
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
FENVALERATE
IDENTITY*
Chemical Name
[S,R]-alpha-cyano-m-phenoxybenzyl
[S,R]-alpha-isopropyl-p-chlorophenylacetate
[S,R]-alpha-cyano-3-phenoxybenzyl
[S,R]-2-(4-chlorophenyl)-3-methyl-1-butyrate
benzenacetic acid
[S,R]-4-chloro-alpha-(1-methylethyl)-[S,R]-cyano (3-phenoxyphenyl)
methyl ester
cyano (3-phenoxyphenyl)methyl-4-chloro-alpha-(1-methylethyl)
benzenacetate
The technical product of fenvalerate contains four optically active
isomers due to two chiral centres present in both alcohol and acid
moieties of the molecule.
Synonyms
Sumicidin(R), Belmark(R), Pydrin(R) S5602, WL43775, SD43775
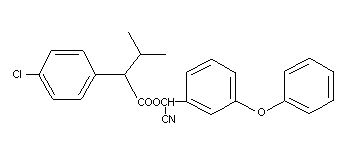
Structural formula
C25H22ClNO3
 * Based on information submitted by Sumitomo Chemical Ltd., Osaka,
Japan and Shell Chemical Ltd., London
Other information on identity and properties
Molecular weight: 419.9
State: Yellow oily liquid at 23~C
Specific gravity: 1.17 g/ml at 23°C
Vapor pressure: 2.1 × 10-6 mm Hg at 70°C
Solubility: (g/l at 20°C)n-hexane 77, xylene >450,
acetone >450, ethanol >450 and methanol >450.
Solubility in water: ca. 2 µg/L
Stability: Stable in most solvents except alcohols at
ambient temperature. Unstable in alkaline
media. No significant breakdown after 100 hours
at 75°C. Decomposed gradually in the range of
150-300°C.
Typical composition of the Technical material % by weight
alpha-cyano-3-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 90-94
alpha-cyano-2-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 1.0-2.5
[Z and E]-2-(4-chlorophenyl)-1-cyano-3-methyl-
1-buten-1-yl-2-(4-chlorophenyl)-3-methyl-butyrate 0.4-2.0
alpha-cyano-3-phenoxybenzyl-2-(2-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
alpha-cyano-3-phenoxybenzyl-2-(3-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
Other related compounds <5.0
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
The results of acute toxicity tests with various animal species are
summarised in Table 1.
Signs of Poisoning
Within four hours of dosing, all animals receiving acutely toxic
levels were restless, developed tremors, piloerection, occasional
diarrhea and an abnormal gait. Following oral administration, animals
recovered rapidly from acute clinical signs of poisoning and were
asymptomatic within 3-4 days. Immediately after exposure, rats show
an abnormal gait which is typical of pyrethroid intoxication. The
animals walk, with hindquarters held up and the hind legs more widely
spaced than normal (splayed). Histological examination of the sciatic
nerve and posterior tibular nerve, after poisoning and for nine days
over the course of recovery, showed axonal breaks, swelling and
vacuolation accompanied by vacuolation and phagocytosis of myelin.
The degree to which myelin was disrupted was dose dependent and was
closely associated with the acute signs of toxicity (Butterworth and
Carter, 1976).
Table 1. Acute toxicity of fenvalerate administered to various animal species
LD50
Species Route Sex Vehicle1 mg/kg Reference
Rat Oral DMSO 451 Walker et al., 1975
Oral PEG: water >3200 Swamitt & Albert, 1977a
Dermal 5000 (24 hr) Okuno et al., 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Mouse Oral M DMSO 200-300 Walker et al., 1975
F 100-200
Oral PEG: water 1202 Summit & Albert, 1977b
Ip M & F Corn oil 85-89 Khoda, et al., 1979
Intravenous Glycerol-formol 65 Albert & Summitt, 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Chinese Oral M DMSO 98 Walker, et al., 1975
hamster F 82
Syrian
hamster Oral PEG: water ca. 760 Hart, 1976a
Dog Oral PEG: water or Doses from 100 Hart, 1976b
corn oil to 1000 mg/kg
were emetic
Rabbit Percutaneous Undiluted 1000-3200 Hine, 1975
Hen Oral >1500 >1500 Milner & Butterworth, 1977
1 PEG = polyethylene glycol;
DMSO = dimethylsulfoxide.
Biochemical Aspects
Absorption, Distribution and Excretion
Fenvalerate, orally administered to rats and mice, was found to be
rapidly absorbed, distributed to a variety of tissues and organs,
metabolized and excreted from the body. The half-life for excretion
in both rodent species was 0.5-0.6 days. Elimination of the
CN-labelled fenvalerate was somewhat slower in both species suggesting
a different pattern of metabolism. Total recovery of the administered
CN-labelled fenvalerate was achieved within 6 days following the acute
administration. Tissue residues following acute administration was
extremely low with the highest concentration being observed in fat,
adrenal gland, skin, hair and in the intestines. High concentrations
of the CN-labelled fenvalerate were noted in the hair and skin which
may account for the data showing that residues of this label were more
slowly excreted from the body (Kaneko and Ohkawa, 1979; Ohkawa, et
al., 1979).
Rats fed 20 ppm in the diet for 28 days were sacrificed and residues
in adipose tissue were examined. Based upon chromatographic and mass
spectral analysis, the residue in fat was characterized as unchanged
fenvalerate containing both distereo isomers (Boyer, 1977a).
Male and female rats fed fenvalerate for 28 days (20 ppm) and placed
on control diets for additional 28 days were examined for tissue
residues and their depletion rates. Maximum residues were reached
rapidly, within 3 weeks of dietary administration. Of the tissues
measured, adipose tissue contained the highest residue. Trace amounts
were observed in other tissues including the brain after 28 days of
treatments. Dissipation of residues from all tissues following the
cessation of treatment was rapid, although with adipose tissue, the
dissipation was slower than with other tissues. At 28 days after the
cessation of dietary fenvalerate, residues were still reported in
adipose tissue, attesting to the slow clearance from this storage
depot (Potter and Arnold, 1977; Potter, 1976).
Metabolism
The metabolic fate of fenvalerate has been examined in rodent species
following acute and subacute oral and dietary administration. In all
cases, the metabolism in both rats and mice and elimination of the
metabolic components was rapid. Fenvalerate undergoes several major
metabolic reactions; cleavage of the ester linkage, hydroxylation in
the acid and alcohol moieties and conversion of the CN group to SCN
and CO2. The resulting metabolite acids and phenols were
subsequently conjugated with glucuronic acid, sulfuric acid or amino
acids. The reactions were similar in both rats and mice with
differences being the nature of the conjugating material and the
quantitative excretion of certain metabolites. Taurine was found to
conjugate with 3-phenoxy-benzoic acid, representing 10-13% of the dose
in mouse urine. This conjugating mechanism was not observed with
rats. In both species, major hydroxylation reactions were noted to
occur in the 4' position of the phenoxybenzoic acid. Hydroxylation
also has been noted in the 2' and 5' positions. Several species
differences in rats and mice were observed with respect to
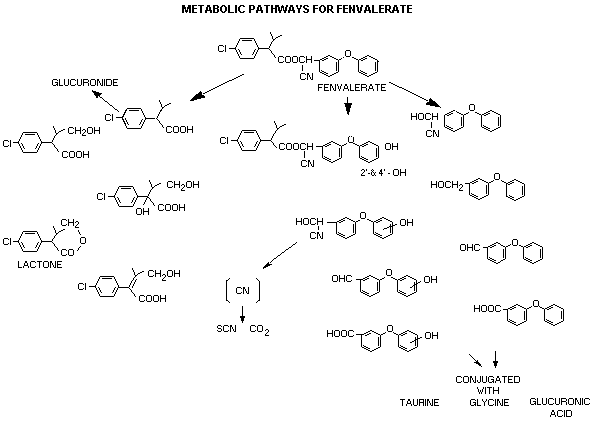
hydroxylation on the phenoxybenzoic acid. Figure 1 gives a schematic
of the metabolites conjugating mechanisms observed in mammals.
Hydroxylated fenvalerate was detected in the faeces of both rats and
mice (Boyer, 1977c; Kaneko and Ohkawa, 1979; Ohkawa, et al., 1979).
The liver of rats fed fenvalerate for 28 days was analyzed for
residues and found to contain 3-phenoxybenzoic acid and the
corresponding 4'-hydroxylated derivative (Boyer, 1977d).
Subcellular fractions of rat liver have been shown to degrade
fenvalerate yielding a wide variety of products, many of which have
been detected in urine and as conjugated products. The most widely
noted were 3-phenoxybenzoic acid and its 4'-hydroxylated derivatives
as well as the corresponding isovaleric acid (Boyer, 1976a; 1976b).
The acute toxicity of the hydrolytic metabolites is presented in Table
2. All metabolites are less toxic than fenvalerate.
Photodecomposition
Photolysis of fenvalerate in solvents by artificial light and as a
thin film on glass or cotton by sunlight yields products resulting
from ester cleavage. The major product in solution was identified as
a photo-induced decarboxylation yielding an unusual product, not known
to occur in mammals.
* Based on information submitted by Sumitomo Chemical Ltd., Osaka,
Japan and Shell Chemical Ltd., London
Other information on identity and properties
Molecular weight: 419.9
State: Yellow oily liquid at 23~C
Specific gravity: 1.17 g/ml at 23°C
Vapor pressure: 2.1 × 10-6 mm Hg at 70°C
Solubility: (g/l at 20°C)n-hexane 77, xylene >450,
acetone >450, ethanol >450 and methanol >450.
Solubility in water: ca. 2 µg/L
Stability: Stable in most solvents except alcohols at
ambient temperature. Unstable in alkaline
media. No significant breakdown after 100 hours
at 75°C. Decomposed gradually in the range of
150-300°C.
Typical composition of the Technical material % by weight
alpha-cyano-3-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 90-94
alpha-cyano-2-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 1.0-2.5
[Z and E]-2-(4-chlorophenyl)-1-cyano-3-methyl-
1-buten-1-yl-2-(4-chlorophenyl)-3-methyl-butyrate 0.4-2.0
alpha-cyano-3-phenoxybenzyl-2-(2-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
alpha-cyano-3-phenoxybenzyl-2-(3-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
Other related compounds <5.0
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
The results of acute toxicity tests with various animal species are
summarised in Table 1.
Signs of Poisoning
Within four hours of dosing, all animals receiving acutely toxic
levels were restless, developed tremors, piloerection, occasional
diarrhea and an abnormal gait. Following oral administration, animals
recovered rapidly from acute clinical signs of poisoning and were
asymptomatic within 3-4 days. Immediately after exposure, rats show
an abnormal gait which is typical of pyrethroid intoxication. The
animals walk, with hindquarters held up and the hind legs more widely
spaced than normal (splayed). Histological examination of the sciatic
nerve and posterior tibular nerve, after poisoning and for nine days
over the course of recovery, showed axonal breaks, swelling and
vacuolation accompanied by vacuolation and phagocytosis of myelin.
The degree to which myelin was disrupted was dose dependent and was
closely associated with the acute signs of toxicity (Butterworth and
Carter, 1976).
Table 1. Acute toxicity of fenvalerate administered to various animal species
LD50
Species Route Sex Vehicle1 mg/kg Reference
Rat Oral DMSO 451 Walker et al., 1975
Oral PEG: water >3200 Swamitt & Albert, 1977a
Dermal 5000 (24 hr) Okuno et al., 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Mouse Oral M DMSO 200-300 Walker et al., 1975
F 100-200
Oral PEG: water 1202 Summit & Albert, 1977b
Ip M & F Corn oil 85-89 Khoda, et al., 1979
Intravenous Glycerol-formol 65 Albert & Summitt, 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Chinese Oral M DMSO 98 Walker, et al., 1975
hamster F 82
Syrian
hamster Oral PEG: water ca. 760 Hart, 1976a
Dog Oral PEG: water or Doses from 100 Hart, 1976b
corn oil to 1000 mg/kg
were emetic
Rabbit Percutaneous Undiluted 1000-3200 Hine, 1975
Hen Oral >1500 >1500 Milner & Butterworth, 1977
1 PEG = polyethylene glycol;
DMSO = dimethylsulfoxide.
Biochemical Aspects
Absorption, Distribution and Excretion
Fenvalerate, orally administered to rats and mice, was found to be
rapidly absorbed, distributed to a variety of tissues and organs,
metabolized and excreted from the body. The half-life for excretion
in both rodent species was 0.5-0.6 days. Elimination of the
CN-labelled fenvalerate was somewhat slower in both species suggesting
a different pattern of metabolism. Total recovery of the administered
CN-labelled fenvalerate was achieved within 6 days following the acute
administration. Tissue residues following acute administration was
extremely low with the highest concentration being observed in fat,
adrenal gland, skin, hair and in the intestines. High concentrations
of the CN-labelled fenvalerate were noted in the hair and skin which
may account for the data showing that residues of this label were more
slowly excreted from the body (Kaneko and Ohkawa, 1979; Ohkawa, et
al., 1979).
Rats fed 20 ppm in the diet for 28 days were sacrificed and residues
in adipose tissue were examined. Based upon chromatographic and mass
spectral analysis, the residue in fat was characterized as unchanged
fenvalerate containing both distereo isomers (Boyer, 1977a).
Male and female rats fed fenvalerate for 28 days (20 ppm) and placed
on control diets for additional 28 days were examined for tissue
residues and their depletion rates. Maximum residues were reached
rapidly, within 3 weeks of dietary administration. Of the tissues
measured, adipose tissue contained the highest residue. Trace amounts
were observed in other tissues including the brain after 28 days of
treatments. Dissipation of residues from all tissues following the
cessation of treatment was rapid, although with adipose tissue, the
dissipation was slower than with other tissues. At 28 days after the
cessation of dietary fenvalerate, residues were still reported in
adipose tissue, attesting to the slow clearance from this storage
depot (Potter and Arnold, 1977; Potter, 1976).
Metabolism
The metabolic fate of fenvalerate has been examined in rodent species
following acute and subacute oral and dietary administration. In all
cases, the metabolism in both rats and mice and elimination of the
metabolic components was rapid. Fenvalerate undergoes several major
metabolic reactions; cleavage of the ester linkage, hydroxylation in
the acid and alcohol moieties and conversion of the CN group to SCN
and CO2. The resulting metabolite acids and phenols were
subsequently conjugated with glucuronic acid, sulfuric acid or amino
acids. The reactions were similar in both rats and mice with
differences being the nature of the conjugating material and the
quantitative excretion of certain metabolites. Taurine was found to
conjugate with 3-phenoxy-benzoic acid, representing 10-13% of the dose
in mouse urine. This conjugating mechanism was not observed with
rats. In both species, major hydroxylation reactions were noted to
occur in the 4' position of the phenoxybenzoic acid. Hydroxylation
also has been noted in the 2' and 5' positions. Several species
differences in rats and mice were observed with respect to
hydroxylation on the phenoxybenzoic acid. Figure 1 gives a schematic
of the metabolites conjugating mechanisms observed in mammals.
Hydroxylated fenvalerate was detected in the faeces of both rats and
mice (Boyer, 1977c; Kaneko and Ohkawa, 1979; Ohkawa, et al., 1979).
The liver of rats fed fenvalerate for 28 days was analyzed for
residues and found to contain 3-phenoxybenzoic acid and the
corresponding 4'-hydroxylated derivative (Boyer, 1977d).
Subcellular fractions of rat liver have been shown to degrade
fenvalerate yielding a wide variety of products, many of which have
been detected in urine and as conjugated products. The most widely
noted were 3-phenoxybenzoic acid and its 4'-hydroxylated derivatives
as well as the corresponding isovaleric acid (Boyer, 1976a; 1976b).
The acute toxicity of the hydrolytic metabolites is presented in Table
2. All metabolites are less toxic than fenvalerate.
Photodecomposition
Photolysis of fenvalerate in solvents by artificial light and as a
thin film on glass or cotton by sunlight yields products resulting
from ester cleavage. The major product in solution was identified as
a photo-induced decarboxylation yielding an unusual product, not known
to occur in mammals.
 Holmstead, et al., 1978)
Effects on Enzymes and Other Biochemical Parameters
Fenvalerate, when fed to adult rats for 14 days did not induce hepatic
microsomal enzyme as measured by the comparative rates of
de-ethylation of an organophosphate pesticide (chlorofenvinphos)
(Creedy and Potter, 1976). Dietary levels of 1000 ppm did not induce
the oxidative O-dealkylation which has been shown to be reflective
of microsomal enzyme induction in rat liver.
Groups of rats (4 to 6 of each sex per group) were administered
fenvalerate orally at dose levels ranging from 0 to 400 mg/kg for 7
consecutive days. Mortality and clinical signs of acute poisoning
were seen only at the highest dose level. A significant neurological
deficit was demonstrated using an inclined plane test (expressed at an
angle at which the animals cannot maintain their hold on an inclining
plane). [See Kaplan and Murphy, 1972]. In addition to functional
deficits, increases in ß-glucuronidase and ß-galactosidase activity in
the posterior tibular nerve and trigeminal ganglia were observed.
Functional motor deficits appeared to coincide with administration on
fenvalerate reaching its maximum effects between day 5-7 of treatment.
Stimulation of lysosomal enzyme activity appears to coincide with
neurological deficits and with sciatic nerve degeneration noted with
acute (high level) intoxication (Dewar, et al., 1977).
Groups of hamsters (5 males and 5 females per group) were administered
fenvalerate at dose levels of 0, 5, 10, 20 and 40 mg/kg for 5
consecutive days. In 3 separate experiments, dose related increases
in ß-glucuronidase and ß-galactosidase activity of peripheral nerves
was observed. Minimal functional deficits were also noted during the
treatment period and for two weeks following treatment. These data
appear to be consistent and to be associated with axonal degeneration
in the peripheral nerve (Dewar, et al., 1978).
METABOLIC PATHWAYS FOR FENVALERATE
Holmstead, et al., 1978)
Effects on Enzymes and Other Biochemical Parameters
Fenvalerate, when fed to adult rats for 14 days did not induce hepatic
microsomal enzyme as measured by the comparative rates of
de-ethylation of an organophosphate pesticide (chlorofenvinphos)
(Creedy and Potter, 1976). Dietary levels of 1000 ppm did not induce
the oxidative O-dealkylation which has been shown to be reflective
of microsomal enzyme induction in rat liver.
Groups of rats (4 to 6 of each sex per group) were administered
fenvalerate orally at dose levels ranging from 0 to 400 mg/kg for 7
consecutive days. Mortality and clinical signs of acute poisoning
were seen only at the highest dose level. A significant neurological
deficit was demonstrated using an inclined plane test (expressed at an
angle at which the animals cannot maintain their hold on an inclining
plane). [See Kaplan and Murphy, 1972]. In addition to functional
deficits, increases in ß-glucuronidase and ß-galactosidase activity in
the posterior tibular nerve and trigeminal ganglia were observed.
Functional motor deficits appeared to coincide with administration on
fenvalerate reaching its maximum effects between day 5-7 of treatment.
Stimulation of lysosomal enzyme activity appears to coincide with
neurological deficits and with sciatic nerve degeneration noted with
acute (high level) intoxication (Dewar, et al., 1977).
Groups of hamsters (5 males and 5 females per group) were administered
fenvalerate at dose levels of 0, 5, 10, 20 and 40 mg/kg for 5
consecutive days. In 3 separate experiments, dose related increases
in ß-glucuronidase and ß-galactosidase activity of peripheral nerves
was observed. Minimal functional deficits were also noted during the
treatment period and for two weeks following treatment. These data
appear to be consistent and to be associated with axonal degeneration
in the peripheral nerve (Dewar, et al., 1978).
METABOLIC PATHWAYS FOR FENVALERATE
 TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of rats (11 male and 22 female rats per group) were fed
fenvalerate in the diet at dosage levels of 0, 1, 5, 25 and 250 ppm.
The animals were fed for 9 weeks prior to mating and initiation of a
standard 3-generation (2 litters per generation) reproduction study.
Fertility, viability, gestation and lactating indices were calculated
for each treatment group compared to control values. Ten male and 10
female weanlings from the F3b litter were examined histologically at
the conclusion of the study.
The mean body weight of the parent of the third generation (F2b
adults) showed a significant reduction at the 250 ppm level. Gross
necropsy revealed kidney changes in these animals which was not
apparently related to fenvalerate. No pathological changes were noted
to account for the weight loss. No effects on reproductive parameters
in any of the three generations were observed in the study.
Histological examination did not show an adverse effect of
fenvalerate. With the exception of weight reduction in the third
generation parents, there was no effect of fenvalerate on any
parameter measured in the study (Beliles, et al., 1978; Stein, 1977).
Teratogenicity
Mouse
Groups of pregnant mice (32 to 33 mice per group) were administered
fenvalerate at dosage levels of O, 5, 15 and 50 mg/kg body weight per
day on days 6 through 15 of gestation in a standard teratogenicity
bioassay. On day 18, groups of 20 mice were sacrificed and fetuses
were removed and examined for somatic and skeletal abnormalities. The
remaining parents were allowed to deliver naturally and the young
maintained for three weeks to weaning to evaluate postnatal deficits.
In addition to the teratogenicity study, two male and two female
weanlings from each dam were maintained for 8 weeks and mated to note
any effects on their reproductive potential. Toxic signs of poisoning
were noted in maternal mice at the high dose level. There was no
significant mortality over the course of the study and no effects were
noted on any of the animals as a result of continuous administration
of fenvalerate. In fetal examinations, there were no somatic or
skeletal changes as noted by internal or external tissue evaluations.
The animals maintained in an abbreviated reproduction study showed no
differences from control value in their ability to reproduce. There
were no changes in the reproduction indicates evaluated with any
animals examined. Under the conditions of this bioassay, fenvalerate
has been shown to have no teratogenic potential in mice (Kohda, et
al., 1976a).
Rabbit
Groups of pregnant rabbits (group size varied from 20 to 31 rabbits)
were administered fenvalerate from day 6 to day 18 of gestation. On
day 28, the rabbits were sacrificed and standard teratogenicity
assessments made with respect to early or late fetal death, viability
and standard somatic and skeletal teratogenic potential. Reduced body
weight of pregnant rabbits was observed with the highest dose level.
There were no significant differences from control values in any of
the parameters measured in the study. Under the conditions of the
study, fenvalerate did not induce a teratogenic event in rabbits (van
der Pauw, et al., 1975).
Special Studies on Mutagenicity
Microorganism
Fenvalerate was examined for its mutagenic potential using a series of
Salmonella typhimurium strains (TA 1535, TA 1538, TA 98 and TA 100) at
dose levels up to 1 mg/ plate. Two strains of Bacillus subtilis (H17
and M45) were tested at concentrations up to 10 mg/disc. The "Ames"
test was performed in the presence and absence of rat activation
systems and with positive and negative chemical controls. The Rec
assay was not evaluated with a metabolic activation system. With both
microbial bioassay systems, fenvalerate was not mutagenic (Suzuki and
Miyamoto, 1976).
In additional trials, fenvalerate did not increase the number of
revertant colonies of S. typhimurim (TA 1535, TA 1537, TA 1538, TA 98
and TA 100) in the presence or absence of a liver subcellular
activation system prepared from 6 different strains of PCB-treated
mice, 3 strains of rat, and the Syrian golden hamster. Fenvalerate,
tested at dosage levels up to 1 mg/plate, was not mutagenic (Suzuki
and Miyamoto, 1977; Suzuki, et al., 1979).
Mouse
Groups of mice were administered fenvalerate orally at doses of 0, 60
and 125 mg/kg body weight and injected intraperitoneally with an
indicator strain of microorganisms in a standard host-mediated assay.
A positive control, dimethylnitrosamine (DMNA), was used in this
study. Fenvalerate did not induce an increase in mutation frequency
of the S. typhimurium indicator while the DMNA significantly
increased the mutation frequency (Suzuki and Miyamoto, 1976).
Groups of mice were administered fenvalerate orally at dose levels of
0, 25 or 50 mg/kg. Immediately after administration, a suspension
culture of yeast Saccharomyces cerviciae was introduced to the
peritoneal cavity. Positive (ethyl methane-sulphonate) and negative
(DMSO) control animals were also used in this standard host-mediated
assay. Five hours after dosing, the yeast cells were recovered and
examined for mitotic gene conversion. No mutagenic effects were
detected in the cells from any of the fenvalerate concentrations
tested (Brooks, 1976).
Dominant Lethal Assay
Mouse
Groups of male mice (10-11 mice per group) were administered
fenvalerate orally at dosage levels of 0, 25, 50 and 100 mg/kg body
weight. Each treated male was mated with 3 virgin females for 7 days.
The procedure was repeated weekly with new females in a standard
dominant lethal assay. The females were sacrificed and examined for
the condition of the fetuses on day 13 of gestation.
There were no significant differences from control values with respect
to the effects of fenvalerate on pregnancy. Foetal implants in
females mated to males during the second week after treatment showed a
significant reduction in viable fetuses. There was also a significant
increase in early foetal deaths occurring in females mated during the
fourth week to males dosed at the highest level (Dean, 1975).
Hamster
Groups of Chinese hamsters (6 male and 6 females per group) were
orally administered fenvalerate (in DMSO) at dose levels of 0, 12.5
and 25 mg/kg on each of two successive days. Animals were sacrificed
8 or 24 hours after dosing and chromosomal preparations were made from
the femoral bone marrow cells. Similar preparations were made with
control animals administered methyl methanesulphonate (50 mg/kg).
Cells from animals administered methyl methanesulphonate showed
substantial numbers of chromatid gaps at 8 hours after dosing but not
at 24 hours. The administration of fenvalerate did not induce
demonstrable chromosomal damage at either sampling interval (Dean and
Senner, 1975).
Special Studies on Neurotoxicity
Hen
Groups of 6 hens were administered fenvalerate orally in dimethyl
sulphoxide at dosage levels of 0 and 1000 mg/kg daily for 5 days. A
positive control of TOCP (0.5 ml/kg) was also included in the study.
After 3 weeks, the fenvalerate and negative control animals were
retreated at the same dosage regimen. At the conclusion of an
additional 3 week observation period, animals were sacrificed and
histological examinations were performed on the central and peripheral
nervous system. All animals receiving TOCP developed readily defined
signs of delayed neurotoxicity and histological lesions were seen in
the sciatic nerve and spinal cord. Clinical signs and histopathologic
lesions were not noted in controls or fenvalerate-treated hens (Milner
and Butterworth, 1977).
Rat
A series of studies was performed to evaluate the neurotoxic potential
(to induce axonal and myelin disruption) of a group of synthetic
pyrethroid esters and of natural pyrethrum. Acute oral administration
of fenvalerate, cypermethrin, resmethrin, permethrin and natural
pyrethrum to rats at very high dosage levels resulted in severe
clinical signs of poisoning and mortality within 24 hours.
Histopathologic lesions were observed in the sciatic nerve with all
compounds tested. At lower levels, fenvalerate (200 mg/kg),
cypermethrin (100 mg/kg), permethrin (200 mg/kg) and pyrethrum (3500
mg/kg) did not show clinical signs of poisoning or histopathologic
lesions (Okuno, et al., 1977a; 1977b).
Groups of 6 male and female rats were fed fenvalerate in the diet at
concentrations of 0 and 2000 ppm for 8 to 10 days, after which the
sciatic nerve was examined for histological signs of degeneration.
All animals exposed to fenvalerate showed typical signs of acute
intoxication including ataxia, tremors and hypersensitivity.
Histological examinations at the end of the feeding interval did not
reveal any adverse effects of fenvalerate on the sciatic nerve (Hand
and Butterworth, 1976).
In a study to evaluate the reversibility of the lesions induced in the
sciatic nerve, rats were administered fenvalerate in the diet at dose
levels of 0 and 3000 ppm for 10 days. Mortality was evident as 60% of
the animals died within the course of the dietary treatment.
Surviving animals were fed normal control diets and examined for 12
weeks following completion of the feeding study. Animals were
sacrificed every 3 weeks and examined histologically for sciatic nerve
disruption. Sciatic nerves of rats sacrificed at 3 weeks on control
diets continued to show swelling and disintegration of axons. At 6
weeks there were no histological lesions. These results were also
observed at the 9 and 12-week intervals suggesting reversibility of
the histopathologic lesion observed following high dose treatment with
fenvalerate (Okuno and Kadota, 1977c).
Antidotal Studies
Following acute poisoning phenobarbital, pentobarbital and
diphenylhydantoin were found to be effective in relieving the acute
signs of intoxication. Phenobarbital (50 mg/kg, ip) prevented tremor,
diphenylhydantoin (100 mg/kg, ip) reduced the toxic reaction and
pentobarbital (35 mg/kg ip) removed the tremor reaction completely
within 30 minutes. Combinations of diphenylhydantoin with either of
the barbiturates was effective in reducing the onset and severity of
tremors while a variety of other agents were not active
(delta-tubocurarine, atropine, meprobamate, diazepam, biperidin and
trimethadione) (Matsubara, et al., 1977).
Special studies in pharmacology
Fenvalerate, administered to dogs in acute dosage rates sufficient to
induce toxic signs of poisoning, showed no consistent cardiovascular
effects as measured by EKG. Respiratory stimulation was noted at high
levels and was not reduced by anesthetic supplements (Urethane/alpha
chlorolos or pentobarbital) (Kirkland and Albert, 1977a; 1977b).
Skin and eye irritancy
Two formulated products were found to be severe eye and skin irritants
when examined in the rabbit. Dermal irritation was evident for 7 days
after a 24-hour exposure and severe conjunctivitis, corneal opacity
and iritis were observed within 30 minutes of an application of 0.2 ml
of the formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation to some degree (Coombs and
Carter, 1975 and 1976).
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
Table 2.
LD50 (mg/kg)
Compound Male Female
Fenvalerate 88.5 85.0
2-(4-Chlorophenyl)isovaleric acid 351 350
3-Phenoxybenzyl alcohol 371 424
3-4'(Hydroxyphenoxyl) benzyl alcohol 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol 876 778
3-Phenoxybenzoic acid 154 169
3-(4'-Hydroxyphenoxy) benzoic acid 783 745
3-(2'-Hydroxyphenoxy) benzoic acid 859 912
3-Phenoxybenzaldehyde 415 416
NaSCN 604 578
All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO (Khoda, et al., 1979).
The acute intraperitoneal toxicity in mice of the proposed
decarboxylated photoproduct was reported to be substantially lower
than that toxicity noted with fenvalerate (Holmstead, et al., 1978).
Short Term Studies
Inhalation
Groups of 4 male and 4 female rats were exposed by inhalation (head
only) to an aerosolized formulation (77 micron particle size)
generated from an aqueous suspension containing 3 grams/litre.
Following a single administration (4 hours) of this non-inhalable
particulate, acute signs of poisoning were noted for a short period,
presumably from oral ingestion of the large particle. There was no
mortality and all animals appeared normal within 3 days following
exposure (Blair and Roderick, 1975).
Groups of rats and mice (10 male and 10 female of each species per
group) were administered fenvalerate by inhalation exposure 3 hours
daily for 4 weeks at concentration levels of 0, 2, 7 and 20 mg/m3.
Animals were exposed to a small, fully respirable particulate (1 to 2
microns) during the course of the study. Mortality was not noted over
the course of the study, but animals at the high dose level showed
acute toxic signs of poisoning. Growth was not affected at any dose
level; nor were hematology and clinical biochemistry parameters.
Gross and microscopic examination of tissues and organs at the
conclusion of the study showed no changes that were related to the
administration of fenvalerate (Khoda, et al., 1976c; Ito, 1976b).
Dermal
Groups of rabbits (7-8 male rabbits per group) were administered
fenvalerate dermally at dose levels of 0, 100 and 400 mg/kg daily for
6 hours (14 exposures were performed over a 22-day period). Mortality
was observed at the high dose level accompanied by severe weight loss,
clinical signs of poisoning and gross dermal effects. Rabbits
tolerated the 100 mg/kg dose with minor local dermatologic effects
(Hine, 1975).
Groups of rabbits (10 male and 10 female rabbits per group, five of
each sex had an occluded skin) were administered fenvalerate dermally
for 6 hours per day, 5 days per week for 3 weeks. The dosage levels
used were 0, 30, 100 and 300 mg/kg body weight. In addition, a
formulated product (an emulsifiable concentrate containing 2.4 pounds
fenvalerate/gallon) was also tested for its dermal toxicity at dosage
levels of 0, 100, 300 and 1000 mg/kg.
Mortality was observed with both treatments predominantly at the high
dosage group. Mortality was preceded by clinical signs of poisoning
primarily in the group exposed to the formulated product. At lower
concentrations, technical fenvalerate was mildly irritating to the
skin upon repeated dermal exposure. The formulation of fenvalerate
and the blank formulation used as a control were severely irritating.
When severe irritation was noted, there were significant effects on
body weight. There were no effects noted on various haematologic,
clinical chemistry and urinalysis parameters related to the presence
of fenvalerate. Gross and microscopic pathologic changes were noted
only at the site of administration and described as acanthosis and
hyperkeratosis of the epidermis. The extent and severity of the
lesions appeared to be dose-related especially with respect to the
formulated product. There were no significant gross or microscopic
effects noted in tissues and organs over the course of the study
(Quinn, et al., 1976).
Dietary
Rat
Groups of rats (12 male and 12 female rats per group) were fed
fenvalerate in the diet at dose levels of 0, 125, 500, 1000 and 2000
ppm for 90 days. Mortality was observed at 2000 ppm while growth and
food consumption were decreased at 1000 ppm and 2000 ppm. With the
exception of an increase In BUN concentration at the two highest dose
levels, hematological and clinical examinations at the conclusion of
the study were normal. Gross and microscopic examinations of tissues
and organs were performed at the conclusion of the study. Increases
in liver to body weight and kidney to body weight ratios were observed
at 500 ppm and above. These gross changes were not accompanied by
observable microscopic changes on histopathological examination.
Sciatic nerves, examined at the conclusion of the study, showed no
myelin or axonal degeneration (Hend and Butterworth, 1975).
Dog
Groups of young adult beagle dogs (4 male and 4 female dogs per group
were fed fenvalerate in the diet at dose levels of 0, 0.5, 0.25, 1.25
and 12.5 mg/kg body weight for 90 days. There were no abnormalities
over the course of the study. Growth, food consumption and behaviour
were normal. Results of clinical laboratory examinations performed 3
times during the course of the study, showed no effects of fenvalerate
in the diet. At the conclusion of the study, data from gross and
microscopic examination of a variety of tissues and organs
substantiated clinical data, again showing no effects of dietary
fenvalerate. Daily administration at a level of 12.5 mg/kg body
weight for a period of 90 days produced no detectable evidence of
toxicologic effects (Hart and Wosu, 1975).
Long Term Studies
Mouse
Groups of mice (from 35 to 47 male and female, ddY strain, mice/group)
were administered fenvalerate in the diet for 78 weeks at dosage
levels of 0, 100, 300, 1000 and 3000 ppm. An interim (3 months) and a
final report showed dose-related effects at 300 ppm and above.
Fenvalerate was not carcinogenic to the ddY strain of mouse (Suzuki
and Kadota, 1976; Ito, 1976a, 1978; Suzuki et al., 1977b).
At the early stages of the study, mortality was evident at the highest
dose level. Growth was reduced at 1000 ppm and above. These effects
were accompanied by excitability, hypersensitivity and other
behavioural changes in the first month of feeding. A variety of
hematological parameters were affected during the first three months
of study, predominantly at the high dose level. Several biochemical
changes were observed including a decrease in alkaline phosphatase, an
increase in blood urea nitrogen, an increase in leucine aminopeptidase
activity and a decrease in cholesterol and glucose occurring at 1000
and 3000 ppm with several of these parameters being affected at 100
ppm (glucose decrease in females). Gross examination of tissues and
organs showed a weight increase in several tissues of both males and
females exposed to the high dose level. Microscopic examination
suggested changes in the liver at the two highest dose levels.
Multiple small necrotic foci in the liver and changes in the
epithelial cells of the proximal convoluted tubules were noted,
apparently related to the presence of fenvalerate in the diet (Suzuki,
et al., 1976; Ito, 1976a).
At the conclusion of the 18-month study, animals were sacrificed for
terminal haematologic and biochemical changes as well as gross and
microscopic examination of tissues and organs. Over the 18 month
study, growth was depressed at 3000 ppm in both males and females.
Behavioural changes noted as transient hypersensitivity occurred at
1000 ppm and above. Increased mortality was also evident at the two
highest dose levels. No compound-related changes were noted with
respect to haematologic parameters, although clinical chemistry
parameters were in some instances substantially increased (SGPT, SGOT,
LDH and LAP activities in both sexes were significantly increased,
predominantly at the two highest dose levels but sporadically at 300
ppm). Gross changes were noted in several organ weights and organ to
body weight ratios primarily in liver, although changes were also
noted in the kidney, heart, spleen, pituitary and ovary at the highest
dose level. These gross changes were not accompanied by
histopathological events with the exception of granulomatous changes
in liver and the mesenteric lymph nodes noted at all treatment groups,
predominantly at the highest two dose levels. Biochemical changes,
noted at the three month sacrifice interval, were not seen at the
conclusion of the study (increased BUN, decreased glucose, etc.).
There were no indications in this study of increased tumorigenicity or
carcinogenicity as a result of fenvalerate (Suzuki, et al., 1977b).
Rat
Groups of rats (15 male and 15 female Wistar rate per group) were fed
at concentrations of O, 50, 150, 500 and 1500 ppm for 15 months in the
diet. There was no mortality in the study attributable to
fenvalerate. Growth, food consumption and behavioural changes were
significantly affected at the highest dose level. Hypersensitivity
was observed at the early stages of the experiment disappearing within
3 months. Growth was significantly depressed in both males and
females at the highest dose level. Food consumption was unaffected
over the course of the study. Clinical examinations, performed at
either one year (urinalysis) or at the conclusion of the study
(hematology, blood biochemistry and gross and microscopic pathology),
showed significant abnormal values in a variety of parameters at the
1500 ppm dose level. No opthamologic effects were noted. Urinalysis
was normal over the course of the study. Haemoglobin concentration
was depressed in males at the highest dose level and the females at
150 ppm and above. Blood biochemistry was significantly altered at
1500 ppm with respect to several parameters (BUN in both males and
females; protein and cholinesterase in females). Gross and
microscopic examination of tissues and organs including specific
sections of sciatic nerve and trigeminal ganglia and nerve showed no
dose-related effects. Generalized inflammatory and degenerative
changes were seen in both control and treated animals. Tumor
incidents were low and not related to the presence of fenvalerate.
There was no suggestion of a carcinogenic potential observed in the
study (Suzuki, et al., 1977a).
Groups of rats (93 male and 93 female Sprague-Dawley rats/group, 183
of each sex were used as the control and an additional 22 of each sex
were used as a separate control and high level group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25, 250 and 500
ppm. There was no mortality associated with the study although growth
was reduced at the 500 ppm dose level. The 500 ppm group and a
separate control group were sacrificed at 26 weeks while the other
animals were maintained for 2 years. There were no significant
effects on food consumption, growth or on behaviour at 250 pm.
Hematology, clinical chemistry tests and urinalyses, performed at
various time intervals over the course of the study on at least 10
animals of each sex at each dose level, showed no dose-related
effects. At the conclusion of the study, organ weight and organ to
body weight ratios were normal. Gross and microscopic examination of
tissues and organs did not differ significantly from controls. Benign
and malignant lesions occurred at random throughout all groups
examined at the end of the study and in those animals that died during
the course of the study. There were no lesions attributable to
fenvalerate. A specific pathology examination of the sciatic nerve of
animals fed 250 ppm was performed. The sciatic nerve was not found to
have been significantly affected by fenvalerate (Gordon and Weir,
1978; Lambert, 1977).
Observations in Man
Fenvalerate was administered dermally to adult men and women to the
skin of the arm or face at dosage levels of approximately 20-40 mg.
Control applications were carried out on the same individuals and
subjective evaluations were performed with respect to dermal
irritation. There was no erythema or other visible skin effects and
an evaluation of the subjective responses suggested no significant
differences between fenvalerate-treated and the control portions of
the body (Hine, 1976).
A double-blind study utilizing 29 male volunteers was performed to
test the skin reaction of formulated fenvalerate. The emulsifiable
concentrated formulation was diluted with water and administered to
one side of the face, on the cheek, with a control formulation applied
to the opposite cheek.
There were no signs of dermatitis noted at 24 hours following
administration. Subjective analyses of irritation or skin sensation
were performed with each individual. Under the conditions of the
study, the formulation of fenvalerate did not produce abnormal skin
sensations. There were no indications that any of the symptoms noted
(which included tingling, burning, stinging, itching, swelling,
numbness or heat) was associated with fenvalerate (Brown and Slomka,
1979).
COMMENTS
Fenvalerate, an ester related to the pyrethroids, currently being
developed for use as an agricultural insecticide, is rapidly absorbed
in mammals, widely distributed, and metabolized. Tissue residues of
fenvalerate and its ester metabolites appear to concentrate to some
degree in adipose tissue. Fenvalerate undergoes several major
metabolic reactions: cleavage of the ester linkage, hydroxylation of
the acid and alcohol moieties, and conversion of the CN group to SCN
and CO2. The metabolic fate in all animals studied appears to be
similar. Photolytic degradation has been shown to produce a
decarboxylated product not known to occur in mammals. Fenvalerate is
moderately toxic when administered by the oral route. The metabolic
and photolysis products are less toxic than the parent ester.
Studies on the mutagenicity and reproductive/teratogenic potential
were negative. Studies on neurotoxicity have shown that, following
high level exposure, rats showed reversible clinical signs of ataxia.
Microscopic examination of the sciatic nerve showed axonal swelling
and myelin disruption. Biochemical studies revealed an increase in
lysosomal enzyme activity (ß-glucuronidase and ß-galactosidase) in
peripheral nerve (see Report Section 3.5). Fenvalerate, when
administered to hens at high levels, did not induce signs of
peripheral neuropathy. No data were available to assess the
susceptibility of humans to this neuropathy.
Short-term and long-term studies have been performed in a variety of
test animals. Fenvalerate is not a carcinogen and in short-term
studies in dogs and in long-term studies in rats and mice dietary
no-effect levels have been observed. A temporary acceptable daily
intake for man was allocated. The concerns of the Meeting over the
potential for bioaccumulation, the neuropathy observed in rodents and
the limited information of the susceptibility to such an effect in man
were the basis for the temporary evaluation and for the request for
further observations in occupationally exposed humans and for
pharmacokinetic data. Further information is desired with respect to
an additional dominant lethal assay to provide verification of
existing data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet equivalent to 11.9 mg/kg body weight
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg body weight
Dog: 12.5 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.06 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenvalerate is a highly active broad spectrum insecticide. It is
particularly effective as a contact and stomach poison against
lepidopterous larvae and also has high activity against Orthoptera,
Hemiptera and Diptera. This activity, together with adequate
stability on foliage, and relatively low toxicity to mammals is likely
to lead to increasing use against pests of agriculture.
The substance can be combined with most other insecticides and
fungicides, but not those with alkaline properties. It is mostly
marketed as 20% and 30% concentrates for aqueous dilution; but
granules, ULV concentrates and powers are also available.
Pre-harvest treatments
Fenvalerate is registered and/or approved in many countries in Europe,
Asia, South and Central America, and Australia. It is used against a
wide range of pests, including tobacco bud worm, bollworms, cut worms,
armyworms, leafrollers, weevils, aphids, thrips, beetles, leaf miners,
moths, bugs, psyllas etc and on a wide range of crops including
fruits, vegetables, beans, cereals, cotton and tobacco. For these
purposes it is applied at from 25 to 300 g/ha depending on the crop.
Other uses
It is registered in Argentina for forestry use against Oiketicus.
It is also being developed for control of insect pests of stored grain
and against insects of importance in public health, animal health and
industry.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Many trials have been carried out on a wide variety of fruits,
vegetables, cereals and oilseeds. Most of the data were generated
under American conditions, supplementary data are available from
several other countries. Table 3 contains the summarised data,
representing several thousand separate analytical data provided by the
principal manufacturers.
Since fenvalerate is not systemic in plants, residues usually are very
low in commodities such as root crops, corn kernels and peas which are
protected from direct contact with the insecticide. Residue levels on
plants normally decline with a half-life of about one to two weeks.
Oilseeds
Following multiple applications at rates up to 0.45 kg/ha, and in over
30 supervised trials in various countries (i.e. higher than the
recommended dosage of up to 0.3. kg/ha). Residues in cottonseed were
generally below 0.1.mg/kg, regardless of the interval between last
treatment and harvest. A residue of 0.21 mg/kg was found in a
Colombian trial 1 day after treatment at the rate of 0.3 kg/ha, and
one trial in U.S.A. showed the residues of 0.12 mg/kg (at a rate of
0.22 kg/ha) and 0.19 mg/kg (0.45 kg/ha) 48 days after treatment. When
the seed cases were separated from kernels, the residues of
fenvalerate almost entirely remained on the seed cases and levels in
the oil products were very low.
All trials on peanuts have been made in U.S.A. When fenvalerate was
applied at rates of up to 0.22 kg/ha the residues in whole nuts did
not exceed 0.1 mg/kg. At the higher rate of 0.45 kg/ha however,
residues of 0.20-0.32 mg/kg were detected 14-21 days after
application. It was shown that residues in the meat and oil did not
exceed the detection limit (0.01 mg/kg) and that the shells carried
almost all the residues found in whole nuts. Even when the peanuts
were harvested 73 days after the treatment at 5 and 10 times higher
rates than the recommended dosage (up to 0.22 kg/ha) the residues in
whole nuts were 0.04 mg/kg and 0.09 mg/kg, respectively. In other
trials at rates up to 0.45 kg/ha the residues in hay, foliage and
vines ranged from 0.01 to 31 mg/kg.
Leafy and stem vegetables
Trials have been carried out in U.S.A. and several countries on
lettuce, celery and brassica vegetables such as broccoli, Brussels
sprouts, cabbage, Chinese cabbage, cauliflower, kale and Chinese kale.
The residues were generally less than 1 mg/kg 5-10 days after
treatment at rates ranging from 0.05 to 0.45 kg/ha. Residues of 1.2
mg/kg were found in cabbage and kale 7 days after treatment. In
celery the maximum residue 7 days after treatment was 1.2 mg/kg where
the application rates were within recommended dosage of 0.22 kg/ha.
When lettuce was treated at rates of up to 0.45 kg/ha and harvested 7
days after treatment, the maximum residue was 1.6 mg/ha at application
rate of 0.10 kg/ha except one trial in France in which 5.5 mg/kg was
found 7 days following application at 0.10 kg/ha.
Fruiting vegetables
Residue data for cucumbers are available from U.S.A. The maximum
residue was 0.47 mg/kg 3 days or later after treatment at rates within
the recommended dosage (up to 0.22 kg/ha).
In trials carried out in various countries, 1 to 9 successive
applications were made to tomatoes at rates up to 0.45 kg/ha. The
tomatoes were harvested at intervals ranging up to 40 days after the
last treatment, and the residues were below 0.5 mg/kg in all samples
taken 7 days or more after the treatment.
Root and tuber vegetables
Trials on sugar beets have been carried out in U.S.A., France, Denmark
and U.K. The maximum residue of 0.03 mg/kg was found in roots 9 days
after a single treatment at a rate of 0.15 kg/ha in the Danish trial.
In other trials the residues were not detectable at the limit of 0.01
mg/kg. In beet tops the maximum residue observed was 5.9 mg/kg, 21
days after treatment of 0.45 kg/ha.
Numerous trials have been carried out on potatoes in U.S.A., Canada,
France and Italy. No residues above the detection limit of 0.01 mg/kg
were found in potatoes harvested at 0-84 days following up to 9
applications at rates of 0.015-0.45 kg/ha.
Alfalfa
Residue data on alfalfa were obtained from the trials in U.S.A. where
single or repeat treatments were made at rates up to 0.45 kg/ha. When
alfalfa was harvested 21 days or later after treatments, the maximum
residue of 5.8 mg/kg was found at an application rate of 0.45 kg/ha.
The residues on dry alfalfa with water content of around 10% were
about 3 times higher than those on green alfalfa.
Legume vegetables
Residue data are available from several countries on legume vegetables
including green beans, dry beans, navy beans, snapbeans, pinto beans,
blackeyed peas, peas and soybeans. Residues in soybeans were
generally not detectable when analyzed more than 15 days after
treatment rates up to 0.45 kg/ha. The maximum residue of 0.06 mg/kg
was found 21 days after treatment at 0.08 kg/ha and 0.20 kg/ha. When
the treatment was made within the recommended dosage (0.22 kg/ha) and
the harvest was made 21 days or later after the treatment, the
residues in soybean plants, hay, straw and trash were below 5 mg/kg
except that one residue of 7.3 mg/kg was found after treatment at 0.20
kg/ha in the Australian trial.
Pome and stone fruits
Trials have been carried out on apples, pears and peaches in U.S.A.,
Canada, Australia, Japan and several European countries. In apples,
at application rates up to 0.72 kg/ha, the residues were less than 2.0
mg/kg 21 days or later after treatment except in a trial in U.S.A.
where 2.9 mg/kg was found 21 days after 7 treatments at 0.22 kg/ha.
In two trials in Japan, where 3 or 6 applications were made at a rate
of 1.4 kg/ha, the residues ranged from 0.92 to 3.5 mg/kg.
In pears the residues generally did not exceed 1 mg/kg following up to
7 applications at rates up to 0.67 kg/ha even when the last treatment
was made close to harvest. In one trial where 3 treatments were made
at a rate of 0.67 kg/ha the residue of 1.3 mg/kg was found 13 days
after the last treatment. In the same trial pears were treated with
fenvalerate at a rate of 1.34 kg/ha, and the residue was 1.9 mg/kg 13
days after 3 treatments.
Peaches were treated at rates of 0.1-0.8 kg/ha. The residues reached
2.3 mg/kg after 3 treatments at a concentration of 0.04% ai. Residues
of 2.2 mg/kg were found after 3 treatments at a rate of 0.8 kg/ha and
after a single treatment at a rate of 0.72 kg/ha.
Small fruits and berries
Trials on grapes have been carried out in U.S.A., Canada, Australia,
France and Italy. Residues did not exceed 0.80 mg/kg 14 days after
treatment at rates up to 0.22 kg/ha except two trials in France and
Italy. In Italian trials a residue of 1.3 mg/kg was found 30 days
after treatment at a rate of 0.2 kg/ha. In U.S.A., trials where
grapes were harvested 21 days after treatment at a rate of 0.11 kg/ha,
the residue of 1.6 mg/kg was reported, although in this trial the
residues 1 day and 14 days after treatment were 0.71 mg/kg and 0.51
mg/kg respectively.
In raspberries and strawberries the residues were less than 0.5 mg/kg
when the crops were harvested 7-23 days after treatment at rates up to
0.225 kg/ha.
Cereal grains, pre-harvest
Trials have been carried out in U.S.A., Canada, Australia and Brazil
on sweet corn, field corn, sorghum and wheat.
In sweet corn the maximum residue in kernels was 0.03 mg/kg, even
though 18 treatments were made with the intervals of 2-7 days and the
crops were harvested 2 days after treatment. The residues in stover
and ensilage ranged from 5.5 to 25 mg/kg. In field corn, the interval
between treatment and sampling was more than 72 days, and fenvalerate
was detected only in stover at a level of 0.07 mg/kg 140 days after
the treatment at a rate of 0.22 mg/kg. In wheat, the maximum residue
was 0.29 mg/kg in grain 13 days after treatment at a rate of 0.14
mg/ha and 15.0 mg/kg in straw 21 days after treatment at a rate of
0.30 kg/ha.
Cereal grains, post-harvest
Fenvalerate has undergone laboratory and silo-scale field trials as a
stored-grain protectant in Australia. Studies for this purpose have
been made on wheat, principally, (Bengston, 1979), barley
(Desmachelier, 1978) and sorghum (Bengston et al, 1979). All the
residue data indicate that fenvalerate is persistent under the
temperature and humidity conditions prevailing in Australian storages.
Large-scale trials have been made on stored wheat and sorghum
(Bengston, 1979 and Bengston et al., 1979a). Because of the strategy
of proposed usage fenvalerate has been applied at 1 mg/kg grain in
conjunction with its synergist, piperonyl butoxide (at 8 mg/kg grain)
and a complementary insecticide, fenitrothion (at l2 mg/kg grain).
Under these conditions of storage the fenvalerate residue declines
slowly: after 9-10 months about 75% of the applied amount remains
(Table 8). Expected residues in whole grain cereals would be less
than 1 mg/kg after storage.
Residues in processed products
Some of the cottonseed, peanuts and soybeans obtained from supervised
trials in U.S.A. and Colombia were subjected to processing to produce
oil products. The occurrence of residues in processed products are
summarized in Table 4.
In U.S.A. trials, the residues in various cottonseed oil products
after processing were less than 0.1 mg/kg. In the Colombian
experiment where a single treatment of 0.3 kg/ha was made one day
before harvest, the residue was 0.16 mg/kg, 0.23 mg/kg, 0.22 mg/kg and
0.18 mg/kg in crude, neutral, bleached and deodorised oil,
respectively, in accordance with a residue of 0.14 mg/kg in the
unprocessed cottonseed. These cottonseeds were separated into kernels
and hulls by a mechanical (simulated commercial) process. Such a
procedure might contribute to the higher residues, since the
mechanical processing might have caused contamination of kernels with
hulls, because when these seeds were separated by hand at the
laboratory, residues in kernels and hulls were 0.02 mg/kg and 0.40
mg/kg, respectively. Cottonseeds which were obtained from American
trials were separated by hand, and processed to oil products and no
residues were detected in extracted kernels.
Peanuts and soybeans which were harvested after treatment at rates of
0.11-0.45 kg/ha were processed to oil products and no residues were
detected in either crude or refined oil, or in extracted meal.
Wine made from grapes which contained up to 0.12 mg/kg of fenvalerate
71-74 days after treatment at rates of 0.075 and 0.15 kg/ha (France)
contained non-detectable residues.
Following post-harvest treatment and storage, fenvalerate residue in
wheat is found principally (68-69%) in the bran which comprises about
15% of the whole grain (Table 9). White flour constitutes about 72%
of the whole grain and contains about 8-9% of its fenvalerate residue.
The remaining residue is in the pollard. Residues in flour are
carried over into bread baked from that flour (Table 7b); there is no
reduction in residue level on a commodity-weight basis. White bread
prepared from treated grain would have about the same residue level of
fenvalerate as white flour, that is about 0.06-0.1 mg/kg on present
usage. The corresponding level for wholemeal bread or flour would be
about 0.5-0.8 mg/kg.
Table 3. Summary of Residues Following Field Trials (1976 to 1979)
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cottonseed Various
countries up to 13 0.0115 20 and Figures covering over 30 supervised trials reach 0.1
0.6 30 mg/kg only in isolated instances.
