
PHORATE
First draft prepared by
M. Caris,
Bureau of Chemical Safety,
Health Canada, Ottawa, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Delayed neuropathy
Studies of metabolites
Enzymes and biochemical parameters
Acute toxicity
Short-term toxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Phorate, an organophosphorus insecticide which inhibits
cholinesterase, was first reviewed by the Joint Meeting in 1977
(Annex I, reference 28). A temporary ADI of 0-0.0002 mg/kg bw was
established in 1982 (Annex I, reference 38). In 1985, after review
of a study of delayed neurotoxicity in chickens, an ADI of 0-0.0002
mg/kg bw was established (Annex I, reference 44).
The purpose of the present evaluation was to review additional
information on toxicity, submitted to complement and update the
database. This included a 90-day study in mice treated in the diet,
14-day and one-year studies of toxicity in dogs treated orally, a
two-generation study of reproductive toxicity in rats, studies of
teratogenicity and preliminary range-finding studies in rats and
rabbits, and studies to determine genotoxicity. In order to
facilitate a comprehensive review of data on the toxicology of
phorate, relevant summaries from previously published monographs and
monograph addenda (Annex I, references 29, 39 and 46) are included
in this monograph.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Male rats given a single oral dose of 2 mg/kg bw of
32P-labelled phorate excreted 35% of the administered radiolabel
in the urine and 3.5% in the faeces within 144 h. Male rats given
six daily doses of 1 mg/kg bw excreted 12% of the administered
radiolabel in the urine and 6% in the faeces within seven days.
Brain, liver and kidney tissues from the latter animals contained
unidentified and largely unextractable residues (Bowman & Casida,
1958; Annex I, reference 29).
(b) Biotransformation
The urine of male rats given daily oral doses of 1 mg/kg bw
contained 17% diethyl phosphoric acid, 80% O,O-diethyl
phosphorothioic acid and 3% O,O-diethyl phosphorodithioic acid.
After 32P-labelled phorate was incubated with rat liver slice
preparations, less than 1% of the radiolabelled compound was
converted to hydrolysis products or unextractable residues. Phorate
sulfoxide, phorate sulfone, phoratoxon sulfoxide and phoratoxon
sulfone were formed (Figure 1) (Bowman & Casida, 1958; Annex I,
reference 29).
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of phorate is summarized in Table 1. Phorate
was extremely toxic to rats when administered by the oral, dermal
and intravenous routes, with acute LD50 values ranging from 1.1 to
10 mg/kg bw. Phorate was also severely toxic to rabbits after dermal
administration and to mice when given either orally or
intraperitoneally.
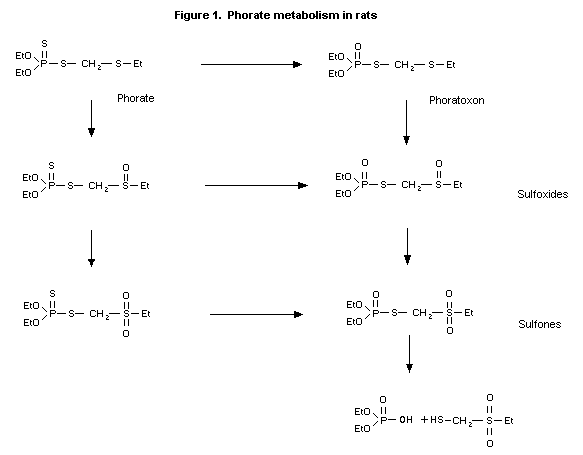 Table 1. Acute toxicity of technical-grade phorate
Species Sex Route LD50 (mg/kg bw per day) Reference
or LC50 (mg/m3)
Mouse NR Oral 11 Blinn, 1982
NR Intraperitoneal 3 Blinn, 1982
Rat NR Oral 1.9-10 Blinn, 1982
M&F Oral M: 2.3; F: 1.1 Gaines, 1969
M&F Oral M: 2.8; F: 1.6 Anon., 1976
M&F Oral M: 3.7; F: 1.4 Newell & Dilley, 1978
NR Dermal 3 Blinn, 1982
M&F Dermal M: 6.2; F: 2.5 Gaines, 1969
M Dermal 5.7 American Cyanamid Co., 1976
M&F Dermal M: 9.3; F: 3.9 Newell & Dilley, 1978
M&F Intravenous M: 2.2; F: 1.2 Newell & Dilley, 1978
M&F Inhalation (1 h) M: 60 Newell & Dilley, 1978
F: 11 Newell & Dilley, 1978
Rabbit M Dermal 5.2 Anon., 1976
From Annex I, references 29 and 39; strains not specified
NR, not reported
(b) Short-term toxicity
Mice
Phorate (purity, 92.1%) was administered to groups of 20 male
and 20 female Crl:CD-1 (ICR)BR outbred Swiss mice at dietary levels
of 0, 1, 3 or 6 ppm, equal to 0.18, 0.55 or 1.10 mg/kg bw per day
for males and 0.23, 0.67 or 1.38 mg/kg bw per day for females, for
13 weeks. A single male mouse treated at the highest dietary level
had convulsions on one occasion during the final week of treatment,
but the relationship of this isolated finding to treatment could not
be ascertained; no other remarkable clinical signs were reported.
Treatment had no effect on survival, food consumption or body-weight
gain. Histopathological examinations were not performed. Plasma,
erythrocyte and brain cholinesterase activities were measured before
terminal sacrifice. Plasma cholinesterase activity was significantly
( p < 0.05) inhibited in animals of each sex at 3 and 6 ppm and in
females at 1 ppm. Erythrocyte cholinesterase levels were
significantly decreased in males and females at the highest dose;
the inhibition, in relation to values in concurrent controls, was
50% in males and 61% in females. A slight, non-significant decrease
in erythrocyte cholinesterase activity (17%) was recorded in females
at 3 ppm. Brain cholinesterase levels, assayed in both halves of the
brain, were significantly ( p < 0.05) decreased in animals of each
sex treated at 3 and 6 ppm, by slightly more than 10% at 3 ppm and
to about 50% of control values at 6 ppm. Minimal inhibition of
plasma cholinesterase levels (15%) in females at 1 ppm was not
considered to represent an adverse effect of treatment, particularly
in the absence of corresponding decreases in erythrocyte and brain
cholinesterase levels. The NOAEL was 1 ppm, equal to 0.18 mg/kg bw
per day, on the basis of significantly reduced brain cholinesterase
activity at dietary levels of 3 and 6 ppm (Trutter, 1990).
Rats
Groups of 50 male and 50 female rats [strain not specified]
were fed phorate (purity, 92%) in the diet at levels of 0, 0.22,
0.66, 2 or 6 ppm, equivalent to 0.01, 0.03, 0.1 or 0.3 mg/kg bw per
day, and groups of 25 male and 25 female rats were fed 12 or 18 ppm,
equivalent to 0.6 or 0.9 mg/kg bw per day. Treatment continued for
13 weeks, and cholinesterase activity was monitored each week.
Occasional episodes of excitability and intermittent tremors were
noted in females given 6 ppm, and animals of each sex receiving 12
and 18 ppm showed severe excitability, intermittent tremors and
ataxia, culminating in death of 50% of animals at 12 ppm and no
survivors at 18 ppm. Levels of 6 ppm and higher produced significant
depression of plasma, erythrocyte and brain cholinesterase activity
in animals of each sex and decreased erythrocyte cholinesterase
activity in females at 2 ppm. In all groups receiving 6 ppm or less,
growth, food consumption, survival and liver and kidney weights and
ratios were within normal limits, and no abnormalities were seen at
gross necropsy or histological examination. The NOAEL was 2 ppm,
equivalent to 0.1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity at 6 ppm and above (Tusing et al.,
1956a; Annex I, reference 29).
Dogs
Groups of two male and one female mongrel dogs received
capsules containing 0, 0.01, 0.05, 0.25 or 1.25 mg/kg bw per day of
phorate (purity, 92%) in corn oil on six days per week for 15 weeks.
Two males received a single dose of 2.5 mg/kg bw. Plasma and
erythrocyte cholinesterase activities were monitored weekly; brain
cholinesterase activity was not measured. At 0.05 mg/kg bw per day,
plasma enzyme activity was significantly depressed. Erythrocyte
cholinesterase activity was not affected during the first 12 weeks
but was depressed slightly (not significantly) during the last three
weeks of the study. Significant decreases in plasma and erythrocyte
enzyme activity were observed at 0.25 mg/kg bw per day, and 1.25
mg/kg bw per day induced total inhibition of plasma cholinesterase
activity and a significant reduction in erythrocyte cholinesterase
activity. All of the dogs administered the single dose of 2.5 mg/kg
bw per day died within 3-4 h; enzyme activity was not determined. No
signs of systemic toxicity were seen, and haematological, clinical
chemistry and urine analyses showed no effects in dogs receiving
0.01 or 0.05 mg/kg bw per day. Histopathological examination
revealed no consistent findings related to the treatment (Tusing et
al., 1956b; Annex I, reference 29).
Groups of three male and three female beagle dogs were fed
phorate in the diet at 0, 0.5 or 1.0 ppm, equivalent to 0.012 or
0.025 mg/kg bw per day, for six weeks. Cholinesterase activity in
plasma and erythrocytes was determined before and every two weeks
during the test. Analyses of variance revealed no significant
difference between the treated and control animals with respect to
plasma and erythrocyte enzyme activities (Kay & Calandra, 1961;
Annex I, reference 29).
In a preliminary range-finding study, groups of two male and
two female beagle dogs were given capsules containing phorate
(purity, 92.1%) in corn oil at doses of 0.01, 0.05, 0.10, 0.25 or
0.50 mg/kg bw per day for 14 days. A control group of three male and
three female dogs was given capsules containing corn oil. Plasma and
erythrocyte cholinesterase levels were measured twice before
treatment, and after 3, 7, 10 and 14 days of treatment; brain
cholinesterase levels were determined in the cerebellum and cerebrum
of each dog at sacrifice. Excessive salivation and tremors were seen
in one female dog given the highest dose, and body-weight gain was
decreased in comparison with the controls in animals of each sex.
Slight decreases in total serum protein were recorded in males and
females at the high dose. Treatment did not affect survival, food
consumption, haematological parameters or organ weights, and gross
pathology showed no effects; histopathological examinations were not
performed. Plasma cholinesterase levels were significantly decreased
in animals of each sex given 0.10 mg/kg bw per day or more and in
males given 0.05 mg/kg bw per day, in comparison with values before
treatment and in control animals, and erythrocyte cholinesterase
activity was significantly (> 20%) inhibited in both males and
females at the highest dose of 0.50 mg/kg bw per day. Brain
cholinesterase activity was inhibited (31-69%) in both males and
females at 0.10 mg/kg bw per day or more. The NOAEL was 0.05 mg/kg
bw per day (Piccirillo et al., 1987).
Phorate (purity, 92.1%) in corn oil was administered orally by
capsule to groups of six male and six female beagle dogs at doses of
0.005, 0.01, 0.05 or 0.25 mg/kg bw per day for one year. Eight male
and eight female controls received capsules containing only corn
oil. Plasma and erythrocyte cholinesterase activities were measured
twice before treatment, at six weeks, at three and six months and at
sacrifice; brain cholinesterase levels were measured in the cerebrum
and cerebellum at termination of treatment. Mild tremors were
observed occasionally in one male and two females during weeks 23-52
of treatment with the high dose. Mean body weights and overall
weight gain (26% less than control) were decreased in males at 0.25
mg/kg bw per day. Ophthalmological examination of all dogs before
treatment, at six months and at one year showed no treatment-related
effects. There were no adverse effects on food consumption or
haematological or urinary parameters. Decreased ( p < 0.05) total
protein levels were recorded at week 6, at three and six months and
at termination of treatment in males treated at 0.25 mg/kg bw per
day. Although the values were reported to be within the range in
historical controls, no supporting data were provided. As the
individual levels of total protein in males at the high dose were
consistently below concurrent values before treatment and in
controls, the finding appeared to be related to treatment. The
decreases ( p < 0.05) in total protein values in females and the
decreased albumin values in males at the high dose were within the
lower limits of control values. Gross and histopathological
examination revealed no lesions that could be attributed to
treatment with phorate. The highest dose induced inhibition of
erythrocyte (> 20%) and brain cholinesterase activity (43-54%);
plasma cholinesterase levels were significantly decreased in animals
of each sex at doses of 0.05 mg/kg bw per day and above. The
decrease in plasma cholinesterase activity at this dose was not
considered to be adverse in the absence of correlative inhibition of
brain and erythrocyte cholinesterase activity and evidence of
clinical toxicity. The NOAEL was 0.05 mg/kg bw per day on the basis
of decreased body weight, significant inhibition of erythrocyte and
brain cholinesterase activity and clinical signs consistent with
cholinergic toxicity at the high dose of 0.25 mg/kg bw per day
(Shellenberger & Tegeris, 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female 41-day-old CD-1 outbred Swiss
albino mice were fed technical-grade phorate (purity, 85.5%) in the
diet at 0, 1, 3 or 6 ppm, equivalent to 0.15, 0.45 or 0.9 mg/kg bw
per day for 18 months. All animals that died or were killed in
moribund condition during the study or were sacrificed terminally
were subjected to gross and histopathological examination. Survival
was not adversely affected: 78-90% of males and 66-74% of females in
the control and treated groups were still alive at the end of the
experiment. The growth of females at 6 ppm was retarded almost
throughout the experiment. All treated animals appeared to eat less
food during the first three weeks and occasionally thereafter, but
no consistent dose-response relationship was seen. Some clinical
signs, such as tremors, hyperactivity and excessive salivation,
occurred at higher incidence and more frequently in animals at the
highest dose than in controls. Gross pathological examination showed
no changes significantly different from those in the controls, and
microscopic evaluation of a wide range of tissues from each animal
revealed no alterations related to treatment. There was no
significant dose-related increase in the incidence of any particular
type of tumour, of animals with tumours, of animals with malignant
tumours or of animals with multiple primary tumours. Although the
incidence of alveolar and bronchiolar adenoma appeared to be
increased in males at 6 ppm (8/50, compared with 3/50 in controls),
this effect was not considered to be related to treatment since the
increase was not statistically significant and this tumour is known
to occur frequently in CD-1 mice. The NOAEL was 3 ppm, equivalent to
0.45 mg/kg bw per day, on the basis of decreased body weight and
clinical signs of toxicity at 6 ppm (Litton Bionetics, 1981a; Annex
I, reference 39).
Rats
Groups of 50 male and 50 female five-week-old Crl:COBS CD(SD)
BR rats were fed dietary levels of technical-grade phorate (purity,
84.5%) at 0, 1, 3 or 6 ppm, equal to 0.05, 0.16 or 0.32 mg/kg bw per
day in males and 0.07, 0.19 or 0.43 mg/kg bw per day in females, for
24 months. The mortality rate appeared to be increased in females at
6 ppm, as only 36% survived to the end of the experiment; however,
more than 60% of animals in all groups, including the controls,
lived at least 90 weeks. The only clinical sign related to treatment
was tremors induced by over-dosing (327% of all the intended doses)
during week 9. Growth was depressed in females at 6 ppm during the
first 26 weeks and again between weeks 74 and 102. Food consumption
showed no consistent dose-response pattern. On haematological
examination, clinical chemistry and urinalysis performed at 6, 12
and 24 months, the only notable findings were decreased values for
erythrocyte counts, haemoglobin and haematocrit in females at the
highest dose at 12 months. Dose-related inhibition (> 20%) of
plasma cholinesterase activity was noted in males at 6 ppm at 12
months, in all treated males at 24 months and in females at both 3
and 6 ppm at all sampling intervals (3, 6, 12 and 24 months).
Erythrocyte cholinesterase activity was not significantly depressed
(< 20%) at any time. The activity of brain cholinesterase was
reduced (> 20%) in males at 6 ppm and in females at 3 ppm or more.
At sacrifice, females given the highest dose had increased
organ:body weight ratios with respect to the adrenals, brain, heart,
liver and spleen. On gross pathological and histopathological
examination of a variety of tissues, the only effect apparently
related to treatment was a significant increase in the incidence of
inflammation and epithelial hyperplasia of the forestomach in
animals of each sex, but particularly in males, at 6 ppm. The most
prevalent types of spontaneous tumours were pituitary adenomas in
animals of each sex and mammary tumours in females. There were no
significant differences between control and treated groups with
regard to incidence, type or time of appearance of tumours. The
marginal no-effect level was 1 ppm, equal to 0.05 mg/kg bw per day
(Litton Bionetics, 1981b; Annex I, reference 39).
(d) Reproductive toxicity
Mice
Groups of eight male and 16 female mice (strain not specified)
were fed phorate (purity unknown) in the diet at levels of 0, 0.6,
1.5 or 3 ppm, equivalent to 0.09, 0.23 or 0.45 mg/kg bw per day, for
three generations, with two litters per generation. There were no
dose-related effects on indices of fertility, gestation, viability
or lactation during the study, but the lactation index was slightly
lowered in the group receiving 3 ppm, to below the control value in
the first mating of the F0 generation, in both matings of the F1
generation and in the second mating of the F2 generation. Gross
and microscopic examination of tissues revealed no consistent
abnormalities related to treatment. The NOAEL was 1.5 ppm,
equivalent to 0.23 mg/kg bw per day, on the basis of decreased
lactation indices at 3 ppm (American Cyanamid Co., 1965; Annex I,
reference 39).
Rats
A two-generation study of reproductive toxicity was conducted
in groups of 25 male and 25 female COBS CD(SD) rats given phorate
(purity, 92.1%) at dietary levels of 0, 1, 2, 4 or 6 ppm, equal to
0.09, 0.17, 0.35 or 0.52 mg/kg bw per day in males and 0.10, 0.20,
0.40 or 0.62 mg/kg bw per day in females, for a minimum of 60 days
before mating, to produce two consecutive litters (F1a and F1b)
of the first (F0) parental generation. Groups of 25 males and 25
females were then selected from the F1b litters to become the
second-generation (F1) parental animals. Owing to a high incidence
of mortality during the post-weaning period among F1b pups at 6
ppm, 30 males and 30 females at this dose were selected as F1
parents. The second-generation parental animals were treated for a
minimum of 100 days before mating to produce two consecutive litters
(F2a and F2b). Animals were exposed continuously to the test
diets before mating and throughout weaning of the offspring in both
generations. Cholinesterase levels were determined in plasma,
erythrocytes and brain from 10 parental F1 rats of each sex in
each group at terminal sacrifice.
A treatment-related decrease in body weight and tremors were
seen at > 4 ppm in parental animals; at 6 ppm, increased
mortality and clinical signs were seen, manifested as convulsive
movements and aggressive behaviour. Routine clinical observation of
the animals revealed an increased incidence of ocular effects at 6
ppm, described as exophthalmus, 'protrusion of tissue off the
cornea' and opacities. Subsequent ophthalmological evaluation
revealed markedly higher incidence and severity of infectious
sequelae affecting the cornea, anterior uveal tract and posterior
segment of the eye in rats at this dose. The increased
susceptibility of rats to ocular disease at the high dose was
considered to be a secondary effect of treatment with phorate.
Plasma cholinesterase levels were significantly decreased in females
at 4 and 6 ppm and in males at 6 ppm. Erythrocyte activity was
inhibited only slightly (10-11%) in animals of each sex at 6 ppm.
Inhibition of brain cholinesterase activity was significant in males
(40%) at 6 ppm and in females at 4 ppm (59%) and 6 ppm (83%). There
was no significant inhibition of brain cholinesterase activity at
the lower dietary levels.
Pup survival during the lactation periods was significantly
reduced in all litters from both parental generations at 6 ppm and
in the F2a litter at 4 ppm. The size of both litters from the F1
generation was slightly decreased at 6 ppm. Mean pup weights were
decreased in both F1a and F1b litters of animals receiving 4 or
6 ppm. The mean weights of pups of the F2a litter were decreased
at 4 ppm (those of the F2b litter were not affected) and in pups
of both F2a and F2b litters at 6 ppm. Anogenital staining was
recorded in F1b and F2b pups at 6 ppm but not in control animals
or in those receiving lower dietary levels. There were no adverse
effects on mating or fertility indices, pregnancy rate, length of
gestation, gestation index or sex distribution ratios, and no gross
morphological alterations or pathological changes were seen in the
reproductive organs at any of the dietary levels studied. The NOAEL
was 2 ppm, equal to 0.17 mg/kg bw per day (Schroeder & Daly, 1991).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 mated female Crl:COBS CD (SD)BR rats were given
technical-grade phorate (purity, 91.7%) by gastric intubaton at 0,
0.125, 0.25 or 0.5 mg/kg bw per day on gestation days 6-15 and were
sacrificed on day 20 of gestation. The fetuses were removed for
gross, skeletal and visceral examination. The pregnancy rate was
comparable in all groups. During the gestation period, 7/23 pregnant
dams at 0.5 mg/kg bw per day, none at 0.25 mg/kg bw per day and 1/24
at 0.125 mg/kg bw per day died. Fetuses at 0.5 mg/kg bw per day had
an increased frequency of enlarged heart (not otherwise
characterized). Clinical signs, body weight and food consumption of
dams during gestation, the number of implantation sites, the number
of resorptions, the number of dead fetuses, mean live litter size,
average fetal weight, sex ratio, and gross, skeletal and visceral
abnormalities of fetuses were not significantly different from those
in the controls. The NOAEL for teratogenicity was 0.25 mg/kg bw per
day (Litton Bionetics, 1978; Annex I, reference 39).
In a preliminary range-finding study, groups of eight mated
female Crl:CDBRVAF/Plus (SD) rats were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.25, 0.5, 0.7 or 0.9
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which it occurred was considered day 0 of gestation.
Treatment with doses > 0.5 mg/kg bw per day was lethal to the
dams, and there were no survivors at these doses after day 12 of
gestation. Overt clinical signs of toxicity preceding death included
twitches, tremors, excessive salivation, exophthalmos, ataxia,
clonic convulsion, and decreased body weight and food intake. Gross
examination of rats which died revealed enlarged and/or congested
adrenal glands. The NOAEL was 0.25 mg/kg bw per day for both
maternal and developmental toxicity (Lochry, 1990a).
In a study of teratogenicity, groups of 24-25 mated female
Crl:CDBRVAF/Plus(SD) rats were treated by gavage with phorate
(purity, 92.1%) in corn oil at doses of 0, 0.1, 0.2, 0.3 or 0.4
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which this occurred was considered day 0 of
gestation. Six of 25 females at 0.4 mg/kg bw per day that died
during days 11-16 of gestation had enlarged adrenal glands. No
deaths were reported at lower doses. Clinical signs of toxicity seen
at 0.4 mg/kg bw per day were an increased number of rats with
tremors, excessive salivation, chromodacryorrhoea and rhinorrhoea,
urine-stained abdominal fur, decreased motor activity, hunched
posture, impaired righting reflex and laboured breathing. Maternal
body weights, corresponding weight gains and food consumption and
fetal body weights were significantly decreased at 0.4 mg/kg bw per
day when compared with control values. Increased incidences of
skeletal variations were observed in fetuses and/or litters at this
dose, which were related to delays in ossification of the
sternebrae, incompletely and unossified pubes and incompletely
ossified ischia. The NOAEL for maternal and developmental toxicity
was 0.3 mg/kg bw per day on the basis of mortality, clinical signs
of toxicity, and significantly decreased body weights and food
consumption in the mothers and decreased fetal body weights and
potentially reversible delays in skeletal ossification at 0.4 mg/kg
bw per day (Lochry, 1990b).
