
ALDICARB
First draft prepared by M M. Watson
Ministry of Agriculture, Fisheries and Food
Harpenden, Hertfordshire, United Kingdom
EXPLANATION
Aldicarb was evaluated for acceptable daily intake by the 1979
and 1982 Joint Meetings (Annex 1, references 32 and 38). An ADI of
0-0.005 mg/kg bw was allocated by the 1982 Joint Meeting. Since that
time additional information has become available, including a new
human volunteer study, and the results of these studies were
reviewed at the present Meeting. In order to facilitate review of
the complete database, summaries of information presented in the
reports of the Joint Meetings of 1979 and 1982 are included in this
monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical Aspects
Absorption, distribution, and excretion
Aldicarb is readily absorbed, distributed widely in the body
and excreted rapidly in mammals. Radiolabelled aldicarb was
administered orally to male rats and residues were analyzed over a
14-day period. Excretion was essentially complete within 4 days
(greater than 95% of the administered dose). The major
concentrations of metabolites were observed to have been excreted
within 24 h of dosing. Four days following acute oral dosing,
residues were not detected in animal tissues. In dogs, the excretion
pattern of aldicarb administered subacutely was similar to that
noted following acute administration in other species. A similar
urinary excretion pattern was observed when aldicarb sulfone and
aldicarb nitrile were administerd to lactating dairy cows either
alone or in combination with one or more aldicarb metabolites.
Following a single acute administration of aldicarb, approximately
83% of the dose was eliminated in the urine within 24 h. A minor
quantity of residue was eliminated in the faeces and small amount of
residues were observed in milk (less than 3% of the administered
dose was observed in milk over a 5-day interval). Aldicarb and/or
aldicarb sulfone administered as a single oral dose to laying hens
were rapidly excreted in the faeces. Minute quantities of terminal
residues were observed in eggs on the first day after treatment, but
the residue level declined rapidly (Knaak et al., 1966; Andrawes
et al., 1967, 1977; Dorough & Ivie, 1968; Sullivan, 1968a,b,c;
Dorough et al., 1970; Hicks et al., 1972; Romine, 1973; Sullivan
& Carpenter, 1974).
A study in goats was conducted to determine the fate, nature
and magnitude of aldicarb residues in urine, milk and tissues. Two
lactating goats received S-methyl-14C-aldicarb administered in
gelatin capsules at a level equivalent to 2.5 ppm in the feed for
ten days. A third goat served as the control. The goats were
sacrificed within 6 to 8 h after the last dose was given. No toxic
effects were noted during the study. Of the applied dose, 61.2% was
eliminated in urine, 11.3% in faeces and 1.1% was secreted in milk.
Only 0.2% of the applied dose was found in the respiratory gases and
< 0.10% in tissues at the end of the ten-day dosing period
(Andrawes & Lee, 1986).
Biotransformation
The metabolic fate of aldicarb has been studied in a variety of
vertebrate and invertebrate species. Minor biotransformation
differences have been found to occur with respect to quantities of
individual metabolites. The basic metabolic profile of aldicarb in
all species examined appears to be the same and is shown in Figure
1. Aldicarb is rapidly oxidized to aldicarb sulfoxide, a relatively
stable metabolite. Aldicarb sulfoxide is slowly degraded by both
oxidative and hydrolytic mechanisms yielding the corresponding
aldicarb sulfone and sulfoxide oxime (Knaak et al., 1966; Metcalf
et al., 1966; Andrawes et al., 1967, 1977; Bull et al., 1967;
Dorough & Ivie, 1968; Sullivan 1968a; Dorough et al., 1970; Hicks
et al., 1972; Sullivan & Carpenter, 1974; Andrawes & Lee, 1986).
Effects on enzymes and other biochemical parameters
As noted with other N-methylcarbamate esters, aldicarb is a
readily reversible inhibitor of cholinesterase activity and in
vitro studies have shown that cholinesterase inhibition induced by
aldicarb and its oxidative metabolites (aldicarb sulfoxide and
aldicarb sulfone) can be rapidly reversed by simple dilution.
Aldicarb sulfoxide was 47 and 25 times more effective in inhibiting
cholinesterase than aldicarb and aldicarb sulfone, respectively,
with an insect enzyme preparation, and 23 and 60 times more
effective, respectively, when using an RBC preparation obtained from
cows (Metcalf et al., 1966; Bull et al., 1967; Chin & Sullivan,
1968).
Toxicological studies
Acute toxicity studies
The acute toxicity of aldicarb and its metabolites has been
studied in a variety of mammalian species. A summary of acute
toxicity data for aldicarb in shown in Table 1 and for the
metabolites in Table 2.
Short-term toxicity studies
Mice
- Aldicarb
Groups of 5 mice/sex were fed aldicarb in the diet at dose
levels of 0, 0.1, 0.3, 0.6 or 1.2 mg/kg bw/day for 7 days. Mortality
was noted in both males and females at the high dose level. Growth
was not affected over the course of the study. Liver and kidney
weights were also unaffected. A dose level of aldicarb equal to 0.65
mg/kg bw/day for females and 0.75 mg/kg bw/day for males was without
substantial effects. The NOAEL in this study was 0.6 mg/kg bw/day
(Weil & Carpenter, 1970e).
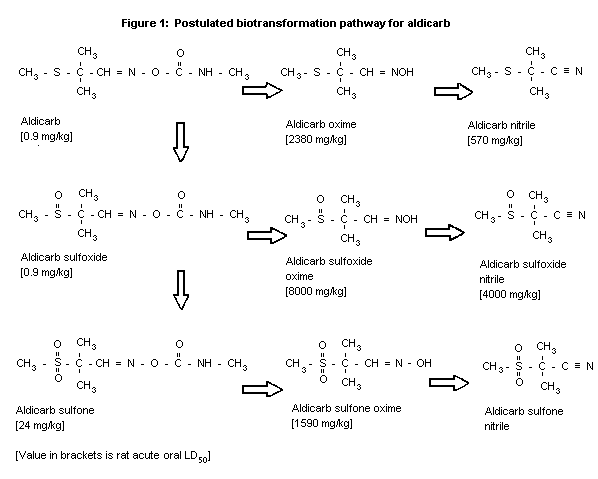 Table 1: Acute toxicity of aldicarb
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Rat M oral corn oil 0.84 West & Carpenter (1966b)
Rat M oral corn oil 0.93 Striegel & Carpenter (1962)
Rat M oral corn oil 0.67-1.23 Carpenter (1963)
Nycum & Carpenter (1968b)
Rat F oral corn oil 0.62-1.07 Carpenter (1963)
Rat F oral gycerol:
ethanol 1.0 WHO (1966)
Rat F ip corn oil 0.71 Carpenter (1963)
Rat M+F ip corn oil 0.44 Carpenter (1963)
Rat M ip PEG 0.37-0.44 Weil & Carpenter (1970a)
Rat M ip ethanol 0.57 Johnson & Carpenter (1966b)
Rat M iv water 0.47 Weil & Carpenter (1970a)
Rat M dermal corn oil >10 Field (1979b)
Rat F dermal DMP 3.2-7.0 WHO (1966)
Rat M dermal water 38.1-44.9 Weil & Carpenter (1968a)
Mouse M oral corn oil 0.382 Weil & Carpenter (1972b)
Mouse M oral corn oil 0.5 Weil & Carpenter (1972c)
Mouse F oral cotton seed 1.5 Dorough et al. (1970)
oil
Mouse F ip cotton seed 0.3 Dorough et al. (1970)
oil
Guinea-pig oral corn oil 1.0 Nycum & Carpenter (1968b)
Rabbit oral propylene 1.3 Nycum & Carpenter (1968b)
glycol
Rabbit M dermal corn oil >10 Field (1979a)
Rabbit M dermal PEG 5.0 Striegel & Carpenter (1962)
Rabbit M dermal dry 141->200 Weil & Carpenter (1968a)
Chicken M oral 9 West & Carpenter (1965)
Table 2: Acute toxicity of aldicarb metabolites
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. nitrile rat oral 570 West & Carpenter (1966b)
A. sulfoxide rat (M) oral corn oil 0.49-1.13 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfone rat (M) oral corn oil 20-25 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfoxide mouse (M) oral corn oil 0.8-1.6 Nycum & Carpenter (1968b)
A. sulfone mouse (M) oral corn oil 25 Nycum & Carpenter (1968b)
A. sulfoxide guinea-pig oral corn oil 0.8-1.8 Nycum & Carpenter (1968b)
A. sulfone guinea-pig oral corn oil >50 Nycum & Carpenter (1968b)
A. sulfoxide rabbit oral corn oil 0.4-1.8 Nycum & Carpenter (1968b)
Table 2 (cont'd)
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. sulfone rabbit oral corn oil 75 Nycum & Carpenter (1968b)
A. sulfoxide rat (M) ip water 0.47 Weil & Carpenter (1970a)
A. sulfoxide rat (M) ip corn oil 0.71 Johnson & Carpenter (1966b)
A. sulfone rat (M) ip PEG 21.2 Weil & Carpenter (1970a)
A. sulfoxide rat (M) iv water 0.47 Weil & Carpenter (1970a)
A. sulfone rat (M) iv water 14.9 Weil & Carpenter (1970a)
A. sulfoxide rabbit dermal water >20 West & Carpenter (1966b)
A. sulfone rabbit dermal water >20 West & Carpenter (1966b)
2-methyl-2-
(methyl-sulfinyl)
propanol-1 rat oral 11 000 Weil & Carpenter (1969b)
OH-methyl A rat oral 42.9 Carpenter (1969)
A. sulfoxide oxime rat (M) oral 8060 Nycum & Carpenter (1968a)
A. sulfone oxime rat (M) oral 1590 Nycum & Carpenter (1968a)
A. sulfoxide nitrile rat (M) oral 4000 Nycum & Carpenter (1968a)
A. sulfone nitrile rat (M) oral 350 Nycum & Carpenter (1968a)
A: aldicarb
Mice
- Aldicarb/aldicarb metabolites
Groups of 3 male and 5 female mice were fed a mixture of
aldicarb and aldicarb sulfone (1:1) in the diet at dose levels of 0,
2, 6, 18, or 36 mg/kg bw/day for 7 days. No mortality was noted at
any dose level, but severe cholinergic signs of poisoning were
observed at the high-dose in males. Depression of growth was
observed at the two highest dose levels. Kidney weight was depressed
at the highest dose and liver weight was reduced substantially at 6
mg/kg bw/day and above in both males and females. The NOAEL in this
study was 2 mg/kg bw/day (Weil & Carpenter, 1970d)
Groups of 5 Charles River CD-1 mice/sex were fed aldicarb
sulfone in the diet at dose levels of 0, 0.15, 0.6, 2.4, 9.6 or 38.4
mg/kg bw/day for 7 days. Significant body-weight decrease was noted
at the highest dose but there were no significant organ weight
changes. Cholinesterase activity was not determined (Weil &
Carpenter, 1974e).
Rats
- Aldicarb
Groups of 5 rats/sex were fed aldicarb in the diet for 7 days
at dose levels of 0, 4, 8 or 16 mg/kg bw/day. Mortality was noted
predominantly at the highest dose at which all males and 2 of 5
females died. One of 5 males also died at 8 mg/kg bw/day. There was
a substantial reduction in body-weight gain noted at all dose
levels. In males, kidney weights were significantly reduced at 8
mg/kg bw/day and liver weight was depressed at 4 and 8 mg/kg bw/day.
In females, both liver and kidney weight was significantly depressed
at all dose levels (Weil & Carpenter, 1970b).
Groups of 5 rats/sex were fed aldicarb in the diet at dose
levels of 0, 0.8, 1.6 or 3.2 mg/kg bw/day for 7 days. Growth was
depressed at 1.6 mg/kg bw/day and above. There was no mortality
attributable to aldicarb. In males, liver weights were depressed in
all treatment groups. In females, liver weights were affected only
at the highest dose, but the liver to body-weight ratio was reduced
at 1.6 mg/kg bw/day and above. Kidney weights were reduced in males
at all dose levels and in females only at the highest dose.
Cholinesterase activity, measured on the day after the conclusion of
feeding, was normal at the highest dose tested with the exception of
plasma cholinesterase which was slightly reduced at the highest
level (Weil & Carpenter, 1969a).
Groups of 10 CFE rats/sex were fed aldicarb in their diet for
93 days at dose levels of 0, 0.02, 0.1 or 0.5 mg/kg bw/day. One rat
per level was killed for cholinesterase (plasma, RBC and brain)
determinations at 1, 2, 4 and 29 days of dosing. Mortality was
increased and body-weight and food consumption were decreased at the
highest level. Histopathology of selected organs was unremarkable
and there were no compound-related effects noted. There were no
consistent dose-related effects on the cholinesterase
determinations, except for plasma cholinesterase depression in both
sexes after 30 days at the highest level. There was no indication of
how soon after feeding the cholinesterase determinations were
performed, which could account for the sporadic results, together
with the small number of animals sampled at each interval. The NOAEL
in this study was 0.1 mg/kg bw/day (Weil & Carpenter, 1963).
Rats
- Aldicarb/aldicarb metabolites
Groups of 5 rats/sex were fed diets containing aldicarb at dose
levels of 0, 0.4, 0.8, 1.6 or 3.2 mg/kg bw/day, or aldicarb
sulfoxide at dose levels of 0, 0.4 or 0.8 mg/kg bw/day, or aldicarb
sulfone, at dose levels of 0, 0.4, 1.0, 2.5, 5 or 20 mg/kg bw/day,
for 7 days. With aldicarb (as with its two major carbamate
metabolites) there was a significant growth depression at the
highest dose. There were no effects noted with respect to liver or
kidney weights. The erythrocyte cholinesterase appeared to be the
most sensitive parameter with all three materials tested. The
NOAELs, based on erythrocyte cholinesterase depression or decreased
body-weight gain over the 7-day treatment period were: aldicarb -
0.8 mg/kg bw/day; aldicarb sulfoxide - 0.4 mg/kg bw/day; and
aldicarb sulfone - 2.5 mg/kg bw/day (Nycum & Carpenter, 1968b).
Groups of 5 rats/sex received in the diet, aldicarb (0.3 mg/kg
bw/day), aldicarb sulfoxide (0.4, 0.8 or 1.6 mg/kg bw/day), aldicarb
sulfone (0.6, 5 or 20 mg/kg bw/day), a 1:1 mixture of aldicarb
sulfoxide and aldicarb sulfone (1.2 mg/kg bw/day) or a control diet.
At the end of the 7 days of feeding, animals were placed on control
diets for one day after which they were sacrificed for
cholinesterase determination and for examination of liver and
kidney. A second one-week feeding trial was performed to compare the
data with other strains of rats. Aldicarb sulfoxide was fed to
groups of 5 male rats at dose levels of 0, 0.4, 0.8 or 1.6 mg/kg
bw/day and aldicarb sulfone was fed to male rats at dose levels of
0, 5 or 20 mg/kg bw/day. As might be expected with the protocol
followed in the study, cholinesterase depression was not observed in
either plasma, erythrocyte or brain at the conclusion of the study.
The second trial using both the same and a different strain of rats
was performed in an effort to explain a slight but non-significant
inhibition of erythrocyte cholinesterase activity measured in the
initial study. Over the course of this study, there were no effects
noted on erythrocyte cholinesterase activity (Weil & Carpenter,
1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.3 or 1.0 mg/kg bw/day and aldicarb sulfone at 0,
2.4 or 16.2 mg/kg bw/day. Animals were sacrificed at 1, 3, 7, 14, 28
and 56 days for cholinesterase analyses. Plasma and erythrocyte
cholinesterase activities were measured at the first three time
intervals. At the last three time intervals, plasma erythrocyte and
brain cholinesterase activities were examined. There was no
mortality, but growth was depressed at the highest dose levels with
both the sulfoxide and sulfone. Rats administered aldicarb sulfoxide
at 1.0 mg/kg bw/day had a slight but significant cholinesterase
depression during the study. There were no effects noted at 0.3
mg/kg bw/day. Cholinesterase depression was marked with the aldicarb
sulfone consistently throughout the study at the highest dose level
of 16.2 mg/kg bw/day (Weil & Cox, 1975).
Groups of 10 Wistar rats/sex were administered aldicarb
sulfoxide and aldicarb sulfone in a 1:1 ratio, in drinking water,
ad libitum, for 29 days. The drinking water levels were 0, 0.075,
0.3, 1.2, 4.8 or 19.2 mg/litre. Plasma and red blood cell
cholinesterase levels were determined after 8, 15 and 29 days of
treatment. Brain cholinesterase was measured at termination. Animals
were continuously exposed to the test substances in water until
blood samples were taken or sacrifice was performed. There was no
mortality and food and water consumption values together with body-
weight gains were significantly decreased in both sexes at the high-
dose level when compared to controls. Significant depression of
plasma, RBC and brain cholinesterase, in both sexes, occurred at the
highest dose level. The data demonstrate that a level of 4.8
mg/litre of aldicarb sulfoxide:aldicarb sulfone in water is without
effect on cholinesterase (plasma, RBC, brain) in either sex. Based
on the actual concentrations being approximately 80% of the nominal
concentrations, the NOAEL was roughly 3.8 mg/litre, equivalent to
0.4 mg/kg bw/day (Mirro et al., 1982).
