
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
FENVALERATE
Explanation
A temporary ADI was established in 1979 when fenvalerate was
first evaluated by the JMPR. The 1981 Joint Meeting reviewed
additional data and requested clarification of the granulomatous
lesions observed in the mouse and of the giant-cell infiltration seen
in the rat. The Meetings also desired further information on the
potential for bioaccumulation and further observations in humans. 1/
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Biotransformation and Excretion
In support of previous findings, fenvalerate was shown to
dissipate from the adipose tissue of Sprague-Dawley rats, dosed
orally with 3 mg/kg of either (2 RS,alpha RS) - or (2S,alpha S) -
fenvalerate, with a half-life of about seven days in each case. The
pyrethroid level in the brain of rats treated intraperitoneally with
2.5 mg/kg (2s,alpha S) - fenvalerate dissipated with a half-life of
about two days (Marei et al., 1982).
TOXICOLOGICAL STUDIES
Special Studies on Microgranulomatous Lesions
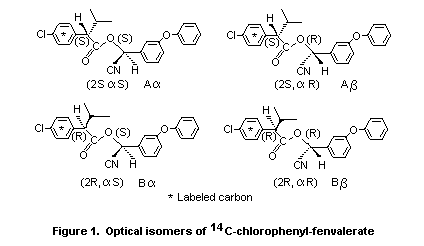
Racemic fenvalerate consists of a mixture of four enantiomers,
designated A (2S,alpha S), Aß(2S,alpha R), B alpha(2R,alpha S) and B
alpha (2R,alpha R), which were employed in the following series of
experiments. 14C-chlorophenyl-fenvalerate enantiomers, as shown in
Figure 1, were also utilized.
 1/ See Annex 2 for FAO and WHO documentation.
Studies with single doses of the individual 14C-chlorophenyl
radiolabelled isomers, orally administered to four male ddy mice at
2.5 mg/kg, showed almost complete excretion in urine and faeces after
6 days, predominantly in urine. Analysis of tissue residues, however,
revealed that the B alpha-isomer gave relatively higher 14C-residues
in most tissues analyzed, especially adrenals, liver, lymph nodes and
spleen.
In a subsequent comparative feeding study, three groups of seven
to ten male ddy mice were separately maintained on a diet containing
500 ppm of each of 14C-chlorophenyl-labelled A alpha-, B alpha- and
Bß-fenvalerate for one and two weeks. The B alpha- isomer resulted in
higher 14C levels in all tissues analyzed than the A alpha- and
Bß-isomers. Radioactivity concentrations were highest in the adrenals,
liver and mesenteric lymph nodes. Furthermore, the predominant
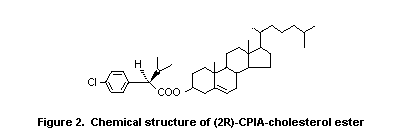
metabolite in these tissues was identified as the ester of (2R)-2-
(4-chlorophenyl) isovaleric acid and cholesterol (2R)-CPIA-cholesterol
ester (see Figure 2). Other compounds identified included unchanged B
alpha- fenvalerate, 2-(4-chlorophenyl)-isovalerate (CPIA) and
2-(4-chlorophenyl)-3-hydroxymethyl-butenoic acid (3-OH-CPIA). In
contrast, the tissues of A alpha- and Bß-treated mice contained
unchanged parent compound, CPIA and 3-OH-CPIA. CPIA-cholesterol ester
was not detectable in any tissues of mice treated with the A
alpha-isomer and was present in only trace amounts in the liver of
mice treated with the Bß-isomer.
1/ See Annex 2 for FAO and WHO documentation.
Studies with single doses of the individual 14C-chlorophenyl
radiolabelled isomers, orally administered to four male ddy mice at
2.5 mg/kg, showed almost complete excretion in urine and faeces after
6 days, predominantly in urine. Analysis of tissue residues, however,
revealed that the B alpha-isomer gave relatively higher 14C-residues
in most tissues analyzed, especially adrenals, liver, lymph nodes and
spleen.
In a subsequent comparative feeding study, three groups of seven
to ten male ddy mice were separately maintained on a diet containing
500 ppm of each of 14C-chlorophenyl-labelled A alpha-, B alpha- and
Bß-fenvalerate for one and two weeks. The B alpha- isomer resulted in
higher 14C levels in all tissues analyzed than the A alpha- and
Bß-isomers. Radioactivity concentrations were highest in the adrenals,
liver and mesenteric lymph nodes. Furthermore, the predominant
metabolite in these tissues was identified as the ester of (2R)-2-
(4-chlorophenyl) isovaleric acid and cholesterol (2R)-CPIA-cholesterol
ester (see Figure 2). Other compounds identified included unchanged B
alpha- fenvalerate, 2-(4-chlorophenyl)-isovalerate (CPIA) and
2-(4-chlorophenyl)-3-hydroxymethyl-butenoic acid (3-OH-CPIA). In
contrast, the tissues of A alpha- and Bß-treated mice contained
unchanged parent compound, CPIA and 3-OH-CPIA. CPIA-cholesterol ester
was not detectable in any tissues of mice treated with the A
alpha-isomer and was present in only trace amounts in the liver of
mice treated with the Bß-isomer.
 (2R)-CPIA-cholesterol ester was also identified in the liver,
spleen, adrenal and ovary of Charles River SD female rats treated with
1 500 ppm 14CO-acid-labelled fenvalerate for two weeks. The
metabolite was identified by co-chromatography with an authentic
standard (TLC,HPLC,GLC) and the identity confirmed by mass
spectrometry.
In a further study, male ddy mice were given a single oral dose
(70 mg/kg) of 14C-chlorophenyl-B alpha isomer and appropriate tissues
were analyzed after sacrifice of seven mice within 24 h of treatment.
CPIA-cholesterol ester was found in intestine, mesenteric lymph nodes,
blood and kidney, but not in the liver, after 30 minutes. After 1 h,
it was detected in all tissues analyzed, including the liver and
spleen.
The accumulation and elimination of fenvalerate was studied in
male ddy mice fed racemic 14C-chlorophenyl-fenvalerate at 300 ppm in
the diet for six weeks and then maintained on basal diet for four
weeks. Six mice were sacrificed at one, two, four and six weeks during
treatment and at two and four weeks post-treatment to permit
determination of 14C-tissue levels. By the sixth week of exposure,
tissue concentrations of radioactivity had reached a plateau in
adrenal, liver, fat, spleen and lymph node. The concentrations of
CPIA-cholesterol ester followed a similar pattern. After cessation of
dosing, tissue residues of both quickly declined in liver, blood,
kidney and skin and more slowly in adrenal, lymph node and spleen.
