
IPRODIONE JMPR 1977
IDENTITY
Iprodione is a recommended common name of APTOR and BSI and a proposed
ISO Standard Common Name.
Chemical name
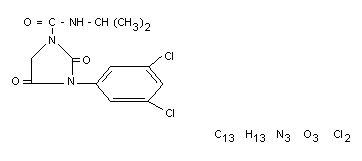
3-(3,5-dichlorophenyl)-N-isopropyl-2,4-dioxoimidazolidine-1-carboxam
ide
Chemical abstracts
1-(1-methylethylaminocarbonyl)-3-
(3,5-diohlorophenylimidazolidine)-2,4-dione
i-isopropylcarbamoyl-3-(3,5-dichlorophenyl)hydantoin
Synonyms
Glycophene, promidione, 26019 RP, ROP, 500 F., NRR 910, LPA 2043
Rovral (R)
Structural formula
 Other Information on Identity and Properties
a) Composition of the technical product
The technical product contains 95% minimum of iprodione. The main
impurities are phenyl hydantoins and bis-isopropyl-1,,3\-urea
(referred to as 32870 R.P.).
b) Physical and chemical properties
Physical state: white, odourless, non-hygroscopic crystals.
Molecular weight: 330.17
Melting point: 136°C
Volatility: not volatile
Vapour Pressure: 2 × 10-7mm. Hg at 20°C
Solubility at 20°C: g/l
water 0.013
ethanol 30
acetonitrile 150
toluene 150
benzene 200
acetone 300
methlyene chloride 500
Formulations
Mainly wettable powder 500 g a.i./kg. Also suspension concentrate 500
g a.i./l and emulsifiable concentrate 200 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Single oral doses of iprodione are rapidly eliminated by rats.
Following a single application of an oral dose of 200 mg/kg, 26% of
the administered dose was eliminated in the urine and 59% in the
faeces within 24 hours after application. The major part of the dose
excreted in the faeces is the parent compound, whereas only 3% of the
administered dose is eliminated unchanged in the urine. Besides the
principal urinary metabolites with a degraded isopropylcarbamoyl group
(about 11% of the dose administered), there are metabolites with
intact hydroxylated or non-hydroxylated aromatic rings. The isomer of
the parent compound accounted for a small proportion of the
metabolites. Residues in the principal organs and tissues did not
exceed 1.5% of the administered dose in rats sacrificed 4 days after
dosage (Laurent and Bays, 1974).
In a similar study rats were dosed once with 100 mg/kg. of
14C-aromatic ring-labelled iprodione; 96 hours after administration
62% of the applied dose was eliminated via the urine and 36% via the
faeces. About 16% was excreted as the parent compound in the faeces:
the remaining radioactivity was mainly in urine in the form of the
desisopropylated derivative (about 20% of the dose) and the
N-(3,5-diachloro-4-hydroxyphenylbiuret) (approx. 13%). Tissues sampled
4 days after dosage contained about 1% of the administered dose.
(Lourer, et al., 1976),
Based on the identified metabolites the reactions that seem to occur
during biotransformation are mainly hydroxylation, oxidation and
desalyklation of the isopropylcarbamoyl group
(-N1-CO-NH-CH(CH3)2->N1-CO-NH2->N1-H).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups of 25-30 rats were orally treated with 0, 100, 200 and 400
mg/kg on Restation days 5 to 15. Females at 400 mg/kg showed reduced
fertility, reduced body weight gain and a dose-related reduction of
food consumption especially during the treatment period. The number of
implantations was also reduced at the highest dose level.
There was no indication for an embryonyic or teratogenic effect of the
test completed (Coquet, 1973a).
Groups of 15-17 New Zealand White rabbits were intubated on gestation
days 6-16 inclusive with 0, 100, 200 or 400 mg/kg. Body weight gain,
over the period of treatment, was slightly reduced at 100 mg/kg, and a
dose-related weight loss occurred at 200 and 400 mg/kg. Food intake
was reduced at 200 mg/kg and above. At 400 mg/kg, 9 of 17 females
died, and only one of the four remaining pregnant animals carried to
term. Foetal loss was increased at 200 mg/kg, and foetal weight was
reduced at 200 mg/kg and above. Multiple malformations occurred in 1
of 68 living foetuses at 200 mg/kg. Minor malformations were noted in
all groups.
Special studies on carcinogenicity
See "Long term studies."
Special study on reproduction
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 and 1000 ppm for
the first 5 weeks of each generation and 0, 250, 500 and 2000 ppm for
the next 8 weeks of treatment. The diet was fed through three
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parental animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendency for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rate of the third generation did not reveal
abnormalities. (Coquet, 1976)
Special study on mutagenicity
Groups of 25 male mice were fed 0, 1500 and 6000 ppm iprodione for 49
days. After termination of the feeding period the male mice were
paired with 2 untreated females for 6 days, followed by a further 2
females for the 6-12 days post-treatment period. The treatment did not
affect body weight, food consumption or fertility of the males. None
of the examined parameters gave any indication of a mutagenic effect
of iprodione (Hastings et al., 1974).
Iprodione showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, reverse mutation tests with and without liner
activation system using E. coli WP2 hcr- and five strains of
Salmonella typhimurium TA and host-mediated assay with S. typimurium G
46 in mice (Shirasu et al., 1976).
Acute Toxicity
TABLE 1. Acute toxicity of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral >2000 Pasquet & Mazuret,
1973
Rat M F Dermal >2500 ibid.
Rat M i.p. 2400 Pasquet &. Mazuret,
1974
F i.p. 1200 ibid.
Mouse M F Oral approx. 4000 Pasquet A Mazuret,
1973
Dog M F Oral >2000 ibid.
Rabbit M F Dermal >1000 ibid.
The signs of toxicity were: loss of reflexes, muscular hypotonia,
sedation and dyspena.
Iprodione did not cause skin or eye-irritation in rabbits.
In the anaesthetized dog iprodione administered at a dose of 300 mg/kg
by the intraduodenal route did not affect the cardiovascular,
respiratory or neurovegetative system (Detaille et al., 1973).
TABLE 2. Acute toxicity of a 50% formulation of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral 8000 Davies & Lowe,
1974
M F Dermal >2000 ibid.
M F 4 h
inhalation 13 mg/l air Pasquet & Mazuret,
1975a
M F Oral 4100 ibid.
Oral 4900 ibid.
M F 4 h inhalation >13/l air Ibid.
Rabbit M F Dermal >2000*) ibid.
*) atoxic
The formulation induced slight irritation in the rabbit eye but had no
irritant effect on the intact or abraded skin of rabbits (Pasquet &
Mazuret, 1975a).
"In the sensitization test with guinea pigs, after 10 applications of
0-3 ml of a 50% iprodione solution, followed 2 weeks later by a
challenge application, no evidence of dermal sensitization was
observed" (Pasquet & Mazuret, 1975b).
Short term studies
Rat
Groups of 15 male and 15 female caesarian originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the diet
for 5 months. No effects were observed on mortality, food consumption,
haematology (as judged by haemoglobin, haematocrit, erythrocyte count,
or total and differential leucocyte count) clinical chemistry (as
judged by BSP, SGOT, SGPT or SAP) or urinalysis. Body weight gain was
slightly reduced (especially in males) at 500 and 1000 ppm. Absolute
(but not relative) heart weight was reduced in males at 500 and 1000
ppm, and absolute kidney weight was reduced at 1000 ppm. In females,
absolute liver and kidney weights were significantly reduced at 500
ppm only. Gross and histopathology were normal at all dose levels. In
a parallel study, dichlozoline, a structurally related compound,
induced cataracts. No such effect was seen with (Ganter et al.,
1973a).
Dog
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 and 7200 ppm for a
period of 3 months. At the top dose level the method of administration
was altered after 6 weeks, to gelatine capsules. The treatment did not
affect mortality. The recorded values of haematological determinations
and urinalyses were within normal limits. As judged by haemoglobin,
haematocrit, reticulocyte erythrocyte count, total and differential
leucocyte count and prothrombine time except for signs of mild anemia
in 1 male and 1 female at 2 months and 1 male at 3 months at the top
dose level. At 7200 ppm a reduction of food consumption was observed,
accompanied by reduced body weight gain. The opththalmosopic
examination of the animals did not reveal any pathological alteration
(Canter and Girard, 1973b). The clinical chemistry determinations
consisted of glucose, urea, cholesterol, bilirubiu, total proteins,
protein electrophoresis, alkaline phosphatase, SGOT, SGPT, LDH, Na+,
C, K+, Cl -, Ca++, P. At 2400 and 7200 ppm a slight increase of SLP was
observed, also a transient increase of SGOT and SGPT after 1 and 2
months of treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, in females at the
dose levels of 2400 ppm and above. At 7200 ppm reduced relative weight
of testes was found, but no histological indication of damage.
The histopathological findings did not reveal any indication of
treatment-related alterations of tissues (Coquet, 1973c).
Long term studies
Mouse
Groups of 60 male and 60 female mice were maintained an a diet
containing the test compound at 0, 200, 500 and 1250 PPM for 18
months. No treatment-related effect on body weight, food consumption
or mortality was found. The recorded values of the haematological
blood chemistry and urinalyses tests performed after 6, 12 and 18
months of the feeding period, were within the physiological range.
Necropsy findings on mice that died during the last 6 months of the
test and on those sacrificed at the termination date showed an
increased number of enlarged lymph nodes in males at 200 ppm. Organ
weight variations occurred sporadically in the various dose groups and
are considered not to be treatment-related. The histopathological
findings failed to reveal abnormal features. The distribution of
neoplastic and non-neoplastic findings did not appear to demonstrate
any significant dose-dependence. The most common tumours were
lymphosarcoma involving the spleen, lymph nodes and thymus (Hastings
and Hullman, 1975).
Rat
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 and 1000 ppm for 24 months. Slight reduction in
body weight gain was observed at 1000 ppm. This was accompanied by
some reduction in food intake. The treatment had no effect on food
consumption, mortality or values of the hematologic, blood chemistry
and urinalyses determinations. Necropsy findings did not reveal any
drug-related gross alteration. Variations in organ weight did not show
a group distribution and seemed not to be related to drug
administration. Histopathology did not indicate a treatment
relationship of neoplastic and non-neoplastic findings. AT 24 months
the most common tumours observed were pituitary adenomas and
adenocarcinoma and fibroadenoma of the mammary glands (Hastings et
al., 1976).
COMMENTS
Iprodione is readily absorbed and rapidly excreted mainly as
metabolites with intact hydantoin-moiety. The compound was not
teratogenic. In a 3-generation study in rats, there was a slight but
statistically significant reduction in postnatal growth at 2000 ppm.
This effect was only marginal at the lower dose of 500 ppm which is
regarded as a no-adverse-effect-level. In a short-term study in dogs
no major effect occurred up to 2400 ppm. Likewise long-term studies in
mice and rats revealed no effects up to 1250 ppm. No ocular
alterations were found in any study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mice: 1250 mg/kg in the diet, equivalent to 160 mg/kg bw
Rat: 500 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.3 mg/kg bw
USE PATTERN
Iprodione is used as a fungicide against a range of fungus diseases,
including Botrytis in vines, black- and red currants, blackberries,
raspberries and vegetables especially lettuce; Botrytis alii on
onions; Rhizocotonia on seed potatoes; seed borne diseases on sugar
beets (Phoma spp.) and cereals. It is also used against Botrytis
and some other fungus diseases on ornamentals.
The compound is used as a foliar spray on several crops, as a post-
harvest dip for fruit, for dipping seed-potatoes and as a seed-
treatment on sugar beet and cereals.
The product is authorized for use on various crops in France, the
Federal Republic of Germany, Greece, the Netherlands and the United
Kingdom. In several other countries the compound is used or included
in testing programmes and it is in course of registration in many
countries including Australia, New Zealand, Japan, USA, Canada,
Israel, and several European countries.
Most of the recommended uses are summarized in Table 3. The
information may not be complete since the use of the compound is
expanding rapidly and more uses may be expected in the near future.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained from supervised trials carried out in
various countries on fruit and vegetables and on some agricultural
crops; they are summarized in Tables 4 to 8 and 10.
Pome and stone fruits (Tables 4 and 5; Rhône Poulenc, 1977a)
Apples
The residue at harvest from pre-harvest treatments at normal
application rates (about 2.25 kg a.i./ha) is about 2 mg/kg. A
combination of such treatments with post-harvest dipping gives rise to
residues of about 6 mg/kg. After repeated applications at about twice
the normal rate residues ranged from 2.9 - 6.5 mg/kg. The residue of
the metabolite RB 30228 (see "Fate of residues") was below 0.15 mg/kg
in these experiments.
Pears
Post-harvest dipping of pears against storage diseases gave rise to
residues of 3.6 - 5 mg/kg.
Peaches
Residues at harvest following applications at the recommended rate
varied between 0.9 and 6 mg/kg. A post-harvest dip adds about 4 mg/kg
to these levels.
Plums
Residues arising from recommended applications varied between 0.6 and
6.8 mg/kg, depending on the pre-harvest intervals observed and the
local conditions. The drying process increased the residue in the
prunes by 0.6 - 1.6 mg/kg.
TABLE 3. Use pattern and recommended pre-harvest intervals of iprodione
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Grapes Botrytis 4 750 Austria 28
" 4 750 France. 15
" 4-5 750 Fed. Rep. of 28
Germany
" 3 3000 Japan 7
" 4 750 Portugal 15
" 4 750 Spain 15
" 4 750 Switzerland
" 4 750 USSR
" 4 750 Yugoslavia
Strawberries Botrytis 4-5 1000 Belgium 15
3 1000 Fed. Rep. of 7
Germany
3 Japan 1
4-5 1000 The Netherlands 14
Pome and Stone fruit
Apples Alternaria about 10 Japan 10
Peaches Monilia 3 Japan 1
Vegetables
Chicory (witloof)
(forcing) Botrytis 1 3 g/m2 Belgium Throughout
Sclerotinia 1 4 g/m2 France the forcing
applied on the top of period
the roots at forcing
TABLE 3. (Continued)
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Cucumbers Botrytis 4 Japan 1
Lettuce Botrytis
Scleotinia 3 750 Belgium 10
(glasshouse)
" 3-4 750 France
" 3 750 Fed. Rep. 21
of Germany (glasshouse)
14
(outdoors)
" 4 750 Japan 14
(also for endive) " 1x 1000-2000 The Netherlands 28
" 2xx 750 The Netherlands 28
Vegetables
Onions Botrytis
Sclerotinia 3 750 Japan 7
cepivorum
Tomatoes Botrytis 4 Japan 1
Alternaria United Kingdom
Agricultural Crops
Beans Sclerotinia 3 Japan 21
Rice Pellicularia 3 Japan 21
TABLE 3. (Continued)
Seed and tuber treatments
Cereal seed
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Barley Helminthosporium 1 60 g a.i./100
kg seed
Wheat Tilletia caries 1 60 g a.i./100
kg seed
Garlic Sclerotinia cepivorum 1 300 g a.i./100 France
kg seed
Potatoes Rhizoctonia solani 1 100-150 g/ France
1000 kg tubers
spraying on
tubers
immediately
before
storage
dipping in
spring before
planting
400 g/100 l
Sugar-beet Phoma spp 1 150 g a.i./kg France.
seed enveloped seed
x= one application at planting.
xx= two applications, the first about a week after planting and a second within two weeks after planting
Berry fruits and currents (Table 6; Rhône-Poulenc, 1977b)
The residue levels at harvest after treatments at normal rates and
observing recommended pre-harvest intervals (10-21 days) were
generally at or below 5 mg/kg on blackcurrants, 2 mg/kg on raspberries
and 6 mg/kg on strawberries.
Grapes
The maximum residues of iprodione on grapes at harvest following
treatment according to good agricultural practice (about 750 g
a.i./ha) were in general not higher than 10 mg/kg. The highest levels
in the unfermented must and the win were 4.4 and 6.4 mg/l
respectively. Some results are shown in Table 10 (Rhône-Poulenc,
1977c). See "Fate of residues", "In storage and processing".
Vegetables (Table 7; Rhône-Poulenc, 1977d)
Chicory (witloof)
The residues in the edible sprouts after a normal period of forcing
and one treatment at the recommended rate did not exceed 1 mg/kg. The
residues in the roots were much higher, with a maximum of about 10
mg/kg. The roots are often used as animal feed.
Cucumbers
Residues on cucumbers treated with 3 kg a.i./ha (twice the normal
rate) were between 0.3 and 2.2 mg/kg.
Lettuce
Residues arising from recommended applications on outdoor lettuce (750
g a.i./ha) varied between 1.7 and 2.5 mg/kg after pro-harvest
intervals of 14-21 days. The residues on glasshouse-grown lettuce are
in general much higher. Three applications of the recommended dosage
gave rise to residues of 6.7 mg/kg after a pre-harvest interval of 39
days, and in other experiments maximum levels of 7.2 mg/kg were found
14 days after the last application. Residue levels of the metabolite
RP 30228 were slightly about the limit of determination; other
metabolites were below it. (Metabolites are identified in the section
"Fate of residues."
Onions
The residues on onions 1 day after application did not exceed 0.2
mg/kg.
