
Pesticide residues in food -- 1999
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
Toxicological evaluations
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Rome, 20-29 September 1999
PERMETHRIN
First draft prepared by
D.B. McGregor
International Agency for Research on Cancer, Lyon, France
Explanation
Evaluation for acceptable daily intake
Biochemical aspects: Absorption, distribution, excretion,
and biotransformation
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Neurotoxicity
Endocrine effects
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Permethrin is a synthetic pyrethroid insecticide. It is an ester
of the dichloro analogue of chrysanthemic acid, chemically identified
as (3-phenoxyphenyl)methyl-(±)- cis-trans-3(2,2-dichloroethyenyl)
-2,2-dimethylcyclopropanecarboxylate. The technical-grade materials
are mixtures of four stereoisomers, although the 1 R, cis isomer is
the most active insecticide. Permethrin is effective against a wide
range of insect pests in agriculture, animal husbandry, and public
health and is used to control residential insects and dust mites. The
insecticidal action of synthetic pyrethroids such as permethrin is due
to interaction with ion channels on axons of the nervous systems of
target species.
Permethrin was evaluated toxicologically by the Meeting in 1979,
1981, and 1982 (Annex 1, references 32, 36, and 38). The 1982 Meeting
established an ADI of 0-0.05 mg/kg bw for the 40:60 cis:trans
mixture of permethrin stereoisomers, since it recognized that mixtures
with different isomeric ratios would require independent evaluation.
The 1987 Meeting (Annex 1, reference 50) included permethrin mixtures
in which the cis:trans ratio is nominally 25:75 in the ADI of 0-0.05
mg/kg bw. Permethrin was reviewed by the present Meeting within the
periodic review programme of the Codex Committee on Pesticide
Residues.
Evaluation for Acceptable Daily Intake
1. Biochemical aspects: Absorption, distribution, excretion, and
biotransformation
Mice
[14C-alcohol-1 RS,cis]Permethrin was applied to a marked
1-cm2 area of the clipped skin of mice at a dose of 1 mg/kg bw
(1 µCi) in 0.1 ml of acetone. The mice were restrained until the
acetone had evaporated and were then placed in metabolism cages. They
were killed 1, 5, 15, 60, 480, and 2880 min after treatment and
examined for the absorption, distribution, and excretion of
permethrin. About 40% of the dose had left the site of application
within 5 min and appeared to move rapidly to other parts of the body
(Shah et al., 1981).
In a study of the metabolism of permethrin in microsomes isolated
from mice, separated cis and trans isomers were used as substrates
for separate or combined esterase and NADPH-dependent oxidase
reactions. Unmetabolized substrate was measured by gas chromatography
and the half-times were measured under pseudo-first-order reaction
conditions. The oxidase activity was similar with the cis and
trans isomers, whereas the esterase activities with 1 R, 1 S, and
1 RS cis-permethrins as substrates were < 4% of those with 1 R,
1 S, and 1 RS trans-permethrins as substrates. The combined oxidase
and esterase activities were three to four times higher when the
trans isomers were used as substrates (Soderlund & Casida, 1977).
trans-Permethrin is hydrolysed by one or more carboxylesterases
located in the soluble fraction of mouse brain homogenates. The
apparent affinity of this activity was greater than that reported for
mouse hepatic carboxylesterase activity, which is primarily
membrane-associated, but the maximum velocity of the reaction was
considerably lower. The authors suggest that the hydrolytic activity
in the brain contributes to the detoxification of permethrin in
mammals (Ghiasuddin & Soderlund, 1984).
Rats
Four male rats were given a single oral dose of one of four
labelled forms of permethrin (radiochemical purity, > 99%),
[14C-acid-1 RS,trans]permethrin,
[14C-alcohol-1 RS,trans]-permethrin,
[14C-acid-1 RS,cis]permethrin, or
[14C-alcohol-1 RS,cis]permethrin, at doses of 4.8 mg/kg for the
acid label and 4.4 mg/kg for the alcohol label. The rats were
maintained in glass metabolism cages, in which urine, faeces, and
carbon dioxide were collected for 12 days after dosing, and were then
killed, and samples of various tissues were analysed for radiolabel.
Faeces were extracted with methanol, and the radiolabel in the
extracts and in urine was determined by direct liquid scintillation
counting. The concentrations in the insoluble faecal residue and in
tissues were determined after combustion. Metabolites in urine and
faeces were isolated and quantified by thin-layer chromatography;
their identification was limited to co-chromatography with synthetic
reference standards.
The rats excreted at least 97% of the dose within 12 days. Those
given the trans isomer excreted 79-82% of the dose in urine and
16-18% in faeces, while rats given the cis isomer excreted 52-54% in
urine and 45-47% in faeces. [14C]carbon dioxide accounted for
> 0.5% of the dose. The concentrations of radiolabel in most
tissues except fat were < 25 µg/kg equivalents of permethrin. The fat
of the rats given acid- and alcohol-labelled cis-permethrin
contained 460 and 620 µg/kg equivalents of permethrin, respectively,
while fat of rats given either labelled form of trans-permethrin
contained a maximum of 86 µg/kg equivalents (Table 1).
Table 1. Distribution of [14C]permethrin residues in rat
excreta and tissues
Sample Percentage of administered dose
Acid label Alcohol label
trans cis trans cis
Urine
0-1 day 57 34 74 44
1-12 days 25 20 58
Total 82 54 79 52
Faeces
0-1 day 9 27 12 26
1-12 days 5 15 220
Unextractable 2 3 43
Total 16 45 18 47
Exhaled air 0.5 0.5 0 0
Total excreta 98.5 99.5 97.0 99.0
Blood < 25* 69 86 115
Bone < 25 < 25 43 < 25
Brain < 25 < 25 < 25 < 25
Fat < 25 460 86 620
Heart < 25 < 25 < 25 < 25
Kidney < 25 < 25 < 25 < 25
Liver < 25 < 25 < 25 < 25
Lung < 25 < 25 < 25 < 25
Muscle < 25 < 25 < 25 46
Spleen < 25 < 25 < 25 < 25
Testes < 25 < 25 < 25 < 25
*Limit of detection
The major urinary metabolite of [14C-acid]permethrin was the
dichlorovinyl acid glucuronide, which accounted for 42% of the dose of
trans-permethrin and 14% of the dose of the cis isomer. The urine
of the rat given the trans isomer also contained free
trans-dichlorovinyl acid (5.6%), trans-hydroxydichlorovinyl acid
(0.3%), cis-hydroxydichlorovinyl acid (1.7%), and the corresponding
glucuronide (0.7%). The urine of the rat given the cis isomer
contained free cis-dichlorovinyl acid (0.7%),
trans-hydroxydichlorovinyl acid (3.3%), cis-hydroxydichlorovinyl
acid (3.5%), and the corresponding glucuronide conjugate (2.0%) and
the lactone form (3.0%) of this metabolite. The remaining unidentified
metabolites each accounted for 0.6% of the dose. The metabolites in
the faeces of the rat given [14C-acid] transpermethrin were the
free trans-dichlorovinyl acid (2.7%), trans-hydroxydichlorovinyl
acid (0.8%), and cis-hydroxydichlorovinyl acid (0.8%). The
metabolites in the faeces of the rat given
[14C-acid] cis-permethrin were the free trans-dichlorovinyl acid
(0.5%), trans-hydroxydichlorovinyl acid (1.5%),
cis-hydroxydichlorovinyl acid (1.2%), the lactone form of
cis-hydroxydichlorovinyl acid (1.1%), and an unknown metabolite that
accounted for 1.7% of the dose (Table 2).
Table 2. Excretion of [14C]acid-labelled metabolites of permethrin and
its ester
Compound Percentage of administered dose
trans isomer cis isomer
Urine Faeces Urine Faeces
Permethrin 0.0 2.8 0.0 6.7
2'-Hydroxypermethrin 0.0 0.0 0.0 0.5
4'-Hydroxypermethrin 0.0 0.0 0.0 2.7
trans-Hydroxypermethrin 0.0 0.0 0.0 2.5
4'-Hydroxy, trans-hydroxypermethrin 0.0 0.0 0.0 3.9
Dichlorovinyl acid
Free 5.6 2.7 0.7 0.5
Glucuronide 42 0.0 14 0.0
Hydroxydichlorovinyl acid
trans isomer 0.3 0.8 3.3 1.5
cis isomer
Free 1.7 0.8 3.5 1.2
Lactone 0.0 0.0 3.0 1.1
Glucuronide 0.7 0.0 2.0 0.0
Unknown
1 0.5 0.6 0.0
2 0.0 1.7
3 0.6
Total 50 7.6 27 23
The major urinary metabolites of [14C-alcohol] trans-permethrin
were 4'-hydroxyphenoxy-benzoic acid sulfate (43%), phenoxybenzoic
acid in the free form (10%) and its glucuronic acid (15%), and
glycine conjugates (4.4%). The urine of the rat given the cis isomer
contained the major metabolite 4'-hydroxyphenoxybenzoic acid sulfate
(29%), free phenoxybenzoic acid (1.1%) and its glucuronic acid (7.0%),
and glycine conjugates (2.0%). 2'-Hydroxyphenoxybenzoic acid sulfate
was also present, representing 2.9% of the dose. Four hydroxylated
forms of permethrin were identified in the faeces of the rats given
[14C alcohol] cis-permethrin, which accounted for 8.5% of the
dose. These metabolites were not present in the faeces of rats given
[14C-alcohol] trans-permethrin, in which the identified metabolites
were phenoxybenzyl alcohol (1.7%) and phenoxybenzoic acid (1.5%).
Unchanged 14C-alcohol-labelled permethrin accounted for 5.3% of the
dose of the trans isomer and 7.3% of that of the cis isomer in the
faeces. The faeces of rats treated with either of the [14C-alcohol]
permethrin isomers contained one to four unknown metabolites which
accounted for 0.7-2.0% of the administered dose (Table 3).
Table 3. Excretion of 14C-alcohol-labelled metabolites of permethrin
Compound Percentage of administered dose
trans isomer cis isomer
Urine Faeces Urine Faeces
Permethrin 0.0 5.3 0.0 7.3
2'-Hydroxypermethrin 0.0 0.0 0.0 0.9
4'-Hydroxypermethrin 0.0 0.0 0.0 2.4
trans-Hydroxypermethrin 0.0 0.0 0.0 1.4
4'-Hydroxy, trans-hydroxypermethrin 0.0 0.0 0.0 3.8
Phenoxybenzyl alcohol 0.0 1.7 0.0 0.0
Phenoxybenzoic acid
Free 10 1.5 1.1 0.0
Glucuronide 15 0.0 7.0 0.0
Glycine 4.4 0.0 2.0 0.0
Hydroxyphenoxybenzoic acid sulfate
2'- 0.0 0.0 2.9 0.0
4'- 43 0.0 29 0.0
Unknown
1 0.7 1.8
2 0.0 1.1
3 2.0
4 2.0
Total 72 9.2 42 23
Thus, both cis- and trans-permethrin are readily excreted by
male rats. Both isomers are retained in fat, the cis isomer being
present at a higher concentration than the trans isomer. cis- and
trans-permethrin are readily hydrolysed in rats to dichlorovinyl
acid and phenoxybenzyl alcohol. The former is excreted mainly as the
glucuronide conjugate, but a small amount undergoes hydroxylation at
one of the geminal dimethyl groups before excretion. The phenoxybenzyl
alcohol is oxidized to phenoxybenzoic acid and is excreted mainly
after further hydroxylation and sulfate conjugation. The results
suggest that the ester group of cis-permethrin is less readily
hydrolysed than that of the trans isomer (Gaughan et al., 1977).
Figure 1 shows the proposed metabolic pathway of permethrin in rats.
In a study conducted according to GLP, two groups of four male
and four female rats were given a single oral dose of 100 mg/kg bw of
permethrin labelled with 14C-cyclopropyl (acid label) or
14C-propyl (alcohol label), and urine and faeces were collected for
7 days. Most of the administered dose (> 87%) was excreted within 24
h in both groups. Faecal elimination was the major route, accounting
for > 71% of the administered dose in both groups by day 7, while
urinary elimination accounted for 18-28% of the dose, for a total of
> 96% of the dose. On day 7, the rats were killed, and samples of
bone, brain, fat, heart, skeletal muscle, whole blood, stomach
including contents, plasma, residual carcass, testis/ovaries, liver,
lung, spleen, kidney, and the intestine including contents were taken
for analysis. The samples were analysed for radiolabel content by
liquid scintillation counting, either directly or after combustion.
The distribution of recovered radiolabel expressed as a
percentage of the administered dose is shown in Table 4. There were no
obvious differences in the excretion patterns of males and females
rats given either labelled form of permethrin. The concentration of
residues in tissues and organs (Table 5) ranged from 0.01 to 11 ppm,
the highest concentration occurring in fat. No large differences in
the distribution of cyclopropyl label were observed between male and
female rats, and there was no difference in the distribution of the
cyclopropyl and phenyl labels in male rats; however, in female rats,
the concentration of the phenyl label was about five times higher than
that of the cyclopropyl label in fat and ovaries (Cameron & Partridge,
1989).
The difference in the results with regard to route of excretion
in these two studies is probably due to the fact that in the earlier
study the rats were given a single low dose, whereas in the later
study the rats were given a high dose and saturation of the absorption
mechanisms in the gastrointestinal tract may have taken place.
A series of experiments was performed to determine the
concentrations of radiolabel in tissues after repeated oral
administration of [14C-phenyl]- or [14C cyclopropyl]permethrin. In
several experiments, male or female rats were given the compounds at
1-10 mg/kg bw for up to 11 weeks and were killed at various times
during and after treatment, and a sample of fat was removed for
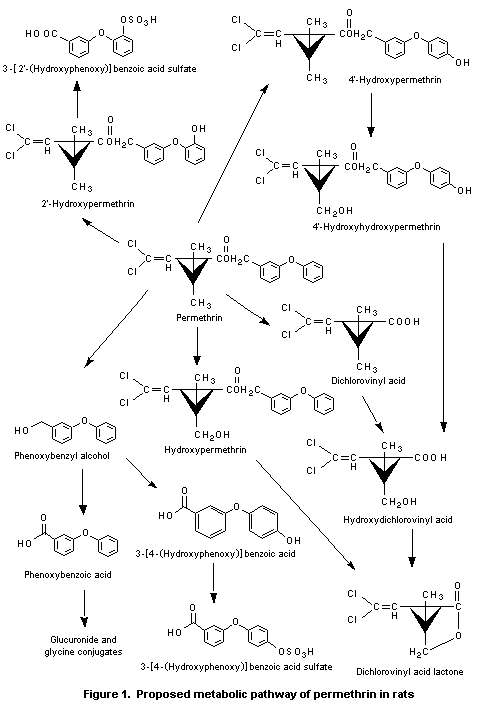 Table 4. Distribution of 14C-labelled permethrin residues in rats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Urine 28 22 19 20
Faeces 71 72. 76 74
Cage wash 2.0 2.5 2.4 2.4
Total excreted 101 96 98 97
Tissues and carcass 0.49 0.30 0.58 0.84
Total recovered after 7 days 101 97 98 98
Table 5. Distribution of 14C-labelled permethrin residues in rat
tissues
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Bone 0.07 0.08 0.14 0.16
Brain 0.18 0.03 0.02 0.01
Fat 6.6 2.4 7.5 11
Heart 0.07 0.06 0.07 0.08
Muscle 0.17 0.13 0.27 0.19
Testis/Ovary 0.30 0.75 0.22 4.7
Liver 0.75 0.33 0.30 0.38
Lung 0.17 0.15 0.15 0.20
Spleen 0.09 0.08 0.13 1.2
Kidney 0.24 0.30 0.38 0.55
Stomach and contents 0.11 0.11 0.25 0.70
Intestine and contents 0.60 0.29 0.38 1.2
Whole blood 0.09 0.05 0.11 0.14
Plasma 0.06 0.04 0.11 0.10
Residual carcass 0.44 0.29 0.63 1.0
analysis. In other experiments, liver, kidneys, brain, and a sample of
muscle were also removed. Groups of male or female rats were also
given single oral doses of 1-6 mg/kg bw for determination of
radiolabel in blood or for whole-body autoradiography. Radiolabel in
tissues and blood was determined after their homogenization and
combustion, while those in fat were identified after solubilization of
the samples in hexane, a clean-up procedure, and thin-layer
chromatography. Sections were prepared for whole-body autoradiography
from animals killed 1, 24, and 96 h after dosing.
The maximum concentration of radiolabel in the fat of rats given
[14C-phenyl]permethrin (1 mg/kg bw; 0.12 µCi) for 77 days was 2.0
ppm. A steady-state concentration was attained within 3 weeks of
dosing. The half-time of elimination of radiolabel from fat was 18
days. The concentrations in liver and kidneys were below the limit of
detection (0.08 ppm) within 1 week of cessation of dosing. No
detectable residues were present in brain or muscle. Similar results
were obtained when [14C-cyclopropyl]permethrin (0.9 mg/kg bw; 0.22
µCi) was given to rats for 3 weeks. The maximum concentration of
radiolabel in fat was 0.72 ppm, and the elimination half-time was
7 days. The highest concentrations of radiolabel in the liver and
kidneys were 0.22 and 0.02 ppm, respectively. Most of the radiolabel
in the fat of rats given [14C-phenyl]permethrin consisted of the
unchanged permethrin, although more cis isomer than trans isomer
was retained. Whole-body autoradiography showed that the radiolabel
was present mainly in the stomach, intestines, liver, kidneys, and fat
24 and 96 h after administration of a single oral dose of
[14C-phenyl]permethrin (6 mg/kg bw; 33 µCi).
[14C-phenyl]Permethrin at a dose of 10 mg/kg bw (20 µCi) was rapidly
absorbed: the maximum concentration (0.34 ppm) was reached in blood
within 1.5 h, and the rate of elimination corresponded to a half-time
of 7 h. Only very low concentrations of radiolabel (< 0.05 ppm) were
detected in the blood of rats given [14C-cyclopropyl]permethrin at
10 mg/kg bw (15 µCi).
The results of these experiments show some retention of
permethrin in the fat of rats after repeated oral administration and
show that the cis isomer is more readily retained than the trans
isomer. Permethrin is, however, readily eliminated from fat. The
concentrations of permethrin and/or its metabolites in other tissues
after repeated administration are relatively low, and the chemical is
not retained after dosing has ceased (Bratt et al., 1977).
In the only study of the metabolism of unlabelled permethrin
(cis:trans ratio, 25:75), a single dose of 460 mg/kg bw was given by
oral gavage and 46 mg/kg bw were given intravenously to fasted male
Sprague-Dawley rats. These doses were reported (Litchfield, 1985) to
be toxic but not lethal. Groups of eight rats were killed 0.25, 0.5,
1, 2, 3, 4, 6, 8, 12, 24, and 48 h after dosing, when blood samples
were collected and plasma separated out. The brain, sciatic nerve, and
liver were collected only from orally treated rats at 0.5, 1, 2, 4, 8,
24, and 48 h. The brains were dissected and the major regions stored
separately. The samples were analysed by high-performance liquid
chromatography for permethrin and its metabolites meta-phenoxybenzyl
alcohol and meta-phenoxybenzoic acid.
The plasma profile of permethrin after intravenous dosing could
be described by a two-compartment open model, with a relatively rapid
distribution phase (half-time, 0.46 h) and a more prolonged
elimination phase (half-time, 8.7 h). The apparent volumes of
distribution were relatively large (elimination V, 0.72 L;
steady-state Vss,0.65 L). After the single oral dose, permethrin
was slowly absorbed (Tmax = 3.5 h when Cmax = 50 mg/ml) and
slowly eliminated (half-time, 12 h). The low total plasma clearance
(0.058 L/h) could explain the latter result. The oral bioavailability,
61%, was relatively low, perhaps due to degradation at the site of
absorption and a first-pass effect. After oral administration, the
concentrations of permethrin were particularly high in brain and and
sciatic nerve; decreasing concentrations were found in sciatic nerve
> hypothalamus > frontal cortex > hippocampus > caudate putamen
> cerebellum > plasma > medulla oblongata > liver. The ratio of
the integrated area under the curve of concentration-time (AUC) for
nervous tissue:plasma ranged from 8.8 in sciatic nerve to 1.2 in
cerebellum but was only 0.44 for liver. The brain regions also showed
the higher AUC values for the metabolites, particularly
meta-phenoxybenzyl alcohol, for which the tissue:plasma ratio was
always > 1.0, whereas this ratio was usually < 0.5 for
meta-phenoxybenxoic acid (Anadón et al., 1991).
Chickens
In a study conducted according to GLP, groups of six laying hens
received capsules containing 1.27 mg of [U-14C-phenyl]permethrin or
[1-14C-cyclopropyl]permethrin, and two hens received a capsule
containing a placebo. The dose was given orally for 7 consecutive days
at a rate equivalent to a dietary intake of 11 ppm. Urine, faeces,
cage wash, and egg samples (separated into whites and yolks) were
collected daily and analysed for radiolabel. All hens were killed 16 h
after the final dose, and samples of breast and thigh muscle, skin and
subcutaneous fat, and the liver were collected at necropsy. The
samples were analysed for radiolabel with or without solubilization or
combustion, followed by liquid scintillation counting.
A mean of 92% of the total dose was excreted (urine, faeces, cage
wash) by day 7 after administration of [14C-cyclopropyl]permethrin,
and a mean of 90% after administration of [14C-phenyl]permethrin.
The mean total was 0.2% in eggs and 0.1% in liver for both groups. The
overall recovery of radiolabel was 93% from the group given the
cyclopropyl abel and 90% from that given phenyl label. At day 6, the
residues in egg yolk reached a maximum mean concentration of 0.27 ppm
with the cyclopropyl label and 0.28 ppm with the phenyl label.
Unchanged permethrin accounted for about 50% of these residues in both
groups. A number of other metabolites were also present in the yolk,
at concentrations of < 0.001 to 0.01 ppm. One of these, present at
about 0.01 ppm, was identified as 4'-hydroxypermethrin. The
concentrations in the egg whites were much lower, ranging from 0.001
to 0.02 ppm for both groups. Unchanged permethrin accounted for about
half of the cyclopropyl-labelled residues, in addition to several
other components, including trans-dichlorovinyl acid (0.002 ppm).
The egg whites from the hens given the phenyl label contained <
0.01 ppm and were therefore not analysed. The concentrations of
radiolabelled residues in breast and leg muscle were 0.01-0.03 ppm.
The breast muscle samples were not analysed further. An average of 47%
of the radiolabel was extractable from leg muscle for both groups.
Permethrin was the major component, at 0.008 ppm, accounting for an
average of 32% in the two groups. Leg muscle from both groups also
contained two metabolites at concentrations of 0.001-0.002 ppm, and
muscle from hens dosed with cyclopropyl label contained one metabolite
at 0.001 ppm (Table 6).
The concentrations of residues in peritoneal fat were 0.37 ppm
for the cyclpropyl label and 0.31 ppm for the phenyl label. Unchanged
permethrin represented about 81% of the total fat residue with both
labels (0.29 ppm for the cyclopropyl and 0.24 ppm for the phenyl
label). The only other extractable component that contained the intact
ester linkage was present at a concentration of about 0.02 ppm and
co-chromatographed with hydroxypermethrin, but it could not be
identified unambiguouly. The total concentrations of radiolabelled
residues in subcutaneous fat (including skin) were 0.18 ppm for the
cyclopropyl label and 0.16 ppm for the phenyl label (Table 6).
The concentrations of residues in the liver were 0.17 ppm for the
cyclopropyl label and 0.29 ppm for the phenyl label. No significant
amounts of unchanged permethrin were present in liver extracts, and no
metabolites containing the intact ester linkage could be
characterized. trans- and cis-dichlorovinyl acid were present at
concentrations of 0.013 and 0.009 ppm, respectively, in livers from
cyclopropyl-treated hens. The corresponding hydrolysis product from
the phenyl label, phenoxybenzoic acid, was not detected. Covalent
binding of radiolabelled residues to tissue protein was substantial,
accounting for 36% (0.057 ppm) of the cyclopropyl-derived and 52%
(0.14 ppm) of the phenyl-derived residues. With both radiolabels, most
of the extractable components were associated with very polar,
uncharacterized material (Table 6).
Analysis of excreta showed that permethrin was extensively
metabolized in hens, yielding many polar components. The excreta
contained metabolites resulting from hydrolysis of the central ester
linkage and from hydroxylation of a geminal dimethyl group attached to
the cyclopropane ring and at the 4' position of the phenoxybenzyl
moiety. Some of the resulting metabolites were further conjugated with
glucuronic acid or sulfate. trans-Dichlorovinyl acid, the
cyclopropyl carboxylic acid resulting directly from hydrolysis of
permethrin, was the major cyclopropyl-labelled metabolite in excreta.
Permethrin was the major component in hens given phenyl-labelled
material (Table 7).
Table 6. Distribution of extractable 14C-labelled residues in tissues of hens
Metabolite Leg muscle Peritoneal fat Liver
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
trans-Dichlorovinyl acid ND ND NA NA ND ND NA NA 8.2 0.013 NA NA
cis-Dichlorovinyl acid ND ND NA NA ND ND NA NA 5.6 0.009 NA NA
Permethrin 31 0.008 34 0.008 78 0.29 77 0.24 ND ND ND ND
Others 19 0.005 10 0.002 5.4a 0.020 6.5a 0.020 73 0.12 66 0.18
Total extractable 49 0.013 44 0.010 84 0.31 84 0.26 86 0.14 66 0.18
ND, not detected; NA, not applicable
a This component corresponds to the retention time of hydroxypermethrin, but low concentrations prevented positive
identification.
Table 7. Distribution of extractable 14C-labelled residues in
excreta of hens
Metabolite Percentage of administered dose
Cyclopropyl label Phenyl label
trans-Dichlorovinyl acid 19 ND
cis-Dichlorovinyl acid 2.2 ND
4'-Hydroxy-hydroxypermethrin 1.9 2.2
4'-Hydroxypermethrin 2.1 0.8
3-Phenoxybenzyl alcohol ND 0.4
Permethrin 16 35
Others 0.3 1.9
Unknown (several polar)a 48 31
Total 89 70
a Fourteen or more unknown metabolites were found.
Permethrin was thus extensively metabolized in hens, resulting in
a large number of metabolites, many of which were polar. The
identified metabolites were formed through hydrolysis of the ester
linkage, hydroxylation of a geminal dimethyl group attached to the
cyclopropane ring, and hydroxylation at the 4' position of the
phenoxybenzyl moiety. Some of the metabolites were further conjugated
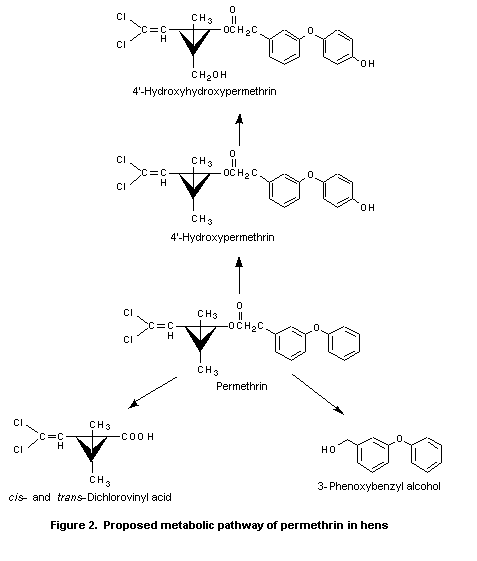
with glucuronic acid and sulfate (Hawkins et al., 1992a). Figure 2
shows the proposed metabolic pathway of permethrin in hens.
Lactating goats
In a study conducted according to GLP, two lactating goats
received oral doses of permethrin labelled at [U-14C-phenyl] or
[1-14C-cyclopropyl] for four consecutive days at a nominal rate of
55 ppm in the diet. The goat given the cyclopropyl label received
capsules containing a mean of 102 mg, and that given the phenyl label
received capsules containing a mean of 122 mg. Urine, faeces, and cage
wash were collected daily and milk was collected twice daily. Blood
samples were taken 16 h after the final dose, before slaughter, and
samples of liver, kidney, bile, omasum, abomasum and contents,
intestines and contents, foreleg and rump muscle, and subcutaneous,
omental, and perirenal fat were taken after slaughter. The samples
were analysed for radiolabel by liquid scintillation counting or by
combustion followed by liquid scintillation counting.
Table 4. Distribution of 14C-labelled permethrin residues in rats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Urine 28 22 19 20
Faeces 71 72. 76 74
Cage wash 2.0 2.5 2.4 2.4
Total excreted 101 96 98 97
Tissues and carcass 0.49 0.30 0.58 0.84
Total recovered after 7 days 101 97 98 98
Table 5. Distribution of 14C-labelled permethrin residues in rat
tissues
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Bone 0.07 0.08 0.14 0.16
Brain 0.18 0.03 0.02 0.01
Fat 6.6 2.4 7.5 11
Heart 0.07 0.06 0.07 0.08
Muscle 0.17 0.13 0.27 0.19
Testis/Ovary 0.30 0.75 0.22 4.7
Liver 0.75 0.33 0.30 0.38
Lung 0.17 0.15 0.15 0.20
Spleen 0.09 0.08 0.13 1.2
Kidney 0.24 0.30 0.38 0.55
Stomach and contents 0.11 0.11 0.25 0.70
Intestine and contents 0.60 0.29 0.38 1.2
Whole blood 0.09 0.05 0.11 0.14
Plasma 0.06 0.04 0.11 0.10
Residual carcass 0.44 0.29 0.63 1.0
analysis. In other experiments, liver, kidneys, brain, and a sample of
muscle were also removed. Groups of male or female rats were also
given single oral doses of 1-6 mg/kg bw for determination of
radiolabel in blood or for whole-body autoradiography. Radiolabel in
tissues and blood was determined after their homogenization and
combustion, while those in fat were identified after solubilization of
the samples in hexane, a clean-up procedure, and thin-layer
chromatography. Sections were prepared for whole-body autoradiography
from animals killed 1, 24, and 96 h after dosing.
The maximum concentration of radiolabel in the fat of rats given
[14C-phenyl]permethrin (1 mg/kg bw; 0.12 µCi) for 77 days was 2.0
ppm. A steady-state concentration was attained within 3 weeks of
dosing. The half-time of elimination of radiolabel from fat was 18
days. The concentrations in liver and kidneys were below the limit of
detection (0.08 ppm) within 1 week of cessation of dosing. No
detectable residues were present in brain or muscle. Similar results
were obtained when [14C-cyclopropyl]permethrin (0.9 mg/kg bw; 0.22
µCi) was given to rats for 3 weeks. The maximum concentration of
radiolabel in fat was 0.72 ppm, and the elimination half-time was
7 days. The highest concentrations of radiolabel in the liver and
kidneys were 0.22 and 0.02 ppm, respectively. Most of the radiolabel
in the fat of rats given [14C-phenyl]permethrin consisted of the
unchanged permethrin, although more cis isomer than trans isomer
was retained. Whole-body autoradiography showed that the radiolabel
was present mainly in the stomach, intestines, liver, kidneys, and fat
24 and 96 h after administration of a single oral dose of
[14C-phenyl]permethrin (6 mg/kg bw; 33 µCi).
[14C-phenyl]Permethrin at a dose of 10 mg/kg bw (20 µCi) was rapidly
absorbed: the maximum concentration (0.34 ppm) was reached in blood
within 1.5 h, and the rate of elimination corresponded to a half-time
of 7 h. Only very low concentrations of radiolabel (< 0.05 ppm) were
detected in the blood of rats given [14C-cyclopropyl]permethrin at
10 mg/kg bw (15 µCi).
The results of these experiments show some retention of
permethrin in the fat of rats after repeated oral administration and
show that the cis isomer is more readily retained than the trans
isomer. Permethrin is, however, readily eliminated from fat. The
concentrations of permethrin and/or its metabolites in other tissues
after repeated administration are relatively low, and the chemical is
not retained after dosing has ceased (Bratt et al., 1977).
In the only study of the metabolism of unlabelled permethrin
(cis:trans ratio, 25:75), a single dose of 460 mg/kg bw was given by
oral gavage and 46 mg/kg bw were given intravenously to fasted male
Sprague-Dawley rats. These doses were reported (Litchfield, 1985) to
be toxic but not lethal. Groups of eight rats were killed 0.25, 0.5,
1, 2, 3, 4, 6, 8, 12, 24, and 48 h after dosing, when blood samples
were collected and plasma separated out. The brain, sciatic nerve, and
liver were collected only from orally treated rats at 0.5, 1, 2, 4, 8,
24, and 48 h. The brains were dissected and the major regions stored
separately. The samples were analysed by high-performance liquid
chromatography for permethrin and its metabolites meta-phenoxybenzyl
alcohol and meta-phenoxybenzoic acid.
The plasma profile of permethrin after intravenous dosing could
be described by a two-compartment open model, with a relatively rapid
distribution phase (half-time, 0.46 h) and a more prolonged
elimination phase (half-time, 8.7 h). The apparent volumes of
distribution were relatively large (elimination V, 0.72 L;
steady-state Vss,0.65 L). After the single oral dose, permethrin
was slowly absorbed (Tmax = 3.5 h when Cmax = 50 mg/ml) and
slowly eliminated (half-time, 12 h). The low total plasma clearance
(0.058 L/h) could explain the latter result. The oral bioavailability,
61%, was relatively low, perhaps due to degradation at the site of
absorption and a first-pass effect. After oral administration, the
concentrations of permethrin were particularly high in brain and and
sciatic nerve; decreasing concentrations were found in sciatic nerve
> hypothalamus > frontal cortex > hippocampus > caudate putamen
> cerebellum > plasma > medulla oblongata > liver. The ratio of
the integrated area under the curve of concentration-time (AUC) for
nervous tissue:plasma ranged from 8.8 in sciatic nerve to 1.2 in
cerebellum but was only 0.44 for liver. The brain regions also showed
the higher AUC values for the metabolites, particularly
meta-phenoxybenzyl alcohol, for which the tissue:plasma ratio was
always > 1.0, whereas this ratio was usually < 0.5 for
meta-phenoxybenxoic acid (Anadón et al., 1991).
Chickens
In a study conducted according to GLP, groups of six laying hens
received capsules containing 1.27 mg of [U-14C-phenyl]permethrin or
[1-14C-cyclopropyl]permethrin, and two hens received a capsule
containing a placebo. The dose was given orally for 7 consecutive days
at a rate equivalent to a dietary intake of 11 ppm. Urine, faeces,
cage wash, and egg samples (separated into whites and yolks) were
collected daily and analysed for radiolabel. All hens were killed 16 h
after the final dose, and samples of breast and thigh muscle, skin and
subcutaneous fat, and the liver were collected at necropsy. The
samples were analysed for radiolabel with or without solubilization or
combustion, followed by liquid scintillation counting.
A mean of 92% of the total dose was excreted (urine, faeces, cage
wash) by day 7 after administration of [14C-cyclopropyl]permethrin,
and a mean of 90% after administration of [14C-phenyl]permethrin.
The mean total was 0.2% in eggs and 0.1% in liver for both groups. The
overall recovery of radiolabel was 93% from the group given the
cyclopropyl abel and 90% from that given phenyl label. At day 6, the
residues in egg yolk reached a maximum mean concentration of 0.27 ppm
with the cyclopropyl label and 0.28 ppm with the phenyl label.
Unchanged permethrin accounted for about 50% of these residues in both
groups. A number of other metabolites were also present in the yolk,
at concentrations of < 0.001 to 0.01 ppm. One of these, present at
about 0.01 ppm, was identified as 4'-hydroxypermethrin. The
concentrations in the egg whites were much lower, ranging from 0.001
to 0.02 ppm for both groups. Unchanged permethrin accounted for about
half of the cyclopropyl-labelled residues, in addition to several
other components, including trans-dichlorovinyl acid (0.002 ppm).
The egg whites from the hens given the phenyl label contained <
0.01 ppm and were therefore not analysed. The concentrations of
radiolabelled residues in breast and leg muscle were 0.01-0.03 ppm.
The breast muscle samples were not analysed further. An average of 47%
of the radiolabel was extractable from leg muscle for both groups.
Permethrin was the major component, at 0.008 ppm, accounting for an
average of 32% in the two groups. Leg muscle from both groups also
contained two metabolites at concentrations of 0.001-0.002 ppm, and
muscle from hens dosed with cyclopropyl label contained one metabolite
at 0.001 ppm (Table 6).
The concentrations of residues in peritoneal fat were 0.37 ppm
for the cyclpropyl label and 0.31 ppm for the phenyl label. Unchanged
permethrin represented about 81% of the total fat residue with both
labels (0.29 ppm for the cyclopropyl and 0.24 ppm for the phenyl
label). The only other extractable component that contained the intact
ester linkage was present at a concentration of about 0.02 ppm and
co-chromatographed with hydroxypermethrin, but it could not be
identified unambiguouly. The total concentrations of radiolabelled
residues in subcutaneous fat (including skin) were 0.18 ppm for the
cyclopropyl label and 0.16 ppm for the phenyl label (Table 6).
The concentrations of residues in the liver were 0.17 ppm for the
cyclopropyl label and 0.29 ppm for the phenyl label. No significant
amounts of unchanged permethrin were present in liver extracts, and no
metabolites containing the intact ester linkage could be
characterized. trans- and cis-dichlorovinyl acid were present at
concentrations of 0.013 and 0.009 ppm, respectively, in livers from
cyclopropyl-treated hens. The corresponding hydrolysis product from
the phenyl label, phenoxybenzoic acid, was not detected. Covalent
binding of radiolabelled residues to tissue protein was substantial,
accounting for 36% (0.057 ppm) of the cyclopropyl-derived and 52%
(0.14 ppm) of the phenyl-derived residues. With both radiolabels, most
of the extractable components were associated with very polar,
uncharacterized material (Table 6).
Analysis of excreta showed that permethrin was extensively
metabolized in hens, yielding many polar components. The excreta
contained metabolites resulting from hydrolysis of the central ester
linkage and from hydroxylation of a geminal dimethyl group attached to
the cyclopropane ring and at the 4' position of the phenoxybenzyl
moiety. Some of the resulting metabolites were further conjugated with
glucuronic acid or sulfate. trans-Dichlorovinyl acid, the
cyclopropyl carboxylic acid resulting directly from hydrolysis of
permethrin, was the major cyclopropyl-labelled metabolite in excreta.
Permethrin was the major component in hens given phenyl-labelled
material (Table 7).
Table 6. Distribution of extractable 14C-labelled residues in tissues of hens
Metabolite Leg muscle Peritoneal fat Liver
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
trans-Dichlorovinyl acid ND ND NA NA ND ND NA NA 8.2 0.013 NA NA
cis-Dichlorovinyl acid ND ND NA NA ND ND NA NA 5.6 0.009 NA NA
Permethrin 31 0.008 34 0.008 78 0.29 77 0.24 ND ND ND ND
Others 19 0.005 10 0.002 5.4a 0.020 6.5a 0.020 73 0.12 66 0.18
Total extractable 49 0.013 44 0.010 84 0.31 84 0.26 86 0.14 66 0.18
ND, not detected; NA, not applicable
a This component corresponds to the retention time of hydroxypermethrin, but low concentrations prevented positive
identification.
Table 7. Distribution of extractable 14C-labelled residues in
excreta of hens
Metabolite Percentage of administered dose
Cyclopropyl label Phenyl label
trans-Dichlorovinyl acid 19 ND
cis-Dichlorovinyl acid 2.2 ND
4'-Hydroxy-hydroxypermethrin 1.9 2.2
4'-Hydroxypermethrin 2.1 0.8
3-Phenoxybenzyl alcohol ND 0.4
Permethrin 16 35
Others 0.3 1.9
Unknown (several polar)a 48 31
Total 89 70
a Fourteen or more unknown metabolites were found.
Permethrin was thus extensively metabolized in hens, resulting in
a large number of metabolites, many of which were polar. The
identified metabolites were formed through hydrolysis of the ester
linkage, hydroxylation of a geminal dimethyl group attached to the
cyclopropane ring, and hydroxylation at the 4' position of the
phenoxybenzyl moiety. Some of the metabolites were further conjugated
with glucuronic acid and sulfate (Hawkins et al., 1992a). Figure 2
shows the proposed metabolic pathway of permethrin in hens.
Lactating goats
In a study conducted according to GLP, two lactating goats
received oral doses of permethrin labelled at [U-14C-phenyl] or
[1-14C-cyclopropyl] for four consecutive days at a nominal rate of
55 ppm in the diet. The goat given the cyclopropyl label received
capsules containing a mean of 102 mg, and that given the phenyl label
received capsules containing a mean of 122 mg. Urine, faeces, and cage
wash were collected daily and milk was collected twice daily. Blood
samples were taken 16 h after the final dose, before slaughter, and
samples of liver, kidney, bile, omasum, abomasum and contents,
intestines and contents, foreleg and rump muscle, and subcutaneous,
omental, and perirenal fat were taken after slaughter. The samples
were analysed for radiolabel by liquid scintillation counting or by
combustion followed by liquid scintillation counting.
 Excretion in urine, faeces, and cage wash accounted for 66% of
the dose after administration of [14C-cyclopropyl]permethrin and 80%
of the dose after administration of [14C-phenyl]permethrin. The
overall recovery was 76% and 81%, respectively. The distribution of
radiolabel as a percentage of the administered dose is given in Table
8, and the total concentrations in tissues are shown in Table 9. The
concentrations of total radiolabelled residues were 0.06 ppm and 0.15
ppm in subcutaneous fat, 0.10 ppm and 0.24 ppm in perirenal fat, and
0.07 ppm and 0.17 ppm in omental fat with the cyclopropyl and phenyl
labels, respectively. In the fat from the phenyl label-treated goat,
extractable radiolabel represented 89% of the total residue and was
associated almost entirely with permethrin. The total concentrations
in muscle were 0.04 ppm and 0.02 ppm with the two labels,
respectively. The extractable portion from the muscle of the goat
given the cyclopropyl label represented 78% and contained three to
four components, including polar material. The extractable portion
from muscle of the goat given the phenyl label represented 55% and
contained two additional components not found with the cyclopropyl
label, one of which co-chromatographed with permethrin.
Table 8. Distribution of 14C-labelled residues in lactating goats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Urine 33 48
Cage wash 0.9 2.8
Faeces 32 29
Milk 0.4 0.5
Liver 0.33 0.17
Kidney 0.04 0.02
Gastrointestinal tract
and contentsa 9.4 Not measured
Total 76 81
a Intestines, omasum, and abomasum only
Table 9. Average distribution of 14C-labelled residues in
goat tissues and milk
Tissue Concentration (µg/g)
Cyclopropyl label Phenyl label
Milka 0.14-0.17 0.24-0.41
Fat
Omental 0.07 0.17
Perirenal 0.10 0.24
Subcutaneous 0.06 0.15
Kidney 1.0 0.78
Liver 1.2 0.91
Muscle (leg and rump) 0.04 0.02
Bileb 9.2 15
Plasmab 0.56 0.19
Whole blood 0.34 0.14
a Mean daily concentration between days 1 and 7
b µg equivalent permethrin per ml
The mean daily residue concentrations in milk, liver, and kidney
samples (Table 10) were 0.14-0.17 ppm with the cyclopropyl label and
0.24-0.41 ppm with the phenyl label. Permethrin was the major
component of the milk extract, accounting for 46% (0.06 ppm) and 56%
(0.17 ppm), respectively. Hydroxypermethrin was identified, at
concentrations of 8.1% (0.011 ppm and) and 2.6% (0.008 ppm),
respectively. Thin-layer chromatography also resolved at least five
unknown components which accounted for 2.6-11% of the total
radiolabelled residues and were common to both goats, indicating that
these components contain an intact ester linkage.
The residue concentrations in liver were 1.18 ppm with the
cyclopropyl label and 0.91 ppm with the phenyl label. The extraction
procedure released approximately 82% and 89% of the radioactive
residues, respectively. Aliquots of the concentrated extract were
analysed by thin-layer and high-performance liquid chromatography and
found to contain at least 13 components. Four identified from the
cyclopropyl-labelled samples were trans-dichlorovinyl acid (9.1%;
0.11 ppm), cis-dichlorovinyl acid (7.0%; 0.083 ppm),
hydroxydichlorovinyl acid (11%; 0.13 ppm), and dichlorovinyl acid
lactone (1%; 0.012 ppm). Two components were identified from the
phenyl-labelled samples as 3-phenoxybenzoic acid (12%; 0.11 ppm) and
4'-hydroxy-3-phenoxybenzoic acid (11%; 0.097 ppm). Additional studies
were conducted on the liver samples (Benner, 1997; Benner et al.,
1996; Skidmore, 1996) to determine if the parent molecule accounted
for a portion of the unknown components seen with the cyclopropyl
Table 10. Distribution of 14C-labelled residues of permethrin in liver, kidney, and milk from lactating goats
Metabolite Liver Kidney Milk
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
Permethrin - - - - - - - - 46 0.06 56
Hydroxypermethrin - - - - - - - - 8.1 0.011 2.6
cis- and trans-Dichlorovinyl acid 16 0.19 - - 26 0.27 - - - - -
Dichlorovinyl acid lactone 1.0 0.012 - - 0.6 0.006 - - - - -
Hydroxydichlorovinyl acid 11 0.13 - - 10 0.11 - - - - -
trans-Dichlorovinyl acid glucuronide - - - - 22 0.23 - - - - -
3-Phenoxybenzoic alcohol 28 0.28
3-Phenoxybenzoic acid - - 7.4 0.07 - - 57 0.44 - - -
4'-Hydroxy-3-phenoxybenzoic alcohol 5.5 0.05
4'-Hydroxy-3-phenoxybenzoic acid - - 3.2 0.03 - - - - - - -
Identified 28 0.34 45 0.43 59 0.61 57 0.44 55 0.071 58
Unknowna 61 34 26 30 30 25
Total 90 79 85 87 84 83
a The unknown is the sum of several components.
label (21%; 0.24 ppm) and the phenyl label (18%; 0.17 ppm). The
unknown component with the cyclopropyl label in liver consisted of a
mixture of several compounds, none of which represented > 5.2% of the
total residue. The results of the reanalysis of the components found
with the phenyl label liver showed that the intact ester linkage of
the parent molecule was not present. The unknown component was
identified as non-polar, base-labile conjugates of 3-phenoxybenzoic
alcohol and 4'-hydroxy-3phenoxybenzoic alcohol. The components were
3-phenoxybenzoic alcohol (28%; 0.28 ppm), 3-phenoxybenzoic acid (7.4%;
0.07 ppm), 4'-hydroxy-3-phenoxybenzoic alcohol (5.5%; 0.05 ppm), and
4'-hydroxy-3-phenoxybenzoic acid (3.2%; 0.03 ppm; Table 10).
The residue concentrations in the kidney were 1.0 ppm for the
cyclopropyl label and 0.78 ppm for the phenyl label. The extraction
procedure released approximately 89% and 93% of the radioactive
residues, respectively. Five components were identified in the
cyclopropyl-labelled samples as trans-dichlorovinyl acid (24%; 0.25
ppm), cis-dichlorovinyl acid (2%; 0.021 ppm), hydroxydichlorovinyl
acid (10%; 0.108 ppm), dichlorovinyl acid lactone (0.6%; 0.006 ppm),
and trans-dichlorovinyl acid glucuronide (22%; 0.23 ppm). One major
component of the phenyl-labelled sample was identified as
3-phenoxybenzoic acid (57%; 0.44 ppm; Table 10; Hawkins et al.,
1992b).
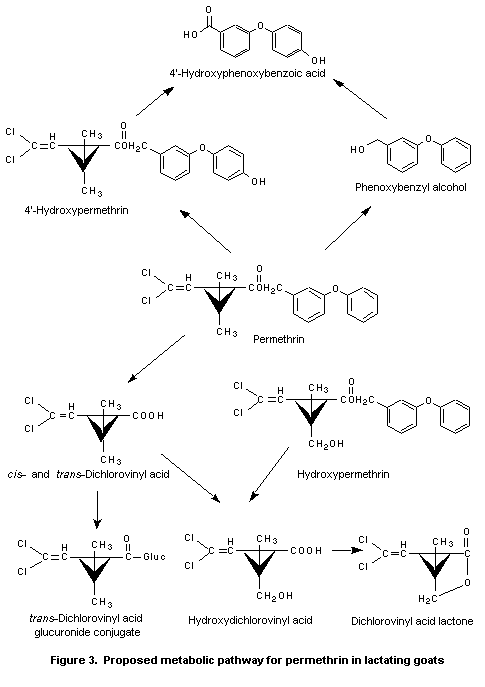
Figure 3 gives the proposed metabolic pathway of permethrin in
lactating goats.
Lactating cows
Four lactating Jersey cows were given three oral doses at 24-h
intervals of about 1 mg/kg bw of [14C] trans- or cis-permethrin
labelled in either the alcohol or the acid moiety. The concentration
of total radiolabel in blood reached a transient peak shortly after
each dose and was maximal after the third dose; it then decreased to
an insignificant level within 2-4 days. Higher blood concentrations
were attained with [14C] trans-permethrin labelled in the acid
moiety than with that labelled in the alcohol moiety. A similar
difference was not seen for cis-permethrin. The radiolabel was
eliminated mainly in the faeces and urine within 12 or 13 days.
Regardless of the position of the label, the trans isomer and its
metabolites were eliminated more rapidly than the cis isomer and its
metabolites. Urinary excretion accounted for about 43% of the
eliminated trans isomer and about 25% of the cis isomer. None of
the radiolabelled preparations resulted in detectable [14C]carbon
dioxide or significant residues in any tissues other than fat and
liver. Although low, the residue concentrations were highest in fat
and were higher after administration of the cis isomer (acid, 1.6%;
alcohol, 0.64%) than the trans isomer (acid, 0.15%; alcohol, 0.40%).
The radiolabel excreted in milk represented < 0.5% of the dose. The
lowest concentration in milk was found with trans-permethrin
labelled in the acid moiety (0.03%) and the highest with
trans-permethrin labelled in the alcohol moiety (0.44%). With all
four labelled preparations, the concentrations of radiolabel in milk
Excretion in urine, faeces, and cage wash accounted for 66% of
the dose after administration of [14C-cyclopropyl]permethrin and 80%
of the dose after administration of [14C-phenyl]permethrin. The
overall recovery was 76% and 81%, respectively. The distribution of
radiolabel as a percentage of the administered dose is given in Table
8, and the total concentrations in tissues are shown in Table 9. The
concentrations of total radiolabelled residues were 0.06 ppm and 0.15
ppm in subcutaneous fat, 0.10 ppm and 0.24 ppm in perirenal fat, and
0.07 ppm and 0.17 ppm in omental fat with the cyclopropyl and phenyl
labels, respectively. In the fat from the phenyl label-treated goat,
extractable radiolabel represented 89% of the total residue and was
associated almost entirely with permethrin. The total concentrations
in muscle were 0.04 ppm and 0.02 ppm with the two labels,
respectively. The extractable portion from the muscle of the goat
given the cyclopropyl label represented 78% and contained three to
four components, including polar material. The extractable portion
from muscle of the goat given the phenyl label represented 55% and
contained two additional components not found with the cyclopropyl
label, one of which co-chromatographed with permethrin.
Table 8. Distribution of 14C-labelled residues in lactating goats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Urine 33 48
Cage wash 0.9 2.8
Faeces 32 29
Milk 0.4 0.5
Liver 0.33 0.17
Kidney 0.04 0.02
Gastrointestinal tract
and contentsa 9.4 Not measured
Total 76 81
a Intestines, omasum, and abomasum only
Table 9. Average distribution of 14C-labelled residues in
goat tissues and milk
Tissue Concentration (µg/g)
Cyclopropyl label Phenyl label
Milka 0.14-0.17 0.24-0.41
Fat
Omental 0.07 0.17
Perirenal 0.10 0.24
Subcutaneous 0.06 0.15
Kidney 1.0 0.78
Liver 1.2 0.91
Muscle (leg and rump) 0.04 0.02
Bileb 9.2 15
Plasmab 0.56 0.19
Whole blood 0.34 0.14
a Mean daily concentration between days 1 and 7
b µg equivalent permethrin per ml
The mean daily residue concentrations in milk, liver, and kidney
samples (Table 10) were 0.14-0.17 ppm with the cyclopropyl label and
0.24-0.41 ppm with the phenyl label. Permethrin was the major
component of the milk extract, accounting for 46% (0.06 ppm) and 56%
(0.17 ppm), respectively. Hydroxypermethrin was identified, at
concentrations of 8.1% (0.011 ppm and) and 2.6% (0.008 ppm),
respectively. Thin-layer chromatography also resolved at least five
unknown components which accounted for 2.6-11% of the total
radiolabelled residues and were common to both goats, indicating that
these components contain an intact ester linkage.
The residue concentrations in liver were 1.18 ppm with the
cyclopropyl label and 0.91 ppm with the phenyl label. The extraction
procedure released approximately 82% and 89% of the radioactive
residues, respectively. Aliquots of the concentrated extract were
analysed by thin-layer and high-performance liquid chromatography and
found to contain at least 13 components. Four identified from the
cyclopropyl-labelled samples were trans-dichlorovinyl acid (9.1%;
0.11 ppm), cis-dichlorovinyl acid (7.0%; 0.083 ppm),
hydroxydichlorovinyl acid (11%; 0.13 ppm), and dichlorovinyl acid
lactone (1%; 0.012 ppm). Two components were identified from the
phenyl-labelled samples as 3-phenoxybenzoic acid (12%; 0.11 ppm) and
4'-hydroxy-3-phenoxybenzoic acid (11%; 0.097 ppm). Additional studies
were conducted on the liver samples (Benner, 1997; Benner et al.,
1996; Skidmore, 1996) to determine if the parent molecule accounted
for a portion of the unknown components seen with the cyclopropyl
Table 10. Distribution of 14C-labelled residues of permethrin in liver, kidney, and milk from lactating goats
Metabolite Liver Kidney Milk
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
Permethrin - - - - - - - - 46 0.06 56
Hydroxypermethrin - - - - - - - - 8.1 0.011 2.6
cis- and trans-Dichlorovinyl acid 16 0.19 - - 26 0.27 - - - - -
Dichlorovinyl acid lactone 1.0 0.012 - - 0.6 0.006 - - - - -
Hydroxydichlorovinyl acid 11 0.13 - - 10 0.11 - - - - -
trans-Dichlorovinyl acid glucuronide - - - - 22 0.23 - - - - -
3-Phenoxybenzoic alcohol 28 0.28
3-Phenoxybenzoic acid - - 7.4 0.07 - - 57 0.44 - - -
4'-Hydroxy-3-phenoxybenzoic alcohol 5.5 0.05
4'-Hydroxy-3-phenoxybenzoic acid - - 3.2 0.03 - - - - - - -
Identified 28 0.34 45 0.43 59 0.61 57 0.44 55 0.071 58
Unknowna 61 34 26 30 30 25
Total 90 79 85 87 84 83
a The unknown is the sum of several components.
label (21%; 0.24 ppm) and the phenyl label (18%; 0.17 ppm). The
unknown component with the cyclopropyl label in liver consisted of a
mixture of several compounds, none of which represented > 5.2% of the
total residue. The results of the reanalysis of the components found
with the phenyl label liver showed that the intact ester linkage of
the parent molecule was not present. The unknown component was
identified as non-polar, base-labile conjugates of 3-phenoxybenzoic
alcohol and 4'-hydroxy-3phenoxybenzoic alcohol. The components were
3-phenoxybenzoic alcohol (28%; 0.28 ppm), 3-phenoxybenzoic acid (7.4%;
0.07 ppm), 4'-hydroxy-3-phenoxybenzoic alcohol (5.5%; 0.05 ppm), and
4'-hydroxy-3-phenoxybenzoic acid (3.2%; 0.03 ppm; Table 10).
The residue concentrations in the kidney were 1.0 ppm for the
cyclopropyl label and 0.78 ppm for the phenyl label. The extraction
procedure released approximately 89% and 93% of the radioactive
residues, respectively. Five components were identified in the
cyclopropyl-labelled samples as trans-dichlorovinyl acid (24%; 0.25
ppm), cis-dichlorovinyl acid (2%; 0.021 ppm), hydroxydichlorovinyl
acid (10%; 0.108 ppm), dichlorovinyl acid lactone (0.6%; 0.006 ppm),
and trans-dichlorovinyl acid glucuronide (22%; 0.23 ppm). One major
component of the phenyl-labelled sample was identified as
3-phenoxybenzoic acid (57%; 0.44 ppm; Table 10; Hawkins et al.,
1992b).
Figure 3 gives the proposed metabolic pathway of permethrin in
lactating goats.
Lactating cows
Four lactating Jersey cows were given three oral doses at 24-h
intervals of about 1 mg/kg bw of [14C] trans- or cis-permethrin
labelled in either the alcohol or the acid moiety. The concentration
of total radiolabel in blood reached a transient peak shortly after
each dose and was maximal after the third dose; it then decreased to
an insignificant level within 2-4 days. Higher blood concentrations
were attained with [14C] trans-permethrin labelled in the acid
moiety than with that labelled in the alcohol moiety. A similar
difference was not seen for cis-permethrin. The radiolabel was
eliminated mainly in the faeces and urine within 12 or 13 days.
Regardless of the position of the label, the trans isomer and its
metabolites were eliminated more rapidly than the cis isomer and its
metabolites. Urinary excretion accounted for about 43% of the
eliminated trans isomer and about 25% of the cis isomer. None of
the radiolabelled preparations resulted in detectable [14C]carbon
dioxide or significant residues in any tissues other than fat and
liver. Although low, the residue concentrations were highest in fat
and were higher after administration of the cis isomer (acid, 1.6%;
alcohol, 0.64%) than the trans isomer (acid, 0.15%; alcohol, 0.40%).
The radiolabel excreted in milk represented < 0.5% of the dose. The
lowest concentration in milk was found with trans-permethrin
labelled in the acid moiety (0.03%) and the highest with
trans-permethrin labelled in the alcohol moiety (0.44%). With all
four labelled preparations, the concentrations of radiolabel in milk
 decreased to < 100 µg/L within 2-4 days after treatment ceased. The
only compound recovered from milk after administration of the acid-
and alcohol-labelled trans isomer was permethrin, whereas with the
cis isomer 85% of the radiolabel was attached to the parent compound
and 15% to trans-hydroxy- cis-permethrin.
The metabolic reactions of permethrin in cows were similar to
those in rats and hens. In cows, the permethrin isomers, their mono-
and di-hydroxy derivatives, and 3-phenoxybenzyl alcohol appeared only
in the faeces, while the cis-hydroxy-chrysanthemic acid lactones
appeared in both faeces and urine. The remaining metabolites occurred
only in urine. Although a slightly larger proportion of cis- than
trans-permethrin was excreted unchanged, the amounts of ester
metabolites were similar, and these metabolites were hydroxylated at
the trans or cis methyl position of the geminal dimethyl group, at
the 4' position of the phenoxybenzyl group, or at both the geminal
dimethyl and phenoxybenzyl groups. The preferred hydroxylation site
for both isomers was the trans methyl group. The major metabolites
of the acid moieties of both isomers were the corresponding
cis-hydroxy-chrysanthemic acid and the corresponding lactone and the
chrysanthemic acid glucuronide, while
trans-hydroxy- cis-chrysanthemic acid was also a major metabolite
of cis-permethrin. The major metabolites of the alcohol moieties of
both isomers were 3-phenoxybenzoic acid-glycine conjugate (3-11% of
the dose), 3-phenoxybenzyl alcohol (8-10%), and 3-phenoxybenzoic
acid-glutamic acid conjugate (12-28%) (Gaughan et al., 1978).
Humans
Two volunteers who ingested about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the dose as
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid in
urine collected over 24 h (Cridland & Weatherley, 1977a,b).
In a study of approximately 350 people who were dusted against
body lice with 30-50 g of powder containing 2.5 or 5.0 g/kg permethrin
(cis:trans ratio, 25:75), the mean amount of permethrin absorbed
during the first 24 h after treatment was estimated to be 14 µg/kg bw
in 19 subjects exposed to powder containing 2.5 g/kg permethrin and 39
µg/kg bw in 15 subjects exposed to 5.0 g/kg. No residue was found in
samples of urine taken 30 and 60 days after treatment (Nassif et al.,
1980).
Four of five workers in Sweden who packed conifer seedlings for
6 h in a tunnel that had been sprayed 1 h earlier with a 2% aqueous
solution of permethrin, resulting in atmospheric concentrations of
0.011-0.085 mg/m3 in the breathing zone, did not excrete detectable
amounts of acid permethrin metabolites in the urine. One very short
person whose face was close to the plants and who had the highest
exposure to permethrin in his breathing zone excreted 0.26 µg/ml
permethrin acid metabolites in his urine the following morning; in the
afternoon, the concentration was below the detection limit of the
method (Kolmodin-Hedman et al., 1982).
Ten patients (five men and five women) with scabies received an
application of about 25 g (range, 21-32 g) of a 5% permethrin cream
over the skin of the whole body except the head and neck. Dermal
absorption of permethrin was calculated from the quantities of
conjugated and unconjugated cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
metabolites of permethrin in the urine. In samples of urine collected
by seven patients 1 and 2 days after application of the permethrin
cream, the mean totals of these metabolites were 410 and 440 µg,
respectively; the mean total in the urine of three patients who
collected urine in the same container for 2 days was 1400 µg. The
urinary concentration of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
varied during the first 48 h from 0.11 to 1.1 µg/ml and that of the
cis isomer from 0.02 to 0.21 µg/ml. This metabolite was still
detectable in the urine of three patients after 1 week and in the
urine of one patient, reported to be an alcoholic, after 2 weeks. The
absorption of permethrin over the first 48 h after application was
estimated from the amount of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
excreted in the urine to be 6 mg (range, 3-11 mg), i.e. 0.5% of the
dose applied (van der Rhee et al., 1989).
Exposure to permethrin was assessed in a survey of 45
professional users of insecticide products, with more than a 100-fold
difference between the average and the highest concentrations. Dermal
contamination was found on 93% of the operators, the greatest
contamination resulting from use of leaky equipment. High airborne
concentrations were linked with use in confined areas. Monitoring of
metabolites in urine showed that systemic uptake occurred, with
concentrations of specific metabolites ranging from none detected to
10 nmol/mmol creatinine of
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid,
19 nmol/mmol creatinine of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 46 nmol/mmol creatinine of 3-phenoxybenzoic acid (Llewellyn
et al., 1996).
The concentrations of permethrin and its metabolites,
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propane carboxylic acid,
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 3-phenoxybenzoic acid, were measured in the urine and plasma
of 30 pest-control workers exposed to pesticides. The concentrations
of permethrin in plasma were below the limit of detection (5 µg/L),
and the urinary concentrations ranged from < 0.5 µg/L to 280 µg/L
(Leng et al., 1997).
Urine samples collected over 24 h from eight pest-control workers
exposed to permethrin were analysed for permethrin metabolites. The
concentrations ranged from 1 to 70 µg/g creatinine (Angerer & Ritter,
1997).
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of orally administered permethrin
in mice and rats (Table 11) demonstrate that two factors that affect
its toxicity are the concentration of the cis isomer and the
vehicle. Permethrin with a cis:trans ratio of 80-100:20-0 is
approximately 7-24 times more toxic than permethrin in which the
cis:trans ratio is 10-25:90-75 when delivered in maize oil, and
permethrin administered in maize oil is four to seven times more toxic
than undiluted permethrin. The alterations in acute toxicity are,
however, greater than can be explained on the basis of the cis
isomer content alone. Thus, a change from a cis:trans ratio of 20:80
to 80:20 in maize oil changed the LD50 value in Wistar rats from
6000 mg/kg bw to 225 mg/kg bw, whereas an LD50 value of about 1000
mg/kg bw would have been expected for the 20:80 material. Frequently
observed clinical signs were convulsions on the day of dosing,
tremors, and hypersensitivity. In animals that died, the
gastrointestinal tract often contained a brown fluid. In male and
female Wistar rats exposed for 4 h to atmospheres containing
permethrin (cis:trans ratio, 40:60), the LC50 value was greater
than a nominal concentration of 24 mg/L (Braun & Killeen, 1976).
