
IPRODIONE
First draft prepared by A. Clevenger
Office of Pesticide Programs
US Environmental Protection Agency
Washington DC USA
EXPLANATION
Iprodione was previously evaluated by the Joint Meeting in 1977
(Annex 1, reference 28) when an ADI of 0-0.3 mg/kg bw was
established. The data reviewed in 1977 consisted of studies of
pharmacokinetics, short-term toxicity in rats and dogs, long-term
toxicity in mice and rats, and special studies on developmental
toxicity, reproduction, and mutagenicity. The present review
evaluates studies made available since the 1977 review. Relevant
portions of the previous monograph have been incorporated into this
toxicological monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
The pharmacokinetics of iprodione following oral administration
was studied using groups (5/sex) of Charles River CD rats. One group
received a single low dose of 50 mg 14C-iprodione/kg bw (uniformly
labelled phenyl ring). A second group received a single high dose of
900 mg 14C-iprodione/kg bw. A third group received multiple doses
(14 daily doses) of 50 mg iprodione/kg bw/day of unlabelled (99.3%
purity) followed by a single dose of 14C-iprodione. Blood, urine,
and faeces were collected for 7 days after treatment at which time
rats were sacrificed and tissues collected.
Following a single low dose, 90% of the administered dose was
eliminated within 4 days primarily in urine. Urinary excretion
accounted for 67% of the administered dose in males and 53% in
females whereas faecal excretion accounted for 25% in males and 39%
in females. Males may have absorbed the dose to a greater extent
than females based on the greater urinary excretion and the larger
area-under-the-curve (blood concentration-time curve) for males.
Peak blood concentrations were reached within 2-4 h. After 7 days,
tissue residues in both sexes were less than 0.7 ppm in any one
tissue and collectively accounted for no more than 0.3% of the
administered dose. Tissue concentrations were highest in the liver
and intestines. The elimination half-life was estimated to be 9 h
for males and 7 h for females based on the blood-concentration time
curves.
Following a single high dose, 90% of the administered dose was
eliminated within 2 days in males and 3 days in females. The single
high dose appeared to be absorbed to a lesser extent than the single
low dose based on the greater faecal excretion of parent compound
following the high dose compared to predominantly urinary excretion
of the single low dose. Faecal excretion accounted for 56% of the
administered high dose in males and 52% in females whereas urinary
excretion accounted for 43% in male and 46% in females. The peak
blood concentration was reached within 6 h and was about three times
the peak concentration following the single low dose. After 7 days,
tissue concentrations were less than 10 ppm in any one tissue and
collectively accounted for no more than 0.2% of the administered
dose. The elimination half-life of the single high dose was about
twice that for the single low dose. The high dose elimination half-
lives were 20 h for males and 13 h for females.
Following multiple low dose exposures, 90% of the administered
dose was eliminated within 3 days, primarily in urine. The excretion
pattern was similar to that for the single low dose. Urinary
excretion accounted for 75% of the administered dose in males and
65% in females whereas faecal excretion accounted for 20% in male
and 28% in females. Tissue concentrations were <1 ppm in any one
tissue (Hallifax, 1989).
The dermal absorption of iprodione was 0.65% in 24 h for male
and female rats. Groups of 3 male and 3 female hairless strain rats
received a dose of 185 mg 14C-iprodione /kg bw of (uniformly
labeled phenyl ring) applied to a 12 cm2 area of the back for 24
h. Ninety to 93% of the dose was recovered from the skin. Of the
0.65% absorbed, 0.45% of the radiolabel was found in urine and
faeces and the remaining 0.15% was present mainly in the carcass
(Laurent et al., 1983).
Biotransformation
Rats
Following a single application of an oral dose of 200 mg
iprodione/kg bw, 26% of the administered dose was eliminated in the
urine and 59% in the faeces within 24 hours after application. The
major portion of the dose excreted in the faeces was the parent
compound, whereas only 3% of the administered dose was eliminated
unchanged in the urine. Besides the principal urinary metabolites
with a degraded isopropylcarbamoyl group (about 11% of the dose
administered), metabolites with intact hydroxylated or non-
hydroxylated aromatic rings were excreted in the urine. The isomer
of the parent compound accounted for a small proportion of the
metabolites. Residues in the principal organs and tissues did not
exceed 1.5% of the administered dose in rats sacrificed 4 days after
dosage (Laurent and Buys, 1974).
In a similar study rats were dosed once with 100 mg/kg of 14C
aromatic ring labelled iprodione; 96 hours after administration 62%
of the applied dose was eliminated via the urine and 36% via the
faeces. About 16% was excreted as the parent compound in the faeces;
the remainig radioactivity was mainly in the urine, in the form of
the desisopropylated derivative (about 20% of the dose) and the N-
(3,5-dichloro-4-hydroxyphenylbiuret) (approx. 13%). Tissues sampled
4 days after dosage contained about 1% of the administered dose
(Laurent, et al, 1976).
The metabolism of iprodione was studied in Charles River CD
rats by analysing the urine and faeces obtained in the
pharmacokinetics study described above (Hallifax, 1989). Metabolite
identity was based on comparison of retention times with reference
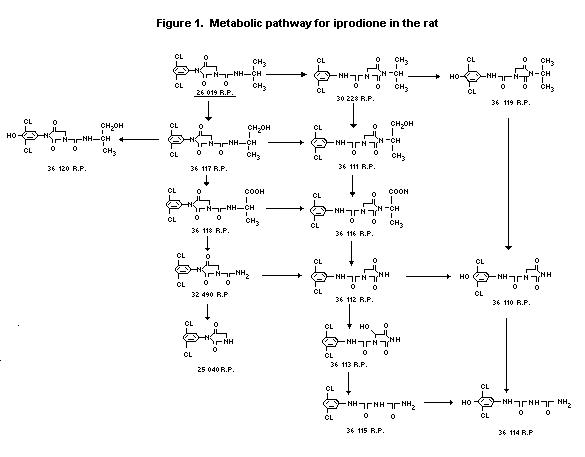
compounds by TLC and HPLC. A proposed metabolism scheme is shown in
Figure 1. Iprodione was extensively metabolized by both males and
females following single low or high doses or repeated low doses.
Males and females of all groups eliminated in the urine relatively
large amounts of a dealkylated metabolite corresponding to reference
compound 32490 RP. The urine of all male and high dose females also
contained large amounts of 36114 RP, a hydantoin ring-opened
metabolite. Two major urinary metabolites, each representing 10-15%
of the administered dose, were unidentified. Females in all groups
eliminated a larger portion of the urinary radiolabel as the parent
compound than males. Most of the extracted radiolabel in the faeces
was the unchanged parent compound with small amounts of the same
metabolites identified in the urine. The faeces of the high dose
group contained relatively more parent compound than the single or
repeated low dose groups, which is consistent with lower absorption
of the high dose (Hallifax, 1989).
Iprodione and three metabolites were identified in hairless
rats (3/sex) treated dermally with a dose (185 mg/kg bw) of 14C-
iprodione for 24 h. Iprodione was found unchanged in the urine,
faeces, and intestines. Metabolite 30228 RP was found in the urine
of males, 32490 was detected in the urine of males and females, and
36114 RP was detected in the urine, faeces, and intestines of males
and females (Laurent et al., 1983).
Humans
Small amounts of the metabolite 32490 RP were detected in the
urine of 3 of 6 workers involved in mixing and diluting the
formulation ROVRAL WP(R) (50% w/w iprodione). Unchanged iprodione
was not detected in the urine (Jones, 1983).
Toxicological studies
Acute toxicity studies
The acute toxicity of iprodione is summarized in Table 1.
Common signs of toxicity include decreased motor activity, ataxia,
tearing, and paralysis. Tonic convulsions were noted in one study in
mice.
 Short-term toxicity studies
Mice
Groups (10-20/sex/dose) of CF-1 mice received iprodione (purity
unspecified) in the diet daily for 28 days at 0, 600, 1900, 6000,
9500, or 15000 ppm (equal to 130, 390, 950, 1500 or 2300 mg/kg
bw/day for males and 120, 420, 1000, 1500 or 2400 mg/kg bw/day for
females). The highest dose produced mortality and depressed body-
weight gain and food consumption. Exposure to 6000 ppm and above
produced ataxia and lethargy during the first week of treatment. The
liver was affected in groups receiving 6000 ppm and above. Absolute
and relative liver weights were increased, livers had a stippled
appearance on gross examination, and the incidence of hepatocyte
vacuolation and focal eosinophilic degeneration was increased. At
15000 ppm granulomatous inflammation, (possibly in response to a
foreign body), was observed in the heart, liver, and kidney. The
NOAEL was 1900 ppm (equal to 390 mg/kg bw/day for males and 420
mg/kg bw/day for females) based on liver effects at 6000 ppm and
above (Stevens, 1974).
Groups of 10 CF-1 Carworth mice/sex received iprodione (purity
unspecified) in the diet daily for 4 weeks at 0, 600, 1900, 6000,
9500 or 15000 ppm, (equal to 115, 366, 1090, 1860 or 4030 mg/kg
bw/day for males - the high dose based on first two weeks only - and
137, 439, 1310, 2090 or 2590 for females). The high dose produced
mortality and depressed body-weight. Depression and ataxia were
observed at 6000 ppm and higher. Relative liver weight was increased
at 6000 ppm and above. Gross necropsy revealed white foci in the
liver of mice receiving 1900 ppm and higher, a stippled appearance
of the liver at 6000 ppm and higher, and liver enlargement at 9500
and 15000 ppm. White foci and granulomatous inflammation were
observed in numerous tissues, primarily at the high dose. One
granulomatous lesion was observed in the liver at 6000 ppm and 5
were observed in the bladder at 9500 ppm. The presence of
spindle-shaped clear spaces in tissues and foreign body type giant
cells suggested a reaction to crystal formation. Liver cell
hypertrophy was increased at 6000 ppm and above. The NOAEL was 600
ppm (equal to 115 mg/kg bw/day for males and 137 mg/kg bw/day for
females) based on gross changes in the liver at 1900 ppm and above
(Huffman, 1974).
Table 1. Acute toxicity of iprodione
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Mouse CD-1 M oral 1870 Takehara et al. (1976b)
F 2670
CD-1 M i.p. 900 Takehara et al. (1976b)
F 625
CD-1 M subcutaneous >6700 Takehara et al. (1976b)
F >6700
Rat CD M oral >2000 Cummins (1989)
F >20002
CD M oral 2060 Takehara et al. (1976a)
F 1530
Wistar M&F oral 3700 Babish (1976a)
CD M i.p. 1330 Takehara et al. (1976a)
F 700
CD M subcutaneous >4500 Takehara et al. (1976a)
F >4500
Sprague-Dawley M inhalation >3.29 Coombs & Clark (1977)
F (4-hr exp) >3.29
Table 1 (cont'd)
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Rabbit New Zeeland white M dermal (24-hr exp) >2000 Plutnick et al. (1988)
F >2000
? ? dermal (?-hr exp) >30 000 Babish (1976b)
Dog Beagle M oral >2000 Pasquet & Mazuret (1974)
F >2000 (FAO/WHO, 1978)
1 Purity of technical iprodione was 95.8%
2 Purity of technical iprodione was 97.9%
The crystal formation in tissues was further evaluated using
groups of 15 CD-1 mice/sex receiving iprodione (93.5%) in the diet
daily for 4 weeks at 0, 1900, 6000, 9500 or 15000 ppm (equivalent to
290, 900, 1400, and 2300 mg/kg bw/day). The two highest dose levels
produced mortality, clinical signs of toxicity, and depressed weight
gain and food consumption. At 6000 ppm and above, crystalline
deposits and effects on the liver were observed. Granulomatous
lesions surrounding crystal deposits were found frequently in the
urinary bladder and occasionally in liver parenchyma, myocardium,
diaphragmatic muscle, and skeletal muscle. It was speculated that
the crystals contained a major metabolite of iprodione, 32490 R.P.,
which was identified in the liver. Liver effects included increased
weight, pale and mottled appearance on gross examination, and
swelling of hepatocytes to form large homogeneous areas in the
centrilobular region of the liver. Histopathological changes in the
testes and spleen were observed at doses levels above 6000 ppm. A
NOAEL of 1900 ppm (equivalent to 290 mg/kg bw/day) was determined
based on crystalline deposits in the urinary bladder, liver
enlargement, and hepatocellular swelling at 6000 ppm and above
(Ganter et al., 1979).
Groups of 10 Crl:CD-1(ICR)BR mice/sex received iprodione (95.7%
purity) in the diet daily for 13 weeks at 0, 1500, 3000, 6000, or
12000 ppm, (equal to 260, 510, 1100 or 2100 mg/kg bw/day for males
and 330, 660, 1300 or 2600 mg/kg bw/day for females). The high dose
was associated with mortality, clinical signs of toxicity, weight
loss, and reduced food consumption. The two highest dose levels
produced crystalline deposits with associated multinucleated cells
in a number of tissues, particularly in the urinary bladder. At the
lowest dose level of 1500 ppm, liver and adrenal gland weights were
increased in females. The incidence and severity of liver cell
enlargement in males and females showed a dose-related increase
beginning with the 1500 ppm dose level. Enlargement and vacuolation
of adrenal cortex cells were increased in treated groups. The
incidence of vacuolation of zona fasciculata cells was 0/10, 1/10,
3/10, 6/10, and 4/9 in females receiving 0, 1500, 3000, 6000 or
12000 ppm, respectively. At 3000 ppm and higher, effects were
observed in the kidney, uterus, ovary, and spleen. The authors
determined that dose levels of 6000 ppm or greater were unsuitable
for a long-term study in mice because of crystal formation and
accompanying effects in the urinary bladder. A NOAEL was not
identified in this study. The lowest dose of 1500 ppm was associated
with enlargement of the liver and adrenal glands and microscopic
changes in these organs (Fryer et al., 1990a).
Rats
Groups of 15 CD/CRJ rats/sex received iprodione (purity
unspecified) in the diet daily for 3 months at 0, 300, 1000, or 3000
ppm, (equal to 21, 70 or 210 mg/kg bw/day for males and 24, 82 or
240 mg/kg bw/day for females). The high dose of 3000 ppm produced
clinical signs of toxicity (i.e. piloerection, rough fur), depressed
food and water consumption, and decreased body-weight (final weight
reduced 20-40%). A number of absolute and relative organ weights
were decreased at the high dose including the liver, spleen, thymus,
kidneys, and heart. Microscopic findings were observed at the high
dose in the liver, spleen, and thymus. Swelling of the zona
glomerulosa of the adrenal gland showed a dose-related increase at
1000 and 3000 ppm in both sexes. The NOAEL was 300 ppm (equal to 21
mg/kg bw/day for males and 24 mg/kg bw/day for females) based on
microscopic changes in the adrenal cortex at 1000 ppm and above
(Itabashi et al., 1978).
Groups of 10 Crl:CD (SD) BR rats/sex received iprodione (95.7%
purity) in the diet daily for up to 13 weeks at 0, 1000, 2000, 3000,
or 5000 ppm (equal to 78, 150 or 250 mg/kg bw/day for males and 89,
180 or 270 mg/kg bw/day for females). The high dose group was
terminated during week 8 due to excessive toxicity which included a
progressive decrease in food intake and body-weight and the death of
one male. Upon examination these animals showed abnormalities in the
liver, adrenal glands, uterus, ovaries, prostate, and seminal
vesicles. Clinical signs (i.e. hunched posture, piloerection,
emaciation) occurred in males and females receiving 3000 ppm. Body-
weight, food intake, and food efficiency were reduced at the 2000
and 3000 ppm. Ovary weight was decreased at 2000 and 3000 ppm, and
uterus weight was reduced at 3000 ppm. Seminal vesicles and the
prostate gland of males receiving 3000 ppm were reported as smaller
than normal but no histological changes were noted.
Histopathological changes were in the adrenal glands, uterus, and
ovaries were increased at 2000 and 3000 ppm. Adrenal gland findings
included enlarged cells of the zona glomerulosa in males and
females and vacuolation of the zone fasciculata mainly in females.
The uterus showed signs of atrophy and ovaries contained reduced
numbers of corpora lutea. A NOAEL of 1000 ppm equal to 78 mg/kg
bw/day for males and 89 mg/kg bw/day for females was determined,
based on a reduced body-weight gain and histopathological changes at
2000 ppm and higher (Fryer et al., 1990b).
Groups of 15 male and 15 female caesarean originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the
diet for 5 months. No effects were observed on mortaility, food
consumption, haematology (as judged by haemoglobin, haematocrit,
erythrocyte count, or total and differential leucocyte count)
clinical chemistry (as judged by BSP, SGOT, SGPT or SAP) or
urinalysis. Body weight gain was slightly reduced (especially in
males) at 500 and 1000 ppm. Absolute (but not relative) heart weight
was reduced in males at 500 and 1000 ppm, and absolute kidney weight
was reduced at 1000 ppm. In females, absolute liver and kidney
weights were significantly reduced at 500 ppm only. Gross and
histopathology were normal at all dose levels. In a parallel study,
dichlozoline, a structurally related compound, induced cataracts. No
such effect was seen with iprodione (Ganter et al., 1973a).
Dogs
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 or 7200 ppm for
a period of 3 months. At the top dose level the method of
administration was altered after 6 weeks, to gelatine capsules. The
treatment did not affect mortality. The recorded values of
haematological determinations and urinalyses were within normal
limits, as judged by haemoglobin, haematocrit, reticulocyte
erythrocyte count, total and differential leucocyte count and
prothrombine time, except for signs of mild anaemia in 1 male and 1
female at 2 months and 1 male at 3 months at the top dose level. At
7200 ppm a reduction of food consumption was observed, accompanied
by reduced body-weight gain. Opththalmosopic examination of the
animals did not reveal any pathological alterations. A transient
increase in SGOT and SGPT was observed after 1 and 2 months of
treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, which was also
observed in females at 2400 ppm and above. At 7200 ppm reduced
relative weight of testes was found, but there was no histological
indication of damage. The histopathological findings did not reveal
any indication of treatment-related alterations of tissues (Coquet,
1973c; Gunter & Girard, 1973).
Groups of 6 beagle dogs/sex received iprodione (96.5% purity)
in the diet daily for 52 weeks at 0, 100, 600, or 3600 ppm, (equal
to 4.1, 24.9 or 145 mg/kg bw/day for males and 4.3, 28.3 or 153
mg/kg bw/day for females). Ophthalmology, haematology, clinical
chemistry, and urinalysis tests were performed periodically over the
course of the study. Slight retinal hyperreflection was noted more
frequently in males receiving 3600 ppm and in females receiving 600
or 3600 ppm. The severity did not increase over time or with dose
level and was not seen consistently in the same animals over time.
Red blood cells were affected at 3600 ppm and possibly at 600 ppm.
Compared to controls, high dose males showed a slight but consistent
decrease in erythrocyte count, haemoglobin, and haematocrit over the
course of the study. Females receiving the high dose showed a less
consistent pattern of anaemia. Platelet count and partial
thromboplastin time were increased over the course of the study in
high dose males and females. The high dose was also associated with
an increase in frequency and severity of erythrocytes containing
Heinz bodies. After 52 weeks, 3/6 control males and 4/6 control
females showed the presence of Heinz bodies compared to all males
and females receiving the high dose. In control dogs the number of
affected erythrocytes was 0-2 per field compared to 6-50 or over 50
per field in dogs receiving the high dose. During the first one-
third of the study, the incidence and severity of Heinz bodies were
also increased in males receiving 600 ppm. No treatment-related
changes were noted in the bone marrow.
Plasma ALP was consistently increased over control values and
was above the normal range in high dose males and females. In high
dose females, serum ALT was transiently elevated early in the study
and LDH was transiently increased late in the study. Total bilirubin
and albumin were slightly elevated during the study particularly in
high dose females. Absolute and relative liver and adrenal gland
weights were increased at the high dose in males and females.
Absolute prostate gland weight was decreased 22% at 600 ppm and 41%
at 3600 ppm. Relative prostate gland weight was also reduced at the
high dose (39%). Two high dose females had enlarged adrenal glands
and one high dose male had a swollen liver on gross examination.
Nematode granulomas were found in various tissues of a number of
control and treated dogs. Treatment-related microscopic changes were
observed in the liver, adrenal glands, and urinary bladder of high
dose males and females. In the urinary bladder, the majority of high
dose males and females had submucosal granulomas and giant cells
containing crystals. The majority of high dose males and females had
fat vacuolation and/or pallid appearance of the adrenal cortex
compared to an absence or lower incidence in controls. Hepatic
centriacinar atrophy was observed in 3/6 males and 4/6 females
receiving the high dose compared to no controls with the lesion.
Pigmented macrophage agglomerates in the liver were more prominent
in the high dose groups than in controls. An additional finding in
females was lipofuscinosis in the proximal convoluted tubules of the
kidney occurring in 2/6, 0/6, 4/5, and 4/6 in the 0, 100, 600, and
3600 ppm groups, respectively. No treatment-related histopathologic
changes were noted in the prostate gland. A NOAEL of 100 ppm, equal
to 4.1 mg/kg bw/day, was determined, 600 ppm representing a minimal
toxic effect level with the majority of toxic changes occurring at
the 3600 ppm dose level (Broadmeadow, 1984).
