
PESTICIDE RESIDUES IN FOOD - 1980
Sponsored jointly by FAO and WHO
EVALUATIONS 1980
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Expert Group on Pesticide Residues
Rome, 6-15 October 1980
PERMETHRIN
Explanation
Permethrin was reviewed at the 1979 Meeting, when a temporary
acceptable daily intake (ADI) of 0.03 mg/kg bodyweight was
recommended. Maximum residue limits (MRL's) were set for residues of
permethrin itself on a wide range of agricultural and horticultural
crops (FAO/WHO, 1980).
Further data on certain aspects of permethrin usage were requested for
future reviews and as a result additional data are now reviewed.
Special note - In this review the term permethrin refers to material
which is nominally 40:60 (±) cis:trans permethrin.
RESIDUES IN FOOD
USE PATTERN
Pre-harvest
Permethrin is a pyrethroid insecticide with a high level of activity
against Lepidopterous pests. It is also effective against a wide
range of Hemiptera, Diptera and Coleoptera. The compound is both a
stomach and a contact insecticide, and shows adulticidal, ovicidal
and, particularly, larvicidal activity. It is also extremely
effective against the great majority of insects resistant to commonly
used insecticides, such as organochlorines and organophosphates.
Unlike natural pyrethrins and earlier synthetic pyrethroids,
permethrin is relatively stable to degradation in sunlight. This
allows it to be used as a practical tool in agriculture. Permethrin
is not plant-systemic and has very little fumigant or translaminar
activity, a programme of sprays is usually required.
Pre-harvest use patterns, including those on forage crops, were
reviewed at the 1979 Meeting (FAO/WHO, 1980). The effective use rates
on various forage crops are shown in Table 1. In reviewing
pre-harvest uses, the 1979 Meeting noted that in several countries
target pests and use patterns are well defined, and pre-harvest
withholding intervals reflecting good agricultural practice in those
outlets are capable of being specified. However in many other
countries, particularly of the third world, and where climatic
conditions are conducive to a rapid build-up of insect infestations,
it is important to make available the full flexibility of a compound
such as permethrin. Therefore in very many countries no pre-harvest
withholding intervals are specified. This in made possible by a
combination of the low effective use rates and resulting comparatively
low residues immediately after spraying. It should also be noted that
permethrin residues on sprayed crop parts decline relatively slowly.
Therefore any benefit of a pre-harvest withholding interval as a means
of reducing residue levels tends to be less than that which accrues,
for example, with those organophosphate or carbamate insecticides that
show comparatively short persistence on sprayed plants.
TABLE 1. Effective Use Rates for Permethrin On Field Crops
Crop Countries Examples of Pests Which May Be Controlled Rates Pre-Harvest
(g ai/ha) Withholding
Interval
Cotton USA Heliothis virescens, H. zea, Anthonomus grandis, 110-220 14 days
Pectinophora goosypii, Lygus spp., Trichoplusia ni,
Bucculatrix thurberiella, Trialeurodes abutilonea,
Aphis gossypii.
Africa Earias, Diparopsis, Heliothis, Pectinophora, 75-200 Non-specified
Empoasca.
Rest of World, Heliothis, Earias, Spodoptera, Pectinophora, 75-200 up to 7 days
including Central/ Cryptophlebia, Diparopsis, Bucculatrix,
South America and Estigmena, Anthonomus, Lygus, Horcias,
Caribbean Trichoplusia, Sacadodes, Eutinobothrus,
Bemisia, Alabama, Dysdercus, Helopeltis,
Frankliniella, Caliothrips.
Soya Brazil Anticarsia gemmatalis, Trichoplusia ni 30-100 60 days
USA Pseudoplusia includens, Trichoplusia ni, 55-100 21 days
Anticarsia gemmatalis, Nezara viridula,
Plattypena scabra, Epilachna varivestis
Heliothis armigera, Laphygma exigua. 110-220 60 days
Alfalfa USA Hypera spp., Lygus spp. 55-220 up to 3 days
Maize Australia Heliothis, Spodoptera, Pyrausta, Sesamia, up to Typically
Canada Peridroma, Pseudaletia, Busseola, Agrotis, 330 1 day
Germany Oscinella
South Africa
USA
Oil seed Rape Western Europe Meligothes spp., Apion spp., Ceutherrhynchus spp. 50-100 (Generally 7
weeks or more)
Sorghum Brazil Spodoptera frugiperda 50 45 days
TABLE 2. Effective Use Rates For Permethrin In Animal Health (Spray Applications)
Maximum No. of Interval between
Target Country Pest Rate applications applications
controlled (g ai/head) per season (days)
Cattle USA Biting and ) ( )
nuisance flies, ) ( 5 ) 14
ticks; ) 1.5 ( )
lice & mites ) ( 2 )
Sheep Biting and ) (
nuisance flies, ) ( 4 21
ticks; ) 2.0 (
keds ) ( 2 14
Pigs Biting and ) ( )
nuisance flies, ) ( 5 )
ticks; ) 0.5 ( ) 14
lice & mites ) ( 2 )
Poultry Biting and ) (
nuisance flies, ) ( 3 28
ticks; ) 0.025 (
lice & mites ) ( 2 14
Buildings, Biting and 0.01 g ai/m2 as necessary as necessary
Breeding sites nuisance flies
TABLE 3. Effective Use Rates For Permethrin in Animal Health (Spray Applications)
Maximum No. of Interval between
Target Country Pest Rate applications applications
Controlled (g ai/m2) per season
Dairies Canada House flies 0.05 as necessary as necessary
Feedlots Stable flies
Stables
Livestock barns
TABLE 4. Effective Use Rates For Permethrin In Animal Health (Dust
Applications)
Target Country Pest Maximum Rate
Controlled (g ai/head)
Cattle USA Biting and 0.175-0.7
Horses nuisance flies,
ticks and lice
Pigs Biting and 0.075-0.3
nuisance flies,
ticks and lice
Post-harvest
Apart from its pre-harvest usage, permethrin has an important
application in the protection of stored grain, being particularly
useful when used in conjunction with organophosphorus (O/P)
insecticides (for example) to control insect species that are
O/P-resistant.
Permethrin has been applied at 1 mg/kg grain in large-scale trials in
Australia. At this level a single application of permethrin,
synergised with piperonyl butoxide, adequately controls the common
strains of Rhyzopertha dominica (the less grain borer), the most
destructive stored grain insect in Australia. When used in conjuction
with 4-6 mg pirimiphos-methyl/kg grain, the common strains of storage
grain insects are controlled for at least 9 months. Pirimiphos-methyl
does not control R. dominica. On the other hand, at the 1 mg/kg
grain rate, permethrin does not control Tribolium species (e.g. T.
castancum, the rust red flour beetle, which is presently the most
prevalent insect pest in Australian stored grain). Laboratory studies
have shown that when used at 4-5 mg/kg grain, a single application of
permethrin does give adequate control of all the commonly occurring
strains of storage insect pests when the grain is dry and cool. This
indicates a future option of varying pyrethroid and organophosphorus
protectant rates, in a complementary manner, to overcome future
problems due to insect resistance, absence of a synergist and/or lack
of a complementary insecticide. The future application rates of
permethrin could be raised above the present 1 mg/kg grain level,
though probably not above 2 mg permethrin/kg grain.
Ectoparasite uses
Permethrin can be used as a dust, spray or dip to control biting and
nuisance flies, ticks, lice, mites and keds, on cattle, goats, horses,
sheep, pigs and poultry (for effective use rates see Tables 2-4). It
can also be used to kill flies in or around agricultural buildings and
on insect breeding or resting sites for the control of house flies
(Musca domestica) and stable flies (Stomoxys calcitrans), for
which it is sold under the trade name `Ectiban' (ICI).
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest uses (forage crops)
A large body of residues data from supervised trials was reviewed at
the 1979 Meeting (FAO/WHO, 1980), which noted that permethrin and its
metabolites are effectively non-systemic in plants. Residues are
highest when crop parts which are consumed are exposed to the spray,
as in the case of forage crops (Table 5). Residue levels decline
comparatively slowly - the 1979 Meeting noted that "half-lives"
ranging from about one to three weeks depending on the crop, had been
demonstrated (FAO/WHO, 1980). The major degradation products are the
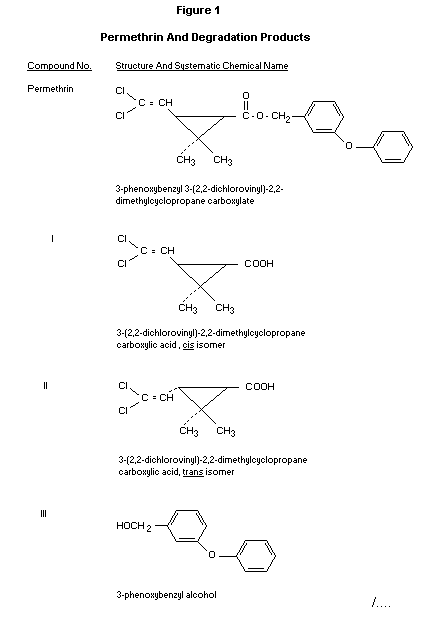
cis and trans isomers of 3-(2,2-dichlorovinyl)-
2,2-dimethylcyclopropane carboxylic acid (DCVA) plus 3-phenoxybenzyl
alcohol (3-PBAlc) (Figure 1), which are present primarily as
conjugates and which are also animal metabolites. Although residue
levels of permethrin, DCVA or 3-phenoxybenzyl alcohol on repeated
applications within the rates and frequency of spraying which are
needed to obtain good insecticidal control. Ground and aerial
applications yielded similar permethrin residue levels in a wide range
of crops (FAO/WHO, 1980).
 In forage crops such as alfalfa, residue levels of the metabolites
DCVA and 3-phenoxybenzyl alcohol are small compared with the
corresponding permethrin residues. This pattern was found on a range
of crops reviewed at the 1979 Joint Meeting which proposed maximum
residue limits based upon permethrin itself only (FAO/WHO, 1980).
In addition to crops grown specifically for forage, livestock feed can
also contain by-products of food processing (e.g. apple pomace, cotton
cake). Residue levels in these commodities are reviewed under "Fate
on Processing".
Post-Harvest Uses
The only significant post-harvest use of permethrin is its application
to bulk stored grain. This has undergone extensive laboratory studies
and silo-scale trials for this purpose in Australia. Since 1977 some
10,000 tons of grain have been treated in silo-scale pilot trials in
five of the Australian States. All the residue studies show that
permethrin is persistent on grain under the conditions of temperature
and moisture content prevailing in Australian storages (Table 6)
(Desmarchelier et al, 1979). Initial residues on grain were about
20% below the level expected from the amount applied. The residue
hardly declined in 9 months storage: after 6-9 months about 80% of the
initial (1 month) residue in grain remains. This level of persistence
is found consistently in studies on wheat, barley and sorghum, and
probably can be generalised for all stored grain (Bengston et al,
1979a, b, c; Desmarchelier et al, 1979). Also, studies show that
the initial ratio of cis/trans isomers is not changed during 8 months
of storage (Table 7) (Simpson, 1979).
Table 5, Sheet 3 (Permethrin Residues On Field Crops [Or Crop Parts] Used For Forage)
Crop Country and Formulations Rate of Volume Number of Interval Lowest Highest Mean Reference
Year Used Application of Spray Applications Between Last Residue Residue Residue
(g ai/ha) (l/ha) Spraying and Determined Determined (mg/kg)
Harvest (mg/kg) (mg/kg)
1. SWEETCORN
Maize USA 25% EC 1101 110-720 8 0-4 days <0.01 0.05 0.02(6) Ussary,
(Zea mays) 1976-8 during 1978b,
Cobs after 210-2201 ground 7-16 0-4 days <0.01 0.01 <0.01(13) 1979a
Kernals applications
Removed 280-450 or 30-135 6-13 0-4 days <0.01 0.12 0.02(11)
[code no. during
A03.1604] aerial
applications
Husks Canada 25% EC 62.5-70 280-1550 2-7 1-7 days 0.06 0.35 0.27(10) Chipman,
[code no. 1975-7 50% EC 30-46 days <0.01 0.33 0.17(2) Inc.,
A03.1604] 100-1051 1-4 1-14 days <0.01 0.35 0.23(13) 1978;
30-43 days <0.01 0.26 0.10(3) Ussary,
140-1501 3-5 3-7 days 0.34 0.36 0.35(2) 1978b,
46 days 0.02(1) 1979a
USA 25% EC 210-2201 110-720 7-8 0-3 days 0.43 14 6.4(7)
1976-8 280-335 6-9 0-14 days 1.0 18 3.7(10)
Stover USA 25% EC 1101 270-600 8 0-4 days 0.99 18 7.2(6) Ussary,
[code no. 1976-9 during 1978b,
A03.1604] 210-2201 ground 7-8 0-3 days 2.5 23 14(8) 1979a
(all data applications
on 24% dry 280-450 or 30-35 6-16 0-14 days 4.6 29 17(12)
matter basis) during
aerial
applications
1 Indicates effective use rates. For use patterns on these crops, see Table 1.
Figures in parentheses are the numbers of results upon which the means are based
TABLE 6. Permethrin Residues In Treated Wheat and Sorghum In Concrete Silos
(Australian Large-Scale Post-Harvest Treatments)
Wheat Data1
Grain conditions Amount Residues (mg/kg)
% moisture air temp. Applied (calc'd) Period of storage (months)
°C2 mg/kg 1 2 3 4 5 6 7 8 9 15
range 8-12 14-35 0.94-1.15 0.70-0.90 0.65-0.83 0.6-0.8 0.70-0.78 0.75-0.80 0.50-0.80 0.75-0.80 0.63-0.65 0.57 0.60-0.70
mean 0.99 0.83 0.75 0.70 0.74 0.74 0.66 0.78 0.64 0.57 0.65
s.d. 0.17 - 0.06 - - 0.14 - - - -
c.v.3 21 - 9 - - 21 - - - -
(Bengston et al, 1979a,b; Desmarchalier et al, 1979)
1 Trials included treatment of 30 tonnes of barley apart from some 10,500 tonnes wheat.
2 Initial temperatures were in the range 25-33°C, at 6 months they were 14-27°C, at 8 months they were 17-22°C. Initial moisture contents were
in the range 9-12%, at 6 months they were 8-12% and at 8 months they were 9-11%. Data are from 1977/78 and 1978/79 large scale trials. Grain
stored in 16 silos (30-1400 tonnes grain) in 5 Australian States.
3 s.d. = standard deviation; c.v. = co-efficient of variation.
Sorghum Data1
Grain Conditions Amount Residues (mg/kg)
% moisture2 air temps. °C2 Applied (calc'd) Period of Storage (months)
mg/kg 1 6 12 17 26
range 11.6 21.2 1.06 0.70-0.80 0.56-0.58 0.62-0.71 0.56-0.57 0.53-0.62
mean 0.75 0.57 0.67 0.57 0.58
(Bengston et al, 1979c)
1 Data from grain stored in a silo (430 tonnes) at one site. 1978 trial.
2 Initial
TABLE 7. Residues Of Cis And Trans Isomers Of Permethrin In Stored Grain
Percentage Proportions In Stored Wheat And Sorghum
Storage Conditions Original % Residues
% moisture air temps. °C1 proportion Period of Storage (weeks)
(nominal) 1 6 13 17 20 26 39
wheat 37 36 35.5 - 38.3 38.8 36.3
cis 10 32 40 63 64 64.5 - 61.7 61.2 63.7
trans 60
sorghum
cis 11.6 21.2 25 20.7 24.6 22.5 21.2 - 21
trans 75 79.3 75.4 77.5 78.8 - 79
(Simpson, 1979)
1 Initial
Ectoparasite uses
Cows were given five whole-body sprays of permethrin at a rate of 1.0
g ai/cow, with 14 days between sprays, using a 5% emulsifiable
concentrate formulation. They were allowed free access to a
self-oiler containing a 0.03 g ai/l solution, ensuring at least two
applications per day for a period of ten weeks, and were housed in
premises that were sprayed at a rate of 0.06 g ai/m2, five sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range that is likely to occur in normal
husbandry practice. Milk samples were taken for analysis before, and
during the ten days after, the fifth application. Only four out of 70
samples of milk analyzed showed any measurable residues of permethrin
or its metabolites cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethyl
cyclopropane carboxylic acid (DCVA), 3-phenoxybenzoic acid (3-PBAcid)
and 3-phenoxybenzylalcohol (3-PBAlc) (Figure 1) (Table 8) (Ussary and
Braithwaite, 1980a).
TABLE 8. Residues of Permethrin And Metabolites In Milk Following
Five
Dermal Applications Of Permethrin At 1.0 g ai/cow
Time after Lowest Highest Mean
Chemical application Residue Residue Residue
(days) (mg/kg) (mg/kg) (mg/kg)
Permethrin 0 <0.01 0.01 0.01 (20)1
1 <0.01 0.01 0.01 (10)
3-7 <0.01 <0.01 <0.01 (30)
10 <0.01 0.01 0.01 (10)
DCVA (I+II) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAcid (IV) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAlc (III) 0-7 <0.01 <0.01 <0.01 (60)
10 <0.01 0.02 0.01 (11)
1 Bracketed figures are numbers of samples
Ussary and Braithwaite, 1980a
Cows were given 6 whole-body sprays of permathrin at a rate of 1.0 g
ai/cow with 14 days between sprays. They were allowed free access to
a self-oiler containing a 0.03 g ai/l solution ensuring at least two
applications per day for a period of ten weeks and were housed in
premises which were sprayed at a rate of 0.06 g ai/m2, six sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range which is likely to occur in normal
husbandry practice. Cows were slaughtered five days after the sixth
application, when permathrin levels in muscle, liver and the kidneys
were low (<0.01 mg/kg). The highest levels of permethrin were 0.10
mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat
respectively (Table 9) (Ussary and Braithwaite, 1980b).
TABLE 9. Residues Of Permethrin In Tissues of Cows Following Dermal
Applications
Tissue Permethrin Residue1
(mg/kg)
Muscle <0.01
Kidney <0.01
Liver <0.01
Intestinal fat 0.05-0.10
Subcutaneous fat 0.03-0.04
1 Limit of determination: 0.01 mg/kg (Ussary and Braithwaite,
1980b)
Pigs were housed in premises treated with mist applications of 0.06 g
permethrin per m3 at 14-day intervals and slaughtered one day after
the sixth application. Permethrin residues were not measurable in
skin, liver or kidney tissues (limit of determination: 0.01 mg/kg) and
only low levels of permethrin were found in the fat and muscle tissues
(0.02 and up to 0.01 mg/kg respectively) (Table 10) (Ussary and
Braithwaite, 1980c).
TABLE 10. Residues of Permethrin Detected in Swine Tissues after Six
Mist Applications of Permethrin to Swine Premises at O.O6 g per m2
Tissue Permethrin Residues
(mg/kg)
Skin <0.01
Muscle <0.01
Liver <O.01
Kidney <0.01
Subcutaneous fat 0.02
Intestinal fat 0.02
(Ussary and Braithwaite, 1980c)
Hens were present at the times of application when premises received
six mist applications of 0.06 g permethrin per m3 at 14-day
intervals. Eggs were collected at intervals up to 50 days following
the first application and hens were sacrificed five days after the
sixth application. Eggs were found to contain permethrin at 0.02
mg/kg on one occasion only. Otherwise, permethrin residues in muscle,
skin, liver and eggs were below 0.01 mg/kg. A residue of 0.02 mg/kg
was detected in fat tissues (Table 11) (Ussary and Braithwaite,
1980d).
TABLE 11. Residues of Permethrin in Eggs and Tissues Chickens
Maintained in Premises Treated with Permethrin
Tissue Permethrin
(mg/kg)
Muscle <0.01
Skin <0.01
Liver <0.01
Fat 0.02
Eggs <0.02
(Ussary and Braithwaite, 1980d)
FATE OF RESIDUES
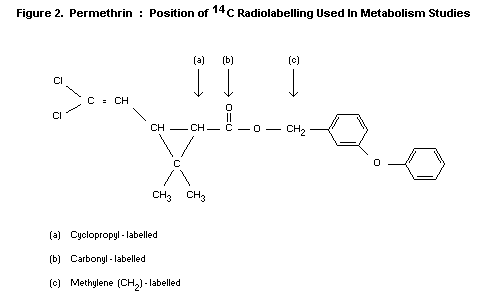
In the text which follows, reference will be made to various sites of
radiolabelling of permethrin. These will refer to 14C-labelling as
shown in Figure 2.
In farm animals
Introduction
Data on absorption, excretion and accumulation after oral dosing of
permethrin were reviewed at the 1979 Meeting (FAO/WHO, 1980).
Permethrin is extensively metabolised and rapidly excreted by cows and
goats after oral administration. Residue levels in milk, muscle and
fat are small, as they are in the skin, muscle and eggs of hens.
Permethrin itself constitutes more than half of the residue in milk,
eggs and fat, and in the muscle of livestock. In all cases, residue
levels decline notably on cessation of exposure.
After dermal application the majority of the small residues detected
are found in the tissues, at or immediately below the site of
application. At least 70% of the total residue in those tissues is
due to permethrin itself. Residues in milk and eggs reach a peak a
few days after dermal application and then decline rapidly.
In forage crops such as alfalfa, residue levels of the metabolites
DCVA and 3-phenoxybenzyl alcohol are small compared with the
corresponding permethrin residues. This pattern was found on a range
of crops reviewed at the 1979 Joint Meeting which proposed maximum
residue limits based upon permethrin itself only (FAO/WHO, 1980).
In addition to crops grown specifically for forage, livestock feed can
also contain by-products of food processing (e.g. apple pomace, cotton
cake). Residue levels in these commodities are reviewed under "Fate
on Processing".
Post-Harvest Uses
The only significant post-harvest use of permethrin is its application
to bulk stored grain. This has undergone extensive laboratory studies
and silo-scale trials for this purpose in Australia. Since 1977 some
10,000 tons of grain have been treated in silo-scale pilot trials in
five of the Australian States. All the residue studies show that
permethrin is persistent on grain under the conditions of temperature
and moisture content prevailing in Australian storages (Table 6)
(Desmarchelier et al, 1979). Initial residues on grain were about
20% below the level expected from the amount applied. The residue
hardly declined in 9 months storage: after 6-9 months about 80% of the
initial (1 month) residue in grain remains. This level of persistence
is found consistently in studies on wheat, barley and sorghum, and
probably can be generalised for all stored grain (Bengston et al,
1979a, b, c; Desmarchelier et al, 1979). Also, studies show that
the initial ratio of cis/trans isomers is not changed during 8 months
of storage (Table 7) (Simpson, 1979).
Table 5, Sheet 3 (Permethrin Residues On Field Crops [Or Crop Parts] Used For Forage)
Crop Country and Formulations Rate of Volume Number of Interval Lowest Highest Mean Reference
Year Used Application of Spray Applications Between Last Residue Residue Residue
(g ai/ha) (l/ha) Spraying and Determined Determined (mg/kg)
Harvest (mg/kg) (mg/kg)
1. SWEETCORN
Maize USA 25% EC 1101 110-720 8 0-4 days <0.01 0.05 0.02(6) Ussary,
(Zea mays) 1976-8 during 1978b,
Cobs after 210-2201 ground 7-16 0-4 days <0.01 0.01 <0.01(13) 1979a
Kernals applications
Removed 280-450 or 30-135 6-13 0-4 days <0.01 0.12 0.02(11)
[code no. during
A03.1604] aerial
applications
Husks Canada 25% EC 62.5-70 280-1550 2-7 1-7 days 0.06 0.35 0.27(10) Chipman,
[code no. 1975-7 50% EC 30-46 days <0.01 0.33 0.17(2) Inc.,
A03.1604] 100-1051 1-4 1-14 days <0.01 0.35 0.23(13) 1978;
30-43 days <0.01 0.26 0.10(3) Ussary,
140-1501 3-5 3-7 days 0.34 0.36 0.35(2) 1978b,
46 days 0.02(1) 1979a
USA 25% EC 210-2201 110-720 7-8 0-3 days 0.43 14 6.4(7)
1976-8 280-335 6-9 0-14 days 1.0 18 3.7(10)
Stover USA 25% EC 1101 270-600 8 0-4 days 0.99 18 7.2(6) Ussary,
[code no. 1976-9 during 1978b,
A03.1604] 210-2201 ground 7-8 0-3 days 2.5 23 14(8) 1979a
(all data applications
on 24% dry 280-450 or 30-35 6-16 0-14 days 4.6 29 17(12)
matter basis) during
aerial
applications
1 Indicates effective use rates. For use patterns on these crops, see Table 1.
Figures in parentheses are the numbers of results upon which the means are based
TABLE 6. Permethrin Residues In Treated Wheat and Sorghum In Concrete Silos
(Australian Large-Scale Post-Harvest Treatments)
Wheat Data1
Grain conditions Amount Residues (mg/kg)
% moisture air temp. Applied (calc'd) Period of storage (months)
°C2 mg/kg 1 2 3 4 5 6 7 8 9 15
range 8-12 14-35 0.94-1.15 0.70-0.90 0.65-0.83 0.6-0.8 0.70-0.78 0.75-0.80 0.50-0.80 0.75-0.80 0.63-0.65 0.57 0.60-0.70
mean 0.99 0.83 0.75 0.70 0.74 0.74 0.66 0.78 0.64 0.57 0.65
s.d. 0.17 - 0.06 - - 0.14 - - - -
c.v.3 21 - 9 - - 21 - - - -
(Bengston et al, 1979a,b; Desmarchalier et al, 1979)
1 Trials included treatment of 30 tonnes of barley apart from some 10,500 tonnes wheat.
2 Initial temperatures were in the range 25-33°C, at 6 months they were 14-27°C, at 8 months they were 17-22°C. Initial moisture contents were
in the range 9-12%, at 6 months they were 8-12% and at 8 months they were 9-11%. Data are from 1977/78 and 1978/79 large scale trials. Grain
stored in 16 silos (30-1400 tonnes grain) in 5 Australian States.
3 s.d. = standard deviation; c.v. = co-efficient of variation.
Sorghum Data1
Grain Conditions Amount Residues (mg/kg)
% moisture2 air temps. °C2 Applied (calc'd) Period of Storage (months)
mg/kg 1 6 12 17 26
range 11.6 21.2 1.06 0.70-0.80 0.56-0.58 0.62-0.71 0.56-0.57 0.53-0.62
mean 0.75 0.57 0.67 0.57 0.58
(Bengston et al, 1979c)
1 Data from grain stored in a silo (430 tonnes) at one site. 1978 trial.
2 Initial
TABLE 7. Residues Of Cis And Trans Isomers Of Permethrin In Stored Grain
Percentage Proportions In Stored Wheat And Sorghum
Storage Conditions Original % Residues
% moisture air temps. °C1 proportion Period of Storage (weeks)
(nominal) 1 6 13 17 20 26 39
wheat 37 36 35.5 - 38.3 38.8 36.3
cis 10 32 40 63 64 64.5 - 61.7 61.2 63.7
trans 60
sorghum
cis 11.6 21.2 25 20.7 24.6 22.5 21.2 - 21
trans 75 79.3 75.4 77.5 78.8 - 79
(Simpson, 1979)
1 Initial
Ectoparasite uses
Cows were given five whole-body sprays of permethrin at a rate of 1.0
g ai/cow, with 14 days between sprays, using a 5% emulsifiable
concentrate formulation. They were allowed free access to a
self-oiler containing a 0.03 g ai/l solution, ensuring at least two
applications per day for a period of ten weeks, and were housed in
premises that were sprayed at a rate of 0.06 g ai/m2, five sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range that is likely to occur in normal
husbandry practice. Milk samples were taken for analysis before, and
during the ten days after, the fifth application. Only four out of 70
samples of milk analyzed showed any measurable residues of permethrin
or its metabolites cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethyl
cyclopropane carboxylic acid (DCVA), 3-phenoxybenzoic acid (3-PBAcid)
and 3-phenoxybenzylalcohol (3-PBAlc) (Figure 1) (Table 8) (Ussary and
Braithwaite, 1980a).
TABLE 8. Residues of Permethrin And Metabolites In Milk Following
Five
Dermal Applications Of Permethrin At 1.0 g ai/cow
Time after Lowest Highest Mean
Chemical application Residue Residue Residue
(days) (mg/kg) (mg/kg) (mg/kg)
Permethrin 0 <0.01 0.01 0.01 (20)1
1 <0.01 0.01 0.01 (10)
3-7 <0.01 <0.01 <0.01 (30)
10 <0.01 0.01 0.01 (10)
DCVA (I+II) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAcid (IV) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAlc (III) 0-7 <0.01 <0.01 <0.01 (60)
10 <0.01 0.02 0.01 (11)
1 Bracketed figures are numbers of samples
Ussary and Braithwaite, 1980a
Cows were given 6 whole-body sprays of permathrin at a rate of 1.0 g
ai/cow with 14 days between sprays. They were allowed free access to
a self-oiler containing a 0.03 g ai/l solution ensuring at least two
applications per day for a period of ten weeks and were housed in
premises which were sprayed at a rate of 0.06 g ai/m2, six sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range which is likely to occur in normal
husbandry practice. Cows were slaughtered five days after the sixth
application, when permathrin levels in muscle, liver and the kidneys
were low (<0.01 mg/kg). The highest levels of permethrin were 0.10
mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat
respectively (Table 9) (Ussary and Braithwaite, 1980b).