Peanuts (whole) U.S.A. 5 0.11 30 <0.01 <0.01
0.22 " <0.01 <0.01
5 0.22 " <0.01 <0.01 <0.01 <0.01
2 0.45 " 0.04 0.12 0.20 0.29
2 0.45 " 0.06 0.18 0.11 0.11
2 0.22 " 0.03
2 0.45 " 0.32
Peanuts (Vines) 5 0.11 " 0.69 0.01 0.02 0.1
5 0.22 " 1.6 0.02 0.04 0.04
2 0.11 " 6.4
2 0.22 " 3.2
2 0.11 " 0.51
2 0.22 " 0.42
Sunflower Australia 1 0.08 " 0.07 0.03
Brussels sprouts U.S.A. 8 0.045 " 0.13
8 0.09 " 0.04
Canada 3 0.15 " 0.85 0.60 0.45
Netherlands 1 0.045 " 0.04
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cabbage U.S.A. 4 0.11 " 0.03
4 0.22 " 0.01
8 0.055 " 0.37
8 0.11 " 0.46
3 0.11 " 1.2 0.82 0.28 0.76
3 0.22 " 2.4 1.2 0.37 0.45
Canada 3 0.15 " 0.25 0.14
3 0.16 " 0.30 0.02 <0.01
Australia 4 0.073 7.3 0.13 0.15 0.05
New Zealand 6 0.15 10 1.2 0.5 0.9
5 0.15 10 1.3 0.6 0.9
Thailand 1 0.07 20 <0.01 <0.01
Japan 3 0.3 20 0.14 0.011 <0.005
6 0.3 20 0.17 0.028 <0.005
Chinese cabbage Netherlands 1 0.05 30 0.52 0.25
Japan 3 0.15 20 0.25 0.13 0.091
5 0.15 20 0.33 0.084 0.061
Kale U.K. 2 0.075 30 1.5 0.88 0.62 0.26
2 0.225 30 1.8 1.2 0.93 0.53
Thailand 1 0.06 20 1.3 0.13
Cauliflower U.S.A. 4 0.11 20 1.5 0.35 0.15
4 0.22 20 1.8 0.61 0.32
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cauliflower Canada 3 0.16 20 0.28 0.02 <0.01
(cont'd)
Thailand 1 0.07 20 0.17 0.1
Broccoli U.S.A. 2 0.11 20 0.77
2 0.22 20 0.69
Canada 3 0.16 20 0.29 0.09 0.06
New Zealand 5 0.15 10 15.3 5.1 2.2
Celery U.S.A. 15 0.22 10 1.5 0.46
15 0.45 10 3.6 1.9
15 0.055 10 0.11 0.20
15 0.11 10 0.37 0.52
15 0.22 10 1.0 1.2
15 0.45 10 2.0 2.6
Lettuce U.S.A. 7 0.11 10 0.21 0.30 0.07
France 1 0.05 10 2.0 0.35 0.11
1 0.10 10 4.0 1.2 0.47
1 0.10 10 6.9 5.5 2.2
1 0.10 10 2.3 0.45 0.30
1 0.10 10 6.2 1.6 0.06
Netherlands 1 0.075 30 2.25 0.67
1 0.075 30 0.25 0.16
Aubergine France 1 0.11 30 0.08 0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cucumbers U.S.A. 5 0.11 30 0.09 0.04 0.03
5 0.22 30 0.48 0.08 0.03
5 0.45 30 0.54 0.07 0.08
Watermelon U.S.A. 4 0.11 30 0.02
4 0.22 30 <0.01
Bell peppers U.S.A. 3 0.11 30 0.06
0.22 30 0.11
0.45 30 0.39
7 0.22 30 0.19
0.45 30 0.34
Green peppers U.S.A. 2 0.45 30 0.02
Squash U.S.A. 5 0.11 30 0.10 0.02 0.01
Tomato U.S.A. 3 0.11 30 0.02 <0.01
0.22 30 0.07 <0.01
0.45 30 0.08 0.01
2 0.11 30 0.1
0.22 30 0.29
0.45 30 0.33
France 1 0.11 30 0.11 0.47 0.13
Netherlands 1 0.22 30 0.31
Spain 2 0.2 30 0.15 0.10 0.15
3 0.2 30 0.35 0.25 0.15
2 0.3 30 0.2 0.1 0.15
3 0.3 30 0.25 0.10 0.15
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Tomato Australia 9 0.01 20 0.16 0.15 0.19
(cont'd) 0.15 20 0.17 0.12 0.29
0.02 20 0.42 0.38 0.28
Mexico 2 0.15 20 0.04 0.03 0.02 0.03
1 0.01 <0.05 <0.05
South Africa 4 0.02 <0.05 <0.05
0.03 0.07 <0.05
0.04 0.06 <0.05
Mexico 1 0.15 30 0.08 0.05 0.03 0.03 0.01
1 0.3 30 0.13 0.13 0.08 0.05 0.02
3 0.3 30 0.31 0.16 0.19
Canary Islands 2 0.15 30 0.25 0.20 0.07
3 0.15 30 0.40 0.30 0.20 0.08 0.01(71)
Sugar Beets Demnark 1 0.15 20 Roots 0.03
Tops 1.2 0.59
U.S.A. 2 0.45 30 Roots <0.01 1.15(71)
Tops 5.9
(Similar findings in France and U.K)
Potatoes U.S.A.,Canada,
(20 trials) France 1-9 0.07-0.45 30 <0.01 <0.01 <0.01 <0.01 <0.01
No detectable residue found in any samples
Alfalfa U.S.A. 1 0.11 30 6.1 3.2 2.6 1.0
(green) 0.22 30 15 11 8.8 1.9
0.45 30 32 24 18 5.8
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Green beans France 1 0.16 30 0.31
0.21 30 0.67 0.48
0.32 0.43
0.43 30 1.7 0.58
Dry Beans U.S.A. 4 0.11 30 0.21
0.22 30 0.13
Navy Beans U.S.A. 4 0.11 30 <0.01
0.22 30 0.01
Australia 1 0.1 20 0.03 0.03
0.2 20 0.06 0.03
Snap beans U.S.A. 3 0.11 30 0.17 0.03
3 0.22 30 0.26 0.05
3 0.45 30 0.67 <0.01
Pinto beans U.S.A. 1 0.11 30 <0.01
0.22 30
Blackeyed peas U.S.A. 2 0.055 30 <0.01
0.11 30 <0.01
Peas U.S.A. 1 0.11 30 <0.01
1 0.22 30 <0.01
1 0.45 30 <0.01
Soybeans U.S.A. 1 0.11 30 0.06 0.01 <0.01 <0.01 <0.01
1 0.22 30 0.05 0.03 0.02 <0.01 <0.01
Brazil 2 0.12 30 <0.01 <0.01
2 0.24 30 <0.01 <0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Soybeans Colombia 4 0.075 30 <0.01
(cont'd) 4 0.30 30 <0.01
Australia 1 0.08 20 0.06
1 0.2 20 0.06
Asparagus bean Thailand 1 0.14 20 0.14 0.04
(A01-0603)
Apples France 1 0.15 30 0.26 0.37 0.40 0.19
Australia 3 0.02% 30 0.80 0.60 0.35
3 0.04% 30 0.90 0.35 0.35
Germany 6 0.0225 30 1.7 2.0 1.0
6 0.045 30 2.8 2.3 2.0 1.9 1.8
6 0.0225 30 0.96 0.84 0.87 0.56 0.61
6 0.045 30 2.4 2.6 1.8 1.5 1.0
6 0.225 30 2.8 2.4 2.1 1.9 1.6
6 0.045 30 4.8 5.8 3.6 1.3 1.3
Japan 3 1.4 20 1.91 1.76 1.88
6 1.4 20 3.21 3.5 2.88
3 0.72 20 0.6 0.26 0.44
3 0.70 20 0.54 0.42 0.40
New Zealand 12 0.008 10 1.0 0.57 0.57 0.37 0.52
South Africa 6 0.024% 20 0.14 0.07 0.05 <0.05
0.03% 20 0.14 0.08 0.09 0.05
0.048% 20 0.36 0.24 0.16 0.1
0.06 20 0.31 0.24 0.18 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Pears U.S.A. 7 0.11 30 0.11 0.08 0.05
7 0.22 30 0.16 0.14 0.08
3 0.67 30 1.3 0.48 0.60
0.24(62)
3 1.34 30 1.9 1.0 0.47
0.28(62)
2 0.67 30 0.54 0.39 0.30 0.15
France 2 0.1 30 0.32 0.23 0.19 0.15
2 0.13 30 0.40 0.30 0.22 0.14
Australia 3 0.02% 30 0.39 0.46 0.31
0.04% 30 0.56 0.60 0.30
South Africa 6 0.024% 30 0.22 0.08 0.06 0.05
0.03% 30 0.26 0.12 0.12 0.05
0.045% 30 0.35 0.24 0.19 0.12
Apricots U.S.A. 2 0.28 30 0.39
0.56 30 0.78
Cherries U.S.A. 3 0.055 30 1.0 0.66
0.11 30 0.93 2.3
0.22 30 2.9 2.6
Peaches U.S.A. 6 0.11 30 0.30 0.27 0.11
0.22 30 0.26 0.23 0.10
Japan 3 0.8 30 0.084 0.045 0.014
6 0.8 30 0.061 0.026 0.016
3 0.6 30 0.054 0.014 0.020
6 0.6 30 0.046 0.017 0.057
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Australia 3 0.02 30 0.52 0.52
0.04 30 1.3 2.3
Plums U.S.A. 2 0.22 30 1.3
0.45 30 1.1
Grapes U.S.A. 5 0.22 30 1.2 1.1 0.80
4 0.11 30 0.31 0.39 0.45
0.22 30 0.71 0.51 1.6
Canada 1 0.078 30 0.25 0.20
France 1 0.075 30 0.30 0.30 0.55 0.40
2 0.075 30 0.65 0.67
1 0.075 30 0.43 0.09 0.06 0.03
Raspberries Canada 1 0.225 30 0.26
3 0.135 30 0.40
Strawberries U.S.A. 1 0.11 30 <0.01 0.11
1 0.22 30 0.38 0.21
Canada 2 0.172 30 0.07
2 0.12 30 0.45
Citrus fruit Japan 3 0.67 30 0.78 0.89
(whole) 6 0.67 30 1.56 1.25
3 0.67 30 1.69 0.91
6 0.67 30 3.63 1.44
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Spain 2 0.15 30 1.1 1.2 0.85 0.75*
1.9 1.2 1.5 0.9
*Juice
<0.01
Kiwi fruit New Zealand 6 0.46 10 3.4 3.2 2.5 2.1
0.92 10 4.1 3.8 3.6 2.2
Sweet corn U.S.A. 18 0.22 30 0.01
(ears) 18 0.28 30 0.02
4 0.11 30 0.02
4 0.22 30 0.02 <0.01(140)
<0.01(164)
Field corn U.S.A. 1 0.22 4 Gran <0.01(164)
(ears) 1 0.11 30 <0.01(136)
1 0.22 30
1 0.45 30
Sorghum U.S.A. 2 0.11 30 0.17
0.22 30 0.32
0.45 30 0.58
Australia 1 0.075 30 4.0 1.09
0.15 30 4.9 1.70
South Africa 1 0.020 20 0.1 0.1 <0.1 <0.1 <0.1
0.030 20 0.2 0.2 0.1 0.1 <0.1
0.040 20 0.2 0.2 0.1 0.1 0.1
0.050 20 0.5 0.3 0.2 0.2 0.1
0.060 20 0.6 0.3 0.3 0.2 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Wheat Argentina 1 0.10 0.08
0.15 0.05
0.20 0.05
0.30 0.10
Canada 1 0.14 0.29 0.12 0.05
Brazil 1 0.09 0.01 0.02 0.01
Almonds U.S.A. 1 0.22 <0.01
Coffee beans Brazil 1 0.10 <0.06
Table 4. Distribution of Residues in Processed Products of Oilseeds
(Crop treated with 30% E.C. fenvalerate in U.S.A. during 1975-1976)
Cottonseed
Rate (kg/ha) 0.11 0.22 0.45 0.22 0.45
No. of applications 14 8 8 8 8
Pre-harvest intervals 30 6 6 48 48
(days)
Residues in....
Ginned cottonseed 0.05 0.01 0.02 0.12 0.19
Delinted cottonseed - - 0.02 0.01
Hulls 0.01 0.02 0.01 0.01 0.01
Solvent extracted meal - 0.01 0.01 0.01 0.01
Crude oil 0.04 0.01 0,01 0.03 0.05
Refined oil 0.05 0.01 0.01 0.04 0.06
Soapstock 0.01 0.01 0.01 0.01 0.01
Peanuts
Rate (kg/ha) 0.11 0.22 0.45
No. of application 2 2 2
Pre-harvest interval (days) 14 14 14
Residues in....
Whole nuts 0.01 0.02 0.02
Nut meats 0.01 0.01 0.01
Shells 0.03 0.04 0.02
Solvent extracted meal 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01
Refined bleached oil 0.01 0.01 0.01
Refined bleached
Hydrogenated oil 0.01 0.01 0.01
Soybeans
Rate (kg/ha) 0.11 0.22 0.45 0.22
No. of application 1 1 1 4
Pre-harvest interval(days) 66 66 66 48
Residues in....
Soybeans 0.01 0.01 0.01 0.02
Hulls 0.01 0.01 0.02 0.06
Solvent extracted meal 0.01 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01 0.01
Soapstock 0.01 0.01 0.01 -
FATE OF RESIDUES
Cows
Three lactating cows were fed daily doses of C-14-fenvalerate
(chlorophenyl ring label) at a level equivalent to 0.11 ppm in the
total diet for 21 days and sacrificed 12 hours after receiving the
last dose. Recovery of radiocarbon was about 65% of the administered
dose of which 29% was recovered in the urine, 36% in the faeces and
less than 1% in the milk. The radiocarbon in the milk ranged from
<0.0006 to 0.0019 µg equivalents/L and reached a plateau after one
week on the treated feed.
Nearly all of the radiocarbon in the milk was present as unchanged
fenvalerate. No detectable radiocarbon was found in the brain, fat,
kidney, liver, lung and muscle. The limit of determination in this
study was 0.02 ppm for fat and 0.01 ppm for other tissues (Potter,
1976a).
In a separate study, three lactating cows were fed 10.9 ppm
C-14-fenvalerate (benzyl ring label) in their total ration for 28
days. The C-14 residues in the milk collected during the last 24 days
of treatment ranged from 0.04 to 0.13 ppm equivalents. The
fenvalerate content of the whole milk ranged from 0.037 to 0.082 mg/kg
during the last 20 days of the test. The cows were sacrificed 12
hours after receiving the last dose. The highest tissue residues were
found in the mesenteric fat 0.74-0.79 mg/kg equivalent. The
gastrocnemius and quadriceps muscle contained up to 0.06 mg/kg
equivalents. A total of 92% of the C-14 administered to the cows was
recovered in the excreta and tissues (Potter and Arnold, 1977;
DeVries, 1976a).
Three lactating Guernsey cows were fed daily doses of C-14-fenvalerate
at 0.15 ppm in their total ration for 21 days. The cows were
sacrificed 12 hours after receiving the last dose. The C-14 in the
milk ranged from 0.0006 to 0.0018 mg/kg equivalents. The C-14
residues in the milk reached a plateau after one week on the treated
feed. Within the experimental error of the measurements all of the
C-14 in the milk was present as fenvalerate. No C-14 was found in
bone, brain, kidney, lung or muscle (limit of detection = 0.004 to
0.007 mg/kg equivalents). Traces of C-14 were found in mesenteric fat
and subcutaneous fat from two cows and in the liver from one cow
(Potter, 1976b).
Lactating cows were fed an equivalent of 0.15 ppm C-14 fenvalerate in
their daily ration for 21 days. Residues of 0.1-0.2 mg/kg of
fenvalerate were found in the cream by TLC and liquid scintillation
counting. These residues were confirmed as fenvalerate by GLC-EC
(Potter, 1976e),
Three dairy cows were each sprayed weekly for three successive weeks
at the estimated rate of 540 mg active material per square metre.
This spraying regime was proposed for ectoparasite control. Samples
of whole milk taken during the treatment period and up to one week
after the last application contained less than 0.01 mg/kg of
fenvalerate. No detectable residue was found in the brain, muscle,
liver and kidney. The fat contained up to 0.2 mg/kg fenvalerate
(DeVries, 1976b).
In a further study (DeVries, 1976c), the same spraying regime was
applied to 10 cows. Residues in cream reached a maximum level of 0.2
mg/kg three days after the last application. The elimination of these
residues followed first order kinetics with a half-life of 4.5 days.
Residues in body fat reached a maximum of 0.4 mg/kg several days after
the last application, and declined thereafter according to first order
kinetics with a half-life of 10.5 days. All other tissues tested were
free of detectable residues.
Fenvalerate was also applied as a spray at a rate of 0.2, 0.4 or 2.0
g/cow. The residues in muscle did not exceed 0.01 mg/kg. The maximum
residue in subcutaneous fat was 0.22 mg/kg 7 days after 3 treatments
at a rate of 2.0 g/cow. The maximum residue in milk was 0.02 mg/kg.
The residues in milk fat reached maximum a few days after treatment
(Noble, 1976a; DeVries, 1976d, 1976e, 1976f, 1976g).
A trial was conducted in Queensland, Australia, to determine the level
and fate of fenvalerate in cows following spray treatment with 0.1%
and 0.2% of fenvalerate at 200 ml per animal. Twenty-seven Hereford
heifers were used in the trial. Twelve animals were drafted to each
treatment group with three being kept as untreated controls. Three
animals in each treatment group were re-treated 7 days after the first
treatment. These animals were then slaughtered and sampled 7 days
following the second treatment to ascertain whether there was any
accumulation. Tissue samples were taken from omental fat, liver,
perirenal fat# kidney and muscle. The residues found in the samples
are indicated in Table 5 (limit of determination 0.01 mg/kg). Only
representative samples of non-fat tissues were analysed when
preliminary results indicated no detectable residues in liver, kidney
and muscle. (Shell Chem. Australia, 1978).
Six dairy cows were selected and randomly divided into two treatment
groups of three animals. Milk samples were collected prior to
treatment. Treatment at the rate of 200 ml/animal was made along the
dorsal midline. One group received spray of approved strength (0.1%
fenvalerate) and the other at double strength (0.2%). One litre
aliquots of milk were collected from each cow at each milking on days
1, 3, 7, and 10 post-treatment. These samples were separated and the
cream and skim milk portions were analysed separately. The results
are given in Table 7. The residues were confined to the butter fat.
A commercial dairy herd was treated with fenvalerate emulsion (0.1%
fenvalerate) at the rate of 200 ml/cow. Samples of the bulked milk
were taken prior to treatment and on days 1, 3, 7 and 10
post-treatment. These samples were separated and the cream and skim
milk analysed for fenvalerate residues. The results are given in
Table 6. The residues were confined to the butter fat.
Table 5. Residues in tissues of 27 Cows following Spray Treatments
(Queensland, Australia)
Residue mg/kg
Treatment of Day Omental Perirenal Liver, kidney
each cow fat fat muscle
untreated 0 <0.02 <0.02 All Samples
untreated 0 <0.02 <0.02 <0.01
untreated 0 <0.02 <0.02
0.2% a.m. 1 0.04 0.06
0.1% 1 0.05 0.03
0.1% 1 <0.02 0.03
0.2% 1 0.06 0.08
0.2% 1 0.08 0.04
0.2% 1 0.04 0.06
0.1% 3 0.02 0.02
0.1% 3 0.04 0.05
0.1% 3 0.02 0.03
0.2% 3 0.03 0.03
0.2% 3 0.04 0.03
0.2% 3 <0.04 <0.02
0.1% 7 0.04 0.04
0.1% 7 0.04 0.03
0.1% 7 0.05 0.10
0.2% 7 0.05 0.06
0.2% 7 0.07 0.05
0.2% 7 0.05 0.08
0.1% 14 0.05 0.06
0.1% 14 0.08 0.07
0 1% 14 0.10 0.08
0.2% 14 0.06 0.07
0.2% 14 0.13 0.07
0.2% 14 0.17 0.08
Table 6. Residues found in Butterfat of Bulked Milk of Commercial
Herds (Each cow treated once with fenvalerate emulsina).
Days after
treatment ppm fenvalerate
0 <0.005
1 0.015
3 0.066
7 0.046
10 0.030
Skim milk contained <0.002 ppm fenvalerate.
Table 7. Residues in Milk From Each of Six Cows Treated Once with Fenvalerate Emulsion
Animals sprayed at 1% Animals sprayed at 2%
Days after
treatment 1 2 3 Mean 4 5 6 Mean
Residues of fenvalerate in mg/kg of moisture-free butter fat
(Pre-treat)
0 (AM) <0.005 <0.005 <0.005 - - - - -
0 (PM) 0.016 0.041 0.006 0.02 0.011 0.008 0.011 0.019
1 (AM) 0.022 0.032 0.026 0.027 0.012 0.034 0.027 0.024
1 (PM) 0.023 0.044 0.025 0.031 0.036 0.029 0.034 0.050
3 (AM) 0.046 0.047 0.030 0.042 0.133 0.059 0.047 0.080
3 (PM) 0.031 0.037 0.019 0.029 0.094 0.050 0.046 0.063
7 (AM) 0.012 0.043 0.022 0.026 0.043 0.025 0.040 0.036
10 (AM) 0.016 0.029 0.022 0.022 0.019 0.029 0.046 0.031
Skim milk (residue after butter fat removal) contained less than 0.002 ppm.
Table 8. Fenvalerate Residues in Treated Wheat and Sorghum Stored in Concrete Silos
Grain Amount of Residues (mg/kg)
fenvalerate
Kind moisture Temperature applied calc'd Storage period (months)
% °C (mg/kg)
0.25 1.5 3 4.5 6.5 8 10
(a) Wheat1
range 10 31-25* 0.9-1.1 0.85-1.16 0.45-0.87 0.76-1.02 0.66-0.82 0.79-0.93 0.74-0.85 0.71-0.76
mean 1.01 1.01 0.7 0.9 0.76 0.86 0.8 0.74
(b) Sorghum2
range 26-24* 0.42-0.55 0.64-0.72 0.53-0.54 0.64-0.69
mean 11.8 0.53 0.68 0.54 0.67
* 1st figures are initial temperatures; 2nd are those 6-9 months later.
Wheat data are from two trial sites, one in each of two Australian States.
1 Bengston, 1979
2 Bengston et al., 1979
Table 9. Distribution of Fenvalerate Residues in Whole Wheat
Fractions and Residue Levels in Bread Baked from the Flour (B.W.
Simpson, 1979)
(a) Distribution of residue between whole grain fractions
Fraction fenvalerate residue
% whole mg/kg % distribution
grain
flour (white) 74.6 0.08 8.5
pollard 14.6 3.3 68.5
bran 10.8 1.5 23
Grain sample: 1.5 kg. It had a residue level of 0.6 mg
fenvalerate/kg.
(b) Residue in bread baked from the ground flour from (a)
Commodity fenvalerate residue (mg/kg)
White flour 0.08-0.09
White bread 0.06-0.1
Wholemeal flour* 0.7-0.8
Wholemeal bread 0.49-0.73
* reconstituted from the fractions (in (a) ) in the original whole
grain proportions.
The processing operations simulated those used in commercial practice.
Table 10. Residues of Fenvalerate Degradation Products in Field-Treated Crops in Canada
S-Phenoxy Fenvalerate Isovaleric "Reverse Decarboxylated
Benzoic Acid Amide Acid Ester" Fenvalerate
Crop Country Dose Rate (×) PHI Fenvalerate WL 44607* WL 47117+ WL 10944* SD53036 SD 54597
Apples Canada 70 g/ha (×1) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
16 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
150 g/ha (×8) 6 weeks 0.29 <0.05 <0.05 <0.05 <0.01 <0.05
6 weeks 0.57 <0.05 <0.05 <0.05 <0.01 <0.05
Pears Canada 30 g/ha (×3) 5 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
70 g/ha (×2) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
Peaches Canada 0.006% 0 weeks 0.4 <0.05 <0.05 <0.05 - -
1 week 0.25 <0.05 <0.05 <0.05 - -
2 weeks 0.10 <0.05 <0.05 <0.05 - -
Grapes Canada 0.006% 0 week 0.45 <0.05 <0.05 <0.05 - -
1 week 0.38 <0.05 <0.05 <0.05 - -
Cabbage Canada 70 g/ha (×7) 2 weeks 0.06 <0.05 <0.05 <0.05 <0.01 <0.05
Cauliflower 70 g/ha 1 day 0.20 <0.05 <0.05 <0.05 - -
14 days 0.02 <0.05 <0.05 <0.05 - -
B. sprouts Canada 70 g/ha (×6) 14 days 0.10 <0.05 <0.05 <0.05 - -
70 g/ha (×7) 1 day 0.14 <0.05 <0.05 <0.05 - -
Lettuce Canada 70 g/ha 7 days <0.01 <0.05 <0.05 <0.05 - -
Tomatoes Canada 150 g/ha (×2) 2 days 0.80 <0.05 <0.05 <0.05 - -
* Includes both free and conjugated forms
+ No conjugates formed
Hens
Mature laying hens were fed 0.03 ppm of C-14-fenvalerate (chlorophenyl
ring label) in their total ration for periods up to 32 days. No
detectable C-14 residues were found in the fat, heart, gizzard, liver,
meat, skin, egg whites and egg yolks (Potter, 1976c).
In a separate study, mature laying hens were fed 0.03 ppm of
C-14-fenvalerate (phenoxyphenyl ring label) in their total ration for
periods up to 32 days. No C-14 residues were detected in the light
meat, dark meat, skin, gizzard, blood or plasma. The residues in egg
yolks ranged from <0.002 to 0.003 mg/kg, fat samples <0.002 to 0.003
mg/kg (Potter and Sauls, 1978).
Laying hens were sprayed twice with a 0.5% emulsible concentrate of
fenvalerate. Fenvalerate residues in the eggs reached maximum levels
of 0.04 mg/kg 6 days after the first application and of 0.14 mg/kg 8
days after the second application. The residue levels in the eggs
decreased by one half in about 22 days. The major tissue depots for
fenvalerate were found to be the skin and fat (DeVries, 1976d).
Plants
Leaves of cotton were treated either once with a formulation of
C-14-fenvalerate (chlorophenyl ring label) at a rate of 240 ug per
leaf, or twice at a rate of 120 ug per leaf per treatment.
Determination of C-14 in extracts showed that the rate of C-14
disappearance from leaves was practically identical between single and
repeated applications, and a disappearance rate of about 50% was 35
days. Thin-layer chromatographic analysis of extracts from the cotton
leaves showed that fenvalerate represents about 50% of the C-14
recovered 101 days after single treatment and 80% of the C-14
recovered 46 days after the last of double treatments (Loeffler, 1976a
and 1976b).
In a separate study, cotton squares were also treated either once with
a formulation of C-14-fenvalerate (chlorophenyl ring label) at a rate
of 240 µg per square or three times at a rate of 120 µg per square per
treatment. Whole seeds isolated two to three months after the first
treatment contained less than 0.05 mg/kg equivalents of fenvalerate.
Single leaves of cotton, located either on the lower or the upper part
of the plants, were treated with an average of 49.7 µg per leaf to
determine the transport of C-14-fenvalerate from the treated to
untreated leaves. After 15 days, no C-14 was found in any of the
untreated leaves both above and below the treated leaves,
demonstrating the inability of fenvalerate or any possible C-14
metabolites to move out of a treated leaf either in the xylem or in
the phloem (Loeffler, 1976 c and d).
When C-14-fenvalerate labelled in either benzyl or chlorophenyl ring
was applied to lettuce grown in boxes (twice at a rate of 10.8 mg/box)
about 70-80% of the C-14 in the mature plants was present as unchanged
fenvalerate 12 days after the second treatment. Fenvalerate
hydrolysed slowly at the ester linkage to give 3-phenoxybenzoic acid
and 2-(4-chlorophenyl) isovaleric acid which then rapidly conjugated
with plant materials (Hitchings, 1977). When the same compounds were
applied to the fruit and leaves of apple trees twice or three times,
the leaves and apples harvested 3 to 4 weeks following the last
treatment were found to contain mainly unchanged fenvalerate (75 -85%
of the total C-14 in leaves and 86-93% of the total C-14 in apples).
After peeling the apples, less than 2% of the C-14 in the apples
remained in the peeled fruit. The major metabolite fraction (11-19%
of the total C-14) in leaves was a mixture of polar compounds which by
hydrolysis produced 3-phenoxybenzoic acid, 3-phenoxybenzaldehyde,
3-phenoxybenzyl alcohol and 2-(4-chlorophenyl) isovaleric acid. Other
minor metabolites identified in leaves and/or apples were des-cyano-,
amide- and 4'-OH-fenvalerate which accounted in total for less than 2%
of the total C-14. (Standen, 1977).
Fenvalerate and the (S)-acid isomer similarly disappeared from
cabbages and bean plants with half-lives of approximately 9 days and
14 days respectively, after foliar application of three C-14-labelled
preparations (Co, Ca and CN) at rates of 17-19 µg (cabbage) and 10 µg
(bean plant) per leaf. On and/or in plants, both compounds underwent
decarboxylation, ester cleavage, hydrolysis of the CN group to CONH2
and COOH groups, hydroxylation at the 2'-and 4'-phenoxy positions,
cleavage of diphenyl ether, conversion of a-cyano-3-phenoxybenzyl
alcohol to the corresponding benzyl alcohol and benzoic acid, and
conjugation of the resulting carboxylic acids and alcohols with sugar.
Very little of the C-14 was transferred to other parts of cabbages and
bean plants. Beans were planted in Kodaira light clay and Katano
sandy loam soils after treatment with 1.0 mg/kg of the CN- and
Ca-labelled preparations. After 30 days, pods and seeds, shoots and
roots were harvested and analyzed for C-14. Although small amounts of
radioactivity were found in roots, traces were present in pods, seeds
and shoots. No parent compound was detected in shoots (Ohkawa et al.,
1979a).
Search for degradation products on field grown crops
A series of trials were conducted in Canada (Shell 1979) to determine
whether and to what extent degradation products occur as free and
conjugated residues in crops grown under typical outdoor conditions.
As indicated in Table 8, none of the known degradation products could
be detected by methods sensitive to 0.05 mg/kg in any of 9 varieties
of fruits and vegetables treated in accordance with label directions.
Fenvalerate itself occurred in most of the samples at levels
consistent with those found in other trials.
Distribution of residues on the plant
From the examination of many plants treated under practical
conditions, it is evident that residues are almost exclusively
confined to those parts of the crop that are directly exposed to the
spray application. As examples, cottonseed kernels and peanut meats
have not contained detectable levels even when quite high amounts were
measured on the whole seeds or nuts (Shell Res. 1974, 1975 a and b,
1976 a and b, Shell Dev. 1976 a and b, 1977a, 1978b).
Trials on cabbage in Australia gave residues at 7.5 mg/kg for outer
discarded leaves and only at 0.05 mg/kg for cabbage hearts 5 days
after treatment at 0.118 kg ai/ha (Microchem., 1977). In another
trial, when tops of sugar beets contained up to 5.9 mg/kg, only trace
residues (0.03 mg/kg or less) were detected in roots (Statens, 1977;
Shell Develop., 1977b). Trials on Japanese radish in Japan showed
residues up to 1.97 mg/kg for leaves and only 0.005-0.04 mg/kg for
roots (Fujita et al., 1978a).
Similarly, in trials with peas, beans, citrus fruit, almonds, sweet
corn and wheat, the main part of the residues occurred on peel, skins,
pods and other exposed parts. Residues in edible portions rarely
exceeded detectable levels. (Shell Dev. 1976, 1977; Fujita et al.,
1978; Japan Food Res. 1977).
Photochemical degradation
When exposed to sunlight on silica gel, glass, soil and in water,
fenvalerate degraded in all these media. After 28 days of exposure to
sunlight about 25% was recovered from silica gel, 48% from soil or
from water, less than 1.3% from glass (FAN 1976).
When a 0.2 M solution of fenvalerate was exposed for 10 hours at 300
nm in quartz SD54597 (Benzenepropanenitrile,
4-chloro-beta-(1-methylethyl)-alpha-(3-phenoxyphenyl)-I was the major
product. Other amounts to less than 1% of the total. At 254 nm
SD53036 (Benzoic acid, 2-phenoxy-, 1-(4-chlorophenyl)-2-methylpropyl
ester) was also formed equivalent to about 3% of SD54597 present and
other products were less than 1% of the total (Ehmann, 1978a and b).
In various solutions (hexane, methanol, and acetonitrile-water; 6/4)
by artificial light (>290 nm) and as a thin film on grass or on
cotton by exposure to sunlight, the major products in solution were
formed via photoinduced decarboxylation. Other products resulted from
cleavage of the ester linkage, probably by a mechanism involving
free-radical intermediates. These were 3-phenoxybenzoyl cyanide,
3-phenoxybenzyl alcohol and 3-phenoxybenzaldehyde from the alcohol
moiety, and 2-(4-chlorophenyl) isovaleric acid and the dimer of the
acid fragment from the acid moiety. Decarboxylated fenvalerate,
2-(4-chlorophenyl) isovaleric acid, 3-phenoxybenzyl alcohol and
3-phenoxybenzaldehyde were also found on cotton. (Holmstead, et al.,
1977, 1978).
Another photodecomposition study reveals that on exposure to sunlight
fenvalerate was rapidly decomposed in distilled water, 2% aqueous
acetone, river and sea water to almost the same extent.
Photodegradation half-life in each aqueous suspension ranged from ca.
4 days in summer to 13-15 days in winter, due to seasonal variation of
sunlight intensity. On soil surfaces, exposed to sunlight, the rate
of photodegradation was dependent upon the soils used, with half-lives
of about 2, 6 and 18 days in Kodaira light clay, Azuchi sandy clay
loam and Katano sandy loam soils, respectively. Thus photodegradation
rate and pathways were dependent on the environmental conditions. The
predominant reactions were decarboxylation and cleavage of ester
linkage in water, while hydration at CN group was dominant on the soil
surface. (Mikami et al., 1979).
Soil
When C14-fenvalerate (chlorophenyl ring label) was applied to steam
sterilized and live sandy loam soil at a concentration of 2.5 ppm, the
amount of solvent extractable C-14 decreased slowly in aerobic and
anaerobic soils and stayed practically constant in sterile soils. The
proportion of intact fenvalerate in the extracts from aerobic and
anaerobic soils decreased to 90% over a three-month period. The
remaining 10% was present in the form of two to three products more
polar than fenvalerate (Loeffler, 1976a). Degradation of the same
labelled compound was examined under aerobic and anaerobic conditions
using three different soils and a steady decrease of recovery in the
organic solvent-extractable fraction were observed both under aerobic
and anaerobic conditions (Lee, 1978). Several of the breakdown
products of fenvalerate formed during the course of disappearance of
the parent compound were identified (Fan 1977). The effects of soil
micro-organisms were investigated and it was demonstrated that
sterilization of soil greatly retards degradation (Ohkawa et al.,
1978; Williams and Brown 1979; Noble, 1976).
The leaching behaviour of fenvalerate from soil was also investigated
(Ohkawa et al., 1978a; Jackson and Roberts, 1976; Kerritt, 1977).
Fish
In tests in which Channel catfish and rainbow trout were exposed to
dilutions of C-14 fenvalerate in water, rates of uptake and
distribution in the bodies of fish were followed and some metabolites
were identified (Potter 1975, 1976; Ohkawa, et al., 1978, 1979). The
results gave no evidence to suggest that problems may arise from the
concentration of residues in the bodies of fish that are used as human
food.
METHODS OF RESIDUE ANALYSIS
GLC equipped with electron-capture detector (ECD) has been used almost
exclusively for detection and quantitation. The ECD exhibits high
sensitivity to fenvalerate, responding to as little as 0.05 ng or even
less (Chapman et al., 1977), which afforded minimum detectability of
0.005-0.02 ppm (Takimoto et al., 1977; Talekar 1977; Shell Dev.
1976a). As a radioactive source, nickel-63 appears more advantageous
than tritium due to the relatively high temperature applied. Although
the alkali flame ionization detector (AFID) was also utilized for
confirmation of residue identity, the response of fenvalerate to
GLC-AFID was found to be about 100 times less than GLC-ECD. The
confirmation can be made by combined gas-liquid chromatography/mass
spectrometry (GLC/MS) using selective ion detection mode at mass peaks
such as 225 amu and 419 amu (Parent ion) (Shell Dev. 1976a).
Since fenvalerate comprises 4 optical isomers due to 2 chiral centres,
its GLC gives either single or resolved peaks depending upon the
column length, the column-packing materials, etc. A single peak
response was obtained by using 45-60 cm-long glass or stainless steel
column packed with 3% OV-101 on Gas-chrom Q (Shell Dev. 1976b), 3%
OV-101/3% Apiezon Grease L on Gas-chrom Q (Takimoto et al., 1977;
Talekar 1977), 5% OV-17 on Chromosorb W AW DMCS (Kato 1979), and 3%
SF-96/3% Apiezon Grease M on Diatomite CLQ (Woodhouse et al., 1979).
On the other hand Lee et al., (1978) observed two completely resolved
peaks for fenvalerate on 1.8 m-long glass column packed with 4%
SE-30/6% QF-1 on Chromosorb W (80/100 mesh) where the RR and SS
isomers with identical chromatographic properties were eluted after
the RS and SR pair. A complete resolution for fenvalerate was also
achieved on 3 m glass column packed with 3% OV-101 on Chromosorb W AW
DMCS (100/120 mesh) (Kato et al., 1978).
When exposed to a relatively high temperature in the GLC system (e.g.
more than 250°C at injection port), fenvalerate may undergo partial
degradation and/or isomerization depending upon the column material,
the glass wool packed into the injection port, and the column-aging
history (Barber 1976a). Therefore, care must be taken in the
simultaneous analysis of the diastereomers. The effects caused by
degradation are reproducible and can be minimized to insignificant
levels for quantitation.
The high-pressure liquid chromatography (HPLC), which permitted less
than 3 ppm of detection limit in one study (McKinney 1975), has been
applied to the residue analysis of fenvalerate. It has been used
successfully to determine residues in cereal whole grains and
commodities from the grains, such as flour and bread (Simpson 1978).
The problems with indeterminate differentiation of fenvalerate
diastereoisomers and breakdown on GLC columns were not found with HPLC
separation. The post-harvest residue data on treated cereals, flour
and bread reported in this monograph have been obtained by HPLC
determination. It was found that the limit of determination was in
the region of 0.02 mg/kg.
The solvent or solvent combinations selected for extraction of
fenvalerate vary according to the nature of substrates. From the
substrates with high moisture contents like fruits, leafy vegetables
and root crops fenvalerate was successfully extracted by chopping and
blending them either with polar solvents, e.g. acetonitrile (Lee et
al., 1978) and methanol-acetonitrile (Takimoto et al., 1977), acetone
(Chapman and Harris, 1978), or with nonpolar-polar solvent mixtures,
e.g. n-hexane-isopropanol (Shell Develop. 1976b) and petroleum
spirit-acetone (Shell Res. 1976). Talekar (1977) adopted a 12-hour
Soxhlet extraction with n-hexane-acetone for chopped cabbage. Dry and
low-moisture products are first fractured or ground to powder, under
freezing if necessary, prior to extraction with either polar, nonpolar
or dual solvent system (Shell Res. 1975). After mixing with anhydrous
sodium sulphate, cottonseeds were fractured, separated from the hull,
and blended with n-hexane (Shell Develop. 1976c), whereas coffee beans
were pulverized and macerated overnight with methanol (Kato 1979).
The blending/extraction with nonpolar solvents like n-hexane resulted
in excellent recoveries of fenvalerate with satisfactory separation
from fatty materials in the case of mammalian tissues, dairy products
and eggs (Shell Develop. 1976a).
Cleanup procedures include partition between immiscible polar and
nonpolar solvents, e.g. acetonitrile and n-hexane, for most extracts
from oily and dehydrated crops as well as from fatty products prior to
further Florisil column chromatography. Chapman and Harris (1978)
partitioned the aqeous acetone extract from a range of vegetables into
n-hexane before proceeding to cleanup on silica or alumina absorbents.
The combined solvent partition-Florisil column chromatography
procedure provided recoveries of 81-95% for eggs, chicken and cow
tissues, milk, and cream spiked at 0.01-0.1 ppm of fenvalerate (Shell
Develop. 1976a), along with 90-95% for cottonseed (Shell Develop.
1975) and 89% for coffee (Kato 1979), both fortified at 0.5 ppm. For
non-oily crops cleanup by either Florisil (activated or deactivated
with water) (Takimoto et al., 1977; Talekar 1977; Lee et al., 1978;
Shell Res. 1976), or silica gel (Lee et al., 1978; Kato et al., 1978)
column chromatography was applied. Over 90% recoveries of fenvalerate
were accomplished for crops including cabbage (Takimoto et al., 1977;
Talekar 1977; Lee et al., 1978); lettuce (Lea et al.,1978), potatoes
(Woodhouse et al., 1979), orange (Fujita et al., 1979), apple,
tomatoes, green peppers, peas and alfalfa (Shell Develop. 1976b) at
fortification levels of 0.01-40 ppm.
For the degradation products and metabolites of fenvalerate, Barber
(1976b) devised the analytical method for detection limits of
0.01-0.02 mg/kg for the residues of 3-phenoxybenzoic acid and
4-cloro-a-(1-methylethyl)-benzeneacetic acid (CMBA) in cottonseed.
(Kato et al., 1979) analyzed CMBA in human urine. The method
permitted detection of CMBA at 0.002 ppm as well as 93-100% recoveries
from the human urine fortified at 0.02-2 mg/kg. Barber (1978a-e) also
developed analytical methods for 1-(4-chlorophenyl)-2-methylpropyl
3-phenoxybenzoate and
4-chloro-B-(1-methylethyl)-a-(3-phenoxyphenyl)-benzenepropanenitrile
in soybeans, pears and apples as well as (aminocarbonyl)
(3-phenoxyphenyl)methyl 4-chloro-a-(1-methylethyl)-benzeneacetate in
potatoes and peanut shells with detection limits at 0.01 mg/kg.
NATIONAL LIMITS
National MRLs brought to the notice of the Meeting are listed (July
1979)
Country Commodity MRL Pre-harvest
(mg/kg) interval, days
Brazil Cotton 0.1 14
Soybeans 0.1 30
U.S.A. Cottonseed 0.2 21
Cattle(fat, meat,
meat by-products) 0.02
Goats (fat, meat,
meat by-products) 0.02
Hogs (fat, meat,
meat by-products) 0.02
Horses (fat, meat,
meat by-products 0.02
Milk (fat) 0.02
Sheep (fat, meat,
meat by-products) 0.02
(temporary)
Lettuce (head) 1 7
Cabbage 2 7
Cauliflower 1 7
Broccoli 0.5 14
Snap beans 0.5 7
(green beans)
Dry beans and 0.5 21
Dry pears
Cucumbers 0.05 10
Squash 0.05 10
Bell peppers 1 10
Tomato 1 7
Field corn 0.05 45
Grapes 1 21
Sweet corn (for
fresh market only) 0.05 3
Soybeans 0.02 21
National Limits, Continued...
Country Commodity Tolerance Pre-harvest
(mg/kg) interval, days
U.S.A.
(temporary)
Peanuts 0.02 21
Potatoes 0.02 21
Pears 1 28
Apples 2 21
Peaches 2 21
Australia Cottonseed, maize,
sweet corn 0.05 7
Pome and stone fruit 1 14
Milk and milk products
(fat basis) 0.2 -
Fat of meat of cattle 0.2 -
Soya beans, navy beans,
mung beans 0.2 21
Oilseed crops 0.2 14
Hungary
(temporary) Apples 1 14
Cherries 1 14
Peaches 1 14
Alfalfa 1 14
Beets 1 14
Cabbage 1 7
South Africa Apples, pears 0.5 14
Tomatoes 0.1 2
Grain sorghum 0.1 28
Cottonseed 0.1 -
New Zealand Brassica vegetables 5 7
Kiwi fruit 3 14
Apples, pears 1 14
Appraisal
Fenvalerate is a new synthetic ester related to the pyrethroids, with
a wide spectrum of efficacy against many species of pests. It is only
slowly degraded in sunlight. It is registered in a number of
countries for use on a variety of crops. The rate of application
ranges from 50-250 g/ha.
Supervised trials have been carried out in many countries and on many
crops. The rate of decline of residues on growing crops is slow, the
half-life ranging from 2 to 3 weeks. On stored cereal grains about
75% of the applied amount is found at the ninth month after treatment.
Fenvalerate does not penetrate significantly into plant tissues nor is
it translocated and there is little or no residue in crop parts not in
direct contact with the insecticide. Most of the residue remains on
the exposed parts of such commodities as cottonseed, peanuts and
fruits and is removed with shells and peels during processing and
preparation. Similarly, after treatment of stored grains some 70% is
in the bran of wheat, with considerably more in the hulls of oats and
rice which are normally removed before consumption.
A wide range of studies have been conducted on the fate of residues in
plants and animals. Residues in plants consist substantially of the
parent compound. In radiolabelled studies the occurrence of free and
conjugated metabolites has been demonstrated but these products have
not been found in field trials at levels above the limit of detection
(<0.05 mg/kg).
When administered or applied to livestock, fenvalerate is degraded and
the degradation products excreted in urine. A small proportion of the
applied dose is transferred to fatty tissues from which it may be
excreted via fat in the milk.
The process of photodecomposition proceeds by oxidation,
decarboxylation, hydration at the Cn-group, cleavage of ester and
diphenyl ether linkages and thence by hydrolysis to produce polar
derivatives of low molecular weight. It varies with exposure and is
fairly slow.
Fenvalerate is readily biodegraded by soil micro-organisms with a
residual half life of from 15 days to 3 months depending on soil
temperature, moisture and organic matter content. Because residues
are tightly bound to soil colloids, there is little likelihood of such
residues being transferred to water of streams through run-off or
leaching. Sprays falling directly onto bodies of water are rapidly
absorbed and inactivated on particulate matter. There is no evidence
of residues in soil being taken up by plants.
Under laboratory conditions it is possible to demonstrate that fish
can absorb the insecticide from water into various body tissues. Due
to the presence of particulate matter, surface absorption and the low
solubility of fenvalerate in water there appears to be little
likelihood of significant uptake of residues by fish under practical
conditions; nor are problems likely to arise from the occurrence of
residues in fish for human consumption.
Residues may be determined by GLC using electron capture detectors.
The ECD exhibits high sensitivity to fenvalerate responding to as
little as 0.05 ng. Polar solvents have been effective for extraction
when used with substrates containing substantial amounts of water.
Non-polar and petroleum solvents have been used with substrates
containing little water. Sweet co-distillation has proved convenient
and effective with lipid substrates. The limit of determination is
0.01 mg/kg.
Several national governments have established maximum residue limits
in a range of commodities. The data available provided a basis for
the Meeting to recommend the following maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits, determined and expressed as
fenvalerate, are recommended:
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Kiwi fruit 5 21
Alfalfa 20 21
Broccoli 2 7
Brussels Sprouts 2 7
Cabbage 2 7
Cauliflower 2 7
Celery 2 7
Lettuce 2 7
Chinese cabbage 1 7
Pome fruits 2 14
Peaches 2 14
Cherries 2 14
Citrus fruit 2 21
Tomatoes 1 7
Small fruits and berries 1 7
Beans (green) 1 7
Beans (dry) 0.5 7
Cottonseed 0.2 7
Cottonseed oil 0.1
Cucumbers 0.2 3
Squash 0.2 3
Soybeans 0.1 21
Sweet corn 0.05 7
Sugar beets 0.05 21
Sunflower seed 0.1 28
Radishes 0.05 21
Potatoes 0.05 7
Peanuts 0.1 7
Cereal grains 5
Wheat bran 5
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Wheat flour (wholemeal) 2
Wheat flour (white) 0.2
Milk 0.01
Milk products (fat basis) 0.2
Fat of meat 0.2
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
fenvalerate and/or metabolites;
2. Observations in humans occupationally exposed to high levels of
fenvalerate to evaluate the potential susceptibility of man to the
neurological disruption noted in rodents.
Desired:
1. An additional dominant lethal assay to reconfirm previous data;
2. Information on the occurrence and fate of photodecomposition
products;
3. Additional data from supervised residue trials on citrus, berry
fruits, beans and several other crops for which data are limited;
4. Results of on-going studies on the level and fate of fenvalerate
residues in stored products especially raw grain and milling
products derived therefrom.
REFERENCES
Albert, J.R. and Summitt, L.M. Intravenous Toxicity of WL 43775
(6-1-0-0) in the Mouse. (1976) Unpublished report from Shell
Development Co., Ltd.,
Barber, G.F.
1976a Separation of SD 43775 isomers for residue analysis.
1976b Residue data for SD 44607 in WL 10944 (acid metabolites of
SD43775 in cottonseed from cotton receiving seven applications of
SD43775, a California study. Shell Development Co.
1978a Residue data for SD 53036 and SD 54597, possible metabolites
of SD 43775, in soybeans receiving one aerial application of SD43775,
a Mississippi study.
1978b Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in whole apples receiving one dormant application of SD43775,
an Oregon study.
1978c Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in pears receiving one dormant application of SD43775, a
Michigan study.
1978d Residue levels of SD47117, a possible metabolite of SD 43775,
in potatoes receiving seven ground spray applications of SD43775, a
Minnesota study.
1978e Residue levels of SD47117, a possible metabolite of SD43775,
in peanut shells from peanuts receiving three aerial applications of
SD43775, an Oklahoma study.
Beliles, R.P., Makris, S.L and Weir, R.J. Three-Generation
Reproduction Study in Rats. (See Stein, 1977 for histopathology data).
(1978) Unpublished report from Litton Bionetics, Inc. submitted by
Sumitomo Chemical Company Ltd.,
Bengston, M. Personal communication from Final Report on Silo Scale
Experiments 1977-1978 to the Australian Wheat Board Working Party on
Grain Protectants. Queensland Department of Primary Industries.
Bengston, M., Davies, R.A.H., Desmachelier, J.M., Phillips, M., and
Simpson B.W. Additional grain protectants for the control of
malathion-resistant insects in stored sorghum. (in manuscript).
Blair D., and Roderick, H. Toxicity studies on the Pyrethroid
Insecticide WL43775, Emulsifiable Concentrate FX3368. Acute
Inhalation Exposure to an Aqueous Spray. (1975) Unpublished report
from Shell Development Co.
Boyer, A.C. Unpublished reports from Shell Development Co. submitted
by Sumitomo Chemical Co.:
1976a Metabolism of WL 43775 by Rat Liver Enzymes.
1976b Metabolism of WL 43775 by Rat Liver Enzymes - Part 2.
1977a Residues in Rat Tissues from Rats Fed C-14-SD 43775.
1977b Identification of Metabolites found in the urine of Rats Fed
C-14-SD 43775.
1977c Identification of Metabolites Found in the Faeces of Rats Fed
C-14-SD 43775.
1977d Identification of Metabolites in the Livers of Rats fed
C-14-SD43775.
Brooks, T.M. Toxicity studies with WL 43775: Mutagenicity Studies
with WL 43775 in the Host-Mediated Assay. (1976) Unpublished report
from Shell Development Co.
Brown, L.J. and Slomka, M.B. Skin Reaction Potential of Use Dialation
(1.33%) PYDRIN(R) EC. (1979) Unpublished report from Shell Development
Co.
Butterworth, S.T.G. and Carter, B.I. Toxicity Studies on the
Insecticide WL 43775: Acute Oral Toxicity and Neuropathological
Effects in Rats. (1976) Unpublished report from Shell Development Co.
Chapman, R.A. and Simmons, H.S. Gas-liquid chromatography of picogram
quantities of pyrethroid insecticides. J. Assoc. Off. Anal. Chem., 60:
977-978.
Chapman, R.A. and Harris, C.R. Extraction and liquid/solid
chromatography for the direct analysis of four pyrethroid insecticides
in crops by gas liquid chromatography. J. Chromatog. 166, 513-518.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Acute Toxicity, Skin Irritancy Potential of the Emulsifiable
Concentrate FX 3368. Unpublished report from Shell Development Co.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Toxicity and Skin and Eye Irritancy Potential of the ULV
Formulation FX 4353. (1976) Unpublished report from Shell Development
Co.
Creedy, C.L. and Potter, D. The Effect of Feeding WL 43775 on the
Microsomal Mono-Oxygenase System of Rat Liver. (1976) Unpublished
report from Shell Development Co.
Dean, B.J. Toxicity Studies with WL 43775: Dominant Lethal Assays in
Male Mice after Single Oral Doses of WL 43775. (1975) Unpublished
report from Shell Development Co.
Dean, B.J. and Senner, R.K. Toxicity Studies with WL 43775:
Chromosome Studies on Bone Narrow Cells of Chinese Hamster after Two
Daily Oral Doses of WL 43775. (1975) Unpublished report from Shell
Development Co.
Desmachelier, J.M. Personal Communication. CSIRO Division of
Entomology. (1979)
DeVries, D.M. Shell Development Co.
1976a Residues of SD 43775 in Milk from Cows fed Radiolabelled
SD43775
1976b Residues in Milk, Hair and Tissues of Cows sprayed with an
Emulsible Concentrate (6-1-3-1ECH)
1976c In Milk, Cream, Hair and Tissues of Cows Sprayed with an
Emulsible Concentrate or Fed 10 ppm in Their Diet
1976d In Eggs and Tissues of Hens Sprayed with an Emulsible
Concentration
1976e In Milk, Hair and Tissues of Cows Sprayed with an Emulsible
Concentration (6-1-3-1ECH)
1976f In Meat and Milk of Cattle Treated Dermally with a Water
Dispersible Liquid Formulation
1976g In Meat, Hair, and Milk Fat of Restrained Cows Treated
Dermally with an Emulsible Concentrated Formation
Dewar, A.J., Moffett, B.J. and Sitton, M.F. Toxicity Studies on the
Insecticide WL 43775: Biochemical and Functional Studies on the
Neurotoxicity of WL 43775 to Rats. (1975) Unpublished report from
Shell Development Co.
Dewar, A.J., Moffett, B.J., Sitton, M.F. and Baker, J.E. Toxicity
Studies on the Insecticide WL 43775: Biochemical and Functional
Studies on the Neurotoxicity of WL 43775 to Chinese Hamsters. (1978)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Three-Month Feeding Study in Rats. (1975)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Short-Term Feeding Study in Rats. (1976)
Unpublished report from Shell Development Co.
Hine, Inc. SD-43775 Toxicology: Acute and Repeated (14-Day) Dermal
Toxicity in the Rabbit. (1975) Unpublished report from Hine, Inc.,
submitted by Sumitomo Chemical Co.
Hine, Inc. Human Skin Testing on SD-43775. (1976) Unpublished report
from Hine, Inc. submitted by Sumitomo Chemical Co.
Hitchings, E.J. The Metabolism of the Pyrethroid Insecticide WL 43775
(Belmark) in Lettuce and Soil under Outdoor Conditions. (1977) Shell
Research Ltd.,
Holmstead, R.L. and Fullmer, D.G. Photodecarboxylation of Cyanohydrin
Esters. Models for Pyrethroid Photodecomposition. J. Agric. Food
Chem., 26, 56
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem., 26, 954
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem. 26, 590-95.
Ito, N. Histopathological Findings of Mice Treated with S5602 for
three Months (see Suzuki, 1976). (1976a) Unpublished report from
Sumitomo Chemical Co. Ltd.,
Histopathological Findings of Mice and Rats Exposed to Mist of S5602
for 4 weeks (see Khoda, 1976c). (1976b) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted by Sumitomo Chemical Co. Ltd.,
Histopathological Findings in Mice Treated with S5602 (see Suzuki, et
al., 1977b). (1978) Unpublished report from Nagoya City University
Medical School submitted by Sumitomo Chemical Co., Ltd.,
Japan Food Research Lab. Analytical Results of S5602 Residues in
Wheat Grain and Wheat Straw (1977)
Kaneko, H., Ohkawa, H. and Miyamoto, J. Comparative Metabolism of
Fenvalerate in Rats and Mice. (1979) Unpublished report from Sumitomo
Chemical Co. Ltd.,
Kaplan, M. and Murphy, S.D. Effect of Acrylamide on Rotor-Rod
Performance and Sciatic Nerve ß-Glucoronidase Activity of Rats.