A second one-year dog study was conducted to determine the
NOAEL between 100 and 600 ppm. Groups of 6 beagle dogs/sex received
iprodione (96% purity) in the diet daily for 52 weeks at 0, 200,
300, 400, or 600 ppm, (equal to 7.8, 12, 18 or 25 mg/kg bw/day for
males and 9.1, 13, 18 or 26 mg/kg bw/day for females). Eye
examinations and haematology were performed during weeks 4, 8, 12,
20, 28, 36, and 52. The adrenal glands, kidneys, and prostate were
the only organs weighed. Kidneys of females and prostate of males
were the only tissues examined microscopically. Iprodione treatment
had no effect on survival, body weight, body-weight gain, food
consumption, or food efficiency. Most dogs treated with iprodione
developed skin lesions consisting of redness and scab formation
occurring primarily in the inguinal, hindlimb, and ventral abdominal
areas. Skin lesions were less frequent and less severe in controls.
The majority of lesions had cleared by study termination. Tests for
mange and fungal infection were negative. It was noted that slight
irritation of the skin was not uncommon in dogs fed powdered chow.
Eye examinations revealed no treatment-related abnormalities.
Haematology parameters were within the normal range for all groups.
In females receiving 600 ppm, erythrocyte count, haemoglobin, and
haematocrit were reduced at week 4 (15%) and at week 36 (10%). In
both males and females receiving 600 ppm, values for these
erythrocyte parameters were consistently lower than control values
through week 36, although the differences were not statistically
significant. Heinz bodies were not observed in any control or
treated animal at any time point. Organ weights (kidney, adrenal
glands, prostate) were unaffected, and no treatment-related
histopathological changes were observed in the kidneys of females or
prostate of males. Based on slight changes in erythrocyte parameters
at 600 ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day (Kangas
et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 60 male and 60 female mice were maintained on a diet
containing the test compound at 0, 200, 500 or 1250 ppm for 18
months. No treatment-related effects on body-weight, food
consumption or mortality were found. The recorded values of the
haematological, blood chemistry and urinalyses tests, performed
after 6, 12 and 18 months of the feeding period, were within the
physiological range. Necropsy findings on mice that died during the
last 6 months of the test and on those sacrificed at the termination
date showed an increased number of enlarged lymph nodes in males at
200 ppm. Organ weight variations occurred sporadically in the
various dose groups which were considered not to be treatment-
related. The histopathological observations failed to reveal
abnormal features. The distribution of neoplastic and non-neoplastic
findings did not appear to demonstrate any significant dose
dependence. The most common tumours were lymphosarcomas involving
the spleen, lymph nodes and thymus (Hastings & Huffmann, 1975).
Rats
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 or 1000 ppm iprodione for 24 months. Slight
reduction in body weight gain was observed at 1000 ppm. This was
accompained by some reduction in food intake. The treatment had no
effect on food consumption, mortality or values of the haematologic,
blood chemistry or urinalyses determinations. Necropsy findings did
not reveal any drug-related gross alterations. Variations in organ
weight did not show a group distribution and did not seem to be
related to drug administration. Histopathology did not indicate a
treatment relationship with neoplastic or non-neoplastic findings.
At 24 months the most common tumours observed were pituitary
adenomas and adenocarcinomas and fibroadenoma of the mammary glands
(Hastings et al., 1976).
Reproduction studies
Rats
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 or 1000 ppm
for the first 5 weeks of each generation and 0, 250, 500 or 2000 ppm
for the next 8 weeks of treatment. The diet was fed through 3
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parent animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendancy for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rats of the third generation did not reveal
abnormalities (Coquet, 1976).
The reproductive toxicity of iprodione was studied in two
successive generations of Crl:CD BR/VAF/Plus rats. The first
parental (F0) animals were mated twice to produce F1a and F1b
litters. F1a animals were mated twice to produce F2a and F2b
litters. Groups (28/sex) received diets containing 0, 300, 1000, or
3000 ppm of iprodione (96.2% purity) beginning at least 10 weeks
prior to mating. The high dose of 3000 ppm was reduced to 2000 ppm
at the time of the first mating of F1a rats because of excessive
toxicity. The administered doses were equal to 17, 55 or 160 mg/kg
bw/day for F0 males; 21, 71 or 210 mg/kg bw/day for F0 females;
20, 68, and 150 mg/kg bw/day for F1 males; and 25, 82 or 190 mg/kg
bw/day for F1 females. Toxicity in adult rats was observed at 1000
ppm and above. Body-weight, body-weight gain, and food consumption
were reduced throughout the treatment period in F0 and F1 males
receiving 3000 ppm and F0 and F1 females receiving 1000 or 3000
ppm. Reproductive performance was unaffected by iprodione exposure.
Offspring toxicity was observed at the high dose. During lactation
(16-21 days of age), F1a and F1b pups exhibited signs of
toxicity including unkempt or hunched appearance, slow movement, and
tremors during the last days of lactation. In both generations,
litter size number of live pups, and pup weight were decreased at
the high dose. The NOAEL was 300 ppm, equal to 21 mg/kg bw/day, for
parental toxicity based on reduced parental body-weight at 1000 ppm
and higher; for embryofetal toxicity, the NOAEL was 1000 ppm, based
on clinical signs, reduced litter size, and reduced pup weight at
3000 ppm (Henwood, 1991).
Special studies on embryo/fetotoxicity
Rats
Groups of 25-30 rats were treated orally with 0, 100, 200 or
400 mg/kg bw/day iprodione on gestation days 5 to 15. Females at 400
mg/kg bw/day showed reduced fertility, reduced body weight gain and
a dose-related reduction in food consumption, especially during the
treatment period. The number of implantations was also reduced at
the highest dose level. There was no indication of an embryotoxic or
teratogenic effect of the test compound (Coquet, 1973a).
In a dose range-finding study in CD pregnant rats, treatment
with 400 and 800 mg/kg bw/day resulted in mortality, weight loss,
and pronounced clinical signs. Treatment with 240 mg/kg bw/day
reduced weight gain and produced clinical signs, and 120 mg/kg
bw/day occasionally produced clinical signs (i.e. flaccid muscles).
The lowest dose of 40 mg/kg bw/day had no effect on maternal health.
It was concluded that dose levels should not exceed 240 mg/kg bw/day
in a definitive developmental toxicity study (Tesh et al., 1986a).
In the definitive study, iprodione (94.2%) was administered by
oral gavage to groups of 25 mated female CD rats at doses of 0, 40,
90, or 200 mg/kg bw/day. Controls received the vehicle (aqueous
methylcellulose). Rats were treated days 6-15 of gestation and
sacrificed on day 21. The administered doses had no adverse effect
on maternal health as assessed by mortality, clinical signs, body-
weight, and food consumption. In the offspring, the incidence of
space between body wall and organs was slightly increased at the
high dose. The incidences were 4.3%, 5.3%, 5.8%, and 11.2% on a
fetal basis and 15%, 20%, 20%, and 32% on a litter basis at 0, 40,
90 and 200 mg/kg bw/day, respectively. The majority of affected
fetuses were small in size. It was noted that this observation was
associated with fetuses of low body-weight in previous studies and
was indicative of slight immaturity. The incidence of small fetuses
(<2.7g) in this study was 2.9%, 5.1%, 4.9%, and 8.0% (fetal basis).
Group mean fetal body-weights were 3.27, 3.18, 3.16, and 3.15 g. The
findings collectively were suggestive of a slight effect on fetal
developmental at the high dose despite the fact that none of the
differences were statistically significant and values at the high
dose were within the historical control range. A NOAEL for maternal
toxicity of 200 mg/kg bw/day and a NOAEL for embryo-fetal toxicity
of 90 mg/kg bw/day were determined, based on slightly delayed fetal
development at 200 mg/kg bw/day. There was no evidence of
teratogenic potential at the highest dose tested (200 mg/kg bw/day)
(Tesh et al., 1986b).
Rabbits
Groups of 15-17 New Zeeland white rabbits were intubated on
gestation days 6-16 inclusive with 0, 100, 200 or 400 mg/kg bw/day
iprodione. Body-weight gain, over the period of treatment, was
slightly reduced at 100 mg/kg bw/day, and a dose-related weight loss
occurred at 200 and 400 mg/kg bw/day. Food intake was reduced at 200
mg/kg bw/day and above. At 400 mg/kg bw/day 9 of 17 females died,
and only one of the four remaining pregnant animals carried to term.
Fetal loss was increased at 200 mg/kg bw/day, and the fetal weight
was reduced at 200 mg/kg bw/day and above. Multiple malformations
occurred in 1 of 68 living fetuses at 200 mg/kg bw/day. Minor
malformations were noted in all groups (Coquet, 1973b).
The developmental toxicity of iprodione was studied in New
Zeeland white rabbits. Iprodione (95.0% purity) was administered by
oral gavage to groups of 18 artificially inseminated females at
doses of 0, 20, 60, or 200 mg/kg bw/day. The control group received
the vehicle (aqueous methylcellulose). Rabbits were treated on days
6-18 of gestation and sacrificed on day 29. Maternal toxicity was
produced at 60 and 200 mg/kg bw/day. The high dose group experienced
weight loss and reduced food consumption during the entire treatment
period. Clinical signs associated with the high dose included hair
loss, diarrhoea, and decreased urination and defecation. The group
receiving 60 mg/kg bw/day experienced slight body-weight loss during
the first six days of treatment compared to positive weight gain by
controls. Seven of 18 females receiving the high dose aborted
compared to 1/18, 0/18, and 1/18 for the 0, 20, and 60 mg/kg bw/day
groups. Ten does receiving the high dose delivered litters, two of
which had totally resorbed litters (compared to 1/13 controls)
resulting in only 8 viable litters at the high dose. A NOAEL of 20
mg/kg bw/day was determined based on depressed maternal weight gain
at 60 mg/kg bw/day and above. The high dose of 200 mg/kg bw/day was
considered an excessive dose for a teratology evaluation. Some
skeletal variations appeared increased at the high dose, but the
incidences were within the historical control range. The NOAEL for
maternal toxicity was 20 mg/kg bw/day based on depressed weight gain
at 60 mg/kg bw/day. The NOAEL for embryo-fetal toxicity was 60 mg/kg
bw/day based on increased abortions and post-implantation losses at
200 mg/kg bw/day (Keets et al., 1985).
Special studies on eye and skin irritation and hypersensitivity
Technical iprodione caused mild, transient eye irritation in
rabbits. The irritation was lessened by washing the eye immediately
after exposure (Babish, 1976c; Bonnette, 1991a).
Technical iprodione is not a dermal irritant in rabbits
(Babish, 1976d; Bonnette, 1991b) or a dermal sensitizer in guinea-
pigs (Trimmer et al., 1988).
Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
Iprodione has been consistently negative in assays for point
mutation, chromosomal aberration, and sister chromatid exchange. A
questionable positive result was reported for DNA damage in
Bacillus subtilis.
Observations in humans
No data available.
Table 2. Results of genotoxicity assays on iprodione
Test system Test object Concentration of Purity Results Reference
iprodione
Ames test (1) S. typhimurium 1-5000 µg/plate dissolved in DMSO 96.2% Negative Lawlor & Valentine (1990)
TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium 25-200µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
TA98, TA100, TA1535, 99.3% (1982a,b)
TA1537, TA1538
S. typhimurium 12.5-250 µg/plate dissolved in DMSO ? Negative Benazet & Cartier (1979)
Ta98, TA100, TA1535,
TA1537
E. coli mutation assay (1) E. coli K12, GY 5057 0.05-1000 µg/ml dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
strain 99.3% (1982a,b)
E. coli, W3110 (pol A+), 12.5-200 µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
p3478 (pol A-) 99.3% (1982a,b)
B. subtilis mutation B. subtilis rec. exc. 20-1670 µg/ml dissolved in DMSO 96.8% Positive Felkner (1985a)
assay (1) pol strains (19 strains) (3)
Saccharomyces cerevisiae Saccharomyces cerevisiae D7 62.5-500 µg/ml dissolved in DMSO 99.3% Negative Bouanchaud & Cartier
mutation assay (1) strain (1982a,b)
(2) Saccharomyces cerevisiae D7 250 µg/ml dissolved in DMSO ? Negative Benazet & Cartier (1979)
strain
CHO/HGPRT mutation assay (1) Chinese hamster ovary cells 5-1500 µg/ml dissolved in DMSO ? Negative Godek et al. (1985)
(CHO-K1-BH4)
In vitro cytogenetics (1) Chinese hamster ovary 15-400 µg/ml dissolved in DMSO ? Negative San Sebastian et al. (1985
cells (CHO-K1-BH4)
Table 2 (cont'd)
Test system Test object Concentration of Purity Results Reference
iprodione
In vitro sister chromatid Chinese hamster ovary cells 5-400 µg/ml dissolved in DMSO ? Negative Felkner (1985b)
exchange (1) (CHO-K1-BH4)
Dominant lethal assay CF-1 mice 0, 1500, 6000 ppm X 49 days ? Negative Hastings et al. (1974)
(WHO, 1978)
(1) Both with and without metabolic activation.
(2) Without metabolic activation.
(3) Positive at highest and lowest doses without metabolic activation. Problem with precipitation of test material, inadequate negative
and positive controls.
COMMENTS
Iprodione is extensively absorbed from the gastrointestinal
tract. It was extensively metabolized and rapidly excreted,
primarily in the urine, although relative faecal excretion of the
parent compound increased at high doses. Higher doses (for example
900 mg/kg bw) appeared to be absorbed to a lesser extent and
eliminated at a slower rate than lower doses (for example 50 mg/kg
bw). The elimination half-life was 7-9 h following a single low dose
and 13-20 h following a single high dose. The metabolic pathways
elucidated in rats involve dealkylation on the isopropyl carbamoyl
chain, hydroxylation of the aromatic ring and rearrangement and
opening of the hydantoin ring.
Iprodione had low acute toxicity by all routes of exposure. The
oral LD50 was greater than 1500 mg/kg bw in mice, rats, and dogs.
The World Health Organization has classified iprodione as unlikely
to present acute hazard in normal use (WHO, 1992).
In three 4-week studies in mice at dietary concentrations of 0,
600, 1900, 6000, 9500, or 15000 ppm, the lowest NOAEL was 600 ppm,
equal to 115 mg/kg bw/day, based on macroscopic hepatic changes at
1900 ppm. At 6000 ppm and above, the test material crystallized in
the tissues. In a 3-month study in mice at dietary concentrations of
0, 1500, 3000, 6000, or 12000 ppm (equal to 260, 510, 1100, and 2100
mg/kg bw/day) an increase in liver and adrenal gland weights and
hypertrophy and/or vacuolation of hepatocytes and adrenal cortical
cells were observed in all treated groups.
In a 3-month study in rats at dietary concentrations of 0, 300,
1000, or 3000 ppm, the NOAEL was 300 ppm, equal to 21 mg/kg bw/day.
Higher doses produced swelling in the zona glomerulosa of the
adrenal cortex. In another 3-month study in rats at dietary
concentrations of 0, 1000, 2000, 3000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 78 mg/kg bw/day, based on reduced body-weight
gain and histopathological changes in the adrenal glands, ovaries
and uterus at 2000 ppm and higher.
In a one-year study in dogs at dietary concentrations of 0,
100, 600, or 3600 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg
bw/day, based on the detection of Heinz bodies in erythrocytes and
decreased prostate gland weight at 600 ppm and higher. In a second
one-year study at dietary concentrations of 0, 200, 300, 400, or 600
ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day, based on
decreased erythrocyte values at 600 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 300, 1000, or 3000/2000 ppm, the NOAEL was 300
ppm, equal to 21 mg/kg bw/day, based on depressed body-weight at
1000 ppm and above. Reproductive performance was unaffected.
Offspring survival and growth were reduced at 3000/2000 ppm.
In a teratology study in rats using gavage doses of 0, 40, 90,
or 200 mg/kg bw/day, the NOAEL for maternal toxicity and
teratogenicity was 200 mg/kg bw/day. The NOAEL for embryofetal
toxicity was 90 mg/kg bw/day, based on slightly delayed fetal
development at 200 mg/kg bw/day. In rabbits administered 0, 20, 60,
or 200 mg/kg bw/day by gavage, the NOAEL for maternal toxicity was
20 mg/kg bw/day based on depressed weight gain at 60 mg/kg bw/day.
The NOAEL for embryofetal toxicity was 60 mg/kg bw/day based on
increased abortions and post-implantation loss at 200 mg/kg bw/day.
No teratogenic effects were found.
After consideration of the available genotoxicity data, the
Meeting concluded that iprodione was not genotoxic.
The former ADI, based on a multi-generation reproduction study
in rats, was revised. The new ADI was based on the results of
several studies, including the reproduction study in rats, the
teratology study in rabbits, and the one-year study in dogs. A
safety factor of 100 was applied to the NOAELs from these studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 600 ppm in the diet, equal to 115 mg/kg bw/day (four-
week study)
Rat: 300 ppm in the diet, equal to 21 mg/kg bw/day
(two-generation reproduction study)
500 ppm in the diet, equivalent to 25 mg/kg bw/day
(reproduction study reviewed by the 1977 Joint
Meeting)
Rabbit: 20 mg/kg bw/day (teratology study, maternal toxicity)
Dog: 400 ppm in the diet, equal to 18 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw/day
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing toxicological studies.
2. Observation in humans.
REFERENCES
Babish, J.C. (1976a). Acute oral toxicity in rats. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976b). Acute dermal toxicity in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976c). Eye irritation test in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976d). Primary skin irritation study with rabbits.
Unpublished report from Food and Drug Research Laboratories, Inc.,
Waverly, New York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Benazet, F. & Cartier, J.R. (1979). Iprodione (26 019 R.P.): In-
vitro mutagenicity study in Salmonella typhimurium (Ames' strains)
and in Saccharomyces cerevisiae (Zimmermann's strain D7).
Unpublished report No. RP/RD/CNG 20 177-E from Rhone-Poulenc, Vitry-
sur-Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Bonnette, K.L. (1991a). Primary eye irritation study in rabbits with
iprodione. Unpublished report No. 3147.109 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bonnette, K.L. (1991b). Primary skin irritation study in rabbits
with iprodione. Unpublished report No. 3147.108 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bouanchaud, D.H. & Cartier, J.R. (1982a). Iprodione (26 019 R.P.):
Supplementary studies of mutagenesis in microorganisms. Unpublished
report No. CRV/CNG 21 465 from Rhône-Poulenc, Vitry-sur-Seine,
France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Bouanchaud, D.H. & Cartier, J.R. (1982b). Iprodione (26 019 R.P.),
technical grade compound: In vitro mutagenesis in microorganisms.
Unpublished report No. CRV/CNG 21 466 from Rhône-Poulenc, Vitry-sur-
Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Broadmeadow, A. (1984). Iprodione: 52-week toxicity study in dietary
administration to beagle dogs. Unpublished report No. 84/RH0022/179
from Life Science Research, Suffolk, England. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Coombs, D.W. & Clark, G.C. (1977). RP 26019 technical: acute
inhalation toxicity-four hour LC50 in rats. Unpublished report No.
RNP 75/775 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Coquet, B. (1973a) Recherche du pouvoir teratogène chez le rat OFA
par voie orale du produit 26019 R.P. Unpublished report from Centre
de Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogène chez le lapin par
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1973c) Essai de toxicité chronique (3 mois) chez le
chien par voie orale du produit 26019 R.P. Unpublished report from
Centre de Recherche et d'Elevage des Onoins, No. 731008.
Coquet, B. (1976) Influence du produit 26019 R.P. sur la
reproduction du rat (essai sur 3 générations). Unpublished report
from Institut Français de Recherches et Essais biologiques, No.
760435 submitted by Rhône-Poulenc.
Cummins, H.A. (1989). Iprodione: acute oral toxicity study in the
rat. Unpublished report No. 89/RHA255/0391 from Life Science
Research, Suffolk, England. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985a). DNA damage in Bacillus subtilis with
iprodione technical. Unpublished report No. 2214 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985b). In vitro sister chromatid exchange in
Chinese hamster ovary cells (CHO). Unpublished report No. PH 319-BO-
001-84 from Borriston Laboratories, Inc., Temple Hill, Maryland,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990a). Iprodione: sub-acute toxicity to mice by
dietary administration for 13 weeks. Unpublished report No. RNP
323/90667 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990b). Iprodione: sub-acute toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RNP
322/90767 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Ganter, P., Pasquet, J., Spicer, E., Girard, P., & Myon, J. (1979).
Iprodione (26 019 R.P.): one month toxicity study in mice by dietary
administration. Unpublished report No. RP/RD/CNG 19 950 from Rhône-
Poulenc, Vitry-sur-Seine, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. & Myon, J. (1973). Produit No. 26019 R.P.
Toxicité à terme (5 mois) chez le rat par voie orale, en comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R,P). Unpublished
report from Laboratoires de Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 17124 submitted by Rhône-
Poulenc.