TABLE 9. Residues Of Permethrin In Tissues of Cows Following Dermal
Applications
Tissue Permethrin Residue1
(mg/kg)
Muscle <0.01
Kidney <0.01
Liver <0.01
Intestinal fat 0.05-0.10
Subcutaneous fat 0.03-0.04
1 Limit of determination: 0.01 mg/kg (Ussary and Braithwaite,
1980b)
Pigs were housed in premises treated with mist applications of 0.06 g
permethrin per m3 at 14-day intervals and slaughtered one day after
the sixth application. Permethrin residues were not measurable in
skin, liver or kidney tissues (limit of determination: 0.01 mg/kg) and
only low levels of permethrin were found in the fat and muscle tissues
(0.02 and up to 0.01 mg/kg respectively) (Table 10) (Ussary and
Braithwaite, 1980c).
TABLE 10. Residues of Permethrin Detected in Swine Tissues after Six
Mist Applications of Permethrin to Swine Premises at O.O6 g per m2
Tissue Permethrin Residues
(mg/kg)
Skin <0.01
Muscle <0.01
Liver <O.01
Kidney <0.01
Subcutaneous fat 0.02
Intestinal fat 0.02
(Ussary and Braithwaite, 1980c)
Hens were present at the times of application when premises received
six mist applications of 0.06 g permethrin per m3 at 14-day
intervals. Eggs were collected at intervals up to 50 days following
the first application and hens were sacrificed five days after the
sixth application. Eggs were found to contain permethrin at 0.02
mg/kg on one occasion only. Otherwise, permethrin residues in muscle,
skin, liver and eggs were below 0.01 mg/kg. A residue of 0.02 mg/kg
was detected in fat tissues (Table 11) (Ussary and Braithwaite,
1980d).
TABLE 11. Residues of Permethrin in Eggs and Tissues Chickens
Maintained in Premises Treated with Permethrin
Tissue Permethrin
(mg/kg)
Muscle <0.01
Skin <0.01
Liver <0.01
Fat 0.02
Eggs <0.02
(Ussary and Braithwaite, 1980d)
FATE OF RESIDUES
In the text which follows, reference will be made to various sites of
radiolabelling of permethrin. These will refer to 14C-labelling as
shown in Figure 2.
In farm animals
Introduction
Data on absorption, excretion and accumulation after oral dosing of
permethrin were reviewed at the 1979 Meeting (FAO/WHO, 1980).
Permethrin is extensively metabolised and rapidly excreted by cows and
goats after oral administration. Residue levels in milk, muscle and
fat are small, as they are in the skin, muscle and eggs of hens.
Permethrin itself constitutes more than half of the residue in milk,
eggs and fat, and in the muscle of livestock. In all cases, residue
levels decline notably on cessation of exposure.
After dermal application the majority of the small residues detected
are found in the tissues, at or immediately below the site of
application. At least 70% of the total residue in those tissues is
due to permethrin itself. Residues in milk and eggs reach a peak a
few days after dermal application and then decline rapidly.
 Cows and goats
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bodyweight, equivalent to approximately 80 mg/kg in the
diet, levels of radioactivity in milk reached a maximum of 0.13 mg
permethrin equivalents/kg after 1-2 days. These declined to less than
0.02 mg/kg after seven days. Levels of radioactivity in the fat were
0.12-0.18 mg permethrin equivalents/kg, after seven days. By fourteen
days these had declined to 0.05-0.06 mg/kg, indicating that the small
residues in fat are also not maintained on cessation of dosing (Bewick
and Leahey, 1976).
Hunt and Gilbert dosed goats orally with either the cis- or
trans-isomers of 14C-labelled permethrin (carbonyl- or
methylene-labelled) at a rate equivalent to approximately 6 mg/kg in
the diet for ten days. Total radioactive residues in the milk reached
a plateau after three days of 0.02-0.05 and <0.01-0.01 mg permethrin
equivalents/kg respectively for the cis- and trans- isomers. The
goats were sacrificed 24 hours after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 12.
Total radioactivity in the fat of animals receiving the cis-isomer
was ten times higher than in those receiving the more readily
hydrolysed trans-isomer (Table 12) (Hunt and Gilbert, 1977).
When cis- and trans-isomers of 14C-labelled permethrin (carbonyl-
and methylene-labelled) were administered orally to lactating Jersey
cows for three consecutive days at approximately 1 mg/kg bodyweight,
radioactivity was largely eliminated from the body in faeces and urine
within 12 or 13 days after the initial treatment. Total
14C-permethrin equivalents in milk were consistently below 0.3 mg/kg
and declined on cessation of exposure. Residues in fat were also low.
Residues recovered from fat and milk were higher when cis-
permethrin rather than trans-permathrin was administered and
consisted almost entirely (>85%) of unmetabolised permethrin.
Total 14C-permethrin equivalents in blood reached a transient peak
shortly after each dose and dropped to insignificant levels within 2-4
days after the last dose (Gaughan et al, 1976, 1978a).
A study with non-radiolabelled permethrin supported the finding that
permethrin itself is the predominant residue in milk. It was also the
major residue in cow muscle. In this study, groups each of three
barren Friesian cows, yielding 9-13 litres of milk per day, were
maintained on diets containing permethrin at approximately 0.2, 1.0,
10 or 50 mg/kg. The permethrin was absorbed on grass nuts. After
28-31 days two cows in each group were sacrificed. The third was
returned to control diet for nine days before sacrifice. Samples of
milk and of meat tissues were analyzed for residues of permethrin,
cis and trans 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II, DCVA) taken together,
3-phenoxybenzylalcohol (III; 3-PBAlc) and 3-phenoxybenzoic acid (IV;
3-PBAcid) (Figure 1) by the gas chromatographic methods reviewed under
"Methods of Residue Analysis".
TABLE 12. Total Radio-labelled Residues In Tissues of Goats Receiving 14C-Labelled Permethrin
Daily Orally For 7-10 Days
Material Dose Level Duration of Period Between Total Residue (mg permethrin
Administered (= mg/kg Administration Last Date And equivalents/kg In
in diet) (days) Sacrifice Fat Muscle Liver Kidney
Permethrin, ‰10 7 4 hours <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis-trans
Permethrin, ‰6 10 24 hours 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin, ‰6 10 24 hours 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
Hunt and Gilbert, 1977
Leahey et al, 1977a
TABLE 13. Characterisation of 14C-Labelled Residues In Liver And Kidney Of Goats
Receiving Permethrin Orally Daily For Seven Days
Radioactivity Soluble in Water
% Of % of Radioactivity % of Radioactivity
Organ Position of Radioactivity Soluble in Diethyl Remaining Water Unextracted
14C-Labelling Soluble in Ether Following Soluble After
Of Permethrin Diethyl Ether Hydrolysis Hydrolyais
Liver Methylene ("Alcohol") 16 41 23 note 1
Cyclopropyl ("Acid") 39 24 36 note 1
Kidney Methylene ("Alcohol") 43 42 <1 6
Cyclopropyl ("Acid") 29 20 22 9
1 liver was almost completely solubilised during refluxing.
Leahey et al, 1977a
Only permethrin itself was detected in milk. Low levels of 0.01-0.06
mg/kg (mean 0.02 mg/kg) and 0.03-0.2 (mean 0.1 mg/kg) were present in
the milk of cows in the 10 and 50 mg/kg groups respectively. These
values are approximately 0.2% of the corresponding dietary levels.
Permethrin residues in milk were less than 0.01 mg/kg at the 0.2 and
1.0 mg/kg dietary inclusion rates. None of the three metabolites was
found in any of the milk samples analyzed (limit of determination:
0.01 mg/kg). Permethrin levels in milk did not accumulate over the
period of the study and they declined rapidly, to below 0.01 mg/kg
after five days, on returning the animals to control diet (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
Permethrin itself was also the major part of the small residue levels
found in adductor, pectoral and cardiac muscle. These small levels
were approximately 0.1% of the corresponding dietary inclusion levels
(Table 14). Permethrin residue levels in peritoneal fat were
invariably higher than in subcutaneous fat (Table 15) (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
As found separately by Leahey et al (below), metabolites rather than
permethrin itself constituted the major part of the residues in liver
and kidney. Again, the levels declined rapidly on cessation of
exposure (Table 14) (Edwards and Iswaren, 1977; Swaine et al,
1980a).
Leahey et al dosed goats orally with 40:60 cis:trans 14C-
labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 mg/kg in the diet for seven days.
Total radioactive residues in the milk reached a plateau of 0.02-0.03
mg permethrin equivalents/kg after five days; 30-50% of this
radioactivity was associated with the butterfat fraction of the milk,
in which total radioactive residues were 0.13-0.27 mg permethrin
equivalents/kg.
The animals were sacrificed four hours after receiving the final dose,
when levels of radiocarbon in meat tissues were as shown in Table 12.
Characterization of the residues in liver and kidney, the two tissues
containing the highest total residues is shown in Table 13. Where
"alcohol"-labelled permethrin was used, approximately 70% of the 14C
in kidney tissue was 3-phenoxybenzoic acid (IV) plus
3-(4'-hydroxyphenoxy) benzoic acid (VI) (Figure 1). Approximately 30%
of the 14C in the liver was due to 3-phenoxybenzyl alcohol (III) plus
3-(4'-hydroxyphenoxy) benzyl alcohol (V). A further 15% was due to
3-phenoxybenzoic acid (IV) plus 3-(4'-hydroxyphenoxy) benzoic acid
VI). Where "acid"-labelled permethrin was used, approximately 10-15%
of the label in liver and kidney was due to the cis and trans
3-(2,2-dichlorovinyl) cyclopropane carboxylic acids (I and II)
(principally the trans isomer). (Leahey et al, 1977a).
TABLE 14. Residues Of Permethrin and Three Major Metabolites in Tissues Of Cows
Receiving Permethrin In The Diet
Nominal Residue (mg/kg) Of
Dietary Feeding Regime Tissue
Inclusion Analysed Cis & Trans
Level Permethrin DCVA 3-PBAlc 3-PBAcid
(mg/kg) (I and II) (III) (IV)
50 "Treated"1 Liver <0.01 0.01-0.04 0.05-0.10 0.03-0.12
Kidney 0.02-0.06 0.07-0.09 0.01-0.09 0.03-0.04
Muscle O.03-0.10 <0.01-0.02 <0.131 <0.01
"Treated plus Liver <0.01 0.01 <0.01 <0.01
Recovery"2 Kidney 0.02 0.06 <0.01 <0.01
Muscle 0.02-0.06 <0.01 <0.01 <0.01
10 "Treated" Liver <0.01 <0.01 0.01-0.03 0.01-0.02
Kidney <0.01-0.01 <0.01-0.02 <0.01-0.01 <0.01-0.02
Muscle <0.01-0.03 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery Kidney 0.01 <0.01 <0.01 0.04
Muscle 0.01-0.02 <0.01 <0.01 <0.01
1.0 "Treated" Liver <0.01 <0.01 <0.01 <0.01
Kidney <0.01 <0.01 <0.01 <0.01-0.01
Muscle <0.01 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery" Kidney 0.01 <0.01 <0.01 0.02
Muscle <0.01 <0.01 <0.01 <0.01
0.2 "Treated" Liver <0.01 - - -
Kidney <0.01 - - -
Muscle <0.01 - - -
"Treated plus Liver <0.01 - - -
Recovery" Kidney 0.01 - - -
Muscle <0.01 - - -
1 "Treated" indicates the animals received treated diet for 28-31 days and were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28 days and were then
returned to untreated diet for a further 9 days before slaughter.
Edwards and Iswaren, 1977; Swaine et al, 1980a
TABLE 15. Residues In Fat Of Cows Receiving Permethrin In The Diet
Nominal Residues (mg/kg) of Permethrin In
Dietary Inclusion
Level (mg/kg) Peritoneal Fat Subcutaneous Fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
(Edwards and Iswaren, 1977)
TABLE 16. Total Radiolabelled Residues In Tissues Of Cows Seven Days
After Receiving A Single Dermal Dose Of 1.25 g of 40:60 Cis:Trans
14C-Labelled Permethrin
Tissue Residue in tissue Residue in tissue
from cow treated from cow treated
with alcohol-labelled with acid-labelled
permethrin, mg/kg1 permethrin µg/kg1
Meat <0.01 <0.01
Subcutaneous fat 0.053 0.033
Perirenal fat 0.046 0.054
Liver 0.080 0.064
Kidney 0.048 0.038
1 Residues expressed as mg/kg permethin equivalents. Bewick et al,
1977.
Permethrin itself also constitutes the major part of the small
residues found in the milk and fat of cows after dermal application.
In one study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). Although the concentration of suspension used
(0.5% w/v) was ten times the maximum that is likely to be used in
practice, the total dose of 40 mg per cow was approximately 1/30 of
the amount that could be applied commercially at any one time. The
dose was placed on an area of 225 cm2.
Levels of radioactivity in the milk and blood reached a maximum during
the first few days after dosing and then declined steadily. Total
residues in milk were always less than 2 µg permethrin equivalents/kg
(i.e. less than 2 ppb) and were below the limit of determination (0.3
µg/kg) by the end of fourteen days following treatment.
Total radiolabelled residues in muscle, liver, kidney and heart were
also small - 0.02 mg permethrin equivalents/kg or below seven days
after treatment - and were considerably smaller after a further seven
days.
Highest residues were found in fat from below the treatment area.
Seven days after treatment the total residue was approximately 0.5 mg
permethrin equivalents/kg, of which at least 80% was due to permethrin
itself. After a further seven days, residues in fat below the site of
application had fallen by about one half; the residue remaining was
again characterised as being due mainly to permethrin itself (Bewick
and Leahey, 1976).
In a second study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 1.25 g per cow applied in a 0.5% w/v
emulsion is at the higher end of the range likely to be used
commercially. The permethrin was brushed onto 25% of the surface area
of the animals.
Levels of radioactivity found in milk were very similar for the two
positions of radiolabelling. Residues reached a peak of approximately
0.01 mg permethrin equivalents/kg after 3-5 days; 89-91% of these
residues could be extracted into butter fat in which the major portion
of the residue (at least 51-68%) was characterised as permethrin
(Leahey and Cameron, 1978).
Seven days after treatment only small residues were present in the
animal tissues. Levels of radioactivity were similar in perirenal and
subcutaneous fat and for the two positions of radiolabelling (Table
16). At least 40% of this residue was characterised as permethrin
(Leahey and Cameron, 1978).
Pigs
Pigs were dosed dermally with 40:60 cis:trans 14C-labelled
permethrin (cyclopropyl or methylene-labelled). Although the
concentration of suspension used (0.5% w/v) was ten times the maximum
that is likely to be used in practice, the total dose of 18 mg per pig
is approximately 1/30 of the amount that could be applied commercially
at any one time. The dose was placed on an area of 100 cm2.
Seven and fourteen days after treatment approximately 1% of the
applied dose remained at the site of application, principally (>95%)
as permethrin. A residue of 0.05 mg permethrin equivalents/kg in fat
immediately below the site of application seven days after treatment
was due essentially to permethrin. After fourteen days, no
radioactive residue was found (limit of determination: 0.012 mg
permethrin equivalents/kg) (Bewick et al, 1977).
A residue of 0.01 mg permethrin equivalents/kg was found in muscle
beneath the site of application seven days after treatment.
Otherwise, no residues were detected in muscle, liver, kidney, heart
or blood either seven or fourteen days after treatment (limit of
determination: approximately 0.001 mg permethrin equivalents/kg)
(Bewick et al, 1977).
Hens
In one study hens received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (cyclopropyl- and methylene-labelled) at a
rate equivalent to 30 mg/kg in the diet. Birds were sacrificed one,
seven and fourteen days after treatment.
Throughout the study, residues in breast muscle, leg muscle and heart
were small (less than 0.04 mg permethrin equivalent/kg). Total
radioactive residues in liver and kidney of 0.22-0.25 mg/kg had
decreased by an order of magnitude after seven days. Residues of
0.20-0.35 mg permethrin equivalents/kg in peritoneal and subcutaneous
fat on the day after treatment also declined during the remainder of
the study, albeit more slowly than in the case of liver and kidney
(Leahey et al, 1977c).
Levels of radioactivity in eggs reached a maximum soon after dosing
(in 1-4 days) and then declined during the remainder of the 14-day
study (Leahey et al, 1977c).
Hens have also been dosed orally with 40:60 cis:trans
14C-permethrin (cyclopropyl- or methylene-labelled) for ten days at a
rate equivalent to approximately 10 mg/kg in the diet or separately
with cis- and trans-isomers (carbonyl- or methylene-labelled) for
three days at a rate equivalent to approximately 80 mg/kg in the diet.
Residues in eggs were present primarily (>75%) in the yolks, in which
radioactivity reached a plateau after 5-8 days of 0.3-0.5 mg
permethrin equivalents/kg in the 10-dose study and 0.6 mg/kg (trans-
isomer administered) or 2.1-2.8 mg/kg (cis-isomer administered) in
the 3-dose study. Permethrin was the major compound identified in the
eggs (52-62%). The cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acids (I and II) and 3-phenoxybenzyl alcohol (III) (Figure 1) were the
major metabolites in eggs, each normally accounting for less than
approximately 10% of the total radioactivity. The carboxylic acids
were present both free and as the glucuronide and taurine conjugates.
Other metabolites arose from hydroxylation in the 4'-position of the
"alcohol" moiety and in the trans-2-methyl moiety in the "acid" part
of the molecule.
The hens were sacrificed four hours after receiving the final dose in
the 10-dose study, when residues were as shown in Table 17. As in the
case of eggs, residues in fat derived from both "acid" and "alcohol"
labels were similar. Permethrin itself represented the major residue
in the fat. Compounds I-III and 3-phenoxybenzoic acid (IV) were also
identified (each less than 10% of the total radioactivity in the fat).
In both muscle and liver, higher residues were detected in hens dosed
with "acid"-labelled permethrin than with "alcohol"-labelled. The
cis- and trans-3-2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II) were the major residues identified in
these tissues. Blood levels declined rapidly during the first 24
hours after administration (Gaughan et al, 1978b; Leahey et al,
1977b).
In a study with non-radiolabelled 40:60 cis:trans permethrin,
groups of 40 laying hens were fed on diets containing approximately
0.4, 3.4 and 33 mg/kg for 28 days and then returned to a control diet
for an additional 14 days. Samples of eggs laid during the study were
analyzed for permethrin residues by the gas chromatographic method
described under "Methods of Residue Analysis". Five hens per group
were sacrificed after 21, 28, 35 and 42 days of the study and tissues
analyzed for permethrin.
At the 0.4 mg/kg dietary inclusion rate no residues of permethrin were
detected on the albumen and yolks of eggs (limit of determination:
0.02 mg/kg) or in the muscle, skin and liver (limit of determination:
0.01 mg/kg). At the higher dietary inclusion rates no permethrin was
detected in egg albumen. In yolks, permethrin residues were up to
0.05 mg/kg and up to 0.64 mg/kg respectively at the 3.4 and 33 mg/kg
treatment levels. Residues did not accumulate and declined rapidly
when feeding finished, reaching non-detectable levels (less than 0.02
mg/kg) before the end of the 14-day recovery period in both cases.
Only small residues (< 0.03 mg/kg) of the metabolites I-IV were found
in eggs during the feeding period. Again these declined on cessation
of exposure. At the 3.4 mg/kg dietary inclusion rate, permethrin
residues in muscle, skin and liver were non-detectable; i.e. less than
0.01 mg/kg. At the 33 mg/kg rate permethrin residues in liver were
also non-detectable. Permethrin was the major constituent of the low
residues in muscle and skin; levels of 0.05-0.08 mg/kg fell to 0.02
mg/kg before the end of the recovery period (Table 18) (Edwards and
Swaine, 1977; Swaine et al, 1980b).
Chickens have also received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 4.5 mg per bird, which is
approximately 20% of the highest rate that is likely to be used
commercially, was brushed on as a 0.1% w/v emulsion to an area between
the rear abdomen and the end of the breast and upper leg. Up to 29.5%
of the applied dose was detected on skin and feathers at the site of
application fourteen days after treatment and was still present (99%)
as permethrin (Leahey et al, 1977).
Total radioactive residues in liver, kidney, heart and muscle were
low-less than 0.02 mg permethrin equivalents/kg one, seven and
fourteen days after treatment, except in muscle below the site of
application which contained up to 0.11 mg permethrin equivalents/kg.
Up to 0.07 mg permethrin equivalents was found in fat at the
application site, 70-88% of the radioactivity in subcutaneous muscle
and fat at the site of application was characterised as permethrin
itself (Leahey et al, 1977c).
Radioactive residues in eggs were all below 0.03 mg permethrin
equivalents/kg, reaching a peak 3-6 days after dosing and then
declining progressively to below 0.01 mg/kg ten days after dosing
(Leahey, et al, 1977c).
When eggshells were painted with a 0.1% w/v emulsion of 40:60
cis:trans 14C-labelled permethrin (combined cyclopropyl- and
methylene-labelling), no radioactivity was detected in either yolks or
albumen one, seven or fourteen days after treatment (limit of
determination: 0.02 mg permethrin equivalents/kg) (Leahey et al,
1977c).
On Processing
The fate of permethrin residues during the processing of cotton,
soybeans, apples, pears, grapes and tomatoes was reviewed at the 1979
Meeting. Residues in the seeds of cotton and beans of soya, and in
their respective processing fractions used in animal feeds, are small
(e.g. well below 0.5 mg/kg) (FAO/WHO, 1980).
Residues in dried apple pomace were 25-30 times the levels in
corresponding whole apples. No residues were determined in apple
juice and apple sauce (limit of determination: 0.01 mg/kg) (Table 19)
(Ussary, 1977a, c).
Residues in wet tomato pulp, which typically contains 25% dry matter,
were in the range 10-50 (mean 25) times the levels in whole tomatoes
(Table 20) (Ussary, 1977d).
During the processing of treated whole wheat grain the permethrin
residue is retained mainly in the bran component although a
significant proportion (12%) remains with the white flour and follows
through almost unchanged into bread (Simpson, 1979).
Permethrin residues in flour from treated whole grain are carried over
into bread baked from that flour. There is no reduction in residue
level on a commodity-weight basis. White flour retains about 12% of
the whole grain residue. The major part (about 62%) of the residue
remains with the bran while about 26% is in the pollard. Therefore,
whilst white bread prepared from treated grain would have a residue of
about 0.15-0.2 mg/kg, the corresponding level in wholemeal bread would
be about 0.7-1 mg/kg (Table 21) (Simpson, 1979).
TABLE 17. Total Radioactive Residues In The Tissues of Hens Receiving
Consecutive Daily Oral Doses Of 14C-Labelled Permethrin
Rate No. of Period Between Residue (mg permethrin equivalents/kg)
Material Administered (expressed as mg/kg Daily Last Dose And In
equivalent in diet) Doses Sacrifice Fat Muscle Liver Kidney
40:60 cis:trans-permethrin
(cyclopropyl-lablled) 10 10 4 hours 0.5-0.6 0.07-0.13 0.9-1.1 N/D.1
(methylene-labelled) 10 10 4 hours 0.3-0.7 0.03 0.4 N/D1
trans-permethrin
(carbonyl-labelled) 80 3 6 days 0.21 <0.06 0.14 0.31
(methylene-labelled) 80 3 6 days 0.18 <0.06 0.08 0.24
cis-permethrin
(carbonyl-labelled) 80 3 6 days 1.0 0.06 0.27 0.34
(methylene-labelled) 80 3 6 days 1.4 <0.06 0.20 0.25
1 N/D indicates not determined.
Gaughan et al, 1978b; Leahey et al, 1977b
TABLE 18. Residues Of Permethrin And Three Major Metabolites In Eggs And Tissues
Of Hens Receiving Permethrin In The Diet Up To 28 Days
Dietary Feeding Regime Product Residue (mg/kg) of
Inclusion Analysed
Level Cis & Trans
(mg/kg) Permethrin DCVA 3-PBAlc 3-PBAcid
(I and II) (III) (IV)
33 "Treated"1 Muscle 0.05-0.08 0.01-0.02 <0.01 <0.01
plus skin
Liver <0.01 <0.01-0.03 <0.01 <0.01
Eggs <0.6 <0.01-0.03 <0.01-0.03 <0.01
"Treated plus Muscle 0.02 <0.01 <0.01 <0.01
Recovery"2 plus skin
Liver <0.01 <0.01 <0.01 <0.02
Eggs <0.02 <0.01 <0.01-0.02 <0.01
3.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.08 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin <0.01 - - -
Liver <0.01 - - -
Eggs <0.03 - - -
0.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.05 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin
Liver <0.01 - - -
Eggs <0.01 - - -
1 "Treated" indicates the animals received treated diet for up to 28 days and
were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28
days and were then returned to untreated diet for up to 14 days before slaughter.
Edwards and Swaine, 1977;
Swaine et al, 1980b
TABLE 19. Permethrin Residues In Commercial Fractions Of Apples: USA: 1976
Volume of Number of Interval Fraction Permethrin Residues
Spray Applications Between Last (mg/kg) after Applying
Applied Spraying and at Stated Rates of mg/kg
(l/ha) Harvest ai in spray
80 mg/kg 160 mg/kg
3150-3600 3-5 38-63 days Mature 0.11-0.40 0.85
apples
Juice <0.01 <0.01
Apple <0.01 -
Sauce
wet 0.74-3.2 6.5
pomace
Dry 3.3-10 22
pomace
Ussary, 1977a, c
TABLE 20. A Comparison Of Permethrin Residues In Processed Tomato Fractions : USA : 1977
Rate of Number of Interval Permethrin Residues (mg/kg) In
Application Applications Between Last
(g ai/ha) Application Whole Tomato Tomato Tomato Tomato
And Harvest Tomatoes Juice Puree Ketchup Pulp
220 2 1 day 0.05 <0.01 0.01 0.01 1.08
3 days 0.08 0.01 0.02 0.01 0.86
7 days 0.04 0.01 0.03 0.02 1.97
440 2 1 day 0.05 <0.01 0.03 0.02 1.60
3 days 0.05 0.01 0.03 0.02 1.45
7 days 0.08 <0.01 0.02 0.01 1.28
Ussary, 1977d
TABLE 21. Distribution Of Permethrin Residues In Whole Wheat
Fractions And Consequent Residue Levels In Bread
Distribution of residue between whole grain fractions1
Permethrin Residue
Fraction % whole grain mg/kg % distribution
Flour (white) 75.1 0.13 12.1
Pollard 11.3 1.9 26.3
Bran 13.6 3.7 61.7
Residue in Bread baked from the ground flour
Commodity Permethrin residue (mg/kg)
White flour 0.13-0.15
White bread 0.13-0.19
Wholemeal flour2 0.81-0.93
Wholemeal bread 0.67-1.00
1 Grain sample 1.5 kg. It had a residue level of 0.65 mg
permathrin/kg after being stored for 9 months.
2 Reconstituted from the fractions in the original whole grain
proportions.
Simpson, 1979
The processing operations simulated those used in commercial practice,
the unbaked bread recipe included potassium bromate and benzoyl
peroxide.
Wholemeal flour was formulated after reconstituting the wheat
fractions in the original ratio.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis (by gas chromatography using
an electron capture detector) for crops treated pre-harvest was
reviewed at the 1979 Meeting (FAO/WHO, 1980).
Both gas chromatography (GC) and high pressure liquid chromatography
(HPLC) have been used to determine permethrin residues in whole cereal
grains and commodities processed from grain (including bread).
The GC procedure described earlier is quite suitable, being able to
determine cis and trans isomers separately or together. Recovery
is essentially quantitative and the limit of determination is about
0.01 mg/kg.
A simple procedure based on a high pressure liquid chromatograph has
also been developed (Simpson, 1978). The limit of determination is a
little better than 0.01 mg/kg. In this procedure the residue is
extracted by cold methanol, partitioned into hexane - and, if
necessary, further cleaned by an acetonitrile partitioning - before
clean-up on an alumina column. The concentrated residue is subjected
to determination by HPLC equipped with a UV detector. This method is
similar to the procedure used by Mourot et al, (1979).
With small modifications, the GC method can be used to determine
permethrin in milk, meat and eggs.
Milk and meat samples are extracted with n-hexane:acetone 4:1. The
acetone is removed by washing with water and the permethrin
partitioned from n-hexane into dimethylformamide. The
dimethylformamide extract is dissolved in 1% aqueous sodium sulphate
and the permethrin back-extracted into n-hexane. The hexane extract
is cleaned up using a Florisil column, and permethrin is determined by
gas chromatography using an electron capture detector. The limit of
determination of the method is 0.01 mg/kg for the combined isomers and
recovery values for samples of meat and milk fortified at 0.01-1.0
mg/kg are normally greater than 70%. Mean recoveries of 89%-92% have
been obtained from milk and 86-88% from tissues of cows and hens
(Edwards and Iswaren, 1977; Edwards and Swaine, 1977). Twenty percent
acetone in hexane has been shown to be an efficient solvent for
extracting permethrin residues from animal tissues (Edwards and
Sapiets, 1978).
The method for permethrin analyses in meat and milk is also applicable
to eggs. These are extracted with n-hexane:acetone 1:1 and the
extract washed with aqueous sodium chloride to remove acetone, and
cleaned up by solvent partition with dimethylformamide and by using a
Florisil column. The limit of determination is 0.02 mg/kg for the
combined isomers and recovery values from yolks and albumen fortified
at 0.01-0.4 mg/kg are generally in the ranges 70-90% and 60-90%
respectively (Edwards and Swaine, 1977).
