
FAO, PL:CP/15
WHO/Food Add./67.32
EVALUATION OF SOME PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party and the WHO Expert Committee on
Pesticide Residues, which met in Geneva, 14-21 November 1966.1
1 Report of a Joint Meeting of the FAO Working Party and the WHO
Expert Committee on Pesticide Residues, FAO Agricultural Studies, in
press; Wld Hlth Org. techn. Rep. Ser., 1967, in press
MALATHION
IDENTITY
Synonyms
carbophos, malathion
Chemical name
S-[1,2-di(ethoxycarbonyl)ethyl] dimethyl phosphorothiolothionate or
S-[1,2-di(ethoxycarbonyl)ethyl] 00-dimethyl phosphorodithioate
Formula
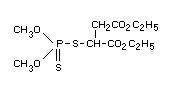 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolyzed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. Malathion or its metabolites were
recovered from eggs from treated hens and milk from cows treated with
malathion (March at al., 1956; O'Brien at al., 1961).
Malathion labelled with 32P was given to a lactating cow at 1.3
mg/kg bodyweight per day for three days. Malathion metabolites were
rapidly excreted in the urine, 69 per cent of the total radioactivity
being excreted in four days after the first dose, after which the
excretion rate decreased. After seven days 77.2 per cent of the dose
was recovered, 69 per cent of which was in the urine, 8 per cent in
the faeces, and 0.2 per cent in the milk. The principal metabolite in
early urine samples was the mono-acid of malathion. In later samples
it was the di-acid. Demethyl malathion was a significant component in
early and late samples. Dimethyl phosphate and
0,0-dimethylphosphorothioate were present in very small amounts. In
the faeces, 85 per cent, of the labelled material was malathion and 12
per cent was malaoxon (O'Brien at al., 1961).
In a lactating cow fed 1.3 mg/kg body-weight daily for three days
there was no significant inhibition of erythrocyte cholinesterase
activity (O'Brien et al., 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro (I50
7 × 10-7) (O'Brien, 1957).
The half time for the conversion in vivo of the reversibly inhibited
form of the dimethylphosphorylated cholinesterase to the irreversibly
inhibited form of this enzyme in the brain of chicken given malathion
has been found to be 2 hours. The same half time was observed in
vitro with the brain homogenate inhibited with paraoxon (Witter &
Gaines, 1963).
After single intraperitoneal or oral doses of malathion, trichlorofon
or dioxathion in rats, an increase in the activities of liver tyrosine
transaminase and alkaline phosphatase, as well as a decrease in the
level of adrenal ascorbic acid were found. Further results of this
experiment support the hypothesis that acute poisoning may produce
metabolic alterations which are mediated through the pituitary-adrenal
system (Murphy, 1966).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazleton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male i.p. 420-474 Hazleton & Holland, 1953
Chicken Oral >850(95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560(95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
In a colony of rats showing an oral LD50 of 925 mg/kg for adults,
the intragastric LD50 for newborn rats was approximately 124 mg/kg
(Lu et al., 1965).
Simultaneous oral administration of malathion and fenitrothion to male
rats resulted in potentiation when one-half the LD50 doses were
given. However, no potentiation was seen when one-tenth the LD50
doses were given (Benes & Cerná, 1966).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm for 6
weeks or after the administration, of 5 oral doses of 500 mg/kg the
production of antibodies against B. pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given malathion at 100 or 500 ppm in
the diet or trichlorofon at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around 100
per cent of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerná, 1965).
In another experiment 95 per cent technical malathion was fed to 3
groups of male rats, 10 animals per group, for 33 days at the levels
of 100, 1000 and 5000 ppm. No sign of toxicity was observed, nor any
deaths. Food intake and weight gain in the groups fed 100 and 1000 ppm
were higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68 per cent of normal
in the 1000 ppm group, and in the 5000 ppm group plasma cholinesterase
activity was 78 per cent and erythrocyte activity 22 per cent of
normal. At all levels no depression of brain cholinesterase activity
was found (American Cyanamid Co., 1955).
Ninety-eight per cent technical malathion was fed to groups of 5 rats
for 8 weeks at levels of 100 and 500 ppm without any inhibition of
whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent technical malathion was fed to 40 male and 40
female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten weeks after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. Ninety-five per cent technical malathion was fed to day-old
chicks for 2 weeks at a level of 10 ppm. For the following 10 weeks
they were divided into groups of 10 and fed 100, 1000 and 5000 ppm in
their diets. The groups on 100 and 1000 ppm behaved normally and
showed a similar growth rate and food consumption to the controls.
Four animals died in the 5000 ppm group, and signs of intoxication and
growth retardation were observed. At necropsy, no pathological lesions
were found. Plasma and brain cholinesterase activities were
significantly lowered in the 5000 ppm group (American Cyanamid Co.,
1955).
In a two-year study, 21 females were fed 250 ppm and 21 females and 6
males 2500 ppm. The 250 ppm group did not differ significantly from
the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The test hens came into production later and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At necropsy no gross or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 6 mg of malathion
in gelatin capsules daily for 32 days. No effect on plasma or
erythrocyte cholinesterase activity could be detected. Five males took
16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks, after the first administration. Maximum depression amounting
to about 25 per cent of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a slight inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted in 10 humans
ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in 5
humans receiving 6 mg EPN for 88 days and 8 mg malathion for the last
44 days, nor in 5 humans ingesting 16 mg malathion for 86 days and 3
mg EPN for the last 41 days. However 10 humans ingesting 6 mg EPN and
16 mg malathion daily for 42 days showed a slight depression of both
the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. Sixty-five per cent technical malathion as a 10 per cent or 25
per cent wettable powder was mixed in the diets of groups of 20 male
rats at the levels 100, 1000 and 5000 ppm, and fed for 2 years. The
mortality rate was not influenced, and at the 2 lower levels weight
gain and food intake were comparable to those of the controls. Five
thousand ppm reduced food intake and decreased weight gain.
Cholinesterase determinations showed no inhibition at the 100 ppm
level; with a diet containing 1000 ppm, 36 per cent inhibition of
cholinesterase activity was found in the plasma, 73 per cent in the
erythrocytes and 37 per cent in the brain, while at the 5000 ppm
level, the plasma samples showed 80 per cent, the erythrocytes 100 per
cent and the brain 77 per cent inhibition. Neither gross nor
microscopic examination revealed any pathological changes attributable
to malathion (American Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent technical malathion was fed as 25 per cent wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10-30 per cent inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95 per cent inhibition of erythrocyte
cholinesterase activity was observed. The 5000 ppm group showed total
inhibition of erythrocyte cholinesterase activity and 60-95 per cent
inhibition of cholinesterase activity in plasma and brain (American
Cyanamid Co., 1955, Hazleton & Holland, 1953).
Ninety-nine per cent technical malathion was fed for 2 years to groups
of 3-4 rats and produced, at 1000 and 5000 ppm levels, inhibition of
erythrocyte cholinesterase activity of the same order as did the 90
per cent compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90 per cent
technical malathion (American Cyanamid Co, 1955, Hazleton & Holland,
1953).
A two-year rat feeding experiment with combinations of six pesticides
(DDT, aldrin, pyrethrin, piperonyl butoxide, 2,4-D and malathion) and
eight flavouring agents (allyl heptylate, anethole, amyl butyrate,
cinnamic aldehyde, citral, ethyl methyl phenyl glycidate, eugenol, and
methyl salicylate) did not show significantly different toxic effects
compared with the effects of the compounds administered separately
(Fitzhugh, 1966).
Comments
The studies are extensive and have been carried out in several species
including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
It would be desirable to carry out reproduction studies in at least
two species, and biochemical studies, particularly with regard to the
influence of other chemicals on the metabolism of malathion.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg/day.
Man. 16 mg a day, equivalent to 0.2 mg/kg/day.
Estimate of acceptable daily intake for man
0.002 mg/kg/body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Malathion is used in many countries against aphids, scales and other
insects on a wide range of fruits and vegetables in agriculture and
horticulture. Crops treated include stone fruits (e.g. plums), pome
fruits (e.g. apples and pears) and soft fruits; carrots, turnips,
tomatoes and leafy vegetables.
Malathion is also used fairly widely in the veterinary field on
poultry (lice, mites, fleas), cattle and pigs (lice and flies).
(b) Post-harvest treatments
Malathion is used on a fairly large range of products during storage.
In some instances, it is applied directly to the raw agricultural
product (e.g. cereals, oilseeds, nuts, beans); in others, its use on
foodstuff (e.g. as a general warehouse spray) is incidental to the
hygiene of storage.
(c) Other uses
Malathion is a common ingredient of pesticides used against various
public health and domestic insect pests (flies, mosquitos, roaches,
etc.). It is also used quite extensively as a home garden insecticide,
when it may be sprayed on to both food crops and ornamental plants.
Tolerances
Product Country Tolerance ppm
General Austria 7
Tolerances (cont'd)
Product Country Tolerance ppm
Cereals Brazil 8
Canada
France
Italy
USA
UK
Cereals Germany 3
India
Cereals Kenya 12.5
Flour France 2
Fruits, green Comeco 5
vegetables (Bulgaria,
Roumania, East
Germany, Poland
Czechoslovakia,
USSR
Apples, pears USA 8
peaches, plums, Canada
tomatoes
Leafy vegetables USA 8
Canada
Meat (beef, pork, USA 4
poultry, etc.) Canada
Residues resulting from supervised trials
(a) Pre-harvest treatments
Many data are available on a variety of food crops from different
rates of application, modes of application, and times between
application and harvest. As examples Waites & Van Middelem (1958)
sprayed turnip tops and collards at various rates and found a maximum
of 3.9 ppm three days after the application. Tew & Sillibourne (1960)
measuring residues in apples and soft fruits, found a half life period
of only 1-1/2 to two days. Under their conditions useful applications
of the pesticide were unlikely to result in residues above 0.5 ppm at
harvest. Eheart (1962) investigated the persistence of various
pesticides on vegetable crops and judged that collards could be
consumed three days after spraying. From these and other data,
including some supplied by manufacturers of malathion, it is possible
to summarize the amounts expected to remain after useful applications
of the insecticide, as follows:
Type of food Pre-harvest Residue
period (ppm)
(days)
Vegetables
Cabbage 2 2 to 30
7 <0.5
Potatoes No residues detected
Lettuce 0 21
7 5
Kale 2 3
7 <0.5
Beans 0 1 to 50
3 0.5
Beet 1 15
4 9
Fruit
Berries (cane) 1 1
3 0.1
Apples and pears 0 5
3 1.5
7 0.5
Cherries and plums 0 Up to 10
7 1.5
Grapes 1 2
7 0.5
Peaches 0 Up to 18
7 3
Tomatoes 0 Up to 6
3 to 4 0.5
(continued)
Type of food Pre-harvest Residue
period (ppm)
(days)
Citrus 1 3.5
7 1.5
21 0.5
Cereals (pre-harvest) 3 Up to 4
7 <0.5
The fairly high levels are from relatively short pre-harvest intervals
and longer intervals result in lower residues. Data are not extensive
for residues from pre-harvest use on cereals. Nevertheless, various
workers have shown that malathion disappears rapidly on plants. For
example, Tomizawa et al. (1960), using labelled malathion, found less
than five per cent remaining on rice plants two days after
application: Koivistoinen (1961) found half life periods of two days
or less for residues on a fairly wide range of plants. Tomizawa & Sato
(1962) examined the mechanisms by which the insecticide disappeared
from rice during growth of the plant. From this work it is evidence
that applications during the growing period and up to seven days from
harvesting of cereals should leave residues which are much lower than
those which are added in many countries during post-harvest
treatments. Pre-harvest treatments therefore are a minor source of
residues in cereals in commerce.