Ganter, P. & Girard, P. (1973). Produit No. 26019 R.P. Examen
histologique de la toxicité oculaire du 26019 R,LO, chez le chien
après un traitement de 3 mois. Unpublished report from Laboratoires
de Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131 submitted by Rhône-Poulenc.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1985). CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report No.
PH 314-BO-001-84 from Borriston Laboratories, Inc., Temple Hill,
Maryland, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Hallifax, D. (1989). Iprodione: absorption, distribution, metabolism
and excretion study in the rat. Unpublished report No.
89/RPM005/1013 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E., Huffman, K.W., & M.A. Gallo (1974). RP 26019:
Dominant lethal mutagenicity in mice. Unpublished report No. SEH
74:69-CH-47 from Hess & Clark, Ashland, Ohio, USA. Submitted to WHO
by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E. & Huffman, K.W. (1975). Chronic toxicologic and
carcinogenic study with RP 26019 in mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:223. Submitted to
WHO by Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C., & Kiggins, E.M. (1976). Chronic
toxicologic and carcinogenic study with RP 26019 in rats.
Unpublished report from Rhodia, Inc., Hess and Clark Division, No.
76:57. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1991). Two-generation reproduction study with
iprodione technical in rats. Unpublished report No. HLA 6224-154
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Huffman, K.W. (1974). Subacute toxicity, RP 26 019, mice.
Unpublished report No. KWH 74:43 from Hess & Clark, Ashland, Ohio,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Itabashi, M., Hayashi, K., Kudo, S., Maruyama, Y., Takahara, K., &
Tajima, M. (1978). Three-month dietary oral toxicity study of 26.019
in rats. Unpublished report from Nippon Institute for Biological
Science, Japan. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Jones, A.G. (1983). Fungicides: Iprodione. Exposure of personnel
mixing ROVRAL WP. Unpublished report No. 428 from May & Baker, Ltd.,
Essex, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Kangas, L. (1991). A 52-week dietary toxicity study of iprodione in
the beagle dog. Unpublished report No. 84296 from Bio-Research
Laboratories Ltd., Senneville, Quebec, Canada. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Keets, S.A., Leist, P.L., Mercieca, M.D., & Clevidence, K.J. (1985).
A teratology study in rabbits with iprodione. Unpublished report No.
WIL-21028 from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Laurent, M. & Buys M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques, Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. & Chabassol, Y. (1976)
Etude du métabolism chez le rat à l'aide du produit marqué au 14C.
Unpublished report from Rhône Poulenc, Direction des Recherches et
du Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-
Seine, C.N.G. An. No. 18548, submitted by Rhône Poulenc.
Laurent, M., Bredenbac, J., Chabassol, Y., & Marlard, M. (1983).
Iprodione. Percutaneous metabolism in the rat. Unpublished report
No. CNG-An 4675-E from Rhône-Poulenc Research, Vitry-sur-Seine,
France. Submitted to WHO by Rhone-Poulenc Agrochimie S.A., Lyon,
France.
Lawlor, T.E. & Valentine, D.C. (1990). Mutagenicity test on
iprodione (technical) in the Salmonella/mammalian-microsome
reverse mutation assay (Ames test) with confirmatory assay.
Unpublished report No. HLA 11092-0-401R from Hazleton Laboratories
America, Inc., Kensington, Maryland, USA. Submitted to WHO by Rhône-
Poulenc Agrochimie S.A., Lyon, France.
Pasquet, J. & Mazuret, A. (1974). 26 019 R.P.: acute toxicity and
local tolerance. Unpublished report No. SUCRP-DSPh 17 746 from
Rhône-Poulenc, Paris, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Plutnick, R.T. & Kapp, R.W. (1988). Iprodione (technical) - acute
dermal limit test in the rabbit. Unpublished report No. 209806 from
Exxon Biomedical Sciences, Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
San Sebastian, J.R., Naismith, R.W., & Matthews, R.J. (1985). In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Unpublished report No. PH 320-BO-001-84 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Stevens, K.R. (1974). Subacute toxicity, RP 26 019. Unpublished
report No. KRS 74:3 from Hess & Clark, Ashland, Ohio, USA. Submitted
to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976a). Acute toxicity of Rovral (26019RP). I. Oral,
intraperitoneal and subcutaneous administration in rats. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976b). Acute toxicity of Rovral (26019RP). II. Oral,
intraperitoneal and subcutaneous administration in mice. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., & Deans, C.F. (1986a). Iprodione
(technical grade): effects of oral administration upon pregnancy in
the rat. 1. Dosage range-finding study. Unpublished report No.
85/RHA063/752 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., Lambert, E.P., Wilby, O.K., & Tesh, S.A.
(1986b). Iprodione (technical grade): teratology study in the rat.
Unpublished report No. 85/RHA064/765 from Life Science Research,
Suffolk, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Trimmer, G.W. (1988). Iprodione (technical) - dermal sensitization
test in the guinea-pig (Buehler method). Unpublished report No.
209821 from Exxon Biomedical Sciences, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Short-term toxicity studies
Mice
Groups (10-20/sex/dose) of CF-1 mice received iprodione (purity
unspecified) in the diet daily for 28 days at 0, 600, 1900, 6000,
9500, or 15000 ppm (equal to 130, 390, 950, 1500 or 2300 mg/kg
bw/day for males and 120, 420, 1000, 1500 or 2400 mg/kg bw/day for
females). The highest dose produced mortality and depressed body-
weight gain and food consumption. Exposure to 6000 ppm and above
produced ataxia and lethargy during the first week of treatment. The
liver was affected in groups receiving 6000 ppm and above. Absolute
and relative liver weights were increased, livers had a stippled
appearance on gross examination, and the incidence of hepatocyte
vacuolation and focal eosinophilic degeneration was increased. At
15000 ppm granulomatous inflammation, (possibly in response to a
foreign body), was observed in the heart, liver, and kidney. The
NOAEL was 1900 ppm (equal to 390 mg/kg bw/day for males and 420
mg/kg bw/day for females) based on liver effects at 6000 ppm and
above (Stevens, 1974).
Groups of 10 CF-1 Carworth mice/sex received iprodione (purity
unspecified) in the diet daily for 4 weeks at 0, 600, 1900, 6000,
9500 or 15000 ppm, (equal to 115, 366, 1090, 1860 or 4030 mg/kg
bw/day for males - the high dose based on first two weeks only - and
137, 439, 1310, 2090 or 2590 for females). The high dose produced
mortality and depressed body-weight. Depression and ataxia were
observed at 6000 ppm and higher. Relative liver weight was increased
at 6000 ppm and above. Gross necropsy revealed white foci in the
liver of mice receiving 1900 ppm and higher, a stippled appearance
of the liver at 6000 ppm and higher, and liver enlargement at 9500
and 15000 ppm. White foci and granulomatous inflammation were
observed in numerous tissues, primarily at the high dose. One
granulomatous lesion was observed in the liver at 6000 ppm and 5
were observed in the bladder at 9500 ppm. The presence of
spindle-shaped clear spaces in tissues and foreign body type giant
cells suggested a reaction to crystal formation. Liver cell
hypertrophy was increased at 6000 ppm and above. The NOAEL was 600
ppm (equal to 115 mg/kg bw/day for males and 137 mg/kg bw/day for
females) based on gross changes in the liver at 1900 ppm and above
(Huffman, 1974).
Table 1. Acute toxicity of iprodione
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Mouse CD-1 M oral 1870 Takehara et al. (1976b)
F 2670
CD-1 M i.p. 900 Takehara et al. (1976b)
F 625
CD-1 M subcutaneous >6700 Takehara et al. (1976b)
F >6700
Rat CD M oral >2000 Cummins (1989)
F >20002
CD M oral 2060 Takehara et al. (1976a)
F 1530
Wistar M&F oral 3700 Babish (1976a)
CD M i.p. 1330 Takehara et al. (1976a)
F 700
CD M subcutaneous >4500 Takehara et al. (1976a)
F >4500
Sprague-Dawley M inhalation >3.29 Coombs & Clark (1977)
F (4-hr exp) >3.29
Table 1 (cont'd)
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Rabbit New Zeeland white M dermal (24-hr exp) >2000 Plutnick et al. (1988)
F >2000
? ? dermal (?-hr exp) >30 000 Babish (1976b)
Dog Beagle M oral >2000 Pasquet & Mazuret (1974)
F >2000 (FAO/WHO, 1978)
1 Purity of technical iprodione was 95.8%
2 Purity of technical iprodione was 97.9%
The crystal formation in tissues was further evaluated using
groups of 15 CD-1 mice/sex receiving iprodione (93.5%) in the diet
daily for 4 weeks at 0, 1900, 6000, 9500 or 15000 ppm (equivalent to
290, 900, 1400, and 2300 mg/kg bw/day). The two highest dose levels
produced mortality, clinical signs of toxicity, and depressed weight
gain and food consumption. At 6000 ppm and above, crystalline
deposits and effects on the liver were observed. Granulomatous
lesions surrounding crystal deposits were found frequently in the
urinary bladder and occasionally in liver parenchyma, myocardium,
diaphragmatic muscle, and skeletal muscle. It was speculated that
the crystals contained a major metabolite of iprodione, 32490 R.P.,
which was identified in the liver. Liver effects included increased
weight, pale and mottled appearance on gross examination, and
swelling of hepatocytes to form large homogeneous areas in the
centrilobular region of the liver. Histopathological changes in the
testes and spleen were observed at doses levels above 6000 ppm. A
NOAEL of 1900 ppm (equivalent to 290 mg/kg bw/day) was determined
based on crystalline deposits in the urinary bladder, liver
enlargement, and hepatocellular swelling at 6000 ppm and above
(Ganter et al., 1979).
Groups of 10 Crl:CD-1(ICR)BR mice/sex received iprodione (95.7%
purity) in the diet daily for 13 weeks at 0, 1500, 3000, 6000, or
12000 ppm, (equal to 260, 510, 1100 or 2100 mg/kg bw/day for males
and 330, 660, 1300 or 2600 mg/kg bw/day for females). The high dose
was associated with mortality, clinical signs of toxicity, weight
loss, and reduced food consumption. The two highest dose levels
produced crystalline deposits with associated multinucleated cells
in a number of tissues, particularly in the urinary bladder. At the
lowest dose level of 1500 ppm, liver and adrenal gland weights were
increased in females. The incidence and severity of liver cell
enlargement in males and females showed a dose-related increase
beginning with the 1500 ppm dose level. Enlargement and vacuolation
of adrenal cortex cells were increased in treated groups. The
incidence of vacuolation of zona fasciculata cells was 0/10, 1/10,
3/10, 6/10, and 4/9 in females receiving 0, 1500, 3000, 6000 or
12000 ppm, respectively. At 3000 ppm and higher, effects were
observed in the kidney, uterus, ovary, and spleen. The authors
determined that dose levels of 6000 ppm or greater were unsuitable
for a long-term study in mice because of crystal formation and
accompanying effects in the urinary bladder. A NOAEL was not
identified in this study. The lowest dose of 1500 ppm was associated
with enlargement of the liver and adrenal glands and microscopic
changes in these organs (Fryer et al., 1990a).
Rats
Groups of 15 CD/CRJ rats/sex received iprodione (purity
unspecified) in the diet daily for 3 months at 0, 300, 1000, or 3000
ppm, (equal to 21, 70 or 210 mg/kg bw/day for males and 24, 82 or
240 mg/kg bw/day for females). The high dose of 3000 ppm produced
clinical signs of toxicity (i.e. piloerection, rough fur), depressed
food and water consumption, and decreased body-weight (final weight
reduced 20-40%). A number of absolute and relative organ weights
were decreased at the high dose including the liver, spleen, thymus,
kidneys, and heart. Microscopic findings were observed at the high
dose in the liver, spleen, and thymus. Swelling of the zona
glomerulosa of the adrenal gland showed a dose-related increase at
1000 and 3000 ppm in both sexes. The NOAEL was 300 ppm (equal to 21
mg/kg bw/day for males and 24 mg/kg bw/day for females) based on
microscopic changes in the adrenal cortex at 1000 ppm and above
(Itabashi et al., 1978).
Groups of 10 Crl:CD (SD) BR rats/sex received iprodione (95.7%
purity) in the diet daily for up to 13 weeks at 0, 1000, 2000, 3000,
or 5000 ppm (equal to 78, 150 or 250 mg/kg bw/day for males and 89,
180 or 270 mg/kg bw/day for females). The high dose group was
terminated during week 8 due to excessive toxicity which included a
progressive decrease in food intake and body-weight and the death of
one male. Upon examination these animals showed abnormalities in the
liver, adrenal glands, uterus, ovaries, prostate, and seminal
vesicles. Clinical signs (i.e. hunched posture, piloerection,
emaciation) occurred in males and females receiving 3000 ppm. Body-
weight, food intake, and food efficiency were reduced at the 2000
and 3000 ppm. Ovary weight was decreased at 2000 and 3000 ppm, and
uterus weight was reduced at 3000 ppm. Seminal vesicles and the
prostate gland of males receiving 3000 ppm were reported as smaller
than normal but no histological changes were noted.
Histopathological changes were in the adrenal glands, uterus, and
ovaries were increased at 2000 and 3000 ppm. Adrenal gland findings
included enlarged cells of the zona glomerulosa in males and
females and vacuolation of the zone fasciculata mainly in females.
The uterus showed signs of atrophy and ovaries contained reduced
numbers of corpora lutea. A NOAEL of 1000 ppm equal to 78 mg/kg
bw/day for males and 89 mg/kg bw/day for females was determined,
based on a reduced body-weight gain and histopathological changes at
2000 ppm and higher (Fryer et al., 1990b).
Groups of 15 male and 15 female caesarean originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the
diet for 5 months. No effects were observed on mortaility, food
consumption, haematology (as judged by haemoglobin, haematocrit,
erythrocyte count, or total and differential leucocyte count)
clinical chemistry (as judged by BSP, SGOT, SGPT or SAP) or
urinalysis. Body weight gain was slightly reduced (especially in
males) at 500 and 1000 ppm. Absolute (but not relative) heart weight
was reduced in males at 500 and 1000 ppm, and absolute kidney weight
was reduced at 1000 ppm. In females, absolute liver and kidney
weights were significantly reduced at 500 ppm only. Gross and
histopathology were normal at all dose levels. In a parallel study,
dichlozoline, a structurally related compound, induced cataracts. No
such effect was seen with iprodione (Ganter et al., 1973a).
Dogs
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 or 7200 ppm for
a period of 3 months. At the top dose level the method of
administration was altered after 6 weeks, to gelatine capsules. The
treatment did not affect mortality. The recorded values of
haematological determinations and urinalyses were within normal
limits, as judged by haemoglobin, haematocrit, reticulocyte
erythrocyte count, total and differential leucocyte count and
prothrombine time, except for signs of mild anaemia in 1 male and 1
female at 2 months and 1 male at 3 months at the top dose level. At
7200 ppm a reduction of food consumption was observed, accompanied
by reduced body-weight gain. Opththalmosopic examination of the
animals did not reveal any pathological alterations. A transient
increase in SGOT and SGPT was observed after 1 and 2 months of
treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, which was also
observed in females at 2400 ppm and above. At 7200 ppm reduced
relative weight of testes was found, but there was no histological
indication of damage. The histopathological findings did not reveal
any indication of treatment-related alterations of tissues (Coquet,
1973c; Gunter & Girard, 1973).
Groups of 6 beagle dogs/sex received iprodione (96.5% purity)
in the diet daily for 52 weeks at 0, 100, 600, or 3600 ppm, (equal
to 4.1, 24.9 or 145 mg/kg bw/day for males and 4.3, 28.3 or 153
mg/kg bw/day for females). Ophthalmology, haematology, clinical
chemistry, and urinalysis tests were performed periodically over the
course of the study. Slight retinal hyperreflection was noted more
frequently in males receiving 3600 ppm and in females receiving 600
or 3600 ppm. The severity did not increase over time or with dose
level and was not seen consistently in the same animals over time.
Red blood cells were affected at 3600 ppm and possibly at 600 ppm.
Compared to controls, high dose males showed a slight but consistent
decrease in erythrocyte count, haemoglobin, and haematocrit over the
course of the study. Females receiving the high dose showed a less
consistent pattern of anaemia. Platelet count and partial
thromboplastin time were increased over the course of the study in
high dose males and females. The high dose was also associated with
an increase in frequency and severity of erythrocytes containing
Heinz bodies. After 52 weeks, 3/6 control males and 4/6 control
females showed the presence of Heinz bodies compared to all males
and females receiving the high dose. In control dogs the number of
affected erythrocytes was 0-2 per field compared to 6-50 or over 50
per field in dogs receiving the high dose. During the first one-
third of the study, the incidence and severity of Heinz bodies were
also increased in males receiving 600 ppm. No treatment-related
changes were noted in the bone marrow.
Plasma ALP was consistently increased over control values and
was above the normal range in high dose males and females. In high
dose females, serum ALT was transiently elevated early in the study
and LDH was transiently increased late in the study. Total bilirubin
and albumin were slightly elevated during the study particularly in
high dose females. Absolute and relative liver and adrenal gland
weights were increased at the high dose in males and females.
Absolute prostate gland weight was decreased 22% at 600 ppm and 41%
at 3600 ppm. Relative prostate gland weight was also reduced at the
high dose (39%). Two high dose females had enlarged adrenal glands
and one high dose male had a swollen liver on gross examination.
Nematode granulomas were found in various tissues of a number of
control and treated dogs. Treatment-related microscopic changes were
observed in the liver, adrenal glands, and urinary bladder of high
dose males and females. In the urinary bladder, the majority of high
dose males and females had submucosal granulomas and giant cells
containing crystals. The majority of high dose males and females had
fat vacuolation and/or pallid appearance of the adrenal cortex
compared to an absence or lower incidence in controls. Hepatic
centriacinar atrophy was observed in 3/6 males and 4/6 females
receiving the high dose compared to no controls with the lesion.
Pigmented macrophage agglomerates in the liver were more prominent
in the high dose groups than in controls. An additional finding in
females was lipofuscinosis in the proximal convoluted tubules of the
kidney occurring in 2/6, 0/6, 4/5, and 4/6 in the 0, 100, 600, and
3600 ppm groups, respectively. No treatment-related histopathologic
changes were noted in the prostate gland. A NOAEL of 100 ppm, equal
to 4.1 mg/kg bw/day, was determined, 600 ppm representing a minimal
toxic effect level with the majority of toxic changes occurring at
the 3600 ppm dose level (Broadmeadow, 1984).
A second one-year dog study was conducted to determine the
NOAEL between 100 and 600 ppm. Groups of 6 beagle dogs/sex received
iprodione (96% purity) in the diet daily for 52 weeks at 0, 200,
300, 400, or 600 ppm, (equal to 7.8, 12, 18 or 25 mg/kg bw/day for
males and 9.1, 13, 18 or 26 mg/kg bw/day for females). Eye
examinations and haematology were performed during weeks 4, 8, 12,
20, 28, 36, and 52. The adrenal glands, kidneys, and prostate were
the only organs weighed. Kidneys of females and prostate of males
were the only tissues examined microscopically. Iprodione treatment
had no effect on survival, body weight, body-weight gain, food
consumption, or food efficiency. Most dogs treated with iprodione
developed skin lesions consisting of redness and scab formation
occurring primarily in the inguinal, hindlimb, and ventral abdominal
areas. Skin lesions were less frequent and less severe in controls.
The majority of lesions had cleared by study termination. Tests for
mange and fungal infection were negative. It was noted that slight
irritation of the skin was not uncommon in dogs fed powdered chow.
Eye examinations revealed no treatment-related abnormalities.
Haematology parameters were within the normal range for all groups.
In females receiving 600 ppm, erythrocyte count, haemoglobin, and
haematocrit were reduced at week 4 (15%) and at week 36 (10%). In
both males and females receiving 600 ppm, values for these
erythrocyte parameters were consistently lower than control values
through week 36, although the differences were not statistically
significant. Heinz bodies were not observed in any control or
treated animal at any time point. Organ weights (kidney, adrenal
glands, prostate) were unaffected, and no treatment-related
histopathological changes were observed in the kidneys of females or
prostate of males. Based on slight changes in erythrocyte parameters
at 600 ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day (Kangas
et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 60 male and 60 female mice were maintained on a diet
containing the test compound at 0, 200, 500 or 1250 ppm for 18
months. No treatment-related effects on body-weight, food
consumption or mortality were found. The recorded values of the
haematological, blood chemistry and urinalyses tests, performed
after 6, 12 and 18 months of the feeding period, were within the
physiological range. Necropsy findings on mice that died during the
last 6 months of the test and on those sacrificed at the termination
date showed an increased number of enlarged lymph nodes in males at
200 ppm. Organ weight variations occurred sporadically in the
various dose groups which were considered not to be treatment-
related. The histopathological observations failed to reveal
abnormal features. The distribution of neoplastic and non-neoplastic
findings did not appear to demonstrate any significant dose
dependence. The most common tumours were lymphosarcomas involving
the spleen, lymph nodes and thymus (Hastings & Huffmann, 1975).