Groups of 15 rats/sex were fed aldicarb sulfoxide in the diet
at dose levels of 0, 0.125, 0.25, 0.5 or 1 mg/kg bw/day for 6
months. Animals were sacrificed at 3 months and at the conclusion of
the study for cholinesterase determinations and for gross and
microscopic examination of liver and kidneys. There was no mortality
noted during the course of the study, although growth, especially in
males, at 0.25 mg/kg bw/day and above was reduced. Cholinesterase
activity was substantially reduced, at the three highest dose
levels, especially in plasma and erythrocytes of males. Gross
examination of liver and kidneys revealed no abnormalities
attributable to aldicarb. The NOAEL in this study was 0.125 mg/kg
bw/day (Weil & Carpenter, 1968b).
In an attempt to resolve the question of cholinesterase
depression and rapid recovery, groups of 5 rats/sex were fed
aldicarb sulfoxide for one week or one week plus one day of control
diets at a dose level of 1 mg/kg bw/day. When the study was
concluded (within one week), animals were sacrificed at 0 and 24 h
after the dietary feeding interval (the 24 h animals were fed
control diets). Cholinesterase depression was noted at the 0 h
sacrifice in erythrocyte and plasma preparations. Administration of
a control diet for one day (24 h sacrifice) completely reversed the
cholinesterase depression noted when animals were sacrificed without
any recovery interval (Weil & Carpenter, 1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.0625, 0.125, 0.25, 0.5 or 1.0 mg/kg bw/day for 3
and 6 months after which some of the animals were sacrificed
immediately and others were placed on a control diet for 24 h prior
to sacrifice and cholinesterase analysis. Cholinesterase activity in
the brain was unaffected by aldicarb sulfoxide. Plasma and
erythrocyte cholinesterase was substantially reduced at the 0 h
sacrifice in both sexes. Males were slightly more sensitive with
depression being noted at 0.25 mg/kg bw/day and above, while with
females depression was noted at 0.5 mg/kg bw/day and above. There
was no cholinesterase depression noted in any of the animals treated
for either 3 months or 6 months when the animals were allowed to
recover from cholinesterase depression for a one day recovery
interval. The NOAEL in this study was 0.125 mg/kg bw/day (Weil &
Carpenter, 1968b).
In a series of studies similar to those reported with aldicarb
sulfoxide, groups of rats were fed aldicarb sulfone at dietary
concentrations at dose levels of 0, 0.2, 0.6, 1.8, 5.4 or 15.2 mg/kg
bw/day for 6 months. Animals were sacrificed at 3 and 6 months for
examination of liver and kidney abnormalities and for evaluation of
cholinesterase activity. Cholinesterase determinations were made at
the end of the 3 and 6 month intervals with rats fed continuously
until sacrifice or rats were allowed to consume a control diet for
24 h prior to sacrifice and determination of cholinesterase
activity. There was no mortality over the course of the study.
Transient but significant growth depression was noted at the highest
level in the longer study. There were no effects noted with respect
to food consumption or on gross and microscopic examinations of
liver and kidneys. Plasma, erythrocyte and brain cholinesterase were
significantlv depressed at 5.4 mg/kg bw/day and above. Erythrocyte
cholinesterase depression was also noted at 1.8 mg/kg bw/day. There
was no cholinesterase depression noted at 0.6 mg/kg bw/day in any of
the tissues examined. In the study to evaluate recovery of
cholinesterase activity, when animals were allowed to equilibrate
for one day on control diets, all depressed cholinesterase values
returned to normal. The NOAEL in this study was 0.6 mg/kg bw/day
(Weil & Carpenter, 1968c).
Groups of rats were fed aldicarb oxime at dose levels of 0,
31.25, 62.5, 125, 250, 500 or 1000 mg/kg bw/day for 7 days. There
was no mortality over the course of the study. Growth was slightly
reduced at the initiation of the study at dose levels of 125 mg/kg
bw/day and above, but at the end of one week only the two highest
dose levels appeared to show a retardation in growth. Gross changes
were noted in both liver and kidneys at the two highest dose levels
in both males and females. The NOAEL in this study was 62.5 mg/kg
bw/day (Weil & Carpenter, 1974b).
Groups of 5 rats/sex were fed the hydrolytic metabolite of
aldicarb (2-methyl-2-(methylsulfinyl)propanol-1) for 7 days at dose
levels of 0, 500 or 1000 mg/kg bw/day. Growth was depressed at both
dose levels in males but only at the highest dose level in females.
In females, while growth was depressed within one day of treatment,
the animals appeared to recover during the rest of the week. There
was no apparent effect on major organs, although, in females at the
highest dose level, kidney weights were slightly depressed.
Cholinesterase activity was not measured (Weil & Carpenter, 1969b).
Rabbits
- Aldicarb
Four groups, each of 5 male rabbits with abraded skin, were
administered aldicarb 10G formulation at dose levels of 0, 5, 10 or
20 mg/kg bw/day, for 6 h per day, 5 days per week, for 15 days.
Water was added periodically during the exposure time to the
dressing containing the aldicarb treatment, simulating a condition
of excess perspiration. One additional group was administered 20
mg/kg bw/day to intact, unabraded skin with no water added to the
dressing. Animals treated with aldicarb under a dry condition with
unabraded skin showed normal weight gains and no apparent effects as
a result of the treatment. Administration of aldicarb under
conditions where the dressing was wet and the skin abraded resulted
in reduced body-weight at a dermal dose of 5 mg/kg bw/day and above.
During the interim where dermal treatment was not applied, recovery
of growth was extremely rapid. Plasma cholinesterase activity was
inhibited at the two highest dose levels. There were no other
adverse effects on haematology or clinical chemical parameters and
limited organ weight analysis and microscopic examination of several
major tissues showed no pathological effects attributable to
aldicarb. Almost identical results were also obtained in a similar
study in rats (Carpenter & Smyth, 1966; Weil & Carpenter, 1968a).
Rabbits
- Aldicarb metabolites
Aldicarb sulfone (technical and 75 WP) were administered to the
unabraded, but closely clipped, ventral surface of rabbits (6
males/group, 5 days/week for 19 applications) at dosages of 4.8
mg/kg bw/day for 75 WP and 3.5, 7.0 or 14.0 mg/kg bw/day for
technical aldicarb sulfone. No mortality, skin irritation or
cholinesterase depression were observed. However, rabbits were
removed from treatment 19 h prior to sampling for cholinesterase
analyses, which could account for the lack of cholinesterase
depression. Body-weight was significantly depressed in the high-dose
level rabbits. There were no reported treatment-related effects on
organ weights and necropsy findings were unremarkable (Weil et
al., 1977).
Dogs
- Aldicarb
Groups of 2 beagle dogs/sex were fed aldicarb at dose levels of
0, 0.2, 0.3 or 0.7 mg/kg bw/day for 7 days. There was no mortality
over the course of the study. Plasma, erythrocyte and brain
cholinesterase activities, measured one day after conclusion of the
dietary treatment, were normal. Gross liver and kidney weights and
organ to body-weight ratios were unaffected by aldicarb in the diet
(Weil & Carpenter, 1973).
Groups of 4 beagle dogs/sex were fed aldicarb in the diet for
five days per week at dose levels of 0, 0.2, 0.3 or 0.7 mg/kg bw/day
for 100 days. There was no mortality over the course of the study
and growth was comparable within all dosage groups. A slightly
decreased testes weight and a slight increase in adrenal weight were
observed at the highest level in males, but there were no effects in
females on any of the tissues and organs examined. Microscopic
analysis did not suggest any abnomalities including tissues where
gross changes had been seen to occur. Cholinesterase values, as well
as other clinical chemistry and haematology parameters, were
unaffected by the presence of aldicarb in the diet, however, the
animals had been removed from aldicarb exposure for 24 to 48 h prior
to cholinesterase analyses. The NOAEL in this study was 0.3 mg/kg
bw/day (Weil & Carpenter, 1974c).
Groups of 3 beagle dogs/sex were fed aldicarb in the diet at
dose levels of 0, 0.025, 0.05 or 0.1 mg/kg bw/day, 5 days per week
for 2 years. There was no mortality over the course of the study and
growth and food consumption data were comparable to control values.
Haematology parameters and clinical chemistry values, evaluated at
five intervals over the course of the study were normal. Plasma and
erythrocyte cholinesterase, evaluated over the course of the study,
did not differ from control values. At the conclusion of the study
brain cholinesterase, while somewhat lower at the high dose level,
was not statistically different from controls. Gross and microscopic
examination of tissues and organs showed no lesions which could be
attributable to the presence of aldicarb in the diet at dose levels
up to and including 0.1 mg/kg bw/day (Weil & Carpenter, 1966c).
A two-week range-finding study was conducted in beagle dogs to
select dose levels for a subsequent one-year dog study. Aldicarb was
fed to groups of 1 male and 1 female at dietary concentrations of 0,
1, 3, 10, 30 or 100 ppm. There was no mortality during the study,
but tremors and slight ataxia were observed at 30 and 100 ppm, and
soft faeces were noted more frequently in these dogs. Food
consumption was depressed in all treated males, particularly at the
highest dose, and slightly depressed in the 100 ppm female. Body-
weight losses were noted in the 100 ppm group and in the 30 ppm
male. Red blood cell and plasma cholinesterase inhibition exceeded
25% at doses of 3 ppm and greater. Brain cholinesterase inhibition
exceeded 25% for the 30 and 100 ppm group males (no calculation was
possible in the female groups due to presumed technical error)
(Hamada et al., 1985b).
As a clear NOEL for cholinesterase depression was not
demonstrated in the first study, a second two-week dietary dose
range-finding study was conducted to select the dose levels for a
subsequent one-year dog study. Aldicarb was fed to groups of 1 male
and 1 female at dietary concentrations of 0, 0.1, 0.3, 1, 3 or 10
ppm. There was no mortality during the study and no
treatment-related clinical signs were observed. Body-weight change
and food consumption were unaffected by treatment. Maximum plasma
and erythrocyte cholinesterase inhibition occurred immediately
following the two-hour feeding and lasted approximately 4 h after
the dosing period. Erythrocyte and plasma cholinesterase inhibition
(> 25% compared to the mean pre-study value) occurred in the 10 ppm
group on both days 7 and 14. There was no inhibition of brain
cholinesterase and no other compound-related effects noted. The
NOAEL in this study was 3 ppm, equal to 0.096 mg/kg bw/day (Hamada,
1987b).
In a five-week study, conducted in order to further investigate
the cholinesterase inhibition dose response curve, aldicarb (99.7%)
was fed at dietary concentrations of 0, 0.35, 0.7, or 2 ppm, to 3
groups of 6 beagle dogs/sex. There were no mortalities nor any
changes in body-weight gain, food consumption, clinical symptoms or
gross pathology indicative of a compound-related effect seen during
the study. Plasma cholinesterase inhibition over 20% when compared
to the control occurred only in the highest dose group. Red blood
cell cholinesterase activity inhibition was not observed in any dose
group and brain cholinesterase activity was not measured. No adverse
effects were observed at 2 ppm (equal to 0.06 mg/kg bw/day) and the
NOEL for any symptoms including plasma cholinesterase depression was
0.7 ppm (equal to 0.02 mg/kg bw/day) (Hamada, 1991).
In a 52-week oral toxicity study in beagle dogs, groups of 5
dogs/sex were fed dietary concentrations of 0, 1, 2, 5 or 10 ppm
aldicarb (95.5%) daily for 52 weeks. The study was designed to
produce maximum cholinesterase depression by limiting feeding time
to two hours per day to mimic a bolus administration of aldicarb.
Erythrocyte and plasma cholinesterase activities were measured from
blood samples collected approximately 2 h after the daily feeding
period to minimise dissociation of the carbamate-cholinesterase-
complex. Aldicarb at up to 10 ppm (equal to 0.241 mg/kg bw/day)
caused no observable effects other than inhibition of cholinesterase
activity. Inhibition of erythrocyte and brain cholinesterase
activity was restricted to dogs receiving 5 or 10 ppm. The NOEL for
plasma cholinesterase inhibition was 1 ppm, equal to 0.027 mg/kg
bw/day, while if the changes in the plasma enzyme activity are
discounted, the NOAEL was 2 ppm, equal to 0.054 mg/kg bw/day
(Hamada, 1988).
An inhalation study was conducted to determine the toxicity of
pyrolysis products of cigarettes made from tobacco pretreated with
aldicarb. Twelve beagle dogs (two animals/sex/dose), which had been
initially conditioned prior to commencement of the test to smoking
10 cigarettes per day via tracheostomy, were exposed to untreated,
low-level and high-level treated cigarettes on a schedule of two
successive cigarettes 5 times per day, 5 days per week for a total
of 19 exposure days. Whole blood cholinesterase was monitored
throughout the study with samples taken before smoking, after 4 and
after 10 cigarettes. All effects observed in this study were
apparently due to inhalation of cigarette smoke. No inhibition of
cholinesterase was seen in any treatment group (Coate et al.,
1982).
Dogs
- Aldicarb metabolites
Groups of 3 beagle dogs/sex were fed aldicarb sulfoxide in the
diet at dose levels of 0, 0.0625, 0.125, 0.25 or 0.5 mg/kg bw/day 5
days per week for 3 months. There was no mortality over the course
of the study. Slight body-weight changes were noted in many of the
dogs at the highest dose level within the first week of treatment
but thereafter the body-weight changes were similar to but lower
than control values. No effects on haematologic and blood chemistry
parameters were observed. Cholinesterase depression measured 24-48 h
after the final exposure was not observed in plasma, erythrocytes or
brain of any of the animals at the conclusion of the study. Gross
and microscopic examination of tissues and organs did not show any
adverse effects attributable to the presence of aldicarb sulfoxide.
The NOAEL in this study was 0.25 mg/kg bw/day (Weil & Carpenter,
1968b).
Groups of 3 dogs/sex were fed aldicarb sulfone in the diet at
dose levels of 0, 0.2, 0.6, 1.8 or 5.4 mg/kg bw/day 5 days per week
for 90 days. There was no mortality over the course of the study.
Slight body-weight depression was noted at the highest dose level,
although the body-weight was not statistically lower than control
levels. There were no effects noted with respect to biochemical and
haematologic parameters and gross and microscopic examination of
tissues and organs revealed no effects attributable to the presence
of aldicarb sulfone in the diet. Cholinesterase depression was not
observed, but dogs were removed from treated diet prior to
cholinesterase determinations. The NOAEL in this study was therefore
greater than 5.4 mg/kg bw/day (Weil & Carpenter, 1968c).
In a two-week range-finding study, aldicarb sulfone was fed to
six groups of 1 male and 1 female beagle dog at dietary
concentrations of 0, 3, 10, 30, 100 or 300 ppm. There was no
mortality during the study, but emesis, tremors and decreased food
consumption were observed in the high-dose group. Inhibition of
cholinesterase (> 25%) occurred at 10 ppm and over in both sexes
for plasma cholinesterase, and 100 ppm and over for red blood cell
and brain cholinesterase (Hamada et al., 1985a).
In a one-year feeding study, aldicarb sulfone was administered
to four groups of 6 beagle dogs/sex at dietary concentrations of 0,
5, 25 or 100 ppm. In this study, samples for cholinesterase
determinations were taken approximately two hours after feeding in
order to measure maximum cholinesterase inhibition. No
treatment-related clinical signs were seen. Body-weight gain was
slightly reduced in the high-dose males and females. There was a
slight decrease in the spleen weights of the mid- and high-dose
females and in the thyroid/parathyroid weight of the high-dose
females when compared to respective control values. These changes
were not seen in the males. Slight treatment-related effects were
noted in the mandibular lymph nodes of the high-dose males and
females, and adrenal cortex of the high-dose females. The NOAEL
based on depression of erythrocyte and plasma cholinesterase
activity was 25 ppm, equal to 0.54 mg/kg bw/day (Hamada, 1987a).
Monkeys
As a result of reports of alleged illness associated with
consumption of watermelons and cucumbers illegally treated or
contaminated with aldicarb in 1985, two studies were conducted to
evaluate the acute toxicity of aldicarb residues in food
commodities. Two crops were chosen for the studies: bananas as a
representative of a registered food crop in the USA, and watermelons
as a representative of a non-registered food crop from the cucurbit
family. (Suggestions had been made that some possible natural
component in cucurbits might synergise aldicarb toxicity). Bananas
were treated with approximately ten times the recommended label rate
and watermelons were treated at 13.44 kg ai/ha, to ensure adequate
residues in the fruit. Each study was performed using 3 Cynomolgus
monkeys/sex, with an additional group of 3 monkeys/sex serving as a
control (total of 12 animals per study). Following analytical
determination of actual residues in the two commodities to be
consumed, (approximately 0.3 ppm in bananas and 5 ppm in
watermelons), intake of the treated crops was adjusted with control
fruit to provide a dose of exactly 0.005 mg aldicarb/kg bw. The
animals were offered the fruit as the first meal of the day, and
consumed it immediately. Individuals were monitored for clinical
signs at regular intervals, and plasma and erythrocyte
cholinesterase activities were measured pre-dose, then at 1, 2, 4,
6, 12, 18 and 24 hours post-feeding. In both studies, there was no
evidence of acute cholinergic distress or any signs of over-exposure
in any animal. Depression of plasma cholinesterase activity was
evident within 1-4 h after ingestion and reached a maximum of 32 to
36% at 2 h after feeding (banana trial) and 36 to 37% at 1 h after
feeding (watermelon trial). Erythrocyte cholinesterase activity was
not depressed in either study. Rapid recovery of all enzyme activity
was observed. There was no indication in these studies that
components in watermelons potentiate aldicarb toxicity (Trutter,
1987a,b).