Investigations of the residues resulting from uses in veterinary
practice have been fairly reassuring. Goulding & Terriere (1959) could
barely detect residues in milk and found none in flesh of cattle
treated for the control of horn fly. Claborn et al. (1960) compared
the effects from this and other insecticides used as sprays on
livestock: only traces of malathion could be detected in milk.
Pasarela et al. (1962) also did not detect malathion in various
tissues of cattle receiving food containing 200 ppm for 41 to 44 days,
although traces were found in the livers of two calves sacrificed
after 14 days. It was also not possible to detect residues in the milk
from cows, each receiving a daily 12 lb ration of dairy chow
containing up to 800 ppm. Adkins & Hair (1965) also were not able to
detect residues after the application of malathion to cattle via back
rubbers.
(b) Post-harvest treatments
As outlined in the second report of the FAO Working Party on Pesticide
Residues (PL/1965/12), malathion is widely used for controlling
insects in stored cereals and residues up to about 8 ppm result from
this usage.
Residues in food moving in commerce
(a) Cereal grains
Samples taken within countries where treatments have been carried out
(e.g. Australia, Britain, USA) show up to 8 ppm, which is the
recommended dosage for effective treatment. This is an average figure
and some spread has been observed due to uneven admixture in some
cases. Lower figures have been found after periods of storage and at
the termination of sea voyages. For example, examinations in the
United Kingdom of 70 samples representing nine shipments from
Australia known to have been treated at between 8 and 10 ppm showed a
mean of 5.1 in the ships before discharging.
Malathion is not very stable, is relatively volatile and soluble in
water. Therefore, it would not be expected to remain long in any
product where it is exposed to air and moisture. On the other hand it
is soluble and would be expected to remain much longer in oily
products. For example, it dissolves in the oil glands of citrus peel
where it may remain for a long period.
Fate of residue during storage and processing
(a) In plants and animals
The rapid losses which occur on the plant before harvesting appear to
be due to a number of factors (Koivistoinen, 1961) including
evaporation, chemical decomposition of surface deposits and metabolism
within the plant. In cereals, metabolism appears to lead to the
formation of thiophosphoric acid and to the mono- and dicarboxylic
acids of malathion.
The fate and toxicity of malathion in the animal body was reviewed in
the report of the first joint meeting of FAO Committee on Pesticides
and the WHO Committee on Pesticide Residues (FAO/WHO 1964) and in the
second report of the FAO Working Party on Pesticide Residues
(PL/1965/12). No significant residues have been found in the milk or
other tissues of animals receiving the pesticide.
Koivistoinen (1961) has shown that an enzyme system in plants rapidly
hydrolyzes malathion similar to that reported by Cook, Blake &
Williams (1957) in liver tissues.
(b) In storage and processing
The losses occurring during the storage of cereals appear to be almost
entirely due to hydrolysis to relatively inert derivatives (Rowlands,
1964, 1965). During the preparation of flour from treated wheat much
of the residue is removed and the residues in the order of one tenth
of those in the whole wheat have been found (Schesser, Priddle &
Ferrell, 1958; Allessandrini, 1965; Acton & Parouchais, 1966). The
main losses apparently occur during the cleaning process. The figure
for certain of the by-products, such as bran, may be greater than
those in the original wheat; but this does not seem very important
bearing in mind that residues of malathion have not been found in the
milk or fat of animals to which the insecticide has been fed.
Malathion is not stable to heating in the presence of moisture,
particularly under neutral or alkaline conditions. It does not readily
stand up to cooking. Alessandrini (1965) found that bread prepared
from flour containing known amounts of malathion had residues of from
eight to 16 per cent of that originally present. She concluded that
the residues would be negligible in bread from flour prepared from
wheat treated at commercial rates. In cooked pasta the residues were
not detectable.
Koivistoinen et al. (1964) investigated the stability of residues
during storage and processing of various fresh fruits and vegetable.
Residues on the surfaces decreased almost as rapidly as on the growing
plants (i.e. 50 per cent loss in from one to two days). Residues were
much more stable on or in deep frozen foods but during the preparation
of juice for storage or of jam from 54 to 86 per cent of the residues
present were destroyed; 1.1 ppm was the highest residue present in any
of this jam.
Methods of residue analysis
A number of methods are available for the determination of malathion
in foodstuffs. For cereals, the method suggested in the Second Report
of the FAO Working Party (PL/1965/12) is suitable. Even though that
method is adequate for the determination of the levels of malathion in
the commodities included in this monograph, there seems good promise
of more sensitive methods for malathion based on gas liquid
chromatography. However, the extraction and clean-up procedures
adequate for gas chromatography of malathion have not been
sufficiently developed for the working party to make a recommendation
at this time. For those interested in using GLC with a thermionic
detector, a paper by Storherr et al. (1964) will be useful. The
methods are sensitive to 0.1 ppm malathion in most foods.
RECOMMENDATIONS FOR TOLERANCES
The range of foodstuffs that could conceivably contain residues of
malathion is very wide. It could include cereal products, various
fruits and vegetables and dairy or other animal products. However,
there is no evidence of any residues being found in meat or dairy
products.
The recommendation for tolerances is as follows:
ppm
Fruit 8.0
Dried fruit 8.0
Nuts 8.0
Citrus 4.0
Cereals and
cereal
products 8.0
Vegetables 6.0
(leafy)
Vegetables 3.0
(other than
leafy)
These values are those resulting from good pest control practice. They
are predicated on a large loss in storage in processing. As an
example, during the storing, transporting and milling of treated
cereals, considerable reductions occur from the initial dosage levels
of about 8 ppm. Cooking results in further substantial losses. The
amounts found in bread or finished pasta made from wheat treated in
this way (i.e. below 0.4 ppm). As these amounts are well within the
permissible level, the residue resulting from good agricultural
practice, viz. 8 ppm, may be accepted as a tolerance for raw cereals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
American Cyanamid Company, New York (1955) Unpublished report
American Cyanamid Company, New York (1960) Unpublished report
Benes, V. & Cerná, V. (1965) Czech. Hyg., 10, 209
Benes, V. & Cerná, V. (1966) VII International Congress of Nutrition,
Hamburg, 1966 (Proceedings - In press)
Benes, V., Pekárek, J. & Cerná, V. (1963) Czech. Hyg., 8, 3
Fitzhugh, O. G. (1966) Canad. med. Ass. J., 94, 598
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol. exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. (1953) Arch. industr. Hyg., 8, 399
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3, 591
March, R. B., Fukuto, T. R., Metcalf, R. L. & Maxon, M. G. (1956) J.
Econ. Ent., 49, 185
Moeller, H. C. & Rider, J. A. (1962) Toxicol. appl. Pharmacol., 4,
123
Murphy, S. D. (1966) Toxicol. appl. Pharmacol., 8, 348
O'Brien, R. D. (1957) J. econ. Ent., 50, 3.59
O'Brien, R. D., Dauterman, W. C., & Niedermeier, R. P. (1961) J.
Agric. Food Chem., 9 (1), 39
Rider, J. A., Moeller, H. C., Swader, J. & Devereaux, G. (1959)
Clinical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1963) Biochem. Pharmacol., 12, 1421
REFERENCES PERTINENT TO AGRICULTURAL DATA
Allessandrini, M. E. (1965) Determination of the persistence and fate
of various insecticides in or on wheat during storage, milling and
during the baking or cooking of the products made from the treated
wheat. [Unpublished work conducted under U.S.D.A., Project No.
E15-AMS-8(a)]
Acton, F. E. & Parouchais, C. (1966) Malathion levels in wheat and
wheat products. Fd. Technol. Aust., 18, 77, 79, 81
Adkins, T. R. & Hair, J. A. (1965) Absence of residues in milk after
dimethoate and malathion were applied with back rubbers to dairy
cattle. J. econ. Ent., 58; (1): 155
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff.
(1960) Meat and milk residues from livestock sprays. J. ag. fd. Chem.,
8: (6). 439-441
Cook, J. W., Blake, J. R. & Williams, M. W. (1957) The enzymatic
hydrolysis of malathion and its inhibition by EPN and other organic
phorphorus compounds. J. Ass. Offic. Agr. Chem., 40: 664-665
Eheart, J. F., Massey, D. H. jr & Dickinson, J. (1962) Persistency of
malathion, parathion, Perthane and Sevin on selected vegetables crops.
Tech. Bull. Va. Agric. Exp. Stat., 157: 8
Goulding, R. L. & Terriere, L. C. (1959) Malathion residues in milk of
dairy cows treated for horn fly control. J. econ. Ent., 52: (2): 341
Koivistoinen, P., Karinpaa, M, K. & Roine, P. (1964) Malathion
residues on fruit treated by dipping. J. ag. food Chem., 12: (6):
551-555
Koivistoinen, P. (1961) Studies on the disappearance of malathion from
plant materials. Ann. Acad. Sci. Fenn. Ser. A. Biol., 14/15: 1-91
Koivistoinen, P., Karinpaa, M. K. & Roine, P. (1964) Stability of
residues in food processing and storage. J. ag. food Chem., 12: (6):
557-560
McFarlane, J. A. & Harris, A. H. (1964) Relative susceptibility to
malathion contamination of six animal feed ingredients. J. Sci. Food
Agric., 15: (9): 612-619
Pasarela, W. R., Brown, R. G. & Shaffer, C. B. (1962) Feeding of
malathion to cattle: Residue analyses of milk and tissue. J. ag. food
Chem., 10 (1): 7-9
Rowlands, D. G. (1964) The degradation of malathion on stored maize
and wheat grains. J. Sci. Food Agric., 15: 114-119
Rowlands, D. G. (1965) The in vitro and in vivo oxidation and
hydrolysis of malathion by wheat grain esterases. J. Sci. Food Agric.,
16: 325-30
Schesser, J. H., Priddle, W. E. & Ferrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion and lindane. J. Econ. Entomol., 51: 516-8
Storherr, R. W., Watts, R. R., Friedman, S. J., Erwin, F. Guiffrida &
Ives, F. (1964) Identification and analyses of five organophosphate
pesticides. Recoveries from crops fortified at different levels. J.
Assoc. Offic. Agric. Chem., 47: 1089-1093
Tew, R. P. & Sillibourne, J. M. (1960) Harvest residues of malathion
on soft fruits and apples. East Malling Res. Stn., Ann. Report, 1960:
48: 116-119
Tomizawa, C. & Sato, T. (1962) Metabolic fate of malathion and methyl
parathion in the Japanese rice plant. Jap. J. App.; Entomol. Zool.,
6: 70-5
Tomizawa, C. & Sato, T. & Kubo, H. (1960) The fate of
organo-phosphorus insecticides sprayed on rice plants. Botyu-Kagaku
[Scientific insect control] 25: 99-105
Waites, R. E. & Van Middelem, C. H. (1958) Residue status of DDT and
malathion on turnip tops, collards and lettuce. J. econ. Ent., 51:
306-308
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolyzed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. Malathion or its metabolites were
recovered from eggs from treated hens and milk from cows treated with
malathion (March at al., 1956; O'Brien at al., 1961).
Malathion labelled with 32P was given to a lactating cow at 1.3
mg/kg bodyweight per day for three days. Malathion metabolites were
rapidly excreted in the urine, 69 per cent of the total radioactivity
being excreted in four days after the first dose, after which the
excretion rate decreased. After seven days 77.2 per cent of the dose
was recovered, 69 per cent of which was in the urine, 8 per cent in
the faeces, and 0.2 per cent in the milk. The principal metabolite in
early urine samples was the mono-acid of malathion. In later samples
it was the di-acid. Demethyl malathion was a significant component in
early and late samples. Dimethyl phosphate and
0,0-dimethylphosphorothioate were present in very small amounts. In
the faeces, 85 per cent, of the labelled material was malathion and 12
per cent was malaoxon (O'Brien at al., 1961).