TABLE 4. Supervised trials of iprodione. Residues in pome and stone fruit (pre-harvest application)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Apples Japan 1975 1 100 5 WP 50% 2.15 1.75
1975 1 100 5 " 2.9 2.25
1975 1 100 5 " 0.38
1975 10 100 5 " 6.5
1975 10 100 5 " (5.75)
1975 10 100 5 " 3.75
1975 10 100 5 " 3.4
1975 10 100 " (1.95)
1975 10 100 5 " 1.7
U.K. 1975 10 100 2.25 " (2.0)
Cherries
Moss Australia 1975 4 1.4 " 8.0
Peaches
Katharine Australia 1976 7 50 1.4 " 5.4
Anne Truly 1976 3 50 0.5 " 1.7
Goldmine 1976 5 50 1.4 " 5.8
Redhaven Canada 1974 4 50 1.0 " 6.5 4.6 4.9
1974 4 50 1.0 " 1.45
Babygold Canada 1974 7 50 1.0 " 2.3 2.0 1.3
1974 6 50 1.0 " 1.8 0.9
Earlired 1974 5 1.0 " 2.5 1.9 2.2 2.2
Redhaven 1974 5 1.0 " 6.1 4.1 3.4 2.9 1.6
Sunhaven 5 1.0 " 7.6 9.0 10.0 8.5
Gifu Japan 1975 7 100 4.0 3.7 2.1
1975 3 100 4.0 " 4.6 2.9
Okayama 1975 2 100 3.0 " 6.3 4.8
1975 3 100 3.0 " 6.8 5.8
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Plums
October Australia 1976 4 1.4 " 2.2
purple
Inra 711 France 1973 4 0.5 SC 0.25 0.26
4 1.0 SC 1.4 0.9
1974 0.5 4.15
3 1.0 6.8
2 0.5 0.2
2 1.0 0.6
* SC = Suspension concentrate
WP = Wettable powder
TABLE 5. Supervised trials of iprodione. Residues in pome and stone fruit (post-harvest application)
Application
Rate
Crop Country Year No. g a.i./100 Formulation Residues (mg/kg) after storage period of (days)
dip 1-2 7 11 85 95
Apples
Cox's U.K. 1973 - - 2.0
1 200 5.8
1 200 4.4
Pears
Conference U.K. 1973 1 200 SC 5.0
1973 1 200 4.8
1973 1 200 3.6
1973 1 200 4.7
1973 1 200
1973 1 200 4.0
Peaches Australia - WP50% 1.7
1 50 WP50% 5.3
1 50 WP50% 5.0
TABLE 6. Supervised trials of iprodione. Residues in berry fruits and currants
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Raspberries France 1974 3 0.8 WP 50% 1.1
Heyton 0.5 0.85
Malling
jewel U.K. 1974 5 1.1 " " 1.55
3 1.1 " " 2.0
4 1.1 " " 7.9 5.0 2.8 2.1
Strawberries Belgium 1974 5 0.75 " " 4.9
Sivetta 5 1.0 " " 6.0
Domunil 5 0.75 " " 1.9
5 1.0 " " 2.5
Redcoat Canada 1974 4 1. " " 3.1 1.8 1.4 0.85
Vista 4 1.1 " " 3.5 2.4 1.4 1.0
Redcoat 4 3.45 2.1 1.6 1.0
Redcoat 3 0.9 0.8 0.2
Red Gauntlet France 1973 4 0.5 " " 0.22
0.75 " " 0.44
1.0 " " 0.66
Immigrante 1974 4 0.75 " " 1.75
4 1.0 " " 2.2
Gorella 1974 4 0.75 " " 0.35
4 1.0 0.44
3 0.75 " " 0.5
3 1.0 " " 1.1
Suprême d'Halles 4 0.5 " " 1.2
4 0.75 " " 1.9
4 1.0 " " 2.75
4 0.5 " " 2.5
4 0.75 " " 3.2
4 1 5.6
Senga Segana Fed. Rep. 1974 3 0.9 " " 5.3 2.7 2.3 1.0 0.4
of Gemany 3 1.25 " " 9.1 3.6 3.4 1.9 0.7
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Senga Segana Netherlands 1974 4 0.75 " " 1.1
1974 5 0.75 " " 26.4 14.2 0.67
Red Gauntlet Switzerland 1974 1 0.75 " " 1.0 0.86 0.2 0.1 0.1
Wädenswill 1974 3 0.75 " " 0.2
1974 3 0.75 " " 0.15
Royal
Sovereign U.K. 1974 4 1.1 " " 1.2 1.6 1.3
Strawberries
Cambridge
Favourite (g) 1974 4 1.1 WP 50% 8.0 6.9 4.5 4.7
Cambridge
Favourite (g) 1974 3 1.1 " " 1.7
Cambridge
Favourite 1974 3 1.0 " " 0.7
Cambridge
Favourite 1976 3 1.6 1.1
Cambridge
Favourite 1976 3 0.75 0.9 0.6
Red Gauntlet 1976 1 0.75 0.6
Red Gauntlet 1976 1 1.0 0.9
Red Gauntlet 1974 3 2.2 1.7
Cambridge
Favourite 1974 3 2.2 0.55
Royal
Sovereign 1974 2 1.0 0.3
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Black
currants
Wellington
Tr. UK 1974 4 1.1 WP 50% 3.9
62 days
Baldwin UK 1974 4 1.1 " " 4.6
62 days
(g) = glasshouse
TABLE 7. Supervised trials of iprodione. Residues in vegetables
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Bean
without pod Japan 1975 1 1 WP 50% 0.05 0.05 0.05
Cucumbers(g) Japan 1975 3 1 0.05 0.05
1975 1 3 WP 50% 1.7 1.0 0.36
1975 3 3 " " 1.6 0.9 0.7
1975 4 3 " " 2.2 1.4 1.2
1975 1 2.5 " " 1.2 1.0 0.24
1975 3 2.5 " " 1.9 1.4 0.3
1975 4 2.5 " " 1.8 1.0 1.0
Lettuce
Val.d'Orge France 1974 6 0.5 " " 0.03
6 0.75 " " 0.03
6 1.0 " " 0.03
6 0.5 EC 200g/l <0.02
6 0.75 " " <0.02
6 1.0 " " 0.04
5 0.5 WP 50% 2.5
5 0.75 " " 1.3
Murkönig Fed. Rep. 3 0.75 " " 15.7 1.8 0.6 0.05 0.05 0.05
Kares of Germany 1976 4 0.75 " " 24.5 9.1 1.7
Reskia 1976 3 0.25 " " 2.6 0.8 0.2
3 0.75 " " 13.2 3.0 1.1
Susan 1976 3 0.5 " " 4.6 1.2 0.4
3 0.75 " " 28 4.3 4.2
Murkönig(g) Fed. Rep. 1976 3 0.25 " " 24 6.9 2.9
of Germany 3 0.75 " " 46 10.5 7.2
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Ravel(g) 1976 3 0.25 " " 26 5.4 2.7
0.75 " " 22 4.8 3.6
Kurume
Br.(g) Japan 1975 2 3 " " 4.6 0.28 0.05
3 3 " " 10.8 0.27 0.1
4 3 " " 7.4 0.3 0.1
Kumamoto(g) Japan 1975 2 1.5 " " 0.48 1.05 0.4
3 1.5 " " 1.9 0.6 1.2
4 1.5 " " 2.25 0.78 1.7
Déciminor(g) Netherlands 1975 2 0.75 " " 8.2 4.9
Vera(g) 1975 2 0.75 " " 9.15 0.05 0.05
Ostinata(g) UK 1974 4 0.56 " " 19 18 14 (24
days)
Val d'Orge 1974 3 0.56 " " 8.8
4 0.56 " " 59 40 41 21
Onions S. Africa 1977 7 50 WP 50% 0.14
0.15
100 " " 0.15
0.24
Sweet
Peppers(g) U.K. 1975 6 4.3
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Tomatoes
Koibuchi (g) Japan 1975 1 2.5 " " 1.25 1.4 1.2 0.8
Koibuchi (g) 1975 3 2.5 " " 5.3 3.4 3.0 2.4
Koibuchi (g) 1975 4 2.5 " " 5.6 5.4 4.3 3.5
1975 3 3.0 " " 2.1 3.3 1.8 2.9
4 3.0 " " 4.6 3.6 4.1 2.8
5 3.0 " " 4.4 4.0 3.8 3.7
Eurocross(g) U.K. 1974 5 50 " " 2.7
6 50 " " 3.8
7 50 " " 4.9
8 50 " " 4.2 2.3
Sonato(g) U.K. 1974 4 50 " " 1.65
5 50 " " 2.3
6 50 " " 2.8
7 50 " " 3.7
8 50 " " 4.2 5.8
5 50 " " 3.1 2.5 2.7
1 1 " " 0.64
(18
days)
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. a.i. Formulation 30-40 40 44-48 59 70 93-104 162
g/m2 S R S R S R S R S R S R S R
Chicory Belgium 1975 1 3 " " 0.41 1.49
1 6 " " 0.36 3.10
3 " " 0.09 2.7
6 " " 0.32 4.4
3 " " 0.77 6.2
6 " " 0.59 10.0
3 " " 0.55 2.7
6 1.0 3.7
France 1974 1 4 " " 0.6
8 " " 1.0 0.07
4 " " 0.25
8 " "
TABLE 7B
Crop Country Year No. Rate Formulation 30-40 40 44-48 59 70 93-104 162
g/100 1g/1000
dip kg S R S R S R S R S R S R S R
Potatoes
pre-plant France 1976 1 200 WP 50% n.d.
treatment 300 " " n.d.
50 " " 0.01
100 " " 0.01
150 " " 0.02
200 " " 3.0 5.0
50 " " 25 25
100 " " 58 67
150 " " 120 140
foliar
spray S.
Africa 1976 5 50 " " <0.02
5 100 " " <0.02
3 50 " " <0.02
3 100 <0.02
WP = wettable povider; EC = emulsion concentrate;
(g) = glasshouse; R = roots (chicory) or tubers (potato); S=sprouts
Peppers and tomatoes
Following applications at normal rates (50 g a.i./100 l) residues of
4.5 - 5 mg/kg were found at harvest after pre-harvest intervals of 3.6
days.
Beans (dry)
After treatment at a dosage rate of 1 kg/a.i./ha, residues in the dry
beans were very low (0.05 - 0.2 mg/kg).
Cereal crops (Table 8; Rhône-Poulenc, 1977e)
Wheat
Two applications at normal rates (1 kg a.i./ha) with a pre-harvest
interval of 73 days did not give rise to measurable residues in the
kernels.
Rice
After treatment during the growing season with relatively high dosages
(1.2 kg a.i./ha), residues of 0.1 - 2.1 mg/kg were found de-husked,
unpolished rise 21 days after the last treatment.
FATE OF RESIDUES
In plants
The fate of iprodione in plants and soil was studied with unlabelled
and 14C-phenyl-labelled products. It was found that when applied to
the leaf surface, iprodione does not appreciably penetrate through the
skin. The residues on the skin had a half-life of 30-60 days, being
slowly converted to
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydentoin, RP 30228), which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including 1-carbamoyl-3-(3.5-
dichlorophenyl) hydantoin (RD 32490) (Rhône-Poulenc, 1973).
Wheat and strawberry plants grown on soil treated with iprodione
(Rhône-Poulenc, 1977f) took up small amounts of the compound (in wheat
0.7-1.3% of the amount applied to the soil surface), which was mainly
found in the leaves and stems (95-99% of the extractable residue).
Within the plant the parent compound was converted to RP 30228, small
amounts of RP 32490 and some more polar unidentified products.
The organosoluble residue in strawberry plants 32 days after a foliar
application at a rate equivalent to 1 kg a.i./ha consisted of 61%
unchanged parent compound and 16% RP 30228. 55 days after foliar
treatment at 2 kg a.i./ha 69% of the residue was iprodione, 7% RP
30228 and 5% RP 32490.
TABLE 8. Supervised trials of iprodione. Residues in cereal crops.
Application Residues (mg/kg) at intervals (days) after application
Rate
Crop Country Year No. Formulation 14-15 21-22 28-30 73 81
g/100 1 kg/ha
Rice Japan 1975 1 100 1.2 WP 50%
grain 0.1 0.1 0.1
straw 16 15 10.3
1975 1 100 1.2 WP 50%
grain 0.3 0.4 0.3
straw 32 12.5 10.5
3 100 1.2 WP 50%
grain 2.1
straw 45
3 100 1.2 WP 50%
grain 1.4
straw 32
3 100 1.2
grain 0.8
straw 49
4 100 1.2 1.8
grain 43
straw
Wheat
Maris
Nimrod UK 1 34 1.0 <0.05
2 34 1.0 <0.05
Jos Cumbier 1 34 1.0 <0.05
2 34 1.0 <0.05
TABLE 9. Nature and Distribution of radio-activity in wheat grown in soil treated with 10 kg/ha. 14C-iprodione
% of total 14C in each plant part as
Unidentified Total 14C
Days after Organo- Water expressed as
treatment Plant part Iprodione RP 30228 RP 32490 soluble soluble Bound iprodione mg/kg
16 roots 49 14 n.d. 21 0 17 20
leaves and 66 6.4 4.4 21 1.5 1.7 20
stem
44 roots 16 15 n.d. 13 1.1 55 31
leaves and 48 9.5 18 20 0.95 4.2 20
stem
89 roots 2.9 8.1 0.8 9.2 0 79 238
leaves and 26 17 14 32 0.22 11 36.7
stem
ears 9.1 3.1 0.1 24 0 56 32
kernels n.d. n. d. n.d. 72 0 28 2.5
Wheat plants were grown on soil treated with excessive dosages of
14C-labelled iprodione and the distribution of the residue in the
plant was studied after 16, 44 and 89 days. The nature and
distribution of the recovered radio-activity is shown in Table 9
(Rhône-Poulenc, 1977f).
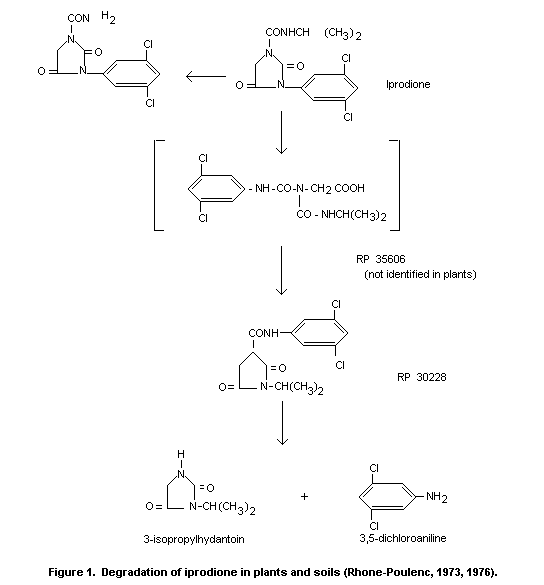
The plant and soil metabolites of iprodione have been identified by
various methods including TLC, GLC, and colorimetric analysis and the
degradation pathway shown in Figure 1 deduced.
In soil
The degradation of residues in soil follows a similar pattern. The
half-life at initial levels of 2 and 5 mg/kg is about 30 days. After
12 months incubation under aerobic conditions at 23-25°C, no more than
3% of the remaining radio-activity was in the form of unchanged
iprodione. Conversion to metabolite RP 30228 proceeded rapidly. The
concentration of RP 30228 reached a maximum (45-55% of the
radio-activity still present) after 80-100 days and then decreased
(Rhône-Poulenc, 1976).
In leaching experiments with radio-labelled iprodione it was shown
that the parent compound was only slightly mobile, remaining in the
0-15 cm layer. The metabolite RP 30228 is less soluble in water than
the parent compound (0.5 mg/l compared to 13 mg/l) and virtually all
remained in the 0-5 cm layer (Rhône-Poulenc 1973, 1976).
In storage and processing
Extensive data were obtained from various countries on the fate of
residues of iprodione during wine making and on the effect of residues
on the fermentation. When grapes containing about 5 mg/kg were used
for wine making, no influence on the fermentation process was found.
This was confirmed in laboratory experiments in which CO2 evolution
and the proportion of viable cells (those susceptible to actidione)
were measured.
In a trial in which the grapes contained 2-10 mg/kg iprodione, the
fermentation process was slightly retarded. It is unlikely that this
effect would be observed under practical conditions of wine making.
During the wine making, iprodione remains fairly stable, but a
considerable part of the residue will be eliminated with the solids
(mavc) during clarification. The residues in wine are generally about
15-25% of those in the grapes. No residues of iprodione were found in
alcohol obtained after distilling wine (Rhône-Poulenc, 1977c; Barre et
al., 1976). Some results are shown in Table 10.
TABLE 10. Residues of iprodione at various stages of vinification
Iprodione, mg/kg in
Country Year Grapes Must Wine Finished
Unfermented Fermented Racked Clarified Wine
France 1974 4.9 2.7 0.98 0.75
3.0 1.7 1.0 0.71-0.84
1975 6.1 2.75 0.8
7.5 4.4 1.45
1975 2.2 1.5 0.34
5.4 0.6
South 1976 1.0 1.3 0.9
Africa 2.6 0.5 1.5
Switzerland 2.3 1.9 0.7
1973 1.7 1.4 0.5
2.9 1.9 0.4
Other Information on Identity and Properties
a) Composition of the technical product
The technical product contains 95% minimum of iprodione. The main
impurities are phenyl hydantoins and bis-isopropyl-1,,3\-urea
(referred to as 32870 R.P.).
b) Physical and chemical properties
Physical state: white, odourless, non-hygroscopic crystals.
Molecular weight: 330.17
Melting point: 136°C
Volatility: not volatile
Vapour Pressure: 2 × 10-7mm. Hg at 20°C
Solubility at 20°C: g/l
water 0.013
ethanol 30
acetonitrile 150
toluene 150
benzene 200
acetone 300
methlyene chloride 500
Formulations
Mainly wettable powder 500 g a.i./kg. Also suspension concentrate 500
g a.i./l and emulsifiable concentrate 200 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Single oral doses of iprodione are rapidly eliminated by rats.
Following a single application of an oral dose of 200 mg/kg, 26% of
the administered dose was eliminated in the urine and 59% in the
faeces within 24 hours after application. The major part of the dose
excreted in the faeces is the parent compound, whereas only 3% of the
administered dose is eliminated unchanged in the urine. Besides the
principal urinary metabolites with a degraded isopropylcarbamoyl group
(about 11% of the dose administered), there are metabolites with
intact hydroxylated or non-hydroxylated aromatic rings. The isomer of
the parent compound accounted for a small proportion of the
metabolites. Residues in the principal organs and tissues did not
exceed 1.5% of the administered dose in rats sacrificed 4 days after
dosage (Laurent and Bays, 1974).
In a similar study rats were dosed once with 100 mg/kg. of
14C-aromatic ring-labelled iprodione; 96 hours after administration
62% of the applied dose was eliminated via the urine and 36% via the
faeces. About 16% was excreted as the parent compound in the faeces:
the remaining radioactivity was mainly in urine in the form of the
desisopropylated derivative (about 20% of the dose) and the
N-(3,5-diachloro-4-hydroxyphenylbiuret) (approx. 13%). Tissues sampled
4 days after dosage contained about 1% of the administered dose.
(Lourer, et al., 1976),
Based on the identified metabolites the reactions that seem to occur
during biotransformation are mainly hydroxylation, oxidation and
desalyklation of the isopropylcarbamoyl group
(-N1-CO-NH-CH(CH3)2->N1-CO-NH2->N1-H).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups of 25-30 rats were orally treated with 0, 100, 200 and 400
mg/kg on Restation days 5 to 15. Females at 400 mg/kg showed reduced
fertility, reduced body weight gain and a dose-related reduction of
food consumption especially during the treatment period. The number of
implantations was also reduced at the highest dose level.
There was no indication for an embryonyic or teratogenic effect of the
test completed (Coquet, 1973a).
Groups of 15-17 New Zealand White rabbits were intubated on gestation
days 6-16 inclusive with 0, 100, 200 or 400 mg/kg. Body weight gain,
over the period of treatment, was slightly reduced at 100 mg/kg, and a
dose-related weight loss occurred at 200 and 400 mg/kg. Food intake
was reduced at 200 mg/kg and above. At 400 mg/kg, 9 of 17 females
died, and only one of the four remaining pregnant animals carried to
term. Foetal loss was increased at 200 mg/kg, and foetal weight was
reduced at 200 mg/kg and above. Multiple malformations occurred in 1
of 68 living foetuses at 200 mg/kg. Minor malformations were noted in
all groups.
Special studies on carcinogenicity
See "Long term studies."
Special study on reproduction
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 and 1000 ppm for
the first 5 weeks of each generation and 0, 250, 500 and 2000 ppm for
the next 8 weeks of treatment. The diet was fed through three
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parental animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendency for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rate of the third generation did not reveal
abnormalities. (Coquet, 1976)
Special study on mutagenicity
Groups of 25 male mice were fed 0, 1500 and 6000 ppm iprodione for 49
days. After termination of the feeding period the male mice were
paired with 2 untreated females for 6 days, followed by a further 2
females for the 6-12 days post-treatment period. The treatment did not
affect body weight, food consumption or fertility of the males. None
of the examined parameters gave any indication of a mutagenic effect
of iprodione (Hastings et al., 1974).
Iprodione showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, reverse mutation tests with and without liner
activation system using E. coli WP2 hcr- and five strains of
Salmonella typhimurium TA and host-mediated assay with S. typimurium G
46 in mice (Shirasu et al., 1976).
Acute Toxicity
TABLE 1. Acute toxicity of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral >2000 Pasquet & Mazuret,
1973
Rat M F Dermal >2500 ibid.
Rat M i.p. 2400 Pasquet &. Mazuret,
1974
F i.p. 1200 ibid.
Mouse M F Oral approx. 4000 Pasquet A Mazuret,
1973
Dog M F Oral >2000 ibid.
Rabbit M F Dermal >1000 ibid.
The signs of toxicity were: loss of reflexes, muscular hypotonia,
sedation and dyspena.
Iprodione did not cause skin or eye-irritation in rabbits.
In the anaesthetized dog iprodione administered at a dose of 300 mg/kg
by the intraduodenal route did not affect the cardiovascular,
respiratory or neurovegetative system (Detaille et al., 1973).
TABLE 2. Acute toxicity of a 50% formulation of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral 8000 Davies & Lowe,
1974
M F Dermal >2000 ibid.
M F 4 h
inhalation 13 mg/l air Pasquet & Mazuret,
1975a
M F Oral 4100 ibid.
Oral 4900 ibid.
M F 4 h inhalation >13/l air Ibid.
Rabbit M F Dermal >2000*) ibid.
*) atoxic
The formulation induced slight irritation in the rabbit eye but had no
irritant effect on the intact or abraded skin of rabbits (Pasquet &
Mazuret, 1975a).
"In the sensitization test with guinea pigs, after 10 applications of
0-3 ml of a 50% iprodione solution, followed 2 weeks later by a
challenge application, no evidence of dermal sensitization was
observed" (Pasquet & Mazuret, 1975b).
Short term studies
Rat
Groups of 15 male and 15 female caesarian originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the diet
for 5 months. No effects were observed on mortality, food consumption,
haematology (as judged by haemoglobin, haematocrit, erythrocyte count,
or total and differential leucocyte count) clinical chemistry (as
judged by BSP, SGOT, SGPT or SAP) or urinalysis. Body weight gain was
slightly reduced (especially in males) at 500 and 1000 ppm. Absolute
(but not relative) heart weight was reduced in males at 500 and 1000
ppm, and absolute kidney weight was reduced at 1000 ppm. In females,
absolute liver and kidney weights were significantly reduced at 500
ppm only. Gross and histopathology were normal at all dose levels. In
a parallel study, dichlozoline, a structurally related compound,
induced cataracts. No such effect was seen with (Ganter et al.,
1973a).
Dog
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 and 7200 ppm for a
period of 3 months. At the top dose level the method of administration
was altered after 6 weeks, to gelatine capsules. The treatment did not
affect mortality. The recorded values of haematological determinations
and urinalyses were within normal limits. As judged by haemoglobin,
haematocrit, reticulocyte erythrocyte count, total and differential
leucocyte count and prothrombine time except for signs of mild anemia
in 1 male and 1 female at 2 months and 1 male at 3 months at the top
dose level. At 7200 ppm a reduction of food consumption was observed,
accompanied by reduced body weight gain. The opththalmosopic
examination of the animals did not reveal any pathological alteration
(Canter and Girard, 1973b). The clinical chemistry determinations
consisted of glucose, urea, cholesterol, bilirubiu, total proteins,
protein electrophoresis, alkaline phosphatase, SGOT, SGPT, LDH, Na+,
C, K+, Cl -, Ca++, P. At 2400 and 7200 ppm a slight increase of SLP was
observed, also a transient increase of SGOT and SGPT after 1 and 2
months of treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, in females at the
dose levels of 2400 ppm and above. At 7200 ppm reduced relative weight
of testes was found, but no histological indication of damage.
The histopathological findings did not reveal any indication of
treatment-related alterations of tissues (Coquet, 1973c).
Long term studies
Mouse
Groups of 60 male and 60 female mice were maintained an a diet
containing the test compound at 0, 200, 500 and 1250 PPM for 18
months. No treatment-related effect on body weight, food consumption
or mortality was found. The recorded values of the haematological
blood chemistry and urinalyses tests performed after 6, 12 and 18
months of the feeding period, were within the physiological range.
Necropsy findings on mice that died during the last 6 months of the
test and on those sacrificed at the termination date showed an
increased number of enlarged lymph nodes in males at 200 ppm. Organ
weight variations occurred sporadically in the various dose groups and
are considered not to be treatment-related. The histopathological
findings failed to reveal abnormal features. The distribution of
neoplastic and non-neoplastic findings did not appear to demonstrate
any significant dose-dependence. The most common tumours were
lymphosarcoma involving the spleen, lymph nodes and thymus (Hastings
and Hullman, 1975).