In male and female New Zealand white rabbits, the dermal LD50
values for cis:trans 55:45 and 40:60 permethrin were > 2000 mg/kg
bw (Braun & Killeen, 1975b; Sauer, 1980b).
(b) Short-term studies of toxicity
Mice
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 39%:56%; purity, 94.7%) was administered in the
diet to groups of 20 male and 20 female Alderley Park mice at
concentrations of 0, 80 (increased to 10 000 ppm in weeks 3 and 4),
200, 400, 1000, 2000, or 4000 ppm in the diet, equivalent to 0, 28,
56, 140, 280, and 560 mg/kg bw per day for 28 days. No deaths
occurred, and no significant clinical signs were observed. Reduced
body-weight gain and poor food utilization were observed in animals
receiving 10 000 ppm. At termination, necropsies were performed on
five mice of each sex from the control group and those given 2000 and
10 000 ppm. Liver weights and the liver:bodyweight ratios were
increased in mice at 2000 and 10 000 ppm, and an increase in the
incidence of eosinophilia of the centrilobular hepatocytes was seen in
all mice receiving 10 000 ppm and two of five female mice receiving
2000 ppm. The NOAEL was 1000 ppm, equivalent to 140 mg/kg bw per day,
on the basis of changes in liver weight at 2000 ppm (Clapp et al.,
1977a).
Table 11. Acute toxicity of orally administered permethrin
Species Strain Sex Vehicle Purity cis:trans LD50 95% CI Reference
(%) ratio (mg/kg bw)
Mouse CF-1 M&F Maize oil 100 10:90 1700 1200-2300 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 95.5 25:75 960 680-1200 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 99.5 40:60 650 420-880 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 100 100:0 230 200-260 Marowitz (1974a)
Rat SD M None 55:45 3600 2400-5200 Sauer (1980c)
F 2300 1800-2900
Rat SD (CD) M Maize oil 41.3: 58.7 1000 880-1200 Cummins & Gardner
F 860 680-1000 (1984a)
Rat SD (CD) M Maize oil 81.1: 18.9 370 280-460 Cummins & Gardner
F 320 240-410 (1984b)
Rat Wistar M&F Maize oil 40:60 1200 860-1500 Braun & Killeen (1975a)
Rat Wistar M&F None 40:60 8900 6000-12 000 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 40:60 1200 1100-1400 Braun & Killeen (1975a)
Rat Long-Evans M&F None 40:60 6000 4200-7800 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 95.5 25:75 1600 1300-2000 Marowitz (1974b)
Rat Wistar F Maize oil 20:80 6000 - Wallwork et al. (1975)
Rat Wistar F Maize oil 30:70 1700 1300-2200 Wallwork et al. (1975)
Rat Wistar F Maize oil 40:60 1300 1000-1600 Wallwork et al. (1975)
Rat Wistar F Maize oil 50:50 1000 730-1400 Wallwork et al. (1975)
Rat Wistar F Maize oil 60:40 440 400-500 Wallwork et al. (1975)
Rat Wistar F Maize oil 80:20 220 200-250 Wallwork et al. (1975)
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 38%:52%; purity, 90.5%) was administered in the
diet to groups of eight Wistar Alderley Park rats of each sex at
concentrations of 0, 200, 500, 1000, 2500, 5000, or 10 000 ppm,
equivalent to 0, 20, 50, 100, 250, 500, and 1000 mg/kg bw per day, for
28 days. All rats receiving 10 000 ppm died within the first 3 days of
the study, and five rats at 5000 ppm had died by day 18. Before death,
the rats showed whole-body tremors, hyperactivity, and piloerection.
The surviving rats receiving 5000 ppm had tremors, hypersensitivity,
piloerection, and urinary incontinence. Rats receiving 2500 ppm had
tremors and piloerection during the first week of treatment and were
hypersensitive throughout the study. Rats receiving 1000 ppm showed
slight tremors on the first day only. No clinical signs were seen
among rats receiving 500 or 200 ppm. Rats receiving 5000 ppm had
decreased body weights, body-weight gains, and food consumption when
compared with controls, while females receiving 2500 ppm had
significantly increased food consumption. Urinary protein excretion
was depresssed in males receiving 5000 ppm. Lymphocytosis was observed
in male rats at doses > 2500 ppm. At the end of the study, the
liver weights and the liver:body weight ratios of rats receiving doses
> 2500 ppm were increased. Histological examination of the livers
showed no remarkable changes. The NOAEL was 500 ppm, equivalent to 50
mg/kg bw per day, on the basis of tremors at 1000 ppm (Clapp et al.,
1977b).
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) wase administered in the diet to groups of six Long
Evans rats of each sex in the diet at concentrations of 0, 2500, 3500,
5000, or 7100 ppm, equal to 0, 250, 350, 5300, and 800 mg/kg bw per
day for males and 0, 270, 380, 550, and 820 mg/kg bw per day for
females, for four weeks. One male and one female receiving the highest
dose died during the first week of treatment. Tremors, the severity of
which was dose-dependent, were observed in most rats receiving doses
> 3500 ppm during the first week of treatment but were subsequently
seen only in animals receiving 7100 ppm and were generally less
severe. Staining of the abdominal fur was also seen in rats at doses
> 3500 ppm, with increasing incidences in weeks 1-4. Decreased body
weight was observed in both males and females treated with doses
> 3500 ppm during the first 2 weeks of treatment. This effect
persisted during weeks 3 and 4 only in females at 7100 ppm. No
histological examination was performed in this preliminary experiment.
The NOAEL was 2500 ppm, equal to 250 mg/kg bw per day, on the basis of
clinical signs and body-weight changes at 3500 ppm (Killeen & Rapp,
1974).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to groups of six male and six female Long
Evans rats in the diet at concentrations of 625, 1250, 2500, 5000, or
7500 ppm for 30 days, equal to 0, 59, 120, 250, and 630 mg/kg bw per
day for males and 0, 69, 130, 280, and 660 mg/kg bw per day for
females: no data on consumption of the compound were available for the
highest concentration. All females and three males receiving 7500 ppm
died within 24 h of the start of treatment, and the remaining three
males in this group had died by the end of the first week. Five of six
males and five of six females at 5000 ppm died during the first week,
but the remaining animals survived the duration of the study. In rats
receiving 2500 ppm, slight-to-moderate tremors and staining of the
anogenital fur were noted throughout most of the study. The surviving
male and female at 5000 ppm showed moderate-to-severe tremors and
staining of the fur in the anogenital region throughout the study. The
body weights of males at 2500 ppm were reduced. No histological
examination was performed in this preliminary experiment. There were
no signs of toxicity at 625 and 1250 ppm. The NOAEL was 1250 ppm,
equal to 120 mg/kg bw per day, on the basis of clinical signs and
body-weight changes at 2500 ppm (Killeen & Rapp, 1975a).
Technical-grade permethrin (cis:trans ratio unstated; purity,
96%) was administered in the diet to groups of six male and six female
Charles River rats (strain not stated) at concentrations of 0 (14
animals of each sex), 30, 100, 300, 1000, or 3000 ppm, equivalent to
0, 3, 10, 30, 100, and 300 mg/kg bw per day, for five weeks. Most of
the observations were restricted to the group receiving 3000 ppm. In
this group, persistent tremors were observed in all males and four
females, body-weight gain was reduced in females by about 7%, the
liver weights adjusted for terminal body weight were increased in
males (15%) and females (13%), and the relative heart weight was
increased in males (8%). Increased prothrombin time and plasma urea
were seen in males and decreased plasma protein in females. Males at
1000 ppm showed an increase in relative liver weight (15%), and
(probably chance) increases in prothrombin time were seen in females
at 30 and 300 ppm. Histological examination revealed renal tubular
mineralization and hydronephrosis as common lesions which were evenly
distributed among the treated groups. No dose-related increase in the
incidence of lesions was found. The NOAEL was 300 ppm, equivalent to
30 mg/kg bw per day, on the basis of increased liver weight at 1000
ppm (Butterworth & Hend, 1976).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to groups of 18 Wistar rats of
each sex at concentrations of 0, 60, 200, 600, or 2000 ppm, equivalent
to 0, 6, 20, 60, and 200 mg/kg bw per day, for 90 days. At that time,
10 rats of each sex per group were killed, and the remaining rats were
maintained without further treatment for an additional 29 days. No
treatment-related deaths occurred, and no treatment-related or
toxicologically significant clinical signs, changes in body weights,
food consumption, or estrus cycle, urinary findings, or blood
anomalies were noted. The absolute and relative weights of the spleen
and lungs were increased in male rats at 2000 ppm, and the relative
and absolute weights of the adrenal glands of female rats at this dose
showed statistically significant decreases. No consistent dose-related
pattern of toxicity was seen. The fat content of the renal cortex was
slightly increased in male rats receiving 600 or 2000 ppm, but this
was not accompanied by morphological alterations. The NOAEL was 600
ppm, equivalent to 60 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm (Williams et al., 1976a).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to 18 Wistar rats of each sex at
concentrations of 0, 200, 600, 2000, or 4000 ppm, equivalent to 0, 20,
60, 200, and 400 mg/kg bw per day, for 90 days. At that time, 10 rats
of each sex per group were killed, and the remainder were maintained
with no further treatment for an additional 36 days. No
treatment-related deaths occurred. The only significant clinical sign
was hypersensitivity in male and female rats receiving 4000 ppm. By
week 3, hypersensitivity was no longer observed in male rats but
continued in the female rats throughout treatment, although the
symptoms disappeared after 3 days without dosing. Males receiving 4000
ppm had decreased body-weight gain, which improved during the recovery
period. A slight-to-moderate decrease in leukocyte count was seen
during the early stages of the study in rats at this dose, but the
values had returned to within the normal range by 90 days. The weight
of the liver showed a slight but significant increase at 90 days but
had returned to normal by the end of the recovery period. The absolute
and relative weights of the thyroids of rats receiving the high dose
showed a slight decrease at 90 days, but no histopathological changes
were found. The NOAEL was 2000 ppm, equivalent to 200 mg/kg bw per
day, on the basis of of hypersensitivity, reduced body weight,
transient leukopenia, and increased liver weights at 4000 ppm
(Williams et al., 1976b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was administered in the
diet to groups of 30 Long Evans rats of each sex at concentrations of
0, 50, 75, 100, or 500 ppm, equivalent to 5, 7.5, 10, and 50 mg/kg bw
per day, for 90 days. There were no significant differences in body
weight, food consumption, or haematological, clinical chemical, or
urinary parameters. At the end of the study, the relative weight of
the liver was increased in male rats at 500 ppm in comparison with
controls, while that of female rats at this dose was significantly
decreased. There was no correlation with clinical or histopathological
findings. No treatment-related histopathological findings were made.
The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis
of increased relative liver weight at 500 ppm (Becci & Parent, 1980).
In a study conducted according to GLP, three batches of
technical-grade permethrin (cis:trans ratio, 36.1%:61.1% to
38.5%:56.2%; purity, 94.1-97.2%) were given in the diet to groups of
eight Wistar-derived rats of each sex at concentrations of 0, 20, 100,
or 1000 ppm, equivalent to 2, 10, and 100 mg/kg bw per day, for 26
weeks, when the rats were killed. The body weight and body-weight gain
of female rats receiving 20 and 100 ppm were similar to those of the
control animals, but females at 1000 ppm gained less weight throughout
treatment, as did males at doses > 100 ppm. There was no overall
effect on food consumption. Animals at 1000 ppm showed statistically
significant increases in liver weight, hepatic aminopyrine
N-demethylase activity, cytochrome P450 content, and smooth
endoplasmic reticulum content. Those at 100 ppm had only slight
hepatic changes, none of the untransformed values being significantly
greater than control levels, although the logarithm of the values for
aminopyrine N-demethylase activity attained significance. The NOAEL
was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
increased liver weight at 1000 ppm, as the changes in aminopyrine
N-demethylase activity were considered not toxicologically relevant
(Hart et al., 1977a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to groups
of 20 Sprague-Dawley rats of each sex by inhalation as an aerosol for
6 h per day, 5 days per week for 13 weeks at concentrations of 0, 125,
250, or 500 mg/m3. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm . At the end of
the 13-week exposure, 10 rats of each sex per group were maintained
without further treatment for an additional 90 days. There were no
treatment-related deaths. Severe tremors and convulsions were
observed in rats at 500 mg/m3, but these signs were no longer seen
by the second week of exposure. Oxygen consumption was monitored
throughout the exposure in order to assess overall metabolic rate. No
differences from controls were found. Hexobarbital-induced sleep times
(220 mg/kg bw intraperitoneally) were reduced in males exposed to 500
mg/m3, indicating the induction of liver enzymes, but the effect was
no longer statistically significant 30 days after exposure or in rats
of the lower concentrations. Body weights and organ-to-body weight
ratios were unaffected. No gross or microscopic treatment-related
pathological changes were observed. The NOAEC was 250 mg/m3 on the
basis of tremors and convulsions at 500 mg/m3 (United States Army,
1978).
Guinea-pigs
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered to groups
of 10 male Hartley guinea-pigs by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol in order to
examine sensitization reactions. There were no treatment-related
deaths or clinical signs during exposure, and the body weight and
organ:body weight ratios were unaffected. No gross or microscopic
treatment-related pathological changes were observed. No sensitization
reaction was seen. The NOAEC was 500 mg/m3, the highest dose tested
(United States Army, 1978).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity. 93.6% and 95.0%) was applied intradermally to groups of
male guinea-pigs as 0.1 ml of a 0.1% (w/v) solution; each batch of
permethrin or 2,4-dinitrochlorobenzene (positive control) contained
one volume of propylene glycol and 29 volumes of saline. Ten
guinea-pigs were challenged with the 0.1% solution, and an additional
five in each group received no prior sensitization but were given a
challenge dose of each batch of test material or the positive control.
The reaction induced by the challenge dose of permethrin was no
greater than that observed in the sensitized animals. The challenge
dose of the positive control produced sensitization reactions in all
of the guinea-pigs in that group (Metker et al., 1977).
Technical-grade permethrin (cis:trans ratio, 25:75; purity not
stated) was tested for skin sensitization potential according to the
method of Magnusson and Kligman. Ten male guinea-pigs received
permethrin, and groups of five male guinea-pigs received the vehicle
or 2,4-dinitrochlorobenzene. All compounds were given as a 1% w/v
solution in corn oil and as a 1% w/v solution in Freund's complete
adjuvant. No reaction to permethrin was seen 5, 24, and 48 h after
dosing, whereas all guinea-pigs given the positive control showed
marked sensitization (Chesher & Malone, 1974).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered by
inhalation as an aerosol to groups of 10 male Hartley guinea-pigs at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol. No
sensitization reaction was seen in any of the guinea-pigs (United
States Army, 1978).
Rabbits
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95.0%) was applied daily to the shaved skin of
groups of eight New Zealand white rabbits for 21 days at doses of 0,
100, 320, or 1000 mg/kg bw per day under an occlusive dressing. Four
rabbits in each group received abrasion at the application site on the
first day. All rabbits were killed on day 10 after the last exposure.
No significant changes were noted in body weights during or after
exposure, and there were no significant changes in organ:body weight
ratios. Moderate irritation of the skin was observed, but the reaction
was not significantly different from controls by day 18. Mild
irritation was still present 10 days after exposure, although it
improved daily. No differences were found in clinical chemical
parameters. There were no treatment-related lesions in the skin or
other tissues or organs. The NOAEL was 1000 mg/kg bw per day, the
highest dose tested (Metker et al., 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio 55:45; purity not stated) was melted and applied
without dilution at a volume of 0.5 ml to the skin of six New Zealand
white rabbits on each of two intact and two abraded sites, for a total
of 2 ml per rabbit. Each site was scored for irritation by the method
of Draize 30-60 min after removal of the occlusive wrap and residual
permethrin and again 24 h and 72 h after dosing. Very slight erythema
was noted on all sites at 24 h, but all irritation had resolved by
72 h. The score for primary dermal irritation was 0.5/8.0, indicating
virtually no irritation (Sauer, 1980c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as a liquid to the abraded skin of 10 New Zealand
white rabbits at a dose of 2000 mg/kg bw and the site was covered with
an occlusive wrap for 24 h. When the wraps were removed, very slight
erythema was noted on three rabbits, and barely perceptible oedema was
seen on one other rabbit (Braun & Killeen, 1975b).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as an aqueous slurry to the skin of six New Zealand
white rabbits as a volume of 0.50 ml of 1 g/ml on an intact and an
abraded site, for a total of 1 ml per rabbit. The sites were covered
with an occlusive wrap for 24 h and scored by the Draize method 24 and
72 h after dosing. Very slight erythema was seen on three abraded and
three intact sites at 24 h, but all irritation had resolved by 72 h.
The score for primary dermal irritation was 0.25, indicating virtually
no irritation (Braun & Killeen, 1975c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was melted, and 0.1 ml
of undiluted material was instilled into the right eye of nine New
Zealand white rabbits. After approximately 20 s, the eyes of three
rabbits were flushed with water for 1 min, and irritation was scored
by the Draize method 24, 48, and 72 h and 4 and 7 days after dosing.
Slight conjunctival redness and chemosis were found in the majority of
the treated eyes, but all irritation had resolved within 4 days. The
maximum score was 7.3/110 for unwashed eyes and 4.0/110 for washed
eyes at 24 h, indicating no irritation (Sauer, 1980d).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was instilled as a liquid into the right eye of nine New
Zealand white rabbits at a volume of 0.10 ml. The eyes of three
rabbits were then flushed for 30 s with 100 ml of warm tap-water and
were scored 1, 24, 48, and 72 h and 4 and 7 days after dosing. Slight
conjunctival redness, chemosis, and discharge were seen in both washed
and unwashed eyes at 1 h. Stippling of the cornea was observed in two
unwashed eyes. All irritation had resolved within 48 h (Braun &
Killeen, 1975d).
Dogs
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to one male and one female beagle dog in
gelatine capsules for 14 days at doses of 0, 125, 250, or 500 mg/kg bw
per day. On the first day of treatment, emesis was observed in four
treated dogs, and thereafter the compound was administered twice daily
in divided doses. Tremors and ataxia were each seen once in the male
treated with 500 mg/kg bw per day during the first week of treatment.
No effect on body weight or food consumption was seen throughout the
study. The NOAEL was 250 mg/kg bw per day on the basis of clinical
signs at 500 mg/kg bw per day (Killeen & Rapp, 1975b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, 94.5%) was administered to groups
of four beagle dogs of each sex in gelatine capsules at doses of 0, 5,
50, or 500 mg/kg bw per day for at least 96 days. Tremors were
observed on isolated occasions in two males and three females at 500
mg/kg bw per day during the exposure. One dog that had tremors also
showed transient narcosis with nystagmus on a single occasion. No
effects attributable to treatment were seen on body weight, food
consumption, clinical chemical, haematological, or urinary parameters,
or histological appearance. The mean absolute and relative (to body
weight) weights of the liver were greater than those of controls in
animals at doses > 50 mg/kg bw per day, but there was no
histological correlate. The increases in absolute liver weights were
11% at 50 and 500 mg/kg bw per day in males and 12% at 500 mg/kg bw
per day in females and were statistically significant. The biological
significance of the effect in males at 50 mg/kg bw per day is obscure
in view of the lack of any additional effect at a dose 10-fold higher.
The NOAEL was 50 mg/kg bw per day on the basis of changes in liver
weight and neurotoxic signs at 500 mg/kg bw per day (Killeen & Rapp,
1976).
Technical-grade permethrin (cis:trans ratio, 54:46; purity,
93.4%) was administered in gelatine capsules to groups of six male and
six female beagle dogs at doses of 0, 5, 50, or 500 mg/kg bw per day
for 90 days. Owing to signs of severe toxicity which increased in
severity and duration, the high dose was lowered to 364 mg/kg bw per
day on day 9 of dosing. The most serious of these signs was seizures
lasting for up to 1 h and characterized by rapid eye twitching with
fasciculations, increased sensitivity to sound, and severe tremors.
Ataxia and aggressive behaviour were also seen in these animals. At
364 mg/kg bw per day, similar signs of toxicity were seen but of
lesser severity and shorter duration. The major signs of toxicity seen
in males at doses > 50 mg/kg bw per day and females at 364 mg/kg bw
per day included impaired gait, ataxia, tremor, muscle twitching,
involuntary limb movements, uncontrolled barking, panting, and
salivation. Tremors and muscle twitching were also seen in females at
50 mg/kg bw per day. No signs of toxicity were observed at the low
dose. Body-weight gain was significantly reduced in males at the high
dose, whereas the body weight and body-weight gain of females were
unaffected by treatment. Serum alkaline phosphatase activity was
increased at 3, 6 10, and 13 weeks among males at the high dose, and
the absolute and relative weights of the liver were increased in males
and females at this dose. Permethrin had no effect on food consumption
or on haematological, urinary, ophthalmological, or histological
parameters. The NOAEL was 5 mg/kg bw per day on the basis of clinical
signs at 50 mg/kg bw per day (Becci et al., 1980).
Technical-grade permethrin (cis:trans ratio, 25:75; purity,
94.5%) was administered in gelatin capsules to groups of four beagle
dogs of each sex at doses of 0, 10, 50, or 250 mg/kg bw per day for at
least 180 days. None of the dogs died during the study. Emesis was
seen in a few treated and control animals, but there were no
treatment-related signs of toxicity, no effect on body weight,
ophthalmological or electrocardiographic parameters, absolute organ
weights, or gross or histopathological appearance. Statistically
significant but sporadic changes in food intake (decreased in dogs at
50 mg/kg bw per day in week 11 and in those at 250 mg/kg bw per day in
weeks 12 and 22), some haematological parameters (decreased packed
cell volume on day 180 and decreased mean corpuscular volume on day 56
at 10 mg/kg bw per day; increased lymphocyte and neutrophil counts at
50 and 250 mg/kg bw per day on day 14), some clinical chemical
parameters (glucose, urea, and sodium concentrations), and increased
relative weights of the liver, heart, and kidneys (by < 17%) were
seen. None of these changes appeared to be related to dose or time and
were not severe enough to be of toxicological importance. Similar
significant changes in blood chemistry were occasionally observed in
the dogs before dosing. The NOAEL was 250 mg/kg bw per day, the
highest dose tested (Reynolds et al., 1978).
In a study conducted according to GLP, permethrin (cis:trans
ratio, 32.3%:60.2%; purity, 92.5%) was administered in corn oil in
gelatin capsules to four groups of six beagle dogs of each sex at
doses of 0, 5, 100, or 1000 mg/kg bw per day for 52 weeks. Body
weights and food consumption were measured, and the dogs were observed
for clinical and behavioural abnormalities. A variety of
haematological and biochemical parameters were measured at intervals
throughout the study. Clinical signs, including convulsions, muscle
tremor, and incoordination, were seen frequently in dogs at 1000 mg/kg
bw per day. The body weights of males at 1000 mg/kg bw per day and
females at doses > 100 mg/kg bw per day were reduced. The weight of
the liver of dogs treated with doses > 100 mg/kg bw was increased,
accompanied by hepatic cellular swelling, consistent with an observed
increase in plasma alkaline phosphatase activity. These findings were
considered to represent an adaptive response and not a toxicological
effect. The dose of 1000 mg/kg bw per day was overtly toxic, resulting
in neurological signs associated in some animals with poor clinical
condition. The NOAEL was 5 mg/kg bw per day on the basis of the
reduction in body weight at 100 mg/kg bw per day (Kalinowski et al.,
1982).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to four
groups of two beagle dogs of each sex by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter of < 1 µm. The dogs
were tested for pulmonary function before and after exposure, and
blood samples were taken weekly for measurements of blood chemistry
and haematology. There were no treatment-related deaths and no toxic
signs were observed. Body weights and organ:body weight ratios were
unaffected, and pulmonary function and blood chemical and
haematological parameters were unchanged. No gross or microscopic,
treatment-related pathological changes were observed. The NOAEC was
500 mg/m3, the highest dose tested (United States Army, 1978).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet to groups of
75 CD-1 mice of each sex for 24 months. Initially, the concentrations
in the diet were 0, 20, 100, and 500 ppm, equivalent to 0, 3, 15, and
75 mg/kg bw per day. The concentration of 500 ppm was changed to 5000
ppm, equivalent to 250 mg/kg bw per day at week 19, but was returned
to 500 ppm at week 21. In addition, at week 21 the concentration of
100 ppm was changed to 4000 ppm, equivalent to 200 mg/kg bw per day,
and the concentrations were 0, 20, 500, and 4000 ppm for the remainder
of the study. During week 65, a problem of animal identification
arose, which detracts from the value of the study; however, the
actions taken to eliminate these mice from the study were carefully
recorded and judged by the US Environmental Protection Agency to be
acceptable, and the study was allowed to continue to its scheduled
conclusion. The rates of death up to 24 months were 43/62 control
males, 41/60 males at the low dose, 46/60 males at the intermediate
dose, 61/67 males at the high dose, 20/50 female controls, 32/60
females at the low dose, 34/62 females at the intermediate dose, and
48/65 at the high dose. The body weight of males at 4000 ppm was
slightly decreased from week 21 to termination, but that of females
and the food consumption of both males and females were unaffected. No
clinical observations were made that correlated with treatment. Mean
haemoglobin, erythrocyte count, and blood glucose concentration were
decreased in animals of each sex at 4000ppm. The mean absolute and
relative (to body weight) weights of the liver were increased for
males receiving 500 ppm and for animals of each sex at 4000 ppm. The
latter showed a greater frequency of non-neoplastic changes including
mononuclear leukocyte infiltrates in some tissues and thrombosis of
the cardiac atrium. There was no significant change in the incidence
of any tumour, and, in particular, the incidence of bronchioalveolar
adenomas was not increased in animals of either sex. The incidences of
hepatomas and hepatocellular carcinomas were slightly increased among
male, but not female, mice (Table 12) (Hogan & Rinehart, 1977; Rapp,
1978).
In view of the problems with the first experiment, a second of
the same design but carried out according to GLP was conducted. The
concentrations in the diet were originally 0, 100, 500, and 2000 ppm,
equivalent to 15, 75, and 300 mg/kg bw per day, and were changed after
2 months to 0, 20, 500, and 2000 ppm, equivalent to 3, 75, and 130
mg/kg bw per day, for males and 20, 2500, and 5000 ppm, equivalent to
3, 380, and 750 mg/kg bw per day, for females. The incidences of
deaths up to 24 months were 55/75 male controls, 48/75 males at the
low dose, 49/75 males at the intermediate dose, 63/75 males at the
Table 12. Incidences of tumours of the lung and liver in mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. livers Neoplasm No. with
examined examined liver
Alveolar Alveolar Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 60 6 0 72 3 4 7
Low 56 7 0 68 1 0 1
Intermediate 53 4 0 68 9 5 14
High 67 5 0 71 4 7 11
Female Control 47 11 0 72 2 0 2
Low 59 8 1 69 1 0 1
Intermediate 60 7 1 67 4 0 4
High 59 9 1 69 1 0 1
From Hogan & Rinehart (1977); Rapp (1978)
high dose, 53/75 female controls, 42/75 females at the low dose, 52/75
females at the intermediate dose, and 53/75 females at the high dose.
The mortality rate among males at the high dose was therefore slightly
greater than that among the control animals. These males also had a
higher incidence than control males of yellow staining in the
anogenital area throughout the study. Treatment had no effect on body
weight, body-weight gain, or food consumption. The differential
leukocyte counts of females at 2500 and 5000 ppm and males at 2000 ppm
were slightly lower than control values, while the segmented
neutrophil counts of males at 2000 ppm were slightly higher than in
controls. A statistically significant decrease in the absolute and
organ:body weight ratio of the testis was seen at 2000 ppm, while
females at doses > 2500 ppm showed statistically significantly
increased absolute liver weights; the liver:body weight ratio was
statistically significant only in females at 5000 ppm.
A dose-dependent increase in the incidence of alveolar-cell
adenomas and carcinomas was found among female mice, and multiple lung
adenomas were often found in the same animal (Table 13). Hepatomas
also occurred more often in female mice at the intermediate and high
doses than controls and mice at the low dose. Hepatoma is considered
to be a spontaneous lesion which occurs in ageing and aged mice
without affecting the mortality rate. The incidence of hepatocellular
carcinoma was notably higher in males at the intermediate dose than in
other treated or control groups (Table 13). The type, incidence,
and/or degree of severity of other neoplasms and all non-neoplastic
histological changes were considered to represent spontaneous lesions
unrelated to treatment. Since the incidences in males and females at
the low dose were close to those in the control group, the increased
incidence of bronchioalveolar adenoma in female mice at the two higher
doses may have been related to treatmen. This conclusion is not,
however, supported by the results of the first study, which was
conducted in the same laboratory with the same strain of mouse, and
the same batch of permethrin and which partially overlapped with the
second one in time. The pulmonary adenomas were thus considered to
have no toxicological significance. The NOAEL for systemic toxicity
was 500 ppm, equivalent to 75 mg/kg bw per day, on the basis of
changes in organ weights at 2000 ppm (Tierney & Rinehart, 1979).
In a study conducted according to GLP, eight batches of
technical-grade permethrin (cis:trans ratios, 44:55 to 36.2:61;
purity, 94.1-98.9%) were administered in the diet of groups of
70 Alderley Park strain mice of each sex at concentrations of 0, 250,
1000, or 2500 ppm, equivalent to 0, 38, 150, and 380 mg/kg bw per day,
for up to 98 weeks. The animals were housed five per sex to a cage.
Ten animals of each sex per group were designated for interim kills at
26 and 52 weeks. Permethrin did not induce clinical signs, and the
mortality rate was unaffected. A treatment-related decrease in
body-weight gain and increased food consumption were noted in males
and females at 2500 ppm. Proliferation of the smooth endoplasmic
reticulum and microbodies and increased eosinophilia in centrilobular
hepatocytes were also observed in most animals at this dose and to a
lesser extent in those at 1000 ppm. These changes had been observed in
Table 13. Incidences of tumours of the lung and liver in a second study of mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. with No. livers Neoplasm No. with
examined lung examined liver
Alveolar Alveolar tumours Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 75 16 7 23 75 8 16 22
Low 75 17 5 20 75 19 12 30
Intermediate 74 20 13 28 75 17 19 34
High 75 17 4 21 75 19 8 25
Female Control 75 10 6 15 74 3 4 6
Low 76 18 7 24 76 4 3 7
Intermediate 75 26 11 35 76 23 3 25
High 75 37 15 44 75 29 2 30
a 26-week staudy (Hart et al., 1977a) and showed no progression in
this 98-week experiment. The kidneys of treated males at all doses
showed a dose-related decrease in vacuolation of the proximal tubular
epithelium, and the effect was statistically significant in males at
250 ppm at 52 weeks (13 ± 6.3 versus 5.4 ± 4.2 vacuoles per
microscope field) but not at 26 weeks (11 ± 6.8 versus 8.4 ± 5.3)
or at 98 weeks (8.4 ± 7.1 versus 7.2 ± 7.0). The changes in the
liver and the kidney were considered to be adaptive characteristics of
no toxicological importance.
No significant increase in the incidence of tumours of unusual
types or in the number of tumour-bearing animals was seen in
comparison with with controls. In male mice, a slight increase in the
incidence of pulmonary adenomas was recognized in the second year of
the study. Up to week 52, one unconfirmed tumour was found among 34
male controls and two in 23 females at the low dose; from week 53
until the end of the study, the incidences in males were 10/36 in
controls, 6/41 at the low dose, 13/40 at the intermediate dose, and 16
(+1 unconfirmed) out of 33 at the high dose. Comparison of controls
versus the high-dose group gives p = 0.09 or, if the unconfirmed
tumour is assumed to have been an adenoma, 0.05 (Fisher's exact test).
This finding was not considered to constitute evidence of a
carcinogenic effect. The NOAEL was 250 ppm, equivalent to 38 mg/kg bw
per day, on the basis of changes in the liver and kidney at 1000 ppm
(Hart et al., 1977b; Ishmael & Litchfield, 1988).
Rats
Technical-grade permethrin of 10 batches (cis:trans ratio,
36.2:61 to 43.9:55; purity, 93.1-98.9%) was administered in the diet
of groups of 60 Alderley Park rats of each sex at concentrations of 0,
500, 1000, or 2500 ppm, equivalent to 0, 25, 50, or 125 mg/kg bw per
day, for up to 104 weeks. Twelve animals of each sex per group were
designated for interim sacrifice at 52 weeks. Treatment did not affect
survival, body weight, body-weight gain, food consumption,
haematological or urinary parameters, or gross or microscopic
appearance. Tremor, piloerection, and hypersensitivity were noted at
2500 ppm during the first 2 weeks of the study. Increased
aminopyrine- N-demethylase activity was observed in all treated males
and in females at doses > 1000 ppm at 52 weeks and in all animals
at the high dose at 104 weeks. Increased liver weights were found in
all treated groups, although the effect did not increase between weeks
52 and 104. The kidneys were heavier than those of controls in males
at 2500 ppm at week 52 and in all treated males at week 104. The
weight of the kidneys of females at doses > 1000 ppm was reduced at
52 weeks and was unaffected at week 104. The increase in kidney weight
in male rats at 104 weeks appeared to be associated with marked
nephropathy in some of these animals. Nephropathy occurs spontaneously
with age in that colony of rats, and the variability of the change in
males at the terminal kill and the absence of this trend in the
females indicate that these effects were not related to treatment.
Treatment had no effect on the incidence of tumour-bearing rats
or of tumours of any particular type. The incidence of centrilobular
hepatocyte hypertrophy was increased in rats at doses > 1000 ppm,
particularly in males, and proliferation of the smooth endoplasmic
reticulum was observed in all treated groups of females and males at
doses > 1000 ppm at week 52; at week 104, the proliferation was
confined to rats at 2500 ppm. These adaptive changes in the liver,
including hepatocyte hypertrophy and the associated increases in liver
weight, microsomal enzyme activity, and smooth endoplasmic reticulum,
were considered to be normal adaptive responses of the liver to
exposure to a xenobiotic and to be toxicologically insignificant. It
is uncertain, however, whether the hepatocyte vacuolation seen in
males and females at 2500 ppm is also part of the physiological
adaptation of the liver. Examination of sciatic nerves revealed no
ultrastructural changes associated with exposure. The NOAEL was 500
ppm, equivalent to 25 mg/kg bw per day, on the basis of the increased
liver hypertrophy at 1000 ppm (Richards et al., 1977; Ishmael &
Litchfield, 1988).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet of groups of
60 Long-Evans rats of each sex at concentrations of 0, 20, 100, or 500
ppm, equivalent to 0, 1, 5, and 25 mg/kg bw per day, for 24 months.
Treatment had no effect on survival. Two females at 500 ppm showed
tremor on day 2 of the study, but tremors were not seen in any other
animal during the remainder of the study. The body weights and food
consumption of treated males were comparable to those of controls. The
mean body weights of females at 500 ppm were slightly lower than
control weights during the first year but were comparable thereafter;
the food consumption of control and treated females was comparable.
The glucose concentration was increased in animals at 500 ppm, in
females at 18 and 24 months and in males at 24 months. The absolute
and relative (to body weight) weights of the ovary of females at 500
ppm were greater than those of controls at the end of the study.
Histological examination of tissues from treated animals revealed no
increase in the incidence of neoplastic or non-neoplastic lesions. The
NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs, changes in body and ovary weights, and clinical
chemical findings at 500 ppm (Braun & Rinehart, 1977; Billups,
1978a,b; Busey, 1978).
(d) Genotoxicity
Technical-grade permethrin was tested in a battery of test
systems in vitro and in vivo (Table 14). It did not increase the
frequency of unscheduled DNA synthesis in human fibroblasts in
vitro or induce DNA repair in Escherichia coli or Bacillus
subtilis. It did not induce gene mutations in bacteria, cultured
mammalian cells, or Drosophila melanogaster, nor recombinational
events (mitotic recombination or gene conversion) in yeast. It did not
induce aneuploidy in D. melanogaster. The evidence for induction of
clastogenic effects in cultured mammalian cells (mainly human
lymphocytes) was equivocal, in that a study of micronucleus induction
Table 14. Results of studies of the genotoxicity of permethrin
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
In vitro
Differential E. coli W3110/ 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity p3478 (1979)
Differential B. subtilis H17/M45 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity (1979)
Reverse mutation S. typhimurium 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
TA100, TA98, (1976)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
TA100, TA98,
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
TA100, TA98, (1979)
TA1537, TA1535
Reverse mutation S. typhimurium 980 µg/plate NR NR Negative ± S9 Bartsch et al.
TA100, TA98 (1980)
Reverse mutation S. typhimurium 5000 µg/plate NR NR Negativeb ± S9 Moriya et al.
TA100, TA98, (1983)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 3000 µg/plate NR 95 Negative ± S9 Pluijmen et al.
TA100, TA98 (1984)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Reverse mutation S. typhimurium 5460 µg/plate NR NR Negative ± S9 Pednekar et al.
TA100, TA98 (1987)
Reverse mutation S. typhimurium 6000 µg/plate NR cis, 99 Negative ± S9 Herrera &
TA100, TA104, trans, 100 Laborda (1988)
TA98, TA97,
TA1538, TA1537,
TA1535
Reverse mutation E. coli WP2 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
Reverse mutation E. coli WP2 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
(1979)
Reverse mutation E. coli WP2 hcr 5000 µg/plate NR NR Negative ± S9 Moriya et al.
(1983)
Gene conversion S, cerevisiae D4, 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
try locus (1976)
Mitotic S. cerevisiae D3 50 000 µg/ml 38:52 90.4 Negative ± S9 Simmon et al.
recombination (1979)
Chromosomal loss D. melanogaster 5 µg/ml feed NR NR Negativea Woodruff et al.
(1983)
Sex-linked recessive D. melanogaster 1.2 µg/ml feed 45:55 91.1 Negativea Mehr et al.
mutation (1988);Gupta et
al. (1990)
Unscheduled DNA Fischer 344 rat 5000 µg/ml 38:52 90.4 Negativea Simmon et al.
synthesis primary hepatocytes (1979)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
Hprt locus
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
OuaR
Gene mutation Mouse lymphoma 94 µg/ml NR NR Negative ± S9 Clive (1977)
L5178Y cells, Tk
locus
Chromosomal Chinese hamster 100 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration ovary cells Equivocal + S9 (1994)
Sister chromatid Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
exchange Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
formation Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 100 µg/ml NR 95 Equivocala Surrallès et al.
formation (1995a)
Micronuclei (with Human lymphocytes 100 µg/ml NR 97 Equivocala Surrallès et al.
inhibition of excision (1995b)
repair)
Micronuclei (with Human lymphocytes 10 µg/ml NR 97 Positivea Surrallès et al.
inhibition of excision (1995b)
repair)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Chromosomal Human lymphocytes 75 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration Equivocal + S9 (1992, 1994)
Inhibition of Chinese hamster 8 µg/ml NR NR Negativea Flodström et al.
gap-junctional lung V79 cells (1988)
intercellular
communication
In vivo
Dominant lethal Male CD-1 mice 452 mg/kg bw NR NR Negative Chesher et al.
mutation × 5 orally (1975)
NR, not reported; S9, 9000 × g supernatant of rat liver
a Not tested in the presence of S9
and chromosomal aberrations in one laboratory showed significant
results, while studies of micronucleus induction in another laboratory
showed negative results, except when the assay was modifed by
inhibition of DNA excision repair with cytosine arabinoside. No tests
of clastogenicity in vivo have been conducted. Permethrin did not
cause dominant lethal mutations in male mice. The Meeting concluded
that permethrin is not mutagenic but that it is clastogenic, at least
in vitro.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) was administered in the diet of groups of 12 male
and 24 female Long-Evans rats at concentrations of 0, 20, or 100 ppm,
equivalent to 0, 1.3, and 6.7 mg/kg bw per day, for three generations,
from F0 until weaning of the F3c generation. Treatment did not
affect the body weights of males or females at any time during the
study, and there was no effect on reproduction, on the number of
births, or on the survival or growth of the offspring. Ophthalmoscopic
examination of all 159 F3c offspring revealed two with
abnormalities: a unilateral cataract in one female in a control litter
and a unilateral coloboma of the choroid at the optic disc of one male
in a high-dose litter. The NOAEL for reproductive and systemic effects
was 100 ppm, equivalent to 6.7 mg/kg bw per day, the highest dose
tested (Schroeder & Rinehart, 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 26:74; purity, 94.5%) was administered in the diet
to groups of 20 Wistar rats of each sex at target doses of 0, 5, 30,
and 180 mg/kg bw per day for three generations, from F0 until
weaning of the F3b generation. The F2 parental rats were then
mated to produce an F3c litter and their pups were removed just
before weaning for teratological examination. There were no
treatment-related effects on body weights of males and females of any
generation and no effect on reproduction or on the number of births or
the survival and growth of the offspring. Offspring in all groups and
in all generations had a few ocular abnormalities which were found
histologically to be abnormalities of the retina and optic nerve,
indicative of glaucoma. The incidence of these findings was low and
not statistically correlated with treatment. The F3b fetuses showed
no treatment-related toxic or teratogenic effects. The NOAEL for
reproductive and systemic effects was 180 mg/kg bw per day, the
highest dose tested (James, 1979).
In a study conducted according to GLP, technical-grade permethrin
of seven batches (nominal cis:trans ratio, 40:60; purity,
94.1-98.9%) was administered in the diet to groups of 12 male and 24
femaleWistar rats at concentrations of 0, 500, 1000, or 2500 ppm,
equivalent to 0, 33, 67, and 170 mg/kg bw per day, for three
generations, from F0 until weaning of the F3b generation. The F2
parental rats were then mated to produce an F3c litter and their
pups were removed just before weaning for teratological examination.
The only clinical sign of toxicity was tremors, which were seen in
parents and pups receiving 1000 or 2500 ppm. The tremors were
transient, and no evidence of neuropathy was found in an extensive
examination of the nervous system of F1 males that had been exposed
in utero and fed the diet continuously for 1 year. No overall
effects on growth or reproductive performance were seen, and there was
no evidence of teratogenicity. Buphthalmia due to the persistent
presence of a pupillary membrane was first seen in the F1b litter
and then in subsequent litters. The incidence of this congenital
lesion was low (< 3%), occurring in two pups in one litter at 500 ppm
litter and in animals at 1000 and 2500 ppm.The effect may have been
due to interaction between permethrin and genetic factors, but it is
more likely to be due to differences in the selection of the F0
parents. Hypertrophy of centrilobular hepatocytes was seen in the
livers of treated F3b pups examined histologically, although no
lesion was seen on gross examination. Liver hypertrophy has been
observed in other studies of permethrin and is considered to be an
adaptive change of no toxicological significance. No NOAEL could be
identified for systemic toxicity, as hypertrophy of centrilobular
hepatocytes in the offspring and tremors in parents and offspring
occurred at all doses. The NOAEL for reproductive toxicity was
2500 ppm, equivalent to 170 mg/kg bw per day, the highest dose tested
(Hodge et al., 1977).
(ii) Developmental toxicity
Mice
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female CD-1
mice on days 6-15 of gestation. The groups consisted of 20 untreated
controls, 23 mice receiving corn oil at 10 ml/kg bw per day, and 22
mice given permethrin at 400 mg/kg bw per day. The animals were
observed daily for clinical signs of toxicity. One treated animal died
on day 17 before termination of pregnancy. Treatment had no
significant effect on weight gain during dosing and pregnancy, and
there was no significant difference in the mean numbers of
implantations among the groups, no significant treatment-related
effect on the numbers of live and normal fetuses or on the numbers of
resorbed, dead, and malformed fetuses, and no treatment-related effect
on individual fetal weights, litter size, or fetal sex ratios. No
gross visceral or skeletal malformations were found in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained death of one treated mouse. The NOAEL for developmental
toxicity was 400 mg/kg bw per day, the only dose tested (James,
1974a).
Rats
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female Wistar
rats on days 6-16 of gestation. The groups consisted of 22 untreated
controls, 23 rats receiving corn oil at 10 ml/kg bw per day, and 23
rats given permethrin at 200 mg/kg bw per day. Two treated rats died
before completion of their pregnancy. Treatment had no significant
effect on weight gain during dosing or pregnancy, and no significant
difference was seen in the mean numbers of corpora lutea or
implantations among the groups, no effect of treatment on the numbers
of live and normal fetuses, on individual fetal weights or litter
size, or on the fetal sex ratio. The numbers of resorbed, dead, and
malformed fetuses were not significantly affected by treatment. No
gross visceral or skeletal malformations were seen in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained deaths of two treated rats. The NOAEL for developmental
toxicity was 200 mg/kg bw per day, the only dose tested (James,
1974b).
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95%), aspirin, and corn oil were administered
orally by gavage to groups of 20 pregnant SpragueDawley rats during
days 6-16 of gestation, at doses of 2 ml/kg bw per day for corn oil,
200 mg/kg bw per day for aspirin, and 4, 41, or 83 mg/kg bw per day
for permethrin. There were no maternal deaths and no treatment-related
effects on the number of resorptions, body weight, length, or fetal
sex ratio. No gross, skeletal, or soft-tissue abnormalities were seen
in any group. The NOAEL for maternal and developmental toxicity was 83
mg/kg bw per day, the highest dose tested (Metker et al., 1977).
Technical-grade permethrin (cis:trans content, 37.5%:57.8%;
purity, 95.3%) diluted in corn oil was administered orally at a volume
of 10 ml/kg bw to groups of 20 pregnant CD ratson days 6-16 of
gestation at doses of 22.5, 71, or 225 mg/kg bw per day. None of the
dams died during the study, and there were no treatment-related
effects on weight gain, food consumption, pregnancy frequency, the
number of corpora lutea, the total number of implantations per
pregnancy, fetal or placental weight, or fetal sex ratios. The NOAEL
for maternal and developmental toxicity was 225 mg/kg bw per day, the
highest dose tested (McGregor & Wickramaratne, 1976).
Rabbits
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage at a volume of 2 ml/kg bw to
groups of female Dutch belted rabbits on days 6-18 of gestation. The
groups consisted of nine untreated controls, six given corn oil, and
seven given permethrin at 400 mg/kg bw per day. One animal in each
group died before the end of the study, but the deaths were not
related to treatment. There was no significant effect on weight gain
during dosing and gestation, no significant difference in the mean
numbers of corpora lutea and implantations, no effect on the numbers
of live and normal fetuses, the numbers of resorbed, dead, and
malformed fetuses, litter size, mean fetal weight, or fetal sex ratio.
No gross skeletal or visceral abnormalities were seen in any group.
The NOAEL for maternal and developmental toxicity was 400 mg/kg, the
highest dose tested (James, 1974c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
92.5%) was administered orally by gavage in 0.5% v/v aqueous Tween 80
to groups of 18 pregnant Dutch rabbits on days 6-18 of gestation at
doses of 600, 1200, or 1800 mg/kg bw per day. Five does at 600 and
1200 mg/kg bw per day and four at 1800 mg/kg bw per day group were
killed when moribund or were found dead. The clinical signs noted
included tremors at 1800 mg/kg bw per day. Many of the females found
dead or killed produced few or no faeces and had more than normal
amounts of fur in the stomach, and hypothermia and salivation were
generally seen. The body weights were reduced in all treated groups
but were statistically significantly lower onlyat 1800 mg/kg bw per
day. A statistically significant increase in post-implantation loss
was seen in animals at 1200 and 1800 mg/kg bw per day, and the number
and percentage of early and late resorptions were higher and the mean
number of live fetuses was lower in animals at these doses. The effect
at 1800 mg/kg bw per day was related to treatment, but animals at 1200
mg/kg bw per day showed a decreased number of implantation sites and a
decrease in the number of corpora lutea. As implantation occurred
before initiation of treatment, the effects at 1200 mg/kg bw per day
are not related. The fetal weights were similar to those of controls.
Evaluation of the fetuses revealed no gross visceral or skeletal
abnormalities. No NOAEL could be identified for maternal toxicity
because deaths, clinical signs, and body weight reduction were seen at
all doses. The NOAEL for developmental toxicity was 1200 mg/kg bw per
day (Richards et al., 1980).
(g) Special studies
(i) Neurotoxicity
Mice
Pyrethroids have been reported to induce two types of neurotoxic
syndrome in mice after intracerebroventricular administration
(Lawrence & Casida, 1982). The first type involves markedly increased
hypersensitivity and hyperactivity, followed quickly by whole-body
tremors and clonic seizures. The mice become prostate 5-20 min after
the onset of these signs, and profound whole-body tremors persist
until death, which usually occurs 5-30 min later. The second type of
syndrome is initially similar to the first but the signs rapidly
progress to include chreothetosis (sinuous writhing) and profuse
salivation, often with tonic seizures shortly before death after 15-45
min. Permethrin induces the second type of response, while
deltamethrin and fenvalerate, for instance, induce the first. Diazepam
at 1 mg/kg bw intraperitoneally did not delay the onset of the second
type of response in male albino mice treated with permethrin
intracerebroventricularly, but a dose of 3 mg/kg bw increased the
LD50 value in mice given (1 R, cis)-permethrin from 0.15 mg/kg bw
to 1.4 mg/kg bw (Gammon et al., 1982).
Technical-grade permethrin (cis:trans ratio, 40:60; purity not
stated) or the separated cis and trans isomers (purity not stated)
were administered in equal volumes of Emulphor and 95% ethanol (total
volume, 0.2 ml/10 mg pyrethroid) intravenously at a volume of 0.1 ml
or intracerebroventricularly at a volume of 0.005 ml to male ICR mice.
All treatments induced hyperactivity, increased sensitivity to
external stimuli, and whole-body tremor, leading to prostration and
ultimately to death. The ED50 values and 95% confidence intervals
for groups of six mice were 20 mg/kg bw (17-22) for the cis isomer,
36 mg/kg bw (33-39) for the technical-grade mixture, and 93 mg/kg bw
(83-100) for the trans isomer after intravenous administration, and
0.09 mg/kg bw (0.06-0.14) for the cis isomer, 0.15 mg/kg bw
(0.13-0.17) for the technical-grade mixture, and 1.1 mg/kg bw
(0.72-1.4) for the trans isomer after intracerebroventricular
administration. These results indicate that most of the activity is
due to the cis isomer and suggest a mainly central site of action.
The effects of a number of drugs that affect neurotransmitter systems
(noradrenaline, dopamine, acetylcholine, gamma-aminobutyric acid
(GABA), and serotonin) were studied on the loss of righting reflex
induced by permethrin given intravenously at a dose of 30 mg/kg bw. It
was not clear whether inhibitory GABA pathways were involved, whereas
the toxicity of permethrin was potentiated by at least some of the
drugs that affect central noradrenergic, cholinergic, or serotonergic
transmission. The involvement of these transmission systems in the
toxicity of permethrin could not be elucidated (Staatz et al., 1982).
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered as a 1.0%
(w/v) solution or suspension in corn oil to 10 Sprague-Dawley CD rats
of each sex at doses of 0, 10, 150, or 300 mg/kg bw. Clinical signs,
body weights, the results of a functional observational battery of
tests, and motor activity were recorded. At the end of the study, the
surviving rats were perfused, and the nervous systems of five rats of
each sex at the high dose and in the control group were examined. One
female at the high dose died on day 0. Treatment-related clinical
signs observed in this group were tremors, staggered gait, splayed
hind limbs, exaggerated hind-limb flexion, and hypersensitivity to
sound. All of the animals had recovered by day 3. There were no
effects on body weight. Rats at the high dose had whole-body tremors,
staggered gait, splayed hind limbs, abnormal posture while moving,
exaggerated hind-limb flexion, and convulsions on the first day of
functional and behavioural testing, but no significant differences
were noted after this time. There was no effect on motor activity.
Neuropathological examination of the nervous system revealed no
treatment-related lesions. The NOAEL for neurotoxicity was 150 mg/kg
bw on the basis of neurotoxic clinical signs and significant changes
in function and behaviour at 300 mg/kg bw per day (Freeman, 1993a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of five Sprague-Dawley CD of each sex at concentrations
of 0, 100, 750, 1500, 3000, 4000, or 5000 ppm for 28 days. Clinical
signs were recorded daily, and body weights were recorded weekly. All
surviving animals underwent gross necropsy on day 28. All rats
receiving 5000 ppm and one female receiving 4000 ppm had died by the
end of day 3. The treatment-related clinical signs included tremors,
splayed hind limbs, staggered gait, and chromorhinorrhoea at
concentrations > 1500 ppm. The clinical signs generally occurred
with increasing frequency with dose, except when pre-empted by death.
No clinical signs were seen among animals receiving doses < 750
ppm. Body weights were significantly reduced at doses > 3000 ppm
during some or all of the study, and body-weight gain was
significantly reduced at 3000 and 4000 ppm. Gross lesions found during
necropsy of animals at doses > 3000 ppm included necrosis of the
tail and feet and reddish-brown fluid in the stomach and intestines.
The NOAEL was 750 ppm, equal to 38 mg/kg bw per day, on the basis of
neurotoxic clinical signs at 1500 ppm (Freeman, 1993b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of 10 Sprague-Dawley rats at concentrations of 0, 250,
1500, or 2500 ppm for 13 weeks. Clinical signs were recorded daily,
and body weights and food consumption were recorded weekly. The
results of a functional observational battery of tests and tests for
motor activity were recorded before exposure and in weeks 4, 8, and 13
of the study. Surviving rats were perfused, and the nervous systems of
five rats of each sex at the high dose and in the control group were
examined microscopically for neuropathological lesions. No
treatment-related deaths occurred during the study, but
treatment-related clinical signs were observed in rats at doses
> 1500 ppm which included staggered gait, splayed hind limbs. and
tremors. These signs generally increased in both frequency and
severity with dose. Reductions in body weight and intermittent
reductions in food consumption were seen among males receiving 2500
ppm. Functional and behavioural testing showed whole-body tremors,
staggered gait, spayed hind limbs, and an abnormal posture during
movement among rats receiving 2500 ppm and to a lesser extent among
those given 1500 ppm. There were no significant differences with
regard to motor activity. No treatment-related lesions were found at
autopsy or on neuropathological examination. The NOAEL for
neurotoxicity was 250 ppm, equal to 15 mg/kg bw per day, on the basis
of neurotoxic clinical signs and significant changes in function and
behaviour at 1500 ppm (Freeman, 1993c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, > 98%) was administered in the
diet of Sprague-Dawley rats at target doses of 100, 200, and 400 mg/kg
bw per day for 90 days. The actual mean doses were 0, 86, 160, and 340
mg/kg bw per day for males and 0, 110, 170, and 350 mg/kg bw per day
for females. The groups consisted of 20 rats of each sex at the high
dose and as untreated controls and 10 rats of each sex at the
intermediate and low doses and as vehicle (acetone) controls. Clinical
signs, body weights, and food consumption were recorded three times a
week. At the end of treatment, half of the animals at the high dose
and of untreated controls were returned to untreated diet and held for
an additional 6 weeks to assess the reversibility of effects. At the
end of the study, the rats were perfused and the nervous systems from
those at the high dose and untreated and vehicle controls were
examined histologically for neuropathological lesions. Neurotoxic
signs were seen in all rats at the high dose and included tremors,
twitching, hyperexcitability, and irritability. The signs occurred
during the first day of treatment and persisted throughout the
90 days. Rats at the intermediate dose had tremors intermittently and
occasional periods of hyperexcitability during the first 2 days of
testing, but not later. When females at the high dose were returned to
untreated diet, all of the signs disappeared within 24 h, whereas in
males the tremors and twitching ceased within 1 day, and the
hyperexcitability and irritability stopped within 2-3 days. Decreased
body-weight gains were noted at the high dose, in females from week 3
and in males from week 11, but these deficits were rapidly corrected
during the recovery period. Neuropathological examination of the
central nervous system and peripheral nerves revealed no
treatment-related lesions. The NOAEL was 86 mg/kg bw per day on the
basis of neurotoxic clinical signs at 160 mg/kg bw per day (United
States Army, 1986).
The peripheral nerves, brain stem, and spinal cord of groups of
five Long-Evans rats of each sex were examined histologically for
abnormalities after exposure to permethrin. The rats were derived from
a 24-month feeding study (Braun & Rinehart, 1977) and from the third
generation of a three-generation study of reproductive toxicity
(Schroeder & Rinehart, 1977), described above, and were 10-11 months
old at the time of autopsy. In addition to standard histological
techniques, the neural material was examined morphometrically for the
number of myelinated fibres per nerve and per square millimetre of
fascicular area and for the frequency distribution of diameters per
nerve and per square millimetre of fascicular area; the morphology of
teased fibres was also evaluated. No structural lesions associated
with exposure of the rats to permethrin at dietary concentrations up
to 500 ppm (equivalent to 25 mg/kg bw per day) were observed in
central or peripheral nerves or in teased fibres of distal sural and
tibial nerves and of the maxillary division of the fifth cranial nerve
(Dyck, 1978).
Chickens
Technical-grade permethrin (cis:trans ratio, 50:50; purity,
96%) was administered by gavage to six adult laying hens at a dose of
1000 mg/kg bw per day for 5 days. After 21 days, the hens were
re-dosed and observed for an additional 21 days. A positive control
group of six hens received an intramuscular injection of a mixture of
atropine sulfate (17 mg/kg bw) and pralidoxime chloride (50 mg/kg bw)
and, 1 h later, a dose of tri- ortho-tolyl phosphate at 0.5 ml/kg bw
by gavage. Six untreated hens were available. No deaths occurred in
the treated or negative control groups during the study, and no
neurological disturbances and no histological lesions were found in
the peripheral or central nervous system. The positive controls showed
lack of coordination, unsteadiness, and loss of balance, which
worsened progressively. Foci of axonal and myelin degeneration were
observed in all positive control birds (Milner & Butterworth, 1977).
Technical-grade permethrin (cis:trans ratio, 36.0%:58.9%;
purity, 94.9%) was administered at a single dose of 15 ml, equal to
9100 mg/kg bw, the maximum possible dose, to 15 hens, which were
observed for 21 days for signs of neurotoxicity. The hens were treated
with an intramuscular injection of atropine and pyridine-2-aldoxime
methane sulfonate, then re-dosed with 15 ml of permethrin and observed
for an additional 21 days. Five hens were given tri- ortho-tolyl
phosphate at 500 mg/kg bw as a positive control, and 10 hens received
water as a negative control. No deaths occurred during the study, and
no signs of neurotoxicity were noted in the treated or negative
control groups. Ataxia, ranging from slight muscular incoordination to
difficulty in standing, was observed in the positive control group. No
treatment-related histopathological lesions of the spinal cord or
sciatic nerve were observed in the treated or negative control groups,
whereas degenerative changes were seen in the spinal cord and sciatic
nerve of hens treated with tri- ortho-tolyl phosphate. An NOAEL for
neurotoxicity could not be identified (Ross, et al., 1977).
(ii) Endocrine effects
The MCF-7 human breast carcinoma cell line was used to study the
oestrogenic potential of permethrin in vitro, pS2 mRNA expression
levels being used as the end-point. Permethrin at 100 µmol/L had no
effect on pS2 expression or on cell proliferation, whereas
17ß-estradiol at a concentration of 10 nmol/L induced a fivefold
increase in pS2 expression (Go et al., 1999).
3. Observations in humans
Twenty-three workers aged 20-52 years who had been exposed to
synthetic pyrethroids and 23 age- and sex-matched control subjects who
had had no contact with pyrethroids were interviewed, examined, and
assessed electrophysiologically. Most of the workers had been exposed
to several different pyrethroids, the more common being cypermethrin,
permethrin, fenvalerate, and fenpropathrin. Nineteen of the subjects
had experienced one or more episodes of abnormal facial sensation
0.5-3 h after exposure which persisted for 0.5-8 h. Thirteen subjects
had experienced more than one episode. There were no abnormal
neurological signs, and the results of electrophysiological studies of
the arms and legs were normal. Three subjects who had had moderate
exposure to permethrin had not developed symptoms, whereas two of them
did so after exposure to fenpropathrin and cypermethrin (Le Quesne et
al., 1980).
Workers who handled seedlings treated with permethrin
(cis:trans ratio, 25:75 for a wettable powder and 40:60 for an
emulsion in organic solvents; both diluted with water to 1-2% before
use) reported irritation of the skin. Of 42 workers who had used the
wettable powder, 12% reported burning and 10% reported blisters. Of 45
workers who had used the emulsion, 2% reporting itching. Irritation of
the upper respiratory tract involving increased nasal secretions and
sneezing was reported by 31% of the workers who had used the wettable
powder and by 2% of those who has used the emulsion (Kolmodin-Hedman
et al., 1982).
Volunteers received applications of 0.05 ml of a field-strength
preparation of technical-grade permethrin (94-96% active ingredient)
in ethanol to an area of 4 cm2 on an ear lobe, a formulation (32-36%
active ingredient) in water to 0.13 mg/cm2, or the solvent and
surfactants; 0.05 ml of the vehicle was applied to the other lobe. No
cutaneous sensation was elicited by the inert ingredients.
Paraesthesia appeared after a latent period of about 30 min, peaked
between 8 and 12 h, and disappeared after about 24 h. Further studies
with a range of doses ahowed that the response was dose-related
(Flannigan et al., 1985).
One of 28 subjects with pediculosis pubis treated with a 1%
permethrin rinse developed mild scrotal erythema and irritation 12 h
after application (Kalter et al., 1987). A group of 435 patients, most
of them children, were treated for pediculosis capitis: approximately
half of the group were treated with a single, 10-min application of
25-50 ml of a cream rinse containing permethrin (1%) and isopropanol
(20%) after towel drying of washed hair, and the remainder were
treated with a liquid product containing pyrethrins (0.3%), piperonyl
butoxide (3%), petroleum distillate (1.2%), and benzyl alcohol (2.4%).
Cutaneous side-effects including pruritus, mild transient skin
burning, stinging sensations, skin tingling, erythema, and scalp rash,
were reported by 7% of the patients in the first group and by 16% of
those in the second (DiNapoli et al., 1988). Similar results and
side-effects were reported by Brandenburg et al. (1986).
An observational study was undertaken in the USA to evaluate the
safety of a cream rinse containing 1% permethrin for treatment of head
louse infestations. Thirty-seven local public health departments
enrolled a total of 38 160 patients for 47 578 treatments with
permethrin and other pediculicides between 1 September 1986 and 31
January 1988. Follow-up information was collected 7-14 days after
treatment at a return visit or by telephone contact. A total of 103
adverse events were reported from 41 955 evaluable treatments. The
rates of reported adverse events were 2.2 per 1000 treatments with
permethrin, 3.4 per 1000 treatments with lindane, and 1.5 per 1000
treatments with other over-the-counter preparations. No serious,
unexpected adverse event was detected in the 18 950 patients treated
with permethrin (Andrews et al., 1992).
Of 10 patients with scabies treated with one application of 25 g
(range, 21-32 g) of a cream containing 5% permethrin, followed by a
thorough washing 8-20 h after treatment, six developed limited,
mild-to-moderate eczema on the scabies-affected skin at one or more
examinations (van der Rhee et al., 1989).
Comments
The metabolism of 14C-permethrin was studied in rats, lactating
goats and cattle, and laying hens. Permethrin was rapidly absorbed,
distributed, and excreted in these species after oral administration.
The metabolism of the pyrethroid was extensive, yielding a vast number
of polar degradates. Ester cleavage, hydroxylation, oxidation, and
ultimately conjugation are the critical biological mechanisms of the
metabolism of permethrin in the species studied. The metabolites that
were common to all species were 4'-hydroxypermethrin, dichlorovinyl
acid, and phenoxybenzyl alcohol. Dichlorovinyl acid and phenoxybenzoic
acid have also been identified in human urine after dermal application
of permethrin.