Groups of 10 pregnant Sprague-Dawley rats were exposed by nose
only in an inhalation chamber to aerosols of technical-grade phorate
(purity, 78-90%) with a count median diameter of 0.57 Ám, generated
from a 1% solution of phorate in xylene, for 1 h per day on days
7-14 of gestation at concentrations of 0.15 ▒ 0.04, 0.4 ▒ 0.15 or
1.94 ▒ 0.48 mg/m3. Three groups of 15 dams were exposed to xylene
or air or to a restricted diet. (The reason for including the last
group was not specified.) All dams were sacrificed on day 20 of
gestation, and fetuses were removed by caesarean section for
visceral and skeletal examination. Five dams at the highest dose
died, and toxic signs (tremors, lachrymation and exophthalmus) were
noted in animals at this level; one of the dead dams had resorbed
the entire litter. Average percentage fetal mortality was markedly
increased at this dose. The summary indicated no treatment-related
effects on the body weight or food consumption of dams during
gestation and no effects on pregnancy rate, average number of
implants, average fetal weight, average number of sternal
ossification centres or the incidence of supernumerary ribs.
Specific information on the incidence of any gross or soft-tissue
abnormalities was not provided (Newell & Dilley, 1978; Annex I,
reference 39).
Rabbits
In a preliminary range-finding study, groups of five mated
female New Zealand white rabbits were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.3, 0.6, 0.9, 1.2 or
1.5 mg/kg bw per day on days 6-18 of gestation. The day on which
coitus with two successive males was observed was designated day 0
of gestation. The incidences of mortality in the six groups were
reported as 0/5, 1/5, 1/5, 1/5, 2/5 and 4/5, respectively. Mean
body-weight loss was seen at 1.2 mg/kg bw per day. Only one female
at the highest dose survived to scheduled sacrifice. Food intake was
generally decreased in the groups receiving 0.3, 0.6, 0.9 and 1.2
mg/kg bw per day, although no clear dose-response relationship was
seen. Increased numbers of resorptions and post-implantation losses
were reported at doses > 0.6 mg/kg bw per day. Decreased mean
fetal body weights and shorter crown-rump lengths were noted at 1.2
mg/kg bw per day. External examination of the fetuses showed no
treatment-related malformations or alterations. The only significant
effects observed at the LOAEL of 0.3 mg/kg bw per day were a single
maternal death and depressed food intake (Schroeder & Daly, 1986).
Groups of 20 mated female New Zealand white rabbits were given
phorate (purity, 92.1%) in corn oil by gavage on days 6-18 of
gestation at doses of 0, 0.15, 0.5, 0.9 or 1.2 mg/kg bw per day. The
day on which coitus with two successive males was observed was
designated day 0 of gestation. At 1.2 mg/kg bw per day, 8/20 females
died during days 14-19 of gestation, one female aborted and another
female delivered prematurely on day 28 of gestation, so that only
10/20 dams survived to scheduled sacrifice. The deaths of two
females at 0.9 and one at 0.5 mg/kg bw per day were also attributed
to treatment with phorate. Anogenital staining was observed at a
higher incidence in females treated with the highest dose. Mean body
weights and corresponding weight gains were decreased at doses >
0.5 mg/kg bw per day. Food consumption was decreased in females
treated at 1.2 mg/kg bw per day. Treatment had no effect on
pre-implantation loss, number of resorptions, number of live
fetuses, fetal body weight or fetal sex ratio. At 1.2 mg/kg bw per
day, all three fetuses in a single litter (five of eight
implantation sites showed early resorptions) had open eyelids,
curved scapulae, an absent supraorbital process, an irregular margin
of the frontals and a displaced anterior fontanel. Although these
anomalies were not observed in the control group, the incidence of
open eyes was within that of historical controls; curved scapulae
had not previously been noted, however, in 1852 fetuses from 269
litters. Ocular and scapular defects were not seen in other fetuses
from dams treated at this dose and were not recorded in any of the
fetuses in the preliminary range-finding study. Although the defects
seen in this single litter may have been a secondary response to the
maternal toxicity of the high dose, the weight of evidence would
suggest that the findings were spurious and perhaps genetically
predicted. There was insufficient evidence to suggest that phorate
is teratogenic to rabbits. The NOAEL for maternal toxicity was 0.15
mg/kg bw per day, as determined by a higher incidence of mortality
and decreased body weights at > 0.5 mg/kg bw per day. In the
absence of embryo- or fetotoxicity, the NOAEL for developmental
toxicity was the highest dose, 1.2 mg/kg bw per day (Schroeder &
Daly, 1987).
(f) Genotoxicity
A battery of assays were performed to test the genotoxicity of
phorate, the results of which are presented in Table 2. Assays for
gene mutation, chromosomal aberrations and other cytogenetics
end-points in vitro and in vivo gave negative results. No
unscheduled DNA synthesis was seen in human fibroblasts, and
dominant lethal mutations were not induced in male mice.
Table 2. Results of tests for the genotoxicity of phorate
End-point Test system Concentration Purity Results Reference
of phorate (%)
In vitro
Reverse S. typhimurium Up to 1000 mg/plate Technical Negativea Simmon et al., 1977b
mutation TA100, 1535, 1537, grade
1538; E. coli WP2
Reverse E. coli p3478, 1 mg (on filter disc) Technical Negativec Simmon et al., 1977b
mutation W3110; B. subtilis per plate grade
Reverse Chinese hamster 30, 40, 50, 80, 100 92.1 Negativec Thilager & Kumarop,
mutation ovary cells, hprt nl/ml 1985
locus 5, 10, 12, 14, 16, 18, Negatived
20 nl/ml
Mitotic S. cerevisiae D3 5% w/v for 4-h Technical Negativea Simmon et al., 1977b
recombination incubation before grade
plating
Unscheduled Human fibroblasts Up to 1 x 10-3 Technical Negativea Simmon et al., 1977b
DNA synthesis WI-38 grade
In vivo
Chromosomal Male and female M: 0 (corn oil), 0.25, 92.1 Negative Ivett & Myhr, 1986
aberration Sprague-Dawley 1.25, 2.5 mg/kg bw
rats, killed after per day; F: 0, 0.13,
6, 18, 30 h 0.63, 1.25 mg/kg bw
per day
Dominant Male mice 0, 5, 10, 20 mg/kg bw Technical Negative Simmon et al., 1977b
lethal per day in diet for grade
mutation 7 weeks, weekly
matings for 8 weeks
a In the presence and absence of metabolic activation
b From Annex 1, reference 46
c In the absence of metabolic activation
d In the presence of metabolic activation
(g) Special studies
Delayed neuropathy in chickens
Groups of six adult hens were fed 0 or 40 ppm phorate (purity
unspecified), equivalent to 5 mg/kg bw per day, in the diet for four
weeks. A third group received 4000 ppm tri- ortho-tolyl phosphate
as a positive control. Each hen was anaesthetized and immediately
perfused with buffered formalin, and sections of brain, lower
thoracic cord and each sciatic nerve were prepared for microscopic
examination. Tri- ortho-tolyl phosphate induced myelin loss in each
hen, but phorate had no adverse effects on nerve fibres or the
myelin sheath (Levinskas et al., 1965; Annex I, reference 29).
Phorate (purity, 89.5%) dissolved in corn oil was administered
by gavage to fasted white Leghorn hens (22-23-months old). The
LD50 by a single oral dose was 14.2 mg/kg bw. Fifty fasted hens
were then given an intramuscular injection of 10 mg/kg bw of
atropine sulfate and 1 h later given a single oral dose of 14.2
mg/kg bw phorate. An additional 15 fasted, atropinized hens were
given corn oil only, and 15 hens that did not receive atropine were
given tri- ortho-tolyl phosphate at 500 mg/kg bw as a positive
control. All surviving hens in the test and vehicle control groups
received the same doses 21 days later, except that atropine sulfate
was administered at 30 mg/kg bw. All hens were observed daily for
mortality, clinical signs and evidence of neurotoxic reactions. Body
weights and food consumption were recorded every three days. All
hens that died during the study and all hens that were killed at
termination of the study at 42 days were subjected to gross
necropsy. Those killed at the end of the study were perfused with
10% neutralized formalin, and brains, vertebral columns (with spinal
cord in situ) and the entire right and left sciatic nerves were
excised and fixed. Microscopic slides of neural tissue were prepared
by taking a sagittal section of the entire brain (corpus striatum,
cerebellum, pons), longitudinal and cross-sections of the cervical,
thoracic and lumbrosacral levels of the spinal cord and both
sagittal and longitudinal sections of the right and left sciatic
nerves. Sections were stained with haematoxylin and eosin, and
replicate sections were stained with luxol fast blue. Tissues from
10 hens in each group were examined histologically.
None of the 15 hens fed corn oil died during the 42-day study,
and all 15 hens given tri- ortho-tolyl phosphate were killed in
extremis on day 16 of the study after clinical signs of
neuropathy, first observed on day 11, became progressively more
severe. These signs included generalized weakness, ataxia and
paralysis of the legs and wings. Of the 50 hens treated with
phorate, 27 died within 24 h of the first dose and 13 more within 24
h of the second dose. Ten hens survived to termination of the study.
Vehicle control and phorate-treated hens had slight generalized
weakness of the limbs, lasting about 2 h, shortly after each
treatment with atropine sulfate; treated hens had slightly more
severe reactions and, in addition, slight to moderate ataxia for up
to 2 h after treatment with phorate. No clinical signs of delayed
neuropathy were observed, however, in any vehicle-control or test
birds. In comparison with vehicle controls, the mean body-weight
gains of tested hens were greater at 0-21 days and smaller at 21-42
days, and the mean feed consumption of test hens was smaller at 0-21
days and greater at 21-42 days. No effects attributable to phorate
were seen at gross necropsy.
Histological examination of neural tissues from
positive-control hens revealed treatment-related lesions involving
the brain, spinal cord and/or sciatic nerves in all 10 birds.
Generally mild to moderate axonal degeneration was observed in the
brains of 4/10 hens, in the spinal cords of 10/10 hens and in the
sciatic nerves of 10/10. In addition, demyelination was observed in
the spinal cords and in the sciatic nerves of 7/10 hens;
Schwann-cell hyperplasia was also observed in the sciatic nerves of
3/10 hens. These lesions were compatible with a delayed neurotoxic
response induced by tri- ortho-tolyl phosphate. Minimal to mild
focal axonal degeneration of the sciatic nerves was noted in 3/10
hens treated with phorate; no axonal degeneration was seen in
vehicle-control hens. The axonal degeneration observed in the
treated birds was associated with interstitial infiltration of
lymphoid cells, which was also observed in other test and
vehicle-control hens. This syndrome, which was distinctly different
from that observed in the positive-control hens, was ascribed to
lesions of a naturally occurring disease (Marek's disease) and was
considered not to be related to treatment with phorate. Treatment
did not induce clinical or histopathological signs indicative of
acute delayed neuropathy (Fletcher, 1984; Annex I, reference 46).
3. Studies on metabolites
(a) Enzymes and biochemical parameters
The metabolites of phorate that inhibit cholinesterase are
phorate sulfoxide and sulfone and the oxygen analogue phoratoxon and
its sulfoxide and sulfone. The negative logarithms of the molar
concentrations of these compounds that induce 50% inhibition of
erythrocyte cholinesterase are as follows: phorate, 3.17; phorate
sulfoxide, 3.35; phorate sulfone, 5.00; phoratoxon, 5.87; phoratoxon
sulfoxide, 6.76; and phoratoxon sulfone, 7.02 (Annex I, reference
29).
(b) Acute toxicity
The acute toxicity of metabolites of phorate was studied in
rats and mice; the results are shown in Table 3. Both the parent
compound and the metabolites are extremely acutely toxic.
Table 3. Acute toxicity of phorate and its metabolites
Compound LD50 in rats (mg/kg bw per day) LD50 in mice (mg/kg bw per day)
Oral Intraperitoneal Oral Intraperitoneal
Phorate 1.9-10 3 11 3
Phorate sulfoxide 2-4 11 7 1
Phorate sulfone 1.8-2.0 27 9 2
Phoratoxon 0.6-0.8 - - -
Phoratoxon sulfoxide 1.4-1.6 1 3 0.02
Phoratoxon sulfone 0.6-0.8 1.8 5 0.4
From Blinn, 1982; Annex I, reference 39
(c) Short-term toxicity
Rats
Groups of 35 male and 35 female rats (strain not specified)
were fed phorate sulfoxide (purity, 92%) at dietary levels of 0.32,
0.8 or 2 ppm, equivalent to 0.02, 0.04 or 0.1 mg/kg bw per day, for
90 days. Fifty males and 50 females served as controls. Brain,
erythrocyte and plasma cholinesterase activities were determined
twice a week. A significant ( p < 0.05) depression of erythrocyte
and brain cholinesterase activity was seen in females at 2 ppm; the
decrease in plasma cholinesterase activity at this dose was less
consistent and was considered to be borderline. Borderline
depression of erythrocyte enzyme activity was seen at 0.8 ppm. At
0.32 ppm, all values were within acceptable statistical limits for
animals of each sex. No effects were observed on other
haematological parameters, organ weights or ratios, and no
consistent histopathological effect was noted that could be
attributed to feeding 2 ppm or less of phorate sulfoxide (Levinskas
et al., 1968; Annex I, reference 29).
Groups of 30 male and 30 female Charles River CD strain rats
(50 male and 50 female controls), 51 days of age, were fed phorate
sulfone (purity, 92%; containing 6% unchanged phorate and about 2%
phorate sulfoxide) in the diet at 0, 0.32, 0.8 or 2 ppm, equivalent
to 0.02, 0.04 or 0.1 mg/kg bw per day, for 90 days. None of the
animals died before the end of the experiment, and there were no
treatment-related changes in appearance or behaviour. Weight gain
and increased food consumption were seen in males fed 0.8 or 2 ppm.
Assay of tissue cholinesterase activity five times during the course
of the experiment showed inhibition (> 20%) of erythrocyte
cholinesterase at 2 ppm in animals of each sex at most time
intervals. Plasma cholinesterase activity was reduced by 23-27% in
males at 2 ppm after one, three and five weeks and by 25-72% in
females at 2 ppm after all sampling intervals; plasma cholinesterase
was also inhibited (39%) in females at 0.8 ppm after one and three
weeks. The activity of brain cholinesterase was reduced (> 20%)
only in females at 2 ppm after three, five and eight weeks. Control
and treated groups did not differ significantly with regard to
values for haematocrit, haemoglobin and total leukocyte count at the
end of the experiment. No treatment-related effects were observed on
the absolute weights of kidneys or liver, and no gross pathological
changes were seen. Histopathological evaluation of a variety of
tissues from five males and five females in the control and
highest-dose groups revealed no morphological alterations
attributable to treatment. The NOAEL was 0.32 ppm, equivalent to
0.02 mg/kg bw per day (Hutchison et al., 1978; Annex I, reference
39).
4. Observations in humans
No relevant information was available.
Comments
The limited data available indicate that phorate given orally
to rats is not readily eliminated, with less than 40% of the
administered radioactivity recovered within six days after
treatment. Urinary metabolites were identified as O,O-diethyl
O-hydrogen phosphoro-thioate, diethyl hydrogen phosphorate and
O,O-diethyl O-hydrogen phosphorodithioate.
Phorate was severely acutely toxic in mice, rats and rabbits
after administration by various routes. WHO (1992) has classified
phorate as extremely hazardous.
In a 13-week study of the toxicity study of dietary levels of
0, 1, 3 and 6 ppm phorate in mice, the NOAEL was 1 ppm, equal to
0.18 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity at dietary levels of 3 ppm and above.
In rats that received phorate in the diet at 0, 0.22, 0.66, 2,
6, 12 or 18 ppm for 13 weeks, the NOAEL was 2 ppm, equivalent to 0.1
mg/kg bw per day, on the basis of inhibition of brain cholinesterase
activity. At 6 ppm, cholinesterase inhibition was associated with
tremors and hyperexcitability and at 12 and 18 ppm, with death.
In a one-year study of toxicity in dogs, phorate was
administered orally in capsules at doses of 0, 0.005, 0.01, 0.05 or
0.25 mg/kg bw per day. The NOAEL was 0.05 mg/kg bw per day, as
determined by decreased body weights, significant inhibition of
erythrocyte and brain cholinesterase activity and clinical signs
consistent with cholinergic toxicity at 0.25 mg/kg bw per day. A
preliminary 14-day range-finding study, a six-week study of dietary
administration and a 15-week study in which phorate was given orally
in capsules supported the findings reported in the one-year study.
Mice and rats were treated with phorate at dietary levels of 0,
1, 3 or 6 ppm for 18 and 24 months, respectively. In mice, the NOAEL
was 3 ppm, equivalent to 0.45 mg/kg bw per day, on the basis of
decreased body weights and increased incidences of tremors,
hyperactivity and excessive salivation at 6 ppm. Cholinesterase
activity was not measured in this study. In the study of toxicity
and carcinogenicity in rats, the NOAEL was 1 ppm, equal to 0.05
mg/kg bw per day, on the basis of significant inhibition of brain
cholinesterase at 3 ppm and above. There was no apparent effect on
erythrocyte cholinesterase activity. Phorate was not carcinogenic
when fed to mice or rats at dietary levels of up to 6 ppm.
A two-generation study of reproductive toxicity in rats given
phorate at dietary levels of 0, 1, 2, 4 or 6 ppm showed no adverse
effects on reproductive parameters. The NOAEL was 2 ppm, equal to
0.17 mg/kg bw per day, on the basis of decreased brain
cholinesterase activity, tremors, decreased parental and pup body
weights and decreased pup survival at 4 ppm and above. In a
three-generation study of reproductive toxicity in mice fed dietary
levels of 0, 0.6, 1.5 or 3 ppm, the NOAEL was 1.5 ppm, equivalent to
0.23 mg/kg bw per day, on the basis of slightly reduced lactation
indices at 3 ppm.
Two studies of teratogenicity were conducted with phorate in
rats, at dose levels of 0, 0.125, 0.25 or 0.5 mg/kg bw per day and
0, 0.1, 0.2, 0.3 or 0.4 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
severe maternal toxicity culminating in death, decreased fetal body
weights and delays in fetal skeletal ossification at 0.4 mg/kg bw
per day. There was no evidence of teratogenic potential at doses as
high as 0.4 mg/kg bw per day. At the maternally lethal dose of 0.5
mg/kg bw per day, there was an increased incidence of fetuses with
enlarged hearts. In rabbits treated with doses of 0, 0.15, 0.5, 0.9
or 1.2 mg/kg bw per day, maternal mortality and decreased body
weight were observed at doses of 0.5 mg/kg bw per day and above,
resulting in an NOAEL of 0.15 mg/kg bw per day. In the absence of
embryo- and fetotoxicity, the NOAEL for developmental toxicity was
the highest dose, 1.2 mg/kg bw per day. Phorate was not teratogenic
to rabbits.
Phorate has been adequately tested for genotoxicity in vitro
and in vivo in a battery of assays. The Meeting concluded that
phorate is not genotoxic.
No clinical or histopathological signs of delayed neuropathy
were seen in chickens.
Ninety-day studies of the toxicity of phorate administered in
the diet were conducted in rats with the cholinesterase-inhibiting
sulfoxide and sulfone metabolites of phorate. The metabolites of
phorate were marginally more potent inhibitors of brain
cholinesterase than phorate in female rats. Brain cholinesterase
activity was inhibited in animals of each sex after administration
of phorate at a dietary level of 6 ppm, but no significant
inhibition was apparent at 2 ppm in either sex. The sulfoxide and
sulfone metabolites of phorate inhibited brain cholinesterase only
in females treated at the highest dietary level, 2 ppm. There were
no significant differences in the erythrocyte cholinesterase
inhibiting activity of the metabolites and the parent compound,
phorate.
An ADI was allocated on the basis of a NOAEL of 0.05 mg/kg bw
per day in the one-year study of toxicity in dogs and the two-year
feeding study in rats. The effect noted in both species was
inhibition of brain cholinesterase activity, which was associated in
dogs with clinical signs consistent with cholinergic toxicity. A
safety factor of 100 was applied.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study
of toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of teratogenicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Adequate studies on absorption, distribution, excretion and
metabolism in rats. Studies known to exist may address this
need, in whole or in part. In order to maintain the ADI, these
data should be submitted in 1995, in time for review in 1996.
2. Observations in humans
References
American Cyanamid Co. (1965) Report on Thimet systemic insecticide:
successive generation studies with mice. Unpublished report from
Central Medical Department Cyanamid. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
American Cyanamid Co. (1976) Thimet soil and systemic insecticide.
Unpublished technical information from Cyanamid International Corp.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Blinn, R.C. (1982) Personal communication to WHO by American Cynamid
Co., Wayne, NJ, USA.
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Fletcher, D.W. (1984) 42-Day neurotoxicity study with phorate in
mature white leghorn chickens. Unpublished report from Bio-life
Associates Ltd, BLAL No. 83 DN 103, AC Protocol No. 981-84-114.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hutchison, E.B., Fegle, H.C., McNerney, J.M. & Levinskas, G.J.
(1978) Thimet(R) systemic insecticide: ninety-day repeated feeding
to albino rats (CL 18,161). Unpublished report from Central Medical
Department, American Cyanamid Co., Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Ivett, J.L. & Myhr, B.C. (1986) Chromosomal aberrations in vivo in
mammalian bone marrow cells on AC 35,024. Second amended report,
Project ID No. 8049, 10947-001. Unpublished report dated 9 January
1986 from Litton Bionetics, Kensington, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Kay, J.H. & Calandra, J.C. (1961) Effects of thimet phorate on
cholinesterase activity in the dog. Unpublished report from
Industrial Bio-test Lans, Inc. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Levinskas, G.J., Morici, B.S. & Shaffer, C.B. (1965) Thimet systemic
insecticide: demyelination studies in white leghorn hens.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Levinskas, G.J., McNerney, J.M. & Fegluy, H.C. (1968) Report on
sulfoxide of Thimet systemic insecticide: ninety-day repeated
feeding to albino rats. Unpublished report from Central Medical
Department, American Cyanamid Co. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1978) Teratology study in rats; Thimet(R)
phorate. Final unpublished report from Litton Bionetics, Inc.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Litton Bionetics (1981a) 18-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1981b) 24-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Lochry, E.A. (1990a) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) pilot study with AC 35,024 in rats. Final
report, Project ID No. 101P-012. Unpublished report dated 19 June
1990 from Argus Research Laboratories Inc., Horsham, PA, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Lochry, E.A. (1990b) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) definitive study with AC 35,024 in rats.
