
PESTICIDE RESIDUES IN FOOD - 1981
Sponsored jointly by FAO and WHO
EVALUATIONS 1981
Food and Agriculture Organization of the United Nations
Rome
FAO PLANT PRODUCTION AND PROTECTION PAPER 42
pesticide residues in food:
1981 evaluations
the monographs
data and recommendations
of the joint meeting
of the
FAO panel of experts on pesticide residues
in food and the environment
and the
WHO expert group on pesticide residues
Geneva, 23 November-2 December 1981
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
Rome 1982
DIFLUBENZURON
IDENTITY
Chemical name
1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)-urea (IUPAC)
N-[(4-chlorophenyl)aminocarbonyl]-2,6 difluorobenzamide
(Chem.abstr. Index)
Synonyms
DIMILIN (R), DU 112307, PH 60-40, TH 6040,
ENT-29054, OMS 1804
Structural formula
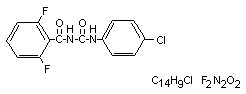 Other information on identity and properties (Keuker 1975)
Molecular weight 310.7
State white, crystalline solid
Melting point (pure compound) 230-232°C
Specific gravity 1.56
Volatility virtually non-volatile
Stability:
- Heat stability after 1 week storage at 50°C, or after
1 day at 100°, no detectable
decomposition.
- Stability in water after 3 weeks at pH 5-4% decomposition;
(0.1 mg/l solution) after 3 weeks at pH 7-8% decomposition;
and after 3 weeks at pH 9-26%
decomposition.
Solubility (g/l at 20°C)
- N-methylpyrolidone 200
- DMSO 120
- DMF 120
- Dioxane 24
- Acetone 6.5
- Acetonitrile 2
- Methanol 0.9
- Dichloromethane 0.6
- Water 0.0002
Partition coefficients
- Dichloromethane/water > 50
- n-Octanol/
water-approximately 5000
Purity of technical product
Diflubenzuron technical contains > 95% pure compound.
Formulations
The main formulation of diflubenzuron is DIMILIN WP-25, a
wettable powder containing 25% of the active ingredient. This is the
formulation that is recommended for use on food crops. The particle
size of the diflubenzuron is defined as 80% smaller than 5 µm. This
formulation has an excellent storage stability; storage for 2 years at
room temperature or 1 year at 54°C did not affect its properties (Popp
1977).
DIMILIN ODC-45 is an oil-dispersible concentrate, containing
450 g diflubenzuron per litre. After dilution with a suitable organic
solvent, this formulation can be applied at ULV rates.
DIMILIN granular formulations are available for the control of
mosquitoes and flies.
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
The intestinal absorption of diflubenzuron in mammals decreases
with increasing dose level. Radio-labelled diflubenzuron, with the
14C label uniformly distributed in the anilino moiety, was
administered by gavage to rats in a single dose of 4, 16, 48, 128 or
1 000 mg/kg bw. Urine was collected in 24 h portions up to 120 h or
144 h. The cumulative excretion in urine as percentage of the dose
decreased from 28% at the 4 mg/kg dose level to only 1% at the
1 000 mg/kg dose level, while total recoveries remained constant
(90%). In rats with cannulated bile ducts, the sum of the excretion in
urine and bile decreased from 42% of the dose at the dose level of
4 mg/kg to about 4% at the dose level of 900 mg/kg (De Lange et al.
1977; Willems et al. 1980).
In mice given a single oral dose of 12.5, 63.5, 202.5 or
925 mg/kg bw, the excretion was almost completed within 48 h. The
cumulative percentage of the dose excreted in the urine decreased from
15% at the dose level of 12.5 mg/kg to approximately 2% at the
925 mg/kg dose level, showing that the relationship between urinary
excretion and dose level in mice is similar to that in rats (De Lange
and Post 1978).
Sheep treated with 500 mg/kg of 14C-diflubenzuron as a single
oral dose eliminated approximately 7% and 5% of the dose in urine and
bile, respectively, during a 4-day post-treatment period. Sheep
receiving a single oral dose of 10 mg/kg eliminated approximately 24%
and 36% of the dose in urine and bile during the same period.
After oral treatment of a lactating cow with radio-labelled
14C-diflubenzuron (10 mg/kg bw) the elimination of radioactivity was
85% in faeces and 16% in urine during a 4-day post-treatment period.
Only about 0.2% of the radio label was secreted into the milk. Maximum
milk residues of 0.8 mg/kg diflubenzuron equivalents were observed in
milk samples collected 24 h after dosing, but radioactivity in milk
had dropped to <0.1 mg/kg by 3 days (Ivie 1978). When applied
dermally, diflubenzuron was not degraded or absorbed through the skin
to any detectable degree in cattle (Ivie 1978), whereas in rabbits
only 0.2% of the dose could be recovered in urine (De Lange 1979).
A pig treated orally with 14C-diflubenzuron (5 mg/kg bw)
excreted 82% of the radioactivity in faeces and 5% in urine in 11 days
(Opdycke 1976).
Chickens receiving a single oral dose of 5 mg/kg of radiolabelled
14C-diflubenzuron excreted the radioactivity in a similar way (91% in
White Leghorn excreta and 82% for Buff Cross chickens after 13 days)
(Opdycke 1976).
Radio-labelled diflubenzuron (14C and 3H) was orally
administered to cats (7 mg/kg bw) on day 10 of a 15-day dosing regimen
of non-radioactive diflubenzuron. Within 72 h after administration, 9%
of the oral dose was excreted in the urine and 77% of the 14C and 71%
of the 3H doses in faeces (Hawkins et al 1980).
In rats, at 72 h after administration of a single oral dose of
5 mg/kg of double-labelled (14C in the anilino moiety and 3H in the
benzoyl moiety) diflubenzuron, 1.3% of the 14C and 3.5% of the 3H
label was retained in the carcasses (De Lange et al 1975). In
expired air of rats that had received radio-labelled diflubenzuron
(14C in the carbonyl group of the benzoyl moiety) 1% of radioactivity
was found (De Lange et al 1975).
Body tissues showed little tendency toward retention of
diflubenzuron. At 4 or 7 days post-treatment appreciable residues
could be detected only in the liver of cow and sheep (Ivie 1978). Low
residues were found also in tissues of pig and chicken. A small
portion of the radioactivity was secreted into eggs of chickens. The
maximum residue level was 0.248 mg/kg 3 days after a single oral dose
of 5 mg/kg bw 14C-diflubenzuron (Opdycke 1976).
The distribution of radioactivity resulting after oral
administration of radio-labelled diflubenzuron (14C in both phenyl
moieties) was studied in cows receiving daily doses in gelatin
capsules over a 28-day period. The daily dose levels equalled 0.05,
0.5, 5.0 and 250 mg/kg feed. No residues in milk were detected at the
two low dose levels. At the 5.0 mg/kg dose level, an average of
0.009 mg/kg of diflubenzuron equivalents was found in the milk: a
plateau was reached between day 4 and day 7 of exposure. After 4 days
of withdrawal, levels were undetectable (<0.0016 mg/kg). At the
250 mg/kg dose level, a plateau was reached in milk by day 2 at a
residue level of 0.20 mg/kg of diflubenzuron equivalents. Total 14C
tissue analyses showed that at dose levels of 0.05, 0.5 and 5 mg/kg
feed only the liver contained detectable, dose-related residues. For
the lowest dose level the radioactivity was just above the limit of
detection. This limit of detection increased with increasing dose
levels in relation to the specific activity of the dosed material.
Withdrawal for 7 days did not result in a substantial decrease in
activity in the liver. At the 250 mg/kg feed, only tested for 7 days
of treatment, residues were found in the kidney and in the liver
(Smith and Merricks 1976a).
In a similar experiment, laying hens were administered daily
dosages equalling 0.05, 0.5 and 5 mg/kg feed of 14C-diflubenzuron for
28 days. A plateau level was reached before day 10 of treatment in
fat, kidney, liver, muscle and eggs. However, the radioactivity in
tissues showed large day-to-day variations. There was a linear
relationship between dose level and plateau level for the kidney,
liver and fat, whereas an exponential relationship was obtained for
eggs. At day 7 of withdrawal, levels in all tissues and eggs were
below the limit of detection (Smith and Merricks 1976b).
Two dairy cows were fed diflubenzuron at rates of 0.25 mg/kg
bw/day or 1.0 mg/kg/day for four months. A third cow received rates
that were increased from 1 via 8 to 16 mg/kg/day, the highest rate
being maintained for three months. In the tissues examined of the
third cow, fat and liver showed residues of 0.2 mg/kg and 0.13 mg/kg
respectively. In the milk of this cow, a residue level of 0.02 mg/kg
could be established at a dose rate of 16 mg/kg (Miller et al
1976a).
The eggs of White Leghorn and Black Sexlinked Cross hens, kept on
a ration containing 10 mg/kg of diflubenzuron, contained plateau
residues of 0.5 to 0.6 or 0.3 to 0.4 mg/kg respectively, from day 9 of
a treatment period of 9 weeks (Miller et al. 1976b).
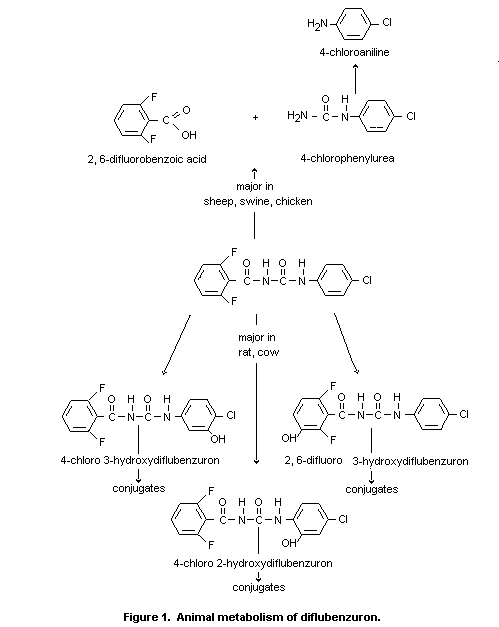
The metabolic fate of diflubenzuron has been studied in various
species. It appears that diflubenzuron is not degraded to any
significant degree within the digestive tract of mammals, as the radio
label eliminated in the faeces of bileduct-cannulated sheep receiving
an oral dose of 14C-diflubenzuron consisted only of the unmetabolized
compound (Ivie 1978). Furthermore, diflubenzuron did not degrade to
any extent when incubated in vitro with digestive tract fluids of
sheep and cattle (Ivie 1978). On the other hand, it was found that no
unchanged diflubenzuron could be detected in urine and bile of orally
dosed rats, sheep, or cattle (Willems et al 1980; Ivie 1978).
In rats and cows, the major metabolic pathway involved
hydroxylation of the phenyl moieties of the intact compound. About 80%
of the metabolites in rat urine were identified as 2,6-difluoro-
3-hydroxybenzuron, 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the ureido
bridge. The major part was excreted as 2,6-difluorobenzoic acid;
4-chlorophenylurea was not detected in bile or urine in a significant
quantity (Willems et al 1980; De Lange et al 1975).
The major metabolite in cow urine was 2,6-difluoro
3-hydroxydiflubenzuron (45%); in addition, relatively small quantities
of 4-chloro-2-hydroxy-(1.6%) and 4-chloro-3-hydroxy-difluorobenzuron
(3.7%) and the scission products 4-chlorophenylurea (0.6%),
2,6-difluorobenzoic acid (6.0%) and 2,6-difluorohippuric acid (6.9%)
were present (Ivie 1978). In contrast to these observations, the
latter two compounds are the major metabolites (approximately 50%) in
sheep urine (Ivie 1978).
In pigs, all of the 14C-diflubenzuron residues extracted from
faeces co-chromatographed with diflubenzuron. In urine, only small
quantities of the cleavage products 2,6-difluoro-benzoic acid,
4-chlorophenylurea and 4-chloroaniline were detected. These data
indicate little metabolism of the compound in swine (Opdycke 1976).
In chickens also, little degradation was observed while the
same metabolic pathway was found (Opdycke 1976). In a detailed
investigation, carried out to find an explanation for the occurrence
of methaemoglobinaemia in diflubenzuron-treated rats, 4-chloroaniline
and compounds reducible to 4-chloroaniline, probably consisting of
N-oxidation products of the aniline, were detected in red blood cells
(De Bree et al 1977).
Metabolism of diflubenzuron in animals is shown in Figure 1.
Effects on enzymes
The activity of the mammalian hexosamine transferases,
responsible for connective tissue glycosaminoglycan formation, was
monitored in adult female mice fed 50, 200, 400, 1 000 and 2 000 mg
diflubenzuron/kg. In these animals the rate of incorporation of
14C-glucose into hyaluronic acid and chondroitin sulphate of skin was
studied. No inhibition was noted. Sulphaemoglobin was demonstrated in
the blood of mice from 200 mg/kg onwards. Recovery was completed after
a 3-week withdrawal period (Bentley et al. 1979).
In another study with rat C-6 astrocytoma cell cultures, 100 nM
(31 ppm) diflubenzuron in the medium did not affect cell morphology or
the rate of cell division. Diflubenzuron did not inhibit the total
production of glycosaminoglycans (Stoolmiller 1978).
Hubbard broiler chickens were fed 0, 2.5 or 250 mg
diflubenzuron/kg feed during 98 days. There was no effect of
diflubenzuron on the incorporation of amino sugar moieties into
mucopolysaccharides of the skin of the animals (Deul and de Jong
1977). The incorporation of 0, 2.5, 25 and 250 mg diflubenzuron/kg in
the diet of male and female 28-day old chickens, for 98 days, did not
affect the hyaluronic acid concentration in the combs (Crookshank
et al. 1978).
Other information on identity and properties (Keuker 1975)
Molecular weight 310.7
State white, crystalline solid
Melting point (pure compound) 230-232°C
Specific gravity 1.56
Volatility virtually non-volatile
Stability:
- Heat stability after 1 week storage at 50°C, or after
1 day at 100°, no detectable
decomposition.
- Stability in water after 3 weeks at pH 5-4% decomposition;
(0.1 mg/l solution) after 3 weeks at pH 7-8% decomposition;
and after 3 weeks at pH 9-26%
decomposition.
Solubility (g/l at 20°C)
- N-methylpyrolidone 200
- DMSO 120
- DMF 120
- Dioxane 24
- Acetone 6.5
- Acetonitrile 2
- Methanol 0.9
- Dichloromethane 0.6
- Water 0.0002
Partition coefficients
- Dichloromethane/water > 50
- n-Octanol/
water-approximately 5000
Purity of technical product
Diflubenzuron technical contains > 95% pure compound.
Formulations
The main formulation of diflubenzuron is DIMILIN WP-25, a
wettable powder containing 25% of the active ingredient. This is the
formulation that is recommended for use on food crops. The particle
size of the diflubenzuron is defined as 80% smaller than 5 µm. This
formulation has an excellent storage stability; storage for 2 years at
room temperature or 1 year at 54°C did not affect its properties (Popp
1977).
DIMILIN ODC-45 is an oil-dispersible concentrate, containing
450 g diflubenzuron per litre. After dilution with a suitable organic
solvent, this formulation can be applied at ULV rates.
DIMILIN granular formulations are available for the control of
mosquitoes and flies.
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
The intestinal absorption of diflubenzuron in mammals decreases
with increasing dose level. Radio-labelled diflubenzuron, with the
14C label uniformly distributed in the anilino moiety, was
administered by gavage to rats in a single dose of 4, 16, 48, 128 or
1 000 mg/kg bw. Urine was collected in 24 h portions up to 120 h or
144 h. The cumulative excretion in urine as percentage of the dose
decreased from 28% at the 4 mg/kg dose level to only 1% at the
1 000 mg/kg dose level, while total recoveries remained constant
(90%). In rats with cannulated bile ducts, the sum of the excretion in
urine and bile decreased from 42% of the dose at the dose level of
4 mg/kg to about 4% at the dose level of 900 mg/kg (De Lange et al.
1977; Willems et al. 1980).
In mice given a single oral dose of 12.5, 63.5, 202.5 or
925 mg/kg bw, the excretion was almost completed within 48 h. The
cumulative percentage of the dose excreted in the urine decreased from
15% at the dose level of 12.5 mg/kg to approximately 2% at the
925 mg/kg dose level, showing that the relationship between urinary
excretion and dose level in mice is similar to that in rats (De Lange
and Post 1978).
Sheep treated with 500 mg/kg of 14C-diflubenzuron as a single
oral dose eliminated approximately 7% and 5% of the dose in urine and
bile, respectively, during a 4-day post-treatment period. Sheep
receiving a single oral dose of 10 mg/kg eliminated approximately 24%
and 36% of the dose in urine and bile during the same period.
After oral treatment of a lactating cow with radio-labelled
14C-diflubenzuron (10 mg/kg bw) the elimination of radioactivity was
85% in faeces and 16% in urine during a 4-day post-treatment period.
Only about 0.2% of the radio label was secreted into the milk. Maximum
milk residues of 0.8 mg/kg diflubenzuron equivalents were observed in
milk samples collected 24 h after dosing, but radioactivity in milk
had dropped to <0.1 mg/kg by 3 days (Ivie 1978). When applied
dermally, diflubenzuron was not degraded or absorbed through the skin
to any detectable degree in cattle (Ivie 1978), whereas in rabbits
only 0.2% of the dose could be recovered in urine (De Lange 1979).
A pig treated orally with 14C-diflubenzuron (5 mg/kg bw)
excreted 82% of the radioactivity in faeces and 5% in urine in 11 days
(Opdycke 1976).
Chickens receiving a single oral dose of 5 mg/kg of radiolabelled
14C-diflubenzuron excreted the radioactivity in a similar way (91% in
White Leghorn excreta and 82% for Buff Cross chickens after 13 days)
(Opdycke 1976).
Radio-labelled diflubenzuron (14C and 3H) was orally
administered to cats (7 mg/kg bw) on day 10 of a 15-day dosing regimen
of non-radioactive diflubenzuron. Within 72 h after administration, 9%
of the oral dose was excreted in the urine and 77% of the 14C and 71%
of the 3H doses in faeces (Hawkins et al 1980).
In rats, at 72 h after administration of a single oral dose of
5 mg/kg of double-labelled (14C in the anilino moiety and 3H in the
benzoyl moiety) diflubenzuron, 1.3% of the 14C and 3.5% of the 3H
label was retained in the carcasses (De Lange et al 1975). In
expired air of rats that had received radio-labelled diflubenzuron
(14C in the carbonyl group of the benzoyl moiety) 1% of radioactivity
was found (De Lange et al 1975).
Body tissues showed little tendency toward retention of
diflubenzuron. At 4 or 7 days post-treatment appreciable residues
could be detected only in the liver of cow and sheep (Ivie 1978). Low
residues were found also in tissues of pig and chicken. A small
portion of the radioactivity was secreted into eggs of chickens. The
maximum residue level was 0.248 mg/kg 3 days after a single oral dose
of 5 mg/kg bw 14C-diflubenzuron (Opdycke 1976).
The distribution of radioactivity resulting after oral
administration of radio-labelled diflubenzuron (14C in both phenyl
moieties) was studied in cows receiving daily doses in gelatin
capsules over a 28-day period. The daily dose levels equalled 0.05,
0.5, 5.0 and 250 mg/kg feed. No residues in milk were detected at the
two low dose levels. At the 5.0 mg/kg dose level, an average of
0.009 mg/kg of diflubenzuron equivalents was found in the milk: a
plateau was reached between day 4 and day 7 of exposure. After 4 days
of withdrawal, levels were undetectable (<0.0016 mg/kg). At the
250 mg/kg dose level, a plateau was reached in milk by day 2 at a
residue level of 0.20 mg/kg of diflubenzuron equivalents. Total 14C
tissue analyses showed that at dose levels of 0.05, 0.5 and 5 mg/kg
feed only the liver contained detectable, dose-related residues. For
the lowest dose level the radioactivity was just above the limit of
detection. This limit of detection increased with increasing dose
levels in relation to the specific activity of the dosed material.
Withdrawal for 7 days did not result in a substantial decrease in
activity in the liver. At the 250 mg/kg feed, only tested for 7 days
of treatment, residues were found in the kidney and in the liver
(Smith and Merricks 1976a).
In a similar experiment, laying hens were administered daily
dosages equalling 0.05, 0.5 and 5 mg/kg feed of 14C-diflubenzuron for
28 days. A plateau level was reached before day 10 of treatment in
fat, kidney, liver, muscle and eggs. However, the radioactivity in
tissues showed large day-to-day variations. There was a linear
relationship between dose level and plateau level for the kidney,
liver and fat, whereas an exponential relationship was obtained for
eggs. At day 7 of withdrawal, levels in all tissues and eggs were
below the limit of detection (Smith and Merricks 1976b).
Two dairy cows were fed diflubenzuron at rates of 0.25 mg/kg
bw/day or 1.0 mg/kg/day for four months. A third cow received rates
that were increased from 1 via 8 to 16 mg/kg/day, the highest rate
being maintained for three months. In the tissues examined of the
third cow, fat and liver showed residues of 0.2 mg/kg and 0.13 mg/kg
respectively. In the milk of this cow, a residue level of 0.02 mg/kg
could be established at a dose rate of 16 mg/kg (Miller et al
1976a).
The eggs of White Leghorn and Black Sexlinked Cross hens, kept on
a ration containing 10 mg/kg of diflubenzuron, contained plateau
residues of 0.5 to 0.6 or 0.3 to 0.4 mg/kg respectively, from day 9 of
a treatment period of 9 weeks (Miller et al. 1976b).
The metabolic fate of diflubenzuron has been studied in various
species. It appears that diflubenzuron is not degraded to any
significant degree within the digestive tract of mammals, as the radio
label eliminated in the faeces of bileduct-cannulated sheep receiving
an oral dose of 14C-diflubenzuron consisted only of the unmetabolized
compound (Ivie 1978). Furthermore, diflubenzuron did not degrade to
any extent when incubated in vitro with digestive tract fluids of
sheep and cattle (Ivie 1978). On the other hand, it was found that no
unchanged diflubenzuron could be detected in urine and bile of orally
dosed rats, sheep, or cattle (Willems et al 1980; Ivie 1978).
In rats and cows, the major metabolic pathway involved
hydroxylation of the phenyl moieties of the intact compound. About 80%
of the metabolites in rat urine were identified as 2,6-difluoro-
3-hydroxybenzuron, 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the ureido
bridge. The major part was excreted as 2,6-difluorobenzoic acid;
4-chlorophenylurea was not detected in bile or urine in a significant
quantity (Willems et al 1980; De Lange et al 1975).
The major metabolite in cow urine was 2,6-difluoro
3-hydroxydiflubenzuron (45%); in addition, relatively small quantities
of 4-chloro-2-hydroxy-(1.6%) and 4-chloro-3-hydroxy-difluorobenzuron
(3.7%) and the scission products 4-chlorophenylurea (0.6%),
2,6-difluorobenzoic acid (6.0%) and 2,6-difluorohippuric acid (6.9%)
were present (Ivie 1978). In contrast to these observations, the
latter two compounds are the major metabolites (approximately 50%) in
sheep urine (Ivie 1978).
In pigs, all of the 14C-diflubenzuron residues extracted from
faeces co-chromatographed with diflubenzuron. In urine, only small
quantities of the cleavage products 2,6-difluoro-benzoic acid,
4-chlorophenylurea and 4-chloroaniline were detected. These data
indicate little metabolism of the compound in swine (Opdycke 1976).
In chickens also, little degradation was observed while the
same metabolic pathway was found (Opdycke 1976). In a detailed
investigation, carried out to find an explanation for the occurrence
of methaemoglobinaemia in diflubenzuron-treated rats, 4-chloroaniline
and compounds reducible to 4-chloroaniline, probably consisting of
N-oxidation products of the aniline, were detected in red blood cells
(De Bree et al 1977).
Metabolism of diflubenzuron in animals is shown in Figure 1.
Effects on enzymes
The activity of the mammalian hexosamine transferases,
responsible for connective tissue glycosaminoglycan formation, was
monitored in adult female mice fed 50, 200, 400, 1 000 and 2 000 mg
diflubenzuron/kg. In these animals the rate of incorporation of
14C-glucose into hyaluronic acid and chondroitin sulphate of skin was
studied. No inhibition was noted. Sulphaemoglobin was demonstrated in
the blood of mice from 200 mg/kg onwards. Recovery was completed after
a 3-week withdrawal period (Bentley et al. 1979).
In another study with rat C-6 astrocytoma cell cultures, 100 nM
(31 ppm) diflubenzuron in the medium did not affect cell morphology or
the rate of cell division. Diflubenzuron did not inhibit the total
production of glycosaminoglycans (Stoolmiller 1978).
Hubbard broiler chickens were fed 0, 2.5 or 250 mg
diflubenzuron/kg feed during 98 days. There was no effect of
diflubenzuron on the incorporation of amino sugar moieties into
mucopolysaccharides of the skin of the animals (Deul and de Jong
1977). The incorporation of 0, 2.5, 25 and 250 mg diflubenzuron/kg in
the diet of male and female 28-day old chickens, for 98 days, did not
affect the hyaluronic acid concentration in the combs (Crookshank
et al. 1978).
 TOXICOLOGICAL STUDIES
Acute toxicity
In none of the acute studies on diflubenzuron was any overt sign
of toxicity observed. In the dermal toxicity study with rats, no
effect on the met- and sulph-haemoglobin values was detected. Results
of acute toxicity studies in rat, mouse and rabbit are summarized in
Table 1.
Loss of activity, catatony, paralysis and severe bradypnoea, were
observed in rats treated with the metabolite 4-chlorophenylurea. At
autopsy, the animals showed congested blood vessels and haemorrhagic
intestines. The rats dosed with 2,6-difluorobenzoic acid showed
symptoms indicative for slight C.N.S. excitation and increased muscle
tone. Results of studies with diflubenzuron metabolites are summarized
in Table 2.
Short-term studies
Mouse
Three groups of 8 male CFLP mice were fed dietary levels of
diflubenzuron for 6 weeks at levels of 0, 16 and 50 mg/kg feed. There
was no clear effect of the treatment on food consumption, body
weight, blood chemistry and macroscopic pathology. The weight of the
spleen was decreased in the highest dose group. In some animals
receiving 50 mg/kg feed, foci of liver cell necrosis with or without
inflammatory cell infiltration were noted. The other organs were not
microscopically examined (Hunter et al 1974).
Male and female mice (40/sex/group) received diflubenzuron in the
diet at a dosage level of 16, 50, 400, 2 000, 10 000 or 50 000 mg/kg
feed during 13 weeks. An additional group of one hundred/sex served as
a control. No compound-related effects were apparent in respect to
clinical signs, survival, growth, food consumption or gross pathology.
Significant treatment-related increases in met- and sulph-haemoglobin
concentrations were noted in all treated groups, except the 16 mg/kg
feed one. At the higher dose levels, a decrease was noted in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects were noted on the weights of liver and
spleen. In the females, adrenal weight was not dose-relatedly
decreased at all dose levels after 7 weeks and increased after 13
weeks at higher dose levels. In males a higher adrenal weight was
observed in comparison to controls. Histopathologically, treatment-
related centrolobular hypertrophy of hepatocytes, with or without cell
TABLE 1. Acute toxicity of diflubenzuron
Species Sex Route LD50 Reference
Rat F,M Oral > 4,640 mg/kg Van Eldik 1973
F,M Dermal >10,000 mg/kg Keet 1976a;Koopman 1977c
F,M Inhalation > 35 mg/kg (6 hr) Berczy et al 1973
Mouse F,M Oral > 4,640 mg/kg Van Eldik 1973;Koopman 1977a
F,M i.p. > 2,150 mg/kg Van Eldik 1974;Koopman 1977b
Rabbit F,M Dermal > 4,000 mg/kg
(50% paste) Davies and Halliday 1974
F,M Inhalation > 30 mg/l (6 hr) Berczy et al 1975
TABLE 2. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50(mg/kg) Reference
4-Chlorophenylurea Rat M Oral 1 080 Koelman-Klaus 1978a
F Oral 1 210 Koelman-Klaus 1978a
2,6-Difluorobenzoic Rat M,F Oral 4 640 Koelman-Klaus 1978b
acid
necrosis, haemosiderosis of the liver and spleen, extramedullary
haematopoiesis and mild chronic hepatitis were observed. The liver
lesions were more severe in males than in females. No no-effect level
was observed (Burdock et al. 1980a; Goodman 1980a).
A 14-week feeding study was performed with groups of 40 male and
40 female HC/CFLP mice (control groups, 96 male and 96 females)
maintained on a diet supplemented with 0, 80, 400, 2 000, 10 000 and
50 000 mg diflubenzuron (purity 97.20%/kg feed.) Half of the number of
mice of both sexes of all groups were sacrificed after 7 weeks. On the
second day of treatment, the majority of mice treated with 50 000 or
10 000 mg/kg feed showed dark eyes and/or prominent caudal blood
vessels. On day 5, blue/grey discolouration of the extremities was
noted for the majority of mice treated with 50 000 mg/kg. In the
course of the study, clinical signs were observed in mice of all
groups, except those of the lowest dose group. Mortality, food
consumption, water consumption and body weight changes were not
clearly affected by the treatment. Results of haematological
investigation showed lower packed cell volume and red blood cell
count at all dose levels except 80 mg/kg feed. The total white blood
cell count, lymphocyte count, haemoglobin concentration, the incidence
of Heinz bodies and the red blood cells were increased in all dose
groups. A treatment-related increase in both met- and sulph-
haemoglobin was recorded in all treated groups at weeks 7 and 14 of
the investigation. Clinical biochemistry revealed higher plasma
glutamic pyruvic transaminase at all dose levels, with the exception
of 80 mg/kg feed. Lower blood cholesterol levels were noted in the
50 000, 10 000 and 2 000 mg/kg feed groups. Macroscopic examination
showed dark discolouration and/or enlargement of the spleen and pale
subcapsular areas of the liver in all dose groups, after both 7 and 14
weeks. Histopathological examination of the spleen revealed increased
haemosiderosis at all dose levels except 80 ppm. In the liver, areas
of focal necrosis and/or fibrosis in the parenchyma, with or without
associated inflammatory cells, fibroblasts or pigment-laden
macrophages, were observed. At higher dose levels, necrotic and fatty
hepatocytes and brown pigment-laden Kupffer cells were found (Colley
et al 1981).
Rat
Groups of 5 male and 5 female rats were fed on a diet containing
diflubenzuron in concentrations of 0, 800, 4 000, 20 000 and
100 000 mg/kg during a four-week period. Behaviour, body weight, food
and water consumption were not clearly affected by the treatment.
There was a dose-related increase in met- and sulph-haemoglobin in the
blood in all treated groups, except for the methaemoglobin value for
the females of the 800 mg/kg dose group. Lower RBC, PCV and
haemoglobin values were observed in both sexes of the 100 000 mg/kg
dose group. Post-mortem examination revealed no changes attributable
to diflubenzuron. For both sexes, relative spleen weights in all test
groups differed significantly from control values. Relative liver
weights were increased in males or females at all dose levels, except
800 mg/kg (Palmer et al. 1977).
Groups of 10 male and 10 female Wistar rats received
diflubenzuron during 13 weeks. The concentrations in the diet were 0,
3.125, 12.5, 50 or 200 mg/kg feed. In addition two groups of 5 males
and 5 females received 0 or 200 mg/kg. These animals were sacrificed
after 6 weeks for clinico-chemical analysis. Behaviour, growth and
food intake were not affected by the treatment. At the highest dose
level, the PCV-value, the haemoglobin concentration and the number of
erythrocytes were decreased, whereas the latter was also lowered in
the 50 mg/kg group. Particularly in the males, the SGOT and SGPT
activity was increased in the highest dose group at the end of the
experiment. Higher testicular weights were recorded in the 200 mg/kg
group. At microscopic examination, a slight increase in the number of
small foci of necrotic parenchymal cells was observed, accompanied by
monocellular inflammatory cell infiltration in the liver of both males
and females of the 50 and 200 mg/kg groups (Kemp et al. 1963a, b).
Diflubenzuron was administered to Sprague-Dawley rats at dietary
levels of 0, 10 000 and 100 000 mg/kg feed for 9 weeks, followed by a
4-week withdrawal period. Each group consisted of 20 male and 20
female animals. After treatment for 9 weeks, group size was reduced to
10 males and 10 females. From week 7 onwards, male and female rats of
both groups showed pallor of extremities and eyes. During the
withdrawal period, no recovery was observed. During or at the end of
the treatment period lower values for red blood cell parameters,
formation of met- and sulph-haemoglobin, higher SGPT-values, and
heavier liver, adrenals and spleen weights were observed. The animals
produced less but more concentrated urine, and iron pigment in the
liver and spleen was also demonstrated. Minor enlargement of
centrolobular hepatocytes in some rats of the highest dose groups was
observed. After the withdrawal period, some recovery, especially of
effects on the liver and methaemoglobin induction, was observed
(Hunter et al. 1979).
Diflubenzuron was administered in the diet to male and female
Sprague-Dawley rats (40/sex/group) at dose levels of 160, 400, 2 000,
10 000 and 50 000 mg/kg feed. An additional group (90 male and 90
female animals) served as a control. No clear treatment-related
effects were noted with respect to mortality, clinical observations,
body weight gain and food consumption. A treatment-related significant
increase in met-haemoglobin was noted in all treated groups. Sulph-
haemoglobin values showed increases from the 2 000 mg/kg group
onwards. For females and males, a significant treatment-related
decrease in haemoglobin and erythrocyte count was observed at all dose
levels at the end of the study. An increase was noted in the
reticulocyte count in all dose groups, except 160 mg/kg, and the
number of Heinz bodies was higher in the 10 000 and 50 000 mg/kg
groups. Analysis of clinical chemistry values and urinalysis revealed
no apparent treatment-related trends. After 7 weeks, spleen weights
were increased in the females at all dose levels, but after 13 weeks
no effect was found at 160 mg/kg. With the exception of the lowest
dose level, all treated groups showed a higher liver weight. The
administration of diflubenzuron resulted in a dose-related increase of
incidence of chronic hepatitis and haemosiderosis of the liver. It was
also associated at all dose levels with haemosiderosis and congestion
of the spleen and mild erythroid hyperplasia of the bone marrow
(Burdock et al 1980b; Goodman 1980b).
Dog
Diflubenzuron was fed to groups of 3 male and 3 female beagle
dogs for 13 weeks at concentrations of 0, 10, 20, 40 and 160 mg/kg in
the diet. No effect of treatment on behaviour, body weight, food and
water consumption was observed. Elevated SAP and SGPT values were
recorded for some dogs receiving 40 or 160 mg diflubenzuron/kg feed.
After 6 weeks, methaemoglobin and other abnormal haemoglobin pigments
were demonstrated in dogs receiving 160 mg/kg. After 12 weeks of
administration, some recovery was observed. Organ weights, gross and
microscopic evaluation did not show treatment-related effects
(Chesterman et al 1974).
Sheep
A 13-week feeding study was carried out with 4 groups of 3 male
and 3 female sheep. The test compound was included in the diet in a
concentration of 0, 500, 2 500 and 10 000 mg diflubenzuron/kg. These
concentrations were fed to the animals in daily amounts of 0.6 during
the first 4 weeks, 0.8 during weeks 5 to 8 and 1.0 kg during the last
5 weeks. After 6 weeks, both the plasma and RBC-cholinesterase
activities were considered to be within normal limits. No treatment-
related effects were observed on food consumption, body weight gain,
haematological parameters and urinalysis. A significant increase in
sulph-haemoglobin was observed in all treated groups at 13 weeks.
After 4 and 8 weeks this effect was only obvious at higher dose
levels. There was also an indication of a treatment-related increase
in methaemoglobin levels. No other clinico-chemical parameters were
affected. The weight of the thyroid was not dose-relatedly decreased
at all dose groups. However, histopathological examination revealed no
abnormalities that could be related to the treatment (Ross et al
1977a,b).
Long-term studies
Mouse
Five groups of 52 male and 52 female CFLP mice were fed
diflubenzuron during 80 weeks. The concentrations in the diet were 0,
4, 8, 16 and 50 mg/kg feed. Behaviour, mortality, food and water
consumption and body weight were not affected by the treatment. The
changes noted on gross and histopathologic examination were common to
both treated and control animals. No treatment-related effects or
significant tumour incidences were found. Although the incidence of
lymphosarcomas in treated female mice, killed after 80 weeks, was
significantly increased (50% level, chi square test) the combined
incidence of lymphosarcomas in treated female mice, sacrificed during
the treatment period and at the termination of the experiment, was not
significantly different (Hunter et al. 1975; Batham and Offer 1977;
Offer 1977).
Rat
Five groups, each composed of 60 male and 60 female Wistar rats,
were fed diflubenzuron during 104 weeks. For the tumorigenicity study,
45 male and 45 female animals were used, whereas 15 male and 15 female
rats constituted the satellite group for the toxicity study. The
concentrations in the diet were 0, 10, 20, 40 and 160 mg/kg feed.
There was no clear treatment-related effect on behaviour, survival,
food consumption, water consumption, body weight gain, efficiency of
food utilization, blood chemistry and urinalysis. Significantly higher
met-haemoglobin levels in both males and females receiving 160 mg
diflubenzuron/kg feed were observed after 52 and 78 weeks. The other
haematological parameters were within normal limits. Organ weights,
gross pathological and microscopic examination of tissues, including
the liver, showed no compound-related effects. There was no indication
of an increase in the number of neoplastic lesions. A no-effect level
of 40 mg diflubenzuron/kg feed was observed (Hunter et al. 1976;
Colley and Offer 1977).
Special studies on met- and sulph-haemoglobin formation
Technical diflubenzuron was administered by gastric intubation to
groups of 10 mice daily for a period of 14 days. The dose levels were
0 (20 animals), 8, 40, 200, 1 000 and 5 000 mg/kg bw. Body weight
measurement and macroscopic evaluation did not reveal any effect of
the treatment. At dose levels of 5 000 and 1 000 mg/kg the percentages
of met-and sulph-haemoglobin and erythrocytes containing Heinz bodies
were increased. No effect could be observed on the met- and sulph-
haemoglobin and on the percentage of erythrocytes containing Heinz
bodies at 200, 40 and 8 mg/kg (Keet 1977b).
A similar experiment was carried out with 2 groups of 15 male
Wistar rats. The animals received doses during 8 consecutive days of
0 or 5 000 mg/kg bw, in 1% tragacanth. There was no effect on the body
weight or the number of Heinz bodies, whereas the bet- and sulph-
haemoglobin levels were only marginally increased from day 1 and 2
respectively (Keet 1977a).
Two groups of 14 male New Zealand White rabbits were fed 0 or
640 mg diflubenzuron/kg feed during 21 days. In the treated group, the
methaemoglobin level was increased from day 5, whereas higher sulph-
haemoglobin levels were observed within 5 h. In a second experiment
with 640 mg/kg feed, the methaemoglobin level was again significantly
increased. Recovery was observed 2 weeks after the treatment was
ceased (Keet 1977c).
Twenty-four male and 24 female cats were divided among five
treatment groups and one control group. They received 30, 70, 100, 300
and 1000 mg diflubenzuron/kg bw per os for 21 days, followed by a
14-day observation period. Sodium nitrite was administered as a
positive control. Diflubenzuron induced a dose-related elevation of
methaemoglobin in females at all dose levels. In males, only 30 and
70 mg/kg did not have a significant effect (maximal effect: 11.8%).
Recovery was slow. Sulph-haemoglobinemia and Heinz body formation were
observed in all treated groups. The haemoglobin concentration, number
of reticulocytes, and organ weights were within normal limits
(Schwartz and Borzelleca 1981).
Special studies on sexual development
Diflubenzuron was incorporated into feed and tested in chickens
for 13 weeks. Fat deposition was greatly increased in females. The
combs, wattles, feathers and voice of males remained undeveloped
throughout the study. Testosterone showed a dose-related decrease
(Smalley 1976 - summary only).
Diflubenzuron was fed to 4 groups of 384 male chickens of a
Hubbard broiler strain at dose levels of 0 (twice), 2.5 and 250 mg/kg
feed. After 28, 56 and 98 days, one third of the number of animals of
each group was killed. No effects were observed on mortality, body
weight, food intake, oestradiol levels in the plasma after 4, 7 and
14 weeks, organ weights, tibia weights and lengths and on gross or
microscopic examination. Only at the end of the study, testosterone
levels in serum were higher in treated groups in comparison to
controls. Both comb and wattle were more developed in the
diflubenzuron groups. However, these differences were not
statistically significant (Keet 1976b).
A parallel study with the same dose levels was carried out with
female broiler chickens. In both diflubenzuron groups, a dose-related
reduction was observed in feed consumption and body weight. Mortality
was increased only at the highest dose level (250 mg/kg feed). In
addition, a significantly increased incidence of leg abnormalities, a
reduced tibia length and relative liver weight were observed. No clear
effect on plasma testosterone was recorded. In the highest dose level,
a marginally decreased plasma oestradiol and reduced comb and wattle
development was observed (Ross et al. 1977c).
In an experiment with 2 groups of 225 female chickens, only one
dose level (250 mg/kg feed) was tested. Animals were sacrificed after
28, 49 and 98 days. The comb and wattle development was normal and no
evidence of treatment-related effects was found. Plasma oestradiol
concentrations were within normal limits (Ross et al. 1979).
Diflubenzuron was administered in the diet to one-day old Mallard
ducks, Leghorn chickens, Nicolas White turkeys and Ring-neck pheasants
for 90 days. The concentrations in the diets were 0, 0.25, 1.25, 2.5,
25 or 250 mg/kg feed. There was no clear treatment-related effect on
serum testosterone levels at days 21, 51 and 90. At the highest dose
level (the only one measured), testosterone levels were decreased in
turkeys and ducks after 42 days. Comb and wattles were not affected by
treatment. The effects on organ weights, including testes, are
difficult to evaluate (Reinert and Cannon 1976).
Young male Long-Evans rats were administered 0, 15, 150 and
300 mg diflubenzuron/kg bw daily during 14 to 96 days by gastric
intubation. The control group contained 15 animals and each test group
8. Diflubenzuron transiently decreased the levels of testosterone in
the plasma at the prepuberal age. No effects on body weight, weight of
testes, prostate, seminal vescicles and adrenals were observed.
Histological examination of the testicular tissues did not reveal any
induced changes (Paten and Santolucito 1980).
Five groups of 38 to 40 Sprague-Dawley rats received
diflubenzuron in their diet at dose levels of 0, 75, 150, 300 or
3 000 mg/kg feed. After 14, 28, 44 and 98 days, one fourth of the
animals were sacrificed. Diflubenzuron had no clear effect on body
weight gain or serum testosterone levels (Booth et al. 1980).
Four pairs of Holstein bull calves received 0 or 1.0 to 2.8 mg
diflubenzuron/kg bw. There was no significant effect of diflubenzuron
on body weight, sperm volume, sperm concentration, libido and serum
testosterone. However, the concentration of testosterone varied
considerably. Histopathological examination of tissues revealed no
significant difference between the treated and control bulls (Miller
et al 1979).
Special studies on mutagenicity
Diflubenzuron
A dominant lethal study was conducted in which 12 male mice per
test group were treated with a single i.p. injection of a suspension
of diflubenzuron in corn oil at levels of 1 000 and 2 000 mg/kg bw.
The control group received corn oil only. Sequential mating of each
male with 3 females per week was conducted for 6 consecutive weeks.
Mating ability of males and numbers of corpora lutea, implantation
sites, resorption sites (early embryonic deaths), and embryos for
treated animals were not different from those for control animals
(Arnold 1974).
Diflubenzuron was examined for mutagenic activity in a series of
in vitro microbiological assays, using the Salmonella typhimurium
strains TA 98, TA 100, TA 1537 and TA 1978. Each plate was run with
and without rat liver homogenate (S-9 mixture) prepared from Aroclor
1254 treated rats. Diflubenzuron, tested at dose levels ranging from
10 to 1 000 µg/plate, was not mutagenic in any of these assays (Bryant
1976).
In similar tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538,
and the Sacchromyces cerevisiae strain D 4. The compound was tested
both in the absence and in the presence of liver S-9 preparations from
Aroclor 1254-induced rats. Diflubenzuron tested at dose levels ranging
from 0.1 to 500 µg/plate did not demonstrate mutagenic activity in any
of these assays (Brusick and Weir 1977a).
In another series of tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535 and TA 1537.
Diflubenzuron, at levels of 10, 100 or 1 000 µg/plate, did not
significantly alter the spontaneous revertant frequency in the four
strains used, either with or without the metabolic activation system
from Aroclor 1254-induced rat liver (McGregor et al 1979).
An in vitro test with mouse lymphoma cells and a micronucleus
test in mice were carried out by the same author. In the in vitro
test with cultured cells, the forward mutation frequency of
TK+/- -> TK-/- in L 517 Y mouse lymphoma cells was tested.
Diflubenzuron at dose levels ranging from 1.2 to 300 µg/ml did not
increase the mutation frequency, either with or without the metabolic
activation system, in the micronucleus test, male mice were given 15,
150 or 1 500 mg/kg bw at 30 h and 6 h before necropsy. Diflubenzuron
did not significantly increase the frequency of micronucleated
erythrocytes in the bone marrow (McGregor et al. 1979).
In another series of tests, diflubenzuron, as well as its
metabolites (2,6-difluorobenzoic acid (DFBA, 4-chlorophenylurea (CPU),
4-chloroaniline), were negative in the S. typhimurium strains TA 98,
TA 100, TA 1535 and TA 1538. The results in the strain TA 1537 were
difficult to interpret (Seuferer et al 1979).
Diflubenzuron was evaluated in a cell transformation test. At
dose levels of 0.02 to 0.312 mg/ml, diflubenzuron did not induce
morphological transformation in BALB/3T3 cells in vitro (Brusick and
Weir 1977b).
Diflubenzuron was evaluated for its ability to induce unscheduled
DNA synthesis in human diploid WI-38 cells blocked in the G1 phase.
The compound was tested at dose levels of 50 to 1 000 µg/ml, both in
the absence and in the presence of liver S-9 preparations of uninduced
mice. Under the conditions of the assay, diflubenzuron did not induce
unscheduled DNA synthesis (Brusick and Weir 1977c).
Diflubenzuron was tested in a transplacental transformation assay
to investigate possible transformation of mammalian cells in culture.
Timed pregnant hamsters were injected intraperitoneally on day 10 of
gestation with solutions of diflubenzuron in dimethylsulphoxide at
dose levels of 10, 200 and 500 mg/kg bw. Three days after injection,
animals were sacrificed and foetal cell cultures were prepared.
Diflubenzuron did not induce morphological transformation or the
ability for growth of colonies. With known carcinogens (benzopyrene
and dimethylnitrosamine), a positive result was obtained (Quarles
et al 1980).
Metabolites
The metabolites 4-chlorophenylurea (CPU), 2,6-difluorobenzoic
acid (DFBA) and 4-chloroaniline were examined for mutagenic activity
in a series of in vitro microbial assays. A spot test at a dose
level of 1 000 µg per spot was carried out with S. typhimurium
strains TA 98, TA 100, TA 1535, TA 1537, TA 1538 and TA 1978, with and
without metabolic activation. A dose response test at dose levels of
10, 100, 500 and 1 000 µg was carried out with TA 98 and TA 100. The
test compounds did not demonstrate mutagenic activity in any of the
assays conducted, except for a weak effect with 500 and 1 000 µg
4-chloroaniline in the TA 98 strain after activation by liver S-9
preparation. At these dose levels, 4-chloroaniline caused a reduction
in the number of colonies, indicating a degree of toxicity to the
bacteria (Dorough 1977).
CPU was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae strain D 4.
The compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to
500 µg/plate, the test compound did not demonstrate mutagenic activity
in any of the assays conducted (Jagannath and Brusick 1977a).
CPU was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration
(312 µg/ml) employed in the concentration range. The other levels were
negative. The results were considered to be an indication of a weak
transforming activity at concentrations near to the level of
cytotoxicity (Matheson and Brusick 1978a).
CPU was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both in the absence and in the presence of liver S-9
preparations of Aroclor 1254-induced mice. CPU at dose levels ranging
from 6.25 to 400 µg/l did not induce unscheduled DNA synthesis
(Matheson et al 1978a).
DFBA was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537, TA 1538 and the S. cerevisiae strain D 4. The
compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to 500
µg/plate, the test compound did not demonstrate mutagenic activity in
any of the assays conducted (Jagannath and Brusick 1977b)
DFBA was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration employed
(2.5 mg/ml). The other levels were negative. These results were
considered to be an indication of weak transforming activity at
concentrations near to the level of cytotoxicity (Matheson and Brusick
1978b).
DFBA was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both with and without metabolic activation. The compound at
dose levels ranging from 75 to 500 µg/ml produced significant
increases in the level of unscheduled DNA synthesis in the presence of
mouse S-9 liver mixture. However, there was no dose response
relationship, 75 µg giving the highest increase and 500 µg the lowest
increase. According to the authors, the results appeared to be on the
plateau of a dose response effect. The compound was considered to be
active under these test conditions (Matheson and Brusick 1978c).
4-Chloroaniline was examined for mutagenic activity in a series
of in vitro microbial assays, using the S. typhimurium strains
TA 98, TA 100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae
strain D 4. The compound was tested with and without liver S-9
preparations from Aroclor 1254-induced rats. The test compound did not
demonstrate mutagenic activity in any of the assays conducted
(Jagannath and Brusick 1977c).
4-Chloroaniline was evaluated in a cell transformation test. At
dose levels ranging from 39 to 625 µg/ml the compound did not induce
morphological transformation in BALB/3T3 cells in vitro (Matheson
et al 1978b).
4-Chloroaniline was tested for its ability to induce unscheduled
DNA synthesis in human WI-38 cells blocked in the G1 phase. The
compound was tested both with and without metabolic activation. At
dose levels of 250 to 1 000 µg/ml, the compound did not induce
unscheduled DNA synthesis (Matheson et al 1978c).
Special studies on reproduction and teratogenicity
Diflubenzuron was fed to 3 groups of 20 male and 20 female rats
at dietary levels of 0, 1 000 and 10 000 mg/kg for one generation and
one litter. The animals were maintained on their respective diets for
60 days prior to mating. There were no clear effects on mating
performance, pregnancy rate, duration of gestation, litter size,
mortality, litter weight, type and distribution of abnormalities.
Diflubenzuron had, among others, a dose-related effect on haemoglobin
(reduced PCV, Hb, total red cells, increased met-haemoglobin, sulph-
haemoglobin, spleen weight, incidence of siderocytes in the spleen and
the occurrence of iron pigment containing Kupffer cells in the liver).
A dose-related effect on the liver was also shown by an increased
weight, SGPT activity and hepatocyte enlargement. Reduced blood
glucose concentrations were recorded in both treated groups. In the
highest dosed group, the offspring showed an increased liver and
spleen weight for both sexes. Microscopically, an increased incidence
of centrolobular hepatocyte enlargement was observed (Palmer et al
1978).
Diflubenzuron was administered in the diet to groups of 20 male
and 20 female rats at concentrations of 0, 10, 20, 40 and 160 mg/kg.
These diets were administered continuously throughout three
generations producing one litter each. As the mating performance was
low in the first generation, a second litter was bred. Parent animals
showed no signs of adverse effects related to treatment. Mating
performance, pregnancy rate and duration of gestation were not
affected. Also, total litter loss, litter size, litter and mean pup
weights, pup mortality and the incidence of abnormalities provided no
evidence of adverse treatment-related effects (Palmer and Hill 1975a).
Groups of 20 pregnant rats were administered diflubenzuron orally
by gavage at levels of 0, 1, 2 or 4 mg/kg bw during days 6 to 15 of
gestation. The parent animals showed no signs of reaction and no
mortalities occurred. Body weight gain and pregnancy rate were
unaffected by treatment. No effects were observed on the number of
viable young, implantations, resorptions and corpora lutea. The
pre-implantation loss, foetal loss, litter weight, foetal weight, the
incidence of major malformations, minor anomalies and skeletal
variants were not dose-relatedly affected (Palmer and Hill 1975b).
Four groups of 13 pregnant New Zealand White rabbits were
administered diflubenzuron orally from day 6 to 18 of gestation at
dose levels of 0, 1, 2 and 4 mg/kg bw. Foetuses were removed on day 29
of pregnancy. There was no effect of the treatment on behaviour, body
weight gain and pregnancy rate. No dose-related differences were
observed on the number of viable young, resorptions, foetal loss,
litter weight and mean foetal weight. The incidence of major
malformations and minor anomalies was not affected by treatment
(Palmer and Hill 1975c).
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller and Booth 1976).
Pregnant female mice were fed a diet containing 50 mg/kg of
diflubenzuron (partly 14C). Some of these mice were sacrificed at
day 17 from conception, the others were allowed to give birth to the
young. The lactating females were kept on treatment and allowed to
suckle their young for 13 days. No radioactivity was detected in the
embryos or young mice (Booth 1977).
RESIDUES IN FOOD
USE PATTERN
Diflubenzuron is a recently-introduced insecticide that
interferes in the deposition of chitin in the insect cuticle through
action on the enzyme chitin synthetase.
Pre-harvest treatments
Diflubenzuron is formulated as a wettable powder containing 25%
of the active ingredient (Dimilin WP-25), or as an oil dispersible
concentrate containing 450 g diflubenzuron per litre. Granular
formulations are also available. Recommended use rates are given with
pre-harvest intervals in Table 3.
Other uses
Diflubenzuron is recommended for the control of flies in animal
husbandry by topical applications to breeding sites and manure heaps.
A further application is on ornamental plants and in forests.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials on the main
crops treated. The dosages in these trials covered recommended and
higher rates. Treatments were made using W-25 wettable powder
formulation, which is the formulation recommended for food crops.