The mass spectrographic technique of multiple ion detection is
suitable for the qualitative and quantitative confirmation of
permethrin residues in crops, milk, eggs and animal tissues following
separation by gas chromatography.
Qualitative confirmation of permethrin residues is given by the
appearance of a peak at the correct retention time for all the
specific m/e values monitored. In addition, the ratios between the
peak heights (or peak areas) given from each m/e value should be
identical to that observed for permethrin analytical standards.
Quantitative confirmation is carried out by comparison of the peak
height or peak area, measured for the most abundant m/e value
recorded, against those obtained with external standards of permethrin
(Swaine and Edwards, 1977).
Residue of both the free and conjugated major plant metabolites of
permethrin, namely cis- and trans- isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
and 3-phenoxybenzyl alcohol (3-PBAlc), can also be determined by gas
chromatography. Samples are extracted with 2:1 methanol:water and
lipids removed by partitioning with dichloromethane. The methanol is
then removed using rotary evaporation, the aqueous solution made 1 N
with HCl and refluxed to free the conjugated residues. The residues
are then extracted by partitioning with n-hexane. The n-hexane is
then removed and the 2,2,2-trichloroethyl ester of DCVA and the
heptafluorobutyryl ester of 3-PBAlc are simultaneously made. The
derivatives are then analyzed by gas-liquid chromatography using
electron capture detection. (Ussary, 1979). Limits of determination
are in the range 0.02-0.10 mg/kg for DCVA and 0.02-0.05 mg/kg for 3-
PBAlc. Recoveries are generally in the range 70-85%. Confirmation of
the metabolite residues is possible using gas chromatography linked to
mass spectrometry using multiple ion detection, similar to the
procedure reviewed above for permethrin (Swaine et al, 1978).
With little modification, this method has also been used to determine
permethrin metabolite residues in meat and milk. Meat tissues are
extracted with 1:1 methanol:water; milk is extracted with 1:1
hexane:acetone. Any permethrin extracted by this procedure is
partitioned out, along with unconjugated residues of 3-PBAlc, into
n-hexane alone (milk) or hexane plus dichloromethane (meat).
Residues of 3-phenoxybenzoic acid (3-PBAcid), both "free" and
conjugated DCVA, and conjugated residues of 3-PBAlc remain in the
aqueous phase which is subjected to a hydrochloric acid hydrolysis to
cleave the conjugates. DCVA and 3-PBAcid are separated from 3-PBAlc
by partition between aqueous sodium hydroxide and dichloromethane.
The PBAlc residues are combined and determined as the
heptafluorobutyryl ester by gas chromatography using an electron
capture detector. DCVA and 3-PBAcid are converted to their methyl
ester derivatives, by treatment with diazomethane, and determined by
gas chromatography linked to mass spectrometry using multiple ion
detection. A limit of determination of 0.01 n/kg has been obtained
routinely for DCVA, 3-PBAlc and 3-PBAcid in meat and milk. Mean
recoveries have been within the range 55-60% from milk and 60-85% from
meat tissues (Swaine et al, 1980).
NATIONAL TOLERANCES
The following national tolerances were reported to the Meeting for
permethrin (total isomers):
Australia Wheat Bran 10 mg/kg
Raw cereals ) 5 mg/kg
Wheat pollard (shorts) )
Brussels sprouts )
Wheat flour(wholemeal) ) 2 mg/kg
Wholemeal bread )
Broccoli )
Cabbage ) 1 mg/kg
Cauliflower )
Wheat flour (white) ) 0.5 mg/kg
White bread )
Tomatoes 0.4 mg/kg
Cotton ) 0.2 mg/kg
Sweetcorn (cobs) )
Eggs ) 0.1 mg/kg
Fat of meat of cattle, )
sheep, pigs and poultry )
Milk and milk products ) 0.05 mg/kg
(fat basis) )
Belgium Eggplants )
Cabbages )
Cucumbers )
Endives )
Fruit crops ) 1 mg/kg
Honeydew melons )
Lettuce )
Peppers )
Tomatoes )
France Maize (whole plants) 0.2 mg/kg
Maize grains 0.1 mg/kg
Holland Endives ) 2 mg/kg
Lettuce )
Apples )
Brassicae ) 1 mg/kg
Pears )
Eggplants )
Cucumbers )
Honeydew melons ) 0.5 mg/kg
Peppers )
Tomatoes )
USA Cotton 0.5 mg/kg
Eggs )
Meat, fat and meat )
by-products of cows, ) 0.05 mg/kg
goats, hogs, horses, )
poultry and sheep )
Venezuela Vegetables ) 10 mg/kg
EVALUATION
APPRAISAL
Permethrin is a pyrethroid insecticide which is effective at low rates
against a wide range of Lepidoptera, Hemiptera, Diptera and
Coleoptera. Unlike natural pyrethrins and earlier synthetic
pyrethrins, permethrin is photostable to a degree that allows it to be
used as an effective tool in agriculture. It shows adulticidal,
ovicidal and particularly larvicidal activity and is effective against
the great majority of insects that. have become resistant to standard
treatments such an organochlorines and organophosphates. A programme
of sprays is usually required.
A particularly valuable usage is in protection of stored grain.
Laboratory and silo-scale studies in Australia have shown that low
levels of permethrin control most of the endemic insect pests of
stored grain, and, when used in conjuction with a synergist and
complementary insecticide, the commonly-occurring strains of all grain
insect pests are controlled. Adequate protection of at least 9 months
storage is achieved by a single application of 1 mg permethrin/kg.
However, for various reasons, it may become necessary in future to use
permethrin without its synergist and/or o/p insecticide. In that
case, higher application rates would be required to maintain the
present level or permethrin efficacy. The same applies if the present
experimental rate is increased for other reasons (e.g. to counter
resistance). A 2 mg/kg grain is likely to be the target concentration
in the near future.
Permethrin can also be used to control various ectoparasites of
cattle, horses, sheep, pigs and poultry. It can be applied either
directly to the animals, or to buildings in which they are housed, or
to insect breeding and resting sites.
Extensive residues data are available from supervised trials. In
pre-harvest uses, residues are highest where a crop part is exposed
directly to the spray, as in the case of many forage crop uses.
Ground and aerial applications yield similar residue levels. Although
residues decline comparatively slowly after spraying, there is no
obvious build-up of residues of permethrin or of its two most
important plant metabolites on repeated application, within the rates
and frequency of permethrin spraying that are needed to obtain good
insecticidal control. The two important plant metabolites are
3-phenoxybenzyl alcohol and the cis and trans isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA),
which are also animal metabolites; levels of these metabolites in
crops are very much smaller than corresponding permethrin residues and
they do not need to be included in routine residue analyses.
Permethrin levels on stored grains also decline slowly. During the
processing of treated whole wheat grain, the permethrin residue is
retained mainly on the bran component, although a significant
proportion (12%) remains with the white flour and follows through
After both oral and dermal administration to livestock, permethrin
itself constitutes the major portion of the residue recovered from
milk, eggs, muscle and fat. After oral administration to goats,
metabolites form the major part of the residue in kidney and liver;
DCVA occurs in both organs, 3-phenoxybenzyl alcohol plus its
4'-hydroxy derivative in liver and 3-phenoxybenzoic acid plus its
4'-hydroxy derivative in liver and kidney.
Following direct application to dairy cattle (1.0 g ai/cow and with
free access to a self-oiler) permethrin levels are low in milk (<0.02
mg/kg) and in muscle, liver and kidney (<0.01 mg/kg). Highest levels
(up to 0.1 mg/kg) have been found in fat.
Cows maintained on a diet containing 50 mg/kg of permethrin yielded
milk containing low levels of residue (0.1 mg/kg), and the level in
muscle was less than 0.1 mg/kg. The residue in milk declined to below
0.01 mg/kg after five days on returning the cows to control diet.
Residues in eggs are also low and contamination of eggs laid during
the treatment of poultry houses will not result in detectable residues
in yolks or albumen.
Permethrin is not detected in the albumen of eggs from hens receiving
the compound at levels up to 33 mg/kg in the diet; levels in yolks are
approximately 2% of corresponding permethrin dietary levels.
In all cases studied, residues of permethrin and its metabolites in
products of animal origin declined notably on cessation of exposure.
Only small residue levels have been found in products of animal origin
following direct application of permethrin to livestock. In view of
the levels of permethrin found in forage crops, such as alfalfa, and
the importance of such crops in animal feedstuffs, it seems likely
that residue levels in products of animal origin will be higher
following forage crop uses rather than from ectoparasite outlets. In
estimating maximum residue levels for products of animals origin,
allowance has also to be made for the dilution effect that will occur
in practice when treated forage is incorporated into livestock diets.
In proposing maximum residue levels on raw grain and milled cereal
products prepared therefrom, careful consideration has been given to
the fact that this insecticide is to be used as a grain protectant and
that residues of the compound are comparatively stable under storage
conditions. Under practical conditions of grain handling and storage,
it is not possible to apply a grain protectant with assurance that the
deposit will be absolutely uniform; there will always be a natural
variation in the level of the deposit resulting from fluctuations in
the flow of grain and of the insecticide. Furthermore, the movement
of bulk grain results in the segregation of the various components of
the bulk with a resultant variation in the level of residues
throughout the mass. Due allowance has been for the amplitude of
these variations and the problems of sampling and analysis.
The meeting examined residue data from supervised storage and feeding
trials reflecting established or proposed good practice. From these
data the meeting was able to estimate the maximum residue levels that
were likely to occur when permethrin was used in practice.
The preferred method of permethrin residue analysis in crops is by gas
chromatography using an electron capture detector. Recoveries are
essentially quantitative and the method has been applied successfully
to a wide range of crops, raw cereal grains and processed products
derived from them, such as flour, bran and bread. A limit of
determination of 0.01 mg/kg (expressed as permethrin cis and trans
isomers). Cis and trans-isomers are capable of being determined
separately by this method. With small modifications, the method has
been applied successfully to the determination of permethrin residues
in meat, milk and eggs, and would with adaptation be suitable for
regulatory purposes.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the residue levels listed below are
temporary pending submission of the "Further Work or Information".
These are recommended as suitable for establishing temporary maximum
residue limits. These levels are additional to those proposed by the
1979 Meeting. These levels refer to the sum of the cis and
trans-isomers of permethrin.
Crop TMRL (mg/kg)
Alfalfa fodder 100) On a dry weight basis
Maize fodder and straw 100)
Apple pomace 50) On a dry weight basis
Soybean fodder 50) On a dry weight basis
Cereal grains 2
Wheat flour (wholemeal) 2
wheat flour (white) 0.5
Wheat bran 10
Carcass meat of
cattle ) For permethrins a portion of
pig ) 1 carcass fat is analyzed and
sheep ) the levels refer to carcass fat.
Meat by-products from:
pigs ) 0.1
sheep )
Poultry meats 0.1
Eggs 0.1
Milk 0.1
FURTHER WORK OR INFORMATION
Recommendations 3, 4, 8, 9 from 1979 meeting remain relevant.
REFERENCES
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Pilot Usage Trials, 1978-79 Working Party On Grain
Protectants", Department of Primary Industries, Queensland, Australia
(unpublished) (1979a).
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Silo Scale Experiments 1977-78 Working Party On
Grain Protectants", Department of Primary Industries, Queensland,
Australia (unpublished) (1979b).
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional Grain Protectants For The Control of
Malathion-Resistant Insects In Stored Sorghum", Department Of Primary
Industries, Entomology Branch, Queensland, Australia (unpublished)
(1979c).
Bewick, D.W. and Leahey, J.P. "Permethrin : Absorption in Cows", ICI
Plant Protection Division Report No. TMJ1357B (unpublished) (1976).
Bewick, D.W., Leahey, J.P. and Saunders, R. "Permethrin Absorption In
Pigs After Dermal Treatment", ICI Plant Protection Division Report No.
TMJ1562A. (unpublished) (1977).
Chipman Inc. Canada "Summary of 1976/77 Permethrin Sweet Corn Residue
Data . (unpublished) (1978).
Desmarchelier, J.M., Bengston, M., Davies, R.A.H., Elder, W.B.,
Hamilton, D., Henning, R., Murray, W., Ridley, E.G., Ripp, E.,
Sieraikowski, C.J., Sticka, R., Snelson, J.T., Thomas, D., Wallbank,
B. and Wilson, A. "Pilot Usage Of The Grain Protectants Fenitrothion
Plus Phenothrin and Pirimiphos-methyl Plus Permethrin", Australian
Wheat Board Working Party on Grain Protectants (in manuscript for
publishing) (1979).
Edwards, M. J. and Iswaren, T.J. "Permethrin: Residue Transfer And
Toxicology Study With Cows Fed Treated Grass Nuts" ICI Plant
Protection Division Report No. TMJ1519/B (unpublished) (1977).
Edwards, M.J. and Swaine, H. "Permethrin: Incorporation of Permethrin
In The diet of Laying Hens: Residues In Eggs And Tissues". ICI Plant
Protection Division Report No. TMJ1520/B (unpublished) (1977).
Edwards, M.J. and Sapiets, A. "Permethrin: The Extraction of the
Pesticide From Animal Tissues". ICI Plant Protection Division Report
No. RJ0009B (unpublished) (1978).
Gaughan, L.C., Unai, T. and Casida, J.E. "Permethrin Metabolism In
Rats And Cows, And In Bean and Cotton Plant", Paper delivered at 172nd
ACS National Meeting, San Francisco (August 1976).
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. "Distribution
And Metabolism Of Trans- And Cis-Permethrin In Lactating Jersey
Cows", J. Agric. Food Chem. 26(3), 613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. "Distribution And
Metabolic Fate of Trans- And Cis- Permethrin In Laying Hens", J.
Agric. Food Chem. 26(6), 1374-1380.
Hunt, L.M. and Gilbert, B.N. "Distribution And Excretion Rates Of
14C-Labelled Permethrin Isomers Administered Orally To Four Lactating
Goats For 10 Days", J. Agric, Food Chem. 25(3), 673.
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
A.G. "Permethrin: Metabolism And Residues In Goats", ICI Plant
Protection Division Report No. TMJ1516B (unpublished) (1977a).
Leahey J.P., Gatehouse, D.M. and Parr, J.S. "Permethrin: Metabolism In
Hens", ICI Plant Protection Division Report No. TMJ1509B (unpublished)
(1977b).
Leahey, J.P., Bewick, D.W., Gatehouse, D.M. and Carpenter, P.K.
"Permethrin: Absorption In Chickens After Dermal And Oral Treatments",
ICI Plant Protection Division Report No. TMJ1481B (unpublished)
(1977c).
Leahey, J.P. and Cameron, A.G. "Permethrin: Residues In Cows After
Dermal Application", ICI Plant Protection Division Report No. RJ0001B
(unpublished) (1978).
Mourot, D., Delephine. B., Boisseau, J. and Gayot, G. "HPLC Of A New
Pyrethroid Insecticide Sumicidin", J. Chromatog. 168, 277-279.
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1978).
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1979).
Swaine, H. and Edwards, M.J. "Confirmation Of Residues Of Permethrin
(PP557) Using Gas Chromatography - Mass Spectrometry In The Multiple
Ion Detection Mode", ICI Plant Protection Division Data (unpublished)
(1977).
Swaine, H., Edwards, M.J. and Ussary, J.P. "Permethin Crop Rotation
Study", ICI Americas Inc Report No. TMU0378/B (unpublished) (1978).
Swaine, H. and Sapiets, A. "Permethrin Residues Summary: Residues In
Crop Samples Analyzed During 1977-8: Part IV: Leaf, Root and Forage
Crops", ICI Plant Protection Division Report No. RJ0081A (unpublished)
(1979).
Swaine, H., Francis, P.D., Rippington, D. and Burke, S.R. "Permethrin
Residue Levels of the Major Metabolites of the Insecticide In the Milk
And Tissues of Cows Fed On A Treated Diet", ICI Plant Protection
Division Report No. RJ0124B (unpublished) (1980a).
Swaine, H., Francis, P.D., Rippington, D., Burke, S.R., Robertson, S.
and Ford, J.P. "Permethrin Metabolites: Eggs And Tissues Of Hens", ICI
Plant Protection Division Residue Data Report No. RD/557/27
(unpublished) (1980b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions of Apples, Wenatchee, Washington, 1976" ICI Americas Inc.
Report No. TMU0306/B (unpublished) (1977a).
Ussary, J.P. "Permethrin Residues In Soybeans", ICI Americas Inc.
Report No. TMU0383/B, (unpublished) (1977b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions Of Apples, Geneva, New York, 1976", ICI Americas Inc. Report
No. TMU0307/B (unpublished) (1977c).
Ussary. J.P. "Permethrin Residues In Tomato Process Fractions", ICI
Americas Inc. Report No. TMU0304/B (unpublished) (1977d).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0388/B (unpublished) (1978a).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0413/B (unpublished) (1978b).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0438/B (unpublished) (1979a).
Ussary, J.P. "Permethrin Residues On Field Corn", ICI Americas Inc.
Report No. TMU0433/B (unpublished) (1979b).
Ussary, J.P. "Permethrin Metabolite Residues On Broccoli", ICI
Americas Inc. Report No. TMU0427/B (unpublished) (1979c).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0461/B (unpublished) (1979d).
Ussary, J.P. and Braithwaite, G.B. "Residues of Permethrin And
Permethrin metabolites in Milk From Ectiban Treated Cows (Trial No.
35NC79-001)", ICI Americas Inc. Report No. TMU0490/B (unpublished)
(1980a).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin and
3-Phenoxybenzyl Alcohol In Cow Tissues (Trial No. 35NC79-001)", ICI
Americas Inc. Report No. TMU0493/B (unpublished) (1980b).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And 3-
Phenoxybenzyl Alcohol In Tissues From Ectiban Treated Swine (Trial No.
35NC79-002)", ICI Americas Inc. Report No. TMU0491/B (unpublished)
(1980c).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And
3-Phenoxybenzyl Alcohol In Tissues And Eggs From Ectiban Treated
Chickens (Trial No. 35NC79-003)", ICI Americas Inc. Report No.
TMU0492/B (unpublished) (1980d).
Cows and goats
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bodyweight, equivalent to approximately 80 mg/kg in the
diet, levels of radioactivity in milk reached a maximum of 0.13 mg
permethrin equivalents/kg after 1-2 days. These declined to less than
0.02 mg/kg after seven days. Levels of radioactivity in the fat were
0.12-0.18 mg permethrin equivalents/kg, after seven days. By fourteen
days these had declined to 0.05-0.06 mg/kg, indicating that the small
residues in fat are also not maintained on cessation of dosing (Bewick
and Leahey, 1976).
Hunt and Gilbert dosed goats orally with either the cis- or
trans-isomers of 14C-labelled permethrin (carbonyl- or
methylene-labelled) at a rate equivalent to approximately 6 mg/kg in
the diet for ten days. Total radioactive residues in the milk reached
a plateau after three days of 0.02-0.05 and <0.01-0.01 mg permethrin
equivalents/kg respectively for the cis- and trans- isomers. The
goats were sacrificed 24 hours after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 12.
Total radioactivity in the fat of animals receiving the cis-isomer
was ten times higher than in those receiving the more readily
hydrolysed trans-isomer (Table 12) (Hunt and Gilbert, 1977).
When cis- and trans-isomers of 14C-labelled permethrin (carbonyl-
and methylene-labelled) were administered orally to lactating Jersey
cows for three consecutive days at approximately 1 mg/kg bodyweight,
radioactivity was largely eliminated from the body in faeces and urine
within 12 or 13 days after the initial treatment. Total
14C-permethrin equivalents in milk were consistently below 0.3 mg/kg
and declined on cessation of exposure. Residues in fat were also low.
Residues recovered from fat and milk were higher when cis-
permethrin rather than trans-permathrin was administered and
consisted almost entirely (>85%) of unmetabolised permethrin.
Total 14C-permethrin equivalents in blood reached a transient peak
shortly after each dose and dropped to insignificant levels within 2-4
days after the last dose (Gaughan et al, 1976, 1978a).
A study with non-radiolabelled permethrin supported the finding that
permethrin itself is the predominant residue in milk. It was also the
major residue in cow muscle. In this study, groups each of three
barren Friesian cows, yielding 9-13 litres of milk per day, were
maintained on diets containing permethrin at approximately 0.2, 1.0,
10 or 50 mg/kg. The permethrin was absorbed on grass nuts. After
28-31 days two cows in each group were sacrificed. The third was
returned to control diet for nine days before sacrifice. Samples of
milk and of meat tissues were analyzed for residues of permethrin,
cis and trans 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II, DCVA) taken together,
3-phenoxybenzylalcohol (III; 3-PBAlc) and 3-phenoxybenzoic acid (IV;
3-PBAcid) (Figure 1) by the gas chromatographic methods reviewed under
"Methods of Residue Analysis".
TABLE 12. Total Radio-labelled Residues In Tissues of Goats Receiving 14C-Labelled Permethrin
Daily Orally For 7-10 Days
Material Dose Level Duration of Period Between Total Residue (mg permethrin
Administered (= mg/kg Administration Last Date And equivalents/kg In
in diet) (days) Sacrifice Fat Muscle Liver Kidney
Permethrin, ‰10 7 4 hours <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis-trans
Permethrin, ‰6 10 24 hours 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin, ‰6 10 24 hours 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
Hunt and Gilbert, 1977
Leahey et al, 1977a
TABLE 13. Characterisation of 14C-Labelled Residues In Liver And Kidney Of Goats
Receiving Permethrin Orally Daily For Seven Days
Radioactivity Soluble in Water
% Of % of Radioactivity % of Radioactivity
Organ Position of Radioactivity Soluble in Diethyl Remaining Water Unextracted
14C-Labelling Soluble in Ether Following Soluble After
Of Permethrin Diethyl Ether Hydrolysis Hydrolyais
Liver Methylene ("Alcohol") 16 41 23 note 1
Cyclopropyl ("Acid") 39 24 36 note 1
Kidney Methylene ("Alcohol") 43 42 <1 6
Cyclopropyl ("Acid") 29 20 22 9
1 liver was almost completely solubilised during refluxing.
Leahey et al, 1977a
Only permethrin itself was detected in milk. Low levels of 0.01-0.06
mg/kg (mean 0.02 mg/kg) and 0.03-0.2 (mean 0.1 mg/kg) were present in
the milk of cows in the 10 and 50 mg/kg groups respectively. These
values are approximately 0.2% of the corresponding dietary levels.
Permethrin residues in milk were less than 0.01 mg/kg at the 0.2 and
1.0 mg/kg dietary inclusion rates. None of the three metabolites was
found in any of the milk samples analyzed (limit of determination:
0.01 mg/kg). Permethrin levels in milk did not accumulate over the
period of the study and they declined rapidly, to below 0.01 mg/kg
after five days, on returning the animals to control diet (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
Permethrin itself was also the major part of the small residue levels
found in adductor, pectoral and cardiac muscle. These small levels
were approximately 0.1% of the corresponding dietary inclusion levels
(Table 14). Permethrin residue levels in peritoneal fat were
invariably higher than in subcutaneous fat (Table 15) (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
As found separately by Leahey et al (below), metabolites rather than
permethrin itself constituted the major part of the residues in liver
and kidney. Again, the levels declined rapidly on cessation of
exposure (Table 14) (Edwards and Iswaren, 1977; Swaine et al,
1980a).
Leahey et al dosed goats orally with 40:60 cis:trans 14C-
labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 mg/kg in the diet for seven days.
Total radioactive residues in the milk reached a plateau of 0.02-0.03
mg permethrin equivalents/kg after five days; 30-50% of this
radioactivity was associated with the butterfat fraction of the milk,
in which total radioactive residues were 0.13-0.27 mg permethrin
equivalents/kg.
The animals were sacrificed four hours after receiving the final dose,
when levels of radiocarbon in meat tissues were as shown in Table 12.
Characterization of the residues in liver and kidney, the two tissues
containing the highest total residues is shown in Table 13. Where
"alcohol"-labelled permethrin was used, approximately 70% of the 14C
in kidney tissue was 3-phenoxybenzoic acid (IV) plus
3-(4'-hydroxyphenoxy) benzoic acid (VI) (Figure 1). Approximately 30%
of the 14C in the liver was due to 3-phenoxybenzyl alcohol (III) plus
3-(4'-hydroxyphenoxy) benzyl alcohol (V). A further 15% was due to
3-phenoxybenzoic acid (IV) plus 3-(4'-hydroxyphenoxy) benzoic acid
VI). Where "acid"-labelled permethrin was used, approximately 10-15%
of the label in liver and kidney was due to the cis and trans
3-(2,2-dichlorovinyl) cyclopropane carboxylic acids (I and II)
(principally the trans isomer). (Leahey et al, 1977a).
TABLE 14. Residues Of Permethrin and Three Major Metabolites in Tissues Of Cows
Receiving Permethrin In The Diet
Nominal Residue (mg/kg) Of
Dietary Feeding Regime Tissue
Inclusion Analysed Cis & Trans
Level Permethrin DCVA 3-PBAlc 3-PBAcid
(mg/kg) (I and II) (III) (IV)
50 "Treated"1 Liver <0.01 0.01-0.04 0.05-0.10 0.03-0.12
Kidney 0.02-0.06 0.07-0.09 0.01-0.09 0.03-0.04
Muscle O.03-0.10 <0.01-0.02 <0.131 <0.01
"Treated plus Liver <0.01 0.01 <0.01 <0.01
Recovery"2 Kidney 0.02 0.06 <0.01 <0.01
Muscle 0.02-0.06 <0.01 <0.01 <0.01
10 "Treated" Liver <0.01 <0.01 0.01-0.03 0.01-0.02
Kidney <0.01-0.01 <0.01-0.02 <0.01-0.01 <0.01-0.02
Muscle <0.01-0.03 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery Kidney 0.01 <0.01 <0.01 0.04
Muscle 0.01-0.02 <0.01 <0.01 <0.01
1.0 "Treated" Liver <0.01 <0.01 <0.01 <0.01
Kidney <0.01 <0.01 <0.01 <0.01-0.01
Muscle <0.01 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery" Kidney 0.01 <0.01 <0.01 0.02
Muscle <0.01 <0.01 <0.01 <0.01
0.2 "Treated" Liver <0.01 - - -
Kidney <0.01 - - -
Muscle <0.01 - - -
"Treated plus Liver <0.01 - - -
Recovery" Kidney 0.01 - - -
Muscle <0.01 - - -
1 "Treated" indicates the animals received treated diet for 28-31 days and were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28 days and were then
returned to untreated diet for a further 9 days before slaughter.
Edwards and Iswaren, 1977; Swaine et al, 1980a
TABLE 15. Residues In Fat Of Cows Receiving Permethrin In The Diet
Nominal Residues (mg/kg) of Permethrin In
Dietary Inclusion
Level (mg/kg) Peritoneal Fat Subcutaneous Fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
(Edwards and Iswaren, 1977)
TABLE 16. Total Radiolabelled Residues In Tissues Of Cows Seven Days
After Receiving A Single Dermal Dose Of 1.25 g of 40:60 Cis:Trans
14C-Labelled Permethrin
Tissue Residue in tissue Residue in tissue
from cow treated from cow treated
with alcohol-labelled with acid-labelled
permethrin, mg/kg1 permethrin µg/kg1
Meat <0.01 <0.01
Subcutaneous fat 0.053 0.033
Perirenal fat 0.046 0.054
Liver 0.080 0.064
Kidney 0.048 0.038
1 Residues expressed as mg/kg permethin equivalents. Bewick et al,
1977.
Permethrin itself also constitutes the major part of the small
residues found in the milk and fat of cows after dermal application.
In one study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). Although the concentration of suspension used
(0.5% w/v) was ten times the maximum that is likely to be used in
practice, the total dose of 40 mg per cow was approximately 1/30 of
the amount that could be applied commercially at any one time. The
dose was placed on an area of 225 cm2.
Levels of radioactivity in the milk and blood reached a maximum during
the first few days after dosing and then declined steadily. Total
residues in milk were always less than 2 µg permethrin equivalents/kg
(i.e. less than 2 ppb) and were below the limit of determination (0.3
µg/kg) by the end of fourteen days following treatment.
Total radiolabelled residues in muscle, liver, kidney and heart were
also small - 0.02 mg permethrin equivalents/kg or below seven days
after treatment - and were considerably smaller after a further seven
days.
Highest residues were found in fat from below the treatment area.
Seven days after treatment the total residue was approximately 0.5 mg
permethrin equivalents/kg, of which at least 80% was due to permethrin
itself. After a further seven days, residues in fat below the site of
application had fallen by about one half; the residue remaining was
again characterised as being due mainly to permethrin itself (Bewick
and Leahey, 1976).
In a second study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 1.25 g per cow applied in a 0.5% w/v
emulsion is at the higher end of the range likely to be used
commercially. The permethrin was brushed onto 25% of the surface area
of the animals.
Levels of radioactivity found in milk were very similar for the two
positions of radiolabelling. Residues reached a peak of approximately
0.01 mg permethrin equivalents/kg after 3-5 days; 89-91% of these
residues could be extracted into butter fat in which the major portion
of the residue (at least 51-68%) was characterised as permethrin
(Leahey and Cameron, 1978).