Rat
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 and 1000 ppm for 24 months. Slight reduction in
body weight gain was observed at 1000 ppm. This was accompanied by
some reduction in food intake. The treatment had no effect on food
consumption, mortality or values of the hematologic, blood chemistry
and urinalyses determinations. Necropsy findings did not reveal any
drug-related gross alteration. Variations in organ weight did not show
a group distribution and seemed not to be related to drug
administration. Histopathology did not indicate a treatment
relationship of neoplastic and non-neoplastic findings. AT 24 months
the most common tumours observed were pituitary adenomas and
adenocarcinoma and fibroadenoma of the mammary glands (Hastings et
al., 1976).
COMMENTS
Iprodione is readily absorbed and rapidly excreted mainly as
metabolites with intact hydantoin-moiety. The compound was not
teratogenic. In a 3-generation study in rats, there was a slight but
statistically significant reduction in postnatal growth at 2000 ppm.
This effect was only marginal at the lower dose of 500 ppm which is
regarded as a no-adverse-effect-level. In a short-term study in dogs
no major effect occurred up to 2400 ppm. Likewise long-term studies in
mice and rats revealed no effects up to 1250 ppm. No ocular
alterations were found in any study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mice: 1250 mg/kg in the diet, equivalent to 160 mg/kg bw
Rat: 500 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.3 mg/kg bw
USE PATTERN
Iprodione is used as a fungicide against a range of fungus diseases,
including Botrytis in vines, black- and red currants, blackberries,
raspberries and vegetables especially lettuce; Botrytis alii on
onions; Rhizocotonia on seed potatoes; seed borne diseases on sugar
beets (Phoma spp.) and cereals. It is also used against Botrytis
and some other fungus diseases on ornamentals.
The compound is used as a foliar spray on several crops, as a post-
harvest dip for fruit, for dipping seed-potatoes and as a seed-
treatment on sugar beet and cereals.
The product is authorized for use on various crops in France, the
Federal Republic of Germany, Greece, the Netherlands and the United
Kingdom. In several other countries the compound is used or included
in testing programmes and it is in course of registration in many
countries including Australia, New Zealand, Japan, USA, Canada,
Israel, and several European countries.
Most of the recommended uses are summarized in Table 3. The
information may not be complete since the use of the compound is
expanding rapidly and more uses may be expected in the near future.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained from supervised trials carried out in
various countries on fruit and vegetables and on some agricultural
crops; they are summarized in Tables 4 to 8 and 10.
Pome and stone fruits (Tables 4 and 5; Rhône Poulenc, 1977a)
Apples
The residue at harvest from pre-harvest treatments at normal
application rates (about 2.25 kg a.i./ha) is about 2 mg/kg. A
combination of such treatments with post-harvest dipping gives rise to
residues of about 6 mg/kg. After repeated applications at about twice
the normal rate residues ranged from 2.9 - 6.5 mg/kg. The residue of
the metabolite RB 30228 (see "Fate of residues") was below 0.15 mg/kg
in these experiments.
Pears
Post-harvest dipping of pears against storage diseases gave rise to
residues of 3.6 - 5 mg/kg.
Peaches
Residues at harvest following applications at the recommended rate
varied between 0.9 and 6 mg/kg. A post-harvest dip adds about 4 mg/kg
to these levels.
Plums
Residues arising from recommended applications varied between 0.6 and
6.8 mg/kg, depending on the pre-harvest intervals observed and the
local conditions. The drying process increased the residue in the
prunes by 0.6 - 1.6 mg/kg.
TABLE 3. Use pattern and recommended pre-harvest intervals of iprodione
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Grapes Botrytis 4 750 Austria 28
" 4 750 France. 15
" 4-5 750 Fed. Rep. of 28
Germany
" 3 3000 Japan 7
" 4 750 Portugal 15
" 4 750 Spain 15
" 4 750 Switzerland
" 4 750 USSR
" 4 750 Yugoslavia
Strawberries Botrytis 4-5 1000 Belgium 15
3 1000 Fed. Rep. of 7
Germany
3 Japan 1
4-5 1000 The Netherlands 14
Pome and Stone fruit
Apples Alternaria about 10 Japan 10
Peaches Monilia 3 Japan 1
Vegetables
Chicory (witloof)
(forcing) Botrytis 1 3 g/m2 Belgium Throughout
Sclerotinia 1 4 g/m2 France the forcing
applied on the top of period
the roots at forcing
TABLE 3. (Continued)
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Cucumbers Botrytis 4 Japan 1
Lettuce Botrytis
Scleotinia 3 750 Belgium 10
(glasshouse)
" 3-4 750 France
" 3 750 Fed. Rep. 21
of Germany (glasshouse)
14
(outdoors)
" 4 750 Japan 14
(also for endive) " 1x 1000-2000 The Netherlands 28
" 2xx 750 The Netherlands 28
Vegetables
Onions Botrytis
Sclerotinia 3 750 Japan 7
cepivorum
Tomatoes Botrytis 4 Japan 1
Alternaria United Kingdom
Agricultural Crops
Beans Sclerotinia 3 Japan 21
Rice Pellicularia 3 Japan 21
TABLE 3. (Continued)
Seed and tuber treatments
Cereal seed
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Barley Helminthosporium 1 60 g a.i./100
kg seed
Wheat Tilletia caries 1 60 g a.i./100
kg seed
Garlic Sclerotinia cepivorum 1 300 g a.i./100 France
kg seed
Potatoes Rhizoctonia solani 1 100-150 g/ France
1000 kg tubers
spraying on
tubers
immediately
before
storage
dipping in
spring before
planting
400 g/100 l
Sugar-beet Phoma spp 1 150 g a.i./kg France.
seed enveloped seed
x= one application at planting.
xx= two applications, the first about a week after planting and a second within two weeks after planting
Berry fruits and currents (Table 6; Rhône-Poulenc, 1977b)
The residue levels at harvest after treatments at normal rates and
observing recommended pre-harvest intervals (10-21 days) were
generally at or below 5 mg/kg on blackcurrants, 2 mg/kg on raspberries
and 6 mg/kg on strawberries.
Grapes
The maximum residues of iprodione on grapes at harvest following
treatment according to good agricultural practice (about 750 g
a.i./ha) were in general not higher than 10 mg/kg. The highest levels
in the unfermented must and the win were 4.4 and 6.4 mg/l
respectively. Some results are shown in Table 10 (Rhône-Poulenc,
1977c). See "Fate of residues", "In storage and processing".
Vegetables (Table 7; Rhône-Poulenc, 1977d)
Chicory (witloof)
The residues in the edible sprouts after a normal period of forcing
and one treatment at the recommended rate did not exceed 1 mg/kg. The
residues in the roots were much higher, with a maximum of about 10
mg/kg. The roots are often used as animal feed.
Cucumbers
Residues on cucumbers treated with 3 kg a.i./ha (twice the normal
rate) were between 0.3 and 2.2 mg/kg.
Lettuce
Residues arising from recommended applications on outdoor lettuce (750
g a.i./ha) varied between 1.7 and 2.5 mg/kg after pro-harvest
intervals of 14-21 days. The residues on glasshouse-grown lettuce are
in general much higher. Three applications of the recommended dosage
gave rise to residues of 6.7 mg/kg after a pre-harvest interval of 39
days, and in other experiments maximum levels of 7.2 mg/kg were found
14 days after the last application. Residue levels of the metabolite
RP 30228 were slightly about the limit of determination; other
metabolites were below it. (Metabolites are identified in the section
"Fate of residues."
Onions
The residues on onions 1 day after application did not exceed 0.2
mg/kg.
TABLE 4. Supervised trials of iprodione. Residues in pome and stone fruit (pre-harvest application)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Apples Japan 1975 1 100 5 WP 50% 2.15 1.75
1975 1 100 5 " 2.9 2.25
1975 1 100 5 " 0.38
1975 10 100 5 " 6.5
1975 10 100 5 " (5.75)
1975 10 100 5 " 3.75
1975 10 100 5 " 3.4
1975 10 100 " (1.95)
1975 10 100 5 " 1.7
U.K. 1975 10 100 2.25 " (2.0)
Cherries
Moss Australia 1975 4 1.4 " 8.0
Peaches
Katharine Australia 1976 7 50 1.4 " 5.4
Anne Truly 1976 3 50 0.5 " 1.7
Goldmine 1976 5 50 1.4 " 5.8
Redhaven Canada 1974 4 50 1.0 " 6.5 4.6 4.9
1974 4 50 1.0 " 1.45
Babygold Canada 1974 7 50 1.0 " 2.3 2.0 1.3
1974 6 50 1.0 " 1.8 0.9
Earlired 1974 5 1.0 " 2.5 1.9 2.2 2.2
Redhaven 1974 5 1.0 " 6.1 4.1 3.4 2.9 1.6
Sunhaven 5 1.0 " 7.6 9.0 10.0 8.5
Gifu Japan 1975 7 100 4.0 3.7 2.1
1975 3 100 4.0 " 4.6 2.9
Okayama 1975 2 100 3.0 " 6.3 4.8
1975 3 100 3.0 " 6.8 5.8
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Plums
October Australia 1976 4 1.4 " 2.2
purple
Inra 711 France 1973 4 0.5 SC 0.25 0.26
4 1.0 SC 1.4 0.9
1974 0.5 4.15
3 1.0 6.8
2 0.5 0.2
2 1.0 0.6
* SC = Suspension concentrate
WP = Wettable powder
TABLE 5. Supervised trials of iprodione. Residues in pome and stone fruit (post-harvest application)
Application
Rate
Crop Country Year No. g a.i./100 Formulation Residues (mg/kg) after storage period of (days)
dip 1-2 7 11 85 95
Apples
Cox's U.K. 1973 - - 2.0
1 200 5.8
1 200 4.4
Pears
Conference U.K. 1973 1 200 SC 5.0
1973 1 200 4.8
1973 1 200 3.6
1973 1 200 4.7
1973 1 200
1973 1 200 4.0
Peaches Australia - WP50% 1.7
1 50 WP50% 5.3
1 50 WP50% 5.0
TABLE 6. Supervised trials of iprodione. Residues in berry fruits and currants
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Raspberries France 1974 3 0.8 WP 50% 1.1
Heyton 0.5 0.85
Malling
jewel U.K. 1974 5 1.1 " " 1.55
3 1.1 " " 2.0
4 1.1 " " 7.9 5.0 2.8 2.1
Strawberries Belgium 1974 5 0.75 " " 4.9
Sivetta 5 1.0 " " 6.0
Domunil 5 0.75 " " 1.9
5 1.0 " " 2.5
Redcoat Canada 1974 4 1. " " 3.1 1.8 1.4 0.85
Vista 4 1.1 " " 3.5 2.4 1.4 1.0
Redcoat 4 3.45 2.1 1.6 1.0
Redcoat 3 0.9 0.8 0.2
Red Gauntlet France 1973 4 0.5 " " 0.22
0.75 " " 0.44
1.0 " " 0.66
Immigrante 1974 4 0.75 " " 1.75
4 1.0 " " 2.2
Gorella 1974 4 0.75 " " 0.35
4 1.0 0.44
3 0.75 " " 0.5
3 1.0 " " 1.1
Suprême d'Halles 4 0.5 " " 1.2
4 0.75 " " 1.9
4 1.0 " " 2.75
4 0.5 " " 2.5
4 0.75 " " 3.2
4 1 5.6
Senga Segana Fed. Rep. 1974 3 0.9 " " 5.3 2.7 2.3 1.0 0.4
of Gemany 3 1.25 " " 9.1 3.6 3.4 1.9 0.7
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Senga Segana Netherlands 1974 4 0.75 " " 1.1
1974 5 0.75 " " 26.4 14.2 0.67
Red Gauntlet Switzerland 1974 1 0.75 " " 1.0 0.86 0.2 0.1 0.1
Wädenswill 1974 3 0.75 " " 0.2
1974 3 0.75 " " 0.15
Royal
Sovereign U.K. 1974 4 1.1 " " 1.2 1.6 1.3
Strawberries
Cambridge
Favourite (g) 1974 4 1.1 WP 50% 8.0 6.9 4.5 4.7
Cambridge
Favourite (g) 1974 3 1.1 " " 1.7
Cambridge
Favourite 1974 3 1.0 " " 0.7
Cambridge
Favourite 1976 3 1.6 1.1
Cambridge
Favourite 1976 3 0.75 0.9 0.6
Red Gauntlet 1976 1 0.75 0.6
Red Gauntlet 1976 1 1.0 0.9
Red Gauntlet 1974 3 2.2 1.7
Cambridge
Favourite 1974 3 2.2 0.55
Royal
Sovereign 1974 2 1.0 0.3
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Black
currants
Wellington
Tr. UK 1974 4 1.1 WP 50% 3.9
62 days
Baldwin UK 1974 4 1.1 " " 4.6
62 days
(g) = glasshouse
TABLE 7. Supervised trials of iprodione. Residues in vegetables
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Bean
without pod Japan 1975 1 1 WP 50% 0.05 0.05 0.05
Cucumbers(g) Japan 1975 3 1 0.05 0.05
1975 1 3 WP 50% 1.7 1.0 0.36
1975 3 3 " " 1.6 0.9 0.7
1975 4 3 " " 2.2 1.4 1.2
1975 1 2.5 " " 1.2 1.0 0.24
1975 3 2.5 " " 1.9 1.4 0.3
1975 4 2.5 " " 1.8 1.0 1.0
Lettuce
Val.d'Orge France 1974 6 0.5 " " 0.03
6 0.75 " " 0.03
6 1.0 " " 0.03
6 0.5 EC 200g/l <0.02
6 0.75 " " <0.02
6 1.0 " " 0.04
5 0.5 WP 50% 2.5
5 0.75 " " 1.3
Murkönig Fed. Rep. 3 0.75 " " 15.7 1.8 0.6 0.05 0.05 0.05
Kares of Germany 1976 4 0.75 " " 24.5 9.1 1.7
Reskia 1976 3 0.25 " " 2.6 0.8 0.2
3 0.75 " " 13.2 3.0 1.1
Susan 1976 3 0.5 " " 4.6 1.2 0.4
3 0.75 " " 28 4.3 4.2
Murkönig(g) Fed. Rep. 1976 3 0.25 " " 24 6.9 2.9
of Germany 3 0.75 " " 46 10.5 7.2
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Ravel(g) 1976 3 0.25 " " 26 5.4 2.7
0.75 " " 22 4.8 3.6
Kurume
Br.(g) Japan 1975 2 3 " " 4.6 0.28 0.05
3 3 " " 10.8 0.27 0.1
4 3 " " 7.4 0.3 0.1
Kumamoto(g) Japan 1975 2 1.5 " " 0.48 1.05 0.4
3 1.5 " " 1.9 0.6 1.2
4 1.5 " " 2.25 0.78 1.7
Déciminor(g) Netherlands 1975 2 0.75 " " 8.2 4.9
Vera(g) 1975 2 0.75 " " 9.15 0.05 0.05
Ostinata(g) UK 1974 4 0.56 " " 19 18 14 (24
days)
Val d'Orge 1974 3 0.56 " " 8.8
4 0.56 " " 59 40 41 21
Onions S. Africa 1977 7 50 WP 50% 0.14
0.15
100 " " 0.15
0.24
Sweet
Peppers(g) U.K. 1975 6 4.3
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Tomatoes
Koibuchi (g) Japan 1975 1 2.5 " " 1.25 1.4 1.2 0.8
Koibuchi (g) 1975 3 2.5 " " 5.3 3.4 3.0 2.4
Koibuchi (g) 1975 4 2.5 " " 5.6 5.4 4.3 3.5
1975 3 3.0 " " 2.1 3.3 1.8 2.9
4 3.0 " " 4.6 3.6 4.1 2.8
5 3.0 " " 4.4 4.0 3.8 3.7
Eurocross(g) U.K. 1974 5 50 " " 2.7
6 50 " " 3.8
7 50 " " 4.9
8 50 " " 4.2 2.3
Sonato(g) U.K. 1974 4 50 " " 1.65
5 50 " " 2.3
6 50 " " 2.8
7 50 " " 3.7
8 50 " " 4.2 5.8
5 50 " " 3.1 2.5 2.7
1 1 " " 0.64
(18
days)
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. a.i. Formulation 30-40 40 44-48 59 70 93-104 162
g/m2 S R S R S R S R S R S R S R
Chicory Belgium 1975 1 3 " " 0.41 1.49
1 6 " " 0.36 3.10
3 " " 0.09 2.7
6 " " 0.32 4.4
3 " " 0.77 6.2
6 " " 0.59 10.0
3 " " 0.55 2.7
6 1.0 3.7
France 1974 1 4 " " 0.6
8 " " 1.0 0.07
4 " " 0.25
8 " "
TABLE 7B
Crop Country Year No. Rate Formulation 30-40 40 44-48 59 70 93-104 162
g/100 1g/1000
dip kg S R S R S R S R S R S R S R
Potatoes
pre-plant France 1976 1 200 WP 50% n.d.
treatment 300 " " n.d.
50 " " 0.01
100 " " 0.01
150 " " 0.02
200 " " 3.0 5.0
50 " " 25 25
100 " " 58 67
150 " " 120 140
foliar
spray S.
Africa 1976 5 50 " " <0.02
5 100 " " <0.02
3 50 " " <0.02
3 100 <0.02
WP = wettable povider; EC = emulsion concentrate;
(g) = glasshouse; R = roots (chicory) or tubers (potato); S=sprouts
Peppers and tomatoes
Following applications at normal rates (50 g a.i./100 l) residues of
4.5 - 5 mg/kg were found at harvest after pre-harvest intervals of 3.6
days.
Beans (dry)
After treatment at a dosage rate of 1 kg/a.i./ha, residues in the dry
beans were very low (0.05 - 0.2 mg/kg).
Cereal crops (Table 8; Rhône-Poulenc, 1977e)
Wheat
Two applications at normal rates (1 kg a.i./ha) with a pre-harvest
interval of 73 days did not give rise to measurable residues in the
kernels.
Rice
After treatment during the growing season with relatively high dosages
(1.2 kg a.i./ha), residues of 0.1 - 2.1 mg/kg were found de-husked,
unpolished rise 21 days after the last treatment.
FATE OF RESIDUES
In plants
The fate of iprodione in plants and soil was studied with unlabelled
and 14C-phenyl-labelled products. It was found that when applied to
the leaf surface, iprodione does not appreciably penetrate through the
skin. The residues on the skin had a half-life of 30-60 days, being
slowly converted to
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydentoin, RP 30228), which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including 1-carbamoyl-3-(3.5-
dichlorophenyl) hydantoin (RD 32490) (Rhône-Poulenc, 1973).
Wheat and strawberry plants grown on soil treated with iprodione
(Rhône-Poulenc, 1977f) took up small amounts of the compound (in wheat
0.7-1.3% of the amount applied to the soil surface), which was mainly
found in the leaves and stems (95-99% of the extractable residue).
Within the plant the parent compound was converted to RP 30228, small
amounts of RP 32490 and some more polar unidentified products.
The organosoluble residue in strawberry plants 32 days after a foliar
application at a rate equivalent to 1 kg a.i./ha consisted of 61%
unchanged parent compound and 16% RP 30228. 55 days after foliar
treatment at 2 kg a.i./ha 69% of the residue was iprodione, 7% RP
30228 and 5% RP 32490.
TABLE 8. Supervised trials of iprodione. Residues in cereal crops.
Application Residues (mg/kg) at intervals (days) after application
Rate
Crop Country Year No. Formulation 14-15 21-22 28-30 73 81
g/100 1 kg/ha
Rice Japan 1975 1 100 1.2 WP 50%
grain 0.1 0.1 0.1
straw 16 15 10.3
1975 1 100 1.2 WP 50%
grain 0.3 0.4 0.3
straw 32 12.5 10.5
3 100 1.2 WP 50%
grain 2.1
straw 45
3 100 1.2 WP 50%
grain 1.4
straw 32
3 100 1.2
grain 0.8
straw 49
4 100 1.2 1.8
grain 43
straw
Wheat
Maris
Nimrod UK 1 34 1.0 <0.05
2 34 1.0 <0.05
Jos Cumbier 1 34 1.0 <0.05
2 34 1.0 <0.05
TABLE 9. Nature and Distribution of radio-activity in wheat grown in soil treated with 10 kg/ha. 14C-iprodione
% of total 14C in each plant part as
Unidentified Total 14C
Days after Organo- Water expressed as
treatment Plant part Iprodione RP 30228 RP 32490 soluble soluble Bound iprodione mg/kg
16 roots 49 14 n.d. 21 0 17 20
leaves and 66 6.4 4.4 21 1.5 1.7 20
stem
44 roots 16 15 n.d. 13 1.1 55 31
leaves and 48 9.5 18 20 0.95 4.2 20
stem
89 roots 2.9 8.1 0.8 9.2 0 79 238
leaves and 26 17 14 32 0.22 11 36.7
stem
ears 9.1 3.1 0.1 24 0 56 32
kernels n.d. n. d. n.d. 72 0 28 2.5
Wheat plants were grown on soil treated with excessive dosages of
14C-labelled iprodione and the distribution of the residue in the
plant was studied after 16, 44 and 89 days. The nature and
distribution of the recovered radio-activity is shown in Table 9
(Rhône-Poulenc, 1977f).
The plant and soil metabolites of iprodione have been identified by
various methods including TLC, GLC, and colorimetric analysis and the
degradation pathway shown in Figure 1 deduced.
In soil
The degradation of residues in soil follows a similar pattern. The
half-life at initial levels of 2 and 5 mg/kg is about 30 days. After
12 months incubation under aerobic conditions at 23-25°C, no more than
3% of the remaining radio-activity was in the form of unchanged
iprodione. Conversion to metabolite RP 30228 proceeded rapidly. The
concentration of RP 30228 reached a maximum (45-55% of the
radio-activity still present) after 80-100 days and then decreased
(Rhône-Poulenc, 1976).
