
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
TRIADIMEFON
Explanation
Triadimefon was evaluated in 1979 and no ADI could be allocated
at that time. Guideline levels were recorded for cereal grains, some
fruits and a number of vegetables, including fruiting vegetables;
guideline levels were also recorded for milk and eggs.*
New information was obtained on the use pattern in various
countries not included in the 1979 Evaluations; additional information
on residues from supervised trials on several crops from other
countries than those covered in the 1979 monograph, and also some
residue information from other crops became available. Information was
also obtained on new or improved methods of analysis for triadimefon
and its main metabolite triadimenol. Data were provided on the fate of
the residues in plants, animals, soil and water and also additional
information on the photodecomposition of the product under various
conditions.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Excretion, storage and metabolism of (benzene ring-UL-14C)-
triadimefon were studied in rats, dairy cattle, pigs and laying hens.
Supplementary metabolic studies were carried out on metabolites of
triadimefon, e.g. triadimenol and triazole (Bayer 1981).
Rat
Rats excreted 75 to 83% of a single oral dose of (benzene ring-
UL-14C)-triadimefon (25 mg/kg bw) within 7 days. Male rats excreted
29.8% of the dose in the urine and 52.7% in the faeces while females
excreted 39.9% in the urine and 34.5% in the faeces. No activity was
found in the expired gases (Fredrickson 1978a).
Each isomer (A and B) of (benzene ring-UL-14C)-triadimenol
was separately administered to rats orally at dose levels of 4 and
25 mg/kg bw. The amounts of the applied activity excreted were 31 to
52% in urine and 37 to 55% in faeces for isomer A, and 14 to 47% in
urine and 44 to 78% in faeces for isomer B. Males excreted more
radioactivity in faeces than in urine, while females excreted slightly
more in urine. No radioactivity was found in expired air (Puhl and
Hurley 1978).
* See Annex II for FAO and WHO documentation.
1,2,4(3(5)-14C)-triazole was administered to rats by the oral,
intravenous and intra-peritoneal routes (Weber et al 1978).
Following oral administration of 1 mg/kg bw, almost 100% of the
radioactivity was absorbed from the gastrointestinal tract. Following
oral or intravenous application of 1 mg/kg bw, about 0.1% of the
applied dose was eliminated with the exhaled air within 30 h. In the
dose range of 0.1 to 100 mg/kg, 92 to 94% of the applied activity was
eliminated renally within 48 h, and 3 to 5% was excreted faecally.
Elimination was dose-proportional and dependent on the route of
administration. Following intraduodenal administration or intravenous
application to rats with a fistulated bile duct, about 12% of the
applied activity was eliminated in the bile within 24 h (Weber
et al 1978).
Following oral administration of 100 mg triadimefon/kg bw,
concentration in blood plasma reached levels of 6 to 8 µg
triadimefon/ml in mice and 1.3 µg triadimefon/ml in rats. The
concentration in mice decreased with a half life of 2.5 h to a level
of 0.9 µg/ml within 8 h (Ritter 1976).
After administration of a 25 mg/kg oral dose of (benzene ring-UL-
14C)-triadimefon to rats, tissue and plasma levels generally peaked
1 to 2 h later. Highest residue levels were found in the fat, reaching
45.0 mg/kg at 4 h in the males and 43.5 mg/kg at 8 h in the females.
Residue levels remaining in the tissues after 7 days were quite low,
with the highest residues being in the liver at 0.14 mg/kg
(Fredrickson 1978a).
Tissues and blood were analysed for 14C at several time-
intervals following administration of a single oral dose of (benzene
ring-UL-14C)-triadimefon isomer A to rats (Puhl and Hurley 1978).
Maximum residue levels were reached at 4 h in females, while in males
several samples had the highest levels at 1 h. Highest residues were
found in fat (ca. 22 to 62 mg triadimenol equivalent/kg), liver
(ca. 41 to 44 mg/kg), skin (ca. 15 to 26 mg/kg) and kidney (ca 13 to
17 mg/kg).
Following administration of 1,2,4-(3(5)-14C)-triazole to rats,
about 50% of the intravenously applied dose of 1 mg/kg bw was present
in the body less gastrointestinal tract (sum of all tissues and
organs) at 8 h post-application. About 1.5% of the administered dose
was present at 3 days post-application. At 6 days post-application,
the amount of activity in the body less gastrointestinal tract had
dropped to below the limit of determination of 0.3%. Throughout the
period of the study, the activity was very largely evenly distributed
in the animal body (Weber et al 1978).
Following administration of (benzene ring-UL-14C)-triadimefon to
rats, a large number of metabolites were found in the urine. The major
metabolite was identified as the triadimenol acid, which was found in
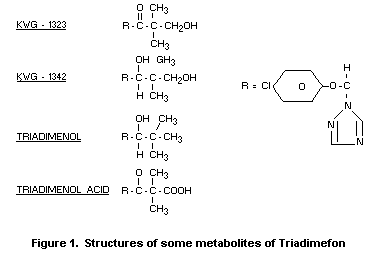
both urine and faeces. KWG 1323 and KWG 1342 were also found in small
amounts. Triadimefon and triadimenol were the major components of the
activity present in plasma, fat and liver (Fredrickson 1978a).
In metabolism studies on rats dosed with isomer A or Isomer B of
(benzene ring-UL-14C)-triadimefon, the metabolites identified in
urine, faeces and tissues (liver, kidneys, fat) were triadimenol acid,
KWG 1342, KW 1323, triadimenol and triadimefon (the latter only in
small amounts in fat). Quantitative differences were observed
according to whether A or isomer B was administered (Puhl and Hurley
1978). Following oral application of 10 mg 1,2,4(3(5)-14C)-triazole
to male rats, the bulk of the radioactivity (>90% of the total amount
of renally eliminated activity) was excreted as unchanged compound
with the urine within 24 h (Ecker 1980).
Pig
Following oral administration of (benzene ring-UL-14C)-
triadimefon (5 g/kg bw) to a pig, 84 to 91% of the activity was
excreted in the urine within the first 48 h and less than 7% in the
faeces (Pither 1978).
Following administration to pigs of 5 mg (benzene ring-UL-14C)-
triadimefon/kg, whole blood and plasma levels peaked at 3 h post-
dosing. Tissue residues collected 3 h following the last of five
consecutive daily oral doses were highest in the kidney (4 mg/kg
triadimefon equivalents) followed by liver (3.14 mg/kg) and fat
(1.00 mg/kg) (Pither 1978).
In studies on the metabolism of (benzene-ring-UL-14C)-
triadimefon in pigs, the metabolites identified in the urine were
conjugated KWG 1342, KWG 1323 and triadimenol acid; in the faeces,
unchanged parent compound was found in addition to the three above
metabolites. In liver, kidney and fat, triadimenol was identified in
addition to the aforementioned metabolites (Pither 1978).
Cow
Following a single oral dose of (benzene ring-UL-14C)-
triadimefon (0.14 mg/kg bw) to a dairy cow, 87% of the dose was
excreted in the urine after 72 h and 6.6% in the faeces. After 5 daily
doses, 0.03 mg/kg triadimefon equivalent was present in the milk
(Fredrickson 1978b).
Tissue residues in a heifer dosed with (benzene ring-UL-14C)-
triadimefon at 10 mg/kg bw were highest in the kidney at 15.00 mg
triadimefon equivalents/kg, followed by fat at 4.01 mg/kg, liver at
3.65 mg/kg and muscle at 0.36 mg/kg.
After 5 daily doses of (benzene ring-UL-14C)-triadimefon at
0.14 mg/kg, the residues in a dairy cow were very low; only the liver
and kidney had significant residues at 0.08 and 0.05 mg triadimefon
equivalent/kg, respectively (Fredrickson 1978b).
In the urine of a dairy cow dosed with (benzene ring-UL-14C)-
triadimefon, the main metabolites found were KWG 1323 and KWG 1342
excreted as conjugates of glucuronic acid; triadimenol acid and an
unidentified polar metabolite were also found in the urine. In the
faeces, triadimenol acid, KWG 1342 and free KWG 1323 were found. In
the tissues, residues were also mainly the triadimenol acid and
conjugates of KWG 1323 and KWG 1342 (Fredrickson 1978b) (Figure 1).
 Hen
When (benzene ring-UL-14C)-triadimefon was administered to
laying hens by a single oral capsular treatment of 2.4 mg/kg, the dose
was eliminated rapidly and 100% was recovered in the excreta 24 h
after treatment (Nye 1979).
Radiocarbon residues in tissues and eggs of laying hens were
determined during a 96-h post-treatment period. The liver contained
0.26 mg triadimefon equivalent/kg 6 h after treatment, the kidney
contained 1.18 mg/kg, breast muscle contained 0.12 mg/kg and abdominal
fat contained 0.30 mg/kg. All tissue radiocarbon levels were well
below 0.01 mg/kg 90 h later. Radiocarbon residues in the eggs were
highest (0.117 mg/kg) 24 h after treatment and had decreased to
0.04 mg/kg 96 h after treatment (Nye 1979).
The nature of the radiocarbon contained in tissues and eggs of
laying hens, as well as that eliminated via the excreta was determined
by Nye (1979). Triadimefon was absorbed by the birds and rapidly
converted to triadimenol acid, which was excreted in its free form.
This metabolite was also the major metabolite isolated in the liver
and kidney. The intermediates in the conversion of triadimefon to the
acid, triadimenol and KWG 1342 were found as major metabolites in the
muscle, fat and eggs. The parent compound was found as the primary
radiocarbon component in the gizzard and abdominal fat.
Catfish
The accumulation and persistence of residues was studied in
Channel catfish (Ictalurus punctatus) continuously exposed to
(benzene ring-UL-14C)-triadimefon for a 28-day period at
concentrations of approximately 10 and 100 ppb in water (Lamb and
Roney 1977). The catfish showed accumulation factors of approximately
7.6 from the 10 ppb level and 6.5 from the 100 ppb level during the
exposure period. The non-edible portions of the catfish contained 74%
and 84% of the extractable 14C residues from the 10 and 100 ppb
levels, respectively. When the catfish were transferred to
uncontaminated water (withdrawal period), approximately 88% of the
accumulated 14C residues were excreted by the fish within 5 h and
approximately 96% were eliminated within 7 to 10 days. Under the
conditions of the study, triadimefon exhibited a low magnitude of
accumulation with a rapid rate of uptake and excretion (Lamb and Roney
1977).
TOXICOLOGICAL STUDIES
Acute toxicity
Triadimefon has a slight (dermal) to moderate (oral,
intraperitoneal) acute toxicity to experimental laboratory animals
(Table 1). The symptoms of poisoning indicate that the target of
TABLE 1. Acute toxicity of triadimefon in animals
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rat M oral ) acetone + 568 ) Thyssen and
Rat F ) oil (1:10) 363 ) Kimmerle 1974
Rat F * oral ) H20 + Cremophor-EL 1126 Flucke and Kimmerle
1977a
Rat M * oral ) 917 Thyssen 1977
Rat M oral ) 830 )
Rat M oral ) 955 )
Rat M oral ) 925 ) "
Rat M oral ) 1000 )
Rat M oral ) 928 )
Rat F * oral ) Polyethylene 1045
Rat M * oral ) glycol 400 1524 )
Rat M oral ) 1940 )
Rat M oral ) 1718
Rat M * oral ) 822 Flucke 1981
Rat M * oral ) 1008 )
Rat M * oral ) 1855 )
Rat F * oral ) 1020 ) Mihail 1980
Rat M oral ) 1245 )
Rat F oral ) 793 )
Mouse M oral ) acetone + oil 989 ) Thyssen and Kimmerle
Mouse F oral ) (1:10) 1071 ) 1974
Mouse M * oral ) polyethylene 732 ) Mihail 1980
Mouse F * oral ) glycol 400 1158 )
Rabbit F oral acetone + oil ca.500 Thyssen and Kimmerle 1974
(1:10)
TABLE 1. (con't)
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rabbit M oral H2O + Cremophor EL 250-500 Mihail 1980
Hen F oral ) acetone _ oil ca. 5000 ) Thyssen and Kimmerle
Quail F oral ) (1:10) >1750,<2500 ) 1974
Duck M * oral ) polyethylene >4000 ) Lamb and Burke 1977
Duck F * oral ) glycol 400 >4000 )
Dog F oral acetone + oil >500 Thyssen and Kimmerle
(1:10) 1974
Rat M i.p.** acetone + oil 321 ) "
Rat F i.p.** (1:10) 293 )
Rat M i.p.** polyethylene 214 ) Mihail 1980
Rat F i.p.** glycol 400 213 )
Rat M dermal acetone + oil >1000 Thyssen and Kimmerle
(1:10) 1974
Rat M dermal) physiol NaCl >5000 ) Mihail 1980
Rat F dermal) solution >5000 )
* = animals fasted; ** i.p. = intraperitoneal
attack is the central nervous system. An influence on the acute
toxicity of the compound undoubtedly is exerted by the liver-damaging
effect at high doses (brittle liver in rats; lobular pattern of liver
in rats and rabbits) and by the local damaging effect on the
gastrointestinal tract (oral application, rat and mouse) and on the
peritoneum (intraperitoneal application, rat).
Comparison of the acute toxicity data for oral application and
for intraperitoneal administration to rats indicates, in the light of
the differences in the LD50 values, the delayed onset of action
following oral application, the long persistence of symptoms and the
long duration of animal mortality occurrence, that absorption from the
gastrointestinal tract is relatively poor and that triadimefon effects
are protracted. The relatively poor absorbability from the
gastrointestinal tract might be of practical significance, as it
suggests that rapid decontamination could be therapeutically effective
in the event of oral poisoning.
The results of the acute toxicity studies did not reveal any
significant sex-specific differences. The oral LD50 values in rats
exhibit a large range. Whereas the LD50 values for administration
of triadimefon in water and Cremophor EL correspond somewhat to those
for administration of the compound in polyethylene glycol 400,
administration in acetone + oil resulted in comparatively higher
toxicity. This may have been due to increased absorption or also to an
additional toxic effect of acetone. The corresponding LD50 values
obtained following administration in aqueous and alcoholic
formulations are usually in the order of 1 000 mg/kg body weight for
oral application to rats (Table 1).
The large range of the LD50 values most probably is associated
with the targets of attack of the compound at toxic doses, such as
damage to the liver and the gastrointestinal tract. These effects may
have had a varying influence on both absorption and detoxification in
the individual animals (Bayer 1981).
Short-term studies
Rat
Groups of 15 male and 15 female Wistar rats received daily oral
triadimefon doses of 3, 10 and 30 mg/kg bw respectively, by gavage for
30 days. The compound was emulsified in acetone and oil (1:10). A
control group of similar size received the acetone + oil mixture only.
The rats were sacrificed 24 h after the final application, and their
tissues were macroscopically examined and weighed. At the end of the
study, blood samples were taken for haematological and clinical
chemical tests. The nine most important tissues were examined
histopathologically. Rats treated with doses of up to and including
30 mg/kg bw did not differ from the controls with respect to
behavioural patterns, body weight development, haematological data,
clinical chemical data, results of urinalyses and histopathological
findings. However, the absolute and relative liver weights showed
a significant increase in male rats treated with doses of 10 and
30 mg/kg bw. In female rats, an increase in the absolute and relative
liver weights was seen only at the dose level of 30 mg/kg bw, and
other organ weights showed no differences. The no-effect dose for rats
treated orally with triadimefon for 30 days was 3 mg/kg for males and
10 mg/kg for females (Thyssen et al 1974).
In a 12-week feeding experiment, groups of 15 male and 15 female
Sprague-Dawley rats were fed triadimefon daily at dietary levels of
0 ppm (control), 50 ppm, 200 ppm, 800 ppm and 2000 ppm, respectively.
Haematological tests, clinical-chemical tests and urinalyses were
performed prior to commencement of test diet administration, after
6 weeks on the test diet and at the end of the treatment period. The
rats were sacrificed and dissected on termination of treatment,
tissues were macroscopically examined and organ weights were measured.
The major tissues were histopathologically examined. Triadimefon
was tolerated at daily dietary concentrations of up to and including
2 000 ppm for the whole of the 12-week period without causing any
symptoms. Haematology, clinical chemistry and organ weight
measurements, as well as gross pathology and histopathology, provided
no indication of toxic effects from administration of the test
compound. Redness and swelling seen macroscopically, especially in the
area of the glandular mucosa of the stomach, could not be confirmed
microscopically (Mohr 1976).
Dog
In a subchronic toxicity study, groups of 4 male and 4 female
beagle dogs were maintained for 13 weeks on a diet containing
triadimefon at concentrations of 0 ppm (control), 150 ppm, 600 ppm and
2 400 ppm, respectively. Clinical inspections, haematological tests,
clinical chemical tests and urinalyses were performed. On termination
of dietary administration, the dogs were sacrificed, dissected and
grossly examined. The most important tissues were weighed. EPN-oxidase
and N-demethylase were measured in liver samples to test for an enzyme
inducing effect. Comprehensive histopathological examinations were
performed on tissues from all dogs. The dietary level of 150 ppm was
tolerated without producing any treatment-related effects. Although
dietary concentrations of 600 ppm and 2 400 ppm caused increased N-
demethylase activities, which was indicative of enzyme induction, this
dose was tolerated without having any other toxic effects. However,
dogs fed a dietary level of 2 400 ppm were affected in several other
respects (e.g., vomiting and reduction in food consumption); hence,
they had a moderate state of nutrition and showed less weight gain
than the other groups. The parameters of the red blood picture were
adversely affected by the treatment. Alkaline phosphatase and
transaminase activities were increased, illustrating a disturbed liver
function. The relative liver weight showed a slight increase in both
sexes in the 2 400 ppm group as compared with the controls. However,
gross pathology and histopathology did not reveal any treatment-
related alterations in the liver or in any other tissues. In the
13-week feeding experiment, the no-effect dietary level for the dog
was 150 ppm (Hoffman and Luckhaus 1974).
In a chronic toxicity study, groups of 4 male and 4 female beagle
dogs were maintained for 104 weeks on a diet containing triadimefon at
concentrations of 0 ppm (control), 100 ppm, 330 ppm, 1 000 ppm (up to
week 54) and 2 000 ppm (from week 55), respectively, of a technical
grade of two batches, purity 88 to 89% and 92 to 97% (90% premix
prepared with highly dispersed silicate). Clinical examinations,
haematological tests, clinical chemical tests and urinalyses were
performed on the dogs. On termination of dietary administration, the
dogs were sacrificed, dissected and grossly examined; their organ
weights were measured and tissues were histopathologically examined.
The results of the study showed that triadimefon dietary
concentrations of up to and including 330 ppm were tolerated for 2
years by both males and females without having any toxic effect. In
the highest dietary concentration group (especially after the dietary
level was raised to 2 000 ppm in week 55) the weight of some dogs of
both sexes was depressed slightly. Clinical chemistry and
histopathology did not reveal any liver damage; however, there were
signs of a treatment-related microsomal enzyme induction at the
highest dietary level. None of the other findings showed any
differences between the treated dogs and the controls (Hoffman and
Gröning 1978).
Long-term studies
Rat
In a 24-month study to evaluate triadimefon for potential chronic
toxicity and carcinogenicity, groups of 50 male and 50 female Wistar
rats were fed dietary levels of 50, 500 and 5 000 ppm, respectively of
a technical grade, composed of 2 batches, one of 88-89%, and the other
of 92-97% (90% premix prepared with a highly dispersed silicate). The
control group, fed only chow, consisted of 100 male and 100 female
rats. For clinical laboratory tests (haematology, clinical chemistry,
urinalysis, enzyme induction assays), 10 rats were additionally used
per group and per sex. After 24 months on the test diet, all survivors
were sacrificed, dissected and grossly examined.
The study revealed no indication of triadimefon having a
carcinogenic effect on rats. With respect to chronic toxicity, the
no-effect dose was found to be 50 ppm for females and 500 ppm for
males. Higher dietary levels had the following effects in the two
sexes. The dietary concentration of 5 000 ppm caused violent motor
reactions in males and females from about week 23. These rats consumed
almost no food while the reactions persisted and, as a result, they
lost a considerable amount of weight and many died. Those that
survived began to consume feed again when the symptoms receded, and
made corresponding weight gains. However, in week 32 and, following
brief improvement, in week 37, these violent motor reactions again
occurred accompanied by refusal of food and considerable loss of
weight. The last of the surviving rats in the 5 000 ppm group were
killed in a moribund condition in week 39. Gross pathology performed
on the 5 000 ppm rats that died and on those sacrificed essentially
disclosed severe mucosal lesions in the stomach, which in most cases
contained no food, accompanied by sometimes severe haemorrhages. These
lesions were considered to have been the cause of the clinical
symptoms observed. From week 85, the body weights of the male rats in
the 500 ppm group were mostly significantly lower than those of the
controls, but the difference was so slight that it was not considered
to have been due to a damaging effect from the test compound. The
growth rate of most of the female rats in the 500 ppm group was
significantly retarded from as early as week 6.
Significantly reduced erythrocyte counts and haemoglobin levels
seen at the dietary levels of 5 000 ppm, and at the end of the study
also in females fed 500 ppm, were indicative of an effect on the blood
or blood-forming tissues. The histopathological examinations revealed
shrunken spleen with reduced haematopoiesis in some of the rats of the
5 000 ppm group. Some of the rats of this group also showed decrease
in haematopoiesis in the marrow. The histopathological examinations
revealed signs of damage to cells of the proximal tubules of the
kidney (vacuolation, desquamation, degeneration) in 5000 ppm rats. In
the lung, increased blood content was seen in the dilated alveolar
vessels. In the stomach of 5 000 ppm rats, increased superficial
ulcerations, haemorrhage of the mucosa and oedemas of the sub-mucosa
were observed. In the adrenals of some rats, congestion of blood
vessels was seen. The dietary level of 5 000 ppm also caused formation
of giant spermatids in some males. In rats fed 5 000 ppm, cholesterol
levels occasionally showed a significant increase, indicative also of
an effect on fat metabolism at this dietary level. The liver weights
showed a statistically significant (p <0.01) and dose-related
increase in the male rats of the 50 and 500 ppm groups and in the
female rats of the 500 ppm group. (On highest dose, 5 000 ppm, all
animals were dead at week 39.)
The statistically significant increased liver weight (p <0.01)
seen in 50 and 500 ppm males and in 500 ppm females was not due to
stimulation of microsomal enzymes, as the N-demethylase activity and
the cytochrome P-450 concentrations measured in the liver at the end
of the experiment were not affected. Thus, the increased liver weights
were considered to have been a non-specific effect of increased
metabolism (Bomhard and Löser 1978a).
Mouse
Triadimefon was evaluated for long-term effects and potential
carcinogenicity in a 24-month study on groups of 50 male and 50
female CF1/W 74 mice fed dietary levels of 0 (control), 50, 300 and
1 800 ppm, respectively, of a technical grade, purity 97% (used as an
approximately 90% premix prepared with a highly disperse silicate).
For clinical laboratory tests, 10 mice were additionally used per
group and per sex. The mice were inspected for clinical symptoms.
Their body weight and food intake were measured. Haematological tests,
clinical chemical tests, organ weight measurements, macroscopic tissue
examinations and comprehensive histological examinations were
performed.
The study revealed no indication of triadimefon having a
carcinogenic effect on mice. No chronic-toxic effects were seen at the
two lower dietary levels of 50 and 300 ppm. Mice fed 1 800 ppm showed
the following alterations, in comparison with the controls. Growth of
both male and female mice was affected. Significantly increased
erythrocyte counts were recorded in both sexes, and increased
haemoglobin and haematocrit values were observed in females. Other
treatment-related alterations seen on termination of the study,
although not present consistently at the intermediate examinations and
necropsies performed after 6 and 12 months, included: distinctly
increased liver weights; increased AP, GOT and GPT activities;
increased incidence of liver swellings, occasionally accompanied by
adhesions of liver lobes in mice that died and in those sacrificed on
termination of the study. At the end of the feeding experiment, a
marked correlation between hyperplastic liver nodules and greatly
increased enzyme activities, indicative of liver damage, was observed
in the 1 800 ppm group. The incidence of liver cell tumours in male
and female mice were as follows:
ppm F M
0 0 2
50 1 2
300 0 1
1800 2 2
An increased incidence of hyperplastic liver nodules, which are
histologically very difficult to differentiate from liver cell tumour,
were also observed with the following incidence in male and female
mice:
ppm F M
0 4 7
50 2 7
300 1 7
1800 15 15
(Bombard and Löser 1980b)
Special studies on reproduction
Rat
Triadimefon was evaluated for its effect on fertility, lactation
performance and development of offspring in a 3-generation
reproduction study in Wistar rats with two matings per generation
(Löser 1979). Each test group consisted of 10 male and 20 female rats
and triadimefon was fed to the rats at dietary concentrations of 0
(control), 50, 300 and 1 800 ppm respectively. At the start of the
study, the F0 generation was ca. 32 to 39 days old and was treated
for 70 days prior to the first mating.
The pups of the F3b generation were sacrificed and
necropsied at an age of 4 weeks, and the major 18 tissues were then
histopathologically examined. Dietary levels of 50 and 300 ppm had no
adverse effect on the reproductive performance of the rats. After 2 of
6 matings, the weight of the pups in the 300 ppm group were
significantly lower than those of the controls during the lactation
period. The dietary level of 1 800 ppm distinctly affected
reproductive performance, as none of the rats became pregnant after
the second mating of the F1b generation. As this dietary level also
distinctly affected the parental rats, evidenced by the growth rate,
there would be no evidence for assuming any primary damaging effect on
reproduction.
Triadimefon did not cause any gross abnormalities or
malformations in rats at the tested dietary concentrations. The
histopathological examinations of the 18 most important tissues from
4-week old pups of the F3b generation revealed no indication of any
tissue alterations due to dietary administration of triadimefon at the
tested concentrations. The dietary concentration of 50 ppm was
tolerated without having any damaging effect whatsoever in the
3-generation reproduction study (Löser 1979).
Hen
Groups of 10 female White Leghorn hens per concentration, which
were mated with one cock per group, were maintained for 4 weeks on a
diet containing triadimefon at concentrations of 0 (control), 10 and
100 ppm, respectively. The hens were inspected for behavioural
patterns, physical appearance and appetite and their body weights and
food intake were measured. Tissues were macroscopically examined and
the 12 most important tissues were histopathologically examined.
Haematological tests were also performed on additional hens maintained
under comparable conditions. The following parameters were measured in
the offspring: number of eggs, egg quality, egg weight, egg size,
thickness of egg shell, weight of egg shell, fertilization rates,
hatch rates. The chicks were inspected for their physical appearance
and behavioural reactions during the first days of their life. The
tested concentrations were tolerated by both the hens and their
offspring without causing any damage whatsoever (Thyssen and Gröning
1978).
Species studies on embryotoxic effects
Rat
Two experiments were performed with groups of 20 pregnant Long
Evans rats dosed daily by gavage with triadimefon in an aqueous
emulsion from gestation day 6 to 15. The first study utilized daily
doses of 10, 30 and 100 mg per kg bw. Each experiment included a
control group dosed only with the vehicle. While the dose of
10 mg/kg/day did not have any harmful effects on the dams, doses of
30 mg/kg/day and above reduced dam weight gain. However, none of the
tested doses caused any behavioural disorders or mortalities. Effects
on progeny were characterized by a slightly increased placenta weight
in the 100 mg/kg group as compared with the controls, and by the
occurrence of occasional cleft palates at 75 mg/kg and 100 mg/kg/day.
Thus, a treatment-related effect and hence a slight teratogenic effect
of triadimefon on rats at oral doses of 75 mg/kg/day and above could
not be completely ruled out. The oral no-effect of triadimefon with
respect to embryonic/foetal development was found to be 50 mg/kg/day
(Machemer 1976b).
Evaluation of triadimefon for embryotoxic effects was also
performed by administering the compound by the route of greatest
relevance to practical conditions, viz. by inhalation. (A hazard from
skin exposure can be ruled out on the ground of the acute toxicity
findings, which did not reveal any toxicologically relevant absorption
through the skin.) For the inhalation evaluation, twenty fertilized
rats per group were exposed for 6 h daily on 10 consecutive days
from gestation day 6 to 15, to analytically measured triadimefon
concentrations of 14 (11 to 18) mg/m3, 33 (28 to 37) mg/m3 and 114
(95 to 132) mg/m3 air in a dynamic flow inhalation apparatus.
Triadimefon was dissolved in a 1:1 mixture of ethanol and polyethylene
glycol 400 and aerosolized in the inhalation chambers. A control group
inhaled the aerosol of the solvent mixture only. While the contration
of 14 mg/m3 air did not affect the dams, concentrations of 33 and
114 mg/m3 slightly reduced dam weight gain during the treatment
period. None of the concentrations adversely affected physical
appearance and behavioural patterns of the dams. Embryonic and foetal
development were not affected by concentrations of up to and including
114 mg/m3. Hence, it was concluded that inhaled triadimefon did not
result in any embryotoxic effects (Machemer and Kimmerle 1976).
Rabbit
Groups of 10 to 13 pregnant Himalayan rabbits received daily oral
doses of triadimefon administered as an aqueous emulsion by gavage at
levels of 0 (control), 5, 15 and 50 mg/kg bw, respectively, from
gestation day 6 to 18. The tested doses had no adverse effects on
physical appearance, behavioural patterns and weight gains of the dams
or on embryonic and foetal development. Oral doses of triadimefon did
not result in toxic or teratogenic effects on rabbits (Machemer
1976c).
Special studies on mutagenic effects
Triadimefon was evaluated for mutagenic potential by the Ames
test on histidine requiring Salmonella typhimurium strains TA 98,
100, 1535, 1537, 1538 and 1950, with and without metabolic activation.
The triadimefon concentrations ranged from 0.1 to 1 000 µg per plate.
The minimal inhibitory concentration of triadimefon on the tester
strains used was 500 µg/ml. Triadimefon did not induce back-mutations
in the test systems, with or without microsome activation, over the
tested concentration range (van Dijck 1976).
Triadimefon was tested for potential DNA-attacking action on
Bacillus subtilis at concentrations of up to 300 µg per plate, by
rec-assay. The compound was also evaluated at concentrations of up to
1 000 µg/plate for potential mutagenicity by the Ames assay using
Salmonella typhimurium strains TA 1535, 1537, 98 and 100, with and
without addition of rat and mouse liver homogenates to the agar. In
these studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Inukai and Iyatomi 1977).
The fungicide was further tested for potential mutagenic effects
by the rec-assay on two Bacillus subtilis strains at concentrations
of up to 2 000 µg/plate, and by the Ames (reversion) assay on the
Salmonella typhimurium strains TA 98 and 100 at concentrations of up
to 5 000 µg/plate, with and without metabolic activation. In these
studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Shirasu et al 1978).
Triadimefon was additionally studied by rec-assay on two
Bacillus subtilis strains at concentrations of up to and including
2 000 µg per plate and by reversion assay on Escherichia coli (WP 2)
and on Salmonella typhimurium strains TA 1535, 1537, 1538, 98 and
100, at concentrations of up to and including 5 000 µg per plate, with
and without metabolic activation by rat liver chromosomes. No
indication of mutagenic activity was found in these studies (Shirasu
et al 1979).
Triadimefon was evaluated for point-mutagenic effects by a
eukaryotic, micro-organism test using Saccharomyces cerevisiae
S 138 and S 211, in comparison with negative (solvent) and positive
controls, with and without metabolic activation by microsomal enzymes
of Aroclor-induced rat livers. Neither a mutagenic nor a
recombinogenic effect was seen at doses extending into the toxic
range, 1 000 µg/plate (Jagannath et al 1980).
A micronucleus test on male and female NMRI mice was performed
with triadimefon. Triadimefon was administered in an aqueous emulsion
at a total oral dose of 400 mg/kg bw applied in two split doses of
200 mg/kg bw each at an interval of 24 h. The thiotepa dose was
2 × 10 mg/kg injected subcutaneously. For comparison, a negative
control group was treated with the vehicle and a positive control
group was treated with thiotepa. Each group consisted of 10 mice;
1 000 polychromatic erythrocytes per mouse were analysed for incidence
of micronuclei. Additionally, the ratio of polychromatic erythrocytes
to normochromatic ones was determined to establish whether the test
compound had any effect on the general activity of bone marrow.
Thiotepa caused a big increase in the number of micronuclei and also
bone marrow depression. Triadimefon did not affect general bone
marrow activity nor did it increase the incidence of micronuclei in
comparison with the control, and hence was deemed to be non-mutagenic
in this system (Machemer 1977).
Twenty male NMRI mice each received a single oral dose of
triadimefon at a level of 200 mg/kg bw, administered as an aqueous
emulsion by gavage. Starting on the day of test compound
administration, a series of 8 matings was performed with the treated
males, each mating period being of 1-week duration; every week, each
male was caged together with 3 fresh untreated virgin females. The
pre-implantation and post-implantation losses of fertilized females
were determined as the criteria for assessment of a mutagenic effect.
(The corpora lutea, total implantations, viable implants and dead
implants were counted.) The results were compared with those obtained
from a control group of similar size. The tested dose was not toxic to
the males and did not affect their fertility. The measured parameters
did not reveal any treatment-related difference between the control
group and the triadimefon-treated group. The dominant lethal test on
the male mouse did not provide any indication of the mutagenicity of
triadimefon at the acute oral dose of 200 mg/kg bw (Machemer 1976a).
Special studies on induction of liver enzymes
Triadimefon was administered daily on 28 consecutive days to
Wistar rats, by gavage in a mixture of acetone and oil at dose levels
of 0 (vehicle), 1, 5 and 25 mg/kg bw, respectively. Each group
consisted of 20 male and 20 female rats; half of them were sacrificed
at the end of the treatment period and the other half were killed
on termination of a 4-week post-treatment observation period.
N-demethylase, O-demethylase and cytochrome P-450 in the rat livers
were measured at the end of the treatment period and on termination of
the post-treatment observation period. The rats were observed for
systemic toxic effects by observing their physical appearance,
behavioural patterns and posture, and their body weights and certain
haematological and clinical chemical parameters were measured. The
rats were necropsied, tissues were macroscopically examined and livers
were weighed. The study revealed that triadimefon at daily doses of 5
and 25 mg/kg bw caused slight induction of microsomal liver enzymes.
The enzyme induction was reversible within the 4-week post-treatment
observation period. None of the other parameters measured and
investigated provided any indication of treatment-related damage
(Mihail and Kaliner 1979).
Special studies on potentiation toxicity
Since triadimefon is used in combination with other fungicidal
compounds, acute combination toxicity studies were performed on Wistar
rats. The compounds tested in combination with triadimefon were
captafol, propineb, tolylfluanid and carbendazim. The oral LD50 of
each of these compounds was first determined. Fifteen female or male
rats were used per dose and the observation period lasted 14 days.
From the LD50 values of the single components the theoretically
expected LD50 of each combination was calculated. For combined
administration, the components were mixed in a percentage ratio
proportional to the LD50 of each and thus applied in equitoxic doses;
thereby, the actual LD50 of the combination was determined. A more
than additive acute toxic effect was not seen for any of the tested
combinations (Flucke and Kimmerle 1977, Thyssen 1977).
OBSERVATIONS IN MAN
Approximately 50 mg of triadimefon was applied to small gauze
pads which were placed for a contact time of 24 h on the skin of the
forearm of each of 8 test persons and the pads were fixed in position
with adhesive strips. Upon removal of the strips and pads, slight
redness of the treated skin area was seen for a brief period in 5 of
the 8 persons. At 24 h post-application, the treated skin areas showed
no variations from the physiological norm in any of the test persons
(Thyssen and Kimmerle 1973).
RESIDUES IN FOOD
USE PATTERN
Triadimefon is effective and is mainly used against powdery
mildews and rusts; it also gives good control of a number of other
diseases e.g. Thielaviopsis on pineapple (post-harvest application).
Triadimefon is registered and marketed in more than 30 countries
in Europe, Africa, America, Asia and Australia; registration is being
sought also in other countries. Details on the uses of triadimefon and
recommendations are given in Table 2. With the exception of the post-
harvest dip of pineapple the product is used as pre-harvest
application. Triadimefon is used also on ornamentals (roses, azaleas),
seed-grass, turf and rubber trees. Sugarcane and sweet potato sets are
dipped in a triadimefon suspension of emulsion prior to planting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on triadimefon residues from supervised residue trials were
obtained on various fruits and vegetables, cereal grains and some
other crops grown under a variety of geographical, climatic conditions
and agricultural practices. All residue figures presented in Table 3
refer to the sum of triadimefon parent compound and its main
metabolite triadimenol.
Fruits and vegetables
The average residue at harvest following the recommended
pre-harvest intervals is in the range of non-detectable (0.01 to
0.05 mg/kg) up to 0.3 mg/kg (maximum residue of 0.4 mg/kg) on most
fruits and vegetables. On red currants somewhat higher residues are
found after the recommended pre-harvest intervals; the average level
at harvest was 0.4 mg/kg (maximum residue in the trials 1.1 mg/kg).
Higher residues were also found on Brussels sprouts (average 0.5,
highest level found 0.9 mg/kg) in trials where the normal pre-harvest
interval was taken into account.
FATE OF RESIDUES
In plants
The behaviour and fate of triadimefon in plants was evaluated.
Studies were carried out on metabolism of (benzene ring-UL-14C)- and
(triazole ring-3,5-14C)-triadimefon in cereals, apple, cucumber and
tomato; translocation of unlabelled (benzene ring-UL-14C)-, and
(triazole ring-3,5-14C)-triadimefon, with the studies being directed
towards elucidation of site of action, mechanism of action and
metabolism; redistribution in the vapour phase after application of
unlabelled and (benzene ring-UL-14C)-triadimefon to plants.
