
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
AZOCYCLOTIN
IDENTITY
Chemical Name: Tri(cyclohexyl)-(1,2,4-Triazol-1-yl)tin or
1-(Tricyclohexylstannyl-1H-1,2,4-triazole)
Synonyms: Peropal, BUE 1452
Structural formula:
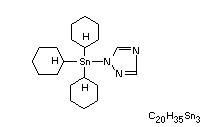 Other Information on Identity and Properties
Molecular Weight: 436.2
Appearance: White Powder
Melting Point: 218.8°C
Vapor Pressure: Less than 5 × 10-5 mbar at 25°C
Solubility: in water <0.25 mg/kg
in cyclohexane )
in isopropanol )
in toluene ) 0-0.01 g
in methylene chloride ) per g solvent
in ligroin )
Minimum degree of purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical azocyclotin was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Azocyclotin is an organotin acaricide effective against spider miters.
It is recommended for the control of strains which are resistant to
other chemical compounds. Azocyclotin gives good control of all
motile stages, i.e. larvae as well as adults. Through trials on mixed
populations, it has been established that azocyclotin has an
ovolarvicidal effect on summer but not on winter eggs.
Azocyclotin is a contact poison, and it is notable for its long
residual activity. It is used on pome and stone fruit, strawberries,
vegetables and grapes. When used at the recommended concentrations,
it displays no phytotoxicity. Azocyclotin is marketed as a 25% and
50% wettable powder, in Argentina, Australia, Austria, Chile, Federal
Republic of Germany, Greece, Iran, Lebanon, Morocco, New Zealand,
Peru, South Africa, Spain and Uruguay. It is used as a foliar spray
on the crops as listed in Table 1. There are no post-harvest uses.
Table 1. Current uses of Azocyclotin
Concentration Number of Pre-harvest
Crop % act. ing. applications interval
Pome and stone 0.025 1-3 14 days
fruit (0.3-0.5 kg/ha)
Strawberry 0.025 1-3 14 days
(0.5 kg/ha)
Vegetables:
Bush beans 0.025 1-2 14 days
(max.3)
Eggplants 0.025 1-2 14 days
(0.375-0.75
kg/ha)
Grapes 0.025 1-2 35 days
(0.5 kg/ha) (max.3-4)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue studies after applications of azocyclotin (I) have been
performed on apple, bush beans, eggplant, damson plums, strawberry and
wine grape (Table 2). The residue data were obtained by the use of an
organotin method (Möllhoff, 1977a) which determines the sum of the
parent compound, azocyclotin (I), and its two metabolites,
tricyclohexyl tin hydroxide (or cyhexatin) (III), and dicyclohexyl tin
oxide (IV) (cfr. Figure 1). Compound IV always represented less than
10% of the total residue.
Residue data obtained by the use of a triazole method (Möllhoff,
1977a) is presented in Table 3, from which it is noted that the
residues of the triazole moiety were analytically non-significant or
below detection limit (i.e. 0.1 - 0.2 mg%kg) shortly after
application. If not otherwise stated, therefore, the following
residue information is obtained by the organotin method.
Apple
After one, two or three applications of 0.3-0.65 kg azocyclotin per
ha, the maximum residue on day 0 was 0.35 mg/kg. Within the course of
three weeks, the residue levels usually dropped down to or below the
limit of determination (0.05 mg/kg).
Bean, kidney
Following three applications of 0.225 kg azocyclotin per ha, the
residue levels declined within three weeks from 0.2-0.3 mg/kg to
n.d.-0.15 mg/kg.
Eggplant
Following one or two applications of azocyclotin at 0.375-0.7 kg/ha,
the initially measured residue level was 0.1-0.45 mg/kg. It decreased
to a level below the limit of determination during the following 30
days, or in several cases during only 14 days.
Damson plums
The maximum residue level measured after three applications of
azocyclotin at 0.5 kg/ha was 0.8 mg/kg, while the residue levels
measured 3 and 4 weeks after the final spray were 0.25, resp. 0.2
mg/kg.
Strawberry
Following two spray applications of azocyclotin at 0.5 kg/ha, the
immediate residue levels were 0.6 mg/kg. No more residues were
detectable 14 days later.
Wine Grape
Following three or four spray applications of azocyclotin at 0.45-0.5
kg/ha, the initial residue level in a series of experiments varied
from 0.85 up to 2.1 mg/kg. Measurements made four weeks after the
final spray showed that the residue level was less than 1 mg/kg
following three applications, but higher following four applications.
FATE OF RESIDUES
A general degradation pathway for azocyclotin is shown in Figure 1,
indicating a gradual and step-wise off-splitting of the rings bound to
the central tin atom, starting with the 1,2,4-triazole moiety. Some
properties of the individual metabolites are given in Table 4.
Table 2. Residues of Azocyclotin from Supervised Trials (Bayer AG)*
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Apple 0.3 kg/ha 1× 7 0.1 - 0.15 France
13-14 <0.05 - 0.05
21 n.d. - <O.05
0.3125 1/2× 0 0.15 - 0.30 Israel
kg/ha 7 0.05 - 0.15
14 <0.05 - 0.05
30 n.d. - n.d.
0.3 - 0.375 2× 14 0.06 - 0.08 Netherlands
kg/ha 21 n.d. - 0.1
28 n.d. - n.d.
0.5 kg/ha 3× 0 0.15 - 0.25 Germany (FRG)
7 0.1 - 0.15
14 0.05 - 0.15
21 n.d. - 0.1
28 n.d. - 0.05
0.625 kg/ha 1/2× 0 0.2 - 0.35 Israel
7 0.15 - 0.3
14 0.05 - 0.2
30 n.d. - <0.05
Beans (Bush
or Kidney) 0.225 kg/ha 3× 0 0.2 - 0.3 Germany (FRG)
7 0.15 - 0.2
14 <0.05 - 0.15
21 n.d. - 0.15
Eggplant 0.375 kg/ha 1/2× 0 0.1 - 0.45 Israel
7 n.d. - 0.15
14 n.d. - n.d.
30 n.d. - n.d.
0.75 kg/ha 1/2× 0 0.1 - 0.45
7 n.d. - 0.2
14 n.d. - 0.1
30 n.d. - n.d.
Table 2. Continued...
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Plum, damson 0.5 kg/ha 3× 0 0.1 - 0.8 Germany (FRG)
7 0.05 - 0.5
14 <0.05 - 0.4
21 <0.05 - 0.25
28 <0.05 - 0.2
Strawberry 0.5 kg/ha 2× 0 0.55 - 0.6 Germany (FRG)
7 0.1 - 0.15
14 n.d. - n.d.
Wine grape 0.3 kg/ha 1× 7 0.3 France
14 0.25
21 0.2
0.45-0.5 3× 0 0.85 - 2.1 Germany (FRG)
kg/ha 14 0.45 - 1.75
21 0.45 - 0.8
28 0.l - 0.8
35 0.1 - 0.9
42 0.1 - 0.15
49 <0.05 - 0.15
0.5 kg/ha 4× 0 1.45 - 2.15
7 1.20 - 2.4
14 1.15 - 1.75
22 1.15 - 1.9
29 1.0 - 1.4
36 1.05 - 1.2
* Results in this table obtained by organotin method (Möllhoff, 1977a).
Table 3. Residues of azocyclotin determined by triazole and organotin methods
Residue
Application Days after Triazole Organotin Country
Crop rate treatment Method Method
Apple 1 × 0.3 kg/ha 7 n.d.- n.d. 0.1 - 0.15 FRG
14 - <0.05 - 0.05
21 - <0.05 - <0.05
1 × 1 kg/ha 0 0.4 - 0.4 - FRG
7 n.d.- <0.1 -
14 n.d. - n.d. -
1 × 0.37-0.75
kg/ha 9 2.32 - New
3 1.28 - Zealand
7 0.43 -
14 0.05 -
Grape 1 × 0.3 kg/ha 7 n.d. 0.3 FRG
14 n.d. 0.25
21 n.d. 0.2
Other Information on Identity and Properties
Molecular Weight: 436.2
Appearance: White Powder
Melting Point: 218.8°C
Vapor Pressure: Less than 5 × 10-5 mbar at 25°C
Solubility: in water <0.25 mg/kg
in cyclohexane )
in isopropanol )
in toluene ) 0-0.01 g
in methylene chloride ) per g solvent
in ligroin )
Minimum degree of purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical azocyclotin was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Azocyclotin is an organotin acaricide effective against spider miters.
It is recommended for the control of strains which are resistant to
other chemical compounds. Azocyclotin gives good control of all
motile stages, i.e. larvae as well as adults. Through trials on mixed
populations, it has been established that azocyclotin has an
ovolarvicidal effect on summer but not on winter eggs.
Azocyclotin is a contact poison, and it is notable for its long
residual activity. It is used on pome and stone fruit, strawberries,
vegetables and grapes. When used at the recommended concentrations,
it displays no phytotoxicity. Azocyclotin is marketed as a 25% and
50% wettable powder, in Argentina, Australia, Austria, Chile, Federal
Republic of Germany, Greece, Iran, Lebanon, Morocco, New Zealand,
Peru, South Africa, Spain and Uruguay. It is used as a foliar spray
on the crops as listed in Table 1. There are no post-harvest uses.
Table 1. Current uses of Azocyclotin
Concentration Number of Pre-harvest
Crop % act. ing. applications interval
Pome and stone 0.025 1-3 14 days
fruit (0.3-0.5 kg/ha)
Strawberry 0.025 1-3 14 days
(0.5 kg/ha)
Vegetables:
Bush beans 0.025 1-2 14 days
(max.3)
Eggplants 0.025 1-2 14 days
(0.375-0.75
kg/ha)
Grapes 0.025 1-2 35 days
(0.5 kg/ha) (max.3-4)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue studies after applications of azocyclotin (I) have been
performed on apple, bush beans, eggplant, damson plums, strawberry and
wine grape (Table 2). The residue data were obtained by the use of an
organotin method (Möllhoff, 1977a) which determines the sum of the
parent compound, azocyclotin (I), and its two metabolites,
tricyclohexyl tin hydroxide (or cyhexatin) (III), and dicyclohexyl tin
oxide (IV) (cfr. Figure 1). Compound IV always represented less than
10% of the total residue.
Residue data obtained by the use of a triazole method (Möllhoff,
1977a) is presented in Table 3, from which it is noted that the
residues of the triazole moiety were analytically non-significant or
below detection limit (i.e. 0.1 - 0.2 mg%kg) shortly after
application. If not otherwise stated, therefore, the following
residue information is obtained by the organotin method.
Apple
After one, two or three applications of 0.3-0.65 kg azocyclotin per
ha, the maximum residue on day 0 was 0.35 mg/kg. Within the course of
three weeks, the residue levels usually dropped down to or below the
limit of determination (0.05 mg/kg).
Bean, kidney
Following three applications of 0.225 kg azocyclotin per ha, the
residue levels declined within three weeks from 0.2-0.3 mg/kg to
n.d.-0.15 mg/kg.
Eggplant
Following one or two applications of azocyclotin at 0.375-0.7 kg/ha,
the initially measured residue level was 0.1-0.45 mg/kg. It decreased
to a level below the limit of determination during the following 30
days, or in several cases during only 14 days.
Damson plums
The maximum residue level measured after three applications of
azocyclotin at 0.5 kg/ha was 0.8 mg/kg, while the residue levels
measured 3 and 4 weeks after the final spray were 0.25, resp. 0.2
mg/kg.
Strawberry
Following two spray applications of azocyclotin at 0.5 kg/ha, the
immediate residue levels were 0.6 mg/kg. No more residues were
detectable 14 days later.
Wine Grape
Following three or four spray applications of azocyclotin at 0.45-0.5
kg/ha, the initial residue level in a series of experiments varied
from 0.85 up to 2.1 mg/kg. Measurements made four weeks after the
final spray showed that the residue level was less than 1 mg/kg
following three applications, but higher following four applications.
FATE OF RESIDUES
A general degradation pathway for azocyclotin is shown in Figure 1,
indicating a gradual and step-wise off-splitting of the rings bound to
the central tin atom, starting with the 1,2,4-triazole moiety. Some
properties of the individual metabolites are given in Table 4.
Table 2. Residues of Azocyclotin from Supervised Trials (Bayer AG)*
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Apple 0.3 kg/ha 1× 7 0.1 - 0.15 France
13-14 <0.05 - 0.05
21 n.d. - <O.05
0.3125 1/2× 0 0.15 - 0.30 Israel
kg/ha 7 0.05 - 0.15
14 <0.05 - 0.05
30 n.d. - n.d.
0.3 - 0.375 2× 14 0.06 - 0.08 Netherlands
kg/ha 21 n.d. - 0.1
28 n.d. - n.d.
0.5 kg/ha 3× 0 0.15 - 0.25 Germany (FRG)
7 0.1 - 0.15
14 0.05 - 0.15
21 n.d. - 0.1
28 n.d. - 0.05
0.625 kg/ha 1/2× 0 0.2 - 0.35 Israel
7 0.15 - 0.3
14 0.05 - 0.2
30 n.d. - <0.05
Beans (Bush
or Kidney) 0.225 kg/ha 3× 0 0.2 - 0.3 Germany (FRG)
7 0.15 - 0.2
14 <0.05 - 0.15
21 n.d. - 0.15
Eggplant 0.375 kg/ha 1/2× 0 0.1 - 0.45 Israel
7 n.d. - 0.15
14 n.d. - n.d.
30 n.d. - n.d.
0.75 kg/ha 1/2× 0 0.1 - 0.45
7 n.d. - 0.2
14 n.d. - 0.1
30 n.d. - n.d.
Table 2. Continued...
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Plum, damson 0.5 kg/ha 3× 0 0.1 - 0.8 Germany (FRG)
7 0.05 - 0.5
14 <0.05 - 0.4
21 <0.05 - 0.25
28 <0.05 - 0.2
Strawberry 0.5 kg/ha 2× 0 0.55 - 0.6 Germany (FRG)
7 0.1 - 0.15
14 n.d. - n.d.