In a lactating cow fed 1.3 mg/kg body-weight daily for three days
there was no significant inhibition of erythrocyte cholinesterase
activity (O'Brien et al., 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro (I50
7 × 10-7) (O'Brien, 1957).
The half time for the conversion in vivo of the reversibly inhibited
form of the dimethylphosphorylated cholinesterase to the irreversibly
inhibited form of this enzyme in the brain of chicken given malathion
has been found to be 2 hours. The same half time was observed in
vitro with the brain homogenate inhibited with paraoxon (Witter &
Gaines, 1963).
After single intraperitoneal or oral doses of malathion, trichlorofon
or dioxathion in rats, an increase in the activities of liver tyrosine
transaminase and alkaline phosphatase, as well as a decrease in the
level of adrenal ascorbic acid were found. Further results of this
experiment support the hypothesis that acute poisoning may produce
metabolic alterations which are mediated through the pituitary-adrenal
system (Murphy, 1966).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazleton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male i.p. 420-474 Hazleton & Holland, 1953
Chicken Oral >850(95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560(95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
In a colony of rats showing an oral LD50 of 925 mg/kg for adults,
the intragastric LD50 for newborn rats was approximately 124 mg/kg
(Lu et al., 1965).
Simultaneous oral administration of malathion and fenitrothion to male
rats resulted in potentiation when one-half the LD50 doses were
given. However, no potentiation was seen when one-tenth the LD50
doses were given (Benes & Cerná, 1966).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm for 6
weeks or after the administration, of 5 oral doses of 500 mg/kg the
production of antibodies against B. pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given malathion at 100 or 500 ppm in
the diet or trichlorofon at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around 100
per cent of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerná, 1965).
In another experiment 95 per cent technical malathion was fed to 3
groups of male rats, 10 animals per group, for 33 days at the levels
of 100, 1000 and 5000 ppm. No sign of toxicity was observed, nor any
deaths. Food intake and weight gain in the groups fed 100 and 1000 ppm
were higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68 per cent of normal
in the 1000 ppm group, and in the 5000 ppm group plasma cholinesterase
activity was 78 per cent and erythrocyte activity 22 per cent of
normal. At all levels no depression of brain cholinesterase activity
was found (American Cyanamid Co., 1955).
Ninety-eight per cent technical malathion was fed to groups of 5 rats
for 8 weeks at levels of 100 and 500 ppm without any inhibition of
whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent technical malathion was fed to 40 male and 40
female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten weeks after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. Ninety-five per cent technical malathion was fed to day-old
chicks for 2 weeks at a level of 10 ppm. For the following 10 weeks
they were divided into groups of 10 and fed 100, 1000 and 5000 ppm in
their diets. The groups on 100 and 1000 ppm behaved normally and
showed a similar growth rate and food consumption to the controls.
Four animals died in the 5000 ppm group, and signs of intoxication and
growth retardation were observed. At necropsy, no pathological lesions
were found. Plasma and brain cholinesterase activities were
significantly lowered in the 5000 ppm group (American Cyanamid Co.,
1955).
In a two-year study, 21 females were fed 250 ppm and 21 females and 6
males 2500 ppm. The 250 ppm group did not differ significantly from
the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The test hens came into production later and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At necropsy no gross or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 6 mg of malathion
in gelatin capsules daily for 32 days. No effect on plasma or
erythrocyte cholinesterase activity could be detected. Five males took
16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks, after the first administration. Maximum depression amounting
to about 25 per cent of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a slight inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted in 10 humans
ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in 5
humans receiving 6 mg EPN for 88 days and 8 mg malathion for the last
44 days, nor in 5 humans ingesting 16 mg malathion for 86 days and 3
mg EPN for the last 41 days. However 10 humans ingesting 6 mg EPN and
16 mg malathion daily for 42 days showed a slight depression of both
the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. Sixty-five per cent technical malathion as a 10 per cent or 25
per cent wettable powder was mixed in the diets of groups of 20 male
rats at the levels 100, 1000 and 5000 ppm, and fed for 2 years. The
mortality rate was not influenced, and at the 2 lower levels weight
gain and food intake were comparable to those of the controls. Five
thousand ppm reduced food intake and decreased weight gain.
Cholinesterase determinations showed no inhibition at the 100 ppm
level; with a diet containing 1000 ppm, 36 per cent inhibition of
cholinesterase activity was found in the plasma, 73 per cent in the
erythrocytes and 37 per cent in the brain, while at the 5000 ppm
level, the plasma samples showed 80 per cent, the erythrocytes 100 per
cent and the brain 77 per cent inhibition. Neither gross nor
microscopic examination revealed any pathological changes attributable
to malathion (American Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent technical malathion was fed as 25 per cent wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10-30 per cent inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95 per cent inhibition of erythrocyte
cholinesterase activity was observed. The 5000 ppm group showed total
inhibition of erythrocyte cholinesterase activity and 60-95 per cent
inhibition of cholinesterase activity in plasma and brain (American
Cyanamid Co., 1955, Hazleton & Holland, 1953).
Ninety-nine per cent technical malathion was fed for 2 years to groups
of 3-4 rats and produced, at 1000 and 5000 ppm levels, inhibition of
erythrocyte cholinesterase activity of the same order as did the 90
per cent compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90 per cent
technical malathion (American Cyanamid Co, 1955, Hazleton & Holland,
1953).
A two-year rat feeding experiment with combinations of six pesticides
(DDT, aldrin, pyrethrin, piperonyl butoxide, 2,4-D and malathion) and
eight flavouring agents (allyl heptylate, anethole, amyl butyrate,
cinnamic aldehyde, citral, ethyl methyl phenyl glycidate, eugenol, and
methyl salicylate) did not show significantly different toxic effects
compared with the effects of the compounds administered separately
(Fitzhugh, 1966).
Comments
The studies are extensive and have been carried out in several species
including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
It would be desirable to carry out reproduction studies in at least
two species, and biochemical studies, particularly with regard to the
influence of other chemicals on the metabolism of malathion.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg/day.
Man. 16 mg a day, equivalent to 0.2 mg/kg/day.
Estimate of acceptable daily intake for man
0.002 mg/kg/body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Malathion is used in many countries against aphids, scales and other
insects on a wide range of fruits and vegetables in agriculture and
horticulture. Crops treated include stone fruits (e.g. plums), pome
fruits (e.g. apples and pears) and soft fruits; carrots, turnips,
tomatoes and leafy vegetables.
Malathion is also used fairly widely in the veterinary field on
poultry (lice, mites, fleas), cattle and pigs (lice and flies).
(b) Post-harvest treatments
Malathion is used on a fairly large range of products during storage.
In some instances, it is applied directly to the raw agricultural
product (e.g. cereals, oilseeds, nuts, beans); in others, its use on
foodstuff (e.g. as a general warehouse spray) is incidental to the
hygiene of storage.
(c) Other uses
Malathion is a common ingredient of pesticides used against various
public health and domestic insect pests (flies, mosquitos, roaches,
etc.). It is also used quite extensively as a home garden insecticide,
when it may be sprayed on to both food crops and ornamental plants.
Tolerances
Product Country Tolerance ppm
General Austria 7
Tolerances (cont'd)
Product Country Tolerance ppm
Cereals Brazil 8
Canada
France
Italy
USA
UK
Cereals Germany 3
India
Cereals Kenya 12.5
Flour France 2
Fruits, green Comeco 5
vegetables (Bulgaria,
Roumania, East
Germany, Poland
Czechoslovakia,
USSR
Apples, pears USA 8
peaches, plums, Canada
tomatoes
Leafy vegetables USA 8
Canada
Meat (beef, pork, USA 4
poultry, etc.) Canada
Residues resulting from supervised trials
(a) Pre-harvest treatments
Many data are available on a variety of food crops from different
rates of application, modes of application, and times between
application and harvest. As examples Waites & Van Middelem (1958)
sprayed turnip tops and collards at various rates and found a maximum
of 3.9 ppm three days after the application. Tew & Sillibourne (1960)
measuring residues in apples and soft fruits, found a half life period
of only 1-1/2 to two days. Under their conditions useful applications
of the pesticide were unlikely to result in residues above 0.5 ppm at
harvest. Eheart (1962) investigated the persistence of various
pesticides on vegetable crops and judged that collards could be
consumed three days after spraying. From these and other data,
including some supplied by manufacturers of malathion, it is possible
to summarize the amounts expected to remain after useful applications
of the insecticide, as follows:
Type of food Pre-harvest Residue
period (ppm)
(days)
Vegetables
Cabbage 2 2 to 30
7 <0.5
Potatoes No residues detected
Lettuce 0 21
7 5
Kale 2 3
7 <0.5
Beans 0 1 to 50
3 0.5
Beet 1 15
4 9
Fruit
Berries (cane) 1 1
3 0.1
Apples and pears 0 5
3 1.5
7 0.5
Cherries and plums 0 Up to 10
7 1.5
Grapes 1 2
7 0.5
Peaches 0 Up to 18
7 3
Tomatoes 0 Up to 6
3 to 4 0.5
(continued)
Type of food Pre-harvest Residue
period (ppm)
(days)
Citrus 1 3.5
7 1.5
21 0.5
Cereals (pre-harvest) 3 Up to 4
7 <0.5
The fairly high levels are from relatively short pre-harvest intervals
and longer intervals result in lower residues. Data are not extensive
for residues from pre-harvest use on cereals. Nevertheless, various
workers have shown that malathion disappears rapidly on plants. For
example, Tomizawa et al. (1960), using labelled malathion, found less
than five per cent remaining on rice plants two days after
application: Koivistoinen (1961) found half life periods of two days
or less for residues on a fairly wide range of plants. Tomizawa & Sato
(1962) examined the mechanisms by which the insecticide disappeared
from rice during growth of the plant. From this work it is evidence
that applications during the growing period and up to seven days from
harvesting of cereals should leave residues which are much lower than
those which are added in many countries during post-harvest
treatments. Pre-harvest treatments therefore are a minor source of
residues in cereals in commerce.
Investigations of the residues resulting from uses in veterinary
practice have been fairly reassuring. Goulding & Terriere (1959) could
barely detect residues in milk and found none in flesh of cattle
treated for the control of horn fly. Claborn et al. (1960) compared
the effects from this and other insecticides used as sprays on
livestock: only traces of malathion could be detected in milk.
Pasarela et al. (1962) also did not detect malathion in various
tissues of cattle receiving food containing 200 ppm for 41 to 44 days,
although traces were found in the livers of two calves sacrificed
after 14 days. It was also not possible to detect residues in the milk
from cows, each receiving a daily 12 lb ration of dairy chow
containing up to 800 ppm. Adkins & Hair (1965) also were not able to
detect residues after the application of malathion to cattle via back
rubbers.
(b) Post-harvest treatments
As outlined in the second report of the FAO Working Party on Pesticide
Residues (PL/1965/12), malathion is widely used for controlling
insects in stored cereals and residues up to about 8 ppm result from
this usage.
Residues in food moving in commerce
(a) Cereal grains
Samples taken within countries where treatments have been carried out
(e.g. Australia, Britain, USA) show up to 8 ppm, which is the
recommended dosage for effective treatment. This is an average figure
and some spread has been observed due to uneven admixture in some
cases. Lower figures have been found after periods of storage and at
the termination of sea voyages. For example, examinations in the
United Kingdom of 70 samples representing nine shipments from
Australia known to have been treated at between 8 and 10 ppm showed a
mean of 5.1 in the ships before discharging.
