
methamidophos by and its leaching from three different soil types. The
absorption by soil was very slight, and it was further decreased by
increasing temperature and decreasing pH. Methamidophos was not
retarded by the soil when passed through soil columns in water. The
chemical was found in soil run-off water only in the first two of five
irrigations. It was degraded in a natural water system outdoors with
half-lives of 15.9 days in water and 7.5 days in silt. It was
concluded that although it was leached, methamidophos was degraded
rapidly in natural water systems.
In fish
Bass fingerlings which had been in water containing 0.01 mg/1 of
methamidophos for eight days, were analyzed. Less than 0.02 mg/kg of
methamidophos was found in all samples (Stanley, 1971 b).
In processing and storage
The effect of simulated commercial processing on methamidophos
residues in tomatoes was investigated by Morris and Olson (1974). The
tomatoes were processed to juice (pressed, pasteurized), to canned
tomatoes (sterilized), to ketchup. (sterilized, pasteurized), to dry
tomato pomace and to dry tomato pulp. The residues in juice, canned
tomatoes and ketchup were smaller than those found in whole tomatoes,
amounting to 89.5, 73.0 and 68.4% respectively, of the residue levels
prior to processing. On the other hand, the residues in dry tomato
pomace and dry tomato pulp were 2.0 and 2.5 times the original levels
respectively.
The effect on residues of washing in a manner simulating
commercial packing practice depended upon the treated crop. After
treatment, the fruits were allowed to stand outdoors for 24 hours.
Whereas washing green peppers and eggplants did not remove any
significant residue, washing tomatoes removed approximately 15% and
washing cucumbers approximately 20% of the methamidophos residue
(Thornton, 1973).
Dehydrated celery flakes were prepared commercially by placing
celery leaves on trays and drying at temperatures of up to 60°C
(Morris, 1975). Dehydration removed 80% of the weight as water, and it
was concluded that the dehydration process did not destroy any of the
methamidophos residue present in fresh celery leaves.
Cottonseed was processed into delinted seed, meal, hulls. and
oil. No residue was found in any of the seed samples or in any of the
fractions from the processed seed. The residue found in the trash
ranged from 0.1 to 2.7 mg/kg (Chevron, 1976).
Rapeseed was processed into oil and meal (Chemagro, 1976). The
following residue levels were measured: rapeseed, 0.02-0.25 mg/kg,
rapeseed oil, n.d.-0.02 mg/kg and rapeseed meal 0.01-0.30 mg/kg.
Leary (1968) conducted a study to determine the stability of
weathered residues of methamidophos on chopped crop samples (broccoli,
lettuce, cabbage, cauliflower, Brussels sprouts) stored deep frozen
for several months. No significant evidence of decomposition was
obtained and there was no indication of any trend towards
decomposition with increased time of storage.
Möllhoff (1971), however, noted that methamidophos residues
decreased by 10-40% in cauliflower samples stored for three months at
-20°C, and by up to 10% in tomatoes stored under the same conditions.
In further studies conducted by Chemagro (1976), to investigate
the effect of frozen storage at about -20°C on methamidophos residues
in peanut meat, peanut forage, sorghum grain, rapeseed, celery and
peppers, decomposition ranged from 0 to 22%.
The effect of frozen storage at about -20°C on methamidophos
residues in cattle tissues and milk and in poultry tissues and eggs
has also been studied (Chemagro, 1976). It was found that
decomposition in poultry liver amounted to 33% after 60 days and in
cattle liver to 85% after 150 days. On the other hand, the residues
remained completely stable in cattle steak, fat and milk, and
decomposition amounted to a maximum of only 13% in eggs, heart and
gizzard of poultry.
METHODS OF RESIDUE ANALYSIS
Methods for the gas-chromatographic determination of
methamidophos have been developed and published in several
modifications, but in practically all cases are based on clean-up
procedures with silica gel columns and GLC with thermionic detection.
Möllhoff (1971) developed a method for determination in plants,
soil and water. Maceration with acetone or an acetone/water mixture is
used for extraction. The limit of determination is generally about
0.01 mg/kg, but for oil seeds only 0.05 mg/kg and for hops and tobacco
0.1 mg/ kg. Recoveries in the 0.01-1.0 mg/kg range vary between 80%
and 100%.
Leary (1971) also determined methamidophos in crops, but with the
use of ethyl acetate for extraction. The GLC instrument in this case
was equipped with a caesium bromide thermionic detector and the method
is claimed to have a limit of determination of 0.003-0.005 mg/kg. At
levels of 0.5-0.1 mg/kg the majority of recovery values were from
90-100%.
Both these methods have been tested for specificity in
conjunction with other organophosphorus insecticides. They were found
to be adequately specific and without interferences from extractives
with a number of different crops (Olson, 1973).
Stanley and co-workers have developed methods for residues in
different forage crops such as hay, peanut meal, sorghum grain and
rape seed (McNamara & Stanley, 1975) and in animal tissues and milk
(Stanley, 1971c). Extractions were with either methanol/chloroform or
acetonitrile and Skellysolve B.
With minor amendments these methods have been found suitable also
for the determination of methamidophos in fish and water with limits
of determination down to 0.02 mg/kg and 0.004 mg/kg respectively
(Stanley 1971 a).
These methods for the determination of methamidophos follow
closely the general pattern for organophosphorus insecticides in
current multi-residue procedures. Undoubtedly, therefore, they will be
suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
TABLE 9. National tolerances reported to the meeting
Country Crop Tolerance, Pre-harvest
mg/kg interval
Australia Capsicums 0.25 14 days
Peaches 0.25 21 days
Tomatoes 0.25 21 days
TABLE 9. (Cont'd.)
Country Crop Tolerance, Pre-harvest
mg/kg interval
Potatoes 0.05 7 days
Canada Broccoli, Brussels
sprouts, lettuce,
peppers 1.0
Cabbage, cauliflower,
cucumbers, eggplant,
tomatoes 0.5
Potatoes, melons negligible
residue
Netherlands Brassica 0.1
Potatoes 0.01x
New Zealand Brassicas, potatoes 0.1
Tomatoes 0.1 3 days
South Africa Peaches 1.0 21 days
Cabbage 1.0 21 days
Potatoes 1.0 14 days
U.S.A. Cauliflower, lettuce
tomatoes 2.0
Broccoli, Brussels
sprouts, cabbage,
peppers, cucumbers,
eggplant 1.0
Melons 0.5
Cottonseed, potatoes 0.1xx
x = limit of determination
xx = negligible residues
APPRAISAL
Methamidophos is a chemically simple organophosphorus insecticide
with broad spectrum effects used to control caterpillars, aphids,
larvae etc. It is active by contact as well as systemically.
Since 1969 methamidophos has been registered in a number of
countries around the world, chiefly in regions with a sub-tropical
climate. Its major uses are pre-harvest treatments of industrial
crops, fruit and vegetables, mainly with spray solutions at rates from
0.5-1.5 kg/ha.
Detailed studies on the metabolic fate of methamidophos indicate
that the compound is readily taken up by plant roots and translocated
with the transpiration stream within the plants, i.e. towards the
edges of the foliage. In studies with the radio-labelled compound, the
further fate has been followed and a complete degradation pathway with
final incorporation into plant constituents is suggested, mainly
through steps involving hydrolytic breakdown.
The degradation of methamidophos in the animal body is also
hydrolytic and there are no signs of accumulation in tissues or
organs. Most of the phosphorus-containing moiety of methamidophos (up
to about 70%) is eliminated via the urine, while a major part of the
carbon (about 40% in the rat) is excreted as CO2 with the breath, the
remainder being found in the urine and faeces.
The results of extensive residue trials on a number of vegetable
and agricultural crops are available. The data confirm methamidophos
as a systemic compound of relatively low persistence. Most of the data
are from trials under practical conditions which are likely to
represent good agricultural practice.
Methamidophos penetrates into the flesh of the fruit in, e.g.,
tomatoes, peppers, eggplants, etc. Washing or peeling such products
therefore does not significantly remove residues. In the frozen
storage of crops treated with methamidophos, residues are found to be
stable for several months.
Methamidophos has been found to be degraded fairly rapidly in
soils and in natural water systems without any signs of accumulation
or concentration in living organisms.
Gas-chromatographic methods making use of thermionic detectors
are available for the determination of methamidopho in various crops
and animal products. They are combined with appropriate extraction and
clean-up procedures and are likely to be suitable for regulatory
purposes.
RECOMMENDATIONS
Maximum residue limits are recommended for methamidophos in the
following commodities at the levels indicated. They refer to the
parent compound.
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Hops 5 Peppers 1
Broccoli 2 Sugar beet leaves 1
Cauliflower 2 Cottonseed 0.1
Celery 2 Potatoes 0.1
Lettuce 2 Rapeseed 0.1
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Tomatoes 2 Sugar beets 0.1
Brussels Meat and fat of
sprouts 1 cattle, goats
Cabbage 1 and sheep 0.01*
Cucumber 1 Milk 0.01*
Eggplant 1
Peaches 1
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies an the teratogenic potential in a suitable
species.
2. Further studies to elucidate the contribution of acephate and
metamidophos alone or in combination in depressing cholinesterase
activity.
3. Further information on the level and fate of residues during
cooking and processing of fruit and vegetables.
REFERENCES
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E.
1971 Mutagenic Study with Monitor Technical (SX No.
171) in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Arnold, D., Keplinger, M.L. and Fancher, O. E. Three Generation
1970 Reproduction Study on SX-171 (Technical RE-9006 -
75%) in Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Bayer Berichte No. 12,13/1972; No. 11,12/1973. Submitted
1972 to the Joint Meeting.
Carlson, D., Vondruska, J. F. and Fancher, O. E. Effects of RE 9006
1969 - 75% SX-171 on the Cholinesterase Activity in the
Beagle Dog. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Chevron Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity of
1968a Monitor Technical in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1908b of Monitor 6S in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, D.H. Acute Oral Toxicity
1968c of RE 9006 (95%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968d of RE 9006 (75%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.D. Acute Dermal Toxicity
1968e of Monitor 6S. Unpublished report from Industrial
Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1968f of Monitor Technical. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968g of RE 9006 (95%) in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968h of RE 9169 (SX-196) in Rats. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
the Chevron Chemical Company.
Cavalli, R.E., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1969 RE 9169 (SX 198) in Rabbits. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
Chevron Chemical Company.
Cavalli, R.E. and Spence, J.A. Effect of Repeated Doses of RE 12420
1970 on Cholinesterase Activity in Rats. Unpublished
report from Standard Oil of California, submitted
to the WHO by Chevron Chemical Company.
Chemagro Reports nos. 44249, 44250 on methamidophos in
1974 soil. Submitted to the Joint Meeting.
Chevron Metabolism of Monitor insecticide by plants.
1968 Chevron report submitted to the Joint Meeting. In
file at FAO, Plant Protection Division.
Crawford, C.R. and Anderson, R.H. The Acute Toxicity of Monitor
1973 Technical in Combination with Marathion to Female
Rats. Unpublished report from Chemagro Division of
Baychem Corporation, submitted to the WHO by
Bayer, A.G.
Crossley, J. and Tutass, H.O. Metabolism of Monitor Insecticide
1969 by rats. Unpublished report no. 721.14 from the
Ortho Division, Chevron Chemical Company,
submitted to the WHO by Bayer, A.G.
Davis, A.C., Bourke, J.B. and Kuhr, R.J. Disappearance of Monitor
1974 Residues from Cole crops. J. Econ. Entomol. 67,
766-768.
Fletcher, D., Jenkins, D.H., Keplinger, M.L. and Fancher, O.E.
1971 Demyelination Study with Monitor (RE 9006)
Technical in Mature Laying Hens. Unpublished study
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Flint, D.R. and Shaw II, H.R. The Mobility and persistence of
1972 MONITOR in soil and water. Report No. 34 483
submitted by Chemagro.
Garofalo, M., Palazzolo, R. and Sanders, R. A Study on the Effect
1973 of Orthene and Monitor on Plasma and Erythrocyte
Cholinesterase Activity in Human Subjects During
Subacute Oral Administration. Unpublished report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Chevron Chemical Company.
Greco, R.A., Lindberg, D.C., Keplinger, M.L. and Fancher, O.E.
1971 Effect of RE 9006 on Plasma and Erythrocyte
Cholinesterase Activity in Beagle Dogs.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Gröning, P. Tamaron. Special Toxicity Studies (Potentiation).
1975 Unpublished report from Institut für Toxikologie,
submitted to the WHO by Bayer, A.G.
Gröning, P. Bayer 17628 (Tamaron) Untersuchung Der Subchronischen
Toxizitat an ratten (3-monate-futterungversuch).
Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Gröning, P. and Lorke, D. Untersuchung der subchronischen toxizitat
1976 an hunden. Unpublished report from the Institut
für Toxikologie, submitted to the WHO by Bayer,
A.G.
Hanna, P.J. and Dyer, K.F. Mutagenicity of Organophosphorus Compounds
1975 in Bacteria and Drosophila. Mut. Res. 28:
405-20.
Horler, D.F., Lubkowitz, J.A., Revilla, A.P., Baruel, J. and Cermeli,
1975 M.M. Uptake and Degradation of Monitor by Tomato
Plants. Pesticides, IUPAC 3rd Int. Congr. Pest.
Chem., Helsinki, 151-156.
Hussain, M., Fukuto, T.R., and Reynolds, H.T. Physical and Chemical
1974 Basis for Systemic Movement of Organophosphorus
Esters in the Cotton Plant. J. Agr. Fd Chem. 22
(2): 225-230.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L.
1971 Teratogenic Study with Monitor Technical in Albino
Rabbits. Unpublished report from Industrial Bio
Test Laboratories, Inc. submitted to the WHO by
Bayer, A.G.
Leary, J.B. Stability of Monitor insecticide residues in frozen
crops and in aqueous solutions. Chevron report
files No. 740.01 and No. 721.2.
Leary, J.B. and Tutass, H.O. Degradation of Monitor insecticide
1968 in soil. Chevron report file No. 721.2.
Leary, J.B. Gas-chromatographic Determination of Monitor
1971 (OS-Dimethyl Phosphoramidothioate) Residues in
Crops. J. Ass. Off. Analyt. Chem. 54 (6):
1396-98.
Lee, H. and Crossley, J. The Fate of Monitor in a Lactating
1972 Ruminant (Goat). Unpublished report from Ortho
Division, Chevron Chemical Company, submitted to
the WHO by Bayer, A.G.
Lindberg, D., Vondruska, J.F. and Fancher, O.E. Two Year Chronic
1969 Oral Toxicity of RE 9006-III, SX-116 in Beagle
Dogs. Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO, by
Bayer, A.G.
Lindberg, D., Keplinger, M.L. and Fancher, O.E. RE 9006 (70%)
1970 SX 171 on the Cholinesterase in the Beagle Dog.
Unpublished study from Industrial Bio-Test
Laboratories Inc., submitted to the WHO by Chevron
Chemical Company.
Lorke, D. and Kimmerle, G. Toxicological Studies on the Active
1967 Ingredient Bayer 71 628. Unpublished report from
the Institut für Toxikologie, submitted to the WHO
by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on Dogs
1970a (Three Month Feeding Experiment). Unpublished
report from Institut für Toxikologie, submitted to
the WHO by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on rats.
1970b Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Magee, P.S. Hydrolysis of Monitor insecticide. Chevron Report
1966 File No. 721.2.
Mastri, C., Keplinger, M.L. and Fancher, O.E. Twenty-one Day
1968 Subacute Dermal Toxicity of Monitor (RE 9006) 75%
Technical SX 171. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Chevron Chemical Company.
McNamara, F.T., and Stanley, C.W. Gas-Chromatographic Method for
1975 the Determination of Residues of MONITOR in Peanut
Meat and Hay, Sorghum Grain, and Rapeseed.
Chemagro Report No. 45.439.
Möllhoff, E. Metode zur gaschromatographischen Bestimmung
1971 von Tamaron-Rückständen in Pflanzen.
Pflanzenschutz-Nachr. 24 (2): 256-262.
Morris, R.A. Effect of Dehydration on Residues of MONITOR
1975 in Celery Leaves. Chemagro Report No. 45.586.
Morris, R.A., and Olson, T.J. The Effect of Processing on MONITOR
1974 Residues in Tomatoes. Chemagro Report No. 41.107.
Olson, T.J. Interference Study on MONITOR (TAMARON) in Various
1973 Crops. Chemagro Reports No. 37.380 and No. 37.392.
Plank, J., Keplinger, M.L. and Fancher, O.E. Paired Feeding Study
1968 of RE 9006 - III SX-116 Albino Rats. Unpublished
report from Industrial Bio-Test Laboratories.
Inc., submitted to the WHO by Chevron Chemical
Company.
Plank, J., Keplinger, M.L. and Fancher, O.E. Ninety-Day
1969 Cholinesterase Study of SX-171 Monitor (RE 9006) -
Albino Rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Bayer, A.G.
Plank, J., Keplinger, M.L. and Fancher, O.E. Two Year Chronic
1970 Oral Toxicity of RE 9006 - III, SX-116 in Albino
Rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Ninety-Day Subacute
1973 Oral Cholinesterase Study with Monitor Technical
in Female Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Schoenig, G., Keplinger, M.L. and Fancher, O.E. Study on the Efficacy
1968 of Atropine Sulfate and 2-PAM C1 as Antidotes for
Monitor (RE 9006) 75% Technical SX-171. Report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971a of MONITOR in Fish and Water. Chemagro Report No.
30.975.
Stanley, C.W. Analysis of Bass and Water for MONITOR. Chemagro
1971b Report No. 30.979.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971c of Residues of MONITOR in Animal Tissues and Milk.
Chemagro Report No. 31.093.
Thornton, J.S. Effect of Washing on Residues in Tomatoes, Cucumbers,
1973 Eggplants and Peppers. Chemagro Reports Nos.
37322, 37539, 37540 and 37541 submitted to the
Joint Meeting. In File at FAO, Plant Protection
Division.
Tucker, B. Acetylcholinesterase Inhibition of Orthene and Ortho
1972 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the WHO by
Chevron Chemical Company.
Tutass, H.O. Microsomal Oxidation of Monitor. Unpublished report
1968a no. 721.2 from the Ortho Division of Chevron
Chemical Company, submitted to the WHO by Bayer,
A.G.
Tutass, H.O. Uptake and Translocation of Monitor Insecticide
1968b by tomato, cabbage and bean plants. Chevron Report
File No. 721.2.
Tutass, H.O. Leaching of Monitor insecticide in soils. Chevron
1968c Report File No. 721.2
Werner, R.A. Systemic insecticidal action of disulfoton and
1972 Monitor in Loblolly pine seedlings. J. Georgia
Entomol. Soc. 7 (1): 64-67.