Long-term toxicity/carcinogenicity studies
Mice
- Aldicarb
Groups of 50 B6C3F1 mice/sex (25 of each sex were used as
controls) were fed aldicarb at dietary concentrations of 0, 2 or 6
ppm for 103 weeks in a carcinogenicity bioassay. A preliminary
dietary study (using 10 mice/sex fed dietary concentrations of
aldicarb of 0, 0.5, 1.0, 2.5, 5, 10, 20 or 40 ppm for 13 weeks)
showed no significant effects on microscopic examinations of the 0,
20 and 40 ppm levels. In the long-term carcinogenicity study there
was no mortality attributable to aldicarb. A variety of benign and
malignant tumours, occurring at different sites in both control and
aldicarb-treated mice, were not unusual for this strain of mice and
were evaluated to be independent of the administration of aldicarb.
Gross and microscopic examination of tissues, organs and all gross
lesions was performed and it was concluded that aldicarb was not
carcinogenic for the B6C3F1 strain of mice of either sex (NIH,
1979).
Groups of 44 CD-1 mice/sex were fed aldicarb at dose levels of
0, 0.1, 0.2, 0.4 or 0.7 mg/kg bw/day for 18 months. Mortality was
evident in males at the two highest dose levels and in females at
the three highest dosage levels during the first two and one-half
months of the study. Following this period, aldicarb was admixed
with the diet in a different manner which appeared to eliminate its
acutely toxic effects. (In the early parts of the study, aldicarb
was mixed in a dry fashion using a finely ground aldicarb
preparation. At the 2.5 month interval, aldicarb was dissolved in
acetone and the acetone-aldicarb solution was dispersed in the diet
at a more uniform rate. It was assumed that consumption of small
crystalline particles of aldicarb may have led to the high mortality
during the initial phases of the study). At the high dose level in
males there was a statistically significant increase in hepatomas
found predominantly in the survivors at the termination of the study
and an increase in lymphoid neoplasias which occured in the mice
that died. None of the male mice surviving at the end of the study
were found to have lymphoid neoplasias. There were no significant
increases in any other types of tumours at dose levels of 0.4 mg/kg
bw/day and below (Weil & Carpenter, 1972c).
Groups of 50 male CD-1 mice were fed aldicarb at dose levels of
0, 0.1, 0.3 or 0.7 mg/kg bw/day in an effort to verify the results
of the previous mouse carcinogenicity bioassay. A group of 150 mice
were used as concurrent controls with a mouse being sacrificed for
each treated animal that died during the course of the study. Diets
were prepared by dissolving aldicarb in acetone and mixing the
solution with the diet. The aldicarb was the same sample as used in
the previous study and the duration of the study was approximately
the same as in the previous trial. There was no mortality observed
in the study as a result of aldicarb in the diet. At the end of 18
months cumulative mortality at all dose levels was the same as noted
in controls. There was no effect of aldicarb on growth in any of the
groups. An examination of the animals that died during the course of
the study and those that were sacrificed at the end of 18 months was
made and the data compared with control values. There was no
significant association between aldicarb in the diet and the
formation of tumours, particularly with respect to the incidence of
hepatomas, lung adenomas, and lymphoid neoplasias. The data were
evaluated with respect to the mice that died, those that survived
the test and the total of all animals. It was concluded that the
administration of aldicarb at levels up to and including 0.7 mg/kg
bw/day for approximately 18 months did not result in a higher than
normal incidence of tumours and the inclusion of aldicarb in the
diet of CD-1 mice did not result in an increased incidence of
carcinogenic response (Weil & Carpenter, 1974d).
Groups of C3H/Hej male mice were administered aldicarb
dissolved in acetone, dermally 3 times a week for 28 months by
applying a brush full of an acetone solution to the shaved back of
the mice. For the first 2 weeks, treatment was performed 3 times a
week using a 0.25% solution in acetone. After 2 weeks this was
reduced to a twice weekly application. This dosing regimen was
maintained for 2 months and further reduced thereafter to a
concentration of 0.125% which was maintained for the rest of the
study. While there was some aldicarb-induced mortality noted over
the course of the study this mortality was not substantially
different from that noted with control applications. There were no
substantial differences with respect to the incidence or onset of
tumours. Two growths, a haemangioma and a thymoma, were noted in the
animals administered aldicarb. Neither of these internal growths was
accompanied by cutaneous papillomas or carcinomas and were
considered to be spontaneous growths unrelated to treatment.
Aldicarb, administered dermally to this sensitive species, did not
induce any incidence of malignancy (Weil & Carpenter, 1966b).
Mice
- Aldicarb metabolites
Groups of Charles River CD-1 mice (50/sex/group) were
administered 0, 0.15, 0.6, 2.4 or 9.6 mg aldicarb sulfone/kg bw/day
via the diet for 18 months. Aldicarb sulfone did not affect tumour
incidence or produce any pathological alteration in this strain of
mouse (Woodside et al., 1977b).
Rats
- Aldicarb
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0, 0.005, 0.025, 0.05 or 0.1 mg/kg bw/day. Additional
groups of 16 rats/sex were maintained for serial sacrifices at 6 and
12 months. There was no mortality, attributable to the presence of
aldicarb. Growth was normal at all dose levels as was consumption of
food and behavioural characteristics. Results of gross examination
of liver and kidney weight at 6 months and at one year did not
differ from control values. Haematologic determinations in the
highest dose level and control groups were normal. Blood and brain
cholinesterase activity, measured at 6 and 12 months, were normal.
Microscopic examination of tissues and organs for histopathologic
occurrences and neoplasms showed the incidence of lesions to be
similar in aldicarb-treated and in control groups. An apparent
no-effect-level in this study is 0.1 mg/kg bw/day (Weil & Carpenter,
1965).
Groups of 50 F344 rats/sex (25/sex for controls) were fed
aldicarb in the diet at dietary concentrations of 0, 2 or 6 ppm for
103 weeks. A preliminary study used 10 rats/sex, with dietary
concentrations of 0, 5, 10, 20, 40, 80, 160 or 320 ppm for 13 weeks.
Microscopic examinations performed on male and female rats of the 0
and 80 ppm dose levels at the conclusion of the preliminary trial
showed no significant effects. In the long-term carcinogenicity
study, there was no mortality attributable to aldicarb in the diet.
A variety of benign and malignant tumours occurring at different
sites in both control and aldicarb treated rats were not unusual for
this strain of rat and were evaluated to be independent of the
administration of aldicarb. Gross and microscopic examination of
tissues, organs and all gross lesions was performed and it was
concluded that aldicarb was not carcinogenic for the F344 strain of
rat of either sex at dietary concentrations of up to 6 ppm (NIH,
1979).
Rats
- Aldicarb/Aldicarb metabolites
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0 or 0.3 mg/kg bw/day. In addition, groups of rats were
fed aldicarb sulfoxide (0.3 or 0.6 mg/kg bw/day), aldicarb sulfone
(0.6 or 2.4 mg/kg bw/day) or a 1:1 mixture of aldicarb sulfoxide and
aldicarb sulfone (0.6 or 1.2 mg/kg bw/day). There was no significant
mortality observed over the course of the study with any of the
individual chemicals or the mixture of sulfoxide and sulfone. In the
initial phases of the study, there was a slightly higher mortality
noted in the high-dose level of aldicarb sulfoxide and in the group
receiving the combined aldicarb sulfoxide and aldicarb sulfone. A
slight increase in mortality was also noted at the latter part of
the study with aldicarb sulfoxide. Growth was slightly depressed at
the high-dose level of the sulfoxide:sulfone mixture, primarily in
males. There were no apparent effects on growth with respect to the
aldicarb, aldicarb sulfoxide or aldicarb sulfone administered alone.
Haematological values observed at various intervals over the course
of the study did not differ from controls. Cholinesterase
determinations were made periodically over the course of the study
(6, 12 and 24 months). Plasma, erythrocyte and brain cholinesterase
were examined only at a 24-h interval after animals were removed
from test diets. There was a slight depression of plasma
cholinesterase noted in males administered the high-dose level of
the combination aldicarb sulfoxide and sulfone at the 24-month
interval. A repeat of the analyses within the final week of the
study showed a slight depression in all chemical groups with respect
to plasma cholinesterase. There were no effects noted at any
interval with respect to red blood cell or brain cholinesterase. The
plasma cholinesterase depression noted at the 24-month interval was
limited to male rats. An evaluation of the incidence of tumours
suggested that there was no statistical difference between treated
and control groups. Gross and microscopic examination of tissues and
organs at various periods over the two-year test interval showed
that these sporadically distributed lesions could not be considered
to be indicative of damage induced by aldicarb, its major
metabolites, or the combination of the sulfoxide and sulfone (Weil &
Carpenter, 1972a).
Reproduction studies
Rats
- Aldicarb
Groups of rats (8 male and 16 female rats per group) were fed
aldicarb at dietary concentrations at doses of 0, 0.05 or 0.1 mg/kg
bw/day for approximately 90 days and mated to initiate a 3-
generation, one litter per generation reproduction study. Dietary
administration was continuous throughout the study. In addition to
the reproduction indices (fertility, gestation, viability and
lactation) the F3 generation was maintained for an additional
period and tissues from these animals were histologically examined
at either weaning or at 90 days of age. In all groups, the
reproduction indices from the aldicarb-treated animals were similar
to the control group. Body-weights of both male and female pups at
weaning were similar to control values as were results of gross and
microscopic examinations of tissues and organs in the F3 weanling
and 90-day old animals. In all three generations, with all criteria
examined, there were no effects of aldicarb on reproduction at a
dose level of 0.1 mg/kg body-weight (Weil & Carpenter, 1964).
Groups of rats (10 male and 20 female per group) were
administered aldicarb in the diet at doses of 0, 0.2, 0.3 or 0.7
mg/kg bw/day for 100 days prior to pairing and mated to initiate an
additional 3-generation (one litter per generation) reproduction
study. Dietary administration was continuous throughout the study. A
larger group was used for the F2 generation (15 male and 25 female
rats) as male pups of this generation were maintained on aldicarb
diets for 148 days and subjected to a (modified) dominant lethal
(mutagenesis) bioassay where they were mated with groups of
untreated virgin females for a period of 10 weeks. Each female in
the group was mated with 2 treated males and allowed to maintain
pregnancy until day 12 when they were sacrificed and examined. There
was some mortality over the course of the study which was associated
with lung infection and not as a result of aldicarb in the diet. At
the high-dose level, body-weights of both male and female F2 pups
were lower than the control values. Overall, there were no effects
on any of the reproduction indices (fertility, gestation, viability,
or lactation). Gross and microscopic examinations of the parents and
pups of the high level and control groups showed no effects
attributable to aldicarb. The dietary dominant lethal mutagenesis
bioassay showed no statistical differences between the
aldicarb-treated rats and controls with respect to early or late
fetal death or any other parameter examined (Weil & Carpenter,
1974a).
Rats
Aldicarb metabolites
Aldicarb sulfone (99.76% pure) was fed to groups of 10 male and
20 female rats at dietary concentrations at doses of 0, 0.6, 2.4 or
9.6 mg/kg bw/day for approximately 100 days. Rats were then mated to
initiate a three-generation, one litter per generation, reproduction
study. Dietary administration was continuous throughout the study.
Male rats fed 9.6 mg/kg bw/day exhibited reduced body-weights. There
were no differences from the control with regard to fertility,
gestation survival or viability indices. It was determined that
aldicarb sulfone, at levels up to and including 9.6 mg/kg bw/day,
was without adverse effects on reproduction under the conditions of
this study (Woodside et al., 1977a).
Special studies on embryo/fetotoxicity
Rats
- Aldicarb
Using a test protocol where both the reproductive and
teratologic potential of aldicarb was evaluated, groups of pregnant
rats were fed aldicarb at dietary concentrations at dose levels of
0, 0.04, 0.2 and 1.0 mg/kg bw/day. Females from each of the dietary
groups were assigned to one of three treatment groups: (1) aldicarb
administered in the diet throughout pregnancy or until pups were
weaned; (2) aldicarb administered in the diet from day 0 to day 7 of
gestation; (3) aldicarb administered in the diet from day 5 to day
15 of gestation. Five or six females from each group were sacrificed
and examined on day 20 of pregnancy and a similar number of females
were allowed to bear, nurse and wean the pups. There were neither
gross manifestations of teratogenicity in any of the pups carried by
females administered aldicarb at dose levels of up to 1 mg/kg bw/day
nor was there apparent interference with the reproductive process by
any of the dosage regimens used in this study. The administration of
aldicarb at dose levels up to and including 1.0 mg/kg bw/day had no
apparent effect on the growth of pregnant females during the course
of the study (Weil & Carpenter, 1966a).
Pregnant Sprague-Dawley rats were given aldicarb by gavage at
levels of 0, 0.125, 0.25 or 0.5 mg/kg bw/day on gestational day 6
through day 15. Maternal toxicity was indicated, at the highest
dose, by reduced weight gain and food intake during the treatment
and post-treatment period as well as by the occurrence of three
maternal deaths and, at the two highest levels, by reduced food
consumption during treatment. Gestational parameters, including
ovarian corpora lutea, number of implantations per litter and sex
ratio were unaffected by treatment. Fetal body-weight per litter was
significantly reduced at 0.5 mg/kg bw/day. There was no increase in
the incidence of external or skeletal malformations. A significantly
increased incidence of dilated lateral ventricles with tissue
depression was observed only at 0.5 mg/kg bw/day. No increased
incidence of malformation was observed in the absence of clear
maternal toxicity nor were these malformations accompanied by more
severe malformations. The NOEL was 0.125 mg/kg bw/day for maternal
toxicity and 0.25 mg/kg bw/day for embryo-fetal toxicity and
teratogenicity (Tyl & Neeper-Bradley, 1988).
Rats
- Aldicarb metabolites
The aldicarb sulfone reproduction study incorporated a
teratology bioassay. The animals were divided into 4 groups and
orally dosed with 0, 0.6, 2.4 or 9.6 mg/kg bw/day at one of the
following time intervals of gestation: 0-20 days, 6-15 days, or 7-9
days. All animals were sacrificed before parturition (day 20) and
the fetuses examined for skeletal and visceral changes. Anomalies
were essentially non-existent and there was no indication of
structural abnormalities produced under the conditions of the study
at levels up to and including 9.6 mg/kg bw/day (Woodside et al.,
1977a).
Rabbit
- Aldicarb
A range-finding study was conducted with aldicarb in rabbits to
determine dosage levels of aldicarb for a teratology study. Pregnant
Dutch belted rabbits (5/dose) were dosed by oral gavage at 0.1,
0.25, 0.5, 0.75 or 1.0 mg/kg bw/day on days 6 to 27 of gestation.
Two does in the high-dose group and one doe in the second highest
group died during the study. Increased incidences of decreased
defecation (all except low-dose group) and soft stools (two highest
dose groups) were noted. Maternal body-weight losses or markedly
decreased maternal body-weight gain during the treatment and
gestation intervals were observed at 0.25 mg/kg bw/day and greater.
Of the uterine parameters measured, there was an increase in the
mean post-implantation loss in the high-dose group. Dosage levels of
0.1, 0.25 and 0.5 mg/kg bw/day were selected for the definitive
study (Aldridge et al., 1983).
Pregnant Dutch belted rabbits (16/dose) were dosed by oral
gavage at 0.1, 0.25, or 0.5 mg/kg bw/day on days 7 to 27 of
gestation. Signs of maternal toxicity in the treated groups included
small amount of stool, pale kidneys and hydroceles on oviducts. A
loss in body-weight occurred in the 0.25 and 0.5 mg/kg bw/day dosage
groups during gestation days 7 to 27. A decline in the number of
viable fetuses per doe was observed in all treatment groups (8.7,
5.0, 6.5 and 6.2 for the control, 0.1, 0.25 and 0.5 mg/kg bw/day
treatment groups, respectively). This can be explained by an
unusually high number of implantations in the control group (9.8,
6.1, 7.2 and 7.8 implantations per doe in the control, 0.1, 0.25 and
0.5 mg/kg bw/day treatment groups, respectively). There was no clear
dose-effect relationship. There were also no increases in
post-implantation losses (1.1, 1.1, 0.7 and 1.7 post-implantation
losses for the control, 0.1, 0.25 and 0.5 mg/kg bw/day treatment
groups, respectively) these figures falling within the historical
control range. There was no meaningful difference in any of the
other developmental parameters nor in the number of developmental
variations or malfunctions between the control and treatment groups.
Aldicarb did not produce a teratogenic response when administered
orally to rabbits by gavage at dosage levels up to 0.5 mg/kg bw/day
(Leng et al., 1983).
