
PHORATE JMPR 1977
IDENTITY
Chemical name
O,O-diethyl s-ethylthiomethyl phosphorodithioate
Synonyms
Thimet (R), timet (USSR), E1 3911, American Cyanamid 03911
L11/6, ENT 24042
Structural formula
S
"
(C2H5O)2 - P-S-CH2-S-C2 H5
C7H17O2PS3
Other information on identity and properties
Technical phorate is a clear pale yellow mobile liquid containing a
minimum of 90% phorate and having a specific gravity of 1.16 at 25°C.
Molecular weight 260.37.
BP -118-120°C at 0.8 mm Hg.
PH -3.3 (1% soln. in 75/25 alcohol/water)
Solubility -approx. 50mg/l in water but miscible with xylene,
vegetable oils, carbon tetrachloride, alcohols, ethers and esters.
Stability - at temperatures up to 25°C the technical grade is known to
be Stable for at least 2 years. Both the technical grade and
formulations are subject to hydrolysis in the presence of moisture and
alkali. Commercial granular formulations are stable for over two years
if kept in closed containers at temperature not exceeding 25°C.
Composition of technical phorate
Full details of the composition of technical phorate were made
available in confidence by the principal manufacturer.
Formulations
Phorate is generally used as 5% and 10% a.i. granules. The technical
material can be coated or adsorbed on a wide range of locally
available carriers: Emulsifiable concentrates containing 60% a.i..are
also available in some countries.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Rats receiving a single oral dose of 2 mg/kg of 32p-labelled phorate
excreted 35% of the administered radioactivity in the urine and 3.5%
in the faeces within 144 hours. Rats receiving 6 daily doses of 1
mg/kg excreted 12% of the total administered radioactivity in the
urine and 6% in the faeces within 7 days. The brain, liver and kidney
tissues from the animals closed with 1 mg/kg contained unidentified
and largely unextractable residues (Bowman and Casida, 1958).
Absorption, distribution and excretion in cows and calves are
described in the section "Fate of residues", "In animals".
Biotransformation
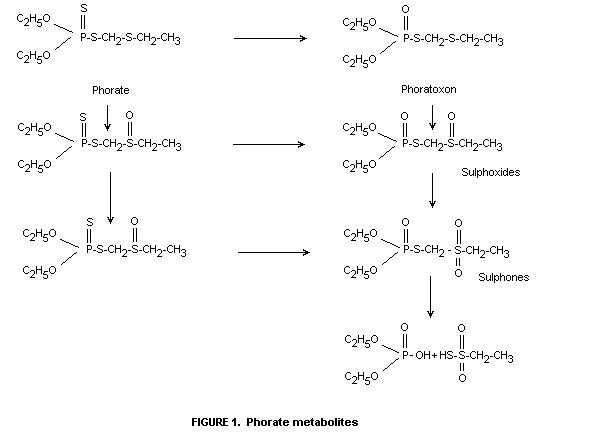
The cholinesterase-inhibiting metabolites of phorate are phorate
sulphoxide and sulphone and the oxygen analogue phoratoxon and its
sulphoxide and sulphone. (See Figure 1.)
The urine of rats receiving daily oral doses of 1 mg/kg contained 17%
diethyl phosphoric acid, 80% 0,0-diethyl phosphorothioic acid and 3%
0,0-diethyl phosphorodithioic acid (Bowman and Casida, 1958).
32p-labelled phorate was incubated with rat liver slice preparations.
Less than 1% of the applied radioactivity was converted to hydrolysis
products or unextractable residue. Phorate sulphoxide, phorate
sulphone, phoratoxon sulphoxide and phoratoxon sulphone were formed
(Bowman and Casida, 1958).
Rats were dosed with atropine and 1.6, 3.2 and 5.5 times the nominal
LD50 for phorate (3.7 mg/kg). Analysis of the blood, by thin layer
chromatography indicated the presence of phorate, phorate sulphoxide
and phorate sulphone. Only phorate sulphone was detected by vapor
phase chromatography (Blinn and Boyd, 1967).
Phorate was rapidly metabolized when incubated in liver homogenates of
four species of animals. Analysis of extracts of rat liver homogenates
by GLC indicated the presence of phorate sulphone and phorate
sulphoxide, but this identification could not be confirmed by TLC (Rao
and McKinley, 1969).
One cow was given a single oral dose of 3.04 mg/kg of phorate to
determine urinary, blood and tissue metabolites. 0,0-diethyl
phosphorothioic acid, 0,0-diethyl phosphoric acid and small amounts of
0,0-diethyl phosphorodithioic acid were detected in the urine. Most of
the radioactivity in the blood and tissues could not be extracted with
 chloroform and acetone (Bowtan and Casida, 1958) (See also "Fate of
residues", "In animals").
Bean plants were dipped into a 500 ppm P-32 phorate solution prior to
planting to determine the metabolism of phorate by plants. The primary
metabolites were phorate sulphoxide and/or phorate sulphone. Small
amounts of phoratoxon sulphoxide, phoratoxon sulphone and unchanged
phorate were also detected. No phoratoxon was detected. The hydrolysis
products formed were phosphoric acid and the diethyl esters of
phosphoric acid, phosphorothioic acid and phosphorodithioic acid
(Bowman and Casida, 1958).
Other work on the biotransformation of phorate in plants is described
in "Fate of residues", "In plants".
Effects on enzymes and other biochemical parameters
The anticholinesterase activities of phorate and its metabolites are
shown below (Table 1).
TABLE 1. Comparison of anticholinesterase activity of phorate
metabolites
Anticholinesterase activity
(p/50)*
Phorate 3.17
Phorate sulphoxide 3.35
Phorate sulphone 5.00
Phoratoxon 5.87
Phoratoxon sulphoxide 6.76
Phoratoxon sulphone 7.02
*p/50 + negative logarithm of molar concentration producing 50%
inhibition of red blood cell cholinesterase.
In rats, at dietary levels above 0.66 ppm, phorate depressed plasma,
erythrocyte and brain enzyme activity (Tusing et al., 1956b). Phorate
sulphoxide at dietary levels greater than 0.32 ppm also produced
depression of cholinesterase in rats in varying degrees during a 90
day studY (Levinskas et al., 1968). In dogs, plasma and erythrocyte
cholinesterase activity was depressed at levels above 0.01 mg/kg/day
(Tusing et al., 1956a). Dietary feeding at 1.0 ppm for 6 weeks
produced no effect in dogs (Kay and Calandra, 1961).
TOXICOLOGICAL STUDIES
Special studies on potentiation
Young rats weighing between 90 and 120 g (5 males/4 dosage levels)
were used to investigate the potentiation of phorate with diazinon,
EPN, azinphos-methyl, malathion, parathion-methyl, parathion,
mevinphos, demeton, tetram and carbophenothion. Oral LD50 values
were determined for each pesticide under identical conditions. Each
pair of compounds was mixed in approximately equitoxic proportions,
i.e., in about the ratios of their LD50's.
Results, given as LD50 in mg/kg are as follows:
Diazinon (283,216); EPN (42.9, 25); azinphos-methyl (18.7, 8);
malathion (329, 178); parathion-methyl (14.2, 11); parathion (12.3,
8); mevinphos (7,4); demeton (6.2,4); tetram (14.2, 5);
carbophenothion (32.5, 29). In the case of every mixture, the observed
LD50 indicating no potentiation (Shaffer et al., 1958).
Special study on neurotoxicity
Groups of 6 adult hens were fed 0 and 40 ppm phorate in the diet for 4
weeks. A third group received 4000 ppm TOCP as a positive control.
Each hen was anesthetized, immediately perfused with buffered
formalin, and sections of brain, lower thoracic cord and each sciatic
nerve prepared for microscipis examination. The TOCP produced myelin
loss in each hen. Phorate produced no adverse effects on nerve fibres
or the myelin sheath (Levinskas et al., 1965).
Special study on mutagenicity
Ten pesticides, including phorate, were tested for dominant lethal
effects. A group of 20 male mice were fed for 7 weeks at three dose
levels of phorate (levels not reported). Reference controls and a
positive control receiving triethylenemelamine in drinking water for 4
weeks were included in the test. Following treatment each male was
mated to two females weekly for 8 weeks. No consistent response
occurred to suggest that phorate in the diet for 7 weeks induced
dominant lethal effects in mice (Jorgenson et al., 1976).
Special study on reproduction and teratology
Groups of mice (8 males and 16 females/group) were fed phorate in the
diet at levels of 0, 0.6, 1.5 and 3.0 ppm during a 3-generation, 2-
litter/generation, reproduction study.
There were no dose related effects on the fertility, gestation,
viability or lactation indices during the study, but there was a
lowering of the lactation index in the 3.0 ppm group. This value fell
below the control value in the first mating of the F0 generation, in
both matings of the F1 and in the second mating of the F2
generation.
Gross and microscopic examination of tissues and cleared pups revealed
no consistent abnormalities related to feeding of phorate (Anonymous,
1965).
Acute Toxicity
TABLE 2. Acute toxicity of phorate
LD50
Species Route, Sex (mg/kg) Reference
Rat Oral M 2.3 Gaines (1969)
F 1.1
Rat Oral M 2.8 Anonymous (1976)
F 1.6
Rat Dermal M 6.2 Gaines (1969)
F 2.5
Rat Dermal M 5.7 Anonymous (1976)
Rabbit Dermal M 5.2
Short term studies
Rat
Groups of rats (50 males and 50 females/group) were fed 92% phorate,
in corn oil, in the diet at levels of 0, 0.22, 0.66, 2.0 and 6.0 ppm
for 13 weeks. Two groups (25 males and 25 females/group) were fed 12
and 18 ppm for 8 and 2 weeks respectively. Cholinesterase activity was
monitored at weekly intervals. Occasional episodes of excitability and
intermittent tremors were noted in the 6.0 ppm female group. Both
sexes receiving the 12 and 18 ppm levels exhibited severe
excitability, intermittent tremors and ataxia culminating in death.
Levels of 6 ppm and higher produced significant depression in both
sexes of plasma, red blood cell and brain activity. At 2 ppm female
red blood cell activity was also depressed. Levels at 0.66 ppm or
below had no effect on cholinesterase activity. Growth, food
consumption, survival, liver and kidney weights and ratios, gross
necropsy and histological findings for all groups at 6 ppm or below
were within normal limits. Levels of 12 and 18 ppm severely affected
growth and survival (Tusing et al., 1956b).
Groups of rats (50 males and 50 females/control and 35 males and 35
females/test groups) were fed the sulphoxide of phorate (92% purity)
for 90 days at levels of 0, 0.32, 0.80 and 2.0 ppm. Brain, erythrocyte
and plasma cholinesterase activities were determined biweekly. At the
2.0 ppm level a significant (P <0.05) depression in erythrocyte and
brain cholinesterase activity occurred in females. Plasma activity
depression at this level was less consistent and judged as borderline.
The 0.8 ppm level produced borderline depression of the erythrocyte
enzyme activity. At 0.32 ppm all values were within acceptable
statistical limits for both sexes. No effects were observed on other
hematological values, organ weights or ratios and no consistent
microscopic pathology was noted which could be attributed to the
feeding of 2 ppm or less of the sulphoxide of phorate (Levinskas et
al., 1968).
Dog
Groups of mongrel dogs (2 males and 1 female/group) received by
capsule 0, 0.01, 0.05, 0.25 and 1.25 mg/kg/day of 92% phorate in corn
oil 6 days/week for 15 weeks. Two males received 2.5 mg/kg as a single
dose. Plasma and red blood cell cholinesterase activities were
monitored at weekly intervals. The 0.05 mg/kg/day level produced a
significant depression in plasma enzyme activity. The red blood cell
activity was not affected for the first 12 weeks but was depressed
slightly (not significantly) during the last 3 weeks of the study.
Significant plasma and red blood cell enzyme depression was produced
by the 0.25 mg/kg/day level. The 1.25 mg/kg/day level produced
complete inhibition of plasma cholinesterase activity and a
significant reduction in red blood cell activity. At the 2.5 mg/kg/day
level all dogs died within 3-4 hours following a single dose and no
enzyme activity determinations were made. No signs of systemic
toxicity in dogs receiving the 0.01 or 0.05 mg/kg/day doses (Gaines,
1969). Histopathological examination revealed no consistent findings
related to the test/material (Tusing et al., 1956a). Beagle dogs (3
males and 3 females/group) were fed in the diet 0, 0.5, or 1.0 ppm
phorate for 6 weeks. Cholinesterase activity of plasma and
erythrocytes was determined for each dog prior to and at biweekly
intervals during the test. Analyses of variance conducted upon plasma
and erythrocyte enzyme activities revealed no significant difference
between the test animal and control animal groups during the test
period (Kay and Calandra, 1961).
Long term studies
No information available.
Observation in humans
No information available.
COMMENTS
Phorate, an organophosphorous pesticide, is a derivative of
dithiophosphoric acid. Less than 40% of a 2 mg/kg oral dose to rats
was excreted in 6 days, and brain, liver and kidney contained
unextractable residues. The major metabolites in both plants and
mammals are the sulphoxides and sulphones of phorate and of its oxygen
analogue, phora-toxon; these have greater anticholinesterase activity
than phorate. Hydrolysis of phorate itself is a minor metabolic
pathway in mammals.
The biochemical data available on phorate are inadequate to determine
whether the oxidative metabolites are retained in mammalian tissues or
organs. Toxicological data submitted are insufficient, except for
cholinesterase inhibition, to determine the short and long term
effects of phorate and its oxidative metabolites. Although the Meeting
considered setting a temporary ADI for humans this could not be
established in the absence of long-term studies. Further studies are
required to evaluate a.) carcinogenic potential, b) teratogenic
potential, c) potential neurotoxicity and d) toxicity of metabolites.
Observations in human subjects are desirable.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phorate is a powerful soil insecticide with excellent systemic
properties. A wide spectrum of insect and free-living nematode pest
problems is controlled by a seed-bed or a side-dressing treatment.
Seed-bed applications protect the germinating seedlings and young crop
stages against a wide range of pests.
Granular formulations of phorate have been extensively field tested
throughout the world since 1954 and are used commercially in many
countries. Major uses are summarized in Table 3. Recommended dosage
rates vary somewhat from country to country, depending mainly on the
pest complex to be controlled and the period of protection required.
Although application is normally made in the furrow at seeding or
planting time, certain pest problems require the application of
granules as a side-dressing at the time of subsequent cultivation.
There are some situations where, in order to control vectors of virus
diseases, it is necessary to make a broadcast treatment with granules
or a foliage application of emulsifiable concentrate to the growing
crop. Preharvest intervals and limitations on grazing vary somewhat
but for the most part they are relatively long because of the residual
life of phorate in treated plants.
In addition to its use on food and forage crops phorate is used on
ornamentals and tobacco and in forest nurseries where sucking insects
are a problem.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive trials have been carried out by the manufacturers and by
independent investigators to determine the level and fate of phorate
residues in virtually every crop on which the insecticide in used.
Copies of trial reports and published scientific papers were provided
to the Meeting by the manufacturers (American Cyanamid, 1977). A
representative selection of the data from these reports has been
summarized in Table 4. The following observations are made on the
detailed data.
TABLE 3. Phorate: use-pattern
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Alfalfa 1 seed treatment 60 days
1.5-2 broadcast 35 days
(grazing)
Barley 1 broadcast-soil incorporated 60 days
Beans 1-1.5 in furrow at planting 60 days
broadcast before flowering -
Brassicas 16g/100m row in furrow at transplanting -
Carrots 1.7-3.3 at drilling time -
TABLE 3. (Continued)
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Cotton 0.2-0.5 seed treatment do not use
seed for food
0.3-1.5 furrow application do not graze
Hops 1-1.5 foliage application 25 days
2-3 band application 42 days
Lettuce 1 at transplanting or
seeding -
1 foliage treatment 18 days
Maize 1-2 in farrow at planting -
1 band application
at cultivation 30 days
1 foliage application 30 days
Onions 12g/100m row in furrow at transplanting -
Peanuts 1-1.5 in furrow at planting do not graze
or feed
2 band application at pegging do not graze
or feed
Peppers 1 in furrow at transplanting -
Potatoes 1.5-3 in furrow at planting 90 days
Sorghum 2 In furrow at planting -
0.5-1 foliage application 28 days
Sugar beets 0.5-1 in furrow at planting -
1-1.5 foliage application 30 days
Sugar cane 4 in furrow at planting -
Tomatoes 1-2 at planting or
transplanting -
Wheat 1 in furrow
1 broadcast 70 days
Sunflower 1-1.5 in furrow at planting -
TABLE 4. Phorate residues resulting from supervised trials.
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
ALFALFA USA 1966 1 1 granule <0.01 <0.01 <0.01 <0.01
(dry) 1 2 10% <0.01 <0.01 <0.01 <0.01
1 4 " 0.06 0.06 0.03 0.02
1 1 " 0.06 0.08 0.71 0.37 0.21
1 2 " 0.01 0.01 0.35 0.46 0.07
1 1 " 0.05 <0.01 0.01 0.01 0.01
1 2 " 0.15 0.02 0.02 0.02 0.01
APPLES Italy 1970 2 1.5 E.C. <0.02 <0.02 <0.02 <0.02
Argentina 1972 1 2 granule 0.01
1 3 " 0.01
BARLEY USA 1967 1 4 " <0.01
(grain) 1 1 " 0.01
BARLEY USA 1967 1 2 " <0.01
(straw) 1 1 "
1 2 0.04
BARLEY USA 1961 1 1 " 0.07 <0.04
(grain)
BEAN, SNAP USA 1966 1 1 " 0.5 0.07 <0.01
USA 1963 1 2 " 0.046 0.016
1 2 " 0.018 0.011
1 4 0.013 0.036
USA 1966 1 1 " 0.5 0.07 <0.01
1 1 <0.01
1 2 " <0.01
COWPEA India 1974 1 2 " 1.46 1.05 0.53
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
EGGPLANT India 1974 1 2 " 1.27 1.01 0.69
MAIZE USA 1970 1 0.5 E.C. 0.068 0.024 <0.020
(plant) 1 " 0.131 0.046 0.007
2 " 0.695 0.110 0.019
S. Africa 1977 1 0.5 granule 0.4 0.1 <0.02 <0.02
1 " 2.8 0.9 0.03 0.03
0.5 " <0.02 <0.02 <0.02
1.0 " 0.06 <0.02 <0.02
0.5 " 0.5 <0.02 <0.02
S. Africa 1977 1.0 granule 0.8 0.03 <0.02
MAIZE S. Africa 1977 1 0.5 " <0.02
(grain) 1.0 " <0.02
Italy 1971 2.5 " <0.02
14 30 40 60 90 120 150
CARROTS UK 1970 2 3 granule 1.01 0.88 0.47 0.64
3 3 " 0.29 0.40 0.39 0.27
2 3 " 0.41 0.41 0.24 0.19
3 1.5 " 0.19 0.09 0.09 0.23
Canada 1975 3 1 E.C. 0.26
4 1 E.C. 0.40
CELERY UK 1971 1 1.2 granule <0.05
1.5 " <0.05
COTTONSEED USA 1969 1 1 E.C.(seed) <0.05
1 1-2 granule <0.05
1 3 " <0-05
1 2 E.C. <0-05
Egypt 1968 5 1 G/E.C. 0.02 0.01
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
GRAPES Mexico 1973 1 4 0.000
1 6 0.000
1 8 0.007
HOPS USA 1968 1 4 granule <0.1 <0.1 <0.1
3 1 E.C. 0.1
LETTUCE USA 1977 1 2 5.14 0.24 0.17 <0.05
1963 1 1 <0-05
1963 1 2 <0.05
1963 2 0.025
PEANUTS USA 1964 2 1+2 granule <0.05 <0-05
(kernels) 2 1+2 " <0.05 <0-05
2 1+2 0.05 0.05
2 1+4 <0.05
1 2 " 0.1 0.08 0.05
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
PEANUT USA 1964 2 1+2 granule 0.05
(foliage)
POTATOES Czechoslovakia 1972 1 1.6 " <0.05
1 2.2 " <0.05
1 4.3 " 0.07
Turkey 1973 1 3.4 <0.05
Mexico 1972 1 1 E.C. 0.004 0.000
1 2 E.C. 0.006 0.000
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
RAPE SEED USA 1971 1 1.5 granule 0.05
RAPE HAY USA 1971 1 1.5 " 0.21
SORGHUM USA 1969 1 1 " <0.05 <0.05
(grain) 1 2 " <0.05 <0.05
SORGHUM USA 1969 1 1 " 0.06 0.06
(fodder) 1 2 " 0.16 0.08
SORGHUM Mexico 1972 1 1 E.C. 0.00 0.00
(grain) 1 2 E.C. 0.02 0.00
MILO USA 1967 1 1 granule <0.01 <0.01 <0.01
2 " <0.01 <0.01 <0.01
4 " 0.22 0.01 <0.01
SOYBEAN USA 1970 1 1 " <0.05 <0-05
2 " <0.05 <0.05
7.5 " <0.05 <0.05
SOYBEAN USA 1970 1 1 " <0.05 <0.05
(foliage) 2 " <0.05 <0.05
7.5 " 0.15 0.19
SUGAR BEET USA 1961 1 1 E.C. 0.05
2 E.C. 0.05
TOMATOES USA 1961 1 1 <0.05 <0.05
2 0.05 0.05
4 0.06
WHEAT USA 1969 1 1 <0.05
(grain) 1 2 <0.05
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
WHEAT USA 1969 1 1 1.4 0.35 0.33
(plant) 1 2 2.7 1.6 3.0
1 1 6.1 0.87 0.07
Alfalfa
Residue results obtained on fresh alfalfa and alfalfa hay grown in
various regions of the United States show that application at twice
the recommended dosage rate of 2 kg/ha does not result in residues in
excess of 0.5 mg/kg in or on fresh alfalfa or 1 mg/kg on alfalfa hay
within 35 days of treatment. Generally the level is less than the
limit of determination (0.01 mg/kg).
The results from a series of trials where phorate was applied to
alfalfa at rates ranging from 2 through 3 to 4 kg/ha indicate that the
half life in green alfalfa is between 8 and 18 days.
Apples
Results from spray trials carried out in Italy where 7.59 of phorate
was applied per tree on one, two and three occasions prior to harvest
show that the residues in apples 30 to 80 days after the last
application were generally less than 0.02 mg/kg of Phorate and
metabolites. One sample from a tree treated 30 days previously showed
0.06 mg/kg phorate sulphone only.
Residue analysis for phorate and metabolites in several varieties of
apples treated in Argentina with phorate granules applied in
incorporation in the soil around the stem at doses ranging from 10-20g
of phorate per tree indicated no residues of parent compound or
metabolite in fruit collected 182 days after application.
Barley
Experiments in the USA where phorate granules were incorporated or
irrigated into the soil at planting time reveal that the residues of
phorate and metabolites in the grain at harvest 60 days later are
generally at or below the limit of determination (0.01 mg/kg). When
applied at twice the recommended rate (1 kg/ha) detectable residues
(0.04 mg/kg) were found in barley straw 60 days later.
Stern (1961) reported that phorate granules applied to barley fields
at the rate of 1 kg/ha one day prior to irrigation, produced
detectable residues in the barley plants (0.07 mg/kg) 14 days later.
The residues had declined below the limit of determination (0.04
mg/kg) by the 28th day following application.
Beans
Extensive field trials carried out during the period 1966-68 on many
varieties of beans under widely differing agricultural and ecological
conditions indicate that the residues are generally well below 0/05
mg\kg 60 days after application. The samples were analysed by the
oxidative cholinesterase inhibition technique which determines phorate
and metabolites. The limit of determination based on control values is
considered to be 0.05 mg/kg. The reports contain results from a few
isolated samples which indicate a total phorate residue of the order
of 0.1 mg/kg.
Carrots
Wit (1960) using analytical methods involving the determination of
total phosphorus showed that, following the application of phorate
granules at the rate of 1.25-1.5 kg/ha, carrots contained from 0.09-
0.19 mg/kg of residues derived from phorate applied 73-132 days
previously.
An extensive experiment carried out in the United Kingdom (Cyanamid
Great Britain Limited, 1971) determined residues of phorate and its
metabolites in carrots following treatment with phorate granules
applied with the seed and at varying times thereafter according to
various treatment codes. The highest residue level was approximately
1mg/kg some 5 weeks after the last treatment, but this declined to
0.64 mg/kg by the end of 4 months. Lower rates produced somewhat lower
residues but significant residues were detected from all treatment
regimes. The residues consisted of phorate and phorate sulphone with
no oxygen analogue or sulphoxide.
Finlayson et al (1976a) studied the distribution of a number of
insecticides used to control carrot rust fly in the peel and pulp of
carrots, measuring also the distribution throughout the length of the
carrot. These investigators found that the bulk of the residue was in
the peel in the top 3cm of the carrot, the total residue in the whole
carrot being of the order of 0.2-0.4 mg/kg. The peel at the top end
contained as much as 7 mg/kg. See also "Fate of residues".
Maskell et al (1974) showed that the application of granules
containing 10% phorate at 1, 2 or 4 kg/ha in a narrow slit together
with carrot seed gave rise to residues which could be reduced by 50%
by placing the granules 2.5cm below the seed. Residues resulting from
the application of 1 kg/ha with the seed or 2 kg/ha 2.5cm below the
seed were not significantly greater than residues in untreated
carrots.
When 4 kg of phorate per ha was applied along with carrot seed the
residue in carrots at harvest 8 months later was 0.4 mg/kg. When the
granules were placed 2.5cm below the seed the residue under
corresponding conditions was only 0.2 mg/kg.
Cottonseed
American Cyanamid Company (1969c) reported the results of 13 trials in
different regions of the USA aimed at determining what residues, if
any, occur in cottonseed following phorate treatment of seed pre-
planting or emulsifiable concentrate as a late season foliar
application. All samples were analysed by the gas-chromatographic
method. The data show less than 0.05 mg/kg phorate in cottonseed
harvested from crops treated at planting time or with a side-dressing.
Late season application with phorate emulsifiable concentrate at rates
up to 2 kg/ha did not show detectable residues in cottonseed from
crops treated 30 days prior to harvest.
Zaki and El-Sayed (1968) showed that residues in mature cottonseed
after spraying mature plants three times with phorate at the rate of 1
kg/ha were only 0.013 mg/kg 14 days after the fourth application of
foliage sprays.
Cowpeas
Satpathy et al (1974) showed that highly significant residues of
phorate could be found in the pods of cowpeas 10 and 20 days after
treatment of the soil with phorate granules applied at the rate of 2
kg/ha. The residues continued to decline with a half life of
approximately 12 days. Phorate residues were relatively more
persistent than those of diazinon and dimethoate but comparable to
disulfoton in cowpeas.
Grapes
The residue analysis of grapes harvested from plants growing in soil
treated with phorate granules at the rate of 4, 6 and 8 kg/ha showed
no residues at the two lower rates and only barely detectable residues
at the highest rate. Treatment had been made by incorporating the
granules into the soil at the time when the grape vines were
sprouting. Grapes were harvested 92 days later (Cyanamid
International, 1973b).
Hops
Kiligemagi and Terriere (1968 a,b) reported an extensive series of
trials designed to determine the level and fate of phorate residues in
hops following the application of phorate granular to the hop beds and
foliar applications at rates from 1 to 5 kg/ha and with pre-harvest
intervals ranging from 15 to 89 days. Using a gas-chromatographic
method specific for phorate and its oxygen analogue sulphone the
investigators were unable to detect, either the parent compound or
metabolites in fresh or dried hops even at the short pre-harvest
interval of 15 days. Aphid and spider mite control were, however,
excellent.
Maize
One of the major uses for phorate ban been the control of a variety of
insects on maize. In the early 1960s the manufacturers carried out,
extensive residue studies in many locations in the USA. Using
oxidative cholinesterase inhibition procedures it was shown that the
insecticide was taken up into the growing plant but that the residues
declined rapidly during the 45 days following application, by which
time residues in the whole plant were not significantly greater than
the apparent phorate residue in untreated control samples. By the time
the grain was ripe, no trace of phorate of other
cholinesterase-inhibiting substances could be found in the grain or
other parts of the plant.
Following the introduction of GLC methods capable of determining
phorate and its metabolites, a number of studies were carried out in
several countries beside the USA. These confirmed that the residue
levels declined to below the limit of determination between 28 and 40
days after the application of phorate granules or spray at rates up to
2 kg/ha. Leuck and Bowman (1970) published the results of one such
study which traced the occurrence and level of phorate and four
metabolites in maize plants treated with 0.5, 1 and 2 kg/ha of phorate
as a spray. These workers showed that the sum of all residues was less
than 1 mg/kg 14 days after the application of all three rates of
phorate.
Peanuts
Investigation in the USA (Diablo Laboritories 1964) show that the
application of phorate granules at planting and subsequent application
at picking does not result in apparent phorate residues different from
these found in untreated controls. The analyses were made using the
oxidative cholinesterase inhibition technique which produces a
positive result in untreated controls of the order of 0.05-0.1 mg/kg
apparent phorate. Samples of kernels from plants treated at the
highest rate did not show any residues higher then the controls when
collected 90 days after the last treatment. Where treatments were
applied 30 to 40 days prior to harvest, a positive residue of the
order of 0.1 mg/kg was found in peanut kernels. The residues in peanut
foliage after 90 days were not significantly different from untreated
controls.
Potatoes
Residue date developed prior to 1960 in support of petitions for the
registration of the use of phorate on potatoes were obtained with a
non-oxidative anti-cholinesterase method of analysis. This procedure
has good sensitivity for the oxygen analogue sulphoxide and sulphone
and, with the long pre-harvest interval involved, was considered
adequate at the time. Extensive residue information on potatoes was
obtained with this method, including results from inordinately high
rates of application, with all findings showing no measurable residues
in mature potatoes at harvest.
Owing to their relatively poor cholinesterase inhibiting properties,
phorate and its intermediary oxidative derivatives such as phorate
sulphoxide or sulphone would not have been measured by the
non-oxidative method had they been present. In 1959, an oxidation step
was introduced into the anti-cholinesterase procedure, which converted
phorate and its partially oxidised metabolites to phorate oxygen
analogue sulphone, and thus measured all toxic phorate residues. At
this stage a method based on the determination of total phosphorus
(Steller and Curry 1964) and including an oxidation step (Blinn 196A)
was utilized for further studies. By this method, which had a limit of
determination of the order of 0.01 mg/kg, it was shown that potatoes
treated at planting with phorate at the maximum recommended rate of 3
kg/ha generally contained 0.05 mg/kg or less when harvested 90 to 120
days later. However, residues as high as 0.15 mg/kg were occasionally
found following the use of phorate at the rate of 3 kg/ha. Following
the use of twice this amount (6 kg/ha) residues ranging up to 0.5 were
reported 100 to 140 days after application (American Cyanamid Company
1966).
In the Netherlands between 1958 and 1960, Wit (1959, 1961)
investigated the residues of systemic insecticides on potatoes, using
analytical methods involving the determination of total phosphorus
(limit of determination 0.1 mg/kg). He found substantial residues of
phorate in potatoes from 3 days to 42 days after application of
phorate granules (1.5-3 kg/ha). These residues ranged up to 3 mg/kg,
with the bulk of the results near 1 mg/kg.
Later Wit (1962a., 1963) using methods that determined phorate and the
sulphoxide metabolite (Laws 1961) showed that residues were less than
0.05 mg/kg 75-110 days after applying phorate granules at 2 + 3 kg/ha.
In 1972 following the development of a highly specific and
highly-sensitive analytical method employing gas-liquid chromatography
for the determination of phorate and phorate metabolites, an extensive
trial was carried out in Czechoslovakia (Cyanamid International
1972a). Residues of phorate or its oxygen analogue sulphone were not
measurable in any treatment. The only detectable residue in the
treated samples was of phorate sulphone, the highest level found being
0.07 mg/kg following the application of 3.4 kg/ha 120 days previously.
A similar field trial was carried out in Turkey (Cyanamid
International, 1973a), where phorate granules were applied at the rate
of 3.4 kg/ha at the time of planting and the potatoes harvested 125
days later. None of the potatoes contained residues of phorate or any
metabolite at levels above the limit of determination (0,01 and
0.02 mg/kg). It was concluded that the total residue of phorate and
metabolites was less than 0.05 mg/kg.
The application of phorate spray to potato Plants at rates to 1 and 2
kg/ha in Mexico (Cyanamid International, 1972b) produced detectable
residues in the tubers which declined from 0.02 mg/kg on the day
following application through 0.002 mg/kg on day 20 to 0 on day 27.
These results indicate that only small amounts of phorate are
translocated from the leaves to the tubers, and that these residues
decompose rapidly.
Sorghum and milo
Extensive trials were carried out in two different regions of the USA
to determine residues of phorate and its metabolites in sorghum fodder
and grain following granular and spray treatments. Samples of fodder
and grain were collected at intervals from 28 to 46 days after
application. The samples were analysed by gas-liquid chromatographic
procedures with a sensitivity of 0.05 mg/kg. All residues were below
0.05 mg/kg except fodder from one location which had been treated with
granular formulations and sampled after 31 and 46 days (American
Cyanamid Company, 1969a).
In an experiment carried out in Mexico, phorate spray was applied to
sorghum at the rate of 1 and 2 kg/ha 28, 21, 14, 7 and 0 days before
harvest, the residues found on the grain immediately after application
on the day of harvest were 0.55 and 0.82 mg/kg respectively. Though of
the order of 0.1 and 0.2 mg/kg could be found on the grain treated 7
days before harvest, that treated 14, 21 or 28 days prior to harvest
contained none as determined by a method sensitive to 0.01 mg/kg.