Seven days after treatment only small residues were present in the
animal tissues. Levels of radioactivity were similar in perirenal and
subcutaneous fat and for the two positions of radiolabelling (Table
16). At least 40% of this residue was characterised as permethrin
(Leahey and Cameron, 1978).
Pigs
Pigs were dosed dermally with 40:60 cis:trans 14C-labelled
permethrin (cyclopropyl or methylene-labelled). Although the
concentration of suspension used (0.5% w/v) was ten times the maximum
that is likely to be used in practice, the total dose of 18 mg per pig
is approximately 1/30 of the amount that could be applied commercially
at any one time. The dose was placed on an area of 100 cm2.
Seven and fourteen days after treatment approximately 1% of the
applied dose remained at the site of application, principally (>95%)
as permethrin. A residue of 0.05 mg permethrin equivalents/kg in fat
immediately below the site of application seven days after treatment
was due essentially to permethrin. After fourteen days, no
radioactive residue was found (limit of determination: 0.012 mg
permethrin equivalents/kg) (Bewick et al, 1977).
A residue of 0.01 mg permethrin equivalents/kg was found in muscle
beneath the site of application seven days after treatment.
Otherwise, no residues were detected in muscle, liver, kidney, heart
or blood either seven or fourteen days after treatment (limit of
determination: approximately 0.001 mg permethrin equivalents/kg)
(Bewick et al, 1977).
Hens
In one study hens received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (cyclopropyl- and methylene-labelled) at a
rate equivalent to 30 mg/kg in the diet. Birds were sacrificed one,
seven and fourteen days after treatment.
Throughout the study, residues in breast muscle, leg muscle and heart
were small (less than 0.04 mg permethrin equivalent/kg). Total
radioactive residues in liver and kidney of 0.22-0.25 mg/kg had
decreased by an order of magnitude after seven days. Residues of
0.20-0.35 mg permethrin equivalents/kg in peritoneal and subcutaneous
fat on the day after treatment also declined during the remainder of
the study, albeit more slowly than in the case of liver and kidney
(Leahey et al, 1977c).
Levels of radioactivity in eggs reached a maximum soon after dosing
(in 1-4 days) and then declined during the remainder of the 14-day
study (Leahey et al, 1977c).
Hens have also been dosed orally with 40:60 cis:trans
14C-permethrin (cyclopropyl- or methylene-labelled) for ten days at a
rate equivalent to approximately 10 mg/kg in the diet or separately
with cis- and trans-isomers (carbonyl- or methylene-labelled) for
three days at a rate equivalent to approximately 80 mg/kg in the diet.
Residues in eggs were present primarily (>75%) in the yolks, in which
radioactivity reached a plateau after 5-8 days of 0.3-0.5 mg
permethrin equivalents/kg in the 10-dose study and 0.6 mg/kg (trans-
isomer administered) or 2.1-2.8 mg/kg (cis-isomer administered) in
the 3-dose study. Permethrin was the major compound identified in the
eggs (52-62%). The cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acids (I and II) and 3-phenoxybenzyl alcohol (III) (Figure 1) were the
major metabolites in eggs, each normally accounting for less than
approximately 10% of the total radioactivity. The carboxylic acids
were present both free and as the glucuronide and taurine conjugates.
Other metabolites arose from hydroxylation in the 4'-position of the
"alcohol" moiety and in the trans-2-methyl moiety in the "acid" part
of the molecule.
The hens were sacrificed four hours after receiving the final dose in
the 10-dose study, when residues were as shown in Table 17. As in the
case of eggs, residues in fat derived from both "acid" and "alcohol"
labels were similar. Permethrin itself represented the major residue
in the fat. Compounds I-III and 3-phenoxybenzoic acid (IV) were also
identified (each less than 10% of the total radioactivity in the fat).
In both muscle and liver, higher residues were detected in hens dosed
with "acid"-labelled permethrin than with "alcohol"-labelled. The
cis- and trans-3-2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II) were the major residues identified in
these tissues. Blood levels declined rapidly during the first 24
hours after administration (Gaughan et al, 1978b; Leahey et al,
1977b).
In a study with non-radiolabelled 40:60 cis:trans permethrin,
groups of 40 laying hens were fed on diets containing approximately
0.4, 3.4 and 33 mg/kg for 28 days and then returned to a control diet
for an additional 14 days. Samples of eggs laid during the study were
analyzed for permethrin residues by the gas chromatographic method
described under "Methods of Residue Analysis". Five hens per group
were sacrificed after 21, 28, 35 and 42 days of the study and tissues
analyzed for permethrin.
At the 0.4 mg/kg dietary inclusion rate no residues of permethrin were
detected on the albumen and yolks of eggs (limit of determination:
0.02 mg/kg) or in the muscle, skin and liver (limit of determination:
0.01 mg/kg). At the higher dietary inclusion rates no permethrin was
detected in egg albumen. In yolks, permethrin residues were up to
0.05 mg/kg and up to 0.64 mg/kg respectively at the 3.4 and 33 mg/kg
treatment levels. Residues did not accumulate and declined rapidly
when feeding finished, reaching non-detectable levels (less than 0.02
mg/kg) before the end of the 14-day recovery period in both cases.
Only small residues (< 0.03 mg/kg) of the metabolites I-IV were found
in eggs during the feeding period. Again these declined on cessation
of exposure. At the 3.4 mg/kg dietary inclusion rate, permethrin
residues in muscle, skin and liver were non-detectable; i.e. less than
0.01 mg/kg. At the 33 mg/kg rate permethrin residues in liver were
also non-detectable. Permethrin was the major constituent of the low
residues in muscle and skin; levels of 0.05-0.08 mg/kg fell to 0.02
mg/kg before the end of the recovery period (Table 18) (Edwards and
Swaine, 1977; Swaine et al, 1980b).
Chickens have also received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 4.5 mg per bird, which is
approximately 20% of the highest rate that is likely to be used
commercially, was brushed on as a 0.1% w/v emulsion to an area between
the rear abdomen and the end of the breast and upper leg. Up to 29.5%
of the applied dose was detected on skin and feathers at the site of
application fourteen days after treatment and was still present (99%)
as permethrin (Leahey et al, 1977).
Total radioactive residues in liver, kidney, heart and muscle were
low-less than 0.02 mg permethrin equivalents/kg one, seven and
fourteen days after treatment, except in muscle below the site of
application which contained up to 0.11 mg permethrin equivalents/kg.
Up to 0.07 mg permethrin equivalents was found in fat at the
application site, 70-88% of the radioactivity in subcutaneous muscle
and fat at the site of application was characterised as permethrin
itself (Leahey et al, 1977c).
Radioactive residues in eggs were all below 0.03 mg permethrin
equivalents/kg, reaching a peak 3-6 days after dosing and then
declining progressively to below 0.01 mg/kg ten days after dosing
(Leahey, et al, 1977c).
When eggshells were painted with a 0.1% w/v emulsion of 40:60
cis:trans 14C-labelled permethrin (combined cyclopropyl- and
methylene-labelling), no radioactivity was detected in either yolks or
albumen one, seven or fourteen days after treatment (limit of
determination: 0.02 mg permethrin equivalents/kg) (Leahey et al,
1977c).
On Processing
The fate of permethrin residues during the processing of cotton,
soybeans, apples, pears, grapes and tomatoes was reviewed at the 1979
Meeting. Residues in the seeds of cotton and beans of soya, and in
their respective processing fractions used in animal feeds, are small
(e.g. well below 0.5 mg/kg) (FAO/WHO, 1980).
Residues in dried apple pomace were 25-30 times the levels in
corresponding whole apples. No residues were determined in apple
juice and apple sauce (limit of determination: 0.01 mg/kg) (Table 19)
(Ussary, 1977a, c).
Residues in wet tomato pulp, which typically contains 25% dry matter,
were in the range 10-50 (mean 25) times the levels in whole tomatoes
(Table 20) (Ussary, 1977d).
During the processing of treated whole wheat grain the permethrin
residue is retained mainly in the bran component although a
significant proportion (12%) remains with the white flour and follows
through almost unchanged into bread (Simpson, 1979).
Permethrin residues in flour from treated whole grain are carried over
into bread baked from that flour. There is no reduction in residue
level on a commodity-weight basis. White flour retains about 12% of
the whole grain residue. The major part (about 62%) of the residue
remains with the bran while about 26% is in the pollard. Therefore,
whilst white bread prepared from treated grain would have a residue of
about 0.15-0.2 mg/kg, the corresponding level in wholemeal bread would
be about 0.7-1 mg/kg (Table 21) (Simpson, 1979).
TABLE 17. Total Radioactive Residues In The Tissues of Hens Receiving
Consecutive Daily Oral Doses Of 14C-Labelled Permethrin
Rate No. of Period Between Residue (mg permethrin equivalents/kg)
Material Administered (expressed as mg/kg Daily Last Dose And In
equivalent in diet) Doses Sacrifice Fat Muscle Liver Kidney
40:60 cis:trans-permethrin
(cyclopropyl-lablled) 10 10 4 hours 0.5-0.6 0.07-0.13 0.9-1.1 N/D.1
(methylene-labelled) 10 10 4 hours 0.3-0.7 0.03 0.4 N/D1
trans-permethrin
(carbonyl-labelled) 80 3 6 days 0.21 <0.06 0.14 0.31
(methylene-labelled) 80 3 6 days 0.18 <0.06 0.08 0.24
cis-permethrin
(carbonyl-labelled) 80 3 6 days 1.0 0.06 0.27 0.34
(methylene-labelled) 80 3 6 days 1.4 <0.06 0.20 0.25
1 N/D indicates not determined.
Gaughan et al, 1978b; Leahey et al, 1977b
TABLE 18. Residues Of Permethrin And Three Major Metabolites In Eggs And Tissues
Of Hens Receiving Permethrin In The Diet Up To 28 Days
Dietary Feeding Regime Product Residue (mg/kg) of
Inclusion Analysed
Level Cis & Trans
(mg/kg) Permethrin DCVA 3-PBAlc 3-PBAcid
(I and II) (III) (IV)
33 "Treated"1 Muscle 0.05-0.08 0.01-0.02 <0.01 <0.01
plus skin
Liver <0.01 <0.01-0.03 <0.01 <0.01
Eggs <0.6 <0.01-0.03 <0.01-0.03 <0.01
"Treated plus Muscle 0.02 <0.01 <0.01 <0.01
Recovery"2 plus skin
Liver <0.01 <0.01 <0.01 <0.02
Eggs <0.02 <0.01 <0.01-0.02 <0.01
3.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.08 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin <0.01 - - -
Liver <0.01 - - -
Eggs <0.03 - - -
0.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.05 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin
Liver <0.01 - - -
Eggs <0.01 - - -
1 "Treated" indicates the animals received treated diet for up to 28 days and
were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28
days and were then returned to untreated diet for up to 14 days before slaughter.
Edwards and Swaine, 1977;
Swaine et al, 1980b
TABLE 19. Permethrin Residues In Commercial Fractions Of Apples: USA: 1976
Volume of Number of Interval Fraction Permethrin Residues
Spray Applications Between Last (mg/kg) after Applying
Applied Spraying and at Stated Rates of mg/kg
(l/ha) Harvest ai in spray
80 mg/kg 160 mg/kg
3150-3600 3-5 38-63 days Mature 0.11-0.40 0.85
apples
Juice <0.01 <0.01
Apple <0.01 -
Sauce
wet 0.74-3.2 6.5
pomace
Dry 3.3-10 22
pomace
Ussary, 1977a, c
TABLE 20. A Comparison Of Permethrin Residues In Processed Tomato Fractions : USA : 1977
Rate of Number of Interval Permethrin Residues (mg/kg) In
Application Applications Between Last
(g ai/ha) Application Whole Tomato Tomato Tomato Tomato
And Harvest Tomatoes Juice Puree Ketchup Pulp
220 2 1 day 0.05 <0.01 0.01 0.01 1.08
3 days 0.08 0.01 0.02 0.01 0.86
7 days 0.04 0.01 0.03 0.02 1.97
440 2 1 day 0.05 <0.01 0.03 0.02 1.60
3 days 0.05 0.01 0.03 0.02 1.45
7 days 0.08 <0.01 0.02 0.01 1.28
Ussary, 1977d
TABLE 21. Distribution Of Permethrin Residues In Whole Wheat
Fractions And Consequent Residue Levels In Bread
Distribution of residue between whole grain fractions1
Permethrin Residue
Fraction % whole grain mg/kg % distribution
Flour (white) 75.1 0.13 12.1
Pollard 11.3 1.9 26.3
Bran 13.6 3.7 61.7
Residue in Bread baked from the ground flour
Commodity Permethrin residue (mg/kg)
White flour 0.13-0.15
White bread 0.13-0.19
Wholemeal flour2 0.81-0.93
Wholemeal bread 0.67-1.00
1 Grain sample 1.5 kg. It had a residue level of 0.65 mg
permathrin/kg after being stored for 9 months.
2 Reconstituted from the fractions in the original whole grain
proportions.
Simpson, 1979
The processing operations simulated those used in commercial practice,
the unbaked bread recipe included potassium bromate and benzoyl
peroxide.
Wholemeal flour was formulated after reconstituting the wheat
fractions in the original ratio.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis (by gas chromatography using
an electron capture detector) for crops treated pre-harvest was
reviewed at the 1979 Meeting (FAO/WHO, 1980).
Both gas chromatography (GC) and high pressure liquid chromatography
(HPLC) have been used to determine permethrin residues in whole cereal
grains and commodities processed from grain (including bread).
The GC procedure described earlier is quite suitable, being able to
determine cis and trans isomers separately or together. Recovery
is essentially quantitative and the limit of determination is about
0.01 mg/kg.
A simple procedure based on a high pressure liquid chromatograph has
also been developed (Simpson, 1978). The limit of determination is a
little better than 0.01 mg/kg. In this procedure the residue is
extracted by cold methanol, partitioned into hexane - and, if
necessary, further cleaned by an acetonitrile partitioning - before
clean-up on an alumina column. The concentrated residue is subjected
to determination by HPLC equipped with a UV detector. This method is
similar to the procedure used by Mourot et al, (1979).
With small modifications, the GC method can be used to determine
permethrin in milk, meat and eggs.
Milk and meat samples are extracted with n-hexane:acetone 4:1. The
acetone is removed by washing with water and the permethrin
partitioned from n-hexane into dimethylformamide. The
dimethylformamide extract is dissolved in 1% aqueous sodium sulphate
and the permethrin back-extracted into n-hexane. The hexane extract
is cleaned up using a Florisil column, and permethrin is determined by
gas chromatography using an electron capture detector. The limit of
determination of the method is 0.01 mg/kg for the combined isomers and
recovery values for samples of meat and milk fortified at 0.01-1.0
mg/kg are normally greater than 70%. Mean recoveries of 89%-92% have
been obtained from milk and 86-88% from tissues of cows and hens
(Edwards and Iswaren, 1977; Edwards and Swaine, 1977). Twenty percent
acetone in hexane has been shown to be an efficient solvent for
extracting permethrin residues from animal tissues (Edwards and
Sapiets, 1978).
The method for permethrin analyses in meat and milk is also applicable
to eggs. These are extracted with n-hexane:acetone 1:1 and the
extract washed with aqueous sodium chloride to remove acetone, and
cleaned up by solvent partition with dimethylformamide and by using a
Florisil column. The limit of determination is 0.02 mg/kg for the
combined isomers and recovery values from yolks and albumen fortified
at 0.01-0.4 mg/kg are generally in the ranges 70-90% and 60-90%
respectively (Edwards and Swaine, 1977).
The mass spectrographic technique of multiple ion detection is
suitable for the qualitative and quantitative confirmation of
permethrin residues in crops, milk, eggs and animal tissues following
separation by gas chromatography.
Qualitative confirmation of permethrin residues is given by the
appearance of a peak at the correct retention time for all the
specific m/e values monitored. In addition, the ratios between the
peak heights (or peak areas) given from each m/e value should be
identical to that observed for permethrin analytical standards.
Quantitative confirmation is carried out by comparison of the peak
height or peak area, measured for the most abundant m/e value
recorded, against those obtained with external standards of permethrin
(Swaine and Edwards, 1977).
Residue of both the free and conjugated major plant metabolites of
permethrin, namely cis- and trans- isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
and 3-phenoxybenzyl alcohol (3-PBAlc), can also be determined by gas
chromatography. Samples are extracted with 2:1 methanol:water and
lipids removed by partitioning with dichloromethane. The methanol is
then removed using rotary evaporation, the aqueous solution made 1 N
with HCl and refluxed to free the conjugated residues. The residues
are then extracted by partitioning with n-hexane. The n-hexane is
then removed and the 2,2,2-trichloroethyl ester of DCVA and the
heptafluorobutyryl ester of 3-PBAlc are simultaneously made. The
derivatives are then analyzed by gas-liquid chromatography using
electron capture detection. (Ussary, 1979). Limits of determination
are in the range 0.02-0.10 mg/kg for DCVA and 0.02-0.05 mg/kg for 3-
PBAlc. Recoveries are generally in the range 70-85%. Confirmation of
the metabolite residues is possible using gas chromatography linked to
mass spectrometry using multiple ion detection, similar to the
procedure reviewed above for permethrin (Swaine et al, 1978).
With little modification, this method has also been used to determine
permethrin metabolite residues in meat and milk. Meat tissues are
extracted with 1:1 methanol:water; milk is extracted with 1:1
hexane:acetone. Any permethrin extracted by this procedure is
partitioned out, along with unconjugated residues of 3-PBAlc, into
n-hexane alone (milk) or hexane plus dichloromethane (meat).
Residues of 3-phenoxybenzoic acid (3-PBAcid), both "free" and
conjugated DCVA, and conjugated residues of 3-PBAlc remain in the
aqueous phase which is subjected to a hydrochloric acid hydrolysis to
cleave the conjugates. DCVA and 3-PBAcid are separated from 3-PBAlc
by partition between aqueous sodium hydroxide and dichloromethane.
The PBAlc residues are combined and determined as the
heptafluorobutyryl ester by gas chromatography using an electron
capture detector. DCVA and 3-PBAcid are converted to their methyl
ester derivatives, by treatment with diazomethane, and determined by
gas chromatography linked to mass spectrometry using multiple ion
detection. A limit of determination of 0.01 n/kg has been obtained
routinely for DCVA, 3-PBAlc and 3-PBAcid in meat and milk. Mean
recoveries have been within the range 55-60% from milk and 60-85% from
meat tissues (Swaine et al, 1980).
NATIONAL TOLERANCES
The following national tolerances were reported to the Meeting for
permethrin (total isomers):
Australia Wheat Bran 10 mg/kg
Raw cereals ) 5 mg/kg
Wheat pollard (shorts) )
Brussels sprouts )
Wheat flour(wholemeal) ) 2 mg/kg
Wholemeal bread )
Broccoli )
Cabbage ) 1 mg/kg
Cauliflower )
Wheat flour (white) ) 0.5 mg/kg
White bread )
Tomatoes 0.4 mg/kg
Cotton ) 0.2 mg/kg
Sweetcorn (cobs) )
Eggs ) 0.1 mg/kg
Fat of meat of cattle, )
sheep, pigs and poultry )
Milk and milk products ) 0.05 mg/kg
(fat basis) )
Belgium Eggplants )
Cabbages )
Cucumbers )
Endives )
Fruit crops ) 1 mg/kg
Honeydew melons )
Lettuce )
Peppers )
Tomatoes )
France Maize (whole plants) 0.2 mg/kg
Maize grains 0.1 mg/kg
Holland Endives ) 2 mg/kg
Lettuce )
Apples )
Brassicae ) 1 mg/kg
Pears )
Eggplants )
Cucumbers )
Honeydew melons ) 0.5 mg/kg
Peppers )
Tomatoes )
USA Cotton 0.5 mg/kg
Eggs )
Meat, fat and meat )
by-products of cows, ) 0.05 mg/kg
goats, hogs, horses, )
poultry and sheep )
Venezuela Vegetables ) 10 mg/kg
EVALUATION
APPRAISAL
Permethrin is a pyrethroid insecticide which is effective at low rates
against a wide range of Lepidoptera, Hemiptera, Diptera and
Coleoptera. Unlike natural pyrethrins and earlier synthetic
pyrethrins, permethrin is photostable to a degree that allows it to be
used as an effective tool in agriculture. It shows adulticidal,
ovicidal and particularly larvicidal activity and is effective against
the great majority of insects that. have become resistant to standard
treatments such an organochlorines and organophosphates. A programme
of sprays is usually required.
A particularly valuable usage is in protection of stored grain.
Laboratory and silo-scale studies in Australia have shown that low
levels of permethrin control most of the endemic insect pests of
stored grain, and, when used in conjuction with a synergist and
complementary insecticide, the commonly-occurring strains of all grain
insect pests are controlled. Adequate protection of at least 9 months
storage is achieved by a single application of 1 mg permethrin/kg.
However, for various reasons, it may become necessary in future to use
permethrin without its synergist and/or o/p insecticide. In that
case, higher application rates would be required to maintain the
present level or permethrin efficacy. The same applies if the present
experimental rate is increased for other reasons (e.g. to counter
resistance). A 2 mg/kg grain is likely to be the target concentration
in the near future.
Permethrin can also be used to control various ectoparasites of
cattle, horses, sheep, pigs and poultry. It can be applied either
directly to the animals, or to buildings in which they are housed, or
to insect breeding and resting sites.
Extensive residues data are available from supervised trials. In
pre-harvest uses, residues are highest where a crop part is exposed
directly to the spray, as in the case of many forage crop uses.
Ground and aerial applications yield similar residue levels. Although
residues decline comparatively slowly after spraying, there is no
obvious build-up of residues of permethrin or of its two most
important plant metabolites on repeated application, within the rates
and frequency of permethrin spraying that are needed to obtain good
insecticidal control. The two important plant metabolites are
3-phenoxybenzyl alcohol and the cis and trans isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA),
which are also animal metabolites; levels of these metabolites in
crops are very much smaller than corresponding permethrin residues and
they do not need to be included in routine residue analyses.
Permethrin levels on stored grains also decline slowly. During the
processing of treated whole wheat grain, the permethrin residue is
retained mainly on the bran component, although a significant
proportion (12%) remains with the white flour and follows through
After both oral and dermal administration to livestock, permethrin
itself constitutes the major portion of the residue recovered from
milk, eggs, muscle and fat. After oral administration to goats,
metabolites form the major part of the residue in kidney and liver;
DCVA occurs in both organs, 3-phenoxybenzyl alcohol plus its
4'-hydroxy derivative in liver and 3-phenoxybenzoic acid plus its
4'-hydroxy derivative in liver and kidney.
Following direct application to dairy cattle (1.0 g ai/cow and with
free access to a self-oiler) permethrin levels are low in milk (<0.02
mg/kg) and in muscle, liver and kidney (<0.01 mg/kg). Highest levels
(up to 0.1 mg/kg) have been found in fat.
Cows maintained on a diet containing 50 mg/kg of permethrin yielded
milk containing low levels of residue (0.1 mg/kg), and the level in
muscle was less than 0.1 mg/kg. The residue in milk declined to below
0.01 mg/kg after five days on returning the cows to control diet.
Residues in eggs are also low and contamination of eggs laid during
the treatment of poultry houses will not result in detectable residues
in yolks or albumen.
Permethrin is not detected in the albumen of eggs from hens receiving
the compound at levels up to 33 mg/kg in the diet; levels in yolks are
approximately 2% of corresponding permethrin dietary levels.
In all cases studied, residues of permethrin and its metabolites in
products of animal origin declined notably on cessation of exposure.
Only small residue levels have been found in products of animal origin
following direct application of permethrin to livestock. In view of
the levels of permethrin found in forage crops, such as alfalfa, and
the importance of such crops in animal feedstuffs, it seems likely
that residue levels in products of animal origin will be higher
following forage crop uses rather than from ectoparasite outlets. In
estimating maximum residue levels for products of animals origin,
allowance has also to be made for the dilution effect that will occur
in practice when treated forage is incorporated into livestock diets.
In proposing maximum residue levels on raw grain and milled cereal
products prepared therefrom, careful consideration has been given to
the fact that this insecticide is to be used as a grain protectant and
that residues of the compound are comparatively stable under storage
conditions. Under practical conditions of grain handling and storage,
it is not possible to apply a grain protectant with assurance that the
deposit will be absolutely uniform; there will always be a natural
variation in the level of the deposit resulting from fluctuations in
the flow of grain and of the insecticide. Furthermore, the movement
of bulk grain results in the segregation of the various components of
the bulk with a resultant variation in the level of residues
throughout the mass. Due allowance has been for the amplitude of
these variations and the problems of sampling and analysis.
The meeting examined residue data from supervised storage and feeding
trials reflecting established or proposed good practice. From these
data the meeting was able to estimate the maximum residue levels that
were likely to occur when permethrin was used in practice.
The preferred method of permethrin residue analysis in crops is by gas
chromatography using an electron capture detector. Recoveries are
essentially quantitative and the method has been applied successfully
to a wide range of crops, raw cereal grains and processed products
derived from them, such as flour, bran and bread. A limit of
determination of 0.01 mg/kg (expressed as permethrin cis and trans
isomers). Cis and trans-isomers are capable of being determined
separately by this method. With small modifications, the method has
been applied successfully to the determination of permethrin residues
in meat, milk and eggs, and would with adaptation be suitable for
regulatory purposes.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the residue levels listed below are
temporary pending submission of the "Further Work or Information".
These are recommended as suitable for establishing temporary maximum
residue limits. These levels are additional to those proposed by the
1979 Meeting. These levels refer to the sum of the cis and
trans-isomers of permethrin.
Crop TMRL (mg/kg)
Alfalfa fodder 100) On a dry weight basis
Maize fodder and straw 100)
Apple pomace 50) On a dry weight basis
Soybean fodder 50) On a dry weight basis
Cereal grains 2
Wheat flour (wholemeal) 2
wheat flour (white) 0.5
Wheat bran 10
Carcass meat of
cattle ) For permethrins a portion of
pig ) 1 carcass fat is analyzed and
sheep ) the levels refer to carcass fat.
Meat by-products from:
pigs ) 0.1
sheep )
Poultry meats 0.1
Eggs 0.1
Milk 0.1
FURTHER WORK OR INFORMATION
Recommendations 3, 4, 8, 9 from 1979 meeting remain relevant.
REFERENCES
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Pilot Usage Trials, 1978-79 Working Party On Grain
Protectants", Department of Primary Industries, Queensland, Australia
(unpublished) (1979a).
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Silo Scale Experiments 1977-78 Working Party On
Grain Protectants", Department of Primary Industries, Queensland,
Australia (unpublished) (1979b).
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional Grain Protectants For The Control of
Malathion-Resistant Insects In Stored Sorghum", Department Of Primary
Industries, Entomology Branch, Queensland, Australia (unpublished)
(1979c).
Bewick, D.W. and Leahey, J.P. "Permethrin : Absorption in Cows", ICI
Plant Protection Division Report No. TMJ1357B (unpublished) (1976).
Bewick, D.W., Leahey, J.P. and Saunders, R. "Permethrin Absorption In
Pigs After Dermal Treatment", ICI Plant Protection Division Report No.
TMJ1562A. (unpublished) (1977).
Chipman Inc. Canada "Summary of 1976/77 Permethrin Sweet Corn Residue
Data . (unpublished) (1978).
Desmarchelier, J.M., Bengston, M., Davies, R.A.H., Elder, W.B.,
Hamilton, D., Henning, R., Murray, W., Ridley, E.G., Ripp, E.,
Sieraikowski, C.J., Sticka, R., Snelson, J.T., Thomas, D., Wallbank,
B. and Wilson, A. "Pilot Usage Of The Grain Protectants Fenitrothion
Plus Phenothrin and Pirimiphos-methyl Plus Permethrin", Australian
Wheat Board Working Party on Grain Protectants (in manuscript for
publishing) (1979).
Edwards, M. J. and Iswaren, T.J. "Permethrin: Residue Transfer And
Toxicology Study With Cows Fed Treated Grass Nuts" ICI Plant
Protection Division Report No. TMJ1519/B (unpublished) (1977).
Edwards, M.J. and Swaine, H. "Permethrin: Incorporation of Permethrin
In The diet of Laying Hens: Residues In Eggs And Tissues". ICI Plant
Protection Division Report No. TMJ1520/B (unpublished) (1977).
Edwards, M.J. and Sapiets, A. "Permethrin: The Extraction of the
Pesticide From Animal Tissues". ICI Plant Protection Division Report
No. RJ0009B (unpublished) (1978).
Gaughan, L.C., Unai, T. and Casida, J.E. "Permethrin Metabolism In
Rats And Cows, And In Bean and Cotton Plant", Paper delivered at 172nd
ACS National Meeting, San Francisco (August 1976).
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. "Distribution
And Metabolism Of Trans- And Cis-Permethrin In Lactating Jersey
Cows", J. Agric. Food Chem. 26(3), 613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. "Distribution And
Metabolic Fate of Trans- And Cis- Permethrin In Laying Hens", J.
Agric. Food Chem. 26(6), 1374-1380.
Hunt, L.M. and Gilbert, B.N. "Distribution And Excretion Rates Of
14C-Labelled Permethrin Isomers Administered Orally To Four Lactating
Goats For 10 Days", J. Agric, Food Chem. 25(3), 673.
