
PESTICIDE RESIDUES IN FOOD - 1979
Sponsored jointly by FAO and WHO
EVALUATIONS 1979
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Geneva, 3-12 December 1979
TRIADIMEFON
IDENTITY
Chemical Name: 1-(4-chlorophenoxy)-3,3-dimethyl-
1-(1,2,4-triazol-1-yl) butan-2-one
Synonyms: Bayleton(R)
MEB 6447
Structural formula: C14H16C1N3O2
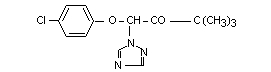 Other Information on identity and properties
Molecular weight: 293.7
Appearance: Colourless crystals
Melting point: 82.3°C (pure active ingredient)
Vapour pressure: <10-6 mbar at 20°C
approx. 2 × 10 -5 mbar at 400C
Solubility: in water 0.007 (pure a.i.)
(g.a.i./100 g solvent in cyclohexanone 60 - 120
at approx. 20°C) in isopropanol 20 - 40
in ligroin (80-110°C) 0 - 1
in methylene chloride >120
in toluene 40 -60
Minimum degree of purity: 90%
Stability: Stable in 0.1 n H2SO4) for 24 hrs
Stable in 0.1 n NaOH ) at 20°C
Impurities in the technical material
Detailed information on the impurities in technical triadimefon was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Triadimefon is a new systemic broad-spectrum fungicide against plant
pathogens, especially powdery mildew, loose smuts and rusts of cereals
and other crops. Its biochemical mechanism of fungicidal effect is
believed to be founded on the inhibition of ergosterine synthesis
(Buchenauer, 1975 and 1976). It has been found to be taken up by
plants from roots as well as through foliar surfaces and to be
transported mostly with transpiration stream. A certain translocation
downwards through the plants, however, is also measurable. Evidence
is also given for a certain vapour phase activity, which is likely to
support greenhouse applications. It is used for the control of
fungicidal diseases in several fruits and vegetables, in clover,
cereals, ornamentals, etc. without observed signs of phytotoxicity.
Triadimefon is registered and marketed for uses in agriculture and
horticulture in a number of countries among which about 25 were
reported to the meeting, representing most of the major agricultural
regions of Africa, America, Asia, Australia and Europe.
Pre-harvest treatments
Triadimefon is used in various formulations including wettable powders
(5 or 25%), emulsifiable concentrates (250 g per litre), dust (0.5%)
or paste (2%) in pre-harvest use patterns and at rates shown in Table
1. Especially promise seems recently to be attached to the use of
triadimefon in the control of mildew on cereal plants growing under
temperate climatic conditions.
Table 1. Some uses of Triadimefon formulations
Crop Pest to be Recommended conc./
controlled dosage
Small fruits mildew 0.0025-0.0% or 0.05-0.15 kg/ha
Strawberry mildew 75 - 100 g/ha
Vegetables mildew 0.0025-0.0125% (field)
0.0015-0.002% (glass house)
100 - 200 g/ha (dusting
Cereal crops mildew 1 - 2 × 125 g/ha
rust 1 × 250 g/ha
Coffee rust 100 - 250 g/ha (protective)
250 - 500 g/ha (eradicative)
Table 1. Continued...
Crop Pest to be Recommended conc./
controlled dosage
Pome fruits mildew 2 × 0.008 - 0.01% (after flowering)
rust or 0.04 - 0.075 kg/ha
Mango mildew 0.01%
or 0.1 - 0.2 kg/ha
Grape mildew 0.0025%
or 0.1 kg/ha (dust)
Tobacco mildew 0.0025 - 0.005%
or 0.04-0.075 kg/ha
Post-harvest uses
No official recommendations have yet been reported for post-harvest
uses of triadimefon, although some experimental trials indicate
potential uses on pineapples.
RESIDUES FROM SUPERVISED TRIALS
Data on triadimefon residues resulting from extensive trials
representing recommended use patterns have been presented by the
originating manufacturer (Bayer AG, Leverkusen). The data includes
controlled field experiments under a variety of geographical and
climatic conditions, as well as examples of greenhouse trials. The
data are presented in the following tables, and were obtained by gas
chromatographic methodology which determines the parent compound,
triadimefon, and its reduced, first metabolite, called triadimenol
(see below under "Rate of Residues"). Results are presented as the
sum of these two compounds, or in certain illustrative examples,
individually.
Fruit and Vegetables
The data in Table 2 summarize results from supervised trials from
various experimental sites in Germany (F.R.), and some other
countries. About a dozen different food crops of commercial interest
are represented in the data, reflecting residue levels which can be
expected after uses in accordance with "good agricultural practice".
In several cases the residue data comprise triadimefon levels as well
as contents of the triadimenol metabolite. Generally, the triadimenol
metabolite is found in levels which are comparable to the parent
compound, but possibly with a slightly greater persistence.
At the time of harvest and following recommended preharvest intervals,
measurable residues in the order of 0.2 - 0.4 mg/kg are found in
paprika and tomato and up to about 1.5 mg/kg in grapes. In hops
(harvested 10 days after treatment and measured after drying) total
residues are significantly higher, i.e. from n.d. up to 8.1 mg/kg (in
one individual sample 12.9 mg/kg). Levels of triadimefon in most of
the remaining examples are generally at or below 0.2 mg/kg.
Cereal plants
Residues of triadimefon, including triadimenol have been determined in
extensive trials on growing cereal plants, e.g. barley, oat and wheat,
with the results which are shown in Table 3. Significant residues are
found in all plants, heads as well as straw parts, and in all
experiments from the tine of last application and during the following
weeks. At the time of harvest, i.e. 6 weeks after last treatment,
residues are still persistent in straw at levels of 2 mg/kg or below.
In one occasional sampling is found 8.2 mg/kg after high dosage
treatment. Separate determination of triadimefon and triadimenol
shows that the majority of residue is in the converted triadimenol
form already at day 14, as shown for one trial in Table 3.
Grains at the time of harvest show no detectable residues in these
experiments.
Pineapples (post-harvest treatment)
Residues in pineapples following a few experimental postharvest
dippings or surface applications are shown in Table 4. A penetration
of triadimefon is hardly noticeable in these results, insofar as only
low residue levels are found in the fruit flesh in comparison with the
contents in peel. A significant conversion, however, of triadimefon
to triadimenol has obviously taken place between dipping and the time
of analyses.
Coffee and tobacco
Trials on residues in beans and shells of coffee and in tobacco leaves
are shown in Table 5. Residues in tobacco leaves vary from 0.7 to
8.5 mg/kg when analysed from 10-28 days after last treatment, and
residues are practically fully converted to triadimenol.
Must and wine after processing
The possible transfer of residues of triadimefon, including
triadimenol from harvested grapes to must and to processed wine is
shown in Table 6. A 50% level may be found in the must when related
to the content in grapes.
Table 2. Residues of Triadimefon from Supervised Trials
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Apple 12 × 0.04 kg/ha 0 <0.06-0.2 FRG
4 <0.06-0.2
7 <0.06-0.15
14 <0.06-0.15
21 n.d.-0.15
28 n.d.-0.2
6 × 0.05 kg/ha 14 n.d.-n.d. n.d.-0.06 FRG
21 n.d.-n.d. n.d.-n.d.
12 × 0.05 kg/ha 0 0.1-0.2 FRG
4 <0.06-0.1
7 <0.06-0.1
14 <0.06-0.1
21 n.d.-0.1
Cucumber 8-1O × 0.07 kg/ha 0 <0.06-0.1 FRG
7 n.d.-0.15
10 n.d.-0.1
Cucumber 4 × 0.075 kg/ha 0 <0.06-0.006 NL
indoor 7 n.d.-n.d.
10 n.d.-n.d.
Currant 4 × 0.2 kg/ha 0 0.65-3.35 FRG
red 4 0.15-0.85
7 0.15-1.05
10 0.1-0.7
14 <0.006-0.65
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Grape 10 × 0.0625 kg/ha 0 0.5 - 1.75 FRG
14 0.15-1.25
21 0.1-1.65
28 0.03-1.4
35 0.03-0.95
10 × 0.05 kg/ha 0 0.8 - 1.0 0.15 - 0.2 FRG
14 0.5 - 0.55 0.07-0.15
21 0.45-0.3 0.1 - 0.1
28 0.25-0.3 0.06-0.07
35 0.25-0.15
38 0.02-0.06
6 × 0.1. kg/ha 0 0.24-0.25 SA
3 0.08-0.09
7 0.03-0.04
14 0.02-0.02
Hops 4 × 0.5-1.0 kg/ha 10 days n.d.-2.6
dried 10 days 2.5-8.11
10 days 2.0-12.9
Melons 8 × 0.03-0.06 kg/ha 0 n.d.-0.25 F
1 n.d.-0.05
4 n.d.-0.15
7 n.d.-0.1
10 n.d.-0.08
2-4 × 0.0375 kg/ha 0 <0.01 <0.02 Jap.
1 <0.01-<0.01 <0.02-<0.02
5 <0.01-<0.01 <0.02-<0.02
Onions 2-3 × 0.0875 kg/ha 10 days <0.02-<0.02 <0.04-<0.04 Jap.
21 days <0.02-<0.02 <0.04-0.04
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Paprika 6 × 0.125 kg/ha 0 0.25-0.4 It.
1 0.15-0.3
4 0.1-0.2
7 0.15-0.25
10 0.08-0.25
Peas (without 2 × 0.03 kg/ha 0 <0.1 SA
pod) 3 <0.1
7 <0.1
14 <0.1
Pumpkin 1 x× 12.5 g per 100/l. 0 0.05-0.05 SA
3 0.01-0.02
5 <0.01-<0.01
Strawberry 3-4x×0.075/0.1 kg/ha 0 0.09-0.15 n.d.-0.06 FRG
1 0.07-0.11 0.04-0.06
4 0.05-0.07 0.05-0.08
7 0.05-0.07 0.07-0.12
10 0.03-0.03 0.05-0.0.8
14 0.02-0.025 0.04-0.10
Sugarbeets 3 x1× 0.125 kg/ha 0 n.d.-n.d. n.d.-n.d. FRG
roots 14 n.d.-n.d. n.d.-n.d.
21 n.d.-n.d. n.d.-n.d.
Sugarbeets 3 x1× 0.125 kg/ha 0 1.21-2.33 0.36-1.10 FRG
leaves 14 n.d.-0.82 n.d.-0.37
21 n.d.-n.d. n.d.-n.d.
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Tomato 2-4 x× 0.075 kg/ha 1 0.03-0.10 <0.02-0.10 Jap.
indoor 5 0.02-0.04 0.04-0.10
10 <0.01-0.02 0.04-0.06
Tomato 6 x× 0.075/0.15 kg/ha 0 0.2-0.4 FRG
1 0.15-0.25
4 0.1-0.1
7 0.1-0.25
11 0.07-0.2
* FRG: Germany (Federal Republic of Germany)
F: France
It: Italy
Jap: Japan
NL: Netherlands
SA : South Africa
Table 3. Residues in cereal crops after triadimefon treatments (FRG)
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Barley 1 × 0.125 kg/ha 0 2.7-3.15 2.6-3.1
(summer) 14 0.11-0.3 0.3-0.45
28 n.d.-0.15 0.2-0.25
35 n.d.-n.d. 0.3-0.4
42 n.d.-n.d. 0.4-0.4
Barley 1 × 0.125 kg/ha 0 4.1-5.2
(S. Africa) 4 1.0-1.2
7 0.7-0.7
14 0.2-0.2
40 n.d.-n.d.
Oats 1 × 0.125 kg/ha 0 0.95-3.85 1.8
14 n.d.-0.3 0.2-0.6
28 n.d.-0.1 n.d.-0.2
35 n.d.-0.2 n.d.-0.15
42 n.d.-n.d. n.d.-0.4
Wheat 1 × 0.125 kg/ha 0 1.2-9.4 1.1-10.45
(summer) 14 0.15-0.95 0.4-1.85
28 <0.05-0.75 0.15-0.75
35 n.d.-0.15 n.d.-1.05
42 n.d.-n.d. <0.15-0.6
2 × 0.125 kg/ha 0 1.15-2.0 2.5-5.0
14 0.2-0.35 1.15-3.55
28 0.1-0.45 0.5-0.9
35 n.d.-0.45 0.1-1.6
42 n.d.-n.d. 0.2-1.1
Table 3. Continued...
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Wheat 1 × 0.25 kg/ha 0 5.0-18.1 3.45-8.55
(summer) 14 0.8-2.35 1.4-3.35
28 0.15-1.3 0.25-1.15
35 n.d.-n.d. 0.65-1.0
42 n.d.-n.d. 0.55-1.85
1 × 0.125 kg/ha 0 6.5-8.6 <0.1-0.71 1.76-4.6 <0.1-0.35
+ 1 × 0.25 kg/ha 14 0.09-0.48 0.13-0.28 0.05-0.19 0.19-0.72
28 n.d.-0.07 n.d.-0.18 n.d.-n.d. n.d.-0.33
35 n.d.-n.d. n.d.-0.20 n.d.-n.d. n.d.-0.44
42 n.d.-n.d. n.d. - n.d. n.d.-n.d. n.d.-0.52
Wheat 1 × 0.125/0.25 kg/ha 0 2.5-3.85 1.3-3.8
(winter) 14 0.2-0.8 0.3-2.1
28 n.d.-0.8 0.4-2.7
35 n.d.-0.95 0.45-3.0
42 n.d.-n.d. 0.85-2.4
2 × 0.25 kg/ha 0 3.1-4.75 5.9-9.95
14 0.4-1.05 1.65-2.75
28 n.d.-1.75 0.45-8.65
35 n.d.-2.55 0.55-6.85
42 n.d.-n.d. 0.45-8.2
* Harvest at day 42 in all experiments
Table 4. Residues from post-harvest treatments of pineapple with
Triadimefon (Bayer AG)
Concentration/ Days after Fruit Residues (mg/kg)
Treatment treatment part Triadimefon Triadimenol
50 ppm/surface 13 days Fruit n.d. n.d.
application Peel 0.05 n.d.
Total <0.03 n.d.
100 ppm/Dipping 13 days Fruit <0.03 <0.06
Peel 1.1
(0.96-1.23) 1.6(1.5-1.7)
Total 0.23 0.33
750 ppm/Dipping 13 days Fruit 0.11 0.06
Peel 6.4 2.6
(6.28-6.5) (2.4-2.8)
Total 1.37 0.55
Table 5. Triadimefon Residues on Coffee and Tobacco (Bayer AG)
Days after Residues (mg/kg)
Crop Application last
treatment Triadimefon Triadimenol TOTAL
Coffee
(Brazil) 3 × 0.25 kg/tree 51 n.d. n.d.
(or 3 × 0.025 kg/ha)(whole bean)
15 × 0.37 kg/tree 32 (beans) - - n.d.
(shell) - - 2.9
8 × 0.75 kg/tree 89 (beans) - - <0.1
(shell) - - 0.45
Tobacco 2 × 0.06/0.09 kg/ha 14 <0.04 7.89-8.48 -
(Japan) 28 <0.04 2.09-2.13 -
2 × 0.075 kg/ha 10 <0.04 2.92 -
23 <0.04 0.70 -
Table 6. Triadimefon residues during wine processing in FRG
Residues (mg/kg)1
1976 1977
Grapes 0.25 - 1.4 0.03 - 0.06
Must 0.02 - 0.65 <0.04 - 0.06
Wine n.d. - 0.45* n.d. - n.d.
1 Sum of triadimefon and triadimenol after GLC-analysis.
* An exceptionally high residue. (average below 0.1 mg/kg).
Foods of animal origin
In feeding experiments with cows, pigs, sheep and poultry, feed was
given containing 56 or 180 mg/kg of triadimefon or triadimenol, and
levels of residues were determined in potential food products at 1 or
8 days after the termination of experimental feedings. Residues
resulting from these experimental feedings are shown in Tables 7 and
8.
The feeding levels are significantly higher than can be expected in
actual feeding practices. However, it is seen that residues from
feeding with triadimefon only occasionally are found in meat from
poultry, sheep and pig, and residues are not detectable in egg or milk
products. After feeding with the reduced metabolite triadimenol
however, residues are found in abdominal fat samples of most animals
at levels of 0.4 to about 7 mg/kg. In egg or milk, including milk
fat, residues are present only at or below the limit of determination.
Residues measured as triadimenol were regularly found in offals from
the slaughtered sheep, pig and poultry. In all cases the triadimenol
metabolite was practically only found irrespective of which of the two
compounds were originally present in the feed.
Table 7. Residues of Triadimefon* in animal products after experimental feeding for 1 week
(Bayer Australia 1978)
Days Residues (mg/kg)
Feeding after Meat Fat Offals
Animal rate feeding
Triadimefon Triadimenol Triadimefon Triadimenol Triadimefon Triadimenol
Feeding of Parent compound, Triadimefon:
Poultry 180 ppm 1 day <0.05 <0.08 <0.25 trace <0.05 2.4
8 days <0.05 <0.08 <0.25 <0.4 <0.05 1.7
Sheep 56 ppm 1 day trace trace <0.2 0.4 <0.1 1.9
8 days <0.1 <0.1 <0.2 0.4 <0.1 1.9
Pig 180 ppm 1 day trace trace <0.65 trace trace 0.2
8 days <0.1 <0.2 <0.65 1.1 <0.1 0.2
Feeding of metabolite, Triadimenol:
Poultry 180 ppm 1 day - <0.08 - trace - 2.1
8 days - <0.08 - <0.4 - 1.8
Sheep 56 ppm 1 day - <0.1 - 2.2 - 3.4
8 days - trace 1.1 (?) 1.1 - 6.5
Pig 180 ppm 1 day - <0.1 - 0.4 - 0.4
8 days - 1.0 - 7.1 - 3.8
* Determined by GLC-analysis of Triadimefon and Triadimenol separately.
Table 8. Residues of Triadimefon *) in milk and eggs after
experimental feeding for one week (Bayer Australia, 1978)
Residues (mg/kg)
Feeding
Product level Triadimefon Triadimenol
After feeding the parent compound, Triadimefon:
Milk, skimmed 168 ppm <0.1 - <0.1 <0.1 - <0.1
Milk fat 168 ppm <0.4 - <0.4 <0.5 - <0.5
Hen's egg 180 ppm <0.1 - <0.1 n.d.- n.d.
After feeding the metabolite, Triadimenol:
Milk, skimmed 168 ppm - <0.1 - <0.1
Milk fat 168 ppm - <0.5 - <0.5
Hen's egg 180 ppm - <0.2 - <0.2
* Determined by GLC-analysis of Triadimefon and Triadimenol
separately.
FATE OF RESIDUES
General remarks
Degradation pathways and metabolite patterns of triadimefon in various
media including soil, microorganisms, water, plants, animals and under
exposure to light have been subject to many investigations from which
illustrative summaries are presented in Figure 1 and Table 9.
The principal metabolite of triadimefon (I) is already mentioned
above,namely triadimenol (III) obtained by reduction of the >C=O
group to >HCOH configuration. Triadimenol is fungicidally active and
seems to give promise as a commercial fungicide in its own rights.
Indications are present that systemic properties of triadimefon may be
ascribed to processes of uptake in the form of triadimefon combined
with translocation of the metabolite, which is then considered to be
the predominant active principle against fungal pests.
Triadimenol occurs in two diastereoisomeric forms, called A and B,
which are determined separately in metabolic studies. Among other
identified metabolites are mentioned cleavage products containing the
triazole- or benzene-rings, i.a. 4-chlorophenol (VIII) from plant
metabolic studies. In animals, hydroxyl-(V,VI) and carboxyl-compounds
(VII) are found, which represent non-disrupted molecule structures,
and which may occur in free forms or as glucoronides.
Other Information on identity and properties
Molecular weight: 293.7
Appearance: Colourless crystals
Melting point: 82.3°C (pure active ingredient)
Vapour pressure: <10-6 mbar at 20°C
approx. 2 × 10 -5 mbar at 400C
Solubility: in water 0.007 (pure a.i.)
(g.a.i./100 g solvent in cyclohexanone 60 - 120
at approx. 20°C) in isopropanol 20 - 40
in ligroin (80-110°C) 0 - 1
in methylene chloride >120
in toluene 40 -60
Minimum degree of purity: 90%
Stability: Stable in 0.1 n H2SO4) for 24 hrs
Stable in 0.1 n NaOH ) at 20°C
Impurities in the technical material
Detailed information on the impurities in technical triadimefon was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Triadimefon is a new systemic broad-spectrum fungicide against plant
pathogens, especially powdery mildew, loose smuts and rusts of cereals
and other crops. Its biochemical mechanism of fungicidal effect is
believed to be founded on the inhibition of ergosterine synthesis
(Buchenauer, 1975 and 1976). It has been found to be taken up by
plants from roots as well as through foliar surfaces and to be
transported mostly with transpiration stream. A certain translocation
downwards through the plants, however, is also measurable. Evidence
is also given for a certain vapour phase activity, which is likely to
support greenhouse applications. It is used for the control of
fungicidal diseases in several fruits and vegetables, in clover,
cereals, ornamentals, etc. without observed signs of phytotoxicity.
Triadimefon is registered and marketed for uses in agriculture and
horticulture in a number of countries among which about 25 were
reported to the meeting, representing most of the major agricultural
regions of Africa, America, Asia, Australia and Europe.
Pre-harvest treatments
Triadimefon is used in various formulations including wettable powders
(5 or 25%), emulsifiable concentrates (250 g per litre), dust (0.5%)
or paste (2%) in pre-harvest use patterns and at rates shown in Table
1. Especially promise seems recently to be attached to the use of
triadimefon in the control of mildew on cereal plants growing under
temperate climatic conditions.
Table 1. Some uses of Triadimefon formulations
Crop Pest to be Recommended conc./
controlled dosage
Small fruits mildew 0.0025-0.0% or 0.05-0.15 kg/ha
Strawberry mildew 75 - 100 g/ha
Vegetables mildew 0.0025-0.0125% (field)
0.0015-0.002% (glass house)
100 - 200 g/ha (dusting
Cereal crops mildew 1 - 2 × 125 g/ha
rust 1 × 250 g/ha
Coffee rust 100 - 250 g/ha (protective)
250 - 500 g/ha (eradicative)
Table 1. Continued...
Crop Pest to be Recommended conc./
controlled dosage
Pome fruits mildew 2 × 0.008 - 0.01% (after flowering)
rust or 0.04 - 0.075 kg/ha
Mango mildew 0.01%
or 0.1 - 0.2 kg/ha
Grape mildew 0.0025%
or 0.1 kg/ha (dust)
Tobacco mildew 0.0025 - 0.005%
or 0.04-0.075 kg/ha
Post-harvest uses
No official recommendations have yet been reported for post-harvest
uses of triadimefon, although some experimental trials indicate
potential uses on pineapples.
RESIDUES FROM SUPERVISED TRIALS
Data on triadimefon residues resulting from extensive trials
representing recommended use patterns have been presented by the
originating manufacturer (Bayer AG, Leverkusen). The data includes
controlled field experiments under a variety of geographical and
climatic conditions, as well as examples of greenhouse trials. The
data are presented in the following tables, and were obtained by gas
chromatographic methodology which determines the parent compound,
triadimefon, and its reduced, first metabolite, called triadimenol
(see below under "Rate of Residues"). Results are presented as the
sum of these two compounds, or in certain illustrative examples,
individually.
Fruit and Vegetables
The data in Table 2 summarize results from supervised trials from
various experimental sites in Germany (F.R.), and some other
countries. About a dozen different food crops of commercial interest
are represented in the data, reflecting residue levels which can be
expected after uses in accordance with "good agricultural practice".
In several cases the residue data comprise triadimefon levels as well
as contents of the triadimenol metabolite. Generally, the triadimenol
metabolite is found in levels which are comparable to the parent
compound, but possibly with a slightly greater persistence.
At the time of harvest and following recommended preharvest intervals,
measurable residues in the order of 0.2 - 0.4 mg/kg are found in
paprika and tomato and up to about 1.5 mg/kg in grapes. In hops
(harvested 10 days after treatment and measured after drying) total
residues are significantly higher, i.e. from n.d. up to 8.1 mg/kg (in
one individual sample 12.9 mg/kg). Levels of triadimefon in most of
the remaining examples are generally at or below 0.2 mg/kg.
Cereal plants
Residues of triadimefon, including triadimenol have been determined in
extensive trials on growing cereal plants, e.g. barley, oat and wheat,
with the results which are shown in Table 3. Significant residues are
found in all plants, heads as well as straw parts, and in all
experiments from the tine of last application and during the following
weeks. At the time of harvest, i.e. 6 weeks after last treatment,
residues are still persistent in straw at levels of 2 mg/kg or below.