Werner, R.A. Absorption, Translocation and Metabolism of Root-
1973 Absorbed 14C Monitor in Loblolly Pine Seedlings.
J. Econ. Entomol. 66 (4): 867-872.
Wolvin, A.A., Palazzolo, R.J. and Fancher, O.E. Neurotoxicity
1968 Study - Chickens Monitor RE 9006, 75% Technical.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
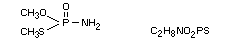 Other information on identity and properties
Molecular weight: 141.1
State: yellowish to colourless crystals
(technical)
colourless crystals (pure)
Melting point: 37 - 39°C (technical)
44.5°C (pure)
Vapour pressure: 3 x 10-4 mm Hg at 30°C (pure)
Density: 1.31
Solubility: Readily soluble in water, alcohols
ketones, aliphatic chlorinated
hydrocarbons; sparingly soluble
in ether; practically insoluble
in petroleum ether.
Stability (alkaline Half-life of 120 hours at pH 9
and acidic conditions and 37°C Half-life of 140 hours at
pH 2
and 40°C
Impurities in the technical product
Maximum level, %
Total impurities 27
OO-Dimethyl phosphoramidothioate 12
Other methyl esters of phosphoramido
and phosphoro-N-methylamidothioates 12
Methyl esters of phosphoric and
phosphorothioic acids 8
Phosphate, sulphate, sulphonium ion,
water 8
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male and female rats were orally administered methamidophos
(32p-labelled) following a two-week preconditioning period where
a daily dose of 0.5 mg/kg was orally administered. Male rats only
were administered 14C-methamidophos with no preconditioning at the
same dose level. In the 14C study (label = S-CH3) absorption and
a distribution was rapid. Elimination of 14C02 within the first
6 hours accounted for 32% of the recovered radioactivity with 9% of
the dose in the urine. Over a 5-day period only 50% of the
administered dose was recovered (39% as 14C02 and the rest
predominantly in urine). The remainder of the 14C was probably
present as residual radioactivity in the body, distributed
randomly. The major tissue residue was found in the polar lipid
fraction with lesser amounts as phospholipids and proteins
(probably incorporated as natural products). Studies with 32p
confirm the rapid absorption and distribution of radioactivity in
rats. The major amount of material recovered following 32p labelled
methamidophos dosing was observed in urine within 1 day. Feces
contained small quantities that were continually excreted (<20% of
the dose was excreted in feces over a 28-day test interval). Thus,
methamidophos is rapidly absorbed and distributed in the body.
Methamidophos and metabolites are excreted predominantly as urinary
products (Crossley and Tutass, 1969).
The metabolic fate of methamidophos in a lactating ruminant
(goat) was essentially the same as reported for the rat. Small
amounts of radioactivity were observed in milk, predominantly as
the parent molecule shortly after dosing. About 1% of the
radioactivity (<0.003 mg/kg) in the milk was methamidophos, which
disappeared rapidly. Distribution of the applied radioactivity in
the goat was rapid, predominantly as exhaled 14C02, in urine
and as radioactivity incorporated into some tissues (muscle, liver,
fat). 24% of the administered radioactivity was excreted within the
dosing period. In tissues, the predominant residue was not the
parent compound as evidenced by solvent partition characteristics
(Lee and Crossley, 1972).
Further details of the fate of metamidophos in the rat and
lactating goat are given in the section "Fate of residues".
Biotransformation
Methamidophos is rapidly degraded primarily by cleavage of the
P-N bond yielding dimethyl phosphoric acid derivatives. Microsomal
preparations in vitro accelerated the hydrolysis of the
phosphoramidate bond (Tutass, 1968a). A qualitative sequence of
metabolism is as follows:
Other information on identity and properties
Molecular weight: 141.1
State: yellowish to colourless crystals
(technical)
colourless crystals (pure)
Melting point: 37 - 39°C (technical)
44.5°C (pure)
Vapour pressure: 3 x 10-4 mm Hg at 30°C (pure)
Density: 1.31
Solubility: Readily soluble in water, alcohols
ketones, aliphatic chlorinated
hydrocarbons; sparingly soluble
in ether; practically insoluble
in petroleum ether.
Stability (alkaline Half-life of 120 hours at pH 9
and acidic conditions and 37°C Half-life of 140 hours at
pH 2
and 40°C
Impurities in the technical product
Maximum level, %
Total impurities 27
OO-Dimethyl phosphoramidothioate 12
Other methyl esters of phosphoramido
and phosphoro-N-methylamidothioates 12
Methyl esters of phosphoric and
phosphorothioic acids 8
Phosphate, sulphate, sulphonium ion,
water 8
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Male and female rats were orally administered methamidophos
(32p-labelled) following a two-week preconditioning period where
a daily dose of 0.5 mg/kg was orally administered. Male rats only
were administered 14C-methamidophos with no preconditioning at the
same dose level. In the 14C study (label = S-CH3) absorption and
a distribution was rapid. Elimination of 14C02 within the first
6 hours accounted for 32% of the recovered radioactivity with 9% of
the dose in the urine. Over a 5-day period only 50% of the
administered dose was recovered (39% as 14C02 and the rest
predominantly in urine). The remainder of the 14C was probably
present as residual radioactivity in the body, distributed
randomly. The major tissue residue was found in the polar lipid
fraction with lesser amounts as phospholipids and proteins
(probably incorporated as natural products). Studies with 32p
confirm the rapid absorption and distribution of radioactivity in
rats. The major amount of material recovered following 32p labelled
methamidophos dosing was observed in urine within 1 day. Feces
contained small quantities that were continually excreted (<20% of
the dose was excreted in feces over a 28-day test interval). Thus,
methamidophos is rapidly absorbed and distributed in the body.
Methamidophos and metabolites are excreted predominantly as urinary
products (Crossley and Tutass, 1969).
The metabolic fate of methamidophos in a lactating ruminant
(goat) was essentially the same as reported for the rat. Small
amounts of radioactivity were observed in milk, predominantly as
the parent molecule shortly after dosing. About 1% of the
radioactivity (<0.003 mg/kg) in the milk was methamidophos, which
disappeared rapidly. Distribution of the applied radioactivity in
the goat was rapid, predominantly as exhaled 14C02, in urine
and as radioactivity incorporated into some tissues (muscle, liver,
fat). 24% of the administered radioactivity was excreted within the
dosing period. In tissues, the predominant residue was not the
parent compound as evidenced by solvent partition characteristics
(Lee and Crossley, 1972).
Further details of the fate of metamidophos in the rat and
lactating goat are given in the section "Fate of residues".
Biotransformation
Methamidophos is rapidly degraded primarily by cleavage of the
P-N bond yielding dimethyl phosphoric acid derivatives. Microsomal
preparations in vitro accelerated the hydrolysis of the
phosphoramidate bond (Tutass, 1968a). A qualitative sequence of
metabolism is as follows:
 (Crossley and Tutass, 1969)
A list of the identified metabolites of methamidophos is given
in Figure 1.
Effects on enzymes and other biochemical parameters
Methamidophos is an active, direct cholinesterase inhibitor.
In blood, cholinesterase depression was observed to be maximum
within 3 hours following oral acute intoxication of male rats.
Cholinesterase activity was maintained at a low level for at least
24 hours following acute poisoning. Three days after treatment, the
enzyme activity of whole blood returned to a level that was almost
normal (Lorke and Kimmerle, 1967).
(Crossley and Tutass, 1969)
A list of the identified metabolites of methamidophos is given
in Figure 1.
Effects on enzymes and other biochemical parameters
Methamidophos is an active, direct cholinesterase inhibitor.
In blood, cholinesterase depression was observed to be maximum
within 3 hours following oral acute intoxication of male rats.
Cholinesterase activity was maintained at a low level for at least
24 hours following acute poisoning. Three days after treatment, the
enzyme activity of whole blood returned to a level that was almost
normal (Lorke and Kimmerle, 1967).
 RBC cholinesterase appears to be more sensitive than plasma
cholinesterase to inhibition as noted by the differing I50 values.
I50(M)
Bovine RBC 3.1 x 10-5
Human Plasma 1.6 x 10-4
(Tucker, 1972)
The sensitivity of RBC relative to plasma cholinesterase to
inhibition by methamidophos was noted in a 3-week dietary study
where methamidophos was fed at 0 and 10 ppm. No cumulative enzyme
depression was observed and RBC was affected slightly more than
plasma (Cavalli and Spence, 1970;) [see acephate - Cavalli and
Spence, 1970f, summary of the data in addendum].
TOXICOLOGICAL STUDIES
Special studies on delayed neurotoxicity
Groups of hens (10 mature laying hens - 1-2 years old/group)
were fed methamidophos in the diet (dosage levels were said to be
0, 0.3, and 3.0 mg/kg body weight) for four weeks. There was a
control of TOCP fed at a dose of 50 mg/kg body weight. No mortality
or severe weight loss was observed and clinical signs of ataxia
were not noted. The TOCP-treated hens developed clinical signs of
ataxia. Histological examination of nervous tissue showed no
abnormalities, even in the TOCP control group (Fletcher et al.,
1971).
In two trials, groups of adult hens (varying in number from 2
to 10/group) were administered methamidophos once orally or by
intraperitoneal injection in the presence or absence of 2-PAM and
atropine. The test protocol used doses equal to and exceeding the
LD50 values in an attempt to establish the neurotoxic potential for
methamidophos. Although mortality was observed, no clinical signs
of delayed neurotoxicity were evident. Histopathological
examinations were not performed (Lorke and Kimmerle, 1967).
A group of six hens each were administered methamidophos
orally at an LD50 dose level of 27.5 mg/kg, observed for 21 days,
re-treated with the same dose and maintained for an additional 21
days. Two separate control groups were used - an untreated control
and a TOCP (500 mg/kg, oral dose) positive control. While some of
the hens (2/6) died from acute toxicity, the survivors of the
methamidophos treatment did not lose weight or show signs of
delayed ataxia. The TOCP treatment group lost weight and showed
positive signs of ataxia within 14 days of poisoning.
Histopathology examination was not performed (Wolvin, et al.,
1968).
Special studies on reproduction
Rat
Groups of rats (8 male and 16 female/group) were fed
methamidophos in the diet at dose levels of 0, 3, 10, and 30 ppm
for 100 days and mated to begin a standard 3 generation, 2
litter/generation reproduction study. Body weights of parental
males (but not females) were reduced but the reduction was slight
and not dose-related. Cholinesterase activity values of the female
animals were measured after the mating trials were concluded. RBC
and plasma cholinesterase depression was evident at 30 ppm in males
and females (male plasma was also depressed at 10 ppm). There were
no significant effects noted on gross or microscopic examinations
of tissues and organs.
Reproductive indices were generally affected at the 30 ppm
dose level. The incidence of pregnancy (fertility index) incidence
of parturition (gestation index) and viability and lactation
indices were reduced at 30 ppm. Numbers of stillborn and
cannibalized pups were increased at the 30 ppm dose but no
suggestions of terata were presented. Histopathologic examination
of the progeny did not reveal any abnormalities. No effects on
reproduction were observed at 10 ppm (Arnold et al., 1970).
Special studies on teratogenesis
Groups of pregnant rabbits (from 17-23 does/group) were
administered methamidophos by gelatin capsule from day 6 through
day 18 of gestation at dosage levels of 0, 0.1, and 0.3 mg/kg. A
positive control (thalidomide, 37.5 mg/kg) was administered to a
group of 27 pregnant does. All animals were sacrificed on day 29 of
gestation. Positive teratogenic signs were observed as expected
with thalidomide. No effects were noted as a result of
administration of methamidophos on growth of does or on fetal
mortality. Fetus weight was normal and skeletal development was
unaffected. One of 63 pups at the 0.3 mg/kg dose level displayed
talipomanus, club hand. This malformation was not observed in 75-79
pups at the low dose or in the controls, but it occurred in 8 of 85
in the positive controls (Ladd et al., 1971). There were no other
obvious defects noted in the study with methamidophos. A larger
number of defects were noted with thalidomide (umbilical hernia,
harelip, cervical elongation, frontal fontanel) that were not seen
with methamidophos. Skeletal examinations of the pups from
methamidophos-treated does were normal.
Special studies on mutagenicity
Groups of mice (12 males/group) were administered
methamidophos by intraperitoneal injection at dosage levels of 0,
1, and 2 mg/kg and mated to virgin females for 6 consecutive weeks
in a dominant lethal test. A positive control group administered
ethylmethane sulfonate (EMS, 400 mg/kg) was included in the study.
Females sacrificed one week after breeding with the treated males
were examined for pregnancy and resorption of fetuses.
Mutagenicity, measured by comparing viable embryos in the test and
control groups, was not evident with methamidophos treatment. A
mutagenic response to EMS was indicated in the first two weeks of
the test (Arnold et al., 1971).
Results of in vitro tests using Salmonella and
Escherichia Spp tester strains were negative when methamidophos was
examined (Hanna and Dyer, 1975).
Special studies on antidotes
Antidotal effects were noted with atropine and atropine +
2-PAM combination administered following intoxication. BH6 and
2-PAM alone were not effective antidotes. Atropine + PAM was more
effective than atropine alone but less effective than atropine +
BH6 (Lorke and Kimmerle, 1967).
The acute oral toxicity of methamidophos in male rats was
reduced 3-fold when animals were administered atropine alone or in
combination with 2-PAM. While 2-PAM alone was not as therapeutic as
atropine it reduced the toxicity by more than two-fold (Schoenig et
al., 1968).
Special studies on potentiation
Simultaneous administration of methamidophos to rats either by
oral gavage or intraperitoneal injection in combination with
malathion showed a significant potentiation (acute interaction in
the toxicity as measured by reduction of the LD50 value). Graduated
doses of malathion and methamidophos by oral administration
resulted in a greater than two-fold potentiation with malathion.
(Gröning, 1975). A slightly less than two-fold increase was
observed when ip injection was the route of administration used
(Crawford and Anderson, 1973). Oral administration of methamidophos
in combination with parathion or EPN did not result in potentiation
of the acute toxicity (Gröning, 1975).
Acute toxicity
TABLE 1. Acute toxicity of methamidophos
Species Sex Route LD50 Reference
(mg/kg)
Rat M Oral 15.6-32.3 Lorke & Kimmerle,
1967; Cavalli
et al., 1968a;
1968b; 1968g.
F Oral 13.0-29.6
M ip 21.3 Lorke & Kimmerle.
1967
F ip 26.4 Lorke & Kimmerle,
1967
TABLE 1. (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
Rat M Dermal
4-hr exp. 110 Lorke & Kimmerle,
7-day exp. 50 1967
Inhalation
1-hr exp. 0.525 mg/L Lorke & Kimmerle,
4-hr exp. 0.162 mg/L 1967
Guinea Pig Oral 30-50 Lorke & Kimmerle,
1967
Mice Oral 16.2 Cavalli et al.,
(Technical) 1968c
Oral (75% 18.0 Cavalli et al.,
Technical) 1968d
Rabbit Oral 10-30 Lorke & Kimmerle,
1967
Dermal 118-125 Cavalli et al.,
1968e; 1968f
Cat Oral 10-30 Lorke & Kimmerle,
1967
Dog Oral 10-30 Lorke & Kimmerle,
1967
Chicken F Oral 25 Lorke & Kimmerle,
1967
F ip 10 Lorke & Kimmerle,
1967
The observed signs of poisoning were typical of
anticholinesterase agents. The signs appeared rapidly following
poisoning (5-20 minutes) and disappeared in 4-6 days. Typical
parasympathomimetic signs of poisoning would be expected.
TABLE 2. Acute toxicity of impurities
LD50
Compound Species Sex Route (mg/kg) Reference
(CH3O)2P(S) Rat M Oral 633 Cavalli et al., 1968h
NH2 (RE9169) F Oral 549 Cavalli et al., 1968h
Signs of poisoning were typical of CNS depression
- paresis, hyporeflexia and anesthesia.
Rabbit Dermal 2,500- Cavalli et al., 1969
5,000
(intact
skin)
1,570 Cavalli et al., 1969
(abraded
skin)
See the monograph on acephate for the toxicity of other products.
Short-term studies
Rat
Groups of rats (15 females/group) were administered methamidophos
orally or by intraperitoneal injection, 5 days/week for 60 days to
examine possible cumulative effects. Oral application consisted of
daily doses of 0, 1.5, 3.0, 6.0, 10.0, and 15.0 mg/kg while ip
application consisted of doses of 0, 1.3, 2.6, 5.3, 8.8, and 13.2
mg/kg. While slight mortality was observed (oral = 3/15, ip = 6/15)
there was no evidence of cumulative action. The cumulative LD50 by
either oral or ip administration was estimated to be > 15 mg/kg body
weight (Lorke & Kimmerle, 1967).
Groups of rats (30 females/treatment group - 90 females were used
as controls) were fed methamidophos in the diet at dosage levels of 0,
0.25, 0.50, 1.00, 2.00 and 4.00 ppm for 13 weeks (a further 4-week
recovery period using a control diet followed this test interval).
There were no deaths or adverse behavioral reactions. Growth, as
evidenced by body weight gain, was normal at all treatment levels.
Cholinesterase depression of plasma, RBC and brain was reported only
in the high dose group.
At 4.0 ppm depression of enzyme activity was obvious in plasma
and RBC, less so in brain. A comparison of the total depression of
cholinesterase activity and recovery during the 4-week control diet
period suggests a no-effect level of 2.0 ppm (Reyna et al., 1973).
Groups of rats (35 males and 35 females/group) were fed
methamidophos in the diet for 13 weeks at dietary levels of 0, 0.3,
1.0, 3.0 and 10.0 ppm. Cholinesterase activity was examined at
periodic intervals using plasma, RBC, and brain tissue. Significant
dose-related depression of plasma cholinesterase activity was noted in
male rats at 3 and 10 ppm and in females at 10 ppm. Decreases in
cholinesterase activity of RBC and brain were noted at 3 and 10 ppm in
both sexes. RBC activity was depressed in both sexes at 10 ppm and in
males only, marginally depressed at 1 and 3 ppm. Recovery of enzyme
activity was noted after one week of control diets following 3 ppm and
at 4 weeks of control diets following 10 ppm (Plank et al., 1969).
Groups of rats (15 males and 15 females/group; 30 of each sex
were used as controls) were fed methamidophos in the diet at dosage
levels of 0, 2, 6, 20, and 60 ppm for 3 months. No mortality or
behavior changes were observed although growth was depressed
significantly at 60 ppm in both sexes. Blood chemistry, hematology,
and urine analysis parameters were normal (bilirubin content of males
only at 20 and 60 ppm was depressed at 90 days, and OCT
(Ornithinecarbamoyl transferase) activity was depressed in females fed
60 ppm at 90 days. These unusual effects were reported to be within
physiological limits). Cholinesterase activity was depressed at 6 ppm
and above in both plasma and RBC (RBC was slightly more sensitive). No
effects were noted at 2 ppm over the 13 week feeding interval. Gross,
macroscopic examination of tissues and organs showed a reduction in
both males and females in the size of several organs; thymus, liver,
spleen, adrenals, and gonads only at 60 ppm. There were no effects
noted at 20 ppm. Microscopic examination of the tissues did not show
any adverse effects related to the presence of methamidophos in the
diet. A no-effect level based on cholinesterase depression is 2 ppm in
the diet (Löser and Lorke, 1970b; Gröning, 1976).