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
A.G. "Permethrin: Metabolism And Residues In Goats", ICI Plant
Protection Division Report No. TMJ1516B (unpublished) (1977a).
Leahey J.P., Gatehouse, D.M. and Parr, J.S. "Permethrin: Metabolism In
Hens", ICI Plant Protection Division Report No. TMJ1509B (unpublished)
(1977b).
Leahey, J.P., Bewick, D.W., Gatehouse, D.M. and Carpenter, P.K.
"Permethrin: Absorption In Chickens After Dermal And Oral Treatments",
ICI Plant Protection Division Report No. TMJ1481B (unpublished)
(1977c).
Leahey, J.P. and Cameron, A.G. "Permethrin: Residues In Cows After
Dermal Application", ICI Plant Protection Division Report No. RJ0001B
(unpublished) (1978).
Mourot, D., Delephine. B., Boisseau, J. and Gayot, G. "HPLC Of A New
Pyrethroid Insecticide Sumicidin", J. Chromatog. 168, 277-279.
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1978).
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1979).
Swaine, H. and Edwards, M.J. "Confirmation Of Residues Of Permethrin
(PP557) Using Gas Chromatography - Mass Spectrometry In The Multiple
Ion Detection Mode", ICI Plant Protection Division Data (unpublished)
(1977).
Swaine, H., Edwards, M.J. and Ussary, J.P. "Permethin Crop Rotation
Study", ICI Americas Inc Report No. TMU0378/B (unpublished) (1978).
Swaine, H. and Sapiets, A. "Permethrin Residues Summary: Residues In
Crop Samples Analyzed During 1977-8: Part IV: Leaf, Root and Forage
Crops", ICI Plant Protection Division Report No. RJ0081A (unpublished)
(1979).
Swaine, H., Francis, P.D., Rippington, D. and Burke, S.R. "Permethrin
Residue Levels of the Major Metabolites of the Insecticide In the Milk
And Tissues of Cows Fed On A Treated Diet", ICI Plant Protection
Division Report No. RJ0124B (unpublished) (1980a).
Swaine, H., Francis, P.D., Rippington, D., Burke, S.R., Robertson, S.
and Ford, J.P. "Permethrin Metabolites: Eggs And Tissues Of Hens", ICI
Plant Protection Division Residue Data Report No. RD/557/27
(unpublished) (1980b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions of Apples, Wenatchee, Washington, 1976" ICI Americas Inc.
Report No. TMU0306/B (unpublished) (1977a).
Ussary, J.P. "Permethrin Residues In Soybeans", ICI Americas Inc.
Report No. TMU0383/B, (unpublished) (1977b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions Of Apples, Geneva, New York, 1976", ICI Americas Inc. Report
No. TMU0307/B (unpublished) (1977c).
Ussary. J.P. "Permethrin Residues In Tomato Process Fractions", ICI
Americas Inc. Report No. TMU0304/B (unpublished) (1977d).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0388/B (unpublished) (1978a).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0413/B (unpublished) (1978b).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0438/B (unpublished) (1979a).
Ussary, J.P. "Permethrin Residues On Field Corn", ICI Americas Inc.
Report No. TMU0433/B (unpublished) (1979b).
Ussary, J.P. "Permethrin Metabolite Residues On Broccoli", ICI
Americas Inc. Report No. TMU0427/B (unpublished) (1979c).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0461/B (unpublished) (1979d).
Ussary, J.P. and Braithwaite, G.B. "Residues of Permethrin And
Permethrin metabolites in Milk From Ectiban Treated Cows (Trial No.
35NC79-001)", ICI Americas Inc. Report No. TMU0490/B (unpublished)
(1980a).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin and
3-Phenoxybenzyl Alcohol In Cow Tissues (Trial No. 35NC79-001)", ICI
Americas Inc. Report No. TMU0493/B (unpublished) (1980b).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And 3-
Phenoxybenzyl Alcohol In Tissues From Ectiban Treated Swine (Trial No.
35NC79-002)", ICI Americas Inc. Report No. TMU0491/B (unpublished)
(1980c).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And
3-Phenoxybenzyl Alcohol In Tissues And Eggs From Ectiban Treated
Chickens (Trial No. 35NC79-003)", ICI Americas Inc. Report No.
TMU0492/B (unpublished) (1980d).

 In forage crops such as alfalfa, residue levels of the metabolites
DCVA and 3-phenoxybenzyl alcohol are small compared with the
corresponding permethrin residues. This pattern was found on a range
of crops reviewed at the 1979 Joint Meeting which proposed maximum
residue limits based upon permethrin itself only (FAO/WHO, 1980).
In addition to crops grown specifically for forage, livestock feed can
also contain by-products of food processing (e.g. apple pomace, cotton
cake). Residue levels in these commodities are reviewed under "Fate
on Processing".
Post-Harvest Uses
The only significant post-harvest use of permethrin is its application
to bulk stored grain. This has undergone extensive laboratory studies
and silo-scale trials for this purpose in Australia. Since 1977 some
10,000 tons of grain have been treated in silo-scale pilot trials in
five of the Australian States. All the residue studies show that
permethrin is persistent on grain under the conditions of temperature
and moisture content prevailing in Australian storages (Table 6)
(Desmarchelier et al, 1979). Initial residues on grain were about
20% below the level expected from the amount applied. The residue
hardly declined in 9 months storage: after 6-9 months about 80% of the
initial (1 month) residue in grain remains. This level of persistence
is found consistently in studies on wheat, barley and sorghum, and
probably can be generalised for all stored grain (Bengston et al,
1979a, b, c; Desmarchelier et al, 1979). Also, studies show that
the initial ratio of cis/trans isomers is not changed during 8 months
of storage (Table 7) (Simpson, 1979).
Table 5, Sheet 3 (Permethrin Residues On Field Crops [Or Crop Parts] Used For Forage)
Crop Country and Formulations Rate of Volume Number of Interval Lowest Highest Mean Reference
Year Used Application of Spray Applications Between Last Residue Residue Residue
(g ai/ha) (l/ha) Spraying and Determined Determined (mg/kg)
Harvest (mg/kg) (mg/kg)
1. SWEETCORN
Maize USA 25% EC 1101 110-720 8 0-4 days <0.01 0.05 0.02(6) Ussary,
(Zea mays) 1976-8 during 1978b,
Cobs after 210-2201 ground 7-16 0-4 days <0.01 0.01 <0.01(13) 1979a
Kernals applications
Removed 280-450 or 30-135 6-13 0-4 days <0.01 0.12 0.02(11)
[code no. during
A03.1604] aerial
applications
Husks Canada 25% EC 62.5-70 280-1550 2-7 1-7 days 0.06 0.35 0.27(10) Chipman,
[code no. 1975-7 50% EC 30-46 days <0.01 0.33 0.17(2) Inc.,
A03.1604] 100-1051 1-4 1-14 days <0.01 0.35 0.23(13) 1978;
30-43 days <0.01 0.26 0.10(3) Ussary,
140-1501 3-5 3-7 days 0.34 0.36 0.35(2) 1978b,
46 days 0.02(1) 1979a
USA 25% EC 210-2201 110-720 7-8 0-3 days 0.43 14 6.4(7)
1976-8 280-335 6-9 0-14 days 1.0 18 3.7(10)
Stover USA 25% EC 1101 270-600 8 0-4 days 0.99 18 7.2(6) Ussary,
[code no. 1976-9 during 1978b,
A03.1604] 210-2201 ground 7-8 0-3 days 2.5 23 14(8) 1979a
(all data applications
on 24% dry 280-450 or 30-35 6-16 0-14 days 4.6 29 17(12)
matter basis) during
aerial
applications
1 Indicates effective use rates. For use patterns on these crops, see Table 1.
Figures in parentheses are the numbers of results upon which the means are based
TABLE 6. Permethrin Residues In Treated Wheat and Sorghum In Concrete Silos
(Australian Large-Scale Post-Harvest Treatments)
Wheat Data1
Grain conditions Amount Residues (mg/kg)
% moisture air temp. Applied (calc'd) Period of storage (months)
°C2 mg/kg 1 2 3 4 5 6 7 8 9 15
range 8-12 14-35 0.94-1.15 0.70-0.90 0.65-0.83 0.6-0.8 0.70-0.78 0.75-0.80 0.50-0.80 0.75-0.80 0.63-0.65 0.57 0.60-0.70
mean 0.99 0.83 0.75 0.70 0.74 0.74 0.66 0.78 0.64 0.57 0.65
s.d. 0.17 - 0.06 - - 0.14 - - - -
c.v.3 21 - 9 - - 21 - - - -
(Bengston et al, 1979a,b; Desmarchalier et al, 1979)
1 Trials included treatment of 30 tonnes of barley apart from some 10,500 tonnes wheat.
2 Initial temperatures were in the range 25-33°C, at 6 months they were 14-27°C, at 8 months they were 17-22°C. Initial moisture contents were
in the range 9-12%, at 6 months they were 8-12% and at 8 months they were 9-11%. Data are from 1977/78 and 1978/79 large scale trials. Grain
stored in 16 silos (30-1400 tonnes grain) in 5 Australian States.
3 s.d. = standard deviation; c.v. = co-efficient of variation.
Sorghum Data1
Grain Conditions Amount Residues (mg/kg)
% moisture2 air temps. °C2 Applied (calc'd) Period of Storage (months)
mg/kg 1 6 12 17 26
range 11.6 21.2 1.06 0.70-0.80 0.56-0.58 0.62-0.71 0.56-0.57 0.53-0.62
mean 0.75 0.57 0.67 0.57 0.58
(Bengston et al, 1979c)
1 Data from grain stored in a silo (430 tonnes) at one site. 1978 trial.
2 Initial
TABLE 7. Residues Of Cis And Trans Isomers Of Permethrin In Stored Grain
Percentage Proportions In Stored Wheat And Sorghum
Storage Conditions Original % Residues
% moisture air temps. °C1 proportion Period of Storage (weeks)
(nominal) 1 6 13 17 20 26 39
wheat 37 36 35.5 - 38.3 38.8 36.3
cis 10 32 40 63 64 64.5 - 61.7 61.2 63.7
trans 60
sorghum
cis 11.6 21.2 25 20.7 24.6 22.5 21.2 - 21
trans 75 79.3 75.4 77.5 78.8 - 79
(Simpson, 1979)
1 Initial
Ectoparasite uses
Cows were given five whole-body sprays of permethrin at a rate of 1.0
g ai/cow, with 14 days between sprays, using a 5% emulsifiable
concentrate formulation. They were allowed free access to a
self-oiler containing a 0.03 g ai/l solution, ensuring at least two
applications per day for a period of ten weeks, and were housed in
premises that were sprayed at a rate of 0.06 g ai/m2, five sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range that is likely to occur in normal
husbandry practice. Milk samples were taken for analysis before, and
during the ten days after, the fifth application. Only four out of 70
samples of milk analyzed showed any measurable residues of permethrin
or its metabolites cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethyl
cyclopropane carboxylic acid (DCVA), 3-phenoxybenzoic acid (3-PBAcid)
and 3-phenoxybenzylalcohol (3-PBAlc) (Figure 1) (Table 8) (Ussary and
Braithwaite, 1980a).
TABLE 8. Residues of Permethrin And Metabolites In Milk Following
Five
Dermal Applications Of Permethrin At 1.0 g ai/cow
Time after Lowest Highest Mean
Chemical application Residue Residue Residue
(days) (mg/kg) (mg/kg) (mg/kg)
Permethrin 0 <0.01 0.01 0.01 (20)1
1 <0.01 0.01 0.01 (10)
3-7 <0.01 <0.01 <0.01 (30)
10 <0.01 0.01 0.01 (10)
DCVA (I+II) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAcid (IV) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAlc (III) 0-7 <0.01 <0.01 <0.01 (60)
10 <0.01 0.02 0.01 (11)
1 Bracketed figures are numbers of samples
Ussary and Braithwaite, 1980a
Cows were given 6 whole-body sprays of permathrin at a rate of 1.0 g
ai/cow with 14 days between sprays. They were allowed free access to
a self-oiler containing a 0.03 g ai/l solution ensuring at least two
applications per day for a period of ten weeks and were housed in
premises which were sprayed at a rate of 0.06 g ai/m2, six sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range which is likely to occur in normal
husbandry practice. Cows were slaughtered five days after the sixth
application, when permathrin levels in muscle, liver and the kidneys
were low (<0.01 mg/kg). The highest levels of permethrin were 0.10
mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat
respectively (Table 9) (Ussary and Braithwaite, 1980b).
TABLE 9. Residues Of Permethrin In Tissues of Cows Following Dermal
Applications
Tissue Permethrin Residue1
(mg/kg)
Muscle <0.01
Kidney <0.01
Liver <0.01
Intestinal fat 0.05-0.10
Subcutaneous fat 0.03-0.04
1 Limit of determination: 0.01 mg/kg (Ussary and Braithwaite,
1980b)
Pigs were housed in premises treated with mist applications of 0.06 g
permethrin per m3 at 14-day intervals and slaughtered one day after
the sixth application. Permethrin residues were not measurable in
skin, liver or kidney tissues (limit of determination: 0.01 mg/kg) and
only low levels of permethrin were found in the fat and muscle tissues
(0.02 and up to 0.01 mg/kg respectively) (Table 10) (Ussary and
Braithwaite, 1980c).
TABLE 10. Residues of Permethrin Detected in Swine Tissues after Six
Mist Applications of Permethrin to Swine Premises at O.O6 g per m2
Tissue Permethrin Residues
(mg/kg)
Skin <0.01
Muscle <0.01
Liver <O.01
Kidney <0.01
Subcutaneous fat 0.02
Intestinal fat 0.02
(Ussary and Braithwaite, 1980c)
Hens were present at the times of application when premises received
six mist applications of 0.06 g permethrin per m3 at 14-day
intervals. Eggs were collected at intervals up to 50 days following
the first application and hens were sacrificed five days after the
sixth application. Eggs were found to contain permethrin at 0.02
mg/kg on one occasion only. Otherwise, permethrin residues in muscle,
skin, liver and eggs were below 0.01 mg/kg. A residue of 0.02 mg/kg
was detected in fat tissues (Table 11) (Ussary and Braithwaite,
1980d).
TABLE 11. Residues of Permethrin in Eggs and Tissues Chickens
Maintained in Premises Treated with Permethrin
Tissue Permethrin
(mg/kg)
Muscle <0.01
Skin <0.01
Liver <0.01
Fat 0.02
Eggs <0.02
(Ussary and Braithwaite, 1980d)
FATE OF RESIDUES
In the text which follows, reference will be made to various sites of
radiolabelling of permethrin. These will refer to 14C-labelling as
shown in Figure 2.
In farm animals
Introduction
Data on absorption, excretion and accumulation after oral dosing of
permethrin were reviewed at the 1979 Meeting (FAO/WHO, 1980).
Permethrin is extensively metabolised and rapidly excreted by cows and
goats after oral administration. Residue levels in milk, muscle and
fat are small, as they are in the skin, muscle and eggs of hens.
Permethrin itself constitutes more than half of the residue in milk,
eggs and fat, and in the muscle of livestock. In all cases, residue
levels decline notably on cessation of exposure.
After dermal application the majority of the small residues detected
are found in the tissues, at or immediately below the site of
application. At least 70% of the total residue in those tissues is
due to permethrin itself. Residues in milk and eggs reach a peak a
few days after dermal application and then decline rapidly.
In forage crops such as alfalfa, residue levels of the metabolites
DCVA and 3-phenoxybenzyl alcohol are small compared with the
corresponding permethrin residues. This pattern was found on a range
of crops reviewed at the 1979 Joint Meeting which proposed maximum
residue limits based upon permethrin itself only (FAO/WHO, 1980).
In addition to crops grown specifically for forage, livestock feed can
also contain by-products of food processing (e.g. apple pomace, cotton
cake). Residue levels in these commodities are reviewed under "Fate
on Processing".
Post-Harvest Uses
The only significant post-harvest use of permethrin is its application
to bulk stored grain. This has undergone extensive laboratory studies
and silo-scale trials for this purpose in Australia. Since 1977 some
10,000 tons of grain have been treated in silo-scale pilot trials in
five of the Australian States. All the residue studies show that
permethrin is persistent on grain under the conditions of temperature
and moisture content prevailing in Australian storages (Table 6)
(Desmarchelier et al, 1979). Initial residues on grain were about
20% below the level expected from the amount applied. The residue
hardly declined in 9 months storage: after 6-9 months about 80% of the
initial (1 month) residue in grain remains. This level of persistence
is found consistently in studies on wheat, barley and sorghum, and
probably can be generalised for all stored grain (Bengston et al,
1979a, b, c; Desmarchelier et al, 1979). Also, studies show that
the initial ratio of cis/trans isomers is not changed during 8 months
of storage (Table 7) (Simpson, 1979).
Table 5, Sheet 3 (Permethrin Residues On Field Crops [Or Crop Parts] Used For Forage)
Crop Country and Formulations Rate of Volume Number of Interval Lowest Highest Mean Reference
Year Used Application of Spray Applications Between Last Residue Residue Residue
(g ai/ha) (l/ha) Spraying and Determined Determined (mg/kg)
Harvest (mg/kg) (mg/kg)
1. SWEETCORN
Maize USA 25% EC 1101 110-720 8 0-4 days <0.01 0.05 0.02(6) Ussary,
(Zea mays) 1976-8 during 1978b,
Cobs after 210-2201 ground 7-16 0-4 days <0.01 0.01 <0.01(13) 1979a
Kernals applications
Removed 280-450 or 30-135 6-13 0-4 days <0.01 0.12 0.02(11)
[code no. during
A03.1604] aerial
applications
Husks Canada 25% EC 62.5-70 280-1550 2-7 1-7 days 0.06 0.35 0.27(10) Chipman,
[code no. 1975-7 50% EC 30-46 days <0.01 0.33 0.17(2) Inc.,
A03.1604] 100-1051 1-4 1-14 days <0.01 0.35 0.23(13) 1978;
30-43 days <0.01 0.26 0.10(3) Ussary,
140-1501 3-5 3-7 days 0.34 0.36 0.35(2) 1978b,
46 days 0.02(1) 1979a
USA 25% EC 210-2201 110-720 7-8 0-3 days 0.43 14 6.4(7)
1976-8 280-335 6-9 0-14 days 1.0 18 3.7(10)
Stover USA 25% EC 1101 270-600 8 0-4 days 0.99 18 7.2(6) Ussary,
[code no. 1976-9 during 1978b,
A03.1604] 210-2201 ground 7-8 0-3 days 2.5 23 14(8) 1979a
(all data applications
on 24% dry 280-450 or 30-35 6-16 0-14 days 4.6 29 17(12)
matter basis) during
aerial
applications
1 Indicates effective use rates. For use patterns on these crops, see Table 1.
Figures in parentheses are the numbers of results upon which the means are based
TABLE 6. Permethrin Residues In Treated Wheat and Sorghum In Concrete Silos
(Australian Large-Scale Post-Harvest Treatments)
Wheat Data1
Grain conditions Amount Residues (mg/kg)
% moisture air temp. Applied (calc'd) Period of storage (months)
°C2 mg/kg 1 2 3 4 5 6 7 8 9 15
range 8-12 14-35 0.94-1.15 0.70-0.90 0.65-0.83 0.6-0.8 0.70-0.78 0.75-0.80 0.50-0.80 0.75-0.80 0.63-0.65 0.57 0.60-0.70
mean 0.99 0.83 0.75 0.70 0.74 0.74 0.66 0.78 0.64 0.57 0.65
s.d. 0.17 - 0.06 - - 0.14 - - - -
c.v.3 21 - 9 - - 21 - - - -
(Bengston et al, 1979a,b; Desmarchalier et al, 1979)
1 Trials included treatment of 30 tonnes of barley apart from some 10,500 tonnes wheat.
2 Initial temperatures were in the range 25-33°C, at 6 months they were 14-27°C, at 8 months they were 17-22°C. Initial moisture contents were
in the range 9-12%, at 6 months they were 8-12% and at 8 months they were 9-11%. Data are from 1977/78 and 1978/79 large scale trials. Grain
stored in 16 silos (30-1400 tonnes grain) in 5 Australian States.
3 s.d. = standard deviation; c.v. = co-efficient of variation.
Sorghum Data1
Grain Conditions Amount Residues (mg/kg)
% moisture2 air temps. °C2 Applied (calc'd) Period of Storage (months)
mg/kg 1 6 12 17 26
range 11.6 21.2 1.06 0.70-0.80 0.56-0.58 0.62-0.71 0.56-0.57 0.53-0.62
mean 0.75 0.57 0.67 0.57 0.58
(Bengston et al, 1979c)
1 Data from grain stored in a silo (430 tonnes) at one site. 1978 trial.
2 Initial
TABLE 7. Residues Of Cis And Trans Isomers Of Permethrin In Stored Grain
Percentage Proportions In Stored Wheat And Sorghum
Storage Conditions Original % Residues
% moisture air temps. °C1 proportion Period of Storage (weeks)
(nominal) 1 6 13 17 20 26 39
wheat 37 36 35.5 - 38.3 38.8 36.3
cis 10 32 40 63 64 64.5 - 61.7 61.2 63.7
trans 60
sorghum
cis 11.6 21.2 25 20.7 24.6 22.5 21.2 - 21
trans 75 79.3 75.4 77.5 78.8 - 79
(Simpson, 1979)
1 Initial
Ectoparasite uses
Cows were given five whole-body sprays of permethrin at a rate of 1.0
g ai/cow, with 14 days between sprays, using a 5% emulsifiable
concentrate formulation. They were allowed free access to a
self-oiler containing a 0.03 g ai/l solution, ensuring at least two
applications per day for a period of ten weeks, and were housed in
premises that were sprayed at a rate of 0.06 g ai/m2, five sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range that is likely to occur in normal
husbandry practice. Milk samples were taken for analysis before, and
during the ten days after, the fifth application. Only four out of 70
samples of milk analyzed showed any measurable residues of permethrin
or its metabolites cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethyl
cyclopropane carboxylic acid (DCVA), 3-phenoxybenzoic acid (3-PBAcid)
and 3-phenoxybenzylalcohol (3-PBAlc) (Figure 1) (Table 8) (Ussary and
Braithwaite, 1980a).
TABLE 8. Residues of Permethrin And Metabolites In Milk Following
Five
Dermal Applications Of Permethrin At 1.0 g ai/cow
Time after Lowest Highest Mean
Chemical application Residue Residue Residue
(days) (mg/kg) (mg/kg) (mg/kg)
Permethrin 0 <0.01 0.01 0.01 (20)1
1 <0.01 0.01 0.01 (10)
3-7 <0.01 <0.01 <0.01 (30)
10 <0.01 0.01 0.01 (10)
DCVA (I+II) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAcid (IV) 0-10 <0.01 <0.01 <0.01 (70)
3-PBAlc (III) 0-7 <0.01 <0.01 <0.01 (60)
10 <0.01 0.02 0.01 (11)
1 Bracketed figures are numbers of samples
Ussary and Braithwaite, 1980a
Cows were given 6 whole-body sprays of permathrin at a rate of 1.0 g
ai/cow with 14 days between sprays. They were allowed free access to
a self-oiler containing a 0.03 g ai/l solution ensuring at least two
applications per day for a period of ten weeks and were housed in
premises which were sprayed at a rate of 0.06 g ai/m2, six sprays
taking place with a 14-day interval between sprays, cows having free
access to the premises during spraying. This degree of exposure is at
the highest end of the range which is likely to occur in normal
husbandry practice. Cows were slaughtered five days after the sixth
application, when permathrin levels in muscle, liver and the kidneys
were low (<0.01 mg/kg). The highest levels of permethrin were 0.10
mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat
respectively (Table 9) (Ussary and Braithwaite, 1980b).
TABLE 9. Residues Of Permethrin In Tissues of Cows Following Dermal
Applications
Tissue Permethrin Residue1
(mg/kg)
Muscle <0.01
Kidney <0.01
Liver <0.01
Intestinal fat 0.05-0.10
Subcutaneous fat 0.03-0.04
1 Limit of determination: 0.01 mg/kg (Ussary and Braithwaite,
1980b)
Pigs were housed in premises treated with mist applications of 0.06 g
permethrin per m3 at 14-day intervals and slaughtered one day after
the sixth application. Permethrin residues were not measurable in
skin, liver or kidney tissues (limit of determination: 0.01 mg/kg) and
only low levels of permethrin were found in the fat and muscle tissues
(0.02 and up to 0.01 mg/kg respectively) (Table 10) (Ussary and
Braithwaite, 1980c).
TABLE 10. Residues of Permethrin Detected in Swine Tissues after Six
Mist Applications of Permethrin to Swine Premises at O.O6 g per m2
Tissue Permethrin Residues
(mg/kg)
Skin <0.01
Muscle <0.01
Liver <O.01
Kidney <0.01
Subcutaneous fat 0.02
Intestinal fat 0.02
(Ussary and Braithwaite, 1980c)
Hens were present at the times of application when premises received
six mist applications of 0.06 g permethrin per m3 at 14-day
intervals. Eggs were collected at intervals up to 50 days following
the first application and hens were sacrificed five days after the
sixth application. Eggs were found to contain permethrin at 0.02
mg/kg on one occasion only. Otherwise, permethrin residues in muscle,
skin, liver and eggs were below 0.01 mg/kg. A residue of 0.02 mg/kg
was detected in fat tissues (Table 11) (Ussary and Braithwaite,
1980d).
TABLE 11. Residues of Permethrin in Eggs and Tissues Chickens
Maintained in Premises Treated with Permethrin
Tissue Permethrin
(mg/kg)
Muscle <0.01
Skin <0.01
Liver <0.01
Fat 0.02
Eggs <0.02
(Ussary and Braithwaite, 1980d)
FATE OF RESIDUES
In the text which follows, reference will be made to various sites of
radiolabelling of permethrin. These will refer to 14C-labelling as
shown in Figure 2.
In farm animals
Introduction
Data on absorption, excretion and accumulation after oral dosing of
permethrin were reviewed at the 1979 Meeting (FAO/WHO, 1980).
Permethrin is extensively metabolised and rapidly excreted by cows and
goats after oral administration. Residue levels in milk, muscle and
fat are small, as they are in the skin, muscle and eggs of hens.
Permethrin itself constitutes more than half of the residue in milk,
eggs and fat, and in the muscle of livestock. In all cases, residue
levels decline notably on cessation of exposure.
After dermal application the majority of the small residues detected
are found in the tissues, at or immediately below the site of
application. At least 70% of the total residue in those tissues is
due to permethrin itself. Residues in milk and eggs reach a peak a
few days after dermal application and then decline rapidly.
 Cows and goats
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bodyweight, equivalent to approximately 80 mg/kg in the
diet, levels of radioactivity in milk reached a maximum of 0.13 mg
permethrin equivalents/kg after 1-2 days. These declined to less than
0.02 mg/kg after seven days. Levels of radioactivity in the fat were
0.12-0.18 mg permethrin equivalents/kg, after seven days. By fourteen
days these had declined to 0.05-0.06 mg/kg, indicating that the small
residues in fat are also not maintained on cessation of dosing (Bewick
and Leahey, 1976).
Hunt and Gilbert dosed goats orally with either the cis- or
trans-isomers of 14C-labelled permethrin (carbonyl- or
methylene-labelled) at a rate equivalent to approximately 6 mg/kg in
the diet for ten days. Total radioactive residues in the milk reached
a plateau after three days of 0.02-0.05 and <0.01-0.01 mg permethrin
equivalents/kg respectively for the cis- and trans- isomers. The
goats were sacrificed 24 hours after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 12.
Total radioactivity in the fat of animals receiving the cis-isomer
was ten times higher than in those receiving the more readily
hydrolysed trans-isomer (Table 12) (Hunt and Gilbert, 1977).
When cis- and trans-isomers of 14C-labelled permethrin (carbonyl-
and methylene-labelled) were administered orally to lactating Jersey
cows for three consecutive days at approximately 1 mg/kg bodyweight,
radioactivity was largely eliminated from the body in faeces and urine
within 12 or 13 days after the initial treatment. Total
14C-permethrin equivalents in milk were consistently below 0.3 mg/kg
and declined on cessation of exposure. Residues in fat were also low.
Residues recovered from fat and milk were higher when cis-
permethrin rather than trans-permathrin was administered and
consisted almost entirely (>85%) of unmetabolised permethrin.
Total 14C-permethrin equivalents in blood reached a transient peak
shortly after each dose and dropped to insignificant levels within 2-4
days after the last dose (Gaughan et al, 1976, 1978a).
A study with non-radiolabelled permethrin supported the finding that
permethrin itself is the predominant residue in milk. It was also the
major residue in cow muscle. In this study, groups each of three
barren Friesian cows, yielding 9-13 litres of milk per day, were
maintained on diets containing permethrin at approximately 0.2, 1.0,
10 or 50 mg/kg. The permethrin was absorbed on grass nuts. After
28-31 days two cows in each group were sacrificed. The third was
returned to control diet for nine days before sacrifice. Samples of
milk and of meat tissues were analyzed for residues of permethrin,
cis and trans 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II, DCVA) taken together,
3-phenoxybenzylalcohol (III; 3-PBAlc) and 3-phenoxybenzoic acid (IV;
3-PBAcid) (Figure 1) by the gas chromatographic methods reviewed under
"Methods of Residue Analysis".