Rats
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 or 1000 ppm iprodione for 24 months. Slight
reduction in body weight gain was observed at 1000 ppm. This was
accompained by some reduction in food intake. The treatment had no
effect on food consumption, mortality or values of the haematologic,
blood chemistry or urinalyses determinations. Necropsy findings did
not reveal any drug-related gross alterations. Variations in organ
weight did not show a group distribution and did not seem to be
related to drug administration. Histopathology did not indicate a
treatment relationship with neoplastic or non-neoplastic findings.
At 24 months the most common tumours observed were pituitary
adenomas and adenocarcinomas and fibroadenoma of the mammary glands
(Hastings et al., 1976).
Reproduction studies
Rats
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 or 1000 ppm
for the first 5 weeks of each generation and 0, 250, 500 or 2000 ppm
for the next 8 weeks of treatment. The diet was fed through 3
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parent animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendancy for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rats of the third generation did not reveal
abnormalities (Coquet, 1976).
The reproductive toxicity of iprodione was studied in two
successive generations of Crl:CD BR/VAF/Plus rats. The first
parental (F0) animals were mated twice to produce F1a and F1b
litters. F1a animals were mated twice to produce F2a and F2b
litters. Groups (28/sex) received diets containing 0, 300, 1000, or
3000 ppm of iprodione (96.2% purity) beginning at least 10 weeks
prior to mating. The high dose of 3000 ppm was reduced to 2000 ppm
at the time of the first mating of F1a rats because of excessive
toxicity. The administered doses were equal to 17, 55 or 160 mg/kg
bw/day for F0 males; 21, 71 or 210 mg/kg bw/day for F0 females;
20, 68, and 150 mg/kg bw/day for F1 males; and 25, 82 or 190 mg/kg
bw/day for F1 females. Toxicity in adult rats was observed at 1000
ppm and above. Body-weight, body-weight gain, and food consumption
were reduced throughout the treatment period in F0 and F1 males
receiving 3000 ppm and F0 and F1 females receiving 1000 or 3000
ppm. Reproductive performance was unaffected by iprodione exposure.
Offspring toxicity was observed at the high dose. During lactation
(16-21 days of age), F1a and F1b pups exhibited signs of
toxicity including unkempt or hunched appearance, slow movement, and
tremors during the last days of lactation. In both generations,
litter size number of live pups, and pup weight were decreased at
the high dose. The NOAEL was 300 ppm, equal to 21 mg/kg bw/day, for
parental toxicity based on reduced parental body-weight at 1000 ppm
and higher; for embryofetal toxicity, the NOAEL was 1000 ppm, based
on clinical signs, reduced litter size, and reduced pup weight at
3000 ppm (Henwood, 1991).
Special studies on embryo/fetotoxicity
Rats
Groups of 25-30 rats were treated orally with 0, 100, 200 or
400 mg/kg bw/day iprodione on gestation days 5 to 15. Females at 400
mg/kg bw/day showed reduced fertility, reduced body weight gain and
a dose-related reduction in food consumption, especially during the
treatment period. The number of implantations was also reduced at
the highest dose level. There was no indication of an embryotoxic or
teratogenic effect of the test compound (Coquet, 1973a).
In a dose range-finding study in CD pregnant rats, treatment
with 400 and 800 mg/kg bw/day resulted in mortality, weight loss,
and pronounced clinical signs. Treatment with 240 mg/kg bw/day
reduced weight gain and produced clinical signs, and 120 mg/kg
bw/day occasionally produced clinical signs (i.e. flaccid muscles).
The lowest dose of 40 mg/kg bw/day had no effect on maternal health.
It was concluded that dose levels should not exceed 240 mg/kg bw/day
in a definitive developmental toxicity study (Tesh et al., 1986a).
In the definitive study, iprodione (94.2%) was administered by
oral gavage to groups of 25 mated female CD rats at doses of 0, 40,
90, or 200 mg/kg bw/day. Controls received the vehicle (aqueous
methylcellulose). Rats were treated days 6-15 of gestation and
sacrificed on day 21. The administered doses had no adverse effect
on maternal health as assessed by mortality, clinical signs, body-
weight, and food consumption. In the offspring, the incidence of
space between body wall and organs was slightly increased at the
high dose. The incidences were 4.3%, 5.3%, 5.8%, and 11.2% on a
fetal basis and 15%, 20%, 20%, and 32% on a litter basis at 0, 40,
90 and 200 mg/kg bw/day, respectively. The majority of affected
fetuses were small in size. It was noted that this observation was
associated with fetuses of low body-weight in previous studies and
was indicative of slight immaturity. The incidence of small fetuses
(<2.7g) in this study was 2.9%, 5.1%, 4.9%, and 8.0% (fetal basis).
Group mean fetal body-weights were 3.27, 3.18, 3.16, and 3.15 g. The
findings collectively were suggestive of a slight effect on fetal
developmental at the high dose despite the fact that none of the
differences were statistically significant and values at the high
dose were within the historical control range. A NOAEL for maternal
toxicity of 200 mg/kg bw/day and a NOAEL for embryo-fetal toxicity
of 90 mg/kg bw/day were determined, based on slightly delayed fetal
development at 200 mg/kg bw/day. There was no evidence of
teratogenic potential at the highest dose tested (200 mg/kg bw/day)
(Tesh et al., 1986b).
Rabbits
Groups of 15-17 New Zeeland white rabbits were intubated on
gestation days 6-16 inclusive with 0, 100, 200 or 400 mg/kg bw/day
iprodione. Body-weight gain, over the period of treatment, was
slightly reduced at 100 mg/kg bw/day, and a dose-related weight loss
occurred at 200 and 400 mg/kg bw/day. Food intake was reduced at 200
mg/kg bw/day and above. At 400 mg/kg bw/day 9 of 17 females died,
and only one of the four remaining pregnant animals carried to term.
Fetal loss was increased at 200 mg/kg bw/day, and the fetal weight
was reduced at 200 mg/kg bw/day and above. Multiple malformations
occurred in 1 of 68 living fetuses at 200 mg/kg bw/day. Minor
malformations were noted in all groups (Coquet, 1973b).
The developmental toxicity of iprodione was studied in New
Zeeland white rabbits. Iprodione (95.0% purity) was administered by
oral gavage to groups of 18 artificially inseminated females at
doses of 0, 20, 60, or 200 mg/kg bw/day. The control group received
the vehicle (aqueous methylcellulose). Rabbits were treated on days
6-18 of gestation and sacrificed on day 29. Maternal toxicity was
produced at 60 and 200 mg/kg bw/day. The high dose group experienced
weight loss and reduced food consumption during the entire treatment
period. Clinical signs associated with the high dose included hair
loss, diarrhoea, and decreased urination and defecation. The group
receiving 60 mg/kg bw/day experienced slight body-weight loss during
the first six days of treatment compared to positive weight gain by
controls. Seven of 18 females receiving the high dose aborted
compared to 1/18, 0/18, and 1/18 for the 0, 20, and 60 mg/kg bw/day
groups. Ten does receiving the high dose delivered litters, two of
which had totally resorbed litters (compared to 1/13 controls)
resulting in only 8 viable litters at the high dose. A NOAEL of 20
mg/kg bw/day was determined based on depressed maternal weight gain
at 60 mg/kg bw/day and above. The high dose of 200 mg/kg bw/day was
considered an excessive dose for a teratology evaluation. Some
skeletal variations appeared increased at the high dose, but the
incidences were within the historical control range. The NOAEL for
maternal toxicity was 20 mg/kg bw/day based on depressed weight gain
at 60 mg/kg bw/day. The NOAEL for embryo-fetal toxicity was 60 mg/kg
bw/day based on increased abortions and post-implantation losses at
200 mg/kg bw/day (Keets et al., 1985).
Special studies on eye and skin irritation and hypersensitivity
Technical iprodione caused mild, transient eye irritation in
rabbits. The irritation was lessened by washing the eye immediately
after exposure (Babish, 1976c; Bonnette, 1991a).
Technical iprodione is not a dermal irritant in rabbits
(Babish, 1976d; Bonnette, 1991b) or a dermal sensitizer in guinea-
pigs (Trimmer et al., 1988).
Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
Iprodione has been consistently negative in assays for point
mutation, chromosomal aberration, and sister chromatid exchange. A
questionable positive result was reported for DNA damage in
Bacillus subtilis.
Observations in humans
No data available.
Table 2. Results of genotoxicity assays on iprodione
Test system Test object Concentration of Purity Results Reference
iprodione
Ames test (1) S. typhimurium 1-5000 µg/plate dissolved in DMSO 96.2% Negative Lawlor & Valentine (1990)
TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium 25-200µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
TA98, TA100, TA1535, 99.3% (1982a,b)
TA1537, TA1538
S. typhimurium 12.5-250 µg/plate dissolved in DMSO ? Negative Benazet & Cartier (1979)
Ta98, TA100, TA1535,
TA1537
E. coli mutation assay (1) E. coli K12, GY 5057 0.05-1000 µg/ml dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
strain 99.3% (1982a,b)
E. coli, W3110 (pol A+), 12.5-200 µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
p3478 (pol A-) 99.3% (1982a,b)
B. subtilis mutation B. subtilis rec. exc. 20-1670 µg/ml dissolved in DMSO 96.8% Positive Felkner (1985a)
assay (1) pol strains (19 strains) (3)
Saccharomyces cerevisiae Saccharomyces cerevisiae D7 62.5-500 µg/ml dissolved in DMSO 99.3% Negative Bouanchaud & Cartier
mutation assay (1) strain (1982a,b)
(2) Saccharomyces cerevisiae D7 250 µg/ml dissolved in DMSO ? Negative Benazet & Cartier (1979)
strain
CHO/HGPRT mutation assay (1) Chinese hamster ovary cells 5-1500 µg/ml dissolved in DMSO ? Negative Godek et al. (1985)
(CHO-K1-BH4)
In vitro cytogenetics (1) Chinese hamster ovary 15-400 µg/ml dissolved in DMSO ? Negative San Sebastian et al. (1985
cells (CHO-K1-BH4)
Table 2 (cont'd)
Test system Test object Concentration of Purity Results Reference
iprodione
In vitro sister chromatid Chinese hamster ovary cells 5-400 µg/ml dissolved in DMSO ? Negative Felkner (1985b)
exchange (1) (CHO-K1-BH4)
Dominant lethal assay CF-1 mice 0, 1500, 6000 ppm X 49 days ? Negative Hastings et al. (1974)
(WHO, 1978)
(1) Both with and without metabolic activation.
(2) Without metabolic activation.
(3) Positive at highest and lowest doses without metabolic activation. Problem with precipitation of test material, inadequate negative
and positive controls.
COMMENTS
Iprodione is extensively absorbed from the gastrointestinal
tract. It was extensively metabolized and rapidly excreted,
primarily in the urine, although relative faecal excretion of the
parent compound increased at high doses. Higher doses (for example
900 mg/kg bw) appeared to be absorbed to a lesser extent and
eliminated at a slower rate than lower doses (for example 50 mg/kg
bw). The elimination half-life was 7-9 h following a single low dose
and 13-20 h following a single high dose. The metabolic pathways
elucidated in rats involve dealkylation on the isopropyl carbamoyl
chain, hydroxylation of the aromatic ring and rearrangement and
opening of the hydantoin ring.
Iprodione had low acute toxicity by all routes of exposure. The
oral LD50 was greater than 1500 mg/kg bw in mice, rats, and dogs.
The World Health Organization has classified iprodione as unlikely
to present acute hazard in normal use (WHO, 1992).
In three 4-week studies in mice at dietary concentrations of 0,
600, 1900, 6000, 9500, or 15000 ppm, the lowest NOAEL was 600 ppm,
equal to 115 mg/kg bw/day, based on macroscopic hepatic changes at
1900 ppm. At 6000 ppm and above, the test material crystallized in
the tissues. In a 3-month study in mice at dietary concentrations of
0, 1500, 3000, 6000, or 12000 ppm (equal to 260, 510, 1100, and 2100
mg/kg bw/day) an increase in liver and adrenal gland weights and
hypertrophy and/or vacuolation of hepatocytes and adrenal cortical
cells were observed in all treated groups.
In a 3-month study in rats at dietary concentrations of 0, 300,
1000, or 3000 ppm, the NOAEL was 300 ppm, equal to 21 mg/kg bw/day.
Higher doses produced swelling in the zona glomerulosa of the
adrenal cortex. In another 3-month study in rats at dietary
concentrations of 0, 1000, 2000, 3000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 78 mg/kg bw/day, based on reduced body-weight
gain and histopathological changes in the adrenal glands, ovaries
and uterus at 2000 ppm and higher.
In a one-year study in dogs at dietary concentrations of 0,
100, 600, or 3600 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg
bw/day, based on the detection of Heinz bodies in erythrocytes and
decreased prostate gland weight at 600 ppm and higher. In a second
one-year study at dietary concentrations of 0, 200, 300, 400, or 600
ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day, based on
decreased erythrocyte values at 600 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 300, 1000, or 3000/2000 ppm, the NOAEL was 300
ppm, equal to 21 mg/kg bw/day, based on depressed body-weight at
1000 ppm and above. Reproductive performance was unaffected.
Offspring survival and growth were reduced at 3000/2000 ppm.
In a teratology study in rats using gavage doses of 0, 40, 90,
or 200 mg/kg bw/day, the NOAEL for maternal toxicity and
teratogenicity was 200 mg/kg bw/day. The NOAEL for embryofetal
toxicity was 90 mg/kg bw/day, based on slightly delayed fetal
development at 200 mg/kg bw/day. In rabbits administered 0, 20, 60,
or 200 mg/kg bw/day by gavage, the NOAEL for maternal toxicity was
20 mg/kg bw/day based on depressed weight gain at 60 mg/kg bw/day.
The NOAEL for embryofetal toxicity was 60 mg/kg bw/day based on
increased abortions and post-implantation loss at 200 mg/kg bw/day.
No teratogenic effects were found.
After consideration of the available genotoxicity data, the
Meeting concluded that iprodione was not genotoxic.
The former ADI, based on a multi-generation reproduction study
in rats, was revised. The new ADI was based on the results of
several studies, including the reproduction study in rats, the
teratology study in rabbits, and the one-year study in dogs. A
safety factor of 100 was applied to the NOAELs from these studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 600 ppm in the diet, equal to 115 mg/kg bw/day (four-
week study)
Rat: 300 ppm in the diet, equal to 21 mg/kg bw/day
(two-generation reproduction study)
500 ppm in the diet, equivalent to 25 mg/kg bw/day
(reproduction study reviewed by the 1977 Joint
Meeting)
Rabbit: 20 mg/kg bw/day (teratology study, maternal toxicity)
Dog: 400 ppm in the diet, equal to 18 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw/day
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing toxicological studies.
2. Observation in humans.
REFERENCES
Babish, J.C. (1976a). Acute oral toxicity in rats. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976b). Acute dermal toxicity in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976c). Eye irritation test in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976d). Primary skin irritation study with rabbits.
Unpublished report from Food and Drug Research Laboratories, Inc.,
Waverly, New York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Benazet, F. & Cartier, J.R. (1979). Iprodione (26 019 R.P.): In-
vitro mutagenicity study in Salmonella typhimurium (Ames' strains)
and in Saccharomyces cerevisiae (Zimmermann's strain D7).
Unpublished report No. RP/RD/CNG 20 177-E from Rhone-Poulenc, Vitry-
sur-Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Bonnette, K.L. (1991a). Primary eye irritation study in rabbits with
iprodione. Unpublished report No. 3147.109 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bonnette, K.L. (1991b). Primary skin irritation study in rabbits
with iprodione. Unpublished report No. 3147.108 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bouanchaud, D.H. & Cartier, J.R. (1982a). Iprodione (26 019 R.P.):
Supplementary studies of mutagenesis in microorganisms. Unpublished
report No. CRV/CNG 21 465 from Rhône-Poulenc, Vitry-sur-Seine,
France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Bouanchaud, D.H. & Cartier, J.R. (1982b). Iprodione (26 019 R.P.),
technical grade compound: In vitro mutagenesis in microorganisms.
Unpublished report No. CRV/CNG 21 466 from Rhône-Poulenc, Vitry-sur-
Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Broadmeadow, A. (1984). Iprodione: 52-week toxicity study in dietary
administration to beagle dogs. Unpublished report No. 84/RH0022/179
from Life Science Research, Suffolk, England. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Coombs, D.W. & Clark, G.C. (1977). RP 26019 technical: acute
inhalation toxicity-four hour LC50 in rats. Unpublished report No.
RNP 75/775 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Coquet, B. (1973a) Recherche du pouvoir teratogène chez le rat OFA
par voie orale du produit 26019 R.P. Unpublished report from Centre
de Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogène chez le lapin par
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1973c) Essai de toxicité chronique (3 mois) chez le
chien par voie orale du produit 26019 R.P. Unpublished report from
Centre de Recherche et d'Elevage des Onoins, No. 731008.
Coquet, B. (1976) Influence du produit 26019 R.P. sur la
reproduction du rat (essai sur 3 générations). Unpublished report
from Institut Français de Recherches et Essais biologiques, No.
760435 submitted by Rhône-Poulenc.
Cummins, H.A. (1989). Iprodione: acute oral toxicity study in the
rat. Unpublished report No. 89/RHA255/0391 from Life Science
Research, Suffolk, England. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985a). DNA damage in Bacillus subtilis with
iprodione technical. Unpublished report No. 2214 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985b). In vitro sister chromatid exchange in
Chinese hamster ovary cells (CHO). Unpublished report No. PH 319-BO-
001-84 from Borriston Laboratories, Inc., Temple Hill, Maryland,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990a). Iprodione: sub-acute toxicity to mice by
dietary administration for 13 weeks. Unpublished report No. RNP
323/90667 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990b). Iprodione: sub-acute toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RNP
322/90767 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Ganter, P., Pasquet, J., Spicer, E., Girard, P., & Myon, J. (1979).
Iprodione (26 019 R.P.): one month toxicity study in mice by dietary
administration. Unpublished report No. RP/RD/CNG 19 950 from Rhône-
Poulenc, Vitry-sur-Seine, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. & Myon, J. (1973). Produit No. 26019 R.P.
Toxicité à terme (5 mois) chez le rat par voie orale, en comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R,P). Unpublished
report from Laboratoires de Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 17124 submitted by Rhône-
Poulenc.
Ganter, P. & Girard, P. (1973). Produit No. 26019 R.P. Examen
histologique de la toxicité oculaire du 26019 R,LO, chez le chien
après un traitement de 3 mois. Unpublished report from Laboratoires
de Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131 submitted by Rhône-Poulenc.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1985). CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report No.
PH 314-BO-001-84 from Borriston Laboratories, Inc., Temple Hill,
Maryland, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Hallifax, D. (1989). Iprodione: absorption, distribution, metabolism
and excretion study in the rat. Unpublished report No.
89/RPM005/1013 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E., Huffman, K.W., & M.A. Gallo (1974). RP 26019:
Dominant lethal mutagenicity in mice. Unpublished report No. SEH
74:69-CH-47 from Hess & Clark, Ashland, Ohio, USA. Submitted to WHO
by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E. & Huffman, K.W. (1975). Chronic toxicologic and
carcinogenic study with RP 26019 in mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:223. Submitted to
WHO by Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C., & Kiggins, E.M. (1976). Chronic
toxicologic and carcinogenic study with RP 26019 in rats.
Unpublished report from Rhodia, Inc., Hess and Clark Division, No.
76:57. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1991). Two-generation reproduction study with
iprodione technical in rats. Unpublished report No. HLA 6224-154
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Huffman, K.W. (1974). Subacute toxicity, RP 26 019, mice.
Unpublished report No. KWH 74:43 from Hess & Clark, Ashland, Ohio,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Itabashi, M., Hayashi, K., Kudo, S., Maruyama, Y., Takahara, K., &
Tajima, M. (1978). Three-month dietary oral toxicity study of 26.019
in rats. Unpublished report from Nippon Institute for Biological
Science, Japan. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Jones, A.G. (1983). Fungicides: Iprodione. Exposure of personnel
mixing ROVRAL WP. Unpublished report No. 428 from May & Baker, Ltd.,
Essex, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Kangas, L. (1991). A 52-week dietary toxicity study of iprodione in
the beagle dog. Unpublished report No. 84296 from Bio-Research
Laboratories Ltd., Senneville, Quebec, Canada. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Keets, S.A., Leist, P.L., Mercieca, M.D., & Clevidence, K.J. (1985).
A teratology study in rabbits with iprodione. Unpublished report No.
WIL-21028 from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Laurent, M. & Buys M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques, Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. & Chabassol, Y. (1976)
Etude du métabolism chez le rat à l'aide du produit marqué au 14C.
Unpublished report from Rhône Poulenc, Direction des Recherches et
du Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-
Seine, C.N.G. An. No. 18548, submitted by Rhône Poulenc.
Laurent, M., Bredenbac, J., Chabassol, Y., & Marlard, M. (1983).
Iprodione. Percutaneous metabolism in the rat. Unpublished report
No. CNG-An 4675-E from Rhône-Poulenc Research, Vitry-sur-Seine,
France. Submitted to WHO by Rhone-Poulenc Agrochimie S.A., Lyon,
France.
Lawlor, T.E. & Valentine, D.C. (1990). Mutagenicity test on
iprodione (technical) in the Salmonella/mammalian-microsome
reverse mutation assay (Ames test) with confirmatory assay.