Final report, Project ID No. 101-012. Unpublished report dated 23
October 1990 from Argus Research Laboratories Inc., Horsham, PA,
USA. Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Newell, G.W. & Dilley, J.V. (1978) Teratology and acute toxicology
of selected chemical pesticides administered by inhalation. US
Environmental Protection Agency Report No. 600/1-78-003, January
1978. Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Piccirillo, V.J., Shellenberger, T.E. & Dauvin, E. M. (1987) 14-Day
range-finding oral toxicity study in the dog with AC 35,024. Revised
final report No. 85013. Unpublished report dated 11 February 1987
from Tegeris Laboratories Inc., Princeton, NJ, USA. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1986) A range-finding teratology study
with phorate in rabbits. Final report, project No. 86-3038.
Unpublished report dated 5 August 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1987) A teratology study with phorate
in rabbits. Final report, project No. 86-3039. Unpublished report
dated 6 April 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1991) A two-generation (two litters)
reproduction study with AC 35,024 to rats. Final report, Project ID
No. 88-3350. Unpublished report dated 23 September 1991 from
Bio/dynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Shellenberger, T.E. & Tegeris, A.S. (1987) One-year oral toxicity
study in purebred beagle dogs with AC 35,024. Final report No.
85015. Unpublished report dated 20 February 1987 from Tegeris
Laboratories Inc., Laurel, MD, USA. Submitted to WHO by American
Cyanamid Co., Princeton, NJ, USA.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1987) Evaluation of
selected pesticides as chemical mutagens in in vitro and in vivo
studies. Unpublished report from Stanford Research Institute, SRI
Report No. LSU-3493, for the US Environmental Protection Agency, EPA
Report No. EPA-600/1-77-028. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Thilagar, A. & Kumarop, V. (1985) Test for chemical induction of
gene mutation at the hgrpt locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Final report,
Study No. 980-85-133. Unpublished report dated 8 August 1985 from
Sitek Research Laboratories, Rockville, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Trutter, J.A. (1990) 13-Week dietary toxicity study in albino mice
with AC 35,024. Final report, Project ID No. 362-201. Unpublished
report dated 16 May 1990 from Hazleton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Tusing, T.W., Kindzin, W., Hanzal, R. & Howard, J. (1956a) Repeated
oral administration (dogs). Experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. & Howard, J.
(1956b) Subacute feeding study--experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Table 1. Acute toxicity of technical-grade phorate
Species Sex Route LD50 (mg/kg bw per day) Reference
or LC50 (mg/m3)
Mouse NR Oral 11 Blinn, 1982
NR Intraperitoneal 3 Blinn, 1982
Rat NR Oral 1.9-10 Blinn, 1982
M&F Oral M: 2.3; F: 1.1 Gaines, 1969
M&F Oral M: 2.8; F: 1.6 Anon., 1976
M&F Oral M: 3.7; F: 1.4 Newell & Dilley, 1978
NR Dermal 3 Blinn, 1982
M&F Dermal M: 6.2; F: 2.5 Gaines, 1969
M Dermal 5.7 American Cyanamid Co., 1976
M&F Dermal M: 9.3; F: 3.9 Newell & Dilley, 1978
M&F Intravenous M: 2.2; F: 1.2 Newell & Dilley, 1978
M&F Inhalation (1 h) M: 60 Newell & Dilley, 1978
F: 11 Newell & Dilley, 1978
Rabbit M Dermal 5.2 Anon., 1976
From Annex I, references 29 and 39; strains not specified
NR, not reported
(b) Short-term toxicity
Mice
Phorate (purity, 92.1%) was administered to groups of 20 male
and 20 female Crl:CD-1 (ICR)BR outbred Swiss mice at dietary levels
of 0, 1, 3 or 6 ppm, equal to 0.18, 0.55 or 1.10 mg/kg bw per day
for males and 0.23, 0.67 or 1.38 mg/kg bw per day for females, for
13 weeks. A single male mouse treated at the highest dietary level
had convulsions on one occasion during the final week of treatment,
but the relationship of this isolated finding to treatment could not
be ascertained; no other remarkable clinical signs were reported.
Treatment had no effect on survival, food consumption or body-weight
gain. Histopathological examinations were not performed. Plasma,
erythrocyte and brain cholinesterase activities were measured before
terminal sacrifice. Plasma cholinesterase activity was significantly
( p < 0.05) inhibited in animals of each sex at 3 and 6 ppm and in
females at 1 ppm. Erythrocyte cholinesterase levels were
significantly decreased in males and females at the highest dose;
the inhibition, in relation to values in concurrent controls, was
50% in males and 61% in females. A slight, non-significant decrease
in erythrocyte cholinesterase activity (17%) was recorded in females
at 3 ppm. Brain cholinesterase levels, assayed in both halves of the
brain, were significantly ( p < 0.05) decreased in animals of each
sex treated at 3 and 6 ppm, by slightly more than 10% at 3 ppm and
to about 50% of control values at 6 ppm. Minimal inhibition of
plasma cholinesterase levels (15%) in females at 1 ppm was not
considered to represent an adverse effect of treatment, particularly
in the absence of corresponding decreases in erythrocyte and brain
cholinesterase levels. The NOAEL was 1 ppm, equal to 0.18 mg/kg bw
per day, on the basis of significantly reduced brain cholinesterase
activity at dietary levels of 3 and 6 ppm (Trutter, 1990).
Rats
Groups of 50 male and 50 female rats [strain not specified]
were fed phorate (purity, 92%) in the diet at levels of 0, 0.22,
0.66, 2 or 6 ppm, equivalent to 0.01, 0.03, 0.1 or 0.3 mg/kg bw per
day, and groups of 25 male and 25 female rats were fed 12 or 18 ppm,
equivalent to 0.6 or 0.9 mg/kg bw per day. Treatment continued for
13 weeks, and cholinesterase activity was monitored each week.
Occasional episodes of excitability and intermittent tremors were
noted in females given 6 ppm, and animals of each sex receiving 12
and 18 ppm showed severe excitability, intermittent tremors and
ataxia, culminating in death of 50% of animals at 12 ppm and no
survivors at 18 ppm. Levels of 6 ppm and higher produced significant
depression of plasma, erythrocyte and brain cholinesterase activity
in animals of each sex and decreased erythrocyte cholinesterase
activity in females at 2 ppm. In all groups receiving 6 ppm or less,
growth, food consumption, survival and liver and kidney weights and
ratios were within normal limits, and no abnormalities were seen at
gross necropsy or histological examination. The NOAEL was 2 ppm,
equivalent to 0.1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity at 6 ppm and above (Tusing et al.,
1956a; Annex I, reference 29).
Dogs
Groups of two male and one female mongrel dogs received
capsules containing 0, 0.01, 0.05, 0.25 or 1.25 mg/kg bw per day of
phorate (purity, 92%) in corn oil on six days per week for 15 weeks.
Two males received a single dose of 2.5 mg/kg bw. Plasma and
erythrocyte cholinesterase activities were monitored weekly; brain
cholinesterase activity was not measured. At 0.05 mg/kg bw per day,
plasma enzyme activity was significantly depressed. Erythrocyte
cholinesterase activity was not affected during the first 12 weeks
but was depressed slightly (not significantly) during the last three
weeks of the study. Significant decreases in plasma and erythrocyte
enzyme activity were observed at 0.25 mg/kg bw per day, and 1.25
mg/kg bw per day induced total inhibition of plasma cholinesterase
activity and a significant reduction in erythrocyte cholinesterase
activity. All of the dogs administered the single dose of 2.5 mg/kg
bw per day died within 3-4 h; enzyme activity was not determined. No
signs of systemic toxicity were seen, and haematological, clinical
chemistry and urine analyses showed no effects in dogs receiving
0.01 or 0.05 mg/kg bw per day. Histopathological examination
revealed no consistent findings related to the treatment (Tusing et
al., 1956b; Annex I, reference 29).
Groups of three male and three female beagle dogs were fed
phorate in the diet at 0, 0.5 or 1.0 ppm, equivalent to 0.012 or
0.025 mg/kg bw per day, for six weeks. Cholinesterase activity in
plasma and erythrocytes was determined before and every two weeks
during the test. Analyses of variance revealed no significant
difference between the treated and control animals with respect to
plasma and erythrocyte enzyme activities (Kay & Calandra, 1961;
Annex I, reference 29).
In a preliminary range-finding study, groups of two male and
two female beagle dogs were given capsules containing phorate
(purity, 92.1%) in corn oil at doses of 0.01, 0.05, 0.10, 0.25 or
0.50 mg/kg bw per day for 14 days. A control group of three male and
three female dogs was given capsules containing corn oil. Plasma and
erythrocyte cholinesterase levels were measured twice before
treatment, and after 3, 7, 10 and 14 days of treatment; brain
cholinesterase levels were determined in the cerebellum and cerebrum
of each dog at sacrifice. Excessive salivation and tremors were seen
in one female dog given the highest dose, and body-weight gain was
decreased in comparison with the controls in animals of each sex.
Slight decreases in total serum protein were recorded in males and
females at the high dose. Treatment did not affect survival, food
consumption, haematological parameters or organ weights, and gross
pathology showed no effects; histopathological examinations were not
performed. Plasma cholinesterase levels were significantly decreased
in animals of each sex given 0.10 mg/kg bw per day or more and in
males given 0.05 mg/kg bw per day, in comparison with values before
treatment and in control animals, and erythrocyte cholinesterase
activity was significantly (> 20%) inhibited in both males and
females at the highest dose of 0.50 mg/kg bw per day. Brain
cholinesterase activity was inhibited (31-69%) in both males and
females at 0.10 mg/kg bw per day or more. The NOAEL was 0.05 mg/kg
bw per day (Piccirillo et al., 1987).
Phorate (purity, 92.1%) in corn oil was administered orally by
capsule to groups of six male and six female beagle dogs at doses of
0.005, 0.01, 0.05 or 0.25 mg/kg bw per day for one year. Eight male
and eight female controls received capsules containing only corn
oil. Plasma and erythrocyte cholinesterase activities were measured
twice before treatment, at six weeks, at three and six months and at
sacrifice; brain cholinesterase levels were measured in the cerebrum
and cerebellum at termination of treatment. Mild tremors were
observed occasionally in one male and two females during weeks 23-52
of treatment with the high dose. Mean body weights and overall
weight gain (26% less than control) were decreased in males at 0.25
mg/kg bw per day. Ophthalmological examination of all dogs before
treatment, at six months and at one year showed no treatment-related
effects. There were no adverse effects on food consumption or
haematological or urinary parameters. Decreased ( p < 0.05) total
protein levels were recorded at week 6, at three and six months and
at termination of treatment in males treated at 0.25 mg/kg bw per
day. Although the values were reported to be within the range in
historical controls, no supporting data were provided. As the
individual levels of total protein in males at the high dose were
consistently below concurrent values before treatment and in
controls, the finding appeared to be related to treatment. The
decreases ( p < 0.05) in total protein values in females and the
decreased albumin values in males at the high dose were within the
lower limits of control values. Gross and histopathological
examination revealed no lesions that could be attributed to
treatment with phorate. The highest dose induced inhibition of
erythrocyte (> 20%) and brain cholinesterase activity (43-54%);
plasma cholinesterase levels were significantly decreased in animals
of each sex at doses of 0.05 mg/kg bw per day and above. The
decrease in plasma cholinesterase activity at this dose was not
considered to be adverse in the absence of correlative inhibition of
brain and erythrocyte cholinesterase activity and evidence of
clinical toxicity. The NOAEL was 0.05 mg/kg bw per day on the basis
of decreased body weight, significant inhibition of erythrocyte and
brain cholinesterase activity and clinical signs consistent with
cholinergic toxicity at the high dose of 0.25 mg/kg bw per day
(Shellenberger & Tegeris, 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female 41-day-old CD-1 outbred Swiss
albino mice were fed technical-grade phorate (purity, 85.5%) in the
diet at 0, 1, 3 or 6 ppm, equivalent to 0.15, 0.45 or 0.9 mg/kg bw
per day for 18 months. All animals that died or were killed in
moribund condition during the study or were sacrificed terminally
were subjected to gross and histopathological examination. Survival
was not adversely affected: 78-90% of males and 66-74% of females in
the control and treated groups were still alive at the end of the
experiment. The growth of females at 6 ppm was retarded almost
throughout the experiment. All treated animals appeared to eat less
food during the first three weeks and occasionally thereafter, but
no consistent dose-response relationship was seen. Some clinical
signs, such as tremors, hyperactivity and excessive salivation,
occurred at higher incidence and more frequently in animals at the
highest dose than in controls. Gross pathological examination showed
no changes significantly different from those in the controls, and
microscopic evaluation of a wide range of tissues from each animal
revealed no alterations related to treatment. There was no
significant dose-related increase in the incidence of any particular
type of tumour, of animals with tumours, of animals with malignant
tumours or of animals with multiple primary tumours. Although the
incidence of alveolar and bronchiolar adenoma appeared to be
increased in males at 6 ppm (8/50, compared with 3/50 in controls),
this effect was not considered to be related to treatment since the
increase was not statistically significant and this tumour is known
to occur frequently in CD-1 mice. The NOAEL was 3 ppm, equivalent to
0.45 mg/kg bw per day, on the basis of decreased body weight and
clinical signs of toxicity at 6 ppm (Litton Bionetics, 1981a; Annex
I, reference 39).
Rats
Groups of 50 male and 50 female five-week-old Crl:COBS CD(SD)
BR rats were fed dietary levels of technical-grade phorate (purity,
84.5%) at 0, 1, 3 or 6 ppm, equal to 0.05, 0.16 or 0.32 mg/kg bw per
day in males and 0.07, 0.19 or 0.43 mg/kg bw per day in females, for
24 months. The mortality rate appeared to be increased in females at
6 ppm, as only 36% survived to the end of the experiment; however,
more than 60% of animals in all groups, including the controls,
lived at least 90 weeks. The only clinical sign related to treatment
was tremors induced by over-dosing (327% of all the intended doses)
during week 9. Growth was depressed in females at 6 ppm during the
first 26 weeks and again between weeks 74 and 102. Food consumption
showed no consistent dose-response pattern. On haematological
examination, clinical chemistry and urinalysis performed at 6, 12
and 24 months, the only notable findings were decreased values for
erythrocyte counts, haemoglobin and haematocrit in females at the
highest dose at 12 months. Dose-related inhibition (> 20%) of
plasma cholinesterase activity was noted in males at 6 ppm at 12
months, in all treated males at 24 months and in females at both 3
and 6 ppm at all sampling intervals (3, 6, 12 and 24 months).
Erythrocyte cholinesterase activity was not significantly depressed
(< 20%) at any time. The activity of brain cholinesterase was
reduced (> 20%) in males at 6 ppm and in females at 3 ppm or more.
At sacrifice, females given the highest dose had increased
organ:body weight ratios with respect to the adrenals, brain, heart,
liver and spleen. On gross pathological and histopathological
examination of a variety of tissues, the only effect apparently
related to treatment was a significant increase in the incidence of
inflammation and epithelial hyperplasia of the forestomach in
animals of each sex, but particularly in males, at 6 ppm. The most
prevalent types of spontaneous tumours were pituitary adenomas in
animals of each sex and mammary tumours in females. There were no
significant differences between control and treated groups with
regard to incidence, type or time of appearance of tumours. The
marginal no-effect level was 1 ppm, equal to 0.05 mg/kg bw per day
(Litton Bionetics, 1981b; Annex I, reference 39).
(d) Reproductive toxicity
Mice
Groups of eight male and 16 female mice (strain not specified)
were fed phorate (purity unknown) in the diet at levels of 0, 0.6,
1.5 or 3 ppm, equivalent to 0.09, 0.23 or 0.45 mg/kg bw per day, for
three generations, with two litters per generation. There were no
dose-related effects on indices of fertility, gestation, viability
or lactation during the study, but the lactation index was slightly
lowered in the group receiving 3 ppm, to below the control value in
the first mating of the F0 generation, in both matings of the F1
generation and in the second mating of the F2 generation. Gross
and microscopic examination of tissues revealed no consistent
abnormalities related to treatment. The NOAEL was 1.5 ppm,
equivalent to 0.23 mg/kg bw per day, on the basis of decreased
lactation indices at 3 ppm (American Cyanamid Co., 1965; Annex I,
reference 39).
Rats
A two-generation study of reproductive toxicity was conducted
in groups of 25 male and 25 female COBS CD(SD) rats given phorate
(purity, 92.1%) at dietary levels of 0, 1, 2, 4 or 6 ppm, equal to
0.09, 0.17, 0.35 or 0.52 mg/kg bw per day in males and 0.10, 0.20,
0.40 or 0.62 mg/kg bw per day in females, for a minimum of 60 days
before mating, to produce two consecutive litters (F1a and F1b)
of the first (F0) parental generation. Groups of 25 males and 25
females were then selected from the F1b litters to become the
second-generation (F1) parental animals. Owing to a high incidence
of mortality during the post-weaning period among F1b pups at 6
ppm, 30 males and 30 females at this dose were selected as F1
parents. The second-generation parental animals were treated for a
minimum of 100 days before mating to produce two consecutive litters
(F2a and F2b). Animals were exposed continuously to the test
diets before mating and throughout weaning of the offspring in both
generations. Cholinesterase levels were determined in plasma,
erythrocytes and brain from 10 parental F1 rats of each sex in
each group at terminal sacrifice.
A treatment-related decrease in body weight and tremors were
seen at > 4 ppm in parental animals; at 6 ppm, increased
mortality and clinical signs were seen, manifested as convulsive
movements and aggressive behaviour. Routine clinical observation of
the animals revealed an increased incidence of ocular effects at 6
ppm, described as exophthalmus, 'protrusion of tissue off the
cornea' and opacities. Subsequent ophthalmological evaluation
revealed markedly higher incidence and severity of infectious
sequelae affecting the cornea, anterior uveal tract and posterior
segment of the eye in rats at this dose. The increased
susceptibility of rats to ocular disease at the high dose was
considered to be a secondary effect of treatment with phorate.
Plasma cholinesterase levels were significantly decreased in females
at 4 and 6 ppm and in males at 6 ppm. Erythrocyte activity was
inhibited only slightly (10-11%) in animals of each sex at 6 ppm.
Inhibition of brain cholinesterase activity was significant in males
(40%) at 6 ppm and in females at 4 ppm (59%) and 6 ppm (83%). There
was no significant inhibition of brain cholinesterase activity at
the lower dietary levels.
Pup survival during the lactation periods was significantly
reduced in all litters from both parental generations at 6 ppm and
in the F2a litter at 4 ppm. The size of both litters from the F1
generation was slightly decreased at 6 ppm. Mean pup weights were
decreased in both F1a and F1b litters of animals receiving 4 or
6 ppm. The mean weights of pups of the F2a litter were decreased
at 4 ppm (those of the F2b litter were not affected) and in pups
of both F2a and F2b litters at 6 ppm. Anogenital staining was
recorded in F1b and F2b pups at 6 ppm but not in control animals
or in those receiving lower dietary levels. There were no adverse
effects on mating or fertility indices, pregnancy rate, length of
gestation, gestation index or sex distribution ratios, and no gross
morphological alterations or pathological changes were seen in the
reproductive organs at any of the dietary levels studied. The NOAEL
was 2 ppm, equal to 0.17 mg/kg bw per day (Schroeder & Daly, 1991).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 mated female Crl:COBS CD (SD)BR rats were given
technical-grade phorate (purity, 91.7%) by gastric intubaton at 0,
0.125, 0.25 or 0.5 mg/kg bw per day on gestation days 6-15 and were
sacrificed on day 20 of gestation. The fetuses were removed for
gross, skeletal and visceral examination. The pregnancy rate was
comparable in all groups. During the gestation period, 7/23 pregnant
dams at 0.5 mg/kg bw per day, none at 0.25 mg/kg bw per day and 1/24
at 0.125 mg/kg bw per day died. Fetuses at 0.5 mg/kg bw per day had
an increased frequency of enlarged heart (not otherwise
characterized). Clinical signs, body weight and food consumption of
dams during gestation, the number of implantation sites, the number
of resorptions, the number of dead fetuses, mean live litter size,
average fetal weight, sex ratio, and gross, skeletal and visceral
abnormalities of fetuses were not significantly different from those
in the controls. The NOAEL for teratogenicity was 0.25 mg/kg bw per
day (Litton Bionetics, 1978; Annex I, reference 39).
In a preliminary range-finding study, groups of eight mated
female Crl:CDBRVAF/Plus (SD) rats were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.25, 0.5, 0.7 or 0.9
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which it occurred was considered day 0 of gestation.
Treatment with doses > 0.5 mg/kg bw per day was lethal to the
dams, and there were no survivors at these doses after day 12 of
gestation. Overt clinical signs of toxicity preceding death included
twitches, tremors, excessive salivation, exophthalmos, ataxia,
clonic convulsion, and decreased body weight and food intake. Gross
examination of rats which died revealed enlarged and/or congested
adrenal glands. The NOAEL was 0.25 mg/kg bw per day for both
maternal and developmental toxicity (Lochry, 1990a).
In a study of teratogenicity, groups of 24-25 mated female
Crl:CDBRVAF/Plus(SD) rats were treated by gavage with phorate
(purity, 92.1%) in corn oil at doses of 0, 0.1, 0.2, 0.3 or 0.4
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which this occurred was considered day 0 of
gestation. Six of 25 females at 0.4 mg/kg bw per day that died
during days 11-16 of gestation had enlarged adrenal glands. No
deaths were reported at lower doses. Clinical signs of toxicity seen
at 0.4 mg/kg bw per day were an increased number of rats with
tremors, excessive salivation, chromodacryorrhoea and rhinorrhoea,
urine-stained abdominal fur, decreased motor activity, hunched
posture, impaired righting reflex and laboured breathing. Maternal
body weights, corresponding weight gains and food consumption and
fetal body weights were significantly decreased at 0.4 mg/kg bw per
day when compared with control values. Increased incidences of
skeletal variations were observed in fetuses and/or litters at this
dose, which were related to delays in ossification of the
sternebrae, incompletely and unossified pubes and incompletely
ossified ischia. The NOAEL for maternal and developmental toxicity
was 0.3 mg/kg bw per day on the basis of mortality, clinical signs
of toxicity, and significantly decreased body weights and food
consumption in the mothers and decreased fetal body weights and
potentially reversible delays in skeletal ossification at 0.4 mg/kg
bw per day (Lochry, 1990b).
Groups of 10 pregnant Sprague-Dawley rats were exposed by nose
only in an inhalation chamber to aerosols of technical-grade phorate
(purity, 78-90%) with a count median diameter of 0.57 Ám, generated
from a 1% solution of phorate in xylene, for 1 h per day on days
7-14 of gestation at concentrations of 0.15 ▒ 0.04, 0.4 ▒ 0.15 or
1.94 ▒ 0.48 mg/m3. Three groups of 15 dams were exposed to xylene
or air or to a restricted diet. (The reason for including the last
group was not specified.) All dams were sacrificed on day 20 of
gestation, and fetuses were removed by caesarean section for
visceral and skeletal examination. Five dams at the highest dose
died, and toxic signs (tremors, lachrymation and exophthalmus) were
noted in animals at this level; one of the dead dams had resorbed
the entire litter. Average percentage fetal mortality was markedly
increased at this dose. The summary indicated no treatment-related
effects on the body weight or food consumption of dams during
gestation and no effects on pregnancy rate, average number of
implants, average fetal weight, average number of sternal
ossification centres or the incidence of supernumerary ribs.