In rats, 96% of an administered dose was recovered in urine and
faeces within 12 days, while the total radiolabelled residues in
tissues accounted for 0.3-0.8% of the dose. Recovery in urine and
faeces within 24 h accounted for about 40% and 25% of the dose of
cis isomer and 65% and 10% of the dose of trans isomer.
respectively. Repeated exposure resulted in temporary accumulation in
fat tissue, but the chemical dissipated rapidly once exposure had
ceased.
In lactating goats and cows dosed orally with permethrin,
recovery in urine and faeces accounted for at least 65% of the dose,
and the total radiolabelled residues in liver and milk samples
represented 0.2-0.5%. Permethrin was extensively metabolized and
readily eliminated after oral administration to laying hens, > 90%
of the administered dose being excreted, while the total radiolabel in
egg and liver samples accounted for 0.1-0.2% of the dose.
The toxicity of permethrin is influenced by many factors
including the cis:trans isomer ratio, the carrier or vehicle, and
the strain of animal used. The cis isomer is considerably more toxic
than the trans isomer. The oral LD50 values in rats ranged from
6000 mg/kg bw for the 20:80 cis:trans isomeric mixture to 220 mg/kg
bw for the 80:20 cis:trans isomeric mixture. Undiluted
technical-grade permethrin (25:75 to 40:60 cis:trans isomeric
mixtures) has low acute toxicity after oral, dermal and inhalation
exposure. It was mildly irritating to the eyes and slightly irritating
to skin. It was not a skin sensitizer when tested by the Magnusson and
Kligman method.
WHO (1999) has classified permethrin as 'moderately hazardous'.
Studies in which rats, mice, rabbits, guinea-pigs, and dogs
received repeated administrations by inhalation, orally, and dermally
showed that the main effects of technical-grade permethrin are on
clinical signs, especially tremor and hyperexcitability, body weight,
and liver weight. In these short-term studies, the NOAEL or NOAEC
values were 250 mg/m3 in a 13-week study in rats exposed by
inhalation; 5 mg/kg bw per day in a 52-week study in which dogs
received the compound in gelatin capsules orally; and 1000 mg/kg bw
per day in a 21-day study in rabbits treated dermally.
In two long-term studies in rats in different laboratories with
different strains, permethrin was not carcinogenic, but the evidence
for carcinogenicity in mice was conflicting. In two studies conducted
in same strain in the same laboratory, permethrin increased the
incidences of lung and liver tumours in one study but not in the
other. The spontaneous background incidence of both these tumour types
is known to be extremely variable. A third study, conducted in a
different mouse strain, gave negative results. Thus, the weight of
evidence supports the conclusion that permethrin has very weak
oncogenic potential, and the probability that it has oncogenic
potential in humans is remote. The NOAEL for long-term toxicity in
rats was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs and changes in body and organ weights and blood
chemistry at 500 ppm. The NOAEL for long-term toxicity in mice was
500 ppm, equivalent to 75 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm.
No genotoxic activity was observed in an adequate battery of
tests for DNA damage and mutagenicity in vitro, but there was
evidence that permethrin can induce chromosomal aberrations in
mammalian cells in vitro. No tests have been carried out in mammals
for DNA damage, mutagenicity, or clastogenicity in vivo. A test for
dominant lethal effects in male mice showed no activity.
In a multigeneration study of reproductive toxicity in rats, the
NOAEL for systemic and reproductive toxicity was 180 mg/kg bw per day.
In a second multigeneration study in rats, an NOAEL could not be
identified for systemic toxicity, as effects were seen at 500 ppm,
equivalent to 33 mg/kg bw per day, the lowest dose tested; the NOAEL
for reproductive toxicity in the same study was 2500 ppm, equivalent
to 170 mg/kg bw per day, the highest dose tested.
In a study of developmental toxicity in rabbits, the NOAEL for
maternal effects was 600 mg/kg bw per day and that for developmental
toxicity was 1200 mg/kg bw per day. In three studies of developmental
toxicity in rats, the NOAEL for maternal toxicity was 83 mg/kg bw per
day and the NOAEL for developmental toxicity was 225 mg/kg bw per day,
the highest dose tested. In a study of developmental toxicity in mice,
no NOAEL was identified for maternal toxicity, whereas the NOAEL for
developmental effects was 400 mg/kg bw per day, the only dose tested.
The Meeting concluded that technical-grade permethrin is not a
reproductive or developmental toxin.
The results of acute and 90-day studies of neurotoxicity in rats
and of an acute study of delayed neurotoxicity in hens showed that
technical-grade permethrin does not induce neuropathological changes.
The NOAEL for neurotoxicity in a study in rats given a single dose was
150 mg/kg bw, on the basis of clinical signs of neurotoxicity and
significant changes in measurements in a functional observational
battery of tests at 300 mg/kg bw. The NOAEL for neurotoxicity in a
13-week study in rats was 15 mg/kg bw per day, on the basis of
clinical signs of neurotoxicity and significant changes in
measurements in the functional observational battery of tests at 90
mg/kg bw per day.
An ADI of 0-0.05 mg/kg bw was established for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60 on the basis of
the NOAEL of 100 ppm, equivalent to 5 mg/kg bw per day, in the 2-year
study in rats, which was based on clinical signs and changes in body
and organ weights and blood chemistry at 500 ppm, and the NOAEL of 5
mg/kg bw per day in a 1-year study in dogs based on reduced body
weight at 100 mg/kg bw per day, and applying a safety factor of 100.
The Meeting concluded that establishment of an acute reference
dose was not necessary because of the low acute toxicity of
technical-grade permethrin.
Toxicological evaluation
Levels that cause no toxic effect (relevant for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60)
Mouse: 500 ppm, equivalent to 75 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
Rat: 100 ppm, equivalent to 5 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
180 mg/kg bw per day (for reproductive toxicity, highest
dose in a three-generation study of reproductive toxicity)
225 mg/kg bw per day (for maternal and developmental
toxicity, highest dose in a study of developmental toxicity)
150 mg/kg bw (single dose in a study of neurotoxicity)
15 mg/kg bw per day (13-week study of neurotoxicity)
Rabbit: 400 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
1200 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity)
Dog: 5 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.05 mg/kg bw (for technical-grade permethrin with cis:trans
ratios of 25:75 to 40:60)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Clarification of the findings of chromosomal aberrations in
vitro and their potential significance in vivo
2. The Meeting was aware of other studies, in particular of acute
toxicity, dermal and ocular irritation, sensitization, and
developmental toxicity in rats that had been made available to
regulatory entities by other sponsors. The continuing support of
permethrin would benefit from the submission of these studies for
review by the Joint Meeting.
Toxicological criteria relevant for estimating guidance values for dietary and non-dietary exposure to permethrin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid and extensive (rat, lactating caprine and bovine, hen)
Distribution Mainly to fat (rat, lactating caprine and bovine, hen)
Potential for accumulation Some accumulation in fat on repeated dosing, rat
Rate and extent of excretion cis-isomer: 40% in urine, 25% in faeces in 24 h in rat
trans-isomer: 65% in urine, 10% in faeces in 24 h in rat
Metabolism in animals Extensive; hydrolysis, hydroxylation, oxidation and conjugation
(rat, lactating caprine and bovine, hen)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 225 (cis:trans ratio, 80:20); 6000 (cis:trans ratio,
20:80) mg/kg bw
Rat, LD50, dermal No data
Rabbit, LD50, dermal 2000 mg/kg bw (cis:trans ratio, 55:45 or 40:60) (highest dose)
Rat, LC50, inhalation > 23.5 mg/l, 4 h (cis:trans ratio, 40:60)
Dermal irritation Slightly irritating to rabbit skin
Ocular irritation Mildly irritating to rabbit eyes
Dermal sensitization No sensitizing potential in guinea-pigs
Short-term toxicity
Target/critical effect Nervous system (rat); liver (mouse, rat, dog)
Lowest relevant oral NOAEL 5 mg/kg bw per day in dog (cis:trans ratio, 32%:52%)
Lowest relevant dermal NOAEL 1000 mg/kg bw per day in rabbit
Lowest relevant inhalation NOAEL 250 mg/m3 in rat (cis:trans ratio, 40:60)
Target/critical effect Nervous system (rat)
Liver (mouse, rat)
Lowest relevant NOAEL Rat, 5 mg/kg bw per day; mouse, 75 mg/kg bw per day
Long-term toxicity and carcinogenicity
Carcinogenicity Not carcinogenic to mouse or rat
Genotoxicity No DNA damage or mutagenicity in vitro; clastogenic
in vitro; not studied in vivo in mammals
Reproductive toxicity
Reproductive target/critical effect None identified
Lowest relevant reproductive NOAEL 180 mg/kg bw per day in rat
Developmental target/critical effect Rat, none identified; rabbit, fetotoxicity
Lowest relevant developmental NOAEL Rat, 225 mg/kg bw per day (cis:trans ratio, 38%:58%);
rabbit, 1200 mg/kg bw per day (cis:trans ratio, 40:60)
Neurotoxicity/Delayed neurotoxicity NOAEL, 150 mg/kg bw, single dose, rats;
NOAEL, 15.5 mg/kg bw per day in a 90-day study, rats
No acute delayed effect in hens (9100 mg/kg bw)
Other toxicological studies
Medical data Paraesthesia
Summary Value Study Safety factor
ADI 0-0.05 mg/kg bw Rat, long-term toxicity, 100
5 mg/kg bw per day
Dog, 1 year, toxicity,
5 mg/kg bw per day
Acute reference dose Unnecessary
References
Andrews, E.B., Joseph, M.C., Magenheim, M.J., Tilson, H.H., Doi, P.A.
& Schultz, M.W. (1992) Postmarketing surveillance study of
permethrin creme rinse. Am. J. Public Health, 82, 857-861.
Angerer, S. & Ritter, A. (1997) Determination of metabolites of
pyrethroids in human urine using solid-phase extraction and gas
chromatography-mass spectrometry. J. Chromatogr. B Biomed.
Sci. Appl., 695, 217-226.
Bartsch, H., Malaveille, C., Camus, A.M., Martel-Planche, G., Brun,
G., Hautefeuille, A., Sabadie, N., Barbin, A., Kuroki, T.,
Drevon, C., Piccoli, C. & Montesano, R. (1980) Validation and
comparative studies on 180 chemicals with S. typhimurium
strains and V79 Chinese hamster cells in the presence of various
metabolizing systems. Mutat. Res., 76, 1-50.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1992)
Cytogenetic effects of permethrin in cultured human lymphocytes.
Mutagenesis, 7, 433-437.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1994)
Induction of structural chromosome aberrations in human
lymphocyte cultures and CHO cells by permethrin. Teratog.
Carcinog. Mutag., 14, 31-38.
Becci, P.J. & Parent, R. (1980) Evaluation of the subchronic toxic
effects of FMC 45801 when administered in the diet to Long Evans
rats over a 90-day period. Unpublished report from Food and Drug
Research Laboratories, Inc., Waverly Research Center, USA, Study
No. 6363. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Becci, P.J., Gephart, L. & Parent, R.A. (1980) 90-day subchronic oral
dosing study with FMC 45801 in beagle dogs. Unpublished report
from Food and Drug Research Laboratories, Inc., Waverly Research
Center, USA, Study No. 6338. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Benner, J. (1997) Permethrin: addendum to MRID numbers 42410001,
43505201, 43962801 and 44196101 submitted in response to EPA CBRS
review of MRID 43962801, goat metabolism -- oral dosing.
Unpublished report from Zeneca Agrochemicals, Bracknell, England,
Project No. HRC/ISN 248/920216sup2. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Benner, J.P., Hamlet, J.M. & Skidmore, M.W. (1996) Addendum to MRID
Nos. 42410001, 43505201 and 43962801, Permethrin: Further
investigation of residues in liver following oral administration
to the goat and radiovalidation of enforcement methods for
analysis of animal tissues. Unpublished report from Zeneca
Agrochemicals (Zeneca Ltd), Bracknell, England, Report No. RJ
2135B. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Billups, L.H. (1978a) Twenty-four month toxicity/carcinogenicity study
of compound FMC 33297 in rats. Histopathologic evaluation of
step-sectioned lungs from male rats. Unpublished report from
Environmental Pathology Services, Rockville, Maryland, USA.,
Study No. 78-6-0049. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA and by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
Billups, L.H. (1978b) Histopathologic evaluation of a twenty-four
month toxicity/carcinogenicity study of compound FMC 33297 in
rats. Unpublished report from Environmental Pathology Services,
Rockville, Maryland, USA., Study No. 77-11-0007. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA and by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Brandenburg, K., Deinard, A.S., DiNapoli, J., Englander, S.J.,
Orthoefer, J. & Wagner, D. (1986) 1% permethrin cream rinse vs
1% lindane shampoo in treating pediculosis capitis. Am. J.
Dis. Child., 140, 894-896.
Bratt, H., Mills, I.H. & Slade, M. (1977) Permethrin: Tissue retention
in rats. Unpublished report from Imperial Chemical Industries
Ltd, Alderley Park, England, Report No. ZO671. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975a) Acute oral toxicity studies in
rats with FMC 33297. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project Nos. 2702-75, 2703-75,
2704-75 and 2705-75. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975b) Acute dermal toxicity in
rabbits. Compound No. FMC 33297. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA, Project No.
2908-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975c) Rabbit primary dermal
irritation. Compound No. FMC 33297. Unpublished report prepared
by Bio/dynamics Inc. East Millstone, New Jersey, USA, Project No.
2909-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975d) Rabbit eye irritation.
Compound No. FMC Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2909-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1976) Acute inhalation. Compound No.
FMC 33297. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2911-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Rinehart, W.E. (1977) Twenty-four month oral
toxicity/carcinogenicity study of FMC 33297 technical in rats.
Unpublished report from Bio/dynamics, Inc., East Millstone, New
Jersey, USA, Project No. 74R-1022. Submitted to WHO by FMC
Corporation, Middleport, New York, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Brusick, D.J. & Weir, R.J. (1976) Mutagenicity evaluation of FMC 33297
(catalyst: titanium isopropylate), C8093-25, MR 51310.
Unpublished report from Litton Bionetics, Inc. Kensington,
Maryland, USA, Project No. 2683. Submitted to WHO by FMC
Corporation, Middleport, New York, USA.
Busey, W.M. (1978) Two-year chronic rat toxicity study FMC-33297.
Unpublished report from Experimental Pathology Laboratories Inc.,
Rockville, Maryland, USA. FMC Project No. NCT 549.32. Revised
pathology report. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Butterworth, S.T.G. & Hend, R.W. (1976) Toxicity studies on the
insecticide WL 43479: A five week feeding study in rats.
Unpublished report from Shell Research Ltd, Sittingbourne,
England, Report No. TLGR.0056.76. Submitted to WHO by Mitchell
Cotts Chemicals, Ltd, Mirfield, England.
Cameron, B.D. & Partridge, S.(1989) The metabolism of
[14C]-permethrin in the rat. Unpublished report from Inveresk
Research International, Musselburgh, Scotland, Report No. 4860.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Chesher, B.C. & Malone, J.C. (1974) Guinea pig sensitisation study
with 21Z73 using the maximization test method. Unpublished report
from Wellcome Research Laboratories, Berkhamsted, England, Lab.
Ref. No. T.L.13-74. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Chesher, B.C., Malone, J.C. & Parker, M.J. (1975) 21Z73, Dominant
lethal study in male mice. Unpublished report from Wellcome
Research Laboratories, Berkhamsted. England, Lab. Ref. No. T.L.
37-75. Submitted to WHO by FMC Corporation, Middleport, New York,
USA.
Clapp, M.J.L., Banham, P.B., Glaister, J.R. & Moyes, A. (1977a) PP557:
28 day feeding study in mice. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/356. Submitted to WHO by FMC Corporation, Middleport, New
York, USA.
Clapp, M.J.L., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W. &
Moyes, A. (1977b) PP557: 28 day feeding study in rats.
Unpublished report from Imperial Chemical Industries, Ltd,
Alderley Edge, England, Report No. CTL/P/355. Submitted to WHO by
FMC Corporation, Middleport, New York, USA.
Clive, D. (1977) Mutagenicity of BW 21Z73 in L5178Y/TK+/- mouse
lymphoma cells with and without exogenous metabolic activation.
Unpublished report from Burroughs Wellcome Co., Research Triangle
Park, North Carolina, USA, Doc. No. TTEP/77/0001. Submitted to
WHO by FMC Corporation, Middleport, New York, USA.
Cridland, J.S. & Weatherley, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)2,2-dimethylcyclopropane carboxylic acid
after oral ingestion of permethrin (NRDC 143) -- A first report.
Unpublished report from The Wellcome Foundation, Ltd, Beckenham,
England, Report No. BDPE-77-1. Submitted to WHO by The Wellcome
Foundation, Ltd, Beckenham, England.
Cridland, J.S. & Weatherley, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial
of the insecticide. Unpublished report from The Wellcome
Foundation, Ltd, Beckenham, England, Report No. BDPE-771.
Submitted to WHO by The Wellcome Foundation, Ltd, Beckenham,
England.
Cummins, H.A. & Gardner, J.R. (1984a) Permethrin: Acute oral toxicity
in the rat. Unpublished report from Life Science Research, Eye,
England, Report No. 84/MCC001/588. Submitted to WHO by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Cummins, H.A. & Gardner, J.R. (1984b) High cis permethrin: (isomer
ratio: 80:20) acute oral toxicity in the rat. Unpublished report
from Life Science Research, Eye, England, Report No.
84/MCC002/587. Submitted to WHO by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
DiNapoli, J.B., Austin, R.D., Englander, S.J., Gomez, M.P. & Barrett,
J.F. (1988) Eradication of head lice with a single treatment.
Am. J. Public Health, 78, 978-980.
Dyck, P.J. (1978) A pathologic and morphometric study of the nervous
system of rats fed compound FMC 33297 (permethrin). Unpublished
report from Peripheral Nerve Laboratory, Mayo Clinic and Mayo
Foundation, New York, New York, USA, Report No. not identified.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Flannigan, S.A., Tucker, S.B., Key, M.M., Ross, C.E., Fairchild, E.J.,
II, Grimes, B.A. & Harrist, R.B. (1985) Synthetic pyrethroid
insecticides: A dermatological evaluation. Br. J. Ind. Med.,
42, 363-372.
Flodström, S., Warngard, L., Ljungquist, S. & Ahlborg, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch.Toxicol., 61, 218-223.
Freeman, C. (1993a) Permethrin technical twenty-eight day
neurotoxicity range-finding study in rats. Unpublished report
from FMC Corporation, Princeton, New Jersey, USA, Study No.
A92-3645. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Freeman, C. (1993b) Permethrin technical subchronic neurotoxicity
screen in rats. Unpublished report from FMC Corporation,
Princeton, New Jersey, USA, Study No. A92-3647. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA
Freeman, C. (1993c) Permethrin technical acute neurotoxicity screen in
rats Unpublished report prepared by FMC Corporation, Princeton,
New Jersey, USA, Study No. A923646. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA
Gammon, D.W., Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. Appl. Pharmacol., 66,
290-296.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1977)
Permethrin metabolism in rats. J. Agric. Food Chem., 25, 9-17.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1978)
Distribution and metabolism of trans- and cis-permethrin in
lactating Jersey cows. J. Agric. Food Chem., 26, 613-618.
Ghiasuddin, S.M. & Soderlund, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. Appl.
Pharmacol., 74, 390-396.
Go, V., Garey, J., Wolff, M.S. & Pogo, B.G. (1999) Estrogenic
potential of certain pyrethroid compounds in the MCF-7 human
breast carcinoma cell line. Environ. Health Perspectives, 107,
173-177.
Gupta, R.K., Mehr, Z.A., Korte, D.W. & Rutledge, L.C. (1990) Mutagenic
potential of permethrin in the Drosophila melanogaster
(Diptera: Drosophilidae) sex-linked recessive lethal test.
J. Econ. Entomol., 83, 721-724.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977a) PP557: Liver hypertrophy study in rats -- Dietary
administration over 26 weeks. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/360. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977b) PP557: Whole life feeding study in mice. Unpublished
report from Imperial Chemical Industries, Ltd, Alderley Park,
England, Report No. CTL/P/359. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, D., Shaw, D. & Latter (1992a) The
metabolism of 14C permethrin in the hen. Unpublished from
Huntingdon Research Centre Ltd, Cambridgeshire, England, Project
No. HRC/ISN 272/920435. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, & Shaw, D. (1992b) The metabolism of 14C
permethrin in the goat. Unpublished report from Huntingdon
Research Centre Ltd, Cambridgeshire, England, Project No. HRC/ISN
248/920216. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Herrera, A. & Laborda, E. (1988) Mutagenic activity in synthetic
pyrethroids in Salmonella typhimurium. Mutagenesis, 3, 509-514.
Herrera, A., Barrueco, C., Caballo, C. & de la Peña E. (1992) Effect
of permethrin on the induction of sister chromatid exchanges and
micronuclei in cultured human lymphocytes. Environ. Mol.
Mutag., 20, 218-222.
Hodge, M.C., Banham, P.B., Glaister, J.R., Richards, D., Taylor, K. &
Weight, T.M. (1977) PP557: 3-Generation reproduction study in
rats. Unpublished report from Imperial Chemical Idustries Ltd,
Alderley Edge, England, Report No. CTL/P/361. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA.
Hogan, G.K. & Rinehart, W.E. (1977) Twenty-four month oral
carcinogenicity study with FMC 33297 technical in mice.
Unpublished report from Bio/dynamics, Inc., East Millstone,New
Jersey, USA, Project No. 74-1100. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Ishmael, J. & Litchfield, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice.
Fundam. Appl. Toxicol., 11, 308-322.
James, D.A. (1974a) Preliminary foetal toxicity study in the mouse
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/12.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1974b) Preliminary foetal toxicity study in the rat given
21Z73 (NRDC 143) orally. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/10.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
James, D.A. (1974c) Preliminary foetal toxicity study in the rabbit
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/19.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 79/3. Submitted
by FMC Corporation, Princeton, New Jersey, USA.
Kalinowski, A.E., Banham, P,B., Chart, I.S., Cook, S.K., Gore, C.W.,
Moreland, S.F. & Woollen, B.H. (1982) Permethrin: One year oral
dosing study in dogs. Unpublished report from Imperial Chemical
Industries Ltd, Alderley Park, England, Report No. CTL/P/647.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Kalter, D.C., Sperber, J., Rosen, T. & Matarasso, S. (1987) Treatment
of pediculosis pubis. Clinical comparison of efficacy and
tolerance of 1% lindane shampoo vs 1% permethrin cream rinse.
Arch. Dermatol., 123, 1315-1319.
Killeen, J.C. & Rapp, W.R. (1974) A four week feeding study of FMC
33297 in rats. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 74S-1024. Submitted to
WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1975a) A thirty day pilot feeding study of
FMC 33297 in rats. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project No. 74-1098. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C., Jr & Rapp, W.R. (1975b) A fourteen-day dose
range-finding study of FMC 33297 in beagle dogs. Unpublished
report from Bio/dynamics Inc., East Millstone, New Jersey, USA,
Project No. 75-1202B. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1976) A three month oral toxicity study of
FMC 33297 in beagle dogs. Unpublished report from Bio/dynamics
Inc., East Millstone, New Jersey, USA, Project No. 75-1188B.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
and by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Kolmodin-Hedman, B., Swensson, Å. & Åkerbolm, N. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50, 27-33.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-activity relationships. Pestic.
Biochem. Physiol., 18, 9-14.
Leng, G., Kuhn, K.H. & Idel, H. (1997) Biological monitoring of
pyrethroids in blood and pyrethroid metabolites in urine:
applications and limitations. Sci. Total Environ., 199,
173-181.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980) Transient
facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurophysiology, 2, 1-11.
Litchfield, M.H. (1985) Toxicity in mammals. In: Leahey, J.P., ed.,
The Pyrethroid Insecticides, London: Taylor & Francis, pp.
99-150.
Llewellyn, D.M., Brazier, A., Brown, R., Cocker, J., Evans, M.L.,
Hampton, J., Nutley, B.P. & White, J. (1996) Occupational
exposure to permethrin during its use as a public hygiene
insecticide. Ann. Occup. Hyg., 40, 499-509.
Marowitz, L.A. (1974a) Comparative acute oral toxicity in mice with
FMC 33297, FMC 37400, FMC 35171 and FMC 30960. Unpublished report
from Bio/dynamics Inc., East Millstone, New Jersey, USA, Project
Nos. 2184-74, 2185-74, 2222-74 and 2238-74. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Marowitz, L.A. (1974b) Acute oral toxicity in rats. Compound No. FMC
33297. Unpublished report from Bio/dynamics Inc., East Millstone,
New Jersey, USA, Project No. 2186-74. Submitted to WHO by FMC
corporation, Princeton, New Jersey, USA.
McGregor, D.B. & Wickramaratne, G.A. (1976) Teratogenicity study in
rats of ICI-PP 557. Unpublished report from Inveresk Research
International, Edinburgh, Scotland, Project No. 404898. Submitted
to FMC Corporation, Princeton, New Jersey, USA
Mehr, Z.A., Justus, J.D., Gupta, R.K. & Korte, D.W., Jr (1988)
Mutagenic potential of permethrin in the Drosophila
melanogaster sex-linked recessive lethal test. Unpublished
report from Letterman Army Institute of Research, Presidio of San
Francisco, California, USA, Report No. 302. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Metker, L., Angerhofer, R.A., Pope, C.R. & Swentzel, K.C. (1977)
Toxicological evaluation of 3-(phenoxyphenyl)methyl
(+)- cis, trans-3,2-(2,2-dichloroethenyl)-2,2dimethylcyclopropa
necarboxylate (permethrin). Unpublished report from the US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 51-0831-78.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: Investigation of the neurotoxic potential of WL
43479 to adult hens. Unpublished report from Shell Toxicology
Laboratory, Sittingbourne, England, Report No. TLGR.OO69.77.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nassif, M., Brooke, J.P., Hutchinson, D.B.A., Kamel, O.M. & Savage,
E.A. (1980) Studies with permethrin against bodylice in Egypt.
Pestic. Sci., 11, 679-684.
Pednekar, M.D., Gandhi, S.R. & Netrawali, M.S. (1987) Evaluation of
mutagenic activities of endosulfan, phosalone, malathion, and
permethrin, before and after metabolic activation, in the Ames
Salmonella test. Bull. Environ. Contam.Toxicol., 38, 925-933.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille
& Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137, 7-15.
Rapp, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC 33297 in mice. Histopathology report. Unpublished report
from McConnell & Rapp, Flemington, New Jersey, USA, FMC
Identification No. ACT 605.35. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Reynolds, J., Piercy, D.W.T., Clampitt, R.B., James, J.A., Thompson,
P.M., Farebrother, D.A. & Dayan, A.D. (1978) Permethrin oral
administration to dogs for 6 months. Unpublished report from The
Wellcome Foundation Ltd, Berkhamsted Hill, England, Doc. No. HEFG
78-14. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
van der Rhee, H.J., Farquhar, J.A. & Vermeulen, N.P.E. (1989) Efficacy
and transdermal absorption of permethrin in scabies patients.
Acta Dermatol. Venereol., 69, 170-182.
Richards, D., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W.,
Pratt, I., Taylor, K. & Weight, T.M. (1977) PP557: Two year
feeding study in rats. Unpublished report from Imperial Chemical
Industries, Ltd, Alderley Edge, England, Report No. CTL/P/357.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Richards, D., Banham, P.B., Kilmartin, M. & Weight, T.M. (1980)
Permethrin: teratogenicity study in the rabbit. Unpublished
report from Imperial Chemical Industries Ltd, Alderley Edge,
England, Report No. CTL/P/523. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E. & Cooke, L.
(1977) Examination of permethrin for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon Research Centre,
Huntingdon, England, Doc. No. ICI/157-NT/77468. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980a) Acute oral toxicity in rats. Test article: FMC 33297
55/45. Unpublished report from Cosmopolitan Safety Evaluation
Laboratory, Inc., Somerville, New Jersey, USA, Study No. 0245A.
submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980b) Acute dermal toxicity study -- Rabbit LD50 test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245B. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980c) Primary dermal irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory Inc., Somerville, New Jersey, USA,
Study No. 0245D. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980d) Primary eye irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245C. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Schroeder, R.E. & Rinehart, W.E. (1977) A three generation
reproduction study of FMC 33297 in rats. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Shah, P.V., Monroe, R.J. & Guthrie, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59, 414-423.
Simmon, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC Corporation compound. Unpublished report from Stanford
Research Institute, Menlo Park, California, USA, Project No.
LSC-4768. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Simmon, V.F., Riccio, E.S., Robinson, D.E. & Mitchell, A.D. (1979) In
vitro microbiological mutagenicity and unscheduled DNA synthesis
studies of fifteen pesticides. Unpublished report from SRI
International, Menlo Park, California, USA, Project No. LSU-3493.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Skidmore, M. (1996) Permethrin: Supplemental information on MRID Nos.
42410001 and 43505201 submitted in response to EPA CBRS review
dated 5 September 1995. Goat metabolism study -- oral dosing.
Unpublished report from Zeneca Ag Products, Bracknell, England,
Project No. HRC/ISN 248/920216sup1. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Soderlund, D.M. & Casida, J.E. (1977) Effects of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pest. Biochem. Physiol., 7, 391-401.
Staatz, C.G., Bloom, A.S. & Lech, J.J. (1982) A pharmacological study
of pyrethroid neurotoxicity in mice. Pest. Biochem. Physiol.,
17, 287-292.
Surrallès, J., Xamena, N., Creus, A., Catalan, J., Norppa, H. &
Marcos, R. (1995a) Induction of micronuclei by five pyrethroid
insecticides in whole-blood and isolated human lymphocyte
cultures. Mutat Res., 341, 169-184.
Surrallès, J., Xamena, N., Creus, A. & Marcos, R. (1995b) The
suitability of the micronucleus assay in human lymphocytes as a
new biomarker of excision repair. Mutat Res., 342, 43-59.
Tierney, W.J. & Rinehart, W.E. (1979) A twenty-four month oral
carcinogencicity study of FMC 33297 in mice. Unpublished report
from Bio/dynamics, East Millstone, New Jersey, USA, Project No.
76-1695. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
United States Army (1978) Subchronic inhalation toxicity of
3-(phenoxyphenyl) methyl
(+) cis, trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane
carboxylate (permethrin). Unpublished report from US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 75-51-0026-80. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
United States Army (1986) Neurotoxicity in rats following subchronic
ingestion of permethrin-treated food. Unpublished report from US
Army Environmental Hygiene Agency, Aberdeen Proving Ground, Study
No. 75-51-0351-87. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Wallwork, L.M., Poll, G.S. & Malone, J.C. (1975) Effect on the rat
oral toxicity of changes in the cis/ trans ratio with 21Z73
(NRDC 143) series. Unpublished report from The Wellcome
Foundation, Berkhamsted, England, Doc. No. HEFG 75-5. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, L.M., Thomson, P.M., Clampitt, R.B. & Malone, J.C. (1976b)
Rat oral 90 day toxicity study. Unpublished report from The
Wellcome Foundation, Berkhamsted and Beckenham, England, Doc. No.
HEFG 76-1. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Williams, L.M., Clampitt, R.D., James, J.A., Reynolds, J. & Woods,
N.C. (1976a) Rat oral 90 day toxicity study. Unpublished report
from TheWellcome Foundation, Berkhamsted, England, Doc. No. HEFG
76-3. Submitted to WHO by FMC Corporation, Princeton, New Jersey,
USA.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster.
Environ. Mutag., 5, 835-846.
decreased to < 100 µg/L within 2-4 days after treatment ceased. The
only compound recovered from milk after administration of the acid-
and alcohol-labelled trans isomer was permethrin, whereas with the
cis isomer 85% of the radiolabel was attached to the parent compound
and 15% to trans-hydroxy- cis-permethrin.
The metabolic reactions of permethrin in cows were similar to
those in rats and hens. In cows, the permethrin isomers, their mono-
and di-hydroxy derivatives, and 3-phenoxybenzyl alcohol appeared only
in the faeces, while the cis-hydroxy-chrysanthemic acid lactones
appeared in both faeces and urine. The remaining metabolites occurred
only in urine. Although a slightly larger proportion of cis- than
trans-permethrin was excreted unchanged, the amounts of ester
metabolites were similar, and these metabolites were hydroxylated at
the trans or cis methyl position of the geminal dimethyl group, at
the 4' position of the phenoxybenzyl group, or at both the geminal
dimethyl and phenoxybenzyl groups. The preferred hydroxylation site
for both isomers was the trans methyl group. The major metabolites
of the acid moieties of both isomers were the corresponding
cis-hydroxy-chrysanthemic acid and the corresponding lactone and the
chrysanthemic acid glucuronide, while
trans-hydroxy- cis-chrysanthemic acid was also a major metabolite
of cis-permethrin. The major metabolites of the alcohol moieties of
both isomers were 3-phenoxybenzoic acid-glycine conjugate (3-11% of
the dose), 3-phenoxybenzyl alcohol (8-10%), and 3-phenoxybenzoic
acid-glutamic acid conjugate (12-28%) (Gaughan et al., 1978).
Humans
Two volunteers who ingested about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the dose as
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid in
urine collected over 24 h (Cridland & Weatherley, 1977a,b).
In a study of approximately 350 people who were dusted against
body lice with 30-50 g of powder containing 2.5 or 5.0 g/kg permethrin
(cis:trans ratio, 25:75), the mean amount of permethrin absorbed
during the first 24 h after treatment was estimated to be 14 µg/kg bw
in 19 subjects exposed to powder containing 2.5 g/kg permethrin and 39
µg/kg bw in 15 subjects exposed to 5.0 g/kg. No residue was found in
samples of urine taken 30 and 60 days after treatment (Nassif et al.,
1980).
Four of five workers in Sweden who packed conifer seedlings for
6 h in a tunnel that had been sprayed 1 h earlier with a 2% aqueous
solution of permethrin, resulting in atmospheric concentrations of
0.011-0.085 mg/m3 in the breathing zone, did not excrete detectable
amounts of acid permethrin metabolites in the urine. One very short
person whose face was close to the plants and who had the highest
exposure to permethrin in his breathing zone excreted 0.26 µg/ml
permethrin acid metabolites in his urine the following morning; in the
afternoon, the concentration was below the detection limit of the
method (Kolmodin-Hedman et al., 1982).
Ten patients (five men and five women) with scabies received an
application of about 25 g (range, 21-32 g) of a 5% permethrin cream
over the skin of the whole body except the head and neck. Dermal
absorption of permethrin was calculated from the quantities of
conjugated and unconjugated cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
metabolites of permethrin in the urine. In samples of urine collected
by seven patients 1 and 2 days after application of the permethrin
cream, the mean totals of these metabolites were 410 and 440 µg,
respectively; the mean total in the urine of three patients who
collected urine in the same container for 2 days was 1400 µg. The
urinary concentration of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
varied during the first 48 h from 0.11 to 1.1 µg/ml and that of the
cis isomer from 0.02 to 0.21 µg/ml. This metabolite was still
detectable in the urine of three patients after 1 week and in the
urine of one patient, reported to be an alcoholic, after 2 weeks. The
absorption of permethrin over the first 48 h after application was
estimated from the amount of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
excreted in the urine to be 6 mg (range, 3-11 mg), i.e. 0.5% of the
dose applied (van der Rhee et al., 1989).
Exposure to permethrin was assessed in a survey of 45
professional users of insecticide products, with more than a 100-fold
difference between the average and the highest concentrations. Dermal
contamination was found on 93% of the operators, the greatest
contamination resulting from use of leaky equipment. High airborne
concentrations were linked with use in confined areas. Monitoring of
metabolites in urine showed that systemic uptake occurred, with
concentrations of specific metabolites ranging from none detected to
10 nmol/mmol creatinine of
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid,
19 nmol/mmol creatinine of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 46 nmol/mmol creatinine of 3-phenoxybenzoic acid (Llewellyn
et al., 1996).
The concentrations of permethrin and its metabolites,
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propane carboxylic acid,
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 3-phenoxybenzoic acid, were measured in the urine and plasma
of 30 pest-control workers exposed to pesticides. The concentrations
of permethrin in plasma were below the limit of detection (5 µg/L),
and the urinary concentrations ranged from < 0.5 µg/L to 280 µg/L
(Leng et al., 1997).
Urine samples collected over 24 h from eight pest-control workers
exposed to permethrin were analysed for permethrin metabolites. The
concentrations ranged from 1 to 70 µg/g creatinine (Angerer & Ritter,
1997).
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of orally administered permethrin
in mice and rats (Table 11) demonstrate that two factors that affect
its toxicity are the concentration of the cis isomer and the
vehicle. Permethrin with a cis:trans ratio of 80-100:20-0 is
approximately 7-24 times more toxic than permethrin in which the
cis:trans ratio is 10-25:90-75 when delivered in maize oil, and
permethrin administered in maize oil is four to seven times more toxic
than undiluted permethrin. The alterations in acute toxicity are,
however, greater than can be explained on the basis of the cis
isomer content alone. Thus, a change from a cis:trans ratio of 20:80
to 80:20 in maize oil changed the LD50 value in Wistar rats from
6000 mg/kg bw to 225 mg/kg bw, whereas an LD50 value of about 1000
mg/kg bw would have been expected for the 20:80 material. Frequently
observed clinical signs were convulsions on the day of dosing,
tremors, and hypersensitivity. In animals that died, the
gastrointestinal tract often contained a brown fluid. In male and
female Wistar rats exposed for 4 h to atmospheres containing
permethrin (cis:trans ratio, 40:60), the LC50 value was greater
than a nominal concentration of 24 mg/L (Braun & Killeen, 1976).
In male and female New Zealand white rabbits, the dermal LD50
values for cis:trans 55:45 and 40:60 permethrin were > 2000 mg/kg
bw (Braun & Killeen, 1975b; Sauer, 1980b).
(b) Short-term studies of toxicity
Mice
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 39%:56%; purity, 94.7%) was administered in the
diet to groups of 20 male and 20 female Alderley Park mice at
concentrations of 0, 80 (increased to 10 000 ppm in weeks 3 and 4),
200, 400, 1000, 2000, or 4000 ppm in the diet, equivalent to 0, 28,
56, 140, 280, and 560 mg/kg bw per day for 28 days. No deaths
occurred, and no significant clinical signs were observed. Reduced
body-weight gain and poor food utilization were observed in animals
receiving 10 000 ppm. At termination, necropsies were performed on
five mice of each sex from the control group and those given 2000 and
10 000 ppm. Liver weights and the liver:bodyweight ratios were
increased in mice at 2000 and 10 000 ppm, and an increase in the
incidence of eosinophilia of the centrilobular hepatocytes was seen in
all mice receiving 10 000 ppm and two of five female mice receiving
2000 ppm. The NOAEL was 1000 ppm, equivalent to 140 mg/kg bw per day,
on the basis of changes in liver weight at 2000 ppm (Clapp et al.,
1977a).
Table 11. Acute toxicity of orally administered permethrin
Species Strain Sex Vehicle Purity cis:trans LD50 95% CI Reference
(%) ratio (mg/kg bw)
Mouse CF-1 M&F Maize oil 100 10:90 1700 1200-2300 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 95.5 25:75 960 680-1200 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 99.5 40:60 650 420-880 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 100 100:0 230 200-260 Marowitz (1974a)
Rat SD M None 55:45 3600 2400-5200 Sauer (1980c)
F 2300 1800-2900
Rat SD (CD) M Maize oil 41.3: 58.7 1000 880-1200 Cummins & Gardner
F 860 680-1000 (1984a)
Rat SD (CD) M Maize oil 81.1: 18.9 370 280-460 Cummins & Gardner
F 320 240-410 (1984b)
Rat Wistar M&F Maize oil 40:60 1200 860-1500 Braun & Killeen (1975a)
Rat Wistar M&F None 40:60 8900 6000-12 000 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 40:60 1200 1100-1400 Braun & Killeen (1975a)
Rat Long-Evans M&F None 40:60 6000 4200-7800 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 95.5 25:75 1600 1300-2000 Marowitz (1974b)
Rat Wistar F Maize oil 20:80 6000 - Wallwork et al. (1975)
Rat Wistar F Maize oil 30:70 1700 1300-2200 Wallwork et al. (1975)
Rat Wistar F Maize oil 40:60 1300 1000-1600 Wallwork et al. (1975)
Rat Wistar F Maize oil 50:50 1000 730-1400 Wallwork et al. (1975)
Rat Wistar F Maize oil 60:40 440 400-500 Wallwork et al. (1975)
Rat Wistar F Maize oil 80:20 220 200-250 Wallwork et al. (1975)
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 38%:52%; purity, 90.5%) was administered in the
diet to groups of eight Wistar Alderley Park rats of each sex at
concentrations of 0, 200, 500, 1000, 2500, 5000, or 10 000 ppm,
equivalent to 0, 20, 50, 100, 250, 500, and 1000 mg/kg bw per day, for
28 days. All rats receiving 10 000 ppm died within the first 3 days of
the study, and five rats at 5000 ppm had died by day 18. Before death,
the rats showed whole-body tremors, hyperactivity, and piloerection.
The surviving rats receiving 5000 ppm had tremors, hypersensitivity,
piloerection, and urinary incontinence. Rats receiving 2500 ppm had
tremors and piloerection during the first week of treatment and were
hypersensitive throughout the study. Rats receiving 1000 ppm showed
slight tremors on the first day only. No clinical signs were seen
among rats receiving 500 or 200 ppm. Rats receiving 5000 ppm had
decreased body weights, body-weight gains, and food consumption when
compared with controls, while females receiving 2500 ppm had
significantly increased food consumption. Urinary protein excretion
was depresssed in males receiving 5000 ppm. Lymphocytosis was observed
in male rats at doses > 2500 ppm. At the end of the study, the
liver weights and the liver:body weight ratios of rats receiving doses
> 2500 ppm were increased. Histological examination of the livers
showed no remarkable changes. The NOAEL was 500 ppm, equivalent to 50
mg/kg bw per day, on the basis of tremors at 1000 ppm (Clapp et al.,
1977b).
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) wase administered in the diet to groups of six Long
Evans rats of each sex in the diet at concentrations of 0, 2500, 3500,
5000, or 7100 ppm, equal to 0, 250, 350, 5300, and 800 mg/kg bw per
day for males and 0, 270, 380, 550, and 820 mg/kg bw per day for
females, for four weeks. One male and one female receiving the highest
dose died during the first week of treatment. Tremors, the severity of
which was dose-dependent, were observed in most rats receiving doses
> 3500 ppm during the first week of treatment but were subsequently
seen only in animals receiving 7100 ppm and were generally less
severe. Staining of the abdominal fur was also seen in rats at doses
> 3500 ppm, with increasing incidences in weeks 1-4. Decreased body
weight was observed in both males and females treated with doses
> 3500 ppm during the first 2 weeks of treatment. This effect
persisted during weeks 3 and 4 only in females at 7100 ppm. No
histological examination was performed in this preliminary experiment.
The NOAEL was 2500 ppm, equal to 250 mg/kg bw per day, on the basis of
clinical signs and body-weight changes at 3500 ppm (Killeen & Rapp,
1974).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to groups of six male and six female Long
Evans rats in the diet at concentrations of 625, 1250, 2500, 5000, or
7500 ppm for 30 days, equal to 0, 59, 120, 250, and 630 mg/kg bw per
day for males and 0, 69, 130, 280, and 660 mg/kg bw per day for
females: no data on consumption of the compound were available for the
highest concentration. All females and three males receiving 7500 ppm
died within 24 h of the start of treatment, and the remaining three
males in this group had died by the end of the first week. Five of six
males and five of six females at 5000 ppm died during the first week,
but the remaining animals survived the duration of the study. In rats
receiving 2500 ppm, slight-to-moderate tremors and staining of the
anogenital fur were noted throughout most of the study. The surviving
male and female at 5000 ppm showed moderate-to-severe tremors and
staining of the fur in the anogenital region throughout the study. The
body weights of males at 2500 ppm were reduced. No histological
examination was performed in this preliminary experiment. There were
no signs of toxicity at 625 and 1250 ppm. The NOAEL was 1250 ppm,
equal to 120 mg/kg bw per day, on the basis of clinical signs and
body-weight changes at 2500 ppm (Killeen & Rapp, 1975a).
Technical-grade permethrin (cis:trans ratio unstated; purity,
96%) was administered in the diet to groups of six male and six female
Charles River rats (strain not stated) at concentrations of 0 (14
animals of each sex), 30, 100, 300, 1000, or 3000 ppm, equivalent to
0, 3, 10, 30, 100, and 300 mg/kg bw per day, for five weeks. Most of
the observations were restricted to the group receiving 3000 ppm. In
this group, persistent tremors were observed in all males and four
females, body-weight gain was reduced in females by about 7%, the
liver weights adjusted for terminal body weight were increased in
males (15%) and females (13%), and the relative heart weight was
increased in males (8%). Increased prothrombin time and plasma urea
were seen in males and decreased plasma protein in females. Males at
1000 ppm showed an increase in relative liver weight (15%), and
(probably chance) increases in prothrombin time were seen in females
at 30 and 300 ppm. Histological examination revealed renal tubular
mineralization and hydronephrosis as common lesions which were evenly
distributed among the treated groups. No dose-related increase in the
incidence of lesions was found. The NOAEL was 300 ppm, equivalent to
30 mg/kg bw per day, on the basis of increased liver weight at 1000
ppm (Butterworth & Hend, 1976).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to groups of 18 Wistar rats of
each sex at concentrations of 0, 60, 200, 600, or 2000 ppm, equivalent
to 0, 6, 20, 60, and 200 mg/kg bw per day, for 90 days. At that time,
10 rats of each sex per group were killed, and the remaining rats were
maintained without further treatment for an additional 29 days. No
treatment-related deaths occurred, and no treatment-related or
toxicologically significant clinical signs, changes in body weights,
food consumption, or estrus cycle, urinary findings, or blood
anomalies were noted. The absolute and relative weights of the spleen
and lungs were increased in male rats at 2000 ppm, and the relative
and absolute weights of the adrenal glands of female rats at this dose
showed statistically significant decreases. No consistent dose-related
pattern of toxicity was seen. The fat content of the renal cortex was
slightly increased in male rats receiving 600 or 2000 ppm, but this
was not accompanied by morphological alterations. The NOAEL was 600
ppm, equivalent to 60 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm (Williams et al., 1976a).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to 18 Wistar rats of each sex at
concentrations of 0, 200, 600, 2000, or 4000 ppm, equivalent to 0, 20,
60, 200, and 400 mg/kg bw per day, for 90 days. At that time, 10 rats
of each sex per group were killed, and the remainder were maintained
with no further treatment for an additional 36 days. No
treatment-related deaths occurred. The only significant clinical sign
was hypersensitivity in male and female rats receiving 4000 ppm. By
week 3, hypersensitivity was no longer observed in male rats but
continued in the female rats throughout treatment, although the
symptoms disappeared after 3 days without dosing. Males receiving 4000
ppm had decreased body-weight gain, which improved during the recovery
period. A slight-to-moderate decrease in leukocyte count was seen
during the early stages of the study in rats at this dose, but the
values had returned to within the normal range by 90 days. The weight
of the liver showed a slight but significant increase at 90 days but
had returned to normal by the end of the recovery period. The absolute
and relative weights of the thyroids of rats receiving the high dose
showed a slight decrease at 90 days, but no histopathological changes
were found. The NOAEL was 2000 ppm, equivalent to 200 mg/kg bw per
day, on the basis of of hypersensitivity, reduced body weight,
transient leukopenia, and increased liver weights at 4000 ppm
(Williams et al., 1976b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was administered in the
diet to groups of 30 Long Evans rats of each sex at concentrations of
0, 50, 75, 100, or 500 ppm, equivalent to 5, 7.5, 10, and 50 mg/kg bw
per day, for 90 days. There were no significant differences in body
weight, food consumption, or haematological, clinical chemical, or
urinary parameters. At the end of the study, the relative weight of
the liver was increased in male rats at 500 ppm in comparison with
controls, while that of female rats at this dose was significantly
decreased. There was no correlation with clinical or histopathological
findings. No treatment-related histopathological findings were made.
The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis
of increased relative liver weight at 500 ppm (Becci & Parent, 1980).
In a study conducted according to GLP, three batches of
technical-grade permethrin (cis:trans ratio, 36.1%:61.1% to
38.5%:56.2%; purity, 94.1-97.2%) were given in the diet to groups of
eight Wistar-derived rats of each sex at concentrations of 0, 20, 100,
or 1000 ppm, equivalent to 2, 10, and 100 mg/kg bw per day, for 26
weeks, when the rats were killed. The body weight and body-weight gain
of female rats receiving 20 and 100 ppm were similar to those of the
control animals, but females at 1000 ppm gained less weight throughout
treatment, as did males at doses > 100 ppm. There was no overall
effect on food consumption. Animals at 1000 ppm showed statistically
significant increases in liver weight, hepatic aminopyrine
N-demethylase activity, cytochrome P450 content, and smooth
endoplasmic reticulum content. Those at 100 ppm had only slight
hepatic changes, none of the untransformed values being significantly
greater than control levels, although the logarithm of the values for
aminopyrine N-demethylase activity attained significance. The NOAEL
was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
increased liver weight at 1000 ppm, as the changes in aminopyrine
N-demethylase activity were considered not toxicologically relevant
(Hart et al., 1977a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to groups
of 20 Sprague-Dawley rats of each sex by inhalation as an aerosol for
6 h per day, 5 days per week for 13 weeks at concentrations of 0, 125,
250, or 500 mg/m3. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm . At the end of
the 13-week exposure, 10 rats of each sex per group were maintained
without further treatment for an additional 90 days. There were no
treatment-related deaths. Severe tremors and convulsions were
observed in rats at 500 mg/m3, but these signs were no longer seen
by the second week of exposure. Oxygen consumption was monitored
throughout the exposure in order to assess overall metabolic rate. No
differences from controls were found. Hexobarbital-induced sleep times
(220 mg/kg bw intraperitoneally) were reduced in males exposed to 500
mg/m3, indicating the induction of liver enzymes, but the effect was
no longer statistically significant 30 days after exposure or in rats
of the lower concentrations. Body weights and organ-to-body weight
ratios were unaffected. No gross or microscopic treatment-related
pathological changes were observed. The NOAEC was 250 mg/m3 on the
basis of tremors and convulsions at 500 mg/m3 (United States Army,
1978).
Guinea-pigs
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered to groups
of 10 male Hartley guinea-pigs by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol in order to
examine sensitization reactions. There were no treatment-related
deaths or clinical signs during exposure, and the body weight and
organ:body weight ratios were unaffected. No gross or microscopic
treatment-related pathological changes were observed. No sensitization
reaction was seen. The NOAEC was 500 mg/m3, the highest dose tested
(United States Army, 1978).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity. 93.6% and 95.0%) was applied intradermally to groups of
male guinea-pigs as 0.1 ml of a 0.1% (w/v) solution; each batch of
permethrin or 2,4-dinitrochlorobenzene (positive control) contained
one volume of propylene glycol and 29 volumes of saline. Ten
guinea-pigs were challenged with the 0.1% solution, and an additional
five in each group received no prior sensitization but were given a
challenge dose of each batch of test material or the positive control.
The reaction induced by the challenge dose of permethrin was no
greater than that observed in the sensitized animals. The challenge
dose of the positive control produced sensitization reactions in all
of the guinea-pigs in that group (Metker et al., 1977).
Technical-grade permethrin (cis:trans ratio, 25:75; purity not
stated) was tested for skin sensitization potential according to the
method of Magnusson and Kligman. Ten male guinea-pigs received
permethrin, and groups of five male guinea-pigs received the vehicle
or 2,4-dinitrochlorobenzene. All compounds were given as a 1% w/v
solution in corn oil and as a 1% w/v solution in Freund's complete
adjuvant. No reaction to permethrin was seen 5, 24, and 48 h after
dosing, whereas all guinea-pigs given the positive control showed
marked sensitization (Chesher & Malone, 1974).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered by
inhalation as an aerosol to groups of 10 male Hartley guinea-pigs at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol. No
sensitization reaction was seen in any of the guinea-pigs (United
States Army, 1978).
Rabbits
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95.0%) was applied daily to the shaved skin of
groups of eight New Zealand white rabbits for 21 days at doses of 0,
100, 320, or 1000 mg/kg bw per day under an occlusive dressing. Four
rabbits in each group received abrasion at the application site on the
first day. All rabbits were killed on day 10 after the last exposure.
No significant changes were noted in body weights during or after
exposure, and there were no significant changes in organ:body weight
ratios. Moderate irritation of the skin was observed, but the reaction
was not significantly different from controls by day 18. Mild
irritation was still present 10 days after exposure, although it
improved daily. No differences were found in clinical chemical
parameters. There were no treatment-related lesions in the skin or
other tissues or organs. The NOAEL was 1000 mg/kg bw per day, the
highest dose tested (Metker et al., 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio 55:45; purity not stated) was melted and applied
without dilution at a volume of 0.5 ml to the skin of six New Zealand
white rabbits on each of two intact and two abraded sites, for a total
of 2 ml per rabbit. Each site was scored for irritation by the method
of Draize 30-60 min after removal of the occlusive wrap and residual
permethrin and again 24 h and 72 h after dosing. Very slight erythema
was noted on all sites at 24 h, but all irritation had resolved by
72 h. The score for primary dermal irritation was 0.5/8.0, indicating
virtually no irritation (Sauer, 1980c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as a liquid to the abraded skin of 10 New Zealand
white rabbits at a dose of 2000 mg/kg bw and the site was covered with
an occlusive wrap for 24 h. When the wraps were removed, very slight
erythema was noted on three rabbits, and barely perceptible oedema was
seen on one other rabbit (Braun & Killeen, 1975b).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as an aqueous slurry to the skin of six New Zealand
white rabbits as a volume of 0.50 ml of 1 g/ml on an intact and an
abraded site, for a total of 1 ml per rabbit. The sites were covered
with an occlusive wrap for 24 h and scored by the Draize method 24 and
72 h after dosing. Very slight erythema was seen on three abraded and
three intact sites at 24 h, but all irritation had resolved by 72 h.
The score for primary dermal irritation was 0.25, indicating virtually
no irritation (Braun & Killeen, 1975c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was melted, and 0.1 ml
of undiluted material was instilled into the right eye of nine New
Zealand white rabbits. After approximately 20 s, the eyes of three
rabbits were flushed with water for 1 min, and irritation was scored
by the Draize method 24, 48, and 72 h and 4 and 7 days after dosing.
Slight conjunctival redness and chemosis were found in the majority of
the treated eyes, but all irritation had resolved within 4 days. The
maximum score was 7.3/110 for unwashed eyes and 4.0/110 for washed
eyes at 24 h, indicating no irritation (Sauer, 1980d).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was instilled as a liquid into the right eye of nine New
Zealand white rabbits at a volume of 0.10 ml. The eyes of three
rabbits were then flushed for 30 s with 100 ml of warm tap-water and
were scored 1, 24, 48, and 72 h and 4 and 7 days after dosing. Slight
conjunctival redness, chemosis, and discharge were seen in both washed
and unwashed eyes at 1 h. Stippling of the cornea was observed in two
unwashed eyes. All irritation had resolved within 48 h (Braun &
Killeen, 1975d).
Dogs
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to one male and one female beagle dog in
gelatine capsules for 14 days at doses of 0, 125, 250, or 500 mg/kg bw
per day. On the first day of treatment, emesis was observed in four
treated dogs, and thereafter the compound was administered twice daily
in divided doses. Tremors and ataxia were each seen once in the male
treated with 500 mg/kg bw per day during the first week of treatment.
No effect on body weight or food consumption was seen throughout the
study. The NOAEL was 250 mg/kg bw per day on the basis of clinical
signs at 500 mg/kg bw per day (Killeen & Rapp, 1975b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, 94.5%) was administered to groups
of four beagle dogs of each sex in gelatine capsules at doses of 0, 5,
50, or 500 mg/kg bw per day for at least 96 days. Tremors were
observed on isolated occasions in two males and three females at 500
mg/kg bw per day during the exposure. One dog that had tremors also
showed transient narcosis with nystagmus on a single occasion. No
effects attributable to treatment were seen on body weight, food
consumption, clinical chemical, haematological, or urinary parameters,
or histological appearance. The mean absolute and relative (to body
weight) weights of the liver were greater than those of controls in
animals at doses > 50 mg/kg bw per day, but there was no
histological correlate. The increases in absolute liver weights were
11% at 50 and 500 mg/kg bw per day in males and 12% at 500 mg/kg bw
per day in females and were statistically significant. The biological
significance of the effect in males at 50 mg/kg bw per day is obscure
in view of the lack of any additional effect at a dose 10-fold higher.
The NOAEL was 50 mg/kg bw per day on the basis of changes in liver
weight and neurotoxic signs at 500 mg/kg bw per day (Killeen & Rapp,
1976).
Technical-grade permethrin (cis:trans ratio, 54:46; purity,
93.4%) was administered in gelatine capsules to groups of six male and
six female beagle dogs at doses of 0, 5, 50, or 500 mg/kg bw per day
for 90 days. Owing to signs of severe toxicity which increased in
severity and duration, the high dose was lowered to 364 mg/kg bw per
day on day 9 of dosing. The most serious of these signs was seizures
lasting for up to 1 h and characterized by rapid eye twitching with
fasciculations, increased sensitivity to sound, and severe tremors.
Ataxia and aggressive behaviour were also seen in these animals. At
364 mg/kg bw per day, similar signs of toxicity were seen but of
lesser severity and shorter duration. The major signs of toxicity seen
in males at doses > 50 mg/kg bw per day and females at 364 mg/kg bw
per day included impaired gait, ataxia, tremor, muscle twitching,
involuntary limb movements, uncontrolled barking, panting, and
salivation. Tremors and muscle twitching were also seen in females at
50 mg/kg bw per day. No signs of toxicity were observed at the low
dose. Body-weight gain was significantly reduced in males at the high
dose, whereas the body weight and body-weight gain of females were
unaffected by treatment. Serum alkaline phosphatase activity was
increased at 3, 6 10, and 13 weeks among males at the high dose, and
the absolute and relative weights of the liver were increased in males
and females at this dose. Permethrin had no effect on food consumption
or on haematological, urinary, ophthalmological, or histological
parameters. The NOAEL was 5 mg/kg bw per day on the basis of clinical
signs at 50 mg/kg bw per day (Becci et al., 1980).
Technical-grade permethrin (cis:trans ratio, 25:75; purity,
94.5%) was administered in gelatin capsules to groups of four beagle
dogs of each sex at doses of 0, 10, 50, or 250 mg/kg bw per day for at
least 180 days. None of the dogs died during the study. Emesis was
seen in a few treated and control animals, but there were no
treatment-related signs of toxicity, no effect on body weight,
ophthalmological or electrocardiographic parameters, absolute organ
weights, or gross or histopathological appearance. Statistically
significant but sporadic changes in food intake (decreased in dogs at
50 mg/kg bw per day in week 11 and in those at 250 mg/kg bw per day in
weeks 12 and 22), some haematological parameters (decreased packed
cell volume on day 180 and decreased mean corpuscular volume on day 56
at 10 mg/kg bw per day; increased lymphocyte and neutrophil counts at
50 and 250 mg/kg bw per day on day 14), some clinical chemical
parameters (glucose, urea, and sodium concentrations), and increased
relative weights of the liver, heart, and kidneys (by < 17%) were
seen. None of these changes appeared to be related to dose or time and
were not severe enough to be of toxicological importance. Similar
significant changes in blood chemistry were occasionally observed in
the dogs before dosing. The NOAEL was 250 mg/kg bw per day, the
highest dose tested (Reynolds et al., 1978).
In a study conducted according to GLP, permethrin (cis:trans
ratio, 32.3%:60.2%; purity, 92.5%) was administered in corn oil in
gelatin capsules to four groups of six beagle dogs of each sex at
doses of 0, 5, 100, or 1000 mg/kg bw per day for 52 weeks. Body
weights and food consumption were measured, and the dogs were observed
for clinical and behavioural abnormalities. A variety of
haematological and biochemical parameters were measured at intervals
throughout the study. Clinical signs, including convulsions, muscle
tremor, and incoordination, were seen frequently in dogs at 1000 mg/kg
bw per day. The body weights of males at 1000 mg/kg bw per day and
females at doses > 100 mg/kg bw per day were reduced. The weight of
the liver of dogs treated with doses > 100 mg/kg bw was increased,
accompanied by hepatic cellular swelling, consistent with an observed
increase in plasma alkaline phosphatase activity. These findings were
considered to represent an adaptive response and not a toxicological
effect. The dose of 1000 mg/kg bw per day was overtly toxic, resulting
in neurological signs associated in some animals with poor clinical
condition. The NOAEL was 5 mg/kg bw per day on the basis of the
reduction in body weight at 100 mg/kg bw per day (Kalinowski et al.,
1982).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to four
groups of two beagle dogs of each sex by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter of < 1 µm. The dogs
were tested for pulmonary function before and after exposure, and
blood samples were taken weekly for measurements of blood chemistry
and haematology. There were no treatment-related deaths and no toxic
signs were observed. Body weights and organ:body weight ratios were
unaffected, and pulmonary function and blood chemical and
haematological parameters were unchanged. No gross or microscopic,
treatment-related pathological changes were observed. The NOAEC was
500 mg/m3, the highest dose tested (United States Army, 1978).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet to groups of
75 CD-1 mice of each sex for 24 months. Initially, the concentrations
in the diet were 0, 20, 100, and 500 ppm, equivalent to 0, 3, 15, and
75 mg/kg bw per day. The concentration of 500 ppm was changed to 5000
ppm, equivalent to 250 mg/kg bw per day at week 19, but was returned
to 500 ppm at week 21. In addition, at week 21 the concentration of
100 ppm was changed to 4000 ppm, equivalent to 200 mg/kg bw per day,
and the concentrations were 0, 20, 500, and 4000 ppm for the remainder
of the study. During week 65, a problem of animal identification
arose, which detracts from the value of the study; however, the
actions taken to eliminate these mice from the study were carefully
recorded and judged by the US Environmental Protection Agency to be
acceptable, and the study was allowed to continue to its scheduled
conclusion. The rates of death up to 24 months were 43/62 control
males, 41/60 males at the low dose, 46/60 males at the intermediate
dose, 61/67 males at the high dose, 20/50 female controls, 32/60
females at the low dose, 34/62 females at the intermediate dose, and
48/65 at the high dose. The body weight of males at 4000 ppm was
slightly decreased from week 21 to termination, but that of females
and the food consumption of both males and females were unaffected. No
clinical observations were made that correlated with treatment. Mean
haemoglobin, erythrocyte count, and blood glucose concentration were
decreased in animals of each sex at 4000ppm. The mean absolute and
relative (to body weight) weights of the liver were increased for
males receiving 500 ppm and for animals of each sex at 4000 ppm. The
latter showed a greater frequency of non-neoplastic changes including
mononuclear leukocyte infiltrates in some tissues and thrombosis of
the cardiac atrium. There was no significant change in the incidence
of any tumour, and, in particular, the incidence of bronchioalveolar
adenomas was not increased in animals of either sex. The incidences of
hepatomas and hepatocellular carcinomas were slightly increased among
male, but not female, mice (Table 12) (Hogan & Rinehart, 1977; Rapp,
1978).
In view of the problems with the first experiment, a second of
the same design but carried out according to GLP was conducted. The
concentrations in the diet were originally 0, 100, 500, and 2000 ppm,
equivalent to 15, 75, and 300 mg/kg bw per day, and were changed after
2 months to 0, 20, 500, and 2000 ppm, equivalent to 3, 75, and 130
mg/kg bw per day, for males and 20, 2500, and 5000 ppm, equivalent to
3, 380, and 750 mg/kg bw per day, for females. The incidences of
deaths up to 24 months were 55/75 male controls, 48/75 males at the
low dose, 49/75 males at the intermediate dose, 63/75 males at the
Table 12. Incidences of tumours of the lung and liver in mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. livers Neoplasm No. with
examined examined liver
Alveolar Alveolar Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 60 6 0 72 3 4 7
Low 56 7 0 68 1 0 1
Intermediate 53 4 0 68 9 5 14
High 67 5 0 71 4 7 11
Female Control 47 11 0 72 2 0 2
Low 59 8 1 69 1 0 1
Intermediate 60 7 1 67 4 0 4
High 59 9 1 69 1 0 1
From Hogan & Rinehart (1977); Rapp (1978)
high dose, 53/75 female controls, 42/75 females at the low dose, 52/75
females at the intermediate dose, and 53/75 females at the high dose.