Unpublished report No. HLA 11092-0-401R from Hazleton Laboratories
America, Inc., Kensington, Maryland, USA. Submitted to WHO by Rhône-
Poulenc Agrochimie S.A., Lyon, France.
Pasquet, J. & Mazuret, A. (1974). 26 019 R.P.: acute toxicity and
local tolerance. Unpublished report No. SUCRP-DSPh 17 746 from
Rhône-Poulenc, Paris, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Plutnick, R.T. & Kapp, R.W. (1988). Iprodione (technical) - acute
dermal limit test in the rabbit. Unpublished report No. 209806 from
Exxon Biomedical Sciences, Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
San Sebastian, J.R., Naismith, R.W., & Matthews, R.J. (1985). In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Unpublished report No. PH 320-BO-001-84 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Stevens, K.R. (1974). Subacute toxicity, RP 26 019. Unpublished
report No. KRS 74:3 from Hess & Clark, Ashland, Ohio, USA. Submitted
to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976a). Acute toxicity of Rovral (26019RP). I. Oral,
intraperitoneal and subcutaneous administration in rats. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976b). Acute toxicity of Rovral (26019RP). II. Oral,
intraperitoneal and subcutaneous administration in mice. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., & Deans, C.F. (1986a). Iprodione
(technical grade): effects of oral administration upon pregnancy in
the rat. 1. Dosage range-finding study. Unpublished report No.
85/RHA063/752 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., Lambert, E.P., Wilby, O.K., & Tesh, S.A.
(1986b). Iprodione (technical grade): teratology study in the rat.
Unpublished report No. 85/RHA064/765 from Life Science Research,
Suffolk, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Trimmer, G.W. (1988). Iprodione (technical) - dermal sensitization
test in the guinea-pig (Buehler method). Unpublished report No.
209821 from Exxon Biomedical Sciences, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Short-term toxicity studies
Mice
Groups (10-20/sex/dose) of CF-1 mice received iprodione (purity
unspecified) in the diet daily for 28 days at 0, 600, 1900, 6000,
9500, or 15000 ppm (equal to 130, 390, 950, 1500 or 2300 mg/kg
bw/day for males and 120, 420, 1000, 1500 or 2400 mg/kg bw/day for
females). The highest dose produced mortality and depressed body-
weight gain and food consumption. Exposure to 6000 ppm and above
produced ataxia and lethargy during the first week of treatment. The
liver was affected in groups receiving 6000 ppm and above. Absolute
and relative liver weights were increased, livers had a stippled
appearance on gross examination, and the incidence of hepatocyte
vacuolation and focal eosinophilic degeneration was increased. At
15000 ppm granulomatous inflammation, (possibly in response to a
foreign body), was observed in the heart, liver, and kidney. The
NOAEL was 1900 ppm (equal to 390 mg/kg bw/day for males and 420
mg/kg bw/day for females) based on liver effects at 6000 ppm and
above (Stevens, 1974).
Groups of 10 CF-1 Carworth mice/sex received iprodione (purity
unspecified) in the diet daily for 4 weeks at 0, 600, 1900, 6000,
9500 or 15000 ppm, (equal to 115, 366, 1090, 1860 or 4030 mg/kg
bw/day for males - the high dose based on first two weeks only - and
137, 439, 1310, 2090 or 2590 for females). The high dose produced
mortality and depressed body-weight. Depression and ataxia were
observed at 6000 ppm and higher. Relative liver weight was increased
at 6000 ppm and above. Gross necropsy revealed white foci in the
liver of mice receiving 1900 ppm and higher, a stippled appearance
of the liver at 6000 ppm and higher, and liver enlargement at 9500
and 15000 ppm. White foci and granulomatous inflammation were
observed in numerous tissues, primarily at the high dose. One
granulomatous lesion was observed in the liver at 6000 ppm and 5
were observed in the bladder at 9500 ppm. The presence of
spindle-shaped clear spaces in tissues and foreign body type giant
cells suggested a reaction to crystal formation. Liver cell
hypertrophy was increased at 6000 ppm and above. The NOAEL was 600
ppm (equal to 115 mg/kg bw/day for males and 137 mg/kg bw/day for
females) based on gross changes in the liver at 1900 ppm and above
(Huffman, 1974).
Table 1. Acute toxicity of iprodione
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Mouse CD-1 M oral 1870 Takehara et al. (1976b)
F 2670
CD-1 M i.p. 900 Takehara et al. (1976b)
F 625
CD-1 M subcutaneous >6700 Takehara et al. (1976b)
F >6700
Rat CD M oral >2000 Cummins (1989)
F >20002
CD M oral 2060 Takehara et al. (1976a)
F 1530
Wistar M&F oral 3700 Babish (1976a)
CD M i.p. 1330 Takehara et al. (1976a)
F 700
CD M subcutaneous >4500 Takehara et al. (1976a)
F >4500
Sprague-Dawley M inhalation >3.29 Coombs & Clark (1977)
F (4-hr exp) >3.29
Table 1 (cont'd)
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Rabbit New Zeeland white M dermal (24-hr exp) >2000 Plutnick et al. (1988)
F >2000
? ? dermal (?-hr exp) >30 000 Babish (1976b)
Dog Beagle M oral >2000 Pasquet & Mazuret (1974)
F >2000 (FAO/WHO, 1978)
1 Purity of technical iprodione was 95.8%
2 Purity of technical iprodione was 97.9%
The crystal formation in tissues was further evaluated using
groups of 15 CD-1 mice/sex receiving iprodione (93.5%) in the diet
daily for 4 weeks at 0, 1900, 6000, 9500 or 15000 ppm (equivalent to
290, 900, 1400, and 2300 mg/kg bw/day). The two highest dose levels
produced mortality, clinical signs of toxicity, and depressed weight
gain and food consumption. At 6000 ppm and above, crystalline
deposits and effects on the liver were observed. Granulomatous
lesions surrounding crystal deposits were found frequently in the
urinary bladder and occasionally in liver parenchyma, myocardium,
diaphragmatic muscle, and skeletal muscle. It was speculated that
the crystals contained a major metabolite of iprodione, 32490 R.P.,
which was identified in the liver. Liver effects included increased
weight, pale and mottled appearance on gross examination, and
swelling of hepatocytes to form large homogeneous areas in the
centrilobular region of the liver. Histopathological changes in the
testes and spleen were observed at doses levels above 6000 ppm. A
NOAEL of 1900 ppm (equivalent to 290 mg/kg bw/day) was determined
based on crystalline deposits in the urinary bladder, liver
enlargement, and hepatocellular swelling at 6000 ppm and above
(Ganter et al., 1979).
Groups of 10 Crl:CD-1(ICR)BR mice/sex received iprodione (95.7%
purity) in the diet daily for 13 weeks at 0, 1500, 3000, 6000, or
12000 ppm, (equal to 260, 510, 1100 or 2100 mg/kg bw/day for males
and 330, 660, 1300 or 2600 mg/kg bw/day for females). The high dose
was associated with mortality, clinical signs of toxicity, weight
loss, and reduced food consumption. The two highest dose levels
produced crystalline deposits with associated multinucleated cells
in a number of tissues, particularly in the urinary bladder. At the
lowest dose level of 1500 ppm, liver and adrenal gland weights were
increased in females. The incidence and severity of liver cell
enlargement in males and females showed a dose-related increase
beginning with the 1500 ppm dose level. Enlargement and vacuolation
of adrenal cortex cells were increased in treated groups. The
incidence of vacuolation of zona fasciculata cells was 0/10, 1/10,
3/10, 6/10, and 4/9 in females receiving 0, 1500, 3000, 6000 or
12000 ppm, respectively. At 3000 ppm and higher, effects were
observed in the kidney, uterus, ovary, and spleen. The authors
determined that dose levels of 6000 ppm or greater were unsuitable
for a long-term study in mice because of crystal formation and
accompanying effects in the urinary bladder. A NOAEL was not
identified in this study. The lowest dose of 1500 ppm was associated
with enlargement of the liver and adrenal glands and microscopic
changes in these organs (Fryer et al., 1990a).
Rats
Groups of 15 CD/CRJ rats/sex received iprodione (purity
unspecified) in the diet daily for 3 months at 0, 300, 1000, or 3000
ppm, (equal to 21, 70 or 210 mg/kg bw/day for males and 24, 82 or
240 mg/kg bw/day for females). The high dose of 3000 ppm produced
clinical signs of toxicity (i.e. piloerection, rough fur), depressed
food and water consumption, and decreased body-weight (final weight
reduced 20-40%). A number of absolute and relative organ weights
were decreased at the high dose including the liver, spleen, thymus,
kidneys, and heart. Microscopic findings were observed at the high
dose in the liver, spleen, and thymus. Swelling of the zona
glomerulosa of the adrenal gland showed a dose-related increase at
1000 and 3000 ppm in both sexes. The NOAEL was 300 ppm (equal to 21
mg/kg bw/day for males and 24 mg/kg bw/day for females) based on
microscopic changes in the adrenal cortex at 1000 ppm and above
(Itabashi et al., 1978).
Groups of 10 Crl:CD (SD) BR rats/sex received iprodione (95.7%
purity) in the diet daily for up to 13 weeks at 0, 1000, 2000, 3000,
or 5000 ppm (equal to 78, 150 or 250 mg/kg bw/day for males and 89,
180 or 270 mg/kg bw/day for females). The high dose group was
terminated during week 8 due to excessive toxicity which included a
progressive decrease in food intake and body-weight and the death of
one male. Upon examination these animals showed abnormalities in the
liver, adrenal glands, uterus, ovaries, prostate, and seminal
vesicles. Clinical signs (i.e. hunched posture, piloerection,
emaciation) occurred in males and females receiving 3000 ppm. Body-
weight, food intake, and food efficiency were reduced at the 2000
and 3000 ppm. Ovary weight was decreased at 2000 and 3000 ppm, and
uterus weight was reduced at 3000 ppm. Seminal vesicles and the
prostate gland of males receiving 3000 ppm were reported as smaller
than normal but no histological changes were noted.
Histopathological changes were in the adrenal glands, uterus, and
ovaries were increased at 2000 and 3000 ppm. Adrenal gland findings
included enlarged cells of the zona glomerulosa in males and
females and vacuolation of the zone fasciculata mainly in females.
The uterus showed signs of atrophy and ovaries contained reduced
numbers of corpora lutea. A NOAEL of 1000 ppm equal to 78 mg/kg
bw/day for males and 89 mg/kg bw/day for females was determined,
based on a reduced body-weight gain and histopathological changes at
2000 ppm and higher (Fryer et al., 1990b).
Groups of 15 male and 15 female caesarean originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the
diet for 5 months. No effects were observed on mortaility, food
consumption, haematology (as judged by haemoglobin, haematocrit,
erythrocyte count, or total and differential leucocyte count)
clinical chemistry (as judged by BSP, SGOT, SGPT or SAP) or
urinalysis. Body weight gain was slightly reduced (especially in
males) at 500 and 1000 ppm. Absolute (but not relative) heart weight
was reduced in males at 500 and 1000 ppm, and absolute kidney weight
was reduced at 1000 ppm. In females, absolute liver and kidney
weights were significantly reduced at 500 ppm only. Gross and
histopathology were normal at all dose levels. In a parallel study,
dichlozoline, a structurally related compound, induced cataracts. No
such effect was seen with iprodione (Ganter et al., 1973a).
Dogs
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 or 7200 ppm for
a period of 3 months. At the top dose level the method of
administration was altered after 6 weeks, to gelatine capsules. The
treatment did not affect mortality. The recorded values of
haematological determinations and urinalyses were within normal
limits, as judged by haemoglobin, haematocrit, reticulocyte
erythrocyte count, total and differential leucocyte count and
prothrombine time, except for signs of mild anaemia in 1 male and 1
female at 2 months and 1 male at 3 months at the top dose level. At
7200 ppm a reduction of food consumption was observed, accompanied
by reduced body-weight gain. Opththalmosopic examination of the
animals did not reveal any pathological alterations. A transient
increase in SGOT and SGPT was observed after 1 and 2 months of
treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, which was also
observed in females at 2400 ppm and above. At 7200 ppm reduced
relative weight of testes was found, but there was no histological
indication of damage. The histopathological findings did not reveal
any indication of treatment-related alterations of tissues (Coquet,
1973c; Gunter & Girard, 1973).
Groups of 6 beagle dogs/sex received iprodione (96.5% purity)
in the diet daily for 52 weeks at 0, 100, 600, or 3600 ppm, (equal
to 4.1, 24.9 or 145 mg/kg bw/day for males and 4.3, 28.3 or 153
mg/kg bw/day for females). Ophthalmology, haematology, clinical
chemistry, and urinalysis tests were performed periodically over the
course of the study. Slight retinal hyperreflection was noted more
frequently in males receiving 3600 ppm and in females receiving 600
or 3600 ppm. The severity did not increase over time or with dose
level and was not seen consistently in the same animals over time.
Red blood cells were affected at 3600 ppm and possibly at 600 ppm.
Compared to controls, high dose males showed a slight but consistent
decrease in erythrocyte count, haemoglobin, and haematocrit over the
course of the study. Females receiving the high dose showed a less
consistent pattern of anaemia. Platelet count and partial
thromboplastin time were increased over the course of the study in
high dose males and females. The high dose was also associated with
an increase in frequency and severity of erythrocytes containing
Heinz bodies. After 52 weeks, 3/6 control males and 4/6 control
females showed the presence of Heinz bodies compared to all males
and females receiving the high dose. In control dogs the number of
affected erythrocytes was 0-2 per field compared to 6-50 or over 50
per field in dogs receiving the high dose. During the first one-
third of the study, the incidence and severity of Heinz bodies were
also increased in males receiving 600 ppm. No treatment-related
changes were noted in the bone marrow.
Plasma ALP was consistently increased over control values and
was above the normal range in high dose males and females. In high
dose females, serum ALT was transiently elevated early in the study
and LDH was transiently increased late in the study. Total bilirubin
and albumin were slightly elevated during the study particularly in
high dose females. Absolute and relative liver and adrenal gland
weights were increased at the high dose in males and females.
Absolute prostate gland weight was decreased 22% at 600 ppm and 41%
at 3600 ppm. Relative prostate gland weight was also reduced at the
high dose (39%). Two high dose females had enlarged adrenal glands
and one high dose male had a swollen liver on gross examination.
Nematode granulomas were found in various tissues of a number of
control and treated dogs. Treatment-related microscopic changes were
observed in the liver, adrenal glands, and urinary bladder of high
dose males and females. In the urinary bladder, the majority of high
dose males and females had submucosal granulomas and giant cells
containing crystals. The majority of high dose males and females had
fat vacuolation and/or pallid appearance of the adrenal cortex
compared to an absence or lower incidence in controls. Hepatic
centriacinar atrophy was observed in 3/6 males and 4/6 females
receiving the high dose compared to no controls with the lesion.
Pigmented macrophage agglomerates in the liver were more prominent
in the high dose groups than in controls. An additional finding in
females was lipofuscinosis in the proximal convoluted tubules of the
kidney occurring in 2/6, 0/6, 4/5, and 4/6 in the 0, 100, 600, and
3600 ppm groups, respectively. No treatment-related histopathologic
changes were noted in the prostate gland. A NOAEL of 100 ppm, equal
to 4.1 mg/kg bw/day, was determined, 600 ppm representing a minimal
toxic effect level with the majority of toxic changes occurring at
the 3600 ppm dose level (Broadmeadow, 1984).
A second one-year dog study was conducted to determine the
NOAEL between 100 and 600 ppm. Groups of 6 beagle dogs/sex received
iprodione (96% purity) in the diet daily for 52 weeks at 0, 200,
300, 400, or 600 ppm, (equal to 7.8, 12, 18 or 25 mg/kg bw/day for
males and 9.1, 13, 18 or 26 mg/kg bw/day for females). Eye
examinations and haematology were performed during weeks 4, 8, 12,
20, 28, 36, and 52. The adrenal glands, kidneys, and prostate were
the only organs weighed. Kidneys of females and prostate of males
were the only tissues examined microscopically. Iprodione treatment
had no effect on survival, body weight, body-weight gain, food
consumption, or food efficiency. Most dogs treated with iprodione
developed skin lesions consisting of redness and scab formation
occurring primarily in the inguinal, hindlimb, and ventral abdominal
areas. Skin lesions were less frequent and less severe in controls.
The majority of lesions had cleared by study termination. Tests for
mange and fungal infection were negative. It was noted that slight
irritation of the skin was not uncommon in dogs fed powdered chow.
Eye examinations revealed no treatment-related abnormalities.
Haematology parameters were within the normal range for all groups.
In females receiving 600 ppm, erythrocyte count, haemoglobin, and
haematocrit were reduced at week 4 (15%) and at week 36 (10%). In
both males and females receiving 600 ppm, values for these
erythrocyte parameters were consistently lower than control values
through week 36, although the differences were not statistically
significant. Heinz bodies were not observed in any control or
treated animal at any time point. Organ weights (kidney, adrenal
glands, prostate) were unaffected, and no treatment-related
histopathological changes were observed in the kidneys of females or
prostate of males. Based on slight changes in erythrocyte parameters
at 600 ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day (Kangas
et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 60 male and 60 female mice were maintained on a diet
containing the test compound at 0, 200, 500 or 1250 ppm for 18
months. No treatment-related effects on body-weight, food
consumption or mortality were found. The recorded values of the
haematological, blood chemistry and urinalyses tests, performed
after 6, 12 and 18 months of the feeding period, were within the
physiological range. Necropsy findings on mice that died during the
last 6 months of the test and on those sacrificed at the termination
date showed an increased number of enlarged lymph nodes in males at
200 ppm. Organ weight variations occurred sporadically in the
various dose groups which were considered not to be treatment-
related. The histopathological observations failed to reveal
abnormal features. The distribution of neoplastic and non-neoplastic
findings did not appear to demonstrate any significant dose
dependence. The most common tumours were lymphosarcomas involving
the spleen, lymph nodes and thymus (Hastings & Huffmann, 1975).
Rats
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 or 1000 ppm iprodione for 24 months. Slight
reduction in body weight gain was observed at 1000 ppm. This was
accompained by some reduction in food intake. The treatment had no
effect on food consumption, mortality or values of the haematologic,
blood chemistry or urinalyses determinations. Necropsy findings did
not reveal any drug-related gross alterations. Variations in organ
weight did not show a group distribution and did not seem to be
related to drug administration. Histopathology did not indicate a
treatment relationship with neoplastic or non-neoplastic findings.
At 24 months the most common tumours observed were pituitary
adenomas and adenocarcinomas and fibroadenoma of the mammary glands
(Hastings et al., 1976).
Reproduction studies
Rats
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 or 1000 ppm
for the first 5 weeks of each generation and 0, 250, 500 or 2000 ppm
for the next 8 weeks of treatment. The diet was fed through 3
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parent animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendancy for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rats of the third generation did not reveal
abnormalities (Coquet, 1976).
The reproductive toxicity of iprodione was studied in two
successive generations of Crl:CD BR/VAF/Plus rats. The first
parental (F0) animals were mated twice to produce F1a and F1b
litters. F1a animals were mated twice to produce F2a and F2b
litters. Groups (28/sex) received diets containing 0, 300, 1000, or
3000 ppm of iprodione (96.2% purity) beginning at least 10 weeks
prior to mating. The high dose of 3000 ppm was reduced to 2000 ppm
at the time of the first mating of F1a rats because of excessive
toxicity. The administered doses were equal to 17, 55 or 160 mg/kg
bw/day for F0 males; 21, 71 or 210 mg/kg bw/day for F0 females;
20, 68, and 150 mg/kg bw/day for F1 males; and 25, 82 or 190 mg/kg
bw/day for F1 females. Toxicity in adult rats was observed at 1000
ppm and above. Body-weight, body-weight gain, and food consumption
were reduced throughout the treatment period in F0 and F1 males
receiving 3000 ppm and F0 and F1 females receiving 1000 or 3000
ppm. Reproductive performance was unaffected by iprodione exposure.
Offspring toxicity was observed at the high dose. During lactation
(16-21 days of age), F1a and F1b pups exhibited signs of
toxicity including unkempt or hunched appearance, slow movement, and
tremors during the last days of lactation. In both generations,
litter size number of live pups, and pup weight were decreased at
the high dose. The NOAEL was 300 ppm, equal to 21 mg/kg bw/day, for
parental toxicity based on reduced parental body-weight at 1000 ppm
and higher; for embryofetal toxicity, the NOAEL was 1000 ppm, based
on clinical signs, reduced litter size, and reduced pup weight at
3000 ppm (Henwood, 1991).
Special studies on embryo/fetotoxicity
Rats
Groups of 25-30 rats were treated orally with 0, 100, 200 or
400 mg/kg bw/day iprodione on gestation days 5 to 15. Females at 400
mg/kg bw/day showed reduced fertility, reduced body weight gain and
a dose-related reduction in food consumption, especially during the
treatment period. The number of implantations was also reduced at
the highest dose level. There was no indication of an embryotoxic or
teratogenic effect of the test compound (Coquet, 1973a).
