
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
PERMETHRIN
Explanation
Permethrin was first evaluated in 1979 when a temporary ADI was
proposed and recommendations were made for MRLs in a wide range of raw
agricultural commodities.* In 1980, the Meeting considered post-
harvest uses of permethrin as a grain protectant insecticide and
recommended MRLs for cereal grains and milled cereal products.
In 1979 further information was required or desired on potential
bioaccumulation of the compound and/or its metabolites; observations
in humans to evaluate possible susceptibility to neurological effects
noted in rodents; results of additional supervised residue trials on
oranges and other citrus varieties in representative citrus-growing
countries; results of additional residue trials on spinach and other
leafy vegetables, meat, milk and eggs; information on world-wide good
agricultural practices (i.e. authorized national use patterns);
information on any future changes in manufacturing processes that
substantially alter the ratio of cis : trans isomers in the technical
grade product; characterization studies on the photodecomposition
products; selected surveys of residues in crops known to have been
treated under practical circumstances.
A number of other matters, outstanding in 1979, were dealt with
at the 1980 Meeting.
Information has been provided to enable the Meeting to consider
each of these topics and several questions that arose at the 13th
Session of CCPR.
Information on identity and properties
The 1979 Meeting listed as Required "Information on any future
changes in manufacturing processes which substantially alter the ratio
of cis- and trans- isomers in the technical grade product". There has
been no change since 1979 in the material sold by the four principal
manufacturers and it is not anticipated that there will be a change in
the foreseeable future. Material of approximately 40:60 cis:trans
isomer ratio is produced most readily by the manufacturing procedures
being used by most of the major manufacturers internationally.
The Meeting recognized that there are at least two manufacturers
producing permethrin with a 25:75 cis:trans ratio and information on
the properties, fate and residues of such isomeric mixture has been
received. The Meeting is therefore able to consider the consequences
of such isomeric mixtures being applied to livestock and starch grain.
* See Annex II for FAO and WHO documentation.
In this review, as in 1979 and 1980, the term "permethrin" refers
to material which is nominally 40:60 (±) cis:trans permethrin, unless
otherwise indicated.
Permethrin (25:75) contains not less than 93% of permethrin with
a cis:trans isomer ratio of approximately 25:75. The Meeting received
details of the composition and concentration of impurities in the
technical material together with relevant specifications and methods
of analysis.
Rickett (1981) reviewed the available information on the
mechanisms and conditions required to obtain isomerization of
permethrin. The following conclusions have been drawn:
1. Interconversions of permethrin isomers involve fission of the 1,3
bond of the cyclopropane ring via a triplet excited state with
energy greater than 60 kcal -1.
2. In solution, the rate of isomerization is solvent-dependent and
is fastest in water. The rate can generally be increased by
adding triplet sensitizers, such as benzophenone or acetone.
3. Isomerization is always accompanied by degradation of the
permethrin. Under field conditions, degradation is a much faster
process and so isomerization is not of practical significance.
4. In the absence of light, no isomerization takes place.
5. When changes is isomer ratios are observed under dark conditions,
these can be explained usually by the lower stability of the
trans isomer to hydrolysis.
Rickett and Knight (1976) studied the photostability of the cis-
and trans- isomers of permethrin and reported that when trans-
permethrin (NRDC 147), cis-permethrin (NRDC 167) and two samples of
the racaemic mixture of varying cis:trans ratio (NRD 143) were coated
onto glass plates at 1g/m2 and exposed to continuous irradiation of
44 000 lux, degradation of cis/trans isomers occurred, tending towards
and equilibrium value of 40:60. This was accompanied by racemization.
Photodegradation in hexane solution produced similar
interconversion. The rate of degradation was markedly reduced when air
was excluded from the solution. A gas chromatography method for
measuring the enantiomeric purity of the acid moiety is described.
Morgan (1979) studied the fate of the two optical isomers
following application of a 25:75 mixture to the hair of cows.
Permethrin (0.1%) was applied to three Friesian cows at a nominal
rate of 0.5 l per animal, using a knapsack sprayer. Analysis of hair
samples collected 1, 7, 14 and 21 days after treatment showed no
significant change in the 25:75 cis/trans isomer ratio of permethrin.
DATA FOR THE ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
Studies have been performed in vivo on a wide range of
mammalian species in order to define better the pharmacokinetics of
permethrin. An oral dose of the compound is quickly absorbed and
extensively metabolized to polar materials, which are rapidly
excreted. Only very small amounts of chemical are taken up by adipose
tissue, and then principally as the less rapidly hydrolysed cis-
isomer of permethrin. On cessation of exposure, permethrin is
eliminated promptly from fat tissues.
Rat
Orally-administered permethrin was taken up rapidly by rats.
After a single dose of 10 mg/kg of 14C-labelled permethrin, a peak
level of radioactivity in blood was observed within 1.5 h, after which
it declined. The half-life of elimination of radioactivity from blood
was approximately 7 h. More than 80% of the orally-administered
radioactivity was excreted in urine and faeces in 48 h, and at least
92% was excreted after 7 days. Differences were noted in the pattern
of excretion of the two isomers: 79 to 82% of the radiolabelled dose
of the more readily hydrolysed trans isomer was excreted in the urine
within 12 days, but only 52 to 54% of the cis isomer was excreted in
the same time.
The major metabolites of permethrin in the rat are excreted in
the urine and are derived from ester cleavage. There are the cis and
trans isomers of 3-(3,3-dichlorovinyl)-2,2-dimethylcyclopropane
carboxylic acid (DCVA), 3-phenoxybenzoic acid and 3-(4-hydroxy-
phenoxy)benzoic acid. These are all excreted principally as polar
conjugates. Hydroxylation at other positions in the 3-phenoxybenzyl
moiety and of the methyl groups attached to the cyclopropane rings
represent only minor routes of metabolism. Only a small proportion of
orally administered permethrin is excreted unchanged (6% in the case
of cis, 3% in the case of trans) and then only in the faeces (FAO/WHO,
1980).
In a bioaccumulation study, female rats were dosed orally with
40:60 cis:trans 14C-cyclopropyl-labelled permethrin daily at
0.8 mg/kg bw/day for up to 21 days. This dose level is equivalent to
approximately 20 ppm in the diet. Groups of three rats were sacrificed
weekly. At the end of three weeks, dosing was terminated and groups of
three animals sacrificed after a further one or two weeks. The highest
mean level of radioactivity found in liver during the dosing period
was 0.22 µg permethrin equivalents/g, and this declined to a non-
detectable level within a week of cessation of dosing (limit of
determination: 0.08 µg permethrin equivalents/g). Levels of
radioactivity in kidney and brain were below 0.08 µg/g throughout the
study. The highest levels of radioactivity found were in adipose
tissues - up to 0.72 µg permethrin equivalents/g. These declined to a
non-detectable level (< 0.08 µg/g) within two weeks after cessation
of dosing (Bratt et al 1977).
Another group of rats was dosed orally with 40:60 cis:trans
14C-methylene-labelled permethrin daily at a dose level of 1 mg/kg
bw/day for up to eleven weeks. Levels of radioactivity in adipose
tissue reached a plateau of approximately 1 to 2 µg permethrin
equivalents/g after three weeks. Approximately 87% of the
radioactivity in the fat was due to permethrin, present principally as
the cis isomer. The half-life of the material in adipose tissue was
about 18 days and elimination was complete within 7 weeks of the
termination of dosing. Plateau levels of 0.2 to 0.7 µg permethrin
equivalents/g in liver and kidney declined to below 0.08 µg/g within a
week of cessation of dosing. Levels of radioactivity in muscle
remained below 0.08 µg/g throughout the study (Bratt et al 1977).
Studies to demonstrate the differences and similarities in
absorption or distribution between the cis- and trans- isomers of NRDC
143, utilizing whole body and radiography in male rats, have been
described (Fairbrother 1977). Following single oral administration of
14C-labelled cis and trans isomers in maize oil, the trans isomer
was better absorbed from the gut than the cis isomer (the blood level
being about 5 times higher 2 h after dosing). Both isomers tended to
remain in the blood; tissues with high blood volumes had the highest
levels of radioactivity. Radioactivity persisted in storage and depot
fat, the cis isomer being taken up more than the trans. Both isomers
were seen in the meninges, but only cis isomer appeared in small
amounts in CNS tissue. Both isomers provided higher levels of
radioactivity 6 h after dosing. Both isomers were excreted in the
urine, but higher concentrations of the cis isomer were found in the
bile. Although the tissue levels as measured by radiometry suggested
that the thymus contained activity, at no time was activity detected
by autoradiography.
The residence time in the body and the metabolic fate of both the
acid and alcohol moieties of (1R, trans)-, (IRS, trans)-, (IR, cis)-
and (IRS, cis)- permethrin were elaborated after these esters were
administered to male albino Sprague-Dawley rats at dosages ranging
from 1.6 to 4.8 mg/kg (Gaughan et al 1977). These trans- and cis-
isomers of permethrin and of their metabolites were almost completely
eliminated from the body within a few days. About 3 and 6% of the
trans- and cis-permethrin doses were excreted in the faeces without
metabolism, due either to incomplete absorption from the G.I. tract or
to enterohepatic circulation. Metabolites retaining the ester linkage
further established the greater in vivo lability of the ester
linkage in the trans- compared with cis-permethrin. The cis- compound
yielded four faecal metabolites, which resulted from hydroxylation at
the 2'-phenoxy, 4'-phenoxy, of 2-trans-methyl position or at both
of the latter two sites. Other significant metabolites were
3-phenoxybenzoic acid (free and glucuronide and glycine conjugates),
the sulphate conjugate of 4'-hydroxy-3-phenoxybenzoic acid, and
sulphate conjugate of 2'-hydroxy-3-phenoxybenzoic acid (from cis-
permethrin only), the trans- and cis-dichlorovinyldimethylcyclopropane
carboxylic acids (free and glucuronide conjugates) and the 2-trans-and
2-cis-hydroxymethyl derivative of each of the aforementioned trans and
cis acids 1 (free and glucuronide conjugates).
Figure 1 illustrates the metabolic pathways for trans-permethrin
and cis-permethrin after oral administration to rats. This figure
depicts the products originating from the (1R)-esters, but the same
products, in nearly the same proportions, result from administration
of the (IRS)-esters. As the rate of mouse microsomal metabolism of
trans-permethrin and cis-permethrin by esterase, oxidase and combined
esterase and oxidase systems is quite similar for the (1R)- and (1S)-
and (1RS)-ester (Soderung and Casida 1976), it was suggested by
Gaughan et al (1977) that it is also likely that (1S, trans)-
permethrin and (1S,cis)-permethrin were also metabolized by the
pathways shown in Figure 1.
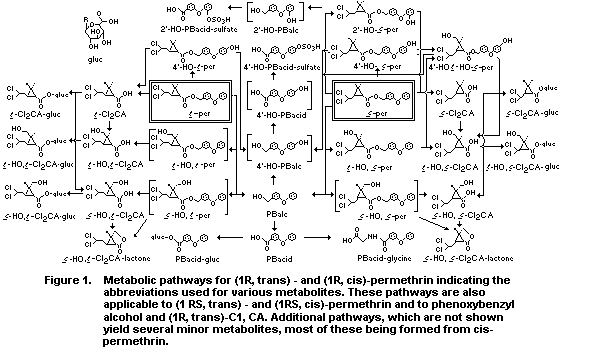 The metabolism of the (1R, trans)- and (1R, cis)-ester of the
active isomers of permethrin following oral administration of 0.5 to
2.9 mg/kg to male Sprague-Dawley rats was examined using compounds
labelled with 14C in the acid or alcohol moieties (Elliot et al
1976). Preliminary results indicated that the organochlorine moiety of
(1R,trans)-and (1R, cis)-permethrin and the (1R, trans)-dichlorovinyl
acid was rapidly and almost completely eliminated from the body and
only traces remained in the fat and liver 4 days after oral
administration.
This ease of elimination is associated with the increased
polarity of the products, which results from rapid in vivo
glucuronidation of the dichlorovinyl acids and, to a lesser extent,
hydroxylation of one of the germinal dimethyl groups. It appeared that
at least some of the hydroxylated acids underwent minor degrees of
conjugation. Much less hydroxylated derivative was formed from the
(1R, trans)-dichlorovinyl acid itself than from the (1R, trans)-
permethrin, indicating that permethrin was hydroxylated to some extent
before hydrolysis. The predominant sites of hydroxylation in the
dichlorovinyl acid appear to be the 2-cis position for (1R, trans)-
permethrin and the 2-trans position for (1R, cis)-permethrin.
(Presumably these methyl groups are sterically favoured at the
hydroxylation site of the microsomal oxidase system.) Neither the
parent isomer nor any metabolites of permethrin remained for an
unusual period or in an unexpected locations in the organs examined.
Four days after treatment, the residues of permethrin and its
metabolites in tissues, determined as the total radiocarbon by
combustion, were below 0.01 ppm permethrin equivalents in blood, bone,
brain, fat, heart, kidney, liver, lungs, muscle, spleen and testes.
The largest persistence was for products derived from the 3-phenoxy-
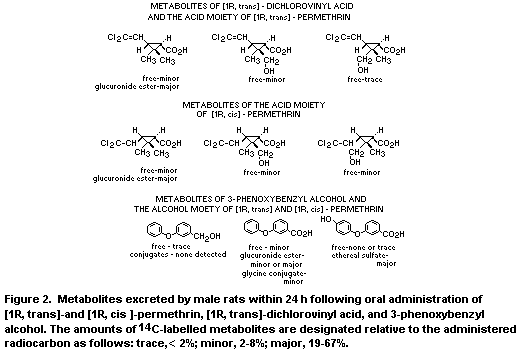
benzyl alcohol in the fat, liver and kidney. Figure 2 illustrates the
structures and relative proportions of the metabolites of (1R, trans)-
and (1R, cis)-permethrin, of (1R,trans)dichlorovinyl acid and of
3,phenoxybenzyl alcohol.
The metabolism of the (1R, trans)- and (1R, cis)-ester of the
active isomers of permethrin following oral administration of 0.5 to
2.9 mg/kg to male Sprague-Dawley rats was examined using compounds
labelled with 14C in the acid or alcohol moieties (Elliot et al
1976). Preliminary results indicated that the organochlorine moiety of
(1R,trans)-and (1R, cis)-permethrin and the (1R, trans)-dichlorovinyl
acid was rapidly and almost completely eliminated from the body and
only traces remained in the fat and liver 4 days after oral
administration.
This ease of elimination is associated with the increased
polarity of the products, which results from rapid in vivo
glucuronidation of the dichlorovinyl acids and, to a lesser extent,
hydroxylation of one of the germinal dimethyl groups. It appeared that
at least some of the hydroxylated acids underwent minor degrees of
conjugation. Much less hydroxylated derivative was formed from the
(1R, trans)-dichlorovinyl acid itself than from the (1R, trans)-
permethrin, indicating that permethrin was hydroxylated to some extent
before hydrolysis. The predominant sites of hydroxylation in the
dichlorovinyl acid appear to be the 2-cis position for (1R, trans)-
permethrin and the 2-trans position for (1R, cis)-permethrin.
(Presumably these methyl groups are sterically favoured at the
hydroxylation site of the microsomal oxidase system.) Neither the
parent isomer nor any metabolites of permethrin remained for an
unusual period or in an unexpected locations in the organs examined.
Four days after treatment, the residues of permethrin and its
metabolites in tissues, determined as the total radiocarbon by
combustion, were below 0.01 ppm permethrin equivalents in blood, bone,
brain, fat, heart, kidney, liver, lungs, muscle, spleen and testes.
The largest persistence was for products derived from the 3-phenoxy-
benzyl alcohol in the fat, liver and kidney. Figure 2 illustrates the
structures and relative proportions of the metabolites of (1R, trans)-
and (1R, cis)-permethrin, of (1R,trans)dichlorovinyl acid and of
3,phenoxybenzyl alcohol.
 Mouse
Groups of 10 male and 10 female mice were fed permethrin in the
diet for 4 weeks at dosage levels of 0, 20, 500 and 4 000 ppm to
compare residue concentrations in adipose tissue with data obtained
from animals fed similar concentrations for 80 weeks. Residue levels
were consistently higher in females than in males. The residue levels
in peritoneal adipose tissue were essentially the same as those seen
in animals fed for over 80 weeks. There was a rapid build-up to
equilibrium levels in mice within 4 weeks of dietary exposure (FAD/WHO
1980).
Dog
Dogs were dosed orally with 40:60 cis:trans 14C-methylene-
labelled permethrin daily at 1 mg/kg bw/day for 10 days. This dose
level is equivalent to approximately 40 ppm in the diet. Levels of
radioactivity in adipose tissue 24 h after termination of dosing did
not exceed 6 ug permethrin equivalents/g. Radioactivity in the fat was
present as permethrin (Bratt and Slade 1977).
Cow
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bw, equivalent to approximately 80 ppm in the diet,
levels of radioactivity in milk reached a maximum of 0.13 µg
permethrin equivalents/g after 1 to 2 days. These declined to less
than 0.02 µg/g after 7 days. Levels of radioactivity in the fat were
0.12 to 0.18 µg permethrin equivalents after 7 days. By 14 days these
had declined to 0.05-0.06 µg/g, indicating that the small residues in
fat are also not maintained on cessation of dosing (Bewick and Leahey
1976).
Radiocarbon from 14C-acid- and 14C-alcohol-labelled preparations
of trans- and cis-permethrin administered orally to lactating Jersey
cows for 3 consecutive days at approximately 1.0 mg/kg was largely
eliminated from the body within 12 or 13 days after the initial
treatment (Gaughan et al 1978a). Milk and fat residues were
relatively low. Total 14C-permethrin equivalents in milk were
consistently below 0.3 µg/g and declined to less than 20% of their
highest values during the 10 days following cessation of exposure.
Residues recovered from fat and milk were higher when cis-permethrin,
rather than trans-permethrin, was administered and consisted almost
entirely (>85%) of unmetabolized permethrin, as well as trace levels
of cis-permethrin hydroxylated at the methyl group trans to the
ester functionality. Major excreted metabolites (each 8 to 28% of
the administered radiocarbon) from both isomers were: the esters
hydroxylated at the trans-methyl group; the acid moieties hydroxylated
at the cis-methyl group and the corresponding gamma-lactones;
3-phenoxybenzyl alcohol; the glutamic acid conjugate of
3-phenoxybenzoic acid. Additionally, 13 excreted metabolites of trans-
permethrin and 10 of cis-permethrin were tentatively identified. Total
14C-permethrin equivalents in blood reached a transient peak shortly
after each dose and dropped to insignificant levels within 2 to 4 days
after the last dose. Figure 3 illustrates the metabolic pathways to
convert trans- and cis-permethrin into more polar derivatives for
excretion. The permthrin isomers, although fat-soluble materials, were
rapidly metabolized and excreted by cows so that relatively little of
these compounds appeared in milk or were retained in tissues for more
than a few days.
Mouse
Groups of 10 male and 10 female mice were fed permethrin in the
diet for 4 weeks at dosage levels of 0, 20, 500 and 4 000 ppm to
compare residue concentrations in adipose tissue with data obtained
from animals fed similar concentrations for 80 weeks. Residue levels
were consistently higher in females than in males. The residue levels
in peritoneal adipose tissue were essentially the same as those seen
in animals fed for over 80 weeks. There was a rapid build-up to
equilibrium levels in mice within 4 weeks of dietary exposure (FAD/WHO
1980).
Dog
Dogs were dosed orally with 40:60 cis:trans 14C-methylene-
labelled permethrin daily at 1 mg/kg bw/day for 10 days. This dose
level is equivalent to approximately 40 ppm in the diet. Levels of
radioactivity in adipose tissue 24 h after termination of dosing did
not exceed 6 ug permethrin equivalents/g. Radioactivity in the fat was
present as permethrin (Bratt and Slade 1977).
Cow
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bw, equivalent to approximately 80 ppm in the diet,
levels of radioactivity in milk reached a maximum of 0.13 µg
permethrin equivalents/g after 1 to 2 days. These declined to less
than 0.02 µg/g after 7 days. Levels of radioactivity in the fat were
0.12 to 0.18 µg permethrin equivalents after 7 days. By 14 days these
had declined to 0.05-0.06 µg/g, indicating that the small residues in
fat are also not maintained on cessation of dosing (Bewick and Leahey
1976).
Radiocarbon from 14C-acid- and 14C-alcohol-labelled preparations
of trans- and cis-permethrin administered orally to lactating Jersey
cows for 3 consecutive days at approximately 1.0 mg/kg was largely
eliminated from the body within 12 or 13 days after the initial
treatment (Gaughan et al 1978a). Milk and fat residues were
relatively low. Total 14C-permethrin equivalents in milk were
consistently below 0.3 µg/g and declined to less than 20% of their
highest values during the 10 days following cessation of exposure.
Residues recovered from fat and milk were higher when cis-permethrin,
rather than trans-permethrin, was administered and consisted almost
entirely (>85%) of unmetabolized permethrin, as well as trace levels
of cis-permethrin hydroxylated at the methyl group trans to the
ester functionality. Major excreted metabolites (each 8 to 28% of
the administered radiocarbon) from both isomers were: the esters
hydroxylated at the trans-methyl group; the acid moieties hydroxylated
at the cis-methyl group and the corresponding gamma-lactones;
3-phenoxybenzyl alcohol; the glutamic acid conjugate of
3-phenoxybenzoic acid. Additionally, 13 excreted metabolites of trans-
permethrin and 10 of cis-permethrin were tentatively identified. Total
14C-permethrin equivalents in blood reached a transient peak shortly
after each dose and dropped to insignificant levels within 2 to 4 days
after the last dose. Figure 3 illustrates the metabolic pathways to
convert trans- and cis-permethrin into more polar derivatives for
excretion. The permthrin isomers, although fat-soluble materials, were
rapidly metabolized and excreted by cows so that relatively little of
these compounds appeared in milk or were retained in tissues for more
than a few days.
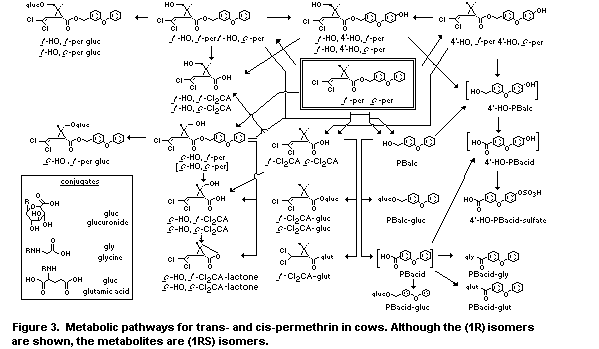 Table 1 lists the radiocarbon recovery in excreta, milk, tissues,
organs and gut contents 12 or 13 days after treatment of three daily
doses of (14C)acid- or (14C)alcohol-trans-and cis-permethrin at
approximately 1 mg/kg for each dose.
TABLE 1. Radiocarbon recovery in excreta, milk, tissues, organs and
gut contents 12 or 13 days after initiating a treatment
schedule consisting of three daily doses of (14C)acid- or
(14C)alcohol-trans- and cis-permethrin at 1 mg/kg for
each dose
Recovery of administered radiocarbon(%)
Sample Trans Cis
Acid Alcohol Acid Alcohol
Urine 38.98 46.71 28.46 22.22
Faeces 51.60 57.24 60.06 75.85
Carbon dioxide1 <0.01 <0.01 <0.01
<0.01
Milk 0.03 0.44 0.26 0.18
Fat 0.15 0.40 1.59 0.64
Liver 0.03 0.04 0.08 0.06
Muscle 0.01 0.04 0.03 0.11
Skin 0.01 0.07 0.04 0.06
Other tissues 0.04 0.02 0.03 0.04
Gut contents <0.01 2.83 0.15 0.05
Total 90.85 107.79 90.70 99.21
1 Collected for 6 days only (includes bile). (Gaughan et al 1978).
Studies in which non-radiolabelled permethrin was administered to
cows support the finding that permethrin itself, rather than its polar
metabolites, consistitutes the predominant part of the small residues
present in milk and fat. Groups of three cows were maintained for 28
to 31 days on diets containing 40:60 cis:trans permethrin at 0.2, 1.0,
10, 50 and 150 ppm in the diet. Two of the cows were then sacrificed
and the third returned to control diet for a further 8 to 9 days
before sacrifice. Permethrin itself constituted more than 80% of the
residue determined in the milk. Mean plateau levels were below
0.01 µg/g at the 0.2 and 1.0 ppm dietary inclusion rates, 0.02 µg/g at
the 10 ppm rate, 0.1 µg/g at the 50 ppm rate and 0.3 µg/g at the
150 ppm rate. Milk was analysed for DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid. The highest metabolite residue detected in the
milk was 0.03 µg/g at the 150 ppm dose rate. In all cases, residue
levels in milk did not accumulate over the period of the study and
they declined rapidly, on returning animals to untreated diet, to
below 0.01 µg/g within five days (Edwards and Iswaran 1977; Swaine and
Sapiets 1981 a,b).
Permethrin itself constituted more than 85% of the residue
determined in adipose tissue (Table 2). Levels in peritoneal fat were
somewhat higher than those in subcutaneous fat, but were still small
(Tables 2 and 3). The small residues in adipose tissue declined
noticeably on cessation of exposure (Table 2).
TABLE 3. Residues in fat of cows receiving permethrin at
rates up to 150 ppm in the diet
Nominal Residues (µg/g) of permethrin in
dietary inclusion
level (ppm) Peritoneal fat Subcutaneous fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
150 2.8 -6.2 0.96-4.3
Permethrin itself was also the major component of the small
residues determined in adductor, pectoral and cardiac muscle. The
residue levels present were approximately 0.1 to 0.2% of corresponding
dietary inclusion levels. As found separately by Leahey et al
(1977) in the goat (see below), DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were major constituents of the residues in liver
and kidney. As with milk and fat residue levels in muscle, liver and
kidney, residues declined rapidly on cessation of exposure of the
animals to permethrin (Edwards and Iswaran 1977; Swaine and Sapiets
1981 a,b).
Goat
Cis-(IRS) and trans-(IRS)-permethrin, radiolabelled with 14C in
either the acid or alcohol moiety, were rapidly metabolized and
excreted after oral administration to lactating Nubian and Nubian-
Saanen cross goats (Ivie and Hunt 1980). In these studies, each of
four goats received 10 successive daily oral doses of one of the four
(14C)-permethrins; each dose ranged from 0.2 to 0.3 mg/kg bw/day,
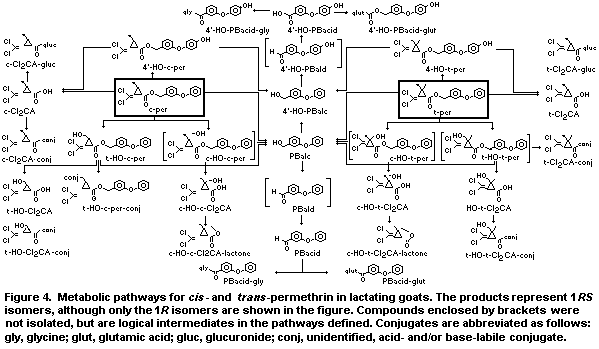
depending on the 14C label and isomer given. Twenty six metabolites
of the permethrin isomers were fully or partly characterized by TLC or
GLC/MS, and several others (enclosed by brackets in Figure 4) although
not isolated are logical intermediates in the pathways defined. In the
TABLE 2. Residues of permethrin and three major metabolites in tissues of cows receiving
permethrin at 150 ppm in the diet
Residue (µg/g) of
Tissue Feeding
analysed regime1 Permethrin Cis + 3-BPAlc 3-PBAcid
Trans DCVA
(I and II) (III) (IV)
Muscle Treated 0.10-0.27 0.04-0.14 0.01-0.12 <0.01-0.05
Treated plus 0.03-0.07 <0.03 <0.01 <0.01
recovery
Subcutaneous Treated 2.9 -4.3 0.14-0.33 0.08-0.24 0.04-0.06
fat
Treated plus 0.96-1.2 <0.01 0.05 0.03-0.04
recovery
Peritoneal Treated 5.4 -6.2 0.16-0.32 0.20-0.29 0.02-0.05
fat
Treated plus 2.8 -3.1 0.09 0.08-0.10 <0.01
recovery
Liver Treated 0.01-0.03 0.18-0.26 0.72-1.1 0.36-0.57
Treated plus <0.01 <0.01 0.01-0.08 0.03-0.08
recovery
Kidney Treated 0.16-0.43 0.37-0.49 0.72-0.91 0.19-0.39
Treated plus 0.04-0.05 <0.01 0.02-0.05 <0.01
recovery
1 Treated - indicates the animals received treated diet for 28-29 days and were then slaughtered.
Treated plus recovery - indicates the animals received treated diet for 28 days and were
then returned to untreated diet for a further 8 days before slaughter.
Table 1 lists the radiocarbon recovery in excreta, milk, tissues,
organs and gut contents 12 or 13 days after treatment of three daily
doses of (14C)acid- or (14C)alcohol-trans-and cis-permethrin at
approximately 1 mg/kg for each dose.
TABLE 1. Radiocarbon recovery in excreta, milk, tissues, organs and
gut contents 12 or 13 days after initiating a treatment
schedule consisting of three daily doses of (14C)acid- or
(14C)alcohol-trans- and cis-permethrin at 1 mg/kg for
each dose
Recovery of administered radiocarbon(%)
Sample Trans Cis
Acid Alcohol Acid Alcohol
Urine 38.98 46.71 28.46 22.22
Faeces 51.60 57.24 60.06 75.85
Carbon dioxide1 <0.01 <0.01 <0.01
<0.01
Milk 0.03 0.44 0.26 0.18
Fat 0.15 0.40 1.59 0.64
Liver 0.03 0.04 0.08 0.06
Muscle 0.01 0.04 0.03 0.11
Skin 0.01 0.07 0.04 0.06
Other tissues 0.04 0.02 0.03 0.04
Gut contents <0.01 2.83 0.15 0.05
Total 90.85 107.79 90.70 99.21
1 Collected for 6 days only (includes bile). (Gaughan et al 1978).
Studies in which non-radiolabelled permethrin was administered to
cows support the finding that permethrin itself, rather than its polar
metabolites, consistitutes the predominant part of the small residues
present in milk and fat. Groups of three cows were maintained for 28
to 31 days on diets containing 40:60 cis:trans permethrin at 0.2, 1.0,
10, 50 and 150 ppm in the diet. Two of the cows were then sacrificed
and the third returned to control diet for a further 8 to 9 days
before sacrifice. Permethrin itself constituted more than 80% of the
residue determined in the milk. Mean plateau levels were below
0.01 µg/g at the 0.2 and 1.0 ppm dietary inclusion rates, 0.02 µg/g at
the 10 ppm rate, 0.1 µg/g at the 50 ppm rate and 0.3 µg/g at the
150 ppm rate. Milk was analysed for DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid. The highest metabolite residue detected in the
milk was 0.03 µg/g at the 150 ppm dose rate. In all cases, residue
levels in milk did not accumulate over the period of the study and
they declined rapidly, on returning animals to untreated diet, to
below 0.01 µg/g within five days (Edwards and Iswaran 1977; Swaine and
Sapiets 1981 a,b).
Permethrin itself constituted more than 85% of the residue
determined in adipose tissue (Table 2). Levels in peritoneal fat were
somewhat higher than those in subcutaneous fat, but were still small
(Tables 2 and 3). The small residues in adipose tissue declined
noticeably on cessation of exposure (Table 2).
TABLE 3. Residues in fat of cows receiving permethrin at
rates up to 150 ppm in the diet
Nominal Residues (µg/g) of permethrin in
dietary inclusion
level (ppm) Peritoneal fat Subcutaneous fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
150 2.8 -6.2 0.96-4.3
Permethrin itself was also the major component of the small
residues determined in adductor, pectoral and cardiac muscle. The
residue levels present were approximately 0.1 to 0.2% of corresponding
dietary inclusion levels. As found separately by Leahey et al
(1977) in the goat (see below), DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were major constituents of the residues in liver
and kidney. As with milk and fat residue levels in muscle, liver and
kidney, residues declined rapidly on cessation of exposure of the
animals to permethrin (Edwards and Iswaran 1977; Swaine and Sapiets
1981 a,b).
Goat
Cis-(IRS) and trans-(IRS)-permethrin, radiolabelled with 14C in
either the acid or alcohol moiety, were rapidly metabolized and
excreted after oral administration to lactating Nubian and Nubian-
Saanen cross goats (Ivie and Hunt 1980). In these studies, each of
four goats received 10 successive daily oral doses of one of the four
(14C)-permethrins; each dose ranged from 0.2 to 0.3 mg/kg bw/day,
depending on the 14C label and isomer given. Twenty six metabolites
of the permethrin isomers were fully or partly characterized by TLC or
GLC/MS, and several others (enclosed by brackets in Figure 4) although
not isolated are logical intermediates in the pathways defined. In the
TABLE 2. Residues of permethrin and three major metabolites in tissues of cows receiving
permethrin at 150 ppm in the diet
Residue (µg/g) of
Tissue Feeding
analysed regime1 Permethrin Cis + 3-BPAlc 3-PBAcid
Trans DCVA
(I and II) (III) (IV)
Muscle Treated 0.10-0.27 0.04-0.14 0.01-0.12 <0.01-0.05
Treated plus 0.03-0.07 <0.03 <0.01 <0.01
recovery
Subcutaneous Treated 2.9 -4.3 0.14-0.33 0.08-0.24 0.04-0.06
fat
Treated plus 0.96-1.2 <0.01 0.05 0.03-0.04
recovery
Peritoneal Treated 5.4 -6.2 0.16-0.32 0.20-0.29 0.02-0.05
fat
Treated plus 2.8 -3.1 0.09 0.08-0.10 <0.01
recovery
Liver Treated 0.01-0.03 0.18-0.26 0.72-1.1 0.36-0.57
Treated plus <0.01 <0.01 0.01-0.08 0.03-0.08
recovery
Kidney Treated 0.16-0.43 0.37-0.49 0.72-0.91 0.19-0.39
Treated plus 0.04-0.05 <0.01 0.02-0.05 <0.01
recovery
1 Treated - indicates the animals received treated diet for 28-29 days and were then slaughtered.
Treated plus recovery - indicates the animals received treated diet for 28 days and were
then returned to untreated diet for a further 8 days before slaughter.
 goat, permethrin was rapidly and extensively degraded via hydrolytic,
oxidative and conjugative reactions, e.g., through hydrolysis of the
ester linkage, hydroxylation of the cis- or trans-methyl of the
germinal dimethyl group and hydroxylation of the 4'-position of the
phenoxybenzyl moiety. Certain of these products were further oxidized
and/or conjugated with glycine, glutamic acid, glucuronic acid, or
other unidentified compounds before excretion.
Unmetabolized permethrin and certain ester metabolites were found
in faeces, milk and fat from the treated goats, but only metabolites
arising from ester hydrolysis were found in the urine.
The patterns of radiocarbon elimination and tissue retention
by these goats were reported earlier by Hunt and Gilbert (1977) and
are briefly summarized in Table 4. Urine was the major route of
radiocarbon excretion in goats treated with (14C-acid) or
(14C-alcohol) preparations of trans-permethrin, but most of the
administered radiocarbon was eliminated via the faeces in cis-
permethrin-treated goats.
TABLE 4. Summary of radiocarbon elimination and residue retention by
lactating goats orally treated for 10 consecutive days with
(14C) alcohol- or (14C)Acid-labelled cis-(1RS)or
trans-(1RS)-permethrin isomers1, 2
Radiocarbon eliminated Tissue residues
Label and isomer cum % dose (ppm)3, 4
faeces urine milk liver kidney fat
(14Cacid)-c-per 67.5 25.8 0.66 0.12 0.05 0.23
(14C-alc)-c-per 51.7 36.4 0.53 0.13 0.05 0.24
(14C-acid)-t-per 15.0 72.1 0.17 0.04 0.03 0.02
(14C-alc)-t-per 12.3 79.4 0.24 0.01 0.03 0.02
1 Data summarized from Hunt and Gilbert (1977).
2 Goats treated with 10 successive daily oral doses of the
appropriate (14C) per isomer (0.2-0.3 mg/kg per d.).
3 Tissues taken 24 h after last dose.
4 Other edible tissues contained lower residues.
Total radioactive residues in milk reached a plateau after
3 days of 0.02 to 0.05 µg permethrin equivalents/g and <0.01 µg/g
respectively for the cis- and trans-isomers. Unmetabolized permethrin
was a major component of the radioactivity in the milk. The goats were
sacrificed 24 h after receiving the final dose. Of the edible tissues
analysed for radiocarbon 24 h after the final permethrin doses, fat,
kidney and liver contained the highest residues. Radiocarbon retained
by the tissues and that secreted into the milk were appreciably higher
in goats treated with cis-permethrin than with trans-permethrin (Table
4, Hunt and Gilbert 1977). Total radioactivity of the fat of the
animals receiving the cis-isomer was ten times higher than those
receiving the more easily hydrolysed trans-isomer and was due mainly
to unmetabolized permethrin (Hunt and Gilbert 1977; Ivie and Hunt
1980).
In another study, goats were orally dosed with 40:60 cis:trans-
14C-labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 ppm in the diet for 7 days. Total
radioactive residues in the milk reached a plateau of 0.02-0.03 µg
permethrin equivalents/g after 5 days. Of this radioactivity, 30 to
50% was associated with the butterfat fraction of the milk in which
total radioactive residues were 0.13-0.27 µg permethrin equivalents/g.
The animals were sacrificed 4 h after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 5. Where
alcohol-labelled permethrin was used, approximately 70% of the
radioactivity in kidney was due to 3-phenoxybenzoic acid plus
3-(4-hydroxyphenoxy)benzyl alcohol. A further 15% was due to
3-phenoxybenzoic acid plus 3-(4-hydroxyphenoxy)benzoic acid. Where
acid-labelled permethrin was used, approximately 10 to 15% of the
label in liver and kidney was due to the cis and trans DCVA,
principally the trans isomer (Leahey et al 1977).
It is important to compare the metabolic fate of permethrin in
the species noted above. With reference to previously published
studies on the fate of (14C)-permethrin in mammals, specifically rats
(Elliott et al 1976; Gaughan et al 1977) and lactating cattle
(Gaughan et al 1978a) the following similarities and differences
between goats and these mammals were noted by Ivie and Hunt (1980).
1) In each of the three species, a greater percentage of an
administered cis-permethrin dose was eliminated in the faeces than was
a trans-permethrin dose, a pattern that appears to be most pronounced
in goats and least in cattle. It was suggested that trans-permethrin
was absorbed more rapidly than cis-permethrin from the G.I. tract, or
alternatively, isomer differences in the rates of biliary excretion of
permethrin and/or its metabolites may account for the above
observations.
2) Although retention of permethrin by tissues and its excretion
into milk of mammals was minimal, cis-permethrin and its metabolites
in rat, lactating goat and lactating cattle were retained by tissues
to a more significant degree than was trans-permethrin.
3) Primary metabolism of permethrin in rats involves attack at five
major sites, including ester cleavage, hydroxylation at the cis- or
trans-methyl of the germinal dimethyl moiety, and hydroxylation at the
2' or 4'- position of the phenoxybenzyl moiety. Ester cleavage and
TABLE 5. Total radiolabelled residues in tissues of goats receiving 14C-labelled permethrin daily orally for 7 to 10 days
Material Dose level Duration of Period between Total residue (µg permethrin equivalents/g)in
administered (ppm in administration last date and
diet) (days) sacrifice (hours) Fat Muscle Liver Kidney
Permethrin -10 7 4 <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis:trans
Permethrin -6 10 24 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin -6 10 24 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
4'-hydroxylation are the major routes of metabolism. Cattle and goats
metabolize permethrin similarly, with the exception that
2'-hydroxylation apparently does not occur in these ruminants.
4) Ester metabolites of permethrin were eliminated primarily through
the faeces of rats, cattle and goats, in contrast to cattle which
eliminated large quantities of ester metabolites of both cis-
permethrin and trans-permethrin in faeces.
5) In each species, conjugation of permethrin metabolites before
urinary excretion was extensive. Rats, cattle and goats eliminated the
acid moiety in urine primarily as conjugates with glucuronic acid. The
alcohol moiety was excreted, mostly as phenoxybenzoic acid glucuronide
or 4'-hydroxyphenoxybenzoic acid-sulphate in rats, but amino acid
conjugates of phenoxybenzoic acid comprised most of the excreted
products in cattle and goats. Although conjugation of phenoxybenzoic
acid with glycine was preferred in goats, conjugation with glutamic
acid was favoured in cattle.
The metabolic fate of permethrin in the goat is similar to that
in the cow. Radioactivity deriving from the oral administration of the
cis-isomer of permethrin is excreted mainly in the faeces, whereas
that deriving from the more readily hydrolysed trans-isomer is
excreted mainly in the urine. Radioactivity in faeces is due
principally to permethrin and, in the case of cis-isomer, to
4'-hydroxypermethrin. the predominant urinary metabolites are DCVA and
3-phenoxybenzoic acid, which are excreted mainly as polar conjugates
(Ivie and Hunt 1980).
The major products of the metabolism of permethrin in the cow are
similar to those in the rat. The most notable differences are that in
the cow a greater percentage of the administered radioactivity is
excreted in faeces as intact ester metabolites, notably as 4'-
hydroxypermethrin. In cow urine, the major degradation product derived
from the alcohol part of the molecule is 3-phenoxybenzoic acid,
whereas in rats it is 3-(4-hydroxyphenoxy) benzoic acid. DCVA is the
major urinary metabolite derived from the acid part of the molecule in
both rats and cows. In all cases, the major urinary metabolites are
excreted as polar conjugates (FAO/WHO 1980).