TABLE 2. Summary of recommended uses of triadimefon
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Fruits
Apple and pear wp 40 - 75 4 - 12 7
Jap. apple wp 150 -200 2 - 3
Jap. pear wp 150 -200 2 - 3
Apricot wp 50 -150 4 - 6 7
Peach wp 50 -150 4 - 6 7
Currant (black,red) wp 50 -150 3 - 4 7
Gooseberry wp 50 -100 3 - 4 7
Grape wp/EC/dust 50-75 (dust 100-200) wingrape 4-10 14-28
tablegrape 4-8 4- 7
Strawberry wp 75 -100 7
Mango wp 100 -200 28
Pineapple wp/EC 0.015-0.07% 1(postharvest
dip) -
Vegetables
Artichoke wp/EC 50 -150 4 - 8 3 - 7
Beans wp/EC 125 -250 3 - 6 7 -14
Pea wp/EC 50 -125 3 - 6 7 -14
Brussels sprout wp/EC 125 -250 3 - 6 14
Onion (Welsh) wp 100 3 10
Cucurbits wp/EC/dust 25-100 (dust100-200) 4 - 10 3 - 7
Pepper wp/EC 50 -150 4 - 8 3 - 7
Tomato wp/EC 50- 150 4 - 8 3 - 7
Grain crops wp/EC 125 -250 1 - 2 21 -35
Sugarbeet wp/EC 125 2 - 3 14
TABLE 2. (con't)
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Coffee
protective wp/EC 100 -250 8 - 12
eradicative wp/EC 250 -500 2 - 4
Hops 200 -500 2 - 6
Tobacco 40 - 75 4 - 6
TABLE 3. Residues of triadimefon/triadimenol from supervised trials
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3 7 14
Fruit
Apple USA 1977 6 20 - 60 wp 50% 0.3 0.2 0.4 0.2 Mobay 1981
1977 6 20 - 60 wp 50% 0.5 0.4 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.2 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.3 0.3 0.4 "
1977 6 340 -560 wp 50% 0.5 0.4 0.3 0.2 "
1978 6 20 - 60 wp 50% 0.3 0.2 0.09 0.08 "
1977 11 340 -560 wp 50% 0.4 0.2 0.1 0.1 "
1977 10 340 -560 wp 50% 0.2 0.1
1977 10 340 -560 wp 50% 0.4 0.3 0.2 0.1 "
1977 10 340 -560 wp 50% 0.4 0.2 "
1977 10 340 -560 wp 50% 0.2 0.1 0.1 0.09 "
1979 6 140 wp 50% 0.7 0.4 "
Pear 1980 6 600 wp 50% 0.6 0.3 0.5 0.3 "
1980 6 600 wp 50% 0.4 0.4 0.3 0.2 "
1980 6 600 wp 50% 0.4 0.5 0.4 0.3 "
1980 10 450 wp 50% 0.3 0.2 0.09 0.06 "
1980 15 12001 wp 50% 0.8 0.5 0.3 0.2 "
Peach 1980 4 12001 wp 50% 3.0 1.6 0.7 "
1980 3 12001 wp 50% 0.1 0.4 0.1 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 3 6/7 14 21
Berry fruits
Currant- Denmark 1979 4 50 wp 5% 0.03 <0.05 <0.02 <0.05 <0.02 <0.05 Bayer 1981
black 1979 4 50 wp 5% 0.06 0.05 0.03 <0.05 <0.02 <0.05 "
France 1979 4 100 wp 5% 0.03 0.2 0.04 0.2 "
1977 4 40 wp 1.25% 0.04 0.14 "
Currant-red Denmark 1979 2 50 wp 5% 0.02 <0.05 <0.02 <0.05 <0.02 <0.05 "
1979 2 50 wp 5% 0.15 <0.05 0.05 <0.05 <0.02 <0.05 "
Raspberry 1979 4 75 wp 5% 0.03 0.05 Bayer 1981
Grape Japan 1979 3 150 wp 5% 0.01 0.4 <0.01 0.2 "
1979 5 150 wp 5% 0.02 0.5 0.01 0.4 "
1979 3 150 wp 5% 0.01 0.4 <0.01 0.3 "
1979 5 150 wp 5% 0.02 0.7 0.01 0.4
USA 1978 3 90 wp 50% 0.06 0.06 0.08 0.2 Mobay 1981
1978 3 110 wp 50% 0.02 0.05 <0.01 <0.01 "
1978 3 110 wp 50% 0.05 0.02 <0.01 <0.01 "
1978 3 150 wp 50% <0.01 0.01 "
1978 3 150 wp 50% 0.06 0.04 <0.01 <0.01 "
1978 3 150 wp 50% 0.03 0.09 <0.01 <0.01 "
1979 3 225 wp 50% <0.01 0.07 "
1979 3 225 wp 50% 0.5 0.4 0.01 0.02 "
1979 3 300 wp 50% 0.2 0.08 "
1979 3 225 wp 50% <0.01 <0.02 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3/5 7 14/15
Fruiting vegetables
Cantaloupes USA 1979 3 130 wp 50% 0.1 0.08 "
1979 3 130 wp 50% 0.1 0.03 "
1979 3 130 wp 50% 0.06 0.1 "
1979 3 130 wp 50% 0.03 0.03 "
Gherkins2 Fed.Rep. 1976 8 60 wp 5% <0.06 <0.06 <0.06 Bayer 1981
of 1976 8 60 wp 5% <0.01 <0.01 <0.06 "
Germany 1975 8 60 wp 5% 0.06 <0.06 <0.01 <0.06 "
1975 8 60 wp 5% 0.06 0.06 <0.06 <0.06 "
Cucumber Mexico 1979 3 125 wp 50% <0.0 <0.01 <0.01 <0.01 Mobay 1981
1979 3 125 wp 50% 0.04 0.05 <0.01 <0.01 "
Tomato Mexico 1979 8 180 wp 25% 0.03 0.05 0.01 0.03 0.01 0.03 "
1979 8 180 wp 25% 0.04 0.05 0.01 0.02 <0.01 0.02 "
1979 8 180 wp 25% 0.04 0.08 0.02 0.04 0.02 0.03 "
1979 8 180 wp 25% 0.07 0.08 0.02 0.06 <0.01 0.01 "
Japan 1977 4 250 wp 25% 0.07 0.08 0.04 0.08 0.022 <0.063 Nitokino
7 250 wp 25% 0.2 0.3 0.09 0.2 0.052 0.23 1981
2 250 wp 25% 0.4 0.2 0.3 0.3 0.22 0.33
4 250 wp 25% 0.4 0.5 0.3 0.6 0.12 0.53
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Other vegetables
Brussels
sprouts UK 1979 3 250 wp 25% <0.05 <0.05 Hazleton 1981
1977 3 125 wp 25% 0.6 0.2 BAY/UK 1981
Chick peas 1980 2 150 wp 25% 0.01 0.02 <0.01 <0.01 <0.01 0.02 Mobay
dry 2 150 wp 25% <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 1981
2 150 wp 25% <0.01 <0.02 <0.01 <0.01 <0.01 <0.01
Leeks UK 1979 4 250 wp 25% <0.05 <0.05 Hazleton 1981
500 wp 25% <0.05 0.08
250 wp 25% 0.09 0.4
500 wp 25% 0.3 0.9
Parsnips UK 1979 3 250 wp 25% 0.05 0.2
3 250 wp 25% 0.01 0.05
Hops UK 1979 8 110-220 wp 5% <0.3 0.8 Bayer 1981
fresh
dry
UK 1979 8 100-200 wp 5% 0.4 1.4
fresh <0.3 1.4 ibid
dry 0.4 1.4
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Fed. Rep.
of
Germany 1979 4 500 wp 5% ibid
fresh 0.9 0.6 2.13 0.83
dry 10.8 4.2 7.23 4.13
Fed.Rep.
of
Germany 1979 4 500 ibid
fresh 2.1 1.0 0.73 0.63
dry 11.4 4.3 1.93 1.73
1 Excessive dosage;
2 Some of the gherkins data were erroneously presented in the 1979 JMPR Evaluations under
cucumbers due to a translation error. Schlangen qurke = cucumber; Einlegequrke = gherkin;
3 After 10 days.
The metabolism of triadimefon (Bayleton(R)) in cereals was
studied in several experiments following application of (benzene ring-
UL-14C)-triadimefon to plants under glass and in field conditions.
The main metabolite formed was triadimenol, which occurs in the
diastereoisomeric forms A and B. On cereal plants, the A form is the
main diastereoisomer. A further metabolism pathway consisted of
oxidation of the tert. butyl group (KWG 1342) and cleavage of the
molecule, with the formation of 4-chlorophenol. The metabolites
triadimenol, and particularly KWG 1342 and 4-chlorophenol, are bound
in the form of glycosides-conjugates, mainly B glycosides. At harvest
the radioactivity still present in straw was distributed as shown in
Table 4.
TABLE 4. Radioactive studies on triadimefon metabolism in cereal
straw at harvest
Metabolite Radioactivity present (%)
Triadimefon unchanged parent 0 - 5
Triadimenol form A 22 - 41
Triadimenol form B 6 - 17
KWG 1342 2 - 16
Polar metabolites1 17 - 37
BUE 2285 less than 1
Free 4-Chlorophenol less than 1
Non-extractable 18 - 32
1 Including the glycosides of triadimenol, KWI 1342 and
4-chlorophenol.
The glycosides are probably fixed in the plants by further
metabolism and are therefore no longer extractable with standard
procedures. The distribution of the radioactivity among the aqueous
and organic phases after extraction of the straw differs between the
(triazole ring-3,5-14C) and the (benzene ring-UL-14C). An explanation
for this may be the different polarities of 4-chlorophenol and
1,2,3-triazole, which can be formed as metabolites of triadimefon
and/or triadimenol. Further studies are underway to elucidate this
phenomena.
In another experiment on summer barley, the non-extractable part
of the residue accounted for higher amounts, i.e. 55-75% (Bayer 1981).
This may be partly due to the application at an earlier stage in plant
development. Following spray application of (triazole ring-3,5-14C)-
triadimefon the metabolites that occurred were the same as those found
following the application of (benzene ring-UL-14C)-triadimefon, the
only difference being that polar metabolites accounted for a higher
proportion when both labels were applied at the same stage in plant
development, i.e. 31% vs 20% (Bayer 1981). For quantitative data
see Table 5. After meed treatment of barley with the pure
diastereoisomeric forms A and B of triadimenol, no conversion of one
form to the other was found in the barley plant (Bayer 1981).
In studies on apple with benzene ring-labelled and triazole-
labelled triadimefon, it was demonstrated that also in this crop
triadimefon was metabolized to triadimenol and in a small amount to an
unidentified compound (Table 6). The rapid decline of the 14C during
the early intervals up to 7 days was attributed to the volatility of
triadimefon parent compound (Mobay 1981). In trials on cucumber and
tomatoes, the fate of (benzene-ring-UL-14C)-triadimefon was studied.
The material was applied as a foliar spray or to the roots. It was
found that in addition to triadimefon and diastereoisomers A and B, a
polar metabolite was formed in the plants, which was shown to be a
glucoside of triadimenol loosely bound to another natural occurring
moiety. It is readily converted to triadimenol glucoside (see Table 6)
(Mobay 1981). It was also shown in these experiments that the
radiocarbon applied to the foliage did not translocate significantly
into neighbouring fruits, in marrow plants triadimenol was identified
as the major metabolite of triadimefon (Clark et al 1978). The
metabolic pathways of triadimefon are illustrated in Figure 2. For
chemical names of the metabolites identified in plants and their
structural formula see Table 9, FAO/WHO 1979, Triadimefon.
Triadimefon was translocated at a relatively fast rate in
anacropetal direction following foliar application to cereals, beans,
cucumber and tomato (Buchenauer 1975, 1976; Bayer 1981; Scheinpflug
et al 1977; Brandes et al 1978; Gasztonyi and Joslpovits 1978) and
also to a larger extent following soil applications to beans and
barley (Buchenauer 1975, 1976). Some authors also observed a slight
basipetal translocation. Gasztonyi and Josepovits (1978) established
that the rate of uptake and subsequently the amount of transport
depended substantially on the mode of leaf treatment, and also found
differences in the amounts of translocated triadimefon according to
plant species.
Gasztonyi and Josepovits (1979) studied the uptake and metabolism
of triadimefon by mycelia of fungi that were sensitive or resistant to
triadimefon. In the fungi, triadimefon accumulated to 20 to 40 fold of
the external concentration, irrespective of the sensitivity of the
fungus. In the course of metabolism, the highly fungitoxic main
metabolite triadimenol was formed; however, in mycelia of sensitive
fungi this transformation was at a high rate, whereas it could not be
demonstrated, or only to a low extent, in resistant strains of fungi.
Based on these observations, triadimefon must be regarded as the
precursor of the active principle, i.e. triadimenol.
Hen
When (benzene ring-UL-14C)-triadimefon was administered to
laying hens by a single oral capsular treatment of 2.4 mg/kg, the dose
was eliminated rapidly and 100% was recovered in the excreta 24 h
after treatment (Nye 1979).
Radiocarbon residues in tissues and eggs of laying hens were
determined during a 96-h post-treatment period. The liver contained
0.26 mg triadimefon equivalent/kg 6 h after treatment, the kidney
contained 1.18 mg/kg, breast muscle contained 0.12 mg/kg and abdominal
fat contained 0.30 mg/kg. All tissue radiocarbon levels were well
below 0.01 mg/kg 90 h later. Radiocarbon residues in the eggs were
highest (0.117 mg/kg) 24 h after treatment and had decreased to
0.04 mg/kg 96 h after treatment (Nye 1979).
The nature of the radiocarbon contained in tissues and eggs of
laying hens, as well as that eliminated via the excreta was determined
by Nye (1979). Triadimefon was absorbed by the birds and rapidly
converted to triadimenol acid, which was excreted in its free form.
This metabolite was also the major metabolite isolated in the liver
and kidney. The intermediates in the conversion of triadimefon to the
acid, triadimenol and KWG 1342 were found as major metabolites in the
muscle, fat and eggs. The parent compound was found as the primary
radiocarbon component in the gizzard and abdominal fat.
Catfish
The accumulation and persistence of residues was studied in
Channel catfish (Ictalurus punctatus) continuously exposed to
(benzene ring-UL-14C)-triadimefon for a 28-day period at
concentrations of approximately 10 and 100 ppb in water (Lamb and
Roney 1977). The catfish showed accumulation factors of approximately
7.6 from the 10 ppb level and 6.5 from the 100 ppb level during the
exposure period. The non-edible portions of the catfish contained 74%
and 84% of the extractable 14C residues from the 10 and 100 ppb
levels, respectively. When the catfish were transferred to
uncontaminated water (withdrawal period), approximately 88% of the
accumulated 14C residues were excreted by the fish within 5 h and
approximately 96% were eliminated within 7 to 10 days. Under the
conditions of the study, triadimefon exhibited a low magnitude of
accumulation with a rapid rate of uptake and excretion (Lamb and Roney
1977).
TOXICOLOGICAL STUDIES
Acute toxicity
Triadimefon has a slight (dermal) to moderate (oral,
intraperitoneal) acute toxicity to experimental laboratory animals
(Table 1). The symptoms of poisoning indicate that the target of
TABLE 1. Acute toxicity of triadimefon in animals
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rat M oral ) acetone + 568 ) Thyssen and
Rat F ) oil (1:10) 363 ) Kimmerle 1974
Rat F * oral ) H20 + Cremophor-EL 1126 Flucke and Kimmerle
1977a
Rat M * oral ) 917 Thyssen 1977
Rat M oral ) 830 )
Rat M oral ) 955 )
Rat M oral ) 925 ) "
Rat M oral ) 1000 )
Rat M oral ) 928 )
Rat F * oral ) Polyethylene 1045
Rat M * oral ) glycol 400 1524 )
Rat M oral ) 1940 )
Rat M oral ) 1718
Rat M * oral ) 822 Flucke 1981
Rat M * oral ) 1008 )
Rat M * oral ) 1855 )
Rat F * oral ) 1020 ) Mihail 1980
Rat M oral ) 1245 )
Rat F oral ) 793 )
Mouse M oral ) acetone + oil 989 ) Thyssen and Kimmerle
Mouse F oral ) (1:10) 1071 ) 1974
Mouse M * oral ) polyethylene 732 ) Mihail 1980
Mouse F * oral ) glycol 400 1158 )
Rabbit F oral acetone + oil ca.500 Thyssen and Kimmerle 1974
(1:10)
TABLE 1. (con't)
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rabbit M oral H2O + Cremophor EL 250-500 Mihail 1980
Hen F oral ) acetone _ oil ca. 5000 ) Thyssen and Kimmerle
Quail F oral ) (1:10) >1750,<2500 ) 1974
Duck M * oral ) polyethylene >4000 ) Lamb and Burke 1977
Duck F * oral ) glycol 400 >4000 )
Dog F oral acetone + oil >500 Thyssen and Kimmerle
(1:10) 1974
Rat M i.p.** acetone + oil 321 ) "
Rat F i.p.** (1:10) 293 )
Rat M i.p.** polyethylene 214 ) Mihail 1980
Rat F i.p.** glycol 400 213 )
Rat M dermal acetone + oil >1000 Thyssen and Kimmerle
(1:10) 1974
Rat M dermal) physiol NaCl >5000 ) Mihail 1980
Rat F dermal) solution >5000 )
* = animals fasted; ** i.p. = intraperitoneal
attack is the central nervous system. An influence on the acute
toxicity of the compound undoubtedly is exerted by the liver-damaging
effect at high doses (brittle liver in rats; lobular pattern of liver
in rats and rabbits) and by the local damaging effect on the
gastrointestinal tract (oral application, rat and mouse) and on the
peritoneum (intraperitoneal application, rat).
Comparison of the acute toxicity data for oral application and
for intraperitoneal administration to rats indicates, in the light of
the differences in the LD50 values, the delayed onset of action
following oral application, the long persistence of symptoms and the
long duration of animal mortality occurrence, that absorption from the
gastrointestinal tract is relatively poor and that triadimefon effects
are protracted. The relatively poor absorbability from the
gastrointestinal tract might be of practical significance, as it
suggests that rapid decontamination could be therapeutically effective
in the event of oral poisoning.
The results of the acute toxicity studies did not reveal any
significant sex-specific differences. The oral LD50 values in rats
exhibit a large range. Whereas the LD50 values for administration
of triadimefon in water and Cremophor EL correspond somewhat to those
for administration of the compound in polyethylene glycol 400,
administration in acetone + oil resulted in comparatively higher
toxicity. This may have been due to increased absorption or also to an
additional toxic effect of acetone. The corresponding LD50 values
obtained following administration in aqueous and alcoholic
formulations are usually in the order of 1 000 mg/kg body weight for
oral application to rats (Table 1).
The large range of the LD50 values most probably is associated
with the targets of attack of the compound at toxic doses, such as
damage to the liver and the gastrointestinal tract. These effects may
have had a varying influence on both absorption and detoxification in
the individual animals (Bayer 1981).
Short-term studies
Rat
Groups of 15 male and 15 female Wistar rats received daily oral
triadimefon doses of 3, 10 and 30 mg/kg bw respectively, by gavage for
30 days. The compound was emulsified in acetone and oil (1:10). A
control group of similar size received the acetone + oil mixture only.
The rats were sacrificed 24 h after the final application, and their
tissues were macroscopically examined and weighed. At the end of the
study, blood samples were taken for haematological and clinical
chemical tests. The nine most important tissues were examined
histopathologically. Rats treated with doses of up to and including
30 mg/kg bw did not differ from the controls with respect to
behavioural patterns, body weight development, haematological data,
clinical chemical data, results of urinalyses and histopathological
findings. However, the absolute and relative liver weights showed
a significant increase in male rats treated with doses of 10 and
30 mg/kg bw. In female rats, an increase in the absolute and relative
liver weights was seen only at the dose level of 30 mg/kg bw, and
other organ weights showed no differences. The no-effect dose for rats
treated orally with triadimefon for 30 days was 3 mg/kg for males and
10 mg/kg for females (Thyssen et al 1974).
In a 12-week feeding experiment, groups of 15 male and 15 female
Sprague-Dawley rats were fed triadimefon daily at dietary levels of
0 ppm (control), 50 ppm, 200 ppm, 800 ppm and 2000 ppm, respectively.
Haematological tests, clinical-chemical tests and urinalyses were
performed prior to commencement of test diet administration, after
6 weeks on the test diet and at the end of the treatment period. The
rats were sacrificed and dissected on termination of treatment,
tissues were macroscopically examined and organ weights were measured.
The major tissues were histopathologically examined. Triadimefon
was tolerated at daily dietary concentrations of up to and including
2 000 ppm for the whole of the 12-week period without causing any
symptoms. Haematology, clinical chemistry and organ weight
measurements, as well as gross pathology and histopathology, provided
no indication of toxic effects from administration of the test
compound. Redness and swelling seen macroscopically, especially in the
area of the glandular mucosa of the stomach, could not be confirmed
microscopically (Mohr 1976).
Dog
In a subchronic toxicity study, groups of 4 male and 4 female
beagle dogs were maintained for 13 weeks on a diet containing
triadimefon at concentrations of 0 ppm (control), 150 ppm, 600 ppm and
2 400 ppm, respectively. Clinical inspections, haematological tests,
clinical chemical tests and urinalyses were performed. On termination
of dietary administration, the dogs were sacrificed, dissected and
grossly examined. The most important tissues were weighed. EPN-oxidase
and N-demethylase were measured in liver samples to test for an enzyme
inducing effect. Comprehensive histopathological examinations were
performed on tissues from all dogs. The dietary level of 150 ppm was
tolerated without producing any treatment-related effects. Although
dietary concentrations of 600 ppm and 2 400 ppm caused increased N-
demethylase activities, which was indicative of enzyme induction, this
dose was tolerated without having any other toxic effects. However,
dogs fed a dietary level of 2 400 ppm were affected in several other
respects (e.g., vomiting and reduction in food consumption); hence,
they had a moderate state of nutrition and showed less weight gain
than the other groups. The parameters of the red blood picture were
adversely affected by the treatment. Alkaline phosphatase and
transaminase activities were increased, illustrating a disturbed liver
function. The relative liver weight showed a slight increase in both
sexes in the 2 400 ppm group as compared with the controls. However,
gross pathology and histopathology did not reveal any treatment-
related alterations in the liver or in any other tissues. In the
13-week feeding experiment, the no-effect dietary level for the dog
was 150 ppm (Hoffman and Luckhaus 1974).
In a chronic toxicity study, groups of 4 male and 4 female beagle
dogs were maintained for 104 weeks on a diet containing triadimefon at
concentrations of 0 ppm (control), 100 ppm, 330 ppm, 1 000 ppm (up to
week 54) and 2 000 ppm (from week 55), respectively, of a technical
grade of two batches, purity 88 to 89% and 92 to 97% (90% premix
prepared with highly dispersed silicate). Clinical examinations,
haematological tests, clinical chemical tests and urinalyses were
performed on the dogs. On termination of dietary administration, the
dogs were sacrificed, dissected and grossly examined; their organ
weights were measured and tissues were histopathologically examined.
The results of the study showed that triadimefon dietary
concentrations of up to and including 330 ppm were tolerated for 2
years by both males and females without having any toxic effect. In
the highest dietary concentration group (especially after the dietary
level was raised to 2 000 ppm in week 55) the weight of some dogs of
both sexes was depressed slightly. Clinical chemistry and
histopathology did not reveal any liver damage; however, there were
signs of a treatment-related microsomal enzyme induction at the
highest dietary level. None of the other findings showed any
differences between the treated dogs and the controls (Hoffman and
Gröning 1978).
Long-term studies
Rat
In a 24-month study to evaluate triadimefon for potential chronic
toxicity and carcinogenicity, groups of 50 male and 50 female Wistar
rats were fed dietary levels of 50, 500 and 5 000 ppm, respectively of
a technical grade, composed of 2 batches, one of 88-89%, and the other
of 92-97% (90% premix prepared with a highly dispersed silicate). The
control group, fed only chow, consisted of 100 male and 100 female
rats. For clinical laboratory tests (haematology, clinical chemistry,
urinalysis, enzyme induction assays), 10 rats were additionally used
per group and per sex. After 24 months on the test diet, all survivors
were sacrificed, dissected and grossly examined.
The study revealed no indication of triadimefon having a
carcinogenic effect on rats. With respect to chronic toxicity, the
no-effect dose was found to be 50 ppm for females and 500 ppm for
males. Higher dietary levels had the following effects in the two
sexes. The dietary concentration of 5 000 ppm caused violent motor
reactions in males and females from about week 23. These rats consumed
almost no food while the reactions persisted and, as a result, they
lost a considerable amount of weight and many died. Those that
survived began to consume feed again when the symptoms receded, and
made corresponding weight gains. However, in week 32 and, following
brief improvement, in week 37, these violent motor reactions again
occurred accompanied by refusal of food and considerable loss of
weight. The last of the surviving rats in the 5 000 ppm group were
killed in a moribund condition in week 39. Gross pathology performed
on the 5 000 ppm rats that died and on those sacrificed essentially
disclosed severe mucosal lesions in the stomach, which in most cases
contained no food, accompanied by sometimes severe haemorrhages. These
lesions were considered to have been the cause of the clinical
symptoms observed. From week 85, the body weights of the male rats in
the 500 ppm group were mostly significantly lower than those of the
controls, but the difference was so slight that it was not considered
to have been due to a damaging effect from the test compound. The
growth rate of most of the female rats in the 500 ppm group was
significantly retarded from as early as week 6.
Significantly reduced erythrocyte counts and haemoglobin levels
seen at the dietary levels of 5 000 ppm, and at the end of the study
also in females fed 500 ppm, were indicative of an effect on the blood
or blood-forming tissues. The histopathological examinations revealed
shrunken spleen with reduced haematopoiesis in some of the rats of the
5 000 ppm group. Some of the rats of this group also showed decrease
in haematopoiesis in the marrow. The histopathological examinations
revealed signs of damage to cells of the proximal tubules of the
kidney (vacuolation, desquamation, degeneration) in 5000 ppm rats. In
the lung, increased blood content was seen in the dilated alveolar
vessels. In the stomach of 5 000 ppm rats, increased superficial
ulcerations, haemorrhage of the mucosa and oedemas of the sub-mucosa
were observed. In the adrenals of some rats, congestion of blood
vessels was seen. The dietary level of 5 000 ppm also caused formation
of giant spermatids in some males. In rats fed 5 000 ppm, cholesterol
levels occasionally showed a significant increase, indicative also of
an effect on fat metabolism at this dietary level. The liver weights
showed a statistically significant (p <0.01) and dose-related
increase in the male rats of the 50 and 500 ppm groups and in the
female rats of the 500 ppm group. (On highest dose, 5 000 ppm, all
animals were dead at week 39.)
The statistically significant increased liver weight (p <0.01)
seen in 50 and 500 ppm males and in 500 ppm females was not due to
stimulation of microsomal enzymes, as the N-demethylase activity and
the cytochrome P-450 concentrations measured in the liver at the end
of the experiment were not affected. Thus, the increased liver weights
were considered to have been a non-specific effect of increased
metabolism (Bomhard and Löser 1978a).
Mouse
Triadimefon was evaluated for long-term effects and potential
carcinogenicity in a 24-month study on groups of 50 male and 50
female CF1/W 74 mice fed dietary levels of 0 (control), 50, 300 and
1 800 ppm, respectively, of a technical grade, purity 97% (used as an
approximately 90% premix prepared with a highly disperse silicate).
For clinical laboratory tests, 10 mice were additionally used per
group and per sex. The mice were inspected for clinical symptoms.
Their body weight and food intake were measured. Haematological tests,
clinical chemical tests, organ weight measurements, macroscopic tissue
examinations and comprehensive histological examinations were
performed.
The study revealed no indication of triadimefon having a
carcinogenic effect on mice. No chronic-toxic effects were seen at the
two lower dietary levels of 50 and 300 ppm. Mice fed 1 800 ppm showed
the following alterations, in comparison with the controls. Growth of
both male and female mice was affected. Significantly increased
erythrocyte counts were recorded in both sexes, and increased
haemoglobin and haematocrit values were observed in females. Other
treatment-related alterations seen on termination of the study,
although not present consistently at the intermediate examinations and
necropsies performed after 6 and 12 months, included: distinctly
increased liver weights; increased AP, GOT and GPT activities;
increased incidence of liver swellings, occasionally accompanied by
adhesions of liver lobes in mice that died and in those sacrificed on
termination of the study. At the end of the feeding experiment, a
marked correlation between hyperplastic liver nodules and greatly
increased enzyme activities, indicative of liver damage, was observed
in the 1 800 ppm group. The incidence of liver cell tumours in male
and female mice were as follows:
ppm F M
0 0 2
50 1 2
300 0 1
1800 2 2
An increased incidence of hyperplastic liver nodules, which are
histologically very difficult to differentiate from liver cell tumour,
were also observed with the following incidence in male and female
mice:
ppm F M
0 4 7
50 2 7
300 1 7
1800 15 15
(Bombard and Löser 1980b)
Special studies on reproduction
Rat
Triadimefon was evaluated for its effect on fertility, lactation
performance and development of offspring in a 3-generation
reproduction study in Wistar rats with two matings per generation
(Löser 1979). Each test group consisted of 10 male and 20 female rats
and triadimefon was fed to the rats at dietary concentrations of 0
(control), 50, 300 and 1 800 ppm respectively. At the start of the
study, the F0 generation was ca. 32 to 39 days old and was treated
for 70 days prior to the first mating.
The pups of the F3b generation were sacrificed and
necropsied at an age of 4 weeks, and the major 18 tissues were then
histopathologically examined. Dietary levels of 50 and 300 ppm had no
adverse effect on the reproductive performance of the rats. After 2 of
6 matings, the weight of the pups in the 300 ppm group were
significantly lower than those of the controls during the lactation
period. The dietary level of 1 800 ppm distinctly affected
reproductive performance, as none of the rats became pregnant after
the second mating of the F1b generation. As this dietary level also
distinctly affected the parental rats, evidenced by the growth rate,
there would be no evidence for assuming any primary damaging effect on
reproduction.
Triadimefon did not cause any gross abnormalities or
malformations in rats at the tested dietary concentrations. The
histopathological examinations of the 18 most important tissues from
4-week old pups of the F3b generation revealed no indication of any
tissue alterations due to dietary administration of triadimefon at the
tested concentrations. The dietary concentration of 50 ppm was
tolerated without having any damaging effect whatsoever in the
3-generation reproduction study (Löser 1979).
Hen
Groups of 10 female White Leghorn hens per concentration, which
were mated with one cock per group, were maintained for 4 weeks on a
diet containing triadimefon at concentrations of 0 (control), 10 and
100 ppm, respectively. The hens were inspected for behavioural
patterns, physical appearance and appetite and their body weights and
food intake were measured. Tissues were macroscopically examined and
the 12 most important tissues were histopathologically examined.
Haematological tests were also performed on additional hens maintained
under comparable conditions. The following parameters were measured in
the offspring: number of eggs, egg quality, egg weight, egg size,
thickness of egg shell, weight of egg shell, fertilization rates,
hatch rates. The chicks were inspected for their physical appearance
and behavioural reactions during the first days of their life. The
tested concentrations were tolerated by both the hens and their
offspring without causing any damage whatsoever (Thyssen and Gröning
1978).
Species studies on embryotoxic effects
Rat
Two experiments were performed with groups of 20 pregnant Long
Evans rats dosed daily by gavage with triadimefon in an aqueous
emulsion from gestation day 6 to 15. The first study utilized daily
doses of 10, 30 and 100 mg per kg bw. Each experiment included a
control group dosed only with the vehicle. While the dose of
10 mg/kg/day did not have any harmful effects on the dams, doses of
30 mg/kg/day and above reduced dam weight gain. However, none of the
tested doses caused any behavioural disorders or mortalities. Effects
on progeny were characterized by a slightly increased placenta weight
in the 100 mg/kg group as compared with the controls, and by the
occurrence of occasional cleft palates at 75 mg/kg and 100 mg/kg/day.
Thus, a treatment-related effect and hence a slight teratogenic effect
of triadimefon on rats at oral doses of 75 mg/kg/day and above could
not be completely ruled out. The oral no-effect of triadimefon with
respect to embryonic/foetal development was found to be 50 mg/kg/day
(Machemer 1976b).
Evaluation of triadimefon for embryotoxic effects was also
performed by administering the compound by the route of greatest
relevance to practical conditions, viz. by inhalation. (A hazard from
skin exposure can be ruled out on the ground of the acute toxicity
findings, which did not reveal any toxicologically relevant absorption
through the skin.) For the inhalation evaluation, twenty fertilized
rats per group were exposed for 6 h daily on 10 consecutive days
from gestation day 6 to 15, to analytically measured triadimefon
concentrations of 14 (11 to 18) mg/m3, 33 (28 to 37) mg/m3 and 114
(95 to 132) mg/m3 air in a dynamic flow inhalation apparatus.
Triadimefon was dissolved in a 1:1 mixture of ethanol and polyethylene
glycol 400 and aerosolized in the inhalation chambers. A control group
inhaled the aerosol of the solvent mixture only. While the contration
of 14 mg/m3 air did not affect the dams, concentrations of 33 and
114 mg/m3 slightly reduced dam weight gain during the treatment
period. None of the concentrations adversely affected physical
appearance and behavioural patterns of the dams. Embryonic and foetal
development were not affected by concentrations of up to and including
114 mg/m3. Hence, it was concluded that inhaled triadimefon did not
result in any embryotoxic effects (Machemer and Kimmerle 1976).
Rabbit
Groups of 10 to 13 pregnant Himalayan rabbits received daily oral
doses of triadimefon administered as an aqueous emulsion by gavage at
levels of 0 (control), 5, 15 and 50 mg/kg bw, respectively, from
gestation day 6 to 18. The tested doses had no adverse effects on
physical appearance, behavioural patterns and weight gains of the dams
or on embryonic and foetal development. Oral doses of triadimefon did
not result in toxic or teratogenic effects on rabbits (Machemer
1976c).
Special studies on mutagenic effects
Triadimefon was evaluated for mutagenic potential by the Ames
test on histidine requiring Salmonella typhimurium strains TA 98,
100, 1535, 1537, 1538 and 1950, with and without metabolic activation.
The triadimefon concentrations ranged from 0.1 to 1 000 µg per plate.
The minimal inhibitory concentration of triadimefon on the tester
strains used was 500 µg/ml. Triadimefon did not induce back-mutations
in the test systems, with or without microsome activation, over the
tested concentration range (van Dijck 1976).
Triadimefon was tested for potential DNA-attacking action on
Bacillus subtilis at concentrations of up to 300 µg per plate, by
rec-assay. The compound was also evaluated at concentrations of up to
1 000 µg/plate for potential mutagenicity by the Ames assay using
Salmonella typhimurium strains TA 1535, 1537, 98 and 100, with and
without addition of rat and mouse liver homogenates to the agar. In
these studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Inukai and Iyatomi 1977).
The fungicide was further tested for potential mutagenic effects
by the rec-assay on two Bacillus subtilis strains at concentrations
of up to 2 000 µg/plate, and by the Ames (reversion) assay on the
Salmonella typhimurium strains TA 98 and 100 at concentrations of up
to 5 000 µg/plate, with and without metabolic activation. In these
studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Shirasu et al 1978).
Triadimefon was additionally studied by rec-assay on two
Bacillus subtilis strains at concentrations of up to and including
2 000 µg per plate and by reversion assay on Escherichia coli (WP 2)
and on Salmonella typhimurium strains TA 1535, 1537, 1538, 98 and
100, at concentrations of up to and including 5 000 µg per plate, with
and without metabolic activation by rat liver chromosomes. No
indication of mutagenic activity was found in these studies (Shirasu
et al 1979).
Triadimefon was evaluated for point-mutagenic effects by a
eukaryotic, micro-organism test using Saccharomyces cerevisiae
S 138 and S 211, in comparison with negative (solvent) and positive
controls, with and without metabolic activation by microsomal enzymes
of Aroclor-induced rat livers. Neither a mutagenic nor a
recombinogenic effect was seen at doses extending into the toxic
range, 1 000 µg/plate (Jagannath et al 1980).
A micronucleus test on male and female NMRI mice was performed
with triadimefon. Triadimefon was administered in an aqueous emulsion
at a total oral dose of 400 mg/kg bw applied in two split doses of
200 mg/kg bw each at an interval of 24 h. The thiotepa dose was
2 × 10 mg/kg injected subcutaneously. For comparison, a negative
control group was treated with the vehicle and a positive control
group was treated with thiotepa. Each group consisted of 10 mice;
1 000 polychromatic erythrocytes per mouse were analysed for incidence
of micronuclei. Additionally, the ratio of polychromatic erythrocytes
to normochromatic ones was determined to establish whether the test
compound had any effect on the general activity of bone marrow.
Thiotepa caused a big increase in the number of micronuclei and also
bone marrow depression. Triadimefon did not affect general bone
marrow activity nor did it increase the incidence of micronuclei in
comparison with the control, and hence was deemed to be non-mutagenic
in this system (Machemer 1977).
Twenty male NMRI mice each received a single oral dose of
triadimefon at a level of 200 mg/kg bw, administered as an aqueous
emulsion by gavage. Starting on the day of test compound
administration, a series of 8 matings was performed with the treated
males, each mating period being of 1-week duration; every week, each
male was caged together with 3 fresh untreated virgin females. The
pre-implantation and post-implantation losses of fertilized females
were determined as the criteria for assessment of a mutagenic effect.
(The corpora lutea, total implantations, viable implants and dead
implants were counted.) The results were compared with those obtained
from a control group of similar size. The tested dose was not toxic to
the males and did not affect their fertility. The measured parameters
did not reveal any treatment-related difference between the control
group and the triadimefon-treated group. The dominant lethal test on
the male mouse did not provide any indication of the mutagenicity of
triadimefon at the acute oral dose of 200 mg/kg bw (Machemer 1976a).
Special studies on induction of liver enzymes
Triadimefon was administered daily on 28 consecutive days to
Wistar rats, by gavage in a mixture of acetone and oil at dose levels
of 0 (vehicle), 1, 5 and 25 mg/kg bw, respectively. Each group
consisted of 20 male and 20 female rats; half of them were sacrificed
at the end of the treatment period and the other half were killed
on termination of a 4-week post-treatment observation period.
N-demethylase, O-demethylase and cytochrome P-450 in the rat livers
were measured at the end of the treatment period and on termination of
the post-treatment observation period. The rats were observed for
systemic toxic effects by observing their physical appearance,
behavioural patterns and posture, and their body weights and certain
haematological and clinical chemical parameters were measured. The
rats were necropsied, tissues were macroscopically examined and livers
were weighed. The study revealed that triadimefon at daily doses of 5
and 25 mg/kg bw caused slight induction of microsomal liver enzymes.
The enzyme induction was reversible within the 4-week post-treatment
observation period. None of the other parameters measured and
investigated provided any indication of treatment-related damage
(Mihail and Kaliner 1979).
Special studies on potentiation toxicity
Since triadimefon is used in combination with other fungicidal
compounds, acute combination toxicity studies were performed on Wistar
rats. The compounds tested in combination with triadimefon were
captafol, propineb, tolylfluanid and carbendazim. The oral LD50 of
each of these compounds was first determined. Fifteen female or male
rats were used per dose and the observation period lasted 14 days.
From the LD50 values of the single components the theoretically
expected LD50 of each combination was calculated. For combined
administration, the components were mixed in a percentage ratio
proportional to the LD50 of each and thus applied in equitoxic doses;
thereby, the actual LD50 of the combination was determined. A more
than additive acute toxic effect was not seen for any of the tested
combinations (Flucke and Kimmerle 1977, Thyssen 1977).
OBSERVATIONS IN MAN
Approximately 50 mg of triadimefon was applied to small gauze
pads which were placed for a contact time of 24 h on the skin of the
forearm of each of 8 test persons and the pads were fixed in position
with adhesive strips. Upon removal of the strips and pads, slight
redness of the treated skin area was seen for a brief period in 5 of
the 8 persons. At 24 h post-application, the treated skin areas showed
no variations from the physiological norm in any of the test persons
(Thyssen and Kimmerle 1973).
RESIDUES IN FOOD
USE PATTERN
Triadimefon is effective and is mainly used against powdery
mildews and rusts; it also gives good control of a number of other
diseases e.g. Thielaviopsis on pineapple (post-harvest application).
Triadimefon is registered and marketed in more than 30 countries
in Europe, Africa, America, Asia and Australia; registration is being
sought also in other countries. Details on the uses of triadimefon and
recommendations are given in Table 2. With the exception of the post-
harvest dip of pineapple the product is used as pre-harvest
application. Triadimefon is used also on ornamentals (roses, azaleas),
seed-grass, turf and rubber trees. Sugarcane and sweet potato sets are
dipped in a triadimefon suspension of emulsion prior to planting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on triadimefon residues from supervised residue trials were
obtained on various fruits and vegetables, cereal grains and some
other crops grown under a variety of geographical, climatic conditions
and agricultural practices. All residue figures presented in Table 3
refer to the sum of triadimefon parent compound and its main
metabolite triadimenol.
Fruits and vegetables
The average residue at harvest following the recommended
pre-harvest intervals is in the range of non-detectable (0.01 to
0.05 mg/kg) up to 0.3 mg/kg (maximum residue of 0.4 mg/kg) on most
fruits and vegetables. On red currants somewhat higher residues are
found after the recommended pre-harvest intervals; the average level
at harvest was 0.4 mg/kg (maximum residue in the trials 1.1 mg/kg).
Higher residues were also found on Brussels sprouts (average 0.5,
highest level found 0.9 mg/kg) in trials where the normal pre-harvest
interval was taken into account.
FATE OF RESIDUES
In plants
The behaviour and fate of triadimefon in plants was evaluated.
Studies were carried out on metabolism of (benzene ring-UL-14C)- and
(triazole ring-3,5-14C)-triadimefon in cereals, apple, cucumber and
tomato; translocation of unlabelled (benzene ring-UL-14C)-, and
(triazole ring-3,5-14C)-triadimefon, with the studies being directed
towards elucidation of site of action, mechanism of action and
metabolism; redistribution in the vapour phase after application of
unlabelled and (benzene ring-UL-14C)-triadimefon to plants.