Special studies on genotoxicity
Results of genotoxicity tests are summarised in Tables 3 and 4
(for in vitro and in vivo tests with aldicarb) and Table 5 (for
in vitro tests with aldicarb metabolites).
Table 3: Results of in vitro genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980c)
TA98,TA100,TA1535,
TA1537,TA1538
Reverse E. coli WP2 nk nk Negative Dunkel et al. (1985)
mutation assay
Reverse S. cerevisiae nk nk Negative Guerzoni et al. (1976)
mutation assay
HGPRT forward CHO cells 1000-5000 µg/ml nk Negative Stankowski et al. (1985a)
mutation assay
SCE Human nk nk Weak Debuyst & Larebeke (1983)
mutation assay lymphocytes Positive
Cytogenetics Human 10-250 µg/ml nk Weak Cid & Matos (1984)
mutation assay lymphocytes Positive
UDS Rat hepatocytes 0.16-5000 µg/ml nk Negative Godek et al. (1984a)
DNA damage S. typhimurium > 500 µg/disc nk Positive Rashid & Mumma (1986)
TA 1538 uvrB
TA 197
nk: not known
Table 4: Results of in vivo genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Micronucleus Mouse 0.001-0.01 93.47% Negative Ivett et al. (1984)
test mg/kg bw
Micronucleus ICR mouse 0.1-0.4 99.7% Negative Ivett (1990)
test mg/kg bw
Dominant Wistar rat 0.2-0.7 99.2% Negative Weil & Carpenter (1974a)
lethal test mg/kg bw
Table 5: Results of in vitro genotoxicity assays on aldicarb metabolites
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980b)
TA98,TA100,TA1535, sulphoxide
TA1537, TA1538
Ames test S. typhimurium 100-10000 µg/plate nk Negative Godek et al. (1980a)
TA98,TA100,TA1535, sulphone
TA1537, TA1538
HGPRT forward CHO cells 500-1500 µg/ml nk Negative Stankowski et al. (1985b)
mutation assay sulphone
Cytogenetics CHO cells 50-500 µg/ml nk Negative San Sebastian et al. (1984)
mutation assay sulphone
UDS Rat hepatocytes 0.1-3000 µg/ml nk Negative Godek et al. (1984b)
sulphone
nk: not known
Special studies on skin and eye irritation and sensitization
Administration of aldicarb to the conjunctival sac of rabbits
did not produce ocular irritation or corneal damage. Ocular
irritation studies were performed at doses that were lethal without
indication of ocular damage. Furthermore, there was no evidence of
dermal irritation when aldicarb was applied to the shaved, abraded
backs of rabbits (Striegel & Carpenter, 1962).
There was no indication of a sensitization reaction induced by
aldicarb. Male guinea-pigs were administered aldicarb by multiple
subdermal applications (0.7 mg/kg body-weight) and re-administered
aldicarb three weeks later by a similar intradermal administration.
There was no suggestion of sensitization in any of the animals
tested (Pozzani & Carpenter, 1968a).
Skin sensitization to aldicarb sulfone (UC 21865, technical) or
a 75% WP formulation was evaluated in albino guinea-pigs (Hartley
Strain) using a modified Landsteiner technique. Neither substance
was determined to be a sensitizer under the conditions of this study
(Conroy & Carpenter, 1977).
Special studies on delayed neurotoxicity
Hens
- Aldicarb
Groups of 6 adult chickens were administered aldicarb as a
single oral dose of 4.5 mg/kg bw or as daily oral doses of 0, 2.25
or 4.5 mg/kg bw/day for 30 days. A positive control group, treated
with 100 mg of TOCP, was used to produce typical delayed neurotoxic
signs of poisoning. While there was some weight loss, which was
correlated with the dose of aldicarb administered, the only
neurological effects attributable to aldicarb were acute signs of
poisoning noted in the first two or three days of treatment. Neither
ataxia nor hind limb paralysis were noted over the course of the
study. Aldicarb did not induce a delayed neurotoxic syndrome similar
to that induced by certain organophosphate esters (Johnson &
Carpenter, 1966a).
Hens
- Aldicarb metabolites
A group of 40 adult white Leghorn hens were intubated to
receive 250 mg/kg bw aldicarb sulfone suspended in maize oil. Two
other groups, each with 10 hens, received either TOCP (500 mg/kg bw)
or maize oil alone, and served as positive and negative control
groups respectively. There were no neurological effects other than
acute cholinergic signs of poisoning in the TOCP dosed group. No
histological examination was performed, owing to the lack of
demonstrated neurotoxic signs. Aldicarb sulfone did not cause
delayed neurotoxic reactions under the conditions of the study
(Babish & Salerno, 1977).
Special studies on behaviour
The effects of acute administration of aldicarb and aldicarb
sulfoxide on avoidance behaviour in rats was compared to a variety
of other carbamate esters. Rats were trained and evaluated for their
ability to avoid electrical shock in standard avoidance behaviour
tests. Aldicarb and aldicarb sulfoxide were administered by
intraperitoneal injection and the rats were evaluated for their
ablity to avoid shocks over a 6 h period following administration.
The lowest beviourally effective dose was found to be 0.266 mg/kg bw
which, when compared to the acute ip LD50 value, was noted to have
a smaller ratio of behaviour effects to acute LD50 than any of the
other carbamates tested. These data suggest that the level of
aldicarb needed to produce measurable avoidance is greater (closer
to a fatal dose and less likely to be achieved at the suggested use
level) than the chemicals to which it was compared. Additionally,
the activity over the 6-h period was seen to rapidly decline again
attesting to the transient nature of the cholinesterase inhibition
(Johnson & Carpenter, 1966b).
Special studies on immune responses
Aldicarb was evaluated for its ability to modulate the immune
response in two strains of mice. The B6C3F1 mouse was chosen
because it is the strain used by the US National Toxicology Program
for immunotoxicology studies, and the hybrid Swiss Webster mouse was
included because aldicarb had been reported to suppress the splenic
plaque-forming cell response to sheep red blood cells in an inverse
dose-response fashion (Olson et al., 1987). An attempt was made to
reproduce these findings and to expand the data base using more
standardised techniques with the B6C3F1 strain. Aldicarb was
administered in the drinking water ad libitum for 34 consecutive
days to female mice of both strains at dosages ranging from 0.1 to
1000 µg/litre in 10-fold increments (equivalent to 0.04 to 364 mg/kg
bw/day). Aldicarb had no effects on body-weights or organ weights,
on numbers or types of circulating white blood cells, or on the
microscopic pathology of the thymus, spleen, liver, kidneys, or
lymph nodes. Also unaffected were the number of antibody forming
cells in the spleen and the amount of circulating antibody in the
blood. Aldicarb had no effect in either strain on in vivo host
resistance to infectious viral challenge, on the capacity of B- and
T-lymphocytes to respond to nonspecific mitogens, or on the ability
of T-lymphocytes to recognize genetically different cell types in a
mixed lymphocyte culture. In this study, subchronic exposure to
aldicarb in the drinking water of mice had no effect on any measured
immunological function or toxicological parameter (Thomas et al.,
1987).
In another study, adult female B6C3F1, mice received
distilled water only or water containing 1.0, 10 or 100 µg/litre of
aldicarb daily for 34 days. To further develop an immune profile of
the compound, following aldicarb exposure, the ability of splenic
natural killer (NK) cells as well as specifically sensitized
cytotoxic T-lymphocytes to lyse YAC-1 lymphoma and, P815 tumour
cells, respectively, was evaluated. To complement the functional
assays, the impact of aldicarb exposure on spleen cell viability,
splenic cellularity and the percentages and absolute numbers of
total T-cells, T-suppressor, T-helper and B-cells was evaluated. The
results of this study demonstrated that aldicarb did not impact upon
the functional ability of interferon-induced splenic NK cells to
lyse YAC-1 lymphoma target cells. Aldicarb had no effect on the
functional capacity of cytotoxic T-lymphocytes to recognize
allogeneic cells in vivo and undergo differentiation and lysis of
these cells in a short-term 51-Cr release assay. The numbers and
percentages of T-cell subpopulations and B-cells in the spleen also
were not significantly affected. In addition, aldicarb had no
significant effect on body-weights, on spleen cellularity or cell
viability or on absolute weights of lymphoid organs. The absence of
statistically significant effects on any of these parameters
indicated that aldicarb did not have adverse effects on the immune
system of mice (Thomas et al., 1990).
Special studies on pesticide antagonistic agents
Following acute oral administration to rats, aldicarb has been
shown to induce a strong muscarinic action at excretory, bronchial
and cardiac nerve sites. A nicotinic effect was also shown to occur
at myoneural junctions. The parasympathetic signs of poisoning were
readily reduced following atropine administration. Administration of
atropine and 2-PAM, alone, or in combination, showed that while
atropine was a more effective antidote, 2-PAM was also active. While
it has been shown that aldicarb elicits a strong muscarinic action
as well as nicotinic action at myoneural sites, the control of signs
of poisoning from both mechanisms appears to be somewhat difficult
to achieve. Atropine has been shown to be an effective antidote to
block the muscarinic effects, but decamethonium, commonly used to
block the nicotinic effects, has been shown to be somewhat
ineffective. Additional studies to influence the nicotinic actions
by such drugs as tubocurare also failed to completely eliminate
nicotinic activity. Further studies confirmed the therapeutic
effects of a variety of oximes (P2S and obidoxime) in reducing the
acute toxic signs of poisoning associated with aldicarb. (Johnson &
Sullivan, 1968a,b; Natoff & Reiff, 1973).
Special studies on pesticide interactions
Aldicarb was administered orally to male rats alone and in
combination with a series of eight organophosphate esters or one
carbamate ester, all anti-cholinesterase agents, to examine the
potential interactive or additive effects. Results of the study,
using proportions of the acute lethal dose of each material alone
and in combination with aldicarb, showed a simple additive effect
with all materials tested. Aldicarb was not found to potentiate the
acute oral toxicity of other anticholinesterase agents. Further
studies were reported on the potential interaction of aldicarb with
alpha-naphthol, aldicarb sulfoxide with aldicarb sulfone and
aldicarb sulfone with parathion administered orally and aldicarb
with alpha-naphthol or with carbaryl administered by the
intraperitoneal route. In no case were any interactions greater tban
the predicted additive effects (West & Carpenter, 1966a; Weil &
Carpenter, 1970a).
Observations in humans
Groups of 4 adult male volunteers were administered aldicarb
orally in aqueous solution at dose levels of 0.025, 0.05 or 0.1
mg/kg body-weight. Clinical signs of poisoning were recorded and
whole blood cholinesterase activity was measured up to 6 h after
administration of the sample. Total urine voided was collected and
aldicarb-excretion patterns for the initial 8 h after dosing were
evaluated. In addition, spot samples were taken at 12 and 24 h.
Acute signs of poisoning, typical of anticholinesterase agents, were
observed at the high-dose level within 1 h after administration of
aldicarb. There were no signs of poisoning observed at the 0.05
mg/kg body-weight dose level. Cholinesterase depression was observed
in all volunteers prodominantly within 1-2 h after treatment. Within
the first six hours of treatment almost all cholinesterase
depression and clinical signs of poisoning were diminished.
Examination of urinary excretion patterns showed that approximately
10% of the administered dose was excreted as carbamates (toxic
residues) within the first 8 h interval. Cholinesterase analyses
confimed the same rapid inhibition and recovery pattern with man as
had been observed in experimental animals (Haines, 1971).
In another study, two additional subjects were administered
aldicarb in water solution at dose levels of 0.05 and 0.26 mg/kg
body-weight. Acute signs of poisoning were recorded at the higher-
dose level and atropine was administered to aid recovery. No signs
of poisoning were recorded with the lower-dose level. Urinary
excretion of carbamate residues within 24 h accounted for
approximately 10% of the administered dose (Cope & Romine, 1973).
A double blind, placebo controlled study has been conducted, in
which aldicarb was given as a single oral dose to healthy male and
female subjects. The doses administered were: placebo (22 subjects -
16 males and 6 females), 0.01 mg/kg bw (8 males), 0.025 mg/kg bw (8
males and 4 females), 0.05 mg/kg bw (8 males and 4 females) and
0.075 mg/kg bw (4 males). Subjects were screened before entry by
general medical history and examination and laboratory tests
including haematology, clinical chemistry and urinalysis. Clinical
measurements were made at intervals before and after dosing. These
included vital signs, (systolic and diastolic blood pressure, pulse
rate), pulmonary function tests, pupil size, electrocardiographs,
salivation and clinical signs of nausea, vomiting, diarrhoea,
sweating, abdominal cramps, involuntary movement and slurred speech.
Samples were taken for urinalysis, clinical chemistry (including red
blood cell and plasma cholinesterase activity) and haematology
evaluation before and after dosing. Urine and blood were collected
for aldicarb analysis. The results of these latter analyses were not
reported. There were no clinically significant changes in vital
signs, pupil size, pulmonary function, ECG's, salivation, clinical
signs, clinical chemistry (apart from cholinesterase), haematology
or urinalysis in the study. Cholinesterase activity in red cells and
plasma was maximally depressed at one hour after dosing and had
recovered by 8 h in all subjects. The fall in activity was dose-
related. Only marginal depression of cholinesterase activity (< 20%)
was seen in erythrocytes from patients treated with 0.01 or 0.025
mg/kg bw and in plasma at 0.01 mg/kg bw. Depression in
cholinesterase activity > 20% was seen in erythrocytes at 0.05 and
0.075 mg/kg bw and in plasma at 0.025, 0.05 and 0.075 mg/kg bw. No
serious adverse events occurred. The minor adverse events which were
recorded were similar to those reported in other volunteer studies.
Only one subject (0.075 mg/kg bw group, actual dose 0.06 mg/kg bw)
developed symptoms which were reported to be related to aldicarb.
The NOAEL in this study should be based on depression (> 20%) in
erythrocyte cholinesterase activity and was thus 0.025 mg/kg bw
(Nimmo et al., 1992).
Fifteen male volunteers participated in a study in Panama to
evaluate human exposure in a banana plantation where Temik-15G was
applied under natural conditions. Temperatures ranged from 24 °C to
32 °C with 80% to 90% relative humidity. The workers used three
different types of hand-held applicators. Blood samples were taken
from all volunteers prior to initiating the test and immediately
following each 6 h working period for estimation of blood
cholinesterase activity. Samples were obtained in the field and
analyzed 1-2 h later in the laboratory. Only two workers showed
greater than 25% reduction in their blood cholinesterase activity
(29% and 50%). Spontaneous reversibility was evident in both cases.
The worker with 50% reduction showed 25% recovery within 3 h. Blood
samples from the other worker indicated 100% recovery 24 h later.
Results of blood analyses indicated that cholinesterase activity was
below the normal range (population) in samples collected from six
workers during the second day of the study. Cholinesterase activity
in all samples taken during the first and third day of the study
were within the normal range. No clinical signs of aldicarb-induced
intoxication were found, although one individual presented symptoms
of nausea, stomachache and headache (Union Carbide, 1979).
A series of human exposure episodes was reported occurring as a
result of a variety of field and glasshouse conditions in an effort
to assess the potential for human harm from exposure under actual
occupational conditions. In several instances, slight blood
cholinesterase depression attested to the actual exposure situation.
Exposure data, as indicated by cholinesterase depression or urinary
excretion, suggested that there was no change in the general health
of workers exposed under any of the working conditions. Although
there were acute clinical signs of poisoning there was no indication
that the workers exposed were harmed once removed from exposure
situation (Williams, 1966; Burrows et al., 1970; Wakefield et
al., 1973; Shrivastava, 1975; Pandey, 1977).
From 1966 to 1979, 133 cases of apparent over-exposure to
aldicarb formulations were reported. Of these cases, 40 were
confirmed aldicarb poisoning episodes where clinical diagnosis
and/or urinalysis for aldicarb and its metabolites were performed.
As has been the case with other carbamate insecticides, the acute
signs of toxicity are rapidly dissipated, although atropine therapy
and hospitalization have been useful therapeutic regimens (Abdalla,
1977, 1979). Several deaths have been reported, but all of these
have been attributed to suicide or gross neglect (Lee, 1984).
California reported that in 1974, 1975 and 1976 a total of 10,
14 and 13 cases of human illnesses were reported, respectively. In
these incidents, people were directly exposed to aldicarb and
illness was brought on by dermal, inhalation and in one instance,
ocular exposure. While most illnesses resulted from aldicarb
exposure in loading or applying the formulated pesticide, some
illness has been reported from the handling of plants and soils
treated with aldicarb (Peoples et al., 1977).
Several misuses of aldicarb on watermelons and cucumbers have
been reported in the literature. Goes et al. (1980) reported on a
suspected food-borne intoxication in Nebraska associated with the
consumption of aldicarb-contaminated hydroponically grown cucumbers.
Two episodes were reported (April 1977: 9 cases; July 1978: 5
cases). Neither cholinesterase determinations nor urine residue
determinations were performed in any of these patients. Four
cucumbers (two in the supermarket and two in the greenhouse were
analyzed and aldicarb content was 6.6 and 10.7 mg/kg (supermarket) 0
and 9.9 mg/kg (greenhouse). No data are available on cucumbers
actually eaten and no attempts to correlate symptoms and aldicarb
consumption were made.