Hen
Radiocarbon from 14C-carbonyl- and 14C-methylene-labelled
preparations of (1RS)-trans- and (1RS)-cis-permethrin administered to
laying hens for three consecutive days at 10 mg/kg for each dose was
largely eliminated from the body within 1 day after the last dose, a
portion as 14CO2 (Gaughan et al 1978b). The excreta contained all
and the eggs most of the following compounds: the unmetabolized
pyrethroids; cis-permethrin hydroxylated at the 4'-position, at the
methyl group trans to the carboxyl, and at both of these sites; the
di-chlorovinyl phenoxybenzoic acid and their 4'-hydroxy derivatives;
sulphate, glucuronide, taurine and other conjugates of these alcohols
and acids (Figure 5). Residues of unmetabolized trans- and cis-
permethrin in fat were 0.15 and 0.93 ppm respectively, at 7 days after
the last dose, and in eggs they reached peak levels of 0.3 and 1.2 ppm
respectively, at 3 to 4 days after the last dose. Almost half of the
residues in eggs were unmetabolized trans- and cis-permethrin in the
yolk and the remainder was a great variety of metabolites in the yolk
and white, including most of those also detected in the excreta.
The preference in hydroxylation site based on identified
metabolites is the same with hens and rats, e.g., phenoxy > cis-
methyl > trans-methyl with cis-permethrin, In contrast, with cows,
both trans- and cis-permethrin have the same preference order of
trans-methyl > cis-methyl = phenoxy.
Metabolites detected in hens, but not in rats or cows, are the
cis isomer of trans-hydroxy permethrin sulphate, the trans isomer of
cis-hydroxy-phenoxybenzyl alcohol-sulphate and Cl2CA-taurine isomers.
Both hens and rats extensively utilize glucuronic acid and
sulphate conjugates in excretion of permethrin metabolites, while
glutamic acid conjugates are most significant in cowsœ
Trout
The distribution and metabolism of the cis- and trans-permethrin
isomers were studied in rainbow trout to evaluate the role of these
parameters in the differential toxicity of permethrin to fish and
mammals (Glickman et al 1981). Both (14C)-permethrin geometrical
isomers were readily taken up and eliminated by rainbow trout and
there appeared to be little difference in the rate of uptake of the
two geometric isomers. Elimination half-lives for (14C)-permethrin
residues in trout tissues, with the exception of fat, were in the
magnitude of hours. High concentrations of a polar metabolite
(glucuronide conjugate of 4'-hydroxypermethrin) were found in bile
within 4 h of cis- and trans-permethrin exposure. Urine contained a
small amount of a polar metabolite that was resistant to hydrolysis by
beta-glucuronidase but was cleaved to some extent by aryl-sulphatase.
The relative absence of permethrin hydrolysis products in trout bile
and the small amount of radioactivity excreted in urine suggested that
the ability of rainbow trout to hydrolyse permethrin in vivo was
minimal. The inability of the rainbow trout to hydrolyse permethrin
rapidly may result in an overall low rate of detoxification, which was
suggested to be a possible factor in the trout's sensitivity to the
compound, particularly the trans-isomer. However, it was also noted
that detoxification may not be the sole factor, as the trout may be
more sensitive physiologically than mammals to permethrin (Glickman
et al 1981).
goat, permethrin was rapidly and extensively degraded via hydrolytic,
oxidative and conjugative reactions, e.g., through hydrolysis of the
ester linkage, hydroxylation of the cis- or trans-methyl of the
germinal dimethyl group and hydroxylation of the 4'-position of the
phenoxybenzyl moiety. Certain of these products were further oxidized
and/or conjugated with glycine, glutamic acid, glucuronic acid, or
other unidentified compounds before excretion.
Unmetabolized permethrin and certain ester metabolites were found
in faeces, milk and fat from the treated goats, but only metabolites
arising from ester hydrolysis were found in the urine.
The patterns of radiocarbon elimination and tissue retention
by these goats were reported earlier by Hunt and Gilbert (1977) and
are briefly summarized in Table 4. Urine was the major route of
radiocarbon excretion in goats treated with (14C-acid) or
(14C-alcohol) preparations of trans-permethrin, but most of the
administered radiocarbon was eliminated via the faeces in cis-
permethrin-treated goats.
TABLE 4. Summary of radiocarbon elimination and residue retention by
lactating goats orally treated for 10 consecutive days with
(14C) alcohol- or (14C)Acid-labelled cis-(1RS)or
trans-(1RS)-permethrin isomers1, 2
Radiocarbon eliminated Tissue residues
Label and isomer cum % dose (ppm)3, 4
faeces urine milk liver kidney fat
(14Cacid)-c-per 67.5 25.8 0.66 0.12 0.05 0.23
(14C-alc)-c-per 51.7 36.4 0.53 0.13 0.05 0.24
(14C-acid)-t-per 15.0 72.1 0.17 0.04 0.03 0.02
(14C-alc)-t-per 12.3 79.4 0.24 0.01 0.03 0.02
1 Data summarized from Hunt and Gilbert (1977).
2 Goats treated with 10 successive daily oral doses of the
appropriate (14C) per isomer (0.2-0.3 mg/kg per d.).
3 Tissues taken 24 h after last dose.
4 Other edible tissues contained lower residues.
Total radioactive residues in milk reached a plateau after
3 days of 0.02 to 0.05 µg permethrin equivalents/g and <0.01 µg/g
respectively for the cis- and trans-isomers. Unmetabolized permethrin
was a major component of the radioactivity in the milk. The goats were
sacrificed 24 h after receiving the final dose. Of the edible tissues
analysed for radiocarbon 24 h after the final permethrin doses, fat,
kidney and liver contained the highest residues. Radiocarbon retained
by the tissues and that secreted into the milk were appreciably higher
in goats treated with cis-permethrin than with trans-permethrin (Table
4, Hunt and Gilbert 1977). Total radioactivity of the fat of the
animals receiving the cis-isomer was ten times higher than those
receiving the more easily hydrolysed trans-isomer and was due mainly
to unmetabolized permethrin (Hunt and Gilbert 1977; Ivie and Hunt
1980).
In another study, goats were orally dosed with 40:60 cis:trans-
14C-labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 ppm in the diet for 7 days. Total
radioactive residues in the milk reached a plateau of 0.02-0.03 µg
permethrin equivalents/g after 5 days. Of this radioactivity, 30 to
50% was associated with the butterfat fraction of the milk in which
total radioactive residues were 0.13-0.27 µg permethrin equivalents/g.
The animals were sacrificed 4 h after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 5. Where
alcohol-labelled permethrin was used, approximately 70% of the
radioactivity in kidney was due to 3-phenoxybenzoic acid plus
3-(4-hydroxyphenoxy)benzyl alcohol. A further 15% was due to
3-phenoxybenzoic acid plus 3-(4-hydroxyphenoxy)benzoic acid. Where
acid-labelled permethrin was used, approximately 10 to 15% of the
label in liver and kidney was due to the cis and trans DCVA,
principally the trans isomer (Leahey et al 1977).
It is important to compare the metabolic fate of permethrin in
the species noted above. With reference to previously published
studies on the fate of (14C)-permethrin in mammals, specifically rats
(Elliott et al 1976; Gaughan et al 1977) and lactating cattle
(Gaughan et al 1978a) the following similarities and differences
between goats and these mammals were noted by Ivie and Hunt (1980).
1) In each of the three species, a greater percentage of an
administered cis-permethrin dose was eliminated in the faeces than was
a trans-permethrin dose, a pattern that appears to be most pronounced
in goats and least in cattle. It was suggested that trans-permethrin
was absorbed more rapidly than cis-permethrin from the G.I. tract, or
alternatively, isomer differences in the rates of biliary excretion of
permethrin and/or its metabolites may account for the above
observations.
2) Although retention of permethrin by tissues and its excretion
into milk of mammals was minimal, cis-permethrin and its metabolites
in rat, lactating goat and lactating cattle were retained by tissues
to a more significant degree than was trans-permethrin.
3) Primary metabolism of permethrin in rats involves attack at five
major sites, including ester cleavage, hydroxylation at the cis- or
trans-methyl of the germinal dimethyl moiety, and hydroxylation at the
2' or 4'- position of the phenoxybenzyl moiety. Ester cleavage and
TABLE 5. Total radiolabelled residues in tissues of goats receiving 14C-labelled permethrin daily orally for 7 to 10 days
Material Dose level Duration of Period between Total residue (µg permethrin equivalents/g)in
administered (ppm in administration last date and
diet) (days) sacrifice (hours) Fat Muscle Liver Kidney
Permethrin -10 7 4 <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis:trans
Permethrin -6 10 24 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin -6 10 24 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
4'-hydroxylation are the major routes of metabolism. Cattle and goats
metabolize permethrin similarly, with the exception that
2'-hydroxylation apparently does not occur in these ruminants.
4) Ester metabolites of permethrin were eliminated primarily through
the faeces of rats, cattle and goats, in contrast to cattle which
eliminated large quantities of ester metabolites of both cis-
permethrin and trans-permethrin in faeces.
5) In each species, conjugation of permethrin metabolites before
urinary excretion was extensive. Rats, cattle and goats eliminated the
acid moiety in urine primarily as conjugates with glucuronic acid. The
alcohol moiety was excreted, mostly as phenoxybenzoic acid glucuronide
or 4'-hydroxyphenoxybenzoic acid-sulphate in rats, but amino acid
conjugates of phenoxybenzoic acid comprised most of the excreted
products in cattle and goats. Although conjugation of phenoxybenzoic
acid with glycine was preferred in goats, conjugation with glutamic
acid was favoured in cattle.
The metabolic fate of permethrin in the goat is similar to that
in the cow. Radioactivity deriving from the oral administration of the
cis-isomer of permethrin is excreted mainly in the faeces, whereas
that deriving from the more readily hydrolysed trans-isomer is
excreted mainly in the urine. Radioactivity in faeces is due
principally to permethrin and, in the case of cis-isomer, to
4'-hydroxypermethrin. the predominant urinary metabolites are DCVA and
3-phenoxybenzoic acid, which are excreted mainly as polar conjugates
(Ivie and Hunt 1980).
The major products of the metabolism of permethrin in the cow are
similar to those in the rat. The most notable differences are that in
the cow a greater percentage of the administered radioactivity is
excreted in faeces as intact ester metabolites, notably as 4'-
hydroxypermethrin. In cow urine, the major degradation product derived
from the alcohol part of the molecule is 3-phenoxybenzoic acid,
whereas in rats it is 3-(4-hydroxyphenoxy) benzoic acid. DCVA is the
major urinary metabolite derived from the acid part of the molecule in
both rats and cows. In all cases, the major urinary metabolites are
excreted as polar conjugates (FAO/WHO 1980).
Hen
Radiocarbon from 14C-carbonyl- and 14C-methylene-labelled
preparations of (1RS)-trans- and (1RS)-cis-permethrin administered to
laying hens for three consecutive days at 10 mg/kg for each dose was
largely eliminated from the body within 1 day after the last dose, a
portion as 14CO2 (Gaughan et al 1978b). The excreta contained all
and the eggs most of the following compounds: the unmetabolized
pyrethroids; cis-permethrin hydroxylated at the 4'-position, at the
methyl group trans to the carboxyl, and at both of these sites; the
di-chlorovinyl phenoxybenzoic acid and their 4'-hydroxy derivatives;
sulphate, glucuronide, taurine and other conjugates of these alcohols
and acids (Figure 5). Residues of unmetabolized trans- and cis-
permethrin in fat were 0.15 and 0.93 ppm respectively, at 7 days after
the last dose, and in eggs they reached peak levels of 0.3 and 1.2 ppm
respectively, at 3 to 4 days after the last dose. Almost half of the
residues in eggs were unmetabolized trans- and cis-permethrin in the
yolk and the remainder was a great variety of metabolites in the yolk
and white, including most of those also detected in the excreta.
The preference in hydroxylation site based on identified
metabolites is the same with hens and rats, e.g., phenoxy > cis-
methyl > trans-methyl with cis-permethrin, In contrast, with cows,
both trans- and cis-permethrin have the same preference order of
trans-methyl > cis-methyl = phenoxy.
Metabolites detected in hens, but not in rats or cows, are the
cis isomer of trans-hydroxy permethrin sulphate, the trans isomer of
cis-hydroxy-phenoxybenzyl alcohol-sulphate and Cl2CA-taurine isomers.
Both hens and rats extensively utilize glucuronic acid and
sulphate conjugates in excretion of permethrin metabolites, while
glutamic acid conjugates are most significant in cowsœ
Trout
The distribution and metabolism of the cis- and trans-permethrin
isomers were studied in rainbow trout to evaluate the role of these
parameters in the differential toxicity of permethrin to fish and
mammals (Glickman et al 1981). Both (14C)-permethrin geometrical
isomers were readily taken up and eliminated by rainbow trout and
there appeared to be little difference in the rate of uptake of the
two geometric isomers. Elimination half-lives for (14C)-permethrin
residues in trout tissues, with the exception of fat, were in the
magnitude of hours. High concentrations of a polar metabolite
(glucuronide conjugate of 4'-hydroxypermethrin) were found in bile
within 4 h of cis- and trans-permethrin exposure. Urine contained a
small amount of a polar metabolite that was resistant to hydrolysis by
beta-glucuronidase but was cleaved to some extent by aryl-sulphatase.
The relative absence of permethrin hydrolysis products in trout bile
and the small amount of radioactivity excreted in urine suggested that
the ability of rainbow trout to hydrolyse permethrin in vivo was
minimal. The inability of the rainbow trout to hydrolyse permethrin
rapidly may result in an overall low rate of detoxification, which was
suggested to be a possible factor in the trout's sensitivity to the
compound, particularly the trans-isomer. However, it was also noted
that detoxification may not be the sole factor, as the trout may be
more sensitive physiologically than mammals to permethrin (Glickman
et al 1981).
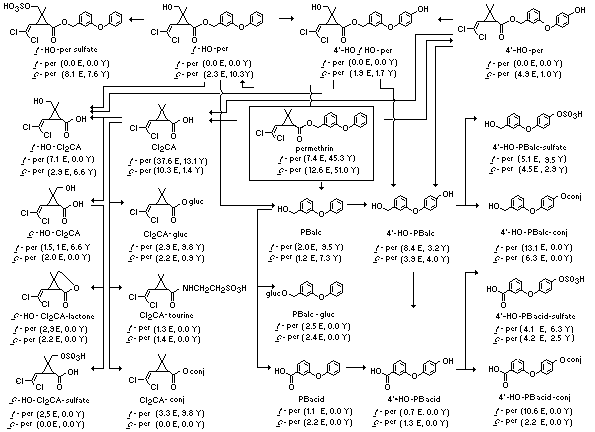 Figure 5. Metabolic pathways for (1 RS)-trans- and
(1 RS)-cis-permethrin in hens showing abbreviations used for
metabolites. Numbers in parentheses are percentage amounts of 14C
for each product derived from trans- and cis-permethrin as
follows: E = products in excreta from the first 3 days of the treatment
schedule relative to total administered 14C (see Table I);
Y = products in egg yolk at days 5 and 6 of the treatment schedule
relative to total 14C content of yolk (see Table III). Ester
products are averages for 14C acid and 14C alcohol preparations
and cleavage products are based on either 14C acid or 14C
alcohol preparations, as appropriate.
Effects on enzymes and other biochemical parameters
Carp liver microsomal esterases hydrolysed trans-permethrin much
more extensively than cis-permethrin, and the same relationship exists
for rainbow trout liver microsomes, although they appeared to be less
active (Glickman et al 1979). There was a strong preference with
both isomers and microsomal mixed-function oxidases of both species
for hydroxylation at the 4'-position of the alcohol moiety as opposed
to other sites. The methyl group trans to the carboxyl was usually
hydroxylated more extensively than the cis-methyl group, the greatest
specificity being with carp microsomes acting on cis-permethrin. The
bile of rainbow trout exposed in vivo to 14C-alcohol-labelled
trans-permethrin contained little or no permethrin but instead
consisted mainly of conjugates cleaved by beta-glucuronidase but not
by aryl-sulphatase (Glickman et al 1979).
The rates of permethrin hydrolysis in rainbow trout and mouse
tissues in vitro were estimated in a recent study (Glickman and Lech
1981). Mouse liver, kidney and plasma, incubated at 37°C, hydrolysed
trans-(14C)-permethrin approximately 166, 38 and 59 times faster,
respectively, than the same rainbow trout tissues incubated at 12°C.
At an incubation temperature of 37°C, trout liver microsomes
hydrolysed trans-permethrin approximately 45 times slower than mouse
liver microsomes. When the total capacity of trout and mouse tissues
to hydrolyse trans-permethrin ions were compared on a whole body
basis, mice hydrolysed trans-permethrin 184 times faster than rainbow
trout (Glickman and Lech 1981),
TOXICOLOGICAL STUDIES
Acute toxicity
(1RS 3RS)-permethrin has an acute oral LD50 of 490 mg/kg for
male and female mice and >5 000 mg/kg for male and female rats
(Miyamoto 1976). Various laboratories and even the same laboratory
(Kadota 1976) reported the rat oral LD50 values of (1RS, 3RS)-
permethrin to range from about 450 to >5 000 mg/kg. (See Table 6).
The individual isomers of permethrin have mouse oral LD50
values as follows: 1R,3S-3 150 mg/kg; 1R, 3R-about 96 mg/kg; 1S,3R and
1S,3S- >5 000 mg/kg (Miyamoto 1976). The (+)-cis-permethrin is more
toxic than the trans-isomer (perhaps due to the easier hydrolysability
of (+)-trans) (Miyamoto 1976). The toxicity of isomer mixtures
approximates that expected if there is no potentiating effect of one
isomer component with another (Ruzo and Casida 1977).
TABLE 6. Acute toxicity of permethrin in animals
Compound Species Sex Route LD501,2 Reference
(mg/kg)
Racemic3 Mouse M Oral 490 Miyamoto 1976
F Oral 490 "
(+)-trans M Oral 3100 "
F Oral 3200 "
(+)-cis M Oral 107 "
F Oral 85 "
(-)-trans M Oral >5000 (0) "
F Oral >5000 (0) "
Racemic Rat M Oral >5000 (20) "
F Oral >5000 (0) "
M,F Dermal >4000 Kadota 1976
M,F Dermal >2000 "
Acute Inhalation Toxicity4 Miyamoto 1976
LC50 mg/m3 Minimum Toxic Dose
(mg/m3)
Racemic Mouse M,F >685 140
Rat M,F >685 140
1 Compound dissolved in corn oil; 0.1 ml/10 g bw and 0.5-1 ml/100 g bw administered
by stomach tube to dd mice and Sprague-Dawley rats respectively;
2 Figures in paretheses indicate the % mortality at the highest dose;
3 The trans, cis ratio of the racemic mixture was 3:2;
4 Experimental conditions: solvent: kerosene; 3 h exposure, air flow 50 l/min.
The effect on the rat acute oral toxicity of changing the
cis/trans ratio from 80% to 20%/80% in six stages was assessed. The
LD50 values for the 80% cis/80% trans mixture gave a value of
approximately 6 000 mg/kg (See Table 7)
The test demonstrated that the high the cis content the greater
the toxicity, both in terms of symptoms and mortality. Where the cis
content was >50%, acute toxic symptoms comprising muscular tremors
were seen with doses of 250 mg/kg or less. When the cis-content was
< 40%, no toxic symptoms were seen after oral doses of 250 mg/kg
(Wallwork et al 1975).
TABLE 7. Acute oral toxicity of permethrin1
Species Permethrin cis/trans ratios Formulation Sex LD50 (mg/kg)
Rat 24/75 maize oil M 1 479
80/20 " F 224.5
60/40 " F 445.3
50/50 " F 1 000
40/60 " F 1 260
30/70 " F 1 684
20/80 " F 6 000
Mouse 25/75 " F 2 690
25/75 " M 2 394
Chick 25/75 " M >4 000
1 References - Wallwork and Malone, 1975; Wallwork et al 1975.
The symptoms of permethrin poisoning in mice and rats include
hypersensitivity, tremor and motor ataxia, sometimes with fibrillation
and salivation (Miyamoto 1976; Ruzo and Casida 1977; Wallwork and
Malone 1975; Wallwork et al 1975).
The subcutaneous and dermal toxicities are very low as compared
with the oral toxicity. Permethrin does not cause eye or skin
irritation or skin sensitizing effects (Ruzo and Casida 1977; Wallwork
and Malone 1975; Wallwork et al 1975).
Short-term studies
Rat
Racemic permethrin was administered to 20 males and 20 females SD
strain rats at dietary concentrations of 375, 750 and 1 500 ppm for 24
weeks. A slight increase of liver weight was often accompanied by
liver hypertrophy and fatty change. At a higher level (3 000 ppm)
there was a slight hypertrophy of hepatoparenchymal cells. These
effects were considered neither indicative nor suggestive of
tumorigenicity (Miyamoto 1976).
In another study, groups of 18 male and 18 female weanling rats
were fed diets containing 0, 200, 600, 2 000 and 4 000 ppm 21Z73 for
90 consecutive days. At 90 days, groups of 20 (10 males and 10
females/group) rats were sacrificed, while the surviving animals were
offered unmedicated diet for a further 36 days, this being the
recovery phase. The no-effect level for 21Z73 in the rat was
determined as 2 000 ppm (equivalent to 175 mg a.i./kg/day) (Williams
1976).
Special studies on neurology
Groups of 10 male and 10 female Sprague-Dawley rats were given a
diet containing 21Z73 for 21 days at doses of 0, 4 000, 6 000 and
9 000 ppm. The permethrin contained 24.4% cis and 74.6% trans isomer.
Severe trembling and abnormal gait appeared in all groups, and the
animals lost weight. No consistent abnormality was found on detailed
histological examination of the brain, spinal cord, dorsal root
ganglia, sciatic and sural nerves, and of terminal motor and sensory
innervation in proximal (thigh) and distal (lumbrical) muscles from
half the animals in the control and 6 000 ppm groups, and from all
animals (term survivors and those dying prematurely) in the 9 000 ppm
group.
It was concluded that the clinical disorders produced in the rat
by 21Z73 were due to a pharmacological effect and not to an anatomical
lesion (Dayan 1980).
Special studies on teratogenicity
Pregnant ICR mice were treated p.o. with racemic permethrin at
dose levels of 15, 50 and 100 mg/kg/day during days 7 to 12 of
gestation. Pups were obtained by caesarean section prior to the
termination of the gestation period and external as well as skeletal
abnormalities were examined. No significant treatment-related effects
were found (Miyamoto 1976).
Special studies on mutagenicity
Bacteria
Racemic, (+)-trans-, (+)-cis-, (-)-trans and (-)-cis-permethrin
dissolved in DMSO were all tested at 10 mg/plate in Escherichia
coli W3623 and W3102 as well as Salmonella typhimurium TA 1535 and
1538 strains and were found to be non-mutagenic (Miyamoto 1976).
In the host-mediated assay (Legator and Malling 1971) both
(+)-trans-permethrin at dose levels of 3 000 and 600 mg/kg, p.o.
and (+)-cis-permethrin at dose levels of 54 and 21 mg/kg were
non-mutagenic (Miyamoto 1976). Table 8 summarizes the system in which
permethrin was found to have no mutagenic activity (Ruzo and Casida
1977).
Table 8 Systems in which permethrin shows no mutagenic activity.
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Bacterial assays, Escherichia W3623trpA: W3102trpE 10.000 a b No N-MethyI-N'-nitro-N
reversion of coli nitrosoguanidine (100); Miyamoto,
tryptophan requirement 4-nitroquinoline N- 1976
oxide (10)a
N-Methyl-N'-nitro-N-
WP2 1-1000 Yes nitrosoguanidine (2) d
N-Methyl-N'-nitro,N,- Miyamoto,
Bacterial assays, Salmonella nitrosoguanidine (100); 1976
reversion of typhimurium TA1535:TA1538 10,000a,b No 4-nitroquinoline-N-
histidine requirement oxide (10)a
N-Methyl-N'-nitro-N-
TA 1535 1-1000a,b Yes nitrosoguanidine (2) d
9-Aminoacridine (100) d
TA1537 1-1000a,b Yes 4-o-Tolylazo-o-
TA 1538. TA98 1-1000a,b Yes toluidine (25) d
Benzo(a)pyrene (20) d
TA 100 1-1000a,b Yes N-Methyl-N'-nitro-N-
Bacterial assays inhibition E. coli W3623 (wild); W3623polA; 10,000 No nitrosoguanidine (100) Miyamoto,
zone of DNA-repair W3623uvrA; W3623recA (100) 1976
deficient mutant as S. typhimurium TA1978 (wild); TA1538uvrB 10,000 No (100)
compared with wild strain Bacillus subtilis H17 (wild); M45recA 10,000 No Streptozotocin (20)
Host-mediated bacterial S. typhimurium G46 600 and 3000
assays, reversion of -mouse (1R,3S)
bacterial histidine 21 and 54
requirement (1R,3R) Ethylmethane sulfonate (620) g
Table 8 (con't)
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Cultured lymphomal Mouse L5178Y/TK+/- 30-125 No 2-Acetylaminofluorene (50) g
cells L5178Y;TK+/- 16-94 Yes Trimethylphosphate (679) i
Dominant lethal system Mouse Charles River CDI 452
a In µg plate.
b Tests on racemic mixture and each individual isomer (1R.3S; 1R,3R: 1S.3R: 1S.3S)
c Tests on racemic mixture.
d Data of G. P. Schoenig (FMC Corporation), unpublished results.
e In mg kg oral dose.
f In µg/ml.
g Data of D. Clive (Wellcome Research Laboratories), unpublished results.
h In mg kg. 5 daily oral doses to male mice.
i Data of B. C. Chesher. J. C. Malone. and M. J. Parker (Wellcome Research Laboratories), unpublished results.
Special studies on interaction
The organophosphorus pesticides profenofos, sulprofos, O-ethyl-O-
(4-nitrophenyl) phenylphosphonothioate (EPN) and S,S,S-tributyl
phosphorotrithioate (DEF) administered intraperitoneally to mice at
0.5 to 5 mg/kg strongly inhibited the liver microsomal esterase(s),
hydrolysing trans-permethrin. Topically applied profenofos, sulprofos
and DEF was much less effective in synergizing the toxicity of trans-
permethrin than that of cis-permethrin to cabbage looper larvae and
house fly adults (Gaughan et al 1980).
Data reviewed by the 1979 Meeting showed that permethrin produces
symptoms indicative of an effect on the nervous system in laboratory
animals. These are manifested clinically by tremoring and ataxia.
These effects are seen only at comparatively high dose levels and are
reversible in those animals that survive high doses. It is only at
lethal or near lethal doses that signs of pathological changes in the
nervous system are observable, with sparse axonal degeneration in the
sciatic nerves of some animals. In a long-term study, no histological
changes were seen in sciatic nerves of rats receiving the high dietary
level of 2 500 ppm permethrin, equivalent to approximately 125 mg
permethrin/kg bw/day daily for two years. Hens receiving a dose of
1 000 mg/kg/day for 5 successive days, and then for another 5 days
three weeks later, and hens receiving the maximum practical single
oral dose of approximately 9 000 mg/kg, showed neither clinical nor
histopathological evidence of neurotoxicity (FAO/WHO 1980; ICI 1981).
OBSERVATIONS ON HUMANS
The 1979 Meeting noted that there was no information on the
relative sensitivity of humans to the peripheral neuropathy noted in
rodent species at very high dose levels. No cases of misuse leading
to acute poisoning in humans have been reported to the major
manufacturers during several seasons of use worldwide. Some laboratory
workers handling natural and synthetic pyrethroids have noticed a
transient sensation in the periorbital area of the face. In a clinical
survey, three subjects who had moderate exposure to permethrin did not
develop these symptoms, which were only found after exposure to other
pyrethroids. Neurological signs and electrophysiological studies in
arms and legs of these subjects were normal (LeQuesne et al 1980).
A survey was conducted in Sweden of 45 subjects handling conifer
seedlings, which were dipped in an EC formulation of 40:60 cis:trans
permethrin that had been diluted with water to 1 to 2% of active
ingredient. One of the subjects mentioned itching of the skin. None
reported burning sensations or paraesthesia in the face. Symptoms were
more marked among subjects handling seedlings treated with WP
formulations of 25:75 cis:trans permethrin or of fenvalerate
(Kolmodin-Hedman et al 1981).
Two volunteers were dosed orally with about 2 and 4 mg permethrin
in order to establish whether "CVA" is a major metabolite in humans,
excreted largely in urine as the unconjugated acid and easily
hydrolysable conjugates, and that therefore a gas chromatographic
analysis of "CVA" concentration in urine could be used to estimate the
amount of absorbed permethrin. The two subjects were shown to excrete
18% and 35% of the theoretical yield of metabolite after a dose of
2 mg, and 39% and 32% after 4 mg. Most of the urinary elimination was
seen during the first 12 h after dosing (Cridland and Weatherley
1977).
An estimate of permethrin (NRDC 143; OMS 182) absorbed by people
employed in a field trial of the insecticide in Kuduna, Nigeria, 7-11
June, has been reported (Cridland and Weatherley 1977). Before the
trial, samples of urine from trial personnel were analysed for CIVA
and creatinine content. By comparison with results from the volunteer
study, using creatinine as a biological internal standard, it was
possible to estimate that not more than 2 mg permethrin was absorbed
by any person handling the insecticide in any 12-h period.
RESIDUES IN FOOD
USE PATTERN
Permethrin was first evaluated at the 1979 Meeting, when MRLs
were recommended for a wide range of commodities on a temporary basis.
These recommendations took account of residue levels on crops
immediately after spraying. For the 1981 Meeting, further information
was required on world-wide good agricultural practices (i.e.
authorized national use patterns). The PHIs that can be recommended
between countries are varied. They tend to be shorter in those
countries in which the majority of the potential use of permethrin
will arise. It was agreed that the Meeting should recommend MRLs to
cover the full worldwide spectrum of good agricultural practices.
Permethrin residues on growing crops decline slowly. They tend to be
rather more variable than for some other compounds. Therefore, the
Meeting agreed to support the process used by the 1979 Meeting, which
took account of permethrin residue levels immediately after spraying.
Permethrin is a photostable synthetic pyrethroid. It possesses an
extremely high level of activity against Lepidoptera. It is also
effective against Hemiptera, Diptera and Coleoptera. It is a stomach
and contact insecticide, with very little fumigant activity.
Permethrin is extremely fast-acting. It is effective against all
growth stages, particularly larvae. It also has significant repellent
action. Permethrin controls many insect strains that have developed
resistance to organophosphorus and organochlorine insecticides. It is
of low mammalian toxicity and yet it is effective against insects at
extremely low rates of application. Unlike earlier pyrethroids, it is
sufficiently photo-stable to be of wide-ranging practical use in
agriculture. It represents a major advance in the insecticide field.
Permethrin is not plant systemic. It has very little fumigant or
translaminar activity. Usually, best results are obtained with good
spray cover, and repeat applications are normally made every 7 to 10
days. However, where conditions are conducive to a rapid build-up of
insect infestations, re-spraying may be needed after as little as 3
days (for example, when controlling Spodoptera littoralis on leaf
brassicae in the Far East). Where a range of rates is quoted for
worldwide use, the higher values are required more frequently in those
climates where infestation pressure is greatest.
The major uses arise in the Americas, Africa and parts of the Far
East, i.e. in hot, often humid conditions, where the pressure of
insect attack is frequently high. Cotton dominates the market
potential for permethrin. Soybeans are also very important in the USA
and Brazil. However, cotton seed and soybeans contain negligible
residues when these crops are sprayed as recommended. Smaller, but
still very important, uses in hot countries are those on horticultural
vegetables and on fruit. These include the human foodstuffs that can
contain noteworthy residues - leafy vegetables, solanaceous fruits,
pip fruits and stone fruits.
Not more than 5% of the potential world usage of permethrin is
associated with Western Europe. There, main outlets are on
horticultural vegetables, top fruit and vines. Typically, use rates of
50 g a.i./ha are effective on leaf brassicae, whereas 100 g a.i./ha is
often needed under the more severe conditions of the Americas, Africa
and the Far East.
In 1979, the group of manufacturers noted the relatively slow
rate at which permethrin residues decline on the sprayed parts of
crops. A table showing "half-lives" of 3 to 29 days was provided. To
persons labelling a product, this slow rate of residue decline
provides altogether different problems from those which pertain when
residues decline quickly. One example of the latter is the compound
pirimicarb. During the first 2 to 3 days after spraying, pirimicarb
levels decline dramatically on many crops. Volatilization is mainly
responsible. This tends to mask other effects that will be present,
including inter-site differences in residue levels or the effects of
photochemical and/or enzymatic degradation. After these first few
days, the rate of residue decline becomes less marked. Thus one can
apply a pre-harvest withholding interval (PHI) of 2 to 3 days in
anticipation of obtaining a substantial decrease in residue levels
routinely on those parts of the crop that are exposed to the spray. In
contrast, permethrin is not volatile. It is comparatively photo-
stable. Residue levels decline slowly. In the absence of a dominant
route of rapid degradation, permethrin residue levels tend to be
somewhat more variable. Undoubtedly, inter-site differences in spray
practice, in weather and even in varieties may contribute to this.
Whatever the reasons, this slow rate of residue decline reduces the
value of a PHI in yielding a substantial reduction in residue levels
on exposed crop parts.
Because of this slow rate of residue decline, and because use
patterns worldwide were still evolving, the 1979 JMPR took account of
residue levels immediately after spraying when proposing MRL values.
Permethrin application is relevant only to crop parts that are sprayed
directly, and are of no consequence to situations in which the edible
part is protected and where residues are negligible (e.g. root
vegetables). Therefore, attention has been focused upon residues in
crops such as vegetables and top fruit.
A survey of crops and of PHIs recommended on them nationally are
summarized in Table 9. Countries that have imposed a PHI of 7 days or
more tend to be ones with more moderate climates and lesser problems
of insect infestation. They are mainly European, but include Brazil,
Peru and Uruguay. Conversely, countries allowing shorter PHIs, or
which do not specify a PHI, on vegetables and on fruit tend to be
those with more severe climates and with greater problems of insect
control. These include the USA, many parts of Latin America, Africa
and the Far East - the countries in which the major potential uses
arise. Noteworthy are the comments received on the PHIs needed on
vegetables in the USA. On lettuce and leaf brassicae, major pests
include loopers, diamondback moth and imported cabbageworm. The crop
yields and quality relate directly to the presence (or absence) of
insects. The cleaner the crop, the higher the number of heads that can
be marketed. Therefore, growers demand a high level of insect control,
which permethrin is capable of giving. Many fresh vegetables are
harvested continuously, on a 1 to 3 day schedule. Many crops go to
processors, who demand standards such that the produce from an entire
field can be discarded if any insects or insect parts are found in any
part of the harvested crop. Permethrin has the quality of causing
loopers to roll into a ball and fall from the plant. There is a need
for an insecticide treatment close to harvest, which permethrin can
fulfill. A similar picture pertains on tomatoes. Fresh market outlets
are supplied on short notice. Growers who sell through brokers often
have to commit field to pre-designated standards of yield and quality
in a crop which is harvested continuously. The US Environmental
Protection Agency has recognized these needs when granting clearances
(as Section 18 Emergency Exemptions) on a wide range of crops (FAO/WHO
1979).
Information on the full range of use patterns is less well-
documented for parts of the Third World. However, it is hard to
believe that USA is the only country in which continuous harvesting
occurs soon after spraying. Clearly, if a crop has to be re-sprayed
every 3 to 7 days, a PHI of more than a few days is not practical.
TABLE 9 Uses of permethrin reported to the Meeting as approved by national governments
Country or Area Crop PHI
(days)
Algeria Apple, pear, brassicae, aubergine, pepper,
tomato, cotton None specified
Argentina Pea, pepper, tomato 1
Alfalfa, cotton,maize 7
Apple, apricot, nectarine, peach,)
pear, plum, quince, soyabean ) 21
Sunflower, sorghum )
Australia Broccoli, Brussels sprout, cabbage, 2
cauliflower, tomate, maize
Austria Cabbage, cauliflower, cucumber, None specified
gherkin, maize, plum, spinach,
turnip, vines
Belgium Aubergine, cabbage, cucumber, 2 (under glass)
endive, lettuce, melon, pepper,
tomato 7 (outdoors)
Bolivia Aubergine, pepper, tomato, cotton Non specified
Brazil Cabbage, cauliflower 3
Tomato, cotton 7
Coffee 30
Maize 45
Soybean 60
Canada Apple, pear, grape (pre-bloom)
Sweet corn 1
Cabbage, cauliflower, Brussels sprout 3
Broccoli 7
Canary Islands Tomato 15
Chile Apple, cherry, damson, peach, None specified
pear, plum, beans, potato,
cabbage, maize
Colombia Aubergine, pepper, tomato, cotton None specified
Costa Rica Cotton None specified
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Cuba Aubergine, tomato, cotton, None specified
chilli pepper
Cyprus Cucumber 5
Beans, pepper, tomato 14
Czechoslovakia Apple 21
Cereals 28
Denmark Pea, beetroot, potato 14
Dominican Republic Brassicae, pepper, tomato, cotton, None specified
oilseed rape
Democratic Republic of Aubergine, cucumber, pumpkins, 4
Germany pepper, tomato
Potato 14
Brassicae, lettuce, spinach, carrot, 21
radish, sugarbeet, swede, turnip
Flax, cereals, oilseed rape None specified
France Grape, apple, pear, lettuce, cabbage 15
Grain crops 40
Federal Republic of Cabbage 7
Germany Apple, pear, hops 14
Oilseed rape 56
Guatemala Cotton, brassicae, potato, maize None specified
Guernsey Aubergine, cucumber, pepper, None specified
tomato (under glass)
Pea, brassicae, aubergine, cucumber, None specified
pepper, tomato (outdoors)
Hong Kong Cabbage, watercress, onion None specified
Hungary Apple, pear, apricot, peach, maize None specified
Indonesia Soybean, cabbage, pepper, oil palm, None specified
cotton
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Iran Pistachios, cotton 7
Israel Pumpkin 10
Jordan Apple, pear, brassicae, aubergine, None specified
okra, pepper, tomato, alfalfa,
cotton
Malaysia Citrus, brassicae, aubergine, pepper, None specified
tomato, oil palm, cocoa, coconut
Mexico Cotton, lettuce, broccoli, Brussels None specified
sprouts, cabbage, cauliflower, maize
Morocco Apple, pear, brassicae, aubergine, None specified
cotton
Netherlands Mushrooms 2
Aubergine, cucumber, honeydew melon, 3
pepper, tomato, courgette
Apple, pear, brassicae, beans, pea, )
horseradish, potato, fennel, leek ) 7
onion, radish, sugarbeet, swede, )
turnip, grape, cherry, plum, strawberry )
currant,gooseberry,blackberry,raspberry,)
rutabaga, potato, fodder beet,poppy seed)
Lettuce, endive, fennel 21
New Zealand Greenhouse fruit and vegetables 2
Brassicae, beans, tomato (outdoor))
and fruit trees other than citrus ) 3
Maize, outdoor fruit and vegetables 7
Fodder crops, pome fruit 14
Citrus fruit, grape 28
Nicaragua Brassicae, aubergine, tomato, cotton, None specified
maize
Pakistan Cotton None specified
Peru Beans, potato, tomato, alfalfa, 7
cotton, maize
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Philippines Banana, rice, mango, cabbage, None specified
cauliflower, tomato
Poland Cucumber, tomato (under glass) 2
Pea, brassicae None specified
Portugal Tomato 2
El Salvador Cotton None specified
South Africa Apple, pear 14
Cotton, maize None specified
Spain Citrus, potato, tomato, cotton 15
Syria Cotton, brassicae, aubergine, okra, None specified
pepper, tomato
Taiwan Cabbage, rice None specified
UK Cucumber, pepper, tomato, None specified
aubergerine, mushroom (fogging indoors).
Apple, pear, cherry, plum, pea, None specified
brassicae, lettuce, celery, courgette,
potato, carrot, swede, turnip, sugarbeet,
grass, cereals, blackcurrants,
redcurrants, gooseberry, raspberry,
strawberry.
Uruguay Apple, peach, plum, pea, beans, 20
soybean, tomato, onion, sorghum,
quince
USA Cotton 14
USSR Apple, cotton None specified
Venezuela Cotton None specified
Cabbage, pepper, tomato, onion 7
Yugoslavia Broccoli, Brussels sprout, cabbage,
cauliflower, tomato, maize 2
One may wish to entertain applying a PHI for toxicological
reasons or owing to knowledge of good agricultural practice. In view
of the slow rate of residue decline, a PHI of 2 to 3 weeks would be
the minimum required to effect a substantial and consistent decline in
residue levels, for toxicological reasons. A few governments have set
PHIs of 2 to 3 weeks; presumably, they must consider that such PHIs
are capable of being observed within the limitations of good
agricultural practice within their countries. Other governments have
set lower PHIs based on a knowledge of good agricultural practice
within their country and of the toxicological and residues data on the
compound. Still other governments have not specified a PHI on the
label.
Post-harvest treatments
In trials in Australia (Halls 1981) permethrin (25:75, cis:trans)
was applied at rates in the range 0.5 to 5.0 mg /kg to wheat of
moisture content 9.5 - 10.5 held in small silos at temperatures that
ranged, due to seasonal effects, from 25°C through 10°C and back to
23°C over a period of 9 months. There was little detectable
degradation of any of the treatments, as indicated in Table 10.
Halls and Periam (1980) reported studies in which two permethrin
(25:75, cis:trans) liquid grain protectant formulations were applied
to wheat, which was then stored for 9 months in metal silos in the UK.
