
PHORATE (addendum)
First draft prepared by
D. Grant & S. Geertsen
Pesticide Evaluation Division, Health Evaluation Division,
Health Canada, Tunney's Pasture, Ottawa, Ontario, Canada
Explanation
Evaluation for acceptable daily intake
Absorption, distribution, and excretion
Biotransformation
Comments
Toxicological evaluation
References
Explanation
Phorate, an organophosphate insecticide that inhibits
cholinesterase, was first reviewed for toxicological effects by the
Joint Meeting in 1977 (Annex 1, reference 28). A temporary ADI of
0-0.0002 mg/kg bw was established in 1982 (Annex 1, reference 38)
In 1994, the Meeting re-evaluated phorate and allocated an ADI of
0-0.0005 mg/kg bw. Because in a limited study in rats it was reported
that less than 40% of the administered 32P label was excreted within
144 h, adequate studies on absorption, distribution, excretion,
and metabolism in rats were requested for review in 1996 (Annex 1,
reference 71). Data available to address this issue are summarized in
this monograph addendum.
Evaluation for acceptable daily intake
1. Absorption, distribution, and excretion
The 1994 review of phorate (Annex 1, reference 73) indicated
that male rats given a single oral dose of 2 mg/kg bw of 32P-labelled
phorate excreted 35% of the administered radiolabel in the urine and
3.5% in the faeces within 144 h. Male rats given six daily doses of
1 mg/kg bw excreted 12% of the administered radiolabel in the urine and
6% in the faeces within seven days. Brain, liver, and kidney tissues
from the latter animals contained unidentified, largely unextractable
residues (Bowman & Casida, 1958).
Male rats were given a single dose of 0.8 mg/kg bw (8 µCi/mg) of
14C-labelled phorate (purity, > 98%; specific activity, 40 µCi/mg;
see Figure 1 for position of 14C) in corn oil by gavage. Most of the
administered dose (77%) was excreted in the urine within the first
24 h after dosing. After eight days, 83% of the administered dose had
been eliminated in the urine and 13% in the faeces. Peak tissue
residue levels were found 6 h after treatment, with 0.37 ppm in blood,
0.29 ppm in kidney, 0.24 ppm in liver, 0.20 ppm in skin, 0.14 ppm in
muscle, and 0.08 ppm in fat. The levels declined throughout the study,
and by 48 h the concentrations of residue in muscle, fat, and skin
were < 0.01 ppm. By 192 h, the residues in liver were only 0.02 ppm
and those in kidney < 0.01 ppm. By the end of the study, 97% of the
administered dose had been recovered from the urine, faeces, body
tissues, and cage rinses. Oxidated, S-methylated, dephosphorylated
metabolites represented 81% of the urinary radiolabel and the parent
compound only 0.5%. Most of the radiolabel extracted from tissue was
in dephosphorylated metabolites (Hussain, 1987).
Figure 1. Position of radiolabel in 14C-phorate
S
"
(C2H5O)2-P-S-14CH2-S-CH2CH3
14C-Phorate (purity, > 98%; see Figure 1 for location of
radiolabel) was rapidly absorbed and excreted by female rats given
a single dose of 0.44 mg/kg bw (40 µCi/mg) in corn oil by gavage.
The urine was the primary route of elimination, with 78% of the
administered dose eliminated within 24 h. Faecal elimination accounted
for only 8% of the administered dose. Peak tissue residue levels
occurred after 6 h, with 0.168 ppm in blood, 0.163 ppm in kidney,
0.142 ppm in liver, 0.109 ppm in skin, 0.100 ppm in muscle, and
0.031 ppm in fat. By 192 h, the liver and kidney residues had dropped
to only 0.008 and 0.010 ppm, respectively, while those in muscle, fat,
skin, and blood residue were below the limit of detection by 48 h.
At the end of the study, > 94% of the administered dose had been
converted to non-phosphorylated metabolites arising from the cleavage
of the phosphorus-sulfur bond, methylation of the liberated thiol
group and oxidation of the resulting divalent sulfur moiety to the
sulfoxide and sulfone (Miller & Wu, 1990).