In leaching experiments with radio-labelled iprodione it was shown
that the parent compound was only slightly mobile, remaining in the
0-15 cm layer. The metabolite RP 30228 is less soluble in water than
the parent compound (0.5 mg/l compared to 13 mg/l) and virtually all
remained in the 0-5 cm layer (Rhône-Poulenc 1973, 1976).
In storage and processing
Extensive data were obtained from various countries on the fate of
residues of iprodione during wine making and on the effect of residues
on the fermentation. When grapes containing about 5 mg/kg were used
for wine making, no influence on the fermentation process was found.
This was confirmed in laboratory experiments in which CO2 evolution
and the proportion of viable cells (those susceptible to actidione)
were measured.
In a trial in which the grapes contained 2-10 mg/kg iprodione, the
fermentation process was slightly retarded. It is unlikely that this
effect would be observed under practical conditions of wine making.
During the wine making, iprodione remains fairly stable, but a
considerable part of the residue will be eliminated with the solids
(mavc) during clarification. The residues in wine are generally about
15-25% of those in the grapes. No residues of iprodione were found in
alcohol obtained after distilling wine (Rhône-Poulenc, 1977c; Barre et
al., 1976). Some results are shown in Table 10.
TABLE 10. Residues of iprodione at various stages of vinification
Iprodione, mg/kg in
Country Year Grapes Must Wine Finished
Unfermented Fermented Racked Clarified Wine
France 1974 4.9 2.7 0.98 0.75
3.0 1.7 1.0 0.71-0.84
1975 6.1 2.75 0.8
7.5 4.4 1.45
1975 2.2 1.5 0.34
5.4 0.6
South 1976 1.0 1.3 0.9
Africa 2.6 0.5 1.5
Switzerland 2.3 1.9 0.7
1973 1.7 1.4 0.5
2.9 1.9 0.4
 METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables. These are suitable or can be adapted for regulatory
purposes. They have been adapted for residue analysis In must and
wine. The limit of determination on most fruits and vegetables is
about 0.01-0.02 mg/kg. Some commodities of plant origin, e.g. prunes
and mare, require a more elaborate clean-up owing to the higher
proportion of interfering plant constituents. The limit of
determination in these commodities is about 0.05-0.1 mg/kg.
No loss of residues was found during storage for more than 1 year at
temperatures of -18°C.(Rhône-Poulenc, 1975a,b).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following maximum residue limits were reported to the Meeting as
established or under consideration. They refer to iprodione, excluding
metabolites.
Country Commodity Maximum residue
limit, mg/kg
Australia Apricots, cherries,
plumes peaches 10
France Grapes 10
Fed. Rep.
of Germany Grapes 10
The Netherlands Lettuce 5
Strawberries 2
New Zealand Apricots, berry fruits,
cherries, grapes,
peaches, plums 10
Switzerland Grapes 7
APPRAISAL
Iprodione is used against a relatively broad range of fungus diseases,
on a wide range of fruits and vegetables.
Its use is authorized, or is in course of registration, for various
crops in a number of countries. It is marketed in the form of a
wettable powder, a suspension concentrate and an emulsifiable
concentrate. The products are mainly used as a spray on the aerial
parts of growing crops, for post-harvest dipping of fruit as a dip for
seed potatoes and as a seed treatment. Application rates vary
according to the crop/disease situation and regional conditions.
Residue data were obtained from supervised trials carried out in
various countries with different climatic conditions. Studies with
unlabelled and 14C-phenyl-labelled products showed that iprodione
does not appreciably penetrate through the plant cuticle. The residue
on the surface of the plants had a half-life of about 30-60 days. It
was converted into
1-(3,5-dichlorophenyloarbamoyl)-3-isopropyl-2,4-dioxoimida-zolidine,
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydantoin, RP 30228, which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including
1-carbamoyl-3-(3,5-dichlorophenyl)hyclantoin (RP 32490).
Wheat and strawberry plants grown on soil treated with iprodione took
up small amounts of the compound (equivalent to 0.7-1.3% of the total
applied to the soil surface). Within the plant the parent compound was
converted into metabolite RP 30228, Small amounts of RP 32490 and some
more polar unidentified products. The degradation of residues in soil
follows the same pattern. The half-life at initial levels of 2 and 5
mg/kg is about 30 days. After 12 months incubation under aerobic
conditions at 23-25°C no more than 3% of the remaining residue is in
the form of unchanged iprodione. The parent compound is only slightly
mobile, remaining mainly in the upper 0-15 cm layer. The metabolits RP
30228 is less soluble in water than the parent compound (0.5 mg/l
compared to 13 mg/l) and virtually all remained in the 0-5 cm layer.
Residues in wine were approximately 15-25% of those on the harvested
grapes.
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables, must and wine, which are suitable or can be adapted for
regulatory purposes. The limit of determination is generally about
0.01-0.02 mg/kg. No loss of residue was found over more than 1 year at
-18°C.
RECOMMENDATIONS
The following maximum residue limits for iprodione on various fruits
and vegetables are recommended. They refer to iprodione, excluding any
metabolites.
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Apples, pears 10 10-14 +
Grapes 10 14-21
Lettuce 10 14-21(281)
Peaches 10 10-14 +
Plums 7 14
Strawberries 7 14
Blackcurrants 5 10-21
Cucumbers 5 3-6
Sweet peppers 5 3-6
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Raspberries 5 10-21
Tomatoes 5 3-7
Rice (hulled, unpolished) 3 21
Chicory (witloof) sprouts 1 throughout forcing
Beans, dry 0.2 14-21
Garlic, onions 0.1 1
1 Glasshouse use.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on the fate of iprodione residues in milk, meat and
eggs when food wastes containing iprodione residues are used as
components of animal feeds.
2. Residue data on grain and straw from supervised trials on cereal
crops treated according to good agricultural practice.
3. Further information about the effects of processing and cooking on
iprodione residues in a range of commodities.
REFERENCES
Barre, P., R. Cordonnier, A. Dugal, L. Lacroixy M. Laurent et M. Buys
(1976) Etude, dens le raisin et le vin, des résidus d'un fongicide
antibotrytis, le 26019 R.P. et leur effects our la fermentation
alcoholique. Progrés Agricole et Viticole 93 (11) 2-8.
Coquet, B. (1973a) Recherche du pouvoir teratogéne chez le rat OFA par
voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogéne chez le lapin pax
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherches et essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1977c) Essai de toxocité chronique (3 mois) chez le chien
par voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onions, No. 731008
Coquet B. (1976) Influence du produit 26019 R.P. sur la reproduction
du rat (Essai sur 3 générations). Unpublished report from Institut
Franc. de Recherche et Essais Biologique, No. 760435 submitted by
Rhône-Poulenc.
Davies, V.A. and Lowe C.Y. (1974) Fungicides: Formulation LPA 2043:
Acute Oral and Dermal Toxicity in the Rat. Unpublished report from May
and Baker Ltd., England, No. RG 2037.
Detaille, J.Y., Pasquet, J., Mazuret, A. and Mondot, S. (1973)
Antifongique 26019 RJ., Phaxmacologie générale chez le chien
anesthésié au pento barbital. Unpublished report des Laboratoires do
Recherches de la Société des Usines Chimiques Rhône-Poulenc, subm.
by Rhône-Poulenc.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. and Myan, J. (1973a) Produit No. 26019 R.P. -
Toxicété à terms (5 mois) chez le rat par voie orale, on comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R.P.). Unpublished
report from Laboratoires do Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 171249 submitted by
Rhône-Poulenc.
Ganter, P. and Girard, P. (1973b) Produit 26019 R.P. - Examen
histologique de la toxicité oculaire du 26019 R.P. chez le chien aprés
un traitement do 3 mois. Unpublished report from Laboratoires de
Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131, submitted by Rhône-Poulenc.
Hastings, S.E., Huffmann, K.W and Gallo, M.A. (1974) Dominant Lethal
Mutagenicity in Mice. Unpublished report from Boss and Clark, Division
of Rhodia Inc., No. SEH 74:69.
Hastings, S.E., Huffman, K.W. (1975) Chronic Toxicologic and
Carcinogenic Study with R.P. 26019 in Mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:113, submitted by
Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C. and Kiggins, E.M. (1976) Chronic
Toxicologic and Carcinogenic Study with R.P. 26019 in Rats.
Unpublished report from Rhodia, Inc., Hess and Clerk Division, No.
76:57, submitted by Rhône-Poulenc.
Laurent, M. and Buys, M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. and Chabassol, Y. (1976)
Etude du métabolism chez le rat á l'aide du produit marqué au 14C.
Unpublished report from Rhône-Poulenc, Direction des Recherches et du
Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-Seine,
C.N.G. -An No. 18548, submitted by Rhône-Poulenc.
Pasquet, J. and Mazuret, A. (1974) Product 26019 R.P. Toxicité aigue
chez le rat par voie intrapéritonéale. Unpublished report from
Laboratoires de Recherches de la Socété des Usines Chimiques
Rhône-Poulenc, No. 174621 submitted by Rhône-Poulenc.
Pasquet J. and Nazuret, A. (1975a) Glycophene (26019 R.P.) -
Formulation LFA 2043 (wettable powder at 50% of glyoophene) Acute
toxicity and Dermal and Ocular tolerance. Unpublished report from
Rhône-Poulenc. No. 18182 submitted by Rhône-Poulenc.
Pasquet J. and Mazuret, A. (1975b) Glycophene (26019 R.P.). Etude de
l'action sensibilisante outanée chez le cobaye. Unpublished report
from Centre Nicolas Grillet, France, No. 18081, submitted by
Rhône-Poulenc.
Pasquet, J., Shirasu, Y., Moriya, M. and Katto, K. (1976) Mutagenicity
Testing on Glycophene in Microbial Systems. Unpublished report from
Institute of Environmental Toxicology, Toxicology Division, submitted
by Rhône-Poulenc.
Rhône-Poulenc (1977a) Dosages de residue dans les fruits a noyau
(prune, Pêche, cerise) at a pepins (poire, pomme). Unpublished report
R.P./R.D./C.N.G. No. 19 157.
Rhône-Poulenc (1977b) Dosage de residue dans les petits fruits
(fraises - framboises - cassis). Unpublished report R.P/R.D./C.N.G.
No. 19 193.
Rhône-Poulenc (1977c) 26019 R.P. Residus dans les raisins, ls mout, le
vin et l'alcohol. Unpublished report R.P./R.D./C.N.G. No. 19 177.
Rhône-Poulenc (1977d) Rovral-26019 RP. Dosages de residue dans les
legumes. Unpublished report R.P./R.D./C.N.G. No. 19 191.
Rhône-Poulenc (1977e) Rovral-26019 RP. Dosages de residue dans les
cereales (blé et riz). Unpublished report R.P/R.D./C.N.G. No. 19 170.
Rhône-Poulenc (1975a) 26019 Methods de dosage des residue dans les
vegetaux et derives. Unpublished report R.P./R.D/C.N.G. -An. No. 2736.
Rhône-Poulenc (1975b) 26019, Discussion at évaluation des performances
de la méthode de dosage des résidus. Unpublished report
R.P./R.D./C.N.G. -An No. 2658.
Rhône-Poulenc (1973) Produit No. 26019 R.P. Metabolisms dans les
vegetaux et dans le sol. Unpublished report S.W. C.R.P. -D.S.Ph.D.S.
An Nord No. 17 374.
Rhône-Poulenc (1977f) Dégradation du 26019 R.P. absorbé par les
racines ou déposé sur les feuilles des vegetaux (fraisiers et blé) a
l'aide de 26019 R.P. marqué au 14C. Unpublished report
R.P./R.D./C.N.G. et C.N.G. An No. 19201.
Rhône-Poulenc (1976) Degradation of RP 26019 in the soil. Treatments
with 2 and 5 ppm 14C labelled product. Unpublished report
R.P./R.D./C.N.G. No. 18785.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables. These are suitable or can be adapted for regulatory
purposes. They have been adapted for residue analysis In must and
wine. The limit of determination on most fruits and vegetables is
about 0.01-0.02 mg/kg. Some commodities of plant origin, e.g. prunes
and mare, require a more elaborate clean-up owing to the higher
proportion of interfering plant constituents. The limit of
determination in these commodities is about 0.05-0.1 mg/kg.
No loss of residues was found during storage for more than 1 year at
temperatures of -18°C.(Rhône-Poulenc, 1975a,b).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following maximum residue limits were reported to the Meeting as
established or under consideration. They refer to iprodione, excluding
metabolites.
Country Commodity Maximum residue
limit, mg/kg
Australia Apricots, cherries,
plumes peaches 10
France Grapes 10
Fed. Rep.
of Germany Grapes 10
The Netherlands Lettuce 5
Strawberries 2
New Zealand Apricots, berry fruits,
cherries, grapes,
peaches, plums 10
Switzerland Grapes 7
APPRAISAL
Iprodione is used against a relatively broad range of fungus diseases,
on a wide range of fruits and vegetables.
Its use is authorized, or is in course of registration, for various
crops in a number of countries. It is marketed in the form of a
wettable powder, a suspension concentrate and an emulsifiable
concentrate. The products are mainly used as a spray on the aerial
parts of growing crops, for post-harvest dipping of fruit as a dip for
seed potatoes and as a seed treatment. Application rates vary
according to the crop/disease situation and regional conditions.
Residue data were obtained from supervised trials carried out in
various countries with different climatic conditions. Studies with
unlabelled and 14C-phenyl-labelled products showed that iprodione
does not appreciably penetrate through the plant cuticle. The residue
on the surface of the plants had a half-life of about 30-60 days. It
was converted into
1-(3,5-dichlorophenyloarbamoyl)-3-isopropyl-2,4-dioxoimida-zolidine,
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydantoin, RP 30228, which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including
1-carbamoyl-3-(3,5-dichlorophenyl)hyclantoin (RP 32490).
Wheat and strawberry plants grown on soil treated with iprodione took
up small amounts of the compound (equivalent to 0.7-1.3% of the total
applied to the soil surface). Within the plant the parent compound was
converted into metabolite RP 30228, Small amounts of RP 32490 and some
more polar unidentified products. The degradation of residues in soil
follows the same pattern. The half-life at initial levels of 2 and 5
mg/kg is about 30 days. After 12 months incubation under aerobic
conditions at 23-25°C no more than 3% of the remaining residue is in
the form of unchanged iprodione. The parent compound is only slightly
mobile, remaining mainly in the upper 0-15 cm layer. The metabolits RP
30228 is less soluble in water than the parent compound (0.5 mg/l
compared to 13 mg/l) and virtually all remained in the 0-5 cm layer.
Residues in wine were approximately 15-25% of those on the harvested
grapes.
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables, must and wine, which are suitable or can be adapted for
regulatory purposes. The limit of determination is generally about
0.01-0.02 mg/kg. No loss of residue was found over more than 1 year at
-18°C.
RECOMMENDATIONS
The following maximum residue limits for iprodione on various fruits
and vegetables are recommended. They refer to iprodione, excluding any
metabolites.
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Apples, pears 10 10-14 +
Grapes 10 14-21
Lettuce 10 14-21(281)
Peaches 10 10-14 +
Plums 7 14
Strawberries 7 14
Blackcurrants 5 10-21
Cucumbers 5 3-6
Sweet peppers 5 3-6
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Raspberries 5 10-21
Tomatoes 5 3-7
Rice (hulled, unpolished) 3 21
Chicory (witloof) sprouts 1 throughout forcing
Beans, dry 0.2 14-21
Garlic, onions 0.1 1
1 Glasshouse use.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on the fate of iprodione residues in milk, meat and
eggs when food wastes containing iprodione residues are used as
components of animal feeds.
2. Residue data on grain and straw from supervised trials on cereal
crops treated according to good agricultural practice.
3. Further information about the effects of processing and cooking on
iprodione residues in a range of commodities.
REFERENCES
Barre, P., R. Cordonnier, A. Dugal, L. Lacroixy M. Laurent et M. Buys
(1976) Etude, dens le raisin et le vin, des résidus d'un fongicide
antibotrytis, le 26019 R.P. et leur effects our la fermentation
alcoholique. Progrés Agricole et Viticole 93 (11) 2-8.
Coquet, B. (1973a) Recherche du pouvoir teratogéne chez le rat OFA par
voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogéne chez le lapin pax
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherches et essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1977c) Essai de toxocité chronique (3 mois) chez le chien
par voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onions, No. 731008
Coquet B. (1976) Influence du produit 26019 R.P. sur la reproduction
du rat (Essai sur 3 générations). Unpublished report from Institut
Franc. de Recherche et Essais Biologique, No. 760435 submitted by
Rhône-Poulenc.
Davies, V.A. and Lowe C.Y. (1974) Fungicides: Formulation LPA 2043:
Acute Oral and Dermal Toxicity in the Rat. Unpublished report from May
and Baker Ltd., England, No. RG 2037.
Detaille, J.Y., Pasquet, J., Mazuret, A. and Mondot, S. (1973)
Antifongique 26019 RJ., Phaxmacologie générale chez le chien
anesthésié au pento barbital. Unpublished report des Laboratoires do
Recherches de la Société des Usines Chimiques Rhône-Poulenc, subm.
by Rhône-Poulenc.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. and Myan, J. (1973a) Produit No. 26019 R.P. -
Toxicété à terms (5 mois) chez le rat par voie orale, on comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R.P.). Unpublished
report from Laboratoires do Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 171249 submitted by
Rhône-Poulenc.
Ganter, P. and Girard, P. (1973b) Produit 26019 R.P. - Examen
histologique de la toxicité oculaire du 26019 R.P. chez le chien aprés
un traitement do 3 mois. Unpublished report from Laboratoires de
Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131, submitted by Rhône-Poulenc.
Hastings, S.E., Huffmann, K.W and Gallo, M.A. (1974) Dominant Lethal
Mutagenicity in Mice. Unpublished report from Boss and Clark, Division
of Rhodia Inc., No. SEH 74:69.
Hastings, S.E., Huffman, K.W. (1975) Chronic Toxicologic and
Carcinogenic Study with R.P. 26019 in Mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:113, submitted by
Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C. and Kiggins, E.M. (1976) Chronic
Toxicologic and Carcinogenic Study with R.P. 26019 in Rats.
Unpublished report from Rhodia, Inc., Hess and Clerk Division, No.
76:57, submitted by Rhône-Poulenc.
Laurent, M. and Buys, M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. and Chabassol, Y. (1976)
Etude du métabolism chez le rat á l'aide du produit marqué au 14C.
Unpublished report from Rhône-Poulenc, Direction des Recherches et du
Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-Seine,
C.N.G. -An No. 18548, submitted by Rhône-Poulenc.
Pasquet, J. and Mazuret, A. (1974) Product 26019 R.P. Toxicité aigue
chez le rat par voie intrapéritonéale. Unpublished report from
Laboratoires de Recherches de la Socété des Usines Chimiques
Rhône-Poulenc, No. 174621 submitted by Rhône-Poulenc.
Pasquet J. and Nazuret, A. (1975a) Glycophene (26019 R.P.) -
Formulation LFA 2043 (wettable powder at 50% of glyoophene) Acute
toxicity and Dermal and Ocular tolerance. Unpublished report from
Rhône-Poulenc. No. 18182 submitted by Rhône-Poulenc.
Pasquet J. and Mazuret, A. (1975b) Glycophene (26019 R.P.). Etude de
l'action sensibilisante outanée chez le cobaye. Unpublished report
from Centre Nicolas Grillet, France, No. 18081, submitted by
Rhône-Poulenc.
Pasquet, J., Shirasu, Y., Moriya, M. and Katto, K. (1976) Mutagenicity
Testing on Glycophene in Microbial Systems. Unpublished report from
Institute of Environmental Toxicology, Toxicology Division, submitted
by Rhône-Poulenc.
Rhône-Poulenc (1977a) Dosages de residue dans les fruits a noyau
(prune, Pêche, cerise) at a pepins (poire, pomme). Unpublished report
R.P./R.D./C.N.G. No. 19 157.
Rhône-Poulenc (1977b) Dosage de residue dans les petits fruits
(fraises - framboises - cassis). Unpublished report R.P/R.D./C.N.G.
No. 19 193.
Rhône-Poulenc (1977c) 26019 R.P. Residus dans les raisins, ls mout, le
vin et l'alcohol. Unpublished report R.P./R.D./C.N.G. No. 19 177.
Rhône-Poulenc (1977d) Rovral-26019 RP. Dosages de residue dans les
legumes. Unpublished report R.P./R.D./C.N.G. No. 19 191.
Rhône-Poulenc (1977e) Rovral-26019 RP. Dosages de residue dans les
cereales (blé et riz). Unpublished report R.P/R.D./C.N.G. No. 19 170.
Rhône-Poulenc (1975a) 26019 Methods de dosage des residue dans les
vegetaux et derives. Unpublished report R.P./R.D/C.N.G. -An. No. 2736.
Rhône-Poulenc (1975b) 26019, Discussion at évaluation des performances
de la méthode de dosage des résidus. Unpublished report
R.P./R.D./C.N.G. -An No. 2658.
Rhône-Poulenc (1973) Produit No. 26019 R.P. Metabolisms dans les
vegetaux et dans le sol. Unpublished report S.W. C.R.P. -D.S.Ph.D.S.
An Nord No. 17 374.
Rhône-Poulenc (1977f) Dégradation du 26019 R.P. absorbé par les
racines ou déposé sur les feuilles des vegetaux (fraisiers et blé) a
l'aide de 26019 R.P. marqué au 14C. Unpublished report
R.P./R.D./C.N.G. et C.N.G. An No. 19201.