TABLE 3. Recommended uses and pre-harvest intervals for diflubenzuron
Use rate Pre-harvest
Crop Country (a.i.) interval (days)
Apple, pear Argentina 0.02% 60
Bulgaria 0.0375% -
France 0.01% 30
German Fed.Rep. 0.02% 28
Greece 0.0125-0.015%1 -
Israel 0.0125% -
Italy 0.0125-0.02% 45
The Netherlands 0.01% 28
Spain 0.01-0.015% 60
South Africa 0.02% -
Switzerland 0.02% 42
UK 150 g/ha 14
Yugoslavia 0.0125-0.02% 30
Brassica leafy
vegetables The Netherlands 0.01% 14
China(Taiwan prov.) 0.017% 22
UK 100 g/ha -
Yugoslavia 200 g/ha -
Cottonseed Egypt 75 g/ha -
Greece 0.01-0.025% -
USA 70 g/ha (6x) -
Mushroom Greece 1 g/m2 -
Korea 1 g/m2 -
The Netherlands 1 g/m2 -
Switzerland 1 g/m2 -
UK 1 g/m2 -
Soybean Brazil 50-75 g/ha 21
Colombia 75-125 g/ha -
USA 35-70 g/ha
Tomato UK 250 g/ha -
Citrus USA2 350 g/ha ?
1 for pears: 0.0125-0.03%;
2 registration applied for.
Apple
Residue data have been obtained from trials in several countries.
Residues on fruit at recommended rates up to 0.02% were usually well
below 1.0 mg/kg at two weeks after the last application. Only three
samples were shown to contain residues from 1.0 to 1.2 mg/kg. The
one sample that contained 3.7 mg/kg is considered to be atypical
(Table 4). In the UK, apples (cv Cox and Egremont Russet) treated with
diflubenzuron 2 weeks before harvest at a dosage rate of 250 g a.i./ha
were shown to be taint-free (Spencer-Jones 1979).
Pear
The residue pattern in pears is very similar to that in apples.
At two weeks after the last application at recommended dosages, the
residues were well below 1.0 mg/kg (Table 4).
Citrus
Residue data have been obtained from numerous trials on orange,
grapefruit and tangerine in the USA. The dosages in these trials
included the recommended rate and also 2x and 4x rates. Residues in
whole fruit were all well below 0.5 mg/kg 1 week after the last
application at the recommended dosage rate. At the 4x dosage rate, the
residue remained generally below 1.0 mg/kg (Table 5).
Examination of peel and pulp separately showed that residues were
exclusively found in the peel when the product was applied at the
recommended rate. Residues in the pulp were all below the detection
limit (0.05 mg/kg). At the 4x use rate, by far the major portion of
the residue was in the peel. Residues in the pulp were either below or
just above the detection limit of 0.05 mg/kg, the highest value found
being 0.07 mg/kg. (Table 6).
Further fractionation of orange and grapefruit showed that the
residue is mainly present in the oil fraction. Here, the residue
ranged from 9.5 to 20 mg/kg at the recommended use rate. At the
4x rate residues were as high as 50 mg/kg. Neither at the recommended
rate nor at the 4x rate could residues be detected in the juice of
both orange and grapefruit, the limit of detection being 0.05 mg/kg
(Table 7).
In several cases, the various citrus fractions were analysed for
residues of the metabolites 4-chlorophenylurea, 4-chloroaniline and
2,6-difluorobenzoic acid. In none of the samples was any residue
detected (limit of detection for all three compounds 0.05 mg/kg)
(Duphar 1975-81).
TABLE 4. Residues following supervised trials in apple and pear1
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Apple
Bramley UK 0.01 1 - 0.22 0.23 0.14
Cox O.P. 0.01 1 - 0.18 0.16 0.20
Egremont R 0.01 1 - 0.27 0.24 0.18
Golden D. Netherlands 0.01 1 - <0.032
Winston 0.01 1 - <0.03
Golden D Europe 0.01 3-5 15-59 0.15 0.16 0.23 0.30
-0.21 -0.34
J. Grieve Netherlands 0.0125 1 - 0.28 0.36 0.13
J. Grieve 0.0125 2 17 1.32 0.94 0.75 0.53
Stark Crimson Italy 0.0125 2 58 0.06
Golden D 0.0125 2 54 0.15
Stark Crimson 0.0125 3 58,34 0.12
Golden D 0.0125 3 58,33 0.05
Jonathan 0.0125 4 35,22,41 0.14
J. Grieve German Fed.Rep. 0.015 1 - 0.43 0.14 0.03 0.09 0.11
Golden D. Europe 0.015 1-8 14-52 0.34 0.78 0.34 0.12 0.23 <0.05
-1,2 -1.1 -0.83
Bramley UK 0.017 1 - 0.17 0.02 0.18 0.19 0.02
Cox O.P. 0.017 1 - 0.09 <0.01 0.22
Egremont R 0.017 1 - 0.17 0.03 0.20
Ida Red 0.017 1 - 0.12
Golden D 0.017 1 - 0.20
McIntosh 0.017 1 - 0.17
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Warner UK 0.017 1 - 0.16
Worcester 0.017 1 - 0.15
Newton 0.017 1 - 0.01
Golden D France 0.01875 3 33,31 0.41
Golden D 0.01875 3 30,27 0.46
Golden D 0.01875 3 29,35 0.43
Golden D 0.01875 4 21,21,28 0.62
Golden D 0.01875 4 20,20,30 3.8
J. Grieve Netherlands 0.02 1 - 0.29 0.21 0.18 0.15 0.13
Golden D 0.02 1 - 0.52 0.31 0.23 0.29
n.s. South Africa 0.02 1 - 0.833 0.32 0.29
n.s. 0.02 1 - 0.784 0.14 0.15 0.23
Cox O.P. Netherlands 0.02 1 - 0.095
S. Boskoop 0.02 1 - 0.146
Ingrid M. 0.02 1 - 0.08
Winston 0.02 1 - 0.097
Golden D 0.02 1 - 0.055
Cox O.P German Fed.Rep. 0.02 1 - 0.258 0.05a
Cox O.P 0.02 1 - 1.568 0.438
Golden D 0.02 1 - 0.738 0.288
Cox O.P. 0.02 2 22 0.538 0.26 0.258 0.19 0.14
Golden D 0.02 2 21 1.268 0.69 0.58 0.53 0.37
Cox O.P. 0.02 2 21 1.878 1.24 0.95 0.51 0.25
Golden D Netherlands 0.02 2 22 <0.03
Gravesteyn Italy 0.02 2 53 0.24
Cox O.P. German Fed.Rep. 0.029 3 21,28 0.84 0.92 0.76 0.72
Cox O.P. 0.029 3 22,28 0.54 0.42 0.40 0.26 0.47
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D 0.02 21,28 1.26 0.89 0.52 0.66 0.63
Golden D Italy 0.02 3 57,56 0.52
Golden D 0.02 3 10,68 0.57
Steymen 0.02 3 58,32 0.40
Golden D Netherlands 0.029 3 40,31 0.45 0.40 0.32 0.29 0.19 0.28
Cox O.P. 0.029 3 34,30 0.30 0.27 0.29 0.24
G. Parmane German Fed.Rep. 0.029 4 29,39,14 0.5210 0.04 0.26 0.30
n.s. South Africa 0.0211 4 28,26,38 1.24
Jonathan Italy 0.02 5 23,15,27,14 0.28
Golden D 0.02 5 34.30,14,28 0.87
Golden D 0.02 5 40,29,15,33 0.77
Golden D France 0.02 5 15,14,16,16 0.27
J. Grieve Netherlands 0.025 1 - 0.67 0.38 0.31
Star-King D Japan 0.025 1 <0.01 0.067
Red-ball 0.025 1 0.087 0.107
n.s. Italy 0.02512 2 62 0.197 0.2313
J. Grieve Netherlands 0.025 2 17 1.14 1.62 0.58 0.87
Golden D 0.025 2 15 0.426
St.Crimson Italy 0.025 2 58 0.10
Golden D 0.025 2 52 0.117
St.Crimson 0.025 3 58,34 0.30
Golden D 0.025 3 52-54,32-49 0.55 0.28
Star-King Japan 0.025 3 0.387
Red-ball 0.025 3 0.207
Jonathan Italy 0.025 4 35,22,41 0.18
Golden D France 0.025 5 28,14,21,21 1.0
Golden D 0.025 6 12-18 0.87
J. Grieve German Fed. Rep. 0.03 1 - 0.73 0.23 0.15 0.15 0.13
Golden D 0.03 1 - 1.0 0.67 0.44 0.43 0.31
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D Netherlands 0.03 1 - 0.056
Cox O.P. 0.03 1 - <0.03
Golden D 0.03 2 15 0.437
Golden D 0.03 2 15-19 0.34 0.32 0.326
n.s. South Africa 0.03 3 28,32 0.26
Golden D 0.03 8 14 2.20
n.s. 0.04 1 - 0.7815 0.38 0.37 0.25
n.s. 0.04 1 - 1.5516 1.25 0.70
Golden D Netherlands 0.04 1 - 0.0514
Winston 0.04 1 - <0.03
Golden D 0.04 2 22-43 0.077
Jonathan 0.04 2 26 <0.05
Gravesteyn Italy 0.04 2 53 0.41
Golden D 0.04 3 10-68 0.83 1.11
Alkmene German Fed. Rep. 0.0417 4 13,16,20 1.13 0.7418 0.63 0.65
Golden D Italy 0.04 5 14-40 1.437
Golden D France 0.04 6 12-18 1.40
Pear Europe 0.01 1-5 17-33 0.1 0.12 0.02
-0.04 -0.31 -0.25
South Africa 0.02 1-3 28-32 0.52 0.11 0.13 0.24
-0.04 -2.64 -1.30 -1.25 -1.31
1 Reference: Duphar 1975-81; 2 4 trials;
3 day 1: 0.63, day 2:0.83, day 6:0.50, day 7: 0.61; 4 day 1: 0.42, day 2: 0.78, day 4: 0.30;
5 average of 6 trials; 6 average of 3 trials;
7 average of 2 trials; 8 average of 2 samples;
9 last spray rate - 0.015; 10 day 0: 0.52, day 7: 0.44;
11 first spray rate-0.025; 12 last spray rate - 0.02;
13 average of 4 trials; 14 average of 5 trials;
15 day 1: 0.78, day 2: 0.11, day 4: 0.91; 16 day 1: 1.55, day 2: 0.72, day 6: 1.15, day 7: 1.39;
17 last spray rate - 0.03; 18 day 9: 0.74, day 14: 0.72.
TABLE 5. Residues following supervised trials in citrus, whole fruit, in Florida and Texas, USA1
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 1 PB 0.07 0.11 0.12 0.13 0.08 0.10
1 PB 0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Temple 1 PB 0.053
1 PB 0.063
Pineapple 1 PB <0.05
1 PB 0.053
Hamlin 1 PB 0.06
1 PB <0.05
Mars 1 PB 0.10
Temple 2 PB,S 0.084
2 PB,S 0.063
Pineapple 2 PB,S 0.093
2 PB,S 0.053
Hamlin 2 PB,S 0.123
2 PB,S <0.05
Mars 2 PB,S 0.16
Temple 3 PB,S,F 0.144 0.13 0.143
3 PB,S,F 0.173 0.13 0.11
Pineapple 3 PB,S,F 0.183 0.18 0.08
3 PB,S,F 0.133 0.10 0.07
Hamlin 3 PB,S,F 0.163 0.20 0.083 0.25
3 PB,S,F 0.10 0.05
Mars 0.35 3 PB,S,F 0.32
Hamlin-pineapple " 3 PB,S,F 0.27 0.29 0.30 0.36 0.21
3 PB,S,F 0.20 0.29 0.22 0.41 0.14
3 PB,S,F 0.05 0.20 0.10 0.11 0.21
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 1 PB 0.054
Marsh 1 PB <0.053
Seedless 1 PB <0.053
Ruby red 1 PB 0.063
1 PB 0.063
Pink 2 PB,S <0.054
Marsh seedless 2 PB,S 0.083
2 PB,S 0.063
Ruby Red 2 PB,S 0.123
Ruby Red 0.35 2 PB,S 0.123
Pink 3 PB,S,F 0.174 0.15 0.093 0.26 0.22
Marsh seedless 3 PB,S,F 0.123 0.073
3 PB,S,F 0.113 0.143
Ruby Red 3 PB,S,F 0.203
3 PB,S,F 0.133
Tangerine Nova 1 PB <0.05
1 PB <0.05
2 PB,S 0.12
2 PB,S 0.09
3 PB,S,F 0.07 0.05
3 PB,S,F 0.09 0.09
Orange Valencia 0.7 1 PB 0.223 0.133 0.163 0.143 0.083 0.153 <0.05
1 PB 0.10 0.11
Mars 1 PB 0.27
2 PB,S 0.28
3 PB,S,F 0.23
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1 PB 0.10
2 PB,S 0.203
3 PB,S,F 0.203
Valencia 1.4 1 PB 0.233 0.113 0.273 0.233 0.123 0.073
1 PB 0.07 0.05
Temple 1 PB <0.053
1 PB 0.07 0.05
Pineapple 1 PB <0.053
1 PB 0.053
Hamlin 1 PB <0.053
Orange Hamlin 1.4 1 PB 0.09
Mars 1 PB 0.18
Grapefruit Pink 1 PB <0.053
1 PB <0.05
Marsh seedless 1 PB <0.053
1 PB 0.343
Ruby Red 1 PB 0.273
Tangerine Nova 1 PB <0.05
1 PB <0.05
Orange Temple 1.4 2 PB,S 0.124
2 PB,S 0.15
Pineapple 2 PB,S 0.173
2 PB,S 0.383
Hamlin 2 PB,S <0.053
Mars 2 PB,S 0.305
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 2 PB,S 1.10 0.26 0.243
2 PB,S 0.23
Marsh seedless 2 PB,S 0.243
2 PB,S 0.353
Ruby Red 2 PB,S 0.233
Tangerine Nova 2 PB,S 0.15
2 PB,S 0.20
Orange Temple 3 PB,S,F 0.384 0.473 0.233 0.91
3 PB,S,F 0.46 0.59
Pineapple 3 PB,S,F 0.253 0.35 0.06
3 PB,S,F 0.573 0.49 0.15
Hamlin 3 PB,S,F 0.713 0.74 0.103 0.44
3 PB,S,F 0.23 0.26
Mars 3 PB,S,F 0.10
Grapefruit Pink 3 PB,S,F 0.393 0.81 0.85 1.11 0.48
3 PB,S,F 0.34 0.65
Marsh seedless 3 PB,S,F 0.533 0.273
3 PB,S,F 0.303 0.613
Ruby Red 3 PB,S,F 0.643
Tangerine Nova 3 PB,S,F 0.16 0.28
3 PB,S,F 0.31 0.24
1 Referenne: Duphar 1975-81;
2 PB = Post Bloom, S = Summer,F = Fall;
3 average of 2 trials;
4 average of 3 Trials;
5 day 70: 0.53 in one trial.
TABLE 6. Residues following supervised trials in citrus, peel and pulp, in Florida and Texas, USA1
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 12 peel 0.10
pulp <0.05
Temple 33 peel 0.574
pulp <0.054
peel 0.21
pulp <0.05
Pineapple 3 peel 0.46
pulp <0.05
3 peel 0.26
pulp <0.05
Hamlin 3 peel 0.40
pulp <0.05
Mars 3 peel 0.50
pulp <0.05
Grapefruit Pink 3 peel 0.58
pulp <0.05
Marsh seedless 3 peel 0.41
pulp <0.05
3 peel 0.84
pulp <0.05
Ruby Red 3 peel 0.614
pulp <0.05
0.355 3 peel 0.934
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 1,4 1 peel 0.09 1.89 1.65 1.83 0.88 0.75
pulp 0.07 0.07 0.07 <0.07 <0.05 <0.05
peel 0.78 0.50 0.70 0.91 0.43 0.15
pulp <0.05 <0.05 <0.05 <0.05 <0.05 (0.05
1 peel 0.29 <0.05
pulp <0.05 <0.05
Orange Temple 1.4 2 peel 0.07
pulp 0.05
Hamlin 2 peel 0.38
pulp <0.05
Temple 3 peel 1.40 2.00
pulp <0.05 <0.05
3 peel 1.30 0.61
pulp <0.05 <0.05
peel 1.20
pulp (0.05
Pineapple 3 peel 0.93 1.8
pulp <0.05 <0.05
3 peel 1.70 0.28
pulp <0.05 <0.05
Hamlin 3 peel 0.20 2.70
pulp <0.05 <0.05
1.45 3 peel 1.3
pulp <0.05
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1.4 1 peel 0.88c
pulp 0.05c
2 peel 0.40c
pulp 0.05
3 peel 0.94
pulp 0.05
Pink 3 peel 2.10
pulp 0.05
3 peel 1.90
pulp 0.07
Marsh seedless 3 peel 0.66
pulp 0.07
3 peel 1.0
pulp 0.05
Tangerine Nova 3 peel 0.18
pulp 0.05
3 peel 0.75
pulp 0.05
1 Reference: Duphar 1975-81;
2 1 Spray Post Bloom;
3 3 sprays: Post Bloom, Summer and Fall;
4 average of 2 trials;
5 plus 0.25% oil.
TABLE 7. Residues following supervised trials in citrus fractions, in Florida, USA1
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Orange (Temple) Whole fruit(unwashed) 0.35 1.4 3 0.13 0.35 14
Whole fruit (washed) 0.09 0.26 14
Chopped peel 0.09 0.13 14
Frits 0.11 0.84 14
Finisher pulp <0.05 <0.05 14
Dried citrus pulp 0.09 0.05 14
Fruit Juice <0.05 <0.05 14
Oil 13.0 28.0 14
Pressed liquor <0.05 0.09 14
Molasses <0.05 0.06 14
Prewash water 0.01 0.02 14
Afterwash water 0.03 0.07 14
Orange (Eamlin) Whole fruit(unwashed) 4 0.28 0.34 2t
Whole fruit(washed) 0.14 0.33 21
Chopped peel 0.06 0.11 21
Frits 0.10 0.55 21
Finisher pulp <0.05 <0.05 21
Dried citrus pulp <0.05 0.66 21
Fruit juice <0.05 <0.05 21
Oil 20.0 50.0 21
Pressed liquor <0.05 0.08 21
Molasses <0.05 0.12 21
Prewash water 0.03 0.06 21
Afterwash water 0.05 0.22 21
TABLE 7. (con't)
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Grapefruit (Pink) Whole fruit (unwashed) 0.35 3 0.09 21
Whole fruit(washed) <0.05 21
Chopped peel 0.08 21
Frits 0.19 21
Finisher pulp <0.05 21
Dried citrus pulp 0.10 21
Fruit juice <0.05 21
Oil 9.50 21
Pressed liquor <0.05 21
Molasses <0.05 21
Prewash water 0.01 21
Afterwash water 0.04 21
Whole fruit (unwashed) 1.4 3 0.09 21
Whole fruit (washed) 0.17 21
Chopped peel 0.11 21
Frits 0.50 21
Finisher pDlp <0.05 21
Dried citrus pulp 0.36 21
Fruit juice <0.05 21
Oil 23.0 21
Pressed liquor <0.05 21
Molasses 0.17 21
Prewash water 0.03 21
Afterwash water 0.17 21
1 Reference: Duphar 1975-81.
In taste tests with orange juice, no off-flavour was detected
which might have been caused by DIMILIN treatment (Braddock 1976b,
1977). Also in the case of grapefruit juice, no adverse effects on
flavour were detected (Braddock 1976a).
Soybean
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended use rate and also much
higher rates. Residues in the soybean seed were generally below the
detection limit, which was 0.05 mg/kg. Only one sample contained a
residue just above this limit, i.e. 0.06 mg/kg. Also at higher rates,
residues remained very low, the highest value found being 0.16 mg/kg.
Fractionation of seed containing <0.05 mg/kg residue did not result
in a detectable residue in any of the fractions. As could be expected
from the foliar stability of diflubenzuron, soybean foliage did
contain residues, the level of which declined with time (Table 8).
In nine trials, rotational crops were grown in fields on which
soybean had been treated with diflubenzuron at both the recommended
and higher rates. In none of the trials could residues be detected
(limit of detection 0.05 mg/kg) in the rotational crops, including
turnips, collards, rye, oats and mustard green. In the samples
analysed for 4-chlorophenylurea, no residue could be detected (limit
of detection 0.05 mg/kg) (Duphar 1975-81).
Residues in soil of soybean fields treated with diflubenzuron
were usually below the limit of detection (0.05 mg/kg), both for the
parent compound and the metabolite 4-chlorophenylurea (CPU).
Diflubenzuron residues never exceeded 0.3 mg/kg and were exclusively
found in the top 7.6 cm of soil. CPU residues did not exceed 0.5 mg/kg
and in only one case could any residue be detected below the top
7.6 cm of soil (Duphar 1975-81).
Cotton
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended rate and also higher ones.
Usually, several applications were made, up to 16 per growing season.
Residues in the cotton seed were generally below the limit of
detection (0.05 mg/kg) (Table 9). Only a few samples contained a
residue slightly above the detection limit, with one high value of
0.17 mg/kg. Fractionation of cotton seed did not result in a
detectable residue of any of the fractions.
In seven trials, rotational crops were grown in fields on which
cotton had been treated with diflubenzuron 15 times during the growing
season either at a rate of 0.067 or 0.280 kg a.i./ha. In none of the
trials could residues of either diflubenzuron or 4-chlorophenylurea
TABLE 8. Residues following supervised trials in soybean, USA1
Application Residues (mg/kg) at intervals (days)
Crop part after application
Rate (a.i.) No.
(kg/ha) 0-7 8-14 15-21 22-28 29-42 43-56 <56
0.034 1-2 <0.05 <0.05 <0.05 <0.05 <0.05
Seed - 0.56 -0.13 -0.16 -0.07 -0.07
Foliage Bragg 0.034 1 0.86 0.49 0.25
Lee 0.034 1 1.10 0.59 0.38
Ranson 0.034 1 2.25 1.40 1.65 0.82
n.s. 0.034 1 0.25
Bragg 0.034 2 0.462 2.6 0.26 0.63
Lee 68 0.034 2 2.1 0.58
Bragg 0.067 1 1.8 0.29 0.33 0.14
Calland 0.067 1 0.16
n.s. 0.280 1 1.6
n.s. 0.280 2 0.23
0.140 1
Seed n.s. <0.05
Hulls <0.05
Meal <0.05
Crude oil <0.05
1 Reference: Duphar 1975-81; 2 Average of 2 trials.
TABLE 9. Residues following supervised trials in cottonseed, USA1
Application Residues (mg/kg) at intervals (days)
after application
Crop part Variety Rate (a.i.) No.
kg/ha 0-7 8-14 15-21 22-28 29-42 43-56 <56
Seed 0.033 1- <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
-0.56 15 -0.07 0.09 -0.05 -0.07 -0.17
Hulls DPL-16 0.140 8 <0.05
Oil <0.05
Meal <0.05
Seed hulls Stoneville 213 0.280 9 <0.05
Meal <0.05
Oil <0.05
Soapstock <0.05
1 Reference: Duphar 1975-81
(limit of detection for both compounds 0.05 mg/kg) be detected in
the rotational crops, including collards, wheat and radish (Duphar
1975-81).
Mushroom
This crop shows a residue pattern different from all the others.
This is undoubtedly due to the different way in which it is grown. The
intimate contact of the mushrooms with the medium in which they are
grown enables the uptake of the metabolite 2,6-difluorobenzoic acid
especially (see also "Fate of residues"). At the recommended dosage
rate of 1 g a.i./m2, the diflubenzuron residue in mushrooms is
generally below 0.1 mg/kg, only one sample contained 0.11 mg/kg.
Also the residue level of 4-chlorophenylurea is very low,
all residues being below 0.1 mg/kg. However, the residues of
2,6-difluorobenzoic acid were considerably higher. At the recommended
use rate they all remain below 1 mg/kg. At higher dosage rates,
somewhat higher residues are found, the highest value being 1.63 mg/kg
at a dosage of 4 g/m2, applied twice. (Table 10).
FATE OF RESIDUES
General comments
The metabolism of diflubenzuron was studied and it was
established that the compound degraded along different lines, as shown
in Figure 2. Breakdown occurred predominantly along line 1, resulting
in 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU). A
minor degradation takes place along lines 2 and 3, resulting in
4-chloroaniline (PCA) and CO2.
TOXICOLOGICAL STUDIES
Acute toxicity
In none of the acute studies on diflubenzuron was any overt sign
of toxicity observed. In the dermal toxicity study with rats, no
effect on the met- and sulph-haemoglobin values was detected. Results
of acute toxicity studies in rat, mouse and rabbit are summarized in
Table 1.
Loss of activity, catatony, paralysis and severe bradypnoea, were
observed in rats treated with the metabolite 4-chlorophenylurea. At
autopsy, the animals showed congested blood vessels and haemorrhagic
intestines. The rats dosed with 2,6-difluorobenzoic acid showed
symptoms indicative for slight C.N.S. excitation and increased muscle
tone. Results of studies with diflubenzuron metabolites are summarized
in Table 2.
Short-term studies
Mouse
Three groups of 8 male CFLP mice were fed dietary levels of
diflubenzuron for 6 weeks at levels of 0, 16 and 50 mg/kg feed. There
was no clear effect of the treatment on food consumption, body
weight, blood chemistry and macroscopic pathology. The weight of the
spleen was decreased in the highest dose group. In some animals
receiving 50 mg/kg feed, foci of liver cell necrosis with or without
inflammatory cell infiltration were noted. The other organs were not
microscopically examined (Hunter et al 1974).
Male and female mice (40/sex/group) received diflubenzuron in the
diet at a dosage level of 16, 50, 400, 2 000, 10 000 or 50 000 mg/kg
feed during 13 weeks. An additional group of one hundred/sex served as
a control. No compound-related effects were apparent in respect to
clinical signs, survival, growth, food consumption or gross pathology.
Significant treatment-related increases in met- and sulph-haemoglobin
concentrations were noted in all treated groups, except the 16 mg/kg
feed one. At the higher dose levels, a decrease was noted in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects were noted on the weights of liver and
spleen. In the females, adrenal weight was not dose-relatedly
decreased at all dose levels after 7 weeks and increased after 13
weeks at higher dose levels. In males a higher adrenal weight was
observed in comparison to controls. Histopathologically, treatment-
related centrolobular hypertrophy of hepatocytes, with or without cell
TABLE 1. Acute toxicity of diflubenzuron
Species Sex Route LD50 Reference
Rat F,M Oral > 4,640 mg/kg Van Eldik 1973
F,M Dermal >10,000 mg/kg Keet 1976a;Koopman 1977c
F,M Inhalation > 35 mg/kg (6 hr) Berczy et al 1973
Mouse F,M Oral > 4,640 mg/kg Van Eldik 1973;Koopman 1977a
F,M i.p. > 2,150 mg/kg Van Eldik 1974;Koopman 1977b
Rabbit F,M Dermal > 4,000 mg/kg
(50% paste) Davies and Halliday 1974
F,M Inhalation > 30 mg/l (6 hr) Berczy et al 1975
TABLE 2. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50(mg/kg) Reference
4-Chlorophenylurea Rat M Oral 1 080 Koelman-Klaus 1978a
F Oral 1 210 Koelman-Klaus 1978a
2,6-Difluorobenzoic Rat M,F Oral 4 640 Koelman-Klaus 1978b
acid
necrosis, haemosiderosis of the liver and spleen, extramedullary
haematopoiesis and mild chronic hepatitis were observed. The liver
lesions were more severe in males than in females. No no-effect level
was observed (Burdock et al. 1980a; Goodman 1980a).
A 14-week feeding study was performed with groups of 40 male and
40 female HC/CFLP mice (control groups, 96 male and 96 females)
maintained on a diet supplemented with 0, 80, 400, 2 000, 10 000 and
50 000 mg diflubenzuron (purity 97.20%/kg feed.) Half of the number of
mice of both sexes of all groups were sacrificed after 7 weeks. On the
second day of treatment, the majority of mice treated with 50 000 or
10 000 mg/kg feed showed dark eyes and/or prominent caudal blood
vessels. On day 5, blue/grey discolouration of the extremities was
noted for the majority of mice treated with 50 000 mg/kg. In the
course of the study, clinical signs were observed in mice of all
groups, except those of the lowest dose group. Mortality, food
consumption, water consumption and body weight changes were not
clearly affected by the treatment. Results of haematological
investigation showed lower packed cell volume and red blood cell
count at all dose levels except 80 mg/kg feed. The total white blood
cell count, lymphocyte count, haemoglobin concentration, the incidence
of Heinz bodies and the red blood cells were increased in all dose
groups. A treatment-related increase in both met- and sulph-
haemoglobin was recorded in all treated groups at weeks 7 and 14 of
the investigation. Clinical biochemistry revealed higher plasma
glutamic pyruvic transaminase at all dose levels, with the exception
of 80 mg/kg feed. Lower blood cholesterol levels were noted in the
50 000, 10 000 and 2 000 mg/kg feed groups. Macroscopic examination
showed dark discolouration and/or enlargement of the spleen and pale
subcapsular areas of the liver in all dose groups, after both 7 and 14
weeks. Histopathological examination of the spleen revealed increased
haemosiderosis at all dose levels except 80 ppm. In the liver, areas
of focal necrosis and/or fibrosis in the parenchyma, with or without
associated inflammatory cells, fibroblasts or pigment-laden
macrophages, were observed. At higher dose levels, necrotic and fatty
hepatocytes and brown pigment-laden Kupffer cells were found (Colley
et al 1981).
Rat
Groups of 5 male and 5 female rats were fed on a diet containing
diflubenzuron in concentrations of 0, 800, 4 000, 20 000 and
100 000 mg/kg during a four-week period. Behaviour, body weight, food
and water consumption were not clearly affected by the treatment.
There was a dose-related increase in met- and sulph-haemoglobin in the
blood in all treated groups, except for the methaemoglobin value for
the females of the 800 mg/kg dose group. Lower RBC, PCV and
haemoglobin values were observed in both sexes of the 100 000 mg/kg
dose group. Post-mortem examination revealed no changes attributable
to diflubenzuron. For both sexes, relative spleen weights in all test
groups differed significantly from control values. Relative liver
weights were increased in males or females at all dose levels, except
800 mg/kg (Palmer et al. 1977).
Groups of 10 male and 10 female Wistar rats received
diflubenzuron during 13 weeks. The concentrations in the diet were 0,
3.125, 12.5, 50 or 200 mg/kg feed. In addition two groups of 5 males
and 5 females received 0 or 200 mg/kg. These animals were sacrificed
after 6 weeks for clinico-chemical analysis. Behaviour, growth and
food intake were not affected by the treatment. At the highest dose
level, the PCV-value, the haemoglobin concentration and the number of
erythrocytes were decreased, whereas the latter was also lowered in
the 50 mg/kg group. Particularly in the males, the SGOT and SGPT
activity was increased in the highest dose group at the end of the
experiment. Higher testicular weights were recorded in the 200 mg/kg
group. At microscopic examination, a slight increase in the number of
small foci of necrotic parenchymal cells was observed, accompanied by
monocellular inflammatory cell infiltration in the liver of both males
and females of the 50 and 200 mg/kg groups (Kemp et al. 1963a, b).
Diflubenzuron was administered to Sprague-Dawley rats at dietary
levels of 0, 10 000 and 100 000 mg/kg feed for 9 weeks, followed by a
4-week withdrawal period. Each group consisted of 20 male and 20
female animals. After treatment for 9 weeks, group size was reduced to
10 males and 10 females. From week 7 onwards, male and female rats of
both groups showed pallor of extremities and eyes. During the
withdrawal period, no recovery was observed. During or at the end of
the treatment period lower values for red blood cell parameters,
formation of met- and sulph-haemoglobin, higher SGPT-values, and
heavier liver, adrenals and spleen weights were observed. The animals
produced less but more concentrated urine, and iron pigment in the
liver and spleen was also demonstrated. Minor enlargement of
centrolobular hepatocytes in some rats of the highest dose groups was
observed. After the withdrawal period, some recovery, especially of
effects on the liver and methaemoglobin induction, was observed
(Hunter et al. 1979).
Diflubenzuron was administered in the diet to male and female
Sprague-Dawley rats (40/sex/group) at dose levels of 160, 400, 2 000,
10 000 and 50 000 mg/kg feed. An additional group (90 male and 90
female animals) served as a control. No clear treatment-related
effects were noted with respect to mortality, clinical observations,
body weight gain and food consumption. A treatment-related significant
increase in met-haemoglobin was noted in all treated groups. Sulph-
haemoglobin values showed increases from the 2 000 mg/kg group
onwards. For females and males, a significant treatment-related
decrease in haemoglobin and erythrocyte count was observed at all dose
levels at the end of the study. An increase was noted in the
reticulocyte count in all dose groups, except 160 mg/kg, and the
number of Heinz bodies was higher in the 10 000 and 50 000 mg/kg
groups. Analysis of clinical chemistry values and urinalysis revealed
no apparent treatment-related trends. After 7 weeks, spleen weights
were increased in the females at all dose levels, but after 13 weeks
no effect was found at 160 mg/kg. With the exception of the lowest
dose level, all treated groups showed a higher liver weight. The
administration of diflubenzuron resulted in a dose-related increase of
incidence of chronic hepatitis and haemosiderosis of the liver. It was
also associated at all dose levels with haemosiderosis and congestion
of the spleen and mild erythroid hyperplasia of the bone marrow
(Burdock et al 1980b; Goodman 1980b).
Dog
Diflubenzuron was fed to groups of 3 male and 3 female beagle
dogs for 13 weeks at concentrations of 0, 10, 20, 40 and 160 mg/kg in
the diet. No effect of treatment on behaviour, body weight, food and
water consumption was observed. Elevated SAP and SGPT values were
recorded for some dogs receiving 40 or 160 mg diflubenzuron/kg feed.
After 6 weeks, methaemoglobin and other abnormal haemoglobin pigments
were demonstrated in dogs receiving 160 mg/kg. After 12 weeks of
administration, some recovery was observed. Organ weights, gross and
microscopic evaluation did not show treatment-related effects
(Chesterman et al 1974).
Sheep
A 13-week feeding study was carried out with 4 groups of 3 male
and 3 female sheep. The test compound was included in the diet in a
concentration of 0, 500, 2 500 and 10 000 mg diflubenzuron/kg. These
concentrations were fed to the animals in daily amounts of 0.6 during
the first 4 weeks, 0.8 during weeks 5 to 8 and 1.0 kg during the last
5 weeks. After 6 weeks, both the plasma and RBC-cholinesterase
activities were considered to be within normal limits. No treatment-
related effects were observed on food consumption, body weight gain,
haematological parameters and urinalysis. A significant increase in
sulph-haemoglobin was observed in all treated groups at 13 weeks.
After 4 and 8 weeks this effect was only obvious at higher dose
levels. There was also an indication of a treatment-related increase
in methaemoglobin levels. No other clinico-chemical parameters were
affected. The weight of the thyroid was not dose-relatedly decreased
at all dose groups. However, histopathological examination revealed no
abnormalities that could be related to the treatment (Ross et al
1977a,b).
Long-term studies
Mouse
Five groups of 52 male and 52 female CFLP mice were fed
diflubenzuron during 80 weeks. The concentrations in the diet were 0,
4, 8, 16 and 50 mg/kg feed. Behaviour, mortality, food and water
consumption and body weight were not affected by the treatment. The
changes noted on gross and histopathologic examination were common to
both treated and control animals. No treatment-related effects or
significant tumour incidences were found. Although the incidence of
lymphosarcomas in treated female mice, killed after 80 weeks, was
significantly increased (50% level, chi square test) the combined
incidence of lymphosarcomas in treated female mice, sacrificed during
the treatment period and at the termination of the experiment, was not
significantly different (Hunter et al. 1975; Batham and Offer 1977;
Offer 1977).
Rat
Five groups, each composed of 60 male and 60 female Wistar rats,
were fed diflubenzuron during 104 weeks. For the tumorigenicity study,
45 male and 45 female animals were used, whereas 15 male and 15 female
rats constituted the satellite group for the toxicity study. The
concentrations in the diet were 0, 10, 20, 40 and 160 mg/kg feed.
There was no clear treatment-related effect on behaviour, survival,
food consumption, water consumption, body weight gain, efficiency of
food utilization, blood chemistry and urinalysis. Significantly higher
met-haemoglobin levels in both males and females receiving 160 mg
diflubenzuron/kg feed were observed after 52 and 78 weeks. The other
haematological parameters were within normal limits. Organ weights,
gross pathological and microscopic examination of tissues, including
the liver, showed no compound-related effects. There was no indication
of an increase in the number of neoplastic lesions. A no-effect level
of 40 mg diflubenzuron/kg feed was observed (Hunter et al. 1976;
Colley and Offer 1977).
Special studies on met- and sulph-haemoglobin formation
Technical diflubenzuron was administered by gastric intubation to
groups of 10 mice daily for a period of 14 days. The dose levels were
0 (20 animals), 8, 40, 200, 1 000 and 5 000 mg/kg bw. Body weight
measurement and macroscopic evaluation did not reveal any effect of
the treatment. At dose levels of 5 000 and 1 000 mg/kg the percentages
of met-and sulph-haemoglobin and erythrocytes containing Heinz bodies
were increased. No effect could be observed on the met- and sulph-
haemoglobin and on the percentage of erythrocytes containing Heinz
bodies at 200, 40 and 8 mg/kg (Keet 1977b).
A similar experiment was carried out with 2 groups of 15 male
Wistar rats. The animals received doses during 8 consecutive days of
0 or 5 000 mg/kg bw, in 1% tragacanth. There was no effect on the body
weight or the number of Heinz bodies, whereas the bet- and sulph-
haemoglobin levels were only marginally increased from day 1 and 2
respectively (Keet 1977a).
Two groups of 14 male New Zealand White rabbits were fed 0 or
640 mg diflubenzuron/kg feed during 21 days. In the treated group, the
methaemoglobin level was increased from day 5, whereas higher sulph-
haemoglobin levels were observed within 5 h. In a second experiment
with 640 mg/kg feed, the methaemoglobin level was again significantly
increased. Recovery was observed 2 weeks after the treatment was
ceased (Keet 1977c).
Twenty-four male and 24 female cats were divided among five
treatment groups and one control group. They received 30, 70, 100, 300
and 1000 mg diflubenzuron/kg bw per os for 21 days, followed by a
14-day observation period. Sodium nitrite was administered as a
positive control. Diflubenzuron induced a dose-related elevation of
methaemoglobin in females at all dose levels. In males, only 30 and
70 mg/kg did not have a significant effect (maximal effect: 11.8%).
Recovery was slow. Sulph-haemoglobinemia and Heinz body formation were
observed in all treated groups. The haemoglobin concentration, number
of reticulocytes, and organ weights were within normal limits
(Schwartz and Borzelleca 1981).
Special studies on sexual development
Diflubenzuron was incorporated into feed and tested in chickens
for 13 weeks. Fat deposition was greatly increased in females. The
combs, wattles, feathers and voice of males remained undeveloped
throughout the study. Testosterone showed a dose-related decrease
(Smalley 1976 - summary only).
Diflubenzuron was fed to 4 groups of 384 male chickens of a
Hubbard broiler strain at dose levels of 0 (twice), 2.5 and 250 mg/kg
feed. After 28, 56 and 98 days, one third of the number of animals of
each group was killed. No effects were observed on mortality, body
weight, food intake, oestradiol levels in the plasma after 4, 7 and
14 weeks, organ weights, tibia weights and lengths and on gross or
microscopic examination. Only at the end of the study, testosterone
levels in serum were higher in treated groups in comparison to
controls. Both comb and wattle were more developed in the
diflubenzuron groups. However, these differences were not
statistically significant (Keet 1976b).
A parallel study with the same dose levels was carried out with
female broiler chickens. In both diflubenzuron groups, a dose-related
reduction was observed in feed consumption and body weight. Mortality
was increased only at the highest dose level (250 mg/kg feed). In
addition, a significantly increased incidence of leg abnormalities, a
reduced tibia length and relative liver weight were observed. No clear
effect on plasma testosterone was recorded. In the highest dose level,
a marginally decreased plasma oestradiol and reduced comb and wattle
development was observed (Ross et al. 1977c).
In an experiment with 2 groups of 225 female chickens, only one
dose level (250 mg/kg feed) was tested. Animals were sacrificed after
28, 49 and 98 days. The comb and wattle development was normal and no
evidence of treatment-related effects was found. Plasma oestradiol
concentrations were within normal limits (Ross et al. 1979).
Diflubenzuron was administered in the diet to one-day old Mallard
ducks, Leghorn chickens, Nicolas White turkeys and Ring-neck pheasants
for 90 days. The concentrations in the diets were 0, 0.25, 1.25, 2.5,
25 or 250 mg/kg feed. There was no clear treatment-related effect on
serum testosterone levels at days 21, 51 and 90. At the highest dose
level (the only one measured), testosterone levels were decreased in
turkeys and ducks after 42 days. Comb and wattles were not affected by
treatment. The effects on organ weights, including testes, are
difficult to evaluate (Reinert and Cannon 1976).
Young male Long-Evans rats were administered 0, 15, 150 and
300 mg diflubenzuron/kg bw daily during 14 to 96 days by gastric
intubation. The control group contained 15 animals and each test group
8. Diflubenzuron transiently decreased the levels of testosterone in
the plasma at the prepuberal age. No effects on body weight, weight of
testes, prostate, seminal vescicles and adrenals were observed.
Histological examination of the testicular tissues did not reveal any
induced changes (Paten and Santolucito 1980).
Five groups of 38 to 40 Sprague-Dawley rats received
diflubenzuron in their diet at dose levels of 0, 75, 150, 300 or
3 000 mg/kg feed. After 14, 28, 44 and 98 days, one fourth of the
animals were sacrificed. Diflubenzuron had no clear effect on body
weight gain or serum testosterone levels (Booth et al. 1980).
Four pairs of Holstein bull calves received 0 or 1.0 to 2.8 mg
diflubenzuron/kg bw. There was no significant effect of diflubenzuron
on body weight, sperm volume, sperm concentration, libido and serum
testosterone. However, the concentration of testosterone varied
considerably. Histopathological examination of tissues revealed no
significant difference between the treated and control bulls (Miller
et al 1979).
Special studies on mutagenicity
Diflubenzuron
A dominant lethal study was conducted in which 12 male mice per
test group were treated with a single i.p. injection of a suspension
of diflubenzuron in corn oil at levels of 1 000 and 2 000 mg/kg bw.
The control group received corn oil only. Sequential mating of each
male with 3 females per week was conducted for 6 consecutive weeks.
Mating ability of males and numbers of corpora lutea, implantation
sites, resorption sites (early embryonic deaths), and embryos for
treated animals were not different from those for control animals
(Arnold 1974).
Diflubenzuron was examined for mutagenic activity in a series of
in vitro microbiological assays, using the Salmonella typhimurium
strains TA 98, TA 100, TA 1537 and TA 1978. Each plate was run with
and without rat liver homogenate (S-9 mixture) prepared from Aroclor
1254 treated rats. Diflubenzuron, tested at dose levels ranging from
10 to 1 000 µg/plate, was not mutagenic in any of these assays (Bryant
1976).
In similar tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538,
and the Sacchromyces cerevisiae strain D 4. The compound was tested
both in the absence and in the presence of liver S-9 preparations from
Aroclor 1254-induced rats. Diflubenzuron tested at dose levels ranging
from 0.1 to 500 µg/plate did not demonstrate mutagenic activity in any
of these assays (Brusick and Weir 1977a).
In another series of tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535 and TA 1537.
Diflubenzuron, at levels of 10, 100 or 1 000 µg/plate, did not
significantly alter the spontaneous revertant frequency in the four
strains used, either with or without the metabolic activation system
from Aroclor 1254-induced rat liver (McGregor et al 1979).
An in vitro test with mouse lymphoma cells and a micronucleus
test in mice were carried out by the same author. In the in vitro
test with cultured cells, the forward mutation frequency of
TK+/- -> TK-/- in L 517 Y mouse lymphoma cells was tested.
Diflubenzuron at dose levels ranging from 1.2 to 300 µg/ml did not
increase the mutation frequency, either with or without the metabolic
activation system, in the micronucleus test, male mice were given 15,
150 or 1 500 mg/kg bw at 30 h and 6 h before necropsy. Diflubenzuron
did not significantly increase the frequency of micronucleated
erythrocytes in the bone marrow (McGregor et al. 1979).
In another series of tests, diflubenzuron, as well as its
metabolites (2,6-difluorobenzoic acid (DFBA, 4-chlorophenylurea (CPU),
4-chloroaniline), were negative in the S. typhimurium strains TA 98,
TA 100, TA 1535 and TA 1538. The results in the strain TA 1537 were
difficult to interpret (Seuferer et al 1979).
Diflubenzuron was evaluated in a cell transformation test. At
dose levels of 0.02 to 0.312 mg/ml, diflubenzuron did not induce
morphological transformation in BALB/3T3 cells in vitro (Brusick and
Weir 1977b).
Diflubenzuron was evaluated for its ability to induce unscheduled
DNA synthesis in human diploid WI-38 cells blocked in the G1 phase.
The compound was tested at dose levels of 50 to 1 000 µg/ml, both in
the absence and in the presence of liver S-9 preparations of uninduced
mice. Under the conditions of the assay, diflubenzuron did not induce
unscheduled DNA synthesis (Brusick and Weir 1977c).
Diflubenzuron was tested in a transplacental transformation assay
to investigate possible transformation of mammalian cells in culture.
Timed pregnant hamsters were injected intraperitoneally on day 10 of
gestation with solutions of diflubenzuron in dimethylsulphoxide at
dose levels of 10, 200 and 500 mg/kg bw. Three days after injection,
animals were sacrificed and foetal cell cultures were prepared.
Diflubenzuron did not induce morphological transformation or the
ability for growth of colonies. With known carcinogens (benzopyrene
and dimethylnitrosamine), a positive result was obtained (Quarles
et al 1980).
Metabolites
The metabolites 4-chlorophenylurea (CPU), 2,6-difluorobenzoic
acid (DFBA) and 4-chloroaniline were examined for mutagenic activity
in a series of in vitro microbial assays. A spot test at a dose
level of 1 000 µg per spot was carried out with S. typhimurium
strains TA 98, TA 100, TA 1535, TA 1537, TA 1538 and TA 1978, with and
without metabolic activation. A dose response test at dose levels of
10, 100, 500 and 1 000 µg was carried out with TA 98 and TA 100. The
test compounds did not demonstrate mutagenic activity in any of the
assays conducted, except for a weak effect with 500 and 1 000 µg
4-chloroaniline in the TA 98 strain after activation by liver S-9
preparation. At these dose levels, 4-chloroaniline caused a reduction
in the number of colonies, indicating a degree of toxicity to the
bacteria (Dorough 1977).
CPU was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae strain D 4.
The compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to
500 µg/plate, the test compound did not demonstrate mutagenic activity
in any of the assays conducted (Jagannath and Brusick 1977a).
CPU was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration
(312 µg/ml) employed in the concentration range. The other levels were
negative. The results were considered to be an indication of a weak
transforming activity at concentrations near to the level of
cytotoxicity (Matheson and Brusick 1978a).
CPU was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both in the absence and in the presence of liver S-9
preparations of Aroclor 1254-induced mice. CPU at dose levels ranging
from 6.25 to 400 µg/l did not induce unscheduled DNA synthesis
(Matheson et al 1978a).
DFBA was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537, TA 1538 and the S. cerevisiae strain D 4. The
compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to 500
µg/plate, the test compound did not demonstrate mutagenic activity in
any of the assays conducted (Jagannath and Brusick 1977b)
DFBA was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration employed
(2.5 mg/ml). The other levels were negative. These results were
considered to be an indication of weak transforming activity at
concentrations near to the level of cytotoxicity (Matheson and Brusick
1978b).
DFBA was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both with and without metabolic activation. The compound at
dose levels ranging from 75 to 500 µg/ml produced significant
increases in the level of unscheduled DNA synthesis in the presence of
mouse S-9 liver mixture. However, there was no dose response
relationship, 75 µg giving the highest increase and 500 µg the lowest
increase. According to the authors, the results appeared to be on the
plateau of a dose response effect. The compound was considered to be
active under these test conditions (Matheson and Brusick 1978c).
4-Chloroaniline was examined for mutagenic activity in a series
of in vitro microbial assays, using the S. typhimurium strains
TA 98, TA 100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae
strain D 4. The compound was tested with and without liver S-9
preparations from Aroclor 1254-induced rats. The test compound did not
demonstrate mutagenic activity in any of the assays conducted
(Jagannath and Brusick 1977c).
4-Chloroaniline was evaluated in a cell transformation test. At
dose levels ranging from 39 to 625 µg/ml the compound did not induce
morphological transformation in BALB/3T3 cells in vitro (Matheson
et al 1978b).
4-Chloroaniline was tested for its ability to induce unscheduled
DNA synthesis in human WI-38 cells blocked in the G1 phase. The
compound was tested both with and without metabolic activation. At
dose levels of 250 to 1 000 µg/ml, the compound did not induce
unscheduled DNA synthesis (Matheson et al 1978c).
Special studies on reproduction and teratogenicity
Diflubenzuron was fed to 3 groups of 20 male and 20 female rats
at dietary levels of 0, 1 000 and 10 000 mg/kg for one generation and
one litter. The animals were maintained on their respective diets for
60 days prior to mating. There were no clear effects on mating
performance, pregnancy rate, duration of gestation, litter size,
mortality, litter weight, type and distribution of abnormalities.
Diflubenzuron had, among others, a dose-related effect on haemoglobin
(reduced PCV, Hb, total red cells, increased met-haemoglobin, sulph-
haemoglobin, spleen weight, incidence of siderocytes in the spleen and
the occurrence of iron pigment containing Kupffer cells in the liver).
A dose-related effect on the liver was also shown by an increased
weight, SGPT activity and hepatocyte enlargement. Reduced blood
glucose concentrations were recorded in both treated groups. In the
highest dosed group, the offspring showed an increased liver and
spleen weight for both sexes. Microscopically, an increased incidence
of centrolobular hepatocyte enlargement was observed (Palmer et al
1978).
Diflubenzuron was administered in the diet to groups of 20 male
and 20 female rats at concentrations of 0, 10, 20, 40 and 160 mg/kg.
These diets were administered continuously throughout three
generations producing one litter each. As the mating performance was
low in the first generation, a second litter was bred. Parent animals
showed no signs of adverse effects related to treatment. Mating
performance, pregnancy rate and duration of gestation were not
affected. Also, total litter loss, litter size, litter and mean pup
weights, pup mortality and the incidence of abnormalities provided no
evidence of adverse treatment-related effects (Palmer and Hill 1975a).
Groups of 20 pregnant rats were administered diflubenzuron orally
by gavage at levels of 0, 1, 2 or 4 mg/kg bw during days 6 to 15 of
gestation. The parent animals showed no signs of reaction and no
mortalities occurred. Body weight gain and pregnancy rate were
unaffected by treatment. No effects were observed on the number of
viable young, implantations, resorptions and corpora lutea. The
pre-implantation loss, foetal loss, litter weight, foetal weight, the
incidence of major malformations, minor anomalies and skeletal
variants were not dose-relatedly affected (Palmer and Hill 1975b).
Four groups of 13 pregnant New Zealand White rabbits were
administered diflubenzuron orally from day 6 to 18 of gestation at
dose levels of 0, 1, 2 and 4 mg/kg bw. Foetuses were removed on day 29
of pregnancy. There was no effect of the treatment on behaviour, body
weight gain and pregnancy rate. No dose-related differences were
observed on the number of viable young, resorptions, foetal loss,
litter weight and mean foetal weight. The incidence of major
malformations and minor anomalies was not affected by treatment
(Palmer and Hill 1975c).
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller and Booth 1976).
Pregnant female mice were fed a diet containing 50 mg/kg of
diflubenzuron (partly 14C). Some of these mice were sacrificed at
day 17 from conception, the others were allowed to give birth to the
young. The lactating females were kept on treatment and allowed to
suckle their young for 13 days. No radioactivity was detected in the
embryos or young mice (Booth 1977).
RESIDUES IN FOOD
USE PATTERN
Diflubenzuron is a recently-introduced insecticide that
interferes in the deposition of chitin in the insect cuticle through
action on the enzyme chitin synthetase.
Pre-harvest treatments
Diflubenzuron is formulated as a wettable powder containing 25%
of the active ingredient (Dimilin WP-25), or as an oil dispersible
concentrate containing 450 g diflubenzuron per litre. Granular
formulations are also available. Recommended use rates are given with
pre-harvest intervals in Table 3.
Other uses
Diflubenzuron is recommended for the control of flies in animal
husbandry by topical applications to breeding sites and manure heaps.
A further application is on ornamental plants and in forests.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials on the main
crops treated. The dosages in these trials covered recommended and
higher rates. Treatments were made using W-25 wettable powder
formulation, which is the formulation recommended for food crops.
TABLE 3. Recommended uses and pre-harvest intervals for diflubenzuron
Use rate Pre-harvest
Crop Country (a.i.) interval (days)
Apple, pear Argentina 0.02% 60
Bulgaria 0.0375% -
France 0.01% 30
German Fed.Rep. 0.02% 28
Greece 0.0125-0.015%1 -
Israel 0.0125% -
Italy 0.0125-0.02% 45
The Netherlands 0.01% 28
Spain 0.01-0.015% 60
South Africa 0.02% -
Switzerland 0.02% 42
UK 150 g/ha 14
Yugoslavia 0.0125-0.02% 30
Brassica leafy
vegetables The Netherlands 0.01% 14
China(Taiwan prov.) 0.017% 22
UK 100 g/ha -
Yugoslavia 200 g/ha -
Cottonseed Egypt 75 g/ha -
Greece 0.01-0.025% -
USA 70 g/ha (6x) -
Mushroom Greece 1 g/m2 -
Korea 1 g/m2 -
The Netherlands 1 g/m2 -
Switzerland 1 g/m2 -
UK 1 g/m2 -
Soybean Brazil 50-75 g/ha 21
Colombia 75-125 g/ha -
USA 35-70 g/ha
Tomato UK 250 g/ha -
Citrus USA2 350 g/ha ?