The mortality rate among males at the high dose was therefore slightly
greater than that among the control animals. These males also had a
higher incidence than control males of yellow staining in the
anogenital area throughout the study. Treatment had no effect on body
weight, body-weight gain, or food consumption. The differential
leukocyte counts of females at 2500 and 5000 ppm and males at 2000 ppm
were slightly lower than control values, while the segmented
neutrophil counts of males at 2000 ppm were slightly higher than in
controls. A statistically significant decrease in the absolute and
organ:body weight ratio of the testis was seen at 2000 ppm, while
females at doses > 2500 ppm showed statistically significantly
increased absolute liver weights; the liver:body weight ratio was
statistically significant only in females at 5000 ppm.
A dose-dependent increase in the incidence of alveolar-cell
adenomas and carcinomas was found among female mice, and multiple lung
adenomas were often found in the same animal (Table 13). Hepatomas
also occurred more often in female mice at the intermediate and high
doses than controls and mice at the low dose. Hepatoma is considered
to be a spontaneous lesion which occurs in ageing and aged mice
without affecting the mortality rate. The incidence of hepatocellular
carcinoma was notably higher in males at the intermediate dose than in
other treated or control groups (Table 13). The type, incidence,
and/or degree of severity of other neoplasms and all non-neoplastic
histological changes were considered to represent spontaneous lesions
unrelated to treatment. Since the incidences in males and females at
the low dose were close to those in the control group, the increased
incidence of bronchioalveolar adenoma in female mice at the two higher
doses may have been related to treatmen. This conclusion is not,
however, supported by the results of the first study, which was
conducted in the same laboratory with the same strain of mouse, and
the same batch of permethrin and which partially overlapped with the
second one in time. The pulmonary adenomas were thus considered to
have no toxicological significance. The NOAEL for systemic toxicity
was 500 ppm, equivalent to 75 mg/kg bw per day, on the basis of
changes in organ weights at 2000 ppm (Tierney & Rinehart, 1979).
In a study conducted according to GLP, eight batches of
technical-grade permethrin (cis:trans ratios, 44:55 to 36.2:61;
purity, 94.1-98.9%) were administered in the diet of groups of
70 Alderley Park strain mice of each sex at concentrations of 0, 250,
1000, or 2500 ppm, equivalent to 0, 38, 150, and 380 mg/kg bw per day,
for up to 98 weeks. The animals were housed five per sex to a cage.
Ten animals of each sex per group were designated for interim kills at
26 and 52 weeks. Permethrin did not induce clinical signs, and the
mortality rate was unaffected. A treatment-related decrease in
body-weight gain and increased food consumption were noted in males
and females at 2500 ppm. Proliferation of the smooth endoplasmic
reticulum and microbodies and increased eosinophilia in centrilobular
hepatocytes were also observed in most animals at this dose and to a
lesser extent in those at 1000 ppm. These changes had been observed in
Table 13. Incidences of tumours of the lung and liver in a second study of mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. with No. livers Neoplasm No. with
examined lung examined liver
Alveolar Alveolar tumours Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 75 16 7 23 75 8 16 22
Low 75 17 5 20 75 19 12 30
Intermediate 74 20 13 28 75 17 19 34
High 75 17 4 21 75 19 8 25
Female Control 75 10 6 15 74 3 4 6
Low 76 18 7 24 76 4 3 7
Intermediate 75 26 11 35 76 23 3 25
High 75 37 15 44 75 29 2 30
a 26-week staudy (Hart et al., 1977a) and showed no progression in
this 98-week experiment. The kidneys of treated males at all doses
showed a dose-related decrease in vacuolation of the proximal tubular
epithelium, and the effect was statistically significant in males at
250 ppm at 52 weeks (13 ± 6.3 versus 5.4 ± 4.2 vacuoles per
microscope field) but not at 26 weeks (11 ± 6.8 versus 8.4 ± 5.3)
or at 98 weeks (8.4 ± 7.1 versus 7.2 ± 7.0). The changes in the
liver and the kidney were considered to be adaptive characteristics of
no toxicological importance.
No significant increase in the incidence of tumours of unusual
types or in the number of tumour-bearing animals was seen in
comparison with with controls. In male mice, a slight increase in the
incidence of pulmonary adenomas was recognized in the second year of
the study. Up to week 52, one unconfirmed tumour was found among 34
male controls and two in 23 females at the low dose; from week 53
until the end of the study, the incidences in males were 10/36 in
controls, 6/41 at the low dose, 13/40 at the intermediate dose, and 16
(+1 unconfirmed) out of 33 at the high dose. Comparison of controls
versus the high-dose group gives p = 0.09 or, if the unconfirmed
tumour is assumed to have been an adenoma, 0.05 (Fisher's exact test).
This finding was not considered to constitute evidence of a
carcinogenic effect. The NOAEL was 250 ppm, equivalent to 38 mg/kg bw
per day, on the basis of changes in the liver and kidney at 1000 ppm
(Hart et al., 1977b; Ishmael & Litchfield, 1988).
Rats
Technical-grade permethrin of 10 batches (cis:trans ratio,
36.2:61 to 43.9:55; purity, 93.1-98.9%) was administered in the diet
of groups of 60 Alderley Park rats of each sex at concentrations of 0,
500, 1000, or 2500 ppm, equivalent to 0, 25, 50, or 125 mg/kg bw per
day, for up to 104 weeks. Twelve animals of each sex per group were
designated for interim sacrifice at 52 weeks. Treatment did not affect
survival, body weight, body-weight gain, food consumption,
haematological or urinary parameters, or gross or microscopic
appearance. Tremor, piloerection, and hypersensitivity were noted at
2500 ppm during the first 2 weeks of the study. Increased
aminopyrine- N-demethylase activity was observed in all treated males
and in females at doses > 1000 ppm at 52 weeks and in all animals
at the high dose at 104 weeks. Increased liver weights were found in
all treated groups, although the effect did not increase between weeks
52 and 104. The kidneys were heavier than those of controls in males
at 2500 ppm at week 52 and in all treated males at week 104. The
weight of the kidneys of females at doses > 1000 ppm was reduced at
52 weeks and was unaffected at week 104. The increase in kidney weight
in male rats at 104 weeks appeared to be associated with marked
nephropathy in some of these animals. Nephropathy occurs spontaneously
with age in that colony of rats, and the variability of the change in
males at the terminal kill and the absence of this trend in the
females indicate that these effects were not related to treatment.
Treatment had no effect on the incidence of tumour-bearing rats
or of tumours of any particular type. The incidence of centrilobular
hepatocyte hypertrophy was increased in rats at doses > 1000 ppm,
particularly in males, and proliferation of the smooth endoplasmic
reticulum was observed in all treated groups of females and males at
doses > 1000 ppm at week 52; at week 104, the proliferation was
confined to rats at 2500 ppm. These adaptive changes in the liver,
including hepatocyte hypertrophy and the associated increases in liver
weight, microsomal enzyme activity, and smooth endoplasmic reticulum,
were considered to be normal adaptive responses of the liver to
exposure to a xenobiotic and to be toxicologically insignificant. It
is uncertain, however, whether the hepatocyte vacuolation seen in
males and females at 2500 ppm is also part of the physiological
adaptation of the liver. Examination of sciatic nerves revealed no
ultrastructural changes associated with exposure. The NOAEL was 500
ppm, equivalent to 25 mg/kg bw per day, on the basis of the increased
liver hypertrophy at 1000 ppm (Richards et al., 1977; Ishmael &
Litchfield, 1988).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet of groups of
60 Long-Evans rats of each sex at concentrations of 0, 20, 100, or 500
ppm, equivalent to 0, 1, 5, and 25 mg/kg bw per day, for 24 months.
Treatment had no effect on survival. Two females at 500 ppm showed
tremor on day 2 of the study, but tremors were not seen in any other
animal during the remainder of the study. The body weights and food
consumption of treated males were comparable to those of controls. The
mean body weights of females at 500 ppm were slightly lower than
control weights during the first year but were comparable thereafter;
the food consumption of control and treated females was comparable.
The glucose concentration was increased in animals at 500 ppm, in
females at 18 and 24 months and in males at 24 months. The absolute
and relative (to body weight) weights of the ovary of females at 500
ppm were greater than those of controls at the end of the study.
Histological examination of tissues from treated animals revealed no
increase in the incidence of neoplastic or non-neoplastic lesions. The
NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs, changes in body and ovary weights, and clinical
chemical findings at 500 ppm (Braun & Rinehart, 1977; Billups,
1978a,b; Busey, 1978).
(d) Genotoxicity
Technical-grade permethrin was tested in a battery of test
systems in vitro and in vivo (Table 14). It did not increase the
frequency of unscheduled DNA synthesis in human fibroblasts in
vitro or induce DNA repair in Escherichia coli or Bacillus
subtilis. It did not induce gene mutations in bacteria, cultured
mammalian cells, or Drosophila melanogaster, nor recombinational
events (mitotic recombination or gene conversion) in yeast. It did not
induce aneuploidy in D. melanogaster. The evidence for induction of
clastogenic effects in cultured mammalian cells (mainly human
lymphocytes) was equivocal, in that a study of micronucleus induction
Table 14. Results of studies of the genotoxicity of permethrin
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
In vitro
Differential E. coli W3110/ 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity p3478 (1979)
Differential B. subtilis H17/M45 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity (1979)
Reverse mutation S. typhimurium 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
TA100, TA98, (1976)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
TA100, TA98,
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
TA100, TA98, (1979)
TA1537, TA1535
Reverse mutation S. typhimurium 980 µg/plate NR NR Negative ± S9 Bartsch et al.
TA100, TA98 (1980)
Reverse mutation S. typhimurium 5000 µg/plate NR NR Negativeb ± S9 Moriya et al.
TA100, TA98, (1983)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 3000 µg/plate NR 95 Negative ± S9 Pluijmen et al.
TA100, TA98 (1984)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Reverse mutation S. typhimurium 5460 µg/plate NR NR Negative ± S9 Pednekar et al.
TA100, TA98 (1987)
Reverse mutation S. typhimurium 6000 µg/plate NR cis, 99 Negative ± S9 Herrera &
TA100, TA104, trans, 100 Laborda (1988)
TA98, TA97,
TA1538, TA1537,
TA1535
Reverse mutation E. coli WP2 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
Reverse mutation E. coli WP2 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
(1979)
Reverse mutation E. coli WP2 hcr 5000 µg/plate NR NR Negative ± S9 Moriya et al.
(1983)
Gene conversion S, cerevisiae D4, 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
try locus (1976)
Mitotic S. cerevisiae D3 50 000 µg/ml 38:52 90.4 Negative ± S9 Simmon et al.
recombination (1979)
Chromosomal loss D. melanogaster 5 µg/ml feed NR NR Negativea Woodruff et al.
(1983)
Sex-linked recessive D. melanogaster 1.2 µg/ml feed 45:55 91.1 Negativea Mehr et al.
mutation (1988);Gupta et
al. (1990)
Unscheduled DNA Fischer 344 rat 5000 µg/ml 38:52 90.4 Negativea Simmon et al.
synthesis primary hepatocytes (1979)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
Hprt locus
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
OuaR
Gene mutation Mouse lymphoma 94 µg/ml NR NR Negative ± S9 Clive (1977)
L5178Y cells, Tk
locus
Chromosomal Chinese hamster 100 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration ovary cells Equivocal + S9 (1994)
Sister chromatid Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
exchange Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
formation Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 100 µg/ml NR 95 Equivocala Surrallès et al.
formation (1995a)
Micronuclei (with Human lymphocytes 100 µg/ml NR 97 Equivocala Surrallès et al.
inhibition of excision (1995b)
repair)
Micronuclei (with Human lymphocytes 10 µg/ml NR 97 Positivea Surrallès et al.
inhibition of excision (1995b)
repair)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Chromosomal Human lymphocytes 75 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration Equivocal + S9 (1992, 1994)
Inhibition of Chinese hamster 8 µg/ml NR NR Negativea Flodström et al.
gap-junctional lung V79 cells (1988)
intercellular
communication
In vivo
Dominant lethal Male CD-1 mice 452 mg/kg bw NR NR Negative Chesher et al.
mutation × 5 orally (1975)
NR, not reported; S9, 9000 × g supernatant of rat liver
a Not tested in the presence of S9
and chromosomal aberrations in one laboratory showed significant
results, while studies of micronucleus induction in another laboratory
showed negative results, except when the assay was modifed by
inhibition of DNA excision repair with cytosine arabinoside. No tests
of clastogenicity in vivo have been conducted. Permethrin did not
cause dominant lethal mutations in male mice. The Meeting concluded
that permethrin is not mutagenic but that it is clastogenic, at least
in vitro.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) was administered in the diet of groups of 12 male
and 24 female Long-Evans rats at concentrations of 0, 20, or 100 ppm,
equivalent to 0, 1.3, and 6.7 mg/kg bw per day, for three generations,
from F0 until weaning of the F3c generation. Treatment did not
affect the body weights of males or females at any time during the
study, and there was no effect on reproduction, on the number of
births, or on the survival or growth of the offspring. Ophthalmoscopic
examination of all 159 F3c offspring revealed two with
abnormalities: a unilateral cataract in one female in a control litter
and a unilateral coloboma of the choroid at the optic disc of one male
in a high-dose litter. The NOAEL for reproductive and systemic effects
was 100 ppm, equivalent to 6.7 mg/kg bw per day, the highest dose
tested (Schroeder & Rinehart, 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 26:74; purity, 94.5%) was administered in the diet
to groups of 20 Wistar rats of each sex at target doses of 0, 5, 30,
and 180 mg/kg bw per day for three generations, from F0 until
weaning of the F3b generation. The F2 parental rats were then
mated to produce an F3c litter and their pups were removed just
before weaning for teratological examination. There were no
treatment-related effects on body weights of males and females of any
generation and no effect on reproduction or on the number of births or
the survival and growth of the offspring. Offspring in all groups and
in all generations had a few ocular abnormalities which were found
histologically to be abnormalities of the retina and optic nerve,
indicative of glaucoma. The incidence of these findings was low and
not statistically correlated with treatment. The F3b fetuses showed
no treatment-related toxic or teratogenic effects. The NOAEL for
reproductive and systemic effects was 180 mg/kg bw per day, the
highest dose tested (James, 1979).
In a study conducted according to GLP, technical-grade permethrin
of seven batches (nominal cis:trans ratio, 40:60; purity,
94.1-98.9%) was administered in the diet to groups of 12 male and 24
femaleWistar rats at concentrations of 0, 500, 1000, or 2500 ppm,
equivalent to 0, 33, 67, and 170 mg/kg bw per day, for three
generations, from F0 until weaning of the F3b generation. The F2
parental rats were then mated to produce an F3c litter and their
pups were removed just before weaning for teratological examination.
The only clinical sign of toxicity was tremors, which were seen in
parents and pups receiving 1000 or 2500 ppm. The tremors were
transient, and no evidence of neuropathy was found in an extensive
examination of the nervous system of F1 males that had been exposed
in utero and fed the diet continuously for 1 year. No overall
effects on growth or reproductive performance were seen, and there was
no evidence of teratogenicity. Buphthalmia due to the persistent
presence of a pupillary membrane was first seen in the F1b litter
and then in subsequent litters. The incidence of this congenital
lesion was low (< 3%), occurring in two pups in one litter at 500 ppm
litter and in animals at 1000 and 2500 ppm.The effect may have been
due to interaction between permethrin and genetic factors, but it is
more likely to be due to differences in the selection of the F0
parents. Hypertrophy of centrilobular hepatocytes was seen in the
livers of treated F3b pups examined histologically, although no
lesion was seen on gross examination. Liver hypertrophy has been
observed in other studies of permethrin and is considered to be an
adaptive change of no toxicological significance. No NOAEL could be
identified for systemic toxicity, as hypertrophy of centrilobular
hepatocytes in the offspring and tremors in parents and offspring
occurred at all doses. The NOAEL for reproductive toxicity was
2500 ppm, equivalent to 170 mg/kg bw per day, the highest dose tested
(Hodge et al., 1977).
(ii) Developmental toxicity
Mice
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female CD-1
mice on days 6-15 of gestation. The groups consisted of 20 untreated
controls, 23 mice receiving corn oil at 10 ml/kg bw per day, and 22
mice given permethrin at 400 mg/kg bw per day. The animals were
observed daily for clinical signs of toxicity. One treated animal died
on day 17 before termination of pregnancy. Treatment had no
significant effect on weight gain during dosing and pregnancy, and
there was no significant difference in the mean numbers of
implantations among the groups, no significant treatment-related
effect on the numbers of live and normal fetuses or on the numbers of
resorbed, dead, and malformed fetuses, and no treatment-related effect
on individual fetal weights, litter size, or fetal sex ratios. No
gross visceral or skeletal malformations were found in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained death of one treated mouse. The NOAEL for developmental
toxicity was 400 mg/kg bw per day, the only dose tested (James,
1974a).
Rats
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female Wistar
rats on days 6-16 of gestation. The groups consisted of 22 untreated
controls, 23 rats receiving corn oil at 10 ml/kg bw per day, and 23
rats given permethrin at 200 mg/kg bw per day. Two treated rats died
before completion of their pregnancy. Treatment had no significant
effect on weight gain during dosing or pregnancy, and no significant
difference was seen in the mean numbers of corpora lutea or
implantations among the groups, no effect of treatment on the numbers
of live and normal fetuses, on individual fetal weights or litter
size, or on the fetal sex ratio. The numbers of resorbed, dead, and
malformed fetuses were not significantly affected by treatment. No
gross visceral or skeletal malformations were seen in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained deaths of two treated rats. The NOAEL for developmental
toxicity was 200 mg/kg bw per day, the only dose tested (James,
1974b).
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95%), aspirin, and corn oil were administered
orally by gavage to groups of 20 pregnant SpragueDawley rats during
days 6-16 of gestation, at doses of 2 ml/kg bw per day for corn oil,
200 mg/kg bw per day for aspirin, and 4, 41, or 83 mg/kg bw per day
for permethrin. There were no maternal deaths and no treatment-related
effects on the number of resorptions, body weight, length, or fetal
sex ratio. No gross, skeletal, or soft-tissue abnormalities were seen
in any group. The NOAEL for maternal and developmental toxicity was 83
mg/kg bw per day, the highest dose tested (Metker et al., 1977).
Technical-grade permethrin (cis:trans content, 37.5%:57.8%;
purity, 95.3%) diluted in corn oil was administered orally at a volume
of 10 ml/kg bw to groups of 20 pregnant CD ratson days 6-16 of
gestation at doses of 22.5, 71, or 225 mg/kg bw per day. None of the
dams died during the study, and there were no treatment-related
effects on weight gain, food consumption, pregnancy frequency, the
number of corpora lutea, the total number of implantations per
pregnancy, fetal or placental weight, or fetal sex ratios. The NOAEL
for maternal and developmental toxicity was 225 mg/kg bw per day, the
highest dose tested (McGregor & Wickramaratne, 1976).
Rabbits
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage at a volume of 2 ml/kg bw to
groups of female Dutch belted rabbits on days 6-18 of gestation. The
groups consisted of nine untreated controls, six given corn oil, and
seven given permethrin at 400 mg/kg bw per day. One animal in each
group died before the end of the study, but the deaths were not
related to treatment. There was no significant effect on weight gain
during dosing and gestation, no significant difference in the mean
numbers of corpora lutea and implantations, no effect on the numbers
of live and normal fetuses, the numbers of resorbed, dead, and
malformed fetuses, litter size, mean fetal weight, or fetal sex ratio.
No gross skeletal or visceral abnormalities were seen in any group.
The NOAEL for maternal and developmental toxicity was 400 mg/kg, the
highest dose tested (James, 1974c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
92.5%) was administered orally by gavage in 0.5% v/v aqueous Tween 80
to groups of 18 pregnant Dutch rabbits on days 6-18 of gestation at
doses of 600, 1200, or 1800 mg/kg bw per day. Five does at 600 and
1200 mg/kg bw per day and four at 1800 mg/kg bw per day group were
killed when moribund or were found dead. The clinical signs noted
included tremors at 1800 mg/kg bw per day. Many of the females found
dead or killed produced few or no faeces and had more than normal
amounts of fur in the stomach, and hypothermia and salivation were
generally seen. The body weights were reduced in all treated groups
but were statistically significantly lower onlyat 1800 mg/kg bw per
day. A statistically significant increase in post-implantation loss
was seen in animals at 1200 and 1800 mg/kg bw per day, and the number
and percentage of early and late resorptions were higher and the mean
number of live fetuses was lower in animals at these doses. The effect
at 1800 mg/kg bw per day was related to treatment, but animals at 1200
mg/kg bw per day showed a decreased number of implantation sites and a
decrease in the number of corpora lutea. As implantation occurred
before initiation of treatment, the effects at 1200 mg/kg bw per day
are not related. The fetal weights were similar to those of controls.
Evaluation of the fetuses revealed no gross visceral or skeletal
abnormalities. No NOAEL could be identified for maternal toxicity
because deaths, clinical signs, and body weight reduction were seen at
all doses. The NOAEL for developmental toxicity was 1200 mg/kg bw per
day (Richards et al., 1980).
(g) Special studies
(i) Neurotoxicity
Mice
Pyrethroids have been reported to induce two types of neurotoxic
syndrome in mice after intracerebroventricular administration
(Lawrence & Casida, 1982). The first type involves markedly increased
hypersensitivity and hyperactivity, followed quickly by whole-body
tremors and clonic seizures. The mice become prostate 5-20 min after
the onset of these signs, and profound whole-body tremors persist
until death, which usually occurs 5-30 min later. The second type of
syndrome is initially similar to the first but the signs rapidly
progress to include chreothetosis (sinuous writhing) and profuse
salivation, often with tonic seizures shortly before death after 15-45
min. Permethrin induces the second type of response, while
deltamethrin and fenvalerate, for instance, induce the first. Diazepam
at 1 mg/kg bw intraperitoneally did not delay the onset of the second
type of response in male albino mice treated with permethrin
intracerebroventricularly, but a dose of 3 mg/kg bw increased the
LD50 value in mice given (1 R, cis)-permethrin from 0.15 mg/kg bw
to 1.4 mg/kg bw (Gammon et al., 1982).
Technical-grade permethrin (cis:trans ratio, 40:60; purity not
stated) or the separated cis and trans isomers (purity not stated)
were administered in equal volumes of Emulphor and 95% ethanol (total
volume, 0.2 ml/10 mg pyrethroid) intravenously at a volume of 0.1 ml
or intracerebroventricularly at a volume of 0.005 ml to male ICR mice.
All treatments induced hyperactivity, increased sensitivity to
external stimuli, and whole-body tremor, leading to prostration and
ultimately to death. The ED50 values and 95% confidence intervals
for groups of six mice were 20 mg/kg bw (17-22) for the cis isomer,
36 mg/kg bw (33-39) for the technical-grade mixture, and 93 mg/kg bw
(83-100) for the trans isomer after intravenous administration, and
0.09 mg/kg bw (0.06-0.14) for the cis isomer, 0.15 mg/kg bw
(0.13-0.17) for the technical-grade mixture, and 1.1 mg/kg bw
(0.72-1.4) for the trans isomer after intracerebroventricular
administration. These results indicate that most of the activity is
due to the cis isomer and suggest a mainly central site of action.
The effects of a number of drugs that affect neurotransmitter systems
(noradrenaline, dopamine, acetylcholine, gamma-aminobutyric acid
(GABA), and serotonin) were studied on the loss of righting reflex
induced by permethrin given intravenously at a dose of 30 mg/kg bw. It
was not clear whether inhibitory GABA pathways were involved, whereas
the toxicity of permethrin was potentiated by at least some of the
drugs that affect central noradrenergic, cholinergic, or serotonergic
transmission. The involvement of these transmission systems in the
toxicity of permethrin could not be elucidated (Staatz et al., 1982).
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered as a 1.0%
(w/v) solution or suspension in corn oil to 10 Sprague-Dawley CD rats
of each sex at doses of 0, 10, 150, or 300 mg/kg bw. Clinical signs,
body weights, the results of a functional observational battery of
tests, and motor activity were recorded. At the end of the study, the
surviving rats were perfused, and the nervous systems of five rats of
each sex at the high dose and in the control group were examined. One
female at the high dose died on day 0. Treatment-related clinical
signs observed in this group were tremors, staggered gait, splayed
hind limbs, exaggerated hind-limb flexion, and hypersensitivity to
sound. All of the animals had recovered by day 3. There were no
effects on body weight. Rats at the high dose had whole-body tremors,
staggered gait, splayed hind limbs, abnormal posture while moving,
exaggerated hind-limb flexion, and convulsions on the first day of
functional and behavioural testing, but no significant differences
were noted after this time. There was no effect on motor activity.
Neuropathological examination of the nervous system revealed no
treatment-related lesions. The NOAEL for neurotoxicity was 150 mg/kg
bw on the basis of neurotoxic clinical signs and significant changes
in function and behaviour at 300 mg/kg bw per day (Freeman, 1993a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of five Sprague-Dawley CD of each sex at concentrations
of 0, 100, 750, 1500, 3000, 4000, or 5000 ppm for 28 days. Clinical
signs were recorded daily, and body weights were recorded weekly. All
surviving animals underwent gross necropsy on day 28. All rats
receiving 5000 ppm and one female receiving 4000 ppm had died by the
end of day 3. The treatment-related clinical signs included tremors,
splayed hind limbs, staggered gait, and chromorhinorrhoea at
concentrations > 1500 ppm. The clinical signs generally occurred
with increasing frequency with dose, except when pre-empted by death.
No clinical signs were seen among animals receiving doses < 750
ppm. Body weights were significantly reduced at doses > 3000 ppm
during some or all of the study, and body-weight gain was
significantly reduced at 3000 and 4000 ppm. Gross lesions found during
necropsy of animals at doses > 3000 ppm included necrosis of the
tail and feet and reddish-brown fluid in the stomach and intestines.
The NOAEL was 750 ppm, equal to 38 mg/kg bw per day, on the basis of
neurotoxic clinical signs at 1500 ppm (Freeman, 1993b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of 10 Sprague-Dawley rats at concentrations of 0, 250,
1500, or 2500 ppm for 13 weeks. Clinical signs were recorded daily,
and body weights and food consumption were recorded weekly. The
results of a functional observational battery of tests and tests for
motor activity were recorded before exposure and in weeks 4, 8, and 13
of the study. Surviving rats were perfused, and the nervous systems of
five rats of each sex at the high dose and in the control group were
examined microscopically for neuropathological lesions. No
treatment-related deaths occurred during the study, but
treatment-related clinical signs were observed in rats at doses
> 1500 ppm which included staggered gait, splayed hind limbs. and
tremors. These signs generally increased in both frequency and
severity with dose. Reductions in body weight and intermittent
reductions in food consumption were seen among males receiving 2500
ppm. Functional and behavioural testing showed whole-body tremors,
staggered gait, spayed hind limbs, and an abnormal posture during
movement among rats receiving 2500 ppm and to a lesser extent among
those given 1500 ppm. There were no significant differences with
regard to motor activity. No treatment-related lesions were found at
autopsy or on neuropathological examination. The NOAEL for
neurotoxicity was 250 ppm, equal to 15 mg/kg bw per day, on the basis
of neurotoxic clinical signs and significant changes in function and
behaviour at 1500 ppm (Freeman, 1993c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, > 98%) was administered in the
diet of Sprague-Dawley rats at target doses of 100, 200, and 400 mg/kg
bw per day for 90 days. The actual mean doses were 0, 86, 160, and 340
mg/kg bw per day for males and 0, 110, 170, and 350 mg/kg bw per day
for females. The groups consisted of 20 rats of each sex at the high
dose and as untreated controls and 10 rats of each sex at the
intermediate and low doses and as vehicle (acetone) controls. Clinical
signs, body weights, and food consumption were recorded three times a
week. At the end of treatment, half of the animals at the high dose
and of untreated controls were returned to untreated diet and held for
an additional 6 weeks to assess the reversibility of effects. At the
end of the study, the rats were perfused and the nervous systems from
those at the high dose and untreated and vehicle controls were
examined histologically for neuropathological lesions. Neurotoxic
signs were seen in all rats at the high dose and included tremors,
twitching, hyperexcitability, and irritability. The signs occurred
during the first day of treatment and persisted throughout the
90 days. Rats at the intermediate dose had tremors intermittently and
occasional periods of hyperexcitability during the first 2 days of
testing, but not later. When females at the high dose were returned to
untreated diet, all of the signs disappeared within 24 h, whereas in
males the tremors and twitching ceased within 1 day, and the
hyperexcitability and irritability stopped within 2-3 days. Decreased
body-weight gains were noted at the high dose, in females from week 3
and in males from week 11, but these deficits were rapidly corrected
during the recovery period. Neuropathological examination of the
central nervous system and peripheral nerves revealed no
treatment-related lesions. The NOAEL was 86 mg/kg bw per day on the
basis of neurotoxic clinical signs at 160 mg/kg bw per day (United
States Army, 1986).
The peripheral nerves, brain stem, and spinal cord of groups of
five Long-Evans rats of each sex were examined histologically for
abnormalities after exposure to permethrin. The rats were derived from
a 24-month feeding study (Braun & Rinehart, 1977) and from the third
generation of a three-generation study of reproductive toxicity
(Schroeder & Rinehart, 1977), described above, and were 10-11 months
old at the time of autopsy. In addition to standard histological
techniques, the neural material was examined morphometrically for the
number of myelinated fibres per nerve and per square millimetre of
fascicular area and for the frequency distribution of diameters per
nerve and per square millimetre of fascicular area; the morphology of
teased fibres was also evaluated. No structural lesions associated
with exposure of the rats to permethrin at dietary concentrations up
to 500 ppm (equivalent to 25 mg/kg bw per day) were observed in
central or peripheral nerves or in teased fibres of distal sural and
tibial nerves and of the maxillary division of the fifth cranial nerve
(Dyck, 1978).
Chickens
Technical-grade permethrin (cis:trans ratio, 50:50; purity,
96%) was administered by gavage to six adult laying hens at a dose of
1000 mg/kg bw per day for 5 days. After 21 days, the hens were
re-dosed and observed for an additional 21 days. A positive control
group of six hens received an intramuscular injection of a mixture of
atropine sulfate (17 mg/kg bw) and pralidoxime chloride (50 mg/kg bw)
and, 1 h later, a dose of tri- ortho-tolyl phosphate at 0.5 ml/kg bw
by gavage. Six untreated hens were available. No deaths occurred in
the treated or negative control groups during the study, and no
neurological disturbances and no histological lesions were found in
the peripheral or central nervous system. The positive controls showed
lack of coordination, unsteadiness, and loss of balance, which
worsened progressively. Foci of axonal and myelin degeneration were
observed in all positive control birds (Milner & Butterworth, 1977).
Technical-grade permethrin (cis:trans ratio, 36.0%:58.9%;
purity, 94.9%) was administered at a single dose of 15 ml, equal to
9100 mg/kg bw, the maximum possible dose, to 15 hens, which were
observed for 21 days for signs of neurotoxicity. The hens were treated
with an intramuscular injection of atropine and pyridine-2-aldoxime
methane sulfonate, then re-dosed with 15 ml of permethrin and observed
for an additional 21 days. Five hens were given tri- ortho-tolyl
phosphate at 500 mg/kg bw as a positive control, and 10 hens received
water as a negative control. No deaths occurred during the study, and
no signs of neurotoxicity were noted in the treated or negative
control groups. Ataxia, ranging from slight muscular incoordination to
difficulty in standing, was observed in the positive control group. No
treatment-related histopathological lesions of the spinal cord or
sciatic nerve were observed in the treated or negative control groups,
whereas degenerative changes were seen in the spinal cord and sciatic
nerve of hens treated with tri- ortho-tolyl phosphate. An NOAEL for
neurotoxicity could not be identified (Ross, et al., 1977).
(ii) Endocrine effects
The MCF-7 human breast carcinoma cell line was used to study the
oestrogenic potential of permethrin in vitro, pS2 mRNA expression
levels being used as the end-point. Permethrin at 100 µmol/L had no
effect on pS2 expression or on cell proliferation, whereas
17ß-estradiol at a concentration of 10 nmol/L induced a fivefold
increase in pS2 expression (Go et al., 1999).
3. Observations in humans
Twenty-three workers aged 20-52 years who had been exposed to
synthetic pyrethroids and 23 age- and sex-matched control subjects who
had had no contact with pyrethroids were interviewed, examined, and
assessed electrophysiologically. Most of the workers had been exposed
to several different pyrethroids, the more common being cypermethrin,
permethrin, fenvalerate, and fenpropathrin. Nineteen of the subjects
had experienced one or more episodes of abnormal facial sensation
0.5-3 h after exposure which persisted for 0.5-8 h. Thirteen subjects
had experienced more than one episode. There were no abnormal
neurological signs, and the results of electrophysiological studies of
the arms and legs were normal. Three subjects who had had moderate
exposure to permethrin had not developed symptoms, whereas two of them
did so after exposure to fenpropathrin and cypermethrin (Le Quesne et
al., 1980).
Workers who handled seedlings treated with permethrin
(cis:trans ratio, 25:75 for a wettable powder and 40:60 for an
emulsion in organic solvents; both diluted with water to 1-2% before
use) reported irritation of the skin. Of 42 workers who had used the
wettable powder, 12% reported burning and 10% reported blisters. Of 45
workers who had used the emulsion, 2% reporting itching. Irritation of
the upper respiratory tract involving increased nasal secretions and
sneezing was reported by 31% of the workers who had used the wettable
powder and by 2% of those who has used the emulsion (Kolmodin-Hedman
et al., 1982).
Volunteers received applications of 0.05 ml of a field-strength
preparation of technical-grade permethrin (94-96% active ingredient)
in ethanol to an area of 4 cm2 on an ear lobe, a formulation (32-36%
active ingredient) in water to 0.13 mg/cm2, or the solvent and
surfactants; 0.05 ml of the vehicle was applied to the other lobe. No
cutaneous sensation was elicited by the inert ingredients.
Paraesthesia appeared after a latent period of about 30 min, peaked
between 8 and 12 h, and disappeared after about 24 h. Further studies
with a range of doses ahowed that the response was dose-related
(Flannigan et al., 1985).
One of 28 subjects with pediculosis pubis treated with a 1%
permethrin rinse developed mild scrotal erythema and irritation 12 h
after application (Kalter et al., 1987). A group of 435 patients, most
of them children, were treated for pediculosis capitis: approximately
half of the group were treated with a single, 10-min application of
25-50 ml of a cream rinse containing permethrin (1%) and isopropanol
(20%) after towel drying of washed hair, and the remainder were
treated with a liquid product containing pyrethrins (0.3%), piperonyl
butoxide (3%), petroleum distillate (1.2%), and benzyl alcohol (2.4%).
Cutaneous side-effects including pruritus, mild transient skin
burning, stinging sensations, skin tingling, erythema, and scalp rash,
were reported by 7% of the patients in the first group and by 16% of
those in the second (DiNapoli et al., 1988). Similar results and
side-effects were reported by Brandenburg et al. (1986).
An observational study was undertaken in the USA to evaluate the
safety of a cream rinse containing 1% permethrin for treatment of head
louse infestations. Thirty-seven local public health departments
enrolled a total of 38 160 patients for 47 578 treatments with
permethrin and other pediculicides between 1 September 1986 and 31
January 1988. Follow-up information was collected 7-14 days after
treatment at a return visit or by telephone contact. A total of 103
adverse events were reported from 41 955 evaluable treatments. The
rates of reported adverse events were 2.2 per 1000 treatments with
permethrin, 3.4 per 1000 treatments with lindane, and 1.5 per 1000
treatments with other over-the-counter preparations. No serious,
unexpected adverse event was detected in the 18 950 patients treated
with permethrin (Andrews et al., 1992).
Of 10 patients with scabies treated with one application of 25 g
(range, 21-32 g) of a cream containing 5% permethrin, followed by a
thorough washing 8-20 h after treatment, six developed limited,
mild-to-moderate eczema on the scabies-affected skin at one or more
examinations (van der Rhee et al., 1989).
Comments
The metabolism of 14C-permethrin was studied in rats, lactating
goats and cattle, and laying hens. Permethrin was rapidly absorbed,
distributed, and excreted in these species after oral administration.
The metabolism of the pyrethroid was extensive, yielding a vast number
of polar degradates. Ester cleavage, hydroxylation, oxidation, and
ultimately conjugation are the critical biological mechanisms of the
metabolism of permethrin in the species studied. The metabolites that
were common to all species were 4'-hydroxypermethrin, dichlorovinyl
acid, and phenoxybenzyl alcohol. Dichlorovinyl acid and phenoxybenzoic
acid have also been identified in human urine after dermal application
of permethrin.
In rats, 96% of an administered dose was recovered in urine and
faeces within 12 days, while the total radiolabelled residues in
tissues accounted for 0.3-0.8% of the dose. Recovery in urine and
faeces within 24 h accounted for about 40% and 25% of the dose of
cis isomer and 65% and 10% of the dose of trans isomer.
respectively. Repeated exposure resulted in temporary accumulation in
fat tissue, but the chemical dissipated rapidly once exposure had
ceased.
In lactating goats and cows dosed orally with permethrin,
recovery in urine and faeces accounted for at least 65% of the dose,
and the total radiolabelled residues in liver and milk samples
represented 0.2-0.5%. Permethrin was extensively metabolized and
readily eliminated after oral administration to laying hens, > 90%
of the administered dose being excreted, while the total radiolabel in
egg and liver samples accounted for 0.1-0.2% of the dose.
The toxicity of permethrin is influenced by many factors
including the cis:trans isomer ratio, the carrier or vehicle, and
the strain of animal used. The cis isomer is considerably more toxic
than the trans isomer. The oral LD50 values in rats ranged from
6000 mg/kg bw for the 20:80 cis:trans isomeric mixture to 220 mg/kg
bw for the 80:20 cis:trans isomeric mixture. Undiluted
technical-grade permethrin (25:75 to 40:60 cis:trans isomeric
mixtures) has low acute toxicity after oral, dermal and inhalation
exposure. It was mildly irritating to the eyes and slightly irritating
to skin. It was not a skin sensitizer when tested by the Magnusson and
Kligman method.
WHO (1999) has classified permethrin as 'moderately hazardous'.
Studies in which rats, mice, rabbits, guinea-pigs, and dogs
received repeated administrations by inhalation, orally, and dermally
showed that the main effects of technical-grade permethrin are on
clinical signs, especially tremor and hyperexcitability, body weight,
and liver weight. In these short-term studies, the NOAEL or NOAEC
values were 250 mg/m3 in a 13-week study in rats exposed by
inhalation; 5 mg/kg bw per day in a 52-week study in which dogs
received the compound in gelatin capsules orally; and 1000 mg/kg bw
per day in a 21-day study in rabbits treated dermally.
In two long-term studies in rats in different laboratories with
different strains, permethrin was not carcinogenic, but the evidence
for carcinogenicity in mice was conflicting. In two studies conducted
in same strain in the same laboratory, permethrin increased the
incidences of lung and liver tumours in one study but not in the
other. The spontaneous background incidence of both these tumour types
is known to be extremely variable. A third study, conducted in a
different mouse strain, gave negative results. Thus, the weight of
evidence supports the conclusion that permethrin has very weak
oncogenic potential, and the probability that it has oncogenic
potential in humans is remote. The NOAEL for long-term toxicity in
rats was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs and changes in body and organ weights and blood
chemistry at 500 ppm. The NOAEL for long-term toxicity in mice was
500 ppm, equivalent to 75 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm.
No genotoxic activity was observed in an adequate battery of
tests for DNA damage and mutagenicity in vitro, but there was
evidence that permethrin can induce chromosomal aberrations in
mammalian cells in vitro. No tests have been carried out in mammals
for DNA damage, mutagenicity, or clastogenicity in vivo. A test for
dominant lethal effects in male mice showed no activity.
In a multigeneration study of reproductive toxicity in rats, the
NOAEL for systemic and reproductive toxicity was 180 mg/kg bw per day.
In a second multigeneration study in rats, an NOAEL could not be
identified for systemic toxicity, as effects were seen at 500 ppm,
equivalent to 33 mg/kg bw per day, the lowest dose tested; the NOAEL
for reproductive toxicity in the same study was 2500 ppm, equivalent
to 170 mg/kg bw per day, the highest dose tested.
In a study of developmental toxicity in rabbits, the NOAEL for
maternal effects was 600 mg/kg bw per day and that for developmental
toxicity was 1200 mg/kg bw per day. In three studies of developmental
toxicity in rats, the NOAEL for maternal toxicity was 83 mg/kg bw per
day and the NOAEL for developmental toxicity was 225 mg/kg bw per day,
the highest dose tested. In a study of developmental toxicity in mice,
no NOAEL was identified for maternal toxicity, whereas the NOAEL for
developmental effects was 400 mg/kg bw per day, the only dose tested.
The Meeting concluded that technical-grade permethrin is not a
reproductive or developmental toxin.
The results of acute and 90-day studies of neurotoxicity in rats
and of an acute study of delayed neurotoxicity in hens showed that
technical-grade permethrin does not induce neuropathological changes.
The NOAEL for neurotoxicity in a study in rats given a single dose was
150 mg/kg bw, on the basis of clinical signs of neurotoxicity and
significant changes in measurements in a functional observational
battery of tests at 300 mg/kg bw. The NOAEL for neurotoxicity in a
13-week study in rats was 15 mg/kg bw per day, on the basis of
clinical signs of neurotoxicity and significant changes in
measurements in the functional observational battery of tests at 90
mg/kg bw per day.
An ADI of 0-0.05 mg/kg bw was established for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60 on the basis of
the NOAEL of 100 ppm, equivalent to 5 mg/kg bw per day, in the 2-year
study in rats, which was based on clinical signs and changes in body
and organ weights and blood chemistry at 500 ppm, and the NOAEL of 5
mg/kg bw per day in a 1-year study in dogs based on reduced body
weight at 100 mg/kg bw per day, and applying a safety factor of 100.
The Meeting concluded that establishment of an acute reference
dose was not necessary because of the low acute toxicity of
technical-grade permethrin.
Toxicological evaluation
Levels that cause no toxic effect (relevant for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60)
Mouse: 500 ppm, equivalent to 75 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
Rat: 100 ppm, equivalent to 5 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
180 mg/kg bw per day (for reproductive toxicity, highest
dose in a three-generation study of reproductive toxicity)
225 mg/kg bw per day (for maternal and developmental
toxicity, highest dose in a study of developmental toxicity)
150 mg/kg bw (single dose in a study of neurotoxicity)
15 mg/kg bw per day (13-week study of neurotoxicity)
Rabbit: 400 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
1200 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity)
Dog: 5 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.05 mg/kg bw (for technical-grade permethrin with cis:trans
ratios of 25:75 to 40:60)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Clarification of the findings of chromosomal aberrations in
vitro and their potential significance in vivo
2. The Meeting was aware of other studies, in particular of acute
toxicity, dermal and ocular irritation, sensitization, and
developmental toxicity in rats that had been made available to
regulatory entities by other sponsors. The continuing support of
permethrin would benefit from the submission of these studies for
review by the Joint Meeting.
Toxicological criteria relevant for estimating guidance values for dietary and non-dietary exposure to permethrin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid and extensive (rat, lactating caprine and bovine, hen)
Distribution Mainly to fat (rat, lactating caprine and bovine, hen)
Potential for accumulation Some accumulation in fat on repeated dosing, rat
Rate and extent of excretion cis-isomer: 40% in urine, 25% in faeces in 24 h in rat
trans-isomer: 65% in urine, 10% in faeces in 24 h in rat
Metabolism in animals Extensive; hydrolysis, hydroxylation, oxidation and conjugation
(rat, lactating caprine and bovine, hen)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 225 (cis:trans ratio, 80:20); 6000 (cis:trans ratio,
20:80) mg/kg bw
Rat, LD50, dermal No data
Rabbit, LD50, dermal 2000 mg/kg bw (cis:trans ratio, 55:45 or 40:60) (highest dose)
Rat, LC50, inhalation > 23.5 mg/l, 4 h (cis:trans ratio, 40:60)
Dermal irritation Slightly irritating to rabbit skin
Ocular irritation Mildly irritating to rabbit eyes
Dermal sensitization No sensitizing potential in guinea-pigs
Short-term toxicity
Target/critical effect Nervous system (rat); liver (mouse, rat, dog)
Lowest relevant oral NOAEL 5 mg/kg bw per day in dog (cis:trans ratio, 32%:52%)
Lowest relevant dermal NOAEL 1000 mg/kg bw per day in rabbit
Lowest relevant inhalation NOAEL 250 mg/m3 in rat (cis:trans ratio, 40:60)
Target/critical effect Nervous system (rat)
Liver (mouse, rat)
Lowest relevant NOAEL Rat, 5 mg/kg bw per day; mouse, 75 mg/kg bw per day
Long-term toxicity and carcinogenicity
Carcinogenicity Not carcinogenic to mouse or rat
Genotoxicity No DNA damage or mutagenicity in vitro; clastogenic
in vitro; not studied in vivo in mammals
Reproductive toxicity
Reproductive target/critical effect None identified
Lowest relevant reproductive NOAEL 180 mg/kg bw per day in rat
Developmental target/critical effect Rat, none identified; rabbit, fetotoxicity
Lowest relevant developmental NOAEL Rat, 225 mg/kg bw per day (cis:trans ratio, 38%:58%);
rabbit, 1200 mg/kg bw per day (cis:trans ratio, 40:60)
Neurotoxicity/Delayed neurotoxicity NOAEL, 150 mg/kg bw, single dose, rats;
NOAEL, 15.5 mg/kg bw per day in a 90-day study, rats
No acute delayed effect in hens (9100 mg/kg bw)
Other toxicological studies
Medical data Paraesthesia
Summary Value Study Safety factor
ADI 0-0.05 mg/kg bw Rat, long-term toxicity, 100
5 mg/kg bw per day
Dog, 1 year, toxicity,
5 mg/kg bw per day
Acute reference dose Unnecessary
References
Andrews, E.B., Joseph, M.C., Magenheim, M.J., Tilson, H.H., Doi, P.A.
& Schultz, M.W. (1992) Postmarketing surveillance study of
permethrin creme rinse. Am. J. Public Health, 82, 857-861.
Angerer, S. & Ritter, A. (1997) Determination of metabolites of
pyrethroids in human urine using solid-phase extraction and gas
chromatography-mass spectrometry. J. Chromatogr. B Biomed.
Sci. Appl., 695, 217-226.
Bartsch, H., Malaveille, C., Camus, A.M., Martel-Planche, G., Brun,
G., Hautefeuille, A., Sabadie, N., Barbin, A., Kuroki, T.,
Drevon, C., Piccoli, C. & Montesano, R. (1980) Validation and
comparative studies on 180 chemicals with S. typhimurium
strains and V79 Chinese hamster cells in the presence of various
metabolizing systems. Mutat. Res., 76, 1-50.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1992)
Cytogenetic effects of permethrin in cultured human lymphocytes.
Mutagenesis, 7, 433-437.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1994)
Induction of structural chromosome aberrations in human
lymphocyte cultures and CHO cells by permethrin. Teratog.
Carcinog. Mutag., 14, 31-38.
Becci, P.J. & Parent, R. (1980) Evaluation of the subchronic toxic
effects of FMC 45801 when administered in the diet to Long Evans
rats over a 90-day period. Unpublished report from Food and Drug
Research Laboratories, Inc., Waverly Research Center, USA, Study
No. 6363. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Becci, P.J., Gephart, L. & Parent, R.A. (1980) 90-day subchronic oral
dosing study with FMC 45801 in beagle dogs. Unpublished report
from Food and Drug Research Laboratories, Inc., Waverly Research
Center, USA, Study No. 6338. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Benner, J. (1997) Permethrin: addendum to MRID numbers 42410001,
43505201, 43962801 and 44196101 submitted in response to EPA CBRS
review of MRID 43962801, goat metabolism -- oral dosing.
Unpublished report from Zeneca Agrochemicals, Bracknell, England,
Project No. HRC/ISN 248/920216sup2. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Benner, J.P., Hamlet, J.M. & Skidmore, M.W. (1996) Addendum to MRID
Nos. 42410001, 43505201 and 43962801, Permethrin: Further
investigation of residues in liver following oral administration
to the goat and radiovalidation of enforcement methods for
analysis of animal tissues. Unpublished report from Zeneca
Agrochemicals (Zeneca Ltd), Bracknell, England, Report No. RJ
2135B. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Billups, L.H. (1978a) Twenty-four month toxicity/carcinogenicity study
of compound FMC 33297 in rats. Histopathologic evaluation of
step-sectioned lungs from male rats. Unpublished report from
Environmental Pathology Services, Rockville, Maryland, USA.,
Study No. 78-6-0049. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA and by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
Billups, L.H. (1978b) Histopathologic evaluation of a twenty-four
month toxicity/carcinogenicity study of compound FMC 33297 in
rats. Unpublished report from Environmental Pathology Services,
Rockville, Maryland, USA., Study No. 77-11-0007. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA and by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Brandenburg, K., Deinard, A.S., DiNapoli, J., Englander, S.J.,
Orthoefer, J. & Wagner, D. (1986) 1% permethrin cream rinse vs
1% lindane shampoo in treating pediculosis capitis. Am. J.
Dis. Child., 140, 894-896.
Bratt, H., Mills, I.H. & Slade, M. (1977) Permethrin: Tissue retention
in rats. Unpublished report from Imperial Chemical Industries
Ltd, Alderley Park, England, Report No. ZO671. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975a) Acute oral toxicity studies in
rats with FMC 33297. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project Nos. 2702-75, 2703-75,
2704-75 and 2705-75. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975b) Acute dermal toxicity in
rabbits. Compound No. FMC 33297. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA, Project No.
2908-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975c) Rabbit primary dermal
irritation. Compound No. FMC 33297. Unpublished report prepared
by Bio/dynamics Inc. East Millstone, New Jersey, USA, Project No.
2909-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975d) Rabbit eye irritation.
Compound No. FMC Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2909-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1976) Acute inhalation. Compound No.
FMC 33297. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2911-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Rinehart, W.E. (1977) Twenty-four month oral
toxicity/carcinogenicity study of FMC 33297 technical in rats.
Unpublished report from Bio/dynamics, Inc., East Millstone, New
Jersey, USA, Project No. 74R-1022. Submitted to WHO by FMC
Corporation, Middleport, New York, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Brusick, D.J. & Weir, R.J. (1976) Mutagenicity evaluation of FMC 33297
(catalyst: titanium isopropylate), C8093-25, MR 51310.
Unpublished report from Litton Bionetics, Inc. Kensington,
Maryland, USA, Project No. 2683. Submitted to WHO by FMC
Corporation, Middleport, New York, USA.
Busey, W.M. (1978) Two-year chronic rat toxicity study FMC-33297.
Unpublished report from Experimental Pathology Laboratories Inc.,
Rockville, Maryland, USA. FMC Project No. NCT 549.32. Revised
pathology report. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Butterworth, S.T.G. & Hend, R.W. (1976) Toxicity studies on the
insecticide WL 43479: A five week feeding study in rats.
Unpublished report from Shell Research Ltd, Sittingbourne,
England, Report No. TLGR.0056.76. Submitted to WHO by Mitchell
Cotts Chemicals, Ltd, Mirfield, England.
Cameron, B.D. & Partridge, S.(1989) The metabolism of
[14C]-permethrin in the rat. Unpublished report from Inveresk
Research International, Musselburgh, Scotland, Report No. 4860.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Chesher, B.C. & Malone, J.C. (1974) Guinea pig sensitisation study
with 21Z73 using the maximization test method. Unpublished report
from Wellcome Research Laboratories, Berkhamsted, England, Lab.
Ref. No. T.L.13-74. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Chesher, B.C., Malone, J.C. & Parker, M.J. (1975) 21Z73, Dominant
lethal study in male mice. Unpublished report from Wellcome
Research Laboratories, Berkhamsted. England, Lab. Ref. No. T.L.
37-75. Submitted to WHO by FMC Corporation, Middleport, New York,
USA.
Clapp, M.J.L., Banham, P.B., Glaister, J.R. & Moyes, A. (1977a) PP557:
28 day feeding study in mice. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/356. Submitted to WHO by FMC Corporation, Middleport, New
York, USA.
Clapp, M.J.L., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W. &
Moyes, A. (1977b) PP557: 28 day feeding study in rats.
Unpublished report from Imperial Chemical Industries, Ltd,
Alderley Edge, England, Report No. CTL/P/355. Submitted to WHO by
FMC Corporation, Middleport, New York, USA.
Clive, D. (1977) Mutagenicity of BW 21Z73 in L5178Y/TK+/- mouse
lymphoma cells with and without exogenous metabolic activation.
Unpublished report from Burroughs Wellcome Co., Research Triangle
Park, North Carolina, USA, Doc. No. TTEP/77/0001. Submitted to
WHO by FMC Corporation, Middleport, New York, USA.
Cridland, J.S. & Weatherley, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)2,2-dimethylcyclopropane carboxylic acid
after oral ingestion of permethrin (NRDC 143) -- A first report.
Unpublished report from The Wellcome Foundation, Ltd, Beckenham,
England, Report No. BDPE-77-1. Submitted to WHO by The Wellcome
Foundation, Ltd, Beckenham, England.
Cridland, J.S. & Weatherley, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial
of the insecticide. Unpublished report from The Wellcome
Foundation, Ltd, Beckenham, England, Report No. BDPE-771.
Submitted to WHO by The Wellcome Foundation, Ltd, Beckenham,
England.
Cummins, H.A. & Gardner, J.R. (1984a) Permethrin: Acute oral toxicity
in the rat. Unpublished report from Life Science Research, Eye,
England, Report No. 84/MCC001/588. Submitted to WHO by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Cummins, H.A. & Gardner, J.R. (1984b) High cis permethrin: (isomer
ratio: 80:20) acute oral toxicity in the rat. Unpublished report
from Life Science Research, Eye, England, Report No.
84/MCC002/587. Submitted to WHO by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
DiNapoli, J.B., Austin, R.D., Englander, S.J., Gomez, M.P. & Barrett,
J.F. (1988) Eradication of head lice with a single treatment.
Am. J. Public Health, 78, 978-980.
Dyck, P.J. (1978) A pathologic and morphometric study of the nervous
system of rats fed compound FMC 33297 (permethrin). Unpublished
report from Peripheral Nerve Laboratory, Mayo Clinic and Mayo
Foundation, New York, New York, USA, Report No. not identified.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Flannigan, S.A., Tucker, S.B., Key, M.M., Ross, C.E., Fairchild, E.J.,
II, Grimes, B.A. & Harrist, R.B. (1985) Synthetic pyrethroid
insecticides: A dermatological evaluation. Br. J. Ind. Med.,
42, 363-372.
Flodström, S., Warngard, L., Ljungquist, S. & Ahlborg, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch.Toxicol., 61, 218-223.
Freeman, C. (1993a) Permethrin technical twenty-eight day
neurotoxicity range-finding study in rats. Unpublished report
from FMC Corporation, Princeton, New Jersey, USA, Study No.
A92-3645. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Freeman, C. (1993b) Permethrin technical subchronic neurotoxicity
screen in rats. Unpublished report from FMC Corporation,
Princeton, New Jersey, USA, Study No. A92-3647. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA
Freeman, C. (1993c) Permethrin technical acute neurotoxicity screen in
rats Unpublished report prepared by FMC Corporation, Princeton,
New Jersey, USA, Study No. A923646. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA
Gammon, D.W., Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. Appl. Pharmacol., 66,
290-296.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1977)
Permethrin metabolism in rats. J. Agric. Food Chem., 25, 9-17.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1978)
Distribution and metabolism of trans- and cis-permethrin in
lactating Jersey cows. J. Agric. Food Chem., 26, 613-618.
Ghiasuddin, S.M. & Soderlund, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. Appl.
Pharmacol., 74, 390-396.
Go, V., Garey, J., Wolff, M.S. & Pogo, B.G. (1999) Estrogenic
potential of certain pyrethroid compounds in the MCF-7 human
breast carcinoma cell line. Environ. Health Perspectives, 107,
173-177.
Gupta, R.K., Mehr, Z.A., Korte, D.W. & Rutledge, L.C. (1990) Mutagenic
potential of permethrin in the Drosophila melanogaster
(Diptera: Drosophilidae) sex-linked recessive lethal test.
J. Econ. Entomol., 83, 721-724.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977a) PP557: Liver hypertrophy study in rats -- Dietary
administration over 26 weeks. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/360. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977b) PP557: Whole life feeding study in mice. Unpublished
report from Imperial Chemical Industries, Ltd, Alderley Park,
England, Report No. CTL/P/359. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, D., Shaw, D. & Latter (1992a) The
metabolism of 14C permethrin in the hen. Unpublished from
Huntingdon Research Centre Ltd, Cambridgeshire, England, Project
No. HRC/ISN 272/920435. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, & Shaw, D. (1992b) The metabolism of 14C
permethrin in the goat. Unpublished report from Huntingdon
Research Centre Ltd, Cambridgeshire, England, Project No. HRC/ISN
248/920216. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Herrera, A. & Laborda, E. (1988) Mutagenic activity in synthetic
pyrethroids in Salmonella typhimurium. Mutagenesis, 3, 509-514.
Herrera, A., Barrueco, C., Caballo, C. & de la Peña E. (1992) Effect
of permethrin on the induction of sister chromatid exchanges and
micronuclei in cultured human lymphocytes. Environ. Mol.
Mutag., 20, 218-222.
Hodge, M.C., Banham, P.B., Glaister, J.R., Richards, D., Taylor, K. &
Weight, T.M. (1977) PP557: 3-Generation reproduction study in
rats. Unpublished report from Imperial Chemical Idustries Ltd,
Alderley Edge, England, Report No. CTL/P/361. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA.
Hogan, G.K. & Rinehart, W.E. (1977) Twenty-four month oral
carcinogenicity study with FMC 33297 technical in mice.
Unpublished report from Bio/dynamics, Inc., East Millstone,New
Jersey, USA, Project No. 74-1100. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Ishmael, J. & Litchfield, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice.
Fundam. Appl. Toxicol., 11, 308-322.
James, D.A. (1974a) Preliminary foetal toxicity study in the mouse
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/12.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1974b) Preliminary foetal toxicity study in the rat given
21Z73 (NRDC 143) orally. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/10.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
James, D.A. (1974c) Preliminary foetal toxicity study in the rabbit
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/19.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 79/3. Submitted
by FMC Corporation, Princeton, New Jersey, USA.
Kalinowski, A.E., Banham, P,B., Chart, I.S., Cook, S.K., Gore, C.W.,
Moreland, S.F. & Woollen, B.H. (1982) Permethrin: One year oral
dosing study in dogs. Unpublished report from Imperial Chemical
Industries Ltd, Alderley Park, England, Report No. CTL/P/647.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Kalter, D.C., Sperber, J., Rosen, T. & Matarasso, S. (1987) Treatment
of pediculosis pubis. Clinical comparison of efficacy and
tolerance of 1% lindane shampoo vs 1% permethrin cream rinse.
Arch. Dermatol., 123, 1315-1319.
Killeen, J.C. & Rapp, W.R. (1974) A four week feeding study of FMC
33297 in rats. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 74S-1024. Submitted to
WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1975a) A thirty day pilot feeding study of
FMC 33297 in rats. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project No. 74-1098. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C., Jr & Rapp, W.R. (1975b) A fourteen-day dose
range-finding study of FMC 33297 in beagle dogs. Unpublished
report from Bio/dynamics Inc., East Millstone, New Jersey, USA,
Project No. 75-1202B. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1976) A three month oral toxicity study of
FMC 33297 in beagle dogs. Unpublished report from Bio/dynamics
Inc., East Millstone, New Jersey, USA, Project No. 75-1188B.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
and by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Kolmodin-Hedman, B., Swensson, Å. & Åkerbolm, N. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50, 27-33.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-activity relationships. Pestic.
Biochem. Physiol., 18, 9-14.
Leng, G., Kuhn, K.H. & Idel, H. (1997) Biological monitoring of
pyrethroids in blood and pyrethroid metabolites in urine:
applications and limitations. Sci. Total Environ., 199,
173-181.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980) Transient
facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurophysiology, 2, 1-11.
Litchfield, M.H. (1985) Toxicity in mammals. In: Leahey, J.P., ed.,
The Pyrethroid Insecticides, London: Taylor & Francis, pp.
99-150.
Llewellyn, D.M., Brazier, A., Brown, R., Cocker, J., Evans, M.L.,
Hampton, J., Nutley, B.P. & White, J. (1996) Occupational
exposure to permethrin during its use as a public hygiene
insecticide. Ann. Occup. Hyg., 40, 499-509.
Marowitz, L.A. (1974a) Comparative acute oral toxicity in mice with
FMC 33297, FMC 37400, FMC 35171 and FMC 30960. Unpublished report
from Bio/dynamics Inc., East Millstone, New Jersey, USA, Project
Nos. 2184-74, 2185-74, 2222-74 and 2238-74. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Marowitz, L.A. (1974b) Acute oral toxicity in rats. Compound No. FMC
33297. Unpublished report from Bio/dynamics Inc., East Millstone,
New Jersey, USA, Project No. 2186-74. Submitted to WHO by FMC
corporation, Princeton, New Jersey, USA.
McGregor, D.B. & Wickramaratne, G.A. (1976) Teratogenicity study in
rats of ICI-PP 557. Unpublished report from Inveresk Research
International, Edinburgh, Scotland, Project No. 404898. Submitted
to FMC Corporation, Princeton, New Jersey, USA
Mehr, Z.A., Justus, J.D., Gupta, R.K. & Korte, D.W., Jr (1988)
Mutagenic potential of permethrin in the Drosophila
melanogaster sex-linked recessive lethal test. Unpublished
report from Letterman Army Institute of Research, Presidio of San
Francisco, California, USA, Report No. 302. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Metker, L., Angerhofer, R.A., Pope, C.R. & Swentzel, K.C. (1977)
Toxicological evaluation of 3-(phenoxyphenyl)methyl
(+)- cis, trans-3,2-(2,2-dichloroethenyl)-2,2dimethylcyclopropa
necarboxylate (permethrin). Unpublished report from the US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 51-0831-78.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: Investigation of the neurotoxic potential of WL
43479 to adult hens. Unpublished report from Shell Toxicology
Laboratory, Sittingbourne, England, Report No. TLGR.OO69.77.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nassif, M., Brooke, J.P., Hutchinson, D.B.A., Kamel, O.M. & Savage,
E.A. (1980) Studies with permethrin against bodylice in Egypt.
Pestic. Sci., 11, 679-684.
Pednekar, M.D., Gandhi, S.R. & Netrawali, M.S. (1987) Evaluation of
mutagenic activities of endosulfan, phosalone, malathion, and
permethrin, before and after metabolic activation, in the Ames
Salmonella test. Bull. Environ. Contam.Toxicol., 38, 925-933.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille
& Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137, 7-15.
Rapp, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC 33297 in mice. Histopathology report. Unpublished report
from McConnell & Rapp, Flemington, New Jersey, USA, FMC
Identification No. ACT 605.35. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Reynolds, J., Piercy, D.W.T., Clampitt, R.B., James, J.A., Thompson,
P.M., Farebrother, D.A. & Dayan, A.D. (1978) Permethrin oral
administration to dogs for 6 months. Unpublished report from The
Wellcome Foundation Ltd, Berkhamsted Hill, England, Doc. No. HEFG
78-14. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
van der Rhee, H.J., Farquhar, J.A. & Vermeulen, N.P.E. (1989) Efficacy
and transdermal absorption of permethrin in scabies patients.