In one occasional sampling is found 8.2 mg/kg after high dosage
treatment. Separate determination of triadimefon and triadimenol
shows that the majority of residue is in the converted triadimenol
form already at day 14, as shown for one trial in Table 3.
Grains at the time of harvest show no detectable residues in these
experiments.
Pineapples (post-harvest treatment)
Residues in pineapples following a few experimental postharvest
dippings or surface applications are shown in Table 4. A penetration
of triadimefon is hardly noticeable in these results, insofar as only
low residue levels are found in the fruit flesh in comparison with the
contents in peel. A significant conversion, however, of triadimefon
to triadimenol has obviously taken place between dipping and the time
of analyses.
Coffee and tobacco
Trials on residues in beans and shells of coffee and in tobacco leaves
are shown in Table 5. Residues in tobacco leaves vary from 0.7 to
8.5 mg/kg when analysed from 10-28 days after last treatment, and
residues are practically fully converted to triadimenol.
Must and wine after processing
The possible transfer of residues of triadimefon, including
triadimenol from harvested grapes to must and to processed wine is
shown in Table 6. A 50% level may be found in the must when related
to the content in grapes.
Table 2. Residues of Triadimefon from Supervised Trials
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Apple 12 × 0.04 kg/ha 0 <0.06-0.2 FRG
4 <0.06-0.2
7 <0.06-0.15
14 <0.06-0.15
21 n.d.-0.15
28 n.d.-0.2
6 × 0.05 kg/ha 14 n.d.-n.d. n.d.-0.06 FRG
21 n.d.-n.d. n.d.-n.d.
12 × 0.05 kg/ha 0 0.1-0.2 FRG
4 <0.06-0.1
7 <0.06-0.1
14 <0.06-0.1
21 n.d.-0.1
Cucumber 8-1O × 0.07 kg/ha 0 <0.06-0.1 FRG
7 n.d.-0.15
10 n.d.-0.1
Cucumber 4 × 0.075 kg/ha 0 <0.06-0.006 NL
indoor 7 n.d.-n.d.
10 n.d.-n.d.
Currant 4 × 0.2 kg/ha 0 0.65-3.35 FRG
red 4 0.15-0.85
7 0.15-1.05
10 0.1-0.7
14 <0.006-0.65
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Grape 10 × 0.0625 kg/ha 0 0.5 - 1.75 FRG
14 0.15-1.25
21 0.1-1.65
28 0.03-1.4
35 0.03-0.95
10 × 0.05 kg/ha 0 0.8 - 1.0 0.15 - 0.2 FRG
14 0.5 - 0.55 0.07-0.15
21 0.45-0.3 0.1 - 0.1
28 0.25-0.3 0.06-0.07
35 0.25-0.15
38 0.02-0.06
6 × 0.1. kg/ha 0 0.24-0.25 SA
3 0.08-0.09
7 0.03-0.04
14 0.02-0.02
Hops 4 × 0.5-1.0 kg/ha 10 days n.d.-2.6
dried 10 days 2.5-8.11
10 days 2.0-12.9
Melons 8 × 0.03-0.06 kg/ha 0 n.d.-0.25 F
1 n.d.-0.05
4 n.d.-0.15
7 n.d.-0.1
10 n.d.-0.08
2-4 × 0.0375 kg/ha 0 <0.01 <0.02 Jap.
1 <0.01-<0.01 <0.02-<0.02
5 <0.01-<0.01 <0.02-<0.02
Onions 2-3 × 0.0875 kg/ha 10 days <0.02-<0.02 <0.04-<0.04 Jap.
21 days <0.02-<0.02 <0.04-0.04
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Paprika 6 × 0.125 kg/ha 0 0.25-0.4 It.
1 0.15-0.3
4 0.1-0.2
7 0.15-0.25
10 0.08-0.25
Peas (without 2 × 0.03 kg/ha 0 <0.1 SA
pod) 3 <0.1
7 <0.1
14 <0.1
Pumpkin 1 x× 12.5 g per 100/l. 0 0.05-0.05 SA
3 0.01-0.02
5 <0.01-<0.01
Strawberry 3-4x×0.075/0.1 kg/ha 0 0.09-0.15 n.d.-0.06 FRG
1 0.07-0.11 0.04-0.06
4 0.05-0.07 0.05-0.08
7 0.05-0.07 0.07-0.12
10 0.03-0.03 0.05-0.0.8
14 0.02-0.025 0.04-0.10
Sugarbeets 3 x1× 0.125 kg/ha 0 n.d.-n.d. n.d.-n.d. FRG
roots 14 n.d.-n.d. n.d.-n.d.
21 n.d.-n.d. n.d.-n.d.
Sugarbeets 3 x1× 0.125 kg/ha 0 1.21-2.33 0.36-1.10 FRG
leaves 14 n.d.-0.82 n.d.-0.37
21 n.d.-n.d. n.d.-n.d.
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Tomato 2-4 x× 0.075 kg/ha 1 0.03-0.10 <0.02-0.10 Jap.
indoor 5 0.02-0.04 0.04-0.10
10 <0.01-0.02 0.04-0.06
Tomato 6 x× 0.075/0.15 kg/ha 0 0.2-0.4 FRG
1 0.15-0.25
4 0.1-0.1
7 0.1-0.25
11 0.07-0.2
* FRG: Germany (Federal Republic of Germany)
F: France
It: Italy
Jap: Japan
NL: Netherlands
SA : South Africa
Table 3. Residues in cereal crops after triadimefon treatments (FRG)
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Barley 1 × 0.125 kg/ha 0 2.7-3.15 2.6-3.1
(summer) 14 0.11-0.3 0.3-0.45
28 n.d.-0.15 0.2-0.25
35 n.d.-n.d. 0.3-0.4
42 n.d.-n.d. 0.4-0.4
Barley 1 × 0.125 kg/ha 0 4.1-5.2
(S. Africa) 4 1.0-1.2
7 0.7-0.7
14 0.2-0.2
40 n.d.-n.d.
Oats 1 × 0.125 kg/ha 0 0.95-3.85 1.8
14 n.d.-0.3 0.2-0.6
28 n.d.-0.1 n.d.-0.2
35 n.d.-0.2 n.d.-0.15
42 n.d.-n.d. n.d.-0.4
Wheat 1 × 0.125 kg/ha 0 1.2-9.4 1.1-10.45
(summer) 14 0.15-0.95 0.4-1.85
28 <0.05-0.75 0.15-0.75
35 n.d.-0.15 n.d.-1.05
42 n.d.-n.d. <0.15-0.6
2 × 0.125 kg/ha 0 1.15-2.0 2.5-5.0
14 0.2-0.35 1.15-3.55
28 0.1-0.45 0.5-0.9
35 n.d.-0.45 0.1-1.6
42 n.d.-n.d. 0.2-1.1
Table 3. Continued...
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Wheat 1 × 0.25 kg/ha 0 5.0-18.1 3.45-8.55
(summer) 14 0.8-2.35 1.4-3.35
28 0.15-1.3 0.25-1.15
35 n.d.-n.d. 0.65-1.0
42 n.d.-n.d. 0.55-1.85
1 × 0.125 kg/ha 0 6.5-8.6 <0.1-0.71 1.76-4.6 <0.1-0.35
+ 1 × 0.25 kg/ha 14 0.09-0.48 0.13-0.28 0.05-0.19 0.19-0.72
28 n.d.-0.07 n.d.-0.18 n.d.-n.d. n.d.-0.33
35 n.d.-n.d. n.d.-0.20 n.d.-n.d. n.d.-0.44
42 n.d.-n.d. n.d. - n.d. n.d.-n.d. n.d.-0.52
Wheat 1 × 0.125/0.25 kg/ha 0 2.5-3.85 1.3-3.8
(winter) 14 0.2-0.8 0.3-2.1
28 n.d.-0.8 0.4-2.7
35 n.d.-0.95 0.45-3.0
42 n.d.-n.d. 0.85-2.4
2 × 0.25 kg/ha 0 3.1-4.75 5.9-9.95
14 0.4-1.05 1.65-2.75
28 n.d.-1.75 0.45-8.65
35 n.d.-2.55 0.55-6.85
42 n.d.-n.d. 0.45-8.2
* Harvest at day 42 in all experiments
Table 4. Residues from post-harvest treatments of pineapple with
Triadimefon (Bayer AG)
Concentration/ Days after Fruit Residues (mg/kg)
Treatment treatment part Triadimefon Triadimenol
50 ppm/surface 13 days Fruit n.d. n.d.
application Peel 0.05 n.d.
Total <0.03 n.d.
100 ppm/Dipping 13 days Fruit <0.03 <0.06
Peel 1.1
(0.96-1.23) 1.6(1.5-1.7)
Total 0.23 0.33
750 ppm/Dipping 13 days Fruit 0.11 0.06
Peel 6.4 2.6
(6.28-6.5) (2.4-2.8)
Total 1.37 0.55
Table 5. Triadimefon Residues on Coffee and Tobacco (Bayer AG)
Days after Residues (mg/kg)
Crop Application last
treatment Triadimefon Triadimenol TOTAL
Coffee
(Brazil) 3 × 0.25 kg/tree 51 n.d. n.d.
(or 3 × 0.025 kg/ha)(whole bean)
15 × 0.37 kg/tree 32 (beans) - - n.d.
(shell) - - 2.9
8 × 0.75 kg/tree 89 (beans) - - <0.1
(shell) - - 0.45
Tobacco 2 × 0.06/0.09 kg/ha 14 <0.04 7.89-8.48 -
(Japan) 28 <0.04 2.09-2.13 -
2 × 0.075 kg/ha 10 <0.04 2.92 -
23 <0.04 0.70 -
Table 6. Triadimefon residues during wine processing in FRG
Residues (mg/kg)1
1976 1977
Grapes 0.25 - 1.4 0.03 - 0.06
Must 0.02 - 0.65 <0.04 - 0.06
Wine n.d. - 0.45* n.d. - n.d.
1 Sum of triadimefon and triadimenol after GLC-analysis.
* An exceptionally high residue. (average below 0.1 mg/kg).
Foods of animal origin
In feeding experiments with cows, pigs, sheep and poultry, feed was
given containing 56 or 180 mg/kg of triadimefon or triadimenol, and
levels of residues were determined in potential food products at 1 or
8 days after the termination of experimental feedings. Residues
resulting from these experimental feedings are shown in Tables 7 and
8.
The feeding levels are significantly higher than can be expected in
actual feeding practices. However, it is seen that residues from
feeding with triadimefon only occasionally are found in meat from
poultry, sheep and pig, and residues are not detectable in egg or milk
products. After feeding with the reduced metabolite triadimenol
however, residues are found in abdominal fat samples of most animals
at levels of 0.4 to about 7 mg/kg. In egg or milk, including milk
fat, residues are present only at or below the limit of determination.
Residues measured as triadimenol were regularly found in offals from
the slaughtered sheep, pig and poultry. In all cases the triadimenol
metabolite was practically only found irrespective of which of the two
compounds were originally present in the feed.
Table 7. Residues of Triadimefon* in animal products after experimental feeding for 1 week
(Bayer Australia 1978)
Days Residues (mg/kg)
Feeding after Meat Fat Offals
Animal rate feeding
Triadimefon Triadimenol Triadimefon Triadimenol Triadimefon Triadimenol
Feeding of Parent compound, Triadimefon:
Poultry 180 ppm 1 day <0.05 <0.08 <0.25 trace <0.05 2.4
8 days <0.05 <0.08 <0.25 <0.4 <0.05 1.7
Sheep 56 ppm 1 day trace trace <0.2 0.4 <0.1 1.9
8 days <0.1 <0.1 <0.2 0.4 <0.1 1.9
Pig 180 ppm 1 day trace trace <0.65 trace trace 0.2
8 days <0.1 <0.2 <0.65 1.1 <0.1 0.2
Feeding of metabolite, Triadimenol:
Poultry 180 ppm 1 day - <0.08 - trace - 2.1
8 days - <0.08 - <0.4 - 1.8
Sheep 56 ppm 1 day - <0.1 - 2.2 - 3.4
8 days - trace 1.1 (?) 1.1 - 6.5
Pig 180 ppm 1 day - <0.1 - 0.4 - 0.4
8 days - 1.0 - 7.1 - 3.8
* Determined by GLC-analysis of Triadimefon and Triadimenol separately.
Table 8. Residues of Triadimefon *) in milk and eggs after
experimental feeding for one week (Bayer Australia, 1978)
Residues (mg/kg)
Feeding
Product level Triadimefon Triadimenol
After feeding the parent compound, Triadimefon:
Milk, skimmed 168 ppm <0.1 - <0.1 <0.1 - <0.1
Milk fat 168 ppm <0.4 - <0.4 <0.5 - <0.5
Hen's egg 180 ppm <0.1 - <0.1 n.d.- n.d.
After feeding the metabolite, Triadimenol:
Milk, skimmed 168 ppm - <0.1 - <0.1
Milk fat 168 ppm - <0.5 - <0.5
Hen's egg 180 ppm - <0.2 - <0.2
* Determined by GLC-analysis of Triadimefon and Triadimenol
separately.
FATE OF RESIDUES
General remarks
Degradation pathways and metabolite patterns of triadimefon in various
media including soil, microorganisms, water, plants, animals and under
exposure to light have been subject to many investigations from which
illustrative summaries are presented in Figure 1 and Table 9.
The principal metabolite of triadimefon (I) is already mentioned
above,namely triadimenol (III) obtained by reduction of the >C=O
group to >HCOH configuration. Triadimenol is fungicidally active and
seems to give promise as a commercial fungicide in its own rights.
Indications are present that systemic properties of triadimefon may be
ascribed to processes of uptake in the form of triadimefon combined
with translocation of the metabolite, which is then considered to be
the predominant active principle against fungal pests.
Triadimenol occurs in two diastereoisomeric forms, called A and B,
which are determined separately in metabolic studies. Among other
identified metabolites are mentioned cleavage products containing the
triazole- or benzene-rings, i.a. 4-chlorophenol (VIII) from plant
metabolic studies. In animals, hydroxyl-(V,VI) and carboxyl-compounds
(VII) are found, which represent non-disrupted molecule structures,
and which may occur in free forms or as glucoronides.
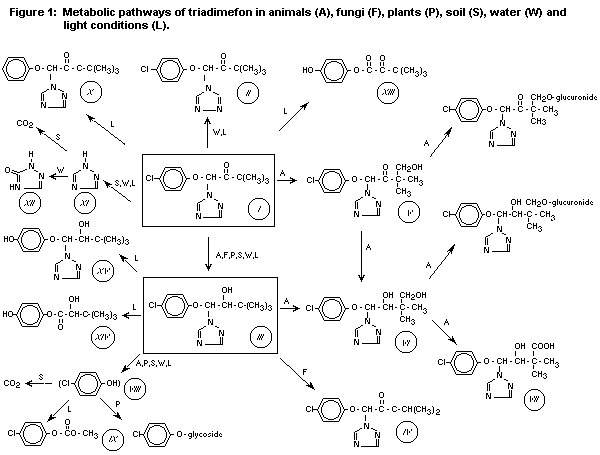 Table 9. Triadimefon and hitherto identified metabolites and their occurrence.
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
Formula
Table 9. Triadimefon and hitherto identified metabolites and their occurrence.
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
Formula  I 1-(4-chlorophenoxy)- Triadimefon X X X X X X
3,3-dimethyl-1-(1H (BAYLETON)
1,2,4-triazole-1-yl)-2
butanone
I 1-(4-chlorophenoxy)- Triadimefon X X X X X X
3,3-dimethyl-1-(1H (BAYLETON)
1,2,4-triazole-1-yl)-2
butanone
 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
II 1-(4-Chlorophenoxy)- symmetrical X
3,3-dimethyl-1-(4H- isomer of I
1,2,4-triazole-4-yl)-
2-butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
II 1-(4-Chlorophenoxy)- symmetrical X
3,3-dimethyl-1-(4H- isomer of I
1,2,4-triazole-4-yl)-
2-butanone
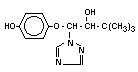
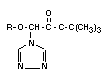 III 1-(4-chlorophenoxy)- Triadimenol X X X X X X
3,3-dimethyl-1- (BAYTAN)
(1H-1,2,4-triazole-1-yl)-
2-butanol
III 1-(4-chlorophenoxy)- Triadimenol X X X X X X
3,3-dimethyl-1- (BAYTAN)
(1H-1,2,4-triazole-1-yl)-
2-butanol
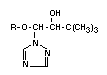 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
IV 1-(4-chlorophenoxy)- isopropyl- X
3-methyl-1-(1H- analogue of I
1,2,4-triazole-1-yl)
2-butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
IV 1-(4-chlorophenoxy)- isopropyl- X
3-methyl-1-(1H- analogue of I
1,2,4-triazole-1-yl)
2-butanone
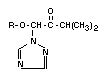 V 1-(4-chlorophenoxy)- KWG 1323 X
3-hydroxymethyl-3-
methyl-1-(1H-1,2,4-
triazole-1-yl)-2-butanone
V 1-(4-chlorophenoxy)- KWG 1323 X
3-hydroxymethyl-3-
methyl-1-(1H-1,2,4-
triazole-1-yl)-2-butanone
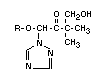 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VI 4-(4-chlorophenoxy)- KWG 1342 X
2,2-dimethyl-4-(1H-
1,2,4-triazole-1-yl)-
1,3-butandiole
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VI 4-(4-chlorophenoxy)- KWG 1342 X
2,2-dimethyl-4-(1H-
1,2,4-triazole-1-yl)-
1,3-butandiole
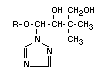 VII 4-(4-chlorophenoxy)- Triadimenol X
3-hydroxy-2,2-dimethyl- acid
4-(1H-1,2,4-triazole-
1-yl)-butane acid
VII 4-(4-chlorophenoxy)- Triadimenol X
3-hydroxy-2,2-dimethyl- acid
4-(1H-1,2,4-triazole-
1-yl)-butane acid
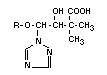 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VIII 4-chlorophenol - X X X X X
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VIII 4-chlorophenol - X X X X X
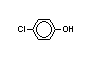 IX 4-chlorophenyl- X
methyl-carbonate -
IX 4-chlorophenyl- X
methyl-carbonate -
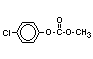 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
X 3,3-dimethyl-l - X
phenoxy-l-(1H-1,2,4
triazole-1-yl)-2-
butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
X 3,3-dimethyl-l - X
phenoxy-l-(1H-1,2,4
triazole-1-yl)-2-
butanone
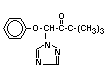 XI 1H-1,2,4-triazole 1,2,4-Triazole X X X
XI 1H-1,2,4-triazole 1,2,4-Triazole X X X
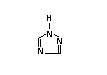 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XII Delta2-1,2,4-triazolin- - X
5-one
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XII Delta2-1,2,4-triazolin- - X
5-one
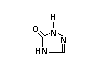 XIII 4-Hydroxyphenyl-2-oxo- X
3,3-dimethyl-butane acid
ester
XIII 4-Hydroxyphenyl-2-oxo- X
3,3-dimethyl-butane acid
ester
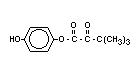 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XIV 4-Hydroxyphenyl-2-hydroxy- - X
3,3-dimethyl-butane acid
ester
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XIV 4-Hydroxyphenyl-2-hydroxy- - X
3,3-dimethyl-butane acid
ester
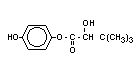 XV 4-Hydroxyphenyl-3,3-di - X
methyl-1-(1H-1,2,4-triazole
1-yl)-2-butanol
XV 4-Hydroxyphenyl-3,3-di - X
methyl-1-(1H-1,2,4-triazole
1-yl)-2-butanol
 Fate in soil
Adsorption and desorption studies determining the Freundlich isotherm
characteristics have been made on triadimefon as well as on
triadimenol in different soils and in different concentrations (Puhl
and Hurley, 1978 a and b, Vogeler, 1978, Frohberger, 1978). The
results of such studies correlate well with laboratory leaching
experiments in standardised procedures (BBA-Merkblatt no. 37) and
TLC-mobility tests from which triadimefon can be classified as a
compound of low mobility in a variety of soil types (Obrist and
Thornton, 1977b, Thornton et al., 1976).
No triadimefon was detected in leachates in the standardised test and
only limited amounts of radiolabelled residues could be determined in
soil layers below 2.5 cm in a balance study on treatment of spring
barley with 14C-labelled triadimefon if measurements were made from
76-427 days after application (Steffens & Wieneke, 1977). In the
leaching studies of Obrist and Thornton (1977b) less than 3% of the
total radioactivity could be traced through 30 cm of aged soil after a
period of 45 days with simulated rain of 12.5 mm per day. None of
this activity was identified as intact triadimefon or as triadimenol.
Half-life determinations in two standard soils gave RL50-values of 60
and 100 days for triadimefon and of 130 and 310 days for triadimenol
(Bayer-reports Nos. 9012/1974 and 9050/1976).
The disappearance rates of triadimefon and triadimenol have been
studied by several groups in German, Japanese and U.S. trials under a
variety of conditions and using a broad range of different soil types
(Mango and Puhl, 1977a; Takase & Yashimoto, 1978; and reported from
Bayer AG and Mobay Chem. Corporation). Variations in RL50-values are
found from about 10 days to 7 months for triadimefon and from about 3
months to 500 days for triadimenol. In summarising such data it is
justified to characterize triadimefon, and especially triadimenol, as
persistent compounds in soil, although it is presently impossible to
evaluate which specific environmental conditions (e.g. soil type,
temperature, etc.) are of greatest significance for the variability in
persistence. Residue levels of triadimefon have been determined in
soil after several years of treatment in cereal fields and apple
orchards (Bayer reports, 1977). The residues consisted mainly of
triadimenol which reached plateau levels (0-10 cm layer) from 0.13
mg/kg to 0.65 mg/kg in the 5th years of trials in apple orchards.
Residue uptake from soil, including crop rotation
Uptake of triadimefon and triadimenol from soil was established in
experiments with barley (Frohberger, 1978) and cloverplants (Fisher
et al., 1979). In the latter study a linear relationship between
amount absorbed and the amount of triadimefon applied to the soil was
found. The uptake of soil residues by later crops have been studied
by Steffen and Wieneke (1977), who used turnip and clover as catch
crops followed by sugarbeet, oats or winter wheat as the main crops in
lysimeters which had originally been treated with radio-labelled
triadimefon on cultures of spring barley. 0.2% of soil residues was
absorbed by the catch crops, while 0.5 - 4.3% was found in the
following main crops. It is assumed from these experiments that
mainly triadimenol is absorbed from the soil, and subsequently
metabolised to polar compounds in the plants.
Soil metabolism
The metabolic conversions and degradation of triadimefon in soil have
been studied by Vogeler (1976a, 1977a and b), Mango & Puhl (1977a) and
Takase & Yashimoto (1978). It is found that a primary reduction to
triadimenol takes place as a microbial reaction, and resulting into
two diastereoisomeric forms, A and B, in ratios which differ from
about 1:1 to 1:4 in the various experiments.
In radio-labelled experiments Mango and Puhl (1977) applied
triadimefon to soils using either benzene-ring or triazole-ring
14C-labelling. After 238 days they found 45-49% of radioactivity in
triadimenol, 7% in triazole and 24% or 5% of 14CO2 from the two ring
systems, respectively, indicating a complete, although gradual and
relatively slow degradation of triadimefon. After application on
cereals, Vogeler in his experiments (using benzene-ring labelling)
found 15-25% and 32-65% of soil radioactivity to be non-extractable.
Fate in microorganisms
As already mentioned, the conversion in soil of triadimefon to
triadimenol under natural environmental conditions is considered to be
a microbial reaction (Mango & Puhl, 1977). This is confirmed in
laboratory shake cultures of Aspergillus niger, which may convert
from 5-32% of triadimefon to triadimenol in 2-5 days, respectively.
No further metabolism of triadimenol was detected in these
experiments. In other experiments (Clark et al., 1978), triadimefon
was incubated with mycelial mats of the fungus, and a different
metabolic pattern was observed as indicated by the findings of an
isopropyl analogue (IV) to triadimefon in the culture by mass
spectrometric analysis. And, in experiments by Pither (1979),
combined bacterial or fungal cultures during 13 days had no detectable
effects on triadimefon, leaving 99% of the parent compound intact in
the cultures.