Rabbit
Groups of rabbits (5 males and 5 females/group) were administered
methamidophos dermally to intact or abraded skin at dose levels of 0,
5, or 10 mg/kg (a 75% technical product). The application was made 6-7
hours/day, 5 days/ week for 3 weeks. Application was to a new site
each day. Mortality occurred in the 10 mg/kg abraded skin group
accompanied by signs of cholinergic stimulation. Hematologic studies,
blood chemistry and urinalysis parameters were unaffected by dermal
application of methamidophos. Gross and microscopic analyses of
tissues and organs were uneventful. Dermatologic reactions, such as
erythema, were minor. The affected skin returned to normal within 1-2
days. Growth was slightly retarded at the 10 mg/kg dose group but was
not accompanied by other adverse signs of poisoning (Mastri et al.,
1968).
Dog
Groups of dogs (3 male and 3 female/group) were administered
methamidophos orally by capsule daily, seven days per week, for 90
days at dosage levels of 0, 0.025, 0.075, and 0.25 mg/kg body weight.
There was no mortality. Growth and food consumption were unaffected.
Red blood cell and plasma cholinesterase activity, examined at several
intervals both before and during treatment, was normal. There was no
depression of cholinesterase noted in this study (Carlson et al.,
1969).
Groups of dogs (3 male and 3 female/group) were used in a repeat
of the above test to examine the 90-day subacute anticholinesterase
effects of methamidophos. Growth was reduced in males at the highest
dose level. In females, a reduced weight gain was observed at all dose
levels. Food consumption was reduced at the highest dose level in both
sexes. Depressed erythrocyte cholinesterase was observed at 0.25 mg/kg
in both males and females. No effects were noted on plasma enzymes. A
no-effect level based on RBC cholinesterase is 0.075 mg/kg (Lindberg
et al., 1970).
Groups of dogs (2 male and 2 female/group) were administered
methamidophos by capsule for periods varying from 21-28 days, at doses
ranging from 0 to 0.6 mg/kg/day. In this study each dog was examined
for 34 weeks to determine a pre-treatment baseline for cholinesterase
activity. Animals were then administered a dose of 0.025 mg/kg for 28
days after which the dose was increased to 0.05 mg/kg. The total
treatment sequence was as follows: 0.025, 0.05, 0.075, and 0.10 mg/kg
each administered for 28 days, followed by 0.125, 0.20, 0.30, 0.40,
0.50, and 0.60 mg/kg each administered for 21 days, after which a
control diet was administered for 28 days. Cholinesterase activity was
measured several times (generally at weekly intervals) during the
course of treatment. In both males and females a dose level was
reached where plasma and RBC cholinesterase activity was depressed. In
males, plasma and RBC cholinesterase was depressed when the dose
regimen reached 0.125 mg/kg. In females, plasma was depressed at a
dose of 0.3 mg/kg while RBC was affected at approximately 0.2 mg/kg.
Based on these data, no effects on cholinesterase would be expected to
occur at levels around 0.1 mg/kg/day. In this study, depression was
considered positive when the average value for cholinesterase activity
declined below the lowest range of the pre-treatment mean (Greco et
al., 1971).
Groups of dogs (2 male and 2 female beagles/group), 3 of each sex
were used as controls) were fed diets containing methamidophos at
dosage levels of 0, 1.5, 5, and 15 ppm for 90 days. There was no
mortality or behavioral change noted over the course of treatment.
Growth and food consumption were normal. There were no effects noted
in blood chemistry, hematology or urinalysis parameters.
Cholinesterase depression (RBC and plasma) was noted at 5 ppm and
above in both sexes (within the first week of dietary administration
at the high dose and after 2 months at the intermediate dose). Gross
macroscopic examination of tissues and calculation of relative organ
weights did not indicate any effects at the highest treatment level. A
dietary no-effect level in this study is 1.5 ppm (Löser and Lorke,
1970a)
Histopathological examination of tissues and organs showed no
effects of methamidophos (Gröning and Lorke, 1976).
Groups of dogs (3 male and 3 female beagles/group) were
administered methamidophos by capsule, orally, for two years at dosage
levels of 0, 0.075, 0.25 and 0.75 mg/kg. There were no deaths or
observable differences in behavior over the two-year study. Growth was
unaffected by methamidophos. There were no effects noted on clinical
chemistry, hematology, or urinalysis parameters recorded.
Cholinesterase activity was not reported. Gross and microscopic
examination of tissues and organs showed no abnormalities (Lindberg et
al., 1969).
Long-term studies
Rat
Groups of rats (45 males and 45 females/group) were fed
methamidophos in the diet at dosage levels of 0, 3, 10, and 30 ppm for
two years. Growth was reduced at the high dose level in males. Growth
in females was unaffected in this study. A separate paired feeding
study (21-day) was conducted where it was noted that growth was
unaffected at 30 ppm. Examination of food consumption data suggested
this effect to be due to diet rejection rather than a direct effect of
methamidophos in the diet. (Plank et al., 1968). In the long-term
study, there were no effects reported on behavior, hematologic blood
chemistry or urinalysis parameters. Gross and microscopic examination
of tissues and organs at 12 or 24 months showed no abnormalities
attributed to methamidophos in the diet. The tumor incidence was
unaffected and a definitive, no-effect level based on somatic changes
would be greater than 30 ppm. Cholinesterase activity was not measured
in the study (Plank et al., 1970).
OBSERVATIONS IN MAN
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. The groups received either a
combination mixture of 1:4 (methamidophos:acephate), 1:9 or a control
dose, daily (divided into 3 equal doses), for periods of 21 days. Each
group was administered the combination at a dose of 0.1 mg/kg/day for
21 days after which the dose was increased (to 0.2 mg/kg/day) and
continued for another 21 days. The protocol called for a total of 4
dose levels (up to 0.4 mg/kg/day) each to be administered for 21 days.
Blood cholinesterase (plasma and RBC) was examined periodically. No
effects were noted on RBC cholinesterase at any treatment level. There
was no substantial depression noted in the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The male
cohort was substantially affected at the 0.3 mg/kg dose and was not
retested at the higher dose level. The group (both males and females)
receiving the higher methamidophos:acephate ratio (1:4) showed
reduction of plasma cholinesterase after two weeks of receiving a dose
of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not used in the 1:4 dose
group. This study indicated the minimal dose necessary to decrease
plasma cholinesterase for the combination of acephate and
methamidophos was 0.2 mg/kg/day of a 1:4 combination of the two
toxicants. No effects with this combination were noted at 0.1 mg/kg
(Garofalo et al., 1973). See the monograph on acephate for a further
amplification of these data.
COMMENTS
Methamidophos, an anticholinesterase organophosphorus ester, is
also a metabolite of acephate. Methamidophos is rapidly absorbed,
distributed, metabolised and excreted in mammals. Bioaccumulation,
based on data on solubility properties, would not be expected to
occur. Biotransformation in mammals results in metabolites of
insignificant toxicological properties. Methamidophos is an acutely
toxic pesticide. The acute signs of poisoning can be relieved with the
aid of atropine (and reactivators, such as 2-PAM). In contrast to the
innocuous metabolites, methamidophos is an active cholinesterase
inhibitor, primarily affecting RBC (and presumably brain)
cholinesterase in animal models. In humans, plasma cholinesterase
appears to be the more sensitive enzyme parameter.
Methamidophos does not induce delayed neurotoxicity although
potentiation of the acute toxicity of malathion has been noted. In
reproduction studies, several parameters were affected at relatively
low levels (the levels were however sufficient to impart
cholinesterase depression in the parents). No terata were induced in
the reproduction study or in special teratological studies in rabbits,
although the dose levels used in this latter study were extremely low.
Mutagenicity tests were negative.
In short and long term studies no significant somatic effects
were noted. Cholinesterase depression was manifested in short term
studies. In 90 day dog studies, cholinesterase depression was observed
at 5 ppm while a dietary level of 1.5 ppm (equivalent to 0.04
mg/kg/day) caused no observable effects. In a 2 year dog study,
somatic effects were not noted at levels of 0.75 mg/kg/day but
cholinesterase activity was not determined. Depression of
cholinesterase activity in rat feeding studies was observed at dietary
levels of 3 ppm and above while 2 ppm was found to induce no
depression of cholinesterase activity. In a controlled study where a
combination dose of methamidophos and acephate was administered to a
group of male and female volunteers, cholinesterase depression was
observed predominantly when a higher methamidophos:acephate ratio was
used (1:4 rather than 1:9). Based on the results of this study a
no-effect level of 0.02 mg/kg was suggested. A higher total
concentration (0.2 mg/kg), administered in a lower ratio (1:9) also
gave no depression of enzyme activity. Based on the no-effect level of
0.04 mg/kg bw in dog an ADI was allocated. The no-effect level in
human studies was further assurance of the adequacy of the numerical
value of the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet equivalent to 0.1 mg/kg bw
Dog: 1.5 ppm in the diet equivalent to 0.04 mg/kg bw
Man: 0.02 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methamidophos is an organophosphorus insecticide with systemic
properties. It is a good stomach poison with further contact poison
action and has good residual activity. It is claimed to be effective
for the control of sucking pests such as aphids, whitefly and spider
mites as well as biting pests such as Laphygma, Prodenia, Trichoplusia
and others.
Methamidophos is used world-wide, chiefly in regions with a
subtropical climate. Formulations based on methamidophos are
registered and sold in more than 40 different countries of the world,
including Egypt, many countries of Latin America, Turkey, U.S.A.,
Canada and several countries of Europe. Methamidophos is marketed in
different emulsifiable concentrate formulations.
The major uses of methamidophos are pre-harvest treatments on
cotton, vegetables, potatoes, maize, sugar beet, stone fruit, tobacco,
hops and ornamentals. Further details on its uses and recommendations
are given in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In vegetables
Residue data are presented in Table 4 on a number of vegetable
crops, namely broccoli, Brussels sprouts, cabbage, cauliflower,
celery, cucumber, eggplant, lettuce, peaches, pepper, potatoes and
tomatoes. Most of the data have been received in detailed as well as
in summarised form from the manufacturers (Chevron Chemagro and
Bayer-reports in file), although with some supplementary material from
other sources (Davis et al., 1974).
Generally vegetable crops are treated with 0.5 - 1 kg/ha of
methamidophos, which leaves residues of a non-persistent nature and
with a well-known distribution pattern, viz. the outer leaves,
especially of leafy vegetables, carry considerably higher residues
than the internal parts of the plants. Some of these outer leaves may
or may not be discarded as "trimmings" when marketed.
With only occasional high values, the data in Table 4 indicate
that residues of methamidophos will rapidly dissipate to low levels.
With appropriate pre-harvest intervals, from 1-3 weeks, the average
residues in all vegetables are usually well below 1 mg/kg.
TABLE 3. Recommended use patterns of methamidophos
Crop Dose, Number Recommended
kg a.i./ha of pre-harvest
treatments interval, days
Cotton 0.05-1.5 3-5 21
Cucumbers 0.4 -0.75 3-4 3
Tomatoes 0.4 -0.75 3-4 3
Cauliflower 0.4 -1.12 3-5 7
(U.S.A. 14)
Brussels sprouts 0.56-1.12 3-5 7
Broccoli 0.56-1.12 3-5 7
Other brassicas 0.4 -1.12 3-5 7
Celery 0.56-1.12 5 21
Lettuce 0.56-1.12 3-4 21
Potatoes 0.5 -1.12 3 7
Maize 0.9 -1.5 3 21
Sugar beet 0.5 -0.6 3-5 28
Stone fruit 0.5 -1.0 2-3 21
(peach)
Tobacco 0.3 -0.6 3-4 14
Hops 0.3 -2.0 4 21
Rape 0.28-0.56 2 10
TABLE 4. Residues of methamidophos in vegetable crops (supervised trials)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Broccoli
heads 0.56-1.12 3-6 2 w n.d. - 2.84 0.45 21 U.S.A.
3 w n.d. - 0.14 0.03 9
4 w n.d. - 0.09 0.02 7
leaves 1.12 3-6 2 w 0.22 - 7.62 2.50 7 U.S.A.
3 w n.d. - 0.88 0.28 7
4 w n.d. - 0.54 0.16 7
total 1.12 3-6 2 w 0.16 - 3.74 1.41 7 U.S.A.
3 w n.d. - 0.57 0.18 7
4 w n.d. - 0.44 0.13 6
Brussels sprouts
heads 1.12 3-5 2 w 0.07 - 0.47 0.26 6 U.S.A.
3 w 0.08 - 0.64 0.27 6
4 w 0.05 - 0.22 0.12 6
leaves 1.12 3-5 2 w 0.16 - 0.17 0.17 2 U.S.A.
3 w 0.06 - 0.21 0.14 2
4 w n.d. - 0.10 0.05 2
total 1.12 3.5 2 w 0.10 - 0.11 0.11 2 U.S.A.
3 w 0.12 0.12 2
4 w 0.05 - 0.08 0.07 2
Cabbage
heads 0.56 - 1.12 2-9 1 w n.d. - 0.7 0.11 11 U.S.A.
2 w n.d. - 0.35 0.04 11 GFR
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Cabbage
savoy 0.3 3 1 w 0.09 1 GFR
2 w 0.07 1
Cauliflower
heads 0.56 - 1.12 3-12 2 w n.d. - 0.45 0.11 16 U.S.A.
3 w n.d. - 0.23 0.06 7 GFR
4 w n.d. - 0.12 0.03 7
leaves 0.56 - 1.12 3-12 2 w 0.1 - 6.28 1.85 8
3 w n.d. - 1.11 0.46 7
4 w n.d. - 0.62 0.24 7
Celery
stalks 1.12 5-7 3 w 0 - 1.55 0.33 6 U.S.A.
tops 1.12 5-7 3 w 0.08 - 6.0 2.59 6
whole plant 1.12 5-7 3 w 0.02 - 2.0 0.74 6
Cucumber 0.6 4-6 0 d 0.19 - 0.45 0.35 3 Mexico
(dust treatment) 1 d 0.13 - 0.44 0.32 3
3 d 0.05 . 0.41 0.24 3
5 d 0.06 - 0.2 0.15 3
7 d 0.03 - 0.31 0.16 3
Eggplant 0.45 - 0.6 7-9 0 d 0.03 - 0.10 0.07 2 Mexico
1 d 0.02 - 0.05 0.04 2
3 d 0.05 - 0.06 0.06 2
5 d 0.03 - 0.04 0.04 2
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Lettuce 7 d 0.05 - 0.06 0.06 2
head (1) 0.56 - 1.12 3-8 2 w 0.01 - 0.68 0.24 12 U.S.A.
3 w n.d. - 0.02 n.d. 5
wrapper
leaves (2) 1.12 3-4 3 w 0.1 - 0.77 0.19 5 U.S.A.
(1) + (2) 1.12 3-4 3 w n.d. - 0.25 0.07 5 U.S.A.
head 1.12 5 2 w n.d. - 1.40 0.21 9 U.S.A.
wrapper 3 w n.d. n.d. 6
leaves (2) 1.12 5 2 w 0.01 - 2.42 0.78 5 U.S.A.
3 w 0.02 - 0.15 0.07 6
(1) + (2) 1.12 5 2 w 0.05 - 0.93 0.31 5 U.S.A.
3 w n.d. - 0.39 0.09 6
Peppers 0.34 - 1.12 4-20 1 w 0.04 - 2.3 1.00 7 U.S.A.
2 w 0.08 - 1.09 0.38 8 Mexico
3 w n.d. - 0.58 0.16 8 ) Australia
4 w n.d. n.d. 4 )
Potatoes 1.12 6-8 1 w n.d. - 0.11 0.04 12 U.S.A.
2 w n.d. n.d. 5 U.S.A.
Canada
Tomatoes 0.5 4 1 d - 0.50 1 Venezuela
3 d - 0.28 1
7 d - 0.22 1
10 d - 0.23 1
14 d - 0.20 1
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
18 d - 0.19 1
Tomatoes 0.6 - 1.33 4-10 0 w 0.10 - 5.07 1.05 17 Australia
1 W 0.08 - 1.83 0.55 18 U.S.A.
2 w 0.03 - 1.62 0.30 18 Venezuela
3 w 0.03 - 0.18 0.09 6
In oil seeds, hops and sugar beets
Rape and cotton plants have been treated with varying amounts of
methamidophos, ranging from 0.28 kg/ha up to an overdose of 2.24 kg/ha
and with up to 4 applications. In these experiments residues in the
harvested seeds ranged from undetectable to 0.06 mg/kg 21-26 days
after the last treatment, and no residues could be detected at 32-72
days after application.
Limited residue data for methamidophos in hops are available.
After six applications with 0.66 kg/ha dried hops contained 3.0 mg/kg
at 17 days. After 3 spray applications with 1.8 kg/ha, the residue
levels of methamidophos amounted to 1.4-2.8 mg/kg at 20 days.
Sugar beets were sprayed on the foliage 4 times with 0.48 kg/ha.
The residue levels 23 days after the last application were 0.01 mg/kg
of methamidophos in the roots and 0.6 mg/kg in the leaves. After 28
days the roots were free of residues, while the tops contained 0.9
mg/kg methamidophos.
In peaches
Peaches were sprayed with 1 kg/ha of methamidophos under German
climatic conditions. 1 week after the second of 2 applications
residues varied from 0.50-1.05 mg/kg (average, 0.73 mg/kg), and 1 week
later they were 0.28-0.75 mg/kg (average 0.45 mg/kg).
FATE OF RESIDUES
The identified metabolites formed from methamidophos are shown in
Figure 1.
In animals
Studies of the absorption, distribution and excretion of
methamidophos in the rat (Crossley and Tutass, 1969), and lactating
goat (Lee and Crossley, 1972) are described in the section
"Biochemical aspects," p. ). Some features of these studies,
particularly the distribution in tissues, are amplified below.
Female and male rats each received a single dose of radiolabelled
methamidophos, administered intragastrically as an aqueous solution
(Crossley and Tutass, 1969). The doses received by each rat were
0.16-0.19 mg of 14C methylthio-labelled methamidophos and 0.21 mg of
32P-labelled methamidophos. In both experiments, the majority of the
applied dose was excreted within 24 hours. This period of fast
elimination in which over 50% of the activity was excreted was
followed by a second phase during which only 1-2% of the applied dose
was eliminated daily.