Rhône-Poulenc (1976) Degradation of RP 26019 in the soil. Treatments
with 2 and 5 ppm 14C labelled product. Unpublished report
R.P./R.D./C.N.G. No. 18785.
 Other Information on Identity and Properties
a) Composition of the technical product
The technical product contains 95% minimum of iprodione. The main
impurities are phenyl hydantoins and bis-isopropyl-1,,3\-urea
(referred to as 32870 R.P.).
b) Physical and chemical properties
Physical state: white, odourless, non-hygroscopic crystals.
Molecular weight: 330.17
Melting point: 136°C
Volatility: not volatile
Vapour Pressure: 2 × 10-7mm. Hg at 20°C
Solubility at 20°C: g/l
water 0.013
ethanol 30
acetonitrile 150
toluene 150
benzene 200
acetone 300
methlyene chloride 500
Formulations
Mainly wettable powder 500 g a.i./kg. Also suspension concentrate 500
g a.i./l and emulsifiable concentrate 200 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Single oral doses of iprodione are rapidly eliminated by rats.
Following a single application of an oral dose of 200 mg/kg, 26% of
the administered dose was eliminated in the urine and 59% in the
faeces within 24 hours after application. The major part of the dose
excreted in the faeces is the parent compound, whereas only 3% of the
administered dose is eliminated unchanged in the urine. Besides the
principal urinary metabolites with a degraded isopropylcarbamoyl group
(about 11% of the dose administered), there are metabolites with
intact hydroxylated or non-hydroxylated aromatic rings. The isomer of
the parent compound accounted for a small proportion of the
metabolites. Residues in the principal organs and tissues did not
exceed 1.5% of the administered dose in rats sacrificed 4 days after
dosage (Laurent and Bays, 1974).
In a similar study rats were dosed once with 100 mg/kg. of
14C-aromatic ring-labelled iprodione; 96 hours after administration
62% of the applied dose was eliminated via the urine and 36% via the
faeces. About 16% was excreted as the parent compound in the faeces:
the remaining radioactivity was mainly in urine in the form of the
desisopropylated derivative (about 20% of the dose) and the
N-(3,5-diachloro-4-hydroxyphenylbiuret) (approx. 13%). Tissues sampled
4 days after dosage contained about 1% of the administered dose.
(Lourer, et al., 1976),
Based on the identified metabolites the reactions that seem to occur
during biotransformation are mainly hydroxylation, oxidation and
desalyklation of the isopropylcarbamoyl group
(-N1-CO-NH-CH(CH3)2->N1-CO-NH2->N1-H).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups of 25-30 rats were orally treated with 0, 100, 200 and 400
mg/kg on Restation days 5 to 15. Females at 400 mg/kg showed reduced
fertility, reduced body weight gain and a dose-related reduction of
food consumption especially during the treatment period. The number of
implantations was also reduced at the highest dose level.
There was no indication for an embryonyic or teratogenic effect of the
test completed (Coquet, 1973a).
Groups of 15-17 New Zealand White rabbits were intubated on gestation
days 6-16 inclusive with 0, 100, 200 or 400 mg/kg. Body weight gain,
over the period of treatment, was slightly reduced at 100 mg/kg, and a
dose-related weight loss occurred at 200 and 400 mg/kg. Food intake
was reduced at 200 mg/kg and above. At 400 mg/kg, 9 of 17 females
died, and only one of the four remaining pregnant animals carried to
term. Foetal loss was increased at 200 mg/kg, and foetal weight was
reduced at 200 mg/kg and above. Multiple malformations occurred in 1
of 68 living foetuses at 200 mg/kg. Minor malformations were noted in
all groups.
Special studies on carcinogenicity
See "Long term studies."
Special study on reproduction
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 and 1000 ppm for
the first 5 weeks of each generation and 0, 250, 500 and 2000 ppm for
the next 8 weeks of treatment. The diet was fed through three
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parental animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendency for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rate of the third generation did not reveal
abnormalities. (Coquet, 1976)
Special study on mutagenicity
Groups of 25 male mice were fed 0, 1500 and 6000 ppm iprodione for 49
days. After termination of the feeding period the male mice were
paired with 2 untreated females for 6 days, followed by a further 2
females for the 6-12 days post-treatment period. The treatment did not
affect body weight, food consumption or fertility of the males. None
of the examined parameters gave any indication of a mutagenic effect
of iprodione (Hastings et al., 1974).
Iprodione showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, reverse mutation tests with and without liner
activation system using E. coli WP2 hcr- and five strains of
Salmonella typhimurium TA and host-mediated assay with S. typimurium G
46 in mice (Shirasu et al., 1976).
Acute Toxicity
TABLE 1. Acute toxicity of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral >2000 Pasquet & Mazuret,
1973
Rat M F Dermal >2500 ibid.
Rat M i.p. 2400 Pasquet &. Mazuret,
1974
F i.p. 1200 ibid.
Mouse M F Oral approx. 4000 Pasquet A Mazuret,
1973
Dog M F Oral >2000 ibid.
Rabbit M F Dermal >1000 ibid.
The signs of toxicity were: loss of reflexes, muscular hypotonia,
sedation and dyspena.
Iprodione did not cause skin or eye-irritation in rabbits.
In the anaesthetized dog iprodione administered at a dose of 300 mg/kg
by the intraduodenal route did not affect the cardiovascular,
respiratory or neurovegetative system (Detaille et al., 1973).
TABLE 2. Acute toxicity of a 50% formulation of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral 8000 Davies & Lowe,
1974
M F Dermal >2000 ibid.
M F 4 h
inhalation 13 mg/l air Pasquet & Mazuret,
1975a
M F Oral 4100 ibid.
Oral 4900 ibid.
M F 4 h inhalation >13/l air Ibid.
Rabbit M F Dermal >2000*) ibid.
*) atoxic
The formulation induced slight irritation in the rabbit eye but had no
irritant effect on the intact or abraded skin of rabbits (Pasquet &
Mazuret, 1975a).
"In the sensitization test with guinea pigs, after 10 applications of
0-3 ml of a 50% iprodione solution, followed 2 weeks later by a
challenge application, no evidence of dermal sensitization was
observed" (Pasquet & Mazuret, 1975b).
Short term studies
Rat
Groups of 15 male and 15 female caesarian originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the diet
for 5 months. No effects were observed on mortality, food consumption,
haematology (as judged by haemoglobin, haematocrit, erythrocyte count,
or total and differential leucocyte count) clinical chemistry (as
judged by BSP, SGOT, SGPT or SAP) or urinalysis. Body weight gain was
slightly reduced (especially in males) at 500 and 1000 ppm. Absolute
(but not relative) heart weight was reduced in males at 500 and 1000
ppm, and absolute kidney weight was reduced at 1000 ppm. In females,
absolute liver and kidney weights were significantly reduced at 500
ppm only. Gross and histopathology were normal at all dose levels. In
a parallel study, dichlozoline, a structurally related compound,
induced cataracts. No such effect was seen with (Ganter et al.,
1973a).
Dog
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 and 7200 ppm for a
period of 3 months. At the top dose level the method of administration
was altered after 6 weeks, to gelatine capsules. The treatment did not
affect mortality. The recorded values of haematological determinations
and urinalyses were within normal limits. As judged by haemoglobin,
haematocrit, reticulocyte erythrocyte count, total and differential
leucocyte count and prothrombine time except for signs of mild anemia
in 1 male and 1 female at 2 months and 1 male at 3 months at the top
dose level. At 7200 ppm a reduction of food consumption was observed,
accompanied by reduced body weight gain. The opththalmosopic
examination of the animals did not reveal any pathological alteration
(Canter and Girard, 1973b). The clinical chemistry determinations
consisted of glucose, urea, cholesterol, bilirubiu, total proteins,
protein electrophoresis, alkaline phosphatase, SGOT, SGPT, LDH, Na+,
C, K+, Cl -, Ca++, P. At 2400 and 7200 ppm a slight increase of SLP was
observed, also a transient increase of SGOT and SGPT after 1 and 2
months of treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, in females at the
dose levels of 2400 ppm and above. At 7200 ppm reduced relative weight
of testes was found, but no histological indication of damage.
The histopathological findings did not reveal any indication of
treatment-related alterations of tissues (Coquet, 1973c).
Long term studies
Mouse
Groups of 60 male and 60 female mice were maintained an a diet
containing the test compound at 0, 200, 500 and 1250 PPM for 18
months. No treatment-related effect on body weight, food consumption
or mortality was found. The recorded values of the haematological
blood chemistry and urinalyses tests performed after 6, 12 and 18
months of the feeding period, were within the physiological range.
Necropsy findings on mice that died during the last 6 months of the
test and on those sacrificed at the termination date showed an
increased number of enlarged lymph nodes in males at 200 ppm. Organ
weight variations occurred sporadically in the various dose groups and
are considered not to be treatment-related. The histopathological
findings failed to reveal abnormal features. The distribution of
neoplastic and non-neoplastic findings did not appear to demonstrate
any significant dose-dependence. The most common tumours were
lymphosarcoma involving the spleen, lymph nodes and thymus (Hastings
and Hullman, 1975).
Rat
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 and 1000 ppm for 24 months. Slight reduction in
body weight gain was observed at 1000 ppm. This was accompanied by
some reduction in food intake. The treatment had no effect on food
consumption, mortality or values of the hematologic, blood chemistry
and urinalyses determinations. Necropsy findings did not reveal any
drug-related gross alteration. Variations in organ weight did not show
a group distribution and seemed not to be related to drug
administration. Histopathology did not indicate a treatment
relationship of neoplastic and non-neoplastic findings. AT 24 months
the most common tumours observed were pituitary adenomas and
adenocarcinoma and fibroadenoma of the mammary glands (Hastings et
al., 1976).
COMMENTS
Iprodione is readily absorbed and rapidly excreted mainly as
metabolites with intact hydantoin-moiety. The compound was not
teratogenic. In a 3-generation study in rats, there was a slight but
statistically significant reduction in postnatal growth at 2000 ppm.
This effect was only marginal at the lower dose of 500 ppm which is
regarded as a no-adverse-effect-level. In a short-term study in dogs
no major effect occurred up to 2400 ppm. Likewise long-term studies in
mice and rats revealed no effects up to 1250 ppm. No ocular
alterations were found in any study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mice: 1250 mg/kg in the diet, equivalent to 160 mg/kg bw
Rat: 500 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.3 mg/kg bw
USE PATTERN
Iprodione is used as a fungicide against a range of fungus diseases,
including Botrytis in vines, black- and red currants, blackberries,
raspberries and vegetables especially lettuce; Botrytis alii on
onions; Rhizocotonia on seed potatoes; seed borne diseases on sugar
beets (Phoma spp.) and cereals. It is also used against Botrytis
and some other fungus diseases on ornamentals.
The compound is used as a foliar spray on several crops, as a post-
harvest dip for fruit, for dipping seed-potatoes and as a seed-
treatment on sugar beet and cereals.
The product is authorized for use on various crops in France, the
Federal Republic of Germany, Greece, the Netherlands and the United
Kingdom. In several other countries the compound is used or included
in testing programmes and it is in course of registration in many
countries including Australia, New Zealand, Japan, USA, Canada,
Israel, and several European countries.
Most of the recommended uses are summarized in Table 3. The
information may not be complete since the use of the compound is
expanding rapidly and more uses may be expected in the near future.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained from supervised trials carried out in
various countries on fruit and vegetables and on some agricultural
crops; they are summarized in Tables 4 to 8 and 10.
Pome and stone fruits (Tables 4 and 5; Rhône Poulenc, 1977a)
Apples
The residue at harvest from pre-harvest treatments at normal
application rates (about 2.25 kg a.i./ha) is about 2 mg/kg. A
combination of such treatments with post-harvest dipping gives rise to
residues of about 6 mg/kg. After repeated applications at about twice
the normal rate residues ranged from 2.9 - 6.5 mg/kg. The residue of
the metabolite RB 30228 (see "Fate of residues") was below 0.15 mg/kg
in these experiments.
Pears
Post-harvest dipping of pears against storage diseases gave rise to
residues of 3.6 - 5 mg/kg.
Peaches
Residues at harvest following applications at the recommended rate
varied between 0.9 and 6 mg/kg. A post-harvest dip adds about 4 mg/kg
to these levels.
Plums
Residues arising from recommended applications varied between 0.6 and
6.8 mg/kg, depending on the pre-harvest intervals observed and the
local conditions. The drying process increased the residue in the
prunes by 0.6 - 1.6 mg/kg.
TABLE 3. Use pattern and recommended pre-harvest intervals of iprodione
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Grapes Botrytis 4 750 Austria 28
" 4 750 France. 15
" 4-5 750 Fed. Rep. of 28
Germany
" 3 3000 Japan 7
" 4 750 Portugal 15
" 4 750 Spain 15
" 4 750 Switzerland
" 4 750 USSR
" 4 750 Yugoslavia
Strawberries Botrytis 4-5 1000 Belgium 15
3 1000 Fed. Rep. of 7
Germany
3 Japan 1
4-5 1000 The Netherlands 14
Pome and Stone fruit
Apples Alternaria about 10 Japan 10
Peaches Monilia 3 Japan 1
Vegetables
Chicory (witloof)
(forcing) Botrytis 1 3 g/m2 Belgium Throughout
Sclerotinia 1 4 g/m2 France the forcing
applied on the top of period
the roots at forcing
TABLE 3. (Continued)
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Cucumbers Botrytis 4 Japan 1
Lettuce Botrytis
Scleotinia 3 750 Belgium 10
(glasshouse)
" 3-4 750 France
" 3 750 Fed. Rep. 21
of Germany (glasshouse)
14
(outdoors)
" 4 750 Japan 14
(also for endive) " 1x 1000-2000 The Netherlands 28
" 2xx 750 The Netherlands 28
Vegetables
Onions Botrytis
Sclerotinia 3 750 Japan 7
cepivorum
Tomatoes Botrytis 4 Japan 1
Alternaria United Kingdom
Agricultural Crops
Beans Sclerotinia 3 Japan 21
Rice Pellicularia 3 Japan 21
TABLE 3. (Continued)
Seed and tuber treatments
Cereal seed
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Barley Helminthosporium 1 60 g a.i./100
kg seed
Wheat Tilletia caries 1 60 g a.i./100
kg seed
Garlic Sclerotinia cepivorum 1 300 g a.i./100 France
kg seed
Potatoes Rhizoctonia solani 1 100-150 g/ France
1000 kg tubers
spraying on
tubers
immediately
before
storage
dipping in
spring before
planting
400 g/100 l
Sugar-beet Phoma spp 1 150 g a.i./kg France.
seed enveloped seed
x= one application at planting.
xx= two applications, the first about a week after planting and a second within two weeks after planting
Berry fruits and currents (Table 6; Rhône-Poulenc, 1977b)
The residue levels at harvest after treatments at normal rates and
observing recommended pre-harvest intervals (10-21 days) were
generally at or below 5 mg/kg on blackcurrants, 2 mg/kg on raspberries
and 6 mg/kg on strawberries.
Grapes
The maximum residues of iprodione on grapes at harvest following
treatment according to good agricultural practice (about 750 g
a.i./ha) were in general not higher than 10 mg/kg. The highest levels
in the unfermented must and the win were 4.4 and 6.4 mg/l
respectively. Some results are shown in Table 10 (Rhône-Poulenc,
1977c). See "Fate of residues", "In storage and processing".
Vegetables (Table 7; Rhône-Poulenc, 1977d)
Chicory (witloof)
The residues in the edible sprouts after a normal period of forcing
and one treatment at the recommended rate did not exceed 1 mg/kg. The
residues in the roots were much higher, with a maximum of about 10
mg/kg. The roots are often used as animal feed.
Cucumbers
Residues on cucumbers treated with 3 kg a.i./ha (twice the normal
rate) were between 0.3 and 2.2 mg/kg.
Lettuce
Residues arising from recommended applications on outdoor lettuce (750
g a.i./ha) varied between 1.7 and 2.5 mg/kg after pro-harvest
intervals of 14-21 days. The residues on glasshouse-grown lettuce are
in general much higher. Three applications of the recommended dosage
gave rise to residues of 6.7 mg/kg after a pre-harvest interval of 39
days, and in other experiments maximum levels of 7.2 mg/kg were found
14 days after the last application. Residue levels of the metabolite
RP 30228 were slightly about the limit of determination; other
metabolites were below it. (Metabolites are identified in the section
"Fate of residues."
Onions
The residues on onions 1 day after application did not exceed 0.2
mg/kg.
TABLE 4. Supervised trials of iprodione. Residues in pome and stone fruit (pre-harvest application)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Apples Japan 1975 1 100 5 WP 50% 2.15 1.75
1975 1 100 5 " 2.9 2.25
1975 1 100 5 " 0.38
1975 10 100 5 " 6.5
1975 10 100 5 " (5.75)
1975 10 100 5 " 3.75
1975 10 100 5 " 3.4
1975 10 100 " (1.95)
1975 10 100 5 " 1.7
U.K. 1975 10 100 2.25 " (2.0)
Cherries
Moss Australia 1975 4 1.4 " 8.0
Peaches
Katharine Australia 1976 7 50 1.4 " 5.4
Anne Truly 1976 3 50 0.5 " 1.7
Goldmine 1976 5 50 1.4 " 5.8
Redhaven Canada 1974 4 50 1.0 " 6.5 4.6 4.9
1974 4 50 1.0 " 1.45
Babygold Canada 1974 7 50 1.0 " 2.3 2.0 1.3
1974 6 50 1.0 " 1.8 0.9
Earlired 1974 5 1.0 " 2.5 1.9 2.2 2.2
Redhaven 1974 5 1.0 " 6.1 4.1 3.4 2.9 1.6
Sunhaven 5 1.0 " 7.6 9.0 10.0 8.5
Gifu Japan 1975 7 100 4.0 3.7 2.1
1975 3 100 4.0 " 4.6 2.9
Okayama 1975 2 100 3.0 " 6.3 4.8
1975 3 100 3.0 " 6.8 5.8
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Plums
October Australia 1976 4 1.4 " 2.2
purple
Inra 711 France 1973 4 0.5 SC 0.25 0.26
4 1.0 SC 1.4 0.9
1974 0.5 4.15
3 1.0 6.8
2 0.5 0.2
2 1.0 0.6
* SC = Suspension concentrate
WP = Wettable powder
TABLE 5. Supervised trials of iprodione. Residues in pome and stone fruit (post-harvest application)
Application
Rate
Crop Country Year No. g a.i./100 Formulation Residues (mg/kg) after storage period of (days)
dip 1-2 7 11 85 95
Apples
Cox's U.K. 1973 - - 2.0
1 200 5.8
1 200 4.4
Pears
Conference U.K. 1973 1 200 SC 5.0
1973 1 200 4.8
1973 1 200 3.6
1973 1 200 4.7
1973 1 200
1973 1 200 4.0
Peaches Australia - WP50% 1.7
1 50 WP50% 5.3
1 50 WP50% 5.0
TABLE 6. Supervised trials of iprodione. Residues in berry fruits and currants
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Raspberries France 1974 3 0.8 WP 50% 1.1
Heyton 0.5 0.85
Malling
jewel U.K. 1974 5 1.1 " " 1.55
3 1.1 " " 2.0
4 1.1 " " 7.9 5.0 2.8 2.1
Strawberries Belgium 1974 5 0.75 " " 4.9
Sivetta 5 1.0 " " 6.0
Domunil 5 0.75 " " 1.9
5 1.0 " " 2.5
Redcoat Canada 1974 4 1. " " 3.1 1.8 1.4 0.85
Vista 4 1.1 " " 3.5 2.4 1.4 1.0
Redcoat 4 3.45 2.1 1.6 1.0
Redcoat 3 0.9 0.8 0.2
Red Gauntlet France 1973 4 0.5 " " 0.22
0.75 " " 0.44
1.0 " " 0.66
Immigrante 1974 4 0.75 " " 1.75
4 1.0 " " 2.2
Gorella 1974 4 0.75 " " 0.35
4 1.0 0.44
3 0.75 " " 0.5
3 1.0 " " 1.1
Suprême d'Halles 4 0.5 " " 1.2
4 0.75 " " 1.9
4 1.0 " " 2.75
4 0.5 " " 2.5
4 0.75 " " 3.2
4 1 5.6
Senga Segana Fed. Rep. 1974 3 0.9 " " 5.3 2.7 2.3 1.0 0.4
of Gemany 3 1.25 " " 9.1 3.6 3.4 1.9 0.7
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Senga Segana Netherlands 1974 4 0.75 " " 1.1
1974 5 0.75 " " 26.4 14.2 0.67
Red Gauntlet Switzerland 1974 1 0.75 " " 1.0 0.86 0.2 0.1 0.1
Wädenswill 1974 3 0.75 " " 0.2
1974 3 0.75 " " 0.15
Royal
Sovereign U.K. 1974 4 1.1 " " 1.2 1.6 1.3
Strawberries
Cambridge
Favourite (g) 1974 4 1.1 WP 50% 8.0 6.9 4.5 4.7
Cambridge
Favourite (g) 1974 3 1.1 " " 1.7
Cambridge
Favourite 1974 3 1.0 " " 0.7
Cambridge
Favourite 1976 3 1.6 1.1
Cambridge
Favourite 1976 3 0.75 0.9 0.6
Red Gauntlet 1976 1 0.75 0.6
Red Gauntlet 1976 1 1.0 0.9
Red Gauntlet 1974 3 2.2 1.7
Cambridge
Favourite 1974 3 2.2 0.55
Royal
Sovereign 1974 2 1.0 0.3
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Black
currants
Wellington
Tr. UK 1974 4 1.1 WP 50% 3.9
62 days
Baldwin UK 1974 4 1.1 " " 4.6
62 days
(g) = glasshouse
TABLE 7. Supervised trials of iprodione. Residues in vegetables
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Bean
without pod Japan 1975 1 1 WP 50% 0.05 0.05 0.05
Cucumbers(g) Japan 1975 3 1 0.05 0.05
1975 1 3 WP 50% 1.7 1.0 0.36
1975 3 3 " " 1.6 0.9 0.7
1975 4 3 " " 2.2 1.4 1.2
1975 1 2.5 " " 1.2 1.0 0.24
1975 3 2.5 " " 1.9 1.4 0.3
1975 4 2.5 " " 1.8 1.0 1.0
Lettuce
Val.d'Orge France 1974 6 0.5 " " 0.03
6 0.75 " " 0.03
6 1.0 " " 0.03
6 0.5 EC 200g/l <0.02
6 0.75 " " <0.02
6 1.0 " " 0.04
5 0.5 WP 50% 2.5
5 0.75 " " 1.3
Murkönig Fed. Rep. 3 0.75 " " 15.7 1.8 0.6 0.05 0.05 0.05
Kares of Germany 1976 4 0.75 " " 24.5 9.1 1.7
Reskia 1976 3 0.25 " " 2.6 0.8 0.2
3 0.75 " " 13.2 3.0 1.1
Susan 1976 3 0.5 " " 4.6 1.2 0.4
3 0.75 " " 28 4.3 4.2
Murkönig(g) Fed. Rep. 1976 3 0.25 " " 24 6.9 2.9
of Germany 3 0.75 " " 46 10.5 7.2
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Ravel(g) 1976 3 0.25 " " 26 5.4 2.7
0.75 " " 22 4.8 3.6
Kurume
Br.(g) Japan 1975 2 3 " " 4.6 0.28 0.05
3 3 " " 10.8 0.27 0.1
4 3 " " 7.4 0.3 0.1
Kumamoto(g) Japan 1975 2 1.5 " " 0.48 1.05 0.4
3 1.5 " " 1.9 0.6 1.2
4 1.5 " " 2.25 0.78 1.7
Déciminor(g) Netherlands 1975 2 0.75 " " 8.2 4.9
Vera(g) 1975 2 0.75 " " 9.15 0.05 0.05
Ostinata(g) UK 1974 4 0.56 " " 19 18 14 (24
days)
Val d'Orge 1974 3 0.56 " " 8.8
4 0.56 " " 59 40 41 21
Onions S. Africa 1977 7 50 WP 50% 0.14
0.15
100 " " 0.15
0.24
Sweet
Peppers(g) U.K. 1975 6 4.3
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Tomatoes
Koibuchi (g) Japan 1975 1 2.5 " " 1.25 1.4 1.2 0.8
Koibuchi (g) 1975 3 2.5 " " 5.3 3.4 3.0 2.4
Koibuchi (g) 1975 4 2.5 " " 5.6 5.4 4.3 3.5
1975 3 3.0 " " 2.1 3.3 1.8 2.9
4 3.0 " " 4.6 3.6 4.1 2.8
5 3.0 " " 4.4 4.0 3.8 3.7
Eurocross(g) U.K. 1974 5 50 " " 2.7
6 50 " " 3.8
7 50 " " 4.9
8 50 " " 4.2 2.3
Sonato(g) U.K. 1974 4 50 " " 1.65
5 50 " " 2.3
6 50 " " 2.8
7 50 " " 3.7
8 50 " " 4.2 5.8
5 50 " " 3.1 2.5 2.7
1 1 " " 0.64
(18
days)
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. a.i. Formulation 30-40 40 44-48 59 70 93-104 162
g/m2 S R S R S R S R S R S R S R
Chicory Belgium 1975 1 3 " " 0.41 1.49
1 6 " " 0.36 3.10
3 " " 0.09 2.7
6 " " 0.32 4.4
3 " " 0.77 6.2
6 " " 0.59 10.0
3 " " 0.55 2.7
6 1.0 3.7
France 1974 1 4 " " 0.6
8 " " 1.0 0.07
4 " " 0.25
8 " "
TABLE 7B
Crop Country Year No. Rate Formulation 30-40 40 44-48 59 70 93-104 162
g/100 1g/1000
dip kg S R S R S R S R S R S R S R
Potatoes
pre-plant France 1976 1 200 WP 50% n.d.