Fate in water
In sterile aqueous buffer solutions, triadimefon is found to be stable
for many weeks at temperatures ranging from 25°C - 45°C and at pH 3,
6, and 9 (Obrist and Thornton, 1976). In a simulated pond
environment, however, a fast degradation was found and metabolites
identified in the following order: triadimenol (III), triadimefon
symmetrical isomer (II), 1,2,4,triazole (XI) and
Delta2-1-2-4-triazolin-5-one (XII) (Obrist et al., 1977a). 30% of
the 14C activity (from benzene-ring labelling) was missing at the end
of the experiment, possibly under formation of 14CO2. The
disappearance rate for the parent compound in these experiments
corresponded to half-lives of 6-8 days in the water phase and 18-20
days in the silt.
Fate in plants
An uptake of triadimefon from soil to plants, e.g. to beans and barley
(Buchenauer, 1975 and 1976) and through foliar surfaces to beans,
cereals, cucumbers and tomato followed by translocation with the
transpiration stream (i.e. acropetally) has been demonstrated in
several studies (Steffens & Führ, 1976; Scheinpflug et al., 1977b;
Brandes et al., 1978; Für et al., 1978; Gasztony & Josepovits,
1978). A slight translocation in the opposite direction, i.e.
basipetal movement, is observed also (cfr. Buchenauer, 1975 and 1976).
A transfer of triadimefon through the vapour phase with a measurable
fungicidal effect and evidence of metabolites in untreated plants is
described in studies from greenhouses (Scheinpflug & Paul, 1977a;
Schlüter, 1977; Scheinpflug et al., 1978; Kraus, 1979) and in closed
glass chambers (Vogeler & Paul, 1976b). Such vapour phase effects are
dependant on the distance between target plant and spray deposits.
As is the case for soil, there is much experimental evidence that
triadimefon is reduced in plants to triadimenol, which is the major
metabolite (up to 80%) and which occurs in the two diastereoisomeric
forms, A and B. Contrasting the situation in soil, however, the
isomer form A is dominating as indicated by the ratios of A:B varying
from 1:1 to 3:1. Besides triadimenol, polar substances (Rf values of
0) and a compound with the Rf value of 0.26 (presumably compound VI
in Table 9) were found.
Results from experimental determinations of various 14C labelled
metabolites and fractions in cereal plants (benzene-ring label) and in
apples (triazole-ring label) are shown in Table 10.
Table 10. Percent distribution of radioactivity in cereals and apples after spray application of 14C-Triadimefon
(recovered radioactivity = 100) (Vogeler, 1976, 1977, 1978; Mango et al., 1977)
EXTRACTABLES
Plant Dose Days
(I = Indoor (a.i.) post RF 0 RF 0.26+ unidentified Triadimenol A Triadimenol B Triadimefon non
F = Field) appl. metabolite extractables
barley (I)
total plant 0 n.d. n.d. n.d. n.d. n.d. 99.5 0.5
" " 5 7.3 n.d. n.d. 57.3 21.8 9.7 3.9
" " 10 15.8 n.d. n.d. 50.3 18.9 7.9 7.1
" " 250 g/ha 28 15.8 n.d. n.d. 45.1 17.4 6.4 15.3
straw 38 15.9 n.d. n.d. 43.6 14.4 10.3 15.8
" 62 16.7 1.9 n.d. 41.0 17.3 4.8 18.3
barley (F)
total plant 0 <1 n.d. n.d. 1 n.d. 92 6
" " 5 13 n.d. n.d. 48 15 12 12
" " 125 g/ha 17 35 n.d. n.d. 32 10 <1 23
straw 28 9 <1 n.d. 12 4 n.d. 75
" 76 8.4 5 n.d. 8.4 3 n.d. 75
wheat (F)
straw 250 g/ha 47 37 2 n.d. 23 6 n.d. 32
apples (F)
peel 28 5.7* n.d. 2.8 14.9 13.6 10.8 5.0
pulp 5.3* n.d. 3.1 11.2 7.1 2.1 0.9
total fruit 15 mg/100ml 11.0* n.d. 5.9 26.1 20.7 13.0 5.9
peel 48 4.8* n.d. 1.5 14.5 14.8 10.4 6.0
pulp 6.4* n.d. 2.1 10.4 9.4 2.1 1.0
total fruit 11.2* n.d. 3.6 24.9 24.2 12.5 7.0
+ presumably compound VI, table 9.
n.d. = not detectable * aqueous phase
The amount of radioactivity in grains and ears was too small for separation by TLC.
FATE IN ANIMALS
Mammals
Following administration of a single oral dose of 14C-triadimefon of
25 mg per kg b.w. to rats, it was found that 75 - 83% was excreted
within 7 days, with 30-40% in the urine and 35-53% in the faeces.
Expiration of 14CO2 was not found (Fredrickson, 1978a). In cows as
well as in pigs (Fredrickson, 1978b; Pither, 1978), 84-91% of
triadimefon doses of 0.14 or 5 mg/kg, respectively, was excreted with
the urine within 48-72 hours after oral administration. Less than 6%
was excreted in faeces.
Evidently, triadimefon is readily absorbed by the animals and
subsequently metabolised in the animal organism in the transitional
period before excretion. Analytical determination of the distribution
between tissues, fat and organs have been made on rats and cows
(Fredrickson, 1978 a and b), pigs (Pither, 1978) and poultry (Nye,
1979).
Results from these studies are summarised in Table 11. This table
shows the peak levels which are found shortly after oral
administration of triadimefon at the dosages which are indicated, and
the remaining residues found after 4-7 days of post-treatment
elimination period.
The metabolic pattern of triadimefon has been followed in most of
these feeding studies with the unequivocal identification of the
metabolites V, VI and VII (cfr. Figure 1 and Table 9) in most tissues
and organs from rat, cow, pig and hen. These metabolites are formed
by stepwise oxidation of the tert. butyl group of triadimefon into
primary alcohol and to carboxylic acid that are eliminated with the
urine, in which they are identified in free forms or as conjugates of
glucoronic acid together with other and unidentified polar
metabolites. As part of these studies it has been found that milk
contained 0.003 mg/kg triadimefon after 5 daily doses of 0.14 mg/kg.
Eggs from hens which had been given one dose of 2.4 mg/kg showed 0.12
mg/kg after 24 hours and 0.04 mg/kg after 96 hours.
Fish
The accumulation and persistence of residues was studied in Channel
catfish (Ictalurus punctatus) under continuous exposure to
radio-labelled triadimefon for a 28-day period at concentrations of
approximately 10 and 100 ppb (µg/l) in water (Lamb and Roney, 1977).
Concentration factors of 7.6 and 6.5, resp. were determined from the
two levels. After transfer to uncontaminated water 88% of the
accumulated 14C residues were excreted by the fish within 5 hours and
approximately 96% within the following 7-10 days.
Table 11. Residues in animal tissues after oral administration of triadimefon.
Days × Residues (mg/kg)*
dose (mg/ Time after Reference
Animal kg b.w.) administr. Muscle Fat Liver Kidney Egg
approx. approx.
Rat 1 × 25 2 - 8 hrs 4.5-8.0 43.5-45.0 26 - 29 11 - 17 Fredrickson
7 days 0.01-0.02 0.02-0.09 0.10-0.14 0.02-0.08 1978a
Cow 1 × 10 1 - 2 hrs. 0.36 4.01 3.65 15.0 Fredrickson
5 × 0.14 1 - 2 hrs. n.d. n.d. 0.08 0.05 1978b
Pig 5 × 5 3 hrs. 0.30 1.0 3.1 4.0 Pither, 1978
Hen 1 × 2.4 6 hrs. 0.12 0.3 0.26 1.18 0.12 Wye, 1979
96 hrs. <0.01 <0.01 <0.01 <0.01 0.04
* Sum of triadimefon and triadimenol after GLC-analysis
Photodegradation
The pattern of photodecomposition of triadimefon and of triadimenol
have been studied in a number of individual tests by exposure to
UV-light of the compounds in solution (or suspension) or on a solid
support, e.g. silicagel plate or silty clay loam soil surfaces
(Vogeler, 1975; Clark et al., 1978; Wilmes, 1978; Nicholas et al.,
1977; Obrist and Thornton, 1978; Takase and Hasebe, 1979). Several of
the reaction products after such exposures are shown in Figure 1 and
Table 9, from which it is evident that the abiotic molecular breakdown
differs considerably from the patterns already described in plant and
animals. Among compounds which are identified in several of the tests
are especially mentioned 1,2,4-triazole (XI), 4-chlorophenol (VIII)
and 4-chlorophenyl methyl carbonate (IX). Also mentioned is the
formation of high molecular polymers as well as fully degraded 14CO2
as the result experimental irradiation.
As has been found in most of the other media, triadimenol proves to be
more stable to photodegradation than triadimefon. An experimental
half-life of 10-12 hours for the latter under specified light
conditions (in water) compares with 36 hours for the triadimenol.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information was reported to the meeting.
METHODS OF RESIDUES ANALYSIS
Residues of triadimefon and its primary metabolite, triadimenol, which
is also a fungicidal chemical, can be measured by gas chromatographic
multiresidue methodology developed by Specht (1977) and modified by
others (Thornton et al., 1977; Thornton and Lloyd, 1977). In
principle this method is based on extraction of the fungicides with
acetone or methanol with addition of a specified amount of water,
followed by partitioning between acetone-water and dichloromethane.
After evaporation of the dichloromethane, the extract is
chromatographed on a Florisil column with 6% (v/v) ethyl ether in
petroleum and 40% (v/v) ethyl acetate in petroleum ether. The second
eluate is concentrated and gas chromatographed using an AFID
(nitrogen-specific) detector.
In this analysis triadimefon, and the two diastereoisomeric forms, A
and B of triadimenol are determined separately. The method is
applicable for the analysis of various plant materials and soils for
which it has been validated and found to have lower limits of
determination of 0.03 mg/kg (triadimefon) and 0.06 mg/kg (triadimenol)
in soil and in plant material containing more than 80% of water, and
0.05 mg/kg (triadimefon) and 0.1-0.15 mg/kg (triadimenol) in grain and
straw of cereals. Recoveries are found as 89-98% in green plants,
80-99% in straw, and 90-99% in grains. Various fruit and vegetables
are quoted for recoveries in ranges from 80-100%.
NATIONAL LIMITS REPORTED TO THE MEETING
National limits and associated pre-harvest intervals which have been
reported to the meeting are shown in Table 12.
APPRAISAL
Triadimefon is a systemic fungicide used against mildew, rust and
other fungi on cereals, vegetables and fruit crops as well as
ornamentals. It is marketed in many countries in formulations of
wettable powders, emulsifiable concentrates, dusts or pastes. It is
often used in foliar spraying programmes with repeated applications at
low dosages. Information on the impurities in technical triadimefon
was reported to the meeting.
It is probable that the systemic properties of triadimefon arise from
uptake of the parent compound through root or plant surfaces, followed
by reduction to and translocation of the major metabolite,
triadimenol, which presumably is the carrier of the fungicidal
activity. Such processes start shortly after application of
triadimefon to plants so that triadimenol dominates the residue
constituent at the end of normal pre-harvest waiting periods. Polar
metabolites, including 4-chlorophenol, as conjugates have been
identified as further metabolites in plant material.
At the time of harvest and following recommended preharvest intervals,
measurable residues up to about 2.0 mg/kg may be found in various
crops, whilst for various other crops the residues are at or below
limit of determination. Whilst the meeting was prepared to recommend
limits for crops in the latter cases, it was considered that the
experimental data for hops, coffee and tobacco, as well as for
pineapples after post-harvest treatments were insufficient. More
information on actual use patterns and related residue data should be
made available to enable recommendations to be made concerning those
crops.
Table 12. National Limits and Preharvest intervals Reported to the
Meeting
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Austria Cereals 1 35
Beets 0.2 35
Grape, pome fruit 1 35
Cucumber, tomato, 1 4
paprika
Belgium Wheat, barley 42
Table 12. Continued...
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Brazil Wheat 0.01 42
Denmark Cereals 28
Pome fruit 14
France Apple 1 7
Melon 1 3
Germany Cereals 35
(F.R.) Cucumber 3
Apple 14
Grape 35
Israel Wheat 42
Netherlands Wheat, barley 42
N. Zealand Apple 49
Cereals 49
Grapes 28
Norway Apple, strawberry 14
Cereals, berries 28
S. Africa Apple 0.05
Barley 0.1 40
Cucumber 0.05 3
Grape 0.05 7
Mango 0.05
Yugoslavia Cereals 35
Cucumber 7
Other crops 21
Metabolism and degradation in animals, soil, microorganisms and water
as well as patterns of abiotic photodecomposition of triadimefon have
been studied extensively, and detailed lists of possible metabolites
and breakdown products are available. Triadimefon and especially
triadimenol are eliminated from tissues of livestock at relatively
fast rates, resulting in residues at or below limit of determination
within one or a few days in meat, milk and egg-products. In fat and
in offals from slaughtered animals, however, significant residues of
triadimenol may be obtained.
Convenient multi-residue methodology based on gas chromatography is
available for the determination of triadimefon and triadimenol in food
and food products.
RECOMMENDATIONS
As no ADI had been allocated the meeting proposed the following
Guideline Level on a basis of taking into account the preharvest
intervals indicated. The GLs refer to the sum of the parent compound
triadimefon and its major metabolite, triadimenol.
Guideline Pre-harvest interval on
Commodity Level(mg/kg) which recommendation is based
Apples 0.2 14 days
Cucumbers (indoor
and outdoor) 0.2 3 days
Currants, red 1 14 days
Eggs 0.1*
Grapes 2 28 days
Melons 0.5 4 days
Milk 0.1*
Onions, spring 0.1* 21 days
Paprika 0.5 3 days
Peas, without pods 0.1* 3 days
Pumpkins 0.1* 3 days
Strawberries 0.2 7 days
Sugar beets, roots 0.1* 21 days
Sugar beets, leaves 0.1* 21 days
Tomatoes, (indoor
and outdoor 0.5 3 days
Grain (barley,
oats, wheat) 0.1* 42 days
Straw (barley,
oat, wheat) 2 42 days
(* At or about the limit of determination)
FURTHER WORK OR INFORMATION
Desirable
1. Feeding studies on livestock animals for establishing
dose-response relationship between feed contents and residues in
animals tissues, especially fat, and including realistic feeding
levels;
2. Further information on residue levels in certain products, such as
coffee and hops and in pineapples after appropriate post harvest
treatments.
REFERENCES
Bayer AG. Various reports on triadimefon residues in fruit,
vegetables and soil, as well as from animal experiments. Bayer AG,
Leverkusen, FRG. (1975/79), Unpublished.
Brandes, W., Steffens, W., Führ, F., Scheinpflug, H. - Weitere
Untersuchungen zur Translokation von 14C-Triadimefon in
Gurkenpflanezen. Pflanzenschutz Nachrichten Bayer 31, 132-144.
Mobay Reports. Various unpublished reports prepared by the Research
and Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, U.S.A.
Buchenauer, H. - Systemisch-fungizide Wirkung und Wirkungsmechanismus
von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-Forstwirtsch.
(Berlin Dahlem) 165, 154-155.
Untorsuchungen zur systemischen Wirkung und zum Einfluss von
RBAYLETON (Triadimefon) auf verschiedene pilzliche
Getreidekrankheiten. Pflanzenschutz-Nachrichten Bayer 29, 267-280.
Clark, T., Clifford, D.R., Deas, A., Gendle, P., Watkins, D.
Photolysis, metabolism and other factors influencing the performance
of triadimefon as a powdery mildew fungicide. Pest. Sci. 9, 497-506.
Fisher, D.J., Pickard, J.A., McKenzie, C.M. - Uptake of the systemic
fungicide triadimefon by clover and its effect on symbiotic nitrogen
fixation. Pest. Sci. 10, 75-82.
Fredrickson, D.R. - Metabolism of BAYLETONTM in Rats. Mobay Report
No. 66 201, June 20, (1978a).
Metabolism of BAYLETONTM in the cow. Mobay Report No. 66, 202,
June 20, (1978b).
Frohberger, P.E. - Zur Frage der Biologischen Aktivität von
BAYLETON-Rück-standen im Boden. BAYER AG.
Pflanzenschutz-Anwendungstechnik, unpublished report April 12, 1978.
Für, F., Paul, V., Steffens, W. and Scheinpflug, H. Translokation von
14C-Triadimefon nach Applikation auf Sommergerste und sein Wirkung
gegen Erysiphe Graminis var. hordei. Pflanzenschutz-Nachrichten Bayer
31, 116-131.
Gasztony, M., Josepovits, G. - Translocation and metabolism of
triadifflefon in different plant species. Acta Phytopathologica
Academiae Scientiarum Hungaricae, 13, 403-415.
Kraus, P. - Untersuchungen zur Wirkung von BAYLETON aus dem Boden über
die Gaphase gegen Erysiphe graminis an Gerste. BAYER AG,
Pflanzenschutz Answendungstechnik, unpublished report, February 8,
1979.
Lamb, D.W., Roney, D.J. - Accumulation and Persistence of Residues in
Channel Catfish Exposed to BAYLETON TM-14C. Mobay Report No. 52 775,
May 4, 1977.
Mango, R.R., Puhl, R.J. - The Aerobic and Anaerobic Soil Metabolism of
BAYLETONTM. Mobay Report No. 51 230, January 12, (1977a).
Mango, R.R., Puhl, R.J. and Thornton, J.S. - The Metabolism of
BAYLETON in Apples. Mobay Report No. 53 621, August 9, (1977b).
Nichols, S.S., Thornton, J.S., Publ, R.J. - Photodecomposition of
BAYLETONTM in Water solution. Mobay Report No. 52 688, April 6,
1977.
Nye, D.E. - The Fate of RBAYLETON-14C in Poultry. Mobay Report No.
67 482, March 8, 1979.
Obrist, J.J., Thornton, J.S. - Stability of BAYLETONTM in Sterile
Aqueous Buffer Solutions. Mobay Report No. 50 799, November 29, 1976.
Obrist, J.J., Publ, R.J. and Thornton, J.S. - The Degradation and Fate
of BAYLETONTM in a simulated pond environment. Mobay Report No. 53
038, May 31, (1977a).
Obrist, J.J. and Thornton, J.S. - Leaching characteristics of
BAYLETONTM on Aged soil. Mobay Report No. 51 232, January 21, 1977b.
Photodecomposition of RBAYTON in Aqueous solutions and on a soil
surface. Mobay Report No. 66 794, October 20, 1978.
Pither, K.M. - Metabolism of BAYLETONTM in Male and female pigs.
Mobay Report No. 66 509, November 29, 1978.
Degradation of RBAYLETON by Mixed cultures of microorganisms.
Mobay Report No. 67 049, January 30, 1979.
Puhl, R.H., Hurley, J.B. - Soil Adsorption and desorption of
BAYLETON-Ring UL14C. Mobay Report No. 66 501, September 11, (1978a).
Soil adsorption and desorption of BAYTON-II. Mobay Report No. 66
745, December 12, (1978b).
Scheinpflug, H. and Paul, V. - On the mode of action of Triadimefon.
Neth. J. Plant Path. 83, (Suppl.1), 105-111. (1978a).
Scheinpflug, H., Paul, V. and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Mitt. Biol.
Bundesanst. Land Forstwirtsch. (Berlin-Dahlem) 178, 178. (1978b).
Scheinpflug, H., Paul V., and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Pflanzenschutz -
Nachrichten Bayer 31, 101-115.
Schlüter, K. - Fungizide Wirkung von Triadimefon in der Gasphase. Z.
Pflanzenkr. Pflanzenschutz 84, 612-614.
Specht, W. - Gas Chromatographic method for determining residues of
the fungicides fuberidazol, fluotrimazole and triadimefon in plants
and soil. Pflanzenschutz-Nachrichten Bayer 30, 1.
Steffens, W., Führ, F. - Versuche zur Retranslokation von Triadimefon.
Kernforschung sanlage Jülich, Arbeitagruppe Radioagronomie,
unpublished report ARA 4/76. (1976).
Steffens, W., Wieneke, J,. - Bilanzversuche mit dem 14C-markierten
Fungizid Triadimefon (MEB 6447) nach Spritzbehandlung von
Sommergerste in Lysimetern mit ungesförten Bodenprofilen, Verbleib in
der behandelten Pflanze, Verlagerung im Boden und Aufnahme durch
Folgefrüchte. Kernforschungsanlage Jülich, Arbeitsgruppe
Radioagronomie, unpublished report ARA 4/77, September. (1977).
Takase, I., Hasebe, K. - Photolysis of Triadimefon in Upland Soils.
Nitokuno Report No. 1093, (RA), March 2, 1979.
Takase, I., Yoshimoto, Y. - Degradation of Triadimefon in Upland soils
under laboratory and field conditions. Nitokuno Report No. 1091 (RA),
December 11, 1978.
Thornton, J.S. - A gas chromatographic method for Bayleton and its
metabolite in apples. Mobay Report No. 54 166, December 30, 1977.
Thornton, J.S., Lloyd, C.M. - A gas chromatographic method for
Bayleton and its metabolite in soil and water. Mobay Report No. 51
231, January 31, 1977.
Thornton, J.S., Hurley, J.B., Obrist, J.J. - Soil thin-layer mobility
of Twenty-four pesticide chemicals. Mobay Report No. 51 0.16, December
15, 1976.
Vogeler, K. BAYER AG, Pflanzenschutz-Anwendungstechnik, unpublished
report. Verhalten von Triadimefon (MEB 6447) unter der Einwirkung von
UV-Licht. RA 364, June 3, 1975.
Der metabolismus von Triadimefon in gerste unter
Gewttchshausbedlingungen und im Boden. RA 272, April 12, (1976a).
Untersuchunge zur Wirkang von Triadimefon über die Gasphase gegen
Echten Mehltau an Gerste. RA 662, August 16, (1976b).
Bericht über die Metabolisierung von 14C-Triadimefon in
Sommergerste und Boden sowie in Folgenpflanzen. RA 813, October 19,
(1977a).
Untersuchungen zur Unwandlung der reinen Diastereoisomeren A und B
von KWG 0519 in das jeweils andere Isomere in Gerstenpflanzen und
Boden. RA 871, November 14, (1977b).
Adsorptionsisothermen von Tiadimenol in verschiedenen Böden. RA 496,
July 3, 1978.
Wilmes, R. - Versuche zum Photoabbau von MEB 6447, MEB 6449 und KWG
0519. BAYER AG, Pflanzenschutz-PIQ, PF-Report No. 1266, August 7,
1978.
Fate in soil
Adsorption and desorption studies determining the Freundlich isotherm
characteristics have been made on triadimefon as well as on
triadimenol in different soils and in different concentrations (Puhl
and Hurley, 1978 a and b, Vogeler, 1978, Frohberger, 1978). The
results of such studies correlate well with laboratory leaching
experiments in standardised procedures (BBA-Merkblatt no. 37) and
TLC-mobility tests from which triadimefon can be classified as a
compound of low mobility in a variety of soil types (Obrist and
Thornton, 1977b, Thornton et al., 1976).
No triadimefon was detected in leachates in the standardised test and
only limited amounts of radiolabelled residues could be determined in
soil layers below 2.5 cm in a balance study on treatment of spring
barley with 14C-labelled triadimefon if measurements were made from
76-427 days after application (Steffens & Wieneke, 1977). In the
leaching studies of Obrist and Thornton (1977b) less than 3% of the
total radioactivity could be traced through 30 cm of aged soil after a
period of 45 days with simulated rain of 12.5 mm per day. None of
this activity was identified as intact triadimefon or as triadimenol.
Half-life determinations in two standard soils gave RL50-values of 60
and 100 days for triadimefon and of 130 and 310 days for triadimenol
(Bayer-reports Nos. 9012/1974 and 9050/1976).
The disappearance rates of triadimefon and triadimenol have been
studied by several groups in German, Japanese and U.S. trials under a
variety of conditions and using a broad range of different soil types
(Mango and Puhl, 1977a; Takase & Yashimoto, 1978; and reported from
Bayer AG and Mobay Chem. Corporation). Variations in RL50-values are
found from about 10 days to 7 months for triadimefon and from about 3
months to 500 days for triadimenol. In summarising such data it is
justified to characterize triadimefon, and especially triadimenol, as
persistent compounds in soil, although it is presently impossible to
evaluate which specific environmental conditions (e.g. soil type,
temperature, etc.) are of greatest significance for the variability in
persistence. Residue levels of triadimefon have been determined in
soil after several years of treatment in cereal fields and apple
orchards (Bayer reports, 1977). The residues consisted mainly of
triadimenol which reached plateau levels (0-10 cm layer) from 0.13
mg/kg to 0.65 mg/kg in the 5th years of trials in apple orchards.