Specific information on the incidence of any gross or soft-tissue
abnormalities was not provided (Newell & Dilley, 1978; Annex I,
reference 39).
Rabbits
In a preliminary range-finding study, groups of five mated
female New Zealand white rabbits were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.3, 0.6, 0.9, 1.2 or
1.5 mg/kg bw per day on days 6-18 of gestation. The day on which
coitus with two successive males was observed was designated day 0
of gestation. The incidences of mortality in the six groups were
reported as 0/5, 1/5, 1/5, 1/5, 2/5 and 4/5, respectively. Mean
body-weight loss was seen at 1.2 mg/kg bw per day. Only one female
at the highest dose survived to scheduled sacrifice. Food intake was
generally decreased in the groups receiving 0.3, 0.6, 0.9 and 1.2
mg/kg bw per day, although no clear dose-response relationship was
seen. Increased numbers of resorptions and post-implantation losses
were reported at doses > 0.6 mg/kg bw per day. Decreased mean
fetal body weights and shorter crown-rump lengths were noted at 1.2
mg/kg bw per day. External examination of the fetuses showed no
treatment-related malformations or alterations. The only significant
effects observed at the LOAEL of 0.3 mg/kg bw per day were a single
maternal death and depressed food intake (Schroeder & Daly, 1986).
Groups of 20 mated female New Zealand white rabbits were given
phorate (purity, 92.1%) in corn oil by gavage on days 6-18 of
gestation at doses of 0, 0.15, 0.5, 0.9 or 1.2 mg/kg bw per day. The
day on which coitus with two successive males was observed was
designated day 0 of gestation. At 1.2 mg/kg bw per day, 8/20 females
died during days 14-19 of gestation, one female aborted and another
female delivered prematurely on day 28 of gestation, so that only
10/20 dams survived to scheduled sacrifice. The deaths of two
females at 0.9 and one at 0.5 mg/kg bw per day were also attributed
to treatment with phorate. Anogenital staining was observed at a
higher incidence in females treated with the highest dose. Mean body
weights and corresponding weight gains were decreased at doses >
0.5 mg/kg bw per day. Food consumption was decreased in females
treated at 1.2 mg/kg bw per day. Treatment had no effect on
pre-implantation loss, number of resorptions, number of live
fetuses, fetal body weight or fetal sex ratio. At 1.2 mg/kg bw per
day, all three fetuses in a single litter (five of eight
implantation sites showed early resorptions) had open eyelids,
curved scapulae, an absent supraorbital process, an irregular margin
of the frontals and a displaced anterior fontanel. Although these
anomalies were not observed in the control group, the incidence of
open eyes was within that of historical controls; curved scapulae
had not previously been noted, however, in 1852 fetuses from 269
litters. Ocular and scapular defects were not seen in other fetuses
from dams treated at this dose and were not recorded in any of the
fetuses in the preliminary range-finding study. Although the defects
seen in this single litter may have been a secondary response to the
maternal toxicity of the high dose, the weight of evidence would
suggest that the findings were spurious and perhaps genetically
predicted. There was insufficient evidence to suggest that phorate
is teratogenic to rabbits. The NOAEL for maternal toxicity was 0.15
mg/kg bw per day, as determined by a higher incidence of mortality
and decreased body weights at > 0.5 mg/kg bw per day. In the
absence of embryo- or fetotoxicity, the NOAEL for developmental
toxicity was the highest dose, 1.2 mg/kg bw per day (Schroeder &
Daly, 1987).
(f) Genotoxicity
A battery of assays were performed to test the genotoxicity of
phorate, the results of which are presented in Table 2. Assays for
gene mutation, chromosomal aberrations and other cytogenetics
end-points in vitro and in vivo gave negative results. No
unscheduled DNA synthesis was seen in human fibroblasts, and
dominant lethal mutations were not induced in male mice.
Table 2. Results of tests for the genotoxicity of phorate
End-point Test system Concentration Purity Results Reference
of phorate (%)
In vitro
Reverse S. typhimurium Up to 1000 mg/plate Technical Negativea Simmon et al., 1977b
mutation TA100, 1535, 1537, grade
1538; E. coli WP2
Reverse E. coli p3478, 1 mg (on filter disc) Technical Negativec Simmon et al., 1977b
mutation W3110; B. subtilis per plate grade
Reverse Chinese hamster 30, 40, 50, 80, 100 92.1 Negativec Thilager & Kumarop,
mutation ovary cells, hprt nl/ml 1985
locus 5, 10, 12, 14, 16, 18, Negatived
20 nl/ml
Mitotic S. cerevisiae D3 5% w/v for 4-h Technical Negativea Simmon et al., 1977b
recombination incubation before grade
plating
Unscheduled Human fibroblasts Up to 1 x 10-3 Technical Negativea Simmon et al., 1977b
DNA synthesis WI-38 grade
In vivo
Chromosomal Male and female M: 0 (corn oil), 0.25, 92.1 Negative Ivett & Myhr, 1986
aberration Sprague-Dawley 1.25, 2.5 mg/kg bw
rats, killed after per day; F: 0, 0.13,
6, 18, 30 h 0.63, 1.25 mg/kg bw
per day
Dominant Male mice 0, 5, 10, 20 mg/kg bw Technical Negative Simmon et al., 1977b
lethal per day in diet for grade
mutation 7 weeks, weekly
matings for 8 weeks
a In the presence and absence of metabolic activation
b From Annex 1, reference 46
c In the absence of metabolic activation
d In the presence of metabolic activation
(g) Special studies
Delayed neuropathy in chickens
Groups of six adult hens were fed 0 or 40 ppm phorate (purity
unspecified), equivalent to 5 mg/kg bw per day, in the diet for four
weeks. A third group received 4000 ppm tri- ortho-tolyl phosphate
as a positive control. Each hen was anaesthetized and immediately
perfused with buffered formalin, and sections of brain, lower
thoracic cord and each sciatic nerve were prepared for microscopic
examination. Tri- ortho-tolyl phosphate induced myelin loss in each
hen, but phorate had no adverse effects on nerve fibres or the
myelin sheath (Levinskas et al., 1965; Annex I, reference 29).
Phorate (purity, 89.5%) dissolved in corn oil was administered
by gavage to fasted white Leghorn hens (22-23-months old). The
LD50 by a single oral dose was 14.2 mg/kg bw. Fifty fasted hens
were then given an intramuscular injection of 10 mg/kg bw of
atropine sulfate and 1 h later given a single oral dose of 14.2
mg/kg bw phorate. An additional 15 fasted, atropinized hens were
given corn oil only, and 15 hens that did not receive atropine were
given tri- ortho-tolyl phosphate at 500 mg/kg bw as a positive
control. All surviving hens in the test and vehicle control groups
received the same doses 21 days later, except that atropine sulfate
was administered at 30 mg/kg bw. All hens were observed daily for
mortality, clinical signs and evidence of neurotoxic reactions. Body
weights and food consumption were recorded every three days. All
hens that died during the study and all hens that were killed at
termination of the study at 42 days were subjected to gross
necropsy. Those killed at the end of the study were perfused with
10% neutralized formalin, and brains, vertebral columns (with spinal
cord in situ) and the entire right and left sciatic nerves were
excised and fixed. Microscopic slides of neural tissue were prepared
by taking a sagittal section of the entire brain (corpus striatum,
cerebellum, pons), longitudinal and cross-sections of the cervical,
thoracic and lumbrosacral levels of the spinal cord and both
sagittal and longitudinal sections of the right and left sciatic
nerves. Sections were stained with haematoxylin and eosin, and
replicate sections were stained with luxol fast blue. Tissues from
10 hens in each group were examined histologically.
None of the 15 hens fed corn oil died during the 42-day study,
and all 15 hens given tri- ortho-tolyl phosphate were killed in
extremis on day 16 of the study after clinical signs of
neuropathy, first observed on day 11, became progressively more
severe. These signs included generalized weakness, ataxia and
paralysis of the legs and wings. Of the 50 hens treated with
phorate, 27 died within 24 h of the first dose and 13 more within 24
h of the second dose. Ten hens survived to termination of the study.
Vehicle control and phorate-treated hens had slight generalized
weakness of the limbs, lasting about 2 h, shortly after each
treatment with atropine sulfate; treated hens had slightly more
severe reactions and, in addition, slight to moderate ataxia for up
to 2 h after treatment with phorate. No clinical signs of delayed
neuropathy were observed, however, in any vehicle-control or test
birds. In comparison with vehicle controls, the mean body-weight
gains of tested hens were greater at 0-21 days and smaller at 21-42
days, and the mean feed consumption of test hens was smaller at 0-21
days and greater at 21-42 days. No effects attributable to phorate
were seen at gross necropsy.
Histological examination of neural tissues from
positive-control hens revealed treatment-related lesions involving
the brain, spinal cord and/or sciatic nerves in all 10 birds.
Generally mild to moderate axonal degeneration was observed in the
brains of 4/10 hens, in the spinal cords of 10/10 hens and in the
sciatic nerves of 10/10. In addition, demyelination was observed in
the spinal cords and in the sciatic nerves of 7/10 hens;
Schwann-cell hyperplasia was also observed in the sciatic nerves of
3/10 hens. These lesions were compatible with a delayed neurotoxic
response induced by tri- ortho-tolyl phosphate. Minimal to mild
focal axonal degeneration of the sciatic nerves was noted in 3/10
hens treated with phorate; no axonal degeneration was seen in
vehicle-control hens. The axonal degeneration observed in the
treated birds was associated with interstitial infiltration of
lymphoid cells, which was also observed in other test and
vehicle-control hens. This syndrome, which was distinctly different
from that observed in the positive-control hens, was ascribed to
lesions of a naturally occurring disease (Marek's disease) and was
considered not to be related to treatment with phorate. Treatment
did not induce clinical or histopathological signs indicative of
acute delayed neuropathy (Fletcher, 1984; Annex I, reference 46).
3. Studies on metabolites
(a) Enzymes and biochemical parameters
The metabolites of phorate that inhibit cholinesterase are
phorate sulfoxide and sulfone and the oxygen analogue phoratoxon and
its sulfoxide and sulfone. The negative logarithms of the molar
concentrations of these compounds that induce 50% inhibition of
erythrocyte cholinesterase are as follows: phorate, 3.17; phorate
sulfoxide, 3.35; phorate sulfone, 5.00; phoratoxon, 5.87; phoratoxon
sulfoxide, 6.76; and phoratoxon sulfone, 7.02 (Annex I, reference
29).
(b) Acute toxicity
The acute toxicity of metabolites of phorate was studied in
rats and mice; the results are shown in Table 3. Both the parent
compound and the metabolites are extremely acutely toxic.
Table 3. Acute toxicity of phorate and its metabolites
Compound LD50 in rats (mg/kg bw per day) LD50 in mice (mg/kg bw per day)
Oral Intraperitoneal Oral Intraperitoneal
Phorate 1.9-10 3 11 3
Phorate sulfoxide 2-4 11 7 1
Phorate sulfone 1.8-2.0 27 9 2
Phoratoxon 0.6-0.8 - - -
Phoratoxon sulfoxide 1.4-1.6 1 3 0.02
Phoratoxon sulfone 0.6-0.8 1.8 5 0.4
From Blinn, 1982; Annex I, reference 39
(c) Short-term toxicity
Rats
Groups of 35 male and 35 female rats (strain not specified)
were fed phorate sulfoxide (purity, 92%) at dietary levels of 0.32,
0.8 or 2 ppm, equivalent to 0.02, 0.04 or 0.1 mg/kg bw per day, for
90 days. Fifty males and 50 females served as controls. Brain,
erythrocyte and plasma cholinesterase activities were determined
twice a week. A significant ( p < 0.05) depression of erythrocyte
and brain cholinesterase activity was seen in females at 2 ppm; the
decrease in plasma cholinesterase activity at this dose was less
consistent and was considered to be borderline. Borderline
depression of erythrocyte enzyme activity was seen at 0.8 ppm. At
0.32 ppm, all values were within acceptable statistical limits for
animals of each sex. No effects were observed on other
haematological parameters, organ weights or ratios, and no
consistent histopathological effect was noted that could be
attributed to feeding 2 ppm or less of phorate sulfoxide (Levinskas
et al., 1968; Annex I, reference 29).
Groups of 30 male and 30 female Charles River CD strain rats
(50 male and 50 female controls), 51 days of age, were fed phorate
sulfone (purity, 92%; containing 6% unchanged phorate and about 2%
phorate sulfoxide) in the diet at 0, 0.32, 0.8 or 2 ppm, equivalent
to 0.02, 0.04 or 0.1 mg/kg bw per day, for 90 days. None of the
animals died before the end of the experiment, and there were no
treatment-related changes in appearance or behaviour. Weight gain
and increased food consumption were seen in males fed 0.8 or 2 ppm.
Assay of tissue cholinesterase activity five times during the course
of the experiment showed inhibition (> 20%) of erythrocyte
cholinesterase at 2 ppm in animals of each sex at most time
intervals. Plasma cholinesterase activity was reduced by 23-27% in
males at 2 ppm after one, three and five weeks and by 25-72% in
females at 2 ppm after all sampling intervals; plasma cholinesterase
was also inhibited (39%) in females at 0.8 ppm after one and three
weeks. The activity of brain cholinesterase was reduced (> 20%)
only in females at 2 ppm after three, five and eight weeks. Control
and treated groups did not differ significantly with regard to
values for haematocrit, haemoglobin and total leukocyte count at the
end of the experiment. No treatment-related effects were observed on
the absolute weights of kidneys or liver, and no gross pathological
changes were seen. Histopathological evaluation of a variety of
tissues from five males and five females in the control and
highest-dose groups revealed no morphological alterations
attributable to treatment. The NOAEL was 0.32 ppm, equivalent to
0.02 mg/kg bw per day (Hutchison et al., 1978; Annex I, reference
39).
4. Observations in humans
No relevant information was available.
Comments
The limited data available indicate that phorate given orally
to rats is not readily eliminated, with less than 40% of the
administered radioactivity recovered within six days after
treatment. Urinary metabolites were identified as O,O-diethyl
O-hydrogen phosphoro-thioate, diethyl hydrogen phosphorate and
O,O-diethyl O-hydrogen phosphorodithioate.
Phorate was severely acutely toxic in mice, rats and rabbits
after administration by various routes. WHO (1992) has classified
phorate as extremely hazardous.
In a 13-week study of the toxicity study of dietary levels of
0, 1, 3 and 6 ppm phorate in mice, the NOAEL was 1 ppm, equal to
0.18 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity at dietary levels of 3 ppm and above.
In rats that received phorate in the diet at 0, 0.22, 0.66, 2,
6, 12 or 18 ppm for 13 weeks, the NOAEL was 2 ppm, equivalent to 0.1
mg/kg bw per day, on the basis of inhibition of brain cholinesterase
activity. At 6 ppm, cholinesterase inhibition was associated with
tremors and hyperexcitability and at 12 and 18 ppm, with death.
In a one-year study of toxicity in dogs, phorate was
administered orally in capsules at doses of 0, 0.005, 0.01, 0.05 or
0.25 mg/kg bw per day. The NOAEL was 0.05 mg/kg bw per day, as
determined by decreased body weights, significant inhibition of
erythrocyte and brain cholinesterase activity and clinical signs
consistent with cholinergic toxicity at 0.25 mg/kg bw per day. A
preliminary 14-day range-finding study, a six-week study of dietary
administration and a 15-week study in which phorate was given orally
in capsules supported the findings reported in the one-year study.
Mice and rats were treated with phorate at dietary levels of 0,
1, 3 or 6 ppm for 18 and 24 months, respectively. In mice, the NOAEL
was 3 ppm, equivalent to 0.45 mg/kg bw per day, on the basis of
decreased body weights and increased incidences of tremors,
hyperactivity and excessive salivation at 6 ppm. Cholinesterase
activity was not measured in this study. In the study of toxicity
and carcinogenicity in rats, the NOAEL was 1 ppm, equal to 0.05
mg/kg bw per day, on the basis of significant inhibition of brain
cholinesterase at 3 ppm and above. There was no apparent effect on
erythrocyte cholinesterase activity. Phorate was not carcinogenic
when fed to mice or rats at dietary levels of up to 6 ppm.
A two-generation study of reproductive toxicity in rats given
phorate at dietary levels of 0, 1, 2, 4 or 6 ppm showed no adverse
effects on reproductive parameters. The NOAEL was 2 ppm, equal to
0.17 mg/kg bw per day, on the basis of decreased brain
cholinesterase activity, tremors, decreased parental and pup body
weights and decreased pup survival at 4 ppm and above. In a
three-generation study of reproductive toxicity in mice fed dietary
levels of 0, 0.6, 1.5 or 3 ppm, the NOAEL was 1.5 ppm, equivalent to
0.23 mg/kg bw per day, on the basis of slightly reduced lactation
indices at 3 ppm.
Two studies of teratogenicity were conducted with phorate in
rats, at dose levels of 0, 0.125, 0.25 or 0.5 mg/kg bw per day and
0, 0.1, 0.2, 0.3 or 0.4 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
severe maternal toxicity culminating in death, decreased fetal body
weights and delays in fetal skeletal ossification at 0.4 mg/kg bw
per day. There was no evidence of teratogenic potential at doses as
high as 0.4 mg/kg bw per day. At the maternally lethal dose of 0.5
mg/kg bw per day, there was an increased incidence of fetuses with
enlarged hearts. In rabbits treated with doses of 0, 0.15, 0.5, 0.9
or 1.2 mg/kg bw per day, maternal mortality and decreased body
weight were observed at doses of 0.5 mg/kg bw per day and above,
resulting in an NOAEL of 0.15 mg/kg bw per day. In the absence of
embryo- and fetotoxicity, the NOAEL for developmental toxicity was
the highest dose, 1.2 mg/kg bw per day. Phorate was not teratogenic
to rabbits.
Phorate has been adequately tested for genotoxicity in vitro
and in vivo in a battery of assays. The Meeting concluded that
phorate is not genotoxic.
No clinical or histopathological signs of delayed neuropathy
were seen in chickens.
Ninety-day studies of the toxicity of phorate administered in
the diet were conducted in rats with the cholinesterase-inhibiting
sulfoxide and sulfone metabolites of phorate. The metabolites of
phorate were marginally more potent inhibitors of brain
cholinesterase than phorate in female rats. Brain cholinesterase
activity was inhibited in animals of each sex after administration
of phorate at a dietary level of 6 ppm, but no significant
inhibition was apparent at 2 ppm in either sex. The sulfoxide and
sulfone metabolites of phorate inhibited brain cholinesterase only
in females treated at the highest dietary level, 2 ppm. There were
no significant differences in the erythrocyte cholinesterase
inhibiting activity of the metabolites and the parent compound,
phorate.
An ADI was allocated on the basis of a NOAEL of 0.05 mg/kg bw
per day in the one-year study of toxicity in dogs and the two-year
feeding study in rats. The effect noted in both species was
inhibition of brain cholinesterase activity, which was associated in
dogs with clinical signs consistent with cholinergic toxicity. A
safety factor of 100 was applied.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study
of toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of teratogenicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Adequate studies on absorption, distribution, excretion and
metabolism in rats. Studies known to exist may address this
need, in whole or in part. In order to maintain the ADI, these
data should be submitted in 1995, in time for review in 1996.
2. Observations in humans
References
American Cyanamid Co. (1965) Report on Thimet systemic insecticide:
successive generation studies with mice. Unpublished report from
Central Medical Department Cyanamid. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
American Cyanamid Co. (1976) Thimet soil and systemic insecticide.
Unpublished technical information from Cyanamid International Corp.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Blinn, R.C. (1982) Personal communication to WHO by American Cynamid
Co., Wayne, NJ, USA.
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Fletcher, D.W. (1984) 42-Day neurotoxicity study with phorate in
mature white leghorn chickens. Unpublished report from Bio-life
Associates Ltd, BLAL No. 83 DN 103, AC Protocol No. 981-84-114.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hutchison, E.B., Fegle, H.C., McNerney, J.M. & Levinskas, G.J.
(1978) Thimet(R) systemic insecticide: ninety-day repeated feeding
to albino rats (CL 18,161). Unpublished report from Central Medical
Department, American Cyanamid Co., Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Ivett, J.L. & Myhr, B.C. (1986) Chromosomal aberrations in vivo in
mammalian bone marrow cells on AC 35,024. Second amended report,
Project ID No. 8049, 10947-001. Unpublished report dated 9 January
1986 from Litton Bionetics, Kensington, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Kay, J.H. & Calandra, J.C. (1961) Effects of thimet phorate on
cholinesterase activity in the dog. Unpublished report from
Industrial Bio-test Lans, Inc. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Levinskas, G.J., Morici, B.S. & Shaffer, C.B. (1965) Thimet systemic
insecticide: demyelination studies in white leghorn hens.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Levinskas, G.J., McNerney, J.M. & Fegluy, H.C. (1968) Report on
sulfoxide of Thimet systemic insecticide: ninety-day repeated
feeding to albino rats. Unpublished report from Central Medical
Department, American Cyanamid Co. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1978) Teratology study in rats; Thimet(R)
phorate. Final unpublished report from Litton Bionetics, Inc.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Litton Bionetics (1981a) 18-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1981b) 24-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Lochry, E.A. (1990a) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) pilot study with AC 35,024 in rats. Final
report, Project ID No. 101P-012. Unpublished report dated 19 June
1990 from Argus Research Laboratories Inc., Horsham, PA, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Lochry, E.A. (1990b) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) definitive study with AC 35,024 in rats.
Final report, Project ID No. 101-012. Unpublished report dated 23
October 1990 from Argus Research Laboratories Inc., Horsham, PA,
USA. Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Newell, G.W. & Dilley, J.V. (1978) Teratology and acute toxicology
of selected chemical pesticides administered by inhalation. US
Environmental Protection Agency Report No. 600/1-78-003, January
1978. Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Piccirillo, V.J., Shellenberger, T.E. & Dauvin, E. M. (1987) 14-Day
range-finding oral toxicity study in the dog with AC 35,024. Revised
final report No. 85013. Unpublished report dated 11 February 1987
from Tegeris Laboratories Inc., Princeton, NJ, USA. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1986) A range-finding teratology study
with phorate in rabbits. Final report, project No. 86-3038.
Unpublished report dated 5 August 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1987) A teratology study with phorate
in rabbits. Final report, project No. 86-3039. Unpublished report
dated 6 April 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1991) A two-generation (two litters)
reproduction study with AC 35,024 to rats. Final report, Project ID
No. 88-3350. Unpublished report dated 23 September 1991 from
Bio/dynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Shellenberger, T.E. & Tegeris, A.S. (1987) One-year oral toxicity
study in purebred beagle dogs with AC 35,024. Final report No.