Soybeans
Studies carried out in the USA (American Cyanamid Company, 1970)
demonstrate that phorate granules used at the rate of 1 and 2 kg/ha
and applied in the furrow or to the side of the seed at planting will
result in no detectable residues (less than 0.05 mg/kg) of phorate
plus its toxic metabolites in soybeans seed at harvest. These same
studies showed no residue in soybeans treated at 7.5 kg/ha at two
separate locations and it is therefore reasonable to assume that no
detectable residues will occur in either soybeans oil or meal
following the use of phorate at the rate of 1-2 kg/ha.
Sugar beet
In 1960 and 1961 the manufacturers carried out or sponsored many field
trials to determine the level and fate of phorate residues in sugar
beet roots and leaves. Neither phorate nor its metabolites were
detected in any samples of sugar beet roots or tops in any trial. The
use of phorate sprays on sugar beet is approved in several countries
and maximum residue limits have been established to permit the feeding
of beet foliage to livestock.
Wit (1962b) reported that sugar beet roots and sugar beet leaves
contained less than 0.1 mg/kg or phorate-derived residues
(phorate + sulphoxides and sulphones) when phorate granules were
applied at the rate of 2-3 kg/ha 120-140 days before harvest.
Tomatoes
Extensive experiments carried out on tomatoes grown in various regions
of the United States during the period 1958-1961 show that following
the recommended application of phorate granules at the rate of 3 kg/ha
at planting time, no significant residues of either phorate or its
oxidative metabolites are found in either mature green or pink ripe
tomatoes. Similar results were also found at the 5 kg/ha. rate. Some
residue is found in the tomato foliage during the first few weeks
following application.
Wheat
Several studies were conducted in 1969 (American Cyanamid Company,
1969b) in the wheat growing areas of the United States to determine
phorate residues in green plants and grain following spring and fall
planting-time applications of phorate granules at rates of 1 and
2 kg/ha. The samples were analysed by GLC methods. The data show
phorate residues to be less than 1 mg/kg in all plant samples
approximately 45 days following application of phorate granules at a
rate of 1 kg/ha. No detectable residues (less than 0.05 mg/kg) were
found in grain harvested 120 to 330 days after treatment.
FATE OF RESIDUES
In animals
32P-phorate was given to a cow by Bowman and Casida (1958) in a
toxic dose of 3.04 mg/kg body weight, the acute symptoms that appeared
being controlled with atropine injections. The blood level increased
within 6-8 hours. After 18-36 hours the content of chloroform-soluble
metabolites in the blood increased slightly. The cow excreted 59% of
the radioactive material in the urine within 72 hours. None of this
was chloroform extractable. The main urinary metabolite was initially
0,0-diethyl phosphorothioic acid and subsequently diethyl phosphoric
acid, excretion of. 0,0-diethyl phosphorodithioic acid was
consistently low over the entire period. Only 0.8% of the applied
radioactivity appeared in the faeces within 96 hours after application
and 10% after 12 hours. The chloroform-soluble products were phorate
sulphoxide and/or sulphone, together with some phorate.
Examination of the cow's tissues 96 hours after feeding showed that
the liver and kidneys contained the highest 32P concentration (10.3
and 5.2 mg/kg phorate equivalents respectively). In contrast, the loin
muscle tissue contained 0.29 mg/kg and the mesenteric fat 0.03 mg/kg
phorate equivalents. The lung contained 1.5 mg/kg. Appreciable amounts
of phorate-derived phosphorus was found in the rumen and omasum walls,
other glands and rib bones. Milk contained 0.07, 0.25, 0.3 and
0.53 mg/kg phorate equivalents at 8, 24, 32 and 56 hours after dosing,
respectively.
Phorate is attacked only slowly by the rumen fluid (Ahmed et al.,
1958).
Phorate was administered to calves at a rate of 0.1 mg/kg for 14 days
to determine the level of phorate residues in liver, kidney, blood,
muscle, heart and fat tissues. No detectable residues ( < 0.1 mg/kg)
were found. Phorate was administered to lactating dairy cows at rates
of 0.05 and 0.1 mg/kg for 14 days. No detectable residues of phorate,
(< 0.02 ppm ) were found in the milk of cows fed 0.05. The milk
from cows fed 0.1 mg/kg contained from <0.02 to 0.05 mg/kg Phorate
(Anonymous, 1961).
Bunyan and Taylor (1966) found practically no phorate in meat and
organs of pheasants after administration of 6.2 to 21.7 mg/kg body
weight. In other investigations with pheasants and pigeons Bunyan et
al (1969) also found no residues after feeding for 14, 28 and 42-day
periods with 100 ppm of phorate in the diet.
Experiments designed to determine the total phorate residues in
chicken tissues and eggs were carried out by American Cyanamid
(1969d). Laying hens were dosed with rations containing 1, 0.3 and
0.1 ppm total phorate (1: 1, phorate: phorate oxygen analogue
sulphone) for 21 consecutive days. The chickens were sacrificed 2-3
hours after the final dose. Egg samples were collected during the
final day of treatment. The samples of muscle, fat, liver, kidney and
eggs were analysed by gas-chromatographic procedures using a caesium
bromide thermionic detector: phorate and its oxidative metabolites
were oxidised to and measured as phorate oxygen analogue sulphone and
calculated as total phorate. The sensitivity of the method for both
phorate and the oxygen analogue sulphone was 0.05 mg/kg. None of the
samples showed any apparent residues of phorate above this level.
In plants
Since 1954, studies of the metabolism of phorate in plants have been
conducted by investigators at American Cyanamid Company and by
researchers in various parts of the world. It has been shown that when
absorbed by plants, phorate is initially and rapidly oxidised to its
sulphoxide and sulphone which in turn are converted to the sulphoxide
and sulphone of the phorate oxygen analogue (Blinn, 1964; Bowmann and
Casida, 1957, 1958; Galley and Foerster, 1976; Krueger, 1975;
Lichtenstein et al., 1974; Metcalf et al., 1957; Zaki and El-Sayed,
1968).
The sequence of oxidative reactions in the metabolism of phorate
following absorption by plants is similar to that in animals and is
given in Figure 1. The oxygen analogue, although presumably formed as
an intermediate, has not been detected.
Bowman and Casida (1957) showed that when phorate was used as a
systemic insecticide for seed treatment of cotton, the metabolites
within the plant consisted of the sulphoxide and sulphone of the
parent compound and of its oxygen analogue. Cotton seeds treated with
phorate at concentrations as high as 32 kg of phorate per 100 kg of
seed showed less than 0.03 mg/kg of phorate or metabolites in the
seeds maturing an plants grown from such treated seed. The residual
persistence following soil and foliage application was studied with
six vegetable crops using radioactive phorate. Table 5 shows the
persistence of radioactivity in vegetable crops treated with
32P-phorate and is derived from the means of values from beans,
beets, cabbage, carrots, lettuce and peas treated by foliage
application and by soil application. Other experiments by Boman and
Casida (1958) on the fate in beans are described previously
(Biotransformation).
Phorate is usually applied as a soil treatment where its outstanding
systemic properties can be utilized to protect the developing plants
from attack by sucking insects for the first few weeks of their
growth. After phorate is applied to the soil and reaches the root zone
of the plant, the insecticide is absorbed by the roots and is
subsequently translocated to aerial parts of the plant. The rate of
movement of phorate from roots to stems and leaves is accelerated by
increased transpiration, and the greatest accumulation of insecticide
in leaves seems to occur under environmental conditions favourable to
transpiration (Hacskaylo et al., 1961). Van Middlelem and Baranowski
(1962) found that the highest concentrations of phorate in tomato
plants usually coincided with preceding periods of relatively high
field temperatures and ample rainfall. Krueger (1975) showed that
soybean root homogenates oxidised phorate to phorate sulphoxide.
Neither the oxygen analogue nor sulphone was detected.
Finlayson at al. (1976a), investigating methods for controlling carrot
rust fly, showed that phorate spray applied over the young carrot
seedlings was readily taken up and held in discrete portions of the
carrot. More than 50% of the residues were located in the top 0-3cm of
the carrot. Total residues in whole carrots generally increased with
higher rates and numbers of applications. Residues were greater in the
peel than in the pulp. Discarding the top 1-2cm of the carrot and
peeling the rest removes most of the residues. See also "Residues
resulting from supervised trials".
In soils
After phorate is applied to the soil, its eventual distribution and
utilization by plants is influenced by various constituents and
qualities of the soil.
In a study of the fate of phorate in soils, Getzin and Chapman (1960)
used radioactive material. One hour after treating various soil types,
these investigators found that 25, 20, and 10 per cent of the
radioactivity applied had been lost through volatilization from sandy
soil, silt-loam and muck respectively. After one hour, little or no
volatilization occurred. They further reported that phorate, when
applied to soil, is partially oxidised, hydrolyzed and bound to the
soil.
It has been shown that phorate moves readily in soil in the vapour
phase (Etheridge and Burt, 1963) as well as in drainage water (Bardner
at al., 1963).
Lindley (1963) reported from field experiments that on mineral soils
less phorate was required for insect control than on peat soils with a
maximum of 35% organic matter, while only partial control of insects
was obtained on soils with more than 35% organic matter, even when
much higher rates of insecticide were used.
Lichtenstein et al, (1973, 1974) showed that while phorate could be
detected in soil, only metabolites were found in plants including
plant roots. Schulz et al. (1973) showed that phorate moved in both
vertical and horizontal directions after a granular band application
of insecticide in soil. Suett (1975) showed that soil temperature had
relatively less effect upon the fate of phorate than on other
soil-incorporated insecticides.
Waller and Dahm (1973) showed that the conversion of phorate to its
sulphoxide in soils is mainly a non-biological process, whereas the
conversion to sulphone is brought about by micro-organisms.
Talekar at al. (1977) in a long-term experiment investigated the
persistence of a number of insecticides following repeated seasonal
application to soil under sub-tropical conditions. They showed that
phorate degraded more rapidly than all the other materials showing
almost complete loss within four months, although measurable
quantities of phorate sulphoxide and sulphone could be detected for
somewhat longer periods.
TABLE 5. Persistence of 32P-phorate and its metabolites in vegetable cropsa.
Days following application
0.1 1 2 4 8 17 32
Foliage application
Phorate equivalentb, mg/kg
Hexane fraction 6.75 2.84 2.49 1.69 0.90 0.44 0.040
Acetone-water fraction 2.60 2.39 1.66 1.45 0.59 0.067 0.003
Hydrolysis productsc 0.28 0.37 0.62 0.85 1.33 0.43 0.27
Unextracted residued 1.44 1.03 0.81 1.62 1.54 1.00 2.28
AntiChE activity, 50% inhibitione
Plant tissue, g 1.3 0.47 0.59 0.54 1.0 >2.2 >2.2
pl50 metabolites 5.44 6.14 6.29 6.62 6.11 <7.22 <7.42
Soil application
Phorate equivalent, mg/kg
Hexane fraction 0.82 0.34 0.40 0.26 0.15 0.049 0.026
Acetone-water fraction 0.12 0.31 0.56 0.36 0.18 0.047 0.005
Hydrolysis products 0.052 0.12 0.13 0.24 0.25 0.21 0.110
Unextracted residue 0.15 0.16 0.42 1.20 0.46 0.11 0.078
AntiChE activity, 50% inhibition
Plant tissue, g 1.8 0.94 1.3 1.2 1.3 >2.9 >3.0
pl50 metabolites 6.89 7.26 6.66 6.72 7.00 7.15 7.22
a Results are average of figures from beans, beets, cabbage, carrots, lettuce and peas in same field plot.
b Radioactivity of phorate or metabolites appearing in fraction indicated, expressed as phorate (mg/kg)
c Water-soluble products not extracted into chloroform.
d ssP remaining in solid portion after both water and chloroform removed.
e Results based on chloroform-soluble metabolites. Amount of plant material containing enough
metabolites for 50% inhibition and pl50 (negative logarithm of molar concentration effecting 50% inhibition)
of these metabolites are reported.
In processing and storage
Leuck and Bowman (1970) showed that residues of phorate and five of
its metabolites remained virtually unaltered when treated corn foliage
was converted into silage and ensiled at 35C° for 30 days.
Askew at al. (1968) examined the effect of 30 minutes boiling on
vegetables containing various organophosphorus pesticide residues.
They demonstrated 100% hydrolysis of phorate residues (2 mg/kg) in
both potatoes and cabbages. They indicated that appreciable quantities
of phorate sulphoxide ware formed during the cooking process, but did
not attempt to quantify the transformation.
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Advice from the US Food and Drug Administration indicates that during
1975 three samples of strawberries were found to contain phorate
residues at levels of 0.07, 0.16 and 1.73 mg/kg. There was no
tolerance for residues of phorate in strawberries.
METHODS OF RESIDUE ANALYSIS
Gas chromatography with an electron-capture detector was used by Egan
et al. (1964) to measure phorate in certain plant tissues. Dewey and
Parker (1965) used an electron-capture detector to measure phorate and
some of its metabolites in soil. Bache and Lisk (1966) reported the
use of an emission spectroscopic detector in soil analysis for phorate
and its metabolites but found very poor chromatographic response for
phoratoxon sulphoxide and sulphone. Mitchell et al. (1968) used gas
chromatography to study the effect of ultra-violet irradiation and
permanganate oxidation on phorate. These workers found it possible to
chromatograph phorate, phoratoxon and phoratoxon sulphone on an
Apiezon L column; quantitative recovery figures were not given,
however. Ruzicka, et al. (1967) used a caesium bromide thermionic
detector with an Apiezon column and found that phorate was not
adequately resolved from phoratoxon, although phoratoxon sulphone
could be resolved. The same workers (1968) later used similar
techniques to measure residues of phorate, phorate sulphoxide and
phorate sulphone on apple leaves. Sans (1967) described a
gas-chromatographic procedure for the separation and measurement of
phorate, but not its metabolites, from various other pesticides.
Nelson (1967) developed a procedure for separating and measuring
phorate, but not its metabolites, from several other organo-phosphate
insecticides in residues on fruits and vegetables.
Bowman et al. (1969b) described procedures for the individual
measurement of phorate and its oxidative metabolites in residues on
corn tissue. These workers used an instrument equipped with a flame
photometric detector and a column of 10% DCL-200 silicone on Gas-Chrom
Q. Recoveries at the 0.05 mg/kg level in excess of 96% were reported
for all of the metabolites except phoratoxon (62% recovery). McLeod et
al. (1969) reported a procedure for the separation and measurement of
phorate and its metabolites from monkey liver homogenates, These
workers used a column packed with 5% diethylene glycol succinate on
Chromosorb W and a flame photometric detector. Recoveries averaging
over 90% were found at fortification levels of the order of 200 mg/kg.
Stanley and Morrison (1969) described the use of the flame photometric
detector with both phosphorus-selective and sulfur-selective filters
to aid in the identification of insecticides; among the compounds
tested was phorate.
Watts and Storherr (1969) and Watts at al. (1969) described clean-up
and gas-chromatographic conditions for the separation and measurement
of many insecticides, including phorate and its metabolites, using a
potassium chloride thermionic detector and a column of 10% DC-200 on
Gas-Chrom Q. Recoveries in excess of 95% were reported for kale
extracts fortified with phorate or its metabolites at levels of 0.1 to
0.3 mg/kg.
Getzin and Shanks (1970) used an instrument equipped with a sodium
sulphate thermionic phosphorus detector and a column of 4% QF-1 on
Gas-Chrom Q to measure phorate and its metabolites in soils. Recovery
values at levels of 20 mg/kg in soil ranged from 64% for phoratoxon
sulphoxide to 96% for phorate.
Higham at al. (1972) have described procedures suitable for the
determination of total phorate-related residues in a wide variety of
sample types. In these procedures, all of the phorate and its
oxidative metabolites present in the residues were converted to
phoratoxon sulphone by oxidation with m-chloroperbenzoic acid as
recommended by Blinn (1964). These procedures employed a
phosphorus-selective detector with a column of 5% DC-200 on Gas-Chrom
Q and were validated at levels down to 0.05 mg/kg in several types of
sample.
Boyd (1976) has reviewed the methods of residue analysis for phorate
and has provided details of methods for all substrates likely to be
encountered in residue analysis. Residues of phorate and its
metabolites are extracted from the prepared sample with chloroform,
chloroform/methanol, methylene chloride or acetonitrile (depending
upon the type of sample) and purified by clean-up procedures
appropriate to the particular type of sample being analysed. All of
the phorate-related residues present are converted to phoratoxon
sulphone by oxidation with m-chloroperbenzoic acid. Measurement is
accomplished by gas-liquid chromotgraphy with an instrument equipped
with a selective phosphorus-sensitive detector. These procedures
appear suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Commodity Tolerance, mg/kg
Argentina lettuce, wheat,
groundnut, rice,
maize 0.1
sugar beet (tuber) 0.3
maize fodder 0.5
sugar beet (leaves) 3
Australia cottonseed, vegetables 0.5
Canada beans, corn, lettuce,
potatoes, rutabagas,
turnips negligible
Germany all plant foods 0.05
Netherlands fruit, vegetables
(not potatoes), spices 0
South Africa all food products 0.05
apples, pears 0.1
China pineapple 0.1
USA sugar beet tops 3
wheat (green fodder) 1.5
alfalfa hay, dried sugar
beet pulp 1
corn forage, hops,
potatoes 0.3
peanut vines and hay,
sugar beet roots 0.3
barley grain, barley
straw, beans, corn grain 0.1
sweet corn, lettuce,
peanuts, rice, sorghum
grain 0.1
sorghum fodder, soybeans,
sugar cane, tomatoes 0.1
cottonseed, wheat grain
and wheat straw 0.05
eggs, meat, fat and meat
by-products of cattle,
goats, hogs, horses, poultry
and sheep 0.05
APPRAISAL
Phorate is a soil insecticide with marked systemic properties widely
used throughout the world since 1954. Maize, cotton, cereal grains and
vegetables are treated with phorate usually at the time of planting,
but sometimes by broadcast treatments of granules or a foliage
application to the growing crop. Pre-harvest intervals are relatively
long and residues in harvested commodities are either absent or
generally do not exceed 0.1 mg/kg.
Carrots are capable of taking up and retaining quantities of phorate
but most of the residue is in the peel or in the crown of the carrot
which in generally removed before processing for consumption. The use
of phorate for the protection of potato crops is important. The
residues at the time of harvest are at or below the limit of
determination.
Soil treatments lead to higher residues than do foliage treatments,
largely because the compound is relatively stable in the soil and in
readily taken up by the plant's root system. In animals phorate is
rapidly hydrolysed and converted into the corresponding sulphoxide and
sulphone and their oxons. No significant amounts of phorate or
metabolites are found in milky eggs or tissues of livestock
administered phorate at levels comparable to those likely to occur in
animal feeds.
The metabolism of phorate in plants is largely similar to that
occurring in animals. Phorate metabolites are relatively stable in
storage and processing. At rates recommended in good agricultural
practice, there appears to be no likelihood of carry-over of residues
in soil affecting subsequent crops.
A method of analysis based on the oxidation of all the phorate-related
residues to the oxygen analogue sulphone followed by measurement by
gas-liquid chromatography is sensitive and specific and appears
suitable for regulatory purposes.
Many countries have established maximum residue limits for phorate
residues.
EVALUATION
In the absence of an ADI, the following guideline levels are recorded.
They refer to the sum of the residues of phorate, phorate sulphoxide,
phorate sulphone and the oxygen analogues of these compounds,
determined as the sulphone of the oxygen analogue and expressed as
phorate.
Commodity Guideline levels, Interval on which
mg/kg guideline levels are
based, days
Alfalfa (dry) 1 35
Barley 0.05 60
Beans 0.1 60
Carrots 0.5 120
Celery 0.1 90
Cottonseed 0.05 120
Cowpea 0.1 60
Eggplant 0.1 60
Grapes 0.05 90
Hops (dried) 0.1 25-40
Lettuce 0.2 60
Maize (green) 0.05 30
Peanuts (kernels) 0.05 60
Potatoes 0.05 120
seed 0.1 90
Sorghum 0.05 30
Soybeans 0.05 120
Sugar beet,
fodder beet 0.05 120
Sugar beet
tops 1 35
Tomatoes 0.1 120
Wheat 0.05 120
Eggs, meat, milk 0.05* 120
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI)
can be established and maximum residue limits (MRL) can be
recommended).
1. Long-term carcinogenicity studies.
2. Teratogenicity studies.
3. Studies on potential neurotoxicity.
4. Studies on the toxicity of metabolites.
DESIRABLE
1. Observation in humans.
REFERENCES
Ahmed, M.K., Casida, J.E., and Nichols, R.E. (1958) Bovine metabolism
of organophosphorus insecticides: significance of rumen fluid with
particular reference to parathion J. Agr. Food Chem. 6, 740.
American Cyanamid Company (1966) Thimet residues in potatoes. Report
No. G102, January 13, 1966.
American Cyanamid Company (1966a) Total Thimet residues in sorghum
fodder and grain - Report No.C188, May 14, 1969.
American Cyanamid Company (1966b) Thimet residues in wheat - Report
No. C215, December 9, 1969.
American Cyanamid Company (1966c) Thimet residues in Cottonseed ;
Report No. C214, December 9, 1969.
American Cyanamid Company (1969d) Total Thimet residues in chicken
tissues Report No. C205 dated November 7, 1970.
American Cyanamid Company (1977) Residue dossier on THIMET
phorate - Vol. I-III.
Anonymous (1965) Report on Thimet Systemic Insecticide: Successive
Generation Studies with Mice. Unpublished report from Central Medical
Department Cyanamid, submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Anonymous (1961) Thimet Phorate Residue Studies in Dairy Animals.
Unpublished report from DIABLO Laboratories, In,., submitted to the
World Health Organization by American Cyanamid Company.
Anonymous (1976) Thimet Soil and Systemic Insecticides Technical
Information published by Cyanamid International Corporation, submitted
to the World Health Organization by American Cyanamid Company.
(Unpublished report)
Askew, J., Mitchell, T.M., Thomson, J. and Wheals, B.B. (1968) The
effect of cooking on organo-phosphorus pesticide residues - J.
Chromat. 32, 417-418.
Bache, C.A., and Lisk, D.J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
chromatography- J. Assoc. Off. Analyt. Chem. 49, 647-650.
Bardner, R., Lord, K.A., and Solly, S.R.B. (1963) Cholinesterase
inhibition method for determining the distribution of organophosphorus
insecticides in soils. Chem. and Ind. 3, 123-124.
Blinn, R.C. (1963) Thin - layer chromatographic isolation and infrared
or colormetric identification of Thimet residues. J. Assoc. Off.
Agric. Chem. 46, (6) 1, 952-96.
Blinn, R.C. (1964) Oxidative conversion of Thimet to its oxygen
analogue sulphone. J. Assoc. Off. Agric. Chem. 47 (4) 641.
Blinn, R.C. and Boyd, J.E. (1967) Thimet R Phorate: Identity of the
Major Phorate Metabolites in the Blood of rats Following oral
Administration of the Insecticide. Unpublished report from the
Research and Development Department of the Agricultural Division of
the American Cyanamid Company, submitted to the World Health
Organization by the American Cyanamid Company.
Bowman M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion disulfoton and phorate in corn, milk grass and
faeces - J. Assoc. Off. Analyt. Chem. 52 (6) 1231-1237.
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969a) GLC determination of
residues of disulfoton, demeton-methyl and their metabolites in
tobacco plants. J. Assos. Off. Analyt. Chem. 52 157-161.
Bowman, M.C., Beroza, M. and Harding, A.J. (1969b) Determination of
Phorate and Five of its Metabolites in Corn. J. Ag. Food Chem. 17
138.
Bowman J.S. and Casida, J.E. (1957) Metabolism of phorate (Thimet) in
plants. J. Ag. Food Chem. 5 (3), 192.
Bowman J.S. and Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants insects and mammals - J. Econ. Entomol. 51 (6)
838-843.
Boyd, J.E. (1976) Thimet (phorate) in Vol VI of Analytical Methods for
Pesticides and Plant Growth Regulators by G. Zweig and G. Sherma
-Academic Press N.Y. p. 493-510.
Brown, M.J. (1975) Improved determination of residues of phorate and
its principal metabolites J. Agric. Food Chem. (2) 334-335.
Bunyan P.J. and Taylor, A. Esterase inhibition of pheasants poisoned
with Thimet. J. Agric. Food Chem. 14, 132.
Bunyan P.J., Jennings, D.M. and Taylor, A. (1969) Organophosphorus
poisoning: Chronic feeding of some common pesticides to pheasants and
pigeons. J. Agric. Food Chem. 17, 1027.
Burns, R.G. (1971) The Loss of phosdrin and phorate insecticides from
a range of soil types - Ball. Env. Contam. and Toxicol. 6 (4)
316-321.
Cyanamid Great Britain Ltd. (1971) Residues of phorate and its
metabolites in carrots. Technical Report No. 106 -16 May 1971.
Cyanamid International (1972a) Residues of phorate and its metabolites
in potatoes - Technical Report No. 179 dated 19 April 1972.
Cyanamid International (1972b) Thimet residue analysis in potato
-Regional Laboratory Report No. 11 dated August 23, 1972.
Cyanamid International (1973a) Thimet and Thimet metabolite residues
in potatoes. Technical Report 240 dated 9 May 1973.
Cyanamid International (1973b) Residue analysis of Thimet and the
oxygen analogue in grapes. Regional Laboratory Report No. 19 September
21, 1973.
Dewey, J.E. and Parker, B.L. (1965) Increase in Toxicity to Drosophila
melanogaster of Phorate, Treated Soils. J. Econ. Entomol. 58, 491.
Diablo Laboratories (1964) Analysis of peanut meats, shells and
foliage for possible residues of total Thimet - Report 4J7511 dated 26
February 1964.
Egan, H., Hammond, E.W, and Thomson, J. (1964) Assoc. Off. Analyt.
Chem. 89, 175.
Etheridge, P. and Burt. P.E. (1963) Vapour phase movement and uptake
of systemic insecticides. Rep. Rothamsted, Exp. Stat. for 1963 Page
138.
Finlayson, D.G., Williams, I.H., Brown, M.J., and Campbell,
C.J. (1976a) Distribution of insecticide residues in carrots at
harvest. J. Aric. Food Chem. 24 (3) 606-608.
Finlayson, D.G., Williams, I.H., Brown, M.J. and Campbell,
E.J. (1976b) Translation and persistence of topically applied diazino
and phorate in beans. Can. J. Plant Sci. 56 947-952.
Galley D.J., and Foerster, L.A. (1976) Distribution and loss of
phorate residues in the foliage of broad bean plants following root
uptake of 14C-labelled phorate. Pestic Sci. 7 301-306.
Gaines T.B. (1969) Acute Toxicity of Pesticides. Toxicol. Appl,
Pharmacol 14, 515-534.
Getzin, L.W. and Chapman R.K. (1960) The fate of phorate in Soils - J.
Econ. Entomol. 53 (1) 47-51.
Getzin, L.W. and Shanks, C.H. (1970) Persistence, Degradation and
Bioactivity of Phorate and its Oxidative Analogues in soil. J. Econ.
Entomol. 63, 52.
Giang, P.A. and Schecter, M.S. (1960) Colorimetric determination of
residues of phorate insecticidally active metabolites. J. Agr. Food
Chem. 8 51-54.
Hacskaylo, J., Lindquist, D.A. and Clark, J.C. (1961) Phorate
accumulation by cotton plants and recovery from soil. J. Econ.
Entomol. 54 (3) 411-414.
Higham, J.W., Peterson, R.P. and Pasarela, N.R. (1972) J. Assoc. Off.
Analyt. Chem. (in press)
Jorgenson, T.A., Rashbrook, C.J. and Newell, G.W. (1976) In vivo
Mutagenesis Investigations of Ten Commercial Pesticides. Abstract No.
41, Fifteenth Annual Meeting, Society of Toxicology, submitted by the
World Health Organization by American Cyanamid Company, Wayne, NJ,
USA.
Kay, J.H. and Calandra, J.C. (1961) Effects of Thimet Phorate on
Cholinesterase Activity in the Dog. Unpublished report from Industrial
Bio-Test Labs., Inc., submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Khajuria, G.N., Jain, H.K. and Dewan, R.S. Residues following the
treatment of sorghum with phorate and dimethoate for the control of
insect pests complex of the crop. Proceedings of the first All-India
Symposium on Progress and Problem in Pesticide - Residue Analysis held
in Ludhiana on 18-19 November 1971 - p. 87-92.
Kiigemagi, O., and Terriere, L.C. (1968a) Residues of phorate in fresh
and dried hops. Report from Oregon State University dated June 10,
1968.
Kiigemagi, O. and Terriere, L.C. (1968b) Residues of phorate and its
metabolites in fresh hops Report from Oregon State University, October
6, 1968.
Krueger, H.R. (1975) Phorate sulfoxidation by plant root extracts.
Pesticide Biochem. & Physiolol. 5, 396-401.
Krueger, H.R., Lindquist, R.K. and Bauerle, W.L. (1973) Phorate and
aldicarb in greenhouse lettuce: Effect of CO2 on residues. J. Econ.
Entomol. 66, 573.
Krueger, H.R. and Mason, J.F. (1973) Phorate and aldicarb: effects of
systemic fungicides on their levels in soyabeans.
Laws (1961) Analyst, 81, 249-255.
Leuck, D.B. and Bowman, M.C. (1970) Residues of phorate and five of
its metabolites. Their presence in Forage Corn and grass - J. Econ,
Entomol. 63 (9) 1838-1842.
Levinskas, G.J., Morici, B.S. and Shaffer, C.B. (1965) Thimet systemic
insecticide: Demyelination studies in white leghorn hens. Unpublished
report from Central Medical Dept. of American Cyanamid Co. submitted
to the World Health Organization by American Cyanamid Co.
Levinskas, G.J., McNerney, J.M. and Fegluy, H.C. (1968) Report on
Sulfoxide of Thimet Systemic Insecticide: Ninety-day Repeated Feeding
to Albino Rats. Unpublished report from Central Medical Department of
American Cyanamid Company, submitted to the World Health Organization
by American Cyanamid Company, Wayne, NJ, USA.
Lichtenstein, E.P., Fuhremann, T.W., Schulz, K.R. and Liang, T.T.,
(1973) Effects of Field application methods on the persistence and
metabolism of phorate in soil, and its translocation into crops. - J.
Econ. Entomol. 66 (4) 863-866.
Lichtenstein, E.P., Fuhremann, T.W. and Schulz, K.R. (1974)
Translocation and metabolism of Phorate as affected by percolating
water in a model soil/plant ecosystem. J. Aric. Food Chem. 22 (6)
991-996.
Lindley, C.D. (1963) The use of phorate granules on potatoes and
carrots Proc. 2nd. Conf. Brit. Insect. & Fangic. Brighton, 1963.
McLeod H.A., Malkins, G. and Rao, S.L.N. (1969) Gas chromatographic
analysis of phorate and five metabolites in monkey liver
homogenates - Bull. Env. Contam. and Toxicol. 4 (4) 224-231.
Maskell, F.E. Gair, R., Williams, J.H., and Cooper, B.J. (1974) Effect
of phorate placement on carrot fly control, seedling density and
terminal residues in the crop. Pestic. Sci. 5 543-548.
Menzer, R.E., Fontanella, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Envir. Contam. & Toxicol. 5 (1) 1-5.
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
Dithio-Systox and Thimet. J. Econ. Entomol. 50 (3) 338-345.
Mitchell, T.H., Razioka, J.H., Thomson, J. and Weals, B.B. (1968) The
chromatographic determination of organophosphorus pesticides. Part
III. The effect of irradiation on the parent compounds. J. Chomat.
32, 17.
Nelson, R.C. (1967) Procedure for nine organic phosphorothioate
pesticide residues on trials and vegetables by using micro-coulometric
gas chromatography. J. Assoc. Off. Analyt Chem. 50: 922-926.
Parker B.L. and Dewey, J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based m toxicity to Drosophilia - J. Econ.
Entomol. 58 (2) 106-111.
Rao, S.L.M. and McKinley, W.J. (1969) Metabolism of Organophosphorous
Insecticides by Liver Homogenates from Different Species. Canadian
Journal of Biochemistry, 47: 1155-1159.
Razicka, J.B., Thomson, J. and Weals, B.B. (1967) J. Chromat. 30,
92.
Razicka, J.B., Thomson, J. and Weals, B.B. (1968) J. Chromat. 33,
430.
Sans, W.W. (1967) Multiple Insecticide Residue Determination Using
Column Chromatography, Chemical Conversion, and Gas Liquid
Chromatography. J. Agr. Food Chem. 15, 192.
Satpathy, J.M., Dash, P.K. and Misra, B. (1974) Insecticidal residues
in some vegetables crops grown in treated soils. Indian J. Agric. Sci,
44 (2) 113-115.
Schulz K.R., Lichtenstein, E.P., Fuhremenn, T.W. and Liang, T.T.
(1973) Movement and Metabolism of phorate under field conditions after
granular band applications J. Econ. Entomol. 66 (4) 873-875.
Shaffer, C.B., West, R. and Golz, H.E. (1958) Thimet: Single Dose
Toxicity to Rats. Joint Toxic Action with Other Phosphates.
Unpublished report from Central Medical Department, American Cyanamid
Company, submitted to the World Health Organization by American
Cyanamid Company, Wayne, NJ, USA.
Singh, K. and Gulati, K.C. (1971) Studies on the persistence of
phorate and di-system in soil and plants. Proceedings of the First
All-India Symposium on Progress and Problems in Pesticide-Residue
Analysis held at Ludhiana on 18-19 November 1971 p. 143-149.
Singh, Kalyan and Gulati, K.C. (1972) Application of enzymatic method
for the estimation of organophosphorus insecticides and their residues
in soil and plant. Part II. Phorate. Indian J. Agr. Chem. 6 103-107.
Stanley, C.W. and Morrison, J.T. (1969) J. Chromatogr. 40, 289.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analogue by total phosphorus determination
after isolation by thin-layer chromatography. J. Assco. Off. Agric.
Chem. 47, (4) 645.