Wine grape 0.3 kg/ha 1× 7 0.3 France
14 0.25
21 0.2
0.45-0.5 3× 0 0.85 - 2.1 Germany (FRG)
kg/ha 14 0.45 - 1.75
21 0.45 - 0.8
28 0.l - 0.8
35 0.1 - 0.9
42 0.1 - 0.15
49 <0.05 - 0.15
0.5 kg/ha 4× 0 1.45 - 2.15
7 1.20 - 2.4
14 1.15 - 1.75
22 1.15 - 1.9
29 1.0 - 1.4
36 1.05 - 1.2
* Results in this table obtained by organotin method (Möllhoff, 1977a).
Table 3. Residues of azocyclotin determined by triazole and organotin methods
Residue
Application Days after Triazole Organotin Country
Crop rate treatment Method Method
Apple 1 × 0.3 kg/ha 7 n.d.- n.d. 0.1 - 0.15 FRG
14 - <0.05 - 0.05
21 - <0.05 - <0.05
1 × 1 kg/ha 0 0.4 - 0.4 - FRG
7 n.d.- <0.1 -
14 n.d. - n.d. -
1 × 0.37-0.75
kg/ha 9 2.32 - New
3 1.28 - Zealand
7 0.43 -
14 0.05 -
Grape 1 × 0.3 kg/ha 7 n.d. 0.3 FRG
14 n.d. 0.25
21 n.d. 0.2
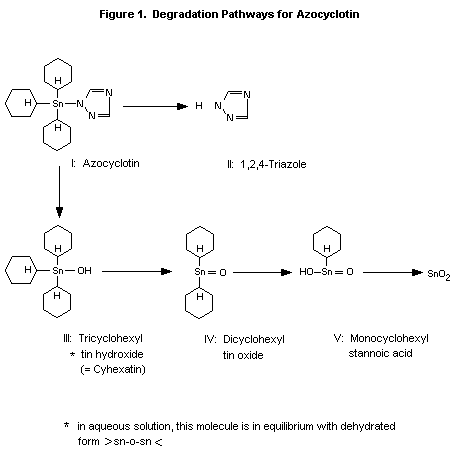 Table 4. Some properties of azocyclotin metabolites
Metabolites (Fig.1)
Azocyclotin II III IV
Melting point 218.8°C 121°C 245°C 291°C
Vapour
pressure at:
20°C) <5 x 10-5 3 X 10-4 not measured
30°C)in mbar - 1 X 10-3
40°C) - 3 X 10-3
50°C) - 1 X 10-2
Solubility in
water (mg/kg) <0.25 readily <1 insoluble
In rats
The meeting is informed that studies on biokinetics and on metabolism
in rats have been conducted with a mixture of
1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyolohexyl-stanny1113-1,2,4-triazole.
In plants
Residue analyses on apples and grapes performed by the triazole method
gave results close to or below the limit of determination (0.1 mg/kg)
one week after treatment of the plants (cfr Table 3). When the
organotin method was used, on the other hand, residues were found for
a longer period (Table 2), indicating that the triazole ring was the
first ligand to be split off from the central tin atom. By referring
to the vapor pressure/temperature gradient of triazole (II) (cfr.
Table 4), it has been suggested that triazole disappearance may be due
to volatilization (Möllhoff, 1977a). In the field studies of Möllhoff
(1977a) it is reported that metabolite IV, dicyclohexyl tin oxide,
occurs only in small amounts in plants material, i.e. less than 10%.
Mass balance and metabolism studies on azocyclotin were performed in a
laboratory ecosystem with bush (i.e. kidney) beans, enclosed in a
quartz bell jar giving 96% light transmission (Wolff et al., 1977a).
The azocyclotin preparation which was used was a well-defined mixture
of 1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyclohexyl-stannyl113-1,2,4-triazole. Forty-four days after the
treatment, the amounts of 14C and 113Sn radioactivity distributed
among the different parts of the system was measured, and 95% of the
applied radioactivity was recovered.
In the exhaust air absorber, less than 0.01% of the applied 113Sn
radioactivity was found as against 41.7% of the originally applied
14C radioactivity. Of the remaining 58.3% of the 14C radioactivity,
43.3% was present on/in the soil, and 9.8% in the plants. Of the
originally applied 113Sn radioactivity, 81.7% was found on or in the
soil and 14.5% on or in the plants. Autoradiographs showed spray
spots caused by 14C radioactivity on the treated leaves, as against
uniform blackening on newly grown plant parts. From these findings
and from the comparison of 14C radioactivity and 113Sn radioactivity
in different plant parts (Table 5), it is evident that a major
proportion of the applied azocyclotin was no longer present as the
unchanged parent compound. There was only little translocation of the
radioactivities in the plants. However, a large proportion of the
14C radioactivity volatilized and was trapped in the absorber.
Thin-layer chromatographic separation of the plant extracts revealed
that the individual azocyclotin metabolites were present in various
plant parts in the following proportions: metabolite III: 47 - 71%,
metabolite IV: 7 - 33%, metabolite V: 5-22% and inorganic Sn: 6-19%
(cfr. Table 6). The structures of these compounds were verified by
comparison of their Rf values from TLC with those of reference
substances, and by the ratios of 113Sn:14C (cfr. Table 6).
In processing
It will be noted from the data presented in Table 7 that when wine
grapes were pressed, about 25% of the residue was carried into the
must. However, the wine made from the must did not contain any
measurable residue amounts of azocyclotin.
In soil
Leaching Studies
Whereas azocyclotin is very sparingly soluble in water, 1,2,4-triazole
(II) displays good solubility. This metabolite, however, is absorbed
from water on soil particles according to soil leaching studies
performed by Möllhoff (1977b). From these studies performed on three
different soils, it was found that neither azocyclotin, nor its
metabolites II, III and IV were detectable in percolated water under
the conditions of the experiments. The limits of determination both
for azocyclotin and for II, III, and IV were at 2% of the applied
amount of parent compound.
Also in the ecosystem planted with bush beans (Wolff et al., 1977a),
vertical movement in the soil was minimal. After 44 days, about 98%
of the applied radioactivity (113Sn + 14C-azocyclotin) was retained
in top soil between 0 and 3 cm, while soil layers below 15 cm showed
no activity at all.
Table 5. Distribution of 113Sn and 14C radioactivities among
different plant parts, soil and exhaust air from laboratory simulation
study after 44 days. (Wolf et al., 1977a).
113Sn (in %) 14C (in %)
Bush (i.e. kidney) beans
Old beans 0.03 0.30
Young beans 0.01 0.03
Leaves + stem 7.92 5.11
Fallen leaves 6.56 4.44
Roots 0.10 0.03
Total in plants 14.62 9.91
Soil 81.7 43.3
Exhaust air <0.01 41.7
Recovered 96.3% 94.9%
Table 6. Metabolite proportions after azocyclotin treatment and ratios of 113Sn:14C-radioactivity in
bean plant extracts (Wolf et al, 1977a)
Origin on
Plant part III: Tricyclohexyl Sn IV: Dicyclohexyl Sn V: Monocyclohexyl Sn TLC-plate*
Old beans 71.7% 17. 3% 4.9% 6.0%
Young beans 47.4% 33.6% 9.5% 9.5%
Leaves + stem 69.5% 7.3% 13.0% 9.6%
Fallen leaves 61.6% 8.4% 15.9% 14.6%
Roots 47.6% 10.9% 22.1% 19.14%
Ratios 113Sn: 14C 113Sn:14C 113Sn:14C 113Sn:14C
Theoretical 1 : 0.92 1 : 0.62 1 : 0.31 1 : 0
Found 1 : 0.91-1.06 1: 0.58-0.84 1: 0.27-0.38 1. 0.03-0.16
* Tin compounds without cyclohexyl rings.
Table 7. Azocyclotin residues during wine processing
(Bayer AG)
Experiment 1 Experiment 2
Azocyclotin (mg/kg)
Grapes 1.05 - 1.2 <0.05 - 0.1
Must 0.25 - 0.9 <0.05 - <0.05
Wine n.d. n.d.
Degradation in soil
Field experiments on three different soils showed that the triazole
component of azocyclotin was eliminated to levels below the limit of
determination (0.1 mg/kg) within 14 days. The organotin residues
measured as the sum of I, III and IV, on the other hand, were degraded
with a half-life of 50 to 90 days. Also following two treatments at a
one-year interval, there was found no extra build-up of soil residues.
(cfr. Table 8).
In studies under laboratory conditions, degradation of azocyclotin in
two different soils indicated half-lives in the order of 250 days,
while in another experiment under the same conditions, half-lives were
found to be 200 days for I and metabolite III, and about one year for
the sum of I, III, IV and V (Wolff et al., 1977b).
Metabolism in soil
In field experiments, azocyclotin (I) itself was measurable only for
one week (Table 3). During the first week, also, up to about 25%
dicyclohexyl tin oxide (IV) formed (Möllhoff, 1977b). Its content
declined thereafter parallel to the disappearance of its immediate
precursor, tricyclohexyl tin hydroxide (III). In laboratory
metabolism studies conducted with azocyclotin dicyclohexyl tin oxide
was formed gradually during 30-60 days and remained thereafter on a
plateau level corresponding to about 15% of the applied azocyclotin
dose.
In another laboratory experiment using a larger amount of soil (500 g)
held in a closed bottle, measurements made nine months after
application of 50 mg azocyclotin/kg showed that 45% of the applied
amount of tin was still present as organotin compounds in the soil.
At this point of time, the analytically determined components triazole
(II) and tricyclohexyl butyl tin (i.e. metabolite III after
butylation) were present in almost stoichiometric proportions of 23.8%
and 25.4%, respectively. Whether azocyclotin or a mixture of triazole
and tricyclohexyl tin hydroxide was present, or whether there was a
balance among I, II and III could not be decided by analysis. From
the fast decline of the triazole proportion in field conditions,
however, it was concluded that triazole under open conditions would
volatilize rapidly (Möllhoff, 1977b).
In the above-mentioned larger-scale laboratory experiment, it was also
found after nine months of storage that of the originally applied
azocyclotin dose, 1O.8% was present as dicyclohexyl tin oxide (IV) and
8.25% as cyclohexyl stannoic acid (V). After derivatization with
butyl lithium these two compounds were measured quantitatively by gas
chromatography, and after separation on capillary columns, they were
identified by mass spectrometry. The same two metabolites (IV and V),
were found in soil nine months after application of the metabolite
dicyclohexyl tin oxide (IV) in amounts corresponding to 36.5% and
11.7% respectively. In the soil metabolism studies of Wolf et al.
(1977b), using a mixture of 14C- and 113Sn-labelled azocyclotin, it
was found that the proportion of 113Sn radioactivity which was
non-extractable never exceeded 6.6% while, on the other hand, the
proportion of non-extractable 14C radioactivity increased towards the
end of the 200-day experiment to 24.5% (cfr Table 9). This is
interpreted as a sign of metabolic incorporation of 14C-carbon into
non-extractable compounds, the identity of which, however, remained
unknown. The proportion of the applied tin to which three cyclohexyl
rings were still bound, declined to a level of 50% during this
experiment.
Fate in water
Degradation of azocyclotin in aquarium water was studied during 150
days in closed bottles at 22 ± 2°C, with azocyclotin added at a level
of 10 mg/kg (Möllhoff, 1977c). The 25% w.p. formulated azocyclotin
was used for this experiment, but despite frequent shaking, the
formulation could not be held continuously in suspension.
At the end of the experimental period, one-third of the applied amount
of azocyclotin still contained all three cyclohexyl rings at the tin
atom (Table 9), while the proportion of triazole still bound to the
tin atom could not be determined analytically. However, formation of
free triazole was observed at a level which on termination of the
experiment was estimated to be 0.41 mg/kg, equivalent to 2.6 mg/kg
when calculated as azocyclotin. In an open system, such free triazole
is expected to disappear by volatilization.
Metabolite IV, dicyclohexyl tin oxide, was determined throughout this
experiment, although in small amounts only, i.e. no more than 0.26
mg/kg.
Table 8. Azocyclotin residues in soil by triazole and organotin
methods (Wolf, et al., 1977b)
Application Days after Azocyclotin (mg/kg)
treatment Triazole Organotin
Method Method
First year
1 × 5 kg/ha 0 2.3 - 3.1 2.3 - 3.1
7 0.48 - 0.65 1.9 - 2.5
14 n.d. - n.d. 1.35 - 1.7
60 - 65 0.3 - 1.25
90 - 92 0.3 - 0.7
120 - 124 0.15 - 0.3
150 - 155 n.d. - 0.2
Second year
1 × 5 kg/ha 0 2.9 3.1
7 0.48 1.9
14 n.d. 1.35
59 0.85
92 0.65
120 0.35
150 0.3
0.3
Table 9. Degradation of Azocyclotin (25% w.p.) in aquarium water
(Möllhoff,1977c)
Storage Analysis by Analysis by
time triazole meth. organotin method
in days
Azocyclotin Tricyclohexyl Dicyclohexyl
+ triazole * tin* tin *
0 8.95 0.26
7 9.0 0.25
28 7.6 7.6 0.33
49 7.9 5.9 0.19
71 5.3 3.2 0.36
91 5.3 2.7 0.29
120 5.3 2.75 0.14
150 5.6 3.0 0.14
* All results calculated as azocyclotin (mg/kg).
METHOD OF RESIDUE ANALYSIS
Residues of azocyclotin can be measured by two different methods
developed by Möllhoff (1977a).
Triazole method (parent compound + metabolite II)
Plant, soil and water samples are extracted with isopropanol followed
by chloroform, and cleanup of extract by precipation. The final
determination is a GLC procedure using thermionic N-detector (AFID)
giving response to the triazole, and with a limit of determination of
0.1 - 0.2 mg/kg, expressed as azocyclotin. Azocyclotin (I) and
1,2,4-triazole (II) cannot be distinguished by this method.