85015. Unpublished report dated 20 February 1987 from Tegeris
Laboratories Inc., Laurel, MD, USA. Submitted to WHO by American
Cyanamid Co., Princeton, NJ, USA.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1987) Evaluation of
selected pesticides as chemical mutagens in in vitro and in vivo
studies. Unpublished report from Stanford Research Institute, SRI
Report No. LSU-3493, for the US Environmental Protection Agency, EPA
Report No. EPA-600/1-77-028. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Thilagar, A. & Kumarop, V. (1985) Test for chemical induction of
gene mutation at the hgrpt locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Final report,
Study No. 980-85-133. Unpublished report dated 8 August 1985 from
Sitek Research Laboratories, Rockville, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Trutter, J.A. (1990) 13-Week dietary toxicity study in albino mice
with AC 35,024. Final report, Project ID No. 362-201. Unpublished
report dated 16 May 1990 from Hazleton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Tusing, T.W., Kindzin, W., Hanzal, R. & Howard, J. (1956a) Repeated
oral administration (dogs). Experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. & Howard, J.
(1956b) Subacute feeding study--experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Table 1. Acute toxicity of technical-grade phorate
Species Sex Route LD50 (mg/kg bw per day) Reference
or LC50 (mg/m3)
Mouse NR Oral 11 Blinn, 1982
NR Intraperitoneal 3 Blinn, 1982
Rat NR Oral 1.9-10 Blinn, 1982
M&F Oral M: 2.3; F: 1.1 Gaines, 1969
M&F Oral M: 2.8; F: 1.6 Anon., 1976
M&F Oral M: 3.7; F: 1.4 Newell & Dilley, 1978
NR Dermal 3 Blinn, 1982
M&F Dermal M: 6.2; F: 2.5 Gaines, 1969
M Dermal 5.7 American Cyanamid Co., 1976
M&F Dermal M: 9.3; F: 3.9 Newell & Dilley, 1978
M&F Intravenous M: 2.2; F: 1.2 Newell & Dilley, 1978
M&F Inhalation (1 h) M: 60 Newell & Dilley, 1978
F: 11 Newell & Dilley, 1978
Rabbit M Dermal 5.2 Anon., 1976
From Annex I, references 29 and 39; strains not specified
NR, not reported
(b) Short-term toxicity
Mice
Phorate (purity, 92.1%) was administered to groups of 20 male
and 20 female Crl:CD-1 (ICR)BR outbred Swiss mice at dietary levels
of 0, 1, 3 or 6 ppm, equal to 0.18, 0.55 or 1.10 mg/kg bw per day
for males and 0.23, 0.67 or 1.38 mg/kg bw per day for females, for
13 weeks. A single male mouse treated at the highest dietary level
had convulsions on one occasion during the final week of treatment,
but the relationship of this isolated finding to treatment could not
be ascertained; no other remarkable clinical signs were reported.
Treatment had no effect on survival, food consumption or body-weight
gain. Histopathological examinations were not performed. Plasma,
erythrocyte and brain cholinesterase activities were measured before
terminal sacrifice. Plasma cholinesterase activity was significantly
( p < 0.05) inhibited in animals of each sex at 3 and 6 ppm and in
females at 1 ppm. Erythrocyte cholinesterase levels were
significantly decreased in males and females at the highest dose;
the inhibition, in relation to values in concurrent controls, was
50% in males and 61% in females. A slight, non-significant decrease
in erythrocyte cholinesterase activity (17%) was recorded in females
at 3 ppm. Brain cholinesterase levels, assayed in both halves of the
brain, were significantly ( p < 0.05) decreased in animals of each
sex treated at 3 and 6 ppm, by slightly more than 10% at 3 ppm and
to about 50% of control values at 6 ppm. Minimal inhibition of
plasma cholinesterase levels (15%) in females at 1 ppm was not
considered to represent an adverse effect of treatment, particularly
in the absence of corresponding decreases in erythrocyte and brain
cholinesterase levels. The NOAEL was 1 ppm, equal to 0.18 mg/kg bw
per day, on the basis of significantly reduced brain cholinesterase
activity at dietary levels of 3 and 6 ppm (Trutter, 1990).
Rats
Groups of 50 male and 50 female rats [strain not specified]
were fed phorate (purity, 92%) in the diet at levels of 0, 0.22,
0.66, 2 or 6 ppm, equivalent to 0.01, 0.03, 0.1 or 0.3 mg/kg bw per
day, and groups of 25 male and 25 female rats were fed 12 or 18 ppm,
equivalent to 0.6 or 0.9 mg/kg bw per day. Treatment continued for
13 weeks, and cholinesterase activity was monitored each week.
Occasional episodes of excitability and intermittent tremors were
noted in females given 6 ppm, and animals of each sex receiving 12
and 18 ppm showed severe excitability, intermittent tremors and
ataxia, culminating in death of 50% of animals at 12 ppm and no
survivors at 18 ppm. Levels of 6 ppm and higher produced significant
depression of plasma, erythrocyte and brain cholinesterase activity
in animals of each sex and decreased erythrocyte cholinesterase
activity in females at 2 ppm. In all groups receiving 6 ppm or less,
growth, food consumption, survival and liver and kidney weights and
ratios were within normal limits, and no abnormalities were seen at
gross necropsy or histological examination. The NOAEL was 2 ppm,
equivalent to 0.1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity at 6 ppm and above (Tusing et al.,
1956a; Annex I, reference 29).
Dogs
Groups of two male and one female mongrel dogs received
capsules containing 0, 0.01, 0.05, 0.25 or 1.25 mg/kg bw per day of
phorate (purity, 92%) in corn oil on six days per week for 15 weeks.
Two males received a single dose of 2.5 mg/kg bw. Plasma and
erythrocyte cholinesterase activities were monitored weekly; brain
cholinesterase activity was not measured. At 0.05 mg/kg bw per day,
plasma enzyme activity was significantly depressed. Erythrocyte
cholinesterase activity was not affected during the first 12 weeks
but was depressed slightly (not significantly) during the last three
weeks of the study. Significant decreases in plasma and erythrocyte
enzyme activity were observed at 0.25 mg/kg bw per day, and 1.25
mg/kg bw per day induced total inhibition of plasma cholinesterase
activity and a significant reduction in erythrocyte cholinesterase
activity. All of the dogs administered the single dose of 2.5 mg/kg
bw per day died within 3-4 h; enzyme activity was not determined. No
signs of systemic toxicity were seen, and haematological, clinical
chemistry and urine analyses showed no effects in dogs receiving
0.01 or 0.05 mg/kg bw per day. Histopathological examination
revealed no consistent findings related to the treatment (Tusing et
al., 1956b; Annex I, reference 29).
Groups of three male and three female beagle dogs were fed
phorate in the diet at 0, 0.5 or 1.0 ppm, equivalent to 0.012 or
0.025 mg/kg bw per day, for six weeks. Cholinesterase activity in
plasma and erythrocytes was determined before and every two weeks
during the test. Analyses of variance revealed no significant
difference between the treated and control animals with respect to
plasma and erythrocyte enzyme activities (Kay & Calandra, 1961;
Annex I, reference 29).
In a preliminary range-finding study, groups of two male and
two female beagle dogs were given capsules containing phorate
(purity, 92.1%) in corn oil at doses of 0.01, 0.05, 0.10, 0.25 or
0.50 mg/kg bw per day for 14 days. A control group of three male and
three female dogs was given capsules containing corn oil. Plasma and
erythrocyte cholinesterase levels were measured twice before
treatment, and after 3, 7, 10 and 14 days of treatment; brain
cholinesterase levels were determined in the cerebellum and cerebrum
of each dog at sacrifice. Excessive salivation and tremors were seen
in one female dog given the highest dose, and body-weight gain was
decreased in comparison with the controls in animals of each sex.
Slight decreases in total serum protein were recorded in males and
females at the high dose. Treatment did not affect survival, food
consumption, haematological parameters or organ weights, and gross
pathology showed no effects; histopathological examinations were not
performed. Plasma cholinesterase levels were significantly decreased
in animals of each sex given 0.10 mg/kg bw per day or more and in
males given 0.05 mg/kg bw per day, in comparison with values before
treatment and in control animals, and erythrocyte cholinesterase
activity was significantly (> 20%) inhibited in both males and
females at the highest dose of 0.50 mg/kg bw per day. Brain
cholinesterase activity was inhibited (31-69%) in both males and
females at 0.10 mg/kg bw per day or more. The NOAEL was 0.05 mg/kg
bw per day (Piccirillo et al., 1987).
Phorate (purity, 92.1%) in corn oil was administered orally by
capsule to groups of six male and six female beagle dogs at doses of
0.005, 0.01, 0.05 or 0.25 mg/kg bw per day for one year. Eight male
and eight female controls received capsules containing only corn
oil. Plasma and erythrocyte cholinesterase activities were measured
twice before treatment, at six weeks, at three and six months and at
sacrifice; brain cholinesterase levels were measured in the cerebrum
and cerebellum at termination of treatment. Mild tremors were
observed occasionally in one male and two females during weeks 23-52
of treatment with the high dose. Mean body weights and overall
weight gain (26% less than control) were decreased in males at 0.25
mg/kg bw per day. Ophthalmological examination of all dogs before
treatment, at six months and at one year showed no treatment-related
effects. There were no adverse effects on food consumption or
haematological or urinary parameters. Decreased ( p < 0.05) total
protein levels were recorded at week 6, at three and six months and
at termination of treatment in males treated at 0.25 mg/kg bw per
day. Although the values were reported to be within the range in
historical controls, no supporting data were provided. As the
individual levels of total protein in males at the high dose were
consistently below concurrent values before treatment and in
controls, the finding appeared to be related to treatment. The
decreases ( p < 0.05) in total protein values in females and the
decreased albumin values in males at the high dose were within the
lower limits of control values. Gross and histopathological
examination revealed no lesions that could be attributed to
treatment with phorate. The highest dose induced inhibition of
erythrocyte (> 20%) and brain cholinesterase activity (43-54%);
plasma cholinesterase levels were significantly decreased in animals
of each sex at doses of 0.05 mg/kg bw per day and above. The
decrease in plasma cholinesterase activity at this dose was not
considered to be adverse in the absence of correlative inhibition of
brain and erythrocyte cholinesterase activity and evidence of
clinical toxicity. The NOAEL was 0.05 mg/kg bw per day on the basis
of decreased body weight, significant inhibition of erythrocyte and
brain cholinesterase activity and clinical signs consistent with
cholinergic toxicity at the high dose of 0.25 mg/kg bw per day
(Shellenberger & Tegeris, 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female 41-day-old CD-1 outbred Swiss
albino mice were fed technical-grade phorate (purity, 85.5%) in the
diet at 0, 1, 3 or 6 ppm, equivalent to 0.15, 0.45 or 0.9 mg/kg bw
per day for 18 months. All animals that died or were killed in
moribund condition during the study or were sacrificed terminally
were subjected to gross and histopathological examination. Survival
was not adversely affected: 78-90% of males and 66-74% of females in
the control and treated groups were still alive at the end of the
experiment. The growth of females at 6 ppm was retarded almost
throughout the experiment. All treated animals appeared to eat less
food during the first three weeks and occasionally thereafter, but
no consistent dose-response relationship was seen. Some clinical
signs, such as tremors, hyperactivity and excessive salivation,
occurred at higher incidence and more frequently in animals at the
highest dose than in controls. Gross pathological examination showed
no changes significantly different from those in the controls, and
microscopic evaluation of a wide range of tissues from each animal
revealed no alterations related to treatment. There was no
significant dose-related increase in the incidence of any particular
type of tumour, of animals with tumours, of animals with malignant
tumours or of animals with multiple primary tumours. Although the
incidence of alveolar and bronchiolar adenoma appeared to be
increased in males at 6 ppm (8/50, compared with 3/50 in controls),
this effect was not considered to be related to treatment since the
increase was not statistically significant and this tumour is known
to occur frequently in CD-1 mice. The NOAEL was 3 ppm, equivalent to
0.45 mg/kg bw per day, on the basis of decreased body weight and
clinical signs of toxicity at 6 ppm (Litton Bionetics, 1981a; Annex
I, reference 39).
Rats
Groups of 50 male and 50 female five-week-old Crl:COBS CD(SD)
BR rats were fed dietary levels of technical-grade phorate (purity,
84.5%) at 0, 1, 3 or 6 ppm, equal to 0.05, 0.16 or 0.32 mg/kg bw per
day in males and 0.07, 0.19 or 0.43 mg/kg bw per day in females, for
24 months. The mortality rate appeared to be increased in females at
6 ppm, as only 36% survived to the end of the experiment; however,
more than 60% of animals in all groups, including the controls,
lived at least 90 weeks. The only clinical sign related to treatment
was tremors induced by over-dosing (327% of all the intended doses)
during week 9. Growth was depressed in females at 6 ppm during the
first 26 weeks and again between weeks 74 and 102. Food consumption
showed no consistent dose-response pattern. On haematological
examination, clinical chemistry and urinalysis performed at 6, 12
and 24 months, the only notable findings were decreased values for
erythrocyte counts, haemoglobin and haematocrit in females at the
highest dose at 12 months. Dose-related inhibition (> 20%) of
plasma cholinesterase activity was noted in males at 6 ppm at 12
months, in all treated males at 24 months and in females at both 3
and 6 ppm at all sampling intervals (3, 6, 12 and 24 months).
Erythrocyte cholinesterase activity was not significantly depressed
(< 20%) at any time. The activity of brain cholinesterase was
reduced (> 20%) in males at 6 ppm and in females at 3 ppm or more.
At sacrifice, females given the highest dose had increased
organ:body weight ratios with respect to the adrenals, brain, heart,
liver and spleen. On gross pathological and histopathological
examination of a variety of tissues, the only effect apparently
related to treatment was a significant increase in the incidence of
inflammation and epithelial hyperplasia of the forestomach in
animals of each sex, but particularly in males, at 6 ppm. The most
prevalent types of spontaneous tumours were pituitary adenomas in
animals of each sex and mammary tumours in females. There were no
significant differences between control and treated groups with
regard to incidence, type or time of appearance of tumours. The
marginal no-effect level was 1 ppm, equal to 0.05 mg/kg bw per day
(Litton Bionetics, 1981b; Annex I, reference 39).
(d) Reproductive toxicity
Mice
Groups of eight male and 16 female mice (strain not specified)
were fed phorate (purity unknown) in the diet at levels of 0, 0.6,
1.5 or 3 ppm, equivalent to 0.09, 0.23 or 0.45 mg/kg bw per day, for
three generations, with two litters per generation. There were no
dose-related effects on indices of fertility, gestation, viability
or lactation during the study, but the lactation index was slightly
lowered in the group receiving 3 ppm, to below the control value in
the first mating of the F0 generation, in both matings of the F1
generation and in the second mating of the F2 generation. Gross
and microscopic examination of tissues revealed no consistent
abnormalities related to treatment. The NOAEL was 1.5 ppm,
equivalent to 0.23 mg/kg bw per day, on the basis of decreased
lactation indices at 3 ppm (American Cyanamid Co., 1965; Annex I,
reference 39).
Rats
A two-generation study of reproductive toxicity was conducted
in groups of 25 male and 25 female COBS CD(SD) rats given phorate
(purity, 92.1%) at dietary levels of 0, 1, 2, 4 or 6 ppm, equal to
0.09, 0.17, 0.35 or 0.52 mg/kg bw per day in males and 0.10, 0.20,
0.40 or 0.62 mg/kg bw per day in females, for a minimum of 60 days
before mating, to produce two consecutive litters (F1a and F1b)
of the first (F0) parental generation. Groups of 25 males and 25
females were then selected from the F1b litters to become the
second-generation (F1) parental animals. Owing to a high incidence
of mortality during the post-weaning period among F1b pups at 6
ppm, 30 males and 30 females at this dose were selected as F1
parents. The second-generation parental animals were treated for a
minimum of 100 days before mating to produce two consecutive litters
(F2a and F2b). Animals were exposed continuously to the test
diets before mating and throughout weaning of the offspring in both
generations. Cholinesterase levels were determined in plasma,
erythrocytes and brain from 10 parental F1 rats of each sex in
each group at terminal sacrifice.
A treatment-related decrease in body weight and tremors were
seen at > 4 ppm in parental animals; at 6 ppm, increased
mortality and clinical signs were seen, manifested as convulsive
movements and aggressive behaviour. Routine clinical observation of
the animals revealed an increased incidence of ocular effects at 6
ppm, described as exophthalmus, 'protrusion of tissue off the
cornea' and opacities. Subsequent ophthalmological evaluation
revealed markedly higher incidence and severity of infectious
sequelae affecting the cornea, anterior uveal tract and posterior
segment of the eye in rats at this dose. The increased
susceptibility of rats to ocular disease at the high dose was
considered to be a secondary effect of treatment with phorate.
Plasma cholinesterase levels were significantly decreased in females
at 4 and 6 ppm and in males at 6 ppm. Erythrocyte activity was
inhibited only slightly (10-11%) in animals of each sex at 6 ppm.
Inhibition of brain cholinesterase activity was significant in males
(40%) at 6 ppm and in females at 4 ppm (59%) and 6 ppm (83%). There
was no significant inhibition of brain cholinesterase activity at
the lower dietary levels.
Pup survival during the lactation periods was significantly
reduced in all litters from both parental generations at 6 ppm and
in the F2a litter at 4 ppm. The size of both litters from the F1
generation was slightly decreased at 6 ppm. Mean pup weights were
decreased in both F1a and F1b litters of animals receiving 4 or
6 ppm. The mean weights of pups of the F2a litter were decreased
at 4 ppm (those of the F2b litter were not affected) and in pups
of both F2a and F2b litters at 6 ppm. Anogenital staining was
recorded in F1b and F2b pups at 6 ppm but not in control animals
or in those receiving lower dietary levels. There were no adverse
effects on mating or fertility indices, pregnancy rate, length of
gestation, gestation index or sex distribution ratios, and no gross
morphological alterations or pathological changes were seen in the
reproductive organs at any of the dietary levels studied. The NOAEL
was 2 ppm, equal to 0.17 mg/kg bw per day (Schroeder & Daly, 1991).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 mated female Crl:COBS CD (SD)BR rats were given
technical-grade phorate (purity, 91.7%) by gastric intubaton at 0,
0.125, 0.25 or 0.5 mg/kg bw per day on gestation days 6-15 and were
sacrificed on day 20 of gestation. The fetuses were removed for
gross, skeletal and visceral examination. The pregnancy rate was
comparable in all groups. During the gestation period, 7/23 pregnant
dams at 0.5 mg/kg bw per day, none at 0.25 mg/kg bw per day and 1/24
at 0.125 mg/kg bw per day died. Fetuses at 0.5 mg/kg bw per day had
an increased frequency of enlarged heart (not otherwise
characterized). Clinical signs, body weight and food consumption of
dams during gestation, the number of implantation sites, the number
of resorptions, the number of dead fetuses, mean live litter size,
average fetal weight, sex ratio, and gross, skeletal and visceral
abnormalities of fetuses were not significantly different from those
in the controls. The NOAEL for teratogenicity was 0.25 mg/kg bw per
day (Litton Bionetics, 1978; Annex I, reference 39).
In a preliminary range-finding study, groups of eight mated
female Crl:CDBRVAF/Plus (SD) rats were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.25, 0.5, 0.7 or 0.9
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which it occurred was considered day 0 of gestation.
Treatment with doses > 0.5 mg/kg bw per day was lethal to the
dams, and there were no survivors at these doses after day 12 of
gestation. Overt clinical signs of toxicity preceding death included
twitches, tremors, excessive salivation, exophthalmos, ataxia,
clonic convulsion, and decreased body weight and food intake. Gross
examination of rats which died revealed enlarged and/or congested
adrenal glands. The NOAEL was 0.25 mg/kg bw per day for both
maternal and developmental toxicity (Lochry, 1990a).
In a study of teratogenicity, groups of 24-25 mated female
Crl:CDBRVAF/Plus(SD) rats were treated by gavage with phorate
(purity, 92.1%) in corn oil at doses of 0, 0.1, 0.2, 0.3 or 0.4
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which this occurred was considered day 0 of
gestation. Six of 25 females at 0.4 mg/kg bw per day that died
during days 11-16 of gestation had enlarged adrenal glands. No
deaths were reported at lower doses. Clinical signs of toxicity seen
at 0.4 mg/kg bw per day were an increased number of rats with
tremors, excessive salivation, chromodacryorrhoea and rhinorrhoea,
urine-stained abdominal fur, decreased motor activity, hunched
posture, impaired righting reflex and laboured breathing. Maternal
body weights, corresponding weight gains and food consumption and
fetal body weights were significantly decreased at 0.4 mg/kg bw per
day when compared with control values. Increased incidences of
skeletal variations were observed in fetuses and/or litters at this
dose, which were related to delays in ossification of the
sternebrae, incompletely and unossified pubes and incompletely
ossified ischia. The NOAEL for maternal and developmental toxicity
was 0.3 mg/kg bw per day on the basis of mortality, clinical signs
of toxicity, and significantly decreased body weights and food
consumption in the mothers and decreased fetal body weights and
potentially reversible delays in skeletal ossification at 0.4 mg/kg
bw per day (Lochry, 1990b).
Groups of 10 pregnant Sprague-Dawley rats were exposed by nose
only in an inhalation chamber to aerosols of technical-grade phorate
(purity, 78-90%) with a count median diameter of 0.57 Ám, generated
from a 1% solution of phorate in xylene, for 1 h per day on days
7-14 of gestation at concentrations of 0.15 ▒ 0.04, 0.4 ▒ 0.15 or
1.94 ▒ 0.48 mg/m3. Three groups of 15 dams were exposed to xylene
or air or to a restricted diet. (The reason for including the last
group was not specified.) All dams were sacrificed on day 20 of
gestation, and fetuses were removed by caesarean section for
visceral and skeletal examination. Five dams at the highest dose
died, and toxic signs (tremors, lachrymation and exophthalmus) were
noted in animals at this level; one of the dead dams had resorbed
the entire litter. Average percentage fetal mortality was markedly
increased at this dose. The summary indicated no treatment-related
effects on the body weight or food consumption of dams during
gestation and no effects on pregnancy rate, average number of
implants, average fetal weight, average number of sternal
ossification centres or the incidence of supernumerary ribs.
Specific information on the incidence of any gross or soft-tissue
abnormalities was not provided (Newell & Dilley, 1978; Annex I,
reference 39).
Rabbits
In a preliminary range-finding study, groups of five mated
female New Zealand white rabbits were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.3, 0.6, 0.9, 1.2 or
1.5 mg/kg bw per day on days 6-18 of gestation. The day on which
coitus with two successive males was observed was designated day 0
of gestation. The incidences of mortality in the six groups were
reported as 0/5, 1/5, 1/5, 1/5, 2/5 and 4/5, respectively. Mean
body-weight loss was seen at 1.2 mg/kg bw per day. Only one female
at the highest dose survived to scheduled sacrifice. Food intake was
generally decreased in the groups receiving 0.3, 0.6, 0.9 and 1.2
mg/kg bw per day, although no clear dose-response relationship was
seen. Increased numbers of resorptions and post-implantation losses
were reported at doses > 0.6 mg/kg bw per day. Decreased mean
fetal body weights and shorter crown-rump lengths were noted at 1.2
mg/kg bw per day. External examination of the fetuses showed no
treatment-related malformations or alterations. The only significant
effects observed at the LOAEL of 0.3 mg/kg bw per day were a single
maternal death and depressed food intake (Schroeder & Daly, 1986).