treatment 300 " " n.d.
50 " " 0.01
100 " " 0.01
150 " " 0.02
200 " " 3.0 5.0
50 " " 25 25
100 " " 58 67
150 " " 120 140
foliar
spray S.
Africa 1976 5 50 " " <0.02
5 100 " " <0.02
3 50 " " <0.02
3 100 <0.02
WP = wettable povider; EC = emulsion concentrate;
(g) = glasshouse; R = roots (chicory) or tubers (potato); S=sprouts
Peppers and tomatoes
Following applications at normal rates (50 g a.i./100 l) residues of
4.5 - 5 mg/kg were found at harvest after pre-harvest intervals of 3.6
days.
Beans (dry)
After treatment at a dosage rate of 1 kg/a.i./ha, residues in the dry
beans were very low (0.05 - 0.2 mg/kg).
Cereal crops (Table 8; Rhône-Poulenc, 1977e)
Wheat
Two applications at normal rates (1 kg a.i./ha) with a pre-harvest
interval of 73 days did not give rise to measurable residues in the
kernels.
Rice
After treatment during the growing season with relatively high dosages
(1.2 kg a.i./ha), residues of 0.1 - 2.1 mg/kg were found de-husked,
unpolished rise 21 days after the last treatment.
FATE OF RESIDUES
In plants
The fate of iprodione in plants and soil was studied with unlabelled
and 14C-phenyl-labelled products. It was found that when applied to
the leaf surface, iprodione does not appreciably penetrate through the
skin. The residues on the skin had a half-life of 30-60 days, being
slowly converted to
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydentoin, RP 30228), which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including 1-carbamoyl-3-(3.5-
dichlorophenyl) hydantoin (RD 32490) (Rhône-Poulenc, 1973).
Wheat and strawberry plants grown on soil treated with iprodione
(Rhône-Poulenc, 1977f) took up small amounts of the compound (in wheat
0.7-1.3% of the amount applied to the soil surface), which was mainly
found in the leaves and stems (95-99% of the extractable residue).
Within the plant the parent compound was converted to RP 30228, small
amounts of RP 32490 and some more polar unidentified products.
The organosoluble residue in strawberry plants 32 days after a foliar
application at a rate equivalent to 1 kg a.i./ha consisted of 61%
unchanged parent compound and 16% RP 30228. 55 days after foliar
treatment at 2 kg a.i./ha 69% of the residue was iprodione, 7% RP
30228 and 5% RP 32490.
TABLE 8. Supervised trials of iprodione. Residues in cereal crops.
Application Residues (mg/kg) at intervals (days) after application
Rate
Crop Country Year No. Formulation 14-15 21-22 28-30 73 81
g/100 1 kg/ha
Rice Japan 1975 1 100 1.2 WP 50%
grain 0.1 0.1 0.1
straw 16 15 10.3
1975 1 100 1.2 WP 50%
grain 0.3 0.4 0.3
straw 32 12.5 10.5
3 100 1.2 WP 50%
grain 2.1
straw 45
3 100 1.2 WP 50%
grain 1.4
straw 32
3 100 1.2
grain 0.8
straw 49
4 100 1.2 1.8
grain 43
straw
Wheat
Maris
Nimrod UK 1 34 1.0 <0.05
2 34 1.0 <0.05
Jos Cumbier 1 34 1.0 <0.05
2 34 1.0 <0.05
TABLE 9. Nature and Distribution of radio-activity in wheat grown in soil treated with 10 kg/ha. 14C-iprodione
% of total 14C in each plant part as
Unidentified Total 14C
Days after Organo- Water expressed as
treatment Plant part Iprodione RP 30228 RP 32490 soluble soluble Bound iprodione mg/kg
16 roots 49 14 n.d. 21 0 17 20
leaves and 66 6.4 4.4 21 1.5 1.7 20
stem
44 roots 16 15 n.d. 13 1.1 55 31
leaves and 48 9.5 18 20 0.95 4.2 20
stem
89 roots 2.9 8.1 0.8 9.2 0 79 238
leaves and 26 17 14 32 0.22 11 36.7
stem
ears 9.1 3.1 0.1 24 0 56 32
kernels n.d. n. d. n.d. 72 0 28 2.5
Wheat plants were grown on soil treated with excessive dosages of
14C-labelled iprodione and the distribution of the residue in the
plant was studied after 16, 44 and 89 days. The nature and
distribution of the recovered radio-activity is shown in Table 9
(Rhône-Poulenc, 1977f).
The plant and soil metabolites of iprodione have been identified by
various methods including TLC, GLC, and colorimetric analysis and the
degradation pathway shown in Figure 1 deduced.
In soil
The degradation of residues in soil follows a similar pattern. The
half-life at initial levels of 2 and 5 mg/kg is about 30 days. After
12 months incubation under aerobic conditions at 23-25°C, no more than
3% of the remaining radio-activity was in the form of unchanged
iprodione. Conversion to metabolite RP 30228 proceeded rapidly. The
concentration of RP 30228 reached a maximum (45-55% of the
radio-activity still present) after 80-100 days and then decreased
(Rhône-Poulenc, 1976).
In leaching experiments with radio-labelled iprodione it was shown
that the parent compound was only slightly mobile, remaining in the
0-15 cm layer. The metabolite RP 30228 is less soluble in water than
the parent compound (0.5 mg/l compared to 13 mg/l) and virtually all
remained in the 0-5 cm layer (Rhône-Poulenc 1973, 1976).
In storage and processing
Extensive data were obtained from various countries on the fate of
residues of iprodione during wine making and on the effect of residues
on the fermentation. When grapes containing about 5 mg/kg were used
for wine making, no influence on the fermentation process was found.
This was confirmed in laboratory experiments in which CO2 evolution
and the proportion of viable cells (those susceptible to actidione)
were measured.
In a trial in which the grapes contained 2-10 mg/kg iprodione, the
fermentation process was slightly retarded. It is unlikely that this
effect would be observed under practical conditions of wine making.
During the wine making, iprodione remains fairly stable, but a
considerable part of the residue will be eliminated with the solids
(mavc) during clarification. The residues in wine are generally about
15-25% of those in the grapes. No residues of iprodione were found in
alcohol obtained after distilling wine (Rhône-Poulenc, 1977c; Barre et
al., 1976). Some results are shown in Table 10.
TABLE 10. Residues of iprodione at various stages of vinification
Iprodione, mg/kg in
Country Year Grapes Must Wine Finished
Unfermented Fermented Racked Clarified Wine
France 1974 4.9 2.7 0.98 0.75
3.0 1.7 1.0 0.71-0.84
1975 6.1 2.75 0.8
7.5 4.4 1.45
1975 2.2 1.5 0.34
5.4 0.6
South 1976 1.0 1.3 0.9
Africa 2.6 0.5 1.5
Switzerland 2.3 1.9 0.7
1973 1.7 1.4 0.5
2.9 1.9 0.4
Other Information on Identity and Properties
a) Composition of the technical product
The technical product contains 95% minimum of iprodione. The main
impurities are phenyl hydantoins and bis-isopropyl-1,,3\-urea
(referred to as 32870 R.P.).
b) Physical and chemical properties
Physical state: white, odourless, non-hygroscopic crystals.
Molecular weight: 330.17
Melting point: 136°C
Volatility: not volatile
Vapour Pressure: 2 × 10-7mm. Hg at 20°C
Solubility at 20°C: g/l
water 0.013
ethanol 30
acetonitrile 150
toluene 150
benzene 200
acetone 300
methlyene chloride 500
Formulations
Mainly wettable powder 500 g a.i./kg. Also suspension concentrate 500
g a.i./l and emulsifiable concentrate 200 g a.i./l.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Single oral doses of iprodione are rapidly eliminated by rats.
Following a single application of an oral dose of 200 mg/kg, 26% of
the administered dose was eliminated in the urine and 59% in the
faeces within 24 hours after application. The major part of the dose
excreted in the faeces is the parent compound, whereas only 3% of the
administered dose is eliminated unchanged in the urine. Besides the
principal urinary metabolites with a degraded isopropylcarbamoyl group
(about 11% of the dose administered), there are metabolites with
intact hydroxylated or non-hydroxylated aromatic rings. The isomer of
the parent compound accounted for a small proportion of the
metabolites. Residues in the principal organs and tissues did not
exceed 1.5% of the administered dose in rats sacrificed 4 days after
dosage (Laurent and Bays, 1974).
In a similar study rats were dosed once with 100 mg/kg. of
14C-aromatic ring-labelled iprodione; 96 hours after administration
62% of the applied dose was eliminated via the urine and 36% via the
faeces. About 16% was excreted as the parent compound in the faeces:
the remaining radioactivity was mainly in urine in the form of the
desisopropylated derivative (about 20% of the dose) and the
N-(3,5-diachloro-4-hydroxyphenylbiuret) (approx. 13%). Tissues sampled
4 days after dosage contained about 1% of the administered dose.
(Lourer, et al., 1976),
Based on the identified metabolites the reactions that seem to occur
during biotransformation are mainly hydroxylation, oxidation and
desalyklation of the isopropylcarbamoyl group
(-N1-CO-NH-CH(CH3)2->N1-CO-NH2->N1-H).
TOXICOLOGICAL STUDIES
Special studies on teratogenicity
Groups of 25-30 rats were orally treated with 0, 100, 200 and 400
mg/kg on Restation days 5 to 15. Females at 400 mg/kg showed reduced
fertility, reduced body weight gain and a dose-related reduction of
food consumption especially during the treatment period. The number of
implantations was also reduced at the highest dose level.
There was no indication for an embryonyic or teratogenic effect of the
test completed (Coquet, 1973a).
Groups of 15-17 New Zealand White rabbits were intubated on gestation
days 6-16 inclusive with 0, 100, 200 or 400 mg/kg. Body weight gain,
over the period of treatment, was slightly reduced at 100 mg/kg, and a
dose-related weight loss occurred at 200 and 400 mg/kg. Food intake
was reduced at 200 mg/kg and above. At 400 mg/kg, 9 of 17 females
died, and only one of the four remaining pregnant animals carried to
term. Foetal loss was increased at 200 mg/kg, and foetal weight was
reduced at 200 mg/kg and above. Multiple malformations occurred in 1
of 68 living foetuses at 200 mg/kg. Minor malformations were noted in
all groups.
Special studies on carcinogenicity
See "Long term studies."
Special study on reproduction
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 and 1000 ppm for
the first 5 weeks of each generation and 0, 250, 500 and 2000 ppm for
the next 8 weeks of treatment. The diet was fed through three
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parental animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendency for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rate of the third generation did not reveal
abnormalities. (Coquet, 1976)
Special study on mutagenicity
Groups of 25 male mice were fed 0, 1500 and 6000 ppm iprodione for 49
days. After termination of the feeding period the male mice were
paired with 2 untreated females for 6 days, followed by a further 2
females for the 6-12 days post-treatment period. The treatment did not
affect body weight, food consumption or fertility of the males. None
of the examined parameters gave any indication of a mutagenic effect
of iprodione (Hastings et al., 1974).
Iprodione showed no mutagenic action in a rec-assay using two strains
of Bacillus subtilis, reverse mutation tests with and without liner
activation system using E. coli WP2 hcr- and five strains of
Salmonella typhimurium TA and host-mediated assay with S. typimurium G
46 in mice (Shirasu et al., 1976).
Acute Toxicity
TABLE 1. Acute toxicity of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral >2000 Pasquet & Mazuret,
1973
Rat M F Dermal >2500 ibid.
Rat M i.p. 2400 Pasquet &. Mazuret,
1974
F i.p. 1200 ibid.
Mouse M F Oral approx. 4000 Pasquet A Mazuret,
1973
Dog M F Oral >2000 ibid.
Rabbit M F Dermal >1000 ibid.
The signs of toxicity were: loss of reflexes, muscular hypotonia,
sedation and dyspena.
Iprodione did not cause skin or eye-irritation in rabbits.
In the anaesthetized dog iprodione administered at a dose of 300 mg/kg
by the intraduodenal route did not affect the cardiovascular,
respiratory or neurovegetative system (Detaille et al., 1973).
TABLE 2. Acute toxicity of a 50% formulation of iprodione
Species Sex Route LD50 References
mg/kg
Rat M F Oral 8000 Davies & Lowe,
1974
M F Dermal >2000 ibid.
M F 4 h
inhalation 13 mg/l air Pasquet & Mazuret,
1975a
M F Oral 4100 ibid.
Oral 4900 ibid.
M F 4 h inhalation >13/l air Ibid.
Rabbit M F Dermal >2000*) ibid.
*) atoxic
The formulation induced slight irritation in the rabbit eye but had no
irritant effect on the intact or abraded skin of rabbits (Pasquet &
Mazuret, 1975a).
"In the sensitization test with guinea pigs, after 10 applications of
0-3 ml of a 50% iprodione solution, followed 2 weeks later by a
challenge application, no evidence of dermal sensitization was
observed" (Pasquet & Mazuret, 1975b).
Short term studies
Rat
Groups of 15 male and 15 female caesarian originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the diet
for 5 months. No effects were observed on mortality, food consumption,
haematology (as judged by haemoglobin, haematocrit, erythrocyte count,
or total and differential leucocyte count) clinical chemistry (as
judged by BSP, SGOT, SGPT or SAP) or urinalysis. Body weight gain was
slightly reduced (especially in males) at 500 and 1000 ppm. Absolute
(but not relative) heart weight was reduced in males at 500 and 1000
ppm, and absolute kidney weight was reduced at 1000 ppm. In females,
absolute liver and kidney weights were significantly reduced at 500
ppm only. Gross and histopathology were normal at all dose levels. In
a parallel study, dichlozoline, a structurally related compound,
induced cataracts. No such effect was seen with (Ganter et al.,
1973a).
Dog
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 and 7200 ppm for a
period of 3 months. At the top dose level the method of administration
was altered after 6 weeks, to gelatine capsules. The treatment did not
affect mortality. The recorded values of haematological determinations
and urinalyses were within normal limits. As judged by haemoglobin,
haematocrit, reticulocyte erythrocyte count, total and differential
leucocyte count and prothrombine time except for signs of mild anemia
in 1 male and 1 female at 2 months and 1 male at 3 months at the top
dose level. At 7200 ppm a reduction of food consumption was observed,
accompanied by reduced body weight gain. The opththalmosopic
examination of the animals did not reveal any pathological alteration
(Canter and Girard, 1973b). The clinical chemistry determinations
consisted of glucose, urea, cholesterol, bilirubiu, total proteins,
protein electrophoresis, alkaline phosphatase, SGOT, SGPT, LDH, Na+,
C, K+, Cl -, Ca++, P. At 2400 and 7200 ppm a slight increase of SLP was
observed, also a transient increase of SGOT and SGPT after 1 and 2
months of treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, in females at the
dose levels of 2400 ppm and above. At 7200 ppm reduced relative weight
of testes was found, but no histological indication of damage.
The histopathological findings did not reveal any indication of
treatment-related alterations of tissues (Coquet, 1973c).
Long term studies
Mouse
Groups of 60 male and 60 female mice were maintained an a diet
containing the test compound at 0, 200, 500 and 1250 PPM for 18
months. No treatment-related effect on body weight, food consumption
or mortality was found. The recorded values of the haematological
blood chemistry and urinalyses tests performed after 6, 12 and 18
months of the feeding period, were within the physiological range.
Necropsy findings on mice that died during the last 6 months of the
test and on those sacrificed at the termination date showed an
increased number of enlarged lymph nodes in males at 200 ppm. Organ
weight variations occurred sporadically in the various dose groups and
are considered not to be treatment-related. The histopathological
findings failed to reveal abnormal features. The distribution of
neoplastic and non-neoplastic findings did not appear to demonstrate
any significant dose-dependence. The most common tumours were
lymphosarcoma involving the spleen, lymph nodes and thymus (Hastings
and Hullman, 1975).
Rat
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 and 1000 ppm for 24 months. Slight reduction in
body weight gain was observed at 1000 ppm. This was accompanied by
some reduction in food intake. The treatment had no effect on food
consumption, mortality or values of the hematologic, blood chemistry
and urinalyses determinations. Necropsy findings did not reveal any
drug-related gross alteration. Variations in organ weight did not show
a group distribution and seemed not to be related to drug
administration. Histopathology did not indicate a treatment
relationship of neoplastic and non-neoplastic findings. AT 24 months
the most common tumours observed were pituitary adenomas and
adenocarcinoma and fibroadenoma of the mammary glands (Hastings et
al., 1976).
COMMENTS
Iprodione is readily absorbed and rapidly excreted mainly as
metabolites with intact hydantoin-moiety. The compound was not
teratogenic. In a 3-generation study in rats, there was a slight but
statistically significant reduction in postnatal growth at 2000 ppm.
This effect was only marginal at the lower dose of 500 ppm which is
regarded as a no-adverse-effect-level. In a short-term study in dogs
no major effect occurred up to 2400 ppm. Likewise long-term studies in
mice and rats revealed no effects up to 1250 ppm. No ocular
alterations were found in any study.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mice: 1250 mg/kg in the diet, equivalent to 160 mg/kg bw
Rat: 500 mg/kg in the diet, equivalent to 25 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.3 mg/kg bw
USE PATTERN
Iprodione is used as a fungicide against a range of fungus diseases,
including Botrytis in vines, black- and red currants, blackberries,
raspberries and vegetables especially lettuce; Botrytis alii on
onions; Rhizocotonia on seed potatoes; seed borne diseases on sugar
beets (Phoma spp.) and cereals. It is also used against Botrytis
and some other fungus diseases on ornamentals.