In a similar study, groups of five male and five female SD-rats
were fed 14C-chlorophenyl-fenvalerate at 25 ppm in the diet for 35
days and then maintained on basal diet for a further 84 days. The
highest concentration of radioactivity accumulated in the following
decreasing order: fat, adrenal, liver, skin, kidney, spleen, blood,
testes. The determination of tissue concentrations enabled the
following half-lives (days) to be estimated: adrenal 84; spleen 33;
blood 9; fat 9; skin 5; liver 1; and kidney 1.
The metabolism of the four enantiomers of fenvalerate was also
studied in vitro using a mouse liver microsomal preparation. The
B alpha- and Bß-isomers were hydrolysed more rapidly than the A alpha-
and Aß-isomers, suggesting that the rate of enzymatic hydrolysis is
more dependent on the chirality of the acid rather than the alcohol
moeity.
In vitro investigations showed that various mouse tissues
produced CPIA-cholesterol ester only from B alpha-fenvalerate,
although a kidney preparation did yield trace amounts from the
Bß-isomer. Kidney, liver and brain microsomal fractions proved most
active in this regard. Free CPIA was not utilized as a substrate.
Further investigations showed that CPIA was also not a substrate
for acyl CoA-cholesterol acyl transferase, and that neither
lecithin-cholesterol acyl transferase nor cholesterol esterase were
implicated in the synthesis of CPIA-cholesterol ester. However, the
observation that alkyl alcohols competed with its formation suggests
that CPIA-cholesterol ester is produced via an ester exchange reaction
catalyzed by microsomal esterases.
Additional studies were made in which racemic fenvalerate and the
four enantiomers were administered to groups of ten male ddy mice in
the diet for 4, 8, 13, 26, 39 and 52 weeks. Dietary concentrations
were varied according to the toxicity of the additive, as tabulated in
Table 1.
TABLE 1. Incidence of Fenvalerate-Induced Granulomatous Changes
Chemical Purity Dosage Feeding period (weeks)
% (ppm) 4 8 13 26 39 52
A 100.0 500 - - 0 0 0 0
A:Aß 95.2 500 - - 0 0 0 0
(1:1)
Racemate 96.1 500 - - 100 100 100 95
B 97.0-99.1 125 0 100 100 - - -
1000 100 100 100 - - -
Bß 99.2-99.7 125 0 0 0 - - -
1000 0 0 0 - - -
Control 0 0 0 0 0 0
Typical granulomatous changes occurred in liver, spleen and lymph
nodes of B alpha-treated groups but not groups treated with the 1:1
mixture of A alpha and Aß or the Bß isomers. The histological
appearance of microgranulomas and giant-cell infiltrations were
observed mainly in the medullary cord of lymph nodes, splenic white
pulp and in the periportal area of the hepatic lobules, but rarely in
midzonal or centrilobular areas.
Electron microscopy showed that the ultrastructure of
granulomatous foci of the B alpha- and racemic-fenvalerate-treated
groups were similar and that there were many activated macrophages and
giant-cells in both liver and lymph nodes. There were no remarkable
changes in the region of the granulomatous cells and little
lymphocytic involvement or fibrosis. Crystalline rods or needles were
included within the cytoplasm of macrophages and giant-cells. These
were also observed in hepatic cells of the B alpha-treated group at 13
weeks. The liver of mice fed 500 ppm racemic fenvalerate for 52 weeks
yielded macrophages or giant-cells with a large number of lysosomes
within the cytoplasm, some of which also contained the cyrstalline
rods; the latter also occurred within hepatocytes from this group. The
crystalline inclusions were never observed in the tissues of mice from
the control group. These studies convincingly demonstrate that the
B alpha-enantiomer of fenvalerate is causally associated with the
granulomatous changes observed.
The identity of the intracellular crystalline rods with
CPIA-cholesterol ester was subsequently established using male ddy
mice fed the B alpha- and Bß-fenvalerate isomers in the diet at
1 000 ppm for eight and 13 weeks. Tritium-labelled 3H-(2R)-CPIA-
cholesterol ester was shown to co-locate in hepatic giant and Kupffer
cells with the previously fed CPIA-cholesterol ester, identified by
positive staining.
In a subsequent study in which the (2RS)-, (2R)- and (2S)-CPIA-
cholesterol esters were administered intravenously to groups of ddy
male mice, the formation of granulomatous changes was observed after
seven days. The liver of all treated mice displayed histologically
identical granulomatous changes, microgranulomas and giant-cell
infiltrates, such as those in fenvalerate-treated mice. The changes
were hardly evident in lymph nodes and spleen. The observation that
all of the enantiomeric esters produced similar changes is
attributable to the common route of administration.
In order to elucidate further the fate of the granulomatous
changes, mice were similarly treated with 10 and 30 mg/kg of the
(2R)-CPIA cholesterol ester and sacrificed at one, four and eight
weeks after injection. Micro-granulomatous changes, including
microgranuloma and giant cell infiltrations, were seen in each case.
Giant cells were seen clearly at the later stages, whereas
microgranulomas were more evident at the early stages. The presence of
the microgranulomas eight weeks after injection is suggestive of
foreign body microgranulomas.
To exclude hypersensitivity as the cause of the
microgranulomatous changes previously observed, groups of five
BALB/cA/nu/nu/SLC female nude mice were fed dietary racemic
fenvalerate at 1 000 and 3 000 ppm in the diet for four weeks.
Histopathological changes typical of the fenvalerate-induced
microgranuloma were observed in all of the treated mice. This study
indicates that granulomatous changes induced by fenvalerate are not
mediated by hypersensitivity reactions (Miyamoto et al., 1984).
More recently a dose-related incidence of hepatic microgranulomas
was found in groups of six male and six female beagles fed fenvalerate
at 0, 250, 500 and 1 000 ppm in the diet for six months. Histiocytic
cell infiltration of mesenteric lymph nodes also occurred in female
dogs fed 500 ppm and 1 000 ppm and in males fed 1 000 ppm.
Multinucleate cells were occasionally seen. The reversibility of these
effects was not studied (Parker et al., 1984).
Special Study on Mutagenicity
Fenvalerate has been shown to be without mutagenicity in
Salmonella typhimurium strains TA 100 and 98 and in V79 Chinese
hamster cells, with and without metabolic activation (Pluijmen
et al., 1984).
OBSERVATIONS IN HUMANS
A field study of 16 workers engaged in agricultural application
of fenvalerate associated exposure with cutaneous symptomatology.