Groups of 20 mated female New Zealand white rabbits were given
phorate (purity, 92.1%) in corn oil by gavage on days 6-18 of
gestation at doses of 0, 0.15, 0.5, 0.9 or 1.2 mg/kg bw per day. The
day on which coitus with two successive males was observed was
designated day 0 of gestation. At 1.2 mg/kg bw per day, 8/20 females
died during days 14-19 of gestation, one female aborted and another
female delivered prematurely on day 28 of gestation, so that only
10/20 dams survived to scheduled sacrifice. The deaths of two
females at 0.9 and one at 0.5 mg/kg bw per day were also attributed
to treatment with phorate. Anogenital staining was observed at a
higher incidence in females treated with the highest dose. Mean body
weights and corresponding weight gains were decreased at doses >
0.5 mg/kg bw per day. Food consumption was decreased in females
treated at 1.2 mg/kg bw per day. Treatment had no effect on
pre-implantation loss, number of resorptions, number of live
fetuses, fetal body weight or fetal sex ratio. At 1.2 mg/kg bw per
day, all three fetuses in a single litter (five of eight
implantation sites showed early resorptions) had open eyelids,
curved scapulae, an absent supraorbital process, an irregular margin
of the frontals and a displaced anterior fontanel. Although these
anomalies were not observed in the control group, the incidence of
open eyes was within that of historical controls; curved scapulae
had not previously been noted, however, in 1852 fetuses from 269
litters. Ocular and scapular defects were not seen in other fetuses
from dams treated at this dose and were not recorded in any of the
fetuses in the preliminary range-finding study. Although the defects
seen in this single litter may have been a secondary response to the
maternal toxicity of the high dose, the weight of evidence would
suggest that the findings were spurious and perhaps genetically
predicted. There was insufficient evidence to suggest that phorate
is teratogenic to rabbits. The NOAEL for maternal toxicity was 0.15
mg/kg bw per day, as determined by a higher incidence of mortality
and decreased body weights at > 0.5 mg/kg bw per day. In the
absence of embryo- or fetotoxicity, the NOAEL for developmental
toxicity was the highest dose, 1.2 mg/kg bw per day (Schroeder &
Daly, 1987).
(f) Genotoxicity
A battery of assays were performed to test the genotoxicity of
phorate, the results of which are presented in Table 2. Assays for
gene mutation, chromosomal aberrations and other cytogenetics
end-points in vitro and in vivo gave negative results. No
unscheduled DNA synthesis was seen in human fibroblasts, and
dominant lethal mutations were not induced in male mice.
Table 2. Results of tests for the genotoxicity of phorate
End-point Test system Concentration Purity Results Reference
of phorate (%)
In vitro
Reverse S. typhimurium Up to 1000 mg/plate Technical Negativea Simmon et al., 1977b
mutation TA100, 1535, 1537, grade
1538; E. coli WP2
Reverse E. coli p3478, 1 mg (on filter disc) Technical Negativec Simmon et al., 1977b
mutation W3110; B. subtilis per plate grade
Reverse Chinese hamster 30, 40, 50, 80, 100 92.1 Negativec Thilager & Kumarop,
mutation ovary cells, hprt nl/ml 1985
locus 5, 10, 12, 14, 16, 18, Negatived
20 nl/ml
Mitotic S. cerevisiae D3 5% w/v for 4-h Technical Negativea Simmon et al., 1977b
recombination incubation before grade
plating
Unscheduled Human fibroblasts Up to 1 x 10-3 Technical Negativea Simmon et al., 1977b
DNA synthesis WI-38 grade
In vivo
Chromosomal Male and female M: 0 (corn oil), 0.25, 92.1 Negative Ivett & Myhr, 1986
aberration Sprague-Dawley 1.25, 2.5 mg/kg bw
rats, killed after per day; F: 0, 0.13,
6, 18, 30 h 0.63, 1.25 mg/kg bw
per day
Dominant Male mice 0, 5, 10, 20 mg/kg bw Technical Negative Simmon et al., 1977b
lethal per day in diet for grade
mutation 7 weeks, weekly
matings for 8 weeks
a In the presence and absence of metabolic activation
b From Annex 1, reference 46
c In the absence of metabolic activation
d In the presence of metabolic activation
(g) Special studies
Delayed neuropathy in chickens
Groups of six adult hens were fed 0 or 40 ppm phorate (purity
unspecified), equivalent to 5 mg/kg bw per day, in the diet for four
weeks. A third group received 4000 ppm tri- ortho-tolyl phosphate
as a positive control. Each hen was anaesthetized and immediately
perfused with buffered formalin, and sections of brain, lower
thoracic cord and each sciatic nerve were prepared for microscopic
examination. Tri- ortho-tolyl phosphate induced myelin loss in each
hen, but phorate had no adverse effects on nerve fibres or the
myelin sheath (Levinskas et al., 1965; Annex I, reference 29).
Phorate (purity, 89.5%) dissolved in corn oil was administered
by gavage to fasted white Leghorn hens (22-23-months old). The
LD50 by a single oral dose was 14.2 mg/kg bw. Fifty fasted hens
were then given an intramuscular injection of 10 mg/kg bw of
atropine sulfate and 1 h later given a single oral dose of 14.2
mg/kg bw phorate. An additional 15 fasted, atropinized hens were
given corn oil only, and 15 hens that did not receive atropine were
given tri- ortho-tolyl phosphate at 500 mg/kg bw as a positive
control. All surviving hens in the test and vehicle control groups
received the same doses 21 days later, except that atropine sulfate
was administered at 30 mg/kg bw. All hens were observed daily for
mortality, clinical signs and evidence of neurotoxic reactions. Body
weights and food consumption were recorded every three days. All
hens that died during the study and all hens that were killed at
termination of the study at 42 days were subjected to gross
necropsy. Those killed at the end of the study were perfused with
10% neutralized formalin, and brains, vertebral columns (with spinal
cord in situ) and the entire right and left sciatic nerves were
excised and fixed. Microscopic slides of neural tissue were prepared
by taking a sagittal section of the entire brain (corpus striatum,
cerebellum, pons), longitudinal and cross-sections of the cervical,
thoracic and lumbrosacral levels of the spinal cord and both
sagittal and longitudinal sections of the right and left sciatic
nerves. Sections were stained with haematoxylin and eosin, and
replicate sections were stained with luxol fast blue. Tissues from
10 hens in each group were examined histologically.
None of the 15 hens fed corn oil died during the 42-day study,
and all 15 hens given tri- ortho-tolyl phosphate were killed in
extremis on day 16 of the study after clinical signs of
neuropathy, first observed on day 11, became progressively more
severe. These signs included generalized weakness, ataxia and
paralysis of the legs and wings. Of the 50 hens treated with
phorate, 27 died within 24 h of the first dose and 13 more within 24
h of the second dose. Ten hens survived to termination of the study.
Vehicle control and phorate-treated hens had slight generalized
weakness of the limbs, lasting about 2 h, shortly after each
treatment with atropine sulfate; treated hens had slightly more
severe reactions and, in addition, slight to moderate ataxia for up
to 2 h after treatment with phorate. No clinical signs of delayed
neuropathy were observed, however, in any vehicle-control or test
birds. In comparison with vehicle controls, the mean body-weight
gains of tested hens were greater at 0-21 days and smaller at 21-42
days, and the mean feed consumption of test hens was smaller at 0-21
days and greater at 21-42 days. No effects attributable to phorate
were seen at gross necropsy.
Histological examination of neural tissues from
positive-control hens revealed treatment-related lesions involving
the brain, spinal cord and/or sciatic nerves in all 10 birds.
Generally mild to moderate axonal degeneration was observed in the
brains of 4/10 hens, in the spinal cords of 10/10 hens and in the
sciatic nerves of 10/10. In addition, demyelination was observed in
the spinal cords and in the sciatic nerves of 7/10 hens;
Schwann-cell hyperplasia was also observed in the sciatic nerves of
3/10 hens. These lesions were compatible with a delayed neurotoxic
response induced by tri- ortho-tolyl phosphate. Minimal to mild
focal axonal degeneration of the sciatic nerves was noted in 3/10
hens treated with phorate; no axonal degeneration was seen in
vehicle-control hens. The axonal degeneration observed in the
treated birds was associated with interstitial infiltration of
lymphoid cells, which was also observed in other test and
vehicle-control hens. This syndrome, which was distinctly different
from that observed in the positive-control hens, was ascribed to
lesions of a naturally occurring disease (Marek's disease) and was
considered not to be related to treatment with phorate. Treatment
did not induce clinical or histopathological signs indicative of
acute delayed neuropathy (Fletcher, 1984; Annex I, reference 46).
3. Studies on metabolites
(a) Enzymes and biochemical parameters
The metabolites of phorate that inhibit cholinesterase are
phorate sulfoxide and sulfone and the oxygen analogue phoratoxon and
its sulfoxide and sulfone. The negative logarithms of the molar
concentrations of these compounds that induce 50% inhibition of
erythrocyte cholinesterase are as follows: phorate, 3.17; phorate
sulfoxide, 3.35; phorate sulfone, 5.00; phoratoxon, 5.87; phoratoxon
sulfoxide, 6.76; and phoratoxon sulfone, 7.02 (Annex I, reference
29).
(b) Acute toxicity
The acute toxicity of metabolites of phorate was studied in
rats and mice; the results are shown in Table 3. Both the parent
compound and the metabolites are extremely acutely toxic.
Table 3. Acute toxicity of phorate and its metabolites
Compound LD50 in rats (mg/kg bw per day) LD50 in mice (mg/kg bw per day)
Oral Intraperitoneal Oral Intraperitoneal
Phorate 1.9-10 3 11 3
Phorate sulfoxide 2-4 11 7 1
Phorate sulfone 1.8-2.0 27 9 2
Phoratoxon 0.6-0.8 - - -
Phoratoxon sulfoxide 1.4-1.6 1 3 0.02
Phoratoxon sulfone 0.6-0.8 1.8 5 0.4
From Blinn, 1982; Annex I, reference 39
(c) Short-term toxicity
Rats
Groups of 35 male and 35 female rats (strain not specified)
were fed phorate sulfoxide (purity, 92%) at dietary levels of 0.32,
0.8 or 2 ppm, equivalent to 0.02, 0.04 or 0.1 mg/kg bw per day, for
90 days. Fifty males and 50 females served as controls. Brain,
erythrocyte and plasma cholinesterase activities were determined
twice a week. A significant ( p < 0.05) depression of erythrocyte
and brain cholinesterase activity was seen in females at 2 ppm; the
decrease in plasma cholinesterase activity at this dose was less
consistent and was considered to be borderline. Borderline
depression of erythrocyte enzyme activity was seen at 0.8 ppm. At
0.32 ppm, all values were within acceptable statistical limits for
animals of each sex. No effects were observed on other
haematological parameters, organ weights or ratios, and no
consistent histopathological effect was noted that could be
attributed to feeding 2 ppm or less of phorate sulfoxide (Levinskas
et al., 1968; Annex I, reference 29).
Groups of 30 male and 30 female Charles River CD strain rats
(50 male and 50 female controls), 51 days of age, were fed phorate
sulfone (purity, 92%; containing 6% unchanged phorate and about 2%
phorate sulfoxide) in the diet at 0, 0.32, 0.8 or 2 ppm, equivalent
to 0.02, 0.04 or 0.1 mg/kg bw per day, for 90 days. None of the
animals died before the end of the experiment, and there were no
treatment-related changes in appearance or behaviour. Weight gain
and increased food consumption were seen in males fed 0.8 or 2 ppm.
Assay of tissue cholinesterase activity five times during the course
of the experiment showed inhibition (> 20%) of erythrocyte
cholinesterase at 2 ppm in animals of each sex at most time
intervals. Plasma cholinesterase activity was reduced by 23-27% in
males at 2 ppm after one, three and five weeks and by 25-72% in
females at 2 ppm after all sampling intervals; plasma cholinesterase
was also inhibited (39%) in females at 0.8 ppm after one and three
weeks. The activity of brain cholinesterase was reduced (> 20%)
only in females at 2 ppm after three, five and eight weeks. Control
and treated groups did not differ significantly with regard to
values for haematocrit, haemoglobin and total leukocyte count at the
end of the experiment. No treatment-related effects were observed on
the absolute weights of kidneys or liver, and no gross pathological
changes were seen. Histopathological evaluation of a variety of
tissues from five males and five females in the control and
highest-dose groups revealed no morphological alterations
attributable to treatment. The NOAEL was 0.32 ppm, equivalent to
0.02 mg/kg bw per day (Hutchison et al., 1978; Annex I, reference
39).
4. Observations in humans
No relevant information was available.
Comments
The limited data available indicate that phorate given orally
to rats is not readily eliminated, with less than 40% of the
administered radioactivity recovered within six days after
treatment. Urinary metabolites were identified as O,O-diethyl
O-hydrogen phosphoro-thioate, diethyl hydrogen phosphorate and
O,O-diethyl O-hydrogen phosphorodithioate.
Phorate was severely acutely toxic in mice, rats and rabbits
after administration by various routes. WHO (1992) has classified
phorate as extremely hazardous.
In a 13-week study of the toxicity study of dietary levels of
0, 1, 3 and 6 ppm phorate in mice, the NOAEL was 1 ppm, equal to
0.18 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity at dietary levels of 3 ppm and above.
In rats that received phorate in the diet at 0, 0.22, 0.66, 2,
6, 12 or 18 ppm for 13 weeks, the NOAEL was 2 ppm, equivalent to 0.1
mg/kg bw per day, on the basis of inhibition of brain cholinesterase
activity. At 6 ppm, cholinesterase inhibition was associated with
tremors and hyperexcitability and at 12 and 18 ppm, with death.
In a one-year study of toxicity in dogs, phorate was
administered orally in capsules at doses of 0, 0.005, 0.01, 0.05 or
0.25 mg/kg bw per day. The NOAEL was 0.05 mg/kg bw per day, as
determined by decreased body weights, significant inhibition of
erythrocyte and brain cholinesterase activity and clinical signs
consistent with cholinergic toxicity at 0.25 mg/kg bw per day. A
preliminary 14-day range-finding study, a six-week study of dietary
administration and a 15-week study in which phorate was given orally
in capsules supported the findings reported in the one-year study.
Mice and rats were treated with phorate at dietary levels of 0,
1, 3 or 6 ppm for 18 and 24 months, respectively. In mice, the NOAEL
was 3 ppm, equivalent to 0.45 mg/kg bw per day, on the basis of
decreased body weights and increased incidences of tremors,
hyperactivity and excessive salivation at 6 ppm. Cholinesterase
activity was not measured in this study. In the study of toxicity
and carcinogenicity in rats, the NOAEL was 1 ppm, equal to 0.05
mg/kg bw per day, on the basis of significant inhibition of brain
cholinesterase at 3 ppm and above. There was no apparent effect on
erythrocyte cholinesterase activity. Phorate was not carcinogenic
when fed to mice or rats at dietary levels of up to 6 ppm.
A two-generation study of reproductive toxicity in rats given
phorate at dietary levels of 0, 1, 2, 4 or 6 ppm showed no adverse
effects on reproductive parameters. The NOAEL was 2 ppm, equal to
0.17 mg/kg bw per day, on the basis of decreased brain
cholinesterase activity, tremors, decreased parental and pup body
weights and decreased pup survival at 4 ppm and above. In a
three-generation study of reproductive toxicity in mice fed dietary
levels of 0, 0.6, 1.5 or 3 ppm, the NOAEL was 1.5 ppm, equivalent to
0.23 mg/kg bw per day, on the basis of slightly reduced lactation
indices at 3 ppm.
Two studies of teratogenicity were conducted with phorate in
rats, at dose levels of 0, 0.125, 0.25 or 0.5 mg/kg bw per day and
0, 0.1, 0.2, 0.3 or 0.4 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
severe maternal toxicity culminating in death, decreased fetal body
weights and delays in fetal skeletal ossification at 0.4 mg/kg bw
per day. There was no evidence of teratogenic potential at doses as
high as 0.4 mg/kg bw per day. At the maternally lethal dose of 0.5
mg/kg bw per day, there was an increased incidence of fetuses with
enlarged hearts. In rabbits treated with doses of 0, 0.15, 0.5, 0.9
or 1.2 mg/kg bw per day, maternal mortality and decreased body
weight were observed at doses of 0.5 mg/kg bw per day and above,
resulting in an NOAEL of 0.15 mg/kg bw per day. In the absence of
embryo- and fetotoxicity, the NOAEL for developmental toxicity was
the highest dose, 1.2 mg/kg bw per day. Phorate was not teratogenic
to rabbits.
Phorate has been adequately tested for genotoxicity in vitro
and in vivo in a battery of assays. The Meeting concluded that
phorate is not genotoxic.
No clinical or histopathological signs of delayed neuropathy
were seen in chickens.
Ninety-day studies of the toxicity of phorate administered in
the diet were conducted in rats with the cholinesterase-inhibiting
sulfoxide and sulfone metabolites of phorate. The metabolites of
phorate were marginally more potent inhibitors of brain
cholinesterase than phorate in female rats. Brain cholinesterase
activity was inhibited in animals of each sex after administration
of phorate at a dietary level of 6 ppm, but no significant
inhibition was apparent at 2 ppm in either sex. The sulfoxide and
sulfone metabolites of phorate inhibited brain cholinesterase only
in females treated at the highest dietary level, 2 ppm. There were
no significant differences in the erythrocyte cholinesterase
inhibiting activity of the metabolites and the parent compound,
phorate.
An ADI was allocated on the basis of a NOAEL of 0.05 mg/kg bw
per day in the one-year study of toxicity in dogs and the two-year
feeding study in rats. The effect noted in both species was
inhibition of brain cholinesterase activity, which was associated in
dogs with clinical signs consistent with cholinergic toxicity. A
safety factor of 100 was applied.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study
of toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of teratogenicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Adequate studies on absorption, distribution, excretion and
metabolism in rats. Studies known to exist may address this
need, in whole or in part. In order to maintain the ADI, these
data should be submitted in 1995, in time for review in 1996.
2. Observations in humans
References
American Cyanamid Co. (1965) Report on Thimet systemic insecticide:
successive generation studies with mice. Unpublished report from
Central Medical Department Cyanamid. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
American Cyanamid Co. (1976) Thimet soil and systemic insecticide.
Unpublished technical information from Cyanamid International Corp.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Blinn, R.C. (1982) Personal communication to WHO by American Cynamid
Co., Wayne, NJ, USA.
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Fletcher, D.W. (1984) 42-Day neurotoxicity study with phorate in
mature white leghorn chickens. Unpublished report from Bio-life
Associates Ltd, BLAL No. 83 DN 103, AC Protocol No. 981-84-114.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hutchison, E.B., Fegle, H.C., McNerney, J.M. & Levinskas, G.J.
(1978) Thimet(R) systemic insecticide: ninety-day repeated feeding
to albino rats (CL 18,161). Unpublished report from Central Medical
Department, American Cyanamid Co., Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Ivett, J.L. & Myhr, B.C. (1986) Chromosomal aberrations in vivo in
mammalian bone marrow cells on AC 35,024. Second amended report,
Project ID No. 8049, 10947-001. Unpublished report dated 9 January
1986 from Litton Bionetics, Kensington, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Kay, J.H. & Calandra, J.C. (1961) Effects of thimet phorate on
cholinesterase activity in the dog. Unpublished report from
Industrial Bio-test Lans, Inc. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Levinskas, G.J., Morici, B.S. & Shaffer, C.B. (1965) Thimet systemic
insecticide: demyelination studies in white leghorn hens.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Levinskas, G.J., McNerney, J.M. & Fegluy, H.C. (1968) Report on
sulfoxide of Thimet systemic insecticide: ninety-day repeated
feeding to albino rats. Unpublished report from Central Medical
Department, American Cyanamid Co. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1978) Teratology study in rats; Thimet(R)
phorate. Final unpublished report from Litton Bionetics, Inc.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Litton Bionetics (1981a) 18-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1981b) 24-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Lochry, E.A. (1990a) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) pilot study with AC 35,024 in rats. Final
report, Project ID No. 101P-012. Unpublished report dated 19 June
1990 from Argus Research Laboratories Inc., Horsham, PA, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Lochry, E.A. (1990b) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) definitive study with AC 35,024 in rats.
Final report, Project ID No. 101-012. Unpublished report dated 23
October 1990 from Argus Research Laboratories Inc., Horsham, PA,
USA. Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Newell, G.W. & Dilley, J.V. (1978) Teratology and acute toxicology
of selected chemical pesticides administered by inhalation. US
Environmental Protection Agency Report No. 600/1-78-003, January
1978. Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Piccirillo, V.J., Shellenberger, T.E. & Dauvin, E. M. (1987) 14-Day
range-finding oral toxicity study in the dog with AC 35,024. Revised
final report No. 85013. Unpublished report dated 11 February 1987
from Tegeris Laboratories Inc., Princeton, NJ, USA. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1986) A range-finding teratology study
with phorate in rabbits. Final report, project No. 86-3038.
Unpublished report dated 5 August 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1987) A teratology study with phorate
in rabbits. Final report, project No. 86-3039. Unpublished report
dated 6 April 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1991) A two-generation (two litters)
reproduction study with AC 35,024 to rats. Final report, Project ID
No. 88-3350. Unpublished report dated 23 September 1991 from
Bio/dynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Shellenberger, T.E. & Tegeris, A.S. (1987) One-year oral toxicity
study in purebred beagle dogs with AC 35,024. Final report No.
85015. Unpublished report dated 20 February 1987 from Tegeris
Laboratories Inc., Laurel, MD, USA. Submitted to WHO by American
Cyanamid Co., Princeton, NJ, USA.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1987) Evaluation of
selected pesticides as chemical mutagens in in vitro and in vivo
studies. Unpublished report from Stanford Research Institute, SRI
Report No. LSU-3493, for the US Environmental Protection Agency, EPA
Report No. EPA-600/1-77-028. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Thilagar, A. & Kumarop, V. (1985) Test for chemical induction of
gene mutation at the hgrpt locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Final report,
Study No. 980-85-133. Unpublished report dated 8 August 1985 from
Sitek Research Laboratories, Rockville, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Trutter, J.A. (1990) 13-Week dietary toxicity study in albino mice
with AC 35,024. Final report, Project ID No. 362-201. Unpublished
report dated 16 May 1990 from Hazleton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Tusing, T.W., Kindzin, W., Hanzal, R. & Howard, J. (1956a) Repeated
oral administration (dogs). Experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. & Howard, J.