Toxicol. Appl. Pharmacol. 22: 259-268.
Kato, S. Analysis of Sumicidin residues in coffee. Analysis
certificate No. 12010695. (1979) Japan Food Research Laboratories.
Kato, T. and Miyamoto, J. Residue Analysis of S-5602 and its optical
isomers in Chinese Cabbage. (1978) Sumitomo Chemical Co.
Kato, T., Ohnishi, J. and Miyamoto, J. The analytical method of
2-(4-chlorophenyl) isovaleric acid, a urinary metabolite of
fenvalerate (Sumicidin(R)). (1979) Sumitomo Chemical Co.
Kirkland, V.L. and Albert, J.R. (1977a) Unpublished Report from Shell
Development Co. Preliminary Pharmacodynamic Investigation of SD 43775
(6-10-0) Administered Intravenously to the Anesthetized Dog: I.
Effects on the Cardiovascular System and Respiration. II.
Electrocardiographic (EKG) Findings.
Khoda, H., Kadota, T. and Miyamoto, J. Unpublished reports from
Sumitomo Chemical Co. Ltd.,:
1976a Teratogenic Study on S5602 in Mice.
1976b Acute Inhalation Toxicity of S3206 and S5602 in Mice and
Rats.
1976c Subacute Inhalation Toxicity Study of S5602 in Mice and Rats.
Khoda, H., Kaneko, H., Ohkawa, H., Kadota, T. and Miyamoto, J. (1979)
Unpublished report from Sumitomo Chemical Co. Ltd., Acute
Intraperitoneal Toxicity of Fenvalerate Metabolites in Mice.
Lampert, P.W. (1977) Letter Report on Long-Term Rat Study (Gordon and
Weir, 1978) from the University of California San Diego School of
Medicine, Department of Pathology submitted by Sumitomo Chemical Co.
Lee, Y.W., Westcott, N.D. and Reichle, R.A. Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871
Loeffler, J.E. From Shell Development Co.:
1976a Disappearance of C-14 from Cotton Leaves Treated with
C-14-(Chlorophenyl)-SD 43775"
1976b Thin Layer Chromatography of SD 43775-Treated Cotton Leaves
1976c Carbon-14 Content of Cotton Seeds after Single and Multiple
Treatment of Squares with C-14-(Chlorophenyl)-SD 43775
1976d Investigation of the Transport of C-
14-(Chlorophenyl)-SD-43775 from Treated to Untreated Leaves on Cotton
Plants
1976e Degradation of C-14-(Chlorophenyl)-SD 43775 during Exposure
to Soil Under Aerobic, Anaerobic, and Sterile Conditions.
Matsubara, T., Suzuki, T., Kadota, T. and Miyamoto, J. Antidotes
Against Poisoning by S5602 in Rats. (1977) Unpublished report from
Sumitomo Chemical Co.
McKinney, W.J. Separation of SD 43775 from hexane extracts of cotton
by liquid chromatography. Shell Development Co.
Microchem Associates:
Report on Residues of WL 43775 in Cabbages, (1977)
Report on Residues of WL 43775 in Navy Beans (1978)
Mikami, N., Takahashi, Hayashi, K. and Miyamoto, J. Photodegradation
of Fenvalerate (Sumicidin(R)) in Water and on Soil Surface. Sumitomo
Chemical Company Ltd.,
Milner, C.K. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticide. Investigation of the Neurotoxic Potential of WL 43775.
(1977) Unpublished report from Shell Development Co.
Noble, P.J. AF1117 Residue Data in Animal Tissues, Milk and Cream.
(1976a) Shell Chemical (Australia) Propriety Ltd.,
Noble, A.S. Soil Percolation Studies with WL 43775. (1976b) Shell
Research Ltd.,
Ohkawa, H., Nambu, K., Inui, H. and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Soil and by Soil Micro-organisms. J.
Pesticide Sci. 3, 129
Ohkawa, H. and Kikuchi, R. Metabolic Fate of Fenvalerate
(Sumicidin(R)) in Rats, Soil, Soil Microorganisms and an Aquatic Model
Ecosystem. Sumitomo Chemical Co., Ltd.,
Ohkawa, H., Nambu, K., Mikami, N., and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Bean Plants and Cabbages. Sumitomo
Chemical Co. Ltd., (1979a)
Ohkawa, H., Kikuchi R. and Miyamoto, J. Bioaccumulation and
Biodegradation of the (S)-Acid Isomer of Fenvalerate (Sumicidin(R)) in
an Aquatic Model Ecosystem. Submitted to J. Pesticide Sci. (1979b)
Ohkawa, H., Kaneko, H., Tsuki, H. and Miyamoto, J. Metabolism of
Fenvalerate (Sumicidin(R)) in rats. J. Pesticide Sci. 4 (2), 143.
Okuno, Y., Kadota, T. and Miyamoto, J. Unpublished report from
Sumitomo Chemical Co.:
1976a. Neurotoxic Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Dermal Applications in Rats.
1976b. Neurotoxic Effects of Some Synthetic Pyrethroids by
Short-Term Feeding in Rats.
1977a. Neurotox Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Oral Administration in Rats.
1977b. Neurotoxic Effects of S5602 and Natural Pyrethrins by Oral
Administration in Rats.
1977c. Recovery of Histopathological Lesions in Rats Caused by
Short-Term Feeding of S5602.
Potter, J.C. (1976) Unpublished reports from Shell Development Co.:
a) Tissues of Rats Fed SD 43775-C-14; b) and c) Milk and tissues of
cows; d) eggs and tissues from laying hens; e) cream from the milk of
cows fed SD 43775 14-C.
Potter, J.C. and Arnold, D.L. Residues of C-14 in Tissues of Rats Fed
SD 43775-C-14 for 28 days. (1977) Unpublished report from Shell
Development Co.
Potter, J.C. and Sauls. Residues of 14-C in eggs and Tissues of
Laying Hens fed 0.03 ppm of SD 43775-14-C (Labelled in Benzene Ring
Adjacent to CN Group). (1978) Shell Development Co.
Quinn R.J., Paa, H., Mastri, C.W., Kinoshita, F.K. and Keplinger, M.L.
21-Day Subacute Dermal Toxicity Study with Technical SD 43775 and SD
43775 (2.4 lb/gal EC) in Albino Rabbits. (1976) Unpublished report
from Industrial Bio-Test Lab submitted by Sumitomo Chemical Co.
Shell Development Co. and Shell Research Ltd., (1975-1978) A numbered
series of 36 unpublished reports with residue data from field trials
on specified crops in different countries.
Simpson, B.W. (1979) Draft report to be published. Queensland
Department of Primary Industries Analytical Chemistry Branch,
Brisbane, Australia.
Standen, M.E. The Metabolism of the Pyrethroid Insecticide WL 43775
in Apple Fruit and Foliage under Outdoor Conditions. (1977) Shell
Research Ltd.,
Stein, A.A. Histopathology Evaluation of Animals from 3-Gene ration
Reproduction Study. (1977) Unpublished report from Microscopy for
Biological Research, Ltd., submitted as part of study cited above
(Beliles et al., 1978) by Sumitomo Chemical Co., Ltd.,
Summit, L.M. and Albert, J.R.
1977a. Oral Lethality of WL 43775 (6-1-0-0) in the Rat (Unpublished
report from Shell Development Co.)
1977b. Determination of the Acute Oral Lethality of WL 43775
(6-1-0-0) in the Male and Female Mouse.
Suzuki, H. and Miyamoto, J.
1976. Unpublished report from Sumitomo Chemical. Studies on
Mutagenicity of S5602 with Bacteria Systems.
1977. Studies on Mutagenicity of Some Pyrethroids on Salmonella
Strains in the Presence of Mouse Hepatic S9 Fractions.
Suzuki, T., Kadota, T. and Miyamoto, J. One Year Chronic Toxicity
Study of S5602 in Mice (Three Month Interim Report). (1976)
Unpublished report from Sumitomo Chemical Co.
Suzuki, H., Kishida, F. and Miyamoto, J. Studies on Mutagenicity of
S5602 in Ames Test in the Presence of Hepatic S9 Fractions from
Several Laboratory Animal Species. (1979) Unpublished report from
Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Fifteen-Month Chronic Toxicity Study of S5602 in Rats. (1977a)
Unpublished report from Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Eighteen-Month Chronic Toxicity Study of S5602 in Mice. (1977b)
Unpublished report from Sumitomo Chemical Co.
Takimoto, Y. and Miyamoto, J. Method for Residue Analysis of S-5602
in Cabbage. (1977) Sumitomo Chemical Co.
Talekar, N.S. Gas-liquid chromatographic determination of
a-cyano-3-phenoxybenzyl a-isopropyl-4-chlorophenylacetate residues
in cabbage. J. Assoc. Off. Anal. Chem., 60: 908-910
van der Pauw, C.L., Dix, K.M., Blanchard, K. and McCarthy, W.V.
Toxicity of WL-43775: Teratological Studies in Rabbits Given WL 43775
Orally. (1975) Unpublished report from Shell Development. Co.
Walker, B.J., Hend, R.W. and Linnett, S. Toxicity Studies on the
Insecticide WL 43775: Summary of Results of Preliminary Experiments.
Unpublished report from Shell Development Co.
Williams, I.H. and Brown, M.J. Persistence of Permethrin and WL 43775
in Soil. J. Agric. Food Chem. 27, 130
Woodhouse, R.N., Almond, R.H. and Anderson, J. Determination of
residues of Sumicidin in potatoes. (1979) Huntingdon Research Centre.
SMO 88/7939.
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of rats (11 male and 22 female rats per group) were fed
fenvalerate in the diet at dosage levels of 0, 1, 5, 25 and 250 ppm.
The animals were fed for 9 weeks prior to mating and initiation of a
standard 3-generation (2 litters per generation) reproduction study.
Fertility, viability, gestation and lactating indices were calculated
for each treatment group compared to control values. Ten male and 10
female weanlings from the F3b litter were examined histologically at
the conclusion of the study.
The mean body weight of the parent of the third generation (F2b
adults) showed a significant reduction at the 250 ppm level. Gross
necropsy revealed kidney changes in these animals which was not
apparently related to fenvalerate. No pathological changes were noted
to account for the weight loss. No effects on reproductive parameters
in any of the three generations were observed in the study.
Histological examination did not show an adverse effect of
fenvalerate. With the exception of weight reduction in the third
generation parents, there was no effect of fenvalerate on any
parameter measured in the study (Beliles, et al., 1978; Stein, 1977).
Teratogenicity
Mouse
Groups of pregnant mice (32 to 33 mice per group) were administered
fenvalerate at dosage levels of O, 5, 15 and 50 mg/kg body weight per
day on days 6 through 15 of gestation in a standard teratogenicity
bioassay. On day 18, groups of 20 mice were sacrificed and fetuses
were removed and examined for somatic and skeletal abnormalities. The
remaining parents were allowed to deliver naturally and the young
maintained for three weeks to weaning to evaluate postnatal deficits.
In addition to the teratogenicity study, two male and two female
weanlings from each dam were maintained for 8 weeks and mated to note
any effects on their reproductive potential. Toxic signs of poisoning
were noted in maternal mice at the high dose level. There was no
significant mortality over the course of the study and no effects were
noted on any of the animals as a result of continuous administration
of fenvalerate. In fetal examinations, there were no somatic or
skeletal changes as noted by internal or external tissue evaluations.
The animals maintained in an abbreviated reproduction study showed no
differences from control value in their ability to reproduce. There
were no changes in the reproduction indicates evaluated with any
animals examined. Under the conditions of this bioassay, fenvalerate
has been shown to have no teratogenic potential in mice (Kohda, et
al., 1976a).
Rabbit
Groups of pregnant rabbits (group size varied from 20 to 31 rabbits)
were administered fenvalerate from day 6 to day 18 of gestation. On
day 28, the rabbits were sacrificed and standard teratogenicity
assessments made with respect to early or late fetal death, viability
and standard somatic and skeletal teratogenic potential. Reduced body
weight of pregnant rabbits was observed with the highest dose level.
There were no significant differences from control values in any of
the parameters measured in the study. Under the conditions of the
study, fenvalerate did not induce a teratogenic event in rabbits (van
der Pauw, et al., 1975).
Special Studies on Mutagenicity
Microorganism
Fenvalerate was examined for its mutagenic potential using a series of
Salmonella typhimurium strains (TA 1535, TA 1538, TA 98 and TA 100) at
dose levels up to 1 mg/ plate. Two strains of Bacillus subtilis (H17
and M45) were tested at concentrations up to 10 mg/disc. The "Ames"
test was performed in the presence and absence of rat activation
systems and with positive and negative chemical controls. The Rec
assay was not evaluated with a metabolic activation system. With both
microbial bioassay systems, fenvalerate was not mutagenic (Suzuki and
Miyamoto, 1976).
In additional trials, fenvalerate did not increase the number of
revertant colonies of S. typhimurim (TA 1535, TA 1537, TA 1538, TA 98
and TA 100) in the presence or absence of a liver subcellular
activation system prepared from 6 different strains of PCB-treated
mice, 3 strains of rat, and the Syrian golden hamster. Fenvalerate,
tested at dosage levels up to 1 mg/plate, was not mutagenic (Suzuki
and Miyamoto, 1977; Suzuki, et al., 1979).
Mouse
Groups of mice were administered fenvalerate orally at doses of 0, 60
and 125 mg/kg body weight and injected intraperitoneally with an
indicator strain of microorganisms in a standard host-mediated assay.
A positive control, dimethylnitrosamine (DMNA), was used in this
study. Fenvalerate did not induce an increase in mutation frequency
of the S. typhimurium indicator while the DMNA significantly
increased the mutation frequency (Suzuki and Miyamoto, 1976).
Groups of mice were administered fenvalerate orally at dose levels of
0, 25 or 50 mg/kg. Immediately after administration, a suspension
culture of yeast Saccharomyces cerviciae was introduced to the
peritoneal cavity. Positive (ethyl methane-sulphonate) and negative
(DMSO) control animals were also used in this standard host-mediated
assay. Five hours after dosing, the yeast cells were recovered and
examined for mitotic gene conversion. No mutagenic effects were
detected in the cells from any of the fenvalerate concentrations
tested (Brooks, 1976).
Dominant Lethal Assay
Mouse
Groups of male mice (10-11 mice per group) were administered
fenvalerate orally at dosage levels of 0, 25, 50 and 100 mg/kg body
weight. Each treated male was mated with 3 virgin females for 7 days.
The procedure was repeated weekly with new females in a standard
dominant lethal assay. The females were sacrificed and examined for
the condition of the fetuses on day 13 of gestation.
There were no significant differences from control values with respect
to the effects of fenvalerate on pregnancy. Foetal implants in
females mated to males during the second week after treatment showed a
significant reduction in viable fetuses. There was also a significant
increase in early foetal deaths occurring in females mated during the
fourth week to males dosed at the highest level (Dean, 1975).
Hamster
Groups of Chinese hamsters (6 male and 6 females per group) were
orally administered fenvalerate (in DMSO) at dose levels of 0, 12.5
and 25 mg/kg on each of two successive days. Animals were sacrificed
8 or 24 hours after dosing and chromosomal preparations were made from
the femoral bone marrow cells. Similar preparations were made with
control animals administered methyl methanesulphonate (50 mg/kg).
Cells from animals administered methyl methanesulphonate showed
substantial numbers of chromatid gaps at 8 hours after dosing but not
at 24 hours. The administration of fenvalerate did not induce
demonstrable chromosomal damage at either sampling interval (Dean and
Senner, 1975).
Special Studies on Neurotoxicity
Hen
Groups of 6 hens were administered fenvalerate orally in dimethyl
sulphoxide at dosage levels of 0 and 1000 mg/kg daily for 5 days. A
positive control of TOCP (0.5 ml/kg) was also included in the study.
After 3 weeks, the fenvalerate and negative control animals were
retreated at the same dosage regimen. At the conclusion of an
additional 3 week observation period, animals were sacrificed and
histological examinations were performed on the central and peripheral
nervous system. All animals receiving TOCP developed readily defined
signs of delayed neurotoxicity and histological lesions were seen in
the sciatic nerve and spinal cord. Clinical signs and histopathologic
lesions were not noted in controls or fenvalerate-treated hens (Milner
and Butterworth, 1977).
Rat
A series of studies was performed to evaluate the neurotoxic potential
(to induce axonal and myelin disruption) of a group of synthetic
pyrethroid esters and of natural pyrethrum. Acute oral administration
of fenvalerate, cypermethrin, resmethrin, permethrin and natural
pyrethrum to rats at very high dosage levels resulted in severe
clinical signs of poisoning and mortality within 24 hours.
Histopathologic lesions were observed in the sciatic nerve with all
compounds tested. At lower levels, fenvalerate (200 mg/kg),
cypermethrin (100 mg/kg), permethrin (200 mg/kg) and pyrethrum (3500
mg/kg) did not show clinical signs of poisoning or histopathologic
lesions (Okuno, et al., 1977a; 1977b).
Groups of 6 male and female rats were fed fenvalerate in the diet at
concentrations of 0 and 2000 ppm for 8 to 10 days, after which the
sciatic nerve was examined for histological signs of degeneration.
All animals exposed to fenvalerate showed typical signs of acute
intoxication including ataxia, tremors and hypersensitivity.
Histological examinations at the end of the feeding interval did not
reveal any adverse effects of fenvalerate on the sciatic nerve (Hand
and Butterworth, 1976).
In a study to evaluate the reversibility of the lesions induced in the
sciatic nerve, rats were administered fenvalerate in the diet at dose
levels of 0 and 3000 ppm for 10 days. Mortality was evident as 60% of
the animals died within the course of the dietary treatment.
Surviving animals were fed normal control diets and examined for 12
weeks following completion of the feeding study. Animals were
sacrificed every 3 weeks and examined histologically for sciatic nerve
disruption. Sciatic nerves of rats sacrificed at 3 weeks on control
diets continued to show swelling and disintegration of axons. At 6
weeks there were no histological lesions. These results were also
observed at the 9 and 12-week intervals suggesting reversibility of
the histopathologic lesion observed following high dose treatment with
fenvalerate (Okuno and Kadota, 1977c).
Antidotal Studies
Following acute poisoning phenobarbital, pentobarbital and
diphenylhydantoin were found to be effective in relieving the acute
signs of intoxication. Phenobarbital (50 mg/kg, ip) prevented tremor,
diphenylhydantoin (100 mg/kg, ip) reduced the toxic reaction and
pentobarbital (35 mg/kg ip) removed the tremor reaction completely
within 30 minutes. Combinations of diphenylhydantoin with either of
the barbiturates was effective in reducing the onset and severity of
tremors while a variety of other agents were not active
(delta-tubocurarine, atropine, meprobamate, diazepam, biperidin and
trimethadione) (Matsubara, et al., 1977).
Special studies in pharmacology
Fenvalerate, administered to dogs in acute dosage rates sufficient to
induce toxic signs of poisoning, showed no consistent cardiovascular
effects as measured by EKG. Respiratory stimulation was noted at high
levels and was not reduced by anesthetic supplements (Urethane/alpha
chlorolos or pentobarbital) (Kirkland and Albert, 1977a; 1977b).
Skin and eye irritancy
Two formulated products were found to be severe eye and skin irritants
when examined in the rabbit. Dermal irritation was evident for 7 days
after a 24-hour exposure and severe conjunctivitis, corneal opacity
and iritis were observed within 30 minutes of an application of 0.2 ml
of the formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation to some degree (Coombs and
Carter, 1975 and 1976).
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
Table 2.
LD50 (mg/kg)
Compound Male Female
Fenvalerate 88.5 85.0
2-(4-Chlorophenyl)isovaleric acid 351 350
3-Phenoxybenzyl alcohol 371 424
3-4'(Hydroxyphenoxyl) benzyl alcohol 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol 876 778
3-Phenoxybenzoic acid 154 169
3-(4'-Hydroxyphenoxy) benzoic acid 783 745
3-(2'-Hydroxyphenoxy) benzoic acid 859 912
3-Phenoxybenzaldehyde 415 416
NaSCN 604 578
All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO (Khoda, et al., 1979).
The acute intraperitoneal toxicity in mice of the proposed
decarboxylated photoproduct was reported to be substantially lower
than that toxicity noted with fenvalerate (Holmstead, et al., 1978).
Short Term Studies
Inhalation
Groups of 4 male and 4 female rats were exposed by inhalation (head
only) to an aerosolized formulation (77 micron particle size)
generated from an aqueous suspension containing 3 grams/litre.
Following a single administration (4 hours) of this non-inhalable
particulate, acute signs of poisoning were noted for a short period,
presumably from oral ingestion of the large particle. There was no
mortality and all animals appeared normal within 3 days following
exposure (Blair and Roderick, 1975).
Groups of rats and mice (10 male and 10 female of each species per
group) were administered fenvalerate by inhalation exposure 3 hours
daily for 4 weeks at concentration levels of 0, 2, 7 and 20 mg/m3.
Animals were exposed to a small, fully respirable particulate (1 to 2
microns) during the course of the study. Mortality was not noted over
the course of the study, but animals at the high dose level showed
acute toxic signs of poisoning. Growth was not affected at any dose
level; nor were hematology and clinical biochemistry parameters.
Gross and microscopic examination of tissues and organs at the
conclusion of the study showed no changes that were related to the
administration of fenvalerate (Khoda, et al., 1976c; Ito, 1976b).
Dermal
Groups of rabbits (7-8 male rabbits per group) were administered
fenvalerate dermally at dose levels of 0, 100 and 400 mg/kg daily for
6 hours (14 exposures were performed over a 22-day period). Mortality
was observed at the high dose level accompanied by severe weight loss,
clinical signs of poisoning and gross dermal effects. Rabbits
tolerated the 100 mg/kg dose with minor local dermatologic effects
(Hine, 1975).
Groups of rabbits (10 male and 10 female rabbits per group, five of
each sex had an occluded skin) were administered fenvalerate dermally
for 6 hours per day, 5 days per week for 3 weeks. The dosage levels
used were 0, 30, 100 and 300 mg/kg body weight. In addition, a
formulated product (an emulsifiable concentrate containing 2.4 pounds
fenvalerate/gallon) was also tested for its dermal toxicity at dosage
levels of 0, 100, 300 and 1000 mg/kg.
Mortality was observed with both treatments predominantly at the high
dosage group. Mortality was preceded by clinical signs of poisoning
primarily in the group exposed to the formulated product. At lower
concentrations, technical fenvalerate was mildly irritating to the
skin upon repeated dermal exposure. The formulation of fenvalerate
and the blank formulation used as a control were severely irritating.
When severe irritation was noted, there were significant effects on
body weight. There were no effects noted on various haematologic,
clinical chemistry and urinalysis parameters related to the presence
of fenvalerate. Gross and microscopic pathologic changes were noted
only at the site of administration and described as acanthosis and
hyperkeratosis of the epidermis. The extent and severity of the
lesions appeared to be dose-related especially with respect to the
formulated product. There were no significant gross or microscopic
effects noted in tissues and organs over the course of the study
(Quinn, et al., 1976).
Dietary
Rat
Groups of rats (12 male and 12 female rats per group) were fed
fenvalerate in the diet at dose levels of 0, 125, 500, 1000 and 2000
ppm for 90 days. Mortality was observed at 2000 ppm while growth and
food consumption were decreased at 1000 ppm and 2000 ppm. With the
exception of an increase In BUN concentration at the two highest dose
levels, hematological and clinical examinations at the conclusion of
the study were normal. Gross and microscopic examinations of tissues
and organs were performed at the conclusion of the study. Increases
in liver to body weight and kidney to body weight ratios were observed
at 500 ppm and above. These gross changes were not accompanied by
observable microscopic changes on histopathological examination.
Sciatic nerves, examined at the conclusion of the study, showed no
myelin or axonal degeneration (Hend and Butterworth, 1975).
Dog
Groups of young adult beagle dogs (4 male and 4 female dogs per group
were fed fenvalerate in the diet at dose levels of 0, 0.5, 0.25, 1.25
and 12.5 mg/kg body weight for 90 days. There were no abnormalities
over the course of the study. Growth, food consumption and behaviour
were normal. Results of clinical laboratory examinations performed 3
times during the course of the study, showed no effects of fenvalerate
in the diet. At the conclusion of the study, data from gross and
microscopic examination of a variety of tissues and organs
substantiated clinical data, again showing no effects of dietary
fenvalerate. Daily administration at a level of 12.5 mg/kg body
weight for a period of 90 days produced no detectable evidence of
toxicologic effects (Hart and Wosu, 1975).
Long Term Studies
Mouse
Groups of mice (from 35 to 47 male and female, ddY strain, mice/group)
were administered fenvalerate in the diet for 78 weeks at dosage
levels of 0, 100, 300, 1000 and 3000 ppm. An interim (3 months) and a
final report showed dose-related effects at 300 ppm and above.
Fenvalerate was not carcinogenic to the ddY strain of mouse (Suzuki
and Kadota, 1976; Ito, 1976a, 1978; Suzuki et al., 1977b).
At the early stages of the study, mortality was evident at the highest
dose level. Growth was reduced at 1000 ppm and above. These effects
were accompanied by excitability, hypersensitivity and other
behavioural changes in the first month of feeding. A variety of
hematological parameters were affected during the first three months
of study, predominantly at the high dose level. Several biochemical
changes were observed including a decrease in alkaline phosphatase, an
increase in blood urea nitrogen, an increase in leucine aminopeptidase
activity and a decrease in cholesterol and glucose occurring at 1000
and 3000 ppm with several of these parameters being affected at 100
ppm (glucose decrease in females). Gross examination of tissues and
organs showed a weight increase in several tissues of both males and
females exposed to the high dose level. Microscopic examination
suggested changes in the liver at the two highest dose levels.
Multiple small necrotic foci in the liver and changes in the
epithelial cells of the proximal convoluted tubules were noted,
apparently related to the presence of fenvalerate in the diet (Suzuki,
et al., 1976; Ito, 1976a).
At the conclusion of the 18-month study, animals were sacrificed for
terminal haematologic and biochemical changes as well as gross and
microscopic examination of tissues and organs. Over the 18 month
study, growth was depressed at 3000 ppm in both males and females.
Behavioural changes noted as transient hypersensitivity occurred at
1000 ppm and above. Increased mortality was also evident at the two
highest dose levels. No compound-related changes were noted with
respect to haematologic parameters, although clinical chemistry
parameters were in some instances substantially increased (SGPT, SGOT,
LDH and LAP activities in both sexes were significantly increased,
predominantly at the two highest dose levels but sporadically at 300
ppm). Gross changes were noted in several organ weights and organ to
body weight ratios primarily in liver, although changes were also
noted in the kidney, heart, spleen, pituitary and ovary at the highest
dose level. These gross changes were not accompanied by
histopathological events with the exception of granulomatous changes
in liver and the mesenteric lymph nodes noted at all treatment groups,
predominantly at the highest two dose levels. Biochemical changes,
noted at the three month sacrifice interval, were not seen at the
conclusion of the study (increased BUN, decreased glucose, etc.).
There were no indications in this study of increased tumorigenicity or
carcinogenicity as a result of fenvalerate (Suzuki, et al., 1977b).
Rat
Groups of rats (15 male and 15 female Wistar rate per group) were fed
at concentrations of O, 50, 150, 500 and 1500 ppm for 15 months in the
diet. There was no mortality in the study attributable to
fenvalerate. Growth, food consumption and behavioural changes were
significantly affected at the highest dose level. Hypersensitivity
was observed at the early stages of the experiment disappearing within
3 months. Growth was significantly depressed in both males and
females at the highest dose level. Food consumption was unaffected
over the course of the study. Clinical examinations, performed at
either one year (urinalysis) or at the conclusion of the study
(hematology, blood biochemistry and gross and microscopic pathology),
showed significant abnormal values in a variety of parameters at the
1500 ppm dose level. No opthamologic effects were noted. Urinalysis
was normal over the course of the study. Haemoglobin concentration
was depressed in males at the highest dose level and the females at
150 ppm and above. Blood biochemistry was significantly altered at
1500 ppm with respect to several parameters (BUN in both males and
females; protein and cholinesterase in females). Gross and
microscopic examination of tissues and organs including specific
sections of sciatic nerve and trigeminal ganglia and nerve showed no
dose-related effects. Generalized inflammatory and degenerative
changes were seen in both control and treated animals. Tumor
incidents were low and not related to the presence of fenvalerate.
There was no suggestion of a carcinogenic potential observed in the
study (Suzuki, et al., 1977a).
Groups of rats (93 male and 93 female Sprague-Dawley rats/group, 183
of each sex were used as the control and an additional 22 of each sex
were used as a separate control and high level group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25, 250 and 500
ppm. There was no mortality associated with the study although growth
was reduced at the 500 ppm dose level. The 500 ppm group and a
separate control group were sacrificed at 26 weeks while the other
animals were maintained for 2 years. There were no significant
effects on food consumption, growth or on behaviour at 250 pm.
Hematology, clinical chemistry tests and urinalyses, performed at
various time intervals over the course of the study on at least 10
animals of each sex at each dose level, showed no dose-related
effects. At the conclusion of the study, organ weight and organ to
body weight ratios were normal. Gross and microscopic examination of
tissues and organs did not differ significantly from controls. Benign
and malignant lesions occurred at random throughout all groups
examined at the end of the study and in those animals that died during
the course of the study. There were no lesions attributable to
fenvalerate. A specific pathology examination of the sciatic nerve of
animals fed 250 ppm was performed. The sciatic nerve was not found to
have been significantly affected by fenvalerate (Gordon and Weir,
1978; Lambert, 1977).
Observations in Man
Fenvalerate was administered dermally to adult men and women to the
skin of the arm or face at dosage levels of approximately 20-40 mg.
Control applications were carried out on the same individuals and
subjective evaluations were performed with respect to dermal
irritation. There was no erythema or other visible skin effects and
an evaluation of the subjective responses suggested no significant
differences between fenvalerate-treated and the control portions of
the body (Hine, 1976).
A double-blind study utilizing 29 male volunteers was performed to
test the skin reaction of formulated fenvalerate. The emulsifiable
concentrated formulation was diluted with water and administered to
one side of the face, on the cheek, with a control formulation applied
to the opposite cheek.
There were no signs of dermatitis noted at 24 hours following
administration. Subjective analyses of irritation or skin sensation
were performed with each individual. Under the conditions of the
study, the formulation of fenvalerate did not produce abnormal skin
sensations. There were no indications that any of the symptoms noted
(which included tingling, burning, stinging, itching, swelling,
numbness or heat) was associated with fenvalerate (Brown and Slomka,
1979).
COMMENTS
Fenvalerate, an ester related to the pyrethroids, currently being
developed for use as an agricultural insecticide, is rapidly absorbed
in mammals, widely distributed, and metabolized. Tissue residues of
fenvalerate and its ester metabolites appear to concentrate to some
degree in adipose tissue. Fenvalerate undergoes several major
metabolic reactions: cleavage of the ester linkage, hydroxylation of
the acid and alcohol moieties, and conversion of the CN group to SCN
and CO2. The metabolic fate in all animals studied appears to be
similar. Photolytic degradation has been shown to produce a
decarboxylated product not known to occur in mammals. Fenvalerate is
moderately toxic when administered by the oral route. The metabolic
and photolysis products are less toxic than the parent ester.
Studies on the mutagenicity and reproductive/teratogenic potential
were negative. Studies on neurotoxicity have shown that, following
high level exposure, rats showed reversible clinical signs of ataxia.
Microscopic examination of the sciatic nerve showed axonal swelling
and myelin disruption. Biochemical studies revealed an increase in
lysosomal enzyme activity (ß-glucuronidase and ß-galactosidase) in
peripheral nerve (see Report Section 3.5). Fenvalerate, when
administered to hens at high levels, did not induce signs of
peripheral neuropathy. No data were available to assess the
susceptibility of humans to this neuropathy.
Short-term and long-term studies have been performed in a variety of
test animals. Fenvalerate is not a carcinogen and in short-term
studies in dogs and in long-term studies in rats and mice dietary
no-effect levels have been observed. A temporary acceptable daily
intake for man was allocated. The concerns of the Meeting over the
potential for bioaccumulation, the neuropathy observed in rodents and
the limited information of the susceptibility to such an effect in man
were the basis for the temporary evaluation and for the request for
further observations in occupationally exposed humans and for
pharmacokinetic data. Further information is desired with respect to
an additional dominant lethal assay to provide verification of
existing data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet equivalent to 11.9 mg/kg body weight
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg body weight
Dog: 12.5 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.06 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenvalerate is a highly active broad spectrum insecticide. It is
particularly effective as a contact and stomach poison against
lepidopterous larvae and also has high activity against Orthoptera,
Hemiptera and Diptera. This activity, together with adequate
stability on foliage, and relatively low toxicity to mammals is likely
to lead to increasing use against pests of agriculture.
The substance can be combined with most other insecticides and
fungicides, but not those with alkaline properties. It is mostly
marketed as 20% and 30% concentrates for aqueous dilution; but
granules, ULV concentrates and powers are also available.
Pre-harvest treatments
Fenvalerate is registered and/or approved in many countries in Europe,
Asia, South and Central America, and Australia. It is used against a
wide range of pests, including tobacco bud worm, bollworms, cut worms,
armyworms, leafrollers, weevils, aphids, thrips, beetles, leaf miners,
moths, bugs, psyllas etc and on a wide range of crops including
fruits, vegetables, beans, cereals, cotton and tobacco. For these
purposes it is applied at from 25 to 300 g/ha depending on the crop.
Other uses
It is registered in Argentina for forestry use against Oiketicus.
It is also being developed for control of insect pests of stored grain
and against insects of importance in public health, animal health and
industry.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Many trials have been carried out on a wide variety of fruits,
vegetables, cereals and oilseeds. Most of the data were generated
under American conditions, supplementary data are available from
several other countries. Table 3 contains the summarised data,
representing several thousand separate analytical data provided by the
principal manufacturers.
Since fenvalerate is not systemic in plants, residues usually are very
low in commodities such as root crops, corn kernels and peas which are
protected from direct contact with the insecticide. Residue levels on
plants normally decline with a half-life of about one to two weeks.
Oilseeds
Following multiple applications at rates up to 0.45 kg/ha, and in over
30 supervised trials in various countries (i.e. higher than the
recommended dosage of up to 0.3. kg/ha). Residues in cottonseed were
generally below 0.1.mg/kg, regardless of the interval between last
treatment and harvest. A residue of 0.21 mg/kg was found in a
Colombian trial 1 day after treatment at the rate of 0.3 kg/ha, and
one trial in U.S.A. showed the residues of 0.12 mg/kg (at a rate of
0.22 kg/ha) and 0.19 mg/kg (0.45 kg/ha) 48 days after treatment. When
the seed cases were separated from kernels, the residues of
fenvalerate almost entirely remained on the seed cases and levels in
the oil products were very low.
All trials on peanuts have been made in U.S.A. When fenvalerate was
applied at rates of up to 0.22 kg/ha the residues in whole nuts did
not exceed 0.1 mg/kg. At the higher rate of 0.45 kg/ha however,
residues of 0.20-0.32 mg/kg were detected 14-21 days after
application. It was shown that residues in the meat and oil did not
exceed the detection limit (0.01 mg/kg) and that the shells carried
almost all the residues found in whole nuts. Even when the peanuts
were harvested 73 days after the treatment at 5 and 10 times higher
rates than the recommended dosage (up to 0.22 kg/ha) the residues in
whole nuts were 0.04 mg/kg and 0.09 mg/kg, respectively. In other
trials at rates up to 0.45 kg/ha the residues in hay, foliage and
vines ranged from 0.01 to 31 mg/kg.
Leafy and stem vegetables
Trials have been carried out in U.S.A. and several countries on
lettuce, celery and brassica vegetables such as broccoli, Brussels
sprouts, cabbage, Chinese cabbage, cauliflower, kale and Chinese kale.
The residues were generally less than 1 mg/kg 5-10 days after
treatment at rates ranging from 0.05 to 0.45 kg/ha. Residues of 1.2
mg/kg were found in cabbage and kale 7 days after treatment. In
celery the maximum residue 7 days after treatment was 1.2 mg/kg where
the application rates were within recommended dosage of 0.22 kg/ha.
When lettuce was treated at rates of up to 0.45 kg/ha and harvested 7
days after treatment, the maximum residue was 1.6 mg/ha at application
rate of 0.10 kg/ha except one trial in France in which 5.5 mg/kg was
found 7 days following application at 0.10 kg/ha.
Fruiting vegetables
Residue data for cucumbers are available from U.S.A. The maximum
residue was 0.47 mg/kg 3 days or later after treatment at rates within
the recommended dosage (up to 0.22 kg/ha).
In trials carried out in various countries, 1 to 9 successive
applications were made to tomatoes at rates up to 0.45 kg/ha. The
tomatoes were harvested at intervals ranging up to 40 days after the
last treatment, and the residues were below 0.5 mg/kg in all samples
taken 7 days or more after the treatment.
Root and tuber vegetables
Trials on sugar beets have been carried out in U.S.A., France, Denmark
and U.K. The maximum residue of 0.03 mg/kg was found in roots 9 days
after a single treatment at a rate of 0.15 kg/ha in the Danish trial.
In other trials the residues were not detectable at the limit of 0.01
mg/kg. In beet tops the maximum residue observed was 5.9 mg/kg, 21
days after treatment of 0.45 kg/ha.
Numerous trials have been carried out on potatoes in U.S.A., Canada,
France and Italy. No residues above the detection limit of 0.01 mg/kg
were found in potatoes harvested at 0-84 days following up to 9
applications at rates of 0.015-0.45 kg/ha.
Alfalfa
Residue data on alfalfa were obtained from the trials in U.S.A. where
single or repeat treatments were made at rates up to 0.45 kg/ha. When
alfalfa was harvested 21 days or later after treatments, the maximum
residue of 5.8 mg/kg was found at an application rate of 0.45 kg/ha.
The residues on dry alfalfa with water content of around 10% were
about 3 times higher than those on green alfalfa.
Legume vegetables
Residue data are available from several countries on legume vegetables
including green beans, dry beans, navy beans, snapbeans, pinto beans,
blackeyed peas, peas and soybeans. Residues in soybeans were
generally not detectable when analyzed more than 15 days after
treatment rates up to 0.45 kg/ha. The maximum residue of 0.06 mg/kg
was found 21 days after treatment at 0.08 kg/ha and 0.20 kg/ha. When
the treatment was made within the recommended dosage (0.22 kg/ha) and
the harvest was made 21 days or later after the treatment, the
residues in soybean plants, hay, straw and trash were below 5 mg/kg
except that one residue of 7.3 mg/kg was found after treatment at 0.20
kg/ha in the Australian trial.
Pome and stone fruits
Trials have been carried out on apples, pears and peaches in U.S.A.,
Canada, Australia, Japan and several European countries. In apples,
at application rates up to 0.72 kg/ha, the residues were less than 2.0
mg/kg 21 days or later after treatment except in a trial in U.S.A.
where 2.9 mg/kg was found 21 days after 7 treatments at 0.22 kg/ha.
In two trials in Japan, where 3 or 6 applications were made at a rate
of 1.4 kg/ha, the residues ranged from 0.92 to 3.5 mg/kg.
In pears the residues generally did not exceed 1 mg/kg following up to
7 applications at rates up to 0.67 kg/ha even when the last treatment
was made close to harvest. In one trial where 3 treatments were made
at a rate of 0.67 kg/ha the residue of 1.3 mg/kg was found 13 days
after the last treatment. In the same trial pears were treated with
fenvalerate at a rate of 1.34 kg/ha, and the residue was 1.9 mg/kg 13
days after 3 treatments.
Peaches were treated at rates of 0.1-0.8 kg/ha. The residues reached
2.3 mg/kg after 3 treatments at a concentration of 0.04% ai. Residues
of 2.2 mg/kg were found after 3 treatments at a rate of 0.8 kg/ha and
after a single treatment at a rate of 0.72 kg/ha.
Small fruits and berries
Trials on grapes have been carried out in U.S.A., Canada, Australia,
France and Italy. Residues did not exceed 0.80 mg/kg 14 days after
treatment at rates up to 0.22 kg/ha except two trials in France and
Italy. In Italian trials a residue of 1.3 mg/kg was found 30 days
after treatment at a rate of 0.2 kg/ha. In U.S.A., trials where
grapes were harvested 21 days after treatment at a rate of 0.11 kg/ha,
the residue of 1.6 mg/kg was reported, although in this trial the
residues 1 day and 14 days after treatment were 0.71 mg/kg and 0.51
mg/kg respectively.
In raspberries and strawberries the residues were less than 0.5 mg/kg
when the crops were harvested 7-23 days after treatment at rates up to
0.225 kg/ha.
Cereal grains, pre-harvest
Trials have been carried out in U.S.A., Canada, Australia and Brazil
on sweet corn, field corn, sorghum and wheat.
In sweet corn the maximum residue in kernels was 0.03 mg/kg, even
though 18 treatments were made with the intervals of 2-7 days and the
crops were harvested 2 days after treatment. The residues in stover
and ensilage ranged from 5.5 to 25 mg/kg. In field corn, the interval
between treatment and sampling was more than 72 days, and fenvalerate
was detected only in stover at a level of 0.07 mg/kg 140 days after
the treatment at a rate of 0.22 mg/kg. In wheat, the maximum residue
was 0.29 mg/kg in grain 13 days after treatment at a rate of 0.14
mg/ha and 15.0 mg/kg in straw 21 days after treatment at a rate of
0.30 kg/ha.
Cereal grains, post-harvest
Fenvalerate has undergone laboratory and silo-scale field trials as a
stored-grain protectant in Australia. Studies for this purpose have
been made on wheat, principally, (Bengston, 1979), barley
(Desmachelier, 1978) and sorghum (Bengston et al, 1979). All the
residue data indicate that fenvalerate is persistent under the
temperature and humidity conditions prevailing in Australian storages.
Large-scale trials have been made on stored wheat and sorghum
(Bengston, 1979 and Bengston et al., 1979a). Because of the strategy
of proposed usage fenvalerate has been applied at 1 mg/kg grain in
conjunction with its synergist, piperonyl butoxide (at 8 mg/kg grain)
and a complementary insecticide, fenitrothion (at l2 mg/kg grain).
Under these conditions of storage the fenvalerate residue declines
slowly: after 9-10 months about 75% of the applied amount remains
(Table 8). Expected residues in whole grain cereals would be less
than 1 mg/kg after storage.
Residues in processed products
Some of the cottonseed, peanuts and soybeans obtained from supervised
trials in U.S.A. and Colombia were subjected to processing to produce
oil products. The occurrence of residues in processed products are
summarized in Table 4.
In U.S.A. trials, the residues in various cottonseed oil products
after processing were less than 0.1 mg/kg. In the Colombian
experiment where a single treatment of 0.3 kg/ha was made one day
before harvest, the residue was 0.16 mg/kg, 0.23 mg/kg, 0.22 mg/kg and
0.18 mg/kg in crude, neutral, bleached and deodorised oil,
respectively, in accordance with a residue of 0.14 mg/kg in the
unprocessed cottonseed. These cottonseeds were separated into kernels
and hulls by a mechanical (simulated commercial) process. Such a
procedure might contribute to the higher residues, since the
mechanical processing might have caused contamination of kernels with
hulls, because when these seeds were separated by hand at the
laboratory, residues in kernels and hulls were 0.02 mg/kg and 0.40
mg/kg, respectively. Cottonseeds which were obtained from American
trials were separated by hand, and processed to oil products and no
residues were detected in extracted kernels.
Peanuts and soybeans which were harvested after treatment at rates of
0.11-0.45 kg/ha were processed to oil products and no residues were
detected in either crude or refined oil, or in extracted meal.
Wine made from grapes which contained up to 0.12 mg/kg of fenvalerate
71-74 days after treatment at rates of 0.075 and 0.15 kg/ha (France)
contained non-detectable residues.
Following post-harvest treatment and storage, fenvalerate residue in
wheat is found principally (68-69%) in the bran which comprises about
15% of the whole grain (Table 9). White flour constitutes about 72%
of the whole grain and contains about 8-9% of its fenvalerate residue.
The remaining residue is in the pollard. Residues in flour are
carried over into bread baked from that flour (Table 7b); there is no
reduction in residue level on a commodity-weight basis. White bread
prepared from treated grain would have about the same residue level of
fenvalerate as white flour, that is about 0.06-0.1 mg/kg on present
usage. The corresponding level for wholemeal bread or flour would be
about 0.5-0.8 mg/kg.
Table 3. Summary of Residues Following Field Trials (1976 to 1979)
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cottonseed Various
countries up to 13 0.0115 20 and Figures covering over 30 supervised trials reach 0.1
0.6 30 mg/kg only in isolated instances.
Peanuts (whole) U.S.A. 5 0.11 30 <0.01 <0.01
0.22 " <0.01 <0.01
5 0.22 " <0.01 <0.01 <0.01 <0.01
2 0.45 " 0.04 0.12 0.20 0.29
2 0.45 " 0.06 0.18 0.11 0.11
2 0.22 " 0.03
2 0.45 " 0.32
Peanuts (Vines) 5 0.11 " 0.69 0.01 0.02 0.1
5 0.22 " 1.6 0.02 0.04 0.04
2 0.11 " 6.4
2 0.22 " 3.2
2 0.11 " 0.51
2 0.22 " 0.42
Sunflower Australia 1 0.08 " 0.07 0.03
Brussels sprouts U.S.A. 8 0.045 " 0.13
8 0.09 " 0.04
Canada 3 0.15 " 0.85 0.60 0.45
Netherlands 1 0.045 " 0.04
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cabbage U.S.A. 4 0.11 " 0.03
4 0.22 " 0.01
8 0.055 " 0.37
8 0.11 " 0.46
3 0.11 " 1.2 0.82 0.28 0.76
3 0.22 " 2.4 1.2 0.37 0.45
Canada 3 0.15 " 0.25 0.14
3 0.16 " 0.30 0.02 <0.01
Australia 4 0.073 7.3 0.13 0.15 0.05
New Zealand 6 0.15 10 1.2 0.5 0.9
5 0.15 10 1.3 0.6 0.9
Thailand 1 0.07 20 <0.01 <0.01
Japan 3 0.3 20 0.14 0.011 <0.005
6 0.3 20 0.17 0.028 <0.005
Chinese cabbage Netherlands 1 0.05 30 0.52 0.25
Japan 3 0.15 20 0.25 0.13 0.091
5 0.15 20 0.33 0.084 0.061
Kale U.K. 2 0.075 30 1.5 0.88 0.62 0.26
2 0.225 30 1.8 1.2 0.93 0.53
Thailand 1 0.06 20 1.3 0.13
Cauliflower U.S.A. 4 0.11 20 1.5 0.35 0.15
4 0.22 20 1.8 0.61 0.32
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cauliflower Canada 3 0.16 20 0.28 0.02 <0.01
(cont'd)
Thailand 1 0.07 20 0.17 0.1
Broccoli U.S.A. 2 0.11 20 0.77
2 0.22 20 0.69
Canada 3 0.16 20 0.29 0.09 0.06
New Zealand 5 0.15 10 15.3 5.1 2.2
Celery U.S.A. 15 0.22 10 1.5 0.46
15 0.45 10 3.6 1.9
15 0.055 10 0.11 0.20
15 0.11 10 0.37 0.52
15 0.22 10 1.0 1.2
15 0.45 10 2.0 2.6
Lettuce U.S.A. 7 0.11 10 0.21 0.30 0.07
France 1 0.05 10 2.0 0.35 0.11
1 0.10 10 4.0 1.2 0.47
1 0.10 10 6.9 5.5 2.2
1 0.10 10 2.3 0.45 0.30
1 0.10 10 6.2 1.6 0.06
Netherlands 1 0.075 30 2.25 0.67
1 0.075 30 0.25 0.16
Aubergine France 1 0.11 30 0.08 0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cucumbers U.S.A. 5 0.11 30 0.09 0.04 0.03
5 0.22 30 0.48 0.08 0.03
5 0.45 30 0.54 0.07 0.08
Watermelon U.S.A. 4 0.11 30 0.02
4 0.22 30 <0.01
Bell peppers U.S.A. 3 0.11 30 0.06
0.22 30 0.11
0.45 30 0.39
7 0.22 30 0.19
0.45 30 0.34
Green peppers U.S.A. 2 0.45 30 0.02
Squash U.S.A. 5 0.11 30 0.10 0.02 0.01
Tomato U.S.A. 3 0.11 30 0.02 <0.01
0.22 30 0.07 <0.01
0.45 30 0.08 0.01
2 0.11 30 0.1
0.22 30 0.29
0.45 30 0.33
France 1 0.11 30 0.11 0.47 0.13
Netherlands 1 0.22 30 0.31
Spain 2 0.2 30 0.15 0.10 0.15
3 0.2 30 0.35 0.25 0.15
2 0.3 30 0.2 0.1 0.15
3 0.3 30 0.25 0.10 0.15
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Tomato Australia 9 0.01 20 0.16 0.15 0.19
(cont'd) 0.15 20 0.17 0.12 0.29
0.02 20 0.42 0.38 0.28
Mexico 2 0.15 20 0.04 0.03 0.02 0.03
1 0.01 <0.05 <0.05
South Africa 4 0.02 <0.05 <0.05
0.03 0.07 <0.05
0.04 0.06 <0.05
Mexico 1 0.15 30 0.08 0.05 0.03 0.03 0.01
1 0.3 30 0.13 0.13 0.08 0.05 0.02
3 0.3 30 0.31 0.16 0.19
Canary Islands 2 0.15 30 0.25 0.20 0.07
3 0.15 30 0.40 0.30 0.20 0.08 0.01(71)
Sugar Beets Demnark 1 0.15 20 Roots 0.03
Tops 1.2 0.59
U.S.A. 2 0.45 30 Roots <0.01 1.15(71)
Tops 5.9
(Similar findings in France and U.K)
Potatoes U.S.A.,Canada,
(20 trials) France 1-9 0.07-0.45 30 <0.01 <0.01 <0.01 <0.01 <0.01
No detectable residue found in any samples
Alfalfa U.S.A. 1 0.11 30 6.1 3.2 2.6 1.0
(green) 0.22 30 15 11 8.8 1.9
0.45 30 32 24 18 5.8
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Green beans France 1 0.16 30 0.31
0.21 30 0.67 0.48
0.32 0.43
0.43 30 1.7 0.58
Dry Beans U.S.A. 4 0.11 30 0.21
0.22 30 0.13
Navy Beans U.S.A. 4 0.11 30 <0.01
0.22 30 0.01
Australia 1 0.1 20 0.03 0.03
0.2 20 0.06 0.03
Snap beans U.S.A. 3 0.11 30 0.17 0.03
3 0.22 30 0.26 0.05
3 0.45 30 0.67 <0.01
Pinto beans U.S.A. 1 0.11 30 <0.01
0.22 30
Blackeyed peas U.S.A. 2 0.055 30 <0.01
0.11 30 <0.01
Peas U.S.A. 1 0.11 30 <0.01
1 0.22 30 <0.01
1 0.45 30 <0.01
Soybeans U.S.A. 1 0.11 30 0.06 0.01 <0.01 <0.01 <0.01
1 0.22 30 0.05 0.03 0.02 <0.01 <0.01
Brazil 2 0.12 30 <0.01 <0.01
2 0.24 30 <0.01 <0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Soybeans Colombia 4 0.075 30 <0.01
(cont'd) 4 0.30 30 <0.01
Australia 1 0.08 20 0.06
1 0.2 20 0.06
Asparagus bean Thailand 1 0.14 20 0.14 0.04
(A01-0603)
Apples France 1 0.15 30 0.26 0.37 0.40 0.19
Australia 3 0.02% 30 0.80 0.60 0.35
3 0.04% 30 0.90 0.35 0.35
Germany 6 0.0225 30 1.7 2.0 1.0
6 0.045 30 2.8 2.3 2.0 1.9 1.8
6 0.0225 30 0.96 0.84 0.87 0.56 0.61
6 0.045 30 2.4 2.6 1.8 1.5 1.0
6 0.225 30 2.8 2.4 2.1 1.9 1.6
6 0.045 30 4.8 5.8 3.6 1.3 1.3
Japan 3 1.4 20 1.91 1.76 1.88
6 1.4 20 3.21 3.5 2.88
3 0.72 20 0.6 0.26 0.44
3 0.70 20 0.54 0.42 0.40
New Zealand 12 0.008 10 1.0 0.57 0.57 0.37 0.52
South Africa 6 0.024% 20 0.14 0.07 0.05 <0.05
0.03% 20 0.14 0.08 0.09 0.05
0.048% 20 0.36 0.24 0.16 0.1
0.06 20 0.31 0.24 0.18 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Pears U.S.A. 7 0.11 30 0.11 0.08 0.05
7 0.22 30 0.16 0.14 0.08
3 0.67 30 1.3 0.48 0.60
0.24(62)
3 1.34 30 1.9 1.0 0.47
0.28(62)
2 0.67 30 0.54 0.39 0.30 0.15
France 2 0.1 30 0.32 0.23 0.19 0.15
2 0.13 30 0.40 0.30 0.22 0.14
Australia 3 0.02% 30 0.39 0.46 0.31
0.04% 30 0.56 0.60 0.30
South Africa 6 0.024% 30 0.22 0.08 0.06 0.05
0.03% 30 0.26 0.12 0.12 0.05
0.045% 30 0.35 0.24 0.19 0.12
Apricots U.S.A. 2 0.28 30 0.39
0.56 30 0.78
Cherries U.S.A. 3 0.055 30 1.0 0.66
0.11 30 0.93 2.3
0.22 30 2.9 2.6
Peaches U.S.A. 6 0.11 30 0.30 0.27 0.11
0.22 30 0.26 0.23 0.10
Japan 3 0.8 30 0.084 0.045 0.014
6 0.8 30 0.061 0.026 0.016
3 0.6 30 0.054 0.014 0.020
6 0.6 30 0.046 0.017 0.057
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Australia 3 0.02 30 0.52 0.52
0.04 30 1.3 2.3
Plums U.S.A. 2 0.22 30 1.3
0.45 30 1.1
Grapes U.S.A. 5 0.22 30 1.2 1.1 0.80
4 0.11 30 0.31 0.39 0.45
0.22 30 0.71 0.51 1.6
Canada 1 0.078 30 0.25 0.20
France 1 0.075 30 0.30 0.30 0.55 0.40
2 0.075 30 0.65 0.67
1 0.075 30 0.43 0.09 0.06 0.03
Raspberries Canada 1 0.225 30 0.26
3 0.135 30 0.40
Strawberries U.S.A. 1 0.11 30 <0.01 0.11
1 0.22 30 0.38 0.21
Canada 2 0.172 30 0.07
2 0.12 30 0.45
Citrus fruit Japan 3 0.67 30 0.78 0.89
(whole) 6 0.67 30 1.56 1.25
3 0.67 30 1.69 0.91
6 0.67 30 3.63 1.44
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Spain 2 0.15 30 1.1 1.2 0.85 0.75*
1.9 1.2 1.5 0.9
*Juice
<0.01
Kiwi fruit New Zealand 6 0.46 10 3.4 3.2 2.5 2.1
0.92 10 4.1 3.8 3.6 2.2
Sweet corn U.S.A. 18 0.22 30 0.01
(ears) 18 0.28 30 0.02
4 0.11 30 0.02
4 0.22 30 0.02 <0.01(140)
<0.01(164)
Field corn U.S.A. 1 0.22 4 Gran <0.01(164)
(ears) 1 0.11 30 <0.01(136)
1 0.22 30
1 0.45 30
Sorghum U.S.A. 2 0.11 30 0.17
0.22 30 0.32
0.45 30 0.58
Australia 1 0.075 30 4.0 1.09
0.15 30 4.9 1.70
South Africa 1 0.020 20 0.1 0.1 <0.1 <0.1 <0.1
0.030 20 0.2 0.2 0.1 0.1 <0.1
0.040 20 0.2 0.2 0.1 0.1 0.1
0.050 20 0.5 0.3 0.2 0.2 0.1
0.060 20 0.6 0.3 0.3 0.2 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Wheat Argentina 1 0.10 0.08
0.15 0.05
0.20 0.05
0.30 0.10
Canada 1 0.14 0.29 0.12 0.05
Brazil 1 0.09 0.01 0.02 0.01
Almonds U.S.A. 1 0.22 <0.01
Coffee beans Brazil 1 0.10 <0.06
Table 4. Distribution of Residues in Processed Products of Oilseeds
(Crop treated with 30% E.C. fenvalerate in U.S.A. during 1975-1976)
Cottonseed
Rate (kg/ha) 0.11 0.22 0.45 0.22 0.45
No. of applications 14 8 8 8 8
Pre-harvest intervals 30 6 6 48 48
(days)
Residues in....