Residue analysis on wheat samples taken at monthly intervals showed no
degradation of the permethrin. There was no detectable degradation of
permethrin by the processes of milling or baking over the storage
period, except possibly in the case of the baking of wholemeal bread
from wheat stored for 6 months or more. The results are given in Table
11. The trials were continued and the results reported later (Halls
1981) indicated that there was no significant degradation 15 months
after treatment.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the 1979 Meeting, residues data were reviewed from supervised
trials on kale and spinach sprayed in the Federal Republic of Germany
in 1977 at 22.5 to 30 g a.i./ha. Having regard to the use pattern data
reviewed at the 1979 Meeting and to the higher use rates that could be
needed under conditions of greater insect pressure in countries
outside of Western Europe, additional trials on kale and spinach were
conducted in the UK in 1980 at a rate of 100 g a.i./ha. Allowing for
these differences in application rates, there is good agreement within
the total data now available. At the 100 g a.i./ha rate, residues
during the first three days after spraying were in the range 1.5 to
4.1 mg/kg on spinach and 1.1 to 3.6 mg/kg on kale (Table 12). The 1979
Meeting noted that the rate of decline in permethrin residue levels on
growing crops is fairly small, with half-life periods ranging from
TABLE 10. Residues of permethrin, piperonyl butoxide and fenitrothion detected on wheat after indicated periods
of storage in Australia
Target Residue analysis (mg/kg)
Compound1 application
rate (mg/kg)
Initial 1 month 2 months 3 months 6 months 8 months 9 months
PM 0.5 0.38 0.35 0.38 0.28 0.39 0.39 0.36
PB 10.0 3.9 4.5 4.4 5.0 5.7 3.8 -
FEN 12.0 7.2 7.2 7.7 6.9 6.9 4.3 4.3
PM 1.0 0.93 0.99 0.88 0.89 0.80 0.75 0.75
PB 10.0 7.9 4.6 4.1 5.5 6.7 4.0 -
FEN 12.0 8.9 7.6 6.6 8.0 7.0 5.8 5.2
PM 2.0 1.90 2.00 2.05 1.59 1.64 1.60 1.68
PM 2.0 1.82 1.76 1.95 2.02 1.88 1.75 2.15
PB 10.0 4.2 3.4 3.0 4.0 4.0 3.5 -
PM 5.0 4.5 4.4 4.9 4.5 5.0 3.6 2.6
PM 5.0 5.1 5.8 6.0 5.1 5.9 5.6 4.5
PB 10.0 5.6 7.3 6.9 6.6 6.4 5.9 -
1 PM = permethrin, PB = piperonyl butoxide, FEN = fenitrothion, - = not analysed.
TABLE 11. Permethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after three,
six or nine months' storage1
Target
application Sampling First- Total
rate period Wholemeal Wholemeal Fine reduction white White
(mg/kg) (months) Wheat flour bread Bran offal flour flour bread
0 0.82 0.80 0.52 4.40 nd1 nd 0.27 0.19
3 0.96 0.65 0.67 3.70 approx. 1 nd nd 0.15
2.0
6 0.95 0.83 0.47 2.90 0.74 0.12 0.17 0.06
(25:75)3 (23:77)3
9 1.09 0.78 0.24 4.00 1.04 0.12 0.25 0.12
0 1.74 1.60 0.95 10.20 nd nd 0.55 0.51
3 2.04 2.25 1.20 10.30 2.40 nd 0.42 0.30
5.0
6 2.32 2.19 0.68 5.90 1.79 0.23 0.34 0.18
(25:75)3 (23:77)3
9 2.14 2.21 0.64 8.00 2.60 0.23 0.60 0.20
1 Analytical results are subject to confidence limits of ± 20%;
2 nd = not detectable (<0.05 mg/kg);
3 cis:trans isomer ratio
TABLE 12. Permethrin residue levels on kale and spinach, UK 1980
Harvest Lowest Highest Mean
Crop Formulation Rate Volume No. of interval residue residue residue
(g a.i./ha) (l/ha) applications (days) (mg/kg) (mg/kg) (mg/kg)
Spinach 25% EC 100 500 1 0 4.1 (1)1
3 1.5 3.6 1.9 (5)
7 0.81(1)
14 0.26(1)
Kale 25% EC 100 500 3 0 3.6 (1)
3 1.1 3.9 2.7 (5)
7 3.9 (1)
(var. acephala) 14 1.3 (1)
1 Number of samples used for calculating mean.
about one to three weeks, depending upon the crop. In the UK trials in
1980, permethrin had an initial half-life of ten days on kale and
three days on spinach (FAO/WHO 1980; Swaine 1981a).
Supervised trials, involving ULV applications to grapefruit and
tangerine were conducted in Spain in 1980, and involving high volume
applications to oranges, lemon and grapefruit in the USA in 1981. The
1979 Meeting noted that in orange, residues were found almost
exclusively in the peel; in edible flesh levels did not exceed
0.01 mg/kg. Similar results have now been found in grapefruit, lemon
and tangerine, in which levels in the edible flesh did not exceed
0.05 mg/kg. Residues on the whole fruit at the use rate normally
recommended (50 g a.i./ha) were within the MRL of 0.5 mg/kg
recommended by the 1979 Meeting (FAO/WHO 1980; Swaine 1981b,
c). Permethrin levels in orange, lemon and grapefruit juice were below
0.05 mg/kg at the use rate normally recommended and did not exceed
0.07 mg/kg at twice that rate (Table 13) (Swaine 1981c). Information
received, from New Zealand, Canada and The Netherlands concerning
residues on apple, grape, orange, kiwi fruit, boysenberry, cabbage,
sweet corn, Brussels sprouts, tomato and cucumber included in Table
14, confirm recommendations made previously. Other limited data on
avocado and cranberry were also received.
FATE OF RESIDUES
Photochemical degradation
One of the major drawbacks in the use of natural pyrethrins and
early synthetic pyrethroids as agricultural insecticides was their
susceptibility to degradation in light and air. Introduction of the
dichlorovinyl group into the acid part of the molecule in place of the
isobutenyl group, common in many early pyrethroids, removes one
possible site of photodegradation.
Deposits of permethrin on glass exposed to daylight near a window
indoors persisted for longer than 3 weeks. Out of doors exposed to UV
and visible light but shielded from wind and rain with temperatures
reaching up to 50°C, deposits lasted for about 10 days. Other
experiments comparing the stability of films exposed near a window
indoors showed 60% undecomposed permethrin after 20 days. Permethrin
remained effective on plywood for more than 12 weeks and under a
sunlamp for more than 26 days. Permethrin formulations also showed
improved stability on plant leaf surfaces, persisting for 10 to 20
days (Elliott et al 1973 a,b).
Photolysis of permethrin in various solvents with artificial
light and on soil in sunlight results primarily in cyclopropane ring
isomerization and ester cleavage to 3-phenoxy-benzyl alcohol and
3-(2,2, dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
TABLE 13. Permethrin residues in Citrus, 1980-81
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Orange USA 24% EC 45 4700 1 0 <0.05 <0.05 0.25 <0.05 Swaine
1981 3 0.07 <0.05 0.15 <0.05 1981c
7 <0.05 <0.05 0.07 <0.05
90 4700 1 0 0.18 <0.05 0.40 <0.05
3 0.13 <0.05 0.40 <0.05
7 0.12 <0.05 0.38 <0.05
Lemon USA 24% EC 45 4700 1 0 0.13 <0.05 0.19 <0.05 Swaine
1981 3 0.08 <0.05 0.23 <0.05 1981c
7 0.10 <0.05 0.23 <0.05
90 4700 1 0 0.42 <0.05 0.57 <0.05
3 0.22 <0.05 0.31 <0.05
7 0.13 <0.05 0.48 <0.05
Grapefruit USA 24% 45 4700 1 0 0.06 <0.05 0.11 <0.05 Swaine
1981 3 <0.05 <0.05 0.14 <0.05 1981c
7 <0.05 <0.05 0.21 (0.05
90 4700 1 0 0.13 0.05 0.28 0.07
3 0.10 <0.05 0.42 <0.05
7 0.07 <0.05 0.27 <0.05
TABLE 13. (con't)
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Grapefruit
Spain 25% EC 50 3.3 2 0 0.08 <0.01 0.26 Swaine
1980 3 0.15 <0.01 0.48 1981b
7 0.12 <0.01 0.39
14 0.11 <0.01 0.35
100 3.3 2 0 0.17 0.01 0.52
3 0.18 0.01 0.53
7 0.14 <0.01 0.45
14 0.12 0.01 0.39
Tangerine Spain 25% EC 50 3.3 2 0 0.49 0.01 1.4 Swaine
1980 3 0.23 <0.01 0.86 1981b
7 0.21 0.02 0.73
14 0.29 <0.01 1.0
100 3.3 2 0 0,18 <0.01 0.65
3 0.13 <0.01 0.47
7 0.44 <0.01 1.7
14 0.52 <0.01 1.8
TABLE 14. Permethrin residues in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 0-1 3 6 9 14 21 28 41
Apple New Zealand 1980 11 5 EC 0.33 0.36 0.31 0.21
11 7.5 EC 0.25 0.7 0.32
7 2.5 EC 0.05 0.06 0.05 N.D.
8 2.5 EC 0.24 0.04 0.16
7 3.75 EC 0.19 0.16 0.16 0.11
Avocado New Zealand 1980 6 2.5 EC 0.09 0.07 0.06 0.04
Grape 7 2.5 EC 0.46 0.37 0.26 0.19
Orange 1 5 EC 0.12skin 0.09skin
nd pulp nd pulp
0.07total 0.05total
1 10 EC nd pulp nd pulp
0.15 Total 0.07 total
0 7 14 28 42 56 84
(a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Kiwi fruit New Zealand 6 1.5 EC 0.34 1.71 0.29 1.43
5 25 EC 0.5 - 0.56 4.22 0.16 - 0.16 - 0.08
6 2.5 EC 0.15 - 0.11 0.34 0.08 0.26 0.03 - nd -
7 2.5 EC 0.25 - 0.34 2.45 0.17 1.21 0.16 - 0.07 - 0.06 -
6 3.0 EC 0.5 2.51 0.41 2.05
6 4.4 EC 2.5 12.5 2.5 12.5 1.9 9.6 1.7 8.8
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation 0 7 14 28 42 56 84
(g a.i./100 l) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Boysenberry 2 2.5 EC 0.26 0.15 0.12 0.07
2 2.5 EC 0.65 0.58 0.34 0.14
2 3.75 EC 0.29 0.32 0.22 0.12
1 2 4 6 9 14 45
Brussels Canada 1976 4 70 EC 0.09
sprout 70 EC 0.09
70 EC 0.05
Cranberry Canada 1977 2 70 EC 0.12(60days)
0.15(" )
0.08(")
Tomato Netherlands 1978 2 76 Smoke 0.58
gener. (0.36-0.75)
1979 1 - " 0.11
August (0.06-0.17)
1979 1 - " <0.01
October
Cucumber 1979 1 - " <0.01
October
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 1 2 4 6 9 14 45
Cabbage New Zealand 1980 1 25 EC 0.55 0.43 0.22 0.17
1 50 EC 0.54 0.78 0.36 0.22
1 100 EC 0.39 0.61 0.44 0.55
Sweet corn 1980 1 50 EC 2.7 nd 2.84 nd 1.04 nd 0.54 nd
foliage foliage foliage fol.
cob cob cob cob
Pasture 1980 1 250 EC 39 13 7 6 4 3
500 EC 45 24 9 9 7 3
750 EC 69 44 29 29 12 10
1000 EC 97 56 35 23 19 15
1500 EC 114 77 56 54 25 24
(See Figure 6). The photolysis of cis and trans isomers of permethrin
has been studied using material 14C-labelled in the carbonyl or
methylene moieties. Both permethrin isomers decomposed under
artificial light (peak outlet lambda 290-320nm) slightly faster in
hexane than in methanol. In each solvent, the cis isomer
photodecomposed approx. 1.6 times faster (T1/2 43 to 58 min) than the
trans isomer. The reacon involved extensive isomerization of the
cyclopropane ring, i.e. interconversion of the trans and cis isomers.
This probably occurred via a triplet energy state forming the
diradical intermediate (cleavage of cyclopropane ring), as the
reaction proceeded in the presence of phenoxybenzaldehyde and
benzophenone acting as photosensitizer, and was efficiently quenched
by 1,3-cyclohexadiene. The isomerization rate increased in the order
methanol < hexane < water, and at equilibrium after 1 to 4 h
irradiation the more thermodynamically stable trans isomer constituted
65 to 70% of the isomer mixture.
Together with the isomerization reaction, ester cleavage was
the major photoreaction in methanol, hexane, water and 2% aqueous
acetone. The major degradation products were DCVA and 3-phenoxybenzyl
alcohol. Other products formed in trace amounts in water included the
monochloropermethrin derivative, and the corresponding monochlorovinyl
acid, formed by reductive dechlorination. Permethrin and
monochloropermethrin do not undergo epoxidation at the dichlorovinyl
or chlorovinyl substituent under normal photooxidative conditions.
Photo oxidation to decarboxylated molecules was negligible with
permethrin (Holmstead et al 1977, 1978).
Exposure of the permethrin isomers on Dunkirk Silt Loam soil for
48 days resulted in approx. 55% loss in sunlight and approx. 35% loss
in the dark. The amount of radioactivity unextractable by 1:1 methanol
and ether was approx. 65% in the dark and approx. 18% in the light.
The unextractable radioactivity appears to be due to microbial
activity rather than to chemical reactions, but irradiation increased
the amount.
On/in the soil, relatively little isomerization at the
cyclopropane ring was encountered as compared with the photolysis in
solution. There was little difference in the amount of the DCVA
detected in the dark or light, and 3-phenoxybenzyl alcohol was the
major cleavage product of the alcohol moiety. Other products detected
in trace amounts were essentially the same as those in solutions
(Holmstead et al 1978).
In soil
The residues of permethrin in an organic soil and in vegetables
grown in soil treated with a granular formulation of permethrin were
studied by means of gas chromatography (Belanger and Hamilton 1979).
Permethrin persisted in the soil for the initial 28 days and then
Figure 5. Metabolic pathways for (1 RS)-trans- and
(1 RS)-cis-permethrin in hens showing abbreviations used for
metabolites. Numbers in parentheses are percentage amounts of 14C
for each product derived from trans- and cis-permethrin as
follows: E = products in excreta from the first 3 days of the treatment
schedule relative to total administered 14C (see Table I);
Y = products in egg yolk at days 5 and 6 of the treatment schedule
relative to total 14C content of yolk (see Table III). Ester
products are averages for 14C acid and 14C alcohol preparations
and cleavage products are based on either 14C acid or 14C
alcohol preparations, as appropriate.
Effects on enzymes and other biochemical parameters
Carp liver microsomal esterases hydrolysed trans-permethrin much
more extensively than cis-permethrin, and the same relationship exists
for rainbow trout liver microsomes, although they appeared to be less
active (Glickman et al 1979). There was a strong preference with
both isomers and microsomal mixed-function oxidases of both species
for hydroxylation at the 4'-position of the alcohol moiety as opposed
to other sites. The methyl group trans to the carboxyl was usually
hydroxylated more extensively than the cis-methyl group, the greatest
specificity being with carp microsomes acting on cis-permethrin. The
bile of rainbow trout exposed in vivo to 14C-alcohol-labelled
trans-permethrin contained little or no permethrin but instead
consisted mainly of conjugates cleaved by beta-glucuronidase but not
by aryl-sulphatase (Glickman et al 1979).
The rates of permethrin hydrolysis in rainbow trout and mouse
tissues in vitro were estimated in a recent study (Glickman and Lech
1981). Mouse liver, kidney and plasma, incubated at 37°C, hydrolysed
trans-(14C)-permethrin approximately 166, 38 and 59 times faster,
respectively, than the same rainbow trout tissues incubated at 12°C.
At an incubation temperature of 37°C, trout liver microsomes
hydrolysed trans-permethrin approximately 45 times slower than mouse
liver microsomes. When the total capacity of trout and mouse tissues
to hydrolyse trans-permethrin ions were compared on a whole body
basis, mice hydrolysed trans-permethrin 184 times faster than rainbow
trout (Glickman and Lech 1981),
TOXICOLOGICAL STUDIES
Acute toxicity
(1RS 3RS)-permethrin has an acute oral LD50 of 490 mg/kg for
male and female mice and >5 000 mg/kg for male and female rats
(Miyamoto 1976). Various laboratories and even the same laboratory
(Kadota 1976) reported the rat oral LD50 values of (1RS, 3RS)-
permethrin to range from about 450 to >5 000 mg/kg. (See Table 6).
The individual isomers of permethrin have mouse oral LD50
values as follows: 1R,3S-3 150 mg/kg; 1R, 3R-about 96 mg/kg; 1S,3R and
1S,3S- >5 000 mg/kg (Miyamoto 1976). The (+)-cis-permethrin is more
toxic than the trans-isomer (perhaps due to the easier hydrolysability
of (+)-trans) (Miyamoto 1976). The toxicity of isomer mixtures
approximates that expected if there is no potentiating effect of one
isomer component with another (Ruzo and Casida 1977).
TABLE 6. Acute toxicity of permethrin in animals
Compound Species Sex Route LD501,2 Reference
(mg/kg)
Racemic3 Mouse M Oral 490 Miyamoto 1976
F Oral 490 "
(+)-trans M Oral 3100 "
F Oral 3200 "
(+)-cis M Oral 107 "
F Oral 85 "
(-)-trans M Oral >5000 (0) "
F Oral >5000 (0) "
Racemic Rat M Oral >5000 (20) "
F Oral >5000 (0) "
M,F Dermal >4000 Kadota 1976
M,F Dermal >2000 "
Acute Inhalation Toxicity4 Miyamoto 1976
LC50 mg/m3 Minimum Toxic Dose
(mg/m3)
Racemic Mouse M,F >685 140
Rat M,F >685 140
1 Compound dissolved in corn oil; 0.1 ml/10 g bw and 0.5-1 ml/100 g bw administered
by stomach tube to dd mice and Sprague-Dawley rats respectively;
2 Figures in paretheses indicate the % mortality at the highest dose;
3 The trans, cis ratio of the racemic mixture was 3:2;
4 Experimental conditions: solvent: kerosene; 3 h exposure, air flow 50 l/min.
The effect on the rat acute oral toxicity of changing the
cis/trans ratio from 80% to 20%/80% in six stages was assessed. The
LD50 values for the 80% cis/80% trans mixture gave a value of
approximately 6 000 mg/kg (See Table 7)
The test demonstrated that the high the cis content the greater
the toxicity, both in terms of symptoms and mortality. Where the cis
content was >50%, acute toxic symptoms comprising muscular tremors
were seen with doses of 250 mg/kg or less. When the cis-content was
< 40%, no toxic symptoms were seen after oral doses of 250 mg/kg
(Wallwork et al 1975).
TABLE 7. Acute oral toxicity of permethrin1
Species Permethrin cis/trans ratios Formulation Sex LD50 (mg/kg)
Rat 24/75 maize oil M 1 479
80/20 " F 224.5
60/40 " F 445.3
50/50 " F 1 000
40/60 " F 1 260
30/70 " F 1 684
20/80 " F 6 000
Mouse 25/75 " F 2 690
25/75 " M 2 394
Chick 25/75 " M >4 000
1 References - Wallwork and Malone, 1975; Wallwork et al 1975.
The symptoms of permethrin poisoning in mice and rats include
hypersensitivity, tremor and motor ataxia, sometimes with fibrillation
and salivation (Miyamoto 1976; Ruzo and Casida 1977; Wallwork and
Malone 1975; Wallwork et al 1975).
The subcutaneous and dermal toxicities are very low as compared
with the oral toxicity. Permethrin does not cause eye or skin
irritation or skin sensitizing effects (Ruzo and Casida 1977; Wallwork
and Malone 1975; Wallwork et al 1975).
Short-term studies
Rat
Racemic permethrin was administered to 20 males and 20 females SD
strain rats at dietary concentrations of 375, 750 and 1 500 ppm for 24
weeks. A slight increase of liver weight was often accompanied by
liver hypertrophy and fatty change. At a higher level (3 000 ppm)
there was a slight hypertrophy of hepatoparenchymal cells. These
effects were considered neither indicative nor suggestive of
tumorigenicity (Miyamoto 1976).
In another study, groups of 18 male and 18 female weanling rats
were fed diets containing 0, 200, 600, 2 000 and 4 000 ppm 21Z73 for
90 consecutive days. At 90 days, groups of 20 (10 males and 10
females/group) rats were sacrificed, while the surviving animals were
offered unmedicated diet for a further 36 days, this being the
recovery phase. The no-effect level for 21Z73 in the rat was
determined as 2 000 ppm (equivalent to 175 mg a.i./kg/day) (Williams
1976).
Special studies on neurology
Groups of 10 male and 10 female Sprague-Dawley rats were given a
diet containing 21Z73 for 21 days at doses of 0, 4 000, 6 000 and
9 000 ppm. The permethrin contained 24.4% cis and 74.6% trans isomer.
Severe trembling and abnormal gait appeared in all groups, and the
animals lost weight. No consistent abnormality was found on detailed
histological examination of the brain, spinal cord, dorsal root
ganglia, sciatic and sural nerves, and of terminal motor and sensory
innervation in proximal (thigh) and distal (lumbrical) muscles from
half the animals in the control and 6 000 ppm groups, and from all
animals (term survivors and those dying prematurely) in the 9 000 ppm
group.
It was concluded that the clinical disorders produced in the rat
by 21Z73 were due to a pharmacological effect and not to an anatomical
lesion (Dayan 1980).
Special studies on teratogenicity
Pregnant ICR mice were treated p.o. with racemic permethrin at
dose levels of 15, 50 and 100 mg/kg/day during days 7 to 12 of
gestation. Pups were obtained by caesarean section prior to the
termination of the gestation period and external as well as skeletal
abnormalities were examined. No significant treatment-related effects
were found (Miyamoto 1976).
Special studies on mutagenicity
Bacteria
Racemic, (+)-trans-, (+)-cis-, (-)-trans and (-)-cis-permethrin
dissolved in DMSO were all tested at 10 mg/plate in Escherichia
coli W3623 and W3102 as well as Salmonella typhimurium TA 1535 and
1538 strains and were found to be non-mutagenic (Miyamoto 1976).
In the host-mediated assay (Legator and Malling 1971) both
(+)-trans-permethrin at dose levels of 3 000 and 600 mg/kg, p.o.
and (+)-cis-permethrin at dose levels of 54 and 21 mg/kg were
non-mutagenic (Miyamoto 1976). Table 8 summarizes the system in which
permethrin was found to have no mutagenic activity (Ruzo and Casida
1977).
Table 8 Systems in which permethrin shows no mutagenic activity.
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Bacterial assays, Escherichia W3623trpA: W3102trpE 10.000 a b No N-MethyI-N'-nitro-N
reversion of coli nitrosoguanidine (100); Miyamoto,
tryptophan requirement 4-nitroquinoline N- 1976
oxide (10)a
N-Methyl-N'-nitro-N-
WP2 1-1000 Yes nitrosoguanidine (2) d
N-Methyl-N'-nitro,N,- Miyamoto,
Bacterial assays, Salmonella nitrosoguanidine (100); 1976
reversion of typhimurium TA1535:TA1538 10,000a,b No 4-nitroquinoline-N-
histidine requirement oxide (10)a
N-Methyl-N'-nitro-N-
TA 1535 1-1000a,b Yes nitrosoguanidine (2) d
9-Aminoacridine (100) d
TA1537 1-1000a,b Yes 4-o-Tolylazo-o-
TA 1538. TA98 1-1000a,b Yes toluidine (25) d
Benzo(a)pyrene (20) d
TA 100 1-1000a,b Yes N-Methyl-N'-nitro-N-
Bacterial assays inhibition E. coli W3623 (wild); W3623polA; 10,000 No nitrosoguanidine (100) Miyamoto,
zone of DNA-repair W3623uvrA; W3623recA (100) 1976
deficient mutant as S. typhimurium TA1978 (wild); TA1538uvrB 10,000 No (100)
compared with wild strain Bacillus subtilis H17 (wild); M45recA 10,000 No Streptozotocin (20)
Host-mediated bacterial S. typhimurium G46 600 and 3000
assays, reversion of -mouse (1R,3S)
bacterial histidine 21 and 54
requirement (1R,3R) Ethylmethane sulfonate (620) g
Table 8 (con't)
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Cultured lymphomal Mouse L5178Y/TK+/- 30-125 No 2-Acetylaminofluorene (50) g
cells L5178Y;TK+/- 16-94 Yes Trimethylphosphate (679) i
Dominant lethal system Mouse Charles River CDI 452
a In µg plate.
b Tests on racemic mixture and each individual isomer (1R.3S; 1R,3R: 1S.3R: 1S.3S)
c Tests on racemic mixture.
d Data of G. P. Schoenig (FMC Corporation), unpublished results.
e In mg kg oral dose.
f In µg/ml.
g Data of D. Clive (Wellcome Research Laboratories), unpublished results.
h In mg kg. 5 daily oral doses to male mice.
i Data of B. C. Chesher. J. C. Malone. and M. J. Parker (Wellcome Research Laboratories), unpublished results.
Special studies on interaction
The organophosphorus pesticides profenofos, sulprofos, O-ethyl-O-
(4-nitrophenyl) phenylphosphonothioate (EPN) and S,S,S-tributyl
phosphorotrithioate (DEF) administered intraperitoneally to mice at
0.5 to 5 mg/kg strongly inhibited the liver microsomal esterase(s),
hydrolysing trans-permethrin. Topically applied profenofos, sulprofos
and DEF was much less effective in synergizing the toxicity of trans-
permethrin than that of cis-permethrin to cabbage looper larvae and
house fly adults (Gaughan et al 1980).
Data reviewed by the 1979 Meeting showed that permethrin produces
symptoms indicative of an effect on the nervous system in laboratory
animals. These are manifested clinically by tremoring and ataxia.
These effects are seen only at comparatively high dose levels and are
reversible in those animals that survive high doses. It is only at
lethal or near lethal doses that signs of pathological changes in the
nervous system are observable, with sparse axonal degeneration in the
sciatic nerves of some animals. In a long-term study, no histological
changes were seen in sciatic nerves of rats receiving the high dietary
level of 2 500 ppm permethrin, equivalent to approximately 125 mg
permethrin/kg bw/day daily for two years. Hens receiving a dose of
1 000 mg/kg/day for 5 successive days, and then for another 5 days
three weeks later, and hens receiving the maximum practical single
oral dose of approximately 9 000 mg/kg, showed neither clinical nor
histopathological evidence of neurotoxicity (FAO/WHO 1980; ICI 1981).
OBSERVATIONS ON HUMANS
The 1979 Meeting noted that there was no information on the
relative sensitivity of humans to the peripheral neuropathy noted in
rodent species at very high dose levels. No cases of misuse leading
to acute poisoning in humans have been reported to the major
manufacturers during several seasons of use worldwide. Some laboratory
workers handling natural and synthetic pyrethroids have noticed a
transient sensation in the periorbital area of the face. In a clinical
survey, three subjects who had moderate exposure to permethrin did not
develop these symptoms, which were only found after exposure to other
pyrethroids. Neurological signs and electrophysiological studies in
arms and legs of these subjects were normal (LeQuesne et al 1980).
A survey was conducted in Sweden of 45 subjects handling conifer
seedlings, which were dipped in an EC formulation of 40:60 cis:trans
permethrin that had been diluted with water to 1 to 2% of active
ingredient. One of the subjects mentioned itching of the skin. None
reported burning sensations or paraesthesia in the face. Symptoms were
more marked among subjects handling seedlings treated with WP
formulations of 25:75 cis:trans permethrin or of fenvalerate
(Kolmodin-Hedman et al 1981).
Two volunteers were dosed orally with about 2 and 4 mg permethrin
in order to establish whether "CVA" is a major metabolite in humans,
excreted largely in urine as the unconjugated acid and easily
hydrolysable conjugates, and that therefore a gas chromatographic
analysis of "CVA" concentration in urine could be used to estimate the
amount of absorbed permethrin. The two subjects were shown to excrete
18% and 35% of the theoretical yield of metabolite after a dose of
2 mg, and 39% and 32% after 4 mg. Most of the urinary elimination was
seen during the first 12 h after dosing (Cridland and Weatherley
1977).
An estimate of permethrin (NRDC 143; OMS 182) absorbed by people
employed in a field trial of the insecticide in Kuduna, Nigeria, 7-11
June, has been reported (Cridland and Weatherley 1977). Before the
trial, samples of urine from trial personnel were analysed for CIVA
and creatinine content. By comparison with results from the volunteer
study, using creatinine as a biological internal standard, it was
possible to estimate that not more than 2 mg permethrin was absorbed
by any person handling the insecticide in any 12-h period.
RESIDUES IN FOOD
USE PATTERN
Permethrin was first evaluated at the 1979 Meeting, when MRLs
were recommended for a wide range of commodities on a temporary basis.
These recommendations took account of residue levels on crops
immediately after spraying. For the 1981 Meeting, further information
was required on world-wide good agricultural practices (i.e.
authorized national use patterns). The PHIs that can be recommended
between countries are varied. They tend to be shorter in those
countries in which the majority of the potential use of permethrin
will arise. It was agreed that the Meeting should recommend MRLs to
cover the full worldwide spectrum of good agricultural practices.
Permethrin residues on growing crops decline slowly. They tend to be
rather more variable than for some other compounds. Therefore, the
Meeting agreed to support the process used by the 1979 Meeting, which
took account of permethrin residue levels immediately after spraying.
Permethrin is a photostable synthetic pyrethroid. It possesses an
extremely high level of activity against Lepidoptera. It is also
effective against Hemiptera, Diptera and Coleoptera. It is a stomach
and contact insecticide, with very little fumigant activity.
Permethrin is extremely fast-acting. It is effective against all
growth stages, particularly larvae. It also has significant repellent
action. Permethrin controls many insect strains that have developed
resistance to organophosphorus and organochlorine insecticides. It is
of low mammalian toxicity and yet it is effective against insects at
extremely low rates of application. Unlike earlier pyrethroids, it is
sufficiently photo-stable to be of wide-ranging practical use in
agriculture. It represents a major advance in the insecticide field.
Permethrin is not plant systemic. It has very little fumigant or
translaminar activity. Usually, best results are obtained with good
spray cover, and repeat applications are normally made every 7 to 10
days. However, where conditions are conducive to a rapid build-up of
insect infestations, re-spraying may be needed after as little as 3
days (for example, when controlling Spodoptera littoralis on leaf
brassicae in the Far East). Where a range of rates is quoted for
worldwide use, the higher values are required more frequently in those
climates where infestation pressure is greatest.
The major uses arise in the Americas, Africa and parts of the Far
East, i.e. in hot, often humid conditions, where the pressure of
insect attack is frequently high. Cotton dominates the market
potential for permethrin. Soybeans are also very important in the USA
and Brazil. However, cotton seed and soybeans contain negligible
residues when these crops are sprayed as recommended. Smaller, but
still very important, uses in hot countries are those on horticultural
vegetables and on fruit. These include the human foodstuffs that can
contain noteworthy residues - leafy vegetables, solanaceous fruits,
pip fruits and stone fruits.
Not more than 5% of the potential world usage of permethrin is
associated with Western Europe. There, main outlets are on
horticultural vegetables, top fruit and vines. Typically, use rates of
50 g a.i./ha are effective on leaf brassicae, whereas 100 g a.i./ha is
often needed under the more severe conditions of the Americas, Africa
and the Far East.
In 1979, the group of manufacturers noted the relatively slow
rate at which permethrin residues decline on the sprayed parts of
crops. A table showing "half-lives" of 3 to 29 days was provided. To
persons labelling a product, this slow rate of residue decline
provides altogether different problems from those which pertain when
residues decline quickly. One example of the latter is the compound
pirimicarb. During the first 2 to 3 days after spraying, pirimicarb
levels decline dramatically on many crops. Volatilization is mainly
responsible. This tends to mask other effects that will be present,
including inter-site differences in residue levels or the effects of
photochemical and/or enzymatic degradation. After these first few
days, the rate of residue decline becomes less marked. Thus one can
apply a pre-harvest withholding interval (PHI) of 2 to 3 days in
anticipation of obtaining a substantial decrease in residue levels
routinely on those parts of the crop that are exposed to the spray. In
contrast, permethrin is not volatile. It is comparatively photo-
stable. Residue levels decline slowly. In the absence of a dominant
route of rapid degradation, permethrin residue levels tend to be
somewhat more variable. Undoubtedly, inter-site differences in spray
practice, in weather and even in varieties may contribute to this.
Whatever the reasons, this slow rate of residue decline reduces the
value of a PHI in yielding a substantial reduction in residue levels
on exposed crop parts.
Because of this slow rate of residue decline, and because use
patterns worldwide were still evolving, the 1979 JMPR took account of
residue levels immediately after spraying when proposing MRL values.
Permethrin application is relevant only to crop parts that are sprayed
directly, and are of no consequence to situations in which the edible
part is protected and where residues are negligible (e.g. root
vegetables). Therefore, attention has been focused upon residues in
crops such as vegetables and top fruit.
A survey of crops and of PHIs recommended on them nationally are
summarized in Table 9. Countries that have imposed a PHI of 7 days or
more tend to be ones with more moderate climates and lesser problems
of insect infestation. They are mainly European, but include Brazil,
Peru and Uruguay. Conversely, countries allowing shorter PHIs, or
which do not specify a PHI, on vegetables and on fruit tend to be
those with more severe climates and with greater problems of insect
control. These include the USA, many parts of Latin America, Africa
and the Far East - the countries in which the major potential uses
arise. Noteworthy are the comments received on the PHIs needed on
vegetables in the USA. On lettuce and leaf brassicae, major pests
include loopers, diamondback moth and imported cabbageworm. The crop
yields and quality relate directly to the presence (or absence) of
insects. The cleaner the crop, the higher the number of heads that can
be marketed. Therefore, growers demand a high level of insect control,
which permethrin is capable of giving. Many fresh vegetables are
harvested continuously, on a 1 to 3 day schedule. Many crops go to
processors, who demand standards such that the produce from an entire
field can be discarded if any insects or insect parts are found in any
part of the harvested crop. Permethrin has the quality of causing
loopers to roll into a ball and fall from the plant. There is a need
for an insecticide treatment close to harvest, which permethrin can
fulfill. A similar picture pertains on tomatoes. Fresh market outlets
are supplied on short notice. Growers who sell through brokers often
have to commit field to pre-designated standards of yield and quality
in a crop which is harvested continuously. The US Environmental
Protection Agency has recognized these needs when granting clearances
(as Section 18 Emergency Exemptions) on a wide range of crops (FAO/WHO
1979).
Information on the full range of use patterns is less well-
documented for parts of the Third World. However, it is hard to
believe that USA is the only country in which continuous harvesting
occurs soon after spraying. Clearly, if a crop has to be re-sprayed
every 3 to 7 days, a PHI of more than a few days is not practical.
TABLE 9 Uses of permethrin reported to the Meeting as approved by national governments
Country or Area Crop PHI
(days)
Algeria Apple, pear, brassicae, aubergine, pepper,
tomato, cotton None specified
Argentina Pea, pepper, tomato 1
Alfalfa, cotton,maize 7
Apple, apricot, nectarine, peach,)
pear, plum, quince, soyabean ) 21
Sunflower, sorghum )
Australia Broccoli, Brussels sprout, cabbage, 2
cauliflower, tomate, maize
Austria Cabbage, cauliflower, cucumber, None specified
gherkin, maize, plum, spinach,
turnip, vines
Belgium Aubergine, cabbage, cucumber, 2 (under glass)
endive, lettuce, melon, pepper,
tomato 7 (outdoors)
Bolivia Aubergine, pepper, tomato, cotton Non specified
Brazil Cabbage, cauliflower 3
Tomato, cotton 7
Coffee 30
Maize 45
Soybean 60
Canada Apple, pear, grape (pre-bloom)
Sweet corn 1
Cabbage, cauliflower, Brussels sprout 3
Broccoli 7
Canary Islands Tomato 15
Chile Apple, cherry, damson, peach, None specified
pear, plum, beans, potato,
cabbage, maize
Colombia Aubergine, pepper, tomato, cotton None specified
Costa Rica Cotton None specified
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Cuba Aubergine, tomato, cotton, None specified
chilli pepper
Cyprus Cucumber 5
Beans, pepper, tomato 14
Czechoslovakia Apple 21
Cereals 28
Denmark Pea, beetroot, potato 14
Dominican Republic Brassicae, pepper, tomato, cotton, None specified
oilseed rape
Democratic Republic of Aubergine, cucumber, pumpkins, 4
Germany pepper, tomato
Potato 14
Brassicae, lettuce, spinach, carrot, 21
radish, sugarbeet, swede, turnip
Flax, cereals, oilseed rape None specified
France Grape, apple, pear, lettuce, cabbage 15
Grain crops 40
Federal Republic of Cabbage 7
Germany Apple, pear, hops 14
Oilseed rape 56
Guatemala Cotton, brassicae, potato, maize None specified
Guernsey Aubergine, cucumber, pepper, None specified
tomato (under glass)
Pea, brassicae, aubergine, cucumber, None specified
pepper, tomato (outdoors)
Hong Kong Cabbage, watercress, onion None specified
Hungary Apple, pear, apricot, peach, maize None specified
Indonesia Soybean, cabbage, pepper, oil palm, None specified
cotton
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Iran Pistachios, cotton 7
Israel Pumpkin 10
Jordan Apple, pear, brassicae, aubergine, None specified
okra, pepper, tomato, alfalfa,
cotton
Malaysia Citrus, brassicae, aubergine, pepper, None specified
tomato, oil palm, cocoa, coconut
Mexico Cotton, lettuce, broccoli, Brussels None specified
sprouts, cabbage, cauliflower, maize
Morocco Apple, pear, brassicae, aubergine, None specified
cotton
Netherlands Mushrooms 2
Aubergine, cucumber, honeydew melon, 3
pepper, tomato, courgette
Apple, pear, brassicae, beans, pea, )
horseradish, potato, fennel, leek ) 7
onion, radish, sugarbeet, swede, )
turnip, grape, cherry, plum, strawberry )
currant,gooseberry,blackberry,raspberry,)
rutabaga, potato, fodder beet,poppy seed)
Lettuce, endive, fennel 21
New Zealand Greenhouse fruit and vegetables 2
Brassicae, beans, tomato (outdoor))
and fruit trees other than citrus ) 3
Maize, outdoor fruit and vegetables 7
Fodder crops, pome fruit 14
Citrus fruit, grape 28
Nicaragua Brassicae, aubergine, tomato, cotton, None specified
maize
Pakistan Cotton None specified
Peru Beans, potato, tomato, alfalfa, 7
cotton, maize
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Philippines Banana, rice, mango, cabbage, None specified
cauliflower, tomato
Poland Cucumber, tomato (under glass) 2
Pea, brassicae None specified
Portugal Tomato 2
El Salvador Cotton None specified
South Africa Apple, pear 14
Cotton, maize None specified
Spain Citrus, potato, tomato, cotton 15
Syria Cotton, brassicae, aubergine, okra, None specified
pepper, tomato
Taiwan Cabbage, rice None specified
UK Cucumber, pepper, tomato, None specified
aubergerine, mushroom (fogging indoors).
Apple, pear, cherry, plum, pea, None specified
brassicae, lettuce, celery, courgette,
potato, carrot, swede, turnip, sugarbeet,
grass, cereals, blackcurrants,
redcurrants, gooseberry, raspberry,
strawberry.
Uruguay Apple, peach, plum, pea, beans, 20
soybean, tomato, onion, sorghum,
quince
USA Cotton 14
USSR Apple, cotton None specified
Venezuela Cotton None specified
Cabbage, pepper, tomato, onion 7
Yugoslavia Broccoli, Brussels sprout, cabbage,
cauliflower, tomato, maize 2
One may wish to entertain applying a PHI for toxicological
reasons or owing to knowledge of good agricultural practice. In view
of the slow rate of residue decline, a PHI of 2 to 3 weeks would be
the minimum required to effect a substantial and consistent decline in
residue levels, for toxicological reasons. A few governments have set
PHIs of 2 to 3 weeks; presumably, they must consider that such PHIs
are capable of being observed within the limitations of good
agricultural practice within their countries. Other governments have
set lower PHIs based on a knowledge of good agricultural practice
within their country and of the toxicological and residues data on the
compound. Still other governments have not specified a PHI on the
label.
Post-harvest treatments
In trials in Australia (Halls 1981) permethrin (25:75, cis:trans)
was applied at rates in the range 0.5 to 5.0 mg /kg to wheat of
moisture content 9.5 - 10.5 held in small silos at temperatures that
ranged, due to seasonal effects, from 25°C through 10°C and back to
23°C over a period of 9 months. There was little detectable
degradation of any of the treatments, as indicated in Table 10.
Halls and Periam (1980) reported studies in which two permethrin
(25:75, cis:trans) liquid grain protectant formulations were applied
to wheat, which was then stored for 9 months in metal silos in the UK.