TABLE 2. Summary of recommended uses of triadimefon
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Fruits
Apple and pear wp 40 - 75 4 - 12 7
Jap. apple wp 150 -200 2 - 3
Jap. pear wp 150 -200 2 - 3
Apricot wp 50 -150 4 - 6 7
Peach wp 50 -150 4 - 6 7
Currant (black,red) wp 50 -150 3 - 4 7
Gooseberry wp 50 -100 3 - 4 7
Grape wp/EC/dust 50-75 (dust 100-200) wingrape 4-10 14-28
tablegrape 4-8 4- 7
Strawberry wp 75 -100 7
Mango wp 100 -200 28
Pineapple wp/EC 0.015-0.07% 1(postharvest
dip) -
Vegetables
Artichoke wp/EC 50 -150 4 - 8 3 - 7
Beans wp/EC 125 -250 3 - 6 7 -14
Pea wp/EC 50 -125 3 - 6 7 -14
Brussels sprout wp/EC 125 -250 3 - 6 14
Onion (Welsh) wp 100 3 10
Cucurbits wp/EC/dust 25-100 (dust100-200) 4 - 10 3 - 7
Pepper wp/EC 50 -150 4 - 8 3 - 7
Tomato wp/EC 50- 150 4 - 8 3 - 7
Grain crops wp/EC 125 -250 1 - 2 21 -35
Sugarbeet wp/EC 125 2 - 3 14
TABLE 2. (con't)
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Coffee
protective wp/EC 100 -250 8 - 12
eradicative wp/EC 250 -500 2 - 4
Hops 200 -500 2 - 6
Tobacco 40 - 75 4 - 6
TABLE 3. Residues of triadimefon/triadimenol from supervised trials
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3 7 14
Fruit
Apple USA 1977 6 20 - 60 wp 50% 0.3 0.2 0.4 0.2 Mobay 1981
1977 6 20 - 60 wp 50% 0.5 0.4 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.2 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.3 0.3 0.4 "
1977 6 340 -560 wp 50% 0.5 0.4 0.3 0.2 "
1978 6 20 - 60 wp 50% 0.3 0.2 0.09 0.08 "
1977 11 340 -560 wp 50% 0.4 0.2 0.1 0.1 "
1977 10 340 -560 wp 50% 0.2 0.1
1977 10 340 -560 wp 50% 0.4 0.3 0.2 0.1 "
1977 10 340 -560 wp 50% 0.4 0.2 "
1977 10 340 -560 wp 50% 0.2 0.1 0.1 0.09 "
1979 6 140 wp 50% 0.7 0.4 "
Pear 1980 6 600 wp 50% 0.6 0.3 0.5 0.3 "
1980 6 600 wp 50% 0.4 0.4 0.3 0.2 "
1980 6 600 wp 50% 0.4 0.5 0.4 0.3 "
1980 10 450 wp 50% 0.3 0.2 0.09 0.06 "
1980 15 12001 wp 50% 0.8 0.5 0.3 0.2 "
Peach 1980 4 12001 wp 50% 3.0 1.6 0.7 "
1980 3 12001 wp 50% 0.1 0.4 0.1 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 3 6/7 14 21
Berry fruits
Currant- Denmark 1979 4 50 wp 5% 0.03 <0.05 <0.02 <0.05 <0.02 <0.05 Bayer 1981
black 1979 4 50 wp 5% 0.06 0.05 0.03 <0.05 <0.02 <0.05 "
France 1979 4 100 wp 5% 0.03 0.2 0.04 0.2 "
1977 4 40 wp 1.25% 0.04 0.14 "
Currant-red Denmark 1979 2 50 wp 5% 0.02 <0.05 <0.02 <0.05 <0.02 <0.05 "
1979 2 50 wp 5% 0.15 <0.05 0.05 <0.05 <0.02 <0.05 "
Raspberry 1979 4 75 wp 5% 0.03 0.05 Bayer 1981
Grape Japan 1979 3 150 wp 5% 0.01 0.4 <0.01 0.2 "
1979 5 150 wp 5% 0.02 0.5 0.01 0.4 "
1979 3 150 wp 5% 0.01 0.4 <0.01 0.3 "
1979 5 150 wp 5% 0.02 0.7 0.01 0.4
USA 1978 3 90 wp 50% 0.06 0.06 0.08 0.2 Mobay 1981
1978 3 110 wp 50% 0.02 0.05 <0.01 <0.01 "
1978 3 110 wp 50% 0.05 0.02 <0.01 <0.01 "
1978 3 150 wp 50% <0.01 0.01 "
1978 3 150 wp 50% 0.06 0.04 <0.01 <0.01 "
1978 3 150 wp 50% 0.03 0.09 <0.01 <0.01 "
1979 3 225 wp 50% <0.01 0.07 "
1979 3 225 wp 50% 0.5 0.4 0.01 0.02 "
1979 3 300 wp 50% 0.2 0.08 "
1979 3 225 wp 50% <0.01 <0.02 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3/5 7 14/15
Fruiting vegetables
Cantaloupes USA 1979 3 130 wp 50% 0.1 0.08 "
1979 3 130 wp 50% 0.1 0.03 "
1979 3 130 wp 50% 0.06 0.1 "
1979 3 130 wp 50% 0.03 0.03 "
Gherkins2 Fed.Rep. 1976 8 60 wp 5% <0.06 <0.06 <0.06 Bayer 1981
of 1976 8 60 wp 5% <0.01 <0.01 <0.06 "
Germany 1975 8 60 wp 5% 0.06 <0.06 <0.01 <0.06 "
1975 8 60 wp 5% 0.06 0.06 <0.06 <0.06 "
Cucumber Mexico 1979 3 125 wp 50% <0.0 <0.01 <0.01 <0.01 Mobay 1981
1979 3 125 wp 50% 0.04 0.05 <0.01 <0.01 "
Tomato Mexico 1979 8 180 wp 25% 0.03 0.05 0.01 0.03 0.01 0.03 "
1979 8 180 wp 25% 0.04 0.05 0.01 0.02 <0.01 0.02 "
1979 8 180 wp 25% 0.04 0.08 0.02 0.04 0.02 0.03 "
1979 8 180 wp 25% 0.07 0.08 0.02 0.06 <0.01 0.01 "
Japan 1977 4 250 wp 25% 0.07 0.08 0.04 0.08 0.022 <0.063 Nitokino
7 250 wp 25% 0.2 0.3 0.09 0.2 0.052 0.23 1981
2 250 wp 25% 0.4 0.2 0.3 0.3 0.22 0.33
4 250 wp 25% 0.4 0.5 0.3 0.6 0.12 0.53
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Other vegetables
Brussels
sprouts UK 1979 3 250 wp 25% <0.05 <0.05 Hazleton 1981
1977 3 125 wp 25% 0.6 0.2 BAY/UK 1981
Chick peas 1980 2 150 wp 25% 0.01 0.02 <0.01 <0.01 <0.01 0.02 Mobay
dry 2 150 wp 25% <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 1981
2 150 wp 25% <0.01 <0.02 <0.01 <0.01 <0.01 <0.01
Leeks UK 1979 4 250 wp 25% <0.05 <0.05 Hazleton 1981
500 wp 25% <0.05 0.08
250 wp 25% 0.09 0.4
500 wp 25% 0.3 0.9
Parsnips UK 1979 3 250 wp 25% 0.05 0.2
3 250 wp 25% 0.01 0.05
Hops UK 1979 8 110-220 wp 5% <0.3 0.8 Bayer 1981
fresh
dry
UK 1979 8 100-200 wp 5% 0.4 1.4
fresh <0.3 1.4 ibid
dry 0.4 1.4
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Fed. Rep.
of
Germany 1979 4 500 wp 5% ibid
fresh 0.9 0.6 2.13 0.83
dry 10.8 4.2 7.23 4.13
Fed.Rep.
of
Germany 1979 4 500 ibid
fresh 2.1 1.0 0.73 0.63
dry 11.4 4.3 1.93 1.73
1 Excessive dosage;
2 Some of the gherkins data were erroneously presented in the 1979 JMPR Evaluations under
cucumbers due to a translation error. Schlangen qurke = cucumber; Einlegequrke = gherkin;
3 After 10 days.
The metabolism of triadimefon (Bayleton(R)) in cereals was
studied in several experiments following application of (benzene ring-
UL-14C)-triadimefon to plants under glass and in field conditions.
The main metabolite formed was triadimenol, which occurs in the
diastereoisomeric forms A and B. On cereal plants, the A form is the
main diastereoisomer. A further metabolism pathway consisted of
oxidation of the tert. butyl group (KWG 1342) and cleavage of the
molecule, with the formation of 4-chlorophenol. The metabolites
triadimenol, and particularly KWG 1342 and 4-chlorophenol, are bound
in the form of glycosides-conjugates, mainly B glycosides. At harvest
the radioactivity still present in straw was distributed as shown in
Table 4.
TABLE 4. Radioactive studies on triadimefon metabolism in cereal
straw at harvest
Metabolite Radioactivity present (%)
Triadimefon unchanged parent 0 - 5
Triadimenol form A 22 - 41
Triadimenol form B 6 - 17
KWG 1342 2 - 16
Polar metabolites1 17 - 37
BUE 2285 less than 1
Free 4-Chlorophenol less than 1
Non-extractable 18 - 32
1 Including the glycosides of triadimenol, KWI 1342 and
4-chlorophenol.
The glycosides are probably fixed in the plants by further
metabolism and are therefore no longer extractable with standard
procedures. The distribution of the radioactivity among the aqueous
and organic phases after extraction of the straw differs between the
(triazole ring-3,5-14C) and the (benzene ring-UL-14C). An explanation
for this may be the different polarities of 4-chlorophenol and
1,2,3-triazole, which can be formed as metabolites of triadimefon
and/or triadimenol. Further studies are underway to elucidate this
phenomena.
In another experiment on summer barley, the non-extractable part
of the residue accounted for higher amounts, i.e. 55-75% (Bayer 1981).
This may be partly due to the application at an earlier stage in plant
development. Following spray application of (triazole ring-3,5-14C)-
triadimefon the metabolites that occurred were the same as those found
following the application of (benzene ring-UL-14C)-triadimefon, the
only difference being that polar metabolites accounted for a higher
proportion when both labels were applied at the same stage in plant
development, i.e. 31% vs 20% (Bayer 1981). For quantitative data
see Table 5. After meed treatment of barley with the pure
diastereoisomeric forms A and B of triadimenol, no conversion of one
form to the other was found in the barley plant (Bayer 1981).
In studies on apple with benzene ring-labelled and triazole-
labelled triadimefon, it was demonstrated that also in this crop
triadimefon was metabolized to triadimenol and in a small amount to an
unidentified compound (Table 6). The rapid decline of the 14C during
the early intervals up to 7 days was attributed to the volatility of
triadimefon parent compound (Mobay 1981). In trials on cucumber and
tomatoes, the fate of (benzene-ring-UL-14C)-triadimefon was studied.
The material was applied as a foliar spray or to the roots. It was
found that in addition to triadimefon and diastereoisomers A and B, a
polar metabolite was formed in the plants, which was shown to be a
glucoside of triadimenol loosely bound to another natural occurring
moiety. It is readily converted to triadimenol glucoside (see Table 6)
(Mobay 1981). It was also shown in these experiments that the
radiocarbon applied to the foliage did not translocate significantly
into neighbouring fruits, in marrow plants triadimenol was identified
as the major metabolite of triadimefon (Clark et al 1978). The
metabolic pathways of triadimefon are illustrated in Figure 2. For
chemical names of the metabolites identified in plants and their
structural formula see Table 9, FAO/WHO 1979, Triadimefon.
Triadimefon was translocated at a relatively fast rate in
anacropetal direction following foliar application to cereals, beans,
cucumber and tomato (Buchenauer 1975, 1976; Bayer 1981; Scheinpflug
et al 1977; Brandes et al 1978; Gasztonyi and Joslpovits 1978) and
also to a larger extent following soil applications to beans and
barley (Buchenauer 1975, 1976). Some authors also observed a slight
basipetal translocation. Gasztonyi and Josepovits (1978) established
that the rate of uptake and subsequently the amount of transport
depended substantially on the mode of leaf treatment, and also found
differences in the amounts of translocated triadimefon according to
plant species.
Gasztonyi and Josepovits (1979) studied the uptake and metabolism
of triadimefon by mycelia of fungi that were sensitive or resistant to
triadimefon. In the fungi, triadimefon accumulated to 20 to 40 fold of
the external concentration, irrespective of the sensitivity of the
fungus. In the course of metabolism, the highly fungitoxic main
metabolite triadimenol was formed; however, in mycelia of sensitive
fungi this transformation was at a high rate, whereas it could not be
demonstrated, or only to a low extent, in resistant strains of fungi.
Based on these observations, triadimefon must be regarded as the
precursor of the active principle, i.e. triadimenol.
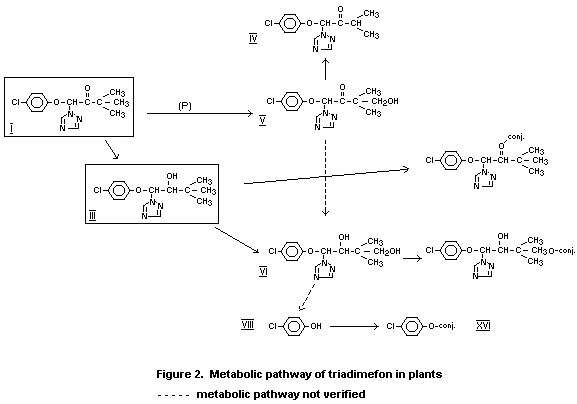 TABLE 5. Percent distribution of radioactivity in cereals after spray application of (14C)-triadimefon (recovered radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(G) benzene ring-
total plant UL-14C 0 n.d.2 n.d. n.d. n.d. 99.5 n.d. 0.5
" " 250g/ha 5 7.3 n.d. 57.3 21.8 9.7 n.d. 3.9
" " 10 15.8 n.d. 50.3 18.9 7.9 n.d. 7.1
" " 28 15.8 n.d. 45.1 17.4 6.4 n.d. 15.3
straw 38 15.9 n.d. 43.6 14.4 10.3 n.d. 15.8
" 62 16.7 1.9 41.0 17.3 4.8 n.d. 18.3
Spring barley(F) benzene ring-
total plant UL-14C 0 <1 n.d. 1 n.d. 92 n.d. 6
" " 125 g/ha 5 13 n.d. 48 15 12 n.d. 12
" " 17 35 n.d. 32 10 <1 n.d. 23
straw 28 9 <1 12 4 n.d. n.d. 75
" 76 8.4 5 8.4 3 n.d. n.d. 75
Spring wheat(F) benzene ring-
straw UL-14C 49 37 2 23 6 n.d. n.d. 32
TABLE 5. (con't)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(F) triazole-ring-
straw 3,4-14C 71 31 8 20 10 1 n.d. 29
250 g/ha
benzene ring-
UL-14C 71 20 16 22 9 1 3 28
250 g/ha
1 G = greenhouse, F = field;
2 n.d. = not detectable. In these studies the amount of radioactivity in the grains
ears was so small that separation by TLC was impossible.
TABLE 6. Percent distribution of radioactivity in apple, tomato and cucumber after spray application of (14C) triadimefon (recovered
radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post aqueous polar Triadimenol Triadimenol Triadimefon Unidentified Non-
appl. phase metabolite3 A B metabolites extractables
Apple (F) triazole ring-
peel 3,5-14C 28 5.7 n.d.2 14.9 13.6 10.8 2.8 5.0
pulp 15 mg/100 ml 5.3 n.d. 11.2 7.1 2.1 3.1 0.9
total fruit 11.0 n.d. 26.1 20.7 13.0 5.9 5.9
peel 48 4.8 n.d. 14.5 14.8 10.4 1.5 6.0
pulp 6.4 n.d. 10.4 9.4 2.1 2.1 1.0
total fruit 11.2 n.d. 24.9 24.2 12.5 3.6 7.0
Tomato (G) benzene ring-
foliage UL-14C 7 7 4 16 3 66 n.d. 3-4
e mg/plant 14 4 5 14 2 71 n.d. 4
(3 treatments) 21 2 22 19 5 44 n.d. 8
fruit 7 6 n.d. 8 14 69 n.d. 2-3
14 15 n.d. 10 18-21 49-52 n.d. 2
Cucumber (G)
foliage 7 7 4 11-12 10 60-61 n.d. 6
14 6 4 16-19 9-10 48-54 n.d. 7
21 7 20 17 12 36 n.d. 8
fruit 7 5 n.d. 8 33 51 n.d. 3
14 11 n.d. 8 32-33 41-43 n.d. 5
1 G = greenhouse, F = field;
2 n.d. = not detectable;
3 glucoside of triadimenol.
In studies following field-scale spraying of spring barley and
spring wheat with (benzene ring-UL-14C)-triadimefon 1.9 to 14.9% and
0.1% of the applied radioactivity was found in the straw and the
kernel respectively of the treated cereals, equivalent to 0.33 to
7.39 mg/kg and 0.01 to 0.04 mg/kg respectively (Bayer 1981). Following
spray treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon, on the other hand, 6.5% and 0.7% of applied radioactivity
was found in straw and grain respectively, equivalent to 3.39 and
0.39 mg/kg of parent triadimefon. Following spray applications of
(benzene ring-UL-14C)-triadimefon in the same trial, the apparent
triadimefon equivalents were 2.92 and 0.16 mg/kg in straw and grain
respectively (Bayer 1981).
In a study, carried out in a closed glass chamber with (benzene
ring-UL-14C)-triadimefon on barley plants, it was observed that the
applied compound moved from treated to un-treated plants, where it was
metabolized in the usual manner (Bayer 1981).
In glasshouse experiments triadimefon displayed vapour phase
activity against powdery mildew on cucumber and barley plants, not
only when it was applied at normal application rates, but also at
substantially lower ones. The effect was dependent on the distance of
the spray deposit (Scheinpflug and Paul 1977; Scheinpflug et al
1978; Schlütter 1977; Bayer 1981). In experiments on apple seedlings
and marrow plants, triadimefon was shown to display vapour phase
activity against powdery mildew (Clark et al 1978).
In animals
Several new data have become available on the fate of triadimefon
in animals. Data on excretion, storage and metabolism are now
available from studies in rats, dairy cattle, pigs and laying hens.
Accumulation of residues was also studied in the Channel catfish
exposed to labelled triadimefon. Feeding experiments were also carried
out on livestock animals, e.g. dairy cattle, pigs, sheep and hens.
Supplementary studies with the main animal metabolite of triadimefon,
triadimenol, were carried out in rats. Radiolabelled triazole was
given to rats in order to obtain data on the fate of this triadimefon
metabolite. The studies on rats are reported under "Biochemical
Aspects".
Cow
Feeding studies were carried out with 1:1 mixture of triadimefon
and triadimenol in dairy cattle. Nine dairy cows (three animals per
dosage) were fed with the mixture by bolus capsule with dosages
equal to 25, 75 and 250 mg/kg bw. The fat contained residues up to
0.029 mg/kg at the 250 mg/kg level and 0.016 mg/kg at the 75 mg/kg
dose level, whereas the residue level in the fat was less than
0.01 mg/kg at the 25 mg/kg feeding level (Mobay 1981). One animal
exposed to the high dose level showed milk residues of 0.0013 mg/kg
triadimefon equivalents on day 27 to 28, and another animal had
triadimenol residues in the milk of 0.001 mg/kg on day 29. All other
animals fed at high level, as well as those fed with the medium level,
showed no milk residues at or above the level of detection of
0.001 mg/kg (Mobay 1981).
Hen
In a feeding study on hens, 16 (4 birds per dosage) were fed a
daily ration for 29 days, containing equal amounts of triadimefon and
triadimenol at total levels in the feed of 10, 25, 75 and 250 mg/kg.
After 29 days of feeding no residues could be detected (<0.01 mg/kg)
in the control and in the feeding levels up to 75 mg/kg included,
except that one of the 4 hens fed at the 75 mg/kg total residue in the
feed showed a triadimenol residue of 0.025 mg/kg in the skin and
0.012 mg/kg in the gizzard. Hens fed at the highest level of 250 mg/kg
total residue in the feed showed maximum residues of 0.02 mg/kg
triadimefon in the fat and 0.038 mg/kg triadimenol in the gizzard, but
no detectable residues in any other tissue. Residues in eggs were
proportional to feeding levels. Maximum total residues in the eggs
(triadimefon and triadimenol) were 0.002, 0.006, 0.011 and 0.050 mg/kg
at feeding levels of 10, 25, 75 and 250 mg/kg in the feed (Mobay
1981).
Other animals
In a study carried out in Australia, seed treated with a mixture
of triadimefon and triadimenol was added to livestock feed, which was
given to cows, pigs, sheep and poultry. After ingestion of feed
containing triadimefon, residue levels at or above the limit of
determination were only present in the skimmed milk, taken while the
animals were being fed. However, residues of triadimenol at or
slightly above the limit of determination were present in the meat,
fat and offal of pigs, fat and offal of sheep and in offal of poultry.
The offal included liver and kidney for pigs and sheep and giblets for
poultry (Bayer 1981).
The metabolic pathway of triadimefon in animals is illustrated in
Figure 3. For chemical names of metabolites shown in Figure 3, see
FAO/WHO 1979, Triadimefon, Table 9. From the information available on
triadimefon in plants, it can be concluded that the same metabolism
pathways and metabolites are found in plants and animals.
In soil
Extensive additional information on the fate of triadimefon and
triadimenol in soil, on the fate in soil micro-organisms and on the
residue uptake from soil, including crop rotation, was made available.
TABLE 5. Percent distribution of radioactivity in cereals after spray application of (14C)-triadimefon (recovered radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(G) benzene ring-
total plant UL-14C 0 n.d.2 n.d. n.d. n.d. 99.5 n.d. 0.5
" " 250g/ha 5 7.3 n.d. 57.3 21.8 9.7 n.d. 3.9
" " 10 15.8 n.d. 50.3 18.9 7.9 n.d. 7.1
" " 28 15.8 n.d. 45.1 17.4 6.4 n.d. 15.3
straw 38 15.9 n.d. 43.6 14.4 10.3 n.d. 15.8
" 62 16.7 1.9 41.0 17.3 4.8 n.d. 18.3
Spring barley(F) benzene ring-
total plant UL-14C 0 <1 n.d. 1 n.d. 92 n.d. 6
" " 125 g/ha 5 13 n.d. 48 15 12 n.d. 12
" " 17 35 n.d. 32 10 <1 n.d. 23
straw 28 9 <1 12 4 n.d. n.d. 75
" 76 8.4 5 8.4 3 n.d. n.d. 75
Spring wheat(F) benzene ring-
straw UL-14C 49 37 2 23 6 n.d. n.d. 32
TABLE 5. (con't)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(F) triazole-ring-
straw 3,4-14C 71 31 8 20 10 1 n.d. 29
250 g/ha
benzene ring-
UL-14C 71 20 16 22 9 1 3 28
250 g/ha
1 G = greenhouse, F = field;
2 n.d. = not detectable. In these studies the amount of radioactivity in the grains
ears was so small that separation by TLC was impossible.
TABLE 6. Percent distribution of radioactivity in apple, tomato and cucumber after spray application of (14C) triadimefon (recovered
radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post aqueous polar Triadimenol Triadimenol Triadimefon Unidentified Non-
appl. phase metabolite3 A B metabolites extractables
Apple (F) triazole ring-
peel 3,5-14C 28 5.7 n.d.2 14.9 13.6 10.8 2.8 5.0
pulp 15 mg/100 ml 5.3 n.d. 11.2 7.1 2.1 3.1 0.9
total fruit 11.0 n.d. 26.1 20.7 13.0 5.9 5.9
peel 48 4.8 n.d. 14.5 14.8 10.4 1.5 6.0
pulp 6.4 n.d. 10.4 9.4 2.1 2.1 1.0
total fruit 11.2 n.d. 24.9 24.2 12.5 3.6 7.0
Tomato (G) benzene ring-
foliage UL-14C 7 7 4 16 3 66 n.d. 3-4
e mg/plant 14 4 5 14 2 71 n.d. 4
(3 treatments) 21 2 22 19 5 44 n.d. 8
fruit 7 6 n.d. 8 14 69 n.d. 2-3
14 15 n.d. 10 18-21 49-52 n.d. 2
Cucumber (G)
foliage 7 7 4 11-12 10 60-61 n.d. 6
14 6 4 16-19 9-10 48-54 n.d. 7
21 7 20 17 12 36 n.d. 8
fruit 7 5 n.d. 8 33 51 n.d. 3
14 11 n.d. 8 32-33 41-43 n.d. 5
1 G = greenhouse, F = field;
2 n.d. = not detectable;
3 glucoside of triadimenol.
In studies following field-scale spraying of spring barley and
spring wheat with (benzene ring-UL-14C)-triadimefon 1.9 to 14.9% and
0.1% of the applied radioactivity was found in the straw and the
kernel respectively of the treated cereals, equivalent to 0.33 to
7.39 mg/kg and 0.01 to 0.04 mg/kg respectively (Bayer 1981). Following
spray treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon, on the other hand, 6.5% and 0.7% of applied radioactivity
was found in straw and grain respectively, equivalent to 3.39 and
0.39 mg/kg of parent triadimefon. Following spray applications of
(benzene ring-UL-14C)-triadimefon in the same trial, the apparent
triadimefon equivalents were 2.92 and 0.16 mg/kg in straw and grain
respectively (Bayer 1981).
In a study, carried out in a closed glass chamber with (benzene
ring-UL-14C)-triadimefon on barley plants, it was observed that the
applied compound moved from treated to un-treated plants, where it was
metabolized in the usual manner (Bayer 1981).
In glasshouse experiments triadimefon displayed vapour phase
activity against powdery mildew on cucumber and barley plants, not
only when it was applied at normal application rates, but also at
substantially lower ones. The effect was dependent on the distance of
the spray deposit (Scheinpflug and Paul 1977; Scheinpflug et al
1978; Schlütter 1977; Bayer 1981). In experiments on apple seedlings
and marrow plants, triadimefon was shown to display vapour phase
activity against powdery mildew (Clark et al 1978).
In animals
Several new data have become available on the fate of triadimefon
in animals. Data on excretion, storage and metabolism are now
available from studies in rats, dairy cattle, pigs and laying hens.
Accumulation of residues was also studied in the Channel catfish
exposed to labelled triadimefon. Feeding experiments were also carried
out on livestock animals, e.g. dairy cattle, pigs, sheep and hens.
Supplementary studies with the main animal metabolite of triadimefon,
triadimenol, were carried out in rats. Radiolabelled triazole was
given to rats in order to obtain data on the fate of this triadimefon
metabolite. The studies on rats are reported under "Biochemical
Aspects".
Cow
Feeding studies were carried out with 1:1 mixture of triadimefon
and triadimenol in dairy cattle. Nine dairy cows (three animals per
dosage) were fed with the mixture by bolus capsule with dosages
equal to 25, 75 and 250 mg/kg bw. The fat contained residues up to
0.029 mg/kg at the 250 mg/kg level and 0.016 mg/kg at the 75 mg/kg
dose level, whereas the residue level in the fat was less than
0.01 mg/kg at the 25 mg/kg feeding level (Mobay 1981). One animal
exposed to the high dose level showed milk residues of 0.0013 mg/kg
triadimefon equivalents on day 27 to 28, and another animal had
triadimenol residues in the milk of 0.001 mg/kg on day 29. All other
animals fed at high level, as well as those fed with the medium level,
showed no milk residues at or above the level of detection of
0.001 mg/kg (Mobay 1981).
Hen
In a feeding study on hens, 16 (4 birds per dosage) were fed a
daily ration for 29 days, containing equal amounts of triadimefon and
triadimenol at total levels in the feed of 10, 25, 75 and 250 mg/kg.
After 29 days of feeding no residues could be detected (<0.01 mg/kg)
in the control and in the feeding levels up to 75 mg/kg included,
except that one of the 4 hens fed at the 75 mg/kg total residue in the
feed showed a triadimenol residue of 0.025 mg/kg in the skin and
0.012 mg/kg in the gizzard. Hens fed at the highest level of 250 mg/kg
total residue in the feed showed maximum residues of 0.02 mg/kg
triadimefon in the fat and 0.038 mg/kg triadimenol in the gizzard, but
no detectable residues in any other tissue. Residues in eggs were
proportional to feeding levels. Maximum total residues in the eggs
(triadimefon and triadimenol) were 0.002, 0.006, 0.011 and 0.050 mg/kg
at feeding levels of 10, 25, 75 and 250 mg/kg in the feed (Mobay
1981).
Other animals
In a study carried out in Australia, seed treated with a mixture
of triadimefon and triadimenol was added to livestock feed, which was
given to cows, pigs, sheep and poultry. After ingestion of feed
containing triadimefon, residue levels at or above the limit of
determination were only present in the skimmed milk, taken while the
animals were being fed. However, residues of triadimenol at or
slightly above the limit of determination were present in the meat,
fat and offal of pigs, fat and offal of sheep and in offal of poultry.
The offal included liver and kidney for pigs and sheep and giblets for
poultry (Bayer 1981).
The metabolic pathway of triadimefon in animals is illustrated in
Figure 3. For chemical names of metabolites shown in Figure 3, see
FAO/WHO 1979, Triadimefon, Table 9. From the information available on
triadimefon in plants, it can be concluded that the same metabolism
pathways and metabolites are found in plants and animals.
In soil
Extensive additional information on the fate of triadimefon and
triadimenol in soil, on the fate in soil micro-organisms and on the
residue uptake from soil, including crop rotation, was made available.
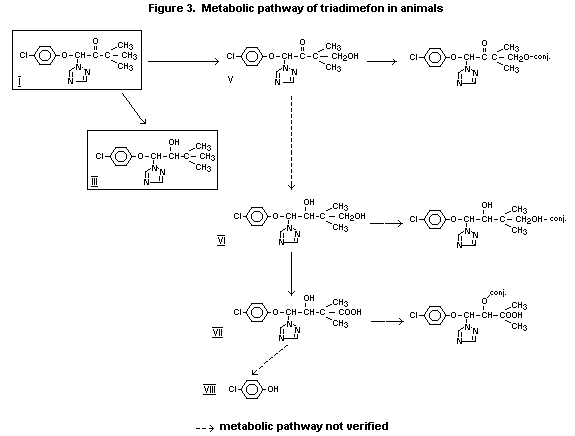 After applying (benzene ring-UL-14C)-triadimefon to spring
barley under field conditions the radioactivity still present in the
soil at harvest contained the metabolites listed in Table 7. The
relatively large proportion of triadimenol diastereoisomer A may be
due to washing from the plant into soil by rainfall (Bayer 1981).
Following treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon under field conditions 4% of 1,2,4-triazole was found in
addition to the above-mentioned metabolites. The percentage of
radioactivity still present in the soil accounted for by polar
metabolites increased to 6%. Including the non-extractables, more than
90% of the radioactivity was identified for both labels (Bayer 1981).
TABLE 7. Metabolites of triadimefon present in soil at harvest of
treated barley
Metabolite Radioactivity (%)
Triadimenol diastereoisomer A 33 - 41
Triadimenol diastereoisomer B 20 - 32
KWG, diastereoisomers A and B 6 - 8
KWG 1732 1
Polar metabolites 7
Non-extractable 10 - 15
In an ongoing three-year field trial on winter wheat using both a
seed treatment and spray of (benzene ring-UL-14C)-triadimenol and
triadimefon, respectively, the proportions reports in Table 8 were
found after the first year (Bayer 1981).
TABLE 8. Metabolites in soil one year after treatment with
triadimefon and triadimenol
Metabolite Radioactivity (%)
Triadimefon 14
Triadimenol diastereoisomer A 36
Triadimenol diastereoisomer B 38
KWG 1640 2
KWG 1732 (XVII) <1
Polar metabolites 2
Non-extractables 8
When triadimenol was applied to soil, it was only slightly
changed by micro-organisms; however, the proportion of diastereoisomer
B increased. This effect can be explained only as resulting from a
redox reaction via triadimefon (Mobay 1981).
When triadimefon was incubated with mycelium mats of
Aspergillus niger a different metabolic pattern was observed than
when the material was added to a shake culture. While some triadimenol
was always present (in amounts up to 4%), as much as 32% of the
triadimefon was converted to the isopropyl analogue (Clark et al
1978). However, in another study the isopropyl analogue was found to
be an artefact of KWG 1342; in this study it was shown that the tert.
butyl group of triadimefon and triadimenol was oxidized to KWG 1342
(VI) and 1323 (V) (Deas and Clifford 1981).
The metabolism of triadimenol by selected soil micro-organisms
was studied (Bayer 1981). Whereas bacteria were unable to metabolize
triadimenol it was shown that some fungi oxidized the tert. butyl
group to KWG 1342.
The metabolic pathways of triadimefon in soil and fungi are
illustrated in Figure 4. For chemical names of metabolites shown in
Figure 4, see FAO/WHO 1979, Triadimefon, Table 9.
In studies with (benzene ring-UL-14C)-triadimefon and (triazole
ring-3,5-14C)-triadimefon carried out on spring barley, the uptake of
residues from soil by crops grown subsequently was studied (Bayer
1981). After the treated cereals had been harvested, turnip and clover
were planted as catch crops, followed by sowing of winter barley,
winter wheat, spring wheat, oats and sugarbeet as main crops.
Following the application of the benzene ring label, the catch crops
absorbed up to 1.1% of the radioactivity present in the soil. Of the
main crops, sugarbeet leaves absorbed up to 4.3%, oat straw 0.5% and
straw of spring and winter wheat and of winter barley up to 1.4%.
These values corresponded with parent equivalents of 0.07, 0.07 and
0.18 mg/kg, respectively.
Following the application of the triazole ring label, larger
amounts of radioactivity were taken up by the main crops, which were
found especially in the storage organs, e.g. sugarbeet roots, spring
wheat kernels. The sugarbeet roots absorbed 2.4% (equivalent to
0.03 mg/kg parent compound) and the spring wheat kernels 1.5%
(0.49 mg/kg parent equivalent) (see Table 9).
After applying (benzene ring-UL-14C)-triadimefon to spring
barley under field conditions the radioactivity still present in the
soil at harvest contained the metabolites listed in Table 7. The
relatively large proportion of triadimenol diastereoisomer A may be
due to washing from the plant into soil by rainfall (Bayer 1981).
Following treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon under field conditions 4% of 1,2,4-triazole was found in
addition to the above-mentioned metabolites. The percentage of
radioactivity still present in the soil accounted for by polar
metabolites increased to 6%. Including the non-extractables, more than
90% of the radioactivity was identified for both labels (Bayer 1981).
TABLE 7. Metabolites of triadimefon present in soil at harvest of
treated barley
Metabolite Radioactivity (%)
Triadimenol diastereoisomer A 33 - 41
Triadimenol diastereoisomer B 20 - 32
KWG, diastereoisomers A and B 6 - 8
KWG 1732 1
Polar metabolites 7
Non-extractable 10 - 15
In an ongoing three-year field trial on winter wheat using both a
seed treatment and spray of (benzene ring-UL-14C)-triadimenol and
triadimefon, respectively, the proportions reports in Table 8 were
found after the first year (Bayer 1981).
TABLE 8. Metabolites in soil one year after treatment with
triadimefon and triadimenol
Metabolite Radioactivity (%)
Triadimefon 14
Triadimenol diastereoisomer A 36
Triadimenol diastereoisomer B 38
KWG 1640 2
KWG 1732 (XVII) <1
Polar metabolites 2
Non-extractables 8
When triadimenol was applied to soil, it was only slightly
changed by micro-organisms; however, the proportion of diastereoisomer
B increased. This effect can be explained only as resulting from a
redox reaction via triadimefon (Mobay 1981).
When triadimefon was incubated with mycelium mats of
Aspergillus niger a different metabolic pattern was observed than
when the material was added to a shake culture. While some triadimenol
was always present (in amounts up to 4%), as much as 32% of the
triadimefon was converted to the isopropyl analogue (Clark et al
1978). However, in another study the isopropyl analogue was found to
be an artefact of KWG 1342; in this study it was shown that the tert.
butyl group of triadimefon and triadimenol was oxidized to KWG 1342
(VI) and 1323 (V) (Deas and Clifford 1981).
The metabolism of triadimenol by selected soil micro-organisms
was studied (Bayer 1981). Whereas bacteria were unable to metabolize
triadimenol it was shown that some fungi oxidized the tert. butyl
group to KWG 1342.
The metabolic pathways of triadimefon in soil and fungi are
illustrated in Figure 4. For chemical names of metabolites shown in
Figure 4, see FAO/WHO 1979, Triadimefon, Table 9.
In studies with (benzene ring-UL-14C)-triadimefon and (triazole
ring-3,5-14C)-triadimefon carried out on spring barley, the uptake of
residues from soil by crops grown subsequently was studied (Bayer
1981). After the treated cereals had been harvested, turnip and clover
were planted as catch crops, followed by sowing of winter barley,
winter wheat, spring wheat, oats and sugarbeet as main crops.
Following the application of the benzene ring label, the catch crops
absorbed up to 1.1% of the radioactivity present in the soil. Of the
main crops, sugarbeet leaves absorbed up to 4.3%, oat straw 0.5% and
straw of spring and winter wheat and of winter barley up to 1.4%.
These values corresponded with parent equivalents of 0.07, 0.07 and
0.18 mg/kg, respectively.
Following the application of the triazole ring label, larger
amounts of radioactivity were taken up by the main crops, which were
found especially in the storage organs, e.g. sugarbeet roots, spring
wheat kernels. The sugarbeet roots absorbed 2.4% (equivalent to
0.03 mg/kg parent compound) and the spring wheat kernels 1.5%
(0.49 mg/kg parent equivalent) (see Table 9).
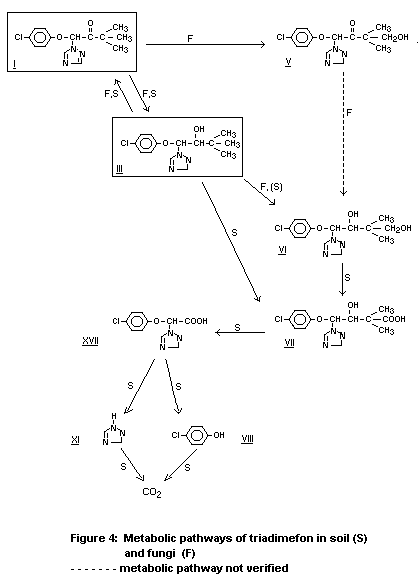 TABLE 9. Residues of triadimefon metabolites in crops after uptake from soil1
Uptake
Catch crop (1)
14C-Label followed by Plant Radioactivity present parent equivalent
main crop (2) and (3) part in soil (%) (mg/kg)
benzene ring- Turnip (1) Leaf 1.1 0.05
UL-14C Edible root 0.2 0.01
Winter wheat (2) Straw 0.6 0.07
Kernel 0.1 <0.01
Sugarbeet (3) Leaf 0.4 0.01
Edible root 0.1 <0.01
Persian clover (1) 1.0 0.06
Winter wheat (2) Straw 0.9 0.07
Kernel 0.1 <0.01
Winter barley (3) Straw 0.3 0.03
Kernel 0.1 <0.01
benzene ring- Sugarbeet (2) Leaf 1.6 0.04
UL-14C Edible root <0.1 <0.01
Spring wheat (3) Straw 0.3 0.04
Kernel <0.1 <0.01
triazole ring- Sugarbeet Leaf 3.8 0.13
3,5-14C Edible root 2.4 0.03
Spring wheat Straw 1.1 0.23
Kernel 1.5 0.49
1 Reference - Bayer 1981.
It is assumed that the rotational crop absorbed mainly
triadimenol from the soil. The crops of winter wheat and winter barley
contained 22 to 35% triadimenol, 3 to 7% KWG 1342, up to 3%
triadimefon and 9 to 27% polar compounds. In turnip and sugarbeet
leaves, on the other hand, KWG 1342 was the main component, accounting
for 38% and 9 to 19% of the radioactivity present (Bayer 1981).
The uptake of residues in rotational glasshouse crops was studied
following the application of triadimefon at concentrations of 2,5,7
and 10 mg/kg on dry weight basis to the soil. In another experiment,
triadimefon was sprayed on bare soil at a rate equivalent to
500 g a.i./ha. In both experiments the rotational crops were grown to
maturity. The residues in radishes and cucumbers planted 14 days after
the treatment of 10 mg/kg dry weight basis were below the limit of
detection (lower than 0.1 mg/kg both for triadimefon and triadimenol).
The residues in radish leaves were about 0.16 mg/kg. The main
pesticide identified in the crop was triadimefon. The triadimefon
parent was either not detected or detected only at very low levels.
The ratio of the diastereoisomers A and B in these crops were 1:2 to
1:3. In the other experiment, radishes, carrots, potatoes, chinese
cabbage and beans were grown in the soil previously treated with
triadimefon. The triadimenol levels in the edible parts of these crops
were below the limit of determination (less than 0.1 mg/kg). In
chinese cabbage, 0.02 mg/kg triadimenol was found, and 0.21 mg/kg in
bean leaves (Mobay 1981).
In storage and processing
Apples containing field weathered residues of (benzene ring-UL-
14C)-triadimefon were stored for 420 days at -10°C. The peel of these
apples was analysed repeatedly over a period of about 14 months.