Hirsch et al. (1987) reported over 300 alleged intoxications
in Canada following ingestion of aldicarb contaminated cucumbers
(residue levels up to 26 mg/kg). In only five cases involving 13
patients, were any remaining portion of the cucumbers consumed still
available for analyses.
Goldman (1990) reported on four outbreaks of aldicarb poisoning
having occurred in California and other states in the USA from 1978
to 1988.
Outbreak 1: watermelons, 1985
Outbreak 2: watermelons, 1987
Outbreak 3: cucumbers, 1988
Outbreak 4: cucumbers, 1978 (corresponds to Goes (1980)
publication).
In the first outbreak, 1376 people allegedly became ill due to
consumption of contaminated watermelons. Of this total, 308 were
considered by the authors themselves as unlikely (235) or incomplete
(73). Nevertheless, only 28 cases were supported by positive
findings of aldicarb residues in the food commodity. All the other
cases were only supported by self-diagnosis.
An epidemiological study of potential adverse health effects of
aldicarb metabolites in drinking water was performed. In two surveys
conducted on Long Island, New York, the major cohorts were selected
on the basis of aldicarb levels in their drinking water. Levels of
contamination were generally 4-12 µg/litre, but a maximum of 400
µg/litre was reported. Questionnaires to determine water and food
consumption, symptoms experienced, and diagnosed illnesses were sent
to 1035 residents of 462 households. Although the initial survey
indicated a possible association between the incidence of diarrhoea
and levels of aldicarb in the water, the follow-up study, focusing
on children, did not confirm this association. No relationship was
detected between food consumption or water source and adverse health
symptoms, and self-reported physician-diagnosed illnesses for
1974-1979 were not significantly related to levels of aldicarb in
the water (Whitlock et al., 1982).
Another survey attempted to relate self-reported symptoms
suggestive of peripheral neuropathy to aldicarb levels in drinking
water in Suffolk County, New York. The response rate was less than
20%. Responses were classified as "probably", "possibly", or
"vaguely" suggestive of a neurologic syndrome. A significant
correlation with aldicarb concentration was obtained only by
combining all three categories of response, including reports of
just one symptom or of symptoms not forming any cohesive syndrome.
The authors concluded that further study was needed (Sterman &
Varma, 1983).
A pilot epidemiological study by Fiore et al. (1986)
evaluated a wide range of clinical immunological parameters in 23
women exposed to aldicarb in their drinking water and in a
non-exposed control group. The groups did not differ in any
immunological parameters except in T8-Lymphocytes: the number was
considered as elevated in five exposed and in one control.
Observation of an elevated stimulation assay response to one of the
large number of antigens (Candida) was not considered as
toxicologically significant, nor was it attributed to aldicarb
exposure.
In a follow-up study, five women of the preceeding group and
still exposed to aldicarb were examined versus previous control
women plus women previously exposed but for whom exposure no longer
occurred (e.g., change in water supply due to addition of a charcoal
filter). According to the authors, this follow-up study confirmed
the previous results, except for increased response to Candida
antigens which was no longer present (Mirkin et al., 1990).
COMMENTS
Aldicarb is rapidly absorbed, widely distributed in the body
and rapidly excreted. Metabolism appears to be similar in all
species studied, aldicarb being rapidly metabolised to aldicarb
sulfoxide, which is more slowly degraded to aldicarb sulfone. All
metabolites are quickly eliminated from the body, 80-90% being
excreted within 24 h. Elimination was complete by the fifth day
after dosing and no bio-accumulation was seen.
Aldicarb has high acute toxicity in a wide variety of mammalian
species. Signs of toxicity are those commonly associated with
acetylcholinesterase inhibition by a carbamate insecticide:
cholinergic signs of poisoning, which are alleviated rapidly on
cessation of exposure. Aldicarb sulfoxide is a more potent inhibitor
of acetylcholinesterase than aldicarb itself, while aldicarb sulfone
is considerably less toxic than either aldicarb or the sulfoxide.
WHO has classified aldicarb as extremely hazardous (WHO, 1992).
Short-term and long-term studies have been performed in rats,
mice and dogs with aldicarb and aldicarb metabolites, both
individually and in combination. Toxicity tests employing mixtures
of aldicarb or aldicarb sulfoxide with aldicarb sulfone are of
interest because aldicarb sulfoxide and aldicarb sulfone are the
terminal residues potentially consumed by humans. Cholinesterase
depression is the most significant indicator of toxicity that can be
evaluated. However, considerable attention must be paid to the
methods of sample collection and determination of cholinesterase
activity. Continuous administration of aldicarb to the test animals
until collection of samples for analysis is important, as is rapid
analysis under carefully controlled conditions.
No-effect levels in the various studies which included
evaluation of cholinesterase inhibition are summarized in Table 6.
No distinction is made in this table for the methods of determining
cholinesterase activity.
It is now considered inappropriate to use no-adverse-effect
levels from many of the earlier repeat-dose studies for the
derivation of an ADI, because animals were not dosed for 24-48 h
prior to collection of tissue samples for measurement of
cholinesterase activity. In the most recent dog studies, which were
conducted in a manner designed to maximize detection of
cholinesterase depression, the overall NOEL was 0.02-0.03 mg/kg
bw/day, but the NOAEL (which discounts inhibition of plasma
cholinesterase only) was 0.05-0.06 mg/kg bw/day.
Results of repeat-dose studies with aldicarb demonstrate that
the method of administering the test material to the test animals
can greatly modify the apparent toxicity of aldicarb and its
metabolites. Mice, rats and dogs have tolerated daily doses equal to
the LD50 incorporated into the diet for 7 days to 2 years. Doses
which caused death in less than 2 h when administered as a bolus,
caused no death and only moderate cholinesterase depression when
given in the diet.
Two dietary carcinogenicity studies have been conducted with
aldicarb in rats and three in mice. A dermal carcinogenicity study
has also been conducted with aldicarb in mice and dietary studies
have been carried out with aldicarb sulfone in mice and aldicarb
sulfoxide in rats. Aldicarb was not carcinogenic in mice and rats.
Two reproduction studies have been conducted in rats with
aldicarb and one with aldicarb sulfone. There were no effects on
reproductive performance at doses up to 0.7 mg/kg bw/day aldicarb or
9.6 mg/kg bw/day aldicarb sulfone. Aldicarb did not display any
teratogenic potential in rats or rabbits in studies which included
maternally toxic doses.
After reviewing the available genotoxicity data, the meeting
concluded that aldicarb, aldicarb sulfoxide and aldicarb sulfone are
not genotoxic.
In a range of special studies in animals (involving delayed
neurotoxicity, behaviour, antagonistic agents and pesticide
interactions) aldicarb displayed no results which gave cause for
concern. There was no evidence of immuno-toxicity in mice in a
number of functional assays of cell-mediated immunity and in host
resistance to respiratory infection.
Epidemiological studies provided no convincing evidence that
aldicarb could significantly alter immunological function in humans.
In addition to the above epidemiological studies, studies
conducted in 1982 and 1983 attempted to correlate any potential
adverse health effects with the occurrence of aldicarb in drinking
water. Although the authors concluded that further study was needed,
there was no clear evidence that aldicarb contamination of drinking
water generally at concentrations of about 4-12 µg/litre, but at a
maximum concentration of 400 µg/litre was related to any health
effects.
The anticholinesterase potential of aldicarb has been
extensively investigated in human. These studies revealed the same
pattern of rapid cholinesterase inhibition and rapid recovery seen
in experimental animals. Transient erythrocyte cholinesterase
depression was seen at single doses of 0.05 mg/kg bw, and the NOAEL
for cholinesterase depression (discounting changes in plasma enzyme
activity) was 0.025 mg/kg bw.
A number of poisoning incidents have been reported in the
agricultural use of aldicarb, but there has been no indication that
the workers exposed were harmed once removed from the exposure
source. Although several deaths have been reported, all of these
have been attributed to suicide or gross neglect.
A number of food-borne aldicarb intoxications have been
reported in the literature. These have all been associated with
misuse and reliable quantification of the dose of aldicarb involved
has always proved difficult, if not impossible.
An ADI was allocated using a 10-fold safety factor applied to
the NOAEL for depression of erythrocyte cholinesterase activity in
human volunteers.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg bw/day (93-day dietary study)
Dog: 0.05 mg/kg bw/day (52-week study)
Human: 0.025 mg/kg bw (double-blind, placebo controlled
volunteer study)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans, including information regarding
the correlation of blood cholinesterase depression and clinical
signs and symptoms.
Table 6: Summary of no effect levels in repeat dose toxicity studies which included
cholinesterase investigations
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Mouse 7 days Aldicarb 0,0.1,0.3,0.6,1.2 0.6 Mortality
Mouse 7 days Aldicarb: 0,2,6,18,36 2 Kidney and liver weight
A. sulfone reduction, growth
1:1 depression
Rat 7 days Aldicarb 0,4,8,16 NE Mortality, growth
depression, kidney and
liver weight reduction
Rat 7 days Aldicarb 0,0.8,1.6,3.2 NE Growth depression,
kidney and liver weight
reduction
Rat 93 days Aldicarb 0,0.02,0.1,0.5 0.1 Mortality, growth
depression
Rat 2 years Aldicarb 0,0.005,0.025, >0.1 No effects seen
0.05,0.1
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Rat 7 days Aldicarb 0,0.4,0.8,1.6,3.2 0.8 Growth depression,
A. sulfoxide 0,0.4,0.8 0.4 RBC cholinesterase
A. sulfone 0,0.4,1.0,2.5,5,20 2.5 depression
Rat 29 days A. sulfoxide: 0,0.0074,0.03, 0.4 Growth depression,
A. sulfone 0.12,0.47,1.67 in brain, plasma and RBC
1:1 drinking water cholinesterase depression
Rat 6 months A. sulfoxide 0,0.125,0.25,0.5,1 0.125 Growth depression,
brain, plasma and RBC
cholinesterase depression
Rat 6 months A. sulfoxide 0,0.0625,0.125, 0.125 Plasma and RBC
0.25,0.5,1.0 cholinesterase depression
Rat 6 months A. sulfone 0,0.2,0.6,1.8, 0.6 Brain, plasma and RBC
5.4,15.2 cholinesterase depression
Rat 7 days A. oxime 0,31.25,62.5,125, 62.5 Growth depression,
250,500,1000 minor liver and kidney
changes
Dog 7 days Aldicarb 0,0.2,0.3,0.7 >0.7 No effects seen
Dog 100 days Aldicarb 0,0.2,0.3,0.7 0.3 Minor organ weight
changes
Dog 2 years Aldicarb 0,0.025,0.05 >0.1 No effects seen
0.1
Dog 2 weeks Aldicarb 0,0.022,0.068, NE Brain, plasma and RBC
cholinesterase
depression
0.192,0.609,1.42
Dog 2 weeks Aldicarb 0,0.003,0.008, 0.096 Brain, plasma and RBC
cholinesterase depression
0.027,0.096,0.28
Dog 5 weeks Aldicarb 0,0.012,0.024, >0.06 No effects seen
0.06
Dog 52 weeks Aldicarb 0,0.027,0.054, 0.054 RBC and plasma
0.131,0.241 cholinesterase depression
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Dog 3 months A. sulfoxide 0,0.0625,0.125, 0.25 Transient growth
0.25,0.5 depression
Dog 3 months A. sulfone 0,0.2,0.6, >5.4 No effects seen
1.8,5.4
Dog 1 year A. sulfone 0,0.11,0.59, 0.11 RBC and plasma
2.25 cholinesterase depression
* mg/kg bw/day equivalence, by dietary administration, unless otherwise stated.
Lowest effect level underlined.
** NOAEL's were tabulated without evaluation of methods of sample collection or method of
determination of cholinesterase activity.
NE Not established
REFERENCES
Abdallah, N.A. (1977) Alleged overexposure cases reported from use
of Temik formulations - 1966 to 1976. Unpublished report from Union
Carbide Corporation.
Abdallah, N.A. (1979) Alleged human overexposure cases reported from
use of Temik formulations - 1976 to 1979. Unpublished report from
Union Carbide Corporation.
Aldridge, D., Schardein, J.L., & Blair, M. (1983) Aldicarb
range-finding teratology study in rabbits. Unpublished report from
International Research and Development Corporation, submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Andrawes, N.R. (1977) The metabolism of (UC 21865) Sulfocarb
pesticide in the rat. Unpublished report from Union Carbide
Corporation.
Andrawes, N.R., Dorough, H.W., & Lindquist, D.A. (1967) degradation
and elimination of Temik in rats. J. Econ. Ent., 60(4): 979-987.
Andrawes, N.R. & Lee R.E. (1986) Temik brand aldicarb pesticide,
metabolism in lactating goats. Unpublished report from Union Carbide
Agricultural Products Company Inc.
Babish, J.G. & Salerno, A. (1977) Neurotoxicity evaluation of UC
21865 in white Leghorn hens (Gallus domesticus). Unpublished
report from Food and Drug Research Laboratories submitted to WHO by
Union Carbide Corporation.
Bull, D.L., Lindquist, D.A., & Coppedge, J.R. (1967) Metabolism of
2-methyl-2-(methylthio)propionaldehyde O-(methylcarbamoyl) oxime
(Temik, US-21149) in insects. J. Agric. Food Chem., 15(4):
610-616.
Burrows, I.E., Haynes, G., & Roberts, N. (1970) Determination of
exposure levels in operators to Temik during field trials.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Carpenter, C.P. (1963) Comparison of the acute toxicity of compound
21149 with several of its analogues. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Carpenter, C.P. (1969) Miscellaneous toxicity studies. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Carpenter, C.P. and Smyth, H.F. (1966) Temik l0G (10.5% Granular
formulation of compound 21149). 15-Day dermal applications to
rabbits. Unpublished Report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Chin, G.H. & Sullivan, L.J. (1968) Some biochemical considerations
of Temik pesticide. Unpublished report from Mellon Institute,
presented to the 156th ACS National Meeting, submitted to WHO by
Union Carbide Corporation.
Cid, M.G. & Matos, E. (1984) Induction of sister-chromatid exchanges
in cultured human lymphocytes by aldicarb, a carbamate pesticide.
Mutat. Res., 138: 175-179.
Coate, W.B., Hardy, R.J., Alsaker, R.D., Dawkins, B.G., Hepner,
K.E., Banos, D.A., & Mense, M.A. (1982) Subacute inhalation toxicity
study of a pesticide residue in dogs. T3, T6 and T10 type
cigarettes. Unpublished report from Hazleton Laboratories America
Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Debuyst, B. & Van Larebeke, N. (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242-243.
Dorough, H.W., Davis, R.B., & Ivie, G.W. (1970) Fate of
Temik-Carbon-14 in lactating cows during a 14-day feeding period.
J. Agr. Food Chem., 18: 135-142
Dorough, H.W. & Ivie, G.W. (1968) Temik-S-35 metabolism in a
lactating cow. J. Agric. Food Chem., 16(3): 460-464
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E. McGregor, D.,
Morteimans, K., Rosenkranz, H.S., & Simmon, V.F. (1985)
Reproducibility of microbial mutagenicity assays: II. Testing of
carcinogens and noncarcinogens in Salmonella typhimurium and
Escherichia coli. Environ. Mutagen., 7 (suppl 5): 1-248.
Field, W.E. (1979a) Acute dermal toxicity in rabbits. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Field, W.E. (1979b) Acute dermal toxicity in rats. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Fiore, M.C., Anderson, H.A., Hong, R., Golubjatnikov, R., Seiser,
J.E., Nordstrom, D., Hanrahan, L., & Belluck, D. (1986) Chronic
exposure to aldicarb-contaminated groundwater and human immune
function. Environ. Res., 41: 633-645.
Godek, E.G., Dolak, M.D., Naismith, R.W., & Matthews, R.J. (1980a)
Ames Salmonella microsome plate test. Aldicarb sulfone.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980b)
Ames Salmonella microsome plate test. Aldicarb sulfoxide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980c)
Ames Salmonella microsome plate test. TEMIK aldicarb pesticide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984a) Rat hepatocyte
primary culture/DNA repair test. Aldicarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984b) Rat hepatocyte
primary culture/DNA repair test. Aldoxycarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Goes, E.H., Savage, E.P., & Gibbons, G. (1980) Suspected foodborne
carbamate pesticide intoxications associated with ingestion of
hydroponic cucumbers. Am. J. Epidemiol., 111: 254-259.
Goldman, L.R., Beller, M., & Jackson, R.J. (1990) Aldicarb food
poisonings in California, 1985-1988: Toxicity estimates for humans.
Arch. Environ. Hlth., 45(3) 141-147.
Guerzoni, M.E., Del Cupolo, L. and Ponti, I. (1976) Attivita
mutagenica degli antiparassitari. Riv. Sci. Teen. Alim. Nutr.,
Urn. 6: 161-165.
Haines, R.G. (1971) Ingestion of aldicarb by human volunteers: a
controlled study of the effect of aldicarb on man. Unpublished
report from Union Carbide Corporation.