This method determines that portion of the molecule which is split off
first from the tin atom, i.e. the 1,2,4-triazole moiety.
Accordingly, residues of azocyclotin measured by the triazole method
usually drop relatively fast below limit of determination, while
remaining organotin compounds can only be determined by method B.
Organotin method (parent compound + metabolites III + IV)
Plant, soil, water and laboratory animal feed samples are macerated
with water and treated with hydrobromic acid in acetone, and
thereafter extracted with hexane. The hexane extracted residue in
ethyl ether is reacted with methyl magnesium chloride and hydrolysed.
The methylated compounds, tricyclohexyl methyl tin and dicyclohexyl
dimethyl tin, which are formed from azocyclotin and metabolites III
and IV are cleaned up on Florisil and gas chromatographed using a
flame photometric detector (FPD) with a 394 nm filter. The limits of
determination are 0.05 - 0.1 mg/kg.
Azocyclotin (I) and cyhexatin (III) cannot be distinguished by this
method, but the method permits determination of the sum of these two
compounds in parallel with determination of metabolite IV
(dicyclohexyl tin oxide).
The proportion of IV in the total residue measured by the organotin
method is usually less than 10% in plants, and between 10 and 20% in
soil, whilst metabolite V (cyclohexyl stannoic acid) is of little
significance as residue. This latter metabolite V could be determined
by the organotin method if chloroform is used instead of hexane for
the extraction. The organotin method has multiresidue method
characteristics as it permits the simultaneous determination of
several chemical entities, in this case partially alkylated or
arylated tin compounds (cfr. Steinmeyer et al., 1965, Heubert &
Wirth, 1975, Figge et al., 1977 and Wright et al., 1979) by
gaschromatographic separations after reaction with methyl magnesium
chloride or with butyl lithium. The latter alkylation agent deserves
special attention because the alkylation seems to proceed very
smoothly producing compounds with molecular weight of the same range.
A more convenient isothermal GLC technique is thereby facilitated.
NATIONAL MRLs REPORTED TO THE MEETING
National Limits and associated pre-harvest intervals which have
been reported to the meeting are shown in Table 10.
Table 10. National MRLs and Safety Intervals
Safety
Country Crop MRL Interval
in mg/kg in days
Chile fruits 15
Cyprus pome and stone fruits,
citrus 21
Germany bush and runner beans 14
F.R.G. pome fruit 14
grapes (except
table grapes) 35
hops 50.0
pome fruits 2.0
wine grapes 2.0
Morocco pome fruit 28
New pipfruit 14
Zealand stone fruit 14
South apples 0.5 3
Africa pears 0.5 3
peaches 0.5 21
APPRAISAL
Azocyclotin is an organotin acaricide used against spider mites on
fruits and vegetables, especially recommended for the control of
strains which are resistant to other chemicals e.g.
organophosphorous compounds. It is formulated in wettable powders (25
and 50%) and marketed in most continents.
In plants, animals, soil and water, azocyclotin (I) is degraded by an
initial off-splitting of 1,2,4-triazole (II) from tricyclohexyl tin
hydroxide (III) identical with cyhexatin, followed by stepwise
formation of cicyclohexyl tin oxide (IV) and monocyclohexyl stannoic
acid (V) through the loss of the three cyclohexyl rings from the
central Sn-atom.
The first degradation step by splitting off of 1,2,4-triazole is a
relatively fast process, and the 1,2,4-triazole moiety disappears to
levels at or below the limit of determination shortly after treatment
of plants or after application to soil or water. The triazole
probably disappears from plants by volatilization.
The remaining organotin compounds, cyhexatin and its further
metabolites, are more persistent, and dissipation of the total
organotin residues is mainly governed by a process of growth dilution
on most plants and vegetables after application. But compounds III,
IV and V are found as simultaneous residues in variable ratios, and
with a limited amount of inorganic Sn also present. In a single study
with soil, inorganic Sn was found at the level of 6-19% of total
residues.
Half-lives of organotin residues in soil varied from 50-90 days in
field studies and from 200 days to one year under laboratory
conditions. Vertical movement or leaching in soils are found to be
minimal or unlikely.
Residues of azocyclotin (as organotin) in apples, beans, eggplants and
strawberry are at or below limit of determination after application at
recommended use levels and appropriate waiting periods, while
significant residues were found in most cases on grapes. After
processing, up to 25% of residues in grapes was carried over to must,
but without any measurable transfer of residues to wine.
Two gas chromatographic methods are available for the determination of
azocyclotin. The one is based on determination of the 1,2,4-triazole
moiety and it will not distinguish azocyclotin (I) from the triazole
(II). Residues by this method usually drop relatively rapidly to
below the limit of determination, after which residue pattern cannot
be distinguished from that which follows treatment with cyhexatin.
The other method is an organotin method developed in recent years,
which permits determination of organotin metabolites III, IV and V
individually, while it will not distinguish azocyclotin (I) from
metabolite III. Metabolite V is of little significance as residue.
It is determined by the same method as I + III and IV, but only after
separate extraction procedure.
RECOMMENDATIONS
The following guideline limits for azocyclotin are recommended on the
basis of data resulting from supervised trials. They refer to the sum
of organotin compounds expressed as azocyclotin.
Limit Pre-harvest interval on which
Commodity (mg/kg) recommendation is based
Apples 0.1* 21 days
Egg plants 0.1* 30 days
Grapes 35 days
Kidney Beans 0.2 21 days
Strawberry 0.1* 14 days
The Meeting realized that recommendations in certain cases are
incompatible with earlier recommendations on cyhexatin from which
azocyclotin residues will be undistinguishable shortly after
application. This may be due to somewhat differing use patterns for
the two compounds, but partly also to the fact that a majority of
earlier data derives from use of another analytical method, i.e.
colorimetric, total Sn-determination. Therefore, it is suggested that
special efforts should be made at the international level to collect
and review information on developed use patterns and residue data
derived from new methodology for cyhexatin.
Further work or information Required
By 1980
As residues from azocyclotin and cyhexatin cannot be distinguished
shortly after application, information on use patterns and on residue
data for both compounds, especially for cyhexatin, should be reviewed
before residue limits for the two pesticides can be harmonized.
REFERENCES
Bayer, A.G. Various reports on azocyclotin residues in fruit,
vegetables and soil (1978), Unpublished.
Figge, K., Koch, J., Lubba, H. - Beitrag zur gaschromatographischen
Analyse von Organozinn-Stabilisatoren für Plyvinylchlorid. J.
Chromatogr. 131, 317-327.
Möllhoff, E. Methode zur gaschromatographischen Bestimmung des
Akarizids Peropal und seiner Metaboliten in Pflanzen, Böden, Wasser
und Kleintierfutter. Pflanzenschutz-Nachr. Bayer 30, 249-263 (1977a).
Abbau und Metabolismus von Peropal im Boden. Bayer AG, Report
RA-11/77 (English version) (1977b).
Abbau von Peropal in Aquarienwasser (geschlossenes System). Bayer
AG; Report RA-815/77 (1977c).
Neubert, G., Wirth, H.O. - Zur Analytik con Organozinnstabilistatoren.
Z. Anal. Chem. 273, 19-23.
Steinmeyer, R.D., Fentiman, A.F., Kahler, E.J., - Analysis of alkyltin
bromides by gas liquid chromatography. Analytical Chem. 37, 520-523.
Wolf, W., Lippert, K.D., Figge, K. - Übber das Verhalten des Akarizids
"Tricyclohexylzinn-1,2,4-triazolid" und seiner Abbauprodukte im
Okosystem "Buschbohnenkulture". Natec, Gesellschaft für
naturwissenschaftlinch-technische Dienste mbH., Projekt NA 76 00 43,
(1977a).
Uber das Abbauverhalten des Akarizids "Tricyclohexylzinn-1,2,4-
triazolid"in Standardboden. Natec, Gesellschaft für naturwissen-,
schaftlich-technische Dienste mbH., Project NA 76 00 43, (1977b).
Wright, B.W., Lee, M.L., Booth, G.M. - Determination of
triphenyltinhydroxide derivatives by capillary GC and Tin-selective
FPD. Journ. High Resolution Chrom. and Chromatography Comm. S. 189.
Table 4. Some properties of azocyclotin metabolites
Metabolites (Fig.1)
Azocyclotin II III IV
Melting point 218.8°C 121°C 245°C 291°C
Vapour
pressure at:
20°C) <5 x 10-5 3 X 10-4 not measured
30°C)in mbar - 1 X 10-3
40°C) - 3 X 10-3
50°C) - 1 X 10-2
Solubility in
water (mg/kg) <0.25 readily <1 insoluble
In rats
The meeting is informed that studies on biokinetics and on metabolism
in rats have been conducted with a mixture of
1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyolohexyl-stanny1113-1,2,4-triazole.
In plants
Residue analyses on apples and grapes performed by the triazole method
gave results close to or below the limit of determination (0.1 mg/kg)
one week after treatment of the plants (cfr Table 3). When the
organotin method was used, on the other hand, residues were found for
a longer period (Table 2), indicating that the triazole ring was the
first ligand to be split off from the central tin atom. By referring
to the vapor pressure/temperature gradient of triazole (II) (cfr.
Table 4), it has been suggested that triazole disappearance may be due
to volatilization (Möllhoff, 1977a). In the field studies of Möllhoff
(1977a) it is reported that metabolite IV, dicyclohexyl tin oxide,
occurs only in small amounts in plants material, i.e. less than 10%.
Mass balance and metabolism studies on azocyclotin were performed in a
laboratory ecosystem with bush (i.e. kidney) beans, enclosed in a
quartz bell jar giving 96% light transmission (Wolff et al., 1977a).
The azocyclotin preparation which was used was a well-defined mixture
of 1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyclohexyl-stannyl113-1,2,4-triazole. Forty-four days after the
treatment, the amounts of 14C and 113Sn radioactivity distributed
among the different parts of the system was measured, and 95% of the
applied radioactivity was recovered.
In the exhaust air absorber, less than 0.01% of the applied 113Sn
radioactivity was found as against 41.7% of the originally applied
14C radioactivity. Of the remaining 58.3% of the 14C radioactivity,
43.3% was present on/in the soil, and 9.8% in the plants. Of the
originally applied 113Sn radioactivity, 81.7% was found on or in the
soil and 14.5% on or in the plants. Autoradiographs showed spray
spots caused by 14C radioactivity on the treated leaves, as against
uniform blackening on newly grown plant parts. From these findings
and from the comparison of 14C radioactivity and 113Sn radioactivity
in different plant parts (Table 5), it is evident that a major
proportion of the applied azocyclotin was no longer present as the
unchanged parent compound. There was only little translocation of the
radioactivities in the plants. However, a large proportion of the
14C radioactivity volatilized and was trapped in the absorber.
Thin-layer chromatographic separation of the plant extracts revealed
that the individual azocyclotin metabolites were present in various
plant parts in the following proportions: metabolite III: 47 - 71%,
metabolite IV: 7 - 33%, metabolite V: 5-22% and inorganic Sn: 6-19%
(cfr. Table 6). The structures of these compounds were verified by
comparison of their Rf values from TLC with those of reference
substances, and by the ratios of 113Sn:14C (cfr. Table 6).
In processing
It will be noted from the data presented in Table 7 that when wine
grapes were pressed, about 25% of the residue was carried into the
must. However, the wine made from the must did not contain any
measurable residue amounts of azocyclotin.
In soil
Leaching Studies
Whereas azocyclotin is very sparingly soluble in water, 1,2,4-triazole
(II) displays good solubility. This metabolite, however, is absorbed
from water on soil particles according to soil leaching studies
performed by Möllhoff (1977b). From these studies performed on three
different soils, it was found that neither azocyclotin, nor its
metabolites II, III and IV were detectable in percolated water under
the conditions of the experiments. The limits of determination both
for azocyclotin and for II, III, and IV were at 2% of the applied
amount of parent compound.
Also in the ecosystem planted with bush beans (Wolff et al., 1977a),
vertical movement in the soil was minimal. After 44 days, about 98%
of the applied radioactivity (113Sn + 14C-azocyclotin) was retained
in top soil between 0 and 3 cm, while soil layers below 15 cm showed
no activity at all.
Table 5. Distribution of 113Sn and 14C radioactivities among
different plant parts, soil and exhaust air from laboratory simulation
study after 44 days. (Wolf et al., 1977a).
113Sn (in %) 14C (in %)
Bush (i.e. kidney) beans
Old beans 0.03 0.30
Young beans 0.01 0.03
Leaves + stem 7.92 5.11
Fallen leaves 6.56 4.44
Roots 0.10 0.03
Total in plants 14.62 9.91
Soil 81.7 43.3
Exhaust air <0.01 41.7
Recovered 96.3% 94.9%
Table 6. Metabolite proportions after azocyclotin treatment and ratios of 113Sn:14C-radioactivity in
bean plant extracts (Wolf et al, 1977a)
Origin on
Plant part III: Tricyclohexyl Sn IV: Dicyclohexyl Sn V: Monocyclohexyl Sn TLC-plate*
Old beans 71.7% 17. 3% 4.9% 6.0%
Young beans 47.4% 33.6% 9.5% 9.5%
Leaves + stem 69.5% 7.3% 13.0% 9.6%
Fallen leaves 61.6% 8.4% 15.9% 14.6%
Roots 47.6% 10.9% 22.1% 19.14%
Ratios 113Sn: 14C 113Sn:14C 113Sn:14C 113Sn:14C
Theoretical 1 : 0.92 1 : 0.62 1 : 0.31 1 : 0
Found 1 : 0.91-1.06 1: 0.58-0.84 1: 0.27-0.38 1. 0.03-0.16
* Tin compounds without cyclohexyl rings.