Stern, V.M. (1971) Summary of Thimet residues in barley. Report from
University of California, Riverside (undated).
Suett, D.L. (1971) Persistence and degradation of chlorfenvinphos,
diazinon, forofos and phorate in soils and their uptake by carrots.
Pestic. Sci. 2, 105.
Suett, D.L. (1974) Uptake of chlorphyrifos and phorate from soil by
carrots as influenced by mode of application and cultivar. Pestic.
Sci. 5, 57-71.
Suett, D.L. (1975) Persistence and degradation of chlorfenvinphos,
chlormephos, disylfoton, phorate, and pirimiphos - ethyl following
spring and late-summer soil application. Pestic. Sci. 6, 385-393.
Talekar, N.S., Lian-Tien Sun, Ehi-Min Lee and Jing-Sing Chen. (1977)
Persistence of some insecticides in subtropical soil. J. Agric. Food
Chem. 25 (25) 348-352.
Tusing, T.W., Kindzin, W., Hanzal, R. and Howard, J. (1956a) Repeated
Oral Administration (Dogs) Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. and Howard, J.
(1956b) Subacute Feeding Study - Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Van Middlelem, C.H. and Baranowski, R.M. (1962) Phorate residues in
tomato fruit and foliage. J. Econ. Entom. 55 (5); 600-603.
Waller, J.B. and Dahm, P.A. Oxidation of phorate in three Iowa soils -
Manuscript from (1973) Iowa State University, Ames, Iowa USA.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for amny organophosphorus pesticide
residues in crop extracts. J. Assoc. Off. Analyt. Chem., 52: 522.
Watts, R.R. and Storherr, R.W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Off. Analyt. Chem. 52, 513-521.
Wit, S.L. (1959) Residu's van systemische middelen bij aardappelen,
etc. - Rijksinstituut voor de Volksgezondheidg Utrecht, FT/6/59 dated
25.4.59.
Wit, S.L. (1961) Residu's van enige systemische insecticiden bij
aardappelen - Rijksinstituut voor de Volksgezondheid, Utrecht,
FT/52/61 dated 29.5.61.
Wit, S.L. (1962a) Onderzoek naar de residu's van enige systemische
middelen bij aardappelen- Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 29/62 dated 29.2.62.
Wit, S.L. (1962b) Onderzoek naar de residu's van Thimet toegepast als
systemisch zaardinsecticide ter bestryding van de vergelingszickte bij
sieten - Rijksinstituut voor de Volksgezondheid, Utreohty Report Tox.
10/62 dated 30.1.62.
Wit, S.L. (1963) Onderzoek naar de residu's van twee systemische
insecticiden bij aardappelen - Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 113/63 dated 25.6.63.
Wit, S.L. (1969) Foraat op zomerwortelen - Rijksinstituut voor de
Volksgezondheid, Utrecht, Report Tox. 59/69 dated May 1969.
Zakil M.M. and El-Sayed, M.M. (1968) Accumulation of systemic
insecticides in mature cotton seeds. Bull. Ent. Soc. Egypt. Econ. Ser.
II 121-127.
chloroform and acetone (Bowtan and Casida, 1958) (See also "Fate of
residues", "In animals").
Bean plants were dipped into a 500 ppm P-32 phorate solution prior to
planting to determine the metabolism of phorate by plants. The primary
metabolites were phorate sulphoxide and/or phorate sulphone. Small
amounts of phoratoxon sulphoxide, phoratoxon sulphone and unchanged
phorate were also detected. No phoratoxon was detected. The hydrolysis
products formed were phosphoric acid and the diethyl esters of
phosphoric acid, phosphorothioic acid and phosphorodithioic acid
(Bowman and Casida, 1958).
Other work on the biotransformation of phorate in plants is described
in "Fate of residues", "In plants".
Effects on enzymes and other biochemical parameters
The anticholinesterase activities of phorate and its metabolites are
shown below (Table 1).
TABLE 1. Comparison of anticholinesterase activity of phorate
metabolites
Anticholinesterase activity
(p/50)*
Phorate 3.17
Phorate sulphoxide 3.35
Phorate sulphone 5.00
Phoratoxon 5.87
Phoratoxon sulphoxide 6.76
Phoratoxon sulphone 7.02
*p/50 + negative logarithm of molar concentration producing 50%
inhibition of red blood cell cholinesterase.
In rats, at dietary levels above 0.66 ppm, phorate depressed plasma,
erythrocyte and brain enzyme activity (Tusing et al., 1956b). Phorate
sulphoxide at dietary levels greater than 0.32 ppm also produced
depression of cholinesterase in rats in varying degrees during a 90
day studY (Levinskas et al., 1968). In dogs, plasma and erythrocyte
cholinesterase activity was depressed at levels above 0.01 mg/kg/day
(Tusing et al., 1956a). Dietary feeding at 1.0 ppm for 6 weeks
produced no effect in dogs (Kay and Calandra, 1961).
TOXICOLOGICAL STUDIES
Special studies on potentiation
Young rats weighing between 90 and 120 g (5 males/4 dosage levels)
were used to investigate the potentiation of phorate with diazinon,
EPN, azinphos-methyl, malathion, parathion-methyl, parathion,
mevinphos, demeton, tetram and carbophenothion. Oral LD50 values
were determined for each pesticide under identical conditions. Each
pair of compounds was mixed in approximately equitoxic proportions,
i.e., in about the ratios of their LD50's.
Results, given as LD50 in mg/kg are as follows:
Diazinon (283,216); EPN (42.9, 25); azinphos-methyl (18.7, 8);
malathion (329, 178); parathion-methyl (14.2, 11); parathion (12.3,
8); mevinphos (7,4); demeton (6.2,4); tetram (14.2, 5);
carbophenothion (32.5, 29). In the case of every mixture, the observed
LD50 indicating no potentiation (Shaffer et al., 1958).
Special study on neurotoxicity
Groups of 6 adult hens were fed 0 and 40 ppm phorate in the diet for 4
weeks. A third group received 4000 ppm TOCP as a positive control.
Each hen was anesthetized, immediately perfused with buffered
formalin, and sections of brain, lower thoracic cord and each sciatic
nerve prepared for microscipis examination. The TOCP produced myelin
loss in each hen. Phorate produced no adverse effects on nerve fibres
or the myelin sheath (Levinskas et al., 1965).
Special study on mutagenicity
Ten pesticides, including phorate, were tested for dominant lethal
effects. A group of 20 male mice were fed for 7 weeks at three dose
levels of phorate (levels not reported). Reference controls and a
positive control receiving triethylenemelamine in drinking water for 4
weeks were included in the test. Following treatment each male was
mated to two females weekly for 8 weeks. No consistent response
occurred to suggest that phorate in the diet for 7 weeks induced
dominant lethal effects in mice (Jorgenson et al., 1976).
Special study on reproduction and teratology
Groups of mice (8 males and 16 females/group) were fed phorate in the
diet at levels of 0, 0.6, 1.5 and 3.0 ppm during a 3-generation, 2-
litter/generation, reproduction study.
There were no dose related effects on the fertility, gestation,
viability or lactation indices during the study, but there was a
lowering of the lactation index in the 3.0 ppm group. This value fell
below the control value in the first mating of the F0 generation, in
both matings of the F1 and in the second mating of the F2
generation.
Gross and microscopic examination of tissues and cleared pups revealed
no consistent abnormalities related to feeding of phorate (Anonymous,
1965).
Acute Toxicity
TABLE 2. Acute toxicity of phorate
LD50
Species Route, Sex (mg/kg) Reference
Rat Oral M 2.3 Gaines (1969)
F 1.1
Rat Oral M 2.8 Anonymous (1976)
F 1.6
Rat Dermal M 6.2 Gaines (1969)
F 2.5
Rat Dermal M 5.7 Anonymous (1976)
Rabbit Dermal M 5.2
Short term studies
Rat
Groups of rats (50 males and 50 females/group) were fed 92% phorate,
in corn oil, in the diet at levels of 0, 0.22, 0.66, 2.0 and 6.0 ppm
for 13 weeks. Two groups (25 males and 25 females/group) were fed 12
and 18 ppm for 8 and 2 weeks respectively. Cholinesterase activity was
monitored at weekly intervals. Occasional episodes of excitability and
intermittent tremors were noted in the 6.0 ppm female group. Both
sexes receiving the 12 and 18 ppm levels exhibited severe
excitability, intermittent tremors and ataxia culminating in death.
Levels of 6 ppm and higher produced significant depression in both
sexes of plasma, red blood cell and brain activity. At 2 ppm female
red blood cell activity was also depressed. Levels at 0.66 ppm or
below had no effect on cholinesterase activity. Growth, food
consumption, survival, liver and kidney weights and ratios, gross
necropsy and histological findings for all groups at 6 ppm or below
were within normal limits. Levels of 12 and 18 ppm severely affected
growth and survival (Tusing et al., 1956b).
Groups of rats (50 males and 50 females/control and 35 males and 35
females/test groups) were fed the sulphoxide of phorate (92% purity)
for 90 days at levels of 0, 0.32, 0.80 and 2.0 ppm. Brain, erythrocyte
and plasma cholinesterase activities were determined biweekly. At the
2.0 ppm level a significant (P <0.05) depression in erythrocyte and
brain cholinesterase activity occurred in females. Plasma activity
depression at this level was less consistent and judged as borderline.
The 0.8 ppm level produced borderline depression of the erythrocyte
enzyme activity. At 0.32 ppm all values were within acceptable
statistical limits for both sexes. No effects were observed on other
hematological values, organ weights or ratios and no consistent
microscopic pathology was noted which could be attributed to the
feeding of 2 ppm or less of the sulphoxide of phorate (Levinskas et
al., 1968).
Dog
Groups of mongrel dogs (2 males and 1 female/group) received by
capsule 0, 0.01, 0.05, 0.25 and 1.25 mg/kg/day of 92% phorate in corn
oil 6 days/week for 15 weeks. Two males received 2.5 mg/kg as a single
dose. Plasma and red blood cell cholinesterase activities were
monitored at weekly intervals. The 0.05 mg/kg/day level produced a
significant depression in plasma enzyme activity. The red blood cell
activity was not affected for the first 12 weeks but was depressed
slightly (not significantly) during the last 3 weeks of the study.
Significant plasma and red blood cell enzyme depression was produced
by the 0.25 mg/kg/day level. The 1.25 mg/kg/day level produced
complete inhibition of plasma cholinesterase activity and a
significant reduction in red blood cell activity. At the 2.5 mg/kg/day
level all dogs died within 3-4 hours following a single dose and no
enzyme activity determinations were made. No signs of systemic
toxicity in dogs receiving the 0.01 or 0.05 mg/kg/day doses (Gaines,
1969). Histopathological examination revealed no consistent findings
related to the test/material (Tusing et al., 1956a). Beagle dogs (3
males and 3 females/group) were fed in the diet 0, 0.5, or 1.0 ppm
phorate for 6 weeks. Cholinesterase activity of plasma and
erythrocytes was determined for each dog prior to and at biweekly
intervals during the test. Analyses of variance conducted upon plasma
and erythrocyte enzyme activities revealed no significant difference
between the test animal and control animal groups during the test
period (Kay and Calandra, 1961).
Long term studies
No information available.
Observation in humans
No information available.
COMMENTS
Phorate, an organophosphorous pesticide, is a derivative of
dithiophosphoric acid. Less than 40% of a 2 mg/kg oral dose to rats
was excreted in 6 days, and brain, liver and kidney contained
unextractable residues. The major metabolites in both plants and
mammals are the sulphoxides and sulphones of phorate and of its oxygen
analogue, phora-toxon; these have greater anticholinesterase activity
than phorate. Hydrolysis of phorate itself is a minor metabolic
pathway in mammals.
The biochemical data available on phorate are inadequate to determine
whether the oxidative metabolites are retained in mammalian tissues or
organs. Toxicological data submitted are insufficient, except for
cholinesterase inhibition, to determine the short and long term
effects of phorate and its oxidative metabolites. Although the Meeting
considered setting a temporary ADI for humans this could not be
established in the absence of long-term studies. Further studies are
required to evaluate a.) carcinogenic potential, b) teratogenic
potential, c) potential neurotoxicity and d) toxicity of metabolites.
Observations in human subjects are desirable.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phorate is a powerful soil insecticide with excellent systemic
properties. A wide spectrum of insect and free-living nematode pest
problems is controlled by a seed-bed or a side-dressing treatment.
Seed-bed applications protect the germinating seedlings and young crop
stages against a wide range of pests.
Granular formulations of phorate have been extensively field tested
throughout the world since 1954 and are used commercially in many
countries. Major uses are summarized in Table 3. Recommended dosage
rates vary somewhat from country to country, depending mainly on the
pest complex to be controlled and the period of protection required.
Although application is normally made in the furrow at seeding or
planting time, certain pest problems require the application of
granules as a side-dressing at the time of subsequent cultivation.
There are some situations where, in order to control vectors of virus
diseases, it is necessary to make a broadcast treatment with granules
or a foliage application of emulsifiable concentrate to the growing
crop. Preharvest intervals and limitations on grazing vary somewhat
but for the most part they are relatively long because of the residual
life of phorate in treated plants.
In addition to its use on food and forage crops phorate is used on
ornamentals and tobacco and in forest nurseries where sucking insects
are a problem.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive trials have been carried out by the manufacturers and by
independent investigators to determine the level and fate of phorate
residues in virtually every crop on which the insecticide in used.
Copies of trial reports and published scientific papers were provided
to the Meeting by the manufacturers (American Cyanamid, 1977). A
representative selection of the data from these reports has been
summarized in Table 4. The following observations are made on the
detailed data.
TABLE 3. Phorate: use-pattern
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Alfalfa 1 seed treatment 60 days
1.5-2 broadcast 35 days
(grazing)
Barley 1 broadcast-soil incorporated 60 days
Beans 1-1.5 in furrow at planting 60 days
broadcast before flowering -
Brassicas 16g/100m row in furrow at transplanting -
Carrots 1.7-3.3 at drilling time -
TABLE 3. (Continued)
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Cotton 0.2-0.5 seed treatment do not use
seed for food
0.3-1.5 furrow application do not graze
Hops 1-1.5 foliage application 25 days
2-3 band application 42 days
Lettuce 1 at transplanting or
seeding -
1 foliage treatment 18 days
Maize 1-2 in farrow at planting -
1 band application
at cultivation 30 days
1 foliage application 30 days
Onions 12g/100m row in furrow at transplanting -
Peanuts 1-1.5 in furrow at planting do not graze
or feed
2 band application at pegging do not graze
or feed
Peppers 1 in furrow at transplanting -
Potatoes 1.5-3 in furrow at planting 90 days
Sorghum 2 In furrow at planting -
0.5-1 foliage application 28 days
Sugar beets 0.5-1 in furrow at planting -
1-1.5 foliage application 30 days
Sugar cane 4 in furrow at planting -
Tomatoes 1-2 at planting or
transplanting -
Wheat 1 in furrow
1 broadcast 70 days
Sunflower 1-1.5 in furrow at planting -
TABLE 4. Phorate residues resulting from supervised trials.
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
ALFALFA USA 1966 1 1 granule <0.01 <0.01 <0.01 <0.01
(dry) 1 2 10% <0.01 <0.01 <0.01 <0.01
1 4 " 0.06 0.06 0.03 0.02
1 1 " 0.06 0.08 0.71 0.37 0.21
1 2 " 0.01 0.01 0.35 0.46 0.07
1 1 " 0.05 <0.01 0.01 0.01 0.01
1 2 " 0.15 0.02 0.02 0.02 0.01
APPLES Italy 1970 2 1.5 E.C. <0.02 <0.02 <0.02 <0.02
Argentina 1972 1 2 granule 0.01
1 3 " 0.01
BARLEY USA 1967 1 4 " <0.01
(grain) 1 1 " 0.01
BARLEY USA 1967 1 2 " <0.01
(straw) 1 1 "
1 2 0.04
BARLEY USA 1961 1 1 " 0.07 <0.04
(grain)
BEAN, SNAP USA 1966 1 1 " 0.5 0.07 <0.01
USA 1963 1 2 " 0.046 0.016
1 2 " 0.018 0.011
1 4 0.013 0.036
USA 1966 1 1 " 0.5 0.07 <0.01
1 1 <0.01
1 2 " <0.01
COWPEA India 1974 1 2 " 1.46 1.05 0.53
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
EGGPLANT India 1974 1 2 " 1.27 1.01 0.69
MAIZE USA 1970 1 0.5 E.C. 0.068 0.024 <0.020
(plant) 1 " 0.131 0.046 0.007
2 " 0.695 0.110 0.019
S. Africa 1977 1 0.5 granule 0.4 0.1 <0.02 <0.02
1 " 2.8 0.9 0.03 0.03
0.5 " <0.02 <0.02 <0.02
1.0 " 0.06 <0.02 <0.02
0.5 " 0.5 <0.02 <0.02
S. Africa 1977 1.0 granule 0.8 0.03 <0.02
MAIZE S. Africa 1977 1 0.5 " <0.02
(grain) 1.0 " <0.02
Italy 1971 2.5 " <0.02
14 30 40 60 90 120 150
CARROTS UK 1970 2 3 granule 1.01 0.88 0.47 0.64
3 3 " 0.29 0.40 0.39 0.27
2 3 " 0.41 0.41 0.24 0.19
3 1.5 " 0.19 0.09 0.09 0.23
Canada 1975 3 1 E.C. 0.26
4 1 E.C. 0.40
CELERY UK 1971 1 1.2 granule <0.05
1.5 " <0.05
COTTONSEED USA 1969 1 1 E.C.(seed) <0.05
1 1-2 granule <0.05
1 3 " <0-05
1 2 E.C. <0-05
Egypt 1968 5 1 G/E.C. 0.02 0.01
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
GRAPES Mexico 1973 1 4 0.000
1 6 0.000
1 8 0.007
HOPS USA 1968 1 4 granule <0.1 <0.1 <0.1
3 1 E.C. 0.1
LETTUCE USA 1977 1 2 5.14 0.24 0.17 <0.05
1963 1 1 <0-05
1963 1 2 <0.05
1963 2 0.025
PEANUTS USA 1964 2 1+2 granule <0.05 <0-05
(kernels) 2 1+2 " <0.05 <0-05
2 1+2 0.05 0.05
2 1+4 <0.05
1 2 " 0.1 0.08 0.05
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
PEANUT USA 1964 2 1+2 granule 0.05
(foliage)
POTATOES Czechoslovakia 1972 1 1.6 " <0.05
1 2.2 " <0.05
1 4.3 " 0.07
Turkey 1973 1 3.4 <0.05
Mexico 1972 1 1 E.C. 0.004 0.000
1 2 E.C. 0.006 0.000
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
RAPE SEED USA 1971 1 1.5 granule 0.05
RAPE HAY USA 1971 1 1.5 " 0.21
SORGHUM USA 1969 1 1 " <0.05 <0.05
(grain) 1 2 " <0.05 <0.05
SORGHUM USA 1969 1 1 " 0.06 0.06
(fodder) 1 2 " 0.16 0.08
SORGHUM Mexico 1972 1 1 E.C. 0.00 0.00
(grain) 1 2 E.C. 0.02 0.00
MILO USA 1967 1 1 granule <0.01 <0.01 <0.01
2 " <0.01 <0.01 <0.01
4 " 0.22 0.01 <0.01
SOYBEAN USA 1970 1 1 " <0.05 <0-05
2 " <0.05 <0.05
7.5 " <0.05 <0.05
SOYBEAN USA 1970 1 1 " <0.05 <0.05
(foliage) 2 " <0.05 <0.05
7.5 " 0.15 0.19
SUGAR BEET USA 1961 1 1 E.C. 0.05
2 E.C. 0.05
TOMATOES USA 1961 1 1 <0.05 <0.05
2 0.05 0.05
4 0.06
WHEAT USA 1969 1 1 <0.05
(grain) 1 2 <0.05
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
WHEAT USA 1969 1 1 1.4 0.35 0.33
(plant) 1 2 2.7 1.6 3.0
1 1 6.1 0.87 0.07
Alfalfa
Residue results obtained on fresh alfalfa and alfalfa hay grown in
various regions of the United States show that application at twice
the recommended dosage rate of 2 kg/ha does not result in residues in
excess of 0.5 mg/kg in or on fresh alfalfa or 1 mg/kg on alfalfa hay
within 35 days of treatment. Generally the level is less than the
limit of determination (0.01 mg/kg).
The results from a series of trials where phorate was applied to
alfalfa at rates ranging from 2 through 3 to 4 kg/ha indicate that the
half life in green alfalfa is between 8 and 18 days.
Apples
Results from spray trials carried out in Italy where 7.59 of phorate
was applied per tree on one, two and three occasions prior to harvest
show that the residues in apples 30 to 80 days after the last
application were generally less than 0.02 mg/kg of Phorate and
metabolites. One sample from a tree treated 30 days previously showed
0.06 mg/kg phorate sulphone only.
Residue analysis for phorate and metabolites in several varieties of
apples treated in Argentina with phorate granules applied in
incorporation in the soil around the stem at doses ranging from 10-20g
of phorate per tree indicated no residues of parent compound or
metabolite in fruit collected 182 days after application.
Barley
Experiments in the USA where phorate granules were incorporated or
irrigated into the soil at planting time reveal that the residues of
phorate and metabolites in the grain at harvest 60 days later are
generally at or below the limit of determination (0.01 mg/kg). When
applied at twice the recommended rate (1 kg/ha) detectable residues
(0.04 mg/kg) were found in barley straw 60 days later.
Stern (1961) reported that phorate granules applied to barley fields
at the rate of 1 kg/ha one day prior to irrigation, produced
detectable residues in the barley plants (0.07 mg/kg) 14 days later.
The residues had declined below the limit of determination (0.04
mg/kg) by the 28th day following application.
Beans
Extensive field trials carried out during the period 1966-68 on many
varieties of beans under widely differing agricultural and ecological
conditions indicate that the residues are generally well below 0/05
mg\kg 60 days after application. The samples were analysed by the
oxidative cholinesterase inhibition technique which determines phorate
and metabolites. The limit of determination based on control values is
considered to be 0.05 mg/kg. The reports contain results from a few
isolated samples which indicate a total phorate residue of the order
of 0.1 mg/kg.
Carrots
Wit (1960) using analytical methods involving the determination of
total phosphorus showed that, following the application of phorate
granules at the rate of 1.25-1.5 kg/ha, carrots contained from 0.09-
0.19 mg/kg of residues derived from phorate applied 73-132 days
previously.
An extensive experiment carried out in the United Kingdom (Cyanamid
Great Britain Limited, 1971) determined residues of phorate and its
metabolites in carrots following treatment with phorate granules
applied with the seed and at varying times thereafter according to
various treatment codes. The highest residue level was approximately
1mg/kg some 5 weeks after the last treatment, but this declined to
0.64 mg/kg by the end of 4 months. Lower rates produced somewhat lower
residues but significant residues were detected from all treatment
regimes. The residues consisted of phorate and phorate sulphone with
no oxygen analogue or sulphoxide.
Finlayson et al (1976a) studied the distribution of a number of
insecticides used to control carrot rust fly in the peel and pulp of
carrots, measuring also the distribution throughout the length of the
carrot. These investigators found that the bulk of the residue was in
the peel in the top 3cm of the carrot, the total residue in the whole
carrot being of the order of 0.2-0.4 mg/kg. The peel at the top end
contained as much as 7 mg/kg. See also "Fate of residues".
Maskell et al (1974) showed that the application of granules
containing 10% phorate at 1, 2 or 4 kg/ha in a narrow slit together
with carrot seed gave rise to residues which could be reduced by 50%
by placing the granules 2.5cm below the seed. Residues resulting from
the application of 1 kg/ha with the seed or 2 kg/ha 2.5cm below the
seed were not significantly greater than residues in untreated
carrots.
When 4 kg of phorate per ha was applied along with carrot seed the
residue in carrots at harvest 8 months later was 0.4 mg/kg. When the
granules were placed 2.5cm below the seed the residue under
corresponding conditions was only 0.2 mg/kg.
Cottonseed
American Cyanamid Company (1969c) reported the results of 13 trials in
different regions of the USA aimed at determining what residues, if
any, occur in cottonseed following phorate treatment of seed pre-
planting or emulsifiable concentrate as a late season foliar
application. All samples were analysed by the gas-chromatographic
method. The data show less than 0.05 mg/kg phorate in cottonseed
harvested from crops treated at planting time or with a side-dressing.
Late season application with phorate emulsifiable concentrate at rates
up to 2 kg/ha did not show detectable residues in cottonseed from
crops treated 30 days prior to harvest.
Zaki and El-Sayed (1968) showed that residues in mature cottonseed
after spraying mature plants three times with phorate at the rate of 1
kg/ha were only 0.013 mg/kg 14 days after the fourth application of
foliage sprays.
Cowpeas
Satpathy et al (1974) showed that highly significant residues of
phorate could be found in the pods of cowpeas 10 and 20 days after
treatment of the soil with phorate granules applied at the rate of 2
kg/ha. The residues continued to decline with a half life of
approximately 12 days. Phorate residues were relatively more
persistent than those of diazinon and dimethoate but comparable to
disulfoton in cowpeas.
Grapes
The residue analysis of grapes harvested from plants growing in soil
treated with phorate granules at the rate of 4, 6 and 8 kg/ha showed
no residues at the two lower rates and only barely detectable residues
at the highest rate. Treatment had been made by incorporating the
granules into the soil at the time when the grape vines were
sprouting. Grapes were harvested 92 days later (Cyanamid
International, 1973b).
Hops
Kiligemagi and Terriere (1968 a,b) reported an extensive series of
trials designed to determine the level and fate of phorate residues in
hops following the application of phorate granular to the hop beds and
foliar applications at rates from 1 to 5 kg/ha and with pre-harvest
intervals ranging from 15 to 89 days. Using a gas-chromatographic
method specific for phorate and its oxygen analogue sulphone the
investigators were unable to detect, either the parent compound or
metabolites in fresh or dried hops even at the short pre-harvest
interval of 15 days. Aphid and spider mite control were, however,
excellent.
Maize
One of the major uses for phorate ban been the control of a variety of
insects on maize. In the early 1960s the manufacturers carried out,
extensive residue studies in many locations in the USA. Using
oxidative cholinesterase inhibition procedures it was shown that the
insecticide was taken up into the growing plant but that the residues
declined rapidly during the 45 days following application, by which
time residues in the whole plant were not significantly greater than
the apparent phorate residue in untreated control samples. By the time
the grain was ripe, no trace of phorate of other
cholinesterase-inhibiting substances could be found in the grain or
other parts of the plant.
Following the introduction of GLC methods capable of determining
phorate and its metabolites, a number of studies were carried out in
several countries beside the USA. These confirmed that the residue
levels declined to below the limit of determination between 28 and 40
days after the application of phorate granules or spray at rates up to
2 kg/ha. Leuck and Bowman (1970) published the results of one such
study which traced the occurrence and level of phorate and four
metabolites in maize plants treated with 0.5, 1 and 2 kg/ha of phorate
as a spray. These workers showed that the sum of all residues was less
than 1 mg/kg 14 days after the application of all three rates of
phorate.
Peanuts
Investigation in the USA (Diablo Laboritories 1964) show that the
application of phorate granules at planting and subsequent application
at picking does not result in apparent phorate residues different from
these found in untreated controls. The analyses were made using the
oxidative cholinesterase inhibition technique which produces a
positive result in untreated controls of the order of 0.05-0.1 mg/kg
apparent phorate. Samples of kernels from plants treated at the
highest rate did not show any residues higher then the controls when
collected 90 days after the last treatment. Where treatments were
applied 30 to 40 days prior to harvest, a positive residue of the
order of 0.1 mg/kg was found in peanut kernels. The residues in peanut
foliage after 90 days were not significantly different from untreated
controls.
Potatoes
Residue date developed prior to 1960 in support of petitions for the
registration of the use of phorate on potatoes were obtained with a
non-oxidative anti-cholinesterase method of analysis. This procedure
has good sensitivity for the oxygen analogue sulphoxide and sulphone
and, with the long pre-harvest interval involved, was considered
adequate at the time. Extensive residue information on potatoes was
obtained with this method, including results from inordinately high
rates of application, with all findings showing no measurable residues
in mature potatoes at harvest.
Owing to their relatively poor cholinesterase inhibiting properties,
phorate and its intermediary oxidative derivatives such as phorate
sulphoxide or sulphone would not have been measured by the
non-oxidative method had they been present. In 1959, an oxidation step
was introduced into the anti-cholinesterase procedure, which converted
phorate and its partially oxidised metabolites to phorate oxygen
analogue sulphone, and thus measured all toxic phorate residues. At
this stage a method based on the determination of total phosphorus
(Steller and Curry 1964) and including an oxidation step (Blinn 196A)
was utilized for further studies. By this method, which had a limit of
determination of the order of 0.01 mg/kg, it was shown that potatoes
treated at planting with phorate at the maximum recommended rate of 3
kg/ha generally contained 0.05 mg/kg or less when harvested 90 to 120
days later. However, residues as high as 0.15 mg/kg were occasionally
found following the use of phorate at the rate of 3 kg/ha. Following
the use of twice this amount (6 kg/ha) residues ranging up to 0.5 were
reported 100 to 140 days after application (American Cyanamid Company
1966).
In the Netherlands between 1958 and 1960, Wit (1959, 1961)
investigated the residues of systemic insecticides on potatoes, using
analytical methods involving the determination of total phosphorus
(limit of determination 0.1 mg/kg). He found substantial residues of
phorate in potatoes from 3 days to 42 days after application of
phorate granules (1.5-3 kg/ha). These residues ranged up to 3 mg/kg,
with the bulk of the results near 1 mg/kg.
Later Wit (1962a., 1963) using methods that determined phorate and the
sulphoxide metabolite (Laws 1961) showed that residues were less than
0.05 mg/kg 75-110 days after applying phorate granules at 2 + 3 kg/ha.
In 1972 following the development of a highly specific and
highly-sensitive analytical method employing gas-liquid chromatography
for the determination of phorate and phorate metabolites, an extensive
trial was carried out in Czechoslovakia (Cyanamid International
1972a). Residues of phorate or its oxygen analogue sulphone were not
measurable in any treatment. The only detectable residue in the
treated samples was of phorate sulphone, the highest level found being
0.07 mg/kg following the application of 3.4 kg/ha 120 days previously.
A similar field trial was carried out in Turkey (Cyanamid
International, 1973a), where phorate granules were applied at the rate
of 3.4 kg/ha at the time of planting and the potatoes harvested 125
days later. None of the potatoes contained residues of phorate or any
metabolite at levels above the limit of determination (0,01 and
0.02 mg/kg). It was concluded that the total residue of phorate and
metabolites was less than 0.05 mg/kg.
The application of phorate spray to potato Plants at rates to 1 and 2
kg/ha in Mexico (Cyanamid International, 1972b) produced detectable
residues in the tubers which declined from 0.02 mg/kg on the day
following application through 0.002 mg/kg on day 20 to 0 on day 27.
These results indicate that only small amounts of phorate are
translocated from the leaves to the tubers, and that these residues
decompose rapidly.
Sorghum and milo
Extensive trials were carried out in two different regions of the USA
to determine residues of phorate and its metabolites in sorghum fodder
and grain following granular and spray treatments. Samples of fodder
and grain were collected at intervals from 28 to 46 days after
application. The samples were analysed by gas-liquid chromatographic
procedures with a sensitivity of 0.05 mg/kg. All residues were below
0.05 mg/kg except fodder from one location which had been treated with
granular formulations and sampled after 31 and 46 days (American
Cyanamid Company, 1969a).
In an experiment carried out in Mexico, phorate spray was applied to
sorghum at the rate of 1 and 2 kg/ha 28, 21, 14, 7 and 0 days before
harvest, the residues found on the grain immediately after application
on the day of harvest were 0.55 and 0.82 mg/kg respectively. Though of
the order of 0.1 and 0.2 mg/kg could be found on the grain treated 7
days before harvest, that treated 14, 21 or 28 days prior to harvest
contained none as determined by a method sensitive to 0.01 mg/kg.
Soybeans
Studies carried out in the USA (American Cyanamid Company, 1970)
demonstrate that phorate granules used at the rate of 1 and 2 kg/ha
and applied in the furrow or to the side of the seed at planting will
result in no detectable residues (less than 0.05 mg/kg) of phorate
plus its toxic metabolites in soybeans seed at harvest. These same
studies showed no residue in soybeans treated at 7.5 kg/ha at two
separate locations and it is therefore reasonable to assume that no
detectable residues will occur in either soybeans oil or meal
following the use of phorate at the rate of 1-2 kg/ha.
Sugar beet
In 1960 and 1961 the manufacturers carried out or sponsored many field
trials to determine the level and fate of phorate residues in sugar
beet roots and leaves. Neither phorate nor its metabolites were
detected in any samples of sugar beet roots or tops in any trial. The
use of phorate sprays on sugar beet is approved in several countries
and maximum residue limits have been established to permit the feeding
of beet foliage to livestock.
Wit (1962b) reported that sugar beet roots and sugar beet leaves
contained less than 0.1 mg/kg or phorate-derived residues
(phorate + sulphoxides and sulphones) when phorate granules were
applied at the rate of 2-3 kg/ha 120-140 days before harvest.
Tomatoes
Extensive experiments carried out on tomatoes grown in various regions
of the United States during the period 1958-1961 show that following
the recommended application of phorate granules at the rate of 3 kg/ha
at planting time, no significant residues of either phorate or its
oxidative metabolites are found in either mature green or pink ripe
tomatoes. Similar results were also found at the 5 kg/ha. rate. Some
residue is found in the tomato foliage during the first few weeks
following application.