Hamada, N.N. (1987a) One-year chronic oral toxicity study in beagle
dogs with aldicarb sulphone technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1987b) Two-week dose range finding oral toxicity study
in beagle dogs with aldicarb technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1988) One-year chronic oral toxicity study in beagle
dogs with aldicarb technical. Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N. (1991) Subchronic toxicity study in beagle dogs with
aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Marshall,
P.M. (1985a) Two-week dose range finding study in beagle dogs -
Aldicarb sulphone (aldoxycarb). Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Williams,
L.D. (1985b) Two-week dose range finding study in beagle dogs -
Aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hicks B.W., Dorough, H.W., & Mehendale, H.M. (1972) Metabolism of
aldicarb pesticide in laying hens. J. Agric. Food Chem., 20(1):
151-156.
Hirsch, G.H., Mori, B.T., Morgan, G.B., Bennett, P.R., & Williams,
B.C. (1987) Report of illness caused by aldicarb-contaminated
cucumbers. Food Addit. Contam., 5: 155-160.
Ivett, J.L. (1990) Mutagenicity test on aldicarb technical in the
mouse bone marrow cytogenetic assay. Unpublished report from
Hazleton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Ivett, J.L., Myhr, B.C., & Lebowitz, H.D. (1984) Mutagenicity
evaluation of aldicarb technical 93.47% in the bone marrow
cytogenetic assay. Unpublished report from Litton Bionetics, Inc.
submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Johnson, H.E. & Carpenter, C.P (1966a) Temik (Technical grade
compound 21149). Demyelination potential in chickens. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Carpenter, C.P (1966b) Temik (technical grade
compound 21149). Comparative behavioural effect in rats. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Sullivan, L.J. (1968a) Studies on an effective
therapy for overdoses of temik, Temik sulfoxide and temik sulfone in
the rat. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Johnson, H.E. and Sullivan, L.J. (1968b) Temik (uc 21149) antidotal
therapy in rats following administration of multiple lethal doses.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Knaak, J.B., Tallant, M.J., & Sullivan, L.J. (1966) The metabolism
of 2-methyl-2-(methylthio)propionaldehyde o-(methylcarbamoyl) oxime
in the rat. J. Agric. Food Chem., 14(6): 573-578
Lee, M.H. & Ransdell, J.F. (1984) A farmworker death due to
pesticide toxicity: a case report. J. Toxicol. Environ. Health,
14: 239-246.
Leng, J.M., Schardein, J.L., & Blair, M. (1983) Aldicarb. Teratology
study in rabbits. Unpublished report from International Research and
Development Corporation submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Burk, J.,
Reynolds, H.T., & Osman, M.F. (1966) Metabolism of
2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyl) oxime in
Plant and Insect. J. Agric. Food Chem., 14(6): 579-584.
Mirkin, I.R., Anderson, H.A., Hanrahan, L., Hong, R., Golubjatnikov,
R., & Belluck, D. (1990) Changes in tlymphocyte distribution
associated with ingestion of aldicarb-contaminated drinking water: a
follow-up study. Environ. Res., 51: 35-50.
Mirro, E.J., DePass, L.R., & Frank, F.R. (1982) Twenty-nine day
water inclusion study in rats. Unpublished report from Bushy Run
Research Laboratory submitted to WHO by Union Carbide Corporation.
Natoff, I.L. & Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl.
Pharmacol., 25: 569-575.
NIH (1979) Bioassay of aldicarb for possible carcinogenicity. US
DHEW Pub.No. (NIH) 79-1391, 103 pages.
Nimmo, W.S., Wyld, P.J., Watson, C.E., & Watson, N (1992) A safety
and tolerability study of aldicarb at various dose levels in healthy
male and female volunteers. Unpublished report from Inveresk
Clinical Research, submitted to WHO by Rhône-Poulenc AGRO, Lyon,
France.
Nycum, J.S. & Carpenter, C.P. (1968a) Toxicity studies on the
probably "non-carbamate" plant metabolites of Temik. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Nycum, J.S. & Carpenter, C.P. (1968b) Toxicity studies on Temik and
related carbamates. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Olson, L.J., Erickson, B.J., Hinsdill, R.D., Wyman, J.A., Porter,
W.P., Binning, L.K., Bidgood, R.C., & Nordheim, E.V. (1987) Aldicarb
immunomodulation in mice: an inverse dose-response to parts per
billion levels in drinking water. Arch. environ. Contam. Toxicol.,
16: 433-439.
Pandey, S.Y. (1977) Human exposure study on Temik 10G. Unpublished
report from Union Carbide India Ltd., submitted to WHO by Union
Carbide Corporation.
Peoples, S.A., Maddy, K.T., & Smith, C.R. (1977) Human health
problems associated with Temik (Aldicarb) in California. Unpublished
report from California Department of Food and Agriculture submitted
to WHO by Union Carbide Corporation.
Pozzani, U.C. & Carpenter, C.P. (1968a) Sensitizing potential in
guinea-pigs as determined by a modified Landsteiner test.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21:
319-334.
Romine, R.R. (1973) Aldicarb pesticide. Aldicarb residues in milk
and meat of cows fed aldicarb sulfoxide and aldicarb sulfone in the
daily diet. Unpublished report from Union Carbide Corporation.
SanSebastian, J.R., Naismith, R.W., & Matthews, R.J. (1984) In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Aldoxycarb technical. Unpublished report from Pharmakon
Research International Inc. Submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Shrivastava, K.N. (1975) Determination of human exposure levels in
commercial application of ternik 10g on potatoes by gloved hand and
hand-operated applicators. Unpublished report from Union Carbide
India Ltd.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985a) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldicarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985b) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldoxycarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Sterman, A.B. & Varma, A. (1983) Evaluating human neurotoxicity of
the pesticide aldicarb: when man becomes the experimental animal.
Neurobehav. Toxicol. Teratol., 5: 493-495.
Striegel, J.A. & Carpenter, C.P. (1962) Range finding test on
compound 21149. Unpublished report from Mellon Institute of
Industrial Research submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. (1968a) The urinary excretion of C-14 equivalents of
Temik in the dog and their metabolic profile as revealed by silica
gel chromatography. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Sullivan, L.J (1968b) Urinary metabolic profiles as determined by
silica gel chromatography of urines from rats and dogs dosed with
Temik sulfone. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Sullivan, L.J (1968c) The excretion of C14 equivalents of Temik
sulfone by the rat after a single peroral dose. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. & Carpenter, C.P. (1974) Preliminary Metabolism of
14C-S-methyl aldicarb nitrile in the rat. Unpublished report from
Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Thomas, P.T., Ratajczak, H.V., Eisenberg, W.C., Furedz-Machacek, M.,
Ketels, K.V., & Barbera, P.W. (1987) Evaluation of host resistance
and immunity in mice exposed to the carbamate pesticide aldicarb.
Fund. Appl. Toxicol., 9: 82-89.
Thomas, P.T., Ratajczak, H.V., Demetral, D., Hagen, K., & Baron, R.
(1990) Aldicarb immunotoxicity: Functional analysis of cell-mediated
immunity and quantitation of lymphocyte sub-populations. Fund.
Appl. Toxicol., 15: 221-230.
Trutter, J.A. (1987a) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residues in bananas. Unpublished report
from Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Trutter, J.A. (1987b) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residue in watermelons. Unpublished
report from Hazleton Laboratories America Inc., submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Tyl, R.W. & Neeper-Bradley, T.L. (1988) Developmental toxicity
evaluation of aldicarb technical administered by gavage to CDO
(Sprague-Dawley) rats. Unpublished report from Bushy Run Research
Centre submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Union Carbide (1979) Volunteer worker's exposure monitoring study
(Panama). Unpublished Report from Union Carbide Corporation.
Wakefield, M., Orme, J.P.R., Ruston, E.A., & Andrews, L. (1973) The
detemination of levels of aldicarb in urine and face masks.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1963) Results of three months of
inclusion of Compound 21149 in the diet of rats. Unpublished Report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1964) Results of a three-generation
reproduction study on rats fed compound 21149 in their diet.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1965) Two-year feeding of compound
21149 in the diet of rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966a) Insecticide Temik. Teratogenic
potential in rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966b) Results of long-term tests for
mouse skin carcinogenicity of three process residues, one epoxide
and three compounds. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966c) Two-year feeding of compound
21149 in the diet of dogs. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968a) Temik 10G-V (10.3% granular
formulation of compound 21149). Acute and 14-day dermal applications
to rabbits. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968b) Temik sulfoxide. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968c) Temik sulfone. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1969a) Purified and technical temik.
Results of feeding in the diets of rats for one week. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1969b) 2-methyl-2-(methylsulfinyl)
propanol-1. Results of feeding in the diets of rats for one week.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970a) Temik and other materials.
Miscellaneous single dose peroral and parenteral LD50 assays and
some joint action studies. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970b) Temik, results of feeding in
the diets of rats for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970c) Temik (T) Temik sulfoxide
(TSO), Temik sulfone (ts02), 1:1 TSO-TS02. Results of feeding in the
diet of rats for 7 days. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970d) 1:1 Temik:Temik sulfone.
results of feeding in the diet of mice for 7 days. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1970e) Temik. Results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972a) Aldicarb (A), Aldicarb
sulfoxide (ASO), Aldicarb sulfone (AS02) and a 1:1 mixture of
ASO:AS02. Two-year feeding in the diet of rats. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972b) Miscellaneous toxicity studies.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972c) Aldicarb, 18-month feeding in
diet of mice. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1973) Aldicarb, seven-day inclusion in
diet of dogs. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974a) Aldicarb. Inclusion in the diet
of rats for three generations and a dominant lethal mutagenesis
test. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974b) Aldicarb Oxime (All). Results
of feeding in the diet of rats for 7 days. Unpublished report from
Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974c) Aldicarb. Inclusion in the
diets of dogs for three months. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974d) Aldicarb, 18-month feeding in
the diet of mice, study II. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974e) UC 21865; results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Cox, E.F. (1975) Aldicarb sulfoxide and aldicarb
sulfone cholinesterase inhibition results after periods of one to
fifty-six days of inclusion in the diets of rats. Unpublished report
from Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Weil, C.S., Conroy, W.J., Condra, N.I., & Cox, E.F. (1977) Aldicarb
sulphone UC 21865. 19-day rabbit skin inunction. Unpublished report
from Mellon institute submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1965) The single dose peroral toxicity
of compounds 20299, 21149, 19786 and 20047a for white Leghorn
cockerels. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1966a) Temik (Compound 21149,
Technical). Joint action with selected organic phosphate and
carbamate pesticides. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P.(1966b) Miscellaneous acute toxicity
data. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Whitlock, N.H., Schuman, S.H., & Loadholt, C.B. (1982) Executive
summary and epidemiologic survey of potential acute health effects
of aldicarb in drinking water - Suffolk County, N.Y. South Carolina
Pesticide Hazard Assessment Program Center, Medical University of
South Carolina, Charleston. Prepared for the Health Effects Branch,
Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency.
WHO (1966) Insecticide Evaluation and Testing Programme, Stage I,
Mammalian Toxicity Report (WHO). Unpublished report from Toxicology
Research Univ., Carshalton, U.K. submitted to WHO by Union Carbide
Corporation.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, F. (1966) Human exposure study in the field application of
Temik 10G on cotton. Unpublished report from Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977a) Inclusion in the
diet of rats for three generations (aldicarb sulphone), dominant
lethal mutagenesis and teratology studies. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977b) Aldicarb sulphone;
18-month feeding in the diet of mice. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Table 1: Acute toxicity of aldicarb
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Rat M oral corn oil 0.84 West & Carpenter (1966b)
Rat M oral corn oil 0.93 Striegel & Carpenter (1962)
Rat M oral corn oil 0.67-1.23 Carpenter (1963)
Nycum & Carpenter (1968b)
Rat F oral corn oil 0.62-1.07 Carpenter (1963)
Rat F oral gycerol:
ethanol 1.0 WHO (1966)
Rat F ip corn oil 0.71 Carpenter (1963)
Rat M+F ip corn oil 0.44 Carpenter (1963)
Rat M ip PEG 0.37-0.44 Weil & Carpenter (1970a)
Rat M ip ethanol 0.57 Johnson & Carpenter (1966b)
Rat M iv water 0.47 Weil & Carpenter (1970a)
Rat M dermal corn oil >10 Field (1979b)
Rat F dermal DMP 3.2-7.0 WHO (1966)
Rat M dermal water 38.1-44.9 Weil & Carpenter (1968a)
Mouse M oral corn oil 0.382 Weil & Carpenter (1972b)
Mouse M oral corn oil 0.5 Weil & Carpenter (1972c)
Mouse F oral cotton seed 1.5 Dorough et al. (1970)
oil
Mouse F ip cotton seed 0.3 Dorough et al. (1970)
oil
Guinea-pig oral corn oil 1.0 Nycum & Carpenter (1968b)
Rabbit oral propylene 1.3 Nycum & Carpenter (1968b)
glycol
Rabbit M dermal corn oil >10 Field (1979a)
Rabbit M dermal PEG 5.0 Striegel & Carpenter (1962)
Rabbit M dermal dry 141->200 Weil & Carpenter (1968a)
Chicken M oral 9 West & Carpenter (1965)
Table 2: Acute toxicity of aldicarb metabolites
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. nitrile rat oral 570 West & Carpenter (1966b)
A. sulfoxide rat (M) oral corn oil 0.49-1.13 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfone rat (M) oral corn oil 20-25 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfoxide mouse (M) oral corn oil 0.8-1.6 Nycum & Carpenter (1968b)
A. sulfone mouse (M) oral corn oil 25 Nycum & Carpenter (1968b)
A. sulfoxide guinea-pig oral corn oil 0.8-1.8 Nycum & Carpenter (1968b)
A. sulfone guinea-pig oral corn oil >50 Nycum & Carpenter (1968b)
A. sulfoxide rabbit oral corn oil 0.4-1.8 Nycum & Carpenter (1968b)
Table 2 (cont'd)
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. sulfone rabbit oral corn oil 75 Nycum & Carpenter (1968b)
A. sulfoxide rat (M) ip water 0.47 Weil & Carpenter (1970a)
A. sulfoxide rat (M) ip corn oil 0.71 Johnson & Carpenter (1966b)
A. sulfone rat (M) ip PEG 21.2 Weil & Carpenter (1970a)
A. sulfoxide rat (M) iv water 0.47 Weil & Carpenter (1970a)
A. sulfone rat (M) iv water 14.9 Weil & Carpenter (1970a)
A. sulfoxide rabbit dermal water >20 West & Carpenter (1966b)
A. sulfone rabbit dermal water >20 West & Carpenter (1966b)
2-methyl-2-
(methyl-sulfinyl)
propanol-1 rat oral 11 000 Weil & Carpenter (1969b)
OH-methyl A rat oral 42.9 Carpenter (1969)
A. sulfoxide oxime rat (M) oral 8060 Nycum & Carpenter (1968a)
A. sulfone oxime rat (M) oral 1590 Nycum & Carpenter (1968a)
A. sulfoxide nitrile rat (M) oral 4000 Nycum & Carpenter (1968a)
A. sulfone nitrile rat (M) oral 350 Nycum & Carpenter (1968a)
A: aldicarb
Mice
- Aldicarb/aldicarb metabolites
Groups of 3 male and 5 female mice were fed a mixture of
aldicarb and aldicarb sulfone (1:1) in the diet at dose levels of 0,
2, 6, 18, or 36 mg/kg bw/day for 7 days. No mortality was noted at
any dose level, but severe cholinergic signs of poisoning were
observed at the high-dose in males. Depression of growth was
observed at the two highest dose levels. Kidney weight was depressed
at the highest dose and liver weight was reduced substantially at 6
mg/kg bw/day and above in both males and females. The NOAEL in this
study was 2 mg/kg bw/day (Weil & Carpenter, 1970d)
Groups of 5 Charles River CD-1 mice/sex were fed aldicarb
sulfone in the diet at dose levels of 0, 0.15, 0.6, 2.4, 9.6 or 38.4
mg/kg bw/day for 7 days. Significant body-weight decrease was noted
at the highest dose but there were no significant organ weight
changes. Cholinesterase activity was not determined (Weil &
Carpenter, 1974e).
Rats
- Aldicarb
Groups of 5 rats/sex were fed aldicarb in the diet for 7 days
at dose levels of 0, 4, 8 or 16 mg/kg bw/day. Mortality was noted
predominantly at the highest dose at which all males and 2 of 5
females died. One of 5 males also died at 8 mg/kg bw/day. There was
a substantial reduction in body-weight gain noted at all dose
levels. In males, kidney weights were significantly reduced at 8
mg/kg bw/day and liver weight was depressed at 4 and 8 mg/kg bw/day.
In females, both liver and kidney weight was significantly depressed
at all dose levels (Weil & Carpenter, 1970b).
Groups of 5 rats/sex were fed aldicarb in the diet at dose
levels of 0, 0.8, 1.6 or 3.2 mg/kg bw/day for 7 days. Growth was
depressed at 1.6 mg/kg bw/day and above. There was no mortality
attributable to aldicarb. In males, liver weights were depressed in
all treatment groups. In females, liver weights were affected only
at the highest dose, but the liver to body-weight ratio was reduced
at 1.6 mg/kg bw/day and above. Kidney weights were reduced in males
at all dose levels and in females only at the highest dose.