Residue analysis on wheat samples taken at monthly intervals showed no
degradation of the permethrin. There was no detectable degradation of
permethrin by the processes of milling or baking over the storage
period, except possibly in the case of the baking of wholemeal bread
from wheat stored for 6 months or more. The results are given in Table
11. The trials were continued and the results reported later (Halls
1981) indicated that there was no significant degradation 15 months
after treatment.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the 1979 Meeting, residues data were reviewed from supervised
trials on kale and spinach sprayed in the Federal Republic of Germany
in 1977 at 22.5 to 30 g a.i./ha. Having regard to the use pattern data
reviewed at the 1979 Meeting and to the higher use rates that could be
needed under conditions of greater insect pressure in countries
outside of Western Europe, additional trials on kale and spinach were
conducted in the UK in 1980 at a rate of 100 g a.i./ha. Allowing for
these differences in application rates, there is good agreement within
the total data now available. At the 100 g a.i./ha rate, residues
during the first three days after spraying were in the range 1.5 to
4.1 mg/kg on spinach and 1.1 to 3.6 mg/kg on kale (Table 12). The 1979
Meeting noted that the rate of decline in permethrin residue levels on
growing crops is fairly small, with half-life periods ranging from
TABLE 10. Residues of permethrin, piperonyl butoxide and fenitrothion detected on wheat after indicated periods
of storage in Australia
Target Residue analysis (mg/kg)
Compound1 application
rate (mg/kg)
Initial 1 month 2 months 3 months 6 months 8 months 9 months
PM 0.5 0.38 0.35 0.38 0.28 0.39 0.39 0.36
PB 10.0 3.9 4.5 4.4 5.0 5.7 3.8 -
FEN 12.0 7.2 7.2 7.7 6.9 6.9 4.3 4.3
PM 1.0 0.93 0.99 0.88 0.89 0.80 0.75 0.75
PB 10.0 7.9 4.6 4.1 5.5 6.7 4.0 -
FEN 12.0 8.9 7.6 6.6 8.0 7.0 5.8 5.2
PM 2.0 1.90 2.00 2.05 1.59 1.64 1.60 1.68
PM 2.0 1.82 1.76 1.95 2.02 1.88 1.75 2.15
PB 10.0 4.2 3.4 3.0 4.0 4.0 3.5 -
PM 5.0 4.5 4.4 4.9 4.5 5.0 3.6 2.6
PM 5.0 5.1 5.8 6.0 5.1 5.9 5.6 4.5
PB 10.0 5.6 7.3 6.9 6.6 6.4 5.9 -
1 PM = permethrin, PB = piperonyl butoxide, FEN = fenitrothion, - = not analysed.
TABLE 11. Permethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after three,
six or nine months' storage1
Target
application Sampling First- Total
rate period Wholemeal Wholemeal Fine reduction white White
(mg/kg) (months) Wheat flour bread Bran offal flour flour bread
0 0.82 0.80 0.52 4.40 nd1 nd 0.27 0.19
3 0.96 0.65 0.67 3.70 approx. 1 nd nd 0.15
2.0
6 0.95 0.83 0.47 2.90 0.74 0.12 0.17 0.06
(25:75)3 (23:77)3
9 1.09 0.78 0.24 4.00 1.04 0.12 0.25 0.12
0 1.74 1.60 0.95 10.20 nd nd 0.55 0.51
3 2.04 2.25 1.20 10.30 2.40 nd 0.42 0.30
5.0
6 2.32 2.19 0.68 5.90 1.79 0.23 0.34 0.18
(25:75)3 (23:77)3
9 2.14 2.21 0.64 8.00 2.60 0.23 0.60 0.20
1 Analytical results are subject to confidence limits of ± 20%;
2 nd = not detectable (<0.05 mg/kg);
3 cis:trans isomer ratio
TABLE 12. Permethrin residue levels on kale and spinach, UK 1980
Harvest Lowest Highest Mean
Crop Formulation Rate Volume No. of interval residue residue residue
(g a.i./ha) (l/ha) applications (days) (mg/kg) (mg/kg) (mg/kg)
Spinach 25% EC 100 500 1 0 4.1 (1)1
3 1.5 3.6 1.9 (5)
7 0.81(1)
14 0.26(1)
Kale 25% EC 100 500 3 0 3.6 (1)
3 1.1 3.9 2.7 (5)
7 3.9 (1)
(var. acephala) 14 1.3 (1)
1 Number of samples used for calculating mean.
about one to three weeks, depending upon the crop. In the UK trials in
1980, permethrin had an initial half-life of ten days on kale and
three days on spinach (FAO/WHO 1980; Swaine 1981a).
Supervised trials, involving ULV applications to grapefruit and
tangerine were conducted in Spain in 1980, and involving high volume
applications to oranges, lemon and grapefruit in the USA in 1981. The
1979 Meeting noted that in orange, residues were found almost
exclusively in the peel; in edible flesh levels did not exceed
0.01 mg/kg. Similar results have now been found in grapefruit, lemon
and tangerine, in which levels in the edible flesh did not exceed
0.05 mg/kg. Residues on the whole fruit at the use rate normally
recommended (50 g a.i./ha) were within the MRL of 0.5 mg/kg
recommended by the 1979 Meeting (FAO/WHO 1980; Swaine 1981b,
c). Permethrin levels in orange, lemon and grapefruit juice were below
0.05 mg/kg at the use rate normally recommended and did not exceed
0.07 mg/kg at twice that rate (Table 13) (Swaine 1981c). Information
received, from New Zealand, Canada and The Netherlands concerning
residues on apple, grape, orange, kiwi fruit, boysenberry, cabbage,
sweet corn, Brussels sprouts, tomato and cucumber included in Table
14, confirm recommendations made previously. Other limited data on
avocado and cranberry were also received.
FATE OF RESIDUES
Photochemical degradation
One of the major drawbacks in the use of natural pyrethrins and
early synthetic pyrethroids as agricultural insecticides was their
susceptibility to degradation in light and air. Introduction of the
dichlorovinyl group into the acid part of the molecule in place of the
isobutenyl group, common in many early pyrethroids, removes one
possible site of photodegradation.
Deposits of permethrin on glass exposed to daylight near a window
indoors persisted for longer than 3 weeks. Out of doors exposed to UV
and visible light but shielded from wind and rain with temperatures
reaching up to 50°C, deposits lasted for about 10 days. Other
experiments comparing the stability of films exposed near a window
indoors showed 60% undecomposed permethrin after 20 days. Permethrin
remained effective on plywood for more than 12 weeks and under a
sunlamp for more than 26 days. Permethrin formulations also showed
improved stability on plant leaf surfaces, persisting for 10 to 20
days (Elliott et al 1973 a,b).
Photolysis of permethrin in various solvents with artificial
light and on soil in sunlight results primarily in cyclopropane ring
isomerization and ester cleavage to 3-phenoxy-benzyl alcohol and
3-(2,2, dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
TABLE 13. Permethrin residues in Citrus, 1980-81
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Orange USA 24% EC 45 4700 1 0 <0.05 <0.05 0.25 <0.05 Swaine
1981 3 0.07 <0.05 0.15 <0.05 1981c
7 <0.05 <0.05 0.07 <0.05
90 4700 1 0 0.18 <0.05 0.40 <0.05
3 0.13 <0.05 0.40 <0.05
7 0.12 <0.05 0.38 <0.05
Lemon USA 24% EC 45 4700 1 0 0.13 <0.05 0.19 <0.05 Swaine
1981 3 0.08 <0.05 0.23 <0.05 1981c
7 0.10 <0.05 0.23 <0.05
90 4700 1 0 0.42 <0.05 0.57 <0.05
3 0.22 <0.05 0.31 <0.05
7 0.13 <0.05 0.48 <0.05
Grapefruit USA 24% 45 4700 1 0 0.06 <0.05 0.11 <0.05 Swaine
1981 3 <0.05 <0.05 0.14 <0.05 1981c
7 <0.05 <0.05 0.21 (0.05
90 4700 1 0 0.13 0.05 0.28 0.07
3 0.10 <0.05 0.42 <0.05
7 0.07 <0.05 0.27 <0.05
TABLE 13. (con't)
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Grapefruit
Spain 25% EC 50 3.3 2 0 0.08 <0.01 0.26 Swaine
1980 3 0.15 <0.01 0.48 1981b
7 0.12 <0.01 0.39
14 0.11 <0.01 0.35
100 3.3 2 0 0.17 0.01 0.52
3 0.18 0.01 0.53
7 0.14 <0.01 0.45
14 0.12 0.01 0.39
Tangerine Spain 25% EC 50 3.3 2 0 0.49 0.01 1.4 Swaine
1980 3 0.23 <0.01 0.86 1981b
7 0.21 0.02 0.73
14 0.29 <0.01 1.0
100 3.3 2 0 0,18 <0.01 0.65
3 0.13 <0.01 0.47
7 0.44 <0.01 1.7
14 0.52 <0.01 1.8
TABLE 14. Permethrin residues in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 0-1 3 6 9 14 21 28 41
Apple New Zealand 1980 11 5 EC 0.33 0.36 0.31 0.21
11 7.5 EC 0.25 0.7 0.32
7 2.5 EC 0.05 0.06 0.05 N.D.
8 2.5 EC 0.24 0.04 0.16
7 3.75 EC 0.19 0.16 0.16 0.11
Avocado New Zealand 1980 6 2.5 EC 0.09 0.07 0.06 0.04
Grape 7 2.5 EC 0.46 0.37 0.26 0.19
Orange 1 5 EC 0.12skin 0.09skin
nd pulp nd pulp
0.07total 0.05total
1 10 EC nd pulp nd pulp
0.15 Total 0.07 total
0 7 14 28 42 56 84
(a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Kiwi fruit New Zealand 6 1.5 EC 0.34 1.71 0.29 1.43
5 25 EC 0.5 - 0.56 4.22 0.16 - 0.16 - 0.08
6 2.5 EC 0.15 - 0.11 0.34 0.08 0.26 0.03 - nd -
7 2.5 EC 0.25 - 0.34 2.45 0.17 1.21 0.16 - 0.07 - 0.06 -
6 3.0 EC 0.5 2.51 0.41 2.05
6 4.4 EC 2.5 12.5 2.5 12.5 1.9 9.6 1.7 8.8
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation 0 7 14 28 42 56 84
(g a.i./100 l) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Boysenberry 2 2.5 EC 0.26 0.15 0.12 0.07
2 2.5 EC 0.65 0.58 0.34 0.14
2 3.75 EC 0.29 0.32 0.22 0.12
1 2 4 6 9 14 45
Brussels Canada 1976 4 70 EC 0.09
sprout 70 EC 0.09
70 EC 0.05
Cranberry Canada 1977 2 70 EC 0.12(60days)
0.15(" )
0.08(")
Tomato Netherlands 1978 2 76 Smoke 0.58
gener. (0.36-0.75)
1979 1 - " 0.11
August (0.06-0.17)
1979 1 - " <0.01
October
Cucumber 1979 1 - " <0.01
October
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 1 2 4 6 9 14 45
Cabbage New Zealand 1980 1 25 EC 0.55 0.43 0.22 0.17
1 50 EC 0.54 0.78 0.36 0.22
1 100 EC 0.39 0.61 0.44 0.55
Sweet corn 1980 1 50 EC 2.7 nd 2.84 nd 1.04 nd 0.54 nd
foliage foliage foliage fol.
cob cob cob cob
Pasture 1980 1 250 EC 39 13 7 6 4 3
500 EC 45 24 9 9 7 3
750 EC 69 44 29 29 12 10
1000 EC 97 56 35 23 19 15
1500 EC 114 77 56 54 25 24
(See Figure 6). The photolysis of cis and trans isomers of permethrin
has been studied using material 14C-labelled in the carbonyl or
methylene moieties. Both permethrin isomers decomposed under
artificial light (peak outlet lambda 290-320nm) slightly faster in
hexane than in methanol. In each solvent, the cis isomer
photodecomposed approx. 1.6 times faster (T1/2 43 to 58 min) than the
trans isomer. The reacon involved extensive isomerization of the
cyclopropane ring, i.e. interconversion of the trans and cis isomers.
This probably occurred via a triplet energy state forming the
diradical intermediate (cleavage of cyclopropane ring), as the
reaction proceeded in the presence of phenoxybenzaldehyde and
benzophenone acting as photosensitizer, and was efficiently quenched
by 1,3-cyclohexadiene. The isomerization rate increased in the order
methanol < hexane < water, and at equilibrium after 1 to 4 h
irradiation the more thermodynamically stable trans isomer constituted
65 to 70% of the isomer mixture.
Together with the isomerization reaction, ester cleavage was
the major photoreaction in methanol, hexane, water and 2% aqueous
acetone. The major degradation products were DCVA and 3-phenoxybenzyl
alcohol. Other products formed in trace amounts in water included the
monochloropermethrin derivative, and the corresponding monochlorovinyl
acid, formed by reductive dechlorination. Permethrin and
monochloropermethrin do not undergo epoxidation at the dichlorovinyl
or chlorovinyl substituent under normal photooxidative conditions.
Photo oxidation to decarboxylated molecules was negligible with
permethrin (Holmstead et al 1977, 1978).
Exposure of the permethrin isomers on Dunkirk Silt Loam soil for
48 days resulted in approx. 55% loss in sunlight and approx. 35% loss
in the dark. The amount of radioactivity unextractable by 1:1 methanol
and ether was approx. 65% in the dark and approx. 18% in the light.
The unextractable radioactivity appears to be due to microbial
activity rather than to chemical reactions, but irradiation increased
the amount.
On/in the soil, relatively little isomerization at the
cyclopropane ring was encountered as compared with the photolysis in
solution. There was little difference in the amount of the DCVA
detected in the dark or light, and 3-phenoxybenzyl alcohol was the
major cleavage product of the alcohol moiety. Other products detected
in trace amounts were essentially the same as those in solutions
(Holmstead et al 1978).
In soil
The residues of permethrin in an organic soil and in vegetables
grown in soil treated with a granular formulation of permethrin were
studied by means of gas chromatography (Belanger and Hamilton 1979).
Permethrin persisted in the soil for the initial 28 days and then
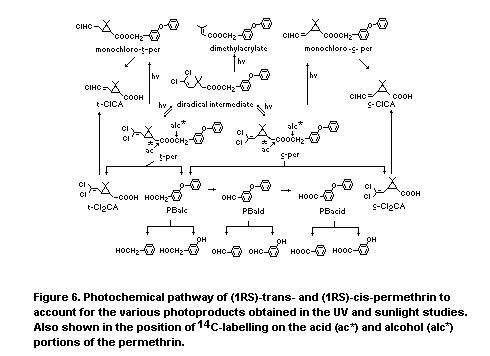 declined slowly during the rest of the season. Permethrin did not
translocate into the edible parts of the vegetables but was present in
the root system of onion and lettuce. Carrot and lettuce yields were
not significantly different from those of controls, but onion yields
were substantially decreased in the presence of permethrin.
Six soils, fortified with permethrin at 1 mg/kg, were incubated
for 16 weeks at temperatures alternating between 20°C for 15 h and
10°C for 9 h. Initially, and at 4-week intervals, soils were sampled
and analysed. In five of the soils, degradation of permethrin was
rapid, resulting in half-lives of approximately 3 weeks. In the other
soil very little degradation occurred, recovery after 16 weeks being
greater than 75%. Sterilization of the two soils in which degradation
was rapid greatly reduced the rate, indicating that microbial
degradation was the chief factor involved (Williams and Brown 1979).
On processing
The 1979 Meeting recommended an MRL of 20 mg/kg in dried tea.
Data are now available on residue levels in the final beverage
produced from tea leaves containing permethrin at four different
levels (in the range approximately 0.5 to 20 mg/kg). An aliquot sample
(5 g) of tea was brewed with 200 cm3 of boiling water, allowed to
cool and filtered. Only very low levels of permethrin were present in
the beverage (0.02-0.08 mg/kg) (Table 15). The residue level in the
beverage, expressed as a percentage of the corresponding residue level
in the dried tea, increased from 0.4%, when leaves containing
approximately 20 mg/kg were used, to 0.8% when leaves containing
approximately 3 mg/kg were used to prepare the beverage (Swaine
1981d). This pattern of data is consistent with the known low
solubility of permethrin in water.
TABLE 15. Permethrin residues in tea and tea leaves
Permethrin residues (mg/kg) Residue level in beverage × 100%
Tea leaves Tea (beverage) Residue level in leaves
18 0.08 0.4
10 0.06 0.6
2.6 0.02 0.8
0.43 <0.01 -
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits summarized in Table 16 have
been reported to the Meeting, in addition to those noted in 1979 and
1980.
TABLE 16. Additional national MRLs reported to the Meeting
mg/kg
Australia
Celery 5
Kiwi fruit 2
Rape seed, sunflower seed 0.2
Fat of meat, linseed, soybeans, mung beans and navy beans 0.1
Sweet corn 0.05 *
Beans 0.5
Water 0.3
Brazil
Cotton 0.5
Tomato 0.3
Cabbage and cauliflower 0.1
Maize 0.1
Soybean, coffee 0.01
France
Apple, pear, grape, lettuce and cabbage 1
Vegetables (other than lettuce and cabbage) 0.5
Wheat 0.5
Hungary
Fruit, vegetables 1
Netherlands
Kiwi fruit 2
Other fruit and vegetables 1
Meat and meat products, mushroom, potato 0.05
Milk and milk products 0.05
New Zealand
Bush and core fruit 1
Beans 0.5
South Africa
Sorghum 0.5
Beans 0.1
EVALUATION
COMMENTS AND APPRAISAL
Permethrin was evaluated by the 1979 and 1980 JMPR, which
considered extensive toxicological data on the compound. The 1979
Meeting required data on the potential bioaccumulation of the compound
and/or its metabolites, as well as observations in humans to evaluate
possible susceptibility to neurological effects noted in rodents,
before a full ADI for permethrin could be established.
The present Meeting considered pharmacokinetic studies on a wide
range of mammalian species. These indicated that permethrin is rapidly
absorbed and metabolized to more polar materials that are excreted.
Only small amounts of permethrin are taken up by adipose tissue and
these are principally of the less-rapidly hydrolysed cis-isomer. On
cessation of exposure, permethrin is promptly eliminated from adipose
tissue.
Information from observations in humans was also available to the
Meeting. This indicated that permethrin did not appear to cause the
transient abnormal facial sensations that are caused by exposure to
other synthetic pyrethroids. No other cause for concern was indicated.
Further studies on teratogenicity, mutagenicity and neurotoxicity
were also evaluated, none of which indicated cause for particular
concern.
The Meeting was of the view that the requirements of the previous
JMPR had been satisfied. However, the Meeting was made aware that
there were in existence at least three further long-term studies,
which were said to suggest possible carcinogenic risk from permethrin.
These studies were not available to the Meeting. In the absence of
these data, the present Meeting felt unable to do other than to extend
the temporary ADI established by the 1979 JMPR.
Residue trials on orange, lemon, grapefruit and tangerine provide
a basis for proposing maximum residue limits in citrus fruit. These
studies confirm that the residue is confined wholly within the peel
and that no residue is detected in flesh or juice.
Data from additional trials on kale and spinach, taken in
conjunction with the information considered in 1979, have enabled the
Meeting to confirm the recommendations for MRLs in spinach and to
propose a higher limit for kale. Information from trials on
boysenberry (dewberry), apple, pear, tomato and cucumber confirmed the
recommendations previously made for MRLs on dewberry and pome fruit.
Extensive information on the use patterns needed and approved for
the use of permethrin in 49 countries show the great diversity in the
pre-harvest interval (PHI). They tend to be much shorter in those
countries in which the majority of the potential use of permethrin
will arise than in countries with a temperate climate and a consequent
lesser threat from insect pests of crops. The slow rate of dissipation
of permethrin residues from plants reduces the significance of a
withholding period between treatment and harvest. The Meeting
considered this feature in conjunction with the low rate of
application and resulting low concentration of residues, and came to
the conclusion that it was appropriate to recommend MRLs based on the
minimum interval between application and harvest.
The Meeting was informed that the largest proportion of the
permethrin used world wide consists of a 40:60 ratio of cis:trans
isomers. It is recognized that products based on a 25:75 ratio of
cis:trans isomers are available. Until information becomes available
from significant usage of such materials in general agriculture/animal
husbandry, the Meeting will confine its recommendations to permethrin
products based principally on the 40:60 ratio (cis:trans isomer) of
permethrin. Data are available on the fate of permethrin on plants
both in the greenhouse and outdoors. Photolysis of permethrin in
various solvents with artificial light and on soil in sunlight
results primarily in cyclopropane ring isomerization and ester
cleavage to 3-phenoxybenzyl alcohol and 3-'2,2-dichlorovinyl)-2,
2-dimethylcyclopropane carboxylic acid (DCVA). These compounds are
also the major metabolites of permethrin on plants. Photoelimination
of carbon dioxide is a negligible route of photochemical degradation
of permethrin.
Questions were raised during the 13th Session of CCPR concerning
the appropriateness of the MRL for permethrin in dry manufactured tea.
Studies have shown that irrespective of the level of residue in the
tea, very little is leached out during the brewing of the beverage.
The Meeting felt that the recommendation should stand.
At the 13th Session of CCPR (1981) the Netherlands commented that
the limits for permethrin on gherkins and squash should preferably be
0.5 mg/kg, in line with those for cucumbers. The Meeting concurs that
the information in the 1979 monographs support this view and
recommendations were amended accordingly.
A number of national authorities have established additional MRLs
for permethrin. These were noted. Further studies in the fate of
permethrin in soil indicate that it is degraded in biologically active
soils and that the residue is not taken up into crops grown in such
treated soil.
The Meeting noted the omission of a recommendation for soybean
oil from the 1979 Evaluations, though the decision was recorded on
page 413.
Level causing no toxicological effect
Rat : 100 ppm in the diet, equivalent to 5.0 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.03 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The following maximum residue limits, determined and expressed as
total permethrin isomers excluding metabolites are recommended:
Commodity MRL (mg/kg)
Citrus fruits 0.5
Kale 5
Gherkins 0.5
Squash 0.5
Soybean oil1 0.1
1 Omitted from the 1979 recommendations
FURTHER WORK OR INFORMATION
Required (by 1982)
Reports of carcinogenicity studies not yet made available to the
JMPR.
Desirable
1. Mutagenicity studies on the metabolite 3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylic acid.
2. Results from further studies of residues in lettuce following
approved use patterns.
REFERENCES
Belanger, A. and Hamilton, H.D. Determination of disulfoton and
1979 permethrin residues in an organic soil and their
translocation into lettuce, onion and carrot. Journal of
Environmental Science and Health, B14(2):213-226.
Bewick, D.W. and Leahey, J.P. Permethrin: absorption in cows. ICI
1976 Plant Protection Division Report no. TMJ1357B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Bratt, H., Mills, I.H. and Slade, M. PP557: tissue retention in the
1977 rat. ICI Central Toxicology Laboratory Report no. CRL/P/352,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Bratt, H. and Slade, M. PP557: Tissue retention in the dog. ICI
1977 Central Toxicology Laboratory Report no. CTL/P/353,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Cridland, J.S. and Weatherley, B.C. Urinary excretion in man of
1977 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid ("CVA") after oral ingestion of permethrin (NRDC 143)-a
first report. Welcome Research Labs Report BDPG 77/1,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
1977 An estimate of permethrin (NRDC 143:OMS1821) absorbed by
people employed in a field trial of the insecticide (Kaduna,
Nigeria, 7-11 June 1977). Wellcome Research Labs. Report
BDPE 77/3, submitted by Wellcome Foundation Ltd. to WHO.
(Unpublished)
Dayan, A.D. 21-day neuropathological study in the Sprague-Dawley rat
1980 of permethrin (21Z73ZJ) administered in the diet. Wellcome
Research Labs. Report BPat.80/48, submitted by Wellcome
Foundation Ltd. to WHO. (Unpublished)
Edwards, M.J. and Iswaran, T.J. Permethrin: residue transfer and
1977 toxicology study with cows fed treated grass nuts, ICI Plant
Protection Division Report No. TMJ1519B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Elliot, M., Farnham, A.W., Janes, N.F., Needham, P.H., Pulman, D.A.
1973a and Stevenson, J.H. A photostable pyrethroid. Nature,
246:169.
1973b NRDC 143: a more stable pyrethroid. Proceedings of the 7th
British Insecticide and Fungicide Conference, p. 721.
Elliot, M., Janes, N.F., Pulman, D.A., Gaughan, L.C., Unai, T. and
1976 Casida, J.E. Radiosynthetis and metabolism in rats of the IR
isomers of the insecticide permethrin. Journal of
Agricultural and Food Chemistry, 24:270-276.
Fairbrother, D.A. NRDC 143. Whole body and radiography study in male
1977 rats. Report no. BPAT 787/3 Wellcome Research Laboratories,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Gaughan, L.E., Unai, T. and Casida, J.E. Permethrin metabolism in
1977 rats. Journal of Agricultural and Food Chemistry, 25:9-17
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. Distribution
1978a and metabolism of trans, and cis-permethrin in lactating
Jersey cows. Journal of Agricultural and Food Chemistry,
26:613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. Distribution and
1978b metabolic fate of trans- and cis-permethrin in laying hens.
Journal of Agricultural and Food Chemistry, 26:1374-1380.
Gaughan, L.C., Engel, J.L. and Casida, J.E. Pesticide interactions:
1980 effect of organo-phosphorus pesticides on the metabolism,
toxicity, and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology,
14:81-85.
Glickman, A.H. and Lech, J.J. Hydrolysis of permethrin, a pyrethroid
1981 insecticide, by rainbow trout and mouse tissues in vitro: a
comparative study. Toxicology and Applied Pharmacology,
60:186-192.
Glickman, A.H., Shono, T., Casida, J.E. and Lech, J.J. In vitro
1979 metabolism of permethrin isomers by carp and rainbow trout
liver microsomes. Journal of Agricultural and Food
Chemistry, 27:1038-1041.
Glickman, A.H., Hamid, A.A.R., Rickert, D.E. and Lech, J.J.
1981 Elimination and metabolism of permethrin isomers in rainbow
trout. Toxicology and Applied Pharmacology, 57:88-98.
Halls, G.R.H. and Periam, A.W. The fate of permethrin residues on
1980 wheat during storage and after milling and baking - results
after 9, 12 and 15 months storage. Wellcome Research Lab.
Report HEFH 80-3. (Unpublished)
Halls, G.R.H. The fate of permethrin residues on wheat during 9 months
1981 storage in Australia, Wellcome Research Laboratories Report
8 HEFH, 81-1. (Unpublished)
Holmstead, R.L., Casida, J.E. and Ruzo, L.O. Photochemical reactions
1977 of pyrethroid insecticides. Paper delivered at 172nd ACS
National Meeting, San Francisco, August 1976.
Holmstead, R.L., Casida, J.E.,Ruzo, L.O. and Fulmer, D.G. Pyrethroid
1978 photodecomposition: permethrin. Journal of Agricultural and
Food Chemistry, 26:590-595.
Hunt, L.M. and Gilbert, B.N. Distribution and excretion rates of 14C
1977 labelled permethrin isomers administered orally to four
lactating goats for 10 days. Journal of Agricultural and
Food Chemistry, 25 (3):673.
ICI.Permethrin: data submitted for review at the 1981 Meeting of WHO
1981 Panel of Experts on pesticide residues in food, to WHO by
Imperial Chemical Industries. (Unpublished)
Ivie, G.W. and Hunt, L.M. Metabolism of cis- and trans-permethrin in
1980 lactating goats. Journal of Agricultural and Food Chemistry,
28:1131-1138.
Jones, B.K. Cypermethrin: bioaccumulation study in the rat. ICI
1981 Central Toxicology Laboratory, Alderley Park Report no.
CTL/P/599, submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Kadota, T. Mammalian toxicological study of permethrin,
1976 3-phenoxybenzyl(+)-cis, trans-2.2-dimethyl-3-
(2,2-dichlorovinyl)-cyclopropane-l-carboxylate. Botyokagaku,
41:43.
Kolmodin-Hedman, B., Swensson, A. and Akerblom, M. Occupational
1981 exposure to some synthetic pyrethroids (permethrin and
fenvalerate) (in press).
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
1977a A.G. Permethrin: metabolism and residues in goats. ICI Plant
Protection Division report no. TMJ1516B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Legator, M.S. and Malling, H.V. The host-mediated assay, a practical
1971 procedure for evaluating potential mutagenic agents in
mammals, In: "Chemical Mutagens" A. Hollaender (ed.) Plenum
Press, New York, p.569.
LeQuesne, P.N., Maxwell, I.C. and Butterworth, T.G. Transient facial
1980 sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2:1.
Miyamoto, J. Degradation, metabolism and toxicity of synthetic
1976 pyrethroids. Environmental Health Perspectives, 14:15-28.
Morgan, D.W.T. A field trial to determine whether there is a change in
1979 the 25:75 cis:trans isomer ratio of permethrin following
application to cattle. Wellcome Research Laboratories Report
HIPH 79-1. (Unpublished)
Rickett, F.E. A review of the isomerization of permethrin. Wellcome
1981 Research Laboratories Report HEFA 81-2. (Unpublished)
Rickett, F.E. and Knight, P.J. Photostability of permethrin isomers.
1976 Wellcome Research Laboratories Report HCDF 76-1.
(Unpublished)
Ruzo, L.O. and Casida, J.E. Metabolism and toxicology of pyrethroids
1977 with dihalovinyl substituents. Environmental Health
Perspective, 21:285-292.
Soderlung, D.M. and Casida, J.E. Results quoted in Gaughan, L.C.,
1976 Unai, T. and Casida, J.E. 1977. (Unpublished)
Swaine, H. Permethrin residue levels on spinach and kale treated with
1981a "Ambush" during 1980 trials in the United Kingdom. ICI Plant
Protection Division Residue Data Report No. 454/PP557B034.
(Unpublished)
Swaine, H. Permethrin residues on grapefruit and tangerine treated
1981b with "Ambush" during 1980 trials in Spain. ICI Plant
Protection Division Residue Data Report No. 492/PP557B-086.
(Unpublished)
1981c Permethrin residues on citrus fruits (lemon, orange,
grapefruit) treated during a 1981 trial in the USA. ICI
Plant Protection Division Residue Data Report No. PP557B042.
(Unpublished)
1981d The transfer of permethrin residue from tea leaves to brewed
tea. ICI Plant Protection Division Residue Data Report no.
PP557B041. (Unpublished)
Swaine, H. and Sapiets, A. Cypermethrin: residue transfer study with
1981a dairy cows fed on a diet containing the insecticide. Report
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
1981b Cypermethrin: residue levels of the major metabolites of the
insecticide in the milk and tissues of dairy cows fed on a
diet containing cypermethrin at 50 mg/kg. Report submitted
by Imperial Chemical Industries to WHO. (Unpublished)
Wallwork, L.M. and Malone, J.C. 21Z73 (25/75). Acute toxicity studies
1975 by various routes of administration in the rat, mouse and
chick. Wellcome Research Laboratories report HEFF75-8,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Wallwork, L.M., Poll, G.S. and Malone, J.C. Effect on the fat oral
1975 toxicity of changes in the cis/trans ratio with 21Z73 (NRDC
143) series, Wellcome Research Labs. Report NEFC75-5,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Williams, L.M. 21Z73, rat oral 90-day study. Wellcome Research Labs.
1976 Report HEFF 76/1, submitted by Wellcome Foundation Ltd. to
WHO. (Unpublished)
Williams, I.H. and Brown, M.J. Persistence of permethrin and WL 43775
1979 in soil. Journal of Agricultural and Food Chemistry,
27(1):130-132.
declined slowly during the rest of the season. Permethrin did not
translocate into the edible parts of the vegetables but was present in
the root system of onion and lettuce. Carrot and lettuce yields were
not significantly different from those of controls, but onion yields
were substantially decreased in the presence of permethrin.
Six soils, fortified with permethrin at 1 mg/kg, were incubated
for 16 weeks at temperatures alternating between 20°C for 15 h and
10°C for 9 h. Initially, and at 4-week intervals, soils were sampled
and analysed. In five of the soils, degradation of permethrin was
rapid, resulting in half-lives of approximately 3 weeks. In the other
soil very little degradation occurred, recovery after 16 weeks being
greater than 75%. Sterilization of the two soils in which degradation
was rapid greatly reduced the rate, indicating that microbial
degradation was the chief factor involved (Williams and Brown 1979).
On processing
The 1979 Meeting recommended an MRL of 20 mg/kg in dried tea.
Data are now available on residue levels in the final beverage
produced from tea leaves containing permethrin at four different
levels (in the range approximately 0.5 to 20 mg/kg). An aliquot sample
(5 g) of tea was brewed with 200 cm3 of boiling water, allowed to
cool and filtered. Only very low levels of permethrin were present in
the beverage (0.02-0.08 mg/kg) (Table 15). The residue level in the
beverage, expressed as a percentage of the corresponding residue level
in the dried tea, increased from 0.4%, when leaves containing
approximately 20 mg/kg were used, to 0.8% when leaves containing
approximately 3 mg/kg were used to prepare the beverage (Swaine
1981d). This pattern of data is consistent with the known low
solubility of permethrin in water.
TABLE 15. Permethrin residues in tea and tea leaves
Permethrin residues (mg/kg) Residue level in beverage × 100%
Tea leaves Tea (beverage) Residue level in leaves
18 0.08 0.4
10 0.06 0.6
2.6 0.02 0.8
0.43 <0.01 -
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits summarized in Table 16 have
been reported to the Meeting, in addition to those noted in 1979 and
1980.
TABLE 16. Additional national MRLs reported to the Meeting
mg/kg
Australia
Celery 5
Kiwi fruit 2
Rape seed, sunflower seed 0.2
Fat of meat, linseed, soybeans, mung beans and navy beans 0.1
Sweet corn 0.05 *
Beans 0.5
Water 0.3
Brazil
Cotton 0.5
Tomato 0.3
Cabbage and cauliflower 0.1
Maize 0.1
Soybean, coffee 0.01
France
Apple, pear, grape, lettuce and cabbage 1
Vegetables (other than lettuce and cabbage) 0.5
Wheat 0.5
Hungary
Fruit, vegetables 1
Netherlands
Kiwi fruit 2
Other fruit and vegetables 1
Meat and meat products, mushroom, potato 0.05
Milk and milk products 0.05
New Zealand
Bush and core fruit 1
Beans 0.5
South Africa
Sorghum 0.5
Beans 0.1
EVALUATION
COMMENTS AND APPRAISAL
Permethrin was evaluated by the 1979 and 1980 JMPR, which
considered extensive toxicological data on the compound. The 1979
Meeting required data on the potential bioaccumulation of the compound
and/or its metabolites, as well as observations in humans to evaluate
possible susceptibility to neurological effects noted in rodents,
before a full ADI for permethrin could be established.
The present Meeting considered pharmacokinetic studies on a wide
range of mammalian species. These indicated that permethrin is rapidly
absorbed and metabolized to more polar materials that are excreted.
Only small amounts of permethrin are taken up by adipose tissue and
these are principally of the less-rapidly hydrolysed cis-isomer. On
cessation of exposure, permethrin is promptly eliminated from adipose
tissue.
Information from observations in humans was also available to the
Meeting. This indicated that permethrin did not appear to cause the
transient abnormal facial sensations that are caused by exposure to
other synthetic pyrethroids. No other cause for concern was indicated.
Further studies on teratogenicity, mutagenicity and neurotoxicity
were also evaluated, none of which indicated cause for particular
concern.
The Meeting was of the view that the requirements of the previous
JMPR had been satisfied. However, the Meeting was made aware that
there were in existence at least three further long-term studies,
which were said to suggest possible carcinogenic risk from permethrin.
These studies were not available to the Meeting. In the absence of
these data, the present Meeting felt unable to do other than to extend
the temporary ADI established by the 1979 JMPR.
Residue trials on orange, lemon, grapefruit and tangerine provide
a basis for proposing maximum residue limits in citrus fruit. These
studies confirm that the residue is confined wholly within the peel
and that no residue is detected in flesh or juice.
Data from additional trials on kale and spinach, taken in
conjunction with the information considered in 1979, have enabled the
Meeting to confirm the recommendations for MRLs in spinach and to
propose a higher limit for kale. Information from trials on
boysenberry (dewberry), apple, pear, tomato and cucumber confirmed the
recommendations previously made for MRLs on dewberry and pome fruit.
Extensive information on the use patterns needed and approved for
the use of permethrin in 49 countries show the great diversity in the
pre-harvest interval (PHI). They tend to be much shorter in those
countries in which the majority of the potential use of permethrin
will arise than in countries with a temperate climate and a consequent
lesser threat from insect pests of crops. The slow rate of dissipation
of permethrin residues from plants reduces the significance of a
withholding period between treatment and harvest. The Meeting
considered this feature in conjunction with the low rate of
application and resulting low concentration of residues, and came to
the conclusion that it was appropriate to recommend MRLs based on the
minimum interval between application and harvest.
The Meeting was informed that the largest proportion of the
permethrin used world wide consists of a 40:60 ratio of cis:trans
isomers. It is recognized that products based on a 25:75 ratio of
cis:trans isomers are available. Until information becomes available
from significant usage of such materials in general agriculture/animal
husbandry, the Meeting will confine its recommendations to permethrin
products based principally on the 40:60 ratio (cis:trans isomer) of
permethrin. Data are available on the fate of permethrin on plants
both in the greenhouse and outdoors. Photolysis of permethrin in
various solvents with artificial light and on soil in sunlight
results primarily in cyclopropane ring isomerization and ester
cleavage to 3-phenoxybenzyl alcohol and 3-'2,2-dichlorovinyl)-2,
2-dimethylcyclopropane carboxylic acid (DCVA). These compounds are
also the major metabolites of permethrin on plants. Photoelimination
of carbon dioxide is a negligible route of photochemical degradation
of permethrin.
Questions were raised during the 13th Session of CCPR concerning
the appropriateness of the MRL for permethrin in dry manufactured tea.
Studies have shown that irrespective of the level of residue in the
tea, very little is leached out during the brewing of the beverage.
The Meeting felt that the recommendation should stand.
At the 13th Session of CCPR (1981) the Netherlands commented that
the limits for permethrin on gherkins and squash should preferably be
0.5 mg/kg, in line with those for cucumbers. The Meeting concurs that
the information in the 1979 monographs support this view and
recommendations were amended accordingly.
A number of national authorities have established additional MRLs
for permethrin. These were noted. Further studies in the fate of
permethrin in soil indicate that it is degraded in biologically active
soils and that the residue is not taken up into crops grown in such
treated soil.
The Meeting noted the omission of a recommendation for soybean
oil from the 1979 Evaluations, though the decision was recorded on
page 413.
Level causing no toxicological effect
Rat : 100 ppm in the diet, equivalent to 5.0 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.03 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The following maximum residue limits, determined and expressed as
total permethrin isomers excluding metabolites are recommended:
Commodity MRL (mg/kg)
Citrus fruits 0.5
Kale 5
Gherkins 0.5
Squash 0.5
Soybean oil1 0.1
1 Omitted from the 1979 recommendations
FURTHER WORK OR INFORMATION
Required (by 1982)
Reports of carcinogenicity studies not yet made available to the
JMPR.
Desirable
1. Mutagenicity studies on the metabolite 3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylic acid.
2. Results from further studies of residues in lettuce following
approved use patterns.
REFERENCES
Belanger, A. and Hamilton, H.D. Determination of disulfoton and
1979 permethrin residues in an organic soil and their
translocation into lettuce, onion and carrot. Journal of
Environmental Science and Health, B14(2):213-226.
Bewick, D.W. and Leahey, J.P. Permethrin: absorption in cows. ICI
1976 Plant Protection Division Report no. TMJ1357B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Bratt, H., Mills, I.H. and Slade, M. PP557: tissue retention in the
1977 rat. ICI Central Toxicology Laboratory Report no. CRL/P/352,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Bratt, H. and Slade, M. PP557: Tissue retention in the dog. ICI
1977 Central Toxicology Laboratory Report no. CTL/P/353,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Cridland, J.S. and Weatherley, B.C. Urinary excretion in man of
1977 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid ("CVA") after oral ingestion of permethrin (NRDC 143)-a
first report. Welcome Research Labs Report BDPG 77/1,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
1977 An estimate of permethrin (NRDC 143:OMS1821) absorbed by
people employed in a field trial of the insecticide (Kaduna,
Nigeria, 7-11 June 1977). Wellcome Research Labs. Report
BDPE 77/3, submitted by Wellcome Foundation Ltd. to WHO.
(Unpublished)
Dayan, A.D. 21-day neuropathological study in the Sprague-Dawley rat
1980 of permethrin (21Z73ZJ) administered in the diet. Wellcome
Research Labs. Report BPat.80/48, submitted by Wellcome
Foundation Ltd. to WHO. (Unpublished)
Edwards, M.J. and Iswaran, T.J. Permethrin: residue transfer and
1977 toxicology study with cows fed treated grass nuts, ICI Plant
Protection Division Report No. TMJ1519B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Elliot, M., Farnham, A.W., Janes, N.F., Needham, P.H., Pulman, D.A.
1973a and Stevenson, J.H. A photostable pyrethroid. Nature,
246:169.
1973b NRDC 143: a more stable pyrethroid. Proceedings of the 7th
British Insecticide and Fungicide Conference, p. 721.
Elliot, M., Janes, N.F., Pulman, D.A., Gaughan, L.C., Unai, T. and
1976 Casida, J.E. Radiosynthetis and metabolism in rats of the IR
isomers of the insecticide permethrin. Journal of
Agricultural and Food Chemistry, 24:270-276.
Fairbrother, D.A. NRDC 143. Whole body and radiography study in male
1977 rats. Report no. BPAT 787/3 Wellcome Research Laboratories,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Gaughan, L.E., Unai, T. and Casida, J.E. Permethrin metabolism in
1977 rats. Journal of Agricultural and Food Chemistry, 25:9-17
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. Distribution
1978a and metabolism of trans, and cis-permethrin in lactating
Jersey cows. Journal of Agricultural and Food Chemistry,
26:613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. Distribution and
1978b metabolic fate of trans- and cis-permethrin in laying hens.
Journal of Agricultural and Food Chemistry, 26:1374-1380.
Gaughan, L.C., Engel, J.L. and Casida, J.E. Pesticide interactions:
1980 effect of organo-phosphorus pesticides on the metabolism,
toxicity, and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology,
14:81-85.