Acta Dermatol. Venereol., 69, 170-182.
Richards, D., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W.,
Pratt, I., Taylor, K. & Weight, T.M. (1977) PP557: Two year
feeding study in rats. Unpublished report from Imperial Chemical
Industries, Ltd, Alderley Edge, England, Report No. CTL/P/357.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Richards, D., Banham, P.B., Kilmartin, M. & Weight, T.M. (1980)
Permethrin: teratogenicity study in the rabbit. Unpublished
report from Imperial Chemical Industries Ltd, Alderley Edge,
England, Report No. CTL/P/523. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E. & Cooke, L.
(1977) Examination of permethrin for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon Research Centre,
Huntingdon, England, Doc. No. ICI/157-NT/77468. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980a) Acute oral toxicity in rats. Test article: FMC 33297
55/45. Unpublished report from Cosmopolitan Safety Evaluation
Laboratory, Inc., Somerville, New Jersey, USA, Study No. 0245A.
submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980b) Acute dermal toxicity study -- Rabbit LD50 test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245B. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980c) Primary dermal irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory Inc., Somerville, New Jersey, USA,
Study No. 0245D. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980d) Primary eye irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245C. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Schroeder, R.E. & Rinehart, W.E. (1977) A three generation
reproduction study of FMC 33297 in rats. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Shah, P.V., Monroe, R.J. & Guthrie, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59, 414-423.
Simmon, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC Corporation compound. Unpublished report from Stanford
Research Institute, Menlo Park, California, USA, Project No.
LSC-4768. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Simmon, V.F., Riccio, E.S., Robinson, D.E. & Mitchell, A.D. (1979) In
vitro microbiological mutagenicity and unscheduled DNA synthesis
studies of fifteen pesticides. Unpublished report from SRI
International, Menlo Park, California, USA, Project No. LSU-3493.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Skidmore, M. (1996) Permethrin: Supplemental information on MRID Nos.
42410001 and 43505201 submitted in response to EPA CBRS review
dated 5 September 1995. Goat metabolism study -- oral dosing.
Unpublished report from Zeneca Ag Products, Bracknell, England,
Project No. HRC/ISN 248/920216sup1. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Soderlund, D.M. & Casida, J.E. (1977) Effects of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pest. Biochem. Physiol., 7, 391-401.
Staatz, C.G., Bloom, A.S. & Lech, J.J. (1982) A pharmacological study
of pyrethroid neurotoxicity in mice. Pest. Biochem. Physiol.,
17, 287-292.
Surrallès, J., Xamena, N., Creus, A., Catalan, J., Norppa, H. &
Marcos, R. (1995a) Induction of micronuclei by five pyrethroid
insecticides in whole-blood and isolated human lymphocyte
cultures. Mutat Res., 341, 169-184.
Surrallès, J., Xamena, N., Creus, A. & Marcos, R. (1995b) The
suitability of the micronucleus assay in human lymphocytes as a
new biomarker of excision repair. Mutat Res., 342, 43-59.
Tierney, W.J. & Rinehart, W.E. (1979) A twenty-four month oral
carcinogencicity study of FMC 33297 in mice. Unpublished report
from Bio/dynamics, East Millstone, New Jersey, USA, Project No.
76-1695. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
United States Army (1978) Subchronic inhalation toxicity of
3-(phenoxyphenyl) methyl
(+) cis, trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane
carboxylate (permethrin). Unpublished report from US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 75-51-0026-80. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
United States Army (1986) Neurotoxicity in rats following subchronic
ingestion of permethrin-treated food. Unpublished report from US
Army Environmental Hygiene Agency, Aberdeen Proving Ground, Study
No. 75-51-0351-87. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Wallwork, L.M., Poll, G.S. & Malone, J.C. (1975) Effect on the rat
oral toxicity of changes in the cis/ trans ratio with 21Z73
(NRDC 143) series. Unpublished report from The Wellcome
Foundation, Berkhamsted, England, Doc. No. HEFG 75-5. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, L.M., Thomson, P.M., Clampitt, R.B. & Malone, J.C. (1976b)
Rat oral 90 day toxicity study. Unpublished report from The
Wellcome Foundation, Berkhamsted and Beckenham, England, Doc. No.
HEFG 76-1. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Williams, L.M., Clampitt, R.D., James, J.A., Reynolds, J. & Woods,
N.C. (1976a) Rat oral 90 day toxicity study. Unpublished report
from TheWellcome Foundation, Berkhamsted, England, Doc. No. HEFG
76-3. Submitted to WHO by FMC Corporation, Princeton, New Jersey,
USA.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster.
Environ. Mutag., 5, 835-846.
 Table 4. Distribution of 14C-labelled permethrin residues in rats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Urine 28 22 19 20
Faeces 71 72. 76 74
Cage wash 2.0 2.5 2.4 2.4
Total excreted 101 96 98 97
Tissues and carcass 0.49 0.30 0.58 0.84
Total recovered after 7 days 101 97 98 98
Table 5. Distribution of 14C-labelled permethrin residues in rat
tissues
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Bone 0.07 0.08 0.14 0.16
Brain 0.18 0.03 0.02 0.01
Fat 6.6 2.4 7.5 11
Heart 0.07 0.06 0.07 0.08
Muscle 0.17 0.13 0.27 0.19
Testis/Ovary 0.30 0.75 0.22 4.7
Liver 0.75 0.33 0.30 0.38
Lung 0.17 0.15 0.15 0.20
Spleen 0.09 0.08 0.13 1.2
Kidney 0.24 0.30 0.38 0.55
Stomach and contents 0.11 0.11 0.25 0.70
Intestine and contents 0.60 0.29 0.38 1.2
Whole blood 0.09 0.05 0.11 0.14
Plasma 0.06 0.04 0.11 0.10
Residual carcass 0.44 0.29 0.63 1.0
analysis. In other experiments, liver, kidneys, brain, and a sample of
muscle were also removed. Groups of male or female rats were also
given single oral doses of 1-6 mg/kg bw for determination of
radiolabel in blood or for whole-body autoradiography. Radiolabel in
tissues and blood was determined after their homogenization and
combustion, while those in fat were identified after solubilization of
the samples in hexane, a clean-up procedure, and thin-layer
chromatography. Sections were prepared for whole-body autoradiography
from animals killed 1, 24, and 96 h after dosing.
The maximum concentration of radiolabel in the fat of rats given
[14C-phenyl]permethrin (1 mg/kg bw; 0.12 µCi) for 77 days was 2.0
ppm. A steady-state concentration was attained within 3 weeks of
dosing. The half-time of elimination of radiolabel from fat was 18
days. The concentrations in liver and kidneys were below the limit of
detection (0.08 ppm) within 1 week of cessation of dosing. No
detectable residues were present in brain or muscle. Similar results
were obtained when [14C-cyclopropyl]permethrin (0.9 mg/kg bw; 0.22
µCi) was given to rats for 3 weeks. The maximum concentration of
radiolabel in fat was 0.72 ppm, and the elimination half-time was
7 days. The highest concentrations of radiolabel in the liver and
kidneys were 0.22 and 0.02 ppm, respectively. Most of the radiolabel
in the fat of rats given [14C-phenyl]permethrin consisted of the
unchanged permethrin, although more cis isomer than trans isomer
was retained. Whole-body autoradiography showed that the radiolabel
was present mainly in the stomach, intestines, liver, kidneys, and fat
24 and 96 h after administration of a single oral dose of
[14C-phenyl]permethrin (6 mg/kg bw; 33 µCi).
[14C-phenyl]Permethrin at a dose of 10 mg/kg bw (20 µCi) was rapidly
absorbed: the maximum concentration (0.34 ppm) was reached in blood
within 1.5 h, and the rate of elimination corresponded to a half-time
of 7 h. Only very low concentrations of radiolabel (< 0.05 ppm) were
detected in the blood of rats given [14C-cyclopropyl]permethrin at
10 mg/kg bw (15 µCi).
The results of these experiments show some retention of
permethrin in the fat of rats after repeated oral administration and
show that the cis isomer is more readily retained than the trans
isomer. Permethrin is, however, readily eliminated from fat. The
concentrations of permethrin and/or its metabolites in other tissues
after repeated administration are relatively low, and the chemical is
not retained after dosing has ceased (Bratt et al., 1977).
In the only study of the metabolism of unlabelled permethrin
(cis:trans ratio, 25:75), a single dose of 460 mg/kg bw was given by
oral gavage and 46 mg/kg bw were given intravenously to fasted male
Sprague-Dawley rats. These doses were reported (Litchfield, 1985) to
be toxic but not lethal. Groups of eight rats were killed 0.25, 0.5,
1, 2, 3, 4, 6, 8, 12, 24, and 48 h after dosing, when blood samples
were collected and plasma separated out. The brain, sciatic nerve, and
liver were collected only from orally treated rats at 0.5, 1, 2, 4, 8,
24, and 48 h. The brains were dissected and the major regions stored
separately. The samples were analysed by high-performance liquid
chromatography for permethrin and its metabolites meta-phenoxybenzyl
alcohol and meta-phenoxybenzoic acid.
The plasma profile of permethrin after intravenous dosing could
be described by a two-compartment open model, with a relatively rapid
distribution phase (half-time, 0.46 h) and a more prolonged
elimination phase (half-time, 8.7 h). The apparent volumes of
distribution were relatively large (elimination V, 0.72 L;
steady-state Vss,0.65 L). After the single oral dose, permethrin
was slowly absorbed (Tmax = 3.5 h when Cmax = 50 mg/ml) and
slowly eliminated (half-time, 12 h). The low total plasma clearance
(0.058 L/h) could explain the latter result. The oral bioavailability,
61%, was relatively low, perhaps due to degradation at the site of
absorption and a first-pass effect. After oral administration, the
concentrations of permethrin were particularly high in brain and and
sciatic nerve; decreasing concentrations were found in sciatic nerve
> hypothalamus > frontal cortex > hippocampus > caudate putamen
> cerebellum > plasma > medulla oblongata > liver. The ratio of
the integrated area under the curve of concentration-time (AUC) for
nervous tissue:plasma ranged from 8.8 in sciatic nerve to 1.2 in
cerebellum but was only 0.44 for liver. The brain regions also showed
the higher AUC values for the metabolites, particularly
meta-phenoxybenzyl alcohol, for which the tissue:plasma ratio was
always > 1.0, whereas this ratio was usually < 0.5 for
meta-phenoxybenxoic acid (Anadón et al., 1991).
Chickens
In a study conducted according to GLP, groups of six laying hens
received capsules containing 1.27 mg of [U-14C-phenyl]permethrin or
[1-14C-cyclopropyl]permethrin, and two hens received a capsule
containing a placebo. The dose was given orally for 7 consecutive days
at a rate equivalent to a dietary intake of 11 ppm. Urine, faeces,
cage wash, and egg samples (separated into whites and yolks) were
collected daily and analysed for radiolabel. All hens were killed 16 h
after the final dose, and samples of breast and thigh muscle, skin and
subcutaneous fat, and the liver were collected at necropsy. The
samples were analysed for radiolabel with or without solubilization or
combustion, followed by liquid scintillation counting.
A mean of 92% of the total dose was excreted (urine, faeces, cage
wash) by day 7 after administration of [14C-cyclopropyl]permethrin,
and a mean of 90% after administration of [14C-phenyl]permethrin.
The mean total was 0.2% in eggs and 0.1% in liver for both groups. The
overall recovery of radiolabel was 93% from the group given the
cyclopropyl abel and 90% from that given phenyl label. At day 6, the
residues in egg yolk reached a maximum mean concentration of 0.27 ppm
with the cyclopropyl label and 0.28 ppm with the phenyl label.
Unchanged permethrin accounted for about 50% of these residues in both
groups. A number of other metabolites were also present in the yolk,
at concentrations of < 0.001 to 0.01 ppm. One of these, present at
about 0.01 ppm, was identified as 4'-hydroxypermethrin. The
concentrations in the egg whites were much lower, ranging from 0.001
to 0.02 ppm for both groups. Unchanged permethrin accounted for about
half of the cyclopropyl-labelled residues, in addition to several
other components, including trans-dichlorovinyl acid (0.002 ppm).
The egg whites from the hens given the phenyl label contained <
0.01 ppm and were therefore not analysed. The concentrations of
radiolabelled residues in breast and leg muscle were 0.01-0.03 ppm.
The breast muscle samples were not analysed further. An average of 47%
of the radiolabel was extractable from leg muscle for both groups.
Permethrin was the major component, at 0.008 ppm, accounting for an
average of 32% in the two groups. Leg muscle from both groups also
contained two metabolites at concentrations of 0.001-0.002 ppm, and
muscle from hens dosed with cyclopropyl label contained one metabolite
at 0.001 ppm (Table 6).
The concentrations of residues in peritoneal fat were 0.37 ppm
for the cyclpropyl label and 0.31 ppm for the phenyl label. Unchanged
permethrin represented about 81% of the total fat residue with both
labels (0.29 ppm for the cyclopropyl and 0.24 ppm for the phenyl
label). The only other extractable component that contained the intact
ester linkage was present at a concentration of about 0.02 ppm and
co-chromatographed with hydroxypermethrin, but it could not be
identified unambiguouly. The total concentrations of radiolabelled
residues in subcutaneous fat (including skin) were 0.18 ppm for the
cyclopropyl label and 0.16 ppm for the phenyl label (Table 6).
The concentrations of residues in the liver were 0.17 ppm for the
cyclopropyl label and 0.29 ppm for the phenyl label. No significant
amounts of unchanged permethrin were present in liver extracts, and no
metabolites containing the intact ester linkage could be
characterized. trans- and cis-dichlorovinyl acid were present at
concentrations of 0.013 and 0.009 ppm, respectively, in livers from
cyclopropyl-treated hens. The corresponding hydrolysis product from
the phenyl label, phenoxybenzoic acid, was not detected. Covalent
binding of radiolabelled residues to tissue protein was substantial,
accounting for 36% (0.057 ppm) of the cyclopropyl-derived and 52%
(0.14 ppm) of the phenyl-derived residues. With both radiolabels, most
of the extractable components were associated with very polar,
uncharacterized material (Table 6).
Analysis of excreta showed that permethrin was extensively
metabolized in hens, yielding many polar components. The excreta
contained metabolites resulting from hydrolysis of the central ester
linkage and from hydroxylation of a geminal dimethyl group attached to
the cyclopropane ring and at the 4' position of the phenoxybenzyl
moiety. Some of the resulting metabolites were further conjugated with
glucuronic acid or sulfate. trans-Dichlorovinyl acid, the
cyclopropyl carboxylic acid resulting directly from hydrolysis of
permethrin, was the major cyclopropyl-labelled metabolite in excreta.
Permethrin was the major component in hens given phenyl-labelled
material (Table 7).
Table 6. Distribution of extractable 14C-labelled residues in tissues of hens
Metabolite Leg muscle Peritoneal fat Liver
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
trans-Dichlorovinyl acid ND ND NA NA ND ND NA NA 8.2 0.013 NA NA
cis-Dichlorovinyl acid ND ND NA NA ND ND NA NA 5.6 0.009 NA NA
Permethrin 31 0.008 34 0.008 78 0.29 77 0.24 ND ND ND ND
Others 19 0.005 10 0.002 5.4a 0.020 6.5a 0.020 73 0.12 66 0.18
Total extractable 49 0.013 44 0.010 84 0.31 84 0.26 86 0.14 66 0.18
ND, not detected; NA, not applicable
a This component corresponds to the retention time of hydroxypermethrin, but low concentrations prevented positive
identification.
Table 7. Distribution of extractable 14C-labelled residues in
excreta of hens
Metabolite Percentage of administered dose
Cyclopropyl label Phenyl label
trans-Dichlorovinyl acid 19 ND
cis-Dichlorovinyl acid 2.2 ND
4'-Hydroxy-hydroxypermethrin 1.9 2.2
4'-Hydroxypermethrin 2.1 0.8
3-Phenoxybenzyl alcohol ND 0.4
Permethrin 16 35
Others 0.3 1.9
Unknown (several polar)a 48 31
Total 89 70
a Fourteen or more unknown metabolites were found.
Permethrin was thus extensively metabolized in hens, resulting in
a large number of metabolites, many of which were polar. The
identified metabolites were formed through hydrolysis of the ester
linkage, hydroxylation of a geminal dimethyl group attached to the
cyclopropane ring, and hydroxylation at the 4' position of the
phenoxybenzyl moiety. Some of the metabolites were further conjugated
with glucuronic acid and sulfate (Hawkins et al., 1992a). Figure 2
shows the proposed metabolic pathway of permethrin in hens.
Lactating goats
In a study conducted according to GLP, two lactating goats
received oral doses of permethrin labelled at [U-14C-phenyl] or
[1-14C-cyclopropyl] for four consecutive days at a nominal rate of
55 ppm in the diet. The goat given the cyclopropyl label received
capsules containing a mean of 102 mg, and that given the phenyl label
received capsules containing a mean of 122 mg. Urine, faeces, and cage
wash were collected daily and milk was collected twice daily. Blood
samples were taken 16 h after the final dose, before slaughter, and
samples of liver, kidney, bile, omasum, abomasum and contents,
intestines and contents, foreleg and rump muscle, and subcutaneous,
omental, and perirenal fat were taken after slaughter. The samples
were analysed for radiolabel by liquid scintillation counting or by
combustion followed by liquid scintillation counting.
Table 4. Distribution of 14C-labelled permethrin residues in rats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Urine 28 22 19 20
Faeces 71 72. 76 74
Cage wash 2.0 2.5 2.4 2.4
Total excreted 101 96 98 97
Tissues and carcass 0.49 0.30 0.58 0.84
Total recovered after 7 days 101 97 98 98
Table 5. Distribution of 14C-labelled permethrin residues in rat
tissues
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Male Female Male Female
Bone 0.07 0.08 0.14 0.16
Brain 0.18 0.03 0.02 0.01
Fat 6.6 2.4 7.5 11
Heart 0.07 0.06 0.07 0.08
Muscle 0.17 0.13 0.27 0.19
Testis/Ovary 0.30 0.75 0.22 4.7
Liver 0.75 0.33 0.30 0.38
Lung 0.17 0.15 0.15 0.20
Spleen 0.09 0.08 0.13 1.2
Kidney 0.24 0.30 0.38 0.55
Stomach and contents 0.11 0.11 0.25 0.70
Intestine and contents 0.60 0.29 0.38 1.2
Whole blood 0.09 0.05 0.11 0.14
Plasma 0.06 0.04 0.11 0.10
Residual carcass 0.44 0.29 0.63 1.0
analysis. In other experiments, liver, kidneys, brain, and a sample of
muscle were also removed. Groups of male or female rats were also
given single oral doses of 1-6 mg/kg bw for determination of
radiolabel in blood or for whole-body autoradiography. Radiolabel in
tissues and blood was determined after their homogenization and
combustion, while those in fat were identified after solubilization of
the samples in hexane, a clean-up procedure, and thin-layer
chromatography. Sections were prepared for whole-body autoradiography
from animals killed 1, 24, and 96 h after dosing.
The maximum concentration of radiolabel in the fat of rats given
[14C-phenyl]permethrin (1 mg/kg bw; 0.12 µCi) for 77 days was 2.0
ppm. A steady-state concentration was attained within 3 weeks of
dosing. The half-time of elimination of radiolabel from fat was 18
days. The concentrations in liver and kidneys were below the limit of
detection (0.08 ppm) within 1 week of cessation of dosing. No
detectable residues were present in brain or muscle. Similar results
were obtained when [14C-cyclopropyl]permethrin (0.9 mg/kg bw; 0.22
µCi) was given to rats for 3 weeks. The maximum concentration of
radiolabel in fat was 0.72 ppm, and the elimination half-time was
7 days. The highest concentrations of radiolabel in the liver and
kidneys were 0.22 and 0.02 ppm, respectively. Most of the radiolabel
in the fat of rats given [14C-phenyl]permethrin consisted of the
unchanged permethrin, although more cis isomer than trans isomer
was retained. Whole-body autoradiography showed that the radiolabel
was present mainly in the stomach, intestines, liver, kidneys, and fat
24 and 96 h after administration of a single oral dose of
[14C-phenyl]permethrin (6 mg/kg bw; 33 µCi).
[14C-phenyl]Permethrin at a dose of 10 mg/kg bw (20 µCi) was rapidly
absorbed: the maximum concentration (0.34 ppm) was reached in blood
within 1.5 h, and the rate of elimination corresponded to a half-time
of 7 h. Only very low concentrations of radiolabel (< 0.05 ppm) were
detected in the blood of rats given [14C-cyclopropyl]permethrin at
10 mg/kg bw (15 µCi).
The results of these experiments show some retention of
permethrin in the fat of rats after repeated oral administration and
show that the cis isomer is more readily retained than the trans
isomer. Permethrin is, however, readily eliminated from fat. The
concentrations of permethrin and/or its metabolites in other tissues
after repeated administration are relatively low, and the chemical is
not retained after dosing has ceased (Bratt et al., 1977).
In the only study of the metabolism of unlabelled permethrin
(cis:trans ratio, 25:75), a single dose of 460 mg/kg bw was given by
oral gavage and 46 mg/kg bw were given intravenously to fasted male
Sprague-Dawley rats. These doses were reported (Litchfield, 1985) to
be toxic but not lethal. Groups of eight rats were killed 0.25, 0.5,
1, 2, 3, 4, 6, 8, 12, 24, and 48 h after dosing, when blood samples
were collected and plasma separated out. The brain, sciatic nerve, and
liver were collected only from orally treated rats at 0.5, 1, 2, 4, 8,
24, and 48 h. The brains were dissected and the major regions stored
separately. The samples were analysed by high-performance liquid
chromatography for permethrin and its metabolites meta-phenoxybenzyl
alcohol and meta-phenoxybenzoic acid.
The plasma profile of permethrin after intravenous dosing could
be described by a two-compartment open model, with a relatively rapid
distribution phase (half-time, 0.46 h) and a more prolonged
elimination phase (half-time, 8.7 h). The apparent volumes of
distribution were relatively large (elimination V, 0.72 L;
steady-state Vss,0.65 L). After the single oral dose, permethrin
was slowly absorbed (Tmax = 3.5 h when Cmax = 50 mg/ml) and
slowly eliminated (half-time, 12 h). The low total plasma clearance
(0.058 L/h) could explain the latter result. The oral bioavailability,
61%, was relatively low, perhaps due to degradation at the site of
absorption and a first-pass effect. After oral administration, the
concentrations of permethrin were particularly high in brain and and
sciatic nerve; decreasing concentrations were found in sciatic nerve
> hypothalamus > frontal cortex > hippocampus > caudate putamen
> cerebellum > plasma > medulla oblongata > liver. The ratio of
the integrated area under the curve of concentration-time (AUC) for
nervous tissue:plasma ranged from 8.8 in sciatic nerve to 1.2 in
cerebellum but was only 0.44 for liver. The brain regions also showed
the higher AUC values for the metabolites, particularly
meta-phenoxybenzyl alcohol, for which the tissue:plasma ratio was
always > 1.0, whereas this ratio was usually < 0.5 for
meta-phenoxybenxoic acid (Anadón et al., 1991).
Chickens
In a study conducted according to GLP, groups of six laying hens
received capsules containing 1.27 mg of [U-14C-phenyl]permethrin or
[1-14C-cyclopropyl]permethrin, and two hens received a capsule
containing a placebo. The dose was given orally for 7 consecutive days
at a rate equivalent to a dietary intake of 11 ppm. Urine, faeces,
cage wash, and egg samples (separated into whites and yolks) were
collected daily and analysed for radiolabel. All hens were killed 16 h
after the final dose, and samples of breast and thigh muscle, skin and
subcutaneous fat, and the liver were collected at necropsy. The
samples were analysed for radiolabel with or without solubilization or
combustion, followed by liquid scintillation counting.
A mean of 92% of the total dose was excreted (urine, faeces, cage
wash) by day 7 after administration of [14C-cyclopropyl]permethrin,
and a mean of 90% after administration of [14C-phenyl]permethrin.
The mean total was 0.2% in eggs and 0.1% in liver for both groups. The
overall recovery of radiolabel was 93% from the group given the
cyclopropyl abel and 90% from that given phenyl label. At day 6, the
residues in egg yolk reached a maximum mean concentration of 0.27 ppm
with the cyclopropyl label and 0.28 ppm with the phenyl label.
Unchanged permethrin accounted for about 50% of these residues in both
groups. A number of other metabolites were also present in the yolk,
at concentrations of < 0.001 to 0.01 ppm. One of these, present at
about 0.01 ppm, was identified as 4'-hydroxypermethrin. The
concentrations in the egg whites were much lower, ranging from 0.001
to 0.02 ppm for both groups. Unchanged permethrin accounted for about
half of the cyclopropyl-labelled residues, in addition to several
other components, including trans-dichlorovinyl acid (0.002 ppm).
The egg whites from the hens given the phenyl label contained <
0.01 ppm and were therefore not analysed. The concentrations of
radiolabelled residues in breast and leg muscle were 0.01-0.03 ppm.
The breast muscle samples were not analysed further. An average of 47%
of the radiolabel was extractable from leg muscle for both groups.
Permethrin was the major component, at 0.008 ppm, accounting for an
average of 32% in the two groups. Leg muscle from both groups also
contained two metabolites at concentrations of 0.001-0.002 ppm, and
muscle from hens dosed with cyclopropyl label contained one metabolite
at 0.001 ppm (Table 6).
The concentrations of residues in peritoneal fat were 0.37 ppm
for the cyclpropyl label and 0.31 ppm for the phenyl label. Unchanged
permethrin represented about 81% of the total fat residue with both
labels (0.29 ppm for the cyclopropyl and 0.24 ppm for the phenyl
label). The only other extractable component that contained the intact
ester linkage was present at a concentration of about 0.02 ppm and
co-chromatographed with hydroxypermethrin, but it could not be
identified unambiguouly. The total concentrations of radiolabelled
residues in subcutaneous fat (including skin) were 0.18 ppm for the
cyclopropyl label and 0.16 ppm for the phenyl label (Table 6).
The concentrations of residues in the liver were 0.17 ppm for the
cyclopropyl label and 0.29 ppm for the phenyl label. No significant
amounts of unchanged permethrin were present in liver extracts, and no
metabolites containing the intact ester linkage could be
characterized. trans- and cis-dichlorovinyl acid were present at
concentrations of 0.013 and 0.009 ppm, respectively, in livers from
cyclopropyl-treated hens. The corresponding hydrolysis product from
the phenyl label, phenoxybenzoic acid, was not detected. Covalent
binding of radiolabelled residues to tissue protein was substantial,
accounting for 36% (0.057 ppm) of the cyclopropyl-derived and 52%
(0.14 ppm) of the phenyl-derived residues. With both radiolabels, most
of the extractable components were associated with very polar,
uncharacterized material (Table 6).
Analysis of excreta showed that permethrin was extensively
metabolized in hens, yielding many polar components. The excreta
contained metabolites resulting from hydrolysis of the central ester
linkage and from hydroxylation of a geminal dimethyl group attached to
the cyclopropane ring and at the 4' position of the phenoxybenzyl
moiety. Some of the resulting metabolites were further conjugated with
glucuronic acid or sulfate. trans-Dichlorovinyl acid, the
cyclopropyl carboxylic acid resulting directly from hydrolysis of
permethrin, was the major cyclopropyl-labelled metabolite in excreta.
Permethrin was the major component in hens given phenyl-labelled
material (Table 7).
Table 6. Distribution of extractable 14C-labelled residues in tissues of hens
Metabolite Leg muscle Peritoneal fat Liver
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
trans-Dichlorovinyl acid ND ND NA NA ND ND NA NA 8.2 0.013 NA NA
cis-Dichlorovinyl acid ND ND NA NA ND ND NA NA 5.6 0.009 NA NA
Permethrin 31 0.008 34 0.008 78 0.29 77 0.24 ND ND ND ND
Others 19 0.005 10 0.002 5.4a 0.020 6.5a 0.020 73 0.12 66 0.18
Total extractable 49 0.013 44 0.010 84 0.31 84 0.26 86 0.14 66 0.18
ND, not detected; NA, not applicable
a This component corresponds to the retention time of hydroxypermethrin, but low concentrations prevented positive
identification.
Table 7. Distribution of extractable 14C-labelled residues in
excreta of hens
Metabolite Percentage of administered dose
Cyclopropyl label Phenyl label
trans-Dichlorovinyl acid 19 ND
cis-Dichlorovinyl acid 2.2 ND
4'-Hydroxy-hydroxypermethrin 1.9 2.2
4'-Hydroxypermethrin 2.1 0.8
3-Phenoxybenzyl alcohol ND 0.4
Permethrin 16 35
Others 0.3 1.9
Unknown (several polar)a 48 31
Total 89 70
a Fourteen or more unknown metabolites were found.
Permethrin was thus extensively metabolized in hens, resulting in
a large number of metabolites, many of which were polar. The
identified metabolites were formed through hydrolysis of the ester
linkage, hydroxylation of a geminal dimethyl group attached to the
cyclopropane ring, and hydroxylation at the 4' position of the
phenoxybenzyl moiety. Some of the metabolites were further conjugated
with glucuronic acid and sulfate (Hawkins et al., 1992a). Figure 2
shows the proposed metabolic pathway of permethrin in hens.
Lactating goats
In a study conducted according to GLP, two lactating goats
received oral doses of permethrin labelled at [U-14C-phenyl] or
[1-14C-cyclopropyl] for four consecutive days at a nominal rate of
55 ppm in the diet. The goat given the cyclopropyl label received
capsules containing a mean of 102 mg, and that given the phenyl label
received capsules containing a mean of 122 mg. Urine, faeces, and cage
wash were collected daily and milk was collected twice daily. Blood
samples were taken 16 h after the final dose, before slaughter, and
samples of liver, kidney, bile, omasum, abomasum and contents,
intestines and contents, foreleg and rump muscle, and subcutaneous,
omental, and perirenal fat were taken after slaughter. The samples
were analysed for radiolabel by liquid scintillation counting or by
combustion followed by liquid scintillation counting.
 Excretion in urine, faeces, and cage wash accounted for 66% of
the dose after administration of [14C-cyclopropyl]permethrin and 80%
of the dose after administration of [14C-phenyl]permethrin. The
overall recovery was 76% and 81%, respectively. The distribution of
radiolabel as a percentage of the administered dose is given in Table
8, and the total concentrations in tissues are shown in Table 9. The
concentrations of total radiolabelled residues were 0.06 ppm and 0.15
ppm in subcutaneous fat, 0.10 ppm and 0.24 ppm in perirenal fat, and
0.07 ppm and 0.17 ppm in omental fat with the cyclopropyl and phenyl
labels, respectively. In the fat from the phenyl label-treated goat,
extractable radiolabel represented 89% of the total residue and was
associated almost entirely with permethrin. The total concentrations
in muscle were 0.04 ppm and 0.02 ppm with the two labels,
respectively. The extractable portion from the muscle of the goat
given the cyclopropyl label represented 78% and contained three to
four components, including polar material. The extractable portion
from muscle of the goat given the phenyl label represented 55% and
contained two additional components not found with the cyclopropyl
label, one of which co-chromatographed with permethrin.
Table 8. Distribution of 14C-labelled residues in lactating goats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Urine 33 48
Cage wash 0.9 2.8
Faeces 32 29
Milk 0.4 0.5
Liver 0.33 0.17
Kidney 0.04 0.02
Gastrointestinal tract
and contentsa 9.4 Not measured
Total 76 81
a Intestines, omasum, and abomasum only
Table 9. Average distribution of 14C-labelled residues in
goat tissues and milk
Tissue Concentration (µg/g)
Cyclopropyl label Phenyl label
Milka 0.14-0.17 0.24-0.41
Fat
Omental 0.07 0.17
Perirenal 0.10 0.24
Subcutaneous 0.06 0.15
Kidney 1.0 0.78
Liver 1.2 0.91
Muscle (leg and rump) 0.04 0.02
Bileb 9.2 15
Plasmab 0.56 0.19
Whole blood 0.34 0.14
a Mean daily concentration between days 1 and 7
b µg equivalent permethrin per ml
The mean daily residue concentrations in milk, liver, and kidney
samples (Table 10) were 0.14-0.17 ppm with the cyclopropyl label and
0.24-0.41 ppm with the phenyl label. Permethrin was the major
component of the milk extract, accounting for 46% (0.06 ppm) and 56%
(0.17 ppm), respectively. Hydroxypermethrin was identified, at
concentrations of 8.1% (0.011 ppm and) and 2.6% (0.008 ppm),
respectively. Thin-layer chromatography also resolved at least five
unknown components which accounted for 2.6-11% of the total
radiolabelled residues and were common to both goats, indicating that
these components contain an intact ester linkage.
The residue concentrations in liver were 1.18 ppm with the
cyclopropyl label and 0.91 ppm with the phenyl label. The extraction
procedure released approximately 82% and 89% of the radioactive
residues, respectively. Aliquots of the concentrated extract were
analysed by thin-layer and high-performance liquid chromatography and
found to contain at least 13 components. Four identified from the
cyclopropyl-labelled samples were trans-dichlorovinyl acid (9.1%;
0.11 ppm), cis-dichlorovinyl acid (7.0%; 0.083 ppm),
hydroxydichlorovinyl acid (11%; 0.13 ppm), and dichlorovinyl acid
lactone (1%; 0.012 ppm). Two components were identified from the
phenyl-labelled samples as 3-phenoxybenzoic acid (12%; 0.11 ppm) and
4'-hydroxy-3-phenoxybenzoic acid (11%; 0.097 ppm). Additional studies
were conducted on the liver samples (Benner, 1997; Benner et al.,
1996; Skidmore, 1996) to determine if the parent molecule accounted
for a portion of the unknown components seen with the cyclopropyl
Table 10. Distribution of 14C-labelled residues of permethrin in liver, kidney, and milk from lactating goats
Metabolite Liver Kidney Milk
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
Permethrin - - - - - - - - 46 0.06 56
Hydroxypermethrin - - - - - - - - 8.1 0.011 2.6
cis- and trans-Dichlorovinyl acid 16 0.19 - - 26 0.27 - - - - -
Dichlorovinyl acid lactone 1.0 0.012 - - 0.6 0.006 - - - - -
Hydroxydichlorovinyl acid 11 0.13 - - 10 0.11 - - - - -
trans-Dichlorovinyl acid glucuronide - - - - 22 0.23 - - - - -
3-Phenoxybenzoic alcohol 28 0.28
3-Phenoxybenzoic acid - - 7.4 0.07 - - 57 0.44 - - -
4'-Hydroxy-3-phenoxybenzoic alcohol 5.5 0.05
4'-Hydroxy-3-phenoxybenzoic acid - - 3.2 0.03 - - - - - - -
Identified 28 0.34 45 0.43 59 0.61 57 0.44 55 0.071 58
Unknowna 61 34 26 30 30 25
Total 90 79 85 87 84 83
a The unknown is the sum of several components.
label (21%; 0.24 ppm) and the phenyl label (18%; 0.17 ppm). The
unknown component with the cyclopropyl label in liver consisted of a
mixture of several compounds, none of which represented > 5.2% of the
total residue. The results of the reanalysis of the components found
with the phenyl label liver showed that the intact ester linkage of
the parent molecule was not present. The unknown component was
identified as non-polar, base-labile conjugates of 3-phenoxybenzoic
alcohol and 4'-hydroxy-3phenoxybenzoic alcohol. The components were
3-phenoxybenzoic alcohol (28%; 0.28 ppm), 3-phenoxybenzoic acid (7.4%;
0.07 ppm), 4'-hydroxy-3-phenoxybenzoic alcohol (5.5%; 0.05 ppm), and
4'-hydroxy-3-phenoxybenzoic acid (3.2%; 0.03 ppm; Table 10).
The residue concentrations in the kidney were 1.0 ppm for the
cyclopropyl label and 0.78 ppm for the phenyl label. The extraction
procedure released approximately 89% and 93% of the radioactive
residues, respectively. Five components were identified in the
cyclopropyl-labelled samples as trans-dichlorovinyl acid (24%; 0.25
ppm), cis-dichlorovinyl acid (2%; 0.021 ppm), hydroxydichlorovinyl
acid (10%; 0.108 ppm), dichlorovinyl acid lactone (0.6%; 0.006 ppm),
and trans-dichlorovinyl acid glucuronide (22%; 0.23 ppm). One major
component of the phenyl-labelled sample was identified as
3-phenoxybenzoic acid (57%; 0.44 ppm; Table 10; Hawkins et al.,
1992b).
Figure 3 gives the proposed metabolic pathway of permethrin in
lactating goats.
Lactating cows
Four lactating Jersey cows were given three oral doses at 24-h
intervals of about 1 mg/kg bw of [14C] trans- or cis-permethrin
labelled in either the alcohol or the acid moiety. The concentration
of total radiolabel in blood reached a transient peak shortly after
each dose and was maximal after the third dose; it then decreased to
an insignificant level within 2-4 days. Higher blood concentrations
were attained with [14C] trans-permethrin labelled in the acid
moiety than with that labelled in the alcohol moiety. A similar
difference was not seen for cis-permethrin. The radiolabel was
eliminated mainly in the faeces and urine within 12 or 13 days.
Regardless of the position of the label, the trans isomer and its
metabolites were eliminated more rapidly than the cis isomer and its
metabolites. Urinary excretion accounted for about 43% of the
eliminated trans isomer and about 25% of the cis isomer. None of
the radiolabelled preparations resulted in detectable [14C]carbon
dioxide or significant residues in any tissues other than fat and
liver. Although low, the residue concentrations were highest in fat
and were higher after administration of the cis isomer (acid, 1.6%;
alcohol, 0.64%) than the trans isomer (acid, 0.15%; alcohol, 0.40%).
The radiolabel excreted in milk represented < 0.5% of the dose. The
lowest concentration in milk was found with trans-permethrin
labelled in the acid moiety (0.03%) and the highest with
trans-permethrin labelled in the alcohol moiety (0.44%). With all
four labelled preparations, the concentrations of radiolabel in milk
Excretion in urine, faeces, and cage wash accounted for 66% of
the dose after administration of [14C-cyclopropyl]permethrin and 80%
of the dose after administration of [14C-phenyl]permethrin. The
overall recovery was 76% and 81%, respectively. The distribution of
radiolabel as a percentage of the administered dose is given in Table
8, and the total concentrations in tissues are shown in Table 9. The
concentrations of total radiolabelled residues were 0.06 ppm and 0.15
ppm in subcutaneous fat, 0.10 ppm and 0.24 ppm in perirenal fat, and
0.07 ppm and 0.17 ppm in omental fat with the cyclopropyl and phenyl
labels, respectively. In the fat from the phenyl label-treated goat,
extractable radiolabel represented 89% of the total residue and was
associated almost entirely with permethrin. The total concentrations
in muscle were 0.04 ppm and 0.02 ppm with the two labels,
respectively. The extractable portion from the muscle of the goat
given the cyclopropyl label represented 78% and contained three to
four components, including polar material. The extractable portion
from muscle of the goat given the phenyl label represented 55% and
contained two additional components not found with the cyclopropyl
label, one of which co-chromatographed with permethrin.
Table 8. Distribution of 14C-labelled residues in lactating goats
Sample Percentage of administered dose
Cyclopropyl label Phenyl label
Urine 33 48
Cage wash 0.9 2.8
Faeces 32 29
Milk 0.4 0.5
Liver 0.33 0.17
Kidney 0.04 0.02
Gastrointestinal tract
and contentsa 9.4 Not measured
Total 76 81
a Intestines, omasum, and abomasum only
Table 9. Average distribution of 14C-labelled residues in
goat tissues and milk
Tissue Concentration (µg/g)
Cyclopropyl label Phenyl label
Milka 0.14-0.17 0.24-0.41
Fat
Omental 0.07 0.17
Perirenal 0.10 0.24
Subcutaneous 0.06 0.15
Kidney 1.0 0.78
Liver 1.2 0.91
Muscle (leg and rump) 0.04 0.02
Bileb 9.2 15
Plasmab 0.56 0.19
Whole blood 0.34 0.14
a Mean daily concentration between days 1 and 7
b µg equivalent permethrin per ml
The mean daily residue concentrations in milk, liver, and kidney
samples (Table 10) were 0.14-0.17 ppm with the cyclopropyl label and
0.24-0.41 ppm with the phenyl label. Permethrin was the major
component of the milk extract, accounting for 46% (0.06 ppm) and 56%
(0.17 ppm), respectively. Hydroxypermethrin was identified, at
concentrations of 8.1% (0.011 ppm and) and 2.6% (0.008 ppm),
respectively. Thin-layer chromatography also resolved at least five
unknown components which accounted for 2.6-11% of the total
radiolabelled residues and were common to both goats, indicating that
these components contain an intact ester linkage.
The residue concentrations in liver were 1.18 ppm with the
cyclopropyl label and 0.91 ppm with the phenyl label. The extraction
procedure released approximately 82% and 89% of the radioactive
residues, respectively. Aliquots of the concentrated extract were
analysed by thin-layer and high-performance liquid chromatography and
found to contain at least 13 components. Four identified from the
cyclopropyl-labelled samples were trans-dichlorovinyl acid (9.1%;
0.11 ppm), cis-dichlorovinyl acid (7.0%; 0.083 ppm),
hydroxydichlorovinyl acid (11%; 0.13 ppm), and dichlorovinyl acid
lactone (1%; 0.012 ppm). Two components were identified from the
phenyl-labelled samples as 3-phenoxybenzoic acid (12%; 0.11 ppm) and
4'-hydroxy-3-phenoxybenzoic acid (11%; 0.097 ppm). Additional studies
were conducted on the liver samples (Benner, 1997; Benner et al.,
1996; Skidmore, 1996) to determine if the parent molecule accounted
for a portion of the unknown components seen with the cyclopropyl
Table 10. Distribution of 14C-labelled residues of permethrin in liver, kidney, and milk from lactating goats
Metabolite Liver Kidney Milk
Cyclopropyl Phenyl Cyclopropyl Phenyl Cyclopropyl Phenyl
% ppm % ppm % ppm % ppm % ppm % ppm
Permethrin - - - - - - - - 46 0.06 56
Hydroxypermethrin - - - - - - - - 8.1 0.011 2.6
cis- and trans-Dichlorovinyl acid 16 0.19 - - 26 0.27 - - - - -
Dichlorovinyl acid lactone 1.0 0.012 - - 0.6 0.006 - - - - -
Hydroxydichlorovinyl acid 11 0.13 - - 10 0.11 - - - - -
trans-Dichlorovinyl acid glucuronide - - - - 22 0.23 - - - - -
3-Phenoxybenzoic alcohol 28 0.28
3-Phenoxybenzoic acid - - 7.4 0.07 - - 57 0.44 - - -
4'-Hydroxy-3-phenoxybenzoic alcohol 5.5 0.05
4'-Hydroxy-3-phenoxybenzoic acid - - 3.2 0.03 - - - - - - -
Identified 28 0.34 45 0.43 59 0.61 57 0.44 55 0.071 58
Unknowna 61 34 26 30 30 25
Total 90 79 85 87 84 83
a The unknown is the sum of several components.
label (21%; 0.24 ppm) and the phenyl label (18%; 0.17 ppm). The
unknown component with the cyclopropyl label in liver consisted of a
mixture of several compounds, none of which represented > 5.2% of the
total residue. The results of the reanalysis of the components found
with the phenyl label liver showed that the intact ester linkage of
the parent molecule was not present. The unknown component was
identified as non-polar, base-labile conjugates of 3-phenoxybenzoic
alcohol and 4'-hydroxy-3phenoxybenzoic alcohol. The components were
3-phenoxybenzoic alcohol (28%; 0.28 ppm), 3-phenoxybenzoic acid (7.4%;
0.07 ppm), 4'-hydroxy-3-phenoxybenzoic alcohol (5.5%; 0.05 ppm), and
4'-hydroxy-3-phenoxybenzoic acid (3.2%; 0.03 ppm; Table 10).
The residue concentrations in the kidney were 1.0 ppm for the
cyclopropyl label and 0.78 ppm for the phenyl label. The extraction
procedure released approximately 89% and 93% of the radioactive
residues, respectively. Five components were identified in the
cyclopropyl-labelled samples as trans-dichlorovinyl acid (24%; 0.25
ppm), cis-dichlorovinyl acid (2%; 0.021 ppm), hydroxydichlorovinyl
acid (10%; 0.108 ppm), dichlorovinyl acid lactone (0.6%; 0.006 ppm),
and trans-dichlorovinyl acid glucuronide (22%; 0.23 ppm). One major
component of the phenyl-labelled sample was identified as
3-phenoxybenzoic acid (57%; 0.44 ppm; Table 10; Hawkins et al.,
1992b).
Figure 3 gives the proposed metabolic pathway of permethrin in
lactating goats.
Lactating cows
Four lactating Jersey cows were given three oral doses at 24-h
intervals of about 1 mg/kg bw of [14C] trans- or cis-permethrin
labelled in either the alcohol or the acid moiety. The concentration
of total radiolabel in blood reached a transient peak shortly after
each dose and was maximal after the third dose; it then decreased to
an insignificant level within 2-4 days. Higher blood concentrations
were attained with [14C] trans-permethrin labelled in the acid
moiety than with that labelled in the alcohol moiety. A similar
difference was not seen for cis-permethrin. The radiolabel was
eliminated mainly in the faeces and urine within 12 or 13 days.
Regardless of the position of the label, the trans isomer and its
metabolites were eliminated more rapidly than the cis isomer and its
metabolites. Urinary excretion accounted for about 43% of the
eliminated trans isomer and about 25% of the cis isomer. None of
the radiolabelled preparations resulted in detectable [14C]carbon
dioxide or significant residues in any tissues other than fat and
liver. Although low, the residue concentrations were highest in fat
and were higher after administration of the cis isomer (acid, 1.6%;
alcohol, 0.64%) than the trans isomer (acid, 0.15%; alcohol, 0.40%).
The radiolabel excreted in milk represented < 0.5% of the dose. The
lowest concentration in milk was found with trans-permethrin
labelled in the acid moiety (0.03%) and the highest with
trans-permethrin labelled in the alcohol moiety (0.44%). With all
four labelled preparations, the concentrations of radiolabel in milk
 decreased to < 100 µg/L within 2-4 days after treatment ceased. The
only compound recovered from milk after administration of the acid-
and alcohol-labelled trans isomer was permethrin, whereas with the
cis isomer 85% of the radiolabel was attached to the parent compound
and 15% to trans-hydroxy- cis-permethrin.
The metabolic reactions of permethrin in cows were similar to
those in rats and hens. In cows, the permethrin isomers, their mono-
and di-hydroxy derivatives, and 3-phenoxybenzyl alcohol appeared only
in the faeces, while the cis-hydroxy-chrysanthemic acid lactones
appeared in both faeces and urine. The remaining metabolites occurred
only in urine. Although a slightly larger proportion of cis- than
trans-permethrin was excreted unchanged, the amounts of ester
metabolites were similar, and these metabolites were hydroxylated at
the trans or cis methyl position of the geminal dimethyl group, at
the 4' position of the phenoxybenzyl group, or at both the geminal
dimethyl and phenoxybenzyl groups. The preferred hydroxylation site
for both isomers was the trans methyl group. The major metabolites
of the acid moieties of both isomers were the corresponding
cis-hydroxy-chrysanthemic acid and the corresponding lactone and the
chrysanthemic acid glucuronide, while
trans-hydroxy- cis-chrysanthemic acid was also a major metabolite
of cis-permethrin. The major metabolites of the alcohol moieties of
both isomers were 3-phenoxybenzoic acid-glycine conjugate (3-11% of
the dose), 3-phenoxybenzyl alcohol (8-10%), and 3-phenoxybenzoic
acid-glutamic acid conjugate (12-28%) (Gaughan et al., 1978).
Humans
Two volunteers who ingested about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the dose as
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid in
urine collected over 24 h (Cridland & Weatherley, 1977a,b).
In a study of approximately 350 people who were dusted against
body lice with 30-50 g of powder containing 2.5 or 5.0 g/kg permethrin
(cis:trans ratio, 25:75), the mean amount of permethrin absorbed
during the first 24 h after treatment was estimated to be 14 µg/kg bw
in 19 subjects exposed to powder containing 2.5 g/kg permethrin and 39
µg/kg bw in 15 subjects exposed to 5.0 g/kg. No residue was found in
samples of urine taken 30 and 60 days after treatment (Nassif et al.,
1980).
Four of five workers in Sweden who packed conifer seedlings for
6 h in a tunnel that had been sprayed 1 h earlier with a 2% aqueous
solution of permethrin, resulting in atmospheric concentrations of
0.011-0.085 mg/m3 in the breathing zone, did not excrete detectable
amounts of acid permethrin metabolites in the urine. One very short
person whose face was close to the plants and who had the highest
exposure to permethrin in his breathing zone excreted 0.26 µg/ml
permethrin acid metabolites in his urine the following morning; in the
afternoon, the concentration was below the detection limit of the
method (Kolmodin-Hedman et al., 1982).
Ten patients (five men and five women) with scabies received an
application of about 25 g (range, 21-32 g) of a 5% permethrin cream
over the skin of the whole body except the head and neck. Dermal
absorption of permethrin was calculated from the quantities of
conjugated and unconjugated cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
metabolites of permethrin in the urine. In samples of urine collected
by seven patients 1 and 2 days after application of the permethrin
cream, the mean totals of these metabolites were 410 and 440 µg,
respectively; the mean total in the urine of three patients who
collected urine in the same container for 2 days was 1400 µg. The
urinary concentration of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
varied during the first 48 h from 0.11 to 1.1 µg/ml and that of the
cis isomer from 0.02 to 0.21 µg/ml. This metabolite was still
detectable in the urine of three patients after 1 week and in the
urine of one patient, reported to be an alcoholic, after 2 weeks. The
absorption of permethrin over the first 48 h after application was
estimated from the amount of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
excreted in the urine to be 6 mg (range, 3-11 mg), i.e. 0.5% of the
dose applied (van der Rhee et al., 1989).
Exposure to permethrin was assessed in a survey of 45
professional users of insecticide products, with more than a 100-fold
difference between the average and the highest concentrations. Dermal
contamination was found on 93% of the operators, the greatest
contamination resulting from use of leaky equipment. High airborne
concentrations were linked with use in confined areas. Monitoring of
metabolites in urine showed that systemic uptake occurred, with
concentrations of specific metabolites ranging from none detected to
10 nmol/mmol creatinine of
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid,
19 nmol/mmol creatinine of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 46 nmol/mmol creatinine of 3-phenoxybenzoic acid (Llewellyn
et al., 1996).
The concentrations of permethrin and its metabolites,
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propane carboxylic acid,
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 3-phenoxybenzoic acid, were measured in the urine and plasma
of 30 pest-control workers exposed to pesticides. The concentrations
of permethrin in plasma were below the limit of detection (5 µg/L),
and the urinary concentrations ranged from < 0.5 µg/L to 280 µg/L
(Leng et al., 1997).
Urine samples collected over 24 h from eight pest-control workers
exposed to permethrin were analysed for permethrin metabolites. The
concentrations ranged from 1 to 70 µg/g creatinine (Angerer & Ritter,
1997).
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of orally administered permethrin
in mice and rats (Table 11) demonstrate that two factors that affect
its toxicity are the concentration of the cis isomer and the
vehicle. Permethrin with a cis:trans ratio of 80-100:20-0 is
approximately 7-24 times more toxic than permethrin in which the
cis:trans ratio is 10-25:90-75 when delivered in maize oil, and
permethrin administered in maize oil is four to seven times more toxic
than undiluted permethrin. The alterations in acute toxicity are,
however, greater than can be explained on the basis of the cis
isomer content alone. Thus, a change from a cis:trans ratio of 20:80
to 80:20 in maize oil changed the LD50 value in Wistar rats from
6000 mg/kg bw to 225 mg/kg bw, whereas an LD50 value of about 1000
mg/kg bw would have been expected for the 20:80 material. Frequently
observed clinical signs were convulsions on the day of dosing,
tremors, and hypersensitivity. In animals that died, the
gastrointestinal tract often contained a brown fluid. In male and
female Wistar rats exposed for 4 h to atmospheres containing
permethrin (cis:trans ratio, 40:60), the LC50 value was greater
than a nominal concentration of 24 mg/L (Braun & Killeen, 1976).
In male and female New Zealand white rabbits, the dermal LD50
values for cis:trans 55:45 and 40:60 permethrin were > 2000 mg/kg
bw (Braun & Killeen, 1975b; Sauer, 1980b).
(b) Short-term studies of toxicity
Mice
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 39%:56%; purity, 94.7%) was administered in the
diet to groups of 20 male and 20 female Alderley Park mice at
concentrations of 0, 80 (increased to 10 000 ppm in weeks 3 and 4),
200, 400, 1000, 2000, or 4000 ppm in the diet, equivalent to 0, 28,
56, 140, 280, and 560 mg/kg bw per day for 28 days. No deaths
occurred, and no significant clinical signs were observed. Reduced
body-weight gain and poor food utilization were observed in animals
receiving 10 000 ppm. At termination, necropsies were performed on
five mice of each sex from the control group and those given 2000 and
10 000 ppm. Liver weights and the liver:bodyweight ratios were
increased in mice at 2000 and 10 000 ppm, and an increase in the
incidence of eosinophilia of the centrilobular hepatocytes was seen in
all mice receiving 10 000 ppm and two of five female mice receiving
2000 ppm. The NOAEL was 1000 ppm, equivalent to 140 mg/kg bw per day,
on the basis of changes in liver weight at 2000 ppm (Clapp et al.,
1977a).
Table 11. Acute toxicity of orally administered permethrin
Species Strain Sex Vehicle Purity cis:trans LD50 95% CI Reference
(%) ratio (mg/kg bw)
Mouse CF-1 M&F Maize oil 100 10:90 1700 1200-2300 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 95.5 25:75 960 680-1200 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 99.5 40:60 650 420-880 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 100 100:0 230 200-260 Marowitz (1974a)
Rat SD M None 55:45 3600 2400-5200 Sauer (1980c)
F 2300 1800-2900
Rat SD (CD) M Maize oil 41.3: 58.7 1000 880-1200 Cummins & Gardner
F 860 680-1000 (1984a)
Rat SD (CD) M Maize oil 81.1: 18.9 370 280-460 Cummins & Gardner
F 320 240-410 (1984b)
Rat Wistar M&F Maize oil 40:60 1200 860-1500 Braun & Killeen (1975a)
Rat Wistar M&F None 40:60 8900 6000-12 000 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 40:60 1200 1100-1400 Braun & Killeen (1975a)
Rat Long-Evans M&F None 40:60 6000 4200-7800 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 95.5 25:75 1600 1300-2000 Marowitz (1974b)
Rat Wistar F Maize oil 20:80 6000 - Wallwork et al. (1975)
Rat Wistar F Maize oil 30:70 1700 1300-2200 Wallwork et al. (1975)
Rat Wistar F Maize oil 40:60 1300 1000-1600 Wallwork et al. (1975)
Rat Wistar F Maize oil 50:50 1000 730-1400 Wallwork et al. (1975)
Rat Wistar F Maize oil 60:40 440 400-500 Wallwork et al. (1975)
Rat Wistar F Maize oil 80:20 220 200-250 Wallwork et al. (1975)
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 38%:52%; purity, 90.5%) was administered in the
diet to groups of eight Wistar Alderley Park rats of each sex at
concentrations of 0, 200, 500, 1000, 2500, 5000, or 10 000 ppm,
equivalent to 0, 20, 50, 100, 250, 500, and 1000 mg/kg bw per day, for
28 days. All rats receiving 10 000 ppm died within the first 3 days of
the study, and five rats at 5000 ppm had died by day 18. Before death,
the rats showed whole-body tremors, hyperactivity, and piloerection.
The surviving rats receiving 5000 ppm had tremors, hypersensitivity,
piloerection, and urinary incontinence. Rats receiving 2500 ppm had
tremors and piloerection during the first week of treatment and were
hypersensitive throughout the study. Rats receiving 1000 ppm showed
slight tremors on the first day only. No clinical signs were seen
among rats receiving 500 or 200 ppm. Rats receiving 5000 ppm had
decreased body weights, body-weight gains, and food consumption when
compared with controls, while females receiving 2500 ppm had
significantly increased food consumption. Urinary protein excretion
was depresssed in males receiving 5000 ppm. Lymphocytosis was observed
in male rats at doses > 2500 ppm. At the end of the study, the
liver weights and the liver:body weight ratios of rats receiving doses
> 2500 ppm were increased. Histological examination of the livers
showed no remarkable changes. The NOAEL was 500 ppm, equivalent to 50
mg/kg bw per day, on the basis of tremors at 1000 ppm (Clapp et al.,
1977b).
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) wase administered in the diet to groups of six Long
Evans rats of each sex in the diet at concentrations of 0, 2500, 3500,
5000, or 7100 ppm, equal to 0, 250, 350, 5300, and 800 mg/kg bw per
day for males and 0, 270, 380, 550, and 820 mg/kg bw per day for
females, for four weeks. One male and one female receiving the highest
dose died during the first week of treatment. Tremors, the severity of
which was dose-dependent, were observed in most rats receiving doses
> 3500 ppm during the first week of treatment but were subsequently
seen only in animals receiving 7100 ppm and were generally less
severe. Staining of the abdominal fur was also seen in rats at doses
> 3500 ppm, with increasing incidences in weeks 1-4. Decreased body
weight was observed in both males and females treated with doses
> 3500 ppm during the first 2 weeks of treatment. This effect
persisted during weeks 3 and 4 only in females at 7100 ppm. No
histological examination was performed in this preliminary experiment.
The NOAEL was 2500 ppm, equal to 250 mg/kg bw per day, on the basis of
clinical signs and body-weight changes at 3500 ppm (Killeen & Rapp,
1974).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to groups of six male and six female Long
Evans rats in the diet at concentrations of 625, 1250, 2500, 5000, or
7500 ppm for 30 days, equal to 0, 59, 120, 250, and 630 mg/kg bw per
day for males and 0, 69, 130, 280, and 660 mg/kg bw per day for
females: no data on consumption of the compound were available for the
highest concentration. All females and three males receiving 7500 ppm
died within 24 h of the start of treatment, and the remaining three
males in this group had died by the end of the first week. Five of six
males and five of six females at 5000 ppm died during the first week,
but the remaining animals survived the duration of the study. In rats
receiving 2500 ppm, slight-to-moderate tremors and staining of the
anogenital fur were noted throughout most of the study. The surviving
male and female at 5000 ppm showed moderate-to-severe tremors and
staining of the fur in the anogenital region throughout the study. The
body weights of males at 2500 ppm were reduced. No histological
examination was performed in this preliminary experiment. There were
no signs of toxicity at 625 and 1250 ppm. The NOAEL was 1250 ppm,
equal to 120 mg/kg bw per day, on the basis of clinical signs and
body-weight changes at 2500 ppm (Killeen & Rapp, 1975a).
Technical-grade permethrin (cis:trans ratio unstated; purity,
96%) was administered in the diet to groups of six male and six female
Charles River rats (strain not stated) at concentrations of 0 (14
animals of each sex), 30, 100, 300, 1000, or 3000 ppm, equivalent to
0, 3, 10, 30, 100, and 300 mg/kg bw per day, for five weeks. Most of
the observations were restricted to the group receiving 3000 ppm. In
this group, persistent tremors were observed in all males and four
females, body-weight gain was reduced in females by about 7%, the
liver weights adjusted for terminal body weight were increased in
males (15%) and females (13%), and the relative heart weight was
increased in males (8%). Increased prothrombin time and plasma urea
were seen in males and decreased plasma protein in females. Males at
1000 ppm showed an increase in relative liver weight (15%), and
(probably chance) increases in prothrombin time were seen in females
at 30 and 300 ppm. Histological examination revealed renal tubular
mineralization and hydronephrosis as common lesions which were evenly
distributed among the treated groups. No dose-related increase in the
incidence of lesions was found. The NOAEL was 300 ppm, equivalent to
30 mg/kg bw per day, on the basis of increased liver weight at 1000
ppm (Butterworth & Hend, 1976).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to groups of 18 Wistar rats of
each sex at concentrations of 0, 60, 200, 600, or 2000 ppm, equivalent
to 0, 6, 20, 60, and 200 mg/kg bw per day, for 90 days. At that time,
10 rats of each sex per group were killed, and the remaining rats were
maintained without further treatment for an additional 29 days. No
treatment-related deaths occurred, and no treatment-related or
toxicologically significant clinical signs, changes in body weights,
food consumption, or estrus cycle, urinary findings, or blood
anomalies were noted. The absolute and relative weights of the spleen
and lungs were increased in male rats at 2000 ppm, and the relative
and absolute weights of the adrenal glands of female rats at this dose
showed statistically significant decreases. No consistent dose-related
pattern of toxicity was seen. The fat content of the renal cortex was
slightly increased in male rats receiving 600 or 2000 ppm, but this
was not accompanied by morphological alterations. The NOAEL was 600
ppm, equivalent to 60 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm (Williams et al., 1976a).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to 18 Wistar rats of each sex at
concentrations of 0, 200, 600, 2000, or 4000 ppm, equivalent to 0, 20,
60, 200, and 400 mg/kg bw per day, for 90 days. At that time, 10 rats
of each sex per group were killed, and the remainder were maintained
with no further treatment for an additional 36 days. No
treatment-related deaths occurred. The only significant clinical sign
was hypersensitivity in male and female rats receiving 4000 ppm. By
week 3, hypersensitivity was no longer observed in male rats but
continued in the female rats throughout treatment, although the
symptoms disappeared after 3 days without dosing. Males receiving 4000
ppm had decreased body-weight gain, which improved during the recovery
period. A slight-to-moderate decrease in leukocyte count was seen
during the early stages of the study in rats at this dose, but the
values had returned to within the normal range by 90 days. The weight
of the liver showed a slight but significant increase at 90 days but
had returned to normal by the end of the recovery period. The absolute
and relative weights of the thyroids of rats receiving the high dose
showed a slight decrease at 90 days, but no histopathological changes
were found. The NOAEL was 2000 ppm, equivalent to 200 mg/kg bw per
day, on the basis of of hypersensitivity, reduced body weight,
transient leukopenia, and increased liver weights at 4000 ppm
(Williams et al., 1976b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was administered in the
diet to groups of 30 Long Evans rats of each sex at concentrations of
0, 50, 75, 100, or 500 ppm, equivalent to 5, 7.5, 10, and 50 mg/kg bw
per day, for 90 days. There were no significant differences in body
weight, food consumption, or haematological, clinical chemical, or
urinary parameters. At the end of the study, the relative weight of
the liver was increased in male rats at 500 ppm in comparison with
controls, while that of female rats at this dose was significantly
decreased. There was no correlation with clinical or histopathological
findings. No treatment-related histopathological findings were made.
The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis
of increased relative liver weight at 500 ppm (Becci & Parent, 1980).