Residue uptake from soil, including crop rotation
Uptake of triadimefon and triadimenol from soil was established in
experiments with barley (Frohberger, 1978) and cloverplants (Fisher
et al., 1979). In the latter study a linear relationship between
amount absorbed and the amount of triadimefon applied to the soil was
found. The uptake of soil residues by later crops have been studied
by Steffen and Wieneke (1977), who used turnip and clover as catch
crops followed by sugarbeet, oats or winter wheat as the main crops in
lysimeters which had originally been treated with radio-labelled
triadimefon on cultures of spring barley. 0.2% of soil residues was
absorbed by the catch crops, while 0.5 - 4.3% was found in the
following main crops. It is assumed from these experiments that
mainly triadimenol is absorbed from the soil, and subsequently
metabolised to polar compounds in the plants.
Soil metabolism
The metabolic conversions and degradation of triadimefon in soil have
been studied by Vogeler (1976a, 1977a and b), Mango & Puhl (1977a) and
Takase & Yashimoto (1978). It is found that a primary reduction to
triadimenol takes place as a microbial reaction, and resulting into
two diastereoisomeric forms, A and B, in ratios which differ from
about 1:1 to 1:4 in the various experiments.
In radio-labelled experiments Mango and Puhl (1977) applied
triadimefon to soils using either benzene-ring or triazole-ring
14C-labelling. After 238 days they found 45-49% of radioactivity in
triadimenol, 7% in triazole and 24% or 5% of 14CO2 from the two ring
systems, respectively, indicating a complete, although gradual and
relatively slow degradation of triadimefon. After application on
cereals, Vogeler in his experiments (using benzene-ring labelling)
found 15-25% and 32-65% of soil radioactivity to be non-extractable.
Fate in microorganisms
As already mentioned, the conversion in soil of triadimefon to
triadimenol under natural environmental conditions is considered to be
a microbial reaction (Mango & Puhl, 1977). This is confirmed in
laboratory shake cultures of Aspergillus niger, which may convert
from 5-32% of triadimefon to triadimenol in 2-5 days, respectively.
No further metabolism of triadimenol was detected in these
experiments. In other experiments (Clark et al., 1978), triadimefon
was incubated with mycelial mats of the fungus, and a different
metabolic pattern was observed as indicated by the findings of an
isopropyl analogue (IV) to triadimefon in the culture by mass
spectrometric analysis. And, in experiments by Pither (1979),
combined bacterial or fungal cultures during 13 days had no detectable
effects on triadimefon, leaving 99% of the parent compound intact in
the cultures.
Fate in water
In sterile aqueous buffer solutions, triadimefon is found to be stable
for many weeks at temperatures ranging from 25°C - 45°C and at pH 3,
6, and 9 (Obrist and Thornton, 1976). In a simulated pond
environment, however, a fast degradation was found and metabolites
identified in the following order: triadimenol (III), triadimefon
symmetrical isomer (II), 1,2,4,triazole (XI) and
Delta2-1-2-4-triazolin-5-one (XII) (Obrist et al., 1977a). 30% of
the 14C activity (from benzene-ring labelling) was missing at the end
of the experiment, possibly under formation of 14CO2. The
disappearance rate for the parent compound in these experiments
corresponded to half-lives of 6-8 days in the water phase and 18-20
days in the silt.
Fate in plants
An uptake of triadimefon from soil to plants, e.g. to beans and barley
(Buchenauer, 1975 and 1976) and through foliar surfaces to beans,
cereals, cucumbers and tomato followed by translocation with the
transpiration stream (i.e. acropetally) has been demonstrated in
several studies (Steffens & Führ, 1976; Scheinpflug et al., 1977b;
Brandes et al., 1978; Für et al., 1978; Gasztony & Josepovits,
1978). A slight translocation in the opposite direction, i.e.
basipetal movement, is observed also (cfr. Buchenauer, 1975 and 1976).
A transfer of triadimefon through the vapour phase with a measurable
fungicidal effect and evidence of metabolites in untreated plants is
described in studies from greenhouses (Scheinpflug & Paul, 1977a;
Schlüter, 1977; Scheinpflug et al., 1978; Kraus, 1979) and in closed
glass chambers (Vogeler & Paul, 1976b). Such vapour phase effects are
dependant on the distance between target plant and spray deposits.
As is the case for soil, there is much experimental evidence that
triadimefon is reduced in plants to triadimenol, which is the major
metabolite (up to 80%) and which occurs in the two diastereoisomeric
forms, A and B. Contrasting the situation in soil, however, the
isomer form A is dominating as indicated by the ratios of A:B varying
from 1:1 to 3:1. Besides triadimenol, polar substances (Rf values of
0) and a compound with the Rf value of 0.26 (presumably compound VI
in Table 9) were found.
Results from experimental determinations of various 14C labelled
metabolites and fractions in cereal plants (benzene-ring label) and in
apples (triazole-ring label) are shown in Table 10.
Table 10. Percent distribution of radioactivity in cereals and apples after spray application of 14C-Triadimefon
(recovered radioactivity = 100) (Vogeler, 1976, 1977, 1978; Mango et al., 1977)
EXTRACTABLES
Plant Dose Days
(I = Indoor (a.i.) post RF 0 RF 0.26+ unidentified Triadimenol A Triadimenol B Triadimefon non
F = Field) appl. metabolite extractables
barley (I)
total plant 0 n.d. n.d. n.d. n.d. n.d. 99.5 0.5
" " 5 7.3 n.d. n.d. 57.3 21.8 9.7 3.9
" " 10 15.8 n.d. n.d. 50.3 18.9 7.9 7.1
" " 250 g/ha 28 15.8 n.d. n.d. 45.1 17.4 6.4 15.3
straw 38 15.9 n.d. n.d. 43.6 14.4 10.3 15.8
" 62 16.7 1.9 n.d. 41.0 17.3 4.8 18.3
barley (F)
total plant 0 <1 n.d. n.d. 1 n.d. 92 6
" " 5 13 n.d. n.d. 48 15 12 12
" " 125 g/ha 17 35 n.d. n.d. 32 10 <1 23
straw 28 9 <1 n.d. 12 4 n.d. 75
" 76 8.4 5 n.d. 8.4 3 n.d. 75
wheat (F)
straw 250 g/ha 47 37 2 n.d. 23 6 n.d. 32
apples (F)
peel 28 5.7* n.d. 2.8 14.9 13.6 10.8 5.0
pulp 5.3* n.d. 3.1 11.2 7.1 2.1 0.9
total fruit 15 mg/100ml 11.0* n.d. 5.9 26.1 20.7 13.0 5.9
peel 48 4.8* n.d. 1.5 14.5 14.8 10.4 6.0
pulp 6.4* n.d. 2.1 10.4 9.4 2.1 1.0
total fruit 11.2* n.d. 3.6 24.9 24.2 12.5 7.0
+ presumably compound VI, table 9.
n.d. = not detectable * aqueous phase
The amount of radioactivity in grains and ears was too small for separation by TLC.
FATE IN ANIMALS
Mammals
Following administration of a single oral dose of 14C-triadimefon of
25 mg per kg b.w. to rats, it was found that 75 - 83% was excreted
within 7 days, with 30-40% in the urine and 35-53% in the faeces.
Expiration of 14CO2 was not found (Fredrickson, 1978a). In cows as
well as in pigs (Fredrickson, 1978b; Pither, 1978), 84-91% of
triadimefon doses of 0.14 or 5 mg/kg, respectively, was excreted with
the urine within 48-72 hours after oral administration. Less than 6%
was excreted in faeces.
Evidently, triadimefon is readily absorbed by the animals and
subsequently metabolised in the animal organism in the transitional
period before excretion. Analytical determination of the distribution
between tissues, fat and organs have been made on rats and cows
(Fredrickson, 1978 a and b), pigs (Pither, 1978) and poultry (Nye,
1979).
Results from these studies are summarised in Table 11. This table
shows the peak levels which are found shortly after oral
administration of triadimefon at the dosages which are indicated, and
the remaining residues found after 4-7 days of post-treatment
elimination period.
The metabolic pattern of triadimefon has been followed in most of
these feeding studies with the unequivocal identification of the
metabolites V, VI and VII (cfr. Figure 1 and Table 9) in most tissues
and organs from rat, cow, pig and hen. These metabolites are formed
by stepwise oxidation of the tert. butyl group of triadimefon into
primary alcohol and to carboxylic acid that are eliminated with the
urine, in which they are identified in free forms or as conjugates of
glucoronic acid together with other and unidentified polar
metabolites. As part of these studies it has been found that milk
contained 0.003 mg/kg triadimefon after 5 daily doses of 0.14 mg/kg.
Eggs from hens which had been given one dose of 2.4 mg/kg showed 0.12
mg/kg after 24 hours and 0.04 mg/kg after 96 hours.
Fish
The accumulation and persistence of residues was studied in Channel
catfish (Ictalurus punctatus) under continuous exposure to
radio-labelled triadimefon for a 28-day period at concentrations of
approximately 10 and 100 ppb (µg/l) in water (Lamb and Roney, 1977).
Concentration factors of 7.6 and 6.5, resp. were determined from the
two levels. After transfer to uncontaminated water 88% of the
accumulated 14C residues were excreted by the fish within 5 hours and
approximately 96% within the following 7-10 days.
Table 11. Residues in animal tissues after oral administration of triadimefon.
Days × Residues (mg/kg)*
dose (mg/ Time after Reference
Animal kg b.w.) administr. Muscle Fat Liver Kidney Egg
approx. approx.
Rat 1 × 25 2 - 8 hrs 4.5-8.0 43.5-45.0 26 - 29 11 - 17 Fredrickson
7 days 0.01-0.02 0.02-0.09 0.10-0.14 0.02-0.08 1978a
Cow 1 × 10 1 - 2 hrs. 0.36 4.01 3.65 15.0 Fredrickson
5 × 0.14 1 - 2 hrs. n.d. n.d. 0.08 0.05 1978b
Pig 5 × 5 3 hrs. 0.30 1.0 3.1 4.0 Pither, 1978
Hen 1 × 2.4 6 hrs. 0.12 0.3 0.26 1.18 0.12 Wye, 1979
96 hrs. <0.01 <0.01 <0.01 <0.01 0.04
* Sum of triadimefon and triadimenol after GLC-analysis
Photodegradation
The pattern of photodecomposition of triadimefon and of triadimenol
have been studied in a number of individual tests by exposure to
UV-light of the compounds in solution (or suspension) or on a solid
support, e.g. silicagel plate or silty clay loam soil surfaces
(Vogeler, 1975; Clark et al., 1978; Wilmes, 1978; Nicholas et al.,
1977; Obrist and Thornton, 1978; Takase and Hasebe, 1979). Several of
the reaction products after such exposures are shown in Figure 1 and
Table 9, from which it is evident that the abiotic molecular breakdown
differs considerably from the patterns already described in plant and
animals. Among compounds which are identified in several of the tests
are especially mentioned 1,2,4-triazole (XI), 4-chlorophenol (VIII)
and 4-chlorophenyl methyl carbonate (IX). Also mentioned is the
formation of high molecular polymers as well as fully degraded 14CO2
as the result experimental irradiation.
As has been found in most of the other media, triadimenol proves to be
more stable to photodegradation than triadimefon. An experimental
half-life of 10-12 hours for the latter under specified light
conditions (in water) compares with 36 hours for the triadimenol.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information was reported to the meeting.
METHODS OF RESIDUES ANALYSIS
Residues of triadimefon and its primary metabolite, triadimenol, which
is also a fungicidal chemical, can be measured by gas chromatographic
multiresidue methodology developed by Specht (1977) and modified by
others (Thornton et al., 1977; Thornton and Lloyd, 1977). In
principle this method is based on extraction of the fungicides with
acetone or methanol with addition of a specified amount of water,
followed by partitioning between acetone-water and dichloromethane.
After evaporation of the dichloromethane, the extract is
chromatographed on a Florisil column with 6% (v/v) ethyl ether in
petroleum and 40% (v/v) ethyl acetate in petroleum ether. The second
eluate is concentrated and gas chromatographed using an AFID
(nitrogen-specific) detector.
In this analysis triadimefon, and the two diastereoisomeric forms, A
and B of triadimenol are determined separately. The method is
applicable for the analysis of various plant materials and soils for
which it has been validated and found to have lower limits of
determination of 0.03 mg/kg (triadimefon) and 0.06 mg/kg (triadimenol)
in soil and in plant material containing more than 80% of water, and
0.05 mg/kg (triadimefon) and 0.1-0.15 mg/kg (triadimenol) in grain and
straw of cereals. Recoveries are found as 89-98% in green plants,
80-99% in straw, and 90-99% in grains. Various fruit and vegetables
are quoted for recoveries in ranges from 80-100%.
NATIONAL LIMITS REPORTED TO THE MEETING
National limits and associated pre-harvest intervals which have been
reported to the meeting are shown in Table 12.
APPRAISAL
Triadimefon is a systemic fungicide used against mildew, rust and
other fungi on cereals, vegetables and fruit crops as well as
ornamentals. It is marketed in many countries in formulations of
wettable powders, emulsifiable concentrates, dusts or pastes. It is
often used in foliar spraying programmes with repeated applications at
low dosages. Information on the impurities in technical triadimefon
was reported to the meeting.
It is probable that the systemic properties of triadimefon arise from
uptake of the parent compound through root or plant surfaces, followed
by reduction to and translocation of the major metabolite,
triadimenol, which presumably is the carrier of the fungicidal
activity. Such processes start shortly after application of
triadimefon to plants so that triadimenol dominates the residue
constituent at the end of normal pre-harvest waiting periods. Polar
metabolites, including 4-chlorophenol, as conjugates have been
identified as further metabolites in plant material.
At the time of harvest and following recommended preharvest intervals,
measurable residues up to about 2.0 mg/kg may be found in various
crops, whilst for various other crops the residues are at or below
limit of determination. Whilst the meeting was prepared to recommend
limits for crops in the latter cases, it was considered that the
experimental data for hops, coffee and tobacco, as well as for
pineapples after post-harvest treatments were insufficient. More
information on actual use patterns and related residue data should be
made available to enable recommendations to be made concerning those
crops.
Table 12. National Limits and Preharvest intervals Reported to the
Meeting
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Austria Cereals 1 35
Beets 0.2 35
Grape, pome fruit 1 35
Cucumber, tomato, 1 4
paprika
Belgium Wheat, barley 42
Table 12. Continued...
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Brazil Wheat 0.01 42
Denmark Cereals 28
Pome fruit 14
France Apple 1 7
Melon 1 3
Germany Cereals 35
(F.R.) Cucumber 3
Apple 14
Grape 35
Israel Wheat 42
Netherlands Wheat, barley 42
N. Zealand Apple 49
Cereals 49
Grapes 28
Norway Apple, strawberry 14
Cereals, berries 28
S. Africa Apple 0.05
Barley 0.1 40
Cucumber 0.05 3
Grape 0.05 7
Mango 0.05
Yugoslavia Cereals 35
Cucumber 7
Other crops 21
Metabolism and degradation in animals, soil, microorganisms and water
as well as patterns of abiotic photodecomposition of triadimefon have
been studied extensively, and detailed lists of possible metabolites
and breakdown products are available. Triadimefon and especially
triadimenol are eliminated from tissues of livestock at relatively
fast rates, resulting in residues at or below limit of determination
within one or a few days in meat, milk and egg-products. In fat and
in offals from slaughtered animals, however, significant residues of
triadimenol may be obtained.
Convenient multi-residue methodology based on gas chromatography is
available for the determination of triadimefon and triadimenol in food
and food products.
RECOMMENDATIONS
As no ADI had been allocated the meeting proposed the following
Guideline Level on a basis of taking into account the preharvest
intervals indicated. The GLs refer to the sum of the parent compound
triadimefon and its major metabolite, triadimenol.
Guideline Pre-harvest interval on
Commodity Level(mg/kg) which recommendation is based
Apples 0.2 14 days
Cucumbers (indoor
and outdoor) 0.2 3 days
Currants, red 1 14 days
Eggs 0.1*
Grapes 2 28 days
Melons 0.5 4 days
Milk 0.1*
Onions, spring 0.1* 21 days
Paprika 0.5 3 days
Peas, without pods 0.1* 3 days
Pumpkins 0.1* 3 days
Strawberries 0.2 7 days
Sugar beets, roots 0.1* 21 days
Sugar beets, leaves 0.1* 21 days
Tomatoes, (indoor
and outdoor 0.5 3 days
Grain (barley,
oats, wheat) 0.1* 42 days
Straw (barley,
oat, wheat) 2 42 days
(* At or about the limit of determination)
FURTHER WORK OR INFORMATION
Desirable
1. Feeding studies on livestock animals for establishing
dose-response relationship between feed contents and residues in
animals tissues, especially fat, and including realistic feeding
levels;
2. Further information on residue levels in certain products, such as
coffee and hops and in pineapples after appropriate post harvest
treatments.
REFERENCES
Bayer AG. Various reports on triadimefon residues in fruit,
vegetables and soil, as well as from animal experiments. Bayer AG,
Leverkusen, FRG. (1975/79), Unpublished.
Brandes, W., Steffens, W., Führ, F., Scheinpflug, H. - Weitere
Untersuchungen zur Translokation von 14C-Triadimefon in
Gurkenpflanezen. Pflanzenschutz Nachrichten Bayer 31, 132-144.
Mobay Reports. Various unpublished reports prepared by the Research
and Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, U.S.A.
Buchenauer, H. - Systemisch-fungizide Wirkung und Wirkungsmechanismus
von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-Forstwirtsch.
(Berlin Dahlem) 165, 154-155.
Untorsuchungen zur systemischen Wirkung und zum Einfluss von
RBAYLETON (Triadimefon) auf verschiedene pilzliche
Getreidekrankheiten. Pflanzenschutz-Nachrichten Bayer 29, 267-280.
Clark, T., Clifford, D.R., Deas, A., Gendle, P., Watkins, D.
Photolysis, metabolism and other factors influencing the performance
of triadimefon as a powdery mildew fungicide. Pest. Sci. 9, 497-506.
Fisher, D.J., Pickard, J.A., McKenzie, C.M. - Uptake of the systemic
fungicide triadimefon by clover and its effect on symbiotic nitrogen
fixation. Pest. Sci. 10, 75-82.
Fredrickson, D.R. - Metabolism of BAYLETONTM in Rats. Mobay Report
No. 66 201, June 20, (1978a).
Metabolism of BAYLETONTM in the cow. Mobay Report No. 66, 202,
June 20, (1978b).
Frohberger, P.E. - Zur Frage der Biologischen Aktivität von
BAYLETON-Rück-standen im Boden. BAYER AG.
Pflanzenschutz-Anwendungstechnik, unpublished report April 12, 1978.
Für, F., Paul, V., Steffens, W. and Scheinpflug, H. Translokation von
14C-Triadimefon nach Applikation auf Sommergerste und sein Wirkung
gegen Erysiphe Graminis var. hordei. Pflanzenschutz-Nachrichten Bayer
31, 116-131.
Gasztony, M., Josepovits, G. - Translocation and metabolism of
triadifflefon in different plant species. Acta Phytopathologica
Academiae Scientiarum Hungaricae, 13, 403-415.
Kraus, P. - Untersuchungen zur Wirkung von BAYLETON aus dem Boden über
die Gaphase gegen Erysiphe graminis an Gerste. BAYER AG,
Pflanzenschutz Answendungstechnik, unpublished report, February 8,
1979.
Lamb, D.W., Roney, D.J. - Accumulation and Persistence of Residues in
Channel Catfish Exposed to BAYLETON TM-14C. Mobay Report No. 52 775,
May 4, 1977.
Mango, R.R., Puhl, R.J. - The Aerobic and Anaerobic Soil Metabolism of
BAYLETONTM. Mobay Report No. 51 230, January 12, (1977a).
Mango, R.R., Puhl, R.J. and Thornton, J.S. - The Metabolism of
BAYLETON in Apples. Mobay Report No. 53 621, August 9, (1977b).
Nichols, S.S., Thornton, J.S., Publ, R.J. - Photodecomposition of
BAYLETONTM in Water solution. Mobay Report No. 52 688, April 6,
1977.
Nye, D.E. - The Fate of RBAYLETON-14C in Poultry. Mobay Report No.
67 482, March 8, 1979.
Obrist, J.J., Thornton, J.S. - Stability of BAYLETONTM in Sterile
Aqueous Buffer Solutions. Mobay Report No. 50 799, November 29, 1976.
Obrist, J.J., Publ, R.J. and Thornton, J.S. - The Degradation and Fate
of BAYLETONTM in a simulated pond environment. Mobay Report No. 53
038, May 31, (1977a).
Obrist, J.J. and Thornton, J.S. - Leaching characteristics of
BAYLETONTM on Aged soil. Mobay Report No. 51 232, January 21, 1977b.
Photodecomposition of RBAYTON in Aqueous solutions and on a soil
surface. Mobay Report No. 66 794, October 20, 1978.
Pither, K.M. - Metabolism of BAYLETONTM in Male and female pigs.
Mobay Report No. 66 509, November 29, 1978.
Degradation of RBAYLETON by Mixed cultures of microorganisms.
Mobay Report No. 67 049, January 30, 1979.
Puhl, R.H., Hurley, J.B. - Soil Adsorption and desorption of
BAYLETON-Ring UL14C. Mobay Report No. 66 501, September 11, (1978a).
Soil adsorption and desorption of BAYTON-II. Mobay Report No. 66
745, December 12, (1978b).
Scheinpflug, H. and Paul, V. - On the mode of action of Triadimefon.
Neth. J. Plant Path. 83, (Suppl.1), 105-111. (1978a).
Scheinpflug, H., Paul, V. and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Mitt. Biol.
Bundesanst. Land Forstwirtsch. (Berlin-Dahlem) 178, 178. (1978b).
Scheinpflug, H., Paul V., and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Pflanzenschutz -
Nachrichten Bayer 31, 101-115.
Schlüter, K. - Fungizide Wirkung von Triadimefon in der Gasphase. Z.
Pflanzenkr. Pflanzenschutz 84, 612-614.
Specht, W. - Gas Chromatographic method for determining residues of
the fungicides fuberidazol, fluotrimazole and triadimefon in plants
and soil. Pflanzenschutz-Nachrichten Bayer 30, 1.
Steffens, W., Führ, F. - Versuche zur Retranslokation von Triadimefon.
Kernforschung sanlage Jülich, Arbeitagruppe Radioagronomie,
unpublished report ARA 4/76. (1976).
Steffens, W., Wieneke, J,. - Bilanzversuche mit dem 14C-markierten
Fungizid Triadimefon (MEB 6447) nach Spritzbehandlung von
Sommergerste in Lysimetern mit ungesförten Bodenprofilen, Verbleib in
der behandelten Pflanze, Verlagerung im Boden und Aufnahme durch
Folgefrüchte. Kernforschungsanlage Jülich, Arbeitsgruppe
Radioagronomie, unpublished report ARA 4/77, September. (1977).
Takase, I., Hasebe, K. - Photolysis of Triadimefon in Upland Soils.
Nitokuno Report No. 1093, (RA), March 2, 1979.
Takase, I., Yoshimoto, Y. - Degradation of Triadimefon in Upland soils
under laboratory and field conditions. Nitokuno Report No. 1091 (RA),
December 11, 1978.
Thornton, J.S. - A gas chromatographic method for Bayleton and its
metabolite in apples. Mobay Report No. 54 166, December 30, 1977.
Thornton, J.S., Lloyd, C.M. - A gas chromatographic method for
Bayleton and its metabolite in soil and water. Mobay Report No. 51
231, January 31, 1977.
Thornton, J.S., Hurley, J.B., Obrist, J.J. - Soil thin-layer mobility
of Twenty-four pesticide chemicals. Mobay Report No. 51 0.16, December
15, 1976.
Vogeler, K. BAYER AG, Pflanzenschutz-Anwendungstechnik, unpublished
report. Verhalten von Triadimefon (MEB 6447) unter der Einwirkung von
UV-Licht. RA 364, June 3, 1975.
Der metabolismus von Triadimefon in gerste unter
Gewttchshausbedlingungen und im Boden. RA 272, April 12, (1976a).
Untersuchunge zur Wirkang von Triadimefon über die Gasphase gegen
Echten Mehltau an Gerste. RA 662, August 16, (1976b).
Bericht über die Metabolisierung von 14C-Triadimefon in
Sommergerste und Boden sowie in Folgenpflanzen. RA 813, October 19,
(1977a).
Untersuchungen zur Unwandlung der reinen Diastereoisomeren A und B
von KWG 0519 in das jeweils andere Isomere in Gerstenpflanzen und
Boden. RA 871, November 14, (1977b).