Ginned cottonseed 0.05 0.01 0.02 0.12 0.19
Delinted cottonseed - - 0.02 0.01
Hulls 0.01 0.02 0.01 0.01 0.01
Solvent extracted meal - 0.01 0.01 0.01 0.01
Crude oil 0.04 0.01 0,01 0.03 0.05
Refined oil 0.05 0.01 0.01 0.04 0.06
Soapstock 0.01 0.01 0.01 0.01 0.01
Peanuts
Rate (kg/ha) 0.11 0.22 0.45
No. of application 2 2 2
Pre-harvest interval (days) 14 14 14
Residues in....
Whole nuts 0.01 0.02 0.02
Nut meats 0.01 0.01 0.01
Shells 0.03 0.04 0.02
Solvent extracted meal 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01
Refined bleached oil 0.01 0.01 0.01
Refined bleached
Hydrogenated oil 0.01 0.01 0.01
Soybeans
Rate (kg/ha) 0.11 0.22 0.45 0.22
No. of application 1 1 1 4
Pre-harvest interval(days) 66 66 66 48
Residues in....
Soybeans 0.01 0.01 0.01 0.02
Hulls 0.01 0.01 0.02 0.06
Solvent extracted meal 0.01 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01 0.01
Soapstock 0.01 0.01 0.01 -
FATE OF RESIDUES
Cows
Three lactating cows were fed daily doses of C-14-fenvalerate
(chlorophenyl ring label) at a level equivalent to 0.11 ppm in the
total diet for 21 days and sacrificed 12 hours after receiving the
last dose. Recovery of radiocarbon was about 65% of the administered
dose of which 29% was recovered in the urine, 36% in the faeces and
less than 1% in the milk. The radiocarbon in the milk ranged from
<0.0006 to 0.0019 µg equivalents/L and reached a plateau after one
week on the treated feed.
Nearly all of the radiocarbon in the milk was present as unchanged
fenvalerate. No detectable radiocarbon was found in the brain, fat,
kidney, liver, lung and muscle. The limit of determination in this
study was 0.02 ppm for fat and 0.01 ppm for other tissues (Potter,
1976a).
In a separate study, three lactating cows were fed 10.9 ppm
C-14-fenvalerate (benzyl ring label) in their total ration for 28
days. The C-14 residues in the milk collected during the last 24 days
of treatment ranged from 0.04 to 0.13 ppm equivalents. The
fenvalerate content of the whole milk ranged from 0.037 to 0.082 mg/kg
during the last 20 days of the test. The cows were sacrificed 12
hours after receiving the last dose. The highest tissue residues were
found in the mesenteric fat 0.74-0.79 mg/kg equivalent. The
gastrocnemius and quadriceps muscle contained up to 0.06 mg/kg
equivalents. A total of 92% of the C-14 administered to the cows was
recovered in the excreta and tissues (Potter and Arnold, 1977;
DeVries, 1976a).
Three lactating Guernsey cows were fed daily doses of C-14-fenvalerate
at 0.15 ppm in their total ration for 21 days. The cows were
sacrificed 12 hours after receiving the last dose. The C-14 in the
milk ranged from 0.0006 to 0.0018 mg/kg equivalents. The C-14
residues in the milk reached a plateau after one week on the treated
feed. Within the experimental error of the measurements all of the
C-14 in the milk was present as fenvalerate. No C-14 was found in
bone, brain, kidney, lung or muscle (limit of detection = 0.004 to
0.007 mg/kg equivalents). Traces of C-14 were found in mesenteric fat
and subcutaneous fat from two cows and in the liver from one cow
(Potter, 1976b).
Lactating cows were fed an equivalent of 0.15 ppm C-14 fenvalerate in
their daily ration for 21 days. Residues of 0.1-0.2 mg/kg of
fenvalerate were found in the cream by TLC and liquid scintillation
counting. These residues were confirmed as fenvalerate by GLC-EC
(Potter, 1976e),
Three dairy cows were each sprayed weekly for three successive weeks
at the estimated rate of 540 mg active material per square metre.
This spraying regime was proposed for ectoparasite control. Samples
of whole milk taken during the treatment period and up to one week
after the last application contained less than 0.01 mg/kg of
fenvalerate. No detectable residue was found in the brain, muscle,
liver and kidney. The fat contained up to 0.2 mg/kg fenvalerate
(DeVries, 1976b).
In a further study (DeVries, 1976c), the same spraying regime was
applied to 10 cows. Residues in cream reached a maximum level of 0.2
mg/kg three days after the last application. The elimination of these
residues followed first order kinetics with a half-life of 4.5 days.
Residues in body fat reached a maximum of 0.4 mg/kg several days after
the last application, and declined thereafter according to first order
kinetics with a half-life of 10.5 days. All other tissues tested were
free of detectable residues.
Fenvalerate was also applied as a spray at a rate of 0.2, 0.4 or 2.0
g/cow. The residues in muscle did not exceed 0.01 mg/kg. The maximum
residue in subcutaneous fat was 0.22 mg/kg 7 days after 3 treatments
at a rate of 2.0 g/cow. The maximum residue in milk was 0.02 mg/kg.
The residues in milk fat reached maximum a few days after treatment
(Noble, 1976a; DeVries, 1976d, 1976e, 1976f, 1976g).
A trial was conducted in Queensland, Australia, to determine the level
and fate of fenvalerate in cows following spray treatment with 0.1%
and 0.2% of fenvalerate at 200 ml per animal. Twenty-seven Hereford
heifers were used in the trial. Twelve animals were drafted to each
treatment group with three being kept as untreated controls. Three
animals in each treatment group were re-treated 7 days after the first
treatment. These animals were then slaughtered and sampled 7 days
following the second treatment to ascertain whether there was any
accumulation. Tissue samples were taken from omental fat, liver,
perirenal fat# kidney and muscle. The residues found in the samples
are indicated in Table 5 (limit of determination 0.01 mg/kg). Only
representative samples of non-fat tissues were analysed when
preliminary results indicated no detectable residues in liver, kidney
and muscle. (Shell Chem. Australia, 1978).
Six dairy cows were selected and randomly divided into two treatment
groups of three animals. Milk samples were collected prior to
treatment. Treatment at the rate of 200 ml/animal was made along the
dorsal midline. One group received spray of approved strength (0.1%
fenvalerate) and the other at double strength (0.2%). One litre
aliquots of milk were collected from each cow at each milking on days
1, 3, 7, and 10 post-treatment. These samples were separated and the
cream and skim milk portions were analysed separately. The results
are given in Table 7. The residues were confined to the butter fat.
A commercial dairy herd was treated with fenvalerate emulsion (0.1%
fenvalerate) at the rate of 200 ml/cow. Samples of the bulked milk
were taken prior to treatment and on days 1, 3, 7 and 10
post-treatment. These samples were separated and the cream and skim
milk analysed for fenvalerate residues. The results are given in
Table 6. The residues were confined to the butter fat.
Table 5. Residues in tissues of 27 Cows following Spray Treatments
(Queensland, Australia)
Residue mg/kg
Treatment of Day Omental Perirenal Liver, kidney
each cow fat fat muscle
untreated 0 <0.02 <0.02 All Samples
untreated 0 <0.02 <0.02 <0.01
untreated 0 <0.02 <0.02
0.2% a.m. 1 0.04 0.06
0.1% 1 0.05 0.03
0.1% 1 <0.02 0.03
0.2% 1 0.06 0.08
0.2% 1 0.08 0.04
0.2% 1 0.04 0.06
0.1% 3 0.02 0.02
0.1% 3 0.04 0.05
0.1% 3 0.02 0.03
0.2% 3 0.03 0.03
0.2% 3 0.04 0.03
0.2% 3 <0.04 <0.02
0.1% 7 0.04 0.04
0.1% 7 0.04 0.03
0.1% 7 0.05 0.10
0.2% 7 0.05 0.06
0.2% 7 0.07 0.05
0.2% 7 0.05 0.08
0.1% 14 0.05 0.06
0.1% 14 0.08 0.07
0 1% 14 0.10 0.08
0.2% 14 0.06 0.07
0.2% 14 0.13 0.07
0.2% 14 0.17 0.08
Table 6. Residues found in Butterfat of Bulked Milk of Commercial
Herds (Each cow treated once with fenvalerate emulsina).
Days after
treatment ppm fenvalerate
0 <0.005
1 0.015
3 0.066
7 0.046
10 0.030
Skim milk contained <0.002 ppm fenvalerate.
Table 7. Residues in Milk From Each of Six Cows Treated Once with Fenvalerate Emulsion
Animals sprayed at 1% Animals sprayed at 2%
Days after
treatment 1 2 3 Mean 4 5 6 Mean
Residues of fenvalerate in mg/kg of moisture-free butter fat
(Pre-treat)
0 (AM) <0.005 <0.005 <0.005 - - - - -
0 (PM) 0.016 0.041 0.006 0.02 0.011 0.008 0.011 0.019
1 (AM) 0.022 0.032 0.026 0.027 0.012 0.034 0.027 0.024
1 (PM) 0.023 0.044 0.025 0.031 0.036 0.029 0.034 0.050
3 (AM) 0.046 0.047 0.030 0.042 0.133 0.059 0.047 0.080
3 (PM) 0.031 0.037 0.019 0.029 0.094 0.050 0.046 0.063
7 (AM) 0.012 0.043 0.022 0.026 0.043 0.025 0.040 0.036
10 (AM) 0.016 0.029 0.022 0.022 0.019 0.029 0.046 0.031
Skim milk (residue after butter fat removal) contained less than 0.002 ppm.
Table 8. Fenvalerate Residues in Treated Wheat and Sorghum Stored in Concrete Silos
Grain Amount of Residues (mg/kg)
fenvalerate
Kind moisture Temperature applied calc'd Storage period (months)
% °C (mg/kg)
0.25 1.5 3 4.5 6.5 8 10
(a) Wheat1
range 10 31-25* 0.9-1.1 0.85-1.16 0.45-0.87 0.76-1.02 0.66-0.82 0.79-0.93 0.74-0.85 0.71-0.76
mean 1.01 1.01 0.7 0.9 0.76 0.86 0.8 0.74
(b) Sorghum2
range 26-24* 0.42-0.55 0.64-0.72 0.53-0.54 0.64-0.69
mean 11.8 0.53 0.68 0.54 0.67
* 1st figures are initial temperatures; 2nd are those 6-9 months later.
Wheat data are from two trial sites, one in each of two Australian States.
1 Bengston, 1979
2 Bengston et al., 1979
Table 9. Distribution of Fenvalerate Residues in Whole Wheat
Fractions and Residue Levels in Bread Baked from the Flour (B.W.
Simpson, 1979)
(a) Distribution of residue between whole grain fractions
Fraction fenvalerate residue
% whole mg/kg % distribution
grain
flour (white) 74.6 0.08 8.5
pollard 14.6 3.3 68.5
bran 10.8 1.5 23
Grain sample: 1.5 kg. It had a residue level of 0.6 mg
fenvalerate/kg.
(b) Residue in bread baked from the ground flour from (a)
Commodity fenvalerate residue (mg/kg)
White flour 0.08-0.09
White bread 0.06-0.1
Wholemeal flour* 0.7-0.8
Wholemeal bread 0.49-0.73
* reconstituted from the fractions (in (a) ) in the original whole
grain proportions.
The processing operations simulated those used in commercial practice.
Table 10. Residues of Fenvalerate Degradation Products in Field-Treated Crops in Canada
S-Phenoxy Fenvalerate Isovaleric "Reverse Decarboxylated
Benzoic Acid Amide Acid Ester" Fenvalerate
Crop Country Dose Rate (×) PHI Fenvalerate WL 44607* WL 47117+ WL 10944* SD53036 SD 54597
Apples Canada 70 g/ha (×1) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
16 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
150 g/ha (×8) 6 weeks 0.29 <0.05 <0.05 <0.05 <0.01 <0.05
6 weeks 0.57 <0.05 <0.05 <0.05 <0.01 <0.05
Pears Canada 30 g/ha (×3) 5 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
70 g/ha (×2) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
Peaches Canada 0.006% 0 weeks 0.4 <0.05 <0.05 <0.05 - -
1 week 0.25 <0.05 <0.05 <0.05 - -
2 weeks 0.10 <0.05 <0.05 <0.05 - -
Grapes Canada 0.006% 0 week 0.45 <0.05 <0.05 <0.05 - -
1 week 0.38 <0.05 <0.05 <0.05 - -
Cabbage Canada 70 g/ha (×7) 2 weeks 0.06 <0.05 <0.05 <0.05 <0.01 <0.05
Cauliflower 70 g/ha 1 day 0.20 <0.05 <0.05 <0.05 - -
14 days 0.02 <0.05 <0.05 <0.05 - -
B. sprouts Canada 70 g/ha (×6) 14 days 0.10 <0.05 <0.05 <0.05 - -
70 g/ha (×7) 1 day 0.14 <0.05 <0.05 <0.05 - -
Lettuce Canada 70 g/ha 7 days <0.01 <0.05 <0.05 <0.05 - -
Tomatoes Canada 150 g/ha (×2) 2 days 0.80 <0.05 <0.05 <0.05 - -
* Includes both free and conjugated forms
+ No conjugates formed
Hens
Mature laying hens were fed 0.03 ppm of C-14-fenvalerate (chlorophenyl
ring label) in their total ration for periods up to 32 days. No
detectable C-14 residues were found in the fat, heart, gizzard, liver,
meat, skin, egg whites and egg yolks (Potter, 1976c).
In a separate study, mature laying hens were fed 0.03 ppm of
C-14-fenvalerate (phenoxyphenyl ring label) in their total ration for
periods up to 32 days. No C-14 residues were detected in the light
meat, dark meat, skin, gizzard, blood or plasma. The residues in egg
yolks ranged from <0.002 to 0.003 mg/kg, fat samples <0.002 to 0.003
mg/kg (Potter and Sauls, 1978).
Laying hens were sprayed twice with a 0.5% emulsible concentrate of
fenvalerate. Fenvalerate residues in the eggs reached maximum levels
of 0.04 mg/kg 6 days after the first application and of 0.14 mg/kg 8
days after the second application. The residue levels in the eggs
decreased by one half in about 22 days. The major tissue depots for
fenvalerate were found to be the skin and fat (DeVries, 1976d).
Plants
Leaves of cotton were treated either once with a formulation of
C-14-fenvalerate (chlorophenyl ring label) at a rate of 240 ug per
leaf, or twice at a rate of 120 ug per leaf per treatment.
Determination of C-14 in extracts showed that the rate of C-14
disappearance from leaves was practically identical between single and
repeated applications, and a disappearance rate of about 50% was 35
days. Thin-layer chromatographic analysis of extracts from the cotton
leaves showed that fenvalerate represents about 50% of the C-14
recovered 101 days after single treatment and 80% of the C-14
recovered 46 days after the last of double treatments (Loeffler, 1976a
and 1976b).
In a separate study, cotton squares were also treated either once with
a formulation of C-14-fenvalerate (chlorophenyl ring label) at a rate
of 240 µg per square or three times at a rate of 120 µg per square per
treatment. Whole seeds isolated two to three months after the first
treatment contained less than 0.05 mg/kg equivalents of fenvalerate.
Single leaves of cotton, located either on the lower or the upper part
of the plants, were treated with an average of 49.7 µg per leaf to
determine the transport of C-14-fenvalerate from the treated to
untreated leaves. After 15 days, no C-14 was found in any of the
untreated leaves both above and below the treated leaves,
demonstrating the inability of fenvalerate or any possible C-14
metabolites to move out of a treated leaf either in the xylem or in
the phloem (Loeffler, 1976 c and d).
When C-14-fenvalerate labelled in either benzyl or chlorophenyl ring
was applied to lettuce grown in boxes (twice at a rate of 10.8 mg/box)
about 70-80% of the C-14 in the mature plants was present as unchanged
fenvalerate 12 days after the second treatment. Fenvalerate
hydrolysed slowly at the ester linkage to give 3-phenoxybenzoic acid
and 2-(4-chlorophenyl) isovaleric acid which then rapidly conjugated
with plant materials (Hitchings, 1977). When the same compounds were
applied to the fruit and leaves of apple trees twice or three times,
the leaves and apples harvested 3 to 4 weeks following the last
treatment were found to contain mainly unchanged fenvalerate (75 -85%
of the total C-14 in leaves and 86-93% of the total C-14 in apples).
After peeling the apples, less than 2% of the C-14 in the apples
remained in the peeled fruit. The major metabolite fraction (11-19%
of the total C-14) in leaves was a mixture of polar compounds which by
hydrolysis produced 3-phenoxybenzoic acid, 3-phenoxybenzaldehyde,
3-phenoxybenzyl alcohol and 2-(4-chlorophenyl) isovaleric acid. Other
minor metabolites identified in leaves and/or apples were des-cyano-,
amide- and 4'-OH-fenvalerate which accounted in total for less than 2%
of the total C-14. (Standen, 1977).
Fenvalerate and the (S)-acid isomer similarly disappeared from
cabbages and bean plants with half-lives of approximately 9 days and
14 days respectively, after foliar application of three C-14-labelled
preparations (Co, Ca and CN) at rates of 17-19 µg (cabbage) and 10 µg
(bean plant) per leaf. On and/or in plants, both compounds underwent
decarboxylation, ester cleavage, hydrolysis of the CN group to CONH2
and COOH groups, hydroxylation at the 2'-and 4'-phenoxy positions,
cleavage of diphenyl ether, conversion of a-cyano-3-phenoxybenzyl
alcohol to the corresponding benzyl alcohol and benzoic acid, and
conjugation of the resulting carboxylic acids and alcohols with sugar.
Very little of the C-14 was transferred to other parts of cabbages and
bean plants. Beans were planted in Kodaira light clay and Katano
sandy loam soils after treatment with 1.0 mg/kg of the CN- and
Ca-labelled preparations. After 30 days, pods and seeds, shoots and
roots were harvested and analyzed for C-14. Although small amounts of
radioactivity were found in roots, traces were present in pods, seeds
and shoots. No parent compound was detected in shoots (Ohkawa et al.,
1979a).
Search for degradation products on field grown crops
A series of trials were conducted in Canada (Shell 1979) to determine
whether and to what extent degradation products occur as free and
conjugated residues in crops grown under typical outdoor conditions.
As indicated in Table 8, none of the known degradation products could
be detected by methods sensitive to 0.05 mg/kg in any of 9 varieties
of fruits and vegetables treated in accordance with label directions.
Fenvalerate itself occurred in most of the samples at levels
consistent with those found in other trials.
Distribution of residues on the plant
From the examination of many plants treated under practical
conditions, it is evident that residues are almost exclusively
confined to those parts of the crop that are directly exposed to the
spray application. As examples, cottonseed kernels and peanut meats
have not contained detectable levels even when quite high amounts were
measured on the whole seeds or nuts (Shell Res. 1974, 1975 a and b,
1976 a and b, Shell Dev. 1976 a and b, 1977a, 1978b).
Trials on cabbage in Australia gave residues at 7.5 mg/kg for outer
discarded leaves and only at 0.05 mg/kg for cabbage hearts 5 days
after treatment at 0.118 kg ai/ha (Microchem., 1977). In another
trial, when tops of sugar beets contained up to 5.9 mg/kg, only trace
residues (0.03 mg/kg or less) were detected in roots (Statens, 1977;
Shell Develop., 1977b). Trials on Japanese radish in Japan showed
residues up to 1.97 mg/kg for leaves and only 0.005-0.04 mg/kg for
roots (Fujita et al., 1978a).
Similarly, in trials with peas, beans, citrus fruit, almonds, sweet
corn and wheat, the main part of the residues occurred on peel, skins,
pods and other exposed parts. Residues in edible portions rarely
exceeded detectable levels. (Shell Dev. 1976, 1977; Fujita et al.,
1978; Japan Food Res. 1977).
Photochemical degradation
When exposed to sunlight on silica gel, glass, soil and in water,
fenvalerate degraded in all these media. After 28 days of exposure to
sunlight about 25% was recovered from silica gel, 48% from soil or
from water, less than 1.3% from glass (FAN 1976).
When a 0.2 M solution of fenvalerate was exposed for 10 hours at 300
nm in quartz SD54597 (Benzenepropanenitrile,
4-chloro-beta-(1-methylethyl)-alpha-(3-phenoxyphenyl)-I was the major
product. Other amounts to less than 1% of the total. At 254 nm
SD53036 (Benzoic acid, 2-phenoxy-, 1-(4-chlorophenyl)-2-methylpropyl
ester) was also formed equivalent to about 3% of SD54597 present and
other products were less than 1% of the total (Ehmann, 1978a and b).
In various solutions (hexane, methanol, and acetonitrile-water; 6/4)
by artificial light (>290 nm) and as a thin film on grass or on
cotton by exposure to sunlight, the major products in solution were
formed via photoinduced decarboxylation. Other products resulted from
cleavage of the ester linkage, probably by a mechanism involving
free-radical intermediates. These were 3-phenoxybenzoyl cyanide,
3-phenoxybenzyl alcohol and 3-phenoxybenzaldehyde from the alcohol
moiety, and 2-(4-chlorophenyl) isovaleric acid and the dimer of the
acid fragment from the acid moiety. Decarboxylated fenvalerate,
2-(4-chlorophenyl) isovaleric acid, 3-phenoxybenzyl alcohol and
3-phenoxybenzaldehyde were also found on cotton. (Holmstead, et al.,
1977, 1978).
Another photodecomposition study reveals that on exposure to sunlight
fenvalerate was rapidly decomposed in distilled water, 2% aqueous
acetone, river and sea water to almost the same extent.
Photodegradation half-life in each aqueous suspension ranged from ca.
4 days in summer to 13-15 days in winter, due to seasonal variation of
sunlight intensity. On soil surfaces, exposed to sunlight, the rate
of photodegradation was dependent upon the soils used, with half-lives
of about 2, 6 and 18 days in Kodaira light clay, Azuchi sandy clay
loam and Katano sandy loam soils, respectively. Thus photodegradation
rate and pathways were dependent on the environmental conditions. The
predominant reactions were decarboxylation and cleavage of ester
linkage in water, while hydration at CN group was dominant on the soil
surface. (Mikami et al., 1979).
Soil
When C14-fenvalerate (chlorophenyl ring label) was applied to steam
sterilized and live sandy loam soil at a concentration of 2.5 ppm, the
amount of solvent extractable C-14 decreased slowly in aerobic and
anaerobic soils and stayed practically constant in sterile soils. The
proportion of intact fenvalerate in the extracts from aerobic and
anaerobic soils decreased to 90% over a three-month period. The
remaining 10% was present in the form of two to three products more
polar than fenvalerate (Loeffler, 1976a). Degradation of the same
labelled compound was examined under aerobic and anaerobic conditions
using three different soils and a steady decrease of recovery in the
organic solvent-extractable fraction were observed both under aerobic
and anaerobic conditions (Lee, 1978). Several of the breakdown
products of fenvalerate formed during the course of disappearance of
the parent compound were identified (Fan 1977). The effects of soil
micro-organisms were investigated and it was demonstrated that
sterilization of soil greatly retards degradation (Ohkawa et al.,
1978; Williams and Brown 1979; Noble, 1976).
The leaching behaviour of fenvalerate from soil was also investigated
(Ohkawa et al., 1978a; Jackson and Roberts, 1976; Kerritt, 1977).
Fish
In tests in which Channel catfish and rainbow trout were exposed to
dilutions of C-14 fenvalerate in water, rates of uptake and
distribution in the bodies of fish were followed and some metabolites
were identified (Potter 1975, 1976; Ohkawa, et al., 1978, 1979). The
results gave no evidence to suggest that problems may arise from the
concentration of residues in the bodies of fish that are used as human
food.
METHODS OF RESIDUE ANALYSIS
GLC equipped with electron-capture detector (ECD) has been used almost
exclusively for detection and quantitation. The ECD exhibits high
sensitivity to fenvalerate, responding to as little as 0.05 ng or even
less (Chapman et al., 1977), which afforded minimum detectability of
0.005-0.02 ppm (Takimoto et al., 1977; Talekar 1977; Shell Dev.
1976a). As a radioactive source, nickel-63 appears more advantageous
than tritium due to the relatively high temperature applied. Although
the alkali flame ionization detector (AFID) was also utilized for
confirmation of residue identity, the response of fenvalerate to
GLC-AFID was found to be about 100 times less than GLC-ECD. The
confirmation can be made by combined gas-liquid chromatography/mass
spectrometry (GLC/MS) using selective ion detection mode at mass peaks
such as 225 amu and 419 amu (Parent ion) (Shell Dev. 1976a).
Since fenvalerate comprises 4 optical isomers due to 2 chiral centres,
its GLC gives either single or resolved peaks depending upon the
column length, the column-packing materials, etc. A single peak
response was obtained by using 45-60 cm-long glass or stainless steel
column packed with 3% OV-101 on Gas-chrom Q (Shell Dev. 1976b), 3%
OV-101/3% Apiezon Grease L on Gas-chrom Q (Takimoto et al., 1977;
Talekar 1977), 5% OV-17 on Chromosorb W AW DMCS (Kato 1979), and 3%
SF-96/3% Apiezon Grease M on Diatomite CLQ (Woodhouse et al., 1979).
On the other hand Lee et al., (1978) observed two completely resolved
peaks for fenvalerate on 1.8 m-long glass column packed with 4%
SE-30/6% QF-1 on Chromosorb W (80/100 mesh) where the RR and SS
isomers with identical chromatographic properties were eluted after
the RS and SR pair. A complete resolution for fenvalerate was also
achieved on 3 m glass column packed with 3% OV-101 on Chromosorb W AW
DMCS (100/120 mesh) (Kato et al., 1978).
When exposed to a relatively high temperature in the GLC system (e.g.
more than 250°C at injection port), fenvalerate may undergo partial
degradation and/or isomerization depending upon the column material,
the glass wool packed into the injection port, and the column-aging
history (Barber 1976a). Therefore, care must be taken in the
simultaneous analysis of the diastereomers. The effects caused by
degradation are reproducible and can be minimized to insignificant
levels for quantitation.
The high-pressure liquid chromatography (HPLC), which permitted less
than 3 ppm of detection limit in one study (McKinney 1975), has been
applied to the residue analysis of fenvalerate. It has been used
successfully to determine residues in cereal whole grains and
commodities from the grains, such as flour and bread (Simpson 1978).
The problems with indeterminate differentiation of fenvalerate
diastereoisomers and breakdown on GLC columns were not found with HPLC
separation. The post-harvest residue data on treated cereals, flour
and bread reported in this monograph have been obtained by HPLC
determination. It was found that the limit of determination was in
the region of 0.02 mg/kg.
The solvent or solvent combinations selected for extraction of
fenvalerate vary according to the nature of substrates. From the
substrates with high moisture contents like fruits, leafy vegetables
and root crops fenvalerate was successfully extracted by chopping and
blending them either with polar solvents, e.g. acetonitrile (Lee et
al., 1978) and methanol-acetonitrile (Takimoto et al., 1977), acetone
(Chapman and Harris, 1978), or with nonpolar-polar solvent mixtures,
e.g. n-hexane-isopropanol (Shell Develop. 1976b) and petroleum
spirit-acetone (Shell Res. 1976). Talekar (1977) adopted a 12-hour
Soxhlet extraction with n-hexane-acetone for chopped cabbage. Dry and
low-moisture products are first fractured or ground to powder, under
freezing if necessary, prior to extraction with either polar, nonpolar
or dual solvent system (Shell Res. 1975). After mixing with anhydrous
sodium sulphate, cottonseeds were fractured, separated from the hull,
and blended with n-hexane (Shell Develop. 1976c), whereas coffee beans
were pulverized and macerated overnight with methanol (Kato 1979).
The blending/extraction with nonpolar solvents like n-hexane resulted
in excellent recoveries of fenvalerate with satisfactory separation
from fatty materials in the case of mammalian tissues, dairy products
and eggs (Shell Develop. 1976a).
Cleanup procedures include partition between immiscible polar and
nonpolar solvents, e.g. acetonitrile and n-hexane, for most extracts
from oily and dehydrated crops as well as from fatty products prior to
further Florisil column chromatography. Chapman and Harris (1978)
partitioned the aqeous acetone extract from a range of vegetables into
n-hexane before proceeding to cleanup on silica or alumina absorbents.
The combined solvent partition-Florisil column chromatography
procedure provided recoveries of 81-95% for eggs, chicken and cow
tissues, milk, and cream spiked at 0.01-0.1 ppm of fenvalerate (Shell
Develop. 1976a), along with 90-95% for cottonseed (Shell Develop.
1975) and 89% for coffee (Kato 1979), both fortified at 0.5 ppm. For
non-oily crops cleanup by either Florisil (activated or deactivated
with water) (Takimoto et al., 1977; Talekar 1977; Lee et al., 1978;
Shell Res. 1976), or silica gel (Lee et al., 1978; Kato et al., 1978)
column chromatography was applied. Over 90% recoveries of fenvalerate
were accomplished for crops including cabbage (Takimoto et al., 1977;
Talekar 1977; Lee et al., 1978); lettuce (Lea et al.,1978), potatoes
(Woodhouse et al., 1979), orange (Fujita et al., 1979), apple,
tomatoes, green peppers, peas and alfalfa (Shell Develop. 1976b) at
fortification levels of 0.01-40 ppm.
For the degradation products and metabolites of fenvalerate, Barber
(1976b) devised the analytical method for detection limits of
0.01-0.02 mg/kg for the residues of 3-phenoxybenzoic acid and
4-cloro-a-(1-methylethyl)-benzeneacetic acid (CMBA) in cottonseed.
(Kato et al., 1979) analyzed CMBA in human urine. The method
permitted detection of CMBA at 0.002 ppm as well as 93-100% recoveries
from the human urine fortified at 0.02-2 mg/kg. Barber (1978a-e) also
developed analytical methods for 1-(4-chlorophenyl)-2-methylpropyl
3-phenoxybenzoate and
4-chloro-B-(1-methylethyl)-a-(3-phenoxyphenyl)-benzenepropanenitrile
in soybeans, pears and apples as well as (aminocarbonyl)
(3-phenoxyphenyl)methyl 4-chloro-a-(1-methylethyl)-benzeneacetate in
potatoes and peanut shells with detection limits at 0.01 mg/kg.
NATIONAL LIMITS
National MRLs brought to the notice of the Meeting are listed (July
1979)
Country Commodity MRL Pre-harvest
(mg/kg) interval, days
Brazil Cotton 0.1 14
Soybeans 0.1 30
U.S.A. Cottonseed 0.2 21
Cattle(fat, meat,
meat by-products) 0.02
Goats (fat, meat,
meat by-products) 0.02
Hogs (fat, meat,
meat by-products) 0.02
Horses (fat, meat,
meat by-products 0.02
Milk (fat) 0.02
Sheep (fat, meat,
meat by-products) 0.02
(temporary)
Lettuce (head) 1 7
Cabbage 2 7
Cauliflower 1 7
Broccoli 0.5 14
Snap beans 0.5 7
(green beans)
Dry beans and 0.5 21
Dry pears
Cucumbers 0.05 10
Squash 0.05 10
Bell peppers 1 10
Tomato 1 7
Field corn 0.05 45
Grapes 1 21
Sweet corn (for
fresh market only) 0.05 3
Soybeans 0.02 21
National Limits, Continued...
Country Commodity Tolerance Pre-harvest
(mg/kg) interval, days
U.S.A.
(temporary)
Peanuts 0.02 21
Potatoes 0.02 21
Pears 1 28
Apples 2 21
Peaches 2 21
Australia Cottonseed, maize,
sweet corn 0.05 7
Pome and stone fruit 1 14
Milk and milk products
(fat basis) 0.2 -
Fat of meat of cattle 0.2 -
Soya beans, navy beans,
mung beans 0.2 21
Oilseed crops 0.2 14
Hungary
(temporary) Apples 1 14
Cherries 1 14
Peaches 1 14
Alfalfa 1 14
Beets 1 14
Cabbage 1 7
South Africa Apples, pears 0.5 14
Tomatoes 0.1 2
Grain sorghum 0.1 28
Cottonseed 0.1 -
New Zealand Brassica vegetables 5 7
Kiwi fruit 3 14
Apples, pears 1 14
Appraisal
Fenvalerate is a new synthetic ester related to the pyrethroids, with
a wide spectrum of efficacy against many species of pests. It is only
slowly degraded in sunlight. It is registered in a number of
countries for use on a variety of crops. The rate of application
ranges from 50-250 g/ha.
Supervised trials have been carried out in many countries and on many
crops. The rate of decline of residues on growing crops is slow, the
half-life ranging from 2 to 3 weeks. On stored cereal grains about
75% of the applied amount is found at the ninth month after treatment.
Fenvalerate does not penetrate significantly into plant tissues nor is
it translocated and there is little or no residue in crop parts not in
direct contact with the insecticide. Most of the residue remains on
the exposed parts of such commodities as cottonseed, peanuts and
fruits and is removed with shells and peels during processing and
preparation. Similarly, after treatment of stored grains some 70% is
in the bran of wheat, with considerably more in the hulls of oats and
rice which are normally removed before consumption.
A wide range of studies have been conducted on the fate of residues in
plants and animals. Residues in plants consist substantially of the
parent compound. In radiolabelled studies the occurrence of free and
conjugated metabolites has been demonstrated but these products have
not been found in field trials at levels above the limit of detection
(<0.05 mg/kg).
When administered or applied to livestock, fenvalerate is degraded and
the degradation products excreted in urine. A small proportion of the
applied dose is transferred to fatty tissues from which it may be
excreted via fat in the milk.
The process of photodecomposition proceeds by oxidation,
decarboxylation, hydration at the Cn-group, cleavage of ester and
diphenyl ether linkages and thence by hydrolysis to produce polar
derivatives of low molecular weight. It varies with exposure and is
fairly slow.
Fenvalerate is readily biodegraded by soil micro-organisms with a
residual half life of from 15 days to 3 months depending on soil
temperature, moisture and organic matter content. Because residues
are tightly bound to soil colloids, there is little likelihood of such
residues being transferred to water of streams through run-off or
leaching. Sprays falling directly onto bodies of water are rapidly
absorbed and inactivated on particulate matter. There is no evidence
of residues in soil being taken up by plants.
Under laboratory conditions it is possible to demonstrate that fish
can absorb the insecticide from water into various body tissues. Due
to the presence of particulate matter, surface absorption and the low
solubility of fenvalerate in water there appears to be little
likelihood of significant uptake of residues by fish under practical
conditions; nor are problems likely to arise from the occurrence of
residues in fish for human consumption.
Residues may be determined by GLC using electron capture detectors.
The ECD exhibits high sensitivity to fenvalerate responding to as
little as 0.05 ng. Polar solvents have been effective for extraction
when used with substrates containing substantial amounts of water.
Non-polar and petroleum solvents have been used with substrates
containing little water. Sweet co-distillation has proved convenient
and effective with lipid substrates. The limit of determination is
0.01 mg/kg.
Several national governments have established maximum residue limits
in a range of commodities. The data available provided a basis for
the Meeting to recommend the following maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits, determined and expressed as
fenvalerate, are recommended:
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Kiwi fruit 5 21
Alfalfa 20 21
Broccoli 2 7
Brussels Sprouts 2 7
Cabbage 2 7
Cauliflower 2 7
Celery 2 7
Lettuce 2 7
Chinese cabbage 1 7
Pome fruits 2 14
Peaches 2 14
Cherries 2 14
Citrus fruit 2 21
Tomatoes 1 7
Small fruits and berries 1 7
Beans (green) 1 7
Beans (dry) 0.5 7
Cottonseed 0.2 7
Cottonseed oil 0.1
Cucumbers 0.2 3
Squash 0.2 3
Soybeans 0.1 21
Sweet corn 0.05 7
Sugar beets 0.05 21
Sunflower seed 0.1 28
Radishes 0.05 21
Potatoes 0.05 7
Peanuts 0.1 7
Cereal grains 5
Wheat bran 5
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Wheat flour (wholemeal) 2
Wheat flour (white) 0.2
Milk 0.01
Milk products (fat basis) 0.2
Fat of meat 0.2
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
fenvalerate and/or metabolites;
2. Observations in humans occupationally exposed to high levels of
fenvalerate to evaluate the potential susceptibility of man to the
neurological disruption noted in rodents.
Desired:
1. An additional dominant lethal assay to reconfirm previous data;
2. Information on the occurrence and fate of photodecomposition
products;
3. Additional data from supervised residue trials on citrus, berry
fruits, beans and several other crops for which data are limited;
4. Results of on-going studies on the level and fate of fenvalerate
residues in stored products especially raw grain and milling
products derived therefrom.
REFERENCES
Albert, J.R. and Summitt, L.M. Intravenous Toxicity of WL 43775
(6-1-0-0) in the Mouse. (1976) Unpublished report from Shell
Development Co., Ltd.,
Barber, G.F.
1976a Separation of SD 43775 isomers for residue analysis.
1976b Residue data for SD 44607 in WL 10944 (acid metabolites of
SD43775 in cottonseed from cotton receiving seven applications of
SD43775, a California study. Shell Development Co.
1978a Residue data for SD 53036 and SD 54597, possible metabolites
of SD 43775, in soybeans receiving one aerial application of SD43775,
a Mississippi study.
1978b Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in whole apples receiving one dormant application of SD43775,
an Oregon study.
1978c Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in pears receiving one dormant application of SD43775, a
Michigan study.
1978d Residue levels of SD47117, a possible metabolite of SD 43775,
in potatoes receiving seven ground spray applications of SD43775, a
Minnesota study.
1978e Residue levels of SD47117, a possible metabolite of SD43775,
in peanut shells from peanuts receiving three aerial applications of
SD43775, an Oklahoma study.
Beliles, R.P., Makris, S.L and Weir, R.J. Three-Generation
Reproduction Study in Rats. (See Stein, 1977 for histopathology data).
(1978) Unpublished report from Litton Bionetics, Inc. submitted by
Sumitomo Chemical Company Ltd.,
Bengston, M. Personal communication from Final Report on Silo Scale
Experiments 1977-1978 to the Australian Wheat Board Working Party on
Grain Protectants. Queensland Department of Primary Industries.
Bengston, M., Davies, R.A.H., Desmachelier, J.M., Phillips, M., and
Simpson B.W. Additional grain protectants for the control of
malathion-resistant insects in stored sorghum. (in manuscript).
Blair D., and Roderick, H. Toxicity studies on the Pyrethroid
Insecticide WL43775, Emulsifiable Concentrate FX3368. Acute
Inhalation Exposure to an Aqueous Spray. (1975) Unpublished report
from Shell Development Co.
Boyer, A.C. Unpublished reports from Shell Development Co. submitted
by Sumitomo Chemical Co.:
1976a Metabolism of WL 43775 by Rat Liver Enzymes.
1976b Metabolism of WL 43775 by Rat Liver Enzymes - Part 2.
1977a Residues in Rat Tissues from Rats Fed C-14-SD 43775.
1977b Identification of Metabolites found in the urine of Rats Fed
C-14-SD 43775.
1977c Identification of Metabolites Found in the Faeces of Rats Fed
C-14-SD 43775.
1977d Identification of Metabolites in the Livers of Rats fed
C-14-SD43775.
Brooks, T.M. Toxicity studies with WL 43775: Mutagenicity Studies
with WL 43775 in the Host-Mediated Assay. (1976) Unpublished report
from Shell Development Co.
Brown, L.J. and Slomka, M.B. Skin Reaction Potential of Use Dialation
(1.33%) PYDRIN(R) EC. (1979) Unpublished report from Shell Development
Co.
Butterworth, S.T.G. and Carter, B.I. Toxicity Studies on the
Insecticide WL 43775: Acute Oral Toxicity and Neuropathological
Effects in Rats. (1976) Unpublished report from Shell Development Co.
Chapman, R.A. and Simmons, H.S. Gas-liquid chromatography of picogram
quantities of pyrethroid insecticides. J. Assoc. Off. Anal. Chem., 60:
977-978.
Chapman, R.A. and Harris, C.R. Extraction and liquid/solid
chromatography for the direct analysis of four pyrethroid insecticides
in crops by gas liquid chromatography. J. Chromatog. 166, 513-518.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Acute Toxicity, Skin Irritancy Potential of the Emulsifiable
Concentrate FX 3368. Unpublished report from Shell Development Co.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Toxicity and Skin and Eye Irritancy Potential of the ULV
Formulation FX 4353. (1976) Unpublished report from Shell Development
Co.
Creedy, C.L. and Potter, D. The Effect of Feeding WL 43775 on the
Microsomal Mono-Oxygenase System of Rat Liver. (1976) Unpublished
report from Shell Development Co.
Dean, B.J. Toxicity Studies with WL 43775: Dominant Lethal Assays in
Male Mice after Single Oral Doses of WL 43775. (1975) Unpublished
report from Shell Development Co.
Dean, B.J. and Senner, R.K. Toxicity Studies with WL 43775:
Chromosome Studies on Bone Narrow Cells of Chinese Hamster after Two
Daily Oral Doses of WL 43775. (1975) Unpublished report from Shell
Development Co.
Desmachelier, J.M. Personal Communication. CSIRO Division of
Entomology. (1979)
DeVries, D.M. Shell Development Co.
1976a Residues of SD 43775 in Milk from Cows fed Radiolabelled
SD43775
1976b Residues in Milk, Hair and Tissues of Cows sprayed with an
Emulsible Concentrate (6-1-3-1ECH)
1976c In Milk, Cream, Hair and Tissues of Cows Sprayed with an
Emulsible Concentrate or Fed 10 ppm in Their Diet
1976d In Eggs and Tissues of Hens Sprayed with an Emulsible
Concentration
1976e In Milk, Hair and Tissues of Cows Sprayed with an Emulsible
Concentration (6-1-3-1ECH)
1976f In Meat and Milk of Cattle Treated Dermally with a Water
Dispersible Liquid Formulation
1976g In Meat, Hair, and Milk Fat of Restrained Cows Treated
Dermally with an Emulsible Concentrated Formation
Dewar, A.J., Moffett, B.J. and Sitton, M.F. Toxicity Studies on the
Insecticide WL 43775: Biochemical and Functional Studies on the
Neurotoxicity of WL 43775 to Rats. (1975) Unpublished report from
Shell Development Co.
Dewar, A.J., Moffett, B.J., Sitton, M.F. and Baker, J.E. Toxicity
Studies on the Insecticide WL 43775: Biochemical and Functional
Studies on the Neurotoxicity of WL 43775 to Chinese Hamsters. (1978)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Three-Month Feeding Study in Rats. (1975)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Short-Term Feeding Study in Rats. (1976)
Unpublished report from Shell Development Co.
Hine, Inc. SD-43775 Toxicology: Acute and Repeated (14-Day) Dermal
Toxicity in the Rabbit. (1975) Unpublished report from Hine, Inc.,
submitted by Sumitomo Chemical Co.
Hine, Inc. Human Skin Testing on SD-43775. (1976) Unpublished report
from Hine, Inc. submitted by Sumitomo Chemical Co.
Hitchings, E.J. The Metabolism of the Pyrethroid Insecticide WL 43775
(Belmark) in Lettuce and Soil under Outdoor Conditions. (1977) Shell
Research Ltd.,
Holmstead, R.L. and Fullmer, D.G. Photodecarboxylation of Cyanohydrin
Esters. Models for Pyrethroid Photodecomposition. J. Agric. Food
Chem., 26, 56
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem., 26, 954
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem. 26, 590-95.
Ito, N. Histopathological Findings of Mice Treated with S5602 for
three Months (see Suzuki, 1976). (1976a) Unpublished report from
Sumitomo Chemical Co. Ltd.,
Histopathological Findings of Mice and Rats Exposed to Mist of S5602
for 4 weeks (see Khoda, 1976c). (1976b) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted by Sumitomo Chemical Co. Ltd.,
Histopathological Findings in Mice Treated with S5602 (see Suzuki, et
al., 1977b). (1978) Unpublished report from Nagoya City University
Medical School submitted by Sumitomo Chemical Co., Ltd.,
Japan Food Research Lab. Analytical Results of S5602 Residues in
Wheat Grain and Wheat Straw (1977)
Kaneko, H., Ohkawa, H. and Miyamoto, J. Comparative Metabolism of
Fenvalerate in Rats and Mice. (1979) Unpublished report from Sumitomo
Chemical Co. Ltd.,
Kaplan, M. and Murphy, S.D. Effect of Acrylamide on Rotor-Rod
Performance and Sciatic Nerve ß-Glucoronidase Activity of Rats.
Toxicol. Appl. Pharmacol. 22: 259-268.
Kato, S. Analysis of Sumicidin residues in coffee. Analysis
certificate No. 12010695. (1979) Japan Food Research Laboratories.
Kato, T. and Miyamoto, J. Residue Analysis of S-5602 and its optical
isomers in Chinese Cabbage. (1978) Sumitomo Chemical Co.
Kato, T., Ohnishi, J. and Miyamoto, J. The analytical method of
2-(4-chlorophenyl) isovaleric acid, a urinary metabolite of
fenvalerate (Sumicidin(R)). (1979) Sumitomo Chemical Co.
Kirkland, V.L. and Albert, J.R. (1977a) Unpublished Report from Shell
Development Co. Preliminary Pharmacodynamic Investigation of SD 43775
(6-10-0) Administered Intravenously to the Anesthetized Dog: I.
Effects on the Cardiovascular System and Respiration. II.
Electrocardiographic (EKG) Findings.
Khoda, H., Kadota, T. and Miyamoto, J. Unpublished reports from
Sumitomo Chemical Co. Ltd.,:
1976a Teratogenic Study on S5602 in Mice.
1976b Acute Inhalation Toxicity of S3206 and S5602 in Mice and
Rats.
1976c Subacute Inhalation Toxicity Study of S5602 in Mice and Rats.
Khoda, H., Kaneko, H., Ohkawa, H., Kadota, T. and Miyamoto, J. (1979)
Unpublished report from Sumitomo Chemical Co. Ltd., Acute
Intraperitoneal Toxicity of Fenvalerate Metabolites in Mice.
Lampert, P.W. (1977) Letter Report on Long-Term Rat Study (Gordon and
Weir, 1978) from the University of California San Diego School of
Medicine, Department of Pathology submitted by Sumitomo Chemical Co.
Lee, Y.W., Westcott, N.D. and Reichle, R.A. Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871
Loeffler, J.E. From Shell Development Co.:
1976a Disappearance of C-14 from Cotton Leaves Treated with
C-14-(Chlorophenyl)-SD 43775"
1976b Thin Layer Chromatography of SD 43775-Treated Cotton Leaves
1976c Carbon-14 Content of Cotton Seeds after Single and Multiple
Treatment of Squares with C-14-(Chlorophenyl)-SD 43775
1976d Investigation of the Transport of C-
14-(Chlorophenyl)-SD-43775 from Treated to Untreated Leaves on Cotton
Plants
1976e Degradation of C-14-(Chlorophenyl)-SD 43775 during Exposure
to Soil Under Aerobic, Anaerobic, and Sterile Conditions.
Matsubara, T., Suzuki, T., Kadota, T. and Miyamoto, J. Antidotes
Against Poisoning by S5602 in Rats. (1977) Unpublished report from
Sumitomo Chemical Co.
McKinney, W.J. Separation of SD 43775 from hexane extracts of cotton
by liquid chromatography. Shell Development Co.
Microchem Associates:
Report on Residues of WL 43775 in Cabbages, (1977)
Report on Residues of WL 43775 in Navy Beans (1978)
Mikami, N., Takahashi, Hayashi, K. and Miyamoto, J. Photodegradation
of Fenvalerate (Sumicidin(R)) in Water and on Soil Surface. Sumitomo
Chemical Company Ltd.,
Milner, C.K. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticide. Investigation of the Neurotoxic Potential of WL 43775.
(1977) Unpublished report from Shell Development Co.
Noble, P.J. AF1117 Residue Data in Animal Tissues, Milk and Cream.
(1976a) Shell Chemical (Australia) Propriety Ltd.,
Noble, A.S. Soil Percolation Studies with WL 43775. (1976b) Shell
Research Ltd.,
Ohkawa, H., Nambu, K., Inui, H. and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Soil and by Soil Micro-organisms. J.
Pesticide Sci. 3, 129
Ohkawa, H. and Kikuchi, R. Metabolic Fate of Fenvalerate
(Sumicidin(R)) in Rats, Soil, Soil Microorganisms and an Aquatic Model
Ecosystem. Sumitomo Chemical Co., Ltd.,
Ohkawa, H., Nambu, K., Mikami, N., and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Bean Plants and Cabbages. Sumitomo
Chemical Co. Ltd., (1979a)
Ohkawa, H., Kikuchi R. and Miyamoto, J. Bioaccumulation and
Biodegradation of the (S)-Acid Isomer of Fenvalerate (Sumicidin(R)) in
an Aquatic Model Ecosystem. Submitted to J. Pesticide Sci. (1979b)
Ohkawa, H., Kaneko, H., Tsuki, H. and Miyamoto, J. Metabolism of
Fenvalerate (Sumicidin(R)) in rats. J. Pesticide Sci. 4 (2), 143.
Okuno, Y., Kadota, T. and Miyamoto, J. Unpublished report from
Sumitomo Chemical Co.:
1976a. Neurotoxic Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Dermal Applications in Rats.
1976b. Neurotoxic Effects of Some Synthetic Pyrethroids by
Short-Term Feeding in Rats.
1977a. Neurotox Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Oral Administration in Rats.
1977b. Neurotoxic Effects of S5602 and Natural Pyrethrins by Oral
Administration in Rats.
1977c. Recovery of Histopathological Lesions in Rats Caused by
Short-Term Feeding of S5602.
Potter, J.C. (1976) Unpublished reports from Shell Development Co.:
a) Tissues of Rats Fed SD 43775-C-14; b) and c) Milk and tissues of
cows; d) eggs and tissues from laying hens; e) cream from the milk of
cows fed SD 43775 14-C.
Potter, J.C. and Arnold, D.L. Residues of C-14 in Tissues of Rats Fed
SD 43775-C-14 for 28 days. (1977) Unpublished report from Shell
Development Co.
Potter, J.C. and Sauls. Residues of 14-C in eggs and Tissues of
Laying Hens fed 0.03 ppm of SD 43775-14-C (Labelled in Benzene Ring
Adjacent to CN Group). (1978) Shell Development Co.
Quinn R.J., Paa, H., Mastri, C.W., Kinoshita, F.K. and Keplinger, M.L.
21-Day Subacute Dermal Toxicity Study with Technical SD 43775 and SD
43775 (2.4 lb/gal EC) in Albino Rabbits. (1976) Unpublished report
from Industrial Bio-Test Lab submitted by Sumitomo Chemical Co.
Shell Development Co. and Shell Research Ltd., (1975-1978) A numbered
series of 36 unpublished reports with residue data from field trials
on specified crops in different countries.
Simpson, B.W. (1979) Draft report to be published. Queensland
Department of Primary Industries Analytical Chemistry Branch,
Brisbane, Australia.
Standen, M.E. The Metabolism of the Pyrethroid Insecticide WL 43775
in Apple Fruit and Foliage under Outdoor Conditions. (1977) Shell
Research Ltd.,
Stein, A.A. Histopathology Evaluation of Animals from 3-Gene ration
Reproduction Study. (1977) Unpublished report from Microscopy for
Biological Research, Ltd., submitted as part of study cited above
(Beliles et al., 1978) by Sumitomo Chemical Co., Ltd.,
Summit, L.M. and Albert, J.R.
1977a. Oral Lethality of WL 43775 (6-1-0-0) in the Rat (Unpublished
report from Shell Development Co.)
1977b. Determination of the Acute Oral Lethality of WL 43775
(6-1-0-0) in the Male and Female Mouse.
Suzuki, H. and Miyamoto, J.
1976. Unpublished report from Sumitomo Chemical. Studies on
Mutagenicity of S5602 with Bacteria Systems.
1977. Studies on Mutagenicity of Some Pyrethroids on Salmonella
Strains in the Presence of Mouse Hepatic S9 Fractions.
Suzuki, T., Kadota, T. and Miyamoto, J. One Year Chronic Toxicity
Study of S5602 in Mice (Three Month Interim Report). (1976)
Unpublished report from Sumitomo Chemical Co.
Suzuki, H., Kishida, F. and Miyamoto, J. Studies on Mutagenicity of
S5602 in Ames Test in the Presence of Hepatic S9 Fractions from
Several Laboratory Animal Species. (1979) Unpublished report from
Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Fifteen-Month Chronic Toxicity Study of S5602 in Rats. (1977a)
Unpublished report from Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Eighteen-Month Chronic Toxicity Study of S5602 in Mice. (1977b)
Unpublished report from Sumitomo Chemical Co.
Takimoto, Y. and Miyamoto, J. Method for Residue Analysis of S-5602
in Cabbage. (1977) Sumitomo Chemical Co.
Talekar, N.S. Gas-liquid chromatographic determination of
a-cyano-3-phenoxybenzyl a-isopropyl-4-chlorophenylacetate residues
in cabbage. J. Assoc. Off. Anal. Chem., 60: 908-910
van der Pauw, C.L., Dix, K.M., Blanchard, K. and McCarthy, W.V.
Toxicity of WL-43775: Teratological Studies in Rabbits Given WL 43775
Orally. (1975) Unpublished report from Shell Development. Co.
Walker, B.J., Hend, R.W. and Linnett, S. Toxicity Studies on the
Insecticide WL 43775: Summary of Results of Preliminary Experiments.
Unpublished report from Shell Development Co.
Williams, I.H. and Brown, M.J. Persistence of Permethrin and WL 43775
in Soil. J. Agric. Food Chem. 27, 130
Woodhouse, R.N., Almond, R.H. and Anderson, J. Determination of
residues of Sumicidin in potatoes. (1979) Huntingdon Research Centre.
SMO 88/7939.
 * Based on information submitted by Sumitomo Chemical Ltd., Osaka,
Japan and Shell Chemical Ltd., London
Other information on identity and properties
Molecular weight: 419.9
State: Yellow oily liquid at 23~C
Specific gravity: 1.17 g/ml at 23°C
Vapor pressure: 2.1 × 10-6 mm Hg at 70°C
Solubility: (g/l at 20°C)n-hexane 77, xylene >450,
acetone >450, ethanol >450 and methanol >450.