In a study conducted according to GLP, three batches of
technical-grade permethrin (cis:trans ratio, 36.1%:61.1% to
38.5%:56.2%; purity, 94.1-97.2%) were given in the diet to groups of
eight Wistar-derived rats of each sex at concentrations of 0, 20, 100,
or 1000 ppm, equivalent to 2, 10, and 100 mg/kg bw per day, for 26
weeks, when the rats were killed. The body weight and body-weight gain
of female rats receiving 20 and 100 ppm were similar to those of the
control animals, but females at 1000 ppm gained less weight throughout
treatment, as did males at doses > 100 ppm. There was no overall
effect on food consumption. Animals at 1000 ppm showed statistically
significant increases in liver weight, hepatic aminopyrine
N-demethylase activity, cytochrome P450 content, and smooth
endoplasmic reticulum content. Those at 100 ppm had only slight
hepatic changes, none of the untransformed values being significantly
greater than control levels, although the logarithm of the values for
aminopyrine N-demethylase activity attained significance. The NOAEL
was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
increased liver weight at 1000 ppm, as the changes in aminopyrine
N-demethylase activity were considered not toxicologically relevant
(Hart et al., 1977a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to groups
of 20 Sprague-Dawley rats of each sex by inhalation as an aerosol for
6 h per day, 5 days per week for 13 weeks at concentrations of 0, 125,
250, or 500 mg/m3. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm . At the end of
the 13-week exposure, 10 rats of each sex per group were maintained
without further treatment for an additional 90 days. There were no
treatment-related deaths. Severe tremors and convulsions were
observed in rats at 500 mg/m3, but these signs were no longer seen
by the second week of exposure. Oxygen consumption was monitored
throughout the exposure in order to assess overall metabolic rate. No
differences from controls were found. Hexobarbital-induced sleep times
(220 mg/kg bw intraperitoneally) were reduced in males exposed to 500
mg/m3, indicating the induction of liver enzymes, but the effect was
no longer statistically significant 30 days after exposure or in rats
of the lower concentrations. Body weights and organ-to-body weight
ratios were unaffected. No gross or microscopic treatment-related
pathological changes were observed. The NOAEC was 250 mg/m3 on the
basis of tremors and convulsions at 500 mg/m3 (United States Army,
1978).
Guinea-pigs
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered to groups
of 10 male Hartley guinea-pigs by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol in order to
examine sensitization reactions. There were no treatment-related
deaths or clinical signs during exposure, and the body weight and
organ:body weight ratios were unaffected. No gross or microscopic
treatment-related pathological changes were observed. No sensitization
reaction was seen. The NOAEC was 500 mg/m3, the highest dose tested
(United States Army, 1978).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity. 93.6% and 95.0%) was applied intradermally to groups of
male guinea-pigs as 0.1 ml of a 0.1% (w/v) solution; each batch of
permethrin or 2,4-dinitrochlorobenzene (positive control) contained
one volume of propylene glycol and 29 volumes of saline. Ten
guinea-pigs were challenged with the 0.1% solution, and an additional
five in each group received no prior sensitization but were given a
challenge dose of each batch of test material or the positive control.
The reaction induced by the challenge dose of permethrin was no
greater than that observed in the sensitized animals. The challenge
dose of the positive control produced sensitization reactions in all
of the guinea-pigs in that group (Metker et al., 1977).
Technical-grade permethrin (cis:trans ratio, 25:75; purity not
stated) was tested for skin sensitization potential according to the
method of Magnusson and Kligman. Ten male guinea-pigs received
permethrin, and groups of five male guinea-pigs received the vehicle
or 2,4-dinitrochlorobenzene. All compounds were given as a 1% w/v
solution in corn oil and as a 1% w/v solution in Freund's complete
adjuvant. No reaction to permethrin was seen 5, 24, and 48 h after
dosing, whereas all guinea-pigs given the positive control showed
marked sensitization (Chesher & Malone, 1974).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered by
inhalation as an aerosol to groups of 10 male Hartley guinea-pigs at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol. No
sensitization reaction was seen in any of the guinea-pigs (United
States Army, 1978).
Rabbits
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95.0%) was applied daily to the shaved skin of
groups of eight New Zealand white rabbits for 21 days at doses of 0,
100, 320, or 1000 mg/kg bw per day under an occlusive dressing. Four
rabbits in each group received abrasion at the application site on the
first day. All rabbits were killed on day 10 after the last exposure.
No significant changes were noted in body weights during or after
exposure, and there were no significant changes in organ:body weight
ratios. Moderate irritation of the skin was observed, but the reaction
was not significantly different from controls by day 18. Mild
irritation was still present 10 days after exposure, although it
improved daily. No differences were found in clinical chemical
parameters. There were no treatment-related lesions in the skin or
other tissues or organs. The NOAEL was 1000 mg/kg bw per day, the
highest dose tested (Metker et al., 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio 55:45; purity not stated) was melted and applied
without dilution at a volume of 0.5 ml to the skin of six New Zealand
white rabbits on each of two intact and two abraded sites, for a total
of 2 ml per rabbit. Each site was scored for irritation by the method
of Draize 30-60 min after removal of the occlusive wrap and residual
permethrin and again 24 h and 72 h after dosing. Very slight erythema
was noted on all sites at 24 h, but all irritation had resolved by
72 h. The score for primary dermal irritation was 0.5/8.0, indicating
virtually no irritation (Sauer, 1980c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as a liquid to the abraded skin of 10 New Zealand
white rabbits at a dose of 2000 mg/kg bw and the site was covered with
an occlusive wrap for 24 h. When the wraps were removed, very slight
erythema was noted on three rabbits, and barely perceptible oedema was
seen on one other rabbit (Braun & Killeen, 1975b).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as an aqueous slurry to the skin of six New Zealand
white rabbits as a volume of 0.50 ml of 1 g/ml on an intact and an
abraded site, for a total of 1 ml per rabbit. The sites were covered
with an occlusive wrap for 24 h and scored by the Draize method 24 and
72 h after dosing. Very slight erythema was seen on three abraded and
three intact sites at 24 h, but all irritation had resolved by 72 h.
The score for primary dermal irritation was 0.25, indicating virtually
no irritation (Braun & Killeen, 1975c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was melted, and 0.1 ml
of undiluted material was instilled into the right eye of nine New
Zealand white rabbits. After approximately 20 s, the eyes of three
rabbits were flushed with water for 1 min, and irritation was scored
by the Draize method 24, 48, and 72 h and 4 and 7 days after dosing.
Slight conjunctival redness and chemosis were found in the majority of
the treated eyes, but all irritation had resolved within 4 days. The
maximum score was 7.3/110 for unwashed eyes and 4.0/110 for washed
eyes at 24 h, indicating no irritation (Sauer, 1980d).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was instilled as a liquid into the right eye of nine New
Zealand white rabbits at a volume of 0.10 ml. The eyes of three
rabbits were then flushed for 30 s with 100 ml of warm tap-water and
were scored 1, 24, 48, and 72 h and 4 and 7 days after dosing. Slight
conjunctival redness, chemosis, and discharge were seen in both washed
and unwashed eyes at 1 h. Stippling of the cornea was observed in two
unwashed eyes. All irritation had resolved within 48 h (Braun &
Killeen, 1975d).
Dogs
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to one male and one female beagle dog in
gelatine capsules for 14 days at doses of 0, 125, 250, or 500 mg/kg bw
per day. On the first day of treatment, emesis was observed in four
treated dogs, and thereafter the compound was administered twice daily
in divided doses. Tremors and ataxia were each seen once in the male
treated with 500 mg/kg bw per day during the first week of treatment.
No effect on body weight or food consumption was seen throughout the
study. The NOAEL was 250 mg/kg bw per day on the basis of clinical
signs at 500 mg/kg bw per day (Killeen & Rapp, 1975b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, 94.5%) was administered to groups
of four beagle dogs of each sex in gelatine capsules at doses of 0, 5,
50, or 500 mg/kg bw per day for at least 96 days. Tremors were
observed on isolated occasions in two males and three females at 500
mg/kg bw per day during the exposure. One dog that had tremors also
showed transient narcosis with nystagmus on a single occasion. No
effects attributable to treatment were seen on body weight, food
consumption, clinical chemical, haematological, or urinary parameters,
or histological appearance. The mean absolute and relative (to body
weight) weights of the liver were greater than those of controls in
animals at doses > 50 mg/kg bw per day, but there was no
histological correlate. The increases in absolute liver weights were
11% at 50 and 500 mg/kg bw per day in males and 12% at 500 mg/kg bw
per day in females and were statistically significant. The biological
significance of the effect in males at 50 mg/kg bw per day is obscure
in view of the lack of any additional effect at a dose 10-fold higher.
The NOAEL was 50 mg/kg bw per day on the basis of changes in liver
weight and neurotoxic signs at 500 mg/kg bw per day (Killeen & Rapp,
1976).
Technical-grade permethrin (cis:trans ratio, 54:46; purity,
93.4%) was administered in gelatine capsules to groups of six male and
six female beagle dogs at doses of 0, 5, 50, or 500 mg/kg bw per day
for 90 days. Owing to signs of severe toxicity which increased in
severity and duration, the high dose was lowered to 364 mg/kg bw per
day on day 9 of dosing. The most serious of these signs was seizures
lasting for up to 1 h and characterized by rapid eye twitching with
fasciculations, increased sensitivity to sound, and severe tremors.
Ataxia and aggressive behaviour were also seen in these animals. At
364 mg/kg bw per day, similar signs of toxicity were seen but of
lesser severity and shorter duration. The major signs of toxicity seen
in males at doses > 50 mg/kg bw per day and females at 364 mg/kg bw
per day included impaired gait, ataxia, tremor, muscle twitching,
involuntary limb movements, uncontrolled barking, panting, and
salivation. Tremors and muscle twitching were also seen in females at
50 mg/kg bw per day. No signs of toxicity were observed at the low
dose. Body-weight gain was significantly reduced in males at the high
dose, whereas the body weight and body-weight gain of females were
unaffected by treatment. Serum alkaline phosphatase activity was
increased at 3, 6 10, and 13 weeks among males at the high dose, and
the absolute and relative weights of the liver were increased in males
and females at this dose. Permethrin had no effect on food consumption
or on haematological, urinary, ophthalmological, or histological
parameters. The NOAEL was 5 mg/kg bw per day on the basis of clinical
signs at 50 mg/kg bw per day (Becci et al., 1980).
Technical-grade permethrin (cis:trans ratio, 25:75; purity,
94.5%) was administered in gelatin capsules to groups of four beagle
dogs of each sex at doses of 0, 10, 50, or 250 mg/kg bw per day for at
least 180 days. None of the dogs died during the study. Emesis was
seen in a few treated and control animals, but there were no
treatment-related signs of toxicity, no effect on body weight,
ophthalmological or electrocardiographic parameters, absolute organ
weights, or gross or histopathological appearance. Statistically
significant but sporadic changes in food intake (decreased in dogs at
50 mg/kg bw per day in week 11 and in those at 250 mg/kg bw per day in
weeks 12 and 22), some haematological parameters (decreased packed
cell volume on day 180 and decreased mean corpuscular volume on day 56
at 10 mg/kg bw per day; increased lymphocyte and neutrophil counts at
50 and 250 mg/kg bw per day on day 14), some clinical chemical
parameters (glucose, urea, and sodium concentrations), and increased
relative weights of the liver, heart, and kidneys (by < 17%) were
seen. None of these changes appeared to be related to dose or time and
were not severe enough to be of toxicological importance. Similar
significant changes in blood chemistry were occasionally observed in
the dogs before dosing. The NOAEL was 250 mg/kg bw per day, the
highest dose tested (Reynolds et al., 1978).
In a study conducted according to GLP, permethrin (cis:trans
ratio, 32.3%:60.2%; purity, 92.5%) was administered in corn oil in
gelatin capsules to four groups of six beagle dogs of each sex at
doses of 0, 5, 100, or 1000 mg/kg bw per day for 52 weeks. Body
weights and food consumption were measured, and the dogs were observed
for clinical and behavioural abnormalities. A variety of
haematological and biochemical parameters were measured at intervals
throughout the study. Clinical signs, including convulsions, muscle
tremor, and incoordination, were seen frequently in dogs at 1000 mg/kg
bw per day. The body weights of males at 1000 mg/kg bw per day and
females at doses > 100 mg/kg bw per day were reduced. The weight of
the liver of dogs treated with doses > 100 mg/kg bw was increased,
accompanied by hepatic cellular swelling, consistent with an observed
increase in plasma alkaline phosphatase activity. These findings were
considered to represent an adaptive response and not a toxicological
effect. The dose of 1000 mg/kg bw per day was overtly toxic, resulting
in neurological signs associated in some animals with poor clinical
condition. The NOAEL was 5 mg/kg bw per day on the basis of the
reduction in body weight at 100 mg/kg bw per day (Kalinowski et al.,
1982).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to four
groups of two beagle dogs of each sex by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter of < 1 µm. The dogs
were tested for pulmonary function before and after exposure, and
blood samples were taken weekly for measurements of blood chemistry
and haematology. There were no treatment-related deaths and no toxic
signs were observed. Body weights and organ:body weight ratios were
unaffected, and pulmonary function and blood chemical and
haematological parameters were unchanged. No gross or microscopic,
treatment-related pathological changes were observed. The NOAEC was
500 mg/m3, the highest dose tested (United States Army, 1978).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet to groups of
75 CD-1 mice of each sex for 24 months. Initially, the concentrations
in the diet were 0, 20, 100, and 500 ppm, equivalent to 0, 3, 15, and
75 mg/kg bw per day. The concentration of 500 ppm was changed to 5000
ppm, equivalent to 250 mg/kg bw per day at week 19, but was returned
to 500 ppm at week 21. In addition, at week 21 the concentration of
100 ppm was changed to 4000 ppm, equivalent to 200 mg/kg bw per day,
and the concentrations were 0, 20, 500, and 4000 ppm for the remainder
of the study. During week 65, a problem of animal identification
arose, which detracts from the value of the study; however, the
actions taken to eliminate these mice from the study were carefully
recorded and judged by the US Environmental Protection Agency to be
acceptable, and the study was allowed to continue to its scheduled
conclusion. The rates of death up to 24 months were 43/62 control
males, 41/60 males at the low dose, 46/60 males at the intermediate
dose, 61/67 males at the high dose, 20/50 female controls, 32/60
females at the low dose, 34/62 females at the intermediate dose, and
48/65 at the high dose. The body weight of males at 4000 ppm was
slightly decreased from week 21 to termination, but that of females
and the food consumption of both males and females were unaffected. No
clinical observations were made that correlated with treatment. Mean
haemoglobin, erythrocyte count, and blood glucose concentration were
decreased in animals of each sex at 4000ppm. The mean absolute and
relative (to body weight) weights of the liver were increased for
males receiving 500 ppm and for animals of each sex at 4000 ppm. The
latter showed a greater frequency of non-neoplastic changes including
mononuclear leukocyte infiltrates in some tissues and thrombosis of
the cardiac atrium. There was no significant change in the incidence
of any tumour, and, in particular, the incidence of bronchioalveolar
adenomas was not increased in animals of either sex. The incidences of
hepatomas and hepatocellular carcinomas were slightly increased among
male, but not female, mice (Table 12) (Hogan & Rinehart, 1977; Rapp,
1978).
In view of the problems with the first experiment, a second of
the same design but carried out according to GLP was conducted. The
concentrations in the diet were originally 0, 100, 500, and 2000 ppm,
equivalent to 15, 75, and 300 mg/kg bw per day, and were changed after
2 months to 0, 20, 500, and 2000 ppm, equivalent to 3, 75, and 130
mg/kg bw per day, for males and 20, 2500, and 5000 ppm, equivalent to
3, 380, and 750 mg/kg bw per day, for females. The incidences of
deaths up to 24 months were 55/75 male controls, 48/75 males at the
low dose, 49/75 males at the intermediate dose, 63/75 males at the
Table 12. Incidences of tumours of the lung and liver in mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. livers Neoplasm No. with
examined examined liver
Alveolar Alveolar Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 60 6 0 72 3 4 7
Low 56 7 0 68 1 0 1
Intermediate 53 4 0 68 9 5 14
High 67 5 0 71 4 7 11
Female Control 47 11 0 72 2 0 2
Low 59 8 1 69 1 0 1
Intermediate 60 7 1 67 4 0 4
High 59 9 1 69 1 0 1
From Hogan & Rinehart (1977); Rapp (1978)
high dose, 53/75 female controls, 42/75 females at the low dose, 52/75
females at the intermediate dose, and 53/75 females at the high dose.
The mortality rate among males at the high dose was therefore slightly
greater than that among the control animals. These males also had a
higher incidence than control males of yellow staining in the
anogenital area throughout the study. Treatment had no effect on body
weight, body-weight gain, or food consumption. The differential
leukocyte counts of females at 2500 and 5000 ppm and males at 2000 ppm
were slightly lower than control values, while the segmented
neutrophil counts of males at 2000 ppm were slightly higher than in
controls. A statistically significant decrease in the absolute and
organ:body weight ratio of the testis was seen at 2000 ppm, while
females at doses > 2500 ppm showed statistically significantly
increased absolute liver weights; the liver:body weight ratio was
statistically significant only in females at 5000 ppm.
A dose-dependent increase in the incidence of alveolar-cell
adenomas and carcinomas was found among female mice, and multiple lung
adenomas were often found in the same animal (Table 13). Hepatomas
also occurred more often in female mice at the intermediate and high
doses than controls and mice at the low dose. Hepatoma is considered
to be a spontaneous lesion which occurs in ageing and aged mice
without affecting the mortality rate. The incidence of hepatocellular
carcinoma was notably higher in males at the intermediate dose than in
other treated or control groups (Table 13). The type, incidence,
and/or degree of severity of other neoplasms and all non-neoplastic
histological changes were considered to represent spontaneous lesions
unrelated to treatment. Since the incidences in males and females at
the low dose were close to those in the control group, the increased
incidence of bronchioalveolar adenoma in female mice at the two higher
doses may have been related to treatmen. This conclusion is not,
however, supported by the results of the first study, which was
conducted in the same laboratory with the same strain of mouse, and
the same batch of permethrin and which partially overlapped with the
second one in time. The pulmonary adenomas were thus considered to
have no toxicological significance. The NOAEL for systemic toxicity
was 500 ppm, equivalent to 75 mg/kg bw per day, on the basis of
changes in organ weights at 2000 ppm (Tierney & Rinehart, 1979).
In a study conducted according to GLP, eight batches of
technical-grade permethrin (cis:trans ratios, 44:55 to 36.2:61;
purity, 94.1-98.9%) were administered in the diet of groups of
70 Alderley Park strain mice of each sex at concentrations of 0, 250,
1000, or 2500 ppm, equivalent to 0, 38, 150, and 380 mg/kg bw per day,
for up to 98 weeks. The animals were housed five per sex to a cage.
Ten animals of each sex per group were designated for interim kills at
26 and 52 weeks. Permethrin did not induce clinical signs, and the
mortality rate was unaffected. A treatment-related decrease in
body-weight gain and increased food consumption were noted in males
and females at 2500 ppm. Proliferation of the smooth endoplasmic
reticulum and microbodies and increased eosinophilia in centrilobular
hepatocytes were also observed in most animals at this dose and to a
lesser extent in those at 1000 ppm. These changes had been observed in
Table 13. Incidences of tumours of the lung and liver in a second study of mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. with No. livers Neoplasm No. with
examined lung examined liver
Alveolar Alveolar tumours Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 75 16 7 23 75 8 16 22
Low 75 17 5 20 75 19 12 30
Intermediate 74 20 13 28 75 17 19 34
High 75 17 4 21 75 19 8 25
Female Control 75 10 6 15 74 3 4 6
Low 76 18 7 24 76 4 3 7
Intermediate 75 26 11 35 76 23 3 25
High 75 37 15 44 75 29 2 30
a 26-week staudy (Hart et al., 1977a) and showed no progression in
this 98-week experiment. The kidneys of treated males at all doses
showed a dose-related decrease in vacuolation of the proximal tubular
epithelium, and the effect was statistically significant in males at
250 ppm at 52 weeks (13 ± 6.3 versus 5.4 ± 4.2 vacuoles per
microscope field) but not at 26 weeks (11 ± 6.8 versus 8.4 ± 5.3)
or at 98 weeks (8.4 ± 7.1 versus 7.2 ± 7.0). The changes in the
liver and the kidney were considered to be adaptive characteristics of
no toxicological importance.
No significant increase in the incidence of tumours of unusual
types or in the number of tumour-bearing animals was seen in
comparison with with controls. In male mice, a slight increase in the
incidence of pulmonary adenomas was recognized in the second year of
the study. Up to week 52, one unconfirmed tumour was found among 34
male controls and two in 23 females at the low dose; from week 53
until the end of the study, the incidences in males were 10/36 in
controls, 6/41 at the low dose, 13/40 at the intermediate dose, and 16
(+1 unconfirmed) out of 33 at the high dose. Comparison of controls
versus the high-dose group gives p = 0.09 or, if the unconfirmed
tumour is assumed to have been an adenoma, 0.05 (Fisher's exact test).
This finding was not considered to constitute evidence of a
carcinogenic effect. The NOAEL was 250 ppm, equivalent to 38 mg/kg bw
per day, on the basis of changes in the liver and kidney at 1000 ppm
(Hart et al., 1977b; Ishmael & Litchfield, 1988).
Rats
Technical-grade permethrin of 10 batches (cis:trans ratio,
36.2:61 to 43.9:55; purity, 93.1-98.9%) was administered in the diet
of groups of 60 Alderley Park rats of each sex at concentrations of 0,
500, 1000, or 2500 ppm, equivalent to 0, 25, 50, or 125 mg/kg bw per
day, for up to 104 weeks. Twelve animals of each sex per group were
designated for interim sacrifice at 52 weeks. Treatment did not affect
survival, body weight, body-weight gain, food consumption,
haematological or urinary parameters, or gross or microscopic
appearance. Tremor, piloerection, and hypersensitivity were noted at
2500 ppm during the first 2 weeks of the study. Increased
aminopyrine- N-demethylase activity was observed in all treated males
and in females at doses > 1000 ppm at 52 weeks and in all animals
at the high dose at 104 weeks. Increased liver weights were found in
all treated groups, although the effect did not increase between weeks
52 and 104. The kidneys were heavier than those of controls in males
at 2500 ppm at week 52 and in all treated males at week 104. The
weight of the kidneys of females at doses > 1000 ppm was reduced at
52 weeks and was unaffected at week 104. The increase in kidney weight
in male rats at 104 weeks appeared to be associated with marked
nephropathy in some of these animals. Nephropathy occurs spontaneously
with age in that colony of rats, and the variability of the change in
males at the terminal kill and the absence of this trend in the
females indicate that these effects were not related to treatment.
Treatment had no effect on the incidence of tumour-bearing rats
or of tumours of any particular type. The incidence of centrilobular
hepatocyte hypertrophy was increased in rats at doses > 1000 ppm,
particularly in males, and proliferation of the smooth endoplasmic
reticulum was observed in all treated groups of females and males at
doses > 1000 ppm at week 52; at week 104, the proliferation was
confined to rats at 2500 ppm. These adaptive changes in the liver,
including hepatocyte hypertrophy and the associated increases in liver
weight, microsomal enzyme activity, and smooth endoplasmic reticulum,
were considered to be normal adaptive responses of the liver to
exposure to a xenobiotic and to be toxicologically insignificant. It
is uncertain, however, whether the hepatocyte vacuolation seen in
males and females at 2500 ppm is also part of the physiological
adaptation of the liver. Examination of sciatic nerves revealed no
ultrastructural changes associated with exposure. The NOAEL was 500
ppm, equivalent to 25 mg/kg bw per day, on the basis of the increased
liver hypertrophy at 1000 ppm (Richards et al., 1977; Ishmael &
Litchfield, 1988).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet of groups of
60 Long-Evans rats of each sex at concentrations of 0, 20, 100, or 500
ppm, equivalent to 0, 1, 5, and 25 mg/kg bw per day, for 24 months.
Treatment had no effect on survival. Two females at 500 ppm showed
tremor on day 2 of the study, but tremors were not seen in any other
animal during the remainder of the study. The body weights and food
consumption of treated males were comparable to those of controls. The
mean body weights of females at 500 ppm were slightly lower than
control weights during the first year but were comparable thereafter;
the food consumption of control and treated females was comparable.
The glucose concentration was increased in animals at 500 ppm, in
females at 18 and 24 months and in males at 24 months. The absolute
and relative (to body weight) weights of the ovary of females at 500
ppm were greater than those of controls at the end of the study.
Histological examination of tissues from treated animals revealed no
increase in the incidence of neoplastic or non-neoplastic lesions. The
NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs, changes in body and ovary weights, and clinical
chemical findings at 500 ppm (Braun & Rinehart, 1977; Billups,
1978a,b; Busey, 1978).
(d) Genotoxicity
Technical-grade permethrin was tested in a battery of test
systems in vitro and in vivo (Table 14). It did not increase the
frequency of unscheduled DNA synthesis in human fibroblasts in
vitro or induce DNA repair in Escherichia coli or Bacillus
subtilis. It did not induce gene mutations in bacteria, cultured
mammalian cells, or Drosophila melanogaster, nor recombinational
events (mitotic recombination or gene conversion) in yeast. It did not
induce aneuploidy in D. melanogaster. The evidence for induction of
clastogenic effects in cultured mammalian cells (mainly human
lymphocytes) was equivocal, in that a study of micronucleus induction
Table 14. Results of studies of the genotoxicity of permethrin
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
In vitro
Differential E. coli W3110/ 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity p3478 (1979)
Differential B. subtilis H17/M45 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity (1979)
Reverse mutation S. typhimurium 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
TA100, TA98, (1976)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
TA100, TA98,
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
TA100, TA98, (1979)
TA1537, TA1535
Reverse mutation S. typhimurium 980 µg/plate NR NR Negative ± S9 Bartsch et al.
TA100, TA98 (1980)
Reverse mutation S. typhimurium 5000 µg/plate NR NR Negativeb ± S9 Moriya et al.
TA100, TA98, (1983)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 3000 µg/plate NR 95 Negative ± S9 Pluijmen et al.
TA100, TA98 (1984)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Reverse mutation S. typhimurium 5460 µg/plate NR NR Negative ± S9 Pednekar et al.
TA100, TA98 (1987)
Reverse mutation S. typhimurium 6000 µg/plate NR cis, 99 Negative ± S9 Herrera &
TA100, TA104, trans, 100 Laborda (1988)
TA98, TA97,
TA1538, TA1537,
TA1535
Reverse mutation E. coli WP2 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
Reverse mutation E. coli WP2 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
(1979)
Reverse mutation E. coli WP2 hcr 5000 µg/plate NR NR Negative ± S9 Moriya et al.
(1983)
Gene conversion S, cerevisiae D4, 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
try locus (1976)
Mitotic S. cerevisiae D3 50 000 µg/ml 38:52 90.4 Negative ± S9 Simmon et al.
recombination (1979)
Chromosomal loss D. melanogaster 5 µg/ml feed NR NR Negativea Woodruff et al.
(1983)
Sex-linked recessive D. melanogaster 1.2 µg/ml feed 45:55 91.1 Negativea Mehr et al.
mutation (1988);Gupta et
al. (1990)
Unscheduled DNA Fischer 344 rat 5000 µg/ml 38:52 90.4 Negativea Simmon et al.
synthesis primary hepatocytes (1979)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
Hprt locus
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
OuaR
Gene mutation Mouse lymphoma 94 µg/ml NR NR Negative ± S9 Clive (1977)
L5178Y cells, Tk
locus
Chromosomal Chinese hamster 100 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration ovary cells Equivocal + S9 (1994)
Sister chromatid Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
exchange Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
formation Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 100 µg/ml NR 95 Equivocala Surrallès et al.
formation (1995a)
Micronuclei (with Human lymphocytes 100 µg/ml NR 97 Equivocala Surrallès et al.
inhibition of excision (1995b)
repair)
Micronuclei (with Human lymphocytes 10 µg/ml NR 97 Positivea Surrallès et al.
inhibition of excision (1995b)
repair)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Chromosomal Human lymphocytes 75 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration Equivocal + S9 (1992, 1994)
Inhibition of Chinese hamster 8 µg/ml NR NR Negativea Flodström et al.
gap-junctional lung V79 cells (1988)
intercellular
communication
In vivo
Dominant lethal Male CD-1 mice 452 mg/kg bw NR NR Negative Chesher et al.
mutation × 5 orally (1975)
NR, not reported; S9, 9000 × g supernatant of rat liver
a Not tested in the presence of S9
and chromosomal aberrations in one laboratory showed significant
results, while studies of micronucleus induction in another laboratory
showed negative results, except when the assay was modifed by
inhibition of DNA excision repair with cytosine arabinoside. No tests
of clastogenicity in vivo have been conducted. Permethrin did not
cause dominant lethal mutations in male mice. The Meeting concluded
that permethrin is not mutagenic but that it is clastogenic, at least
in vitro.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) was administered in the diet of groups of 12 male
and 24 female Long-Evans rats at concentrations of 0, 20, or 100 ppm,
equivalent to 0, 1.3, and 6.7 mg/kg bw per day, for three generations,
from F0 until weaning of the F3c generation. Treatment did not
affect the body weights of males or females at any time during the
study, and there was no effect on reproduction, on the number of
births, or on the survival or growth of the offspring. Ophthalmoscopic
examination of all 159 F3c offspring revealed two with
abnormalities: a unilateral cataract in one female in a control litter
and a unilateral coloboma of the choroid at the optic disc of one male
in a high-dose litter. The NOAEL for reproductive and systemic effects
was 100 ppm, equivalent to 6.7 mg/kg bw per day, the highest dose
tested (Schroeder & Rinehart, 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 26:74; purity, 94.5%) was administered in the diet
to groups of 20 Wistar rats of each sex at target doses of 0, 5, 30,
and 180 mg/kg bw per day for three generations, from F0 until
weaning of the F3b generation. The F2 parental rats were then
mated to produce an F3c litter and their pups were removed just
before weaning for teratological examination. There were no
treatment-related effects on body weights of males and females of any
generation and no effect on reproduction or on the number of births or
the survival and growth of the offspring. Offspring in all groups and
in all generations had a few ocular abnormalities which were found
histologically to be abnormalities of the retina and optic nerve,
indicative of glaucoma. The incidence of these findings was low and
not statistically correlated with treatment. The F3b fetuses showed
no treatment-related toxic or teratogenic effects. The NOAEL for
reproductive and systemic effects was 180 mg/kg bw per day, the
highest dose tested (James, 1979).
In a study conducted according to GLP, technical-grade permethrin
of seven batches (nominal cis:trans ratio, 40:60; purity,
94.1-98.9%) was administered in the diet to groups of 12 male and 24
femaleWistar rats at concentrations of 0, 500, 1000, or 2500 ppm,
equivalent to 0, 33, 67, and 170 mg/kg bw per day, for three
generations, from F0 until weaning of the F3b generation. The F2
parental rats were then mated to produce an F3c litter and their
pups were removed just before weaning for teratological examination.
The only clinical sign of toxicity was tremors, which were seen in
parents and pups receiving 1000 or 2500 ppm. The tremors were
transient, and no evidence of neuropathy was found in an extensive
examination of the nervous system of F1 males that had been exposed
in utero and fed the diet continuously for 1 year. No overall
effects on growth or reproductive performance were seen, and there was
no evidence of teratogenicity. Buphthalmia due to the persistent
presence of a pupillary membrane was first seen in the F1b litter
and then in subsequent litters. The incidence of this congenital
lesion was low (< 3%), occurring in two pups in one litter at 500 ppm
litter and in animals at 1000 and 2500 ppm.The effect may have been
due to interaction between permethrin and genetic factors, but it is
more likely to be due to differences in the selection of the F0
parents. Hypertrophy of centrilobular hepatocytes was seen in the
livers of treated F3b pups examined histologically, although no
lesion was seen on gross examination. Liver hypertrophy has been
observed in other studies of permethrin and is considered to be an
adaptive change of no toxicological significance. No NOAEL could be
identified for systemic toxicity, as hypertrophy of centrilobular
hepatocytes in the offspring and tremors in parents and offspring
occurred at all doses. The NOAEL for reproductive toxicity was
2500 ppm, equivalent to 170 mg/kg bw per day, the highest dose tested
(Hodge et al., 1977).
(ii) Developmental toxicity
Mice
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female CD-1
mice on days 6-15 of gestation. The groups consisted of 20 untreated
controls, 23 mice receiving corn oil at 10 ml/kg bw per day, and 22
mice given permethrin at 400 mg/kg bw per day. The animals were
observed daily for clinical signs of toxicity. One treated animal died
on day 17 before termination of pregnancy. Treatment had no
significant effect on weight gain during dosing and pregnancy, and
there was no significant difference in the mean numbers of
implantations among the groups, no significant treatment-related
effect on the numbers of live and normal fetuses or on the numbers of
resorbed, dead, and malformed fetuses, and no treatment-related effect
on individual fetal weights, litter size, or fetal sex ratios. No
gross visceral or skeletal malformations were found in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained death of one treated mouse. The NOAEL for developmental
toxicity was 400 mg/kg bw per day, the only dose tested (James,
1974a).
Rats
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female Wistar
rats on days 6-16 of gestation. The groups consisted of 22 untreated
controls, 23 rats receiving corn oil at 10 ml/kg bw per day, and 23
rats given permethrin at 200 mg/kg bw per day. Two treated rats died
before completion of their pregnancy. Treatment had no significant
effect on weight gain during dosing or pregnancy, and no significant
difference was seen in the mean numbers of corpora lutea or
implantations among the groups, no effect of treatment on the numbers
of live and normal fetuses, on individual fetal weights or litter
size, or on the fetal sex ratio. The numbers of resorbed, dead, and
malformed fetuses were not significantly affected by treatment. No
gross visceral or skeletal malformations were seen in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained deaths of two treated rats. The NOAEL for developmental
toxicity was 200 mg/kg bw per day, the only dose tested (James,
1974b).
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95%), aspirin, and corn oil were administered
orally by gavage to groups of 20 pregnant SpragueDawley rats during
days 6-16 of gestation, at doses of 2 ml/kg bw per day for corn oil,
200 mg/kg bw per day for aspirin, and 4, 41, or 83 mg/kg bw per day
for permethrin. There were no maternal deaths and no treatment-related
effects on the number of resorptions, body weight, length, or fetal
sex ratio. No gross, skeletal, or soft-tissue abnormalities were seen
in any group. The NOAEL for maternal and developmental toxicity was 83
mg/kg bw per day, the highest dose tested (Metker et al., 1977).
Technical-grade permethrin (cis:trans content, 37.5%:57.8%;
purity, 95.3%) diluted in corn oil was administered orally at a volume
of 10 ml/kg bw to groups of 20 pregnant CD ratson days 6-16 of
gestation at doses of 22.5, 71, or 225 mg/kg bw per day. None of the
dams died during the study, and there were no treatment-related
effects on weight gain, food consumption, pregnancy frequency, the
number of corpora lutea, the total number of implantations per
pregnancy, fetal or placental weight, or fetal sex ratios. The NOAEL
for maternal and developmental toxicity was 225 mg/kg bw per day, the
highest dose tested (McGregor & Wickramaratne, 1976).
Rabbits
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage at a volume of 2 ml/kg bw to
groups of female Dutch belted rabbits on days 6-18 of gestation. The
groups consisted of nine untreated controls, six given corn oil, and
seven given permethrin at 400 mg/kg bw per day. One animal in each
group died before the end of the study, but the deaths were not
related to treatment. There was no significant effect on weight gain
during dosing and gestation, no significant difference in the mean
numbers of corpora lutea and implantations, no effect on the numbers
of live and normal fetuses, the numbers of resorbed, dead, and
malformed fetuses, litter size, mean fetal weight, or fetal sex ratio.
No gross skeletal or visceral abnormalities were seen in any group.
The NOAEL for maternal and developmental toxicity was 400 mg/kg, the
highest dose tested (James, 1974c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
92.5%) was administered orally by gavage in 0.5% v/v aqueous Tween 80
to groups of 18 pregnant Dutch rabbits on days 6-18 of gestation at
doses of 600, 1200, or 1800 mg/kg bw per day. Five does at 600 and
1200 mg/kg bw per day and four at 1800 mg/kg bw per day group were
killed when moribund or were found dead. The clinical signs noted
included tremors at 1800 mg/kg bw per day. Many of the females found
dead or killed produced few or no faeces and had more than normal
amounts of fur in the stomach, and hypothermia and salivation were
generally seen. The body weights were reduced in all treated groups
but were statistically significantly lower onlyat 1800 mg/kg bw per
day. A statistically significant increase in post-implantation loss
was seen in animals at 1200 and 1800 mg/kg bw per day, and the number
and percentage of early and late resorptions were higher and the mean
number of live fetuses was lower in animals at these doses. The effect
at 1800 mg/kg bw per day was related to treatment, but animals at 1200
mg/kg bw per day showed a decreased number of implantation sites and a
decrease in the number of corpora lutea. As implantation occurred
before initiation of treatment, the effects at 1200 mg/kg bw per day
are not related. The fetal weights were similar to those of controls.
Evaluation of the fetuses revealed no gross visceral or skeletal
abnormalities. No NOAEL could be identified for maternal toxicity
because deaths, clinical signs, and body weight reduction were seen at
all doses. The NOAEL for developmental toxicity was 1200 mg/kg bw per
day (Richards et al., 1980).
(g) Special studies
(i) Neurotoxicity
Mice
Pyrethroids have been reported to induce two types of neurotoxic
syndrome in mice after intracerebroventricular administration
(Lawrence & Casida, 1982). The first type involves markedly increased
hypersensitivity and hyperactivity, followed quickly by whole-body
tremors and clonic seizures. The mice become prostate 5-20 min after
the onset of these signs, and profound whole-body tremors persist
until death, which usually occurs 5-30 min later. The second type of
syndrome is initially similar to the first but the signs rapidly
progress to include chreothetosis (sinuous writhing) and profuse
salivation, often with tonic seizures shortly before death after 15-45
min. Permethrin induces the second type of response, while
deltamethrin and fenvalerate, for instance, induce the first. Diazepam
at 1 mg/kg bw intraperitoneally did not delay the onset of the second
type of response in male albino mice treated with permethrin
intracerebroventricularly, but a dose of 3 mg/kg bw increased the
LD50 value in mice given (1 R, cis)-permethrin from 0.15 mg/kg bw
to 1.4 mg/kg bw (Gammon et al., 1982).
Technical-grade permethrin (cis:trans ratio, 40:60; purity not
stated) or the separated cis and trans isomers (purity not stated)
were administered in equal volumes of Emulphor and 95% ethanol (total
volume, 0.2 ml/10 mg pyrethroid) intravenously at a volume of 0.1 ml
or intracerebroventricularly at a volume of 0.005 ml to male ICR mice.
All treatments induced hyperactivity, increased sensitivity to
external stimuli, and whole-body tremor, leading to prostration and
ultimately to death. The ED50 values and 95% confidence intervals
for groups of six mice were 20 mg/kg bw (17-22) for the cis isomer,
36 mg/kg bw (33-39) for the technical-grade mixture, and 93 mg/kg bw
(83-100) for the trans isomer after intravenous administration, and
0.09 mg/kg bw (0.06-0.14) for the cis isomer, 0.15 mg/kg bw
(0.13-0.17) for the technical-grade mixture, and 1.1 mg/kg bw
(0.72-1.4) for the trans isomer after intracerebroventricular
administration. These results indicate that most of the activity is
due to the cis isomer and suggest a mainly central site of action.
The effects of a number of drugs that affect neurotransmitter systems
(noradrenaline, dopamine, acetylcholine, gamma-aminobutyric acid
(GABA), and serotonin) were studied on the loss of righting reflex
induced by permethrin given intravenously at a dose of 30 mg/kg bw. It
was not clear whether inhibitory GABA pathways were involved, whereas
the toxicity of permethrin was potentiated by at least some of the
drugs that affect central noradrenergic, cholinergic, or serotonergic
transmission. The involvement of these transmission systems in the
toxicity of permethrin could not be elucidated (Staatz et al., 1982).
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered as a 1.0%
(w/v) solution or suspension in corn oil to 10 Sprague-Dawley CD rats
of each sex at doses of 0, 10, 150, or 300 mg/kg bw. Clinical signs,
body weights, the results of a functional observational battery of
tests, and motor activity were recorded. At the end of the study, the
surviving rats were perfused, and the nervous systems of five rats of
each sex at the high dose and in the control group were examined. One
female at the high dose died on day 0. Treatment-related clinical
signs observed in this group were tremors, staggered gait, splayed
hind limbs, exaggerated hind-limb flexion, and hypersensitivity to
sound. All of the animals had recovered by day 3. There were no
effects on body weight. Rats at the high dose had whole-body tremors,
staggered gait, splayed hind limbs, abnormal posture while moving,
exaggerated hind-limb flexion, and convulsions on the first day of
functional and behavioural testing, but no significant differences
were noted after this time. There was no effect on motor activity.
Neuropathological examination of the nervous system revealed no
treatment-related lesions. The NOAEL for neurotoxicity was 150 mg/kg
bw on the basis of neurotoxic clinical signs and significant changes
in function and behaviour at 300 mg/kg bw per day (Freeman, 1993a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of five Sprague-Dawley CD of each sex at concentrations
of 0, 100, 750, 1500, 3000, 4000, or 5000 ppm for 28 days. Clinical
signs were recorded daily, and body weights were recorded weekly. All
surviving animals underwent gross necropsy on day 28. All rats
receiving 5000 ppm and one female receiving 4000 ppm had died by the
end of day 3. The treatment-related clinical signs included tremors,
splayed hind limbs, staggered gait, and chromorhinorrhoea at
concentrations > 1500 ppm. The clinical signs generally occurred
with increasing frequency with dose, except when pre-empted by death.
No clinical signs were seen among animals receiving doses < 750
ppm. Body weights were significantly reduced at doses > 3000 ppm
during some or all of the study, and body-weight gain was
significantly reduced at 3000 and 4000 ppm. Gross lesions found during
necropsy of animals at doses > 3000 ppm included necrosis of the
tail and feet and reddish-brown fluid in the stomach and intestines.
The NOAEL was 750 ppm, equal to 38 mg/kg bw per day, on the basis of
neurotoxic clinical signs at 1500 ppm (Freeman, 1993b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of 10 Sprague-Dawley rats at concentrations of 0, 250,
1500, or 2500 ppm for 13 weeks. Clinical signs were recorded daily,
and body weights and food consumption were recorded weekly. The
results of a functional observational battery of tests and tests for
motor activity were recorded before exposure and in weeks 4, 8, and 13
of the study. Surviving rats were perfused, and the nervous systems of
five rats of each sex at the high dose and in the control group were
examined microscopically for neuropathological lesions. No
treatment-related deaths occurred during the study, but
treatment-related clinical signs were observed in rats at doses
> 1500 ppm which included staggered gait, splayed hind limbs. and
tremors. These signs generally increased in both frequency and
severity with dose. Reductions in body weight and intermittent
reductions in food consumption were seen among males receiving 2500
ppm. Functional and behavioural testing showed whole-body tremors,
staggered gait, spayed hind limbs, and an abnormal posture during
movement among rats receiving 2500 ppm and to a lesser extent among
those given 1500 ppm. There were no significant differences with
regard to motor activity. No treatment-related lesions were found at
autopsy or on neuropathological examination. The NOAEL for
neurotoxicity was 250 ppm, equal to 15 mg/kg bw per day, on the basis
of neurotoxic clinical signs and significant changes in function and
behaviour at 1500 ppm (Freeman, 1993c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, > 98%) was administered in the
diet of Sprague-Dawley rats at target doses of 100, 200, and 400 mg/kg
bw per day for 90 days. The actual mean doses were 0, 86, 160, and 340
mg/kg bw per day for males and 0, 110, 170, and 350 mg/kg bw per day
for females. The groups consisted of 20 rats of each sex at the high
dose and as untreated controls and 10 rats of each sex at the
intermediate and low doses and as vehicle (acetone) controls. Clinical
signs, body weights, and food consumption were recorded three times a
week. At the end of treatment, half of the animals at the high dose
and of untreated controls were returned to untreated diet and held for
an additional 6 weeks to assess the reversibility of effects. At the
end of the study, the rats were perfused and the nervous systems from
those at the high dose and untreated and vehicle controls were
examined histologically for neuropathological lesions. Neurotoxic
signs were seen in all rats at the high dose and included tremors,
twitching, hyperexcitability, and irritability. The signs occurred
during the first day of treatment and persisted throughout the
90 days. Rats at the intermediate dose had tremors intermittently and
occasional periods of hyperexcitability during the first 2 days of
testing, but not later. When females at the high dose were returned to
untreated diet, all of the signs disappeared within 24 h, whereas in
males the tremors and twitching ceased within 1 day, and the
hyperexcitability and irritability stopped within 2-3 days. Decreased
body-weight gains were noted at the high dose, in females from week 3
and in males from week 11, but these deficits were rapidly corrected
during the recovery period. Neuropathological examination of the
central nervous system and peripheral nerves revealed no
treatment-related lesions. The NOAEL was 86 mg/kg bw per day on the
basis of neurotoxic clinical signs at 160 mg/kg bw per day (United
States Army, 1986).
The peripheral nerves, brain stem, and spinal cord of groups of
five Long-Evans rats of each sex were examined histologically for
abnormalities after exposure to permethrin. The rats were derived from
a 24-month feeding study (Braun & Rinehart, 1977) and from the third
generation of a three-generation study of reproductive toxicity
(Schroeder & Rinehart, 1977), described above, and were 10-11 months
old at the time of autopsy. In addition to standard histological
techniques, the neural material was examined morphometrically for the
number of myelinated fibres per nerve and per square millimetre of
fascicular area and for the frequency distribution of diameters per
nerve and per square millimetre of fascicular area; the morphology of
teased fibres was also evaluated. No structural lesions associated
with exposure of the rats to permethrin at dietary concentrations up
to 500 ppm (equivalent to 25 mg/kg bw per day) were observed in
central or peripheral nerves or in teased fibres of distal sural and
tibial nerves and of the maxillary division of the fifth cranial nerve
(Dyck, 1978).
Chickens
Technical-grade permethrin (cis:trans ratio, 50:50; purity,
96%) was administered by gavage to six adult laying hens at a dose of
1000 mg/kg bw per day for 5 days. After 21 days, the hens were
re-dosed and observed for an additional 21 days. A positive control
group of six hens received an intramuscular injection of a mixture of
atropine sulfate (17 mg/kg bw) and pralidoxime chloride (50 mg/kg bw)
and, 1 h later, a dose of tri- ortho-tolyl phosphate at 0.5 ml/kg bw
by gavage. Six untreated hens were available. No deaths occurred in
the treated or negative control groups during the study, and no
neurological disturbances and no histological lesions were found in
the peripheral or central nervous system. The positive controls showed
lack of coordination, unsteadiness, and loss of balance, which
worsened progressively. Foci of axonal and myelin degeneration were
observed in all positive control birds (Milner & Butterworth, 1977).
Technical-grade permethrin (cis:trans ratio, 36.0%:58.9%;
purity, 94.9%) was administered at a single dose of 15 ml, equal to
9100 mg/kg bw, the maximum possible dose, to 15 hens, which were
observed for 21 days for signs of neurotoxicity. The hens were treated
with an intramuscular injection of atropine and pyridine-2-aldoxime
methane sulfonate, then re-dosed with 15 ml of permethrin and observed
for an additional 21 days. Five hens were given tri- ortho-tolyl
phosphate at 500 mg/kg bw as a positive control, and 10 hens received
water as a negative control. No deaths occurred during the study, and
no signs of neurotoxicity were noted in the treated or negative
control groups. Ataxia, ranging from slight muscular incoordination to
difficulty in standing, was observed in the positive control group. No
treatment-related histopathological lesions of the spinal cord or
sciatic nerve were observed in the treated or negative control groups,
whereas degenerative changes were seen in the spinal cord and sciatic
nerve of hens treated with tri- ortho-tolyl phosphate. An NOAEL for
neurotoxicity could not be identified (Ross, et al., 1977).
(ii) Endocrine effects
The MCF-7 human breast carcinoma cell line was used to study the
oestrogenic potential of permethrin in vitro, pS2 mRNA expression
levels being used as the end-point. Permethrin at 100 µmol/L had no
effect on pS2 expression or on cell proliferation, whereas
17ß-estradiol at a concentration of 10 nmol/L induced a fivefold
increase in pS2 expression (Go et al., 1999).
3. Observations in humans
Twenty-three workers aged 20-52 years who had been exposed to
synthetic pyrethroids and 23 age- and sex-matched control subjects who
had had no contact with pyrethroids were interviewed, examined, and
assessed electrophysiologically. Most of the workers had been exposed
to several different pyrethroids, the more common being cypermethrin,
permethrin, fenvalerate, and fenpropathrin. Nineteen of the subjects
had experienced one or more episodes of abnormal facial sensation
0.5-3 h after exposure which persisted for 0.5-8 h. Thirteen subjects
had experienced more than one episode. There were no abnormal
neurological signs, and the results of electrophysiological studies of
the arms and legs were normal. Three subjects who had had moderate
exposure to permethrin had not developed symptoms, whereas two of them
did so after exposure to fenpropathrin and cypermethrin (Le Quesne et
al., 1980).
Workers who handled seedlings treated with permethrin
(cis:trans ratio, 25:75 for a wettable powder and 40:60 for an
emulsion in organic solvents; both diluted with water to 1-2% before
use) reported irritation of the skin. Of 42 workers who had used the
wettable powder, 12% reported burning and 10% reported blisters. Of 45
workers who had used the emulsion, 2% reporting itching. Irritation of
the upper respiratory tract involving increased nasal secretions and
sneezing was reported by 31% of the workers who had used the wettable
powder and by 2% of those who has used the emulsion (Kolmodin-Hedman
et al., 1982).
Volunteers received applications of 0.05 ml of a field-strength
preparation of technical-grade permethrin (94-96% active ingredient)
in ethanol to an area of 4 cm2 on an ear lobe, a formulation (32-36%
active ingredient) in water to 0.13 mg/cm2, or the solvent and
surfactants; 0.05 ml of the vehicle was applied to the other lobe. No
cutaneous sensation was elicited by the inert ingredients.
Paraesthesia appeared after a latent period of about 30 min, peaked
between 8 and 12 h, and disappeared after about 24 h. Further studies
with a range of doses ahowed that the response was dose-related
(Flannigan et al., 1985).
One of 28 subjects with pediculosis pubis treated with a 1%
permethrin rinse developed mild scrotal erythema and irritation 12 h
after application (Kalter et al., 1987). A group of 435 patients, most
of them children, were treated for pediculosis capitis: approximately
half of the group were treated with a single, 10-min application of
25-50 ml of a cream rinse containing permethrin (1%) and isopropanol
(20%) after towel drying of washed hair, and the remainder were
treated with a liquid product containing pyrethrins (0.3%), piperonyl
butoxide (3%), petroleum distillate (1.2%), and benzyl alcohol (2.4%).
Cutaneous side-effects including pruritus, mild transient skin
burning, stinging sensations, skin tingling, erythema, and scalp rash,
were reported by 7% of the patients in the first group and by 16% of
those in the second (DiNapoli et al., 1988). Similar results and
side-effects were reported by Brandenburg et al. (1986).
An observational study was undertaken in the USA to evaluate the
safety of a cream rinse containing 1% permethrin for treatment of head
louse infestations. Thirty-seven local public health departments
enrolled a total of 38 160 patients for 47 578 treatments with
permethrin and other pediculicides between 1 September 1986 and 31
January 1988. Follow-up information was collected 7-14 days after
treatment at a return visit or by telephone contact. A total of 103
adverse events were reported from 41 955 evaluable treatments. The
rates of reported adverse events were 2.2 per 1000 treatments with
permethrin, 3.4 per 1000 treatments with lindane, and 1.5 per 1000
treatments with other over-the-counter preparations. No serious,
unexpected adverse event was detected in the 18 950 patients treated
with permethrin (Andrews et al., 1992).
Of 10 patients with scabies treated with one application of 25 g
(range, 21-32 g) of a cream containing 5% permethrin, followed by a
thorough washing 8-20 h after treatment, six developed limited,
mild-to-moderate eczema on the scabies-affected skin at one or more
examinations (van der Rhee et al., 1989).
Comments
The metabolism of 14C-permethrin was studied in rats, lactating
goats and cattle, and laying hens. Permethrin was rapidly absorbed,
distributed, and excreted in these species after oral administration.
The metabolism of the pyrethroid was extensive, yielding a vast number
of polar degradates. Ester cleavage, hydroxylation, oxidation, and
ultimately conjugation are the critical biological mechanisms of the
metabolism of permethrin in the species studied. The metabolites that
were common to all species were 4'-hydroxypermethrin, dichlorovinyl
acid, and phenoxybenzyl alcohol. Dichlorovinyl acid and phenoxybenzoic
acid have also been identified in human urine after dermal application
of permethrin.
In rats, 96% of an administered dose was recovered in urine and
faeces within 12 days, while the total radiolabelled residues in
tissues accounted for 0.3-0.8% of the dose. Recovery in urine and
faeces within 24 h accounted for about 40% and 25% of the dose of
cis isomer and 65% and 10% of the dose of trans isomer.
respectively. Repeated exposure resulted in temporary accumulation in
fat tissue, but the chemical dissipated rapidly once exposure had
ceased.
In lactating goats and cows dosed orally with permethrin,
recovery in urine and faeces accounted for at least 65% of the dose,
and the total radiolabelled residues in liver and milk samples
represented 0.2-0.5%. Permethrin was extensively metabolized and
readily eliminated after oral administration to laying hens, > 90%
of the administered dose being excreted, while the total radiolabel in
egg and liver samples accounted for 0.1-0.2% of the dose.
The toxicity of permethrin is influenced by many factors
including the cis:trans isomer ratio, the carrier or vehicle, and
the strain of animal used. The cis isomer is considerably more toxic
than the trans isomer. The oral LD50 values in rats ranged from
6000 mg/kg bw for the 20:80 cis:trans isomeric mixture to 220 mg/kg
bw for the 80:20 cis:trans isomeric mixture. Undiluted
technical-grade permethrin (25:75 to 40:60 cis:trans isomeric
mixtures) has low acute toxicity after oral, dermal and inhalation
exposure. It was mildly irritating to the eyes and slightly irritating
to skin. It was not a skin sensitizer when tested by the Magnusson and
Kligman method.
WHO (1999) has classified permethrin as 'moderately hazardous'.
Studies in which rats, mice, rabbits, guinea-pigs, and dogs
received repeated administrations by inhalation, orally, and dermally
showed that the main effects of technical-grade permethrin are on
clinical signs, especially tremor and hyperexcitability, body weight,
and liver weight. In these short-term studies, the NOAEL or NOAEC
values were 250 mg/m3 in a 13-week study in rats exposed by
inhalation; 5 mg/kg bw per day in a 52-week study in which dogs
received the compound in gelatin capsules orally; and 1000 mg/kg bw
per day in a 21-day study in rabbits treated dermally.
In two long-term studies in rats in different laboratories with
different strains, permethrin was not carcinogenic, but the evidence
for carcinogenicity in mice was conflicting. In two studies conducted
in same strain in the same laboratory, permethrin increased the
incidences of lung and liver tumours in one study but not in the
other. The spontaneous background incidence of both these tumour types
is known to be extremely variable. A third study, conducted in a
different mouse strain, gave negative results. Thus, the weight of
evidence supports the conclusion that permethrin has very weak
oncogenic potential, and the probability that it has oncogenic
potential in humans is remote. The NOAEL for long-term toxicity in
rats was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs and changes in body and organ weights and blood
chemistry at 500 ppm. The NOAEL for long-term toxicity in mice was
500 ppm, equivalent to 75 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm.
No genotoxic activity was observed in an adequate battery of
tests for DNA damage and mutagenicity in vitro, but there was
evidence that permethrin can induce chromosomal aberrations in
mammalian cells in vitro. No tests have been carried out in mammals
for DNA damage, mutagenicity, or clastogenicity in vivo. A test for
dominant lethal effects in male mice showed no activity.
In a multigeneration study of reproductive toxicity in rats, the
NOAEL for systemic and reproductive toxicity was 180 mg/kg bw per day.
In a second multigeneration study in rats, an NOAEL could not be
identified for systemic toxicity, as effects were seen at 500 ppm,
equivalent to 33 mg/kg bw per day, the lowest dose tested; the NOAEL
for reproductive toxicity in the same study was 2500 ppm, equivalent
to 170 mg/kg bw per day, the highest dose tested.
In a study of developmental toxicity in rabbits, the NOAEL for
maternal effects was 600 mg/kg bw per day and that for developmental
toxicity was 1200 mg/kg bw per day. In three studies of developmental
toxicity in rats, the NOAEL for maternal toxicity was 83 mg/kg bw per
day and the NOAEL for developmental toxicity was 225 mg/kg bw per day,
the highest dose tested. In a study of developmental toxicity in mice,
no NOAEL was identified for maternal toxicity, whereas the NOAEL for
developmental effects was 400 mg/kg bw per day, the only dose tested.
The Meeting concluded that technical-grade permethrin is not a
reproductive or developmental toxin.
The results of acute and 90-day studies of neurotoxicity in rats
and of an acute study of delayed neurotoxicity in hens showed that
technical-grade permethrin does not induce neuropathological changes.
The NOAEL for neurotoxicity in a study in rats given a single dose was
150 mg/kg bw, on the basis of clinical signs of neurotoxicity and
significant changes in measurements in a functional observational
battery of tests at 300 mg/kg bw. The NOAEL for neurotoxicity in a
13-week study in rats was 15 mg/kg bw per day, on the basis of
clinical signs of neurotoxicity and significant changes in
measurements in the functional observational battery of tests at 90
mg/kg bw per day.
An ADI of 0-0.05 mg/kg bw was established for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60 on the basis of
the NOAEL of 100 ppm, equivalent to 5 mg/kg bw per day, in the 2-year
study in rats, which was based on clinical signs and changes in body
and organ weights and blood chemistry at 500 ppm, and the NOAEL of 5
mg/kg bw per day in a 1-year study in dogs based on reduced body
weight at 100 mg/kg bw per day, and applying a safety factor of 100.
The Meeting concluded that establishment of an acute reference
dose was not necessary because of the low acute toxicity of
technical-grade permethrin.
Toxicological evaluation
Levels that cause no toxic effect (relevant for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60)
Mouse: 500 ppm, equivalent to 75 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
Rat: 100 ppm, equivalent to 5 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
180 mg/kg bw per day (for reproductive toxicity, highest
dose in a three-generation study of reproductive toxicity)
225 mg/kg bw per day (for maternal and developmental
toxicity, highest dose in a study of developmental toxicity)
150 mg/kg bw (single dose in a study of neurotoxicity)
15 mg/kg bw per day (13-week study of neurotoxicity)
Rabbit: 400 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
1200 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity)
Dog: 5 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.05 mg/kg bw (for technical-grade permethrin with cis:trans
ratios of 25:75 to 40:60)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Clarification of the findings of chromosomal aberrations in
vitro and their potential significance in vivo
2. The Meeting was aware of other studies, in particular of acute
toxicity, dermal and ocular irritation, sensitization, and
developmental toxicity in rats that had been made available to
regulatory entities by other sponsors. The continuing support of
permethrin would benefit from the submission of these studies for
review by the Joint Meeting.
Toxicological criteria relevant for estimating guidance values for dietary and non-dietary exposure to permethrin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid and extensive (rat, lactating caprine and bovine, hen)
Distribution Mainly to fat (rat, lactating caprine and bovine, hen)
Potential for accumulation Some accumulation in fat on repeated dosing, rat
Rate and extent of excretion cis-isomer: 40% in urine, 25% in faeces in 24 h in rat
trans-isomer: 65% in urine, 10% in faeces in 24 h in rat
Metabolism in animals Extensive; hydrolysis, hydroxylation, oxidation and conjugation
(rat, lactating caprine and bovine, hen)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 225 (cis:trans ratio, 80:20); 6000 (cis:trans ratio,
20:80) mg/kg bw
Rat, LD50, dermal No data
Rabbit, LD50, dermal 2000 mg/kg bw (cis:trans ratio, 55:45 or 40:60) (highest dose)
Rat, LC50, inhalation > 23.5 mg/l, 4 h (cis:trans ratio, 40:60)
Dermal irritation Slightly irritating to rabbit skin
Ocular irritation Mildly irritating to rabbit eyes
Dermal sensitization No sensitizing potential in guinea-pigs
Short-term toxicity
Target/critical effect Nervous system (rat); liver (mouse, rat, dog)
Lowest relevant oral NOAEL 5 mg/kg bw per day in dog (cis:trans ratio, 32%:52%)
Lowest relevant dermal NOAEL 1000 mg/kg bw per day in rabbit
Lowest relevant inhalation NOAEL 250 mg/m3 in rat (cis:trans ratio, 40:60)
Target/critical effect Nervous system (rat)
Liver (mouse, rat)
Lowest relevant NOAEL Rat, 5 mg/kg bw per day; mouse, 75 mg/kg bw per day
Long-term toxicity and carcinogenicity
Carcinogenicity Not carcinogenic to mouse or rat
Genotoxicity No DNA damage or mutagenicity in vitro; clastogenic
in vitro; not studied in vivo in mammals
Reproductive toxicity
Reproductive target/critical effect None identified
Lowest relevant reproductive NOAEL 180 mg/kg bw per day in rat
Developmental target/critical effect Rat, none identified; rabbit, fetotoxicity
Lowest relevant developmental NOAEL Rat, 225 mg/kg bw per day (cis:trans ratio, 38%:58%);
rabbit, 1200 mg/kg bw per day (cis:trans ratio, 40:60)
Neurotoxicity/Delayed neurotoxicity NOAEL, 150 mg/kg bw, single dose, rats;
NOAEL, 15.5 mg/kg bw per day in a 90-day study, rats
No acute delayed effect in hens (9100 mg/kg bw)
Other toxicological studies
Medical data Paraesthesia
Summary Value Study Safety factor
ADI 0-0.05 mg/kg bw Rat, long-term toxicity, 100
5 mg/kg bw per day
Dog, 1 year, toxicity,
5 mg/kg bw per day
Acute reference dose Unnecessary
References
Andrews, E.B., Joseph, M.C., Magenheim, M.J., Tilson, H.H., Doi, P.A.
& Schultz, M.W. (1992) Postmarketing surveillance study of
permethrin creme rinse. Am. J. Public Health, 82, 857-861.
Angerer, S. & Ritter, A. (1997) Determination of metabolites of
pyrethroids in human urine using solid-phase extraction and gas
chromatography-mass spectrometry. J. Chromatogr. B Biomed.
Sci. Appl., 695, 217-226.
Bartsch, H., Malaveille, C., Camus, A.M., Martel-Planche, G., Brun,
G., Hautefeuille, A., Sabadie, N., Barbin, A., Kuroki, T.,
Drevon, C., Piccoli, C. & Montesano, R. (1980) Validation and
comparative studies on 180 chemicals with S. typhimurium
strains and V79 Chinese hamster cells in the presence of various
metabolizing systems. Mutat. Res., 76, 1-50.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1992)
Cytogenetic effects of permethrin in cultured human lymphocytes.
Mutagenesis, 7, 433-437.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1994)
Induction of structural chromosome aberrations in human
lymphocyte cultures and CHO cells by permethrin. Teratog.
Carcinog. Mutag., 14, 31-38.
Becci, P.J. & Parent, R. (1980) Evaluation of the subchronic toxic
effects of FMC 45801 when administered in the diet to Long Evans
rats over a 90-day period. Unpublished report from Food and Drug
Research Laboratories, Inc., Waverly Research Center, USA, Study
No. 6363. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Becci, P.J., Gephart, L. & Parent, R.A. (1980) 90-day subchronic oral
dosing study with FMC 45801 in beagle dogs. Unpublished report
from Food and Drug Research Laboratories, Inc., Waverly Research
Center, USA, Study No. 6338. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Benner, J. (1997) Permethrin: addendum to MRID numbers 42410001,
43505201, 43962801 and 44196101 submitted in response to EPA CBRS
review of MRID 43962801, goat metabolism -- oral dosing.