2. Biotransformation
The 1994 review of phorate (Annex 1, reference 73) indicated that
the urine of male rats given daily doses of 1 mg/kg bw contained 17%
diethyl phosphoric acid, 80% O,O-diethylphosphorothioic acid, and 3%
O,O-diethyl phosphorodithioic acid. When 32P-labelled phorate was
incubated with rat liver slice preparations, < 1% of the radiolabelled
compound was converted to hydrolysis products or unextractable
residues. Phorate sulfoxide, phorate sulfone, phoratoxon sufoxide,
and phoratoxon sulfone were formed (Bowman & Casida, 1958).
In male rats given a single dose of 0.8 mg/kg bw 14C-phorate,
the main urinary metabolites were the non-phosphorylated CL 180,298
(43%), CL 180,296 (28%), and CL 180,297 (9.6%) (see Figure 2 and Table
1 for identity of metabolites). Phosphorylated metabolites (CL 18,061,
CL 18,161, CL 18,162, CL 18,177, and CL 4,259) accounted for < 15% of
the recovered urinary metabolites, and unmetabolized parent compound
accounted for < 1%. The main residues in liver, kidney, and muscle
were also the non-phosphorylated metabolites, accounting for > 68,
79, and 83% of the tissue metabolites, respectively (Hussain, 1987).
In female rats given a single dose of 0.44 mg/kg bw 14C-phorate,
the main urinary metabolites were non-phosphorylated CL 180,298 (43%),
CL 180,296 (24%), CL 325,959 (15%), and CL 180,297 (4.6%). The main
tissue metabolites were also non-phosphorylated, accounting for
> 35, 24, and 74% of the metabolites in liver, kidney, and muscle,
respectively. No phosphorylated metabolites were identified in
the urine or tissue samples. Faecal samples contained primarily
unmetabolized parent compound (33%) and the phosphorylated metabolites
CL 18,177 (24%) CL 18,161 (8.8%), CL 18,162 (5.5%), and CL 4,259
(4.3%). The proposed metabolic pathway is presented in Figure 2, and
the chemical names of the metabolites are given in Table 1 (Miller &
Wu, 1990).
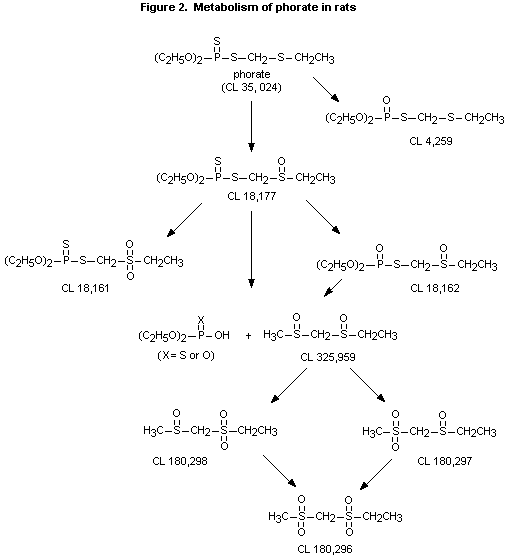 Table 1. Identity of metabolites of phorate
Metabolite no. Chemical name
CL 35,024 Phosphorodithioic acid, O,O-diethyl S-(ethylthio)methylester
(parent compound)
CL 18,162 Phosphorothioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,177 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,061 Phosphorothioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 4,259 Phosphorothioic acid, O,O-diethyl S-(ethylthio)methylester
CL 18,161 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 180,298 Sulfoxide, (ethylsulfonyl)methyl
CL 180,297 Sulfoxide, ethyl(methylsulfonyl)methyl
CL 180,296 Methane, (ethylsulfonyl)(methylsulfonyl)-
CL 325,959 Methane, (ethylsulfinyl)(methylsulfinyl)-
From Miller & Wu (1990)
Comments
14C-Labelled phorate was rapidly absorbed and excreted by rats
after a single dose in corn oil given by gavage. The urine was
the primary route of elimination, with approximately 80% of the
administered radiolabel excreted within 24 h; faecal elimination
accounted for about 10% of the label.