Solubility in water: ca. 2 µg/L
Stability: Stable in most solvents except alcohols at
ambient temperature. Unstable in alkaline
media. No significant breakdown after 100 hours
at 75°C. Decomposed gradually in the range of
150-300°C.
Typical composition of the Technical material % by weight
alpha-cyano-3-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 90-94
alpha-cyano-2-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 1.0-2.5
[Z and E]-2-(4-chlorophenyl)-1-cyano-3-methyl-
1-buten-1-yl-2-(4-chlorophenyl)-3-methyl-butyrate 0.4-2.0
alpha-cyano-3-phenoxybenzyl-2-(2-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
alpha-cyano-3-phenoxybenzyl-2-(3-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
Other related compounds <5.0
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
The results of acute toxicity tests with various animal species are
summarised in Table 1.
Signs of Poisoning
Within four hours of dosing, all animals receiving acutely toxic
levels were restless, developed tremors, piloerection, occasional
diarrhea and an abnormal gait. Following oral administration, animals
recovered rapidly from acute clinical signs of poisoning and were
asymptomatic within 3-4 days. Immediately after exposure, rats show
an abnormal gait which is typical of pyrethroid intoxication. The
animals walk, with hindquarters held up and the hind legs more widely
spaced than normal (splayed). Histological examination of the sciatic
nerve and posterior tibular nerve, after poisoning and for nine days
over the course of recovery, showed axonal breaks, swelling and
vacuolation accompanied by vacuolation and phagocytosis of myelin.
The degree to which myelin was disrupted was dose dependent and was
closely associated with the acute signs of toxicity (Butterworth and
Carter, 1976).
Table 1. Acute toxicity of fenvalerate administered to various animal species
LD50
Species Route Sex Vehicle1 mg/kg Reference
Rat Oral DMSO 451 Walker et al., 1975
Oral PEG: water >3200 Swamitt & Albert, 1977a
Dermal 5000 (24 hr) Okuno et al., 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Mouse Oral M DMSO 200-300 Walker et al., 1975
F 100-200
Oral PEG: water 1202 Summit & Albert, 1977b
Ip M & F Corn oil 85-89 Khoda, et al., 1979
Intravenous Glycerol-formol 65 Albert & Summitt, 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Chinese Oral M DMSO 98 Walker, et al., 1975
hamster F 82
Syrian
hamster Oral PEG: water ca. 760 Hart, 1976a
Dog Oral PEG: water or Doses from 100 Hart, 1976b
corn oil to 1000 mg/kg
were emetic
Rabbit Percutaneous Undiluted 1000-3200 Hine, 1975
Hen Oral >1500 >1500 Milner & Butterworth, 1977
1 PEG = polyethylene glycol;
DMSO = dimethylsulfoxide.
Biochemical Aspects
Absorption, Distribution and Excretion
Fenvalerate, orally administered to rats and mice, was found to be
rapidly absorbed, distributed to a variety of tissues and organs,
metabolized and excreted from the body. The half-life for excretion
in both rodent species was 0.5-0.6 days. Elimination of the
CN-labelled fenvalerate was somewhat slower in both species suggesting
a different pattern of metabolism. Total recovery of the administered
CN-labelled fenvalerate was achieved within 6 days following the acute
administration. Tissue residues following acute administration was
extremely low with the highest concentration being observed in fat,
adrenal gland, skin, hair and in the intestines. High concentrations
of the CN-labelled fenvalerate were noted in the hair and skin which
may account for the data showing that residues of this label were more
slowly excreted from the body (Kaneko and Ohkawa, 1979; Ohkawa, et
al., 1979).
Rats fed 20 ppm in the diet for 28 days were sacrificed and residues
in adipose tissue were examined. Based upon chromatographic and mass
spectral analysis, the residue in fat was characterized as unchanged
fenvalerate containing both distereo isomers (Boyer, 1977a).
Male and female rats fed fenvalerate for 28 days (20 ppm) and placed
on control diets for additional 28 days were examined for tissue
residues and their depletion rates. Maximum residues were reached
rapidly, within 3 weeks of dietary administration. Of the tissues
measured, adipose tissue contained the highest residue. Trace amounts
were observed in other tissues including the brain after 28 days of
treatments. Dissipation of residues from all tissues following the
cessation of treatment was rapid, although with adipose tissue, the
dissipation was slower than with other tissues. At 28 days after the
cessation of dietary fenvalerate, residues were still reported in
adipose tissue, attesting to the slow clearance from this storage
depot (Potter and Arnold, 1977; Potter, 1976).
Metabolism
The metabolic fate of fenvalerate has been examined in rodent species
following acute and subacute oral and dietary administration. In all
cases, the metabolism in both rats and mice and elimination of the
metabolic components was rapid. Fenvalerate undergoes several major
metabolic reactions; cleavage of the ester linkage, hydroxylation in
the acid and alcohol moieties and conversion of the CN group to SCN
and CO2. The resulting metabolite acids and phenols were
subsequently conjugated with glucuronic acid, sulfuric acid or amino
acids. The reactions were similar in both rats and mice with
differences being the nature of the conjugating material and the
quantitative excretion of certain metabolites. Taurine was found to
conjugate with 3-phenoxy-benzoic acid, representing 10-13% of the dose
in mouse urine. This conjugating mechanism was not observed with
rats. In both species, major hydroxylation reactions were noted to
occur in the 4' position of the phenoxybenzoic acid. Hydroxylation
also has been noted in the 2' and 5' positions. Several species
differences in rats and mice were observed with respect to
hydroxylation on the phenoxybenzoic acid. Figure 1 gives a schematic
of the metabolites conjugating mechanisms observed in mammals.
Hydroxylated fenvalerate was detected in the faeces of both rats and
mice (Boyer, 1977c; Kaneko and Ohkawa, 1979; Ohkawa, et al., 1979).
The liver of rats fed fenvalerate for 28 days was analyzed for
residues and found to contain 3-phenoxybenzoic acid and the
corresponding 4'-hydroxylated derivative (Boyer, 1977d).
Subcellular fractions of rat liver have been shown to degrade
fenvalerate yielding a wide variety of products, many of which have
been detected in urine and as conjugated products. The most widely
noted were 3-phenoxybenzoic acid and its 4'-hydroxylated derivatives
as well as the corresponding isovaleric acid (Boyer, 1976a; 1976b).
The acute toxicity of the hydrolytic metabolites is presented in Table
2. All metabolites are less toxic than fenvalerate.
Photodecomposition
Photolysis of fenvalerate in solvents by artificial light and as a
thin film on glass or cotton by sunlight yields products resulting
from ester cleavage. The major product in solution was identified as
a photo-induced decarboxylation yielding an unusual product, not known
to occur in mammals.
* Based on information submitted by Sumitomo Chemical Ltd., Osaka,
Japan and Shell Chemical Ltd., London
Other information on identity and properties
Molecular weight: 419.9
State: Yellow oily liquid at 23~C
Specific gravity: 1.17 g/ml at 23°C
Vapor pressure: 2.1 × 10-6 mm Hg at 70°C
Solubility: (g/l at 20°C)n-hexane 77, xylene >450,
acetone >450, ethanol >450 and methanol >450.
Solubility in water: ca. 2 µg/L
Stability: Stable in most solvents except alcohols at
ambient temperature. Unstable in alkaline
media. No significant breakdown after 100 hours
at 75°C. Decomposed gradually in the range of
150-300°C.
Typical composition of the Technical material % by weight
alpha-cyano-3-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 90-94
alpha-cyano-2-phenoxybenzyl-2-(4-chlorophenyl)-
3-methyl-1-butyrate 1.0-2.5
[Z and E]-2-(4-chlorophenyl)-1-cyano-3-methyl-
1-buten-1-yl-2-(4-chlorophenyl)-3-methyl-butyrate 0.4-2.0
alpha-cyano-3-phenoxybenzyl-2-(2-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
alpha-cyano-3-phenoxybenzyl-2-(3-chlorophenyl)-
3-methyl-1-butyrate 0.5-1.0
Other related compounds <5.0
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Acute Toxicity
The results of acute toxicity tests with various animal species are
summarised in Table 1.
Signs of Poisoning
Within four hours of dosing, all animals receiving acutely toxic
levels were restless, developed tremors, piloerection, occasional
diarrhea and an abnormal gait. Following oral administration, animals
recovered rapidly from acute clinical signs of poisoning and were
asymptomatic within 3-4 days. Immediately after exposure, rats show
an abnormal gait which is typical of pyrethroid intoxication. The
animals walk, with hindquarters held up and the hind legs more widely
spaced than normal (splayed). Histological examination of the sciatic
nerve and posterior tibular nerve, after poisoning and for nine days
over the course of recovery, showed axonal breaks, swelling and
vacuolation accompanied by vacuolation and phagocytosis of myelin.
The degree to which myelin was disrupted was dose dependent and was
closely associated with the acute signs of toxicity (Butterworth and
Carter, 1976).
Table 1. Acute toxicity of fenvalerate administered to various animal species
LD50
Species Route Sex Vehicle1 mg/kg Reference
Rat Oral DMSO 451 Walker et al., 1975
Oral PEG: water >3200 Swamitt & Albert, 1977a
Dermal 5000 (24 hr) Okuno et al., 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Mouse Oral M DMSO 200-300 Walker et al., 1975
F 100-200
Oral PEG: water 1202 Summit & Albert, 1977b
Ip M & F Corn oil 85-89 Khoda, et al., 1979
Intravenous Glycerol-formol 65 Albert & Summitt, 1976
Inhalation M & F Water >101 mg/m3 (3hr) Kohda et al., 1976b
Chinese Oral M DMSO 98 Walker, et al., 1975
hamster F 82
Syrian
hamster Oral PEG: water ca. 760 Hart, 1976a
Dog Oral PEG: water or Doses from 100 Hart, 1976b
corn oil to 1000 mg/kg
were emetic
Rabbit Percutaneous Undiluted 1000-3200 Hine, 1975
Hen Oral >1500 >1500 Milner & Butterworth, 1977
1 PEG = polyethylene glycol;
DMSO = dimethylsulfoxide.
Biochemical Aspects
Absorption, Distribution and Excretion
Fenvalerate, orally administered to rats and mice, was found to be
rapidly absorbed, distributed to a variety of tissues and organs,
metabolized and excreted from the body. The half-life for excretion
in both rodent species was 0.5-0.6 days. Elimination of the
CN-labelled fenvalerate was somewhat slower in both species suggesting
a different pattern of metabolism. Total recovery of the administered
CN-labelled fenvalerate was achieved within 6 days following the acute
administration. Tissue residues following acute administration was
extremely low with the highest concentration being observed in fat,
adrenal gland, skin, hair and in the intestines. High concentrations
of the CN-labelled fenvalerate were noted in the hair and skin which
may account for the data showing that residues of this label were more
slowly excreted from the body (Kaneko and Ohkawa, 1979; Ohkawa, et
al., 1979).
Rats fed 20 ppm in the diet for 28 days were sacrificed and residues
in adipose tissue were examined. Based upon chromatographic and mass
spectral analysis, the residue in fat was characterized as unchanged
fenvalerate containing both distereo isomers (Boyer, 1977a).
Male and female rats fed fenvalerate for 28 days (20 ppm) and placed
on control diets for additional 28 days were examined for tissue
residues and their depletion rates. Maximum residues were reached
rapidly, within 3 weeks of dietary administration. Of the tissues
measured, adipose tissue contained the highest residue. Trace amounts
were observed in other tissues including the brain after 28 days of
treatments. Dissipation of residues from all tissues following the
cessation of treatment was rapid, although with adipose tissue, the
dissipation was slower than with other tissues. At 28 days after the
cessation of dietary fenvalerate, residues were still reported in
adipose tissue, attesting to the slow clearance from this storage
depot (Potter and Arnold, 1977; Potter, 1976).
Metabolism
The metabolic fate of fenvalerate has been examined in rodent species
following acute and subacute oral and dietary administration. In all
cases, the metabolism in both rats and mice and elimination of the
metabolic components was rapid. Fenvalerate undergoes several major
metabolic reactions; cleavage of the ester linkage, hydroxylation in
the acid and alcohol moieties and conversion of the CN group to SCN
and CO2. The resulting metabolite acids and phenols were
subsequently conjugated with glucuronic acid, sulfuric acid or amino
acids. The reactions were similar in both rats and mice with
differences being the nature of the conjugating material and the
quantitative excretion of certain metabolites. Taurine was found to
conjugate with 3-phenoxy-benzoic acid, representing 10-13% of the dose
in mouse urine. This conjugating mechanism was not observed with
rats. In both species, major hydroxylation reactions were noted to
occur in the 4' position of the phenoxybenzoic acid. Hydroxylation
also has been noted in the 2' and 5' positions. Several species
differences in rats and mice were observed with respect to
hydroxylation on the phenoxybenzoic acid. Figure 1 gives a schematic
of the metabolites conjugating mechanisms observed in mammals.
Hydroxylated fenvalerate was detected in the faeces of both rats and
mice (Boyer, 1977c; Kaneko and Ohkawa, 1979; Ohkawa, et al., 1979).
The liver of rats fed fenvalerate for 28 days was analyzed for
residues and found to contain 3-phenoxybenzoic acid and the
corresponding 4'-hydroxylated derivative (Boyer, 1977d).
Subcellular fractions of rat liver have been shown to degrade
fenvalerate yielding a wide variety of products, many of which have
been detected in urine and as conjugated products. The most widely
noted were 3-phenoxybenzoic acid and its 4'-hydroxylated derivatives
as well as the corresponding isovaleric acid (Boyer, 1976a; 1976b).
The acute toxicity of the hydrolytic metabolites is presented in Table
2. All metabolites are less toxic than fenvalerate.
Photodecomposition
Photolysis of fenvalerate in solvents by artificial light and as a
thin film on glass or cotton by sunlight yields products resulting
from ester cleavage. The major product in solution was identified as
a photo-induced decarboxylation yielding an unusual product, not known
to occur in mammals.
 Holmstead, et al., 1978)
Effects on Enzymes and Other Biochemical Parameters
Fenvalerate, when fed to adult rats for 14 days did not induce hepatic
microsomal enzyme as measured by the comparative rates of
de-ethylation of an organophosphate pesticide (chlorofenvinphos)
(Creedy and Potter, 1976). Dietary levels of 1000 ppm did not induce
the oxidative O-dealkylation which has been shown to be reflective
of microsomal enzyme induction in rat liver.
Groups of rats (4 to 6 of each sex per group) were administered
fenvalerate orally at dose levels ranging from 0 to 400 mg/kg for 7
consecutive days. Mortality and clinical signs of acute poisoning
were seen only at the highest dose level. A significant neurological
deficit was demonstrated using an inclined plane test (expressed at an
angle at which the animals cannot maintain their hold on an inclining
plane). [See Kaplan and Murphy, 1972]. In addition to functional
deficits, increases in ß-glucuronidase and ß-galactosidase activity in
the posterior tibular nerve and trigeminal ganglia were observed.
Functional motor deficits appeared to coincide with administration on
fenvalerate reaching its maximum effects between day 5-7 of treatment.
Stimulation of lysosomal enzyme activity appears to coincide with
neurological deficits and with sciatic nerve degeneration noted with
acute (high level) intoxication (Dewar, et al., 1977).
Groups of hamsters (5 males and 5 females per group) were administered
fenvalerate at dose levels of 0, 5, 10, 20 and 40 mg/kg for 5
consecutive days. In 3 separate experiments, dose related increases
in ß-glucuronidase and ß-galactosidase activity of peripheral nerves
was observed. Minimal functional deficits were also noted during the
treatment period and for two weeks following treatment. These data
appear to be consistent and to be associated with axonal degeneration
in the peripheral nerve (Dewar, et al., 1978).
METABOLIC PATHWAYS FOR FENVALERATE
Holmstead, et al., 1978)
Effects on Enzymes and Other Biochemical Parameters
Fenvalerate, when fed to adult rats for 14 days did not induce hepatic
microsomal enzyme as measured by the comparative rates of
de-ethylation of an organophosphate pesticide (chlorofenvinphos)
(Creedy and Potter, 1976). Dietary levels of 1000 ppm did not induce
the oxidative O-dealkylation which has been shown to be reflective
of microsomal enzyme induction in rat liver.
Groups of rats (4 to 6 of each sex per group) were administered
fenvalerate orally at dose levels ranging from 0 to 400 mg/kg for 7
consecutive days. Mortality and clinical signs of acute poisoning
were seen only at the highest dose level. A significant neurological
deficit was demonstrated using an inclined plane test (expressed at an
angle at which the animals cannot maintain their hold on an inclining
plane). [See Kaplan and Murphy, 1972]. In addition to functional
deficits, increases in ß-glucuronidase and ß-galactosidase activity in
the posterior tibular nerve and trigeminal ganglia were observed.
Functional motor deficits appeared to coincide with administration on
fenvalerate reaching its maximum effects between day 5-7 of treatment.
Stimulation of lysosomal enzyme activity appears to coincide with
neurological deficits and with sciatic nerve degeneration noted with
acute (high level) intoxication (Dewar, et al., 1977).
Groups of hamsters (5 males and 5 females per group) were administered
fenvalerate at dose levels of 0, 5, 10, 20 and 40 mg/kg for 5
consecutive days. In 3 separate experiments, dose related increases
in ß-glucuronidase and ß-galactosidase activity of peripheral nerves
was observed. Minimal functional deficits were also noted during the
treatment period and for two weeks following treatment. These data
appear to be consistent and to be associated with axonal degeneration
in the peripheral nerve (Dewar, et al., 1978).
METABOLIC PATHWAYS FOR FENVALERATE
 TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of rats (11 male and 22 female rats per group) were fed
fenvalerate in the diet at dosage levels of 0, 1, 5, 25 and 250 ppm.
The animals were fed for 9 weeks prior to mating and initiation of a
standard 3-generation (2 litters per generation) reproduction study.
Fertility, viability, gestation and lactating indices were calculated
for each treatment group compared to control values. Ten male and 10
female weanlings from the F3b litter were examined histologically at
the conclusion of the study.
The mean body weight of the parent of the third generation (F2b
adults) showed a significant reduction at the 250 ppm level. Gross
necropsy revealed kidney changes in these animals which was not
apparently related to fenvalerate. No pathological changes were noted
to account for the weight loss. No effects on reproductive parameters
in any of the three generations were observed in the study.
Histological examination did not show an adverse effect of
fenvalerate. With the exception of weight reduction in the third
generation parents, there was no effect of fenvalerate on any
parameter measured in the study (Beliles, et al., 1978; Stein, 1977).
Teratogenicity
Mouse
Groups of pregnant mice (32 to 33 mice per group) were administered
fenvalerate at dosage levels of O, 5, 15 and 50 mg/kg body weight per
day on days 6 through 15 of gestation in a standard teratogenicity
bioassay. On day 18, groups of 20 mice were sacrificed and fetuses
were removed and examined for somatic and skeletal abnormalities. The
remaining parents were allowed to deliver naturally and the young
maintained for three weeks to weaning to evaluate postnatal deficits.
In addition to the teratogenicity study, two male and two female
weanlings from each dam were maintained for 8 weeks and mated to note
any effects on their reproductive potential. Toxic signs of poisoning
were noted in maternal mice at the high dose level. There was no
significant mortality over the course of the study and no effects were
noted on any of the animals as a result of continuous administration
of fenvalerate. In fetal examinations, there were no somatic or
skeletal changes as noted by internal or external tissue evaluations.
The animals maintained in an abbreviated reproduction study showed no
differences from control value in their ability to reproduce. There
were no changes in the reproduction indicates evaluated with any
animals examined. Under the conditions of this bioassay, fenvalerate
has been shown to have no teratogenic potential in mice (Kohda, et
al., 1976a).
Rabbit
Groups of pregnant rabbits (group size varied from 20 to 31 rabbits)
were administered fenvalerate from day 6 to day 18 of gestation. On
day 28, the rabbits were sacrificed and standard teratogenicity
assessments made with respect to early or late fetal death, viability
and standard somatic and skeletal teratogenic potential. Reduced body
weight of pregnant rabbits was observed with the highest dose level.
There were no significant differences from control values in any of
the parameters measured in the study. Under the conditions of the
study, fenvalerate did not induce a teratogenic event in rabbits (van
der Pauw, et al., 1975).
Special Studies on Mutagenicity
Microorganism
Fenvalerate was examined for its mutagenic potential using a series of
Salmonella typhimurium strains (TA 1535, TA 1538, TA 98 and TA 100) at
dose levels up to 1 mg/ plate. Two strains of Bacillus subtilis (H17
and M45) were tested at concentrations up to 10 mg/disc. The "Ames"
test was performed in the presence and absence of rat activation
systems and with positive and negative chemical controls. The Rec
assay was not evaluated with a metabolic activation system. With both
microbial bioassay systems, fenvalerate was not mutagenic (Suzuki and
Miyamoto, 1976).
In additional trials, fenvalerate did not increase the number of
revertant colonies of S. typhimurim (TA 1535, TA 1537, TA 1538, TA 98
and TA 100) in the presence or absence of a liver subcellular
activation system prepared from 6 different strains of PCB-treated
mice, 3 strains of rat, and the Syrian golden hamster. Fenvalerate,
tested at dosage levels up to 1 mg/plate, was not mutagenic (Suzuki
and Miyamoto, 1977; Suzuki, et al., 1979).
Mouse
Groups of mice were administered fenvalerate orally at doses of 0, 60
and 125 mg/kg body weight and injected intraperitoneally with an
indicator strain of microorganisms in a standard host-mediated assay.
A positive control, dimethylnitrosamine (DMNA), was used in this
study. Fenvalerate did not induce an increase in mutation frequency
of the S. typhimurium indicator while the DMNA significantly
increased the mutation frequency (Suzuki and Miyamoto, 1976).
Groups of mice were administered fenvalerate orally at dose levels of
0, 25 or 50 mg/kg. Immediately after administration, a suspension
culture of yeast Saccharomyces cerviciae was introduced to the
peritoneal cavity. Positive (ethyl methane-sulphonate) and negative
(DMSO) control animals were also used in this standard host-mediated
assay. Five hours after dosing, the yeast cells were recovered and
examined for mitotic gene conversion. No mutagenic effects were
detected in the cells from any of the fenvalerate concentrations
tested (Brooks, 1976).
Dominant Lethal Assay
Mouse
Groups of male mice (10-11 mice per group) were administered
fenvalerate orally at dosage levels of 0, 25, 50 and 100 mg/kg body
weight. Each treated male was mated with 3 virgin females for 7 days.
The procedure was repeated weekly with new females in a standard
dominant lethal assay. The females were sacrificed and examined for
the condition of the fetuses on day 13 of gestation.
There were no significant differences from control values with respect
to the effects of fenvalerate on pregnancy. Foetal implants in
females mated to males during the second week after treatment showed a
significant reduction in viable fetuses. There was also a significant
increase in early foetal deaths occurring in females mated during the
fourth week to males dosed at the highest level (Dean, 1975).
Hamster
Groups of Chinese hamsters (6 male and 6 females per group) were
orally administered fenvalerate (in DMSO) at dose levels of 0, 12.5
and 25 mg/kg on each of two successive days. Animals were sacrificed
8 or 24 hours after dosing and chromosomal preparations were made from
the femoral bone marrow cells. Similar preparations were made with
control animals administered methyl methanesulphonate (50 mg/kg).
Cells from animals administered methyl methanesulphonate showed
substantial numbers of chromatid gaps at 8 hours after dosing but not
at 24 hours. The administration of fenvalerate did not induce
demonstrable chromosomal damage at either sampling interval (Dean and
Senner, 1975).
Special Studies on Neurotoxicity
Hen
Groups of 6 hens were administered fenvalerate orally in dimethyl
sulphoxide at dosage levels of 0 and 1000 mg/kg daily for 5 days. A
positive control of TOCP (0.5 ml/kg) was also included in the study.
After 3 weeks, the fenvalerate and negative control animals were
retreated at the same dosage regimen. At the conclusion of an
additional 3 week observation period, animals were sacrificed and
histological examinations were performed on the central and peripheral
nervous system. All animals receiving TOCP developed readily defined
signs of delayed neurotoxicity and histological lesions were seen in
the sciatic nerve and spinal cord. Clinical signs and histopathologic
lesions were not noted in controls or fenvalerate-treated hens (Milner
and Butterworth, 1977).
Rat
A series of studies was performed to evaluate the neurotoxic potential
(to induce axonal and myelin disruption) of a group of synthetic
pyrethroid esters and of natural pyrethrum. Acute oral administration
of fenvalerate, cypermethrin, resmethrin, permethrin and natural
pyrethrum to rats at very high dosage levels resulted in severe
clinical signs of poisoning and mortality within 24 hours.
Histopathologic lesions were observed in the sciatic nerve with all
compounds tested. At lower levels, fenvalerate (200 mg/kg),
cypermethrin (100 mg/kg), permethrin (200 mg/kg) and pyrethrum (3500
mg/kg) did not show clinical signs of poisoning or histopathologic
lesions (Okuno, et al., 1977a; 1977b).
Groups of 6 male and female rats were fed fenvalerate in the diet at
concentrations of 0 and 2000 ppm for 8 to 10 days, after which the
sciatic nerve was examined for histological signs of degeneration.
All animals exposed to fenvalerate showed typical signs of acute
intoxication including ataxia, tremors and hypersensitivity.
Histological examinations at the end of the feeding interval did not
reveal any adverse effects of fenvalerate on the sciatic nerve (Hand
and Butterworth, 1976).
In a study to evaluate the reversibility of the lesions induced in the
sciatic nerve, rats were administered fenvalerate in the diet at dose
levels of 0 and 3000 ppm for 10 days. Mortality was evident as 60% of
the animals died within the course of the dietary treatment.
Surviving animals were fed normal control diets and examined for 12
weeks following completion of the feeding study. Animals were
sacrificed every 3 weeks and examined histologically for sciatic nerve
disruption. Sciatic nerves of rats sacrificed at 3 weeks on control
diets continued to show swelling and disintegration of axons. At 6
weeks there were no histological lesions. These results were also
observed at the 9 and 12-week intervals suggesting reversibility of
the histopathologic lesion observed following high dose treatment with
fenvalerate (Okuno and Kadota, 1977c).
Antidotal Studies
Following acute poisoning phenobarbital, pentobarbital and
diphenylhydantoin were found to be effective in relieving the acute
signs of intoxication. Phenobarbital (50 mg/kg, ip) prevented tremor,
diphenylhydantoin (100 mg/kg, ip) reduced the toxic reaction and
pentobarbital (35 mg/kg ip) removed the tremor reaction completely
within 30 minutes. Combinations of diphenylhydantoin with either of
the barbiturates was effective in reducing the onset and severity of
tremors while a variety of other agents were not active
(delta-tubocurarine, atropine, meprobamate, diazepam, biperidin and
trimethadione) (Matsubara, et al., 1977).
Special studies in pharmacology
Fenvalerate, administered to dogs in acute dosage rates sufficient to
induce toxic signs of poisoning, showed no consistent cardiovascular
effects as measured by EKG. Respiratory stimulation was noted at high
levels and was not reduced by anesthetic supplements (Urethane/alpha
chlorolos or pentobarbital) (Kirkland and Albert, 1977a; 1977b).
Skin and eye irritancy
Two formulated products were found to be severe eye and skin irritants
when examined in the rabbit. Dermal irritation was evident for 7 days
after a 24-hour exposure and severe conjunctivitis, corneal opacity
and iritis were observed within 30 minutes of an application of 0.2 ml
of the formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation to some degree (Coombs and
Carter, 1975 and 1976).
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
Table 2.
LD50 (mg/kg)
Compound Male Female
Fenvalerate 88.5 85.0
2-(4-Chlorophenyl)isovaleric acid 351 350
3-Phenoxybenzyl alcohol 371 424
3-4'(Hydroxyphenoxyl) benzyl alcohol 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol 876 778
3-Phenoxybenzoic acid 154 169
3-(4'-Hydroxyphenoxy) benzoic acid 783 745
3-(2'-Hydroxyphenoxy) benzoic acid 859 912
3-Phenoxybenzaldehyde 415 416
NaSCN 604 578
All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO (Khoda, et al., 1979).
The acute intraperitoneal toxicity in mice of the proposed
decarboxylated photoproduct was reported to be substantially lower
than that toxicity noted with fenvalerate (Holmstead, et al., 1978).
Short Term Studies
Inhalation
Groups of 4 male and 4 female rats were exposed by inhalation (head
only) to an aerosolized formulation (77 micron particle size)
generated from an aqueous suspension containing 3 grams/litre.
Following a single administration (4 hours) of this non-inhalable
particulate, acute signs of poisoning were noted for a short period,
presumably from oral ingestion of the large particle. There was no
mortality and all animals appeared normal within 3 days following
exposure (Blair and Roderick, 1975).
Groups of rats and mice (10 male and 10 female of each species per
group) were administered fenvalerate by inhalation exposure 3 hours
daily for 4 weeks at concentration levels of 0, 2, 7 and 20 mg/m3.
Animals were exposed to a small, fully respirable particulate (1 to 2
microns) during the course of the study. Mortality was not noted over
the course of the study, but animals at the high dose level showed
acute toxic signs of poisoning. Growth was not affected at any dose
level; nor were hematology and clinical biochemistry parameters.
Gross and microscopic examination of tissues and organs at the
conclusion of the study showed no changes that were related to the
administration of fenvalerate (Khoda, et al., 1976c; Ito, 1976b).
Dermal
Groups of rabbits (7-8 male rabbits per group) were administered
fenvalerate dermally at dose levels of 0, 100 and 400 mg/kg daily for
6 hours (14 exposures were performed over a 22-day period). Mortality
was observed at the high dose level accompanied by severe weight loss,
clinical signs of poisoning and gross dermal effects. Rabbits
tolerated the 100 mg/kg dose with minor local dermatologic effects
(Hine, 1975).
Groups of rabbits (10 male and 10 female rabbits per group, five of
each sex had an occluded skin) were administered fenvalerate dermally
for 6 hours per day, 5 days per week for 3 weeks. The dosage levels
used were 0, 30, 100 and 300 mg/kg body weight. In addition, a
formulated product (an emulsifiable concentrate containing 2.4 pounds
fenvalerate/gallon) was also tested for its dermal toxicity at dosage
levels of 0, 100, 300 and 1000 mg/kg.
Mortality was observed with both treatments predominantly at the high
dosage group. Mortality was preceded by clinical signs of poisoning
primarily in the group exposed to the formulated product. At lower
concentrations, technical fenvalerate was mildly irritating to the
skin upon repeated dermal exposure. The formulation of fenvalerate
and the blank formulation used as a control were severely irritating.
When severe irritation was noted, there were significant effects on
body weight. There were no effects noted on various haematologic,
clinical chemistry and urinalysis parameters related to the presence
of fenvalerate. Gross and microscopic pathologic changes were noted
only at the site of administration and described as acanthosis and
hyperkeratosis of the epidermis. The extent and severity of the
lesions appeared to be dose-related especially with respect to the
formulated product. There were no significant gross or microscopic
effects noted in tissues and organs over the course of the study
(Quinn, et al., 1976).
Dietary
Rat
Groups of rats (12 male and 12 female rats per group) were fed
fenvalerate in the diet at dose levels of 0, 125, 500, 1000 and 2000
ppm for 90 days. Mortality was observed at 2000 ppm while growth and
food consumption were decreased at 1000 ppm and 2000 ppm. With the
exception of an increase In BUN concentration at the two highest dose
levels, hematological and clinical examinations at the conclusion of
the study were normal. Gross and microscopic examinations of tissues
and organs were performed at the conclusion of the study. Increases
in liver to body weight and kidney to body weight ratios were observed
at 500 ppm and above. These gross changes were not accompanied by
observable microscopic changes on histopathological examination.
Sciatic nerves, examined at the conclusion of the study, showed no
myelin or axonal degeneration (Hend and Butterworth, 1975).
Dog
Groups of young adult beagle dogs (4 male and 4 female dogs per group
were fed fenvalerate in the diet at dose levels of 0, 0.5, 0.25, 1.25
and 12.5 mg/kg body weight for 90 days. There were no abnormalities
over the course of the study. Growth, food consumption and behaviour
were normal. Results of clinical laboratory examinations performed 3
times during the course of the study, showed no effects of fenvalerate
in the diet. At the conclusion of the study, data from gross and
microscopic examination of a variety of tissues and organs
substantiated clinical data, again showing no effects of dietary
fenvalerate. Daily administration at a level of 12.5 mg/kg body
weight for a period of 90 days produced no detectable evidence of
toxicologic effects (Hart and Wosu, 1975).
Long Term Studies
Mouse
Groups of mice (from 35 to 47 male and female, ddY strain, mice/group)
were administered fenvalerate in the diet for 78 weeks at dosage
levels of 0, 100, 300, 1000 and 3000 ppm. An interim (3 months) and a
final report showed dose-related effects at 300 ppm and above.
Fenvalerate was not carcinogenic to the ddY strain of mouse (Suzuki
and Kadota, 1976; Ito, 1976a, 1978; Suzuki et al., 1977b).
At the early stages of the study, mortality was evident at the highest
dose level. Growth was reduced at 1000 ppm and above. These effects
were accompanied by excitability, hypersensitivity and other
behavioural changes in the first month of feeding. A variety of
hematological parameters were affected during the first three months
of study, predominantly at the high dose level. Several biochemical
changes were observed including a decrease in alkaline phosphatase, an
increase in blood urea nitrogen, an increase in leucine aminopeptidase
activity and a decrease in cholesterol and glucose occurring at 1000
and 3000 ppm with several of these parameters being affected at 100
ppm (glucose decrease in females). Gross examination of tissues and
organs showed a weight increase in several tissues of both males and
females exposed to the high dose level. Microscopic examination
suggested changes in the liver at the two highest dose levels.
Multiple small necrotic foci in the liver and changes in the
epithelial cells of the proximal convoluted tubules were noted,
apparently related to the presence of fenvalerate in the diet (Suzuki,
et al., 1976; Ito, 1976a).
At the conclusion of the 18-month study, animals were sacrificed for
terminal haematologic and biochemical changes as well as gross and
microscopic examination of tissues and organs. Over the 18 month
study, growth was depressed at 3000 ppm in both males and females.
Behavioural changes noted as transient hypersensitivity occurred at
1000 ppm and above. Increased mortality was also evident at the two
highest dose levels. No compound-related changes were noted with
respect to haematologic parameters, although clinical chemistry
parameters were in some instances substantially increased (SGPT, SGOT,
LDH and LAP activities in both sexes were significantly increased,
predominantly at the two highest dose levels but sporadically at 300
ppm). Gross changes were noted in several organ weights and organ to
body weight ratios primarily in liver, although changes were also
noted in the kidney, heart, spleen, pituitary and ovary at the highest
dose level. These gross changes were not accompanied by
histopathological events with the exception of granulomatous changes
in liver and the mesenteric lymph nodes noted at all treatment groups,
predominantly at the highest two dose levels. Biochemical changes,
noted at the three month sacrifice interval, were not seen at the
conclusion of the study (increased BUN, decreased glucose, etc.).
There were no indications in this study of increased tumorigenicity or
carcinogenicity as a result of fenvalerate (Suzuki, et al., 1977b).
Rat
Groups of rats (15 male and 15 female Wistar rate per group) were fed
at concentrations of O, 50, 150, 500 and 1500 ppm for 15 months in the
diet. There was no mortality in the study attributable to
fenvalerate. Growth, food consumption and behavioural changes were
significantly affected at the highest dose level. Hypersensitivity
was observed at the early stages of the experiment disappearing within
3 months. Growth was significantly depressed in both males and
females at the highest dose level. Food consumption was unaffected
over the course of the study. Clinical examinations, performed at
either one year (urinalysis) or at the conclusion of the study
(hematology, blood biochemistry and gross and microscopic pathology),
showed significant abnormal values in a variety of parameters at the
1500 ppm dose level. No opthamologic effects were noted. Urinalysis
was normal over the course of the study. Haemoglobin concentration
was depressed in males at the highest dose level and the females at
150 ppm and above. Blood biochemistry was significantly altered at
1500 ppm with respect to several parameters (BUN in both males and
females; protein and cholinesterase in females). Gross and
microscopic examination of tissues and organs including specific
sections of sciatic nerve and trigeminal ganglia and nerve showed no
dose-related effects. Generalized inflammatory and degenerative
changes were seen in both control and treated animals. Tumor
incidents were low and not related to the presence of fenvalerate.
There was no suggestion of a carcinogenic potential observed in the
study (Suzuki, et al., 1977a).
Groups of rats (93 male and 93 female Sprague-Dawley rats/group, 183
of each sex were used as the control and an additional 22 of each sex
were used as a separate control and high level group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25, 250 and 500
ppm. There was no mortality associated with the study although growth
was reduced at the 500 ppm dose level. The 500 ppm group and a
separate control group were sacrificed at 26 weeks while the other
animals were maintained for 2 years. There were no significant
effects on food consumption, growth or on behaviour at 250 pm.
Hematology, clinical chemistry tests and urinalyses, performed at
various time intervals over the course of the study on at least 10
animals of each sex at each dose level, showed no dose-related
effects. At the conclusion of the study, organ weight and organ to
body weight ratios were normal. Gross and microscopic examination of
tissues and organs did not differ significantly from controls. Benign
and malignant lesions occurred at random throughout all groups
examined at the end of the study and in those animals that died during
the course of the study. There were no lesions attributable to
fenvalerate. A specific pathology examination of the sciatic nerve of
animals fed 250 ppm was performed. The sciatic nerve was not found to
have been significantly affected by fenvalerate (Gordon and Weir,
1978; Lambert, 1977).
Observations in Man
Fenvalerate was administered dermally to adult men and women to the
skin of the arm or face at dosage levels of approximately 20-40 mg.
Control applications were carried out on the same individuals and
subjective evaluations were performed with respect to dermal
irritation. There was no erythema or other visible skin effects and
an evaluation of the subjective responses suggested no significant
differences between fenvalerate-treated and the control portions of
the body (Hine, 1976).
A double-blind study utilizing 29 male volunteers was performed to
test the skin reaction of formulated fenvalerate. The emulsifiable
concentrated formulation was diluted with water and administered to
one side of the face, on the cheek, with a control formulation applied
to the opposite cheek.
There were no signs of dermatitis noted at 24 hours following
administration. Subjective analyses of irritation or skin sensation
were performed with each individual. Under the conditions of the
study, the formulation of fenvalerate did not produce abnormal skin
sensations. There were no indications that any of the symptoms noted
(which included tingling, burning, stinging, itching, swelling,
numbness or heat) was associated with fenvalerate (Brown and Slomka,
1979).
COMMENTS
Fenvalerate, an ester related to the pyrethroids, currently being
developed for use as an agricultural insecticide, is rapidly absorbed
in mammals, widely distributed, and metabolized. Tissue residues of
fenvalerate and its ester metabolites appear to concentrate to some
degree in adipose tissue. Fenvalerate undergoes several major
metabolic reactions: cleavage of the ester linkage, hydroxylation of
the acid and alcohol moieties, and conversion of the CN group to SCN
and CO2. The metabolic fate in all animals studied appears to be
similar. Photolytic degradation has been shown to produce a
decarboxylated product not known to occur in mammals. Fenvalerate is
moderately toxic when administered by the oral route. The metabolic
and photolysis products are less toxic than the parent ester.
Studies on the mutagenicity and reproductive/teratogenic potential
were negative. Studies on neurotoxicity have shown that, following
high level exposure, rats showed reversible clinical signs of ataxia.
Microscopic examination of the sciatic nerve showed axonal swelling
and myelin disruption. Biochemical studies revealed an increase in
lysosomal enzyme activity (ß-glucuronidase and ß-galactosidase) in
peripheral nerve (see Report Section 3.5). Fenvalerate, when
administered to hens at high levels, did not induce signs of
peripheral neuropathy. No data were available to assess the
susceptibility of humans to this neuropathy.
Short-term and long-term studies have been performed in a variety of
test animals. Fenvalerate is not a carcinogen and in short-term
studies in dogs and in long-term studies in rats and mice dietary
no-effect levels have been observed. A temporary acceptable daily
intake for man was allocated. The concerns of the Meeting over the
potential for bioaccumulation, the neuropathy observed in rodents and
the limited information of the susceptibility to such an effect in man
were the basis for the temporary evaluation and for the request for
further observations in occupationally exposed humans and for
pharmacokinetic data. Further information is desired with respect to
an additional dominant lethal assay to provide verification of
existing data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet equivalent to 11.9 mg/kg body weight
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg body weight
Dog: 12.5 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.06 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenvalerate is a highly active broad spectrum insecticide. It is
particularly effective as a contact and stomach poison against
lepidopterous larvae and also has high activity against Orthoptera,
Hemiptera and Diptera. This activity, together with adequate
stability on foliage, and relatively low toxicity to mammals is likely
to lead to increasing use against pests of agriculture.
The substance can be combined with most other insecticides and
fungicides, but not those with alkaline properties. It is mostly
marketed as 20% and 30% concentrates for aqueous dilution; but
granules, ULV concentrates and powers are also available.
Pre-harvest treatments
Fenvalerate is registered and/or approved in many countries in Europe,
Asia, South and Central America, and Australia. It is used against a
wide range of pests, including tobacco bud worm, bollworms, cut worms,
armyworms, leafrollers, weevils, aphids, thrips, beetles, leaf miners,
moths, bugs, psyllas etc and on a wide range of crops including
fruits, vegetables, beans, cereals, cotton and tobacco. For these
purposes it is applied at from 25 to 300 g/ha depending on the crop.
Other uses
It is registered in Argentina for forestry use against Oiketicus.
It is also being developed for control of insect pests of stored grain
and against insects of importance in public health, animal health and
industry.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Many trials have been carried out on a wide variety of fruits,
vegetables, cereals and oilseeds. Most of the data were generated
under American conditions, supplementary data are available from
several other countries. Table 3 contains the summarised data,
representing several thousand separate analytical data provided by the
principal manufacturers.
Since fenvalerate is not systemic in plants, residues usually are very
low in commodities such as root crops, corn kernels and peas which are
protected from direct contact with the insecticide. Residue levels on
plants normally decline with a half-life of about one to two weeks.
Oilseeds
Following multiple applications at rates up to 0.45 kg/ha, and in over
30 supervised trials in various countries (i.e. higher than the
recommended dosage of up to 0.3. kg/ha). Residues in cottonseed were
generally below 0.1.mg/kg, regardless of the interval between last
treatment and harvest. A residue of 0.21 mg/kg was found in a
Colombian trial 1 day after treatment at the rate of 0.3 kg/ha, and
one trial in U.S.A. showed the residues of 0.12 mg/kg (at a rate of
0.22 kg/ha) and 0.19 mg/kg (0.45 kg/ha) 48 days after treatment. When
the seed cases were separated from kernels, the residues of
fenvalerate almost entirely remained on the seed cases and levels in
the oil products were very low.
All trials on peanuts have been made in U.S.A. When fenvalerate was
applied at rates of up to 0.22 kg/ha the residues in whole nuts did
not exceed 0.1 mg/kg. At the higher rate of 0.45 kg/ha however,
residues of 0.20-0.32 mg/kg were detected 14-21 days after
application. It was shown that residues in the meat and oil did not
exceed the detection limit (0.01 mg/kg) and that the shells carried
almost all the residues found in whole nuts. Even when the peanuts
were harvested 73 days after the treatment at 5 and 10 times higher
rates than the recommended dosage (up to 0.22 kg/ha) the residues in
whole nuts were 0.04 mg/kg and 0.09 mg/kg, respectively. In other
trials at rates up to 0.45 kg/ha the residues in hay, foliage and
vines ranged from 0.01 to 31 mg/kg.
Leafy and stem vegetables
Trials have been carried out in U.S.A. and several countries on
lettuce, celery and brassica vegetables such as broccoli, Brussels
sprouts, cabbage, Chinese cabbage, cauliflower, kale and Chinese kale.
The residues were generally less than 1 mg/kg 5-10 days after
treatment at rates ranging from 0.05 to 0.45 kg/ha. Residues of 1.2
mg/kg were found in cabbage and kale 7 days after treatment. In
celery the maximum residue 7 days after treatment was 1.2 mg/kg where
the application rates were within recommended dosage of 0.22 kg/ha.
When lettuce was treated at rates of up to 0.45 kg/ha and harvested 7
days after treatment, the maximum residue was 1.6 mg/ha at application
rate of 0.10 kg/ha except one trial in France in which 5.5 mg/kg was
found 7 days following application at 0.10 kg/ha.
Fruiting vegetables
Residue data for cucumbers are available from U.S.A. The maximum
residue was 0.47 mg/kg 3 days or later after treatment at rates within
the recommended dosage (up to 0.22 kg/ha).
In trials carried out in various countries, 1 to 9 successive
applications were made to tomatoes at rates up to 0.45 kg/ha. The
tomatoes were harvested at intervals ranging up to 40 days after the
last treatment, and the residues were below 0.5 mg/kg in all samples
taken 7 days or more after the treatment.
Root and tuber vegetables
Trials on sugar beets have been carried out in U.S.A., France, Denmark
and U.K. The maximum residue of 0.03 mg/kg was found in roots 9 days
after a single treatment at a rate of 0.15 kg/ha in the Danish trial.
In other trials the residues were not detectable at the limit of 0.01
mg/kg. In beet tops the maximum residue observed was 5.9 mg/kg, 21
days after treatment of 0.45 kg/ha.
Numerous trials have been carried out on potatoes in U.S.A., Canada,
France and Italy. No residues above the detection limit of 0.01 mg/kg
were found in potatoes harvested at 0-84 days following up to 9
applications at rates of 0.015-0.45 kg/ha.
Alfalfa
Residue data on alfalfa were obtained from the trials in U.S.A. where
single or repeat treatments were made at rates up to 0.45 kg/ha. When
alfalfa was harvested 21 days or later after treatments, the maximum
residue of 5.8 mg/kg was found at an application rate of 0.45 kg/ha.
The residues on dry alfalfa with water content of around 10% were
about 3 times higher than those on green alfalfa.
Legume vegetables
Residue data are available from several countries on legume vegetables
including green beans, dry beans, navy beans, snapbeans, pinto beans,
blackeyed peas, peas and soybeans. Residues in soybeans were
generally not detectable when analyzed more than 15 days after
treatment rates up to 0.45 kg/ha. The maximum residue of 0.06 mg/kg
was found 21 days after treatment at 0.08 kg/ha and 0.20 kg/ha. When
the treatment was made within the recommended dosage (0.22 kg/ha) and
the harvest was made 21 days or later after the treatment, the
residues in soybean plants, hay, straw and trash were below 5 mg/kg
except that one residue of 7.3 mg/kg was found after treatment at 0.20
kg/ha in the Australian trial.
Pome and stone fruits
Trials have been carried out on apples, pears and peaches in U.S.A.,
Canada, Australia, Japan and several European countries. In apples,
at application rates up to 0.72 kg/ha, the residues were less than 2.0
mg/kg 21 days or later after treatment except in a trial in U.S.A.
where 2.9 mg/kg was found 21 days after 7 treatments at 0.22 kg/ha.
In two trials in Japan, where 3 or 6 applications were made at a rate
of 1.4 kg/ha, the residues ranged from 0.92 to 3.5 mg/kg.
In pears the residues generally did not exceed 1 mg/kg following up to
7 applications at rates up to 0.67 kg/ha even when the last treatment
was made close to harvest. In one trial where 3 treatments were made
at a rate of 0.67 kg/ha the residue of 1.3 mg/kg was found 13 days
after the last treatment. In the same trial pears were treated with
fenvalerate at a rate of 1.34 kg/ha, and the residue was 1.9 mg/kg 13
days after 3 treatments.
Peaches were treated at rates of 0.1-0.8 kg/ha. The residues reached
2.3 mg/kg after 3 treatments at a concentration of 0.04% ai. Residues
of 2.2 mg/kg were found after 3 treatments at a rate of 0.8 kg/ha and
after a single treatment at a rate of 0.72 kg/ha.
Small fruits and berries
Trials on grapes have been carried out in U.S.A., Canada, Australia,
France and Italy. Residues did not exceed 0.80 mg/kg 14 days after
treatment at rates up to 0.22 kg/ha except two trials in France and
Italy. In Italian trials a residue of 1.3 mg/kg was found 30 days
after treatment at a rate of 0.2 kg/ha. In U.S.A., trials where
grapes were harvested 21 days after treatment at a rate of 0.11 kg/ha,
the residue of 1.6 mg/kg was reported, although in this trial the
residues 1 day and 14 days after treatment were 0.71 mg/kg and 0.51
mg/kg respectively.
In raspberries and strawberries the residues were less than 0.5 mg/kg
when the crops were harvested 7-23 days after treatment at rates up to
0.225 kg/ha.
Cereal grains, pre-harvest
Trials have been carried out in U.S.A., Canada, Australia and Brazil
on sweet corn, field corn, sorghum and wheat.
In sweet corn the maximum residue in kernels was 0.03 mg/kg, even
though 18 treatments were made with the intervals of 2-7 days and the
crops were harvested 2 days after treatment. The residues in stover
and ensilage ranged from 5.5 to 25 mg/kg. In field corn, the interval
between treatment and sampling was more than 72 days, and fenvalerate
was detected only in stover at a level of 0.07 mg/kg 140 days after
the treatment at a rate of 0.22 mg/kg. In wheat, the maximum residue
was 0.29 mg/kg in grain 13 days after treatment at a rate of 0.14
mg/ha and 15.0 mg/kg in straw 21 days after treatment at a rate of
0.30 kg/ha.
Cereal grains, post-harvest
Fenvalerate has undergone laboratory and silo-scale field trials as a
stored-grain protectant in Australia. Studies for this purpose have
been made on wheat, principally, (Bengston, 1979), barley
(Desmachelier, 1978) and sorghum (Bengston et al, 1979). All the
residue data indicate that fenvalerate is persistent under the
temperature and humidity conditions prevailing in Australian storages.
Large-scale trials have been made on stored wheat and sorghum
(Bengston, 1979 and Bengston et al., 1979a). Because of the strategy
of proposed usage fenvalerate has been applied at 1 mg/kg grain in
conjunction with its synergist, piperonyl butoxide (at 8 mg/kg grain)
and a complementary insecticide, fenitrothion (at l2 mg/kg grain).
Under these conditions of storage the fenvalerate residue declines
slowly: after 9-10 months about 75% of the applied amount remains
(Table 8). Expected residues in whole grain cereals would be less
than 1 mg/kg after storage.
Residues in processed products
Some of the cottonseed, peanuts and soybeans obtained from supervised
trials in U.S.A. and Colombia were subjected to processing to produce
oil products. The occurrence of residues in processed products are
summarized in Table 4.
In U.S.A. trials, the residues in various cottonseed oil products
after processing were less than 0.1 mg/kg. In the Colombian
experiment where a single treatment of 0.3 kg/ha was made one day
before harvest, the residue was 0.16 mg/kg, 0.23 mg/kg, 0.22 mg/kg and
0.18 mg/kg in crude, neutral, bleached and deodorised oil,
respectively, in accordance with a residue of 0.14 mg/kg in the
unprocessed cottonseed. These cottonseeds were separated into kernels
and hulls by a mechanical (simulated commercial) process. Such a
procedure might contribute to the higher residues, since the
mechanical processing might have caused contamination of kernels with
hulls, because when these seeds were separated by hand at the
laboratory, residues in kernels and hulls were 0.02 mg/kg and 0.40
mg/kg, respectively. Cottonseeds which were obtained from American
trials were separated by hand, and processed to oil products and no
residues were detected in extracted kernels.
Peanuts and soybeans which were harvested after treatment at rates of
0.11-0.45 kg/ha were processed to oil products and no residues were
detected in either crude or refined oil, or in extracted meal.
Wine made from grapes which contained up to 0.12 mg/kg of fenvalerate
71-74 days after treatment at rates of 0.075 and 0.15 kg/ha (France)
contained non-detectable residues.
Following post-harvest treatment and storage, fenvalerate residue in
wheat is found principally (68-69%) in the bran which comprises about
15% of the whole grain (Table 9). White flour constitutes about 72%
of the whole grain and contains about 8-9% of its fenvalerate residue.
The remaining residue is in the pollard. Residues in flour are
carried over into bread baked from that flour (Table 7b); there is no
reduction in residue level on a commodity-weight basis. White bread
prepared from treated grain would have about the same residue level of
fenvalerate as white flour, that is about 0.06-0.1 mg/kg on present
usage. The corresponding level for wholemeal bread or flour would be
about 0.5-0.8 mg/kg.
Table 3. Summary of Residues Following Field Trials (1976 to 1979)
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cottonseed Various
countries up to 13 0.0115 20 and Figures covering over 30 supervised trials reach 0.1
0.6 30 mg/kg only in isolated instances.