The compound is used as a foliar spray on several crops, as a post-
harvest dip for fruit, for dipping seed-potatoes and as a seed-
treatment on sugar beet and cereals.
The product is authorized for use on various crops in France, the
Federal Republic of Germany, Greece, the Netherlands and the United
Kingdom. In several other countries the compound is used or included
in testing programmes and it is in course of registration in many
countries including Australia, New Zealand, Japan, USA, Canada,
Israel, and several European countries.
Most of the recommended uses are summarized in Table 3. The
information may not be complete since the use of the compound is
expanding rapidly and more uses may be expected in the near future.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive data were obtained from supervised trials carried out in
various countries on fruit and vegetables and on some agricultural
crops; they are summarized in Tables 4 to 8 and 10.
Pome and stone fruits (Tables 4 and 5; Rhône Poulenc, 1977a)
Apples
The residue at harvest from pre-harvest treatments at normal
application rates (about 2.25 kg a.i./ha) is about 2 mg/kg. A
combination of such treatments with post-harvest dipping gives rise to
residues of about 6 mg/kg. After repeated applications at about twice
the normal rate residues ranged from 2.9 - 6.5 mg/kg. The residue of
the metabolite RB 30228 (see "Fate of residues") was below 0.15 mg/kg
in these experiments.
Pears
Post-harvest dipping of pears against storage diseases gave rise to
residues of 3.6 - 5 mg/kg.
Peaches
Residues at harvest following applications at the recommended rate
varied between 0.9 and 6 mg/kg. A post-harvest dip adds about 4 mg/kg
to these levels.
Plums
Residues arising from recommended applications varied between 0.6 and
6.8 mg/kg, depending on the pre-harvest intervals observed and the
local conditions. The drying process increased the residue in the
prunes by 0.6 - 1.6 mg/kg.
TABLE 3. Use pattern and recommended pre-harvest intervals of iprodione
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Grapes Botrytis 4 750 Austria 28
" 4 750 France. 15
" 4-5 750 Fed. Rep. of 28
Germany
" 3 3000 Japan 7
" 4 750 Portugal 15
" 4 750 Spain 15
" 4 750 Switzerland
" 4 750 USSR
" 4 750 Yugoslavia
Strawberries Botrytis 4-5 1000 Belgium 15
3 1000 Fed. Rep. of 7
Germany
3 Japan 1
4-5 1000 The Netherlands 14
Pome and Stone fruit
Apples Alternaria about 10 Japan 10
Peaches Monilia 3 Japan 1
Vegetables
Chicory (witloof)
(forcing) Botrytis 1 3 g/m2 Belgium Throughout
Sclerotinia 1 4 g/m2 France the forcing
applied on the top of period
the roots at forcing
TABLE 3. (Continued)
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Cucumbers Botrytis 4 Japan 1
Lettuce Botrytis
Scleotinia 3 750 Belgium 10
(glasshouse)
" 3-4 750 France
" 3 750 Fed. Rep. 21
of Germany (glasshouse)
14
(outdoors)
" 4 750 Japan 14
(also for endive) " 1x 1000-2000 The Netherlands 28
" 2xx 750 The Netherlands 28
Vegetables
Onions Botrytis
Sclerotinia 3 750 Japan 7
cepivorum
Tomatoes Botrytis 4 Japan 1
Alternaria United Kingdom
Agricultural Crops
Beans Sclerotinia 3 Japan 21
Rice Pellicularia 3 Japan 21
TABLE 3. (Continued)
Seed and tuber treatments
Cereal seed
Pre-harvest
Crop Disease Application intervals
No. of treatments Rate Country Days
g a.i./ha
Barley Helminthosporium 1 60 g a.i./100
kg seed
Wheat Tilletia caries 1 60 g a.i./100
kg seed
Garlic Sclerotinia cepivorum 1 300 g a.i./100 France
kg seed
Potatoes Rhizoctonia solani 1 100-150 g/ France
1000 kg tubers
spraying on
tubers
immediately
before
storage
dipping in
spring before
planting
400 g/100 l
Sugar-beet Phoma spp 1 150 g a.i./kg France.
seed enveloped seed
x= one application at planting.
xx= two applications, the first about a week after planting and a second within two weeks after planting
Berry fruits and currents (Table 6; Rhône-Poulenc, 1977b)
The residue levels at harvest after treatments at normal rates and
observing recommended pre-harvest intervals (10-21 days) were
generally at or below 5 mg/kg on blackcurrants, 2 mg/kg on raspberries
and 6 mg/kg on strawberries.
Grapes
The maximum residues of iprodione on grapes at harvest following
treatment according to good agricultural practice (about 750 g
a.i./ha) were in general not higher than 10 mg/kg. The highest levels
in the unfermented must and the win were 4.4 and 6.4 mg/l
respectively. Some results are shown in Table 10 (Rhône-Poulenc,
1977c). See "Fate of residues", "In storage and processing".
Vegetables (Table 7; Rhône-Poulenc, 1977d)
Chicory (witloof)
The residues in the edible sprouts after a normal period of forcing
and one treatment at the recommended rate did not exceed 1 mg/kg. The
residues in the roots were much higher, with a maximum of about 10
mg/kg. The roots are often used as animal feed.
Cucumbers
Residues on cucumbers treated with 3 kg a.i./ha (twice the normal
rate) were between 0.3 and 2.2 mg/kg.
Lettuce
Residues arising from recommended applications on outdoor lettuce (750
g a.i./ha) varied between 1.7 and 2.5 mg/kg after pro-harvest
intervals of 14-21 days. The residues on glasshouse-grown lettuce are
in general much higher. Three applications of the recommended dosage
gave rise to residues of 6.7 mg/kg after a pre-harvest interval of 39
days, and in other experiments maximum levels of 7.2 mg/kg were found
14 days after the last application. Residue levels of the metabolite
RP 30228 were slightly about the limit of determination; other
metabolites were below it. (Metabolites are identified in the section
"Fate of residues."
Onions
The residues on onions 1 day after application did not exceed 0.2
mg/kg.
TABLE 4. Supervised trials of iprodione. Residues in pome and stone fruit (pre-harvest application)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Apples Japan 1975 1 100 5 WP 50% 2.15 1.75
1975 1 100 5 " 2.9 2.25
1975 1 100 5 " 0.38
1975 10 100 5 " 6.5
1975 10 100 5 " (5.75)
1975 10 100 5 " 3.75
1975 10 100 5 " 3.4
1975 10 100 " (1.95)
1975 10 100 5 " 1.7
U.K. 1975 10 100 2.25 " (2.0)
Cherries
Moss Australia 1975 4 1.4 " 8.0
Peaches
Katharine Australia 1976 7 50 1.4 " 5.4
Anne Truly 1976 3 50 0.5 " 1.7
Goldmine 1976 5 50 1.4 " 5.8
Redhaven Canada 1974 4 50 1.0 " 6.5 4.6 4.9
1974 4 50 1.0 " 1.45
Babygold Canada 1974 7 50 1.0 " 2.3 2.0 1.3
1974 6 50 1.0 " 1.8 0.9
Earlired 1974 5 1.0 " 2.5 1.9 2.2 2.2
Redhaven 1974 5 1.0 " 6.1 4.1 3.4 2.9 1.6
Sunhaven 5 1.0 " 7.6 9.0 10.0 8.5
Gifu Japan 1975 7 100 4.0 3.7 2.1
1975 3 100 4.0 " 4.6 2.9
Okayama 1975 2 100 3.0 " 6.3 4.8
1975 3 100 3.0 " 6.8 5.8
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) often application
Crop Country Year No Rate Formulation* 0-2 3-6 7-10 13-14 30-35 40 50
g/100 1
kg/ha (20-22)
Plums
October Australia 1976 4 1.4 " 2.2
purple
Inra 711 France 1973 4 0.5 SC 0.25 0.26
4 1.0 SC 1.4 0.9
1974 0.5 4.15
3 1.0 6.8
2 0.5 0.2
2 1.0 0.6
* SC = Suspension concentrate
WP = Wettable powder
TABLE 5. Supervised trials of iprodione. Residues in pome and stone fruit (post-harvest application)
Application
Rate
Crop Country Year No. g a.i./100 Formulation Residues (mg/kg) after storage period of (days)
dip 1-2 7 11 85 95
Apples
Cox's U.K. 1973 - - 2.0
1 200 5.8
1 200 4.4
Pears
Conference U.K. 1973 1 200 SC 5.0
1973 1 200 4.8
1973 1 200 3.6
1973 1 200 4.7
1973 1 200
1973 1 200 4.0
Peaches Australia - WP50% 1.7
1 50 WP50% 5.3
1 50 WP50% 5.0
TABLE 6. Supervised trials of iprodione. Residues in berry fruits and currants
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Raspberries France 1974 3 0.8 WP 50% 1.1
Heyton 0.5 0.85
Malling
jewel U.K. 1974 5 1.1 " " 1.55
3 1.1 " " 2.0
4 1.1 " " 7.9 5.0 2.8 2.1
Strawberries Belgium 1974 5 0.75 " " 4.9
Sivetta 5 1.0 " " 6.0
Domunil 5 0.75 " " 1.9
5 1.0 " " 2.5
Redcoat Canada 1974 4 1. " " 3.1 1.8 1.4 0.85
Vista 4 1.1 " " 3.5 2.4 1.4 1.0
Redcoat 4 3.45 2.1 1.6 1.0
Redcoat 3 0.9 0.8 0.2
Red Gauntlet France 1973 4 0.5 " " 0.22
0.75 " " 0.44
1.0 " " 0.66
Immigrante 1974 4 0.75 " " 1.75
4 1.0 " " 2.2
Gorella 1974 4 0.75 " " 0.35
4 1.0 0.44
3 0.75 " " 0.5
3 1.0 " " 1.1
Suprême d'Halles 4 0.5 " " 1.2
4 0.75 " " 1.9
4 1.0 " " 2.75
4 0.5 " " 2.5
4 0.75 " " 3.2
4 1 5.6
Senga Segana Fed. Rep. 1974 3 0.9 " " 5.3 2.7 2.3 1.0 0.4
of Gemany 3 1.25 " " 9.1 3.6 3.4 1.9 0.7
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Senga Segana Netherlands 1974 4 0.75 " " 1.1
1974 5 0.75 " " 26.4 14.2 0.67
Red Gauntlet Switzerland 1974 1 0.75 " " 1.0 0.86 0.2 0.1 0.1
Wädenswill 1974 3 0.75 " " 0.2
1974 3 0.75 " " 0.15
Royal
Sovereign U.K. 1974 4 1.1 " " 1.2 1.6 1.3
Strawberries
Cambridge
Favourite (g) 1974 4 1.1 WP 50% 8.0 6.9 4.5 4.7
Cambridge
Favourite (g) 1974 3 1.1 " " 1.7
Cambridge
Favourite 1974 3 1.0 " " 0.7
Cambridge
Favourite 1976 3 1.6 1.1
Cambridge
Favourite 1976 3 0.75 0.9 0.6
Red Gauntlet 1976 1 0.75 0.6
Red Gauntlet 1976 1 1.0 0.9
Red Gauntlet 1974 3 2.2 1.7
Cambridge
Favourite 1974 3 2.2 0.55
Royal
Sovereign 1974 2 1.0 0.3
TABLE 6. (Continued)
Application Residues (mg/kg) at intervals (days) after application
Crop Country Year No. Rate Formulation 0-1 3-4 7-10 13-17 18-23 28-30 33-36
g/100
1 kg/ha
Black
currants
Wellington
Tr. UK 1974 4 1.1 WP 50% 3.9
62 days
Baldwin UK 1974 4 1.1 " " 4.6
62 days
(g) = glasshouse
TABLE 7. Supervised trials of iprodione. Residues in vegetables
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Bean
without pod Japan 1975 1 1 WP 50% 0.05 0.05 0.05
Cucumbers(g) Japan 1975 3 1 0.05 0.05
1975 1 3 WP 50% 1.7 1.0 0.36
1975 3 3 " " 1.6 0.9 0.7
1975 4 3 " " 2.2 1.4 1.2
1975 1 2.5 " " 1.2 1.0 0.24
1975 3 2.5 " " 1.9 1.4 0.3
1975 4 2.5 " " 1.8 1.0 1.0
Lettuce
Val.d'Orge France 1974 6 0.5 " " 0.03
6 0.75 " " 0.03
6 1.0 " " 0.03
6 0.5 EC 200g/l <0.02
6 0.75 " " <0.02
6 1.0 " " 0.04
5 0.5 WP 50% 2.5
5 0.75 " " 1.3
Murkönig Fed. Rep. 3 0.75 " " 15.7 1.8 0.6 0.05 0.05 0.05
Kares of Germany 1976 4 0.75 " " 24.5 9.1 1.7
Reskia 1976 3 0.25 " " 2.6 0.8 0.2
3 0.75 " " 13.2 3.0 1.1
Susan 1976 3 0.5 " " 4.6 1.2 0.4
3 0.75 " " 28 4.3 4.2
Murkönig(g) Fed. Rep. 1976 3 0.25 " " 24 6.9 2.9
of Germany 3 0.75 " " 46 10.5 7.2
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Ravel(g) 1976 3 0.25 " " 26 5.4 2.7
0.75 " " 22 4.8 3.6
Kurume
Br.(g) Japan 1975 2 3 " " 4.6 0.28 0.05
3 3 " " 10.8 0.27 0.1
4 3 " " 7.4 0.3 0.1
Kumamoto(g) Japan 1975 2 1.5 " " 0.48 1.05 0.4
3 1.5 " " 1.9 0.6 1.2
4 1.5 " " 2.25 0.78 1.7
Déciminor(g) Netherlands 1975 2 0.75 " " 8.2 4.9
Vera(g) 1975 2 0.75 " " 9.15 0.05 0.05
Ostinata(g) UK 1974 4 0.56 " " 19 18 14 (24
days)
Val d'Orge 1974 3 0.56 " " 8.8
4 0.56 " " 59 40 41 21
Onions S. Africa 1977 7 50 WP 50% 0.14
0.15
100 " " 0.15
0.24
Sweet
Peppers(g) U.K. 1975 6 4.3
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. 1kg/ha Formulation 0-1 3-4 5-7 10-14 21-22 28-30 31-35
g/100
Tomatoes
Koibuchi (g) Japan 1975 1 2.5 " " 1.25 1.4 1.2 0.8
Koibuchi (g) 1975 3 2.5 " " 5.3 3.4 3.0 2.4
Koibuchi (g) 1975 4 2.5 " " 5.6 5.4 4.3 3.5
1975 3 3.0 " " 2.1 3.3 1.8 2.9
4 3.0 " " 4.6 3.6 4.1 2.8
5 3.0 " " 4.4 4.0 3.8 3.7
Eurocross(g) U.K. 1974 5 50 " " 2.7
6 50 " " 3.8
7 50 " " 4.9
8 50 " " 4.2 2.3
Sonato(g) U.K. 1974 4 50 " " 1.65
5 50 " " 2.3
6 50 " " 2.8
7 50 " " 3.7
8 50 " " 4.2 5.8
5 50 " " 3.1 2.5 2.7
1 1 " " 0.64
(18
days)
TABLE 7. (Continued)
Application Residues (mg/kg) at interval (days) after application
Rate
Crop Country Year No. a.i. Formulation 30-40 40 44-48 59 70 93-104 162
g/m2 S R S R S R S R S R S R S R
Chicory Belgium 1975 1 3 " " 0.41 1.49
1 6 " " 0.36 3.10
3 " " 0.09 2.7
6 " " 0.32 4.4
3 " " 0.77 6.2
6 " " 0.59 10.0
3 " " 0.55 2.7
6 1.0 3.7
France 1974 1 4 " " 0.6
8 " " 1.0 0.07
4 " " 0.25
8 " "
TABLE 7B
Crop Country Year No. Rate Formulation 30-40 40 44-48 59 70 93-104 162
g/100 1g/1000
dip kg S R S R S R S R S R S R S R
Potatoes
pre-plant France 1976 1 200 WP 50% n.d.
treatment 300 " " n.d.
50 " " 0.01
100 " " 0.01
150 " " 0.02
200 " " 3.0 5.0
50 " " 25 25
100 " " 58 67
150 " " 120 140
foliar
spray S.
Africa 1976 5 50 " " <0.02
5 100 " " <0.02
3 50 " " <0.02
3 100 <0.02
WP = wettable povider; EC = emulsion concentrate;
(g) = glasshouse; R = roots (chicory) or tubers (potato); S=sprouts
Peppers and tomatoes
Following applications at normal rates (50 g a.i./100 l) residues of
4.5 - 5 mg/kg were found at harvest after pre-harvest intervals of 3.6
days.
Beans (dry)
After treatment at a dosage rate of 1 kg/a.i./ha, residues in the dry
beans were very low (0.05 - 0.2 mg/kg).
Cereal crops (Table 8; Rhône-Poulenc, 1977e)
Wheat
Two applications at normal rates (1 kg a.i./ha) with a pre-harvest
interval of 73 days did not give rise to measurable residues in the
kernels.
Rice
After treatment during the growing season with relatively high dosages
(1.2 kg a.i./ha), residues of 0.1 - 2.1 mg/kg were found de-husked,
unpolished rise 21 days after the last treatment.
FATE OF RESIDUES
In plants
The fate of iprodione in plants and soil was studied with unlabelled
and 14C-phenyl-labelled products. It was found that when applied to
the leaf surface, iprodione does not appreciably penetrate through the
skin. The residues on the skin had a half-life of 30-60 days, being
slowly converted to
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydentoin, RP 30228), which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including 1-carbamoyl-3-(3.5-
dichlorophenyl) hydantoin (RD 32490) (Rhône-Poulenc, 1973).
Wheat and strawberry plants grown on soil treated with iprodione
(Rhône-Poulenc, 1977f) took up small amounts of the compound (in wheat
0.7-1.3% of the amount applied to the soil surface), which was mainly
found in the leaves and stems (95-99% of the extractable residue).
Within the plant the parent compound was converted to RP 30228, small
amounts of RP 32490 and some more polar unidentified products.
The organosoluble residue in strawberry plants 32 days after a foliar
application at a rate equivalent to 1 kg a.i./ha consisted of 61%
unchanged parent compound and 16% RP 30228. 55 days after foliar
treatment at 2 kg a.i./ha 69% of the residue was iprodione, 7% RP
30228 and 5% RP 32490.
TABLE 8. Supervised trials of iprodione. Residues in cereal crops.
Application Residues (mg/kg) at intervals (days) after application
Rate
Crop Country Year No. Formulation 14-15 21-22 28-30 73 81
g/100 1 kg/ha
Rice Japan 1975 1 100 1.2 WP 50%
grain 0.1 0.1 0.1
straw 16 15 10.3
1975 1 100 1.2 WP 50%
grain 0.3 0.4 0.3
straw 32 12.5 10.5
3 100 1.2 WP 50%
grain 2.1
straw 45
3 100 1.2 WP 50%
grain 1.4
straw 32
3 100 1.2
grain 0.8
straw 49
4 100 1.2 1.8
grain 43
straw
Wheat
Maris
Nimrod UK 1 34 1.0 <0.05
2 34 1.0 <0.05
Jos Cumbier 1 34 1.0 <0.05
2 34 1.0 <0.05
TABLE 9. Nature and Distribution of radio-activity in wheat grown in soil treated with 10 kg/ha. 14C-iprodione
% of total 14C in each plant part as
Unidentified Total 14C
Days after Organo- Water expressed as
treatment Plant part Iprodione RP 30228 RP 32490 soluble soluble Bound iprodione mg/kg
16 roots 49 14 n.d. 21 0 17 20
leaves and 66 6.4 4.4 21 1.5 1.7 20
stem
44 roots 16 15 n.d. 13 1.1 55 31
leaves and 48 9.5 18 20 0.95 4.2 20
stem
89 roots 2.9 8.1 0.8 9.2 0 79 238
leaves and 26 17 14 32 0.22 11 36.7
stem
ears 9.1 3.1 0.1 24 0 56 32
kernels n.d. n. d. n.d. 72 0 28 2.5
Wheat plants were grown on soil treated with excessive dosages of
14C-labelled iprodione and the distribution of the residue in the
plant was studied after 16, 44 and 89 days. The nature and
distribution of the recovered radio-activity is shown in Table 9
(Rhône-Poulenc, 1977f).
The plant and soil metabolites of iprodione have been identified by
various methods including TLC, GLC, and colorimetric analysis and the
degradation pathway shown in Figure 1 deduced.
In soil
The degradation of residues in soil follows a similar pattern. The
half-life at initial levels of 2 and 5 mg/kg is about 30 days. After
12 months incubation under aerobic conditions at 23-25°C, no more than
3% of the remaining radio-activity was in the form of unchanged
iprodione. Conversion to metabolite RP 30228 proceeded rapidly. The
concentration of RP 30228 reached a maximum (45-55% of the
radio-activity still present) after 80-100 days and then decreased
(Rhône-Poulenc, 1976).
In leaching experiments with radio-labelled iprodione it was shown
that the parent compound was only slightly mobile, remaining in the
0-15 cm layer. The metabolite RP 30228 is less soluble in water than
the parent compound (0.5 mg/l compared to 13 mg/l) and virtually all
remained in the 0-5 cm layer (Rhône-Poulenc 1973, 1976).
In storage and processing
Extensive data were obtained from various countries on the fate of
residues of iprodione during wine making and on the effect of residues
on the fermentation. When grapes containing about 5 mg/kg were used
for wine making, no influence on the fermentation process was found.