The current studies showed essentially total excretion of 14C
label after 192 h. The Meeting concluded that phorate and its
metabolites are rapidly excreted and that accumulation of a toxic
metabolite is not a concern. Thus, the new data did not indicate
that the ADI allocated in 1994 should be reassessed. The ADI of
0-0.0005 mg/kg bw allocated on the basis of an NOAEL of 0.05 mg/kg bw
per day in a one-year study of toxicity in dogs and a two-year study of
toxicity and carcinogenicity in rats, with a 100-fold safety factor,
was confirmed.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study of
toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of developmental toxicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
References
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Hussain, M. (1987) Thimet(R) insecticide, prorate (CL 35,024):
Disposition and metabolic fate of carbon-14 labelled CL 35,024 in the
rat. Unpublished report from American Cyanamid Co. Report No. PD-M
Volume 27-59. Submitted to WHO by American Cyanamid Co., Princeton,
NJ, USA.
Miller, P. & Wu, D. (1990) Phorate (CL 35,024): Absorption,
disposition, elimination and metabolic fate of carbon-14 CL 35,024 in
the female rat. Unpublished report from XenoBiotic Laboratories Inc.,
American Cyanamid Co. XBL Report No. RPT0043, PD-M Volume 27-59.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Table 1. Identity of metabolites of phorate
Metabolite no. Chemical name
CL 35,024 Phosphorodithioic acid, O,O-diethyl S-(ethylthio)methylester
(parent compound)
CL 18,162 Phosphorothioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,177 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,061 Phosphorothioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 4,259 Phosphorothioic acid, O,O-diethyl S-(ethylthio)methylester
CL 18,161 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 180,298 Sulfoxide, (ethylsulfonyl)methyl
CL 180,297 Sulfoxide, ethyl(methylsulfonyl)methyl
CL 180,296 Methane, (ethylsulfonyl)(methylsulfonyl)-
CL 325,959 Methane, (ethylsulfinyl)(methylsulfinyl)-
From Miller & Wu (1990)
Comments
14C-Labelled phorate was rapidly absorbed and excreted by rats
after a single dose in corn oil given by gavage. The urine was
the primary route of elimination, with approximately 80% of the
administered radiolabel excreted within 24 h; faecal elimination
accounted for about 10% of the label.
The current studies showed essentially total excretion of 14C
label after 192 h. The Meeting concluded that phorate and its
metabolites are rapidly excreted and that accumulation of a toxic
metabolite is not a concern. Thus, the new data did not indicate
that the ADI allocated in 1994 should be reassessed. The ADI of
0-0.0005 mg/kg bw allocated on the basis of an NOAEL of 0.05 mg/kg bw
per day in a one-year study of toxicity in dogs and a two-year study of
toxicity and carcinogenicity in rats, with a 100-fold safety factor,
was confirmed.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study of
toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of developmental toxicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
References
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Hussain, M. (1987) Thimet(R) insecticide, prorate (CL 35,024):
Disposition and metabolic fate of carbon-14 labelled CL 35,024 in the
rat. Unpublished report from American Cyanamid Co. Report No. PD-M
Volume 27-59. Submitted to WHO by American Cyanamid Co., Princeton,
NJ, USA.
Miller, P. & Wu, D. (1990) Phorate (CL 35,024): Absorption,
disposition, elimination and metabolic fate of carbon-14 CL 35,024 in
the female rat. Unpublished report from XenoBiotic Laboratories Inc.,
American Cyanamid Co. XBL Report No. RPT0043, PD-M Volume 27-59.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
 Table 1. Identity of metabolites of phorate
Metabolite no. Chemical name
CL 35,024 Phosphorodithioic acid, O,O-diethyl S-(ethylthio)methylester
(parent compound)
CL 18,162 Phosphorothioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,177 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,061 Phosphorothioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 4,259 Phosphorothioic acid, O,O-diethyl S-(ethylthio)methylester
CL 18,161 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 180,298 Sulfoxide, (ethylsulfonyl)methyl
CL 180,297 Sulfoxide, ethyl(methylsulfonyl)methyl
CL 180,296 Methane, (ethylsulfonyl)(methylsulfonyl)-
CL 325,959 Methane, (ethylsulfinyl)(methylsulfinyl)-
From Miller & Wu (1990)
Comments
14C-Labelled phorate was rapidly absorbed and excreted by rats
after a single dose in corn oil given by gavage. The urine was
the primary route of elimination, with approximately 80% of the
administered radiolabel excreted within 24 h; faecal elimination
accounted for about 10% of the label.