In the experiments with 14C-labelled methamidophos, the major
excretion route was via the breath (approx. 39% of the applied dose);
urine and faeces contained 11.1% and 1.5% respectively. In the
experiments with 32P-labelled methamidophos, on the other hand, the
majority of radioactivity was eliminated in the urine (until day 14),
and up to about 20% was eliminated in the faeces. There was no
accumulation in tissues or organs (see Table 5).
TABLE 5. Distribution of radioactivity in tissues and organs of
rats after dosing with labelled methamidophos (Crossley
and Tutass, 1969)
Radioactivity, % of applied dose
Organ/ 14C 32P
tissue female rats male rats female rats
Day 5 - 9 Day 1 Day 28 Day 1 Day 28
Carcass 21.9 9.9 6.7 11.3 4.4
Liver 0.4 6.9 0.1 5.6 0.1
Kidney 0.1 0.5 0.0 0.4 0.0
Heart 0.3 0.1 0.0 0.1 0.0
Lung 0.1 - - - -
Fat - 0.1 0.0 0.2 0.0
Muscle - 0.2 0.1 0.3 0.1
Total 22.6 17.4 6.9 17.4 4.6
The degradation of methamidophos in the animal body is hydrolytic
in nature (Crossley & Tutass, 1969; Tutass, 1968). The degradation
sequence (Crossley & Tutass, 1969), is shown above
("Biotransformation"). Data on the quantitative distribution of
residues 32P residues in the urine are presented in Table 6.
In the faeces, the majority of radioactivity was not extractable
with either water or acetone. That which was extractable showed the
same degradation profile as in the urine. The non-extractable
radioactivity was in all probability incorporated into natural
biochemical constituents.
TABLE 6. Distribution of 32P in urine of rats dosed with
32P-methamidophos (Crossley and Tutass, 1969)
32P, % of total in sample
Metabolite* Male rats Female rats
Day 1 Day 2 Day 1 Day 2
methamidophos (I) 40 10 40 trace
OS-dimethyl
phosphorothioate (II) 5 - 5 -
methylphosphoric
acid (III) 40 25 40 30
phosphoric acid (IV) 15 65 15 70
* Numbers in parenthesis refer to Figure 1.
S-methyl-14C-methamidophos was given orally to a goat in order
to assess its fate in a lactating ruminant (Lee and Crossley, 1972).
The goat received 3.75 mg. of 14C methamidophos daily after the
morning milking for 7 consecutive days. Methamidophos was rapidly
metabolized and about half of the radioactivity rapidly excreted. This
excreted radioactivity was distributed mainly between the breath and
urine and, to a lesser extent, the faeces and milk. The activity that
remained in the animal after the initial rapid excretion was
eliminated at a slower but constant rate. None of the radioactivity
remaining in the body was concentrated in any one organ, although
slightly higher levels were found in the liver than elsewhere. The
distribution of radioactivity in the goat is summarized in Table 7.
The results are essentially the same as those found in the rat study
(Table 5), although a somewhat higher proportion was excreted in the
breath of the rats.
TABLE 7. Distribution of radioactivity in goat dosed with 14C-methamidophos expressed as
percentage of applied dose or mg/kg methamidophos equivalents (Lee and Crossley, 1972)
Sample Dosing Period Recovery Period Total Blood and Tissues
(days 0 - 8) (days 9 - 17) Excreted
% % % % mg/kg
Urine 17.24 0.63 17.87 Bloodx 5.87 -
Faeces 4.00 0.98 4.98 Liver 3.93 0.22
Milk 2.49 0.74 3.23 Kidney 0.61 0.16
Total 23.73 2.35 26.08 Heart 0.61 0.16
Mammary 0.82 0.10
Brain 0.21 0.08
Fatxx 4.13 0.16
Musclexxx 33.10 0.16
Total recovered = 75% Total 49.28 1.04
x Assuming blood to be 10% of the body weight
xx Assuming fat to be 5% of the body weight
xxx Assuming muscle to be 40% of the body weight
TLC of urinary samples revealed the presence of methamidophos and
the first metabolite OS-dimethyl phosphorothioate (II in Figure 1). In
the sample taken 12 hours after the third dose, 7% of the activity in
the urine was found to be parent compound and 16% metabolite II.
Extraction procedures applied to the samples of tissues, blood, milk,
urine and faeces extracted only small proportions of their activity,
indicating that the main source of activity was not methamidophos. The
maximum possible level of methamidophos in milk estimated on this
basis was 0.008 mg/kg. The maximum content in the milk found by direct
analysis was 0.003 mg/kg (about 1% of the total activity in the milk).
Since the data obtained from the goat are similar to those from the
rats; it is believed that the non-extractable activity in the goat
tissues, milk, etc. is due to the incorporation of 14C into the
natural constituents of the body, as in rats.
In plants
Methamidophos is an insecticide with systemic activity (Werner,
1972). The physical and chemical basis of systemic movement in the
cotton plant was investigated by Hussain et al. (1974).
Root and leaf treatment of tomato, cabbage and bean plants
demonstrated that 14C-methamidophos is readily taken up by roots and
leaves and transported with the transpiration water towards the
margins of leaves, while little is retained in the stem and petioles.
The labelled compound accumulating at the margins of leaves is
essentially only methamidophos, while in the centre of the leaf some
more polar compounds are present, apparently as the result of
metabolic degradation. The uptake of methamidophos by roots appears to
be independent of pH within the physiological range of 3.5-7.5
(Tutass, 1968b).
Horler et al., (1975) showed that in tomato plants there is only
slight movement of the intact insecticide from treated leaves to the
fruit. After rapid penetration of 14C-methamidophos or/and
32P-methamidophos into tomato leaves, the residue in leaves and fruit
in the initial phase has a biological half-life of 7-10 days. However,
especially in the fruit, after this initial period the rate slows to
give a half-life of about 6 weeks (Horler et al., 1975). The authors
assume that intact insecticide is lost from fruits and leaves by a
mechanism associated with transpiration, rather than by metabolism and
translocation.
Werner (1973) also reports rapid absorption of
methylthio-14C-methamidophos by the roots of Loblolly pine seedlings.
After 96 hours exposure, an accumulation of radioactivity was evident
in the needle tips. Besides unchanged methamidophos, 3 compounds were
isolated from the seedlings. One was identified as the major
metabolite, OS-dimethyl phosphorothioate (II, Figure 1), and in
addition two unidentified compounds were detected, of which one
(Unknown 1) was probably phosphoric acid (VI, Figure 1).
Methamidophos, II and Unknown 1 were detected in the roots, stems and
needles, but Unknown 2 only in the needles, of seedlings treated for
72 and 96 hours (see Table 8).
TABLE 8. Percentage distribution of radioactive methamidophos
and 14C-compounds from TLC and GLC of pine seedling
extracts (Werner, 1973)
Seedling exposure period (h)
Compound* Plant part 6 12 24 48 72 96
I (methamidophos) Roots 80.0 46.0 40.0 35.7 3.3 0.9
Stem 0 0 1.6 1.0 1.1 1.7
Needles 0 31.0 27.9 26.3 22.0 30.5
II (OS-dimethyl Roots 20.0 3.8 1.6 1.0 3.3 3.5
phosphorothioate)
Stem 0 3.8 13.1 6.1 11.0 10.4
Needles 0 46.0 26.2 18.4 16.5 17.4
Unknown 1 Roots 0 0 1.6 1.0 2.2 1.7
Stem 0 3.8 4.9 3.1 4.4 2.6
Needles 0 19.2 18.1 17.4 19.7 10.4
Unknown 2 Roots 0 0 0 0 0 0
Stem 0 0 0 0 0 0
Needles 0 0 0 0 16.5 20.9
* Roman numerals refer to Figure 1.
Experiments with methylthio-14C-methamidophos on cabbages and
tomatoes as well as on plant tissue cultures of sweet potatoes and
tobacco have shown that degradation occurs chiefly by hydrolysis
(Chevron, 1968). Hydrolysis first occurs at the P-N bond to yield
OS-dimethyl phosphorothioate (II, Figure 1), which was consistently
detected as a metabolite in all tissues investigated. Continued,
though somewhat slower, hydrolysis of II apparently leads to cleavage
of CH3S-P and CH3O-P bonds yielding VII and III as major products.
Quantitative data on the residues of these metabolites are not given.
The final fate of the radiolabel is its incorporation into plant
constituents such as pigments, amino acids, carbohydrates and
structural material (up to 19% of the activity in the sample of
cabbage plants).
In soil
Methamidophos is degraded at a relatively fast rate in soils. In
laboratory experiments, Leary and Tutass (1968) found half-lives of
1.9 days in silt soil, 4.8 days in loam soil and 6.1 days in sandy
soil. Sterilization of silt soil by autoclaving resulted in a
considerable increase in the half-life, to about 6 weeks.
In laboratory experiments conducted by Horler et al. (1975),
methamidophos was also found to undergo rapid degradation. In basic
soils (pH 7.40 and 7.75), only 3-7% of the original activity was found
after 4 days; in an acid soil (pH 4.30), 25% was present after 9 days.
In the basic soils, there were differences in the degradation of the
metabolites. In clay-loam soil (pH 7.40), the metabolite degraded at a
slower rate than in loam soil (pH 7.75). Since both soils had a
similar pH, such differences may be connected with the fact that
microorganisms play a role in the decomposition.
In degradation experiments with field soils sampled from depths
of 0-10 cm, and from depths of 0-15 cm and 15-30 cm, it was noted that
at sampling on the day of application to the sail, only a fraction of
the applied methamidophos was still present (Bayer, 1972; Chemagro,
1974). Within one week, the residue dropped further to 10% of the
level measured on the day of application. At depths of 15-30 cm, only
very small amounts of residue (maximum of 0.04 mg/kg) were found in
the soil, and no accumulation was noted after several applications.
Leary and Tutass (1968) found that metabolic degradation of
S-methyl-14-methamidophos in silt soil under aerobic conditions
resulted after 3 days in the evolution of 70% of the applied dose as
14CO2. On the other hand, only 7.6% was evolved as 14CO2 under
aerobic conditions, with 84.6% of the applied activity remaining in
the soil. Up to about 30% of the activity that remained in the soil
was not acetone-extractable. Most of the extractable radioactivity was
in the form of OS-dimethyl phosphorothioate (II, Figure 1), while
minor amounts were in the form of methamidophos, amino acids and
carbohydrates. Quantitative data on these constituents were not given.
Horler et al (1975) also identified OS-dimethylphosphorothioate
(II) as the major metabolite in soil. However, they also found very
small amounts of V, VI and III in clay loam soil. In loam soil, VI was
not found. It is stated that the loss of the methoxy group results in
the evolution of CO2. Quantitative data are not given.
In water
The hydrolysis of methamidophos was examined at elevated
temperatures in aqueous solutions as a function of pH (Leary, 1968;
Magee, 1966). In solutions with a pH below 2, the half-life was a
matter of hours. At pH 9, the half-life was 2.6 days at 25°C and 1.5
days at 37°C. In mildly acidic or neutral solutions, methamidophos
showed remarkable stability at temperatures up to 80°C (less than 5%
hydrolysis in 25 hours). The P-NH2 bond is broken first, followed by
hydrolysis of the CH3SP grouping.
Leaching experiments with methamidophos and three soil types
(muck, sandy loam and loam) (Tutass, 1968c), showed little retention
of the compound. Soil type apparently had only a minor effect upon
retention. The compound was least retained in loam.
Experiments to study the leaching behaviour of methamidophos with
a standardized soil column technique revealed that about two-thirds of
the compound was leached out of sandy and sandy loam soils while only
about one-fifth was leached out of a soil with an increased content of
organic matter (Bayer, 1972).
Flint and Shaw (1972) also studied the absorption of
methamidophos by and its leaching from three different soil types. The
absorption by soil was very slight, and it was further decreased by
increasing temperature and decreasing pH. Methamidophos was not
retarded by the soil when passed through soil columns in water. The
chemical was found in soil run-off water only in the first two of five
irrigations. It was degraded in a natural water system outdoors with
half-lives of 15.9 days in water and 7.5 days in silt. It was
concluded that although it was leached, methamidophos was degraded
rapidly in natural water systems.
In fish
Bass fingerlings which had been in water containing 0.01 mg/1 of
methamidophos for eight days, were analyzed. Less than 0.02 mg/kg of
methamidophos was found in all samples (Stanley, 1971 b).
In processing and storage
The effect of simulated commercial processing on methamidophos
residues in tomatoes was investigated by Morris and Olson (1974). The
tomatoes were processed to juice (pressed, pasteurized), to canned
tomatoes (sterilized), to ketchup. (sterilized, pasteurized), to dry
tomato pomace and to dry tomato pulp. The residues in juice, canned
tomatoes and ketchup were smaller than those found in whole tomatoes,
amounting to 89.5, 73.0 and 68.4% respectively, of the residue levels
prior to processing. On the other hand, the residues in dry tomato
pomace and dry tomato pulp were 2.0 and 2.5 times the original levels
respectively.
The effect on residues of washing in a manner simulating
commercial packing practice depended upon the treated crop. After
treatment, the fruits were allowed to stand outdoors for 24 hours.
Whereas washing green peppers and eggplants did not remove any
significant residue, washing tomatoes removed approximately 15% and
washing cucumbers approximately 20% of the methamidophos residue
(Thornton, 1973).
Dehydrated celery flakes were prepared commercially by placing
celery leaves on trays and drying at temperatures of up to 60°C
(Morris, 1975). Dehydration removed 80% of the weight as water, and it
was concluded that the dehydration process did not destroy any of the
methamidophos residue present in fresh celery leaves.
Cottonseed was processed into delinted seed, meal, hulls. and
oil. No residue was found in any of the seed samples or in any of the
fractions from the processed seed. The residue found in the trash
ranged from 0.1 to 2.7 mg/kg (Chevron, 1976).
Rapeseed was processed into oil and meal (Chemagro, 1976). The
following residue levels were measured: rapeseed, 0.02-0.25 mg/kg,
rapeseed oil, n.d.-0.02 mg/kg and rapeseed meal 0.01-0.30 mg/kg.
Leary (1968) conducted a study to determine the stability of
weathered residues of methamidophos on chopped crop samples (broccoli,
lettuce, cabbage, cauliflower, Brussels sprouts) stored deep frozen
for several months. No significant evidence of decomposition was
obtained and there was no indication of any trend towards
decomposition with increased time of storage.
Möllhoff (1971), however, noted that methamidophos residues
decreased by 10-40% in cauliflower samples stored for three months at
-20°C, and by up to 10% in tomatoes stored under the same conditions.
In further studies conducted by Chemagro (1976), to investigate
the effect of frozen storage at about -20°C on methamidophos residues
in peanut meat, peanut forage, sorghum grain, rapeseed, celery and
peppers, decomposition ranged from 0 to 22%.
The effect of frozen storage at about -20°C on methamidophos
residues in cattle tissues and milk and in poultry tissues and eggs
has also been studied (Chemagro, 1976). It was found that
decomposition in poultry liver amounted to 33% after 60 days and in
cattle liver to 85% after 150 days. On the other hand, the residues
remained completely stable in cattle steak, fat and milk, and
decomposition amounted to a maximum of only 13% in eggs, heart and
gizzard of poultry.
METHODS OF RESIDUE ANALYSIS
Methods for the gas-chromatographic determination of
methamidophos have been developed and published in several
modifications, but in practically all cases are based on clean-up
procedures with silica gel columns and GLC with thermionic detection.
Möllhoff (1971) developed a method for determination in plants,
soil and water. Maceration with acetone or an acetone/water mixture is
used for extraction. The limit of determination is generally about
0.01 mg/kg, but for oil seeds only 0.05 mg/kg and for hops and tobacco
0.1 mg/ kg. Recoveries in the 0.01-1.0 mg/kg range vary between 80%
and 100%.
Leary (1971) also determined methamidophos in crops, but with the
use of ethyl acetate for extraction. The GLC instrument in this case
was equipped with a caesium bromide thermionic detector and the method
is claimed to have a limit of determination of 0.003-0.005 mg/kg. At
levels of 0.5-0.1 mg/kg the majority of recovery values were from
90-100%.
Both these methods have been tested for specificity in
conjunction with other organophosphorus insecticides. They were found
to be adequately specific and without interferences from extractives
with a number of different crops (Olson, 1973).
Stanley and co-workers have developed methods for residues in
different forage crops such as hay, peanut meal, sorghum grain and
rape seed (McNamara & Stanley, 1975) and in animal tissues and milk
(Stanley, 1971c). Extractions were with either methanol/chloroform or
acetonitrile and Skellysolve B.
With minor amendments these methods have been found suitable also
for the determination of methamidophos in fish and water with limits
of determination down to 0.02 mg/kg and 0.004 mg/kg respectively
(Stanley 1971 a).
These methods for the determination of methamidophos follow
closely the general pattern for organophosphorus insecticides in
current multi-residue procedures. Undoubtedly, therefore, they will be
suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
TABLE 9. National tolerances reported to the meeting
Country Crop Tolerance, Pre-harvest
mg/kg interval
Australia Capsicums 0.25 14 days
Peaches 0.25 21 days
Tomatoes 0.25 21 days
TABLE 9. (Cont'd.)
Country Crop Tolerance, Pre-harvest
mg/kg interval
Potatoes 0.05 7 days
Canada Broccoli, Brussels
sprouts, lettuce,
peppers 1.0
Cabbage, cauliflower,
cucumbers, eggplant,
tomatoes 0.5
Potatoes, melons negligible
residue
Netherlands Brassica 0.1
Potatoes 0.01x
New Zealand Brassicas, potatoes 0.1
Tomatoes 0.1 3 days
South Africa Peaches 1.0 21 days
Cabbage 1.0 21 days
Potatoes 1.0 14 days
U.S.A. Cauliflower, lettuce
tomatoes 2.0
Broccoli, Brussels
sprouts, cabbage,
peppers, cucumbers,
eggplant 1.0
Melons 0.5
Cottonseed, potatoes 0.1xx
x = limit of determination
xx = negligible residues
APPRAISAL
Methamidophos is a chemically simple organophosphorus insecticide
with broad spectrum effects used to control caterpillars, aphids,
larvae etc. It is active by contact as well as systemically.
Since 1969 methamidophos has been registered in a number of
countries around the world, chiefly in regions with a sub-tropical
climate. Its major uses are pre-harvest treatments of industrial
crops, fruit and vegetables, mainly with spray solutions at rates from
0.5-1.5 kg/ha.
Detailed studies on the metabolic fate of methamidophos indicate
that the compound is readily taken up by plant roots and translocated
with the transpiration stream within the plants, i.e. towards the
edges of the foliage. In studies with the radio-labelled compound, the
further fate has been followed and a complete degradation pathway with
final incorporation into plant constituents is suggested, mainly
through steps involving hydrolytic breakdown.