Adsorptionsisothermen von Tiadimenol in verschiedenen Böden. RA 496,
July 3, 1978.
Wilmes, R. - Versuche zum Photoabbau von MEB 6447, MEB 6449 und KWG
0519. BAYER AG, Pflanzenschutz-PIQ, PF-Report No. 1266, August 7,
1978.
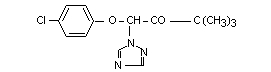 Other Information on identity and properties
Molecular weight: 293.7
Appearance: Colourless crystals
Melting point: 82.3°C (pure active ingredient)
Vapour pressure: <10-6 mbar at 20°C
approx. 2 × 10 -5 mbar at 400C
Solubility: in water 0.007 (pure a.i.)
(g.a.i./100 g solvent in cyclohexanone 60 - 120
at approx. 20°C) in isopropanol 20 - 40
in ligroin (80-110°C) 0 - 1
in methylene chloride >120
in toluene 40 -60
Minimum degree of purity: 90%
Stability: Stable in 0.1 n H2SO4) for 24 hrs
Stable in 0.1 n NaOH ) at 20°C
Impurities in the technical material
Detailed information on the impurities in technical triadimefon was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Triadimefon is a new systemic broad-spectrum fungicide against plant
pathogens, especially powdery mildew, loose smuts and rusts of cereals
and other crops. Its biochemical mechanism of fungicidal effect is
believed to be founded on the inhibition of ergosterine synthesis
(Buchenauer, 1975 and 1976). It has been found to be taken up by
plants from roots as well as through foliar surfaces and to be
transported mostly with transpiration stream. A certain translocation
downwards through the plants, however, is also measurable. Evidence
is also given for a certain vapour phase activity, which is likely to
support greenhouse applications. It is used for the control of
fungicidal diseases in several fruits and vegetables, in clover,
cereals, ornamentals, etc. without observed signs of phytotoxicity.
Triadimefon is registered and marketed for uses in agriculture and
horticulture in a number of countries among which about 25 were
reported to the meeting, representing most of the major agricultural
regions of Africa, America, Asia, Australia and Europe.
Pre-harvest treatments
Triadimefon is used in various formulations including wettable powders
(5 or 25%), emulsifiable concentrates (250 g per litre), dust (0.5%)
or paste (2%) in pre-harvest use patterns and at rates shown in Table
1. Especially promise seems recently to be attached to the use of
triadimefon in the control of mildew on cereal plants growing under
temperate climatic conditions.
Table 1. Some uses of Triadimefon formulations
Crop Pest to be Recommended conc./
controlled dosage
Small fruits mildew 0.0025-0.0% or 0.05-0.15 kg/ha
Strawberry mildew 75 - 100 g/ha
Vegetables mildew 0.0025-0.0125% (field)
0.0015-0.002% (glass house)
100 - 200 g/ha (dusting
Cereal crops mildew 1 - 2 × 125 g/ha
rust 1 × 250 g/ha
Coffee rust 100 - 250 g/ha (protective)
250 - 500 g/ha (eradicative)
Table 1. Continued...
Crop Pest to be Recommended conc./
controlled dosage
Pome fruits mildew 2 × 0.008 - 0.01% (after flowering)
rust or 0.04 - 0.075 kg/ha
Mango mildew 0.01%
or 0.1 - 0.2 kg/ha
Grape mildew 0.0025%
or 0.1 kg/ha (dust)
Tobacco mildew 0.0025 - 0.005%
or 0.04-0.075 kg/ha
Post-harvest uses
No official recommendations have yet been reported for post-harvest
uses of triadimefon, although some experimental trials indicate
potential uses on pineapples.
RESIDUES FROM SUPERVISED TRIALS
Data on triadimefon residues resulting from extensive trials
representing recommended use patterns have been presented by the
originating manufacturer (Bayer AG, Leverkusen). The data includes
controlled field experiments under a variety of geographical and
climatic conditions, as well as examples of greenhouse trials. The
data are presented in the following tables, and were obtained by gas
chromatographic methodology which determines the parent compound,
triadimefon, and its reduced, first metabolite, called triadimenol
(see below under "Rate of Residues"). Results are presented as the
sum of these two compounds, or in certain illustrative examples,
individually.
Fruit and Vegetables
The data in Table 2 summarize results from supervised trials from
various experimental sites in Germany (F.R.), and some other
countries. About a dozen different food crops of commercial interest
are represented in the data, reflecting residue levels which can be
expected after uses in accordance with "good agricultural practice".
In several cases the residue data comprise triadimefon levels as well
as contents of the triadimenol metabolite. Generally, the triadimenol
metabolite is found in levels which are comparable to the parent
compound, but possibly with a slightly greater persistence.
At the time of harvest and following recommended preharvest intervals,
measurable residues in the order of 0.2 - 0.4 mg/kg are found in
paprika and tomato and up to about 1.5 mg/kg in grapes. In hops
(harvested 10 days after treatment and measured after drying) total
residues are significantly higher, i.e. from n.d. up to 8.1 mg/kg (in
one individual sample 12.9 mg/kg). Levels of triadimefon in most of
the remaining examples are generally at or below 0.2 mg/kg.
Cereal plants
Residues of triadimefon, including triadimenol have been determined in
extensive trials on growing cereal plants, e.g. barley, oat and wheat,
with the results which are shown in Table 3. Significant residues are
found in all plants, heads as well as straw parts, and in all
experiments from the tine of last application and during the following
weeks. At the time of harvest, i.e. 6 weeks after last treatment,
residues are still persistent in straw at levels of 2 mg/kg or below.
In one occasional sampling is found 8.2 mg/kg after high dosage
treatment. Separate determination of triadimefon and triadimenol
shows that the majority of residue is in the converted triadimenol
form already at day 14, as shown for one trial in Table 3.
Grains at the time of harvest show no detectable residues in these
experiments.
Pineapples (post-harvest treatment)
Residues in pineapples following a few experimental postharvest
dippings or surface applications are shown in Table 4. A penetration
of triadimefon is hardly noticeable in these results, insofar as only
low residue levels are found in the fruit flesh in comparison with the
contents in peel. A significant conversion, however, of triadimefon
to triadimenol has obviously taken place between dipping and the time
of analyses.
Coffee and tobacco
Trials on residues in beans and shells of coffee and in tobacco leaves
are shown in Table 5. Residues in tobacco leaves vary from 0.7 to
8.5 mg/kg when analysed from 10-28 days after last treatment, and
residues are practically fully converted to triadimenol.
Must and wine after processing
The possible transfer of residues of triadimefon, including
triadimenol from harvested grapes to must and to processed wine is
shown in Table 6. A 50% level may be found in the must when related
to the content in grapes.
Table 2. Residues of Triadimefon from Supervised Trials
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Apple 12 × 0.04 kg/ha 0 <0.06-0.2 FRG
4 <0.06-0.2
7 <0.06-0.15
14 <0.06-0.15
21 n.d.-0.15
28 n.d.-0.2
6 × 0.05 kg/ha 14 n.d.-n.d. n.d.-0.06 FRG
21 n.d.-n.d. n.d.-n.d.
12 × 0.05 kg/ha 0 0.1-0.2 FRG
4 <0.06-0.1
7 <0.06-0.1
14 <0.06-0.1
21 n.d.-0.1
Cucumber 8-1O × 0.07 kg/ha 0 <0.06-0.1 FRG
7 n.d.-0.15
10 n.d.-0.1
Cucumber 4 × 0.075 kg/ha 0 <0.06-0.006 NL
indoor 7 n.d.-n.d.
10 n.d.-n.d.
Currant 4 × 0.2 kg/ha 0 0.65-3.35 FRG
red 4 0.15-0.85
7 0.15-1.05
10 0.1-0.7
14 <0.006-0.65
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Grape 10 × 0.0625 kg/ha 0 0.5 - 1.75 FRG
14 0.15-1.25
21 0.1-1.65
28 0.03-1.4
35 0.03-0.95
10 × 0.05 kg/ha 0 0.8 - 1.0 0.15 - 0.2 FRG
14 0.5 - 0.55 0.07-0.15
21 0.45-0.3 0.1 - 0.1
28 0.25-0.3 0.06-0.07
35 0.25-0.15
38 0.02-0.06
6 × 0.1. kg/ha 0 0.24-0.25 SA
3 0.08-0.09
7 0.03-0.04
14 0.02-0.02
Hops 4 × 0.5-1.0 kg/ha 10 days n.d.-2.6
dried 10 days 2.5-8.11
10 days 2.0-12.9
Melons 8 × 0.03-0.06 kg/ha 0 n.d.-0.25 F
1 n.d.-0.05
4 n.d.-0.15
7 n.d.-0.1
10 n.d.-0.08
2-4 × 0.0375 kg/ha 0 <0.01 <0.02 Jap.
1 <0.01-<0.01 <0.02-<0.02
5 <0.01-<0.01 <0.02-<0.02
Onions 2-3 × 0.0875 kg/ha 10 days <0.02-<0.02 <0.04-<0.04 Jap.
21 days <0.02-<0.02 <0.04-0.04
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Paprika 6 × 0.125 kg/ha 0 0.25-0.4 It.
1 0.15-0.3
4 0.1-0.2
7 0.15-0.25
10 0.08-0.25
Peas (without 2 × 0.03 kg/ha 0 <0.1 SA
pod) 3 <0.1
7 <0.1
14 <0.1
Pumpkin 1 x× 12.5 g per 100/l. 0 0.05-0.05 SA
3 0.01-0.02
5 <0.01-<0.01
Strawberry 3-4x×0.075/0.1 kg/ha 0 0.09-0.15 n.d.-0.06 FRG
1 0.07-0.11 0.04-0.06
4 0.05-0.07 0.05-0.08
7 0.05-0.07 0.07-0.12
10 0.03-0.03 0.05-0.0.8
14 0.02-0.025 0.04-0.10
Sugarbeets 3 x1× 0.125 kg/ha 0 n.d.-n.d. n.d.-n.d. FRG
roots 14 n.d.-n.d. n.d.-n.d.
21 n.d.-n.d. n.d.-n.d.
Sugarbeets 3 x1× 0.125 kg/ha 0 1.21-2.33 0.36-1.10 FRG
leaves 14 n.d.-0.82 n.d.-0.37
21 n.d.-n.d. n.d.-n.d.
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Tomato 2-4 x× 0.075 kg/ha 1 0.03-0.10 <0.02-0.10 Jap.
indoor 5 0.02-0.04 0.04-0.10
10 <0.01-0.02 0.04-0.06
Tomato 6 x× 0.075/0.15 kg/ha 0 0.2-0.4 FRG
1 0.15-0.25
4 0.1-0.1
7 0.1-0.25
11 0.07-0.2
* FRG: Germany (Federal Republic of Germany)
F: France
It: Italy
Jap: Japan
NL: Netherlands
SA : South Africa
Table 3. Residues in cereal crops after triadimefon treatments (FRG)
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Barley 1 × 0.125 kg/ha 0 2.7-3.15 2.6-3.1
(summer) 14 0.11-0.3 0.3-0.45
28 n.d.-0.15 0.2-0.25
35 n.d.-n.d. 0.3-0.4
42 n.d.-n.d. 0.4-0.4
Barley 1 × 0.125 kg/ha 0 4.1-5.2
(S. Africa) 4 1.0-1.2
7 0.7-0.7
14 0.2-0.2
40 n.d.-n.d.
Oats 1 × 0.125 kg/ha 0 0.95-3.85 1.8
14 n.d.-0.3 0.2-0.6
28 n.d.-0.1 n.d.-0.2
35 n.d.-0.2 n.d.-0.15
42 n.d.-n.d. n.d.-0.4
Wheat 1 × 0.125 kg/ha 0 1.2-9.4 1.1-10.45
(summer) 14 0.15-0.95 0.4-1.85
28 <0.05-0.75 0.15-0.75
35 n.d.-0.15 n.d.-1.05
42 n.d.-n.d. <0.15-0.6
2 × 0.125 kg/ha 0 1.15-2.0 2.5-5.0
14 0.2-0.35 1.15-3.55
28 0.1-0.45 0.5-0.9
35 n.d.-0.45 0.1-1.6
42 n.d.-n.d. 0.2-1.1
Table 3. Continued...
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Wheat 1 × 0.25 kg/ha 0 5.0-18.1 3.45-8.55
(summer) 14 0.8-2.35 1.4-3.35
28 0.15-1.3 0.25-1.15
35 n.d.-n.d. 0.65-1.0
42 n.d.-n.d. 0.55-1.85
1 × 0.125 kg/ha 0 6.5-8.6 <0.1-0.71 1.76-4.6 <0.1-0.35
+ 1 × 0.25 kg/ha 14 0.09-0.48 0.13-0.28 0.05-0.19 0.19-0.72
28 n.d.-0.07 n.d.-0.18 n.d.-n.d. n.d.-0.33
35 n.d.-n.d. n.d.-0.20 n.d.-n.d. n.d.-0.44
42 n.d.-n.d. n.d. - n.d. n.d.-n.d. n.d.-0.52
Wheat 1 × 0.125/0.25 kg/ha 0 2.5-3.85 1.3-3.8
(winter) 14 0.2-0.8 0.3-2.1
28 n.d.-0.8 0.4-2.7
35 n.d.-0.95 0.45-3.0
42 n.d.-n.d. 0.85-2.4
2 × 0.25 kg/ha 0 3.1-4.75 5.9-9.95
14 0.4-1.05 1.65-2.75
28 n.d.-1.75 0.45-8.65
35 n.d.-2.55 0.55-6.85
42 n.d.-n.d. 0.45-8.2
* Harvest at day 42 in all experiments
Table 4. Residues from post-harvest treatments of pineapple with
Triadimefon (Bayer AG)
Concentration/ Days after Fruit Residues (mg/kg)
Treatment treatment part Triadimefon Triadimenol
50 ppm/surface 13 days Fruit n.d. n.d.
application Peel 0.05 n.d.
Total <0.03 n.d.
100 ppm/Dipping 13 days Fruit <0.03 <0.06
Peel 1.1
(0.96-1.23) 1.6(1.5-1.7)
Total 0.23 0.33
750 ppm/Dipping 13 days Fruit 0.11 0.06
Peel 6.4 2.6
(6.28-6.5) (2.4-2.8)
Total 1.37 0.55
Table 5. Triadimefon Residues on Coffee and Tobacco (Bayer AG)
Days after Residues (mg/kg)
Crop Application last
treatment Triadimefon Triadimenol TOTAL
Coffee
(Brazil) 3 × 0.25 kg/tree 51 n.d. n.d.
(or 3 × 0.025 kg/ha)(whole bean)
15 × 0.37 kg/tree 32 (beans) - - n.d.
(shell) - - 2.9
8 × 0.75 kg/tree 89 (beans) - - <0.1
(shell) - - 0.45
Tobacco 2 × 0.06/0.09 kg/ha 14 <0.04 7.89-8.48 -
(Japan) 28 <0.04 2.09-2.13 -
2 × 0.075 kg/ha 10 <0.04 2.92 -
23 <0.04 0.70 -
Table 6. Triadimefon residues during wine processing in FRG
Residues (mg/kg)1
1976 1977
Grapes 0.25 - 1.4 0.03 - 0.06
Must 0.02 - 0.65 <0.04 - 0.06
Wine n.d. - 0.45* n.d. - n.d.
1 Sum of triadimefon and triadimenol after GLC-analysis.
* An exceptionally high residue. (average below 0.1 mg/kg).
Foods of animal origin
In feeding experiments with cows, pigs, sheep and poultry, feed was
given containing 56 or 180 mg/kg of triadimefon or triadimenol, and
levels of residues were determined in potential food products at 1 or
8 days after the termination of experimental feedings. Residues
resulting from these experimental feedings are shown in Tables 7 and
8.
The feeding levels are significantly higher than can be expected in
actual feeding practices. However, it is seen that residues from
feeding with triadimefon only occasionally are found in meat from
poultry, sheep and pig, and residues are not detectable in egg or milk
products. After feeding with the reduced metabolite triadimenol
however, residues are found in abdominal fat samples of most animals
at levels of 0.4 to about 7 mg/kg. In egg or milk, including milk
fat, residues are present only at or below the limit of determination.
Residues measured as triadimenol were regularly found in offals from
the slaughtered sheep, pig and poultry. In all cases the triadimenol
metabolite was practically only found irrespective of which of the two
compounds were originally present in the feed.
Table 7. Residues of Triadimefon* in animal products after experimental feeding for 1 week
(Bayer Australia 1978)
Days Residues (mg/kg)
Feeding after Meat Fat Offals
Animal rate feeding
Triadimefon Triadimenol Triadimefon Triadimenol Triadimefon Triadimenol
Feeding of Parent compound, Triadimefon:
Poultry 180 ppm 1 day <0.05 <0.08 <0.25 trace <0.05 2.4
8 days <0.05 <0.08 <0.25 <0.4 <0.05 1.7
Sheep 56 ppm 1 day trace trace <0.2 0.4 <0.1 1.9
8 days <0.1 <0.1 <0.2 0.4 <0.1 1.9
Pig 180 ppm 1 day trace trace <0.65 trace trace 0.2
8 days <0.1 <0.2 <0.65 1.1 <0.1 0.2
Feeding of metabolite, Triadimenol:
Poultry 180 ppm 1 day - <0.08 - trace - 2.1
8 days - <0.08 - <0.4 - 1.8
Sheep 56 ppm 1 day - <0.1 - 2.2 - 3.4
8 days - trace 1.1 (?) 1.1 - 6.5
Pig 180 ppm 1 day - <0.1 - 0.4 - 0.4
8 days - 1.0 - 7.1 - 3.8
* Determined by GLC-analysis of Triadimefon and Triadimenol separately.
Table 8. Residues of Triadimefon *) in milk and eggs after
experimental feeding for one week (Bayer Australia, 1978)
Residues (mg/kg)
Feeding
Product level Triadimefon Triadimenol
After feeding the parent compound, Triadimefon:
Milk, skimmed 168 ppm <0.1 - <0.1 <0.1 - <0.1
Milk fat 168 ppm <0.4 - <0.4 <0.5 - <0.5
Hen's egg 180 ppm <0.1 - <0.1 n.d.- n.d.
After feeding the metabolite, Triadimenol:
Milk, skimmed 168 ppm - <0.1 - <0.1
Milk fat 168 ppm - <0.5 - <0.5
Hen's egg 180 ppm - <0.2 - <0.2
* Determined by GLC-analysis of Triadimefon and Triadimenol
separately.
FATE OF RESIDUES
General remarks
Degradation pathways and metabolite patterns of triadimefon in various
media including soil, microorganisms, water, plants, animals and under
exposure to light have been subject to many investigations from which
illustrative summaries are presented in Figure 1 and Table 9.
The principal metabolite of triadimefon (I) is already mentioned
above,namely triadimenol (III) obtained by reduction of the >C=O
group to >HCOH configuration. Triadimenol is fungicidally active and
seems to give promise as a commercial fungicide in its own rights.
Indications are present that systemic properties of triadimefon may be
ascribed to processes of uptake in the form of triadimefon combined
with translocation of the metabolite, which is then considered to be
the predominant active principle against fungal pests.
Triadimenol occurs in two diastereoisomeric forms, called A and B,
which are determined separately in metabolic studies. Among other
identified metabolites are mentioned cleavage products containing the
triazole- or benzene-rings, i.a. 4-chlorophenol (VIII) from plant
metabolic studies. In animals, hydroxyl-(V,VI) and carboxyl-compounds
(VII) are found, which represent non-disrupted molecule structures,
and which may occur in free forms or as glucoronides.
Other Information on identity and properties
Molecular weight: 293.7
Appearance: Colourless crystals
Melting point: 82.3°C (pure active ingredient)
Vapour pressure: <10-6 mbar at 20°C
approx. 2 × 10 -5 mbar at 400C
Solubility: in water 0.007 (pure a.i.)
(g.a.i./100 g solvent in cyclohexanone 60 - 120
at approx. 20°C) in isopropanol 20 - 40
in ligroin (80-110°C) 0 - 1
in methylene chloride >120
in toluene 40 -60
Minimum degree of purity: 90%
Stability: Stable in 0.1 n H2SO4) for 24 hrs
Stable in 0.1 n NaOH ) at 20°C
Impurities in the technical material
Detailed information on the impurities in technical triadimefon was
reported to the meeting.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Triadimefon is a new systemic broad-spectrum fungicide against plant
pathogens, especially powdery mildew, loose smuts and rusts of cereals
and other crops. Its biochemical mechanism of fungicidal effect is
believed to be founded on the inhibition of ergosterine synthesis
(Buchenauer, 1975 and 1976). It has been found to be taken up by
plants from roots as well as through foliar surfaces and to be
transported mostly with transpiration stream. A certain translocation
downwards through the plants, however, is also measurable. Evidence
is also given for a certain vapour phase activity, which is likely to
support greenhouse applications. It is used for the control of
fungicidal diseases in several fruits and vegetables, in clover,
cereals, ornamentals, etc. without observed signs of phytotoxicity.
Triadimefon is registered and marketed for uses in agriculture and
horticulture in a number of countries among which about 25 were
reported to the meeting, representing most of the major agricultural
regions of Africa, America, Asia, Australia and Europe.
Pre-harvest treatments
Triadimefon is used in various formulations including wettable powders
(5 or 25%), emulsifiable concentrates (250 g per litre), dust (0.5%)
or paste (2%) in pre-harvest use patterns and at rates shown in Table
1. Especially promise seems recently to be attached to the use of
triadimefon in the control of mildew on cereal plants growing under
temperate climatic conditions.
Table 1. Some uses of Triadimefon formulations
Crop Pest to be Recommended conc./
controlled dosage
Small fruits mildew 0.0025-0.0% or 0.05-0.15 kg/ha
Strawberry mildew 75 - 100 g/ha
Vegetables mildew 0.0025-0.0125% (field)
0.0015-0.002% (glass house)
100 - 200 g/ha (dusting
Cereal crops mildew 1 - 2 × 125 g/ha
rust 1 × 250 g/ha
Coffee rust 100 - 250 g/ha (protective)
250 - 500 g/ha (eradicative)
Table 1. Continued...
Crop Pest to be Recommended conc./
controlled dosage
Pome fruits mildew 2 × 0.008 - 0.01% (after flowering)
rust or 0.04 - 0.075 kg/ha
Mango mildew 0.01%
or 0.1 - 0.2 kg/ha
Grape mildew 0.0025%
or 0.1 kg/ha (dust)
Tobacco mildew 0.0025 - 0.005%
or 0.04-0.075 kg/ha
Post-harvest uses
No official recommendations have yet been reported for post-harvest
uses of triadimefon, although some experimental trials indicate
potential uses on pineapples.
RESIDUES FROM SUPERVISED TRIALS
Data on triadimefon residues resulting from extensive trials
representing recommended use patterns have been presented by the
originating manufacturer (Bayer AG, Leverkusen). The data includes
controlled field experiments under a variety of geographical and
climatic conditions, as well as examples of greenhouse trials. The
data are presented in the following tables, and were obtained by gas
chromatographic methodology which determines the parent compound,
triadimefon, and its reduced, first metabolite, called triadimenol
(see below under "Rate of Residues"). Results are presented as the
sum of these two compounds, or in certain illustrative examples,
individually.
Fruit and Vegetables
The data in Table 2 summarize results from supervised trials from
various experimental sites in Germany (F.R.), and some other
countries. About a dozen different food crops of commercial interest
are represented in the data, reflecting residue levels which can be
expected after uses in accordance with "good agricultural practice".
In several cases the residue data comprise triadimefon levels as well
as contents of the triadimenol metabolite. Generally, the triadimenol
metabolite is found in levels which are comparable to the parent
compound, but possibly with a slightly greater persistence.
At the time of harvest and following recommended preharvest intervals,
measurable residues in the order of 0.2 - 0.4 mg/kg are found in
paprika and tomato and up to about 1.5 mg/kg in grapes. In hops
(harvested 10 days after treatment and measured after drying) total
residues are significantly higher, i.e. from n.d. up to 8.1 mg/kg (in
one individual sample 12.9 mg/kg). Levels of triadimefon in most of
the remaining examples are generally at or below 0.2 mg/kg.
Cereal plants
Residues of triadimefon, including triadimenol have been determined in
extensive trials on growing cereal plants, e.g. barley, oat and wheat,
with the results which are shown in Table 3. Significant residues are
found in all plants, heads as well as straw parts, and in all
experiments from the tine of last application and during the following
weeks. At the time of harvest, i.e. 6 weeks after last treatment,
residues are still persistent in straw at levels of 2 mg/kg or below.