Glickman, A.H. and Lech, J.J. Hydrolysis of permethrin, a pyrethroid
1981 insecticide, by rainbow trout and mouse tissues in vitro: a
comparative study. Toxicology and Applied Pharmacology,
60:186-192.
Glickman, A.H., Shono, T., Casida, J.E. and Lech, J.J. In vitro
1979 metabolism of permethrin isomers by carp and rainbow trout
liver microsomes. Journal of Agricultural and Food
Chemistry, 27:1038-1041.
Glickman, A.H., Hamid, A.A.R., Rickert, D.E. and Lech, J.J.
1981 Elimination and metabolism of permethrin isomers in rainbow
trout. Toxicology and Applied Pharmacology, 57:88-98.
Halls, G.R.H. and Periam, A.W. The fate of permethrin residues on
1980 wheat during storage and after milling and baking - results
after 9, 12 and 15 months storage. Wellcome Research Lab.
Report HEFH 80-3. (Unpublished)
Halls, G.R.H. The fate of permethrin residues on wheat during 9 months
1981 storage in Australia, Wellcome Research Laboratories Report
8 HEFH, 81-1. (Unpublished)
Holmstead, R.L., Casida, J.E. and Ruzo, L.O. Photochemical reactions
1977 of pyrethroid insecticides. Paper delivered at 172nd ACS
National Meeting, San Francisco, August 1976.
Holmstead, R.L., Casida, J.E.,Ruzo, L.O. and Fulmer, D.G. Pyrethroid
1978 photodecomposition: permethrin. Journal of Agricultural and
Food Chemistry, 26:590-595.
Hunt, L.M. and Gilbert, B.N. Distribution and excretion rates of 14C
1977 labelled permethrin isomers administered orally to four
lactating goats for 10 days. Journal of Agricultural and
Food Chemistry, 25 (3):673.
ICI.Permethrin: data submitted for review at the 1981 Meeting of WHO
1981 Panel of Experts on pesticide residues in food, to WHO by
Imperial Chemical Industries. (Unpublished)
Ivie, G.W. and Hunt, L.M. Metabolism of cis- and trans-permethrin in
1980 lactating goats. Journal of Agricultural and Food Chemistry,
28:1131-1138.
Jones, B.K. Cypermethrin: bioaccumulation study in the rat. ICI
1981 Central Toxicology Laboratory, Alderley Park Report no.
CTL/P/599, submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Kadota, T. Mammalian toxicological study of permethrin,
1976 3-phenoxybenzyl(+)-cis, trans-2.2-dimethyl-3-
(2,2-dichlorovinyl)-cyclopropane-l-carboxylate. Botyokagaku,
41:43.
Kolmodin-Hedman, B., Swensson, A. and Akerblom, M. Occupational
1981 exposure to some synthetic pyrethroids (permethrin and
fenvalerate) (in press).
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
1977a A.G. Permethrin: metabolism and residues in goats. ICI Plant
Protection Division report no. TMJ1516B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Legator, M.S. and Malling, H.V. The host-mediated assay, a practical
1971 procedure for evaluating potential mutagenic agents in
mammals, In: "Chemical Mutagens" A. Hollaender (ed.) Plenum
Press, New York, p.569.
LeQuesne, P.N., Maxwell, I.C. and Butterworth, T.G. Transient facial
1980 sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2:1.
Miyamoto, J. Degradation, metabolism and toxicity of synthetic
1976 pyrethroids. Environmental Health Perspectives, 14:15-28.
Morgan, D.W.T. A field trial to determine whether there is a change in
1979 the 25:75 cis:trans isomer ratio of permethrin following
application to cattle. Wellcome Research Laboratories Report
HIPH 79-1. (Unpublished)
Rickett, F.E. A review of the isomerization of permethrin. Wellcome
1981 Research Laboratories Report HEFA 81-2. (Unpublished)
Rickett, F.E. and Knight, P.J. Photostability of permethrin isomers.
1976 Wellcome Research Laboratories Report HCDF 76-1.
(Unpublished)
Ruzo, L.O. and Casida, J.E. Metabolism and toxicology of pyrethroids
1977 with dihalovinyl substituents. Environmental Health
Perspective, 21:285-292.
Soderlung, D.M. and Casida, J.E. Results quoted in Gaughan, L.C.,
1976 Unai, T. and Casida, J.E. 1977. (Unpublished)
Swaine, H. Permethrin residue levels on spinach and kale treated with
1981a "Ambush" during 1980 trials in the United Kingdom. ICI Plant
Protection Division Residue Data Report No. 454/PP557B034.
(Unpublished)
Swaine, H. Permethrin residues on grapefruit and tangerine treated
1981b with "Ambush" during 1980 trials in Spain. ICI Plant
Protection Division Residue Data Report No. 492/PP557B-086.
(Unpublished)
1981c Permethrin residues on citrus fruits (lemon, orange,
grapefruit) treated during a 1981 trial in the USA. ICI
Plant Protection Division Residue Data Report No. PP557B042.
(Unpublished)
1981d The transfer of permethrin residue from tea leaves to brewed
tea. ICI Plant Protection Division Residue Data Report no.
PP557B041. (Unpublished)
Swaine, H. and Sapiets, A. Cypermethrin: residue transfer study with
1981a dairy cows fed on a diet containing the insecticide. Report
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
1981b Cypermethrin: residue levels of the major metabolites of the
insecticide in the milk and tissues of dairy cows fed on a
diet containing cypermethrin at 50 mg/kg. Report submitted
by Imperial Chemical Industries to WHO. (Unpublished)
Wallwork, L.M. and Malone, J.C. 21Z73 (25/75). Acute toxicity studies
1975 by various routes of administration in the rat, mouse and
chick. Wellcome Research Laboratories report HEFF75-8,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Wallwork, L.M., Poll, G.S. and Malone, J.C. Effect on the fat oral
1975 toxicity of changes in the cis/trans ratio with 21Z73 (NRDC
143) series, Wellcome Research Labs. Report NEFC75-5,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Williams, L.M. 21Z73, rat oral 90-day study. Wellcome Research Labs.
1976 Report HEFF 76/1, submitted by Wellcome Foundation Ltd. to
WHO. (Unpublished)
Williams, I.H. and Brown, M.J. Persistence of permethrin and WL 43775
1979 in soil. Journal of Agricultural and Food Chemistry,
27(1):130-132.
 The metabolism of the (1R, trans)- and (1R, cis)-ester of the
active isomers of permethrin following oral administration of 0.5 to
2.9 mg/kg to male Sprague-Dawley rats was examined using compounds
labelled with 14C in the acid or alcohol moieties (Elliot et al
1976). Preliminary results indicated that the organochlorine moiety of
(1R,trans)-and (1R, cis)-permethrin and the (1R, trans)-dichlorovinyl
acid was rapidly and almost completely eliminated from the body and
only traces remained in the fat and liver 4 days after oral
administration.
This ease of elimination is associated with the increased
polarity of the products, which results from rapid in vivo
glucuronidation of the dichlorovinyl acids and, to a lesser extent,
hydroxylation of one of the germinal dimethyl groups. It appeared that
at least some of the hydroxylated acids underwent minor degrees of
conjugation. Much less hydroxylated derivative was formed from the
(1R, trans)-dichlorovinyl acid itself than from the (1R, trans)-
permethrin, indicating that permethrin was hydroxylated to some extent
before hydrolysis. The predominant sites of hydroxylation in the
dichlorovinyl acid appear to be the 2-cis position for (1R, trans)-
permethrin and the 2-trans position for (1R, cis)-permethrin.
(Presumably these methyl groups are sterically favoured at the
hydroxylation site of the microsomal oxidase system.) Neither the
parent isomer nor any metabolites of permethrin remained for an
unusual period or in an unexpected locations in the organs examined.
Four days after treatment, the residues of permethrin and its
metabolites in tissues, determined as the total radiocarbon by
combustion, were below 0.01 ppm permethrin equivalents in blood, bone,
brain, fat, heart, kidney, liver, lungs, muscle, spleen and testes.
The largest persistence was for products derived from the 3-phenoxy-
benzyl alcohol in the fat, liver and kidney. Figure 2 illustrates the
structures and relative proportions of the metabolites of (1R, trans)-
and (1R, cis)-permethrin, of (1R,trans)dichlorovinyl acid and of
3,phenoxybenzyl alcohol.
The metabolism of the (1R, trans)- and (1R, cis)-ester of the
active isomers of permethrin following oral administration of 0.5 to
2.9 mg/kg to male Sprague-Dawley rats was examined using compounds
labelled with 14C in the acid or alcohol moieties (Elliot et al
1976). Preliminary results indicated that the organochlorine moiety of
(1R,trans)-and (1R, cis)-permethrin and the (1R, trans)-dichlorovinyl
acid was rapidly and almost completely eliminated from the body and
only traces remained in the fat and liver 4 days after oral
administration.
This ease of elimination is associated with the increased
polarity of the products, which results from rapid in vivo
glucuronidation of the dichlorovinyl acids and, to a lesser extent,
hydroxylation of one of the germinal dimethyl groups. It appeared that
at least some of the hydroxylated acids underwent minor degrees of
conjugation. Much less hydroxylated derivative was formed from the
(1R, trans)-dichlorovinyl acid itself than from the (1R, trans)-
permethrin, indicating that permethrin was hydroxylated to some extent
before hydrolysis. The predominant sites of hydroxylation in the
dichlorovinyl acid appear to be the 2-cis position for (1R, trans)-
permethrin and the 2-trans position for (1R, cis)-permethrin.
(Presumably these methyl groups are sterically favoured at the
hydroxylation site of the microsomal oxidase system.) Neither the
parent isomer nor any metabolites of permethrin remained for an
unusual period or in an unexpected locations in the organs examined.
Four days after treatment, the residues of permethrin and its
metabolites in tissues, determined as the total radiocarbon by
combustion, were below 0.01 ppm permethrin equivalents in blood, bone,
brain, fat, heart, kidney, liver, lungs, muscle, spleen and testes.
The largest persistence was for products derived from the 3-phenoxy-
benzyl alcohol in the fat, liver and kidney. Figure 2 illustrates the
structures and relative proportions of the metabolites of (1R, trans)-
and (1R, cis)-permethrin, of (1R,trans)dichlorovinyl acid and of
3,phenoxybenzyl alcohol.
 Mouse
Groups of 10 male and 10 female mice were fed permethrin in the
diet for 4 weeks at dosage levels of 0, 20, 500 and 4 000 ppm to
compare residue concentrations in adipose tissue with data obtained
from animals fed similar concentrations for 80 weeks. Residue levels
were consistently higher in females than in males. The residue levels
in peritoneal adipose tissue were essentially the same as those seen
in animals fed for over 80 weeks. There was a rapid build-up to
equilibrium levels in mice within 4 weeks of dietary exposure (FAD/WHO
1980).
Dog
Dogs were dosed orally with 40:60 cis:trans 14C-methylene-
labelled permethrin daily at 1 mg/kg bw/day for 10 days. This dose
level is equivalent to approximately 40 ppm in the diet. Levels of
radioactivity in adipose tissue 24 h after termination of dosing did
not exceed 6 ug permethrin equivalents/g. Radioactivity in the fat was
present as permethrin (Bratt and Slade 1977).
Cow
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bw, equivalent to approximately 80 ppm in the diet,
levels of radioactivity in milk reached a maximum of 0.13 µg
permethrin equivalents/g after 1 to 2 days. These declined to less
than 0.02 µg/g after 7 days. Levels of radioactivity in the fat were
0.12 to 0.18 µg permethrin equivalents after 7 days. By 14 days these
had declined to 0.05-0.06 µg/g, indicating that the small residues in
fat are also not maintained on cessation of dosing (Bewick and Leahey
1976).
Radiocarbon from 14C-acid- and 14C-alcohol-labelled preparations
of trans- and cis-permethrin administered orally to lactating Jersey
cows for 3 consecutive days at approximately 1.0 mg/kg was largely
eliminated from the body within 12 or 13 days after the initial
treatment (Gaughan et al 1978a). Milk and fat residues were
relatively low. Total 14C-permethrin equivalents in milk were
consistently below 0.3 µg/g and declined to less than 20% of their
highest values during the 10 days following cessation of exposure.
Residues recovered from fat and milk were higher when cis-permethrin,
rather than trans-permethrin, was administered and consisted almost
entirely (>85%) of unmetabolized permethrin, as well as trace levels
of cis-permethrin hydroxylated at the methyl group trans to the
ester functionality. Major excreted metabolites (each 8 to 28% of
the administered radiocarbon) from both isomers were: the esters
hydroxylated at the trans-methyl group; the acid moieties hydroxylated
at the cis-methyl group and the corresponding gamma-lactones;
3-phenoxybenzyl alcohol; the glutamic acid conjugate of
3-phenoxybenzoic acid. Additionally, 13 excreted metabolites of trans-
permethrin and 10 of cis-permethrin were tentatively identified. Total
14C-permethrin equivalents in blood reached a transient peak shortly
after each dose and dropped to insignificant levels within 2 to 4 days
after the last dose. Figure 3 illustrates the metabolic pathways to
convert trans- and cis-permethrin into more polar derivatives for
excretion. The permthrin isomers, although fat-soluble materials, were
rapidly metabolized and excreted by cows so that relatively little of
these compounds appeared in milk or were retained in tissues for more
than a few days.
Mouse
Groups of 10 male and 10 female mice were fed permethrin in the
diet for 4 weeks at dosage levels of 0, 20, 500 and 4 000 ppm to
compare residue concentrations in adipose tissue with data obtained
from animals fed similar concentrations for 80 weeks. Residue levels
were consistently higher in females than in males. The residue levels
in peritoneal adipose tissue were essentially the same as those seen
in animals fed for over 80 weeks. There was a rapid build-up to
equilibrium levels in mice within 4 weeks of dietary exposure (FAD/WHO
1980).
Dog
Dogs were dosed orally with 40:60 cis:trans 14C-methylene-
labelled permethrin daily at 1 mg/kg bw/day for 10 days. This dose
level is equivalent to approximately 40 ppm in the diet. Levels of
radioactivity in adipose tissue 24 h after termination of dosing did
not exceed 6 ug permethrin equivalents/g. Radioactivity in the fat was
present as permethrin (Bratt and Slade 1977).
Cow
When cows received a single oral dose of 40:60 cis:trans
14C-labelled permethrin (either cyclopropyl- or methylene-labelled)
at 2.5 mg/kg bw, equivalent to approximately 80 ppm in the diet,
levels of radioactivity in milk reached a maximum of 0.13 µg
permethrin equivalents/g after 1 to 2 days. These declined to less
than 0.02 µg/g after 7 days. Levels of radioactivity in the fat were
0.12 to 0.18 µg permethrin equivalents after 7 days. By 14 days these
had declined to 0.05-0.06 µg/g, indicating that the small residues in
fat are also not maintained on cessation of dosing (Bewick and Leahey
1976).
Radiocarbon from 14C-acid- and 14C-alcohol-labelled preparations
of trans- and cis-permethrin administered orally to lactating Jersey
cows for 3 consecutive days at approximately 1.0 mg/kg was largely
eliminated from the body within 12 or 13 days after the initial
treatment (Gaughan et al 1978a). Milk and fat residues were
relatively low. Total 14C-permethrin equivalents in milk were
consistently below 0.3 µg/g and declined to less than 20% of their
highest values during the 10 days following cessation of exposure.
Residues recovered from fat and milk were higher when cis-permethrin,
rather than trans-permethrin, was administered and consisted almost
entirely (>85%) of unmetabolized permethrin, as well as trace levels
of cis-permethrin hydroxylated at the methyl group trans to the
ester functionality. Major excreted metabolites (each 8 to 28% of
the administered radiocarbon) from both isomers were: the esters
hydroxylated at the trans-methyl group; the acid moieties hydroxylated
at the cis-methyl group and the corresponding gamma-lactones;
3-phenoxybenzyl alcohol; the glutamic acid conjugate of
3-phenoxybenzoic acid. Additionally, 13 excreted metabolites of trans-
permethrin and 10 of cis-permethrin were tentatively identified. Total
14C-permethrin equivalents in blood reached a transient peak shortly
after each dose and dropped to insignificant levels within 2 to 4 days
after the last dose. Figure 3 illustrates the metabolic pathways to
convert trans- and cis-permethrin into more polar derivatives for
excretion. The permthrin isomers, although fat-soluble materials, were
rapidly metabolized and excreted by cows so that relatively little of
these compounds appeared in milk or were retained in tissues for more
than a few days.
 Table 1 lists the radiocarbon recovery in excreta, milk, tissues,
organs and gut contents 12 or 13 days after treatment of three daily
doses of (14C)acid- or (14C)alcohol-trans-and cis-permethrin at
approximately 1 mg/kg for each dose.
TABLE 1. Radiocarbon recovery in excreta, milk, tissues, organs and
gut contents 12 or 13 days after initiating a treatment
schedule consisting of three daily doses of (14C)acid- or
(14C)alcohol-trans- and cis-permethrin at 1 mg/kg for
each dose
Recovery of administered radiocarbon(%)
Sample Trans Cis
Acid Alcohol Acid Alcohol
Urine 38.98 46.71 28.46 22.22
Faeces 51.60 57.24 60.06 75.85
Carbon dioxide1 <0.01 <0.01 <0.01
<0.01
Milk 0.03 0.44 0.26 0.18
Fat 0.15 0.40 1.59 0.64
Liver 0.03 0.04 0.08 0.06
Muscle 0.01 0.04 0.03 0.11
Skin 0.01 0.07 0.04 0.06
Other tissues 0.04 0.02 0.03 0.04
Gut contents <0.01 2.83 0.15 0.05
Total 90.85 107.79 90.70 99.21
1 Collected for 6 days only (includes bile). (Gaughan et al 1978).
Studies in which non-radiolabelled permethrin was administered to
cows support the finding that permethrin itself, rather than its polar
metabolites, consistitutes the predominant part of the small residues
present in milk and fat. Groups of three cows were maintained for 28
to 31 days on diets containing 40:60 cis:trans permethrin at 0.2, 1.0,
10, 50 and 150 ppm in the diet. Two of the cows were then sacrificed
and the third returned to control diet for a further 8 to 9 days
before sacrifice. Permethrin itself constituted more than 80% of the
residue determined in the milk. Mean plateau levels were below
0.01 µg/g at the 0.2 and 1.0 ppm dietary inclusion rates, 0.02 µg/g at
the 10 ppm rate, 0.1 µg/g at the 50 ppm rate and 0.3 µg/g at the
150 ppm rate. Milk was analysed for DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid. The highest metabolite residue detected in the
milk was 0.03 µg/g at the 150 ppm dose rate. In all cases, residue
levels in milk did not accumulate over the period of the study and
they declined rapidly, on returning animals to untreated diet, to
below 0.01 µg/g within five days (Edwards and Iswaran 1977; Swaine and
Sapiets 1981 a,b).
Permethrin itself constituted more than 85% of the residue
determined in adipose tissue (Table 2). Levels in peritoneal fat were
somewhat higher than those in subcutaneous fat, but were still small
(Tables 2 and 3). The small residues in adipose tissue declined
noticeably on cessation of exposure (Table 2).
TABLE 3. Residues in fat of cows receiving permethrin at
rates up to 150 ppm in the diet
Nominal Residues (µg/g) of permethrin in
dietary inclusion
level (ppm) Peritoneal fat Subcutaneous fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
150 2.8 -6.2 0.96-4.3
Permethrin itself was also the major component of the small
residues determined in adductor, pectoral and cardiac muscle. The
residue levels present were approximately 0.1 to 0.2% of corresponding
dietary inclusion levels. As found separately by Leahey et al
(1977) in the goat (see below), DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were major constituents of the residues in liver
and kidney. As with milk and fat residue levels in muscle, liver and
kidney, residues declined rapidly on cessation of exposure of the
animals to permethrin (Edwards and Iswaran 1977; Swaine and Sapiets
1981 a,b).
Goat
Cis-(IRS) and trans-(IRS)-permethrin, radiolabelled with 14C in
either the acid or alcohol moiety, were rapidly metabolized and
excreted after oral administration to lactating Nubian and Nubian-
Saanen cross goats (Ivie and Hunt 1980). In these studies, each of
four goats received 10 successive daily oral doses of one of the four
(14C)-permethrins; each dose ranged from 0.2 to 0.3 mg/kg bw/day,
depending on the 14C label and isomer given. Twenty six metabolites
of the permethrin isomers were fully or partly characterized by TLC or
GLC/MS, and several others (enclosed by brackets in Figure 4) although
not isolated are logical intermediates in the pathways defined. In the
TABLE 2. Residues of permethrin and three major metabolites in tissues of cows receiving
permethrin at 150 ppm in the diet
Residue (µg/g) of
Tissue Feeding
analysed regime1 Permethrin Cis + 3-BPAlc 3-PBAcid
Trans DCVA
(I and II) (III) (IV)
Muscle Treated 0.10-0.27 0.04-0.14 0.01-0.12 <0.01-0.05
Treated plus 0.03-0.07 <0.03 <0.01 <0.01
recovery
Subcutaneous Treated 2.9 -4.3 0.14-0.33 0.08-0.24 0.04-0.06
fat
Treated plus 0.96-1.2 <0.01 0.05 0.03-0.04
recovery
Peritoneal Treated 5.4 -6.2 0.16-0.32 0.20-0.29 0.02-0.05
fat
Treated plus 2.8 -3.1 0.09 0.08-0.10 <0.01
recovery
Liver Treated 0.01-0.03 0.18-0.26 0.72-1.1 0.36-0.57
Treated plus <0.01 <0.01 0.01-0.08 0.03-0.08
recovery
Kidney Treated 0.16-0.43 0.37-0.49 0.72-0.91 0.19-0.39
Treated plus 0.04-0.05 <0.01 0.02-0.05 <0.01
recovery
1 Treated - indicates the animals received treated diet for 28-29 days and were then slaughtered.
Treated plus recovery - indicates the animals received treated diet for 28 days and were
then returned to untreated diet for a further 8 days before slaughter.
Table 1 lists the radiocarbon recovery in excreta, milk, tissues,
organs and gut contents 12 or 13 days after treatment of three daily
doses of (14C)acid- or (14C)alcohol-trans-and cis-permethrin at
approximately 1 mg/kg for each dose.
TABLE 1. Radiocarbon recovery in excreta, milk, tissues, organs and
gut contents 12 or 13 days after initiating a treatment
schedule consisting of three daily doses of (14C)acid- or
(14C)alcohol-trans- and cis-permethrin at 1 mg/kg for
each dose
Recovery of administered radiocarbon(%)
Sample Trans Cis
Acid Alcohol Acid Alcohol
Urine 38.98 46.71 28.46 22.22
Faeces 51.60 57.24 60.06 75.85
Carbon dioxide1 <0.01 <0.01 <0.01
<0.01
Milk 0.03 0.44 0.26 0.18
Fat 0.15 0.40 1.59 0.64
Liver 0.03 0.04 0.08 0.06
Muscle 0.01 0.04 0.03 0.11
Skin 0.01 0.07 0.04 0.06
Other tissues 0.04 0.02 0.03 0.04
Gut contents <0.01 2.83 0.15 0.05
Total 90.85 107.79 90.70 99.21
1 Collected for 6 days only (includes bile). (Gaughan et al 1978).
Studies in which non-radiolabelled permethrin was administered to
cows support the finding that permethrin itself, rather than its polar
metabolites, consistitutes the predominant part of the small residues
present in milk and fat. Groups of three cows were maintained for 28
to 31 days on diets containing 40:60 cis:trans permethrin at 0.2, 1.0,
10, 50 and 150 ppm in the diet. Two of the cows were then sacrificed
and the third returned to control diet for a further 8 to 9 days
before sacrifice. Permethrin itself constituted more than 80% of the
residue determined in the milk. Mean plateau levels were below
0.01 µg/g at the 0.2 and 1.0 ppm dietary inclusion rates, 0.02 µg/g at
the 10 ppm rate, 0.1 µg/g at the 50 ppm rate and 0.3 µg/g at the
150 ppm rate. Milk was analysed for DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid. The highest metabolite residue detected in the
milk was 0.03 µg/g at the 150 ppm dose rate. In all cases, residue
levels in milk did not accumulate over the period of the study and
they declined rapidly, on returning animals to untreated diet, to
below 0.01 µg/g within five days (Edwards and Iswaran 1977; Swaine and
Sapiets 1981 a,b).
Permethrin itself constituted more than 85% of the residue
determined in adipose tissue (Table 2). Levels in peritoneal fat were
somewhat higher than those in subcutaneous fat, but were still small
(Tables 2 and 3). The small residues in adipose tissue declined
noticeably on cessation of exposure (Table 2).
TABLE 3. Residues in fat of cows receiving permethrin at
rates up to 150 ppm in the diet
Nominal Residues (µg/g) of permethrin in
dietary inclusion
level (ppm) Peritoneal fat Subcutaneous fat
0.2 <0.01-0.04 <0.01
1.0 0.01-0.02 <0.01
10 0.02-0.25 <0.01-0.09
50 0.46-1.1 0.10-0.42
150 2.8 -6.2 0.96-4.3
Permethrin itself was also the major component of the small
residues determined in adductor, pectoral and cardiac muscle. The
residue levels present were approximately 0.1 to 0.2% of corresponding
dietary inclusion levels. As found separately by Leahey et al
(1977) in the goat (see below), DCVA, 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid were major constituents of the residues in liver
and kidney. As with milk and fat residue levels in muscle, liver and
kidney, residues declined rapidly on cessation of exposure of the
animals to permethrin (Edwards and Iswaran 1977; Swaine and Sapiets
1981 a,b).
Goat
Cis-(IRS) and trans-(IRS)-permethrin, radiolabelled with 14C in
either the acid or alcohol moiety, were rapidly metabolized and
excreted after oral administration to lactating Nubian and Nubian-
Saanen cross goats (Ivie and Hunt 1980). In these studies, each of
four goats received 10 successive daily oral doses of one of the four
(14C)-permethrins; each dose ranged from 0.2 to 0.3 mg/kg bw/day,
depending on the 14C label and isomer given. Twenty six metabolites
of the permethrin isomers were fully or partly characterized by TLC or
GLC/MS, and several others (enclosed by brackets in Figure 4) although
not isolated are logical intermediates in the pathways defined. In the
TABLE 2. Residues of permethrin and three major metabolites in tissues of cows receiving
permethrin at 150 ppm in the diet
Residue (µg/g) of
Tissue Feeding
analysed regime1 Permethrin Cis + 3-BPAlc 3-PBAcid
Trans DCVA
(I and II) (III) (IV)
Muscle Treated 0.10-0.27 0.04-0.14 0.01-0.12 <0.01-0.05
Treated plus 0.03-0.07 <0.03 <0.01 <0.01
recovery
Subcutaneous Treated 2.9 -4.3 0.14-0.33 0.08-0.24 0.04-0.06
fat
Treated plus 0.96-1.2 <0.01 0.05 0.03-0.04
recovery
Peritoneal Treated 5.4 -6.2 0.16-0.32 0.20-0.29 0.02-0.05
fat
Treated plus 2.8 -3.1 0.09 0.08-0.10 <0.01
recovery
Liver Treated 0.01-0.03 0.18-0.26 0.72-1.1 0.36-0.57
Treated plus <0.01 <0.01 0.01-0.08 0.03-0.08
recovery
Kidney Treated 0.16-0.43 0.37-0.49 0.72-0.91 0.19-0.39
Treated plus 0.04-0.05 <0.01 0.02-0.05 <0.01
recovery
1 Treated - indicates the animals received treated diet for 28-29 days and were then slaughtered.
Treated plus recovery - indicates the animals received treated diet for 28 days and were
then returned to untreated diet for a further 8 days before slaughter.
 goat, permethrin was rapidly and extensively degraded via hydrolytic,
oxidative and conjugative reactions, e.g., through hydrolysis of the
ester linkage, hydroxylation of the cis- or trans-methyl of the
germinal dimethyl group and hydroxylation of the 4'-position of the
phenoxybenzyl moiety. Certain of these products were further oxidized
and/or conjugated with glycine, glutamic acid, glucuronic acid, or
other unidentified compounds before excretion.
Unmetabolized permethrin and certain ester metabolites were found
in faeces, milk and fat from the treated goats, but only metabolites
arising from ester hydrolysis were found in the urine.
The patterns of radiocarbon elimination and tissue retention
by these goats were reported earlier by Hunt and Gilbert (1977) and
are briefly summarized in Table 4. Urine was the major route of
radiocarbon excretion in goats treated with (14C-acid) or
(14C-alcohol) preparations of trans-permethrin, but most of the
administered radiocarbon was eliminated via the faeces in cis-
permethrin-treated goats.
TABLE 4. Summary of radiocarbon elimination and residue retention by
lactating goats orally treated for 10 consecutive days with
(14C) alcohol- or (14C)Acid-labelled cis-(1RS)or
trans-(1RS)-permethrin isomers1, 2
Radiocarbon eliminated Tissue residues
Label and isomer cum % dose (ppm)3, 4
faeces urine milk liver kidney fat
(14Cacid)-c-per 67.5 25.8 0.66 0.12 0.05 0.23
(14C-alc)-c-per 51.7 36.4 0.53 0.13 0.05 0.24
(14C-acid)-t-per 15.0 72.1 0.17 0.04 0.03 0.02
(14C-alc)-t-per 12.3 79.4 0.24 0.01 0.03 0.02
1 Data summarized from Hunt and Gilbert (1977).
2 Goats treated with 10 successive daily oral doses of the
appropriate (14C) per isomer (0.2-0.3 mg/kg per d.).
3 Tissues taken 24 h after last dose.
4 Other edible tissues contained lower residues.
Total radioactive residues in milk reached a plateau after
3 days of 0.02 to 0.05 µg permethrin equivalents/g and <0.01 µg/g
respectively for the cis- and trans-isomers. Unmetabolized permethrin
was a major component of the radioactivity in the milk. The goats were
sacrificed 24 h after receiving the final dose. Of the edible tissues
analysed for radiocarbon 24 h after the final permethrin doses, fat,
kidney and liver contained the highest residues. Radiocarbon retained
by the tissues and that secreted into the milk were appreciably higher
in goats treated with cis-permethrin than with trans-permethrin (Table
4, Hunt and Gilbert 1977). Total radioactivity of the fat of the
animals receiving the cis-isomer was ten times higher than those
receiving the more easily hydrolysed trans-isomer and was due mainly
to unmetabolized permethrin (Hunt and Gilbert 1977; Ivie and Hunt
1980).
In another study, goats were orally dosed with 40:60 cis:trans-
14C-labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 ppm in the diet for 7 days. Total
radioactive residues in the milk reached a plateau of 0.02-0.03 µg
permethrin equivalents/g after 5 days. Of this radioactivity, 30 to
50% was associated with the butterfat fraction of the milk in which
total radioactive residues were 0.13-0.27 µg permethrin equivalents/g.
The animals were sacrificed 4 h after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 5. Where
alcohol-labelled permethrin was used, approximately 70% of the
radioactivity in kidney was due to 3-phenoxybenzoic acid plus
3-(4-hydroxyphenoxy)benzyl alcohol. A further 15% was due to
3-phenoxybenzoic acid plus 3-(4-hydroxyphenoxy)benzoic acid. Where
acid-labelled permethrin was used, approximately 10 to 15% of the
label in liver and kidney was due to the cis and trans DCVA,
principally the trans isomer (Leahey et al 1977).
It is important to compare the metabolic fate of permethrin in
the species noted above. With reference to previously published
studies on the fate of (14C)-permethrin in mammals, specifically rats
(Elliott et al 1976; Gaughan et al 1977) and lactating cattle
(Gaughan et al 1978a) the following similarities and differences
between goats and these mammals were noted by Ivie and Hunt (1980).
1) In each of the three species, a greater percentage of an
administered cis-permethrin dose was eliminated in the faeces than was
a trans-permethrin dose, a pattern that appears to be most pronounced
in goats and least in cattle. It was suggested that trans-permethrin
was absorbed more rapidly than cis-permethrin from the G.I. tract, or
alternatively, isomer differences in the rates of biliary excretion of
permethrin and/or its metabolites may account for the above
observations.
2) Although retention of permethrin by tissues and its excretion
into milk of mammals was minimal, cis-permethrin and its metabolites
in rat, lactating goat and lactating cattle were retained by tissues
to a more significant degree than was trans-permethrin.
3) Primary metabolism of permethrin in rats involves attack at five
major sites, including ester cleavage, hydroxylation at the cis- or
trans-methyl of the germinal dimethyl moiety, and hydroxylation at the
2' or 4'- position of the phenoxybenzyl moiety. Ester cleavage and
TABLE 5. Total radiolabelled residues in tissues of goats receiving 14C-labelled permethrin daily orally for 7 to 10 days
Material Dose level Duration of Period between Total residue (µg permethrin equivalents/g)in
administered (ppm in administration last date and
diet) (days) sacrifice (hours) Fat Muscle Liver Kidney
Permethrin -10 7 4 <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis:trans
Permethrin -6 10 24 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin -6 10 24 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
4'-hydroxylation are the major routes of metabolism. Cattle and goats
metabolize permethrin similarly, with the exception that
2'-hydroxylation apparently does not occur in these ruminants.
4) Ester metabolites of permethrin were eliminated primarily through
the faeces of rats, cattle and goats, in contrast to cattle which
eliminated large quantities of ester metabolites of both cis-
permethrin and trans-permethrin in faeces.
5) In each species, conjugation of permethrin metabolites before
urinary excretion was extensive. Rats, cattle and goats eliminated the
acid moiety in urine primarily as conjugates with glucuronic acid. The
alcohol moiety was excreted, mostly as phenoxybenzoic acid glucuronide
or 4'-hydroxyphenoxybenzoic acid-sulphate in rats, but amino acid
conjugates of phenoxybenzoic acid comprised most of the excreted
products in cattle and goats. Although conjugation of phenoxybenzoic
acid with glycine was preferred in goats, conjugation with glutamic
acid was favoured in cattle.
The metabolic fate of permethrin in the goat is similar to that
in the cow. Radioactivity deriving from the oral administration of the
cis-isomer of permethrin is excreted mainly in the faeces, whereas
that deriving from the more readily hydrolysed trans-isomer is
excreted mainly in the urine. Radioactivity in faeces is due
principally to permethrin and, in the case of cis-isomer, to
4'-hydroxypermethrin. the predominant urinary metabolites are DCVA and
3-phenoxybenzoic acid, which are excreted mainly as polar conjugates
(Ivie and Hunt 1980).
The major products of the metabolism of permethrin in the cow are
similar to those in the rat. The most notable differences are that in
the cow a greater percentage of the administered radioactivity is
excreted in faeces as intact ester metabolites, notably as 4'-
hydroxypermethrin. In cow urine, the major degradation product derived
from the alcohol part of the molecule is 3-phenoxybenzoic acid,
whereas in rats it is 3-(4-hydroxyphenoxy) benzoic acid. DCVA is the
major urinary metabolite derived from the acid part of the molecule in
both rats and cows. In all cases, the major urinary metabolites are
excreted as polar conjugates (FAO/WHO 1980).
Hen
Radiocarbon from 14C-carbonyl- and 14C-methylene-labelled
preparations of (1RS)-trans- and (1RS)-cis-permethrin administered to
laying hens for three consecutive days at 10 mg/kg for each dose was
largely eliminated from the body within 1 day after the last dose, a
portion as 14CO2 (Gaughan et al 1978b). The excreta contained all
and the eggs most of the following compounds: the unmetabolized
pyrethroids; cis-permethrin hydroxylated at the 4'-position, at the
methyl group trans to the carboxyl, and at both of these sites; the
di-chlorovinyl phenoxybenzoic acid and their 4'-hydroxy derivatives;
sulphate, glucuronide, taurine and other conjugates of these alcohols
and acids (Figure 5). Residues of unmetabolized trans- and cis-
permethrin in fat were 0.15 and 0.93 ppm respectively, at 7 days after
the last dose, and in eggs they reached peak levels of 0.3 and 1.2 ppm
respectively, at 3 to 4 days after the last dose. Almost half of the
residues in eggs were unmetabolized trans- and cis-permethrin in the
yolk and the remainder was a great variety of metabolites in the yolk
and white, including most of those also detected in the excreta.
The preference in hydroxylation site based on identified
metabolites is the same with hens and rats, e.g., phenoxy > cis-
methyl > trans-methyl with cis-permethrin, In contrast, with cows,
both trans- and cis-permethrin have the same preference order of
trans-methyl > cis-methyl = phenoxy.
Metabolites detected in hens, but not in rats or cows, are the
cis isomer of trans-hydroxy permethrin sulphate, the trans isomer of
cis-hydroxy-phenoxybenzyl alcohol-sulphate and Cl2CA-taurine isomers.
Both hens and rats extensively utilize glucuronic acid and
sulphate conjugates in excretion of permethrin metabolites, while
glutamic acid conjugates are most significant in cowsœ
Trout
The distribution and metabolism of the cis- and trans-permethrin
isomers were studied in rainbow trout to evaluate the role of these
parameters in the differential toxicity of permethrin to fish and
mammals (Glickman et al 1981). Both (14C)-permethrin geometrical
isomers were readily taken up and eliminated by rainbow trout and
there appeared to be little difference in the rate of uptake of the
two geometric isomers. Elimination half-lives for (14C)-permethrin
residues in trout tissues, with the exception of fat, were in the
magnitude of hours. High concentrations of a polar metabolite
(glucuronide conjugate of 4'-hydroxypermethrin) were found in bile
within 4 h of cis- and trans-permethrin exposure. Urine contained a
small amount of a polar metabolite that was resistant to hydrolysis by
beta-glucuronidase but was cleaved to some extent by aryl-sulphatase.
The relative absence of permethrin hydrolysis products in trout bile
and the small amount of radioactivity excreted in urine suggested that
the ability of rainbow trout to hydrolyse permethrin in vivo was
minimal. The inability of the rainbow trout to hydrolyse permethrin
rapidly may result in an overall low rate of detoxification, which was
suggested to be a possible factor in the trout's sensitivity to the
compound, particularly the trans-isomer. However, it was also noted
that detoxification may not be the sole factor, as the trout may be
more sensitive physiologically than mammals to permethrin (Glickman
et al 1981).
goat, permethrin was rapidly and extensively degraded via hydrolytic,
oxidative and conjugative reactions, e.g., through hydrolysis of the
ester linkage, hydroxylation of the cis- or trans-methyl of the
germinal dimethyl group and hydroxylation of the 4'-position of the
phenoxybenzyl moiety. Certain of these products were further oxidized
and/or conjugated with glycine, glutamic acid, glucuronic acid, or
other unidentified compounds before excretion.
Unmetabolized permethrin and certain ester metabolites were found
in faeces, milk and fat from the treated goats, but only metabolites
arising from ester hydrolysis were found in the urine.
The patterns of radiocarbon elimination and tissue retention
by these goats were reported earlier by Hunt and Gilbert (1977) and
are briefly summarized in Table 4. Urine was the major route of
radiocarbon excretion in goats treated with (14C-acid) or
(14C-alcohol) preparations of trans-permethrin, but most of the
administered radiocarbon was eliminated via the faeces in cis-
permethrin-treated goats.
TABLE 4. Summary of radiocarbon elimination and residue retention by
lactating goats orally treated for 10 consecutive days with
(14C) alcohol- or (14C)Acid-labelled cis-(1RS)or
trans-(1RS)-permethrin isomers1, 2
Radiocarbon eliminated Tissue residues
Label and isomer cum % dose (ppm)3, 4
faeces urine milk liver kidney fat
(14Cacid)-c-per 67.5 25.8 0.66 0.12 0.05 0.23
(14C-alc)-c-per 51.7 36.4 0.53 0.13 0.05 0.24
(14C-acid)-t-per 15.0 72.1 0.17 0.04 0.03 0.02
(14C-alc)-t-per 12.3 79.4 0.24 0.01 0.03 0.02
1 Data summarized from Hunt and Gilbert (1977).
2 Goats treated with 10 successive daily oral doses of the
appropriate (14C) per isomer (0.2-0.3 mg/kg per d.).
3 Tissues taken 24 h after last dose.
4 Other edible tissues contained lower residues.
Total radioactive residues in milk reached a plateau after
3 days of 0.02 to 0.05 µg permethrin equivalents/g and <0.01 µg/g
respectively for the cis- and trans-isomers. Unmetabolized permethrin
was a major component of the radioactivity in the milk. The goats were
sacrificed 24 h after receiving the final dose. Of the edible tissues
analysed for radiocarbon 24 h after the final permethrin doses, fat,
kidney and liver contained the highest residues. Radiocarbon retained
by the tissues and that secreted into the milk were appreciably higher
in goats treated with cis-permethrin than with trans-permethrin (Table
4, Hunt and Gilbert 1977). Total radioactivity of the fat of the
animals receiving the cis-isomer was ten times higher than those
receiving the more easily hydrolysed trans-isomer and was due mainly
to unmetabolized permethrin (Hunt and Gilbert 1977; Ivie and Hunt
1980).
In another study, goats were orally dosed with 40:60 cis:trans-
14C-labelled permethrin (cyclopropyl or methylene-labelled) at a rate
equivalent to approximately 10 ppm in the diet for 7 days. Total
radioactive residues in the milk reached a plateau of 0.02-0.03 µg
permethrin equivalents/g after 5 days. Of this radioactivity, 30 to
50% was associated with the butterfat fraction of the milk in which
total radioactive residues were 0.13-0.27 µg permethrin equivalents/g.