Essentially no change was seen in amount or percentage distribution of
triadimefon or triadimenol during this period (Mobay 1981).
The same results were obtained in a study to investigate the
effect of frozen storage (-0°C to -10°C) on soil residues of
triadimefon and triadimenol in a loam soil. No residue decrease was
found during 516 days of cold storage.
In a Mobay (1981) study, 20 kg of apples were processed under
simulated industry conditions. Almost all residues were found in the
peel. The highest concentration of residues was found in the wet
pomace (about 4 times higher than in fresh fruit), but further drying
caused a residue decrease resulting in a dry product with residues
approximately twice that found in fresh whole fruit.
After processing wine grapes containing weathered triadimefon
residues, practically no residues were present in the wine
(<0.01 mg/kg) except in one sample with 0.5 mg/kg (Bayer 1981; Mobay
1981).
After processing of hops, which were sprayed during the growing
season at excessive rates, no residues were found in beer (0.05 mg/kg
limit of determination) (Bayer 1981).
Field treated wheat grain containing 0.3 mg/kg and 0.2 mg/kg
triadimefon and triadimenol respectively was processed in a Buhler
roller mill. Highest residues were found in the bran (triadimefon and
triadimenol, both 0.8 mg/kg). Lesser amounts were found in the shorts
(both 0.2 mg/kg) and only trace amounts in the flour (Mobay 1981).
In water
Benzene ring-UL-14C)-triadimenol added to water did not show
apparent degradation at various temperatures and pH levels 4, 7 and 9,
temp. 20°C and 40°C. After 32 days, 97% of the added radioactivity was
still present, mainly as unchanged triadimenol (Mobay 1981). However,
degradation of triadimefon was observed at 70°C in tap water, and
reached approximately 90% in 8 weeks. The salts present were
responsible for this degradation, especially C++ salts accelerated
further hydrolysis (Bayer 1981).
In contrast with the data obtained in buffer solutions, it was
found that both (benzene ring-UL-14C)-triadimefon and (triazole ring-
3,5-14C)-triadimefon showed rapid degradation in a simulated natural
pond environment. Triadimefon had a half-life of 6 to 8 days in the
water phase and 18 to 20 days in the silt phase. Triadimenol was the
main metabolite during the experiment; the following minor metabolites
were identified: triadimefon, symmetrical isomer, 1,2,4 triazole and
delta2 -1,2,4-triazoline-5-one (Mobay 1981).
METHODS OF RESIDUE ANALYSIS
Residues of triadimefon and triadimenol can be measured by gas
chromatography using a nitrogen-specific alkali flame detector (AFID).
Some methods suitable for plant material and soil are presented in
FAO/WHO (1979). The specificity of the method for apples described in
FAO/WHO (1979), was demonstrated in an interference study (Mobay
1981).
A new multi-residue method was developed involving clean-up by
gel permeation chromatography and mini silica gel column
chromatography. Following extraction of plant material with
acetone/water and soil with methanol/water 70:30, the extract is
shaken in dichloromethane and then cleaned up with ethylacetate/
cylohexane by gel permeation chromatography. Additional clean-up is
performed in some cases using mini silica gel column chromatography
(Specht and Tillkes 1980). The method was slightly modified by Mobay
Chemical Corporation (Mobay 1981). A similar method was developed by
Nitokono (1981).
The limits of determination of the various methods for plant
material referred to above are about 0.01 to 0.1 mg/kg, depending on
the commodity.
A method for determining triadimefon and triadimenol in grape
juice and wine using capillary gas chromatography has become
available; the sample is passed through a column packed with XAD-2
resin (Nickless et al 1981). The limit of determination is 0.005 to
0.01 mg/kg.
Methods for the determination of triadimefon and triadimenol in
animal material, such as cattle tissues, milk, poultry and eggs, have
been developed. After extraction of the samples with methanol
(methanol/acetone for milk), it is passed through a XAD-4 resin
column, then cleaned up by partition between dichloromethanol and
water, partition between acetonitrile and hexane and finally by
passing through a Florisil column (Mobay 1981). The limits of
determination were 0.05 to 0.1 mg/kg for cattle tissues, 0.005 to
0.01 mg/kg for milk and about 0.005 to 0.01 mg/kg for poultry and
eggs. The specificity of the method was demonstrated in an
interference study (Mobay 1981). The method can be modified, using
GLC/MS, which enables the determination of triadimefon and all known
metabolites, free or conjugated, in cattle tissues and milk (Mobay
1981).
NATIONAL MAXIMUM RESIDUE LIMITS
Additional maximum residue limits have been reported to the
Meeting, together with recommended pre-harvest intervals (Table 10).
EVALUATION
COMMENTS AND APPRAISAL
Triadimefon is rapidly and readily absorbed from the
gastrointestinal tract. The route of excretion appears to vary
between sexes in the rat, where excretion is relatively slow.
Excretion is rapid in cows and pigs and extremely rapid in hens.
The compound is metabolized in all species, the major excreted
metabolite being triadimenol.
In studies designed to evaluate triadimefon for cumulative
toxicity, the liver appeared to be the major target organ. The liver
weight was affected in the dog, rat and mouse, the effect observed
being correlated in the dog and mouse with biochemical signs of liver
damage (e.g. increased transaminase activity levels in plasma). With
increasing doses, the first signs of a hepatotropic effect are
considered to be due to the increased microsomal liver enzyme
activities, as shown by the findings obtained in rats and dogs. The
TABLE 10. National maximum residue limits reported to the Meeting
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Australia Apple 1
Cucurbits 0.2 7
Grape 2 14
Raw cereals 0.5 28
Eggs, milk 0.1
Meat, meat products 0.05
Austria Pome fruit 35
Grape 35
Cucumber, pepper,
tomato 1 4
Other vegetables 14
Cereals 11 35
Sugarbeet 0.21 35
Belgium Barley, wheat 0.052 42
Apple 0.052 14
Cucumber, gherkin 0.052 3
Brazil Wheat 0.1 42
Bulgaria Apple 14
Grape 14
Sugarbeet 14
Tobacco 14
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Czechoslovakia Apple 28
Grape 28
Denmark Fruits (including small
fruits) 14
Cucumber 14
Wheat, barley 28
Fed.Rep.of Germany Apple and pear 0.53 14
Grape 33 35
Strawberry 0.23
Cucumber 0.53 3
Cereals 0.53
Hops 15 14
Other commodities of
plant origin 0.1
Israel Apple 0.05 14
Grape 0.1 21
Cucumber, melon, pumpkin,
pepper, tomato ,eggplant 3
Wheat 42
Netherlands Apple 0.1 14
Cereals 0.05 42
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Portugal Apple 56
Grape (wine) 28
Grape (table) 21
Cucumber (including gherkin)
melon, pumpkin 3
Pea 7
Pepper 7
South Africa Apple, pear 0.05 28
Grape (table 0.05 7 resp 28-424
Grape (wine) 0.05 7 resp 28-424
Mango 0.05 28
Cucurbits 0.05 3
Pea 0.05 1
Spain Fruits, except grape 15
Grape 15-214
Artichoke 15
Cucumber, melon 15
Tomato
Sweden Fruits (including small
fruits and berries) 28
Switzerland Apple 0.1 21
Grape 0.5 21
Wine 0.5
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
UK Apple 14
Blackcurrant, gooseberry 14
Grape 14
Brussels sprout 14
1 Provisional;
2 Under consideration;
3 Proposed levels;
4 According to formulation.
only species showing histopathological changes was the mouse, at the
highest tested dose level. The most sensitive species with respect to
enzyme induction was the rat, which reacted to minimum oral daily
doses of 5 mg/kg bw. In the rat, the liver microsomal enzyme induction
was rapidly reversible within 4 weeks.
The other toxicological target was the haemapoietic system as
indicated by the results obtained in rats and dogs. Other effects
(only found in the rat) were observed in the kidney and testis and on
cholesterol levels, especially in the chronic toxicity study.
Moreover, these effects only appeared at the extremely high dose of
5 000 ppm in the diet (250 mg/kg bw), which caused excessive mucosal
lesions in the stomach and hence undoubtedly nutritional disorders.
Triadimefon was not mutagenic when tested with or without metabolic
activation in various strains of Salmonella, B; subtilis, E. coli
and S. cerevisiae.
The long-term studies on two rodent species did not provide any
indication of triadimefon having carcinogenic potential.
The information on the use pattern and pre-harvest intervals in
several countries was updated, and new information was obtained on use
patterns, pre-harvest intervals and national maximum residue limits in
other countries.
Additional data were obtained on residues from supervised trials,
some from countries not included in FAO/WHO 1979, on various fruits,
vegetables, cereals and some other crops, e.g. apple, red currant,
grape, strawberry, cucumber, melon, pepper, tomato, onion, pea, cereal
crops and sugarbeet. New information was obtained on residues from
supervised trials on black currant, raspberry, leek, parsnip, hops,
sweet potato and sugarcane.
Additional information was obtained on the fate of triadimefon
residues in plants, animals (including livestock), soils (including
microorganisms) and water, especially with regard to the further
degradation of the main plant and animal metabolite of triadimefon,
triadimenol. New information was also obtained on the fate of the
triazole part of the triadimefon molecule in plants, animals, soil and
water from studies with (triazole ring-3,5-14C)-triadimefon,
(triazole ring-3,5-14C)-triadimenol, (diastereoisomer A and B) and
1,2,4 (3,5-14C)-triazole. The latter three compounds are main
(triadimenol A and B) or minor plant and animal metabolites of
triadimefon.
Information on new or improved methods of residue analysis was
made available to the Meeting. Most of the methods are based on the
determination of the residue of triadimefon and triadimenol by gas
chromatography using a nitrogen-specific alkali flame detector(AFID).
The methods were modified for the determination of the triadimefon/
triadimenol residues in grape juice, wine and animal material (such as
cattle and poultry tissues, milk and eggs). The specificity of the
methods for triadimefon and triadimenol in plant material, such as
apples, meat and milk, has been demonstrated in interference studies.
The methods now available are suitable or can be adapted for
regulatory purposes.
Level causing no toxicological effects
Mouse : 300 ppm in diet, equivalent to 40 mg/kg bw/day
Rat : 50 ppm in diet, equivalent to 2.5 mg/kg bw/day
Dog : 330 ppm in diet, equivalent to 8.25 mg/kg bw/day
Estimated temporary admissible daily intake for man
0 - 0.01 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
The additional residue data evaluated by the Meeting in
conjunction with the data provided to the 1979 JMPR confirm the limits
proposed in 1979 for red currant, grape, strawberry, cucumber, melon,
pepper, tomato, onion, pea, cereal grains and coffee. The following
temporary maximum residue limits, expressed as the sum of triadimefon
and triadimenol, are recommended. The data presented on residues in
pineapple, Brussels sprout and leek are not sufficient to consider a
maximum residue limit.
Pre-harvest interval
Temporary MRL on which recommendation
Commodity (mg/kg) is based (days)
Apple 0.5 14
Black currant 1 7
Raspberry 0.2 7
Hops (dry) 15 14
Coffee beans 0.11
Meat 0.11
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
Clarification of the effects on liver in mice and rats.
Desirable
1. Clarification of effects on the central nervous system observed
in acute studies.
2. Information on the use pattern (concentration, dipping time) of
the post-harvest treatment of pineapple and residues arising from
this use.
REFERENCES
Bayer. Reports submitted to FAO and WHO by Bayer AG.(Unpublished)
1981
Bomhard, E. and Löser, E. Chronic toxicity study on rats (two-year
1978a feeding experiment, 1 August. Report no. 7707, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
1978b Chronic toxicity study on mice (two-year feeding experiment)
1 August. Report no. 9344, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Brandes, W., Steffens, W., Führ, F. and Scheinpflug H. Weitere Un
1978 tersuchungen zur Translokation von 14C-Triadimefon.
Pflanzenschutz-Nachrichten Bayer, 31:132-144.
Buchenauer, H. Systemisch-fungizide Wirkung und Wirkungs mechanismus
1975 von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-
Forst wirtsch. (Berlin-Dahlem), 165: 154-155.
1976 Studies on the systemic activity of Bayleton (triadimefon)
and its effects against certain fungal diseases of cereals.
Pflanzenschutz-Nachrichten, 29:266-280 (English edition)
Clark, T., Clifford, D.R., Deas, A., Gendle, P. and Watkins, D.
1978 Photolysis, metabolism and other factors influencing the
performance of triadimefon as a powdery mildew fungicide.
Pesticide Science, 9:497-506.
Deas, A.H.B. and Clifford, D.R. Metabolism of the 1,2,4-
1981 triazolylmethane fungicides, triadimefon, triadimenol and
diclobutrazol by Aspergillus niger (Van Tiegh). (In press)
Ecker, W. Biotransformation of 1,2,4-[3(5)-14C]triazol in rats.
1980 Bayer AG, Institut für Pharmakokinetik, Elberfeld, Pharma
Report no. 9478 (PF-Report no. 1471), 14 October 1980,
submitted by Bayer AG to WHO. (Unpublished)
Flucke, W. Bestimmung der akuten Toxizität (LD50) 16 February, Bayer
1981 AG, Institut für Toxikologie, report submitted by Bayer AG
to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Propineb (LH 30/Z)-Triadimefon (MEB 6447)
1977a Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7065, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Triadimefon (MEB 6447) und Captafol,
1977b Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7069, Bayer AG, Institut für Toxikoligie, report submitted
by Bayer AG to WHO. (Unpublished)
1977c Triadimefon (MEB 6447) und Tolylfluanid (KUE 13 183 b),
Untersuchungen zur akuten Toxizitüt nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr
7304, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Fredrickson, D.R. Metabolism of BAYLETONTM in rats. Mobay report no.
1978a 66201, 20 June 1978 (revised: 21 November 1979), submitted
by Bayer AG to WHO. (Unpublished)
1978b Metabolism of BAYLETONTM in cow. Mobay report no. 66202,
20 June 1978 (revised 21 November 1979), submitted by Bayer
AG to WHO. (Unpublished)
Gasztonyi, M. and Josepovits, G. The activation of triadimefon and its
1979 role in the selectivity of fungicide action. Pesticide
Science, 10:57-65.
Hoffman, K. and Gröning, P. Long-term toxicity study on dogs (two-year
1978 feeding study), 24 October. Report no. 7882, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Hoffman, K. and Luckhaus, G. Subchronic toxicity study on dogs,
1974 (thirteen-week feeding experiment) 16 December. Report no.
5071, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Inukai, H. and Iyatomi, A. Mutagenicity test on bacterial systems, 19
1977 December. Report no. 68, Nitokuno, Laboratory of Toxicology,
Japan, report submitted by Bayer AG to WHO. (Unpublished)
Jagannath, D.R., Brusick, and Hoorn, A.J.W. Mutagenicity evaluation of
1980 MEB 6447 in the reverse mutation induction assay, January.
R-Bericht nr. 1675, Litton Bionetics Inc., USA, report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Burke, M.A. Acute oral toxicity of BAYLETONTM (formerly
1977 BAY MEB 6447) technical to adult mallard ducks, 11 May.
Report no. 52872, Mobay Chemical Corp., Agr. Div., submitted
by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Roney, D.J. Accumulation and persistence of residues in
1977 channel catfish exposed to BAYLETONTM-14C (formerly BAY
MEB 6447), Mobay Report no. 52775, 4 May, submitted by Bayer
AG to WHO. (Unpublished)
Löser, E. Multigeneration reproduction study on rats, 12 April. Report
1979 no. 8297, Bayer AG, Institut für Toxikologie, submitted by
Bayer AG to WHO. (Unpublished)
Machemer, L. Dominant lethal study on male mice to test for mutagenic
1976a effects, 27 January. Report no. 5837, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
1976b Evaluation of embryotoxic and teratogenic effects on rats
following oral administration, 27 August. Report no. 6294,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
1976c Evaluation of embryotoxic and teratogenic effects on rabbits
following oral administration, 30 August. Report no. 6297,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
Machemer, L. Micronucleus test on mice to evaluate MEB 6447 for
1977 mutagenic effects, 23 February. Report no. 6622, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Machemer, L. and Kimmerle, G. Evaluation of MEB 6447 (triadimefon) for
1976 embryotoxic and teratogenic effects on rats following
inhalation in dynamic flow apparatus, 30 August. Report no.
6298, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Mihail, F. Acute toxicity studies, 27 June. Report no. 9277, Bayer AG,
1980 Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Mihail, F. and Kaliner, G. Subakuter oraler Kumulationsversuch an
1979 Ratten, 20 February, Bericht nr. 8195, Bayer AG, Institut
für Toxikologie, report submitted by Bayer AG to WHO.
(Unpublished)
Mobay Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Mohr, U. Subchronic toxicity study on rats (twelve-week feeding
1976 experiment), 5 November. Report no. R840a, Medizinische
Hochschule Hannover, Abteilung für, experimentelle
Pathologie, submitted by Bayer AG to WHO. (Unpublished)
Nickless, G., Spitzer, T. and Pickard, J.A. Determination of
1981 triadimefon in grape juice and wine using capillary gas
chromatography. Journal of Chromatography, 208: 409-413.
Nitokuno. Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Nye, D.E. The fate of BAYLETON-14C in poultry. Mobay Report no. 67482,
1979 8 March, submitted by Bayer AG to WHO. (Unpublished)
Pither, K.M. Metabolism of BAYLETONTM in male and female pigs. Mobay
1978 report no. 66509, 7 August (revised: 29 November 1978)
submitted by Bayer AG to WHO. (Unpublished)
Puhl, R.J. and Hurley, J.B. The metabolism and excretion of BAYTANTM
1978 by rats. Mobay Report no. 66489, 14 August 1978 (revised 26
September 1979 and 4 February 1980), submitted by Bayer AG
to WHO. (Unpublished)
Ritter, W. BAY e 8364 (MEB 6447): Pharmacokinetics in mice and rats.
1976 Bayer AG, Pharma-Institut für Pharmakokinetik, report no.
6458, 29 October, 1976, submitted by Bayer AG to WHO.
(Unpublished)
Scheinpflug, H. and Paul, V. On the mode of action of triadimefon.
1977 Netherlands Journal of Plant Pathology, 83(suppl.):105-111.
Scheinpflug, H., Paul, V. and Kraus, P. Untersuchungen zur
1977 Wirkungsweise von (R)Bayleton bei Getreidekrunkheiten. Mitt.
Biol. Bundesanst. Lund-Forstwirtschaft (Berlin-Dahlem)
178:178.
1978 Studies on the mode of action of (R)Bayleton against cereal
diseases. Pflanzenschutz Nach richten Bayer, 31:101-115.
Schlütter, K. Fungizine Wirkung von Triadimefon in der Gasphase. Z.
1977 Pflanzenkrank. Pflanzenschutz, 84:612-614.
Shirasu, Y., Moriya, M. and Koyashiki, R. Triadimefon-mutagenicity
1978 test on bacterial systems, 18 July. Department of
Toxicology, Institute of Environmental Toxicology, Japan,
report submitted by Bayer AG to WHO. (Unpublished)
Shirasu, Y., Moriya, M. and Miyazawa, T. Triadimefon-mutagenicity test
1979 of bacterial systems 27 October. Department of Toxicology,
Institute of Environmental Toxicology, Japan, report
submitted by Bayer AG to WHO. (Unpublished)
Specht, W. and Tillkes, M. Gaschromatographische Bestimmung von
Rückständen an Pflunzenbehandlungsmitteln nach Clean-up Uber
Gelchromatographic und mini-Kieselgel Säulenchromatographic.
Pflanzenschutz-Nachrichten Bayer, 33:61-85.
Thyssen, J. Studies on acute combination toxicity of triadimefon and
1977 carbadazim, 22 November. Report no. 7119, Bayer AG, Institut
für Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Thyssen, J. and Gröning, P. Fütterungsversuche mit Hühnern unter
1978 besonderer Berücksichtigung einer Möglichen Beeinflussung
der Fortpflanzung, 15 November. Bericht n.7924, Bayer AG,
Institut für Toxikologie, report submitted by Bayer AG to
WHO. (Unpublished)
Thyssen, J. and Kimmerle, G. MEB 6447-Acute toxicity studies, 3
1979 January, Report no.4416, Bayer AG, Institut für Toxikologie,
submitted by Bayer AG to WHO.(Unpublished)
Thyssen, J., Kimmerle, G. and Luckhaus, G. MEB 6447 - subacute
1974 toxicity studies, 25 January. Report no. 4464, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
van Dijck, P. Evaluation of Bayleton (MEB 6447, triadimefon) for
1976 mutagenic potential by the Ames test with histidine-
auxotrophic Salmonella typhimurium strains, 27 September.
Laboratorium voor Hygiene, Leuven (Belgium), report
submitted by Bayer AG to WHO. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. 1,2,4-triazol-14C.
1978 Biokinetische Untersuchungen an Ratten. Bayer AG, Institut
für Pharmakokinetik, Elberfeld, Pharma Report no. 7920, 13
November 1978, submitted by Bayer AG to WHO. (Unpublished)
TABLE 9. Residues of triadimefon metabolites in crops after uptake from soil1
Uptake
Catch crop (1)
14C-Label followed by Plant Radioactivity present parent equivalent
main crop (2) and (3) part in soil (%) (mg/kg)
benzene ring- Turnip (1) Leaf 1.1 0.05
UL-14C Edible root 0.2 0.01
Winter wheat (2) Straw 0.6 0.07
Kernel 0.1 <0.01
Sugarbeet (3) Leaf 0.4 0.01
Edible root 0.1 <0.01
Persian clover (1) 1.0 0.06
Winter wheat (2) Straw 0.9 0.07
Kernel 0.1 <0.01
Winter barley (3) Straw 0.3 0.03
Kernel 0.1 <0.01
benzene ring- Sugarbeet (2) Leaf 1.6 0.04
UL-14C Edible root <0.1 <0.01
Spring wheat (3) Straw 0.3 0.04
Kernel <0.1 <0.01
triazole ring- Sugarbeet Leaf 3.8 0.13
3,5-14C Edible root 2.4 0.03
Spring wheat Straw 1.1 0.23
Kernel 1.5 0.49
1 Reference - Bayer 1981.
It is assumed that the rotational crop absorbed mainly
triadimenol from the soil. The crops of winter wheat and winter barley
contained 22 to 35% triadimenol, 3 to 7% KWG 1342, up to 3%
triadimefon and 9 to 27% polar compounds. In turnip and sugarbeet
leaves, on the other hand, KWG 1342 was the main component, accounting
for 38% and 9 to 19% of the radioactivity present (Bayer 1981).
The uptake of residues in rotational glasshouse crops was studied
following the application of triadimefon at concentrations of 2,5,7
and 10 mg/kg on dry weight basis to the soil. In another experiment,
triadimefon was sprayed on bare soil at a rate equivalent to
500 g a.i./ha. In both experiments the rotational crops were grown to
maturity. The residues in radishes and cucumbers planted 14 days after
the treatment of 10 mg/kg dry weight basis were below the limit of
detection (lower than 0.1 mg/kg both for triadimefon and triadimenol).
The residues in radish leaves were about 0.16 mg/kg. The main
pesticide identified in the crop was triadimefon. The triadimefon
parent was either not detected or detected only at very low levels.
The ratio of the diastereoisomers A and B in these crops were 1:2 to
1:3. In the other experiment, radishes, carrots, potatoes, chinese
cabbage and beans were grown in the soil previously treated with
triadimefon. The triadimenol levels in the edible parts of these crops
were below the limit of determination (less than 0.1 mg/kg). In
chinese cabbage, 0.02 mg/kg triadimenol was found, and 0.21 mg/kg in
bean leaves (Mobay 1981).
In storage and processing
Apples containing field weathered residues of (benzene ring-UL-
14C)-triadimefon were stored for 420 days at -10°C. The peel of these
apples was analysed repeatedly over a period of about 14 months.
Essentially no change was seen in amount or percentage distribution of
triadimefon or triadimenol during this period (Mobay 1981).
The same results were obtained in a study to investigate the
effect of frozen storage (-0°C to -10°C) on soil residues of
triadimefon and triadimenol in a loam soil. No residue decrease was
found during 516 days of cold storage.
In a Mobay (1981) study, 20 kg of apples were processed under
simulated industry conditions. Almost all residues were found in the
peel. The highest concentration of residues was found in the wet
pomace (about 4 times higher than in fresh fruit), but further drying
caused a residue decrease resulting in a dry product with residues
approximately twice that found in fresh whole fruit.
After processing wine grapes containing weathered triadimefon
residues, practically no residues were present in the wine
(<0.01 mg/kg) except in one sample with 0.5 mg/kg (Bayer 1981; Mobay
1981).
After processing of hops, which were sprayed during the growing
season at excessive rates, no residues were found in beer (0.05 mg/kg
limit of determination) (Bayer 1981).
Field treated wheat grain containing 0.3 mg/kg and 0.2 mg/kg
triadimefon and triadimenol respectively was processed in a Buhler
roller mill. Highest residues were found in the bran (triadimefon and
triadimenol, both 0.8 mg/kg). Lesser amounts were found in the shorts
(both 0.2 mg/kg) and only trace amounts in the flour (Mobay 1981).
In water
Benzene ring-UL-14C)-triadimenol added to water did not show
apparent degradation at various temperatures and pH levels 4, 7 and 9,
temp. 20°C and 40°C. After 32 days, 97% of the added radioactivity was
still present, mainly as unchanged triadimenol (Mobay 1981). However,
degradation of triadimefon was observed at 70°C in tap water, and
reached approximately 90% in 8 weeks. The salts present were
responsible for this degradation, especially C++ salts accelerated
further hydrolysis (Bayer 1981).
In contrast with the data obtained in buffer solutions, it was
found that both (benzene ring-UL-14C)-triadimefon and (triazole ring-
3,5-14C)-triadimefon showed rapid degradation in a simulated natural
pond environment. Triadimefon had a half-life of 6 to 8 days in the
water phase and 18 to 20 days in the silt phase. Triadimenol was the
main metabolite during the experiment; the following minor metabolites
were identified: triadimefon, symmetrical isomer, 1,2,4 triazole and
delta2 -1,2,4-triazoline-5-one (Mobay 1981).
METHODS OF RESIDUE ANALYSIS
Residues of triadimefon and triadimenol can be measured by gas
chromatography using a nitrogen-specific alkali flame detector (AFID).
Some methods suitable for plant material and soil are presented in
FAO/WHO (1979). The specificity of the method for apples described in
FAO/WHO (1979), was demonstrated in an interference study (Mobay
1981).
A new multi-residue method was developed involving clean-up by
gel permeation chromatography and mini silica gel column
chromatography. Following extraction of plant material with
acetone/water and soil with methanol/water 70:30, the extract is
shaken in dichloromethane and then cleaned up with ethylacetate/
cylohexane by gel permeation chromatography. Additional clean-up is
performed in some cases using mini silica gel column chromatography
(Specht and Tillkes 1980). The method was slightly modified by Mobay
Chemical Corporation (Mobay 1981). A similar method was developed by
Nitokono (1981).
The limits of determination of the various methods for plant
material referred to above are about 0.01 to 0.1 mg/kg, depending on
the commodity.
A method for determining triadimefon and triadimenol in grape
juice and wine using capillary gas chromatography has become
available; the sample is passed through a column packed with XAD-2
resin (Nickless et al 1981). The limit of determination is 0.005 to
0.01 mg/kg.
Methods for the determination of triadimefon and triadimenol in
animal material, such as cattle tissues, milk, poultry and eggs, have
been developed. After extraction of the samples with methanol
(methanol/acetone for milk), it is passed through a XAD-4 resin
column, then cleaned up by partition between dichloromethanol and
water, partition between acetonitrile and hexane and finally by
passing through a Florisil column (Mobay 1981). The limits of
determination were 0.05 to 0.1 mg/kg for cattle tissues, 0.005 to
0.01 mg/kg for milk and about 0.005 to 0.01 mg/kg for poultry and
eggs. The specificity of the method was demonstrated in an
interference study (Mobay 1981). The method can be modified, using
GLC/MS, which enables the determination of triadimefon and all known
metabolites, free or conjugated, in cattle tissues and milk (Mobay
1981).
NATIONAL MAXIMUM RESIDUE LIMITS
Additional maximum residue limits have been reported to the
Meeting, together with recommended pre-harvest intervals (Table 10).
EVALUATION
COMMENTS AND APPRAISAL
Triadimefon is rapidly and readily absorbed from the
gastrointestinal tract. The route of excretion appears to vary
between sexes in the rat, where excretion is relatively slow.
Excretion is rapid in cows and pigs and extremely rapid in hens.
The compound is metabolized in all species, the major excreted
metabolite being triadimenol.
In studies designed to evaluate triadimefon for cumulative
toxicity, the liver appeared to be the major target organ. The liver
weight was affected in the dog, rat and mouse, the effect observed
being correlated in the dog and mouse with biochemical signs of liver
damage (e.g. increased transaminase activity levels in plasma). With
increasing doses, the first signs of a hepatotropic effect are
considered to be due to the increased microsomal liver enzyme
activities, as shown by the findings obtained in rats and dogs. The
TABLE 10. National maximum residue limits reported to the Meeting
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Australia Apple 1
Cucurbits 0.2 7
Grape 2 14
Raw cereals 0.5 28
Eggs, milk 0.1
Meat, meat products 0.05
Austria Pome fruit 35
Grape 35
Cucumber, pepper,
tomato 1 4
Other vegetables 14
Cereals 11 35
Sugarbeet 0.21 35
Belgium Barley, wheat 0.052 42
Apple 0.052 14
Cucumber, gherkin 0.052 3
Brazil Wheat 0.1 42
Bulgaria Apple 14
Grape 14
Sugarbeet 14
Tobacco 14
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Czechoslovakia Apple 28
Grape 28
Denmark Fruits (including small
fruits) 14
Cucumber 14
Wheat, barley 28
Fed.Rep.of Germany Apple and pear 0.53 14
Grape 33 35
Strawberry 0.23
Cucumber 0.53 3
Cereals 0.53
Hops 15 14
Other commodities of
plant origin 0.1
Israel Apple 0.05 14
Grape 0.1 21
Cucumber, melon, pumpkin,
pepper, tomato ,eggplant 3
Wheat 42
Netherlands Apple 0.1 14
Cereals 0.05 42
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Portugal Apple 56
Grape (wine) 28
Grape (table) 21
Cucumber (including gherkin)
melon, pumpkin 3
Pea 7
Pepper 7
South Africa Apple, pear 0.05 28
Grape (table 0.05 7 resp 28-424
Grape (wine) 0.05 7 resp 28-424
Mango 0.05 28
Cucurbits 0.05 3
Pea 0.05 1
Spain Fruits, except grape 15
Grape 15-214
Artichoke 15
Cucumber, melon 15
Tomato
Sweden Fruits (including small
fruits and berries) 28
Switzerland Apple 0.1 21
Grape 0.5 21
Wine 0.5
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
UK Apple 14
Blackcurrant, gooseberry 14
Grape 14
Brussels sprout 14
1 Provisional;
2 Under consideration;
3 Proposed levels;
4 According to formulation.
only species showing histopathological changes was the mouse, at the
highest tested dose level. The most sensitive species with respect to
enzyme induction was the rat, which reacted to minimum oral daily
doses of 5 mg/kg bw. In the rat, the liver microsomal enzyme induction
was rapidly reversible within 4 weeks.
The other toxicological target was the haemapoietic system as
indicated by the results obtained in rats and dogs. Other effects
(only found in the rat) were observed in the kidney and testis and on
cholesterol levels, especially in the chronic toxicity study.
Moreover, these effects only appeared at the extremely high dose of
5 000 ppm in the diet (250 mg/kg bw), which caused excessive mucosal
lesions in the stomach and hence undoubtedly nutritional disorders.
Triadimefon was not mutagenic when tested with or without metabolic
activation in various strains of Salmonella, B; subtilis, E. coli
and S. cerevisiae.
The long-term studies on two rodent species did not provide any
indication of triadimefon having carcinogenic potential.
The information on the use pattern and pre-harvest intervals in
several countries was updated, and new information was obtained on use
patterns, pre-harvest intervals and national maximum residue limits in
other countries.
Additional data were obtained on residues from supervised trials,
some from countries not included in FAO/WHO 1979, on various fruits,
vegetables, cereals and some other crops, e.g. apple, red currant,
grape, strawberry, cucumber, melon, pepper, tomato, onion, pea, cereal
crops and sugarbeet. New information was obtained on residues from
supervised trials on black currant, raspberry, leek, parsnip, hops,
sweet potato and sugarcane.
Additional information was obtained on the fate of triadimefon
residues in plants, animals (including livestock), soils (including
microorganisms) and water, especially with regard to the further
degradation of the main plant and animal metabolite of triadimefon,
triadimenol. New information was also obtained on the fate of the
triazole part of the triadimefon molecule in plants, animals, soil and
water from studies with (triazole ring-3,5-14C)-triadimefon,
(triazole ring-3,5-14C)-triadimenol, (diastereoisomer A and B) and
1,2,4 (3,5-14C)-triazole. The latter three compounds are main
(triadimenol A and B) or minor plant and animal metabolites of
triadimefon.
Information on new or improved methods of residue analysis was
made available to the Meeting. Most of the methods are based on the
determination of the residue of triadimefon and triadimenol by gas
chromatography using a nitrogen-specific alkali flame detector(AFID).
The methods were modified for the determination of the triadimefon/
triadimenol residues in grape juice, wine and animal material (such as
cattle and poultry tissues, milk and eggs). The specificity of the
methods for triadimefon and triadimenol in plant material, such as
apples, meat and milk, has been demonstrated in interference studies.
The methods now available are suitable or can be adapted for
regulatory purposes.
Level causing no toxicological effects
Mouse : 300 ppm in diet, equivalent to 40 mg/kg bw/day
Rat : 50 ppm in diet, equivalent to 2.5 mg/kg bw/day
Dog : 330 ppm in diet, equivalent to 8.25 mg/kg bw/day
Estimated temporary admissible daily intake for man
0 - 0.01 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
The additional residue data evaluated by the Meeting in
conjunction with the data provided to the 1979 JMPR confirm the limits
proposed in 1979 for red currant, grape, strawberry, cucumber, melon,
pepper, tomato, onion, pea, cereal grains and coffee. The following
temporary maximum residue limits, expressed as the sum of triadimefon
and triadimenol, are recommended. The data presented on residues in
pineapple, Brussels sprout and leek are not sufficient to consider a
maximum residue limit.
Pre-harvest interval
Temporary MRL on which recommendation
Commodity (mg/kg) is based (days)
Apple 0.5 14
Black currant 1 7
Raspberry 0.2 7
Hops (dry) 15 14
Coffee beans 0.11
Meat 0.11
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
Clarification of the effects on liver in mice and rats.
Desirable
1. Clarification of effects on the central nervous system observed
in acute studies.
2. Information on the use pattern (concentration, dipping time) of
the post-harvest treatment of pineapple and residues arising from
this use.
REFERENCES
Bayer. Reports submitted to FAO and WHO by Bayer AG.(Unpublished)
1981
Bomhard, E. and Löser, E. Chronic toxicity study on rats (two-year
1978a feeding experiment, 1 August. Report no. 7707, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
1978b Chronic toxicity study on mice (two-year feeding experiment)
1 August. Report no. 9344, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Brandes, W., Steffens, W., Führ, F. and Scheinpflug H. Weitere Un
1978 tersuchungen zur Translokation von 14C-Triadimefon.
Pflanzenschutz-Nachrichten Bayer, 31:132-144.
Buchenauer, H. Systemisch-fungizide Wirkung und Wirkungs mechanismus
1975 von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-
Forst wirtsch. (Berlin-Dahlem), 165: 154-155.
1976 Studies on the systemic activity of Bayleton (triadimefon)
and its effects against certain fungal diseases of cereals.
Pflanzenschutz-Nachrichten, 29:266-280 (English edition)
Clark, T., Clifford, D.R., Deas, A., Gendle, P. and Watkins, D.
1978 Photolysis, metabolism and other factors influencing the
performance of triadimefon as a powdery mildew fungicide.
Pesticide Science, 9:497-506.
Deas, A.H.B. and Clifford, D.R. Metabolism of the 1,2,4-
1981 triazolylmethane fungicides, triadimefon, triadimenol and
diclobutrazol by Aspergillus niger (Van Tiegh). (In press)
Ecker, W. Biotransformation of 1,2,4-[3(5)-14C]triazol in rats.
1980 Bayer AG, Institut für Pharmakokinetik, Elberfeld, Pharma
Report no. 9478 (PF-Report no. 1471), 14 October 1980,
submitted by Bayer AG to WHO. (Unpublished)
Flucke, W. Bestimmung der akuten Toxizität (LD50) 16 February, Bayer
1981 AG, Institut für Toxikologie, report submitted by Bayer AG
to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Propineb (LH 30/Z)-Triadimefon (MEB 6447)
1977a Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7065, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Triadimefon (MEB 6447) und Captafol,
1977b Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7069, Bayer AG, Institut für Toxikoligie, report submitted
by Bayer AG to WHO. (Unpublished)
1977c Triadimefon (MEB 6447) und Tolylfluanid (KUE 13 183 b),
Untersuchungen zur akuten Toxizitüt nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr
7304, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Fredrickson, D.R. Metabolism of BAYLETONTM in rats. Mobay report no.
1978a 66201, 20 June 1978 (revised: 21 November 1979), submitted
by Bayer AG to WHO. (Unpublished)
1978b Metabolism of BAYLETONTM in cow. Mobay report no. 66202,
20 June 1978 (revised 21 November 1979), submitted by Bayer
AG to WHO. (Unpublished)
Gasztonyi, M. and Josepovits, G. The activation of triadimefon and its
1979 role in the selectivity of fungicide action. Pesticide
Science, 10:57-65.
Hoffman, K. and Gröning, P. Long-term toxicity study on dogs (two-year
1978 feeding study), 24 October. Report no. 7882, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Hoffman, K. and Luckhaus, G. Subchronic toxicity study on dogs,
1974 (thirteen-week feeding experiment) 16 December. Report no.
5071, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Inukai, H. and Iyatomi, A. Mutagenicity test on bacterial systems, 19
1977 December. Report no. 68, Nitokuno, Laboratory of Toxicology,
Japan, report submitted by Bayer AG to WHO. (Unpublished)
Jagannath, D.R., Brusick, and Hoorn, A.J.W. Mutagenicity evaluation of
1980 MEB 6447 in the reverse mutation induction assay, January.
R-Bericht nr. 1675, Litton Bionetics Inc., USA, report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Burke, M.A. Acute oral toxicity of BAYLETONTM (formerly
1977 BAY MEB 6447) technical to adult mallard ducks, 11 May.
Report no. 52872, Mobay Chemical Corp., Agr. Div., submitted
by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Roney, D.J. Accumulation and persistence of residues in
1977 channel catfish exposed to BAYLETONTM-14C (formerly BAY
MEB 6447), Mobay Report no. 52775, 4 May, submitted by Bayer
AG to WHO. (Unpublished)
Löser, E. Multigeneration reproduction study on rats, 12 April. Report
1979 no. 8297, Bayer AG, Institut für Toxikologie, submitted by
Bayer AG to WHO. (Unpublished)
Machemer, L. Dominant lethal study on male mice to test for mutagenic
1976a effects, 27 January. Report no. 5837, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
1976b Evaluation of embryotoxic and teratogenic effects on rats
following oral administration, 27 August. Report no. 6294,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
1976c Evaluation of embryotoxic and teratogenic effects on rabbits
following oral administration, 30 August. Report no. 6297,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
Machemer, L. Micronucleus test on mice to evaluate MEB 6447 for
1977 mutagenic effects, 23 February. Report no. 6622, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Machemer, L. and Kimmerle, G. Evaluation of MEB 6447 (triadimefon) for
1976 embryotoxic and teratogenic effects on rats following
inhalation in dynamic flow apparatus, 30 August. Report no.
6298, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Mihail, F. Acute toxicity studies, 27 June. Report no. 9277, Bayer AG,
1980 Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Mihail, F. and Kaliner, G. Subakuter oraler Kumulationsversuch an
1979 Ratten, 20 February, Bericht nr. 8195, Bayer AG, Institut
für Toxikologie, report submitted by Bayer AG to WHO.
(Unpublished)
Mobay Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Mohr, U. Subchronic toxicity study on rats (twelve-week feeding
1976 experiment), 5 November. Report no. R840a, Medizinische
Hochschule Hannover, Abteilung für, experimentelle
Pathologie, submitted by Bayer AG to WHO. (Unpublished)
Nickless, G., Spitzer, T. and Pickard, J.A. Determination of
1981 triadimefon in grape juice and wine using capillary gas
chromatography. Journal of Chromatography, 208: 409-413.