In one occasional sampling is found 8.2 mg/kg after high dosage
treatment. Separate determination of triadimefon and triadimenol
shows that the majority of residue is in the converted triadimenol
form already at day 14, as shown for one trial in Table 3.
Grains at the time of harvest show no detectable residues in these
experiments.
Pineapples (post-harvest treatment)
Residues in pineapples following a few experimental postharvest
dippings or surface applications are shown in Table 4. A penetration
of triadimefon is hardly noticeable in these results, insofar as only
low residue levels are found in the fruit flesh in comparison with the
contents in peel. A significant conversion, however, of triadimefon
to triadimenol has obviously taken place between dipping and the time
of analyses.
Coffee and tobacco
Trials on residues in beans and shells of coffee and in tobacco leaves
are shown in Table 5. Residues in tobacco leaves vary from 0.7 to
8.5 mg/kg when analysed from 10-28 days after last treatment, and
residues are practically fully converted to triadimenol.
Must and wine after processing
The possible transfer of residues of triadimefon, including
triadimenol from harvested grapes to must and to processed wine is
shown in Table 6. A 50% level may be found in the must when related
to the content in grapes.
Table 2. Residues of Triadimefon from Supervised Trials
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Apple 12 × 0.04 kg/ha 0 <0.06-0.2 FRG
4 <0.06-0.2
7 <0.06-0.15
14 <0.06-0.15
21 n.d.-0.15
28 n.d.-0.2
6 × 0.05 kg/ha 14 n.d.-n.d. n.d.-0.06 FRG
21 n.d.-n.d. n.d.-n.d.
12 × 0.05 kg/ha 0 0.1-0.2 FRG
4 <0.06-0.1
7 <0.06-0.1
14 <0.06-0.1
21 n.d.-0.1
Cucumber 8-1O × 0.07 kg/ha 0 <0.06-0.1 FRG
7 n.d.-0.15
10 n.d.-0.1
Cucumber 4 × 0.075 kg/ha 0 <0.06-0.006 NL
indoor 7 n.d.-n.d.
10 n.d.-n.d.
Currant 4 × 0.2 kg/ha 0 0.65-3.35 FRG
red 4 0.15-0.85
7 0.15-1.05
10 0.1-0.7
14 <0.006-0.65
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Grape 10 × 0.0625 kg/ha 0 0.5 - 1.75 FRG
14 0.15-1.25
21 0.1-1.65
28 0.03-1.4
35 0.03-0.95
10 × 0.05 kg/ha 0 0.8 - 1.0 0.15 - 0.2 FRG
14 0.5 - 0.55 0.07-0.15
21 0.45-0.3 0.1 - 0.1
28 0.25-0.3 0.06-0.07
35 0.25-0.15
38 0.02-0.06
6 × 0.1. kg/ha 0 0.24-0.25 SA
3 0.08-0.09
7 0.03-0.04
14 0.02-0.02
Hops 4 × 0.5-1.0 kg/ha 10 days n.d.-2.6
dried 10 days 2.5-8.11
10 days 2.0-12.9
Melons 8 × 0.03-0.06 kg/ha 0 n.d.-0.25 F
1 n.d.-0.05
4 n.d.-0.15
7 n.d.-0.1
10 n.d.-0.08
2-4 × 0.0375 kg/ha 0 <0.01 <0.02 Jap.
1 <0.01-<0.01 <0.02-<0.02
5 <0.01-<0.01 <0.02-<0.02
Onions 2-3 × 0.0875 kg/ha 10 days <0.02-<0.02 <0.04-<0.04 Jap.
21 days <0.02-<0.02 <0.04-0.04
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Paprika 6 × 0.125 kg/ha 0 0.25-0.4 It.
1 0.15-0.3
4 0.1-0.2
7 0.15-0.25
10 0.08-0.25
Peas (without 2 × 0.03 kg/ha 0 <0.1 SA
pod) 3 <0.1
7 <0.1
14 <0.1
Pumpkin 1 x× 12.5 g per 100/l. 0 0.05-0.05 SA
3 0.01-0.02
5 <0.01-<0.01
Strawberry 3-4x×0.075/0.1 kg/ha 0 0.09-0.15 n.d.-0.06 FRG
1 0.07-0.11 0.04-0.06
4 0.05-0.07 0.05-0.08
7 0.05-0.07 0.07-0.12
10 0.03-0.03 0.05-0.0.8
14 0.02-0.025 0.04-0.10
Sugarbeets 3 x1× 0.125 kg/ha 0 n.d.-n.d. n.d.-n.d. FRG
roots 14 n.d.-n.d. n.d.-n.d.
21 n.d.-n.d. n.d.-n.d.
Sugarbeets 3 x1× 0.125 kg/ha 0 1.21-2.33 0.36-1.10 FRG
leaves 14 n.d.-0.82 n.d.-0.37
21 n.d.-n.d. n.d.-n.d.
Table 2. Continued...
Days after Residues (mg/kg)
Crop Application last Triadimefon Triadimenol Country
No. × Rate treatment Total
Tomato 2-4 x× 0.075 kg/ha 1 0.03-0.10 <0.02-0.10 Jap.
indoor 5 0.02-0.04 0.04-0.10
10 <0.01-0.02 0.04-0.06
Tomato 6 x× 0.075/0.15 kg/ha 0 0.2-0.4 FRG
1 0.15-0.25
4 0.1-0.1
7 0.1-0.25
11 0.07-0.2
* FRG: Germany (Federal Republic of Germany)
F: France
It: Italy
Jap: Japan
NL: Netherlands
SA : South Africa
Table 3. Residues in cereal crops after triadimefon treatments (FRG)
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Barley 1 × 0.125 kg/ha 0 2.7-3.15 2.6-3.1
(summer) 14 0.11-0.3 0.3-0.45
28 n.d.-0.15 0.2-0.25
35 n.d.-n.d. 0.3-0.4
42 n.d.-n.d. 0.4-0.4
Barley 1 × 0.125 kg/ha 0 4.1-5.2
(S. Africa) 4 1.0-1.2
7 0.7-0.7
14 0.2-0.2
40 n.d.-n.d.
Oats 1 × 0.125 kg/ha 0 0.95-3.85 1.8
14 n.d.-0.3 0.2-0.6
28 n.d.-0.1 n.d.-0.2
35 n.d.-0.2 n.d.-0.15
42 n.d.-n.d. n.d.-0.4
Wheat 1 × 0.125 kg/ha 0 1.2-9.4 1.1-10.45
(summer) 14 0.15-0.95 0.4-1.85
28 <0.05-0.75 0.15-0.75
35 n.d.-0.15 n.d.-1.05
42 n.d.-n.d. <0.15-0.6
2 × 0.125 kg/ha 0 1.15-2.0 2.5-5.0
14 0.2-0.35 1.15-3.55
28 0.1-0.45 0.5-0.9
35 n.d.-0.45 0.1-1.6
42 n.d.-n.d. 0.2-1.1
Table 3. Continued...
Residues (mg/kg)
Application Days after Heads (grains) Straw
Crop No. × Rate last Triadimefon Triadimenol Triadimefon Triadimenol
treatment* Total Total
Wheat 1 × 0.25 kg/ha 0 5.0-18.1 3.45-8.55
(summer) 14 0.8-2.35 1.4-3.35
28 0.15-1.3 0.25-1.15
35 n.d.-n.d. 0.65-1.0
42 n.d.-n.d. 0.55-1.85
1 × 0.125 kg/ha 0 6.5-8.6 <0.1-0.71 1.76-4.6 <0.1-0.35
+ 1 × 0.25 kg/ha 14 0.09-0.48 0.13-0.28 0.05-0.19 0.19-0.72
28 n.d.-0.07 n.d.-0.18 n.d.-n.d. n.d.-0.33
35 n.d.-n.d. n.d.-0.20 n.d.-n.d. n.d.-0.44
42 n.d.-n.d. n.d. - n.d. n.d.-n.d. n.d.-0.52
Wheat 1 × 0.125/0.25 kg/ha 0 2.5-3.85 1.3-3.8
(winter) 14 0.2-0.8 0.3-2.1
28 n.d.-0.8 0.4-2.7
35 n.d.-0.95 0.45-3.0
42 n.d.-n.d. 0.85-2.4
2 × 0.25 kg/ha 0 3.1-4.75 5.9-9.95
14 0.4-1.05 1.65-2.75
28 n.d.-1.75 0.45-8.65
35 n.d.-2.55 0.55-6.85
42 n.d.-n.d. 0.45-8.2
* Harvest at day 42 in all experiments
Table 4. Residues from post-harvest treatments of pineapple with
Triadimefon (Bayer AG)
Concentration/ Days after Fruit Residues (mg/kg)
Treatment treatment part Triadimefon Triadimenol
50 ppm/surface 13 days Fruit n.d. n.d.
application Peel 0.05 n.d.
Total <0.03 n.d.
100 ppm/Dipping 13 days Fruit <0.03 <0.06
Peel 1.1
(0.96-1.23) 1.6(1.5-1.7)
Total 0.23 0.33
750 ppm/Dipping 13 days Fruit 0.11 0.06
Peel 6.4 2.6
(6.28-6.5) (2.4-2.8)
Total 1.37 0.55
Table 5. Triadimefon Residues on Coffee and Tobacco (Bayer AG)
Days after Residues (mg/kg)
Crop Application last
treatment Triadimefon Triadimenol TOTAL
Coffee
(Brazil) 3 × 0.25 kg/tree 51 n.d. n.d.
(or 3 × 0.025 kg/ha)(whole bean)
15 × 0.37 kg/tree 32 (beans) - - n.d.
(shell) - - 2.9
8 × 0.75 kg/tree 89 (beans) - - <0.1
(shell) - - 0.45
Tobacco 2 × 0.06/0.09 kg/ha 14 <0.04 7.89-8.48 -
(Japan) 28 <0.04 2.09-2.13 -
2 × 0.075 kg/ha 10 <0.04 2.92 -
23 <0.04 0.70 -
Table 6. Triadimefon residues during wine processing in FRG
Residues (mg/kg)1
1976 1977
Grapes 0.25 - 1.4 0.03 - 0.06
Must 0.02 - 0.65 <0.04 - 0.06
Wine n.d. - 0.45* n.d. - n.d.
1 Sum of triadimefon and triadimenol after GLC-analysis.
* An exceptionally high residue. (average below 0.1 mg/kg).
Foods of animal origin
In feeding experiments with cows, pigs, sheep and poultry, feed was
given containing 56 or 180 mg/kg of triadimefon or triadimenol, and
levels of residues were determined in potential food products at 1 or
8 days after the termination of experimental feedings. Residues
resulting from these experimental feedings are shown in Tables 7 and
8.
The feeding levels are significantly higher than can be expected in
actual feeding practices. However, it is seen that residues from
feeding with triadimefon only occasionally are found in meat from
poultry, sheep and pig, and residues are not detectable in egg or milk
products. After feeding with the reduced metabolite triadimenol
however, residues are found in abdominal fat samples of most animals
at levels of 0.4 to about 7 mg/kg. In egg or milk, including milk
fat, residues are present only at or below the limit of determination.
Residues measured as triadimenol were regularly found in offals from
the slaughtered sheep, pig and poultry. In all cases the triadimenol
metabolite was practically only found irrespective of which of the two
compounds were originally present in the feed.
Table 7. Residues of Triadimefon* in animal products after experimental feeding for 1 week
(Bayer Australia 1978)
Days Residues (mg/kg)
Feeding after Meat Fat Offals
Animal rate feeding
Triadimefon Triadimenol Triadimefon Triadimenol Triadimefon Triadimenol
Feeding of Parent compound, Triadimefon:
Poultry 180 ppm 1 day <0.05 <0.08 <0.25 trace <0.05 2.4
8 days <0.05 <0.08 <0.25 <0.4 <0.05 1.7
Sheep 56 ppm 1 day trace trace <0.2 0.4 <0.1 1.9
8 days <0.1 <0.1 <0.2 0.4 <0.1 1.9
Pig 180 ppm 1 day trace trace <0.65 trace trace 0.2
8 days <0.1 <0.2 <0.65 1.1 <0.1 0.2
Feeding of metabolite, Triadimenol:
Poultry 180 ppm 1 day - <0.08 - trace - 2.1
8 days - <0.08 - <0.4 - 1.8
Sheep 56 ppm 1 day - <0.1 - 2.2 - 3.4
8 days - trace 1.1 (?) 1.1 - 6.5
Pig 180 ppm 1 day - <0.1 - 0.4 - 0.4
8 days - 1.0 - 7.1 - 3.8
* Determined by GLC-analysis of Triadimefon and Triadimenol separately.
Table 8. Residues of Triadimefon *) in milk and eggs after
experimental feeding for one week (Bayer Australia, 1978)
Residues (mg/kg)
Feeding
Product level Triadimefon Triadimenol
After feeding the parent compound, Triadimefon:
Milk, skimmed 168 ppm <0.1 - <0.1 <0.1 - <0.1
Milk fat 168 ppm <0.4 - <0.4 <0.5 - <0.5
Hen's egg 180 ppm <0.1 - <0.1 n.d.- n.d.
After feeding the metabolite, Triadimenol:
Milk, skimmed 168 ppm - <0.1 - <0.1
Milk fat 168 ppm - <0.5 - <0.5
Hen's egg 180 ppm - <0.2 - <0.2
* Determined by GLC-analysis of Triadimefon and Triadimenol
separately.
FATE OF RESIDUES
General remarks
Degradation pathways and metabolite patterns of triadimefon in various
media including soil, microorganisms, water, plants, animals and under
exposure to light have been subject to many investigations from which
illustrative summaries are presented in Figure 1 and Table 9.
The principal metabolite of triadimefon (I) is already mentioned
above,namely triadimenol (III) obtained by reduction of the >C=O
group to >HCOH configuration. Triadimenol is fungicidally active and
seems to give promise as a commercial fungicide in its own rights.
Indications are present that systemic properties of triadimefon may be
ascribed to processes of uptake in the form of triadimefon combined
with translocation of the metabolite, which is then considered to be
the predominant active principle against fungal pests.
Triadimenol occurs in two diastereoisomeric forms, called A and B,
which are determined separately in metabolic studies. Among other
identified metabolites are mentioned cleavage products containing the
triazole- or benzene-rings, i.a. 4-chlorophenol (VIII) from plant
metabolic studies. In animals, hydroxyl-(V,VI) and carboxyl-compounds
(VII) are found, which represent non-disrupted molecule structures,
and which may occur in free forms or as glucoronides.
 Table 9. Triadimefon and hitherto identified metabolites and their occurrence.
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
Formula
Table 9. Triadimefon and hitherto identified metabolites and their occurrence.
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
Formula  I 1-(4-chlorophenoxy)- Triadimefon X X X X X X
3,3-dimethyl-1-(1H (BAYLETON)
1,2,4-triazole-1-yl)-2
butanone
I 1-(4-chlorophenoxy)- Triadimefon X X X X X X
3,3-dimethyl-1-(1H (BAYLETON)
1,2,4-triazole-1-yl)-2
butanone
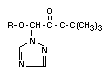 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
II 1-(4-Chlorophenoxy)- symmetrical X
3,3-dimethyl-1-(4H- isomer of I
1,2,4-triazole-4-yl)-
2-butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
II 1-(4-Chlorophenoxy)- symmetrical X
3,3-dimethyl-1-(4H- isomer of I
1,2,4-triazole-4-yl)-
2-butanone
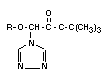 III 1-(4-chlorophenoxy)- Triadimenol X X X X X X
3,3-dimethyl-1- (BAYTAN)
(1H-1,2,4-triazole-1-yl)-
2-butanol
III 1-(4-chlorophenoxy)- Triadimenol X X X X X X
3,3-dimethyl-1- (BAYTAN)
(1H-1,2,4-triazole-1-yl)-
2-butanol
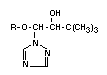 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
IV 1-(4-chlorophenoxy)- isopropyl- X
3-methyl-1-(1H- analogue of I
1,2,4-triazole-1-yl)
2-butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
IV 1-(4-chlorophenoxy)- isopropyl- X
3-methyl-1-(1H- analogue of I
1,2,4-triazole-1-yl)
2-butanone
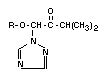 V 1-(4-chlorophenoxy)- KWG 1323 X
3-hydroxymethyl-3-
methyl-1-(1H-1,2,4-
triazole-1-yl)-2-butanone
V 1-(4-chlorophenoxy)- KWG 1323 X
3-hydroxymethyl-3-
methyl-1-(1H-1,2,4-
triazole-1-yl)-2-butanone
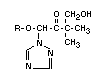 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VI 4-(4-chlorophenoxy)- KWG 1342 X
2,2-dimethyl-4-(1H-
1,2,4-triazole-1-yl)-
1,3-butandiole
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VI 4-(4-chlorophenoxy)- KWG 1342 X
2,2-dimethyl-4-(1H-
1,2,4-triazole-1-yl)-
1,3-butandiole
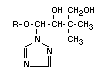 VII 4-(4-chlorophenoxy)- Triadimenol X
3-hydroxy-2,2-dimethyl- acid
4-(1H-1,2,4-triazole-
1-yl)-butane acid
VII 4-(4-chlorophenoxy)- Triadimenol X
3-hydroxy-2,2-dimethyl- acid
4-(1H-1,2,4-triazole-
1-yl)-butane acid
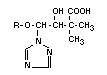 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VIII 4-chlorophenol - X X X X X
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
VIII 4-chlorophenol - X X X X X
 IX 4-chlorophenyl- X
methyl-carbonate -
IX 4-chlorophenyl- X
methyl-carbonate -
 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
X 3,3-dimethyl-l - X
phenoxy-l-(1H-1,2,4
triazole-1-yl)-2-
butanone
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
X 3,3-dimethyl-l - X
phenoxy-l-(1H-1,2,4
triazole-1-yl)-2-
butanone
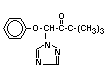 XI 1H-1,2,4-triazole 1,2,4-Triazole X X X
XI 1H-1,2,4-triazole 1,2,4-Triazole X X X
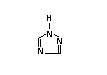 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XII Delta2-1,2,4-triazolin- - X
5-one
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XII Delta2-1,2,4-triazolin- - X
5-one
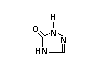 XIII 4-Hydroxyphenyl-2-oxo- X
3,3-dimethyl-butane acid
ester
XIII 4-Hydroxyphenyl-2-oxo- X
3,3-dimethyl-butane acid
ester
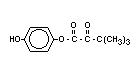 Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XIV 4-Hydroxyphenyl-2-hydroxy- - X
3,3-dimethyl-butane acid
ester
Table 9. Continued...
No. Designation Designation Occurrence
used in text
Soil Fungi Water Plants Animals Light
XIV 4-Hydroxyphenyl-2-hydroxy- - X
3,3-dimethyl-butane acid
ester
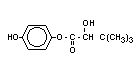 XV 4-Hydroxyphenyl-3,3-di - X
methyl-1-(1H-1,2,4-triazole
1-yl)-2-butanol
XV 4-Hydroxyphenyl-3,3-di - X
methyl-1-(1H-1,2,4-triazole
1-yl)-2-butanol
 Fate in soil
Adsorption and desorption studies determining the Freundlich isotherm
characteristics have been made on triadimefon as well as on
triadimenol in different soils and in different concentrations (Puhl
and Hurley, 1978 a and b, Vogeler, 1978, Frohberger, 1978). The
results of such studies correlate well with laboratory leaching
experiments in standardised procedures (BBA-Merkblatt no. 37) and
TLC-mobility tests from which triadimefon can be classified as a
compound of low mobility in a variety of soil types (Obrist and
Thornton, 1977b, Thornton et al., 1976).
No triadimefon was detected in leachates in the standardised test and
only limited amounts of radiolabelled residues could be determined in
soil layers below 2.5 cm in a balance study on treatment of spring
barley with 14C-labelled triadimefon if measurements were made from
76-427 days after application (Steffens & Wieneke, 1977). In the
leaching studies of Obrist and Thornton (1977b) less than 3% of the
total radioactivity could be traced through 30 cm of aged soil after a
period of 45 days with simulated rain of 12.5 mm per day. None of
this activity was identified as intact triadimefon or as triadimenol.
Half-life determinations in two standard soils gave RL50-values of 60
and 100 days for triadimefon and of 130 and 310 days for triadimenol
(Bayer-reports Nos. 9012/1974 and 9050/1976).
The disappearance rates of triadimefon and triadimenol have been
studied by several groups in German, Japanese and U.S. trials under a
variety of conditions and using a broad range of different soil types
(Mango and Puhl, 1977a; Takase & Yashimoto, 1978; and reported from
Bayer AG and Mobay Chem. Corporation). Variations in RL50-values are
found from about 10 days to 7 months for triadimefon and from about 3
months to 500 days for triadimenol. In summarising such data it is
justified to characterize triadimefon, and especially triadimenol, as
persistent compounds in soil, although it is presently impossible to
evaluate which specific environmental conditions (e.g. soil type,
temperature, etc.) are of greatest significance for the variability in
persistence. Residue levels of triadimefon have been determined in
soil after several years of treatment in cereal fields and apple
orchards (Bayer reports, 1977). The residues consisted mainly of
triadimenol which reached plateau levels (0-10 cm layer) from 0.13
mg/kg to 0.65 mg/kg in the 5th years of trials in apple orchards.
Residue uptake from soil, including crop rotation
Uptake of triadimefon and triadimenol from soil was established in
experiments with barley (Frohberger, 1978) and cloverplants (Fisher
et al., 1979). In the latter study a linear relationship between
amount absorbed and the amount of triadimefon applied to the soil was
found. The uptake of soil residues by later crops have been studied
by Steffen and Wieneke (1977), who used turnip and clover as catch
crops followed by sugarbeet, oats or winter wheat as the main crops in
lysimeters which had originally been treated with radio-labelled
triadimefon on cultures of spring barley. 0.2% of soil residues was
absorbed by the catch crops, while 0.5 - 4.3% was found in the
following main crops. It is assumed from these experiments that
mainly triadimenol is absorbed from the soil, and subsequently
metabolised to polar compounds in the plants.
Soil metabolism
The metabolic conversions and degradation of triadimefon in soil have
been studied by Vogeler (1976a, 1977a and b), Mango & Puhl (1977a) and
Takase & Yashimoto (1978). It is found that a primary reduction to
triadimenol takes place as a microbial reaction, and resulting into
two diastereoisomeric forms, A and B, in ratios which differ from
about 1:1 to 1:4 in the various experiments.
In radio-labelled experiments Mango and Puhl (1977) applied
triadimefon to soils using either benzene-ring or triazole-ring
14C-labelling. After 238 days they found 45-49% of radioactivity in
triadimenol, 7% in triazole and 24% or 5% of 14CO2 from the two ring
systems, respectively, indicating a complete, although gradual and
relatively slow degradation of triadimefon. After application on
cereals, Vogeler in his experiments (using benzene-ring labelling)
found 15-25% and 32-65% of soil radioactivity to be non-extractable.
Fate in microorganisms
As already mentioned, the conversion in soil of triadimefon to
triadimenol under natural environmental conditions is considered to be
a microbial reaction (Mango & Puhl, 1977). This is confirmed in
laboratory shake cultures of Aspergillus niger, which may convert
from 5-32% of triadimefon to triadimenol in 2-5 days, respectively.
No further metabolism of triadimenol was detected in these
experiments. In other experiments (Clark et al., 1978), triadimefon
was incubated with mycelial mats of the fungus, and a different
metabolic pattern was observed as indicated by the findings of an
isopropyl analogue (IV) to triadimefon in the culture by mass
spectrometric analysis. And, in experiments by Pither (1979),
combined bacterial or fungal cultures during 13 days had no detectable
effects on triadimefon, leaving 99% of the parent compound intact in
the cultures.
Fate in water
In sterile aqueous buffer solutions, triadimefon is found to be stable
for many weeks at temperatures ranging from 25°C - 45°C and at pH 3,
6, and 9 (Obrist and Thornton, 1976). In a simulated pond
environment, however, a fast degradation was found and metabolites
identified in the following order: triadimenol (III), triadimefon
symmetrical isomer (II), 1,2,4,triazole (XI) and
Delta2-1-2-4-triazolin-5-one (XII) (Obrist et al., 1977a). 30% of
the 14C activity (from benzene-ring labelling) was missing at the end
of the experiment, possibly under formation of 14CO2. The
disappearance rate for the parent compound in these experiments
corresponded to half-lives of 6-8 days in the water phase and 18-20
days in the silt.