Table 7. Azocyclotin residues during wine processing
(Bayer AG)
Experiment 1 Experiment 2
Azocyclotin (mg/kg)
Grapes 1.05 - 1.2 <0.05 - 0.1
Must 0.25 - 0.9 <0.05 - <0.05
Wine n.d. n.d.
Degradation in soil
Field experiments on three different soils showed that the triazole
component of azocyclotin was eliminated to levels below the limit of
determination (0.1 mg/kg) within 14 days. The organotin residues
measured as the sum of I, III and IV, on the other hand, were degraded
with a half-life of 50 to 90 days. Also following two treatments at a
one-year interval, there was found no extra build-up of soil residues.
(cfr. Table 8).
In studies under laboratory conditions, degradation of azocyclotin in
two different soils indicated half-lives in the order of 250 days,
while in another experiment under the same conditions, half-lives were
found to be 200 days for I and metabolite III, and about one year for
the sum of I, III, IV and V (Wolff et al., 1977b).
Metabolism in soil
In field experiments, azocyclotin (I) itself was measurable only for
one week (Table 3). During the first week, also, up to about 25%
dicyclohexyl tin oxide (IV) formed (Möllhoff, 1977b). Its content
declined thereafter parallel to the disappearance of its immediate
precursor, tricyclohexyl tin hydroxide (III). In laboratory
metabolism studies conducted with azocyclotin dicyclohexyl tin oxide
was formed gradually during 30-60 days and remained thereafter on a
plateau level corresponding to about 15% of the applied azocyclotin
dose.
In another laboratory experiment using a larger amount of soil (500 g)
held in a closed bottle, measurements made nine months after
application of 50 mg azocyclotin/kg showed that 45% of the applied
amount of tin was still present as organotin compounds in the soil.
At this point of time, the analytically determined components triazole
(II) and tricyclohexyl butyl tin (i.e. metabolite III after
butylation) were present in almost stoichiometric proportions of 23.8%
and 25.4%, respectively. Whether azocyclotin or a mixture of triazole
and tricyclohexyl tin hydroxide was present, or whether there was a
balance among I, II and III could not be decided by analysis. From
the fast decline of the triazole proportion in field conditions,
however, it was concluded that triazole under open conditions would
volatilize rapidly (Möllhoff, 1977b).
In the above-mentioned larger-scale laboratory experiment, it was also
found after nine months of storage that of the originally applied
azocyclotin dose, 1O.8% was present as dicyclohexyl tin oxide (IV) and
8.25% as cyclohexyl stannoic acid (V). After derivatization with
butyl lithium these two compounds were measured quantitatively by gas
chromatography, and after separation on capillary columns, they were
identified by mass spectrometry. The same two metabolites (IV and V),
were found in soil nine months after application of the metabolite
dicyclohexyl tin oxide (IV) in amounts corresponding to 36.5% and
11.7% respectively. In the soil metabolism studies of Wolf et al.
(1977b), using a mixture of 14C- and 113Sn-labelled azocyclotin, it
was found that the proportion of 113Sn radioactivity which was
non-extractable never exceeded 6.6% while, on the other hand, the
proportion of non-extractable 14C radioactivity increased towards the
end of the 200-day experiment to 24.5% (cfr Table 9). This is
interpreted as a sign of metabolic incorporation of 14C-carbon into
non-extractable compounds, the identity of which, however, remained
unknown. The proportion of the applied tin to which three cyclohexyl
rings were still bound, declined to a level of 50% during this
experiment.
Fate in water
Degradation of azocyclotin in aquarium water was studied during 150
days in closed bottles at 22 ± 2°C, with azocyclotin added at a level
of 10 mg/kg (Möllhoff, 1977c). The 25% w.p. formulated azocyclotin
was used for this experiment, but despite frequent shaking, the
formulation could not be held continuously in suspension.
At the end of the experimental period, one-third of the applied amount
of azocyclotin still contained all three cyclohexyl rings at the tin
atom (Table 9), while the proportion of triazole still bound to the
tin atom could not be determined analytically. However, formation of
free triazole was observed at a level which on termination of the
experiment was estimated to be 0.41 mg/kg, equivalent to 2.6 mg/kg
when calculated as azocyclotin. In an open system, such free triazole
is expected to disappear by volatilization.
Metabolite IV, dicyclohexyl tin oxide, was determined throughout this
experiment, although in small amounts only, i.e. no more than 0.26
mg/kg.
Table 8. Azocyclotin residues in soil by triazole and organotin
methods (Wolf, et al., 1977b)
Application Days after Azocyclotin (mg/kg)
treatment Triazole Organotin
Method Method
First year
1 × 5 kg/ha 0 2.3 - 3.1 2.3 - 3.1
7 0.48 - 0.65 1.9 - 2.5
14 n.d. - n.d. 1.35 - 1.7
60 - 65 0.3 - 1.25
90 - 92 0.3 - 0.7
120 - 124 0.15 - 0.3
150 - 155 n.d. - 0.2
Second year
1 × 5 kg/ha 0 2.9 3.1
7 0.48 1.9
14 n.d. 1.35
59 0.85
92 0.65
120 0.35
150 0.3
0.3
Table 9. Degradation of Azocyclotin (25% w.p.) in aquarium water
(Möllhoff,1977c)
Storage Analysis by Analysis by
time triazole meth. organotin method
in days
Azocyclotin Tricyclohexyl Dicyclohexyl
+ triazole * tin* tin *
0 8.95 0.26
7 9.0 0.25
28 7.6 7.6 0.33
49 7.9 5.9 0.19
71 5.3 3.2 0.36
91 5.3 2.7 0.29
120 5.3 2.75 0.14
150 5.6 3.0 0.14
* All results calculated as azocyclotin (mg/kg).
METHOD OF RESIDUE ANALYSIS
Residues of azocyclotin can be measured by two different methods
developed by Möllhoff (1977a).
Triazole method (parent compound + metabolite II)
Plant, soil and water samples are extracted with isopropanol followed
by chloroform, and cleanup of extract by precipation. The final
determination is a GLC procedure using thermionic N-detector (AFID)
giving response to the triazole, and with a limit of determination of
0.1 - 0.2 mg/kg, expressed as azocyclotin. Azocyclotin (I) and
1,2,4-triazole (II) cannot be distinguished by this method.
This method determines that portion of the molecule which is split off
first from the tin atom, i.e. the 1,2,4-triazole moiety.
Accordingly, residues of azocyclotin measured by the triazole method
usually drop relatively fast below limit of determination, while
remaining organotin compounds can only be determined by method B.
Organotin method (parent compound + metabolites III + IV)
Plant, soil, water and laboratory animal feed samples are macerated
with water and treated with hydrobromic acid in acetone, and
thereafter extracted with hexane. The hexane extracted residue in
ethyl ether is reacted with methyl magnesium chloride and hydrolysed.
The methylated compounds, tricyclohexyl methyl tin and dicyclohexyl
dimethyl tin, which are formed from azocyclotin and metabolites III
and IV are cleaned up on Florisil and gas chromatographed using a
flame photometric detector (FPD) with a 394 nm filter. The limits of
determination are 0.05 - 0.1 mg/kg.
Azocyclotin (I) and cyhexatin (III) cannot be distinguished by this
method, but the method permits determination of the sum of these two
compounds in parallel with determination of metabolite IV
(dicyclohexyl tin oxide).
The proportion of IV in the total residue measured by the organotin
method is usually less than 10% in plants, and between 10 and 20% in
soil, whilst metabolite V (cyclohexyl stannoic acid) is of little
significance as residue. This latter metabolite V could be determined
by the organotin method if chloroform is used instead of hexane for
the extraction. The organotin method has multiresidue method
characteristics as it permits the simultaneous determination of
several chemical entities, in this case partially alkylated or
arylated tin compounds (cfr. Steinmeyer et al., 1965, Heubert &
Wirth, 1975, Figge et al., 1977 and Wright et al., 1979) by
gaschromatographic separations after reaction with methyl magnesium
chloride or with butyl lithium. The latter alkylation agent deserves
special attention because the alkylation seems to proceed very
smoothly producing compounds with molecular weight of the same range.
A more convenient isothermal GLC technique is thereby facilitated.
NATIONAL MRLs REPORTED TO THE MEETING
National Limits and associated pre-harvest intervals which have
been reported to the meeting are shown in Table 10.
Table 10. National MRLs and Safety Intervals
Safety
Country Crop MRL Interval
in mg/kg in days
Chile fruits 15
Cyprus pome and stone fruits,
citrus 21
Germany bush and runner beans 14
F.R.G. pome fruit 14
grapes (except
table grapes) 35
hops 50.0
pome fruits 2.0
wine grapes 2.0
Morocco pome fruit 28
New pipfruit 14
Zealand stone fruit 14
South apples 0.5 3
Africa pears 0.5 3
peaches 0.5 21
APPRAISAL
Azocyclotin is an organotin acaricide used against spider mites on
fruits and vegetables, especially recommended for the control of
strains which are resistant to other chemicals e.g.
organophosphorous compounds. It is formulated in wettable powders (25
and 50%) and marketed in most continents.
In plants, animals, soil and water, azocyclotin (I) is degraded by an
initial off-splitting of 1,2,4-triazole (II) from tricyclohexyl tin
hydroxide (III) identical with cyhexatin, followed by stepwise
formation of cicyclohexyl tin oxide (IV) and monocyclohexyl stannoic
acid (V) through the loss of the three cyclohexyl rings from the
central Sn-atom.
The first degradation step by splitting off of 1,2,4-triazole is a
relatively fast process, and the 1,2,4-triazole moiety disappears to
levels at or below the limit of determination shortly after treatment
of plants or after application to soil or water. The triazole
probably disappears from plants by volatilization.
The remaining organotin compounds, cyhexatin and its further
metabolites, are more persistent, and dissipation of the total
organotin residues is mainly governed by a process of growth dilution
on most plants and vegetables after application. But compounds III,
IV and V are found as simultaneous residues in variable ratios, and
with a limited amount of inorganic Sn also present. In a single study
with soil, inorganic Sn was found at the level of 6-19% of total
residues.
Half-lives of organotin residues in soil varied from 50-90 days in
field studies and from 200 days to one year under laboratory
conditions. Vertical movement or leaching in soils are found to be
minimal or unlikely.
Residues of azocyclotin (as organotin) in apples, beans, eggplants and
strawberry are at or below limit of determination after application at
recommended use levels and appropriate waiting periods, while
significant residues were found in most cases on grapes. After
processing, up to 25% of residues in grapes was carried over to must,
but without any measurable transfer of residues to wine.
Two gas chromatographic methods are available for the determination of
azocyclotin. The one is based on determination of the 1,2,4-triazole
moiety and it will not distinguish azocyclotin (I) from the triazole
(II). Residues by this method usually drop relatively rapidly to
below the limit of determination, after which residue pattern cannot
be distinguished from that which follows treatment with cyhexatin.
The other method is an organotin method developed in recent years,
which permits determination of organotin metabolites III, IV and V
individually, while it will not distinguish azocyclotin (I) from
metabolite III. Metabolite V is of little significance as residue.
It is determined by the same method as I + III and IV, but only after
separate extraction procedure.
RECOMMENDATIONS
The following guideline limits for azocyclotin are recommended on the
basis of data resulting from supervised trials. They refer to the sum
of organotin compounds expressed as azocyclotin.
Limit Pre-harvest interval on which
Commodity (mg/kg) recommendation is based
Apples 0.1* 21 days
Egg plants 0.1* 30 days
Grapes 35 days
Kidney Beans 0.2 21 days
Strawberry 0.1* 14 days
The Meeting realized that recommendations in certain cases are
incompatible with earlier recommendations on cyhexatin from which
azocyclotin residues will be undistinguishable shortly after
application. This may be due to somewhat differing use patterns for
the two compounds, but partly also to the fact that a majority of
earlier data derives from use of another analytical method, i.e.
colorimetric, total Sn-determination. Therefore, it is suggested that
special efforts should be made at the international level to collect
and review information on developed use patterns and residue data
derived from new methodology for cyhexatin.
Further work or information Required
By 1980
As residues from azocyclotin and cyhexatin cannot be distinguished
shortly after application, information on use patterns and on residue
data for both compounds, especially for cyhexatin, should be reviewed
before residue limits for the two pesticides can be harmonized.
REFERENCES
Bayer, A.G. Various reports on azocyclotin residues in fruit,
vegetables and soil (1978), Unpublished.
Figge, K., Koch, J., Lubba, H. - Beitrag zur gaschromatographischen
Analyse von Organozinn-Stabilisatoren für Plyvinylchlorid. J.
Chromatogr. 131, 317-327.
Möllhoff, E. Methode zur gaschromatographischen Bestimmung des
Akarizids Peropal und seiner Metaboliten in Pflanzen, Böden, Wasser
und Kleintierfutter. Pflanzenschutz-Nachr. Bayer 30, 249-263 (1977a).
Abbau und Metabolismus von Peropal im Boden. Bayer AG, Report
RA-11/77 (English version) (1977b).
Abbau von Peropal in Aquarienwasser (geschlossenes System). Bayer
AG; Report RA-815/77 (1977c).
Neubert, G., Wirth, H.O. - Zur Analytik con Organozinnstabilistatoren.
Z. Anal. Chem. 273, 19-23.
Steinmeyer, R.D., Fentiman, A.F., Kahler, E.J., - Analysis of alkyltin
bromides by gas liquid chromatography. Analytical Chem. 37, 520-523.
Wolf, W., Lippert, K.D., Figge, K. - Übber das Verhalten des Akarizids
"Tricyclohexylzinn-1,2,4-triazolid" und seiner Abbauprodukte im
Okosystem "Buschbohnenkulture". Natec, Gesellschaft für
naturwissenschaftlinch-technische Dienste mbH., Projekt NA 76 00 43,
(1977a).