Malathion is not very stable, is relatively volatile and soluble in
water. Therefore, it would not be expected to remain long in any
product where it is exposed to air and moisture. On the other hand it
is soluble and would be expected to remain much longer in oily
products. For example, it dissolves in the oil glands of citrus peel
where it may remain for a long period.
Fate of residue during storage and processing
(a) In plants and animals
The rapid losses which occur on the plant before harvesting appear to
be due to a number of factors (Koivistoinen, 1961) including
evaporation, chemical decomposition of surface deposits and metabolism
within the plant. In cereals, metabolism appears to lead to the
formation of thiophosphoric acid and to the mono- and dicarboxylic
acids of malathion.
The fate and toxicity of malathion in the animal body was reviewed in
the report of the first joint meeting of FAO Committee on Pesticides
and the WHO Committee on Pesticide Residues (FAO/WHO 1964) and in the
second report of the FAO Working Party on Pesticide Residues
(PL/1965/12). No significant residues have been found in the milk or
other tissues of animals receiving the pesticide.
Koivistoinen (1961) has shown that an enzyme system in plants rapidly
hydrolyzes malathion similar to that reported by Cook, Blake &
Williams (1957) in liver tissues.
(b) In storage and processing
The losses occurring during the storage of cereals appear to be almost
entirely due to hydrolysis to relatively inert derivatives (Rowlands,
1964, 1965). During the preparation of flour from treated wheat much
of the residue is removed and the residues in the order of one tenth
of those in the whole wheat have been found (Schesser, Priddle &
Ferrell, 1958; Allessandrini, 1965; Acton & Parouchais, 1966). The
main losses apparently occur during the cleaning process. The figure
for certain of the by-products, such as bran, may be greater than
those in the original wheat; but this does not seem very important
bearing in mind that residues of malathion have not been found in the
milk or fat of animals to which the insecticide has been fed.
Malathion is not stable to heating in the presence of moisture,
particularly under neutral or alkaline conditions. It does not readily
stand up to cooking. Alessandrini (1965) found that bread prepared
from flour containing known amounts of malathion had residues of from
eight to 16 per cent of that originally present. She concluded that
the residues would be negligible in bread from flour prepared from
wheat treated at commercial rates. In cooked pasta the residues were
not detectable.
Koivistoinen et al. (1964) investigated the stability of residues
during storage and processing of various fresh fruits and vegetable.
Residues on the surfaces decreased almost as rapidly as on the growing
plants (i.e. 50 per cent loss in from one to two days). Residues were
much more stable on or in deep frozen foods but during the preparation
of juice for storage or of jam from 54 to 86 per cent of the residues
present were destroyed; 1.1 ppm was the highest residue present in any
of this jam.
Methods of residue analysis
A number of methods are available for the determination of malathion
in foodstuffs. For cereals, the method suggested in the Second Report
of the FAO Working Party (PL/1965/12) is suitable. Even though that
method is adequate for the determination of the levels of malathion in
the commodities included in this monograph, there seems good promise
of more sensitive methods for malathion based on gas liquid
chromatography. However, the extraction and clean-up procedures
adequate for gas chromatography of malathion have not been
sufficiently developed for the working party to make a recommendation
at this time. For those interested in using GLC with a thermionic
detector, a paper by Storherr et al. (1964) will be useful. The
methods are sensitive to 0.1 ppm malathion in most foods.
RECOMMENDATIONS FOR TOLERANCES
The range of foodstuffs that could conceivably contain residues of
malathion is very wide. It could include cereal products, various
fruits and vegetables and dairy or other animal products. However,
there is no evidence of any residues being found in meat or dairy
products.
The recommendation for tolerances is as follows:
ppm
Fruit 8.0
Dried fruit 8.0
Nuts 8.0
Citrus 4.0
Cereals and
cereal
products 8.0
Vegetables 6.0
(leafy)
Vegetables 3.0
(other than
leafy)
These values are those resulting from good pest control practice. They
are predicated on a large loss in storage in processing. As an
example, during the storing, transporting and milling of treated
cereals, considerable reductions occur from the initial dosage levels
of about 8 ppm. Cooking results in further substantial losses. The
amounts found in bread or finished pasta made from wheat treated in
this way (i.e. below 0.4 ppm). As these amounts are well within the
permissible level, the residue resulting from good agricultural
practice, viz. 8 ppm, may be accepted as a tolerance for raw cereals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
American Cyanamid Company, New York (1955) Unpublished report
American Cyanamid Company, New York (1960) Unpublished report
Benes, V. & Cerná, V. (1965) Czech. Hyg., 10, 209
Benes, V. & Cerná, V. (1966) VII International Congress of Nutrition,
Hamburg, 1966 (Proceedings - In press)
Benes, V., Pekárek, J. & Cerná, V. (1963) Czech. Hyg., 8, 3
Fitzhugh, O. G. (1966) Canad. med. Ass. J., 94, 598
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol. exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. (1953) Arch. industr. Hyg., 8, 399
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3, 591
March, R. B., Fukuto, T. R., Metcalf, R. L. & Maxon, M. G. (1956) J.
Econ. Ent., 49, 185
Moeller, H. C. & Rider, J. A. (1962) Toxicol. appl. Pharmacol., 4,
123
Murphy, S. D. (1966) Toxicol. appl. Pharmacol., 8, 348
O'Brien, R. D. (1957) J. econ. Ent., 50, 3.59
O'Brien, R. D., Dauterman, W. C., & Niedermeier, R. P. (1961) J.
Agric. Food Chem., 9 (1), 39
Rider, J. A., Moeller, H. C., Swader, J. & Devereaux, G. (1959)
Clinical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1963) Biochem. Pharmacol., 12, 1421
REFERENCES PERTINENT TO AGRICULTURAL DATA
Allessandrini, M. E. (1965) Determination of the persistence and fate
of various insecticides in or on wheat during storage, milling and
during the baking or cooking of the products made from the treated
wheat. [Unpublished work conducted under U.S.D.A., Project No.
E15-AMS-8(a)]
Acton, F. E. & Parouchais, C. (1966) Malathion levels in wheat and
wheat products. Fd. Technol. Aust., 18, 77, 79, 81
Adkins, T. R. & Hair, J. A. (1965) Absence of residues in milk after
dimethoate and malathion were applied with back rubbers to dairy
cattle. J. econ. Ent., 58; (1): 155
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff.
(1960) Meat and milk residues from livestock sprays. J. ag. fd. Chem.,
8: (6). 439-441
Cook, J. W., Blake, J. R. & Williams, M. W. (1957) The enzymatic
hydrolysis of malathion and its inhibition by EPN and other organic
phorphorus compounds. J. Ass. Offic. Agr. Chem., 40: 664-665
Eheart, J. F., Massey, D. H. jr & Dickinson, J. (1962) Persistency of
malathion, parathion, Perthane and Sevin on selected vegetables crops.
Tech. Bull. Va. Agric. Exp. Stat., 157: 8
Goulding, R. L. & Terriere, L. C. (1959) Malathion residues in milk of
dairy cows treated for horn fly control. J. econ. Ent., 52: (2): 341
Koivistoinen, P., Karinpaa, M, K. & Roine, P. (1964) Malathion
residues on fruit treated by dipping. J. ag. food Chem., 12: (6):
551-555
Koivistoinen, P. (1961) Studies on the disappearance of malathion from
plant materials. Ann. Acad. Sci. Fenn. Ser. A. Biol., 14/15: 1-91
Koivistoinen, P., Karinpaa, M. K. & Roine, P. (1964) Stability of
residues in food processing and storage. J. ag. food Chem., 12: (6):
557-560
McFarlane, J. A. & Harris, A. H. (1964) Relative susceptibility to
malathion contamination of six animal feed ingredients. J. Sci. Food
Agric., 15: (9): 612-619
Pasarela, W. R., Brown, R. G. & Shaffer, C. B. (1962) Feeding of
malathion to cattle: Residue analyses of milk and tissue. J. ag. food
Chem., 10 (1): 7-9
Rowlands, D. G. (1964) The degradation of malathion on stored maize
and wheat grains. J. Sci. Food Agric., 15: 114-119
Rowlands, D. G. (1965) The in vitro and in vivo oxidation and
hydrolysis of malathion by wheat grain esterases. J. Sci. Food Agric.,
16: 325-30
Schesser, J. H., Priddle, W. E. & Ferrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion and lindane. J. Econ. Entomol., 51: 516-8
Storherr, R. W., Watts, R. R., Friedman, S. J., Erwin, F. Guiffrida &
Ives, F. (1964) Identification and analyses of five organophosphate
pesticides. Recoveries from crops fortified at different levels. J.
Assoc. Offic. Agric. Chem., 47: 1089-1093
Tew, R. P. & Sillibourne, J. M. (1960) Harvest residues of malathion
on soft fruits and apples. East Malling Res. Stn., Ann. Report, 1960:
48: 116-119
Tomizawa, C. & Sato, T. (1962) Metabolic fate of malathion and methyl
parathion in the Japanese rice plant. Jap. J. App.; Entomol. Zool.,
6: 70-5
Tomizawa, C. & Sato, T. & Kubo, H. (1960) The fate of
organo-phosphorus insecticides sprayed on rice plants. Botyu-Kagaku
[Scientific insect control] 25: 99-105
Waites, R. E. & Van Middelem, C. H. (1958) Residue status of DDT and
malathion on turnip tops, collards and lettuce. J. econ. Ent., 51:
306-308
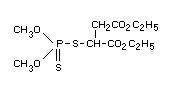 BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolyzed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. Malathion or its metabolites were
recovered from eggs from treated hens and milk from cows treated with
malathion (March at al., 1956; O'Brien at al., 1961).
Malathion labelled with 32P was given to a lactating cow at 1.3
mg/kg bodyweight per day for three days. Malathion metabolites were
rapidly excreted in the urine, 69 per cent of the total radioactivity
being excreted in four days after the first dose, after which the
excretion rate decreased. After seven days 77.2 per cent of the dose
was recovered, 69 per cent of which was in the urine, 8 per cent in
the faeces, and 0.2 per cent in the milk. The principal metabolite in
early urine samples was the mono-acid of malathion. In later samples
it was the di-acid. Demethyl malathion was a significant component in
early and late samples. Dimethyl phosphate and
0,0-dimethylphosphorothioate were present in very small amounts. In
the faeces, 85 per cent, of the labelled material was malathion and 12
per cent was malaoxon (O'Brien at al., 1961).
In a lactating cow fed 1.3 mg/kg body-weight daily for three days
there was no significant inhibition of erythrocyte cholinesterase
activity (O'Brien et al., 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro (I50
7 × 10-7) (O'Brien, 1957).
The half time for the conversion in vivo of the reversibly inhibited
form of the dimethylphosphorylated cholinesterase to the irreversibly
inhibited form of this enzyme in the brain of chicken given malathion
has been found to be 2 hours. The same half time was observed in
vitro with the brain homogenate inhibited with paraoxon (Witter &
Gaines, 1963).
After single intraperitoneal or oral doses of malathion, trichlorofon
or dioxathion in rats, an increase in the activities of liver tyrosine
transaminase and alkaline phosphatase, as well as a decrease in the
level of adrenal ascorbic acid were found. Further results of this
experiment support the hypothesis that acute poisoning may produce
metabolic alterations which are mediated through the pituitary-adrenal
system (Murphy, 1966).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazleton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male i.p. 420-474 Hazleton & Holland, 1953
Chicken Oral >850(95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560(95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
In a colony of rats showing an oral LD50 of 925 mg/kg for adults,
the intragastric LD50 for newborn rats was approximately 124 mg/kg
(Lu et al., 1965).