The animals were sacrificed 4 h after receiving the final dose, when
levels of radiocarbon in meat tissues were as shown in Table 5. Where
alcohol-labelled permethrin was used, approximately 70% of the
radioactivity in kidney was due to 3-phenoxybenzoic acid plus
3-(4-hydroxyphenoxy)benzyl alcohol. A further 15% was due to
3-phenoxybenzoic acid plus 3-(4-hydroxyphenoxy)benzoic acid. Where
acid-labelled permethrin was used, approximately 10 to 15% of the
label in liver and kidney was due to the cis and trans DCVA,
principally the trans isomer (Leahey et al 1977).
It is important to compare the metabolic fate of permethrin in
the species noted above. With reference to previously published
studies on the fate of (14C)-permethrin in mammals, specifically rats
(Elliott et al 1976; Gaughan et al 1977) and lactating cattle
(Gaughan et al 1978a) the following similarities and differences
between goats and these mammals were noted by Ivie and Hunt (1980).
1) In each of the three species, a greater percentage of an
administered cis-permethrin dose was eliminated in the faeces than was
a trans-permethrin dose, a pattern that appears to be most pronounced
in goats and least in cattle. It was suggested that trans-permethrin
was absorbed more rapidly than cis-permethrin from the G.I. tract, or
alternatively, isomer differences in the rates of biliary excretion of
permethrin and/or its metabolites may account for the above
observations.
2) Although retention of permethrin by tissues and its excretion
into milk of mammals was minimal, cis-permethrin and its metabolites
in rat, lactating goat and lactating cattle were retained by tissues
to a more significant degree than was trans-permethrin.
3) Primary metabolism of permethrin in rats involves attack at five
major sites, including ester cleavage, hydroxylation at the cis- or
trans-methyl of the germinal dimethyl moiety, and hydroxylation at the
2' or 4'- position of the phenoxybenzyl moiety. Ester cleavage and
TABLE 5. Total radiolabelled residues in tissues of goats receiving 14C-labelled permethrin daily orally for 7 to 10 days
Material Dose level Duration of Period between Total residue (µg permethrin equivalents/g)in
administered (ppm in administration last date and
diet) (days) sacrifice (hours) Fat Muscle Liver Kidney
Permethrin -10 7 4 <0.01 <0.014 0.12-0.34 0.31-0.41
40:60 cis:trans
Permethrin -6 10 24 0.01-0.03 <0.01 0.01-0.04 0.03
trans-isomer
Permethrin -6 10 24 0.22-0.25 <0.01 0.12-0.13 0.05
cis-isomer
4'-hydroxylation are the major routes of metabolism. Cattle and goats
metabolize permethrin similarly, with the exception that
2'-hydroxylation apparently does not occur in these ruminants.
4) Ester metabolites of permethrin were eliminated primarily through
the faeces of rats, cattle and goats, in contrast to cattle which
eliminated large quantities of ester metabolites of both cis-
permethrin and trans-permethrin in faeces.
5) In each species, conjugation of permethrin metabolites before
urinary excretion was extensive. Rats, cattle and goats eliminated the
acid moiety in urine primarily as conjugates with glucuronic acid. The
alcohol moiety was excreted, mostly as phenoxybenzoic acid glucuronide
or 4'-hydroxyphenoxybenzoic acid-sulphate in rats, but amino acid
conjugates of phenoxybenzoic acid comprised most of the excreted
products in cattle and goats. Although conjugation of phenoxybenzoic
acid with glycine was preferred in goats, conjugation with glutamic
acid was favoured in cattle.
The metabolic fate of permethrin in the goat is similar to that
in the cow. Radioactivity deriving from the oral administration of the
cis-isomer of permethrin is excreted mainly in the faeces, whereas
that deriving from the more readily hydrolysed trans-isomer is
excreted mainly in the urine. Radioactivity in faeces is due
principally to permethrin and, in the case of cis-isomer, to
4'-hydroxypermethrin. the predominant urinary metabolites are DCVA and
3-phenoxybenzoic acid, which are excreted mainly as polar conjugates
(Ivie and Hunt 1980).
The major products of the metabolism of permethrin in the cow are
similar to those in the rat. The most notable differences are that in
the cow a greater percentage of the administered radioactivity is
excreted in faeces as intact ester metabolites, notably as 4'-
hydroxypermethrin. In cow urine, the major degradation product derived
from the alcohol part of the molecule is 3-phenoxybenzoic acid,
whereas in rats it is 3-(4-hydroxyphenoxy) benzoic acid. DCVA is the
major urinary metabolite derived from the acid part of the molecule in
both rats and cows. In all cases, the major urinary metabolites are
excreted as polar conjugates (FAO/WHO 1980).
Hen
Radiocarbon from 14C-carbonyl- and 14C-methylene-labelled
preparations of (1RS)-trans- and (1RS)-cis-permethrin administered to
laying hens for three consecutive days at 10 mg/kg for each dose was
largely eliminated from the body within 1 day after the last dose, a
portion as 14CO2 (Gaughan et al 1978b). The excreta contained all
and the eggs most of the following compounds: the unmetabolized
pyrethroids; cis-permethrin hydroxylated at the 4'-position, at the
methyl group trans to the carboxyl, and at both of these sites; the
di-chlorovinyl phenoxybenzoic acid and their 4'-hydroxy derivatives;
sulphate, glucuronide, taurine and other conjugates of these alcohols
and acids (Figure 5). Residues of unmetabolized trans- and cis-
permethrin in fat were 0.15 and 0.93 ppm respectively, at 7 days after
the last dose, and in eggs they reached peak levels of 0.3 and 1.2 ppm
respectively, at 3 to 4 days after the last dose. Almost half of the
residues in eggs were unmetabolized trans- and cis-permethrin in the
yolk and the remainder was a great variety of metabolites in the yolk
and white, including most of those also detected in the excreta.
The preference in hydroxylation site based on identified
metabolites is the same with hens and rats, e.g., phenoxy > cis-
methyl > trans-methyl with cis-permethrin, In contrast, with cows,
both trans- and cis-permethrin have the same preference order of
trans-methyl > cis-methyl = phenoxy.
Metabolites detected in hens, but not in rats or cows, are the
cis isomer of trans-hydroxy permethrin sulphate, the trans isomer of
cis-hydroxy-phenoxybenzyl alcohol-sulphate and Cl2CA-taurine isomers.
Both hens and rats extensively utilize glucuronic acid and
sulphate conjugates in excretion of permethrin metabolites, while
glutamic acid conjugates are most significant in cowsœ
Trout
The distribution and metabolism of the cis- and trans-permethrin
isomers were studied in rainbow trout to evaluate the role of these
parameters in the differential toxicity of permethrin to fish and
mammals (Glickman et al 1981). Both (14C)-permethrin geometrical
isomers were readily taken up and eliminated by rainbow trout and
there appeared to be little difference in the rate of uptake of the
two geometric isomers. Elimination half-lives for (14C)-permethrin
residues in trout tissues, with the exception of fat, were in the
magnitude of hours. High concentrations of a polar metabolite
(glucuronide conjugate of 4'-hydroxypermethrin) were found in bile
within 4 h of cis- and trans-permethrin exposure. Urine contained a
small amount of a polar metabolite that was resistant to hydrolysis by
beta-glucuronidase but was cleaved to some extent by aryl-sulphatase.
The relative absence of permethrin hydrolysis products in trout bile
and the small amount of radioactivity excreted in urine suggested that
the ability of rainbow trout to hydrolyse permethrin in vivo was
minimal. The inability of the rainbow trout to hydrolyse permethrin
rapidly may result in an overall low rate of detoxification, which was
suggested to be a possible factor in the trout's sensitivity to the
compound, particularly the trans-isomer. However, it was also noted
that detoxification may not be the sole factor, as the trout may be
more sensitive physiologically than mammals to permethrin (Glickman
et al 1981).
 Figure 5. Metabolic pathways for (1 RS)-trans- and
(1 RS)-cis-permethrin in hens showing abbreviations used for
metabolites. Numbers in parentheses are percentage amounts of 14C
for each product derived from trans- and cis-permethrin as
follows: E = products in excreta from the first 3 days of the treatment
schedule relative to total administered 14C (see Table I);
Y = products in egg yolk at days 5 and 6 of the treatment schedule
relative to total 14C content of yolk (see Table III). Ester
products are averages for 14C acid and 14C alcohol preparations
and cleavage products are based on either 14C acid or 14C
alcohol preparations, as appropriate.
Effects on enzymes and other biochemical parameters
Carp liver microsomal esterases hydrolysed trans-permethrin much
more extensively than cis-permethrin, and the same relationship exists
for rainbow trout liver microsomes, although they appeared to be less
active (Glickman et al 1979). There was a strong preference with
both isomers and microsomal mixed-function oxidases of both species
for hydroxylation at the 4'-position of the alcohol moiety as opposed
to other sites. The methyl group trans to the carboxyl was usually
hydroxylated more extensively than the cis-methyl group, the greatest
specificity being with carp microsomes acting on cis-permethrin. The
bile of rainbow trout exposed in vivo to 14C-alcohol-labelled
trans-permethrin contained little or no permethrin but instead
consisted mainly of conjugates cleaved by beta-glucuronidase but not
by aryl-sulphatase (Glickman et al 1979).
The rates of permethrin hydrolysis in rainbow trout and mouse
tissues in vitro were estimated in a recent study (Glickman and Lech
1981). Mouse liver, kidney and plasma, incubated at 37°C, hydrolysed
trans-(14C)-permethrin approximately 166, 38 and 59 times faster,
respectively, than the same rainbow trout tissues incubated at 12°C.
At an incubation temperature of 37°C, trout liver microsomes
hydrolysed trans-permethrin approximately 45 times slower than mouse
liver microsomes. When the total capacity of trout and mouse tissues
to hydrolyse trans-permethrin ions were compared on a whole body
basis, mice hydrolysed trans-permethrin 184 times faster than rainbow
trout (Glickman and Lech 1981),
TOXICOLOGICAL STUDIES
Acute toxicity
(1RS 3RS)-permethrin has an acute oral LD50 of 490 mg/kg for
male and female mice and >5 000 mg/kg for male and female rats
(Miyamoto 1976). Various laboratories and even the same laboratory
(Kadota 1976) reported the rat oral LD50 values of (1RS, 3RS)-
permethrin to range from about 450 to >5 000 mg/kg. (See Table 6).
The individual isomers of permethrin have mouse oral LD50
values as follows: 1R,3S-3 150 mg/kg; 1R, 3R-about 96 mg/kg; 1S,3R and
1S,3S- >5 000 mg/kg (Miyamoto 1976). The (+)-cis-permethrin is more
toxic than the trans-isomer (perhaps due to the easier hydrolysability
of (+)-trans) (Miyamoto 1976). The toxicity of isomer mixtures
approximates that expected if there is no potentiating effect of one
isomer component with another (Ruzo and Casida 1977).
TABLE 6. Acute toxicity of permethrin in animals
Compound Species Sex Route LD501,2 Reference
(mg/kg)
Racemic3 Mouse M Oral 490 Miyamoto 1976
F Oral 490 "
(+)-trans M Oral 3100 "
F Oral 3200 "
(+)-cis M Oral 107 "
F Oral 85 "
(-)-trans M Oral >5000 (0) "
F Oral >5000 (0) "
Racemic Rat M Oral >5000 (20) "
F Oral >5000 (0) "
M,F Dermal >4000 Kadota 1976
M,F Dermal >2000 "
Acute Inhalation Toxicity4 Miyamoto 1976
LC50 mg/m3 Minimum Toxic Dose
(mg/m3)
Racemic Mouse M,F >685 140
Rat M,F >685 140
1 Compound dissolved in corn oil; 0.1 ml/10 g bw and 0.5-1 ml/100 g bw administered
by stomach tube to dd mice and Sprague-Dawley rats respectively;
2 Figures in paretheses indicate the % mortality at the highest dose;
3 The trans, cis ratio of the racemic mixture was 3:2;
4 Experimental conditions: solvent: kerosene; 3 h exposure, air flow 50 l/min.
The effect on the rat acute oral toxicity of changing the
cis/trans ratio from 80% to 20%/80% in six stages was assessed. The
LD50 values for the 80% cis/80% trans mixture gave a value of
approximately 6 000 mg/kg (See Table 7)
The test demonstrated that the high the cis content the greater
the toxicity, both in terms of symptoms and mortality. Where the cis
content was >50%, acute toxic symptoms comprising muscular tremors
were seen with doses of 250 mg/kg or less. When the cis-content was
< 40%, no toxic symptoms were seen after oral doses of 250 mg/kg
(Wallwork et al 1975).
TABLE 7. Acute oral toxicity of permethrin1
Species Permethrin cis/trans ratios Formulation Sex LD50 (mg/kg)
Rat 24/75 maize oil M 1 479
80/20 " F 224.5
60/40 " F 445.3
50/50 " F 1 000
40/60 " F 1 260
30/70 " F 1 684
20/80 " F 6 000
Mouse 25/75 " F 2 690
25/75 " M 2 394
Chick 25/75 " M >4 000
1 References - Wallwork and Malone, 1975; Wallwork et al 1975.
The symptoms of permethrin poisoning in mice and rats include
hypersensitivity, tremor and motor ataxia, sometimes with fibrillation
and salivation (Miyamoto 1976; Ruzo and Casida 1977; Wallwork and
Malone 1975; Wallwork et al 1975).
The subcutaneous and dermal toxicities are very low as compared
with the oral toxicity. Permethrin does not cause eye or skin
irritation or skin sensitizing effects (Ruzo and Casida 1977; Wallwork
and Malone 1975; Wallwork et al 1975).
Short-term studies
Rat
Racemic permethrin was administered to 20 males and 20 females SD
strain rats at dietary concentrations of 375, 750 and 1 500 ppm for 24
weeks. A slight increase of liver weight was often accompanied by
liver hypertrophy and fatty change. At a higher level (3 000 ppm)
there was a slight hypertrophy of hepatoparenchymal cells. These
effects were considered neither indicative nor suggestive of
tumorigenicity (Miyamoto 1976).
In another study, groups of 18 male and 18 female weanling rats
were fed diets containing 0, 200, 600, 2 000 and 4 000 ppm 21Z73 for
90 consecutive days. At 90 days, groups of 20 (10 males and 10
females/group) rats were sacrificed, while the surviving animals were
offered unmedicated diet for a further 36 days, this being the
recovery phase. The no-effect level for 21Z73 in the rat was
determined as 2 000 ppm (equivalent to 175 mg a.i./kg/day) (Williams
1976).
Special studies on neurology
Groups of 10 male and 10 female Sprague-Dawley rats were given a
diet containing 21Z73 for 21 days at doses of 0, 4 000, 6 000 and
9 000 ppm. The permethrin contained 24.4% cis and 74.6% trans isomer.
Severe trembling and abnormal gait appeared in all groups, and the
animals lost weight. No consistent abnormality was found on detailed
histological examination of the brain, spinal cord, dorsal root
ganglia, sciatic and sural nerves, and of terminal motor and sensory
innervation in proximal (thigh) and distal (lumbrical) muscles from
half the animals in the control and 6 000 ppm groups, and from all
animals (term survivors and those dying prematurely) in the 9 000 ppm
group.
It was concluded that the clinical disorders produced in the rat
by 21Z73 were due to a pharmacological effect and not to an anatomical
lesion (Dayan 1980).
Special studies on teratogenicity
Pregnant ICR mice were treated p.o. with racemic permethrin at
dose levels of 15, 50 and 100 mg/kg/day during days 7 to 12 of
gestation. Pups were obtained by caesarean section prior to the
termination of the gestation period and external as well as skeletal
abnormalities were examined. No significant treatment-related effects
were found (Miyamoto 1976).
Special studies on mutagenicity
Bacteria
Racemic, (+)-trans-, (+)-cis-, (-)-trans and (-)-cis-permethrin
dissolved in DMSO were all tested at 10 mg/plate in Escherichia
coli W3623 and W3102 as well as Salmonella typhimurium TA 1535 and
1538 strains and were found to be non-mutagenic (Miyamoto 1976).
In the host-mediated assay (Legator and Malling 1971) both
(+)-trans-permethrin at dose levels of 3 000 and 600 mg/kg, p.o.
and (+)-cis-permethrin at dose levels of 54 and 21 mg/kg were
non-mutagenic (Miyamoto 1976). Table 8 summarizes the system in which
permethrin was found to have no mutagenic activity (Ruzo and Casida
1977).
Table 8 Systems in which permethrin shows no mutagenic activity.
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Bacterial assays, Escherichia W3623trpA: W3102trpE 10.000 a b No N-MethyI-N'-nitro-N
reversion of coli nitrosoguanidine (100); Miyamoto,
tryptophan requirement 4-nitroquinoline N- 1976
oxide (10)a
N-Methyl-N'-nitro-N-
WP2 1-1000 Yes nitrosoguanidine (2) d
N-Methyl-N'-nitro,N,- Miyamoto,
Bacterial assays, Salmonella nitrosoguanidine (100); 1976
reversion of typhimurium TA1535:TA1538 10,000a,b No 4-nitroquinoline-N-
histidine requirement oxide (10)a
N-Methyl-N'-nitro-N-
TA 1535 1-1000a,b Yes nitrosoguanidine (2) d
9-Aminoacridine (100) d
TA1537 1-1000a,b Yes 4-o-Tolylazo-o-
TA 1538. TA98 1-1000a,b Yes toluidine (25) d
Benzo(a)pyrene (20) d
TA 100 1-1000a,b Yes N-Methyl-N'-nitro-N-
Bacterial assays inhibition E. coli W3623 (wild); W3623polA; 10,000 No nitrosoguanidine (100) Miyamoto,
zone of DNA-repair W3623uvrA; W3623recA (100) 1976
deficient mutant as S. typhimurium TA1978 (wild); TA1538uvrB 10,000 No (100)
compared with wild strain Bacillus subtilis H17 (wild); M45recA 10,000 No Streptozotocin (20)
Host-mediated bacterial S. typhimurium G46 600 and 3000
assays, reversion of -mouse (1R,3S)
bacterial histidine 21 and 54
requirement (1R,3R) Ethylmethane sulfonate (620) g
Table 8 (con't)
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Cultured lymphomal Mouse L5178Y/TK+/- 30-125 No 2-Acetylaminofluorene (50) g
cells L5178Y;TK+/- 16-94 Yes Trimethylphosphate (679) i
Dominant lethal system Mouse Charles River CDI 452
a In µg plate.
b Tests on racemic mixture and each individual isomer (1R.3S; 1R,3R: 1S.3R: 1S.3S)
c Tests on racemic mixture.
d Data of G. P. Schoenig (FMC Corporation), unpublished results.
e In mg kg oral dose.
f In µg/ml.
g Data of D. Clive (Wellcome Research Laboratories), unpublished results.
h In mg kg. 5 daily oral doses to male mice.
i Data of B. C. Chesher. J. C. Malone. and M. J. Parker (Wellcome Research Laboratories), unpublished results.
Special studies on interaction
The organophosphorus pesticides profenofos, sulprofos, O-ethyl-O-
(4-nitrophenyl) phenylphosphonothioate (EPN) and S,S,S-tributyl
phosphorotrithioate (DEF) administered intraperitoneally to mice at
0.5 to 5 mg/kg strongly inhibited the liver microsomal esterase(s),
hydrolysing trans-permethrin. Topically applied profenofos, sulprofos
and DEF was much less effective in synergizing the toxicity of trans-
permethrin than that of cis-permethrin to cabbage looper larvae and
house fly adults (Gaughan et al 1980).
Data reviewed by the 1979 Meeting showed that permethrin produces
symptoms indicative of an effect on the nervous system in laboratory
animals. These are manifested clinically by tremoring and ataxia.
These effects are seen only at comparatively high dose levels and are
reversible in those animals that survive high doses. It is only at
lethal or near lethal doses that signs of pathological changes in the
nervous system are observable, with sparse axonal degeneration in the
sciatic nerves of some animals. In a long-term study, no histological
changes were seen in sciatic nerves of rats receiving the high dietary
level of 2 500 ppm permethrin, equivalent to approximately 125 mg
permethrin/kg bw/day daily for two years. Hens receiving a dose of
1 000 mg/kg/day for 5 successive days, and then for another 5 days
three weeks later, and hens receiving the maximum practical single
oral dose of approximately 9 000 mg/kg, showed neither clinical nor
histopathological evidence of neurotoxicity (FAO/WHO 1980; ICI 1981).
OBSERVATIONS ON HUMANS
The 1979 Meeting noted that there was no information on the
relative sensitivity of humans to the peripheral neuropathy noted in
rodent species at very high dose levels. No cases of misuse leading
to acute poisoning in humans have been reported to the major
manufacturers during several seasons of use worldwide. Some laboratory
workers handling natural and synthetic pyrethroids have noticed a
transient sensation in the periorbital area of the face. In a clinical
survey, three subjects who had moderate exposure to permethrin did not
develop these symptoms, which were only found after exposure to other
pyrethroids. Neurological signs and electrophysiological studies in
arms and legs of these subjects were normal (LeQuesne et al 1980).
A survey was conducted in Sweden of 45 subjects handling conifer
seedlings, which were dipped in an EC formulation of 40:60 cis:trans
permethrin that had been diluted with water to 1 to 2% of active
ingredient. One of the subjects mentioned itching of the skin. None
reported burning sensations or paraesthesia in the face. Symptoms were
more marked among subjects handling seedlings treated with WP
formulations of 25:75 cis:trans permethrin or of fenvalerate
(Kolmodin-Hedman et al 1981).
Two volunteers were dosed orally with about 2 and 4 mg permethrin
in order to establish whether "CVA" is a major metabolite in humans,
excreted largely in urine as the unconjugated acid and easily
hydrolysable conjugates, and that therefore a gas chromatographic
analysis of "CVA" concentration in urine could be used to estimate the
amount of absorbed permethrin. The two subjects were shown to excrete
18% and 35% of the theoretical yield of metabolite after a dose of
2 mg, and 39% and 32% after 4 mg. Most of the urinary elimination was
seen during the first 12 h after dosing (Cridland and Weatherley
1977).
An estimate of permethrin (NRDC 143; OMS 182) absorbed by people
employed in a field trial of the insecticide in Kuduna, Nigeria, 7-11
June, has been reported (Cridland and Weatherley 1977). Before the
trial, samples of urine from trial personnel were analysed for CIVA
and creatinine content. By comparison with results from the volunteer
study, using creatinine as a biological internal standard, it was
possible to estimate that not more than 2 mg permethrin was absorbed
by any person handling the insecticide in any 12-h period.
RESIDUES IN FOOD
USE PATTERN
Permethrin was first evaluated at the 1979 Meeting, when MRLs
were recommended for a wide range of commodities on a temporary basis.
These recommendations took account of residue levels on crops
immediately after spraying. For the 1981 Meeting, further information
was required on world-wide good agricultural practices (i.e.
authorized national use patterns). The PHIs that can be recommended
between countries are varied. They tend to be shorter in those
countries in which the majority of the potential use of permethrin
will arise. It was agreed that the Meeting should recommend MRLs to
cover the full worldwide spectrum of good agricultural practices.
Permethrin residues on growing crops decline slowly. They tend to be
rather more variable than for some other compounds. Therefore, the
Meeting agreed to support the process used by the 1979 Meeting, which
took account of permethrin residue levels immediately after spraying.
Permethrin is a photostable synthetic pyrethroid. It possesses an
extremely high level of activity against Lepidoptera. It is also
effective against Hemiptera, Diptera and Coleoptera. It is a stomach
and contact insecticide, with very little fumigant activity.
Permethrin is extremely fast-acting. It is effective against all
growth stages, particularly larvae. It also has significant repellent
action. Permethrin controls many insect strains that have developed
resistance to organophosphorus and organochlorine insecticides. It is
of low mammalian toxicity and yet it is effective against insects at
extremely low rates of application. Unlike earlier pyrethroids, it is
sufficiently photo-stable to be of wide-ranging practical use in
agriculture. It represents a major advance in the insecticide field.
Permethrin is not plant systemic. It has very little fumigant or
translaminar activity. Usually, best results are obtained with good
spray cover, and repeat applications are normally made every 7 to 10
days. However, where conditions are conducive to a rapid build-up of
insect infestations, re-spraying may be needed after as little as 3
days (for example, when controlling Spodoptera littoralis on leaf
brassicae in the Far East). Where a range of rates is quoted for
worldwide use, the higher values are required more frequently in those
climates where infestation pressure is greatest.
The major uses arise in the Americas, Africa and parts of the Far
East, i.e. in hot, often humid conditions, where the pressure of
insect attack is frequently high. Cotton dominates the market
potential for permethrin. Soybeans are also very important in the USA
and Brazil. However, cotton seed and soybeans contain negligible
residues when these crops are sprayed as recommended. Smaller, but
still very important, uses in hot countries are those on horticultural
vegetables and on fruit. These include the human foodstuffs that can
contain noteworthy residues - leafy vegetables, solanaceous fruits,
pip fruits and stone fruits.
Not more than 5% of the potential world usage of permethrin is
associated with Western Europe. There, main outlets are on
horticultural vegetables, top fruit and vines. Typically, use rates of
50 g a.i./ha are effective on leaf brassicae, whereas 100 g a.i./ha is
often needed under the more severe conditions of the Americas, Africa
and the Far East.
In 1979, the group of manufacturers noted the relatively slow
rate at which permethrin residues decline on the sprayed parts of
crops. A table showing "half-lives" of 3 to 29 days was provided. To
persons labelling a product, this slow rate of residue decline
provides altogether different problems from those which pertain when
residues decline quickly. One example of the latter is the compound
pirimicarb. During the first 2 to 3 days after spraying, pirimicarb
levels decline dramatically on many crops. Volatilization is mainly
responsible. This tends to mask other effects that will be present,
including inter-site differences in residue levels or the effects of
photochemical and/or enzymatic degradation. After these first few
days, the rate of residue decline becomes less marked. Thus one can
apply a pre-harvest withholding interval (PHI) of 2 to 3 days in
anticipation of obtaining a substantial decrease in residue levels
routinely on those parts of the crop that are exposed to the spray. In
contrast, permethrin is not volatile. It is comparatively photo-
stable. Residue levels decline slowly. In the absence of a dominant
route of rapid degradation, permethrin residue levels tend to be
somewhat more variable. Undoubtedly, inter-site differences in spray
practice, in weather and even in varieties may contribute to this.
Whatever the reasons, this slow rate of residue decline reduces the
value of a PHI in yielding a substantial reduction in residue levels
on exposed crop parts.
Because of this slow rate of residue decline, and because use
patterns worldwide were still evolving, the 1979 JMPR took account of
residue levels immediately after spraying when proposing MRL values.
Permethrin application is relevant only to crop parts that are sprayed
directly, and are of no consequence to situations in which the edible
part is protected and where residues are negligible (e.g. root
vegetables). Therefore, attention has been focused upon residues in
crops such as vegetables and top fruit.
A survey of crops and of PHIs recommended on them nationally are
summarized in Table 9. Countries that have imposed a PHI of 7 days or
more tend to be ones with more moderate climates and lesser problems
of insect infestation. They are mainly European, but include Brazil,
Peru and Uruguay. Conversely, countries allowing shorter PHIs, or
which do not specify a PHI, on vegetables and on fruit tend to be
those with more severe climates and with greater problems of insect
control. These include the USA, many parts of Latin America, Africa
and the Far East - the countries in which the major potential uses
arise. Noteworthy are the comments received on the PHIs needed on
vegetables in the USA. On lettuce and leaf brassicae, major pests
include loopers, diamondback moth and imported cabbageworm. The crop
yields and quality relate directly to the presence (or absence) of
insects. The cleaner the crop, the higher the number of heads that can
be marketed. Therefore, growers demand a high level of insect control,
which permethrin is capable of giving. Many fresh vegetables are
harvested continuously, on a 1 to 3 day schedule. Many crops go to
processors, who demand standards such that the produce from an entire
field can be discarded if any insects or insect parts are found in any
part of the harvested crop. Permethrin has the quality of causing
loopers to roll into a ball and fall from the plant. There is a need
for an insecticide treatment close to harvest, which permethrin can
fulfill. A similar picture pertains on tomatoes. Fresh market outlets
are supplied on short notice. Growers who sell through brokers often
have to commit field to pre-designated standards of yield and quality
in a crop which is harvested continuously. The US Environmental
Protection Agency has recognized these needs when granting clearances
(as Section 18 Emergency Exemptions) on a wide range of crops (FAO/WHO
1979).
Information on the full range of use patterns is less well-
documented for parts of the Third World. However, it is hard to
believe that USA is the only country in which continuous harvesting
occurs soon after spraying. Clearly, if a crop has to be re-sprayed
every 3 to 7 days, a PHI of more than a few days is not practical.
TABLE 9 Uses of permethrin reported to the Meeting as approved by national governments
Country or Area Crop PHI
(days)
Algeria Apple, pear, brassicae, aubergine, pepper,
tomato, cotton None specified
Argentina Pea, pepper, tomato 1
Alfalfa, cotton,maize 7
Apple, apricot, nectarine, peach,)
pear, plum, quince, soyabean ) 21
Sunflower, sorghum )
Australia Broccoli, Brussels sprout, cabbage, 2
cauliflower, tomate, maize
Austria Cabbage, cauliflower, cucumber, None specified
gherkin, maize, plum, spinach,
turnip, vines
Belgium Aubergine, cabbage, cucumber, 2 (under glass)
endive, lettuce, melon, pepper,
tomato 7 (outdoors)
Bolivia Aubergine, pepper, tomato, cotton Non specified
Brazil Cabbage, cauliflower 3
Tomato, cotton 7
Coffee 30
Maize 45
Soybean 60
Canada Apple, pear, grape (pre-bloom)
Sweet corn 1
Cabbage, cauliflower, Brussels sprout 3
Broccoli 7
Canary Islands Tomato 15
Chile Apple, cherry, damson, peach, None specified
pear, plum, beans, potato,
cabbage, maize
Colombia Aubergine, pepper, tomato, cotton None specified
Costa Rica Cotton None specified
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Cuba Aubergine, tomato, cotton, None specified
chilli pepper
Cyprus Cucumber 5
Beans, pepper, tomato 14
Czechoslovakia Apple 21
Cereals 28
Denmark Pea, beetroot, potato 14
Dominican Republic Brassicae, pepper, tomato, cotton, None specified
oilseed rape
Democratic Republic of Aubergine, cucumber, pumpkins, 4
Germany pepper, tomato
Potato 14
Brassicae, lettuce, spinach, carrot, 21
radish, sugarbeet, swede, turnip
Flax, cereals, oilseed rape None specified
France Grape, apple, pear, lettuce, cabbage 15
Grain crops 40
Federal Republic of Cabbage 7
Germany Apple, pear, hops 14
Oilseed rape 56
Guatemala Cotton, brassicae, potato, maize None specified
Guernsey Aubergine, cucumber, pepper, None specified
tomato (under glass)
Pea, brassicae, aubergine, cucumber, None specified
pepper, tomato (outdoors)
Hong Kong Cabbage, watercress, onion None specified
Hungary Apple, pear, apricot, peach, maize None specified
Indonesia Soybean, cabbage, pepper, oil palm, None specified
cotton
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Iran Pistachios, cotton 7
Israel Pumpkin 10
Jordan Apple, pear, brassicae, aubergine, None specified
okra, pepper, tomato, alfalfa,
cotton
Malaysia Citrus, brassicae, aubergine, pepper, None specified
tomato, oil palm, cocoa, coconut
Mexico Cotton, lettuce, broccoli, Brussels None specified
sprouts, cabbage, cauliflower, maize
Morocco Apple, pear, brassicae, aubergine, None specified
cotton
Netherlands Mushrooms 2
Aubergine, cucumber, honeydew melon, 3
pepper, tomato, courgette
Apple, pear, brassicae, beans, pea, )
horseradish, potato, fennel, leek ) 7
onion, radish, sugarbeet, swede, )
turnip, grape, cherry, plum, strawberry )
currant,gooseberry,blackberry,raspberry,)
rutabaga, potato, fodder beet,poppy seed)
Lettuce, endive, fennel 21
New Zealand Greenhouse fruit and vegetables 2
Brassicae, beans, tomato (outdoor))
and fruit trees other than citrus ) 3
Maize, outdoor fruit and vegetables 7
Fodder crops, pome fruit 14
Citrus fruit, grape 28
Nicaragua Brassicae, aubergine, tomato, cotton, None specified
maize
Pakistan Cotton None specified
Peru Beans, potato, tomato, alfalfa, 7
cotton, maize
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Philippines Banana, rice, mango, cabbage, None specified
cauliflower, tomato
Poland Cucumber, tomato (under glass) 2
Pea, brassicae None specified
Portugal Tomato 2
El Salvador Cotton None specified
South Africa Apple, pear 14
Cotton, maize None specified
Spain Citrus, potato, tomato, cotton 15
Syria Cotton, brassicae, aubergine, okra, None specified
pepper, tomato
Taiwan Cabbage, rice None specified
UK Cucumber, pepper, tomato, None specified
aubergerine, mushroom (fogging indoors).
Apple, pear, cherry, plum, pea, None specified
brassicae, lettuce, celery, courgette,
potato, carrot, swede, turnip, sugarbeet,
grass, cereals, blackcurrants,
redcurrants, gooseberry, raspberry,
strawberry.
Uruguay Apple, peach, plum, pea, beans, 20
soybean, tomato, onion, sorghum,
quince
USA Cotton 14
USSR Apple, cotton None specified
Venezuela Cotton None specified
Cabbage, pepper, tomato, onion 7
Yugoslavia Broccoli, Brussels sprout, cabbage,
cauliflower, tomato, maize 2
One may wish to entertain applying a PHI for toxicological
reasons or owing to knowledge of good agricultural practice. In view
of the slow rate of residue decline, a PHI of 2 to 3 weeks would be
the minimum required to effect a substantial and consistent decline in
residue levels, for toxicological reasons. A few governments have set
PHIs of 2 to 3 weeks; presumably, they must consider that such PHIs
are capable of being observed within the limitations of good
agricultural practice within their countries. Other governments have
set lower PHIs based on a knowledge of good agricultural practice
within their country and of the toxicological and residues data on the
compound. Still other governments have not specified a PHI on the
label.
Post-harvest treatments
In trials in Australia (Halls 1981) permethrin (25:75, cis:trans)
was applied at rates in the range 0.5 to 5.0 mg /kg to wheat of
moisture content 9.5 - 10.5 held in small silos at temperatures that
ranged, due to seasonal effects, from 25°C through 10°C and back to
23°C over a period of 9 months. There was little detectable
degradation of any of the treatments, as indicated in Table 10.
Halls and Periam (1980) reported studies in which two permethrin
(25:75, cis:trans) liquid grain protectant formulations were applied
to wheat, which was then stored for 9 months in metal silos in the UK.
Residue analysis on wheat samples taken at monthly intervals showed no
degradation of the permethrin. There was no detectable degradation of
permethrin by the processes of milling or baking over the storage
period, except possibly in the case of the baking of wholemeal bread
from wheat stored for 6 months or more. The results are given in Table
11. The trials were continued and the results reported later (Halls
1981) indicated that there was no significant degradation 15 months
after treatment.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the 1979 Meeting, residues data were reviewed from supervised
trials on kale and spinach sprayed in the Federal Republic of Germany
in 1977 at 22.5 to 30 g a.i./ha. Having regard to the use pattern data
reviewed at the 1979 Meeting and to the higher use rates that could be
needed under conditions of greater insect pressure in countries
outside of Western Europe, additional trials on kale and spinach were
conducted in the UK in 1980 at a rate of 100 g a.i./ha. Allowing for
these differences in application rates, there is good agreement within
the total data now available. At the 100 g a.i./ha rate, residues
during the first three days after spraying were in the range 1.5 to
4.1 mg/kg on spinach and 1.1 to 3.6 mg/kg on kale (Table 12). The 1979
Meeting noted that the rate of decline in permethrin residue levels on
growing crops is fairly small, with half-life periods ranging from
TABLE 10. Residues of permethrin, piperonyl butoxide and fenitrothion detected on wheat after indicated periods
of storage in Australia
Target Residue analysis (mg/kg)
Compound1 application
rate (mg/kg)
Initial 1 month 2 months 3 months 6 months 8 months 9 months
PM 0.5 0.38 0.35 0.38 0.28 0.39 0.39 0.36
PB 10.0 3.9 4.5 4.4 5.0 5.7 3.8 -
FEN 12.0 7.2 7.2 7.7 6.9 6.9 4.3 4.3
PM 1.0 0.93 0.99 0.88 0.89 0.80 0.75 0.75
PB 10.0 7.9 4.6 4.1 5.5 6.7 4.0 -
FEN 12.0 8.9 7.6 6.6 8.0 7.0 5.8 5.2
PM 2.0 1.90 2.00 2.05 1.59 1.64 1.60 1.68
PM 2.0 1.82 1.76 1.95 2.02 1.88 1.75 2.15
PB 10.0 4.2 3.4 3.0 4.0 4.0 3.5 -
PM 5.0 4.5 4.4 4.9 4.5 5.0 3.6 2.6
PM 5.0 5.1 5.8 6.0 5.1 5.9 5.6 4.5
PB 10.0 5.6 7.3 6.9 6.6 6.4 5.9 -
1 PM = permethrin, PB = piperonyl butoxide, FEN = fenitrothion, - = not analysed.
TABLE 11. Permethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after three,
six or nine months' storage1
Target
application Sampling First- Total
rate period Wholemeal Wholemeal Fine reduction white White
(mg/kg) (months) Wheat flour bread Bran offal flour flour bread
0 0.82 0.80 0.52 4.40 nd1 nd 0.27 0.19
3 0.96 0.65 0.67 3.70 approx. 1 nd nd 0.15
2.0
6 0.95 0.83 0.47 2.90 0.74 0.12 0.17 0.06
(25:75)3 (23:77)3
9 1.09 0.78 0.24 4.00 1.04 0.12 0.25 0.12
0 1.74 1.60 0.95 10.20 nd nd 0.55 0.51
3 2.04 2.25 1.20 10.30 2.40 nd 0.42 0.30
5.0
6 2.32 2.19 0.68 5.90 1.79 0.23 0.34 0.18
(25:75)3 (23:77)3
9 2.14 2.21 0.64 8.00 2.60 0.23 0.60 0.20
1 Analytical results are subject to confidence limits of ± 20%;
2 nd = not detectable (<0.05 mg/kg);
3 cis:trans isomer ratio
TABLE 12. Permethrin residue levels on kale and spinach, UK 1980
Harvest Lowest Highest Mean
Crop Formulation Rate Volume No. of interval residue residue residue
(g a.i./ha) (l/ha) applications (days) (mg/kg) (mg/kg) (mg/kg)
Spinach 25% EC 100 500 1 0 4.1 (1)1
3 1.5 3.6 1.9 (5)
7 0.81(1)
14 0.26(1)
Kale 25% EC 100 500 3 0 3.6 (1)
3 1.1 3.9 2.7 (5)
7 3.9 (1)
(var. acephala) 14 1.3 (1)
1 Number of samples used for calculating mean.
about one to three weeks, depending upon the crop. In the UK trials in
1980, permethrin had an initial half-life of ten days on kale and
three days on spinach (FAO/WHO 1980; Swaine 1981a).
Supervised trials, involving ULV applications to grapefruit and
tangerine were conducted in Spain in 1980, and involving high volume
applications to oranges, lemon and grapefruit in the USA in 1981. The
1979 Meeting noted that in orange, residues were found almost
exclusively in the peel; in edible flesh levels did not exceed
0.01 mg/kg. Similar results have now been found in grapefruit, lemon
and tangerine, in which levels in the edible flesh did not exceed
0.05 mg/kg. Residues on the whole fruit at the use rate normally
recommended (50 g a.i./ha) were within the MRL of 0.5 mg/kg
recommended by the 1979 Meeting (FAO/WHO 1980; Swaine 1981b,
c). Permethrin levels in orange, lemon and grapefruit juice were below
0.05 mg/kg at the use rate normally recommended and did not exceed
0.07 mg/kg at twice that rate (Table 13) (Swaine 1981c). Information
received, from New Zealand, Canada and The Netherlands concerning
residues on apple, grape, orange, kiwi fruit, boysenberry, cabbage,
sweet corn, Brussels sprouts, tomato and cucumber included in Table
14, confirm recommendations made previously. Other limited data on
avocado and cranberry were also received.
FATE OF RESIDUES
Photochemical degradation
One of the major drawbacks in the use of natural pyrethrins and
early synthetic pyrethroids as agricultural insecticides was their
susceptibility to degradation in light and air. Introduction of the
dichlorovinyl group into the acid part of the molecule in place of the
isobutenyl group, common in many early pyrethroids, removes one
possible site of photodegradation.
Deposits of permethrin on glass exposed to daylight near a window
indoors persisted for longer than 3 weeks. Out of doors exposed to UV
and visible light but shielded from wind and rain with temperatures
reaching up to 50°C, deposits lasted for about 10 days. Other
experiments comparing the stability of films exposed near a window
indoors showed 60% undecomposed permethrin after 20 days. Permethrin
remained effective on plywood for more than 12 weeks and under a
sunlamp for more than 26 days. Permethrin formulations also showed
improved stability on plant leaf surfaces, persisting for 10 to 20
days (Elliott et al 1973 a,b).