Nitokuno. Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Nye, D.E. The fate of BAYLETON-14C in poultry. Mobay Report no. 67482,
1979 8 March, submitted by Bayer AG to WHO. (Unpublished)
Pither, K.M. Metabolism of BAYLETONTM in male and female pigs. Mobay
1978 report no. 66509, 7 August (revised: 29 November 1978)
submitted by Bayer AG to WHO. (Unpublished)
Puhl, R.J. and Hurley, J.B. The metabolism and excretion of BAYTANTM
1978 by rats. Mobay Report no. 66489, 14 August 1978 (revised 26
September 1979 and 4 February 1980), submitted by Bayer AG
to WHO. (Unpublished)
Ritter, W. BAY e 8364 (MEB 6447): Pharmacokinetics in mice and rats.
1976 Bayer AG, Pharma-Institut für Pharmakokinetik, report no.
6458, 29 October, 1976, submitted by Bayer AG to WHO.
(Unpublished)
Scheinpflug, H. and Paul, V. On the mode of action of triadimefon.
1977 Netherlands Journal of Plant Pathology, 83(suppl.):105-111.
Scheinpflug, H., Paul, V. and Kraus, P. Untersuchungen zur
1977 Wirkungsweise von (R)Bayleton bei Getreidekrunkheiten. Mitt.
Biol. Bundesanst. Lund-Forstwirtschaft (Berlin-Dahlem)
178:178.
1978 Studies on the mode of action of (R)Bayleton against cereal
diseases. Pflanzenschutz Nach richten Bayer, 31:101-115.
Schlütter, K. Fungizine Wirkung von Triadimefon in der Gasphase. Z.
1977 Pflanzenkrank. Pflanzenschutz, 84:612-614.
Shirasu, Y., Moriya, M. and Koyashiki, R. Triadimefon-mutagenicity
1978 test on bacterial systems, 18 July. Department of
Toxicology, Institute of Environmental Toxicology, Japan,
report submitted by Bayer AG to WHO. (Unpublished)
Shirasu, Y., Moriya, M. and Miyazawa, T. Triadimefon-mutagenicity test
1979 of bacterial systems 27 October. Department of Toxicology,
Institute of Environmental Toxicology, Japan, report
submitted by Bayer AG to WHO. (Unpublished)
Specht, W. and Tillkes, M. Gaschromatographische Bestimmung von
Rückständen an Pflunzenbehandlungsmitteln nach Clean-up Uber
Gelchromatographic und mini-Kieselgel Säulenchromatographic.
Pflanzenschutz-Nachrichten Bayer, 33:61-85.
Thyssen, J. Studies on acute combination toxicity of triadimefon and
1977 carbadazim, 22 November. Report no. 7119, Bayer AG, Institut
für Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Thyssen, J. and Gröning, P. Fütterungsversuche mit Hühnern unter
1978 besonderer Berücksichtigung einer Möglichen Beeinflussung
der Fortpflanzung, 15 November. Bericht n.7924, Bayer AG,
Institut für Toxikologie, report submitted by Bayer AG to
WHO. (Unpublished)
Thyssen, J. and Kimmerle, G. MEB 6447-Acute toxicity studies, 3
1979 January, Report no.4416, Bayer AG, Institut für Toxikologie,
submitted by Bayer AG to WHO.(Unpublished)
Thyssen, J., Kimmerle, G. and Luckhaus, G. MEB 6447 - subacute
1974 toxicity studies, 25 January. Report no. 4464, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
van Dijck, P. Evaluation of Bayleton (MEB 6447, triadimefon) for
1976 mutagenic potential by the Ames test with histidine-
auxotrophic Salmonella typhimurium strains, 27 September.
Laboratorium voor Hygiene, Leuven (Belgium), report
submitted by Bayer AG to WHO. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. 1,2,4-triazol-14C.
1978 Biokinetische Untersuchungen an Ratten. Bayer AG, Institut
für Pharmakokinetik, Elberfeld, Pharma Report no. 7920, 13
November 1978, submitted by Bayer AG to WHO. (Unpublished)
 Hen
When (benzene ring-UL-14C)-triadimefon was administered to
laying hens by a single oral capsular treatment of 2.4 mg/kg, the dose
was eliminated rapidly and 100% was recovered in the excreta 24 h
after treatment (Nye 1979).
Radiocarbon residues in tissues and eggs of laying hens were
determined during a 96-h post-treatment period. The liver contained
0.26 mg triadimefon equivalent/kg 6 h after treatment, the kidney
contained 1.18 mg/kg, breast muscle contained 0.12 mg/kg and abdominal
fat contained 0.30 mg/kg. All tissue radiocarbon levels were well
below 0.01 mg/kg 90 h later. Radiocarbon residues in the eggs were
highest (0.117 mg/kg) 24 h after treatment and had decreased to
0.04 mg/kg 96 h after treatment (Nye 1979).
The nature of the radiocarbon contained in tissues and eggs of
laying hens, as well as that eliminated via the excreta was determined
by Nye (1979). Triadimefon was absorbed by the birds and rapidly
converted to triadimenol acid, which was excreted in its free form.
This metabolite was also the major metabolite isolated in the liver
and kidney. The intermediates in the conversion of triadimefon to the
acid, triadimenol and KWG 1342 were found as major metabolites in the
muscle, fat and eggs. The parent compound was found as the primary
radiocarbon component in the gizzard and abdominal fat.
Catfish
The accumulation and persistence of residues was studied in
Channel catfish (Ictalurus punctatus) continuously exposed to
(benzene ring-UL-14C)-triadimefon for a 28-day period at
concentrations of approximately 10 and 100 ppb in water (Lamb and
Roney 1977). The catfish showed accumulation factors of approximately
7.6 from the 10 ppb level and 6.5 from the 100 ppb level during the
exposure period. The non-edible portions of the catfish contained 74%
and 84% of the extractable 14C residues from the 10 and 100 ppb
levels, respectively. When the catfish were transferred to
uncontaminated water (withdrawal period), approximately 88% of the
accumulated 14C residues were excreted by the fish within 5 h and
approximately 96% were eliminated within 7 to 10 days. Under the
conditions of the study, triadimefon exhibited a low magnitude of
accumulation with a rapid rate of uptake and excretion (Lamb and Roney
1977).
TOXICOLOGICAL STUDIES
Acute toxicity
Triadimefon has a slight (dermal) to moderate (oral,
intraperitoneal) acute toxicity to experimental laboratory animals
(Table 1). The symptoms of poisoning indicate that the target of
TABLE 1. Acute toxicity of triadimefon in animals
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rat M oral ) acetone + 568 ) Thyssen and
Rat F ) oil (1:10) 363 ) Kimmerle 1974
Rat F * oral ) H20 + Cremophor-EL 1126 Flucke and Kimmerle
1977a
Rat M * oral ) 917 Thyssen 1977
Rat M oral ) 830 )
Rat M oral ) 955 )
Rat M oral ) 925 ) "
Rat M oral ) 1000 )
Rat M oral ) 928 )
Rat F * oral ) Polyethylene 1045
Rat M * oral ) glycol 400 1524 )
Rat M oral ) 1940 )
Rat M oral ) 1718
Rat M * oral ) 822 Flucke 1981
Rat M * oral ) 1008 )
Rat M * oral ) 1855 )
Rat F * oral ) 1020 ) Mihail 1980
Rat M oral ) 1245 )
Rat F oral ) 793 )
Mouse M oral ) acetone + oil 989 ) Thyssen and Kimmerle
Mouse F oral ) (1:10) 1071 ) 1974
Mouse M * oral ) polyethylene 732 ) Mihail 1980
Mouse F * oral ) glycol 400 1158 )
Rabbit F oral acetone + oil ca.500 Thyssen and Kimmerle 1974
(1:10)
TABLE 1. (con't)
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rabbit M oral H2O + Cremophor EL 250-500 Mihail 1980
Hen F oral ) acetone _ oil ca. 5000 ) Thyssen and Kimmerle
Quail F oral ) (1:10) >1750,<2500 ) 1974
Duck M * oral ) polyethylene >4000 ) Lamb and Burke 1977
Duck F * oral ) glycol 400 >4000 )
Dog F oral acetone + oil >500 Thyssen and Kimmerle
(1:10) 1974
Rat M i.p.** acetone + oil 321 ) "
Rat F i.p.** (1:10) 293 )
Rat M i.p.** polyethylene 214 ) Mihail 1980
Rat F i.p.** glycol 400 213 )
Rat M dermal acetone + oil >1000 Thyssen and Kimmerle
(1:10) 1974
Rat M dermal) physiol NaCl >5000 ) Mihail 1980
Rat F dermal) solution >5000 )
* = animals fasted; ** i.p. = intraperitoneal
attack is the central nervous system. An influence on the acute
toxicity of the compound undoubtedly is exerted by the liver-damaging
effect at high doses (brittle liver in rats; lobular pattern of liver
in rats and rabbits) and by the local damaging effect on the
gastrointestinal tract (oral application, rat and mouse) and on the
peritoneum (intraperitoneal application, rat).
Comparison of the acute toxicity data for oral application and
for intraperitoneal administration to rats indicates, in the light of
the differences in the LD50 values, the delayed onset of action
following oral application, the long persistence of symptoms and the
long duration of animal mortality occurrence, that absorption from the
gastrointestinal tract is relatively poor and that triadimefon effects
are protracted. The relatively poor absorbability from the
gastrointestinal tract might be of practical significance, as it
suggests that rapid decontamination could be therapeutically effective
in the event of oral poisoning.
The results of the acute toxicity studies did not reveal any
significant sex-specific differences. The oral LD50 values in rats
exhibit a large range. Whereas the LD50 values for administration
of triadimefon in water and Cremophor EL correspond somewhat to those
for administration of the compound in polyethylene glycol 400,
administration in acetone + oil resulted in comparatively higher
toxicity. This may have been due to increased absorption or also to an
additional toxic effect of acetone. The corresponding LD50 values
obtained following administration in aqueous and alcoholic
formulations are usually in the order of 1 000 mg/kg body weight for
oral application to rats (Table 1).
The large range of the LD50 values most probably is associated
with the targets of attack of the compound at toxic doses, such as
damage to the liver and the gastrointestinal tract. These effects may
have had a varying influence on both absorption and detoxification in
the individual animals (Bayer 1981).
Short-term studies
Rat
Groups of 15 male and 15 female Wistar rats received daily oral
triadimefon doses of 3, 10 and 30 mg/kg bw respectively, by gavage for
30 days. The compound was emulsified in acetone and oil (1:10). A
control group of similar size received the acetone + oil mixture only.
The rats were sacrificed 24 h after the final application, and their
tissues were macroscopically examined and weighed. At the end of the
study, blood samples were taken for haematological and clinical
chemical tests. The nine most important tissues were examined
histopathologically. Rats treated with doses of up to and including
30 mg/kg bw did not differ from the controls with respect to
behavioural patterns, body weight development, haematological data,
clinical chemical data, results of urinalyses and histopathological
findings. However, the absolute and relative liver weights showed
a significant increase in male rats treated with doses of 10 and
30 mg/kg bw. In female rats, an increase in the absolute and relative
liver weights was seen only at the dose level of 30 mg/kg bw, and
other organ weights showed no differences. The no-effect dose for rats
treated orally with triadimefon for 30 days was 3 mg/kg for males and
10 mg/kg for females (Thyssen et al 1974).
In a 12-week feeding experiment, groups of 15 male and 15 female
Sprague-Dawley rats were fed triadimefon daily at dietary levels of
0 ppm (control), 50 ppm, 200 ppm, 800 ppm and 2000 ppm, respectively.
Haematological tests, clinical-chemical tests and urinalyses were
performed prior to commencement of test diet administration, after
6 weeks on the test diet and at the end of the treatment period. The
rats were sacrificed and dissected on termination of treatment,
tissues were macroscopically examined and organ weights were measured.
The major tissues were histopathologically examined. Triadimefon
was tolerated at daily dietary concentrations of up to and including
2 000 ppm for the whole of the 12-week period without causing any
symptoms. Haematology, clinical chemistry and organ weight
measurements, as well as gross pathology and histopathology, provided
no indication of toxic effects from administration of the test
compound. Redness and swelling seen macroscopically, especially in the
area of the glandular mucosa of the stomach, could not be confirmed
microscopically (Mohr 1976).
Dog
In a subchronic toxicity study, groups of 4 male and 4 female
beagle dogs were maintained for 13 weeks on a diet containing
triadimefon at concentrations of 0 ppm (control), 150 ppm, 600 ppm and
2 400 ppm, respectively. Clinical inspections, haematological tests,
clinical chemical tests and urinalyses were performed. On termination
of dietary administration, the dogs were sacrificed, dissected and
grossly examined. The most important tissues were weighed. EPN-oxidase
and N-demethylase were measured in liver samples to test for an enzyme
inducing effect. Comprehensive histopathological examinations were
performed on tissues from all dogs. The dietary level of 150 ppm was
tolerated without producing any treatment-related effects. Although
dietary concentrations of 600 ppm and 2 400 ppm caused increased N-
demethylase activities, which was indicative of enzyme induction, this
dose was tolerated without having any other toxic effects. However,
dogs fed a dietary level of 2 400 ppm were affected in several other
respects (e.g., vomiting and reduction in food consumption); hence,
they had a moderate state of nutrition and showed less weight gain
than the other groups. The parameters of the red blood picture were
adversely affected by the treatment. Alkaline phosphatase and
transaminase activities were increased, illustrating a disturbed liver
function. The relative liver weight showed a slight increase in both
sexes in the 2 400 ppm group as compared with the controls. However,
gross pathology and histopathology did not reveal any treatment-
related alterations in the liver or in any other tissues. In the
13-week feeding experiment, the no-effect dietary level for the dog
was 150 ppm (Hoffman and Luckhaus 1974).
In a chronic toxicity study, groups of 4 male and 4 female beagle
dogs were maintained for 104 weeks on a diet containing triadimefon at
concentrations of 0 ppm (control), 100 ppm, 330 ppm, 1 000 ppm (up to
week 54) and 2 000 ppm (from week 55), respectively, of a technical
grade of two batches, purity 88 to 89% and 92 to 97% (90% premix
prepared with highly dispersed silicate). Clinical examinations,
haematological tests, clinical chemical tests and urinalyses were
performed on the dogs. On termination of dietary administration, the
dogs were sacrificed, dissected and grossly examined; their organ
weights were measured and tissues were histopathologically examined.
The results of the study showed that triadimefon dietary
concentrations of up to and including 330 ppm were tolerated for 2
years by both males and females without having any toxic effect. In
the highest dietary concentration group (especially after the dietary
level was raised to 2 000 ppm in week 55) the weight of some dogs of
both sexes was depressed slightly. Clinical chemistry and
histopathology did not reveal any liver damage; however, there were
signs of a treatment-related microsomal enzyme induction at the
highest dietary level. None of the other findings showed any
differences between the treated dogs and the controls (Hoffman and
Gröning 1978).
Long-term studies
Rat
In a 24-month study to evaluate triadimefon for potential chronic
toxicity and carcinogenicity, groups of 50 male and 50 female Wistar
rats were fed dietary levels of 50, 500 and 5 000 ppm, respectively of
a technical grade, composed of 2 batches, one of 88-89%, and the other
of 92-97% (90% premix prepared with a highly dispersed silicate). The
control group, fed only chow, consisted of 100 male and 100 female
rats. For clinical laboratory tests (haematology, clinical chemistry,
urinalysis, enzyme induction assays), 10 rats were additionally used
per group and per sex. After 24 months on the test diet, all survivors
were sacrificed, dissected and grossly examined.
The study revealed no indication of triadimefon having a
carcinogenic effect on rats. With respect to chronic toxicity, the
no-effect dose was found to be 50 ppm for females and 500 ppm for
males. Higher dietary levels had the following effects in the two
sexes. The dietary concentration of 5 000 ppm caused violent motor
reactions in males and females from about week 23. These rats consumed
almost no food while the reactions persisted and, as a result, they
lost a considerable amount of weight and many died. Those that
survived began to consume feed again when the symptoms receded, and
made corresponding weight gains. However, in week 32 and, following
brief improvement, in week 37, these violent motor reactions again
occurred accompanied by refusal of food and considerable loss of
weight. The last of the surviving rats in the 5 000 ppm group were
killed in a moribund condition in week 39. Gross pathology performed
on the 5 000 ppm rats that died and on those sacrificed essentially
disclosed severe mucosal lesions in the stomach, which in most cases
contained no food, accompanied by sometimes severe haemorrhages. These
lesions were considered to have been the cause of the clinical
symptoms observed. From week 85, the body weights of the male rats in
the 500 ppm group were mostly significantly lower than those of the
controls, but the difference was so slight that it was not considered
to have been due to a damaging effect from the test compound. The
growth rate of most of the female rats in the 500 ppm group was
significantly retarded from as early as week 6.
Significantly reduced erythrocyte counts and haemoglobin levels
seen at the dietary levels of 5 000 ppm, and at the end of the study
also in females fed 500 ppm, were indicative of an effect on the blood
or blood-forming tissues. The histopathological examinations revealed
shrunken spleen with reduced haematopoiesis in some of the rats of the
5 000 ppm group. Some of the rats of this group also showed decrease
in haematopoiesis in the marrow. The histopathological examinations
revealed signs of damage to cells of the proximal tubules of the
kidney (vacuolation, desquamation, degeneration) in 5000 ppm rats. In
the lung, increased blood content was seen in the dilated alveolar
vessels. In the stomach of 5 000 ppm rats, increased superficial
ulcerations, haemorrhage of the mucosa and oedemas of the sub-mucosa
were observed. In the adrenals of some rats, congestion of blood
vessels was seen. The dietary level of 5 000 ppm also caused formation
of giant spermatids in some males. In rats fed 5 000 ppm, cholesterol
levels occasionally showed a significant increase, indicative also of
an effect on fat metabolism at this dietary level. The liver weights
showed a statistically significant (p <0.01) and dose-related
increase in the male rats of the 50 and 500 ppm groups and in the
female rats of the 500 ppm group. (On highest dose, 5 000 ppm, all
animals were dead at week 39.)
The statistically significant increased liver weight (p <0.01)
seen in 50 and 500 ppm males and in 500 ppm females was not due to
stimulation of microsomal enzymes, as the N-demethylase activity and
the cytochrome P-450 concentrations measured in the liver at the end
of the experiment were not affected. Thus, the increased liver weights
were considered to have been a non-specific effect of increased
metabolism (Bomhard and Löser 1978a).
Mouse
Triadimefon was evaluated for long-term effects and potential
carcinogenicity in a 24-month study on groups of 50 male and 50
female CF1/W 74 mice fed dietary levels of 0 (control), 50, 300 and
1 800 ppm, respectively, of a technical grade, purity 97% (used as an
approximately 90% premix prepared with a highly disperse silicate).
For clinical laboratory tests, 10 mice were additionally used per
group and per sex. The mice were inspected for clinical symptoms.
Their body weight and food intake were measured. Haematological tests,
clinical chemical tests, organ weight measurements, macroscopic tissue
examinations and comprehensive histological examinations were
performed.
The study revealed no indication of triadimefon having a
carcinogenic effect on mice. No chronic-toxic effects were seen at the
two lower dietary levels of 50 and 300 ppm. Mice fed 1 800 ppm showed
the following alterations, in comparison with the controls. Growth of
both male and female mice was affected. Significantly increased
erythrocyte counts were recorded in both sexes, and increased
haemoglobin and haematocrit values were observed in females. Other
treatment-related alterations seen on termination of the study,
although not present consistently at the intermediate examinations and
necropsies performed after 6 and 12 months, included: distinctly
increased liver weights; increased AP, GOT and GPT activities;
increased incidence of liver swellings, occasionally accompanied by
adhesions of liver lobes in mice that died and in those sacrificed on
termination of the study. At the end of the feeding experiment, a
marked correlation between hyperplastic liver nodules and greatly
increased enzyme activities, indicative of liver damage, was observed
in the 1 800 ppm group. The incidence of liver cell tumours in male
and female mice were as follows:
ppm F M
0 0 2
50 1 2
300 0 1
1800 2 2
An increased incidence of hyperplastic liver nodules, which are
histologically very difficult to differentiate from liver cell tumour,
were also observed with the following incidence in male and female
mice:
ppm F M
0 4 7
50 2 7
300 1 7
1800 15 15
(Bombard and Löser 1980b)
Special studies on reproduction
Rat
Triadimefon was evaluated for its effect on fertility, lactation
performance and development of offspring in a 3-generation
reproduction study in Wistar rats with two matings per generation
(Löser 1979). Each test group consisted of 10 male and 20 female rats
and triadimefon was fed to the rats at dietary concentrations of 0
(control), 50, 300 and 1 800 ppm respectively. At the start of the
study, the F0 generation was ca. 32 to 39 days old and was treated
for 70 days prior to the first mating.
The pups of the F3b generation were sacrificed and
necropsied at an age of 4 weeks, and the major 18 tissues were then
histopathologically examined. Dietary levels of 50 and 300 ppm had no
adverse effect on the reproductive performance of the rats. After 2 of
6 matings, the weight of the pups in the 300 ppm group were
significantly lower than those of the controls during the lactation
period. The dietary level of 1 800 ppm distinctly affected
reproductive performance, as none of the rats became pregnant after
the second mating of the F1b generation. As this dietary level also
distinctly affected the parental rats, evidenced by the growth rate,
there would be no evidence for assuming any primary damaging effect on
reproduction.
Triadimefon did not cause any gross abnormalities or
malformations in rats at the tested dietary concentrations. The
histopathological examinations of the 18 most important tissues from
4-week old pups of the F3b generation revealed no indication of any
tissue alterations due to dietary administration of triadimefon at the
tested concentrations. The dietary concentration of 50 ppm was
tolerated without having any damaging effect whatsoever in the
3-generation reproduction study (Löser 1979).
Hen
Groups of 10 female White Leghorn hens per concentration, which
were mated with one cock per group, were maintained for 4 weeks on a
diet containing triadimefon at concentrations of 0 (control), 10 and
100 ppm, respectively. The hens were inspected for behavioural
patterns, physical appearance and appetite and their body weights and
food intake were measured. Tissues were macroscopically examined and
the 12 most important tissues were histopathologically examined.
Haematological tests were also performed on additional hens maintained
under comparable conditions. The following parameters were measured in
the offspring: number of eggs, egg quality, egg weight, egg size,
thickness of egg shell, weight of egg shell, fertilization rates,
hatch rates. The chicks were inspected for their physical appearance
and behavioural reactions during the first days of their life. The
tested concentrations were tolerated by both the hens and their
offspring without causing any damage whatsoever (Thyssen and Gröning
1978).
Species studies on embryotoxic effects
Rat
Two experiments were performed with groups of 20 pregnant Long
Evans rats dosed daily by gavage with triadimefon in an aqueous
emulsion from gestation day 6 to 15. The first study utilized daily
doses of 10, 30 and 100 mg per kg bw. Each experiment included a
control group dosed only with the vehicle. While the dose of
10 mg/kg/day did not have any harmful effects on the dams, doses of
30 mg/kg/day and above reduced dam weight gain. However, none of the
tested doses caused any behavioural disorders or mortalities. Effects
on progeny were characterized by a slightly increased placenta weight
in the 100 mg/kg group as compared with the controls, and by the
occurrence of occasional cleft palates at 75 mg/kg and 100 mg/kg/day.
Thus, a treatment-related effect and hence a slight teratogenic effect
of triadimefon on rats at oral doses of 75 mg/kg/day and above could
not be completely ruled out. The oral no-effect of triadimefon with
respect to embryonic/foetal development was found to be 50 mg/kg/day
(Machemer 1976b).
Evaluation of triadimefon for embryotoxic effects was also
performed by administering the compound by the route of greatest
relevance to practical conditions, viz. by inhalation. (A hazard from
skin exposure can be ruled out on the ground of the acute toxicity
findings, which did not reveal any toxicologically relevant absorption
through the skin.) For the inhalation evaluation, twenty fertilized
rats per group were exposed for 6 h daily on 10 consecutive days
from gestation day 6 to 15, to analytically measured triadimefon
concentrations of 14 (11 to 18) mg/m3, 33 (28 to 37) mg/m3 and 114
(95 to 132) mg/m3 air in a dynamic flow inhalation apparatus.
Triadimefon was dissolved in a 1:1 mixture of ethanol and polyethylene
glycol 400 and aerosolized in the inhalation chambers. A control group
inhaled the aerosol of the solvent mixture only. While the contration
of 14 mg/m3 air did not affect the dams, concentrations of 33 and
114 mg/m3 slightly reduced dam weight gain during the treatment
period. None of the concentrations adversely affected physical
appearance and behavioural patterns of the dams. Embryonic and foetal
development were not affected by concentrations of up to and including
114 mg/m3. Hence, it was concluded that inhaled triadimefon did not
result in any embryotoxic effects (Machemer and Kimmerle 1976).
Rabbit
Groups of 10 to 13 pregnant Himalayan rabbits received daily oral
doses of triadimefon administered as an aqueous emulsion by gavage at
levels of 0 (control), 5, 15 and 50 mg/kg bw, respectively, from
gestation day 6 to 18. The tested doses had no adverse effects on
physical appearance, behavioural patterns and weight gains of the dams
or on embryonic and foetal development. Oral doses of triadimefon did
not result in toxic or teratogenic effects on rabbits (Machemer
1976c).
Special studies on mutagenic effects
Triadimefon was evaluated for mutagenic potential by the Ames
test on histidine requiring Salmonella typhimurium strains TA 98,
100, 1535, 1537, 1538 and 1950, with and without metabolic activation.
The triadimefon concentrations ranged from 0.1 to 1 000 µg per plate.
The minimal inhibitory concentration of triadimefon on the tester
strains used was 500 µg/ml. Triadimefon did not induce back-mutations
in the test systems, with or without microsome activation, over the
tested concentration range (van Dijck 1976).
Triadimefon was tested for potential DNA-attacking action on
Bacillus subtilis at concentrations of up to 300 µg per plate, by
rec-assay. The compound was also evaluated at concentrations of up to
1 000 µg/plate for potential mutagenicity by the Ames assay using
Salmonella typhimurium strains TA 1535, 1537, 98 and 100, with and
without addition of rat and mouse liver homogenates to the agar. In
these studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Inukai and Iyatomi 1977).
The fungicide was further tested for potential mutagenic effects
by the rec-assay on two Bacillus subtilis strains at concentrations
of up to 2 000 µg/plate, and by the Ames (reversion) assay on the
Salmonella typhimurium strains TA 98 and 100 at concentrations of up
to 5 000 µg/plate, with and without metabolic activation. In these
studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Shirasu et al 1978).
Triadimefon was additionally studied by rec-assay on two
Bacillus subtilis strains at concentrations of up to and including
2 000 µg per plate and by reversion assay on Escherichia coli (WP 2)
and on Salmonella typhimurium strains TA 1535, 1537, 1538, 98 and
100, at concentrations of up to and including 5 000 µg per plate, with
and without metabolic activation by rat liver chromosomes. No
indication of mutagenic activity was found in these studies (Shirasu
et al 1979).
Triadimefon was evaluated for point-mutagenic effects by a
eukaryotic, micro-organism test using Saccharomyces cerevisiae
S 138 and S 211, in comparison with negative (solvent) and positive
controls, with and without metabolic activation by microsomal enzymes
of Aroclor-induced rat livers. Neither a mutagenic nor a
recombinogenic effect was seen at doses extending into the toxic
range, 1 000 µg/plate (Jagannath et al 1980).
A micronucleus test on male and female NMRI mice was performed
with triadimefon. Triadimefon was administered in an aqueous emulsion
at a total oral dose of 400 mg/kg bw applied in two split doses of
200 mg/kg bw each at an interval of 24 h. The thiotepa dose was
2 × 10 mg/kg injected subcutaneously. For comparison, a negative
control group was treated with the vehicle and a positive control
group was treated with thiotepa. Each group consisted of 10 mice;
1 000 polychromatic erythrocytes per mouse were analysed for incidence
of micronuclei. Additionally, the ratio of polychromatic erythrocytes
to normochromatic ones was determined to establish whether the test
compound had any effect on the general activity of bone marrow.
Thiotepa caused a big increase in the number of micronuclei and also
bone marrow depression. Triadimefon did not affect general bone
marrow activity nor did it increase the incidence of micronuclei in
comparison with the control, and hence was deemed to be non-mutagenic
in this system (Machemer 1977).
Twenty male NMRI mice each received a single oral dose of
triadimefon at a level of 200 mg/kg bw, administered as an aqueous
emulsion by gavage. Starting on the day of test compound
administration, a series of 8 matings was performed with the treated
males, each mating period being of 1-week duration; every week, each
male was caged together with 3 fresh untreated virgin females. The
pre-implantation and post-implantation losses of fertilized females
were determined as the criteria for assessment of a mutagenic effect.
(The corpora lutea, total implantations, viable implants and dead
implants were counted.) The results were compared with those obtained
from a control group of similar size. The tested dose was not toxic to
the males and did not affect their fertility. The measured parameters
did not reveal any treatment-related difference between the control
group and the triadimefon-treated group. The dominant lethal test on
the male mouse did not provide any indication of the mutagenicity of
triadimefon at the acute oral dose of 200 mg/kg bw (Machemer 1976a).
Special studies on induction of liver enzymes
Triadimefon was administered daily on 28 consecutive days to
Wistar rats, by gavage in a mixture of acetone and oil at dose levels
of 0 (vehicle), 1, 5 and 25 mg/kg bw, respectively. Each group
consisted of 20 male and 20 female rats; half of them were sacrificed
at the end of the treatment period and the other half were killed
on termination of a 4-week post-treatment observation period.
N-demethylase, O-demethylase and cytochrome P-450 in the rat livers
were measured at the end of the treatment period and on termination of
the post-treatment observation period. The rats were observed for
systemic toxic effects by observing their physical appearance,
behavioural patterns and posture, and their body weights and certain
haematological and clinical chemical parameters were measured. The
rats were necropsied, tissues were macroscopically examined and livers
were weighed. The study revealed that triadimefon at daily doses of 5
and 25 mg/kg bw caused slight induction of microsomal liver enzymes.
The enzyme induction was reversible within the 4-week post-treatment
observation period. None of the other parameters measured and
investigated provided any indication of treatment-related damage
(Mihail and Kaliner 1979).
Special studies on potentiation toxicity
Since triadimefon is used in combination with other fungicidal
compounds, acute combination toxicity studies were performed on Wistar
rats. The compounds tested in combination with triadimefon were
captafol, propineb, tolylfluanid and carbendazim. The oral LD50 of
each of these compounds was first determined. Fifteen female or male
rats were used per dose and the observation period lasted 14 days.
From the LD50 values of the single components the theoretically
expected LD50 of each combination was calculated. For combined
administration, the components were mixed in a percentage ratio
proportional to the LD50 of each and thus applied in equitoxic doses;
thereby, the actual LD50 of the combination was determined. A more
than additive acute toxic effect was not seen for any of the tested
combinations (Flucke and Kimmerle 1977, Thyssen 1977).
OBSERVATIONS IN MAN
Approximately 50 mg of triadimefon was applied to small gauze
pads which were placed for a contact time of 24 h on the skin of the
forearm of each of 8 test persons and the pads were fixed in position
with adhesive strips. Upon removal of the strips and pads, slight
redness of the treated skin area was seen for a brief period in 5 of
the 8 persons. At 24 h post-application, the treated skin areas showed
no variations from the physiological norm in any of the test persons
(Thyssen and Kimmerle 1973).
RESIDUES IN FOOD
USE PATTERN
Triadimefon is effective and is mainly used against powdery
mildews and rusts; it also gives good control of a number of other
diseases e.g. Thielaviopsis on pineapple (post-harvest application).
Triadimefon is registered and marketed in more than 30 countries
in Europe, Africa, America, Asia and Australia; registration is being
sought also in other countries. Details on the uses of triadimefon and
recommendations are given in Table 2. With the exception of the post-
harvest dip of pineapple the product is used as pre-harvest
application. Triadimefon is used also on ornamentals (roses, azaleas),
seed-grass, turf and rubber trees. Sugarcane and sweet potato sets are
dipped in a triadimefon suspension of emulsion prior to planting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on triadimefon residues from supervised residue trials were
obtained on various fruits and vegetables, cereal grains and some
other crops grown under a variety of geographical, climatic conditions
and agricultural practices. All residue figures presented in Table 3
refer to the sum of triadimefon parent compound and its main
metabolite triadimenol.
Fruits and vegetables
The average residue at harvest following the recommended
pre-harvest intervals is in the range of non-detectable (0.01 to
0.05 mg/kg) up to 0.3 mg/kg (maximum residue of 0.4 mg/kg) on most
fruits and vegetables. On red currants somewhat higher residues are
found after the recommended pre-harvest intervals; the average level
at harvest was 0.4 mg/kg (maximum residue in the trials 1.1 mg/kg).
Higher residues were also found on Brussels sprouts (average 0.5,
highest level found 0.9 mg/kg) in trials where the normal pre-harvest
interval was taken into account.
FATE OF RESIDUES
In plants
The behaviour and fate of triadimefon in plants was evaluated.
Studies were carried out on metabolism of (benzene ring-UL-14C)- and
(triazole ring-3,5-14C)-triadimefon in cereals, apple, cucumber and
tomato; translocation of unlabelled (benzene ring-UL-14C)-, and
(triazole ring-3,5-14C)-triadimefon, with the studies being directed
towards elucidation of site of action, mechanism of action and
metabolism; redistribution in the vapour phase after application of
unlabelled and (benzene ring-UL-14C)-triadimefon to plants.
TABLE 2. Summary of recommended uses of triadimefon
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Fruits
Apple and pear wp 40 - 75 4 - 12 7
Jap. apple wp 150 -200 2 - 3
Jap. pear wp 150 -200 2 - 3
Apricot wp 50 -150 4 - 6 7
Peach wp 50 -150 4 - 6 7
Currant (black,red) wp 50 -150 3 - 4 7
Gooseberry wp 50 -100 3 - 4 7
Grape wp/EC/dust 50-75 (dust 100-200) wingrape 4-10 14-28
tablegrape 4-8 4- 7
Strawberry wp 75 -100 7
Mango wp 100 -200 28
Pineapple wp/EC 0.015-0.07% 1(postharvest
dip) -
Vegetables
Artichoke wp/EC 50 -150 4 - 8 3 - 7
Beans wp/EC 125 -250 3 - 6 7 -14
Pea wp/EC 50 -125 3 - 6 7 -14
Brussels sprout wp/EC 125 -250 3 - 6 14
Onion (Welsh) wp 100 3 10
Cucurbits wp/EC/dust 25-100 (dust100-200) 4 - 10 3 - 7
Pepper wp/EC 50 -150 4 - 8 3 - 7
Tomato wp/EC 50- 150 4 - 8 3 - 7
Grain crops wp/EC 125 -250 1 - 2 21 -35
Sugarbeet wp/EC 125 2 - 3 14
TABLE 2. (con't)
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Coffee
protective wp/EC 100 -250 8 - 12
eradicative wp/EC 250 -500 2 - 4
Hops 200 -500 2 - 6
Tobacco 40 - 75 4 - 6
TABLE 3. Residues of triadimefon/triadimenol from supervised trials
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3 7 14
Fruit
Apple USA 1977 6 20 - 60 wp 50% 0.3 0.2 0.4 0.2 Mobay 1981
1977 6 20 - 60 wp 50% 0.5 0.4 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.2 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.3 0.3 0.4 "
1977 6 340 -560 wp 50% 0.5 0.4 0.3 0.2 "
1978 6 20 - 60 wp 50% 0.3 0.2 0.09 0.08 "
1977 11 340 -560 wp 50% 0.4 0.2 0.1 0.1 "
1977 10 340 -560 wp 50% 0.2 0.1
1977 10 340 -560 wp 50% 0.4 0.3 0.2 0.1 "
1977 10 340 -560 wp 50% 0.4 0.2 "
1977 10 340 -560 wp 50% 0.2 0.1 0.1 0.09 "
1979 6 140 wp 50% 0.7 0.4 "
Pear 1980 6 600 wp 50% 0.6 0.3 0.5 0.3 "
1980 6 600 wp 50% 0.4 0.4 0.3 0.2 "
1980 6 600 wp 50% 0.4 0.5 0.4 0.3 "
1980 10 450 wp 50% 0.3 0.2 0.09 0.06 "
1980 15 12001 wp 50% 0.8 0.5 0.3 0.2 "
Peach 1980 4 12001 wp 50% 3.0 1.6 0.7 "
1980 3 12001 wp 50% 0.1 0.4 0.1 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 3 6/7 14 21
Berry fruits
Currant- Denmark 1979 4 50 wp 5% 0.03 <0.05 <0.02 <0.05 <0.02 <0.05 Bayer 1981
black 1979 4 50 wp 5% 0.06 0.05 0.03 <0.05 <0.02 <0.05 "
France 1979 4 100 wp 5% 0.03 0.2 0.04 0.2 "
1977 4 40 wp 1.25% 0.04 0.14 "
Currant-red Denmark 1979 2 50 wp 5% 0.02 <0.05 <0.02 <0.05 <0.02 <0.05 "
1979 2 50 wp 5% 0.15 <0.05 0.05 <0.05 <0.02 <0.05 "
Raspberry 1979 4 75 wp 5% 0.03 0.05 Bayer 1981
Grape Japan 1979 3 150 wp 5% 0.01 0.4 <0.01 0.2 "
1979 5 150 wp 5% 0.02 0.5 0.01 0.4 "
1979 3 150 wp 5% 0.01 0.4 <0.01 0.3 "
1979 5 150 wp 5% 0.02 0.7 0.01 0.4
USA 1978 3 90 wp 50% 0.06 0.06 0.08 0.2 Mobay 1981
1978 3 110 wp 50% 0.02 0.05 <0.01 <0.01 "
1978 3 110 wp 50% 0.05 0.02 <0.01 <0.01 "
1978 3 150 wp 50% <0.01 0.01 "
1978 3 150 wp 50% 0.06 0.04 <0.01 <0.01 "
1978 3 150 wp 50% 0.03 0.09 <0.01 <0.01 "
1979 3 225 wp 50% <0.01 0.07 "
1979 3 225 wp 50% 0.5 0.4 0.01 0.02 "
1979 3 300 wp 50% 0.2 0.08 "
1979 3 225 wp 50% <0.01 <0.02 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3/5 7 14/15
Fruiting vegetables
Cantaloupes USA 1979 3 130 wp 50% 0.1 0.08 "
1979 3 130 wp 50% 0.1 0.03 "
1979 3 130 wp 50% 0.06 0.1 "
1979 3 130 wp 50% 0.03 0.03 "
Gherkins2 Fed.Rep. 1976 8 60 wp 5% <0.06 <0.06 <0.06 Bayer 1981
of 1976 8 60 wp 5% <0.01 <0.01 <0.06 "
Germany 1975 8 60 wp 5% 0.06 <0.06 <0.01 <0.06 "
1975 8 60 wp 5% 0.06 0.06 <0.06 <0.06 "
Cucumber Mexico 1979 3 125 wp 50% <0.0 <0.01 <0.01 <0.01 Mobay 1981
1979 3 125 wp 50% 0.04 0.05 <0.01 <0.01 "
Tomato Mexico 1979 8 180 wp 25% 0.03 0.05 0.01 0.03 0.01 0.03 "
1979 8 180 wp 25% 0.04 0.05 0.01 0.02 <0.01 0.02 "
1979 8 180 wp 25% 0.04 0.08 0.02 0.04 0.02 0.03 "
1979 8 180 wp 25% 0.07 0.08 0.02 0.06 <0.01 0.01 "
Japan 1977 4 250 wp 25% 0.07 0.08 0.04 0.08 0.022 <0.063 Nitokino
7 250 wp 25% 0.2 0.3 0.09 0.2 0.052 0.23 1981
2 250 wp 25% 0.4 0.2 0.3 0.3 0.22 0.33
4 250 wp 25% 0.4 0.5 0.3 0.6 0.12 0.53
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Other vegetables
Brussels
sprouts UK 1979 3 250 wp 25% <0.05 <0.05 Hazleton 1981
1977 3 125 wp 25% 0.6 0.2 BAY/UK 1981
Chick peas 1980 2 150 wp 25% 0.01 0.02 <0.01 <0.01 <0.01 0.02 Mobay
dry 2 150 wp 25% <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 1981
2 150 wp 25% <0.01 <0.02 <0.01 <0.01 <0.01 <0.01
Leeks UK 1979 4 250 wp 25% <0.05 <0.05 Hazleton 1981
500 wp 25% <0.05 0.08
250 wp 25% 0.09 0.4
500 wp 25% 0.3 0.9
Parsnips UK 1979 3 250 wp 25% 0.05 0.2
3 250 wp 25% 0.01 0.05
Hops UK 1979 8 110-220 wp 5% <0.3 0.8 Bayer 1981
fresh
dry
UK 1979 8 100-200 wp 5% 0.4 1.4
fresh <0.3 1.4 ibid
dry 0.4 1.4
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Fed. Rep.
of
Germany 1979 4 500 wp 5% ibid
fresh 0.9 0.6 2.13 0.83
dry 10.8 4.2 7.23 4.13
Fed.Rep.
of
Germany 1979 4 500 ibid
fresh 2.1 1.0 0.73 0.63
dry 11.4 4.3 1.93 1.73
1 Excessive dosage;
2 Some of the gherkins data were erroneously presented in the 1979 JMPR Evaluations under
cucumbers due to a translation error. Schlangen qurke = cucumber; Einlegequrke = gherkin;
3 After 10 days.
The metabolism of triadimefon (Bayleton(R)) in cereals was
studied in several experiments following application of (benzene ring-
UL-14C)-triadimefon to plants under glass and in field conditions.