Uber das Abbauverhalten des Akarizids "Tricyclohexylzinn-1,2,4-
triazolid"in Standardboden. Natec, Gesellschaft für naturwissen-,
schaftlich-technische Dienste mbH., Project NA 76 00 43, (1977b).
Wright, B.W., Lee, M.L., Booth, G.M. - Determination of
triphenyltinhydroxide derivatives by capillary GC and Tin-selective
FPD. Journ. High Resolution Chrom. and Chromatography Comm. S. 189.
 Other Information on Identity and Properties
Molecular Weight: 436.2
Appearance: White Powder
Melting Point: 218.8°C
Vapor Pressure: Less than 5 × 10-5 mbar at 25°C
Solubility: in water <0.25 mg/kg
in cyclohexane )
in isopropanol )
in toluene ) 0-0.01 g
in methylene chloride ) per g solvent
in ligroin )
Minimum degree of purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical azocyclotin was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Azocyclotin is an organotin acaricide effective against spider miters.
It is recommended for the control of strains which are resistant to
other chemical compounds. Azocyclotin gives good control of all
motile stages, i.e. larvae as well as adults. Through trials on mixed
populations, it has been established that azocyclotin has an
ovolarvicidal effect on summer but not on winter eggs.
Azocyclotin is a contact poison, and it is notable for its long
residual activity. It is used on pome and stone fruit, strawberries,
vegetables and grapes. When used at the recommended concentrations,
it displays no phytotoxicity. Azocyclotin is marketed as a 25% and
50% wettable powder, in Argentina, Australia, Austria, Chile, Federal
Republic of Germany, Greece, Iran, Lebanon, Morocco, New Zealand,
Peru, South Africa, Spain and Uruguay. It is used as a foliar spray
on the crops as listed in Table 1. There are no post-harvest uses.
Table 1. Current uses of Azocyclotin
Concentration Number of Pre-harvest
Crop % act. ing. applications interval
Pome and stone 0.025 1-3 14 days
fruit (0.3-0.5 kg/ha)
Strawberry 0.025 1-3 14 days
(0.5 kg/ha)
Vegetables:
Bush beans 0.025 1-2 14 days
(max.3)
Eggplants 0.025 1-2 14 days
(0.375-0.75
kg/ha)
Grapes 0.025 1-2 35 days
(0.5 kg/ha) (max.3-4)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue studies after applications of azocyclotin (I) have been
performed on apple, bush beans, eggplant, damson plums, strawberry and
wine grape (Table 2). The residue data were obtained by the use of an
organotin method (Möllhoff, 1977a) which determines the sum of the
parent compound, azocyclotin (I), and its two metabolites,
tricyclohexyl tin hydroxide (or cyhexatin) (III), and dicyclohexyl tin
oxide (IV) (cfr. Figure 1). Compound IV always represented less than
10% of the total residue.
Residue data obtained by the use of a triazole method (Möllhoff,
1977a) is presented in Table 3, from which it is noted that the
residues of the triazole moiety were analytically non-significant or
below detection limit (i.e. 0.1 - 0.2 mg%kg) shortly after
application. If not otherwise stated, therefore, the following
residue information is obtained by the organotin method.
Apple
After one, two or three applications of 0.3-0.65 kg azocyclotin per
ha, the maximum residue on day 0 was 0.35 mg/kg. Within the course of
three weeks, the residue levels usually dropped down to or below the
limit of determination (0.05 mg/kg).
Bean, kidney
Following three applications of 0.225 kg azocyclotin per ha, the
residue levels declined within three weeks from 0.2-0.3 mg/kg to
n.d.-0.15 mg/kg.
Eggplant
Following one or two applications of azocyclotin at 0.375-0.7 kg/ha,
the initially measured residue level was 0.1-0.45 mg/kg. It decreased
to a level below the limit of determination during the following 30
days, or in several cases during only 14 days.
Damson plums
The maximum residue level measured after three applications of
azocyclotin at 0.5 kg/ha was 0.8 mg/kg, while the residue levels
measured 3 and 4 weeks after the final spray were 0.25, resp. 0.2
mg/kg.
Strawberry
Following two spray applications of azocyclotin at 0.5 kg/ha, the
immediate residue levels were 0.6 mg/kg. No more residues were
detectable 14 days later.
Wine Grape
Following three or four spray applications of azocyclotin at 0.45-0.5
kg/ha, the initial residue level in a series of experiments varied
from 0.85 up to 2.1 mg/kg. Measurements made four weeks after the
final spray showed that the residue level was less than 1 mg/kg
following three applications, but higher following four applications.
FATE OF RESIDUES
A general degradation pathway for azocyclotin is shown in Figure 1,
indicating a gradual and step-wise off-splitting of the rings bound to
the central tin atom, starting with the 1,2,4-triazole moiety. Some
properties of the individual metabolites are given in Table 4.
Table 2. Residues of Azocyclotin from Supervised Trials (Bayer AG)*
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Apple 0.3 kg/ha 1× 7 0.1 - 0.15 France
13-14 <0.05 - 0.05
21 n.d. - <O.05
0.3125 1/2× 0 0.15 - 0.30 Israel
kg/ha 7 0.05 - 0.15
14 <0.05 - 0.05
30 n.d. - n.d.
0.3 - 0.375 2× 14 0.06 - 0.08 Netherlands
kg/ha 21 n.d. - 0.1
28 n.d. - n.d.
0.5 kg/ha 3× 0 0.15 - 0.25 Germany (FRG)
7 0.1 - 0.15
14 0.05 - 0.15
21 n.d. - 0.1
28 n.d. - 0.05
0.625 kg/ha 1/2× 0 0.2 - 0.35 Israel
7 0.15 - 0.3
14 0.05 - 0.2
30 n.d. - <0.05
Beans (Bush
or Kidney) 0.225 kg/ha 3× 0 0.2 - 0.3 Germany (FRG)
7 0.15 - 0.2
14 <0.05 - 0.15
21 n.d. - 0.15
Eggplant 0.375 kg/ha 1/2× 0 0.1 - 0.45 Israel
7 n.d. - 0.15
14 n.d. - n.d.
30 n.d. - n.d.
0.75 kg/ha 1/2× 0 0.1 - 0.45
7 n.d. - 0.2
14 n.d. - 0.1
30 n.d. - n.d.
Table 2. Continued...
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Plum, damson 0.5 kg/ha 3× 0 0.1 - 0.8 Germany (FRG)
7 0.05 - 0.5
14 <0.05 - 0.4
21 <0.05 - 0.25
28 <0.05 - 0.2
Strawberry 0.5 kg/ha 2× 0 0.55 - 0.6 Germany (FRG)
7 0.1 - 0.15
14 n.d. - n.d.
Wine grape 0.3 kg/ha 1× 7 0.3 France
14 0.25
21 0.2
0.45-0.5 3× 0 0.85 - 2.1 Germany (FRG)
kg/ha 14 0.45 - 1.75
21 0.45 - 0.8
28 0.l - 0.8
35 0.1 - 0.9
42 0.1 - 0.15
49 <0.05 - 0.15
0.5 kg/ha 4× 0 1.45 - 2.15
7 1.20 - 2.4
14 1.15 - 1.75
22 1.15 - 1.9
29 1.0 - 1.4
36 1.05 - 1.2
* Results in this table obtained by organotin method (Möllhoff, 1977a).
Table 3. Residues of azocyclotin determined by triazole and organotin methods
Residue
Application Days after Triazole Organotin Country
Crop rate treatment Method Method
Apple 1 × 0.3 kg/ha 7 n.d.- n.d. 0.1 - 0.15 FRG
14 - <0.05 - 0.05
21 - <0.05 - <0.05
1 × 1 kg/ha 0 0.4 - 0.4 - FRG
7 n.d.- <0.1 -
14 n.d. - n.d. -
1 × 0.37-0.75
kg/ha 9 2.32 - New
3 1.28 - Zealand
7 0.43 -
14 0.05 -
Grape 1 × 0.3 kg/ha 7 n.d. 0.3 FRG
14 n.d. 0.25
21 n.d. 0.2
Other Information on Identity and Properties
Molecular Weight: 436.2
Appearance: White Powder
Melting Point: 218.8°C
Vapor Pressure: Less than 5 × 10-5 mbar at 25°C
Solubility: in water <0.25 mg/kg
in cyclohexane )
in isopropanol )
in toluene ) 0-0.01 g
in methylene chloride ) per g solvent
in ligroin )
Minimum degree of purity: 90%
Impurities in the technical material
Detailed information on the impurities in technical azocyclotin was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Azocyclotin is an organotin acaricide effective against spider miters.
It is recommended for the control of strains which are resistant to
other chemical compounds. Azocyclotin gives good control of all
motile stages, i.e. larvae as well as adults. Through trials on mixed
populations, it has been established that azocyclotin has an
ovolarvicidal effect on summer but not on winter eggs.
Azocyclotin is a contact poison, and it is notable for its long
residual activity. It is used on pome and stone fruit, strawberries,
vegetables and grapes. When used at the recommended concentrations,
it displays no phytotoxicity. Azocyclotin is marketed as a 25% and
50% wettable powder, in Argentina, Australia, Austria, Chile, Federal
Republic of Germany, Greece, Iran, Lebanon, Morocco, New Zealand,
Peru, South Africa, Spain and Uruguay. It is used as a foliar spray
on the crops as listed in Table 1. There are no post-harvest uses.
Table 1. Current uses of Azocyclotin
Concentration Number of Pre-harvest
Crop % act. ing. applications interval
Pome and stone 0.025 1-3 14 days
fruit (0.3-0.5 kg/ha)
Strawberry 0.025 1-3 14 days
(0.5 kg/ha)
Vegetables:
Bush beans 0.025 1-2 14 days
(max.3)
Eggplants 0.025 1-2 14 days
(0.375-0.75
kg/ha)
Grapes 0.025 1-2 35 days
(0.5 kg/ha) (max.3-4)
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue studies after applications of azocyclotin (I) have been
performed on apple, bush beans, eggplant, damson plums, strawberry and
wine grape (Table 2). The residue data were obtained by the use of an
organotin method (Möllhoff, 1977a) which determines the sum of the
parent compound, azocyclotin (I), and its two metabolites,
tricyclohexyl tin hydroxide (or cyhexatin) (III), and dicyclohexyl tin
oxide (IV) (cfr. Figure 1). Compound IV always represented less than
10% of the total residue.
Residue data obtained by the use of a triazole method (Möllhoff,
1977a) is presented in Table 3, from which it is noted that the
residues of the triazole moiety were analytically non-significant or
below detection limit (i.e. 0.1 - 0.2 mg%kg) shortly after
application. If not otherwise stated, therefore, the following
residue information is obtained by the organotin method.
Apple
After one, two or three applications of 0.3-0.65 kg azocyclotin per
ha, the maximum residue on day 0 was 0.35 mg/kg. Within the course of
three weeks, the residue levels usually dropped down to or below the
limit of determination (0.05 mg/kg).
Bean, kidney
Following three applications of 0.225 kg azocyclotin per ha, the
residue levels declined within three weeks from 0.2-0.3 mg/kg to
n.d.-0.15 mg/kg.
Eggplant
Following one or two applications of azocyclotin at 0.375-0.7 kg/ha,
the initially measured residue level was 0.1-0.45 mg/kg. It decreased
to a level below the limit of determination during the following 30
days, or in several cases during only 14 days.
Damson plums
The maximum residue level measured after three applications of
azocyclotin at 0.5 kg/ha was 0.8 mg/kg, while the residue levels
measured 3 and 4 weeks after the final spray were 0.25, resp. 0.2
mg/kg.
Strawberry
Following two spray applications of azocyclotin at 0.5 kg/ha, the
immediate residue levels were 0.6 mg/kg. No more residues were
detectable 14 days later.
Wine Grape
Following three or four spray applications of azocyclotin at 0.45-0.5
kg/ha, the initial residue level in a series of experiments varied
from 0.85 up to 2.1 mg/kg. Measurements made four weeks after the
final spray showed that the residue level was less than 1 mg/kg
following three applications, but higher following four applications.
FATE OF RESIDUES
A general degradation pathway for azocyclotin is shown in Figure 1,
indicating a gradual and step-wise off-splitting of the rings bound to
the central tin atom, starting with the 1,2,4-triazole moiety. Some
properties of the individual metabolites are given in Table 4.
Table 2. Residues of Azocyclotin from Supervised Trials (Bayer AG)*
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Apple 0.3 kg/ha 1× 7 0.1 - 0.15 France
13-14 <0.05 - 0.05
21 n.d. - <O.05
0.3125 1/2× 0 0.15 - 0.30 Israel
kg/ha 7 0.05 - 0.15
14 <0.05 - 0.05
30 n.d. - n.d.
0.3 - 0.375 2× 14 0.06 - 0.08 Netherlands
kg/ha 21 n.d. - 0.1
28 n.d. - n.d.
0.5 kg/ha 3× 0 0.15 - 0.25 Germany (FRG)
7 0.1 - 0.15
14 0.05 - 0.15
21 n.d. - 0.1
28 n.d. - 0.05
0.625 kg/ha 1/2× 0 0.2 - 0.35 Israel
7 0.15 - 0.3
14 0.05 - 0.2
30 n.d. - <0.05
Beans (Bush
or Kidney) 0.225 kg/ha 3× 0 0.2 - 0.3 Germany (FRG)
7 0.15 - 0.2
14 <0.05 - 0.15
21 n.d. - 0.15
Eggplant 0.375 kg/ha 1/2× 0 0.1 - 0.45 Israel
7 n.d. - 0.15
14 n.d. - n.d.
30 n.d. - n.d.
0.75 kg/ha 1/2× 0 0.1 - 0.45
7 n.d. - 0.2
14 n.d. - 0.1
30 n.d. - n.d.
Table 2. Continued...