Simultaneous oral administration of malathion and fenitrothion to male
rats resulted in potentiation when one-half the LD50 doses were
given. However, no potentiation was seen when one-tenth the LD50
doses were given (Benes & Cerná, 1966).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm for 6
weeks or after the administration, of 5 oral doses of 500 mg/kg the
production of antibodies against B. pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given malathion at 100 or 500 ppm in
the diet or trichlorofon at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around 100
per cent of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerná, 1965).
In another experiment 95 per cent technical malathion was fed to 3
groups of male rats, 10 animals per group, for 33 days at the levels
of 100, 1000 and 5000 ppm. No sign of toxicity was observed, nor any
deaths. Food intake and weight gain in the groups fed 100 and 1000 ppm
were higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68 per cent of normal
in the 1000 ppm group, and in the 5000 ppm group plasma cholinesterase
activity was 78 per cent and erythrocyte activity 22 per cent of
normal. At all levels no depression of brain cholinesterase activity
was found (American Cyanamid Co., 1955).
Ninety-eight per cent technical malathion was fed to groups of 5 rats
for 8 weeks at levels of 100 and 500 ppm without any inhibition of
whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent technical malathion was fed to 40 male and 40
female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten weeks after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. Ninety-five per cent technical malathion was fed to day-old
chicks for 2 weeks at a level of 10 ppm. For the following 10 weeks
they were divided into groups of 10 and fed 100, 1000 and 5000 ppm in
their diets. The groups on 100 and 1000 ppm behaved normally and
showed a similar growth rate and food consumption to the controls.
Four animals died in the 5000 ppm group, and signs of intoxication and
growth retardation were observed. At necropsy, no pathological lesions
were found. Plasma and brain cholinesterase activities were
significantly lowered in the 5000 ppm group (American Cyanamid Co.,
1955).
In a two-year study, 21 females were fed 250 ppm and 21 females and 6
males 2500 ppm. The 250 ppm group did not differ significantly from
the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The test hens came into production later and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At necropsy no gross or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 6 mg of malathion
in gelatin capsules daily for 32 days. No effect on plasma or
erythrocyte cholinesterase activity could be detected. Five males took
16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks, after the first administration. Maximum depression amounting
to about 25 per cent of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a slight inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted in 10 humans
ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in 5
humans receiving 6 mg EPN for 88 days and 8 mg malathion for the last
44 days, nor in 5 humans ingesting 16 mg malathion for 86 days and 3
mg EPN for the last 41 days. However 10 humans ingesting 6 mg EPN and
16 mg malathion daily for 42 days showed a slight depression of both
the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. Sixty-five per cent technical malathion as a 10 per cent or 25
per cent wettable powder was mixed in the diets of groups of 20 male
rats at the levels 100, 1000 and 5000 ppm, and fed for 2 years. The
mortality rate was not influenced, and at the 2 lower levels weight
gain and food intake were comparable to those of the controls. Five
thousand ppm reduced food intake and decreased weight gain.
Cholinesterase determinations showed no inhibition at the 100 ppm
level; with a diet containing 1000 ppm, 36 per cent inhibition of
cholinesterase activity was found in the plasma, 73 per cent in the
erythrocytes and 37 per cent in the brain, while at the 5000 ppm
level, the plasma samples showed 80 per cent, the erythrocytes 100 per
cent and the brain 77 per cent inhibition. Neither gross nor
microscopic examination revealed any pathological changes attributable
to malathion (American Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent technical malathion was fed as 25 per cent wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10-30 per cent inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95 per cent inhibition of erythrocyte
cholinesterase activity was observed. The 5000 ppm group showed total
inhibition of erythrocyte cholinesterase activity and 60-95 per cent
inhibition of cholinesterase activity in plasma and brain (American
Cyanamid Co., 1955, Hazleton & Holland, 1953).
Ninety-nine per cent technical malathion was fed for 2 years to groups
of 3-4 rats and produced, at 1000 and 5000 ppm levels, inhibition of
erythrocyte cholinesterase activity of the same order as did the 90
per cent compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90 per cent
technical malathion (American Cyanamid Co, 1955, Hazleton & Holland,
1953).
A two-year rat feeding experiment with combinations of six pesticides
(DDT, aldrin, pyrethrin, piperonyl butoxide, 2,4-D and malathion) and
eight flavouring agents (allyl heptylate, anethole, amyl butyrate,
cinnamic aldehyde, citral, ethyl methyl phenyl glycidate, eugenol, and
methyl salicylate) did not show significantly different toxic effects
compared with the effects of the compounds administered separately
(Fitzhugh, 1966).
Comments
The studies are extensive and have been carried out in several species
including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
It would be desirable to carry out reproduction studies in at least
two species, and biochemical studies, particularly with regard to the
influence of other chemicals on the metabolism of malathion.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg/day.
Man. 16 mg a day, equivalent to 0.2 mg/kg/day.
Estimate of acceptable daily intake for man
0.002 mg/kg/body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Malathion is used in many countries against aphids, scales and other
insects on a wide range of fruits and vegetables in agriculture and
horticulture. Crops treated include stone fruits (e.g. plums), pome
fruits (e.g. apples and pears) and soft fruits; carrots, turnips,
tomatoes and leafy vegetables.
Malathion is also used fairly widely in the veterinary field on
poultry (lice, mites, fleas), cattle and pigs (lice and flies).
(b) Post-harvest treatments
Malathion is used on a fairly large range of products during storage.
In some instances, it is applied directly to the raw agricultural
product (e.g. cereals, oilseeds, nuts, beans); in others, its use on
foodstuff (e.g. as a general warehouse spray) is incidental to the
hygiene of storage.
(c) Other uses
Malathion is a common ingredient of pesticides used against various
public health and domestic insect pests (flies, mosquitos, roaches,
etc.). It is also used quite extensively as a home garden insecticide,
when it may be sprayed on to both food crops and ornamental plants.
Tolerances
Product Country Tolerance ppm
General Austria 7
Tolerances (cont'd)
Product Country Tolerance ppm
Cereals Brazil 8
Canada
France
Italy
USA
UK
Cereals Germany 3
India
Cereals Kenya 12.5
Flour France 2
Fruits, green Comeco 5
vegetables (Bulgaria,
Roumania, East
Germany, Poland
Czechoslovakia,
USSR
Apples, pears USA 8
peaches, plums, Canada
tomatoes
Leafy vegetables USA 8
Canada
Meat (beef, pork, USA 4
poultry, etc.) Canada
Residues resulting from supervised trials
(a) Pre-harvest treatments
Many data are available on a variety of food crops from different
rates of application, modes of application, and times between
application and harvest. As examples Waites & Van Middelem (1958)
sprayed turnip tops and collards at various rates and found a maximum
of 3.9 ppm three days after the application. Tew & Sillibourne (1960)
measuring residues in apples and soft fruits, found a half life period
of only 1-1/2 to two days. Under their conditions useful applications
of the pesticide were unlikely to result in residues above 0.5 ppm at
harvest. Eheart (1962) investigated the persistence of various
pesticides on vegetable crops and judged that collards could be
consumed three days after spraying. From these and other data,
including some supplied by manufacturers of malathion, it is possible
to summarize the amounts expected to remain after useful applications
of the insecticide, as follows:
Type of food Pre-harvest Residue
period (ppm)
(days)
Vegetables
Cabbage 2 2 to 30
7 <0.5
Potatoes No residues detected
Lettuce 0 21
7 5
Kale 2 3
7 <0.5
Beans 0 1 to 50
3 0.5
Beet 1 15
4 9
Fruit
Berries (cane) 1 1
3 0.1
Apples and pears 0 5
3 1.5
7 0.5
Cherries and plums 0 Up to 10
7 1.5
Grapes 1 2
7 0.5
Peaches 0 Up to 18
7 3
Tomatoes 0 Up to 6
3 to 4 0.5
(continued)
Type of food Pre-harvest Residue
period (ppm)
(days)
Citrus 1 3.5
7 1.5
21 0.5
Cereals (pre-harvest) 3 Up to 4
7 <0.5
The fairly high levels are from relatively short pre-harvest intervals
and longer intervals result in lower residues. Data are not extensive
for residues from pre-harvest use on cereals. Nevertheless, various
workers have shown that malathion disappears rapidly on plants. For
example, Tomizawa et al. (1960), using labelled malathion, found less
than five per cent remaining on rice plants two days after
application: Koivistoinen (1961) found half life periods of two days
or less for residues on a fairly wide range of plants. Tomizawa & Sato
(1962) examined the mechanisms by which the insecticide disappeared
from rice during growth of the plant. From this work it is evidence
that applications during the growing period and up to seven days from
harvesting of cereals should leave residues which are much lower than
those which are added in many countries during post-harvest
treatments. Pre-harvest treatments therefore are a minor source of
residues in cereals in commerce.
Investigations of the residues resulting from uses in veterinary
practice have been fairly reassuring. Goulding & Terriere (1959) could
barely detect residues in milk and found none in flesh of cattle
treated for the control of horn fly. Claborn et al. (1960) compared
the effects from this and other insecticides used as sprays on
livestock: only traces of malathion could be detected in milk.
Pasarela et al. (1962) also did not detect malathion in various
tissues of cattle receiving food containing 200 ppm for 41 to 44 days,
although traces were found in the livers of two calves sacrificed
after 14 days. It was also not possible to detect residues in the milk
from cows, each receiving a daily 12 lb ration of dairy chow
containing up to 800 ppm. Adkins & Hair (1965) also were not able to
detect residues after the application of malathion to cattle via back
rubbers.
(b) Post-harvest treatments
As outlined in the second report of the FAO Working Party on Pesticide
Residues (PL/1965/12), malathion is widely used for controlling
insects in stored cereals and residues up to about 8 ppm result from
this usage.
Residues in food moving in commerce
(a) Cereal grains
Samples taken within countries where treatments have been carried out
(e.g. Australia, Britain, USA) show up to 8 ppm, which is the
recommended dosage for effective treatment. This is an average figure
and some spread has been observed due to uneven admixture in some
cases. Lower figures have been found after periods of storage and at
the termination of sea voyages. For example, examinations in the
United Kingdom of 70 samples representing nine shipments from
Australia known to have been treated at between 8 and 10 ppm showed a
mean of 5.1 in the ships before discharging.
Malathion is not very stable, is relatively volatile and soluble in
water. Therefore, it would not be expected to remain long in any
product where it is exposed to air and moisture. On the other hand it
is soluble and would be expected to remain much longer in oily
products. For example, it dissolves in the oil glands of citrus peel
where it may remain for a long period.
Fate of residue during storage and processing
(a) In plants and animals
The rapid losses which occur on the plant before harvesting appear to
be due to a number of factors (Koivistoinen, 1961) including
evaporation, chemical decomposition of surface deposits and metabolism
within the plant. In cereals, metabolism appears to lead to the
formation of thiophosphoric acid and to the mono- and dicarboxylic
acids of malathion.
The fate and toxicity of malathion in the animal body was reviewed in
the report of the first joint meeting of FAO Committee on Pesticides
and the WHO Committee on Pesticide Residues (FAO/WHO 1964) and in the
second report of the FAO Working Party on Pesticide Residues
(PL/1965/12). No significant residues have been found in the milk or
other tissues of animals receiving the pesticide.
Koivistoinen (1961) has shown that an enzyme system in plants rapidly
hydrolyzes malathion similar to that reported by Cook, Blake &
Williams (1957) in liver tissues.