Paraesthesia usually developed at exposed body sites after some hours
and the symptoms then progressed from a mild itch to a stinging
sensation and peaked with numbness. Sweating, exposure to sun or heat
or the application of water to the exposed site aggravated these
symptoms. Normal sensation returned with 24 h of cessation of exposure
(Tucker & Flannigan, 1983).
COMMENTS
The data required by the 1981 meeting have been received and
evaluated. The limited data provided on the tissue distribution of
fenvalerate confirm the rapid dissipation from fatty tissue,
indicating a minimal potential of the compound for bioaccumulation.
A series of elegant studies convincingly demonstrate the
relationship between th B alpha-isomer of fenvalerate and the
occurrence of the granulomata in mice, and of giant cell infiltration
in rats. The mechanism has been demonstrated to be a type of foreign
body response due to the deposition of crystals of the cholesterol
2-(4-chlorophenyl)-isovalerate ester in the tissues. Although no
dose-response relationship was determined, there is no reason to
question the previously established no-observable-effect levels in
rodents.
A recent study in dogs indicates that similar microgranulomatous
lesions are formed in this species following fenvalerate
administration in the diet. There is no reason to doubt that the
mechanism involved in the formation of these lesions is similar to
that described for the formation of similar lesions in the rodents.
Limited mutagenicity studies were negative.
Observations in humans are limited to field exposure and indicate
that fenvalerate causes similar cutaneous sensations to those induced
by other synthetic pyrethroids, possibly as a result of local effects.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 30 ppm in the diet, equivalent to 3.5 mg/kg bw
Rat: 150 ppm in the diet, equivalent to 7.5 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Submission of carcinogenicity studies with fenvalerate
commissioned by IARC and NTP.
2. Determination of the no-effect level in dog with respect to
granulomata formation and full details of the recently published
six-month feeding study of fenvalerate in dogs.
Desirable
Observations in humans.
REFERENCES
Marei A.E. - S.M., Ruzo, L.O. & Casida J.E. Analysis and persistence
1982 of permethrin, cypermethrin deltamethrin and fenvalerate in
the fat and brain of treated rats. J. Agric. Food Chem.,
30: 558-562.
Parker, C.M. et al. Six-month feeding study of fenvalerate in dogs.
1984 Fundam. Appl. Toxicol., 4: 577.
Pluijmen M. et al. Lack of mutagenicity of synthetic pyrethroids in
1984 Salmonella typhimurium strains and in V79 Chinese hamster
cells. Mutat. Res., 137: 7-15.
Tucker, S.B. & Flannigan S.A. Cutaneous effects from occupational
1983 exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
Miyamoto, J., Matsuo, M., Okuno, Y. & Kaneko, H. Studies on formation
1984 of microgranulomatous lesions including giant cell
infiltration found in mice and rats treated with
fenvalerate. Laboratory of Biochemistry and Toxicology,
Takarazuka Research Centre Technical Report AT-40-0370
submitted by Sumitomo Chemical Co. Ltd., to WHO.
(Unpublished)
(2R)-CPIA-cholesterol ester was also identified in the liver,
spleen, adrenal and ovary of Charles River SD female rats treated with
1 500 ppm 14CO-acid-labelled fenvalerate for two weeks. The
metabolite was identified by co-chromatography with an authentic
standard (TLC,HPLC,GLC) and the identity confirmed by mass
spectrometry.
In a further study, male ddy mice were given a single oral dose
(70 mg/kg) of 14C-chlorophenyl-B alpha isomer and appropriate tissues
were analyzed after sacrifice of seven mice within 24 h of treatment.
CPIA-cholesterol ester was found in intestine, mesenteric lymph nodes,
blood and kidney, but not in the liver, after 30 minutes. After 1 h,
it was detected in all tissues analyzed, including the liver and
spleen.
The accumulation and elimination of fenvalerate was studied in
male ddy mice fed racemic 14C-chlorophenyl-fenvalerate at 300 ppm in
the diet for six weeks and then maintained on basal diet for four
weeks. Six mice were sacrificed at one, two, four and six weeks during
treatment and at two and four weeks post-treatment to permit
determination of 14C-tissue levels. By the sixth week of exposure,
tissue concentrations of radioactivity had reached a plateau in
adrenal, liver, fat, spleen and lymph node. The concentrations of
CPIA-cholesterol ester followed a similar pattern. After cessation of
dosing, tissue residues of both quickly declined in liver, blood,
kidney and skin and more slowly in adrenal, lymph node and spleen.
In a similar study, groups of five male and five female SD-rats
were fed 14C-chlorophenyl-fenvalerate at 25 ppm in the diet for 35
days and then maintained on basal diet for a further 84 days. The
highest concentration of radioactivity accumulated in the following
decreasing order: fat, adrenal, liver, skin, kidney, spleen, blood,
testes. The determination of tissue concentrations enabled the
following half-lives (days) to be estimated: adrenal 84; spleen 33;
blood 9; fat 9; skin 5; liver 1; and kidney 1.
The metabolism of the four enantiomers of fenvalerate was also
studied in vitro using a mouse liver microsomal preparation. The
B alpha- and Bß-isomers were hydrolysed more rapidly than the A alpha-
and Aß-isomers, suggesting that the rate of enzymatic hydrolysis is
more dependent on the chirality of the acid rather than the alcohol
moeity.
In vitro investigations showed that various mouse tissues
produced CPIA-cholesterol ester only from B alpha-fenvalerate,
although a kidney preparation did yield trace amounts from the
Bß-isomer. Kidney, liver and brain microsomal fractions proved most
active in this regard. Free CPIA was not utilized as a substrate.
Further investigations showed that CPIA was also not a substrate
for acyl CoA-cholesterol acyl transferase, and that neither
lecithin-cholesterol acyl transferase nor cholesterol esterase were
implicated in the synthesis of CPIA-cholesterol ester. However, the
observation that alkyl alcohols competed with its formation suggests
that CPIA-cholesterol ester is produced via an ester exchange reaction
catalyzed by microsomal esterases.
Additional studies were made in which racemic fenvalerate and the
four enantiomers were administered to groups of ten male ddy mice in
the diet for 4, 8, 13, 26, 39 and 52 weeks. Dietary concentrations
were varied according to the toxicity of the additive, as tabulated in
Table 1.