This was confirmed in laboratory experiments in which CO2 evolution
and the proportion of viable cells (those susceptible to actidione)
were measured.
In a trial in which the grapes contained 2-10 mg/kg iprodione, the
fermentation process was slightly retarded. It is unlikely that this
effect would be observed under practical conditions of wine making.
During the wine making, iprodione remains fairly stable, but a
considerable part of the residue will be eliminated with the solids
(mavc) during clarification. The residues in wine are generally about
15-25% of those in the grapes. No residues of iprodione were found in
alcohol obtained after distilling wine (Rhône-Poulenc, 1977c; Barre et
al., 1976). Some results are shown in Table 10.
TABLE 10. Residues of iprodione at various stages of vinification
Iprodione, mg/kg in
Country Year Grapes Must Wine Finished
Unfermented Fermented Racked Clarified Wine
France 1974 4.9 2.7 0.98 0.75
3.0 1.7 1.0 0.71-0.84
1975 6.1 2.75 0.8
7.5 4.4 1.45
1975 2.2 1.5 0.34
5.4 0.6
South 1976 1.0 1.3 0.9
Africa 2.6 0.5 1.5
Switzerland 2.3 1.9 0.7
1973 1.7 1.4 0.5
2.9 1.9 0.4
 METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables. These are suitable or can be adapted for regulatory
purposes. They have been adapted for residue analysis In must and
wine. The limit of determination on most fruits and vegetables is
about 0.01-0.02 mg/kg. Some commodities of plant origin, e.g. prunes
and mare, require a more elaborate clean-up owing to the higher
proportion of interfering plant constituents. The limit of
determination in these commodities is about 0.05-0.1 mg/kg.
No loss of residues was found during storage for more than 1 year at
temperatures of -18°C.(Rhône-Poulenc, 1975a,b).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following maximum residue limits were reported to the Meeting as
established or under consideration. They refer to iprodione, excluding
metabolites.
Country Commodity Maximum residue
limit, mg/kg
Australia Apricots, cherries,
plumes peaches 10
France Grapes 10
Fed. Rep.
of Germany Grapes 10
The Netherlands Lettuce 5
Strawberries 2
New Zealand Apricots, berry fruits,
cherries, grapes,
peaches, plums 10
Switzerland Grapes 7
APPRAISAL
Iprodione is used against a relatively broad range of fungus diseases,
on a wide range of fruits and vegetables.
Its use is authorized, or is in course of registration, for various
crops in a number of countries. It is marketed in the form of a
wettable powder, a suspension concentrate and an emulsifiable
concentrate. The products are mainly used as a spray on the aerial
parts of growing crops, for post-harvest dipping of fruit as a dip for
seed potatoes and as a seed treatment. Application rates vary
according to the crop/disease situation and regional conditions.
Residue data were obtained from supervised trials carried out in
various countries with different climatic conditions. Studies with
unlabelled and 14C-phenyl-labelled products showed that iprodione
does not appreciably penetrate through the plant cuticle. The residue
on the surface of the plants had a half-life of about 30-60 days. It
was converted into
1-(3,5-dichlorophenyloarbamoyl)-3-isopropyl-2,4-dioxoimida-zolidine,
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydantoin, RP 30228, which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including
1-carbamoyl-3-(3,5-dichlorophenyl)hyclantoin (RP 32490).
Wheat and strawberry plants grown on soil treated with iprodione took
up small amounts of the compound (equivalent to 0.7-1.3% of the total
applied to the soil surface). Within the plant the parent compound was
converted into metabolite RP 30228, Small amounts of RP 32490 and some
more polar unidentified products. The degradation of residues in soil
follows the same pattern. The half-life at initial levels of 2 and 5
mg/kg is about 30 days. After 12 months incubation under aerobic
conditions at 23-25°C no more than 3% of the remaining residue is in
the form of unchanged iprodione. The parent compound is only slightly
mobile, remaining mainly in the upper 0-15 cm layer. The metabolits RP
30228 is less soluble in water than the parent compound (0.5 mg/l
compared to 13 mg/l) and virtually all remained in the 0-5 cm layer.
Residues in wine were approximately 15-25% of those on the harvested
grapes.
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables, must and wine, which are suitable or can be adapted for
regulatory purposes. The limit of determination is generally about
0.01-0.02 mg/kg. No loss of residue was found over more than 1 year at
-18°C.
RECOMMENDATIONS
The following maximum residue limits for iprodione on various fruits
and vegetables are recommended. They refer to iprodione, excluding any
metabolites.
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Apples, pears 10 10-14 +
Grapes 10 14-21
Lettuce 10 14-21(281)
Peaches 10 10-14 +
Plums 7 14
Strawberries 7 14
Blackcurrants 5 10-21
Cucumbers 5 3-6
Sweet peppers 5 3-6
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Raspberries 5 10-21
Tomatoes 5 3-7
Rice (hulled, unpolished) 3 21
Chicory (witloof) sprouts 1 throughout forcing
Beans, dry 0.2 14-21
Garlic, onions 0.1 1
1 Glasshouse use.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on the fate of iprodione residues in milk, meat and
eggs when food wastes containing iprodione residues are used as
components of animal feeds.
2. Residue data on grain and straw from supervised trials on cereal
crops treated according to good agricultural practice.
3. Further information about the effects of processing and cooking on
iprodione residues in a range of commodities.
REFERENCES
Barre, P., R. Cordonnier, A. Dugal, L. Lacroixy M. Laurent et M. Buys
(1976) Etude, dens le raisin et le vin, des résidus d'un fongicide
antibotrytis, le 26019 R.P. et leur effects our la fermentation
alcoholique. Progrés Agricole et Viticole 93 (11) 2-8.
Coquet, B. (1973a) Recherche du pouvoir teratogéne chez le rat OFA par
voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogéne chez le lapin pax
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherches et essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1977c) Essai de toxocité chronique (3 mois) chez le chien
par voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onions, No. 731008
Coquet B. (1976) Influence du produit 26019 R.P. sur la reproduction
du rat (Essai sur 3 générations). Unpublished report from Institut
Franc. de Recherche et Essais Biologique, No. 760435 submitted by
Rhône-Poulenc.
Davies, V.A. and Lowe C.Y. (1974) Fungicides: Formulation LPA 2043:
Acute Oral and Dermal Toxicity in the Rat. Unpublished report from May
and Baker Ltd., England, No. RG 2037.
Detaille, J.Y., Pasquet, J., Mazuret, A. and Mondot, S. (1973)
Antifongique 26019 RJ., Phaxmacologie générale chez le chien
anesthésié au pento barbital. Unpublished report des Laboratoires do
Recherches de la Société des Usines Chimiques Rhône-Poulenc, subm.
by Rhône-Poulenc.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. and Myan, J. (1973a) Produit No. 26019 R.P. -
Toxicété à terms (5 mois) chez le rat par voie orale, on comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R.P.). Unpublished
report from Laboratoires do Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 171249 submitted by
Rhône-Poulenc.
Ganter, P. and Girard, P. (1973b) Produit 26019 R.P. - Examen
histologique de la toxicité oculaire du 26019 R.P. chez le chien aprés
un traitement do 3 mois. Unpublished report from Laboratoires de
Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131, submitted by Rhône-Poulenc.
Hastings, S.E., Huffmann, K.W and Gallo, M.A. (1974) Dominant Lethal
Mutagenicity in Mice. Unpublished report from Boss and Clark, Division
of Rhodia Inc., No. SEH 74:69.
Hastings, S.E., Huffman, K.W. (1975) Chronic Toxicologic and
Carcinogenic Study with R.P. 26019 in Mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:113, submitted by
Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C. and Kiggins, E.M. (1976) Chronic
Toxicologic and Carcinogenic Study with R.P. 26019 in Rats.
Unpublished report from Rhodia, Inc., Hess and Clerk Division, No.
76:57, submitted by Rhône-Poulenc.
Laurent, M. and Buys, M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. and Chabassol, Y. (1976)
Etude du métabolism chez le rat á l'aide du produit marqué au 14C.
Unpublished report from Rhône-Poulenc, Direction des Recherches et du
Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-Seine,
C.N.G. -An No. 18548, submitted by Rhône-Poulenc.
Pasquet, J. and Mazuret, A. (1974) Product 26019 R.P. Toxicité aigue
chez le rat par voie intrapéritonéale. Unpublished report from
Laboratoires de Recherches de la Socété des Usines Chimiques
Rhône-Poulenc, No. 174621 submitted by Rhône-Poulenc.
Pasquet J. and Nazuret, A. (1975a) Glycophene (26019 R.P.) -
Formulation LFA 2043 (wettable powder at 50% of glyoophene) Acute
toxicity and Dermal and Ocular tolerance. Unpublished report from
Rhône-Poulenc. No. 18182 submitted by Rhône-Poulenc.
Pasquet J. and Mazuret, A. (1975b) Glycophene (26019 R.P.). Etude de
l'action sensibilisante outanée chez le cobaye. Unpublished report
from Centre Nicolas Grillet, France, No. 18081, submitted by
Rhône-Poulenc.
Pasquet, J., Shirasu, Y., Moriya, M. and Katto, K. (1976) Mutagenicity
Testing on Glycophene in Microbial Systems. Unpublished report from
Institute of Environmental Toxicology, Toxicology Division, submitted
by Rhône-Poulenc.
Rhône-Poulenc (1977a) Dosages de residue dans les fruits a noyau
(prune, Pêche, cerise) at a pepins (poire, pomme). Unpublished report
R.P./R.D./C.N.G. No. 19 157.
Rhône-Poulenc (1977b) Dosage de residue dans les petits fruits
(fraises - framboises - cassis). Unpublished report R.P/R.D./C.N.G.
No. 19 193.
Rhône-Poulenc (1977c) 26019 R.P. Residus dans les raisins, ls mout, le
vin et l'alcohol. Unpublished report R.P./R.D./C.N.G. No. 19 177.
Rhône-Poulenc (1977d) Rovral-26019 RP. Dosages de residue dans les
legumes. Unpublished report R.P./R.D./C.N.G. No. 19 191.
Rhône-Poulenc (1977e) Rovral-26019 RP. Dosages de residue dans les
cereales (blé et riz). Unpublished report R.P/R.D./C.N.G. No. 19 170.
Rhône-Poulenc (1975a) 26019 Methods de dosage des residue dans les
vegetaux et derives. Unpublished report R.P./R.D/C.N.G. -An. No. 2736.
Rhône-Poulenc (1975b) 26019, Discussion at évaluation des performances
de la méthode de dosage des résidus. Unpublished report
R.P./R.D./C.N.G. -An No. 2658.
Rhône-Poulenc (1973) Produit No. 26019 R.P. Metabolisms dans les
vegetaux et dans le sol. Unpublished report S.W. C.R.P. -D.S.Ph.D.S.
An Nord No. 17 374.
Rhône-Poulenc (1977f) Dégradation du 26019 R.P. absorbé par les
racines ou déposé sur les feuilles des vegetaux (fraisiers et blé) a
l'aide de 26019 R.P. marqué au 14C. Unpublished report
R.P./R.D./C.N.G. et C.N.G. An No. 19201.
Rhône-Poulenc (1976) Degradation of RP 26019 in the soil. Treatments
with 2 and 5 ppm 14C labelled product. Unpublished report
R.P./R.D./C.N.G. No. 18785.
METHODS OF RESIDUE ANALYSIS
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables. These are suitable or can be adapted for regulatory
purposes. They have been adapted for residue analysis In must and
wine. The limit of determination on most fruits and vegetables is
about 0.01-0.02 mg/kg. Some commodities of plant origin, e.g. prunes
and mare, require a more elaborate clean-up owing to the higher
proportion of interfering plant constituents. The limit of
determination in these commodities is about 0.05-0.1 mg/kg.
No loss of residues was found during storage for more than 1 year at
temperatures of -18°C.(Rhône-Poulenc, 1975a,b).
NATIONAL TOLERANCES REPORTED TO THE MEETING
The following maximum residue limits were reported to the Meeting as
established or under consideration. They refer to iprodione, excluding
metabolites.
Country Commodity Maximum residue
limit, mg/kg
Australia Apricots, cherries,
plumes peaches 10
France Grapes 10
Fed. Rep.
of Germany Grapes 10
The Netherlands Lettuce 5
Strawberries 2
New Zealand Apricots, berry fruits,
cherries, grapes,
peaches, plums 10
Switzerland Grapes 7
APPRAISAL
Iprodione is used against a relatively broad range of fungus diseases,
on a wide range of fruits and vegetables.
Its use is authorized, or is in course of registration, for various
crops in a number of countries. It is marketed in the form of a
wettable powder, a suspension concentrate and an emulsifiable
concentrate. The products are mainly used as a spray on the aerial
parts of growing crops, for post-harvest dipping of fruit as a dip for
seed potatoes and as a seed treatment. Application rates vary
according to the crop/disease situation and regional conditions.
Residue data were obtained from supervised trials carried out in
various countries with different climatic conditions. Studies with
unlabelled and 14C-phenyl-labelled products showed that iprodione
does not appreciably penetrate through the plant cuticle. The residue
on the surface of the plants had a half-life of about 30-60 days. It
was converted into
1-(3,5-dichlorophenyloarbamoyl)-3-isopropyl-2,4-dioxoimida-zolidine,
1-(3,5-dichlorophenylcarbamoyl)-3-isopropylhydantoin, RP 30228, which
represented up to 35% of the remaining residue. 2-5% of this residue
consisted of minor degradation products, including
1-carbamoyl-3-(3,5-dichlorophenyl)hyclantoin (RP 32490).
Wheat and strawberry plants grown on soil treated with iprodione took
up small amounts of the compound (equivalent to 0.7-1.3% of the total
applied to the soil surface). Within the plant the parent compound was
converted into metabolite RP 30228, Small amounts of RP 32490 and some
more polar unidentified products. The degradation of residues in soil
follows the same pattern. The half-life at initial levels of 2 and 5
mg/kg is about 30 days. After 12 months incubation under aerobic
conditions at 23-25°C no more than 3% of the remaining residue is in
the form of unchanged iprodione. The parent compound is only slightly
mobile, remaining mainly in the upper 0-15 cm layer. The metabolits RP
30228 is less soluble in water than the parent compound (0.5 mg/l
compared to 13 mg/l) and virtually all remained in the 0-5 cm layer.
Residues in wine were approximately 15-25% of those on the harvested
grapes.
Gas-chromatographic methods using electron capture detectors have been
developed for the analysis of residues in several fruits and
vegetables, must and wine, which are suitable or can be adapted for
regulatory purposes. The limit of determination is generally about
0.01-0.02 mg/kg. No loss of residue was found over more than 1 year at
-18°C.
RECOMMENDATIONS
The following maximum residue limits for iprodione on various fruits
and vegetables are recommended. They refer to iprodione, excluding any
metabolites.
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Apples, pears 10 10-14 +
Grapes 10 14-21
Lettuce 10 14-21(281)
Peaches 10 10-14 +
Plums 7 14
Strawberries 7 14
Blackcurrants 5 10-21
Cucumbers 5 3-6
Sweet peppers 5 3-6
Commodity Limit, mg/kg pre-harvest interval
on which
recommendations are based post-harvest
treatment
Raspberries 5 10-21
Tomatoes 5 3-7
Rice (hulled, unpolished) 3 21
Chicory (witloof) sprouts 1 throughout forcing
Beans, dry 0.2 14-21
Garlic, onions 0.1 1
1 Glasshouse use.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Information on the fate of iprodione residues in milk, meat and
eggs when food wastes containing iprodione residues are used as
components of animal feeds.
2. Residue data on grain and straw from supervised trials on cereal
crops treated according to good agricultural practice.
3. Further information about the effects of processing and cooking on
iprodione residues in a range of commodities.
REFERENCES
Barre, P., R. Cordonnier, A. Dugal, L. Lacroixy M. Laurent et M. Buys
(1976) Etude, dens le raisin et le vin, des résidus d'un fongicide
antibotrytis, le 26019 R.P. et leur effects our la fermentation
alcoholique. Progrés Agricole et Viticole 93 (11) 2-8.
Coquet, B. (1973a) Recherche du pouvoir teratogéne chez le rat OFA par
voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogéne chez le lapin pax
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherches et essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1977c) Essai de toxocité chronique (3 mois) chez le chien
par voie orals du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Elevage des Onions, No. 731008
Coquet B. (1976) Influence du produit 26019 R.P. sur la reproduction
du rat (Essai sur 3 générations). Unpublished report from Institut
Franc. de Recherche et Essais Biologique, No. 760435 submitted by
Rhône-Poulenc.
Davies, V.A. and Lowe C.Y. (1974) Fungicides: Formulation LPA 2043:
Acute Oral and Dermal Toxicity in the Rat. Unpublished report from May
and Baker Ltd., England, No. RG 2037.
Detaille, J.Y., Pasquet, J., Mazuret, A. and Mondot, S. (1973)
Antifongique 26019 RJ., Phaxmacologie générale chez le chien
anesthésié au pento barbital. Unpublished report des Laboratoires do
Recherches de la Société des Usines Chimiques Rhône-Poulenc, subm.
by Rhône-Poulenc.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. and Myan, J. (1973a) Produit No. 26019 R.P. -
Toxicété à terms (5 mois) chez le rat par voie orale, on comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R.P.). Unpublished
report from Laboratoires do Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 171249 submitted by
Rhône-Poulenc.
Ganter, P. and Girard, P. (1973b) Produit 26019 R.P. - Examen
histologique de la toxicité oculaire du 26019 R.P. chez le chien aprés
un traitement do 3 mois. Unpublished report from Laboratoires de
Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131, submitted by Rhône-Poulenc.
Hastings, S.E., Huffmann, K.W and Gallo, M.A. (1974) Dominant Lethal
Mutagenicity in Mice. Unpublished report from Boss and Clark, Division
of Rhodia Inc., No. SEH 74:69.
Hastings, S.E., Huffman, K.W. (1975) Chronic Toxicologic and
Carcinogenic Study with R.P. 26019 in Mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:113, submitted by
Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C. and Kiggins, E.M. (1976) Chronic
Toxicologic and Carcinogenic Study with R.P. 26019 in Rats.
Unpublished report from Rhodia, Inc., Hess and Clerk Division, No.
76:57, submitted by Rhône-Poulenc.
Laurent, M. and Buys, M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. and Chabassol, Y. (1976)
Etude du métabolism chez le rat á l'aide du produit marqué au 14C.
Unpublished report from Rhône-Poulenc, Direction des Recherches et du
Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-Seine,
C.N.G. -An No. 18548, submitted by Rhône-Poulenc.
Pasquet, J. and Mazuret, A. (1974) Product 26019 R.P. Toxicité aigue
chez le rat par voie intrapéritonéale. Unpublished report from
Laboratoires de Recherches de la Socété des Usines Chimiques
Rhône-Poulenc, No. 174621 submitted by Rhône-Poulenc.
Pasquet J. and Nazuret, A. (1975a) Glycophene (26019 R.P.) -
Formulation LFA 2043 (wettable powder at 50% of glyoophene) Acute
toxicity and Dermal and Ocular tolerance. Unpublished report from
Rhône-Poulenc. No. 18182 submitted by Rhône-Poulenc.
Pasquet J. and Mazuret, A. (1975b) Glycophene (26019 R.P.). Etude de
l'action sensibilisante outanée chez le cobaye. Unpublished report
from Centre Nicolas Grillet, France, No. 18081, submitted by
Rhône-Poulenc.
Pasquet, J., Shirasu, Y., Moriya, M. and Katto, K. (1976) Mutagenicity
Testing on Glycophene in Microbial Systems. Unpublished report from
Institute of Environmental Toxicology, Toxicology Division, submitted
by Rhône-Poulenc.
Rhône-Poulenc (1977a) Dosages de residue dans les fruits a noyau
(prune, Pêche, cerise) at a pepins (poire, pomme). Unpublished report
R.P./R.D./C.N.G. No. 19 157.
Rhône-Poulenc (1977b) Dosage de residue dans les petits fruits
(fraises - framboises - cassis). Unpublished report R.P/R.D./C.N.G.
No. 19 193.
Rhône-Poulenc (1977c) 26019 R.P. Residus dans les raisins, ls mout, le
vin et l'alcohol. Unpublished report R.P./R.D./C.N.G. No. 19 177.
Rhône-Poulenc (1977d) Rovral-26019 RP. Dosages de residue dans les
legumes. Unpublished report R.P./R.D./C.N.G. No. 19 191.
Rhône-Poulenc (1977e) Rovral-26019 RP. Dosages de residue dans les
cereales (blé et riz). Unpublished report R.P/R.D./C.N.G. No. 19 170.
Rhône-Poulenc (1975a) 26019 Methods de dosage des residue dans les
vegetaux et derives. Unpublished report R.P./R.D/C.N.G. -An. No. 2736.
Rhône-Poulenc (1975b) 26019, Discussion at évaluation des performances
de la méthode de dosage des résidus. Unpublished report
R.P./R.D./C.N.G. -An No. 2658.
Rhône-Poulenc (1973) Produit No. 26019 R.P. Metabolisms dans les
vegetaux et dans le sol. Unpublished report S.W. C.R.P. -D.S.Ph.D.S.
An Nord No. 17 374.
Rhône-Poulenc (1977f) Dégradation du 26019 R.P. absorbé par les
racines ou déposé sur les feuilles des vegetaux (fraisiers et blé) a
l'aide de 26019 R.P. marqué au 14C. Unpublished report
R.P./R.D./C.N.G. et C.N.G. An No. 19201.
Rhône-Poulenc (1976) Degradation of RP 26019 in the soil. Treatments
with 2 and 5 ppm 14C labelled product. Unpublished report
R.P./R.D./C.N.G. No. 18785.