The degradation of methamidophos in the animal body is also
hydrolytic and there are no signs of accumulation in tissues or
organs. Most of the phosphorus-containing moiety of methamidophos (up
to about 70%) is eliminated via the urine, while a major part of the
carbon (about 40% in the rat) is excreted as CO2 with the breath, the
remainder being found in the urine and faeces.
The results of extensive residue trials on a number of vegetable
and agricultural crops are available. The data confirm methamidophos
as a systemic compound of relatively low persistence. Most of the data
are from trials under practical conditions which are likely to
represent good agricultural practice.
Methamidophos penetrates into the flesh of the fruit in, e.g.,
tomatoes, peppers, eggplants, etc. Washing or peeling such products
therefore does not significantly remove residues. In the frozen
storage of crops treated with methamidophos, residues are found to be
stable for several months.
Methamidophos has been found to be degraded fairly rapidly in
soils and in natural water systems without any signs of accumulation
or concentration in living organisms.
Gas-chromatographic methods making use of thermionic detectors
are available for the determination of methamidopho in various crops
and animal products. They are combined with appropriate extraction and
clean-up procedures and are likely to be suitable for regulatory
purposes.
RECOMMENDATIONS
Maximum residue limits are recommended for methamidophos in the
following commodities at the levels indicated. They refer to the
parent compound.
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Hops 5 Peppers 1
Broccoli 2 Sugar beet leaves 1
Cauliflower 2 Cottonseed 0.1
Celery 2 Potatoes 0.1
Lettuce 2 Rapeseed 0.1
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Tomatoes 2 Sugar beets 0.1
Brussels Meat and fat of
sprouts 1 cattle, goats
Cabbage 1 and sheep 0.01*
Cucumber 1 Milk 0.01*
Eggplant 1
Peaches 1
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies an the teratogenic potential in a suitable
species.
2. Further studies to elucidate the contribution of acephate and
metamidophos alone or in combination in depressing cholinesterase
activity.
3. Further information on the level and fate of residues during
cooking and processing of fruit and vegetables.
REFERENCES
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E.
1971 Mutagenic Study with Monitor Technical (SX No.
171) in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Arnold, D., Keplinger, M.L. and Fancher, O. E. Three Generation
1970 Reproduction Study on SX-171 (Technical RE-9006 -
75%) in Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Bayer Berichte No. 12,13/1972; No. 11,12/1973. Submitted
1972 to the Joint Meeting.
Carlson, D., Vondruska, J. F. and Fancher, O. E. Effects of RE 9006
1969 - 75% SX-171 on the Cholinesterase Activity in the
Beagle Dog. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Chevron Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity of
1968a Monitor Technical in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1908b of Monitor 6S in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, D.H. Acute Oral Toxicity
1968c of RE 9006 (95%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968d of RE 9006 (75%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.D. Acute Dermal Toxicity
1968e of Monitor 6S. Unpublished report from Industrial
Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1968f of Monitor Technical. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968g of RE 9006 (95%) in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968h of RE 9169 (SX-196) in Rats. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
the Chevron Chemical Company.
Cavalli, R.E., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1969 RE 9169 (SX 198) in Rabbits. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
Chevron Chemical Company.
Cavalli, R.E. and Spence, J.A. Effect of Repeated Doses of RE 12420
1970 on Cholinesterase Activity in Rats. Unpublished
report from Standard Oil of California, submitted
to the WHO by Chevron Chemical Company.
Chemagro Reports nos. 44249, 44250 on methamidophos in
1974 soil. Submitted to the Joint Meeting.
Chevron Metabolism of Monitor insecticide by plants.
1968 Chevron report submitted to the Joint Meeting. In
file at FAO, Plant Protection Division.
Crawford, C.R. and Anderson, R.H. The Acute Toxicity of Monitor
1973 Technical in Combination with Marathion to Female
Rats. Unpublished report from Chemagro Division of
Baychem Corporation, submitted to the WHO by
Bayer, A.G.
Crossley, J. and Tutass, H.O. Metabolism of Monitor Insecticide
1969 by rats. Unpublished report no. 721.14 from the
Ortho Division, Chevron Chemical Company,
submitted to the WHO by Bayer, A.G.
Davis, A.C., Bourke, J.B. and Kuhr, R.J. Disappearance of Monitor
1974 Residues from Cole crops. J. Econ. Entomol. 67,
766-768.
Fletcher, D., Jenkins, D.H., Keplinger, M.L. and Fancher, O.E.
1971 Demyelination Study with Monitor (RE 9006)
Technical in Mature Laying Hens. Unpublished study
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Flint, D.R. and Shaw II, H.R. The Mobility and persistence of
1972 MONITOR in soil and water. Report No. 34 483
submitted by Chemagro.
Garofalo, M., Palazzolo, R. and Sanders, R. A Study on the Effect
1973 of Orthene and Monitor on Plasma and Erythrocyte
Cholinesterase Activity in Human Subjects During
Subacute Oral Administration. Unpublished report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Chevron Chemical Company.
Greco, R.A., Lindberg, D.C., Keplinger, M.L. and Fancher, O.E.
1971 Effect of RE 9006 on Plasma and Erythrocyte
Cholinesterase Activity in Beagle Dogs.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Gröning, P. Tamaron. Special Toxicity Studies (Potentiation).
1975 Unpublished report from Institut für Toxikologie,
submitted to the WHO by Bayer, A.G.
Gröning, P. Bayer 17628 (Tamaron) Untersuchung Der Subchronischen
Toxizitat an ratten (3-monate-futterungversuch).
Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Gröning, P. and Lorke, D. Untersuchung der subchronischen toxizitat
1976 an hunden. Unpublished report from the Institut
für Toxikologie, submitted to the WHO by Bayer,
A.G.
Hanna, P.J. and Dyer, K.F. Mutagenicity of Organophosphorus Compounds
1975 in Bacteria and Drosophila. Mut. Res. 28:
405-20.
Horler, D.F., Lubkowitz, J.A., Revilla, A.P., Baruel, J. and Cermeli,
1975 M.M. Uptake and Degradation of Monitor by Tomato
Plants. Pesticides, IUPAC 3rd Int. Congr. Pest.
Chem., Helsinki, 151-156.
Hussain, M., Fukuto, T.R., and Reynolds, H.T. Physical and Chemical
1974 Basis for Systemic Movement of Organophosphorus
Esters in the Cotton Plant. J. Agr. Fd Chem. 22
(2): 225-230.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L.
1971 Teratogenic Study with Monitor Technical in Albino
Rabbits. Unpublished report from Industrial Bio
Test Laboratories, Inc. submitted to the WHO by
Bayer, A.G.
Leary, J.B. Stability of Monitor insecticide residues in frozen
crops and in aqueous solutions. Chevron report
files No. 740.01 and No. 721.2.
Leary, J.B. and Tutass, H.O. Degradation of Monitor insecticide
1968 in soil. Chevron report file No. 721.2.
Leary, J.B. Gas-chromatographic Determination of Monitor
1971 (OS-Dimethyl Phosphoramidothioate) Residues in
Crops. J. Ass. Off. Analyt. Chem. 54 (6):
1396-98.
Lee, H. and Crossley, J. The Fate of Monitor in a Lactating
1972 Ruminant (Goat). Unpublished report from Ortho
Division, Chevron Chemical Company, submitted to
the WHO by Bayer, A.G.
Lindberg, D., Vondruska, J.F. and Fancher, O.E. Two Year Chronic
1969 Oral Toxicity of RE 9006-III, SX-116 in Beagle
Dogs. Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO, by
Bayer, A.G.
Lindberg, D., Keplinger, M.L. and Fancher, O.E. RE 9006 (70%)
1970 SX 171 on the Cholinesterase in the Beagle Dog.
Unpublished study from Industrial Bio-Test
Laboratories Inc., submitted to the WHO by Chevron
Chemical Company.
Lorke, D. and Kimmerle, G. Toxicological Studies on the Active
1967 Ingredient Bayer 71 628. Unpublished report from
the Institut für Toxikologie, submitted to the WHO
by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on Dogs
1970a (Three Month Feeding Experiment). Unpublished
report from Institut für Toxikologie, submitted to
the WHO by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on rats.
1970b Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Magee, P.S. Hydrolysis of Monitor insecticide. Chevron Report
1966 File No. 721.2.
Mastri, C., Keplinger, M.L. and Fancher, O.E. Twenty-one Day
1968 Subacute Dermal Toxicity of Monitor (RE 9006) 75%
Technical SX 171. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Chevron Chemical Company.
McNamara, F.T., and Stanley, C.W. Gas-Chromatographic Method for
1975 the Determination of Residues of MONITOR in Peanut
Meat and Hay, Sorghum Grain, and Rapeseed.
Chemagro Report No. 45.439.
Möllhoff, E. Metode zur gaschromatographischen Bestimmung
1971 von Tamaron-Rückständen in Pflanzen.
Pflanzenschutz-Nachr. 24 (2): 256-262.
Morris, R.A. Effect of Dehydration on Residues of MONITOR
1975 in Celery Leaves. Chemagro Report No. 45.586.
Morris, R.A., and Olson, T.J. The Effect of Processing on MONITOR
1974 Residues in Tomatoes. Chemagro Report No. 41.107.
Olson, T.J. Interference Study on MONITOR (TAMARON) in Various
1973 Crops. Chemagro Reports No. 37.380 and No. 37.392.
Plank, J., Keplinger, M.L. and Fancher, O.E. Paired Feeding Study
1968 of RE 9006 - III SX-116 Albino Rats. Unpublished
report from Industrial Bio-Test Laboratories.
Inc., submitted to the WHO by Chevron Chemical
Company.
Plank, J., Keplinger, M.L. and Fancher, O.E. Ninety-Day
1969 Cholinesterase Study of SX-171 Monitor (RE 9006) -
Albino Rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Bayer, A.G.
Plank, J., Keplinger, M.L. and Fancher, O.E. Two Year Chronic
1970 Oral Toxicity of RE 9006 - III, SX-116 in Albino
Rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Ninety-Day Subacute
1973 Oral Cholinesterase Study with Monitor Technical
in Female Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Schoenig, G., Keplinger, M.L. and Fancher, O.E. Study on the Efficacy
1968 of Atropine Sulfate and 2-PAM C1 as Antidotes for
Monitor (RE 9006) 75% Technical SX-171. Report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971a of MONITOR in Fish and Water. Chemagro Report No.
30.975.
Stanley, C.W. Analysis of Bass and Water for MONITOR. Chemagro
1971b Report No. 30.979.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971c of Residues of MONITOR in Animal Tissues and Milk.
Chemagro Report No. 31.093.
Thornton, J.S. Effect of Washing on Residues in Tomatoes, Cucumbers,
1973 Eggplants and Peppers. Chemagro Reports Nos.
37322, 37539, 37540 and 37541 submitted to the
Joint Meeting. In File at FAO, Plant Protection
Division.
Tucker, B. Acetylcholinesterase Inhibition of Orthene and Ortho
1972 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the WHO by
Chevron Chemical Company.
Tutass, H.O. Microsomal Oxidation of Monitor. Unpublished report
1968a no. 721.2 from the Ortho Division of Chevron
Chemical Company, submitted to the WHO by Bayer,
A.G.
Tutass, H.O. Uptake and Translocation of Monitor Insecticide
1968b by tomato, cabbage and bean plants. Chevron Report
File No. 721.2.
Tutass, H.O. Leaching of Monitor insecticide in soils. Chevron
1968c Report File No. 721.2
Werner, R.A. Systemic insecticidal action of disulfoton and
1972 Monitor in Loblolly pine seedlings. J. Georgia
Entomol. Soc. 7 (1): 64-67.
Werner, R.A. Absorption, Translocation and Metabolism of Root-
1973 Absorbed 14C Monitor in Loblolly Pine Seedlings.
J. Econ. Entomol. 66 (4): 867-872.
Wolvin, A.A., Palazzolo, R.J. and Fancher, O.E. Neurotoxicity
1968 Study - Chickens Monitor RE 9006, 75% Technical.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
RBC cholinesterase appears to be more sensitive than plasma
cholinesterase to inhibition as noted by the differing I50 values.
I50(M)
Bovine RBC 3.1 x 10-5
Human Plasma 1.6 x 10-4
(Tucker, 1972)
The sensitivity of RBC relative to plasma cholinesterase to
inhibition by methamidophos was noted in a 3-week dietary study
where methamidophos was fed at 0 and 10 ppm. No cumulative enzyme
depression was observed and RBC was affected slightly more than
plasma (Cavalli and Spence, 1970;) [see acephate - Cavalli and
Spence, 1970f, summary of the data in addendum].
TOXICOLOGICAL STUDIES
Special studies on delayed neurotoxicity
Groups of hens (10 mature laying hens - 1-2 years old/group)
were fed methamidophos in the diet (dosage levels were said to be
0, 0.3, and 3.0 mg/kg body weight) for four weeks. There was a
control of TOCP fed at a dose of 50 mg/kg body weight. No mortality
or severe weight loss was observed and clinical signs of ataxia
were not noted. The TOCP-treated hens developed clinical signs of
ataxia. Histological examination of nervous tissue showed no
abnormalities, even in the TOCP control group (Fletcher et al.,
1971).
In two trials, groups of adult hens (varying in number from 2
to 10/group) were administered methamidophos once orally or by
intraperitoneal injection in the presence or absence of 2-PAM and
atropine. The test protocol used doses equal to and exceeding the
LD50 values in an attempt to establish the neurotoxic potential for
methamidophos. Although mortality was observed, no clinical signs
of delayed neurotoxicity were evident. Histopathological
examinations were not performed (Lorke and Kimmerle, 1967).
A group of six hens each were administered methamidophos
orally at an LD50 dose level of 27.5 mg/kg, observed for 21 days,
re-treated with the same dose and maintained for an additional 21
days. Two separate control groups were used - an untreated control
and a TOCP (500 mg/kg, oral dose) positive control. While some of
the hens (2/6) died from acute toxicity, the survivors of the
methamidophos treatment did not lose weight or show signs of
delayed ataxia. The TOCP treatment group lost weight and showed
positive signs of ataxia within 14 days of poisoning.
Histopathology examination was not performed (Wolvin, et al.,
1968).
Special studies on reproduction
Rat
Groups of rats (8 male and 16 female/group) were fed
methamidophos in the diet at dose levels of 0, 3, 10, and 30 ppm
for 100 days and mated to begin a standard 3 generation, 2
litter/generation reproduction study. Body weights of parental
males (but not females) were reduced but the reduction was slight
and not dose-related. Cholinesterase activity values of the female
animals were measured after the mating trials were concluded. RBC
and plasma cholinesterase depression was evident at 30 ppm in males
and females (male plasma was also depressed at 10 ppm). There were
no significant effects noted on gross or microscopic examinations
of tissues and organs.
Reproductive indices were generally affected at the 30 ppm
dose level. The incidence of pregnancy (fertility index) incidence
of parturition (gestation index) and viability and lactation
indices were reduced at 30 ppm. Numbers of stillborn and
cannibalized pups were increased at the 30 ppm dose but no
suggestions of terata were presented. Histopathologic examination
of the progeny did not reveal any abnormalities. No effects on
reproduction were observed at 10 ppm (Arnold et al., 1970).
Special studies on teratogenesis
Groups of pregnant rabbits (from 17-23 does/group) were
administered methamidophos by gelatin capsule from day 6 through
day 18 of gestation at dosage levels of 0, 0.1, and 0.3 mg/kg. A
positive control (thalidomide, 37.5 mg/kg) was administered to a
group of 27 pregnant does. All animals were sacrificed on day 29 of
gestation. Positive teratogenic signs were observed as expected
with thalidomide. No effects were noted as a result of
administration of methamidophos on growth of does or on fetal
mortality. Fetus weight was normal and skeletal development was
unaffected. One of 63 pups at the 0.3 mg/kg dose level displayed
talipomanus, club hand. This malformation was not observed in 75-79
pups at the low dose or in the controls, but it occurred in 8 of 85
in the positive controls (Ladd et al., 1971). There were no other
obvious defects noted in the study with methamidophos. A larger
number of defects were noted with thalidomide (umbilical hernia,
harelip, cervical elongation, frontal fontanel) that were not seen
with methamidophos. Skeletal examinations of the pups from
methamidophos-treated does were normal.
Special studies on mutagenicity
Groups of mice (12 males/group) were administered
methamidophos by intraperitoneal injection at dosage levels of 0,
1, and 2 mg/kg and mated to virgin females for 6 consecutive weeks
in a dominant lethal test. A positive control group administered
ethylmethane sulfonate (EMS, 400 mg/kg) was included in the study.
Females sacrificed one week after breeding with the treated males
were examined for pregnancy and resorption of fetuses.
Mutagenicity, measured by comparing viable embryos in the test and
control groups, was not evident with methamidophos treatment. A
mutagenic response to EMS was indicated in the first two weeks of
the test (Arnold et al., 1971).
Results of in vitro tests using Salmonella and
Escherichia Spp tester strains were negative when methamidophos was
examined (Hanna and Dyer, 1975).
Special studies on antidotes
Antidotal effects were noted with atropine and atropine +
2-PAM combination administered following intoxication. BH6 and
2-PAM alone were not effective antidotes. Atropine + PAM was more
effective than atropine alone but less effective than atropine +
BH6 (Lorke and Kimmerle, 1967).
The acute oral toxicity of methamidophos in male rats was
reduced 3-fold when animals were administered atropine alone or in
combination with 2-PAM. While 2-PAM alone was not as therapeutic as
atropine it reduced the toxicity by more than two-fold (Schoenig et
al., 1968).
Special studies on potentiation
Simultaneous administration of methamidophos to rats either by
oral gavage or intraperitoneal injection in combination with
malathion showed a significant potentiation (acute interaction in
the toxicity as measured by reduction of the LD50 value). Graduated
doses of malathion and methamidophos by oral administration
resulted in a greater than two-fold potentiation with malathion.
(Gröning, 1975). A slightly less than two-fold increase was
observed when ip injection was the route of administration used
(Crawford and Anderson, 1973). Oral administration of methamidophos
in combination with parathion or EPN did not result in potentiation
of the acute toxicity (Gröning, 1975).
Acute toxicity
TABLE 1. Acute toxicity of methamidophos
Species Sex Route LD50 Reference
(mg/kg)
Rat M Oral 15.6-32.3 Lorke & Kimmerle,
1967; Cavalli
et al., 1968a;
1968b; 1968g.
F Oral 13.0-29.6
M ip 21.3 Lorke & Kimmerle.
1967
F ip 26.4 Lorke & Kimmerle,
1967
TABLE 1. (Cont'd.)