TABLE 1. Incidence of Fenvalerate-Induced Granulomatous Changes
Chemical Purity Dosage Feeding period (weeks)
% (ppm) 4 8 13 26 39 52
A 100.0 500 - - 0 0 0 0
A:Aß 95.2 500 - - 0 0 0 0
(1:1)
Racemate 96.1 500 - - 100 100 100 95
B 97.0-99.1 125 0 100 100 - - -
1000 100 100 100 - - -
Bß 99.2-99.7 125 0 0 0 - - -
1000 0 0 0 - - -
Control 0 0 0 0 0 0
Typical granulomatous changes occurred in liver, spleen and lymph
nodes of B alpha-treated groups but not groups treated with the 1:1
mixture of A alpha and Aß or the Bß isomers. The histological
appearance of microgranulomas and giant-cell infiltrations were
observed mainly in the medullary cord of lymph nodes, splenic white
pulp and in the periportal area of the hepatic lobules, but rarely in
midzonal or centrilobular areas.
Electron microscopy showed that the ultrastructure of
granulomatous foci of the B alpha- and racemic-fenvalerate-treated
groups were similar and that there were many activated macrophages and
giant-cells in both liver and lymph nodes. There were no remarkable
changes in the region of the granulomatous cells and little
lymphocytic involvement or fibrosis. Crystalline rods or needles were
included within the cytoplasm of macrophages and giant-cells. These
were also observed in hepatic cells of the B alpha-treated group at 13
weeks. The liver of mice fed 500 ppm racemic fenvalerate for 52 weeks
yielded macrophages or giant-cells with a large number of lysosomes
within the cytoplasm, some of which also contained the cyrstalline
rods; the latter also occurred within hepatocytes from this group. The
crystalline inclusions were never observed in the tissues of mice from
the control group. These studies convincingly demonstrate that the
B alpha-enantiomer of fenvalerate is causally associated with the
granulomatous changes observed.
The identity of the intracellular crystalline rods with
CPIA-cholesterol ester was subsequently established using male ddy
mice fed the B alpha- and Bß-fenvalerate isomers in the diet at
1 000 ppm for eight and 13 weeks. Tritium-labelled 3H-(2R)-CPIA-
cholesterol ester was shown to co-locate in hepatic giant and Kupffer
cells with the previously fed CPIA-cholesterol ester, identified by
positive staining.
In a subsequent study in which the (2RS)-, (2R)- and (2S)-CPIA-
cholesterol esters were administered intravenously to groups of ddy
male mice, the formation of granulomatous changes was observed after
seven days. The liver of all treated mice displayed histologically
identical granulomatous changes, microgranulomas and giant-cell
infiltrates, such as those in fenvalerate-treated mice. The changes
were hardly evident in lymph nodes and spleen. The observation that
all of the enantiomeric esters produced similar changes is
attributable to the common route of administration.
In order to elucidate further the fate of the granulomatous
changes, mice were similarly treated with 10 and 30 mg/kg of the
(2R)-CPIA cholesterol ester and sacrificed at one, four and eight
weeks after injection. Micro-granulomatous changes, including
microgranuloma and giant cell infiltrations, were seen in each case.
Giant cells were seen clearly at the later stages, whereas
microgranulomas were more evident at the early stages. The presence of
the microgranulomas eight weeks after injection is suggestive of
foreign body microgranulomas.
To exclude hypersensitivity as the cause of the
microgranulomatous changes previously observed, groups of five
BALB/cA/nu/nu/SLC female nude mice were fed dietary racemic
fenvalerate at 1 000 and 3 000 ppm in the diet for four weeks.
Histopathological changes typical of the fenvalerate-induced
microgranuloma were observed in all of the treated mice. This study
indicates that granulomatous changes induced by fenvalerate are not
mediated by hypersensitivity reactions (Miyamoto et al., 1984).
More recently a dose-related incidence of hepatic microgranulomas
was found in groups of six male and six female beagles fed fenvalerate
at 0, 250, 500 and 1 000 ppm in the diet for six months. Histiocytic
cell infiltration of mesenteric lymph nodes also occurred in female
dogs fed 500 ppm and 1 000 ppm and in males fed 1 000 ppm.
Multinucleate cells were occasionally seen. The reversibility of these
effects was not studied (Parker et al., 1984).
Special Study on Mutagenicity
Fenvalerate has been shown to be without mutagenicity in
Salmonella typhimurium strains TA 100 and 98 and in V79 Chinese
hamster cells, with and without metabolic activation (Pluijmen
et al., 1984).
OBSERVATIONS IN HUMANS
A field study of 16 workers engaged in agricultural application
of fenvalerate associated exposure with cutaneous symptomatology.
Paraesthesia usually developed at exposed body sites after some hours
and the symptoms then progressed from a mild itch to a stinging
sensation and peaked with numbness. Sweating, exposure to sun or heat
or the application of water to the exposed site aggravated these
symptoms. Normal sensation returned with 24 h of cessation of exposure
(Tucker & Flannigan, 1983).
COMMENTS
The data required by the 1981 meeting have been received and
evaluated. The limited data provided on the tissue distribution of
fenvalerate confirm the rapid dissipation from fatty tissue,
indicating a minimal potential of the compound for bioaccumulation.
A series of elegant studies convincingly demonstrate the
relationship between th B alpha-isomer of fenvalerate and the
occurrence of the granulomata in mice, and of giant cell infiltration
in rats. The mechanism has been demonstrated to be a type of foreign
body response due to the deposition of crystals of the cholesterol
2-(4-chlorophenyl)-isovalerate ester in the tissues. Although no
dose-response relationship was determined, there is no reason to
question the previously established no-observable-effect levels in
rodents.
A recent study in dogs indicates that similar microgranulomatous
lesions are formed in this species following fenvalerate
administration in the diet. There is no reason to doubt that the
mechanism involved in the formation of these lesions is similar to
that described for the formation of similar lesions in the rodents.
Limited mutagenicity studies were negative.
Observations in humans are limited to field exposure and indicate
that fenvalerate causes similar cutaneous sensations to those induced
by other synthetic pyrethroids, possibly as a result of local effects.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 30 ppm in the diet, equivalent to 3.5 mg/kg bw
Rat: 150 ppm in the diet, equivalent to 7.5 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Submission of carcinogenicity studies with fenvalerate
commissioned by IARC and NTP.
2. Determination of the no-effect level in dog with respect to
granulomata formation and full details of the recently published
six-month feeding study of fenvalerate in dogs.
Desirable
Observations in humans.
REFERENCES
Marei A.E. - S.M., Ruzo, L.O. & Casida J.E. Analysis and persistence
1982 of permethrin, cypermethrin deltamethrin and fenvalerate in
the fat and brain of treated rats. J. Agric. Food Chem.,
30: 558-562.