Cholinesterase activity, measured on the day after the conclusion of
feeding, was normal at the highest dose tested with the exception of
plasma cholinesterase which was slightly reduced at the highest
level (Weil & Carpenter, 1969a).
Groups of 10 CFE rats/sex were fed aldicarb in their diet for
93 days at dose levels of 0, 0.02, 0.1 or 0.5 mg/kg bw/day. One rat
per level was killed for cholinesterase (plasma, RBC and brain)
determinations at 1, 2, 4 and 29 days of dosing. Mortality was
increased and body-weight and food consumption were decreased at the
highest level. Histopathology of selected organs was unremarkable
and there were no compound-related effects noted. There were no
consistent dose-related effects on the cholinesterase
determinations, except for plasma cholinesterase depression in both
sexes after 30 days at the highest level. There was no indication of
how soon after feeding the cholinesterase determinations were
performed, which could account for the sporadic results, together
with the small number of animals sampled at each interval. The NOAEL
in this study was 0.1 mg/kg bw/day (Weil & Carpenter, 1963).
Rats
- Aldicarb/aldicarb metabolites
Groups of 5 rats/sex were fed diets containing aldicarb at dose
levels of 0, 0.4, 0.8, 1.6 or 3.2 mg/kg bw/day, or aldicarb
sulfoxide at dose levels of 0, 0.4 or 0.8 mg/kg bw/day, or aldicarb
sulfone, at dose levels of 0, 0.4, 1.0, 2.5, 5 or 20 mg/kg bw/day,
for 7 days. With aldicarb (as with its two major carbamate
metabolites) there was a significant growth depression at the
highest dose. There were no effects noted with respect to liver or
kidney weights. The erythrocyte cholinesterase appeared to be the
most sensitive parameter with all three materials tested. The
NOAELs, based on erythrocyte cholinesterase depression or decreased
body-weight gain over the 7-day treatment period were: aldicarb -
0.8 mg/kg bw/day; aldicarb sulfoxide - 0.4 mg/kg bw/day; and
aldicarb sulfone - 2.5 mg/kg bw/day (Nycum & Carpenter, 1968b).
Groups of 5 rats/sex received in the diet, aldicarb (0.3 mg/kg
bw/day), aldicarb sulfoxide (0.4, 0.8 or 1.6 mg/kg bw/day), aldicarb
sulfone (0.6, 5 or 20 mg/kg bw/day), a 1:1 mixture of aldicarb
sulfoxide and aldicarb sulfone (1.2 mg/kg bw/day) or a control diet.
At the end of the 7 days of feeding, animals were placed on control
diets for one day after which they were sacrificed for
cholinesterase determination and for examination of liver and
kidney. A second one-week feeding trial was performed to compare the
data with other strains of rats. Aldicarb sulfoxide was fed to
groups of 5 male rats at dose levels of 0, 0.4, 0.8 or 1.6 mg/kg
bw/day and aldicarb sulfone was fed to male rats at dose levels of
0, 5 or 20 mg/kg bw/day. As might be expected with the protocol
followed in the study, cholinesterase depression was not observed in
either plasma, erythrocyte or brain at the conclusion of the study.
The second trial using both the same and a different strain of rats
was performed in an effort to explain a slight but non-significant
inhibition of erythrocyte cholinesterase activity measured in the
initial study. Over the course of this study, there were no effects
noted on erythrocyte cholinesterase activity (Weil & Carpenter,
1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.3 or 1.0 mg/kg bw/day and aldicarb sulfone at 0,
2.4 or 16.2 mg/kg bw/day. Animals were sacrificed at 1, 3, 7, 14, 28
and 56 days for cholinesterase analyses. Plasma and erythrocyte
cholinesterase activities were measured at the first three time
intervals. At the last three time intervals, plasma erythrocyte and
brain cholinesterase activities were examined. There was no
mortality, but growth was depressed at the highest dose levels with
both the sulfoxide and sulfone. Rats administered aldicarb sulfoxide
at 1.0 mg/kg bw/day had a slight but significant cholinesterase
depression during the study. There were no effects noted at 0.3
mg/kg bw/day. Cholinesterase depression was marked with the aldicarb
sulfone consistently throughout the study at the highest dose level
of 16.2 mg/kg bw/day (Weil & Cox, 1975).
Groups of 10 Wistar rats/sex were administered aldicarb
sulfoxide and aldicarb sulfone in a 1:1 ratio, in drinking water,
ad libitum, for 29 days. The drinking water levels were 0, 0.075,
0.3, 1.2, 4.8 or 19.2 mg/litre. Plasma and red blood cell
cholinesterase levels were determined after 8, 15 and 29 days of
treatment. Brain cholinesterase was measured at termination. Animals
were continuously exposed to the test substances in water until
blood samples were taken or sacrifice was performed. There was no
mortality and food and water consumption values together with body-
weight gains were significantly decreased in both sexes at the high-
dose level when compared to controls. Significant depression of
plasma, RBC and brain cholinesterase, in both sexes, occurred at the
highest dose level. The data demonstrate that a level of 4.8
mg/litre of aldicarb sulfoxide:aldicarb sulfone in water is without
effect on cholinesterase (plasma, RBC, brain) in either sex. Based
on the actual concentrations being approximately 80% of the nominal
concentrations, the NOAEL was roughly 3.8 mg/litre, equivalent to
0.4 mg/kg bw/day (Mirro et al., 1982).
Groups of 15 rats/sex were fed aldicarb sulfoxide in the diet
at dose levels of 0, 0.125, 0.25, 0.5 or 1 mg/kg bw/day for 6
months. Animals were sacrificed at 3 months and at the conclusion of
the study for cholinesterase determinations and for gross and
microscopic examination of liver and kidneys. There was no mortality
noted during the course of the study, although growth, especially in
males, at 0.25 mg/kg bw/day and above was reduced. Cholinesterase
activity was substantially reduced, at the three highest dose
levels, especially in plasma and erythrocytes of males. Gross
examination of liver and kidneys revealed no abnormalities
attributable to aldicarb. The NOAEL in this study was 0.125 mg/kg
bw/day (Weil & Carpenter, 1968b).
In an attempt to resolve the question of cholinesterase
depression and rapid recovery, groups of 5 rats/sex were fed
aldicarb sulfoxide for one week or one week plus one day of control
diets at a dose level of 1 mg/kg bw/day. When the study was
concluded (within one week), animals were sacrificed at 0 and 24 h
after the dietary feeding interval (the 24 h animals were fed
control diets). Cholinesterase depression was noted at the 0 h
sacrifice in erythrocyte and plasma preparations. Administration of
a control diet for one day (24 h sacrifice) completely reversed the
cholinesterase depression noted when animals were sacrificed without
any recovery interval (Weil & Carpenter, 1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.0625, 0.125, 0.25, 0.5 or 1.0 mg/kg bw/day for 3
and 6 months after which some of the animals were sacrificed
immediately and others were placed on a control diet for 24 h prior
to sacrifice and cholinesterase analysis. Cholinesterase activity in
the brain was unaffected by aldicarb sulfoxide. Plasma and
erythrocyte cholinesterase was substantially reduced at the 0 h
sacrifice in both sexes. Males were slightly more sensitive with
depression being noted at 0.25 mg/kg bw/day and above, while with
females depression was noted at 0.5 mg/kg bw/day and above. There
was no cholinesterase depression noted in any of the animals treated
for either 3 months or 6 months when the animals were allowed to
recover from cholinesterase depression for a one day recovery
interval. The NOAEL in this study was 0.125 mg/kg bw/day (Weil &
Carpenter, 1968b).
In a series of studies similar to those reported with aldicarb
sulfoxide, groups of rats were fed aldicarb sulfone at dietary
concentrations at dose levels of 0, 0.2, 0.6, 1.8, 5.4 or 15.2 mg/kg
bw/day for 6 months. Animals were sacrificed at 3 and 6 months for
examination of liver and kidney abnormalities and for evaluation of
cholinesterase activity. Cholinesterase determinations were made at
the end of the 3 and 6 month intervals with rats fed continuously
until sacrifice or rats were allowed to consume a control diet for
24 h prior to sacrifice and determination of cholinesterase
activity. There was no mortality over the course of the study.
Transient but significant growth depression was noted at the highest
level in the longer study. There were no effects noted with respect
to food consumption or on gross and microscopic examinations of
liver and kidneys. Plasma, erythrocyte and brain cholinesterase were
significantlv depressed at 5.4 mg/kg bw/day and above. Erythrocyte
cholinesterase depression was also noted at 1.8 mg/kg bw/day. There
was no cholinesterase depression noted at 0.6 mg/kg bw/day in any of
the tissues examined. In the study to evaluate recovery of
cholinesterase activity, when animals were allowed to equilibrate
for one day on control diets, all depressed cholinesterase values
returned to normal. The NOAEL in this study was 0.6 mg/kg bw/day
(Weil & Carpenter, 1968c).
Groups of rats were fed aldicarb oxime at dose levels of 0,
31.25, 62.5, 125, 250, 500 or 1000 mg/kg bw/day for 7 days. There
was no mortality over the course of the study. Growth was slightly
reduced at the initiation of the study at dose levels of 125 mg/kg
bw/day and above, but at the end of one week only the two highest
dose levels appeared to show a retardation in growth. Gross changes
were noted in both liver and kidneys at the two highest dose levels
in both males and females. The NOAEL in this study was 62.5 mg/kg
bw/day (Weil & Carpenter, 1974b).
Groups of 5 rats/sex were fed the hydrolytic metabolite of
aldicarb (2-methyl-2-(methylsulfinyl)propanol-1) for 7 days at dose
levels of 0, 500 or 1000 mg/kg bw/day. Growth was depressed at both
dose levels in males but only at the highest dose level in females.
In females, while growth was depressed within one day of treatment,
the animals appeared to recover during the rest of the week. There
was no apparent effect on major organs, although, in females at the
highest dose level, kidney weights were slightly depressed.
Cholinesterase activity was not measured (Weil & Carpenter, 1969b).
Rabbits
- Aldicarb
Four groups, each of 5 male rabbits with abraded skin, were
administered aldicarb 10G formulation at dose levels of 0, 5, 10 or
20 mg/kg bw/day, for 6 h per day, 5 days per week, for 15 days.
Water was added periodically during the exposure time to the
dressing containing the aldicarb treatment, simulating a condition
of excess perspiration. One additional group was administered 20
mg/kg bw/day to intact, unabraded skin with no water added to the
dressing. Animals treated with aldicarb under a dry condition with
unabraded skin showed normal weight gains and no apparent effects as
a result of the treatment. Administration of aldicarb under
conditions where the dressing was wet and the skin abraded resulted
in reduced body-weight at a dermal dose of 5 mg/kg bw/day and above.
During the interim where dermal treatment was not applied, recovery
of growth was extremely rapid. Plasma cholinesterase activity was
inhibited at the two highest dose levels. There were no other
adverse effects on haematology or clinical chemical parameters and
limited organ weight analysis and microscopic examination of several
major tissues showed no pathological effects attributable to
aldicarb. Almost identical results were also obtained in a similar
study in rats (Carpenter & Smyth, 1966; Weil & Carpenter, 1968a).
Rabbits
- Aldicarb metabolites
Aldicarb sulfone (technical and 75 WP) were administered to the
unabraded, but closely clipped, ventral surface of rabbits (6
males/group, 5 days/week for 19 applications) at dosages of 4.8
mg/kg bw/day for 75 WP and 3.5, 7.0 or 14.0 mg/kg bw/day for
technical aldicarb sulfone. No mortality, skin irritation or
cholinesterase depression were observed. However, rabbits were
removed from treatment 19 h prior to sampling for cholinesterase
analyses, which could account for the lack of cholinesterase
depression. Body-weight was significantly depressed in the high-dose
level rabbits. There were no reported treatment-related effects on
organ weights and necropsy findings were unremarkable (Weil et
al., 1977).
Dogs
- Aldicarb
Groups of 2 beagle dogs/sex were fed aldicarb at dose levels of
0, 0.2, 0.3 or 0.7 mg/kg bw/day for 7 days. There was no mortality
over the course of the study. Plasma, erythrocyte and brain
cholinesterase activities, measured one day after conclusion of the
dietary treatment, were normal. Gross liver and kidney weights and
organ to body-weight ratios were unaffected by aldicarb in the diet
(Weil & Carpenter, 1973).
Groups of 4 beagle dogs/sex were fed aldicarb in the diet for
five days per week at dose levels of 0, 0.2, 0.3 or 0.7 mg/kg bw/day
for 100 days. There was no mortality over the course of the study
and growth was comparable within all dosage groups. A slightly
decreased testes weight and a slight increase in adrenal weight were
observed at the highest level in males, but there were no effects in
females on any of the tissues and organs examined. Microscopic
analysis did not suggest any abnomalities including tissues where
gross changes had been seen to occur. Cholinesterase values, as well
as other clinical chemistry and haematology parameters, were
unaffected by the presence of aldicarb in the diet, however, the
animals had been removed from aldicarb exposure for 24 to 48 h prior
to cholinesterase analyses. The NOAEL in this study was 0.3 mg/kg
bw/day (Weil & Carpenter, 1974c).
Groups of 3 beagle dogs/sex were fed aldicarb in the diet at
dose levels of 0, 0.025, 0.05 or 0.1 mg/kg bw/day, 5 days per week
for 2 years. There was no mortality over the course of the study and
growth and food consumption data were comparable to control values.
Haematology parameters and clinical chemistry values, evaluated at
five intervals over the course of the study were normal. Plasma and
erythrocyte cholinesterase, evaluated over the course of the study,
did not differ from control values. At the conclusion of the study
brain cholinesterase, while somewhat lower at the high dose level,
was not statistically different from controls. Gross and microscopic
examination of tissues and organs showed no lesions which could be
attributable to the presence of aldicarb in the diet at dose levels
up to and including 0.1 mg/kg bw/day (Weil & Carpenter, 1966c).
A two-week range-finding study was conducted in beagle dogs to
select dose levels for a subsequent one-year dog study. Aldicarb was
fed to groups of 1 male and 1 female at dietary concentrations of 0,
1, 3, 10, 30 or 100 ppm. There was no mortality during the study,
but tremors and slight ataxia were observed at 30 and 100 ppm, and
soft faeces were noted more frequently in these dogs. Food
consumption was depressed in all treated males, particularly at the
highest dose, and slightly depressed in the 100 ppm female. Body-
weight losses were noted in the 100 ppm group and in the 30 ppm
male. Red blood cell and plasma cholinesterase inhibition exceeded
25% at doses of 3 ppm and greater. Brain cholinesterase inhibition
exceeded 25% for the 30 and 100 ppm group males (no calculation was
possible in the female groups due to presumed technical error)
(Hamada et al., 1985b).
As a clear NOEL for cholinesterase depression was not
demonstrated in the first study, a second two-week dietary dose
range-finding study was conducted to select the dose levels for a
subsequent one-year dog study. Aldicarb was fed to groups of 1 male
and 1 female at dietary concentrations of 0, 0.1, 0.3, 1, 3 or 10
ppm. There was no mortality during the study and no
treatment-related clinical signs were observed. Body-weight change
and food consumption were unaffected by treatment. Maximum plasma
and erythrocyte cholinesterase inhibition occurred immediately
following the two-hour feeding and lasted approximately 4 h after
the dosing period. Erythrocyte and plasma cholinesterase inhibition
(> 25% compared to the mean pre-study value) occurred in the 10 ppm
group on both days 7 and 14. There was no inhibition of brain
cholinesterase and no other compound-related effects noted. The
NOAEL in this study was 3 ppm, equal to 0.096 mg/kg bw/day (Hamada,
1987b).
In a five-week study, conducted in order to further investigate
the cholinesterase inhibition dose response curve, aldicarb (99.7%)
was fed at dietary concentrations of 0, 0.35, 0.7, or 2 ppm, to 3
groups of 6 beagle dogs/sex. There were no mortalities nor any
changes in body-weight gain, food consumption, clinical symptoms or
gross pathology indicative of a compound-related effect seen during
the study. Plasma cholinesterase inhibition over 20% when compared
to the control occurred only in the highest dose group. Red blood
cell cholinesterase activity inhibition was not observed in any dose
group and brain cholinesterase activity was not measured. No adverse
effects were observed at 2 ppm (equal to 0.06 mg/kg bw/day) and the
NOEL for any symptoms including plasma cholinesterase depression was
0.7 ppm (equal to 0.02 mg/kg bw/day) (Hamada, 1991).
In a 52-week oral toxicity study in beagle dogs, groups of 5
dogs/sex were fed dietary concentrations of 0, 1, 2, 5 or 10 ppm
aldicarb (95.5%) daily for 52 weeks. The study was designed to
produce maximum cholinesterase depression by limiting feeding time
to two hours per day to mimic a bolus administration of aldicarb.
Erythrocyte and plasma cholinesterase activities were measured from
blood samples collected approximately 2 h after the daily feeding
period to minimise dissociation of the carbamate-cholinesterase-
complex. Aldicarb at up to 10 ppm (equal to 0.241 mg/kg bw/day)
caused no observable effects other than inhibition of cholinesterase
activity. Inhibition of erythrocyte and brain cholinesterase
activity was restricted to dogs receiving 5 or 10 ppm. The NOEL for
plasma cholinesterase inhibition was 1 ppm, equal to 0.027 mg/kg
bw/day, while if the changes in the plasma enzyme activity are
discounted, the NOAEL was 2 ppm, equal to 0.054 mg/kg bw/day
(Hamada, 1988).