Species Sex Route LD50 Reference
(mg/kg)
Rat M Dermal
4-hr exp. 110 Lorke & Kimmerle,
7-day exp. 50 1967
Inhalation
1-hr exp. 0.525 mg/L Lorke & Kimmerle,
4-hr exp. 0.162 mg/L 1967
Guinea Pig Oral 30-50 Lorke & Kimmerle,
1967
Mice Oral 16.2 Cavalli et al.,
(Technical) 1968c
Oral (75% 18.0 Cavalli et al.,
Technical) 1968d
Rabbit Oral 10-30 Lorke & Kimmerle,
1967
Dermal 118-125 Cavalli et al.,
1968e; 1968f
Cat Oral 10-30 Lorke & Kimmerle,
1967
Dog Oral 10-30 Lorke & Kimmerle,
1967
Chicken F Oral 25 Lorke & Kimmerle,
1967
F ip 10 Lorke & Kimmerle,
1967
The observed signs of poisoning were typical of
anticholinesterase agents. The signs appeared rapidly following
poisoning (5-20 minutes) and disappeared in 4-6 days. Typical
parasympathomimetic signs of poisoning would be expected.
TABLE 2. Acute toxicity of impurities
LD50
Compound Species Sex Route (mg/kg) Reference
(CH3O)2P(S) Rat M Oral 633 Cavalli et al., 1968h
NH2 (RE9169) F Oral 549 Cavalli et al., 1968h
Signs of poisoning were typical of CNS depression
- paresis, hyporeflexia and anesthesia.
Rabbit Dermal 2,500- Cavalli et al., 1969
5,000
(intact
skin)
1,570 Cavalli et al., 1969
(abraded
skin)
See the monograph on acephate for the toxicity of other products.
Short-term studies
Rat
Groups of rats (15 females/group) were administered methamidophos
orally or by intraperitoneal injection, 5 days/week for 60 days to
examine possible cumulative effects. Oral application consisted of
daily doses of 0, 1.5, 3.0, 6.0, 10.0, and 15.0 mg/kg while ip
application consisted of doses of 0, 1.3, 2.6, 5.3, 8.8, and 13.2
mg/kg. While slight mortality was observed (oral = 3/15, ip = 6/15)
there was no evidence of cumulative action. The cumulative LD50 by
either oral or ip administration was estimated to be > 15 mg/kg body
weight (Lorke & Kimmerle, 1967).
Groups of rats (30 females/treatment group - 90 females were used
as controls) were fed methamidophos in the diet at dosage levels of 0,
0.25, 0.50, 1.00, 2.00 and 4.00 ppm for 13 weeks (a further 4-week
recovery period using a control diet followed this test interval).
There were no deaths or adverse behavioral reactions. Growth, as
evidenced by body weight gain, was normal at all treatment levels.
Cholinesterase depression of plasma, RBC and brain was reported only
in the high dose group.
At 4.0 ppm depression of enzyme activity was obvious in plasma
and RBC, less so in brain. A comparison of the total depression of
cholinesterase activity and recovery during the 4-week control diet
period suggests a no-effect level of 2.0 ppm (Reyna et al., 1973).
Groups of rats (35 males and 35 females/group) were fed
methamidophos in the diet for 13 weeks at dietary levels of 0, 0.3,
1.0, 3.0 and 10.0 ppm. Cholinesterase activity was examined at
periodic intervals using plasma, RBC, and brain tissue. Significant
dose-related depression of plasma cholinesterase activity was noted in
male rats at 3 and 10 ppm and in females at 10 ppm. Decreases in
cholinesterase activity of RBC and brain were noted at 3 and 10 ppm in
both sexes. RBC activity was depressed in both sexes at 10 ppm and in
males only, marginally depressed at 1 and 3 ppm. Recovery of enzyme
activity was noted after one week of control diets following 3 ppm and
at 4 weeks of control diets following 10 ppm (Plank et al., 1969).
Groups of rats (15 males and 15 females/group; 30 of each sex
were used as controls) were fed methamidophos in the diet at dosage
levels of 0, 2, 6, 20, and 60 ppm for 3 months. No mortality or
behavior changes were observed although growth was depressed
significantly at 60 ppm in both sexes. Blood chemistry, hematology,
and urine analysis parameters were normal (bilirubin content of males
only at 20 and 60 ppm was depressed at 90 days, and OCT
(Ornithinecarbamoyl transferase) activity was depressed in females fed
60 ppm at 90 days. These unusual effects were reported to be within
physiological limits). Cholinesterase activity was depressed at 6 ppm
and above in both plasma and RBC (RBC was slightly more sensitive). No
effects were noted at 2 ppm over the 13 week feeding interval. Gross,
macroscopic examination of tissues and organs showed a reduction in
both males and females in the size of several organs; thymus, liver,
spleen, adrenals, and gonads only at 60 ppm. There were no effects
noted at 20 ppm. Microscopic examination of the tissues did not show
any adverse effects related to the presence of methamidophos in the
diet. A no-effect level based on cholinesterase depression is 2 ppm in
the diet (Löser and Lorke, 1970b; Gröning, 1976).
Rabbit
Groups of rabbits (5 males and 5 females/group) were administered
methamidophos dermally to intact or abraded skin at dose levels of 0,
5, or 10 mg/kg (a 75% technical product). The application was made 6-7
hours/day, 5 days/ week for 3 weeks. Application was to a new site
each day. Mortality occurred in the 10 mg/kg abraded skin group
accompanied by signs of cholinergic stimulation. Hematologic studies,
blood chemistry and urinalysis parameters were unaffected by dermal
application of methamidophos. Gross and microscopic analyses of
tissues and organs were uneventful. Dermatologic reactions, such as
erythema, were minor. The affected skin returned to normal within 1-2
days. Growth was slightly retarded at the 10 mg/kg dose group but was
not accompanied by other adverse signs of poisoning (Mastri et al.,
1968).
Dog
Groups of dogs (3 male and 3 female/group) were administered
methamidophos orally by capsule daily, seven days per week, for 90
days at dosage levels of 0, 0.025, 0.075, and 0.25 mg/kg body weight.
There was no mortality. Growth and food consumption were unaffected.
Red blood cell and plasma cholinesterase activity, examined at several
intervals both before and during treatment, was normal. There was no
depression of cholinesterase noted in this study (Carlson et al.,
1969).
Groups of dogs (3 male and 3 female/group) were used in a repeat
of the above test to examine the 90-day subacute anticholinesterase
effects of methamidophos. Growth was reduced in males at the highest
dose level. In females, a reduced weight gain was observed at all dose
levels. Food consumption was reduced at the highest dose level in both
sexes. Depressed erythrocyte cholinesterase was observed at 0.25 mg/kg
in both males and females. No effects were noted on plasma enzymes. A
no-effect level based on RBC cholinesterase is 0.075 mg/kg (Lindberg
et al., 1970).
Groups of dogs (2 male and 2 female/group) were administered
methamidophos by capsule for periods varying from 21-28 days, at doses
ranging from 0 to 0.6 mg/kg/day. In this study each dog was examined
for 34 weeks to determine a pre-treatment baseline for cholinesterase
activity. Animals were then administered a dose of 0.025 mg/kg for 28
days after which the dose was increased to 0.05 mg/kg. The total
treatment sequence was as follows: 0.025, 0.05, 0.075, and 0.10 mg/kg
each administered for 28 days, followed by 0.125, 0.20, 0.30, 0.40,
0.50, and 0.60 mg/kg each administered for 21 days, after which a
control diet was administered for 28 days. Cholinesterase activity was
measured several times (generally at weekly intervals) during the
course of treatment. In both males and females a dose level was
reached where plasma and RBC cholinesterase activity was depressed. In
males, plasma and RBC cholinesterase was depressed when the dose
regimen reached 0.125 mg/kg. In females, plasma was depressed at a
dose of 0.3 mg/kg while RBC was affected at approximately 0.2 mg/kg.
Based on these data, no effects on cholinesterase would be expected to
occur at levels around 0.1 mg/kg/day. In this study, depression was
considered positive when the average value for cholinesterase activity
declined below the lowest range of the pre-treatment mean (Greco et
al., 1971).
Groups of dogs (2 male and 2 female beagles/group), 3 of each sex
were used as controls) were fed diets containing methamidophos at
dosage levels of 0, 1.5, 5, and 15 ppm for 90 days. There was no
mortality or behavioral change noted over the course of treatment.
Growth and food consumption were normal. There were no effects noted
in blood chemistry, hematology or urinalysis parameters.
Cholinesterase depression (RBC and plasma) was noted at 5 ppm and
above in both sexes (within the first week of dietary administration
at the high dose and after 2 months at the intermediate dose). Gross
macroscopic examination of tissues and calculation of relative organ
weights did not indicate any effects at the highest treatment level. A
dietary no-effect level in this study is 1.5 ppm (Löser and Lorke,
1970a)
Histopathological examination of tissues and organs showed no
effects of methamidophos (Gröning and Lorke, 1976).
Groups of dogs (3 male and 3 female beagles/group) were
administered methamidophos by capsule, orally, for two years at dosage
levels of 0, 0.075, 0.25 and 0.75 mg/kg. There were no deaths or
observable differences in behavior over the two-year study. Growth was
unaffected by methamidophos. There were no effects noted on clinical
chemistry, hematology, or urinalysis parameters recorded.
Cholinesterase activity was not reported. Gross and microscopic
examination of tissues and organs showed no abnormalities (Lindberg et
al., 1969).
Long-term studies
Rat
Groups of rats (45 males and 45 females/group) were fed
methamidophos in the diet at dosage levels of 0, 3, 10, and 30 ppm for
two years. Growth was reduced at the high dose level in males. Growth
in females was unaffected in this study. A separate paired feeding
study (21-day) was conducted where it was noted that growth was
unaffected at 30 ppm. Examination of food consumption data suggested
this effect to be due to diet rejection rather than a direct effect of
methamidophos in the diet. (Plank et al., 1968). In the long-term
study, there were no effects reported on behavior, hematologic blood
chemistry or urinalysis parameters. Gross and microscopic examination
of tissues and organs at 12 or 24 months showed no abnormalities
attributed to methamidophos in the diet. The tumor incidence was
unaffected and a definitive, no-effect level based on somatic changes
would be greater than 30 ppm. Cholinesterase activity was not measured
in the study (Plank et al., 1970).
OBSERVATIONS IN MAN
A study was conducted where controlled doses of combinations of
acephate and methamidophos were orally administered to people for
periods up to 21 days. A cohort of 14 people were divided into 3
groups containing either 4 or 6 people. The groups received either a
combination mixture of 1:4 (methamidophos:acephate), 1:9 or a control
dose, daily (divided into 3 equal doses), for periods of 21 days. Each
group was administered the combination at a dose of 0.1 mg/kg/day for
21 days after which the dose was increased (to 0.2 mg/kg/day) and
continued for another 21 days. The protocol called for a total of 4
dose levels (up to 0.4 mg/kg/day) each to be administered for 21 days.
Blood cholinesterase (plasma and RBC) was examined periodically. No
effects were noted on RBC cholinesterase at any treatment level. There
was no substantial depression noted in the female group receiving the
1:9 combination tested at the highest dose (0.4 mg/kg/day). The male
cohort was substantially affected at the 0.3 mg/kg dose and was not
retested at the higher dose level. The group (both males and females)
receiving the higher methamidophos:acephate ratio (1:4) showed
reduction of plasma cholinesterase after two weeks of receiving a dose
of 0.2 mg/kg/day. The 0.3 mg/kg regimen was not used in the 1:4 dose
group. This study indicated the minimal dose necessary to decrease
plasma cholinesterase for the combination of acephate and
methamidophos was 0.2 mg/kg/day of a 1:4 combination of the two
toxicants. No effects with this combination were noted at 0.1 mg/kg
(Garofalo et al., 1973). See the monograph on acephate for a further
amplification of these data.
COMMENTS
Methamidophos, an anticholinesterase organophosphorus ester, is
also a metabolite of acephate. Methamidophos is rapidly absorbed,
distributed, metabolised and excreted in mammals. Bioaccumulation,
based on data on solubility properties, would not be expected to
occur. Biotransformation in mammals results in metabolites of
insignificant toxicological properties. Methamidophos is an acutely
toxic pesticide. The acute signs of poisoning can be relieved with the
aid of atropine (and reactivators, such as 2-PAM). In contrast to the
innocuous metabolites, methamidophos is an active cholinesterase
inhibitor, primarily affecting RBC (and presumably brain)
cholinesterase in animal models. In humans, plasma cholinesterase
appears to be the more sensitive enzyme parameter.
Methamidophos does not induce delayed neurotoxicity although
potentiation of the acute toxicity of malathion has been noted. In
reproduction studies, several parameters were affected at relatively
low levels (the levels were however sufficient to impart
cholinesterase depression in the parents). No terata were induced in
the reproduction study or in special teratological studies in rabbits,
although the dose levels used in this latter study were extremely low.
Mutagenicity tests were negative.
In short and long term studies no significant somatic effects
were noted. Cholinesterase depression was manifested in short term
studies. In 90 day dog studies, cholinesterase depression was observed
at 5 ppm while a dietary level of 1.5 ppm (equivalent to 0.04
mg/kg/day) caused no observable effects. In a 2 year dog study,
somatic effects were not noted at levels of 0.75 mg/kg/day but
cholinesterase activity was not determined. Depression of
cholinesterase activity in rat feeding studies was observed at dietary
levels of 3 ppm and above while 2 ppm was found to induce no
depression of cholinesterase activity. In a controlled study where a
combination dose of methamidophos and acephate was administered to a
group of male and female volunteers, cholinesterase depression was
observed predominantly when a higher methamidophos:acephate ratio was
used (1:4 rather than 1:9). Based on the results of this study a
no-effect level of 0.02 mg/kg was suggested. A higher total
concentration (0.2 mg/kg), administered in a lower ratio (1:9) also
gave no depression of enzyme activity. Based on the no-effect level of
0.04 mg/kg bw in dog an ADI was allocated. The no-effect level in
human studies was further assurance of the adequacy of the numerical
value of the ADI.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 2 ppm in the diet equivalent to 0.1 mg/kg bw
Dog: 1.5 ppm in the diet equivalent to 0.04 mg/kg bw
Man: 0.02 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0 - 0.002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methamidophos is an organophosphorus insecticide with systemic
properties. It is a good stomach poison with further contact poison
action and has good residual activity. It is claimed to be effective
for the control of sucking pests such as aphids, whitefly and spider
mites as well as biting pests such as Laphygma, Prodenia, Trichoplusia
and others.
Methamidophos is used world-wide, chiefly in regions with a
subtropical climate. Formulations based on methamidophos are
registered and sold in more than 40 different countries of the world,
including Egypt, many countries of Latin America, Turkey, U.S.A.,
Canada and several countries of Europe. Methamidophos is marketed in
different emulsifiable concentrate formulations.
The major uses of methamidophos are pre-harvest treatments on
cotton, vegetables, potatoes, maize, sugar beet, stone fruit, tobacco,
hops and ornamentals. Further details on its uses and recommendations
are given in Table 3.
RESIDUES RESULTING FROM SUPERVISED TRIALS
In vegetables
Residue data are presented in Table 4 on a number of vegetable
crops, namely broccoli, Brussels sprouts, cabbage, cauliflower,
celery, cucumber, eggplant, lettuce, peaches, pepper, potatoes and
tomatoes. Most of the data have been received in detailed as well as
in summarised form from the manufacturers (Chevron Chemagro and
Bayer-reports in file), although with some supplementary material from
other sources (Davis et al., 1974).
Generally vegetable crops are treated with 0.5 - 1 kg/ha of
methamidophos, which leaves residues of a non-persistent nature and
with a well-known distribution pattern, viz. the outer leaves,
especially of leafy vegetables, carry considerably higher residues
than the internal parts of the plants. Some of these outer leaves may
or may not be discarded as "trimmings" when marketed.
With only occasional high values, the data in Table 4 indicate
that residues of methamidophos will rapidly dissipate to low levels.
With appropriate pre-harvest intervals, from 1-3 weeks, the average
residues in all vegetables are usually well below 1 mg/kg.
TABLE 3. Recommended use patterns of methamidophos
Crop Dose, Number Recommended
kg a.i./ha of pre-harvest
treatments interval, days
Cotton 0.05-1.5 3-5 21
Cucumbers 0.4 -0.75 3-4 3
Tomatoes 0.4 -0.75 3-4 3
Cauliflower 0.4 -1.12 3-5 7
(U.S.A. 14)
Brussels sprouts 0.56-1.12 3-5 7
Broccoli 0.56-1.12 3-5 7
Other brassicas 0.4 -1.12 3-5 7
Celery 0.56-1.12 5 21
Lettuce 0.56-1.12 3-4 21
Potatoes 0.5 -1.12 3 7
Maize 0.9 -1.5 3 21
Sugar beet 0.5 -0.6 3-5 28
Stone fruit 0.5 -1.0 2-3 21
(peach)
Tobacco 0.3 -0.6 3-4 14
Hops 0.3 -2.0 4 21
Rape 0.28-0.56 2 10
TABLE 4. Residues of methamidophos in vegetable crops (supervised trials)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Broccoli
heads 0.56-1.12 3-6 2 w n.d. - 2.84 0.45 21 U.S.A.
3 w n.d. - 0.14 0.03 9
4 w n.d. - 0.09 0.02 7
leaves 1.12 3-6 2 w 0.22 - 7.62 2.50 7 U.S.A.
3 w n.d. - 0.88 0.28 7
4 w n.d. - 0.54 0.16 7
total 1.12 3-6 2 w 0.16 - 3.74 1.41 7 U.S.A.
3 w n.d. - 0.57 0.18 7
4 w n.d. - 0.44 0.13 6
Brussels sprouts
heads 1.12 3-5 2 w 0.07 - 0.47 0.26 6 U.S.A.
3 w 0.08 - 0.64 0.27 6
4 w 0.05 - 0.22 0.12 6
leaves 1.12 3-5 2 w 0.16 - 0.17 0.17 2 U.S.A.
3 w 0.06 - 0.21 0.14 2
4 w n.d. - 0.10 0.05 2
total 1.12 3.5 2 w 0.10 - 0.11 0.11 2 U.S.A.
3 w 0.12 0.12 2
4 w 0.05 - 0.08 0.07 2
Cabbage
heads 0.56 - 1.12 2-9 1 w n.d. - 0.7 0.11 11 U.S.A.
2 w n.d. - 0.35 0.04 11 GFR
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Cabbage
savoy 0.3 3 1 w 0.09 1 GFR
2 w 0.07 1
Cauliflower
heads 0.56 - 1.12 3-12 2 w n.d. - 0.45 0.11 16 U.S.A.