Parker, C.M. et al. Six-month feeding study of fenvalerate in dogs.
1984 Fundam. Appl. Toxicol., 4: 577.
Pluijmen M. et al. Lack of mutagenicity of synthetic pyrethroids in
1984 Salmonella typhimurium strains and in V79 Chinese hamster
cells. Mutat. Res., 137: 7-15.
Tucker, S.B. & Flannigan S.A. Cutaneous effects from occupational
1983 exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
Miyamoto, J., Matsuo, M., Okuno, Y. & Kaneko, H. Studies on formation
1984 of microgranulomatous lesions including giant cell
infiltration found in mice and rats treated with
fenvalerate. Laboratory of Biochemistry and Toxicology,
Takarazuka Research Centre Technical Report AT-40-0370
submitted by Sumitomo Chemical Co. Ltd., to WHO.
(Unpublished)
 1/ See Annex 2 for FAO and WHO documentation.
Studies with single doses of the individual 14C-chlorophenyl
radiolabelled isomers, orally administered to four male ddy mice at
2.5 mg/kg, showed almost complete excretion in urine and faeces after
6 days, predominantly in urine. Analysis of tissue residues, however,
revealed that the B alpha-isomer gave relatively higher 14C-residues
in most tissues analyzed, especially adrenals, liver, lymph nodes and
spleen.
In a subsequent comparative feeding study, three groups of seven
to ten male ddy mice were separately maintained on a diet containing
500 ppm of each of 14C-chlorophenyl-labelled A alpha-, B alpha- and
Bß-fenvalerate for one and two weeks. The B alpha- isomer resulted in
higher 14C levels in all tissues analyzed than the A alpha- and
Bß-isomers. Radioactivity concentrations were highest in the adrenals,
liver and mesenteric lymph nodes. Furthermore, the predominant
metabolite in these tissues was identified as the ester of (2R)-2-
(4-chlorophenyl) isovaleric acid and cholesterol (2R)-CPIA-cholesterol
ester (see Figure 2). Other compounds identified included unchanged B
alpha- fenvalerate, 2-(4-chlorophenyl)-isovalerate (CPIA) and
2-(4-chlorophenyl)-3-hydroxymethyl-butenoic acid (3-OH-CPIA). In
contrast, the tissues of A alpha- and Bß-treated mice contained
unchanged parent compound, CPIA and 3-OH-CPIA. CPIA-cholesterol ester
was not detectable in any tissues of mice treated with the A
alpha-isomer and was present in only trace amounts in the liver of
mice treated with the Bß-isomer.
1/ See Annex 2 for FAO and WHO documentation.
Studies with single doses of the individual 14C-chlorophenyl
radiolabelled isomers, orally administered to four male ddy mice at
2.5 mg/kg, showed almost complete excretion in urine and faeces after
6 days, predominantly in urine. Analysis of tissue residues, however,
revealed that the B alpha-isomer gave relatively higher 14C-residues
in most tissues analyzed, especially adrenals, liver, lymph nodes and
spleen.
In a subsequent comparative feeding study, three groups of seven
to ten male ddy mice were separately maintained on a diet containing
500 ppm of each of 14C-chlorophenyl-labelled A alpha-, B alpha- and
Bß-fenvalerate for one and two weeks. The B alpha- isomer resulted in
higher 14C levels in all tissues analyzed than the A alpha- and
Bß-isomers. Radioactivity concentrations were highest in the adrenals,
liver and mesenteric lymph nodes. Furthermore, the predominant
metabolite in these tissues was identified as the ester of (2R)-2-
(4-chlorophenyl) isovaleric acid and cholesterol (2R)-CPIA-cholesterol
ester (see Figure 2). Other compounds identified included unchanged B
alpha- fenvalerate, 2-(4-chlorophenyl)-isovalerate (CPIA) and
2-(4-chlorophenyl)-3-hydroxymethyl-butenoic acid (3-OH-CPIA). In
contrast, the tissues of A alpha- and Bß-treated mice contained
unchanged parent compound, CPIA and 3-OH-CPIA. CPIA-cholesterol ester
was not detectable in any tissues of mice treated with the A
alpha-isomer and was present in only trace amounts in the liver of
mice treated with the Bß-isomer.
 (2R)-CPIA-cholesterol ester was also identified in the liver,
spleen, adrenal and ovary of Charles River SD female rats treated with
1 500 ppm 14CO-acid-labelled fenvalerate for two weeks. The
metabolite was identified by co-chromatography with an authentic
standard (TLC,HPLC,GLC) and the identity confirmed by mass
spectrometry.
In a further study, male ddy mice were given a single oral dose
(70 mg/kg) of 14C-chlorophenyl-B alpha isomer and appropriate tissues
were analyzed after sacrifice of seven mice within 24 h of treatment.
CPIA-cholesterol ester was found in intestine, mesenteric lymph nodes,
blood and kidney, but not in the liver, after 30 minutes. After 1 h,
it was detected in all tissues analyzed, including the liver and
spleen.
The accumulation and elimination of fenvalerate was studied in
male ddy mice fed racemic 14C-chlorophenyl-fenvalerate at 300 ppm in
the diet for six weeks and then maintained on basal diet for four
weeks. Six mice were sacrificed at one, two, four and six weeks during
treatment and at two and four weeks post-treatment to permit
determination of 14C-tissue levels. By the sixth week of exposure,
tissue concentrations of radioactivity had reached a plateau in
adrenal, liver, fat, spleen and lymph node. The concentrations of
CPIA-cholesterol ester followed a similar pattern. After cessation of
dosing, tissue residues of both quickly declined in liver, blood,
kidney and skin and more slowly in adrenal, lymph node and spleen.
In a similar study, groups of five male and five female SD-rats
were fed 14C-chlorophenyl-fenvalerate at 25 ppm in the diet for 35
days and then maintained on basal diet for a further 84 days. The
highest concentration of radioactivity accumulated in the following
decreasing order: fat, adrenal, liver, skin, kidney, spleen, blood,
testes. The determination of tissue concentrations enabled the
following half-lives (days) to be estimated: adrenal 84; spleen 33;
blood 9; fat 9; skin 5; liver 1; and kidney 1.
The metabolism of the four enantiomers of fenvalerate was also
studied in vitro using a mouse liver microsomal preparation. The
B alpha- and Bß-isomers were hydrolysed more rapidly than the A alpha-
and Aß-isomers, suggesting that the rate of enzymatic hydrolysis is
more dependent on the chirality of the acid rather than the alcohol
moeity.