1 for pears: 0.0125-0.03%;
2 registration applied for.
Apple
Residue data have been obtained from trials in several countries.
Residues on fruit at recommended rates up to 0.02% were usually well
below 1.0 mg/kg at two weeks after the last application. Only three
samples were shown to contain residues from 1.0 to 1.2 mg/kg. The
one sample that contained 3.7 mg/kg is considered to be atypical
(Table 4). In the UK, apples (cv Cox and Egremont Russet) treated with
diflubenzuron 2 weeks before harvest at a dosage rate of 250 g a.i./ha
were shown to be taint-free (Spencer-Jones 1979).
Pear
The residue pattern in pears is very similar to that in apples.
At two weeks after the last application at recommended dosages, the
residues were well below 1.0 mg/kg (Table 4).
Citrus
Residue data have been obtained from numerous trials on orange,
grapefruit and tangerine in the USA. The dosages in these trials
included the recommended rate and also 2x and 4x rates. Residues in
whole fruit were all well below 0.5 mg/kg 1 week after the last
application at the recommended dosage rate. At the 4x dosage rate, the
residue remained generally below 1.0 mg/kg (Table 5).
Examination of peel and pulp separately showed that residues were
exclusively found in the peel when the product was applied at the
recommended rate. Residues in the pulp were all below the detection
limit (0.05 mg/kg). At the 4x use rate, by far the major portion of
the residue was in the peel. Residues in the pulp were either below or
just above the detection limit of 0.05 mg/kg, the highest value found
being 0.07 mg/kg. (Table 6).
Further fractionation of orange and grapefruit showed that the
residue is mainly present in the oil fraction. Here, the residue
ranged from 9.5 to 20 mg/kg at the recommended use rate. At the
4x rate residues were as high as 50 mg/kg. Neither at the recommended
rate nor at the 4x rate could residues be detected in the juice of
both orange and grapefruit, the limit of detection being 0.05 mg/kg
(Table 7).
In several cases, the various citrus fractions were analysed for
residues of the metabolites 4-chlorophenylurea, 4-chloroaniline and
2,6-difluorobenzoic acid. In none of the samples was any residue
detected (limit of detection for all three compounds 0.05 mg/kg)
(Duphar 1975-81).
TABLE 4. Residues following supervised trials in apple and pear1
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Apple
Bramley UK 0.01 1 - 0.22 0.23 0.14
Cox O.P. 0.01 1 - 0.18 0.16 0.20
Egremont R 0.01 1 - 0.27 0.24 0.18
Golden D. Netherlands 0.01 1 - <0.032
Winston 0.01 1 - <0.03
Golden D Europe 0.01 3-5 15-59 0.15 0.16 0.23 0.30
-0.21 -0.34
J. Grieve Netherlands 0.0125 1 - 0.28 0.36 0.13
J. Grieve 0.0125 2 17 1.32 0.94 0.75 0.53
Stark Crimson Italy 0.0125 2 58 0.06
Golden D 0.0125 2 54 0.15
Stark Crimson 0.0125 3 58,34 0.12
Golden D 0.0125 3 58,33 0.05
Jonathan 0.0125 4 35,22,41 0.14
J. Grieve German Fed.Rep. 0.015 1 - 0.43 0.14 0.03 0.09 0.11
Golden D. Europe 0.015 1-8 14-52 0.34 0.78 0.34 0.12 0.23 <0.05
-1,2 -1.1 -0.83
Bramley UK 0.017 1 - 0.17 0.02 0.18 0.19 0.02
Cox O.P. 0.017 1 - 0.09 <0.01 0.22
Egremont R 0.017 1 - 0.17 0.03 0.20
Ida Red 0.017 1 - 0.12
Golden D 0.017 1 - 0.20
McIntosh 0.017 1 - 0.17
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Warner UK 0.017 1 - 0.16
Worcester 0.017 1 - 0.15
Newton 0.017 1 - 0.01
Golden D France 0.01875 3 33,31 0.41
Golden D 0.01875 3 30,27 0.46
Golden D 0.01875 3 29,35 0.43
Golden D 0.01875 4 21,21,28 0.62
Golden D 0.01875 4 20,20,30 3.8
J. Grieve Netherlands 0.02 1 - 0.29 0.21 0.18 0.15 0.13
Golden D 0.02 1 - 0.52 0.31 0.23 0.29
n.s. South Africa 0.02 1 - 0.833 0.32 0.29
n.s. 0.02 1 - 0.784 0.14 0.15 0.23
Cox O.P. Netherlands 0.02 1 - 0.095
S. Boskoop 0.02 1 - 0.146
Ingrid M. 0.02 1 - 0.08
Winston 0.02 1 - 0.097
Golden D 0.02 1 - 0.055
Cox O.P German Fed.Rep. 0.02 1 - 0.258 0.05a
Cox O.P 0.02 1 - 1.568 0.438
Golden D 0.02 1 - 0.738 0.288
Cox O.P. 0.02 2 22 0.538 0.26 0.258 0.19 0.14
Golden D 0.02 2 21 1.268 0.69 0.58 0.53 0.37
Cox O.P. 0.02 2 21 1.878 1.24 0.95 0.51 0.25
Golden D Netherlands 0.02 2 22 <0.03
Gravesteyn Italy 0.02 2 53 0.24
Cox O.P. German Fed.Rep. 0.029 3 21,28 0.84 0.92 0.76 0.72
Cox O.P. 0.029 3 22,28 0.54 0.42 0.40 0.26 0.47
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D 0.02 21,28 1.26 0.89 0.52 0.66 0.63
Golden D Italy 0.02 3 57,56 0.52
Golden D 0.02 3 10,68 0.57
Steymen 0.02 3 58,32 0.40
Golden D Netherlands 0.029 3 40,31 0.45 0.40 0.32 0.29 0.19 0.28
Cox O.P. 0.029 3 34,30 0.30 0.27 0.29 0.24
G. Parmane German Fed.Rep. 0.029 4 29,39,14 0.5210 0.04 0.26 0.30
n.s. South Africa 0.0211 4 28,26,38 1.24
Jonathan Italy 0.02 5 23,15,27,14 0.28
Golden D 0.02 5 34.30,14,28 0.87
Golden D 0.02 5 40,29,15,33 0.77
Golden D France 0.02 5 15,14,16,16 0.27
J. Grieve Netherlands 0.025 1 - 0.67 0.38 0.31
Star-King D Japan 0.025 1 <0.01 0.067
Red-ball 0.025 1 0.087 0.107
n.s. Italy 0.02512 2 62 0.197 0.2313
J. Grieve Netherlands 0.025 2 17 1.14 1.62 0.58 0.87
Golden D 0.025 2 15 0.426
St.Crimson Italy 0.025 2 58 0.10
Golden D 0.025 2 52 0.117
St.Crimson 0.025 3 58,34 0.30
Golden D 0.025 3 52-54,32-49 0.55 0.28
Star-King Japan 0.025 3 0.387
Red-ball 0.025 3 0.207
Jonathan Italy 0.025 4 35,22,41 0.18
Golden D France 0.025 5 28,14,21,21 1.0
Golden D 0.025 6 12-18 0.87
J. Grieve German Fed. Rep. 0.03 1 - 0.73 0.23 0.15 0.15 0.13
Golden D 0.03 1 - 1.0 0.67 0.44 0.43 0.31
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D Netherlands 0.03 1 - 0.056
Cox O.P. 0.03 1 - <0.03
Golden D 0.03 2 15 0.437
Golden D 0.03 2 15-19 0.34 0.32 0.326
n.s. South Africa 0.03 3 28,32 0.26
Golden D 0.03 8 14 2.20
n.s. 0.04 1 - 0.7815 0.38 0.37 0.25
n.s. 0.04 1 - 1.5516 1.25 0.70
Golden D Netherlands 0.04 1 - 0.0514
Winston 0.04 1 - <0.03
Golden D 0.04 2 22-43 0.077
Jonathan 0.04 2 26 <0.05
Gravesteyn Italy 0.04 2 53 0.41
Golden D 0.04 3 10-68 0.83 1.11
Alkmene German Fed. Rep. 0.0417 4 13,16,20 1.13 0.7418 0.63 0.65
Golden D Italy 0.04 5 14-40 1.437
Golden D France 0.04 6 12-18 1.40
Pear Europe 0.01 1-5 17-33 0.1 0.12 0.02
-0.04 -0.31 -0.25
South Africa 0.02 1-3 28-32 0.52 0.11 0.13 0.24
-0.04 -2.64 -1.30 -1.25 -1.31
1 Reference: Duphar 1975-81; 2 4 trials;
3 day 1: 0.63, day 2:0.83, day 6:0.50, day 7: 0.61; 4 day 1: 0.42, day 2: 0.78, day 4: 0.30;
5 average of 6 trials; 6 average of 3 trials;
7 average of 2 trials; 8 average of 2 samples;
9 last spray rate - 0.015; 10 day 0: 0.52, day 7: 0.44;
11 first spray rate-0.025; 12 last spray rate - 0.02;
13 average of 4 trials; 14 average of 5 trials;
15 day 1: 0.78, day 2: 0.11, day 4: 0.91; 16 day 1: 1.55, day 2: 0.72, day 6: 1.15, day 7: 1.39;
17 last spray rate - 0.03; 18 day 9: 0.74, day 14: 0.72.
TABLE 5. Residues following supervised trials in citrus, whole fruit, in Florida and Texas, USA1
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 1 PB 0.07 0.11 0.12 0.13 0.08 0.10
1 PB 0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Temple 1 PB 0.053
1 PB 0.063
Pineapple 1 PB <0.05
1 PB 0.053
Hamlin 1 PB 0.06
1 PB <0.05
Mars 1 PB 0.10
Temple 2 PB,S 0.084
2 PB,S 0.063
Pineapple 2 PB,S 0.093
2 PB,S 0.053
Hamlin 2 PB,S 0.123
2 PB,S <0.05
Mars 2 PB,S 0.16
Temple 3 PB,S,F 0.144 0.13 0.143
3 PB,S,F 0.173 0.13 0.11
Pineapple 3 PB,S,F 0.183 0.18 0.08
3 PB,S,F 0.133 0.10 0.07
Hamlin 3 PB,S,F 0.163 0.20 0.083 0.25
3 PB,S,F 0.10 0.05
Mars 0.35 3 PB,S,F 0.32
Hamlin-pineapple " 3 PB,S,F 0.27 0.29 0.30 0.36 0.21
3 PB,S,F 0.20 0.29 0.22 0.41 0.14
3 PB,S,F 0.05 0.20 0.10 0.11 0.21
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 1 PB 0.054
Marsh 1 PB <0.053
Seedless 1 PB <0.053
Ruby red 1 PB 0.063
1 PB 0.063
Pink 2 PB,S <0.054
Marsh seedless 2 PB,S 0.083
2 PB,S 0.063
Ruby Red 2 PB,S 0.123
Ruby Red 0.35 2 PB,S 0.123
Pink 3 PB,S,F 0.174 0.15 0.093 0.26 0.22
Marsh seedless 3 PB,S,F 0.123 0.073
3 PB,S,F 0.113 0.143
Ruby Red 3 PB,S,F 0.203
3 PB,S,F 0.133
Tangerine Nova 1 PB <0.05
1 PB <0.05
2 PB,S 0.12
2 PB,S 0.09
3 PB,S,F 0.07 0.05
3 PB,S,F 0.09 0.09
Orange Valencia 0.7 1 PB 0.223 0.133 0.163 0.143 0.083 0.153 <0.05
1 PB 0.10 0.11
Mars 1 PB 0.27
2 PB,S 0.28
3 PB,S,F 0.23
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1 PB 0.10
2 PB,S 0.203
3 PB,S,F 0.203
Valencia 1.4 1 PB 0.233 0.113 0.273 0.233 0.123 0.073
1 PB 0.07 0.05
Temple 1 PB <0.053
1 PB 0.07 0.05
Pineapple 1 PB <0.053
1 PB 0.053
Hamlin 1 PB <0.053
Orange Hamlin 1.4 1 PB 0.09
Mars 1 PB 0.18
Grapefruit Pink 1 PB <0.053
1 PB <0.05
Marsh seedless 1 PB <0.053
1 PB 0.343
Ruby Red 1 PB 0.273
Tangerine Nova 1 PB <0.05
1 PB <0.05
Orange Temple 1.4 2 PB,S 0.124
2 PB,S 0.15
Pineapple 2 PB,S 0.173
2 PB,S 0.383
Hamlin 2 PB,S <0.053
Mars 2 PB,S 0.305
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 2 PB,S 1.10 0.26 0.243
2 PB,S 0.23
Marsh seedless 2 PB,S 0.243
2 PB,S 0.353
Ruby Red 2 PB,S 0.233
Tangerine Nova 2 PB,S 0.15
2 PB,S 0.20
Orange Temple 3 PB,S,F 0.384 0.473 0.233 0.91
3 PB,S,F 0.46 0.59
Pineapple 3 PB,S,F 0.253 0.35 0.06
3 PB,S,F 0.573 0.49 0.15
Hamlin 3 PB,S,F 0.713 0.74 0.103 0.44
3 PB,S,F 0.23 0.26
Mars 3 PB,S,F 0.10
Grapefruit Pink 3 PB,S,F 0.393 0.81 0.85 1.11 0.48
3 PB,S,F 0.34 0.65
Marsh seedless 3 PB,S,F 0.533 0.273
3 PB,S,F 0.303 0.613
Ruby Red 3 PB,S,F 0.643
Tangerine Nova 3 PB,S,F 0.16 0.28
3 PB,S,F 0.31 0.24
1 Referenne: Duphar 1975-81;
2 PB = Post Bloom, S = Summer,F = Fall;
3 average of 2 trials;
4 average of 3 Trials;
5 day 70: 0.53 in one trial.
TABLE 6. Residues following supervised trials in citrus, peel and pulp, in Florida and Texas, USA1
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 12 peel 0.10
pulp <0.05
Temple 33 peel 0.574
pulp <0.054
peel 0.21
pulp <0.05
Pineapple 3 peel 0.46
pulp <0.05
3 peel 0.26
pulp <0.05
Hamlin 3 peel 0.40
pulp <0.05
Mars 3 peel 0.50
pulp <0.05
Grapefruit Pink 3 peel 0.58
pulp <0.05
Marsh seedless 3 peel 0.41
pulp <0.05
3 peel 0.84
pulp <0.05
Ruby Red 3 peel 0.614
pulp <0.05
0.355 3 peel 0.934
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 1,4 1 peel 0.09 1.89 1.65 1.83 0.88 0.75
pulp 0.07 0.07 0.07 <0.07 <0.05 <0.05
peel 0.78 0.50 0.70 0.91 0.43 0.15
pulp <0.05 <0.05 <0.05 <0.05 <0.05 (0.05
1 peel 0.29 <0.05
pulp <0.05 <0.05
Orange Temple 1.4 2 peel 0.07
pulp 0.05
Hamlin 2 peel 0.38
pulp <0.05
Temple 3 peel 1.40 2.00
pulp <0.05 <0.05
3 peel 1.30 0.61
pulp <0.05 <0.05
peel 1.20
pulp (0.05
Pineapple 3 peel 0.93 1.8
pulp <0.05 <0.05
3 peel 1.70 0.28
pulp <0.05 <0.05
Hamlin 3 peel 0.20 2.70
pulp <0.05 <0.05
1.45 3 peel 1.3
pulp <0.05
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1.4 1 peel 0.88c
pulp 0.05c
2 peel 0.40c
pulp 0.05
3 peel 0.94
pulp 0.05
Pink 3 peel 2.10
pulp 0.05
3 peel 1.90
pulp 0.07
Marsh seedless 3 peel 0.66
pulp 0.07
3 peel 1.0
pulp 0.05
Tangerine Nova 3 peel 0.18
pulp 0.05
3 peel 0.75
pulp 0.05
1 Reference: Duphar 1975-81;
2 1 Spray Post Bloom;
3 3 sprays: Post Bloom, Summer and Fall;
4 average of 2 trials;
5 plus 0.25% oil.
TABLE 7. Residues following supervised trials in citrus fractions, in Florida, USA1
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Orange (Temple) Whole fruit(unwashed) 0.35 1.4 3 0.13 0.35 14
Whole fruit (washed) 0.09 0.26 14
Chopped peel 0.09 0.13 14
Frits 0.11 0.84 14
Finisher pulp <0.05 <0.05 14
Dried citrus pulp 0.09 0.05 14
Fruit Juice <0.05 <0.05 14
Oil 13.0 28.0 14
Pressed liquor <0.05 0.09 14
Molasses <0.05 0.06 14
Prewash water 0.01 0.02 14
Afterwash water 0.03 0.07 14
Orange (Eamlin) Whole fruit(unwashed) 4 0.28 0.34 2t
Whole fruit(washed) 0.14 0.33 21
Chopped peel 0.06 0.11 21
Frits 0.10 0.55 21
Finisher pulp <0.05 <0.05 21
Dried citrus pulp <0.05 0.66 21
Fruit juice <0.05 <0.05 21
Oil 20.0 50.0 21
Pressed liquor <0.05 0.08 21
Molasses <0.05 0.12 21
Prewash water 0.03 0.06 21
Afterwash water 0.05 0.22 21
TABLE 7. (con't)
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Grapefruit (Pink) Whole fruit (unwashed) 0.35 3 0.09 21
Whole fruit(washed) <0.05 21
Chopped peel 0.08 21
Frits 0.19 21
Finisher pulp <0.05 21
Dried citrus pulp 0.10 21
Fruit juice <0.05 21
Oil 9.50 21
Pressed liquor <0.05 21
Molasses <0.05 21
Prewash water 0.01 21
Afterwash water 0.04 21
Whole fruit (unwashed) 1.4 3 0.09 21
Whole fruit (washed) 0.17 21
Chopped peel 0.11 21
Frits 0.50 21
Finisher pDlp <0.05 21
Dried citrus pulp 0.36 21
Fruit juice <0.05 21
Oil 23.0 21
Pressed liquor <0.05 21
Molasses 0.17 21
Prewash water 0.03 21
Afterwash water 0.17 21
1 Reference: Duphar 1975-81.
In taste tests with orange juice, no off-flavour was detected
which might have been caused by DIMILIN treatment (Braddock 1976b,
1977). Also in the case of grapefruit juice, no adverse effects on
flavour were detected (Braddock 1976a).
Soybean
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended use rate and also much
higher rates. Residues in the soybean seed were generally below the
detection limit, which was 0.05 mg/kg. Only one sample contained a
residue just above this limit, i.e. 0.06 mg/kg. Also at higher rates,
residues remained very low, the highest value found being 0.16 mg/kg.
Fractionation of seed containing <0.05 mg/kg residue did not result
in a detectable residue in any of the fractions. As could be expected
from the foliar stability of diflubenzuron, soybean foliage did
contain residues, the level of which declined with time (Table 8).
In nine trials, rotational crops were grown in fields on which
soybean had been treated with diflubenzuron at both the recommended
and higher rates. In none of the trials could residues be detected
(limit of detection 0.05 mg/kg) in the rotational crops, including
turnips, collards, rye, oats and mustard green. In the samples
analysed for 4-chlorophenylurea, no residue could be detected (limit
of detection 0.05 mg/kg) (Duphar 1975-81).
Residues in soil of soybean fields treated with diflubenzuron
were usually below the limit of detection (0.05 mg/kg), both for the
parent compound and the metabolite 4-chlorophenylurea (CPU).
Diflubenzuron residues never exceeded 0.3 mg/kg and were exclusively
found in the top 7.6 cm of soil. CPU residues did not exceed 0.5 mg/kg
and in only one case could any residue be detected below the top
7.6 cm of soil (Duphar 1975-81).
Cotton
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended rate and also higher ones.
Usually, several applications were made, up to 16 per growing season.
Residues in the cotton seed were generally below the limit of
detection (0.05 mg/kg) (Table 9). Only a few samples contained a
residue slightly above the detection limit, with one high value of
0.17 mg/kg. Fractionation of cotton seed did not result in a
detectable residue of any of the fractions.
In seven trials, rotational crops were grown in fields on which
cotton had been treated with diflubenzuron 15 times during the growing
season either at a rate of 0.067 or 0.280 kg a.i./ha. In none of the
trials could residues of either diflubenzuron or 4-chlorophenylurea
TABLE 8. Residues following supervised trials in soybean, USA1
Application Residues (mg/kg) at intervals (days)
Crop part after application
Rate (a.i.) No.
(kg/ha) 0-7 8-14 15-21 22-28 29-42 43-56 <56
0.034 1-2 <0.05 <0.05 <0.05 <0.05 <0.05
Seed - 0.56 -0.13 -0.16 -0.07 -0.07
Foliage Bragg 0.034 1 0.86 0.49 0.25
Lee 0.034 1 1.10 0.59 0.38
Ranson 0.034 1 2.25 1.40 1.65 0.82
n.s. 0.034 1 0.25
Bragg 0.034 2 0.462 2.6 0.26 0.63
Lee 68 0.034 2 2.1 0.58
Bragg 0.067 1 1.8 0.29 0.33 0.14
Calland 0.067 1 0.16
n.s. 0.280 1 1.6
n.s. 0.280 2 0.23
0.140 1
Seed n.s. <0.05
Hulls <0.05
Meal <0.05
Crude oil <0.05
1 Reference: Duphar 1975-81; 2 Average of 2 trials.
TABLE 9. Residues following supervised trials in cottonseed, USA1
Application Residues (mg/kg) at intervals (days)
after application
Crop part Variety Rate (a.i.) No.
kg/ha 0-7 8-14 15-21 22-28 29-42 43-56 <56
Seed 0.033 1- <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
-0.56 15 -0.07 0.09 -0.05 -0.07 -0.17
Hulls DPL-16 0.140 8 <0.05
Oil <0.05
Meal <0.05
Seed hulls Stoneville 213 0.280 9 <0.05
Meal <0.05
Oil <0.05
Soapstock <0.05
1 Reference: Duphar 1975-81
(limit of detection for both compounds 0.05 mg/kg) be detected in
the rotational crops, including collards, wheat and radish (Duphar
1975-81).
Mushroom
This crop shows a residue pattern different from all the others.
This is undoubtedly due to the different way in which it is grown. The
intimate contact of the mushrooms with the medium in which they are
grown enables the uptake of the metabolite 2,6-difluorobenzoic acid
especially (see also "Fate of residues"). At the recommended dosage
rate of 1 g a.i./m2, the diflubenzuron residue in mushrooms is
generally below 0.1 mg/kg, only one sample contained 0.11 mg/kg.
Also the residue level of 4-chlorophenylurea is very low,
all residues being below 0.1 mg/kg. However, the residues of
2,6-difluorobenzoic acid were considerably higher. At the recommended
use rate they all remain below 1 mg/kg. At higher dosage rates,
somewhat higher residues are found, the highest value being 1.63 mg/kg
at a dosage of 4 g/m2, applied twice. (Table 10).
FATE OF RESIDUES
General comments
The metabolism of diflubenzuron was studied and it was
established that the compound degraded along different lines, as shown
in Figure 2. Breakdown occurred predominantly along line 1, resulting
in 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU). A
minor degradation takes place along lines 2 and 3, resulting in
4-chloroaniline (PCA) and CO2.
 In plants
The fate of radio-labelled diflubenzuron (14C and 3H) has been
studied on corn, soybean, cabbage and apple in the greenhouse after
foliar application. No significant degradation or translocation was
observed for 16 weeks after application (Nimmo and De Wilde 1974;
Nimmo et al 1978). Similar results were obtained on pine needles
(Nimmo and De Wilde 1980).
TABLE 10. Residues following supervised trials in mushrooms1
Application Interval Residues (mg/kg) at intervals (days) after last application
Country between
Rate applications diflubenzuron CPU
(g a.i./m2) No. (days) 0-21 22-28 29-35 36-43 >43 0-21 22-28 29-35 36-43 >43
Netherlands 0.5 2 12 0.04 <0.01 <0.01 <0.01 <0.01 0.02 0.01 0.02 0.02 0.02
1.0 1 2 0.04 0.02 0.05 0.02 0.02 0.02 0.03 0.06 0.04 0.04
1.0 2 3 0.06 0.03 0.02 0.04 <0.11 <0.02 0.02 0.04 0.03 0.03
2.0 2 2 0.05 0.05 0.06 0.04 0.05 <0.02 0.02 0.07 0.06 0.06
4.0 2 2 0.11 0.08 0.10 0.06 0.07 <0.02 0.03 0.07 0.07 0.04
UK 10 mg/kg 1 <0.03 <0.03 <0.01 <0.01
30 mg/kg 1 <0.03 <0.03 <0.01 <0.01
100 mg/kg 1 <0.03 <0.03 <0.01 <0.01
Residues (mg/kg) at intervals (days)
Application Interval after last application
Country between
Rate applications DFBA
(g a.i./m) No. (days) 0-21 22-28 23-35 36-43 <43
Netherlands 0.5 2 12 0.27 <0.01 0.24 0.16 0.14
1.0 1 2 0.45 0.16 0.38 0.36 0.43
1.0 2 3 0.40 0.90 0.59 0.66 0.31
2.0 2 2 0.53 1.42 0.94 0.60 0.95
4.0 2 2 1.2 1.57 1.63 1.16 1.2
UK 10 mg/kg 1 0.19 0.31
30 mg/kg 1 0.44 0.60
100 mg/kg 1 0.50 0.80
1 Reference: Duphar 1975-81;
2 Average of 2 trials;
3 average of 4 trials.
Also under greenhouse conditions, diflubenzuron (14C) was
applied to cotton plants. Absorption, translocation and metabolism
were not significant over the trial period of 48 days (Mansager
et al 1979).
Under field conditions, it was demonstrated that there is very
little absorption or degradation of 14C-diflubenzuron on cotton
foliage (Bull and Ivie 1978).
The fate of diflubenzuron was also studied following application
to soybeans under field conditions. Again, it was found that there is
no significant absorption, translocation or metabolism of
diflubenzuron (Gustafson and Wargo 1976).
When cotton plants with diflubenzuron residues are incorporated
into the soil in the fall, there is little degradation of
diflubenzuron during the winter. However, with the onset of higher
temperatures, the residues declined rapidly (Bull and Ivie 1978).
This, probably, is caused by an increased accessibility of the residue
as a result of the progressive decay of the plant material with which
it was associated (Bull 1980).
Similarly, the degradation of diflubenzuron residues on oak leaf
litter is a rather slow process, the half life being roughly 6 to 9
months (Willems 1981),
Also in other organic substrates, the degradation of
diflubenzuron is considerably slower than in soil. This has been
demonstrated in chicken manure (half life time ca. 4 months), calf
manure (half life time ca. 6 months) and in a mushroom growth medium,
consisting mainly of horse and chicken manure (30-50% degradation in
one month). In these substrates, as in soil, the main metabolites are
2,6-difluorobenzoic acid and 4-chlorophenylurea (Nimmo and De Wilde
1977 a, b).
In animals
The metabolic fate of diflubenzuron has been studied in a variety
of vertebrate and invertebrate species (See section on "Biochemical
aspects").
There seems to be conclusive evidence that diflubenzuron is not
degraded within the digestive tract of mammals to any significant
degree. The portion of diflubenzuron that is absorbed from the
digestive tract is strongly dose-related. After absorption,
diflubenzuron is completely metabolized before excretion.
Metabolic pathways of diflubenzuron in insects seem to be
essentially the same as in mammals (Chang and Stokes 1979; Ivie and
Wright 1978; Chang 1978; Pimprikar 1977; Bull and Ivie 1980).
In soil
The fate of diflubenzuron has been studied in seven agricultural
soils and three hydro-soils, including the soil types recommended by
the US Environmental Protection Agency and the German Biologische
Bundes-Anstalt. In these studies, the compound was mixed through the
soil at a concentration of 1 mg/kg. From the results, the following
conclusions could be drawn:
a) The rate of degradation is strongly dependent on the
particle size of the diflubenzuron. At an average particle
size of approximately 10 µm, the half life in various soils
ranged from 8 to 16 weeks, whereas at a mean particle size
of about 2 µm the half life was 0.5 to 1 week.
b) The half life in different types of soil varied only
slightly.
c) The metabolic pathways of diflubenzuron in soil are
summarized in Figure 3.
The main metabolic pathway (over 90%) is hydrolysis, which
produces 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU).
A very minor pathway is hydrolysis of the other carbon-nitrogen bond,
producing 4-chloroaniline (PCA) and 2,6-difluorobenzamide, which is
rapidly converted into the corresponding acid, DFBA. DFBA is rapidly
degraded further, the half life being approximately 4 weeks. The first
step is a decarboxylation, followed by ring degradation. CPU was found
to be converted into bound residues with a half life of 5 to 10 weeks.
In the bound residue, after 2 months, both CPU and PCA are present in
roughly equal amounts. As PCA is generated directly from diflubenzuron
in only small amounts, there must be degradation of CPU into PCA. Free
PCA was not found in these studies (Nimmo and De Wilde 1975a; Verloop
and Ferrell 1977). This can be explained by the rapid binding to the
soil, found for anilines in general and PCA in particular (Hsu and
Bartha 1974; Moreale and Van Bladel 1976; Bollag et al 1978).
In a sandy loam soil, it was demonstrated that the degradation of
diflubenzuron is of a microbiological nature: in an experiment with
normal soil, after 4 weeks only 2% of the initial amount of
diflubenzuron was still present, whereas in a steam-sterilized soil,
94% of the diflubenzuron was unchanged (Nimmo and De Wilde 1975a).
Studies in the laboratory (Helling 1975; Rieck 1975; Bull and
Shaver 1980) and in the field (Danhaus et al 1976; Bull and Ivie
1978) have shown that diflubenzuron has a very low mobility in the
soil. Under field conditions, by far the major portion remains in the
top 7.5 cm of soil.
In plants
The fate of radio-labelled diflubenzuron (14C and 3H) has been
studied on corn, soybean, cabbage and apple in the greenhouse after
foliar application. No significant degradation or translocation was
observed for 16 weeks after application (Nimmo and De Wilde 1974;
Nimmo et al 1978). Similar results were obtained on pine needles
(Nimmo and De Wilde 1980).
TABLE 10. Residues following supervised trials in mushrooms1
Application Interval Residues (mg/kg) at intervals (days) after last application
Country between
Rate applications diflubenzuron CPU
(g a.i./m2) No. (days) 0-21 22-28 29-35 36-43 >43 0-21 22-28 29-35 36-43 >43
Netherlands 0.5 2 12 0.04 <0.01 <0.01 <0.01 <0.01 0.02 0.01 0.02 0.02 0.02
1.0 1 2 0.04 0.02 0.05 0.02 0.02 0.02 0.03 0.06 0.04 0.04
1.0 2 3 0.06 0.03 0.02 0.04 <0.11 <0.02 0.02 0.04 0.03 0.03
2.0 2 2 0.05 0.05 0.06 0.04 0.05 <0.02 0.02 0.07 0.06 0.06
4.0 2 2 0.11 0.08 0.10 0.06 0.07 <0.02 0.03 0.07 0.07 0.04
UK 10 mg/kg 1 <0.03 <0.03 <0.01 <0.01
30 mg/kg 1 <0.03 <0.03 <0.01 <0.01
100 mg/kg 1 <0.03 <0.03 <0.01 <0.01
Residues (mg/kg) at intervals (days)
Application Interval after last application
Country between
Rate applications DFBA
(g a.i./m) No. (days) 0-21 22-28 23-35 36-43 <43
Netherlands 0.5 2 12 0.27 <0.01 0.24 0.16 0.14
1.0 1 2 0.45 0.16 0.38 0.36 0.43
1.0 2 3 0.40 0.90 0.59 0.66 0.31
2.0 2 2 0.53 1.42 0.94 0.60 0.95
4.0 2 2 1.2 1.57 1.63 1.16 1.2
UK 10 mg/kg 1 0.19 0.31
30 mg/kg 1 0.44 0.60
100 mg/kg 1 0.50 0.80
1 Reference: Duphar 1975-81;
2 Average of 2 trials;
3 average of 4 trials.
Also under greenhouse conditions, diflubenzuron (14C) was
applied to cotton plants. Absorption, translocation and metabolism
were not significant over the trial period of 48 days (Mansager
et al 1979).
Under field conditions, it was demonstrated that there is very
little absorption or degradation of 14C-diflubenzuron on cotton
foliage (Bull and Ivie 1978).
The fate of diflubenzuron was also studied following application
to soybeans under field conditions. Again, it was found that there is
no significant absorption, translocation or metabolism of
diflubenzuron (Gustafson and Wargo 1976).
When cotton plants with diflubenzuron residues are incorporated
into the soil in the fall, there is little degradation of
diflubenzuron during the winter. However, with the onset of higher
temperatures, the residues declined rapidly (Bull and Ivie 1978).
This, probably, is caused by an increased accessibility of the residue
as a result of the progressive decay of the plant material with which
it was associated (Bull 1980).
Similarly, the degradation of diflubenzuron residues on oak leaf
litter is a rather slow process, the half life being roughly 6 to 9
months (Willems 1981),
Also in other organic substrates, the degradation of
diflubenzuron is considerably slower than in soil. This has been
demonstrated in chicken manure (half life time ca. 4 months), calf
manure (half life time ca. 6 months) and in a mushroom growth medium,
consisting mainly of horse and chicken manure (30-50% degradation in
one month). In these substrates, as in soil, the main metabolites are
2,6-difluorobenzoic acid and 4-chlorophenylurea (Nimmo and De Wilde
1977 a, b).
In animals
The metabolic fate of diflubenzuron has been studied in a variety
of vertebrate and invertebrate species (See section on "Biochemical
aspects").
There seems to be conclusive evidence that diflubenzuron is not
degraded within the digestive tract of mammals to any significant
degree. The portion of diflubenzuron that is absorbed from the
digestive tract is strongly dose-related. After absorption,
diflubenzuron is completely metabolized before excretion.
Metabolic pathways of diflubenzuron in insects seem to be
essentially the same as in mammals (Chang and Stokes 1979; Ivie and
Wright 1978; Chang 1978; Pimprikar 1977; Bull and Ivie 1980).
In soil
The fate of diflubenzuron has been studied in seven agricultural
soils and three hydro-soils, including the soil types recommended by
the US Environmental Protection Agency and the German Biologische
Bundes-Anstalt. In these studies, the compound was mixed through the
soil at a concentration of 1 mg/kg. From the results, the following
conclusions could be drawn:
a) The rate of degradation is strongly dependent on the
particle size of the diflubenzuron. At an average particle
size of approximately 10 µm, the half life in various soils
ranged from 8 to 16 weeks, whereas at a mean particle size
of about 2 µm the half life was 0.5 to 1 week.
b) The half life in different types of soil varied only
slightly.
c) The metabolic pathways of diflubenzuron in soil are
summarized in Figure 3.
The main metabolic pathway (over 90%) is hydrolysis, which
produces 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU).
A very minor pathway is hydrolysis of the other carbon-nitrogen bond,
producing 4-chloroaniline (PCA) and 2,6-difluorobenzamide, which is
rapidly converted into the corresponding acid, DFBA. DFBA is rapidly
degraded further, the half life being approximately 4 weeks. The first
step is a decarboxylation, followed by ring degradation. CPU was found
to be converted into bound residues with a half life of 5 to 10 weeks.
In the bound residue, after 2 months, both CPU and PCA are present in
roughly equal amounts. As PCA is generated directly from diflubenzuron
in only small amounts, there must be degradation of CPU into PCA. Free
PCA was not found in these studies (Nimmo and De Wilde 1975a; Verloop
and Ferrell 1977). This can be explained by the rapid binding to the
soil, found for anilines in general and PCA in particular (Hsu and
Bartha 1974; Moreale and Van Bladel 1976; Bollag et al 1978).
In a sandy loam soil, it was demonstrated that the degradation of
diflubenzuron is of a microbiological nature: in an experiment with
normal soil, after 4 weeks only 2% of the initial amount of
diflubenzuron was still present, whereas in a steam-sterilized soil,
94% of the diflubenzuron was unchanged (Nimmo and De Wilde 1975a).
Studies in the laboratory (Helling 1975; Rieck 1975; Bull and
Shaver 1980) and in the field (Danhaus et al 1976; Bull and Ivie
1978) have shown that diflubenzuron has a very low mobility in the
soil. Under field conditions, by far the major portion remains in the
top 7.5 cm of soil.
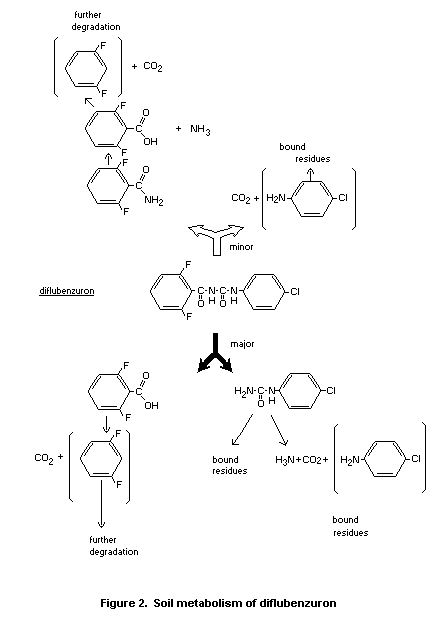 In model experiments, in which plants were grown on nutrient
solutions, it was shown that the soil metabolite 4-chlorphenylurea was
rapidly taken up and transported by tomatoes and broad beans, with
little biotransformation (Van den Berg 1978a).
In similar experiments, it was found that the other main soil
metabolite, 2,6-difluorobenzoic acid, is rapidly decarboxylated under
the influence of tomato roots, with very little uptake and
transportation (Van den Berg 1978b).
Under laboratory conditions, seedlings of various crops,
transplanted into soil treated with radioactive diflubenzuron, took up
only small amounts of radioactivity (Nimmo and De Wilde 1976a,
Mansager et al. 1979).
Also, when diflubenzuron was applied to soil in which the
seedlings were already present, the uptake by wheat and rice was
low, the residue in the leaves amounting to up to 0.5 mg/kg of
diflubenzuron equivalents. This residue consisted mainly of
4-chlorophenylurea and of polar conjugates. Very low levels of
residues were found in the wheat grain (Nimmo and De Wilde 1976b).
The rotational crop uptake of 14C residues following soil
application of diflubenzuron was studied under field conditions. The
experimental plots received two dosages of 66 g a.i./ha with an
interval of 15 days. Wheat, onion and cabbage were planted 2 months
after the last application. Samples of foliage were collected 2.5
months after planting. Radioactivity in plant tissue was below the
level of 0.01 mg/kg of diflubenzuron equivalents (Danhaus and Sieck
1976).
In another field study, 14C-diflubenzuron was applied to cotton
during the 1976 cotton season. One plot received 6 applications
(6A-plot) of 70 g a.i./ha, a second plot received 10 applications
(10A-plot) of this dosage. Radioactive cotton plant residues were
incorporated into the soil in November 1976. During the spring
of 1977, rotational crops were planted in this soil. Levels of
radioactive residues in these crops were generally very low. The 10A
plot gave somewhat higher residues than the 6A plot. The radioactivity
in all cases was below the level that would correspond to 0.05 mg/kg
of diflubenzuron (Bull and Ivie 1978).
A special case of uptake of diflubenzuron and its metabolites is
found in growing mushrooms in a diflubenzuron-treated medium. The rate
of degradation of diflubenzuron in this medium is much slower than in
soil. However, the metabolic pathway is the same one and the main
metabolites are 4-chlorophenylurea (CPU) and 2,6-difluorobenzoic acid
(DFBA). Both metabolites are taken up by the mushrooms. From a medium
treated with 2 g/m2 of radioactive diflubenzuron, the residues in
mushrooms reached levels of 0.1 to 0.6 mg/kg of CPU and 1 to 3 mg/kg
of DFBA (Nimmo and De Wilde 1977a).
Degradation in water
The degradation of diflubenzuron was studied at 20°C in sterile
water at various pH levels. There appears to be little degradation
under neutral or acidic conditions. However, at higher pH levels
there is a pH-dependent rate of degradation; at pH 9 the half life
is about 6 weeks and at pH 12 the half life was about 1.5 weeks.
The main degradation products were 2,6-difluorobenzoic acid and
4-chlorophenylurea (Nimmo and De Wilde 1975b).
Similar results were found with dilute solutions in distilled
water kept at 36°C. The degradation followed the same pathway; the
rate of degradation was again strongly pH dependent; the half life
times were shorter than at 20°C. Under these conditions, no
p-chloroaniline could be detected as a degradation product of
diflubenzuron (Ivie et al. 1980).
Heat catalysed degradation of diflubenzuron in an aqueous medium
is far more complex, and several degradation products are found that
are not formed, or hardly formed, under moderate conditions. One of
these products arises through expulsion of HF from the diflubenzuron
molecule, with concomittant cyclization:
In model experiments, in which plants were grown on nutrient
solutions, it was shown that the soil metabolite 4-chlorphenylurea was
rapidly taken up and transported by tomatoes and broad beans, with
little biotransformation (Van den Berg 1978a).
In similar experiments, it was found that the other main soil
metabolite, 2,6-difluorobenzoic acid, is rapidly decarboxylated under
the influence of tomato roots, with very little uptake and
transportation (Van den Berg 1978b).
Under laboratory conditions, seedlings of various crops,
transplanted into soil treated with radioactive diflubenzuron, took up
only small amounts of radioactivity (Nimmo and De Wilde 1976a,
Mansager et al. 1979).
Also, when diflubenzuron was applied to soil in which the
seedlings were already present, the uptake by wheat and rice was
low, the residue in the leaves amounting to up to 0.5 mg/kg of
diflubenzuron equivalents. This residue consisted mainly of
4-chlorophenylurea and of polar conjugates. Very low levels of
residues were found in the wheat grain (Nimmo and De Wilde 1976b).
The rotational crop uptake of 14C residues following soil
application of diflubenzuron was studied under field conditions. The
experimental plots received two dosages of 66 g a.i./ha with an
interval of 15 days. Wheat, onion and cabbage were planted 2 months
after the last application. Samples of foliage were collected 2.5
months after planting. Radioactivity in plant tissue was below the
level of 0.01 mg/kg of diflubenzuron equivalents (Danhaus and Sieck
1976).
In another field study, 14C-diflubenzuron was applied to cotton
during the 1976 cotton season. One plot received 6 applications
(6A-plot) of 70 g a.i./ha, a second plot received 10 applications
(10A-plot) of this dosage. Radioactive cotton plant residues were
incorporated into the soil in November 1976. During the spring
of 1977, rotational crops were planted in this soil. Levels of
radioactive residues in these crops were generally very low. The 10A
plot gave somewhat higher residues than the 6A plot. The radioactivity
in all cases was below the level that would correspond to 0.05 mg/kg
of diflubenzuron (Bull and Ivie 1978).
A special case of uptake of diflubenzuron and its metabolites is
found in growing mushrooms in a diflubenzuron-treated medium. The rate
of degradation of diflubenzuron in this medium is much slower than in
soil. However, the metabolic pathway is the same one and the main
metabolites are 4-chlorophenylurea (CPU) and 2,6-difluorobenzoic acid
(DFBA). Both metabolites are taken up by the mushrooms. From a medium
treated with 2 g/m2 of radioactive diflubenzuron, the residues in
mushrooms reached levels of 0.1 to 0.6 mg/kg of CPU and 1 to 3 mg/kg
of DFBA (Nimmo and De Wilde 1977a).
Degradation in water
The degradation of diflubenzuron was studied at 20°C in sterile
water at various pH levels. There appears to be little degradation
under neutral or acidic conditions. However, at higher pH levels
there is a pH-dependent rate of degradation; at pH 9 the half life
is about 6 weeks and at pH 12 the half life was about 1.5 weeks.
The main degradation products were 2,6-difluorobenzoic acid and
4-chlorophenylurea (Nimmo and De Wilde 1975b).
Similar results were found with dilute solutions in distilled
water kept at 36°C. The degradation followed the same pathway; the
rate of degradation was again strongly pH dependent; the half life
times were shorter than at 20°C. Under these conditions, no
p-chloroaniline could be detected as a degradation product of
diflubenzuron (Ivie et al. 1980).
Heat catalysed degradation of diflubenzuron in an aqueous medium
is far more complex, and several degradation products are found that
are not formed, or hardly formed, under moderate conditions. One of
these products arises through expulsion of HF from the diflubenzuron
molecule, with concomittant cyclization:
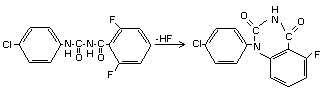 The resulting compound co-chromatographs with 4-chloroaniline in
a number of TLC solvent systems, which may lead to confusion (Ivie
et al 1980; Maas et al. 1980).
Under field conditions, diflubenzuron was demonstrated to
degrade rapidly, 4-chlorophenylurea being formed as a metabolite.
Diflubenzuron residues generally could not be detected (limit of
detection 0.002 mg/l) at 72 h after application of up to 110 g/ha of
field water (Schaefer and Dupras 1976).
When diflubenzuron was applied to field water, the compound
degraded to 4-chlorophenylurea. Small amounts of 4-chloroaniline were
apparent, but this was only a minor degradation product. Fish
initially accumulated diflubenzuron from water, but the tissue
concentration declined steadily with time. The fish tissue did contain
moderate amounts of 4-chlorophenylurea but only trace levels of
4-chloroaniline (Schaeffer et al 1980).
Photodecomposition
Crystalline diflubenzuron was radiated for 24 h on a glass sheet
at a 10 µg/cm2 level, by a mlu 300 W lamp. Decomposition was less
than 4%.
METHODS OF RESIDUE ANALYSIS
The recommended procedure for analyses of diflubenzuron residues
in a wide range of matrices, including soil, sediment, water, fish,
milk, eggs, animal tissues, agricultural crops and manure, involves an
extraction of the residue from the sample with a suitable solvent
clean-up of the extract by column chromatography, followed by
detection with high pressure liquid chromatography (HPLC) (Di Prima
et al. 1978; Buisman et al. 1977).
An alternative method for analyses of residues of diflubenzuron,
4-chlorophenylurea, and 4-chloroaniline in citrus and its process
fractions utilizes detection by gas chromatography (Cannizzaro 1978).
This method can also be applied in the analysis of residues of
diflubenzuron in soybean foliage, seed and process fractions (Di Prima
1976 a, b).
A GLC-ECD determination can also be carried out after hydrolysis
and derivatization with heptafluorobutyric anhydride (Rabenor
et al 1978).
National maximum residue levels reported to the Meeting
Recommended national MRLs and pre-harvest intervals are given in
Table 11.
TABLE 11. National maximum residue levels of diflubenzuron
Crop Country MRL Pre-harvest
(mg/kg) interval (days)
Apple, pear Argentina - 60
France 1.0 30
German Fed. Rep. 1.0 28
Italy 0.5 45
The Netherlands 1.0 28
Spain - 60
Switzerland 1.0 42
UK - 14
Yugoslavia - 30
Brassica leafy
vegetables The Netherlands 1.0 14
China (Taiwan province) - 22
German Fed. Rep. 1.0 -
Cottonseed USA 0.2 -
Mushroom The Netherlands 1.0 -
German Fed. Rep. 0.2 -
Switzerland 0.5 -
Soybean Brazil - 21
Eggs, milk, meat
and meat products USA 0.05 -
EVALUATION
COMMENTS AND APPRAISAL
The biotransformation of diflubenzuron has been evaluated in
several species. The major metabolites excreted by cow and rat result
from hydroxylation of the difluorobenzoyl moiety and the chlorophenyl
ring. In sheep and pigs, scission of the ureido bridge is the major
metabolic route: the major metabolites are 2,6-difluorobenzoic acid
and 4-chlorophenylurea. The extent of intestinal absorption of
diflubenzuron is generally low and decreases with increasing dose
levels. There is no indication of bioaccumulation in body tissues.
Diflubenzuron has a low acute toxicity. Short-term and long-term
studies in mice, rats, rabbits, cats, dogs and sheep show, in most
studies, dose-related increases in met-and sulph-haemoglobin. At high
dose levels haematocrit, haemoglobin and erythrocyte counts were
decreased, whereas reticulocyte and Heinz body counts were increased.
The effects, indicative of increased erythrocyte destruction, were
accompanied by increases in liver and spleen weight, concomitant with
haemosiderosis. It appears probably that met-haemoglobin formation is
the result of N-oxidation of 4-chloroaniline.
Several studies on chickens have been performed to investigate
the effects of diflubenzuron on sexual development and on testosterone
levels. Studies on sexual development failed to show any indications
of adverse effects, except in one case, where marginal decreases in
oestradiol and reduced comb and wattle development were observed.
Similar studies conducted in rats and bulls showed no significant
effects.
No adverse effects were noted in two reproduction studies in the
rat, in rabbit, rat and mouse teratogenicity and mouse and rat
carcinogenicity studies.
Mutagenicity studies were negative, as were in vivo and
in vitro mutagenicity studies on the metabolites, except in the case
of a cell transformation study, which was weakly positive for both 4-
chlorophenylurea and 2,6-difluorobenzoic acid.
Based on the most sensitive toxicological parameter, met-
haemoglobin formation, the no-effect level in rats was 40 ppm in the
diet (equivalent to 2 mg/kg bw/day) and in dogs 40 ppm in the diet
(equivalent to 1 mg/kg bw/day). In the long-term mouse study, this
parameter was not measured. The duration of the dog study was
unacceptable for use in ADI estimations. However, this species appears
to be the most susceptible with respect to met-haemoglobin production.
To allow for this, a higher safety factor was applied to the no-effect
level in the rat study.
Diflubenzuron is a recently-introduced insecticide, which is
registered for use in many countries as a foliar spray on pome fruits,
brassica leafy vegetables, cotton, soybean, tomato and citrus.
The rate of application ranges from 35 to 200 g/ha or as sprays
of concentrations of 0.01 to 0.04%. It is also used for the control of
flies in breeding sites, such as manure heaps and in mushroom growing,
and for control of insects on ornamental plants and in forests.
Diflubenzuron interferes in the deposition of chitin in the
insect cuticule through an influence on the enzyme chitin synthetase.
Because of its mode of action, diflubenzuron destroys insects slowly
and it is therefore necessary to treat crops well before harvest.
Where repeated applications are necessary, the intervals between
treatments are generally of the order of 28 days.
Extensive data of supervised trials from many countries were
available to the Meeting. Apples were treated at recommended rates up
to 0.02%; residues are well below 1 mg/kg at two weeks after the last
application. Pears follow the same pattern as apples. Citrus (whole
fruit) is at a level below 0.5 mg/kg after one week following the last
application of the recommended dosage rate. Examination of peel and
pulp separately showed that residues were exclusively found in the
peel. Residues in the pulp were all below the limit of determination
(0.05 mg/kg).
Residues in soybean seed and cottonseed were generally below the
limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron treated soil, rather
high contents of the metabolite DFBA are taken up from the soil. At
recommended dosage rates, the parent compound, diflubenzuron, is found
at a level below 0.1 mg/kg, while the metabolite 2,6-difluorobenzoic
acid ranges at a 1 mg/kg level.
Generally it may be established that when plants are treated at
recommended dosage rates, residues are below 2 mg/kg immediately after
application and that the rate of decline in residue concentration is
relatively slow over a period of about 56 days.
Degradation of diflubenzuron in animals and soil results
in the following metabolites: 2,6-difluorobenzoic acid (DFBA),
4-chlorophenylurea (CPU) and 4-chloroaniline (PCA). In soil the rate
of degradation is strongly dependent on the particle size of
diflubenzuron. The main metabolic pathway is by hydrolysis (>90%),
leading to DFBA and CPU. The compound does not penetrate into plant
tissue and residues are only present on those parts directly exposed
during the application. After application in plants, diflubenzuron is
not metabolized to any practical extent.
Diflubenzuron is not degraded in the digestive tract of mammals
to any significant degree, but when absorbed, it is metabolized before
excretion. After a high level treatment of cows, 0.02 mg/kg of the
parent compound were found in milk. Good agricultural practice would
not lead to residues higher than the limit of determination
(<0.02 mg/kg). Slightly higher residues, but still at the limit of
determination, were found in poultry meat and eggs.
Analytical methods for the parent compound and metabolites are
available. Depending on the nature of the substrate, diflubenzuron is
extracted with acetonitrile, ethylacetate or hexane. Clean-up involves
liquid-liquid partition and column chromatography, followed by HPLC or
GLCECD after an additional derivatization with heptafluorobutyric
anhydride. DFBA is determined after extraction and esterification
(diazomethane) by GLC-MS. The limit of determination is 0.05 mg/kg in
both cases.
Level causing no toxicological effect
Rat : 40 ppm in the diet, equivalent to 2 mg/kg bw/day
Estimation of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
Maximum residue levels were estimated for several crops. Since a
temporary ADI was estimated, the Meeting recommended these levels as
suitable for establishing temporary MRLs. They refer to diflubenzuron
alone; metabolites are not included.
Crop MRL (mg/kg)
Apple 1
Brussels sprouts 1
Cabbage 1
Citrus fruit 1
Cottonseed 0.2
Mushroom 0.2
Pear 1
Plum 1
Soybean 0.1
Carcass meat 0.05 *
Eggs 0.05 *
Milk 0.05 *
Meat byproducts 0.05 *
Poultry meat 0.05 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A dog study of adequate duration.