The main metabolite formed was triadimenol, which occurs in the
diastereoisomeric forms A and B. On cereal plants, the A form is the
main diastereoisomer. A further metabolism pathway consisted of
oxidation of the tert. butyl group (KWG 1342) and cleavage of the
molecule, with the formation of 4-chlorophenol. The metabolites
triadimenol, and particularly KWG 1342 and 4-chlorophenol, are bound
in the form of glycosides-conjugates, mainly B glycosides. At harvest
the radioactivity still present in straw was distributed as shown in
Table 4.
TABLE 4. Radioactive studies on triadimefon metabolism in cereal
straw at harvest
Metabolite Radioactivity present (%)
Triadimefon unchanged parent 0 - 5
Triadimenol form A 22 - 41
Triadimenol form B 6 - 17
KWG 1342 2 - 16
Polar metabolites1 17 - 37
BUE 2285 less than 1
Free 4-Chlorophenol less than 1
Non-extractable 18 - 32
1 Including the glycosides of triadimenol, KWI 1342 and
4-chlorophenol.
The glycosides are probably fixed in the plants by further
metabolism and are therefore no longer extractable with standard
procedures. The distribution of the radioactivity among the aqueous
and organic phases after extraction of the straw differs between the
(triazole ring-3,5-14C) and the (benzene ring-UL-14C). An explanation
for this may be the different polarities of 4-chlorophenol and
1,2,3-triazole, which can be formed as metabolites of triadimefon
and/or triadimenol. Further studies are underway to elucidate this
phenomena.
In another experiment on summer barley, the non-extractable part
of the residue accounted for higher amounts, i.e. 55-75% (Bayer 1981).
This may be partly due to the application at an earlier stage in plant
development. Following spray application of (triazole ring-3,5-14C)-
triadimefon the metabolites that occurred were the same as those found
following the application of (benzene ring-UL-14C)-triadimefon, the
only difference being that polar metabolites accounted for a higher
proportion when both labels were applied at the same stage in plant
development, i.e. 31% vs 20% (Bayer 1981). For quantitative data
see Table 5. After meed treatment of barley with the pure
diastereoisomeric forms A and B of triadimenol, no conversion of one
form to the other was found in the barley plant (Bayer 1981).
In studies on apple with benzene ring-labelled and triazole-
labelled triadimefon, it was demonstrated that also in this crop
triadimefon was metabolized to triadimenol and in a small amount to an
unidentified compound (Table 6). The rapid decline of the 14C during
the early intervals up to 7 days was attributed to the volatility of
triadimefon parent compound (Mobay 1981). In trials on cucumber and
tomatoes, the fate of (benzene-ring-UL-14C)-triadimefon was studied.
The material was applied as a foliar spray or to the roots. It was
found that in addition to triadimefon and diastereoisomers A and B, a
polar metabolite was formed in the plants, which was shown to be a
glucoside of triadimenol loosely bound to another natural occurring
moiety. It is readily converted to triadimenol glucoside (see Table 6)
(Mobay 1981). It was also shown in these experiments that the
radiocarbon applied to the foliage did not translocate significantly
into neighbouring fruits, in marrow plants triadimenol was identified
as the major metabolite of triadimefon (Clark et al 1978). The
metabolic pathways of triadimefon are illustrated in Figure 2. For
chemical names of the metabolites identified in plants and their
structural formula see Table 9, FAO/WHO 1979, Triadimefon.
Triadimefon was translocated at a relatively fast rate in
anacropetal direction following foliar application to cereals, beans,
cucumber and tomato (Buchenauer 1975, 1976; Bayer 1981; Scheinpflug
et al 1977; Brandes et al 1978; Gasztonyi and Joslpovits 1978) and
also to a larger extent following soil applications to beans and
barley (Buchenauer 1975, 1976). Some authors also observed a slight
basipetal translocation. Gasztonyi and Josepovits (1978) established
that the rate of uptake and subsequently the amount of transport
depended substantially on the mode of leaf treatment, and also found
differences in the amounts of translocated triadimefon according to
plant species.
Gasztonyi and Josepovits (1979) studied the uptake and metabolism
of triadimefon by mycelia of fungi that were sensitive or resistant to
triadimefon. In the fungi, triadimefon accumulated to 20 to 40 fold of
the external concentration, irrespective of the sensitivity of the
fungus. In the course of metabolism, the highly fungitoxic main
metabolite triadimenol was formed; however, in mycelia of sensitive
fungi this transformation was at a high rate, whereas it could not be
demonstrated, or only to a low extent, in resistant strains of fungi.
Based on these observations, triadimefon must be regarded as the
precursor of the active principle, i.e. triadimenol.
Hen
When (benzene ring-UL-14C)-triadimefon was administered to
laying hens by a single oral capsular treatment of 2.4 mg/kg, the dose
was eliminated rapidly and 100% was recovered in the excreta 24 h
after treatment (Nye 1979).
Radiocarbon residues in tissues and eggs of laying hens were
determined during a 96-h post-treatment period. The liver contained
0.26 mg triadimefon equivalent/kg 6 h after treatment, the kidney
contained 1.18 mg/kg, breast muscle contained 0.12 mg/kg and abdominal
fat contained 0.30 mg/kg. All tissue radiocarbon levels were well
below 0.01 mg/kg 90 h later. Radiocarbon residues in the eggs were
highest (0.117 mg/kg) 24 h after treatment and had decreased to
0.04 mg/kg 96 h after treatment (Nye 1979).
The nature of the radiocarbon contained in tissues and eggs of
laying hens, as well as that eliminated via the excreta was determined
by Nye (1979). Triadimefon was absorbed by the birds and rapidly
converted to triadimenol acid, which was excreted in its free form.
This metabolite was also the major metabolite isolated in the liver
and kidney. The intermediates in the conversion of triadimefon to the
acid, triadimenol and KWG 1342 were found as major metabolites in the
muscle, fat and eggs. The parent compound was found as the primary
radiocarbon component in the gizzard and abdominal fat.
Catfish
The accumulation and persistence of residues was studied in
Channel catfish (Ictalurus punctatus) continuously exposed to
(benzene ring-UL-14C)-triadimefon for a 28-day period at
concentrations of approximately 10 and 100 ppb in water (Lamb and
Roney 1977). The catfish showed accumulation factors of approximately
7.6 from the 10 ppb level and 6.5 from the 100 ppb level during the
exposure period. The non-edible portions of the catfish contained 74%
and 84% of the extractable 14C residues from the 10 and 100 ppb
levels, respectively. When the catfish were transferred to
uncontaminated water (withdrawal period), approximately 88% of the
accumulated 14C residues were excreted by the fish within 5 h and
approximately 96% were eliminated within 7 to 10 days. Under the
conditions of the study, triadimefon exhibited a low magnitude of
accumulation with a rapid rate of uptake and excretion (Lamb and Roney
1977).
TOXICOLOGICAL STUDIES
Acute toxicity
Triadimefon has a slight (dermal) to moderate (oral,
intraperitoneal) acute toxicity to experimental laboratory animals
(Table 1). The symptoms of poisoning indicate that the target of
TABLE 1. Acute toxicity of triadimefon in animals
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rat M oral ) acetone + 568 ) Thyssen and
Rat F ) oil (1:10) 363 ) Kimmerle 1974
Rat F * oral ) H20 + Cremophor-EL 1126 Flucke and Kimmerle
1977a
Rat M * oral ) 917 Thyssen 1977
Rat M oral ) 830 )
Rat M oral ) 955 )
Rat M oral ) 925 ) "
Rat M oral ) 1000 )
Rat M oral ) 928 )
Rat F * oral ) Polyethylene 1045
Rat M * oral ) glycol 400 1524 )
Rat M oral ) 1940 )
Rat M oral ) 1718
Rat M * oral ) 822 Flucke 1981
Rat M * oral ) 1008 )
Rat M * oral ) 1855 )
Rat F * oral ) 1020 ) Mihail 1980
Rat M oral ) 1245 )
Rat F oral ) 793 )
Mouse M oral ) acetone + oil 989 ) Thyssen and Kimmerle
Mouse F oral ) (1:10) 1071 ) 1974
Mouse M * oral ) polyethylene 732 ) Mihail 1980
Mouse F * oral ) glycol 400 1158 )
Rabbit F oral acetone + oil ca.500 Thyssen and Kimmerle 1974
(1:10)
TABLE 1. (con't)
Animal Route LD50 (mg/kg bw,
species/ of Vehicle 14-day Reference
sex appl. observation)
Rabbit M oral H2O + Cremophor EL 250-500 Mihail 1980
Hen F oral ) acetone _ oil ca. 5000 ) Thyssen and Kimmerle
Quail F oral ) (1:10) >1750,<2500 ) 1974
Duck M * oral ) polyethylene >4000 ) Lamb and Burke 1977
Duck F * oral ) glycol 400 >4000 )
Dog F oral acetone + oil >500 Thyssen and Kimmerle
(1:10) 1974
Rat M i.p.** acetone + oil 321 ) "
Rat F i.p.** (1:10) 293 )
Rat M i.p.** polyethylene 214 ) Mihail 1980
Rat F i.p.** glycol 400 213 )
Rat M dermal acetone + oil >1000 Thyssen and Kimmerle
(1:10) 1974
Rat M dermal) physiol NaCl >5000 ) Mihail 1980
Rat F dermal) solution >5000 )
* = animals fasted; ** i.p. = intraperitoneal
attack is the central nervous system. An influence on the acute
toxicity of the compound undoubtedly is exerted by the liver-damaging
effect at high doses (brittle liver in rats; lobular pattern of liver
in rats and rabbits) and by the local damaging effect on the
gastrointestinal tract (oral application, rat and mouse) and on the
peritoneum (intraperitoneal application, rat).
Comparison of the acute toxicity data for oral application and
for intraperitoneal administration to rats indicates, in the light of
the differences in the LD50 values, the delayed onset of action
following oral application, the long persistence of symptoms and the
long duration of animal mortality occurrence, that absorption from the
gastrointestinal tract is relatively poor and that triadimefon effects
are protracted. The relatively poor absorbability from the
gastrointestinal tract might be of practical significance, as it
suggests that rapid decontamination could be therapeutically effective
in the event of oral poisoning.
The results of the acute toxicity studies did not reveal any
significant sex-specific differences. The oral LD50 values in rats
exhibit a large range. Whereas the LD50 values for administration
of triadimefon in water and Cremophor EL correspond somewhat to those
for administration of the compound in polyethylene glycol 400,
administration in acetone + oil resulted in comparatively higher
toxicity. This may have been due to increased absorption or also to an
additional toxic effect of acetone. The corresponding LD50 values
obtained following administration in aqueous and alcoholic
formulations are usually in the order of 1 000 mg/kg body weight for
oral application to rats (Table 1).
The large range of the LD50 values most probably is associated
with the targets of attack of the compound at toxic doses, such as
damage to the liver and the gastrointestinal tract. These effects may
have had a varying influence on both absorption and detoxification in
the individual animals (Bayer 1981).
Short-term studies
Rat
Groups of 15 male and 15 female Wistar rats received daily oral
triadimefon doses of 3, 10 and 30 mg/kg bw respectively, by gavage for
30 days. The compound was emulsified in acetone and oil (1:10). A
control group of similar size received the acetone + oil mixture only.
The rats were sacrificed 24 h after the final application, and their
tissues were macroscopically examined and weighed. At the end of the
study, blood samples were taken for haematological and clinical
chemical tests. The nine most important tissues were examined
histopathologically. Rats treated with doses of up to and including
30 mg/kg bw did not differ from the controls with respect to
behavioural patterns, body weight development, haematological data,
clinical chemical data, results of urinalyses and histopathological
findings. However, the absolute and relative liver weights showed
a significant increase in male rats treated with doses of 10 and
30 mg/kg bw. In female rats, an increase in the absolute and relative
liver weights was seen only at the dose level of 30 mg/kg bw, and
other organ weights showed no differences. The no-effect dose for rats
treated orally with triadimefon for 30 days was 3 mg/kg for males and
10 mg/kg for females (Thyssen et al 1974).
In a 12-week feeding experiment, groups of 15 male and 15 female
Sprague-Dawley rats were fed triadimefon daily at dietary levels of
0 ppm (control), 50 ppm, 200 ppm, 800 ppm and 2000 ppm, respectively.
Haematological tests, clinical-chemical tests and urinalyses were
performed prior to commencement of test diet administration, after
6 weeks on the test diet and at the end of the treatment period. The
rats were sacrificed and dissected on termination of treatment,
tissues were macroscopically examined and organ weights were measured.
The major tissues were histopathologically examined. Triadimefon
was tolerated at daily dietary concentrations of up to and including
2 000 ppm for the whole of the 12-week period without causing any
symptoms. Haematology, clinical chemistry and organ weight
measurements, as well as gross pathology and histopathology, provided
no indication of toxic effects from administration of the test
compound. Redness and swelling seen macroscopically, especially in the
area of the glandular mucosa of the stomach, could not be confirmed
microscopically (Mohr 1976).
Dog
In a subchronic toxicity study, groups of 4 male and 4 female
beagle dogs were maintained for 13 weeks on a diet containing
triadimefon at concentrations of 0 ppm (control), 150 ppm, 600 ppm and
2 400 ppm, respectively. Clinical inspections, haematological tests,
clinical chemical tests and urinalyses were performed. On termination
of dietary administration, the dogs were sacrificed, dissected and
grossly examined. The most important tissues were weighed. EPN-oxidase
and N-demethylase were measured in liver samples to test for an enzyme
inducing effect. Comprehensive histopathological examinations were
performed on tissues from all dogs. The dietary level of 150 ppm was
tolerated without producing any treatment-related effects. Although
dietary concentrations of 600 ppm and 2 400 ppm caused increased N-
demethylase activities, which was indicative of enzyme induction, this
dose was tolerated without having any other toxic effects. However,
dogs fed a dietary level of 2 400 ppm were affected in several other
respects (e.g., vomiting and reduction in food consumption); hence,
they had a moderate state of nutrition and showed less weight gain
than the other groups. The parameters of the red blood picture were
adversely affected by the treatment. Alkaline phosphatase and
transaminase activities were increased, illustrating a disturbed liver
function. The relative liver weight showed a slight increase in both
sexes in the 2 400 ppm group as compared with the controls. However,
gross pathology and histopathology did not reveal any treatment-
related alterations in the liver or in any other tissues. In the
13-week feeding experiment, the no-effect dietary level for the dog
was 150 ppm (Hoffman and Luckhaus 1974).
In a chronic toxicity study, groups of 4 male and 4 female beagle
dogs were maintained for 104 weeks on a diet containing triadimefon at
concentrations of 0 ppm (control), 100 ppm, 330 ppm, 1 000 ppm (up to
week 54) and 2 000 ppm (from week 55), respectively, of a technical
grade of two batches, purity 88 to 89% and 92 to 97% (90% premix
prepared with highly dispersed silicate). Clinical examinations,
haematological tests, clinical chemical tests and urinalyses were
performed on the dogs. On termination of dietary administration, the
dogs were sacrificed, dissected and grossly examined; their organ
weights were measured and tissues were histopathologically examined.
The results of the study showed that triadimefon dietary
concentrations of up to and including 330 ppm were tolerated for 2
years by both males and females without having any toxic effect. In
the highest dietary concentration group (especially after the dietary
level was raised to 2 000 ppm in week 55) the weight of some dogs of
both sexes was depressed slightly. Clinical chemistry and
histopathology did not reveal any liver damage; however, there were
signs of a treatment-related microsomal enzyme induction at the
highest dietary level. None of the other findings showed any
differences between the treated dogs and the controls (Hoffman and
Gröning 1978).
Long-term studies
Rat
In a 24-month study to evaluate triadimefon for potential chronic
toxicity and carcinogenicity, groups of 50 male and 50 female Wistar
rats were fed dietary levels of 50, 500 and 5 000 ppm, respectively of
a technical grade, composed of 2 batches, one of 88-89%, and the other
of 92-97% (90% premix prepared with a highly dispersed silicate). The
control group, fed only chow, consisted of 100 male and 100 female
rats. For clinical laboratory tests (haematology, clinical chemistry,
urinalysis, enzyme induction assays), 10 rats were additionally used
per group and per sex. After 24 months on the test diet, all survivors
were sacrificed, dissected and grossly examined.
The study revealed no indication of triadimefon having a
carcinogenic effect on rats. With respect to chronic toxicity, the
no-effect dose was found to be 50 ppm for females and 500 ppm for
males. Higher dietary levels had the following effects in the two
sexes. The dietary concentration of 5 000 ppm caused violent motor
reactions in males and females from about week 23. These rats consumed
almost no food while the reactions persisted and, as a result, they
lost a considerable amount of weight and many died. Those that
survived began to consume feed again when the symptoms receded, and
made corresponding weight gains. However, in week 32 and, following
brief improvement, in week 37, these violent motor reactions again
occurred accompanied by refusal of food and considerable loss of
weight. The last of the surviving rats in the 5 000 ppm group were
killed in a moribund condition in week 39. Gross pathology performed
on the 5 000 ppm rats that died and on those sacrificed essentially
disclosed severe mucosal lesions in the stomach, which in most cases
contained no food, accompanied by sometimes severe haemorrhages. These
lesions were considered to have been the cause of the clinical
symptoms observed. From week 85, the body weights of the male rats in
the 500 ppm group were mostly significantly lower than those of the
controls, but the difference was so slight that it was not considered
to have been due to a damaging effect from the test compound. The
growth rate of most of the female rats in the 500 ppm group was
significantly retarded from as early as week 6.
Significantly reduced erythrocyte counts and haemoglobin levels
seen at the dietary levels of 5 000 ppm, and at the end of the study
also in females fed 500 ppm, were indicative of an effect on the blood
or blood-forming tissues. The histopathological examinations revealed
shrunken spleen with reduced haematopoiesis in some of the rats of the
5 000 ppm group. Some of the rats of this group also showed decrease
in haematopoiesis in the marrow. The histopathological examinations
revealed signs of damage to cells of the proximal tubules of the
kidney (vacuolation, desquamation, degeneration) in 5000 ppm rats. In
the lung, increased blood content was seen in the dilated alveolar
vessels. In the stomach of 5 000 ppm rats, increased superficial
ulcerations, haemorrhage of the mucosa and oedemas of the sub-mucosa
were observed. In the adrenals of some rats, congestion of blood
vessels was seen. The dietary level of 5 000 ppm also caused formation
of giant spermatids in some males. In rats fed 5 000 ppm, cholesterol
levels occasionally showed a significant increase, indicative also of
an effect on fat metabolism at this dietary level. The liver weights
showed a statistically significant (p <0.01) and dose-related
increase in the male rats of the 50 and 500 ppm groups and in the
female rats of the 500 ppm group. (On highest dose, 5 000 ppm, all
animals were dead at week 39.)
The statistically significant increased liver weight (p <0.01)
seen in 50 and 500 ppm males and in 500 ppm females was not due to
stimulation of microsomal enzymes, as the N-demethylase activity and
the cytochrome P-450 concentrations measured in the liver at the end
of the experiment were not affected. Thus, the increased liver weights
were considered to have been a non-specific effect of increased
metabolism (Bomhard and Löser 1978a).
Mouse
Triadimefon was evaluated for long-term effects and potential
carcinogenicity in a 24-month study on groups of 50 male and 50
female CF1/W 74 mice fed dietary levels of 0 (control), 50, 300 and
1 800 ppm, respectively, of a technical grade, purity 97% (used as an
approximately 90% premix prepared with a highly disperse silicate).
For clinical laboratory tests, 10 mice were additionally used per
group and per sex. The mice were inspected for clinical symptoms.
Their body weight and food intake were measured. Haematological tests,
clinical chemical tests, organ weight measurements, macroscopic tissue
examinations and comprehensive histological examinations were
performed.
The study revealed no indication of triadimefon having a
carcinogenic effect on mice. No chronic-toxic effects were seen at the
two lower dietary levels of 50 and 300 ppm. Mice fed 1 800 ppm showed
the following alterations, in comparison with the controls. Growth of
both male and female mice was affected. Significantly increased
erythrocyte counts were recorded in both sexes, and increased
haemoglobin and haematocrit values were observed in females. Other
treatment-related alterations seen on termination of the study,
although not present consistently at the intermediate examinations and
necropsies performed after 6 and 12 months, included: distinctly
increased liver weights; increased AP, GOT and GPT activities;
increased incidence of liver swellings, occasionally accompanied by
adhesions of liver lobes in mice that died and in those sacrificed on
termination of the study. At the end of the feeding experiment, a
marked correlation between hyperplastic liver nodules and greatly
increased enzyme activities, indicative of liver damage, was observed
in the 1 800 ppm group. The incidence of liver cell tumours in male
and female mice were as follows:
ppm F M
0 0 2
50 1 2
300 0 1
1800 2 2
An increased incidence of hyperplastic liver nodules, which are
histologically very difficult to differentiate from liver cell tumour,
were also observed with the following incidence in male and female
mice:
ppm F M
0 4 7
50 2 7
300 1 7
1800 15 15
(Bombard and Löser 1980b)
Special studies on reproduction
Rat
Triadimefon was evaluated for its effect on fertility, lactation
performance and development of offspring in a 3-generation
reproduction study in Wistar rats with two matings per generation
(Löser 1979). Each test group consisted of 10 male and 20 female rats
and triadimefon was fed to the rats at dietary concentrations of 0
(control), 50, 300 and 1 800 ppm respectively. At the start of the
study, the F0 generation was ca. 32 to 39 days old and was treated
for 70 days prior to the first mating.
The pups of the F3b generation were sacrificed and
necropsied at an age of 4 weeks, and the major 18 tissues were then
histopathologically examined. Dietary levels of 50 and 300 ppm had no
adverse effect on the reproductive performance of the rats. After 2 of
6 matings, the weight of the pups in the 300 ppm group were
significantly lower than those of the controls during the lactation
period. The dietary level of 1 800 ppm distinctly affected
reproductive performance, as none of the rats became pregnant after
the second mating of the F1b generation. As this dietary level also
distinctly affected the parental rats, evidenced by the growth rate,
there would be no evidence for assuming any primary damaging effect on
reproduction.
Triadimefon did not cause any gross abnormalities or
malformations in rats at the tested dietary concentrations. The
histopathological examinations of the 18 most important tissues from
4-week old pups of the F3b generation revealed no indication of any
tissue alterations due to dietary administration of triadimefon at the
tested concentrations. The dietary concentration of 50 ppm was
tolerated without having any damaging effect whatsoever in the
3-generation reproduction study (Löser 1979).
Hen
Groups of 10 female White Leghorn hens per concentration, which
were mated with one cock per group, were maintained for 4 weeks on a
diet containing triadimefon at concentrations of 0 (control), 10 and
100 ppm, respectively. The hens were inspected for behavioural
patterns, physical appearance and appetite and their body weights and
food intake were measured. Tissues were macroscopically examined and
the 12 most important tissues were histopathologically examined.
Haematological tests were also performed on additional hens maintained
under comparable conditions. The following parameters were measured in
the offspring: number of eggs, egg quality, egg weight, egg size,
thickness of egg shell, weight of egg shell, fertilization rates,
hatch rates. The chicks were inspected for their physical appearance
and behavioural reactions during the first days of their life. The
tested concentrations were tolerated by both the hens and their
offspring without causing any damage whatsoever (Thyssen and Gröning
1978).
Species studies on embryotoxic effects
Rat
Two experiments were performed with groups of 20 pregnant Long
Evans rats dosed daily by gavage with triadimefon in an aqueous
emulsion from gestation day 6 to 15. The first study utilized daily
doses of 10, 30 and 100 mg per kg bw. Each experiment included a
control group dosed only with the vehicle. While the dose of
10 mg/kg/day did not have any harmful effects on the dams, doses of
30 mg/kg/day and above reduced dam weight gain. However, none of the
tested doses caused any behavioural disorders or mortalities. Effects
on progeny were characterized by a slightly increased placenta weight
in the 100 mg/kg group as compared with the controls, and by the
occurrence of occasional cleft palates at 75 mg/kg and 100 mg/kg/day.
Thus, a treatment-related effect and hence a slight teratogenic effect
of triadimefon on rats at oral doses of 75 mg/kg/day and above could
not be completely ruled out. The oral no-effect of triadimefon with
respect to embryonic/foetal development was found to be 50 mg/kg/day
(Machemer 1976b).
Evaluation of triadimefon for embryotoxic effects was also
performed by administering the compound by the route of greatest
relevance to practical conditions, viz. by inhalation. (A hazard from
skin exposure can be ruled out on the ground of the acute toxicity
findings, which did not reveal any toxicologically relevant absorption
through the skin.) For the inhalation evaluation, twenty fertilized
rats per group were exposed for 6 h daily on 10 consecutive days
from gestation day 6 to 15, to analytically measured triadimefon
concentrations of 14 (11 to 18) mg/m3, 33 (28 to 37) mg/m3 and 114
(95 to 132) mg/m3 air in a dynamic flow inhalation apparatus.
Triadimefon was dissolved in a 1:1 mixture of ethanol and polyethylene
glycol 400 and aerosolized in the inhalation chambers. A control group
inhaled the aerosol of the solvent mixture only. While the contration
of 14 mg/m3 air did not affect the dams, concentrations of 33 and
114 mg/m3 slightly reduced dam weight gain during the treatment
period. None of the concentrations adversely affected physical
appearance and behavioural patterns of the dams. Embryonic and foetal
development were not affected by concentrations of up to and including
114 mg/m3. Hence, it was concluded that inhaled triadimefon did not
result in any embryotoxic effects (Machemer and Kimmerle 1976).
Rabbit
Groups of 10 to 13 pregnant Himalayan rabbits received daily oral
doses of triadimefon administered as an aqueous emulsion by gavage at
levels of 0 (control), 5, 15 and 50 mg/kg bw, respectively, from
gestation day 6 to 18. The tested doses had no adverse effects on
physical appearance, behavioural patterns and weight gains of the dams
or on embryonic and foetal development. Oral doses of triadimefon did
not result in toxic or teratogenic effects on rabbits (Machemer
1976c).
Special studies on mutagenic effects
Triadimefon was evaluated for mutagenic potential by the Ames
test on histidine requiring Salmonella typhimurium strains TA 98,
100, 1535, 1537, 1538 and 1950, with and without metabolic activation.
The triadimefon concentrations ranged from 0.1 to 1 000 µg per plate.
The minimal inhibitory concentration of triadimefon on the tester
strains used was 500 µg/ml. Triadimefon did not induce back-mutations
in the test systems, with or without microsome activation, over the
tested concentration range (van Dijck 1976).
Triadimefon was tested for potential DNA-attacking action on
Bacillus subtilis at concentrations of up to 300 µg per plate, by
rec-assay. The compound was also evaluated at concentrations of up to
1 000 µg/plate for potential mutagenicity by the Ames assay using
Salmonella typhimurium strains TA 1535, 1537, 98 and 100, with and
without addition of rat and mouse liver homogenates to the agar. In
these studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Inukai and Iyatomi 1977).
The fungicide was further tested for potential mutagenic effects
by the rec-assay on two Bacillus subtilis strains at concentrations
of up to 2 000 µg/plate, and by the Ames (reversion) assay on the
Salmonella typhimurium strains TA 98 and 100 at concentrations of up
to 5 000 µg/plate, with and without metabolic activation. In these
studies, triadimefon had neither a DNA-attacking action nor a
mutagenic effect (Shirasu et al 1978).
Triadimefon was additionally studied by rec-assay on two
Bacillus subtilis strains at concentrations of up to and including
2 000 µg per plate and by reversion assay on Escherichia coli (WP 2)
and on Salmonella typhimurium strains TA 1535, 1537, 1538, 98 and
100, at concentrations of up to and including 5 000 µg per plate, with
and without metabolic activation by rat liver chromosomes. No
indication of mutagenic activity was found in these studies (Shirasu
et al 1979).
Triadimefon was evaluated for point-mutagenic effects by a
eukaryotic, micro-organism test using Saccharomyces cerevisiae
S 138 and S 211, in comparison with negative (solvent) and positive
controls, with and without metabolic activation by microsomal enzymes
of Aroclor-induced rat livers. Neither a mutagenic nor a
recombinogenic effect was seen at doses extending into the toxic
range, 1 000 µg/plate (Jagannath et al 1980).
A micronucleus test on male and female NMRI mice was performed
with triadimefon. Triadimefon was administered in an aqueous emulsion
at a total oral dose of 400 mg/kg bw applied in two split doses of
200 mg/kg bw each at an interval of 24 h. The thiotepa dose was
2 × 10 mg/kg injected subcutaneously. For comparison, a negative
control group was treated with the vehicle and a positive control
group was treated with thiotepa. Each group consisted of 10 mice;
1 000 polychromatic erythrocytes per mouse were analysed for incidence
of micronuclei. Additionally, the ratio of polychromatic erythrocytes
to normochromatic ones was determined to establish whether the test
compound had any effect on the general activity of bone marrow.
Thiotepa caused a big increase in the number of micronuclei and also
bone marrow depression. Triadimefon did not affect general bone
marrow activity nor did it increase the incidence of micronuclei in
comparison with the control, and hence was deemed to be non-mutagenic
in this system (Machemer 1977).
Twenty male NMRI mice each received a single oral dose of
triadimefon at a level of 200 mg/kg bw, administered as an aqueous
emulsion by gavage. Starting on the day of test compound
administration, a series of 8 matings was performed with the treated
males, each mating period being of 1-week duration; every week, each
male was caged together with 3 fresh untreated virgin females. The
pre-implantation and post-implantation losses of fertilized females
were determined as the criteria for assessment of a mutagenic effect.
(The corpora lutea, total implantations, viable implants and dead
implants were counted.) The results were compared with those obtained
from a control group of similar size. The tested dose was not toxic to
the males and did not affect their fertility. The measured parameters
did not reveal any treatment-related difference between the control
group and the triadimefon-treated group. The dominant lethal test on
the male mouse did not provide any indication of the mutagenicity of
triadimefon at the acute oral dose of 200 mg/kg bw (Machemer 1976a).
Special studies on induction of liver enzymes
Triadimefon was administered daily on 28 consecutive days to
Wistar rats, by gavage in a mixture of acetone and oil at dose levels
of 0 (vehicle), 1, 5 and 25 mg/kg bw, respectively. Each group
consisted of 20 male and 20 female rats; half of them were sacrificed
at the end of the treatment period and the other half were killed
on termination of a 4-week post-treatment observation period.
N-demethylase, O-demethylase and cytochrome P-450 in the rat livers
were measured at the end of the treatment period and on termination of
the post-treatment observation period. The rats were observed for
systemic toxic effects by observing their physical appearance,
behavioural patterns and posture, and their body weights and certain
haematological and clinical chemical parameters were measured. The
rats were necropsied, tissues were macroscopically examined and livers
were weighed. The study revealed that triadimefon at daily doses of 5
and 25 mg/kg bw caused slight induction of microsomal liver enzymes.
The enzyme induction was reversible within the 4-week post-treatment
observation period. None of the other parameters measured and
investigated provided any indication of treatment-related damage
(Mihail and Kaliner 1979).
Special studies on potentiation toxicity
Since triadimefon is used in combination with other fungicidal
compounds, acute combination toxicity studies were performed on Wistar
rats. The compounds tested in combination with triadimefon were
captafol, propineb, tolylfluanid and carbendazim. The oral LD50 of
each of these compounds was first determined. Fifteen female or male
rats were used per dose and the observation period lasted 14 days.
From the LD50 values of the single components the theoretically
expected LD50 of each combination was calculated. For combined
administration, the components were mixed in a percentage ratio
proportional to the LD50 of each and thus applied in equitoxic doses;
thereby, the actual LD50 of the combination was determined. A more
than additive acute toxic effect was not seen for any of the tested
combinations (Flucke and Kimmerle 1977, Thyssen 1977).
OBSERVATIONS IN MAN
Approximately 50 mg of triadimefon was applied to small gauze
pads which were placed for a contact time of 24 h on the skin of the
forearm of each of 8 test persons and the pads were fixed in position
with adhesive strips. Upon removal of the strips and pads, slight
redness of the treated skin area was seen for a brief period in 5 of
the 8 persons. At 24 h post-application, the treated skin areas showed
no variations from the physiological norm in any of the test persons
(Thyssen and Kimmerle 1973).
RESIDUES IN FOOD
USE PATTERN
Triadimefon is effective and is mainly used against powdery
mildews and rusts; it also gives good control of a number of other
diseases e.g. Thielaviopsis on pineapple (post-harvest application).
Triadimefon is registered and marketed in more than 30 countries
in Europe, Africa, America, Asia and Australia; registration is being
sought also in other countries. Details on the uses of triadimefon and
recommendations are given in Table 2. With the exception of the post-
harvest dip of pineapple the product is used as pre-harvest
application. Triadimefon is used also on ornamentals (roses, azaleas),
seed-grass, turf and rubber trees. Sugarcane and sweet potato sets are
dipped in a triadimefon suspension of emulsion prior to planting.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Data on triadimefon residues from supervised residue trials were
obtained on various fruits and vegetables, cereal grains and some
other crops grown under a variety of geographical, climatic conditions
and agricultural practices. All residue figures presented in Table 3
refer to the sum of triadimefon parent compound and its main
metabolite triadimenol.
Fruits and vegetables
The average residue at harvest following the recommended
pre-harvest intervals is in the range of non-detectable (0.01 to
0.05 mg/kg) up to 0.3 mg/kg (maximum residue of 0.4 mg/kg) on most
fruits and vegetables. On red currants somewhat higher residues are
found after the recommended pre-harvest intervals; the average level
at harvest was 0.4 mg/kg (maximum residue in the trials 1.1 mg/kg).
Higher residues were also found on Brussels sprouts (average 0.5,
highest level found 0.9 mg/kg) in trials where the normal pre-harvest
interval was taken into account.
FATE OF RESIDUES
In plants
The behaviour and fate of triadimefon in plants was evaluated.
Studies were carried out on metabolism of (benzene ring-UL-14C)- and
(triazole ring-3,5-14C)-triadimefon in cereals, apple, cucumber and
tomato; translocation of unlabelled (benzene ring-UL-14C)-, and
(triazole ring-3,5-14C)-triadimefon, with the studies being directed
towards elucidation of site of action, mechanism of action and
metabolism; redistribution in the vapour phase after application of
unlabelled and (benzene ring-UL-14C)-triadimefon to plants.
TABLE 2. Summary of recommended uses of triadimefon
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Fruits
Apple and pear wp 40 - 75 4 - 12 7
Jap. apple wp 150 -200 2 - 3
Jap. pear wp 150 -200 2 - 3
Apricot wp 50 -150 4 - 6 7
Peach wp 50 -150 4 - 6 7
Currant (black,red) wp 50 -150 3 - 4 7
Gooseberry wp 50 -100 3 - 4 7
Grape wp/EC/dust 50-75 (dust 100-200) wingrape 4-10 14-28
tablegrape 4-8 4- 7
Strawberry wp 75 -100 7
Mango wp 100 -200 28
Pineapple wp/EC 0.015-0.07% 1(postharvest
dip) -
Vegetables
Artichoke wp/EC 50 -150 4 - 8 3 - 7
Beans wp/EC 125 -250 3 - 6 7 -14
Pea wp/EC 50 -125 3 - 6 7 -14
Brussels sprout wp/EC 125 -250 3 - 6 14
Onion (Welsh) wp 100 3 10
Cucurbits wp/EC/dust 25-100 (dust100-200) 4 - 10 3 - 7
Pepper wp/EC 50 -150 4 - 8 3 - 7
Tomato wp/EC 50- 150 4 - 8 3 - 7
Grain crops wp/EC 125 -250 1 - 2 21 -35
Sugarbeet wp/EC 125 2 - 3 14
TABLE 2. (con't)
Applications Recommended
Crop or Formulation Rate pre-harvest
Commodity (a.i./ha) No. intervals (days)
Coffee
protective wp/EC 100 -250 8 - 12
eradicative wp/EC 250 -500 2 - 4
Hops 200 -500 2 - 6
Tobacco 40 - 75 4 - 6
TABLE 3. Residues of triadimefon/triadimenol from supervised trials
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3 7 14
Fruit
Apple USA 1977 6 20 - 60 wp 50% 0.3 0.2 0.4 0.2 Mobay 1981
1977 6 20 - 60 wp 50% 0.5 0.4 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.2 0.3 0.2 "
1977 6 20 - 60 wp 50% 0.3 0.3 0.3 0.4 "
1977 6 340 -560 wp 50% 0.5 0.4 0.3 0.2 "
1978 6 20 - 60 wp 50% 0.3 0.2 0.09 0.08 "
1977 11 340 -560 wp 50% 0.4 0.2 0.1 0.1 "
1977 10 340 -560 wp 50% 0.2 0.1
1977 10 340 -560 wp 50% 0.4 0.3 0.2 0.1 "
1977 10 340 -560 wp 50% 0.4 0.2 "
1977 10 340 -560 wp 50% 0.2 0.1 0.1 0.09 "
1979 6 140 wp 50% 0.7 0.4 "
Pear 1980 6 600 wp 50% 0.6 0.3 0.5 0.3 "
1980 6 600 wp 50% 0.4 0.4 0.3 0.2 "
1980 6 600 wp 50% 0.4 0.5 0.4 0.3 "
1980 10 450 wp 50% 0.3 0.2 0.09 0.06 "
1980 15 12001 wp 50% 0.8 0.5 0.3 0.2 "
Peach 1980 4 12001 wp 50% 3.0 1.6 0.7 "
1980 3 12001 wp 50% 0.1 0.4 0.1 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 3 6/7 14 21
Berry fruits
Currant- Denmark 1979 4 50 wp 5% 0.03 <0.05 <0.02 <0.05 <0.02 <0.05 Bayer 1981
black 1979 4 50 wp 5% 0.06 0.05 0.03 <0.05 <0.02 <0.05 "
France 1979 4 100 wp 5% 0.03 0.2 0.04 0.2 "
1977 4 40 wp 1.25% 0.04 0.14 "
Currant-red Denmark 1979 2 50 wp 5% 0.02 <0.05 <0.02 <0.05 <0.02 <0.05 "
1979 2 50 wp 5% 0.15 <0.05 0.05 <0.05 <0.02 <0.05 "
Raspberry 1979 4 75 wp 5% 0.03 0.05 Bayer 1981
Grape Japan 1979 3 150 wp 5% 0.01 0.4 <0.01 0.2 "
1979 5 150 wp 5% 0.02 0.5 0.01 0.4 "
1979 3 150 wp 5% 0.01 0.4 <0.01 0.3 "
1979 5 150 wp 5% 0.02 0.7 0.01 0.4
USA 1978 3 90 wp 50% 0.06 0.06 0.08 0.2 Mobay 1981
1978 3 110 wp 50% 0.02 0.05 <0.01 <0.01 "
1978 3 110 wp 50% 0.05 0.02 <0.01 <0.01 "
1978 3 150 wp 50% <0.01 0.01 "
1978 3 150 wp 50% 0.06 0.04 <0.01 <0.01 "
1978 3 150 wp 50% 0.03 0.09 <0.01 <0.01 "
1979 3 225 wp 50% <0.01 0.07 "
1979 3 225 wp 50% 0.5 0.4 0.01 0.02 "
1979 3 300 wp 50% 0.2 0.08 "
1979 3 225 wp 50% <0.01 <0.02 "
TABLE 3. (con't)
Application Residues (mg/kg) at intervals (days)
Rate after harvest
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 0/1 3/5 7 14/15
Fruiting vegetables
Cantaloupes USA 1979 3 130 wp 50% 0.1 0.08 "
1979 3 130 wp 50% 0.1 0.03 "
1979 3 130 wp 50% 0.06 0.1 "
1979 3 130 wp 50% 0.03 0.03 "
Gherkins2 Fed.Rep. 1976 8 60 wp 5% <0.06 <0.06 <0.06 Bayer 1981
of 1976 8 60 wp 5% <0.01 <0.01 <0.06 "
Germany 1975 8 60 wp 5% 0.06 <0.06 <0.01 <0.06 "
1975 8 60 wp 5% 0.06 0.06 <0.06 <0.06 "
Cucumber Mexico 1979 3 125 wp 50% <0.0 <0.01 <0.01 <0.01 Mobay 1981
1979 3 125 wp 50% 0.04 0.05 <0.01 <0.01 "
Tomato Mexico 1979 8 180 wp 25% 0.03 0.05 0.01 0.03 0.01 0.03 "
1979 8 180 wp 25% 0.04 0.05 0.01 0.02 <0.01 0.02 "
1979 8 180 wp 25% 0.04 0.08 0.02 0.04 0.02 0.03 "
1979 8 180 wp 25% 0.07 0.08 0.02 0.06 <0.01 0.01 "
Japan 1977 4 250 wp 25% 0.07 0.08 0.04 0.08 0.022 <0.063 Nitokino
7 250 wp 25% 0.2 0.3 0.09 0.2 0.052 0.23 1981
2 250 wp 25% 0.4 0.2 0.3 0.3 0.22 0.33
4 250 wp 25% 0.4 0.5 0.3 0.6 0.12 0.53
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Other vegetables
Brussels
sprouts UK 1979 3 250 wp 25% <0.05 <0.05 Hazleton 1981
1977 3 125 wp 25% 0.6 0.2 BAY/UK 1981
Chick peas 1980 2 150 wp 25% 0.01 0.02 <0.01 <0.01 <0.01 0.02 Mobay
dry 2 150 wp 25% <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 1981
2 150 wp 25% <0.01 <0.02 <0.01 <0.01 <0.01 <0.01
Leeks UK 1979 4 250 wp 25% <0.05 <0.05 Hazleton 1981
500 wp 25% <0.05 0.08
250 wp 25% 0.09 0.4
500 wp 25% 0.3 0.9
Parsnips UK 1979 3 250 wp 25% 0.05 0.2
3 250 wp 25% 0.01 0.05
Hops UK 1979 8 110-220 wp 5% <0.3 0.8 Bayer 1981
fresh
dry
UK 1979 8 100-200 wp 5% 0.4 1.4
fresh <0.3 1.4 ibid
dry 0.4 1.4
TABLE 3. (con't)
Application Residues of triadimefon/triadimenol (mg/kg) at
Rate intervals(days)after harvest(1 figure = sum of both)
Crop Country Year No. g a.i./ha Formulation Reference
(g/100 L) 7 12/14 20/21
Fed. Rep.
of
Germany 1979 4 500 wp 5% ibid
fresh 0.9 0.6 2.13 0.83
dry 10.8 4.2 7.23 4.13
Fed.Rep.
of
Germany 1979 4 500 ibid
fresh 2.1 1.0 0.73 0.63
dry 11.4 4.3 1.93 1.73
1 Excessive dosage;
2 Some of the gherkins data were erroneously presented in the 1979 JMPR Evaluations under
cucumbers due to a translation error. Schlangen qurke = cucumber; Einlegequrke = gherkin;
3 After 10 days.
The metabolism of triadimefon (Bayleton(R)) in cereals was
studied in several experiments following application of (benzene ring-
UL-14C)-triadimefon to plants under glass and in field conditions.