In vitro investigations showed that various mouse tissues
produced CPIA-cholesterol ester only from B alpha-fenvalerate,
although a kidney preparation did yield trace amounts from the
Bß-isomer. Kidney, liver and brain microsomal fractions proved most
active in this regard. Free CPIA was not utilized as a substrate.
Further investigations showed that CPIA was also not a substrate
for acyl CoA-cholesterol acyl transferase, and that neither
lecithin-cholesterol acyl transferase nor cholesterol esterase were
implicated in the synthesis of CPIA-cholesterol ester. However, the
observation that alkyl alcohols competed with its formation suggests
that CPIA-cholesterol ester is produced via an ester exchange reaction
catalyzed by microsomal esterases.
Additional studies were made in which racemic fenvalerate and the
four enantiomers were administered to groups of ten male ddy mice in
the diet for 4, 8, 13, 26, 39 and 52 weeks. Dietary concentrations
were varied according to the toxicity of the additive, as tabulated in
Table 1.
TABLE 1. Incidence of Fenvalerate-Induced Granulomatous Changes
Chemical Purity Dosage Feeding period (weeks)
% (ppm) 4 8 13 26 39 52
A 100.0 500 - - 0 0 0 0
A:Aß 95.2 500 - - 0 0 0 0
(1:1)
Racemate 96.1 500 - - 100 100 100 95
B 97.0-99.1 125 0 100 100 - - -
1000 100 100 100 - - -
Bß 99.2-99.7 125 0 0 0 - - -
1000 0 0 0 - - -
Control 0 0 0 0 0 0
Typical granulomatous changes occurred in liver, spleen and lymph
nodes of B alpha-treated groups but not groups treated with the 1:1
mixture of A alpha and Aß or the Bß isomers. The histological
appearance of microgranulomas and giant-cell infiltrations were
observed mainly in the medullary cord of lymph nodes, splenic white
pulp and in the periportal area of the hepatic lobules, but rarely in
midzonal or centrilobular areas.
Electron microscopy showed that the ultrastructure of
granulomatous foci of the B alpha- and racemic-fenvalerate-treated
groups were similar and that there were many activated macrophages and
giant-cells in both liver and lymph nodes. There were no remarkable
changes in the region of the granulomatous cells and little
lymphocytic involvement or fibrosis. Crystalline rods or needles were
included within the cytoplasm of macrophages and giant-cells. These
were also observed in hepatic cells of the B alpha-treated group at 13
weeks. The liver of mice fed 500 ppm racemic fenvalerate for 52 weeks
yielded macrophages or giant-cells with a large number of lysosomes
within the cytoplasm, some of which also contained the cyrstalline
rods; the latter also occurred within hepatocytes from this group. The
crystalline inclusions were never observed in the tissues of mice from
the control group. These studies convincingly demonstrate that the
B alpha-enantiomer of fenvalerate is causally associated with the
granulomatous changes observed.
The identity of the intracellular crystalline rods with
CPIA-cholesterol ester was subsequently established using male ddy
mice fed the B alpha- and Bß-fenvalerate isomers in the diet at
1 000 ppm for eight and 13 weeks. Tritium-labelled 3H-(2R)-CPIA-
cholesterol ester was shown to co-locate in hepatic giant and Kupffer
cells with the previously fed CPIA-cholesterol ester, identified by
positive staining.
In a subsequent study in which the (2RS)-, (2R)- and (2S)-CPIA-
cholesterol esters were administered intravenously to groups of ddy
male mice, the formation of granulomatous changes was observed after
seven days. The liver of all treated mice displayed histologically
identical granulomatous changes, microgranulomas and giant-cell
infiltrates, such as those in fenvalerate-treated mice. The changes
were hardly evident in lymph nodes and spleen. The observation that
all of the enantiomeric esters produced similar changes is
attributable to the common route of administration.
In order to elucidate further the fate of the granulomatous
changes, mice were similarly treated with 10 and 30 mg/kg of the
(2R)-CPIA cholesterol ester and sacrificed at one, four and eight
weeks after injection. Micro-granulomatous changes, including
microgranuloma and giant cell infiltrations, were seen in each case.
Giant cells were seen clearly at the later stages, whereas
microgranulomas were more evident at the early stages. The presence of
the microgranulomas eight weeks after injection is suggestive of
foreign body microgranulomas.
To exclude hypersensitivity as the cause of the
microgranulomatous changes previously observed, groups of five
BALB/cA/nu/nu/SLC female nude mice were fed dietary racemic
fenvalerate at 1 000 and 3 000 ppm in the diet for four weeks.
Histopathological changes typical of the fenvalerate-induced
microgranuloma were observed in all of the treated mice. This study
indicates that granulomatous changes induced by fenvalerate are not
mediated by hypersensitivity reactions (Miyamoto et al., 1984).
More recently a dose-related incidence of hepatic microgranulomas
was found in groups of six male and six female beagles fed fenvalerate
at 0, 250, 500 and 1 000 ppm in the diet for six months. Histiocytic
cell infiltration of mesenteric lymph nodes also occurred in female
dogs fed 500 ppm and 1 000 ppm and in males fed 1 000 ppm.
Multinucleate cells were occasionally seen. The reversibility of these
effects was not studied (Parker et al., 1984).
Special Study on Mutagenicity
Fenvalerate has been shown to be without mutagenicity in
Salmonella typhimurium strains TA 100 and 98 and in V79 Chinese
hamster cells, with and without metabolic activation (Pluijmen
et al., 1984).
OBSERVATIONS IN HUMANS
A field study of 16 workers engaged in agricultural application
of fenvalerate associated exposure with cutaneous symptomatology.
Paraesthesia usually developed at exposed body sites after some hours
and the symptoms then progressed from a mild itch to a stinging
sensation and peaked with numbness. Sweating, exposure to sun or heat
or the application of water to the exposed site aggravated these
symptoms. Normal sensation returned with 24 h of cessation of exposure
(Tucker & Flannigan, 1983).
COMMENTS
The data required by the 1981 meeting have been received and
evaluated. The limited data provided on the tissue distribution of
fenvalerate confirm the rapid dissipation from fatty tissue,
indicating a minimal potential of the compound for bioaccumulation.
A series of elegant studies convincingly demonstrate the
relationship between th B alpha-isomer of fenvalerate and the
occurrence of the granulomata in mice, and of giant cell infiltration
in rats. The mechanism has been demonstrated to be a type of foreign
body response due to the deposition of crystals of the cholesterol
2-(4-chlorophenyl)-isovalerate ester in the tissues. Although no
dose-response relationship was determined, there is no reason to
question the previously established no-observable-effect levels in
rodents.