3 w n.d. - 0.23 0.06 7 GFR
4 w n.d. - 0.12 0.03 7
leaves 0.56 - 1.12 3-12 2 w 0.1 - 6.28 1.85 8
3 w n.d. - 1.11 0.46 7
4 w n.d. - 0.62 0.24 7
Celery
stalks 1.12 5-7 3 w 0 - 1.55 0.33 6 U.S.A.
tops 1.12 5-7 3 w 0.08 - 6.0 2.59 6
whole plant 1.12 5-7 3 w 0.02 - 2.0 0.74 6
Cucumber 0.6 4-6 0 d 0.19 - 0.45 0.35 3 Mexico
(dust treatment) 1 d 0.13 - 0.44 0.32 3
3 d 0.05 . 0.41 0.24 3
5 d 0.06 - 0.2 0.15 3
7 d 0.03 - 0.31 0.16 3
Eggplant 0.45 - 0.6 7-9 0 d 0.03 - 0.10 0.07 2 Mexico
1 d 0.02 - 0.05 0.04 2
3 d 0.05 - 0.06 0.06 2
5 d 0.03 - 0.04 0.04 2
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
Lettuce 7 d 0.05 - 0.06 0.06 2
head (1) 0.56 - 1.12 3-8 2 w 0.01 - 0.68 0.24 12 U.S.A.
3 w n.d. - 0.02 n.d. 5
wrapper
leaves (2) 1.12 3-4 3 w 0.1 - 0.77 0.19 5 U.S.A.
(1) + (2) 1.12 3-4 3 w n.d. - 0.25 0.07 5 U.S.A.
head 1.12 5 2 w n.d. - 1.40 0.21 9 U.S.A.
wrapper 3 w n.d. n.d. 6
leaves (2) 1.12 5 2 w 0.01 - 2.42 0.78 5 U.S.A.
3 w 0.02 - 0.15 0.07 6
(1) + (2) 1.12 5 2 w 0.05 - 0.93 0.31 5 U.S.A.
3 w n.d. - 0.39 0.09 6
Peppers 0.34 - 1.12 4-20 1 w 0.04 - 2.3 1.00 7 U.S.A.
2 w 0.08 - 1.09 0.38 8 Mexico
3 w n.d. - 0.58 0.16 8 ) Australia
4 w n.d. n.d. 4 )
Potatoes 1.12 6-8 1 w n.d. - 0.11 0.04 12 U.S.A.
2 w n.d. n.d. 5 U.S.A.
Canada
Tomatoes 0.5 4 1 d - 0.50 1 Venezuela
3 d - 0.28 1
7 d - 0.22 1
10 d - 0.23 1
14 d - 0.20 1
TABLE 4. (Cont'd.)
Crop Dose, Number Time after Residues (mg/kg) Number Country
kg/ha of final range average of
treatments application values
(w=weeks-d=days)
18 d - 0.19 1
Tomatoes 0.6 - 1.33 4-10 0 w 0.10 - 5.07 1.05 17 Australia
1 W 0.08 - 1.83 0.55 18 U.S.A.
2 w 0.03 - 1.62 0.30 18 Venezuela
3 w 0.03 - 0.18 0.09 6
In oil seeds, hops and sugar beets
Rape and cotton plants have been treated with varying amounts of
methamidophos, ranging from 0.28 kg/ha up to an overdose of 2.24 kg/ha
and with up to 4 applications. In these experiments residues in the
harvested seeds ranged from undetectable to 0.06 mg/kg 21-26 days
after the last treatment, and no residues could be detected at 32-72
days after application.
Limited residue data for methamidophos in hops are available.
After six applications with 0.66 kg/ha dried hops contained 3.0 mg/kg
at 17 days. After 3 spray applications with 1.8 kg/ha, the residue
levels of methamidophos amounted to 1.4-2.8 mg/kg at 20 days.
Sugar beets were sprayed on the foliage 4 times with 0.48 kg/ha.
The residue levels 23 days after the last application were 0.01 mg/kg
of methamidophos in the roots and 0.6 mg/kg in the leaves. After 28
days the roots were free of residues, while the tops contained 0.9
mg/kg methamidophos.
In peaches
Peaches were sprayed with 1 kg/ha of methamidophos under German
climatic conditions. 1 week after the second of 2 applications
residues varied from 0.50-1.05 mg/kg (average, 0.73 mg/kg), and 1 week
later they were 0.28-0.75 mg/kg (average 0.45 mg/kg).
FATE OF RESIDUES
The identified metabolites formed from methamidophos are shown in
Figure 1.
In animals
Studies of the absorption, distribution and excretion of
methamidophos in the rat (Crossley and Tutass, 1969), and lactating
goat (Lee and Crossley, 1972) are described in the section
"Biochemical aspects," p. ). Some features of these studies,
particularly the distribution in tissues, are amplified below.
Female and male rats each received a single dose of radiolabelled
methamidophos, administered intragastrically as an aqueous solution
(Crossley and Tutass, 1969). The doses received by each rat were
0.16-0.19 mg of 14C methylthio-labelled methamidophos and 0.21 mg of
32P-labelled methamidophos. In both experiments, the majority of the
applied dose was excreted within 24 hours. This period of fast
elimination in which over 50% of the activity was excreted was
followed by a second phase during which only 1-2% of the applied dose
was eliminated daily.
In the experiments with 14C-labelled methamidophos, the major
excretion route was via the breath (approx. 39% of the applied dose);
urine and faeces contained 11.1% and 1.5% respectively. In the
experiments with 32P-labelled methamidophos, on the other hand, the
majority of radioactivity was eliminated in the urine (until day 14),
and up to about 20% was eliminated in the faeces. There was no
accumulation in tissues or organs (see Table 5).
TABLE 5. Distribution of radioactivity in tissues and organs of
rats after dosing with labelled methamidophos (Crossley
and Tutass, 1969)
Radioactivity, % of applied dose
Organ/ 14C 32P
tissue female rats male rats female rats
Day 5 - 9 Day 1 Day 28 Day 1 Day 28
Carcass 21.9 9.9 6.7 11.3 4.4
Liver 0.4 6.9 0.1 5.6 0.1
Kidney 0.1 0.5 0.0 0.4 0.0
Heart 0.3 0.1 0.0 0.1 0.0
Lung 0.1 - - - -
Fat - 0.1 0.0 0.2 0.0
Muscle - 0.2 0.1 0.3 0.1
Total 22.6 17.4 6.9 17.4 4.6
The degradation of methamidophos in the animal body is hydrolytic
in nature (Crossley & Tutass, 1969; Tutass, 1968). The degradation
sequence (Crossley & Tutass, 1969), is shown above
("Biotransformation"). Data on the quantitative distribution of
residues 32P residues in the urine are presented in Table 6.
In the faeces, the majority of radioactivity was not extractable
with either water or acetone. That which was extractable showed the
same degradation profile as in the urine. The non-extractable
radioactivity was in all probability incorporated into natural
biochemical constituents.
TABLE 6. Distribution of 32P in urine of rats dosed with
32P-methamidophos (Crossley and Tutass, 1969)
32P, % of total in sample
Metabolite* Male rats Female rats
Day 1 Day 2 Day 1 Day 2
methamidophos (I) 40 10 40 trace
OS-dimethyl
phosphorothioate (II) 5 - 5 -
methylphosphoric
acid (III) 40 25 40 30
phosphoric acid (IV) 15 65 15 70
* Numbers in parenthesis refer to Figure 1.
S-methyl-14C-methamidophos was given orally to a goat in order
to assess its fate in a lactating ruminant (Lee and Crossley, 1972).
The goat received 3.75 mg. of 14C methamidophos daily after the
morning milking for 7 consecutive days. Methamidophos was rapidly
metabolized and about half of the radioactivity rapidly excreted. This
excreted radioactivity was distributed mainly between the breath and
urine and, to a lesser extent, the faeces and milk. The activity that
remained in the animal after the initial rapid excretion was
eliminated at a slower but constant rate. None of the radioactivity
remaining in the body was concentrated in any one organ, although
slightly higher levels were found in the liver than elsewhere. The
distribution of radioactivity in the goat is summarized in Table 7.
The results are essentially the same as those found in the rat study
(Table 5), although a somewhat higher proportion was excreted in the
breath of the rats.
TABLE 7. Distribution of radioactivity in goat dosed with 14C-methamidophos expressed as
percentage of applied dose or mg/kg methamidophos equivalents (Lee and Crossley, 1972)
Sample Dosing Period Recovery Period Total Blood and Tissues
(days 0 - 8) (days 9 - 17) Excreted
% % % % mg/kg
Urine 17.24 0.63 17.87 Bloodx 5.87 -
Faeces 4.00 0.98 4.98 Liver 3.93 0.22
Milk 2.49 0.74 3.23 Kidney 0.61 0.16
Total 23.73 2.35 26.08 Heart 0.61 0.16
Mammary 0.82 0.10
Brain 0.21 0.08
Fatxx 4.13 0.16
Musclexxx 33.10 0.16
Total recovered = 75% Total 49.28 1.04
x Assuming blood to be 10% of the body weight
xx Assuming fat to be 5% of the body weight
xxx Assuming muscle to be 40% of the body weight
TLC of urinary samples revealed the presence of methamidophos and
the first metabolite OS-dimethyl phosphorothioate (II in Figure 1). In
the sample taken 12 hours after the third dose, 7% of the activity in
the urine was found to be parent compound and 16% metabolite II.
Extraction procedures applied to the samples of tissues, blood, milk,
urine and faeces extracted only small proportions of their activity,
indicating that the main source of activity was not methamidophos. The
maximum possible level of methamidophos in milk estimated on this
basis was 0.008 mg/kg. The maximum content in the milk found by direct
analysis was 0.003 mg/kg (about 1% of the total activity in the milk).
Since the data obtained from the goat are similar to those from the
rats; it is believed that the non-extractable activity in the goat
tissues, milk, etc. is due to the incorporation of 14C into the
natural constituents of the body, as in rats.
In plants
Methamidophos is an insecticide with systemic activity (Werner,
1972). The physical and chemical basis of systemic movement in the
cotton plant was investigated by Hussain et al. (1974).
Root and leaf treatment of tomato, cabbage and bean plants
demonstrated that 14C-methamidophos is readily taken up by roots and
leaves and transported with the transpiration water towards the
margins of leaves, while little is retained in the stem and petioles.
The labelled compound accumulating at the margins of leaves is
essentially only methamidophos, while in the centre of the leaf some
more polar compounds are present, apparently as the result of
metabolic degradation. The uptake of methamidophos by roots appears to
be independent of pH within the physiological range of 3.5-7.5
(Tutass, 1968b).
Horler et al., (1975) showed that in tomato plants there is only
slight movement of the intact insecticide from treated leaves to the
fruit. After rapid penetration of 14C-methamidophos or/and
32P-methamidophos into tomato leaves, the residue in leaves and fruit
in the initial phase has a biological half-life of 7-10 days. However,
especially in the fruit, after this initial period the rate slows to
give a half-life of about 6 weeks (Horler et al., 1975). The authors
assume that intact insecticide is lost from fruits and leaves by a
mechanism associated with transpiration, rather than by metabolism and
translocation.
Werner (1973) also reports rapid absorption of
methylthio-14C-methamidophos by the roots of Loblolly pine seedlings.
After 96 hours exposure, an accumulation of radioactivity was evident
in the needle tips. Besides unchanged methamidophos, 3 compounds were
isolated from the seedlings. One was identified as the major
metabolite, OS-dimethyl phosphorothioate (II, Figure 1), and in
addition two unidentified compounds were detected, of which one
(Unknown 1) was probably phosphoric acid (VI, Figure 1).
Methamidophos, II and Unknown 1 were detected in the roots, stems and
needles, but Unknown 2 only in the needles, of seedlings treated for
72 and 96 hours (see Table 8).
TABLE 8. Percentage distribution of radioactive methamidophos
and 14C-compounds from TLC and GLC of pine seedling
extracts (Werner, 1973)
Seedling exposure period (h)
Compound* Plant part 6 12 24 48 72 96
I (methamidophos) Roots 80.0 46.0 40.0 35.7 3.3 0.9
Stem 0 0 1.6 1.0 1.1 1.7
Needles 0 31.0 27.9 26.3 22.0 30.5
II (OS-dimethyl Roots 20.0 3.8 1.6 1.0 3.3 3.5
phosphorothioate)
Stem 0 3.8 13.1 6.1 11.0 10.4
Needles 0 46.0 26.2 18.4 16.5 17.4
Unknown 1 Roots 0 0 1.6 1.0 2.2 1.7
Stem 0 3.8 4.9 3.1 4.4 2.6
Needles 0 19.2 18.1 17.4 19.7 10.4
Unknown 2 Roots 0 0 0 0 0 0
Stem 0 0 0 0 0 0
Needles 0 0 0 0 16.5 20.9
* Roman numerals refer to Figure 1.
Experiments with methylthio-14C-methamidophos on cabbages and
tomatoes as well as on plant tissue cultures of sweet potatoes and
tobacco have shown that degradation occurs chiefly by hydrolysis
(Chevron, 1968). Hydrolysis first occurs at the P-N bond to yield
OS-dimethyl phosphorothioate (II, Figure 1), which was consistently
detected as a metabolite in all tissues investigated. Continued,
though somewhat slower, hydrolysis of II apparently leads to cleavage
of CH3S-P and CH3O-P bonds yielding VII and III as major products.
Quantitative data on the residues of these metabolites are not given.
The final fate of the radiolabel is its incorporation into plant
constituents such as pigments, amino acids, carbohydrates and
structural material (up to 19% of the activity in the sample of
cabbage plants).
In soil
Methamidophos is degraded at a relatively fast rate in soils. In
laboratory experiments, Leary and Tutass (1968) found half-lives of
1.9 days in silt soil, 4.8 days in loam soil and 6.1 days in sandy
soil. Sterilization of silt soil by autoclaving resulted in a
considerable increase in the half-life, to about 6 weeks.
In laboratory experiments conducted by Horler et al. (1975),
methamidophos was also found to undergo rapid degradation. In basic
soils (pH 7.40 and 7.75), only 3-7% of the original activity was found
after 4 days; in an acid soil (pH 4.30), 25% was present after 9 days.
In the basic soils, there were differences in the degradation of the
metabolites. In clay-loam soil (pH 7.40), the metabolite degraded at a
slower rate than in loam soil (pH 7.75). Since both soils had a
similar pH, such differences may be connected with the fact that
microorganisms play a role in the decomposition.
In degradation experiments with field soils sampled from depths
of 0-10 cm, and from depths of 0-15 cm and 15-30 cm, it was noted that
at sampling on the day of application to the sail, only a fraction of
the applied methamidophos was still present (Bayer, 1972; Chemagro,
1974). Within one week, the residue dropped further to 10% of the
level measured on the day of application. At depths of 15-30 cm, only
very small amounts of residue (maximum of 0.04 mg/kg) were found in
the soil, and no accumulation was noted after several applications.
Leary and Tutass (1968) found that metabolic degradation of
S-methyl-14-methamidophos in silt soil under aerobic conditions
resulted after 3 days in the evolution of 70% of the applied dose as
14CO2. On the other hand, only 7.6% was evolved as 14CO2 under
aerobic conditions, with 84.6% of the applied activity remaining in
the soil. Up to about 30% of the activity that remained in the soil
was not acetone-extractable. Most of the extractable radioactivity was
in the form of OS-dimethyl phosphorothioate (II, Figure 1), while
minor amounts were in the form of methamidophos, amino acids and
carbohydrates. Quantitative data on these constituents were not given.
Horler et al (1975) also identified OS-dimethylphosphorothioate
(II) as the major metabolite in soil. However, they also found very
small amounts of V, VI and III in clay loam soil. In loam soil, VI was
not found. It is stated that the loss of the methoxy group results in
the evolution of CO2. Quantitative data are not given.
In water
The hydrolysis of methamidophos was examined at elevated
temperatures in aqueous solutions as a function of pH (Leary, 1968;
Magee, 1966). In solutions with a pH below 2, the half-life was a
matter of hours. At pH 9, the half-life was 2.6 days at 25°C and 1.5
days at 37°C. In mildly acidic or neutral solutions, methamidophos
showed remarkable stability at temperatures up to 80°C (less than 5%
hydrolysis in 25 hours). The P-NH2 bond is broken first, followed by
hydrolysis of the CH3SP grouping.
Leaching experiments with methamidophos and three soil types
(muck, sandy loam and loam) (Tutass, 1968c), showed little retention
of the compound. Soil type apparently had only a minor effect upon
retention. The compound was least retained in loam.
Experiments to study the leaching behaviour of methamidophos with
a standardized soil column technique revealed that about two-thirds of
the compound was leached out of sandy and sandy loam soils while only
about one-fifth was leached out of a soil with an increased content of
organic matter (Bayer, 1972).
Flint and Shaw (1972) also studied the absorption of
methamidophos by and its leaching from three different soil types. The
absorption by soil was very slight, and it was further decreased by
increasing temperature and decreasing pH. Methamidophos was not
retarded by the soil when passed through soil columns in water. The
chemical was found in soil run-off water only in the first two of five
irrigations. It was degraded in a natural water system outdoors with
half-lives of 15.9 days in water and 7.5 days in silt. It was
concluded that although it was leached, methamidophos was degraded
rapidly in natural water systems.
In fish
Bass fingerlings which had been in water containing 0.01 mg/1 of
methamidophos for eight days, were analyzed. Less than 0.02 mg/kg of
methamidophos was found in all samples (Stanley, 1971 b).
In processing and storage
The effect of simulated commercial processing on methamidophos
residues in tomatoes was investigated by Morris and Olson (1974). The
tomatoes were processed to juice (pressed, pasteurized), to canned
tomatoes (sterilized), to ketchup. (sterilized, pasteurized), to dry
tomato pomace and to dry tomato pulp. The residues in juice, canned
tomatoes and ketchup were smaller than those found in whole tomatoes,
amounting to 89.5, 73.0 and 68.4% respectively, of the residue levels
prior to processing. On the other hand, the residues in dry tomato
pomace and dry tomato pulp were 2.0 and 2.5 times the original levels
respectively.
The effect on residues of washing in a manner simulating
commercial packing practice depended upon the treated crop. After
treatment, the fruits were allowed to stand outdoors for 24 hours.
Whereas washing green peppers and eggplants did not remove any
significant residue, washing tomatoes removed approximately 15% and
washing cucumbers approximately 20% of the methamidophos residue
(Thornton, 1973).
Dehydrated celery flakes were prepared commercially by placing
celery leaves on trays and drying at temperatures of up to 60°C
(Morris, 1975). Dehydration removed 80% of the weight as water, and it
was concluded that the dehydration process did not destroy any of the
methamidophos residue present in fresh celery leaves.