Photolysis of permethrin in various solvents with artificial
light and on soil in sunlight results primarily in cyclopropane ring
isomerization and ester cleavage to 3-phenoxy-benzyl alcohol and
3-(2,2, dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
TABLE 13. Permethrin residues in Citrus, 1980-81
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Orange USA 24% EC 45 4700 1 0 <0.05 <0.05 0.25 <0.05 Swaine
1981 3 0.07 <0.05 0.15 <0.05 1981c
7 <0.05 <0.05 0.07 <0.05
90 4700 1 0 0.18 <0.05 0.40 <0.05
3 0.13 <0.05 0.40 <0.05
7 0.12 <0.05 0.38 <0.05
Lemon USA 24% EC 45 4700 1 0 0.13 <0.05 0.19 <0.05 Swaine
1981 3 0.08 <0.05 0.23 <0.05 1981c
7 0.10 <0.05 0.23 <0.05
90 4700 1 0 0.42 <0.05 0.57 <0.05
3 0.22 <0.05 0.31 <0.05
7 0.13 <0.05 0.48 <0.05
Grapefruit USA 24% 45 4700 1 0 0.06 <0.05 0.11 <0.05 Swaine
1981 3 <0.05 <0.05 0.14 <0.05 1981c
7 <0.05 <0.05 0.21 (0.05
90 4700 1 0 0.13 0.05 0.28 0.07
3 0.10 <0.05 0.42 <0.05
7 0.07 <0.05 0.27 <0.05
TABLE 13. (con't)
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Grapefruit
Spain 25% EC 50 3.3 2 0 0.08 <0.01 0.26 Swaine
1980 3 0.15 <0.01 0.48 1981b
7 0.12 <0.01 0.39
14 0.11 <0.01 0.35
100 3.3 2 0 0.17 0.01 0.52
3 0.18 0.01 0.53
7 0.14 <0.01 0.45
14 0.12 0.01 0.39
Tangerine Spain 25% EC 50 3.3 2 0 0.49 0.01 1.4 Swaine
1980 3 0.23 <0.01 0.86 1981b
7 0.21 0.02 0.73
14 0.29 <0.01 1.0
100 3.3 2 0 0,18 <0.01 0.65
3 0.13 <0.01 0.47
7 0.44 <0.01 1.7
14 0.52 <0.01 1.8
TABLE 14. Permethrin residues in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 0-1 3 6 9 14 21 28 41
Apple New Zealand 1980 11 5 EC 0.33 0.36 0.31 0.21
11 7.5 EC 0.25 0.7 0.32
7 2.5 EC 0.05 0.06 0.05 N.D.
8 2.5 EC 0.24 0.04 0.16
7 3.75 EC 0.19 0.16 0.16 0.11
Avocado New Zealand 1980 6 2.5 EC 0.09 0.07 0.06 0.04
Grape 7 2.5 EC 0.46 0.37 0.26 0.19
Orange 1 5 EC 0.12skin 0.09skin
nd pulp nd pulp
0.07total 0.05total
1 10 EC nd pulp nd pulp
0.15 Total 0.07 total
0 7 14 28 42 56 84
(a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Kiwi fruit New Zealand 6 1.5 EC 0.34 1.71 0.29 1.43
5 25 EC 0.5 - 0.56 4.22 0.16 - 0.16 - 0.08
6 2.5 EC 0.15 - 0.11 0.34 0.08 0.26 0.03 - nd -
7 2.5 EC 0.25 - 0.34 2.45 0.17 1.21 0.16 - 0.07 - 0.06 -
6 3.0 EC 0.5 2.51 0.41 2.05
6 4.4 EC 2.5 12.5 2.5 12.5 1.9 9.6 1.7 8.8
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation 0 7 14 28 42 56 84
(g a.i./100 l) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Boysenberry 2 2.5 EC 0.26 0.15 0.12 0.07
2 2.5 EC 0.65 0.58 0.34 0.14
2 3.75 EC 0.29 0.32 0.22 0.12
1 2 4 6 9 14 45
Brussels Canada 1976 4 70 EC 0.09
sprout 70 EC 0.09
70 EC 0.05
Cranberry Canada 1977 2 70 EC 0.12(60days)
0.15(" )
0.08(")
Tomato Netherlands 1978 2 76 Smoke 0.58
gener. (0.36-0.75)
1979 1 - " 0.11
August (0.06-0.17)
1979 1 - " <0.01
October
Cucumber 1979 1 - " <0.01
October
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 1 2 4 6 9 14 45
Cabbage New Zealand 1980 1 25 EC 0.55 0.43 0.22 0.17
1 50 EC 0.54 0.78 0.36 0.22
1 100 EC 0.39 0.61 0.44 0.55
Sweet corn 1980 1 50 EC 2.7 nd 2.84 nd 1.04 nd 0.54 nd
foliage foliage foliage fol.
cob cob cob cob
Pasture 1980 1 250 EC 39 13 7 6 4 3
500 EC 45 24 9 9 7 3
750 EC 69 44 29 29 12 10
1000 EC 97 56 35 23 19 15
1500 EC 114 77 56 54 25 24
(See Figure 6). The photolysis of cis and trans isomers of permethrin
has been studied using material 14C-labelled in the carbonyl or
methylene moieties. Both permethrin isomers decomposed under
artificial light (peak outlet lambda 290-320nm) slightly faster in
hexane than in methanol. In each solvent, the cis isomer
photodecomposed approx. 1.6 times faster (T1/2 43 to 58 min) than the
trans isomer. The reacon involved extensive isomerization of the
cyclopropane ring, i.e. interconversion of the trans and cis isomers.
This probably occurred via a triplet energy state forming the
diradical intermediate (cleavage of cyclopropane ring), as the
reaction proceeded in the presence of phenoxybenzaldehyde and
benzophenone acting as photosensitizer, and was efficiently quenched
by 1,3-cyclohexadiene. The isomerization rate increased in the order
methanol < hexane < water, and at equilibrium after 1 to 4 h
irradiation the more thermodynamically stable trans isomer constituted
65 to 70% of the isomer mixture.
Together with the isomerization reaction, ester cleavage was
the major photoreaction in methanol, hexane, water and 2% aqueous
acetone. The major degradation products were DCVA and 3-phenoxybenzyl
alcohol. Other products formed in trace amounts in water included the
monochloropermethrin derivative, and the corresponding monochlorovinyl
acid, formed by reductive dechlorination. Permethrin and
monochloropermethrin do not undergo epoxidation at the dichlorovinyl
or chlorovinyl substituent under normal photooxidative conditions.
Photo oxidation to decarboxylated molecules was negligible with
permethrin (Holmstead et al 1977, 1978).
Exposure of the permethrin isomers on Dunkirk Silt Loam soil for
48 days resulted in approx. 55% loss in sunlight and approx. 35% loss
in the dark. The amount of radioactivity unextractable by 1:1 methanol
and ether was approx. 65% in the dark and approx. 18% in the light.
The unextractable radioactivity appears to be due to microbial
activity rather than to chemical reactions, but irradiation increased
the amount.
On/in the soil, relatively little isomerization at the
cyclopropane ring was encountered as compared with the photolysis in
solution. There was little difference in the amount of the DCVA
detected in the dark or light, and 3-phenoxybenzyl alcohol was the
major cleavage product of the alcohol moiety. Other products detected
in trace amounts were essentially the same as those in solutions
(Holmstead et al 1978).
In soil
The residues of permethrin in an organic soil and in vegetables
grown in soil treated with a granular formulation of permethrin were
studied by means of gas chromatography (Belanger and Hamilton 1979).
Permethrin persisted in the soil for the initial 28 days and then
Figure 5. Metabolic pathways for (1 RS)-trans- and
(1 RS)-cis-permethrin in hens showing abbreviations used for
metabolites. Numbers in parentheses are percentage amounts of 14C
for each product derived from trans- and cis-permethrin as
follows: E = products in excreta from the first 3 days of the treatment
schedule relative to total administered 14C (see Table I);
Y = products in egg yolk at days 5 and 6 of the treatment schedule
relative to total 14C content of yolk (see Table III). Ester
products are averages for 14C acid and 14C alcohol preparations
and cleavage products are based on either 14C acid or 14C
alcohol preparations, as appropriate.
Effects on enzymes and other biochemical parameters
Carp liver microsomal esterases hydrolysed trans-permethrin much
more extensively than cis-permethrin, and the same relationship exists
for rainbow trout liver microsomes, although they appeared to be less
active (Glickman et al 1979). There was a strong preference with
both isomers and microsomal mixed-function oxidases of both species
for hydroxylation at the 4'-position of the alcohol moiety as opposed
to other sites. The methyl group trans to the carboxyl was usually
hydroxylated more extensively than the cis-methyl group, the greatest
specificity being with carp microsomes acting on cis-permethrin. The
bile of rainbow trout exposed in vivo to 14C-alcohol-labelled
trans-permethrin contained little or no permethrin but instead
consisted mainly of conjugates cleaved by beta-glucuronidase but not
by aryl-sulphatase (Glickman et al 1979).
The rates of permethrin hydrolysis in rainbow trout and mouse
tissues in vitro were estimated in a recent study (Glickman and Lech
1981). Mouse liver, kidney and plasma, incubated at 37°C, hydrolysed
trans-(14C)-permethrin approximately 166, 38 and 59 times faster,
respectively, than the same rainbow trout tissues incubated at 12°C.
At an incubation temperature of 37°C, trout liver microsomes
hydrolysed trans-permethrin approximately 45 times slower than mouse
liver microsomes. When the total capacity of trout and mouse tissues
to hydrolyse trans-permethrin ions were compared on a whole body
basis, mice hydrolysed trans-permethrin 184 times faster than rainbow
trout (Glickman and Lech 1981),
TOXICOLOGICAL STUDIES
Acute toxicity
(1RS 3RS)-permethrin has an acute oral LD50 of 490 mg/kg for
male and female mice and >5 000 mg/kg for male and female rats
(Miyamoto 1976). Various laboratories and even the same laboratory
(Kadota 1976) reported the rat oral LD50 values of (1RS, 3RS)-
permethrin to range from about 450 to >5 000 mg/kg. (See Table 6).
The individual isomers of permethrin have mouse oral LD50
values as follows: 1R,3S-3 150 mg/kg; 1R, 3R-about 96 mg/kg; 1S,3R and
1S,3S- >5 000 mg/kg (Miyamoto 1976). The (+)-cis-permethrin is more
toxic than the trans-isomer (perhaps due to the easier hydrolysability
of (+)-trans) (Miyamoto 1976). The toxicity of isomer mixtures
approximates that expected if there is no potentiating effect of one
isomer component with another (Ruzo and Casida 1977).
TABLE 6. Acute toxicity of permethrin in animals
Compound Species Sex Route LD501,2 Reference
(mg/kg)
Racemic3 Mouse M Oral 490 Miyamoto 1976
F Oral 490 "
(+)-trans M Oral 3100 "
F Oral 3200 "
(+)-cis M Oral 107 "
F Oral 85 "
(-)-trans M Oral >5000 (0) "
F Oral >5000 (0) "
Racemic Rat M Oral >5000 (20) "
F Oral >5000 (0) "
M,F Dermal >4000 Kadota 1976
M,F Dermal >2000 "
Acute Inhalation Toxicity4 Miyamoto 1976
LC50 mg/m3 Minimum Toxic Dose
(mg/m3)
Racemic Mouse M,F >685 140
Rat M,F >685 140
1 Compound dissolved in corn oil; 0.1 ml/10 g bw and 0.5-1 ml/100 g bw administered
by stomach tube to dd mice and Sprague-Dawley rats respectively;
2 Figures in paretheses indicate the % mortality at the highest dose;
3 The trans, cis ratio of the racemic mixture was 3:2;
4 Experimental conditions: solvent: kerosene; 3 h exposure, air flow 50 l/min.
The effect on the rat acute oral toxicity of changing the
cis/trans ratio from 80% to 20%/80% in six stages was assessed. The
LD50 values for the 80% cis/80% trans mixture gave a value of
approximately 6 000 mg/kg (See Table 7)
The test demonstrated that the high the cis content the greater
the toxicity, both in terms of symptoms and mortality. Where the cis
content was >50%, acute toxic symptoms comprising muscular tremors
were seen with doses of 250 mg/kg or less. When the cis-content was
< 40%, no toxic symptoms were seen after oral doses of 250 mg/kg
(Wallwork et al 1975).
TABLE 7. Acute oral toxicity of permethrin1
Species Permethrin cis/trans ratios Formulation Sex LD50 (mg/kg)
Rat 24/75 maize oil M 1 479
80/20 " F 224.5
60/40 " F 445.3
50/50 " F 1 000
40/60 " F 1 260
30/70 " F 1 684
20/80 " F 6 000
Mouse 25/75 " F 2 690
25/75 " M 2 394
Chick 25/75 " M >4 000
1 References - Wallwork and Malone, 1975; Wallwork et al 1975.
The symptoms of permethrin poisoning in mice and rats include
hypersensitivity, tremor and motor ataxia, sometimes with fibrillation
and salivation (Miyamoto 1976; Ruzo and Casida 1977; Wallwork and
Malone 1975; Wallwork et al 1975).
The subcutaneous and dermal toxicities are very low as compared
with the oral toxicity. Permethrin does not cause eye or skin
irritation or skin sensitizing effects (Ruzo and Casida 1977; Wallwork
and Malone 1975; Wallwork et al 1975).
Short-term studies
Rat
Racemic permethrin was administered to 20 males and 20 females SD
strain rats at dietary concentrations of 375, 750 and 1 500 ppm for 24
weeks. A slight increase of liver weight was often accompanied by
liver hypertrophy and fatty change. At a higher level (3 000 ppm)
there was a slight hypertrophy of hepatoparenchymal cells. These
effects were considered neither indicative nor suggestive of
tumorigenicity (Miyamoto 1976).
In another study, groups of 18 male and 18 female weanling rats
were fed diets containing 0, 200, 600, 2 000 and 4 000 ppm 21Z73 for
90 consecutive days. At 90 days, groups of 20 (10 males and 10
females/group) rats were sacrificed, while the surviving animals were
offered unmedicated diet for a further 36 days, this being the
recovery phase. The no-effect level for 21Z73 in the rat was
determined as 2 000 ppm (equivalent to 175 mg a.i./kg/day) (Williams
1976).
Special studies on neurology
Groups of 10 male and 10 female Sprague-Dawley rats were given a
diet containing 21Z73 for 21 days at doses of 0, 4 000, 6 000 and
9 000 ppm. The permethrin contained 24.4% cis and 74.6% trans isomer.
Severe trembling and abnormal gait appeared in all groups, and the
animals lost weight. No consistent abnormality was found on detailed
histological examination of the brain, spinal cord, dorsal root
ganglia, sciatic and sural nerves, and of terminal motor and sensory
innervation in proximal (thigh) and distal (lumbrical) muscles from
half the animals in the control and 6 000 ppm groups, and from all
animals (term survivors and those dying prematurely) in the 9 000 ppm
group.
It was concluded that the clinical disorders produced in the rat
by 21Z73 were due to a pharmacological effect and not to an anatomical
lesion (Dayan 1980).
Special studies on teratogenicity
Pregnant ICR mice were treated p.o. with racemic permethrin at
dose levels of 15, 50 and 100 mg/kg/day during days 7 to 12 of
gestation. Pups were obtained by caesarean section prior to the
termination of the gestation period and external as well as skeletal
abnormalities were examined. No significant treatment-related effects
were found (Miyamoto 1976).
Special studies on mutagenicity
Bacteria
Racemic, (+)-trans-, (+)-cis-, (-)-trans and (-)-cis-permethrin
dissolved in DMSO were all tested at 10 mg/plate in Escherichia
coli W3623 and W3102 as well as Salmonella typhimurium TA 1535 and
1538 strains and were found to be non-mutagenic (Miyamoto 1976).
In the host-mediated assay (Legator and Malling 1971) both
(+)-trans-permethrin at dose levels of 3 000 and 600 mg/kg, p.o.
and (+)-cis-permethrin at dose levels of 54 and 21 mg/kg were
non-mutagenic (Miyamoto 1976). Table 8 summarizes the system in which
permethrin was found to have no mutagenic activity (Ruzo and Casida
1977).
Table 8 Systems in which permethrin shows no mutagenic activity.
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Bacterial assays, Escherichia W3623trpA: W3102trpE 10.000 a b No N-MethyI-N'-nitro-N
reversion of coli nitrosoguanidine (100); Miyamoto,
tryptophan requirement 4-nitroquinoline N- 1976
oxide (10)a
N-Methyl-N'-nitro-N-
WP2 1-1000 Yes nitrosoguanidine (2) d
N-Methyl-N'-nitro,N,- Miyamoto,
Bacterial assays, Salmonella nitrosoguanidine (100); 1976
reversion of typhimurium TA1535:TA1538 10,000a,b No 4-nitroquinoline-N-
histidine requirement oxide (10)a
N-Methyl-N'-nitro-N-
TA 1535 1-1000a,b Yes nitrosoguanidine (2) d
9-Aminoacridine (100) d
TA1537 1-1000a,b Yes 4-o-Tolylazo-o-
TA 1538. TA98 1-1000a,b Yes toluidine (25) d
Benzo(a)pyrene (20) d
TA 100 1-1000a,b Yes N-Methyl-N'-nitro-N-
Bacterial assays inhibition E. coli W3623 (wild); W3623polA; 10,000 No nitrosoguanidine (100) Miyamoto,
zone of DNA-repair W3623uvrA; W3623recA (100) 1976
deficient mutant as S. typhimurium TA1978 (wild); TA1538uvrB 10,000 No (100)
compared with wild strain Bacillus subtilis H17 (wild); M45recA 10,000 No Streptozotocin (20)
Host-mediated bacterial S. typhimurium G46 600 and 3000
assays, reversion of -mouse (1R,3S)
bacterial histidine 21 and 54
requirement (1R,3R) Ethylmethane sulfonate (620) g
Table 8 (con't)
Metabolic
Permethrin activation Positive control
Method Species Strain amount system and amount References
Cultured lymphomal Mouse L5178Y/TK+/- 30-125 No 2-Acetylaminofluorene (50) g
cells L5178Y;TK+/- 16-94 Yes Trimethylphosphate (679) i
Dominant lethal system Mouse Charles River CDI 452
a In µg plate.
b Tests on racemic mixture and each individual isomer (1R.3S; 1R,3R: 1S.3R: 1S.3S)
c Tests on racemic mixture.
d Data of G. P. Schoenig (FMC Corporation), unpublished results.
e In mg kg oral dose.
f In µg/ml.
g Data of D. Clive (Wellcome Research Laboratories), unpublished results.
h In mg kg. 5 daily oral doses to male mice.
i Data of B. C. Chesher. J. C. Malone. and M. J. Parker (Wellcome Research Laboratories), unpublished results.
Special studies on interaction
The organophosphorus pesticides profenofos, sulprofos, O-ethyl-O-
(4-nitrophenyl) phenylphosphonothioate (EPN) and S,S,S-tributyl
phosphorotrithioate (DEF) administered intraperitoneally to mice at
0.5 to 5 mg/kg strongly inhibited the liver microsomal esterase(s),
hydrolysing trans-permethrin. Topically applied profenofos, sulprofos
and DEF was much less effective in synergizing the toxicity of trans-
permethrin than that of cis-permethrin to cabbage looper larvae and
house fly adults (Gaughan et al 1980).
Data reviewed by the 1979 Meeting showed that permethrin produces
symptoms indicative of an effect on the nervous system in laboratory
animals. These are manifested clinically by tremoring and ataxia.
These effects are seen only at comparatively high dose levels and are
reversible in those animals that survive high doses. It is only at
lethal or near lethal doses that signs of pathological changes in the
nervous system are observable, with sparse axonal degeneration in the
sciatic nerves of some animals. In a long-term study, no histological
changes were seen in sciatic nerves of rats receiving the high dietary
level of 2 500 ppm permethrin, equivalent to approximately 125 mg
permethrin/kg bw/day daily for two years. Hens receiving a dose of
1 000 mg/kg/day for 5 successive days, and then for another 5 days
three weeks later, and hens receiving the maximum practical single
oral dose of approximately 9 000 mg/kg, showed neither clinical nor
histopathological evidence of neurotoxicity (FAO/WHO 1980; ICI 1981).
OBSERVATIONS ON HUMANS
The 1979 Meeting noted that there was no information on the
relative sensitivity of humans to the peripheral neuropathy noted in
rodent species at very high dose levels. No cases of misuse leading
to acute poisoning in humans have been reported to the major
manufacturers during several seasons of use worldwide. Some laboratory
workers handling natural and synthetic pyrethroids have noticed a
transient sensation in the periorbital area of the face. In a clinical
survey, three subjects who had moderate exposure to permethrin did not
develop these symptoms, which were only found after exposure to other
pyrethroids. Neurological signs and electrophysiological studies in
arms and legs of these subjects were normal (LeQuesne et al 1980).
A survey was conducted in Sweden of 45 subjects handling conifer
seedlings, which were dipped in an EC formulation of 40:60 cis:trans
permethrin that had been diluted with water to 1 to 2% of active
ingredient. One of the subjects mentioned itching of the skin. None
reported burning sensations or paraesthesia in the face. Symptoms were
more marked among subjects handling seedlings treated with WP
formulations of 25:75 cis:trans permethrin or of fenvalerate
(Kolmodin-Hedman et al 1981).
Two volunteers were dosed orally with about 2 and 4 mg permethrin
in order to establish whether "CVA" is a major metabolite in humans,
excreted largely in urine as the unconjugated acid and easily
hydrolysable conjugates, and that therefore a gas chromatographic
analysis of "CVA" concentration in urine could be used to estimate the
amount of absorbed permethrin. The two subjects were shown to excrete
18% and 35% of the theoretical yield of metabolite after a dose of
2 mg, and 39% and 32% after 4 mg. Most of the urinary elimination was
seen during the first 12 h after dosing (Cridland and Weatherley
1977).
An estimate of permethrin (NRDC 143; OMS 182) absorbed by people
employed in a field trial of the insecticide in Kuduna, Nigeria, 7-11
June, has been reported (Cridland and Weatherley 1977). Before the
trial, samples of urine from trial personnel were analysed for CIVA
and creatinine content. By comparison with results from the volunteer
study, using creatinine as a biological internal standard, it was
possible to estimate that not more than 2 mg permethrin was absorbed
by any person handling the insecticide in any 12-h period.
RESIDUES IN FOOD
USE PATTERN
Permethrin was first evaluated at the 1979 Meeting, when MRLs
were recommended for a wide range of commodities on a temporary basis.
These recommendations took account of residue levels on crops
immediately after spraying. For the 1981 Meeting, further information
was required on world-wide good agricultural practices (i.e.
authorized national use patterns). The PHIs that can be recommended
between countries are varied. They tend to be shorter in those
countries in which the majority of the potential use of permethrin
will arise. It was agreed that the Meeting should recommend MRLs to
cover the full worldwide spectrum of good agricultural practices.
Permethrin residues on growing crops decline slowly. They tend to be
rather more variable than for some other compounds. Therefore, the
Meeting agreed to support the process used by the 1979 Meeting, which
took account of permethrin residue levels immediately after spraying.
Permethrin is a photostable synthetic pyrethroid. It possesses an
extremely high level of activity against Lepidoptera. It is also
effective against Hemiptera, Diptera and Coleoptera. It is a stomach
and contact insecticide, with very little fumigant activity.
Permethrin is extremely fast-acting. It is effective against all
growth stages, particularly larvae. It also has significant repellent
action. Permethrin controls many insect strains that have developed
resistance to organophosphorus and organochlorine insecticides. It is
of low mammalian toxicity and yet it is effective against insects at
extremely low rates of application. Unlike earlier pyrethroids, it is
sufficiently photo-stable to be of wide-ranging practical use in
agriculture. It represents a major advance in the insecticide field.
Permethrin is not plant systemic. It has very little fumigant or
translaminar activity. Usually, best results are obtained with good
spray cover, and repeat applications are normally made every 7 to 10
days. However, where conditions are conducive to a rapid build-up of
insect infestations, re-spraying may be needed after as little as 3
days (for example, when controlling Spodoptera littoralis on leaf
brassicae in the Far East). Where a range of rates is quoted for
worldwide use, the higher values are required more frequently in those
climates where infestation pressure is greatest.
The major uses arise in the Americas, Africa and parts of the Far
East, i.e. in hot, often humid conditions, where the pressure of
insect attack is frequently high. Cotton dominates the market
potential for permethrin. Soybeans are also very important in the USA
and Brazil. However, cotton seed and soybeans contain negligible
residues when these crops are sprayed as recommended. Smaller, but
still very important, uses in hot countries are those on horticultural
vegetables and on fruit. These include the human foodstuffs that can
contain noteworthy residues - leafy vegetables, solanaceous fruits,
pip fruits and stone fruits.
Not more than 5% of the potential world usage of permethrin is
associated with Western Europe. There, main outlets are on
horticultural vegetables, top fruit and vines. Typically, use rates of
50 g a.i./ha are effective on leaf brassicae, whereas 100 g a.i./ha is
often needed under the more severe conditions of the Americas, Africa
and the Far East.
In 1979, the group of manufacturers noted the relatively slow
rate at which permethrin residues decline on the sprayed parts of
crops. A table showing "half-lives" of 3 to 29 days was provided. To
persons labelling a product, this slow rate of residue decline
provides altogether different problems from those which pertain when
residues decline quickly. One example of the latter is the compound
pirimicarb. During the first 2 to 3 days after spraying, pirimicarb
levels decline dramatically on many crops. Volatilization is mainly
responsible. This tends to mask other effects that will be present,
including inter-site differences in residue levels or the effects of
photochemical and/or enzymatic degradation. After these first few
days, the rate of residue decline becomes less marked. Thus one can
apply a pre-harvest withholding interval (PHI) of 2 to 3 days in
anticipation of obtaining a substantial decrease in residue levels
routinely on those parts of the crop that are exposed to the spray. In
contrast, permethrin is not volatile. It is comparatively photo-
stable. Residue levels decline slowly. In the absence of a dominant
route of rapid degradation, permethrin residue levels tend to be
somewhat more variable. Undoubtedly, inter-site differences in spray
practice, in weather and even in varieties may contribute to this.
Whatever the reasons, this slow rate of residue decline reduces the
value of a PHI in yielding a substantial reduction in residue levels
on exposed crop parts.
Because of this slow rate of residue decline, and because use
patterns worldwide were still evolving, the 1979 JMPR took account of
residue levels immediately after spraying when proposing MRL values.
Permethrin application is relevant only to crop parts that are sprayed
directly, and are of no consequence to situations in which the edible
part is protected and where residues are negligible (e.g. root
vegetables). Therefore, attention has been focused upon residues in
crops such as vegetables and top fruit.
A survey of crops and of PHIs recommended on them nationally are
summarized in Table 9. Countries that have imposed a PHI of 7 days or
more tend to be ones with more moderate climates and lesser problems
of insect infestation. They are mainly European, but include Brazil,
Peru and Uruguay. Conversely, countries allowing shorter PHIs, or
which do not specify a PHI, on vegetables and on fruit tend to be
those with more severe climates and with greater problems of insect
control. These include the USA, many parts of Latin America, Africa
and the Far East - the countries in which the major potential uses
arise. Noteworthy are the comments received on the PHIs needed on
vegetables in the USA. On lettuce and leaf brassicae, major pests
include loopers, diamondback moth and imported cabbageworm. The crop
yields and quality relate directly to the presence (or absence) of
insects. The cleaner the crop, the higher the number of heads that can
be marketed. Therefore, growers demand a high level of insect control,
which permethrin is capable of giving. Many fresh vegetables are
harvested continuously, on a 1 to 3 day schedule. Many crops go to
processors, who demand standards such that the produce from an entire
field can be discarded if any insects or insect parts are found in any
part of the harvested crop. Permethrin has the quality of causing
loopers to roll into a ball and fall from the plant. There is a need
for an insecticide treatment close to harvest, which permethrin can
fulfill. A similar picture pertains on tomatoes. Fresh market outlets
are supplied on short notice. Growers who sell through brokers often
have to commit field to pre-designated standards of yield and quality
in a crop which is harvested continuously. The US Environmental
Protection Agency has recognized these needs when granting clearances
(as Section 18 Emergency Exemptions) on a wide range of crops (FAO/WHO
1979).
Information on the full range of use patterns is less well-
documented for parts of the Third World. However, it is hard to
believe that USA is the only country in which continuous harvesting
occurs soon after spraying. Clearly, if a crop has to be re-sprayed
every 3 to 7 days, a PHI of more than a few days is not practical.
TABLE 9 Uses of permethrin reported to the Meeting as approved by national governments
Country or Area Crop PHI
(days)
Algeria Apple, pear, brassicae, aubergine, pepper,
tomato, cotton None specified
Argentina Pea, pepper, tomato 1
Alfalfa, cotton,maize 7
Apple, apricot, nectarine, peach,)
pear, plum, quince, soyabean ) 21
Sunflower, sorghum )
Australia Broccoli, Brussels sprout, cabbage, 2
cauliflower, tomate, maize
Austria Cabbage, cauliflower, cucumber, None specified
gherkin, maize, plum, spinach,
turnip, vines
Belgium Aubergine, cabbage, cucumber, 2 (under glass)
endive, lettuce, melon, pepper,
tomato 7 (outdoors)
Bolivia Aubergine, pepper, tomato, cotton Non specified
Brazil Cabbage, cauliflower 3
Tomato, cotton 7
Coffee 30
Maize 45
Soybean 60
Canada Apple, pear, grape (pre-bloom)
Sweet corn 1
Cabbage, cauliflower, Brussels sprout 3
Broccoli 7
Canary Islands Tomato 15
Chile Apple, cherry, damson, peach, None specified
pear, plum, beans, potato,
cabbage, maize
Colombia Aubergine, pepper, tomato, cotton None specified
Costa Rica Cotton None specified
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Cuba Aubergine, tomato, cotton, None specified
chilli pepper
Cyprus Cucumber 5
Beans, pepper, tomato 14
Czechoslovakia Apple 21
Cereals 28
Denmark Pea, beetroot, potato 14
Dominican Republic Brassicae, pepper, tomato, cotton, None specified
oilseed rape
Democratic Republic of Aubergine, cucumber, pumpkins, 4
Germany pepper, tomato
Potato 14
Brassicae, lettuce, spinach, carrot, 21
radish, sugarbeet, swede, turnip
Flax, cereals, oilseed rape None specified
France Grape, apple, pear, lettuce, cabbage 15
Grain crops 40
Federal Republic of Cabbage 7
Germany Apple, pear, hops 14
Oilseed rape 56
Guatemala Cotton, brassicae, potato, maize None specified
Guernsey Aubergine, cucumber, pepper, None specified
tomato (under glass)
Pea, brassicae, aubergine, cucumber, None specified
pepper, tomato (outdoors)
Hong Kong Cabbage, watercress, onion None specified
Hungary Apple, pear, apricot, peach, maize None specified
Indonesia Soybean, cabbage, pepper, oil palm, None specified
cotton
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Iran Pistachios, cotton 7
Israel Pumpkin 10
Jordan Apple, pear, brassicae, aubergine, None specified
okra, pepper, tomato, alfalfa,
cotton
Malaysia Citrus, brassicae, aubergine, pepper, None specified
tomato, oil palm, cocoa, coconut
Mexico Cotton, lettuce, broccoli, Brussels None specified
sprouts, cabbage, cauliflower, maize
Morocco Apple, pear, brassicae, aubergine, None specified
cotton
Netherlands Mushrooms 2
Aubergine, cucumber, honeydew melon, 3
pepper, tomato, courgette
Apple, pear, brassicae, beans, pea, )
horseradish, potato, fennel, leek ) 7
onion, radish, sugarbeet, swede, )
turnip, grape, cherry, plum, strawberry )
currant,gooseberry,blackberry,raspberry,)
rutabaga, potato, fodder beet,poppy seed)
Lettuce, endive, fennel 21
New Zealand Greenhouse fruit and vegetables 2
Brassicae, beans, tomato (outdoor))
and fruit trees other than citrus ) 3
Maize, outdoor fruit and vegetables 7
Fodder crops, pome fruit 14
Citrus fruit, grape 28
Nicaragua Brassicae, aubergine, tomato, cotton, None specified
maize
Pakistan Cotton None specified
Peru Beans, potato, tomato, alfalfa, 7
cotton, maize
TABLE 9 (con't)
Country or Area Crop PHI
(days)
Philippines Banana, rice, mango, cabbage, None specified
cauliflower, tomato
Poland Cucumber, tomato (under glass) 2
Pea, brassicae None specified
Portugal Tomato 2
El Salvador Cotton None specified
South Africa Apple, pear 14
Cotton, maize None specified
Spain Citrus, potato, tomato, cotton 15
Syria Cotton, brassicae, aubergine, okra, None specified
pepper, tomato
Taiwan Cabbage, rice None specified
UK Cucumber, pepper, tomato, None specified
aubergerine, mushroom (fogging indoors).
Apple, pear, cherry, plum, pea, None specified
brassicae, lettuce, celery, courgette,
potato, carrot, swede, turnip, sugarbeet,
grass, cereals, blackcurrants,
redcurrants, gooseberry, raspberry,
strawberry.
Uruguay Apple, peach, plum, pea, beans, 20
soybean, tomato, onion, sorghum,
quince
USA Cotton 14
USSR Apple, cotton None specified
Venezuela Cotton None specified
Cabbage, pepper, tomato, onion 7
Yugoslavia Broccoli, Brussels sprout, cabbage,
cauliflower, tomato, maize 2
One may wish to entertain applying a PHI for toxicological
reasons or owing to knowledge of good agricultural practice. In view
of the slow rate of residue decline, a PHI of 2 to 3 weeks would be
the minimum required to effect a substantial and consistent decline in
residue levels, for toxicological reasons. A few governments have set
PHIs of 2 to 3 weeks; presumably, they must consider that such PHIs
are capable of being observed within the limitations of good
agricultural practice within their countries. Other governments have
set lower PHIs based on a knowledge of good agricultural practice
within their country and of the toxicological and residues data on the
compound. Still other governments have not specified a PHI on the
label.
Post-harvest treatments
In trials in Australia (Halls 1981) permethrin (25:75, cis:trans)
was applied at rates in the range 0.5 to 5.0 mg /kg to wheat of
moisture content 9.5 - 10.5 held in small silos at temperatures that
ranged, due to seasonal effects, from 25°C through 10°C and back to
23°C over a period of 9 months. There was little detectable
degradation of any of the treatments, as indicated in Table 10.
Halls and Periam (1980) reported studies in which two permethrin
(25:75, cis:trans) liquid grain protectant formulations were applied
to wheat, which was then stored for 9 months in metal silos in the UK.
Residue analysis on wheat samples taken at monthly intervals showed no
degradation of the permethrin. There was no detectable degradation of
permethrin by the processes of milling or baking over the storage
period, except possibly in the case of the baking of wholemeal bread
from wheat stored for 6 months or more. The results are given in Table
11. The trials were continued and the results reported later (Halls
1981) indicated that there was no significant degradation 15 months
after treatment.
RESIDUES RESULTING FROM SUPERVISED TRIALS
At the 1979 Meeting, residues data were reviewed from supervised
trials on kale and spinach sprayed in the Federal Republic of Germany
in 1977 at 22.5 to 30 g a.i./ha. Having regard to the use pattern data
reviewed at the 1979 Meeting and to the higher use rates that could be
needed under conditions of greater insect pressure in countries
outside of Western Europe, additional trials on kale and spinach were
conducted in the UK in 1980 at a rate of 100 g a.i./ha. Allowing for
these differences in application rates, there is good agreement within
the total data now available. At the 100 g a.i./ha rate, residues
during the first three days after spraying were in the range 1.5 to
4.1 mg/kg on spinach and 1.1 to 3.6 mg/kg on kale (Table 12). The 1979
Meeting noted that the rate of decline in permethrin residue levels on
growing crops is fairly small, with half-life periods ranging from
TABLE 10. Residues of permethrin, piperonyl butoxide and fenitrothion detected on wheat after indicated periods
of storage in Australia
Target Residue analysis (mg/kg)
Compound1 application
rate (mg/kg)
Initial 1 month 2 months 3 months 6 months 8 months 9 months
PM 0.5 0.38 0.35 0.38 0.28 0.39 0.39 0.36
PB 10.0 3.9 4.5 4.4 5.0 5.7 3.8 -
FEN 12.0 7.2 7.2 7.7 6.9 6.9 4.3 4.3
PM 1.0 0.93 0.99 0.88 0.89 0.80 0.75 0.75
PB 10.0 7.9 4.6 4.1 5.5 6.7 4.0 -
FEN 12.0 8.9 7.6 6.6 8.0 7.0 5.8 5.2
PM 2.0 1.90 2.00 2.05 1.59 1.64 1.60 1.68
PM 2.0 1.82 1.76 1.95 2.02 1.88 1.75 2.15
PB 10.0 4.2 3.4 3.0 4.0 4.0 3.5 -
PM 5.0 4.5 4.4 4.9 4.5 5.0 3.6 2.6
PM 5.0 5.1 5.8 6.0 5.1 5.9 5.6 4.5
PB 10.0 5.6 7.3 6.9 6.6 6.4 5.9 -
1 PM = permethrin, PB = piperonyl butoxide, FEN = fenitrothion, - = not analysed.
TABLE 11. Permethrin residues on milling fractions and bread made from freshly treated wheat and from wheat after three,
six or nine months' storage1
Target
application Sampling First- Total
rate period Wholemeal Wholemeal Fine reduction white White
(mg/kg) (months) Wheat flour bread Bran offal flour flour bread
0 0.82 0.80 0.52 4.40 nd1 nd 0.27 0.19
3 0.96 0.65 0.67 3.70 approx. 1 nd nd 0.15
2.0
6 0.95 0.83 0.47 2.90 0.74 0.12 0.17 0.06
(25:75)3 (23:77)3
9 1.09 0.78 0.24 4.00 1.04 0.12 0.25 0.12
0 1.74 1.60 0.95 10.20 nd nd 0.55 0.51
3 2.04 2.25 1.20 10.30 2.40 nd 0.42 0.30
5.0
6 2.32 2.19 0.68 5.90 1.79 0.23 0.34 0.18
(25:75)3 (23:77)3
9 2.14 2.21 0.64 8.00 2.60 0.23 0.60 0.20
1 Analytical results are subject to confidence limits of ± 20%;
2 nd = not detectable (<0.05 mg/kg);
3 cis:trans isomer ratio
TABLE 12. Permethrin residue levels on kale and spinach, UK 1980
Harvest Lowest Highest Mean
Crop Formulation Rate Volume No. of interval residue residue residue
(g a.i./ha) (l/ha) applications (days) (mg/kg) (mg/kg) (mg/kg)
Spinach 25% EC 100 500 1 0 4.1 (1)1
3 1.5 3.6 1.9 (5)
7 0.81(1)
14 0.26(1)
Kale 25% EC 100 500 3 0 3.6 (1)
3 1.1 3.9 2.7 (5)
7 3.9 (1)
(var. acephala) 14 1.3 (1)
1 Number of samples used for calculating mean.
about one to three weeks, depending upon the crop. In the UK trials in
1980, permethrin had an initial half-life of ten days on kale and
three days on spinach (FAO/WHO 1980; Swaine 1981a).
Supervised trials, involving ULV applications to grapefruit and
tangerine were conducted in Spain in 1980, and involving high volume
applications to oranges, lemon and grapefruit in the USA in 1981. The
1979 Meeting noted that in orange, residues were found almost
exclusively in the peel; in edible flesh levels did not exceed
0.01 mg/kg. Similar results have now been found in grapefruit, lemon
and tangerine, in which levels in the edible flesh did not exceed
0.05 mg/kg. Residues on the whole fruit at the use rate normally
recommended (50 g a.i./ha) were within the MRL of 0.5 mg/kg
recommended by the 1979 Meeting (FAO/WHO 1980; Swaine 1981b,
c). Permethrin levels in orange, lemon and grapefruit juice were below
0.05 mg/kg at the use rate normally recommended and did not exceed
0.07 mg/kg at twice that rate (Table 13) (Swaine 1981c). Information
received, from New Zealand, Canada and The Netherlands concerning
residues on apple, grape, orange, kiwi fruit, boysenberry, cabbage,
sweet corn, Brussels sprouts, tomato and cucumber included in Table
14, confirm recommendations made previously. Other limited data on
avocado and cranberry were also received.
FATE OF RESIDUES
Photochemical degradation
One of the major drawbacks in the use of natural pyrethrins and
early synthetic pyrethroids as agricultural insecticides was their
susceptibility to degradation in light and air. Introduction of the
dichlorovinyl group into the acid part of the molecule in place of the
isobutenyl group, common in many early pyrethroids, removes one
possible site of photodegradation.
Deposits of permethrin on glass exposed to daylight near a window
indoors persisted for longer than 3 weeks. Out of doors exposed to UV
and visible light but shielded from wind and rain with temperatures
reaching up to 50°C, deposits lasted for about 10 days. Other
experiments comparing the stability of films exposed near a window
indoors showed 60% undecomposed permethrin after 20 days. Permethrin
remained effective on plywood for more than 12 weeks and under a
sunlamp for more than 26 days. Permethrin formulations also showed
improved stability on plant leaf surfaces, persisting for 10 to 20
days (Elliott et al 1973 a,b).