In a dose range-finding study in CD pregnant rats, treatment
with 400 and 800 mg/kg bw/day resulted in mortality, weight loss,
and pronounced clinical signs. Treatment with 240 mg/kg bw/day
reduced weight gain and produced clinical signs, and 120 mg/kg
bw/day occasionally produced clinical signs (i.e. flaccid muscles).
The lowest dose of 40 mg/kg bw/day had no effect on maternal health.
It was concluded that dose levels should not exceed 240 mg/kg bw/day
in a definitive developmental toxicity study (Tesh et al., 1986a).
In the definitive study, iprodione (94.2%) was administered by
oral gavage to groups of 25 mated female CD rats at doses of 0, 40,
90, or 200 mg/kg bw/day. Controls received the vehicle (aqueous
methylcellulose). Rats were treated days 6-15 of gestation and
sacrificed on day 21. The administered doses had no adverse effect
on maternal health as assessed by mortality, clinical signs, body-
weight, and food consumption. In the offspring, the incidence of
space between body wall and organs was slightly increased at the
high dose. The incidences were 4.3%, 5.3%, 5.8%, and 11.2% on a
fetal basis and 15%, 20%, 20%, and 32% on a litter basis at 0, 40,
90 and 200 mg/kg bw/day, respectively. The majority of affected
fetuses were small in size. It was noted that this observation was
associated with fetuses of low body-weight in previous studies and
was indicative of slight immaturity. The incidence of small fetuses
(<2.7g) in this study was 2.9%, 5.1%, 4.9%, and 8.0% (fetal basis).
Group mean fetal body-weights were 3.27, 3.18, 3.16, and 3.15 g. The
findings collectively were suggestive of a slight effect on fetal
developmental at the high dose despite the fact that none of the
differences were statistically significant and values at the high
dose were within the historical control range. A NOAEL for maternal
toxicity of 200 mg/kg bw/day and a NOAEL for embryo-fetal toxicity
of 90 mg/kg bw/day were determined, based on slightly delayed fetal
development at 200 mg/kg bw/day. There was no evidence of
teratogenic potential at the highest dose tested (200 mg/kg bw/day)
(Tesh et al., 1986b).
Rabbits
Groups of 15-17 New Zeeland white rabbits were intubated on
gestation days 6-16 inclusive with 0, 100, 200 or 400 mg/kg bw/day
iprodione. Body-weight gain, over the period of treatment, was
slightly reduced at 100 mg/kg bw/day, and a dose-related weight loss
occurred at 200 and 400 mg/kg bw/day. Food intake was reduced at 200
mg/kg bw/day and above. At 400 mg/kg bw/day 9 of 17 females died,
and only one of the four remaining pregnant animals carried to term.
Fetal loss was increased at 200 mg/kg bw/day, and the fetal weight
was reduced at 200 mg/kg bw/day and above. Multiple malformations
occurred in 1 of 68 living fetuses at 200 mg/kg bw/day. Minor
malformations were noted in all groups (Coquet, 1973b).
The developmental toxicity of iprodione was studied in New
Zeeland white rabbits. Iprodione (95.0% purity) was administered by
oral gavage to groups of 18 artificially inseminated females at
doses of 0, 20, 60, or 200 mg/kg bw/day. The control group received
the vehicle (aqueous methylcellulose). Rabbits were treated on days
6-18 of gestation and sacrificed on day 29. Maternal toxicity was
produced at 60 and 200 mg/kg bw/day. The high dose group experienced
weight loss and reduced food consumption during the entire treatment
period. Clinical signs associated with the high dose included hair
loss, diarrhoea, and decreased urination and defecation. The group
receiving 60 mg/kg bw/day experienced slight body-weight loss during
the first six days of treatment compared to positive weight gain by
controls. Seven of 18 females receiving the high dose aborted
compared to 1/18, 0/18, and 1/18 for the 0, 20, and 60 mg/kg bw/day
groups. Ten does receiving the high dose delivered litters, two of
which had totally resorbed litters (compared to 1/13 controls)
resulting in only 8 viable litters at the high dose. A NOAEL of 20
mg/kg bw/day was determined based on depressed maternal weight gain
at 60 mg/kg bw/day and above. The high dose of 200 mg/kg bw/day was
considered an excessive dose for a teratology evaluation. Some
skeletal variations appeared increased at the high dose, but the
incidences were within the historical control range. The NOAEL for
maternal toxicity was 20 mg/kg bw/day based on depressed weight gain
at 60 mg/kg bw/day. The NOAEL for embryo-fetal toxicity was 60 mg/kg
bw/day based on increased abortions and post-implantation losses at
200 mg/kg bw/day (Keets et al., 1985).
Special studies on eye and skin irritation and hypersensitivity
Technical iprodione caused mild, transient eye irritation in
rabbits. The irritation was lessened by washing the eye immediately
after exposure (Babish, 1976c; Bonnette, 1991a).
Technical iprodione is not a dermal irritant in rabbits
(Babish, 1976d; Bonnette, 1991b) or a dermal sensitizer in guinea-
pigs (Trimmer et al., 1988).
Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
Iprodione has been consistently negative in assays for point
mutation, chromosomal aberration, and sister chromatid exchange. A
questionable positive result was reported for DNA damage in
Bacillus subtilis.
Observations in humans
No data available.
Table 2. Results of genotoxicity assays on iprodione
Test system Test object Concentration of Purity Results Reference
iprodione
Ames test (1) S. typhimurium 1-5000 µg/plate dissolved in DMSO 96.2% Negative Lawlor & Valentine (1990)
TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium 25-200µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
TA98, TA100, TA1535, 99.3% (1982a,b)
TA1537, TA1538
S. typhimurium 12.5-250 µg/plate dissolved in DMSO ? Negative Benazet & Cartier (1979)
Ta98, TA100, TA1535,
TA1537
E. coli mutation assay (1) E. coli K12, GY 5057 0.05-1000 µg/ml dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
strain 99.3% (1982a,b)
E. coli, W3110 (pol A+), 12.5-200 µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
p3478 (pol A-) 99.3% (1982a,b)
B. subtilis mutation B. subtilis rec. exc. 20-1670 µg/ml dissolved in DMSO 96.8% Positive Felkner (1985a)
assay (1) pol strains (19 strains) (3)
Saccharomyces cerevisiae Saccharomyces cerevisiae D7 62.5-500 µg/ml dissolved in DMSO 99.3% Negative Bouanchaud & Cartier
mutation assay (1) strain (1982a,b)
(2) Saccharomyces cerevisiae D7 250 µg/ml dissolved in DMSO ? Negative Benazet & Cartier (1979)
strain
CHO/HGPRT mutation assay (1) Chinese hamster ovary cells 5-1500 µg/ml dissolved in DMSO ? Negative Godek et al. (1985)
(CHO-K1-BH4)
In vitro cytogenetics (1) Chinese hamster ovary 15-400 µg/ml dissolved in DMSO ? Negative San Sebastian et al. (1985
cells (CHO-K1-BH4)
Table 2 (cont'd)
Test system Test object Concentration of Purity Results Reference
iprodione
In vitro sister chromatid Chinese hamster ovary cells 5-400 µg/ml dissolved in DMSO ? Negative Felkner (1985b)
exchange (1) (CHO-K1-BH4)
Dominant lethal assay CF-1 mice 0, 1500, 6000 ppm X 49 days ? Negative Hastings et al. (1974)
(WHO, 1978)
(1) Both with and without metabolic activation.
(2) Without metabolic activation.
(3) Positive at highest and lowest doses without metabolic activation. Problem with precipitation of test material, inadequate negative
and positive controls.
COMMENTS
Iprodione is extensively absorbed from the gastrointestinal
tract. It was extensively metabolized and rapidly excreted,
primarily in the urine, although relative faecal excretion of the
parent compound increased at high doses. Higher doses (for example
900 mg/kg bw) appeared to be absorbed to a lesser extent and
eliminated at a slower rate than lower doses (for example 50 mg/kg
bw). The elimination half-life was 7-9 h following a single low dose
and 13-20 h following a single high dose. The metabolic pathways
elucidated in rats involve dealkylation on the isopropyl carbamoyl
chain, hydroxylation of the aromatic ring and rearrangement and
opening of the hydantoin ring.
Iprodione had low acute toxicity by all routes of exposure. The
oral LD50 was greater than 1500 mg/kg bw in mice, rats, and dogs.
The World Health Organization has classified iprodione as unlikely
to present acute hazard in normal use (WHO, 1992).
In three 4-week studies in mice at dietary concentrations of 0,
600, 1900, 6000, 9500, or 15000 ppm, the lowest NOAEL was 600 ppm,
equal to 115 mg/kg bw/day, based on macroscopic hepatic changes at
1900 ppm. At 6000 ppm and above, the test material crystallized in
the tissues. In a 3-month study in mice at dietary concentrations of
0, 1500, 3000, 6000, or 12000 ppm (equal to 260, 510, 1100, and 2100
mg/kg bw/day) an increase in liver and adrenal gland weights and
hypertrophy and/or vacuolation of hepatocytes and adrenal cortical
cells were observed in all treated groups.
In a 3-month study in rats at dietary concentrations of 0, 300,
1000, or 3000 ppm, the NOAEL was 300 ppm, equal to 21 mg/kg bw/day.
Higher doses produced swelling in the zona glomerulosa of the
adrenal cortex. In another 3-month study in rats at dietary
concentrations of 0, 1000, 2000, 3000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 78 mg/kg bw/day, based on reduced body-weight
gain and histopathological changes in the adrenal glands, ovaries
and uterus at 2000 ppm and higher.
In a one-year study in dogs at dietary concentrations of 0,
100, 600, or 3600 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg
bw/day, based on the detection of Heinz bodies in erythrocytes and
decreased prostate gland weight at 600 ppm and higher. In a second
one-year study at dietary concentrations of 0, 200, 300, 400, or 600
ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day, based on
decreased erythrocyte values at 600 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 300, 1000, or 3000/2000 ppm, the NOAEL was 300
ppm, equal to 21 mg/kg bw/day, based on depressed body-weight at
1000 ppm and above. Reproductive performance was unaffected.
Offspring survival and growth were reduced at 3000/2000 ppm.
In a teratology study in rats using gavage doses of 0, 40, 90,
or 200 mg/kg bw/day, the NOAEL for maternal toxicity and
teratogenicity was 200 mg/kg bw/day. The NOAEL for embryofetal
toxicity was 90 mg/kg bw/day, based on slightly delayed fetal
development at 200 mg/kg bw/day. In rabbits administered 0, 20, 60,
or 200 mg/kg bw/day by gavage, the NOAEL for maternal toxicity was
20 mg/kg bw/day based on depressed weight gain at 60 mg/kg bw/day.
The NOAEL for embryofetal toxicity was 60 mg/kg bw/day based on
increased abortions and post-implantation loss at 200 mg/kg bw/day.
No teratogenic effects were found.
After consideration of the available genotoxicity data, the
Meeting concluded that iprodione was not genotoxic.
The former ADI, based on a multi-generation reproduction study
in rats, was revised. The new ADI was based on the results of
several studies, including the reproduction study in rats, the
teratology study in rabbits, and the one-year study in dogs. A
safety factor of 100 was applied to the NOAELs from these studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 600 ppm in the diet, equal to 115 mg/kg bw/day (four-
week study)
Rat: 300 ppm in the diet, equal to 21 mg/kg bw/day
(two-generation reproduction study)
500 ppm in the diet, equivalent to 25 mg/kg bw/day
(reproduction study reviewed by the 1977 Joint
Meeting)
Rabbit: 20 mg/kg bw/day (teratology study, maternal toxicity)
Dog: 400 ppm in the diet, equal to 18 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw/day
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing toxicological studies.
2. Observation in humans.
REFERENCES
Babish, J.C. (1976a). Acute oral toxicity in rats. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976b). Acute dermal toxicity in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976c). Eye irritation test in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976d). Primary skin irritation study with rabbits.
Unpublished report from Food and Drug Research Laboratories, Inc.,
Waverly, New York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Benazet, F. & Cartier, J.R. (1979). Iprodione (26 019 R.P.): In-
vitro mutagenicity study in Salmonella typhimurium (Ames' strains)
and in Saccharomyces cerevisiae (Zimmermann's strain D7).
Unpublished report No. RP/RD/CNG 20 177-E from Rhone-Poulenc, Vitry-
sur-Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Bonnette, K.L. (1991a). Primary eye irritation study in rabbits with
iprodione. Unpublished report No. 3147.109 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bonnette, K.L. (1991b). Primary skin irritation study in rabbits
with iprodione. Unpublished report No. 3147.108 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bouanchaud, D.H. & Cartier, J.R. (1982a). Iprodione (26 019 R.P.):
Supplementary studies of mutagenesis in microorganisms. Unpublished
report No. CRV/CNG 21 465 from Rhône-Poulenc, Vitry-sur-Seine,
France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Bouanchaud, D.H. & Cartier, J.R. (1982b). Iprodione (26 019 R.P.),
technical grade compound: In vitro mutagenesis in microorganisms.
Unpublished report No. CRV/CNG 21 466 from Rhône-Poulenc, Vitry-sur-
Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Broadmeadow, A. (1984). Iprodione: 52-week toxicity study in dietary
administration to beagle dogs. Unpublished report No. 84/RH0022/179
from Life Science Research, Suffolk, England. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Coombs, D.W. & Clark, G.C. (1977). RP 26019 technical: acute
inhalation toxicity-four hour LC50 in rats. Unpublished report No.
RNP 75/775 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Coquet, B. (1973a) Recherche du pouvoir teratogène chez le rat OFA
par voie orale du produit 26019 R.P. Unpublished report from Centre
de Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogène chez le lapin par
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1973c) Essai de toxicité chronique (3 mois) chez le
chien par voie orale du produit 26019 R.P. Unpublished report from
Centre de Recherche et d'Elevage des Onoins, No. 731008.
Coquet, B. (1976) Influence du produit 26019 R.P. sur la
reproduction du rat (essai sur 3 générations). Unpublished report
from Institut Français de Recherches et Essais biologiques, No.
760435 submitted by Rhône-Poulenc.
Cummins, H.A. (1989). Iprodione: acute oral toxicity study in the
rat. Unpublished report No. 89/RHA255/0391 from Life Science
Research, Suffolk, England. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985a). DNA damage in Bacillus subtilis with
iprodione technical. Unpublished report No. 2214 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985b). In vitro sister chromatid exchange in
Chinese hamster ovary cells (CHO). Unpublished report No. PH 319-BO-
001-84 from Borriston Laboratories, Inc., Temple Hill, Maryland,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990a). Iprodione: sub-acute toxicity to mice by
dietary administration for 13 weeks. Unpublished report No. RNP
323/90667 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990b). Iprodione: sub-acute toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RNP
322/90767 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Ganter, P., Pasquet, J., Spicer, E., Girard, P., & Myon, J. (1979).
Iprodione (26 019 R.P.): one month toxicity study in mice by dietary
administration. Unpublished report No. RP/RD/CNG 19 950 from Rhône-
Poulenc, Vitry-sur-Seine, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. & Myon, J. (1973). Produit No. 26019 R.P.
Toxicité à terme (5 mois) chez le rat par voie orale, en comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R,P). Unpublished
report from Laboratoires de Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 17124 submitted by Rhône-
Poulenc.
Ganter, P. & Girard, P. (1973). Produit No. 26019 R.P. Examen
histologique de la toxicité oculaire du 26019 R,LO, chez le chien
après un traitement de 3 mois. Unpublished report from Laboratoires
de Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131 submitted by Rhône-Poulenc.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1985). CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report No.
PH 314-BO-001-84 from Borriston Laboratories, Inc., Temple Hill,
Maryland, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Hallifax, D. (1989). Iprodione: absorption, distribution, metabolism
and excretion study in the rat. Unpublished report No.
89/RPM005/1013 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E., Huffman, K.W., & M.A. Gallo (1974). RP 26019:
Dominant lethal mutagenicity in mice. Unpublished report No. SEH
74:69-CH-47 from Hess & Clark, Ashland, Ohio, USA. Submitted to WHO
by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E. & Huffman, K.W. (1975). Chronic toxicologic and
carcinogenic study with RP 26019 in mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:223. Submitted to
WHO by Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C., & Kiggins, E.M. (1976). Chronic
toxicologic and carcinogenic study with RP 26019 in rats.
Unpublished report from Rhodia, Inc., Hess and Clark Division, No.
76:57. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1991). Two-generation reproduction study with
iprodione technical in rats. Unpublished report No. HLA 6224-154
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Huffman, K.W. (1974). Subacute toxicity, RP 26 019, mice.
Unpublished report No. KWH 74:43 from Hess & Clark, Ashland, Ohio,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Itabashi, M., Hayashi, K., Kudo, S., Maruyama, Y., Takahara, K., &
Tajima, M. (1978). Three-month dietary oral toxicity study of 26.019
in rats. Unpublished report from Nippon Institute for Biological
Science, Japan. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Jones, A.G. (1983). Fungicides: Iprodione. Exposure of personnel
mixing ROVRAL WP. Unpublished report No. 428 from May & Baker, Ltd.,
Essex, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Kangas, L. (1991). A 52-week dietary toxicity study of iprodione in
the beagle dog. Unpublished report No. 84296 from Bio-Research
Laboratories Ltd., Senneville, Quebec, Canada. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Keets, S.A., Leist, P.L., Mercieca, M.D., & Clevidence, K.J. (1985).
A teratology study in rabbits with iprodione. Unpublished report No.
WIL-21028 from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Laurent, M. & Buys M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques, Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. & Chabassol, Y. (1976)
Etude du métabolism chez le rat à l'aide du produit marqué au 14C.
Unpublished report from Rhône Poulenc, Direction des Recherches et
du Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-
Seine, C.N.G. An. No. 18548, submitted by Rhône Poulenc.
Laurent, M., Bredenbac, J., Chabassol, Y., & Marlard, M. (1983).
Iprodione. Percutaneous metabolism in the rat. Unpublished report
No. CNG-An 4675-E from Rhône-Poulenc Research, Vitry-sur-Seine,
France. Submitted to WHO by Rhone-Poulenc Agrochimie S.A., Lyon,
France.
Lawlor, T.E. & Valentine, D.C. (1990). Mutagenicity test on
iprodione (technical) in the Salmonella/mammalian-microsome
reverse mutation assay (Ames test) with confirmatory assay.
Unpublished report No. HLA 11092-0-401R from Hazleton Laboratories
America, Inc., Kensington, Maryland, USA. Submitted to WHO by Rhône-
Poulenc Agrochimie S.A., Lyon, France.
Pasquet, J. & Mazuret, A. (1974). 26 019 R.P.: acute toxicity and
local tolerance. Unpublished report No. SUCRP-DSPh 17 746 from
Rhône-Poulenc, Paris, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Plutnick, R.T. & Kapp, R.W. (1988). Iprodione (technical) - acute
dermal limit test in the rabbit. Unpublished report No. 209806 from
Exxon Biomedical Sciences, Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
San Sebastian, J.R., Naismith, R.W., & Matthews, R.J. (1985). In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Unpublished report No. PH 320-BO-001-84 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Stevens, K.R. (1974). Subacute toxicity, RP 26 019. Unpublished
report No. KRS 74:3 from Hess & Clark, Ashland, Ohio, USA. Submitted
to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976a). Acute toxicity of Rovral (26019RP). I. Oral,
intraperitoneal and subcutaneous administration in rats. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976b). Acute toxicity of Rovral (26019RP). II. Oral,
intraperitoneal and subcutaneous administration in mice. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., & Deans, C.F. (1986a). Iprodione
(technical grade): effects of oral administration upon pregnancy in
the rat. 1. Dosage range-finding study. Unpublished report No.
85/RHA063/752 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., Lambert, E.P., Wilby, O.K., & Tesh, S.A.
(1986b). Iprodione (technical grade): teratology study in the rat.
Unpublished report No. 85/RHA064/765 from Life Science Research,
Suffolk, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Trimmer, G.W. (1988). Iprodione (technical) - dermal sensitization
test in the guinea-pig (Buehler method). Unpublished report No.
209821 from Exxon Biomedical Sciences, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Short-term toxicity studies
Mice
Groups (10-20/sex/dose) of CF-1 mice received iprodione (purity
unspecified) in the diet daily for 28 days at 0, 600, 1900, 6000,
9500, or 15000 ppm (equal to 130, 390, 950, 1500 or 2300 mg/kg
bw/day for males and 120, 420, 1000, 1500 or 2400 mg/kg bw/day for
females). The highest dose produced mortality and depressed body-
weight gain and food consumption. Exposure to 6000 ppm and above
produced ataxia and lethargy during the first week of treatment. The
liver was affected in groups receiving 6000 ppm and above. Absolute
and relative liver weights were increased, livers had a stippled
appearance on gross examination, and the incidence of hepatocyte
vacuolation and focal eosinophilic degeneration was increased. At
15000 ppm granulomatous inflammation, (possibly in response to a
foreign body), was observed in the heart, liver, and kidney. The
NOAEL was 1900 ppm (equal to 390 mg/kg bw/day for males and 420
mg/kg bw/day for females) based on liver effects at 6000 ppm and
above (Stevens, 1974).