Application Days after last Residues Country
Crop Rate Frequency Treatment (mg/kg)
Plum, damson 0.5 kg/ha 3× 0 0.1 - 0.8 Germany (FRG)
7 0.05 - 0.5
14 <0.05 - 0.4
21 <0.05 - 0.25
28 <0.05 - 0.2
Strawberry 0.5 kg/ha 2× 0 0.55 - 0.6 Germany (FRG)
7 0.1 - 0.15
14 n.d. - n.d.
Wine grape 0.3 kg/ha 1× 7 0.3 France
14 0.25
21 0.2
0.45-0.5 3× 0 0.85 - 2.1 Germany (FRG)
kg/ha 14 0.45 - 1.75
21 0.45 - 0.8
28 0.l - 0.8
35 0.1 - 0.9
42 0.1 - 0.15
49 <0.05 - 0.15
0.5 kg/ha 4× 0 1.45 - 2.15
7 1.20 - 2.4
14 1.15 - 1.75
22 1.15 - 1.9
29 1.0 - 1.4
36 1.05 - 1.2
* Results in this table obtained by organotin method (Möllhoff, 1977a).
Table 3. Residues of azocyclotin determined by triazole and organotin methods
Residue
Application Days after Triazole Organotin Country
Crop rate treatment Method Method
Apple 1 × 0.3 kg/ha 7 n.d.- n.d. 0.1 - 0.15 FRG
14 - <0.05 - 0.05
21 - <0.05 - <0.05
1 × 1 kg/ha 0 0.4 - 0.4 - FRG
7 n.d.- <0.1 -
14 n.d. - n.d. -
1 × 0.37-0.75
kg/ha 9 2.32 - New
3 1.28 - Zealand
7 0.43 -
14 0.05 -
Grape 1 × 0.3 kg/ha 7 n.d. 0.3 FRG
14 n.d. 0.25
21 n.d. 0.2
 Table 4. Some properties of azocyclotin metabolites
Metabolites (Fig.1)
Azocyclotin II III IV
Melting point 218.8°C 121°C 245°C 291°C
Vapour
pressure at:
20°C) <5 x 10-5 3 X 10-4 not measured
30°C)in mbar - 1 X 10-3
40°C) - 3 X 10-3
50°C) - 1 X 10-2
Solubility in
water (mg/kg) <0.25 readily <1 insoluble
In rats
The meeting is informed that studies on biokinetics and on metabolism
in rats have been conducted with a mixture of
1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyolohexyl-stanny1113-1,2,4-triazole.
In plants
Residue analyses on apples and grapes performed by the triazole method
gave results close to or below the limit of determination (0.1 mg/kg)
one week after treatment of the plants (cfr Table 3). When the
organotin method was used, on the other hand, residues were found for
a longer period (Table 2), indicating that the triazole ring was the
first ligand to be split off from the central tin atom. By referring
to the vapor pressure/temperature gradient of triazole (II) (cfr.
Table 4), it has been suggested that triazole disappearance may be due
to volatilization (Möllhoff, 1977a). In the field studies of Möllhoff
(1977a) it is reported that metabolite IV, dicyclohexyl tin oxide,
occurs only in small amounts in plants material, i.e. less than 10%.
Mass balance and metabolism studies on azocyclotin were performed in a
laboratory ecosystem with bush (i.e. kidney) beans, enclosed in a
quartz bell jar giving 96% light transmission (Wolff et al., 1977a).
The azocyclotin preparation which was used was a well-defined mixture
of 1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyclohexyl-stannyl113-1,2,4-triazole. Forty-four days after the
treatment, the amounts of 14C and 113Sn radioactivity distributed
among the different parts of the system was measured, and 95% of the
applied radioactivity was recovered.
In the exhaust air absorber, less than 0.01% of the applied 113Sn
radioactivity was found as against 41.7% of the originally applied
14C radioactivity. Of the remaining 58.3% of the 14C radioactivity,
43.3% was present on/in the soil, and 9.8% in the plants. Of the
originally applied 113Sn radioactivity, 81.7% was found on or in the
soil and 14.5% on or in the plants. Autoradiographs showed spray
spots caused by 14C radioactivity on the treated leaves, as against
uniform blackening on newly grown plant parts. From these findings
and from the comparison of 14C radioactivity and 113Sn radioactivity
in different plant parts (Table 5), it is evident that a major
proportion of the applied azocyclotin was no longer present as the
unchanged parent compound. There was only little translocation of the
radioactivities in the plants. However, a large proportion of the
14C radioactivity volatilized and was trapped in the absorber.
Thin-layer chromatographic separation of the plant extracts revealed
that the individual azocyclotin metabolites were present in various
plant parts in the following proportions: metabolite III: 47 - 71%,
metabolite IV: 7 - 33%, metabolite V: 5-22% and inorganic Sn: 6-19%
(cfr. Table 6). The structures of these compounds were verified by
comparison of their Rf values from TLC with those of reference
substances, and by the ratios of 113Sn:14C (cfr. Table 6).
In processing
It will be noted from the data presented in Table 7 that when wine
grapes were pressed, about 25% of the residue was carried into the
must. However, the wine made from the must did not contain any
measurable residue amounts of azocyclotin.
In soil
Leaching Studies
Whereas azocyclotin is very sparingly soluble in water, 1,2,4-triazole
(II) displays good solubility. This metabolite, however, is absorbed
from water on soil particles according to soil leaching studies
performed by Möllhoff (1977b). From these studies performed on three
different soils, it was found that neither azocyclotin, nor its
metabolites II, III and IV were detectable in percolated water under
the conditions of the experiments. The limits of determination both
for azocyclotin and for II, III, and IV were at 2% of the applied
amount of parent compound.
Also in the ecosystem planted with bush beans (Wolff et al., 1977a),
vertical movement in the soil was minimal. After 44 days, about 98%
of the applied radioactivity (113Sn + 14C-azocyclotin) was retained
in top soil between 0 and 3 cm, while soil layers below 15 cm showed
no activity at all.
Table 5. Distribution of 113Sn and 14C radioactivities among
different plant parts, soil and exhaust air from laboratory simulation
study after 44 days. (Wolf et al., 1977a).
113Sn (in %) 14C (in %)
Bush (i.e. kidney) beans
Old beans 0.03 0.30
Young beans 0.01 0.03
Leaves + stem 7.92 5.11
Fallen leaves 6.56 4.44
Roots 0.10 0.03
Total in plants 14.62 9.91
Soil 81.7 43.3
Exhaust air <0.01 41.7
Recovered 96.3% 94.9%
Table 6. Metabolite proportions after azocyclotin treatment and ratios of 113Sn:14C-radioactivity in
bean plant extracts (Wolf et al, 1977a)
Origin on
Plant part III: Tricyclohexyl Sn IV: Dicyclohexyl Sn V: Monocyclohexyl Sn TLC-plate*
Old beans 71.7% 17. 3% 4.9% 6.0%
Young beans 47.4% 33.6% 9.5% 9.5%
Leaves + stem 69.5% 7.3% 13.0% 9.6%
Fallen leaves 61.6% 8.4% 15.9% 14.6%
Roots 47.6% 10.9% 22.1% 19.14%
Ratios 113Sn: 14C 113Sn:14C 113Sn:14C 113Sn:14C
Theoretical 1 : 0.92 1 : 0.62 1 : 0.31 1 : 0
Found 1 : 0.91-1.06 1: 0.58-0.84 1: 0.27-0.38 1. 0.03-0.16
* Tin compounds without cyclohexyl rings.
Table 7. Azocyclotin residues during wine processing
(Bayer AG)
Experiment 1 Experiment 2
Azocyclotin (mg/kg)
Grapes 1.05 - 1.2 <0.05 - 0.1
Must 0.25 - 0.9 <0.05 - <0.05
Wine n.d. n.d.
Degradation in soil
Field experiments on three different soils showed that the triazole
component of azocyclotin was eliminated to levels below the limit of
determination (0.1 mg/kg) within 14 days. The organotin residues
measured as the sum of I, III and IV, on the other hand, were degraded
with a half-life of 50 to 90 days. Also following two treatments at a
one-year interval, there was found no extra build-up of soil residues.
(cfr. Table 8).
In studies under laboratory conditions, degradation of azocyclotin in
two different soils indicated half-lives in the order of 250 days,
while in another experiment under the same conditions, half-lives were
found to be 200 days for I and metabolite III, and about one year for
the sum of I, III, IV and V (Wolff et al., 1977b).
Metabolism in soil
In field experiments, azocyclotin (I) itself was measurable only for
one week (Table 3). During the first week, also, up to about 25%
dicyclohexyl tin oxide (IV) formed (Möllhoff, 1977b). Its content
declined thereafter parallel to the disappearance of its immediate
precursor, tricyclohexyl tin hydroxide (III). In laboratory
metabolism studies conducted with azocyclotin dicyclohexyl tin oxide
was formed gradually during 30-60 days and remained thereafter on a
plateau level corresponding to about 15% of the applied azocyclotin
dose.
In another laboratory experiment using a larger amount of soil (500 g)
held in a closed bottle, measurements made nine months after
application of 50 mg azocyclotin/kg showed that 45% of the applied
amount of tin was still present as organotin compounds in the soil.
At this point of time, the analytically determined components triazole
(II) and tricyclohexyl butyl tin (i.e. metabolite III after
butylation) were present in almost stoichiometric proportions of 23.8%
and 25.4%, respectively. Whether azocyclotin or a mixture of triazole
and tricyclohexyl tin hydroxide was present, or whether there was a
balance among I, II and III could not be decided by analysis. From
the fast decline of the triazole proportion in field conditions,
however, it was concluded that triazole under open conditions would
volatilize rapidly (Möllhoff, 1977b).
In the above-mentioned larger-scale laboratory experiment, it was also
found after nine months of storage that of the originally applied
azocyclotin dose, 1O.8% was present as dicyclohexyl tin oxide (IV) and
8.25% as cyclohexyl stannoic acid (V). After derivatization with
butyl lithium these two compounds were measured quantitatively by gas
chromatography, and after separation on capillary columns, they were
identified by mass spectrometry. The same two metabolites (IV and V),
were found in soil nine months after application of the metabolite
dicyclohexyl tin oxide (IV) in amounts corresponding to 36.5% and
11.7% respectively. In the soil metabolism studies of Wolf et al.
(1977b), using a mixture of 14C- and 113Sn-labelled azocyclotin, it
was found that the proportion of 113Sn radioactivity which was
non-extractable never exceeded 6.6% while, on the other hand, the
proportion of non-extractable 14C radioactivity increased towards the
end of the 200-day experiment to 24.5% (cfr Table 9). This is
interpreted as a sign of metabolic incorporation of 14C-carbon into
non-extractable compounds, the identity of which, however, remained
unknown. The proportion of the applied tin to which three cyclohexyl
rings were still bound, declined to a level of 50% during this
experiment.
Fate in water
Degradation of azocyclotin in aquarium water was studied during 150
days in closed bottles at 22 ± 2°C, with azocyclotin added at a level
of 10 mg/kg (Möllhoff, 1977c). The 25% w.p. formulated azocyclotin
was used for this experiment, but despite frequent shaking, the
formulation could not be held continuously in suspension.
At the end of the experimental period, one-third of the applied amount
of azocyclotin still contained all three cyclohexyl rings at the tin
atom (Table 9), while the proportion of triazole still bound to the
tin atom could not be determined analytically. However, formation of
free triazole was observed at a level which on termination of the
experiment was estimated to be 0.41 mg/kg, equivalent to 2.6 mg/kg
when calculated as azocyclotin. In an open system, such free triazole
is expected to disappear by volatilization.
Metabolite IV, dicyclohexyl tin oxide, was determined throughout this
experiment, although in small amounts only, i.e. no more than 0.26
mg/kg.
Table 8. Azocyclotin residues in soil by triazole and organotin
methods (Wolf, et al., 1977b)
Application Days after Azocyclotin (mg/kg)
treatment Triazole Organotin
Method Method
First year
1 × 5 kg/ha 0 2.3 - 3.1 2.3 - 3.1
7 0.48 - 0.65 1.9 - 2.5
14 n.d. - n.d. 1.35 - 1.7
60 - 65 0.3 - 1.25
90 - 92 0.3 - 0.7
120 - 124 0.15 - 0.3
150 - 155 n.d. - 0.2
Second year
1 × 5 kg/ha 0 2.9 3.1
7 0.48 1.9
14 n.d. 1.35
59 0.85
92 0.65
120 0.35
150 0.3
0.3
Table 9. Degradation of Azocyclotin (25% w.p.) in aquarium water
(Möllhoff,1977c)
Storage Analysis by Analysis by
time triazole meth. organotin method
in days
Azocyclotin Tricyclohexyl Dicyclohexyl
+ triazole * tin* tin *
0 8.95 0.26
7 9.0 0.25
28 7.6 7.6 0.33
49 7.9 5.9 0.19
71 5.3 3.2 0.36
91 5.3 2.7 0.29
120 5.3 2.75 0.14
150 5.6 3.0 0.14
* All results calculated as azocyclotin (mg/kg).
METHOD OF RESIDUE ANALYSIS
Residues of azocyclotin can be measured by two different methods
developed by Möllhoff (1977a).
Triazole method (parent compound + metabolite II)
Plant, soil and water samples are extracted with isopropanol followed
by chloroform, and cleanup of extract by precipation. The final
determination is a GLC procedure using thermionic N-detector (AFID)
giving response to the triazole, and with a limit of determination of
0.1 - 0.2 mg/kg, expressed as azocyclotin. Azocyclotin (I) and
1,2,4-triazole (II) cannot be distinguished by this method.
This method determines that portion of the molecule which is split off
first from the tin atom, i.e. the 1,2,4-triazole moiety.
Accordingly, residues of azocyclotin measured by the triazole method
usually drop relatively fast below limit of determination, while
remaining organotin compounds can only be determined by method B.