(1956b) Subacute feeding study--experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Table 1. Acute toxicity of technical-grade phorate
Species Sex Route LD50 (mg/kg bw per day) Reference
or LC50 (mg/m3)
Mouse NR Oral 11 Blinn, 1982
NR Intraperitoneal 3 Blinn, 1982
Rat NR Oral 1.9-10 Blinn, 1982
M&F Oral M: 2.3; F: 1.1 Gaines, 1969
M&F Oral M: 2.8; F: 1.6 Anon., 1976
M&F Oral M: 3.7; F: 1.4 Newell & Dilley, 1978
NR Dermal 3 Blinn, 1982
M&F Dermal M: 6.2; F: 2.5 Gaines, 1969
M Dermal 5.7 American Cyanamid Co., 1976
M&F Dermal M: 9.3; F: 3.9 Newell & Dilley, 1978
M&F Intravenous M: 2.2; F: 1.2 Newell & Dilley, 1978
M&F Inhalation (1 h) M: 60 Newell & Dilley, 1978
F: 11 Newell & Dilley, 1978
Rabbit M Dermal 5.2 Anon., 1976
From Annex I, references 29 and 39; strains not specified
NR, not reported
(b) Short-term toxicity
Mice
Phorate (purity, 92.1%) was administered to groups of 20 male
and 20 female Crl:CD-1 (ICR)BR outbred Swiss mice at dietary levels
of 0, 1, 3 or 6 ppm, equal to 0.18, 0.55 or 1.10 mg/kg bw per day
for males and 0.23, 0.67 or 1.38 mg/kg bw per day for females, for
13 weeks. A single male mouse treated at the highest dietary level
had convulsions on one occasion during the final week of treatment,
but the relationship of this isolated finding to treatment could not
be ascertained; no other remarkable clinical signs were reported.
Treatment had no effect on survival, food consumption or body-weight
gain. Histopathological examinations were not performed. Plasma,
erythrocyte and brain cholinesterase activities were measured before
terminal sacrifice. Plasma cholinesterase activity was significantly
( p < 0.05) inhibited in animals of each sex at 3 and 6 ppm and in
females at 1 ppm. Erythrocyte cholinesterase levels were
significantly decreased in males and females at the highest dose;
the inhibition, in relation to values in concurrent controls, was
50% in males and 61% in females. A slight, non-significant decrease
in erythrocyte cholinesterase activity (17%) was recorded in females
at 3 ppm. Brain cholinesterase levels, assayed in both halves of the
brain, were significantly ( p < 0.05) decreased in animals of each
sex treated at 3 and 6 ppm, by slightly more than 10% at 3 ppm and
to about 50% of control values at 6 ppm. Minimal inhibition of
plasma cholinesterase levels (15%) in females at 1 ppm was not
considered to represent an adverse effect of treatment, particularly
in the absence of corresponding decreases in erythrocyte and brain
cholinesterase levels. The NOAEL was 1 ppm, equal to 0.18 mg/kg bw
per day, on the basis of significantly reduced brain cholinesterase
activity at dietary levels of 3 and 6 ppm (Trutter, 1990).
Rats
Groups of 50 male and 50 female rats [strain not specified]
were fed phorate (purity, 92%) in the diet at levels of 0, 0.22,
0.66, 2 or 6 ppm, equivalent to 0.01, 0.03, 0.1 or 0.3 mg/kg bw per
day, and groups of 25 male and 25 female rats were fed 12 or 18 ppm,
equivalent to 0.6 or 0.9 mg/kg bw per day. Treatment continued for
13 weeks, and cholinesterase activity was monitored each week.
Occasional episodes of excitability and intermittent tremors were
noted in females given 6 ppm, and animals of each sex receiving 12
and 18 ppm showed severe excitability, intermittent tremors and
ataxia, culminating in death of 50% of animals at 12 ppm and no
survivors at 18 ppm. Levels of 6 ppm and higher produced significant
depression of plasma, erythrocyte and brain cholinesterase activity
in animals of each sex and decreased erythrocyte cholinesterase
activity in females at 2 ppm. In all groups receiving 6 ppm or less,
growth, food consumption, survival and liver and kidney weights and
ratios were within normal limits, and no abnormalities were seen at
gross necropsy or histological examination. The NOAEL was 2 ppm,
equivalent to 0.1 mg/kg bw per day, on the basis of inhibition of
brain cholinesterase activity at 6 ppm and above (Tusing et al.,
1956a; Annex I, reference 29).
Dogs
Groups of two male and one female mongrel dogs received
capsules containing 0, 0.01, 0.05, 0.25 or 1.25 mg/kg bw per day of
phorate (purity, 92%) in corn oil on six days per week for 15 weeks.
Two males received a single dose of 2.5 mg/kg bw. Plasma and
erythrocyte cholinesterase activities were monitored weekly; brain
cholinesterase activity was not measured. At 0.05 mg/kg bw per day,
plasma enzyme activity was significantly depressed. Erythrocyte
cholinesterase activity was not affected during the first 12 weeks
but was depressed slightly (not significantly) during the last three
weeks of the study. Significant decreases in plasma and erythrocyte
enzyme activity were observed at 0.25 mg/kg bw per day, and 1.25
mg/kg bw per day induced total inhibition of plasma cholinesterase
activity and a significant reduction in erythrocyte cholinesterase
activity. All of the dogs administered the single dose of 2.5 mg/kg
bw per day died within 3-4 h; enzyme activity was not determined. No
signs of systemic toxicity were seen, and haematological, clinical
chemistry and urine analyses showed no effects in dogs receiving
0.01 or 0.05 mg/kg bw per day. Histopathological examination
revealed no consistent findings related to the treatment (Tusing et
al., 1956b; Annex I, reference 29).
Groups of three male and three female beagle dogs were fed
phorate in the diet at 0, 0.5 or 1.0 ppm, equivalent to 0.012 or
0.025 mg/kg bw per day, for six weeks. Cholinesterase activity in
plasma and erythrocytes was determined before and every two weeks
during the test. Analyses of variance revealed no significant
difference between the treated and control animals with respect to
plasma and erythrocyte enzyme activities (Kay & Calandra, 1961;
Annex I, reference 29).
In a preliminary range-finding study, groups of two male and
two female beagle dogs were given capsules containing phorate
(purity, 92.1%) in corn oil at doses of 0.01, 0.05, 0.10, 0.25 or
0.50 mg/kg bw per day for 14 days. A control group of three male and
three female dogs was given capsules containing corn oil. Plasma and
erythrocyte cholinesterase levels were measured twice before
treatment, and after 3, 7, 10 and 14 days of treatment; brain
cholinesterase levels were determined in the cerebellum and cerebrum
of each dog at sacrifice. Excessive salivation and tremors were seen
in one female dog given the highest dose, and body-weight gain was
decreased in comparison with the controls in animals of each sex.
Slight decreases in total serum protein were recorded in males and
females at the high dose. Treatment did not affect survival, food
consumption, haematological parameters or organ weights, and gross
pathology showed no effects; histopathological examinations were not
performed. Plasma cholinesterase levels were significantly decreased
in animals of each sex given 0.10 mg/kg bw per day or more and in
males given 0.05 mg/kg bw per day, in comparison with values before
treatment and in control animals, and erythrocyte cholinesterase
activity was significantly (> 20%) inhibited in both males and
females at the highest dose of 0.50 mg/kg bw per day. Brain
cholinesterase activity was inhibited (31-69%) in both males and
females at 0.10 mg/kg bw per day or more. The NOAEL was 0.05 mg/kg
bw per day (Piccirillo et al., 1987).
Phorate (purity, 92.1%) in corn oil was administered orally by
capsule to groups of six male and six female beagle dogs at doses of
0.005, 0.01, 0.05 or 0.25 mg/kg bw per day for one year. Eight male
and eight female controls received capsules containing only corn
oil. Plasma and erythrocyte cholinesterase activities were measured
twice before treatment, at six weeks, at three and six months and at
sacrifice; brain cholinesterase levels were measured in the cerebrum
and cerebellum at termination of treatment. Mild tremors were
observed occasionally in one male and two females during weeks 23-52
of treatment with the high dose. Mean body weights and overall
weight gain (26% less than control) were decreased in males at 0.25
mg/kg bw per day. Ophthalmological examination of all dogs before
treatment, at six months and at one year showed no treatment-related
effects. There were no adverse effects on food consumption or
haematological or urinary parameters. Decreased ( p < 0.05) total
protein levels were recorded at week 6, at three and six months and
at termination of treatment in males treated at 0.25 mg/kg bw per
day. Although the values were reported to be within the range in
historical controls, no supporting data were provided. As the
individual levels of total protein in males at the high dose were
consistently below concurrent values before treatment and in
controls, the finding appeared to be related to treatment. The
decreases ( p < 0.05) in total protein values in females and the
decreased albumin values in males at the high dose were within the
lower limits of control values. Gross and histopathological
examination revealed no lesions that could be attributed to
treatment with phorate. The highest dose induced inhibition of
erythrocyte (> 20%) and brain cholinesterase activity (43-54%);
plasma cholinesterase levels were significantly decreased in animals
of each sex at doses of 0.05 mg/kg bw per day and above. The
decrease in plasma cholinesterase activity at this dose was not
considered to be adverse in the absence of correlative inhibition of
brain and erythrocyte cholinesterase activity and evidence of
clinical toxicity. The NOAEL was 0.05 mg/kg bw per day on the basis
of decreased body weight, significant inhibition of erythrocyte and
brain cholinesterase activity and clinical signs consistent with
cholinergic toxicity at the high dose of 0.25 mg/kg bw per day
(Shellenberger & Tegeris, 1987).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female 41-day-old CD-1 outbred Swiss
albino mice were fed technical-grade phorate (purity, 85.5%) in the
diet at 0, 1, 3 or 6 ppm, equivalent to 0.15, 0.45 or 0.9 mg/kg bw
per day for 18 months. All animals that died or were killed in
moribund condition during the study or were sacrificed terminally
were subjected to gross and histopathological examination. Survival
was not adversely affected: 78-90% of males and 66-74% of females in
the control and treated groups were still alive at the end of the
experiment. The growth of females at 6 ppm was retarded almost
throughout the experiment. All treated animals appeared to eat less
food during the first three weeks and occasionally thereafter, but
no consistent dose-response relationship was seen. Some clinical
signs, such as tremors, hyperactivity and excessive salivation,
occurred at higher incidence and more frequently in animals at the
highest dose than in controls. Gross pathological examination showed
no changes significantly different from those in the controls, and
microscopic evaluation of a wide range of tissues from each animal
revealed no alterations related to treatment. There was no
significant dose-related increase in the incidence of any particular
type of tumour, of animals with tumours, of animals with malignant
tumours or of animals with multiple primary tumours. Although the
incidence of alveolar and bronchiolar adenoma appeared to be
increased in males at 6 ppm (8/50, compared with 3/50 in controls),
this effect was not considered to be related to treatment since the
increase was not statistically significant and this tumour is known
to occur frequently in CD-1 mice. The NOAEL was 3 ppm, equivalent to
0.45 mg/kg bw per day, on the basis of decreased body weight and
clinical signs of toxicity at 6 ppm (Litton Bionetics, 1981a; Annex
I, reference 39).
Rats
Groups of 50 male and 50 female five-week-old Crl:COBS CD(SD)
BR rats were fed dietary levels of technical-grade phorate (purity,
84.5%) at 0, 1, 3 or 6 ppm, equal to 0.05, 0.16 or 0.32 mg/kg bw per
day in males and 0.07, 0.19 or 0.43 mg/kg bw per day in females, for
24 months. The mortality rate appeared to be increased in females at
6 ppm, as only 36% survived to the end of the experiment; however,
more than 60% of animals in all groups, including the controls,
lived at least 90 weeks. The only clinical sign related to treatment
was tremors induced by over-dosing (327% of all the intended doses)
during week 9. Growth was depressed in females at 6 ppm during the
first 26 weeks and again between weeks 74 and 102. Food consumption
showed no consistent dose-response pattern. On haematological
examination, clinical chemistry and urinalysis performed at 6, 12
and 24 months, the only notable findings were decreased values for
erythrocyte counts, haemoglobin and haematocrit in females at the
highest dose at 12 months. Dose-related inhibition (> 20%) of
plasma cholinesterase activity was noted in males at 6 ppm at 12
months, in all treated males at 24 months and in females at both 3
and 6 ppm at all sampling intervals (3, 6, 12 and 24 months).
Erythrocyte cholinesterase activity was not significantly depressed
(< 20%) at any time. The activity of brain cholinesterase was
reduced (> 20%) in males at 6 ppm and in females at 3 ppm or more.
At sacrifice, females given the highest dose had increased
organ:body weight ratios with respect to the adrenals, brain, heart,
liver and spleen. On gross pathological and histopathological
examination of a variety of tissues, the only effect apparently
related to treatment was a significant increase in the incidence of
inflammation and epithelial hyperplasia of the forestomach in
animals of each sex, but particularly in males, at 6 ppm. The most
prevalent types of spontaneous tumours were pituitary adenomas in
animals of each sex and mammary tumours in females. There were no
significant differences between control and treated groups with
regard to incidence, type or time of appearance of tumours. The
marginal no-effect level was 1 ppm, equal to 0.05 mg/kg bw per day
(Litton Bionetics, 1981b; Annex I, reference 39).
(d) Reproductive toxicity
Mice
Groups of eight male and 16 female mice (strain not specified)
were fed phorate (purity unknown) in the diet at levels of 0, 0.6,
1.5 or 3 ppm, equivalent to 0.09, 0.23 or 0.45 mg/kg bw per day, for
three generations, with two litters per generation. There were no
dose-related effects on indices of fertility, gestation, viability
or lactation during the study, but the lactation index was slightly
lowered in the group receiving 3 ppm, to below the control value in
the first mating of the F0 generation, in both matings of the F1
generation and in the second mating of the F2 generation. Gross
and microscopic examination of tissues revealed no consistent
abnormalities related to treatment. The NOAEL was 1.5 ppm,
equivalent to 0.23 mg/kg bw per day, on the basis of decreased
lactation indices at 3 ppm (American Cyanamid Co., 1965; Annex I,
reference 39).
Rats
A two-generation study of reproductive toxicity was conducted
in groups of 25 male and 25 female COBS CD(SD) rats given phorate
(purity, 92.1%) at dietary levels of 0, 1, 2, 4 or 6 ppm, equal to
0.09, 0.17, 0.35 or 0.52 mg/kg bw per day in males and 0.10, 0.20,
0.40 or 0.62 mg/kg bw per day in females, for a minimum of 60 days
before mating, to produce two consecutive litters (F1a and F1b)
of the first (F0) parental generation. Groups of 25 males and 25
females were then selected from the F1b litters to become the
second-generation (F1) parental animals. Owing to a high incidence
of mortality during the post-weaning period among F1b pups at 6
ppm, 30 males and 30 females at this dose were selected as F1
parents. The second-generation parental animals were treated for a
minimum of 100 days before mating to produce two consecutive litters
(F2a and F2b). Animals were exposed continuously to the test
diets before mating and throughout weaning of the offspring in both
generations. Cholinesterase levels were determined in plasma,
erythrocytes and brain from 10 parental F1 rats of each sex in
each group at terminal sacrifice.
A treatment-related decrease in body weight and tremors were
seen at > 4 ppm in parental animals; at 6 ppm, increased
mortality and clinical signs were seen, manifested as convulsive
movements and aggressive behaviour. Routine clinical observation of
the animals revealed an increased incidence of ocular effects at 6
ppm, described as exophthalmus, 'protrusion of tissue off the
cornea' and opacities. Subsequent ophthalmological evaluation
revealed markedly higher incidence and severity of infectious
sequelae affecting the cornea, anterior uveal tract and posterior
segment of the eye in rats at this dose. The increased
susceptibility of rats to ocular disease at the high dose was
considered to be a secondary effect of treatment with phorate.
Plasma cholinesterase levels were significantly decreased in females
at 4 and 6 ppm and in males at 6 ppm. Erythrocyte activity was
inhibited only slightly (10-11%) in animals of each sex at 6 ppm.
Inhibition of brain cholinesterase activity was significant in males
(40%) at 6 ppm and in females at 4 ppm (59%) and 6 ppm (83%). There
was no significant inhibition of brain cholinesterase activity at
the lower dietary levels.
Pup survival during the lactation periods was significantly
reduced in all litters from both parental generations at 6 ppm and
in the F2a litter at 4 ppm. The size of both litters from the F1
generation was slightly decreased at 6 ppm. Mean pup weights were
decreased in both F1a and F1b litters of animals receiving 4 or
6 ppm. The mean weights of pups of the F2a litter were decreased
at 4 ppm (those of the F2b litter were not affected) and in pups
of both F2a and F2b litters at 6 ppm. Anogenital staining was
recorded in F1b and F2b pups at 6 ppm but not in control animals
or in those receiving lower dietary levels. There were no adverse
effects on mating or fertility indices, pregnancy rate, length of
gestation, gestation index or sex distribution ratios, and no gross
morphological alterations or pathological changes were seen in the
reproductive organs at any of the dietary levels studied. The NOAEL
was 2 ppm, equal to 0.17 mg/kg bw per day (Schroeder & Daly, 1991).
(e) Embryotoxicity and teratogenicity
Rats
Groups of 25 mated female Crl:COBS CD (SD)BR rats were given
technical-grade phorate (purity, 91.7%) by gastric intubaton at 0,
0.125, 0.25 or 0.5 mg/kg bw per day on gestation days 6-15 and were
sacrificed on day 20 of gestation. The fetuses were removed for
gross, skeletal and visceral examination. The pregnancy rate was
comparable in all groups. During the gestation period, 7/23 pregnant
dams at 0.5 mg/kg bw per day, none at 0.25 mg/kg bw per day and 1/24
at 0.125 mg/kg bw per day died. Fetuses at 0.5 mg/kg bw per day had
an increased frequency of enlarged heart (not otherwise
characterized). Clinical signs, body weight and food consumption of
dams during gestation, the number of implantation sites, the number
of resorptions, the number of dead fetuses, mean live litter size,
average fetal weight, sex ratio, and gross, skeletal and visceral
abnormalities of fetuses were not significantly different from those
in the controls. The NOAEL for teratogenicity was 0.25 mg/kg bw per
day (Litton Bionetics, 1978; Annex I, reference 39).
In a preliminary range-finding study, groups of eight mated
female Crl:CDBRVAF/Plus (SD) rats were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.25, 0.5, 0.7 or 0.9
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which it occurred was considered day 0 of gestation.
Treatment with doses > 0.5 mg/kg bw per day was lethal to the
dams, and there were no survivors at these doses after day 12 of
gestation. Overt clinical signs of toxicity preceding death included
twitches, tremors, excessive salivation, exophthalmos, ataxia,
clonic convulsion, and decreased body weight and food intake. Gross
examination of rats which died revealed enlarged and/or congested
adrenal glands. The NOAEL was 0.25 mg/kg bw per day for both
maternal and developmental toxicity (Lochry, 1990a).
In a study of teratogenicity, groups of 24-25 mated female
Crl:CDBRVAF/Plus(SD) rats were treated by gavage with phorate
(purity, 92.1%) in corn oil at doses of 0, 0.1, 0.2, 0.3 or 0.4
mg/kg bw per day on days 6-15 of gestation. The presence of
spermatozoa or a copulatory plug was considered evidence of mating,
and the day on which this occurred was considered day 0 of
gestation. Six of 25 females at 0.4 mg/kg bw per day that died
during days 11-16 of gestation had enlarged adrenal glands. No
deaths were reported at lower doses. Clinical signs of toxicity seen
at 0.4 mg/kg bw per day were an increased number of rats with
tremors, excessive salivation, chromodacryorrhoea and rhinorrhoea,
urine-stained abdominal fur, decreased motor activity, hunched
posture, impaired righting reflex and laboured breathing. Maternal
body weights, corresponding weight gains and food consumption and
fetal body weights were significantly decreased at 0.4 mg/kg bw per
day when compared with control values. Increased incidences of
skeletal variations were observed in fetuses and/or litters at this
dose, which were related to delays in ossification of the
sternebrae, incompletely and unossified pubes and incompletely
ossified ischia. The NOAEL for maternal and developmental toxicity
was 0.3 mg/kg bw per day on the basis of mortality, clinical signs
of toxicity, and significantly decreased body weights and food
consumption in the mothers and decreased fetal body weights and
potentially reversible delays in skeletal ossification at 0.4 mg/kg
bw per day (Lochry, 1990b).
Groups of 10 pregnant Sprague-Dawley rats were exposed by nose
only in an inhalation chamber to aerosols of technical-grade phorate
(purity, 78-90%) with a count median diameter of 0.57 Ám, generated
from a 1% solution of phorate in xylene, for 1 h per day on days
7-14 of gestation at concentrations of 0.15 ▒ 0.04, 0.4 ▒ 0.15 or
1.94 ▒ 0.48 mg/m3. Three groups of 15 dams were exposed to xylene
or air or to a restricted diet. (The reason for including the last
group was not specified.) All dams were sacrificed on day 20 of
gestation, and fetuses were removed by caesarean section for
visceral and skeletal examination. Five dams at the highest dose
died, and toxic signs (tremors, lachrymation and exophthalmus) were
noted in animals at this level; one of the dead dams had resorbed
the entire litter. Average percentage fetal mortality was markedly
increased at this dose. The summary indicated no treatment-related
effects on the body weight or food consumption of dams during
gestation and no effects on pregnancy rate, average number of
implants, average fetal weight, average number of sternal
ossification centres or the incidence of supernumerary ribs.
Specific information on the incidence of any gross or soft-tissue
abnormalities was not provided (Newell & Dilley, 1978; Annex I,
reference 39).
Rabbits
In a preliminary range-finding study, groups of five mated
female New Zealand white rabbits were administered phorate (purity,
92.1%) in corn oil by gavage at doses of 0, 0.3, 0.6, 0.9, 1.2 or
1.5 mg/kg bw per day on days 6-18 of gestation. The day on which
coitus with two successive males was observed was designated day 0
of gestation. The incidences of mortality in the six groups were
reported as 0/5, 1/5, 1/5, 1/5, 2/5 and 4/5, respectively. Mean
body-weight loss was seen at 1.2 mg/kg bw per day. Only one female
at the highest dose survived to scheduled sacrifice. Food intake was
generally decreased in the groups receiving 0.3, 0.6, 0.9 and 1.2
mg/kg bw per day, although no clear dose-response relationship was
seen. Increased numbers of resorptions and post-implantation losses
were reported at doses > 0.6 mg/kg bw per day. Decreased mean
fetal body weights and shorter crown-rump lengths were noted at 1.2
mg/kg bw per day. External examination of the fetuses showed no
treatment-related malformations or alterations. The only significant
effects observed at the LOAEL of 0.3 mg/kg bw per day were a single
maternal death and depressed food intake (Schroeder & Daly, 1986).
Groups of 20 mated female New Zealand white rabbits were given
phorate (purity, 92.1%) in corn oil by gavage on days 6-18 of
gestation at doses of 0, 0.15, 0.5, 0.9 or 1.2 mg/kg bw per day. The
day on which coitus with two successive males was observed was
designated day 0 of gestation. At 1.2 mg/kg bw per day, 8/20 females
died during days 14-19 of gestation, one female aborted and another
female delivered prematurely on day 28 of gestation, so that only
10/20 dams survived to scheduled sacrifice. The deaths of two
females at 0.9 and one at 0.5 mg/kg bw per day were also attributed
to treatment with phorate. Anogenital staining was observed at a
higher incidence in females treated with the highest dose. Mean body
weights and corresponding weight gains were decreased at doses >
0.5 mg/kg bw per day. Food consumption was decreased in females
treated at 1.2 mg/kg bw per day. Treatment had no effect on
pre-implantation loss, number of resorptions, number of live
fetuses, fetal body weight or fetal sex ratio. At 1.2 mg/kg bw per
day, all three fetuses in a single litter (five of eight
implantation sites showed early resorptions) had open eyelids,
curved scapulae, an absent supraorbital process, an irregular margin
of the frontals and a displaced anterior fontanel. Although these
anomalies were not observed in the control group, the incidence of
open eyes was within that of historical controls; curved scapulae
had not previously been noted, however, in 1852 fetuses from 269
litters. Ocular and scapular defects were not seen in other fetuses
from dams treated at this dose and were not recorded in any of the
fetuses in the preliminary range-finding study. Although the defects
seen in this single litter may have been a secondary response to the
maternal toxicity of the high dose, the weight of evidence would
suggest that the findings were spurious and perhaps genetically
predicted. There was insufficient evidence to suggest that phorate
is teratogenic to rabbits. The NOAEL for maternal toxicity was 0.15
mg/kg bw per day, as determined by a higher incidence of mortality
and decreased body weights at > 0.5 mg/kg bw per day. In the
absence of embryo- or fetotoxicity, the NOAEL for developmental
toxicity was the highest dose, 1.2 mg/kg bw per day (Schroeder &
Daly, 1987).