2. Results of an ongoing carcinogenicity study.
Desirable
1. Observations in humans, particularly with regard to met-
haemoglobin formation.
2. Information about the possible occurrence of metabolite DFBA
content in milk, meat and eggs, as the existing residue data
apply to the parent compound only.
REFERENCES
Arnold, D.A. Mutagenic study with TH 6040 in albino mice. Industrial
1974 Bio-test Laboratories Report no. 622-05068. (Unpublished)
Batham, P. and Offer, J.M. Tumorigenicity of DU 112307 to mice:
1977 dietary administration for 80 weeks. Revaluated pathological
data. Addendum to, Huntingdon Research Centre Report no
PDR170/75685. (Unpublished)
Bentley, J.P., Weber, G.H. and Gould, D. The effect of diflubenzuron
1979 feeding on glycosaminoglycan and sulph-haemoglobin
biosynthesis in mice. Pesticide Biochemistry and Physiology,
10:162.
Berczy, Z.S. et al. Acute inhalation toxicity to the rat of DU 112307
1973 technical grade powder. Huntingdon Research Centre Report
no. PDR74/73849. (Unpublished)
1975 Acute inhalation toxicity to the rabbit of DU 112307
technical grade powder. Huntingdon Research Centre Report
no. PDR198/74988. (Unpublished)
Bollag, J.M., Blattman, P. and Laanis, T. Absorption and
1978 transformation of four substituted anilines in soil. Journal
of Agricultural and Food Chemistry, 26:1302.
Booth, G.M. et al. The effect of diflubenzuron on rat serum
1980 testosterone, weight and food consumption. Brigham Young
University, Provo, Utah, USA, 5 Feb. (Unpublished)
Booth, G.M. Biochemical effects of diflubenzuron on mouse embryos.
1977 Brigham Young University, Provo, Utah, USA. (Unpublished)
Braddock. Taste test for "Dimilin" Ruby Red grapefruit juice.
1976a University of Florida, Gainsville, USA, 18 October.
(Unpublished)
Braddock. Taste test for "Dimilin" Hamlet orange juice. University of
1976b Florida, Gainesville, USA, 23 November. (Unpublished)
Braddock. Taste test for "Dimilin" Temple orange juice. University of
1977 Florida, Gainesville, USA, 2 February. (Unpublished)
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of diflubenzuron
1977a technical, batch FL44/60521. Litton Bionetics Project no.
2683. (Unpublished)
1977b Evaluation of diflubenzuron. in vitro malignant
transformation in BALB/3T3 cells. Litton Bionetics Project
no. 2688. (Unpublished)
1977c Evaluation of diflubenzuron: unscheduled DNA synthesis in
WI-38 cells. Litton Bionetics Project no. 2688.
(Unpublished)
Bryant, H. Activity of TH 6040 in the Ames Salmonella typhimurium
1976 mutagenesis assay. University of Kentucky, Lexington, USA.
(Unpublished)
Buisman, P. et al. Residue anlysemethod van diflubenzuron in gewas,
1977 grond en water. Duphar Rept. no. 56630/70/77 (in Dutch,
English summary). (Unpublished)
Bull, D.L. Fate of diflubenzuron after application to cotton and the
1980 boll weevil, South-western Entomology Supplement, nos 1
and 2.
Bull, D.L. and Ivie, G.W. Fate of diflubenzuron in cotton, soil and
1978 rotational crops. Journal of Agricultural and Food
Chemistry, 26:515.
1980 Activity and fate of diflubenzuron and certain derivatives
in the boll weevil. Pesticide Biochemistry and Physiology,
13:41.
Bull, D.L. and Shaver, T.N. Fate of potassium-3,4-dichloro-5
1980 isothiazole-carboxylate in soil. Journal of Agricultural and
Food Chemistry, 28:982.
Burdock, G.A. et al. Ninety-day subchronic toxicity study in mice:
1980a diflubenzuron technical. Hazleton Project no. 533-120.
(Unpublished)
1980b Subchronic dietary toxicity study in rats, diflubenzuron.
Hazleton Project no. 533-119. (Unpublished)
Cannizzaro, R.D. Determination of diflubenzuron, 4-chlorophenylurea,
1978 and 4-chloroaniline in citrus and process fractions by gas
chromatography. Analytical Method no.22A, Thompson-Hayward
Chemical Co. (Unpublished)
Chang, S.C. Conjugation: the major metabolic pathway of 14C
1978 diflubenzuron in the house-fly. Journal of Economic
Entomology, 71:31.
Chang, S.C. and Stokes, J.B. Conjugation: the major metabolic pathway
1979 of 14C-diflubenzuron in the boll weevil. Journal of
Economic Entomology, 72:15.
Chesterman, H. et al. DU 112307, toxicity in repeated dietary
1974 administration to beagle dogs. Huntingdon Research Centre
Report no. PDR169/74157. (Unpublished)
Colley, J. and Offer, J.M. Effects of DU 112307 in dietary
1977 administration to rats for 104 weeks, revaluated
pathological data. Huntingdon Research Centre, addendum to
HRC Report no. PDR171/75995. (Unpublished)
Colley, J.C. et al. The effects of dietary administration of
1981 diflubenzuron to male and female HC/CFLP mice for 13 weeks,
vol. I and II. Huntingdon Research Centre Report no.
PDR/294/80185. (Unpublished)
Crookshank, H.R. et al. Effect of diflubenzuron (Dimilin: TH6040) on
1978 the hyaluronic acid concentration in chicken comb. Poultry
Science, 57:804.
Davies, R.F. and Halliday, J.C. Acute percutaneous toxicity to rabbits
1974 of DU 112307 (technical). Huntingdon Research Centre Report
no.2171/D175/73. (Unpublished)
Danhaus, R.G. and Sieck, R.F. Rotation crop uptake of 14C residues
1976 following soil application of Dimilin. ADC project no. 222.
(Unpublished)
Danhaus, R.G. et al. 14C-Dimilin field soil leaching study. ADC project
1976 no. 205. (Unpublished)
De Bree, H. et al. Diflubenzuron: analysis of metabolites connected
1977 with met-haemoglobinemia. Duphar Report no. 56654/8/77.
(Unpublished)
De Lange, N. et al. PH 60-40: Excretion of radioactivity and
1974 metabolite patterns in rats following oral administration.
Duphar Report no. 56654/20/74. (Unpublished)
1975 Diflubenzuron (PH 60-40): Balance studies in the rat, and
identification of urinary metabolites. Duphar Report no.
56654/22/75. (Unpublished)
1977 Diflubenzuron: Intestinal absorption in the rat in relation
to dosage level. Duphar Report no. 56654/10/77.
(Unpublished)
De Lange, N. Diflubenzuron: Dermal absorption in the rabbit. Duphar
1979 Report no. 56654/9/79. (Unpublished)
De Lange, N. and Post, L.C. Diflubenzuron: intestinal absorption in
1978 the mouse in relation to dosage level. Duphar Report no.
56654/4/78. (Unpublished)
Deul, D.H. and De Jong, B.J. The possible influence of DU 112307 on
1977 the in vivo synthesis of hyaluronic acid in chicken skin.
Duphar Report no. 56635/1/77. (Unpublished)
Di Prima, S.J. Determination of diflubenzuron residues in soybean
1976a foliage and seed by gas chromatography. Analytical Method
no. 10, Thompson-Hayward Chemical Co. (Unpublished)
1976b Determination of diflubenzuron residues in soybean process
fractions by gas chromatography. Method no. 13, Thompson-
Hayward Chemical Company. (Unpublished)
1978 Analysis of diflubenzuron residues in environmental samples
by high-pressure liquid chromatography. Journal of
Agricultural and Food Chemistry, 26:968.
Dorough, H.W. Screening of selected Thompson Hayward chemicals for
1977 activity in the Ames Salmonella mutagenic test. University
of Kentucky, Lexington, USA. (Unpublished)
Duphar. Reports on residues of diflubenzuron. In apples, reports nos.
1975-81 30/39/75, 30/6/A/75, 30/22A/76, 30/44/76, 30/45/76,
30/53/76, 30/10/77, 30/11/77, 30/13/77, 30/14/77, 30/84/77,
30/22/78, 30/38/79, 30/76/80, In pears, reports nos.
30/91/76,30/84/77, 30/96/77, 30/97/77. In brassica leafy
vegetables reports nos. 30/48/76,30/91/77, 30/73/78. In
soybeans, reports nos. 83/06/81, SR35. In other crops,
reports nos. 30/72/77, 30/102/77, 30/129/77, 30/130/77,
30/40/78, 30/9/79, 30/43/80,83/05/81, 83/07/81, K/R/003/77,
K/R/004/77, K3R/005/77, K/R/006/77, K/R/007/77.(Unpublished)
Goodman, D.G. Histopathologic evaluation of mice administered
1980a diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
1980b Histopathologic evaluation of rats administered
diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
Gustafson, D.E. and Wargo, J.P. Fate of Dimilin following application
1976 to soybeans. Analytical Development Corporation Project 222.
(Unpublished)
Hawkins, D.R. Excretion of radioactivity after oral administration of
1980 3H/14C-diflubenzuron to cats. Huntingdon Research Centre
Report no. PDR/302/80443. (Unpublished)
Helling, C.S. Soil mobility of three Thompson-Hayward pesticides.
1975 ARS/USDA Beltsville, USA. (Unpublished)
Hsu, T.S. and Bartha, R. Interaction of pesticide-derived
1974 chloroaniline residues with soil organic matter. Soil
Science, 16:444.
Hunter, B. et al. DU 112307, preliminary assessment of the toxicity to
1974 male mice in dietary administration for 6 weeks. Huntingdon
Research Centre Report no.PDR/174/74199. (Unpublished)
Hunter, B. et al. Tumorigenicity of DU 112307 to mice, dietary
1975 administration for 80 weeks. Huntingdon Research Centre
Report no. PDR/170/75685. (Unpublished)
1976 Effects of DU 112307 in dietary administration to rats for
104 weeks. Huntingdon Research Centre Report no.
PDR/171/75945. (Unpublished)
1979 DU 112307, toxicity to rats in dietary administration for
nine weeks followed by a four-week withdrawal period.
Huntingdon Research Centre Report no. PDR/248/77883
(Unpublished)
Ivie, G.W. Fate of diflubenzuron in cattle and sheep. Journal of
1978 Agricultural and Food Chemistry, 26:81.
Ivie, G.W. and Wright, J.E. Fate of diflubenzuron in the stable fly
1978 and house fly. Journal of Agricultural and Food Chemistry,
26:90.
Ivie, G.W. Fate of diflubenzuron in water. Journal of Agricultural and
1980 Food Chemistry, 28: 330.
Jagannath, D.R. and Brusick, D.J. Mutagenicity evaluation of
1977a 4-chlorophenylurea. Litton Bionetics Project no. 20838.
(Unpublished)
1977b Mutagenic evaluation of 2,6-difluorobenzoic acid. Litton
Bionetics Project no.20838. (Unpublished)
1977c Mutagenicity evaluation of 4-chloroaniline. Litton Bionetics
Project no. 20838. (Unpublished)
Keet, C.M.J.F. Effects of DU 112307 technical after dietary
1976b administration to male Hubbard Broiler chickens for 14
weeks. Duphar Report no. 56645/25/76. (Unpublished)
1977a The met-haemoglobin, sulph-haemoglobin and Heinz body
forming properties of DU 112307 after oral administration to
male rats during 8 days. Duphar Report no. 56645/15/77.
(Unpublished)
1977b The effect of DU 112307 (technical) in male mice after daily
oral administration for a period of 14 days on body weight,
met-haemoglobin, sulph-haemoglobin and Heinz body formation
and on gross pathology. Duphar Report no. 56645/33/77.
(Unpublished)
1977c The met-haemoglobin and sulph-haemoglobin forming properties
of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Duphar Report no. 56645/2/77.
(Unpublished)
Kemp, A. et al. Dietary administration of DU 112307 to male and
1973a female rats for 3 months. Duphar Report no. 56645/13A/73.
(Unpublished)
1973b Appendix III to report no. 56645/13A/73, individual data:
dietary administration of DU 112307 to male and female rats
for 3 months. Duphar Report no. 56645/13B/73. (Unpublished)
Keuker, H. Diflubenzuron, physical and chemical properties. Duphar
1975 Report no. 56630/107/75. (Unpublished)
Koelman-Klaus, H.J.S. Acute oral toxicity study of p-chlorophenylurea
1978a in male and female rats. Duphar Report no. 56645/9/78.
(Unpublished)
1978b Acute oral toxicity study of 2,6-difluorobenzoic acid in
male and female rats. Duphar Report no. 56645/25/78.
(Unpublished)
Koopman, T.S.M. Acute oral toxicity study with DU 112307 (technical)
1977a in mice. Duphar Report no. 56645/4/77. (Unpublished)
1977b Acute intra-peritoneal toxicity study with DU 112307
(technical) in mice. Duphar Report no. 56645/5/77.
(Unpublished)
1977c Acute dermal toxicity study with DU 112307 (technical) in
rats. Duphar Report no. 56645/7/77. (Unpublished)
Maas, W. et al. Benzoylphenylurea insecticides. In:R. Wegler (Ed.),
1980 Chemie der Pflanezen-schutz-und Schädlingsbekämpfungs-
mittel, Band 6. Springer Verlag, Heidelberg, 1980.
Mansager, E.R. et al. Fate of 14C-diflubenzuron on cotton and in soil.
1979 Pesticide Biochemistry and Physiology, 12: 172.
Matheson, D.W. and Brusick, D.J. Evaluation of 4-chlorophenylurea: In
1978a vitro malignant transformation in BALB/3T3/cells. Litton
Bionetics Project no. 20840. (Unpublished)
1978b Evaluation of 2,6-difluorobenzoic acid: in vitro malignant
transformation in BALB/3T3/cells. Litton Bionetics Project
no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 2,6-difluorobenzoic acid in the
unscheduled DNA synthesis in human WI-38 cell assay. Litton
Bionetics Project no. 20840. (Unpublished)
Matheson, D.W. et al. Mutagenicity evaluation of 4-chlorophenylurea in
1978a the unscheduled DNA synthesis in human WI-38 cells assay.
Litton Bionetics Project no. 20840. (Unpublished)
1978b Mutagenic evaluation of 4-chloroaniline in the in vitro
transformation of BALB/3T3 cells assay. Litton Bionetics
Project no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 4-chloroaniline in the
unscheduled DNA synthesis in human WI-38 cells assay. Litton
Bionetics Project no. 20840. (Unpublished)
McGregor, J.T. et al. Mutagenicity tests of diflubenzuron in the
1979 micronucleus test in mice, the L5178Y mouse lymphoma forward
mutation assay, and the Ames Salmonella Reserve mutation
test. Mutation Research, 66:45.
Miller, R.W. et al. Feeding TH-6040 to cattle: Residues in tissues and
1976a milk and breakdown in manure. Journal of Agricultural and
Food Chemistry, 24:687.
1976b Effects of feeding TF-6040 to two breeds of chickens.
Journal of Economic Entomology, 69:741.
Miller, R.W., Cecil, H.C., Carey, A.M., Corley, C. and Kiddy, C.A.
1979 Effects of feeding diflubenzuron to young male Holstein
cattle. Bulletin of Environmental Contamination and
Toxicology, 23:482.
Moreale, A. and Van Bladel, R. Influence of soil properties on
1976 absorption of pesticide-derived aniline and p-chloroaniline.
Journal of Soil Science, 27:48.
Nimmo, W.B. and De Wilde, P.C. Fate of diflubenzuron on leaves of
1974 corn, soybean, cabbage and apples. Duphar Report no.
56635/16A/74. (Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in soil
1975a and natural water. Duphar Report no. 56635/37/1975.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in sterile
1975b water at pH 5,7, 9 and 12. Duphar Report no. 56635/32/75.
(Unpublished)
Nimmo, W. B. and De Wilde, P.C. Re-uptake from diflubenzuron treated
1976a soil by soybean, maize and tomato. Duphar Report no.
56635/4/76. (Unpublished)
1976b Uptake of diflubenzuron and its metabolites from the soil by
rice wheat. Duphar Report no. 56635/8/76. (Unpublished)
Nimmo W.B. and De Wilde, P.C. Degradation of diflubenzuron in mushroom
1977a growth medium and uptake of its metabolites in the
mushrooms. Duphar Report no. 56635/21A/77. (Unpublished)
1977b Degradation of diflubenzuron in cow and chicken manure.
Duphar Report no.56635/27/77. (Unpublished)
Nimmo, W.B. et al. Fate of diflubenzuron applied to leaves and fruits
1978 of apples trees. Duphar Report no. 56635/33/78.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. The fate of 14C-diflubenzuron on pine.
1980 Duphar Report no. 56635/18/79. (Unpublished)
Offer, J.M. Tumorigenicity of DU 112307 to mice, dietary
1977 administration for 80 weeks. Addendum: histological
examination of additional tissues, Huntingdon Research
Centre Report PDR/250, addendum to PDR/170/75685.
(Unpublished)
Opdycke, J.C. Metabolism and fate of diflubenzuron in chickens and
1976 swine. M. Sc.Thesis, Graduate School University of Maryland,
College Park, USA.
Palmer, A.K. and Hill, P.A. Effect of DU 112307 on reproduction
1975a function of multiple generations in the rat. Huntingdon
Research Centre Report no. PDR/17375954. (Unpublished)
1975b Effect of DU 112307 on pregnancy of the rat. Huntingdon
Research Centre Report no. PDR/192/74978. (Unpublished)
1975c Effect of DU 112307 on pregnancy of the New Zealand white
rabbit. Huntingdon Research Centre Report no. PDR/193/74937.
(Unpublished)
Palmer, A.K. et al. Preliminary assessment of the effect of DU 112307
1977 on the rat. Huntingdon Research Centre Report no.
PDR/243/77208. (Unpublished)
1978 Effect of dietary administration of DU 112307 on
reproduction function of one generation in the rat.
Huntingdon Research Centre Report no. PDR/244/78653.
(Unpublished)
Patel, Y.M. and Santolucito, J.A. Selected toxicological studies of
1980 Dimilin in weanling male rats. EPA-600/3-80-031.
(Unpublished)
Pimprikar, G.D. Mechanisms of resistance to Dimilin in Musca
1977 domestica. Ph.D. Thesis University of California, Riverside,
USA.
Popp, F. Storage stability of Dimilin 25W, Duphar Report no.
1977 56623/6/77. (Unpublished)
Quarles, J.M., Norman, J.O. and Kubena, L.F. Absence of transformation
1980 by diflubenzuron in a host-mediated transplacental
carcinogen assay. Bulletin of Environmental Contamination
and Toxicology, 25:252.
Rabenor, B., De Wilde, P.C., De Boer, F.G., Korven, P.K., Di Prima, S.
1978 J. and Canizzaro, R.D. In: G. Zweig (Ed.), Determination of
diflubenzuron residues using GLC. Analytical Methods for
Pesticides and Plant Growth Regulators. Vol. 10. Academic
Press, New York, p. 57-72.
Rieck, C.E. Soil column leaching study with Dimilin. University of
1975 Kentucky, Lexington, USA. (Unpublished)
Reinert, H.K. and Cannon, G.E. The evaluation of TH 6040 in four avian
1976 species. Cannon Laboratories Report no. 5E-8680A-D.
(Unpublished)
Ross, D.B. et al. DU 112307, 13-week oral toxicity study in sheep.
1977a Huntingdon Research Centre Report no. PDR/299/77226.
(Unpublished)
1977b Blood red cell and plasma cholinesterase value following
six-week inclusion of DU 112307 in the diet of sheep.
Huntingdon Research Centre Report no. PDR/229A/77225.
(Unpublished)
1977c The effects of DU 112307 on female chickens following
dietary inclusion for 98 days. Huntingdon Research Centre
Report no. PDR/240/77183. (Unpublished)
Ross, D.B. et al. The effects of DU 112307 on female chickens
1979 following dietary inclusion for 98 days, (second study).
Huntingdon Research Centre Report No. PDR/251/781030.
(Unpublished)
Schaefer, c. and Dupras, E.F. Factors affecting the stability in water
1976 and the persistence of Dimilin in field waters. Journal of
Agricultural and Food Chemistry, 24:733.
Schaefer, C. et al. The occurrence of p-chloroaniline and p-
1980 chlorophenylurea from degradation of diflubenzuron in water
and fish. Proc. 48th Annual Conference of California
Mosquito & Vector Control Association, Anaheim, USA.
Schwartz, S.L. and Borzelleca, J.F. Effects of diflubenzuron on met-
1981 haemoglobin, sulph-haemoglobin and Heinz body production in
cats. Georgetown University, Washington, D.C., USA
(Unpublished)
Seegmiller, R.E. and Booth, G.M. Teratogenic and biochemical effects
1976 of TH 6040 and chick embryos. Brigham Young University,
Provo, Utah, USA. (Unpublished)
Seuferer, S.L., Braymer, H.D. and Dunn, J.J. Metabolism of
1979 diflubenzuron by soil organisms and mutagenicity of the
metabolites. Pesticide Biochemistry and Physiology, 10: 174.
Smalley, H.E. Comparative toxicology of some insect growth regulators.
1976 Clinical Toxicology, 9:27.
Smith, K.S. and Merricks, D.L. TH 6040:Tissue residue and metabolism
1976a study in dairy cows. Cannon Labs, lab.no. 5E-7372 (March
29). (Unpublished)
1976b TH 6040: tissue residue and metabolism study in poultry.
Cannon Labs, lab.no. 5E-7372 (March 12). (Unpublished)
Spencer-Jones, D.H. Letter from Duphar Midox to Duphar, 25 May.
1979
Stoolmiller, A.C. Toxicological study on the effect of diflubenzuron
1978 on rat C-6 astrocytoma cells In vitro. Gen. Pharmac:, 9:11.
Van den Berg, G. Uptake, translocation and metabolism of
1978a 4-chlorophenylurea in tomato and broad bean. Duphar Report
no. 56635/5/67. (Unpublished)
1978b Uptake and decarboxylation of 2,6-difluorobenzoid acid in
tomato. Duphar Report no. 56635/4/78. (Unpublished)
Van Eldik, A. Acute toxicity studies with DU 112307 in mice and rats.
1973 Duphar Report no. 56645/14/73. (Unpublished)
1974 Acute toxicity studies with DU 112307 (technical) in mice
after intra-peritoneal administration. Duphar Report no.
56645/1/74. (Unpublished)
Verlopp, A. and Ferrell, C.D. Benzoylphenylureas, a new group of
1977 larvicides interfering with chitin deposition. American
Chemical Society Symposium.
Willems, A.C.M. et al. Diflubenzuron: Intestinal absorption and
1980 metabolism in the rat. Xenobiotica, 10:103.
The resulting compound co-chromatographs with 4-chloroaniline in
a number of TLC solvent systems, which may lead to confusion (Ivie
et al 1980; Maas et al. 1980).
Under field conditions, diflubenzuron was demonstrated to
degrade rapidly, 4-chlorophenylurea being formed as a metabolite.
Diflubenzuron residues generally could not be detected (limit of
detection 0.002 mg/l) at 72 h after application of up to 110 g/ha of
field water (Schaefer and Dupras 1976).
When diflubenzuron was applied to field water, the compound
degraded to 4-chlorophenylurea. Small amounts of 4-chloroaniline were
apparent, but this was only a minor degradation product. Fish
initially accumulated diflubenzuron from water, but the tissue
concentration declined steadily with time. The fish tissue did contain
moderate amounts of 4-chlorophenylurea but only trace levels of
4-chloroaniline (Schaeffer et al 1980).
Photodecomposition
Crystalline diflubenzuron was radiated for 24 h on a glass sheet
at a 10 µg/cm2 level, by a mlu 300 W lamp. Decomposition was less
than 4%.
METHODS OF RESIDUE ANALYSIS
The recommended procedure for analyses of diflubenzuron residues
in a wide range of matrices, including soil, sediment, water, fish,
milk, eggs, animal tissues, agricultural crops and manure, involves an
extraction of the residue from the sample with a suitable solvent
clean-up of the extract by column chromatography, followed by
detection with high pressure liquid chromatography (HPLC) (Di Prima
et al. 1978; Buisman et al. 1977).
An alternative method for analyses of residues of diflubenzuron,
4-chlorophenylurea, and 4-chloroaniline in citrus and its process
fractions utilizes detection by gas chromatography (Cannizzaro 1978).
This method can also be applied in the analysis of residues of
diflubenzuron in soybean foliage, seed and process fractions (Di Prima
1976 a, b).
A GLC-ECD determination can also be carried out after hydrolysis
and derivatization with heptafluorobutyric anhydride (Rabenor
et al 1978).
National maximum residue levels reported to the Meeting
Recommended national MRLs and pre-harvest intervals are given in
Table 11.
TABLE 11. National maximum residue levels of diflubenzuron
Crop Country MRL Pre-harvest
(mg/kg) interval (days)
Apple, pear Argentina - 60
France 1.0 30
German Fed. Rep. 1.0 28
Italy 0.5 45
The Netherlands 1.0 28
Spain - 60
Switzerland 1.0 42
UK - 14
Yugoslavia - 30
Brassica leafy
vegetables The Netherlands 1.0 14
China (Taiwan province) - 22
German Fed. Rep. 1.0 -
Cottonseed USA 0.2 -
Mushroom The Netherlands 1.0 -
German Fed. Rep. 0.2 -
Switzerland 0.5 -
Soybean Brazil - 21
Eggs, milk, meat
and meat products USA 0.05 -
EVALUATION
COMMENTS AND APPRAISAL
The biotransformation of diflubenzuron has been evaluated in
several species. The major metabolites excreted by cow and rat result
from hydroxylation of the difluorobenzoyl moiety and the chlorophenyl
ring. In sheep and pigs, scission of the ureido bridge is the major
metabolic route: the major metabolites are 2,6-difluorobenzoic acid
and 4-chlorophenylurea. The extent of intestinal absorption of
diflubenzuron is generally low and decreases with increasing dose
levels. There is no indication of bioaccumulation in body tissues.
Diflubenzuron has a low acute toxicity. Short-term and long-term
studies in mice, rats, rabbits, cats, dogs and sheep show, in most
studies, dose-related increases in met-and sulph-haemoglobin. At high
dose levels haematocrit, haemoglobin and erythrocyte counts were
decreased, whereas reticulocyte and Heinz body counts were increased.
The effects, indicative of increased erythrocyte destruction, were
accompanied by increases in liver and spleen weight, concomitant with
haemosiderosis. It appears probably that met-haemoglobin formation is
the result of N-oxidation of 4-chloroaniline.
Several studies on chickens have been performed to investigate
the effects of diflubenzuron on sexual development and on testosterone
levels. Studies on sexual development failed to show any indications
of adverse effects, except in one case, where marginal decreases in
oestradiol and reduced comb and wattle development were observed.
Similar studies conducted in rats and bulls showed no significant
effects.
No adverse effects were noted in two reproduction studies in the
rat, in rabbit, rat and mouse teratogenicity and mouse and rat
carcinogenicity studies.
Mutagenicity studies were negative, as were in vivo and
in vitro mutagenicity studies on the metabolites, except in the case
of a cell transformation study, which was weakly positive for both 4-
chlorophenylurea and 2,6-difluorobenzoic acid.
Based on the most sensitive toxicological parameter, met-
haemoglobin formation, the no-effect level in rats was 40 ppm in the
diet (equivalent to 2 mg/kg bw/day) and in dogs 40 ppm in the diet
(equivalent to 1 mg/kg bw/day). In the long-term mouse study, this
parameter was not measured. The duration of the dog study was
unacceptable for use in ADI estimations. However, this species appears
to be the most susceptible with respect to met-haemoglobin production.
To allow for this, a higher safety factor was applied to the no-effect
level in the rat study.
Diflubenzuron is a recently-introduced insecticide, which is
registered for use in many countries as a foliar spray on pome fruits,
brassica leafy vegetables, cotton, soybean, tomato and citrus.
The rate of application ranges from 35 to 200 g/ha or as sprays
of concentrations of 0.01 to 0.04%. It is also used for the control of
flies in breeding sites, such as manure heaps and in mushroom growing,
and for control of insects on ornamental plants and in forests.
Diflubenzuron interferes in the deposition of chitin in the
insect cuticule through an influence on the enzyme chitin synthetase.
Because of its mode of action, diflubenzuron destroys insects slowly
and it is therefore necessary to treat crops well before harvest.
Where repeated applications are necessary, the intervals between
treatments are generally of the order of 28 days.
Extensive data of supervised trials from many countries were
available to the Meeting. Apples were treated at recommended rates up
to 0.02%; residues are well below 1 mg/kg at two weeks after the last
application. Pears follow the same pattern as apples. Citrus (whole
fruit) is at a level below 0.5 mg/kg after one week following the last
application of the recommended dosage rate. Examination of peel and
pulp separately showed that residues were exclusively found in the
peel. Residues in the pulp were all below the limit of determination
(0.05 mg/kg).
Residues in soybean seed and cottonseed were generally below the
limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron treated soil, rather
high contents of the metabolite DFBA are taken up from the soil. At
recommended dosage rates, the parent compound, diflubenzuron, is found
at a level below 0.1 mg/kg, while the metabolite 2,6-difluorobenzoic
acid ranges at a 1 mg/kg level.
Generally it may be established that when plants are treated at
recommended dosage rates, residues are below 2 mg/kg immediately after
application and that the rate of decline in residue concentration is
relatively slow over a period of about 56 days.
Degradation of diflubenzuron in animals and soil results
in the following metabolites: 2,6-difluorobenzoic acid (DFBA),
4-chlorophenylurea (CPU) and 4-chloroaniline (PCA). In soil the rate
of degradation is strongly dependent on the particle size of
diflubenzuron. The main metabolic pathway is by hydrolysis (>90%),
leading to DFBA and CPU. The compound does not penetrate into plant
tissue and residues are only present on those parts directly exposed
during the application. After application in plants, diflubenzuron is
not metabolized to any practical extent.
Diflubenzuron is not degraded in the digestive tract of mammals
to any significant degree, but when absorbed, it is metabolized before
excretion. After a high level treatment of cows, 0.02 mg/kg of the
parent compound were found in milk. Good agricultural practice would
not lead to residues higher than the limit of determination
(<0.02 mg/kg). Slightly higher residues, but still at the limit of
determination, were found in poultry meat and eggs.
Analytical methods for the parent compound and metabolites are
available. Depending on the nature of the substrate, diflubenzuron is
extracted with acetonitrile, ethylacetate or hexane. Clean-up involves
liquid-liquid partition and column chromatography, followed by HPLC or
GLCECD after an additional derivatization with heptafluorobutyric
anhydride. DFBA is determined after extraction and esterification
(diazomethane) by GLC-MS. The limit of determination is 0.05 mg/kg in
both cases.
Level causing no toxicological effect
Rat : 40 ppm in the diet, equivalent to 2 mg/kg bw/day
Estimation of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
Maximum residue levels were estimated for several crops. Since a
temporary ADI was estimated, the Meeting recommended these levels as
suitable for establishing temporary MRLs. They refer to diflubenzuron
alone; metabolites are not included.
Crop MRL (mg/kg)
Apple 1
Brussels sprouts 1
Cabbage 1
Citrus fruit 1
Cottonseed 0.2
Mushroom 0.2
Pear 1
Plum 1
Soybean 0.1
Carcass meat 0.05 *
Eggs 0.05 *
Milk 0.05 *
Meat byproducts 0.05 *
Poultry meat 0.05 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A dog study of adequate duration.
2. Results of an ongoing carcinogenicity study.
Desirable
1. Observations in humans, particularly with regard to met-
haemoglobin formation.
2. Information about the possible occurrence of metabolite DFBA
content in milk, meat and eggs, as the existing residue data
apply to the parent compound only.
REFERENCES
Arnold, D.A. Mutagenic study with TH 6040 in albino mice. Industrial
1974 Bio-test Laboratories Report no. 622-05068. (Unpublished)
Batham, P. and Offer, J.M. Tumorigenicity of DU 112307 to mice:
1977 dietary administration for 80 weeks. Revaluated pathological
data. Addendum to, Huntingdon Research Centre Report no
PDR170/75685. (Unpublished)
Bentley, J.P., Weber, G.H. and Gould, D. The effect of diflubenzuron
1979 feeding on glycosaminoglycan and sulph-haemoglobin
biosynthesis in mice. Pesticide Biochemistry and Physiology,
10:162.
Berczy, Z.S. et al. Acute inhalation toxicity to the rat of DU 112307
1973 technical grade powder. Huntingdon Research Centre Report
no. PDR74/73849. (Unpublished)
1975 Acute inhalation toxicity to the rabbit of DU 112307
technical grade powder. Huntingdon Research Centre Report
no. PDR198/74988. (Unpublished)
Bollag, J.M., Blattman, P. and Laanis, T. Absorption and
1978 transformation of four substituted anilines in soil. Journal
of Agricultural and Food Chemistry, 26:1302.
Booth, G.M. et al. The effect of diflubenzuron on rat serum
1980 testosterone, weight and food consumption. Brigham Young
University, Provo, Utah, USA, 5 Feb. (Unpublished)
Booth, G.M. Biochemical effects of diflubenzuron on mouse embryos.
1977 Brigham Young University, Provo, Utah, USA. (Unpublished)
Braddock. Taste test for "Dimilin" Ruby Red grapefruit juice.
1976a University of Florida, Gainsville, USA, 18 October.
(Unpublished)
Braddock. Taste test for "Dimilin" Hamlet orange juice. University of
1976b Florida, Gainesville, USA, 23 November. (Unpublished)
Braddock. Taste test for "Dimilin" Temple orange juice. University of
1977 Florida, Gainesville, USA, 2 February. (Unpublished)
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of diflubenzuron
1977a technical, batch FL44/60521. Litton Bionetics Project no.
2683. (Unpublished)
1977b Evaluation of diflubenzuron. in vitro malignant
transformation in BALB/3T3 cells. Litton Bionetics Project
no. 2688. (Unpublished)
1977c Evaluation of diflubenzuron: unscheduled DNA synthesis in
WI-38 cells. Litton Bionetics Project no. 2688.
(Unpublished)
Bryant, H. Activity of TH 6040 in the Ames Salmonella typhimurium
1976 mutagenesis assay. University of Kentucky, Lexington, USA.
(Unpublished)
Buisman, P. et al. Residue anlysemethod van diflubenzuron in gewas,
1977 grond en water. Duphar Rept. no. 56630/70/77 (in Dutch,
English summary). (Unpublished)
Bull, D.L. Fate of diflubenzuron after application to cotton and the
1980 boll weevil, South-western Entomology Supplement, nos 1
and 2.
Bull, D.L. and Ivie, G.W. Fate of diflubenzuron in cotton, soil and
1978 rotational crops. Journal of Agricultural and Food
Chemistry, 26:515.
1980 Activity and fate of diflubenzuron and certain derivatives
in the boll weevil. Pesticide Biochemistry and Physiology,
13:41.
Bull, D.L. and Shaver, T.N. Fate of potassium-3,4-dichloro-5
1980 isothiazole-carboxylate in soil. Journal of Agricultural and
Food Chemistry, 28:982.
Burdock, G.A. et al. Ninety-day subchronic toxicity study in mice:
1980a diflubenzuron technical. Hazleton Project no. 533-120.
(Unpublished)
1980b Subchronic dietary toxicity study in rats, diflubenzuron.
Hazleton Project no. 533-119. (Unpublished)
Cannizzaro, R.D. Determination of diflubenzuron, 4-chlorophenylurea,
1978 and 4-chloroaniline in citrus and process fractions by gas
chromatography. Analytical Method no.22A, Thompson-Hayward
Chemical Co. (Unpublished)
Chang, S.C. Conjugation: the major metabolic pathway of 14C
1978 diflubenzuron in the house-fly. Journal of Economic
Entomology, 71:31.
Chang, S.C. and Stokes, J.B. Conjugation: the major metabolic pathway
1979 of 14C-diflubenzuron in the boll weevil. Journal of
Economic Entomology, 72:15.
Chesterman, H. et al. DU 112307, toxicity in repeated dietary
1974 administration to beagle dogs. Huntingdon Research Centre
Report no. PDR169/74157. (Unpublished)
Colley, J. and Offer, J.M. Effects of DU 112307 in dietary
1977 administration to rats for 104 weeks, revaluated
pathological data. Huntingdon Research Centre, addendum to
HRC Report no. PDR171/75995. (Unpublished)
Colley, J.C. et al. The effects of dietary administration of
1981 diflubenzuron to male and female HC/CFLP mice for 13 weeks,
vol. I and II. Huntingdon Research Centre Report no.
PDR/294/80185. (Unpublished)
Crookshank, H.R. et al. Effect of diflubenzuron (Dimilin: TH6040) on
1978 the hyaluronic acid concentration in chicken comb. Poultry
Science, 57:804.
Davies, R.F. and Halliday, J.C. Acute percutaneous toxicity to rabbits
1974 of DU 112307 (technical). Huntingdon Research Centre Report
no.2171/D175/73. (Unpublished)
Danhaus, R.G. and Sieck, R.F. Rotation crop uptake of 14C residues
1976 following soil application of Dimilin. ADC project no. 222.
(Unpublished)
Danhaus, R.G. et al. 14C-Dimilin field soil leaching study. ADC project
1976 no. 205. (Unpublished)
De Bree, H. et al. Diflubenzuron: analysis of metabolites connected
1977 with met-haemoglobinemia. Duphar Report no. 56654/8/77.
(Unpublished)
De Lange, N. et al. PH 60-40: Excretion of radioactivity and
1974 metabolite patterns in rats following oral administration.
Duphar Report no. 56654/20/74. (Unpublished)
1975 Diflubenzuron (PH 60-40): Balance studies in the rat, and
identification of urinary metabolites. Duphar Report no.
56654/22/75. (Unpublished)
1977 Diflubenzuron: Intestinal absorption in the rat in relation
to dosage level. Duphar Report no. 56654/10/77.
(Unpublished)
De Lange, N. Diflubenzuron: Dermal absorption in the rabbit. Duphar
1979 Report no. 56654/9/79. (Unpublished)
De Lange, N. and Post, L.C. Diflubenzuron: intestinal absorption in
1978 the mouse in relation to dosage level. Duphar Report no.
56654/4/78. (Unpublished)
Deul, D.H. and De Jong, B.J. The possible influence of DU 112307 on
1977 the in vivo synthesis of hyaluronic acid in chicken skin.
Duphar Report no. 56635/1/77. (Unpublished)
Di Prima, S.J. Determination of diflubenzuron residues in soybean
1976a foliage and seed by gas chromatography. Analytical Method
no. 10, Thompson-Hayward Chemical Co. (Unpublished)
1976b Determination of diflubenzuron residues in soybean process
fractions by gas chromatography. Method no. 13, Thompson-
Hayward Chemical Company. (Unpublished)
1978 Analysis of diflubenzuron residues in environmental samples
by high-pressure liquid chromatography. Journal of
Agricultural and Food Chemistry, 26:968.
Dorough, H.W. Screening of selected Thompson Hayward chemicals for
1977 activity in the Ames Salmonella mutagenic test. University
of Kentucky, Lexington, USA. (Unpublished)
Duphar. Reports on residues of diflubenzuron. In apples, reports nos.
1975-81 30/39/75, 30/6/A/75, 30/22A/76, 30/44/76, 30/45/76,
30/53/76, 30/10/77, 30/11/77, 30/13/77, 30/14/77, 30/84/77,
30/22/78, 30/38/79, 30/76/80, In pears, reports nos.
30/91/76,30/84/77, 30/96/77, 30/97/77. In brassica leafy
vegetables reports nos. 30/48/76,30/91/77, 30/73/78. In
soybeans, reports nos. 83/06/81, SR35. In other crops,
reports nos. 30/72/77, 30/102/77, 30/129/77, 30/130/77,
30/40/78, 30/9/79, 30/43/80,83/05/81, 83/07/81, K/R/003/77,
K/R/004/77, K3R/005/77, K/R/006/77, K/R/007/77.(Unpublished)
Goodman, D.G. Histopathologic evaluation of mice administered
1980a diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
1980b Histopathologic evaluation of rats administered
diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
Gustafson, D.E. and Wargo, J.P. Fate of Dimilin following application
1976 to soybeans. Analytical Development Corporation Project 222.
(Unpublished)
Hawkins, D.R. Excretion of radioactivity after oral administration of
1980 3H/14C-diflubenzuron to cats. Huntingdon Research Centre
Report no. PDR/302/80443. (Unpublished)
Helling, C.S. Soil mobility of three Thompson-Hayward pesticides.
1975 ARS/USDA Beltsville, USA. (Unpublished)
Hsu, T.S. and Bartha, R. Interaction of pesticide-derived
1974 chloroaniline residues with soil organic matter. Soil
Science, 16:444.
Hunter, B. et al. DU 112307, preliminary assessment of the toxicity to
1974 male mice in dietary administration for 6 weeks. Huntingdon
Research Centre Report no.PDR/174/74199. (Unpublished)
Hunter, B. et al. Tumorigenicity of DU 112307 to mice, dietary
1975 administration for 80 weeks. Huntingdon Research Centre
Report no. PDR/170/75685. (Unpublished)
1976 Effects of DU 112307 in dietary administration to rats for
104 weeks. Huntingdon Research Centre Report no.
PDR/171/75945. (Unpublished)
1979 DU 112307, toxicity to rats in dietary administration for
nine weeks followed by a four-week withdrawal period.
Huntingdon Research Centre Report no. PDR/248/77883
(Unpublished)
Ivie, G.W. Fate of diflubenzuron in cattle and sheep. Journal of
1978 Agricultural and Food Chemistry, 26:81.
Ivie, G.W. and Wright, J.E. Fate of diflubenzuron in the stable fly
1978 and house fly. Journal of Agricultural and Food Chemistry,
26:90.
Ivie, G.W. Fate of diflubenzuron in water. Journal of Agricultural and
1980 Food Chemistry, 28: 330.
Jagannath, D.R. and Brusick, D.J. Mutagenicity evaluation of
1977a 4-chlorophenylurea. Litton Bionetics Project no. 20838.
(Unpublished)
1977b Mutagenic evaluation of 2,6-difluorobenzoic acid. Litton
Bionetics Project no.20838. (Unpublished)
1977c Mutagenicity evaluation of 4-chloroaniline. Litton Bionetics
Project no. 20838. (Unpublished)
Keet, C.M.J.F. Effects of DU 112307 technical after dietary
1976b administration to male Hubbard Broiler chickens for 14
weeks. Duphar Report no. 56645/25/76. (Unpublished)
1977a The met-haemoglobin, sulph-haemoglobin and Heinz body
forming properties of DU 112307 after oral administration to
male rats during 8 days. Duphar Report no. 56645/15/77.
(Unpublished)
1977b The effect of DU 112307 (technical) in male mice after daily
oral administration for a period of 14 days on body weight,
met-haemoglobin, sulph-haemoglobin and Heinz body formation
and on gross pathology. Duphar Report no. 56645/33/77.
(Unpublished)
1977c The met-haemoglobin and sulph-haemoglobin forming properties
of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Duphar Report no. 56645/2/77.
(Unpublished)
Kemp, A. et al. Dietary administration of DU 112307 to male and
1973a female rats for 3 months. Duphar Report no. 56645/13A/73.
(Unpublished)
1973b Appendix III to report no. 56645/13A/73, individual data:
dietary administration of DU 112307 to male and female rats
for 3 months. Duphar Report no. 56645/13B/73. (Unpublished)
Keuker, H. Diflubenzuron, physical and chemical properties. Duphar
1975 Report no. 56630/107/75. (Unpublished)
Koelman-Klaus, H.J.S. Acute oral toxicity study of p-chlorophenylurea
1978a in male and female rats. Duphar Report no. 56645/9/78.
(Unpublished)
1978b Acute oral toxicity study of 2,6-difluorobenzoic acid in
male and female rats. Duphar Report no. 56645/25/78.
(Unpublished)
Koopman, T.S.M. Acute oral toxicity study with DU 112307 (technical)
1977a in mice. Duphar Report no. 56645/4/77. (Unpublished)
1977b Acute intra-peritoneal toxicity study with DU 112307
(technical) in mice. Duphar Report no. 56645/5/77.
(Unpublished)
1977c Acute dermal toxicity study with DU 112307 (technical) in
rats. Duphar Report no. 56645/7/77. (Unpublished)
Maas, W. et al. Benzoylphenylurea insecticides. In:R. Wegler (Ed.),
1980 Chemie der Pflanezen-schutz-und Schädlingsbekämpfungs-
mittel, Band 6. Springer Verlag, Heidelberg, 1980.
Mansager, E.R. et al. Fate of 14C-diflubenzuron on cotton and in soil.
1979 Pesticide Biochemistry and Physiology, 12: 172.
Matheson, D.W. and Brusick, D.J. Evaluation of 4-chlorophenylurea: In
1978a vitro malignant transformation in BALB/3T3/cells. Litton
Bionetics Project no. 20840. (Unpublished)
1978b Evaluation of 2,6-difluorobenzoic acid: in vitro malignant
transformation in BALB/3T3/cells. Litton Bionetics Project
no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 2,6-difluorobenzoic acid in the
unscheduled DNA synthesis in human WI-38 cell assay. Litton
Bionetics Project no. 20840. (Unpublished)
Matheson, D.W. et al. Mutagenicity evaluation of 4-chlorophenylurea in
1978a the unscheduled DNA synthesis in human WI-38 cells assay.
Litton Bionetics Project no. 20840. (Unpublished)
1978b Mutagenic evaluation of 4-chloroaniline in the in vitro
transformation of BALB/3T3 cells assay. Litton Bionetics
Project no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 4-chloroaniline in the
unscheduled DNA synthesis in human WI-38 cells assay. Litton
Bionetics Project no. 20840. (Unpublished)
McGregor, J.T. et al. Mutagenicity tests of diflubenzuron in the
1979 micronucleus test in mice, the L5178Y mouse lymphoma forward
mutation assay, and the Ames Salmonella Reserve mutation
test. Mutation Research, 66:45.
Miller, R.W. et al. Feeding TH-6040 to cattle: Residues in tissues and
1976a milk and breakdown in manure. Journal of Agricultural and
Food Chemistry, 24:687.
1976b Effects of feeding TF-6040 to two breeds of chickens.
Journal of Economic Entomology, 69:741.
Miller, R.W., Cecil, H.C., Carey, A.M., Corley, C. and Kiddy, C.A.
1979 Effects of feeding diflubenzuron to young male Holstein
cattle. Bulletin of Environmental Contamination and
Toxicology, 23:482.
Moreale, A. and Van Bladel, R. Influence of soil properties on
1976 absorption of pesticide-derived aniline and p-chloroaniline.
Journal of Soil Science, 27:48.
Nimmo, W.B. and De Wilde, P.C. Fate of diflubenzuron on leaves of
1974 corn, soybean, cabbage and apples. Duphar Report no.
56635/16A/74. (Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in soil
1975a and natural water. Duphar Report no. 56635/37/1975.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in sterile
1975b water at pH 5,7, 9 and 12. Duphar Report no. 56635/32/75.
(Unpublished)
Nimmo, W. B. and De Wilde, P.C. Re-uptake from diflubenzuron treated
1976a soil by soybean, maize and tomato. Duphar Report no.
56635/4/76. (Unpublished)
1976b Uptake of diflubenzuron and its metabolites from the soil by
rice wheat. Duphar Report no. 56635/8/76. (Unpublished)
Nimmo W.B. and De Wilde, P.C. Degradation of diflubenzuron in mushroom
1977a growth medium and uptake of its metabolites in the
mushrooms. Duphar Report no. 56635/21A/77. (Unpublished)
1977b Degradation of diflubenzuron in cow and chicken manure.
Duphar Report no.56635/27/77. (Unpublished)
Nimmo, W.B. et al. Fate of diflubenzuron applied to leaves and fruits
1978 of apples trees. Duphar Report no. 56635/33/78.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. The fate of 14C-diflubenzuron on pine.
1980 Duphar Report no. 56635/18/79. (Unpublished)
Offer, J.M. Tumorigenicity of DU 112307 to mice, dietary
1977 administration for 80 weeks. Addendum: histological
examination of additional tissues, Huntingdon Research
Centre Report PDR/250, addendum to PDR/170/75685.
(Unpublished)
Opdycke, J.C. Metabolism and fate of diflubenzuron in chickens and
1976 swine. M. Sc.Thesis, Graduate School University of Maryland,
College Park, USA.
Palmer, A.K. and Hill, P.A. Effect of DU 112307 on reproduction
1975a function of multiple generations in the rat. Huntingdon
Research Centre Report no. PDR/17375954. (Unpublished)
1975b Effect of DU 112307 on pregnancy of the rat. Huntingdon
Research Centre Report no. PDR/192/74978. (Unpublished)
1975c Effect of DU 112307 on pregnancy of the New Zealand white
rabbit. Huntingdon Research Centre Report no. PDR/193/74937.
(Unpublished)
Palmer, A.K. et al. Preliminary assessment of the effect of DU 112307
1977 on the rat. Huntingdon Research Centre Report no.
PDR/243/77208. (Unpublished)
1978 Effect of dietary administration of DU 112307 on
reproduction function of one generation in the rat.
Huntingdon Research Centre Report no. PDR/244/78653.
(Unpublished)
Patel, Y.M. and Santolucito, J.A. Selected toxicological studies of
1980 Dimilin in weanling male rats. EPA-600/3-80-031.
(Unpublished)
Pimprikar, G.D. Mechanisms of resistance to Dimilin in Musca
1977 domestica. Ph.D. Thesis University of California, Riverside,
USA.
Popp, F. Storage stability of Dimilin 25W, Duphar Report no.
1977 56623/6/77. (Unpublished)
Quarles, J.M., Norman, J.O. and Kubena, L.F. Absence of transformation
1980 by diflubenzuron in a host-mediated transplacental
carcinogen assay. Bulletin of Environmental Contamination
and Toxicology, 25:252.
Rabenor, B., De Wilde, P.C., De Boer, F.G., Korven, P.K., Di Prima, S.
1978 J. and Canizzaro, R.D. In: G. Zweig (Ed.), Determination of
diflubenzuron residues using GLC. Analytical Methods for
Pesticides and Plant Growth Regulators. Vol. 10. Academic
Press, New York, p. 57-72.
Rieck, C.E. Soil column leaching study with Dimilin. University of
1975 Kentucky, Lexington, USA. (Unpublished)
Reinert, H.K. and Cannon, G.E. The evaluation of TH 6040 in four avian
1976 species. Cannon Laboratories Report no. 5E-8680A-D.
(Unpublished)
Ross, D.B. et al. DU 112307, 13-week oral toxicity study in sheep.
1977a Huntingdon Research Centre Report no. PDR/299/77226.
(Unpublished)
1977b Blood red cell and plasma cholinesterase value following
six-week inclusion of DU 112307 in the diet of sheep.
Huntingdon Research Centre Report no. PDR/229A/77225.
(Unpublished)
1977c The effects of DU 112307 on female chickens following
dietary inclusion for 98 days. Huntingdon Research Centre
Report no. PDR/240/77183. (Unpublished)
Ross, D.B. et al. The effects of DU 112307 on female chickens
1979 following dietary inclusion for 98 days, (second study).
Huntingdon Research Centre Report No. PDR/251/781030.
(Unpublished)
Schaefer, c. and Dupras, E.F. Factors affecting the stability in water
1976 and the persistence of Dimilin in field waters. Journal of
Agricultural and Food Chemistry, 24:733.
Schaefer, C. et al. The occurrence of p-chloroaniline and p-
1980 chlorophenylurea from degradation of diflubenzuron in water
and fish. Proc. 48th Annual Conference of California
Mosquito & Vector Control Association, Anaheim, USA.
Schwartz, S.L. and Borzelleca, J.F. Effects of diflubenzuron on met-
1981 haemoglobin, sulph-haemoglobin and Heinz body production in
cats. Georgetown University, Washington, D.C., USA
(Unpublished)
Seegmiller, R.E. and Booth, G.M. Teratogenic and biochemical effects
1976 of TH 6040 and chick embryos. Brigham Young University,
Provo, Utah, USA. (Unpublished)
Seuferer, S.L., Braymer, H.D. and Dunn, J.J. Metabolism of
1979 diflubenzuron by soil organisms and mutagenicity of the
metabolites. Pesticide Biochemistry and Physiology, 10: 174.
Smalley, H.E. Comparative toxicology of some insect growth regulators.
1976 Clinical Toxicology, 9:27.
Smith, K.S. and Merricks, D.L. TH 6040:Tissue residue and metabolism
1976a study in dairy cows. Cannon Labs, lab.no. 5E-7372 (March
29). (Unpublished)
1976b TH 6040: tissue residue and metabolism study in poultry.
Cannon Labs, lab.no. 5E-7372 (March 12). (Unpublished)
Spencer-Jones, D.H. Letter from Duphar Midox to Duphar, 25 May.
1979
Stoolmiller, A.C. Toxicological study on the effect of diflubenzuron
1978 on rat C-6 astrocytoma cells In vitro. Gen. Pharmac:, 9:11.
Van den Berg, G. Uptake, translocation and metabolism of
1978a 4-chlorophenylurea in tomato and broad bean. Duphar Report
no. 56635/5/67. (Unpublished)
1978b Uptake and decarboxylation of 2,6-difluorobenzoid acid in
tomato. Duphar Report no. 56635/4/78. (Unpublished)
Van Eldik, A. Acute toxicity studies with DU 112307 in mice and rats.
1973 Duphar Report no. 56645/14/73. (Unpublished)
1974 Acute toxicity studies with DU 112307 (technical) in mice
after intra-peritoneal administration. Duphar Report no.