(b) In storage and processing
The losses occurring during the storage of cereals appear to be almost
entirely due to hydrolysis to relatively inert derivatives (Rowlands,
1964, 1965). During the preparation of flour from treated wheat much
of the residue is removed and the residues in the order of one tenth
of those in the whole wheat have been found (Schesser, Priddle &
Ferrell, 1958; Allessandrini, 1965; Acton & Parouchais, 1966). The
main losses apparently occur during the cleaning process. The figure
for certain of the by-products, such as bran, may be greater than
those in the original wheat; but this does not seem very important
bearing in mind that residues of malathion have not been found in the
milk or fat of animals to which the insecticide has been fed.
Malathion is not stable to heating in the presence of moisture,
particularly under neutral or alkaline conditions. It does not readily
stand up to cooking. Alessandrini (1965) found that bread prepared
from flour containing known amounts of malathion had residues of from
eight to 16 per cent of that originally present. She concluded that
the residues would be negligible in bread from flour prepared from
wheat treated at commercial rates. In cooked pasta the residues were
not detectable.
Koivistoinen et al. (1964) investigated the stability of residues
during storage and processing of various fresh fruits and vegetable.
Residues on the surfaces decreased almost as rapidly as on the growing
plants (i.e. 50 per cent loss in from one to two days). Residues were
much more stable on or in deep frozen foods but during the preparation
of juice for storage or of jam from 54 to 86 per cent of the residues
present were destroyed; 1.1 ppm was the highest residue present in any
of this jam.
Methods of residue analysis
A number of methods are available for the determination of malathion
in foodstuffs. For cereals, the method suggested in the Second Report
of the FAO Working Party (PL/1965/12) is suitable. Even though that
method is adequate for the determination of the levels of malathion in
the commodities included in this monograph, there seems good promise
of more sensitive methods for malathion based on gas liquid
chromatography. However, the extraction and clean-up procedures
adequate for gas chromatography of malathion have not been
sufficiently developed for the working party to make a recommendation
at this time. For those interested in using GLC with a thermionic
detector, a paper by Storherr et al. (1964) will be useful. The
methods are sensitive to 0.1 ppm malathion in most foods.
RECOMMENDATIONS FOR TOLERANCES
The range of foodstuffs that could conceivably contain residues of
malathion is very wide. It could include cereal products, various
fruits and vegetables and dairy or other animal products. However,
there is no evidence of any residues being found in meat or dairy
products.
The recommendation for tolerances is as follows:
ppm
Fruit 8.0
Dried fruit 8.0
Nuts 8.0
Citrus 4.0
Cereals and
cereal
products 8.0
Vegetables 6.0
(leafy)
Vegetables 3.0
(other than
leafy)
These values are those resulting from good pest control practice. They
are predicated on a large loss in storage in processing. As an
example, during the storing, transporting and milling of treated
cereals, considerable reductions occur from the initial dosage levels
of about 8 ppm. Cooking results in further substantial losses. The
amounts found in bread or finished pasta made from wheat treated in
this way (i.e. below 0.4 ppm). As these amounts are well within the
permissible level, the residue resulting from good agricultural
practice, viz. 8 ppm, may be accepted as a tolerance for raw cereals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
American Cyanamid Company, New York (1955) Unpublished report
American Cyanamid Company, New York (1960) Unpublished report
Benes, V. & Cerná, V. (1965) Czech. Hyg., 10, 209
Benes, V. & Cerná, V. (1966) VII International Congress of Nutrition,
Hamburg, 1966 (Proceedings - In press)
Benes, V., Pekárek, J. & Cerná, V. (1963) Czech. Hyg., 8, 3
Fitzhugh, O. G. (1966) Canad. med. Ass. J., 94, 598
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol. exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. (1953) Arch. industr. Hyg., 8, 399
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3, 591
March, R. B., Fukuto, T. R., Metcalf, R. L. & Maxon, M. G. (1956) J.
Econ. Ent., 49, 185
Moeller, H. C. & Rider, J. A. (1962) Toxicol. appl. Pharmacol., 4,
123
Murphy, S. D. (1966) Toxicol. appl. Pharmacol., 8, 348
O'Brien, R. D. (1957) J. econ. Ent., 50, 3.59
O'Brien, R. D., Dauterman, W. C., & Niedermeier, R. P. (1961) J.
Agric. Food Chem., 9 (1), 39
Rider, J. A., Moeller, H. C., Swader, J. & Devereaux, G. (1959)
Clinical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1963) Biochem. Pharmacol., 12, 1421
REFERENCES PERTINENT TO AGRICULTURAL DATA
Allessandrini, M. E. (1965) Determination of the persistence and fate
of various insecticides in or on wheat during storage, milling and
during the baking or cooking of the products made from the treated
wheat. [Unpublished work conducted under U.S.D.A., Project No.
E15-AMS-8(a)]
Acton, F. E. & Parouchais, C. (1966) Malathion levels in wheat and
wheat products. Fd. Technol. Aust., 18, 77, 79, 81
Adkins, T. R. & Hair, J. A. (1965) Absence of residues in milk after
dimethoate and malathion were applied with back rubbers to dairy
cattle. J. econ. Ent., 58; (1): 155
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff.
(1960) Meat and milk residues from livestock sprays. J. ag. fd. Chem.,
8: (6). 439-441
Cook, J. W., Blake, J. R. & Williams, M. W. (1957) The enzymatic
hydrolysis of malathion and its inhibition by EPN and other organic
phorphorus compounds. J. Ass. Offic. Agr. Chem., 40: 664-665
Eheart, J. F., Massey, D. H. jr & Dickinson, J. (1962) Persistency of
malathion, parathion, Perthane and Sevin on selected vegetables crops.
Tech. Bull. Va. Agric. Exp. Stat., 157: 8
Goulding, R. L. & Terriere, L. C. (1959) Malathion residues in milk of
dairy cows treated for horn fly control. J. econ. Ent., 52: (2): 341
Koivistoinen, P., Karinpaa, M, K. & Roine, P. (1964) Malathion
residues on fruit treated by dipping. J. ag. food Chem., 12: (6):
551-555
Koivistoinen, P. (1961) Studies on the disappearance of malathion from
plant materials. Ann. Acad. Sci. Fenn. Ser. A. Biol., 14/15: 1-91
Koivistoinen, P., Karinpaa, M. K. & Roine, P. (1964) Stability of
residues in food processing and storage. J. ag. food Chem., 12: (6):
557-560
McFarlane, J. A. & Harris, A. H. (1964) Relative susceptibility to
malathion contamination of six animal feed ingredients. J. Sci. Food
Agric., 15: (9): 612-619
Pasarela, W. R., Brown, R. G. & Shaffer, C. B. (1962) Feeding of
malathion to cattle: Residue analyses of milk and tissue. J. ag. food
Chem., 10 (1): 7-9
Rowlands, D. G. (1964) The degradation of malathion on stored maize
and wheat grains. J. Sci. Food Agric., 15: 114-119
Rowlands, D. G. (1965) The in vitro and in vivo oxidation and
hydrolysis of malathion by wheat grain esterases. J. Sci. Food Agric.,
16: 325-30
Schesser, J. H., Priddle, W. E. & Ferrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion and lindane. J. Econ. Entomol., 51: 516-8
Storherr, R. W., Watts, R. R., Friedman, S. J., Erwin, F. Guiffrida &
Ives, F. (1964) Identification and analyses of five organophosphate
pesticides. Recoveries from crops fortified at different levels. J.
Assoc. Offic. Agric. Chem., 47: 1089-1093
Tew, R. P. & Sillibourne, J. M. (1960) Harvest residues of malathion
on soft fruits and apples. East Malling Res. Stn., Ann. Report, 1960:
48: 116-119
Tomizawa, C. & Sato, T. (1962) Metabolic fate of malathion and methyl
parathion in the Japanese rice plant. Jap. J. App.; Entomol. Zool.,
6: 70-5
Tomizawa, C. & Sato, T. & Kubo, H. (1960) The fate of
organo-phosphorus insecticides sprayed on rice plants. Botyu-Kagaku
[Scientific insect control] 25: 99-105
Waites, R. E. & Van Middelem, C. H. (1958) Residue status of DDT and
malathion on turnip tops, collards and lettuce. J. econ. Ent., 51:
306-308
BIOLOGICAL DATA AND TOXICOLOGICAL EVALUATION
Biochemical aspects
Malathion is rapidly absorbed from the intestinal tract. Its
metabolism has been studied in the hen, mouse, rat, cow and man.
Malathion is oxidized to malaoxon, the active form of the compound,
and is also hydrolyzed to less toxic metabolites. Six to eight
metabolites have been found, the main ones being in the urine,
malathion mono- and di-acids. Malathion or its metabolites were
recovered from eggs from treated hens and milk from cows treated with
malathion (March at al., 1956; O'Brien at al., 1961).
Malathion labelled with 32P was given to a lactating cow at 1.3
mg/kg bodyweight per day for three days. Malathion metabolites were
rapidly excreted in the urine, 69 per cent of the total radioactivity
being excreted in four days after the first dose, after which the
excretion rate decreased. After seven days 77.2 per cent of the dose
was recovered, 69 per cent of which was in the urine, 8 per cent in
the faeces, and 0.2 per cent in the milk. The principal metabolite in
early urine samples was the mono-acid of malathion. In later samples
it was the di-acid. Demethyl malathion was a significant component in
early and late samples. Dimethyl phosphate and
0,0-dimethylphosphorothioate were present in very small amounts. In
the faeces, 85 per cent, of the labelled material was malathion and 12
per cent was malaoxon (O'Brien at al., 1961).
In a lactating cow fed 1.3 mg/kg body-weight daily for three days
there was no significant inhibition of erythrocyte cholinesterase
activity (O'Brien et al., 1961).
Malaoxon is a cholinesterase inhibitor in vivo and in vitro (I50
7 × 10-7) (O'Brien, 1957).
The half time for the conversion in vivo of the reversibly inhibited
form of the dimethylphosphorylated cholinesterase to the irreversibly
inhibited form of this enzyme in the brain of chicken given malathion
has been found to be 2 hours. The same half time was observed in
vitro with the brain homogenate inhibited with paraoxon (Witter &
Gaines, 1963).
After single intraperitoneal or oral doses of malathion, trichlorofon
or dioxathion in rats, an increase in the activities of liver tyrosine
transaminase and alkaline phosphatase, as well as a decrease in the
level of adrenal ascorbic acid were found. Further results of this
experiment support the hypothesis that acute poisoning may produce
metabolic alterations which are mediated through the pituitary-adrenal
system (Murphy, 1966).
Simultaneous administration of malathion and ethyl p-nitrophenyl
thionobenzenephosphate (EPN) results in a potentiation of the
cholinesterase inhibitory effect of malathion in the mouse, rat and
dog (Frawley et al., 1957).
Acute toxicity
Animal Route LD50 References
mg/kg body-weight
90% 99%
technical technical
Rat, male Oral 940-1156* 4700-5843* American Cyanamid Co., 1955
Hazleton & Holland, 1953
Rat, male Oral 390-480* 1400-1845* American Cyanamid Co., 1955
Frawley et al., 1957
Hazleton & Holland, 1953
Mouse, male Oral 720-886 3300-4060 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male Oral 2700-3320 American Cyanamid Co., 1955
Hazleton & Holland, 1953
Mouse, male i.p. 420-474 Hazleton & Holland, 1953
Chicken Oral >850(95%) American Cyanamid Co., 1955
Calf Oral 80 (95%) American Cyanamid Co., 1955
Cow Oral 560(95%) American Cyanamid Co., 1955
* Differences due to use of different vehicles.
In a colony of rats showing an oral LD50 of 925 mg/kg for adults,
the intragastric LD50 for newborn rats was approximately 124 mg/kg
(Lu et al., 1965).