Wheat
Several studies were conducted in 1969 (American Cyanamid Company,
1969b) in the wheat growing areas of the United States to determine
phorate residues in green plants and grain following spring and fall
planting-time applications of phorate granules at rates of 1 and
2 kg/ha. The samples were analysed by GLC methods. The data show
phorate residues to be less than 1 mg/kg in all plant samples
approximately 45 days following application of phorate granules at a
rate of 1 kg/ha. No detectable residues (less than 0.05 mg/kg) were
found in grain harvested 120 to 330 days after treatment.
FATE OF RESIDUES
In animals
32P-phorate was given to a cow by Bowman and Casida (1958) in a
toxic dose of 3.04 mg/kg body weight, the acute symptoms that appeared
being controlled with atropine injections. The blood level increased
within 6-8 hours. After 18-36 hours the content of chloroform-soluble
metabolites in the blood increased slightly. The cow excreted 59% of
the radioactive material in the urine within 72 hours. None of this
was chloroform extractable. The main urinary metabolite was initially
0,0-diethyl phosphorothioic acid and subsequently diethyl phosphoric
acid, excretion of. 0,0-diethyl phosphorodithioic acid was
consistently low over the entire period. Only 0.8% of the applied
radioactivity appeared in the faeces within 96 hours after application
and 10% after 12 hours. The chloroform-soluble products were phorate
sulphoxide and/or sulphone, together with some phorate.
Examination of the cow's tissues 96 hours after feeding showed that
the liver and kidneys contained the highest 32P concentration (10.3
and 5.2 mg/kg phorate equivalents respectively). In contrast, the loin
muscle tissue contained 0.29 mg/kg and the mesenteric fat 0.03 mg/kg
phorate equivalents. The lung contained 1.5 mg/kg. Appreciable amounts
of phorate-derived phosphorus was found in the rumen and omasum walls,
other glands and rib bones. Milk contained 0.07, 0.25, 0.3 and
0.53 mg/kg phorate equivalents at 8, 24, 32 and 56 hours after dosing,
respectively.
Phorate is attacked only slowly by the rumen fluid (Ahmed et al.,
1958).
Phorate was administered to calves at a rate of 0.1 mg/kg for 14 days
to determine the level of phorate residues in liver, kidney, blood,
muscle, heart and fat tissues. No detectable residues ( < 0.1 mg/kg)
were found. Phorate was administered to lactating dairy cows at rates
of 0.05 and 0.1 mg/kg for 14 days. No detectable residues of phorate,
(< 0.02 ppm ) were found in the milk of cows fed 0.05. The milk
from cows fed 0.1 mg/kg contained from <0.02 to 0.05 mg/kg Phorate
(Anonymous, 1961).
Bunyan and Taylor (1966) found practically no phorate in meat and
organs of pheasants after administration of 6.2 to 21.7 mg/kg body
weight. In other investigations with pheasants and pigeons Bunyan et
al (1969) also found no residues after feeding for 14, 28 and 42-day
periods with 100 ppm of phorate in the diet.
Experiments designed to determine the total phorate residues in
chicken tissues and eggs were carried out by American Cyanamid
(1969d). Laying hens were dosed with rations containing 1, 0.3 and
0.1 ppm total phorate (1: 1, phorate: phorate oxygen analogue
sulphone) for 21 consecutive days. The chickens were sacrificed 2-3
hours after the final dose. Egg samples were collected during the
final day of treatment. The samples of muscle, fat, liver, kidney and
eggs were analysed by gas-chromatographic procedures using a caesium
bromide thermionic detector: phorate and its oxidative metabolites
were oxidised to and measured as phorate oxygen analogue sulphone and
calculated as total phorate. The sensitivity of the method for both
phorate and the oxygen analogue sulphone was 0.05 mg/kg. None of the
samples showed any apparent residues of phorate above this level.
In plants
Since 1954, studies of the metabolism of phorate in plants have been
conducted by investigators at American Cyanamid Company and by
researchers in various parts of the world. It has been shown that when
absorbed by plants, phorate is initially and rapidly oxidised to its
sulphoxide and sulphone which in turn are converted to the sulphoxide
and sulphone of the phorate oxygen analogue (Blinn, 1964; Bowmann and
Casida, 1957, 1958; Galley and Foerster, 1976; Krueger, 1975;
Lichtenstein et al., 1974; Metcalf et al., 1957; Zaki and El-Sayed,
1968).
The sequence of oxidative reactions in the metabolism of phorate
following absorption by plants is similar to that in animals and is
given in Figure 1. The oxygen analogue, although presumably formed as
an intermediate, has not been detected.
Bowman and Casida (1957) showed that when phorate was used as a
systemic insecticide for seed treatment of cotton, the metabolites
within the plant consisted of the sulphoxide and sulphone of the
parent compound and of its oxygen analogue. Cotton seeds treated with
phorate at concentrations as high as 32 kg of phorate per 100 kg of
seed showed less than 0.03 mg/kg of phorate or metabolites in the
seeds maturing an plants grown from such treated seed. The residual
persistence following soil and foliage application was studied with
six vegetable crops using radioactive phorate. Table 5 shows the
persistence of radioactivity in vegetable crops treated with
32P-phorate and is derived from the means of values from beans,
beets, cabbage, carrots, lettuce and peas treated by foliage
application and by soil application. Other experiments by Boman and
Casida (1958) on the fate in beans are described previously
(Biotransformation).
Phorate is usually applied as a soil treatment where its outstanding
systemic properties can be utilized to protect the developing plants
from attack by sucking insects for the first few weeks of their
growth. After phorate is applied to the soil and reaches the root zone
of the plant, the insecticide is absorbed by the roots and is
subsequently translocated to aerial parts of the plant. The rate of
movement of phorate from roots to stems and leaves is accelerated by
increased transpiration, and the greatest accumulation of insecticide
in leaves seems to occur under environmental conditions favourable to
transpiration (Hacskaylo et al., 1961). Van Middlelem and Baranowski
(1962) found that the highest concentrations of phorate in tomato
plants usually coincided with preceding periods of relatively high
field temperatures and ample rainfall. Krueger (1975) showed that
soybean root homogenates oxidised phorate to phorate sulphoxide.
Neither the oxygen analogue nor sulphone was detected.
Finlayson at al. (1976a), investigating methods for controlling carrot
rust fly, showed that phorate spray applied over the young carrot
seedlings was readily taken up and held in discrete portions of the
carrot. More than 50% of the residues were located in the top 0-3cm of
the carrot. Total residues in whole carrots generally increased with
higher rates and numbers of applications. Residues were greater in the
peel than in the pulp. Discarding the top 1-2cm of the carrot and
peeling the rest removes most of the residues. See also "Residues
resulting from supervised trials".
In soils
After phorate is applied to the soil, its eventual distribution and
utilization by plants is influenced by various constituents and
qualities of the soil.
In a study of the fate of phorate in soils, Getzin and Chapman (1960)
used radioactive material. One hour after treating various soil types,
these investigators found that 25, 20, and 10 per cent of the
radioactivity applied had been lost through volatilization from sandy
soil, silt-loam and muck respectively. After one hour, little or no
volatilization occurred. They further reported that phorate, when
applied to soil, is partially oxidised, hydrolyzed and bound to the
soil.
It has been shown that phorate moves readily in soil in the vapour
phase (Etheridge and Burt, 1963) as well as in drainage water (Bardner
at al., 1963).
Lindley (1963) reported from field experiments that on mineral soils
less phorate was required for insect control than on peat soils with a
maximum of 35% organic matter, while only partial control of insects
was obtained on soils with more than 35% organic matter, even when
much higher rates of insecticide were used.
Lichtenstein et al, (1973, 1974) showed that while phorate could be
detected in soil, only metabolites were found in plants including
plant roots. Schulz et al. (1973) showed that phorate moved in both
vertical and horizontal directions after a granular band application
of insecticide in soil. Suett (1975) showed that soil temperature had
relatively less effect upon the fate of phorate than on other
soil-incorporated insecticides.
Waller and Dahm (1973) showed that the conversion of phorate to its
sulphoxide in soils is mainly a non-biological process, whereas the
conversion to sulphone is brought about by micro-organisms.
Talekar at al. (1977) in a long-term experiment investigated the
persistence of a number of insecticides following repeated seasonal
application to soil under sub-tropical conditions. They showed that
phorate degraded more rapidly than all the other materials showing
almost complete loss within four months, although measurable
quantities of phorate sulphoxide and sulphone could be detected for
somewhat longer periods.
TABLE 5. Persistence of 32P-phorate and its metabolites in vegetable cropsa.
Days following application
0.1 1 2 4 8 17 32
Foliage application
Phorate equivalentb, mg/kg
Hexane fraction 6.75 2.84 2.49 1.69 0.90 0.44 0.040
Acetone-water fraction 2.60 2.39 1.66 1.45 0.59 0.067 0.003
Hydrolysis productsc 0.28 0.37 0.62 0.85 1.33 0.43 0.27
Unextracted residued 1.44 1.03 0.81 1.62 1.54 1.00 2.28
AntiChE activity, 50% inhibitione
Plant tissue, g 1.3 0.47 0.59 0.54 1.0 >2.2 >2.2
pl50 metabolites 5.44 6.14 6.29 6.62 6.11 <7.22 <7.42
Soil application
Phorate equivalent, mg/kg
Hexane fraction 0.82 0.34 0.40 0.26 0.15 0.049 0.026
Acetone-water fraction 0.12 0.31 0.56 0.36 0.18 0.047 0.005
Hydrolysis products 0.052 0.12 0.13 0.24 0.25 0.21 0.110
Unextracted residue 0.15 0.16 0.42 1.20 0.46 0.11 0.078
AntiChE activity, 50% inhibition
Plant tissue, g 1.8 0.94 1.3 1.2 1.3 >2.9 >3.0
pl50 metabolites 6.89 7.26 6.66 6.72 7.00 7.15 7.22
a Results are average of figures from beans, beets, cabbage, carrots, lettuce and peas in same field plot.
b Radioactivity of phorate or metabolites appearing in fraction indicated, expressed as phorate (mg/kg)
c Water-soluble products not extracted into chloroform.
d ssP remaining in solid portion after both water and chloroform removed.
e Results based on chloroform-soluble metabolites. Amount of plant material containing enough
metabolites for 50% inhibition and pl50 (negative logarithm of molar concentration effecting 50% inhibition)
of these metabolites are reported.
In processing and storage
Leuck and Bowman (1970) showed that residues of phorate and five of
its metabolites remained virtually unaltered when treated corn foliage
was converted into silage and ensiled at 35C° for 30 days.
Askew at al. (1968) examined the effect of 30 minutes boiling on
vegetables containing various organophosphorus pesticide residues.
They demonstrated 100% hydrolysis of phorate residues (2 mg/kg) in
both potatoes and cabbages. They indicated that appreciable quantities
of phorate sulphoxide ware formed during the cooking process, but did
not attempt to quantify the transformation.
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Advice from the US Food and Drug Administration indicates that during
1975 three samples of strawberries were found to contain phorate
residues at levels of 0.07, 0.16 and 1.73 mg/kg. There was no
tolerance for residues of phorate in strawberries.
METHODS OF RESIDUE ANALYSIS
Gas chromatography with an electron-capture detector was used by Egan
et al. (1964) to measure phorate in certain plant tissues. Dewey and
Parker (1965) used an electron-capture detector to measure phorate and
some of its metabolites in soil. Bache and Lisk (1966) reported the
use of an emission spectroscopic detector in soil analysis for phorate
and its metabolites but found very poor chromatographic response for
phoratoxon sulphoxide and sulphone. Mitchell et al. (1968) used gas
chromatography to study the effect of ultra-violet irradiation and
permanganate oxidation on phorate. These workers found it possible to
chromatograph phorate, phoratoxon and phoratoxon sulphone on an
Apiezon L column; quantitative recovery figures were not given,
however. Ruzicka, et al. (1967) used a caesium bromide thermionic
detector with an Apiezon column and found that phorate was not
adequately resolved from phoratoxon, although phoratoxon sulphone
could be resolved. The same workers (1968) later used similar
techniques to measure residues of phorate, phorate sulphoxide and
phorate sulphone on apple leaves. Sans (1967) described a
gas-chromatographic procedure for the separation and measurement of
phorate, but not its metabolites, from various other pesticides.
Nelson (1967) developed a procedure for separating and measuring
phorate, but not its metabolites, from several other organo-phosphate
insecticides in residues on fruits and vegetables.
Bowman et al. (1969b) described procedures for the individual
measurement of phorate and its oxidative metabolites in residues on
corn tissue. These workers used an instrument equipped with a flame
photometric detector and a column of 10% DCL-200 silicone on Gas-Chrom
Q. Recoveries at the 0.05 mg/kg level in excess of 96% were reported
for all of the metabolites except phoratoxon (62% recovery). McLeod et
al. (1969) reported a procedure for the separation and measurement of
phorate and its metabolites from monkey liver homogenates, These
workers used a column packed with 5% diethylene glycol succinate on
Chromosorb W and a flame photometric detector. Recoveries averaging
over 90% were found at fortification levels of the order of 200 mg/kg.
Stanley and Morrison (1969) described the use of the flame photometric
detector with both phosphorus-selective and sulfur-selective filters
to aid in the identification of insecticides; among the compounds
tested was phorate.
Watts and Storherr (1969) and Watts at al. (1969) described clean-up
and gas-chromatographic conditions for the separation and measurement
of many insecticides, including phorate and its metabolites, using a
potassium chloride thermionic detector and a column of 10% DC-200 on
Gas-Chrom Q. Recoveries in excess of 95% were reported for kale
extracts fortified with phorate or its metabolites at levels of 0.1 to
0.3 mg/kg.
Getzin and Shanks (1970) used an instrument equipped with a sodium
sulphate thermionic phosphorus detector and a column of 4% QF-1 on
Gas-Chrom Q to measure phorate and its metabolites in soils. Recovery
values at levels of 20 mg/kg in soil ranged from 64% for phoratoxon
sulphoxide to 96% for phorate.
Higham at al. (1972) have described procedures suitable for the
determination of total phorate-related residues in a wide variety of
sample types. In these procedures, all of the phorate and its
oxidative metabolites present in the residues were converted to
phoratoxon sulphone by oxidation with m-chloroperbenzoic acid as
recommended by Blinn (1964). These procedures employed a
phosphorus-selective detector with a column of 5% DC-200 on Gas-Chrom
Q and were validated at levels down to 0.05 mg/kg in several types of
sample.
Boyd (1976) has reviewed the methods of residue analysis for phorate
and has provided details of methods for all substrates likely to be
encountered in residue analysis. Residues of phorate and its
metabolites are extracted from the prepared sample with chloroform,
chloroform/methanol, methylene chloride or acetonitrile (depending
upon the type of sample) and purified by clean-up procedures
appropriate to the particular type of sample being analysed. All of
the phorate-related residues present are converted to phoratoxon
sulphone by oxidation with m-chloroperbenzoic acid. Measurement is
accomplished by gas-liquid chromotgraphy with an instrument equipped
with a selective phosphorus-sensitive detector. These procedures
appear suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Commodity Tolerance, mg/kg
Argentina lettuce, wheat,
groundnut, rice,
maize 0.1
sugar beet (tuber) 0.3
maize fodder 0.5
sugar beet (leaves) 3
Australia cottonseed, vegetables 0.5
Canada beans, corn, lettuce,
potatoes, rutabagas,
turnips negligible
Germany all plant foods 0.05
Netherlands fruit, vegetables
(not potatoes), spices 0
South Africa all food products 0.05
apples, pears 0.1
China pineapple 0.1
USA sugar beet tops 3
wheat (green fodder) 1.5
alfalfa hay, dried sugar
beet pulp 1
corn forage, hops,
potatoes 0.3
peanut vines and hay,
sugar beet roots 0.3
barley grain, barley
straw, beans, corn grain 0.1
sweet corn, lettuce,
peanuts, rice, sorghum
grain 0.1
sorghum fodder, soybeans,
sugar cane, tomatoes 0.1
cottonseed, wheat grain
and wheat straw 0.05
eggs, meat, fat and meat
by-products of cattle,
goats, hogs, horses, poultry
and sheep 0.05
APPRAISAL
Phorate is a soil insecticide with marked systemic properties widely
used throughout the world since 1954. Maize, cotton, cereal grains and
vegetables are treated with phorate usually at the time of planting,
but sometimes by broadcast treatments of granules or a foliage
application to the growing crop. Pre-harvest intervals are relatively
long and residues in harvested commodities are either absent or
generally do not exceed 0.1 mg/kg.
Carrots are capable of taking up and retaining quantities of phorate
but most of the residue is in the peel or in the crown of the carrot
which in generally removed before processing for consumption. The use
of phorate for the protection of potato crops is important. The
residues at the time of harvest are at or below the limit of
determination.
Soil treatments lead to higher residues than do foliage treatments,
largely because the compound is relatively stable in the soil and in
readily taken up by the plant's root system. In animals phorate is
rapidly hydrolysed and converted into the corresponding sulphoxide and
sulphone and their oxons. No significant amounts of phorate or
metabolites are found in milky eggs or tissues of livestock
administered phorate at levels comparable to those likely to occur in
animal feeds.
The metabolism of phorate in plants is largely similar to that
occurring in animals. Phorate metabolites are relatively stable in
storage and processing. At rates recommended in good agricultural
practice, there appears to be no likelihood of carry-over of residues
in soil affecting subsequent crops.
A method of analysis based on the oxidation of all the phorate-related
residues to the oxygen analogue sulphone followed by measurement by
gas-liquid chromatography is sensitive and specific and appears
suitable for regulatory purposes.
Many countries have established maximum residue limits for phorate
residues.
EVALUATION
In the absence of an ADI, the following guideline levels are recorded.
They refer to the sum of the residues of phorate, phorate sulphoxide,
phorate sulphone and the oxygen analogues of these compounds,
determined as the sulphone of the oxygen analogue and expressed as
phorate.
Commodity Guideline levels, Interval on which
mg/kg guideline levels are
based, days
Alfalfa (dry) 1 35
Barley 0.05 60
Beans 0.1 60
Carrots 0.5 120
Celery 0.1 90
Cottonseed 0.05 120
Cowpea 0.1 60
Eggplant 0.1 60
Grapes 0.05 90
Hops (dried) 0.1 25-40
Lettuce 0.2 60
Maize (green) 0.05 30
Peanuts (kernels) 0.05 60
Potatoes 0.05 120
seed 0.1 90
Sorghum 0.05 30
Soybeans 0.05 120
Sugar beet,
fodder beet 0.05 120
Sugar beet
tops 1 35
Tomatoes 0.1 120
Wheat 0.05 120
Eggs, meat, milk 0.05* 120
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI)
can be established and maximum residue limits (MRL) can be
recommended).
1. Long-term carcinogenicity studies.
2. Teratogenicity studies.
3. Studies on potential neurotoxicity.
4. Studies on the toxicity of metabolites.
DESIRABLE
1. Observation in humans.
REFERENCES
Ahmed, M.K., Casida, J.E., and Nichols, R.E. (1958) Bovine metabolism
of organophosphorus insecticides: significance of rumen fluid with
particular reference to parathion J. Agr. Food Chem. 6, 740.
American Cyanamid Company (1966) Thimet residues in potatoes. Report
No. G102, January 13, 1966.
American Cyanamid Company (1966a) Total Thimet residues in sorghum
fodder and grain - Report No.C188, May 14, 1969.
American Cyanamid Company (1966b) Thimet residues in wheat - Report
No. C215, December 9, 1969.
American Cyanamid Company (1966c) Thimet residues in Cottonseed ;
Report No. C214, December 9, 1969.
American Cyanamid Company (1969d) Total Thimet residues in chicken
tissues Report No. C205 dated November 7, 1970.
American Cyanamid Company (1977) Residue dossier on THIMET
phorate - Vol. I-III.
Anonymous (1965) Report on Thimet Systemic Insecticide: Successive
Generation Studies with Mice. Unpublished report from Central Medical
Department Cyanamid, submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Anonymous (1961) Thimet Phorate Residue Studies in Dairy Animals.
Unpublished report from DIABLO Laboratories, In,., submitted to the
World Health Organization by American Cyanamid Company.
Anonymous (1976) Thimet Soil and Systemic Insecticides Technical
Information published by Cyanamid International Corporation, submitted
to the World Health Organization by American Cyanamid Company.
(Unpublished report)
Askew, J., Mitchell, T.M., Thomson, J. and Wheals, B.B. (1968) The
effect of cooking on organo-phosphorus pesticide residues - J.
Chromat. 32, 417-418.
Bache, C.A., and Lisk, D.J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
chromatography- J. Assoc. Off. Analyt. Chem. 49, 647-650.
Bardner, R., Lord, K.A., and Solly, S.R.B. (1963) Cholinesterase
inhibition method for determining the distribution of organophosphorus
insecticides in soils. Chem. and Ind. 3, 123-124.
Blinn, R.C. (1963) Thin - layer chromatographic isolation and infrared
or colormetric identification of Thimet residues. J. Assoc. Off.
Agric. Chem. 46, (6) 1, 952-96.
Blinn, R.C. (1964) Oxidative conversion of Thimet to its oxygen
analogue sulphone. J. Assoc. Off. Agric. Chem. 47 (4) 641.
Blinn, R.C. and Boyd, J.E. (1967) Thimet R Phorate: Identity of the
Major Phorate Metabolites in the Blood of rats Following oral
Administration of the Insecticide. Unpublished report from the
Research and Development Department of the Agricultural Division of
the American Cyanamid Company, submitted to the World Health
Organization by the American Cyanamid Company.
Bowman M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion disulfoton and phorate in corn, milk grass and
faeces - J. Assoc. Off. Analyt. Chem. 52 (6) 1231-1237.
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969a) GLC determination of
residues of disulfoton, demeton-methyl and their metabolites in
tobacco plants. J. Assos. Off. Analyt. Chem. 52 157-161.
Bowman, M.C., Beroza, M. and Harding, A.J. (1969b) Determination of
Phorate and Five of its Metabolites in Corn. J. Ag. Food Chem. 17
138.
Bowman J.S. and Casida, J.E. (1957) Metabolism of phorate (Thimet) in
plants. J. Ag. Food Chem. 5 (3), 192.
Bowman J.S. and Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants insects and mammals - J. Econ. Entomol. 51 (6)
838-843.
Boyd, J.E. (1976) Thimet (phorate) in Vol VI of Analytical Methods for
Pesticides and Plant Growth Regulators by G. Zweig and G. Sherma
-Academic Press N.Y. p. 493-510.
Brown, M.J. (1975) Improved determination of residues of phorate and
its principal metabolites J. Agric. Food Chem. (2) 334-335.
Bunyan P.J. and Taylor, A. Esterase inhibition of pheasants poisoned
with Thimet. J. Agric. Food Chem. 14, 132.
Bunyan P.J., Jennings, D.M. and Taylor, A. (1969) Organophosphorus
poisoning: Chronic feeding of some common pesticides to pheasants and
pigeons. J. Agric. Food Chem. 17, 1027.
Burns, R.G. (1971) The Loss of phosdrin and phorate insecticides from
a range of soil types - Ball. Env. Contam. and Toxicol. 6 (4)
316-321.
Cyanamid Great Britain Ltd. (1971) Residues of phorate and its
metabolites in carrots. Technical Report No. 106 -16 May 1971.
Cyanamid International (1972a) Residues of phorate and its metabolites
in potatoes - Technical Report No. 179 dated 19 April 1972.
Cyanamid International (1972b) Thimet residue analysis in potato
-Regional Laboratory Report No. 11 dated August 23, 1972.
Cyanamid International (1973a) Thimet and Thimet metabolite residues
in potatoes. Technical Report 240 dated 9 May 1973.
Cyanamid International (1973b) Residue analysis of Thimet and the
oxygen analogue in grapes. Regional Laboratory Report No. 19 September
21, 1973.
Dewey, J.E. and Parker, B.L. (1965) Increase in Toxicity to Drosophila
melanogaster of Phorate, Treated Soils. J. Econ. Entomol. 58, 491.
Diablo Laboratories (1964) Analysis of peanut meats, shells and
foliage for possible residues of total Thimet - Report 4J7511 dated 26
February 1964.
Egan, H., Hammond, E.W, and Thomson, J. (1964) Assoc. Off. Analyt.
Chem. 89, 175.
Etheridge, P. and Burt. P.E. (1963) Vapour phase movement and uptake
of systemic insecticides. Rep. Rothamsted, Exp. Stat. for 1963 Page
138.
Finlayson, D.G., Williams, I.H., Brown, M.J., and Campbell,
C.J. (1976a) Distribution of insecticide residues in carrots at
harvest. J. Aric. Food Chem. 24 (3) 606-608.
Finlayson, D.G., Williams, I.H., Brown, M.J. and Campbell,
E.J. (1976b) Translation and persistence of topically applied diazino
and phorate in beans. Can. J. Plant Sci. 56 947-952.
Galley D.J., and Foerster, L.A. (1976) Distribution and loss of
phorate residues in the foliage of broad bean plants following root
uptake of 14C-labelled phorate. Pestic Sci. 7 301-306.
Gaines T.B. (1969) Acute Toxicity of Pesticides. Toxicol. Appl,
Pharmacol 14, 515-534.
Getzin, L.W. and Chapman R.K. (1960) The fate of phorate in Soils - J.
Econ. Entomol. 53 (1) 47-51.
Getzin, L.W. and Shanks, C.H. (1970) Persistence, Degradation and
Bioactivity of Phorate and its Oxidative Analogues in soil. J. Econ.
Entomol. 63, 52.
Giang, P.A. and Schecter, M.S. (1960) Colorimetric determination of
residues of phorate insecticidally active metabolites. J. Agr. Food
Chem. 8 51-54.
Hacskaylo, J., Lindquist, D.A. and Clark, J.C. (1961) Phorate
accumulation by cotton plants and recovery from soil. J. Econ.
Entomol. 54 (3) 411-414.
Higham, J.W., Peterson, R.P. and Pasarela, N.R. (1972) J. Assoc. Off.
Analyt. Chem. (in press)
Jorgenson, T.A., Rashbrook, C.J. and Newell, G.W. (1976) In vivo
Mutagenesis Investigations of Ten Commercial Pesticides. Abstract No.
41, Fifteenth Annual Meeting, Society of Toxicology, submitted by the
World Health Organization by American Cyanamid Company, Wayne, NJ,
USA.
Kay, J.H. and Calandra, J.C. (1961) Effects of Thimet Phorate on
Cholinesterase Activity in the Dog. Unpublished report from Industrial
Bio-Test Labs., Inc., submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Khajuria, G.N., Jain, H.K. and Dewan, R.S. Residues following the
treatment of sorghum with phorate and dimethoate for the control of
insect pests complex of the crop. Proceedings of the first All-India
Symposium on Progress and Problem in Pesticide - Residue Analysis held
in Ludhiana on 18-19 November 1971 - p. 87-92.
Kiigemagi, O., and Terriere, L.C. (1968a) Residues of phorate in fresh
and dried hops. Report from Oregon State University dated June 10,
1968.
Kiigemagi, O. and Terriere, L.C. (1968b) Residues of phorate and its
metabolites in fresh hops Report from Oregon State University, October
6, 1968.
Krueger, H.R. (1975) Phorate sulfoxidation by plant root extracts.
Pesticide Biochem. & Physiolol. 5, 396-401.
Krueger, H.R., Lindquist, R.K. and Bauerle, W.L. (1973) Phorate and
aldicarb in greenhouse lettuce: Effect of CO2 on residues. J. Econ.
Entomol. 66, 573.
Krueger, H.R. and Mason, J.F. (1973) Phorate and aldicarb: effects of
systemic fungicides on their levels in soyabeans.
Laws (1961) Analyst, 81, 249-255.
Leuck, D.B. and Bowman, M.C. (1970) Residues of phorate and five of
its metabolites. Their presence in Forage Corn and grass - J. Econ,
Entomol. 63 (9) 1838-1842.
Levinskas, G.J., Morici, B.S. and Shaffer, C.B. (1965) Thimet systemic
insecticide: Demyelination studies in white leghorn hens. Unpublished
report from Central Medical Dept. of American Cyanamid Co. submitted
to the World Health Organization by American Cyanamid Co.
Levinskas, G.J., McNerney, J.M. and Fegluy, H.C. (1968) Report on
Sulfoxide of Thimet Systemic Insecticide: Ninety-day Repeated Feeding
to Albino Rats. Unpublished report from Central Medical Department of
American Cyanamid Company, submitted to the World Health Organization
by American Cyanamid Company, Wayne, NJ, USA.
Lichtenstein, E.P., Fuhremann, T.W., Schulz, K.R. and Liang, T.T.,
(1973) Effects of Field application methods on the persistence and
metabolism of phorate in soil, and its translocation into crops. - J.
Econ. Entomol. 66 (4) 863-866.
Lichtenstein, E.P., Fuhremann, T.W. and Schulz, K.R. (1974)
Translocation and metabolism of Phorate as affected by percolating
water in a model soil/plant ecosystem. J. Aric. Food Chem. 22 (6)
991-996.
Lindley, C.D. (1963) The use of phorate granules on potatoes and
carrots Proc. 2nd. Conf. Brit. Insect. & Fangic. Brighton, 1963.
McLeod H.A., Malkins, G. and Rao, S.L.N. (1969) Gas chromatographic
analysis of phorate and five metabolites in monkey liver
homogenates - Bull. Env. Contam. and Toxicol. 4 (4) 224-231.
Maskell, F.E. Gair, R., Williams, J.H., and Cooper, B.J. (1974) Effect
of phorate placement on carrot fly control, seedling density and
terminal residues in the crop. Pestic. Sci. 5 543-548.
Menzer, R.E., Fontanella, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Envir. Contam. & Toxicol. 5 (1) 1-5.
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
Dithio-Systox and Thimet. J. Econ. Entomol. 50 (3) 338-345.
Mitchell, T.H., Razioka, J.H., Thomson, J. and Weals, B.B. (1968) The
chromatographic determination of organophosphorus pesticides. Part
III. The effect of irradiation on the parent compounds. J. Chomat.
32, 17.
Nelson, R.C. (1967) Procedure for nine organic phosphorothioate
pesticide residues on trials and vegetables by using micro-coulometric
gas chromatography. J. Assoc. Off. Analyt Chem. 50: 922-926.
Parker B.L. and Dewey, J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based m toxicity to Drosophilia - J. Econ.
Entomol. 58 (2) 106-111.
Rao, S.L.M. and McKinley, W.J. (1969) Metabolism of Organophosphorous
Insecticides by Liver Homogenates from Different Species. Canadian
Journal of Biochemistry, 47: 1155-1159.
Razicka, J.B., Thomson, J. and Weals, B.B. (1967) J. Chromat. 30,
92.
Razicka, J.B., Thomson, J. and Weals, B.B. (1968) J. Chromat. 33,
430.
Sans, W.W. (1967) Multiple Insecticide Residue Determination Using
Column Chromatography, Chemical Conversion, and Gas Liquid
Chromatography. J. Agr. Food Chem. 15, 192.
Satpathy, J.M., Dash, P.K. and Misra, B. (1974) Insecticidal residues
in some vegetables crops grown in treated soils. Indian J. Agric. Sci,
44 (2) 113-115.
Schulz K.R., Lichtenstein, E.P., Fuhremenn, T.W. and Liang, T.T.
(1973) Movement and Metabolism of phorate under field conditions after
granular band applications J. Econ. Entomol. 66 (4) 873-875.
Shaffer, C.B., West, R. and Golz, H.E. (1958) Thimet: Single Dose
Toxicity to Rats. Joint Toxic Action with Other Phosphates.
Unpublished report from Central Medical Department, American Cyanamid
Company, submitted to the World Health Organization by American
Cyanamid Company, Wayne, NJ, USA.
Singh, K. and Gulati, K.C. (1971) Studies on the persistence of
phorate and di-system in soil and plants. Proceedings of the First
All-India Symposium on Progress and Problems in Pesticide-Residue
Analysis held at Ludhiana on 18-19 November 1971 p. 143-149.
Singh, Kalyan and Gulati, K.C. (1972) Application of enzymatic method
for the estimation of organophosphorus insecticides and their residues
in soil and plant. Part II. Phorate. Indian J. Agr. Chem. 6 103-107.
Stanley, C.W. and Morrison, J.T. (1969) J. Chromatogr. 40, 289.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analogue by total phosphorus determination
after isolation by thin-layer chromatography. J. Assco. Off. Agric.
Chem. 47, (4) 645.
Stern, V.M. (1971) Summary of Thimet residues in barley. Report from
University of California, Riverside (undated).
Suett, D.L. (1971) Persistence and degradation of chlorfenvinphos,
diazinon, forofos and phorate in soils and their uptake by carrots.
Pestic. Sci. 2, 105.
Suett, D.L. (1974) Uptake of chlorphyrifos and phorate from soil by
carrots as influenced by mode of application and cultivar. Pestic.
Sci. 5, 57-71.
Suett, D.L. (1975) Persistence and degradation of chlorfenvinphos,
chlormephos, disylfoton, phorate, and pirimiphos - ethyl following
spring and late-summer soil application. Pestic. Sci. 6, 385-393.
Talekar, N.S., Lian-Tien Sun, Ehi-Min Lee and Jing-Sing Chen. (1977)
Persistence of some insecticides in subtropical soil. J. Agric. Food
Chem. 25 (25) 348-352.
Tusing, T.W., Kindzin, W., Hanzal, R. and Howard, J. (1956a) Repeated
Oral Administration (Dogs) Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. and Howard, J.
(1956b) Subacute Feeding Study - Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Van Middlelem, C.H. and Baranowski, R.M. (1962) Phorate residues in
tomato fruit and foliage. J. Econ. Entom. 55 (5); 600-603.
Waller, J.B. and Dahm, P.A. Oxidation of phorate in three Iowa soils -
Manuscript from (1973) Iowa State University, Ames, Iowa USA.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for amny organophosphorus pesticide
residues in crop extracts. J. Assoc. Off. Analyt. Chem., 52: 522.
Watts, R.R. and Storherr, R.W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Off. Analyt. Chem. 52, 513-521.