56645/1/74. (Unpublished)
Verlopp, A. and Ferrell, C.D. Benzoylphenylureas, a new group of
1977 larvicides interfering with chitin deposition. American
Chemical Society Symposium.
Willems, A.C.M. et al. Diflubenzuron: Intestinal absorption and
1980 metabolism in the rat. Xenobiotica, 10:103.
 Other information on identity and properties (Keuker 1975)
Molecular weight 310.7
State white, crystalline solid
Melting point (pure compound) 230-232°C
Specific gravity 1.56
Volatility virtually non-volatile
Stability:
- Heat stability after 1 week storage at 50°C, or after
1 day at 100°, no detectable
decomposition.
- Stability in water after 3 weeks at pH 5-4% decomposition;
(0.1 mg/l solution) after 3 weeks at pH 7-8% decomposition;
and after 3 weeks at pH 9-26%
decomposition.
Solubility (g/l at 20°C)
- N-methylpyrolidone 200
- DMSO 120
- DMF 120
- Dioxane 24
- Acetone 6.5
- Acetonitrile 2
- Methanol 0.9
- Dichloromethane 0.6
- Water 0.0002
Partition coefficients
- Dichloromethane/water > 50
- n-Octanol/
water-approximately 5000
Purity of technical product
Diflubenzuron technical contains > 95% pure compound.
Formulations
The main formulation of diflubenzuron is DIMILIN WP-25, a
wettable powder containing 25% of the active ingredient. This is the
formulation that is recommended for use on food crops. The particle
size of the diflubenzuron is defined as 80% smaller than 5 µm. This
formulation has an excellent storage stability; storage for 2 years at
room temperature or 1 year at 54°C did not affect its properties (Popp
1977).
DIMILIN ODC-45 is an oil-dispersible concentrate, containing
450 g diflubenzuron per litre. After dilution with a suitable organic
solvent, this formulation can be applied at ULV rates.
DIMILIN granular formulations are available for the control of
mosquitoes and flies.
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
The intestinal absorption of diflubenzuron in mammals decreases
with increasing dose level. Radio-labelled diflubenzuron, with the
14C label uniformly distributed in the anilino moiety, was
administered by gavage to rats in a single dose of 4, 16, 48, 128 or
1 000 mg/kg bw. Urine was collected in 24 h portions up to 120 h or
144 h. The cumulative excretion in urine as percentage of the dose
decreased from 28% at the 4 mg/kg dose level to only 1% at the
1 000 mg/kg dose level, while total recoveries remained constant
(90%). In rats with cannulated bile ducts, the sum of the excretion in
urine and bile decreased from 42% of the dose at the dose level of
4 mg/kg to about 4% at the dose level of 900 mg/kg (De Lange et al.
1977; Willems et al. 1980).
In mice given a single oral dose of 12.5, 63.5, 202.5 or
925 mg/kg bw, the excretion was almost completed within 48 h. The
cumulative percentage of the dose excreted in the urine decreased from
15% at the dose level of 12.5 mg/kg to approximately 2% at the
925 mg/kg dose level, showing that the relationship between urinary
excretion and dose level in mice is similar to that in rats (De Lange
and Post 1978).
Sheep treated with 500 mg/kg of 14C-diflubenzuron as a single
oral dose eliminated approximately 7% and 5% of the dose in urine and
bile, respectively, during a 4-day post-treatment period. Sheep
receiving a single oral dose of 10 mg/kg eliminated approximately 24%
and 36% of the dose in urine and bile during the same period.
After oral treatment of a lactating cow with radio-labelled
14C-diflubenzuron (10 mg/kg bw) the elimination of radioactivity was
85% in faeces and 16% in urine during a 4-day post-treatment period.
Only about 0.2% of the radio label was secreted into the milk. Maximum
milk residues of 0.8 mg/kg diflubenzuron equivalents were observed in
milk samples collected 24 h after dosing, but radioactivity in milk
had dropped to <0.1 mg/kg by 3 days (Ivie 1978). When applied
dermally, diflubenzuron was not degraded or absorbed through the skin
to any detectable degree in cattle (Ivie 1978), whereas in rabbits
only 0.2% of the dose could be recovered in urine (De Lange 1979).
A pig treated orally with 14C-diflubenzuron (5 mg/kg bw)
excreted 82% of the radioactivity in faeces and 5% in urine in 11 days
(Opdycke 1976).
Chickens receiving a single oral dose of 5 mg/kg of radiolabelled
14C-diflubenzuron excreted the radioactivity in a similar way (91% in
White Leghorn excreta and 82% for Buff Cross chickens after 13 days)
(Opdycke 1976).
Radio-labelled diflubenzuron (14C and 3H) was orally
administered to cats (7 mg/kg bw) on day 10 of a 15-day dosing regimen
of non-radioactive diflubenzuron. Within 72 h after administration, 9%
of the oral dose was excreted in the urine and 77% of the 14C and 71%
of the 3H doses in faeces (Hawkins et al 1980).
In rats, at 72 h after administration of a single oral dose of
5 mg/kg of double-labelled (14C in the anilino moiety and 3H in the
benzoyl moiety) diflubenzuron, 1.3% of the 14C and 3.5% of the 3H
label was retained in the carcasses (De Lange et al 1975). In
expired air of rats that had received radio-labelled diflubenzuron
(14C in the carbonyl group of the benzoyl moiety) 1% of radioactivity
was found (De Lange et al 1975).
Body tissues showed little tendency toward retention of
diflubenzuron. At 4 or 7 days post-treatment appreciable residues
could be detected only in the liver of cow and sheep (Ivie 1978). Low
residues were found also in tissues of pig and chicken. A small
portion of the radioactivity was secreted into eggs of chickens. The
maximum residue level was 0.248 mg/kg 3 days after a single oral dose
of 5 mg/kg bw 14C-diflubenzuron (Opdycke 1976).
The distribution of radioactivity resulting after oral
administration of radio-labelled diflubenzuron (14C in both phenyl
moieties) was studied in cows receiving daily doses in gelatin
capsules over a 28-day period. The daily dose levels equalled 0.05,
0.5, 5.0 and 250 mg/kg feed. No residues in milk were detected at the
two low dose levels. At the 5.0 mg/kg dose level, an average of
0.009 mg/kg of diflubenzuron equivalents was found in the milk: a
plateau was reached between day 4 and day 7 of exposure. After 4 days
of withdrawal, levels were undetectable (<0.0016 mg/kg). At the
250 mg/kg dose level, a plateau was reached in milk by day 2 at a
residue level of 0.20 mg/kg of diflubenzuron equivalents. Total 14C
tissue analyses showed that at dose levels of 0.05, 0.5 and 5 mg/kg
feed only the liver contained detectable, dose-related residues. For
the lowest dose level the radioactivity was just above the limit of
detection. This limit of detection increased with increasing dose
levels in relation to the specific activity of the dosed material.
Withdrawal for 7 days did not result in a substantial decrease in
activity in the liver. At the 250 mg/kg feed, only tested for 7 days
of treatment, residues were found in the kidney and in the liver
(Smith and Merricks 1976a).
In a similar experiment, laying hens were administered daily
dosages equalling 0.05, 0.5 and 5 mg/kg feed of 14C-diflubenzuron for
28 days. A plateau level was reached before day 10 of treatment in
fat, kidney, liver, muscle and eggs. However, the radioactivity in
tissues showed large day-to-day variations. There was a linear
relationship between dose level and plateau level for the kidney,
liver and fat, whereas an exponential relationship was obtained for
eggs. At day 7 of withdrawal, levels in all tissues and eggs were
below the limit of detection (Smith and Merricks 1976b).
Two dairy cows were fed diflubenzuron at rates of 0.25 mg/kg
bw/day or 1.0 mg/kg/day for four months. A third cow received rates
that were increased from 1 via 8 to 16 mg/kg/day, the highest rate
being maintained for three months. In the tissues examined of the
third cow, fat and liver showed residues of 0.2 mg/kg and 0.13 mg/kg
respectively. In the milk of this cow, a residue level of 0.02 mg/kg
could be established at a dose rate of 16 mg/kg (Miller et al
1976a).
The eggs of White Leghorn and Black Sexlinked Cross hens, kept on
a ration containing 10 mg/kg of diflubenzuron, contained plateau
residues of 0.5 to 0.6 or 0.3 to 0.4 mg/kg respectively, from day 9 of
a treatment period of 9 weeks (Miller et al. 1976b).
The metabolic fate of diflubenzuron has been studied in various
species. It appears that diflubenzuron is not degraded to any
significant degree within the digestive tract of mammals, as the radio
label eliminated in the faeces of bileduct-cannulated sheep receiving
an oral dose of 14C-diflubenzuron consisted only of the unmetabolized
compound (Ivie 1978). Furthermore, diflubenzuron did not degrade to
any extent when incubated in vitro with digestive tract fluids of
sheep and cattle (Ivie 1978). On the other hand, it was found that no
unchanged diflubenzuron could be detected in urine and bile of orally
dosed rats, sheep, or cattle (Willems et al 1980; Ivie 1978).
In rats and cows, the major metabolic pathway involved
hydroxylation of the phenyl moieties of the intact compound. About 80%
of the metabolites in rat urine were identified as 2,6-difluoro-
3-hydroxybenzuron, 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the ureido
bridge. The major part was excreted as 2,6-difluorobenzoic acid;
4-chlorophenylurea was not detected in bile or urine in a significant
quantity (Willems et al 1980; De Lange et al 1975).
The major metabolite in cow urine was 2,6-difluoro
3-hydroxydiflubenzuron (45%); in addition, relatively small quantities
of 4-chloro-2-hydroxy-(1.6%) and 4-chloro-3-hydroxy-difluorobenzuron
(3.7%) and the scission products 4-chlorophenylurea (0.6%),
2,6-difluorobenzoic acid (6.0%) and 2,6-difluorohippuric acid (6.9%)
were present (Ivie 1978). In contrast to these observations, the
latter two compounds are the major metabolites (approximately 50%) in
sheep urine (Ivie 1978).
In pigs, all of the 14C-diflubenzuron residues extracted from
faeces co-chromatographed with diflubenzuron. In urine, only small
quantities of the cleavage products 2,6-difluoro-benzoic acid,
4-chlorophenylurea and 4-chloroaniline were detected. These data
indicate little metabolism of the compound in swine (Opdycke 1976).
In chickens also, little degradation was observed while the
same metabolic pathway was found (Opdycke 1976). In a detailed
investigation, carried out to find an explanation for the occurrence
of methaemoglobinaemia in diflubenzuron-treated rats, 4-chloroaniline
and compounds reducible to 4-chloroaniline, probably consisting of
N-oxidation products of the aniline, were detected in red blood cells
(De Bree et al 1977).
Metabolism of diflubenzuron in animals is shown in Figure 1.
Effects on enzymes
The activity of the mammalian hexosamine transferases,
responsible for connective tissue glycosaminoglycan formation, was
monitored in adult female mice fed 50, 200, 400, 1 000 and 2 000 mg
diflubenzuron/kg. In these animals the rate of incorporation of
14C-glucose into hyaluronic acid and chondroitin sulphate of skin was
studied. No inhibition was noted. Sulphaemoglobin was demonstrated in
the blood of mice from 200 mg/kg onwards. Recovery was completed after
a 3-week withdrawal period (Bentley et al. 1979).
In another study with rat C-6 astrocytoma cell cultures, 100 nM
(31 ppm) diflubenzuron in the medium did not affect cell morphology or
the rate of cell division. Diflubenzuron did not inhibit the total
production of glycosaminoglycans (Stoolmiller 1978).
Hubbard broiler chickens were fed 0, 2.5 or 250 mg
diflubenzuron/kg feed during 98 days. There was no effect of
diflubenzuron on the incorporation of amino sugar moieties into
mucopolysaccharides of the skin of the animals (Deul and de Jong
1977). The incorporation of 0, 2.5, 25 and 250 mg diflubenzuron/kg in
the diet of male and female 28-day old chickens, for 98 days, did not
affect the hyaluronic acid concentration in the combs (Crookshank
et al. 1978).
Other information on identity and properties (Keuker 1975)
Molecular weight 310.7
State white, crystalline solid
Melting point (pure compound) 230-232°C
Specific gravity 1.56
Volatility virtually non-volatile
Stability:
- Heat stability after 1 week storage at 50°C, or after
1 day at 100°, no detectable
decomposition.
- Stability in water after 3 weeks at pH 5-4% decomposition;
(0.1 mg/l solution) after 3 weeks at pH 7-8% decomposition;
and after 3 weeks at pH 9-26%
decomposition.
Solubility (g/l at 20°C)
- N-methylpyrolidone 200
- DMSO 120
- DMF 120
- Dioxane 24
- Acetone 6.5
- Acetonitrile 2
- Methanol 0.9
- Dichloromethane 0.6
- Water 0.0002
Partition coefficients
- Dichloromethane/water > 50
- n-Octanol/
water-approximately 5000
Purity of technical product
Diflubenzuron technical contains > 95% pure compound.
Formulations
The main formulation of diflubenzuron is DIMILIN WP-25, a
wettable powder containing 25% of the active ingredient. This is the
formulation that is recommended for use on food crops. The particle
size of the diflubenzuron is defined as 80% smaller than 5 µm. This
formulation has an excellent storage stability; storage for 2 years at
room temperature or 1 year at 54°C did not affect its properties (Popp
1977).
DIMILIN ODC-45 is an oil-dispersible concentrate, containing
450 g diflubenzuron per litre. After dilution with a suitable organic
solvent, this formulation can be applied at ULV rates.
DIMILIN granular formulations are available for the control of
mosquitoes and flies.
DATA FOR ESTIMATION OF ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution, biotransformation and excretion
The intestinal absorption of diflubenzuron in mammals decreases
with increasing dose level. Radio-labelled diflubenzuron, with the
14C label uniformly distributed in the anilino moiety, was
administered by gavage to rats in a single dose of 4, 16, 48, 128 or
1 000 mg/kg bw. Urine was collected in 24 h portions up to 120 h or
144 h. The cumulative excretion in urine as percentage of the dose
decreased from 28% at the 4 mg/kg dose level to only 1% at the
1 000 mg/kg dose level, while total recoveries remained constant
(90%). In rats with cannulated bile ducts, the sum of the excretion in
urine and bile decreased from 42% of the dose at the dose level of
4 mg/kg to about 4% at the dose level of 900 mg/kg (De Lange et al.
1977; Willems et al. 1980).
In mice given a single oral dose of 12.5, 63.5, 202.5 or
925 mg/kg bw, the excretion was almost completed within 48 h. The
cumulative percentage of the dose excreted in the urine decreased from
15% at the dose level of 12.5 mg/kg to approximately 2% at the
925 mg/kg dose level, showing that the relationship between urinary
excretion and dose level in mice is similar to that in rats (De Lange
and Post 1978).
Sheep treated with 500 mg/kg of 14C-diflubenzuron as a single
oral dose eliminated approximately 7% and 5% of the dose in urine and
bile, respectively, during a 4-day post-treatment period. Sheep
receiving a single oral dose of 10 mg/kg eliminated approximately 24%
and 36% of the dose in urine and bile during the same period.
After oral treatment of a lactating cow with radio-labelled
14C-diflubenzuron (10 mg/kg bw) the elimination of radioactivity was
85% in faeces and 16% in urine during a 4-day post-treatment period.
Only about 0.2% of the radio label was secreted into the milk. Maximum
milk residues of 0.8 mg/kg diflubenzuron equivalents were observed in
milk samples collected 24 h after dosing, but radioactivity in milk
had dropped to <0.1 mg/kg by 3 days (Ivie 1978). When applied
dermally, diflubenzuron was not degraded or absorbed through the skin
to any detectable degree in cattle (Ivie 1978), whereas in rabbits
only 0.2% of the dose could be recovered in urine (De Lange 1979).
A pig treated orally with 14C-diflubenzuron (5 mg/kg bw)
excreted 82% of the radioactivity in faeces and 5% in urine in 11 days
(Opdycke 1976).
Chickens receiving a single oral dose of 5 mg/kg of radiolabelled
14C-diflubenzuron excreted the radioactivity in a similar way (91% in
White Leghorn excreta and 82% for Buff Cross chickens after 13 days)
(Opdycke 1976).
Radio-labelled diflubenzuron (14C and 3H) was orally
administered to cats (7 mg/kg bw) on day 10 of a 15-day dosing regimen
of non-radioactive diflubenzuron. Within 72 h after administration, 9%
of the oral dose was excreted in the urine and 77% of the 14C and 71%
of the 3H doses in faeces (Hawkins et al 1980).
In rats, at 72 h after administration of a single oral dose of
5 mg/kg of double-labelled (14C in the anilino moiety and 3H in the
benzoyl moiety) diflubenzuron, 1.3% of the 14C and 3.5% of the 3H
label was retained in the carcasses (De Lange et al 1975). In
expired air of rats that had received radio-labelled diflubenzuron
(14C in the carbonyl group of the benzoyl moiety) 1% of radioactivity
was found (De Lange et al 1975).
Body tissues showed little tendency toward retention of
diflubenzuron. At 4 or 7 days post-treatment appreciable residues
could be detected only in the liver of cow and sheep (Ivie 1978). Low
residues were found also in tissues of pig and chicken. A small
portion of the radioactivity was secreted into eggs of chickens. The
maximum residue level was 0.248 mg/kg 3 days after a single oral dose
of 5 mg/kg bw 14C-diflubenzuron (Opdycke 1976).
The distribution of radioactivity resulting after oral
administration of radio-labelled diflubenzuron (14C in both phenyl
moieties) was studied in cows receiving daily doses in gelatin
capsules over a 28-day period. The daily dose levels equalled 0.05,
0.5, 5.0 and 250 mg/kg feed. No residues in milk were detected at the
two low dose levels. At the 5.0 mg/kg dose level, an average of
0.009 mg/kg of diflubenzuron equivalents was found in the milk: a
plateau was reached between day 4 and day 7 of exposure. After 4 days
of withdrawal, levels were undetectable (<0.0016 mg/kg). At the
250 mg/kg dose level, a plateau was reached in milk by day 2 at a
residue level of 0.20 mg/kg of diflubenzuron equivalents. Total 14C
tissue analyses showed that at dose levels of 0.05, 0.5 and 5 mg/kg
feed only the liver contained detectable, dose-related residues. For
the lowest dose level the radioactivity was just above the limit of
detection. This limit of detection increased with increasing dose
levels in relation to the specific activity of the dosed material.
Withdrawal for 7 days did not result in a substantial decrease in
activity in the liver. At the 250 mg/kg feed, only tested for 7 days
of treatment, residues were found in the kidney and in the liver
(Smith and Merricks 1976a).
In a similar experiment, laying hens were administered daily
dosages equalling 0.05, 0.5 and 5 mg/kg feed of 14C-diflubenzuron for
28 days. A plateau level was reached before day 10 of treatment in
fat, kidney, liver, muscle and eggs. However, the radioactivity in
tissues showed large day-to-day variations. There was a linear
relationship between dose level and plateau level for the kidney,
liver and fat, whereas an exponential relationship was obtained for
eggs. At day 7 of withdrawal, levels in all tissues and eggs were
below the limit of detection (Smith and Merricks 1976b).
Two dairy cows were fed diflubenzuron at rates of 0.25 mg/kg
bw/day or 1.0 mg/kg/day for four months. A third cow received rates
that were increased from 1 via 8 to 16 mg/kg/day, the highest rate
being maintained for three months. In the tissues examined of the
third cow, fat and liver showed residues of 0.2 mg/kg and 0.13 mg/kg
respectively. In the milk of this cow, a residue level of 0.02 mg/kg
could be established at a dose rate of 16 mg/kg (Miller et al
1976a).
The eggs of White Leghorn and Black Sexlinked Cross hens, kept on
a ration containing 10 mg/kg of diflubenzuron, contained plateau
residues of 0.5 to 0.6 or 0.3 to 0.4 mg/kg respectively, from day 9 of
a treatment period of 9 weeks (Miller et al. 1976b).
The metabolic fate of diflubenzuron has been studied in various
species. It appears that diflubenzuron is not degraded to any
significant degree within the digestive tract of mammals, as the radio
label eliminated in the faeces of bileduct-cannulated sheep receiving
an oral dose of 14C-diflubenzuron consisted only of the unmetabolized
compound (Ivie 1978). Furthermore, diflubenzuron did not degrade to
any extent when incubated in vitro with digestive tract fluids of
sheep and cattle (Ivie 1978). On the other hand, it was found that no
unchanged diflubenzuron could be detected in urine and bile of orally
dosed rats, sheep, or cattle (Willems et al 1980; Ivie 1978).
In rats and cows, the major metabolic pathway involved
hydroxylation of the phenyl moieties of the intact compound. About 80%
of the metabolites in rat urine were identified as 2,6-difluoro-
3-hydroxybenzuron, 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the ureido
bridge. The major part was excreted as 2,6-difluorobenzoic acid;
4-chlorophenylurea was not detected in bile or urine in a significant
quantity (Willems et al 1980; De Lange et al 1975).
The major metabolite in cow urine was 2,6-difluoro
3-hydroxydiflubenzuron (45%); in addition, relatively small quantities
of 4-chloro-2-hydroxy-(1.6%) and 4-chloro-3-hydroxy-difluorobenzuron
(3.7%) and the scission products 4-chlorophenylurea (0.6%),
2,6-difluorobenzoic acid (6.0%) and 2,6-difluorohippuric acid (6.9%)
were present (Ivie 1978). In contrast to these observations, the
latter two compounds are the major metabolites (approximately 50%) in
sheep urine (Ivie 1978).
In pigs, all of the 14C-diflubenzuron residues extracted from
faeces co-chromatographed with diflubenzuron. In urine, only small
quantities of the cleavage products 2,6-difluoro-benzoic acid,
4-chlorophenylurea and 4-chloroaniline were detected. These data
indicate little metabolism of the compound in swine (Opdycke 1976).
In chickens also, little degradation was observed while the
same metabolic pathway was found (Opdycke 1976). In a detailed
investigation, carried out to find an explanation for the occurrence
of methaemoglobinaemia in diflubenzuron-treated rats, 4-chloroaniline
and compounds reducible to 4-chloroaniline, probably consisting of
N-oxidation products of the aniline, were detected in red blood cells
(De Bree et al 1977).
Metabolism of diflubenzuron in animals is shown in Figure 1.
Effects on enzymes
The activity of the mammalian hexosamine transferases,
responsible for connective tissue glycosaminoglycan formation, was
monitored in adult female mice fed 50, 200, 400, 1 000 and 2 000 mg
diflubenzuron/kg. In these animals the rate of incorporation of
14C-glucose into hyaluronic acid and chondroitin sulphate of skin was
studied. No inhibition was noted. Sulphaemoglobin was demonstrated in
the blood of mice from 200 mg/kg onwards. Recovery was completed after
a 3-week withdrawal period (Bentley et al. 1979).
In another study with rat C-6 astrocytoma cell cultures, 100 nM
(31 ppm) diflubenzuron in the medium did not affect cell morphology or
the rate of cell division. Diflubenzuron did not inhibit the total
production of glycosaminoglycans (Stoolmiller 1978).
Hubbard broiler chickens were fed 0, 2.5 or 250 mg
diflubenzuron/kg feed during 98 days. There was no effect of
diflubenzuron on the incorporation of amino sugar moieties into
mucopolysaccharides of the skin of the animals (Deul and de Jong
1977). The incorporation of 0, 2.5, 25 and 250 mg diflubenzuron/kg in
the diet of male and female 28-day old chickens, for 98 days, did not
affect the hyaluronic acid concentration in the combs (Crookshank
et al. 1978).
 TOXICOLOGICAL STUDIES
Acute toxicity
In none of the acute studies on diflubenzuron was any overt sign
of toxicity observed. In the dermal toxicity study with rats, no
effect on the met- and sulph-haemoglobin values was detected. Results
of acute toxicity studies in rat, mouse and rabbit are summarized in
Table 1.
Loss of activity, catatony, paralysis and severe bradypnoea, were
observed in rats treated with the metabolite 4-chlorophenylurea. At
autopsy, the animals showed congested blood vessels and haemorrhagic
intestines. The rats dosed with 2,6-difluorobenzoic acid showed
symptoms indicative for slight C.N.S. excitation and increased muscle
tone. Results of studies with diflubenzuron metabolites are summarized
in Table 2.
Short-term studies
Mouse
Three groups of 8 male CFLP mice were fed dietary levels of
diflubenzuron for 6 weeks at levels of 0, 16 and 50 mg/kg feed. There
was no clear effect of the treatment on food consumption, body
weight, blood chemistry and macroscopic pathology. The weight of the
spleen was decreased in the highest dose group. In some animals
receiving 50 mg/kg feed, foci of liver cell necrosis with or without
inflammatory cell infiltration were noted. The other organs were not
microscopically examined (Hunter et al 1974).
Male and female mice (40/sex/group) received diflubenzuron in the
diet at a dosage level of 16, 50, 400, 2 000, 10 000 or 50 000 mg/kg
feed during 13 weeks. An additional group of one hundred/sex served as
a control. No compound-related effects were apparent in respect to
clinical signs, survival, growth, food consumption or gross pathology.
Significant treatment-related increases in met- and sulph-haemoglobin
concentrations were noted in all treated groups, except the 16 mg/kg
feed one. At the higher dose levels, a decrease was noted in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects were noted on the weights of liver and
spleen. In the females, adrenal weight was not dose-relatedly
decreased at all dose levels after 7 weeks and increased after 13
weeks at higher dose levels. In males a higher adrenal weight was
observed in comparison to controls. Histopathologically, treatment-
related centrolobular hypertrophy of hepatocytes, with or without cell
TABLE 1. Acute toxicity of diflubenzuron
Species Sex Route LD50 Reference
Rat F,M Oral > 4,640 mg/kg Van Eldik 1973
F,M Dermal >10,000 mg/kg Keet 1976a;Koopman 1977c
F,M Inhalation > 35 mg/kg (6 hr) Berczy et al 1973
Mouse F,M Oral > 4,640 mg/kg Van Eldik 1973;Koopman 1977a
F,M i.p. > 2,150 mg/kg Van Eldik 1974;Koopman 1977b
Rabbit F,M Dermal > 4,000 mg/kg
(50% paste) Davies and Halliday 1974
F,M Inhalation > 30 mg/l (6 hr) Berczy et al 1975
TABLE 2. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50(mg/kg) Reference
4-Chlorophenylurea Rat M Oral 1 080 Koelman-Klaus 1978a
F Oral 1 210 Koelman-Klaus 1978a
2,6-Difluorobenzoic Rat M,F Oral 4 640 Koelman-Klaus 1978b
acid
necrosis, haemosiderosis of the liver and spleen, extramedullary
haematopoiesis and mild chronic hepatitis were observed. The liver
lesions were more severe in males than in females. No no-effect level
was observed (Burdock et al. 1980a; Goodman 1980a).
A 14-week feeding study was performed with groups of 40 male and
40 female HC/CFLP mice (control groups, 96 male and 96 females)
maintained on a diet supplemented with 0, 80, 400, 2 000, 10 000 and
50 000 mg diflubenzuron (purity 97.20%/kg feed.) Half of the number of
mice of both sexes of all groups were sacrificed after 7 weeks. On the
second day of treatment, the majority of mice treated with 50 000 or
10 000 mg/kg feed showed dark eyes and/or prominent caudal blood
vessels. On day 5, blue/grey discolouration of the extremities was
noted for the majority of mice treated with 50 000 mg/kg. In the
course of the study, clinical signs were observed in mice of all
groups, except those of the lowest dose group. Mortality, food
consumption, water consumption and body weight changes were not
clearly affected by the treatment. Results of haematological
investigation showed lower packed cell volume and red blood cell
count at all dose levels except 80 mg/kg feed. The total white blood
cell count, lymphocyte count, haemoglobin concentration, the incidence
of Heinz bodies and the red blood cells were increased in all dose
groups. A treatment-related increase in both met- and sulph-
haemoglobin was recorded in all treated groups at weeks 7 and 14 of
the investigation. Clinical biochemistry revealed higher plasma
glutamic pyruvic transaminase at all dose levels, with the exception
of 80 mg/kg feed. Lower blood cholesterol levels were noted in the
50 000, 10 000 and 2 000 mg/kg feed groups. Macroscopic examination
showed dark discolouration and/or enlargement of the spleen and pale
subcapsular areas of the liver in all dose groups, after both 7 and 14
weeks. Histopathological examination of the spleen revealed increased
haemosiderosis at all dose levels except 80 ppm. In the liver, areas
of focal necrosis and/or fibrosis in the parenchyma, with or without
associated inflammatory cells, fibroblasts or pigment-laden
macrophages, were observed. At higher dose levels, necrotic and fatty
hepatocytes and brown pigment-laden Kupffer cells were found (Colley
et al 1981).
Rat
Groups of 5 male and 5 female rats were fed on a diet containing
diflubenzuron in concentrations of 0, 800, 4 000, 20 000 and
100 000 mg/kg during a four-week period. Behaviour, body weight, food
and water consumption were not clearly affected by the treatment.
There was a dose-related increase in met- and sulph-haemoglobin in the
blood in all treated groups, except for the methaemoglobin value for
the females of the 800 mg/kg dose group. Lower RBC, PCV and
haemoglobin values were observed in both sexes of the 100 000 mg/kg
dose group. Post-mortem examination revealed no changes attributable
to diflubenzuron. For both sexes, relative spleen weights in all test
groups differed significantly from control values. Relative liver
weights were increased in males or females at all dose levels, except
800 mg/kg (Palmer et al. 1977).
Groups of 10 male and 10 female Wistar rats received
diflubenzuron during 13 weeks. The concentrations in the diet were 0,
3.125, 12.5, 50 or 200 mg/kg feed. In addition two groups of 5 males
and 5 females received 0 or 200 mg/kg. These animals were sacrificed
after 6 weeks for clinico-chemical analysis. Behaviour, growth and
food intake were not affected by the treatment. At the highest dose
level, the PCV-value, the haemoglobin concentration and the number of
erythrocytes were decreased, whereas the latter was also lowered in
the 50 mg/kg group. Particularly in the males, the SGOT and SGPT
activity was increased in the highest dose group at the end of the
experiment. Higher testicular weights were recorded in the 200 mg/kg
group. At microscopic examination, a slight increase in the number of
small foci of necrotic parenchymal cells was observed, accompanied by
monocellular inflammatory cell infiltration in the liver of both males
and females of the 50 and 200 mg/kg groups (Kemp et al. 1963a, b).
Diflubenzuron was administered to Sprague-Dawley rats at dietary
levels of 0, 10 000 and 100 000 mg/kg feed for 9 weeks, followed by a
4-week withdrawal period. Each group consisted of 20 male and 20
female animals. After treatment for 9 weeks, group size was reduced to
10 males and 10 females. From week 7 onwards, male and female rats of
both groups showed pallor of extremities and eyes. During the
withdrawal period, no recovery was observed. During or at the end of
the treatment period lower values for red blood cell parameters,
formation of met- and sulph-haemoglobin, higher SGPT-values, and
heavier liver, adrenals and spleen weights were observed. The animals
produced less but more concentrated urine, and iron pigment in the
liver and spleen was also demonstrated. Minor enlargement of
centrolobular hepatocytes in some rats of the highest dose groups was
observed. After the withdrawal period, some recovery, especially of
effects on the liver and methaemoglobin induction, was observed
(Hunter et al. 1979).
Diflubenzuron was administered in the diet to male and female
Sprague-Dawley rats (40/sex/group) at dose levels of 160, 400, 2 000,
10 000 and 50 000 mg/kg feed. An additional group (90 male and 90
female animals) served as a control. No clear treatment-related
effects were noted with respect to mortality, clinical observations,
body weight gain and food consumption. A treatment-related significant
increase in met-haemoglobin was noted in all treated groups. Sulph-
haemoglobin values showed increases from the 2 000 mg/kg group
onwards. For females and males, a significant treatment-related
decrease in haemoglobin and erythrocyte count was observed at all dose
levels at the end of the study. An increase was noted in the
reticulocyte count in all dose groups, except 160 mg/kg, and the
number of Heinz bodies was higher in the 10 000 and 50 000 mg/kg
groups. Analysis of clinical chemistry values and urinalysis revealed
no apparent treatment-related trends. After 7 weeks, spleen weights
were increased in the females at all dose levels, but after 13 weeks
no effect was found at 160 mg/kg. With the exception of the lowest
dose level, all treated groups showed a higher liver weight. The
administration of diflubenzuron resulted in a dose-related increase of
incidence of chronic hepatitis and haemosiderosis of the liver. It was
also associated at all dose levels with haemosiderosis and congestion
of the spleen and mild erythroid hyperplasia of the bone marrow
(Burdock et al 1980b; Goodman 1980b).
Dog
Diflubenzuron was fed to groups of 3 male and 3 female beagle
dogs for 13 weeks at concentrations of 0, 10, 20, 40 and 160 mg/kg in
the diet. No effect of treatment on behaviour, body weight, food and
water consumption was observed. Elevated SAP and SGPT values were
recorded for some dogs receiving 40 or 160 mg diflubenzuron/kg feed.
After 6 weeks, methaemoglobin and other abnormal haemoglobin pigments
were demonstrated in dogs receiving 160 mg/kg. After 12 weeks of
administration, some recovery was observed. Organ weights, gross and
microscopic evaluation did not show treatment-related effects
(Chesterman et al 1974).
Sheep
A 13-week feeding study was carried out with 4 groups of 3 male
and 3 female sheep. The test compound was included in the diet in a
concentration of 0, 500, 2 500 and 10 000 mg diflubenzuron/kg. These
concentrations were fed to the animals in daily amounts of 0.6 during
the first 4 weeks, 0.8 during weeks 5 to 8 and 1.0 kg during the last
5 weeks. After 6 weeks, both the plasma and RBC-cholinesterase
activities were considered to be within normal limits. No treatment-
related effects were observed on food consumption, body weight gain,
haematological parameters and urinalysis. A significant increase in
sulph-haemoglobin was observed in all treated groups at 13 weeks.
After 4 and 8 weeks this effect was only obvious at higher dose
levels. There was also an indication of a treatment-related increase
in methaemoglobin levels. No other clinico-chemical parameters were
affected. The weight of the thyroid was not dose-relatedly decreased
at all dose groups. However, histopathological examination revealed no
abnormalities that could be related to the treatment (Ross et al
1977a,b).
Long-term studies
Mouse
Five groups of 52 male and 52 female CFLP mice were fed
diflubenzuron during 80 weeks. The concentrations in the diet were 0,
4, 8, 16 and 50 mg/kg feed. Behaviour, mortality, food and water
consumption and body weight were not affected by the treatment. The
changes noted on gross and histopathologic examination were common to
both treated and control animals. No treatment-related effects or
significant tumour incidences were found. Although the incidence of
lymphosarcomas in treated female mice, killed after 80 weeks, was
significantly increased (50% level, chi square test) the combined
incidence of lymphosarcomas in treated female mice, sacrificed during
the treatment period and at the termination of the experiment, was not
significantly different (Hunter et al. 1975; Batham and Offer 1977;
Offer 1977).
Rat
Five groups, each composed of 60 male and 60 female Wistar rats,
were fed diflubenzuron during 104 weeks. For the tumorigenicity study,
45 male and 45 female animals were used, whereas 15 male and 15 female
rats constituted the satellite group for the toxicity study. The
concentrations in the diet were 0, 10, 20, 40 and 160 mg/kg feed.
There was no clear treatment-related effect on behaviour, survival,
food consumption, water consumption, body weight gain, efficiency of
food utilization, blood chemistry and urinalysis. Significantly higher
met-haemoglobin levels in both males and females receiving 160 mg
diflubenzuron/kg feed were observed after 52 and 78 weeks. The other
haematological parameters were within normal limits. Organ weights,
gross pathological and microscopic examination of tissues, including
the liver, showed no compound-related effects. There was no indication
of an increase in the number of neoplastic lesions. A no-effect level
of 40 mg diflubenzuron/kg feed was observed (Hunter et al. 1976;
Colley and Offer 1977).
Special studies on met- and sulph-haemoglobin formation
Technical diflubenzuron was administered by gastric intubation to
groups of 10 mice daily for a period of 14 days. The dose levels were
0 (20 animals), 8, 40, 200, 1 000 and 5 000 mg/kg bw. Body weight
measurement and macroscopic evaluation did not reveal any effect of
the treatment. At dose levels of 5 000 and 1 000 mg/kg the percentages
of met-and sulph-haemoglobin and erythrocytes containing Heinz bodies
were increased. No effect could be observed on the met- and sulph-
haemoglobin and on the percentage of erythrocytes containing Heinz
bodies at 200, 40 and 8 mg/kg (Keet 1977b).
A similar experiment was carried out with 2 groups of 15 male
Wistar rats. The animals received doses during 8 consecutive days of
0 or 5 000 mg/kg bw, in 1% tragacanth. There was no effect on the body
weight or the number of Heinz bodies, whereas the bet- and sulph-
haemoglobin levels were only marginally increased from day 1 and 2
respectively (Keet 1977a).
Two groups of 14 male New Zealand White rabbits were fed 0 or
640 mg diflubenzuron/kg feed during 21 days. In the treated group, the
methaemoglobin level was increased from day 5, whereas higher sulph-
haemoglobin levels were observed within 5 h. In a second experiment
with 640 mg/kg feed, the methaemoglobin level was again significantly
increased. Recovery was observed 2 weeks after the treatment was
ceased (Keet 1977c).
Twenty-four male and 24 female cats were divided among five
treatment groups and one control group. They received 30, 70, 100, 300
and 1000 mg diflubenzuron/kg bw per os for 21 days, followed by a
14-day observation period. Sodium nitrite was administered as a
positive control. Diflubenzuron induced a dose-related elevation of
methaemoglobin in females at all dose levels. In males, only 30 and
70 mg/kg did not have a significant effect (maximal effect: 11.8%).
Recovery was slow. Sulph-haemoglobinemia and Heinz body formation were
observed in all treated groups. The haemoglobin concentration, number
of reticulocytes, and organ weights were within normal limits
(Schwartz and Borzelleca 1981).
Special studies on sexual development
Diflubenzuron was incorporated into feed and tested in chickens
for 13 weeks. Fat deposition was greatly increased in females. The
combs, wattles, feathers and voice of males remained undeveloped
throughout the study. Testosterone showed a dose-related decrease
(Smalley 1976 - summary only).
Diflubenzuron was fed to 4 groups of 384 male chickens of a
Hubbard broiler strain at dose levels of 0 (twice), 2.5 and 250 mg/kg
feed. After 28, 56 and 98 days, one third of the number of animals of
each group was killed. No effects were observed on mortality, body
weight, food intake, oestradiol levels in the plasma after 4, 7 and
14 weeks, organ weights, tibia weights and lengths and on gross or
microscopic examination. Only at the end of the study, testosterone
levels in serum were higher in treated groups in comparison to
controls. Both comb and wattle were more developed in the
diflubenzuron groups. However, these differences were not
statistically significant (Keet 1976b).
A parallel study with the same dose levels was carried out with
female broiler chickens. In both diflubenzuron groups, a dose-related
reduction was observed in feed consumption and body weight. Mortality
was increased only at the highest dose level (250 mg/kg feed). In
addition, a significantly increased incidence of leg abnormalities, a
reduced tibia length and relative liver weight were observed. No clear
effect on plasma testosterone was recorded. In the highest dose level,
a marginally decreased plasma oestradiol and reduced comb and wattle
development was observed (Ross et al. 1977c).
In an experiment with 2 groups of 225 female chickens, only one
dose level (250 mg/kg feed) was tested. Animals were sacrificed after
28, 49 and 98 days. The comb and wattle development was normal and no
evidence of treatment-related effects was found. Plasma oestradiol
concentrations were within normal limits (Ross et al. 1979).
Diflubenzuron was administered in the diet to one-day old Mallard
ducks, Leghorn chickens, Nicolas White turkeys and Ring-neck pheasants
for 90 days. The concentrations in the diets were 0, 0.25, 1.25, 2.5,
25 or 250 mg/kg feed. There was no clear treatment-related effect on
serum testosterone levels at days 21, 51 and 90. At the highest dose
level (the only one measured), testosterone levels were decreased in
turkeys and ducks after 42 days. Comb and wattles were not affected by
treatment. The effects on organ weights, including testes, are
difficult to evaluate (Reinert and Cannon 1976).
Young male Long-Evans rats were administered 0, 15, 150 and
300 mg diflubenzuron/kg bw daily during 14 to 96 days by gastric
intubation. The control group contained 15 animals and each test group
8. Diflubenzuron transiently decreased the levels of testosterone in
the plasma at the prepuberal age. No effects on body weight, weight of
testes, prostate, seminal vescicles and adrenals were observed.
Histological examination of the testicular tissues did not reveal any
induced changes (Paten and Santolucito 1980).
Five groups of 38 to 40 Sprague-Dawley rats received
diflubenzuron in their diet at dose levels of 0, 75, 150, 300 or
3 000 mg/kg feed. After 14, 28, 44 and 98 days, one fourth of the
animals were sacrificed. Diflubenzuron had no clear effect on body
weight gain or serum testosterone levels (Booth et al. 1980).
Four pairs of Holstein bull calves received 0 or 1.0 to 2.8 mg
diflubenzuron/kg bw. There was no significant effect of diflubenzuron
on body weight, sperm volume, sperm concentration, libido and serum
testosterone. However, the concentration of testosterone varied
considerably. Histopathological examination of tissues revealed no
significant difference between the treated and control bulls (Miller
et al 1979).
Special studies on mutagenicity
Diflubenzuron
A dominant lethal study was conducted in which 12 male mice per
test group were treated with a single i.p. injection of a suspension
of diflubenzuron in corn oil at levels of 1 000 and 2 000 mg/kg bw.
The control group received corn oil only. Sequential mating of each
male with 3 females per week was conducted for 6 consecutive weeks.
Mating ability of males and numbers of corpora lutea, implantation
sites, resorption sites (early embryonic deaths), and embryos for
treated animals were not different from those for control animals
(Arnold 1974).
Diflubenzuron was examined for mutagenic activity in a series of
in vitro microbiological assays, using the Salmonella typhimurium
strains TA 98, TA 100, TA 1537 and TA 1978. Each plate was run with
and without rat liver homogenate (S-9 mixture) prepared from Aroclor
1254 treated rats. Diflubenzuron, tested at dose levels ranging from
10 to 1 000 µg/plate, was not mutagenic in any of these assays (Bryant
1976).
In similar tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538,
and the Sacchromyces cerevisiae strain D 4. The compound was tested
both in the absence and in the presence of liver S-9 preparations from
Aroclor 1254-induced rats. Diflubenzuron tested at dose levels ranging
from 0.1 to 500 µg/plate did not demonstrate mutagenic activity in any
of these assays (Brusick and Weir 1977a).
In another series of tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535 and TA 1537.
Diflubenzuron, at levels of 10, 100 or 1 000 µg/plate, did not
significantly alter the spontaneous revertant frequency in the four
strains used, either with or without the metabolic activation system
from Aroclor 1254-induced rat liver (McGregor et al 1979).
An in vitro test with mouse lymphoma cells and a micronucleus
test in mice were carried out by the same author. In the in vitro
test with cultured cells, the forward mutation frequency of
TK+/- -> TK-/- in L 517 Y mouse lymphoma cells was tested.
Diflubenzuron at dose levels ranging from 1.2 to 300 µg/ml did not
increase the mutation frequency, either with or without the metabolic
activation system, in the micronucleus test, male mice were given 15,
150 or 1 500 mg/kg bw at 30 h and 6 h before necropsy. Diflubenzuron
did not significantly increase the frequency of micronucleated
erythrocytes in the bone marrow (McGregor et al. 1979).
In another series of tests, diflubenzuron, as well as its
metabolites (2,6-difluorobenzoic acid (DFBA, 4-chlorophenylurea (CPU),
4-chloroaniline), were negative in the S. typhimurium strains TA 98,
TA 100, TA 1535 and TA 1538. The results in the strain TA 1537 were
difficult to interpret (Seuferer et al 1979).
Diflubenzuron was evaluated in a cell transformation test. At
dose levels of 0.02 to 0.312 mg/ml, diflubenzuron did not induce
morphological transformation in BALB/3T3 cells in vitro (Brusick and
Weir 1977b).
Diflubenzuron was evaluated for its ability to induce unscheduled
DNA synthesis in human diploid WI-38 cells blocked in the G1 phase.
The compound was tested at dose levels of 50 to 1 000 µg/ml, both in
the absence and in the presence of liver S-9 preparations of uninduced
mice. Under the conditions of the assay, diflubenzuron did not induce
unscheduled DNA synthesis (Brusick and Weir 1977c).
Diflubenzuron was tested in a transplacental transformation assay
to investigate possible transformation of mammalian cells in culture.
Timed pregnant hamsters were injected intraperitoneally on day 10 of
gestation with solutions of diflubenzuron in dimethylsulphoxide at
dose levels of 10, 200 and 500 mg/kg bw. Three days after injection,
animals were sacrificed and foetal cell cultures were prepared.
Diflubenzuron did not induce morphological transformation or the
ability for growth of colonies. With known carcinogens (benzopyrene
and dimethylnitrosamine), a positive result was obtained (Quarles
et al 1980).
Metabolites
The metabolites 4-chlorophenylurea (CPU), 2,6-difluorobenzoic
acid (DFBA) and 4-chloroaniline were examined for mutagenic activity
in a series of in vitro microbial assays. A spot test at a dose
level of 1 000 µg per spot was carried out with S. typhimurium
strains TA 98, TA 100, TA 1535, TA 1537, TA 1538 and TA 1978, with and
without metabolic activation. A dose response test at dose levels of
10, 100, 500 and 1 000 µg was carried out with TA 98 and TA 100. The
test compounds did not demonstrate mutagenic activity in any of the
assays conducted, except for a weak effect with 500 and 1 000 µg
4-chloroaniline in the TA 98 strain after activation by liver S-9
preparation. At these dose levels, 4-chloroaniline caused a reduction
in the number of colonies, indicating a degree of toxicity to the
bacteria (Dorough 1977).
CPU was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae strain D 4.
The compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to
500 µg/plate, the test compound did not demonstrate mutagenic activity
in any of the assays conducted (Jagannath and Brusick 1977a).
CPU was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration
(312 µg/ml) employed in the concentration range. The other levels were
negative. The results were considered to be an indication of a weak
transforming activity at concentrations near to the level of
cytotoxicity (Matheson and Brusick 1978a).
CPU was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both in the absence and in the presence of liver S-9
preparations of Aroclor 1254-induced mice. CPU at dose levels ranging
from 6.25 to 400 µg/l did not induce unscheduled DNA synthesis
(Matheson et al 1978a).
DFBA was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537, TA 1538 and the S. cerevisiae strain D 4. The
compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to 500
µg/plate, the test compound did not demonstrate mutagenic activity in
any of the assays conducted (Jagannath and Brusick 1977b)
DFBA was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration employed
(2.5 mg/ml). The other levels were negative. These results were
considered to be an indication of weak transforming activity at
concentrations near to the level of cytotoxicity (Matheson and Brusick
1978b).
DFBA was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both with and without metabolic activation. The compound at
dose levels ranging from 75 to 500 µg/ml produced significant
increases in the level of unscheduled DNA synthesis in the presence of
mouse S-9 liver mixture. However, there was no dose response
relationship, 75 µg giving the highest increase and 500 µg the lowest
increase. According to the authors, the results appeared to be on the
plateau of a dose response effect. The compound was considered to be
active under these test conditions (Matheson and Brusick 1978c).
4-Chloroaniline was examined for mutagenic activity in a series
of in vitro microbial assays, using the S. typhimurium strains
TA 98, TA 100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae
strain D 4. The compound was tested with and without liver S-9
preparations from Aroclor 1254-induced rats. The test compound did not
demonstrate mutagenic activity in any of the assays conducted
(Jagannath and Brusick 1977c).
4-Chloroaniline was evaluated in a cell transformation test. At
dose levels ranging from 39 to 625 µg/ml the compound did not induce
morphological transformation in BALB/3T3 cells in vitro (Matheson
et al 1978b).
4-Chloroaniline was tested for its ability to induce unscheduled
DNA synthesis in human WI-38 cells blocked in the G1 phase. The
compound was tested both with and without metabolic activation. At
dose levels of 250 to 1 000 µg/ml, the compound did not induce
unscheduled DNA synthesis (Matheson et al 1978c).
Special studies on reproduction and teratogenicity
Diflubenzuron was fed to 3 groups of 20 male and 20 female rats
at dietary levels of 0, 1 000 and 10 000 mg/kg for one generation and
one litter. The animals were maintained on their respective diets for
60 days prior to mating. There were no clear effects on mating
performance, pregnancy rate, duration of gestation, litter size,
mortality, litter weight, type and distribution of abnormalities.
Diflubenzuron had, among others, a dose-related effect on haemoglobin
(reduced PCV, Hb, total red cells, increased met-haemoglobin, sulph-
haemoglobin, spleen weight, incidence of siderocytes in the spleen and
the occurrence of iron pigment containing Kupffer cells in the liver).
A dose-related effect on the liver was also shown by an increased
weight, SGPT activity and hepatocyte enlargement. Reduced blood
glucose concentrations were recorded in both treated groups. In the
highest dosed group, the offspring showed an increased liver and
spleen weight for both sexes. Microscopically, an increased incidence
of centrolobular hepatocyte enlargement was observed (Palmer et al
1978).
Diflubenzuron was administered in the diet to groups of 20 male
and 20 female rats at concentrations of 0, 10, 20, 40 and 160 mg/kg.
These diets were administered continuously throughout three
generations producing one litter each. As the mating performance was
low in the first generation, a second litter was bred. Parent animals
showed no signs of adverse effects related to treatment. Mating
performance, pregnancy rate and duration of gestation were not
affected. Also, total litter loss, litter size, litter and mean pup
weights, pup mortality and the incidence of abnormalities provided no
evidence of adverse treatment-related effects (Palmer and Hill 1975a).
Groups of 20 pregnant rats were administered diflubenzuron orally
by gavage at levels of 0, 1, 2 or 4 mg/kg bw during days 6 to 15 of
gestation. The parent animals showed no signs of reaction and no
mortalities occurred. Body weight gain and pregnancy rate were
unaffected by treatment. No effects were observed on the number of
viable young, implantations, resorptions and corpora lutea. The
pre-implantation loss, foetal loss, litter weight, foetal weight, the
incidence of major malformations, minor anomalies and skeletal
variants were not dose-relatedly affected (Palmer and Hill 1975b).
Four groups of 13 pregnant New Zealand White rabbits were
administered diflubenzuron orally from day 6 to 18 of gestation at
dose levels of 0, 1, 2 and 4 mg/kg bw. Foetuses were removed on day 29
of pregnancy. There was no effect of the treatment on behaviour, body
weight gain and pregnancy rate. No dose-related differences were
observed on the number of viable young, resorptions, foetal loss,
litter weight and mean foetal weight. The incidence of major
malformations and minor anomalies was not affected by treatment
(Palmer and Hill 1975c).
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller and Booth 1976).
Pregnant female mice were fed a diet containing 50 mg/kg of
diflubenzuron (partly 14C). Some of these mice were sacrificed at
day 17 from conception, the others were allowed to give birth to the
young. The lactating females were kept on treatment and allowed to
suckle their young for 13 days. No radioactivity was detected in the
embryos or young mice (Booth 1977).
RESIDUES IN FOOD
USE PATTERN
Diflubenzuron is a recently-introduced insecticide that
interferes in the deposition of chitin in the insect cuticle through
action on the enzyme chitin synthetase.
Pre-harvest treatments
Diflubenzuron is formulated as a wettable powder containing 25%
of the active ingredient (Dimilin WP-25), or as an oil dispersible
concentrate containing 450 g diflubenzuron per litre. Granular
formulations are also available. Recommended use rates are given with
pre-harvest intervals in Table 3.
Other uses
Diflubenzuron is recommended for the control of flies in animal
husbandry by topical applications to breeding sites and manure heaps.
A further application is on ornamental plants and in forests.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials on the main
crops treated. The dosages in these trials covered recommended and
higher rates. Treatments were made using W-25 wettable powder
formulation, which is the formulation recommended for food crops.
TABLE 3. Recommended uses and pre-harvest intervals for diflubenzuron
Use rate Pre-harvest
Crop Country (a.i.) interval (days)
Apple, pear Argentina 0.02% 60
Bulgaria 0.0375% -
France 0.01% 30
German Fed.Rep. 0.02% 28
Greece 0.0125-0.015%1 -
Israel 0.0125% -
Italy 0.0125-0.02% 45
The Netherlands 0.01% 28
Spain 0.01-0.015% 60
South Africa 0.02% -
Switzerland 0.02% 42
UK 150 g/ha 14
Yugoslavia 0.0125-0.02% 30
Brassica leafy
vegetables The Netherlands 0.01% 14
China(Taiwan prov.) 0.017% 22
UK 100 g/ha -
Yugoslavia 200 g/ha -
Cottonseed Egypt 75 g/ha -
Greece 0.01-0.025% -
USA 70 g/ha (6x) -
Mushroom Greece 1 g/m2 -
Korea 1 g/m2 -
The Netherlands 1 g/m2 -
Switzerland 1 g/m2 -
UK 1 g/m2 -
Soybean Brazil 50-75 g/ha 21
Colombia 75-125 g/ha -
USA 35-70 g/ha
Tomato UK 250 g/ha -
Citrus USA2 350 g/ha ?
1 for pears: 0.0125-0.03%;
2 registration applied for.
Apple
Residue data have been obtained from trials in several countries.