Fate in plants
An uptake of triadimefon from soil to plants, e.g. to beans and barley
(Buchenauer, 1975 and 1976) and through foliar surfaces to beans,
cereals, cucumbers and tomato followed by translocation with the
transpiration stream (i.e. acropetally) has been demonstrated in
several studies (Steffens & Führ, 1976; Scheinpflug et al., 1977b;
Brandes et al., 1978; Für et al., 1978; Gasztony & Josepovits,
1978). A slight translocation in the opposite direction, i.e.
basipetal movement, is observed also (cfr. Buchenauer, 1975 and 1976).
A transfer of triadimefon through the vapour phase with a measurable
fungicidal effect and evidence of metabolites in untreated plants is
described in studies from greenhouses (Scheinpflug & Paul, 1977a;
Schlüter, 1977; Scheinpflug et al., 1978; Kraus, 1979) and in closed
glass chambers (Vogeler & Paul, 1976b). Such vapour phase effects are
dependant on the distance between target plant and spray deposits.
As is the case for soil, there is much experimental evidence that
triadimefon is reduced in plants to triadimenol, which is the major
metabolite (up to 80%) and which occurs in the two diastereoisomeric
forms, A and B. Contrasting the situation in soil, however, the
isomer form A is dominating as indicated by the ratios of A:B varying
from 1:1 to 3:1. Besides triadimenol, polar substances (Rf values of
0) and a compound with the Rf value of 0.26 (presumably compound VI
in Table 9) were found.
Results from experimental determinations of various 14C labelled
metabolites and fractions in cereal plants (benzene-ring label) and in
apples (triazole-ring label) are shown in Table 10.
Table 10. Percent distribution of radioactivity in cereals and apples after spray application of 14C-Triadimefon
(recovered radioactivity = 100) (Vogeler, 1976, 1977, 1978; Mango et al., 1977)
EXTRACTABLES
Plant Dose Days
(I = Indoor (a.i.) post RF 0 RF 0.26+ unidentified Triadimenol A Triadimenol B Triadimefon non
F = Field) appl. metabolite extractables
barley (I)
total plant 0 n.d. n.d. n.d. n.d. n.d. 99.5 0.5
" " 5 7.3 n.d. n.d. 57.3 21.8 9.7 3.9
" " 10 15.8 n.d. n.d. 50.3 18.9 7.9 7.1
" " 250 g/ha 28 15.8 n.d. n.d. 45.1 17.4 6.4 15.3
straw 38 15.9 n.d. n.d. 43.6 14.4 10.3 15.8
" 62 16.7 1.9 n.d. 41.0 17.3 4.8 18.3
barley (F)
total plant 0 <1 n.d. n.d. 1 n.d. 92 6
" " 5 13 n.d. n.d. 48 15 12 12
" " 125 g/ha 17 35 n.d. n.d. 32 10 <1 23
straw 28 9 <1 n.d. 12 4 n.d. 75
" 76 8.4 5 n.d. 8.4 3 n.d. 75
wheat (F)
straw 250 g/ha 47 37 2 n.d. 23 6 n.d. 32
apples (F)
peel 28 5.7* n.d. 2.8 14.9 13.6 10.8 5.0
pulp 5.3* n.d. 3.1 11.2 7.1 2.1 0.9
total fruit 15 mg/100ml 11.0* n.d. 5.9 26.1 20.7 13.0 5.9
peel 48 4.8* n.d. 1.5 14.5 14.8 10.4 6.0
pulp 6.4* n.d. 2.1 10.4 9.4 2.1 1.0
total fruit 11.2* n.d. 3.6 24.9 24.2 12.5 7.0
+ presumably compound VI, table 9.
n.d. = not detectable * aqueous phase
The amount of radioactivity in grains and ears was too small for separation by TLC.
FATE IN ANIMALS
Mammals
Following administration of a single oral dose of 14C-triadimefon of
25 mg per kg b.w. to rats, it was found that 75 - 83% was excreted
within 7 days, with 30-40% in the urine and 35-53% in the faeces.
Expiration of 14CO2 was not found (Fredrickson, 1978a). In cows as
well as in pigs (Fredrickson, 1978b; Pither, 1978), 84-91% of
triadimefon doses of 0.14 or 5 mg/kg, respectively, was excreted with
the urine within 48-72 hours after oral administration. Less than 6%
was excreted in faeces.
Evidently, triadimefon is readily absorbed by the animals and
subsequently metabolised in the animal organism in the transitional
period before excretion. Analytical determination of the distribution
between tissues, fat and organs have been made on rats and cows
(Fredrickson, 1978 a and b), pigs (Pither, 1978) and poultry (Nye,
1979).
Results from these studies are summarised in Table 11. This table
shows the peak levels which are found shortly after oral
administration of triadimefon at the dosages which are indicated, and
the remaining residues found after 4-7 days of post-treatment
elimination period.
The metabolic pattern of triadimefon has been followed in most of
these feeding studies with the unequivocal identification of the
metabolites V, VI and VII (cfr. Figure 1 and Table 9) in most tissues
and organs from rat, cow, pig and hen. These metabolites are formed
by stepwise oxidation of the tert. butyl group of triadimefon into
primary alcohol and to carboxylic acid that are eliminated with the
urine, in which they are identified in free forms or as conjugates of
glucoronic acid together with other and unidentified polar
metabolites. As part of these studies it has been found that milk
contained 0.003 mg/kg triadimefon after 5 daily doses of 0.14 mg/kg.
Eggs from hens which had been given one dose of 2.4 mg/kg showed 0.12
mg/kg after 24 hours and 0.04 mg/kg after 96 hours.
Fish
The accumulation and persistence of residues was studied in Channel
catfish (Ictalurus punctatus) under continuous exposure to
radio-labelled triadimefon for a 28-day period at concentrations of
approximately 10 and 100 ppb (µg/l) in water (Lamb and Roney, 1977).
Concentration factors of 7.6 and 6.5, resp. were determined from the
two levels. After transfer to uncontaminated water 88% of the
accumulated 14C residues were excreted by the fish within 5 hours and
approximately 96% within the following 7-10 days.
Table 11. Residues in animal tissues after oral administration of triadimefon.
Days × Residues (mg/kg)*
dose (mg/ Time after Reference
Animal kg b.w.) administr. Muscle Fat Liver Kidney Egg
approx. approx.
Rat 1 × 25 2 - 8 hrs 4.5-8.0 43.5-45.0 26 - 29 11 - 17 Fredrickson
7 days 0.01-0.02 0.02-0.09 0.10-0.14 0.02-0.08 1978a
Cow 1 × 10 1 - 2 hrs. 0.36 4.01 3.65 15.0 Fredrickson
5 × 0.14 1 - 2 hrs. n.d. n.d. 0.08 0.05 1978b
Pig 5 × 5 3 hrs. 0.30 1.0 3.1 4.0 Pither, 1978
Hen 1 × 2.4 6 hrs. 0.12 0.3 0.26 1.18 0.12 Wye, 1979
96 hrs. <0.01 <0.01 <0.01 <0.01 0.04
* Sum of triadimefon and triadimenol after GLC-analysis
Photodegradation
The pattern of photodecomposition of triadimefon and of triadimenol
have been studied in a number of individual tests by exposure to
UV-light of the compounds in solution (or suspension) or on a solid
support, e.g. silicagel plate or silty clay loam soil surfaces
(Vogeler, 1975; Clark et al., 1978; Wilmes, 1978; Nicholas et al.,
1977; Obrist and Thornton, 1978; Takase and Hasebe, 1979). Several of
the reaction products after such exposures are shown in Figure 1 and
Table 9, from which it is evident that the abiotic molecular breakdown
differs considerably from the patterns already described in plant and
animals. Among compounds which are identified in several of the tests
are especially mentioned 1,2,4-triazole (XI), 4-chlorophenol (VIII)
and 4-chlorophenyl methyl carbonate (IX). Also mentioned is the
formation of high molecular polymers as well as fully degraded 14CO2
as the result experimental irradiation.
As has been found in most of the other media, triadimenol proves to be
more stable to photodegradation than triadimefon. An experimental
half-life of 10-12 hours for the latter under specified light
conditions (in water) compares with 36 hours for the triadimenol.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information was reported to the meeting.
METHODS OF RESIDUES ANALYSIS
Residues of triadimefon and its primary metabolite, triadimenol, which
is also a fungicidal chemical, can be measured by gas chromatographic
multiresidue methodology developed by Specht (1977) and modified by
others (Thornton et al., 1977; Thornton and Lloyd, 1977). In
principle this method is based on extraction of the fungicides with
acetone or methanol with addition of a specified amount of water,
followed by partitioning between acetone-water and dichloromethane.
After evaporation of the dichloromethane, the extract is
chromatographed on a Florisil column with 6% (v/v) ethyl ether in
petroleum and 40% (v/v) ethyl acetate in petroleum ether. The second
eluate is concentrated and gas chromatographed using an AFID
(nitrogen-specific) detector.
In this analysis triadimefon, and the two diastereoisomeric forms, A
and B of triadimenol are determined separately. The method is
applicable for the analysis of various plant materials and soils for
which it has been validated and found to have lower limits of
determination of 0.03 mg/kg (triadimefon) and 0.06 mg/kg (triadimenol)
in soil and in plant material containing more than 80% of water, and
0.05 mg/kg (triadimefon) and 0.1-0.15 mg/kg (triadimenol) in grain and
straw of cereals. Recoveries are found as 89-98% in green plants,
80-99% in straw, and 90-99% in grains. Various fruit and vegetables
are quoted for recoveries in ranges from 80-100%.
NATIONAL LIMITS REPORTED TO THE MEETING
National limits and associated pre-harvest intervals which have been
reported to the meeting are shown in Table 12.
APPRAISAL
Triadimefon is a systemic fungicide used against mildew, rust and
other fungi on cereals, vegetables and fruit crops as well as
ornamentals. It is marketed in many countries in formulations of
wettable powders, emulsifiable concentrates, dusts or pastes. It is
often used in foliar spraying programmes with repeated applications at
low dosages. Information on the impurities in technical triadimefon
was reported to the meeting.
It is probable that the systemic properties of triadimefon arise from
uptake of the parent compound through root or plant surfaces, followed
by reduction to and translocation of the major metabolite,
triadimenol, which presumably is the carrier of the fungicidal
activity. Such processes start shortly after application of
triadimefon to plants so that triadimenol dominates the residue
constituent at the end of normal pre-harvest waiting periods. Polar
metabolites, including 4-chlorophenol, as conjugates have been
identified as further metabolites in plant material.
At the time of harvest and following recommended preharvest intervals,
measurable residues up to about 2.0 mg/kg may be found in various
crops, whilst for various other crops the residues are at or below
limit of determination. Whilst the meeting was prepared to recommend
limits for crops in the latter cases, it was considered that the
experimental data for hops, coffee and tobacco, as well as for
pineapples after post-harvest treatments were insufficient. More
information on actual use patterns and related residue data should be
made available to enable recommendations to be made concerning those
crops.
Table 12. National Limits and Preharvest intervals Reported to the
Meeting
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Austria Cereals 1 35
Beets 0.2 35
Grape, pome fruit 1 35
Cucumber, tomato, 1 4
paprika
Belgium Wheat, barley 42
Table 12. Continued...
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Brazil Wheat 0.01 42
Denmark Cereals 28
Pome fruit 14
France Apple 1 7
Melon 1 3
Germany Cereals 35
(F.R.) Cucumber 3
Apple 14
Grape 35
Israel Wheat 42
Netherlands Wheat, barley 42
N. Zealand Apple 49
Cereals 49
Grapes 28
Norway Apple, strawberry 14
Cereals, berries 28
S. Africa Apple 0.05
Barley 0.1 40
Cucumber 0.05 3
Grape 0.05 7
Mango 0.05
Yugoslavia Cereals 35
Cucumber 7
Other crops 21
Metabolism and degradation in animals, soil, microorganisms and water
as well as patterns of abiotic photodecomposition of triadimefon have
been studied extensively, and detailed lists of possible metabolites
and breakdown products are available. Triadimefon and especially
triadimenol are eliminated from tissues of livestock at relatively
fast rates, resulting in residues at or below limit of determination
within one or a few days in meat, milk and egg-products. In fat and
in offals from slaughtered animals, however, significant residues of
triadimenol may be obtained.
Convenient multi-residue methodology based on gas chromatography is
available for the determination of triadimefon and triadimenol in food
and food products.
RECOMMENDATIONS
As no ADI had been allocated the meeting proposed the following
Guideline Level on a basis of taking into account the preharvest
intervals indicated. The GLs refer to the sum of the parent compound
triadimefon and its major metabolite, triadimenol.
Guideline Pre-harvest interval on
Commodity Level(mg/kg) which recommendation is based
Apples 0.2 14 days
Cucumbers (indoor
and outdoor) 0.2 3 days
Currants, red 1 14 days
Eggs 0.1*
Grapes 2 28 days
Melons 0.5 4 days
Milk 0.1*
Onions, spring 0.1* 21 days
Paprika 0.5 3 days
Peas, without pods 0.1* 3 days
Pumpkins 0.1* 3 days
Strawberries 0.2 7 days
Sugar beets, roots 0.1* 21 days
Sugar beets, leaves 0.1* 21 days
Tomatoes, (indoor
and outdoor 0.5 3 days
Grain (barley,
oats, wheat) 0.1* 42 days
Straw (barley,
oat, wheat) 2 42 days
(* At or about the limit of determination)
FURTHER WORK OR INFORMATION
Desirable
1. Feeding studies on livestock animals for establishing
dose-response relationship between feed contents and residues in
animals tissues, especially fat, and including realistic feeding
levels;
2. Further information on residue levels in certain products, such as
coffee and hops and in pineapples after appropriate post harvest
treatments.
REFERENCES
Bayer AG. Various reports on triadimefon residues in fruit,
vegetables and soil, as well as from animal experiments. Bayer AG,
Leverkusen, FRG. (1975/79), Unpublished.
Brandes, W., Steffens, W., Führ, F., Scheinpflug, H. - Weitere
Untersuchungen zur Translokation von 14C-Triadimefon in
Gurkenpflanezen. Pflanzenschutz Nachrichten Bayer 31, 132-144.
Mobay Reports. Various unpublished reports prepared by the Research
and Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, U.S.A.
Buchenauer, H. - Systemisch-fungizide Wirkung und Wirkungsmechanismus
von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-Forstwirtsch.
(Berlin Dahlem) 165, 154-155.
Untorsuchungen zur systemischen Wirkung und zum Einfluss von
RBAYLETON (Triadimefon) auf verschiedene pilzliche
Getreidekrankheiten. Pflanzenschutz-Nachrichten Bayer 29, 267-280.
Clark, T., Clifford, D.R., Deas, A., Gendle, P., Watkins, D.
Photolysis, metabolism and other factors influencing the performance
of triadimefon as a powdery mildew fungicide. Pest. Sci. 9, 497-506.
Fisher, D.J., Pickard, J.A., McKenzie, C.M. - Uptake of the systemic
fungicide triadimefon by clover and its effect on symbiotic nitrogen
fixation. Pest. Sci. 10, 75-82.
Fredrickson, D.R. - Metabolism of BAYLETONTM in Rats. Mobay Report
No. 66 201, June 20, (1978a).
Metabolism of BAYLETONTM in the cow. Mobay Report No. 66, 202,
June 20, (1978b).
Frohberger, P.E. - Zur Frage der Biologischen Aktivität von
BAYLETON-Rück-standen im Boden. BAYER AG.
Pflanzenschutz-Anwendungstechnik, unpublished report April 12, 1978.
Für, F., Paul, V., Steffens, W. and Scheinpflug, H. Translokation von
14C-Triadimefon nach Applikation auf Sommergerste und sein Wirkung
gegen Erysiphe Graminis var. hordei. Pflanzenschutz-Nachrichten Bayer
31, 116-131.
Gasztony, M., Josepovits, G. - Translocation and metabolism of
triadifflefon in different plant species. Acta Phytopathologica
Academiae Scientiarum Hungaricae, 13, 403-415.
Kraus, P. - Untersuchungen zur Wirkung von BAYLETON aus dem Boden über
die Gaphase gegen Erysiphe graminis an Gerste. BAYER AG,
Pflanzenschutz Answendungstechnik, unpublished report, February 8,
1979.
Lamb, D.W., Roney, D.J. - Accumulation and Persistence of Residues in
Channel Catfish Exposed to BAYLETON TM-14C. Mobay Report No. 52 775,
May 4, 1977.
Mango, R.R., Puhl, R.J. - The Aerobic and Anaerobic Soil Metabolism of
BAYLETONTM. Mobay Report No. 51 230, January 12, (1977a).
Mango, R.R., Puhl, R.J. and Thornton, J.S. - The Metabolism of
BAYLETON in Apples. Mobay Report No. 53 621, August 9, (1977b).
Nichols, S.S., Thornton, J.S., Publ, R.J. - Photodecomposition of
BAYLETONTM in Water solution. Mobay Report No. 52 688, April 6,
1977.
Nye, D.E. - The Fate of RBAYLETON-14C in Poultry. Mobay Report No.
67 482, March 8, 1979.
Obrist, J.J., Thornton, J.S. - Stability of BAYLETONTM in Sterile
Aqueous Buffer Solutions. Mobay Report No. 50 799, November 29, 1976.
Obrist, J.J., Publ, R.J. and Thornton, J.S. - The Degradation and Fate
of BAYLETONTM in a simulated pond environment. Mobay Report No. 53
038, May 31, (1977a).
Obrist, J.J. and Thornton, J.S. - Leaching characteristics of
BAYLETONTM on Aged soil. Mobay Report No. 51 232, January 21, 1977b.
Photodecomposition of RBAYTON in Aqueous solutions and on a soil
surface. Mobay Report No. 66 794, October 20, 1978.
Pither, K.M. - Metabolism of BAYLETONTM in Male and female pigs.
Mobay Report No. 66 509, November 29, 1978.
Degradation of RBAYLETON by Mixed cultures of microorganisms.
Mobay Report No. 67 049, January 30, 1979.
Puhl, R.H., Hurley, J.B. - Soil Adsorption and desorption of
BAYLETON-Ring UL14C. Mobay Report No. 66 501, September 11, (1978a).
Soil adsorption and desorption of BAYTON-II. Mobay Report No. 66
745, December 12, (1978b).
Scheinpflug, H. and Paul, V. - On the mode of action of Triadimefon.
Neth. J. Plant Path. 83, (Suppl.1), 105-111. (1978a).
Scheinpflug, H., Paul, V. and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Mitt. Biol.
Bundesanst. Land Forstwirtsch. (Berlin-Dahlem) 178, 178. (1978b).
Scheinpflug, H., Paul V., and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Pflanzenschutz -
Nachrichten Bayer 31, 101-115.
Schlüter, K. - Fungizide Wirkung von Triadimefon in der Gasphase. Z.
Pflanzenkr. Pflanzenschutz 84, 612-614.
Specht, W. - Gas Chromatographic method for determining residues of
the fungicides fuberidazol, fluotrimazole and triadimefon in plants
and soil. Pflanzenschutz-Nachrichten Bayer 30, 1.
Steffens, W., Führ, F. - Versuche zur Retranslokation von Triadimefon.
Kernforschung sanlage Jülich, Arbeitagruppe Radioagronomie,
unpublished report ARA 4/76. (1976).
Steffens, W., Wieneke, J,. - Bilanzversuche mit dem 14C-markierten
Fungizid Triadimefon (MEB 6447) nach Spritzbehandlung von
Sommergerste in Lysimetern mit ungesförten Bodenprofilen, Verbleib in
der behandelten Pflanze, Verlagerung im Boden und Aufnahme durch
Folgefrüchte. Kernforschungsanlage Jülich, Arbeitsgruppe
Radioagronomie, unpublished report ARA 4/77, September. (1977).
Takase, I., Hasebe, K. - Photolysis of Triadimefon in Upland Soils.
Nitokuno Report No. 1093, (RA), March 2, 1979.
Takase, I., Yoshimoto, Y. - Degradation of Triadimefon in Upland soils
under laboratory and field conditions. Nitokuno Report No. 1091 (RA),
December 11, 1978.
Thornton, J.S. - A gas chromatographic method for Bayleton and its
metabolite in apples. Mobay Report No. 54 166, December 30, 1977.
Thornton, J.S., Lloyd, C.M. - A gas chromatographic method for
Bayleton and its metabolite in soil and water. Mobay Report No. 51
231, January 31, 1977.
Thornton, J.S., Hurley, J.B., Obrist, J.J. - Soil thin-layer mobility
of Twenty-four pesticide chemicals. Mobay Report No. 51 0.16, December
15, 1976.
Vogeler, K. BAYER AG, Pflanzenschutz-Anwendungstechnik, unpublished
report. Verhalten von Triadimefon (MEB 6447) unter der Einwirkung von
UV-Licht. RA 364, June 3, 1975.
Der metabolismus von Triadimefon in gerste unter
Gewttchshausbedlingungen und im Boden. RA 272, April 12, (1976a).
Untersuchunge zur Wirkang von Triadimefon über die Gasphase gegen
Echten Mehltau an Gerste. RA 662, August 16, (1976b).
Bericht über die Metabolisierung von 14C-Triadimefon in
Sommergerste und Boden sowie in Folgenpflanzen. RA 813, October 19,
(1977a).
Untersuchungen zur Unwandlung der reinen Diastereoisomeren A und B
von KWG 0519 in das jeweils andere Isomere in Gerstenpflanzen und
Boden. RA 871, November 14, (1977b).
Adsorptionsisothermen von Tiadimenol in verschiedenen Böden. RA 496,
July 3, 1978.
Wilmes, R. - Versuche zum Photoabbau von MEB 6447, MEB 6449 und KWG
0519. BAYER AG, Pflanzenschutz-PIQ, PF-Report No. 1266, August 7,
1978.
Fate in soil
Adsorption and desorption studies determining the Freundlich isotherm
characteristics have been made on triadimefon as well as on
triadimenol in different soils and in different concentrations (Puhl
and Hurley, 1978 a and b, Vogeler, 1978, Frohberger, 1978). The
results of such studies correlate well with laboratory leaching
experiments in standardised procedures (BBA-Merkblatt no. 37) and
TLC-mobility tests from which triadimefon can be classified as a
compound of low mobility in a variety of soil types (Obrist and
Thornton, 1977b, Thornton et al., 1976).
No triadimefon was detected in leachates in the standardised test and
only limited amounts of radiolabelled residues could be determined in
soil layers below 2.5 cm in a balance study on treatment of spring
barley with 14C-labelled triadimefon if measurements were made from
76-427 days after application (Steffens & Wieneke, 1977). In the
leaching studies of Obrist and Thornton (1977b) less than 3% of the
total radioactivity could be traced through 30 cm of aged soil after a
period of 45 days with simulated rain of 12.5 mm per day. None of
this activity was identified as intact triadimefon or as triadimenol.
Half-life determinations in two standard soils gave RL50-values of 60
and 100 days for triadimefon and of 130 and 310 days for triadimenol
(Bayer-reports Nos. 9012/1974 and 9050/1976).
The disappearance rates of triadimefon and triadimenol have been
studied by several groups in German, Japanese and U.S. trials under a
variety of conditions and using a broad range of different soil types
(Mango and Puhl, 1977a; Takase & Yashimoto, 1978; and reported from
Bayer AG and Mobay Chem. Corporation). Variations in RL50-values are
found from about 10 days to 7 months for triadimefon and from about 3
months to 500 days for triadimenol. In summarising such data it is
justified to characterize triadimefon, and especially triadimenol, as
persistent compounds in soil, although it is presently impossible to
evaluate which specific environmental conditions (e.g. soil type,
temperature, etc.) are of greatest significance for the variability in
persistence. Residue levels of triadimefon have been determined in
soil after several years of treatment in cereal fields and apple
orchards (Bayer reports, 1977). The residues consisted mainly of
triadimenol which reached plateau levels (0-10 cm layer) from 0.13
mg/kg to 0.65 mg/kg in the 5th years of trials in apple orchards.
Residue uptake from soil, including crop rotation
Uptake of triadimefon and triadimenol from soil was established in
experiments with barley (Frohberger, 1978) and cloverplants (Fisher
et al., 1979). In the latter study a linear relationship between
amount absorbed and the amount of triadimefon applied to the soil was
found. The uptake of soil residues by later crops have been studied
by Steffen and Wieneke (1977), who used turnip and clover as catch
crops followed by sugarbeet, oats or winter wheat as the main crops in
lysimeters which had originally been treated with radio-labelled
triadimefon on cultures of spring barley. 0.2% of soil residues was
absorbed by the catch crops, while 0.5 - 4.3% was found in the
following main crops. It is assumed from these experiments that
mainly triadimenol is absorbed from the soil, and subsequently
metabolised to polar compounds in the plants.