Organotin method (parent compound + metabolites III + IV)
Plant, soil, water and laboratory animal feed samples are macerated
with water and treated with hydrobromic acid in acetone, and
thereafter extracted with hexane. The hexane extracted residue in
ethyl ether is reacted with methyl magnesium chloride and hydrolysed.
The methylated compounds, tricyclohexyl methyl tin and dicyclohexyl
dimethyl tin, which are formed from azocyclotin and metabolites III
and IV are cleaned up on Florisil and gas chromatographed using a
flame photometric detector (FPD) with a 394 nm filter. The limits of
determination are 0.05 - 0.1 mg/kg.
Azocyclotin (I) and cyhexatin (III) cannot be distinguished by this
method, but the method permits determination of the sum of these two
compounds in parallel with determination of metabolite IV
(dicyclohexyl tin oxide).
The proportion of IV in the total residue measured by the organotin
method is usually less than 10% in plants, and between 10 and 20% in
soil, whilst metabolite V (cyclohexyl stannoic acid) is of little
significance as residue. This latter metabolite V could be determined
by the organotin method if chloroform is used instead of hexane for
the extraction. The organotin method has multiresidue method
characteristics as it permits the simultaneous determination of
several chemical entities, in this case partially alkylated or
arylated tin compounds (cfr. Steinmeyer et al., 1965, Heubert &
Wirth, 1975, Figge et al., 1977 and Wright et al., 1979) by
gaschromatographic separations after reaction with methyl magnesium
chloride or with butyl lithium. The latter alkylation agent deserves
special attention because the alkylation seems to proceed very
smoothly producing compounds with molecular weight of the same range.
A more convenient isothermal GLC technique is thereby facilitated.
NATIONAL MRLs REPORTED TO THE MEETING
National Limits and associated pre-harvest intervals which have
been reported to the meeting are shown in Table 10.
Table 10. National MRLs and Safety Intervals
Safety
Country Crop MRL Interval
in mg/kg in days
Chile fruits 15
Cyprus pome and stone fruits,
citrus 21
Germany bush and runner beans 14
F.R.G. pome fruit 14
grapes (except
table grapes) 35
hops 50.0
pome fruits 2.0
wine grapes 2.0
Morocco pome fruit 28
New pipfruit 14
Zealand stone fruit 14
South apples 0.5 3
Africa pears 0.5 3
peaches 0.5 21
APPRAISAL
Azocyclotin is an organotin acaricide used against spider mites on
fruits and vegetables, especially recommended for the control of
strains which are resistant to other chemicals e.g.
organophosphorous compounds. It is formulated in wettable powders (25
and 50%) and marketed in most continents.
In plants, animals, soil and water, azocyclotin (I) is degraded by an
initial off-splitting of 1,2,4-triazole (II) from tricyclohexyl tin
hydroxide (III) identical with cyhexatin, followed by stepwise
formation of cicyclohexyl tin oxide (IV) and monocyclohexyl stannoic
acid (V) through the loss of the three cyclohexyl rings from the
central Sn-atom.
The first degradation step by splitting off of 1,2,4-triazole is a
relatively fast process, and the 1,2,4-triazole moiety disappears to
levels at or below the limit of determination shortly after treatment
of plants or after application to soil or water. The triazole
probably disappears from plants by volatilization.
The remaining organotin compounds, cyhexatin and its further
metabolites, are more persistent, and dissipation of the total
organotin residues is mainly governed by a process of growth dilution
on most plants and vegetables after application. But compounds III,
IV and V are found as simultaneous residues in variable ratios, and
with a limited amount of inorganic Sn also present. In a single study
with soil, inorganic Sn was found at the level of 6-19% of total
residues.
Half-lives of organotin residues in soil varied from 50-90 days in
field studies and from 200 days to one year under laboratory
conditions. Vertical movement or leaching in soils are found to be
minimal or unlikely.
Residues of azocyclotin (as organotin) in apples, beans, eggplants and
strawberry are at or below limit of determination after application at
recommended use levels and appropriate waiting periods, while
significant residues were found in most cases on grapes. After
processing, up to 25% of residues in grapes was carried over to must,
but without any measurable transfer of residues to wine.
Two gas chromatographic methods are available for the determination of
azocyclotin. The one is based on determination of the 1,2,4-triazole
moiety and it will not distinguish azocyclotin (I) from the triazole
(II). Residues by this method usually drop relatively rapidly to
below the limit of determination, after which residue pattern cannot
be distinguished from that which follows treatment with cyhexatin.
The other method is an organotin method developed in recent years,
which permits determination of organotin metabolites III, IV and V
individually, while it will not distinguish azocyclotin (I) from
metabolite III. Metabolite V is of little significance as residue.
It is determined by the same method as I + III and IV, but only after
separate extraction procedure.
RECOMMENDATIONS
The following guideline limits for azocyclotin are recommended on the
basis of data resulting from supervised trials. They refer to the sum
of organotin compounds expressed as azocyclotin.
Limit Pre-harvest interval on which
Commodity (mg/kg) recommendation is based
Apples 0.1* 21 days
Egg plants 0.1* 30 days
Grapes 35 days
Kidney Beans 0.2 21 days
Strawberry 0.1* 14 days
The Meeting realized that recommendations in certain cases are
incompatible with earlier recommendations on cyhexatin from which
azocyclotin residues will be undistinguishable shortly after
application. This may be due to somewhat differing use patterns for
the two compounds, but partly also to the fact that a majority of
earlier data derives from use of another analytical method, i.e.
colorimetric, total Sn-determination. Therefore, it is suggested that
special efforts should be made at the international level to collect
and review information on developed use patterns and residue data
derived from new methodology for cyhexatin.
Further work or information Required
By 1980
As residues from azocyclotin and cyhexatin cannot be distinguished
shortly after application, information on use patterns and on residue
data for both compounds, especially for cyhexatin, should be reviewed
before residue limits for the two pesticides can be harmonized.
REFERENCES
Bayer, A.G. Various reports on azocyclotin residues in fruit,
vegetables and soil (1978), Unpublished.
Figge, K., Koch, J., Lubba, H. - Beitrag zur gaschromatographischen
Analyse von Organozinn-Stabilisatoren für Plyvinylchlorid. J.
Chromatogr. 131, 317-327.
Möllhoff, E. Methode zur gaschromatographischen Bestimmung des
Akarizids Peropal und seiner Metaboliten in Pflanzen, Böden, Wasser
und Kleintierfutter. Pflanzenschutz-Nachr. Bayer 30, 249-263 (1977a).
Abbau und Metabolismus von Peropal im Boden. Bayer AG, Report
RA-11/77 (English version) (1977b).
Abbau von Peropal in Aquarienwasser (geschlossenes System). Bayer
AG; Report RA-815/77 (1977c).
Neubert, G., Wirth, H.O. - Zur Analytik con Organozinnstabilistatoren.
Z. Anal. Chem. 273, 19-23.
Steinmeyer, R.D., Fentiman, A.F., Kahler, E.J., - Analysis of alkyltin
bromides by gas liquid chromatography. Analytical Chem. 37, 520-523.
Wolf, W., Lippert, K.D., Figge, K. - Übber das Verhalten des Akarizids
"Tricyclohexylzinn-1,2,4-triazolid" und seiner Abbauprodukte im
Okosystem "Buschbohnenkulture". Natec, Gesellschaft für
naturwissenschaftlinch-technische Dienste mbH., Projekt NA 76 00 43,
(1977a).
Uber das Abbauverhalten des Akarizids "Tricyclohexylzinn-1,2,4-
triazolid"in Standardboden. Natec, Gesellschaft für naturwissen-,
schaftlich-technische Dienste mbH., Project NA 76 00 43, (1977b).
Wright, B.W., Lee, M.L., Booth, G.M. - Determination of
triphenyltinhydroxide derivatives by capillary GC and Tin-selective
FPD. Journ. High Resolution Chrom. and Chromatography Comm. S. 189.
Table 4. Some properties of azocyclotin metabolites
Metabolites (Fig.1)
Azocyclotin II III IV
Melting point 218.8°C 121°C 245°C 291°C
Vapour
pressure at:
20°C) <5 x 10-5 3 X 10-4 not measured
30°C)in mbar - 1 X 10-3
40°C) - 3 X 10-3
50°C) - 1 X 10-2
Solubility in
water (mg/kg) <0.25 readily <1 insoluble
In rats
The meeting is informed that studies on biokinetics and on metabolism
in rats have been conducted with a mixture of
1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyolohexyl-stanny1113-1,2,4-triazole.
In plants
Residue analyses on apples and grapes performed by the triazole method
gave results close to or below the limit of determination (0.1 mg/kg)
one week after treatment of the plants (cfr Table 3). When the
organotin method was used, on the other hand, residues were found for
a longer period (Table 2), indicating that the triazole ring was the
first ligand to be split off from the central tin atom. By referring
to the vapor pressure/temperature gradient of triazole (II) (cfr.
Table 4), it has been suggested that triazole disappearance may be due
to volatilization (Möllhoff, 1977a). In the field studies of Möllhoff
(1977a) it is reported that metabolite IV, dicyclohexyl tin oxide,
occurs only in small amounts in plants material, i.e. less than 10%.
Mass balance and metabolism studies on azocyclotin were performed in a
laboratory ecosystem with bush (i.e. kidney) beans, enclosed in a
quartz bell jar giving 96% light transmission (Wolff et al., 1977a).
The azocyclotin preparation which was used was a well-defined mixture
of 1-tricyclohexyl-14C-stannyl-1,2,4-triazole and
1-tricyclohexyl-stannyl113-1,2,4-triazole. Forty-four days after the
treatment, the amounts of 14C and 113Sn radioactivity distributed
among the different parts of the system was measured, and 95% of the
applied radioactivity was recovered.
In the exhaust air absorber, less than 0.01% of the applied 113Sn
radioactivity was found as against 41.7% of the originally applied
14C radioactivity. Of the remaining 58.3% of the 14C radioactivity,
43.3% was present on/in the soil, and 9.8% in the plants. Of the
originally applied 113Sn radioactivity, 81.7% was found on or in the
soil and 14.5% on or in the plants. Autoradiographs showed spray
spots caused by 14C radioactivity on the treated leaves, as against
uniform blackening on newly grown plant parts. From these findings
and from the comparison of 14C radioactivity and 113Sn radioactivity
in different plant parts (Table 5), it is evident that a major
proportion of the applied azocyclotin was no longer present as the
unchanged parent compound. There was only little translocation of the
radioactivities in the plants. However, a large proportion of the
14C radioactivity volatilized and was trapped in the absorber.
Thin-layer chromatographic separation of the plant extracts revealed
that the individual azocyclotin metabolites were present in various
plant parts in the following proportions: metabolite III: 47 - 71%,
metabolite IV: 7 - 33%, metabolite V: 5-22% and inorganic Sn: 6-19%
(cfr. Table 6). The structures of these compounds were verified by
comparison of their Rf values from TLC with those of reference
substances, and by the ratios of 113Sn:14C (cfr. Table 6).
In processing
It will be noted from the data presented in Table 7 that when wine
grapes were pressed, about 25% of the residue was carried into the
must. However, the wine made from the must did not contain any
measurable residue amounts of azocyclotin.
In soil
Leaching Studies
Whereas azocyclotin is very sparingly soluble in water, 1,2,4-triazole
(II) displays good solubility. This metabolite, however, is absorbed
from water on soil particles according to soil leaching studies
performed by Möllhoff (1977b). From these studies performed on three
different soils, it was found that neither azocyclotin, nor its
metabolites II, III and IV were detectable in percolated water under
the conditions of the experiments. The limits of determination both
for azocyclotin and for II, III, and IV were at 2% of the applied
amount of parent compound.
Also in the ecosystem planted with bush beans (Wolff et al., 1977a),
vertical movement in the soil was minimal. After 44 days, about 98%
of the applied radioactivity (113Sn + 14C-azocyclotin) was retained
in top soil between 0 and 3 cm, while soil layers below 15 cm showed
no activity at all.
Table 5. Distribution of 113Sn and 14C radioactivities among
different plant parts, soil and exhaust air from laboratory simulation
study after 44 days. (Wolf et al., 1977a).
113Sn (in %) 14C (in %)
Bush (i.e. kidney) beans
Old beans 0.03 0.30
Young beans 0.01 0.03
Leaves + stem 7.92 5.11
Fallen leaves 6.56 4.44
Roots 0.10 0.03
Total in plants 14.62 9.91
Soil 81.7 43.3
Exhaust air <0.01 41.7
Recovered 96.3% 94.9%
Table 6. Metabolite proportions after azocyclotin treatment and ratios of 113Sn:14C-radioactivity in
bean plant extracts (Wolf et al, 1977a)
Origin on
Plant part III: Tricyclohexyl Sn IV: Dicyclohexyl Sn V: Monocyclohexyl Sn TLC-plate*
Old beans 71.7% 17. 3% 4.9% 6.0%
Young beans 47.4% 33.6% 9.5% 9.5%
Leaves + stem 69.5% 7.3% 13.0% 9.6%
Fallen leaves 61.6% 8.4% 15.9% 14.6%
Roots 47.6% 10.9% 22.1% 19.14%
Ratios 113Sn: 14C 113Sn:14C 113Sn:14C 113Sn:14C
Theoretical 1 : 0.92 1 : 0.62 1 : 0.31 1 : 0
Found 1 : 0.91-1.06 1: 0.58-0.84 1: 0.27-0.38 1. 0.03-0.16
* Tin compounds without cyclohexyl rings.
Table 7. Azocyclotin residues during wine processing
(Bayer AG)
Experiment 1 Experiment 2
Azocyclotin (mg/kg)
Grapes 1.05 - 1.2 <0.05 - 0.1
Must 0.25 - 0.9 <0.05 - <0.05
Wine n.d. n.d.