Residues on fruit at recommended rates up to 0.02% were usually well
below 1.0 mg/kg at two weeks after the last application. Only three
samples were shown to contain residues from 1.0 to 1.2 mg/kg. The
one sample that contained 3.7 mg/kg is considered to be atypical
(Table 4). In the UK, apples (cv Cox and Egremont Russet) treated with
diflubenzuron 2 weeks before harvest at a dosage rate of 250 g a.i./ha
were shown to be taint-free (Spencer-Jones 1979).
Pear
The residue pattern in pears is very similar to that in apples.
At two weeks after the last application at recommended dosages, the
residues were well below 1.0 mg/kg (Table 4).
Citrus
Residue data have been obtained from numerous trials on orange,
grapefruit and tangerine in the USA. The dosages in these trials
included the recommended rate and also 2x and 4x rates. Residues in
whole fruit were all well below 0.5 mg/kg 1 week after the last
application at the recommended dosage rate. At the 4x dosage rate, the
residue remained generally below 1.0 mg/kg (Table 5).
Examination of peel and pulp separately showed that residues were
exclusively found in the peel when the product was applied at the
recommended rate. Residues in the pulp were all below the detection
limit (0.05 mg/kg). At the 4x use rate, by far the major portion of
the residue was in the peel. Residues in the pulp were either below or
just above the detection limit of 0.05 mg/kg, the highest value found
being 0.07 mg/kg. (Table 6).
Further fractionation of orange and grapefruit showed that the
residue is mainly present in the oil fraction. Here, the residue
ranged from 9.5 to 20 mg/kg at the recommended use rate. At the
4x rate residues were as high as 50 mg/kg. Neither at the recommended
rate nor at the 4x rate could residues be detected in the juice of
both orange and grapefruit, the limit of detection being 0.05 mg/kg
(Table 7).
In several cases, the various citrus fractions were analysed for
residues of the metabolites 4-chlorophenylurea, 4-chloroaniline and
2,6-difluorobenzoic acid. In none of the samples was any residue
detected (limit of detection for all three compounds 0.05 mg/kg)
(Duphar 1975-81).
TABLE 4. Residues following supervised trials in apple and pear1
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Apple
Bramley UK 0.01 1 - 0.22 0.23 0.14
Cox O.P. 0.01 1 - 0.18 0.16 0.20
Egremont R 0.01 1 - 0.27 0.24 0.18
Golden D. Netherlands 0.01 1 - <0.032
Winston 0.01 1 - <0.03
Golden D Europe 0.01 3-5 15-59 0.15 0.16 0.23 0.30
-0.21 -0.34
J. Grieve Netherlands 0.0125 1 - 0.28 0.36 0.13
J. Grieve 0.0125 2 17 1.32 0.94 0.75 0.53
Stark Crimson Italy 0.0125 2 58 0.06
Golden D 0.0125 2 54 0.15
Stark Crimson 0.0125 3 58,34 0.12
Golden D 0.0125 3 58,33 0.05
Jonathan 0.0125 4 35,22,41 0.14
J. Grieve German Fed.Rep. 0.015 1 - 0.43 0.14 0.03 0.09 0.11
Golden D. Europe 0.015 1-8 14-52 0.34 0.78 0.34 0.12 0.23 <0.05
-1,2 -1.1 -0.83
Bramley UK 0.017 1 - 0.17 0.02 0.18 0.19 0.02
Cox O.P. 0.017 1 - 0.09 <0.01 0.22
Egremont R 0.017 1 - 0.17 0.03 0.20
Ida Red 0.017 1 - 0.12
Golden D 0.017 1 - 0.20
McIntosh 0.017 1 - 0.17
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Warner UK 0.017 1 - 0.16
Worcester 0.017 1 - 0.15
Newton 0.017 1 - 0.01
Golden D France 0.01875 3 33,31 0.41
Golden D 0.01875 3 30,27 0.46
Golden D 0.01875 3 29,35 0.43
Golden D 0.01875 4 21,21,28 0.62
Golden D 0.01875 4 20,20,30 3.8
J. Grieve Netherlands 0.02 1 - 0.29 0.21 0.18 0.15 0.13
Golden D 0.02 1 - 0.52 0.31 0.23 0.29
n.s. South Africa 0.02 1 - 0.833 0.32 0.29
n.s. 0.02 1 - 0.784 0.14 0.15 0.23
Cox O.P. Netherlands 0.02 1 - 0.095
S. Boskoop 0.02 1 - 0.146
Ingrid M. 0.02 1 - 0.08
Winston 0.02 1 - 0.097
Golden D 0.02 1 - 0.055
Cox O.P German Fed.Rep. 0.02 1 - 0.258 0.05a
Cox O.P 0.02 1 - 1.568 0.438
Golden D 0.02 1 - 0.738 0.288
Cox O.P. 0.02 2 22 0.538 0.26 0.258 0.19 0.14
Golden D 0.02 2 21 1.268 0.69 0.58 0.53 0.37
Cox O.P. 0.02 2 21 1.878 1.24 0.95 0.51 0.25
Golden D Netherlands 0.02 2 22 <0.03
Gravesteyn Italy 0.02 2 53 0.24
Cox O.P. German Fed.Rep. 0.029 3 21,28 0.84 0.92 0.76 0.72
Cox O.P. 0.029 3 22,28 0.54 0.42 0.40 0.26 0.47
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D 0.02 21,28 1.26 0.89 0.52 0.66 0.63
Golden D Italy 0.02 3 57,56 0.52
Golden D 0.02 3 10,68 0.57
Steymen 0.02 3 58,32 0.40
Golden D Netherlands 0.029 3 40,31 0.45 0.40 0.32 0.29 0.19 0.28
Cox O.P. 0.029 3 34,30 0.30 0.27 0.29 0.24
G. Parmane German Fed.Rep. 0.029 4 29,39,14 0.5210 0.04 0.26 0.30
n.s. South Africa 0.0211 4 28,26,38 1.24
Jonathan Italy 0.02 5 23,15,27,14 0.28
Golden D 0.02 5 34.30,14,28 0.87
Golden D 0.02 5 40,29,15,33 0.77
Golden D France 0.02 5 15,14,16,16 0.27
J. Grieve Netherlands 0.025 1 - 0.67 0.38 0.31
Star-King D Japan 0.025 1 <0.01 0.067
Red-ball 0.025 1 0.087 0.107
n.s. Italy 0.02512 2 62 0.197 0.2313
J. Grieve Netherlands 0.025 2 17 1.14 1.62 0.58 0.87
Golden D 0.025 2 15 0.426
St.Crimson Italy 0.025 2 58 0.10
Golden D 0.025 2 52 0.117
St.Crimson 0.025 3 58,34 0.30
Golden D 0.025 3 52-54,32-49 0.55 0.28
Star-King Japan 0.025 3 0.387
Red-ball 0.025 3 0.207
Jonathan Italy 0.025 4 35,22,41 0.18
Golden D France 0.025 5 28,14,21,21 1.0
Golden D 0.025 6 12-18 0.87
J. Grieve German Fed. Rep. 0.03 1 - 0.73 0.23 0.15 0.15 0.13
Golden D 0.03 1 - 1.0 0.67 0.44 0.43 0.31
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D Netherlands 0.03 1 - 0.056
Cox O.P. 0.03 1 - <0.03
Golden D 0.03 2 15 0.437
Golden D 0.03 2 15-19 0.34 0.32 0.326
n.s. South Africa 0.03 3 28,32 0.26
Golden D 0.03 8 14 2.20
n.s. 0.04 1 - 0.7815 0.38 0.37 0.25
n.s. 0.04 1 - 1.5516 1.25 0.70
Golden D Netherlands 0.04 1 - 0.0514
Winston 0.04 1 - <0.03
Golden D 0.04 2 22-43 0.077
Jonathan 0.04 2 26 <0.05
Gravesteyn Italy 0.04 2 53 0.41
Golden D 0.04 3 10-68 0.83 1.11
Alkmene German Fed. Rep. 0.0417 4 13,16,20 1.13 0.7418 0.63 0.65
Golden D Italy 0.04 5 14-40 1.437
Golden D France 0.04 6 12-18 1.40
Pear Europe 0.01 1-5 17-33 0.1 0.12 0.02
-0.04 -0.31 -0.25
South Africa 0.02 1-3 28-32 0.52 0.11 0.13 0.24
-0.04 -2.64 -1.30 -1.25 -1.31
1 Reference: Duphar 1975-81; 2 4 trials;
3 day 1: 0.63, day 2:0.83, day 6:0.50, day 7: 0.61; 4 day 1: 0.42, day 2: 0.78, day 4: 0.30;
5 average of 6 trials; 6 average of 3 trials;
7 average of 2 trials; 8 average of 2 samples;
9 last spray rate - 0.015; 10 day 0: 0.52, day 7: 0.44;
11 first spray rate-0.025; 12 last spray rate - 0.02;
13 average of 4 trials; 14 average of 5 trials;
15 day 1: 0.78, day 2: 0.11, day 4: 0.91; 16 day 1: 1.55, day 2: 0.72, day 6: 1.15, day 7: 1.39;
17 last spray rate - 0.03; 18 day 9: 0.74, day 14: 0.72.
TABLE 5. Residues following supervised trials in citrus, whole fruit, in Florida and Texas, USA1
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 1 PB 0.07 0.11 0.12 0.13 0.08 0.10
1 PB 0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Temple 1 PB 0.053
1 PB 0.063
Pineapple 1 PB <0.05
1 PB 0.053
Hamlin 1 PB 0.06
1 PB <0.05
Mars 1 PB 0.10
Temple 2 PB,S 0.084
2 PB,S 0.063
Pineapple 2 PB,S 0.093
2 PB,S 0.053
Hamlin 2 PB,S 0.123
2 PB,S <0.05
Mars 2 PB,S 0.16
Temple 3 PB,S,F 0.144 0.13 0.143
3 PB,S,F 0.173 0.13 0.11
Pineapple 3 PB,S,F 0.183 0.18 0.08
3 PB,S,F 0.133 0.10 0.07
Hamlin 3 PB,S,F 0.163 0.20 0.083 0.25
3 PB,S,F 0.10 0.05
Mars 0.35 3 PB,S,F 0.32
Hamlin-pineapple " 3 PB,S,F 0.27 0.29 0.30 0.36 0.21
3 PB,S,F 0.20 0.29 0.22 0.41 0.14
3 PB,S,F 0.05 0.20 0.10 0.11 0.21
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 1 PB 0.054
Marsh 1 PB <0.053
Seedless 1 PB <0.053
Ruby red 1 PB 0.063
1 PB 0.063
Pink 2 PB,S <0.054
Marsh seedless 2 PB,S 0.083
2 PB,S 0.063
Ruby Red 2 PB,S 0.123
Ruby Red 0.35 2 PB,S 0.123
Pink 3 PB,S,F 0.174 0.15 0.093 0.26 0.22
Marsh seedless 3 PB,S,F 0.123 0.073
3 PB,S,F 0.113 0.143
Ruby Red 3 PB,S,F 0.203
3 PB,S,F 0.133
Tangerine Nova 1 PB <0.05
1 PB <0.05
2 PB,S 0.12
2 PB,S 0.09
3 PB,S,F 0.07 0.05
3 PB,S,F 0.09 0.09
Orange Valencia 0.7 1 PB 0.223 0.133 0.163 0.143 0.083 0.153 <0.05
1 PB 0.10 0.11
Mars 1 PB 0.27
2 PB,S 0.28
3 PB,S,F 0.23
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1 PB 0.10
2 PB,S 0.203
3 PB,S,F 0.203
Valencia 1.4 1 PB 0.233 0.113 0.273 0.233 0.123 0.073
1 PB 0.07 0.05
Temple 1 PB <0.053
1 PB 0.07 0.05
Pineapple 1 PB <0.053
1 PB 0.053
Hamlin 1 PB <0.053
Orange Hamlin 1.4 1 PB 0.09
Mars 1 PB 0.18
Grapefruit Pink 1 PB <0.053
1 PB <0.05
Marsh seedless 1 PB <0.053
1 PB 0.343
Ruby Red 1 PB 0.273
Tangerine Nova 1 PB <0.05
1 PB <0.05
Orange Temple 1.4 2 PB,S 0.124
2 PB,S 0.15
Pineapple 2 PB,S 0.173
2 PB,S 0.383
Hamlin 2 PB,S <0.053
Mars 2 PB,S 0.305
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 2 PB,S 1.10 0.26 0.243
2 PB,S 0.23
Marsh seedless 2 PB,S 0.243
2 PB,S 0.353
Ruby Red 2 PB,S 0.233
Tangerine Nova 2 PB,S 0.15
2 PB,S 0.20
Orange Temple 3 PB,S,F 0.384 0.473 0.233 0.91
3 PB,S,F 0.46 0.59
Pineapple 3 PB,S,F 0.253 0.35 0.06
3 PB,S,F 0.573 0.49 0.15
Hamlin 3 PB,S,F 0.713 0.74 0.103 0.44
3 PB,S,F 0.23 0.26
Mars 3 PB,S,F 0.10
Grapefruit Pink 3 PB,S,F 0.393 0.81 0.85 1.11 0.48
3 PB,S,F 0.34 0.65
Marsh seedless 3 PB,S,F 0.533 0.273
3 PB,S,F 0.303 0.613
Ruby Red 3 PB,S,F 0.643
Tangerine Nova 3 PB,S,F 0.16 0.28
3 PB,S,F 0.31 0.24
1 Referenne: Duphar 1975-81;
2 PB = Post Bloom, S = Summer,F = Fall;
3 average of 2 trials;
4 average of 3 Trials;
5 day 70: 0.53 in one trial.
TABLE 6. Residues following supervised trials in citrus, peel and pulp, in Florida and Texas, USA1
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 12 peel 0.10
pulp <0.05
Temple 33 peel 0.574
pulp <0.054
peel 0.21
pulp <0.05
Pineapple 3 peel 0.46
pulp <0.05
3 peel 0.26
pulp <0.05
Hamlin 3 peel 0.40
pulp <0.05
Mars 3 peel 0.50
pulp <0.05
Grapefruit Pink 3 peel 0.58
pulp <0.05
Marsh seedless 3 peel 0.41
pulp <0.05
3 peel 0.84
pulp <0.05
Ruby Red 3 peel 0.614
pulp <0.05
0.355 3 peel 0.934
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 1,4 1 peel 0.09 1.89 1.65 1.83 0.88 0.75
pulp 0.07 0.07 0.07 <0.07 <0.05 <0.05
peel 0.78 0.50 0.70 0.91 0.43 0.15
pulp <0.05 <0.05 <0.05 <0.05 <0.05 (0.05
1 peel 0.29 <0.05
pulp <0.05 <0.05
Orange Temple 1.4 2 peel 0.07
pulp 0.05
Hamlin 2 peel 0.38
pulp <0.05
Temple 3 peel 1.40 2.00
pulp <0.05 <0.05
3 peel 1.30 0.61
pulp <0.05 <0.05
peel 1.20
pulp (0.05
Pineapple 3 peel 0.93 1.8
pulp <0.05 <0.05
3 peel 1.70 0.28
pulp <0.05 <0.05
Hamlin 3 peel 0.20 2.70
pulp <0.05 <0.05
1.45 3 peel 1.3
pulp <0.05
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1.4 1 peel 0.88c
pulp 0.05c
2 peel 0.40c
pulp 0.05
3 peel 0.94
pulp 0.05
Pink 3 peel 2.10
pulp 0.05
3 peel 1.90
pulp 0.07
Marsh seedless 3 peel 0.66
pulp 0.07
3 peel 1.0
pulp 0.05
Tangerine Nova 3 peel 0.18
pulp 0.05
3 peel 0.75
pulp 0.05
1 Reference: Duphar 1975-81;
2 1 Spray Post Bloom;
3 3 sprays: Post Bloom, Summer and Fall;
4 average of 2 trials;
5 plus 0.25% oil.
TABLE 7. Residues following supervised trials in citrus fractions, in Florida, USA1
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Orange (Temple) Whole fruit(unwashed) 0.35 1.4 3 0.13 0.35 14
Whole fruit (washed) 0.09 0.26 14
Chopped peel 0.09 0.13 14
Frits 0.11 0.84 14
Finisher pulp <0.05 <0.05 14
Dried citrus pulp 0.09 0.05 14
Fruit Juice <0.05 <0.05 14
Oil 13.0 28.0 14
Pressed liquor <0.05 0.09 14
Molasses <0.05 0.06 14
Prewash water 0.01 0.02 14
Afterwash water 0.03 0.07 14
Orange (Eamlin) Whole fruit(unwashed) 4 0.28 0.34 2t
Whole fruit(washed) 0.14 0.33 21
Chopped peel 0.06 0.11 21
Frits 0.10 0.55 21
Finisher pulp <0.05 <0.05 21
Dried citrus pulp <0.05 0.66 21
Fruit juice <0.05 <0.05 21
Oil 20.0 50.0 21
Pressed liquor <0.05 0.08 21
Molasses <0.05 0.12 21
Prewash water 0.03 0.06 21
Afterwash water 0.05 0.22 21
TABLE 7. (con't)
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Grapefruit (Pink) Whole fruit (unwashed) 0.35 3 0.09 21
Whole fruit(washed) <0.05 21
Chopped peel 0.08 21
Frits 0.19 21
Finisher pulp <0.05 21
Dried citrus pulp 0.10 21
Fruit juice <0.05 21
Oil 9.50 21
Pressed liquor <0.05 21
Molasses <0.05 21
Prewash water 0.01 21
Afterwash water 0.04 21
Whole fruit (unwashed) 1.4 3 0.09 21
Whole fruit (washed) 0.17 21
Chopped peel 0.11 21
Frits 0.50 21
Finisher pDlp <0.05 21
Dried citrus pulp 0.36 21
Fruit juice <0.05 21
Oil 23.0 21
Pressed liquor <0.05 21
Molasses 0.17 21
Prewash water 0.03 21
Afterwash water 0.17 21
1 Reference: Duphar 1975-81.
In taste tests with orange juice, no off-flavour was detected
which might have been caused by DIMILIN treatment (Braddock 1976b,
1977). Also in the case of grapefruit juice, no adverse effects on
flavour were detected (Braddock 1976a).
Soybean
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended use rate and also much
higher rates. Residues in the soybean seed were generally below the
detection limit, which was 0.05 mg/kg. Only one sample contained a
residue just above this limit, i.e. 0.06 mg/kg. Also at higher rates,
residues remained very low, the highest value found being 0.16 mg/kg.
Fractionation of seed containing <0.05 mg/kg residue did not result
in a detectable residue in any of the fractions. As could be expected
from the foliar stability of diflubenzuron, soybean foliage did
contain residues, the level of which declined with time (Table 8).
In nine trials, rotational crops were grown in fields on which
soybean had been treated with diflubenzuron at both the recommended
and higher rates. In none of the trials could residues be detected
(limit of detection 0.05 mg/kg) in the rotational crops, including
turnips, collards, rye, oats and mustard green. In the samples
analysed for 4-chlorophenylurea, no residue could be detected (limit
of detection 0.05 mg/kg) (Duphar 1975-81).
Residues in soil of soybean fields treated with diflubenzuron
were usually below the limit of detection (0.05 mg/kg), both for the
parent compound and the metabolite 4-chlorophenylurea (CPU).
Diflubenzuron residues never exceeded 0.3 mg/kg and were exclusively
found in the top 7.6 cm of soil. CPU residues did not exceed 0.5 mg/kg
and in only one case could any residue be detected below the top
7.6 cm of soil (Duphar 1975-81).
Cotton
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended rate and also higher ones.
Usually, several applications were made, up to 16 per growing season.
Residues in the cotton seed were generally below the limit of
detection (0.05 mg/kg) (Table 9). Only a few samples contained a
residue slightly above the detection limit, with one high value of
0.17 mg/kg. Fractionation of cotton seed did not result in a
detectable residue of any of the fractions.
In seven trials, rotational crops were grown in fields on which
cotton had been treated with diflubenzuron 15 times during the growing
season either at a rate of 0.067 or 0.280 kg a.i./ha. In none of the
trials could residues of either diflubenzuron or 4-chlorophenylurea
TABLE 8. Residues following supervised trials in soybean, USA1
Application Residues (mg/kg) at intervals (days)
Crop part after application
Rate (a.i.) No.
(kg/ha) 0-7 8-14 15-21 22-28 29-42 43-56 <56
0.034 1-2 <0.05 <0.05 <0.05 <0.05 <0.05
Seed - 0.56 -0.13 -0.16 -0.07 -0.07
Foliage Bragg 0.034 1 0.86 0.49 0.25
Lee 0.034 1 1.10 0.59 0.38
Ranson 0.034 1 2.25 1.40 1.65 0.82
n.s. 0.034 1 0.25
Bragg 0.034 2 0.462 2.6 0.26 0.63
Lee 68 0.034 2 2.1 0.58
Bragg 0.067 1 1.8 0.29 0.33 0.14
Calland 0.067 1 0.16
n.s. 0.280 1 1.6
n.s. 0.280 2 0.23
0.140 1
Seed n.s. <0.05
Hulls <0.05
Meal <0.05
Crude oil <0.05
1 Reference: Duphar 1975-81; 2 Average of 2 trials.
TABLE 9. Residues following supervised trials in cottonseed, USA1
Application Residues (mg/kg) at intervals (days)
after application
Crop part Variety Rate (a.i.) No.
kg/ha 0-7 8-14 15-21 22-28 29-42 43-56 <56
Seed 0.033 1- <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
-0.56 15 -0.07 0.09 -0.05 -0.07 -0.17
Hulls DPL-16 0.140 8 <0.05
Oil <0.05
Meal <0.05
Seed hulls Stoneville 213 0.280 9 <0.05
Meal <0.05
Oil <0.05
Soapstock <0.05
1 Reference: Duphar 1975-81
(limit of detection for both compounds 0.05 mg/kg) be detected in
the rotational crops, including collards, wheat and radish (Duphar
1975-81).
Mushroom
This crop shows a residue pattern different from all the others.
This is undoubtedly due to the different way in which it is grown. The
intimate contact of the mushrooms with the medium in which they are
grown enables the uptake of the metabolite 2,6-difluorobenzoic acid
especially (see also "Fate of residues"). At the recommended dosage
rate of 1 g a.i./m2, the diflubenzuron residue in mushrooms is
generally below 0.1 mg/kg, only one sample contained 0.11 mg/kg.
Also the residue level of 4-chlorophenylurea is very low,
all residues being below 0.1 mg/kg. However, the residues of
2,6-difluorobenzoic acid were considerably higher. At the recommended
use rate they all remain below 1 mg/kg. At higher dosage rates,
somewhat higher residues are found, the highest value being 1.63 mg/kg
at a dosage of 4 g/m2, applied twice. (Table 10).
FATE OF RESIDUES
General comments
The metabolism of diflubenzuron was studied and it was
established that the compound degraded along different lines, as shown
in Figure 2. Breakdown occurred predominantly along line 1, resulting
in 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU). A
minor degradation takes place along lines 2 and 3, resulting in
4-chloroaniline (PCA) and CO2.
TOXICOLOGICAL STUDIES
Acute toxicity
In none of the acute studies on diflubenzuron was any overt sign
of toxicity observed. In the dermal toxicity study with rats, no
effect on the met- and sulph-haemoglobin values was detected. Results
of acute toxicity studies in rat, mouse and rabbit are summarized in
Table 1.
Loss of activity, catatony, paralysis and severe bradypnoea, were
observed in rats treated with the metabolite 4-chlorophenylurea. At
autopsy, the animals showed congested blood vessels and haemorrhagic
intestines. The rats dosed with 2,6-difluorobenzoic acid showed
symptoms indicative for slight C.N.S. excitation and increased muscle
tone. Results of studies with diflubenzuron metabolites are summarized
in Table 2.
Short-term studies
Mouse
Three groups of 8 male CFLP mice were fed dietary levels of
diflubenzuron for 6 weeks at levels of 0, 16 and 50 mg/kg feed. There
was no clear effect of the treatment on food consumption, body
weight, blood chemistry and macroscopic pathology. The weight of the
spleen was decreased in the highest dose group. In some animals
receiving 50 mg/kg feed, foci of liver cell necrosis with or without
inflammatory cell infiltration were noted. The other organs were not
microscopically examined (Hunter et al 1974).
Male and female mice (40/sex/group) received diflubenzuron in the
diet at a dosage level of 16, 50, 400, 2 000, 10 000 or 50 000 mg/kg
feed during 13 weeks. An additional group of one hundred/sex served as
a control. No compound-related effects were apparent in respect to
clinical signs, survival, growth, food consumption or gross pathology.
Significant treatment-related increases in met- and sulph-haemoglobin
concentrations were noted in all treated groups, except the 16 mg/kg
feed one. At the higher dose levels, a decrease was noted in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects were noted on the weights of liver and
spleen. In the females, adrenal weight was not dose-relatedly
decreased at all dose levels after 7 weeks and increased after 13
weeks at higher dose levels. In males a higher adrenal weight was
observed in comparison to controls. Histopathologically, treatment-
related centrolobular hypertrophy of hepatocytes, with or without cell
TABLE 1. Acute toxicity of diflubenzuron
Species Sex Route LD50 Reference
Rat F,M Oral > 4,640 mg/kg Van Eldik 1973
F,M Dermal >10,000 mg/kg Keet 1976a;Koopman 1977c
F,M Inhalation > 35 mg/kg (6 hr) Berczy et al 1973
Mouse F,M Oral > 4,640 mg/kg Van Eldik 1973;Koopman 1977a
F,M i.p. > 2,150 mg/kg Van Eldik 1974;Koopman 1977b
Rabbit F,M Dermal > 4,000 mg/kg
(50% paste) Davies and Halliday 1974
F,M Inhalation > 30 mg/l (6 hr) Berczy et al 1975
TABLE 2. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50(mg/kg) Reference
4-Chlorophenylurea Rat M Oral 1 080 Koelman-Klaus 1978a
F Oral 1 210 Koelman-Klaus 1978a
2,6-Difluorobenzoic Rat M,F Oral 4 640 Koelman-Klaus 1978b
acid
necrosis, haemosiderosis of the liver and spleen, extramedullary
haematopoiesis and mild chronic hepatitis were observed. The liver
lesions were more severe in males than in females. No no-effect level
was observed (Burdock et al. 1980a; Goodman 1980a).
A 14-week feeding study was performed with groups of 40 male and
40 female HC/CFLP mice (control groups, 96 male and 96 females)
maintained on a diet supplemented with 0, 80, 400, 2 000, 10 000 and
50 000 mg diflubenzuron (purity 97.20%/kg feed.) Half of the number of
mice of both sexes of all groups were sacrificed after 7 weeks. On the
second day of treatment, the majority of mice treated with 50 000 or
10 000 mg/kg feed showed dark eyes and/or prominent caudal blood
vessels. On day 5, blue/grey discolouration of the extremities was
noted for the majority of mice treated with 50 000 mg/kg. In the
course of the study, clinical signs were observed in mice of all
groups, except those of the lowest dose group. Mortality, food
consumption, water consumption and body weight changes were not
clearly affected by the treatment. Results of haematological
investigation showed lower packed cell volume and red blood cell
count at all dose levels except 80 mg/kg feed. The total white blood
cell count, lymphocyte count, haemoglobin concentration, the incidence
of Heinz bodies and the red blood cells were increased in all dose
groups. A treatment-related increase in both met- and sulph-
haemoglobin was recorded in all treated groups at weeks 7 and 14 of
the investigation. Clinical biochemistry revealed higher plasma
glutamic pyruvic transaminase at all dose levels, with the exception
of 80 mg/kg feed. Lower blood cholesterol levels were noted in the
50 000, 10 000 and 2 000 mg/kg feed groups. Macroscopic examination
showed dark discolouration and/or enlargement of the spleen and pale
subcapsular areas of the liver in all dose groups, after both 7 and 14
weeks. Histopathological examination of the spleen revealed increased
haemosiderosis at all dose levels except 80 ppm. In the liver, areas
of focal necrosis and/or fibrosis in the parenchyma, with or without
associated inflammatory cells, fibroblasts or pigment-laden
macrophages, were observed. At higher dose levels, necrotic and fatty
hepatocytes and brown pigment-laden Kupffer cells were found (Colley
et al 1981).
Rat
Groups of 5 male and 5 female rats were fed on a diet containing
diflubenzuron in concentrations of 0, 800, 4 000, 20 000 and
100 000 mg/kg during a four-week period. Behaviour, body weight, food
and water consumption were not clearly affected by the treatment.
There was a dose-related increase in met- and sulph-haemoglobin in the
blood in all treated groups, except for the methaemoglobin value for
the females of the 800 mg/kg dose group. Lower RBC, PCV and
haemoglobin values were observed in both sexes of the 100 000 mg/kg
dose group. Post-mortem examination revealed no changes attributable
to diflubenzuron. For both sexes, relative spleen weights in all test
groups differed significantly from control values. Relative liver
weights were increased in males or females at all dose levels, except
800 mg/kg (Palmer et al. 1977).
Groups of 10 male and 10 female Wistar rats received
diflubenzuron during 13 weeks. The concentrations in the diet were 0,
3.125, 12.5, 50 or 200 mg/kg feed. In addition two groups of 5 males
and 5 females received 0 or 200 mg/kg. These animals were sacrificed
after 6 weeks for clinico-chemical analysis. Behaviour, growth and
food intake were not affected by the treatment. At the highest dose
level, the PCV-value, the haemoglobin concentration and the number of
erythrocytes were decreased, whereas the latter was also lowered in
the 50 mg/kg group. Particularly in the males, the SGOT and SGPT
activity was increased in the highest dose group at the end of the
experiment. Higher testicular weights were recorded in the 200 mg/kg
group. At microscopic examination, a slight increase in the number of
small foci of necrotic parenchymal cells was observed, accompanied by
monocellular inflammatory cell infiltration in the liver of both males
and females of the 50 and 200 mg/kg groups (Kemp et al. 1963a, b).
Diflubenzuron was administered to Sprague-Dawley rats at dietary
levels of 0, 10 000 and 100 000 mg/kg feed for 9 weeks, followed by a
4-week withdrawal period. Each group consisted of 20 male and 20
female animals. After treatment for 9 weeks, group size was reduced to
10 males and 10 females. From week 7 onwards, male and female rats of
both groups showed pallor of extremities and eyes. During the
withdrawal period, no recovery was observed. During or at the end of
the treatment period lower values for red blood cell parameters,
formation of met- and sulph-haemoglobin, higher SGPT-values, and
heavier liver, adrenals and spleen weights were observed. The animals
produced less but more concentrated urine, and iron pigment in the
liver and spleen was also demonstrated. Minor enlargement of
centrolobular hepatocytes in some rats of the highest dose groups was
observed. After the withdrawal period, some recovery, especially of
effects on the liver and methaemoglobin induction, was observed
(Hunter et al. 1979).
Diflubenzuron was administered in the diet to male and female
Sprague-Dawley rats (40/sex/group) at dose levels of 160, 400, 2 000,
10 000 and 50 000 mg/kg feed. An additional group (90 male and 90
female animals) served as a control. No clear treatment-related
effects were noted with respect to mortality, clinical observations,
body weight gain and food consumption. A treatment-related significant
increase in met-haemoglobin was noted in all treated groups. Sulph-
haemoglobin values showed increases from the 2 000 mg/kg group
onwards. For females and males, a significant treatment-related
decrease in haemoglobin and erythrocyte count was observed at all dose
levels at the end of the study. An increase was noted in the
reticulocyte count in all dose groups, except 160 mg/kg, and the
number of Heinz bodies was higher in the 10 000 and 50 000 mg/kg
groups. Analysis of clinical chemistry values and urinalysis revealed
no apparent treatment-related trends. After 7 weeks, spleen weights
were increased in the females at all dose levels, but after 13 weeks
no effect was found at 160 mg/kg. With the exception of the lowest
dose level, all treated groups showed a higher liver weight. The
administration of diflubenzuron resulted in a dose-related increase of
incidence of chronic hepatitis and haemosiderosis of the liver. It was
also associated at all dose levels with haemosiderosis and congestion
of the spleen and mild erythroid hyperplasia of the bone marrow
(Burdock et al 1980b; Goodman 1980b).
Dog
Diflubenzuron was fed to groups of 3 male and 3 female beagle
dogs for 13 weeks at concentrations of 0, 10, 20, 40 and 160 mg/kg in
the diet. No effect of treatment on behaviour, body weight, food and
water consumption was observed. Elevated SAP and SGPT values were
recorded for some dogs receiving 40 or 160 mg diflubenzuron/kg feed.
After 6 weeks, methaemoglobin and other abnormal haemoglobin pigments
were demonstrated in dogs receiving 160 mg/kg. After 12 weeks of
administration, some recovery was observed. Organ weights, gross and
microscopic evaluation did not show treatment-related effects
(Chesterman et al 1974).
Sheep
A 13-week feeding study was carried out with 4 groups of 3 male
and 3 female sheep. The test compound was included in the diet in a
concentration of 0, 500, 2 500 and 10 000 mg diflubenzuron/kg. These
concentrations were fed to the animals in daily amounts of 0.6 during
the first 4 weeks, 0.8 during weeks 5 to 8 and 1.0 kg during the last
5 weeks. After 6 weeks, both the plasma and RBC-cholinesterase
activities were considered to be within normal limits. No treatment-
related effects were observed on food consumption, body weight gain,
haematological parameters and urinalysis. A significant increase in
sulph-haemoglobin was observed in all treated groups at 13 weeks.
After 4 and 8 weeks this effect was only obvious at higher dose
levels. There was also an indication of a treatment-related increase
in methaemoglobin levels. No other clinico-chemical parameters were
affected. The weight of the thyroid was not dose-relatedly decreased
at all dose groups. However, histopathological examination revealed no
abnormalities that could be related to the treatment (Ross et al
1977a,b).
Long-term studies
Mouse
Five groups of 52 male and 52 female CFLP mice were fed
diflubenzuron during 80 weeks. The concentrations in the diet were 0,
4, 8, 16 and 50 mg/kg feed. Behaviour, mortality, food and water
consumption and body weight were not affected by the treatment. The
changes noted on gross and histopathologic examination were common to
both treated and control animals. No treatment-related effects or
significant tumour incidences were found. Although the incidence of
lymphosarcomas in treated female mice, killed after 80 weeks, was
significantly increased (50% level, chi square test) the combined
incidence of lymphosarcomas in treated female mice, sacrificed during
the treatment period and at the termination of the experiment, was not
significantly different (Hunter et al. 1975; Batham and Offer 1977;
Offer 1977).
Rat
Five groups, each composed of 60 male and 60 female Wistar rats,
were fed diflubenzuron during 104 weeks. For the tumorigenicity study,
45 male and 45 female animals were used, whereas 15 male and 15 female
rats constituted the satellite group for the toxicity study. The
concentrations in the diet were 0, 10, 20, 40 and 160 mg/kg feed.
There was no clear treatment-related effect on behaviour, survival,
food consumption, water consumption, body weight gain, efficiency of
food utilization, blood chemistry and urinalysis. Significantly higher
met-haemoglobin levels in both males and females receiving 160 mg
diflubenzuron/kg feed were observed after 52 and 78 weeks. The other
haematological parameters were within normal limits. Organ weights,
gross pathological and microscopic examination of tissues, including
the liver, showed no compound-related effects. There was no indication
of an increase in the number of neoplastic lesions. A no-effect level
of 40 mg diflubenzuron/kg feed was observed (Hunter et al. 1976;
Colley and Offer 1977).
Special studies on met- and sulph-haemoglobin formation
Technical diflubenzuron was administered by gastric intubation to
groups of 10 mice daily for a period of 14 days. The dose levels were
0 (20 animals), 8, 40, 200, 1 000 and 5 000 mg/kg bw. Body weight
measurement and macroscopic evaluation did not reveal any effect of
the treatment. At dose levels of 5 000 and 1 000 mg/kg the percentages
of met-and sulph-haemoglobin and erythrocytes containing Heinz bodies
were increased. No effect could be observed on the met- and sulph-
haemoglobin and on the percentage of erythrocytes containing Heinz
bodies at 200, 40 and 8 mg/kg (Keet 1977b).
A similar experiment was carried out with 2 groups of 15 male
Wistar rats. The animals received doses during 8 consecutive days of
0 or 5 000 mg/kg bw, in 1% tragacanth. There was no effect on the body
weight or the number of Heinz bodies, whereas the bet- and sulph-
haemoglobin levels were only marginally increased from day 1 and 2
respectively (Keet 1977a).
Two groups of 14 male New Zealand White rabbits were fed 0 or
640 mg diflubenzuron/kg feed during 21 days. In the treated group, the
methaemoglobin level was increased from day 5, whereas higher sulph-
haemoglobin levels were observed within 5 h. In a second experiment
with 640 mg/kg feed, the methaemoglobin level was again significantly
increased. Recovery was observed 2 weeks after the treatment was
ceased (Keet 1977c).
Twenty-four male and 24 female cats were divided among five
treatment groups and one control group. They received 30, 70, 100, 300
and 1000 mg diflubenzuron/kg bw per os for 21 days, followed by a
14-day observation period. Sodium nitrite was administered as a
positive control. Diflubenzuron induced a dose-related elevation of
methaemoglobin in females at all dose levels. In males, only 30 and
70 mg/kg did not have a significant effect (maximal effect: 11.8%).
Recovery was slow. Sulph-haemoglobinemia and Heinz body formation were
observed in all treated groups. The haemoglobin concentration, number
of reticulocytes, and organ weights were within normal limits
(Schwartz and Borzelleca 1981).
Special studies on sexual development
Diflubenzuron was incorporated into feed and tested in chickens
for 13 weeks. Fat deposition was greatly increased in females. The
combs, wattles, feathers and voice of males remained undeveloped
throughout the study. Testosterone showed a dose-related decrease
(Smalley 1976 - summary only).
Diflubenzuron was fed to 4 groups of 384 male chickens of a
Hubbard broiler strain at dose levels of 0 (twice), 2.5 and 250 mg/kg
feed. After 28, 56 and 98 days, one third of the number of animals of
each group was killed. No effects were observed on mortality, body
weight, food intake, oestradiol levels in the plasma after 4, 7 and
14 weeks, organ weights, tibia weights and lengths and on gross or
microscopic examination. Only at the end of the study, testosterone
levels in serum were higher in treated groups in comparison to
controls. Both comb and wattle were more developed in the
diflubenzuron groups. However, these differences were not
statistically significant (Keet 1976b).
A parallel study with the same dose levels was carried out with
female broiler chickens. In both diflubenzuron groups, a dose-related
reduction was observed in feed consumption and body weight. Mortality
was increased only at the highest dose level (250 mg/kg feed). In
addition, a significantly increased incidence of leg abnormalities, a
reduced tibia length and relative liver weight were observed. No clear
effect on plasma testosterone was recorded. In the highest dose level,
a marginally decreased plasma oestradiol and reduced comb and wattle
development was observed (Ross et al. 1977c).
In an experiment with 2 groups of 225 female chickens, only one
dose level (250 mg/kg feed) was tested. Animals were sacrificed after
28, 49 and 98 days. The comb and wattle development was normal and no
evidence of treatment-related effects was found. Plasma oestradiol
concentrations were within normal limits (Ross et al. 1979).
Diflubenzuron was administered in the diet to one-day old Mallard
ducks, Leghorn chickens, Nicolas White turkeys and Ring-neck pheasants
for 90 days. The concentrations in the diets were 0, 0.25, 1.25, 2.5,
25 or 250 mg/kg feed. There was no clear treatment-related effect on
serum testosterone levels at days 21, 51 and 90. At the highest dose
level (the only one measured), testosterone levels were decreased in
turkeys and ducks after 42 days. Comb and wattles were not affected by
treatment. The effects on organ weights, including testes, are
difficult to evaluate (Reinert and Cannon 1976).
Young male Long-Evans rats were administered 0, 15, 150 and
300 mg diflubenzuron/kg bw daily during 14 to 96 days by gastric
intubation. The control group contained 15 animals and each test group
8. Diflubenzuron transiently decreased the levels of testosterone in
the plasma at the prepuberal age. No effects on body weight, weight of
testes, prostate, seminal vescicles and adrenals were observed.
Histological examination of the testicular tissues did not reveal any
induced changes (Paten and Santolucito 1980).
Five groups of 38 to 40 Sprague-Dawley rats received
diflubenzuron in their diet at dose levels of 0, 75, 150, 300 or
3 000 mg/kg feed. After 14, 28, 44 and 98 days, one fourth of the
animals were sacrificed. Diflubenzuron had no clear effect on body
weight gain or serum testosterone levels (Booth et al. 1980).
Four pairs of Holstein bull calves received 0 or 1.0 to 2.8 mg
diflubenzuron/kg bw. There was no significant effect of diflubenzuron
on body weight, sperm volume, sperm concentration, libido and serum
testosterone. However, the concentration of testosterone varied
considerably. Histopathological examination of tissues revealed no
significant difference between the treated and control bulls (Miller
et al 1979).
Special studies on mutagenicity
Diflubenzuron
A dominant lethal study was conducted in which 12 male mice per
test group were treated with a single i.p. injection of a suspension
of diflubenzuron in corn oil at levels of 1 000 and 2 000 mg/kg bw.
The control group received corn oil only. Sequential mating of each
male with 3 females per week was conducted for 6 consecutive weeks.
Mating ability of males and numbers of corpora lutea, implantation
sites, resorption sites (early embryonic deaths), and embryos for
treated animals were not different from those for control animals
(Arnold 1974).
Diflubenzuron was examined for mutagenic activity in a series of
in vitro microbiological assays, using the Salmonella typhimurium
strains TA 98, TA 100, TA 1537 and TA 1978. Each plate was run with
and without rat liver homogenate (S-9 mixture) prepared from Aroclor
1254 treated rats. Diflubenzuron, tested at dose levels ranging from
10 to 1 000 µg/plate, was not mutagenic in any of these assays (Bryant
1976).
In similar tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538,
and the Sacchromyces cerevisiae strain D 4. The compound was tested
both in the absence and in the presence of liver S-9 preparations from
Aroclor 1254-induced rats. Diflubenzuron tested at dose levels ranging
from 0.1 to 500 µg/plate did not demonstrate mutagenic activity in any
of these assays (Brusick and Weir 1977a).
In another series of tests, diflubenzuron was studied in the
S. typhimurium strains TA 98, TA 100, TA 1535 and TA 1537.
Diflubenzuron, at levels of 10, 100 or 1 000 µg/plate, did not
significantly alter the spontaneous revertant frequency in the four
strains used, either with or without the metabolic activation system
from Aroclor 1254-induced rat liver (McGregor et al 1979).
An in vitro test with mouse lymphoma cells and a micronucleus
test in mice were carried out by the same author. In the in vitro
test with cultured cells, the forward mutation frequency of
TK+/- -> TK-/- in L 517 Y mouse lymphoma cells was tested.
Diflubenzuron at dose levels ranging from 1.2 to 300 µg/ml did not
increase the mutation frequency, either with or without the metabolic
activation system, in the micronucleus test, male mice were given 15,
150 or 1 500 mg/kg bw at 30 h and 6 h before necropsy. Diflubenzuron
did not significantly increase the frequency of micronucleated
erythrocytes in the bone marrow (McGregor et al. 1979).
In another series of tests, diflubenzuron, as well as its
metabolites (2,6-difluorobenzoic acid (DFBA, 4-chlorophenylurea (CPU),
4-chloroaniline), were negative in the S. typhimurium strains TA 98,
TA 100, TA 1535 and TA 1538. The results in the strain TA 1537 were
difficult to interpret (Seuferer et al 1979).
Diflubenzuron was evaluated in a cell transformation test. At
dose levels of 0.02 to 0.312 mg/ml, diflubenzuron did not induce
morphological transformation in BALB/3T3 cells in vitro (Brusick and
Weir 1977b).
Diflubenzuron was evaluated for its ability to induce unscheduled
DNA synthesis in human diploid WI-38 cells blocked in the G1 phase.
The compound was tested at dose levels of 50 to 1 000 µg/ml, both in
the absence and in the presence of liver S-9 preparations of uninduced
mice. Under the conditions of the assay, diflubenzuron did not induce
unscheduled DNA synthesis (Brusick and Weir 1977c).
Diflubenzuron was tested in a transplacental transformation assay
to investigate possible transformation of mammalian cells in culture.
Timed pregnant hamsters were injected intraperitoneally on day 10 of
gestation with solutions of diflubenzuron in dimethylsulphoxide at
dose levels of 10, 200 and 500 mg/kg bw. Three days after injection,
animals were sacrificed and foetal cell cultures were prepared.
Diflubenzuron did not induce morphological transformation or the
ability for growth of colonies. With known carcinogens (benzopyrene
and dimethylnitrosamine), a positive result was obtained (Quarles
et al 1980).
Metabolites
The metabolites 4-chlorophenylurea (CPU), 2,6-difluorobenzoic
acid (DFBA) and 4-chloroaniline were examined for mutagenic activity
in a series of in vitro microbial assays. A spot test at a dose
level of 1 000 µg per spot was carried out with S. typhimurium
strains TA 98, TA 100, TA 1535, TA 1537, TA 1538 and TA 1978, with and
without metabolic activation. A dose response test at dose levels of
10, 100, 500 and 1 000 µg was carried out with TA 98 and TA 100. The
test compounds did not demonstrate mutagenic activity in any of the
assays conducted, except for a weak effect with 500 and 1 000 µg
4-chloroaniline in the TA 98 strain after activation by liver S-9
preparation. At these dose levels, 4-chloroaniline caused a reduction
in the number of colonies, indicating a degree of toxicity to the
bacteria (Dorough 1977).
CPU was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae strain D 4.
The compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to
500 µg/plate, the test compound did not demonstrate mutagenic activity
in any of the assays conducted (Jagannath and Brusick 1977a).
CPU was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration
(312 µg/ml) employed in the concentration range. The other levels were
negative. The results were considered to be an indication of a weak
transforming activity at concentrations near to the level of
cytotoxicity (Matheson and Brusick 1978a).
CPU was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both in the absence and in the presence of liver S-9
preparations of Aroclor 1254-induced mice. CPU at dose levels ranging
from 6.25 to 400 µg/l did not induce unscheduled DNA synthesis
(Matheson et al 1978a).
DFBA was examined for mutagenic activity in a series of in
vitro microbial assays, using the S. typhimurium strains TA 98, TA
100, TA 1535, TA 1537, TA 1538 and the S. cerevisiae strain D 4. The
compound was tested both with and without liver S-9 preparations
from Aroclor 1254-induced rats. At dose levels ranging from 0.1 to 500
µg/plate, the test compound did not demonstrate mutagenic activity in
any of the assays conducted (Jagannath and Brusick 1977b)
DFBA was evaluated in a cell transformation test. The test
material induced a significant increase in morphological
transformation in BALB/3T3 cells at the highest concentration employed
(2.5 mg/ml). The other levels were negative. These results were
considered to be an indication of weak transforming activity at
concentrations near to the level of cytotoxicity (Matheson and Brusick
1978b).
DFBA was evaluated for its ability to induce unscheduled DNA
synthesis in human WI-38 cells blocked in the G1 phase. The compound
was tested both with and without metabolic activation. The compound at
dose levels ranging from 75 to 500 µg/ml produced significant
increases in the level of unscheduled DNA synthesis in the presence of
mouse S-9 liver mixture. However, there was no dose response
relationship, 75 µg giving the highest increase and 500 µg the lowest
increase. According to the authors, the results appeared to be on the
plateau of a dose response effect. The compound was considered to be
active under these test conditions (Matheson and Brusick 1978c).
4-Chloroaniline was examined for mutagenic activity in a series
of in vitro microbial assays, using the S. typhimurium strains
TA 98, TA 100, TA 1535, TA 1537 and TA 1538, and the S. cerevisiae
strain D 4. The compound was tested with and without liver S-9
preparations from Aroclor 1254-induced rats. The test compound did not
demonstrate mutagenic activity in any of the assays conducted
(Jagannath and Brusick 1977c).
4-Chloroaniline was evaluated in a cell transformation test. At
dose levels ranging from 39 to 625 µg/ml the compound did not induce
morphological transformation in BALB/3T3 cells in vitro (Matheson
et al 1978b).
4-Chloroaniline was tested for its ability to induce unscheduled
DNA synthesis in human WI-38 cells blocked in the G1 phase. The
compound was tested both with and without metabolic activation. At
dose levels of 250 to 1 000 µg/ml, the compound did not induce
unscheduled DNA synthesis (Matheson et al 1978c).
Special studies on reproduction and teratogenicity
Diflubenzuron was fed to 3 groups of 20 male and 20 female rats
at dietary levels of 0, 1 000 and 10 000 mg/kg for one generation and
one litter. The animals were maintained on their respective diets for
60 days prior to mating. There were no clear effects on mating
performance, pregnancy rate, duration of gestation, litter size,
mortality, litter weight, type and distribution of abnormalities.
Diflubenzuron had, among others, a dose-related effect on haemoglobin
(reduced PCV, Hb, total red cells, increased met-haemoglobin, sulph-
haemoglobin, spleen weight, incidence of siderocytes in the spleen and
the occurrence of iron pigment containing Kupffer cells in the liver).
A dose-related effect on the liver was also shown by an increased
weight, SGPT activity and hepatocyte enlargement. Reduced blood
glucose concentrations were recorded in both treated groups. In the
highest dosed group, the offspring showed an increased liver and
spleen weight for both sexes. Microscopically, an increased incidence
of centrolobular hepatocyte enlargement was observed (Palmer et al
1978).
Diflubenzuron was administered in the diet to groups of 20 male
and 20 female rats at concentrations of 0, 10, 20, 40 and 160 mg/kg.
These diets were administered continuously throughout three
generations producing one litter each. As the mating performance was
low in the first generation, a second litter was bred. Parent animals
showed no signs of adverse effects related to treatment. Mating
performance, pregnancy rate and duration of gestation were not
affected. Also, total litter loss, litter size, litter and mean pup
weights, pup mortality and the incidence of abnormalities provided no
evidence of adverse treatment-related effects (Palmer and Hill 1975a).
Groups of 20 pregnant rats were administered diflubenzuron orally
by gavage at levels of 0, 1, 2 or 4 mg/kg bw during days 6 to 15 of
gestation. The parent animals showed no signs of reaction and no
mortalities occurred. Body weight gain and pregnancy rate were
unaffected by treatment. No effects were observed on the number of
viable young, implantations, resorptions and corpora lutea. The
pre-implantation loss, foetal loss, litter weight, foetal weight, the
incidence of major malformations, minor anomalies and skeletal
variants were not dose-relatedly affected (Palmer and Hill 1975b).
Four groups of 13 pregnant New Zealand White rabbits were
administered diflubenzuron orally from day 6 to 18 of gestation at
dose levels of 0, 1, 2 and 4 mg/kg bw. Foetuses were removed on day 29
of pregnancy. There was no effect of the treatment on behaviour, body
weight gain and pregnancy rate. No dose-related differences were
observed on the number of viable young, resorptions, foetal loss,
litter weight and mean foetal weight. The incidence of major
malformations and minor anomalies was not affected by treatment
(Palmer and Hill 1975c).
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller and Booth 1976).
Pregnant female mice were fed a diet containing 50 mg/kg of
diflubenzuron (partly 14C). Some of these mice were sacrificed at
day 17 from conception, the others were allowed to give birth to the
young. The lactating females were kept on treatment and allowed to
suckle their young for 13 days. No radioactivity was detected in the
embryos or young mice (Booth 1977).
RESIDUES IN FOOD
USE PATTERN
Diflubenzuron is a recently-introduced insecticide that
interferes in the deposition of chitin in the insect cuticle through
action on the enzyme chitin synthetase.
Pre-harvest treatments
Diflubenzuron is formulated as a wettable powder containing 25%
of the active ingredient (Dimilin WP-25), or as an oil dispersible
concentrate containing 450 g diflubenzuron per litre. Granular
formulations are also available. Recommended use rates are given with
pre-harvest intervals in Table 3.
Other uses
Diflubenzuron is recommended for the control of flies in animal
husbandry by topical applications to breeding sites and manure heaps.
A further application is on ornamental plants and in forests.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained from numerous trials on the main
crops treated. The dosages in these trials covered recommended and
higher rates. Treatments were made using W-25 wettable powder
formulation, which is the formulation recommended for food crops.
TABLE 3. Recommended uses and pre-harvest intervals for diflubenzuron
Use rate Pre-harvest
Crop Country (a.i.) interval (days)
Apple, pear Argentina 0.02% 60
Bulgaria 0.0375% -
France 0.01% 30
German Fed.Rep. 0.02% 28
Greece 0.0125-0.015%1 -
Israel 0.0125% -
Italy 0.0125-0.02% 45
The Netherlands 0.01% 28
Spain 0.01-0.015% 60
South Africa 0.02% -
Switzerland 0.02% 42
UK 150 g/ha 14
Yugoslavia 0.0125-0.02% 30
Brassica leafy
vegetables The Netherlands 0.01% 14
China(Taiwan prov.) 0.017% 22
UK 100 g/ha -
Yugoslavia 200 g/ha -
Cottonseed Egypt 75 g/ha -
Greece 0.01-0.025% -
USA 70 g/ha (6x) -
Mushroom Greece 1 g/m2 -
Korea 1 g/m2 -
The Netherlands 1 g/m2 -
Switzerland 1 g/m2 -
UK 1 g/m2 -
Soybean Brazil 50-75 g/ha 21
Colombia 75-125 g/ha -
USA 35-70 g/ha
Tomato UK 250 g/ha -
Citrus USA2 350 g/ha ?
1 for pears: 0.0125-0.03%;
2 registration applied for.
Apple
Residue data have been obtained from trials in several countries.