Peanuts (whole) U.S.A. 5 0.11 30 <0.01 <0.01
0.22 " <0.01 <0.01
5 0.22 " <0.01 <0.01 <0.01 <0.01
2 0.45 " 0.04 0.12 0.20 0.29
2 0.45 " 0.06 0.18 0.11 0.11
2 0.22 " 0.03
2 0.45 " 0.32
Peanuts (Vines) 5 0.11 " 0.69 0.01 0.02 0.1
5 0.22 " 1.6 0.02 0.04 0.04
2 0.11 " 6.4
2 0.22 " 3.2
2 0.11 " 0.51
2 0.22 " 0.42
Sunflower Australia 1 0.08 " 0.07 0.03
Brussels sprouts U.S.A. 8 0.045 " 0.13
8 0.09 " 0.04
Canada 3 0.15 " 0.85 0.60 0.45
Netherlands 1 0.045 " 0.04
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cabbage U.S.A. 4 0.11 " 0.03
4 0.22 " 0.01
8 0.055 " 0.37
8 0.11 " 0.46
3 0.11 " 1.2 0.82 0.28 0.76
3 0.22 " 2.4 1.2 0.37 0.45
Canada 3 0.15 " 0.25 0.14
3 0.16 " 0.30 0.02 <0.01
Australia 4 0.073 7.3 0.13 0.15 0.05
New Zealand 6 0.15 10 1.2 0.5 0.9
5 0.15 10 1.3 0.6 0.9
Thailand 1 0.07 20 <0.01 <0.01
Japan 3 0.3 20 0.14 0.011 <0.005
6 0.3 20 0.17 0.028 <0.005
Chinese cabbage Netherlands 1 0.05 30 0.52 0.25
Japan 3 0.15 20 0.25 0.13 0.091
5 0.15 20 0.33 0.084 0.061
Kale U.K. 2 0.075 30 1.5 0.88 0.62 0.26
2 0.225 30 1.8 1.2 0.93 0.53
Thailand 1 0.06 20 1.3 0.13
Cauliflower U.S.A. 4 0.11 20 1.5 0.35 0.15
4 0.22 20 1.8 0.61 0.32
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cauliflower Canada 3 0.16 20 0.28 0.02 <0.01
(cont'd)
Thailand 1 0.07 20 0.17 0.1
Broccoli U.S.A. 2 0.11 20 0.77
2 0.22 20 0.69
Canada 3 0.16 20 0.29 0.09 0.06
New Zealand 5 0.15 10 15.3 5.1 2.2
Celery U.S.A. 15 0.22 10 1.5 0.46
15 0.45 10 3.6 1.9
15 0.055 10 0.11 0.20
15 0.11 10 0.37 0.52
15 0.22 10 1.0 1.2
15 0.45 10 2.0 2.6
Lettuce U.S.A. 7 0.11 10 0.21 0.30 0.07
France 1 0.05 10 2.0 0.35 0.11
1 0.10 10 4.0 1.2 0.47
1 0.10 10 6.9 5.5 2.2
1 0.10 10 2.3 0.45 0.30
1 0.10 10 6.2 1.6 0.06
Netherlands 1 0.075 30 2.25 0.67
1 0.075 30 0.25 0.16
Aubergine France 1 0.11 30 0.08 0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cucumbers U.S.A. 5 0.11 30 0.09 0.04 0.03
5 0.22 30 0.48 0.08 0.03
5 0.45 30 0.54 0.07 0.08
Watermelon U.S.A. 4 0.11 30 0.02
4 0.22 30 <0.01
Bell peppers U.S.A. 3 0.11 30 0.06
0.22 30 0.11
0.45 30 0.39
7 0.22 30 0.19
0.45 30 0.34
Green peppers U.S.A. 2 0.45 30 0.02
Squash U.S.A. 5 0.11 30 0.10 0.02 0.01
Tomato U.S.A. 3 0.11 30 0.02 <0.01
0.22 30 0.07 <0.01
0.45 30 0.08 0.01
2 0.11 30 0.1
0.22 30 0.29
0.45 30 0.33
France 1 0.11 30 0.11 0.47 0.13
Netherlands 1 0.22 30 0.31
Spain 2 0.2 30 0.15 0.10 0.15
3 0.2 30 0.35 0.25 0.15
2 0.3 30 0.2 0.1 0.15
3 0.3 30 0.25 0.10 0.15
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Tomato Australia 9 0.01 20 0.16 0.15 0.19
(cont'd) 0.15 20 0.17 0.12 0.29
0.02 20 0.42 0.38 0.28
Mexico 2 0.15 20 0.04 0.03 0.02 0.03
1 0.01 <0.05 <0.05
South Africa 4 0.02 <0.05 <0.05
0.03 0.07 <0.05
0.04 0.06 <0.05
Mexico 1 0.15 30 0.08 0.05 0.03 0.03 0.01
1 0.3 30 0.13 0.13 0.08 0.05 0.02
3 0.3 30 0.31 0.16 0.19
Canary Islands 2 0.15 30 0.25 0.20 0.07
3 0.15 30 0.40 0.30 0.20 0.08 0.01(71)
Sugar Beets Demnark 1 0.15 20 Roots 0.03
Tops 1.2 0.59
U.S.A. 2 0.45 30 Roots <0.01 1.15(71)
Tops 5.9
(Similar findings in France and U.K)
Potatoes U.S.A.,Canada,
(20 trials) France 1-9 0.07-0.45 30 <0.01 <0.01 <0.01 <0.01 <0.01
No detectable residue found in any samples
Alfalfa U.S.A. 1 0.11 30 6.1 3.2 2.6 1.0
(green) 0.22 30 15 11 8.8 1.9
0.45 30 32 24 18 5.8
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Green beans France 1 0.16 30 0.31
0.21 30 0.67 0.48
0.32 0.43
0.43 30 1.7 0.58
Dry Beans U.S.A. 4 0.11 30 0.21
0.22 30 0.13
Navy Beans U.S.A. 4 0.11 30 <0.01
0.22 30 0.01
Australia 1 0.1 20 0.03 0.03
0.2 20 0.06 0.03
Snap beans U.S.A. 3 0.11 30 0.17 0.03
3 0.22 30 0.26 0.05
3 0.45 30 0.67 <0.01
Pinto beans U.S.A. 1 0.11 30 <0.01
0.22 30
Blackeyed peas U.S.A. 2 0.055 30 <0.01
0.11 30 <0.01
Peas U.S.A. 1 0.11 30 <0.01
1 0.22 30 <0.01
1 0.45 30 <0.01
Soybeans U.S.A. 1 0.11 30 0.06 0.01 <0.01 <0.01 <0.01
1 0.22 30 0.05 0.03 0.02 <0.01 <0.01
Brazil 2 0.12 30 <0.01 <0.01
2 0.24 30 <0.01 <0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Soybeans Colombia 4 0.075 30 <0.01
(cont'd) 4 0.30 30 <0.01
Australia 1 0.08 20 0.06
1 0.2 20 0.06
Asparagus bean Thailand 1 0.14 20 0.14 0.04
(A01-0603)
Apples France 1 0.15 30 0.26 0.37 0.40 0.19
Australia 3 0.02% 30 0.80 0.60 0.35
3 0.04% 30 0.90 0.35 0.35
Germany 6 0.0225 30 1.7 2.0 1.0
6 0.045 30 2.8 2.3 2.0 1.9 1.8
6 0.0225 30 0.96 0.84 0.87 0.56 0.61
6 0.045 30 2.4 2.6 1.8 1.5 1.0
6 0.225 30 2.8 2.4 2.1 1.9 1.6
6 0.045 30 4.8 5.8 3.6 1.3 1.3
Japan 3 1.4 20 1.91 1.76 1.88
6 1.4 20 3.21 3.5 2.88
3 0.72 20 0.6 0.26 0.44
3 0.70 20 0.54 0.42 0.40
New Zealand 12 0.008 10 1.0 0.57 0.57 0.37 0.52
South Africa 6 0.024% 20 0.14 0.07 0.05 <0.05
0.03% 20 0.14 0.08 0.09 0.05
0.048% 20 0.36 0.24 0.16 0.1
0.06 20 0.31 0.24 0.18 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Pears U.S.A. 7 0.11 30 0.11 0.08 0.05
7 0.22 30 0.16 0.14 0.08
3 0.67 30 1.3 0.48 0.60
0.24(62)
3 1.34 30 1.9 1.0 0.47
0.28(62)
2 0.67 30 0.54 0.39 0.30 0.15
France 2 0.1 30 0.32 0.23 0.19 0.15
2 0.13 30 0.40 0.30 0.22 0.14
Australia 3 0.02% 30 0.39 0.46 0.31
0.04% 30 0.56 0.60 0.30
South Africa 6 0.024% 30 0.22 0.08 0.06 0.05
0.03% 30 0.26 0.12 0.12 0.05
0.045% 30 0.35 0.24 0.19 0.12
Apricots U.S.A. 2 0.28 30 0.39
0.56 30 0.78
Cherries U.S.A. 3 0.055 30 1.0 0.66
0.11 30 0.93 2.3
0.22 30 2.9 2.6
Peaches U.S.A. 6 0.11 30 0.30 0.27 0.11
0.22 30 0.26 0.23 0.10
Japan 3 0.8 30 0.084 0.045 0.014
6 0.8 30 0.061 0.026 0.016
3 0.6 30 0.054 0.014 0.020
6 0.6 30 0.046 0.017 0.057
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Australia 3 0.02 30 0.52 0.52
0.04 30 1.3 2.3
Plums U.S.A. 2 0.22 30 1.3
0.45 30 1.1
Grapes U.S.A. 5 0.22 30 1.2 1.1 0.80
4 0.11 30 0.31 0.39 0.45
0.22 30 0.71 0.51 1.6
Canada 1 0.078 30 0.25 0.20
France 1 0.075 30 0.30 0.30 0.55 0.40
2 0.075 30 0.65 0.67
1 0.075 30 0.43 0.09 0.06 0.03
Raspberries Canada 1 0.225 30 0.26
3 0.135 30 0.40
Strawberries U.S.A. 1 0.11 30 <0.01 0.11
1 0.22 30 0.38 0.21
Canada 2 0.172 30 0.07
2 0.12 30 0.45
Citrus fruit Japan 3 0.67 30 0.78 0.89
(whole) 6 0.67 30 1.56 1.25
3 0.67 30 1.69 0.91
6 0.67 30 3.63 1.44
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Spain 2 0.15 30 1.1 1.2 0.85 0.75*
1.9 1.2 1.5 0.9
*Juice
<0.01
Kiwi fruit New Zealand 6 0.46 10 3.4 3.2 2.5 2.1
0.92 10 4.1 3.8 3.6 2.2
Sweet corn U.S.A. 18 0.22 30 0.01
(ears) 18 0.28 30 0.02
4 0.11 30 0.02
4 0.22 30 0.02 <0.01(140)
<0.01(164)
Field corn U.S.A. 1 0.22 4 Gran <0.01(164)
(ears) 1 0.11 30 <0.01(136)
1 0.22 30
1 0.45 30
Sorghum U.S.A. 2 0.11 30 0.17
0.22 30 0.32
0.45 30 0.58
Australia 1 0.075 30 4.0 1.09
0.15 30 4.9 1.70
South Africa 1 0.020 20 0.1 0.1 <0.1 <0.1 <0.1
0.030 20 0.2 0.2 0.1 0.1 <0.1
0.040 20 0.2 0.2 0.1 0.1 0.1
0.050 20 0.5 0.3 0.2 0.2 0.1
0.060 20 0.6 0.3 0.3 0.2 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Wheat Argentina 1 0.10 0.08
0.15 0.05
0.20 0.05
0.30 0.10
Canada 1 0.14 0.29 0.12 0.05
Brazil 1 0.09 0.01 0.02 0.01
Almonds U.S.A. 1 0.22 <0.01
Coffee beans Brazil 1 0.10 <0.06
Table 4. Distribution of Residues in Processed Products of Oilseeds
(Crop treated with 30% E.C. fenvalerate in U.S.A. during 1975-1976)
Cottonseed
Rate (kg/ha) 0.11 0.22 0.45 0.22 0.45
No. of applications 14 8 8 8 8
Pre-harvest intervals 30 6 6 48 48
(days)
Residues in....
Ginned cottonseed 0.05 0.01 0.02 0.12 0.19
Delinted cottonseed - - 0.02 0.01
Hulls 0.01 0.02 0.01 0.01 0.01
Solvent extracted meal - 0.01 0.01 0.01 0.01
Crude oil 0.04 0.01 0,01 0.03 0.05
Refined oil 0.05 0.01 0.01 0.04 0.06
Soapstock 0.01 0.01 0.01 0.01 0.01
Peanuts
Rate (kg/ha) 0.11 0.22 0.45
No. of application 2 2 2
Pre-harvest interval (days) 14 14 14
Residues in....
Whole nuts 0.01 0.02 0.02
Nut meats 0.01 0.01 0.01
Shells 0.03 0.04 0.02
Solvent extracted meal 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01
Refined bleached oil 0.01 0.01 0.01
Refined bleached
Hydrogenated oil 0.01 0.01 0.01
Soybeans
Rate (kg/ha) 0.11 0.22 0.45 0.22
No. of application 1 1 1 4
Pre-harvest interval(days) 66 66 66 48
Residues in....
Soybeans 0.01 0.01 0.01 0.02
Hulls 0.01 0.01 0.02 0.06
Solvent extracted meal 0.01 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01 0.01
Soapstock 0.01 0.01 0.01 -
FATE OF RESIDUES
Cows
Three lactating cows were fed daily doses of C-14-fenvalerate
(chlorophenyl ring label) at a level equivalent to 0.11 ppm in the
total diet for 21 days and sacrificed 12 hours after receiving the
last dose. Recovery of radiocarbon was about 65% of the administered
dose of which 29% was recovered in the urine, 36% in the faeces and
less than 1% in the milk. The radiocarbon in the milk ranged from
<0.0006 to 0.0019 µg equivalents/L and reached a plateau after one
week on the treated feed.
Nearly all of the radiocarbon in the milk was present as unchanged
fenvalerate. No detectable radiocarbon was found in the brain, fat,
kidney, liver, lung and muscle. The limit of determination in this
study was 0.02 ppm for fat and 0.01 ppm for other tissues (Potter,
1976a).
In a separate study, three lactating cows were fed 10.9 ppm
C-14-fenvalerate (benzyl ring label) in their total ration for 28
days. The C-14 residues in the milk collected during the last 24 days
of treatment ranged from 0.04 to 0.13 ppm equivalents. The
fenvalerate content of the whole milk ranged from 0.037 to 0.082 mg/kg
during the last 20 days of the test. The cows were sacrificed 12
hours after receiving the last dose. The highest tissue residues were
found in the mesenteric fat 0.74-0.79 mg/kg equivalent. The
gastrocnemius and quadriceps muscle contained up to 0.06 mg/kg
equivalents. A total of 92% of the C-14 administered to the cows was
recovered in the excreta and tissues (Potter and Arnold, 1977;
DeVries, 1976a).
Three lactating Guernsey cows were fed daily doses of C-14-fenvalerate
at 0.15 ppm in their total ration for 21 days. The cows were
sacrificed 12 hours after receiving the last dose. The C-14 in the
milk ranged from 0.0006 to 0.0018 mg/kg equivalents. The C-14
residues in the milk reached a plateau after one week on the treated
feed. Within the experimental error of the measurements all of the
C-14 in the milk was present as fenvalerate. No C-14 was found in
bone, brain, kidney, lung or muscle (limit of detection = 0.004 to
0.007 mg/kg equivalents). Traces of C-14 were found in mesenteric fat
and subcutaneous fat from two cows and in the liver from one cow
(Potter, 1976b).
Lactating cows were fed an equivalent of 0.15 ppm C-14 fenvalerate in
their daily ration for 21 days. Residues of 0.1-0.2 mg/kg of
fenvalerate were found in the cream by TLC and liquid scintillation
counting. These residues were confirmed as fenvalerate by GLC-EC
(Potter, 1976e),
Three dairy cows were each sprayed weekly for three successive weeks
at the estimated rate of 540 mg active material per square metre.
This spraying regime was proposed for ectoparasite control. Samples
of whole milk taken during the treatment period and up to one week
after the last application contained less than 0.01 mg/kg of
fenvalerate. No detectable residue was found in the brain, muscle,
liver and kidney. The fat contained up to 0.2 mg/kg fenvalerate
(DeVries, 1976b).
In a further study (DeVries, 1976c), the same spraying regime was
applied to 10 cows. Residues in cream reached a maximum level of 0.2
mg/kg three days after the last application. The elimination of these
residues followed first order kinetics with a half-life of 4.5 days.
Residues in body fat reached a maximum of 0.4 mg/kg several days after
the last application, and declined thereafter according to first order
kinetics with a half-life of 10.5 days. All other tissues tested were
free of detectable residues.
Fenvalerate was also applied as a spray at a rate of 0.2, 0.4 or 2.0
g/cow. The residues in muscle did not exceed 0.01 mg/kg. The maximum
residue in subcutaneous fat was 0.22 mg/kg 7 days after 3 treatments
at a rate of 2.0 g/cow. The maximum residue in milk was 0.02 mg/kg.
The residues in milk fat reached maximum a few days after treatment
(Noble, 1976a; DeVries, 1976d, 1976e, 1976f, 1976g).
A trial was conducted in Queensland, Australia, to determine the level
and fate of fenvalerate in cows following spray treatment with 0.1%
and 0.2% of fenvalerate at 200 ml per animal. Twenty-seven Hereford
heifers were used in the trial. Twelve animals were drafted to each
treatment group with three being kept as untreated controls. Three
animals in each treatment group were re-treated 7 days after the first
treatment. These animals were then slaughtered and sampled 7 days
following the second treatment to ascertain whether there was any
accumulation. Tissue samples were taken from omental fat, liver,
perirenal fat# kidney and muscle. The residues found in the samples
are indicated in Table 5 (limit of determination 0.01 mg/kg). Only
representative samples of non-fat tissues were analysed when
preliminary results indicated no detectable residues in liver, kidney
and muscle. (Shell Chem. Australia, 1978).
Six dairy cows were selected and randomly divided into two treatment
groups of three animals. Milk samples were collected prior to
treatment. Treatment at the rate of 200 ml/animal was made along the
dorsal midline. One group received spray of approved strength (0.1%
fenvalerate) and the other at double strength (0.2%). One litre
aliquots of milk were collected from each cow at each milking on days
1, 3, 7, and 10 post-treatment. These samples were separated and the
cream and skim milk portions were analysed separately. The results
are given in Table 7. The residues were confined to the butter fat.
A commercial dairy herd was treated with fenvalerate emulsion (0.1%
fenvalerate) at the rate of 200 ml/cow. Samples of the bulked milk
were taken prior to treatment and on days 1, 3, 7 and 10
post-treatment. These samples were separated and the cream and skim
milk analysed for fenvalerate residues. The results are given in
Table 6. The residues were confined to the butter fat.
Table 5. Residues in tissues of 27 Cows following Spray Treatments
(Queensland, Australia)
Residue mg/kg
Treatment of Day Omental Perirenal Liver, kidney
each cow fat fat muscle
untreated 0 <0.02 <0.02 All Samples
untreated 0 <0.02 <0.02 <0.01
untreated 0 <0.02 <0.02
0.2% a.m. 1 0.04 0.06
0.1% 1 0.05 0.03
0.1% 1 <0.02 0.03
0.2% 1 0.06 0.08
0.2% 1 0.08 0.04
0.2% 1 0.04 0.06
0.1% 3 0.02 0.02
0.1% 3 0.04 0.05
0.1% 3 0.02 0.03
0.2% 3 0.03 0.03
0.2% 3 0.04 0.03
0.2% 3 <0.04 <0.02
0.1% 7 0.04 0.04
0.1% 7 0.04 0.03
0.1% 7 0.05 0.10
0.2% 7 0.05 0.06
0.2% 7 0.07 0.05
0.2% 7 0.05 0.08
0.1% 14 0.05 0.06
0.1% 14 0.08 0.07
0 1% 14 0.10 0.08
0.2% 14 0.06 0.07
0.2% 14 0.13 0.07
0.2% 14 0.17 0.08
Table 6. Residues found in Butterfat of Bulked Milk of Commercial
Herds (Each cow treated once with fenvalerate emulsina).
Days after
treatment ppm fenvalerate
0 <0.005
1 0.015
3 0.066
7 0.046
10 0.030
Skim milk contained <0.002 ppm fenvalerate.
Table 7. Residues in Milk From Each of Six Cows Treated Once with Fenvalerate Emulsion
Animals sprayed at 1% Animals sprayed at 2%
Days after
treatment 1 2 3 Mean 4 5 6 Mean
Residues of fenvalerate in mg/kg of moisture-free butter fat
(Pre-treat)
0 (AM) <0.005 <0.005 <0.005 - - - - -
0 (PM) 0.016 0.041 0.006 0.02 0.011 0.008 0.011 0.019
1 (AM) 0.022 0.032 0.026 0.027 0.012 0.034 0.027 0.024
1 (PM) 0.023 0.044 0.025 0.031 0.036 0.029 0.034 0.050
3 (AM) 0.046 0.047 0.030 0.042 0.133 0.059 0.047 0.080
3 (PM) 0.031 0.037 0.019 0.029 0.094 0.050 0.046 0.063
7 (AM) 0.012 0.043 0.022 0.026 0.043 0.025 0.040 0.036
10 (AM) 0.016 0.029 0.022 0.022 0.019 0.029 0.046 0.031
Skim milk (residue after butter fat removal) contained less than 0.002 ppm.
Table 8. Fenvalerate Residues in Treated Wheat and Sorghum Stored in Concrete Silos
Grain Amount of Residues (mg/kg)
fenvalerate
Kind moisture Temperature applied calc'd Storage period (months)
% °C (mg/kg)
0.25 1.5 3 4.5 6.5 8 10
(a) Wheat1
range 10 31-25* 0.9-1.1 0.85-1.16 0.45-0.87 0.76-1.02 0.66-0.82 0.79-0.93 0.74-0.85 0.71-0.76
mean 1.01 1.01 0.7 0.9 0.76 0.86 0.8 0.74
(b) Sorghum2
range 26-24* 0.42-0.55 0.64-0.72 0.53-0.54 0.64-0.69
mean 11.8 0.53 0.68 0.54 0.67
* 1st figures are initial temperatures; 2nd are those 6-9 months later.
Wheat data are from two trial sites, one in each of two Australian States.
1 Bengston, 1979
2 Bengston et al., 1979
Table 9. Distribution of Fenvalerate Residues in Whole Wheat
Fractions and Residue Levels in Bread Baked from the Flour (B.W.
Simpson, 1979)
(a) Distribution of residue between whole grain fractions
Fraction fenvalerate residue
% whole mg/kg % distribution
grain
flour (white) 74.6 0.08 8.5
pollard 14.6 3.3 68.5
bran 10.8 1.5 23
Grain sample: 1.5 kg. It had a residue level of 0.6 mg
fenvalerate/kg.
(b) Residue in bread baked from the ground flour from (a)
Commodity fenvalerate residue (mg/kg)
White flour 0.08-0.09
White bread 0.06-0.1
Wholemeal flour* 0.7-0.8
Wholemeal bread 0.49-0.73
* reconstituted from the fractions (in (a) ) in the original whole
grain proportions.
The processing operations simulated those used in commercial practice.
Table 10. Residues of Fenvalerate Degradation Products in Field-Treated Crops in Canada
S-Phenoxy Fenvalerate Isovaleric "Reverse Decarboxylated
Benzoic Acid Amide Acid Ester" Fenvalerate
Crop Country Dose Rate (×) PHI Fenvalerate WL 44607* WL 47117+ WL 10944* SD53036 SD 54597
Apples Canada 70 g/ha (×1) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
16 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
150 g/ha (×8) 6 weeks 0.29 <0.05 <0.05 <0.05 <0.01 <0.05
6 weeks 0.57 <0.05 <0.05 <0.05 <0.01 <0.05
Pears Canada 30 g/ha (×3) 5 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
70 g/ha (×2) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
Peaches Canada 0.006% 0 weeks 0.4 <0.05 <0.05 <0.05 - -
1 week 0.25 <0.05 <0.05 <0.05 - -
2 weeks 0.10 <0.05 <0.05 <0.05 - -
Grapes Canada 0.006% 0 week 0.45 <0.05 <0.05 <0.05 - -
1 week 0.38 <0.05 <0.05 <0.05 - -
Cabbage Canada 70 g/ha (×7) 2 weeks 0.06 <0.05 <0.05 <0.05 <0.01 <0.05
Cauliflower 70 g/ha 1 day 0.20 <0.05 <0.05 <0.05 - -
14 days 0.02 <0.05 <0.05 <0.05 - -
B. sprouts Canada 70 g/ha (×6) 14 days 0.10 <0.05 <0.05 <0.05 - -
70 g/ha (×7) 1 day 0.14 <0.05 <0.05 <0.05 - -
Lettuce Canada 70 g/ha 7 days <0.01 <0.05 <0.05 <0.05 - -
Tomatoes Canada 150 g/ha (×2) 2 days 0.80 <0.05 <0.05 <0.05 - -
* Includes both free and conjugated forms
+ No conjugates formed
Hens
Mature laying hens were fed 0.03 ppm of C-14-fenvalerate (chlorophenyl
ring label) in their total ration for periods up to 32 days. No
detectable C-14 residues were found in the fat, heart, gizzard, liver,
meat, skin, egg whites and egg yolks (Potter, 1976c).
In a separate study, mature laying hens were fed 0.03 ppm of
C-14-fenvalerate (phenoxyphenyl ring label) in their total ration for
periods up to 32 days. No C-14 residues were detected in the light
meat, dark meat, skin, gizzard, blood or plasma. The residues in egg
yolks ranged from <0.002 to 0.003 mg/kg, fat samples <0.002 to 0.003
mg/kg (Potter and Sauls, 1978).
Laying hens were sprayed twice with a 0.5% emulsible concentrate of
fenvalerate. Fenvalerate residues in the eggs reached maximum levels
of 0.04 mg/kg 6 days after the first application and of 0.14 mg/kg 8
days after the second application. The residue levels in the eggs
decreased by one half in about 22 days. The major tissue depots for
fenvalerate were found to be the skin and fat (DeVries, 1976d).
Plants
Leaves of cotton were treated either once with a formulation of
C-14-fenvalerate (chlorophenyl ring label) at a rate of 240 ug per
leaf, or twice at a rate of 120 ug per leaf per treatment.
Determination of C-14 in extracts showed that the rate of C-14
disappearance from leaves was practically identical between single and
repeated applications, and a disappearance rate of about 50% was 35
days. Thin-layer chromatographic analysis of extracts from the cotton
leaves showed that fenvalerate represents about 50% of the C-14
recovered 101 days after single treatment and 80% of the C-14
recovered 46 days after the last of double treatments (Loeffler, 1976a
and 1976b).
In a separate study, cotton squares were also treated either once with
a formulation of C-14-fenvalerate (chlorophenyl ring label) at a rate
of 240 µg per square or three times at a rate of 120 µg per square per
treatment. Whole seeds isolated two to three months after the first
treatment contained less than 0.05 mg/kg equivalents of fenvalerate.
Single leaves of cotton, located either on the lower or the upper part
of the plants, were treated with an average of 49.7 µg per leaf to
determine the transport of C-14-fenvalerate from the treated to
untreated leaves. After 15 days, no C-14 was found in any of the
untreated leaves both above and below the treated leaves,
demonstrating the inability of fenvalerate or any possible C-14
metabolites to move out of a treated leaf either in the xylem or in
the phloem (Loeffler, 1976 c and d).
When C-14-fenvalerate labelled in either benzyl or chlorophenyl ring
was applied to lettuce grown in boxes (twice at a rate of 10.8 mg/box)
about 70-80% of the C-14 in the mature plants was present as unchanged
fenvalerate 12 days after the second treatment. Fenvalerate
hydrolysed slowly at the ester linkage to give 3-phenoxybenzoic acid
and 2-(4-chlorophenyl) isovaleric acid which then rapidly conjugated
with plant materials (Hitchings, 1977). When the same compounds were
applied to the fruit and leaves of apple trees twice or three times,
the leaves and apples harvested 3 to 4 weeks following the last
treatment were found to contain mainly unchanged fenvalerate (75 -85%
of the total C-14 in leaves and 86-93% of the total C-14 in apples).
After peeling the apples, less than 2% of the C-14 in the apples
remained in the peeled fruit. The major metabolite fraction (11-19%
of the total C-14) in leaves was a mixture of polar compounds which by
hydrolysis produced 3-phenoxybenzoic acid, 3-phenoxybenzaldehyde,
3-phenoxybenzyl alcohol and 2-(4-chlorophenyl) isovaleric acid. Other
minor metabolites identified in leaves and/or apples were des-cyano-,
amide- and 4'-OH-fenvalerate which accounted in total for less than 2%
of the total C-14. (Standen, 1977).
Fenvalerate and the (S)-acid isomer similarly disappeared from
cabbages and bean plants with half-lives of approximately 9 days and
14 days respectively, after foliar application of three C-14-labelled
preparations (Co, Ca and CN) at rates of 17-19 µg (cabbage) and 10 µg
(bean plant) per leaf. On and/or in plants, both compounds underwent
decarboxylation, ester cleavage, hydrolysis of the CN group to CONH2
and COOH groups, hydroxylation at the 2'-and 4'-phenoxy positions,
cleavage of diphenyl ether, conversion of a-cyano-3-phenoxybenzyl
alcohol to the corresponding benzyl alcohol and benzoic acid, and
conjugation of the resulting carboxylic acids and alcohols with sugar.
Very little of the C-14 was transferred to other parts of cabbages and
bean plants. Beans were planted in Kodaira light clay and Katano
sandy loam soils after treatment with 1.0 mg/kg of the CN- and
Ca-labelled preparations. After 30 days, pods and seeds, shoots and
roots were harvested and analyzed for C-14. Although small amounts of
radioactivity were found in roots, traces were present in pods, seeds
and shoots. No parent compound was detected in shoots (Ohkawa et al.,
1979a).
Search for degradation products on field grown crops
A series of trials were conducted in Canada (Shell 1979) to determine
whether and to what extent degradation products occur as free and
conjugated residues in crops grown under typical outdoor conditions.
As indicated in Table 8, none of the known degradation products could
be detected by methods sensitive to 0.05 mg/kg in any of 9 varieties
of fruits and vegetables treated in accordance with label directions.
Fenvalerate itself occurred in most of the samples at levels
consistent with those found in other trials.
Distribution of residues on the plant
From the examination of many plants treated under practical
conditions, it is evident that residues are almost exclusively
confined to those parts of the crop that are directly exposed to the
spray application. As examples, cottonseed kernels and peanut meats
have not contained detectable levels even when quite high amounts were
measured on the whole seeds or nuts (Shell Res. 1974, 1975 a and b,
1976 a and b, Shell Dev. 1976 a and b, 1977a, 1978b).
Trials on cabbage in Australia gave residues at 7.5 mg/kg for outer
discarded leaves and only at 0.05 mg/kg for cabbage hearts 5 days
after treatment at 0.118 kg ai/ha (Microchem., 1977). In another
trial, when tops of sugar beets contained up to 5.9 mg/kg, only trace
residues (0.03 mg/kg or less) were detected in roots (Statens, 1977;
Shell Develop., 1977b). Trials on Japanese radish in Japan showed
residues up to 1.97 mg/kg for leaves and only 0.005-0.04 mg/kg for
roots (Fujita et al., 1978a).
Similarly, in trials with peas, beans, citrus fruit, almonds, sweet
corn and wheat, the main part of the residues occurred on peel, skins,
pods and other exposed parts. Residues in edible portions rarely
exceeded detectable levels. (Shell Dev. 1976, 1977; Fujita et al.,
1978; Japan Food Res. 1977).
Photochemical degradation
When exposed to sunlight on silica gel, glass, soil and in water,
fenvalerate degraded in all these media. After 28 days of exposure to
sunlight about 25% was recovered from silica gel, 48% from soil or
from water, less than 1.3% from glass (FAN 1976).
When a 0.2 M solution of fenvalerate was exposed for 10 hours at 300
nm in quartz SD54597 (Benzenepropanenitrile,
4-chloro-beta-(1-methylethyl)-alpha-(3-phenoxyphenyl)-I was the major
product. Other amounts to less than 1% of the total. At 254 nm
SD53036 (Benzoic acid, 2-phenoxy-, 1-(4-chlorophenyl)-2-methylpropyl
ester) was also formed equivalent to about 3% of SD54597 present and
other products were less than 1% of the total (Ehmann, 1978a and b).
In various solutions (hexane, methanol, and acetonitrile-water; 6/4)
by artificial light (>290 nm) and as a thin film on grass or on
cotton by exposure to sunlight, the major products in solution were
formed via photoinduced decarboxylation. Other products resulted from
cleavage of the ester linkage, probably by a mechanism involving
free-radical intermediates. These were 3-phenoxybenzoyl cyanide,
3-phenoxybenzyl alcohol and 3-phenoxybenzaldehyde from the alcohol
moiety, and 2-(4-chlorophenyl) isovaleric acid and the dimer of the
acid fragment from the acid moiety. Decarboxylated fenvalerate,
2-(4-chlorophenyl) isovaleric acid, 3-phenoxybenzyl alcohol and
3-phenoxybenzaldehyde were also found on cotton. (Holmstead, et al.,
1977, 1978).
Another photodecomposition study reveals that on exposure to sunlight
fenvalerate was rapidly decomposed in distilled water, 2% aqueous
acetone, river and sea water to almost the same extent.
Photodegradation half-life in each aqueous suspension ranged from ca.
4 days in summer to 13-15 days in winter, due to seasonal variation of
sunlight intensity. On soil surfaces, exposed to sunlight, the rate
of photodegradation was dependent upon the soils used, with half-lives
of about 2, 6 and 18 days in Kodaira light clay, Azuchi sandy clay
loam and Katano sandy loam soils, respectively. Thus photodegradation
rate and pathways were dependent on the environmental conditions. The
predominant reactions were decarboxylation and cleavage of ester
linkage in water, while hydration at CN group was dominant on the soil
surface. (Mikami et al., 1979).
Soil
When C14-fenvalerate (chlorophenyl ring label) was applied to steam
sterilized and live sandy loam soil at a concentration of 2.5 ppm, the
amount of solvent extractable C-14 decreased slowly in aerobic and
anaerobic soils and stayed practically constant in sterile soils. The
proportion of intact fenvalerate in the extracts from aerobic and
anaerobic soils decreased to 90% over a three-month period. The
remaining 10% was present in the form of two to three products more
polar than fenvalerate (Loeffler, 1976a). Degradation of the same
labelled compound was examined under aerobic and anaerobic conditions
using three different soils and a steady decrease of recovery in the
organic solvent-extractable fraction were observed both under aerobic
and anaerobic conditions (Lee, 1978). Several of the breakdown
products of fenvalerate formed during the course of disappearance of
the parent compound were identified (Fan 1977). The effects of soil
micro-organisms were investigated and it was demonstrated that
sterilization of soil greatly retards degradation (Ohkawa et al.,
1978; Williams and Brown 1979; Noble, 1976).
The leaching behaviour of fenvalerate from soil was also investigated
(Ohkawa et al., 1978a; Jackson and Roberts, 1976; Kerritt, 1977).
Fish
In tests in which Channel catfish and rainbow trout were exposed to
dilutions of C-14 fenvalerate in water, rates of uptake and
distribution in the bodies of fish were followed and some metabolites
were identified (Potter 1975, 1976; Ohkawa, et al., 1978, 1979). The
results gave no evidence to suggest that problems may arise from the
concentration of residues in the bodies of fish that are used as human
food.
METHODS OF RESIDUE ANALYSIS
GLC equipped with electron-capture detector (ECD) has been used almost
exclusively for detection and quantitation. The ECD exhibits high
sensitivity to fenvalerate, responding to as little as 0.05 ng or even
less (Chapman et al., 1977), which afforded minimum detectability of
0.005-0.02 ppm (Takimoto et al., 1977; Talekar 1977; Shell Dev.
1976a). As a radioactive source, nickel-63 appears more advantageous
than tritium due to the relatively high temperature applied. Although
the alkali flame ionization detector (AFID) was also utilized for
confirmation of residue identity, the response of fenvalerate to
GLC-AFID was found to be about 100 times less than GLC-ECD. The
confirmation can be made by combined gas-liquid chromatography/mass
spectrometry (GLC/MS) using selective ion detection mode at mass peaks
such as 225 amu and 419 amu (Parent ion) (Shell Dev. 1976a).
Since fenvalerate comprises 4 optical isomers due to 2 chiral centres,
its GLC gives either single or resolved peaks depending upon the
column length, the column-packing materials, etc. A single peak
response was obtained by using 45-60 cm-long glass or stainless steel
column packed with 3% OV-101 on Gas-chrom Q (Shell Dev. 1976b), 3%
OV-101/3% Apiezon Grease L on Gas-chrom Q (Takimoto et al., 1977;
Talekar 1977), 5% OV-17 on Chromosorb W AW DMCS (Kato 1979), and 3%
SF-96/3% Apiezon Grease M on Diatomite CLQ (Woodhouse et al., 1979).
On the other hand Lee et al., (1978) observed two completely resolved
peaks for fenvalerate on 1.8 m-long glass column packed with 4%
SE-30/6% QF-1 on Chromosorb W (80/100 mesh) where the RR and SS
isomers with identical chromatographic properties were eluted after
the RS and SR pair. A complete resolution for fenvalerate was also
achieved on 3 m glass column packed with 3% OV-101 on Chromosorb W AW
DMCS (100/120 mesh) (Kato et al., 1978).
When exposed to a relatively high temperature in the GLC system (e.g.
more than 250°C at injection port), fenvalerate may undergo partial
degradation and/or isomerization depending upon the column material,
the glass wool packed into the injection port, and the column-aging
history (Barber 1976a). Therefore, care must be taken in the
simultaneous analysis of the diastereomers. The effects caused by
degradation are reproducible and can be minimized to insignificant
levels for quantitation.
The high-pressure liquid chromatography (HPLC), which permitted less
than 3 ppm of detection limit in one study (McKinney 1975), has been
applied to the residue analysis of fenvalerate. It has been used
successfully to determine residues in cereal whole grains and
commodities from the grains, such as flour and bread (Simpson 1978).
The problems with indeterminate differentiation of fenvalerate
diastereoisomers and breakdown on GLC columns were not found with HPLC
separation. The post-harvest residue data on treated cereals, flour
and bread reported in this monograph have been obtained by HPLC
determination. It was found that the limit of determination was in
the region of 0.02 mg/kg.
The solvent or solvent combinations selected for extraction of
fenvalerate vary according to the nature of substrates. From the
substrates with high moisture contents like fruits, leafy vegetables
and root crops fenvalerate was successfully extracted by chopping and
blending them either with polar solvents, e.g. acetonitrile (Lee et
al., 1978) and methanol-acetonitrile (Takimoto et al., 1977), acetone
(Chapman and Harris, 1978), or with nonpolar-polar solvent mixtures,
e.g. n-hexane-isopropanol (Shell Develop. 1976b) and petroleum
spirit-acetone (Shell Res. 1976). Talekar (1977) adopted a 12-hour
Soxhlet extraction with n-hexane-acetone for chopped cabbage. Dry and
low-moisture products are first fractured or ground to powder, under
freezing if necessary, prior to extraction with either polar, nonpolar
or dual solvent system (Shell Res. 1975). After mixing with anhydrous
sodium sulphate, cottonseeds were fractured, separated from the hull,
and blended with n-hexane (Shell Develop. 1976c), whereas coffee beans
were pulverized and macerated overnight with methanol (Kato 1979).
The blending/extraction with nonpolar solvents like n-hexane resulted
in excellent recoveries of fenvalerate with satisfactory separation
from fatty materials in the case of mammalian tissues, dairy products
and eggs (Shell Develop. 1976a).
Cleanup procedures include partition between immiscible polar and
nonpolar solvents, e.g. acetonitrile and n-hexane, for most extracts
from oily and dehydrated crops as well as from fatty products prior to
further Florisil column chromatography. Chapman and Harris (1978)
partitioned the aqeous acetone extract from a range of vegetables into
n-hexane before proceeding to cleanup on silica or alumina absorbents.
The combined solvent partition-Florisil column chromatography
procedure provided recoveries of 81-95% for eggs, chicken and cow
tissues, milk, and cream spiked at 0.01-0.1 ppm of fenvalerate (Shell
Develop. 1976a), along with 90-95% for cottonseed (Shell Develop.
1975) and 89% for coffee (Kato 1979), both fortified at 0.5 ppm. For
non-oily crops cleanup by either Florisil (activated or deactivated
with water) (Takimoto et al., 1977; Talekar 1977; Lee et al., 1978;
Shell Res. 1976), or silica gel (Lee et al., 1978; Kato et al., 1978)
column chromatography was applied. Over 90% recoveries of fenvalerate
were accomplished for crops including cabbage (Takimoto et al., 1977;
Talekar 1977; Lee et al., 1978); lettuce (Lea et al.,1978), potatoes
(Woodhouse et al., 1979), orange (Fujita et al., 1979), apple,
tomatoes, green peppers, peas and alfalfa (Shell Develop. 1976b) at
fortification levels of 0.01-40 ppm.
For the degradation products and metabolites of fenvalerate, Barber
(1976b) devised the analytical method for detection limits of
0.01-0.02 mg/kg for the residues of 3-phenoxybenzoic acid and
4-cloro-a-(1-methylethyl)-benzeneacetic acid (CMBA) in cottonseed.
(Kato et al., 1979) analyzed CMBA in human urine. The method
permitted detection of CMBA at 0.002 ppm as well as 93-100% recoveries
from the human urine fortified at 0.02-2 mg/kg. Barber (1978a-e) also
developed analytical methods for 1-(4-chlorophenyl)-2-methylpropyl
3-phenoxybenzoate and
4-chloro-B-(1-methylethyl)-a-(3-phenoxyphenyl)-benzenepropanenitrile
in soybeans, pears and apples as well as (aminocarbonyl)
(3-phenoxyphenyl)methyl 4-chloro-a-(1-methylethyl)-benzeneacetate in
potatoes and peanut shells with detection limits at 0.01 mg/kg.
NATIONAL LIMITS
National MRLs brought to the notice of the Meeting are listed (July
1979)
Country Commodity MRL Pre-harvest
(mg/kg) interval, days
Brazil Cotton 0.1 14
Soybeans 0.1 30
U.S.A. Cottonseed 0.2 21
Cattle(fat, meat,
meat by-products) 0.02
Goats (fat, meat,
meat by-products) 0.02
Hogs (fat, meat,
meat by-products) 0.02
Horses (fat, meat,
meat by-products 0.02
Milk (fat) 0.02
Sheep (fat, meat,
meat by-products) 0.02
(temporary)
Lettuce (head) 1 7
Cabbage 2 7
Cauliflower 1 7
Broccoli 0.5 14
Snap beans 0.5 7
(green beans)
Dry beans and 0.5 21
Dry pears
Cucumbers 0.05 10
Squash 0.05 10
Bell peppers 1 10
Tomato 1 7
Field corn 0.05 45
Grapes 1 21
Sweet corn (for
fresh market only) 0.05 3
Soybeans 0.02 21
National Limits, Continued...
Country Commodity Tolerance Pre-harvest
(mg/kg) interval, days
U.S.A.
(temporary)
Peanuts 0.02 21
Potatoes 0.02 21
Pears 1 28
Apples 2 21
Peaches 2 21
Australia Cottonseed, maize,
sweet corn 0.05 7
Pome and stone fruit 1 14
Milk and milk products
(fat basis) 0.2 -
Fat of meat of cattle 0.2 -
Soya beans, navy beans,
mung beans 0.2 21
Oilseed crops 0.2 14
Hungary
(temporary) Apples 1 14
Cherries 1 14
Peaches 1 14
Alfalfa 1 14
Beets 1 14
Cabbage 1 7
South Africa Apples, pears 0.5 14
Tomatoes 0.1 2
Grain sorghum 0.1 28
Cottonseed 0.1 -
New Zealand Brassica vegetables 5 7
Kiwi fruit 3 14
Apples, pears 1 14
Appraisal
Fenvalerate is a new synthetic ester related to the pyrethroids, with
a wide spectrum of efficacy against many species of pests. It is only
slowly degraded in sunlight. It is registered in a number of
countries for use on a variety of crops. The rate of application
ranges from 50-250 g/ha.
Supervised trials have been carried out in many countries and on many
crops. The rate of decline of residues on growing crops is slow, the
half-life ranging from 2 to 3 weeks. On stored cereal grains about
75% of the applied amount is found at the ninth month after treatment.
Fenvalerate does not penetrate significantly into plant tissues nor is
it translocated and there is little or no residue in crop parts not in
direct contact with the insecticide. Most of the residue remains on
the exposed parts of such commodities as cottonseed, peanuts and
fruits and is removed with shells and peels during processing and
preparation. Similarly, after treatment of stored grains some 70% is
in the bran of wheat, with considerably more in the hulls of oats and
rice which are normally removed before consumption.
A wide range of studies have been conducted on the fate of residues in
plants and animals. Residues in plants consist substantially of the
parent compound. In radiolabelled studies the occurrence of free and
conjugated metabolites has been demonstrated but these products have
not been found in field trials at levels above the limit of detection
(<0.05 mg/kg).
When administered or applied to livestock, fenvalerate is degraded and
the degradation products excreted in urine. A small proportion of the
applied dose is transferred to fatty tissues from which it may be
excreted via fat in the milk.
The process of photodecomposition proceeds by oxidation,
decarboxylation, hydration at the Cn-group, cleavage of ester and
diphenyl ether linkages and thence by hydrolysis to produce polar
derivatives of low molecular weight. It varies with exposure and is
fairly slow.
Fenvalerate is readily biodegraded by soil micro-organisms with a
residual half life of from 15 days to 3 months depending on soil
temperature, moisture and organic matter content. Because residues
are tightly bound to soil colloids, there is little likelihood of such
residues being transferred to water of streams through run-off or
leaching. Sprays falling directly onto bodies of water are rapidly
absorbed and inactivated on particulate matter. There is no evidence
of residues in soil being taken up by plants.
Under laboratory conditions it is possible to demonstrate that fish
can absorb the insecticide from water into various body tissues. Due
to the presence of particulate matter, surface absorption and the low
solubility of fenvalerate in water there appears to be little
likelihood of significant uptake of residues by fish under practical
conditions; nor are problems likely to arise from the occurrence of
residues in fish for human consumption.
Residues may be determined by GLC using electron capture detectors.
The ECD exhibits high sensitivity to fenvalerate responding to as
little as 0.05 ng. Polar solvents have been effective for extraction
when used with substrates containing substantial amounts of water.
Non-polar and petroleum solvents have been used with substrates
containing little water. Sweet co-distillation has proved convenient
and effective with lipid substrates. The limit of determination is
0.01 mg/kg.
Several national governments have established maximum residue limits
in a range of commodities. The data available provided a basis for
the Meeting to recommend the following maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits, determined and expressed as
fenvalerate, are recommended:
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Kiwi fruit 5 21
Alfalfa 20 21
Broccoli 2 7
Brussels Sprouts 2 7
Cabbage 2 7
Cauliflower 2 7
Celery 2 7
Lettuce 2 7
Chinese cabbage 1 7
Pome fruits 2 14
Peaches 2 14
Cherries 2 14
Citrus fruit 2 21
Tomatoes 1 7
Small fruits and berries 1 7
Beans (green) 1 7
Beans (dry) 0.5 7
Cottonseed 0.2 7
Cottonseed oil 0.1
Cucumbers 0.2 3
Squash 0.2 3
Soybeans 0.1 21
Sweet corn 0.05 7
Sugar beets 0.05 21
Sunflower seed 0.1 28
Radishes 0.05 21
Potatoes 0.05 7
Peanuts 0.1 7
Cereal grains 5
Wheat bran 5
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Wheat flour (wholemeal) 2
Wheat flour (white) 0.2
Milk 0.01
Milk products (fat basis) 0.2
Fat of meat 0.2
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
fenvalerate and/or metabolites;
2. Observations in humans occupationally exposed to high levels of
fenvalerate to evaluate the potential susceptibility of man to the
neurological disruption noted in rodents.
Desired:
1. An additional dominant lethal assay to reconfirm previous data;
2. Information on the occurrence and fate of photodecomposition
products;
3. Additional data from supervised residue trials on citrus, berry
fruits, beans and several other crops for which data are limited;
4. Results of on-going studies on the level and fate of fenvalerate
residues in stored products especially raw grain and milling
products derived therefrom.
REFERENCES
Albert, J.R. and Summitt, L.M. Intravenous Toxicity of WL 43775
(6-1-0-0) in the Mouse. (1976) Unpublished report from Shell
Development Co., Ltd.,
Barber, G.F.
1976a Separation of SD 43775 isomers for residue analysis.
1976b Residue data for SD 44607 in WL 10944 (acid metabolites of
SD43775 in cottonseed from cotton receiving seven applications of
SD43775, a California study. Shell Development Co.
1978a Residue data for SD 53036 and SD 54597, possible metabolites
of SD 43775, in soybeans receiving one aerial application of SD43775,
a Mississippi study.
1978b Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in whole apples receiving one dormant application of SD43775,
an Oregon study.
1978c Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in pears receiving one dormant application of SD43775, a
Michigan study.
1978d Residue levels of SD47117, a possible metabolite of SD 43775,
in potatoes receiving seven ground spray applications of SD43775, a
Minnesota study.
1978e Residue levels of SD47117, a possible metabolite of SD43775,
in peanut shells from peanuts receiving three aerial applications of
SD43775, an Oklahoma study.
Beliles, R.P., Makris, S.L and Weir, R.J. Three-Generation
Reproduction Study in Rats. (See Stein, 1977 for histopathology data).
(1978) Unpublished report from Litton Bionetics, Inc. submitted by
Sumitomo Chemical Company Ltd.,
Bengston, M. Personal communication from Final Report on Silo Scale
Experiments 1977-1978 to the Australian Wheat Board Working Party on
Grain Protectants. Queensland Department of Primary Industries.
Bengston, M., Davies, R.A.H., Desmachelier, J.M., Phillips, M., and
Simpson B.W. Additional grain protectants for the control of
malathion-resistant insects in stored sorghum. (in manuscript).
Blair D., and Roderick, H. Toxicity studies on the Pyrethroid
Insecticide WL43775, Emulsifiable Concentrate FX3368. Acute
Inhalation Exposure to an Aqueous Spray. (1975) Unpublished report
from Shell Development Co.
Boyer, A.C. Unpublished reports from Shell Development Co. submitted
by Sumitomo Chemical Co.:
1976a Metabolism of WL 43775 by Rat Liver Enzymes.
1976b Metabolism of WL 43775 by Rat Liver Enzymes - Part 2.
1977a Residues in Rat Tissues from Rats Fed C-14-SD 43775.
1977b Identification of Metabolites found in the urine of Rats Fed
C-14-SD 43775.
1977c Identification of Metabolites Found in the Faeces of Rats Fed
C-14-SD 43775.
1977d Identification of Metabolites in the Livers of Rats fed
C-14-SD43775.
Brooks, T.M. Toxicity studies with WL 43775: Mutagenicity Studies
with WL 43775 in the Host-Mediated Assay. (1976) Unpublished report
from Shell Development Co.
Brown, L.J. and Slomka, M.B. Skin Reaction Potential of Use Dialation
(1.33%) PYDRIN(R) EC. (1979) Unpublished report from Shell Development
Co.
Butterworth, S.T.G. and Carter, B.I. Toxicity Studies on the
Insecticide WL 43775: Acute Oral Toxicity and Neuropathological
Effects in Rats. (1976) Unpublished report from Shell Development Co.
Chapman, R.A. and Simmons, H.S. Gas-liquid chromatography of picogram
quantities of pyrethroid insecticides. J. Assoc. Off. Anal. Chem., 60:
977-978.
Chapman, R.A. and Harris, C.R. Extraction and liquid/solid
chromatography for the direct analysis of four pyrethroid insecticides
in crops by gas liquid chromatography. J. Chromatog. 166, 513-518.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Acute Toxicity, Skin Irritancy Potential of the Emulsifiable
Concentrate FX 3368. Unpublished report from Shell Development Co.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Toxicity and Skin and Eye Irritancy Potential of the ULV
Formulation FX 4353. (1976) Unpublished report from Shell Development
Co.
Creedy, C.L. and Potter, D. The Effect of Feeding WL 43775 on the
Microsomal Mono-Oxygenase System of Rat Liver. (1976) Unpublished
report from Shell Development Co.
Dean, B.J. Toxicity Studies with WL 43775: Dominant Lethal Assays in
Male Mice after Single Oral Doses of WL 43775. (1975) Unpublished
report from Shell Development Co.
Dean, B.J. and Senner, R.K. Toxicity Studies with WL 43775:
Chromosome Studies on Bone Narrow Cells of Chinese Hamster after Two
Daily Oral Doses of WL 43775. (1975) Unpublished report from Shell
Development Co.
Desmachelier, J.M. Personal Communication. CSIRO Division of
Entomology. (1979)
DeVries, D.M. Shell Development Co.
1976a Residues of SD 43775 in Milk from Cows fed Radiolabelled
SD43775
1976b Residues in Milk, Hair and Tissues of Cows sprayed with an
Emulsible Concentrate (6-1-3-1ECH)
1976c In Milk, Cream, Hair and Tissues of Cows Sprayed with an
Emulsible Concentrate or Fed 10 ppm in Their Diet
1976d In Eggs and Tissues of Hens Sprayed with an Emulsible
Concentration
1976e In Milk, Hair and Tissues of Cows Sprayed with an Emulsible
Concentration (6-1-3-1ECH)
1976f In Meat and Milk of Cattle Treated Dermally with a Water
Dispersible Liquid Formulation
1976g In Meat, Hair, and Milk Fat of Restrained Cows Treated
Dermally with an Emulsible Concentrated Formation
Dewar, A.J., Moffett, B.J. and Sitton, M.F. Toxicity Studies on the
Insecticide WL 43775: Biochemical and Functional Studies on the
Neurotoxicity of WL 43775 to Rats. (1975) Unpublished report from
Shell Development Co.
Dewar, A.J., Moffett, B.J., Sitton, M.F. and Baker, J.E. Toxicity
Studies on the Insecticide WL 43775: Biochemical and Functional
Studies on the Neurotoxicity of WL 43775 to Chinese Hamsters. (1978)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Three-Month Feeding Study in Rats. (1975)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Short-Term Feeding Study in Rats. (1976)
Unpublished report from Shell Development Co.
Hine, Inc. SD-43775 Toxicology: Acute and Repeated (14-Day) Dermal
Toxicity in the Rabbit. (1975) Unpublished report from Hine, Inc.,
submitted by Sumitomo Chemical Co.
Hine, Inc. Human Skin Testing on SD-43775. (1976) Unpublished report
from Hine, Inc. submitted by Sumitomo Chemical Co.
Hitchings, E.J. The Metabolism of the Pyrethroid Insecticide WL 43775
(Belmark) in Lettuce and Soil under Outdoor Conditions. (1977) Shell
Research Ltd.,
Holmstead, R.L. and Fullmer, D.G. Photodecarboxylation of Cyanohydrin
Esters. Models for Pyrethroid Photodecomposition. J. Agric. Food
Chem., 26, 56
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem., 26, 954
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem. 26, 590-95.
Ito, N. Histopathological Findings of Mice Treated with S5602 for
three Months (see Suzuki, 1976). (1976a) Unpublished report from
Sumitomo Chemical Co. Ltd.,
Histopathological Findings of Mice and Rats Exposed to Mist of S5602
for 4 weeks (see Khoda, 1976c). (1976b) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted by Sumitomo Chemical Co. Ltd.,
Histopathological Findings in Mice Treated with S5602 (see Suzuki, et
al., 1977b). (1978) Unpublished report from Nagoya City University
Medical School submitted by Sumitomo Chemical Co., Ltd.,
Japan Food Research Lab. Analytical Results of S5602 Residues in
Wheat Grain and Wheat Straw (1977)
Kaneko, H., Ohkawa, H. and Miyamoto, J. Comparative Metabolism of
Fenvalerate in Rats and Mice. (1979) Unpublished report from Sumitomo
Chemical Co. Ltd.,
Kaplan, M. and Murphy, S.D. Effect of Acrylamide on Rotor-Rod
Performance and Sciatic Nerve ß-Glucoronidase Activity of Rats.
Toxicol. Appl. Pharmacol. 22: 259-268.
Kato, S. Analysis of Sumicidin residues in coffee. Analysis
certificate No. 12010695. (1979) Japan Food Research Laboratories.
Kato, T. and Miyamoto, J. Residue Analysis of S-5602 and its optical
isomers in Chinese Cabbage. (1978) Sumitomo Chemical Co.
Kato, T., Ohnishi, J. and Miyamoto, J. The analytical method of
2-(4-chlorophenyl) isovaleric acid, a urinary metabolite of
fenvalerate (Sumicidin(R)). (1979) Sumitomo Chemical Co.
Kirkland, V.L. and Albert, J.R. (1977a) Unpublished Report from Shell
Development Co. Preliminary Pharmacodynamic Investigation of SD 43775
(6-10-0) Administered Intravenously to the Anesthetized Dog: I.
Effects on the Cardiovascular System and Respiration. II.
Electrocardiographic (EKG) Findings.
Khoda, H., Kadota, T. and Miyamoto, J. Unpublished reports from
Sumitomo Chemical Co. Ltd.,:
1976a Teratogenic Study on S5602 in Mice.
1976b Acute Inhalation Toxicity of S3206 and S5602 in Mice and
Rats.
1976c Subacute Inhalation Toxicity Study of S5602 in Mice and Rats.
Khoda, H., Kaneko, H., Ohkawa, H., Kadota, T. and Miyamoto, J. (1979)
Unpublished report from Sumitomo Chemical Co. Ltd., Acute
Intraperitoneal Toxicity of Fenvalerate Metabolites in Mice.