A recent study in dogs indicates that similar microgranulomatous
lesions are formed in this species following fenvalerate
administration in the diet. There is no reason to doubt that the
mechanism involved in the formation of these lesions is similar to
that described for the formation of similar lesions in the rodents.
Limited mutagenicity studies were negative.
Observations in humans are limited to field exposure and indicate
that fenvalerate causes similar cutaneous sensations to those induced
by other synthetic pyrethroids, possibly as a result of local effects.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 30 ppm in the diet, equivalent to 3.5 mg/kg bw
Rat: 150 ppm in the diet, equivalent to 7.5 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Submission of carcinogenicity studies with fenvalerate
commissioned by IARC and NTP.
2. Determination of the no-effect level in dog with respect to
granulomata formation and full details of the recently published
six-month feeding study of fenvalerate in dogs.
Desirable
Observations in humans.
REFERENCES
Marei A.E. - S.M., Ruzo, L.O. & Casida J.E. Analysis and persistence
1982 of permethrin, cypermethrin deltamethrin and fenvalerate in
the fat and brain of treated rats. J. Agric. Food Chem.,
30: 558-562.
Parker, C.M. et al. Six-month feeding study of fenvalerate in dogs.
1984 Fundam. Appl. Toxicol., 4: 577.
Pluijmen M. et al. Lack of mutagenicity of synthetic pyrethroids in
1984 Salmonella typhimurium strains and in V79 Chinese hamster
cells. Mutat. Res., 137: 7-15.
Tucker, S.B. & Flannigan S.A. Cutaneous effects from occupational
1983 exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
Miyamoto, J., Matsuo, M., Okuno, Y. & Kaneko, H. Studies on formation
1984 of microgranulomatous lesions including giant cell
infiltration found in mice and rats treated with
fenvalerate. Laboratory of Biochemistry and Toxicology,
Takarazuka Research Centre Technical Report AT-40-0370
submitted by Sumitomo Chemical Co. Ltd., to WHO.
(Unpublished)
(2R)-CPIA-cholesterol ester was also identified in the liver,
spleen, adrenal and ovary of Charles River SD female rats treated with
1 500 ppm 14CO-acid-labelled fenvalerate for two weeks. The
metabolite was identified by co-chromatography with an authentic
standard (TLC,HPLC,GLC) and the identity confirmed by mass
spectrometry.
In a further study, male ddy mice were given a single oral dose
(70 mg/kg) of 14C-chlorophenyl-B alpha isomer and appropriate tissues
were analyzed after sacrifice of seven mice within 24 h of treatment.
CPIA-cholesterol ester was found in intestine, mesenteric lymph nodes,
blood and kidney, but not in the liver, after 30 minutes. After 1 h,
it was detected in all tissues analyzed, including the liver and
spleen.
The accumulation and elimination of fenvalerate was studied in
male ddy mice fed racemic 14C-chlorophenyl-fenvalerate at 300 ppm in
the diet for six weeks and then maintained on basal diet for four
weeks. Six mice were sacrificed at one, two, four and six weeks during
treatment and at two and four weeks post-treatment to permit
determination of 14C-tissue levels. By the sixth week of exposure,
tissue concentrations of radioactivity had reached a plateau in
adrenal, liver, fat, spleen and lymph node. The concentrations of
CPIA-cholesterol ester followed a similar pattern. After cessation of
dosing, tissue residues of both quickly declined in liver, blood,
kidney and skin and more slowly in adrenal, lymph node and spleen.
In a similar study, groups of five male and five female SD-rats
were fed 14C-chlorophenyl-fenvalerate at 25 ppm in the diet for 35
days and then maintained on basal diet for a further 84 days. The
highest concentration of radioactivity accumulated in the following
decreasing order: fat, adrenal, liver, skin, kidney, spleen, blood,
testes. The determination of tissue concentrations enabled the
following half-lives (days) to be estimated: adrenal 84; spleen 33;
blood 9; fat 9; skin 5; liver 1; and kidney 1.
The metabolism of the four enantiomers of fenvalerate was also
studied in vitro using a mouse liver microsomal preparation. The
B alpha- and Bß-isomers were hydrolysed more rapidly than the A alpha-
and Aß-isomers, suggesting that the rate of enzymatic hydrolysis is
more dependent on the chirality of the acid rather than the alcohol
moeity.
In vitro investigations showed that various mouse tissues
produced CPIA-cholesterol ester only from B alpha-fenvalerate,
although a kidney preparation did yield trace amounts from the
Bß-isomer. Kidney, liver and brain microsomal fractions proved most
active in this regard. Free CPIA was not utilized as a substrate.
Further investigations showed that CPIA was also not a substrate
for acyl CoA-cholesterol acyl transferase, and that neither
lecithin-cholesterol acyl transferase nor cholesterol esterase were
implicated in the synthesis of CPIA-cholesterol ester. However, the
observation that alkyl alcohols competed with its formation suggests
that CPIA-cholesterol ester is produced via an ester exchange reaction
catalyzed by microsomal esterases.
Additional studies were made in which racemic fenvalerate and the
four enantiomers were administered to groups of ten male ddy mice in
the diet for 4, 8, 13, 26, 39 and 52 weeks. Dietary concentrations
were varied according to the toxicity of the additive, as tabulated in
Table 1.
TABLE 1. Incidence of Fenvalerate-Induced Granulomatous Changes
Chemical Purity Dosage Feeding period (weeks)
% (ppm) 4 8 13 26 39 52
A 100.0 500 - - 0 0 0 0
A:Aß 95.2 500 - - 0 0 0 0
(1:1)
Racemate 96.1 500 - - 100 100 100 95
B 97.0-99.1 125 0 100 100 - - -
1000 100 100 100 - - -
Bß 99.2-99.7 125 0 0 0 - - -
1000 0 0 0 - - -
Control 0 0 0 0 0 0
Typical granulomatous changes occurred in liver, spleen and lymph
nodes of B alpha-treated groups but not groups treated with the 1:1
mixture of A alpha and Aß or the Bß isomers. The histological
appearance of microgranulomas and giant-cell infiltrations were
observed mainly in the medullary cord of lymph nodes, splenic white
pulp and in the periportal area of the hepatic lobules, but rarely in
midzonal or centrilobular areas.