(f) Genotoxicity
A battery of assays were performed to test the genotoxicity of
phorate, the results of which are presented in Table 2. Assays for
gene mutation, chromosomal aberrations and other cytogenetics
end-points in vitro and in vivo gave negative results. No
unscheduled DNA synthesis was seen in human fibroblasts, and
dominant lethal mutations were not induced in male mice.
Table 2. Results of tests for the genotoxicity of phorate
End-point Test system Concentration Purity Results Reference
of phorate (%)
In vitro
Reverse S. typhimurium Up to 1000 mg/plate Technical Negativea Simmon et al., 1977b
mutation TA100, 1535, 1537, grade
1538; E. coli WP2
Reverse E. coli p3478, 1 mg (on filter disc) Technical Negativec Simmon et al., 1977b
mutation W3110; B. subtilis per plate grade
Reverse Chinese hamster 30, 40, 50, 80, 100 92.1 Negativec Thilager & Kumarop,
mutation ovary cells, hprt nl/ml 1985
locus 5, 10, 12, 14, 16, 18, Negatived
20 nl/ml
Mitotic S. cerevisiae D3 5% w/v for 4-h Technical Negativea Simmon et al., 1977b
recombination incubation before grade
plating
Unscheduled Human fibroblasts Up to 1 x 10-3 Technical Negativea Simmon et al., 1977b
DNA synthesis WI-38 grade
In vivo
Chromosomal Male and female M: 0 (corn oil), 0.25, 92.1 Negative Ivett & Myhr, 1986
aberration Sprague-Dawley 1.25, 2.5 mg/kg bw
rats, killed after per day; F: 0, 0.13,
6, 18, 30 h 0.63, 1.25 mg/kg bw
per day
Dominant Male mice 0, 5, 10, 20 mg/kg bw Technical Negative Simmon et al., 1977b
lethal per day in diet for grade
mutation 7 weeks, weekly
matings for 8 weeks
a In the presence and absence of metabolic activation
b From Annex 1, reference 46
c In the absence of metabolic activation
d In the presence of metabolic activation
(g) Special studies
Delayed neuropathy in chickens
Groups of six adult hens were fed 0 or 40 ppm phorate (purity
unspecified), equivalent to 5 mg/kg bw per day, in the diet for four
weeks. A third group received 4000 ppm tri- ortho-tolyl phosphate
as a positive control. Each hen was anaesthetized and immediately
perfused with buffered formalin, and sections of brain, lower
thoracic cord and each sciatic nerve were prepared for microscopic
examination. Tri- ortho-tolyl phosphate induced myelin loss in each
hen, but phorate had no adverse effects on nerve fibres or the
myelin sheath (Levinskas et al., 1965; Annex I, reference 29).
Phorate (purity, 89.5%) dissolved in corn oil was administered
by gavage to fasted white Leghorn hens (22-23-months old). The
LD50 by a single oral dose was 14.2 mg/kg bw. Fifty fasted hens
were then given an intramuscular injection of 10 mg/kg bw of
atropine sulfate and 1 h later given a single oral dose of 14.2
mg/kg bw phorate. An additional 15 fasted, atropinized hens were
given corn oil only, and 15 hens that did not receive atropine were
given tri- ortho-tolyl phosphate at 500 mg/kg bw as a positive
control. All surviving hens in the test and vehicle control groups
received the same doses 21 days later, except that atropine sulfate
was administered at 30 mg/kg bw. All hens were observed daily for
mortality, clinical signs and evidence of neurotoxic reactions. Body
weights and food consumption were recorded every three days. All
hens that died during the study and all hens that were killed at
termination of the study at 42 days were subjected to gross
necropsy. Those killed at the end of the study were perfused with
10% neutralized formalin, and brains, vertebral columns (with spinal
cord in situ) and the entire right and left sciatic nerves were
excised and fixed. Microscopic slides of neural tissue were prepared
by taking a sagittal section of the entire brain (corpus striatum,
cerebellum, pons), longitudinal and cross-sections of the cervical,
thoracic and lumbrosacral levels of the spinal cord and both
sagittal and longitudinal sections of the right and left sciatic
nerves. Sections were stained with haematoxylin and eosin, and
replicate sections were stained with luxol fast blue. Tissues from
10 hens in each group were examined histologically.
None of the 15 hens fed corn oil died during the 42-day study,
and all 15 hens given tri- ortho-tolyl phosphate were killed in
extremis on day 16 of the study after clinical signs of
neuropathy, first observed on day 11, became progressively more
severe. These signs included generalized weakness, ataxia and
paralysis of the legs and wings. Of the 50 hens treated with
phorate, 27 died within 24 h of the first dose and 13 more within 24
h of the second dose. Ten hens survived to termination of the study.
Vehicle control and phorate-treated hens had slight generalized
weakness of the limbs, lasting about 2 h, shortly after each
treatment with atropine sulfate; treated hens had slightly more
severe reactions and, in addition, slight to moderate ataxia for up
to 2 h after treatment with phorate. No clinical signs of delayed
neuropathy were observed, however, in any vehicle-control or test
birds. In comparison with vehicle controls, the mean body-weight
gains of tested hens were greater at 0-21 days and smaller at 21-42
days, and the mean feed consumption of test hens was smaller at 0-21
days and greater at 21-42 days. No effects attributable to phorate
were seen at gross necropsy.
Histological examination of neural tissues from
positive-control hens revealed treatment-related lesions involving
the brain, spinal cord and/or sciatic nerves in all 10 birds.
Generally mild to moderate axonal degeneration was observed in the
brains of 4/10 hens, in the spinal cords of 10/10 hens and in the
sciatic nerves of 10/10. In addition, demyelination was observed in
the spinal cords and in the sciatic nerves of 7/10 hens;
Schwann-cell hyperplasia was also observed in the sciatic nerves of
3/10 hens. These lesions were compatible with a delayed neurotoxic
response induced by tri- ortho-tolyl phosphate. Minimal to mild
focal axonal degeneration of the sciatic nerves was noted in 3/10
hens treated with phorate; no axonal degeneration was seen in
vehicle-control hens. The axonal degeneration observed in the
treated birds was associated with interstitial infiltration of
lymphoid cells, which was also observed in other test and
vehicle-control hens. This syndrome, which was distinctly different
from that observed in the positive-control hens, was ascribed to
lesions of a naturally occurring disease (Marek's disease) and was
considered not to be related to treatment with phorate. Treatment
did not induce clinical or histopathological signs indicative of
acute delayed neuropathy (Fletcher, 1984; Annex I, reference 46).
3. Studies on metabolites
(a) Enzymes and biochemical parameters
The metabolites of phorate that inhibit cholinesterase are
phorate sulfoxide and sulfone and the oxygen analogue phoratoxon and
its sulfoxide and sulfone. The negative logarithms of the molar
concentrations of these compounds that induce 50% inhibition of
erythrocyte cholinesterase are as follows: phorate, 3.17; phorate
sulfoxide, 3.35; phorate sulfone, 5.00; phoratoxon, 5.87; phoratoxon
sulfoxide, 6.76; and phoratoxon sulfone, 7.02 (Annex I, reference
29).
(b) Acute toxicity
The acute toxicity of metabolites of phorate was studied in
rats and mice; the results are shown in Table 3. Both the parent
compound and the metabolites are extremely acutely toxic.
Table 3. Acute toxicity of phorate and its metabolites
Compound LD50 in rats (mg/kg bw per day) LD50 in mice (mg/kg bw per day)
Oral Intraperitoneal Oral Intraperitoneal
Phorate 1.9-10 3 11 3
Phorate sulfoxide 2-4 11 7 1
Phorate sulfone 1.8-2.0 27 9 2
Phoratoxon 0.6-0.8 - - -
Phoratoxon sulfoxide 1.4-1.6 1 3 0.02
Phoratoxon sulfone 0.6-0.8 1.8 5 0.4
From Blinn, 1982; Annex I, reference 39
(c) Short-term toxicity
Rats
Groups of 35 male and 35 female rats (strain not specified)
were fed phorate sulfoxide (purity, 92%) at dietary levels of 0.32,
0.8 or 2 ppm, equivalent to 0.02, 0.04 or 0.1 mg/kg bw per day, for
90 days. Fifty males and 50 females served as controls. Brain,
erythrocyte and plasma cholinesterase activities were determined
twice a week. A significant ( p < 0.05) depression of erythrocyte
and brain cholinesterase activity was seen in females at 2 ppm; the
decrease in plasma cholinesterase activity at this dose was less
consistent and was considered to be borderline. Borderline
depression of erythrocyte enzyme activity was seen at 0.8 ppm. At
0.32 ppm, all values were within acceptable statistical limits for
animals of each sex. No effects were observed on other
haematological parameters, organ weights or ratios, and no
consistent histopathological effect was noted that could be
attributed to feeding 2 ppm or less of phorate sulfoxide (Levinskas
et al., 1968; Annex I, reference 29).
Groups of 30 male and 30 female Charles River CD strain rats
(50 male and 50 female controls), 51 days of age, were fed phorate
sulfone (purity, 92%; containing 6% unchanged phorate and about 2%
phorate sulfoxide) in the diet at 0, 0.32, 0.8 or 2 ppm, equivalent
to 0.02, 0.04 or 0.1 mg/kg bw per day, for 90 days. None of the
animals died before the end of the experiment, and there were no
treatment-related changes in appearance or behaviour. Weight gain
and increased food consumption were seen in males fed 0.8 or 2 ppm.
Assay of tissue cholinesterase activity five times during the course
of the experiment showed inhibition (> 20%) of erythrocyte
cholinesterase at 2 ppm in animals of each sex at most time
intervals. Plasma cholinesterase activity was reduced by 23-27% in
males at 2 ppm after one, three and five weeks and by 25-72% in
females at 2 ppm after all sampling intervals; plasma cholinesterase
was also inhibited (39%) in females at 0.8 ppm after one and three
weeks. The activity of brain cholinesterase was reduced (> 20%)
only in females at 2 ppm after three, five and eight weeks. Control
and treated groups did not differ significantly with regard to
values for haematocrit, haemoglobin and total leukocyte count at the
end of the experiment. No treatment-related effects were observed on
the absolute weights of kidneys or liver, and no gross pathological
changes were seen. Histopathological evaluation of a variety of
tissues from five males and five females in the control and
highest-dose groups revealed no morphological alterations
attributable to treatment. The NOAEL was 0.32 ppm, equivalent to
0.02 mg/kg bw per day (Hutchison et al., 1978; Annex I, reference
39).
4. Observations in humans
No relevant information was available.
Comments
The limited data available indicate that phorate given orally
to rats is not readily eliminated, with less than 40% of the
administered radioactivity recovered within six days after
treatment. Urinary metabolites were identified as O,O-diethyl
O-hydrogen phosphoro-thioate, diethyl hydrogen phosphorate and
O,O-diethyl O-hydrogen phosphorodithioate.
Phorate was severely acutely toxic in mice, rats and rabbits
after administration by various routes. WHO (1992) has classified
phorate as extremely hazardous.
In a 13-week study of the toxicity study of dietary levels of
0, 1, 3 and 6 ppm phorate in mice, the NOAEL was 1 ppm, equal to
0.18 mg/kg bw per day, on the basis of inhibition of brain
cholinesterase activity at dietary levels of 3 ppm and above.
In rats that received phorate in the diet at 0, 0.22, 0.66, 2,
6, 12 or 18 ppm for 13 weeks, the NOAEL was 2 ppm, equivalent to 0.1
mg/kg bw per day, on the basis of inhibition of brain cholinesterase
activity. At 6 ppm, cholinesterase inhibition was associated with
tremors and hyperexcitability and at 12 and 18 ppm, with death.
In a one-year study of toxicity in dogs, phorate was
administered orally in capsules at doses of 0, 0.005, 0.01, 0.05 or
0.25 mg/kg bw per day. The NOAEL was 0.05 mg/kg bw per day, as
determined by decreased body weights, significant inhibition of
erythrocyte and brain cholinesterase activity and clinical signs
consistent with cholinergic toxicity at 0.25 mg/kg bw per day. A
preliminary 14-day range-finding study, a six-week study of dietary
administration and a 15-week study in which phorate was given orally
in capsules supported the findings reported in the one-year study.
Mice and rats were treated with phorate at dietary levels of 0,
1, 3 or 6 ppm for 18 and 24 months, respectively. In mice, the NOAEL
was 3 ppm, equivalent to 0.45 mg/kg bw per day, on the basis of
decreased body weights and increased incidences of tremors,
hyperactivity and excessive salivation at 6 ppm. Cholinesterase
activity was not measured in this study. In the study of toxicity
and carcinogenicity in rats, the NOAEL was 1 ppm, equal to 0.05
mg/kg bw per day, on the basis of significant inhibition of brain
cholinesterase at 3 ppm and above. There was no apparent effect on
erythrocyte cholinesterase activity. Phorate was not carcinogenic
when fed to mice or rats at dietary levels of up to 6 ppm.
A two-generation study of reproductive toxicity in rats given
phorate at dietary levels of 0, 1, 2, 4 or 6 ppm showed no adverse
effects on reproductive parameters. The NOAEL was 2 ppm, equal to
0.17 mg/kg bw per day, on the basis of decreased brain
cholinesterase activity, tremors, decreased parental and pup body
weights and decreased pup survival at 4 ppm and above. In a
three-generation study of reproductive toxicity in mice fed dietary
levels of 0, 0.6, 1.5 or 3 ppm, the NOAEL was 1.5 ppm, equivalent to
0.23 mg/kg bw per day, on the basis of slightly reduced lactation
indices at 3 ppm.
Two studies of teratogenicity were conducted with phorate in
rats, at dose levels of 0, 0.125, 0.25 or 0.5 mg/kg bw per day and
0, 0.1, 0.2, 0.3 or 0.4 mg/kg bw per day. The NOAEL for maternal and
developmental toxicity was 0.3 mg/kg bw per day on the basis of
severe maternal toxicity culminating in death, decreased fetal body
weights and delays in fetal skeletal ossification at 0.4 mg/kg bw
per day. There was no evidence of teratogenic potential at doses as
high as 0.4 mg/kg bw per day. At the maternally lethal dose of 0.5
mg/kg bw per day, there was an increased incidence of fetuses with
enlarged hearts. In rabbits treated with doses of 0, 0.15, 0.5, 0.9
or 1.2 mg/kg bw per day, maternal mortality and decreased body
weight were observed at doses of 0.5 mg/kg bw per day and above,
resulting in an NOAEL of 0.15 mg/kg bw per day. In the absence of
embryo- and fetotoxicity, the NOAEL for developmental toxicity was
the highest dose, 1.2 mg/kg bw per day. Phorate was not teratogenic
to rabbits.
Phorate has been adequately tested for genotoxicity in vitro
and in vivo in a battery of assays. The Meeting concluded that
phorate is not genotoxic.
No clinical or histopathological signs of delayed neuropathy
were seen in chickens.
Ninety-day studies of the toxicity of phorate administered in
the diet were conducted in rats with the cholinesterase-inhibiting
sulfoxide and sulfone metabolites of phorate. The metabolites of
phorate were marginally more potent inhibitors of brain
cholinesterase than phorate in female rats. Brain cholinesterase
activity was inhibited in animals of each sex after administration
of phorate at a dietary level of 6 ppm, but no significant
inhibition was apparent at 2 ppm in either sex. The sulfoxide and
sulfone metabolites of phorate inhibited brain cholinesterase only
in females treated at the highest dietary level, 2 ppm. There were
no significant differences in the erythrocyte cholinesterase
inhibiting activity of the metabolites and the parent compound,
phorate.
An ADI was allocated on the basis of a NOAEL of 0.05 mg/kg bw
per day in the one-year study of toxicity in dogs and the two-year
feeding study in rats. The effect noted in both species was
inhibition of brain cholinesterase activity, which was associated in
dogs with clinical signs consistent with cholinergic toxicity. A
safety factor of 100 was applied.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study
of toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of teratogenicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Adequate studies on absorption, distribution, excretion and
metabolism in rats. Studies known to exist may address this
need, in whole or in part. In order to maintain the ADI, these
data should be submitted in 1995, in time for review in 1996.
2. Observations in humans
References
American Cyanamid Co. (1965) Report on Thimet systemic insecticide:
successive generation studies with mice. Unpublished report from
Central Medical Department Cyanamid. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
American Cyanamid Co. (1976) Thimet soil and systemic insecticide.
Unpublished technical information from Cyanamid International Corp.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Blinn, R.C. (1982) Personal communication to WHO by American Cynamid
Co., Wayne, NJ, USA.
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Fletcher, D.W. (1984) 42-Day neurotoxicity study with phorate in
mature white leghorn chickens. Unpublished report from Bio-life
Associates Ltd, BLAL No. 83 DN 103, AC Protocol No. 981-84-114.
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Gaines, T.B. (1969) Acute toxicity of pesticides. Toxicol. Appl.
Pharmacol., 14, 515-534.
Hutchison, E.B., Fegle, H.C., McNerney, J.M. & Levinskas, G.J.
(1978) Thimet(R) systemic insecticide: ninety-day repeated feeding
to albino rats (CL 18,161). Unpublished report from Central Medical
Department, American Cyanamid Co., Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Ivett, J.L. & Myhr, B.C. (1986) Chromosomal aberrations in vivo in
mammalian bone marrow cells on AC 35,024. Second amended report,
Project ID No. 8049, 10947-001. Unpublished report dated 9 January
1986 from Litton Bionetics, Kensington, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Kay, J.H. & Calandra, J.C. (1961) Effects of thimet phorate on
cholinesterase activity in the dog. Unpublished report from
Industrial Bio-test Lans, Inc. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Levinskas, G.J., Morici, B.S. & Shaffer, C.B. (1965) Thimet systemic
insecticide: demyelination studies in white leghorn hens.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Levinskas, G.J., McNerney, J.M. & Fegluy, H.C. (1968) Report on
sulfoxide of Thimet systemic insecticide: ninety-day repeated
feeding to albino rats. Unpublished report from Central Medical
Department, American Cyanamid Co. Submitted to WHO by American
Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1978) Teratology study in rats; Thimet(R)
phorate. Final unpublished report from Litton Bionetics, Inc.
Unpublished report from Central Medical Department, American
Cyanamid Co. Submitted to WHO by American Cynamid Co., Wayne, NJ,
USA.
Litton Bionetics (1981a) 18-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Litton Bionetics (1981b) 24-Month chronic toxicity and potential
carcinogenicity study in mice. Phorate. Final unpublished report
from Central Medical Department, American Cyanamid Co. Submitted to
WHO by American Cynamid Co., Wayne, NJ, USA.
Lochry, E.A. (1990a) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) pilot study with AC 35,024 in rats. Final
report, Project ID No. 101P-012. Unpublished report dated 19 June
1990 from Argus Research Laboratories Inc., Horsham, PA, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Lochry, E.A. (1990b) An oral developmental toxicity (embryo-fetal
toxicity/teratogenicity) definitive study with AC 35,024 in rats.
Final report, Project ID No. 101-012. Unpublished report dated 23
October 1990 from Argus Research Laboratories Inc., Horsham, PA,
USA. Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Newell, G.W. & Dilley, J.V. (1978) Teratology and acute toxicology
of selected chemical pesticides administered by inhalation. US
Environmental Protection Agency Report No. 600/1-78-003, January
1978. Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Piccirillo, V.J., Shellenberger, T.E. & Dauvin, E. M. (1987) 14-Day
range-finding oral toxicity study in the dog with AC 35,024. Revised
final report No. 85013. Unpublished report dated 11 February 1987
from Tegeris Laboratories Inc., Princeton, NJ, USA. Submitted to WHO
by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1986) A range-finding teratology study
with phorate in rabbits. Final report, project No. 86-3038.
Unpublished report dated 5 August 1986 from Bio/dynamics Inc., East
Millstone, NJ, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1987) A teratology study with phorate
in rabbits. Final report, project No. 86-3039. Unpublished report
dated 6 April 1987 from Bio/dynamics Inc., East Millstone, NJ, USA.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Schroeder, R.E. & Daly, I.W. (1991) A two-generation (two litters)
reproduction study with AC 35,024 to rats. Final report, Project ID
No. 88-3350. Unpublished report dated 23 September 1991 from
Bio/dynamics Inc., East Millstone, NJ, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Shellenberger, T.E. & Tegeris, A.S. (1987) One-year oral toxicity
study in purebred beagle dogs with AC 35,024. Final report No.
85015. Unpublished report dated 20 February 1987 from Tegeris
Laboratories Inc., Laurel, MD, USA. Submitted to WHO by American
Cyanamid Co., Princeton, NJ, USA.
Simmon, V.F., Mitchell, A.D. & Jorgenson, T.A. (1987) Evaluation of
selected pesticides as chemical mutagens in in vitro and in vivo
studies. Unpublished report from Stanford Research Institute, SRI
Report No. LSU-3493, for the US Environmental Protection Agency, EPA
Report No. EPA-600/1-77-028. Submitted to WHO by American Cynamid
Co., Wayne, NJ, USA.
Thilagar, A. & Kumarop, V. (1985) Test for chemical induction of
gene mutation at the hgrpt locus in cultured Chinese hamster ovary
(CHO) cells with and without metabolic activation. Final report,
Study No. 980-85-133. Unpublished report dated 8 August 1985 from
Sitek Research Laboratories, Rockville, MD, USA. Submitted to WHO by
American Cyanamid Co., Princeton, NJ, USA.
Trutter, J.A. (1990) 13-Week dietary toxicity study in albino mice
with AC 35,024. Final report, Project ID No. 362-201. Unpublished
report dated 16 May 1990 from Hazleton Laboratories America Inc.,
Vienna, VA, USA. Submitted to WHO by American Cyanamid Co.,
Princeton, NJ, USA.
Tusing, T.W., Kindzin, W., Hanzal, R. & Howard, J. (1956a) Repeated
oral administration (dogs). Experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
Tusing, T.W., Paynter, O.E., Kindzin, M., Hanzal, R. & Howard, J.
(1956b) Subacute feeding study--experimental insecticide 3911, 92%.
Unpublished report from Hazleton Labs, Inc. Submitted to WHO by
Submitted to WHO by American Cynamid Co., Wayne, NJ, USA.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