Wit, S.L. (1959) Residu's van systemische middelen bij aardappelen,
etc. - Rijksinstituut voor de Volksgezondheidg Utrecht, FT/6/59 dated
25.4.59.
Wit, S.L. (1961) Residu's van enige systemische insecticiden bij
aardappelen - Rijksinstituut voor de Volksgezondheid, Utrecht,
FT/52/61 dated 29.5.61.
Wit, S.L. (1962a) Onderzoek naar de residu's van enige systemische
middelen bij aardappelen- Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 29/62 dated 29.2.62.
Wit, S.L. (1962b) Onderzoek naar de residu's van Thimet toegepast als
systemisch zaardinsecticide ter bestryding van de vergelingszickte bij
sieten - Rijksinstituut voor de Volksgezondheid, Utreohty Report Tox.
10/62 dated 30.1.62.
Wit, S.L. (1963) Onderzoek naar de residu's van twee systemische
insecticiden bij aardappelen - Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 113/63 dated 25.6.63.
Wit, S.L. (1969) Foraat op zomerwortelen - Rijksinstituut voor de
Volksgezondheid, Utrecht, Report Tox. 59/69 dated May 1969.
Zakil M.M. and El-Sayed, M.M. (1968) Accumulation of systemic
insecticides in mature cotton seeds. Bull. Ent. Soc. Egypt. Econ. Ser.
II 121-127.
 chloroform and acetone (Bowtan and Casida, 1958) (See also "Fate of
residues", "In animals").
Bean plants were dipped into a 500 ppm P-32 phorate solution prior to
planting to determine the metabolism of phorate by plants. The primary
metabolites were phorate sulphoxide and/or phorate sulphone. Small
amounts of phoratoxon sulphoxide, phoratoxon sulphone and unchanged
phorate were also detected. No phoratoxon was detected. The hydrolysis
products formed were phosphoric acid and the diethyl esters of
phosphoric acid, phosphorothioic acid and phosphorodithioic acid
(Bowman and Casida, 1958).
Other work on the biotransformation of phorate in plants is described
in "Fate of residues", "In plants".
Effects on enzymes and other biochemical parameters
The anticholinesterase activities of phorate and its metabolites are
shown below (Table 1).
TABLE 1. Comparison of anticholinesterase activity of phorate
metabolites
Anticholinesterase activity
(p/50)*
Phorate 3.17
Phorate sulphoxide 3.35
Phorate sulphone 5.00
Phoratoxon 5.87
Phoratoxon sulphoxide 6.76
Phoratoxon sulphone 7.02
*p/50 + negative logarithm of molar concentration producing 50%
inhibition of red blood cell cholinesterase.
In rats, at dietary levels above 0.66 ppm, phorate depressed plasma,
erythrocyte and brain enzyme activity (Tusing et al., 1956b). Phorate
sulphoxide at dietary levels greater than 0.32 ppm also produced
depression of cholinesterase in rats in varying degrees during a 90
day studY (Levinskas et al., 1968). In dogs, plasma and erythrocyte
cholinesterase activity was depressed at levels above 0.01 mg/kg/day
(Tusing et al., 1956a). Dietary feeding at 1.0 ppm for 6 weeks
produced no effect in dogs (Kay and Calandra, 1961).
TOXICOLOGICAL STUDIES
Special studies on potentiation
Young rats weighing between 90 and 120 g (5 males/4 dosage levels)
were used to investigate the potentiation of phorate with diazinon,
EPN, azinphos-methyl, malathion, parathion-methyl, parathion,
mevinphos, demeton, tetram and carbophenothion. Oral LD50 values
were determined for each pesticide under identical conditions. Each
pair of compounds was mixed in approximately equitoxic proportions,
i.e., in about the ratios of their LD50's.
Results, given as LD50 in mg/kg are as follows:
Diazinon (283,216); EPN (42.9, 25); azinphos-methyl (18.7, 8);
malathion (329, 178); parathion-methyl (14.2, 11); parathion (12.3,
8); mevinphos (7,4); demeton (6.2,4); tetram (14.2, 5);
carbophenothion (32.5, 29). In the case of every mixture, the observed
LD50 indicating no potentiation (Shaffer et al., 1958).
Special study on neurotoxicity
Groups of 6 adult hens were fed 0 and 40 ppm phorate in the diet for 4
weeks. A third group received 4000 ppm TOCP as a positive control.
Each hen was anesthetized, immediately perfused with buffered
formalin, and sections of brain, lower thoracic cord and each sciatic
nerve prepared for microscipis examination. The TOCP produced myelin
loss in each hen. Phorate produced no adverse effects on nerve fibres
or the myelin sheath (Levinskas et al., 1965).
Special study on mutagenicity
Ten pesticides, including phorate, were tested for dominant lethal
effects. A group of 20 male mice were fed for 7 weeks at three dose
levels of phorate (levels not reported). Reference controls and a
positive control receiving triethylenemelamine in drinking water for 4
weeks were included in the test. Following treatment each male was
mated to two females weekly for 8 weeks. No consistent response
occurred to suggest that phorate in the diet for 7 weeks induced
dominant lethal effects in mice (Jorgenson et al., 1976).
Special study on reproduction and teratology
Groups of mice (8 males and 16 females/group) were fed phorate in the
diet at levels of 0, 0.6, 1.5 and 3.0 ppm during a 3-generation, 2-
litter/generation, reproduction study.
There were no dose related effects on the fertility, gestation,
viability or lactation indices during the study, but there was a
lowering of the lactation index in the 3.0 ppm group. This value fell
below the control value in the first mating of the F0 generation, in
both matings of the F1 and in the second mating of the F2
generation.
Gross and microscopic examination of tissues and cleared pups revealed
no consistent abnormalities related to feeding of phorate (Anonymous,
1965).
Acute Toxicity
TABLE 2. Acute toxicity of phorate
LD50
Species Route, Sex (mg/kg) Reference
Rat Oral M 2.3 Gaines (1969)
F 1.1
Rat Oral M 2.8 Anonymous (1976)
F 1.6
Rat Dermal M 6.2 Gaines (1969)
F 2.5
Rat Dermal M 5.7 Anonymous (1976)
Rabbit Dermal M 5.2
Short term studies
Rat
Groups of rats (50 males and 50 females/group) were fed 92% phorate,
in corn oil, in the diet at levels of 0, 0.22, 0.66, 2.0 and 6.0 ppm
for 13 weeks. Two groups (25 males and 25 females/group) were fed 12
and 18 ppm for 8 and 2 weeks respectively. Cholinesterase activity was
monitored at weekly intervals. Occasional episodes of excitability and
intermittent tremors were noted in the 6.0 ppm female group. Both
sexes receiving the 12 and 18 ppm levels exhibited severe
excitability, intermittent tremors and ataxia culminating in death.
Levels of 6 ppm and higher produced significant depression in both
sexes of plasma, red blood cell and brain activity. At 2 ppm female
red blood cell activity was also depressed. Levels at 0.66 ppm or
below had no effect on cholinesterase activity. Growth, food
consumption, survival, liver and kidney weights and ratios, gross
necropsy and histological findings for all groups at 6 ppm or below
were within normal limits. Levels of 12 and 18 ppm severely affected
growth and survival (Tusing et al., 1956b).
Groups of rats (50 males and 50 females/control and 35 males and 35
females/test groups) were fed the sulphoxide of phorate (92% purity)
for 90 days at levels of 0, 0.32, 0.80 and 2.0 ppm. Brain, erythrocyte
and plasma cholinesterase activities were determined biweekly. At the
2.0 ppm level a significant (P <0.05) depression in erythrocyte and
brain cholinesterase activity occurred in females. Plasma activity
depression at this level was less consistent and judged as borderline.
The 0.8 ppm level produced borderline depression of the erythrocyte
enzyme activity. At 0.32 ppm all values were within acceptable
statistical limits for both sexes. No effects were observed on other
hematological values, organ weights or ratios and no consistent
microscopic pathology was noted which could be attributed to the
feeding of 2 ppm or less of the sulphoxide of phorate (Levinskas et
al., 1968).
Dog
Groups of mongrel dogs (2 males and 1 female/group) received by
capsule 0, 0.01, 0.05, 0.25 and 1.25 mg/kg/day of 92% phorate in corn
oil 6 days/week for 15 weeks. Two males received 2.5 mg/kg as a single
dose. Plasma and red blood cell cholinesterase activities were
monitored at weekly intervals. The 0.05 mg/kg/day level produced a
significant depression in plasma enzyme activity. The red blood cell
activity was not affected for the first 12 weeks but was depressed
slightly (not significantly) during the last 3 weeks of the study.
Significant plasma and red blood cell enzyme depression was produced
by the 0.25 mg/kg/day level. The 1.25 mg/kg/day level produced
complete inhibition of plasma cholinesterase activity and a
significant reduction in red blood cell activity. At the 2.5 mg/kg/day
level all dogs died within 3-4 hours following a single dose and no
enzyme activity determinations were made. No signs of systemic
toxicity in dogs receiving the 0.01 or 0.05 mg/kg/day doses (Gaines,
1969). Histopathological examination revealed no consistent findings
related to the test/material (Tusing et al., 1956a). Beagle dogs (3
males and 3 females/group) were fed in the diet 0, 0.5, or 1.0 ppm
phorate for 6 weeks. Cholinesterase activity of plasma and
erythrocytes was determined for each dog prior to and at biweekly
intervals during the test. Analyses of variance conducted upon plasma
and erythrocyte enzyme activities revealed no significant difference
between the test animal and control animal groups during the test
period (Kay and Calandra, 1961).
Long term studies
No information available.
Observation in humans
No information available.
COMMENTS
Phorate, an organophosphorous pesticide, is a derivative of
dithiophosphoric acid. Less than 40% of a 2 mg/kg oral dose to rats
was excreted in 6 days, and brain, liver and kidney contained
unextractable residues. The major metabolites in both plants and
mammals are the sulphoxides and sulphones of phorate and of its oxygen
analogue, phora-toxon; these have greater anticholinesterase activity
than phorate. Hydrolysis of phorate itself is a minor metabolic
pathway in mammals.
The biochemical data available on phorate are inadequate to determine
whether the oxidative metabolites are retained in mammalian tissues or
organs. Toxicological data submitted are insufficient, except for
cholinesterase inhibition, to determine the short and long term
effects of phorate and its oxidative metabolites. Although the Meeting
considered setting a temporary ADI for humans this could not be
established in the absence of long-term studies. Further studies are
required to evaluate a.) carcinogenic potential, b) teratogenic
potential, c) potential neurotoxicity and d) toxicity of metabolites.
Observations in human subjects are desirable.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phorate is a powerful soil insecticide with excellent systemic
properties. A wide spectrum of insect and free-living nematode pest
problems is controlled by a seed-bed or a side-dressing treatment.
Seed-bed applications protect the germinating seedlings and young crop
stages against a wide range of pests.
Granular formulations of phorate have been extensively field tested
throughout the world since 1954 and are used commercially in many
countries. Major uses are summarized in Table 3. Recommended dosage
rates vary somewhat from country to country, depending mainly on the
pest complex to be controlled and the period of protection required.
Although application is normally made in the furrow at seeding or
planting time, certain pest problems require the application of
granules as a side-dressing at the time of subsequent cultivation.
There are some situations where, in order to control vectors of virus
diseases, it is necessary to make a broadcast treatment with granules
or a foliage application of emulsifiable concentrate to the growing
crop. Preharvest intervals and limitations on grazing vary somewhat
but for the most part they are relatively long because of the residual
life of phorate in treated plants.
In addition to its use on food and forage crops phorate is used on
ornamentals and tobacco and in forest nurseries where sucking insects
are a problem.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive trials have been carried out by the manufacturers and by
independent investigators to determine the level and fate of phorate
residues in virtually every crop on which the insecticide in used.
Copies of trial reports and published scientific papers were provided
to the Meeting by the manufacturers (American Cyanamid, 1977). A
representative selection of the data from these reports has been
summarized in Table 4. The following observations are made on the
detailed data.
TABLE 3. Phorate: use-pattern
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Alfalfa 1 seed treatment 60 days
1.5-2 broadcast 35 days
(grazing)
Barley 1 broadcast-soil incorporated 60 days
Beans 1-1.5 in furrow at planting 60 days
broadcast before flowering -
Brassicas 16g/100m row in furrow at transplanting -
Carrots 1.7-3.3 at drilling time -
TABLE 3. (Continued)
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Cotton 0.2-0.5 seed treatment do not use
seed for food
0.3-1.5 furrow application do not graze
Hops 1-1.5 foliage application 25 days
2-3 band application 42 days
Lettuce 1 at transplanting or
seeding -
1 foliage treatment 18 days
Maize 1-2 in farrow at planting -
1 band application
at cultivation 30 days
1 foliage application 30 days
Onions 12g/100m row in furrow at transplanting -
Peanuts 1-1.5 in furrow at planting do not graze
or feed
2 band application at pegging do not graze
or feed
Peppers 1 in furrow at transplanting -
Potatoes 1.5-3 in furrow at planting 90 days
Sorghum 2 In furrow at planting -
0.5-1 foliage application 28 days
Sugar beets 0.5-1 in furrow at planting -
1-1.5 foliage application 30 days
Sugar cane 4 in furrow at planting -
Tomatoes 1-2 at planting or
transplanting -
Wheat 1 in furrow
1 broadcast 70 days
Sunflower 1-1.5 in furrow at planting -
TABLE 4. Phorate residues resulting from supervised trials.
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
ALFALFA USA 1966 1 1 granule <0.01 <0.01 <0.01 <0.01
(dry) 1 2 10% <0.01 <0.01 <0.01 <0.01
1 4 " 0.06 0.06 0.03 0.02
1 1 " 0.06 0.08 0.71 0.37 0.21
1 2 " 0.01 0.01 0.35 0.46 0.07
1 1 " 0.05 <0.01 0.01 0.01 0.01
1 2 " 0.15 0.02 0.02 0.02 0.01
APPLES Italy 1970 2 1.5 E.C. <0.02 <0.02 <0.02 <0.02
Argentina 1972 1 2 granule 0.01
1 3 " 0.01
BARLEY USA 1967 1 4 " <0.01
(grain) 1 1 " 0.01
BARLEY USA 1967 1 2 " <0.01
(straw) 1 1 "
1 2 0.04
BARLEY USA 1961 1 1 " 0.07 <0.04
(grain)
BEAN, SNAP USA 1966 1 1 " 0.5 0.07 <0.01
USA 1963 1 2 " 0.046 0.016
1 2 " 0.018 0.011
1 4 0.013 0.036
USA 1966 1 1 " 0.5 0.07 <0.01
1 1 <0.01
1 2 " <0.01
COWPEA India 1974 1 2 " 1.46 1.05 0.53
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
EGGPLANT India 1974 1 2 " 1.27 1.01 0.69
MAIZE USA 1970 1 0.5 E.C. 0.068 0.024 <0.020
(plant) 1 " 0.131 0.046 0.007
2 " 0.695 0.110 0.019
S. Africa 1977 1 0.5 granule 0.4 0.1 <0.02 <0.02
1 " 2.8 0.9 0.03 0.03
0.5 " <0.02 <0.02 <0.02
1.0 " 0.06 <0.02 <0.02
0.5 " 0.5 <0.02 <0.02
S. Africa 1977 1.0 granule 0.8 0.03 <0.02
MAIZE S. Africa 1977 1 0.5 " <0.02
(grain) 1.0 " <0.02
Italy 1971 2.5 " <0.02
14 30 40 60 90 120 150
CARROTS UK 1970 2 3 granule 1.01 0.88 0.47 0.64
3 3 " 0.29 0.40 0.39 0.27
2 3 " 0.41 0.41 0.24 0.19
3 1.5 " 0.19 0.09 0.09 0.23
Canada 1975 3 1 E.C. 0.26
4 1 E.C. 0.40
CELERY UK 1971 1 1.2 granule <0.05
1.5 " <0.05
COTTONSEED USA 1969 1 1 E.C.(seed) <0.05
1 1-2 granule <0.05
1 3 " <0-05
1 2 E.C. <0-05
Egypt 1968 5 1 G/E.C. 0.02 0.01
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
GRAPES Mexico 1973 1 4 0.000
1 6 0.000
1 8 0.007
HOPS USA 1968 1 4 granule <0.1 <0.1 <0.1
3 1 E.C. 0.1
LETTUCE USA 1977 1 2 5.14 0.24 0.17 <0.05
1963 1 1 <0-05
1963 1 2 <0.05
1963 2 0.025
PEANUTS USA 1964 2 1+2 granule <0.05 <0-05
(kernels) 2 1+2 " <0.05 <0-05
2 1+2 0.05 0.05
2 1+4 <0.05
1 2 " 0.1 0.08 0.05
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
PEANUT USA 1964 2 1+2 granule 0.05
(foliage)
POTATOES Czechoslovakia 1972 1 1.6 " <0.05
1 2.2 " <0.05
1 4.3 " 0.07
Turkey 1973 1 3.4 <0.05
Mexico 1972 1 1 E.C. 0.004 0.000
1 2 E.C. 0.006 0.000
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
RAPE SEED USA 1971 1 1.5 granule 0.05
RAPE HAY USA 1971 1 1.5 " 0.21
SORGHUM USA 1969 1 1 " <0.05 <0.05
(grain) 1 2 " <0.05 <0.05
SORGHUM USA 1969 1 1 " 0.06 0.06
(fodder) 1 2 " 0.16 0.08
SORGHUM Mexico 1972 1 1 E.C. 0.00 0.00
(grain) 1 2 E.C. 0.02 0.00
MILO USA 1967 1 1 granule <0.01 <0.01 <0.01
2 " <0.01 <0.01 <0.01
4 " 0.22 0.01 <0.01
SOYBEAN USA 1970 1 1 " <0.05 <0-05
2 " <0.05 <0.05
7.5 " <0.05 <0.05
SOYBEAN USA 1970 1 1 " <0.05 <0.05
(foliage) 2 " <0.05 <0.05
7.5 " 0.15 0.19
SUGAR BEET USA 1961 1 1 E.C. 0.05
2 E.C. 0.05
TOMATOES USA 1961 1 1 <0.05 <0.05
2 0.05 0.05
4 0.06
WHEAT USA 1969 1 1 <0.05
(grain) 1 2 <0.05
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
WHEAT USA 1969 1 1 1.4 0.35 0.33
(plant) 1 2 2.7 1.6 3.0
1 1 6.1 0.87 0.07
Alfalfa
Residue results obtained on fresh alfalfa and alfalfa hay grown in
various regions of the United States show that application at twice
the recommended dosage rate of 2 kg/ha does not result in residues in
excess of 0.5 mg/kg in or on fresh alfalfa or 1 mg/kg on alfalfa hay
within 35 days of treatment. Generally the level is less than the
limit of determination (0.01 mg/kg).
The results from a series of trials where phorate was applied to
alfalfa at rates ranging from 2 through 3 to 4 kg/ha indicate that the
half life in green alfalfa is between 8 and 18 days.
Apples
Results from spray trials carried out in Italy where 7.59 of phorate
was applied per tree on one, two and three occasions prior to harvest
show that the residues in apples 30 to 80 days after the last
application were generally less than 0.02 mg/kg of Phorate and
metabolites. One sample from a tree treated 30 days previously showed
0.06 mg/kg phorate sulphone only.
Residue analysis for phorate and metabolites in several varieties of
apples treated in Argentina with phorate granules applied in
incorporation in the soil around the stem at doses ranging from 10-20g
of phorate per tree indicated no residues of parent compound or
metabolite in fruit collected 182 days after application.
Barley
Experiments in the USA where phorate granules were incorporated or
irrigated into the soil at planting time reveal that the residues of
phorate and metabolites in the grain at harvest 60 days later are
generally at or below the limit of determination (0.01 mg/kg). When
applied at twice the recommended rate (1 kg/ha) detectable residues
(0.04 mg/kg) were found in barley straw 60 days later.
Stern (1961) reported that phorate granules applied to barley fields
at the rate of 1 kg/ha one day prior to irrigation, produced
detectable residues in the barley plants (0.07 mg/kg) 14 days later.
The residues had declined below the limit of determination (0.04
mg/kg) by the 28th day following application.
Beans
Extensive field trials carried out during the period 1966-68 on many
varieties of beans under widely differing agricultural and ecological
conditions indicate that the residues are generally well below 0/05
mg\kg 60 days after application. The samples were analysed by the
oxidative cholinesterase inhibition technique which determines phorate
and metabolites. The limit of determination based on control values is
considered to be 0.05 mg/kg. The reports contain results from a few
isolated samples which indicate a total phorate residue of the order
of 0.1 mg/kg.
Carrots
Wit (1960) using analytical methods involving the determination of
total phosphorus showed that, following the application of phorate
granules at the rate of 1.25-1.5 kg/ha, carrots contained from 0.09-
0.19 mg/kg of residues derived from phorate applied 73-132 days
previously.
An extensive experiment carried out in the United Kingdom (Cyanamid
Great Britain Limited, 1971) determined residues of phorate and its
metabolites in carrots following treatment with phorate granules
applied with the seed and at varying times thereafter according to
various treatment codes. The highest residue level was approximately
1mg/kg some 5 weeks after the last treatment, but this declined to
0.64 mg/kg by the end of 4 months. Lower rates produced somewhat lower
residues but significant residues were detected from all treatment
regimes. The residues consisted of phorate and phorate sulphone with
no oxygen analogue or sulphoxide.
Finlayson et al (1976a) studied the distribution of a number of
insecticides used to control carrot rust fly in the peel and pulp of
carrots, measuring also the distribution throughout the length of the
carrot. These investigators found that the bulk of the residue was in
the peel in the top 3cm of the carrot, the total residue in the whole
carrot being of the order of 0.2-0.4 mg/kg. The peel at the top end
contained as much as 7 mg/kg. See also "Fate of residues".
Maskell et al (1974) showed that the application of granules
containing 10% phorate at 1, 2 or 4 kg/ha in a narrow slit together
with carrot seed gave rise to residues which could be reduced by 50%
by placing the granules 2.5cm below the seed. Residues resulting from
the application of 1 kg/ha with the seed or 2 kg/ha 2.5cm below the
seed were not significantly greater than residues in untreated
carrots.
When 4 kg of phorate per ha was applied along with carrot seed the
residue in carrots at harvest 8 months later was 0.4 mg/kg. When the
granules were placed 2.5cm below the seed the residue under
corresponding conditions was only 0.2 mg/kg.
Cottonseed
American Cyanamid Company (1969c) reported the results of 13 trials in
different regions of the USA aimed at determining what residues, if
any, occur in cottonseed following phorate treatment of seed pre-
planting or emulsifiable concentrate as a late season foliar
application. All samples were analysed by the gas-chromatographic
method. The data show less than 0.05 mg/kg phorate in cottonseed
harvested from crops treated at planting time or with a side-dressing.
Late season application with phorate emulsifiable concentrate at rates
up to 2 kg/ha did not show detectable residues in cottonseed from
crops treated 30 days prior to harvest.
Zaki and El-Sayed (1968) showed that residues in mature cottonseed
after spraying mature plants three times with phorate at the rate of 1
kg/ha were only 0.013 mg/kg 14 days after the fourth application of
foliage sprays.
Cowpeas
Satpathy et al (1974) showed that highly significant residues of
phorate could be found in the pods of cowpeas 10 and 20 days after
treatment of the soil with phorate granules applied at the rate of 2
kg/ha. The residues continued to decline with a half life of
approximately 12 days. Phorate residues were relatively more
persistent than those of diazinon and dimethoate but comparable to
disulfoton in cowpeas.
Grapes
The residue analysis of grapes harvested from plants growing in soil
treated with phorate granules at the rate of 4, 6 and 8 kg/ha showed
no residues at the two lower rates and only barely detectable residues
at the highest rate. Treatment had been made by incorporating the
granules into the soil at the time when the grape vines were
sprouting. Grapes were harvested 92 days later (Cyanamid
International, 1973b).
Hops
Kiligemagi and Terriere (1968 a,b) reported an extensive series of
trials designed to determine the level and fate of phorate residues in
hops following the application of phorate granular to the hop beds and
foliar applications at rates from 1 to 5 kg/ha and with pre-harvest
intervals ranging from 15 to 89 days. Using a gas-chromatographic
method specific for phorate and its oxygen analogue sulphone the
investigators were unable to detect, either the parent compound or
metabolites in fresh or dried hops even at the short pre-harvest
interval of 15 days. Aphid and spider mite control were, however,
excellent.
Maize
One of the major uses for phorate ban been the control of a variety of
insects on maize. In the early 1960s the manufacturers carried out,
extensive residue studies in many locations in the USA. Using
oxidative cholinesterase inhibition procedures it was shown that the
insecticide was taken up into the growing plant but that the residues
declined rapidly during the 45 days following application, by which
time residues in the whole plant were not significantly greater than
the apparent phorate residue in untreated control samples. By the time
the grain was ripe, no trace of phorate of other
cholinesterase-inhibiting substances could be found in the grain or
other parts of the plant.
Following the introduction of GLC methods capable of determining
phorate and its metabolites, a number of studies were carried out in
several countries beside the USA. These confirmed that the residue
levels declined to below the limit of determination between 28 and 40
days after the application of phorate granules or spray at rates up to
2 kg/ha. Leuck and Bowman (1970) published the results of one such
study which traced the occurrence and level of phorate and four
metabolites in maize plants treated with 0.5, 1 and 2 kg/ha of phorate
as a spray. These workers showed that the sum of all residues was less
than 1 mg/kg 14 days after the application of all three rates of
phorate.
Peanuts
Investigation in the USA (Diablo Laboritories 1964) show that the
application of phorate granules at planting and subsequent application
at picking does not result in apparent phorate residues different from
these found in untreated controls. The analyses were made using the
oxidative cholinesterase inhibition technique which produces a
positive result in untreated controls of the order of 0.05-0.1 mg/kg
apparent phorate. Samples of kernels from plants treated at the
highest rate did not show any residues higher then the controls when
collected 90 days after the last treatment. Where treatments were
applied 30 to 40 days prior to harvest, a positive residue of the
order of 0.1 mg/kg was found in peanut kernels. The residues in peanut
foliage after 90 days were not significantly different from untreated
controls.
Potatoes
Residue date developed prior to 1960 in support of petitions for the
registration of the use of phorate on potatoes were obtained with a
non-oxidative anti-cholinesterase method of analysis. This procedure
has good sensitivity for the oxygen analogue sulphoxide and sulphone
and, with the long pre-harvest interval involved, was considered
adequate at the time. Extensive residue information on potatoes was
obtained with this method, including results from inordinately high
rates of application, with all findings showing no measurable residues
in mature potatoes at harvest.
Owing to their relatively poor cholinesterase inhibiting properties,
phorate and its intermediary oxidative derivatives such as phorate
sulphoxide or sulphone would not have been measured by the
non-oxidative method had they been present. In 1959, an oxidation step
was introduced into the anti-cholinesterase procedure, which converted
phorate and its partially oxidised metabolites to phorate oxygen
analogue sulphone, and thus measured all toxic phorate residues. At
this stage a method based on the determination of total phosphorus
(Steller and Curry 1964) and including an oxidation step (Blinn 196A)
was utilized for further studies. By this method, which had a limit of
determination of the order of 0.01 mg/kg, it was shown that potatoes
treated at planting with phorate at the maximum recommended rate of 3
kg/ha generally contained 0.05 mg/kg or less when harvested 90 to 120
days later. However, residues as high as 0.15 mg/kg were occasionally
found following the use of phorate at the rate of 3 kg/ha. Following
the use of twice this amount (6 kg/ha) residues ranging up to 0.5 were
reported 100 to 140 days after application (American Cyanamid Company
1966).
In the Netherlands between 1958 and 1960, Wit (1959, 1961)
investigated the residues of systemic insecticides on potatoes, using
analytical methods involving the determination of total phosphorus
(limit of determination 0.1 mg/kg). He found substantial residues of
phorate in potatoes from 3 days to 42 days after application of
phorate granules (1.5-3 kg/ha). These residues ranged up to 3 mg/kg,
with the bulk of the results near 1 mg/kg.
Later Wit (1962a., 1963) using methods that determined phorate and the
sulphoxide metabolite (Laws 1961) showed that residues were less than
0.05 mg/kg 75-110 days after applying phorate granules at 2 + 3 kg/ha.
In 1972 following the development of a highly specific and
highly-sensitive analytical method employing gas-liquid chromatography
for the determination of phorate and phorate metabolites, an extensive
trial was carried out in Czechoslovakia (Cyanamid International
1972a). Residues of phorate or its oxygen analogue sulphone were not
measurable in any treatment. The only detectable residue in the
treated samples was of phorate sulphone, the highest level found being
0.07 mg/kg following the application of 3.4 kg/ha 120 days previously.
A similar field trial was carried out in Turkey (Cyanamid
International, 1973a), where phorate granules were applied at the rate
of 3.4 kg/ha at the time of planting and the potatoes harvested 125
days later. None of the potatoes contained residues of phorate or any
metabolite at levels above the limit of determination (0,01 and
0.02 mg/kg). It was concluded that the total residue of phorate and
metabolites was less than 0.05 mg/kg.
The application of phorate spray to potato Plants at rates to 1 and 2
kg/ha in Mexico (Cyanamid International, 1972b) produced detectable
residues in the tubers which declined from 0.02 mg/kg on the day
following application through 0.002 mg/kg on day 20 to 0 on day 27.
These results indicate that only small amounts of phorate are
translocated from the leaves to the tubers, and that these residues
decompose rapidly.
Sorghum and milo
Extensive trials were carried out in two different regions of the USA
to determine residues of phorate and its metabolites in sorghum fodder
and grain following granular and spray treatments. Samples of fodder
and grain were collected at intervals from 28 to 46 days after
application. The samples were analysed by gas-liquid chromatographic
procedures with a sensitivity of 0.05 mg/kg. All residues were below
0.05 mg/kg except fodder from one location which had been treated with
granular formulations and sampled after 31 and 46 days (American
Cyanamid Company, 1969a).
In an experiment carried out in Mexico, phorate spray was applied to
sorghum at the rate of 1 and 2 kg/ha 28, 21, 14, 7 and 0 days before
harvest, the residues found on the grain immediately after application
on the day of harvest were 0.55 and 0.82 mg/kg respectively. Though of
the order of 0.1 and 0.2 mg/kg could be found on the grain treated 7
days before harvest, that treated 14, 21 or 28 days prior to harvest
contained none as determined by a method sensitive to 0.01 mg/kg.
Soybeans
Studies carried out in the USA (American Cyanamid Company, 1970)
demonstrate that phorate granules used at the rate of 1 and 2 kg/ha
and applied in the furrow or to the side of the seed at planting will
result in no detectable residues (less than 0.05 mg/kg) of phorate
plus its toxic metabolites in soybeans seed at harvest. These same
studies showed no residue in soybeans treated at 7.5 kg/ha at two
separate locations and it is therefore reasonable to assume that no
detectable residues will occur in either soybeans oil or meal
following the use of phorate at the rate of 1-2 kg/ha.
Sugar beet
In 1960 and 1961 the manufacturers carried out or sponsored many field
trials to determine the level and fate of phorate residues in sugar
beet roots and leaves. Neither phorate nor its metabolites were
detected in any samples of sugar beet roots or tops in any trial. The
use of phorate sprays on sugar beet is approved in several countries
and maximum residue limits have been established to permit the feeding
of beet foliage to livestock.
Wit (1962b) reported that sugar beet roots and sugar beet leaves
contained less than 0.1 mg/kg or phorate-derived residues
(phorate + sulphoxides and sulphones) when phorate granules were
applied at the rate of 2-3 kg/ha 120-140 days before harvest.
Tomatoes
Extensive experiments carried out on tomatoes grown in various regions
of the United States during the period 1958-1961 show that following
the recommended application of phorate granules at the rate of 3 kg/ha
at planting time, no significant residues of either phorate or its
oxidative metabolites are found in either mature green or pink ripe
tomatoes. Similar results were also found at the 5 kg/ha. rate. Some
residue is found in the tomato foliage during the first few weeks
following application.
Wheat
Several studies were conducted in 1969 (American Cyanamid Company,
1969b) in the wheat growing areas of the United States to determine
phorate residues in green plants and grain following spring and fall
planting-time applications of phorate granules at rates of 1 and
2 kg/ha. The samples were analysed by GLC methods. The data show
phorate residues to be less than 1 mg/kg in all plant samples
approximately 45 days following application of phorate granules at a
rate of 1 kg/ha. No detectable residues (less than 0.05 mg/kg) were
found in grain harvested 120 to 330 days after treatment.
FATE OF RESIDUES
In animals
32P-phorate was given to a cow by Bowman and Casida (1958) in a
toxic dose of 3.04 mg/kg body weight, the acute symptoms that appeared
being controlled with atropine injections. The blood level increased
within 6-8 hours. After 18-36 hours the content of chloroform-soluble
metabolites in the blood increased slightly. The cow excreted 59% of
the radioactive material in the urine within 72 hours. None of this
was chloroform extractable. The main urinary metabolite was initially
0,0-diethyl phosphorothioic acid and subsequently diethyl phosphoric
acid, excretion of. 0,0-diethyl phosphorodithioic acid was
consistently low over the entire period. Only 0.8% of the applied
radioactivity appeared in the faeces within 96 hours after application
and 10% after 12 hours. The chloroform-soluble products were phorate
sulphoxide and/or sulphone, together with some phorate.
Examination of the cow's tissues 96 hours after feeding showed that
the liver and kidneys contained the highest 32P concentration (10.3
and 5.2 mg/kg phorate equivalents respectively). In contrast, the loin
muscle tissue contained 0.29 mg/kg and the mesenteric fat 0.03 mg/kg
phorate equivalents. The lung contained 1.5 mg/kg. Appreciable amounts
of phorate-derived phosphorus was found in the rumen and omasum walls,
other glands and rib bones. Milk contained 0.07, 0.25, 0.3 and
0.53 mg/kg phorate equivalents at 8, 24, 32 and 56 hours after dosing,
respectively.