Residues on fruit at recommended rates up to 0.02% were usually well
below 1.0 mg/kg at two weeks after the last application. Only three
samples were shown to contain residues from 1.0 to 1.2 mg/kg. The
one sample that contained 3.7 mg/kg is considered to be atypical
(Table 4). In the UK, apples (cv Cox and Egremont Russet) treated with
diflubenzuron 2 weeks before harvest at a dosage rate of 250 g a.i./ha
were shown to be taint-free (Spencer-Jones 1979).
Pear
The residue pattern in pears is very similar to that in apples.
At two weeks after the last application at recommended dosages, the
residues were well below 1.0 mg/kg (Table 4).
Citrus
Residue data have been obtained from numerous trials on orange,
grapefruit and tangerine in the USA. The dosages in these trials
included the recommended rate and also 2x and 4x rates. Residues in
whole fruit were all well below 0.5 mg/kg 1 week after the last
application at the recommended dosage rate. At the 4x dosage rate, the
residue remained generally below 1.0 mg/kg (Table 5).
Examination of peel and pulp separately showed that residues were
exclusively found in the peel when the product was applied at the
recommended rate. Residues in the pulp were all below the detection
limit (0.05 mg/kg). At the 4x use rate, by far the major portion of
the residue was in the peel. Residues in the pulp were either below or
just above the detection limit of 0.05 mg/kg, the highest value found
being 0.07 mg/kg. (Table 6).
Further fractionation of orange and grapefruit showed that the
residue is mainly present in the oil fraction. Here, the residue
ranged from 9.5 to 20 mg/kg at the recommended use rate. At the
4x rate residues were as high as 50 mg/kg. Neither at the recommended
rate nor at the 4x rate could residues be detected in the juice of
both orange and grapefruit, the limit of detection being 0.05 mg/kg
(Table 7).
In several cases, the various citrus fractions were analysed for
residues of the metabolites 4-chlorophenylurea, 4-chloroaniline and
2,6-difluorobenzoic acid. In none of the samples was any residue
detected (limit of detection for all three compounds 0.05 mg/kg)
(Duphar 1975-81).
TABLE 4. Residues following supervised trials in apple and pear1
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Apple
Bramley UK 0.01 1 - 0.22 0.23 0.14
Cox O.P. 0.01 1 - 0.18 0.16 0.20
Egremont R 0.01 1 - 0.27 0.24 0.18
Golden D. Netherlands 0.01 1 - <0.032
Winston 0.01 1 - <0.03
Golden D Europe 0.01 3-5 15-59 0.15 0.16 0.23 0.30
-0.21 -0.34
J. Grieve Netherlands 0.0125 1 - 0.28 0.36 0.13
J. Grieve 0.0125 2 17 1.32 0.94 0.75 0.53
Stark Crimson Italy 0.0125 2 58 0.06
Golden D 0.0125 2 54 0.15
Stark Crimson 0.0125 3 58,34 0.12
Golden D 0.0125 3 58,33 0.05
Jonathan 0.0125 4 35,22,41 0.14
J. Grieve German Fed.Rep. 0.015 1 - 0.43 0.14 0.03 0.09 0.11
Golden D. Europe 0.015 1-8 14-52 0.34 0.78 0.34 0.12 0.23 <0.05
-1,2 -1.1 -0.83
Bramley UK 0.017 1 - 0.17 0.02 0.18 0.19 0.02
Cox O.P. 0.017 1 - 0.09 <0.01 0.22
Egremont R 0.017 1 - 0.17 0.03 0.20
Ida Red 0.017 1 - 0.12
Golden D 0.017 1 - 0.20
McIntosh 0.017 1 - 0.17
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Warner UK 0.017 1 - 0.16
Worcester 0.017 1 - 0.15
Newton 0.017 1 - 0.01
Golden D France 0.01875 3 33,31 0.41
Golden D 0.01875 3 30,27 0.46
Golden D 0.01875 3 29,35 0.43
Golden D 0.01875 4 21,21,28 0.62
Golden D 0.01875 4 20,20,30 3.8
J. Grieve Netherlands 0.02 1 - 0.29 0.21 0.18 0.15 0.13
Golden D 0.02 1 - 0.52 0.31 0.23 0.29
n.s. South Africa 0.02 1 - 0.833 0.32 0.29
n.s. 0.02 1 - 0.784 0.14 0.15 0.23
Cox O.P. Netherlands 0.02 1 - 0.095
S. Boskoop 0.02 1 - 0.146
Ingrid M. 0.02 1 - 0.08
Winston 0.02 1 - 0.097
Golden D 0.02 1 - 0.055
Cox O.P German Fed.Rep. 0.02 1 - 0.258 0.05a
Cox O.P 0.02 1 - 1.568 0.438
Golden D 0.02 1 - 0.738 0.288
Cox O.P. 0.02 2 22 0.538 0.26 0.258 0.19 0.14
Golden D 0.02 2 21 1.268 0.69 0.58 0.53 0.37
Cox O.P. 0.02 2 21 1.878 1.24 0.95 0.51 0.25
Golden D Netherlands 0.02 2 22 <0.03
Gravesteyn Italy 0.02 2 53 0.24
Cox O.P. German Fed.Rep. 0.029 3 21,28 0.84 0.92 0.76 0.72
Cox O.P. 0.029 3 22,28 0.54 0.42 0.40 0.26 0.47
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D 0.02 21,28 1.26 0.89 0.52 0.66 0.63
Golden D Italy 0.02 3 57,56 0.52
Golden D 0.02 3 10,68 0.57
Steymen 0.02 3 58,32 0.40
Golden D Netherlands 0.029 3 40,31 0.45 0.40 0.32 0.29 0.19 0.28
Cox O.P. 0.029 3 34,30 0.30 0.27 0.29 0.24
G. Parmane German Fed.Rep. 0.029 4 29,39,14 0.5210 0.04 0.26 0.30
n.s. South Africa 0.0211 4 28,26,38 1.24
Jonathan Italy 0.02 5 23,15,27,14 0.28
Golden D 0.02 5 34.30,14,28 0.87
Golden D 0.02 5 40,29,15,33 0.77
Golden D France 0.02 5 15,14,16,16 0.27
J. Grieve Netherlands 0.025 1 - 0.67 0.38 0.31
Star-King D Japan 0.025 1 <0.01 0.067
Red-ball 0.025 1 0.087 0.107
n.s. Italy 0.02512 2 62 0.197 0.2313
J. Grieve Netherlands 0.025 2 17 1.14 1.62 0.58 0.87
Golden D 0.025 2 15 0.426
St.Crimson Italy 0.025 2 58 0.10
Golden D 0.025 2 52 0.117
St.Crimson 0.025 3 58,34 0.30
Golden D 0.025 3 52-54,32-49 0.55 0.28
Star-King Japan 0.025 3 0.387
Red-ball 0.025 3 0.207
Jonathan Italy 0.025 4 35,22,41 0.18
Golden D France 0.025 5 28,14,21,21 1.0
Golden D 0.025 6 12-18 0.87
J. Grieve German Fed. Rep. 0.03 1 - 0.73 0.23 0.15 0.15 0.13
Golden D 0.03 1 - 1.0 0.67 0.44 0.43 0.31
TABLE 4. (con't)
Application Interval Residues (mg/kg) at intervals (days)
Crop between after last application
and variety Country Rate No. applications
(%) (days) 0-7 8-14 15-21 22-28 29-42 43-56 >56
Golden D Netherlands 0.03 1 - 0.056
Cox O.P. 0.03 1 - <0.03
Golden D 0.03 2 15 0.437
Golden D 0.03 2 15-19 0.34 0.32 0.326
n.s. South Africa 0.03 3 28,32 0.26
Golden D 0.03 8 14 2.20
n.s. 0.04 1 - 0.7815 0.38 0.37 0.25
n.s. 0.04 1 - 1.5516 1.25 0.70
Golden D Netherlands 0.04 1 - 0.0514
Winston 0.04 1 - <0.03
Golden D 0.04 2 22-43 0.077
Jonathan 0.04 2 26 <0.05
Gravesteyn Italy 0.04 2 53 0.41
Golden D 0.04 3 10-68 0.83 1.11
Alkmene German Fed. Rep. 0.0417 4 13,16,20 1.13 0.7418 0.63 0.65
Golden D Italy 0.04 5 14-40 1.437
Golden D France 0.04 6 12-18 1.40
Pear Europe 0.01 1-5 17-33 0.1 0.12 0.02
-0.04 -0.31 -0.25
South Africa 0.02 1-3 28-32 0.52 0.11 0.13 0.24
-0.04 -2.64 -1.30 -1.25 -1.31
1 Reference: Duphar 1975-81; 2 4 trials;
3 day 1: 0.63, day 2:0.83, day 6:0.50, day 7: 0.61; 4 day 1: 0.42, day 2: 0.78, day 4: 0.30;
5 average of 6 trials; 6 average of 3 trials;
7 average of 2 trials; 8 average of 2 samples;
9 last spray rate - 0.015; 10 day 0: 0.52, day 7: 0.44;
11 first spray rate-0.025; 12 last spray rate - 0.02;
13 average of 4 trials; 14 average of 5 trials;
15 day 1: 0.78, day 2: 0.11, day 4: 0.91; 16 day 1: 1.55, day 2: 0.72, day 6: 1.15, day 7: 1.39;
17 last spray rate - 0.03; 18 day 9: 0.74, day 14: 0.72.
TABLE 5. Residues following supervised trials in citrus, whole fruit, in Florida and Texas, USA1
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 1 PB 0.07 0.11 0.12 0.13 0.08 0.10
1 PB 0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Temple 1 PB 0.053
1 PB 0.063
Pineapple 1 PB <0.05
1 PB 0.053
Hamlin 1 PB 0.06
1 PB <0.05
Mars 1 PB 0.10
Temple 2 PB,S 0.084
2 PB,S 0.063
Pineapple 2 PB,S 0.093
2 PB,S 0.053
Hamlin 2 PB,S 0.123
2 PB,S <0.05
Mars 2 PB,S 0.16
Temple 3 PB,S,F 0.144 0.13 0.143
3 PB,S,F 0.173 0.13 0.11
Pineapple 3 PB,S,F 0.183 0.18 0.08
3 PB,S,F 0.133 0.10 0.07
Hamlin 3 PB,S,F 0.163 0.20 0.083 0.25
3 PB,S,F 0.10 0.05
Mars 0.35 3 PB,S,F 0.32
Hamlin-pineapple " 3 PB,S,F 0.27 0.29 0.30 0.36 0.21
3 PB,S,F 0.20 0.29 0.22 0.41 0.14
3 PB,S,F 0.05 0.20 0.10 0.11 0.21
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 1 PB 0.054
Marsh 1 PB <0.053
Seedless 1 PB <0.053
Ruby red 1 PB 0.063
1 PB 0.063
Pink 2 PB,S <0.054
Marsh seedless 2 PB,S 0.083
2 PB,S 0.063
Ruby Red 2 PB,S 0.123
Ruby Red 0.35 2 PB,S 0.123
Pink 3 PB,S,F 0.174 0.15 0.093 0.26 0.22
Marsh seedless 3 PB,S,F 0.123 0.073
3 PB,S,F 0.113 0.143
Ruby Red 3 PB,S,F 0.203
3 PB,S,F 0.133
Tangerine Nova 1 PB <0.05
1 PB <0.05
2 PB,S 0.12
2 PB,S 0.09
3 PB,S,F 0.07 0.05
3 PB,S,F 0.09 0.09
Orange Valencia 0.7 1 PB 0.223 0.133 0.163 0.143 0.083 0.153 <0.05
1 PB 0.10 0.11
Mars 1 PB 0.27
2 PB,S 0.28
3 PB,S,F 0.23
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1 PB 0.10
2 PB,S 0.203
3 PB,S,F 0.203
Valencia 1.4 1 PB 0.233 0.113 0.273 0.233 0.123 0.073
1 PB 0.07 0.05
Temple 1 PB <0.053
1 PB 0.07 0.05
Pineapple 1 PB <0.053
1 PB 0.053
Hamlin 1 PB <0.053
Orange Hamlin 1.4 1 PB 0.09
Mars 1 PB 0.18
Grapefruit Pink 1 PB <0.053
1 PB <0.05
Marsh seedless 1 PB <0.053
1 PB 0.343
Ruby Red 1 PB 0.273
Tangerine Nova 1 PB <0.05
1 PB <0.05
Orange Temple 1.4 2 PB,S 0.124
2 PB,S 0.15
Pineapple 2 PB,S 0.173
2 PB,S 0.383
Hamlin 2 PB,S <0.053
Mars 2 PB,S 0.305
TABLE 5. (con't)
Fruit type and Variety Application Residues (mg/kg) at intervals (days)
after application
Rate
(kg/ha) No. Times2 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Pink 2 PB,S 1.10 0.26 0.243
2 PB,S 0.23
Marsh seedless 2 PB,S 0.243
2 PB,S 0.353
Ruby Red 2 PB,S 0.233
Tangerine Nova 2 PB,S 0.15
2 PB,S 0.20
Orange Temple 3 PB,S,F 0.384 0.473 0.233 0.91
3 PB,S,F 0.46 0.59
Pineapple 3 PB,S,F 0.253 0.35 0.06
3 PB,S,F 0.573 0.49 0.15
Hamlin 3 PB,S,F 0.713 0.74 0.103 0.44
3 PB,S,F 0.23 0.26
Mars 3 PB,S,F 0.10
Grapefruit Pink 3 PB,S,F 0.393 0.81 0.85 1.11 0.48
3 PB,S,F 0.34 0.65
Marsh seedless 3 PB,S,F 0.533 0.273
3 PB,S,F 0.303 0.613
Ruby Red 3 PB,S,F 0.643
Tangerine Nova 3 PB,S,F 0.16 0.28
3 PB,S,F 0.31 0.24
1 Referenne: Duphar 1975-81;
2 PB = Post Bloom, S = Summer,F = Fall;
3 average of 2 trials;
4 average of 3 Trials;
5 day 70: 0.53 in one trial.
TABLE 6. Residues following supervised trials in citrus, peel and pulp, in Florida and Texas, USA1
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 0.35 12 peel 0.10
pulp <0.05
Temple 33 peel 0.574
pulp <0.054
peel 0.21
pulp <0.05
Pineapple 3 peel 0.46
pulp <0.05
3 peel 0.26
pulp <0.05
Hamlin 3 peel 0.40
pulp <0.05
Mars 3 peel 0.50
pulp <0.05
Grapefruit Pink 3 peel 0.58
pulp <0.05
Marsh seedless 3 peel 0.41
pulp <0.05
3 peel 0.84
pulp <0.05
Ruby Red 3 peel 0.614
pulp <0.05
0.355 3 peel 0.934
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Orange Valencia 1,4 1 peel 0.09 1.89 1.65 1.83 0.88 0.75
pulp 0.07 0.07 0.07 <0.07 <0.05 <0.05
peel 0.78 0.50 0.70 0.91 0.43 0.15
pulp <0.05 <0.05 <0.05 <0.05 <0.05 (0.05
1 peel 0.29 <0.05
pulp <0.05 <0.05
Orange Temple 1.4 2 peel 0.07
pulp 0.05
Hamlin 2 peel 0.38
pulp <0.05
Temple 3 peel 1.40 2.00
pulp <0.05 <0.05
3 peel 1.30 0.61
pulp <0.05 <0.05
peel 1.20
pulp (0.05
Pineapple 3 peel 0.93 1.8
pulp <0.05 <0.05
3 peel 1.70 0.28
pulp <0.05 <0.05
Hamlin 3 peel 0.20 2.70
pulp <0.05 <0.05
1.45 3 peel 1.3
pulp <0.05
TABLE 6. (con't)
Application Residues (mg/kg) at intervals (days) after application
Fruit type and Variety Rate Citrus
(kg/ha No. fraction 0-7 8-14 15-21 22-28 29-42 43-56 >56
Grapefruit Ruby Red 1.4 1 peel 0.88c
pulp 0.05c
2 peel 0.40c
pulp 0.05
3 peel 0.94
pulp 0.05
Pink 3 peel 2.10
pulp 0.05
3 peel 1.90
pulp 0.07
Marsh seedless 3 peel 0.66
pulp 0.07
3 peel 1.0
pulp 0.05
Tangerine Nova 3 peel 0.18
pulp 0.05
3 peel 0.75
pulp 0.05
1 Reference: Duphar 1975-81;
2 1 Spray Post Bloom;
3 3 sprays: Post Bloom, Summer and Fall;
4 average of 2 trials;
5 plus 0.25% oil.
TABLE 7. Residues following supervised trials in citrus fractions, in Florida, USA1
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Orange (Temple) Whole fruit(unwashed) 0.35 1.4 3 0.13 0.35 14
Whole fruit (washed) 0.09 0.26 14
Chopped peel 0.09 0.13 14
Frits 0.11 0.84 14
Finisher pulp <0.05 <0.05 14
Dried citrus pulp 0.09 0.05 14
Fruit Juice <0.05 <0.05 14
Oil 13.0 28.0 14
Pressed liquor <0.05 0.09 14
Molasses <0.05 0.06 14
Prewash water 0.01 0.02 14
Afterwash water 0.03 0.07 14
Orange (Eamlin) Whole fruit(unwashed) 4 0.28 0.34 2t
Whole fruit(washed) 0.14 0.33 21
Chopped peel 0.06 0.11 21
Frits 0.10 0.55 21
Finisher pulp <0.05 <0.05 21
Dried citrus pulp <0.05 0.66 21
Fruit juice <0.05 <0.05 21
Oil 20.0 50.0 21
Pressed liquor <0.05 0.08 21
Molasses <0.05 0.12 21
Prewash water 0.03 0.06 21
Afterwash water 0.05 0.22 21
TABLE 7. (con't)
Residue found Interval after
Fruit type Sample type Rate Treatment last application
(kg/ha) No. (mg/kg) (days)
Grapefruit (Pink) Whole fruit (unwashed) 0.35 3 0.09 21
Whole fruit(washed) <0.05 21
Chopped peel 0.08 21
Frits 0.19 21
Finisher pulp <0.05 21
Dried citrus pulp 0.10 21
Fruit juice <0.05 21
Oil 9.50 21
Pressed liquor <0.05 21
Molasses <0.05 21
Prewash water 0.01 21
Afterwash water 0.04 21
Whole fruit (unwashed) 1.4 3 0.09 21
Whole fruit (washed) 0.17 21
Chopped peel 0.11 21
Frits 0.50 21
Finisher pDlp <0.05 21
Dried citrus pulp 0.36 21
Fruit juice <0.05 21
Oil 23.0 21
Pressed liquor <0.05 21
Molasses 0.17 21
Prewash water 0.03 21
Afterwash water 0.17 21
1 Reference: Duphar 1975-81.
In taste tests with orange juice, no off-flavour was detected
which might have been caused by DIMILIN treatment (Braddock 1976b,
1977). Also in the case of grapefruit juice, no adverse effects on
flavour were detected (Braddock 1976a).
Soybean
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended use rate and also much
higher rates. Residues in the soybean seed were generally below the
detection limit, which was 0.05 mg/kg. Only one sample contained a
residue just above this limit, i.e. 0.06 mg/kg. Also at higher rates,
residues remained very low, the highest value found being 0.16 mg/kg.
Fractionation of seed containing <0.05 mg/kg residue did not result
in a detectable residue in any of the fractions. As could be expected
from the foliar stability of diflubenzuron, soybean foliage did
contain residues, the level of which declined with time (Table 8).
In nine trials, rotational crops were grown in fields on which
soybean had been treated with diflubenzuron at both the recommended
and higher rates. In none of the trials could residues be detected
(limit of detection 0.05 mg/kg) in the rotational crops, including
turnips, collards, rye, oats and mustard green. In the samples
analysed for 4-chlorophenylurea, no residue could be detected (limit
of detection 0.05 mg/kg) (Duphar 1975-81).
Residues in soil of soybean fields treated with diflubenzuron
were usually below the limit of detection (0.05 mg/kg), both for the
parent compound and the metabolite 4-chlorophenylurea (CPU).
Diflubenzuron residues never exceeded 0.3 mg/kg and were exclusively
found in the top 7.6 cm of soil. CPU residues did not exceed 0.5 mg/kg
and in only one case could any residue be detected below the top
7.6 cm of soil (Duphar 1975-81).
Cotton
Residue data were obtained from numerous trials, mainly in the
USA. The dosages included the recommended rate and also higher ones.
Usually, several applications were made, up to 16 per growing season.
Residues in the cotton seed were generally below the limit of
detection (0.05 mg/kg) (Table 9). Only a few samples contained a
residue slightly above the detection limit, with one high value of
0.17 mg/kg. Fractionation of cotton seed did not result in a
detectable residue of any of the fractions.
In seven trials, rotational crops were grown in fields on which
cotton had been treated with diflubenzuron 15 times during the growing
season either at a rate of 0.067 or 0.280 kg a.i./ha. In none of the
trials could residues of either diflubenzuron or 4-chlorophenylurea
TABLE 8. Residues following supervised trials in soybean, USA1
Application Residues (mg/kg) at intervals (days)
Crop part after application
Rate (a.i.) No.
(kg/ha) 0-7 8-14 15-21 22-28 29-42 43-56 <56
0.034 1-2 <0.05 <0.05 <0.05 <0.05 <0.05
Seed - 0.56 -0.13 -0.16 -0.07 -0.07
Foliage Bragg 0.034 1 0.86 0.49 0.25
Lee 0.034 1 1.10 0.59 0.38
Ranson 0.034 1 2.25 1.40 1.65 0.82
n.s. 0.034 1 0.25
Bragg 0.034 2 0.462 2.6 0.26 0.63
Lee 68 0.034 2 2.1 0.58
Bragg 0.067 1 1.8 0.29 0.33 0.14
Calland 0.067 1 0.16
n.s. 0.280 1 1.6
n.s. 0.280 2 0.23
0.140 1
Seed n.s. <0.05
Hulls <0.05
Meal <0.05
Crude oil <0.05
1 Reference: Duphar 1975-81; 2 Average of 2 trials.
TABLE 9. Residues following supervised trials in cottonseed, USA1
Application Residues (mg/kg) at intervals (days)
after application
Crop part Variety Rate (a.i.) No.
kg/ha 0-7 8-14 15-21 22-28 29-42 43-56 <56
Seed 0.033 1- <0.05 <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
-0.56 15 -0.07 0.09 -0.05 -0.07 -0.17
Hulls DPL-16 0.140 8 <0.05
Oil <0.05
Meal <0.05
Seed hulls Stoneville 213 0.280 9 <0.05
Meal <0.05
Oil <0.05
Soapstock <0.05
1 Reference: Duphar 1975-81
(limit of detection for both compounds 0.05 mg/kg) be detected in
the rotational crops, including collards, wheat and radish (Duphar
1975-81).
Mushroom
This crop shows a residue pattern different from all the others.
This is undoubtedly due to the different way in which it is grown. The
intimate contact of the mushrooms with the medium in which they are
grown enables the uptake of the metabolite 2,6-difluorobenzoic acid
especially (see also "Fate of residues"). At the recommended dosage
rate of 1 g a.i./m2, the diflubenzuron residue in mushrooms is
generally below 0.1 mg/kg, only one sample contained 0.11 mg/kg.
Also the residue level of 4-chlorophenylurea is very low,
all residues being below 0.1 mg/kg. However, the residues of
2,6-difluorobenzoic acid were considerably higher. At the recommended
use rate they all remain below 1 mg/kg. At higher dosage rates,
somewhat higher residues are found, the highest value being 1.63 mg/kg
at a dosage of 4 g/m2, applied twice. (Table 10).
FATE OF RESIDUES
General comments
The metabolism of diflubenzuron was studied and it was
established that the compound degraded along different lines, as shown
in Figure 2. Breakdown occurred predominantly along line 1, resulting
in 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU). A
minor degradation takes place along lines 2 and 3, resulting in
4-chloroaniline (PCA) and CO2.
 In plants
The fate of radio-labelled diflubenzuron (14C and 3H) has been
studied on corn, soybean, cabbage and apple in the greenhouse after
foliar application. No significant degradation or translocation was
observed for 16 weeks after application (Nimmo and De Wilde 1974;
Nimmo et al 1978). Similar results were obtained on pine needles
(Nimmo and De Wilde 1980).
TABLE 10. Residues following supervised trials in mushrooms1
Application Interval Residues (mg/kg) at intervals (days) after last application
Country between
Rate applications diflubenzuron CPU
(g a.i./m2) No. (days) 0-21 22-28 29-35 36-43 >43 0-21 22-28 29-35 36-43 >43
Netherlands 0.5 2 12 0.04 <0.01 <0.01 <0.01 <0.01 0.02 0.01 0.02 0.02 0.02
1.0 1 2 0.04 0.02 0.05 0.02 0.02 0.02 0.03 0.06 0.04 0.04
1.0 2 3 0.06 0.03 0.02 0.04 <0.11 <0.02 0.02 0.04 0.03 0.03
2.0 2 2 0.05 0.05 0.06 0.04 0.05 <0.02 0.02 0.07 0.06 0.06
4.0 2 2 0.11 0.08 0.10 0.06 0.07 <0.02 0.03 0.07 0.07 0.04
UK 10 mg/kg 1 <0.03 <0.03 <0.01 <0.01
30 mg/kg 1 <0.03 <0.03 <0.01 <0.01
100 mg/kg 1 <0.03 <0.03 <0.01 <0.01
Residues (mg/kg) at intervals (days)
Application Interval after last application
Country between
Rate applications DFBA
(g a.i./m) No. (days) 0-21 22-28 23-35 36-43 <43
Netherlands 0.5 2 12 0.27 <0.01 0.24 0.16 0.14
1.0 1 2 0.45 0.16 0.38 0.36 0.43
1.0 2 3 0.40 0.90 0.59 0.66 0.31
2.0 2 2 0.53 1.42 0.94 0.60 0.95
4.0 2 2 1.2 1.57 1.63 1.16 1.2
UK 10 mg/kg 1 0.19 0.31
30 mg/kg 1 0.44 0.60
100 mg/kg 1 0.50 0.80
1 Reference: Duphar 1975-81;
2 Average of 2 trials;
3 average of 4 trials.
Also under greenhouse conditions, diflubenzuron (14C) was
applied to cotton plants. Absorption, translocation and metabolism
were not significant over the trial period of 48 days (Mansager
et al 1979).
Under field conditions, it was demonstrated that there is very
little absorption or degradation of 14C-diflubenzuron on cotton
foliage (Bull and Ivie 1978).
The fate of diflubenzuron was also studied following application
to soybeans under field conditions. Again, it was found that there is
no significant absorption, translocation or metabolism of
diflubenzuron (Gustafson and Wargo 1976).
When cotton plants with diflubenzuron residues are incorporated
into the soil in the fall, there is little degradation of
diflubenzuron during the winter. However, with the onset of higher
temperatures, the residues declined rapidly (Bull and Ivie 1978).
This, probably, is caused by an increased accessibility of the residue
as a result of the progressive decay of the plant material with which
it was associated (Bull 1980).
Similarly, the degradation of diflubenzuron residues on oak leaf
litter is a rather slow process, the half life being roughly 6 to 9
months (Willems 1981),
Also in other organic substrates, the degradation of
diflubenzuron is considerably slower than in soil. This has been
demonstrated in chicken manure (half life time ca. 4 months), calf
manure (half life time ca. 6 months) and in a mushroom growth medium,
consisting mainly of horse and chicken manure (30-50% degradation in
one month). In these substrates, as in soil, the main metabolites are
2,6-difluorobenzoic acid and 4-chlorophenylurea (Nimmo and De Wilde
1977 a, b).
In animals
The metabolic fate of diflubenzuron has been studied in a variety
of vertebrate and invertebrate species (See section on "Biochemical
aspects").
There seems to be conclusive evidence that diflubenzuron is not
degraded within the digestive tract of mammals to any significant
degree. The portion of diflubenzuron that is absorbed from the
digestive tract is strongly dose-related. After absorption,
diflubenzuron is completely metabolized before excretion.
Metabolic pathways of diflubenzuron in insects seem to be
essentially the same as in mammals (Chang and Stokes 1979; Ivie and
Wright 1978; Chang 1978; Pimprikar 1977; Bull and Ivie 1980).
In soil
The fate of diflubenzuron has been studied in seven agricultural
soils and three hydro-soils, including the soil types recommended by
the US Environmental Protection Agency and the German Biologische
Bundes-Anstalt. In these studies, the compound was mixed through the
soil at a concentration of 1 mg/kg. From the results, the following
conclusions could be drawn:
a) The rate of degradation is strongly dependent on the
particle size of the diflubenzuron. At an average particle
size of approximately 10 µm, the half life in various soils
ranged from 8 to 16 weeks, whereas at a mean particle size
of about 2 µm the half life was 0.5 to 1 week.
b) The half life in different types of soil varied only
slightly.
c) The metabolic pathways of diflubenzuron in soil are
summarized in Figure 3.
The main metabolic pathway (over 90%) is hydrolysis, which
produces 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU).
A very minor pathway is hydrolysis of the other carbon-nitrogen bond,
producing 4-chloroaniline (PCA) and 2,6-difluorobenzamide, which is
rapidly converted into the corresponding acid, DFBA. DFBA is rapidly
degraded further, the half life being approximately 4 weeks. The first
step is a decarboxylation, followed by ring degradation. CPU was found
to be converted into bound residues with a half life of 5 to 10 weeks.
In the bound residue, after 2 months, both CPU and PCA are present in
roughly equal amounts. As PCA is generated directly from diflubenzuron
in only small amounts, there must be degradation of CPU into PCA. Free
PCA was not found in these studies (Nimmo and De Wilde 1975a; Verloop
and Ferrell 1977). This can be explained by the rapid binding to the
soil, found for anilines in general and PCA in particular (Hsu and
Bartha 1974; Moreale and Van Bladel 1976; Bollag et al 1978).
In a sandy loam soil, it was demonstrated that the degradation of
diflubenzuron is of a microbiological nature: in an experiment with
normal soil, after 4 weeks only 2% of the initial amount of
diflubenzuron was still present, whereas in a steam-sterilized soil,
94% of the diflubenzuron was unchanged (Nimmo and De Wilde 1975a).
Studies in the laboratory (Helling 1975; Rieck 1975; Bull and
Shaver 1980) and in the field (Danhaus et al 1976; Bull and Ivie
1978) have shown that diflubenzuron has a very low mobility in the
soil. Under field conditions, by far the major portion remains in the
top 7.5 cm of soil.
In plants
The fate of radio-labelled diflubenzuron (14C and 3H) has been
studied on corn, soybean, cabbage and apple in the greenhouse after
foliar application. No significant degradation or translocation was
observed for 16 weeks after application (Nimmo and De Wilde 1974;
Nimmo et al 1978). Similar results were obtained on pine needles
(Nimmo and De Wilde 1980).
TABLE 10. Residues following supervised trials in mushrooms1
Application Interval Residues (mg/kg) at intervals (days) after last application
Country between
Rate applications diflubenzuron CPU
(g a.i./m2) No. (days) 0-21 22-28 29-35 36-43 >43 0-21 22-28 29-35 36-43 >43
Netherlands 0.5 2 12 0.04 <0.01 <0.01 <0.01 <0.01 0.02 0.01 0.02 0.02 0.02
1.0 1 2 0.04 0.02 0.05 0.02 0.02 0.02 0.03 0.06 0.04 0.04
1.0 2 3 0.06 0.03 0.02 0.04 <0.11 <0.02 0.02 0.04 0.03 0.03
2.0 2 2 0.05 0.05 0.06 0.04 0.05 <0.02 0.02 0.07 0.06 0.06
4.0 2 2 0.11 0.08 0.10 0.06 0.07 <0.02 0.03 0.07 0.07 0.04
UK 10 mg/kg 1 <0.03 <0.03 <0.01 <0.01
30 mg/kg 1 <0.03 <0.03 <0.01 <0.01
100 mg/kg 1 <0.03 <0.03 <0.01 <0.01
Residues (mg/kg) at intervals (days)
Application Interval after last application
Country between
Rate applications DFBA
(g a.i./m) No. (days) 0-21 22-28 23-35 36-43 <43
Netherlands 0.5 2 12 0.27 <0.01 0.24 0.16 0.14
1.0 1 2 0.45 0.16 0.38 0.36 0.43
1.0 2 3 0.40 0.90 0.59 0.66 0.31
2.0 2 2 0.53 1.42 0.94 0.60 0.95
4.0 2 2 1.2 1.57 1.63 1.16 1.2
UK 10 mg/kg 1 0.19 0.31
30 mg/kg 1 0.44 0.60
100 mg/kg 1 0.50 0.80
1 Reference: Duphar 1975-81;
2 Average of 2 trials;
3 average of 4 trials.
Also under greenhouse conditions, diflubenzuron (14C) was
applied to cotton plants. Absorption, translocation and metabolism
were not significant over the trial period of 48 days (Mansager
et al 1979).
Under field conditions, it was demonstrated that there is very
little absorption or degradation of 14C-diflubenzuron on cotton
foliage (Bull and Ivie 1978).
The fate of diflubenzuron was also studied following application
to soybeans under field conditions. Again, it was found that there is
no significant absorption, translocation or metabolism of
diflubenzuron (Gustafson and Wargo 1976).
When cotton plants with diflubenzuron residues are incorporated
into the soil in the fall, there is little degradation of
diflubenzuron during the winter. However, with the onset of higher
temperatures, the residues declined rapidly (Bull and Ivie 1978).
This, probably, is caused by an increased accessibility of the residue
as a result of the progressive decay of the plant material with which
it was associated (Bull 1980).
Similarly, the degradation of diflubenzuron residues on oak leaf
litter is a rather slow process, the half life being roughly 6 to 9
months (Willems 1981),
Also in other organic substrates, the degradation of
diflubenzuron is considerably slower than in soil. This has been
demonstrated in chicken manure (half life time ca. 4 months), calf
manure (half life time ca. 6 months) and in a mushroom growth medium,
consisting mainly of horse and chicken manure (30-50% degradation in
one month). In these substrates, as in soil, the main metabolites are
2,6-difluorobenzoic acid and 4-chlorophenylurea (Nimmo and De Wilde
1977 a, b).
In animals
The metabolic fate of diflubenzuron has been studied in a variety
of vertebrate and invertebrate species (See section on "Biochemical
aspects").
There seems to be conclusive evidence that diflubenzuron is not
degraded within the digestive tract of mammals to any significant
degree. The portion of diflubenzuron that is absorbed from the
digestive tract is strongly dose-related. After absorption,
diflubenzuron is completely metabolized before excretion.
Metabolic pathways of diflubenzuron in insects seem to be
essentially the same as in mammals (Chang and Stokes 1979; Ivie and
Wright 1978; Chang 1978; Pimprikar 1977; Bull and Ivie 1980).
In soil
The fate of diflubenzuron has been studied in seven agricultural
soils and three hydro-soils, including the soil types recommended by
the US Environmental Protection Agency and the German Biologische
Bundes-Anstalt. In these studies, the compound was mixed through the
soil at a concentration of 1 mg/kg. From the results, the following
conclusions could be drawn:
a) The rate of degradation is strongly dependent on the
particle size of the diflubenzuron. At an average particle
size of approximately 10 µm, the half life in various soils
ranged from 8 to 16 weeks, whereas at a mean particle size
of about 2 µm the half life was 0.5 to 1 week.
b) The half life in different types of soil varied only
slightly.
c) The metabolic pathways of diflubenzuron in soil are
summarized in Figure 3.
The main metabolic pathway (over 90%) is hydrolysis, which
produces 2,6-difluorobenzoic acid (DFBA) and 4-chlorophenylurea (CPU).
A very minor pathway is hydrolysis of the other carbon-nitrogen bond,
producing 4-chloroaniline (PCA) and 2,6-difluorobenzamide, which is
rapidly converted into the corresponding acid, DFBA. DFBA is rapidly
degraded further, the half life being approximately 4 weeks. The first
step is a decarboxylation, followed by ring degradation. CPU was found
to be converted into bound residues with a half life of 5 to 10 weeks.
In the bound residue, after 2 months, both CPU and PCA are present in
roughly equal amounts. As PCA is generated directly from diflubenzuron
in only small amounts, there must be degradation of CPU into PCA. Free
PCA was not found in these studies (Nimmo and De Wilde 1975a; Verloop
and Ferrell 1977). This can be explained by the rapid binding to the
soil, found for anilines in general and PCA in particular (Hsu and
Bartha 1974; Moreale and Van Bladel 1976; Bollag et al 1978).
In a sandy loam soil, it was demonstrated that the degradation of
diflubenzuron is of a microbiological nature: in an experiment with
normal soil, after 4 weeks only 2% of the initial amount of
diflubenzuron was still present, whereas in a steam-sterilized soil,
94% of the diflubenzuron was unchanged (Nimmo and De Wilde 1975a).
Studies in the laboratory (Helling 1975; Rieck 1975; Bull and
Shaver 1980) and in the field (Danhaus et al 1976; Bull and Ivie
1978) have shown that diflubenzuron has a very low mobility in the
soil. Under field conditions, by far the major portion remains in the
top 7.5 cm of soil.
 In model experiments, in which plants were grown on nutrient
solutions, it was shown that the soil metabolite 4-chlorphenylurea was
rapidly taken up and transported by tomatoes and broad beans, with
little biotransformation (Van den Berg 1978a).
In similar experiments, it was found that the other main soil
metabolite, 2,6-difluorobenzoic acid, is rapidly decarboxylated under
the influence of tomato roots, with very little uptake and
transportation (Van den Berg 1978b).
Under laboratory conditions, seedlings of various crops,
transplanted into soil treated with radioactive diflubenzuron, took up
only small amounts of radioactivity (Nimmo and De Wilde 1976a,
Mansager et al. 1979).
Also, when diflubenzuron was applied to soil in which the
seedlings were already present, the uptake by wheat and rice was
low, the residue in the leaves amounting to up to 0.5 mg/kg of
diflubenzuron equivalents. This residue consisted mainly of
4-chlorophenylurea and of polar conjugates. Very low levels of
residues were found in the wheat grain (Nimmo and De Wilde 1976b).
The rotational crop uptake of 14C residues following soil
application of diflubenzuron was studied under field conditions. The
experimental plots received two dosages of 66 g a.i./ha with an
interval of 15 days. Wheat, onion and cabbage were planted 2 months
after the last application. Samples of foliage were collected 2.5
months after planting. Radioactivity in plant tissue was below the
level of 0.01 mg/kg of diflubenzuron equivalents (Danhaus and Sieck
1976).
In another field study, 14C-diflubenzuron was applied to cotton
during the 1976 cotton season. One plot received 6 applications
(6A-plot) of 70 g a.i./ha, a second plot received 10 applications
(10A-plot) of this dosage. Radioactive cotton plant residues were
incorporated into the soil in November 1976. During the spring
of 1977, rotational crops were planted in this soil. Levels of
radioactive residues in these crops were generally very low. The 10A
plot gave somewhat higher residues than the 6A plot. The radioactivity
in all cases was below the level that would correspond to 0.05 mg/kg
of diflubenzuron (Bull and Ivie 1978).
A special case of uptake of diflubenzuron and its metabolites is
found in growing mushrooms in a diflubenzuron-treated medium. The rate
of degradation of diflubenzuron in this medium is much slower than in
soil. However, the metabolic pathway is the same one and the main
metabolites are 4-chlorophenylurea (CPU) and 2,6-difluorobenzoic acid
(DFBA). Both metabolites are taken up by the mushrooms. From a medium
treated with 2 g/m2 of radioactive diflubenzuron, the residues in
mushrooms reached levels of 0.1 to 0.6 mg/kg of CPU and 1 to 3 mg/kg
of DFBA (Nimmo and De Wilde 1977a).
Degradation in water
The degradation of diflubenzuron was studied at 20°C in sterile
water at various pH levels. There appears to be little degradation
under neutral or acidic conditions. However, at higher pH levels
there is a pH-dependent rate of degradation; at pH 9 the half life
is about 6 weeks and at pH 12 the half life was about 1.5 weeks.
The main degradation products were 2,6-difluorobenzoic acid and
4-chlorophenylurea (Nimmo and De Wilde 1975b).
Similar results were found with dilute solutions in distilled
water kept at 36°C. The degradation followed the same pathway; the
rate of degradation was again strongly pH dependent; the half life
times were shorter than at 20°C. Under these conditions, no
p-chloroaniline could be detected as a degradation product of
diflubenzuron (Ivie et al. 1980).
Heat catalysed degradation of diflubenzuron in an aqueous medium
is far more complex, and several degradation products are found that
are not formed, or hardly formed, under moderate conditions. One of
these products arises through expulsion of HF from the diflubenzuron
molecule, with concomittant cyclization:
In model experiments, in which plants were grown on nutrient
solutions, it was shown that the soil metabolite 4-chlorphenylurea was
rapidly taken up and transported by tomatoes and broad beans, with
little biotransformation (Van den Berg 1978a).
In similar experiments, it was found that the other main soil
metabolite, 2,6-difluorobenzoic acid, is rapidly decarboxylated under
the influence of tomato roots, with very little uptake and
transportation (Van den Berg 1978b).
Under laboratory conditions, seedlings of various crops,
transplanted into soil treated with radioactive diflubenzuron, took up
only small amounts of radioactivity (Nimmo and De Wilde 1976a,
Mansager et al. 1979).
Also, when diflubenzuron was applied to soil in which the
seedlings were already present, the uptake by wheat and rice was
low, the residue in the leaves amounting to up to 0.5 mg/kg of
diflubenzuron equivalents. This residue consisted mainly of
4-chlorophenylurea and of polar conjugates. Very low levels of
residues were found in the wheat grain (Nimmo and De Wilde 1976b).
The rotational crop uptake of 14C residues following soil
application of diflubenzuron was studied under field conditions. The
experimental plots received two dosages of 66 g a.i./ha with an
interval of 15 days. Wheat, onion and cabbage were planted 2 months
after the last application. Samples of foliage were collected 2.5
months after planting. Radioactivity in plant tissue was below the
level of 0.01 mg/kg of diflubenzuron equivalents (Danhaus and Sieck
1976).
In another field study, 14C-diflubenzuron was applied to cotton
during the 1976 cotton season. One plot received 6 applications
(6A-plot) of 70 g a.i./ha, a second plot received 10 applications
(10A-plot) of this dosage. Radioactive cotton plant residues were
incorporated into the soil in November 1976. During the spring
of 1977, rotational crops were planted in this soil. Levels of
radioactive residues in these crops were generally very low. The 10A
plot gave somewhat higher residues than the 6A plot. The radioactivity
in all cases was below the level that would correspond to 0.05 mg/kg
of diflubenzuron (Bull and Ivie 1978).
A special case of uptake of diflubenzuron and its metabolites is
found in growing mushrooms in a diflubenzuron-treated medium. The rate
of degradation of diflubenzuron in this medium is much slower than in
soil. However, the metabolic pathway is the same one and the main
metabolites are 4-chlorophenylurea (CPU) and 2,6-difluorobenzoic acid
(DFBA). Both metabolites are taken up by the mushrooms. From a medium
treated with 2 g/m2 of radioactive diflubenzuron, the residues in
mushrooms reached levels of 0.1 to 0.6 mg/kg of CPU and 1 to 3 mg/kg
of DFBA (Nimmo and De Wilde 1977a).
Degradation in water
The degradation of diflubenzuron was studied at 20°C in sterile
water at various pH levels. There appears to be little degradation
under neutral or acidic conditions. However, at higher pH levels
there is a pH-dependent rate of degradation; at pH 9 the half life
is about 6 weeks and at pH 12 the half life was about 1.5 weeks.
The main degradation products were 2,6-difluorobenzoic acid and
4-chlorophenylurea (Nimmo and De Wilde 1975b).
Similar results were found with dilute solutions in distilled
water kept at 36°C. The degradation followed the same pathway; the
rate of degradation was again strongly pH dependent; the half life
times were shorter than at 20°C. Under these conditions, no
p-chloroaniline could be detected as a degradation product of
diflubenzuron (Ivie et al. 1980).
Heat catalysed degradation of diflubenzuron in an aqueous medium
is far more complex, and several degradation products are found that
are not formed, or hardly formed, under moderate conditions. One of
these products arises through expulsion of HF from the diflubenzuron
molecule, with concomittant cyclization:
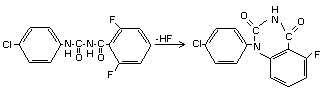 The resulting compound co-chromatographs with 4-chloroaniline in
a number of TLC solvent systems, which may lead to confusion (Ivie
et al 1980; Maas et al. 1980).
Under field conditions, diflubenzuron was demonstrated to
degrade rapidly, 4-chlorophenylurea being formed as a metabolite.
Diflubenzuron residues generally could not be detected (limit of
detection 0.002 mg/l) at 72 h after application of up to 110 g/ha of
field water (Schaefer and Dupras 1976).
When diflubenzuron was applied to field water, the compound
degraded to 4-chlorophenylurea. Small amounts of 4-chloroaniline were
apparent, but this was only a minor degradation product. Fish
initially accumulated diflubenzuron from water, but the tissue
concentration declined steadily with time. The fish tissue did contain
moderate amounts of 4-chlorophenylurea but only trace levels of
4-chloroaniline (Schaeffer et al 1980).
Photodecomposition
Crystalline diflubenzuron was radiated for 24 h on a glass sheet
at a 10 µg/cm2 level, by a mlu 300 W lamp. Decomposition was less
than 4%.
METHODS OF RESIDUE ANALYSIS
The recommended procedure for analyses of diflubenzuron residues
in a wide range of matrices, including soil, sediment, water, fish,
milk, eggs, animal tissues, agricultural crops and manure, involves an
extraction of the residue from the sample with a suitable solvent
clean-up of the extract by column chromatography, followed by
detection with high pressure liquid chromatography (HPLC) (Di Prima
et al. 1978; Buisman et al. 1977).
An alternative method for analyses of residues of diflubenzuron,
4-chlorophenylurea, and 4-chloroaniline in citrus and its process
fractions utilizes detection by gas chromatography (Cannizzaro 1978).
This method can also be applied in the analysis of residues of
diflubenzuron in soybean foliage, seed and process fractions (Di Prima
1976 a, b).
A GLC-ECD determination can also be carried out after hydrolysis
and derivatization with heptafluorobutyric anhydride (Rabenor
et al 1978).
National maximum residue levels reported to the Meeting
Recommended national MRLs and pre-harvest intervals are given in
Table 11.
TABLE 11. National maximum residue levels of diflubenzuron
Crop Country MRL Pre-harvest
(mg/kg) interval (days)
Apple, pear Argentina - 60
France 1.0 30
German Fed. Rep. 1.0 28
Italy 0.5 45
The Netherlands 1.0 28
Spain - 60
Switzerland 1.0 42
UK - 14
Yugoslavia - 30
Brassica leafy
vegetables The Netherlands 1.0 14
China (Taiwan province) - 22
German Fed. Rep. 1.0 -
Cottonseed USA 0.2 -
Mushroom The Netherlands 1.0 -
German Fed. Rep. 0.2 -
Switzerland 0.5 -
Soybean Brazil - 21
Eggs, milk, meat
and meat products USA 0.05 -
EVALUATION
COMMENTS AND APPRAISAL
The biotransformation of diflubenzuron has been evaluated in
several species. The major metabolites excreted by cow and rat result
from hydroxylation of the difluorobenzoyl moiety and the chlorophenyl
ring. In sheep and pigs, scission of the ureido bridge is the major
metabolic route: the major metabolites are 2,6-difluorobenzoic acid
and 4-chlorophenylurea. The extent of intestinal absorption of
diflubenzuron is generally low and decreases with increasing dose
levels. There is no indication of bioaccumulation in body tissues.
Diflubenzuron has a low acute toxicity. Short-term and long-term
studies in mice, rats, rabbits, cats, dogs and sheep show, in most
studies, dose-related increases in met-and sulph-haemoglobin. At high
dose levels haematocrit, haemoglobin and erythrocyte counts were
decreased, whereas reticulocyte and Heinz body counts were increased.
The effects, indicative of increased erythrocyte destruction, were
accompanied by increases in liver and spleen weight, concomitant with
haemosiderosis. It appears probably that met-haemoglobin formation is
the result of N-oxidation of 4-chloroaniline.
Several studies on chickens have been performed to investigate
the effects of diflubenzuron on sexual development and on testosterone
levels. Studies on sexual development failed to show any indications
of adverse effects, except in one case, where marginal decreases in
oestradiol and reduced comb and wattle development were observed.
Similar studies conducted in rats and bulls showed no significant
effects.
No adverse effects were noted in two reproduction studies in the
rat, in rabbit, rat and mouse teratogenicity and mouse and rat
carcinogenicity studies.
Mutagenicity studies were negative, as were in vivo and
in vitro mutagenicity studies on the metabolites, except in the case
of a cell transformation study, which was weakly positive for both 4-
chlorophenylurea and 2,6-difluorobenzoic acid.
Based on the most sensitive toxicological parameter, met-
haemoglobin formation, the no-effect level in rats was 40 ppm in the
diet (equivalent to 2 mg/kg bw/day) and in dogs 40 ppm in the diet
(equivalent to 1 mg/kg bw/day). In the long-term mouse study, this
parameter was not measured. The duration of the dog study was
unacceptable for use in ADI estimations. However, this species appears
to be the most susceptible with respect to met-haemoglobin production.
To allow for this, a higher safety factor was applied to the no-effect
level in the rat study.
Diflubenzuron is a recently-introduced insecticide, which is
registered for use in many countries as a foliar spray on pome fruits,
brassica leafy vegetables, cotton, soybean, tomato and citrus.
The rate of application ranges from 35 to 200 g/ha or as sprays
of concentrations of 0.01 to 0.04%. It is also used for the control of
flies in breeding sites, such as manure heaps and in mushroom growing,
and for control of insects on ornamental plants and in forests.
Diflubenzuron interferes in the deposition of chitin in the
insect cuticule through an influence on the enzyme chitin synthetase.
Because of its mode of action, diflubenzuron destroys insects slowly
and it is therefore necessary to treat crops well before harvest.
Where repeated applications are necessary, the intervals between
treatments are generally of the order of 28 days.
Extensive data of supervised trials from many countries were
available to the Meeting. Apples were treated at recommended rates up
to 0.02%; residues are well below 1 mg/kg at two weeks after the last
application. Pears follow the same pattern as apples. Citrus (whole
fruit) is at a level below 0.5 mg/kg after one week following the last
application of the recommended dosage rate. Examination of peel and
pulp separately showed that residues were exclusively found in the
peel. Residues in the pulp were all below the limit of determination
(0.05 mg/kg).
Residues in soybean seed and cottonseed were generally below the
limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron treated soil, rather
high contents of the metabolite DFBA are taken up from the soil. At
recommended dosage rates, the parent compound, diflubenzuron, is found
at a level below 0.1 mg/kg, while the metabolite 2,6-difluorobenzoic
acid ranges at a 1 mg/kg level.
Generally it may be established that when plants are treated at
recommended dosage rates, residues are below 2 mg/kg immediately after
application and that the rate of decline in residue concentration is
relatively slow over a period of about 56 days.
Degradation of diflubenzuron in animals and soil results
in the following metabolites: 2,6-difluorobenzoic acid (DFBA),
4-chlorophenylurea (CPU) and 4-chloroaniline (PCA). In soil the rate
of degradation is strongly dependent on the particle size of
diflubenzuron. The main metabolic pathway is by hydrolysis (>90%),
leading to DFBA and CPU. The compound does not penetrate into plant
tissue and residues are only present on those parts directly exposed
during the application. After application in plants, diflubenzuron is
not metabolized to any practical extent.
Diflubenzuron is not degraded in the digestive tract of mammals
to any significant degree, but when absorbed, it is metabolized before
excretion. After a high level treatment of cows, 0.02 mg/kg of the
parent compound were found in milk. Good agricultural practice would
not lead to residues higher than the limit of determination
(<0.02 mg/kg). Slightly higher residues, but still at the limit of
determination, were found in poultry meat and eggs.
Analytical methods for the parent compound and metabolites are
available. Depending on the nature of the substrate, diflubenzuron is
extracted with acetonitrile, ethylacetate or hexane. Clean-up involves
liquid-liquid partition and column chromatography, followed by HPLC or
GLCECD after an additional derivatization with heptafluorobutyric
anhydride. DFBA is determined after extraction and esterification
(diazomethane) by GLC-MS. The limit of determination is 0.05 mg/kg in
both cases.
Level causing no toxicological effect
Rat : 40 ppm in the diet, equivalent to 2 mg/kg bw/day
Estimation of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
Maximum residue levels were estimated for several crops. Since a
temporary ADI was estimated, the Meeting recommended these levels as
suitable for establishing temporary MRLs. They refer to diflubenzuron
alone; metabolites are not included.
Crop MRL (mg/kg)
Apple 1
Brussels sprouts 1
Cabbage 1
Citrus fruit 1
Cottonseed 0.2
Mushroom 0.2
Pear 1
Plum 1
Soybean 0.1
Carcass meat 0.05 *
Eggs 0.05 *
Milk 0.05 *
Meat byproducts 0.05 *
Poultry meat 0.05 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A dog study of adequate duration.
2. Results of an ongoing carcinogenicity study.
Desirable
1. Observations in humans, particularly with regard to met-
haemoglobin formation.
2. Information about the possible occurrence of metabolite DFBA
content in milk, meat and eggs, as the existing residue data
apply to the parent compound only.
REFERENCES
Arnold, D.A. Mutagenic study with TH 6040 in albino mice. Industrial
1974 Bio-test Laboratories Report no. 622-05068. (Unpublished)
Batham, P. and Offer, J.M. Tumorigenicity of DU 112307 to mice:
1977 dietary administration for 80 weeks. Revaluated pathological
data. Addendum to, Huntingdon Research Centre Report no
PDR170/75685. (Unpublished)
Bentley, J.P., Weber, G.H. and Gould, D. The effect of diflubenzuron
1979 feeding on glycosaminoglycan and sulph-haemoglobin
biosynthesis in mice. Pesticide Biochemistry and Physiology,
10:162.