TABLE 12. Total Radio-labelled Residues In Tissues of Goats Receiving 14C-Labelled Permethrin
Daily Orally For 7-10 Days
Material Dose Level Duration of Period Between Total Residue (mg permethrin
Administered (= mg/kg Administration Last Date And equivalents/kg In
in diet) (days) Sacrifice Fat Muscle Liver Kidney
Permethrin, ‰10 7 4 hours <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis-trans
Permethrin, ‰6 10 24 hours 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin, ‰6 10 24 hours 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
Hunt and Gilbert, 1977
Leahey et al, 1977a
TABLE 13. Characterisation of 14C-Labelled Residues In Liver And Kidney Of Goats
Receiving Permethrin Orally Daily For Seven Days
Radioactivity Soluble in Water
% Of % of Radioactivity % of Radioactivity
Organ Position of Radioactivity Soluble in Diethyl Remaining Water Unextracted
14C-Labelling Soluble in Ether Following Soluble After
Of Permethrin Diethyl Ether Hydrolysis Hydrolyais
Liver Methylene ("Alcohol") 16 41 23 note 1
Cyclopropyl ("Acid") 39 24 36 note 1
Kidney Methylene ("Alcohol") 43 42 <1 6
Cyclopropyl ("Acid") 29 20 22 9
1 liver was almost completely solubilised during refluxing.
Leahey et al, 1977a
Only permethrin itself was detected in milk. Low levels of 0.01-0.06
mg/kg (mean 0.02 mg/kg) and 0.03-0.2 (mean 0.1 mg/kg) were present in
the milk of cows in the 10 and 50 mg/kg groups respectively. These
values are approximately 0.2% of the corresponding dietary levels.
Permethrin residues in milk were less than 0.01 mg/kg at the 0.2 and
1.0 mg/kg dietary inclusion rates. None of the three metabolites was
found in any of the milk samples analyzed (limit of determination:
0.01 mg/kg). Permethrin levels in milk did not accumulate over the
period of the study and they declined rapidly, to below 0.01 mg/kg
after five days, on returning the animals to control diet (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
Permethrin itself was also the major part of the small residue levels
found in adductor, pectoral and cardiac muscle. These small levels
were approximately 0.1% of the corresponding dietary inclusion levels
(Table 14). Permethrin residue levels in peritoneal fat were
invariably higher than in subcutaneous fat (Table 15) (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
As found separately by Leahey et al (below), metabolites rather than
permethrin itself constituted the major part of the residues in liver
and kidney. Again, the levels declined rapidly on cessation of
exposure (Table 14) (Edwards and Iswaren, 1977; Swaine et al,
1980a).
Leahey et al dosed goats orally with 40:60 cis:trans 14C-
labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 mg/kg in the diet for seven days.
Total radioactive residues in the milk reached a plateau of 0.02-0.03
mg permethrin equivalents/kg after five days; 30-50% of this
radioactivity was associated with the butterfat fraction of the milk,
in which total radioactive residues were 0.13-0.27 mg permethrin
equivalents/kg.
The animals were sacrificed four hours after receiving the final dose,
when levels of radiocarbon in meat tissues were as shown in Table 12.
Characterization of the residues in liver and kidney, the two tissues
containing the highest total residues is shown in Table 13. Where
"alcohol"-labelled permethrin was used, approximately 70% of the 14C
in kidney tissue was 3-phenoxybenzoic acid (IV) plus
3-(4'-hydroxyphenoxy) benzoic acid (VI) (Figure 1). Approximately 30%
of the 14C in the liver was due to 3-phenoxybenzyl alcohol (III) plus
3-(4'-hydroxyphenoxy) benzyl alcohol (V). A further 15% was due to
3-phenoxybenzoic acid (IV) plus 3-(4'-hydroxyphenoxy) benzoic acid
VI). Where "acid"-labelled permethrin was used, approximately 10-15%
of the label in liver and kidney was due to the cis and trans
3-(2,2-dichlorovinyl) cyclopropane carboxylic acids (I and II)
(principally the trans isomer). (Leahey et al, 1977a).
TABLE 14. Residues Of Permethrin and Three Major Metabolites in Tissues Of Cows
Receiving Permethrin In The Diet
Nominal Residue (mg/kg) Of
Dietary Feeding Regime Tissue
Inclusion Analysed Cis & Trans
Level Permethrin DCVA 3-PBAlc 3-PBAcid
(mg/kg) (I and II) (III) (IV)
50 "Treated"1 Liver <0.01 0.01-0.04 0.05-0.10 0.03-0.12
Kidney 0.02-0.06 0.07-0.09 0.01-0.09 0.03-0.04
Muscle O.03-0.10 <0.01-0.02 <0.131 <0.01
"Treated plus Liver <0.01 0.01 <0.01 <0.01
Recovery"2 Kidney 0.02 0.06 <0.01 <0.01
Muscle 0.02-0.06 <0.01 <0.01 <0.01
10 "Treated" Liver <0.01 <0.01 0.01-0.03 0.01-0.02
Kidney <0.01-0.01 <0.01-0.02 <0.01-0.01 <0.01-0.02
Muscle <0.01-0.03 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery Kidney 0.01 <0.01 <0.01 0.04
Muscle 0.01-0.02 <0.01 <0.01 <0.01
1.0 "Treated" Liver <0.01 <0.01 <0.01 <0.01
Kidney <0.01 <0.01 <0.01 <0.01-0.01
Muscle <0.01 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery" Kidney 0.01 <0.01 <0.01 0.02
Muscle <0.01 <0.01 <0.01 <0.01
0.2 "Treated" Liver <0.01 - - -
Kidney <0.01 - - -
Muscle <0.01 - - -
"Treated plus Liver <0.01 - - -
Recovery" Kidney 0.01 - - -
Muscle <0.01 - - -
1 "Treated" indicates the animals received treated diet for 28-31 days and were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28 days and were then
returned to untreated diet for a further 9 days before slaughter.
Edwards and Iswaren, 1977; Swaine et al, 1980a
TABLE 15. Residues In Fat Of Cows Receiving Permethrin In The Diet
Nominal Residues (mg/kg) of Permethrin In
Dietary Inclusion
Level (mg/kg) Peritoneal Fat Subcutaneous Fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
(Edwards and Iswaren, 1977)
TABLE 16. Total Radiolabelled Residues In Tissues Of Cows Seven Days
After Receiving A Single Dermal Dose Of 1.25 g of 40:60 Cis:Trans
14C-Labelled Permethrin
Tissue Residue in tissue Residue in tissue
from cow treated from cow treated
with alcohol-labelled with acid-labelled
permethrin, mg/kg1 permethrin µg/kg1
Meat <0.01 <0.01
Subcutaneous fat 0.053 0.033
Perirenal fat 0.046 0.054
Liver 0.080 0.064
Kidney 0.048 0.038
1 Residues expressed as mg/kg permethin equivalents. Bewick et al,
1977.
Permethrin itself also constitutes the major part of the small
residues found in the milk and fat of cows after dermal application.
In one study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). Although the concentration of suspension used
(0.5% w/v) was ten times the maximum that is likely to be used in
practice, the total dose of 40 mg per cow was approximately 1/30 of
the amount that could be applied commercially at any one time. The
dose was placed on an area of 225 cm2.
Levels of radioactivity in the milk and blood reached a maximum during
the first few days after dosing and then declined steadily. Total
residues in milk were always less than 2 µg permethrin equivalents/kg
(i.e. less than 2 ppb) and were below the limit of determination (0.3
µg/kg) by the end of fourteen days following treatment.
Total radiolabelled residues in muscle, liver, kidney and heart were
also small - 0.02 mg permethrin equivalents/kg or below seven days
after treatment - and were considerably smaller after a further seven
days.
Highest residues were found in fat from below the treatment area.
Seven days after treatment the total residue was approximately 0.5 mg
permethrin equivalents/kg, of which at least 80% was due to permethrin
itself. After a further seven days, residues in fat below the site of
application had fallen by about one half; the residue remaining was
again characterised as being due mainly to permethrin itself (Bewick
and Leahey, 1976).
In a second study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 1.25 g per cow applied in a 0.5% w/v
emulsion is at the higher end of the range likely to be used
commercially. The permethrin was brushed onto 25% of the surface area
of the animals.
Levels of radioactivity found in milk were very similar for the two
positions of radiolabelling. Residues reached a peak of approximately
0.01 mg permethrin equivalents/kg after 3-5 days; 89-91% of these
residues could be extracted into butter fat in which the major portion
of the residue (at least 51-68%) was characterised as permethrin
(Leahey and Cameron, 1978).
Seven days after treatment only small residues were present in the
animal tissues. Levels of radioactivity were similar in perirenal and
subcutaneous fat and for the two positions of radiolabelling (Table
16). At least 40% of this residue was characterised as permethrin
(Leahey and Cameron, 1978).
Pigs
Pigs were dosed dermally with 40:60 cis:trans 14C-labelled
permethrin (cyclopropyl or methylene-labelled). Although the
concentration of suspension used (0.5% w/v) was ten times the maximum
that is likely to be used in practice, the total dose of 18 mg per pig
is approximately 1/30 of the amount that could be applied commercially
at any one time. The dose was placed on an area of 100 cm2.
Seven and fourteen days after treatment approximately 1% of the
applied dose remained at the site of application, principally (>95%)
as permethrin. A residue of 0.05 mg permethrin equivalents/kg in fat
immediately below the site of application seven days after treatment
was due essentially to permethrin. After fourteen days, no
radioactive residue was found (limit of determination: 0.012 mg
permethrin equivalents/kg) (Bewick et al, 1977).
A residue of 0.01 mg permethrin equivalents/kg was found in muscle
beneath the site of application seven days after treatment.
Otherwise, no residues were detected in muscle, liver, kidney, heart
or blood either seven or fourteen days after treatment (limit of
determination: approximately 0.001 mg permethrin equivalents/kg)
(Bewick et al, 1977).
Hens
In one study hens received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (cyclopropyl- and methylene-labelled) at a
rate equivalent to 30 mg/kg in the diet. Birds were sacrificed one,
seven and fourteen days after treatment.
Throughout the study, residues in breast muscle, leg muscle and heart
were small (less than 0.04 mg permethrin equivalent/kg). Total
radioactive residues in liver and kidney of 0.22-0.25 mg/kg had
decreased by an order of magnitude after seven days. Residues of
0.20-0.35 mg permethrin equivalents/kg in peritoneal and subcutaneous
fat on the day after treatment also declined during the remainder of
the study, albeit more slowly than in the case of liver and kidney
(Leahey et al, 1977c).
Levels of radioactivity in eggs reached a maximum soon after dosing
(in 1-4 days) and then declined during the remainder of the 14-day
study (Leahey et al, 1977c).
Hens have also been dosed orally with 40:60 cis:trans
14C-permethrin (cyclopropyl- or methylene-labelled) for ten days at a
rate equivalent to approximately 10 mg/kg in the diet or separately
with cis- and trans-isomers (carbonyl- or methylene-labelled) for
three days at a rate equivalent to approximately 80 mg/kg in the diet.
Residues in eggs were present primarily (>75%) in the yolks, in which
radioactivity reached a plateau after 5-8 days of 0.3-0.5 mg
permethrin equivalents/kg in the 10-dose study and 0.6 mg/kg (trans-
isomer administered) or 2.1-2.8 mg/kg (cis-isomer administered) in
the 3-dose study. Permethrin was the major compound identified in the
eggs (52-62%). The cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acids (I and II) and 3-phenoxybenzyl alcohol (III) (Figure 1) were the
major metabolites in eggs, each normally accounting for less than
approximately 10% of the total radioactivity. The carboxylic acids
were present both free and as the glucuronide and taurine conjugates.
Other metabolites arose from hydroxylation in the 4'-position of the
"alcohol" moiety and in the trans-2-methyl moiety in the "acid" part
of the molecule.
The hens were sacrificed four hours after receiving the final dose in
the 10-dose study, when residues were as shown in Table 17. As in the
case of eggs, residues in fat derived from both "acid" and "alcohol"
labels were similar. Permethrin itself represented the major residue
in the fat. Compounds I-III and 3-phenoxybenzoic acid (IV) were also
identified (each less than 10% of the total radioactivity in the fat).
In both muscle and liver, higher residues were detected in hens dosed
with "acid"-labelled permethrin than with "alcohol"-labelled. The
cis- and trans-3-2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II) were the major residues identified in
these tissues. Blood levels declined rapidly during the first 24
hours after administration (Gaughan et al, 1978b; Leahey et al,
1977b).
In a study with non-radiolabelled 40:60 cis:trans permethrin,
groups of 40 laying hens were fed on diets containing approximately
0.4, 3.4 and 33 mg/kg for 28 days and then returned to a control diet
for an additional 14 days. Samples of eggs laid during the study were
analyzed for permethrin residues by the gas chromatographic method
described under "Methods of Residue Analysis". Five hens per group
were sacrificed after 21, 28, 35 and 42 days of the study and tissues
analyzed for permethrin.
At the 0.4 mg/kg dietary inclusion rate no residues of permethrin were
detected on the albumen and yolks of eggs (limit of determination:
0.02 mg/kg) or in the muscle, skin and liver (limit of determination:
0.01 mg/kg). At the higher dietary inclusion rates no permethrin was
detected in egg albumen. In yolks, permethrin residues were up to
0.05 mg/kg and up to 0.64 mg/kg respectively at the 3.4 and 33 mg/kg
treatment levels. Residues did not accumulate and declined rapidly
when feeding finished, reaching non-detectable levels (less than 0.02
mg/kg) before the end of the 14-day recovery period in both cases.
Only small residues (< 0.03 mg/kg) of the metabolites I-IV were found
in eggs during the feeding period. Again these declined on cessation
of exposure. At the 3.4 mg/kg dietary inclusion rate, permethrin
residues in muscle, skin and liver were non-detectable; i.e. less than
0.01 mg/kg. At the 33 mg/kg rate permethrin residues in liver were
also non-detectable. Permethrin was the major constituent of the low
residues in muscle and skin; levels of 0.05-0.08 mg/kg fell to 0.02
mg/kg before the end of the recovery period (Table 18) (Edwards and
Swaine, 1977; Swaine et al, 1980b).
Chickens have also received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 4.5 mg per bird, which is
approximately 20% of the highest rate that is likely to be used
commercially, was brushed on as a 0.1% w/v emulsion to an area between
the rear abdomen and the end of the breast and upper leg. Up to 29.5%
of the applied dose was detected on skin and feathers at the site of
application fourteen days after treatment and was still present (99%)
as permethrin (Leahey et al, 1977).
Total radioactive residues in liver, kidney, heart and muscle were
low-less than 0.02 mg permethrin equivalents/kg one, seven and
fourteen days after treatment, except in muscle below the site of
application which contained up to 0.11 mg permethrin equivalents/kg.
Up to 0.07 mg permethrin equivalents was found in fat at the
application site, 70-88% of the radioactivity in subcutaneous muscle
and fat at the site of application was characterised as permethrin
itself (Leahey et al, 1977c).
Radioactive residues in eggs were all below 0.03 mg permethrin
equivalents/kg, reaching a peak 3-6 days after dosing and then
declining progressively to below 0.01 mg/kg ten days after dosing
(Leahey, et al, 1977c).
When eggshells were painted with a 0.1% w/v emulsion of 40:60
cis:trans 14C-labelled permethrin (combined cyclopropyl- and
methylene-labelling), no radioactivity was detected in either yolks or
albumen one, seven or fourteen days after treatment (limit of
determination: 0.02 mg permethrin equivalents/kg) (Leahey et al,
1977c).
On Processing
The fate of permethrin residues during the processing of cotton,
soybeans, apples, pears, grapes and tomatoes was reviewed at the 1979
Meeting. Residues in the seeds of cotton and beans of soya, and in
their respective processing fractions used in animal feeds, are small
(e.g. well below 0.5 mg/kg) (FAO/WHO, 1980).
Residues in dried apple pomace were 25-30 times the levels in
corresponding whole apples. No residues were determined in apple
juice and apple sauce (limit of determination: 0.01 mg/kg) (Table 19)
(Ussary, 1977a, c).
Residues in wet tomato pulp, which typically contains 25% dry matter,
were in the range 10-50 (mean 25) times the levels in whole tomatoes
(Table 20) (Ussary, 1977d).
During the processing of treated whole wheat grain the permethrin
residue is retained mainly in the bran component although a
significant proportion (12%) remains with the white flour and follows
through almost unchanged into bread (Simpson, 1979).
Permethrin residues in flour from treated whole grain are carried over
into bread baked from that flour. There is no reduction in residue
level on a commodity-weight basis. White flour retains about 12% of
the whole grain residue. The major part (about 62%) of the residue
remains with the bran while about 26% is in the pollard. Therefore,
whilst white bread prepared from treated grain would have a residue of
about 0.15-0.2 mg/kg, the corresponding level in wholemeal bread would
be about 0.7-1 mg/kg (Table 21) (Simpson, 1979).
TABLE 17. Total Radioactive Residues In The Tissues of Hens Receiving
Consecutive Daily Oral Doses Of 14C-Labelled Permethrin
Rate No. of Period Between Residue (mg permethrin equivalents/kg)
Material Administered (expressed as mg/kg Daily Last Dose And In
equivalent in diet) Doses Sacrifice Fat Muscle Liver Kidney
40:60 cis:trans-permethrin
(cyclopropyl-lablled) 10 10 4 hours 0.5-0.6 0.07-0.13 0.9-1.1 N/D.1
(methylene-labelled) 10 10 4 hours 0.3-0.7 0.03 0.4 N/D1
trans-permethrin
(carbonyl-labelled) 80 3 6 days 0.21 <0.06 0.14 0.31
(methylene-labelled) 80 3 6 days 0.18 <0.06 0.08 0.24
cis-permethrin
(carbonyl-labelled) 80 3 6 days 1.0 0.06 0.27 0.34
(methylene-labelled) 80 3 6 days 1.4 <0.06 0.20 0.25
1 N/D indicates not determined.
Gaughan et al, 1978b; Leahey et al, 1977b
TABLE 18. Residues Of Permethrin And Three Major Metabolites In Eggs And Tissues
Of Hens Receiving Permethrin In The Diet Up To 28 Days
Dietary Feeding Regime Product Residue (mg/kg) of
Inclusion Analysed
Level Cis & Trans
(mg/kg) Permethrin DCVA 3-PBAlc 3-PBAcid
(I and II) (III) (IV)
33 "Treated"1 Muscle 0.05-0.08 0.01-0.02 <0.01 <0.01
plus skin
Liver <0.01 <0.01-0.03 <0.01 <0.01
Eggs <0.6 <0.01-0.03 <0.01-0.03 <0.01
"Treated plus Muscle 0.02 <0.01 <0.01 <0.01
Recovery"2 plus skin
Liver <0.01 <0.01 <0.01 <0.02
Eggs <0.02 <0.01 <0.01-0.02 <0.01
3.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.08 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin <0.01 - - -
Liver <0.01 - - -
Eggs <0.03 - - -
0.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.05 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin
Liver <0.01 - - -
Eggs <0.01 - - -
1 "Treated" indicates the animals received treated diet for up to 28 days and
were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28
days and were then returned to untreated diet for up to 14 days before slaughter.
Edwards and Swaine, 1977;
Swaine et al, 1980b
TABLE 19. Permethrin Residues In Commercial Fractions Of Apples: USA: 1976
Volume of Number of Interval Fraction Permethrin Residues
Spray Applications Between Last (mg/kg) after Applying
Applied Spraying and at Stated Rates of mg/kg
(l/ha) Harvest ai in spray
80 mg/kg 160 mg/kg
3150-3600 3-5 38-63 days Mature 0.11-0.40 0.85
apples
Juice <0.01 <0.01
Apple <0.01 -
Sauce
wet 0.74-3.2 6.5
pomace
Dry 3.3-10 22
pomace
Ussary, 1977a, c
TABLE 20. A Comparison Of Permethrin Residues In Processed Tomato Fractions : USA : 1977
Rate of Number of Interval Permethrin Residues (mg/kg) In
Application Applications Between Last
(g ai/ha) Application Whole Tomato Tomato Tomato Tomato
And Harvest Tomatoes Juice Puree Ketchup Pulp
220 2 1 day 0.05 <0.01 0.01 0.01 1.08
3 days 0.08 0.01 0.02 0.01 0.86
7 days 0.04 0.01 0.03 0.02 1.97
440 2 1 day 0.05 <0.01 0.03 0.02 1.60
3 days 0.05 0.01 0.03 0.02 1.45
7 days 0.08 <0.01 0.02 0.01 1.28
Ussary, 1977d
TABLE 21. Distribution Of Permethrin Residues In Whole Wheat
Fractions And Consequent Residue Levels In Bread
Distribution of residue between whole grain fractions1
Permethrin Residue
Fraction % whole grain mg/kg % distribution
Flour (white) 75.1 0.13 12.1
Pollard 11.3 1.9 26.3
Bran 13.6 3.7 61.7
Residue in Bread baked from the ground flour
Commodity Permethrin residue (mg/kg)
White flour 0.13-0.15
White bread 0.13-0.19
Wholemeal flour2 0.81-0.93
Wholemeal bread 0.67-1.00
1 Grain sample 1.5 kg. It had a residue level of 0.65 mg
permathrin/kg after being stored for 9 months.
2 Reconstituted from the fractions in the original whole grain
proportions.
Simpson, 1979
The processing operations simulated those used in commercial practice,
the unbaked bread recipe included potassium bromate and benzoyl
peroxide.
Wholemeal flour was formulated after reconstituting the wheat
fractions in the original ratio.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis (by gas chromatography using
an electron capture detector) for crops treated pre-harvest was
reviewed at the 1979 Meeting (FAO/WHO, 1980).
Both gas chromatography (GC) and high pressure liquid chromatography
(HPLC) have been used to determine permethrin residues in whole cereal
grains and commodities processed from grain (including bread).
The GC procedure described earlier is quite suitable, being able to
determine cis and trans isomers separately or together. Recovery
is essentially quantitative and the limit of determination is about
0.01 mg/kg.
A simple procedure based on a high pressure liquid chromatograph has
also been developed (Simpson, 1978). The limit of determination is a
little better than 0.01 mg/kg. In this procedure the residue is
extracted by cold methanol, partitioned into hexane - and, if
necessary, further cleaned by an acetonitrile partitioning - before
clean-up on an alumina column. The concentrated residue is subjected
to determination by HPLC equipped with a UV detector. This method is
similar to the procedure used by Mourot et al, (1979).
With small modifications, the GC method can be used to determine
permethrin in milk, meat and eggs.
Milk and meat samples are extracted with n-hexane:acetone 4:1. The
acetone is removed by washing with water and the permethrin
partitioned from n-hexane into dimethylformamide. The
dimethylformamide extract is dissolved in 1% aqueous sodium sulphate
and the permethrin back-extracted into n-hexane. The hexane extract
is cleaned up using a Florisil column, and permethrin is determined by
gas chromatography using an electron capture detector. The limit of
determination of the method is 0.01 mg/kg for the combined isomers and
recovery values for samples of meat and milk fortified at 0.01-1.0
mg/kg are normally greater than 70%. Mean recoveries of 89%-92% have
been obtained from milk and 86-88% from tissues of cows and hens
(Edwards and Iswaren, 1977; Edwards and Swaine, 1977). Twenty percent
acetone in hexane has been shown to be an efficient solvent for
extracting permethrin residues from animal tissues (Edwards and
Sapiets, 1978).
The method for permethrin analyses in meat and milk is also applicable
to eggs. These are extracted with n-hexane:acetone 1:1 and the
extract washed with aqueous sodium chloride to remove acetone, and
cleaned up by solvent partition with dimethylformamide and by using a
Florisil column. The limit of determination is 0.02 mg/kg for the
combined isomers and recovery values from yolks and albumen fortified
at 0.01-0.4 mg/kg are generally in the ranges 70-90% and 60-90%
respectively (Edwards and Swaine, 1977).
The mass spectrographic technique of multiple ion detection is
suitable for the qualitative and quantitative confirmation of
permethrin residues in crops, milk, eggs and animal tissues following
separation by gas chromatography.
Qualitative confirmation of permethrin residues is given by the
appearance of a peak at the correct retention time for all the
specific m/e values monitored. In addition, the ratios between the
peak heights (or peak areas) given from each m/e value should be
identical to that observed for permethrin analytical standards.
Quantitative confirmation is carried out by comparison of the peak
height or peak area, measured for the most abundant m/e value
recorded, against those obtained with external standards of permethrin
(Swaine and Edwards, 1977).
Residue of both the free and conjugated major plant metabolites of
permethrin, namely cis- and trans- isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
and 3-phenoxybenzyl alcohol (3-PBAlc), can also be determined by gas
chromatography. Samples are extracted with 2:1 methanol:water and
lipids removed by partitioning with dichloromethane. The methanol is
then removed using rotary evaporation, the aqueous solution made 1 N
with HCl and refluxed to free the conjugated residues. The residues
are then extracted by partitioning with n-hexane. The n-hexane is
then removed and the 2,2,2-trichloroethyl ester of DCVA and the
heptafluorobutyryl ester of 3-PBAlc are simultaneously made. The
derivatives are then analyzed by gas-liquid chromatography using
electron capture detection. (Ussary, 1979). Limits of determination
are in the range 0.02-0.10 mg/kg for DCVA and 0.02-0.05 mg/kg for 3-
PBAlc. Recoveries are generally in the range 70-85%. Confirmation of
the metabolite residues is possible using gas chromatography linked to
mass spectrometry using multiple ion detection, similar to the
procedure reviewed above for permethrin (Swaine et al, 1978).
With little modification, this method has also been used to determine
permethrin metabolite residues in meat and milk. Meat tissues are
extracted with 1:1 methanol:water; milk is extracted with 1:1
hexane:acetone. Any permethrin extracted by this procedure is
partitioned out, along with unconjugated residues of 3-PBAlc, into
n-hexane alone (milk) or hexane plus dichloromethane (meat).
Residues of 3-phenoxybenzoic acid (3-PBAcid), both "free" and
conjugated DCVA, and conjugated residues of 3-PBAlc remain in the
aqueous phase which is subjected to a hydrochloric acid hydrolysis to
cleave the conjugates. DCVA and 3-PBAcid are separated from 3-PBAlc
by partition between aqueous sodium hydroxide and dichloromethane.
The PBAlc residues are combined and determined as the
heptafluorobutyryl ester by gas chromatography using an electron
capture detector. DCVA and 3-PBAcid are converted to their methyl
ester derivatives, by treatment with diazomethane, and determined by
gas chromatography linked to mass spectrometry using multiple ion
detection. A limit of determination of 0.01 n/kg has been obtained
routinely for DCVA, 3-PBAlc and 3-PBAcid in meat and milk. Mean
recoveries have been within the range 55-60% from milk and 60-85% from
meat tissues (Swaine et al, 1980).
NATIONAL TOLERANCES
The following national tolerances were reported to the Meeting for
permethrin (total isomers):
Australia Wheat Bran 10 mg/kg
Raw cereals ) 5 mg/kg
Wheat pollard (shorts) )
Brussels sprouts )
Wheat flour(wholemeal) ) 2 mg/kg
Wholemeal bread )
Broccoli )
Cabbage ) 1 mg/kg
Cauliflower )
Wheat flour (white) ) 0.5 mg/kg
White bread )
Tomatoes 0.4 mg/kg
Cotton ) 0.2 mg/kg
Sweetcorn (cobs) )
Eggs ) 0.1 mg/kg
Fat of meat of cattle, )
sheep, pigs and poultry )
Milk and milk products ) 0.05 mg/kg
(fat basis) )
Belgium Eggplants )
Cabbages )
Cucumbers )
Endives )
Fruit crops ) 1 mg/kg
Honeydew melons )
Lettuce )
Peppers )
Tomatoes )
France Maize (whole plants) 0.2 mg/kg
Maize grains 0.1 mg/kg
Holland Endives ) 2 mg/kg
Lettuce )
Apples )
Brassicae ) 1 mg/kg
Pears )
Eggplants )
Cucumbers )
Honeydew melons ) 0.5 mg/kg
Peppers )
Tomatoes )
USA Cotton 0.5 mg/kg
Eggs )
Meat, fat and meat )
by-products of cows, ) 0.05 mg/kg
goats, hogs, horses, )
poultry and sheep )
Venezuela Vegetables ) 10 mg/kg
EVALUATION
APPRAISAL
Permethrin is a pyrethroid insecticide which is effective at low rates
against a wide range of Lepidoptera, Hemiptera, Diptera and
Coleoptera. Unlike natural pyrethrins and earlier synthetic
pyrethrins, permethrin is photostable to a degree that allows it to be
used as an effective tool in agriculture. It shows adulticidal,
ovicidal and particularly larvicidal activity and is effective against
the great majority of insects that. have become resistant to standard
treatments such an organochlorines and organophosphates. A programme
of sprays is usually required.
A particularly valuable usage is in protection of stored grain.
Laboratory and silo-scale studies in Australia have shown that low
levels of permethrin control most of the endemic insect pests of
stored grain, and, when used in conjuction with a synergist and
complementary insecticide, the commonly-occurring strains of all grain
insect pests are controlled. Adequate protection of at least 9 months
storage is achieved by a single application of 1 mg permethrin/kg.
However, for various reasons, it may become necessary in future to use
permethrin without its synergist and/or o/p insecticide. In that
case, higher application rates would be required to maintain the
present level or permethrin efficacy. The same applies if the present
experimental rate is increased for other reasons (e.g. to counter
resistance). A 2 mg/kg grain is likely to be the target concentration
in the near future.