The main metabolite formed was triadimenol, which occurs in the
diastereoisomeric forms A and B. On cereal plants, the A form is the
main diastereoisomer. A further metabolism pathway consisted of
oxidation of the tert. butyl group (KWG 1342) and cleavage of the
molecule, with the formation of 4-chlorophenol. The metabolites
triadimenol, and particularly KWG 1342 and 4-chlorophenol, are bound
in the form of glycosides-conjugates, mainly B glycosides. At harvest
the radioactivity still present in straw was distributed as shown in
Table 4.
TABLE 4. Radioactive studies on triadimefon metabolism in cereal
straw at harvest
Metabolite Radioactivity present (%)
Triadimefon unchanged parent 0 - 5
Triadimenol form A 22 - 41
Triadimenol form B 6 - 17
KWG 1342 2 - 16
Polar metabolites1 17 - 37
BUE 2285 less than 1
Free 4-Chlorophenol less than 1
Non-extractable 18 - 32
1 Including the glycosides of triadimenol, KWI 1342 and
4-chlorophenol.
The glycosides are probably fixed in the plants by further
metabolism and are therefore no longer extractable with standard
procedures. The distribution of the radioactivity among the aqueous
and organic phases after extraction of the straw differs between the
(triazole ring-3,5-14C) and the (benzene ring-UL-14C). An explanation
for this may be the different polarities of 4-chlorophenol and
1,2,3-triazole, which can be formed as metabolites of triadimefon
and/or triadimenol. Further studies are underway to elucidate this
phenomena.
In another experiment on summer barley, the non-extractable part
of the residue accounted for higher amounts, i.e. 55-75% (Bayer 1981).
This may be partly due to the application at an earlier stage in plant
development. Following spray application of (triazole ring-3,5-14C)-
triadimefon the metabolites that occurred were the same as those found
following the application of (benzene ring-UL-14C)-triadimefon, the
only difference being that polar metabolites accounted for a higher
proportion when both labels were applied at the same stage in plant
development, i.e. 31% vs 20% (Bayer 1981). For quantitative data
see Table 5. After meed treatment of barley with the pure
diastereoisomeric forms A and B of triadimenol, no conversion of one
form to the other was found in the barley plant (Bayer 1981).
In studies on apple with benzene ring-labelled and triazole-
labelled triadimefon, it was demonstrated that also in this crop
triadimefon was metabolized to triadimenol and in a small amount to an
unidentified compound (Table 6). The rapid decline of the 14C during
the early intervals up to 7 days was attributed to the volatility of
triadimefon parent compound (Mobay 1981). In trials on cucumber and
tomatoes, the fate of (benzene-ring-UL-14C)-triadimefon was studied.
The material was applied as a foliar spray or to the roots. It was
found that in addition to triadimefon and diastereoisomers A and B, a
polar metabolite was formed in the plants, which was shown to be a
glucoside of triadimenol loosely bound to another natural occurring
moiety. It is readily converted to triadimenol glucoside (see Table 6)
(Mobay 1981). It was also shown in these experiments that the
radiocarbon applied to the foliage did not translocate significantly
into neighbouring fruits, in marrow plants triadimenol was identified
as the major metabolite of triadimefon (Clark et al 1978). The
metabolic pathways of triadimefon are illustrated in Figure 2. For
chemical names of the metabolites identified in plants and their
structural formula see Table 9, FAO/WHO 1979, Triadimefon.
Triadimefon was translocated at a relatively fast rate in
anacropetal direction following foliar application to cereals, beans,
cucumber and tomato (Buchenauer 1975, 1976; Bayer 1981; Scheinpflug
et al 1977; Brandes et al 1978; Gasztonyi and Joslpovits 1978) and
also to a larger extent following soil applications to beans and
barley (Buchenauer 1975, 1976). Some authors also observed a slight
basipetal translocation. Gasztonyi and Josepovits (1978) established
that the rate of uptake and subsequently the amount of transport
depended substantially on the mode of leaf treatment, and also found
differences in the amounts of translocated triadimefon according to
plant species.
Gasztonyi and Josepovits (1979) studied the uptake and metabolism
of triadimefon by mycelia of fungi that were sensitive or resistant to
triadimefon. In the fungi, triadimefon accumulated to 20 to 40 fold of
the external concentration, irrespective of the sensitivity of the
fungus. In the course of metabolism, the highly fungitoxic main
metabolite triadimenol was formed; however, in mycelia of sensitive
fungi this transformation was at a high rate, whereas it could not be
demonstrated, or only to a low extent, in resistant strains of fungi.
Based on these observations, triadimefon must be regarded as the
precursor of the active principle, i.e. triadimenol.
 TABLE 5. Percent distribution of radioactivity in cereals after spray application of (14C)-triadimefon (recovered radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(G) benzene ring-
total plant UL-14C 0 n.d.2 n.d. n.d. n.d. 99.5 n.d. 0.5
" " 250g/ha 5 7.3 n.d. 57.3 21.8 9.7 n.d. 3.9
" " 10 15.8 n.d. 50.3 18.9 7.9 n.d. 7.1
" " 28 15.8 n.d. 45.1 17.4 6.4 n.d. 15.3
straw 38 15.9 n.d. 43.6 14.4 10.3 n.d. 15.8
" 62 16.7 1.9 41.0 17.3 4.8 n.d. 18.3
Spring barley(F) benzene ring-
total plant UL-14C 0 <1 n.d. 1 n.d. 92 n.d. 6
" " 125 g/ha 5 13 n.d. 48 15 12 n.d. 12
" " 17 35 n.d. 32 10 <1 n.d. 23
straw 28 9 <1 12 4 n.d. n.d. 75
" 76 8.4 5 8.4 3 n.d. n.d. 75
Spring wheat(F) benzene ring-
straw UL-14C 49 37 2 23 6 n.d. n.d. 32
TABLE 5. (con't)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(F) triazole-ring-
straw 3,4-14C 71 31 8 20 10 1 n.d. 29
250 g/ha
benzene ring-
UL-14C 71 20 16 22 9 1 3 28
250 g/ha
1 G = greenhouse, F = field;
2 n.d. = not detectable. In these studies the amount of radioactivity in the grains
ears was so small that separation by TLC was impossible.
TABLE 6. Percent distribution of radioactivity in apple, tomato and cucumber after spray application of (14C) triadimefon (recovered
radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post aqueous polar Triadimenol Triadimenol Triadimefon Unidentified Non-
appl. phase metabolite3 A B metabolites extractables
Apple (F) triazole ring-
peel 3,5-14C 28 5.7 n.d.2 14.9 13.6 10.8 2.8 5.0
pulp 15 mg/100 ml 5.3 n.d. 11.2 7.1 2.1 3.1 0.9
total fruit 11.0 n.d. 26.1 20.7 13.0 5.9 5.9
peel 48 4.8 n.d. 14.5 14.8 10.4 1.5 6.0
pulp 6.4 n.d. 10.4 9.4 2.1 2.1 1.0
total fruit 11.2 n.d. 24.9 24.2 12.5 3.6 7.0
Tomato (G) benzene ring-
foliage UL-14C 7 7 4 16 3 66 n.d. 3-4
e mg/plant 14 4 5 14 2 71 n.d. 4
(3 treatments) 21 2 22 19 5 44 n.d. 8
fruit 7 6 n.d. 8 14 69 n.d. 2-3
14 15 n.d. 10 18-21 49-52 n.d. 2
Cucumber (G)
foliage 7 7 4 11-12 10 60-61 n.d. 6
14 6 4 16-19 9-10 48-54 n.d. 7
21 7 20 17 12 36 n.d. 8
fruit 7 5 n.d. 8 33 51 n.d. 3
14 11 n.d. 8 32-33 41-43 n.d. 5
1 G = greenhouse, F = field;
2 n.d. = not detectable;
3 glucoside of triadimenol.
In studies following field-scale spraying of spring barley and
spring wheat with (benzene ring-UL-14C)-triadimefon 1.9 to 14.9% and
0.1% of the applied radioactivity was found in the straw and the
kernel respectively of the treated cereals, equivalent to 0.33 to
7.39 mg/kg and 0.01 to 0.04 mg/kg respectively (Bayer 1981). Following
spray treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon, on the other hand, 6.5% and 0.7% of applied radioactivity
was found in straw and grain respectively, equivalent to 3.39 and
0.39 mg/kg of parent triadimefon. Following spray applications of
(benzene ring-UL-14C)-triadimefon in the same trial, the apparent
triadimefon equivalents were 2.92 and 0.16 mg/kg in straw and grain
respectively (Bayer 1981).
In a study, carried out in a closed glass chamber with (benzene
ring-UL-14C)-triadimefon on barley plants, it was observed that the
applied compound moved from treated to un-treated plants, where it was
metabolized in the usual manner (Bayer 1981).
In glasshouse experiments triadimefon displayed vapour phase
activity against powdery mildew on cucumber and barley plants, not
only when it was applied at normal application rates, but also at
substantially lower ones. The effect was dependent on the distance of
the spray deposit (Scheinpflug and Paul 1977; Scheinpflug et al
1978; Schlütter 1977; Bayer 1981). In experiments on apple seedlings
and marrow plants, triadimefon was shown to display vapour phase
activity against powdery mildew (Clark et al 1978).
In animals
Several new data have become available on the fate of triadimefon
in animals. Data on excretion, storage and metabolism are now
available from studies in rats, dairy cattle, pigs and laying hens.
Accumulation of residues was also studied in the Channel catfish
exposed to labelled triadimefon. Feeding experiments were also carried
out on livestock animals, e.g. dairy cattle, pigs, sheep and hens.
Supplementary studies with the main animal metabolite of triadimefon,
triadimenol, were carried out in rats. Radiolabelled triazole was
given to rats in order to obtain data on the fate of this triadimefon
metabolite. The studies on rats are reported under "Biochemical
Aspects".
Cow
Feeding studies were carried out with 1:1 mixture of triadimefon
and triadimenol in dairy cattle. Nine dairy cows (three animals per
dosage) were fed with the mixture by bolus capsule with dosages
equal to 25, 75 and 250 mg/kg bw. The fat contained residues up to
0.029 mg/kg at the 250 mg/kg level and 0.016 mg/kg at the 75 mg/kg
dose level, whereas the residue level in the fat was less than
0.01 mg/kg at the 25 mg/kg feeding level (Mobay 1981). One animal
exposed to the high dose level showed milk residues of 0.0013 mg/kg
triadimefon equivalents on day 27 to 28, and another animal had
triadimenol residues in the milk of 0.001 mg/kg on day 29. All other
animals fed at high level, as well as those fed with the medium level,
showed no milk residues at or above the level of detection of
0.001 mg/kg (Mobay 1981).
Hen
In a feeding study on hens, 16 (4 birds per dosage) were fed a
daily ration for 29 days, containing equal amounts of triadimefon and
triadimenol at total levels in the feed of 10, 25, 75 and 250 mg/kg.
After 29 days of feeding no residues could be detected (<0.01 mg/kg)
in the control and in the feeding levels up to 75 mg/kg included,
except that one of the 4 hens fed at the 75 mg/kg total residue in the
feed showed a triadimenol residue of 0.025 mg/kg in the skin and
0.012 mg/kg in the gizzard. Hens fed at the highest level of 250 mg/kg
total residue in the feed showed maximum residues of 0.02 mg/kg
triadimefon in the fat and 0.038 mg/kg triadimenol in the gizzard, but
no detectable residues in any other tissue. Residues in eggs were
proportional to feeding levels. Maximum total residues in the eggs
(triadimefon and triadimenol) were 0.002, 0.006, 0.011 and 0.050 mg/kg
at feeding levels of 10, 25, 75 and 250 mg/kg in the feed (Mobay
1981).
Other animals
In a study carried out in Australia, seed treated with a mixture
of triadimefon and triadimenol was added to livestock feed, which was
given to cows, pigs, sheep and poultry. After ingestion of feed
containing triadimefon, residue levels at or above the limit of
determination were only present in the skimmed milk, taken while the
animals were being fed. However, residues of triadimenol at or
slightly above the limit of determination were present in the meat,
fat and offal of pigs, fat and offal of sheep and in offal of poultry.
The offal included liver and kidney for pigs and sheep and giblets for
poultry (Bayer 1981).
The metabolic pathway of triadimefon in animals is illustrated in
Figure 3. For chemical names of metabolites shown in Figure 3, see
FAO/WHO 1979, Triadimefon, Table 9. From the information available on
triadimefon in plants, it can be concluded that the same metabolism
pathways and metabolites are found in plants and animals.
In soil
Extensive additional information on the fate of triadimefon and
triadimenol in soil, on the fate in soil micro-organisms and on the
residue uptake from soil, including crop rotation, was made available.
TABLE 5. Percent distribution of radioactivity in cereals after spray application of (14C)-triadimefon (recovered radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(G) benzene ring-
total plant UL-14C 0 n.d.2 n.d. n.d. n.d. 99.5 n.d. 0.5
" " 250g/ha 5 7.3 n.d. 57.3 21.8 9.7 n.d. 3.9
" " 10 15.8 n.d. 50.3 18.9 7.9 n.d. 7.1
" " 28 15.8 n.d. 45.1 17.4 6.4 n.d. 15.3
straw 38 15.9 n.d. 43.6 14.4 10.3 n.d. 15.8
" 62 16.7 1.9 41.0 17.3 4.8 n.d. 18.3
Spring barley(F) benzene ring-
total plant UL-14C 0 <1 n.d. 1 n.d. 92 n.d. 6
" " 125 g/ha 5 13 n.d. 48 15 12 n.d. 12
" " 17 35 n.d. 32 10 <1 n.d. 23
straw 28 9 <1 12 4 n.d. n.d. 75
" 76 8.4 5 8.4 3 n.d. n.d. 75
Spring wheat(F) benzene ring-
straw UL-14C 49 37 2 23 6 n.d. n.d. 32
TABLE 5. (con't)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post polar KWG Triadimenol Triadimenol Unidentified Non-
appl. compounds 1342 A B Triadimefon metabolites extractables
Spring barley(F) triazole-ring-
straw 3,4-14C 71 31 8 20 10 1 n.d. 29
250 g/ha
benzene ring-
UL-14C 71 20 16 22 9 1 3 28
250 g/ha
1 G = greenhouse, F = field;
2 n.d. = not detectable. In these studies the amount of radioactivity in the grains
ears was so small that separation by TLC was impossible.
TABLE 6. Percent distribution of radioactivity in apple, tomato and cucumber after spray application of (14C) triadimefon (recovered
radioactivity = 100)
Metabolites extracted
Analysed1 Dose Days
material (a.i.) post aqueous polar Triadimenol Triadimenol Triadimefon Unidentified Non-
appl. phase metabolite3 A B metabolites extractables
Apple (F) triazole ring-
peel 3,5-14C 28 5.7 n.d.2 14.9 13.6 10.8 2.8 5.0
pulp 15 mg/100 ml 5.3 n.d. 11.2 7.1 2.1 3.1 0.9
total fruit 11.0 n.d. 26.1 20.7 13.0 5.9 5.9
peel 48 4.8 n.d. 14.5 14.8 10.4 1.5 6.0
pulp 6.4 n.d. 10.4 9.4 2.1 2.1 1.0
total fruit 11.2 n.d. 24.9 24.2 12.5 3.6 7.0
Tomato (G) benzene ring-
foliage UL-14C 7 7 4 16 3 66 n.d. 3-4
e mg/plant 14 4 5 14 2 71 n.d. 4
(3 treatments) 21 2 22 19 5 44 n.d. 8
fruit 7 6 n.d. 8 14 69 n.d. 2-3
14 15 n.d. 10 18-21 49-52 n.d. 2
Cucumber (G)
foliage 7 7 4 11-12 10 60-61 n.d. 6
14 6 4 16-19 9-10 48-54 n.d. 7
21 7 20 17 12 36 n.d. 8
fruit 7 5 n.d. 8 33 51 n.d. 3
14 11 n.d. 8 32-33 41-43 n.d. 5
1 G = greenhouse, F = field;
2 n.d. = not detectable;
3 glucoside of triadimenol.
In studies following field-scale spraying of spring barley and
spring wheat with (benzene ring-UL-14C)-triadimefon 1.9 to 14.9% and
0.1% of the applied radioactivity was found in the straw and the
kernel respectively of the treated cereals, equivalent to 0.33 to
7.39 mg/kg and 0.01 to 0.04 mg/kg respectively (Bayer 1981). Following
spray treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon, on the other hand, 6.5% and 0.7% of applied radioactivity
was found in straw and grain respectively, equivalent to 3.39 and
0.39 mg/kg of parent triadimefon. Following spray applications of
(benzene ring-UL-14C)-triadimefon in the same trial, the apparent
triadimefon equivalents were 2.92 and 0.16 mg/kg in straw and grain
respectively (Bayer 1981).
In a study, carried out in a closed glass chamber with (benzene
ring-UL-14C)-triadimefon on barley plants, it was observed that the
applied compound moved from treated to un-treated plants, where it was
metabolized in the usual manner (Bayer 1981).
In glasshouse experiments triadimefon displayed vapour phase
activity against powdery mildew on cucumber and barley plants, not
only when it was applied at normal application rates, but also at
substantially lower ones. The effect was dependent on the distance of
the spray deposit (Scheinpflug and Paul 1977; Scheinpflug et al
1978; Schlütter 1977; Bayer 1981). In experiments on apple seedlings
and marrow plants, triadimefon was shown to display vapour phase
activity against powdery mildew (Clark et al 1978).
In animals
Several new data have become available on the fate of triadimefon
in animals. Data on excretion, storage and metabolism are now
available from studies in rats, dairy cattle, pigs and laying hens.
Accumulation of residues was also studied in the Channel catfish
exposed to labelled triadimefon. Feeding experiments were also carried
out on livestock animals, e.g. dairy cattle, pigs, sheep and hens.
Supplementary studies with the main animal metabolite of triadimefon,
triadimenol, were carried out in rats. Radiolabelled triazole was
given to rats in order to obtain data on the fate of this triadimefon
metabolite. The studies on rats are reported under "Biochemical
Aspects".
Cow
Feeding studies were carried out with 1:1 mixture of triadimefon
and triadimenol in dairy cattle. Nine dairy cows (three animals per
dosage) were fed with the mixture by bolus capsule with dosages
equal to 25, 75 and 250 mg/kg bw. The fat contained residues up to
0.029 mg/kg at the 250 mg/kg level and 0.016 mg/kg at the 75 mg/kg
dose level, whereas the residue level in the fat was less than
0.01 mg/kg at the 25 mg/kg feeding level (Mobay 1981). One animal
exposed to the high dose level showed milk residues of 0.0013 mg/kg
triadimefon equivalents on day 27 to 28, and another animal had
triadimenol residues in the milk of 0.001 mg/kg on day 29. All other
animals fed at high level, as well as those fed with the medium level,
showed no milk residues at or above the level of detection of
0.001 mg/kg (Mobay 1981).
Hen
In a feeding study on hens, 16 (4 birds per dosage) were fed a
daily ration for 29 days, containing equal amounts of triadimefon and
triadimenol at total levels in the feed of 10, 25, 75 and 250 mg/kg.
After 29 days of feeding no residues could be detected (<0.01 mg/kg)
in the control and in the feeding levels up to 75 mg/kg included,
except that one of the 4 hens fed at the 75 mg/kg total residue in the
feed showed a triadimenol residue of 0.025 mg/kg in the skin and
0.012 mg/kg in the gizzard. Hens fed at the highest level of 250 mg/kg
total residue in the feed showed maximum residues of 0.02 mg/kg
triadimefon in the fat and 0.038 mg/kg triadimenol in the gizzard, but
no detectable residues in any other tissue. Residues in eggs were
proportional to feeding levels. Maximum total residues in the eggs
(triadimefon and triadimenol) were 0.002, 0.006, 0.011 and 0.050 mg/kg
at feeding levels of 10, 25, 75 and 250 mg/kg in the feed (Mobay
1981).
Other animals
In a study carried out in Australia, seed treated with a mixture
of triadimefon and triadimenol was added to livestock feed, which was
given to cows, pigs, sheep and poultry. After ingestion of feed
containing triadimefon, residue levels at or above the limit of
determination were only present in the skimmed milk, taken while the
animals were being fed. However, residues of triadimenol at or
slightly above the limit of determination were present in the meat,
fat and offal of pigs, fat and offal of sheep and in offal of poultry.
The offal included liver and kidney for pigs and sheep and giblets for
poultry (Bayer 1981).
The metabolic pathway of triadimefon in animals is illustrated in
Figure 3. For chemical names of metabolites shown in Figure 3, see
FAO/WHO 1979, Triadimefon, Table 9. From the information available on
triadimefon in plants, it can be concluded that the same metabolism
pathways and metabolites are found in plants and animals.
In soil
Extensive additional information on the fate of triadimefon and
triadimenol in soil, on the fate in soil micro-organisms and on the
residue uptake from soil, including crop rotation, was made available.
 After applying (benzene ring-UL-14C)-triadimefon to spring
barley under field conditions the radioactivity still present in the
soil at harvest contained the metabolites listed in Table 7. The
relatively large proportion of triadimenol diastereoisomer A may be
due to washing from the plant into soil by rainfall (Bayer 1981).
Following treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon under field conditions 4% of 1,2,4-triazole was found in
addition to the above-mentioned metabolites. The percentage of
radioactivity still present in the soil accounted for by polar
metabolites increased to 6%. Including the non-extractables, more than
90% of the radioactivity was identified for both labels (Bayer 1981).
TABLE 7. Metabolites of triadimefon present in soil at harvest of
treated barley
Metabolite Radioactivity (%)
Triadimenol diastereoisomer A 33 - 41
Triadimenol diastereoisomer B 20 - 32
KWG, diastereoisomers A and B 6 - 8
KWG 1732 1
Polar metabolites 7
Non-extractable 10 - 15
In an ongoing three-year field trial on winter wheat using both a
seed treatment and spray of (benzene ring-UL-14C)-triadimenol and
triadimefon, respectively, the proportions reports in Table 8 were
found after the first year (Bayer 1981).
TABLE 8. Metabolites in soil one year after treatment with
triadimefon and triadimenol
Metabolite Radioactivity (%)
Triadimefon 14
Triadimenol diastereoisomer A 36
Triadimenol diastereoisomer B 38
KWG 1640 2
KWG 1732 (XVII) <1
Polar metabolites 2
Non-extractables 8
When triadimenol was applied to soil, it was only slightly
changed by micro-organisms; however, the proportion of diastereoisomer
B increased. This effect can be explained only as resulting from a
redox reaction via triadimefon (Mobay 1981).
When triadimefon was incubated with mycelium mats of
Aspergillus niger a different metabolic pattern was observed than
when the material was added to a shake culture. While some triadimenol
was always present (in amounts up to 4%), as much as 32% of the
triadimefon was converted to the isopropyl analogue (Clark et al
1978). However, in another study the isopropyl analogue was found to
be an artefact of KWG 1342; in this study it was shown that the tert.
butyl group of triadimefon and triadimenol was oxidized to KWG 1342
(VI) and 1323 (V) (Deas and Clifford 1981).
The metabolism of triadimenol by selected soil micro-organisms
was studied (Bayer 1981). Whereas bacteria were unable to metabolize
triadimenol it was shown that some fungi oxidized the tert. butyl
group to KWG 1342.
The metabolic pathways of triadimefon in soil and fungi are
illustrated in Figure 4. For chemical names of metabolites shown in
Figure 4, see FAO/WHO 1979, Triadimefon, Table 9.
In studies with (benzene ring-UL-14C)-triadimefon and (triazole
ring-3,5-14C)-triadimefon carried out on spring barley, the uptake of
residues from soil by crops grown subsequently was studied (Bayer
1981). After the treated cereals had been harvested, turnip and clover
were planted as catch crops, followed by sowing of winter barley,
winter wheat, spring wheat, oats and sugarbeet as main crops.
Following the application of the benzene ring label, the catch crops
absorbed up to 1.1% of the radioactivity present in the soil. Of the
main crops, sugarbeet leaves absorbed up to 4.3%, oat straw 0.5% and
straw of spring and winter wheat and of winter barley up to 1.4%.
These values corresponded with parent equivalents of 0.07, 0.07 and
0.18 mg/kg, respectively.
Following the application of the triazole ring label, larger
amounts of radioactivity were taken up by the main crops, which were
found especially in the storage organs, e.g. sugarbeet roots, spring
wheat kernels. The sugarbeet roots absorbed 2.4% (equivalent to
0.03 mg/kg parent compound) and the spring wheat kernels 1.5%
(0.49 mg/kg parent equivalent) (see Table 9).
After applying (benzene ring-UL-14C)-triadimefon to spring
barley under field conditions the radioactivity still present in the
soil at harvest contained the metabolites listed in Table 7. The
relatively large proportion of triadimenol diastereoisomer A may be
due to washing from the plant into soil by rainfall (Bayer 1981).
Following treatment of spring barley with (triazole ring-3,5-14C)-
triadimefon under field conditions 4% of 1,2,4-triazole was found in
addition to the above-mentioned metabolites. The percentage of
radioactivity still present in the soil accounted for by polar
metabolites increased to 6%. Including the non-extractables, more than
90% of the radioactivity was identified for both labels (Bayer 1981).
TABLE 7. Metabolites of triadimefon present in soil at harvest of
treated barley
Metabolite Radioactivity (%)
Triadimenol diastereoisomer A 33 - 41
Triadimenol diastereoisomer B 20 - 32
KWG, diastereoisomers A and B 6 - 8
KWG 1732 1
Polar metabolites 7
Non-extractable 10 - 15
In an ongoing three-year field trial on winter wheat using both a
seed treatment and spray of (benzene ring-UL-14C)-triadimenol and
triadimefon, respectively, the proportions reports in Table 8 were
found after the first year (Bayer 1981).
TABLE 8. Metabolites in soil one year after treatment with
triadimefon and triadimenol
Metabolite Radioactivity (%)
Triadimefon 14
Triadimenol diastereoisomer A 36
Triadimenol diastereoisomer B 38
KWG 1640 2
KWG 1732 (XVII) <1
Polar metabolites 2
Non-extractables 8
When triadimenol was applied to soil, it was only slightly
changed by micro-organisms; however, the proportion of diastereoisomer
B increased. This effect can be explained only as resulting from a
redox reaction via triadimefon (Mobay 1981).
When triadimefon was incubated with mycelium mats of
Aspergillus niger a different metabolic pattern was observed than
when the material was added to a shake culture. While some triadimenol
was always present (in amounts up to 4%), as much as 32% of the
triadimefon was converted to the isopropyl analogue (Clark et al
1978). However, in another study the isopropyl analogue was found to
be an artefact of KWG 1342; in this study it was shown that the tert.
butyl group of triadimefon and triadimenol was oxidized to KWG 1342
(VI) and 1323 (V) (Deas and Clifford 1981).
The metabolism of triadimenol by selected soil micro-organisms
was studied (Bayer 1981). Whereas bacteria were unable to metabolize
triadimenol it was shown that some fungi oxidized the tert. butyl
group to KWG 1342.
The metabolic pathways of triadimefon in soil and fungi are
illustrated in Figure 4. For chemical names of metabolites shown in
Figure 4, see FAO/WHO 1979, Triadimefon, Table 9.
In studies with (benzene ring-UL-14C)-triadimefon and (triazole
ring-3,5-14C)-triadimefon carried out on spring barley, the uptake of
residues from soil by crops grown subsequently was studied (Bayer
1981). After the treated cereals had been harvested, turnip and clover
were planted as catch crops, followed by sowing of winter barley,
winter wheat, spring wheat, oats and sugarbeet as main crops.
Following the application of the benzene ring label, the catch crops
absorbed up to 1.1% of the radioactivity present in the soil. Of the
main crops, sugarbeet leaves absorbed up to 4.3%, oat straw 0.5% and
straw of spring and winter wheat and of winter barley up to 1.4%.
These values corresponded with parent equivalents of 0.07, 0.07 and
0.18 mg/kg, respectively.
Following the application of the triazole ring label, larger
amounts of radioactivity were taken up by the main crops, which were
found especially in the storage organs, e.g. sugarbeet roots, spring
wheat kernels. The sugarbeet roots absorbed 2.4% (equivalent to
0.03 mg/kg parent compound) and the spring wheat kernels 1.5%
(0.49 mg/kg parent equivalent) (see Table 9).
 TABLE 9. Residues of triadimefon metabolites in crops after uptake from soil1
Uptake
Catch crop (1)
14C-Label followed by Plant Radioactivity present parent equivalent
main crop (2) and (3) part in soil (%) (mg/kg)
benzene ring- Turnip (1) Leaf 1.1 0.05
UL-14C Edible root 0.2 0.01
Winter wheat (2) Straw 0.6 0.07
Kernel 0.1 <0.01
Sugarbeet (3) Leaf 0.4 0.01
Edible root 0.1 <0.01
Persian clover (1) 1.0 0.06
Winter wheat (2) Straw 0.9 0.07
Kernel 0.1 <0.01
Winter barley (3) Straw 0.3 0.03
Kernel 0.1 <0.01
benzene ring- Sugarbeet (2) Leaf 1.6 0.04
UL-14C Edible root <0.1 <0.01
Spring wheat (3) Straw 0.3 0.04
Kernel <0.1 <0.01
triazole ring- Sugarbeet Leaf 3.8 0.13
3,5-14C Edible root 2.4 0.03
Spring wheat Straw 1.1 0.23
Kernel 1.5 0.49
1 Reference - Bayer 1981.
It is assumed that the rotational crop absorbed mainly
triadimenol from the soil. The crops of winter wheat and winter barley
contained 22 to 35% triadimenol, 3 to 7% KWG 1342, up to 3%
triadimefon and 9 to 27% polar compounds. In turnip and sugarbeet
leaves, on the other hand, KWG 1342 was the main component, accounting
for 38% and 9 to 19% of the radioactivity present (Bayer 1981).
The uptake of residues in rotational glasshouse crops was studied
following the application of triadimefon at concentrations of 2,5,7
and 10 mg/kg on dry weight basis to the soil. In another experiment,
triadimefon was sprayed on bare soil at a rate equivalent to
500 g a.i./ha. In both experiments the rotational crops were grown to
maturity. The residues in radishes and cucumbers planted 14 days after
the treatment of 10 mg/kg dry weight basis were below the limit of
detection (lower than 0.1 mg/kg both for triadimefon and triadimenol).
The residues in radish leaves were about 0.16 mg/kg. The main
pesticide identified in the crop was triadimefon. The triadimefon
parent was either not detected or detected only at very low levels.
The ratio of the diastereoisomers A and B in these crops were 1:2 to
1:3. In the other experiment, radishes, carrots, potatoes, chinese
cabbage and beans were grown in the soil previously treated with
triadimefon. The triadimenol levels in the edible parts of these crops
were below the limit of determination (less than 0.1 mg/kg). In
chinese cabbage, 0.02 mg/kg triadimenol was found, and 0.21 mg/kg in
bean leaves (Mobay 1981).
In storage and processing
Apples containing field weathered residues of (benzene ring-UL-
14C)-triadimefon were stored for 420 days at -10°C. The peel of these
apples was analysed repeatedly over a period of about 14 months.
Essentially no change was seen in amount or percentage distribution of
triadimefon or triadimenol during this period (Mobay 1981).
The same results were obtained in a study to investigate the
effect of frozen storage (-0°C to -10°C) on soil residues of
triadimefon and triadimenol in a loam soil. No residue decrease was
found during 516 days of cold storage.
In a Mobay (1981) study, 20 kg of apples were processed under
simulated industry conditions. Almost all residues were found in the
peel. The highest concentration of residues was found in the wet
pomace (about 4 times higher than in fresh fruit), but further drying
caused a residue decrease resulting in a dry product with residues
approximately twice that found in fresh whole fruit.
After processing wine grapes containing weathered triadimefon
residues, practically no residues were present in the wine
(<0.01 mg/kg) except in one sample with 0.5 mg/kg (Bayer 1981; Mobay
1981).
After processing of hops, which were sprayed during the growing
season at excessive rates, no residues were found in beer (0.05 mg/kg
limit of determination) (Bayer 1981).
Field treated wheat grain containing 0.3 mg/kg and 0.2 mg/kg
triadimefon and triadimenol respectively was processed in a Buhler
roller mill. Highest residues were found in the bran (triadimefon and
triadimenol, both 0.8 mg/kg). Lesser amounts were found in the shorts
(both 0.2 mg/kg) and only trace amounts in the flour (Mobay 1981).
In water
Benzene ring-UL-14C)-triadimenol added to water did not show
apparent degradation at various temperatures and pH levels 4, 7 and 9,
temp. 20°C and 40°C. After 32 days, 97% of the added radioactivity was
still present, mainly as unchanged triadimenol (Mobay 1981). However,
degradation of triadimefon was observed at 70°C in tap water, and
reached approximately 90% in 8 weeks. The salts present were
responsible for this degradation, especially C++ salts accelerated
further hydrolysis (Bayer 1981).
In contrast with the data obtained in buffer solutions, it was
found that both (benzene ring-UL-14C)-triadimefon and (triazole ring-
3,5-14C)-triadimefon showed rapid degradation in a simulated natural
pond environment. Triadimefon had a half-life of 6 to 8 days in the
water phase and 18 to 20 days in the silt phase. Triadimenol was the
main metabolite during the experiment; the following minor metabolites
were identified: triadimefon, symmetrical isomer, 1,2,4 triazole and
delta2 -1,2,4-triazoline-5-one (Mobay 1981).
METHODS OF RESIDUE ANALYSIS
Residues of triadimefon and triadimenol can be measured by gas
chromatography using a nitrogen-specific alkali flame detector (AFID).
Some methods suitable for plant material and soil are presented in
FAO/WHO (1979). The specificity of the method for apples described in
FAO/WHO (1979), was demonstrated in an interference study (Mobay
1981).
A new multi-residue method was developed involving clean-up by
gel permeation chromatography and mini silica gel column
chromatography. Following extraction of plant material with
acetone/water and soil with methanol/water 70:30, the extract is
shaken in dichloromethane and then cleaned up with ethylacetate/
cylohexane by gel permeation chromatography. Additional clean-up is
performed in some cases using mini silica gel column chromatography
(Specht and Tillkes 1980). The method was slightly modified by Mobay
Chemical Corporation (Mobay 1981). A similar method was developed by
Nitokono (1981).
The limits of determination of the various methods for plant
material referred to above are about 0.01 to 0.1 mg/kg, depending on
the commodity.
A method for determining triadimefon and triadimenol in grape
juice and wine using capillary gas chromatography has become
available; the sample is passed through a column packed with XAD-2
resin (Nickless et al 1981). The limit of determination is 0.005 to
0.01 mg/kg.
Methods for the determination of triadimefon and triadimenol in
animal material, such as cattle tissues, milk, poultry and eggs, have
been developed. After extraction of the samples with methanol
(methanol/acetone for milk), it is passed through a XAD-4 resin
column, then cleaned up by partition between dichloromethanol and
water, partition between acetonitrile and hexane and finally by
passing through a Florisil column (Mobay 1981). The limits of
determination were 0.05 to 0.1 mg/kg for cattle tissues, 0.005 to
0.01 mg/kg for milk and about 0.005 to 0.01 mg/kg for poultry and
eggs. The specificity of the method was demonstrated in an
interference study (Mobay 1981). The method can be modified, using
GLC/MS, which enables the determination of triadimefon and all known
metabolites, free or conjugated, in cattle tissues and milk (Mobay
1981).
NATIONAL MAXIMUM RESIDUE LIMITS
Additional maximum residue limits have been reported to the
Meeting, together with recommended pre-harvest intervals (Table 10).
EVALUATION
COMMENTS AND APPRAISAL
Triadimefon is rapidly and readily absorbed from the
gastrointestinal tract. The route of excretion appears to vary
between sexes in the rat, where excretion is relatively slow.
Excretion is rapid in cows and pigs and extremely rapid in hens.
The compound is metabolized in all species, the major excreted
metabolite being triadimenol.