Lampert, P.W. (1977) Letter Report on Long-Term Rat Study (Gordon and
Weir, 1978) from the University of California San Diego School of
Medicine, Department of Pathology submitted by Sumitomo Chemical Co.
Lee, Y.W., Westcott, N.D. and Reichle, R.A. Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871
Loeffler, J.E. From Shell Development Co.:
1976a Disappearance of C-14 from Cotton Leaves Treated with
C-14-(Chlorophenyl)-SD 43775"
1976b Thin Layer Chromatography of SD 43775-Treated Cotton Leaves
1976c Carbon-14 Content of Cotton Seeds after Single and Multiple
Treatment of Squares with C-14-(Chlorophenyl)-SD 43775
1976d Investigation of the Transport of C-
14-(Chlorophenyl)-SD-43775 from Treated to Untreated Leaves on Cotton
Plants
1976e Degradation of C-14-(Chlorophenyl)-SD 43775 during Exposure
to Soil Under Aerobic, Anaerobic, and Sterile Conditions.
Matsubara, T., Suzuki, T., Kadota, T. and Miyamoto, J. Antidotes
Against Poisoning by S5602 in Rats. (1977) Unpublished report from
Sumitomo Chemical Co.
McKinney, W.J. Separation of SD 43775 from hexane extracts of cotton
by liquid chromatography. Shell Development Co.
Microchem Associates:
Report on Residues of WL 43775 in Cabbages, (1977)
Report on Residues of WL 43775 in Navy Beans (1978)
Mikami, N., Takahashi, Hayashi, K. and Miyamoto, J. Photodegradation
of Fenvalerate (Sumicidin(R)) in Water and on Soil Surface. Sumitomo
Chemical Company Ltd.,
Milner, C.K. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticide. Investigation of the Neurotoxic Potential of WL 43775.
(1977) Unpublished report from Shell Development Co.
Noble, P.J. AF1117 Residue Data in Animal Tissues, Milk and Cream.
(1976a) Shell Chemical (Australia) Propriety Ltd.,
Noble, A.S. Soil Percolation Studies with WL 43775. (1976b) Shell
Research Ltd.,
Ohkawa, H., Nambu, K., Inui, H. and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Soil and by Soil Micro-organisms. J.
Pesticide Sci. 3, 129
Ohkawa, H. and Kikuchi, R. Metabolic Fate of Fenvalerate
(Sumicidin(R)) in Rats, Soil, Soil Microorganisms and an Aquatic Model
Ecosystem. Sumitomo Chemical Co., Ltd.,
Ohkawa, H., Nambu, K., Mikami, N., and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Bean Plants and Cabbages. Sumitomo
Chemical Co. Ltd., (1979a)
Ohkawa, H., Kikuchi R. and Miyamoto, J. Bioaccumulation and
Biodegradation of the (S)-Acid Isomer of Fenvalerate (Sumicidin(R)) in
an Aquatic Model Ecosystem. Submitted to J. Pesticide Sci. (1979b)
Ohkawa, H., Kaneko, H., Tsuki, H. and Miyamoto, J. Metabolism of
Fenvalerate (Sumicidin(R)) in rats. J. Pesticide Sci. 4 (2), 143.
Okuno, Y., Kadota, T. and Miyamoto, J. Unpublished report from
Sumitomo Chemical Co.:
1976a. Neurotoxic Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Dermal Applications in Rats.
1976b. Neurotoxic Effects of Some Synthetic Pyrethroids by
Short-Term Feeding in Rats.
1977a. Neurotox Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Oral Administration in Rats.
1977b. Neurotoxic Effects of S5602 and Natural Pyrethrins by Oral
Administration in Rats.
1977c. Recovery of Histopathological Lesions in Rats Caused by
Short-Term Feeding of S5602.
Potter, J.C. (1976) Unpublished reports from Shell Development Co.:
a) Tissues of Rats Fed SD 43775-C-14; b) and c) Milk and tissues of
cows; d) eggs and tissues from laying hens; e) cream from the milk of
cows fed SD 43775 14-C.
Potter, J.C. and Arnold, D.L. Residues of C-14 in Tissues of Rats Fed
SD 43775-C-14 for 28 days. (1977) Unpublished report from Shell
Development Co.
Potter, J.C. and Sauls. Residues of 14-C in eggs and Tissues of
Laying Hens fed 0.03 ppm of SD 43775-14-C (Labelled in Benzene Ring
Adjacent to CN Group). (1978) Shell Development Co.
Quinn R.J., Paa, H., Mastri, C.W., Kinoshita, F.K. and Keplinger, M.L.
21-Day Subacute Dermal Toxicity Study with Technical SD 43775 and SD
43775 (2.4 lb/gal EC) in Albino Rabbits. (1976) Unpublished report
from Industrial Bio-Test Lab submitted by Sumitomo Chemical Co.
Shell Development Co. and Shell Research Ltd., (1975-1978) A numbered
series of 36 unpublished reports with residue data from field trials
on specified crops in different countries.
Simpson, B.W. (1979) Draft report to be published. Queensland
Department of Primary Industries Analytical Chemistry Branch,
Brisbane, Australia.
Standen, M.E. The Metabolism of the Pyrethroid Insecticide WL 43775
in Apple Fruit and Foliage under Outdoor Conditions. (1977) Shell
Research Ltd.,
Stein, A.A. Histopathology Evaluation of Animals from 3-Gene ration
Reproduction Study. (1977) Unpublished report from Microscopy for
Biological Research, Ltd., submitted as part of study cited above
(Beliles et al., 1978) by Sumitomo Chemical Co., Ltd.,
Summit, L.M. and Albert, J.R.
1977a. Oral Lethality of WL 43775 (6-1-0-0) in the Rat (Unpublished
report from Shell Development Co.)
1977b. Determination of the Acute Oral Lethality of WL 43775
(6-1-0-0) in the Male and Female Mouse.
Suzuki, H. and Miyamoto, J.
1976. Unpublished report from Sumitomo Chemical. Studies on
Mutagenicity of S5602 with Bacteria Systems.
1977. Studies on Mutagenicity of Some Pyrethroids on Salmonella
Strains in the Presence of Mouse Hepatic S9 Fractions.
Suzuki, T., Kadota, T. and Miyamoto, J. One Year Chronic Toxicity
Study of S5602 in Mice (Three Month Interim Report). (1976)
Unpublished report from Sumitomo Chemical Co.
Suzuki, H., Kishida, F. and Miyamoto, J. Studies on Mutagenicity of
S5602 in Ames Test in the Presence of Hepatic S9 Fractions from
Several Laboratory Animal Species. (1979) Unpublished report from
Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Fifteen-Month Chronic Toxicity Study of S5602 in Rats. (1977a)
Unpublished report from Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Eighteen-Month Chronic Toxicity Study of S5602 in Mice. (1977b)
Unpublished report from Sumitomo Chemical Co.
Takimoto, Y. and Miyamoto, J. Method for Residue Analysis of S-5602
in Cabbage. (1977) Sumitomo Chemical Co.
Talekar, N.S. Gas-liquid chromatographic determination of
a-cyano-3-phenoxybenzyl a-isopropyl-4-chlorophenylacetate residues
in cabbage. J. Assoc. Off. Anal. Chem., 60: 908-910
van der Pauw, C.L., Dix, K.M., Blanchard, K. and McCarthy, W.V.
Toxicity of WL-43775: Teratological Studies in Rabbits Given WL 43775
Orally. (1975) Unpublished report from Shell Development. Co.
Walker, B.J., Hend, R.W. and Linnett, S. Toxicity Studies on the
Insecticide WL 43775: Summary of Results of Preliminary Experiments.
Unpublished report from Shell Development Co.
Williams, I.H. and Brown, M.J. Persistence of Permethrin and WL 43775
in Soil. J. Agric. Food Chem. 27, 130
Woodhouse, R.N., Almond, R.H. and Anderson, J. Determination of
residues of Sumicidin in potatoes. (1979) Huntingdon Research Centre.
SMO 88/7939.
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
Groups of rats (11 male and 22 female rats per group) were fed
fenvalerate in the diet at dosage levels of 0, 1, 5, 25 and 250 ppm.
The animals were fed for 9 weeks prior to mating and initiation of a
standard 3-generation (2 litters per generation) reproduction study.
Fertility, viability, gestation and lactating indices were calculated
for each treatment group compared to control values. Ten male and 10
female weanlings from the F3b litter were examined histologically at
the conclusion of the study.
The mean body weight of the parent of the third generation (F2b
adults) showed a significant reduction at the 250 ppm level. Gross
necropsy revealed kidney changes in these animals which was not
apparently related to fenvalerate. No pathological changes were noted
to account for the weight loss. No effects on reproductive parameters
in any of the three generations were observed in the study.
Histological examination did not show an adverse effect of
fenvalerate. With the exception of weight reduction in the third
generation parents, there was no effect of fenvalerate on any
parameter measured in the study (Beliles, et al., 1978; Stein, 1977).
Teratogenicity
Mouse
Groups of pregnant mice (32 to 33 mice per group) were administered
fenvalerate at dosage levels of O, 5, 15 and 50 mg/kg body weight per
day on days 6 through 15 of gestation in a standard teratogenicity
bioassay. On day 18, groups of 20 mice were sacrificed and fetuses
were removed and examined for somatic and skeletal abnormalities. The
remaining parents were allowed to deliver naturally and the young
maintained for three weeks to weaning to evaluate postnatal deficits.
In addition to the teratogenicity study, two male and two female
weanlings from each dam were maintained for 8 weeks and mated to note
any effects on their reproductive potential. Toxic signs of poisoning
were noted in maternal mice at the high dose level. There was no
significant mortality over the course of the study and no effects were
noted on any of the animals as a result of continuous administration
of fenvalerate. In fetal examinations, there were no somatic or
skeletal changes as noted by internal or external tissue evaluations.
The animals maintained in an abbreviated reproduction study showed no
differences from control value in their ability to reproduce. There
were no changes in the reproduction indicates evaluated with any
animals examined. Under the conditions of this bioassay, fenvalerate
has been shown to have no teratogenic potential in mice (Kohda, et
al., 1976a).
Rabbit
Groups of pregnant rabbits (group size varied from 20 to 31 rabbits)
were administered fenvalerate from day 6 to day 18 of gestation. On
day 28, the rabbits were sacrificed and standard teratogenicity
assessments made with respect to early or late fetal death, viability
and standard somatic and skeletal teratogenic potential. Reduced body
weight of pregnant rabbits was observed with the highest dose level.
There were no significant differences from control values in any of
the parameters measured in the study. Under the conditions of the
study, fenvalerate did not induce a teratogenic event in rabbits (van
der Pauw, et al., 1975).
Special Studies on Mutagenicity
Microorganism
Fenvalerate was examined for its mutagenic potential using a series of
Salmonella typhimurium strains (TA 1535, TA 1538, TA 98 and TA 100) at
dose levels up to 1 mg/ plate. Two strains of Bacillus subtilis (H17
and M45) were tested at concentrations up to 10 mg/disc. The "Ames"
test was performed in the presence and absence of rat activation
systems and with positive and negative chemical controls. The Rec
assay was not evaluated with a metabolic activation system. With both
microbial bioassay systems, fenvalerate was not mutagenic (Suzuki and
Miyamoto, 1976).
In additional trials, fenvalerate did not increase the number of
revertant colonies of S. typhimurim (TA 1535, TA 1537, TA 1538, TA 98
and TA 100) in the presence or absence of a liver subcellular
activation system prepared from 6 different strains of PCB-treated
mice, 3 strains of rat, and the Syrian golden hamster. Fenvalerate,
tested at dosage levels up to 1 mg/plate, was not mutagenic (Suzuki
and Miyamoto, 1977; Suzuki, et al., 1979).
Mouse
Groups of mice were administered fenvalerate orally at doses of 0, 60
and 125 mg/kg body weight and injected intraperitoneally with an
indicator strain of microorganisms in a standard host-mediated assay.
A positive control, dimethylnitrosamine (DMNA), was used in this
study. Fenvalerate did not induce an increase in mutation frequency
of the S. typhimurium indicator while the DMNA significantly
increased the mutation frequency (Suzuki and Miyamoto, 1976).
Groups of mice were administered fenvalerate orally at dose levels of
0, 25 or 50 mg/kg. Immediately after administration, a suspension
culture of yeast Saccharomyces cerviciae was introduced to the
peritoneal cavity. Positive (ethyl methane-sulphonate) and negative
(DMSO) control animals were also used in this standard host-mediated
assay. Five hours after dosing, the yeast cells were recovered and
examined for mitotic gene conversion. No mutagenic effects were
detected in the cells from any of the fenvalerate concentrations
tested (Brooks, 1976).
Dominant Lethal Assay
Mouse
Groups of male mice (10-11 mice per group) were administered
fenvalerate orally at dosage levels of 0, 25, 50 and 100 mg/kg body
weight. Each treated male was mated with 3 virgin females for 7 days.
The procedure was repeated weekly with new females in a standard
dominant lethal assay. The females were sacrificed and examined for
the condition of the fetuses on day 13 of gestation.
There were no significant differences from control values with respect
to the effects of fenvalerate on pregnancy. Foetal implants in
females mated to males during the second week after treatment showed a
significant reduction in viable fetuses. There was also a significant
increase in early foetal deaths occurring in females mated during the
fourth week to males dosed at the highest level (Dean, 1975).
Hamster
Groups of Chinese hamsters (6 male and 6 females per group) were
orally administered fenvalerate (in DMSO) at dose levels of 0, 12.5
and 25 mg/kg on each of two successive days. Animals were sacrificed
8 or 24 hours after dosing and chromosomal preparations were made from
the femoral bone marrow cells. Similar preparations were made with
control animals administered methyl methanesulphonate (50 mg/kg).
Cells from animals administered methyl methanesulphonate showed
substantial numbers of chromatid gaps at 8 hours after dosing but not
at 24 hours. The administration of fenvalerate did not induce
demonstrable chromosomal damage at either sampling interval (Dean and
Senner, 1975).
Special Studies on Neurotoxicity
Hen
Groups of 6 hens were administered fenvalerate orally in dimethyl
sulphoxide at dosage levels of 0 and 1000 mg/kg daily for 5 days. A
positive control of TOCP (0.5 ml/kg) was also included in the study.
After 3 weeks, the fenvalerate and negative control animals were
retreated at the same dosage regimen. At the conclusion of an
additional 3 week observation period, animals were sacrificed and
histological examinations were performed on the central and peripheral
nervous system. All animals receiving TOCP developed readily defined
signs of delayed neurotoxicity and histological lesions were seen in
the sciatic nerve and spinal cord. Clinical signs and histopathologic
lesions were not noted in controls or fenvalerate-treated hens (Milner
and Butterworth, 1977).
Rat
A series of studies was performed to evaluate the neurotoxic potential
(to induce axonal and myelin disruption) of a group of synthetic
pyrethroid esters and of natural pyrethrum. Acute oral administration
of fenvalerate, cypermethrin, resmethrin, permethrin and natural
pyrethrum to rats at very high dosage levels resulted in severe
clinical signs of poisoning and mortality within 24 hours.
Histopathologic lesions were observed in the sciatic nerve with all
compounds tested. At lower levels, fenvalerate (200 mg/kg),
cypermethrin (100 mg/kg), permethrin (200 mg/kg) and pyrethrum (3500
mg/kg) did not show clinical signs of poisoning or histopathologic
lesions (Okuno, et al., 1977a; 1977b).
Groups of 6 male and female rats were fed fenvalerate in the diet at
concentrations of 0 and 2000 ppm for 8 to 10 days, after which the
sciatic nerve was examined for histological signs of degeneration.
All animals exposed to fenvalerate showed typical signs of acute
intoxication including ataxia, tremors and hypersensitivity.
Histological examinations at the end of the feeding interval did not
reveal any adverse effects of fenvalerate on the sciatic nerve (Hand
and Butterworth, 1976).
In a study to evaluate the reversibility of the lesions induced in the
sciatic nerve, rats were administered fenvalerate in the diet at dose
levels of 0 and 3000 ppm for 10 days. Mortality was evident as 60% of
the animals died within the course of the dietary treatment.
Surviving animals were fed normal control diets and examined for 12
weeks following completion of the feeding study. Animals were
sacrificed every 3 weeks and examined histologically for sciatic nerve
disruption. Sciatic nerves of rats sacrificed at 3 weeks on control
diets continued to show swelling and disintegration of axons. At 6
weeks there were no histological lesions. These results were also
observed at the 9 and 12-week intervals suggesting reversibility of
the histopathologic lesion observed following high dose treatment with
fenvalerate (Okuno and Kadota, 1977c).
Antidotal Studies
Following acute poisoning phenobarbital, pentobarbital and
diphenylhydantoin were found to be effective in relieving the acute
signs of intoxication. Phenobarbital (50 mg/kg, ip) prevented tremor,
diphenylhydantoin (100 mg/kg, ip) reduced the toxic reaction and
pentobarbital (35 mg/kg ip) removed the tremor reaction completely
within 30 minutes. Combinations of diphenylhydantoin with either of
the barbiturates was effective in reducing the onset and severity of
tremors while a variety of other agents were not active
(delta-tubocurarine, atropine, meprobamate, diazepam, biperidin and
trimethadione) (Matsubara, et al., 1977).
Special studies in pharmacology
Fenvalerate, administered to dogs in acute dosage rates sufficient to
induce toxic signs of poisoning, showed no consistent cardiovascular
effects as measured by EKG. Respiratory stimulation was noted at high
levels and was not reduced by anesthetic supplements (Urethane/alpha
chlorolos or pentobarbital) (Kirkland and Albert, 1977a; 1977b).
Skin and eye irritancy
Two formulated products were found to be severe eye and skin irritants
when examined in the rabbit. Dermal irritation was evident for 7 days
after a 24-hour exposure and severe conjunctivitis, corneal opacity
and iritis were observed within 30 minutes of an application of 0.2 ml
of the formulation to the conjunctival sac. Irrigation of the eye
after treatment reduced the irritation to some degree (Coombs and
Carter, 1975 and 1976).
Acute intraperitoneal toxicity of fenvalerate metabolites in mice
Table 2.
LD50 (mg/kg)
Compound Male Female
Fenvalerate 88.5 85.0
2-(4-Chlorophenyl)isovaleric acid 351 350
3-Phenoxybenzyl alcohol 371 424
3-4'(Hydroxyphenoxyl) benzyl alcohol 750-1000 750-1000
3-(2'-Hydroxyphenoxyl) benzyl alcohol 876 778
3-Phenoxybenzoic acid 154 169
3-(4'-Hydroxyphenoxy) benzoic acid 783 745
3-(2'-Hydroxyphenoxy) benzoic acid 859 912
3-Phenoxybenzaldehyde 415 416
NaSCN 604 578
All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO (Khoda, et al., 1979).
The acute intraperitoneal toxicity in mice of the proposed
decarboxylated photoproduct was reported to be substantially lower
than that toxicity noted with fenvalerate (Holmstead, et al., 1978).
Short Term Studies
Inhalation
Groups of 4 male and 4 female rats were exposed by inhalation (head
only) to an aerosolized formulation (77 micron particle size)
generated from an aqueous suspension containing 3 grams/litre.
Following a single administration (4 hours) of this non-inhalable
particulate, acute signs of poisoning were noted for a short period,
presumably from oral ingestion of the large particle. There was no
mortality and all animals appeared normal within 3 days following
exposure (Blair and Roderick, 1975).
Groups of rats and mice (10 male and 10 female of each species per
group) were administered fenvalerate by inhalation exposure 3 hours
daily for 4 weeks at concentration levels of 0, 2, 7 and 20 mg/m3.
Animals were exposed to a small, fully respirable particulate (1 to 2
microns) during the course of the study. Mortality was not noted over
the course of the study, but animals at the high dose level showed
acute toxic signs of poisoning. Growth was not affected at any dose
level; nor were hematology and clinical biochemistry parameters.
Gross and microscopic examination of tissues and organs at the
conclusion of the study showed no changes that were related to the
administration of fenvalerate (Khoda, et al., 1976c; Ito, 1976b).
Dermal
Groups of rabbits (7-8 male rabbits per group) were administered
fenvalerate dermally at dose levels of 0, 100 and 400 mg/kg daily for
6 hours (14 exposures were performed over a 22-day period). Mortality
was observed at the high dose level accompanied by severe weight loss,
clinical signs of poisoning and gross dermal effects. Rabbits
tolerated the 100 mg/kg dose with minor local dermatologic effects
(Hine, 1975).
Groups of rabbits (10 male and 10 female rabbits per group, five of
each sex had an occluded skin) were administered fenvalerate dermally
for 6 hours per day, 5 days per week for 3 weeks. The dosage levels
used were 0, 30, 100 and 300 mg/kg body weight. In addition, a
formulated product (an emulsifiable concentrate containing 2.4 pounds
fenvalerate/gallon) was also tested for its dermal toxicity at dosage
levels of 0, 100, 300 and 1000 mg/kg.
Mortality was observed with both treatments predominantly at the high
dosage group. Mortality was preceded by clinical signs of poisoning
primarily in the group exposed to the formulated product. At lower
concentrations, technical fenvalerate was mildly irritating to the
skin upon repeated dermal exposure. The formulation of fenvalerate
and the blank formulation used as a control were severely irritating.
When severe irritation was noted, there were significant effects on
body weight. There were no effects noted on various haematologic,
clinical chemistry and urinalysis parameters related to the presence
of fenvalerate. Gross and microscopic pathologic changes were noted
only at the site of administration and described as acanthosis and
hyperkeratosis of the epidermis. The extent and severity of the
lesions appeared to be dose-related especially with respect to the
formulated product. There were no significant gross or microscopic
effects noted in tissues and organs over the course of the study
(Quinn, et al., 1976).
Dietary
Rat
Groups of rats (12 male and 12 female rats per group) were fed
fenvalerate in the diet at dose levels of 0, 125, 500, 1000 and 2000
ppm for 90 days. Mortality was observed at 2000 ppm while growth and
food consumption were decreased at 1000 ppm and 2000 ppm. With the
exception of an increase In BUN concentration at the two highest dose
levels, hematological and clinical examinations at the conclusion of
the study were normal. Gross and microscopic examinations of tissues
and organs were performed at the conclusion of the study. Increases
in liver to body weight and kidney to body weight ratios were observed
at 500 ppm and above. These gross changes were not accompanied by
observable microscopic changes on histopathological examination.
Sciatic nerves, examined at the conclusion of the study, showed no
myelin or axonal degeneration (Hend and Butterworth, 1975).
Dog
Groups of young adult beagle dogs (4 male and 4 female dogs per group
were fed fenvalerate in the diet at dose levels of 0, 0.5, 0.25, 1.25
and 12.5 mg/kg body weight for 90 days. There were no abnormalities
over the course of the study. Growth, food consumption and behaviour
were normal. Results of clinical laboratory examinations performed 3
times during the course of the study, showed no effects of fenvalerate
in the diet. At the conclusion of the study, data from gross and
microscopic examination of a variety of tissues and organs
substantiated clinical data, again showing no effects of dietary
fenvalerate. Daily administration at a level of 12.5 mg/kg body
weight for a period of 90 days produced no detectable evidence of
toxicologic effects (Hart and Wosu, 1975).
Long Term Studies
Mouse
Groups of mice (from 35 to 47 male and female, ddY strain, mice/group)
were administered fenvalerate in the diet for 78 weeks at dosage
levels of 0, 100, 300, 1000 and 3000 ppm. An interim (3 months) and a
final report showed dose-related effects at 300 ppm and above.
Fenvalerate was not carcinogenic to the ddY strain of mouse (Suzuki
and Kadota, 1976; Ito, 1976a, 1978; Suzuki et al., 1977b).
At the early stages of the study, mortality was evident at the highest
dose level. Growth was reduced at 1000 ppm and above. These effects
were accompanied by excitability, hypersensitivity and other
behavioural changes in the first month of feeding. A variety of
hematological parameters were affected during the first three months
of study, predominantly at the high dose level. Several biochemical
changes were observed including a decrease in alkaline phosphatase, an
increase in blood urea nitrogen, an increase in leucine aminopeptidase
activity and a decrease in cholesterol and glucose occurring at 1000
and 3000 ppm with several of these parameters being affected at 100
ppm (glucose decrease in females). Gross examination of tissues and
organs showed a weight increase in several tissues of both males and
females exposed to the high dose level. Microscopic examination
suggested changes in the liver at the two highest dose levels.
Multiple small necrotic foci in the liver and changes in the
epithelial cells of the proximal convoluted tubules were noted,
apparently related to the presence of fenvalerate in the diet (Suzuki,
et al., 1976; Ito, 1976a).
At the conclusion of the 18-month study, animals were sacrificed for
terminal haematologic and biochemical changes as well as gross and
microscopic examination of tissues and organs. Over the 18 month
study, growth was depressed at 3000 ppm in both males and females.
Behavioural changes noted as transient hypersensitivity occurred at
1000 ppm and above. Increased mortality was also evident at the two
highest dose levels. No compound-related changes were noted with
respect to haematologic parameters, although clinical chemistry
parameters were in some instances substantially increased (SGPT, SGOT,
LDH and LAP activities in both sexes were significantly increased,
predominantly at the two highest dose levels but sporadically at 300
ppm). Gross changes were noted in several organ weights and organ to
body weight ratios primarily in liver, although changes were also
noted in the kidney, heart, spleen, pituitary and ovary at the highest
dose level. These gross changes were not accompanied by
histopathological events with the exception of granulomatous changes
in liver and the mesenteric lymph nodes noted at all treatment groups,
predominantly at the highest two dose levels. Biochemical changes,
noted at the three month sacrifice interval, were not seen at the
conclusion of the study (increased BUN, decreased glucose, etc.).
There were no indications in this study of increased tumorigenicity or
carcinogenicity as a result of fenvalerate (Suzuki, et al., 1977b).
Rat
Groups of rats (15 male and 15 female Wistar rate per group) were fed
at concentrations of O, 50, 150, 500 and 1500 ppm for 15 months in the
diet. There was no mortality in the study attributable to
fenvalerate. Growth, food consumption and behavioural changes were
significantly affected at the highest dose level. Hypersensitivity
was observed at the early stages of the experiment disappearing within
3 months. Growth was significantly depressed in both males and
females at the highest dose level. Food consumption was unaffected
over the course of the study. Clinical examinations, performed at
either one year (urinalysis) or at the conclusion of the study
(hematology, blood biochemistry and gross and microscopic pathology),
showed significant abnormal values in a variety of parameters at the
1500 ppm dose level. No opthamologic effects were noted. Urinalysis
was normal over the course of the study. Haemoglobin concentration
was depressed in males at the highest dose level and the females at
150 ppm and above. Blood biochemistry was significantly altered at
1500 ppm with respect to several parameters (BUN in both males and
females; protein and cholinesterase in females). Gross and
microscopic examination of tissues and organs including specific
sections of sciatic nerve and trigeminal ganglia and nerve showed no
dose-related effects. Generalized inflammatory and degenerative
changes were seen in both control and treated animals. Tumor
incidents were low and not related to the presence of fenvalerate.
There was no suggestion of a carcinogenic potential observed in the
study (Suzuki, et al., 1977a).
Groups of rats (93 male and 93 female Sprague-Dawley rats/group, 183
of each sex were used as the control and an additional 22 of each sex
were used as a separate control and high level group) were fed
fenvalerate in the diet at dose levels of 0, 1, 5, 25, 250 and 500
ppm. There was no mortality associated with the study although growth
was reduced at the 500 ppm dose level. The 500 ppm group and a
separate control group were sacrificed at 26 weeks while the other
animals were maintained for 2 years. There were no significant
effects on food consumption, growth or on behaviour at 250 pm.
Hematology, clinical chemistry tests and urinalyses, performed at
various time intervals over the course of the study on at least 10
animals of each sex at each dose level, showed no dose-related
effects. At the conclusion of the study, organ weight and organ to
body weight ratios were normal. Gross and microscopic examination of
tissues and organs did not differ significantly from controls. Benign
and malignant lesions occurred at random throughout all groups
examined at the end of the study and in those animals that died during
the course of the study. There were no lesions attributable to
fenvalerate. A specific pathology examination of the sciatic nerve of
animals fed 250 ppm was performed. The sciatic nerve was not found to
have been significantly affected by fenvalerate (Gordon and Weir,
1978; Lambert, 1977).
Observations in Man
Fenvalerate was administered dermally to adult men and women to the
skin of the arm or face at dosage levels of approximately 20-40 mg.
Control applications were carried out on the same individuals and
subjective evaluations were performed with respect to dermal
irritation. There was no erythema or other visible skin effects and
an evaluation of the subjective responses suggested no significant
differences between fenvalerate-treated and the control portions of
the body (Hine, 1976).
A double-blind study utilizing 29 male volunteers was performed to
test the skin reaction of formulated fenvalerate. The emulsifiable
concentrated formulation was diluted with water and administered to
one side of the face, on the cheek, with a control formulation applied
to the opposite cheek.
There were no signs of dermatitis noted at 24 hours following
administration. Subjective analyses of irritation or skin sensation
were performed with each individual. Under the conditions of the
study, the formulation of fenvalerate did not produce abnormal skin
sensations. There were no indications that any of the symptoms noted
(which included tingling, burning, stinging, itching, swelling,
numbness or heat) was associated with fenvalerate (Brown and Slomka,
1979).
COMMENTS
Fenvalerate, an ester related to the pyrethroids, currently being
developed for use as an agricultural insecticide, is rapidly absorbed
in mammals, widely distributed, and metabolized. Tissue residues of
fenvalerate and its ester metabolites appear to concentrate to some
degree in adipose tissue. Fenvalerate undergoes several major
metabolic reactions: cleavage of the ester linkage, hydroxylation of
the acid and alcohol moieties, and conversion of the CN group to SCN
and CO2. The metabolic fate in all animals studied appears to be
similar. Photolytic degradation has been shown to produce a
decarboxylated product not known to occur in mammals. Fenvalerate is
moderately toxic when administered by the oral route. The metabolic
and photolysis products are less toxic than the parent ester.
Studies on the mutagenicity and reproductive/teratogenic potential
were negative. Studies on neurotoxicity have shown that, following
high level exposure, rats showed reversible clinical signs of ataxia.
Microscopic examination of the sciatic nerve showed axonal swelling
and myelin disruption. Biochemical studies revealed an increase in
lysosomal enzyme activity (ß-glucuronidase and ß-galactosidase) in
peripheral nerve (see Report Section 3.5). Fenvalerate, when
administered to hens at high levels, did not induce signs of
peripheral neuropathy. No data were available to assess the
susceptibility of humans to this neuropathy.
Short-term and long-term studies have been performed in a variety of
test animals. Fenvalerate is not a carcinogen and in short-term
studies in dogs and in long-term studies in rats and mice dietary
no-effect levels have been observed. A temporary acceptable daily
intake for man was allocated. The concerns of the Meeting over the
potential for bioaccumulation, the neuropathy observed in rodents and
the limited information of the susceptibility to such an effect in man
were the basis for the temporary evaluation and for the request for
further observations in occupationally exposed humans and for
pharmacokinetic data. Further information is desired with respect to
an additional dominant lethal assay to provide verification of
existing data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effects
Mouse: 100 ppm in the diet equivalent to 11.9 mg/kg body weight
Rat: 250 ppm in the diet equivalent to 12.5 mg/kg body weight
Dog: 12.5 mg/kg body weight
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR MAN
0-0.06 mg/kg body weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Fenvalerate is a highly active broad spectrum insecticide. It is
particularly effective as a contact and stomach poison against
lepidopterous larvae and also has high activity against Orthoptera,
Hemiptera and Diptera. This activity, together with adequate
stability on foliage, and relatively low toxicity to mammals is likely
to lead to increasing use against pests of agriculture.
The substance can be combined with most other insecticides and
fungicides, but not those with alkaline properties. It is mostly
marketed as 20% and 30% concentrates for aqueous dilution; but
granules, ULV concentrates and powers are also available.
Pre-harvest treatments
Fenvalerate is registered and/or approved in many countries in Europe,
Asia, South and Central America, and Australia. It is used against a
wide range of pests, including tobacco bud worm, bollworms, cut worms,
armyworms, leafrollers, weevils, aphids, thrips, beetles, leaf miners,
moths, bugs, psyllas etc and on a wide range of crops including
fruits, vegetables, beans, cereals, cotton and tobacco. For these
purposes it is applied at from 25 to 300 g/ha depending on the crop.
Other uses
It is registered in Argentina for forestry use against Oiketicus.
It is also being developed for control of insect pests of stored grain
and against insects of importance in public health, animal health and
industry.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses
Many trials have been carried out on a wide variety of fruits,
vegetables, cereals and oilseeds. Most of the data were generated
under American conditions, supplementary data are available from
several other countries. Table 3 contains the summarised data,
representing several thousand separate analytical data provided by the
principal manufacturers.
Since fenvalerate is not systemic in plants, residues usually are very
low in commodities such as root crops, corn kernels and peas which are
protected from direct contact with the insecticide. Residue levels on
plants normally decline with a half-life of about one to two weeks.
Oilseeds
Following multiple applications at rates up to 0.45 kg/ha, and in over
30 supervised trials in various countries (i.e. higher than the
recommended dosage of up to 0.3. kg/ha). Residues in cottonseed were
generally below 0.1.mg/kg, regardless of the interval between last
treatment and harvest. A residue of 0.21 mg/kg was found in a
Colombian trial 1 day after treatment at the rate of 0.3 kg/ha, and
one trial in U.S.A. showed the residues of 0.12 mg/kg (at a rate of
0.22 kg/ha) and 0.19 mg/kg (0.45 kg/ha) 48 days after treatment. When
the seed cases were separated from kernels, the residues of
fenvalerate almost entirely remained on the seed cases and levels in
the oil products were very low.
All trials on peanuts have been made in U.S.A. When fenvalerate was
applied at rates of up to 0.22 kg/ha the residues in whole nuts did
not exceed 0.1 mg/kg. At the higher rate of 0.45 kg/ha however,
residues of 0.20-0.32 mg/kg were detected 14-21 days after
application. It was shown that residues in the meat and oil did not
exceed the detection limit (0.01 mg/kg) and that the shells carried
almost all the residues found in whole nuts. Even when the peanuts
were harvested 73 days after the treatment at 5 and 10 times higher
rates than the recommended dosage (up to 0.22 kg/ha) the residues in
whole nuts were 0.04 mg/kg and 0.09 mg/kg, respectively. In other
trials at rates up to 0.45 kg/ha the residues in hay, foliage and
vines ranged from 0.01 to 31 mg/kg.
Leafy and stem vegetables
Trials have been carried out in U.S.A. and several countries on
lettuce, celery and brassica vegetables such as broccoli, Brussels
sprouts, cabbage, Chinese cabbage, cauliflower, kale and Chinese kale.
The residues were generally less than 1 mg/kg 5-10 days after
treatment at rates ranging from 0.05 to 0.45 kg/ha. Residues of 1.2
mg/kg were found in cabbage and kale 7 days after treatment. In
celery the maximum residue 7 days after treatment was 1.2 mg/kg where
the application rates were within recommended dosage of 0.22 kg/ha.
When lettuce was treated at rates of up to 0.45 kg/ha and harvested 7
days after treatment, the maximum residue was 1.6 mg/ha at application
rate of 0.10 kg/ha except one trial in France in which 5.5 mg/kg was
found 7 days following application at 0.10 kg/ha.
Fruiting vegetables
Residue data for cucumbers are available from U.S.A. The maximum
residue was 0.47 mg/kg 3 days or later after treatment at rates within
the recommended dosage (up to 0.22 kg/ha).
In trials carried out in various countries, 1 to 9 successive
applications were made to tomatoes at rates up to 0.45 kg/ha. The
tomatoes were harvested at intervals ranging up to 40 days after the
last treatment, and the residues were below 0.5 mg/kg in all samples
taken 7 days or more after the treatment.
Root and tuber vegetables
Trials on sugar beets have been carried out in U.S.A., France, Denmark
and U.K. The maximum residue of 0.03 mg/kg was found in roots 9 days
after a single treatment at a rate of 0.15 kg/ha in the Danish trial.
In other trials the residues were not detectable at the limit of 0.01
mg/kg. In beet tops the maximum residue observed was 5.9 mg/kg, 21
days after treatment of 0.45 kg/ha.
Numerous trials have been carried out on potatoes in U.S.A., Canada,
France and Italy. No residues above the detection limit of 0.01 mg/kg
were found in potatoes harvested at 0-84 days following up to 9
applications at rates of 0.015-0.45 kg/ha.
Alfalfa
Residue data on alfalfa were obtained from the trials in U.S.A. where
single or repeat treatments were made at rates up to 0.45 kg/ha. When
alfalfa was harvested 21 days or later after treatments, the maximum
residue of 5.8 mg/kg was found at an application rate of 0.45 kg/ha.
The residues on dry alfalfa with water content of around 10% were
about 3 times higher than those on green alfalfa.
Legume vegetables
Residue data are available from several countries on legume vegetables
including green beans, dry beans, navy beans, snapbeans, pinto beans,
blackeyed peas, peas and soybeans. Residues in soybeans were
generally not detectable when analyzed more than 15 days after
treatment rates up to 0.45 kg/ha. The maximum residue of 0.06 mg/kg
was found 21 days after treatment at 0.08 kg/ha and 0.20 kg/ha. When
the treatment was made within the recommended dosage (0.22 kg/ha) and
the harvest was made 21 days or later after the treatment, the
residues in soybean plants, hay, straw and trash were below 5 mg/kg
except that one residue of 7.3 mg/kg was found after treatment at 0.20
kg/ha in the Australian trial.
Pome and stone fruits
Trials have been carried out on apples, pears and peaches in U.S.A.,
Canada, Australia, Japan and several European countries. In apples,
at application rates up to 0.72 kg/ha, the residues were less than 2.0
mg/kg 21 days or later after treatment except in a trial in U.S.A.
where 2.9 mg/kg was found 21 days after 7 treatments at 0.22 kg/ha.
In two trials in Japan, where 3 or 6 applications were made at a rate
of 1.4 kg/ha, the residues ranged from 0.92 to 3.5 mg/kg.
In pears the residues generally did not exceed 1 mg/kg following up to
7 applications at rates up to 0.67 kg/ha even when the last treatment
was made close to harvest. In one trial where 3 treatments were made
at a rate of 0.67 kg/ha the residue of 1.3 mg/kg was found 13 days
after the last treatment. In the same trial pears were treated with
fenvalerate at a rate of 1.34 kg/ha, and the residue was 1.9 mg/kg 13
days after 3 treatments.
Peaches were treated at rates of 0.1-0.8 kg/ha. The residues reached
2.3 mg/kg after 3 treatments at a concentration of 0.04% ai. Residues
of 2.2 mg/kg were found after 3 treatments at a rate of 0.8 kg/ha and
after a single treatment at a rate of 0.72 kg/ha.
Small fruits and berries
Trials on grapes have been carried out in U.S.A., Canada, Australia,
France and Italy. Residues did not exceed 0.80 mg/kg 14 days after
treatment at rates up to 0.22 kg/ha except two trials in France and
Italy. In Italian trials a residue of 1.3 mg/kg was found 30 days
after treatment at a rate of 0.2 kg/ha. In U.S.A., trials where
grapes were harvested 21 days after treatment at a rate of 0.11 kg/ha,
the residue of 1.6 mg/kg was reported, although in this trial the
residues 1 day and 14 days after treatment were 0.71 mg/kg and 0.51
mg/kg respectively.
In raspberries and strawberries the residues were less than 0.5 mg/kg
when the crops were harvested 7-23 days after treatment at rates up to
0.225 kg/ha.
Cereal grains, pre-harvest
Trials have been carried out in U.S.A., Canada, Australia and Brazil
on sweet corn, field corn, sorghum and wheat.
In sweet corn the maximum residue in kernels was 0.03 mg/kg, even
though 18 treatments were made with the intervals of 2-7 days and the
crops were harvested 2 days after treatment. The residues in stover
and ensilage ranged from 5.5 to 25 mg/kg. In field corn, the interval
between treatment and sampling was more than 72 days, and fenvalerate
was detected only in stover at a level of 0.07 mg/kg 140 days after
the treatment at a rate of 0.22 mg/kg. In wheat, the maximum residue
was 0.29 mg/kg in grain 13 days after treatment at a rate of 0.14
mg/ha and 15.0 mg/kg in straw 21 days after treatment at a rate of
0.30 kg/ha.
Cereal grains, post-harvest
Fenvalerate has undergone laboratory and silo-scale field trials as a
stored-grain protectant in Australia. Studies for this purpose have
been made on wheat, principally, (Bengston, 1979), barley
(Desmachelier, 1978) and sorghum (Bengston et al, 1979). All the
residue data indicate that fenvalerate is persistent under the
temperature and humidity conditions prevailing in Australian storages.
Large-scale trials have been made on stored wheat and sorghum
(Bengston, 1979 and Bengston et al., 1979a). Because of the strategy
of proposed usage fenvalerate has been applied at 1 mg/kg grain in
conjunction with its synergist, piperonyl butoxide (at 8 mg/kg grain)
and a complementary insecticide, fenitrothion (at l2 mg/kg grain).
Under these conditions of storage the fenvalerate residue declines
slowly: after 9-10 months about 75% of the applied amount remains
(Table 8). Expected residues in whole grain cereals would be less
than 1 mg/kg after storage.
Residues in processed products
Some of the cottonseed, peanuts and soybeans obtained from supervised
trials in U.S.A. and Colombia were subjected to processing to produce
oil products. The occurrence of residues in processed products are
summarized in Table 4.
In U.S.A. trials, the residues in various cottonseed oil products
after processing were less than 0.1 mg/kg. In the Colombian
experiment where a single treatment of 0.3 kg/ha was made one day
before harvest, the residue was 0.16 mg/kg, 0.23 mg/kg, 0.22 mg/kg and
0.18 mg/kg in crude, neutral, bleached and deodorised oil,
respectively, in accordance with a residue of 0.14 mg/kg in the
unprocessed cottonseed. These cottonseeds were separated into kernels
and hulls by a mechanical (simulated commercial) process. Such a
procedure might contribute to the higher residues, since the
mechanical processing might have caused contamination of kernels with
hulls, because when these seeds were separated by hand at the
laboratory, residues in kernels and hulls were 0.02 mg/kg and 0.40
mg/kg, respectively. Cottonseeds which were obtained from American
trials were separated by hand, and processed to oil products and no
residues were detected in extracted kernels.
Peanuts and soybeans which were harvested after treatment at rates of
0.11-0.45 kg/ha were processed to oil products and no residues were
detected in either crude or refined oil, or in extracted meal.
Wine made from grapes which contained up to 0.12 mg/kg of fenvalerate
71-74 days after treatment at rates of 0.075 and 0.15 kg/ha (France)
contained non-detectable residues.
Following post-harvest treatment and storage, fenvalerate residue in
wheat is found principally (68-69%) in the bran which comprises about
15% of the whole grain (Table 9). White flour constitutes about 72%
of the whole grain and contains about 8-9% of its fenvalerate residue.
The remaining residue is in the pollard. Residues in flour are
carried over into bread baked from that flour (Table 7b); there is no
reduction in residue level on a commodity-weight basis. White bread
prepared from treated grain would have about the same residue level of
fenvalerate as white flour, that is about 0.06-0.1 mg/kg on present
usage. The corresponding level for wholemeal bread or flour would be
about 0.5-0.8 mg/kg.
Table 3. Summary of Residues Following Field Trials (1976 to 1979)
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cottonseed Various
countries up to 13 0.0115 20 and Figures covering over 30 supervised trials reach 0.1
0.6 30 mg/kg only in isolated instances.
Peanuts (whole) U.S.A. 5 0.11 30 <0.01 <0.01
0.22 " <0.01 <0.01
5 0.22 " <0.01 <0.01 <0.01 <0.01
2 0.45 " 0.04 0.12 0.20 0.29
2 0.45 " 0.06 0.18 0.11 0.11
2 0.22 " 0.03
2 0.45 " 0.32
Peanuts (Vines) 5 0.11 " 0.69 0.01 0.02 0.1
5 0.22 " 1.6 0.02 0.04 0.04
2 0.11 " 6.4
2 0.22 " 3.2
2 0.11 " 0.51
2 0.22 " 0.42
Sunflower Australia 1 0.08 " 0.07 0.03
Brussels sprouts U.S.A. 8 0.045 " 0.13
8 0.09 " 0.04
Canada 3 0.15 " 0.85 0.60 0.45
Netherlands 1 0.045 " 0.04
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cabbage U.S.A. 4 0.11 " 0.03
4 0.22 " 0.01
8 0.055 " 0.37
8 0.11 " 0.46
3 0.11 " 1.2 0.82 0.28 0.76
3 0.22 " 2.4 1.2 0.37 0.45
Canada 3 0.15 " 0.25 0.14
3 0.16 " 0.30 0.02 <0.01
Australia 4 0.073 7.3 0.13 0.15 0.05
New Zealand 6 0.15 10 1.2 0.5 0.9
5 0.15 10 1.3 0.6 0.9
Thailand 1 0.07 20 <0.01 <0.01
Japan 3 0.3 20 0.14 0.011 <0.005
6 0.3 20 0.17 0.028 <0.005
Chinese cabbage Netherlands 1 0.05 30 0.52 0.25
Japan 3 0.15 20 0.25 0.13 0.091
5 0.15 20 0.33 0.084 0.061
Kale U.K. 2 0.075 30 1.5 0.88 0.62 0.26
2 0.225 30 1.8 1.2 0.93 0.53
Thailand 1 0.06 20 1.3 0.13
Cauliflower U.S.A. 4 0.11 20 1.5 0.35 0.15
4 0.22 20 1.8 0.61 0.32
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cauliflower Canada 3 0.16 20 0.28 0.02 <0.01
(cont'd)
Thailand 1 0.07 20 0.17 0.1
Broccoli U.S.A. 2 0.11 20 0.77
2 0.22 20 0.69
Canada 3 0.16 20 0.29 0.09 0.06
New Zealand 5 0.15 10 15.3 5.1 2.2
Celery U.S.A. 15 0.22 10 1.5 0.46
15 0.45 10 3.6 1.9
15 0.055 10 0.11 0.20
15 0.11 10 0.37 0.52
15 0.22 10 1.0 1.2
15 0.45 10 2.0 2.6
Lettuce U.S.A. 7 0.11 10 0.21 0.30 0.07
France 1 0.05 10 2.0 0.35 0.11
1 0.10 10 4.0 1.2 0.47
1 0.10 10 6.9 5.5 2.2
1 0.10 10 2.3 0.45 0.30
1 0.10 10 6.2 1.6 0.06
Netherlands 1 0.075 30 2.25 0.67
1 0.075 30 0.25 0.16
Aubergine France 1 0.11 30 0.08 0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Cucumbers U.S.A. 5 0.11 30 0.09 0.04 0.03
5 0.22 30 0.48 0.08 0.03
5 0.45 30 0.54 0.07 0.08
Watermelon U.S.A. 4 0.11 30 0.02
4 0.22 30 <0.01
Bell peppers U.S.A. 3 0.11 30 0.06
0.22 30 0.11
0.45 30 0.39
7 0.22 30 0.19
0.45 30 0.34
Green peppers U.S.A. 2 0.45 30 0.02
Squash U.S.A. 5 0.11 30 0.10 0.02 0.01
Tomato U.S.A. 3 0.11 30 0.02 <0.01
0.22 30 0.07 <0.01
0.45 30 0.08 0.01
2 0.11 30 0.1
0.22 30 0.29
0.45 30 0.33
France 1 0.11 30 0.11 0.47 0.13
Netherlands 1 0.22 30 0.31
Spain 2 0.2 30 0.15 0.10 0.15
3 0.2 30 0.35 0.25 0.15
2 0.3 30 0.2 0.1 0.15
3 0.3 30 0.25 0.10 0.15
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Tomato Australia 9 0.01 20 0.16 0.15 0.19
(cont'd) 0.15 20 0.17 0.12 0.29
0.02 20 0.42 0.38 0.28
Mexico 2 0.15 20 0.04 0.03 0.02 0.03
1 0.01 <0.05 <0.05
South Africa 4 0.02 <0.05 <0.05
0.03 0.07 <0.05
0.04 0.06 <0.05
Mexico 1 0.15 30 0.08 0.05 0.03 0.03 0.01
1 0.3 30 0.13 0.13 0.08 0.05 0.02
3 0.3 30 0.31 0.16 0.19
Canary Islands 2 0.15 30 0.25 0.20 0.07
3 0.15 30 0.40 0.30 0.20 0.08 0.01(71)
Sugar Beets Demnark 1 0.15 20 Roots 0.03
Tops 1.2 0.59
U.S.A. 2 0.45 30 Roots <0.01 1.15(71)
Tops 5.9
(Similar findings in France and U.K)
Potatoes U.S.A.,Canada,
(20 trials) France 1-9 0.07-0.45 30 <0.01 <0.01 <0.01 <0.01 <0.01
No detectable residue found in any samples
Alfalfa U.S.A. 1 0.11 30 6.1 3.2 2.6 1.0
(green) 0.22 30 15 11 8.8 1.9
0.45 30 32 24 18 5.8
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Green beans France 1 0.16 30 0.31
0.21 30 0.67 0.48
0.32 0.43
0.43 30 1.7 0.58
Dry Beans U.S.A. 4 0.11 30 0.21
0.22 30 0.13
Navy Beans U.S.A. 4 0.11 30 <0.01
0.22 30 0.01
Australia 1 0.1 20 0.03 0.03
0.2 20 0.06 0.03
Snap beans U.S.A. 3 0.11 30 0.17 0.03
3 0.22 30 0.26 0.05
3 0.45 30 0.67 <0.01
Pinto beans U.S.A. 1 0.11 30 <0.01
0.22 30
Blackeyed peas U.S.A. 2 0.055 30 <0.01
0.11 30 <0.01
Peas U.S.A. 1 0.11 30 <0.01
1 0.22 30 <0.01
1 0.45 30 <0.01
Soybeans U.S.A. 1 0.11 30 0.06 0.01 <0.01 <0.01 <0.01
1 0.22 30 0.05 0.03 0.02 <0.01 <0.01
Brazil 2 0.12 30 <0.01 <0.01
2 0.24 30 <0.01 <0.01
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Soybeans Colombia 4 0.075 30 <0.01
(cont'd) 4 0.30 30 <0.01
Australia 1 0.08 20 0.06
1 0.2 20 0.06
Asparagus bean Thailand 1 0.14 20 0.14 0.04
(A01-0603)
Apples France 1 0.15 30 0.26 0.37 0.40 0.19
Australia 3 0.02% 30 0.80 0.60 0.35
3 0.04% 30 0.90 0.35 0.35
Germany 6 0.0225 30 1.7 2.0 1.0
6 0.045 30 2.8 2.3 2.0 1.9 1.8
6 0.0225 30 0.96 0.84 0.87 0.56 0.61
6 0.045 30 2.4 2.6 1.8 1.5 1.0
6 0.225 30 2.8 2.4 2.1 1.9 1.6
6 0.045 30 4.8 5.8 3.6 1.3 1.3
Japan 3 1.4 20 1.91 1.76 1.88
6 1.4 20 3.21 3.5 2.88
3 0.72 20 0.6 0.26 0.44
3 0.70 20 0.54 0.42 0.40
New Zealand 12 0.008 10 1.0 0.57 0.57 0.37 0.52
South Africa 6 0.024% 20 0.14 0.07 0.05 <0.05
0.03% 20 0.14 0.08 0.09 0.05
0.048% 20 0.36 0.24 0.16 0.1
0.06 20 0.31 0.24 0.18 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Pears U.S.A. 7 0.11 30 0.11 0.08 0.05
7 0.22 30 0.16 0.14 0.08
3 0.67 30 1.3 0.48 0.60
0.24(62)
3 1.34 30 1.9 1.0 0.47
0.28(62)
2 0.67 30 0.54 0.39 0.30 0.15
France 2 0.1 30 0.32 0.23 0.19 0.15
2 0.13 30 0.40 0.30 0.22 0.14
Australia 3 0.02% 30 0.39 0.46 0.31
0.04% 30 0.56 0.60 0.30
South Africa 6 0.024% 30 0.22 0.08 0.06 0.05
0.03% 30 0.26 0.12 0.12 0.05
0.045% 30 0.35 0.24 0.19 0.12
Apricots U.S.A. 2 0.28 30 0.39
0.56 30 0.78
Cherries U.S.A. 3 0.055 30 1.0 0.66
0.11 30 0.93 2.3
0.22 30 2.9 2.6
Peaches U.S.A. 6 0.11 30 0.30 0.27 0.11
0.22 30 0.26 0.23 0.10
Japan 3 0.8 30 0.084 0.045 0.014
6 0.8 30 0.061 0.026 0.016
3 0.6 30 0.054 0.014 0.020
6 0.6 30 0.046 0.017 0.057
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Australia 3 0.02 30 0.52 0.52
0.04 30 1.3 2.3
Plums U.S.A. 2 0.22 30 1.3
0.45 30 1.1
Grapes U.S.A. 5 0.22 30 1.2 1.1 0.80
4 0.11 30 0.31 0.39 0.45
0.22 30 0.71 0.51 1.6
Canada 1 0.078 30 0.25 0.20
France 1 0.075 30 0.30 0.30 0.55 0.40
2 0.075 30 0.65 0.67
1 0.075 30 0.43 0.09 0.06 0.03
Raspberries Canada 1 0.225 30 0.26
3 0.135 30 0.40
Strawberries U.S.A. 1 0.11 30 <0.01 0.11
1 0.22 30 0.38 0.21
Canada 2 0.172 30 0.07
2 0.12 30 0.45
Citrus fruit Japan 3 0.67 30 0.78 0.89
(whole) 6 0.67 30 1.56 1.25
3 0.67 30 1.69 0.91
6 0.67 30 3.63 1.44
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Spain 2 0.15 30 1.1 1.2 0.85 0.75*
1.9 1.2 1.5 0.9
*Juice
<0.01
Kiwi fruit New Zealand 6 0.46 10 3.4 3.2 2.5 2.1
0.92 10 4.1 3.8 3.6 2.2
Sweet corn U.S.A. 18 0.22 30 0.01
(ears) 18 0.28 30 0.02
4 0.11 30 0.02
4 0.22 30 0.02 <0.01(140)
<0.01(164)
Field corn U.S.A. 1 0.22 4 Gran <0.01(164)
(ears) 1 0.11 30 <0.01(136)
1 0.22 30
1 0.45 30
Sorghum U.S.A. 2 0.11 30 0.17
0.22 30 0.32
0.45 30 0.58
Australia 1 0.075 30 4.0 1.09
0.15 30 4.9 1.70
South Africa 1 0.020 20 0.1 0.1 <0.1 <0.1 <0.1
0.030 20 0.2 0.2 0.1 0.1 <0.1
0.040 20 0.2 0.2 0.1 0.1 0.1
0.050 20 0.5 0.3 0.2 0.2 0.1
0.060 20 0.6 0.3 0.3 0.2 0.1
Table 3. Continued...
Applications Residues in mg/kg, at intervals (days) after application
Crop Country no. rate EC formulation
kg a.i./ha % 0 7 14 21 28 56
Wheat Argentina 1 0.10 0.08
0.15 0.05
0.20 0.05
0.30 0.10
Canada 1 0.14 0.29 0.12 0.05
Brazil 1 0.09 0.01 0.02 0.01
Almonds U.S.A. 1 0.22 <0.01
Coffee beans Brazil 1 0.10 <0.06
Table 4. Distribution of Residues in Processed Products of Oilseeds
(Crop treated with 30% E.C. fenvalerate in U.S.A. during 1975-1976)
Cottonseed
Rate (kg/ha) 0.11 0.22 0.45 0.22 0.45
No. of applications 14 8 8 8 8
Pre-harvest intervals 30 6 6 48 48
(days)
Residues in....