Permethrin can also be used to control various ectoparasites of
cattle, horses, sheep, pigs and poultry. It can be applied either
directly to the animals, or to buildings in which they are housed, or
to insect breeding and resting sites.
Extensive residues data are available from supervised trials. In
pre-harvest uses, residues are highest where a crop part is exposed
directly to the spray, as in the case of many forage crop uses.
Ground and aerial applications yield similar residue levels. Although
residues decline comparatively slowly after spraying, there is no
obvious build-up of residues of permethrin or of its two most
important plant metabolites on repeated application, within the rates
and frequency of permethrin spraying that are needed to obtain good
insecticidal control. The two important plant metabolites are
3-phenoxybenzyl alcohol and the cis and trans isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA),
which are also animal metabolites; levels of these metabolites in
crops are very much smaller than corresponding permethrin residues and
they do not need to be included in routine residue analyses.
Permethrin levels on stored grains also decline slowly. During the
processing of treated whole wheat grain, the permethrin residue is
retained mainly on the bran component, although a significant
proportion (12%) remains with the white flour and follows through
After both oral and dermal administration to livestock, permethrin
itself constitutes the major portion of the residue recovered from
milk, eggs, muscle and fat. After oral administration to goats,
metabolites form the major part of the residue in kidney and liver;
DCVA occurs in both organs, 3-phenoxybenzyl alcohol plus its
4'-hydroxy derivative in liver and 3-phenoxybenzoic acid plus its
4'-hydroxy derivative in liver and kidney.
Following direct application to dairy cattle (1.0 g ai/cow and with
free access to a self-oiler) permethrin levels are low in milk (<0.02
mg/kg) and in muscle, liver and kidney (<0.01 mg/kg). Highest levels
(up to 0.1 mg/kg) have been found in fat.
Cows maintained on a diet containing 50 mg/kg of permethrin yielded
milk containing low levels of residue (0.1 mg/kg), and the level in
muscle was less than 0.1 mg/kg. The residue in milk declined to below
0.01 mg/kg after five days on returning the cows to control diet.
Residues in eggs are also low and contamination of eggs laid during
the treatment of poultry houses will not result in detectable residues
in yolks or albumen.
Permethrin is not detected in the albumen of eggs from hens receiving
the compound at levels up to 33 mg/kg in the diet; levels in yolks are
approximately 2% of corresponding permethrin dietary levels.
In all cases studied, residues of permethrin and its metabolites in
products of animal origin declined notably on cessation of exposure.
Only small residue levels have been found in products of animal origin
following direct application of permethrin to livestock. In view of
the levels of permethrin found in forage crops, such as alfalfa, and
the importance of such crops in animal feedstuffs, it seems likely
that residue levels in products of animal origin will be higher
following forage crop uses rather than from ectoparasite outlets. In
estimating maximum residue levels for products of animals origin,
allowance has also to be made for the dilution effect that will occur
in practice when treated forage is incorporated into livestock diets.
In proposing maximum residue levels on raw grain and milled cereal
products prepared therefrom, careful consideration has been given to
the fact that this insecticide is to be used as a grain protectant and
that residues of the compound are comparatively stable under storage
conditions. Under practical conditions of grain handling and storage,
it is not possible to apply a grain protectant with assurance that the
deposit will be absolutely uniform; there will always be a natural
variation in the level of the deposit resulting from fluctuations in
the flow of grain and of the insecticide. Furthermore, the movement
of bulk grain results in the segregation of the various components of
the bulk with a resultant variation in the level of residues
throughout the mass. Due allowance has been for the amplitude of
these variations and the problems of sampling and analysis.
The meeting examined residue data from supervised storage and feeding
trials reflecting established or proposed good practice. From these
data the meeting was able to estimate the maximum residue levels that
were likely to occur when permethrin was used in practice.
The preferred method of permethrin residue analysis in crops is by gas
chromatography using an electron capture detector. Recoveries are
essentially quantitative and the method has been applied successfully
to a wide range of crops, raw cereal grains and processed products
derived from them, such as flour, bran and bread. A limit of
determination of 0.01 mg/kg (expressed as permethrin cis and trans
isomers). Cis and trans-isomers are capable of being determined
separately by this method. With small modifications, the method has
been applied successfully to the determination of permethrin residues
in meat, milk and eggs, and would with adaptation be suitable for
regulatory purposes.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the residue levels listed below are
temporary pending submission of the "Further Work or Information".
These are recommended as suitable for establishing temporary maximum
residue limits. These levels are additional to those proposed by the
1979 Meeting. These levels refer to the sum of the cis and
trans-isomers of permethrin.
Crop TMRL (mg/kg)
Alfalfa fodder 100) On a dry weight basis
Maize fodder and straw 100)
Apple pomace 50) On a dry weight basis
Soybean fodder 50) On a dry weight basis
Cereal grains 2
Wheat flour (wholemeal) 2
wheat flour (white) 0.5
Wheat bran 10
Carcass meat of
cattle ) For permethrins a portion of
pig ) 1 carcass fat is analyzed and
sheep ) the levels refer to carcass fat.
Meat by-products from:
pigs ) 0.1
sheep )
Poultry meats 0.1
Eggs 0.1
Milk 0.1
FURTHER WORK OR INFORMATION
Recommendations 3, 4, 8, 9 from 1979 meeting remain relevant.
REFERENCES
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Pilot Usage Trials, 1978-79 Working Party On Grain
Protectants", Department of Primary Industries, Queensland, Australia
(unpublished) (1979a).
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Silo Scale Experiments 1977-78 Working Party On
Grain Protectants", Department of Primary Industries, Queensland,
Australia (unpublished) (1979b).
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional Grain Protectants For The Control of
Malathion-Resistant Insects In Stored Sorghum", Department Of Primary
Industries, Entomology Branch, Queensland, Australia (unpublished)
(1979c).
Bewick, D.W. and Leahey, J.P. "Permethrin : Absorption in Cows", ICI
Plant Protection Division Report No. TMJ1357B (unpublished) (1976).
Bewick, D.W., Leahey, J.P. and Saunders, R. "Permethrin Absorption In
Pigs After Dermal Treatment", ICI Plant Protection Division Report No.
TMJ1562A. (unpublished) (1977).
Chipman Inc. Canada "Summary of 1976/77 Permethrin Sweet Corn Residue
Data . (unpublished) (1978).
Desmarchelier, J.M., Bengston, M., Davies, R.A.H., Elder, W.B.,
Hamilton, D., Henning, R., Murray, W., Ridley, E.G., Ripp, E.,
Sieraikowski, C.J., Sticka, R., Snelson, J.T., Thomas, D., Wallbank,
B. and Wilson, A. "Pilot Usage Of The Grain Protectants Fenitrothion
Plus Phenothrin and Pirimiphos-methyl Plus Permethrin", Australian
Wheat Board Working Party on Grain Protectants (in manuscript for
publishing) (1979).
Edwards, M. J. and Iswaren, T.J. "Permethrin: Residue Transfer And
Toxicology Study With Cows Fed Treated Grass Nuts" ICI Plant
Protection Division Report No. TMJ1519/B (unpublished) (1977).
Edwards, M.J. and Swaine, H. "Permethrin: Incorporation of Permethrin
In The diet of Laying Hens: Residues In Eggs And Tissues". ICI Plant
Protection Division Report No. TMJ1520/B (unpublished) (1977).
Edwards, M.J. and Sapiets, A. "Permethrin: The Extraction of the
Pesticide From Animal Tissues". ICI Plant Protection Division Report
No. RJ0009B (unpublished) (1978).
Gaughan, L.C., Unai, T. and Casida, J.E. "Permethrin Metabolism In
Rats And Cows, And In Bean and Cotton Plant", Paper delivered at 172nd
ACS National Meeting, San Francisco (August 1976).
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. "Distribution
And Metabolism Of Trans- And Cis-Permethrin In Lactating Jersey
Cows", J. Agric. Food Chem. 26(3), 613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. "Distribution And
Metabolic Fate of Trans- And Cis- Permethrin In Laying Hens", J.
Agric. Food Chem. 26(6), 1374-1380.
Hunt, L.M. and Gilbert, B.N. "Distribution And Excretion Rates Of
14C-Labelled Permethrin Isomers Administered Orally To Four Lactating
Goats For 10 Days", J. Agric, Food Chem. 25(3), 673.
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
A.G. "Permethrin: Metabolism And Residues In Goats", ICI Plant
Protection Division Report No. TMJ1516B (unpublished) (1977a).
Leahey J.P., Gatehouse, D.M. and Parr, J.S. "Permethrin: Metabolism In
Hens", ICI Plant Protection Division Report No. TMJ1509B (unpublished)
(1977b).
Leahey, J.P., Bewick, D.W., Gatehouse, D.M. and Carpenter, P.K.
"Permethrin: Absorption In Chickens After Dermal And Oral Treatments",
ICI Plant Protection Division Report No. TMJ1481B (unpublished)
(1977c).
Leahey, J.P. and Cameron, A.G. "Permethrin: Residues In Cows After
Dermal Application", ICI Plant Protection Division Report No. RJ0001B
(unpublished) (1978).
Mourot, D., Delephine. B., Boisseau, J. and Gayot, G. "HPLC Of A New
Pyrethroid Insecticide Sumicidin", J. Chromatog. 168, 277-279.
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1978).
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1979).
Swaine, H. and Edwards, M.J. "Confirmation Of Residues Of Permethrin
(PP557) Using Gas Chromatography - Mass Spectrometry In The Multiple
Ion Detection Mode", ICI Plant Protection Division Data (unpublished)
(1977).
Swaine, H., Edwards, M.J. and Ussary, J.P. "Permethin Crop Rotation
Study", ICI Americas Inc Report No. TMU0378/B (unpublished) (1978).
Swaine, H. and Sapiets, A. "Permethrin Residues Summary: Residues In
Crop Samples Analyzed During 1977-8: Part IV: Leaf, Root and Forage
Crops", ICI Plant Protection Division Report No. RJ0081A (unpublished)
(1979).
Swaine, H., Francis, P.D., Rippington, D. and Burke, S.R. "Permethrin
Residue Levels of the Major Metabolites of the Insecticide In the Milk
And Tissues of Cows Fed On A Treated Diet", ICI Plant Protection
Division Report No. RJ0124B (unpublished) (1980a).
Swaine, H., Francis, P.D., Rippington, D., Burke, S.R., Robertson, S.
and Ford, J.P. "Permethrin Metabolites: Eggs And Tissues Of Hens", ICI
Plant Protection Division Residue Data Report No. RD/557/27
(unpublished) (1980b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions of Apples, Wenatchee, Washington, 1976" ICI Americas Inc.
Report No. TMU0306/B (unpublished) (1977a).
Ussary, J.P. "Permethrin Residues In Soybeans", ICI Americas Inc.
Report No. TMU0383/B, (unpublished) (1977b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions Of Apples, Geneva, New York, 1976", ICI Americas Inc. Report
No. TMU0307/B (unpublished) (1977c).
Ussary. J.P. "Permethrin Residues In Tomato Process Fractions", ICI
Americas Inc. Report No. TMU0304/B (unpublished) (1977d).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0388/B (unpublished) (1978a).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0413/B (unpublished) (1978b).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0438/B (unpublished) (1979a).
Ussary, J.P. "Permethrin Residues On Field Corn", ICI Americas Inc.
Report No. TMU0433/B (unpublished) (1979b).
Ussary, J.P. "Permethrin Metabolite Residues On Broccoli", ICI
Americas Inc. Report No. TMU0427/B (unpublished) (1979c).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0461/B (unpublished) (1979d).
Ussary, J.P. and Braithwaite, G.B. "Residues of Permethrin And
Permethrin metabolites in Milk From Ectiban Treated Cows (Trial No.
35NC79-001)", ICI Americas Inc. Report No. TMU0490/B (unpublished)
(1980a).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin and
3-Phenoxybenzyl Alcohol In Cow Tissues (Trial No. 35NC79-001)", ICI
Americas Inc. Report No. TMU0493/B (unpublished) (1980b).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And 3-
Phenoxybenzyl Alcohol In Tissues From Ectiban Treated Swine (Trial No.
35NC79-002)", ICI Americas Inc. Report No. TMU0491/B (unpublished)
(1980c).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And
3-Phenoxybenzyl Alcohol In Tissues And Eggs From Ectiban Treated
Chickens (Trial No. 35NC79-003)", ICI Americas Inc. Report No.
TMU0492/B (unpublished) (1980d).
Cows and goats
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bodyweight, equivalent to approximately 80 mg/kg in the
diet, levels of radioactivity in milk reached a maximum of 0.13 mg
permethrin equivalents/kg after 1-2 days. These declined to less than
0.02 mg/kg after seven days. Levels of radioactivity in the fat were
0.12-0.18 mg permethrin equivalents/kg, after seven days. By fourteen
days these had declined to 0.05-0.06 mg/kg, indicating that the small
residues in fat are also not maintained on cessation of dosing (Bewick
and Leahey, 1976).
Hunt and Gilbert dosed goats orally with either the cis- or
trans-isomers of 14C-labelled permethrin (carbonyl- or
methylene-labelled) at a rate equivalent to approximately 6 mg/kg in
the diet for ten days. Total radioactive residues in the milk reached
a plateau after three days of 0.02-0.05 and <0.01-0.01 mg permethrin
equivalents/kg respectively for the cis- and trans- isomers. The
goats were sacrificed 24 hours after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 12.
Total radioactivity in the fat of animals receiving the cis-isomer
was ten times higher than in those receiving the more readily
hydrolysed trans-isomer (Table 12) (Hunt and Gilbert, 1977).
When cis- and trans-isomers of 14C-labelled permethrin (carbonyl-
and methylene-labelled) were administered orally to lactating Jersey
cows for three consecutive days at approximately 1 mg/kg bodyweight,
radioactivity was largely eliminated from the body in faeces and urine
within 12 or 13 days after the initial treatment. Total
14C-permethrin equivalents in milk were consistently below 0.3 mg/kg
and declined on cessation of exposure. Residues in fat were also low.
Residues recovered from fat and milk were higher when cis-
permethrin rather than trans-permathrin was administered and
consisted almost entirely (>85%) of unmetabolised permethrin.
Total 14C-permethrin equivalents in blood reached a transient peak
shortly after each dose and dropped to insignificant levels within 2-4
days after the last dose (Gaughan et al, 1976, 1978a).
A study with non-radiolabelled permethrin supported the finding that
permethrin itself is the predominant residue in milk. It was also the
major residue in cow muscle. In this study, groups each of three
barren Friesian cows, yielding 9-13 litres of milk per day, were
maintained on diets containing permethrin at approximately 0.2, 1.0,
10 or 50 mg/kg. The permethrin was absorbed on grass nuts. After
28-31 days two cows in each group were sacrificed. The third was
returned to control diet for nine days before sacrifice. Samples of
milk and of meat tissues were analyzed for residues of permethrin,
cis and trans 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II, DCVA) taken together,
3-phenoxybenzylalcohol (III; 3-PBAlc) and 3-phenoxybenzoic acid (IV;
3-PBAcid) (Figure 1) by the gas chromatographic methods reviewed under
"Methods of Residue Analysis".
TABLE 12. Total Radio-labelled Residues In Tissues of Goats Receiving 14C-Labelled Permethrin
Daily Orally For 7-10 Days
Material Dose Level Duration of Period Between Total Residue (mg permethrin
Administered (= mg/kg Administration Last Date And equivalents/kg In
in diet) (days) Sacrifice Fat Muscle Liver Kidney
Permethrin, ‰10 7 4 hours <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis-trans
Permethrin, ‰6 10 24 hours 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin, ‰6 10 24 hours 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
Hunt and Gilbert, 1977
Leahey et al, 1977a
TABLE 13. Characterisation of 14C-Labelled Residues In Liver And Kidney Of Goats
Receiving Permethrin Orally Daily For Seven Days
Radioactivity Soluble in Water
% Of % of Radioactivity % of Radioactivity
Organ Position of Radioactivity Soluble in Diethyl Remaining Water Unextracted
14C-Labelling Soluble in Ether Following Soluble After
Of Permethrin Diethyl Ether Hydrolysis Hydrolyais
Liver Methylene ("Alcohol") 16 41 23 note 1
Cyclopropyl ("Acid") 39 24 36 note 1
Kidney Methylene ("Alcohol") 43 42 <1 6
Cyclopropyl ("Acid") 29 20 22 9
1 liver was almost completely solubilised during refluxing.
Leahey et al, 1977a
Only permethrin itself was detected in milk. Low levels of 0.01-0.06
mg/kg (mean 0.02 mg/kg) and 0.03-0.2 (mean 0.1 mg/kg) were present in
the milk of cows in the 10 and 50 mg/kg groups respectively. These
values are approximately 0.2% of the corresponding dietary levels.
Permethrin residues in milk were less than 0.01 mg/kg at the 0.2 and
1.0 mg/kg dietary inclusion rates. None of the three metabolites was
found in any of the milk samples analyzed (limit of determination:
0.01 mg/kg). Permethrin levels in milk did not accumulate over the
period of the study and they declined rapidly, to below 0.01 mg/kg
after five days, on returning the animals to control diet (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
Permethrin itself was also the major part of the small residue levels
found in adductor, pectoral and cardiac muscle. These small levels
were approximately 0.1% of the corresponding dietary inclusion levels
(Table 14). Permethrin residue levels in peritoneal fat were
invariably higher than in subcutaneous fat (Table 15) (Edwards and
Iswaren, 1977; Swaine et al, 1980a).
As found separately by Leahey et al (below), metabolites rather than
permethrin itself constituted the major part of the residues in liver
and kidney. Again, the levels declined rapidly on cessation of
exposure (Table 14) (Edwards and Iswaren, 1977; Swaine et al,
1980a).
Leahey et al dosed goats orally with 40:60 cis:trans 14C-
labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 mg/kg in the diet for seven days.
Total radioactive residues in the milk reached a plateau of 0.02-0.03
mg permethrin equivalents/kg after five days; 30-50% of this
radioactivity was associated with the butterfat fraction of the milk,
in which total radioactive residues were 0.13-0.27 mg permethrin
equivalents/kg.
The animals were sacrificed four hours after receiving the final dose,
when levels of radiocarbon in meat tissues were as shown in Table 12.
Characterization of the residues in liver and kidney, the two tissues
containing the highest total residues is shown in Table 13. Where
"alcohol"-labelled permethrin was used, approximately 70% of the 14C
in kidney tissue was 3-phenoxybenzoic acid (IV) plus
3-(4'-hydroxyphenoxy) benzoic acid (VI) (Figure 1). Approximately 30%
of the 14C in the liver was due to 3-phenoxybenzyl alcohol (III) plus
3-(4'-hydroxyphenoxy) benzyl alcohol (V). A further 15% was due to
3-phenoxybenzoic acid (IV) plus 3-(4'-hydroxyphenoxy) benzoic acid
VI). Where "acid"-labelled permethrin was used, approximately 10-15%
of the label in liver and kidney was due to the cis and trans
3-(2,2-dichlorovinyl) cyclopropane carboxylic acids (I and II)
(principally the trans isomer). (Leahey et al, 1977a).
TABLE 14. Residues Of Permethrin and Three Major Metabolites in Tissues Of Cows
Receiving Permethrin In The Diet
Nominal Residue (mg/kg) Of
Dietary Feeding Regime Tissue
Inclusion Analysed Cis & Trans
Level Permethrin DCVA 3-PBAlc 3-PBAcid
(mg/kg) (I and II) (III) (IV)
50 "Treated"1 Liver <0.01 0.01-0.04 0.05-0.10 0.03-0.12
Kidney 0.02-0.06 0.07-0.09 0.01-0.09 0.03-0.04
Muscle O.03-0.10 <0.01-0.02 <0.131 <0.01
"Treated plus Liver <0.01 0.01 <0.01 <0.01
Recovery"2 Kidney 0.02 0.06 <0.01 <0.01
Muscle 0.02-0.06 <0.01 <0.01 <0.01
10 "Treated" Liver <0.01 <0.01 0.01-0.03 0.01-0.02
Kidney <0.01-0.01 <0.01-0.02 <0.01-0.01 <0.01-0.02
Muscle <0.01-0.03 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery Kidney 0.01 <0.01 <0.01 0.04
Muscle 0.01-0.02 <0.01 <0.01 <0.01
1.0 "Treated" Liver <0.01 <0.01 <0.01 <0.01
Kidney <0.01 <0.01 <0.01 <0.01-0.01
Muscle <0.01 <0.01 <0.01 <0.01
"Treated plus Liver <0.01 <0.01 <0.01 <0.01
Recovery" Kidney 0.01 <0.01 <0.01 0.02
Muscle <0.01 <0.01 <0.01 <0.01
0.2 "Treated" Liver <0.01 - - -
Kidney <0.01 - - -
Muscle <0.01 - - -
"Treated plus Liver <0.01 - - -
Recovery" Kidney 0.01 - - -
Muscle <0.01 - - -
1 "Treated" indicates the animals received treated diet for 28-31 days and were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28 days and were then
returned to untreated diet for a further 9 days before slaughter.
Edwards and Iswaren, 1977; Swaine et al, 1980a
TABLE 15. Residues In Fat Of Cows Receiving Permethrin In The Diet
Nominal Residues (mg/kg) of Permethrin In
Dietary Inclusion
Level (mg/kg) Peritoneal Fat Subcutaneous Fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
(Edwards and Iswaren, 1977)
TABLE 16. Total Radiolabelled Residues In Tissues Of Cows Seven Days
After Receiving A Single Dermal Dose Of 1.25 g of 40:60 Cis:Trans
14C-Labelled Permethrin
Tissue Residue in tissue Residue in tissue
from cow treated from cow treated
with alcohol-labelled with acid-labelled
permethrin, mg/kg1 permethrin µg/kg1
Meat <0.01 <0.01
Subcutaneous fat 0.053 0.033
Perirenal fat 0.046 0.054
Liver 0.080 0.064
Kidney 0.048 0.038
1 Residues expressed as mg/kg permethin equivalents. Bewick et al,
1977.
Permethrin itself also constitutes the major part of the small
residues found in the milk and fat of cows after dermal application.
In one study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). Although the concentration of suspension used
(0.5% w/v) was ten times the maximum that is likely to be used in
practice, the total dose of 40 mg per cow was approximately 1/30 of
the amount that could be applied commercially at any one time. The
dose was placed on an area of 225 cm2.
Levels of radioactivity in the milk and blood reached a maximum during
the first few days after dosing and then declined steadily. Total
residues in milk were always less than 2 µg permethrin equivalents/kg
(i.e. less than 2 ppb) and were below the limit of determination (0.3
µg/kg) by the end of fourteen days following treatment.
Total radiolabelled residues in muscle, liver, kidney and heart were
also small - 0.02 mg permethrin equivalents/kg or below seven days
after treatment - and were considerably smaller after a further seven
days.
Highest residues were found in fat from below the treatment area.
Seven days after treatment the total residue was approximately 0.5 mg
permethrin equivalents/kg, of which at least 80% was due to permethrin
itself. After a further seven days, residues in fat below the site of
application had fallen by about one half; the residue remaining was
again characterised as being due mainly to permethrin itself (Bewick
and Leahey, 1976).
In a second study, cows received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 1.25 g per cow applied in a 0.5% w/v
emulsion is at the higher end of the range likely to be used
commercially. The permethrin was brushed onto 25% of the surface area
of the animals.
Levels of radioactivity found in milk were very similar for the two
positions of radiolabelling. Residues reached a peak of approximately
0.01 mg permethrin equivalents/kg after 3-5 days; 89-91% of these
residues could be extracted into butter fat in which the major portion
of the residue (at least 51-68%) was characterised as permethrin
(Leahey and Cameron, 1978).
Seven days after treatment only small residues were present in the
animal tissues. Levels of radioactivity were similar in perirenal and
subcutaneous fat and for the two positions of radiolabelling (Table
16). At least 40% of this residue was characterised as permethrin
(Leahey and Cameron, 1978).
Pigs
Pigs were dosed dermally with 40:60 cis:trans 14C-labelled
permethrin (cyclopropyl or methylene-labelled). Although the
concentration of suspension used (0.5% w/v) was ten times the maximum
that is likely to be used in practice, the total dose of 18 mg per pig
is approximately 1/30 of the amount that could be applied commercially
at any one time. The dose was placed on an area of 100 cm2.
Seven and fourteen days after treatment approximately 1% of the
applied dose remained at the site of application, principally (>95%)
as permethrin. A residue of 0.05 mg permethrin equivalents/kg in fat
immediately below the site of application seven days after treatment
was due essentially to permethrin. After fourteen days, no
radioactive residue was found (limit of determination: 0.012 mg
permethrin equivalents/kg) (Bewick et al, 1977).
A residue of 0.01 mg permethrin equivalents/kg was found in muscle
beneath the site of application seven days after treatment.
Otherwise, no residues were detected in muscle, liver, kidney, heart
or blood either seven or fourteen days after treatment (limit of
determination: approximately 0.001 mg permethrin equivalents/kg)
(Bewick et al, 1977).
Hens
In one study hens received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (cyclopropyl- and methylene-labelled) at a
rate equivalent to 30 mg/kg in the diet. Birds were sacrificed one,
seven and fourteen days after treatment.
Throughout the study, residues in breast muscle, leg muscle and heart
were small (less than 0.04 mg permethrin equivalent/kg). Total
radioactive residues in liver and kidney of 0.22-0.25 mg/kg had
decreased by an order of magnitude after seven days. Residues of
0.20-0.35 mg permethrin equivalents/kg in peritoneal and subcutaneous
fat on the day after treatment also declined during the remainder of
the study, albeit more slowly than in the case of liver and kidney
(Leahey et al, 1977c).
Levels of radioactivity in eggs reached a maximum soon after dosing
(in 1-4 days) and then declined during the remainder of the 14-day
study (Leahey et al, 1977c).
Hens have also been dosed orally with 40:60 cis:trans
14C-permethrin (cyclopropyl- or methylene-labelled) for ten days at a
rate equivalent to approximately 10 mg/kg in the diet or separately
with cis- and trans-isomers (carbonyl- or methylene-labelled) for
three days at a rate equivalent to approximately 80 mg/kg in the diet.
Residues in eggs were present primarily (>75%) in the yolks, in which
radioactivity reached a plateau after 5-8 days of 0.3-0.5 mg
permethrin equivalents/kg in the 10-dose study and 0.6 mg/kg (trans-
isomer administered) or 2.1-2.8 mg/kg (cis-isomer administered) in
the 3-dose study. Permethrin was the major compound identified in the
eggs (52-62%). The cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acids (I and II) and 3-phenoxybenzyl alcohol (III) (Figure 1) were the
major metabolites in eggs, each normally accounting for less than
approximately 10% of the total radioactivity. The carboxylic acids
were present both free and as the glucuronide and taurine conjugates.
Other metabolites arose from hydroxylation in the 4'-position of the
"alcohol" moiety and in the trans-2-methyl moiety in the "acid" part
of the molecule.
The hens were sacrificed four hours after receiving the final dose in
the 10-dose study, when residues were as shown in Table 17. As in the
case of eggs, residues in fat derived from both "acid" and "alcohol"
labels were similar. Permethrin itself represented the major residue
in the fat. Compounds I-III and 3-phenoxybenzoic acid (IV) were also
identified (each less than 10% of the total radioactivity in the fat).
In both muscle and liver, higher residues were detected in hens dosed
with "acid"-labelled permethrin than with "alcohol"-labelled. The
cis- and trans-3-2,2-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acids (I and II) were the major residues identified in
these tissues. Blood levels declined rapidly during the first 24
hours after administration (Gaughan et al, 1978b; Leahey et al,
1977b).
In a study with non-radiolabelled 40:60 cis:trans permethrin,
groups of 40 laying hens were fed on diets containing approximately
0.4, 3.4 and 33 mg/kg for 28 days and then returned to a control diet
for an additional 14 days. Samples of eggs laid during the study were
analyzed for permethrin residues by the gas chromatographic method
described under "Methods of Residue Analysis". Five hens per group
were sacrificed after 21, 28, 35 and 42 days of the study and tissues
analyzed for permethrin.
At the 0.4 mg/kg dietary inclusion rate no residues of permethrin were
detected on the albumen and yolks of eggs (limit of determination:
0.02 mg/kg) or in the muscle, skin and liver (limit of determination:
0.01 mg/kg). At the higher dietary inclusion rates no permethrin was
detected in egg albumen. In yolks, permethrin residues were up to
0.05 mg/kg and up to 0.64 mg/kg respectively at the 3.4 and 33 mg/kg
treatment levels. Residues did not accumulate and declined rapidly
when feeding finished, reaching non-detectable levels (less than 0.02
mg/kg) before the end of the 14-day recovery period in both cases.
Only small residues (< 0.03 mg/kg) of the metabolites I-IV were found
in eggs during the feeding period. Again these declined on cessation
of exposure. At the 3.4 mg/kg dietary inclusion rate, permethrin
residues in muscle, skin and liver were non-detectable; i.e. less than
0.01 mg/kg. At the 33 mg/kg rate permethrin residues in liver were
also non-detectable. Permethrin was the major constituent of the low
residues in muscle and skin; levels of 0.05-0.08 mg/kg fell to 0.02
mg/kg before the end of the recovery period (Table 18) (Edwards and
Swaine, 1977; Swaine et al, 1980b).