In studies designed to evaluate triadimefon for cumulative
toxicity, the liver appeared to be the major target organ. The liver
weight was affected in the dog, rat and mouse, the effect observed
being correlated in the dog and mouse with biochemical signs of liver
damage (e.g. increased transaminase activity levels in plasma). With
increasing doses, the first signs of a hepatotropic effect are
considered to be due to the increased microsomal liver enzyme
activities, as shown by the findings obtained in rats and dogs. The
TABLE 10. National maximum residue limits reported to the Meeting
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Australia Apple 1
Cucurbits 0.2 7
Grape 2 14
Raw cereals 0.5 28
Eggs, milk 0.1
Meat, meat products 0.05
Austria Pome fruit 35
Grape 35
Cucumber, pepper,
tomato 1 4
Other vegetables 14
Cereals 11 35
Sugarbeet 0.21 35
Belgium Barley, wheat 0.052 42
Apple 0.052 14
Cucumber, gherkin 0.052 3
Brazil Wheat 0.1 42
Bulgaria Apple 14
Grape 14
Sugarbeet 14
Tobacco 14
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Czechoslovakia Apple 28
Grape 28
Denmark Fruits (including small
fruits) 14
Cucumber 14
Wheat, barley 28
Fed.Rep.of Germany Apple and pear 0.53 14
Grape 33 35
Strawberry 0.23
Cucumber 0.53 3
Cereals 0.53
Hops 15 14
Other commodities of
plant origin 0.1
Israel Apple 0.05 14
Grape 0.1 21
Cucumber, melon, pumpkin,
pepper, tomato ,eggplant 3
Wheat 42
Netherlands Apple 0.1 14
Cereals 0.05 42
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Portugal Apple 56
Grape (wine) 28
Grape (table) 21
Cucumber (including gherkin)
melon, pumpkin 3
Pea 7
Pepper 7
South Africa Apple, pear 0.05 28
Grape (table 0.05 7 resp 28-424
Grape (wine) 0.05 7 resp 28-424
Mango 0.05 28
Cucurbits 0.05 3
Pea 0.05 1
Spain Fruits, except grape 15
Grape 15-214
Artichoke 15
Cucumber, melon 15
Tomato
Sweden Fruits (including small
fruits and berries) 28
Switzerland Apple 0.1 21
Grape 0.5 21
Wine 0.5
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
UK Apple 14
Blackcurrant, gooseberry 14
Grape 14
Brussels sprout 14
1 Provisional;
2 Under consideration;
3 Proposed levels;
4 According to formulation.
only species showing histopathological changes was the mouse, at the
highest tested dose level. The most sensitive species with respect to
enzyme induction was the rat, which reacted to minimum oral daily
doses of 5 mg/kg bw. In the rat, the liver microsomal enzyme induction
was rapidly reversible within 4 weeks.
The other toxicological target was the haemapoietic system as
indicated by the results obtained in rats and dogs. Other effects
(only found in the rat) were observed in the kidney and testis and on
cholesterol levels, especially in the chronic toxicity study.
Moreover, these effects only appeared at the extremely high dose of
5 000 ppm in the diet (250 mg/kg bw), which caused excessive mucosal
lesions in the stomach and hence undoubtedly nutritional disorders.
Triadimefon was not mutagenic when tested with or without metabolic
activation in various strains of Salmonella, B; subtilis, E. coli
and S. cerevisiae.
The long-term studies on two rodent species did not provide any
indication of triadimefon having carcinogenic potential.
The information on the use pattern and pre-harvest intervals in
several countries was updated, and new information was obtained on use
patterns, pre-harvest intervals and national maximum residue limits in
other countries.
Additional data were obtained on residues from supervised trials,
some from countries not included in FAO/WHO 1979, on various fruits,
vegetables, cereals and some other crops, e.g. apple, red currant,
grape, strawberry, cucumber, melon, pepper, tomato, onion, pea, cereal
crops and sugarbeet. New information was obtained on residues from
supervised trials on black currant, raspberry, leek, parsnip, hops,
sweet potato and sugarcane.
Additional information was obtained on the fate of triadimefon
residues in plants, animals (including livestock), soils (including
microorganisms) and water, especially with regard to the further
degradation of the main plant and animal metabolite of triadimefon,
triadimenol. New information was also obtained on the fate of the
triazole part of the triadimefon molecule in plants, animals, soil and
water from studies with (triazole ring-3,5-14C)-triadimefon,
(triazole ring-3,5-14C)-triadimenol, (diastereoisomer A and B) and
1,2,4 (3,5-14C)-triazole. The latter three compounds are main
(triadimenol A and B) or minor plant and animal metabolites of
triadimefon.
Information on new or improved methods of residue analysis was
made available to the Meeting. Most of the methods are based on the
determination of the residue of triadimefon and triadimenol by gas
chromatography using a nitrogen-specific alkali flame detector(AFID).
The methods were modified for the determination of the triadimefon/
triadimenol residues in grape juice, wine and animal material (such as
cattle and poultry tissues, milk and eggs). The specificity of the
methods for triadimefon and triadimenol in plant material, such as
apples, meat and milk, has been demonstrated in interference studies.
The methods now available are suitable or can be adapted for
regulatory purposes.
Level causing no toxicological effects
Mouse : 300 ppm in diet, equivalent to 40 mg/kg bw/day
Rat : 50 ppm in diet, equivalent to 2.5 mg/kg bw/day
Dog : 330 ppm in diet, equivalent to 8.25 mg/kg bw/day
Estimated temporary admissible daily intake for man
0 - 0.01 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
The additional residue data evaluated by the Meeting in
conjunction with the data provided to the 1979 JMPR confirm the limits
proposed in 1979 for red currant, grape, strawberry, cucumber, melon,
pepper, tomato, onion, pea, cereal grains and coffee. The following
temporary maximum residue limits, expressed as the sum of triadimefon
and triadimenol, are recommended. The data presented on residues in
pineapple, Brussels sprout and leek are not sufficient to consider a
maximum residue limit.
Pre-harvest interval
Temporary MRL on which recommendation
Commodity (mg/kg) is based (days)
Apple 0.5 14
Black currant 1 7
Raspberry 0.2 7
Hops (dry) 15 14
Coffee beans 0.11
Meat 0.11
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
Clarification of the effects on liver in mice and rats.
Desirable
1. Clarification of effects on the central nervous system observed
in acute studies.
2. Information on the use pattern (concentration, dipping time) of
the post-harvest treatment of pineapple and residues arising from
this use.
REFERENCES
Bayer. Reports submitted to FAO and WHO by Bayer AG.(Unpublished)
1981
Bomhard, E. and Löser, E. Chronic toxicity study on rats (two-year
1978a feeding experiment, 1 August. Report no. 7707, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
1978b Chronic toxicity study on mice (two-year feeding experiment)
1 August. Report no. 9344, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Brandes, W., Steffens, W., Führ, F. and Scheinpflug H. Weitere Un
1978 tersuchungen zur Translokation von 14C-Triadimefon.
Pflanzenschutz-Nachrichten Bayer, 31:132-144.
Buchenauer, H. Systemisch-fungizide Wirkung und Wirkungs mechanismus
1975 von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-
Forst wirtsch. (Berlin-Dahlem), 165: 154-155.
1976 Studies on the systemic activity of Bayleton (triadimefon)
and its effects against certain fungal diseases of cereals.
Pflanzenschutz-Nachrichten, 29:266-280 (English edition)
Clark, T., Clifford, D.R., Deas, A., Gendle, P. and Watkins, D.
1978 Photolysis, metabolism and other factors influencing the
performance of triadimefon as a powdery mildew fungicide.
Pesticide Science, 9:497-506.
Deas, A.H.B. and Clifford, D.R. Metabolism of the 1,2,4-
1981 triazolylmethane fungicides, triadimefon, triadimenol and
diclobutrazol by Aspergillus niger (Van Tiegh). (In press)
Ecker, W. Biotransformation of 1,2,4-[3(5)-14C]triazol in rats.
1980 Bayer AG, Institut für Pharmakokinetik, Elberfeld, Pharma
Report no. 9478 (PF-Report no. 1471), 14 October 1980,
submitted by Bayer AG to WHO. (Unpublished)
Flucke, W. Bestimmung der akuten Toxizität (LD50) 16 February, Bayer
1981 AG, Institut für Toxikologie, report submitted by Bayer AG
to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Propineb (LH 30/Z)-Triadimefon (MEB 6447)
1977a Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7065, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Triadimefon (MEB 6447) und Captafol,
1977b Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7069, Bayer AG, Institut für Toxikoligie, report submitted
by Bayer AG to WHO. (Unpublished)
1977c Triadimefon (MEB 6447) und Tolylfluanid (KUE 13 183 b),
Untersuchungen zur akuten Toxizitüt nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr
7304, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Fredrickson, D.R. Metabolism of BAYLETONTM in rats. Mobay report no.
1978a 66201, 20 June 1978 (revised: 21 November 1979), submitted
by Bayer AG to WHO. (Unpublished)
1978b Metabolism of BAYLETONTM in cow. Mobay report no. 66202,
20 June 1978 (revised 21 November 1979), submitted by Bayer
AG to WHO. (Unpublished)
Gasztonyi, M. and Josepovits, G. The activation of triadimefon and its
1979 role in the selectivity of fungicide action. Pesticide
Science, 10:57-65.
Hoffman, K. and Gröning, P. Long-term toxicity study on dogs (two-year
1978 feeding study), 24 October. Report no. 7882, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Hoffman, K. and Luckhaus, G. Subchronic toxicity study on dogs,
1974 (thirteen-week feeding experiment) 16 December. Report no.
5071, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Inukai, H. and Iyatomi, A. Mutagenicity test on bacterial systems, 19
1977 December. Report no. 68, Nitokuno, Laboratory of Toxicology,
Japan, report submitted by Bayer AG to WHO. (Unpublished)
Jagannath, D.R., Brusick, and Hoorn, A.J.W. Mutagenicity evaluation of
1980 MEB 6447 in the reverse mutation induction assay, January.
R-Bericht nr. 1675, Litton Bionetics Inc., USA, report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Burke, M.A. Acute oral toxicity of BAYLETONTM (formerly
1977 BAY MEB 6447) technical to adult mallard ducks, 11 May.
Report no. 52872, Mobay Chemical Corp., Agr. Div., submitted
by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Roney, D.J. Accumulation and persistence of residues in
1977 channel catfish exposed to BAYLETONTM-14C (formerly BAY
MEB 6447), Mobay Report no. 52775, 4 May, submitted by Bayer
AG to WHO. (Unpublished)
Löser, E. Multigeneration reproduction study on rats, 12 April. Report
1979 no. 8297, Bayer AG, Institut für Toxikologie, submitted by
Bayer AG to WHO. (Unpublished)
Machemer, L. Dominant lethal study on male mice to test for mutagenic
1976a effects, 27 January. Report no. 5837, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
1976b Evaluation of embryotoxic and teratogenic effects on rats
following oral administration, 27 August. Report no. 6294,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
1976c Evaluation of embryotoxic and teratogenic effects on rabbits
following oral administration, 30 August. Report no. 6297,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
Machemer, L. Micronucleus test on mice to evaluate MEB 6447 for
1977 mutagenic effects, 23 February. Report no. 6622, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Machemer, L. and Kimmerle, G. Evaluation of MEB 6447 (triadimefon) for
1976 embryotoxic and teratogenic effects on rats following
inhalation in dynamic flow apparatus, 30 August. Report no.
6298, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Mihail, F. Acute toxicity studies, 27 June. Report no. 9277, Bayer AG,
1980 Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Mihail, F. and Kaliner, G. Subakuter oraler Kumulationsversuch an
1979 Ratten, 20 February, Bericht nr. 8195, Bayer AG, Institut
für Toxikologie, report submitted by Bayer AG to WHO.
(Unpublished)
Mobay Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Mohr, U. Subchronic toxicity study on rats (twelve-week feeding
1976 experiment), 5 November. Report no. R840a, Medizinische
Hochschule Hannover, Abteilung für, experimentelle
Pathologie, submitted by Bayer AG to WHO. (Unpublished)
Nickless, G., Spitzer, T. and Pickard, J.A. Determination of
1981 triadimefon in grape juice and wine using capillary gas
chromatography. Journal of Chromatography, 208: 409-413.
Nitokuno. Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Nye, D.E. The fate of BAYLETON-14C in poultry. Mobay Report no. 67482,
1979 8 March, submitted by Bayer AG to WHO. (Unpublished)
Pither, K.M. Metabolism of BAYLETONTM in male and female pigs. Mobay
1978 report no. 66509, 7 August (revised: 29 November 1978)
submitted by Bayer AG to WHO. (Unpublished)
Puhl, R.J. and Hurley, J.B. The metabolism and excretion of BAYTANTM
1978 by rats. Mobay Report no. 66489, 14 August 1978 (revised 26
September 1979 and 4 February 1980), submitted by Bayer AG
to WHO. (Unpublished)
Ritter, W. BAY e 8364 (MEB 6447): Pharmacokinetics in mice and rats.
1976 Bayer AG, Pharma-Institut für Pharmakokinetik, report no.
6458, 29 October, 1976, submitted by Bayer AG to WHO.
(Unpublished)
Scheinpflug, H. and Paul, V. On the mode of action of triadimefon.
1977 Netherlands Journal of Plant Pathology, 83(suppl.):105-111.
Scheinpflug, H., Paul, V. and Kraus, P. Untersuchungen zur
1977 Wirkungsweise von (R)Bayleton bei Getreidekrunkheiten. Mitt.
Biol. Bundesanst. Lund-Forstwirtschaft (Berlin-Dahlem)
178:178.
1978 Studies on the mode of action of (R)Bayleton against cereal
diseases. Pflanzenschutz Nach richten Bayer, 31:101-115.
Schlütter, K. Fungizine Wirkung von Triadimefon in der Gasphase. Z.
1977 Pflanzenkrank. Pflanzenschutz, 84:612-614.
Shirasu, Y., Moriya, M. and Koyashiki, R. Triadimefon-mutagenicity
1978 test on bacterial systems, 18 July. Department of
Toxicology, Institute of Environmental Toxicology, Japan,
report submitted by Bayer AG to WHO. (Unpublished)
Shirasu, Y., Moriya, M. and Miyazawa, T. Triadimefon-mutagenicity test
1979 of bacterial systems 27 October. Department of Toxicology,
Institute of Environmental Toxicology, Japan, report
submitted by Bayer AG to WHO. (Unpublished)
Specht, W. and Tillkes, M. Gaschromatographische Bestimmung von
Rückständen an Pflunzenbehandlungsmitteln nach Clean-up Uber
Gelchromatographic und mini-Kieselgel Säulenchromatographic.
Pflanzenschutz-Nachrichten Bayer, 33:61-85.
Thyssen, J. Studies on acute combination toxicity of triadimefon and
1977 carbadazim, 22 November. Report no. 7119, Bayer AG, Institut
für Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Thyssen, J. and Gröning, P. Fütterungsversuche mit Hühnern unter
1978 besonderer Berücksichtigung einer Möglichen Beeinflussung
der Fortpflanzung, 15 November. Bericht n.7924, Bayer AG,
Institut für Toxikologie, report submitted by Bayer AG to
WHO. (Unpublished)
Thyssen, J. and Kimmerle, G. MEB 6447-Acute toxicity studies, 3
1979 January, Report no.4416, Bayer AG, Institut für Toxikologie,
submitted by Bayer AG to WHO.(Unpublished)
Thyssen, J., Kimmerle, G. and Luckhaus, G. MEB 6447 - subacute
1974 toxicity studies, 25 January. Report no. 4464, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
van Dijck, P. Evaluation of Bayleton (MEB 6447, triadimefon) for
1976 mutagenic potential by the Ames test with histidine-
auxotrophic Salmonella typhimurium strains, 27 September.
Laboratorium voor Hygiene, Leuven (Belgium), report
submitted by Bayer AG to WHO. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. 1,2,4-triazol-14C.
1978 Biokinetische Untersuchungen an Ratten. Bayer AG, Institut
für Pharmakokinetik, Elberfeld, Pharma Report no. 7920, 13
November 1978, submitted by Bayer AG to WHO. (Unpublished)
TABLE 9. Residues of triadimefon metabolites in crops after uptake from soil1
Uptake
Catch crop (1)
14C-Label followed by Plant Radioactivity present parent equivalent
main crop (2) and (3) part in soil (%) (mg/kg)
benzene ring- Turnip (1) Leaf 1.1 0.05
UL-14C Edible root 0.2 0.01
Winter wheat (2) Straw 0.6 0.07
Kernel 0.1 <0.01
Sugarbeet (3) Leaf 0.4 0.01
Edible root 0.1 <0.01
Persian clover (1) 1.0 0.06
Winter wheat (2) Straw 0.9 0.07
Kernel 0.1 <0.01
Winter barley (3) Straw 0.3 0.03
Kernel 0.1 <0.01
benzene ring- Sugarbeet (2) Leaf 1.6 0.04
UL-14C Edible root <0.1 <0.01
Spring wheat (3) Straw 0.3 0.04
Kernel <0.1 <0.01
triazole ring- Sugarbeet Leaf 3.8 0.13
3,5-14C Edible root 2.4 0.03
Spring wheat Straw 1.1 0.23
Kernel 1.5 0.49
1 Reference - Bayer 1981.
It is assumed that the rotational crop absorbed mainly
triadimenol from the soil. The crops of winter wheat and winter barley
contained 22 to 35% triadimenol, 3 to 7% KWG 1342, up to 3%
triadimefon and 9 to 27% polar compounds. In turnip and sugarbeet
leaves, on the other hand, KWG 1342 was the main component, accounting
for 38% and 9 to 19% of the radioactivity present (Bayer 1981).
The uptake of residues in rotational glasshouse crops was studied
following the application of triadimefon at concentrations of 2,5,7
and 10 mg/kg on dry weight basis to the soil. In another experiment,
triadimefon was sprayed on bare soil at a rate equivalent to
500 g a.i./ha. In both experiments the rotational crops were grown to
maturity. The residues in radishes and cucumbers planted 14 days after
the treatment of 10 mg/kg dry weight basis were below the limit of
detection (lower than 0.1 mg/kg both for triadimefon and triadimenol).
The residues in radish leaves were about 0.16 mg/kg. The main
pesticide identified in the crop was triadimefon. The triadimefon
parent was either not detected or detected only at very low levels.
The ratio of the diastereoisomers A and B in these crops were 1:2 to
1:3. In the other experiment, radishes, carrots, potatoes, chinese
cabbage and beans were grown in the soil previously treated with
triadimefon. The triadimenol levels in the edible parts of these crops
were below the limit of determination (less than 0.1 mg/kg). In
chinese cabbage, 0.02 mg/kg triadimenol was found, and 0.21 mg/kg in
bean leaves (Mobay 1981).
In storage and processing
Apples containing field weathered residues of (benzene ring-UL-
14C)-triadimefon were stored for 420 days at -10°C. The peel of these
apples was analysed repeatedly over a period of about 14 months.
Essentially no change was seen in amount or percentage distribution of
triadimefon or triadimenol during this period (Mobay 1981).
The same results were obtained in a study to investigate the
effect of frozen storage (-0°C to -10°C) on soil residues of
triadimefon and triadimenol in a loam soil. No residue decrease was
found during 516 days of cold storage.
In a Mobay (1981) study, 20 kg of apples were processed under
simulated industry conditions. Almost all residues were found in the
peel. The highest concentration of residues was found in the wet
pomace (about 4 times higher than in fresh fruit), but further drying
caused a residue decrease resulting in a dry product with residues
approximately twice that found in fresh whole fruit.
After processing wine grapes containing weathered triadimefon
residues, practically no residues were present in the wine
(<0.01 mg/kg) except in one sample with 0.5 mg/kg (Bayer 1981; Mobay
1981).
After processing of hops, which were sprayed during the growing
season at excessive rates, no residues were found in beer (0.05 mg/kg
limit of determination) (Bayer 1981).
Field treated wheat grain containing 0.3 mg/kg and 0.2 mg/kg
triadimefon and triadimenol respectively was processed in a Buhler
roller mill. Highest residues were found in the bran (triadimefon and
triadimenol, both 0.8 mg/kg). Lesser amounts were found in the shorts
(both 0.2 mg/kg) and only trace amounts in the flour (Mobay 1981).
In water
Benzene ring-UL-14C)-triadimenol added to water did not show
apparent degradation at various temperatures and pH levels 4, 7 and 9,
temp. 20°C and 40°C. After 32 days, 97% of the added radioactivity was
still present, mainly as unchanged triadimenol (Mobay 1981). However,
degradation of triadimefon was observed at 70°C in tap water, and
reached approximately 90% in 8 weeks. The salts present were
responsible for this degradation, especially C++ salts accelerated
further hydrolysis (Bayer 1981).
In contrast with the data obtained in buffer solutions, it was
found that both (benzene ring-UL-14C)-triadimefon and (triazole ring-
3,5-14C)-triadimefon showed rapid degradation in a simulated natural
pond environment. Triadimefon had a half-life of 6 to 8 days in the
water phase and 18 to 20 days in the silt phase. Triadimenol was the
main metabolite during the experiment; the following minor metabolites
were identified: triadimefon, symmetrical isomer, 1,2,4 triazole and
delta2 -1,2,4-triazoline-5-one (Mobay 1981).
METHODS OF RESIDUE ANALYSIS
Residues of triadimefon and triadimenol can be measured by gas
chromatography using a nitrogen-specific alkali flame detector (AFID).
Some methods suitable for plant material and soil are presented in
FAO/WHO (1979). The specificity of the method for apples described in
FAO/WHO (1979), was demonstrated in an interference study (Mobay
1981).
A new multi-residue method was developed involving clean-up by
gel permeation chromatography and mini silica gel column
chromatography. Following extraction of plant material with
acetone/water and soil with methanol/water 70:30, the extract is
shaken in dichloromethane and then cleaned up with ethylacetate/
cylohexane by gel permeation chromatography. Additional clean-up is
performed in some cases using mini silica gel column chromatography
(Specht and Tillkes 1980). The method was slightly modified by Mobay
Chemical Corporation (Mobay 1981). A similar method was developed by
Nitokono (1981).
The limits of determination of the various methods for plant
material referred to above are about 0.01 to 0.1 mg/kg, depending on
the commodity.
A method for determining triadimefon and triadimenol in grape
juice and wine using capillary gas chromatography has become
available; the sample is passed through a column packed with XAD-2
resin (Nickless et al 1981). The limit of determination is 0.005 to
0.01 mg/kg.
Methods for the determination of triadimefon and triadimenol in
animal material, such as cattle tissues, milk, poultry and eggs, have
been developed. After extraction of the samples with methanol
(methanol/acetone for milk), it is passed through a XAD-4 resin
column, then cleaned up by partition between dichloromethanol and
water, partition between acetonitrile and hexane and finally by
passing through a Florisil column (Mobay 1981). The limits of
determination were 0.05 to 0.1 mg/kg for cattle tissues, 0.005 to
0.01 mg/kg for milk and about 0.005 to 0.01 mg/kg for poultry and
eggs. The specificity of the method was demonstrated in an
interference study (Mobay 1981). The method can be modified, using
GLC/MS, which enables the determination of triadimefon and all known
metabolites, free or conjugated, in cattle tissues and milk (Mobay
1981).
NATIONAL MAXIMUM RESIDUE LIMITS
Additional maximum residue limits have been reported to the
Meeting, together with recommended pre-harvest intervals (Table 10).
EVALUATION
COMMENTS AND APPRAISAL
Triadimefon is rapidly and readily absorbed from the
gastrointestinal tract. The route of excretion appears to vary
between sexes in the rat, where excretion is relatively slow.
Excretion is rapid in cows and pigs and extremely rapid in hens.
The compound is metabolized in all species, the major excreted
metabolite being triadimenol.
In studies designed to evaluate triadimefon for cumulative
toxicity, the liver appeared to be the major target organ. The liver
weight was affected in the dog, rat and mouse, the effect observed
being correlated in the dog and mouse with biochemical signs of liver
damage (e.g. increased transaminase activity levels in plasma). With
increasing doses, the first signs of a hepatotropic effect are
considered to be due to the increased microsomal liver enzyme
activities, as shown by the findings obtained in rats and dogs. The
TABLE 10. National maximum residue limits reported to the Meeting
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Australia Apple 1
Cucurbits 0.2 7
Grape 2 14
Raw cereals 0.5 28
Eggs, milk 0.1
Meat, meat products 0.05
Austria Pome fruit 35
Grape 35
Cucumber, pepper,
tomato 1 4
Other vegetables 14
Cereals 11 35
Sugarbeet 0.21 35
Belgium Barley, wheat 0.052 42
Apple 0.052 14
Cucumber, gherkin 0.052 3
Brazil Wheat 0.1 42
Bulgaria Apple 14
Grape 14
Sugarbeet 14
Tobacco 14
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Czechoslovakia Apple 28
Grape 28
Denmark Fruits (including small
fruits) 14
Cucumber 14
Wheat, barley 28
Fed.Rep.of Germany Apple and pear 0.53 14
Grape 33 35
Strawberry 0.23
Cucumber 0.53 3
Cereals 0.53
Hops 15 14
Other commodities of
plant origin 0.1
Israel Apple 0.05 14
Grape 0.1 21
Cucumber, melon, pumpkin,
pepper, tomato ,eggplant 3
Wheat 42
Netherlands Apple 0.1 14
Cereals 0.05 42
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
Portugal Apple 56
Grape (wine) 28
Grape (table) 21
Cucumber (including gherkin)
melon, pumpkin 3
Pea 7
Pepper 7
South Africa Apple, pear 0.05 28
Grape (table 0.05 7 resp 28-424
Grape (wine) 0.05 7 resp 28-424
Mango 0.05 28
Cucurbits 0.05 3
Pea 0.05 1
Spain Fruits, except grape 15
Grape 15-214
Artichoke 15
Cucumber, melon 15
Tomato
Sweden Fruits (including small
fruits and berries) 28
Switzerland Apple 0.1 21
Grape 0.5 21
Wine 0.5
TABLE 10. (con't)
Recommended
Country Crop/commodity Maximum residue pre-harvest
limit (mg/kg) interval (days)
UK Apple 14
Blackcurrant, gooseberry 14
Grape 14
Brussels sprout 14
1 Provisional;
2 Under consideration;
3 Proposed levels;
4 According to formulation.
only species showing histopathological changes was the mouse, at the
highest tested dose level. The most sensitive species with respect to
enzyme induction was the rat, which reacted to minimum oral daily
doses of 5 mg/kg bw. In the rat, the liver microsomal enzyme induction
was rapidly reversible within 4 weeks.
The other toxicological target was the haemapoietic system as
indicated by the results obtained in rats and dogs. Other effects
(only found in the rat) were observed in the kidney and testis and on
cholesterol levels, especially in the chronic toxicity study.
Moreover, these effects only appeared at the extremely high dose of
5 000 ppm in the diet (250 mg/kg bw), which caused excessive mucosal
lesions in the stomach and hence undoubtedly nutritional disorders.
Triadimefon was not mutagenic when tested with or without metabolic
activation in various strains of Salmonella, B; subtilis, E. coli
and S. cerevisiae.
The long-term studies on two rodent species did not provide any
indication of triadimefon having carcinogenic potential.
The information on the use pattern and pre-harvest intervals in
several countries was updated, and new information was obtained on use
patterns, pre-harvest intervals and national maximum residue limits in
other countries.
Additional data were obtained on residues from supervised trials,
some from countries not included in FAO/WHO 1979, on various fruits,
vegetables, cereals and some other crops, e.g. apple, red currant,
grape, strawberry, cucumber, melon, pepper, tomato, onion, pea, cereal
crops and sugarbeet. New information was obtained on residues from
supervised trials on black currant, raspberry, leek, parsnip, hops,
sweet potato and sugarcane.
Additional information was obtained on the fate of triadimefon
residues in plants, animals (including livestock), soils (including
microorganisms) and water, especially with regard to the further
degradation of the main plant and animal metabolite of triadimefon,
triadimenol. New information was also obtained on the fate of the
triazole part of the triadimefon molecule in plants, animals, soil and
water from studies with (triazole ring-3,5-14C)-triadimefon,
(triazole ring-3,5-14C)-triadimenol, (diastereoisomer A and B) and
1,2,4 (3,5-14C)-triazole. The latter three compounds are main
(triadimenol A and B) or minor plant and animal metabolites of
triadimefon.
Information on new or improved methods of residue analysis was
made available to the Meeting. Most of the methods are based on the
determination of the residue of triadimefon and triadimenol by gas
chromatography using a nitrogen-specific alkali flame detector(AFID).
The methods were modified for the determination of the triadimefon/
triadimenol residues in grape juice, wine and animal material (such as
cattle and poultry tissues, milk and eggs). The specificity of the
methods for triadimefon and triadimenol in plant material, such as
apples, meat and milk, has been demonstrated in interference studies.
The methods now available are suitable or can be adapted for
regulatory purposes.
Level causing no toxicological effects
Mouse : 300 ppm in diet, equivalent to 40 mg/kg bw/day
Rat : 50 ppm in diet, equivalent to 2.5 mg/kg bw/day
Dog : 330 ppm in diet, equivalent to 8.25 mg/kg bw/day
Estimated temporary admissible daily intake for man
0 - 0.01 mg/kg bw.
RECOMMENDATIONS OF RESIDUE LIMITS
The additional residue data evaluated by the Meeting in
conjunction with the data provided to the 1979 JMPR confirm the limits
proposed in 1979 for red currant, grape, strawberry, cucumber, melon,
pepper, tomato, onion, pea, cereal grains and coffee. The following
temporary maximum residue limits, expressed as the sum of triadimefon
and triadimenol, are recommended. The data presented on residues in
pineapple, Brussels sprout and leek are not sufficient to consider a
maximum residue limit.
Pre-harvest interval
Temporary MRL on which recommendation
Commodity (mg/kg) is based (days)
Apple 0.5 14
Black currant 1 7
Raspberry 0.2 7
Hops (dry) 15 14
Coffee beans 0.11
Meat 0.11
1 At or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1983)
Clarification of the effects on liver in mice and rats.
Desirable
1. Clarification of effects on the central nervous system observed
in acute studies.
2. Information on the use pattern (concentration, dipping time) of
the post-harvest treatment of pineapple and residues arising from
this use.
REFERENCES
Bayer. Reports submitted to FAO and WHO by Bayer AG.(Unpublished)
1981
Bomhard, E. and Löser, E. Chronic toxicity study on rats (two-year
1978a feeding experiment, 1 August. Report no. 7707, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
1978b Chronic toxicity study on mice (two-year feeding experiment)
1 August. Report no. 9344, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Brandes, W., Steffens, W., Führ, F. and Scheinpflug H. Weitere Un
1978 tersuchungen zur Translokation von 14C-Triadimefon.
Pflanzenschutz-Nachrichten Bayer, 31:132-144.
Buchenauer, H. Systemisch-fungizide Wirkung und Wirkungs mechanismus
1975 von Triadimefon (MEB 6447). Mitt. Biol. Bundesanst. Land-
Forst wirtsch. (Berlin-Dahlem), 165: 154-155.
1976 Studies on the systemic activity of Bayleton (triadimefon)
and its effects against certain fungal diseases of cereals.
Pflanzenschutz-Nachrichten, 29:266-280 (English edition)
Clark, T., Clifford, D.R., Deas, A., Gendle, P. and Watkins, D.
1978 Photolysis, metabolism and other factors influencing the
performance of triadimefon as a powdery mildew fungicide.
Pesticide Science, 9:497-506.
Deas, A.H.B. and Clifford, D.R. Metabolism of the 1,2,4-
1981 triazolylmethane fungicides, triadimefon, triadimenol and
diclobutrazol by Aspergillus niger (Van Tiegh). (In press)
Ecker, W. Biotransformation of 1,2,4-[3(5)-14C]triazol in rats.
1980 Bayer AG, Institut für Pharmakokinetik, Elberfeld, Pharma
Report no. 9478 (PF-Report no. 1471), 14 October 1980,
submitted by Bayer AG to WHO. (Unpublished)
Flucke, W. Bestimmung der akuten Toxizität (LD50) 16 February, Bayer
1981 AG, Institut für Toxikologie, report submitted by Bayer AG
to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Propineb (LH 30/Z)-Triadimefon (MEB 6447)
1977a Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7065, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Flucke, W. and Kimmerle, G. Triadimefon (MEB 6447) und Captafol,
1977b Untersuchungen zur akuten Toxizität nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr.
7069, Bayer AG, Institut für Toxikoligie, report submitted
by Bayer AG to WHO. (Unpublished)
1977c Triadimefon (MEB 6447) und Tolylfluanid (KUE 13 183 b),
Untersuchungen zur akuten Toxizitüt nach gleichzeitiger
Verabreichung beider Wirkstoffe, 25 Oktober, Bericht nr
7304, Bayer AG, Institut für Toxikologie, report submitted
by Bayer AG to WHO. (Unpublished)
Fredrickson, D.R. Metabolism of BAYLETONTM in rats. Mobay report no.
1978a 66201, 20 June 1978 (revised: 21 November 1979), submitted
by Bayer AG to WHO. (Unpublished)
1978b Metabolism of BAYLETONTM in cow. Mobay report no. 66202,
20 June 1978 (revised 21 November 1979), submitted by Bayer
AG to WHO. (Unpublished)
Gasztonyi, M. and Josepovits, G. The activation of triadimefon and its
1979 role in the selectivity of fungicide action. Pesticide
Science, 10:57-65.
Hoffman, K. and Gröning, P. Long-term toxicity study on dogs (two-year
1978 feeding study), 24 October. Report no. 7882, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Hoffman, K. and Luckhaus, G. Subchronic toxicity study on dogs,
1974 (thirteen-week feeding experiment) 16 December. Report no.
5071, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Inukai, H. and Iyatomi, A. Mutagenicity test on bacterial systems, 19
1977 December. Report no. 68, Nitokuno, Laboratory of Toxicology,
Japan, report submitted by Bayer AG to WHO. (Unpublished)
Jagannath, D.R., Brusick, and Hoorn, A.J.W. Mutagenicity evaluation of
1980 MEB 6447 in the reverse mutation induction assay, January.
R-Bericht nr. 1675, Litton Bionetics Inc., USA, report
submitted by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Burke, M.A. Acute oral toxicity of BAYLETONTM (formerly
1977 BAY MEB 6447) technical to adult mallard ducks, 11 May.
Report no. 52872, Mobay Chemical Corp., Agr. Div., submitted
by Bayer AG to WHO. (Unpublished)
Lamb, D.W. and Roney, D.J. Accumulation and persistence of residues in
1977 channel catfish exposed to BAYLETONTM-14C (formerly BAY
MEB 6447), Mobay Report no. 52775, 4 May, submitted by Bayer
AG to WHO. (Unpublished)
Löser, E. Multigeneration reproduction study on rats, 12 April. Report
1979 no. 8297, Bayer AG, Institut für Toxikologie, submitted by
Bayer AG to WHO. (Unpublished)
Machemer, L. Dominant lethal study on male mice to test for mutagenic
1976a effects, 27 January. Report no. 5837, Bayer AG, Institut für
Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
1976b Evaluation of embryotoxic and teratogenic effects on rats
following oral administration, 27 August. Report no. 6294,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
1976c Evaluation of embryotoxic and teratogenic effects on rabbits
following oral administration, 30 August. Report no. 6297,
Bayer AG, Institut für Toxikologie, submitted by Bayer AG to
WHO. (Unpublished)
Machemer, L. Micronucleus test on mice to evaluate MEB 6447 for
1977 mutagenic effects, 23 February. Report no. 6622, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Machemer, L. and Kimmerle, G. Evaluation of MEB 6447 (triadimefon) for
1976 embryotoxic and teratogenic effects on rats following
inhalation in dynamic flow apparatus, 30 August. Report no.
6298, Bayer AG, Institut für Toxikologie, submitted by Bayer
AG to WHO. (Unpublished)
Mihail, F. Acute toxicity studies, 27 June. Report no. 9277, Bayer AG,
1980 Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
Mihail, F. and Kaliner, G. Subakuter oraler Kumulationsversuch an
1979 Ratten, 20 February, Bericht nr. 8195, Bayer AG, Institut
für Toxikologie, report submitted by Bayer AG to WHO.
(Unpublished)
Mobay Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Mohr, U. Subchronic toxicity study on rats (twelve-week feeding
1976 experiment), 5 November. Report no. R840a, Medizinische
Hochschule Hannover, Abteilung für, experimentelle
Pathologie, submitted by Bayer AG to WHO. (Unpublished)
Nickless, G., Spitzer, T. and Pickard, J.A. Determination of
1981 triadimefon in grape juice and wine using capillary gas
chromatography. Journal of Chromatography, 208: 409-413.
Nitokuno. Reports submitted to FAO by Bayer AG. (Unpublished)
1981
Nye, D.E. The fate of BAYLETON-14C in poultry. Mobay Report no. 67482,
1979 8 March, submitted by Bayer AG to WHO. (Unpublished)
Pither, K.M. Metabolism of BAYLETONTM in male and female pigs. Mobay
1978 report no. 66509, 7 August (revised: 29 November 1978)
submitted by Bayer AG to WHO. (Unpublished)
Puhl, R.J. and Hurley, J.B. The metabolism and excretion of BAYTANTM
1978 by rats. Mobay Report no. 66489, 14 August 1978 (revised 26
September 1979 and 4 February 1980), submitted by Bayer AG
to WHO. (Unpublished)
Ritter, W. BAY e 8364 (MEB 6447): Pharmacokinetics in mice and rats.
1976 Bayer AG, Pharma-Institut für Pharmakokinetik, report no.
6458, 29 October, 1976, submitted by Bayer AG to WHO.
(Unpublished)
Scheinpflug, H. and Paul, V. On the mode of action of triadimefon.
1977 Netherlands Journal of Plant Pathology, 83(suppl.):105-111.
Scheinpflug, H., Paul, V. and Kraus, P. Untersuchungen zur
1977 Wirkungsweise von (R)Bayleton bei Getreidekrunkheiten. Mitt.
Biol. Bundesanst. Lund-Forstwirtschaft (Berlin-Dahlem)
178:178.
1978 Studies on the mode of action of (R)Bayleton against cereal
diseases. Pflanzenschutz Nach richten Bayer, 31:101-115.
Schlütter, K. Fungizine Wirkung von Triadimefon in der Gasphase. Z.
1977 Pflanzenkrank. Pflanzenschutz, 84:612-614.
Shirasu, Y., Moriya, M. and Koyashiki, R. Triadimefon-mutagenicity
1978 test on bacterial systems, 18 July. Department of
Toxicology, Institute of Environmental Toxicology, Japan,
report submitted by Bayer AG to WHO. (Unpublished)
Shirasu, Y., Moriya, M. and Miyazawa, T. Triadimefon-mutagenicity test
1979 of bacterial systems 27 October. Department of Toxicology,
Institute of Environmental Toxicology, Japan, report
submitted by Bayer AG to WHO. (Unpublished)
Specht, W. and Tillkes, M. Gaschromatographische Bestimmung von
Rückständen an Pflunzenbehandlungsmitteln nach Clean-up Uber
Gelchromatographic und mini-Kieselgel Säulenchromatographic.
Pflanzenschutz-Nachrichten Bayer, 33:61-85.
Thyssen, J. Studies on acute combination toxicity of triadimefon and
1977 carbadazim, 22 November. Report no. 7119, Bayer AG, Institut
für Toxikologie, submitted by Bayer AG to WHO. (Unpublished)
Thyssen, J. and Gröning, P. Fütterungsversuche mit Hühnern unter
1978 besonderer Berücksichtigung einer Möglichen Beeinflussung
der Fortpflanzung, 15 November. Bericht n.7924, Bayer AG,
Institut für Toxikologie, report submitted by Bayer AG to
WHO. (Unpublished)
Thyssen, J. and Kimmerle, G. MEB 6447-Acute toxicity studies, 3
1979 January, Report no.4416, Bayer AG, Institut für Toxikologie,
submitted by Bayer AG to WHO.(Unpublished)
Thyssen, J., Kimmerle, G. and Luckhaus, G. MEB 6447 - subacute
1974 toxicity studies, 25 January. Report no. 4464, Bayer AG,
Institut für Toxikologie, submitted by Bayer AG to WHO.
(Unpublished)
van Dijck, P. Evaluation of Bayleton (MEB 6447, triadimefon) for
1976 mutagenic potential by the Ames test with histidine-
auxotrophic Salmonella typhimurium strains, 27 September.
Laboratorium voor Hygiene, Leuven (Belgium), report
submitted by Bayer AG to WHO. (Unpublished)
Weber, H., Patzschke, K. and Wegner, L.A. 1,2,4-triazol-14C.
1978 Biokinetische Untersuchungen an Ratten. Bayer AG, Institut
für Pharmakokinetik, Elberfeld, Pharma Report no. 7920, 13
November 1978, submitted by Bayer AG to WHO. (Unpublished)