Simultaneous oral administration of malathion and fenitrothion to male
rats resulted in potentiation when one-half the LD50 doses were
given. However, no potentiation was seen when one-tenth the LD50
doses were given (Benes & Cerná, 1966).
Short-term studies
Mouse. When malathion was added to the diet as 500 or 5000 ppm for 6
weeks or after the administration, of 5 oral doses of 500 mg/kg the
production of antibodies against B. pertussis was not affected
(Benes et al., 1963).
Rat. Groups of 10 males were given malathion at 100 or 500 ppm in
the diet or trichlorofon at 60 or 300 ppm for 6 weeks and this was
followed by the administration of both compounds at the same time.
During the experiment erythrocyte cholinesterase fluctuated around 100
per cent of the initial values. At the end of the experiment, in
comparison with the control group, the adrenals weighed more and
showed hypertrophy of both cortex and medulla, the intensity of which
was related to the concentration of the two substances in the diet
(Benes & Cerná, 1965).
In another experiment 95 per cent technical malathion was fed to 3
groups of male rats, 10 animals per group, for 33 days at the levels
of 100, 1000 and 5000 ppm. No sign of toxicity was observed, nor any
deaths. Food intake and weight gain in the groups fed 100 and 1000 ppm
were higher than in the control group; groups fed 5000 ppm showed no
difference from the controls. Cholinesterase activity was determined
in 6 animals from each group. Activity was normal in the 100 ppm
group. Erythrocyte cholinesterase activity was 68 per cent of normal
in the 1000 ppm group, and in the 5000 ppm group plasma cholinesterase
activity was 78 per cent and erythrocyte activity 22 per cent of
normal. At all levels no depression of brain cholinesterase activity
was found (American Cyanamid Co., 1955).
Ninety-eight per cent technical malathion was fed to groups of 5 rats
for 8 weeks at levels of 100 and 500 ppm without any inhibition of
whole-blood cholinesterase activity (Frawley et al., 1957).
Ninety-five per cent technical malathion was fed to 40 male and 40
female rats for 5 months in a daily dose of 240 mg/kg body-weight
(4000 ppm in the diet). Growth was normal and no signs of intoxication
occurred. Ten weeks after the beginning of the experiment, 18 females
and 12 males were used for breeding. The average litter size from the
treated females was smaller than in the controls and the number of
newborn alive after 7 and 21 days was about half the number in the
litters of the controls (Kalow & Marton, 1961).
Chick. Ninety-five per cent technical malathion was fed to day-old
chicks for 2 weeks at a level of 10 ppm. For the following 10 weeks
they were divided into groups of 10 and fed 100, 1000 and 5000 ppm in
their diets. The groups on 100 and 1000 ppm behaved normally and
showed a similar growth rate and food consumption to the controls.
Four animals died in the 5000 ppm group, and signs of intoxication and
growth retardation were observed. At necropsy, no pathological lesions
were found. Plasma and brain cholinesterase activities were
significantly lowered in the 5000 ppm group (American Cyanamid Co.,
1955).
In a two-year study, 21 females were fed 250 ppm and 21 females and 6
males 2500 ppm. The 250 ppm group did not differ significantly from
the controls. At the 2500 ppm level a decrease in plasma
cholinesterase activity was found between the 195th and 465th day of
experiment. The test hens came into production later and laid slightly
fewer eggs, but the hatchability was not influenced. The offspring
showed no deformities. At necropsy no gross or microscopical lesions
were found (American Cyanamid Co., 1960).
Man. Five male volunteers, 23-36 years old, took 6 mg of malathion
in gelatin capsules daily for 32 days. No effect on plasma or
erythrocyte cholinesterase activity could be detected. Five males took
16 mg daily for 47 days, also without any significant effect on
cholinesterase activity. A daily dose of 24 mg taken by 5 males for 56
days was followed by depression of the plasma cholinesterase activity
2 weeks, after the first administration. Maximum depression amounting
to about 25 per cent of the plasma cholinesterase activity occurred
approximately 3 weeks after the cessation of administration. No
clinically manifest side-effects were reported. Simultaneous intake of
16 mg of malathion and 5 mg of EPN per day caused a slight inhibition
of cholinesterase activity (Moeller & Rider, 1962).
No plasma or RBC cholinesterase depression was noted in 10 humans
ingesting 3 mg EPN or 8 mg malathion daily for 32 days, nor in 5
humans receiving 6 mg EPN for 88 days and 8 mg malathion for the last
44 days, nor in 5 humans ingesting 16 mg malathion for 86 days and 3
mg EPN for the last 41 days. However 10 humans ingesting 6 mg EPN and
16 mg malathion daily for 42 days showed a slight depression of both
the plasma and the RBC cholinesterase (Rider et al., 1959).
Long-term studies
Rat. Sixty-five per cent technical malathion as a 10 per cent or 25
per cent wettable powder was mixed in the diets of groups of 20 male
rats at the levels 100, 1000 and 5000 ppm, and fed for 2 years. The
mortality rate was not influenced, and at the 2 lower levels weight
gain and food intake were comparable to those of the controls. Five
thousand ppm reduced food intake and decreased weight gain.
Cholinesterase determinations showed no inhibition at the 100 ppm
level; with a diet containing 1000 ppm, 36 per cent inhibition of
cholinesterase activity was found in the plasma, 73 per cent in the
erythrocytes and 37 per cent in the brain, while at the 5000 ppm
level, the plasma samples showed 80 per cent, the erythrocytes 100 per
cent and the brain 77 per cent inhibition. Neither gross nor
microscopic examination revealed any pathological changes attributable
to malathion (American Cyanamid Co., 1955; Hazleton & Holland, 1953).
Ninety per cent technical malathion was fed as 25 per cent wettable
powder in the diet to 20 males at a concentration of 100 ppm, to 20
males and 10 females at 1000 ppm, and to 20 males at 5000 ppm for 2
years. Mortality rate, growth response and food intake were not
influenced by any of these diets, except that there was some growth
retardation when the concentration was 5000 ppm. Terminal
cholinesterase determinations revealed 10-30 per cent inhibition of
cholinesterase activity in the plasma, erythrocytes and brain at 100
ppm. At 1000 ppm, 60-95 per cent inhibition of erythrocyte
cholinesterase activity was observed. The 5000 ppm group showed total
inhibition of erythrocyte cholinesterase activity and 60-95 per cent
inhibition of cholinesterase activity in plasma and brain (American
Cyanamid Co., 1955, Hazleton & Holland, 1953).
Ninety-nine per cent technical malathion was fed for 2 years to groups
of 3-4 rats and produced, at 1000 and 5000 ppm levels, inhibition of
erythrocyte cholinesterase activity of the same order as did the 90
per cent compound. The decrease in plasma and brain cholinesterase
activity, however, was much less than that produced by 90 per cent
technical malathion (American Cyanamid Co, 1955, Hazleton & Holland,
1953).
A two-year rat feeding experiment with combinations of six pesticides
(DDT, aldrin, pyrethrin, piperonyl butoxide, 2,4-D and malathion) and
eight flavouring agents (allyl heptylate, anethole, amyl butyrate,
cinnamic aldehyde, citral, ethyl methyl phenyl glycidate, eugenol, and
methyl salicylate) did not show significantly different toxic effects
compared with the effects of the compounds administered separately
(Fitzhugh, 1966).
Comments
The studies are extensive and have been carried out in several species
including man.
In view of the very high doses used in the short-term breeding
experiments in the rat, the results of these experiments were not
taken into account in arriving at the maximum acceptable daily intake
for man.
It would be desirable to carry out reproduction studies in at least
two species, and biochemical studies, particularly with regard to the
influence of other chemicals on the metabolism of malathion.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat. 100 ppm in the diet, equivalent to 5 mg/kg/day.
Man. 16 mg a day, equivalent to 0.2 mg/kg/day.
Estimate of acceptable daily intake for man
0.002 mg/kg/body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
Use pattern
(a) Pre-harvest treatments
Malathion is used in many countries against aphids, scales and other
insects on a wide range of fruits and vegetables in agriculture and
horticulture. Crops treated include stone fruits (e.g. plums), pome
fruits (e.g. apples and pears) and soft fruits; carrots, turnips,
tomatoes and leafy vegetables.
Malathion is also used fairly widely in the veterinary field on
poultry (lice, mites, fleas), cattle and pigs (lice and flies).
(b) Post-harvest treatments
Malathion is used on a fairly large range of products during storage.
In some instances, it is applied directly to the raw agricultural
product (e.g. cereals, oilseeds, nuts, beans); in others, its use on
foodstuff (e.g. as a general warehouse spray) is incidental to the
hygiene of storage.
(c) Other uses
Malathion is a common ingredient of pesticides used against various
public health and domestic insect pests (flies, mosquitos, roaches,
etc.). It is also used quite extensively as a home garden insecticide,
when it may be sprayed on to both food crops and ornamental plants.
Tolerances
Product Country Tolerance ppm
General Austria 7
Tolerances (cont'd)
Product Country Tolerance ppm
Cereals Brazil 8
Canada
France
Italy
USA
UK
Cereals Germany 3
India
Cereals Kenya 12.5
Flour France 2
Fruits, green Comeco 5
vegetables (Bulgaria,
Roumania, East
Germany, Poland
Czechoslovakia,
USSR
Apples, pears USA 8
peaches, plums, Canada
tomatoes
Leafy vegetables USA 8
Canada
Meat (beef, pork, USA 4
poultry, etc.) Canada
Residues resulting from supervised trials
(a) Pre-harvest treatments
Many data are available on a variety of food crops from different
rates of application, modes of application, and times between
application and harvest. As examples Waites & Van Middelem (1958)
sprayed turnip tops and collards at various rates and found a maximum
of 3.9 ppm three days after the application. Tew & Sillibourne (1960)
measuring residues in apples and soft fruits, found a half life period
of only 1-1/2 to two days. Under their conditions useful applications
of the pesticide were unlikely to result in residues above 0.5 ppm at
harvest. Eheart (1962) investigated the persistence of various
pesticides on vegetable crops and judged that collards could be
consumed three days after spraying. From these and other data,
including some supplied by manufacturers of malathion, it is possible
to summarize the amounts expected to remain after useful applications
of the insecticide, as follows:
Type of food Pre-harvest Residue
period (ppm)
(days)
Vegetables
Cabbage 2 2 to 30
7 <0.5
Potatoes No residues detected
Lettuce 0 21
7 5
Kale 2 3
7 <0.5
Beans 0 1 to 50
3 0.5
Beet 1 15
4 9
Fruit
Berries (cane) 1 1
3 0.1
Apples and pears 0 5
3 1.5
7 0.5
Cherries and plums 0 Up to 10
7 1.5
Grapes 1 2
7 0.5
Peaches 0 Up to 18
7 3
Tomatoes 0 Up to 6
3 to 4 0.5
(continued)
Type of food Pre-harvest Residue
period (ppm)
(days)
Citrus 1 3.5
7 1.5
21 0.5
Cereals (pre-harvest) 3 Up to 4
7 <0.5
The fairly high levels are from relatively short pre-harvest intervals
and longer intervals result in lower residues. Data are not extensive
for residues from pre-harvest use on cereals. Nevertheless, various
workers have shown that malathion disappears rapidly on plants. For
example, Tomizawa et al. (1960), using labelled malathion, found less
than five per cent remaining on rice plants two days after
application: Koivistoinen (1961) found half life periods of two days
or less for residues on a fairly wide range of plants. Tomizawa & Sato
(1962) examined the mechanisms by which the insecticide disappeared
from rice during growth of the plant. From this work it is evidence
that applications during the growing period and up to seven days from
harvesting of cereals should leave residues which are much lower than
those which are added in many countries during post-harvest
treatments. Pre-harvest treatments therefore are a minor source of
residues in cereals in commerce.