Groups of 10 CF-1 Carworth mice/sex received iprodione (purity
unspecified) in the diet daily for 4 weeks at 0, 600, 1900, 6000,
9500 or 15000 ppm, (equal to 115, 366, 1090, 1860 or 4030 mg/kg
bw/day for males - the high dose based on first two weeks only - and
137, 439, 1310, 2090 or 2590 for females). The high dose produced
mortality and depressed body-weight. Depression and ataxia were
observed at 6000 ppm and higher. Relative liver weight was increased
at 6000 ppm and above. Gross necropsy revealed white foci in the
liver of mice receiving 1900 ppm and higher, a stippled appearance
of the liver at 6000 ppm and higher, and liver enlargement at 9500
and 15000 ppm. White foci and granulomatous inflammation were
observed in numerous tissues, primarily at the high dose. One
granulomatous lesion was observed in the liver at 6000 ppm and 5
were observed in the bladder at 9500 ppm. The presence of
spindle-shaped clear spaces in tissues and foreign body type giant
cells suggested a reaction to crystal formation. Liver cell
hypertrophy was increased at 6000 ppm and above. The NOAEL was 600
ppm (equal to 115 mg/kg bw/day for males and 137 mg/kg bw/day for
females) based on gross changes in the liver at 1900 ppm and above
(Huffman, 1974).
Table 1. Acute toxicity of iprodione
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Mouse CD-1 M oral 1870 Takehara et al. (1976b)
F 2670
CD-1 M i.p. 900 Takehara et al. (1976b)
F 625
CD-1 M subcutaneous >6700 Takehara et al. (1976b)
F >6700
Rat CD M oral >2000 Cummins (1989)
F >20002
CD M oral 2060 Takehara et al. (1976a)
F 1530
Wistar M&F oral 3700 Babish (1976a)
CD M i.p. 1330 Takehara et al. (1976a)
F 700
CD M subcutaneous >4500 Takehara et al. (1976a)
F >4500
Sprague-Dawley M inhalation >3.29 Coombs & Clark (1977)
F (4-hr exp) >3.29
Table 1 (cont'd)
Species Strain Sex Route LD50 LC50 Reference
mg/kg bw mg/l
Rabbit New Zeeland white M dermal (24-hr exp) >2000 Plutnick et al. (1988)
F >2000
? ? dermal (?-hr exp) >30 000 Babish (1976b)
Dog Beagle M oral >2000 Pasquet & Mazuret (1974)
F >2000 (FAO/WHO, 1978)
1 Purity of technical iprodione was 95.8%
2 Purity of technical iprodione was 97.9%
The crystal formation in tissues was further evaluated using
groups of 15 CD-1 mice/sex receiving iprodione (93.5%) in the diet
daily for 4 weeks at 0, 1900, 6000, 9500 or 15000 ppm (equivalent to
290, 900, 1400, and 2300 mg/kg bw/day). The two highest dose levels
produced mortality, clinical signs of toxicity, and depressed weight
gain and food consumption. At 6000 ppm and above, crystalline
deposits and effects on the liver were observed. Granulomatous
lesions surrounding crystal deposits were found frequently in the
urinary bladder and occasionally in liver parenchyma, myocardium,
diaphragmatic muscle, and skeletal muscle. It was speculated that
the crystals contained a major metabolite of iprodione, 32490 R.P.,
which was identified in the liver. Liver effects included increased
weight, pale and mottled appearance on gross examination, and
swelling of hepatocytes to form large homogeneous areas in the
centrilobular region of the liver. Histopathological changes in the
testes and spleen were observed at doses levels above 6000 ppm. A
NOAEL of 1900 ppm (equivalent to 290 mg/kg bw/day) was determined
based on crystalline deposits in the urinary bladder, liver
enlargement, and hepatocellular swelling at 6000 ppm and above
(Ganter et al., 1979).
Groups of 10 Crl:CD-1(ICR)BR mice/sex received iprodione (95.7%
purity) in the diet daily for 13 weeks at 0, 1500, 3000, 6000, or
12000 ppm, (equal to 260, 510, 1100 or 2100 mg/kg bw/day for males
and 330, 660, 1300 or 2600 mg/kg bw/day for females). The high dose
was associated with mortality, clinical signs of toxicity, weight
loss, and reduced food consumption. The two highest dose levels
produced crystalline deposits with associated multinucleated cells
in a number of tissues, particularly in the urinary bladder. At the
lowest dose level of 1500 ppm, liver and adrenal gland weights were
increased in females. The incidence and severity of liver cell
enlargement in males and females showed a dose-related increase
beginning with the 1500 ppm dose level. Enlargement and vacuolation
of adrenal cortex cells were increased in treated groups. The
incidence of vacuolation of zona fasciculata cells was 0/10, 1/10,
3/10, 6/10, and 4/9 in females receiving 0, 1500, 3000, 6000 or
12000 ppm, respectively. At 3000 ppm and higher, effects were
observed in the kidney, uterus, ovary, and spleen. The authors
determined that dose levels of 6000 ppm or greater were unsuitable
for a long-term study in mice because of crystal formation and
accompanying effects in the urinary bladder. A NOAEL was not
identified in this study. The lowest dose of 1500 ppm was associated
with enlargement of the liver and adrenal glands and microscopic
changes in these organs (Fryer et al., 1990a).
Rats
Groups of 15 CD/CRJ rats/sex received iprodione (purity
unspecified) in the diet daily for 3 months at 0, 300, 1000, or 3000
ppm, (equal to 21, 70 or 210 mg/kg bw/day for males and 24, 82 or
240 mg/kg bw/day for females). The high dose of 3000 ppm produced
clinical signs of toxicity (i.e. piloerection, rough fur), depressed
food and water consumption, and decreased body-weight (final weight
reduced 20-40%). A number of absolute and relative organ weights
were decreased at the high dose including the liver, spleen, thymus,
kidneys, and heart. Microscopic findings were observed at the high
dose in the liver, spleen, and thymus. Swelling of the zona
glomerulosa of the adrenal gland showed a dose-related increase at
1000 and 3000 ppm in both sexes. The NOAEL was 300 ppm (equal to 21
mg/kg bw/day for males and 24 mg/kg bw/day for females) based on
microscopic changes in the adrenal cortex at 1000 ppm and above
(Itabashi et al., 1978).
Groups of 10 Crl:CD (SD) BR rats/sex received iprodione (95.7%
purity) in the diet daily for up to 13 weeks at 0, 1000, 2000, 3000,
or 5000 ppm (equal to 78, 150 or 250 mg/kg bw/day for males and 89,
180 or 270 mg/kg bw/day for females). The high dose group was
terminated during week 8 due to excessive toxicity which included a
progressive decrease in food intake and body-weight and the death of
one male. Upon examination these animals showed abnormalities in the
liver, adrenal glands, uterus, ovaries, prostate, and seminal
vesicles. Clinical signs (i.e. hunched posture, piloerection,
emaciation) occurred in males and females receiving 3000 ppm. Body-
weight, food intake, and food efficiency were reduced at the 2000
and 3000 ppm. Ovary weight was decreased at 2000 and 3000 ppm, and
uterus weight was reduced at 3000 ppm. Seminal vesicles and the
prostate gland of males receiving 3000 ppm were reported as smaller
than normal but no histological changes were noted.
Histopathological changes were in the adrenal glands, uterus, and
ovaries were increased at 2000 and 3000 ppm. Adrenal gland findings
included enlarged cells of the zona glomerulosa in males and
females and vacuolation of the zone fasciculata mainly in females.
The uterus showed signs of atrophy and ovaries contained reduced
numbers of corpora lutea. A NOAEL of 1000 ppm equal to 78 mg/kg
bw/day for males and 89 mg/kg bw/day for females was determined,
based on a reduced body-weight gain and histopathological changes at
2000 ppm and higher (Fryer et al., 1990b).
Groups of 15 male and 15 female caesarean originated, barrier
sustained, rats were fed 0, 150, 500 or 1000 ppm iprodione in the
diet for 5 months. No effects were observed on mortaility, food
consumption, haematology (as judged by haemoglobin, haematocrit,
erythrocyte count, or total and differential leucocyte count)
clinical chemistry (as judged by BSP, SGOT, SGPT or SAP) or
urinalysis. Body weight gain was slightly reduced (especially in
males) at 500 and 1000 ppm. Absolute (but not relative) heart weight
was reduced in males at 500 and 1000 ppm, and absolute kidney weight
was reduced at 1000 ppm. In females, absolute liver and kidney
weights were significantly reduced at 500 ppm only. Gross and
histopathology were normal at all dose levels. In a parallel study,
dichlozoline, a structurally related compound, induced cataracts. No
such effect was seen with iprodione (Ganter et al., 1973a).
Dogs
Groups of 2 male and 2 female dogs were maintained on a diet
containing iprodione at dose levels of 0, 800, 2400 or 7200 ppm for
a period of 3 months. At the top dose level the method of
administration was altered after 6 weeks, to gelatine capsules. The
treatment did not affect mortality. The recorded values of
haematological determinations and urinalyses were within normal
limits, as judged by haemoglobin, haematocrit, reticulocyte
erythrocyte count, total and differential leucocyte count and
prothrombine time, except for signs of mild anaemia in 1 male and 1
female at 2 months and 1 male at 3 months at the top dose level. At
7200 ppm a reduction of food consumption was observed, accompanied
by reduced body-weight gain. Opththalmosopic examination of the
animals did not reveal any pathological alterations. A transient
increase in SGOT and SGPT was observed after 1 and 2 months of
treatment at 7200 ppm. In treated male rats a dose-dependent
increase of relative liver weights was observed, which was also
observed in females at 2400 ppm and above. At 7200 ppm reduced
relative weight of testes was found, but there was no histological
indication of damage. The histopathological findings did not reveal
any indication of treatment-related alterations of tissues (Coquet,
1973c; Gunter & Girard, 1973).
Groups of 6 beagle dogs/sex received iprodione (96.5% purity)
in the diet daily for 52 weeks at 0, 100, 600, or 3600 ppm, (equal
to 4.1, 24.9 or 145 mg/kg bw/day for males and 4.3, 28.3 or 153
mg/kg bw/day for females). Ophthalmology, haematology, clinical
chemistry, and urinalysis tests were performed periodically over the
course of the study. Slight retinal hyperreflection was noted more
frequently in males receiving 3600 ppm and in females receiving 600
or 3600 ppm. The severity did not increase over time or with dose
level and was not seen consistently in the same animals over time.
Red blood cells were affected at 3600 ppm and possibly at 600 ppm.
Compared to controls, high dose males showed a slight but consistent
decrease in erythrocyte count, haemoglobin, and haematocrit over the
course of the study. Females receiving the high dose showed a less
consistent pattern of anaemia. Platelet count and partial
thromboplastin time were increased over the course of the study in
high dose males and females. The high dose was also associated with
an increase in frequency and severity of erythrocytes containing
Heinz bodies. After 52 weeks, 3/6 control males and 4/6 control
females showed the presence of Heinz bodies compared to all males
and females receiving the high dose. In control dogs the number of
affected erythrocytes was 0-2 per field compared to 6-50 or over 50
per field in dogs receiving the high dose. During the first one-
third of the study, the incidence and severity of Heinz bodies were
also increased in males receiving 600 ppm. No treatment-related
changes were noted in the bone marrow.
Plasma ALP was consistently increased over control values and
was above the normal range in high dose males and females. In high
dose females, serum ALT was transiently elevated early in the study
and LDH was transiently increased late in the study. Total bilirubin
and albumin were slightly elevated during the study particularly in
high dose females. Absolute and relative liver and adrenal gland
weights were increased at the high dose in males and females.
Absolute prostate gland weight was decreased 22% at 600 ppm and 41%
at 3600 ppm. Relative prostate gland weight was also reduced at the
high dose (39%). Two high dose females had enlarged adrenal glands
and one high dose male had a swollen liver on gross examination.
Nematode granulomas were found in various tissues of a number of
control and treated dogs. Treatment-related microscopic changes were
observed in the liver, adrenal glands, and urinary bladder of high
dose males and females. In the urinary bladder, the majority of high
dose males and females had submucosal granulomas and giant cells
containing crystals. The majority of high dose males and females had
fat vacuolation and/or pallid appearance of the adrenal cortex
compared to an absence or lower incidence in controls. Hepatic
centriacinar atrophy was observed in 3/6 males and 4/6 females
receiving the high dose compared to no controls with the lesion.
Pigmented macrophage agglomerates in the liver were more prominent
in the high dose groups than in controls. An additional finding in
females was lipofuscinosis in the proximal convoluted tubules of the
kidney occurring in 2/6, 0/6, 4/5, and 4/6 in the 0, 100, 600, and
3600 ppm groups, respectively. No treatment-related histopathologic
changes were noted in the prostate gland. A NOAEL of 100 ppm, equal
to 4.1 mg/kg bw/day, was determined, 600 ppm representing a minimal
toxic effect level with the majority of toxic changes occurring at
the 3600 ppm dose level (Broadmeadow, 1984).
A second one-year dog study was conducted to determine the
NOAEL between 100 and 600 ppm. Groups of 6 beagle dogs/sex received
iprodione (96% purity) in the diet daily for 52 weeks at 0, 200,
300, 400, or 600 ppm, (equal to 7.8, 12, 18 or 25 mg/kg bw/day for
males and 9.1, 13, 18 or 26 mg/kg bw/day for females). Eye
examinations and haematology were performed during weeks 4, 8, 12,
20, 28, 36, and 52. The adrenal glands, kidneys, and prostate were
the only organs weighed. Kidneys of females and prostate of males
were the only tissues examined microscopically. Iprodione treatment
had no effect on survival, body weight, body-weight gain, food
consumption, or food efficiency. Most dogs treated with iprodione
developed skin lesions consisting of redness and scab formation
occurring primarily in the inguinal, hindlimb, and ventral abdominal
areas. Skin lesions were less frequent and less severe in controls.
The majority of lesions had cleared by study termination. Tests for
mange and fungal infection were negative. It was noted that slight
irritation of the skin was not uncommon in dogs fed powdered chow.
Eye examinations revealed no treatment-related abnormalities.
Haematology parameters were within the normal range for all groups.
In females receiving 600 ppm, erythrocyte count, haemoglobin, and
haematocrit were reduced at week 4 (15%) and at week 36 (10%). In
both males and females receiving 600 ppm, values for these
erythrocyte parameters were consistently lower than control values
through week 36, although the differences were not statistically
significant. Heinz bodies were not observed in any control or
treated animal at any time point. Organ weights (kidney, adrenal
glands, prostate) were unaffected, and no treatment-related
histopathological changes were observed in the kidneys of females or
prostate of males. Based on slight changes in erythrocyte parameters
at 600 ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day (Kangas
et al., 1991).
Long-term toxicity/carcinogenicity studies
Mice
Groups of 60 male and 60 female mice were maintained on a diet
containing the test compound at 0, 200, 500 or 1250 ppm for 18
months. No treatment-related effects on body-weight, food
consumption or mortality were found. The recorded values of the
haematological, blood chemistry and urinalyses tests, performed
after 6, 12 and 18 months of the feeding period, were within the
physiological range. Necropsy findings on mice that died during the
last 6 months of the test and on those sacrificed at the termination
date showed an increased number of enlarged lymph nodes in males at
200 ppm. Organ weight variations occurred sporadically in the
various dose groups which were considered not to be treatment-
related. The histopathological observations failed to reveal
abnormal features. The distribution of neoplastic and non-neoplastic
findings did not appear to demonstrate any significant dose
dependence. The most common tumours were lymphosarcomas involving
the spleen, lymph nodes and thymus (Hastings & Huffmann, 1975).
Rats
Groups of 60 male and 60 female rats were maintained on a diet
containing 0, 125, 250 or 1000 ppm iprodione for 24 months. Slight
reduction in body weight gain was observed at 1000 ppm. This was
accompained by some reduction in food intake. The treatment had no
effect on food consumption, mortality or values of the haematologic,
blood chemistry or urinalyses determinations. Necropsy findings did
not reveal any drug-related gross alterations. Variations in organ
weight did not show a group distribution and did not seem to be
related to drug administration. Histopathology did not indicate a
treatment relationship with neoplastic or non-neoplastic findings.
At 24 months the most common tumours observed were pituitary
adenomas and adenocarcinomas and fibroadenoma of the mammary glands
(Hastings et al., 1976).
Reproduction studies
Rats
Groups of 10 male and 20 female rats were maintained on a diet
containing iprodione at concentrations of 0, 125, 250 or 1000 ppm
for the first 5 weeks of each generation and 0, 250, 500 or 2000 ppm
for the next 8 weeks of treatment. The diet was fed through 3
generations. The treatment did not affect the growth rate, food
consumption, mortality or fertility of the parent animals. The
number of living delivered pups of the females treated with 2000 ppm
was slightly reduced and the post-natal growth of the pups was
slightly retarded. There was also a tendancy for growth reduction at
500 ppm. Autopsy findings and microscopic examination of the major
organs performed in rats of the third generation did not reveal
abnormalities (Coquet, 1976).
The reproductive toxicity of iprodione was studied in two
successive generations of Crl:CD BR/VAF/Plus rats. The first
parental (F0) animals were mated twice to produce F1a and F1b
litters. F1a animals were mated twice to produce F2a and F2b
litters. Groups (28/sex) received diets containing 0, 300, 1000, or
3000 ppm of iprodione (96.2% purity) beginning at least 10 weeks
prior to mating. The high dose of 3000 ppm was reduced to 2000 ppm
at the time of the first mating of F1a rats because of excessive
toxicity. The administered doses were equal to 17, 55 or 160 mg/kg
bw/day for F0 males; 21, 71 or 210 mg/kg bw/day for F0 females;
20, 68, and 150 mg/kg bw/day for F1 males; and 25, 82 or 190 mg/kg
bw/day for F1 females. Toxicity in adult rats was observed at 1000
ppm and above. Body-weight, body-weight gain, and food consumption
were reduced throughout the treatment period in F0 and F1 males
receiving 3000 ppm and F0 and F1 females receiving 1000 or 3000
ppm. Reproductive performance was unaffected by iprodione exposure.
Offspring toxicity was observed at the high dose. During lactation
(16-21 days of age), F1a and F1b pups exhibited signs of
toxicity including unkempt or hunched appearance, slow movement, and
tremors during the last days of lactation. In both generations,
litter size number of live pups, and pup weight were decreased at
the high dose. The NOAEL was 300 ppm, equal to 21 mg/kg bw/day, for
parental toxicity based on reduced parental body-weight at 1000 ppm
and higher; for embryofetal toxicity, the NOAEL was 1000 ppm, based
on clinical signs, reduced litter size, and reduced pup weight at
3000 ppm (Henwood, 1991).
Special studies on embryo/fetotoxicity
Rats
Groups of 25-30 rats were treated orally with 0, 100, 200 or
400 mg/kg bw/day iprodione on gestation days 5 to 15. Females at 400
mg/kg bw/day showed reduced fertility, reduced body weight gain and
a dose-related reduction in food consumption, especially during the
treatment period. The number of implantations was also reduced at
the highest dose level. There was no indication of an embryotoxic or
teratogenic effect of the test compound (Coquet, 1973a).
In a dose range-finding study in CD pregnant rats, treatment
with 400 and 800 mg/kg bw/day resulted in mortality, weight loss,
and pronounced clinical signs. Treatment with 240 mg/kg bw/day
reduced weight gain and produced clinical signs, and 120 mg/kg
bw/day occasionally produced clinical signs (i.e. flaccid muscles).
The lowest dose of 40 mg/kg bw/day had no effect on maternal health.
It was concluded that dose levels should not exceed 240 mg/kg bw/day
in a definitive developmental toxicity study (Tesh et al., 1986a).
In the definitive study, iprodione (94.2%) was administered by
oral gavage to groups of 25 mated female CD rats at doses of 0, 40,
90, or 200 mg/kg bw/day. Controls received the vehicle (aqueous
methylcellulose). Rats were treated days 6-15 of gestation and
sacrificed on day 21. The administered doses had no adverse effect
on maternal health as assessed by mortality, clinical signs, body-
weight, and food consumption. In the offspring, the incidence of
space between body wall and organs was slightly increased at the
high dose. The incidences were 4.3%, 5.3%, 5.8%, and 11.2% on a
fetal basis and 15%, 20%, 20%, and 32% on a litter basis at 0, 40,
90 and 200 mg/kg bw/day, respectively. The majority of affected
fetuses were small in size. It was noted that this observation was
associated with fetuses of low body-weight in previous studies and
was indicative of slight immaturity. The incidence of small fetuses
(<2.7g) in this study was 2.9%, 5.1%, 4.9%, and 8.0% (fetal basis).
Group mean fetal body-weights were 3.27, 3.18, 3.16, and 3.15 g. The
findings collectively were suggestive of a slight effect on fetal
developmental at the high dose despite the fact that none of the
differences were statistically significant and values at the high
dose were within the historical control range. A NOAEL for maternal
toxicity of 200 mg/kg bw/day and a NOAEL for embryo-fetal toxicity
of 90 mg/kg bw/day were determined, based on slightly delayed fetal
development at 200 mg/kg bw/day. There was no evidence of
teratogenic potential at the highest dose tested (200 mg/kg bw/day)
(Tesh et al., 1986b).