Photolysis of permethrin in various solvents with artificial
light and on soil in sunlight results primarily in cyclopropane ring
isomerization and ester cleavage to 3-phenoxy-benzyl alcohol and
3-(2,2, dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCVA)
TABLE 13. Permethrin residues in Citrus, 1980-81
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Orange USA 24% EC 45 4700 1 0 <0.05 <0.05 0.25 <0.05 Swaine
1981 3 0.07 <0.05 0.15 <0.05 1981c
7 <0.05 <0.05 0.07 <0.05
90 4700 1 0 0.18 <0.05 0.40 <0.05
3 0.13 <0.05 0.40 <0.05
7 0.12 <0.05 0.38 <0.05
Lemon USA 24% EC 45 4700 1 0 0.13 <0.05 0.19 <0.05 Swaine
1981 3 0.08 <0.05 0.23 <0.05 1981c
7 0.10 <0.05 0.23 <0.05
90 4700 1 0 0.42 <0.05 0.57 <0.05
3 0.22 <0.05 0.31 <0.05
7 0.13 <0.05 0.48 <0.05
Grapefruit USA 24% 45 4700 1 0 0.06 <0.05 0.11 <0.05 Swaine
1981 3 <0.05 <0.05 0.14 <0.05 1981c
7 <0.05 <0.05 0.21 (0.05
90 4700 1 0 0.13 0.05 0.28 0.07
3 0.10 <0.05 0.42 <0.05
7 0.07 <0.05 0.27 <0.05
TABLE 13. (con't)
No. Harvest Residue levels(mg/kg) in
Crop Country Formulation Rate Volume of interval
Year (g a.i./ha) (l/ha) applications (days) Whole Reference
fruit Flesh Peel Juice
Grapefruit
Spain 25% EC 50 3.3 2 0 0.08 <0.01 0.26 Swaine
1980 3 0.15 <0.01 0.48 1981b
7 0.12 <0.01 0.39
14 0.11 <0.01 0.35
100 3.3 2 0 0.17 0.01 0.52
3 0.18 0.01 0.53
7 0.14 <0.01 0.45
14 0.12 0.01 0.39
Tangerine Spain 25% EC 50 3.3 2 0 0.49 0.01 1.4 Swaine
1980 3 0.23 <0.01 0.86 1981b
7 0.21 0.02 0.73
14 0.29 <0.01 1.0
100 3.3 2 0 0,18 <0.01 0.65
3 0.13 <0.01 0.47
7 0.44 <0.01 1.7
14 0.52 <0.01 1.8
TABLE 14. Permethrin residues in various crops
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 0-1 3 6 9 14 21 28 41
Apple New Zealand 1980 11 5 EC 0.33 0.36 0.31 0.21
11 7.5 EC 0.25 0.7 0.32
7 2.5 EC 0.05 0.06 0.05 N.D.
8 2.5 EC 0.24 0.04 0.16
7 3.75 EC 0.19 0.16 0.16 0.11
Avocado New Zealand 1980 6 2.5 EC 0.09 0.07 0.06 0.04
Grape 7 2.5 EC 0.46 0.37 0.26 0.19
Orange 1 5 EC 0.12skin 0.09skin
nd pulp nd pulp
0.07total 0.05total
1 10 EC nd pulp nd pulp
0.15 Total 0.07 total
0 7 14 28 42 56 84
(a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Kiwi fruit New Zealand 6 1.5 EC 0.34 1.71 0.29 1.43
5 25 EC 0.5 - 0.56 4.22 0.16 - 0.16 - 0.08
6 2.5 EC 0.15 - 0.11 0.34 0.08 0.26 0.03 - nd -
7 2.5 EC 0.25 - 0.34 2.45 0.17 1.21 0.16 - 0.07 - 0.06 -
6 3.0 EC 0.5 2.51 0.41 2.05
6 4.4 EC 2.5 12.5 2.5 12.5 1.9 9.6 1.7 8.8
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation 0 7 14 28 42 56 84
(g a.i./100 l) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b) (a) (b)
Boysenberry 2 2.5 EC 0.26 0.15 0.12 0.07
2 2.5 EC 0.65 0.58 0.34 0.14
2 3.75 EC 0.29 0.32 0.22 0.12
1 2 4 6 9 14 45
Brussels Canada 1976 4 70 EC 0.09
sprout 70 EC 0.09
70 EC 0.05
Cranberry Canada 1977 2 70 EC 0.12(60days)
0.15(" )
0.08(")
Tomato Netherlands 1978 2 76 Smoke 0.58
gener. (0.36-0.75)
1979 1 - " 0.11
August (0.06-0.17)
1979 1 - " <0.01
October
Cucumber 1979 1 - " <0.01
October
TABLE 14. (con't)
Application Residues (mg/kg) at intervals(days) after application
Crop Country Year No. Rate Formulation
(g a.i./100 l) 1 2 4 6 9 14 45
Cabbage New Zealand 1980 1 25 EC 0.55 0.43 0.22 0.17
1 50 EC 0.54 0.78 0.36 0.22
1 100 EC 0.39 0.61 0.44 0.55
Sweet corn 1980 1 50 EC 2.7 nd 2.84 nd 1.04 nd 0.54 nd
foliage foliage foliage fol.
cob cob cob cob
Pasture 1980 1 250 EC 39 13 7 6 4 3
500 EC 45 24 9 9 7 3
750 EC 69 44 29 29 12 10
1000 EC 97 56 35 23 19 15
1500 EC 114 77 56 54 25 24
(See Figure 6). The photolysis of cis and trans isomers of permethrin
has been studied using material 14C-labelled in the carbonyl or
methylene moieties. Both permethrin isomers decomposed under
artificial light (peak outlet lambda 290-320nm) slightly faster in
hexane than in methanol. In each solvent, the cis isomer
photodecomposed approx. 1.6 times faster (T1/2 43 to 58 min) than the
trans isomer. The reacon involved extensive isomerization of the
cyclopropane ring, i.e. interconversion of the trans and cis isomers.
This probably occurred via a triplet energy state forming the
diradical intermediate (cleavage of cyclopropane ring), as the
reaction proceeded in the presence of phenoxybenzaldehyde and
benzophenone acting as photosensitizer, and was efficiently quenched
by 1,3-cyclohexadiene. The isomerization rate increased in the order
methanol < hexane < water, and at equilibrium after 1 to 4 h
irradiation the more thermodynamically stable trans isomer constituted
65 to 70% of the isomer mixture.
Together with the isomerization reaction, ester cleavage was
the major photoreaction in methanol, hexane, water and 2% aqueous
acetone. The major degradation products were DCVA and 3-phenoxybenzyl
alcohol. Other products formed in trace amounts in water included the
monochloropermethrin derivative, and the corresponding monochlorovinyl
acid, formed by reductive dechlorination. Permethrin and
monochloropermethrin do not undergo epoxidation at the dichlorovinyl
or chlorovinyl substituent under normal photooxidative conditions.
Photo oxidation to decarboxylated molecules was negligible with
permethrin (Holmstead et al 1977, 1978).
Exposure of the permethrin isomers on Dunkirk Silt Loam soil for
48 days resulted in approx. 55% loss in sunlight and approx. 35% loss
in the dark. The amount of radioactivity unextractable by 1:1 methanol
and ether was approx. 65% in the dark and approx. 18% in the light.
The unextractable radioactivity appears to be due to microbial
activity rather than to chemical reactions, but irradiation increased
the amount.
On/in the soil, relatively little isomerization at the
cyclopropane ring was encountered as compared with the photolysis in
solution. There was little difference in the amount of the DCVA
detected in the dark or light, and 3-phenoxybenzyl alcohol was the
major cleavage product of the alcohol moiety. Other products detected
in trace amounts were essentially the same as those in solutions
(Holmstead et al 1978).
In soil
The residues of permethrin in an organic soil and in vegetables
grown in soil treated with a granular formulation of permethrin were
studied by means of gas chromatography (Belanger and Hamilton 1979).
Permethrin persisted in the soil for the initial 28 days and then
 declined slowly during the rest of the season. Permethrin did not
translocate into the edible parts of the vegetables but was present in
the root system of onion and lettuce. Carrot and lettuce yields were
not significantly different from those of controls, but onion yields
were substantially decreased in the presence of permethrin.
Six soils, fortified with permethrin at 1 mg/kg, were incubated
for 16 weeks at temperatures alternating between 20°C for 15 h and
10°C for 9 h. Initially, and at 4-week intervals, soils were sampled
and analysed. In five of the soils, degradation of permethrin was
rapid, resulting in half-lives of approximately 3 weeks. In the other
soil very little degradation occurred, recovery after 16 weeks being
greater than 75%. Sterilization of the two soils in which degradation
was rapid greatly reduced the rate, indicating that microbial
degradation was the chief factor involved (Williams and Brown 1979).
On processing
The 1979 Meeting recommended an MRL of 20 mg/kg in dried tea.
Data are now available on residue levels in the final beverage
produced from tea leaves containing permethrin at four different
levels (in the range approximately 0.5 to 20 mg/kg). An aliquot sample
(5 g) of tea was brewed with 200 cm3 of boiling water, allowed to
cool and filtered. Only very low levels of permethrin were present in
the beverage (0.02-0.08 mg/kg) (Table 15). The residue level in the
beverage, expressed as a percentage of the corresponding residue level
in the dried tea, increased from 0.4%, when leaves containing
approximately 20 mg/kg were used, to 0.8% when leaves containing
approximately 3 mg/kg were used to prepare the beverage (Swaine
1981d). This pattern of data is consistent with the known low
solubility of permethrin in water.
TABLE 15. Permethrin residues in tea and tea leaves
Permethrin residues (mg/kg) Residue level in beverage × 100%
Tea leaves Tea (beverage) Residue level in leaves
18 0.08 0.4
10 0.06 0.6
2.6 0.02 0.8
0.43 <0.01 -
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits summarized in Table 16 have
been reported to the Meeting, in addition to those noted in 1979 and
1980.
TABLE 16. Additional national MRLs reported to the Meeting
mg/kg
Australia
Celery 5
Kiwi fruit 2
Rape seed, sunflower seed 0.2
Fat of meat, linseed, soybeans, mung beans and navy beans 0.1
Sweet corn 0.05 *
Beans 0.5
Water 0.3
Brazil
Cotton 0.5
Tomato 0.3
Cabbage and cauliflower 0.1
Maize 0.1
Soybean, coffee 0.01
France
Apple, pear, grape, lettuce and cabbage 1
Vegetables (other than lettuce and cabbage) 0.5
Wheat 0.5
Hungary
Fruit, vegetables 1
Netherlands
Kiwi fruit 2
Other fruit and vegetables 1
Meat and meat products, mushroom, potato 0.05
Milk and milk products 0.05
New Zealand
Bush and core fruit 1
Beans 0.5
South Africa
Sorghum 0.5
Beans 0.1
EVALUATION
COMMENTS AND APPRAISAL
Permethrin was evaluated by the 1979 and 1980 JMPR, which
considered extensive toxicological data on the compound. The 1979
Meeting required data on the potential bioaccumulation of the compound
and/or its metabolites, as well as observations in humans to evaluate
possible susceptibility to neurological effects noted in rodents,
before a full ADI for permethrin could be established.
The present Meeting considered pharmacokinetic studies on a wide
range of mammalian species. These indicated that permethrin is rapidly
absorbed and metabolized to more polar materials that are excreted.
Only small amounts of permethrin are taken up by adipose tissue and
these are principally of the less-rapidly hydrolysed cis-isomer. On
cessation of exposure, permethrin is promptly eliminated from adipose
tissue.
Information from observations in humans was also available to the
Meeting. This indicated that permethrin did not appear to cause the
transient abnormal facial sensations that are caused by exposure to
other synthetic pyrethroids. No other cause for concern was indicated.
Further studies on teratogenicity, mutagenicity and neurotoxicity
were also evaluated, none of which indicated cause for particular
concern.
The Meeting was of the view that the requirements of the previous
JMPR had been satisfied. However, the Meeting was made aware that
there were in existence at least three further long-term studies,
which were said to suggest possible carcinogenic risk from permethrin.
These studies were not available to the Meeting. In the absence of
these data, the present Meeting felt unable to do other than to extend
the temporary ADI established by the 1979 JMPR.
Residue trials on orange, lemon, grapefruit and tangerine provide
a basis for proposing maximum residue limits in citrus fruit. These
studies confirm that the residue is confined wholly within the peel
and that no residue is detected in flesh or juice.
Data from additional trials on kale and spinach, taken in
conjunction with the information considered in 1979, have enabled the
Meeting to confirm the recommendations for MRLs in spinach and to
propose a higher limit for kale. Information from trials on
boysenberry (dewberry), apple, pear, tomato and cucumber confirmed the
recommendations previously made for MRLs on dewberry and pome fruit.
Extensive information on the use patterns needed and approved for
the use of permethrin in 49 countries show the great diversity in the
pre-harvest interval (PHI). They tend to be much shorter in those
countries in which the majority of the potential use of permethrin
will arise than in countries with a temperate climate and a consequent
lesser threat from insect pests of crops. The slow rate of dissipation
of permethrin residues from plants reduces the significance of a
withholding period between treatment and harvest. The Meeting
considered this feature in conjunction with the low rate of
application and resulting low concentration of residues, and came to
the conclusion that it was appropriate to recommend MRLs based on the
minimum interval between application and harvest.
The Meeting was informed that the largest proportion of the
permethrin used world wide consists of a 40:60 ratio of cis:trans
isomers. It is recognized that products based on a 25:75 ratio of
cis:trans isomers are available. Until information becomes available
from significant usage of such materials in general agriculture/animal
husbandry, the Meeting will confine its recommendations to permethrin
products based principally on the 40:60 ratio (cis:trans isomer) of
permethrin. Data are available on the fate of permethrin on plants
both in the greenhouse and outdoors. Photolysis of permethrin in
various solvents with artificial light and on soil in sunlight
results primarily in cyclopropane ring isomerization and ester
cleavage to 3-phenoxybenzyl alcohol and 3-'2,2-dichlorovinyl)-2,
2-dimethylcyclopropane carboxylic acid (DCVA). These compounds are
also the major metabolites of permethrin on plants. Photoelimination
of carbon dioxide is a negligible route of photochemical degradation
of permethrin.
Questions were raised during the 13th Session of CCPR concerning
the appropriateness of the MRL for permethrin in dry manufactured tea.
Studies have shown that irrespective of the level of residue in the
tea, very little is leached out during the brewing of the beverage.
The Meeting felt that the recommendation should stand.
At the 13th Session of CCPR (1981) the Netherlands commented that
the limits for permethrin on gherkins and squash should preferably be
0.5 mg/kg, in line with those for cucumbers. The Meeting concurs that
the information in the 1979 monographs support this view and
recommendations were amended accordingly.
A number of national authorities have established additional MRLs
for permethrin. These were noted. Further studies in the fate of
permethrin in soil indicate that it is degraded in biologically active
soils and that the residue is not taken up into crops grown in such
treated soil.
The Meeting noted the omission of a recommendation for soybean
oil from the 1979 Evaluations, though the decision was recorded on
page 413.
Level causing no toxicological effect
Rat : 100 ppm in the diet, equivalent to 5.0 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.03 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The following maximum residue limits, determined and expressed as
total permethrin isomers excluding metabolites are recommended:
Commodity MRL (mg/kg)
Citrus fruits 0.5
Kale 5
Gherkins 0.5
Squash 0.5
Soybean oil1 0.1
1 Omitted from the 1979 recommendations
FURTHER WORK OR INFORMATION
Required (by 1982)
Reports of carcinogenicity studies not yet made available to the
JMPR.
Desirable
1. Mutagenicity studies on the metabolite 3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylic acid.
2. Results from further studies of residues in lettuce following
approved use patterns.
REFERENCES
Belanger, A. and Hamilton, H.D. Determination of disulfoton and
1979 permethrin residues in an organic soil and their
translocation into lettuce, onion and carrot. Journal of
Environmental Science and Health, B14(2):213-226.
Bewick, D.W. and Leahey, J.P. Permethrin: absorption in cows. ICI
1976 Plant Protection Division Report no. TMJ1357B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Bratt, H., Mills, I.H. and Slade, M. PP557: tissue retention in the
1977 rat. ICI Central Toxicology Laboratory Report no. CRL/P/352,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Bratt, H. and Slade, M. PP557: Tissue retention in the dog. ICI
1977 Central Toxicology Laboratory Report no. CTL/P/353,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Cridland, J.S. and Weatherley, B.C. Urinary excretion in man of
1977 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid ("CVA") after oral ingestion of permethrin (NRDC 143)-a
first report. Welcome Research Labs Report BDPG 77/1,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
1977 An estimate of permethrin (NRDC 143:OMS1821) absorbed by
people employed in a field trial of the insecticide (Kaduna,
Nigeria, 7-11 June 1977). Wellcome Research Labs. Report
BDPE 77/3, submitted by Wellcome Foundation Ltd. to WHO.
(Unpublished)
Dayan, A.D. 21-day neuropathological study in the Sprague-Dawley rat
1980 of permethrin (21Z73ZJ) administered in the diet. Wellcome
Research Labs. Report BPat.80/48, submitted by Wellcome
Foundation Ltd. to WHO. (Unpublished)
Edwards, M.J. and Iswaran, T.J. Permethrin: residue transfer and
1977 toxicology study with cows fed treated grass nuts, ICI Plant
Protection Division Report No. TMJ1519B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Elliot, M., Farnham, A.W., Janes, N.F., Needham, P.H., Pulman, D.A.
1973a and Stevenson, J.H. A photostable pyrethroid. Nature,
246:169.
1973b NRDC 143: a more stable pyrethroid. Proceedings of the 7th
British Insecticide and Fungicide Conference, p. 721.
Elliot, M., Janes, N.F., Pulman, D.A., Gaughan, L.C., Unai, T. and
1976 Casida, J.E. Radiosynthetis and metabolism in rats of the IR
isomers of the insecticide permethrin. Journal of
Agricultural and Food Chemistry, 24:270-276.
Fairbrother, D.A. NRDC 143. Whole body and radiography study in male
1977 rats. Report no. BPAT 787/3 Wellcome Research Laboratories,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Gaughan, L.E., Unai, T. and Casida, J.E. Permethrin metabolism in
1977 rats. Journal of Agricultural and Food Chemistry, 25:9-17
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. Distribution
1978a and metabolism of trans, and cis-permethrin in lactating
Jersey cows. Journal of Agricultural and Food Chemistry,
26:613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. Distribution and
1978b metabolic fate of trans- and cis-permethrin in laying hens.
Journal of Agricultural and Food Chemistry, 26:1374-1380.
Gaughan, L.C., Engel, J.L. and Casida, J.E. Pesticide interactions:
1980 effect of organo-phosphorus pesticides on the metabolism,
toxicity, and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology,
14:81-85.
Glickman, A.H. and Lech, J.J. Hydrolysis of permethrin, a pyrethroid
1981 insecticide, by rainbow trout and mouse tissues in vitro: a
comparative study. Toxicology and Applied Pharmacology,
60:186-192.
Glickman, A.H., Shono, T., Casida, J.E. and Lech, J.J. In vitro
1979 metabolism of permethrin isomers by carp and rainbow trout
liver microsomes. Journal of Agricultural and Food
Chemistry, 27:1038-1041.
Glickman, A.H., Hamid, A.A.R., Rickert, D.E. and Lech, J.J.
1981 Elimination and metabolism of permethrin isomers in rainbow
trout. Toxicology and Applied Pharmacology, 57:88-98.
Halls, G.R.H. and Periam, A.W. The fate of permethrin residues on
1980 wheat during storage and after milling and baking - results
after 9, 12 and 15 months storage. Wellcome Research Lab.
Report HEFH 80-3. (Unpublished)
Halls, G.R.H. The fate of permethrin residues on wheat during 9 months
1981 storage in Australia, Wellcome Research Laboratories Report
8 HEFH, 81-1. (Unpublished)
Holmstead, R.L., Casida, J.E. and Ruzo, L.O. Photochemical reactions
1977 of pyrethroid insecticides. Paper delivered at 172nd ACS
National Meeting, San Francisco, August 1976.
Holmstead, R.L., Casida, J.E.,Ruzo, L.O. and Fulmer, D.G. Pyrethroid
1978 photodecomposition: permethrin. Journal of Agricultural and
Food Chemistry, 26:590-595.
Hunt, L.M. and Gilbert, B.N. Distribution and excretion rates of 14C
1977 labelled permethrin isomers administered orally to four
lactating goats for 10 days. Journal of Agricultural and
Food Chemistry, 25 (3):673.
ICI.Permethrin: data submitted for review at the 1981 Meeting of WHO
1981 Panel of Experts on pesticide residues in food, to WHO by
Imperial Chemical Industries. (Unpublished)
Ivie, G.W. and Hunt, L.M. Metabolism of cis- and trans-permethrin in
1980 lactating goats. Journal of Agricultural and Food Chemistry,
28:1131-1138.
Jones, B.K. Cypermethrin: bioaccumulation study in the rat. ICI
1981 Central Toxicology Laboratory, Alderley Park Report no.
CTL/P/599, submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Kadota, T. Mammalian toxicological study of permethrin,
1976 3-phenoxybenzyl(+)-cis, trans-2.2-dimethyl-3-
(2,2-dichlorovinyl)-cyclopropane-l-carboxylate. Botyokagaku,
41:43.
Kolmodin-Hedman, B., Swensson, A. and Akerblom, M. Occupational
1981 exposure to some synthetic pyrethroids (permethrin and
fenvalerate) (in press).
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
1977a A.G. Permethrin: metabolism and residues in goats. ICI Plant
Protection Division report no. TMJ1516B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Legator, M.S. and Malling, H.V. The host-mediated assay, a practical
1971 procedure for evaluating potential mutagenic agents in
mammals, In: "Chemical Mutagens" A. Hollaender (ed.) Plenum
Press, New York, p.569.
LeQuesne, P.N., Maxwell, I.C. and Butterworth, T.G. Transient facial
1980 sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2:1.
Miyamoto, J. Degradation, metabolism and toxicity of synthetic
1976 pyrethroids. Environmental Health Perspectives, 14:15-28.
Morgan, D.W.T. A field trial to determine whether there is a change in
1979 the 25:75 cis:trans isomer ratio of permethrin following
application to cattle. Wellcome Research Laboratories Report
HIPH 79-1. (Unpublished)
Rickett, F.E. A review of the isomerization of permethrin. Wellcome
1981 Research Laboratories Report HEFA 81-2. (Unpublished)
Rickett, F.E. and Knight, P.J. Photostability of permethrin isomers.
1976 Wellcome Research Laboratories Report HCDF 76-1.
(Unpublished)
Ruzo, L.O. and Casida, J.E. Metabolism and toxicology of pyrethroids
1977 with dihalovinyl substituents. Environmental Health
Perspective, 21:285-292.
Soderlung, D.M. and Casida, J.E. Results quoted in Gaughan, L.C.,
1976 Unai, T. and Casida, J.E. 1977. (Unpublished)
Swaine, H. Permethrin residue levels on spinach and kale treated with
1981a "Ambush" during 1980 trials in the United Kingdom. ICI Plant
Protection Division Residue Data Report No. 454/PP557B034.
(Unpublished)
Swaine, H. Permethrin residues on grapefruit and tangerine treated
1981b with "Ambush" during 1980 trials in Spain. ICI Plant
Protection Division Residue Data Report No. 492/PP557B-086.
(Unpublished)
1981c Permethrin residues on citrus fruits (lemon, orange,
grapefruit) treated during a 1981 trial in the USA. ICI
Plant Protection Division Residue Data Report No. PP557B042.
(Unpublished)
1981d The transfer of permethrin residue from tea leaves to brewed
tea. ICI Plant Protection Division Residue Data Report no.
PP557B041. (Unpublished)
Swaine, H. and Sapiets, A. Cypermethrin: residue transfer study with
1981a dairy cows fed on a diet containing the insecticide. Report
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
1981b Cypermethrin: residue levels of the major metabolites of the
insecticide in the milk and tissues of dairy cows fed on a
diet containing cypermethrin at 50 mg/kg. Report submitted
by Imperial Chemical Industries to WHO. (Unpublished)
Wallwork, L.M. and Malone, J.C. 21Z73 (25/75). Acute toxicity studies
1975 by various routes of administration in the rat, mouse and
chick. Wellcome Research Laboratories report HEFF75-8,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Wallwork, L.M., Poll, G.S. and Malone, J.C. Effect on the fat oral
1975 toxicity of changes in the cis/trans ratio with 21Z73 (NRDC
143) series, Wellcome Research Labs. Report NEFC75-5,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Williams, L.M. 21Z73, rat oral 90-day study. Wellcome Research Labs.
1976 Report HEFF 76/1, submitted by Wellcome Foundation Ltd. to
WHO. (Unpublished)
Williams, I.H. and Brown, M.J. Persistence of permethrin and WL 43775
1979 in soil. Journal of Agricultural and Food Chemistry,
27(1):130-132.
declined slowly during the rest of the season. Permethrin did not
translocate into the edible parts of the vegetables but was present in
the root system of onion and lettuce. Carrot and lettuce yields were
not significantly different from those of controls, but onion yields
were substantially decreased in the presence of permethrin.
Six soils, fortified with permethrin at 1 mg/kg, were incubated
for 16 weeks at temperatures alternating between 20°C for 15 h and
10°C for 9 h. Initially, and at 4-week intervals, soils were sampled
and analysed. In five of the soils, degradation of permethrin was
rapid, resulting in half-lives of approximately 3 weeks. In the other
soil very little degradation occurred, recovery after 16 weeks being
greater than 75%. Sterilization of the two soils in which degradation
was rapid greatly reduced the rate, indicating that microbial
degradation was the chief factor involved (Williams and Brown 1979).
On processing
The 1979 Meeting recommended an MRL of 20 mg/kg in dried tea.
Data are now available on residue levels in the final beverage
produced from tea leaves containing permethrin at four different
levels (in the range approximately 0.5 to 20 mg/kg). An aliquot sample
(5 g) of tea was brewed with 200 cm3 of boiling water, allowed to
cool and filtered. Only very low levels of permethrin were present in
the beverage (0.02-0.08 mg/kg) (Table 15). The residue level in the
beverage, expressed as a percentage of the corresponding residue level
in the dried tea, increased from 0.4%, when leaves containing
approximately 20 mg/kg were used, to 0.8% when leaves containing
approximately 3 mg/kg were used to prepare the beverage (Swaine
1981d). This pattern of data is consistent with the known low
solubility of permethrin in water.
TABLE 15. Permethrin residues in tea and tea leaves
Permethrin residues (mg/kg) Residue level in beverage × 100%
Tea leaves Tea (beverage) Residue level in leaves
18 0.08 0.4
10 0.06 0.6
2.6 0.02 0.8
0.43 <0.01 -
NATIONAL MAXIMUM RESIDUE LIMITS
The national maximum residue limits summarized in Table 16 have
been reported to the Meeting, in addition to those noted in 1979 and
1980.
TABLE 16. Additional national MRLs reported to the Meeting
mg/kg
Australia
Celery 5
Kiwi fruit 2
Rape seed, sunflower seed 0.2
Fat of meat, linseed, soybeans, mung beans and navy beans 0.1
Sweet corn 0.05 *
Beans 0.5
Water 0.3
Brazil
Cotton 0.5
Tomato 0.3
Cabbage and cauliflower 0.1
Maize 0.1
Soybean, coffee 0.01
France
Apple, pear, grape, lettuce and cabbage 1
Vegetables (other than lettuce and cabbage) 0.5
Wheat 0.5
Hungary
Fruit, vegetables 1
Netherlands
Kiwi fruit 2
Other fruit and vegetables 1
Meat and meat products, mushroom, potato 0.05
Milk and milk products 0.05
New Zealand
Bush and core fruit 1
Beans 0.5
South Africa
Sorghum 0.5
Beans 0.1
EVALUATION
COMMENTS AND APPRAISAL
Permethrin was evaluated by the 1979 and 1980 JMPR, which
considered extensive toxicological data on the compound. The 1979
Meeting required data on the potential bioaccumulation of the compound
and/or its metabolites, as well as observations in humans to evaluate
possible susceptibility to neurological effects noted in rodents,
before a full ADI for permethrin could be established.
The present Meeting considered pharmacokinetic studies on a wide
range of mammalian species. These indicated that permethrin is rapidly
absorbed and metabolized to more polar materials that are excreted.
Only small amounts of permethrin are taken up by adipose tissue and
these are principally of the less-rapidly hydrolysed cis-isomer. On
cessation of exposure, permethrin is promptly eliminated from adipose
tissue.
Information from observations in humans was also available to the
Meeting. This indicated that permethrin did not appear to cause the
transient abnormal facial sensations that are caused by exposure to
other synthetic pyrethroids. No other cause for concern was indicated.
Further studies on teratogenicity, mutagenicity and neurotoxicity
were also evaluated, none of which indicated cause for particular
concern.
The Meeting was of the view that the requirements of the previous
JMPR had been satisfied. However, the Meeting was made aware that
there were in existence at least three further long-term studies,
which were said to suggest possible carcinogenic risk from permethrin.
These studies were not available to the Meeting. In the absence of
these data, the present Meeting felt unable to do other than to extend
the temporary ADI established by the 1979 JMPR.
Residue trials on orange, lemon, grapefruit and tangerine provide
a basis for proposing maximum residue limits in citrus fruit. These
studies confirm that the residue is confined wholly within the peel
and that no residue is detected in flesh or juice.
Data from additional trials on kale and spinach, taken in
conjunction with the information considered in 1979, have enabled the
Meeting to confirm the recommendations for MRLs in spinach and to
propose a higher limit for kale. Information from trials on
boysenberry (dewberry), apple, pear, tomato and cucumber confirmed the
recommendations previously made for MRLs on dewberry and pome fruit.
Extensive information on the use patterns needed and approved for
the use of permethrin in 49 countries show the great diversity in the
pre-harvest interval (PHI). They tend to be much shorter in those
countries in which the majority of the potential use of permethrin
will arise than in countries with a temperate climate and a consequent
lesser threat from insect pests of crops. The slow rate of dissipation
of permethrin residues from plants reduces the significance of a
withholding period between treatment and harvest. The Meeting
considered this feature in conjunction with the low rate of
application and resulting low concentration of residues, and came to
the conclusion that it was appropriate to recommend MRLs based on the
minimum interval between application and harvest.
The Meeting was informed that the largest proportion of the
permethrin used world wide consists of a 40:60 ratio of cis:trans
isomers. It is recognized that products based on a 25:75 ratio of
cis:trans isomers are available. Until information becomes available
from significant usage of such materials in general agriculture/animal
husbandry, the Meeting will confine its recommendations to permethrin
products based principally on the 40:60 ratio (cis:trans isomer) of
permethrin. Data are available on the fate of permethrin on plants
both in the greenhouse and outdoors. Photolysis of permethrin in
various solvents with artificial light and on soil in sunlight
results primarily in cyclopropane ring isomerization and ester
cleavage to 3-phenoxybenzyl alcohol and 3-'2,2-dichlorovinyl)-2,
2-dimethylcyclopropane carboxylic acid (DCVA). These compounds are
also the major metabolites of permethrin on plants. Photoelimination
of carbon dioxide is a negligible route of photochemical degradation
of permethrin.
Questions were raised during the 13th Session of CCPR concerning
the appropriateness of the MRL for permethrin in dry manufactured tea.
Studies have shown that irrespective of the level of residue in the
tea, very little is leached out during the brewing of the beverage.
The Meeting felt that the recommendation should stand.
At the 13th Session of CCPR (1981) the Netherlands commented that
the limits for permethrin on gherkins and squash should preferably be
0.5 mg/kg, in line with those for cucumbers. The Meeting concurs that
the information in the 1979 monographs support this view and
recommendations were amended accordingly.
A number of national authorities have established additional MRLs
for permethrin. These were noted. Further studies in the fate of
permethrin in soil indicate that it is degraded in biologically active
soils and that the residue is not taken up into crops grown in such
treated soil.
The Meeting noted the omission of a recommendation for soybean
oil from the 1979 Evaluations, though the decision was recorded on
page 413.
Level causing no toxicological effect
Rat : 100 ppm in the diet, equivalent to 5.0 mg/kg bw/day
Estimate of temporary acceptable daily intake for man
0 - 0.03 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
The following maximum residue limits, determined and expressed as
total permethrin isomers excluding metabolites are recommended:
Commodity MRL (mg/kg)
Citrus fruits 0.5
Kale 5
Gherkins 0.5
Squash 0.5
Soybean oil1 0.1
1 Omitted from the 1979 recommendations
FURTHER WORK OR INFORMATION
Required (by 1982)
Reports of carcinogenicity studies not yet made available to the
JMPR.
Desirable
1. Mutagenicity studies on the metabolite 3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylic acid.
2. Results from further studies of residues in lettuce following
approved use patterns.
REFERENCES
Belanger, A. and Hamilton, H.D. Determination of disulfoton and
1979 permethrin residues in an organic soil and their
translocation into lettuce, onion and carrot. Journal of
Environmental Science and Health, B14(2):213-226.
Bewick, D.W. and Leahey, J.P. Permethrin: absorption in cows. ICI
1976 Plant Protection Division Report no. TMJ1357B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Bratt, H., Mills, I.H. and Slade, M. PP557: tissue retention in the
1977 rat. ICI Central Toxicology Laboratory Report no. CRL/P/352,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Bratt, H. and Slade, M. PP557: Tissue retention in the dog. ICI
1977 Central Toxicology Laboratory Report no. CTL/P/353,
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Cridland, J.S. and Weatherley, B.C. Urinary excretion in man of
1977 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic
acid ("CVA") after oral ingestion of permethrin (NRDC 143)-a
first report. Welcome Research Labs Report BDPG 77/1,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
1977 An estimate of permethrin (NRDC 143:OMS1821) absorbed by
people employed in a field trial of the insecticide (Kaduna,
Nigeria, 7-11 June 1977). Wellcome Research Labs. Report
BDPE 77/3, submitted by Wellcome Foundation Ltd. to WHO.
(Unpublished)
Dayan, A.D. 21-day neuropathological study in the Sprague-Dawley rat
1980 of permethrin (21Z73ZJ) administered in the diet. Wellcome
Research Labs. Report BPat.80/48, submitted by Wellcome
Foundation Ltd. to WHO. (Unpublished)
Edwards, M.J. and Iswaran, T.J. Permethrin: residue transfer and
1977 toxicology study with cows fed treated grass nuts, ICI Plant
Protection Division Report No. TMJ1519B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Elliot, M., Farnham, A.W., Janes, N.F., Needham, P.H., Pulman, D.A.
1973a and Stevenson, J.H. A photostable pyrethroid. Nature,
246:169.
1973b NRDC 143: a more stable pyrethroid. Proceedings of the 7th
British Insecticide and Fungicide Conference, p. 721.
Elliot, M., Janes, N.F., Pulman, D.A., Gaughan, L.C., Unai, T. and
1976 Casida, J.E. Radiosynthetis and metabolism in rats of the IR
isomers of the insecticide permethrin. Journal of
Agricultural and Food Chemistry, 24:270-276.
Fairbrother, D.A. NRDC 143. Whole body and radiography study in male
1977 rats. Report no. BPAT 787/3 Wellcome Research Laboratories,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Gaughan, L.E., Unai, T. and Casida, J.E. Permethrin metabolism in
1977 rats. Journal of Agricultural and Food Chemistry, 25:9-17
Gaughan, L.C., Ackerman, M.E., Unai, T. and Casida, J.E. Distribution
1978a and metabolism of trans, and cis-permethrin in lactating
Jersey cows. Journal of Agricultural and Food Chemistry,
26:613-618.
Gaughan, L.C., Robinson, R.A. and Casida, J.E. Distribution and
1978b metabolic fate of trans- and cis-permethrin in laying hens.
Journal of Agricultural and Food Chemistry, 26:1374-1380.
Gaughan, L.C., Engel, J.L. and Casida, J.E. Pesticide interactions:
1980 effect of organo-phosphorus pesticides on the metabolism,
toxicity, and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology,
14:81-85.
Glickman, A.H. and Lech, J.J. Hydrolysis of permethrin, a pyrethroid
1981 insecticide, by rainbow trout and mouse tissues in vitro: a
comparative study. Toxicology and Applied Pharmacology,
60:186-192.
Glickman, A.H., Shono, T., Casida, J.E. and Lech, J.J. In vitro
1979 metabolism of permethrin isomers by carp and rainbow trout
liver microsomes. Journal of Agricultural and Food
Chemistry, 27:1038-1041.
Glickman, A.H., Hamid, A.A.R., Rickert, D.E. and Lech, J.J.
1981 Elimination and metabolism of permethrin isomers in rainbow
trout. Toxicology and Applied Pharmacology, 57:88-98.
Halls, G.R.H. and Periam, A.W. The fate of permethrin residues on
1980 wheat during storage and after milling and baking - results
after 9, 12 and 15 months storage. Wellcome Research Lab.
Report HEFH 80-3. (Unpublished)
Halls, G.R.H. The fate of permethrin residues on wheat during 9 months
1981 storage in Australia, Wellcome Research Laboratories Report
8 HEFH, 81-1. (Unpublished)
Holmstead, R.L., Casida, J.E. and Ruzo, L.O. Photochemical reactions
1977 of pyrethroid insecticides. Paper delivered at 172nd ACS
National Meeting, San Francisco, August 1976.
Holmstead, R.L., Casida, J.E.,Ruzo, L.O. and Fulmer, D.G. Pyrethroid
1978 photodecomposition: permethrin. Journal of Agricultural and
Food Chemistry, 26:590-595.
Hunt, L.M. and Gilbert, B.N. Distribution and excretion rates of 14C
1977 labelled permethrin isomers administered orally to four
lactating goats for 10 days. Journal of Agricultural and
Food Chemistry, 25 (3):673.
ICI.Permethrin: data submitted for review at the 1981 Meeting of WHO
1981 Panel of Experts on pesticide residues in food, to WHO by
Imperial Chemical Industries. (Unpublished)
Ivie, G.W. and Hunt, L.M. Metabolism of cis- and trans-permethrin in
1980 lactating goats. Journal of Agricultural and Food Chemistry,
28:1131-1138.
Jones, B.K. Cypermethrin: bioaccumulation study in the rat. ICI
1981 Central Toxicology Laboratory, Alderley Park Report no.
CTL/P/599, submitted by Imperial Chemical Industries to WHO.
(Unpublished)
Kadota, T. Mammalian toxicological study of permethrin,
1976 3-phenoxybenzyl(+)-cis, trans-2.2-dimethyl-3-
(2,2-dichlorovinyl)-cyclopropane-l-carboxylate. Botyokagaku,
41:43.
Kolmodin-Hedman, B., Swensson, A. and Akerblom, M. Occupational
1981 exposure to some synthetic pyrethroids (permethrin and
fenvalerate) (in press).
Leahey, J.P., Bewick, D., Carpenter, P.K., Parr, J.S. and Cameron,
1977a A.G. Permethrin: metabolism and residues in goats. ICI Plant
Protection Division report no. TMJ1516B, submitted by
Imperial Chemical Industries to WHO. (Unpublished)
Legator, M.S. and Malling, H.V. The host-mediated assay, a practical
1971 procedure for evaluating potential mutagenic agents in
mammals, In: "Chemical Mutagens" A. Hollaender (ed.) Plenum
Press, New York, p.569.
LeQuesne, P.N., Maxwell, I.C. and Butterworth, T.G. Transient facial
1980 sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electrophysiological assessment.
Neurotoxicology, 2:1.
Miyamoto, J. Degradation, metabolism and toxicity of synthetic
1976 pyrethroids. Environmental Health Perspectives, 14:15-28.
Morgan, D.W.T. A field trial to determine whether there is a change in
1979 the 25:75 cis:trans isomer ratio of permethrin following
application to cattle. Wellcome Research Laboratories Report
HIPH 79-1. (Unpublished)
Rickett, F.E. A review of the isomerization of permethrin. Wellcome
1981 Research Laboratories Report HEFA 81-2. (Unpublished)
Rickett, F.E. and Knight, P.J. Photostability of permethrin isomers.
1976 Wellcome Research Laboratories Report HCDF 76-1.
(Unpublished)
Ruzo, L.O. and Casida, J.E. Metabolism and toxicology of pyrethroids
1977 with dihalovinyl substituents. Environmental Health
Perspective, 21:285-292.
Soderlung, D.M. and Casida, J.E. Results quoted in Gaughan, L.C.,
1976 Unai, T. and Casida, J.E. 1977. (Unpublished)
Swaine, H. Permethrin residue levels on spinach and kale treated with
1981a "Ambush" during 1980 trials in the United Kingdom. ICI Plant
Protection Division Residue Data Report No. 454/PP557B034.
(Unpublished)
Swaine, H. Permethrin residues on grapefruit and tangerine treated
1981b with "Ambush" during 1980 trials in Spain. ICI Plant
Protection Division Residue Data Report No. 492/PP557B-086.
(Unpublished)
1981c Permethrin residues on citrus fruits (lemon, orange,
grapefruit) treated during a 1981 trial in the USA. ICI
Plant Protection Division Residue Data Report No. PP557B042.
(Unpublished)
1981d The transfer of permethrin residue from tea leaves to brewed
tea. ICI Plant Protection Division Residue Data Report no.
PP557B041. (Unpublished)
Swaine, H. and Sapiets, A. Cypermethrin: residue transfer study with
1981a dairy cows fed on a diet containing the insecticide. Report
submitted by Imperial Chemical Industries to WHO.
(Unpublished)
1981b Cypermethrin: residue levels of the major metabolites of the
insecticide in the milk and tissues of dairy cows fed on a
diet containing cypermethrin at 50 mg/kg. Report submitted
by Imperial Chemical Industries to WHO. (Unpublished)
Wallwork, L.M. and Malone, J.C. 21Z73 (25/75). Acute toxicity studies
1975 by various routes of administration in the rat, mouse and
chick. Wellcome Research Laboratories report HEFF75-8,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Wallwork, L.M., Poll, G.S. and Malone, J.C. Effect on the fat oral
1975 toxicity of changes in the cis/trans ratio with 21Z73 (NRDC
143) series, Wellcome Research Labs. Report NEFC75-5,
submitted by Wellcome Foundation Ltd. to WHO. (Unpublished)
Williams, L.M. 21Z73, rat oral 90-day study. Wellcome Research Labs.
1976 Report HEFF 76/1, submitted by Wellcome Foundation Ltd. to
WHO. (Unpublished)
Williams, I.H. and Brown, M.J. Persistence of permethrin and WL 43775
1979 in soil. Journal of Agricultural and Food Chemistry,
27(1):130-132.