Ginned cottonseed 0.05 0.01 0.02 0.12 0.19
Delinted cottonseed - - 0.02 0.01
Hulls 0.01 0.02 0.01 0.01 0.01
Solvent extracted meal - 0.01 0.01 0.01 0.01
Crude oil 0.04 0.01 0,01 0.03 0.05
Refined oil 0.05 0.01 0.01 0.04 0.06
Soapstock 0.01 0.01 0.01 0.01 0.01
Peanuts
Rate (kg/ha) 0.11 0.22 0.45
No. of application 2 2 2
Pre-harvest interval (days) 14 14 14
Residues in....
Whole nuts 0.01 0.02 0.02
Nut meats 0.01 0.01 0.01
Shells 0.03 0.04 0.02
Solvent extracted meal 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01
Refined bleached oil 0.01 0.01 0.01
Refined bleached
Hydrogenated oil 0.01 0.01 0.01
Soybeans
Rate (kg/ha) 0.11 0.22 0.45 0.22
No. of application 1 1 1 4
Pre-harvest interval(days) 66 66 66 48
Residues in....
Soybeans 0.01 0.01 0.01 0.02
Hulls 0.01 0.01 0.02 0.06
Solvent extracted meal 0.01 0.01 0.01 0.01
Crude oil 0.01 0.01 0.01 0.01
Soapstock 0.01 0.01 0.01 -
FATE OF RESIDUES
Cows
Three lactating cows were fed daily doses of C-14-fenvalerate
(chlorophenyl ring label) at a level equivalent to 0.11 ppm in the
total diet for 21 days and sacrificed 12 hours after receiving the
last dose. Recovery of radiocarbon was about 65% of the administered
dose of which 29% was recovered in the urine, 36% in the faeces and
less than 1% in the milk. The radiocarbon in the milk ranged from
<0.0006 to 0.0019 µg equivalents/L and reached a plateau after one
week on the treated feed.
Nearly all of the radiocarbon in the milk was present as unchanged
fenvalerate. No detectable radiocarbon was found in the brain, fat,
kidney, liver, lung and muscle. The limit of determination in this
study was 0.02 ppm for fat and 0.01 ppm for other tissues (Potter,
1976a).
In a separate study, three lactating cows were fed 10.9 ppm
C-14-fenvalerate (benzyl ring label) in their total ration for 28
days. The C-14 residues in the milk collected during the last 24 days
of treatment ranged from 0.04 to 0.13 ppm equivalents. The
fenvalerate content of the whole milk ranged from 0.037 to 0.082 mg/kg
during the last 20 days of the test. The cows were sacrificed 12
hours after receiving the last dose. The highest tissue residues were
found in the mesenteric fat 0.74-0.79 mg/kg equivalent. The
gastrocnemius and quadriceps muscle contained up to 0.06 mg/kg
equivalents. A total of 92% of the C-14 administered to the cows was
recovered in the excreta and tissues (Potter and Arnold, 1977;
DeVries, 1976a).
Three lactating Guernsey cows were fed daily doses of C-14-fenvalerate
at 0.15 ppm in their total ration for 21 days. The cows were
sacrificed 12 hours after receiving the last dose. The C-14 in the
milk ranged from 0.0006 to 0.0018 mg/kg equivalents. The C-14
residues in the milk reached a plateau after one week on the treated
feed. Within the experimental error of the measurements all of the
C-14 in the milk was present as fenvalerate. No C-14 was found in
bone, brain, kidney, lung or muscle (limit of detection = 0.004 to
0.007 mg/kg equivalents). Traces of C-14 were found in mesenteric fat
and subcutaneous fat from two cows and in the liver from one cow
(Potter, 1976b).
Lactating cows were fed an equivalent of 0.15 ppm C-14 fenvalerate in
their daily ration for 21 days. Residues of 0.1-0.2 mg/kg of
fenvalerate were found in the cream by TLC and liquid scintillation
counting. These residues were confirmed as fenvalerate by GLC-EC
(Potter, 1976e),
Three dairy cows were each sprayed weekly for three successive weeks
at the estimated rate of 540 mg active material per square metre.
This spraying regime was proposed for ectoparasite control. Samples
of whole milk taken during the treatment period and up to one week
after the last application contained less than 0.01 mg/kg of
fenvalerate. No detectable residue was found in the brain, muscle,
liver and kidney. The fat contained up to 0.2 mg/kg fenvalerate
(DeVries, 1976b).
In a further study (DeVries, 1976c), the same spraying regime was
applied to 10 cows. Residues in cream reached a maximum level of 0.2
mg/kg three days after the last application. The elimination of these
residues followed first order kinetics with a half-life of 4.5 days.
Residues in body fat reached a maximum of 0.4 mg/kg several days after
the last application, and declined thereafter according to first order
kinetics with a half-life of 10.5 days. All other tissues tested were
free of detectable residues.
Fenvalerate was also applied as a spray at a rate of 0.2, 0.4 or 2.0
g/cow. The residues in muscle did not exceed 0.01 mg/kg. The maximum
residue in subcutaneous fat was 0.22 mg/kg 7 days after 3 treatments
at a rate of 2.0 g/cow. The maximum residue in milk was 0.02 mg/kg.
The residues in milk fat reached maximum a few days after treatment
(Noble, 1976a; DeVries, 1976d, 1976e, 1976f, 1976g).
A trial was conducted in Queensland, Australia, to determine the level
and fate of fenvalerate in cows following spray treatment with 0.1%
and 0.2% of fenvalerate at 200 ml per animal. Twenty-seven Hereford
heifers were used in the trial. Twelve animals were drafted to each
treatment group with three being kept as untreated controls. Three
animals in each treatment group were re-treated 7 days after the first
treatment. These animals were then slaughtered and sampled 7 days
following the second treatment to ascertain whether there was any
accumulation. Tissue samples were taken from omental fat, liver,
perirenal fat# kidney and muscle. The residues found in the samples
are indicated in Table 5 (limit of determination 0.01 mg/kg). Only
representative samples of non-fat tissues were analysed when
preliminary results indicated no detectable residues in liver, kidney
and muscle. (Shell Chem. Australia, 1978).
Six dairy cows were selected and randomly divided into two treatment
groups of three animals. Milk samples were collected prior to
treatment. Treatment at the rate of 200 ml/animal was made along the
dorsal midline. One group received spray of approved strength (0.1%
fenvalerate) and the other at double strength (0.2%). One litre
aliquots of milk were collected from each cow at each milking on days
1, 3, 7, and 10 post-treatment. These samples were separated and the
cream and skim milk portions were analysed separately. The results
are given in Table 7. The residues were confined to the butter fat.
A commercial dairy herd was treated with fenvalerate emulsion (0.1%
fenvalerate) at the rate of 200 ml/cow. Samples of the bulked milk
were taken prior to treatment and on days 1, 3, 7 and 10
post-treatment. These samples were separated and the cream and skim
milk analysed for fenvalerate residues. The results are given in
Table 6. The residues were confined to the butter fat.
Table 5. Residues in tissues of 27 Cows following Spray Treatments
(Queensland, Australia)
Residue mg/kg
Treatment of Day Omental Perirenal Liver, kidney
each cow fat fat muscle
untreated 0 <0.02 <0.02 All Samples
untreated 0 <0.02 <0.02 <0.01
untreated 0 <0.02 <0.02
0.2% a.m. 1 0.04 0.06
0.1% 1 0.05 0.03
0.1% 1 <0.02 0.03
0.2% 1 0.06 0.08
0.2% 1 0.08 0.04
0.2% 1 0.04 0.06
0.1% 3 0.02 0.02
0.1% 3 0.04 0.05
0.1% 3 0.02 0.03
0.2% 3 0.03 0.03
0.2% 3 0.04 0.03
0.2% 3 <0.04 <0.02
0.1% 7 0.04 0.04
0.1% 7 0.04 0.03
0.1% 7 0.05 0.10
0.2% 7 0.05 0.06
0.2% 7 0.07 0.05
0.2% 7 0.05 0.08
0.1% 14 0.05 0.06
0.1% 14 0.08 0.07
0 1% 14 0.10 0.08
0.2% 14 0.06 0.07
0.2% 14 0.13 0.07
0.2% 14 0.17 0.08
Table 6. Residues found in Butterfat of Bulked Milk of Commercial
Herds (Each cow treated once with fenvalerate emulsina).
Days after
treatment ppm fenvalerate
0 <0.005
1 0.015
3 0.066
7 0.046
10 0.030
Skim milk contained <0.002 ppm fenvalerate.
Table 7. Residues in Milk From Each of Six Cows Treated Once with Fenvalerate Emulsion
Animals sprayed at 1% Animals sprayed at 2%
Days after
treatment 1 2 3 Mean 4 5 6 Mean
Residues of fenvalerate in mg/kg of moisture-free butter fat
(Pre-treat)
0 (AM) <0.005 <0.005 <0.005 - - - - -
0 (PM) 0.016 0.041 0.006 0.02 0.011 0.008 0.011 0.019
1 (AM) 0.022 0.032 0.026 0.027 0.012 0.034 0.027 0.024
1 (PM) 0.023 0.044 0.025 0.031 0.036 0.029 0.034 0.050
3 (AM) 0.046 0.047 0.030 0.042 0.133 0.059 0.047 0.080
3 (PM) 0.031 0.037 0.019 0.029 0.094 0.050 0.046 0.063
7 (AM) 0.012 0.043 0.022 0.026 0.043 0.025 0.040 0.036
10 (AM) 0.016 0.029 0.022 0.022 0.019 0.029 0.046 0.031
Skim milk (residue after butter fat removal) contained less than 0.002 ppm.
Table 8. Fenvalerate Residues in Treated Wheat and Sorghum Stored in Concrete Silos
Grain Amount of Residues (mg/kg)
fenvalerate
Kind moisture Temperature applied calc'd Storage period (months)
% °C (mg/kg)
0.25 1.5 3 4.5 6.5 8 10
(a) Wheat1
range 10 31-25* 0.9-1.1 0.85-1.16 0.45-0.87 0.76-1.02 0.66-0.82 0.79-0.93 0.74-0.85 0.71-0.76
mean 1.01 1.01 0.7 0.9 0.76 0.86 0.8 0.74
(b) Sorghum2
range 26-24* 0.42-0.55 0.64-0.72 0.53-0.54 0.64-0.69
mean 11.8 0.53 0.68 0.54 0.67
* 1st figures are initial temperatures; 2nd are those 6-9 months later.
Wheat data are from two trial sites, one in each of two Australian States.
1 Bengston, 1979
2 Bengston et al., 1979
Table 9. Distribution of Fenvalerate Residues in Whole Wheat
Fractions and Residue Levels in Bread Baked from the Flour (B.W.
Simpson, 1979)
(a) Distribution of residue between whole grain fractions
Fraction fenvalerate residue
% whole mg/kg % distribution
grain
flour (white) 74.6 0.08 8.5
pollard 14.6 3.3 68.5
bran 10.8 1.5 23
Grain sample: 1.5 kg. It had a residue level of 0.6 mg
fenvalerate/kg.
(b) Residue in bread baked from the ground flour from (a)
Commodity fenvalerate residue (mg/kg)
White flour 0.08-0.09
White bread 0.06-0.1
Wholemeal flour* 0.7-0.8
Wholemeal bread 0.49-0.73
* reconstituted from the fractions (in (a) ) in the original whole
grain proportions.
The processing operations simulated those used in commercial practice.
Table 10. Residues of Fenvalerate Degradation Products in Field-Treated Crops in Canada
S-Phenoxy Fenvalerate Isovaleric "Reverse Decarboxylated
Benzoic Acid Amide Acid Ester" Fenvalerate
Crop Country Dose Rate (×) PHI Fenvalerate WL 44607* WL 47117+ WL 10944* SD53036 SD 54597
Apples Canada 70 g/ha (×1) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
16 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
150 g/ha (×8) 6 weeks 0.29 <0.05 <0.05 <0.05 <0.01 <0.05
6 weeks 0.57 <0.05 <0.05 <0.05 <0.01 <0.05
Pears Canada 30 g/ha (×3) 5 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
70 g/ha (×2) 12 weeks <0.01 <0.05 <0.05 <0.05 <0.01 <0.05
Peaches Canada 0.006% 0 weeks 0.4 <0.05 <0.05 <0.05 - -
1 week 0.25 <0.05 <0.05 <0.05 - -
2 weeks 0.10 <0.05 <0.05 <0.05 - -
Grapes Canada 0.006% 0 week 0.45 <0.05 <0.05 <0.05 - -
1 week 0.38 <0.05 <0.05 <0.05 - -
Cabbage Canada 70 g/ha (×7) 2 weeks 0.06 <0.05 <0.05 <0.05 <0.01 <0.05
Cauliflower 70 g/ha 1 day 0.20 <0.05 <0.05 <0.05 - -
14 days 0.02 <0.05 <0.05 <0.05 - -
B. sprouts Canada 70 g/ha (×6) 14 days 0.10 <0.05 <0.05 <0.05 - -
70 g/ha (×7) 1 day 0.14 <0.05 <0.05 <0.05 - -
Lettuce Canada 70 g/ha 7 days <0.01 <0.05 <0.05 <0.05 - -
Tomatoes Canada 150 g/ha (×2) 2 days 0.80 <0.05 <0.05 <0.05 - -
* Includes both free and conjugated forms
+ No conjugates formed
Hens
Mature laying hens were fed 0.03 ppm of C-14-fenvalerate (chlorophenyl
ring label) in their total ration for periods up to 32 days. No
detectable C-14 residues were found in the fat, heart, gizzard, liver,
meat, skin, egg whites and egg yolks (Potter, 1976c).
In a separate study, mature laying hens were fed 0.03 ppm of
C-14-fenvalerate (phenoxyphenyl ring label) in their total ration for
periods up to 32 days. No C-14 residues were detected in the light
meat, dark meat, skin, gizzard, blood or plasma. The residues in egg
yolks ranged from <0.002 to 0.003 mg/kg, fat samples <0.002 to 0.003
mg/kg (Potter and Sauls, 1978).
Laying hens were sprayed twice with a 0.5% emulsible concentrate of
fenvalerate. Fenvalerate residues in the eggs reached maximum levels
of 0.04 mg/kg 6 days after the first application and of 0.14 mg/kg 8
days after the second application. The residue levels in the eggs
decreased by one half in about 22 days. The major tissue depots for
fenvalerate were found to be the skin and fat (DeVries, 1976d).
Plants
Leaves of cotton were treated either once with a formulation of
C-14-fenvalerate (chlorophenyl ring label) at a rate of 240 ug per
leaf, or twice at a rate of 120 ug per leaf per treatment.
Determination of C-14 in extracts showed that the rate of C-14
disappearance from leaves was practically identical between single and
repeated applications, and a disappearance rate of about 50% was 35
days. Thin-layer chromatographic analysis of extracts from the cotton
leaves showed that fenvalerate represents about 50% of the C-14
recovered 101 days after single treatment and 80% of the C-14
recovered 46 days after the last of double treatments (Loeffler, 1976a
and 1976b).
In a separate study, cotton squares were also treated either once with
a formulation of C-14-fenvalerate (chlorophenyl ring label) at a rate
of 240 µg per square or three times at a rate of 120 µg per square per
treatment. Whole seeds isolated two to three months after the first
treatment contained less than 0.05 mg/kg equivalents of fenvalerate.
Single leaves of cotton, located either on the lower or the upper part
of the plants, were treated with an average of 49.7 µg per leaf to
determine the transport of C-14-fenvalerate from the treated to
untreated leaves. After 15 days, no C-14 was found in any of the
untreated leaves both above and below the treated leaves,
demonstrating the inability of fenvalerate or any possible C-14
metabolites to move out of a treated leaf either in the xylem or in
the phloem (Loeffler, 1976 c and d).
When C-14-fenvalerate labelled in either benzyl or chlorophenyl ring
was applied to lettuce grown in boxes (twice at a rate of 10.8 mg/box)
about 70-80% of the C-14 in the mature plants was present as unchanged
fenvalerate 12 days after the second treatment. Fenvalerate
hydrolysed slowly at the ester linkage to give 3-phenoxybenzoic acid
and 2-(4-chlorophenyl) isovaleric acid which then rapidly conjugated
with plant materials (Hitchings, 1977). When the same compounds were
applied to the fruit and leaves of apple trees twice or three times,
the leaves and apples harvested 3 to 4 weeks following the last
treatment were found to contain mainly unchanged fenvalerate (75 -85%
of the total C-14 in leaves and 86-93% of the total C-14 in apples).
After peeling the apples, less than 2% of the C-14 in the apples
remained in the peeled fruit. The major metabolite fraction (11-19%
of the total C-14) in leaves was a mixture of polar compounds which by
hydrolysis produced 3-phenoxybenzoic acid, 3-phenoxybenzaldehyde,
3-phenoxybenzyl alcohol and 2-(4-chlorophenyl) isovaleric acid. Other
minor metabolites identified in leaves and/or apples were des-cyano-,
amide- and 4'-OH-fenvalerate which accounted in total for less than 2%
of the total C-14. (Standen, 1977).
Fenvalerate and the (S)-acid isomer similarly disappeared from
cabbages and bean plants with half-lives of approximately 9 days and
14 days respectively, after foliar application of three C-14-labelled
preparations (Co, Ca and CN) at rates of 17-19 µg (cabbage) and 10 µg
(bean plant) per leaf. On and/or in plants, both compounds underwent
decarboxylation, ester cleavage, hydrolysis of the CN group to CONH2
and COOH groups, hydroxylation at the 2'-and 4'-phenoxy positions,
cleavage of diphenyl ether, conversion of a-cyano-3-phenoxybenzyl
alcohol to the corresponding benzyl alcohol and benzoic acid, and
conjugation of the resulting carboxylic acids and alcohols with sugar.
Very little of the C-14 was transferred to other parts of cabbages and
bean plants. Beans were planted in Kodaira light clay and Katano
sandy loam soils after treatment with 1.0 mg/kg of the CN- and
Ca-labelled preparations. After 30 days, pods and seeds, shoots and
roots were harvested and analyzed for C-14. Although small amounts of
radioactivity were found in roots, traces were present in pods, seeds
and shoots. No parent compound was detected in shoots (Ohkawa et al.,
1979a).
Search for degradation products on field grown crops
A series of trials were conducted in Canada (Shell 1979) to determine
whether and to what extent degradation products occur as free and
conjugated residues in crops grown under typical outdoor conditions.
As indicated in Table 8, none of the known degradation products could
be detected by methods sensitive to 0.05 mg/kg in any of 9 varieties
of fruits and vegetables treated in accordance with label directions.
Fenvalerate itself occurred in most of the samples at levels
consistent with those found in other trials.
Distribution of residues on the plant
From the examination of many plants treated under practical
conditions, it is evident that residues are almost exclusively
confined to those parts of the crop that are directly exposed to the
spray application. As examples, cottonseed kernels and peanut meats
have not contained detectable levels even when quite high amounts were
measured on the whole seeds or nuts (Shell Res. 1974, 1975 a and b,
1976 a and b, Shell Dev. 1976 a and b, 1977a, 1978b).
Trials on cabbage in Australia gave residues at 7.5 mg/kg for outer
discarded leaves and only at 0.05 mg/kg for cabbage hearts 5 days
after treatment at 0.118 kg ai/ha (Microchem., 1977). In another
trial, when tops of sugar beets contained up to 5.9 mg/kg, only trace
residues (0.03 mg/kg or less) were detected in roots (Statens, 1977;
Shell Develop., 1977b). Trials on Japanese radish in Japan showed
residues up to 1.97 mg/kg for leaves and only 0.005-0.04 mg/kg for
roots (Fujita et al., 1978a).
Similarly, in trials with peas, beans, citrus fruit, almonds, sweet
corn and wheat, the main part of the residues occurred on peel, skins,
pods and other exposed parts. Residues in edible portions rarely
exceeded detectable levels. (Shell Dev. 1976, 1977; Fujita et al.,
1978; Japan Food Res. 1977).
Photochemical degradation
When exposed to sunlight on silica gel, glass, soil and in water,
fenvalerate degraded in all these media. After 28 days of exposure to
sunlight about 25% was recovered from silica gel, 48% from soil or
from water, less than 1.3% from glass (FAN 1976).
When a 0.2 M solution of fenvalerate was exposed for 10 hours at 300
nm in quartz SD54597 (Benzenepropanenitrile,
4-chloro-beta-(1-methylethyl)-alpha-(3-phenoxyphenyl)-I was the major
product. Other amounts to less than 1% of the total. At 254 nm
SD53036 (Benzoic acid, 2-phenoxy-, 1-(4-chlorophenyl)-2-methylpropyl
ester) was also formed equivalent to about 3% of SD54597 present and
other products were less than 1% of the total (Ehmann, 1978a and b).
In various solutions (hexane, methanol, and acetonitrile-water; 6/4)
by artificial light (>290 nm) and as a thin film on grass or on
cotton by exposure to sunlight, the major products in solution were
formed via photoinduced decarboxylation. Other products resulted from
cleavage of the ester linkage, probably by a mechanism involving
free-radical intermediates. These were 3-phenoxybenzoyl cyanide,
3-phenoxybenzyl alcohol and 3-phenoxybenzaldehyde from the alcohol
moiety, and 2-(4-chlorophenyl) isovaleric acid and the dimer of the
acid fragment from the acid moiety. Decarboxylated fenvalerate,
2-(4-chlorophenyl) isovaleric acid, 3-phenoxybenzyl alcohol and
3-phenoxybenzaldehyde were also found on cotton. (Holmstead, et al.,
1977, 1978).
Another photodecomposition study reveals that on exposure to sunlight
fenvalerate was rapidly decomposed in distilled water, 2% aqueous
acetone, river and sea water to almost the same extent.
Photodegradation half-life in each aqueous suspension ranged from ca.
4 days in summer to 13-15 days in winter, due to seasonal variation of
sunlight intensity. On soil surfaces, exposed to sunlight, the rate
of photodegradation was dependent upon the soils used, with half-lives
of about 2, 6 and 18 days in Kodaira light clay, Azuchi sandy clay
loam and Katano sandy loam soils, respectively. Thus photodegradation
rate and pathways were dependent on the environmental conditions. The
predominant reactions were decarboxylation and cleavage of ester
linkage in water, while hydration at CN group was dominant on the soil
surface. (Mikami et al., 1979).
Soil
When C14-fenvalerate (chlorophenyl ring label) was applied to steam
sterilized and live sandy loam soil at a concentration of 2.5 ppm, the
amount of solvent extractable C-14 decreased slowly in aerobic and
anaerobic soils and stayed practically constant in sterile soils. The
proportion of intact fenvalerate in the extracts from aerobic and
anaerobic soils decreased to 90% over a three-month period. The
remaining 10% was present in the form of two to three products more
polar than fenvalerate (Loeffler, 1976a). Degradation of the same
labelled compound was examined under aerobic and anaerobic conditions
using three different soils and a steady decrease of recovery in the
organic solvent-extractable fraction were observed both under aerobic
and anaerobic conditions (Lee, 1978). Several of the breakdown
products of fenvalerate formed during the course of disappearance of
the parent compound were identified (Fan 1977). The effects of soil
micro-organisms were investigated and it was demonstrated that
sterilization of soil greatly retards degradation (Ohkawa et al.,
1978; Williams and Brown 1979; Noble, 1976).
The leaching behaviour of fenvalerate from soil was also investigated
(Ohkawa et al., 1978a; Jackson and Roberts, 1976; Kerritt, 1977).
Fish
In tests in which Channel catfish and rainbow trout were exposed to
dilutions of C-14 fenvalerate in water, rates of uptake and
distribution in the bodies of fish were followed and some metabolites
were identified (Potter 1975, 1976; Ohkawa, et al., 1978, 1979). The
results gave no evidence to suggest that problems may arise from the
concentration of residues in the bodies of fish that are used as human
food.
METHODS OF RESIDUE ANALYSIS
GLC equipped with electron-capture detector (ECD) has been used almost
exclusively for detection and quantitation. The ECD exhibits high
sensitivity to fenvalerate, responding to as little as 0.05 ng or even
less (Chapman et al., 1977), which afforded minimum detectability of
0.005-0.02 ppm (Takimoto et al., 1977; Talekar 1977; Shell Dev.
1976a). As a radioactive source, nickel-63 appears more advantageous
than tritium due to the relatively high temperature applied. Although
the alkali flame ionization detector (AFID) was also utilized for
confirmation of residue identity, the response of fenvalerate to
GLC-AFID was found to be about 100 times less than GLC-ECD. The
confirmation can be made by combined gas-liquid chromatography/mass
spectrometry (GLC/MS) using selective ion detection mode at mass peaks
such as 225 amu and 419 amu (Parent ion) (Shell Dev. 1976a).
Since fenvalerate comprises 4 optical isomers due to 2 chiral centres,
its GLC gives either single or resolved peaks depending upon the
column length, the column-packing materials, etc. A single peak
response was obtained by using 45-60 cm-long glass or stainless steel
column packed with 3% OV-101 on Gas-chrom Q (Shell Dev. 1976b), 3%
OV-101/3% Apiezon Grease L on Gas-chrom Q (Takimoto et al., 1977;
Talekar 1977), 5% OV-17 on Chromosorb W AW DMCS (Kato 1979), and 3%
SF-96/3% Apiezon Grease M on Diatomite CLQ (Woodhouse et al., 1979).
On the other hand Lee et al., (1978) observed two completely resolved
peaks for fenvalerate on 1.8 m-long glass column packed with 4%
SE-30/6% QF-1 on Chromosorb W (80/100 mesh) where the RR and SS
isomers with identical chromatographic properties were eluted after
the RS and SR pair. A complete resolution for fenvalerate was also
achieved on 3 m glass column packed with 3% OV-101 on Chromosorb W AW
DMCS (100/120 mesh) (Kato et al., 1978).
When exposed to a relatively high temperature in the GLC system (e.g.
more than 250°C at injection port), fenvalerate may undergo partial
degradation and/or isomerization depending upon the column material,
the glass wool packed into the injection port, and the column-aging
history (Barber 1976a). Therefore, care must be taken in the
simultaneous analysis of the diastereomers. The effects caused by
degradation are reproducible and can be minimized to insignificant
levels for quantitation.
The high-pressure liquid chromatography (HPLC), which permitted less
than 3 ppm of detection limit in one study (McKinney 1975), has been
applied to the residue analysis of fenvalerate. It has been used
successfully to determine residues in cereal whole grains and
commodities from the grains, such as flour and bread (Simpson 1978).
The problems with indeterminate differentiation of fenvalerate
diastereoisomers and breakdown on GLC columns were not found with HPLC
separation. The post-harvest residue data on treated cereals, flour
and bread reported in this monograph have been obtained by HPLC
determination. It was found that the limit of determination was in
the region of 0.02 mg/kg.
The solvent or solvent combinations selected for extraction of
fenvalerate vary according to the nature of substrates. From the
substrates with high moisture contents like fruits, leafy vegetables
and root crops fenvalerate was successfully extracted by chopping and
blending them either with polar solvents, e.g. acetonitrile (Lee et
al., 1978) and methanol-acetonitrile (Takimoto et al., 1977), acetone
(Chapman and Harris, 1978), or with nonpolar-polar solvent mixtures,
e.g. n-hexane-isopropanol (Shell Develop. 1976b) and petroleum
spirit-acetone (Shell Res. 1976). Talekar (1977) adopted a 12-hour
Soxhlet extraction with n-hexane-acetone for chopped cabbage. Dry and
low-moisture products are first fractured or ground to powder, under
freezing if necessary, prior to extraction with either polar, nonpolar
or dual solvent system (Shell Res. 1975). After mixing with anhydrous
sodium sulphate, cottonseeds were fractured, separated from the hull,
and blended with n-hexane (Shell Develop. 1976c), whereas coffee beans
were pulverized and macerated overnight with methanol (Kato 1979).
The blending/extraction with nonpolar solvents like n-hexane resulted
in excellent recoveries of fenvalerate with satisfactory separation
from fatty materials in the case of mammalian tissues, dairy products
and eggs (Shell Develop. 1976a).
Cleanup procedures include partition between immiscible polar and
nonpolar solvents, e.g. acetonitrile and n-hexane, for most extracts
from oily and dehydrated crops as well as from fatty products prior to
further Florisil column chromatography. Chapman and Harris (1978)
partitioned the aqeous acetone extract from a range of vegetables into
n-hexane before proceeding to cleanup on silica or alumina absorbents.
The combined solvent partition-Florisil column chromatography
procedure provided recoveries of 81-95% for eggs, chicken and cow
tissues, milk, and cream spiked at 0.01-0.1 ppm of fenvalerate (Shell
Develop. 1976a), along with 90-95% for cottonseed (Shell Develop.
1975) and 89% for coffee (Kato 1979), both fortified at 0.5 ppm. For
non-oily crops cleanup by either Florisil (activated or deactivated
with water) (Takimoto et al., 1977; Talekar 1977; Lee et al., 1978;
Shell Res. 1976), or silica gel (Lee et al., 1978; Kato et al., 1978)
column chromatography was applied. Over 90% recoveries of fenvalerate
were accomplished for crops including cabbage (Takimoto et al., 1977;
Talekar 1977; Lee et al., 1978); lettuce (Lea et al.,1978), potatoes
(Woodhouse et al., 1979), orange (Fujita et al., 1979), apple,
tomatoes, green peppers, peas and alfalfa (Shell Develop. 1976b) at
fortification levels of 0.01-40 ppm.
For the degradation products and metabolites of fenvalerate, Barber
(1976b) devised the analytical method for detection limits of
0.01-0.02 mg/kg for the residues of 3-phenoxybenzoic acid and
4-cloro-a-(1-methylethyl)-benzeneacetic acid (CMBA) in cottonseed.
(Kato et al., 1979) analyzed CMBA in human urine. The method
permitted detection of CMBA at 0.002 ppm as well as 93-100% recoveries
from the human urine fortified at 0.02-2 mg/kg. Barber (1978a-e) also
developed analytical methods for 1-(4-chlorophenyl)-2-methylpropyl
3-phenoxybenzoate and
4-chloro-B-(1-methylethyl)-a-(3-phenoxyphenyl)-benzenepropanenitrile
in soybeans, pears and apples as well as (aminocarbonyl)
(3-phenoxyphenyl)methyl 4-chloro-a-(1-methylethyl)-benzeneacetate in
potatoes and peanut shells with detection limits at 0.01 mg/kg.
NATIONAL LIMITS
National MRLs brought to the notice of the Meeting are listed (July
1979)
Country Commodity MRL Pre-harvest
(mg/kg) interval, days
Brazil Cotton 0.1 14
Soybeans 0.1 30
U.S.A. Cottonseed 0.2 21
Cattle(fat, meat,
meat by-products) 0.02
Goats (fat, meat,
meat by-products) 0.02
Hogs (fat, meat,
meat by-products) 0.02
Horses (fat, meat,
meat by-products 0.02
Milk (fat) 0.02
Sheep (fat, meat,
meat by-products) 0.02
(temporary)
Lettuce (head) 1 7
Cabbage 2 7
Cauliflower 1 7
Broccoli 0.5 14
Snap beans 0.5 7
(green beans)
Dry beans and 0.5 21
Dry pears
Cucumbers 0.05 10
Squash 0.05 10
Bell peppers 1 10
Tomato 1 7
Field corn 0.05 45
Grapes 1 21
Sweet corn (for
fresh market only) 0.05 3
Soybeans 0.02 21
National Limits, Continued...
Country Commodity Tolerance Pre-harvest
(mg/kg) interval, days
U.S.A.
(temporary)
Peanuts 0.02 21
Potatoes 0.02 21
Pears 1 28
Apples 2 21
Peaches 2 21
Australia Cottonseed, maize,
sweet corn 0.05 7
Pome and stone fruit 1 14
Milk and milk products
(fat basis) 0.2 -
Fat of meat of cattle 0.2 -
Soya beans, navy beans,
mung beans 0.2 21
Oilseed crops 0.2 14
Hungary
(temporary) Apples 1 14
Cherries 1 14
Peaches 1 14
Alfalfa 1 14
Beets 1 14
Cabbage 1 7
South Africa Apples, pears 0.5 14
Tomatoes 0.1 2
Grain sorghum 0.1 28
Cottonseed 0.1 -
New Zealand Brassica vegetables 5 7
Kiwi fruit 3 14
Apples, pears 1 14
Appraisal
Fenvalerate is a new synthetic ester related to the pyrethroids, with
a wide spectrum of efficacy against many species of pests. It is only
slowly degraded in sunlight. It is registered in a number of
countries for use on a variety of crops. The rate of application
ranges from 50-250 g/ha.
Supervised trials have been carried out in many countries and on many
crops. The rate of decline of residues on growing crops is slow, the
half-life ranging from 2 to 3 weeks. On stored cereal grains about
75% of the applied amount is found at the ninth month after treatment.
Fenvalerate does not penetrate significantly into plant tissues nor is
it translocated and there is little or no residue in crop parts not in
direct contact with the insecticide. Most of the residue remains on
the exposed parts of such commodities as cottonseed, peanuts and
fruits and is removed with shells and peels during processing and
preparation. Similarly, after treatment of stored grains some 70% is
in the bran of wheat, with considerably more in the hulls of oats and
rice which are normally removed before consumption.
A wide range of studies have been conducted on the fate of residues in
plants and animals. Residues in plants consist substantially of the
parent compound. In radiolabelled studies the occurrence of free and
conjugated metabolites has been demonstrated but these products have
not been found in field trials at levels above the limit of detection
(<0.05 mg/kg).
When administered or applied to livestock, fenvalerate is degraded and
the degradation products excreted in urine. A small proportion of the
applied dose is transferred to fatty tissues from which it may be
excreted via fat in the milk.
The process of photodecomposition proceeds by oxidation,
decarboxylation, hydration at the Cn-group, cleavage of ester and
diphenyl ether linkages and thence by hydrolysis to produce polar
derivatives of low molecular weight. It varies with exposure and is
fairly slow.
Fenvalerate is readily biodegraded by soil micro-organisms with a
residual half life of from 15 days to 3 months depending on soil
temperature, moisture and organic matter content. Because residues
are tightly bound to soil colloids, there is little likelihood of such
residues being transferred to water of streams through run-off or
leaching. Sprays falling directly onto bodies of water are rapidly
absorbed and inactivated on particulate matter. There is no evidence
of residues in soil being taken up by plants.
Under laboratory conditions it is possible to demonstrate that fish
can absorb the insecticide from water into various body tissues. Due
to the presence of particulate matter, surface absorption and the low
solubility of fenvalerate in water there appears to be little
likelihood of significant uptake of residues by fish under practical
conditions; nor are problems likely to arise from the occurrence of
residues in fish for human consumption.
Residues may be determined by GLC using electron capture detectors.
The ECD exhibits high sensitivity to fenvalerate responding to as
little as 0.05 ng. Polar solvents have been effective for extraction
when used with substrates containing substantial amounts of water.
Non-polar and petroleum solvents have been used with substrates
containing little water. Sweet co-distillation has proved convenient
and effective with lipid substrates. The limit of determination is
0.01 mg/kg.
Several national governments have established maximum residue limits
in a range of commodities. The data available provided a basis for
the Meeting to recommend the following maximum residue limits.
RECOMMENDATIONS
The following maximum residue limits, determined and expressed as
fenvalerate, are recommended:
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Kiwi fruit 5 21
Alfalfa 20 21
Broccoli 2 7
Brussels Sprouts 2 7
Cabbage 2 7
Cauliflower 2 7
Celery 2 7
Lettuce 2 7
Chinese cabbage 1 7
Pome fruits 2 14
Peaches 2 14
Cherries 2 14
Citrus fruit 2 21
Tomatoes 1 7
Small fruits and berries 1 7
Beans (green) 1 7
Beans (dry) 0.5 7
Cottonseed 0.2 7
Cottonseed oil 0.1
Cucumbers 0.2 3
Squash 0.2 3
Soybeans 0.1 21
Sweet corn 0.05 7
Sugar beets 0.05 21
Sunflower seed 0.1 28
Radishes 0.05 21
Potatoes 0.05 7
Peanuts 0.1 7
Cereal grains 5
Wheat bran 5
Pre-harvest interval
(days) on which
MRL recommendations are
Crop (mg/kg) based
Wheat flour (wholemeal) 2
Wheat flour (white) 0.2
Milk 0.01
Milk products (fat basis) 0.2
Fat of meat 0.2
FURTHER WORK OR INFORMATION
Required by 1981:
1. Pharmacokinetic data on the potential bioaccumulation of
fenvalerate and/or metabolites;
2. Observations in humans occupationally exposed to high levels of
fenvalerate to evaluate the potential susceptibility of man to the
neurological disruption noted in rodents.
Desired:
1. An additional dominant lethal assay to reconfirm previous data;
2. Information on the occurrence and fate of photodecomposition
products;
3. Additional data from supervised residue trials on citrus, berry
fruits, beans and several other crops for which data are limited;
4. Results of on-going studies on the level and fate of fenvalerate
residues in stored products especially raw grain and milling
products derived therefrom.
REFERENCES
Albert, J.R. and Summitt, L.M. Intravenous Toxicity of WL 43775
(6-1-0-0) in the Mouse. (1976) Unpublished report from Shell
Development Co., Ltd.,
Barber, G.F.
1976a Separation of SD 43775 isomers for residue analysis.
1976b Residue data for SD 44607 in WL 10944 (acid metabolites of
SD43775 in cottonseed from cotton receiving seven applications of
SD43775, a California study. Shell Development Co.
1978a Residue data for SD 53036 and SD 54597, possible metabolites
of SD 43775, in soybeans receiving one aerial application of SD43775,
a Mississippi study.
1978b Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in whole apples receiving one dormant application of SD43775,
an Oregon study.
1978c Residue data for SD53036 and SD54597, possible metabolites of
SD43775, in pears receiving one dormant application of SD43775, a
Michigan study.
1978d Residue levels of SD47117, a possible metabolite of SD 43775,
in potatoes receiving seven ground spray applications of SD43775, a
Minnesota study.
1978e Residue levels of SD47117, a possible metabolite of SD43775,
in peanut shells from peanuts receiving three aerial applications of
SD43775, an Oklahoma study.
Beliles, R.P., Makris, S.L and Weir, R.J. Three-Generation
Reproduction Study in Rats. (See Stein, 1977 for histopathology data).
(1978) Unpublished report from Litton Bionetics, Inc. submitted by
Sumitomo Chemical Company Ltd.,
Bengston, M. Personal communication from Final Report on Silo Scale
Experiments 1977-1978 to the Australian Wheat Board Working Party on
Grain Protectants. Queensland Department of Primary Industries.
Bengston, M., Davies, R.A.H., Desmachelier, J.M., Phillips, M., and
Simpson B.W. Additional grain protectants for the control of
malathion-resistant insects in stored sorghum. (in manuscript).
Blair D., and Roderick, H. Toxicity studies on the Pyrethroid
Insecticide WL43775, Emulsifiable Concentrate FX3368. Acute
Inhalation Exposure to an Aqueous Spray. (1975) Unpublished report
from Shell Development Co.
Boyer, A.C. Unpublished reports from Shell Development Co. submitted
by Sumitomo Chemical Co.:
1976a Metabolism of WL 43775 by Rat Liver Enzymes.
1976b Metabolism of WL 43775 by Rat Liver Enzymes - Part 2.
1977a Residues in Rat Tissues from Rats Fed C-14-SD 43775.
1977b Identification of Metabolites found in the urine of Rats Fed
C-14-SD 43775.
1977c Identification of Metabolites Found in the Faeces of Rats Fed
C-14-SD 43775.
1977d Identification of Metabolites in the Livers of Rats fed
C-14-SD43775.
Brooks, T.M. Toxicity studies with WL 43775: Mutagenicity Studies
with WL 43775 in the Host-Mediated Assay. (1976) Unpublished report
from Shell Development Co.
Brown, L.J. and Slomka, M.B. Skin Reaction Potential of Use Dialation
(1.33%) PYDRIN(R) EC. (1979) Unpublished report from Shell Development
Co.
Butterworth, S.T.G. and Carter, B.I. Toxicity Studies on the
Insecticide WL 43775: Acute Oral Toxicity and Neuropathological
Effects in Rats. (1976) Unpublished report from Shell Development Co.
Chapman, R.A. and Simmons, H.S. Gas-liquid chromatography of picogram
quantities of pyrethroid insecticides. J. Assoc. Off. Anal. Chem., 60:
977-978.
Chapman, R.A. and Harris, C.R. Extraction and liquid/solid
chromatography for the direct analysis of four pyrethroid insecticides
in crops by gas liquid chromatography. J. Chromatog. 166, 513-518.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Acute Toxicity, Skin Irritancy Potential of the Emulsifiable
Concentrate FX 3368. Unpublished report from Shell Development Co.
Coombs, A.D. and Carter, B.I. Toxicity Studies on the Insecticide
WL43775: Toxicity and Skin and Eye Irritancy Potential of the ULV
Formulation FX 4353. (1976) Unpublished report from Shell Development
Co.
Creedy, C.L. and Potter, D. The Effect of Feeding WL 43775 on the
Microsomal Mono-Oxygenase System of Rat Liver. (1976) Unpublished
report from Shell Development Co.
Dean, B.J. Toxicity Studies with WL 43775: Dominant Lethal Assays in
Male Mice after Single Oral Doses of WL 43775. (1975) Unpublished
report from Shell Development Co.
Dean, B.J. and Senner, R.K. Toxicity Studies with WL 43775:
Chromosome Studies on Bone Narrow Cells of Chinese Hamster after Two
Daily Oral Doses of WL 43775. (1975) Unpublished report from Shell
Development Co.
Desmachelier, J.M. Personal Communication. CSIRO Division of
Entomology. (1979)
DeVries, D.M. Shell Development Co.
1976a Residues of SD 43775 in Milk from Cows fed Radiolabelled
SD43775
1976b Residues in Milk, Hair and Tissues of Cows sprayed with an
Emulsible Concentrate (6-1-3-1ECH)
1976c In Milk, Cream, Hair and Tissues of Cows Sprayed with an
Emulsible Concentrate or Fed 10 ppm in Their Diet
1976d In Eggs and Tissues of Hens Sprayed with an Emulsible
Concentration
1976e In Milk, Hair and Tissues of Cows Sprayed with an Emulsible
Concentration (6-1-3-1ECH)
1976f In Meat and Milk of Cattle Treated Dermally with a Water
Dispersible Liquid Formulation
1976g In Meat, Hair, and Milk Fat of Restrained Cows Treated
Dermally with an Emulsible Concentrated Formation
Dewar, A.J., Moffett, B.J. and Sitton, M.F. Toxicity Studies on the
Insecticide WL 43775: Biochemical and Functional Studies on the
Neurotoxicity of WL 43775 to Rats. (1975) Unpublished report from
Shell Development Co.
Dewar, A.J., Moffett, B.J., Sitton, M.F. and Baker, J.E. Toxicity
Studies on the Insecticide WL 43775: Biochemical and Functional
Studies on the Neurotoxicity of WL 43775 to Chinese Hamsters. (1978)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Three-Month Feeding Study in Rats. (1975)
Unpublished report from Shell Development Co.
Hend, R.W. and Butterworth, S.T.G. Toxicity Studies of the
Insecticide WL 43775: A Short-Term Feeding Study in Rats. (1976)
Unpublished report from Shell Development Co.
Hine, Inc. SD-43775 Toxicology: Acute and Repeated (14-Day) Dermal
Toxicity in the Rabbit. (1975) Unpublished report from Hine, Inc.,
submitted by Sumitomo Chemical Co.
Hine, Inc. Human Skin Testing on SD-43775. (1976) Unpublished report
from Hine, Inc. submitted by Sumitomo Chemical Co.
Hitchings, E.J. The Metabolism of the Pyrethroid Insecticide WL 43775
(Belmark) in Lettuce and Soil under Outdoor Conditions. (1977) Shell
Research Ltd.,
Holmstead, R.L. and Fullmer, D.G. Photodecarboxylation of Cyanohydrin
Esters. Models for Pyrethroid Photodecomposition. J. Agric. Food
Chem., 26, 56
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem., 26, 954
Holmstead, R.L., Fullmer, D.G. and Ruzo, L.O. Pyrethroid
Photodecomposition: Pydrin. J. Agric. Food Chem. 26, 590-95.
Ito, N. Histopathological Findings of Mice Treated with S5602 for
three Months (see Suzuki, 1976). (1976a) Unpublished report from
Sumitomo Chemical Co. Ltd.,
Histopathological Findings of Mice and Rats Exposed to Mist of S5602
for 4 weeks (see Khoda, 1976c). (1976b) Unpublished report from
Sumitomo Chemical Co., Ltd., submitted by Sumitomo Chemical Co. Ltd.,
Histopathological Findings in Mice Treated with S5602 (see Suzuki, et
al., 1977b). (1978) Unpublished report from Nagoya City University
Medical School submitted by Sumitomo Chemical Co., Ltd.,
Japan Food Research Lab. Analytical Results of S5602 Residues in
Wheat Grain and Wheat Straw (1977)
Kaneko, H., Ohkawa, H. and Miyamoto, J. Comparative Metabolism of
Fenvalerate in Rats and Mice. (1979) Unpublished report from Sumitomo
Chemical Co. Ltd.,
Kaplan, M. and Murphy, S.D. Effect of Acrylamide on Rotor-Rod
Performance and Sciatic Nerve ß-Glucoronidase Activity of Rats.
Toxicol. Appl. Pharmacol. 22: 259-268.
Kato, S. Analysis of Sumicidin residues in coffee. Analysis
certificate No. 12010695. (1979) Japan Food Research Laboratories.
Kato, T. and Miyamoto, J. Residue Analysis of S-5602 and its optical
isomers in Chinese Cabbage. (1978) Sumitomo Chemical Co.
Kato, T., Ohnishi, J. and Miyamoto, J. The analytical method of
2-(4-chlorophenyl) isovaleric acid, a urinary metabolite of
fenvalerate (Sumicidin(R)). (1979) Sumitomo Chemical Co.
Kirkland, V.L. and Albert, J.R. (1977a) Unpublished Report from Shell
Development Co. Preliminary Pharmacodynamic Investigation of SD 43775
(6-10-0) Administered Intravenously to the Anesthetized Dog: I.
Effects on the Cardiovascular System and Respiration. II.
Electrocardiographic (EKG) Findings.
Khoda, H., Kadota, T. and Miyamoto, J. Unpublished reports from
Sumitomo Chemical Co. Ltd.,:
1976a Teratogenic Study on S5602 in Mice.
1976b Acute Inhalation Toxicity of S3206 and S5602 in Mice and
Rats.
1976c Subacute Inhalation Toxicity Study of S5602 in Mice and Rats.
Khoda, H., Kaneko, H., Ohkawa, H., Kadota, T. and Miyamoto, J. (1979)
Unpublished report from Sumitomo Chemical Co. Ltd., Acute
Intraperitoneal Toxicity of Fenvalerate Metabolites in Mice.
Lampert, P.W. (1977) Letter Report on Long-Term Rat Study (Gordon and
Weir, 1978) from the University of California San Diego School of
Medicine, Department of Pathology submitted by Sumitomo Chemical Co.
Lee, Y.W., Westcott, N.D. and Reichle, R.A. Gas-liquid
chromatographic determination of Pydrin, a synthetic pyrethroid, in
cabbage and lettuce. J. Assoc. Off. Anal. Chem., 61: 869-871
Loeffler, J.E. From Shell Development Co.:
1976a Disappearance of C-14 from Cotton Leaves Treated with
C-14-(Chlorophenyl)-SD 43775"
1976b Thin Layer Chromatography of SD 43775-Treated Cotton Leaves
1976c Carbon-14 Content of Cotton Seeds after Single and Multiple
Treatment of Squares with C-14-(Chlorophenyl)-SD 43775
1976d Investigation of the Transport of C-
14-(Chlorophenyl)-SD-43775 from Treated to Untreated Leaves on Cotton
Plants
1976e Degradation of C-14-(Chlorophenyl)-SD 43775 during Exposure
to Soil Under Aerobic, Anaerobic, and Sterile Conditions.
Matsubara, T., Suzuki, T., Kadota, T. and Miyamoto, J. Antidotes
Against Poisoning by S5602 in Rats. (1977) Unpublished report from
Sumitomo Chemical Co.
McKinney, W.J. Separation of SD 43775 from hexane extracts of cotton
by liquid chromatography. Shell Development Co.
Microchem Associates:
Report on Residues of WL 43775 in Cabbages, (1977)
Report on Residues of WL 43775 in Navy Beans (1978)
Mikami, N., Takahashi, Hayashi, K. and Miyamoto, J. Photodegradation
of Fenvalerate (Sumicidin(R)) in Water and on Soil Surface. Sumitomo
Chemical Company Ltd.,
Milner, C.K. and Butterworth, S.T.G. Toxicity of Pyrethroid
Insecticide. Investigation of the Neurotoxic Potential of WL 43775.
(1977) Unpublished report from Shell Development Co.
Noble, P.J. AF1117 Residue Data in Animal Tissues, Milk and Cream.
(1976a) Shell Chemical (Australia) Propriety Ltd.,
Noble, A.S. Soil Percolation Studies with WL 43775. (1976b) Shell
Research Ltd.,
Ohkawa, H., Nambu, K., Inui, H. and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Soil and by Soil Micro-organisms. J.
Pesticide Sci. 3, 129
Ohkawa, H. and Kikuchi, R. Metabolic Fate of Fenvalerate
(Sumicidin(R)) in Rats, Soil, Soil Microorganisms and an Aquatic Model
Ecosystem. Sumitomo Chemical Co., Ltd.,
Ohkawa, H., Nambu, K., Mikami, N., and Miyamoto, J. Metabolic Fate of
Fenvalerate (Sumicidin(R)) in Bean Plants and Cabbages. Sumitomo
Chemical Co. Ltd., (1979a)
Ohkawa, H., Kikuchi R. and Miyamoto, J. Bioaccumulation and
Biodegradation of the (S)-Acid Isomer of Fenvalerate (Sumicidin(R)) in
an Aquatic Model Ecosystem. Submitted to J. Pesticide Sci. (1979b)
Ohkawa, H., Kaneko, H., Tsuki, H. and Miyamoto, J. Metabolism of
Fenvalerate (Sumicidin(R)) in rats. J. Pesticide Sci. 4 (2), 143.
Okuno, Y., Kadota, T. and Miyamoto, J. Unpublished report from
Sumitomo Chemical Co.:
1976a. Neurotoxic Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Dermal Applications in Rats.
1976b. Neurotoxic Effects of Some Synthetic Pyrethroids by
Short-Term Feeding in Rats.
1977a. Neurotox Effects of Some Synthetic Pyrethroids and Natural
Pyrethrins by Oral Administration in Rats.
1977b. Neurotoxic Effects of S5602 and Natural Pyrethrins by Oral
Administration in Rats.
1977c. Recovery of Histopathological Lesions in Rats Caused by
Short-Term Feeding of S5602.
Potter, J.C. (1976) Unpublished reports from Shell Development Co.:
a) Tissues of Rats Fed SD 43775-C-14; b) and c) Milk and tissues of
cows; d) eggs and tissues from laying hens; e) cream from the milk of
cows fed SD 43775 14-C.
Potter, J.C. and Arnold, D.L. Residues of C-14 in Tissues of Rats Fed
SD 43775-C-14 for 28 days. (1977) Unpublished report from Shell
Development Co.
Potter, J.C. and Sauls. Residues of 14-C in eggs and Tissues of
Laying Hens fed 0.03 ppm of SD 43775-14-C (Labelled in Benzene Ring
Adjacent to CN Group). (1978) Shell Development Co.
Quinn R.J., Paa, H., Mastri, C.W., Kinoshita, F.K. and Keplinger, M.L.
21-Day Subacute Dermal Toxicity Study with Technical SD 43775 and SD
43775 (2.4 lb/gal EC) in Albino Rabbits. (1976) Unpublished report
from Industrial Bio-Test Lab submitted by Sumitomo Chemical Co.
Shell Development Co. and Shell Research Ltd., (1975-1978) A numbered
series of 36 unpublished reports with residue data from field trials
on specified crops in different countries.
Simpson, B.W. (1979) Draft report to be published. Queensland
Department of Primary Industries Analytical Chemistry Branch,
Brisbane, Australia.
Standen, M.E. The Metabolism of the Pyrethroid Insecticide WL 43775
in Apple Fruit and Foliage under Outdoor Conditions. (1977) Shell
Research Ltd.,
Stein, A.A. Histopathology Evaluation of Animals from 3-Gene ration
Reproduction Study. (1977) Unpublished report from Microscopy for
Biological Research, Ltd., submitted as part of study cited above
(Beliles et al., 1978) by Sumitomo Chemical Co., Ltd.,
Summit, L.M. and Albert, J.R.
1977a. Oral Lethality of WL 43775 (6-1-0-0) in the Rat (Unpublished
report from Shell Development Co.)
1977b. Determination of the Acute Oral Lethality of WL 43775
(6-1-0-0) in the Male and Female Mouse.
Suzuki, H. and Miyamoto, J.
1976. Unpublished report from Sumitomo Chemical. Studies on
Mutagenicity of S5602 with Bacteria Systems.
1977. Studies on Mutagenicity of Some Pyrethroids on Salmonella
Strains in the Presence of Mouse Hepatic S9 Fractions.
Suzuki, T., Kadota, T. and Miyamoto, J. One Year Chronic Toxicity
Study of S5602 in Mice (Three Month Interim Report). (1976)
Unpublished report from Sumitomo Chemical Co.
Suzuki, H., Kishida, F. and Miyamoto, J. Studies on Mutagenicity of
S5602 in Ames Test in the Presence of Hepatic S9 Fractions from
Several Laboratory Animal Species. (1979) Unpublished report from
Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Fifteen-Month Chronic Toxicity Study of S5602 in Rats. (1977a)
Unpublished report from Sumitomo Chemical Co.
Suzuki, T., Okuno, Y., Hiromori, T., Ito, S., Kadota, T. and Miyamoto,
J. Eighteen-Month Chronic Toxicity Study of S5602 in Mice. (1977b)
Unpublished report from Sumitomo Chemical Co.
Takimoto, Y. and Miyamoto, J. Method for Residue Analysis of S-5602
in Cabbage. (1977) Sumitomo Chemical Co.
Talekar, N.S. Gas-liquid chromatographic determination of
a-cyano-3-phenoxybenzyl a-isopropyl-4-chlorophenylacetate residues
in cabbage. J. Assoc. Off. Anal. Chem., 60: 908-910
van der Pauw, C.L., Dix, K.M., Blanchard, K. and McCarthy, W.V.
Toxicity of WL-43775: Teratological Studies in Rabbits Given WL 43775
Orally. (1975) Unpublished report from Shell Development. Co.
Walker, B.J., Hend, R.W. and Linnett, S. Toxicity Studies on the
Insecticide WL 43775: Summary of Results of Preliminary Experiments.
Unpublished report from Shell Development Co.
Williams, I.H. and Brown, M.J. Persistence of Permethrin and WL 43775
in Soil. J. Agric. Food Chem. 27, 130
Woodhouse, R.N., Almond, R.H. and Anderson, J. Determination of
residues of Sumicidin in potatoes. (1979) Huntingdon Research Centre.
SMO 88/7939.