Phorate is attacked only slowly by the rumen fluid (Ahmed et al.,
1958).
Phorate was administered to calves at a rate of 0.1 mg/kg for 14 days
to determine the level of phorate residues in liver, kidney, blood,
muscle, heart and fat tissues. No detectable residues ( < 0.1 mg/kg)
were found. Phorate was administered to lactating dairy cows at rates
of 0.05 and 0.1 mg/kg for 14 days. No detectable residues of phorate,
(< 0.02 ppm ) were found in the milk of cows fed 0.05. The milk
from cows fed 0.1 mg/kg contained from <0.02 to 0.05 mg/kg Phorate
(Anonymous, 1961).
Bunyan and Taylor (1966) found practically no phorate in meat and
organs of pheasants after administration of 6.2 to 21.7 mg/kg body
weight. In other investigations with pheasants and pigeons Bunyan et
al (1969) also found no residues after feeding for 14, 28 and 42-day
periods with 100 ppm of phorate in the diet.
Experiments designed to determine the total phorate residues in
chicken tissues and eggs were carried out by American Cyanamid
(1969d). Laying hens were dosed with rations containing 1, 0.3 and
0.1 ppm total phorate (1: 1, phorate: phorate oxygen analogue
sulphone) for 21 consecutive days. The chickens were sacrificed 2-3
hours after the final dose. Egg samples were collected during the
final day of treatment. The samples of muscle, fat, liver, kidney and
eggs were analysed by gas-chromatographic procedures using a caesium
bromide thermionic detector: phorate and its oxidative metabolites
were oxidised to and measured as phorate oxygen analogue sulphone and
calculated as total phorate. The sensitivity of the method for both
phorate and the oxygen analogue sulphone was 0.05 mg/kg. None of the
samples showed any apparent residues of phorate above this level.
In plants
Since 1954, studies of the metabolism of phorate in plants have been
conducted by investigators at American Cyanamid Company and by
researchers in various parts of the world. It has been shown that when
absorbed by plants, phorate is initially and rapidly oxidised to its
sulphoxide and sulphone which in turn are converted to the sulphoxide
and sulphone of the phorate oxygen analogue (Blinn, 1964; Bowmann and
Casida, 1957, 1958; Galley and Foerster, 1976; Krueger, 1975;
Lichtenstein et al., 1974; Metcalf et al., 1957; Zaki and El-Sayed,
1968).
The sequence of oxidative reactions in the metabolism of phorate
following absorption by plants is similar to that in animals and is
given in Figure 1. The oxygen analogue, although presumably formed as
an intermediate, has not been detected.
Bowman and Casida (1957) showed that when phorate was used as a
systemic insecticide for seed treatment of cotton, the metabolites
within the plant consisted of the sulphoxide and sulphone of the
parent compound and of its oxygen analogue. Cotton seeds treated with
phorate at concentrations as high as 32 kg of phorate per 100 kg of
seed showed less than 0.03 mg/kg of phorate or metabolites in the
seeds maturing an plants grown from such treated seed. The residual
persistence following soil and foliage application was studied with
six vegetable crops using radioactive phorate. Table 5 shows the
persistence of radioactivity in vegetable crops treated with
32P-phorate and is derived from the means of values from beans,
beets, cabbage, carrots, lettuce and peas treated by foliage
application and by soil application. Other experiments by Boman and
Casida (1958) on the fate in beans are described previously
(Biotransformation).
Phorate is usually applied as a soil treatment where its outstanding
systemic properties can be utilized to protect the developing plants
from attack by sucking insects for the first few weeks of their
growth. After phorate is applied to the soil and reaches the root zone
of the plant, the insecticide is absorbed by the roots and is
subsequently translocated to aerial parts of the plant. The rate of
movement of phorate from roots to stems and leaves is accelerated by
increased transpiration, and the greatest accumulation of insecticide
in leaves seems to occur under environmental conditions favourable to
transpiration (Hacskaylo et al., 1961). Van Middlelem and Baranowski
(1962) found that the highest concentrations of phorate in tomato
plants usually coincided with preceding periods of relatively high
field temperatures and ample rainfall. Krueger (1975) showed that
soybean root homogenates oxidised phorate to phorate sulphoxide.
Neither the oxygen analogue nor sulphone was detected.
Finlayson at al. (1976a), investigating methods for controlling carrot
rust fly, showed that phorate spray applied over the young carrot
seedlings was readily taken up and held in discrete portions of the
carrot. More than 50% of the residues were located in the top 0-3cm of
the carrot. Total residues in whole carrots generally increased with
higher rates and numbers of applications. Residues were greater in the
peel than in the pulp. Discarding the top 1-2cm of the carrot and
peeling the rest removes most of the residues. See also "Residues
resulting from supervised trials".
In soils
After phorate is applied to the soil, its eventual distribution and
utilization by plants is influenced by various constituents and
qualities of the soil.
In a study of the fate of phorate in soils, Getzin and Chapman (1960)
used radioactive material. One hour after treating various soil types,
these investigators found that 25, 20, and 10 per cent of the
radioactivity applied had been lost through volatilization from sandy
soil, silt-loam and muck respectively. After one hour, little or no
volatilization occurred. They further reported that phorate, when
applied to soil, is partially oxidised, hydrolyzed and bound to the
soil.
It has been shown that phorate moves readily in soil in the vapour
phase (Etheridge and Burt, 1963) as well as in drainage water (Bardner
at al., 1963).
Lindley (1963) reported from field experiments that on mineral soils
less phorate was required for insect control than on peat soils with a
maximum of 35% organic matter, while only partial control of insects
was obtained on soils with more than 35% organic matter, even when
much higher rates of insecticide were used.
Lichtenstein et al, (1973, 1974) showed that while phorate could be
detected in soil, only metabolites were found in plants including
plant roots. Schulz et al. (1973) showed that phorate moved in both
vertical and horizontal directions after a granular band application
of insecticide in soil. Suett (1975) showed that soil temperature had
relatively less effect upon the fate of phorate than on other
soil-incorporated insecticides.
Waller and Dahm (1973) showed that the conversion of phorate to its
sulphoxide in soils is mainly a non-biological process, whereas the
conversion to sulphone is brought about by micro-organisms.
Talekar at al. (1977) in a long-term experiment investigated the
persistence of a number of insecticides following repeated seasonal
application to soil under sub-tropical conditions. They showed that
phorate degraded more rapidly than all the other materials showing
almost complete loss within four months, although measurable
quantities of phorate sulphoxide and sulphone could be detected for
somewhat longer periods.
TABLE 5. Persistence of 32P-phorate and its metabolites in vegetable cropsa.
Days following application
0.1 1 2 4 8 17 32
Foliage application
Phorate equivalentb, mg/kg
Hexane fraction 6.75 2.84 2.49 1.69 0.90 0.44 0.040
Acetone-water fraction 2.60 2.39 1.66 1.45 0.59 0.067 0.003
Hydrolysis productsc 0.28 0.37 0.62 0.85 1.33 0.43 0.27
Unextracted residued 1.44 1.03 0.81 1.62 1.54 1.00 2.28
AntiChE activity, 50% inhibitione
Plant tissue, g 1.3 0.47 0.59 0.54 1.0 >2.2 >2.2
pl50 metabolites 5.44 6.14 6.29 6.62 6.11 <7.22 <7.42
Soil application
Phorate equivalent, mg/kg
Hexane fraction 0.82 0.34 0.40 0.26 0.15 0.049 0.026
Acetone-water fraction 0.12 0.31 0.56 0.36 0.18 0.047 0.005
Hydrolysis products 0.052 0.12 0.13 0.24 0.25 0.21 0.110
Unextracted residue 0.15 0.16 0.42 1.20 0.46 0.11 0.078
AntiChE activity, 50% inhibition
Plant tissue, g 1.8 0.94 1.3 1.2 1.3 >2.9 >3.0
pl50 metabolites 6.89 7.26 6.66 6.72 7.00 7.15 7.22
a Results are average of figures from beans, beets, cabbage, carrots, lettuce and peas in same field plot.
b Radioactivity of phorate or metabolites appearing in fraction indicated, expressed as phorate (mg/kg)
c Water-soluble products not extracted into chloroform.
d ssP remaining in solid portion after both water and chloroform removed.
e Results based on chloroform-soluble metabolites. Amount of plant material containing enough
metabolites for 50% inhibition and pl50 (negative logarithm of molar concentration effecting 50% inhibition)
of these metabolites are reported.
In processing and storage
Leuck and Bowman (1970) showed that residues of phorate and five of
its metabolites remained virtually unaltered when treated corn foliage
was converted into silage and ensiled at 35C° for 30 days.
Askew at al. (1968) examined the effect of 30 minutes boiling on
vegetables containing various organophosphorus pesticide residues.
They demonstrated 100% hydrolysis of phorate residues (2 mg/kg) in
both potatoes and cabbages. They indicated that appreciable quantities
of phorate sulphoxide ware formed during the cooking process, but did
not attempt to quantify the transformation.
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Advice from the US Food and Drug Administration indicates that during
1975 three samples of strawberries were found to contain phorate
residues at levels of 0.07, 0.16 and 1.73 mg/kg. There was no
tolerance for residues of phorate in strawberries.
METHODS OF RESIDUE ANALYSIS
Gas chromatography with an electron-capture detector was used by Egan
et al. (1964) to measure phorate in certain plant tissues. Dewey and
Parker (1965) used an electron-capture detector to measure phorate and
some of its metabolites in soil. Bache and Lisk (1966) reported the
use of an emission spectroscopic detector in soil analysis for phorate
and its metabolites but found very poor chromatographic response for
phoratoxon sulphoxide and sulphone. Mitchell et al. (1968) used gas
chromatography to study the effect of ultra-violet irradiation and
permanganate oxidation on phorate. These workers found it possible to
chromatograph phorate, phoratoxon and phoratoxon sulphone on an
Apiezon L column; quantitative recovery figures were not given,
however. Ruzicka, et al. (1967) used a caesium bromide thermionic
detector with an Apiezon column and found that phorate was not
adequately resolved from phoratoxon, although phoratoxon sulphone
could be resolved. The same workers (1968) later used similar
techniques to measure residues of phorate, phorate sulphoxide and
phorate sulphone on apple leaves. Sans (1967) described a
gas-chromatographic procedure for the separation and measurement of
phorate, but not its metabolites, from various other pesticides.
Nelson (1967) developed a procedure for separating and measuring
phorate, but not its metabolites, from several other organo-phosphate
insecticides in residues on fruits and vegetables.
Bowman et al. (1969b) described procedures for the individual
measurement of phorate and its oxidative metabolites in residues on
corn tissue. These workers used an instrument equipped with a flame
photometric detector and a column of 10% DCL-200 silicone on Gas-Chrom
Q. Recoveries at the 0.05 mg/kg level in excess of 96% were reported
for all of the metabolites except phoratoxon (62% recovery). McLeod et
al. (1969) reported a procedure for the separation and measurement of
phorate and its metabolites from monkey liver homogenates, These
workers used a column packed with 5% diethylene glycol succinate on
Chromosorb W and a flame photometric detector. Recoveries averaging
over 90% were found at fortification levels of the order of 200 mg/kg.
Stanley and Morrison (1969) described the use of the flame photometric
detector with both phosphorus-selective and sulfur-selective filters
to aid in the identification of insecticides; among the compounds
tested was phorate.
Watts and Storherr (1969) and Watts at al. (1969) described clean-up
and gas-chromatographic conditions for the separation and measurement
of many insecticides, including phorate and its metabolites, using a
potassium chloride thermionic detector and a column of 10% DC-200 on
Gas-Chrom Q. Recoveries in excess of 95% were reported for kale
extracts fortified with phorate or its metabolites at levels of 0.1 to
0.3 mg/kg.
Getzin and Shanks (1970) used an instrument equipped with a sodium
sulphate thermionic phosphorus detector and a column of 4% QF-1 on
Gas-Chrom Q to measure phorate and its metabolites in soils. Recovery
values at levels of 20 mg/kg in soil ranged from 64% for phoratoxon
sulphoxide to 96% for phorate.
Higham at al. (1972) have described procedures suitable for the
determination of total phorate-related residues in a wide variety of
sample types. In these procedures, all of the phorate and its
oxidative metabolites present in the residues were converted to
phoratoxon sulphone by oxidation with m-chloroperbenzoic acid as
recommended by Blinn (1964). These procedures employed a
phosphorus-selective detector with a column of 5% DC-200 on Gas-Chrom
Q and were validated at levels down to 0.05 mg/kg in several types of
sample.
Boyd (1976) has reviewed the methods of residue analysis for phorate
and has provided details of methods for all substrates likely to be
encountered in residue analysis. Residues of phorate and its
metabolites are extracted from the prepared sample with chloroform,
chloroform/methanol, methylene chloride or acetonitrile (depending
upon the type of sample) and purified by clean-up procedures
appropriate to the particular type of sample being analysed. All of
the phorate-related residues present are converted to phoratoxon
sulphone by oxidation with m-chloroperbenzoic acid. Measurement is
accomplished by gas-liquid chromotgraphy with an instrument equipped
with a selective phosphorus-sensitive detector. These procedures
appear suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Commodity Tolerance, mg/kg
Argentina lettuce, wheat,
groundnut, rice,
maize 0.1
sugar beet (tuber) 0.3
maize fodder 0.5
sugar beet (leaves) 3
Australia cottonseed, vegetables 0.5
Canada beans, corn, lettuce,
potatoes, rutabagas,
turnips negligible
Germany all plant foods 0.05
Netherlands fruit, vegetables
(not potatoes), spices 0
South Africa all food products 0.05
apples, pears 0.1
China pineapple 0.1
USA sugar beet tops 3
wheat (green fodder) 1.5
alfalfa hay, dried sugar
beet pulp 1
corn forage, hops,
potatoes 0.3
peanut vines and hay,
sugar beet roots 0.3
barley grain, barley
straw, beans, corn grain 0.1
sweet corn, lettuce,
peanuts, rice, sorghum
grain 0.1
sorghum fodder, soybeans,
sugar cane, tomatoes 0.1
cottonseed, wheat grain
and wheat straw 0.05
eggs, meat, fat and meat
by-products of cattle,
goats, hogs, horses, poultry
and sheep 0.05
APPRAISAL
Phorate is a soil insecticide with marked systemic properties widely
used throughout the world since 1954. Maize, cotton, cereal grains and
vegetables are treated with phorate usually at the time of planting,
but sometimes by broadcast treatments of granules or a foliage
application to the growing crop. Pre-harvest intervals are relatively
long and residues in harvested commodities are either absent or
generally do not exceed 0.1 mg/kg.
Carrots are capable of taking up and retaining quantities of phorate
but most of the residue is in the peel or in the crown of the carrot
which in generally removed before processing for consumption. The use
of phorate for the protection of potato crops is important. The
residues at the time of harvest are at or below the limit of
determination.
Soil treatments lead to higher residues than do foliage treatments,
largely because the compound is relatively stable in the soil and in
readily taken up by the plant's root system. In animals phorate is
rapidly hydrolysed and converted into the corresponding sulphoxide and
sulphone and their oxons. No significant amounts of phorate or
metabolites are found in milky eggs or tissues of livestock
administered phorate at levels comparable to those likely to occur in
animal feeds.
The metabolism of phorate in plants is largely similar to that
occurring in animals. Phorate metabolites are relatively stable in
storage and processing. At rates recommended in good agricultural
practice, there appears to be no likelihood of carry-over of residues
in soil affecting subsequent crops.
A method of analysis based on the oxidation of all the phorate-related
residues to the oxygen analogue sulphone followed by measurement by
gas-liquid chromatography is sensitive and specific and appears
suitable for regulatory purposes.
Many countries have established maximum residue limits for phorate
residues.
EVALUATION
In the absence of an ADI, the following guideline levels are recorded.
They refer to the sum of the residues of phorate, phorate sulphoxide,
phorate sulphone and the oxygen analogues of these compounds,
determined as the sulphone of the oxygen analogue and expressed as
phorate.
Commodity Guideline levels, Interval on which
mg/kg guideline levels are
based, days
Alfalfa (dry) 1 35
Barley 0.05 60
Beans 0.1 60
Carrots 0.5 120
Celery 0.1 90
Cottonseed 0.05 120
Cowpea 0.1 60
Eggplant 0.1 60
Grapes 0.05 90
Hops (dried) 0.1 25-40
Lettuce 0.2 60
Maize (green) 0.05 30
Peanuts (kernels) 0.05 60
Potatoes 0.05 120
seed 0.1 90
Sorghum 0.05 30
Soybeans 0.05 120
Sugar beet,
fodder beet 0.05 120
Sugar beet
tops 1 35
Tomatoes 0.1 120
Wheat 0.05 120
Eggs, meat, milk 0.05* 120
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI)
can be established and maximum residue limits (MRL) can be
recommended).
1. Long-term carcinogenicity studies.
2. Teratogenicity studies.
3. Studies on potential neurotoxicity.
4. Studies on the toxicity of metabolites.
DESIRABLE
1. Observation in humans.
REFERENCES
Ahmed, M.K., Casida, J.E., and Nichols, R.E. (1958) Bovine metabolism
of organophosphorus insecticides: significance of rumen fluid with
particular reference to parathion J. Agr. Food Chem. 6, 740.
American Cyanamid Company (1966) Thimet residues in potatoes. Report
No. G102, January 13, 1966.
American Cyanamid Company (1966a) Total Thimet residues in sorghum
fodder and grain - Report No.C188, May 14, 1969.
American Cyanamid Company (1966b) Thimet residues in wheat - Report
No. C215, December 9, 1969.
American Cyanamid Company (1966c) Thimet residues in Cottonseed ;
Report No. C214, December 9, 1969.
American Cyanamid Company (1969d) Total Thimet residues in chicken
tissues Report No. C205 dated November 7, 1970.
American Cyanamid Company (1977) Residue dossier on THIMET
phorate - Vol. I-III.
Anonymous (1965) Report on Thimet Systemic Insecticide: Successive
Generation Studies with Mice. Unpublished report from Central Medical
Department Cyanamid, submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Anonymous (1961) Thimet Phorate Residue Studies in Dairy Animals.
Unpublished report from DIABLO Laboratories, In,., submitted to the
World Health Organization by American Cyanamid Company.
Anonymous (1976) Thimet Soil and Systemic Insecticides Technical
Information published by Cyanamid International Corporation, submitted
to the World Health Organization by American Cyanamid Company.
(Unpublished report)
Askew, J., Mitchell, T.M., Thomson, J. and Wheals, B.B. (1968) The
effect of cooking on organo-phosphorus pesticide residues - J.
Chromat. 32, 417-418.
Bache, C.A., and Lisk, D.J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
chromatography- J. Assoc. Off. Analyt. Chem. 49, 647-650.
Bardner, R., Lord, K.A., and Solly, S.R.B. (1963) Cholinesterase
inhibition method for determining the distribution of organophosphorus
insecticides in soils. Chem. and Ind. 3, 123-124.
Blinn, R.C. (1963) Thin - layer chromatographic isolation and infrared
or colormetric identification of Thimet residues. J. Assoc. Off.
Agric. Chem. 46, (6) 1, 952-96.
Blinn, R.C. (1964) Oxidative conversion of Thimet to its oxygen
analogue sulphone. J. Assoc. Off. Agric. Chem. 47 (4) 641.
Blinn, R.C. and Boyd, J.E. (1967) Thimet R Phorate: Identity of the
Major Phorate Metabolites in the Blood of rats Following oral
Administration of the Insecticide. Unpublished report from the
Research and Development Department of the Agricultural Division of
the American Cyanamid Company, submitted to the World Health
Organization by the American Cyanamid Company.
Bowman M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion disulfoton and phorate in corn, milk grass and
faeces - J. Assoc. Off. Analyt. Chem. 52 (6) 1231-1237.
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969a) GLC determination of
residues of disulfoton, demeton-methyl and their metabolites in
tobacco plants. J. Assos. Off. Analyt. Chem. 52 157-161.
Bowman, M.C., Beroza, M. and Harding, A.J. (1969b) Determination of
Phorate and Five of its Metabolites in Corn. J. Ag. Food Chem. 17
138.
Bowman J.S. and Casida, J.E. (1957) Metabolism of phorate (Thimet) in
plants. J. Ag. Food Chem. 5 (3), 192.
Bowman J.S. and Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants insects and mammals - J. Econ. Entomol. 51 (6)
838-843.
Boyd, J.E. (1976) Thimet (phorate) in Vol VI of Analytical Methods for
Pesticides and Plant Growth Regulators by G. Zweig and G. Sherma
-Academic Press N.Y. p. 493-510.
Brown, M.J. (1975) Improved determination of residues of phorate and
its principal metabolites J. Agric. Food Chem. (2) 334-335.
Bunyan P.J. and Taylor, A. Esterase inhibition of pheasants poisoned
with Thimet. J. Agric. Food Chem. 14, 132.
Bunyan P.J., Jennings, D.M. and Taylor, A. (1969) Organophosphorus
poisoning: Chronic feeding of some common pesticides to pheasants and
pigeons. J. Agric. Food Chem. 17, 1027.
Burns, R.G. (1971) The Loss of phosdrin and phorate insecticides from
a range of soil types - Ball. Env. Contam. and Toxicol. 6 (4)
316-321.
Cyanamid Great Britain Ltd. (1971) Residues of phorate and its
metabolites in carrots. Technical Report No. 106 -16 May 1971.
Cyanamid International (1972a) Residues of phorate and its metabolites
in potatoes - Technical Report No. 179 dated 19 April 1972.
Cyanamid International (1972b) Thimet residue analysis in potato
-Regional Laboratory Report No. 11 dated August 23, 1972.
Cyanamid International (1973a) Thimet and Thimet metabolite residues
in potatoes. Technical Report 240 dated 9 May 1973.
Cyanamid International (1973b) Residue analysis of Thimet and the
oxygen analogue in grapes. Regional Laboratory Report No. 19 September
21, 1973.
Dewey, J.E. and Parker, B.L. (1965) Increase in Toxicity to Drosophila
melanogaster of Phorate, Treated Soils. J. Econ. Entomol. 58, 491.
Diablo Laboratories (1964) Analysis of peanut meats, shells and
foliage for possible residues of total Thimet - Report 4J7511 dated 26
February 1964.
Egan, H., Hammond, E.W, and Thomson, J. (1964) Assoc. Off. Analyt.
Chem. 89, 175.
Etheridge, P. and Burt. P.E. (1963) Vapour phase movement and uptake
of systemic insecticides. Rep. Rothamsted, Exp. Stat. for 1963 Page
138.
Finlayson, D.G., Williams, I.H., Brown, M.J., and Campbell,
C.J. (1976a) Distribution of insecticide residues in carrots at
harvest. J. Aric. Food Chem. 24 (3) 606-608.
Finlayson, D.G., Williams, I.H., Brown, M.J. and Campbell,
E.J. (1976b) Translation and persistence of topically applied diazino
and phorate in beans. Can. J. Plant Sci. 56 947-952.
Galley D.J., and Foerster, L.A. (1976) Distribution and loss of
phorate residues in the foliage of broad bean plants following root
uptake of 14C-labelled phorate. Pestic Sci. 7 301-306.
Gaines T.B. (1969) Acute Toxicity of Pesticides. Toxicol. Appl,
Pharmacol 14, 515-534.
Getzin, L.W. and Chapman R.K. (1960) The fate of phorate in Soils - J.
Econ. Entomol. 53 (1) 47-51.
Getzin, L.W. and Shanks, C.H. (1970) Persistence, Degradation and
Bioactivity of Phorate and its Oxidative Analogues in soil. J. Econ.
Entomol. 63, 52.
Giang, P.A. and Schecter, M.S. (1960) Colorimetric determination of
residues of phorate insecticidally active metabolites. J. Agr. Food
Chem. 8 51-54.
Hacskaylo, J., Lindquist, D.A. and Clark, J.C. (1961) Phorate
accumulation by cotton plants and recovery from soil. J. Econ.
Entomol. 54 (3) 411-414.
Higham, J.W., Peterson, R.P. and Pasarela, N.R. (1972) J. Assoc. Off.
Analyt. Chem. (in press)
Jorgenson, T.A., Rashbrook, C.J. and Newell, G.W. (1976) In vivo
Mutagenesis Investigations of Ten Commercial Pesticides. Abstract No.
41, Fifteenth Annual Meeting, Society of Toxicology, submitted by the
World Health Organization by American Cyanamid Company, Wayne, NJ,
USA.
Kay, J.H. and Calandra, J.C. (1961) Effects of Thimet Phorate on
Cholinesterase Activity in the Dog. Unpublished report from Industrial
Bio-Test Labs., Inc., submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Khajuria, G.N., Jain, H.K. and Dewan, R.S. Residues following the
treatment of sorghum with phorate and dimethoate for the control of
insect pests complex of the crop. Proceedings of the first All-India
Symposium on Progress and Problem in Pesticide - Residue Analysis held
in Ludhiana on 18-19 November 1971 - p. 87-92.
Kiigemagi, O., and Terriere, L.C. (1968a) Residues of phorate in fresh
and dried hops. Report from Oregon State University dated June 10,
1968.
Kiigemagi, O. and Terriere, L.C. (1968b) Residues of phorate and its
metabolites in fresh hops Report from Oregon State University, October
6, 1968.
Krueger, H.R. (1975) Phorate sulfoxidation by plant root extracts.
Pesticide Biochem. & Physiolol. 5, 396-401.
Krueger, H.R., Lindquist, R.K. and Bauerle, W.L. (1973) Phorate and
aldicarb in greenhouse lettuce: Effect of CO2 on residues. J. Econ.
Entomol. 66, 573.
Krueger, H.R. and Mason, J.F. (1973) Phorate and aldicarb: effects of
systemic fungicides on their levels in soyabeans.
Laws (1961) Analyst, 81, 249-255.
Leuck, D.B. and Bowman, M.C. (1970) Residues of phorate and five of
its metabolites. Their presence in Forage Corn and grass - J. Econ,
Entomol. 63 (9) 1838-1842.
Levinskas, G.J., Morici, B.S. and Shaffer, C.B. (1965) Thimet systemic
insecticide: Demyelination studies in white leghorn hens. Unpublished
report from Central Medical Dept. of American Cyanamid Co. submitted
to the World Health Organization by American Cyanamid Co.
Levinskas, G.J., McNerney, J.M. and Fegluy, H.C. (1968) Report on
Sulfoxide of Thimet Systemic Insecticide: Ninety-day Repeated Feeding
to Albino Rats. Unpublished report from Central Medical Department of
American Cyanamid Company, submitted to the World Health Organization
by American Cyanamid Company, Wayne, NJ, USA.
Lichtenstein, E.P., Fuhremann, T.W., Schulz, K.R. and Liang, T.T.,
(1973) Effects of Field application methods on the persistence and
metabolism of phorate in soil, and its translocation into crops. - J.
Econ. Entomol. 66 (4) 863-866.
Lichtenstein, E.P., Fuhremann, T.W. and Schulz, K.R. (1974)
Translocation and metabolism of Phorate as affected by percolating
water in a model soil/plant ecosystem. J. Aric. Food Chem. 22 (6)
991-996.
Lindley, C.D. (1963) The use of phorate granules on potatoes and
carrots Proc. 2nd. Conf. Brit. Insect. & Fangic. Brighton, 1963.
McLeod H.A., Malkins, G. and Rao, S.L.N. (1969) Gas chromatographic
analysis of phorate and five metabolites in monkey liver
homogenates - Bull. Env. Contam. and Toxicol. 4 (4) 224-231.
Maskell, F.E. Gair, R., Williams, J.H., and Cooper, B.J. (1974) Effect
of phorate placement on carrot fly control, seedling density and
terminal residues in the crop. Pestic. Sci. 5 543-548.
Menzer, R.E., Fontanella, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Envir. Contam. & Toxicol. 5 (1) 1-5.
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
Dithio-Systox and Thimet. J. Econ. Entomol. 50 (3) 338-345.
Mitchell, T.H., Razioka, J.H., Thomson, J. and Weals, B.B. (1968) The
chromatographic determination of organophosphorus pesticides. Part
III. The effect of irradiation on the parent compounds. J. Chomat.
32, 17.
Nelson, R.C. (1967) Procedure for nine organic phosphorothioate
pesticide residues on trials and vegetables by using micro-coulometric
gas chromatography. J. Assoc. Off. Analyt Chem. 50: 922-926.
Parker B.L. and Dewey, J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based m toxicity to Drosophilia - J. Econ.
Entomol. 58 (2) 106-111.
Rao, S.L.M. and McKinley, W.J. (1969) Metabolism of Organophosphorous
Insecticides by Liver Homogenates from Different Species. Canadian
Journal of Biochemistry, 47: 1155-1159.
Razicka, J.B., Thomson, J. and Weals, B.B. (1967) J. Chromat. 30,
92.
Razicka, J.B., Thomson, J. and Weals, B.B. (1968) J. Chromat. 33,
430.
Sans, W.W. (1967) Multiple Insecticide Residue Determination Using
Column Chromatography, Chemical Conversion, and Gas Liquid
Chromatography. J. Agr. Food Chem. 15, 192.
Satpathy, J.M., Dash, P.K. and Misra, B. (1974) Insecticidal residues
in some vegetables crops grown in treated soils. Indian J. Agric. Sci,
44 (2) 113-115.
Schulz K.R., Lichtenstein, E.P., Fuhremenn, T.W. and Liang, T.T.
(1973) Movement and Metabolism of phorate under field conditions after
granular band applications J. Econ. Entomol. 66 (4) 873-875.
Shaffer, C.B., West, R. and Golz, H.E. (1958) Thimet: Single Dose
Toxicity to Rats. Joint Toxic Action with Other Phosphates.
Unpublished report from Central Medical Department, American Cyanamid
Company, submitted to the World Health Organization by American
Cyanamid Company, Wayne, NJ, USA.
Singh, K. and Gulati, K.C. (1971) Studies on the persistence of
phorate and di-system in soil and plants. Proceedings of the First
All-India Symposium on Progress and Problems in Pesticide-Residue
Analysis held at Ludhiana on 18-19 November 1971 p. 143-149.
Singh, Kalyan and Gulati, K.C. (1972) Application of enzymatic method
for the estimation of organophosphorus insecticides and their residues
in soil and plant. Part II. Phorate. Indian J. Agr. Chem. 6 103-107.
Stanley, C.W. and Morrison, J.T. (1969) J. Chromatogr. 40, 289.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analogue by total phosphorus determination
after isolation by thin-layer chromatography. J. Assco. Off. Agric.
Chem. 47, (4) 645.
Stern, V.M. (1971) Summary of Thimet residues in barley. Report from
University of California, Riverside (undated).
Suett, D.L. (1971) Persistence and degradation of chlorfenvinphos,
diazinon, forofos and phorate in soils and their uptake by carrots.
Pestic. Sci. 2, 105.
Suett, D.L. (1974) Uptake of chlorphyrifos and phorate from soil by
carrots as influenced by mode of application and cultivar. Pestic.
Sci. 5, 57-71.
Suett, D.L. (1975) Persistence and degradation of chlorfenvinphos,
chlormephos, disylfoton, phorate, and pirimiphos - ethyl following
spring and late-summer soil application. Pestic. Sci. 6, 385-393.
Talekar, N.S., Lian-Tien Sun, Ehi-Min Lee and Jing-Sing Chen. (1977)
Persistence of some insecticides in subtropical soil. J. Agric. Food
Chem. 25 (25) 348-352.
Tusing, T.W., Kindzin, W., Hanzal, R. and Howard, J. (1956a) Repeated
Oral Administration (Dogs) Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. and Howard, J.
(1956b) Subacute Feeding Study - Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Van Middlelem, C.H. and Baranowski, R.M. (1962) Phorate residues in
tomato fruit and foliage. J. Econ. Entom. 55 (5); 600-603.
Waller, J.B. and Dahm, P.A. Oxidation of phorate in three Iowa soils -
Manuscript from (1973) Iowa State University, Ames, Iowa USA.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for amny organophosphorus pesticide
residues in crop extracts. J. Assoc. Off. Analyt. Chem., 52: 522.
Watts, R.R. and Storherr, R.W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Off. Analyt. Chem. 52, 513-521.
Wit, S.L. (1959) Residu's van systemische middelen bij aardappelen,
etc. - Rijksinstituut voor de Volksgezondheidg Utrecht, FT/6/59 dated
25.4.59.
Wit, S.L. (1961) Residu's van enige systemische insecticiden bij
aardappelen - Rijksinstituut voor de Volksgezondheid, Utrecht,
FT/52/61 dated 29.5.61.
Wit, S.L. (1962a) Onderzoek naar de residu's van enige systemische
middelen bij aardappelen- Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 29/62 dated 29.2.62.
Wit, S.L. (1962b) Onderzoek naar de residu's van Thimet toegepast als
systemisch zaardinsecticide ter bestryding van de vergelingszickte bij
sieten - Rijksinstituut voor de Volksgezondheid, Utreohty Report Tox.
10/62 dated 30.1.62.
Wit, S.L. (1963) Onderzoek naar de residu's van twee systemische
insecticiden bij aardappelen - Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 113/63 dated 25.6.63.
Wit, S.L. (1969) Foraat op zomerwortelen - Rijksinstituut voor de
Volksgezondheid, Utrecht, Report Tox. 59/69 dated May 1969.
Zakil M.M. and El-Sayed, M.M. (1968) Accumulation of systemic
insecticides in mature cotton seeds. Bull. Ent. Soc. Egypt. Econ. Ser.
II 121-127.
chloroform and acetone (Bowtan and Casida, 1958) (See also "Fate of
residues", "In animals").