Investigations of the residues resulting from uses in veterinary
practice have been fairly reassuring. Goulding & Terriere (1959) could
barely detect residues in milk and found none in flesh of cattle
treated for the control of horn fly. Claborn et al. (1960) compared
the effects from this and other insecticides used as sprays on
livestock: only traces of malathion could be detected in milk.
Pasarela et al. (1962) also did not detect malathion in various
tissues of cattle receiving food containing 200 ppm for 41 to 44 days,
although traces were found in the livers of two calves sacrificed
after 14 days. It was also not possible to detect residues in the milk
from cows, each receiving a daily 12 lb ration of dairy chow
containing up to 800 ppm. Adkins & Hair (1965) also were not able to
detect residues after the application of malathion to cattle via back
rubbers.
(b) Post-harvest treatments
As outlined in the second report of the FAO Working Party on Pesticide
Residues (PL/1965/12), malathion is widely used for controlling
insects in stored cereals and residues up to about 8 ppm result from
this usage.
Residues in food moving in commerce
(a) Cereal grains
Samples taken within countries where treatments have been carried out
(e.g. Australia, Britain, USA) show up to 8 ppm, which is the
recommended dosage for effective treatment. This is an average figure
and some spread has been observed due to uneven admixture in some
cases. Lower figures have been found after periods of storage and at
the termination of sea voyages. For example, examinations in the
United Kingdom of 70 samples representing nine shipments from
Australia known to have been treated at between 8 and 10 ppm showed a
mean of 5.1 in the ships before discharging.
Malathion is not very stable, is relatively volatile and soluble in
water. Therefore, it would not be expected to remain long in any
product where it is exposed to air and moisture. On the other hand it
is soluble and would be expected to remain much longer in oily
products. For example, it dissolves in the oil glands of citrus peel
where it may remain for a long period.
Fate of residue during storage and processing
(a) In plants and animals
The rapid losses which occur on the plant before harvesting appear to
be due to a number of factors (Koivistoinen, 1961) including
evaporation, chemical decomposition of surface deposits and metabolism
within the plant. In cereals, metabolism appears to lead to the
formation of thiophosphoric acid and to the mono- and dicarboxylic
acids of malathion.
The fate and toxicity of malathion in the animal body was reviewed in
the report of the first joint meeting of FAO Committee on Pesticides
and the WHO Committee on Pesticide Residues (FAO/WHO 1964) and in the
second report of the FAO Working Party on Pesticide Residues
(PL/1965/12). No significant residues have been found in the milk or
other tissues of animals receiving the pesticide.
Koivistoinen (1961) has shown that an enzyme system in plants rapidly
hydrolyzes malathion similar to that reported by Cook, Blake &
Williams (1957) in liver tissues.
(b) In storage and processing
The losses occurring during the storage of cereals appear to be almost
entirely due to hydrolysis to relatively inert derivatives (Rowlands,
1964, 1965). During the preparation of flour from treated wheat much
of the residue is removed and the residues in the order of one tenth
of those in the whole wheat have been found (Schesser, Priddle &
Ferrell, 1958; Allessandrini, 1965; Acton & Parouchais, 1966). The
main losses apparently occur during the cleaning process. The figure
for certain of the by-products, such as bran, may be greater than
those in the original wheat; but this does not seem very important
bearing in mind that residues of malathion have not been found in the
milk or fat of animals to which the insecticide has been fed.
Malathion is not stable to heating in the presence of moisture,
particularly under neutral or alkaline conditions. It does not readily
stand up to cooking. Alessandrini (1965) found that bread prepared
from flour containing known amounts of malathion had residues of from
eight to 16 per cent of that originally present. She concluded that
the residues would be negligible in bread from flour prepared from
wheat treated at commercial rates. In cooked pasta the residues were
not detectable.
Koivistoinen et al. (1964) investigated the stability of residues
during storage and processing of various fresh fruits and vegetable.
Residues on the surfaces decreased almost as rapidly as on the growing
plants (i.e. 50 per cent loss in from one to two days). Residues were
much more stable on or in deep frozen foods but during the preparation
of juice for storage or of jam from 54 to 86 per cent of the residues
present were destroyed; 1.1 ppm was the highest residue present in any
of this jam.
Methods of residue analysis
A number of methods are available for the determination of malathion
in foodstuffs. For cereals, the method suggested in the Second Report
of the FAO Working Party (PL/1965/12) is suitable. Even though that
method is adequate for the determination of the levels of malathion in
the commodities included in this monograph, there seems good promise
of more sensitive methods for malathion based on gas liquid
chromatography. However, the extraction and clean-up procedures
adequate for gas chromatography of malathion have not been
sufficiently developed for the working party to make a recommendation
at this time. For those interested in using GLC with a thermionic
detector, a paper by Storherr et al. (1964) will be useful. The
methods are sensitive to 0.1 ppm malathion in most foods.
RECOMMENDATIONS FOR TOLERANCES
The range of foodstuffs that could conceivably contain residues of
malathion is very wide. It could include cereal products, various
fruits and vegetables and dairy or other animal products. However,
there is no evidence of any residues being found in meat or dairy
products.
The recommendation for tolerances is as follows:
ppm
Fruit 8.0
Dried fruit 8.0
Nuts 8.0
Citrus 4.0
Cereals and
cereal
products 8.0
Vegetables 6.0
(leafy)
Vegetables 3.0
(other than
leafy)
These values are those resulting from good pest control practice. They
are predicated on a large loss in storage in processing. As an
example, during the storing, transporting and milling of treated
cereals, considerable reductions occur from the initial dosage levels
of about 8 ppm. Cooking results in further substantial losses. The
amounts found in bread or finished pasta made from wheat treated in
this way (i.e. below 0.4 ppm). As these amounts are well within the
permissible level, the residue resulting from good agricultural
practice, viz. 8 ppm, may be accepted as a tolerance for raw cereals.
REFERENCES PERTINENT TO BIOLOGICAL DATA
American Cyanamid Company, New York (1955) Unpublished report
American Cyanamid Company, New York (1960) Unpublished report
Benes, V. & Cerná, V. (1965) Czech. Hyg., 10, 209
Benes, V. & Cerná, V. (1966) VII International Congress of Nutrition,
Hamburg, 1966 (Proceedings - In press)
Benes, V., Pekárek, J. & Cerná, V. (1963) Czech. Hyg., 8, 3
Fitzhugh, O. G. (1966) Canad. med. Ass. J., 94, 598
Frawley, J. P., Fuyat, H. N., Hagan, E. C., Blake, J. R. & Fitzhugh,
O. G. (1957) J. Pharmacol. exp. Ther., 121, 96
Hazleton, L. W. & Holland, E. (1953) Arch. industr. Hyg., 8, 399
Kalow, W. & Marton, A. (1961) Nature, 192, 464
Lu, F. C., Jessup, D. C. & Lavallée, A. (1965) Food & Cosmetics
Toxicol., 3, 591
March, R. B., Fukuto, T. R., Metcalf, R. L. & Maxon, M. G. (1956) J.
Econ. Ent., 49, 185
Moeller, H. C. & Rider, J. A. (1962) Toxicol. appl. Pharmacol., 4,
123
Murphy, S. D. (1966) Toxicol. appl. Pharmacol., 8, 348
O'Brien, R. D. (1957) J. econ. Ent., 50, 3.59
O'Brien, R. D., Dauterman, W. C., & Niedermeier, R. P. (1961) J.
Agric. Food Chem., 9 (1), 39
Rider, J. A., Moeller, H. C., Swader, J. & Devereaux, G. (1959)
Clinical Res., 7, 81
Witter, R. F. & Gaines, T. B. (1963) Biochem. Pharmacol., 12, 1421
REFERENCES PERTINENT TO AGRICULTURAL DATA
Allessandrini, M. E. (1965) Determination of the persistence and fate
of various insecticides in or on wheat during storage, milling and
during the baking or cooking of the products made from the treated
wheat. [Unpublished work conducted under U.S.D.A., Project No.
E15-AMS-8(a)]
Acton, F. E. & Parouchais, C. (1966) Malathion levels in wheat and
wheat products. Fd. Technol. Aust., 18, 77, 79, 81
Adkins, T. R. & Hair, J. A. (1965) Absence of residues in milk after
dimethoate and malathion were applied with back rubbers to dairy
cattle. J. econ. Ent., 58; (1): 155
Claborn, H. V., Bushland, R. C., Mann, H. D., Ivey, M. C. & Radeleff.
(1960) Meat and milk residues from livestock sprays. J. ag. fd. Chem.,
8: (6). 439-441
Cook, J. W., Blake, J. R. & Williams, M. W. (1957) The enzymatic
hydrolysis of malathion and its inhibition by EPN and other organic
phorphorus compounds. J. Ass. Offic. Agr. Chem., 40: 664-665
Eheart, J. F., Massey, D. H. jr & Dickinson, J. (1962) Persistency of
malathion, parathion, Perthane and Sevin on selected vegetables crops.
Tech. Bull. Va. Agric. Exp. Stat., 157: 8
Goulding, R. L. & Terriere, L. C. (1959) Malathion residues in milk of
dairy cows treated for horn fly control. J. econ. Ent., 52: (2): 341
Koivistoinen, P., Karinpaa, M, K. & Roine, P. (1964) Malathion
residues on fruit treated by dipping. J. ag. food Chem., 12: (6):
551-555
Koivistoinen, P. (1961) Studies on the disappearance of malathion from
plant materials. Ann. Acad. Sci. Fenn. Ser. A. Biol., 14/15: 1-91
Koivistoinen, P., Karinpaa, M. K. & Roine, P. (1964) Stability of
residues in food processing and storage. J. ag. food Chem., 12: (6):
557-560
McFarlane, J. A. & Harris, A. H. (1964) Relative susceptibility to
malathion contamination of six animal feed ingredients. J. Sci. Food
Agric., 15: (9): 612-619
Pasarela, W. R., Brown, R. G. & Shaffer, C. B. (1962) Feeding of
malathion to cattle: Residue analyses of milk and tissue. J. ag. food
Chem., 10 (1): 7-9
Rowlands, D. G. (1964) The degradation of malathion on stored maize
and wheat grains. J. Sci. Food Agric., 15: 114-119
Rowlands, D. G. (1965) The in vitro and in vivo oxidation and
hydrolysis of malathion by wheat grain esterases. J. Sci. Food Agric.,
16: 325-30
Schesser, J. H., Priddle, W. E. & Ferrell, E. P. (1958) Insecticidal
residues in milling fractions from wheat treated with methoxychlor,
malathion and lindane. J. Econ. Entomol., 51: 516-8
Storherr, R. W., Watts, R. R., Friedman, S. J., Erwin, F. Guiffrida &
Ives, F. (1964) Identification and analyses of five organophosphate
pesticides. Recoveries from crops fortified at different levels. J.
Assoc. Offic. Agric. Chem., 47: 1089-1093
Tew, R. P. & Sillibourne, J. M. (1960) Harvest residues of malathion
on soft fruits and apples. East Malling Res. Stn., Ann. Report, 1960:
48: 116-119
Tomizawa, C. & Sato, T. (1962) Metabolic fate of malathion and methyl
parathion in the Japanese rice plant. Jap. J. App.; Entomol. Zool.,
6: 70-5
Tomizawa, C. & Sato, T. & Kubo, H. (1960) The fate of
organo-phosphorus insecticides sprayed on rice plants. Botyu-Kagaku
[Scientific insect control] 25: 99-105
Waites, R. E. & Van Middelem, C. H. (1958) Residue status of DDT and
malathion on turnip tops, collards and lettuce. J. econ. Ent., 51:
306-308