Chickens have also received a single dermal dose of 40:60
cis:trans 14C-labelled permethrin (cyclopropyl- or
methylene-labelled). The dose of 4.5 mg per bird, which is
approximately 20% of the highest rate that is likely to be used
commercially, was brushed on as a 0.1% w/v emulsion to an area between
the rear abdomen and the end of the breast and upper leg. Up to 29.5%
of the applied dose was detected on skin and feathers at the site of
application fourteen days after treatment and was still present (99%)
as permethrin (Leahey et al, 1977).
Total radioactive residues in liver, kidney, heart and muscle were
low-less than 0.02 mg permethrin equivalents/kg one, seven and
fourteen days after treatment, except in muscle below the site of
application which contained up to 0.11 mg permethrin equivalents/kg.
Up to 0.07 mg permethrin equivalents was found in fat at the
application site, 70-88% of the radioactivity in subcutaneous muscle
and fat at the site of application was characterised as permethrin
itself (Leahey et al, 1977c).
Radioactive residues in eggs were all below 0.03 mg permethrin
equivalents/kg, reaching a peak 3-6 days after dosing and then
declining progressively to below 0.01 mg/kg ten days after dosing
(Leahey, et al, 1977c).
When eggshells were painted with a 0.1% w/v emulsion of 40:60
cis:trans 14C-labelled permethrin (combined cyclopropyl- and
methylene-labelling), no radioactivity was detected in either yolks or
albumen one, seven or fourteen days after treatment (limit of
determination: 0.02 mg permethrin equivalents/kg) (Leahey et al,
1977c).
On Processing
The fate of permethrin residues during the processing of cotton,
soybeans, apples, pears, grapes and tomatoes was reviewed at the 1979
Meeting. Residues in the seeds of cotton and beans of soya, and in
their respective processing fractions used in animal feeds, are small
(e.g. well below 0.5 mg/kg) (FAO/WHO, 1980).
Residues in dried apple pomace were 25-30 times the levels in
corresponding whole apples. No residues were determined in apple
juice and apple sauce (limit of determination: 0.01 mg/kg) (Table 19)
(Ussary, 1977a, c).
Residues in wet tomato pulp, which typically contains 25% dry matter,
were in the range 10-50 (mean 25) times the levels in whole tomatoes
(Table 20) (Ussary, 1977d).
During the processing of treated whole wheat grain the permethrin
residue is retained mainly in the bran component although a
significant proportion (12%) remains with the white flour and follows
through almost unchanged into bread (Simpson, 1979).
Permethrin residues in flour from treated whole grain are carried over
into bread baked from that flour. There is no reduction in residue
level on a commodity-weight basis. White flour retains about 12% of
the whole grain residue. The major part (about 62%) of the residue
remains with the bran while about 26% is in the pollard. Therefore,
whilst white bread prepared from treated grain would have a residue of
about 0.15-0.2 mg/kg, the corresponding level in wholemeal bread would
be about 0.7-1 mg/kg (Table 21) (Simpson, 1979).
TABLE 17. Total Radioactive Residues In The Tissues of Hens Receiving
Consecutive Daily Oral Doses Of 14C-Labelled Permethrin
Rate No. of Period Between Residue (mg permethrin equivalents/kg)
Material Administered (expressed as mg/kg Daily Last Dose And In
equivalent in diet) Doses Sacrifice Fat Muscle Liver Kidney
40:60 cis:trans-permethrin
(cyclopropyl-lablled) 10 10 4 hours 0.5-0.6 0.07-0.13 0.9-1.1 N/D.1
(methylene-labelled) 10 10 4 hours 0.3-0.7 0.03 0.4 N/D1
trans-permethrin
(carbonyl-labelled) 80 3 6 days 0.21 <0.06 0.14 0.31
(methylene-labelled) 80 3 6 days 0.18 <0.06 0.08 0.24
cis-permethrin
(carbonyl-labelled) 80 3 6 days 1.0 0.06 0.27 0.34
(methylene-labelled) 80 3 6 days 1.4 <0.06 0.20 0.25
1 N/D indicates not determined.
Gaughan et al, 1978b; Leahey et al, 1977b
TABLE 18. Residues Of Permethrin And Three Major Metabolites In Eggs And Tissues
Of Hens Receiving Permethrin In The Diet Up To 28 Days
Dietary Feeding Regime Product Residue (mg/kg) of
Inclusion Analysed
Level Cis & Trans
(mg/kg) Permethrin DCVA 3-PBAlc 3-PBAcid
(I and II) (III) (IV)
33 "Treated"1 Muscle 0.05-0.08 0.01-0.02 <0.01 <0.01
plus skin
Liver <0.01 <0.01-0.03 <0.01 <0.01
Eggs <0.6 <0.01-0.03 <0.01-0.03 <0.01
"Treated plus Muscle 0.02 <0.01 <0.01 <0.01
Recovery"2 plus skin
Liver <0.01 <0.01 <0.01 <0.02
Eggs <0.02 <0.01 <0.01-0.02 <0.01
3.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.08 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin <0.01 - - -
Liver <0.01 - - -
Eggs <0.03 - - -
0.4 "Treated" Muscle <0.01 - - -
plus skin
Liver <0.01 - - -
Eggs <0.05 - - -
"Treated plus Muscle <0.01 - - -
Recovery" plus skin
Liver <0.01 - - -
Eggs <0.01 - - -
1 "Treated" indicates the animals received treated diet for up to 28 days and
were then slaughtered.
2 "Treated plus Recovery" indicates the animals received treated diet for 28
days and were then returned to untreated diet for up to 14 days before slaughter.
Edwards and Swaine, 1977;
Swaine et al, 1980b
TABLE 19. Permethrin Residues In Commercial Fractions Of Apples: USA: 1976
Volume of Number of Interval Fraction Permethrin Residues
Spray Applications Between Last (mg/kg) after Applying
Applied Spraying and at Stated Rates of mg/kg
(l/ha) Harvest ai in spray
80 mg/kg 160 mg/kg
3150-3600 3-5 38-63 days Mature 0.11-0.40 0.85
apples
Juice <0.01 <0.01
Apple <0.01 -
Sauce
wet 0.74-3.2 6.5
pomace
Dry 3.3-10 22
pomace
Ussary, 1977a, c
TABLE 20. A Comparison Of Permethrin Residues In Processed Tomato Fractions : USA : 1977
Rate of Number of Interval Permethrin Residues (mg/kg) In
Application Applications Between Last
(g ai/ha) Application Whole Tomato Tomato Tomato Tomato
And Harvest Tomatoes Juice Puree Ketchup Pulp
220 2 1 day 0.05 <0.01 0.01 0.01 1.08
3 days 0.08 0.01 0.02 0.01 0.86
7 days 0.04 0.01 0.03 0.02 1.97
440 2 1 day 0.05 <0.01 0.03 0.02 1.60
3 days 0.05 0.01 0.03 0.02 1.45
7 days 0.08 <0.01 0.02 0.01 1.28
Ussary, 1977d
TABLE 21. Distribution Of Permethrin Residues In Whole Wheat
Fractions And Consequent Residue Levels In Bread
Distribution of residue between whole grain fractions1
Permethrin Residue
Fraction % whole grain mg/kg % distribution
Flour (white) 75.1 0.13 12.1
Pollard 11.3 1.9 26.3
Bran 13.6 3.7 61.7
Residue in Bread baked from the ground flour
Commodity Permethrin residue (mg/kg)
White flour 0.13-0.15
White bread 0.13-0.19
Wholemeal flour2 0.81-0.93
Wholemeal bread 0.67-1.00
1 Grain sample 1.5 kg. It had a residue level of 0.65 mg
permathrin/kg after being stored for 9 months.
2 Reconstituted from the fractions in the original whole grain
proportions.
Simpson, 1979
The processing operations simulated those used in commercial practice,
the unbaked bread recipe included potassium bromate and benzoyl
peroxide.
Wholemeal flour was formulated after reconstituting the wheat
fractions in the original ratio.
METHODS OF RESIDUE ANALYSIS
The preferred method of residue analysis (by gas chromatography using
an electron capture detector) for crops treated pre-harvest was
reviewed at the 1979 Meeting (FAO/WHO, 1980).
Both gas chromatography (GC) and high pressure liquid chromatography
(HPLC) have been used to determine permethrin residues in whole cereal
grains and commodities processed from grain (including bread).
The GC procedure described earlier is quite suitable, being able to
determine cis and trans isomers separately or together. Recovery
is essentially quantitative and the limit of determination is about
0.01 mg/kg.
A simple procedure based on a high pressure liquid chromatograph has
also been developed (Simpson, 1978). The limit of determination is a
little better than 0.01 mg/kg. In this procedure the residue is
extracted by cold methanol, partitioned into hexane - and, if
necessary, further cleaned by an acetonitrile partitioning - before
clean-up on an alumina column. The concentrated residue is subjected
to determination by HPLC equipped with a UV detector. This method is
similar to the procedure used by Mourot et al, (1979).
With small modifications, the GC method can be used to determine
permethrin in milk, meat and eggs.
Milk and meat samples are extracted with n-hexane:acetone 4:1. The
acetone is removed by washing with water and the permethrin
partitioned from n-hexane into dimethylformamide. The
dimethylformamide extract is dissolved in 1% aqueous sodium sulphate
and the permethrin back-extracted into n-hexane. The hexane extract
is cleaned up using a Florisil column, and permethrin is determined by
gas chromatography using an electron capture detector. The limit of
determination of the method is 0.01 mg/kg for the combined isomers and
recovery values for samples of meat and milk fortified at 0.01-1.0
mg/kg are normally greater than 70%. Mean recoveries of 89%-92% have
been obtained from milk and 86-88% from tissues of cows and hens
(Edwards and Iswaren, 1977; Edwards and Swaine, 1977). Twenty percent
acetone in hexane has been shown to be an efficient solvent for
extracting permethrin residues from animal tissues (Edwards and
Sapiets, 1978).
The method for permethrin analyses in meat and milk is also applicable
to eggs. These are extracted with n-hexane:acetone 1:1 and the
extract washed with aqueous sodium chloride to remove acetone, and
cleaned up by solvent partition with dimethylformamide and by using a
Florisil column. The limit of determination is 0.02 mg/kg for the
combined isomers and recovery values from yolks and albumen fortified
at 0.01-0.4 mg/kg are generally in the ranges 70-90% and 60-90%
respectively (Edwards and Swaine, 1977).
The mass spectrographic technique of multiple ion detection is
suitable for the qualitative and quantitative confirmation of
permethrin residues in crops, milk, eggs and animal tissues following
separation by gas chromatography.
Qualitative confirmation of permethrin residues is given by the
appearance of a peak at the correct retention time for all the
specific m/e values monitored. In addition, the ratios between the
peak heights (or peak areas) given from each m/e value should be
identical to that observed for permethrin analytical standards.
Quantitative confirmation is carried out by comparison of the peak
height or peak area, measured for the most abundant m/e value
recorded, against those obtained with external standards of permethrin
(Swaine and Edwards, 1977).
Residue of both the free and conjugated major plant metabolites of
permethrin, namely cis- and trans- isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
and 3-phenoxybenzyl alcohol (3-PBAlc), can also be determined by gas
chromatography. Samples are extracted with 2:1 methanol:water and
lipids removed by partitioning with dichloromethane. The methanol is
then removed using rotary evaporation, the aqueous solution made 1 N
with HCl and refluxed to free the conjugated residues. The residues
are then extracted by partitioning with n-hexane. The n-hexane is
then removed and the 2,2,2-trichloroethyl ester of DCVA and the
heptafluorobutyryl ester of 3-PBAlc are simultaneously made. The
derivatives are then analyzed by gas-liquid chromatography using
electron capture detection. (Ussary, 1979). Limits of determination
are in the range 0.02-0.10 mg/kg for DCVA and 0.02-0.05 mg/kg for 3-
PBAlc. Recoveries are generally in the range 70-85%. Confirmation of
the metabolite residues is possible using gas chromatography linked to
mass spectrometry using multiple ion detection, similar to the
procedure reviewed above for permethrin (Swaine et al, 1978).
With little modification, this method has also been used to determine
permethrin metabolite residues in meat and milk. Meat tissues are
extracted with 1:1 methanol:water; milk is extracted with 1:1
hexane:acetone. Any permethrin extracted by this procedure is
partitioned out, along with unconjugated residues of 3-PBAlc, into
n-hexane alone (milk) or hexane plus dichloromethane (meat).
Residues of 3-phenoxybenzoic acid (3-PBAcid), both "free" and
conjugated DCVA, and conjugated residues of 3-PBAlc remain in the
aqueous phase which is subjected to a hydrochloric acid hydrolysis to
cleave the conjugates. DCVA and 3-PBAcid are separated from 3-PBAlc
by partition between aqueous sodium hydroxide and dichloromethane.
The PBAlc residues are combined and determined as the
heptafluorobutyryl ester by gas chromatography using an electron
capture detector. DCVA and 3-PBAcid are converted to their methyl
ester derivatives, by treatment with diazomethane, and determined by
gas chromatography linked to mass spectrometry using multiple ion
detection. A limit of determination of 0.01 n/kg has been obtained
routinely for DCVA, 3-PBAlc and 3-PBAcid in meat and milk. Mean
recoveries have been within the range 55-60% from milk and 60-85% from
meat tissues (Swaine et al, 1980).
NATIONAL TOLERANCES
The following national tolerances were reported to the Meeting for
permethrin (total isomers):
Australia Wheat Bran 10 mg/kg
Raw cereals ) 5 mg/kg
Wheat pollard (shorts) )
Brussels sprouts )
Wheat flour(wholemeal) ) 2 mg/kg
Wholemeal bread )
Broccoli )
Cabbage ) 1 mg/kg
Cauliflower )
Wheat flour (white) ) 0.5 mg/kg
White bread )
Tomatoes 0.4 mg/kg
Cotton ) 0.2 mg/kg
Sweetcorn (cobs) )
Eggs ) 0.1 mg/kg
Fat of meat of cattle, )
sheep, pigs and poultry )
Milk and milk products ) 0.05 mg/kg
(fat basis) )
Belgium Eggplants )
Cabbages )
Cucumbers )
Endives )
Fruit crops ) 1 mg/kg
Honeydew melons )
Lettuce )
Peppers )
Tomatoes )
France Maize (whole plants) 0.2 mg/kg
Maize grains 0.1 mg/kg
Holland Endives ) 2 mg/kg
Lettuce )
Apples )
Brassicae ) 1 mg/kg
Pears )
Eggplants )
Cucumbers )
Honeydew melons ) 0.5 mg/kg
Peppers )
Tomatoes )
USA Cotton 0.5 mg/kg
Eggs )
Meat, fat and meat )
by-products of cows, ) 0.05 mg/kg
goats, hogs, horses, )
poultry and sheep )
Venezuela Vegetables ) 10 mg/kg
EVALUATION
APPRAISAL
Permethrin is a pyrethroid insecticide which is effective at low rates
against a wide range of Lepidoptera, Hemiptera, Diptera and
Coleoptera. Unlike natural pyrethrins and earlier synthetic
pyrethrins, permethrin is photostable to a degree that allows it to be
used as an effective tool in agriculture. It shows adulticidal,
ovicidal and particularly larvicidal activity and is effective against
the great majority of insects that. have become resistant to standard
treatments such an organochlorines and organophosphates. A programme
of sprays is usually required.
A particularly valuable usage is in protection of stored grain.
Laboratory and silo-scale studies in Australia have shown that low
levels of permethrin control most of the endemic insect pests of
stored grain, and, when used in conjuction with a synergist and
complementary insecticide, the commonly-occurring strains of all grain
insect pests are controlled. Adequate protection of at least 9 months
storage is achieved by a single application of 1 mg permethrin/kg.
However, for various reasons, it may become necessary in future to use
permethrin without its synergist and/or o/p insecticide. In that
case, higher application rates would be required to maintain the
present level or permethrin efficacy. The same applies if the present
experimental rate is increased for other reasons (e.g. to counter
resistance). A 2 mg/kg grain is likely to be the target concentration
in the near future.
Permethrin can also be used to control various ectoparasites of
cattle, horses, sheep, pigs and poultry. It can be applied either
directly to the animals, or to buildings in which they are housed, or
to insect breeding and resting sites.
Extensive residues data are available from supervised trials. In
pre-harvest uses, residues are highest where a crop part is exposed
directly to the spray, as in the case of many forage crop uses.
Ground and aerial applications yield similar residue levels. Although
residues decline comparatively slowly after spraying, there is no
obvious build-up of residues of permethrin or of its two most
important plant metabolites on repeated application, within the rates
and frequency of permethrin spraying that are needed to obtain good
insecticidal control. The two important plant metabolites are
3-phenoxybenzyl alcohol and the cis and trans isomers of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA),
which are also animal metabolites; levels of these metabolites in
crops are very much smaller than corresponding permethrin residues and
they do not need to be included in routine residue analyses.
Permethrin levels on stored grains also decline slowly. During the
processing of treated whole wheat grain, the permethrin residue is
retained mainly on the bran component, although a significant
proportion (12%) remains with the white flour and follows through
After both oral and dermal administration to livestock, permethrin
itself constitutes the major portion of the residue recovered from
milk, eggs, muscle and fat. After oral administration to goats,
metabolites form the major part of the residue in kidney and liver;
DCVA occurs in both organs, 3-phenoxybenzyl alcohol plus its
4'-hydroxy derivative in liver and 3-phenoxybenzoic acid plus its
4'-hydroxy derivative in liver and kidney.
Following direct application to dairy cattle (1.0 g ai/cow and with
free access to a self-oiler) permethrin levels are low in milk (<0.02
mg/kg) and in muscle, liver and kidney (<0.01 mg/kg). Highest levels
(up to 0.1 mg/kg) have been found in fat.
Cows maintained on a diet containing 50 mg/kg of permethrin yielded
milk containing low levels of residue (0.1 mg/kg), and the level in
muscle was less than 0.1 mg/kg. The residue in milk declined to below
0.01 mg/kg after five days on returning the cows to control diet.
Residues in eggs are also low and contamination of eggs laid during
the treatment of poultry houses will not result in detectable residues
in yolks or albumen.
Permethrin is not detected in the albumen of eggs from hens receiving
the compound at levels up to 33 mg/kg in the diet; levels in yolks are
approximately 2% of corresponding permethrin dietary levels.
In all cases studied, residues of permethrin and its metabolites in
products of animal origin declined notably on cessation of exposure.
Only small residue levels have been found in products of animal origin
following direct application of permethrin to livestock. In view of
the levels of permethrin found in forage crops, such as alfalfa, and
the importance of such crops in animal feedstuffs, it seems likely
that residue levels in products of animal origin will be higher
following forage crop uses rather than from ectoparasite outlets. In
estimating maximum residue levels for products of animals origin,
allowance has also to be made for the dilution effect that will occur
in practice when treated forage is incorporated into livestock diets.
In proposing maximum residue levels on raw grain and milled cereal
products prepared therefrom, careful consideration has been given to
the fact that this insecticide is to be used as a grain protectant and
that residues of the compound are comparatively stable under storage
conditions. Under practical conditions of grain handling and storage,
it is not possible to apply a grain protectant with assurance that the
deposit will be absolutely uniform; there will always be a natural
variation in the level of the deposit resulting from fluctuations in
the flow of grain and of the insecticide. Furthermore, the movement
of bulk grain results in the segregation of the various components of
the bulk with a resultant variation in the level of residues
throughout the mass. Due allowance has been for the amplitude of
these variations and the problems of sampling and analysis.
The meeting examined residue data from supervised storage and feeding
trials reflecting established or proposed good practice. From these
data the meeting was able to estimate the maximum residue levels that
were likely to occur when permethrin was used in practice.
The preferred method of permethrin residue analysis in crops is by gas
chromatography using an electron capture detector. Recoveries are
essentially quantitative and the method has been applied successfully
to a wide range of crops, raw cereal grains and processed products
derived from them, such as flour, bran and bread. A limit of
determination of 0.01 mg/kg (expressed as permethrin cis and trans
isomers). Cis and trans-isomers are capable of being determined
separately by this method. With small modifications, the method has
been applied successfully to the determination of permethrin residues
in meat, milk and eggs, and would with adaptation be suitable for
regulatory purposes.
RECOMMENDATIONS OF RESIDUES LIMITS
The meeting concludes that the residue levels listed below are
temporary pending submission of the "Further Work or Information".
These are recommended as suitable for establishing temporary maximum
residue limits. These levels are additional to those proposed by the
1979 Meeting. These levels refer to the sum of the cis and
trans-isomers of permethrin.
Crop TMRL (mg/kg)
Alfalfa fodder 100) On a dry weight basis
Maize fodder and straw 100)
Apple pomace 50) On a dry weight basis
Soybean fodder 50) On a dry weight basis
Cereal grains 2
Wheat flour (wholemeal) 2
wheat flour (white) 0.5
Wheat bran 10
Carcass meat of
cattle ) For permethrins a portion of
pig ) 1 carcass fat is analyzed and
sheep ) the levels refer to carcass fat.
Meat by-products from:
pigs ) 0.1
sheep )
Poultry meats 0.1
Eggs 0.1
Milk 0.1
FURTHER WORK OR INFORMATION
Recommendations 3, 4, 8, 9 from 1979 meeting remain relevant.
REFERENCES
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Pilot Usage Trials, 1978-79 Working Party On Grain
Protectants", Department of Primary Industries, Queensland, Australia
(unpublished) (1979a).
Bengston, M., Davis, D., Desmarchelier, J.M., Elder, W.B., Henning,
R., Minett, W., Murray, W., Ridley, E.G., Ripp, B.E., Sieraikowski,
C.J., Sticka, R., Snelson, J.T., Thomas D., Wallbank, B.E. and Wilson,
A. "Final Report On Silo Scale Experiments 1977-78 Working Party On
Grain Protectants", Department of Primary Industries, Queensland,
Australia (unpublished) (1979b).
Bengston, M., Davies, R.A.H., Desmarchelier, J.M., Phillips, M. and
Simpson, B.J. "Additional Grain Protectants For The Control of
Malathion-Resistant Insects In Stored Sorghum", Department Of Primary
Industries, Entomology Branch, Queensland, Australia (unpublished)
(1979c).
Bewick, D.W. and Leahey, J.P. "Permethrin : Absorption in Cows", ICI
Plant Protection Division Report No. TMJ1357B (unpublished) (1976).
Bewick, D.W., Leahey, J.P. and Saunders, R. "Permethrin Absorption In
Pigs After Dermal Treatment", ICI Plant Protection Division Report No.
TMJ1562A. (unpublished) (1977).
Chipman Inc. Canada "Summary of 1976/77 Permethrin Sweet Corn Residue
Data . (unpublished) (1978).
Desmarchelier, J.M., Bengston, M., Davies, R.A.H., Elder, W.B.,
Hamilton, D., Henning, R., Murray, W., Ridley, E.G., Ripp, E.,
Sieraikowski, C.J., Sticka, R., Snelson, J.T., Thomas, D., Wallbank,
B. and Wilson, A. "Pilot Usage Of The Grain Protectants Fenitrothion
Plus Phenothrin and Pirimiphos-methyl Plus Permethrin", Australian
Wheat Board Working Party on Grain Protectants (in manuscript for
publishing) (1979).
Edwards, M. J. and Iswaren, T.J. "Permethrin: Residue Transfer And
Toxicology Study With Cows Fed Treated Grass Nuts" ICI Plant
Protection Division Report No. TMJ1519/B (unpublished) (1977).
Edwards, M.J. and Swaine, H. "Permethrin: Incorporation of Permethrin
In The diet of Laying Hens: Residues In Eggs And Tissues". ICI Plant
Protection Division Report No. TMJ1520/B (unpublished) (1977).
Edwards, M.J. and Sapiets, A. "Permethrin: The Extraction of the
Pesticide From Animal Tissues". ICI Plant Protection Division Report
No. RJ0009B (unpublished) (1978).
Gaughan, L.C., Unai, T. and Casida, J.E. "Permethrin Metabolism In
Rats And Cows, And In Bean and Cotton Plant", Paper delivered at 172nd
ACS National Meeting, San Francisco (August 1976).
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. "Distribution
And Metabolism Of Trans- And Cis-Permethrin In Lactating Jersey
Cows", J. Agric. Food Chem. 26(3), 613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. "Distribution And
Metabolic Fate of Trans- And Cis- Permethrin In Laying Hens", J.
Agric. Food Chem. 26(6), 1374-1380.
Hunt, L.M. and Gilbert, B.N. "Distribution And Excretion Rates Of
14C-Labelled Permethrin Isomers Administered Orally To Four Lactating
Goats For 10 Days", J. Agric, Food Chem. 25(3), 673.
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
A.G. "Permethrin: Metabolism And Residues In Goats", ICI Plant
Protection Division Report No. TMJ1516B (unpublished) (1977a).
Leahey J.P., Gatehouse, D.M. and Parr, J.S. "Permethrin: Metabolism In
Hens", ICI Plant Protection Division Report No. TMJ1509B (unpublished)
(1977b).
Leahey, J.P., Bewick, D.W., Gatehouse, D.M. and Carpenter, P.K.
"Permethrin: Absorption In Chickens After Dermal And Oral Treatments",
ICI Plant Protection Division Report No. TMJ1481B (unpublished)
(1977c).
Leahey, J.P. and Cameron, A.G. "Permethrin: Residues In Cows After
Dermal Application", ICI Plant Protection Division Report No. RJ0001B
(unpublished) (1978).
Mourot, D., Delephine. B., Boisseau, J. and Gayot, G. "HPLC Of A New
Pyrethroid Insecticide Sumicidin", J. Chromatog. 168, 277-279.
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1978).
Simpson, B.W. Queensland Department Of Primary Industries Agricultural
Chemistry Branch, Queensland, Australia (unpublished data) (1979).
Swaine, H. and Edwards, M.J. "Confirmation Of Residues Of Permethrin
(PP557) Using Gas Chromatography - Mass Spectrometry In The Multiple
Ion Detection Mode", ICI Plant Protection Division Data (unpublished)
(1977).
Swaine, H., Edwards, M.J. and Ussary, J.P. "Permethin Crop Rotation
Study", ICI Americas Inc Report No. TMU0378/B (unpublished) (1978).
Swaine, H. and Sapiets, A. "Permethrin Residues Summary: Residues In
Crop Samples Analyzed During 1977-8: Part IV: Leaf, Root and Forage
Crops", ICI Plant Protection Division Report No. RJ0081A (unpublished)
(1979).
Swaine, H., Francis, P.D., Rippington, D. and Burke, S.R. "Permethrin
Residue Levels of the Major Metabolites of the Insecticide In the Milk
And Tissues of Cows Fed On A Treated Diet", ICI Plant Protection
Division Report No. RJ0124B (unpublished) (1980a).
Swaine, H., Francis, P.D., Rippington, D., Burke, S.R., Robertson, S.
and Ford, J.P. "Permethrin Metabolites: Eggs And Tissues Of Hens", ICI
Plant Protection Division Residue Data Report No. RD/557/27
(unpublished) (1980b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions of Apples, Wenatchee, Washington, 1976" ICI Americas Inc.
Report No. TMU0306/B (unpublished) (1977a).
Ussary, J.P. "Permethrin Residues In Soybeans", ICI Americas Inc.
Report No. TMU0383/B, (unpublished) (1977b).
Ussary, J.P. "Permethrin Residues In The Commercial Processing
Fractions Of Apples, Geneva, New York, 1976", ICI Americas Inc. Report
No. TMU0307/B (unpublished) (1977c).
Ussary. J.P. "Permethrin Residues In Tomato Process Fractions", ICI
Americas Inc. Report No. TMU0304/B (unpublished) (1977d).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0388/B (unpublished) (1978a).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0413/B (unpublished) (1978b).
Ussary, J.P. "Permethrin Residues On Sweet Corn", ICI Americas Inc.
Report No. TMU0438/B (unpublished) (1979a).
Ussary, J.P. "Permethrin Residues On Field Corn", ICI Americas Inc.
Report No. TMU0433/B (unpublished) (1979b).
Ussary, J.P. "Permethrin Metabolite Residues On Broccoli", ICI
Americas Inc. Report No. TMU0427/B (unpublished) (1979c).
Ussary, J.P. "Permethrin Residues On Alfalfa", ICI Americas Inc.
Report No. TMU0461/B (unpublished) (1979d).
Ussary, J.P. and Braithwaite, G.B. "Residues of Permethrin And
Permethrin metabolites in Milk From Ectiban Treated Cows (Trial No.
35NC79-001)", ICI Americas Inc. Report No. TMU0490/B (unpublished)
(1980a).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin and
3-Phenoxybenzyl Alcohol In Cow Tissues (Trial No. 35NC79-001)", ICI
Americas Inc. Report No. TMU0493/B (unpublished) (1980b).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And 3-
Phenoxybenzyl Alcohol In Tissues From Ectiban Treated Swine (Trial No.
35NC79-002)", ICI Americas Inc. Report No. TMU0491/B (unpublished)
(1980c).
Ussary, J.P. and Braithwaite, G.B. "Residues Of Permethrin And
3-Phenoxybenzyl Alcohol In Tissues And Eggs From Ectiban Treated
Chickens (Trial No. 35NC79-003)", ICI Americas Inc. Report No.
TMU0492/B (unpublished) (1980d).