Bean plants were dipped into a 500 ppm P-32 phorate solution prior to
planting to determine the metabolism of phorate by plants. The primary
metabolites were phorate sulphoxide and/or phorate sulphone. Small
amounts of phoratoxon sulphoxide, phoratoxon sulphone and unchanged
phorate were also detected. No phoratoxon was detected. The hydrolysis
products formed were phosphoric acid and the diethyl esters of
phosphoric acid, phosphorothioic acid and phosphorodithioic acid
(Bowman and Casida, 1958).
Other work on the biotransformation of phorate in plants is described
in "Fate of residues", "In plants".
Effects on enzymes and other biochemical parameters
The anticholinesterase activities of phorate and its metabolites are
shown below (Table 1).
TABLE 1. Comparison of anticholinesterase activity of phorate
metabolites
Anticholinesterase activity
(p/50)*
Phorate 3.17
Phorate sulphoxide 3.35
Phorate sulphone 5.00
Phoratoxon 5.87
Phoratoxon sulphoxide 6.76
Phoratoxon sulphone 7.02
*p/50 + negative logarithm of molar concentration producing 50%
inhibition of red blood cell cholinesterase.
In rats, at dietary levels above 0.66 ppm, phorate depressed plasma,
erythrocyte and brain enzyme activity (Tusing et al., 1956b). Phorate
sulphoxide at dietary levels greater than 0.32 ppm also produced
depression of cholinesterase in rats in varying degrees during a 90
day studY (Levinskas et al., 1968). In dogs, plasma and erythrocyte
cholinesterase activity was depressed at levels above 0.01 mg/kg/day
(Tusing et al., 1956a). Dietary feeding at 1.0 ppm for 6 weeks
produced no effect in dogs (Kay and Calandra, 1961).
TOXICOLOGICAL STUDIES
Special studies on potentiation
Young rats weighing between 90 and 120 g (5 males/4 dosage levels)
were used to investigate the potentiation of phorate with diazinon,
EPN, azinphos-methyl, malathion, parathion-methyl, parathion,
mevinphos, demeton, tetram and carbophenothion. Oral LD50 values
were determined for each pesticide under identical conditions. Each
pair of compounds was mixed in approximately equitoxic proportions,
i.e., in about the ratios of their LD50's.
Results, given as LD50 in mg/kg are as follows:
Diazinon (283,216); EPN (42.9, 25); azinphos-methyl (18.7, 8);
malathion (329, 178); parathion-methyl (14.2, 11); parathion (12.3,
8); mevinphos (7,4); demeton (6.2,4); tetram (14.2, 5);
carbophenothion (32.5, 29). In the case of every mixture, the observed
LD50 indicating no potentiation (Shaffer et al., 1958).
Special study on neurotoxicity
Groups of 6 adult hens were fed 0 and 40 ppm phorate in the diet for 4
weeks. A third group received 4000 ppm TOCP as a positive control.
Each hen was anesthetized, immediately perfused with buffered
formalin, and sections of brain, lower thoracic cord and each sciatic
nerve prepared for microscipis examination. The TOCP produced myelin
loss in each hen. Phorate produced no adverse effects on nerve fibres
or the myelin sheath (Levinskas et al., 1965).
Special study on mutagenicity
Ten pesticides, including phorate, were tested for dominant lethal
effects. A group of 20 male mice were fed for 7 weeks at three dose
levels of phorate (levels not reported). Reference controls and a
positive control receiving triethylenemelamine in drinking water for 4
weeks were included in the test. Following treatment each male was
mated to two females weekly for 8 weeks. No consistent response
occurred to suggest that phorate in the diet for 7 weeks induced
dominant lethal effects in mice (Jorgenson et al., 1976).
Special study on reproduction and teratology
Groups of mice (8 males and 16 females/group) were fed phorate in the
diet at levels of 0, 0.6, 1.5 and 3.0 ppm during a 3-generation, 2-
litter/generation, reproduction study.
There were no dose related effects on the fertility, gestation,
viability or lactation indices during the study, but there was a
lowering of the lactation index in the 3.0 ppm group. This value fell
below the control value in the first mating of the F0 generation, in
both matings of the F1 and in the second mating of the F2
generation.
Gross and microscopic examination of tissues and cleared pups revealed
no consistent abnormalities related to feeding of phorate (Anonymous,
1965).
Acute Toxicity
TABLE 2. Acute toxicity of phorate
LD50
Species Route, Sex (mg/kg) Reference
Rat Oral M 2.3 Gaines (1969)
F 1.1
Rat Oral M 2.8 Anonymous (1976)
F 1.6
Rat Dermal M 6.2 Gaines (1969)
F 2.5
Rat Dermal M 5.7 Anonymous (1976)
Rabbit Dermal M 5.2
Short term studies
Rat
Groups of rats (50 males and 50 females/group) were fed 92% phorate,
in corn oil, in the diet at levels of 0, 0.22, 0.66, 2.0 and 6.0 ppm
for 13 weeks. Two groups (25 males and 25 females/group) were fed 12
and 18 ppm for 8 and 2 weeks respectively. Cholinesterase activity was
monitored at weekly intervals. Occasional episodes of excitability and
intermittent tremors were noted in the 6.0 ppm female group. Both
sexes receiving the 12 and 18 ppm levels exhibited severe
excitability, intermittent tremors and ataxia culminating in death.
Levels of 6 ppm and higher produced significant depression in both
sexes of plasma, red blood cell and brain activity. At 2 ppm female
red blood cell activity was also depressed. Levels at 0.66 ppm or
below had no effect on cholinesterase activity. Growth, food
consumption, survival, liver and kidney weights and ratios, gross
necropsy and histological findings for all groups at 6 ppm or below
were within normal limits. Levels of 12 and 18 ppm severely affected
growth and survival (Tusing et al., 1956b).
Groups of rats (50 males and 50 females/control and 35 males and 35
females/test groups) were fed the sulphoxide of phorate (92% purity)
for 90 days at levels of 0, 0.32, 0.80 and 2.0 ppm. Brain, erythrocyte
and plasma cholinesterase activities were determined biweekly. At the
2.0 ppm level a significant (P <0.05) depression in erythrocyte and
brain cholinesterase activity occurred in females. Plasma activity
depression at this level was less consistent and judged as borderline.
The 0.8 ppm level produced borderline depression of the erythrocyte
enzyme activity. At 0.32 ppm all values were within acceptable
statistical limits for both sexes. No effects were observed on other
hematological values, organ weights or ratios and no consistent
microscopic pathology was noted which could be attributed to the
feeding of 2 ppm or less of the sulphoxide of phorate (Levinskas et
al., 1968).
Dog
Groups of mongrel dogs (2 males and 1 female/group) received by
capsule 0, 0.01, 0.05, 0.25 and 1.25 mg/kg/day of 92% phorate in corn
oil 6 days/week for 15 weeks. Two males received 2.5 mg/kg as a single
dose. Plasma and red blood cell cholinesterase activities were
monitored at weekly intervals. The 0.05 mg/kg/day level produced a
significant depression in plasma enzyme activity. The red blood cell
activity was not affected for the first 12 weeks but was depressed
slightly (not significantly) during the last 3 weeks of the study.
Significant plasma and red blood cell enzyme depression was produced
by the 0.25 mg/kg/day level. The 1.25 mg/kg/day level produced
complete inhibition of plasma cholinesterase activity and a
significant reduction in red blood cell activity. At the 2.5 mg/kg/day
level all dogs died within 3-4 hours following a single dose and no
enzyme activity determinations were made. No signs of systemic
toxicity in dogs receiving the 0.01 or 0.05 mg/kg/day doses (Gaines,
1969). Histopathological examination revealed no consistent findings
related to the test/material (Tusing et al., 1956a). Beagle dogs (3
males and 3 females/group) were fed in the diet 0, 0.5, or 1.0 ppm
phorate for 6 weeks. Cholinesterase activity of plasma and
erythrocytes was determined for each dog prior to and at biweekly
intervals during the test. Analyses of variance conducted upon plasma
and erythrocyte enzyme activities revealed no significant difference
between the test animal and control animal groups during the test
period (Kay and Calandra, 1961).
Long term studies
No information available.
Observation in humans
No information available.
COMMENTS
Phorate, an organophosphorous pesticide, is a derivative of
dithiophosphoric acid. Less than 40% of a 2 mg/kg oral dose to rats
was excreted in 6 days, and brain, liver and kidney contained
unextractable residues. The major metabolites in both plants and
mammals are the sulphoxides and sulphones of phorate and of its oxygen
analogue, phora-toxon; these have greater anticholinesterase activity
than phorate. Hydrolysis of phorate itself is a minor metabolic
pathway in mammals.
The biochemical data available on phorate are inadequate to determine
whether the oxidative metabolites are retained in mammalian tissues or
organs. Toxicological data submitted are insufficient, except for
cholinesterase inhibition, to determine the short and long term
effects of phorate and its oxidative metabolites. Although the Meeting
considered setting a temporary ADI for humans this could not be
established in the absence of long-term studies. Further studies are
required to evaluate a.) carcinogenic potential, b) teratogenic
potential, c) potential neurotoxicity and d) toxicity of metabolites.
Observations in human subjects are desirable.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Phorate is a powerful soil insecticide with excellent systemic
properties. A wide spectrum of insect and free-living nematode pest
problems is controlled by a seed-bed or a side-dressing treatment.
Seed-bed applications protect the germinating seedlings and young crop
stages against a wide range of pests.
Granular formulations of phorate have been extensively field tested
throughout the world since 1954 and are used commercially in many
countries. Major uses are summarized in Table 3. Recommended dosage
rates vary somewhat from country to country, depending mainly on the
pest complex to be controlled and the period of protection required.
Although application is normally made in the furrow at seeding or
planting time, certain pest problems require the application of
granules as a side-dressing at the time of subsequent cultivation.
There are some situations where, in order to control vectors of virus
diseases, it is necessary to make a broadcast treatment with granules
or a foliage application of emulsifiable concentrate to the growing
crop. Preharvest intervals and limitations on grazing vary somewhat
but for the most part they are relatively long because of the residual
life of phorate in treated plants.
In addition to its use on food and forage crops phorate is used on
ornamentals and tobacco and in forest nurseries where sucking insects
are a problem.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Extensive trials have been carried out by the manufacturers and by
independent investigators to determine the level and fate of phorate
residues in virtually every crop on which the insecticide in used.
Copies of trial reports and published scientific papers were provided
to the Meeting by the manufacturers (American Cyanamid, 1977). A
representative selection of the data from these reports has been
summarized in Table 4. The following observations are made on the
detailed data.
TABLE 3. Phorate: use-pattern
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Alfalfa 1 seed treatment 60 days
1.5-2 broadcast 35 days
(grazing)
Barley 1 broadcast-soil incorporated 60 days
Beans 1-1.5 in furrow at planting 60 days
broadcast before flowering -
Brassicas 16g/100m row in furrow at transplanting -
Carrots 1.7-3.3 at drilling time -
TABLE 3. (Continued)
Crop Dosage(kg/ha) When and Where Applied Limitations
and
pre-harvest
Intervals
Cotton 0.2-0.5 seed treatment do not use
seed for food
0.3-1.5 furrow application do not graze
Hops 1-1.5 foliage application 25 days
2-3 band application 42 days
Lettuce 1 at transplanting or
seeding -
1 foliage treatment 18 days
Maize 1-2 in farrow at planting -
1 band application
at cultivation 30 days
1 foliage application 30 days
Onions 12g/100m row in furrow at transplanting -
Peanuts 1-1.5 in furrow at planting do not graze
or feed
2 band application at pegging do not graze
or feed
Peppers 1 in furrow at transplanting -
Potatoes 1.5-3 in furrow at planting 90 days
Sorghum 2 In furrow at planting -
0.5-1 foliage application 28 days
Sugar beets 0.5-1 in furrow at planting -
1-1.5 foliage application 30 days
Sugar cane 4 in furrow at planting -
Tomatoes 1-2 at planting or
transplanting -
Wheat 1 in furrow
1 broadcast 70 days
Sunflower 1-1.5 in furrow at planting -
TABLE 4. Phorate residues resulting from supervised trials.
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
ALFALFA USA 1966 1 1 granule <0.01 <0.01 <0.01 <0.01
(dry) 1 2 10% <0.01 <0.01 <0.01 <0.01
1 4 " 0.06 0.06 0.03 0.02
1 1 " 0.06 0.08 0.71 0.37 0.21
1 2 " 0.01 0.01 0.35 0.46 0.07
1 1 " 0.05 <0.01 0.01 0.01 0.01
1 2 " 0.15 0.02 0.02 0.02 0.01
APPLES Italy 1970 2 1.5 E.C. <0.02 <0.02 <0.02 <0.02
Argentina 1972 1 2 granule 0.01
1 3 " 0.01
BARLEY USA 1967 1 4 " <0.01
(grain) 1 1 " 0.01
BARLEY USA 1967 1 2 " <0.01
(straw) 1 1 "
1 2 0.04
BARLEY USA 1961 1 1 " 0.07 <0.04
(grain)
BEAN, SNAP USA 1966 1 1 " 0.5 0.07 <0.01
USA 1963 1 2 " 0.046 0.016
1 2 " 0.018 0.011
1 4 0.013 0.036
USA 1966 1 1 " 0.5 0.07 <0.01
1 1 <0.01
1 2 " <0.01
COWPEA India 1974 1 2 " 1.46 1.05 0.53
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
EGGPLANT India 1974 1 2 " 1.27 1.01 0.69
MAIZE USA 1970 1 0.5 E.C. 0.068 0.024 <0.020
(plant) 1 " 0.131 0.046 0.007
2 " 0.695 0.110 0.019
S. Africa 1977 1 0.5 granule 0.4 0.1 <0.02 <0.02
1 " 2.8 0.9 0.03 0.03
0.5 " <0.02 <0.02 <0.02
1.0 " 0.06 <0.02 <0.02
0.5 " 0.5 <0.02 <0.02
S. Africa 1977 1.0 granule 0.8 0.03 <0.02
MAIZE S. Africa 1977 1 0.5 " <0.02
(grain) 1.0 " <0.02
Italy 1971 2.5 " <0.02
14 30 40 60 90 120 150
CARROTS UK 1970 2 3 granule 1.01 0.88 0.47 0.64
3 3 " 0.29 0.40 0.39 0.27
2 3 " 0.41 0.41 0.24 0.19
3 1.5 " 0.19 0.09 0.09 0.23
Canada 1975 3 1 E.C. 0.26
4 1 E.C. 0.40
CELERY UK 1971 1 1.2 granule <0.05
1.5 " <0.05
COTTONSEED USA 1969 1 1 E.C.(seed) <0.05
1 1-2 granule <0.05
1 3 " <0-05
1 2 E.C. <0-05
Egypt 1968 5 1 G/E.C. 0.02 0.01
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 21 28 35 45 60 90
no. kg
a.i./ha
GRAPES Mexico 1973 1 4 0.000
1 6 0.000
1 8 0.007
HOPS USA 1968 1 4 granule <0.1 <0.1 <0.1
3 1 E.C. 0.1
LETTUCE USA 1977 1 2 5.14 0.24 0.17 <0.05
1963 1 1 <0-05
1963 1 2 <0.05
1963 2 0.025
PEANUTS USA 1964 2 1+2 granule <0.05 <0-05
(kernels) 2 1+2 " <0.05 <0-05
2 1+2 0.05 0.05
2 1+4 <0.05
1 2 " 0.1 0.08 0.05
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
PEANUT USA 1964 2 1+2 granule 0.05
(foliage)
POTATOES Czechoslovakia 1972 1 1.6 " <0.05
1 2.2 " <0.05
1 4.3 " 0.07
Turkey 1973 1 3.4 <0.05
Mexico 1972 1 1 E.C. 0.004 0.000
1 2 E.C. 0.006 0.000
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
RAPE SEED USA 1971 1 1.5 granule 0.05
RAPE HAY USA 1971 1 1.5 " 0.21
SORGHUM USA 1969 1 1 " <0.05 <0.05
(grain) 1 2 " <0.05 <0.05
SORGHUM USA 1969 1 1 " 0.06 0.06
(fodder) 1 2 " 0.16 0.08
SORGHUM Mexico 1972 1 1 E.C. 0.00 0.00
(grain) 1 2 E.C. 0.02 0.00
MILO USA 1967 1 1 granule <0.01 <0.01 <0.01
2 " <0.01 <0.01 <0.01
4 " 0.22 0.01 <0.01
SOYBEAN USA 1970 1 1 " <0.05 <0-05
2 " <0.05 <0.05
7.5 " <0.05 <0.05
SOYBEAN USA 1970 1 1 " <0.05 <0.05
(foliage) 2 " <0.05 <0.05
7.5 " 0.15 0.19
SUGAR BEET USA 1961 1 1 E.C. 0.05
2 E.C. 0.05
TOMATOES USA 1961 1 1 <0.05 <0.05
2 0.05 0.05
4 0.06
WHEAT USA 1969 1 1 <0.05
(grain) 1 2 <0.05
TABLE 4. (Continued)
Application Residues (mg/kg) at intervals (days) after application
rate
Crop Country Year formulation 14 30 40 60 90 120 150
no. kg
a.i./ha
WHEAT USA 1969 1 1 1.4 0.35 0.33
(plant) 1 2 2.7 1.6 3.0
1 1 6.1 0.87 0.07
Alfalfa
Residue results obtained on fresh alfalfa and alfalfa hay grown in
various regions of the United States show that application at twice
the recommended dosage rate of 2 kg/ha does not result in residues in
excess of 0.5 mg/kg in or on fresh alfalfa or 1 mg/kg on alfalfa hay
within 35 days of treatment. Generally the level is less than the
limit of determination (0.01 mg/kg).
The results from a series of trials where phorate was applied to
alfalfa at rates ranging from 2 through 3 to 4 kg/ha indicate that the
half life in green alfalfa is between 8 and 18 days.
Apples
Results from spray trials carried out in Italy where 7.59 of phorate
was applied per tree on one, two and three occasions prior to harvest
show that the residues in apples 30 to 80 days after the last
application were generally less than 0.02 mg/kg of Phorate and
metabolites. One sample from a tree treated 30 days previously showed
0.06 mg/kg phorate sulphone only.
Residue analysis for phorate and metabolites in several varieties of
apples treated in Argentina with phorate granules applied in
incorporation in the soil around the stem at doses ranging from 10-20g
of phorate per tree indicated no residues of parent compound or
metabolite in fruit collected 182 days after application.
Barley
Experiments in the USA where phorate granules were incorporated or
irrigated into the soil at planting time reveal that the residues of
phorate and metabolites in the grain at harvest 60 days later are
generally at or below the limit of determination (0.01 mg/kg). When
applied at twice the recommended rate (1 kg/ha) detectable residues
(0.04 mg/kg) were found in barley straw 60 days later.
Stern (1961) reported that phorate granules applied to barley fields
at the rate of 1 kg/ha one day prior to irrigation, produced
detectable residues in the barley plants (0.07 mg/kg) 14 days later.
The residues had declined below the limit of determination (0.04
mg/kg) by the 28th day following application.
Beans
Extensive field trials carried out during the period 1966-68 on many
varieties of beans under widely differing agricultural and ecological
conditions indicate that the residues are generally well below 0/05
mg\kg 60 days after application. The samples were analysed by the
oxidative cholinesterase inhibition technique which determines phorate
and metabolites. The limit of determination based on control values is
considered to be 0.05 mg/kg. The reports contain results from a few
isolated samples which indicate a total phorate residue of the order
of 0.1 mg/kg.
Carrots
Wit (1960) using analytical methods involving the determination of
total phosphorus showed that, following the application of phorate
granules at the rate of 1.25-1.5 kg/ha, carrots contained from 0.09-
0.19 mg/kg of residues derived from phorate applied 73-132 days
previously.
An extensive experiment carried out in the United Kingdom (Cyanamid
Great Britain Limited, 1971) determined residues of phorate and its
metabolites in carrots following treatment with phorate granules
applied with the seed and at varying times thereafter according to
various treatment codes. The highest residue level was approximately
1mg/kg some 5 weeks after the last treatment, but this declined to
0.64 mg/kg by the end of 4 months. Lower rates produced somewhat lower
residues but significant residues were detected from all treatment
regimes. The residues consisted of phorate and phorate sulphone with
no oxygen analogue or sulphoxide.
Finlayson et al (1976a) studied the distribution of a number of
insecticides used to control carrot rust fly in the peel and pulp of
carrots, measuring also the distribution throughout the length of the
carrot. These investigators found that the bulk of the residue was in
the peel in the top 3cm of the carrot, the total residue in the whole
carrot being of the order of 0.2-0.4 mg/kg. The peel at the top end
contained as much as 7 mg/kg. See also "Fate of residues".
Maskell et al (1974) showed that the application of granules
containing 10% phorate at 1, 2 or 4 kg/ha in a narrow slit together
with carrot seed gave rise to residues which could be reduced by 50%
by placing the granules 2.5cm below the seed. Residues resulting from
the application of 1 kg/ha with the seed or 2 kg/ha 2.5cm below the
seed were not significantly greater than residues in untreated
carrots.
When 4 kg of phorate per ha was applied along with carrot seed the
residue in carrots at harvest 8 months later was 0.4 mg/kg. When the
granules were placed 2.5cm below the seed the residue under
corresponding conditions was only 0.2 mg/kg.
Cottonseed
American Cyanamid Company (1969c) reported the results of 13 trials in
different regions of the USA aimed at determining what residues, if
any, occur in cottonseed following phorate treatment of seed pre-
planting or emulsifiable concentrate as a late season foliar
application. All samples were analysed by the gas-chromatographic
method. The data show less than 0.05 mg/kg phorate in cottonseed
harvested from crops treated at planting time or with a side-dressing.
Late season application with phorate emulsifiable concentrate at rates
up to 2 kg/ha did not show detectable residues in cottonseed from
crops treated 30 days prior to harvest.
Zaki and El-Sayed (1968) showed that residues in mature cottonseed
after spraying mature plants three times with phorate at the rate of 1
kg/ha were only 0.013 mg/kg 14 days after the fourth application of
foliage sprays.
Cowpeas
Satpathy et al (1974) showed that highly significant residues of
phorate could be found in the pods of cowpeas 10 and 20 days after
treatment of the soil with phorate granules applied at the rate of 2
kg/ha. The residues continued to decline with a half life of
approximately 12 days. Phorate residues were relatively more
persistent than those of diazinon and dimethoate but comparable to
disulfoton in cowpeas.
Grapes
The residue analysis of grapes harvested from plants growing in soil
treated with phorate granules at the rate of 4, 6 and 8 kg/ha showed
no residues at the two lower rates and only barely detectable residues
at the highest rate. Treatment had been made by incorporating the
granules into the soil at the time when the grape vines were
sprouting. Grapes were harvested 92 days later (Cyanamid
International, 1973b).
Hops
Kiligemagi and Terriere (1968 a,b) reported an extensive series of
trials designed to determine the level and fate of phorate residues in
hops following the application of phorate granular to the hop beds and
foliar applications at rates from 1 to 5 kg/ha and with pre-harvest
intervals ranging from 15 to 89 days. Using a gas-chromatographic
method specific for phorate and its oxygen analogue sulphone the
investigators were unable to detect, either the parent compound or
metabolites in fresh or dried hops even at the short pre-harvest
interval of 15 days. Aphid and spider mite control were, however,
excellent.
Maize
One of the major uses for phorate ban been the control of a variety of
insects on maize. In the early 1960s the manufacturers carried out,
extensive residue studies in many locations in the USA. Using
oxidative cholinesterase inhibition procedures it was shown that the
insecticide was taken up into the growing plant but that the residues
declined rapidly during the 45 days following application, by which
time residues in the whole plant were not significantly greater than
the apparent phorate residue in untreated control samples. By the time
the grain was ripe, no trace of phorate of other
cholinesterase-inhibiting substances could be found in the grain or
other parts of the plant.
Following the introduction of GLC methods capable of determining
phorate and its metabolites, a number of studies were carried out in
several countries beside the USA. These confirmed that the residue
levels declined to below the limit of determination between 28 and 40
days after the application of phorate granules or spray at rates up to
2 kg/ha. Leuck and Bowman (1970) published the results of one such
study which traced the occurrence and level of phorate and four
metabolites in maize plants treated with 0.5, 1 and 2 kg/ha of phorate
as a spray. These workers showed that the sum of all residues was less
than 1 mg/kg 14 days after the application of all three rates of
phorate.
Peanuts
Investigation in the USA (Diablo Laboritories 1964) show that the
application of phorate granules at planting and subsequent application
at picking does not result in apparent phorate residues different from
these found in untreated controls. The analyses were made using the
oxidative cholinesterase inhibition technique which produces a
positive result in untreated controls of the order of 0.05-0.1 mg/kg
apparent phorate. Samples of kernels from plants treated at the
highest rate did not show any residues higher then the controls when
collected 90 days after the last treatment. Where treatments were
applied 30 to 40 days prior to harvest, a positive residue of the
order of 0.1 mg/kg was found in peanut kernels. The residues in peanut
foliage after 90 days were not significantly different from untreated
controls.
Potatoes
Residue date developed prior to 1960 in support of petitions for the
registration of the use of phorate on potatoes were obtained with a
non-oxidative anti-cholinesterase method of analysis. This procedure
has good sensitivity for the oxygen analogue sulphoxide and sulphone
and, with the long pre-harvest interval involved, was considered
adequate at the time. Extensive residue information on potatoes was
obtained with this method, including results from inordinately high
rates of application, with all findings showing no measurable residues
in mature potatoes at harvest.
Owing to their relatively poor cholinesterase inhibiting properties,
phorate and its intermediary oxidative derivatives such as phorate
sulphoxide or sulphone would not have been measured by the
non-oxidative method had they been present. In 1959, an oxidation step
was introduced into the anti-cholinesterase procedure, which converted
phorate and its partially oxidised metabolites to phorate oxygen
analogue sulphone, and thus measured all toxic phorate residues. At
this stage a method based on the determination of total phosphorus
(Steller and Curry 1964) and including an oxidation step (Blinn 196A)
was utilized for further studies. By this method, which had a limit of
determination of the order of 0.01 mg/kg, it was shown that potatoes
treated at planting with phorate at the maximum recommended rate of 3
kg/ha generally contained 0.05 mg/kg or less when harvested 90 to 120
days later. However, residues as high as 0.15 mg/kg were occasionally
found following the use of phorate at the rate of 3 kg/ha. Following
the use of twice this amount (6 kg/ha) residues ranging up to 0.5 were
reported 100 to 140 days after application (American Cyanamid Company
1966).
In the Netherlands between 1958 and 1960, Wit (1959, 1961)
investigated the residues of systemic insecticides on potatoes, using
analytical methods involving the determination of total phosphorus
(limit of determination 0.1 mg/kg). He found substantial residues of
phorate in potatoes from 3 days to 42 days after application of
phorate granules (1.5-3 kg/ha). These residues ranged up to 3 mg/kg,
with the bulk of the results near 1 mg/kg.
Later Wit (1962a., 1963) using methods that determined phorate and the
sulphoxide metabolite (Laws 1961) showed that residues were less than
0.05 mg/kg 75-110 days after applying phorate granules at 2 + 3 kg/ha.
In 1972 following the development of a highly specific and
highly-sensitive analytical method employing gas-liquid chromatography
for the determination of phorate and phorate metabolites, an extensive
trial was carried out in Czechoslovakia (Cyanamid International
1972a). Residues of phorate or its oxygen analogue sulphone were not
measurable in any treatment. The only detectable residue in the
treated samples was of phorate sulphone, the highest level found being
0.07 mg/kg following the application of 3.4 kg/ha 120 days previously.
A similar field trial was carried out in Turkey (Cyanamid
International, 1973a), where phorate granules were applied at the rate
of 3.4 kg/ha at the time of planting and the potatoes harvested 125
days later. None of the potatoes contained residues of phorate or any
metabolite at levels above the limit of determination (0,01 and
0.02 mg/kg). It was concluded that the total residue of phorate and
metabolites was less than 0.05 mg/kg.
The application of phorate spray to potato Plants at rates to 1 and 2
kg/ha in Mexico (Cyanamid International, 1972b) produced detectable
residues in the tubers which declined from 0.02 mg/kg on the day
following application through 0.002 mg/kg on day 20 to 0 on day 27.
These results indicate that only small amounts of phorate are
translocated from the leaves to the tubers, and that these residues
decompose rapidly.
Sorghum and milo
Extensive trials were carried out in two different regions of the USA
to determine residues of phorate and its metabolites in sorghum fodder
and grain following granular and spray treatments. Samples of fodder
and grain were collected at intervals from 28 to 46 days after
application. The samples were analysed by gas-liquid chromatographic
procedures with a sensitivity of 0.05 mg/kg. All residues were below
0.05 mg/kg except fodder from one location which had been treated with
granular formulations and sampled after 31 and 46 days (American
Cyanamid Company, 1969a).
In an experiment carried out in Mexico, phorate spray was applied to
sorghum at the rate of 1 and 2 kg/ha 28, 21, 14, 7 and 0 days before
harvest, the residues found on the grain immediately after application
on the day of harvest were 0.55 and 0.82 mg/kg respectively. Though of
the order of 0.1 and 0.2 mg/kg could be found on the grain treated 7
days before harvest, that treated 14, 21 or 28 days prior to harvest
contained none as determined by a method sensitive to 0.01 mg/kg.
Soybeans
Studies carried out in the USA (American Cyanamid Company, 1970)
demonstrate that phorate granules used at the rate of 1 and 2 kg/ha
and applied in the furrow or to the side of the seed at planting will
result in no detectable residues (less than 0.05 mg/kg) of phorate
plus its toxic metabolites in soybeans seed at harvest. These same
studies showed no residue in soybeans treated at 7.5 kg/ha at two
separate locations and it is therefore reasonable to assume that no
detectable residues will occur in either soybeans oil or meal
following the use of phorate at the rate of 1-2 kg/ha.
Sugar beet
In 1960 and 1961 the manufacturers carried out or sponsored many field
trials to determine the level and fate of phorate residues in sugar
beet roots and leaves. Neither phorate nor its metabolites were
detected in any samples of sugar beet roots or tops in any trial. The
use of phorate sprays on sugar beet is approved in several countries
and maximum residue limits have been established to permit the feeding
of beet foliage to livestock.
Wit (1962b) reported that sugar beet roots and sugar beet leaves
contained less than 0.1 mg/kg or phorate-derived residues
(phorate + sulphoxides and sulphones) when phorate granules were
applied at the rate of 2-3 kg/ha 120-140 days before harvest.
Tomatoes
Extensive experiments carried out on tomatoes grown in various regions
of the United States during the period 1958-1961 show that following
the recommended application of phorate granules at the rate of 3 kg/ha
at planting time, no significant residues of either phorate or its
oxidative metabolites are found in either mature green or pink ripe
tomatoes. Similar results were also found at the 5 kg/ha. rate. Some
residue is found in the tomato foliage during the first few weeks
following application.
Wheat
Several studies were conducted in 1969 (American Cyanamid Company,
1969b) in the wheat growing areas of the United States to determine
phorate residues in green plants and grain following spring and fall
planting-time applications of phorate granules at rates of 1 and
2 kg/ha. The samples were analysed by GLC methods. The data show
phorate residues to be less than 1 mg/kg in all plant samples
approximately 45 days following application of phorate granules at a
rate of 1 kg/ha. No detectable residues (less than 0.05 mg/kg) were
found in grain harvested 120 to 330 days after treatment.
FATE OF RESIDUES
In animals
32P-phorate was given to a cow by Bowman and Casida (1958) in a
toxic dose of 3.04 mg/kg body weight, the acute symptoms that appeared
being controlled with atropine injections. The blood level increased
within 6-8 hours. After 18-36 hours the content of chloroform-soluble
metabolites in the blood increased slightly. The cow excreted 59% of
the radioactive material in the urine within 72 hours. None of this
was chloroform extractable. The main urinary metabolite was initially
0,0-diethyl phosphorothioic acid and subsequently diethyl phosphoric
acid, excretion of. 0,0-diethyl phosphorodithioic acid was
consistently low over the entire period. Only 0.8% of the applied
radioactivity appeared in the faeces within 96 hours after application
and 10% after 12 hours. The chloroform-soluble products were phorate
sulphoxide and/or sulphone, together with some phorate.
Examination of the cow's tissues 96 hours after feeding showed that
the liver and kidneys contained the highest 32P concentration (10.3
and 5.2 mg/kg phorate equivalents respectively). In contrast, the loin
muscle tissue contained 0.29 mg/kg and the mesenteric fat 0.03 mg/kg
phorate equivalents. The lung contained 1.5 mg/kg. Appreciable amounts
of phorate-derived phosphorus was found in the rumen and omasum walls,
other glands and rib bones. Milk contained 0.07, 0.25, 0.3 and
0.53 mg/kg phorate equivalents at 8, 24, 32 and 56 hours after dosing,
respectively.
Phorate is attacked only slowly by the rumen fluid (Ahmed et al.,
1958).
Phorate was administered to calves at a rate of 0.1 mg/kg for 14 days
to determine the level of phorate residues in liver, kidney, blood,
muscle, heart and fat tissues. No detectable residues ( < 0.1 mg/kg)
were found. Phorate was administered to lactating dairy cows at rates
of 0.05 and 0.1 mg/kg for 14 days. No detectable residues of phorate,
(< 0.02 ppm ) were found in the milk of cows fed 0.05. The milk
from cows fed 0.1 mg/kg contained from <0.02 to 0.05 mg/kg Phorate
(Anonymous, 1961).
Bunyan and Taylor (1966) found practically no phorate in meat and
organs of pheasants after administration of 6.2 to 21.7 mg/kg body
weight. In other investigations with pheasants and pigeons Bunyan et
al (1969) also found no residues after feeding for 14, 28 and 42-day
periods with 100 ppm of phorate in the diet.