Rabbits
Groups of 15-17 New Zeeland white rabbits were intubated on
gestation days 6-16 inclusive with 0, 100, 200 or 400 mg/kg bw/day
iprodione. Body-weight gain, over the period of treatment, was
slightly reduced at 100 mg/kg bw/day, and a dose-related weight loss
occurred at 200 and 400 mg/kg bw/day. Food intake was reduced at 200
mg/kg bw/day and above. At 400 mg/kg bw/day 9 of 17 females died,
and only one of the four remaining pregnant animals carried to term.
Fetal loss was increased at 200 mg/kg bw/day, and the fetal weight
was reduced at 200 mg/kg bw/day and above. Multiple malformations
occurred in 1 of 68 living fetuses at 200 mg/kg bw/day. Minor
malformations were noted in all groups (Coquet, 1973b).
The developmental toxicity of iprodione was studied in New
Zeeland white rabbits. Iprodione (95.0% purity) was administered by
oral gavage to groups of 18 artificially inseminated females at
doses of 0, 20, 60, or 200 mg/kg bw/day. The control group received
the vehicle (aqueous methylcellulose). Rabbits were treated on days
6-18 of gestation and sacrificed on day 29. Maternal toxicity was
produced at 60 and 200 mg/kg bw/day. The high dose group experienced
weight loss and reduced food consumption during the entire treatment
period. Clinical signs associated with the high dose included hair
loss, diarrhoea, and decreased urination and defecation. The group
receiving 60 mg/kg bw/day experienced slight body-weight loss during
the first six days of treatment compared to positive weight gain by
controls. Seven of 18 females receiving the high dose aborted
compared to 1/18, 0/18, and 1/18 for the 0, 20, and 60 mg/kg bw/day
groups. Ten does receiving the high dose delivered litters, two of
which had totally resorbed litters (compared to 1/13 controls)
resulting in only 8 viable litters at the high dose. A NOAEL of 20
mg/kg bw/day was determined based on depressed maternal weight gain
at 60 mg/kg bw/day and above. The high dose of 200 mg/kg bw/day was
considered an excessive dose for a teratology evaluation. Some
skeletal variations appeared increased at the high dose, but the
incidences were within the historical control range. The NOAEL for
maternal toxicity was 20 mg/kg bw/day based on depressed weight gain
at 60 mg/kg bw/day. The NOAEL for embryo-fetal toxicity was 60 mg/kg
bw/day based on increased abortions and post-implantation losses at
200 mg/kg bw/day (Keets et al., 1985).
Special studies on eye and skin irritation and hypersensitivity
Technical iprodione caused mild, transient eye irritation in
rabbits. The irritation was lessened by washing the eye immediately
after exposure (Babish, 1976c; Bonnette, 1991a).
Technical iprodione is not a dermal irritant in rabbits
(Babish, 1976d; Bonnette, 1991b) or a dermal sensitizer in guinea-
pigs (Trimmer et al., 1988).
Special studies on genotoxicity
The results of genotoxicity studies are summarized in Table 2.
Iprodione has been consistently negative in assays for point
mutation, chromosomal aberration, and sister chromatid exchange. A
questionable positive result was reported for DNA damage in
Bacillus subtilis.
Observations in humans
No data available.
Table 2. Results of genotoxicity assays on iprodione
Test system Test object Concentration of Purity Results Reference
iprodione
Ames test (1) S. typhimurium 1-5000 µg/plate dissolved in DMSO 96.2% Negative Lawlor & Valentine (1990)
TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium 25-200µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
TA98, TA100, TA1535, 99.3% (1982a,b)
TA1537, TA1538
S. typhimurium 12.5-250 µg/plate dissolved in DMSO ? Negative Benazet & Cartier (1979)
Ta98, TA100, TA1535,
TA1537
E. coli mutation assay (1) E. coli K12, GY 5057 0.05-1000 µg/ml dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
strain 99.3% (1982a,b)
E. coli, W3110 (pol A+), 12.5-200 µg/plate dissolved in DMSO 95.1 Negative Bouanchaud & Cartier
p3478 (pol A-) 99.3% (1982a,b)
B. subtilis mutation B. subtilis rec. exc. 20-1670 µg/ml dissolved in DMSO 96.8% Positive Felkner (1985a)
assay (1) pol strains (19 strains) (3)
Saccharomyces cerevisiae Saccharomyces cerevisiae D7 62.5-500 µg/ml dissolved in DMSO 99.3% Negative Bouanchaud & Cartier
mutation assay (1) strain (1982a,b)
(2) Saccharomyces cerevisiae D7 250 µg/ml dissolved in DMSO ? Negative Benazet & Cartier (1979)
strain
CHO/HGPRT mutation assay (1) Chinese hamster ovary cells 5-1500 µg/ml dissolved in DMSO ? Negative Godek et al. (1985)
(CHO-K1-BH4)
In vitro cytogenetics (1) Chinese hamster ovary 15-400 µg/ml dissolved in DMSO ? Negative San Sebastian et al. (1985
cells (CHO-K1-BH4)
Table 2 (cont'd)
Test system Test object Concentration of Purity Results Reference
iprodione
In vitro sister chromatid Chinese hamster ovary cells 5-400 µg/ml dissolved in DMSO ? Negative Felkner (1985b)
exchange (1) (CHO-K1-BH4)
Dominant lethal assay CF-1 mice 0, 1500, 6000 ppm X 49 days ? Negative Hastings et al. (1974)
(WHO, 1978)
(1) Both with and without metabolic activation.
(2) Without metabolic activation.
(3) Positive at highest and lowest doses without metabolic activation. Problem with precipitation of test material, inadequate negative
and positive controls.
COMMENTS
Iprodione is extensively absorbed from the gastrointestinal
tract. It was extensively metabolized and rapidly excreted,
primarily in the urine, although relative faecal excretion of the
parent compound increased at high doses. Higher doses (for example
900 mg/kg bw) appeared to be absorbed to a lesser extent and
eliminated at a slower rate than lower doses (for example 50 mg/kg
bw). The elimination half-life was 7-9 h following a single low dose
and 13-20 h following a single high dose. The metabolic pathways
elucidated in rats involve dealkylation on the isopropyl carbamoyl
chain, hydroxylation of the aromatic ring and rearrangement and
opening of the hydantoin ring.
Iprodione had low acute toxicity by all routes of exposure. The
oral LD50 was greater than 1500 mg/kg bw in mice, rats, and dogs.
The World Health Organization has classified iprodione as unlikely
to present acute hazard in normal use (WHO, 1992).
In three 4-week studies in mice at dietary concentrations of 0,
600, 1900, 6000, 9500, or 15000 ppm, the lowest NOAEL was 600 ppm,
equal to 115 mg/kg bw/day, based on macroscopic hepatic changes at
1900 ppm. At 6000 ppm and above, the test material crystallized in
the tissues. In a 3-month study in mice at dietary concentrations of
0, 1500, 3000, 6000, or 12000 ppm (equal to 260, 510, 1100, and 2100
mg/kg bw/day) an increase in liver and adrenal gland weights and
hypertrophy and/or vacuolation of hepatocytes and adrenal cortical
cells were observed in all treated groups.
In a 3-month study in rats at dietary concentrations of 0, 300,
1000, or 3000 ppm, the NOAEL was 300 ppm, equal to 21 mg/kg bw/day.
Higher doses produced swelling in the zona glomerulosa of the
adrenal cortex. In another 3-month study in rats at dietary
concentrations of 0, 1000, 2000, 3000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 78 mg/kg bw/day, based on reduced body-weight
gain and histopathological changes in the adrenal glands, ovaries
and uterus at 2000 ppm and higher.
In a one-year study in dogs at dietary concentrations of 0,
100, 600, or 3600 ppm, the NOAEL was 100 ppm, equal to 4.1 mg/kg
bw/day, based on the detection of Heinz bodies in erythrocytes and
decreased prostate gland weight at 600 ppm and higher. In a second
one-year study at dietary concentrations of 0, 200, 300, 400, or 600
ppm, the NOAEL was 400 ppm, equal to 18 mg/kg bw/day, based on
decreased erythrocyte values at 600 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 300, 1000, or 3000/2000 ppm, the NOAEL was 300
ppm, equal to 21 mg/kg bw/day, based on depressed body-weight at
1000 ppm and above. Reproductive performance was unaffected.
Offspring survival and growth were reduced at 3000/2000 ppm.
In a teratology study in rats using gavage doses of 0, 40, 90,
or 200 mg/kg bw/day, the NOAEL for maternal toxicity and
teratogenicity was 200 mg/kg bw/day. The NOAEL for embryofetal
toxicity was 90 mg/kg bw/day, based on slightly delayed fetal
development at 200 mg/kg bw/day. In rabbits administered 0, 20, 60,
or 200 mg/kg bw/day by gavage, the NOAEL for maternal toxicity was
20 mg/kg bw/day based on depressed weight gain at 60 mg/kg bw/day.
The NOAEL for embryofetal toxicity was 60 mg/kg bw/day based on
increased abortions and post-implantation loss at 200 mg/kg bw/day.
No teratogenic effects were found.
After consideration of the available genotoxicity data, the
Meeting concluded that iprodione was not genotoxic.
The former ADI, based on a multi-generation reproduction study
in rats, was revised. The new ADI was based on the results of
several studies, including the reproduction study in rats, the
teratology study in rabbits, and the one-year study in dogs. A
safety factor of 100 was applied to the NOAELs from these studies.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 600 ppm in the diet, equal to 115 mg/kg bw/day (four-
week study)
Rat: 300 ppm in the diet, equal to 21 mg/kg bw/day
(two-generation reproduction study)
500 ppm in the diet, equivalent to 25 mg/kg bw/day
(reproduction study reviewed by the 1977 Joint
Meeting)
Rabbit: 20 mg/kg bw/day (teratology study, maternal toxicity)
Dog: 400 ppm in the diet, equal to 18 mg/kg bw/day (one-
year study)
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw/day
Studies which will provide information valuable in the continued
evaluation of the compound
1. Ongoing toxicological studies.
2. Observation in humans.
REFERENCES
Babish, J.C. (1976a). Acute oral toxicity in rats. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976b). Acute dermal toxicity in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976c). Eye irritation test in rabbits. Unpublished
report from Food and Drug Research Laboratories, Inc., Waverly, New
York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Babish, J.C. (1976d). Primary skin irritation study with rabbits.
Unpublished report from Food and Drug Research Laboratories, Inc.,
Waverly, New York, USA. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Benazet, F. & Cartier, J.R. (1979). Iprodione (26 019 R.P.): In-
vitro mutagenicity study in Salmonella typhimurium (Ames' strains)
and in Saccharomyces cerevisiae (Zimmermann's strain D7).
Unpublished report No. RP/RD/CNG 20 177-E from Rhone-Poulenc, Vitry-
sur-Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie
S.A., Lyon, France.
Bonnette, K.L. (1991a). Primary eye irritation study in rabbits with
iprodione. Unpublished report No. 3147.109 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bonnette, K.L. (1991b). Primary skin irritation study in rabbits
with iprodione. Unpublished report No. 3147.108 from Springborn
Laboratories, Inc., Spencerville, Ohio, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Bouanchaud, D.H. & Cartier, J.R. (1982a). Iprodione (26 019 R.P.):
Supplementary studies of mutagenesis in microorganisms. Unpublished
report No. CRV/CNG 21 465 from Rhône-Poulenc, Vitry-sur-Seine,
France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Bouanchaud, D.H. & Cartier, J.R. (1982b). Iprodione (26 019 R.P.),
technical grade compound: In vitro mutagenesis in microorganisms.
Unpublished report No. CRV/CNG 21 466 from Rhône-Poulenc, Vitry-sur-
Seine, France. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Broadmeadow, A. (1984). Iprodione: 52-week toxicity study in dietary
administration to beagle dogs. Unpublished report No. 84/RH0022/179
from Life Science Research, Suffolk, England. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Coombs, D.W. & Clark, G.C. (1977). RP 26019 technical: acute
inhalation toxicity-four hour LC50 in rats. Unpublished report No.
RNP 75/775 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Coquet, B. (1973a) Recherche du pouvoir teratogène chez le rat OFA
par voie orale du produit 26019 R.P. Unpublished report from Centre
de Recherche et d'Elevage des Onoins, No. 731016.
Coquet, B. (1973b) Recherche du pouvoir teratogène chez le lapin par
voie orale du produit 26019 R.P. Unpublished report from Centre de
Recherche et d'Essais biologiques, Les Onoins, No. 730925.
Coquet, B. (1973c) Essai de toxicité chronique (3 mois) chez le
chien par voie orale du produit 26019 R.P. Unpublished report from
Centre de Recherche et d'Elevage des Onoins, No. 731008.
Coquet, B. (1976) Influence du produit 26019 R.P. sur la
reproduction du rat (essai sur 3 générations). Unpublished report
from Institut Français de Recherches et Essais biologiques, No.
760435 submitted by Rhône-Poulenc.
Cummins, H.A. (1989). Iprodione: acute oral toxicity study in the
rat. Unpublished report No. 89/RHA255/0391 from Life Science
Research, Suffolk, England. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985a). DNA damage in Bacillus subtilis with
iprodione technical. Unpublished report No. 2214 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Felkner, I.C. (1985b). In vitro sister chromatid exchange in
Chinese hamster ovary cells (CHO). Unpublished report No. PH 319-BO-
001-84 from Borriston Laboratories, Inc., Temple Hill, Maryland,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990a). Iprodione: sub-acute toxicity to mice by
dietary administration for 13 weeks. Unpublished report No. RNP
323/90667 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Fryer, S.E. (1990b). Iprodione: sub-acute toxicity to rats by
dietary administration for 13 weeks. Unpublished report No. RNP
322/90767 from Huntingdon Research Centre Ltd., Cambridgeshire,
England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Ganter, P., Pasquet, J., Spicer, E., Girard, P., & Myon, J. (1979).
Iprodione (26 019 R.P.): one month toxicity study in mice by dietary
administration. Unpublished report No. RP/RD/CNG 19 950 from Rhône-
Poulenc, Vitry-sur-Seine, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Ganter, P., Julou, L., Pascal, S., Pasquet, J., Populaire, P.,
Delesque, M., LeBail, R. & Myon, J. (1973). Produit No. 26019 R.P.
Toxicité à terme (5 mois) chez le rat par voie orale, en comparaison
avec la dichlozoline (Selex, N.D. Sumitomo = 23319 R,P). Unpublished
report from Laboratoires de Recherches de la Société des Usines
Chimiques Rhône-Poulenc, No. D.S.Ph. No. 17124 submitted by Rhône-
Poulenc.
Ganter, P. & Girard, P. (1973). Produit No. 26019 R.P. Examen
histologique de la toxicité oculaire du 26019 R,LO, chez le chien
après un traitement de 3 mois. Unpublished report from Laboratoires
de Recherches de la Société des Usines Chimiques Rhône-Poulenc, No.
D.S.Ph. No. 17131 submitted by Rhône-Poulenc.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1985). CHO/HGPRT
mammalian cell forward gene mutation assay. Unpublished report No.
PH 314-BO-001-84 from Borriston Laboratories, Inc., Temple Hill,
Maryland, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Hallifax, D. (1989). Iprodione: absorption, distribution, metabolism
and excretion study in the rat. Unpublished report No.
89/RPM005/1013 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E., Huffman, K.W., & M.A. Gallo (1974). RP 26019:
Dominant lethal mutagenicity in mice. Unpublished report No. SEH
74:69-CH-47 from Hess & Clark, Ashland, Ohio, USA. Submitted to WHO
by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Hastings, S.E. & Huffman, K.W. (1975). Chronic toxicologic and
carcinogenic study with RP 26019 in mice. Unpublished report from
Rhodia, Inc., Hess and Clark Division, No. SEH 75:223. Submitted to
WHO by Rhône-Poulenc.
Hastings, S.E., Winbigler, J.C., & Kiggins, E.M. (1976). Chronic
toxicologic and carcinogenic study with RP 26019 in rats.
Unpublished report from Rhodia, Inc., Hess and Clark Division, No.
76:57. Submitted to WHO by Rhône-Poulenc.
Henwood, S.M. (1991). Two-generation reproduction study with
iprodione technical in rats. Unpublished report No. HLA 6224-154
from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Huffman, K.W. (1974). Subacute toxicity, RP 26 019, mice.
Unpublished report No. KWH 74:43 from Hess & Clark, Ashland, Ohio,
USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon,
France.
Itabashi, M., Hayashi, K., Kudo, S., Maruyama, Y., Takahara, K., &
Tajima, M. (1978). Three-month dietary oral toxicity study of 26.019
in rats. Unpublished report from Nippon Institute for Biological
Science, Japan. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Jones, A.G. (1983). Fungicides: Iprodione. Exposure of personnel
mixing ROVRAL WP. Unpublished report No. 428 from May & Baker, Ltd.,
Essex, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Kangas, L. (1991). A 52-week dietary toxicity study of iprodione in
the beagle dog. Unpublished report No. 84296 from Bio-Research
Laboratories Ltd., Senneville, Quebec, Canada. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Keets, S.A., Leist, P.L., Mercieca, M.D., & Clevidence, K.J. (1985).
A teratology study in rabbits with iprodione. Unpublished report No.
WIL-21028 from WIL Research Laboratories, Inc., Ashland, Ohio, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Laurent, M. & Buys M. (1974) Etude du métabolisme chez le rat.
Unpublished report from Société des Usines chimiques, Rhône-Poulenc.
Laurent, M., Brunie, B., Buys, M., Heusse, D. & Chabassol, Y. (1976)
Etude du métabolism chez le rat à l'aide du produit marqué au 14C.
Unpublished report from Rhône Poulenc, Direction des Recherches et
du Développement, Centre de Recherches Nicolas Grillet, Vitry-sur-
Seine, C.N.G. An. No. 18548, submitted by Rhône Poulenc.
Laurent, M., Bredenbac, J., Chabassol, Y., & Marlard, M. (1983).
Iprodione. Percutaneous metabolism in the rat. Unpublished report
No. CNG-An 4675-E from Rhône-Poulenc Research, Vitry-sur-Seine,
France. Submitted to WHO by Rhone-Poulenc Agrochimie S.A., Lyon,
France.
Lawlor, T.E. & Valentine, D.C. (1990). Mutagenicity test on
iprodione (technical) in the Salmonella/mammalian-microsome
reverse mutation assay (Ames test) with confirmatory assay.
Unpublished report No. HLA 11092-0-401R from Hazleton Laboratories
America, Inc., Kensington, Maryland, USA. Submitted to WHO by Rhône-
Poulenc Agrochimie S.A., Lyon, France.
Pasquet, J. & Mazuret, A. (1974). 26 019 R.P.: acute toxicity and
local tolerance. Unpublished report No. SUCRP-DSPh 17 746 from
Rhône-Poulenc, Paris, France. Submitted to WHO by Rhône-Poulenc
Agrochimie S.A., Lyon, France.
Plutnick, R.T. & Kapp, R.W. (1988). Iprodione (technical) - acute
dermal limit test in the rabbit. Unpublished report No. 209806 from
Exxon Biomedical Sciences, Inc., East Millstone, New Jersey, USA.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
San Sebastian, J.R., Naismith, R.W., & Matthews, R.J. (1985). In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Unpublished report No. PH 320-BO-001-84 from Borriston
Laboratories, Inc., Temple Hill, Maryland, USA. Submitted to WHO by
Rhône-Poulenc Agrochimie S.A., Lyon, France.
Stevens, K.R. (1974). Subacute toxicity, RP 26 019. Unpublished
report No. KRS 74:3 from Hess & Clark, Ashland, Ohio, USA. Submitted
to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976a). Acute toxicity of Rovral (26019RP). I. Oral,
intraperitoneal and subcutaneous administration in rats. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Takehara, K., Itabashi, M., Hayashi, K., Kudow, S., Kawakubo, A., &
Tajima, M. (1976b). Acute toxicity of Rovral (26019RP). II. Oral,
intraperitoneal and subcutaneous administration in mice. Unpublished
report from Nippon Institute for Biological Science, Japan.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., & Deans, C.F. (1986a). Iprodione
(technical grade): effects of oral administration upon pregnancy in
the rat. 1. Dosage range-finding study. Unpublished report No.
85/RHA063/752 from Life Science Research, Suffolk, England.
Submitted to WHO by Rhône-Poulenc Agrochimie S.A., Lyon, France.
Tesh, J.M., McAnulty, P.A., Lambert, E.P., Wilby, O.K., & Tesh, S.A.
(1986b). Iprodione (technical grade): teratology study in the rat.
Unpublished report No. 85/RHA064/765 from Life Science Research,
Suffolk, England. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
Trimmer, G.W. (1988). Iprodione (technical) - dermal sensitization
test in the guinea-pig (Buehler method). Unpublished report No.
209821 from Exxon Biomedical Sciences, Inc., East Millstone, New
Jersey, USA. Submitted to WHO by Rhône-Poulenc Agrochimie S.A.,
Lyon, France.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