Electron microscopy showed that the ultrastructure of
granulomatous foci of the B alpha- and racemic-fenvalerate-treated
groups were similar and that there were many activated macrophages and
giant-cells in both liver and lymph nodes. There were no remarkable
changes in the region of the granulomatous cells and little
lymphocytic involvement or fibrosis. Crystalline rods or needles were
included within the cytoplasm of macrophages and giant-cells. These
were also observed in hepatic cells of the B alpha-treated group at 13
weeks. The liver of mice fed 500 ppm racemic fenvalerate for 52 weeks
yielded macrophages or giant-cells with a large number of lysosomes
within the cytoplasm, some of which also contained the cyrstalline
rods; the latter also occurred within hepatocytes from this group. The
crystalline inclusions were never observed in the tissues of mice from
the control group. These studies convincingly demonstrate that the
B alpha-enantiomer of fenvalerate is causally associated with the
granulomatous changes observed.
The identity of the intracellular crystalline rods with
CPIA-cholesterol ester was subsequently established using male ddy
mice fed the B alpha- and Bß-fenvalerate isomers in the diet at
1 000 ppm for eight and 13 weeks. Tritium-labelled 3H-(2R)-CPIA-
cholesterol ester was shown to co-locate in hepatic giant and Kupffer
cells with the previously fed CPIA-cholesterol ester, identified by
positive staining.
In a subsequent study in which the (2RS)-, (2R)- and (2S)-CPIA-
cholesterol esters were administered intravenously to groups of ddy
male mice, the formation of granulomatous changes was observed after
seven days. The liver of all treated mice displayed histologically
identical granulomatous changes, microgranulomas and giant-cell
infiltrates, such as those in fenvalerate-treated mice. The changes
were hardly evident in lymph nodes and spleen. The observation that
all of the enantiomeric esters produced similar changes is
attributable to the common route of administration.
In order to elucidate further the fate of the granulomatous
changes, mice were similarly treated with 10 and 30 mg/kg of the
(2R)-CPIA cholesterol ester and sacrificed at one, four and eight
weeks after injection. Micro-granulomatous changes, including
microgranuloma and giant cell infiltrations, were seen in each case.
Giant cells were seen clearly at the later stages, whereas
microgranulomas were more evident at the early stages. The presence of
the microgranulomas eight weeks after injection is suggestive of
foreign body microgranulomas.
To exclude hypersensitivity as the cause of the
microgranulomatous changes previously observed, groups of five
BALB/cA/nu/nu/SLC female nude mice were fed dietary racemic
fenvalerate at 1 000 and 3 000 ppm in the diet for four weeks.
Histopathological changes typical of the fenvalerate-induced
microgranuloma were observed in all of the treated mice. This study
indicates that granulomatous changes induced by fenvalerate are not
mediated by hypersensitivity reactions (Miyamoto et al., 1984).
More recently a dose-related incidence of hepatic microgranulomas
was found in groups of six male and six female beagles fed fenvalerate
at 0, 250, 500 and 1 000 ppm in the diet for six months. Histiocytic
cell infiltration of mesenteric lymph nodes also occurred in female
dogs fed 500 ppm and 1 000 ppm and in males fed 1 000 ppm.
Multinucleate cells were occasionally seen. The reversibility of these
effects was not studied (Parker et al., 1984).
Special Study on Mutagenicity
Fenvalerate has been shown to be without mutagenicity in
Salmonella typhimurium strains TA 100 and 98 and in V79 Chinese
hamster cells, with and without metabolic activation (Pluijmen
et al., 1984).
OBSERVATIONS IN HUMANS
A field study of 16 workers engaged in agricultural application
of fenvalerate associated exposure with cutaneous symptomatology.
Paraesthesia usually developed at exposed body sites after some hours
and the symptoms then progressed from a mild itch to a stinging
sensation and peaked with numbness. Sweating, exposure to sun or heat
or the application of water to the exposed site aggravated these
symptoms. Normal sensation returned with 24 h of cessation of exposure
(Tucker & Flannigan, 1983).
COMMENTS
The data required by the 1981 meeting have been received and
evaluated. The limited data provided on the tissue distribution of
fenvalerate confirm the rapid dissipation from fatty tissue,
indicating a minimal potential of the compound for bioaccumulation.
A series of elegant studies convincingly demonstrate the
relationship between th B alpha-isomer of fenvalerate and the
occurrence of the granulomata in mice, and of giant cell infiltration
in rats. The mechanism has been demonstrated to be a type of foreign
body response due to the deposition of crystals of the cholesterol
2-(4-chlorophenyl)-isovalerate ester in the tissues. Although no
dose-response relationship was determined, there is no reason to
question the previously established no-observable-effect levels in
rodents.
A recent study in dogs indicates that similar microgranulomatous
lesions are formed in this species following fenvalerate
administration in the diet. There is no reason to doubt that the
mechanism involved in the formation of these lesions is similar to
that described for the formation of similar lesions in the rodents.
Limited mutagenicity studies were negative.
Observations in humans are limited to field exposure and indicate
that fenvalerate causes similar cutaneous sensations to those induced
by other synthetic pyrethroids, possibly as a result of local effects.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Mouse: 30 ppm in the diet, equivalent to 3.5 mg/kg bw
Rat: 150 ppm in the diet, equivalent to 7.5 mg/kg bw
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.02 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1986)
1. Submission of carcinogenicity studies with fenvalerate
commissioned by IARC and NTP.
2. Determination of the no-effect level in dog with respect to
granulomata formation and full details of the recently published
six-month feeding study of fenvalerate in dogs.
Desirable
Observations in humans.
REFERENCES
Marei A.E. - S.M., Ruzo, L.O. & Casida J.E. Analysis and persistence
1982 of permethrin, cypermethrin deltamethrin and fenvalerate in
the fat and brain of treated rats. J. Agric. Food Chem.,
30: 558-562.
Parker, C.M. et al. Six-month feeding study of fenvalerate in dogs.
1984 Fundam. Appl. Toxicol., 4: 577.
Pluijmen M. et al. Lack of mutagenicity of synthetic pyrethroids in
1984 Salmonella typhimurium strains and in V79 Chinese hamster
cells. Mutat. Res., 137: 7-15.
Tucker, S.B. & Flannigan S.A. Cutaneous effects from occupational
1983 exposure to fenvalerate. Arch. Toxicol., 54: 195-202.
Miyamoto, J., Matsuo, M., Okuno, Y. & Kaneko, H. Studies on formation
1984 of microgranulomatous lesions including giant cell
infiltration found in mice and rats treated with
fenvalerate. Laboratory of Biochemistry and Toxicology,
Takarazuka Research Centre Technical Report AT-40-0370
submitted by Sumitomo Chemical Co. Ltd., to WHO.
(Unpublished)