Experiments designed to determine the total phorate residues in
chicken tissues and eggs were carried out by American Cyanamid
(1969d). Laying hens were dosed with rations containing 1, 0.3 and
0.1 ppm total phorate (1: 1, phorate: phorate oxygen analogue
sulphone) for 21 consecutive days. The chickens were sacrificed 2-3
hours after the final dose. Egg samples were collected during the
final day of treatment. The samples of muscle, fat, liver, kidney and
eggs were analysed by gas-chromatographic procedures using a caesium
bromide thermionic detector: phorate and its oxidative metabolites
were oxidised to and measured as phorate oxygen analogue sulphone and
calculated as total phorate. The sensitivity of the method for both
phorate and the oxygen analogue sulphone was 0.05 mg/kg. None of the
samples showed any apparent residues of phorate above this level.
In plants
Since 1954, studies of the metabolism of phorate in plants have been
conducted by investigators at American Cyanamid Company and by
researchers in various parts of the world. It has been shown that when
absorbed by plants, phorate is initially and rapidly oxidised to its
sulphoxide and sulphone which in turn are converted to the sulphoxide
and sulphone of the phorate oxygen analogue (Blinn, 1964; Bowmann and
Casida, 1957, 1958; Galley and Foerster, 1976; Krueger, 1975;
Lichtenstein et al., 1974; Metcalf et al., 1957; Zaki and El-Sayed,
1968).
The sequence of oxidative reactions in the metabolism of phorate
following absorption by plants is similar to that in animals and is
given in Figure 1. The oxygen analogue, although presumably formed as
an intermediate, has not been detected.
Bowman and Casida (1957) showed that when phorate was used as a
systemic insecticide for seed treatment of cotton, the metabolites
within the plant consisted of the sulphoxide and sulphone of the
parent compound and of its oxygen analogue. Cotton seeds treated with
phorate at concentrations as high as 32 kg of phorate per 100 kg of
seed showed less than 0.03 mg/kg of phorate or metabolites in the
seeds maturing an plants grown from such treated seed. The residual
persistence following soil and foliage application was studied with
six vegetable crops using radioactive phorate. Table 5 shows the
persistence of radioactivity in vegetable crops treated with
32P-phorate and is derived from the means of values from beans,
beets, cabbage, carrots, lettuce and peas treated by foliage
application and by soil application. Other experiments by Boman and
Casida (1958) on the fate in beans are described previously
(Biotransformation).
Phorate is usually applied as a soil treatment where its outstanding
systemic properties can be utilized to protect the developing plants
from attack by sucking insects for the first few weeks of their
growth. After phorate is applied to the soil and reaches the root zone
of the plant, the insecticide is absorbed by the roots and is
subsequently translocated to aerial parts of the plant. The rate of
movement of phorate from roots to stems and leaves is accelerated by
increased transpiration, and the greatest accumulation of insecticide
in leaves seems to occur under environmental conditions favourable to
transpiration (Hacskaylo et al., 1961). Van Middlelem and Baranowski
(1962) found that the highest concentrations of phorate in tomato
plants usually coincided with preceding periods of relatively high
field temperatures and ample rainfall. Krueger (1975) showed that
soybean root homogenates oxidised phorate to phorate sulphoxide.
Neither the oxygen analogue nor sulphone was detected.
Finlayson at al. (1976a), investigating methods for controlling carrot
rust fly, showed that phorate spray applied over the young carrot
seedlings was readily taken up and held in discrete portions of the
carrot. More than 50% of the residues were located in the top 0-3cm of
the carrot. Total residues in whole carrots generally increased with
higher rates and numbers of applications. Residues were greater in the
peel than in the pulp. Discarding the top 1-2cm of the carrot and
peeling the rest removes most of the residues. See also "Residues
resulting from supervised trials".
In soils
After phorate is applied to the soil, its eventual distribution and
utilization by plants is influenced by various constituents and
qualities of the soil.
In a study of the fate of phorate in soils, Getzin and Chapman (1960)
used radioactive material. One hour after treating various soil types,
these investigators found that 25, 20, and 10 per cent of the
radioactivity applied had been lost through volatilization from sandy
soil, silt-loam and muck respectively. After one hour, little or no
volatilization occurred. They further reported that phorate, when
applied to soil, is partially oxidised, hydrolyzed and bound to the
soil.
It has been shown that phorate moves readily in soil in the vapour
phase (Etheridge and Burt, 1963) as well as in drainage water (Bardner
at al., 1963).
Lindley (1963) reported from field experiments that on mineral soils
less phorate was required for insect control than on peat soils with a
maximum of 35% organic matter, while only partial control of insects
was obtained on soils with more than 35% organic matter, even when
much higher rates of insecticide were used.
Lichtenstein et al, (1973, 1974) showed that while phorate could be
detected in soil, only metabolites were found in plants including
plant roots. Schulz et al. (1973) showed that phorate moved in both
vertical and horizontal directions after a granular band application
of insecticide in soil. Suett (1975) showed that soil temperature had
relatively less effect upon the fate of phorate than on other
soil-incorporated insecticides.
Waller and Dahm (1973) showed that the conversion of phorate to its
sulphoxide in soils is mainly a non-biological process, whereas the
conversion to sulphone is brought about by micro-organisms.
Talekar at al. (1977) in a long-term experiment investigated the
persistence of a number of insecticides following repeated seasonal
application to soil under sub-tropical conditions. They showed that
phorate degraded more rapidly than all the other materials showing
almost complete loss within four months, although measurable
quantities of phorate sulphoxide and sulphone could be detected for
somewhat longer periods.
TABLE 5. Persistence of 32P-phorate and its metabolites in vegetable cropsa.
Days following application
0.1 1 2 4 8 17 32
Foliage application
Phorate equivalentb, mg/kg
Hexane fraction 6.75 2.84 2.49 1.69 0.90 0.44 0.040
Acetone-water fraction 2.60 2.39 1.66 1.45 0.59 0.067 0.003
Hydrolysis productsc 0.28 0.37 0.62 0.85 1.33 0.43 0.27
Unextracted residued 1.44 1.03 0.81 1.62 1.54 1.00 2.28
AntiChE activity, 50% inhibitione
Plant tissue, g 1.3 0.47 0.59 0.54 1.0 >2.2 >2.2
pl50 metabolites 5.44 6.14 6.29 6.62 6.11 <7.22 <7.42
Soil application
Phorate equivalent, mg/kg
Hexane fraction 0.82 0.34 0.40 0.26 0.15 0.049 0.026
Acetone-water fraction 0.12 0.31 0.56 0.36 0.18 0.047 0.005
Hydrolysis products 0.052 0.12 0.13 0.24 0.25 0.21 0.110
Unextracted residue 0.15 0.16 0.42 1.20 0.46 0.11 0.078
AntiChE activity, 50% inhibition
Plant tissue, g 1.8 0.94 1.3 1.2 1.3 >2.9 >3.0
pl50 metabolites 6.89 7.26 6.66 6.72 7.00 7.15 7.22
a Results are average of figures from beans, beets, cabbage, carrots, lettuce and peas in same field plot.
b Radioactivity of phorate or metabolites appearing in fraction indicated, expressed as phorate (mg/kg)
c Water-soluble products not extracted into chloroform.
d ssP remaining in solid portion after both water and chloroform removed.
e Results based on chloroform-soluble metabolites. Amount of plant material containing enough
metabolites for 50% inhibition and pl50 (negative logarithm of molar concentration effecting 50% inhibition)
of these metabolites are reported.
In processing and storage
Leuck and Bowman (1970) showed that residues of phorate and five of
its metabolites remained virtually unaltered when treated corn foliage
was converted into silage and ensiled at 35C° for 30 days.
Askew at al. (1968) examined the effect of 30 minutes boiling on
vegetables containing various organophosphorus pesticide residues.
They demonstrated 100% hydrolysis of phorate residues (2 mg/kg) in
both potatoes and cabbages. They indicated that appreciable quantities
of phorate sulphoxide ware formed during the cooking process, but did
not attempt to quantify the transformation.
EVIDENCE OF RESIDUES IN COMMERCE OR AT CONSUMPTION
Advice from the US Food and Drug Administration indicates that during
1975 three samples of strawberries were found to contain phorate
residues at levels of 0.07, 0.16 and 1.73 mg/kg. There was no
tolerance for residues of phorate in strawberries.
METHODS OF RESIDUE ANALYSIS
Gas chromatography with an electron-capture detector was used by Egan
et al. (1964) to measure phorate in certain plant tissues. Dewey and
Parker (1965) used an electron-capture detector to measure phorate and
some of its metabolites in soil. Bache and Lisk (1966) reported the
use of an emission spectroscopic detector in soil analysis for phorate
and its metabolites but found very poor chromatographic response for
phoratoxon sulphoxide and sulphone. Mitchell et al. (1968) used gas
chromatography to study the effect of ultra-violet irradiation and
permanganate oxidation on phorate. These workers found it possible to
chromatograph phorate, phoratoxon and phoratoxon sulphone on an
Apiezon L column; quantitative recovery figures were not given,
however. Ruzicka, et al. (1967) used a caesium bromide thermionic
detector with an Apiezon column and found that phorate was not
adequately resolved from phoratoxon, although phoratoxon sulphone
could be resolved. The same workers (1968) later used similar
techniques to measure residues of phorate, phorate sulphoxide and
phorate sulphone on apple leaves. Sans (1967) described a
gas-chromatographic procedure for the separation and measurement of
phorate, but not its metabolites, from various other pesticides.
Nelson (1967) developed a procedure for separating and measuring
phorate, but not its metabolites, from several other organo-phosphate
insecticides in residues on fruits and vegetables.
Bowman et al. (1969b) described procedures for the individual
measurement of phorate and its oxidative metabolites in residues on
corn tissue. These workers used an instrument equipped with a flame
photometric detector and a column of 10% DCL-200 silicone on Gas-Chrom
Q. Recoveries at the 0.05 mg/kg level in excess of 96% were reported
for all of the metabolites except phoratoxon (62% recovery). McLeod et
al. (1969) reported a procedure for the separation and measurement of
phorate and its metabolites from monkey liver homogenates, These
workers used a column packed with 5% diethylene glycol succinate on
Chromosorb W and a flame photometric detector. Recoveries averaging
over 90% were found at fortification levels of the order of 200 mg/kg.
Stanley and Morrison (1969) described the use of the flame photometric
detector with both phosphorus-selective and sulfur-selective filters
to aid in the identification of insecticides; among the compounds
tested was phorate.
Watts and Storherr (1969) and Watts at al. (1969) described clean-up
and gas-chromatographic conditions for the separation and measurement
of many insecticides, including phorate and its metabolites, using a
potassium chloride thermionic detector and a column of 10% DC-200 on
Gas-Chrom Q. Recoveries in excess of 95% were reported for kale
extracts fortified with phorate or its metabolites at levels of 0.1 to
0.3 mg/kg.
Getzin and Shanks (1970) used an instrument equipped with a sodium
sulphate thermionic phosphorus detector and a column of 4% QF-1 on
Gas-Chrom Q to measure phorate and its metabolites in soils. Recovery
values at levels of 20 mg/kg in soil ranged from 64% for phoratoxon
sulphoxide to 96% for phorate.
Higham at al. (1972) have described procedures suitable for the
determination of total phorate-related residues in a wide variety of
sample types. In these procedures, all of the phorate and its
oxidative metabolites present in the residues were converted to
phoratoxon sulphone by oxidation with m-chloroperbenzoic acid as
recommended by Blinn (1964). These procedures employed a
phosphorus-selective detector with a column of 5% DC-200 on Gas-Chrom
Q and were validated at levels down to 0.05 mg/kg in several types of
sample.
Boyd (1976) has reviewed the methods of residue analysis for phorate
and has provided details of methods for all substrates likely to be
encountered in residue analysis. Residues of phorate and its
metabolites are extracted from the prepared sample with chloroform,
chloroform/methanol, methylene chloride or acetonitrile (depending
upon the type of sample) and purified by clean-up procedures
appropriate to the particular type of sample being analysed. All of
the phorate-related residues present are converted to phoratoxon
sulphone by oxidation with m-chloroperbenzoic acid. Measurement is
accomplished by gas-liquid chromotgraphy with an instrument equipped
with a selective phosphorus-sensitive detector. These procedures
appear suitable for regulatory purposes.
NATIONAL TOLERANCES REPORTED TO THE MEETING
Country Commodity Tolerance, mg/kg
Argentina lettuce, wheat,
groundnut, rice,
maize 0.1
sugar beet (tuber) 0.3
maize fodder 0.5
sugar beet (leaves) 3
Australia cottonseed, vegetables 0.5
Canada beans, corn, lettuce,
potatoes, rutabagas,
turnips negligible
Germany all plant foods 0.05
Netherlands fruit, vegetables
(not potatoes), spices 0
South Africa all food products 0.05
apples, pears 0.1
China pineapple 0.1
USA sugar beet tops 3
wheat (green fodder) 1.5
alfalfa hay, dried sugar
beet pulp 1
corn forage, hops,
potatoes 0.3
peanut vines and hay,
sugar beet roots 0.3
barley grain, barley
straw, beans, corn grain 0.1
sweet corn, lettuce,
peanuts, rice, sorghum
grain 0.1
sorghum fodder, soybeans,
sugar cane, tomatoes 0.1
cottonseed, wheat grain
and wheat straw 0.05
eggs, meat, fat and meat
by-products of cattle,
goats, hogs, horses, poultry
and sheep 0.05
APPRAISAL
Phorate is a soil insecticide with marked systemic properties widely
used throughout the world since 1954. Maize, cotton, cereal grains and
vegetables are treated with phorate usually at the time of planting,
but sometimes by broadcast treatments of granules or a foliage
application to the growing crop. Pre-harvest intervals are relatively
long and residues in harvested commodities are either absent or
generally do not exceed 0.1 mg/kg.
Carrots are capable of taking up and retaining quantities of phorate
but most of the residue is in the peel or in the crown of the carrot
which in generally removed before processing for consumption. The use
of phorate for the protection of potato crops is important. The
residues at the time of harvest are at or below the limit of
determination.
Soil treatments lead to higher residues than do foliage treatments,
largely because the compound is relatively stable in the soil and in
readily taken up by the plant's root system. In animals phorate is
rapidly hydrolysed and converted into the corresponding sulphoxide and
sulphone and their oxons. No significant amounts of phorate or
metabolites are found in milky eggs or tissues of livestock
administered phorate at levels comparable to those likely to occur in
animal feeds.
The metabolism of phorate in plants is largely similar to that
occurring in animals. Phorate metabolites are relatively stable in
storage and processing. At rates recommended in good agricultural
practice, there appears to be no likelihood of carry-over of residues
in soil affecting subsequent crops.
A method of analysis based on the oxidation of all the phorate-related
residues to the oxygen analogue sulphone followed by measurement by
gas-liquid chromatography is sensitive and specific and appears
suitable for regulatory purposes.
Many countries have established maximum residue limits for phorate
residues.
EVALUATION
In the absence of an ADI, the following guideline levels are recorded.
They refer to the sum of the residues of phorate, phorate sulphoxide,
phorate sulphone and the oxygen analogues of these compounds,
determined as the sulphone of the oxygen analogue and expressed as
phorate.
Commodity Guideline levels, Interval on which
mg/kg guideline levels are
based, days
Alfalfa (dry) 1 35
Barley 0.05 60
Beans 0.1 60
Carrots 0.5 120
Celery 0.1 90
Cottonseed 0.05 120
Cowpea 0.1 60
Eggplant 0.1 60
Grapes 0.05 90
Hops (dried) 0.1 25-40
Lettuce 0.2 60
Maize (green) 0.05 30
Peanuts (kernels) 0.05 60
Potatoes 0.05 120
seed 0.1 90
Sorghum 0.05 30
Soybeans 0.05 120
Sugar beet,
fodder beet 0.05 120
Sugar beet
tops 1 35
Tomatoes 0.1 120
Wheat 0.05 120
Eggs, meat, milk 0.05* 120
* at or about the limit of determination
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake for humans (ADI)
can be established and maximum residue limits (MRL) can be
recommended).
1. Long-term carcinogenicity studies.
2. Teratogenicity studies.
3. Studies on potential neurotoxicity.
4. Studies on the toxicity of metabolites.
DESIRABLE
1. Observation in humans.
REFERENCES
Ahmed, M.K., Casida, J.E., and Nichols, R.E. (1958) Bovine metabolism
of organophosphorus insecticides: significance of rumen fluid with
particular reference to parathion J. Agr. Food Chem. 6, 740.
American Cyanamid Company (1966) Thimet residues in potatoes. Report
No. G102, January 13, 1966.
American Cyanamid Company (1966a) Total Thimet residues in sorghum
fodder and grain - Report No.C188, May 14, 1969.
American Cyanamid Company (1966b) Thimet residues in wheat - Report
No. C215, December 9, 1969.
American Cyanamid Company (1966c) Thimet residues in Cottonseed ;
Report No. C214, December 9, 1969.
American Cyanamid Company (1969d) Total Thimet residues in chicken
tissues Report No. C205 dated November 7, 1970.
American Cyanamid Company (1977) Residue dossier on THIMET
phorate - Vol. I-III.
Anonymous (1965) Report on Thimet Systemic Insecticide: Successive
Generation Studies with Mice. Unpublished report from Central Medical
Department Cyanamid, submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Anonymous (1961) Thimet Phorate Residue Studies in Dairy Animals.
Unpublished report from DIABLO Laboratories, In,., submitted to the
World Health Organization by American Cyanamid Company.
Anonymous (1976) Thimet Soil and Systemic Insecticides Technical
Information published by Cyanamid International Corporation, submitted
to the World Health Organization by American Cyanamid Company.
(Unpublished report)
Askew, J., Mitchell, T.M., Thomson, J. and Wheals, B.B. (1968) The
effect of cooking on organo-phosphorus pesticide residues - J.
Chromat. 32, 417-418.
Bache, C.A., and Lisk, D.J. (1966) Determination of oxidative
metabolites of dimethoate and Thimet in soil by emission spectroscopic
chromatography- J. Assoc. Off. Analyt. Chem. 49, 647-650.
Bardner, R., Lord, K.A., and Solly, S.R.B. (1963) Cholinesterase
inhibition method for determining the distribution of organophosphorus
insecticides in soils. Chem. and Ind. 3, 123-124.
Blinn, R.C. (1963) Thin - layer chromatographic isolation and infrared
or colormetric identification of Thimet residues. J. Assoc. Off.
Agric. Chem. 46, (6) 1, 952-96.
Blinn, R.C. (1964) Oxidative conversion of Thimet to its oxygen
analogue sulphone. J. Assoc. Off. Agric. Chem. 47 (4) 641.
Blinn, R.C. and Boyd, J.E. (1967) Thimet R Phorate: Identity of the
Major Phorate Metabolites in the Blood of rats Following oral
Administration of the Insecticide. Unpublished report from the
Research and Development Department of the Agricultural Division of
the American Cyanamid Company, submitted to the World Health
Organization by the American Cyanamid Company.
Bowman M.C. and Beroza, M. (1969) Rapid GLC method for determining
residues of fenthion disulfoton and phorate in corn, milk grass and
faeces - J. Assoc. Off. Analyt. Chem. 52 (6) 1231-1237.
Bowman, M.C., Beroza, M. and Gentry, C.R. (1969a) GLC determination of
residues of disulfoton, demeton-methyl and their metabolites in
tobacco plants. J. Assos. Off. Analyt. Chem. 52 157-161.
Bowman, M.C., Beroza, M. and Harding, A.J. (1969b) Determination of
Phorate and Five of its Metabolites in Corn. J. Ag. Food Chem. 17
138.
Bowman J.S. and Casida, J.E. (1957) Metabolism of phorate (Thimet) in
plants. J. Ag. Food Chem. 5 (3), 192.
Bowman J.S. and Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants insects and mammals - J. Econ. Entomol. 51 (6)
838-843.
Boyd, J.E. (1976) Thimet (phorate) in Vol VI of Analytical Methods for
Pesticides and Plant Growth Regulators by G. Zweig and G. Sherma
-Academic Press N.Y. p. 493-510.
Brown, M.J. (1975) Improved determination of residues of phorate and
its principal metabolites J. Agric. Food Chem. (2) 334-335.
Bunyan P.J. and Taylor, A. Esterase inhibition of pheasants poisoned
with Thimet. J. Agric. Food Chem. 14, 132.
Bunyan P.J., Jennings, D.M. and Taylor, A. (1969) Organophosphorus
poisoning: Chronic feeding of some common pesticides to pheasants and
pigeons. J. Agric. Food Chem. 17, 1027.
Burns, R.G. (1971) The Loss of phosdrin and phorate insecticides from
a range of soil types - Ball. Env. Contam. and Toxicol. 6 (4)
316-321.
Cyanamid Great Britain Ltd. (1971) Residues of phorate and its
metabolites in carrots. Technical Report No. 106 -16 May 1971.
Cyanamid International (1972a) Residues of phorate and its metabolites
in potatoes - Technical Report No. 179 dated 19 April 1972.
Cyanamid International (1972b) Thimet residue analysis in potato
-Regional Laboratory Report No. 11 dated August 23, 1972.
Cyanamid International (1973a) Thimet and Thimet metabolite residues
in potatoes. Technical Report 240 dated 9 May 1973.
Cyanamid International (1973b) Residue analysis of Thimet and the
oxygen analogue in grapes. Regional Laboratory Report No. 19 September
21, 1973.
Dewey, J.E. and Parker, B.L. (1965) Increase in Toxicity to Drosophila
melanogaster of Phorate, Treated Soils. J. Econ. Entomol. 58, 491.
Diablo Laboratories (1964) Analysis of peanut meats, shells and
foliage for possible residues of total Thimet - Report 4J7511 dated 26
February 1964.
Egan, H., Hammond, E.W, and Thomson, J. (1964) Assoc. Off. Analyt.
Chem. 89, 175.
Etheridge, P. and Burt. P.E. (1963) Vapour phase movement and uptake
of systemic insecticides. Rep. Rothamsted, Exp. Stat. for 1963 Page
138.
Finlayson, D.G., Williams, I.H., Brown, M.J., and Campbell,
C.J. (1976a) Distribution of insecticide residues in carrots at
harvest. J. Aric. Food Chem. 24 (3) 606-608.
Finlayson, D.G., Williams, I.H., Brown, M.J. and Campbell,
E.J. (1976b) Translation and persistence of topically applied diazino
and phorate in beans. Can. J. Plant Sci. 56 947-952.
Galley D.J., and Foerster, L.A. (1976) Distribution and loss of
phorate residues in the foliage of broad bean plants following root
uptake of 14C-labelled phorate. Pestic Sci. 7 301-306.
Gaines T.B. (1969) Acute Toxicity of Pesticides. Toxicol. Appl,
Pharmacol 14, 515-534.
Getzin, L.W. and Chapman R.K. (1960) The fate of phorate in Soils - J.
Econ. Entomol. 53 (1) 47-51.
Getzin, L.W. and Shanks, C.H. (1970) Persistence, Degradation and
Bioactivity of Phorate and its Oxidative Analogues in soil. J. Econ.
Entomol. 63, 52.
Giang, P.A. and Schecter, M.S. (1960) Colorimetric determination of
residues of phorate insecticidally active metabolites. J. Agr. Food
Chem. 8 51-54.
Hacskaylo, J., Lindquist, D.A. and Clark, J.C. (1961) Phorate
accumulation by cotton plants and recovery from soil. J. Econ.
Entomol. 54 (3) 411-414.
Higham, J.W., Peterson, R.P. and Pasarela, N.R. (1972) J. Assoc. Off.
Analyt. Chem. (in press)
Jorgenson, T.A., Rashbrook, C.J. and Newell, G.W. (1976) In vivo
Mutagenesis Investigations of Ten Commercial Pesticides. Abstract No.
41, Fifteenth Annual Meeting, Society of Toxicology, submitted by the
World Health Organization by American Cyanamid Company, Wayne, NJ,
USA.
Kay, J.H. and Calandra, J.C. (1961) Effects of Thimet Phorate on
Cholinesterase Activity in the Dog. Unpublished report from Industrial
Bio-Test Labs., Inc., submitted to the World Health Organization by
American Cyanamid Company, Wayne, NJ, USA.
Khajuria, G.N., Jain, H.K. and Dewan, R.S. Residues following the
treatment of sorghum with phorate and dimethoate for the control of
insect pests complex of the crop. Proceedings of the first All-India
Symposium on Progress and Problem in Pesticide - Residue Analysis held
in Ludhiana on 18-19 November 1971 - p. 87-92.
Kiigemagi, O., and Terriere, L.C. (1968a) Residues of phorate in fresh
and dried hops. Report from Oregon State University dated June 10,
1968.
Kiigemagi, O. and Terriere, L.C. (1968b) Residues of phorate and its
metabolites in fresh hops Report from Oregon State University, October
6, 1968.
Krueger, H.R. (1975) Phorate sulfoxidation by plant root extracts.
Pesticide Biochem. & Physiolol. 5, 396-401.
Krueger, H.R., Lindquist, R.K. and Bauerle, W.L. (1973) Phorate and
aldicarb in greenhouse lettuce: Effect of CO2 on residues. J. Econ.
Entomol. 66, 573.
Krueger, H.R. and Mason, J.F. (1973) Phorate and aldicarb: effects of
systemic fungicides on their levels in soyabeans.
Laws (1961) Analyst, 81, 249-255.
Leuck, D.B. and Bowman, M.C. (1970) Residues of phorate and five of
its metabolites. Their presence in Forage Corn and grass - J. Econ,
Entomol. 63 (9) 1838-1842.
Levinskas, G.J., Morici, B.S. and Shaffer, C.B. (1965) Thimet systemic
insecticide: Demyelination studies in white leghorn hens. Unpublished
report from Central Medical Dept. of American Cyanamid Co. submitted
to the World Health Organization by American Cyanamid Co.
Levinskas, G.J., McNerney, J.M. and Fegluy, H.C. (1968) Report on
Sulfoxide of Thimet Systemic Insecticide: Ninety-day Repeated Feeding
to Albino Rats. Unpublished report from Central Medical Department of
American Cyanamid Company, submitted to the World Health Organization
by American Cyanamid Company, Wayne, NJ, USA.
Lichtenstein, E.P., Fuhremann, T.W., Schulz, K.R. and Liang, T.T.,
(1973) Effects of Field application methods on the persistence and
metabolism of phorate in soil, and its translocation into crops. - J.
Econ. Entomol. 66 (4) 863-866.
Lichtenstein, E.P., Fuhremann, T.W. and Schulz, K.R. (1974)
Translocation and metabolism of Phorate as affected by percolating
water in a model soil/plant ecosystem. J. Aric. Food Chem. 22 (6)
991-996.
Lindley, C.D. (1963) The use of phorate granules on potatoes and
carrots Proc. 2nd. Conf. Brit. Insect. & Fangic. Brighton, 1963.
McLeod H.A., Malkins, G. and Rao, S.L.N. (1969) Gas chromatographic
analysis of phorate and five metabolites in monkey liver
homogenates - Bull. Env. Contam. and Toxicol. 4 (4) 224-231.
Maskell, F.E. Gair, R., Williams, J.H., and Cooper, B.J. (1974) Effect
of phorate placement on carrot fly control, seedling density and
terminal residues in the crop. Pestic. Sci. 5 543-548.
Menzer, R.E., Fontanella, E.L. and Ditman, L.P. (1970) Degradation of
disulfoton and phorate in soil influenced by environmental factors and
soil type. Bull. Envir. Contam. & Toxicol. 5 (1) 1-5.
Metcalf, R.L., Fukuto, T.R. and March, R.B. (1957) Plant metabolism of
Dithio-Systox and Thimet. J. Econ. Entomol. 50 (3) 338-345.
Mitchell, T.H., Razioka, J.H., Thomson, J. and Weals, B.B. (1968) The
chromatographic determination of organophosphorus pesticides. Part
III. The effect of irradiation on the parent compounds. J. Chomat.
32, 17.
Nelson, R.C. (1967) Procedure for nine organic phosphorothioate
pesticide residues on trials and vegetables by using micro-coulometric
gas chromatography. J. Assoc. Off. Analyt Chem. 50: 922-926.
Parker B.L. and Dewey, J.E. (1965) Decline of phorate and dimethoate
residues in treated soils based m toxicity to Drosophilia - J. Econ.
Entomol. 58 (2) 106-111.
Rao, S.L.M. and McKinley, W.J. (1969) Metabolism of Organophosphorous
Insecticides by Liver Homogenates from Different Species. Canadian
Journal of Biochemistry, 47: 1155-1159.
Razicka, J.B., Thomson, J. and Weals, B.B. (1967) J. Chromat. 30,
92.
Razicka, J.B., Thomson, J. and Weals, B.B. (1968) J. Chromat. 33,
430.
Sans, W.W. (1967) Multiple Insecticide Residue Determination Using
Column Chromatography, Chemical Conversion, and Gas Liquid
Chromatography. J. Agr. Food Chem. 15, 192.
Satpathy, J.M., Dash, P.K. and Misra, B. (1974) Insecticidal residues
in some vegetables crops grown in treated soils. Indian J. Agric. Sci,
44 (2) 113-115.
Schulz K.R., Lichtenstein, E.P., Fuhremenn, T.W. and Liang, T.T.
(1973) Movement and Metabolism of phorate under field conditions after
granular band applications J. Econ. Entomol. 66 (4) 873-875.
Shaffer, C.B., West, R. and Golz, H.E. (1958) Thimet: Single Dose
Toxicity to Rats. Joint Toxic Action with Other Phosphates.
Unpublished report from Central Medical Department, American Cyanamid
Company, submitted to the World Health Organization by American
Cyanamid Company, Wayne, NJ, USA.
Singh, K. and Gulati, K.C. (1971) Studies on the persistence of
phorate and di-system in soil and plants. Proceedings of the First
All-India Symposium on Progress and Problems in Pesticide-Residue
Analysis held at Ludhiana on 18-19 November 1971 p. 143-149.
Singh, Kalyan and Gulati, K.C. (1972) Application of enzymatic method
for the estimation of organophosphorus insecticides and their residues
in soil and plant. Part II. Phorate. Indian J. Agr. Chem. 6 103-107.
Stanley, C.W. and Morrison, J.T. (1969) J. Chromatogr. 40, 289.
Steller, W.A. and Curry, A.N. (1964) Measurement of residues of Cygon
insecticide and its oxygen analogue by total phosphorus determination
after isolation by thin-layer chromatography. J. Assco. Off. Agric.
Chem. 47, (4) 645.
Stern, V.M. (1971) Summary of Thimet residues in barley. Report from
University of California, Riverside (undated).
Suett, D.L. (1971) Persistence and degradation of chlorfenvinphos,
diazinon, forofos and phorate in soils and their uptake by carrots.
Pestic. Sci. 2, 105.
Suett, D.L. (1974) Uptake of chlorphyrifos and phorate from soil by
carrots as influenced by mode of application and cultivar. Pestic.
Sci. 5, 57-71.
Suett, D.L. (1975) Persistence and degradation of chlorfenvinphos,
chlormephos, disylfoton, phorate, and pirimiphos - ethyl following
spring and late-summer soil application. Pestic. Sci. 6, 385-393.
Talekar, N.S., Lian-Tien Sun, Ehi-Min Lee and Jing-Sing Chen. (1977)
Persistence of some insecticides in subtropical soil. J. Agric. Food
Chem. 25 (25) 348-352.
Tusing, T.W., Kindzin, W., Hanzal, R. and Howard, J. (1956a) Repeated
Oral Administration (Dogs) Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. and Howard, J.
(1956b) Subacute Feeding Study - Experimental Insecticide 3911, 92%.
Unpublished report from Hazelton Labs., Inc., submitted to the World
Health Organization by American Cyanamid Company, Wayne, NJ, USA.
Van Middlelem, C.H. and Baranowski, R.M. (1962) Phorate residues in
tomato fruit and foliage. J. Econ. Entom. 55 (5); 600-603.
Waller, J.B. and Dahm, P.A. Oxidation of phorate in three Iowa soils -
Manuscript from (1973) Iowa State University, Ames, Iowa USA.
Watts, R.R., Storherr, R.W., Pardue, J.R. and Osgood, T. (1969)
Charcoal column clean-up method for amny organophosphorus pesticide
residues in crop extracts. J. Assoc. Off. Analyt. Chem., 52: 522.
Watts, R.R. and Storherr, R.W. (1969) Gas chromatography of
organophosphorus pesticides: retention times and response data on
three columns. J. Assoc. Off. Analyt. Chem. 52, 513-521.
Wit, S.L. (1959) Residu's van systemische middelen bij aardappelen,
etc. - Rijksinstituut voor de Volksgezondheidg Utrecht, FT/6/59 dated
25.4.59.
Wit, S.L. (1961) Residu's van enige systemische insecticiden bij
aardappelen - Rijksinstituut voor de Volksgezondheid, Utrecht,
FT/52/61 dated 29.5.61.
Wit, S.L. (1962a) Onderzoek naar de residu's van enige systemische
middelen bij aardappelen- Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 29/62 dated 29.2.62.
Wit, S.L. (1962b) Onderzoek naar de residu's van Thimet toegepast als
systemisch zaardinsecticide ter bestryding van de vergelingszickte bij
sieten - Rijksinstituut voor de Volksgezondheid, Utreohty Report Tox.
10/62 dated 30.1.62.
Wit, S.L. (1963) Onderzoek naar de residu's van twee systemische
insecticiden bij aardappelen - Rijksinstituut voor de Volksgezondheid,
Utrecht, Report Tox. 113/63 dated 25.6.63.
Wit, S.L. (1969) Foraat op zomerwortelen - Rijksinstituut voor de
Volksgezondheid, Utrecht, Report Tox. 59/69 dated May 1969.
Zakil M.M. and El-Sayed, M.M. (1968) Accumulation of systemic
insecticides in mature cotton seeds. Bull. Ent. Soc. Egypt. Econ. Ser.
II 121-127.