Unpublished report from Zeneca Agrochemicals, Bracknell, England,
Project No. HRC/ISN 248/920216sup2. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Benner, J.P., Hamlet, J.M. & Skidmore, M.W. (1996) Addendum to MRID
Nos. 42410001, 43505201 and 43962801, Permethrin: Further
investigation of residues in liver following oral administration
to the goat and radiovalidation of enforcement methods for
analysis of animal tissues. Unpublished report from Zeneca
Agrochemicals (Zeneca Ltd), Bracknell, England, Report No. RJ
2135B. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Billups, L.H. (1978a) Twenty-four month toxicity/carcinogenicity study
of compound FMC 33297 in rats. Histopathologic evaluation of
step-sectioned lungs from male rats. Unpublished report from
Environmental Pathology Services, Rockville, Maryland, USA.,
Study No. 78-6-0049. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA and by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
Billups, L.H. (1978b) Histopathologic evaluation of a twenty-four
month toxicity/carcinogenicity study of compound FMC 33297 in
rats. Unpublished report from Environmental Pathology Services,
Rockville, Maryland, USA., Study No. 77-11-0007. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA and by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Brandenburg, K., Deinard, A.S., DiNapoli, J., Englander, S.J.,
Orthoefer, J. & Wagner, D. (1986) 1% permethrin cream rinse vs
1% lindane shampoo in treating pediculosis capitis. Am. J.
Dis. Child., 140, 894-896.
Bratt, H., Mills, I.H. & Slade, M. (1977) Permethrin: Tissue retention
in rats. Unpublished report from Imperial Chemical Industries
Ltd, Alderley Park, England, Report No. ZO671. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975a) Acute oral toxicity studies in
rats with FMC 33297. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project Nos. 2702-75, 2703-75,
2704-75 and 2705-75. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975b) Acute dermal toxicity in
rabbits. Compound No. FMC 33297. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA, Project No.
2908-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975c) Rabbit primary dermal
irritation. Compound No. FMC 33297. Unpublished report prepared
by Bio/dynamics Inc. East Millstone, New Jersey, USA, Project No.
2909-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975d) Rabbit eye irritation.
Compound No. FMC Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2909-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1976) Acute inhalation. Compound No.
FMC 33297. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2911-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Rinehart, W.E. (1977) Twenty-four month oral
toxicity/carcinogenicity study of FMC 33297 technical in rats.
Unpublished report from Bio/dynamics, Inc., East Millstone, New
Jersey, USA, Project No. 74R-1022. Submitted to WHO by FMC
Corporation, Middleport, New York, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Brusick, D.J. & Weir, R.J. (1976) Mutagenicity evaluation of FMC 33297
(catalyst: titanium isopropylate), C8093-25, MR 51310.
Unpublished report from Litton Bionetics, Inc. Kensington,
Maryland, USA, Project No. 2683. Submitted to WHO by FMC
Corporation, Middleport, New York, USA.
Busey, W.M. (1978) Two-year chronic rat toxicity study FMC-33297.
Unpublished report from Experimental Pathology Laboratories Inc.,
Rockville, Maryland, USA. FMC Project No. NCT 549.32. Revised
pathology report. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Butterworth, S.T.G. & Hend, R.W. (1976) Toxicity studies on the
insecticide WL 43479: A five week feeding study in rats.
Unpublished report from Shell Research Ltd, Sittingbourne,
England, Report No. TLGR.0056.76. Submitted to WHO by Mitchell
Cotts Chemicals, Ltd, Mirfield, England.
Cameron, B.D. & Partridge, S.(1989) The metabolism of
[14C]-permethrin in the rat. Unpublished report from Inveresk
Research International, Musselburgh, Scotland, Report No. 4860.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Chesher, B.C. & Malone, J.C. (1974) Guinea pig sensitisation study
with 21Z73 using the maximization test method. Unpublished report
from Wellcome Research Laboratories, Berkhamsted, England, Lab.
Ref. No. T.L.13-74. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Chesher, B.C., Malone, J.C. & Parker, M.J. (1975) 21Z73, Dominant
lethal study in male mice. Unpublished report from Wellcome
Research Laboratories, Berkhamsted. England, Lab. Ref. No. T.L.
37-75. Submitted to WHO by FMC Corporation, Middleport, New York,
USA.
Clapp, M.J.L., Banham, P.B., Glaister, J.R. & Moyes, A. (1977a) PP557:
28 day feeding study in mice. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/356. Submitted to WHO by FMC Corporation, Middleport, New
York, USA.
Clapp, M.J.L., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W. &
Moyes, A. (1977b) PP557: 28 day feeding study in rats.
Unpublished report from Imperial Chemical Industries, Ltd,
Alderley Edge, England, Report No. CTL/P/355. Submitted to WHO by
FMC Corporation, Middleport, New York, USA.
Clive, D. (1977) Mutagenicity of BW 21Z73 in L5178Y/TK+/- mouse
lymphoma cells with and without exogenous metabolic activation.
Unpublished report from Burroughs Wellcome Co., Research Triangle
Park, North Carolina, USA, Doc. No. TTEP/77/0001. Submitted to
WHO by FMC Corporation, Middleport, New York, USA.
Cridland, J.S. & Weatherley, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)2,2-dimethylcyclopropane carboxylic acid
after oral ingestion of permethrin (NRDC 143) -- A first report.
Unpublished report from The Wellcome Foundation, Ltd, Beckenham,
England, Report No. BDPE-77-1. Submitted to WHO by The Wellcome
Foundation, Ltd, Beckenham, England.
Cridland, J.S. & Weatherley, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial
of the insecticide. Unpublished report from The Wellcome
Foundation, Ltd, Beckenham, England, Report No. BDPE-771.
Submitted to WHO by The Wellcome Foundation, Ltd, Beckenham,
England.
Cummins, H.A. & Gardner, J.R. (1984a) Permethrin: Acute oral toxicity
in the rat. Unpublished report from Life Science Research, Eye,
England, Report No. 84/MCC001/588. Submitted to WHO by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Cummins, H.A. & Gardner, J.R. (1984b) High cis permethrin: (isomer
ratio: 80:20) acute oral toxicity in the rat. Unpublished report
from Life Science Research, Eye, England, Report No.
84/MCC002/587. Submitted to WHO by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
DiNapoli, J.B., Austin, R.D., Englander, S.J., Gomez, M.P. & Barrett,
J.F. (1988) Eradication of head lice with a single treatment.
Am. J. Public Health, 78, 978-980.
Dyck, P.J. (1978) A pathologic and morphometric study of the nervous
system of rats fed compound FMC 33297 (permethrin). Unpublished
report from Peripheral Nerve Laboratory, Mayo Clinic and Mayo
Foundation, New York, New York, USA, Report No. not identified.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Flannigan, S.A., Tucker, S.B., Key, M.M., Ross, C.E., Fairchild, E.J.,
II, Grimes, B.A. & Harrist, R.B. (1985) Synthetic pyrethroid
insecticides: A dermatological evaluation. Br. J. Ind. Med.,
42, 363-372.
Flodström, S., Warngard, L., Ljungquist, S. & Ahlborg, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch.Toxicol., 61, 218-223.
Freeman, C. (1993a) Permethrin technical twenty-eight day
neurotoxicity range-finding study in rats. Unpublished report
from FMC Corporation, Princeton, New Jersey, USA, Study No.
A92-3645. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Freeman, C. (1993b) Permethrin technical subchronic neurotoxicity
screen in rats. Unpublished report from FMC Corporation,
Princeton, New Jersey, USA, Study No. A92-3647. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA
Freeman, C. (1993c) Permethrin technical acute neurotoxicity screen in
rats Unpublished report prepared by FMC Corporation, Princeton,
New Jersey, USA, Study No. A923646. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA
Gammon, D.W., Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. Appl. Pharmacol., 66,
290-296.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1977)
Permethrin metabolism in rats. J. Agric. Food Chem., 25, 9-17.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1978)
Distribution and metabolism of trans- and cis-permethrin in
lactating Jersey cows. J. Agric. Food Chem., 26, 613-618.
Ghiasuddin, S.M. & Soderlund, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. Appl.
Pharmacol., 74, 390-396.
Go, V., Garey, J., Wolff, M.S. & Pogo, B.G. (1999) Estrogenic
potential of certain pyrethroid compounds in the MCF-7 human
breast carcinoma cell line. Environ. Health Perspectives, 107,
173-177.
Gupta, R.K., Mehr, Z.A., Korte, D.W. & Rutledge, L.C. (1990) Mutagenic
potential of permethrin in the Drosophila melanogaster
(Diptera: Drosophilidae) sex-linked recessive lethal test.
J. Econ. Entomol., 83, 721-724.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977a) PP557: Liver hypertrophy study in rats -- Dietary
administration over 26 weeks. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/360. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977b) PP557: Whole life feeding study in mice. Unpublished
report from Imperial Chemical Industries, Ltd, Alderley Park,
England, Report No. CTL/P/359. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, D., Shaw, D. & Latter (1992a) The
metabolism of 14C permethrin in the hen. Unpublished from
Huntingdon Research Centre Ltd, Cambridgeshire, England, Project
No. HRC/ISN 272/920435. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, & Shaw, D. (1992b) The metabolism of 14C
permethrin in the goat. Unpublished report from Huntingdon
Research Centre Ltd, Cambridgeshire, England, Project No. HRC/ISN
248/920216. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Herrera, A. & Laborda, E. (1988) Mutagenic activity in synthetic
pyrethroids in Salmonella typhimurium. Mutagenesis, 3, 509-514.
Herrera, A., Barrueco, C., Caballo, C. & de la Peña E. (1992) Effect
of permethrin on the induction of sister chromatid exchanges and
micronuclei in cultured human lymphocytes. Environ. Mol.
Mutag., 20, 218-222.
Hodge, M.C., Banham, P.B., Glaister, J.R., Richards, D., Taylor, K. &
Weight, T.M. (1977) PP557: 3-Generation reproduction study in
rats. Unpublished report from Imperial Chemical Idustries Ltd,
Alderley Edge, England, Report No. CTL/P/361. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA.
Hogan, G.K. & Rinehart, W.E. (1977) Twenty-four month oral
carcinogenicity study with FMC 33297 technical in mice.
Unpublished report from Bio/dynamics, Inc., East Millstone,New
Jersey, USA, Project No. 74-1100. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Ishmael, J. & Litchfield, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice.
Fundam. Appl. Toxicol., 11, 308-322.
James, D.A. (1974a) Preliminary foetal toxicity study in the mouse
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/12.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1974b) Preliminary foetal toxicity study in the rat given
21Z73 (NRDC 143) orally. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/10.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
James, D.A. (1974c) Preliminary foetal toxicity study in the rabbit
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/19.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 79/3. Submitted
by FMC Corporation, Princeton, New Jersey, USA.
Kalinowski, A.E., Banham, P,B., Chart, I.S., Cook, S.K., Gore, C.W.,
Moreland, S.F. & Woollen, B.H. (1982) Permethrin: One year oral
dosing study in dogs. Unpublished report from Imperial Chemical
Industries Ltd, Alderley Park, England, Report No. CTL/P/647.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Kalter, D.C., Sperber, J., Rosen, T. & Matarasso, S. (1987) Treatment
of pediculosis pubis. Clinical comparison of efficacy and
tolerance of 1% lindane shampoo vs 1% permethrin cream rinse.
Arch. Dermatol., 123, 1315-1319.
Killeen, J.C. & Rapp, W.R. (1974) A four week feeding study of FMC
33297 in rats. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 74S-1024. Submitted to
WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1975a) A thirty day pilot feeding study of
FMC 33297 in rats. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project No. 74-1098. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C., Jr & Rapp, W.R. (1975b) A fourteen-day dose
range-finding study of FMC 33297 in beagle dogs. Unpublished
report from Bio/dynamics Inc., East Millstone, New Jersey, USA,
Project No. 75-1202B. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1976) A three month oral toxicity study of
FMC 33297 in beagle dogs. Unpublished report from Bio/dynamics
Inc., East Millstone, New Jersey, USA, Project No. 75-1188B.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
and by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Kolmodin-Hedman, B., Swensson, Å. & Åkerbolm, N. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50, 27-33.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-activity relationships. Pestic.
Biochem. Physiol., 18, 9-14.
Leng, G., Kuhn, K.H. & Idel, H. (1997) Biological monitoring of
pyrethroids in blood and pyrethroid metabolites in urine:
applications and limitations. Sci. Total Environ., 199,
173-181.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980) Transient
facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurophysiology, 2, 1-11.
Litchfield, M.H. (1985) Toxicity in mammals. In: Leahey, J.P., ed.,
The Pyrethroid Insecticides, London: Taylor & Francis, pp.
99-150.
Llewellyn, D.M., Brazier, A., Brown, R., Cocker, J., Evans, M.L.,
Hampton, J., Nutley, B.P. & White, J. (1996) Occupational
exposure to permethrin during its use as a public hygiene
insecticide. Ann. Occup. Hyg., 40, 499-509.
Marowitz, L.A. (1974a) Comparative acute oral toxicity in mice with
FMC 33297, FMC 37400, FMC 35171 and FMC 30960. Unpublished report
from Bio/dynamics Inc., East Millstone, New Jersey, USA, Project
Nos. 2184-74, 2185-74, 2222-74 and 2238-74. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Marowitz, L.A. (1974b) Acute oral toxicity in rats. Compound No. FMC
33297. Unpublished report from Bio/dynamics Inc., East Millstone,
New Jersey, USA, Project No. 2186-74. Submitted to WHO by FMC
corporation, Princeton, New Jersey, USA.
McGregor, D.B. & Wickramaratne, G.A. (1976) Teratogenicity study in
rats of ICI-PP 557. Unpublished report from Inveresk Research
International, Edinburgh, Scotland, Project No. 404898. Submitted
to FMC Corporation, Princeton, New Jersey, USA
Mehr, Z.A., Justus, J.D., Gupta, R.K. & Korte, D.W., Jr (1988)
Mutagenic potential of permethrin in the Drosophila
melanogaster sex-linked recessive lethal test. Unpublished
report from Letterman Army Institute of Research, Presidio of San
Francisco, California, USA, Report No. 302. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Metker, L., Angerhofer, R.A., Pope, C.R. & Swentzel, K.C. (1977)
Toxicological evaluation of 3-(phenoxyphenyl)methyl
(+)- cis, trans-3,2-(2,2-dichloroethenyl)-2,2dimethylcyclopropa
necarboxylate (permethrin). Unpublished report from the US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 51-0831-78.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: Investigation of the neurotoxic potential of WL
43479 to adult hens. Unpublished report from Shell Toxicology
Laboratory, Sittingbourne, England, Report No. TLGR.OO69.77.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nassif, M., Brooke, J.P., Hutchinson, D.B.A., Kamel, O.M. & Savage,
E.A. (1980) Studies with permethrin against bodylice in Egypt.
Pestic. Sci., 11, 679-684.
Pednekar, M.D., Gandhi, S.R. & Netrawali, M.S. (1987) Evaluation of
mutagenic activities of endosulfan, phosalone, malathion, and
permethrin, before and after metabolic activation, in the Ames
Salmonella test. Bull. Environ. Contam.Toxicol., 38, 925-933.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille
& Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137, 7-15.
Rapp, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC 33297 in mice. Histopathology report. Unpublished report
from McConnell & Rapp, Flemington, New Jersey, USA, FMC
Identification No. ACT 605.35. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Reynolds, J., Piercy, D.W.T., Clampitt, R.B., James, J.A., Thompson,
P.M., Farebrother, D.A. & Dayan, A.D. (1978) Permethrin oral
administration to dogs for 6 months. Unpublished report from The
Wellcome Foundation Ltd, Berkhamsted Hill, England, Doc. No. HEFG
78-14. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
van der Rhee, H.J., Farquhar, J.A. & Vermeulen, N.P.E. (1989) Efficacy
and transdermal absorption of permethrin in scabies patients.
Acta Dermatol. Venereol., 69, 170-182.
Richards, D., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W.,
Pratt, I., Taylor, K. & Weight, T.M. (1977) PP557: Two year
feeding study in rats. Unpublished report from Imperial Chemical
Industries, Ltd, Alderley Edge, England, Report No. CTL/P/357.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Richards, D., Banham, P.B., Kilmartin, M. & Weight, T.M. (1980)
Permethrin: teratogenicity study in the rabbit. Unpublished
report from Imperial Chemical Industries Ltd, Alderley Edge,
England, Report No. CTL/P/523. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E. & Cooke, L.
(1977) Examination of permethrin for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon Research Centre,
Huntingdon, England, Doc. No. ICI/157-NT/77468. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980a) Acute oral toxicity in rats. Test article: FMC 33297
55/45. Unpublished report from Cosmopolitan Safety Evaluation
Laboratory, Inc., Somerville, New Jersey, USA, Study No. 0245A.
submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980b) Acute dermal toxicity study -- Rabbit LD50 test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245B. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980c) Primary dermal irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory Inc., Somerville, New Jersey, USA,
Study No. 0245D. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980d) Primary eye irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245C. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Schroeder, R.E. & Rinehart, W.E. (1977) A three generation
reproduction study of FMC 33297 in rats. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Shah, P.V., Monroe, R.J. & Guthrie, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59, 414-423.
Simmon, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC Corporation compound. Unpublished report from Stanford
Research Institute, Menlo Park, California, USA, Project No.
LSC-4768. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Simmon, V.F., Riccio, E.S., Robinson, D.E. & Mitchell, A.D. (1979) In
vitro microbiological mutagenicity and unscheduled DNA synthesis
studies of fifteen pesticides. Unpublished report from SRI
International, Menlo Park, California, USA, Project No. LSU-3493.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Skidmore, M. (1996) Permethrin: Supplemental information on MRID Nos.
42410001 and 43505201 submitted in response to EPA CBRS review
dated 5 September 1995. Goat metabolism study -- oral dosing.
Unpublished report from Zeneca Ag Products, Bracknell, England,
Project No. HRC/ISN 248/920216sup1. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Soderlund, D.M. & Casida, J.E. (1977) Effects of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pest. Biochem. Physiol., 7, 391-401.
Staatz, C.G., Bloom, A.S. & Lech, J.J. (1982) A pharmacological study
of pyrethroid neurotoxicity in mice. Pest. Biochem. Physiol.,
17, 287-292.
Surrallès, J., Xamena, N., Creus, A., Catalan, J., Norppa, H. &
Marcos, R. (1995a) Induction of micronuclei by five pyrethroid
insecticides in whole-blood and isolated human lymphocyte
cultures. Mutat Res., 341, 169-184.
Surrallès, J., Xamena, N., Creus, A. & Marcos, R. (1995b) The
suitability of the micronucleus assay in human lymphocytes as a
new biomarker of excision repair. Mutat Res., 342, 43-59.
Tierney, W.J. & Rinehart, W.E. (1979) A twenty-four month oral
carcinogencicity study of FMC 33297 in mice. Unpublished report
from Bio/dynamics, East Millstone, New Jersey, USA, Project No.
76-1695. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
United States Army (1978) Subchronic inhalation toxicity of
3-(phenoxyphenyl) methyl
(+) cis, trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane
carboxylate (permethrin). Unpublished report from US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 75-51-0026-80. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
United States Army (1986) Neurotoxicity in rats following subchronic
ingestion of permethrin-treated food. Unpublished report from US
Army Environmental Hygiene Agency, Aberdeen Proving Ground, Study
No. 75-51-0351-87. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Wallwork, L.M., Poll, G.S. & Malone, J.C. (1975) Effect on the rat
oral toxicity of changes in the cis/ trans ratio with 21Z73
(NRDC 143) series. Unpublished report from The Wellcome
Foundation, Berkhamsted, England, Doc. No. HEFG 75-5. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, L.M., Thomson, P.M., Clampitt, R.B. & Malone, J.C. (1976b)
Rat oral 90 day toxicity study. Unpublished report from The
Wellcome Foundation, Berkhamsted and Beckenham, England, Doc. No.
HEFG 76-1. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Williams, L.M., Clampitt, R.D., James, J.A., Reynolds, J. & Woods,
N.C. (1976a) Rat oral 90 day toxicity study. Unpublished report
from TheWellcome Foundation, Berkhamsted, England, Doc. No. HEFG
76-3. Submitted to WHO by FMC Corporation, Princeton, New Jersey,
USA.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster.
Environ. Mutag., 5, 835-846.
decreased to < 100 µg/L within 2-4 days after treatment ceased. The
only compound recovered from milk after administration of the acid-
and alcohol-labelled trans isomer was permethrin, whereas with the
cis isomer 85% of the radiolabel was attached to the parent compound
and 15% to trans-hydroxy- cis-permethrin.
The metabolic reactions of permethrin in cows were similar to
those in rats and hens. In cows, the permethrin isomers, their mono-
and di-hydroxy derivatives, and 3-phenoxybenzyl alcohol appeared only
in the faeces, while the cis-hydroxy-chrysanthemic acid lactones
appeared in both faeces and urine. The remaining metabolites occurred
only in urine. Although a slightly larger proportion of cis- than
trans-permethrin was excreted unchanged, the amounts of ester
metabolites were similar, and these metabolites were hydroxylated at
the trans or cis methyl position of the geminal dimethyl group, at
the 4' position of the phenoxybenzyl group, or at both the geminal
dimethyl and phenoxybenzyl groups. The preferred hydroxylation site
for both isomers was the trans methyl group. The major metabolites
of the acid moieties of both isomers were the corresponding
cis-hydroxy-chrysanthemic acid and the corresponding lactone and the
chrysanthemic acid glucuronide, while
trans-hydroxy- cis-chrysanthemic acid was also a major metabolite
of cis-permethrin. The major metabolites of the alcohol moieties of
both isomers were 3-phenoxybenzoic acid-glycine conjugate (3-11% of
the dose), 3-phenoxybenzyl alcohol (8-10%), and 3-phenoxybenzoic
acid-glutamic acid conjugate (12-28%) (Gaughan et al., 1978).
Humans
Two volunteers who ingested about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the dose as
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid in
urine collected over 24 h (Cridland & Weatherley, 1977a,b).
In a study of approximately 350 people who were dusted against
body lice with 30-50 g of powder containing 2.5 or 5.0 g/kg permethrin
(cis:trans ratio, 25:75), the mean amount of permethrin absorbed
during the first 24 h after treatment was estimated to be 14 µg/kg bw
in 19 subjects exposed to powder containing 2.5 g/kg permethrin and 39
µg/kg bw in 15 subjects exposed to 5.0 g/kg. No residue was found in
samples of urine taken 30 and 60 days after treatment (Nassif et al.,
1980).
Four of five workers in Sweden who packed conifer seedlings for
6 h in a tunnel that had been sprayed 1 h earlier with a 2% aqueous
solution of permethrin, resulting in atmospheric concentrations of
0.011-0.085 mg/m3 in the breathing zone, did not excrete detectable
amounts of acid permethrin metabolites in the urine. One very short
person whose face was close to the plants and who had the highest
exposure to permethrin in his breathing zone excreted 0.26 µg/ml
permethrin acid metabolites in his urine the following morning; in the
afternoon, the concentration was below the detection limit of the
method (Kolmodin-Hedman et al., 1982).
Ten patients (five men and five women) with scabies received an
application of about 25 g (range, 21-32 g) of a 5% permethrin cream
over the skin of the whole body except the head and neck. Dermal
absorption of permethrin was calculated from the quantities of
conjugated and unconjugated cis- and
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
metabolites of permethrin in the urine. In samples of urine collected
by seven patients 1 and 2 days after application of the permethrin
cream, the mean totals of these metabolites were 410 and 440 µg,
respectively; the mean total in the urine of three patients who
collected urine in the same container for 2 days was 1400 µg. The
urinary concentration of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
varied during the first 48 h from 0.11 to 1.1 µg/ml and that of the
cis isomer from 0.02 to 0.21 µg/ml. This metabolite was still
detectable in the urine of three patients after 1 week and in the
urine of one patient, reported to be an alcoholic, after 2 weeks. The
absorption of permethrin over the first 48 h after application was
estimated from the amount of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid
excreted in the urine to be 6 mg (range, 3-11 mg), i.e. 0.5% of the
dose applied (van der Rhee et al., 1989).
Exposure to permethrin was assessed in a survey of 45
professional users of insecticide products, with more than a 100-fold
difference between the average and the highest concentrations. Dermal
contamination was found on 93% of the operators, the greatest
contamination resulting from use of leaky equipment. High airborne
concentrations were linked with use in confined areas. Monitoring of
metabolites in urine showed that systemic uptake occurred, with
concentrations of specific metabolites ranging from none detected to
10 nmol/mmol creatinine of
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid,
19 nmol/mmol creatinine of
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 46 nmol/mmol creatinine of 3-phenoxybenzoic acid (Llewellyn
et al., 1996).
The concentrations of permethrin and its metabolites,
cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propane carboxylic acid,
trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid, and 3-phenoxybenzoic acid, were measured in the urine and plasma
of 30 pest-control workers exposed to pesticides. The concentrations
of permethrin in plasma were below the limit of detection (5 µg/L),
and the urinary concentrations ranged from < 0.5 µg/L to 280 µg/L
(Leng et al., 1997).
Urine samples collected over 24 h from eight pest-control workers
exposed to permethrin were analysed for permethrin metabolites. The
concentrations ranged from 1 to 70 µg/g creatinine (Angerer & Ritter,
1997).
2. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of orally administered permethrin
in mice and rats (Table 11) demonstrate that two factors that affect
its toxicity are the concentration of the cis isomer and the
vehicle. Permethrin with a cis:trans ratio of 80-100:20-0 is
approximately 7-24 times more toxic than permethrin in which the
cis:trans ratio is 10-25:90-75 when delivered in maize oil, and
permethrin administered in maize oil is four to seven times more toxic
than undiluted permethrin. The alterations in acute toxicity are,
however, greater than can be explained on the basis of the cis
isomer content alone. Thus, a change from a cis:trans ratio of 20:80
to 80:20 in maize oil changed the LD50 value in Wistar rats from
6000 mg/kg bw to 225 mg/kg bw, whereas an LD50 value of about 1000
mg/kg bw would have been expected for the 20:80 material. Frequently
observed clinical signs were convulsions on the day of dosing,
tremors, and hypersensitivity. In animals that died, the
gastrointestinal tract often contained a brown fluid. In male and
female Wistar rats exposed for 4 h to atmospheres containing
permethrin (cis:trans ratio, 40:60), the LC50 value was greater
than a nominal concentration of 24 mg/L (Braun & Killeen, 1976).
In male and female New Zealand white rabbits, the dermal LD50
values for cis:trans 55:45 and 40:60 permethrin were > 2000 mg/kg
bw (Braun & Killeen, 1975b; Sauer, 1980b).
(b) Short-term studies of toxicity
Mice
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 39%:56%; purity, 94.7%) was administered in the
diet to groups of 20 male and 20 female Alderley Park mice at
concentrations of 0, 80 (increased to 10 000 ppm in weeks 3 and 4),
200, 400, 1000, 2000, or 4000 ppm in the diet, equivalent to 0, 28,
56, 140, 280, and 560 mg/kg bw per day for 28 days. No deaths
occurred, and no significant clinical signs were observed. Reduced
body-weight gain and poor food utilization were observed in animals
receiving 10 000 ppm. At termination, necropsies were performed on
five mice of each sex from the control group and those given 2000 and
10 000 ppm. Liver weights and the liver:bodyweight ratios were
increased in mice at 2000 and 10 000 ppm, and an increase in the
incidence of eosinophilia of the centrilobular hepatocytes was seen in
all mice receiving 10 000 ppm and two of five female mice receiving
2000 ppm. The NOAEL was 1000 ppm, equivalent to 140 mg/kg bw per day,
on the basis of changes in liver weight at 2000 ppm (Clapp et al.,
1977a).
Table 11. Acute toxicity of orally administered permethrin
Species Strain Sex Vehicle Purity cis:trans LD50 95% CI Reference
(%) ratio (mg/kg bw)
Mouse CF-1 M&F Maize oil 100 10:90 1700 1200-2300 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 95.5 25:75 960 680-1200 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 99.5 40:60 650 420-880 Marowitz (1974a)
Mouse CF-1 M&F Maize oil 100 100:0 230 200-260 Marowitz (1974a)
Rat SD M None 55:45 3600 2400-5200 Sauer (1980c)
F 2300 1800-2900
Rat SD (CD) M Maize oil 41.3: 58.7 1000 880-1200 Cummins & Gardner
F 860 680-1000 (1984a)
Rat SD (CD) M Maize oil 81.1: 18.9 370 280-460 Cummins & Gardner
F 320 240-410 (1984b)
Rat Wistar M&F Maize oil 40:60 1200 860-1500 Braun & Killeen (1975a)
Rat Wistar M&F None 40:60 8900 6000-12 000 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 40:60 1200 1100-1400 Braun & Killeen (1975a)
Rat Long-Evans M&F None 40:60 6000 4200-7800 Braun & Killeen (1975a)
Rat Long-Evans M&F Maize oil 95.5 25:75 1600 1300-2000 Marowitz (1974b)
Rat Wistar F Maize oil 20:80 6000 - Wallwork et al. (1975)
Rat Wistar F Maize oil 30:70 1700 1300-2200 Wallwork et al. (1975)
Rat Wistar F Maize oil 40:60 1300 1000-1600 Wallwork et al. (1975)
Rat Wistar F Maize oil 50:50 1000 730-1400 Wallwork et al. (1975)
Rat Wistar F Maize oil 60:40 440 400-500 Wallwork et al. (1975)
Rat Wistar F Maize oil 80:20 220 200-250 Wallwork et al. (1975)
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 38%:52%; purity, 90.5%) was administered in the
diet to groups of eight Wistar Alderley Park rats of each sex at
concentrations of 0, 200, 500, 1000, 2500, 5000, or 10 000 ppm,
equivalent to 0, 20, 50, 100, 250, 500, and 1000 mg/kg bw per day, for
28 days. All rats receiving 10 000 ppm died within the first 3 days of
the study, and five rats at 5000 ppm had died by day 18. Before death,
the rats showed whole-body tremors, hyperactivity, and piloerection.
The surviving rats receiving 5000 ppm had tremors, hypersensitivity,
piloerection, and urinary incontinence. Rats receiving 2500 ppm had
tremors and piloerection during the first week of treatment and were
hypersensitive throughout the study. Rats receiving 1000 ppm showed
slight tremors on the first day only. No clinical signs were seen
among rats receiving 500 or 200 ppm. Rats receiving 5000 ppm had
decreased body weights, body-weight gains, and food consumption when
compared with controls, while females receiving 2500 ppm had
significantly increased food consumption. Urinary protein excretion
was depresssed in males receiving 5000 ppm. Lymphocytosis was observed
in male rats at doses > 2500 ppm. At the end of the study, the
liver weights and the liver:body weight ratios of rats receiving doses
> 2500 ppm were increased. Histological examination of the livers
showed no remarkable changes. The NOAEL was 500 ppm, equivalent to 50
mg/kg bw per day, on the basis of tremors at 1000 ppm (Clapp et al.,
1977b).
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) wase administered in the diet to groups of six Long
Evans rats of each sex in the diet at concentrations of 0, 2500, 3500,
5000, or 7100 ppm, equal to 0, 250, 350, 5300, and 800 mg/kg bw per
day for males and 0, 270, 380, 550, and 820 mg/kg bw per day for
females, for four weeks. One male and one female receiving the highest
dose died during the first week of treatment. Tremors, the severity of
which was dose-dependent, were observed in most rats receiving doses
> 3500 ppm during the first week of treatment but were subsequently
seen only in animals receiving 7100 ppm and were generally less
severe. Staining of the abdominal fur was also seen in rats at doses
> 3500 ppm, with increasing incidences in weeks 1-4. Decreased body
weight was observed in both males and females treated with doses
> 3500 ppm during the first 2 weeks of treatment. This effect
persisted during weeks 3 and 4 only in females at 7100 ppm. No
histological examination was performed in this preliminary experiment.
The NOAEL was 2500 ppm, equal to 250 mg/kg bw per day, on the basis of
clinical signs and body-weight changes at 3500 ppm (Killeen & Rapp,
1974).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to groups of six male and six female Long
Evans rats in the diet at concentrations of 625, 1250, 2500, 5000, or
7500 ppm for 30 days, equal to 0, 59, 120, 250, and 630 mg/kg bw per
day for males and 0, 69, 130, 280, and 660 mg/kg bw per day for
females: no data on consumption of the compound were available for the
highest concentration. All females and three males receiving 7500 ppm
died within 24 h of the start of treatment, and the remaining three
males in this group had died by the end of the first week. Five of six
males and five of six females at 5000 ppm died during the first week,
but the remaining animals survived the duration of the study. In rats
receiving 2500 ppm, slight-to-moderate tremors and staining of the
anogenital fur were noted throughout most of the study. The surviving
male and female at 5000 ppm showed moderate-to-severe tremors and
staining of the fur in the anogenital region throughout the study. The
body weights of males at 2500 ppm were reduced. No histological
examination was performed in this preliminary experiment. There were
no signs of toxicity at 625 and 1250 ppm. The NOAEL was 1250 ppm,
equal to 120 mg/kg bw per day, on the basis of clinical signs and
body-weight changes at 2500 ppm (Killeen & Rapp, 1975a).
Technical-grade permethrin (cis:trans ratio unstated; purity,
96%) was administered in the diet to groups of six male and six female
Charles River rats (strain not stated) at concentrations of 0 (14
animals of each sex), 30, 100, 300, 1000, or 3000 ppm, equivalent to
0, 3, 10, 30, 100, and 300 mg/kg bw per day, for five weeks. Most of
the observations were restricted to the group receiving 3000 ppm. In
this group, persistent tremors were observed in all males and four
females, body-weight gain was reduced in females by about 7%, the
liver weights adjusted for terminal body weight were increased in
males (15%) and females (13%), and the relative heart weight was
increased in males (8%). Increased prothrombin time and plasma urea
were seen in males and decreased plasma protein in females. Males at
1000 ppm showed an increase in relative liver weight (15%), and
(probably chance) increases in prothrombin time were seen in females
at 30 and 300 ppm. Histological examination revealed renal tubular
mineralization and hydronephrosis as common lesions which were evenly
distributed among the treated groups. No dose-related increase in the
incidence of lesions was found. The NOAEL was 300 ppm, equivalent to
30 mg/kg bw per day, on the basis of increased liver weight at 1000
ppm (Butterworth & Hend, 1976).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to groups of 18 Wistar rats of
each sex at concentrations of 0, 60, 200, 600, or 2000 ppm, equivalent
to 0, 6, 20, 60, and 200 mg/kg bw per day, for 90 days. At that time,
10 rats of each sex per group were killed, and the remaining rats were
maintained without further treatment for an additional 29 days. No
treatment-related deaths occurred, and no treatment-related or
toxicologically significant clinical signs, changes in body weights,
food consumption, or estrus cycle, urinary findings, or blood
anomalies were noted. The absolute and relative weights of the spleen
and lungs were increased in male rats at 2000 ppm, and the relative
and absolute weights of the adrenal glands of female rats at this dose
showed statistically significant decreases. No consistent dose-related
pattern of toxicity was seen. The fat content of the renal cortex was
slightly increased in male rats receiving 600 or 2000 ppm, but this
was not accompanied by morphological alterations. The NOAEL was 600
ppm, equivalent to 60 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm (Williams et al., 1976a).
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered in the diet to 18 Wistar rats of each sex at
concentrations of 0, 200, 600, 2000, or 4000 ppm, equivalent to 0, 20,
60, 200, and 400 mg/kg bw per day, for 90 days. At that time, 10 rats
of each sex per group were killed, and the remainder were maintained
with no further treatment for an additional 36 days. No
treatment-related deaths occurred. The only significant clinical sign
was hypersensitivity in male and female rats receiving 4000 ppm. By
week 3, hypersensitivity was no longer observed in male rats but
continued in the female rats throughout treatment, although the
symptoms disappeared after 3 days without dosing. Males receiving 4000
ppm had decreased body-weight gain, which improved during the recovery
period. A slight-to-moderate decrease in leukocyte count was seen
during the early stages of the study in rats at this dose, but the
values had returned to within the normal range by 90 days. The weight
of the liver showed a slight but significant increase at 90 days but
had returned to normal by the end of the recovery period. The absolute
and relative weights of the thyroids of rats receiving the high dose
showed a slight decrease at 90 days, but no histopathological changes
were found. The NOAEL was 2000 ppm, equivalent to 200 mg/kg bw per
day, on the basis of of hypersensitivity, reduced body weight,
transient leukopenia, and increased liver weights at 4000 ppm
(Williams et al., 1976b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was administered in the
diet to groups of 30 Long Evans rats of each sex at concentrations of
0, 50, 75, 100, or 500 ppm, equivalent to 5, 7.5, 10, and 50 mg/kg bw
per day, for 90 days. There were no significant differences in body
weight, food consumption, or haematological, clinical chemical, or
urinary parameters. At the end of the study, the relative weight of
the liver was increased in male rats at 500 ppm in comparison with
controls, while that of female rats at this dose was significantly
decreased. There was no correlation with clinical or histopathological
findings. No treatment-related histopathological findings were made.
The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis
of increased relative liver weight at 500 ppm (Becci & Parent, 1980).
In a study conducted according to GLP, three batches of
technical-grade permethrin (cis:trans ratio, 36.1%:61.1% to
38.5%:56.2%; purity, 94.1-97.2%) were given in the diet to groups of
eight Wistar-derived rats of each sex at concentrations of 0, 20, 100,
or 1000 ppm, equivalent to 2, 10, and 100 mg/kg bw per day, for 26
weeks, when the rats were killed. The body weight and body-weight gain
of female rats receiving 20 and 100 ppm were similar to those of the
control animals, but females at 1000 ppm gained less weight throughout
treatment, as did males at doses > 100 ppm. There was no overall
effect on food consumption. Animals at 1000 ppm showed statistically
significant increases in liver weight, hepatic aminopyrine
N-demethylase activity, cytochrome P450 content, and smooth
endoplasmic reticulum content. Those at 100 ppm had only slight
hepatic changes, none of the untransformed values being significantly
greater than control levels, although the logarithm of the values for
aminopyrine N-demethylase activity attained significance. The NOAEL
was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
increased liver weight at 1000 ppm, as the changes in aminopyrine
N-demethylase activity were considered not toxicologically relevant
(Hart et al., 1977a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to groups
of 20 Sprague-Dawley rats of each sex by inhalation as an aerosol for
6 h per day, 5 days per week for 13 weeks at concentrations of 0, 125,
250, or 500 mg/m3. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm . At the end of
the 13-week exposure, 10 rats of each sex per group were maintained
without further treatment for an additional 90 days. There were no
treatment-related deaths. Severe tremors and convulsions were
observed in rats at 500 mg/m3, but these signs were no longer seen
by the second week of exposure. Oxygen consumption was monitored
throughout the exposure in order to assess overall metabolic rate. No
differences from controls were found. Hexobarbital-induced sleep times
(220 mg/kg bw intraperitoneally) were reduced in males exposed to 500
mg/m3, indicating the induction of liver enzymes, but the effect was
no longer statistically significant 30 days after exposure or in rats
of the lower concentrations. Body weights and organ-to-body weight
ratios were unaffected. No gross or microscopic treatment-related
pathological changes were observed. The NOAEC was 250 mg/m3 on the
basis of tremors and convulsions at 500 mg/m3 (United States Army,
1978).
Guinea-pigs
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered to groups
of 10 male Hartley guinea-pigs by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol in order to
examine sensitization reactions. There were no treatment-related
deaths or clinical signs during exposure, and the body weight and
organ:body weight ratios were unaffected. No gross or microscopic
treatment-related pathological changes were observed. No sensitization
reaction was seen. The NOAEC was 500 mg/m3, the highest dose tested
(United States Army, 1978).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity. 93.6% and 95.0%) was applied intradermally to groups of
male guinea-pigs as 0.1 ml of a 0.1% (w/v) solution; each batch of
permethrin or 2,4-dinitrochlorobenzene (positive control) contained
one volume of propylene glycol and 29 volumes of saline. Ten
guinea-pigs were challenged with the 0.1% solution, and an additional
five in each group received no prior sensitization but were given a
challenge dose of each batch of test material or the positive control.
The reaction induced by the challenge dose of permethrin was no
greater than that observed in the sensitized animals. The challenge
dose of the positive control produced sensitization reactions in all
of the guinea-pigs in that group (Metker et al., 1977).
Technical-grade permethrin (cis:trans ratio, 25:75; purity not
stated) was tested for skin sensitization potential according to the
method of Magnusson and Kligman. Ten male guinea-pigs received
permethrin, and groups of five male guinea-pigs received the vehicle
or 2,4-dinitrochlorobenzene. All compounds were given as a 1% w/v
solution in corn oil and as a 1% w/v solution in Freund's complete
adjuvant. No reaction to permethrin was seen 5, 24, and 48 h after
dosing, whereas all guinea-pigs given the positive control showed
marked sensitization (Chesher & Malone, 1974).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7 %) was administered by
inhalation as an aerosol to groups of 10 male Hartley guinea-pigs at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter < 1 µm. The guinea-pigs
were held for 14 days after the last exposure and challenged with an
intradermal injection of permethrin in 3% propylene glycol. No
sensitization reaction was seen in any of the guinea-pigs (United
States Army, 1978).
Rabbits
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95.0%) was applied daily to the shaved skin of
groups of eight New Zealand white rabbits for 21 days at doses of 0,
100, 320, or 1000 mg/kg bw per day under an occlusive dressing. Four
rabbits in each group received abrasion at the application site on the
first day. All rabbits were killed on day 10 after the last exposure.
No significant changes were noted in body weights during or after
exposure, and there were no significant changes in organ:body weight
ratios. Moderate irritation of the skin was observed, but the reaction
was not significantly different from controls by day 18. Mild
irritation was still present 10 days after exposure, although it
improved daily. No differences were found in clinical chemical
parameters. There were no treatment-related lesions in the skin or
other tissues or organs. The NOAEL was 1000 mg/kg bw per day, the
highest dose tested (Metker et al., 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio 55:45; purity not stated) was melted and applied
without dilution at a volume of 0.5 ml to the skin of six New Zealand
white rabbits on each of two intact and two abraded sites, for a total
of 2 ml per rabbit. Each site was scored for irritation by the method
of Draize 30-60 min after removal of the occlusive wrap and residual
permethrin and again 24 h and 72 h after dosing. Very slight erythema
was noted on all sites at 24 h, but all irritation had resolved by
72 h. The score for primary dermal irritation was 0.5/8.0, indicating
virtually no irritation (Sauer, 1980c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as a liquid to the abraded skin of 10 New Zealand
white rabbits at a dose of 2000 mg/kg bw and the site was covered with
an occlusive wrap for 24 h. When the wraps were removed, very slight
erythema was noted on three rabbits, and barely perceptible oedema was
seen on one other rabbit (Braun & Killeen, 1975b).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was applied as an aqueous slurry to the skin of six New Zealand
white rabbits as a volume of 0.50 ml of 1 g/ml on an intact and an
abraded site, for a total of 1 ml per rabbit. The sites were covered
with an occlusive wrap for 24 h and scored by the Draize method 24 and
72 h after dosing. Very slight erythema was seen on three abraded and
three intact sites at 24 h, but all irritation had resolved by 72 h.
The score for primary dermal irritation was 0.25, indicating virtually
no irritation (Braun & Killeen, 1975c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 55:45; purity not stated) was melted, and 0.1 ml
of undiluted material was instilled into the right eye of nine New
Zealand white rabbits. After approximately 20 s, the eyes of three
rabbits were flushed with water for 1 min, and irritation was scored
by the Draize method 24, 48, and 72 h and 4 and 7 days after dosing.
Slight conjunctival redness and chemosis were found in the majority of
the treated eyes, but all irritation had resolved within 4 days. The
maximum score was 7.3/110 for unwashed eyes and 4.0/110 for washed
eyes at 24 h, indicating no irritation (Sauer, 1980d).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was instilled as a liquid into the right eye of nine New
Zealand white rabbits at a volume of 0.10 ml. The eyes of three
rabbits were then flushed for 30 s with 100 ml of warm tap-water and
were scored 1, 24, 48, and 72 h and 4 and 7 days after dosing. Slight
conjunctival redness, chemosis, and discharge were seen in both washed
and unwashed eyes at 1 h. Stippling of the cornea was observed in two
unwashed eyes. All irritation had resolved within 48 h (Braun &
Killeen, 1975d).
Dogs
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
94.5%) was administered to one male and one female beagle dog in
gelatine capsules for 14 days at doses of 0, 125, 250, or 500 mg/kg bw
per day. On the first day of treatment, emesis was observed in four
treated dogs, and thereafter the compound was administered twice daily
in divided doses. Tremors and ataxia were each seen once in the male
treated with 500 mg/kg bw per day during the first week of treatment.
No effect on body weight or food consumption was seen throughout the
study. The NOAEL was 250 mg/kg bw per day on the basis of clinical
signs at 500 mg/kg bw per day (Killeen & Rapp, 1975b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, 94.5%) was administered to groups
of four beagle dogs of each sex in gelatine capsules at doses of 0, 5,
50, or 500 mg/kg bw per day for at least 96 days. Tremors were
observed on isolated occasions in two males and three females at 500
mg/kg bw per day during the exposure. One dog that had tremors also
showed transient narcosis with nystagmus on a single occasion. No
effects attributable to treatment were seen on body weight, food
consumption, clinical chemical, haematological, or urinary parameters,
or histological appearance. The mean absolute and relative (to body
weight) weights of the liver were greater than those of controls in
animals at doses > 50 mg/kg bw per day, but there was no
histological correlate. The increases in absolute liver weights were
11% at 50 and 500 mg/kg bw per day in males and 12% at 500 mg/kg bw
per day in females and were statistically significant. The biological
significance of the effect in males at 50 mg/kg bw per day is obscure
in view of the lack of any additional effect at a dose 10-fold higher.
The NOAEL was 50 mg/kg bw per day on the basis of changes in liver
weight and neurotoxic signs at 500 mg/kg bw per day (Killeen & Rapp,
1976).
Technical-grade permethrin (cis:trans ratio, 54:46; purity,
93.4%) was administered in gelatine capsules to groups of six male and
six female beagle dogs at doses of 0, 5, 50, or 500 mg/kg bw per day
for 90 days. Owing to signs of severe toxicity which increased in
severity and duration, the high dose was lowered to 364 mg/kg bw per
day on day 9 of dosing. The most serious of these signs was seizures
lasting for up to 1 h and characterized by rapid eye twitching with
fasciculations, increased sensitivity to sound, and severe tremors.
Ataxia and aggressive behaviour were also seen in these animals. At
364 mg/kg bw per day, similar signs of toxicity were seen but of
lesser severity and shorter duration. The major signs of toxicity seen
in males at doses > 50 mg/kg bw per day and females at 364 mg/kg bw
per day included impaired gait, ataxia, tremor, muscle twitching,
involuntary limb movements, uncontrolled barking, panting, and
salivation. Tremors and muscle twitching were also seen in females at
50 mg/kg bw per day. No signs of toxicity were observed at the low
dose. Body-weight gain was significantly reduced in males at the high
dose, whereas the body weight and body-weight gain of females were
unaffected by treatment. Serum alkaline phosphatase activity was
increased at 3, 6 10, and 13 weeks among males at the high dose, and
the absolute and relative weights of the liver were increased in males
and females at this dose. Permethrin had no effect on food consumption
or on haematological, urinary, ophthalmological, or histological
parameters. The NOAEL was 5 mg/kg bw per day on the basis of clinical
signs at 50 mg/kg bw per day (Becci et al., 1980).
Technical-grade permethrin (cis:trans ratio, 25:75; purity,
94.5%) was administered in gelatin capsules to groups of four beagle
dogs of each sex at doses of 0, 10, 50, or 250 mg/kg bw per day for at
least 180 days. None of the dogs died during the study. Emesis was
seen in a few treated and control animals, but there were no
treatment-related signs of toxicity, no effect on body weight,
ophthalmological or electrocardiographic parameters, absolute organ
weights, or gross or histopathological appearance. Statistically
significant but sporadic changes in food intake (decreased in dogs at
50 mg/kg bw per day in week 11 and in those at 250 mg/kg bw per day in
weeks 12 and 22), some haematological parameters (decreased packed
cell volume on day 180 and decreased mean corpuscular volume on day 56
at 10 mg/kg bw per day; increased lymphocyte and neutrophil counts at
50 and 250 mg/kg bw per day on day 14), some clinical chemical
parameters (glucose, urea, and sodium concentrations), and increased
relative weights of the liver, heart, and kidneys (by < 17%) were
seen. None of these changes appeared to be related to dose or time and
were not severe enough to be of toxicological importance. Similar
significant changes in blood chemistry were occasionally observed in
the dogs before dosing. The NOAEL was 250 mg/kg bw per day, the
highest dose tested (Reynolds et al., 1978).
In a study conducted according to GLP, permethrin (cis:trans
ratio, 32.3%:60.2%; purity, 92.5%) was administered in corn oil in
gelatin capsules to four groups of six beagle dogs of each sex at
doses of 0, 5, 100, or 1000 mg/kg bw per day for 52 weeks. Body
weights and food consumption were measured, and the dogs were observed
for clinical and behavioural abnormalities. A variety of
haematological and biochemical parameters were measured at intervals
throughout the study. Clinical signs, including convulsions, muscle
tremor, and incoordination, were seen frequently in dogs at 1000 mg/kg
bw per day. The body weights of males at 1000 mg/kg bw per day and
females at doses > 100 mg/kg bw per day were reduced. The weight of
the liver of dogs treated with doses > 100 mg/kg bw was increased,
accompanied by hepatic cellular swelling, consistent with an observed
increase in plasma alkaline phosphatase activity. These findings were
considered to represent an adaptive response and not a toxicological
effect. The dose of 1000 mg/kg bw per day was overtly toxic, resulting
in neurological signs associated in some animals with poor clinical
condition. The NOAEL was 5 mg/kg bw per day on the basis of the
reduction in body weight at 100 mg/kg bw per day (Kalinowski et al.,
1982).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 60:40; purity, 92.7%) was administered to four
groups of two beagle dogs of each sex by inhalation as an aerosol at
concentrations of 0, 125, 250, or 500 mg/m3 for 6 h per day, 5 days
per week for 13 weeks. The mass median diameter of the aerosol was 5.1
µm, and 85% of the particles had a diameter of < 1 µm. The dogs
were tested for pulmonary function before and after exposure, and
blood samples were taken weekly for measurements of blood chemistry
and haematology. There were no treatment-related deaths and no toxic
signs were observed. Body weights and organ:body weight ratios were
unaffected, and pulmonary function and blood chemical and
haematological parameters were unchanged. No gross or microscopic,
treatment-related pathological changes were observed. The NOAEC was
500 mg/m3, the highest dose tested (United States Army, 1978).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet to groups of
75 CD-1 mice of each sex for 24 months. Initially, the concentrations
in the diet were 0, 20, 100, and 500 ppm, equivalent to 0, 3, 15, and
75 mg/kg bw per day. The concentration of 500 ppm was changed to 5000
ppm, equivalent to 250 mg/kg bw per day at week 19, but was returned
to 500 ppm at week 21. In addition, at week 21 the concentration of
100 ppm was changed to 4000 ppm, equivalent to 200 mg/kg bw per day,
and the concentrations were 0, 20, 500, and 4000 ppm for the remainder
of the study. During week 65, a problem of animal identification
arose, which detracts from the value of the study; however, the
actions taken to eliminate these mice from the study were carefully
recorded and judged by the US Environmental Protection Agency to be
acceptable, and the study was allowed to continue to its scheduled
conclusion. The rates of death up to 24 months were 43/62 control
males, 41/60 males at the low dose, 46/60 males at the intermediate
dose, 61/67 males at the high dose, 20/50 female controls, 32/60
females at the low dose, 34/62 females at the intermediate dose, and
48/65 at the high dose. The body weight of males at 4000 ppm was
slightly decreased from week 21 to termination, but that of females
and the food consumption of both males and females were unaffected. No
clinical observations were made that correlated with treatment. Mean
haemoglobin, erythrocyte count, and blood glucose concentration were
decreased in animals of each sex at 4000ppm. The mean absolute and
relative (to body weight) weights of the liver were increased for
males receiving 500 ppm and for animals of each sex at 4000 ppm. The
latter showed a greater frequency of non-neoplastic changes including
mononuclear leukocyte infiltrates in some tissues and thrombosis of
the cardiac atrium. There was no significant change in the incidence
of any tumour, and, in particular, the incidence of bronchioalveolar
adenomas was not increased in animals of either sex. The incidences of
hepatomas and hepatocellular carcinomas were slightly increased among
male, but not female, mice (Table 12) (Hogan & Rinehart, 1977; Rapp,
1978).
In view of the problems with the first experiment, a second of
the same design but carried out according to GLP was conducted. The
concentrations in the diet were originally 0, 100, 500, and 2000 ppm,
equivalent to 15, 75, and 300 mg/kg bw per day, and were changed after
2 months to 0, 20, 500, and 2000 ppm, equivalent to 3, 75, and 130
mg/kg bw per day, for males and 20, 2500, and 5000 ppm, equivalent to
3, 380, and 750 mg/kg bw per day, for females. The incidences of
deaths up to 24 months were 55/75 male controls, 48/75 males at the
low dose, 49/75 males at the intermediate dose, 63/75 males at the
Table 12. Incidences of tumours of the lung and liver in mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. livers Neoplasm No. with
examined examined liver
Alveolar Alveolar Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 60 6 0 72 3 4 7
Low 56 7 0 68 1 0 1
Intermediate 53 4 0 68 9 5 14
High 67 5 0 71 4 7 11
Female Control 47 11 0 72 2 0 2
Low 59 8 1 69 1 0 1
Intermediate 60 7 1 67 4 0 4
High 59 9 1 69 1 0 1
From Hogan & Rinehart (1977); Rapp (1978)
high dose, 53/75 female controls, 42/75 females at the low dose, 52/75
females at the intermediate dose, and 53/75 females at the high dose.
The mortality rate among males at the high dose was therefore slightly
greater than that among the control animals. These males also had a
higher incidence than control males of yellow staining in the
anogenital area throughout the study. Treatment had no effect on body
weight, body-weight gain, or food consumption. The differential
leukocyte counts of females at 2500 and 5000 ppm and males at 2000 ppm
were slightly lower than control values, while the segmented
neutrophil counts of males at 2000 ppm were slightly higher than in
controls. A statistically significant decrease in the absolute and
organ:body weight ratio of the testis was seen at 2000 ppm, while
females at doses > 2500 ppm showed statistically significantly
increased absolute liver weights; the liver:body weight ratio was
statistically significant only in females at 5000 ppm.
A dose-dependent increase in the incidence of alveolar-cell
adenomas and carcinomas was found among female mice, and multiple lung
adenomas were often found in the same animal (Table 13). Hepatomas
also occurred more often in female mice at the intermediate and high
doses than controls and mice at the low dose. Hepatoma is considered
to be a spontaneous lesion which occurs in ageing and aged mice
without affecting the mortality rate. The incidence of hepatocellular
carcinoma was notably higher in males at the intermediate dose than in
other treated or control groups (Table 13). The type, incidence,
and/or degree of severity of other neoplasms and all non-neoplastic
histological changes were considered to represent spontaneous lesions
unrelated to treatment. Since the incidences in males and females at
the low dose were close to those in the control group, the increased
incidence of bronchioalveolar adenoma in female mice at the two higher
doses may have been related to treatmen. This conclusion is not,
however, supported by the results of the first study, which was
conducted in the same laboratory with the same strain of mouse, and
the same batch of permethrin and which partially overlapped with the
second one in time. The pulmonary adenomas were thus considered to
have no toxicological significance. The NOAEL for systemic toxicity
was 500 ppm, equivalent to 75 mg/kg bw per day, on the basis of
changes in organ weights at 2000 ppm (Tierney & Rinehart, 1979).
In a study conducted according to GLP, eight batches of
technical-grade permethrin (cis:trans ratios, 44:55 to 36.2:61;
purity, 94.1-98.9%) were administered in the diet of groups of
70 Alderley Park strain mice of each sex at concentrations of 0, 250,
1000, or 2500 ppm, equivalent to 0, 38, 150, and 380 mg/kg bw per day,
for up to 98 weeks. The animals were housed five per sex to a cage.
Ten animals of each sex per group were designated for interim kills at
26 and 52 weeks. Permethrin did not induce clinical signs, and the
mortality rate was unaffected. A treatment-related decrease in
body-weight gain and increased food consumption were noted in males
and females at 2500 ppm. Proliferation of the smooth endoplasmic
reticulum and microbodies and increased eosinophilia in centrilobular
hepatocytes were also observed in most animals at this dose and to a
lesser extent in those at 1000 ppm. These changes had been observed in
Table 13. Incidences of tumours of the lung and liver in a second study of mice fed diets containing permethrin
Sex Dose No. lungs Neoplasm No. with No. livers Neoplasm No. with
examined lung examined liver
Alveolar Alveolar tumours Hepatoma Hepatocellular tumours
adenoma carcinoma carcinoma
Male Control 75 16 7 23 75 8 16 22
Low 75 17 5 20 75 19 12 30
Intermediate 74 20 13 28 75 17 19 34
High 75 17 4 21 75 19 8 25
Female Control 75 10 6 15 74 3 4 6
Low 76 18 7 24 76 4 3 7
Intermediate 75 26 11 35 76 23 3 25
High 75 37 15 44 75 29 2 30
a 26-week staudy (Hart et al., 1977a) and showed no progression in
this 98-week experiment. The kidneys of treated males at all doses
showed a dose-related decrease in vacuolation of the proximal tubular
epithelium, and the effect was statistically significant in males at
250 ppm at 52 weeks (13 ± 6.3 versus 5.4 ± 4.2 vacuoles per
microscope field) but not at 26 weeks (11 ± 6.8 versus 8.4 ± 5.3)
or at 98 weeks (8.4 ± 7.1 versus 7.2 ± 7.0). The changes in the
liver and the kidney were considered to be adaptive characteristics of
no toxicological importance.
No significant increase in the incidence of tumours of unusual
types or in the number of tumour-bearing animals was seen in
comparison with with controls. In male mice, a slight increase in the
incidence of pulmonary adenomas was recognized in the second year of
the study. Up to week 52, one unconfirmed tumour was found among 34
male controls and two in 23 females at the low dose; from week 53
until the end of the study, the incidences in males were 10/36 in
controls, 6/41 at the low dose, 13/40 at the intermediate dose, and 16
(+1 unconfirmed) out of 33 at the high dose. Comparison of controls
versus the high-dose group gives p = 0.09 or, if the unconfirmed
tumour is assumed to have been an adenoma, 0.05 (Fisher's exact test).
This finding was not considered to constitute evidence of a
carcinogenic effect. The NOAEL was 250 ppm, equivalent to 38 mg/kg bw
per day, on the basis of changes in the liver and kidney at 1000 ppm
(Hart et al., 1977b; Ishmael & Litchfield, 1988).
Rats
Technical-grade permethrin of 10 batches (cis:trans ratio,
36.2:61 to 43.9:55; purity, 93.1-98.9%) was administered in the diet
of groups of 60 Alderley Park rats of each sex at concentrations of 0,
500, 1000, or 2500 ppm, equivalent to 0, 25, 50, or 125 mg/kg bw per
day, for up to 104 weeks. Twelve animals of each sex per group were
designated for interim sacrifice at 52 weeks. Treatment did not affect
survival, body weight, body-weight gain, food consumption,
haematological or urinary parameters, or gross or microscopic
appearance. Tremor, piloerection, and hypersensitivity were noted at
2500 ppm during the first 2 weeks of the study. Increased
aminopyrine- N-demethylase activity was observed in all treated males
and in females at doses > 1000 ppm at 52 weeks and in all animals
at the high dose at 104 weeks. Increased liver weights were found in
all treated groups, although the effect did not increase between weeks
52 and 104. The kidneys were heavier than those of controls in males
at 2500 ppm at week 52 and in all treated males at week 104. The
weight of the kidneys of females at doses > 1000 ppm was reduced at
52 weeks and was unaffected at week 104. The increase in kidney weight
in male rats at 104 weeks appeared to be associated with marked
nephropathy in some of these animals. Nephropathy occurs spontaneously
with age in that colony of rats, and the variability of the change in
males at the terminal kill and the absence of this trend in the
females indicate that these effects were not related to treatment.
Treatment had no effect on the incidence of tumour-bearing rats
or of tumours of any particular type. The incidence of centrilobular
hepatocyte hypertrophy was increased in rats at doses > 1000 ppm,
particularly in males, and proliferation of the smooth endoplasmic
reticulum was observed in all treated groups of females and males at
doses > 1000 ppm at week 52; at week 104, the proliferation was
confined to rats at 2500 ppm. These adaptive changes in the liver,
including hepatocyte hypertrophy and the associated increases in liver
weight, microsomal enzyme activity, and smooth endoplasmic reticulum,
were considered to be normal adaptive responses of the liver to
exposure to a xenobiotic and to be toxicologically insignificant. It
is uncertain, however, whether the hepatocyte vacuolation seen in
males and females at 2500 ppm is also part of the physiological
adaptation of the liver. Examination of sciatic nerves revealed no
ultrastructural changes associated with exposure. The NOAEL was 500
ppm, equivalent to 25 mg/kg bw per day, on the basis of the increased
liver hypertrophy at 1000 ppm (Richards et al., 1977; Ishmael &
Litchfield, 1988).
Technical-grade permethrin of two batches (cis:trans ratio,
40:60; purity, 94.5-96.7%) was administered in the diet of groups of
60 Long-Evans rats of each sex at concentrations of 0, 20, 100, or 500
ppm, equivalent to 0, 1, 5, and 25 mg/kg bw per day, for 24 months.
Treatment had no effect on survival. Two females at 500 ppm showed
tremor on day 2 of the study, but tremors were not seen in any other
animal during the remainder of the study. The body weights and food
consumption of treated males were comparable to those of controls. The
mean body weights of females at 500 ppm were slightly lower than
control weights during the first year but were comparable thereafter;
the food consumption of control and treated females was comparable.
The glucose concentration was increased in animals at 500 ppm, in
females at 18 and 24 months and in males at 24 months. The absolute
and relative (to body weight) weights of the ovary of females at 500
ppm were greater than those of controls at the end of the study.
Histological examination of tissues from treated animals revealed no
increase in the incidence of neoplastic or non-neoplastic lesions. The
NOAEL was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs, changes in body and ovary weights, and clinical
chemical findings at 500 ppm (Braun & Rinehart, 1977; Billups,
1978a,b; Busey, 1978).
(d) Genotoxicity
Technical-grade permethrin was tested in a battery of test
systems in vitro and in vivo (Table 14). It did not increase the
frequency of unscheduled DNA synthesis in human fibroblasts in
vitro or induce DNA repair in Escherichia coli or Bacillus
subtilis. It did not induce gene mutations in bacteria, cultured
mammalian cells, or Drosophila melanogaster, nor recombinational
events (mitotic recombination or gene conversion) in yeast. It did not
induce aneuploidy in D. melanogaster. The evidence for induction of
clastogenic effects in cultured mammalian cells (mainly human
lymphocytes) was equivocal, in that a study of micronucleus induction
Table 14. Results of studies of the genotoxicity of permethrin
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
In vitro
Differential E. coli W3110/ 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity p3478 (1979)
Differential B. subtilis H17/M45 5000 µg/disc 38:52 90.4 Negative ± S9 Simmon et al.
toxicity (1979)
Reverse mutation S. typhimurium 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
TA100, TA98, (1976)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
TA100, TA98,
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
TA100, TA98, (1979)
TA1537, TA1535
Reverse mutation S. typhimurium 980 µg/plate NR NR Negative ± S9 Bartsch et al.
TA100, TA98 (1980)
Reverse mutation S. typhimurium 5000 µg/plate NR NR Negativeb ± S9 Moriya et al.
TA100, TA98, (1983)
TA1538, TA1537,
TA1535
Reverse mutation S. typhimurium 3000 µg/plate NR 95 Negative ± S9 Pluijmen et al.
TA100, TA98 (1984)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Reverse mutation S. typhimurium 5460 µg/plate NR NR Negative ± S9 Pednekar et al.
TA100, TA98 (1987)
Reverse mutation S. typhimurium 6000 µg/plate NR cis, 99 Negative ± S9 Herrera &
TA100, TA104, trans, 100 Laborda (1988)
TA98, TA97,
TA1538, TA1537,
TA1535
Reverse mutation E. coli WP2 1000 µg/plate NR 95.7 Negative ± S9 Simmon (1976)
Reverse mutation E. coli WP2 7500 µg/plate 38:52 90.4 Negative ± S9 Simmon et al.