Soil metabolism
The metabolic conversions and degradation of triadimefon in soil have
been studied by Vogeler (1976a, 1977a and b), Mango & Puhl (1977a) and
Takase & Yashimoto (1978). It is found that a primary reduction to
triadimenol takes place as a microbial reaction, and resulting into
two diastereoisomeric forms, A and B, in ratios which differ from
about 1:1 to 1:4 in the various experiments.
In radio-labelled experiments Mango and Puhl (1977) applied
triadimefon to soils using either benzene-ring or triazole-ring
14C-labelling. After 238 days they found 45-49% of radioactivity in
triadimenol, 7% in triazole and 24% or 5% of 14CO2 from the two ring
systems, respectively, indicating a complete, although gradual and
relatively slow degradation of triadimefon. After application on
cereals, Vogeler in his experiments (using benzene-ring labelling)
found 15-25% and 32-65% of soil radioactivity to be non-extractable.
Fate in microorganisms
As already mentioned, the conversion in soil of triadimefon to
triadimenol under natural environmental conditions is considered to be
a microbial reaction (Mango & Puhl, 1977). This is confirmed in
laboratory shake cultures of Aspergillus niger, which may convert
from 5-32% of triadimefon to triadimenol in 2-5 days, respectively.
No further metabolism of triadimenol was detected in these
experiments. In other experiments (Clark et al., 1978), triadimefon
was incubated with mycelial mats of the fungus, and a different
metabolic pattern was observed as indicated by the findings of an
isopropyl analogue (IV) to triadimefon in the culture by mass
spectrometric analysis. And, in experiments by Pither (1979),
combined bacterial or fungal cultures during 13 days had no detectable
effects on triadimefon, leaving 99% of the parent compound intact in
the cultures.
Fate in water
In sterile aqueous buffer solutions, triadimefon is found to be stable
for many weeks at temperatures ranging from 25°C - 45°C and at pH 3,
6, and 9 (Obrist and Thornton, 1976). In a simulated pond
environment, however, a fast degradation was found and metabolites
identified in the following order: triadimenol (III), triadimefon
symmetrical isomer (II), 1,2,4,triazole (XI) and
Delta2-1-2-4-triazolin-5-one (XII) (Obrist et al., 1977a). 30% of
the 14C activity (from benzene-ring labelling) was missing at the end
of the experiment, possibly under formation of 14CO2. The
disappearance rate for the parent compound in these experiments
corresponded to half-lives of 6-8 days in the water phase and 18-20
days in the silt.
Fate in plants
An uptake of triadimefon from soil to plants, e.g. to beans and barley
(Buchenauer, 1975 and 1976) and through foliar surfaces to beans,
cereals, cucumbers and tomato followed by translocation with the
transpiration stream (i.e. acropetally) has been demonstrated in
several studies (Steffens & Führ, 1976; Scheinpflug et al., 1977b;
Brandes et al., 1978; Für et al., 1978; Gasztony & Josepovits,
1978). A slight translocation in the opposite direction, i.e.
basipetal movement, is observed also (cfr. Buchenauer, 1975 and 1976).
A transfer of triadimefon through the vapour phase with a measurable
fungicidal effect and evidence of metabolites in untreated plants is
described in studies from greenhouses (Scheinpflug & Paul, 1977a;
Schlüter, 1977; Scheinpflug et al., 1978; Kraus, 1979) and in closed
glass chambers (Vogeler & Paul, 1976b). Such vapour phase effects are
dependant on the distance between target plant and spray deposits.
As is the case for soil, there is much experimental evidence that
triadimefon is reduced in plants to triadimenol, which is the major
metabolite (up to 80%) and which occurs in the two diastereoisomeric
forms, A and B. Contrasting the situation in soil, however, the
isomer form A is dominating as indicated by the ratios of A:B varying
from 1:1 to 3:1. Besides triadimenol, polar substances (Rf values of
0) and a compound with the Rf value of 0.26 (presumably compound VI
in Table 9) were found.
Results from experimental determinations of various 14C labelled
metabolites and fractions in cereal plants (benzene-ring label) and in
apples (triazole-ring label) are shown in Table 10.
Table 10. Percent distribution of radioactivity in cereals and apples after spray application of 14C-Triadimefon
(recovered radioactivity = 100) (Vogeler, 1976, 1977, 1978; Mango et al., 1977)
EXTRACTABLES
Plant Dose Days
(I = Indoor (a.i.) post RF 0 RF 0.26+ unidentified Triadimenol A Triadimenol B Triadimefon non
F = Field) appl. metabolite extractables
barley (I)
total plant 0 n.d. n.d. n.d. n.d. n.d. 99.5 0.5
" " 5 7.3 n.d. n.d. 57.3 21.8 9.7 3.9
" " 10 15.8 n.d. n.d. 50.3 18.9 7.9 7.1
" " 250 g/ha 28 15.8 n.d. n.d. 45.1 17.4 6.4 15.3
straw 38 15.9 n.d. n.d. 43.6 14.4 10.3 15.8
" 62 16.7 1.9 n.d. 41.0 17.3 4.8 18.3
barley (F)
total plant 0 <1 n.d. n.d. 1 n.d. 92 6
" " 5 13 n.d. n.d. 48 15 12 12
" " 125 g/ha 17 35 n.d. n.d. 32 10 <1 23
straw 28 9 <1 n.d. 12 4 n.d. 75
" 76 8.4 5 n.d. 8.4 3 n.d. 75
wheat (F)
straw 250 g/ha 47 37 2 n.d. 23 6 n.d. 32
apples (F)
peel 28 5.7* n.d. 2.8 14.9 13.6 10.8 5.0
pulp 5.3* n.d. 3.1 11.2 7.1 2.1 0.9
total fruit 15 mg/100ml 11.0* n.d. 5.9 26.1 20.7 13.0 5.9
peel 48 4.8* n.d. 1.5 14.5 14.8 10.4 6.0
pulp 6.4* n.d. 2.1 10.4 9.4 2.1 1.0
total fruit 11.2* n.d. 3.6 24.9 24.2 12.5 7.0
+ presumably compound VI, table 9.
n.d. = not detectable * aqueous phase
The amount of radioactivity in grains and ears was too small for separation by TLC.
FATE IN ANIMALS
Mammals
Following administration of a single oral dose of 14C-triadimefon of
25 mg per kg b.w. to rats, it was found that 75 - 83% was excreted
within 7 days, with 30-40% in the urine and 35-53% in the faeces.
Expiration of 14CO2 was not found (Fredrickson, 1978a). In cows as
well as in pigs (Fredrickson, 1978b; Pither, 1978), 84-91% of
triadimefon doses of 0.14 or 5 mg/kg, respectively, was excreted with
the urine within 48-72 hours after oral administration. Less than 6%
was excreted in faeces.
Evidently, triadimefon is readily absorbed by the animals and
subsequently metabolised in the animal organism in the transitional
period before excretion. Analytical determination of the distribution
between tissues, fat and organs have been made on rats and cows
(Fredrickson, 1978 a and b), pigs (Pither, 1978) and poultry (Nye,
1979).
Results from these studies are summarised in Table 11. This table
shows the peak levels which are found shortly after oral
administration of triadimefon at the dosages which are indicated, and
the remaining residues found after 4-7 days of post-treatment
elimination period.
The metabolic pattern of triadimefon has been followed in most of
these feeding studies with the unequivocal identification of the
metabolites V, VI and VII (cfr. Figure 1 and Table 9) in most tissues
and organs from rat, cow, pig and hen. These metabolites are formed
by stepwise oxidation of the tert. butyl group of triadimefon into
primary alcohol and to carboxylic acid that are eliminated with the
urine, in which they are identified in free forms or as conjugates of
glucoronic acid together with other and unidentified polar
metabolites. As part of these studies it has been found that milk
contained 0.003 mg/kg triadimefon after 5 daily doses of 0.14 mg/kg.
Eggs from hens which had been given one dose of 2.4 mg/kg showed 0.12
mg/kg after 24 hours and 0.04 mg/kg after 96 hours.
Fish
The accumulation and persistence of residues was studied in Channel
catfish (Ictalurus punctatus) under continuous exposure to
radio-labelled triadimefon for a 28-day period at concentrations of
approximately 10 and 100 ppb (µg/l) in water (Lamb and Roney, 1977).
Concentration factors of 7.6 and 6.5, resp. were determined from the
two levels. After transfer to uncontaminated water 88% of the
accumulated 14C residues were excreted by the fish within 5 hours and
approximately 96% within the following 7-10 days.
Table 11. Residues in animal tissues after oral administration of triadimefon.
Days × Residues (mg/kg)*
dose (mg/ Time after Reference
Animal kg b.w.) administr. Muscle Fat Liver Kidney Egg
approx. approx.
Rat 1 × 25 2 - 8 hrs 4.5-8.0 43.5-45.0 26 - 29 11 - 17 Fredrickson
7 days 0.01-0.02 0.02-0.09 0.10-0.14 0.02-0.08 1978a
Cow 1 × 10 1 - 2 hrs. 0.36 4.01 3.65 15.0 Fredrickson
5 × 0.14 1 - 2 hrs. n.d. n.d. 0.08 0.05 1978b
Pig 5 × 5 3 hrs. 0.30 1.0 3.1 4.0 Pither, 1978
Hen 1 × 2.4 6 hrs. 0.12 0.3 0.26 1.18 0.12 Wye, 1979
96 hrs. <0.01 <0.01 <0.01 <0.01 0.04
* Sum of triadimefon and triadimenol after GLC-analysis
Photodegradation
The pattern of photodecomposition of triadimefon and of triadimenol
have been studied in a number of individual tests by exposure to
UV-light of the compounds in solution (or suspension) or on a solid
support, e.g. silicagel plate or silty clay loam soil surfaces
(Vogeler, 1975; Clark et al., 1978; Wilmes, 1978; Nicholas et al.,
1977; Obrist and Thornton, 1978; Takase and Hasebe, 1979). Several of
the reaction products after such exposures are shown in Figure 1 and
Table 9, from which it is evident that the abiotic molecular breakdown
differs considerably from the patterns already described in plant and
animals. Among compounds which are identified in several of the tests
are especially mentioned 1,2,4-triazole (XI), 4-chlorophenol (VIII)
and 4-chlorophenyl methyl carbonate (IX). Also mentioned is the
formation of high molecular polymers as well as fully degraded 14CO2
as the result experimental irradiation.
As has been found in most of the other media, triadimenol proves to be
more stable to photodegradation than triadimefon. An experimental
half-life of 10-12 hours for the latter under specified light
conditions (in water) compares with 36 hours for the triadimenol.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT
CONSUMPTION
No information was reported to the meeting.
METHODS OF RESIDUES ANALYSIS
Residues of triadimefon and its primary metabolite, triadimenol, which
is also a fungicidal chemical, can be measured by gas chromatographic
multiresidue methodology developed by Specht (1977) and modified by
others (Thornton et al., 1977; Thornton and Lloyd, 1977). In
principle this method is based on extraction of the fungicides with
acetone or methanol with addition of a specified amount of water,
followed by partitioning between acetone-water and dichloromethane.
After evaporation of the dichloromethane, the extract is
chromatographed on a Florisil column with 6% (v/v) ethyl ether in
petroleum and 40% (v/v) ethyl acetate in petroleum ether. The second
eluate is concentrated and gas chromatographed using an AFID
(nitrogen-specific) detector.
In this analysis triadimefon, and the two diastereoisomeric forms, A
and B of triadimenol are determined separately. The method is
applicable for the analysis of various plant materials and soils for
which it has been validated and found to have lower limits of
determination of 0.03 mg/kg (triadimefon) and 0.06 mg/kg (triadimenol)
in soil and in plant material containing more than 80% of water, and
0.05 mg/kg (triadimefon) and 0.1-0.15 mg/kg (triadimenol) in grain and
straw of cereals. Recoveries are found as 89-98% in green plants,
80-99% in straw, and 90-99% in grains. Various fruit and vegetables
are quoted for recoveries in ranges from 80-100%.
NATIONAL LIMITS REPORTED TO THE MEETING
National limits and associated pre-harvest intervals which have been
reported to the meeting are shown in Table 12.
APPRAISAL
Triadimefon is a systemic fungicide used against mildew, rust and
other fungi on cereals, vegetables and fruit crops as well as
ornamentals. It is marketed in many countries in formulations of
wettable powders, emulsifiable concentrates, dusts or pastes. It is
often used in foliar spraying programmes with repeated applications at
low dosages. Information on the impurities in technical triadimefon
was reported to the meeting.
It is probable that the systemic properties of triadimefon arise from
uptake of the parent compound through root or plant surfaces, followed
by reduction to and translocation of the major metabolite,
triadimenol, which presumably is the carrier of the fungicidal
activity. Such processes start shortly after application of
triadimefon to plants so that triadimenol dominates the residue
constituent at the end of normal pre-harvest waiting periods. Polar
metabolites, including 4-chlorophenol, as conjugates have been
identified as further metabolites in plant material.
At the time of harvest and following recommended preharvest intervals,
measurable residues up to about 2.0 mg/kg may be found in various
crops, whilst for various other crops the residues are at or below
limit of determination. Whilst the meeting was prepared to recommend
limits for crops in the latter cases, it was considered that the
experimental data for hops, coffee and tobacco, as well as for
pineapples after post-harvest treatments were insufficient. More
information on actual use patterns and related residue data should be
made available to enable recommendations to be made concerning those
crops.
Table 12. National Limits and Preharvest intervals Reported to the
Meeting
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Austria Cereals 1 35
Beets 0.2 35
Grape, pome fruit 1 35
Cucumber, tomato, 1 4
paprika
Belgium Wheat, barley 42
Table 12. Continued...
Limit Pre-harvest
Country Crop (mg/kg) interval (days)
Brazil Wheat 0.01 42
Denmark Cereals 28
Pome fruit 14
France Apple 1 7
Melon 1 3
Germany Cereals 35
(F.R.) Cucumber 3
Apple 14
Grape 35
Israel Wheat 42
Netherlands Wheat, barley 42
N. Zealand Apple 49
Cereals 49
Grapes 28
Norway Apple, strawberry 14
Cereals, berries 28
S. Africa Apple 0.05
Barley 0.1 40
Cucumber 0.05 3
Grape 0.05 7
Mango 0.05
Yugoslavia Cereals 35
Cucumber 7
Other crops 21
Metabolism and degradation in animals, soil, microorganisms and water
as well as patterns of abiotic photodecomposition of triadimefon have
been studied extensively, and detailed lists of possible metabolites
and breakdown products are available. Triadimefon and especially
triadimenol are eliminated from tissues of livestock at relatively
fast rates, resulting in residues at or below limit of determination
within one or a few days in meat, milk and egg-products. In fat and
in offals from slaughtered animals, however, significant residues of
triadimenol may be obtained.
Convenient multi-residue methodology based on gas chromatography is
available for the determination of triadimefon and triadimenol in food
and food products.
RECOMMENDATIONS
As no ADI had been allocated the meeting proposed the following
Guideline Level on a basis of taking into account the preharvest
intervals indicated. The GLs refer to the sum of the parent compound
triadimefon and its major metabolite, triadimenol.
Guideline Pre-harvest interval on
Commodity Level(mg/kg) which recommendation is based
Apples 0.2 14 days
Cucumbers (indoor
and outdoor) 0.2 3 days
Currants, red 1 14 days
Eggs 0.1*
Grapes 2 28 days
Melons 0.5 4 days
Milk 0.1*
Onions, spring 0.1* 21 days
Paprika 0.5 3 days
Peas, without pods 0.1* 3 days
Pumpkins 0.1* 3 days
Strawberries 0.2 7 days
Sugar beets, roots 0.1* 21 days
Sugar beets, leaves 0.1* 21 days
Tomatoes, (indoor
and outdoor 0.5 3 days
Grain (barley,
oats, wheat) 0.1* 42 days
Straw (barley,
oat, wheat) 2 42 days
(* At or about the limit of determination)
FURTHER WORK OR INFORMATION
Desirable
1. Feeding studies on livestock animals for establishing
dose-response relationship between feed contents and residues in
animals tissues, especially fat, and including realistic feeding
levels;
2. Further information on residue levels in certain products, such as
coffee and hops and in pineapples after appropriate post harvest
treatments.
REFERENCES
Bayer AG. Various reports on triadimefon residues in fruit,
vegetables and soil, as well as from animal experiments. Bayer AG,
Leverkusen, FRG. (1975/79), Unpublished.
Brandes, W., Steffens, W., Führ, F., Scheinpflug, H. - Weitere
Untersuchungen zur Translokation von 14C-Triadimefon in
Gurkenpflanezen. Pflanzenschutz Nachrichten Bayer 31, 132-144.
Mobay Reports. Various unpublished reports prepared by the Research
and Development Dept., Agricultural Chemicals Division, Mobay Chemical
Corporation, Kansas City, Mo. 64 120, U.S.A.
Buchenauer, H. - Systemisch-fungizide Wirkung und Wirkungsmechanismus
von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-Forstwirtsch.
(Berlin Dahlem) 165, 154-155.
Untorsuchungen zur systemischen Wirkung und zum Einfluss von
RBAYLETON (Triadimefon) auf verschiedene pilzliche
Getreidekrankheiten. Pflanzenschutz-Nachrichten Bayer 29, 267-280.
Clark, T., Clifford, D.R., Deas, A., Gendle, P., Watkins, D.
Photolysis, metabolism and other factors influencing the performance
of triadimefon as a powdery mildew fungicide. Pest. Sci. 9, 497-506.
Fisher, D.J., Pickard, J.A., McKenzie, C.M. - Uptake of the systemic
fungicide triadimefon by clover and its effect on symbiotic nitrogen
fixation. Pest. Sci. 10, 75-82.
Fredrickson, D.R. - Metabolism of BAYLETONTM in Rats. Mobay Report
No. 66 201, June 20, (1978a).
Metabolism of BAYLETONTM in the cow. Mobay Report No. 66, 202,
June 20, (1978b).
Frohberger, P.E. - Zur Frage der Biologischen Aktivität von
BAYLETON-Rück-standen im Boden. BAYER AG.
Pflanzenschutz-Anwendungstechnik, unpublished report April 12, 1978.
Für, F., Paul, V., Steffens, W. and Scheinpflug, H. Translokation von
14C-Triadimefon nach Applikation auf Sommergerste und sein Wirkung
gegen Erysiphe Graminis var. hordei. Pflanzenschutz-Nachrichten Bayer
31, 116-131.
Gasztony, M., Josepovits, G. - Translocation and metabolism of
triadifflefon in different plant species. Acta Phytopathologica
Academiae Scientiarum Hungaricae, 13, 403-415.
Kraus, P. - Untersuchungen zur Wirkung von BAYLETON aus dem Boden über
die Gaphase gegen Erysiphe graminis an Gerste. BAYER AG,
Pflanzenschutz Answendungstechnik, unpublished report, February 8,
1979.
Lamb, D.W., Roney, D.J. - Accumulation and Persistence of Residues in
Channel Catfish Exposed to BAYLETON TM-14C. Mobay Report No. 52 775,
May 4, 1977.
Mango, R.R., Puhl, R.J. - The Aerobic and Anaerobic Soil Metabolism of
BAYLETONTM. Mobay Report No. 51 230, January 12, (1977a).
Mango, R.R., Puhl, R.J. and Thornton, J.S. - The Metabolism of
BAYLETON in Apples. Mobay Report No. 53 621, August 9, (1977b).
Nichols, S.S., Thornton, J.S., Publ, R.J. - Photodecomposition of
BAYLETONTM in Water solution. Mobay Report No. 52 688, April 6,
1977.
Nye, D.E. - The Fate of RBAYLETON-14C in Poultry. Mobay Report No.
67 482, March 8, 1979.
Obrist, J.J., Thornton, J.S. - Stability of BAYLETONTM in Sterile
Aqueous Buffer Solutions. Mobay Report No. 50 799, November 29, 1976.
Obrist, J.J., Publ, R.J. and Thornton, J.S. - The Degradation and Fate
of BAYLETONTM in a simulated pond environment. Mobay Report No. 53
038, May 31, (1977a).
Obrist, J.J. and Thornton, J.S. - Leaching characteristics of
BAYLETONTM on Aged soil. Mobay Report No. 51 232, January 21, 1977b.
Photodecomposition of RBAYTON in Aqueous solutions and on a soil
surface. Mobay Report No. 66 794, October 20, 1978.
Pither, K.M. - Metabolism of BAYLETONTM in Male and female pigs.
Mobay Report No. 66 509, November 29, 1978.
Degradation of RBAYLETON by Mixed cultures of microorganisms.
Mobay Report No. 67 049, January 30, 1979.
Puhl, R.H., Hurley, J.B. - Soil Adsorption and desorption of
BAYLETON-Ring UL14C. Mobay Report No. 66 501, September 11, (1978a).
Soil adsorption and desorption of BAYTON-II. Mobay Report No. 66
745, December 12, (1978b).
Scheinpflug, H. and Paul, V. - On the mode of action of Triadimefon.
Neth. J. Plant Path. 83, (Suppl.1), 105-111. (1978a).
Scheinpflug, H., Paul, V. and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Mitt. Biol.
Bundesanst. Land Forstwirtsch. (Berlin-Dahlem) 178, 178. (1978b).
Scheinpflug, H., Paul V., and Kraus, P. - Untersuchungen zur
Wirkungsweise von BAYLETON bei Getreidekrankheiten. Pflanzenschutz -
Nachrichten Bayer 31, 101-115.
Schlüter, K. - Fungizide Wirkung von Triadimefon in der Gasphase. Z.
Pflanzenkr. Pflanzenschutz 84, 612-614.
Specht, W. - Gas Chromatographic method for determining residues of
the fungicides fuberidazol, fluotrimazole and triadimefon in plants
and soil. Pflanzenschutz-Nachrichten Bayer 30, 1.
Steffens, W., Führ, F. - Versuche zur Retranslokation von Triadimefon.
Kernforschung sanlage Jülich, Arbeitagruppe Radioagronomie,
unpublished report ARA 4/76. (1976).
Steffens, W., Wieneke, J,. - Bilanzversuche mit dem 14C-markierten
Fungizid Triadimefon (MEB 6447) nach Spritzbehandlung von
Sommergerste in Lysimetern mit ungesförten Bodenprofilen, Verbleib in
der behandelten Pflanze, Verlagerung im Boden und Aufnahme durch
Folgefrüchte. Kernforschungsanlage Jülich, Arbeitsgruppe
Radioagronomie, unpublished report ARA 4/77, September. (1977).
Takase, I., Hasebe, K. - Photolysis of Triadimefon in Upland Soils.
Nitokuno Report No. 1093, (RA), March 2, 1979.
Takase, I., Yoshimoto, Y. - Degradation of Triadimefon in Upland soils
under laboratory and field conditions. Nitokuno Report No. 1091 (RA),
December 11, 1978.
Thornton, J.S. - A gas chromatographic method for Bayleton and its
metabolite in apples. Mobay Report No. 54 166, December 30, 1977.
Thornton, J.S., Lloyd, C.M. - A gas chromatographic method for
Bayleton and its metabolite in soil and water. Mobay Report No. 51
231, January 31, 1977.
Thornton, J.S., Hurley, J.B., Obrist, J.J. - Soil thin-layer mobility
of Twenty-four pesticide chemicals. Mobay Report No. 51 0.16, December
15, 1976.
Vogeler, K. BAYER AG, Pflanzenschutz-Anwendungstechnik, unpublished
report. Verhalten von Triadimefon (MEB 6447) unter der Einwirkung von
UV-Licht. RA 364, June 3, 1975.
Der metabolismus von Triadimefon in gerste unter
Gewttchshausbedlingungen und im Boden. RA 272, April 12, (1976a).
Untersuchunge zur Wirkang von Triadimefon über die Gasphase gegen
Echten Mehltau an Gerste. RA 662, August 16, (1976b).
Bericht über die Metabolisierung von 14C-Triadimefon in
Sommergerste und Boden sowie in Folgenpflanzen. RA 813, October 19,
(1977a).
Untersuchungen zur Unwandlung der reinen Diastereoisomeren A und B
von KWG 0519 in das jeweils andere Isomere in Gerstenpflanzen und
Boden. RA 871, November 14, (1977b).
Adsorptionsisothermen von Tiadimenol in verschiedenen Böden. RA 496,
July 3, 1978.
Wilmes, R. - Versuche zum Photoabbau von MEB 6447, MEB 6449 und KWG
0519. BAYER AG, Pflanzenschutz-PIQ, PF-Report No. 1266, August 7,
1978.