Berczy, Z.S. et al. Acute inhalation toxicity to the rat of DU 112307
1973 technical grade powder. Huntingdon Research Centre Report
no. PDR74/73849. (Unpublished)
1975 Acute inhalation toxicity to the rabbit of DU 112307
technical grade powder. Huntingdon Research Centre Report
no. PDR198/74988. (Unpublished)
Bollag, J.M., Blattman, P. and Laanis, T. Absorption and
1978 transformation of four substituted anilines in soil. Journal
of Agricultural and Food Chemistry, 26:1302.
Booth, G.M. et al. The effect of diflubenzuron on rat serum
1980 testosterone, weight and food consumption. Brigham Young
University, Provo, Utah, USA, 5 Feb. (Unpublished)
Booth, G.M. Biochemical effects of diflubenzuron on mouse embryos.
1977 Brigham Young University, Provo, Utah, USA. (Unpublished)
Braddock. Taste test for "Dimilin" Ruby Red grapefruit juice.
1976a University of Florida, Gainsville, USA, 18 October.
(Unpublished)
Braddock. Taste test for "Dimilin" Hamlet orange juice. University of
1976b Florida, Gainesville, USA, 23 November. (Unpublished)
Braddock. Taste test for "Dimilin" Temple orange juice. University of
1977 Florida, Gainesville, USA, 2 February. (Unpublished)
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of diflubenzuron
1977a technical, batch FL44/60521. Litton Bionetics Project no.
2683. (Unpublished)
1977b Evaluation of diflubenzuron. in vitro malignant
transformation in BALB/3T3 cells. Litton Bionetics Project
no. 2688. (Unpublished)
1977c Evaluation of diflubenzuron: unscheduled DNA synthesis in
WI-38 cells. Litton Bionetics Project no. 2688.
(Unpublished)
Bryant, H. Activity of TH 6040 in the Ames Salmonella typhimurium
1976 mutagenesis assay. University of Kentucky, Lexington, USA.
(Unpublished)
Buisman, P. et al. Residue anlysemethod van diflubenzuron in gewas,
1977 grond en water. Duphar Rept. no. 56630/70/77 (in Dutch,
English summary). (Unpublished)
Bull, D.L. Fate of diflubenzuron after application to cotton and the
1980 boll weevil, South-western Entomology Supplement, nos 1
and 2.
Bull, D.L. and Ivie, G.W. Fate of diflubenzuron in cotton, soil and
1978 rotational crops. Journal of Agricultural and Food
Chemistry, 26:515.
1980 Activity and fate of diflubenzuron and certain derivatives
in the boll weevil. Pesticide Biochemistry and Physiology,
13:41.
Bull, D.L. and Shaver, T.N. Fate of potassium-3,4-dichloro-5
1980 isothiazole-carboxylate in soil. Journal of Agricultural and
Food Chemistry, 28:982.
Burdock, G.A. et al. Ninety-day subchronic toxicity study in mice:
1980a diflubenzuron technical. Hazleton Project no. 533-120.
(Unpublished)
1980b Subchronic dietary toxicity study in rats, diflubenzuron.
Hazleton Project no. 533-119. (Unpublished)
Cannizzaro, R.D. Determination of diflubenzuron, 4-chlorophenylurea,
1978 and 4-chloroaniline in citrus and process fractions by gas
chromatography. Analytical Method no.22A, Thompson-Hayward
Chemical Co. (Unpublished)
Chang, S.C. Conjugation: the major metabolic pathway of 14C
1978 diflubenzuron in the house-fly. Journal of Economic
Entomology, 71:31.
Chang, S.C. and Stokes, J.B. Conjugation: the major metabolic pathway
1979 of 14C-diflubenzuron in the boll weevil. Journal of
Economic Entomology, 72:15.
Chesterman, H. et al. DU 112307, toxicity in repeated dietary
1974 administration to beagle dogs. Huntingdon Research Centre
Report no. PDR169/74157. (Unpublished)
Colley, J. and Offer, J.M. Effects of DU 112307 in dietary
1977 administration to rats for 104 weeks, revaluated
pathological data. Huntingdon Research Centre, addendum to
HRC Report no. PDR171/75995. (Unpublished)
Colley, J.C. et al. The effects of dietary administration of
1981 diflubenzuron to male and female HC/CFLP mice for 13 weeks,
vol. I and II. Huntingdon Research Centre Report no.
PDR/294/80185. (Unpublished)
Crookshank, H.R. et al. Effect of diflubenzuron (Dimilin: TH6040) on
1978 the hyaluronic acid concentration in chicken comb. Poultry
Science, 57:804.
Davies, R.F. and Halliday, J.C. Acute percutaneous toxicity to rabbits
1974 of DU 112307 (technical). Huntingdon Research Centre Report
no.2171/D175/73. (Unpublished)
Danhaus, R.G. and Sieck, R.F. Rotation crop uptake of 14C residues
1976 following soil application of Dimilin. ADC project no. 222.
(Unpublished)
Danhaus, R.G. et al. 14C-Dimilin field soil leaching study. ADC project
1976 no. 205. (Unpublished)
De Bree, H. et al. Diflubenzuron: analysis of metabolites connected
1977 with met-haemoglobinemia. Duphar Report no. 56654/8/77.
(Unpublished)
De Lange, N. et al. PH 60-40: Excretion of radioactivity and
1974 metabolite patterns in rats following oral administration.
Duphar Report no. 56654/20/74. (Unpublished)
1975 Diflubenzuron (PH 60-40): Balance studies in the rat, and
identification of urinary metabolites. Duphar Report no.
56654/22/75. (Unpublished)
1977 Diflubenzuron: Intestinal absorption in the rat in relation
to dosage level. Duphar Report no. 56654/10/77.
(Unpublished)
De Lange, N. Diflubenzuron: Dermal absorption in the rabbit. Duphar
1979 Report no. 56654/9/79. (Unpublished)
De Lange, N. and Post, L.C. Diflubenzuron: intestinal absorption in
1978 the mouse in relation to dosage level. Duphar Report no.
56654/4/78. (Unpublished)
Deul, D.H. and De Jong, B.J. The possible influence of DU 112307 on
1977 the in vivo synthesis of hyaluronic acid in chicken skin.
Duphar Report no. 56635/1/77. (Unpublished)
Di Prima, S.J. Determination of diflubenzuron residues in soybean
1976a foliage and seed by gas chromatography. Analytical Method
no. 10, Thompson-Hayward Chemical Co. (Unpublished)
1976b Determination of diflubenzuron residues in soybean process
fractions by gas chromatography. Method no. 13, Thompson-
Hayward Chemical Company. (Unpublished)
1978 Analysis of diflubenzuron residues in environmental samples
by high-pressure liquid chromatography. Journal of
Agricultural and Food Chemistry, 26:968.
Dorough, H.W. Screening of selected Thompson Hayward chemicals for
1977 activity in the Ames Salmonella mutagenic test. University
of Kentucky, Lexington, USA. (Unpublished)
Duphar. Reports on residues of diflubenzuron. In apples, reports nos.
1975-81 30/39/75, 30/6/A/75, 30/22A/76, 30/44/76, 30/45/76,
30/53/76, 30/10/77, 30/11/77, 30/13/77, 30/14/77, 30/84/77,
30/22/78, 30/38/79, 30/76/80, In pears, reports nos.
30/91/76,30/84/77, 30/96/77, 30/97/77. In brassica leafy
vegetables reports nos. 30/48/76,30/91/77, 30/73/78. In
soybeans, reports nos. 83/06/81, SR35. In other crops,
reports nos. 30/72/77, 30/102/77, 30/129/77, 30/130/77,
30/40/78, 30/9/79, 30/43/80,83/05/81, 83/07/81, K/R/003/77,
K/R/004/77, K3R/005/77, K/R/006/77, K/R/007/77.(Unpublished)
Goodman, D.G. Histopathologic evaluation of mice administered
1980a diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
1980b Histopathologic evaluation of rats administered
diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
Gustafson, D.E. and Wargo, J.P. Fate of Dimilin following application
1976 to soybeans. Analytical Development Corporation Project 222.
(Unpublished)
Hawkins, D.R. Excretion of radioactivity after oral administration of
1980 3H/14C-diflubenzuron to cats. Huntingdon Research Centre
Report no. PDR/302/80443. (Unpublished)
Helling, C.S. Soil mobility of three Thompson-Hayward pesticides.
1975 ARS/USDA Beltsville, USA. (Unpublished)
Hsu, T.S. and Bartha, R. Interaction of pesticide-derived
1974 chloroaniline residues with soil organic matter. Soil
Science, 16:444.
Hunter, B. et al. DU 112307, preliminary assessment of the toxicity to
1974 male mice in dietary administration for 6 weeks. Huntingdon
Research Centre Report no.PDR/174/74199. (Unpublished)
Hunter, B. et al. Tumorigenicity of DU 112307 to mice, dietary
1975 administration for 80 weeks. Huntingdon Research Centre
Report no. PDR/170/75685. (Unpublished)
1976 Effects of DU 112307 in dietary administration to rats for
104 weeks. Huntingdon Research Centre Report no.
PDR/171/75945. (Unpublished)
1979 DU 112307, toxicity to rats in dietary administration for
nine weeks followed by a four-week withdrawal period.
Huntingdon Research Centre Report no. PDR/248/77883
(Unpublished)
Ivie, G.W. Fate of diflubenzuron in cattle and sheep. Journal of
1978 Agricultural and Food Chemistry, 26:81.
Ivie, G.W. and Wright, J.E. Fate of diflubenzuron in the stable fly
1978 and house fly. Journal of Agricultural and Food Chemistry,
26:90.
Ivie, G.W. Fate of diflubenzuron in water. Journal of Agricultural and
1980 Food Chemistry, 28: 330.
Jagannath, D.R. and Brusick, D.J. Mutagenicity evaluation of
1977a 4-chlorophenylurea. Litton Bionetics Project no. 20838.
(Unpublished)
1977b Mutagenic evaluation of 2,6-difluorobenzoic acid. Litton
Bionetics Project no.20838. (Unpublished)
1977c Mutagenicity evaluation of 4-chloroaniline. Litton Bionetics
Project no. 20838. (Unpublished)
Keet, C.M.J.F. Effects of DU 112307 technical after dietary
1976b administration to male Hubbard Broiler chickens for 14
weeks. Duphar Report no. 56645/25/76. (Unpublished)
1977a The met-haemoglobin, sulph-haemoglobin and Heinz body
forming properties of DU 112307 after oral administration to
male rats during 8 days. Duphar Report no. 56645/15/77.
(Unpublished)
1977b The effect of DU 112307 (technical) in male mice after daily
oral administration for a period of 14 days on body weight,
met-haemoglobin, sulph-haemoglobin and Heinz body formation
and on gross pathology. Duphar Report no. 56645/33/77.
(Unpublished)
1977c The met-haemoglobin and sulph-haemoglobin forming properties
of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Duphar Report no. 56645/2/77.
(Unpublished)
Kemp, A. et al. Dietary administration of DU 112307 to male and
1973a female rats for 3 months. Duphar Report no. 56645/13A/73.
(Unpublished)
1973b Appendix III to report no. 56645/13A/73, individual data:
dietary administration of DU 112307 to male and female rats
for 3 months. Duphar Report no. 56645/13B/73. (Unpublished)
Keuker, H. Diflubenzuron, physical and chemical properties. Duphar
1975 Report no. 56630/107/75. (Unpublished)
Koelman-Klaus, H.J.S. Acute oral toxicity study of p-chlorophenylurea
1978a in male and female rats. Duphar Report no. 56645/9/78.
(Unpublished)
1978b Acute oral toxicity study of 2,6-difluorobenzoic acid in
male and female rats. Duphar Report no. 56645/25/78.
(Unpublished)
Koopman, T.S.M. Acute oral toxicity study with DU 112307 (technical)
1977a in mice. Duphar Report no. 56645/4/77. (Unpublished)
1977b Acute intra-peritoneal toxicity study with DU 112307
(technical) in mice. Duphar Report no. 56645/5/77.
(Unpublished)
1977c Acute dermal toxicity study with DU 112307 (technical) in
rats. Duphar Report no. 56645/7/77. (Unpublished)
Maas, W. et al. Benzoylphenylurea insecticides. In:R. Wegler (Ed.),
1980 Chemie der Pflanezen-schutz-und Schädlingsbekämpfungs-
mittel, Band 6. Springer Verlag, Heidelberg, 1980.
Mansager, E.R. et al. Fate of 14C-diflubenzuron on cotton and in soil.
1979 Pesticide Biochemistry and Physiology, 12: 172.
Matheson, D.W. and Brusick, D.J. Evaluation of 4-chlorophenylurea: In
1978a vitro malignant transformation in BALB/3T3/cells. Litton
Bionetics Project no. 20840. (Unpublished)
1978b Evaluation of 2,6-difluorobenzoic acid: in vitro malignant
transformation in BALB/3T3/cells. Litton Bionetics Project
no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 2,6-difluorobenzoic acid in the
unscheduled DNA synthesis in human WI-38 cell assay. Litton
Bionetics Project no. 20840. (Unpublished)
Matheson, D.W. et al. Mutagenicity evaluation of 4-chlorophenylurea in
1978a the unscheduled DNA synthesis in human WI-38 cells assay.
Litton Bionetics Project no. 20840. (Unpublished)
1978b Mutagenic evaluation of 4-chloroaniline in the in vitro
transformation of BALB/3T3 cells assay. Litton Bionetics
Project no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 4-chloroaniline in the
unscheduled DNA synthesis in human WI-38 cells assay. Litton
Bionetics Project no. 20840. (Unpublished)
McGregor, J.T. et al. Mutagenicity tests of diflubenzuron in the
1979 micronucleus test in mice, the L5178Y mouse lymphoma forward
mutation assay, and the Ames Salmonella Reserve mutation
test. Mutation Research, 66:45.
Miller, R.W. et al. Feeding TH-6040 to cattle: Residues in tissues and
1976a milk and breakdown in manure. Journal of Agricultural and
Food Chemistry, 24:687.
1976b Effects of feeding TF-6040 to two breeds of chickens.
Journal of Economic Entomology, 69:741.
Miller, R.W., Cecil, H.C., Carey, A.M., Corley, C. and Kiddy, C.A.
1979 Effects of feeding diflubenzuron to young male Holstein
cattle. Bulletin of Environmental Contamination and
Toxicology, 23:482.
Moreale, A. and Van Bladel, R. Influence of soil properties on
1976 absorption of pesticide-derived aniline and p-chloroaniline.
Journal of Soil Science, 27:48.
Nimmo, W.B. and De Wilde, P.C. Fate of diflubenzuron on leaves of
1974 corn, soybean, cabbage and apples. Duphar Report no.
56635/16A/74. (Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in soil
1975a and natural water. Duphar Report no. 56635/37/1975.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in sterile
1975b water at pH 5,7, 9 and 12. Duphar Report no. 56635/32/75.
(Unpublished)
Nimmo, W. B. and De Wilde, P.C. Re-uptake from diflubenzuron treated
1976a soil by soybean, maize and tomato. Duphar Report no.
56635/4/76. (Unpublished)
1976b Uptake of diflubenzuron and its metabolites from the soil by
rice wheat. Duphar Report no. 56635/8/76. (Unpublished)
Nimmo W.B. and De Wilde, P.C. Degradation of diflubenzuron in mushroom
1977a growth medium and uptake of its metabolites in the
mushrooms. Duphar Report no. 56635/21A/77. (Unpublished)
1977b Degradation of diflubenzuron in cow and chicken manure.
Duphar Report no.56635/27/77. (Unpublished)
Nimmo, W.B. et al. Fate of diflubenzuron applied to leaves and fruits
1978 of apples trees. Duphar Report no. 56635/33/78.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. The fate of 14C-diflubenzuron on pine.
1980 Duphar Report no. 56635/18/79. (Unpublished)
Offer, J.M. Tumorigenicity of DU 112307 to mice, dietary
1977 administration for 80 weeks. Addendum: histological
examination of additional tissues, Huntingdon Research
Centre Report PDR/250, addendum to PDR/170/75685.
(Unpublished)
Opdycke, J.C. Metabolism and fate of diflubenzuron in chickens and
1976 swine. M. Sc.Thesis, Graduate School University of Maryland,
College Park, USA.
Palmer, A.K. and Hill, P.A. Effect of DU 112307 on reproduction
1975a function of multiple generations in the rat. Huntingdon
Research Centre Report no. PDR/17375954. (Unpublished)
1975b Effect of DU 112307 on pregnancy of the rat. Huntingdon
Research Centre Report no. PDR/192/74978. (Unpublished)
1975c Effect of DU 112307 on pregnancy of the New Zealand white
rabbit. Huntingdon Research Centre Report no. PDR/193/74937.
(Unpublished)
Palmer, A.K. et al. Preliminary assessment of the effect of DU 112307
1977 on the rat. Huntingdon Research Centre Report no.
PDR/243/77208. (Unpublished)
1978 Effect of dietary administration of DU 112307 on
reproduction function of one generation in the rat.
Huntingdon Research Centre Report no. PDR/244/78653.
(Unpublished)
Patel, Y.M. and Santolucito, J.A. Selected toxicological studies of
1980 Dimilin in weanling male rats. EPA-600/3-80-031.
(Unpublished)
Pimprikar, G.D. Mechanisms of resistance to Dimilin in Musca
1977 domestica. Ph.D. Thesis University of California, Riverside,
USA.
Popp, F. Storage stability of Dimilin 25W, Duphar Report no.
1977 56623/6/77. (Unpublished)
Quarles, J.M., Norman, J.O. and Kubena, L.F. Absence of transformation
1980 by diflubenzuron in a host-mediated transplacental
carcinogen assay. Bulletin of Environmental Contamination
and Toxicology, 25:252.
Rabenor, B., De Wilde, P.C., De Boer, F.G., Korven, P.K., Di Prima, S.
1978 J. and Canizzaro, R.D. In: G. Zweig (Ed.), Determination of
diflubenzuron residues using GLC. Analytical Methods for
Pesticides and Plant Growth Regulators. Vol. 10. Academic
Press, New York, p. 57-72.
Rieck, C.E. Soil column leaching study with Dimilin. University of
1975 Kentucky, Lexington, USA. (Unpublished)
Reinert, H.K. and Cannon, G.E. The evaluation of TH 6040 in four avian
1976 species. Cannon Laboratories Report no. 5E-8680A-D.
(Unpublished)
Ross, D.B. et al. DU 112307, 13-week oral toxicity study in sheep.
1977a Huntingdon Research Centre Report no. PDR/299/77226.
(Unpublished)
1977b Blood red cell and plasma cholinesterase value following
six-week inclusion of DU 112307 in the diet of sheep.
Huntingdon Research Centre Report no. PDR/229A/77225.
(Unpublished)
1977c The effects of DU 112307 on female chickens following
dietary inclusion for 98 days. Huntingdon Research Centre
Report no. PDR/240/77183. (Unpublished)
Ross, D.B. et al. The effects of DU 112307 on female chickens
1979 following dietary inclusion for 98 days, (second study).
Huntingdon Research Centre Report No. PDR/251/781030.
(Unpublished)
Schaefer, c. and Dupras, E.F. Factors affecting the stability in water
1976 and the persistence of Dimilin in field waters. Journal of
Agricultural and Food Chemistry, 24:733.
Schaefer, C. et al. The occurrence of p-chloroaniline and p-
1980 chlorophenylurea from degradation of diflubenzuron in water
and fish. Proc. 48th Annual Conference of California
Mosquito & Vector Control Association, Anaheim, USA.
Schwartz, S.L. and Borzelleca, J.F. Effects of diflubenzuron on met-
1981 haemoglobin, sulph-haemoglobin and Heinz body production in
cats. Georgetown University, Washington, D.C., USA
(Unpublished)
Seegmiller, R.E. and Booth, G.M. Teratogenic and biochemical effects
1976 of TH 6040 and chick embryos. Brigham Young University,
Provo, Utah, USA. (Unpublished)
Seuferer, S.L., Braymer, H.D. and Dunn, J.J. Metabolism of
1979 diflubenzuron by soil organisms and mutagenicity of the
metabolites. Pesticide Biochemistry and Physiology, 10: 174.
Smalley, H.E. Comparative toxicology of some insect growth regulators.
1976 Clinical Toxicology, 9:27.
Smith, K.S. and Merricks, D.L. TH 6040:Tissue residue and metabolism
1976a study in dairy cows. Cannon Labs, lab.no. 5E-7372 (March
29). (Unpublished)
1976b TH 6040: tissue residue and metabolism study in poultry.
Cannon Labs, lab.no. 5E-7372 (March 12). (Unpublished)
Spencer-Jones, D.H. Letter from Duphar Midox to Duphar, 25 May.
1979
Stoolmiller, A.C. Toxicological study on the effect of diflubenzuron
1978 on rat C-6 astrocytoma cells In vitro. Gen. Pharmac:, 9:11.
Van den Berg, G. Uptake, translocation and metabolism of
1978a 4-chlorophenylurea in tomato and broad bean. Duphar Report
no. 56635/5/67. (Unpublished)
1978b Uptake and decarboxylation of 2,6-difluorobenzoid acid in
tomato. Duphar Report no. 56635/4/78. (Unpublished)
Van Eldik, A. Acute toxicity studies with DU 112307 in mice and rats.
1973 Duphar Report no. 56645/14/73. (Unpublished)
1974 Acute toxicity studies with DU 112307 (technical) in mice
after intra-peritoneal administration. Duphar Report no.
56645/1/74. (Unpublished)
Verlopp, A. and Ferrell, C.D. Benzoylphenylureas, a new group of
1977 larvicides interfering with chitin deposition. American
Chemical Society Symposium.
Willems, A.C.M. et al. Diflubenzuron: Intestinal absorption and
1980 metabolism in the rat. Xenobiotica, 10:103.
The resulting compound co-chromatographs with 4-chloroaniline in
a number of TLC solvent systems, which may lead to confusion (Ivie
et al 1980; Maas et al. 1980).
Under field conditions, diflubenzuron was demonstrated to
degrade rapidly, 4-chlorophenylurea being formed as a metabolite.
Diflubenzuron residues generally could not be detected (limit of
detection 0.002 mg/l) at 72 h after application of up to 110 g/ha of
field water (Schaefer and Dupras 1976).
When diflubenzuron was applied to field water, the compound
degraded to 4-chlorophenylurea. Small amounts of 4-chloroaniline were
apparent, but this was only a minor degradation product. Fish
initially accumulated diflubenzuron from water, but the tissue
concentration declined steadily with time. The fish tissue did contain
moderate amounts of 4-chlorophenylurea but only trace levels of
4-chloroaniline (Schaeffer et al 1980).
Photodecomposition
Crystalline diflubenzuron was radiated for 24 h on a glass sheet
at a 10 µg/cm2 level, by a mlu 300 W lamp. Decomposition was less
than 4%.
METHODS OF RESIDUE ANALYSIS
The recommended procedure for analyses of diflubenzuron residues
in a wide range of matrices, including soil, sediment, water, fish,
milk, eggs, animal tissues, agricultural crops and manure, involves an
extraction of the residue from the sample with a suitable solvent
clean-up of the extract by column chromatography, followed by
detection with high pressure liquid chromatography (HPLC) (Di Prima
et al. 1978; Buisman et al. 1977).
An alternative method for analyses of residues of diflubenzuron,
4-chlorophenylurea, and 4-chloroaniline in citrus and its process
fractions utilizes detection by gas chromatography (Cannizzaro 1978).
This method can also be applied in the analysis of residues of
diflubenzuron in soybean foliage, seed and process fractions (Di Prima
1976 a, b).
A GLC-ECD determination can also be carried out after hydrolysis
and derivatization with heptafluorobutyric anhydride (Rabenor
et al 1978).
National maximum residue levels reported to the Meeting
Recommended national MRLs and pre-harvest intervals are given in
Table 11.
TABLE 11. National maximum residue levels of diflubenzuron
Crop Country MRL Pre-harvest
(mg/kg) interval (days)
Apple, pear Argentina - 60
France 1.0 30
German Fed. Rep. 1.0 28
Italy 0.5 45
The Netherlands 1.0 28
Spain - 60
Switzerland 1.0 42
UK - 14
Yugoslavia - 30
Brassica leafy
vegetables The Netherlands 1.0 14
China (Taiwan province) - 22
German Fed. Rep. 1.0 -
Cottonseed USA 0.2 -
Mushroom The Netherlands 1.0 -
German Fed. Rep. 0.2 -
Switzerland 0.5 -
Soybean Brazil - 21
Eggs, milk, meat
and meat products USA 0.05 -
EVALUATION
COMMENTS AND APPRAISAL
The biotransformation of diflubenzuron has been evaluated in
several species. The major metabolites excreted by cow and rat result
from hydroxylation of the difluorobenzoyl moiety and the chlorophenyl
ring. In sheep and pigs, scission of the ureido bridge is the major
metabolic route: the major metabolites are 2,6-difluorobenzoic acid
and 4-chlorophenylurea. The extent of intestinal absorption of
diflubenzuron is generally low and decreases with increasing dose
levels. There is no indication of bioaccumulation in body tissues.
Diflubenzuron has a low acute toxicity. Short-term and long-term
studies in mice, rats, rabbits, cats, dogs and sheep show, in most
studies, dose-related increases in met-and sulph-haemoglobin. At high
dose levels haematocrit, haemoglobin and erythrocyte counts were
decreased, whereas reticulocyte and Heinz body counts were increased.
The effects, indicative of increased erythrocyte destruction, were
accompanied by increases in liver and spleen weight, concomitant with
haemosiderosis. It appears probably that met-haemoglobin formation is
the result of N-oxidation of 4-chloroaniline.
Several studies on chickens have been performed to investigate
the effects of diflubenzuron on sexual development and on testosterone
levels. Studies on sexual development failed to show any indications
of adverse effects, except in one case, where marginal decreases in
oestradiol and reduced comb and wattle development were observed.
Similar studies conducted in rats and bulls showed no significant
effects.
No adverse effects were noted in two reproduction studies in the
rat, in rabbit, rat and mouse teratogenicity and mouse and rat
carcinogenicity studies.
Mutagenicity studies were negative, as were in vivo and
in vitro mutagenicity studies on the metabolites, except in the case
of a cell transformation study, which was weakly positive for both 4-
chlorophenylurea and 2,6-difluorobenzoic acid.
Based on the most sensitive toxicological parameter, met-
haemoglobin formation, the no-effect level in rats was 40 ppm in the
diet (equivalent to 2 mg/kg bw/day) and in dogs 40 ppm in the diet
(equivalent to 1 mg/kg bw/day). In the long-term mouse study, this
parameter was not measured. The duration of the dog study was
unacceptable for use in ADI estimations. However, this species appears
to be the most susceptible with respect to met-haemoglobin production.
To allow for this, a higher safety factor was applied to the no-effect
level in the rat study.
Diflubenzuron is a recently-introduced insecticide, which is
registered for use in many countries as a foliar spray on pome fruits,
brassica leafy vegetables, cotton, soybean, tomato and citrus.
The rate of application ranges from 35 to 200 g/ha or as sprays
of concentrations of 0.01 to 0.04%. It is also used for the control of
flies in breeding sites, such as manure heaps and in mushroom growing,
and for control of insects on ornamental plants and in forests.
Diflubenzuron interferes in the deposition of chitin in the
insect cuticule through an influence on the enzyme chitin synthetase.
Because of its mode of action, diflubenzuron destroys insects slowly
and it is therefore necessary to treat crops well before harvest.
Where repeated applications are necessary, the intervals between
treatments are generally of the order of 28 days.
Extensive data of supervised trials from many countries were
available to the Meeting. Apples were treated at recommended rates up
to 0.02%; residues are well below 1 mg/kg at two weeks after the last
application. Pears follow the same pattern as apples. Citrus (whole
fruit) is at a level below 0.5 mg/kg after one week following the last
application of the recommended dosage rate. Examination of peel and
pulp separately showed that residues were exclusively found in the
peel. Residues in the pulp were all below the limit of determination
(0.05 mg/kg).
Residues in soybean seed and cottonseed were generally below the
limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron treated soil, rather
high contents of the metabolite DFBA are taken up from the soil. At
recommended dosage rates, the parent compound, diflubenzuron, is found
at a level below 0.1 mg/kg, while the metabolite 2,6-difluorobenzoic
acid ranges at a 1 mg/kg level.
Generally it may be established that when plants are treated at
recommended dosage rates, residues are below 2 mg/kg immediately after
application and that the rate of decline in residue concentration is
relatively slow over a period of about 56 days.
Degradation of diflubenzuron in animals and soil results
in the following metabolites: 2,6-difluorobenzoic acid (DFBA),
4-chlorophenylurea (CPU) and 4-chloroaniline (PCA). In soil the rate
of degradation is strongly dependent on the particle size of
diflubenzuron. The main metabolic pathway is by hydrolysis (>90%),
leading to DFBA and CPU. The compound does not penetrate into plant
tissue and residues are only present on those parts directly exposed
during the application. After application in plants, diflubenzuron is
not metabolized to any practical extent.
Diflubenzuron is not degraded in the digestive tract of mammals
to any significant degree, but when absorbed, it is metabolized before
excretion. After a high level treatment of cows, 0.02 mg/kg of the
parent compound were found in milk. Good agricultural practice would
not lead to residues higher than the limit of determination
(<0.02 mg/kg). Slightly higher residues, but still at the limit of
determination, were found in poultry meat and eggs.
Analytical methods for the parent compound and metabolites are
available. Depending on the nature of the substrate, diflubenzuron is
extracted with acetonitrile, ethylacetate or hexane. Clean-up involves
liquid-liquid partition and column chromatography, followed by HPLC or
GLCECD after an additional derivatization with heptafluorobutyric
anhydride. DFBA is determined after extraction and esterification
(diazomethane) by GLC-MS. The limit of determination is 0.05 mg/kg in
both cases.
Level causing no toxicological effect
Rat : 40 ppm in the diet, equivalent to 2 mg/kg bw/day
Estimation of temporary acceptable daily intake for man
0 - 0.004 mg/kg bw
RECOMMENDATIONS OF RESIDUE LIMITS
Maximum residue levels were estimated for several crops. Since a
temporary ADI was estimated, the Meeting recommended these levels as
suitable for establishing temporary MRLs. They refer to diflubenzuron
alone; metabolites are not included.
Crop MRL (mg/kg)
Apple 1
Brussels sprouts 1
Cabbage 1
Citrus fruit 1
Cottonseed 0.2
Mushroom 0.2
Pear 1
Plum 1
Soybean 0.1
Carcass meat 0.05 *
Eggs 0.05 *
Milk 0.05 *
Meat byproducts 0.05 *
Poultry meat 0.05 *
* Level at or about the limit of determination.
FURTHER WORK OR INFORMATION
Required (by 1984)
1. A dog study of adequate duration.
2. Results of an ongoing carcinogenicity study.
Desirable
1. Observations in humans, particularly with regard to met-
haemoglobin formation.
2. Information about the possible occurrence of metabolite DFBA
content in milk, meat and eggs, as the existing residue data
apply to the parent compound only.
REFERENCES
Arnold, D.A. Mutagenic study with TH 6040 in albino mice. Industrial
1974 Bio-test Laboratories Report no. 622-05068. (Unpublished)
Batham, P. and Offer, J.M. Tumorigenicity of DU 112307 to mice:
1977 dietary administration for 80 weeks. Revaluated pathological
data. Addendum to, Huntingdon Research Centre Report no
PDR170/75685. (Unpublished)
Bentley, J.P., Weber, G.H. and Gould, D. The effect of diflubenzuron
1979 feeding on glycosaminoglycan and sulph-haemoglobin
biosynthesis in mice. Pesticide Biochemistry and Physiology,
10:162.
Berczy, Z.S. et al. Acute inhalation toxicity to the rat of DU 112307
1973 technical grade powder. Huntingdon Research Centre Report
no. PDR74/73849. (Unpublished)
1975 Acute inhalation toxicity to the rabbit of DU 112307
technical grade powder. Huntingdon Research Centre Report
no. PDR198/74988. (Unpublished)
Bollag, J.M., Blattman, P. and Laanis, T. Absorption and
1978 transformation of four substituted anilines in soil. Journal
of Agricultural and Food Chemistry, 26:1302.
Booth, G.M. et al. The effect of diflubenzuron on rat serum
1980 testosterone, weight and food consumption. Brigham Young
University, Provo, Utah, USA, 5 Feb. (Unpublished)
Booth, G.M. Biochemical effects of diflubenzuron on mouse embryos.
1977 Brigham Young University, Provo, Utah, USA. (Unpublished)
Braddock. Taste test for "Dimilin" Ruby Red grapefruit juice.
1976a University of Florida, Gainsville, USA, 18 October.
(Unpublished)
Braddock. Taste test for "Dimilin" Hamlet orange juice. University of
1976b Florida, Gainesville, USA, 23 November. (Unpublished)
Braddock. Taste test for "Dimilin" Temple orange juice. University of
1977 Florida, Gainesville, USA, 2 February. (Unpublished)
Brusick, D.J. and Weir, R.J. Mutagenicity evaluation of diflubenzuron
1977a technical, batch FL44/60521. Litton Bionetics Project no.
2683. (Unpublished)
1977b Evaluation of diflubenzuron. in vitro malignant
transformation in BALB/3T3 cells. Litton Bionetics Project
no. 2688. (Unpublished)
1977c Evaluation of diflubenzuron: unscheduled DNA synthesis in
WI-38 cells. Litton Bionetics Project no. 2688.
(Unpublished)
Bryant, H. Activity of TH 6040 in the Ames Salmonella typhimurium
1976 mutagenesis assay. University of Kentucky, Lexington, USA.
(Unpublished)
Buisman, P. et al. Residue anlysemethod van diflubenzuron in gewas,
1977 grond en water. Duphar Rept. no. 56630/70/77 (in Dutch,
English summary). (Unpublished)
Bull, D.L. Fate of diflubenzuron after application to cotton and the
1980 boll weevil, South-western Entomology Supplement, nos 1
and 2.
Bull, D.L. and Ivie, G.W. Fate of diflubenzuron in cotton, soil and
1978 rotational crops. Journal of Agricultural and Food
Chemistry, 26:515.
1980 Activity and fate of diflubenzuron and certain derivatives
in the boll weevil. Pesticide Biochemistry and Physiology,
13:41.
Bull, D.L. and Shaver, T.N. Fate of potassium-3,4-dichloro-5
1980 isothiazole-carboxylate in soil. Journal of Agricultural and
Food Chemistry, 28:982.
Burdock, G.A. et al. Ninety-day subchronic toxicity study in mice:
1980a diflubenzuron technical. Hazleton Project no. 533-120.
(Unpublished)
1980b Subchronic dietary toxicity study in rats, diflubenzuron.
Hazleton Project no. 533-119. (Unpublished)
Cannizzaro, R.D. Determination of diflubenzuron, 4-chlorophenylurea,
1978 and 4-chloroaniline in citrus and process fractions by gas
chromatography. Analytical Method no.22A, Thompson-Hayward
Chemical Co. (Unpublished)
Chang, S.C. Conjugation: the major metabolic pathway of 14C
1978 diflubenzuron in the house-fly. Journal of Economic
Entomology, 71:31.
Chang, S.C. and Stokes, J.B. Conjugation: the major metabolic pathway
1979 of 14C-diflubenzuron in the boll weevil. Journal of
Economic Entomology, 72:15.
Chesterman, H. et al. DU 112307, toxicity in repeated dietary
1974 administration to beagle dogs. Huntingdon Research Centre
Report no. PDR169/74157. (Unpublished)
Colley, J. and Offer, J.M. Effects of DU 112307 in dietary
1977 administration to rats for 104 weeks, revaluated
pathological data. Huntingdon Research Centre, addendum to
HRC Report no. PDR171/75995. (Unpublished)
Colley, J.C. et al. The effects of dietary administration of
1981 diflubenzuron to male and female HC/CFLP mice for 13 weeks,
vol. I and II. Huntingdon Research Centre Report no.
PDR/294/80185. (Unpublished)
Crookshank, H.R. et al. Effect of diflubenzuron (Dimilin: TH6040) on
1978 the hyaluronic acid concentration in chicken comb. Poultry
Science, 57:804.
Davies, R.F. and Halliday, J.C. Acute percutaneous toxicity to rabbits
1974 of DU 112307 (technical). Huntingdon Research Centre Report
no.2171/D175/73. (Unpublished)
Danhaus, R.G. and Sieck, R.F. Rotation crop uptake of 14C residues
1976 following soil application of Dimilin. ADC project no. 222.
(Unpublished)
Danhaus, R.G. et al. 14C-Dimilin field soil leaching study. ADC project
1976 no. 205. (Unpublished)
De Bree, H. et al. Diflubenzuron: analysis of metabolites connected
1977 with met-haemoglobinemia. Duphar Report no. 56654/8/77.
(Unpublished)
De Lange, N. et al. PH 60-40: Excretion of radioactivity and
1974 metabolite patterns in rats following oral administration.
Duphar Report no. 56654/20/74. (Unpublished)
1975 Diflubenzuron (PH 60-40): Balance studies in the rat, and
identification of urinary metabolites. Duphar Report no.
56654/22/75. (Unpublished)
1977 Diflubenzuron: Intestinal absorption in the rat in relation
to dosage level. Duphar Report no. 56654/10/77.
(Unpublished)
De Lange, N. Diflubenzuron: Dermal absorption in the rabbit. Duphar
1979 Report no. 56654/9/79. (Unpublished)
De Lange, N. and Post, L.C. Diflubenzuron: intestinal absorption in
1978 the mouse in relation to dosage level. Duphar Report no.
56654/4/78. (Unpublished)
Deul, D.H. and De Jong, B.J. The possible influence of DU 112307 on
1977 the in vivo synthesis of hyaluronic acid in chicken skin.
Duphar Report no. 56635/1/77. (Unpublished)
Di Prima, S.J. Determination of diflubenzuron residues in soybean
1976a foliage and seed by gas chromatography. Analytical Method
no. 10, Thompson-Hayward Chemical Co. (Unpublished)
1976b Determination of diflubenzuron residues in soybean process
fractions by gas chromatography. Method no. 13, Thompson-
Hayward Chemical Company. (Unpublished)
1978 Analysis of diflubenzuron residues in environmental samples
by high-pressure liquid chromatography. Journal of
Agricultural and Food Chemistry, 26:968.
Dorough, H.W. Screening of selected Thompson Hayward chemicals for
1977 activity in the Ames Salmonella mutagenic test. University
of Kentucky, Lexington, USA. (Unpublished)
Duphar. Reports on residues of diflubenzuron. In apples, reports nos.
1975-81 30/39/75, 30/6/A/75, 30/22A/76, 30/44/76, 30/45/76,
30/53/76, 30/10/77, 30/11/77, 30/13/77, 30/14/77, 30/84/77,
30/22/78, 30/38/79, 30/76/80, In pears, reports nos.
30/91/76,30/84/77, 30/96/77, 30/97/77. In brassica leafy
vegetables reports nos. 30/48/76,30/91/77, 30/73/78. In
soybeans, reports nos. 83/06/81, SR35. In other crops,
reports nos. 30/72/77, 30/102/77, 30/129/77, 30/130/77,
30/40/78, 30/9/79, 30/43/80,83/05/81, 83/07/81, K/R/003/77,
K/R/004/77, K3R/005/77, K/R/006/77, K/R/007/77.(Unpublished)
Goodman, D.G. Histopathologic evaluation of mice administered
1980a diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
1980b Histopathologic evaluation of rats administered
diflubenzuron in the diet. Clement Associates. Submitted by
Duphar. (Unpublished)
Gustafson, D.E. and Wargo, J.P. Fate of Dimilin following application
1976 to soybeans. Analytical Development Corporation Project 222.
(Unpublished)
Hawkins, D.R. Excretion of radioactivity after oral administration of
1980 3H/14C-diflubenzuron to cats. Huntingdon Research Centre
Report no. PDR/302/80443. (Unpublished)
Helling, C.S. Soil mobility of three Thompson-Hayward pesticides.
1975 ARS/USDA Beltsville, USA. (Unpublished)
Hsu, T.S. and Bartha, R. Interaction of pesticide-derived
1974 chloroaniline residues with soil organic matter. Soil
Science, 16:444.
Hunter, B. et al. DU 112307, preliminary assessment of the toxicity to
1974 male mice in dietary administration for 6 weeks. Huntingdon
Research Centre Report no.PDR/174/74199. (Unpublished)
Hunter, B. et al. Tumorigenicity of DU 112307 to mice, dietary
1975 administration for 80 weeks. Huntingdon Research Centre
Report no. PDR/170/75685. (Unpublished)
1976 Effects of DU 112307 in dietary administration to rats for
104 weeks. Huntingdon Research Centre Report no.
PDR/171/75945. (Unpublished)
1979 DU 112307, toxicity to rats in dietary administration for
nine weeks followed by a four-week withdrawal period.
Huntingdon Research Centre Report no. PDR/248/77883
(Unpublished)
Ivie, G.W. Fate of diflubenzuron in cattle and sheep. Journal of
1978 Agricultural and Food Chemistry, 26:81.
Ivie, G.W. and Wright, J.E. Fate of diflubenzuron in the stable fly
1978 and house fly. Journal of Agricultural and Food Chemistry,
26:90.
Ivie, G.W. Fate of diflubenzuron in water. Journal of Agricultural and
1980 Food Chemistry, 28: 330.
Jagannath, D.R. and Brusick, D.J. Mutagenicity evaluation of
1977a 4-chlorophenylurea. Litton Bionetics Project no. 20838.
(Unpublished)
1977b Mutagenic evaluation of 2,6-difluorobenzoic acid. Litton
Bionetics Project no.20838. (Unpublished)
1977c Mutagenicity evaluation of 4-chloroaniline. Litton Bionetics
Project no. 20838. (Unpublished)
Keet, C.M.J.F. Effects of DU 112307 technical after dietary
1976b administration to male Hubbard Broiler chickens for 14
weeks. Duphar Report no. 56645/25/76. (Unpublished)
1977a The met-haemoglobin, sulph-haemoglobin and Heinz body
forming properties of DU 112307 after oral administration to
male rats during 8 days. Duphar Report no. 56645/15/77.
(Unpublished)
1977b The effect of DU 112307 (technical) in male mice after daily
oral administration for a period of 14 days on body weight,
met-haemoglobin, sulph-haemoglobin and Heinz body formation
and on gross pathology. Duphar Report no. 56645/33/77.
(Unpublished)
1977c The met-haemoglobin and sulph-haemoglobin forming properties
of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Duphar Report no. 56645/2/77.
(Unpublished)
Kemp, A. et al. Dietary administration of DU 112307 to male and
1973a female rats for 3 months. Duphar Report no. 56645/13A/73.
(Unpublished)
1973b Appendix III to report no. 56645/13A/73, individual data:
dietary administration of DU 112307 to male and female rats
for 3 months. Duphar Report no. 56645/13B/73. (Unpublished)
Keuker, H. Diflubenzuron, physical and chemical properties. Duphar
1975 Report no. 56630/107/75. (Unpublished)
Koelman-Klaus, H.J.S. Acute oral toxicity study of p-chlorophenylurea
1978a in male and female rats. Duphar Report no. 56645/9/78.
(Unpublished)
1978b Acute oral toxicity study of 2,6-difluorobenzoic acid in
male and female rats. Duphar Report no. 56645/25/78.
(Unpublished)
Koopman, T.S.M. Acute oral toxicity study with DU 112307 (technical)
1977a in mice. Duphar Report no. 56645/4/77. (Unpublished)
1977b Acute intra-peritoneal toxicity study with DU 112307
(technical) in mice. Duphar Report no. 56645/5/77.
(Unpublished)
1977c Acute dermal toxicity study with DU 112307 (technical) in
rats. Duphar Report no. 56645/7/77. (Unpublished)
Maas, W. et al. Benzoylphenylurea insecticides. In:R. Wegler (Ed.),
1980 Chemie der Pflanezen-schutz-und Schädlingsbekämpfungs-
mittel, Band 6. Springer Verlag, Heidelberg, 1980.
Mansager, E.R. et al. Fate of 14C-diflubenzuron on cotton and in soil.
1979 Pesticide Biochemistry and Physiology, 12: 172.
Matheson, D.W. and Brusick, D.J. Evaluation of 4-chlorophenylurea: In
1978a vitro malignant transformation in BALB/3T3/cells. Litton
Bionetics Project no. 20840. (Unpublished)
1978b Evaluation of 2,6-difluorobenzoic acid: in vitro malignant
transformation in BALB/3T3/cells. Litton Bionetics Project
no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 2,6-difluorobenzoic acid in the
unscheduled DNA synthesis in human WI-38 cell assay. Litton
Bionetics Project no. 20840. (Unpublished)
Matheson, D.W. et al. Mutagenicity evaluation of 4-chlorophenylurea in
1978a the unscheduled DNA synthesis in human WI-38 cells assay.
Litton Bionetics Project no. 20840. (Unpublished)
1978b Mutagenic evaluation of 4-chloroaniline in the in vitro
transformation of BALB/3T3 cells assay. Litton Bionetics
Project no. 20840. (Unpublished)
1978c Mutagenicity evaluation of 4-chloroaniline in the
unscheduled DNA synthesis in human WI-38 cells assay. Litton
Bionetics Project no. 20840. (Unpublished)
McGregor, J.T. et al. Mutagenicity tests of diflubenzuron in the
1979 micronucleus test in mice, the L5178Y mouse lymphoma forward
mutation assay, and the Ames Salmonella Reserve mutation
test. Mutation Research, 66:45.
Miller, R.W. et al. Feeding TH-6040 to cattle: Residues in tissues and
1976a milk and breakdown in manure. Journal of Agricultural and
Food Chemistry, 24:687.
1976b Effects of feeding TF-6040 to two breeds of chickens.
Journal of Economic Entomology, 69:741.
Miller, R.W., Cecil, H.C., Carey, A.M., Corley, C. and Kiddy, C.A.
1979 Effects of feeding diflubenzuron to young male Holstein
cattle. Bulletin of Environmental Contamination and
Toxicology, 23:482.
Moreale, A. and Van Bladel, R. Influence of soil properties on
1976 absorption of pesticide-derived aniline and p-chloroaniline.
Journal of Soil Science, 27:48.
Nimmo, W.B. and De Wilde, P.C. Fate of diflubenzuron on leaves of
1974 corn, soybean, cabbage and apples. Duphar Report no.
56635/16A/74. (Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in soil
1975a and natural water. Duphar Report no. 56635/37/1975.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. Degradation of diflubenzuron in sterile
1975b water at pH 5,7, 9 and 12. Duphar Report no. 56635/32/75.
(Unpublished)
Nimmo, W. B. and De Wilde, P.C. Re-uptake from diflubenzuron treated
1976a soil by soybean, maize and tomato. Duphar Report no.
56635/4/76. (Unpublished)
1976b Uptake of diflubenzuron and its metabolites from the soil by
rice wheat. Duphar Report no. 56635/8/76. (Unpublished)
Nimmo W.B. and De Wilde, P.C. Degradation of diflubenzuron in mushroom
1977a growth medium and uptake of its metabolites in the
mushrooms. Duphar Report no. 56635/21A/77. (Unpublished)
1977b Degradation of diflubenzuron in cow and chicken manure.
Duphar Report no.56635/27/77. (Unpublished)
Nimmo, W.B. et al. Fate of diflubenzuron applied to leaves and fruits
1978 of apples trees. Duphar Report no. 56635/33/78.
(Unpublished)
Nimmo, W.B. and De Wilde, P.C. The fate of 14C-diflubenzuron on pine.
1980 Duphar Report no. 56635/18/79. (Unpublished)
Offer, J.M. Tumorigenicity of DU 112307 to mice, dietary
1977 administration for 80 weeks. Addendum: histological
examination of additional tissues, Huntingdon Research
Centre Report PDR/250, addendum to PDR/170/75685.
(Unpublished)
Opdycke, J.C. Metabolism and fate of diflubenzuron in chickens and
1976 swine. M. Sc.Thesis, Graduate School University of Maryland,
College Park, USA.
Palmer, A.K. and Hill, P.A. Effect of DU 112307 on reproduction
1975a function of multiple generations in the rat. Huntingdon
Research Centre Report no. PDR/17375954. (Unpublished)
1975b Effect of DU 112307 on pregnancy of the rat. Huntingdon
Research Centre Report no. PDR/192/74978. (Unpublished)
1975c Effect of DU 112307 on pregnancy of the New Zealand white
rabbit. Huntingdon Research Centre Report no. PDR/193/74937.
(Unpublished)
Palmer, A.K. et al. Preliminary assessment of the effect of DU 112307
1977 on the rat. Huntingdon Research Centre Report no.
PDR/243/77208. (Unpublished)
1978 Effect of dietary administration of DU 112307 on
reproduction function of one generation in the rat.
Huntingdon Research Centre Report no. PDR/244/78653.
(Unpublished)
Patel, Y.M. and Santolucito, J.A. Selected toxicological studies of
1980 Dimilin in weanling male rats. EPA-600/3-80-031.
(Unpublished)
Pimprikar, G.D. Mechanisms of resistance to Dimilin in Musca
1977 domestica. Ph.D. Thesis University of California, Riverside,
USA.
Popp, F. Storage stability of Dimilin 25W, Duphar Report no.
1977 56623/6/77. (Unpublished)
Quarles, J.M., Norman, J.O. and Kubena, L.F. Absence of transformation
1980 by diflubenzuron in a host-mediated transplacental
carcinogen assay. Bulletin of Environmental Contamination
and Toxicology, 25:252.
Rabenor, B., De Wilde, P.C., De Boer, F.G., Korven, P.K., Di Prima, S.
1978 J. and Canizzaro, R.D. In: G. Zweig (Ed.), Determination of
diflubenzuron residues using GLC. Analytical Methods for
Pesticides and Plant Growth Regulators. Vol. 10. Academic
Press, New York, p. 57-72.
Rieck, C.E. Soil column leaching study with Dimilin. University of
1975 Kentucky, Lexington, USA. (Unpublished)
Reinert, H.K. and Cannon, G.E. The evaluation of TH 6040 in four avian
1976 species. Cannon Laboratories Report no. 5E-8680A-D.
(Unpublished)
Ross, D.B. et al. DU 112307, 13-week oral toxicity study in sheep.
1977a Huntingdon Research Centre Report no. PDR/299/77226.
(Unpublished)
1977b Blood red cell and plasma cholinesterase value following
six-week inclusion of DU 112307 in the diet of sheep.
Huntingdon Research Centre Report no. PDR/229A/77225.
(Unpublished)
1977c The effects of DU 112307 on female chickens following
dietary inclusion for 98 days. Huntingdon Research Centre
Report no. PDR/240/77183. (Unpublished)
Ross, D.B. et al. The effects of DU 112307 on female chickens
1979 following dietary inclusion for 98 days, (second study).
Huntingdon Research Centre Report No. PDR/251/781030.
(Unpublished)
Schaefer, c. and Dupras, E.F. Factors affecting the stability in water
1976 and the persistence of Dimilin in field waters. Journal of
Agricultural and Food Chemistry, 24:733.
Schaefer, C. et al. The occurrence of p-chloroaniline and p-
1980 chlorophenylurea from degradation of diflubenzuron in water
and fish. Proc. 48th Annual Conference of California
Mosquito & Vector Control Association, Anaheim, USA.
Schwartz, S.L. and Borzelleca, J.F. Effects of diflubenzuron on met-
1981 haemoglobin, sulph-haemoglobin and Heinz body production in
cats. Georgetown University, Washington, D.C., USA
(Unpublished)
Seegmiller, R.E. and Booth, G.M. Teratogenic and biochemical effects
1976 of TH 6040 and chick embryos. Brigham Young University,
Provo, Utah, USA. (Unpublished)
Seuferer, S.L., Braymer, H.D. and Dunn, J.J. Metabolism of
1979 diflubenzuron by soil organisms and mutagenicity of the
metabolites. Pesticide Biochemistry and Physiology, 10: 174.
Smalley, H.E. Comparative toxicology of some insect growth regulators.
1976 Clinical Toxicology, 9:27.
Smith, K.S. and Merricks, D.L. TH 6040:Tissue residue and metabolism
1976a study in dairy cows. Cannon Labs, lab.no. 5E-7372 (March
29). (Unpublished)
1976b TH 6040: tissue residue and metabolism study in poultry.
Cannon Labs, lab.no. 5E-7372 (March 12). (Unpublished)
Spencer-Jones, D.H. Letter from Duphar Midox to Duphar, 25 May.
1979
Stoolmiller, A.C. Toxicological study on the effect of diflubenzuron
1978 on rat C-6 astrocytoma cells In vitro. Gen. Pharmac:, 9:11.
Van den Berg, G. Uptake, translocation and metabolism of
1978a 4-chlorophenylurea in tomato and broad bean. Duphar Report
no. 56635/5/67. (Unpublished)
1978b Uptake and decarboxylation of 2,6-difluorobenzoid acid in
tomato. Duphar Report no. 56635/4/78. (Unpublished)
Van Eldik, A. Acute toxicity studies with DU 112307 in mice and rats.
1973 Duphar Report no. 56645/14/73. (Unpublished)
1974 Acute toxicity studies with DU 112307 (technical) in mice
after intra-peritoneal administration. Duphar Report no.
56645/1/74. (Unpublished)
Verlopp, A. and Ferrell, C.D. Benzoylphenylureas, a new group of
1977 larvicides interfering with chitin deposition. American
Chemical Society Symposium.
Willems, A.C.M. et al. Diflubenzuron: Intestinal absorption and
1980 metabolism in the rat. Xenobiotica, 10:103.