(1979)
Reverse mutation E. coli WP2 hcr 5000 µg/plate NR NR Negative ± S9 Moriya et al.
(1983)
Gene conversion S, cerevisiae D4, 5 µl/plate 44:56 93.6 Negative ± S9 Brusick & Weir
try locus (1976)
Mitotic S. cerevisiae D3 50 000 µg/ml 38:52 90.4 Negative ± S9 Simmon et al.
recombination (1979)
Chromosomal loss D. melanogaster 5 µg/ml feed NR NR Negativea Woodruff et al.
(1983)
Sex-linked recessive D. melanogaster 1.2 µg/ml feed 45:55 91.1 Negativea Mehr et al.
mutation (1988);Gupta et
al. (1990)
Unscheduled DNA Fischer 344 rat 5000 µg/ml 38:52 90.4 Negativea Simmon et al.
synthesis primary hepatocytes (1979)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
Hprt locus
Gene mutation Chinese hamster 40 µg/ml NR 95 Negative ± S9 Pluijmen et al.
lung V79 cells, (1984)
OuaR
Gene mutation Mouse lymphoma 94 µg/ml NR NR Negative ± S9 Clive (1977)
L5178Y cells, Tk
locus
Chromosomal Chinese hamster 100 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration ovary cells Equivocal + S9 (1994)
Sister chromatid Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
exchange Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 50 µg/ml NR 99.5 Positive - S9 Barrueco et al.
formation Equivocal + S9 (1992); Herrera
et al. (1992)
Micronucleus Human lymphocytes 100 µg/ml NR 95 Equivocala Surrallès et al.
formation (1995a)
Micronuclei (with Human lymphocytes 100 µg/ml NR 97 Equivocala Surrallès et al.
inhibition of excision (1995b)
repair)
Micronuclei (with Human lymphocytes 10 µg/ml NR 97 Positivea Surrallès et al.
inhibition of excision (1995b)
repair)
Table 14. (continued)
End-point Test object Concentration cis:trans Purity Result Reference
ratio (%)
Chromosomal Human lymphocytes 75 µg/ml NR 99.5 Positive - S9 Barrueco et al.
aberration Equivocal + S9 (1992, 1994)
Inhibition of Chinese hamster 8 µg/ml NR NR Negativea Flodström et al.
gap-junctional lung V79 cells (1988)
intercellular
communication
In vivo
Dominant lethal Male CD-1 mice 452 mg/kg bw NR NR Negative Chesher et al.
mutation × 5 orally (1975)
NR, not reported; S9, 9000 × g supernatant of rat liver
a Not tested in the presence of S9
and chromosomal aberrations in one laboratory showed significant
results, while studies of micronucleus induction in another laboratory
showed negative results, except when the assay was modifed by
inhibition of DNA excision repair with cytosine arabinoside. No tests
of clastogenicity in vivo have been conducted. Permethrin did not
cause dominant lethal mutations in male mice. The Meeting concluded
that permethrin is not mutagenic but that it is clastogenic, at least
in vitro.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Technical-grade permethrin of two batches (cis:trans ratio and
purity not stated) was administered in the diet of groups of 12 male
and 24 female Long-Evans rats at concentrations of 0, 20, or 100 ppm,
equivalent to 0, 1.3, and 6.7 mg/kg bw per day, for three generations,
from F0 until weaning of the F3c generation. Treatment did not
affect the body weights of males or females at any time during the
study, and there was no effect on reproduction, on the number of
births, or on the survival or growth of the offspring. Ophthalmoscopic
examination of all 159 F3c offspring revealed two with
abnormalities: a unilateral cataract in one female in a control litter
and a unilateral coloboma of the choroid at the optic disc of one male
in a high-dose litter. The NOAEL for reproductive and systemic effects
was 100 ppm, equivalent to 6.7 mg/kg bw per day, the highest dose
tested (Schroeder & Rinehart, 1977).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 26:74; purity, 94.5%) was administered in the diet
to groups of 20 Wistar rats of each sex at target doses of 0, 5, 30,
and 180 mg/kg bw per day for three generations, from F0 until
weaning of the F3b generation. The F2 parental rats were then
mated to produce an F3c litter and their pups were removed just
before weaning for teratological examination. There were no
treatment-related effects on body weights of males and females of any
generation and no effect on reproduction or on the number of births or
the survival and growth of the offspring. Offspring in all groups and
in all generations had a few ocular abnormalities which were found
histologically to be abnormalities of the retina and optic nerve,
indicative of glaucoma. The incidence of these findings was low and
not statistically correlated with treatment. The F3b fetuses showed
no treatment-related toxic or teratogenic effects. The NOAEL for
reproductive and systemic effects was 180 mg/kg bw per day, the
highest dose tested (James, 1979).
In a study conducted according to GLP, technical-grade permethrin
of seven batches (nominal cis:trans ratio, 40:60; purity,
94.1-98.9%) was administered in the diet to groups of 12 male and 24
femaleWistar rats at concentrations of 0, 500, 1000, or 2500 ppm,
equivalent to 0, 33, 67, and 170 mg/kg bw per day, for three
generations, from F0 until weaning of the F3b generation. The F2
parental rats were then mated to produce an F3c litter and their
pups were removed just before weaning for teratological examination.
The only clinical sign of toxicity was tremors, which were seen in
parents and pups receiving 1000 or 2500 ppm. The tremors were
transient, and no evidence of neuropathy was found in an extensive
examination of the nervous system of F1 males that had been exposed
in utero and fed the diet continuously for 1 year. No overall
effects on growth or reproductive performance were seen, and there was
no evidence of teratogenicity. Buphthalmia due to the persistent
presence of a pupillary membrane was first seen in the F1b litter
and then in subsequent litters. The incidence of this congenital
lesion was low (< 3%), occurring in two pups in one litter at 500 ppm
litter and in animals at 1000 and 2500 ppm.The effect may have been
due to interaction between permethrin and genetic factors, but it is
more likely to be due to differences in the selection of the F0
parents. Hypertrophy of centrilobular hepatocytes was seen in the
livers of treated F3b pups examined histologically, although no
lesion was seen on gross examination. Liver hypertrophy has been
observed in other studies of permethrin and is considered to be an
adaptive change of no toxicological significance. No NOAEL could be
identified for systemic toxicity, as hypertrophy of centrilobular
hepatocytes in the offspring and tremors in parents and offspring
occurred at all doses. The NOAEL for reproductive toxicity was
2500 ppm, equivalent to 170 mg/kg bw per day, the highest dose tested
(Hodge et al., 1977).
(ii) Developmental toxicity
Mice
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female CD-1
mice on days 6-15 of gestation. The groups consisted of 20 untreated
controls, 23 mice receiving corn oil at 10 ml/kg bw per day, and 22
mice given permethrin at 400 mg/kg bw per day. The animals were
observed daily for clinical signs of toxicity. One treated animal died
on day 17 before termination of pregnancy. Treatment had no
significant effect on weight gain during dosing and pregnancy, and
there was no significant difference in the mean numbers of
implantations among the groups, no significant treatment-related
effect on the numbers of live and normal fetuses or on the numbers of
resorbed, dead, and malformed fetuses, and no treatment-related effect
on individual fetal weights, litter size, or fetal sex ratios. No
gross visceral or skeletal malformations were found in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained death of one treated mouse. The NOAEL for developmental
toxicity was 400 mg/kg bw per day, the only dose tested (James,
1974a).
Rats
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage to groups of female Wistar
rats on days 6-16 of gestation. The groups consisted of 22 untreated
controls, 23 rats receiving corn oil at 10 ml/kg bw per day, and 23
rats given permethrin at 200 mg/kg bw per day. Two treated rats died
before completion of their pregnancy. Treatment had no significant
effect on weight gain during dosing or pregnancy, and no significant
difference was seen in the mean numbers of corpora lutea or
implantations among the groups, no effect of treatment on the numbers
of live and normal fetuses, on individual fetal weights or litter
size, or on the fetal sex ratio. The numbers of resorbed, dead, and
malformed fetuses were not significantly affected by treatment. No
gross visceral or skeletal malformations were seen in any group. No
NOAEL could be identified for maternal toxicity because of the
unexplained deaths of two treated rats. The NOAEL for developmental
toxicity was 200 mg/kg bw per day, the only dose tested (James,
1974b).
Technical-grade permethrin (cis:trans ratio, 44:56 to
46.5:53.5; purity, 92.4-95%), aspirin, and corn oil were administered
orally by gavage to groups of 20 pregnant SpragueDawley rats during
days 6-16 of gestation, at doses of 2 ml/kg bw per day for corn oil,
200 mg/kg bw per day for aspirin, and 4, 41, or 83 mg/kg bw per day
for permethrin. There were no maternal deaths and no treatment-related
effects on the number of resorptions, body weight, length, or fetal
sex ratio. No gross, skeletal, or soft-tissue abnormalities were seen
in any group. The NOAEL for maternal and developmental toxicity was 83
mg/kg bw per day, the highest dose tested (Metker et al., 1977).
Technical-grade permethrin (cis:trans content, 37.5%:57.8%;
purity, 95.3%) diluted in corn oil was administered orally at a volume
of 10 ml/kg bw to groups of 20 pregnant CD ratson days 6-16 of
gestation at doses of 22.5, 71, or 225 mg/kg bw per day. None of the
dams died during the study, and there were no treatment-related
effects on weight gain, food consumption, pregnancy frequency, the
number of corpora lutea, the total number of implantations per
pregnancy, fetal or placental weight, or fetal sex ratios. The NOAEL
for maternal and developmental toxicity was 225 mg/kg bw per day, the
highest dose tested (McGregor & Wickramaratne, 1976).
Rabbits
Technical-grade permethrin (cis:trans ratio and purity not
stated) was administered orally by gavage at a volume of 2 ml/kg bw to
groups of female Dutch belted rabbits on days 6-18 of gestation. The
groups consisted of nine untreated controls, six given corn oil, and
seven given permethrin at 400 mg/kg bw per day. One animal in each
group died before the end of the study, but the deaths were not
related to treatment. There was no significant effect on weight gain
during dosing and gestation, no significant difference in the mean
numbers of corpora lutea and implantations, no effect on the numbers
of live and normal fetuses, the numbers of resorbed, dead, and
malformed fetuses, litter size, mean fetal weight, or fetal sex ratio.
No gross skeletal or visceral abnormalities were seen in any group.
The NOAEL for maternal and developmental toxicity was 400 mg/kg, the
highest dose tested (James, 1974c).
Technical-grade permethrin (cis:trans ratio, 40:60; purity,
92.5%) was administered orally by gavage in 0.5% v/v aqueous Tween 80
to groups of 18 pregnant Dutch rabbits on days 6-18 of gestation at
doses of 600, 1200, or 1800 mg/kg bw per day. Five does at 600 and
1200 mg/kg bw per day and four at 1800 mg/kg bw per day group were
killed when moribund or were found dead. The clinical signs noted
included tremors at 1800 mg/kg bw per day. Many of the females found
dead or killed produced few or no faeces and had more than normal
amounts of fur in the stomach, and hypothermia and salivation were
generally seen. The body weights were reduced in all treated groups
but were statistically significantly lower onlyat 1800 mg/kg bw per
day. A statistically significant increase in post-implantation loss
was seen in animals at 1200 and 1800 mg/kg bw per day, and the number
and percentage of early and late resorptions were higher and the mean
number of live fetuses was lower in animals at these doses. The effect
at 1800 mg/kg bw per day was related to treatment, but animals at 1200
mg/kg bw per day showed a decreased number of implantation sites and a
decrease in the number of corpora lutea. As implantation occurred
before initiation of treatment, the effects at 1200 mg/kg bw per day
are not related. The fetal weights were similar to those of controls.
Evaluation of the fetuses revealed no gross visceral or skeletal
abnormalities. No NOAEL could be identified for maternal toxicity
because deaths, clinical signs, and body weight reduction were seen at
all doses. The NOAEL for developmental toxicity was 1200 mg/kg bw per
day (Richards et al., 1980).
(g) Special studies
(i) Neurotoxicity
Mice
Pyrethroids have been reported to induce two types of neurotoxic
syndrome in mice after intracerebroventricular administration
(Lawrence & Casida, 1982). The first type involves markedly increased
hypersensitivity and hyperactivity, followed quickly by whole-body
tremors and clonic seizures. The mice become prostate 5-20 min after
the onset of these signs, and profound whole-body tremors persist
until death, which usually occurs 5-30 min later. The second type of
syndrome is initially similar to the first but the signs rapidly
progress to include chreothetosis (sinuous writhing) and profuse
salivation, often with tonic seizures shortly before death after 15-45
min. Permethrin induces the second type of response, while
deltamethrin and fenvalerate, for instance, induce the first. Diazepam
at 1 mg/kg bw intraperitoneally did not delay the onset of the second
type of response in male albino mice treated with permethrin
intracerebroventricularly, but a dose of 3 mg/kg bw increased the
LD50 value in mice given (1 R, cis)-permethrin from 0.15 mg/kg bw
to 1.4 mg/kg bw (Gammon et al., 1982).
Technical-grade permethrin (cis:trans ratio, 40:60; purity not
stated) or the separated cis and trans isomers (purity not stated)
were administered in equal volumes of Emulphor and 95% ethanol (total
volume, 0.2 ml/10 mg pyrethroid) intravenously at a volume of 0.1 ml
or intracerebroventricularly at a volume of 0.005 ml to male ICR mice.
All treatments induced hyperactivity, increased sensitivity to
external stimuli, and whole-body tremor, leading to prostration and
ultimately to death. The ED50 values and 95% confidence intervals
for groups of six mice were 20 mg/kg bw (17-22) for the cis isomer,
36 mg/kg bw (33-39) for the technical-grade mixture, and 93 mg/kg bw
(83-100) for the trans isomer after intravenous administration, and
0.09 mg/kg bw (0.06-0.14) for the cis isomer, 0.15 mg/kg bw
(0.13-0.17) for the technical-grade mixture, and 1.1 mg/kg bw
(0.72-1.4) for the trans isomer after intracerebroventricular
administration. These results indicate that most of the activity is
due to the cis isomer and suggest a mainly central site of action.
The effects of a number of drugs that affect neurotransmitter systems
(noradrenaline, dopamine, acetylcholine, gamma-aminobutyric acid
(GABA), and serotonin) were studied on the loss of righting reflex
induced by permethrin given intravenously at a dose of 30 mg/kg bw. It
was not clear whether inhibitory GABA pathways were involved, whereas
the toxicity of permethrin was potentiated by at least some of the
drugs that affect central noradrenergic, cholinergic, or serotonergic
transmission. The involvement of these transmission systems in the
toxicity of permethrin could not be elucidated (Staatz et al., 1982).
Rats
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered as a 1.0%
(w/v) solution or suspension in corn oil to 10 Sprague-Dawley CD rats
of each sex at doses of 0, 10, 150, or 300 mg/kg bw. Clinical signs,
body weights, the results of a functional observational battery of
tests, and motor activity were recorded. At the end of the study, the
surviving rats were perfused, and the nervous systems of five rats of
each sex at the high dose and in the control group were examined. One
female at the high dose died on day 0. Treatment-related clinical
signs observed in this group were tremors, staggered gait, splayed
hind limbs, exaggerated hind-limb flexion, and hypersensitivity to
sound. All of the animals had recovered by day 3. There were no
effects on body weight. Rats at the high dose had whole-body tremors,
staggered gait, splayed hind limbs, abnormal posture while moving,
exaggerated hind-limb flexion, and convulsions on the first day of
functional and behavioural testing, but no significant differences
were noted after this time. There was no effect on motor activity.
Neuropathological examination of the nervous system revealed no
treatment-related lesions. The NOAEL for neurotoxicity was 150 mg/kg
bw on the basis of neurotoxic clinical signs and significant changes
in function and behaviour at 300 mg/kg bw per day (Freeman, 1993a).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of five Sprague-Dawley CD of each sex at concentrations
of 0, 100, 750, 1500, 3000, 4000, or 5000 ppm for 28 days. Clinical
signs were recorded daily, and body weights were recorded weekly. All
surviving animals underwent gross necropsy on day 28. All rats
receiving 5000 ppm and one female receiving 4000 ppm had died by the
end of day 3. The treatment-related clinical signs included tremors,
splayed hind limbs, staggered gait, and chromorhinorrhoea at
concentrations > 1500 ppm. The clinical signs generally occurred
with increasing frequency with dose, except when pre-empted by death.
No clinical signs were seen among animals receiving doses < 750
ppm. Body weights were significantly reduced at doses > 3000 ppm
during some or all of the study, and body-weight gain was
significantly reduced at 3000 and 4000 ppm. Gross lesions found during
necropsy of animals at doses > 3000 ppm included necrosis of the
tail and feet and reddish-brown fluid in the stomach and intestines.
The NOAEL was 750 ppm, equal to 38 mg/kg bw per day, on the basis of
neurotoxic clinical signs at 1500 ppm (Freeman, 1993b).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 36%:59%; purity, 95.3%) was administered in the
diet of groups of 10 Sprague-Dawley rats at concentrations of 0, 250,
1500, or 2500 ppm for 13 weeks. Clinical signs were recorded daily,
and body weights and food consumption were recorded weekly. The
results of a functional observational battery of tests and tests for
motor activity were recorded before exposure and in weeks 4, 8, and 13
of the study. Surviving rats were perfused, and the nervous systems of
five rats of each sex at the high dose and in the control group were
examined microscopically for neuropathological lesions. No
treatment-related deaths occurred during the study, but
treatment-related clinical signs were observed in rats at doses
> 1500 ppm which included staggered gait, splayed hind limbs. and
tremors. These signs generally increased in both frequency and
severity with dose. Reductions in body weight and intermittent
reductions in food consumption were seen among males receiving 2500
ppm. Functional and behavioural testing showed whole-body tremors,
staggered gait, spayed hind limbs, and an abnormal posture during
movement among rats receiving 2500 ppm and to a lesser extent among
those given 1500 ppm. There were no significant differences with
regard to motor activity. No treatment-related lesions were found at
autopsy or on neuropathological examination. The NOAEL for
neurotoxicity was 250 ppm, equal to 15 mg/kg bw per day, on the basis
of neurotoxic clinical signs and significant changes in function and
behaviour at 1500 ppm (Freeman, 1993c).
In a study conducted according to GLP, technical-grade permethrin
(cis:trans ratio, 40:60; purity, > 98%) was administered in the
diet of Sprague-Dawley rats at target doses of 100, 200, and 400 mg/kg
bw per day for 90 days. The actual mean doses were 0, 86, 160, and 340
mg/kg bw per day for males and 0, 110, 170, and 350 mg/kg bw per day
for females. The groups consisted of 20 rats of each sex at the high
dose and as untreated controls and 10 rats of each sex at the
intermediate and low doses and as vehicle (acetone) controls. Clinical
signs, body weights, and food consumption were recorded three times a
week. At the end of treatment, half of the animals at the high dose
and of untreated controls were returned to untreated diet and held for
an additional 6 weeks to assess the reversibility of effects. At the
end of the study, the rats were perfused and the nervous systems from
those at the high dose and untreated and vehicle controls were
examined histologically for neuropathological lesions. Neurotoxic
signs were seen in all rats at the high dose and included tremors,
twitching, hyperexcitability, and irritability. The signs occurred
during the first day of treatment and persisted throughout the
90 days. Rats at the intermediate dose had tremors intermittently and
occasional periods of hyperexcitability during the first 2 days of
testing, but not later. When females at the high dose were returned to
untreated diet, all of the signs disappeared within 24 h, whereas in
males the tremors and twitching ceased within 1 day, and the
hyperexcitability and irritability stopped within 2-3 days. Decreased
body-weight gains were noted at the high dose, in females from week 3
and in males from week 11, but these deficits were rapidly corrected
during the recovery period. Neuropathological examination of the
central nervous system and peripheral nerves revealed no
treatment-related lesions. The NOAEL was 86 mg/kg bw per day on the
basis of neurotoxic clinical signs at 160 mg/kg bw per day (United
States Army, 1986).
The peripheral nerves, brain stem, and spinal cord of groups of
five Long-Evans rats of each sex were examined histologically for
abnormalities after exposure to permethrin. The rats were derived from
a 24-month feeding study (Braun & Rinehart, 1977) and from the third
generation of a three-generation study of reproductive toxicity
(Schroeder & Rinehart, 1977), described above, and were 10-11 months
old at the time of autopsy. In addition to standard histological
techniques, the neural material was examined morphometrically for the
number of myelinated fibres per nerve and per square millimetre of
fascicular area and for the frequency distribution of diameters per
nerve and per square millimetre of fascicular area; the morphology of
teased fibres was also evaluated. No structural lesions associated
with exposure of the rats to permethrin at dietary concentrations up
to 500 ppm (equivalent to 25 mg/kg bw per day) were observed in
central or peripheral nerves or in teased fibres of distal sural and
tibial nerves and of the maxillary division of the fifth cranial nerve
(Dyck, 1978).
Chickens
Technical-grade permethrin (cis:trans ratio, 50:50; purity,
96%) was administered by gavage to six adult laying hens at a dose of
1000 mg/kg bw per day for 5 days. After 21 days, the hens were
re-dosed and observed for an additional 21 days. A positive control
group of six hens received an intramuscular injection of a mixture of
atropine sulfate (17 mg/kg bw) and pralidoxime chloride (50 mg/kg bw)
and, 1 h later, a dose of tri- ortho-tolyl phosphate at 0.5 ml/kg bw
by gavage. Six untreated hens were available. No deaths occurred in
the treated or negative control groups during the study, and no
neurological disturbances and no histological lesions were found in
the peripheral or central nervous system. The positive controls showed
lack of coordination, unsteadiness, and loss of balance, which
worsened progressively. Foci of axonal and myelin degeneration were
observed in all positive control birds (Milner & Butterworth, 1977).
Technical-grade permethrin (cis:trans ratio, 36.0%:58.9%;
purity, 94.9%) was administered at a single dose of 15 ml, equal to
9100 mg/kg bw, the maximum possible dose, to 15 hens, which were
observed for 21 days for signs of neurotoxicity. The hens were treated
with an intramuscular injection of atropine and pyridine-2-aldoxime
methane sulfonate, then re-dosed with 15 ml of permethrin and observed
for an additional 21 days. Five hens were given tri- ortho-tolyl
phosphate at 500 mg/kg bw as a positive control, and 10 hens received
water as a negative control. No deaths occurred during the study, and
no signs of neurotoxicity were noted in the treated or negative
control groups. Ataxia, ranging from slight muscular incoordination to
difficulty in standing, was observed in the positive control group. No
treatment-related histopathological lesions of the spinal cord or
sciatic nerve were observed in the treated or negative control groups,
whereas degenerative changes were seen in the spinal cord and sciatic
nerve of hens treated with tri- ortho-tolyl phosphate. An NOAEL for
neurotoxicity could not be identified (Ross, et al., 1977).
(ii) Endocrine effects
The MCF-7 human breast carcinoma cell line was used to study the
oestrogenic potential of permethrin in vitro, pS2 mRNA expression
levels being used as the end-point. Permethrin at 100 µmol/L had no
effect on pS2 expression or on cell proliferation, whereas
17ß-estradiol at a concentration of 10 nmol/L induced a fivefold
increase in pS2 expression (Go et al., 1999).
3. Observations in humans
Twenty-three workers aged 20-52 years who had been exposed to
synthetic pyrethroids and 23 age- and sex-matched control subjects who
had had no contact with pyrethroids were interviewed, examined, and
assessed electrophysiologically. Most of the workers had been exposed
to several different pyrethroids, the more common being cypermethrin,
permethrin, fenvalerate, and fenpropathrin. Nineteen of the subjects
had experienced one or more episodes of abnormal facial sensation
0.5-3 h after exposure which persisted for 0.5-8 h. Thirteen subjects
had experienced more than one episode. There were no abnormal
neurological signs, and the results of electrophysiological studies of
the arms and legs were normal. Three subjects who had had moderate
exposure to permethrin had not developed symptoms, whereas two of them
did so after exposure to fenpropathrin and cypermethrin (Le Quesne et
al., 1980).
Workers who handled seedlings treated with permethrin
(cis:trans ratio, 25:75 for a wettable powder and 40:60 for an
emulsion in organic solvents; both diluted with water to 1-2% before
use) reported irritation of the skin. Of 42 workers who had used the
wettable powder, 12% reported burning and 10% reported blisters. Of 45
workers who had used the emulsion, 2% reporting itching. Irritation of
the upper respiratory tract involving increased nasal secretions and
sneezing was reported by 31% of the workers who had used the wettable
powder and by 2% of those who has used the emulsion (Kolmodin-Hedman
et al., 1982).
Volunteers received applications of 0.05 ml of a field-strength
preparation of technical-grade permethrin (94-96% active ingredient)
in ethanol to an area of 4 cm2 on an ear lobe, a formulation (32-36%
active ingredient) in water to 0.13 mg/cm2, or the solvent and
surfactants; 0.05 ml of the vehicle was applied to the other lobe. No
cutaneous sensation was elicited by the inert ingredients.
Paraesthesia appeared after a latent period of about 30 min, peaked
between 8 and 12 h, and disappeared after about 24 h. Further studies
with a range of doses ahowed that the response was dose-related
(Flannigan et al., 1985).
One of 28 subjects with pediculosis pubis treated with a 1%
permethrin rinse developed mild scrotal erythema and irritation 12 h
after application (Kalter et al., 1987). A group of 435 patients, most
of them children, were treated for pediculosis capitis: approximately
half of the group were treated with a single, 10-min application of
25-50 ml of a cream rinse containing permethrin (1%) and isopropanol
(20%) after towel drying of washed hair, and the remainder were
treated with a liquid product containing pyrethrins (0.3%), piperonyl
butoxide (3%), petroleum distillate (1.2%), and benzyl alcohol (2.4%).
Cutaneous side-effects including pruritus, mild transient skin
burning, stinging sensations, skin tingling, erythema, and scalp rash,
were reported by 7% of the patients in the first group and by 16% of
those in the second (DiNapoli et al., 1988). Similar results and
side-effects were reported by Brandenburg et al. (1986).
An observational study was undertaken in the USA to evaluate the
safety of a cream rinse containing 1% permethrin for treatment of head
louse infestations. Thirty-seven local public health departments
enrolled a total of 38 160 patients for 47 578 treatments with
permethrin and other pediculicides between 1 September 1986 and 31
January 1988. Follow-up information was collected 7-14 days after
treatment at a return visit or by telephone contact. A total of 103
adverse events were reported from 41 955 evaluable treatments. The
rates of reported adverse events were 2.2 per 1000 treatments with
permethrin, 3.4 per 1000 treatments with lindane, and 1.5 per 1000
treatments with other over-the-counter preparations. No serious,
unexpected adverse event was detected in the 18 950 patients treated
with permethrin (Andrews et al., 1992).
Of 10 patients with scabies treated with one application of 25 g
(range, 21-32 g) of a cream containing 5% permethrin, followed by a
thorough washing 8-20 h after treatment, six developed limited,
mild-to-moderate eczema on the scabies-affected skin at one or more
examinations (van der Rhee et al., 1989).
Comments
The metabolism of 14C-permethrin was studied in rats, lactating
goats and cattle, and laying hens. Permethrin was rapidly absorbed,
distributed, and excreted in these species after oral administration.
The metabolism of the pyrethroid was extensive, yielding a vast number
of polar degradates. Ester cleavage, hydroxylation, oxidation, and
ultimately conjugation are the critical biological mechanisms of the
metabolism of permethrin in the species studied. The metabolites that
were common to all species were 4'-hydroxypermethrin, dichlorovinyl
acid, and phenoxybenzyl alcohol. Dichlorovinyl acid and phenoxybenzoic
acid have also been identified in human urine after dermal application
of permethrin.
In rats, 96% of an administered dose was recovered in urine and
faeces within 12 days, while the total radiolabelled residues in
tissues accounted for 0.3-0.8% of the dose. Recovery in urine and
faeces within 24 h accounted for about 40% and 25% of the dose of
cis isomer and 65% and 10% of the dose of trans isomer.
respectively. Repeated exposure resulted in temporary accumulation in
fat tissue, but the chemical dissipated rapidly once exposure had
ceased.
In lactating goats and cows dosed orally with permethrin,
recovery in urine and faeces accounted for at least 65% of the dose,
and the total radiolabelled residues in liver and milk samples
represented 0.2-0.5%. Permethrin was extensively metabolized and
readily eliminated after oral administration to laying hens, > 90%
of the administered dose being excreted, while the total radiolabel in
egg and liver samples accounted for 0.1-0.2% of the dose.
The toxicity of permethrin is influenced by many factors
including the cis:trans isomer ratio, the carrier or vehicle, and
the strain of animal used. The cis isomer is considerably more toxic
than the trans isomer. The oral LD50 values in rats ranged from
6000 mg/kg bw for the 20:80 cis:trans isomeric mixture to 220 mg/kg
bw for the 80:20 cis:trans isomeric mixture. Undiluted
technical-grade permethrin (25:75 to 40:60 cis:trans isomeric
mixtures) has low acute toxicity after oral, dermal and inhalation
exposure. It was mildly irritating to the eyes and slightly irritating
to skin. It was not a skin sensitizer when tested by the Magnusson and
Kligman method.
WHO (1999) has classified permethrin as 'moderately hazardous'.
Studies in which rats, mice, rabbits, guinea-pigs, and dogs
received repeated administrations by inhalation, orally, and dermally
showed that the main effects of technical-grade permethrin are on
clinical signs, especially tremor and hyperexcitability, body weight,
and liver weight. In these short-term studies, the NOAEL or NOAEC
values were 250 mg/m3 in a 13-week study in rats exposed by
inhalation; 5 mg/kg bw per day in a 52-week study in which dogs
received the compound in gelatin capsules orally; and 1000 mg/kg bw
per day in a 21-day study in rabbits treated dermally.
In two long-term studies in rats in different laboratories with
different strains, permethrin was not carcinogenic, but the evidence
for carcinogenicity in mice was conflicting. In two studies conducted
in same strain in the same laboratory, permethrin increased the
incidences of lung and liver tumours in one study but not in the
other. The spontaneous background incidence of both these tumour types
is known to be extremely variable. A third study, conducted in a
different mouse strain, gave negative results. Thus, the weight of
evidence supports the conclusion that permethrin has very weak
oncogenic potential, and the probability that it has oncogenic
potential in humans is remote. The NOAEL for long-term toxicity in
rats was 100 ppm, equivalent to 5 mg/kg bw per day, on the basis of
clinical signs and changes in body and organ weights and blood
chemistry at 500 ppm. The NOAEL for long-term toxicity in mice was
500 ppm, equivalent to 75 mg/kg bw per day, on the basis of changes in
organ weights at 2000 ppm.
No genotoxic activity was observed in an adequate battery of
tests for DNA damage and mutagenicity in vitro, but there was
evidence that permethrin can induce chromosomal aberrations in
mammalian cells in vitro. No tests have been carried out in mammals
for DNA damage, mutagenicity, or clastogenicity in vivo. A test for
dominant lethal effects in male mice showed no activity.
In a multigeneration study of reproductive toxicity in rats, the
NOAEL for systemic and reproductive toxicity was 180 mg/kg bw per day.
In a second multigeneration study in rats, an NOAEL could not be
identified for systemic toxicity, as effects were seen at 500 ppm,
equivalent to 33 mg/kg bw per day, the lowest dose tested; the NOAEL
for reproductive toxicity in the same study was 2500 ppm, equivalent
to 170 mg/kg bw per day, the highest dose tested.
In a study of developmental toxicity in rabbits, the NOAEL for
maternal effects was 600 mg/kg bw per day and that for developmental
toxicity was 1200 mg/kg bw per day. In three studies of developmental
toxicity in rats, the NOAEL for maternal toxicity was 83 mg/kg bw per
day and the NOAEL for developmental toxicity was 225 mg/kg bw per day,
the highest dose tested. In a study of developmental toxicity in mice,
no NOAEL was identified for maternal toxicity, whereas the NOAEL for
developmental effects was 400 mg/kg bw per day, the only dose tested.
The Meeting concluded that technical-grade permethrin is not a
reproductive or developmental toxin.
The results of acute and 90-day studies of neurotoxicity in rats
and of an acute study of delayed neurotoxicity in hens showed that
technical-grade permethrin does not induce neuropathological changes.
The NOAEL for neurotoxicity in a study in rats given a single dose was
150 mg/kg bw, on the basis of clinical signs of neurotoxicity and
significant changes in measurements in a functional observational
battery of tests at 300 mg/kg bw. The NOAEL for neurotoxicity in a
13-week study in rats was 15 mg/kg bw per day, on the basis of
clinical signs of neurotoxicity and significant changes in
measurements in the functional observational battery of tests at 90
mg/kg bw per day.
An ADI of 0-0.05 mg/kg bw was established for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60 on the basis of
the NOAEL of 100 ppm, equivalent to 5 mg/kg bw per day, in the 2-year
study in rats, which was based on clinical signs and changes in body
and organ weights and blood chemistry at 500 ppm, and the NOAEL of 5
mg/kg bw per day in a 1-year study in dogs based on reduced body
weight at 100 mg/kg bw per day, and applying a safety factor of 100.
The Meeting concluded that establishment of an acute reference
dose was not necessary because of the low acute toxicity of
technical-grade permethrin.
Toxicological evaluation
Levels that cause no toxic effect (relevant for technical-grade
permethrin with cis:trans ratios of 25:75 to 40:60)
Mouse: 500 ppm, equivalent to 75 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
Rat: 100 ppm, equivalent to 5 mg/kg bw per day (2-year study of
toxicity and carcinogenicity)
180 mg/kg bw per day (for reproductive toxicity, highest
dose in a three-generation study of reproductive toxicity)
225 mg/kg bw per day (for maternal and developmental
toxicity, highest dose in a study of developmental toxicity)
150 mg/kg bw (single dose in a study of neurotoxicity)
15 mg/kg bw per day (13-week study of neurotoxicity)
Rabbit: 400 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
1200 mg/kg bw per day (developmental toxicity in a study of
developmental toxicity)
Dog: 5 mg/kg bw per day (1-year study of toxicity)
Estimate of acceptable daily intake for humans
0-0.05 mg/kg bw (for technical-grade permethrin with cis:trans
ratios of 25:75 to 40:60)
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for continued
evaluation of the compound
1. Clarification of the findings of chromosomal aberrations in
vitro and their potential significance in vivo
2. The Meeting was aware of other studies, in particular of acute
toxicity, dermal and ocular irritation, sensitization, and
developmental toxicity in rats that had been made available to
regulatory entities by other sponsors. The continuing support of
permethrin would benefit from the submission of these studies for
review by the Joint Meeting.
Toxicological criteria relevant for estimating guidance values for dietary and non-dietary exposure to permethrin
Absorption, distribution, excretion and metabolism in mammals
Rate and extent of absorption Rapid and extensive (rat, lactating caprine and bovine, hen)
Distribution Mainly to fat (rat, lactating caprine and bovine, hen)
Potential for accumulation Some accumulation in fat on repeated dosing, rat
Rate and extent of excretion cis-isomer: 40% in urine, 25% in faeces in 24 h in rat
trans-isomer: 65% in urine, 10% in faeces in 24 h in rat
Metabolism in animals Extensive; hydrolysis, hydroxylation, oxidation and conjugation
(rat, lactating caprine and bovine, hen)
Toxicologically significant compounds Parent compound
(animals, plants and environment)
Acute toxicity
Rat, LD50, oral 225 (cis:trans ratio, 80:20); 6000 (cis:trans ratio,
20:80) mg/kg bw
Rat, LD50, dermal No data
Rabbit, LD50, dermal 2000 mg/kg bw (cis:trans ratio, 55:45 or 40:60) (highest dose)
Rat, LC50, inhalation > 23.5 mg/l, 4 h (cis:trans ratio, 40:60)
Dermal irritation Slightly irritating to rabbit skin
Ocular irritation Mildly irritating to rabbit eyes
Dermal sensitization No sensitizing potential in guinea-pigs
Short-term toxicity
Target/critical effect Nervous system (rat); liver (mouse, rat, dog)
Lowest relevant oral NOAEL 5 mg/kg bw per day in dog (cis:trans ratio, 32%:52%)
Lowest relevant dermal NOAEL 1000 mg/kg bw per day in rabbit
Lowest relevant inhalation NOAEL 250 mg/m3 in rat (cis:trans ratio, 40:60)
Target/critical effect Nervous system (rat)
Liver (mouse, rat)
Lowest relevant NOAEL Rat, 5 mg/kg bw per day; mouse, 75 mg/kg bw per day
Long-term toxicity and carcinogenicity
Carcinogenicity Not carcinogenic to mouse or rat
Genotoxicity No DNA damage or mutagenicity in vitro; clastogenic
in vitro; not studied in vivo in mammals
Reproductive toxicity
Reproductive target/critical effect None identified
Lowest relevant reproductive NOAEL 180 mg/kg bw per day in rat
Developmental target/critical effect Rat, none identified; rabbit, fetotoxicity
Lowest relevant developmental NOAEL Rat, 225 mg/kg bw per day (cis:trans ratio, 38%:58%);
rabbit, 1200 mg/kg bw per day (cis:trans ratio, 40:60)
Neurotoxicity/Delayed neurotoxicity NOAEL, 150 mg/kg bw, single dose, rats;
NOAEL, 15.5 mg/kg bw per day in a 90-day study, rats
No acute delayed effect in hens (9100 mg/kg bw)
Other toxicological studies
Medical data Paraesthesia
Summary Value Study Safety factor
ADI 0-0.05 mg/kg bw Rat, long-term toxicity, 100
5 mg/kg bw per day
Dog, 1 year, toxicity,
5 mg/kg bw per day
Acute reference dose Unnecessary
References
Andrews, E.B., Joseph, M.C., Magenheim, M.J., Tilson, H.H., Doi, P.A.
& Schultz, M.W. (1992) Postmarketing surveillance study of
permethrin creme rinse. Am. J. Public Health, 82, 857-861.
Angerer, S. & Ritter, A. (1997) Determination of metabolites of
pyrethroids in human urine using solid-phase extraction and gas
chromatography-mass spectrometry. J. Chromatogr. B Biomed.
Sci. Appl., 695, 217-226.
Bartsch, H., Malaveille, C., Camus, A.M., Martel-Planche, G., Brun,
G., Hautefeuille, A., Sabadie, N., Barbin, A., Kuroki, T.,
Drevon, C., Piccoli, C. & Montesano, R. (1980) Validation and
comparative studies on 180 chemicals with S. typhimurium
strains and V79 Chinese hamster cells in the presence of various
metabolizing systems. Mutat. Res., 76, 1-50.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1992)
Cytogenetic effects of permethrin in cultured human lymphocytes.
Mutagenesis, 7, 433-437.
Barrueco, C., Herrera, A., Caballo, C. & de la Peña, E. (1994)
Induction of structural chromosome aberrations in human
lymphocyte cultures and CHO cells by permethrin. Teratog.
Carcinog. Mutag., 14, 31-38.
Becci, P.J. & Parent, R. (1980) Evaluation of the subchronic toxic
effects of FMC 45801 when administered in the diet to Long Evans
rats over a 90-day period. Unpublished report from Food and Drug
Research Laboratories, Inc., Waverly Research Center, USA, Study
No. 6363. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Becci, P.J., Gephart, L. & Parent, R.A. (1980) 90-day subchronic oral
dosing study with FMC 45801 in beagle dogs. Unpublished report
from Food and Drug Research Laboratories, Inc., Waverly Research
Center, USA, Study No. 6338. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Benner, J. (1997) Permethrin: addendum to MRID numbers 42410001,
43505201, 43962801 and 44196101 submitted in response to EPA CBRS
review of MRID 43962801, goat metabolism -- oral dosing.
Unpublished report from Zeneca Agrochemicals, Bracknell, England,
Project No. HRC/ISN 248/920216sup2. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Benner, J.P., Hamlet, J.M. & Skidmore, M.W. (1996) Addendum to MRID
Nos. 42410001, 43505201 and 43962801, Permethrin: Further
investigation of residues in liver following oral administration
to the goat and radiovalidation of enforcement methods for
analysis of animal tissues. Unpublished report from Zeneca
Agrochemicals (Zeneca Ltd), Bracknell, England, Report No. RJ
2135B. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Billups, L.H. (1978a) Twenty-four month toxicity/carcinogenicity study
of compound FMC 33297 in rats. Histopathologic evaluation of
step-sectioned lungs from male rats. Unpublished report from
Environmental Pathology Services, Rockville, Maryland, USA.,
Study No. 78-6-0049. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA and by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
Billups, L.H. (1978b) Histopathologic evaluation of a twenty-four
month toxicity/carcinogenicity study of compound FMC 33297 in
rats. Unpublished report from Environmental Pathology Services,
Rockville, Maryland, USA., Study No. 77-11-0007. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA and by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Brandenburg, K., Deinard, A.S., DiNapoli, J., Englander, S.J.,
Orthoefer, J. & Wagner, D. (1986) 1% permethrin cream rinse vs
1% lindane shampoo in treating pediculosis capitis. Am. J.
Dis. Child., 140, 894-896.
Bratt, H., Mills, I.H. & Slade, M. (1977) Permethrin: Tissue retention
in rats. Unpublished report from Imperial Chemical Industries
Ltd, Alderley Park, England, Report No. ZO671. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975a) Acute oral toxicity studies in
rats with FMC 33297. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project Nos. 2702-75, 2703-75,
2704-75 and 2705-75. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975b) Acute dermal toxicity in
rabbits. Compound No. FMC 33297. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA, Project No.
2908-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975c) Rabbit primary dermal
irritation. Compound No. FMC 33297. Unpublished report prepared
by Bio/dynamics Inc. East Millstone, New Jersey, USA, Project No.
2909-75. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1975d) Rabbit eye irritation.
Compound No. FMC Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2909-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Killeen, J.C., Jr (1976) Acute inhalation. Compound No.
FMC 33297. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 2911-75. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Braun, W.G. & Rinehart, W.E. (1977) Twenty-four month oral
toxicity/carcinogenicity study of FMC 33297 technical in rats.
Unpublished report from Bio/dynamics, Inc., East Millstone, New
Jersey, USA, Project No. 74R-1022. Submitted to WHO by FMC
Corporation, Middleport, New York, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Brusick, D.J. & Weir, R.J. (1976) Mutagenicity evaluation of FMC 33297
(catalyst: titanium isopropylate), C8093-25, MR 51310.
Unpublished report from Litton Bionetics, Inc. Kensington,
Maryland, USA, Project No. 2683. Submitted to WHO by FMC
Corporation, Middleport, New York, USA.
Busey, W.M. (1978) Two-year chronic rat toxicity study FMC-33297.
Unpublished report from Experimental Pathology Laboratories Inc.,
Rockville, Maryland, USA. FMC Project No. NCT 549.32. Revised
pathology report. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Butterworth, S.T.G. & Hend, R.W. (1976) Toxicity studies on the
insecticide WL 43479: A five week feeding study in rats.
Unpublished report from Shell Research Ltd, Sittingbourne,
England, Report No. TLGR.0056.76. Submitted to WHO by Mitchell
Cotts Chemicals, Ltd, Mirfield, England.
Cameron, B.D. & Partridge, S.(1989) The metabolism of
[14C]-permethrin in the rat. Unpublished report from Inveresk
Research International, Musselburgh, Scotland, Report No. 4860.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Chesher, B.C. & Malone, J.C. (1974) Guinea pig sensitisation study
with 21Z73 using the maximization test method. Unpublished report
from Wellcome Research Laboratories, Berkhamsted, England, Lab.
Ref. No. T.L.13-74. Submitted to WHO by FMC Corporation,
Middleport, New York, USA.
Chesher, B.C., Malone, J.C. & Parker, M.J. (1975) 21Z73, Dominant
lethal study in male mice. Unpublished report from Wellcome
Research Laboratories, Berkhamsted. England, Lab. Ref. No. T.L.
37-75. Submitted to WHO by FMC Corporation, Middleport, New York,
USA.
Clapp, M.J.L., Banham, P.B., Glaister, J.R. & Moyes, A. (1977a) PP557:
28 day feeding study in mice. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/356. Submitted to WHO by FMC Corporation, Middleport, New
York, USA.
Clapp, M.J.L., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W. &
Moyes, A. (1977b) PP557: 28 day feeding study in rats.
Unpublished report from Imperial Chemical Industries, Ltd,
Alderley Edge, England, Report No. CTL/P/355. Submitted to WHO by
FMC Corporation, Middleport, New York, USA.
Clive, D. (1977) Mutagenicity of BW 21Z73 in L5178Y/TK+/- mouse
lymphoma cells with and without exogenous metabolic activation.
Unpublished report from Burroughs Wellcome Co., Research Triangle
Park, North Carolina, USA, Doc. No. TTEP/77/0001. Submitted to
WHO by FMC Corporation, Middleport, New York, USA.
Cridland, J.S. & Weatherley, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)2,2-dimethylcyclopropane carboxylic acid
after oral ingestion of permethrin (NRDC 143) -- A first report.
Unpublished report from The Wellcome Foundation, Ltd, Beckenham,
England, Report No. BDPE-77-1. Submitted to WHO by The Wellcome
Foundation, Ltd, Beckenham, England.
Cridland, J.S. & Weatherley, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial
of the insecticide. Unpublished report from The Wellcome
Foundation, Ltd, Beckenham, England, Report No. BDPE-771.
Submitted to WHO by The Wellcome Foundation, Ltd, Beckenham,
England.
Cummins, H.A. & Gardner, J.R. (1984a) Permethrin: Acute oral toxicity
in the rat. Unpublished report from Life Science Research, Eye,
England, Report No. 84/MCC001/588. Submitted to WHO by Mitchell
Cotts Chemicals Ltd, Mirfield, England.
Cummins, H.A. & Gardner, J.R. (1984b) High cis permethrin: (isomer
ratio: 80:20) acute oral toxicity in the rat. Unpublished report
from Life Science Research, Eye, England, Report No.
84/MCC002/587. Submitted to WHO by Mitchell Cotts Chemicals Ltd,
Mirfield, England.
DiNapoli, J.B., Austin, R.D., Englander, S.J., Gomez, M.P. & Barrett,
J.F. (1988) Eradication of head lice with a single treatment.
Am. J. Public Health, 78, 978-980.
Dyck, P.J. (1978) A pathologic and morphometric study of the nervous
system of rats fed compound FMC 33297 (permethrin). Unpublished
report from Peripheral Nerve Laboratory, Mayo Clinic and Mayo
Foundation, New York, New York, USA, Report No. not identified.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Flannigan, S.A., Tucker, S.B., Key, M.M., Ross, C.E., Fairchild, E.J.,
II, Grimes, B.A. & Harrist, R.B. (1985) Synthetic pyrethroid
insecticides: A dermatological evaluation. Br. J. Ind. Med.,
42, 363-372.
Flodström, S., Warngard, L., Ljungquist, S. & Ahlborg, U.G. (1988)
Inhibition of metabolic cooperation in vitro and enhancement of
enzyme altered foci incidence in rat liver by the pyrethroid
insecticide fenvalerate. Arch.Toxicol., 61, 218-223.
Freeman, C. (1993a) Permethrin technical twenty-eight day
neurotoxicity range-finding study in rats. Unpublished report
from FMC Corporation, Princeton, New Jersey, USA, Study No.
A92-3645. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Freeman, C. (1993b) Permethrin technical subchronic neurotoxicity
screen in rats. Unpublished report from FMC Corporation,
Princeton, New Jersey, USA, Study No. A92-3647. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA
Freeman, C. (1993c) Permethrin technical acute neurotoxicity screen in
rats Unpublished report prepared by FMC Corporation, Princeton,
New Jersey, USA, Study No. A923646. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA
Gammon, D.W., Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid
toxicology: Protective effects of diazepam and phenobarbital in
the mouse and the cockroach. Toxicol. Appl. Pharmacol., 66,
290-296.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1977)
Permethrin metabolism in rats. J. Agric. Food Chem., 25, 9-17.
Gaughan, L.C., Ackerman, M.E., Unai, T. & Casida, J.E. (1978)
Distribution and metabolism of trans- and cis-permethrin in
lactating Jersey cows. J. Agric. Food Chem., 26, 613-618.
Ghiasuddin, S.M. & Soderlund, D.M. (1984) Hydrolysis of pyrethroid
insecticides by soluble mouse brain esterases. Toxicol. Appl.
Pharmacol., 74, 390-396.
Go, V., Garey, J., Wolff, M.S. & Pogo, B.G. (1999) Estrogenic
potential of certain pyrethroid compounds in the MCF-7 human
breast carcinoma cell line. Environ. Health Perspectives, 107,
173-177.
Gupta, R.K., Mehr, Z.A., Korte, D.W. & Rutledge, L.C. (1990) Mutagenic
potential of permethrin in the Drosophila melanogaster
(Diptera: Drosophilidae) sex-linked recessive lethal test.
J. Econ. Entomol., 83, 721-724.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977a) PP557: Liver hypertrophy study in rats -- Dietary
administration over 26 weeks. Unpublished report from Imperial
Chemical Industries, Ltd, Alderley Edge, England, Report No.
CTL/P/360. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Hart, D., Banham, P.B., Glaister, J.R., Pratt, I. & Weight, T.M.
(1977b) PP557: Whole life feeding study in mice. Unpublished
report from Imperial Chemical Industries, Ltd, Alderley Park,
England, Report No. CTL/P/359. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, D., Shaw, D. & Latter (1992a) The
metabolism of 14C permethrin in the hen. Unpublished from
Huntingdon Research Centre Ltd, Cambridgeshire, England, Project
No. HRC/ISN 272/920435. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Hawkins, D.R., Kirkpatrick, & Shaw, D. (1992b) The metabolism of 14C
permethrin in the goat. Unpublished report from Huntingdon
Research Centre Ltd, Cambridgeshire, England, Project No. HRC/ISN
248/920216. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Herrera, A. & Laborda, E. (1988) Mutagenic activity in synthetic
pyrethroids in Salmonella typhimurium. Mutagenesis, 3, 509-514.
Herrera, A., Barrueco, C., Caballo, C. & de la Peña E. (1992) Effect
of permethrin on the induction of sister chromatid exchanges and
micronuclei in cultured human lymphocytes. Environ. Mol.
Mutag., 20, 218-222.
Hodge, M.C., Banham, P.B., Glaister, J.R., Richards, D., Taylor, K. &
Weight, T.M. (1977) PP557: 3-Generation reproduction study in
rats. Unpublished report from Imperial Chemical Idustries Ltd,
Alderley Edge, England, Report No. CTL/P/361. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA.
Hogan, G.K. & Rinehart, W.E. (1977) Twenty-four month oral
carcinogenicity study with FMC 33297 technical in mice.
Unpublished report from Bio/dynamics, Inc., East Millstone,New
Jersey, USA, Project No. 74-1100. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Ishmael, J. & Litchfield, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice.
Fundam. Appl. Toxicol., 11, 308-322.
James, D.A. (1974a) Preliminary foetal toxicity study in the mouse
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/12.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1974b) Preliminary foetal toxicity study in the rat given
21Z73 (NRDC 143) orally. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/10.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
James, D.A. (1974c) Preliminary foetal toxicity study in the rabbit
given 21Z73 (NRDC 143) orally. Unpublished report from The
Wellcome Foundation Ltd, Beckenham, England, Doc. No. BPAT 74/19.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
James, D.A. (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat. Unpublished report from The Wellcome
Foundation Ltd, Beckenham, England, Doc. No. BPAT 79/3. Submitted
by FMC Corporation, Princeton, New Jersey, USA.
Kalinowski, A.E., Banham, P,B., Chart, I.S., Cook, S.K., Gore, C.W.,
Moreland, S.F. & Woollen, B.H. (1982) Permethrin: One year oral
dosing study in dogs. Unpublished report from Imperial Chemical
Industries Ltd, Alderley Park, England, Report No. CTL/P/647.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Kalter, D.C., Sperber, J., Rosen, T. & Matarasso, S. (1987) Treatment
of pediculosis pubis. Clinical comparison of efficacy and
tolerance of 1% lindane shampoo vs 1% permethrin cream rinse.
Arch. Dermatol., 123, 1315-1319.
Killeen, J.C. & Rapp, W.R. (1974) A four week feeding study of FMC
33297 in rats. Unpublished report from Bio/dynamics Inc., East
Millstone, New Jersey, USA, Project No. 74S-1024. Submitted to
WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1975a) A thirty day pilot feeding study of
FMC 33297 in rats. Unpublished report from Bio/dynamics Inc.,
East Millstone, New Jersey, USA, Project No. 74-1098. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
Killeen, J.C., Jr & Rapp, W.R. (1975b) A fourteen-day dose
range-finding study of FMC 33297 in beagle dogs. Unpublished
report from Bio/dynamics Inc., East Millstone, New Jersey, USA,
Project No. 75-1202B. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Killeen, J.C. & Rapp, W.R. (1976) A three month oral toxicity study of
FMC 33297 in beagle dogs. Unpublished report from Bio/dynamics
Inc., East Millstone, New Jersey, USA, Project No. 75-1188B.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA
and by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Kolmodin-Hedman, B., Swensson, Å. & Åkerbolm, N. (1982) Occupational
exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50, 27-33.
Lawrence, L.J. & Casida, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-activity relationships. Pestic.
Biochem. Physiol., 18, 9-14.
Leng, G., Kuhn, K.H. & Idel, H. (1997) Biological monitoring of
pyrethroids in blood and pyrethroid metabolites in urine:
applications and limitations. Sci. Total Environ., 199,
173-181.
Le Quesne, P.M., Maxwell, I.C. & Butterworth, S.T.G. (1980) Transient
facial sensory symptoms following exposure to synthetic
pyrethroids: A clinical and electrophysiological assessment.
Neurophysiology, 2, 1-11.
Litchfield, M.H. (1985) Toxicity in mammals. In: Leahey, J.P., ed.,
The Pyrethroid Insecticides, London: Taylor & Francis, pp.
99-150.
Llewellyn, D.M., Brazier, A., Brown, R., Cocker, J., Evans, M.L.,
Hampton, J., Nutley, B.P. & White, J. (1996) Occupational
exposure to permethrin during its use as a public hygiene
insecticide. Ann. Occup. Hyg., 40, 499-509.
Marowitz, L.A. (1974a) Comparative acute oral toxicity in mice with
FMC 33297, FMC 37400, FMC 35171 and FMC 30960. Unpublished report
from Bio/dynamics Inc., East Millstone, New Jersey, USA, Project
Nos. 2184-74, 2185-74, 2222-74 and 2238-74. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Marowitz, L.A. (1974b) Acute oral toxicity in rats. Compound No. FMC
33297. Unpublished report from Bio/dynamics Inc., East Millstone,
New Jersey, USA, Project No. 2186-74. Submitted to WHO by FMC
corporation, Princeton, New Jersey, USA.
McGregor, D.B. & Wickramaratne, G.A. (1976) Teratogenicity study in
rats of ICI-PP 557. Unpublished report from Inveresk Research
International, Edinburgh, Scotland, Project No. 404898. Submitted
to FMC Corporation, Princeton, New Jersey, USA
Mehr, Z.A., Justus, J.D., Gupta, R.K. & Korte, D.W., Jr (1988)
Mutagenic potential of permethrin in the Drosophila
melanogaster sex-linked recessive lethal test. Unpublished
report from Letterman Army Institute of Research, Presidio of San
Francisco, California, USA, Report No. 302. Submitted to WHO by
FMC Corporation, Princeton, New Jersey, USA
Metker, L., Angerhofer, R.A., Pope, C.R. & Swentzel, K.C. (1977)
Toxicological evaluation of 3-(phenoxyphenyl)methyl
(+)- cis, trans-3,2-(2,2-dichloroethenyl)-2,2dimethylcyclopropa
necarboxylate (permethrin). Unpublished report from the US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 51-0831-78.
Milner, C.K. & Butterworth, S.T.G. (1977) Toxicity of pyrethroid
insecticides: Investigation of the neurotoxic potential of WL
43479 to adult hens. Unpublished report from Shell Toxicology
Laboratory, Sittingbourne, England, Report No. TLGR.OO69.77.
Submitted to WHO by Mitchell Cotts Chemicals, Mirfield, England.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Nassif, M., Brooke, J.P., Hutchinson, D.B.A., Kamel, O.M. & Savage,
E.A. (1980) Studies with permethrin against bodylice in Egypt.
Pestic. Sci., 11, 679-684.
Pednekar, M.D., Gandhi, S.R. & Netrawali, M.S. (1987) Evaluation of
mutagenic activities of endosulfan, phosalone, malathion, and
permethrin, before and after metabolic activation, in the Ames
Salmonella test. Bull. Environ. Contam.Toxicol., 38, 925-933.
Pluijmen, M., Drevon, C., Montesano, R., Malaveille, C., Hautefeuille
& Bartsch, H. (1984) Lack of mutagenicity of synthetic
pyrethroids in Salmonella typhimurium strains and in V79
Chinese hamster cells. Mutat. Res., 137, 7-15.
Rapp, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC 33297 in mice. Histopathology report. Unpublished report
from McConnell & Rapp, Flemington, New Jersey, USA, FMC
Identification No. ACT 605.35. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Reynolds, J., Piercy, D.W.T., Clampitt, R.B., James, J.A., Thompson,
P.M., Farebrother, D.A. & Dayan, A.D. (1978) Permethrin oral
administration to dogs for 6 months. Unpublished report from The
Wellcome Foundation Ltd, Berkhamsted Hill, England, Doc. No. HEFG
78-14. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
van der Rhee, H.J., Farquhar, J.A. & Vermeulen, N.P.E. (1989) Efficacy
and transdermal absorption of permethrin in scabies patients.
Acta Dermatol. Venereol., 69, 170-182.
Richards, D., Banham, P.B., Chart, I.S., Glaister, J.R., Gore, C.W.,
Pratt, I., Taylor, K. & Weight, T.M. (1977) PP557: Two year
feeding study in rats. Unpublished report from Imperial Chemical
Industries, Ltd, Alderley Edge, England, Report No. CTL/P/357.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Richards, D., Banham, P.B., Kilmartin, M. & Weight, T.M. (1980)
Permethrin: teratogenicity study in the rabbit. Unpublished
report from Imperial Chemical Industries Ltd, Alderley Edge,
England, Report No. CTL/P/523. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
Ross, D.B., Roberts, N.L., Cameron, M.McD., Prentice, D.E. & Cooke, L.
(1977) Examination of permethrin for neurotoxicity in the
domestic hen. Unpublished report from Huntingdon Research Centre,
Huntingdon, England, Doc. No. ICI/157-NT/77468. Submitted to WHO
by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980a) Acute oral toxicity in rats. Test article: FMC 33297
55/45. Unpublished report from Cosmopolitan Safety Evaluation
Laboratory, Inc., Somerville, New Jersey, USA, Study No. 0245A.
submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Sauer, C. (1980b) Acute dermal toxicity study -- Rabbit LD50 test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245B. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980c) Primary dermal irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory Inc., Somerville, New Jersey, USA,
Study No. 0245D. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Sauer, C. (1980d) Primary eye irritation study in rabbits. Test
article: FMC 33297 55/45. Unpublished report from Cosmopolitan
Safety Evaluation Laboratory, Inc., Somerville, New Jersey, USA,
Study No. 0245C. Submitted to WHO by FMC Corporation, Princeton,
New Jersey, USA.
Schroeder, R.E. & Rinehart, W.E. (1977) A three generation
reproduction study of FMC 33297 in rats. Unpublished report from
Bio/dynamics Inc., East Millstone, New Jersey, USA. Submitted to
WHO by Mitchell Cotts Chemicals Ltd, Mirfield, England.
Shah, P.V., Monroe, R.J. & Guthrie, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. Appl.
Pharmacol., 59, 414-423.
Simmon, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC Corporation compound. Unpublished report from Stanford
Research Institute, Menlo Park, California, USA, Project No.
LSC-4768. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Simmon, V.F., Riccio, E.S., Robinson, D.E. & Mitchell, A.D. (1979) In
vitro microbiological mutagenicity and unscheduled DNA synthesis
studies of fifteen pesticides. Unpublished report from SRI
International, Menlo Park, California, USA, Project No. LSU-3493.
Submitted to WHO by FMC Corporation, Princeton, New Jersey, USA.
Skidmore, M. (1996) Permethrin: Supplemental information on MRID Nos.
42410001 and 43505201 submitted in response to EPA CBRS review
dated 5 September 1995. Goat metabolism study -- oral dosing.
Unpublished report from Zeneca Ag Products, Bracknell, England,
Project No. HRC/ISN 248/920216sup1. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA.
Soderlund, D.M. & Casida, J.E. (1977) Effects of pyrethroid structure
on rates of hydrolysis and oxidation by mouse liver microsomal
enzymes. Pest. Biochem. Physiol., 7, 391-401.
Staatz, C.G., Bloom, A.S. & Lech, J.J. (1982) A pharmacological study
of pyrethroid neurotoxicity in mice. Pest. Biochem. Physiol.,
17, 287-292.
Surrallès, J., Xamena, N., Creus, A., Catalan, J., Norppa, H. &
Marcos, R. (1995a) Induction of micronuclei by five pyrethroid
insecticides in whole-blood and isolated human lymphocyte
cultures. Mutat Res., 341, 169-184.
Surrallès, J., Xamena, N., Creus, A. & Marcos, R. (1995b) The
suitability of the micronucleus assay in human lymphocytes as a
new biomarker of excision repair. Mutat Res., 342, 43-59.
Tierney, W.J. & Rinehart, W.E. (1979) A twenty-four month oral
carcinogencicity study of FMC 33297 in mice. Unpublished report
from Bio/dynamics, East Millstone, New Jersey, USA, Project No.
76-1695. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
United States Army (1978) Subchronic inhalation toxicity of
3-(phenoxyphenyl) methyl
(+) cis, trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane
carboxylate (permethrin). Unpublished report from US Army
Environmental Hygiene Agency, Aberdeen Proving Ground, Maryland,
USA, Study No. 75-51-0026-80. Submitted to WHO by FMC
Corporation, Princeton, New Jersey, USA and by Mitchell Cotts
Chemicals Ltd, Mirfield, England.
United States Army (1986) Neurotoxicity in rats following subchronic
ingestion of permethrin-treated food. Unpublished report from US
Army Environmental Hygiene Agency, Aberdeen Proving Ground, Study
No. 75-51-0351-87. Submitted to WHO by FMC Corporation,
Princeton, New Jersey, USA.
Wallwork, L.M., Poll, G.S. & Malone, J.C. (1975) Effect on the rat
oral toxicity of changes in the cis/ trans ratio with 21Z73
(NRDC 143) series. Unpublished report from The Wellcome
Foundation, Berkhamsted, England, Doc. No. HEFG 75-5. Submitted
to WHO by FMC Corporation, Princeton, New Jersey, USA.
WHO (1999) Recommended Classification of Pesticides by Hazard and
Guidelines to Classification 1998-1999 (WHO/PCS/98.21/Rev. 1),
Geneva, International Programme on Chemical Safety.
Williams, L.M., Thomson, P.M., Clampitt, R.B. & Malone, J.C. (1976b)
Rat oral 90 day toxicity study. Unpublished report from The
Wellcome Foundation, Berkhamsted and Beckenham, England, Doc. No.
HEFG 76-1. Submitted to WHO by FMC Corporation, Princeton, New
Jersey, USA.
Williams, L.M., Clampitt, R.D., James, J.A., Reynolds, J. & Woods,
N.C. (1976a) Rat oral 90 day toxicity study. Unpublished report
from TheWellcome Foundation, Berkhamsted, England, Doc. No. HEFG
76-3. Submitted to WHO by FMC Corporation, Princeton, New Jersey,
USA.
Woodruff, R.C., Phillips, J.P. & Irwin, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster.
Environ. Mutag., 5, 835-846.