Degradation in soil
Field experiments on three different soils showed that the triazole
component of azocyclotin was eliminated to levels below the limit of
determination (0.1 mg/kg) within 14 days. The organotin residues
measured as the sum of I, III and IV, on the other hand, were degraded
with a half-life of 50 to 90 days. Also following two treatments at a
one-year interval, there was found no extra build-up of soil residues.
(cfr. Table 8).
In studies under laboratory conditions, degradation of azocyclotin in
two different soils indicated half-lives in the order of 250 days,
while in another experiment under the same conditions, half-lives were
found to be 200 days for I and metabolite III, and about one year for
the sum of I, III, IV and V (Wolff et al., 1977b).
Metabolism in soil
In field experiments, azocyclotin (I) itself was measurable only for
one week (Table 3). During the first week, also, up to about 25%
dicyclohexyl tin oxide (IV) formed (Möllhoff, 1977b). Its content
declined thereafter parallel to the disappearance of its immediate
precursor, tricyclohexyl tin hydroxide (III). In laboratory
metabolism studies conducted with azocyclotin dicyclohexyl tin oxide
was formed gradually during 30-60 days and remained thereafter on a
plateau level corresponding to about 15% of the applied azocyclotin
dose.
In another laboratory experiment using a larger amount of soil (500 g)
held in a closed bottle, measurements made nine months after
application of 50 mg azocyclotin/kg showed that 45% of the applied
amount of tin was still present as organotin compounds in the soil.
At this point of time, the analytically determined components triazole
(II) and tricyclohexyl butyl tin (i.e. metabolite III after
butylation) were present in almost stoichiometric proportions of 23.8%
and 25.4%, respectively. Whether azocyclotin or a mixture of triazole
and tricyclohexyl tin hydroxide was present, or whether there was a
balance among I, II and III could not be decided by analysis. From
the fast decline of the triazole proportion in field conditions,
however, it was concluded that triazole under open conditions would
volatilize rapidly (Möllhoff, 1977b).
In the above-mentioned larger-scale laboratory experiment, it was also
found after nine months of storage that of the originally applied
azocyclotin dose, 1O.8% was present as dicyclohexyl tin oxide (IV) and
8.25% as cyclohexyl stannoic acid (V). After derivatization with
butyl lithium these two compounds were measured quantitatively by gas
chromatography, and after separation on capillary columns, they were
identified by mass spectrometry. The same two metabolites (IV and V),
were found in soil nine months after application of the metabolite
dicyclohexyl tin oxide (IV) in amounts corresponding to 36.5% and
11.7% respectively. In the soil metabolism studies of Wolf et al.
(1977b), using a mixture of 14C- and 113Sn-labelled azocyclotin, it
was found that the proportion of 113Sn radioactivity which was
non-extractable never exceeded 6.6% while, on the other hand, the
proportion of non-extractable 14C radioactivity increased towards the
end of the 200-day experiment to 24.5% (cfr Table 9). This is
interpreted as a sign of metabolic incorporation of 14C-carbon into
non-extractable compounds, the identity of which, however, remained
unknown. The proportion of the applied tin to which three cyclohexyl
rings were still bound, declined to a level of 50% during this
experiment.
Fate in water
Degradation of azocyclotin in aquarium water was studied during 150
days in closed bottles at 22 ± 2°C, with azocyclotin added at a level
of 10 mg/kg (Möllhoff, 1977c). The 25% w.p. formulated azocyclotin
was used for this experiment, but despite frequent shaking, the
formulation could not be held continuously in suspension.
At the end of the experimental period, one-third of the applied amount
of azocyclotin still contained all three cyclohexyl rings at the tin
atom (Table 9), while the proportion of triazole still bound to the
tin atom could not be determined analytically. However, formation of
free triazole was observed at a level which on termination of the
experiment was estimated to be 0.41 mg/kg, equivalent to 2.6 mg/kg
when calculated as azocyclotin. In an open system, such free triazole
is expected to disappear by volatilization.
Metabolite IV, dicyclohexyl tin oxide, was determined throughout this
experiment, although in small amounts only, i.e. no more than 0.26
mg/kg.
Table 8. Azocyclotin residues in soil by triazole and organotin
methods (Wolf, et al., 1977b)
Application Days after Azocyclotin (mg/kg)
treatment Triazole Organotin
Method Method
First year
1 × 5 kg/ha 0 2.3 - 3.1 2.3 - 3.1
7 0.48 - 0.65 1.9 - 2.5
14 n.d. - n.d. 1.35 - 1.7
60 - 65 0.3 - 1.25
90 - 92 0.3 - 0.7
120 - 124 0.15 - 0.3
150 - 155 n.d. - 0.2
Second year
1 × 5 kg/ha 0 2.9 3.1
7 0.48 1.9
14 n.d. 1.35
59 0.85
92 0.65
120 0.35
150 0.3
0.3
Table 9. Degradation of Azocyclotin (25% w.p.) in aquarium water
(Möllhoff,1977c)
Storage Analysis by Analysis by
time triazole meth. organotin method
in days
Azocyclotin Tricyclohexyl Dicyclohexyl
+ triazole * tin* tin *
0 8.95 0.26
7 9.0 0.25
28 7.6 7.6 0.33
49 7.9 5.9 0.19
71 5.3 3.2 0.36
91 5.3 2.7 0.29
120 5.3 2.75 0.14
150 5.6 3.0 0.14
* All results calculated as azocyclotin (mg/kg).
METHOD OF RESIDUE ANALYSIS
Residues of azocyclotin can be measured by two different methods
developed by Möllhoff (1977a).
Triazole method (parent compound + metabolite II)
Plant, soil and water samples are extracted with isopropanol followed
by chloroform, and cleanup of extract by precipation. The final
determination is a GLC procedure using thermionic N-detector (AFID)
giving response to the triazole, and with a limit of determination of
0.1 - 0.2 mg/kg, expressed as azocyclotin. Azocyclotin (I) and
1,2,4-triazole (II) cannot be distinguished by this method.
This method determines that portion of the molecule which is split off
first from the tin atom, i.e. the 1,2,4-triazole moiety.
Accordingly, residues of azocyclotin measured by the triazole method
usually drop relatively fast below limit of determination, while
remaining organotin compounds can only be determined by method B.
Organotin method (parent compound + metabolites III + IV)
Plant, soil, water and laboratory animal feed samples are macerated
with water and treated with hydrobromic acid in acetone, and
thereafter extracted with hexane. The hexane extracted residue in
ethyl ether is reacted with methyl magnesium chloride and hydrolysed.
The methylated compounds, tricyclohexyl methyl tin and dicyclohexyl
dimethyl tin, which are formed from azocyclotin and metabolites III
and IV are cleaned up on Florisil and gas chromatographed using a
flame photometric detector (FPD) with a 394 nm filter. The limits of
determination are 0.05 - 0.1 mg/kg.
Azocyclotin (I) and cyhexatin (III) cannot be distinguished by this
method, but the method permits determination of the sum of these two
compounds in parallel with determination of metabolite IV
(dicyclohexyl tin oxide).
The proportion of IV in the total residue measured by the organotin
method is usually less than 10% in plants, and between 10 and 20% in
soil, whilst metabolite V (cyclohexyl stannoic acid) is of little
significance as residue. This latter metabolite V could be determined
by the organotin method if chloroform is used instead of hexane for
the extraction. The organotin method has multiresidue method
characteristics as it permits the simultaneous determination of
several chemical entities, in this case partially alkylated or
arylated tin compounds (cfr. Steinmeyer et al., 1965, Heubert &
Wirth, 1975, Figge et al., 1977 and Wright et al., 1979) by
gaschromatographic separations after reaction with methyl magnesium
chloride or with butyl lithium. The latter alkylation agent deserves
special attention because the alkylation seems to proceed very
smoothly producing compounds with molecular weight of the same range.
A more convenient isothermal GLC technique is thereby facilitated.
NATIONAL MRLs REPORTED TO THE MEETING
National Limits and associated pre-harvest intervals which have
been reported to the meeting are shown in Table 10.
Table 10. National MRLs and Safety Intervals
Safety
Country Crop MRL Interval
in mg/kg in days
Chile fruits 15
Cyprus pome and stone fruits,
citrus 21
Germany bush and runner beans 14
F.R.G. pome fruit 14
grapes (except
table grapes) 35
hops 50.0
pome fruits 2.0
wine grapes 2.0
Morocco pome fruit 28
New pipfruit 14
Zealand stone fruit 14
South apples 0.5 3
Africa pears 0.5 3
peaches 0.5 21
APPRAISAL
Azocyclotin is an organotin acaricide used against spider mites on
fruits and vegetables, especially recommended for the control of
strains which are resistant to other chemicals e.g.
organophosphorous compounds. It is formulated in wettable powders (25
and 50%) and marketed in most continents.
In plants, animals, soil and water, azocyclotin (I) is degraded by an
initial off-splitting of 1,2,4-triazole (II) from tricyclohexyl tin
hydroxide (III) identical with cyhexatin, followed by stepwise
formation of cicyclohexyl tin oxide (IV) and monocyclohexyl stannoic
acid (V) through the loss of the three cyclohexyl rings from the
central Sn-atom.
The first degradation step by splitting off of 1,2,4-triazole is a
relatively fast process, and the 1,2,4-triazole moiety disappears to
levels at or below the limit of determination shortly after treatment
of plants or after application to soil or water. The triazole
probably disappears from plants by volatilization.
The remaining organotin compounds, cyhexatin and its further
metabolites, are more persistent, and dissipation of the total
organotin residues is mainly governed by a process of growth dilution
on most plants and vegetables after application. But compounds III,
IV and V are found as simultaneous residues in variable ratios, and
with a limited amount of inorganic Sn also present. In a single study
with soil, inorganic Sn was found at the level of 6-19% of total
residues.
Half-lives of organotin residues in soil varied from 50-90 days in
field studies and from 200 days to one year under laboratory
conditions. Vertical movement or leaching in soils are found to be
minimal or unlikely.
Residues of azocyclotin (as organotin) in apples, beans, eggplants and
strawberry are at or below limit of determination after application at
recommended use levels and appropriate waiting periods, while
significant residues were found in most cases on grapes. After
processing, up to 25% of residues in grapes was carried over to must,
but without any measurable transfer of residues to wine.
Two gas chromatographic methods are available for the determination of
azocyclotin. The one is based on determination of the 1,2,4-triazole
moiety and it will not distinguish azocyclotin (I) from the triazole
(II). Residues by this method usually drop relatively rapidly to
below the limit of determination, after which residue pattern cannot
be distinguished from that which follows treatment with cyhexatin.
The other method is an organotin method developed in recent years,
which permits determination of organotin metabolites III, IV and V
individually, while it will not distinguish azocyclotin (I) from
metabolite III. Metabolite V is of little significance as residue.
It is determined by the same method as I + III and IV, but only after
separate extraction procedure.
RECOMMENDATIONS
The following guideline limits for azocyclotin are recommended on the
basis of data resulting from supervised trials. They refer to the sum
of organotin compounds expressed as azocyclotin.
Limit Pre-harvest interval on which
Commodity (mg/kg) recommendation is based
Apples 0.1* 21 days
Egg plants 0.1* 30 days
Grapes 35 days
Kidney Beans 0.2 21 days
Strawberry 0.1* 14 days
The Meeting realized that recommendations in certain cases are
incompatible with earlier recommendations on cyhexatin from which
azocyclotin residues will be undistinguishable shortly after
application. This may be due to somewhat differing use patterns for
the two compounds, but partly also to the fact that a majority of
earlier data derives from use of another analytical method, i.e.
colorimetric, total Sn-determination. Therefore, it is suggested that
special efforts should be made at the international level to collect
and review information on developed use patterns and residue data
derived from new methodology for cyhexatin.
Further work or information Required
By 1980
As residues from azocyclotin and cyhexatin cannot be distinguished
shortly after application, information on use patterns and on residue
data for both compounds, especially for cyhexatin, should be reviewed
before residue limits for the two pesticides can be harmonized.
REFERENCES
Bayer, A.G. Various reports on azocyclotin residues in fruit,
vegetables and soil (1978), Unpublished.
Figge, K., Koch, J., Lubba, H. - Beitrag zur gaschromatographischen
Analyse von Organozinn-Stabilisatoren für Plyvinylchlorid. J.
Chromatogr. 131, 317-327.
Möllhoff, E. Methode zur gaschromatographischen Bestimmung des
Akarizids Peropal und seiner Metaboliten in Pflanzen, Böden, Wasser
und Kleintierfutter. Pflanzenschutz-Nachr. Bayer 30, 249-263 (1977a).
Abbau und Metabolismus von Peropal im Boden. Bayer AG, Report
RA-11/77 (English version) (1977b).
Abbau von Peropal in Aquarienwasser (geschlossenes System). Bayer
AG; Report RA-815/77 (1977c).
Neubert, G., Wirth, H.O. - Zur Analytik con Organozinnstabilistatoren.
Z. Anal. Chem. 273, 19-23.
Steinmeyer, R.D., Fentiman, A.F., Kahler, E.J., - Analysis of alkyltin
bromides by gas liquid chromatography. Analytical Chem. 37, 520-523.
Wolf, W., Lippert, K.D., Figge, K. - Übber das Verhalten des Akarizids
"Tricyclohexylzinn-1,2,4-triazolid" und seiner Abbauprodukte im
Okosystem "Buschbohnenkulture". Natec, Gesellschaft für
naturwissenschaftlinch-technische Dienste mbH., Projekt NA 76 00 43,
(1977a).
Uber das Abbauverhalten des Akarizids "Tricyclohexylzinn-1,2,4-
triazolid"in Standardboden. Natec, Gesellschaft für naturwissen-,
schaftlich-technische Dienste mbH., Project NA 76 00 43, (1977b).
Wright, B.W., Lee, M.L., Booth, G.M. - Determination of
triphenyltinhydroxide derivatives by capillary GC and Tin-selective
FPD. Journ. High Resolution Chrom. and Chromatography Comm. S. 189.