The current studies showed essentially total excretion of 14C
label after 192 h. The Meeting concluded that phorate and its
metabolites are rapidly excreted and that accumulation of a toxic
metabolite is not a concern. Thus, the new data did not indicate
that the ADI allocated in 1994 should be reassessed. The ADI of
0-0.0005 mg/kg bw allocated on the basis of an NOAEL of 0.05 mg/kg bw
per day in a one-year study of toxicity in dogs and a two-year study of
toxicity and carcinogenicity in rats, with a 100-fold safety factor,
was confirmed.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study of
toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of developmental toxicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
References
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Hussain, M. (1987) Thimet(R) insecticide, prorate (CL 35,024):
Disposition and metabolic fate of carbon-14 labelled CL 35,024 in the
rat. Unpublished report from American Cyanamid Co. Report No. PD-M
Volume 27-59. Submitted to WHO by American Cyanamid Co., Princeton,
NJ, USA.
Miller, P. & Wu, D. (1990) Phorate (CL 35,024): Absorption,
disposition, elimination and metabolic fate of carbon-14 CL 35,024 in
the female rat. Unpublished report from XenoBiotic Laboratories Inc.,
American Cyanamid Co. XBL Report No. RPT0043, PD-M Volume 27-59.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
Table 1. Identity of metabolites of phorate
Metabolite no. Chemical name
CL 35,024 Phosphorodithioic acid, O,O-diethyl S-(ethylthio)methylester
(parent compound)
CL 18,162 Phosphorothioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,177 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfinyl)methylester
CL 18,061 Phosphorothioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 4,259 Phosphorothioic acid, O,O-diethyl S-(ethylthio)methylester
CL 18,161 Phosphorodithioic acid, O,O-diethyl S-(ethylsulfonyl)methylester
CL 180,298 Sulfoxide, (ethylsulfonyl)methyl
CL 180,297 Sulfoxide, ethyl(methylsulfonyl)methyl
CL 180,296 Methane, (ethylsulfonyl)(methylsulfonyl)-
CL 325,959 Methane, (ethylsulfinyl)(methylsulfinyl)-
From Miller & Wu (1990)
Comments
14C-Labelled phorate was rapidly absorbed and excreted by rats
after a single dose in corn oil given by gavage. The urine was
the primary route of elimination, with approximately 80% of the
administered radiolabel excreted within 24 h; faecal elimination
accounted for about 10% of the label.
The current studies showed essentially total excretion of 14C
label after 192 h. The Meeting concluded that phorate and its
metabolites are rapidly excreted and that accumulation of a toxic
metabolite is not a concern. Thus, the new data did not indicate
that the ADI allocated in 1994 should be reassessed. The ADI of
0-0.0005 mg/kg bw allocated on the basis of an NOAEL of 0.05 mg/kg bw
per day in a one-year study of toxicity in dogs and a two-year study of
toxicity and carcinogenicity in rats, with a 100-fold safety factor,
was confirmed.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 1 ppm, equal to 0.18 mg/kg bw per day (13-week study of
toxicity)
Rat: 1 ppm, equal to 0.05 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
Rabbit: 0.15 mg/kg bw per day (study of developmental toxicity)
Dog: 0.05 mg/kg bw per day (one-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Further observations in humans
References
Bowman, J.S. & Casida, J.E. (1958) Further studies on the metabolism
of Thimet by plants, insects and mammals. J. Econ. Entomol., 51,
838-843.
Hussain, M. (1987) Thimet(R) insecticide, prorate (CL 35,024):
Disposition and metabolic fate of carbon-14 labelled CL 35,024 in the
rat. Unpublished report from American Cyanamid Co. Report No. PD-M
Volume 27-59. Submitted to WHO by American Cyanamid Co., Princeton,
NJ, USA.
Miller, P. & Wu, D. (1990) Phorate (CL 35,024): Absorption,
disposition, elimination and metabolic fate of carbon-14 CL 35,024 in
the female rat. Unpublished report from XenoBiotic Laboratories Inc.,
American Cyanamid Co. XBL Report No. RPT0043, PD-M Volume 27-59.
Submitted to WHO by American Cyanamid Co., Princeton, NJ, USA.