Cottonseed was processed into delinted seed, meal, hulls. and
oil. No residue was found in any of the seed samples or in any of the
fractions from the processed seed. The residue found in the trash
ranged from 0.1 to 2.7 mg/kg (Chevron, 1976).
Rapeseed was processed into oil and meal (Chemagro, 1976). The
following residue levels were measured: rapeseed, 0.02-0.25 mg/kg,
rapeseed oil, n.d.-0.02 mg/kg and rapeseed meal 0.01-0.30 mg/kg.
Leary (1968) conducted a study to determine the stability of
weathered residues of methamidophos on chopped crop samples (broccoli,
lettuce, cabbage, cauliflower, Brussels sprouts) stored deep frozen
for several months. No significant evidence of decomposition was
obtained and there was no indication of any trend towards
decomposition with increased time of storage.
Möllhoff (1971), however, noted that methamidophos residues
decreased by 10-40% in cauliflower samples stored for three months at
-20°C, and by up to 10% in tomatoes stored under the same conditions.
In further studies conducted by Chemagro (1976), to investigate
the effect of frozen storage at about -20°C on methamidophos residues
in peanut meat, peanut forage, sorghum grain, rapeseed, celery and
peppers, decomposition ranged from 0 to 22%.
The effect of frozen storage at about -20°C on methamidophos
residues in cattle tissues and milk and in poultry tissues and eggs
has also been studied (Chemagro, 1976). It was found that
decomposition in poultry liver amounted to 33% after 60 days and in
cattle liver to 85% after 150 days. On the other hand, the residues
remained completely stable in cattle steak, fat and milk, and
decomposition amounted to a maximum of only 13% in eggs, heart and
gizzard of poultry.
METHODS OF RESIDUE ANALYSIS
Methods for the gas-chromatographic determination of
methamidophos have been developed and published in several
modifications, but in practically all cases are based on clean-up
procedures with silica gel columns and GLC with thermionic detection.
Möllhoff (1971) developed a method for determination in plants,
soil and water. Maceration with acetone or an acetone/water mixture is
used for extraction. The limit of determination is generally about
0.01 mg/kg, but for oil seeds only 0.05 mg/kg and for hops and tobacco
0.1 mg/ kg. Recoveries in the 0.01-1.0 mg/kg range vary between 80%
and 100%.
Leary (1971) also determined methamidophos in crops, but with the
use of ethyl acetate for extraction. The GLC instrument in this case
was equipped with a caesium bromide thermionic detector and the method
is claimed to have a limit of determination of 0.003-0.005 mg/kg. At
levels of 0.5-0.1 mg/kg the majority of recovery values were from
90-100%.
Both these methods have been tested for specificity in
conjunction with other organophosphorus insecticides. They were found
to be adequately specific and without interferences from extractives
with a number of different crops (Olson, 1973).
Stanley and co-workers have developed methods for residues in
different forage crops such as hay, peanut meal, sorghum grain and
rape seed (McNamara & Stanley, 1975) and in animal tissues and milk
(Stanley, 1971c). Extractions were with either methanol/chloroform or
acetonitrile and Skellysolve B.
With minor amendments these methods have been found suitable also
for the determination of methamidophos in fish and water with limits
of determination down to 0.02 mg/kg and 0.004 mg/kg respectively
(Stanley 1971 a).
These methods for the determination of methamidophos follow
closely the general pattern for organophosphorus insecticides in
current multi-residue procedures. Undoubtedly, therefore, they will be
suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
TABLE 9. National tolerances reported to the meeting
Country Crop Tolerance, Pre-harvest
mg/kg interval
Australia Capsicums 0.25 14 days
Peaches 0.25 21 days
Tomatoes 0.25 21 days
TABLE 9. (Cont'd.)
Country Crop Tolerance, Pre-harvest
mg/kg interval
Potatoes 0.05 7 days
Canada Broccoli, Brussels
sprouts, lettuce,
peppers 1.0
Cabbage, cauliflower,
cucumbers, eggplant,
tomatoes 0.5
Potatoes, melons negligible
residue
Netherlands Brassica 0.1
Potatoes 0.01x
New Zealand Brassicas, potatoes 0.1
Tomatoes 0.1 3 days
South Africa Peaches 1.0 21 days
Cabbage 1.0 21 days
Potatoes 1.0 14 days
U.S.A. Cauliflower, lettuce
tomatoes 2.0
Broccoli, Brussels
sprouts, cabbage,
peppers, cucumbers,
eggplant 1.0
Melons 0.5
Cottonseed, potatoes 0.1xx
x = limit of determination
xx = negligible residues
APPRAISAL
Methamidophos is a chemically simple organophosphorus insecticide
with broad spectrum effects used to control caterpillars, aphids,
larvae etc. It is active by contact as well as systemically.
Since 1969 methamidophos has been registered in a number of
countries around the world, chiefly in regions with a sub-tropical
climate. Its major uses are pre-harvest treatments of industrial
crops, fruit and vegetables, mainly with spray solutions at rates from
0.5-1.5 kg/ha.
Detailed studies on the metabolic fate of methamidophos indicate
that the compound is readily taken up by plant roots and translocated
with the transpiration stream within the plants, i.e. towards the
edges of the foliage. In studies with the radio-labelled compound, the
further fate has been followed and a complete degradation pathway with
final incorporation into plant constituents is suggested, mainly
through steps involving hydrolytic breakdown.
The degradation of methamidophos in the animal body is also
hydrolytic and there are no signs of accumulation in tissues or
organs. Most of the phosphorus-containing moiety of methamidophos (up
to about 70%) is eliminated via the urine, while a major part of the
carbon (about 40% in the rat) is excreted as CO2 with the breath, the
remainder being found in the urine and faeces.
The results of extensive residue trials on a number of vegetable
and agricultural crops are available. The data confirm methamidophos
as a systemic compound of relatively low persistence. Most of the data
are from trials under practical conditions which are likely to
represent good agricultural practice.
Methamidophos penetrates into the flesh of the fruit in, e.g.,
tomatoes, peppers, eggplants, etc. Washing or peeling such products
therefore does not significantly remove residues. In the frozen
storage of crops treated with methamidophos, residues are found to be
stable for several months.
Methamidophos has been found to be degraded fairly rapidly in
soils and in natural water systems without any signs of accumulation
or concentration in living organisms.
Gas-chromatographic methods making use of thermionic detectors
are available for the determination of methamidopho in various crops
and animal products. They are combined with appropriate extraction and
clean-up procedures and are likely to be suitable for regulatory
purposes.
RECOMMENDATIONS
Maximum residue limits are recommended for methamidophos in the
following commodities at the levels indicated. They refer to the
parent compound.
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Hops 5 Peppers 1
Broccoli 2 Sugar beet leaves 1
Cauliflower 2 Cottonseed 0.1
Celery 2 Potatoes 0.1
Lettuce 2 Rapeseed 0.1
Limit, Limit,
Commodity mg/kg Commodity mg/kg
Tomatoes 2 Sugar beets 0.1
Brussels Meat and fat of
sprouts 1 cattle, goats
Cabbage 1 and sheep 0.01*
Cucumber 1 Milk 0.01*
Eggplant 1
Peaches 1
* At or about the limit of determination
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further studies an the teratogenic potential in a suitable
species.
2. Further studies to elucidate the contribution of acephate and
metamidophos alone or in combination in depressing cholinesterase
activity.
3. Further information on the level and fate of residues during
cooking and processing of fruit and vegetables.
REFERENCES
Arnold, D., Kennedy, G., Keplinger, M.L. and Fancher, O.E.
1971 Mutagenic Study with Monitor Technical (SX No.
171) in Albino Mice. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Arnold, D., Keplinger, M.L. and Fancher, O. E. Three Generation
1970 Reproduction Study on SX-171 (Technical RE-9006 -
75%) in Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Bayer Berichte No. 12,13/1972; No. 11,12/1973. Submitted
1972 to the Joint Meeting.
Carlson, D., Vondruska, J. F. and Fancher, O. E. Effects of RE 9006
1969 - 75% SX-171 on the Cholinesterase Activity in the
Beagle Dog. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Chevron Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity of
1968a Monitor Technical in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1908b of Monitor 6S in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, D.H. Acute Oral Toxicity
1968c of RE 9006 (95%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968d of RE 9006 (75%) in Mice. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.D. Acute Dermal Toxicity
1968e of Monitor 6S. Unpublished report from Industrial
Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1968f of Monitor Technical. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Bayer, A.G.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968g of RE 9006 (95%) in Rats. Unpublished report from
Industrial Hygiene and Toxicology, Standard Oil of
California, submitted to the WHO by Chevron
Chemical Company.
Cavalli, R.D., Hallesy, D.W. and Spence, J.A. Acute Oral Toxicity
1968h of RE 9169 (SX-196) in Rats. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
the Chevron Chemical Company.
Cavalli, R.E., Hallesy, D.W. and Spence, J.A. Acute Dermal Toxicity
1969 RE 9169 (SX 198) in Rabbits. Unpublished report
from Industrial Hygiene and Toxicology, Standard
Oil Company of California, submitted to the WHO by
Chevron Chemical Company.
Cavalli, R.E. and Spence, J.A. Effect of Repeated Doses of RE 12420
1970 on Cholinesterase Activity in Rats. Unpublished
report from Standard Oil of California, submitted
to the WHO by Chevron Chemical Company.
Chemagro Reports nos. 44249, 44250 on methamidophos in
1974 soil. Submitted to the Joint Meeting.
Chevron Metabolism of Monitor insecticide by plants.
1968 Chevron report submitted to the Joint Meeting. In
file at FAO, Plant Protection Division.
Crawford, C.R. and Anderson, R.H. The Acute Toxicity of Monitor
1973 Technical in Combination with Marathion to Female
Rats. Unpublished report from Chemagro Division of
Baychem Corporation, submitted to the WHO by
Bayer, A.G.
Crossley, J. and Tutass, H.O. Metabolism of Monitor Insecticide
1969 by rats. Unpublished report no. 721.14 from the
Ortho Division, Chevron Chemical Company,
submitted to the WHO by Bayer, A.G.
Davis, A.C., Bourke, J.B. and Kuhr, R.J. Disappearance of Monitor
1974 Residues from Cole crops. J. Econ. Entomol. 67,
766-768.
Fletcher, D., Jenkins, D.H., Keplinger, M.L. and Fancher, O.E.
1971 Demyelination Study with Monitor (RE 9006)
Technical in Mature Laying Hens. Unpublished study
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Flint, D.R. and Shaw II, H.R. The Mobility and persistence of
1972 MONITOR in soil and water. Report No. 34 483
submitted by Chemagro.
Garofalo, M., Palazzolo, R. and Sanders, R. A Study on the Effect
1973 of Orthene and Monitor on Plasma and Erythrocyte
Cholinesterase Activity in Human Subjects During
Subacute Oral Administration. Unpublished report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Chevron Chemical Company.
Greco, R.A., Lindberg, D.C., Keplinger, M.L. and Fancher, O.E.
1971 Effect of RE 9006 on Plasma and Erythrocyte
Cholinesterase Activity in Beagle Dogs.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Gröning, P. Tamaron. Special Toxicity Studies (Potentiation).
1975 Unpublished report from Institut für Toxikologie,
submitted to the WHO by Bayer, A.G.
Gröning, P. Bayer 17628 (Tamaron) Untersuchung Der Subchronischen
Toxizitat an ratten (3-monate-futterungversuch).
Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Gröning, P. and Lorke, D. Untersuchung der subchronischen toxizitat
1976 an hunden. Unpublished report from the Institut
für Toxikologie, submitted to the WHO by Bayer,
A.G.
Hanna, P.J. and Dyer, K.F. Mutagenicity of Organophosphorus Compounds
1975 in Bacteria and Drosophila. Mut. Res. 28:
405-20.
Horler, D.F., Lubkowitz, J.A., Revilla, A.P., Baruel, J. and Cermeli,
1975 M.M. Uptake and Degradation of Monitor by Tomato
Plants. Pesticides, IUPAC 3rd Int. Congr. Pest.
Chem., Helsinki, 151-156.
Hussain, M., Fukuto, T.R., and Reynolds, H.T. Physical and Chemical
1974 Basis for Systemic Movement of Organophosphorus
Esters in the Cotton Plant. J. Agr. Fd Chem. 22
(2): 225-230.
Ladd, R., Jenkins, D.H., Wright, P.L. and Keplinger, M.L.
1971 Teratogenic Study with Monitor Technical in Albino
Rabbits. Unpublished report from Industrial Bio
Test Laboratories, Inc. submitted to the WHO by
Bayer, A.G.
Leary, J.B. Stability of Monitor insecticide residues in frozen
crops and in aqueous solutions. Chevron report
files No. 740.01 and No. 721.2.
Leary, J.B. and Tutass, H.O. Degradation of Monitor insecticide
1968 in soil. Chevron report file No. 721.2.
Leary, J.B. Gas-chromatographic Determination of Monitor
1971 (OS-Dimethyl Phosphoramidothioate) Residues in
Crops. J. Ass. Off. Analyt. Chem. 54 (6):
1396-98.
Lee, H. and Crossley, J. The Fate of Monitor in a Lactating
1972 Ruminant (Goat). Unpublished report from Ortho
Division, Chevron Chemical Company, submitted to
the WHO by Bayer, A.G.
Lindberg, D., Vondruska, J.F. and Fancher, O.E. Two Year Chronic
1969 Oral Toxicity of RE 9006-III, SX-116 in Beagle
Dogs. Unpublished study from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO, by
Bayer, A.G.
Lindberg, D., Keplinger, M.L. and Fancher, O.E. RE 9006 (70%)
1970 SX 171 on the Cholinesterase in the Beagle Dog.
Unpublished study from Industrial Bio-Test
Laboratories Inc., submitted to the WHO by Chevron
Chemical Company.
Lorke, D. and Kimmerle, G. Toxicological Studies on the Active
1967 Ingredient Bayer 71 628. Unpublished report from
the Institut für Toxikologie, submitted to the WHO
by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on Dogs
1970a (Three Month Feeding Experiment). Unpublished
report from Institut für Toxikologie, submitted to
the WHO by Bayer, A.G.
Löser, E. and Lorke, D. Subchronic Toxicological Studies on rats.
1970b Unpublished report from the Institut für
Toxikologie, submitted to the WHO by Bayer, A.G.
Magee, P.S. Hydrolysis of Monitor insecticide. Chevron Report
1966 File No. 721.2.
Mastri, C., Keplinger, M.L. and Fancher, O.E. Twenty-one Day
1968 Subacute Dermal Toxicity of Monitor (RE 9006) 75%
Technical SX 171. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Chevron Chemical Company.
McNamara, F.T., and Stanley, C.W. Gas-Chromatographic Method for
1975 the Determination of Residues of MONITOR in Peanut
Meat and Hay, Sorghum Grain, and Rapeseed.
Chemagro Report No. 45.439.
Möllhoff, E. Metode zur gaschromatographischen Bestimmung
1971 von Tamaron-Rückständen in Pflanzen.
Pflanzenschutz-Nachr. 24 (2): 256-262.
Morris, R.A. Effect of Dehydration on Residues of MONITOR
1975 in Celery Leaves. Chemagro Report No. 45.586.
Morris, R.A., and Olson, T.J. The Effect of Processing on MONITOR
1974 Residues in Tomatoes. Chemagro Report No. 41.107.
Olson, T.J. Interference Study on MONITOR (TAMARON) in Various
1973 Crops. Chemagro Reports No. 37.380 and No. 37.392.
Plank, J., Keplinger, M.L. and Fancher, O.E. Paired Feeding Study
1968 of RE 9006 - III SX-116 Albino Rats. Unpublished
report from Industrial Bio-Test Laboratories.
Inc., submitted to the WHO by Chevron Chemical
Company.
Plank, J., Keplinger, M.L. and Fancher, O.E. Ninety-Day
1969 Cholinesterase Study of SX-171 Monitor (RE 9006) -
Albino Rats. Unpublished report from Industrial
Bio-Test Laboratories, Inc., submitted to the WHO
by Bayer, A.G.
Plank, J., Keplinger, M.L. and Fancher, O.E. Two Year Chronic
1970 Oral Toxicity of RE 9006 - III, SX-116 in Albino
Rats. Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
Reyna, M.S., Kennedy, G.L. and Keplinger, M.L. Ninety-Day Subacute
1973 Oral Cholinesterase Study with Monitor Technical
in Female Albino Rats. Unpublished report from
Industrial Bio-Test Laboratories, Inc., submitted
to the WHO by Bayer, A.G.
Schoenig, G., Keplinger, M.L. and Fancher, O.E. Study on the Efficacy
1968 of Atropine Sulfate and 2-PAM C1 as Antidotes for
Monitor (RE 9006) 75% Technical SX-171. Report
from Industrial Bio-Test Laboratories, Inc.,
submitted to the WHO by Bayer, A.G.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971a of MONITOR in Fish and Water. Chemagro Report No.
30.975.
Stanley, C.W. Analysis of Bass and Water for MONITOR. Chemagro
1971b Report No. 30.979.
Stanley, C.W. A Gas Chromatographic Method for the Determination
1971c of Residues of MONITOR in Animal Tissues and Milk.
Chemagro Report No. 31.093.
Thornton, J.S. Effect of Washing on Residues in Tomatoes, Cucumbers,
1973 Eggplants and Peppers. Chemagro Reports Nos.
37322, 37539, 37540 and 37541 submitted to the
Joint Meeting. In File at FAO, Plant Protection
Division.
Tucker, B. Acetylcholinesterase Inhibition of Orthene and Ortho
1972 9006. Unpublished report from Ortho Division of
Chevron Chemical Company, submitted to the WHO by
Chevron Chemical Company.
Tutass, H.O. Microsomal Oxidation of Monitor. Unpublished report
1968a no. 721.2 from the Ortho Division of Chevron
Chemical Company, submitted to the WHO by Bayer,
A.G.
Tutass, H.O. Uptake and Translocation of Monitor Insecticide
1968b by tomato, cabbage and bean plants. Chevron Report
File No. 721.2.
Tutass, H.O. Leaching of Monitor insecticide in soils. Chevron
1968c Report File No. 721.2
Werner, R.A. Systemic insecticidal action of disulfoton and
1972 Monitor in Loblolly pine seedlings. J. Georgia
Entomol. Soc. 7 (1): 64-67.
Werner, R.A. Absorption, Translocation and Metabolism of Root-
1973 Absorbed 14C Monitor in Loblolly Pine Seedlings.
J. Econ. Entomol. 66 (4): 867-872.
Wolvin, A.A., Palazzolo, R.J. and Fancher, O.E. Neurotoxicity
1968 Study - Chickens Monitor RE 9006, 75% Technical.
Unpublished report from Industrial Bio-Test
Laboratories, Inc., submitted to the WHO by Bayer,
A.G.