An inhalation study was conducted to determine the toxicity of
pyrolysis products of cigarettes made from tobacco pretreated with
aldicarb. Twelve beagle dogs (two animals/sex/dose), which had been
initially conditioned prior to commencement of the test to smoking
10 cigarettes per day via tracheostomy, were exposed to untreated,
low-level and high-level treated cigarettes on a schedule of two
successive cigarettes 5 times per day, 5 days per week for a total
of 19 exposure days. Whole blood cholinesterase was monitored
throughout the study with samples taken before smoking, after 4 and
after 10 cigarettes. All effects observed in this study were
apparently due to inhalation of cigarette smoke. No inhibition of
cholinesterase was seen in any treatment group (Coate et al.,
1982).
Dogs
- Aldicarb metabolites
Groups of 3 beagle dogs/sex were fed aldicarb sulfoxide in the
diet at dose levels of 0, 0.0625, 0.125, 0.25 or 0.5 mg/kg bw/day 5
days per week for 3 months. There was no mortality over the course
of the study. Slight body-weight changes were noted in many of the
dogs at the highest dose level within the first week of treatment
but thereafter the body-weight changes were similar to but lower
than control values. No effects on haematologic and blood chemistry
parameters were observed. Cholinesterase depression measured 24-48 h
after the final exposure was not observed in plasma, erythrocytes or
brain of any of the animals at the conclusion of the study. Gross
and microscopic examination of tissues and organs did not show any
adverse effects attributable to the presence of aldicarb sulfoxide.
The NOAEL in this study was 0.25 mg/kg bw/day (Weil & Carpenter,
1968b).
Groups of 3 dogs/sex were fed aldicarb sulfone in the diet at
dose levels of 0, 0.2, 0.6, 1.8 or 5.4 mg/kg bw/day 5 days per week
for 90 days. There was no mortality over the course of the study.
Slight body-weight depression was noted at the highest dose level,
although the body-weight was not statistically lower than control
levels. There were no effects noted with respect to biochemical and
haematologic parameters and gross and microscopic examination of
tissues and organs revealed no effects attributable to the presence
of aldicarb sulfone in the diet. Cholinesterase depression was not
observed, but dogs were removed from treated diet prior to
cholinesterase determinations. The NOAEL in this study was therefore
greater than 5.4 mg/kg bw/day (Weil & Carpenter, 1968c).
In a two-week range-finding study, aldicarb sulfone was fed to
six groups of 1 male and 1 female beagle dog at dietary
concentrations of 0, 3, 10, 30, 100 or 300 ppm. There was no
mortality during the study, but emesis, tremors and decreased food
consumption were observed in the high-dose group. Inhibition of
cholinesterase (> 25%) occurred at 10 ppm and over in both sexes
for plasma cholinesterase, and 100 ppm and over for red blood cell
and brain cholinesterase (Hamada et al., 1985a).
In a one-year feeding study, aldicarb sulfone was administered
to four groups of 6 beagle dogs/sex at dietary concentrations of 0,
5, 25 or 100 ppm. In this study, samples for cholinesterase
determinations were taken approximately two hours after feeding in
order to measure maximum cholinesterase inhibition. No
treatment-related clinical signs were seen. Body-weight gain was
slightly reduced in the high-dose males and females. There was a
slight decrease in the spleen weights of the mid- and high-dose
females and in the thyroid/parathyroid weight of the high-dose
females when compared to respective control values. These changes
were not seen in the males. Slight treatment-related effects were
noted in the mandibular lymph nodes of the high-dose males and
females, and adrenal cortex of the high-dose females. The NOAEL
based on depression of erythrocyte and plasma cholinesterase
activity was 25 ppm, equal to 0.54 mg/kg bw/day (Hamada, 1987a).
Monkeys
As a result of reports of alleged illness associated with
consumption of watermelons and cucumbers illegally treated or
contaminated with aldicarb in 1985, two studies were conducted to
evaluate the acute toxicity of aldicarb residues in food
commodities. Two crops were chosen for the studies: bananas as a
representative of a registered food crop in the USA, and watermelons
as a representative of a non-registered food crop from the cucurbit
family. (Suggestions had been made that some possible natural
component in cucurbits might synergise aldicarb toxicity). Bananas
were treated with approximately ten times the recommended label rate
and watermelons were treated at 13.44 kg ai/ha, to ensure adequate
residues in the fruit. Each study was performed using 3 Cynomolgus
monkeys/sex, with an additional group of 3 monkeys/sex serving as a
control (total of 12 animals per study). Following analytical
determination of actual residues in the two commodities to be
consumed, (approximately 0.3 ppm in bananas and 5 ppm in
watermelons), intake of the treated crops was adjusted with control
fruit to provide a dose of exactly 0.005 mg aldicarb/kg bw. The
animals were offered the fruit as the first meal of the day, and
consumed it immediately. Individuals were monitored for clinical
signs at regular intervals, and plasma and erythrocyte
cholinesterase activities were measured pre-dose, then at 1, 2, 4,
6, 12, 18 and 24 hours post-feeding. In both studies, there was no
evidence of acute cholinergic distress or any signs of over-exposure
in any animal. Depression of plasma cholinesterase activity was
evident within 1-4 h after ingestion and reached a maximum of 32 to
36% at 2 h after feeding (banana trial) and 36 to 37% at 1 h after
feeding (watermelon trial). Erythrocyte cholinesterase activity was
not depressed in either study. Rapid recovery of all enzyme activity
was observed. There was no indication in these studies that
components in watermelons potentiate aldicarb toxicity (Trutter,
1987a,b).
Long-term toxicity/carcinogenicity studies
Mice
- Aldicarb
Groups of 50 B6C3F1 mice/sex (25 of each sex were used as
controls) were fed aldicarb at dietary concentrations of 0, 2 or 6
ppm for 103 weeks in a carcinogenicity bioassay. A preliminary
dietary study (using 10 mice/sex fed dietary concentrations of
aldicarb of 0, 0.5, 1.0, 2.5, 5, 10, 20 or 40 ppm for 13 weeks)
showed no significant effects on microscopic examinations of the 0,
20 and 40 ppm levels. In the long-term carcinogenicity study there
was no mortality attributable to aldicarb. A variety of benign and
malignant tumours, occurring at different sites in both control and
aldicarb-treated mice, were not unusual for this strain of mice and
were evaluated to be independent of the administration of aldicarb.
Gross and microscopic examination of tissues, organs and all gross
lesions was performed and it was concluded that aldicarb was not
carcinogenic for the B6C3F1 strain of mice of either sex (NIH,
1979).
Groups of 44 CD-1 mice/sex were fed aldicarb at dose levels of
0, 0.1, 0.2, 0.4 or 0.7 mg/kg bw/day for 18 months. Mortality was
evident in males at the two highest dose levels and in females at
the three highest dosage levels during the first two and one-half
months of the study. Following this period, aldicarb was admixed
with the diet in a different manner which appeared to eliminate its
acutely toxic effects. (In the early parts of the study, aldicarb
was mixed in a dry fashion using a finely ground aldicarb
preparation. At the 2.5 month interval, aldicarb was dissolved in
acetone and the acetone-aldicarb solution was dispersed in the diet
at a more uniform rate. It was assumed that consumption of small
crystalline particles of aldicarb may have led to the high mortality
during the initial phases of the study). At the high dose level in
males there was a statistically significant increase in hepatomas
found predominantly in the survivors at the termination of the study
and an increase in lymphoid neoplasias which occured in the mice
that died. None of the male mice surviving at the end of the study
were found to have lymphoid neoplasias. There were no significant
increases in any other types of tumours at dose levels of 0.4 mg/kg
bw/day and below (Weil & Carpenter, 1972c).
Groups of 50 male CD-1 mice were fed aldicarb at dose levels of
0, 0.1, 0.3 or 0.7 mg/kg bw/day in an effort to verify the results
of the previous mouse carcinogenicity bioassay. A group of 150 mice
were used as concurrent controls with a mouse being sacrificed for
each treated animal that died during the course of the study. Diets
were prepared by dissolving aldicarb in acetone and mixing the
solution with the diet. The aldicarb was the same sample as used in
the previous study and the duration of the study was approximately
the same as in the previous trial. There was no mortality observed
in the study as a result of aldicarb in the diet. At the end of 18
months cumulative mortality at all dose levels was the same as noted
in controls. There was no effect of aldicarb on growth in any of the
groups. An examination of the animals that died during the course of
the study and those that were sacrificed at the end of 18 months was
made and the data compared with control values. There was no
significant association between aldicarb in the diet and the
formation of tumours, particularly with respect to the incidence of
hepatomas, lung adenomas, and lymphoid neoplasias. The data were
evaluated with respect to the mice that died, those that survived
the test and the total of all animals. It was concluded that the
administration of aldicarb at levels up to and including 0.7 mg/kg
bw/day for approximately 18 months did not result in a higher than
normal incidence of tumours and the inclusion of aldicarb in the
diet of CD-1 mice did not result in an increased incidence of
carcinogenic response (Weil & Carpenter, 1974d).
Groups of C3H/Hej male mice were administered aldicarb
dissolved in acetone, dermally 3 times a week for 28 months by
applying a brush full of an acetone solution to the shaved back of
the mice. For the first 2 weeks, treatment was performed 3 times a
week using a 0.25% solution in acetone. After 2 weeks this was
reduced to a twice weekly application. This dosing regimen was
maintained for 2 months and further reduced thereafter to a
concentration of 0.125% which was maintained for the rest of the
study. While there was some aldicarb-induced mortality noted over
the course of the study this mortality was not substantially
different from that noted with control applications. There were no
substantial differences with respect to the incidence or onset of
tumours. Two growths, a haemangioma and a thymoma, were noted in the
animals administered aldicarb. Neither of these internal growths was
accompanied by cutaneous papillomas or carcinomas and were
considered to be spontaneous growths unrelated to treatment.
Aldicarb, administered dermally to this sensitive species, did not
induce any incidence of malignancy (Weil & Carpenter, 1966b).
Mice
- Aldicarb metabolites
Groups of Charles River CD-1 mice (50/sex/group) were
administered 0, 0.15, 0.6, 2.4 or 9.6 mg aldicarb sulfone/kg bw/day
via the diet for 18 months. Aldicarb sulfone did not affect tumour
incidence or produce any pathological alteration in this strain of
mouse (Woodside et al., 1977b).
Rats
- Aldicarb
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0, 0.005, 0.025, 0.05 or 0.1 mg/kg bw/day. Additional
groups of 16 rats/sex were maintained for serial sacrifices at 6 and
12 months. There was no mortality, attributable to the presence of
aldicarb. Growth was normal at all dose levels as was consumption of
food and behavioural characteristics. Results of gross examination
of liver and kidney weight at 6 months and at one year did not
differ from control values. Haematologic determinations in the
highest dose level and control groups were normal. Blood and brain
cholinesterase activity, measured at 6 and 12 months, were normal.
Microscopic examination of tissues and organs for histopathologic
occurrences and neoplasms showed the incidence of lesions to be
similar in aldicarb-treated and in control groups. An apparent
no-effect-level in this study is 0.1 mg/kg bw/day (Weil & Carpenter,
1965).
Groups of 50 F344 rats/sex (25/sex for controls) were fed
aldicarb in the diet at dietary concentrations of 0, 2 or 6 ppm for
103 weeks. A preliminary study used 10 rats/sex, with dietary
concentrations of 0, 5, 10, 20, 40, 80, 160 or 320 ppm for 13 weeks.
Microscopic examinations performed on male and female rats of the 0
and 80 ppm dose levels at the conclusion of the preliminary trial
showed no significant effects. In the long-term carcinogenicity
study, there was no mortality attributable to aldicarb in the diet.
A variety of benign and malignant tumours occurring at different
sites in both control and aldicarb treated rats were not unusual for
this strain of rat and were evaluated to be independent of the
administration of aldicarb. Gross and microscopic examination of
tissues, organs and all gross lesions was performed and it was
concluded that aldicarb was not carcinogenic for the F344 strain of
rat of either sex at dietary concentrations of up to 6 ppm (NIH,
1979).
Rats
- Aldicarb/Aldicarb metabolites
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0 or 0.3 mg/kg bw/day. In addition, groups of rats were
fed aldicarb sulfoxide (0.3 or 0.6 mg/kg bw/day), aldicarb sulfone
(0.6 or 2.4 mg/kg bw/day) or a 1:1 mixture of aldicarb sulfoxide and
aldicarb sulfone (0.6 or 1.2 mg/kg bw/day). There was no significant
mortality observed over the course of the study with any of the
individual chemicals or the mixture of sulfoxide and sulfone. In the
initial phases of the study, there was a slightly higher mortality
noted in the high-dose level of aldicarb sulfoxide and in the group
receiving the combined aldicarb sulfoxide and aldicarb sulfone. A
slight increase in mortality was also noted at the latter part of
the study with aldicarb sulfoxide. Growth was slightly depressed at
the high-dose level of the sulfoxide:sulfone mixture, primarily in
males. There were no apparent effects on growth with respect to the
aldicarb, aldicarb sulfoxide or aldicarb sulfone administered alone.
Haematological values observed at various intervals over the course
of the study did not differ from controls. Cholinesterase
determinations were made periodically over the course of the study
(6, 12 and 24 months). Plasma, erythrocyte and brain cholinesterase
were examined only at a 24-h interval after animals were removed
from test diets. There was a slight depression of plasma
cholinesterase noted in males administered the high-dose level of
the combination aldicarb sulfoxide and sulfone at the 24-month
interval. A repeat of the analyses within the final week of the
study showed a slight depression in all chemical groups with respect
to plasma cholinesterase. There were no effects noted at any
interval with respect to red blood cell or brain cholinesterase. The
plasma cholinesterase depression noted at the 24-month interval was
limited to male rats. An evaluation of the incidence of tumours
suggested that there was no statistical difference between treated
and control groups. Gross and microscopic examination of tissues and
organs at various periods over the two-year test interval showed
that these sporadically distributed lesions could not be considered
to be indicative of damage induced by aldicarb, its major
metabolites, or the combination of the sulfoxide and sulfone (Weil &
Carpenter, 1972a).
Reproduction studies
Rats
- Aldicarb
Groups of rats (8 male and 16 female rats per group) were fed
aldicarb at dietary concentrations at doses of 0, 0.05 or 0.1 mg/kg
bw/day for approximately 90 days and mated to initiate a 3-
generation, one litter per generation reproduction study. Dietary
administration was continuous throughout the study. In addition to
the reproduction indices (fertility, gestation, viability and
lactation) the F3 generation was maintained for an additional
period and tissues from these animals were histologically examined
at either weaning or at 90 days of age. In all groups, the
reproduction indices from the aldicarb-treated animals were similar
to the control group. Body-weights of both male and female pups at
weaning were similar to control values as were results of gross and
microscopic examinations of tissues and organs in the F3 weanling
and 90-day old animals. In all three generations, with all criteria
examined, there were no effects of aldicarb on reproduction at a
dose level of 0.1 mg/kg body-weight (Weil & Carpenter, 1964).
Groups of rats (10 male and 20 female per group) were
administered aldicarb in the diet at doses of 0, 0.2, 0.3 or 0.7
mg/kg bw/day for 100 days prior to pairing and mated to initiate an
additional 3-generation (one litter per generation) reproduction
study. Dietary administration was continuous throughout the study. A
larger group was used for the F2 generation (15 male and 25 female
rats) as male pups of this generation were maintained on aldicarb
diets for 148 days and subjected to a (modified) dominant lethal
(mutagenesis) bioassay where they were mated with groups of
untreated virgin females for a period of 10 weeks. Each female in
the group was mated with 2 treated males and allowed to maintain
pregnancy until day 12 when they were sacrificed and examined. There
was some mortality over the course of the study which was associated
with lung infection and not as a result of aldicarb in the diet. At
the high-dose level, body-weights of both male and female F2 pups
were lower than the control values. Overall, there were no effects
on any of the reproduction indices (fertility, gestation, viability,
or lactation). Gross and microscopic examinations of the parents and
pups of the high level and control groups showed no effects
attributable to aldicarb. The dietary dominant lethal mutagenesis
bioassay showed no statistical differences between the
aldicarb-treated rats and controls with respect to early or late
fetal death or any other parameter examined (Weil & Carpenter,
1974a).
Rats
Aldicarb metabolites
Aldicarb sulfone (99.76% pure) was fed to groups of 10 male and
20 female rats at dietary concentrations at doses of 0, 0.6, 2.4 or
9.6 mg/kg bw/day for approximately 100 days. Rats were then mated to
initiate a three-generation, one litter per generation, reproduction
study. Dietary administration was continuous throughout the study.
Male rats fed 9.6 mg/kg bw/day exhibited reduced body-weights. There
were no differences from the control with regard to fertility,
gestation survival or viability indices. It was determined that
aldicarb sulfone, at levels up to and including 9.6 mg/kg bw/day,
was without adverse effects on reproduction under the conditions of
this study (Woodside et al., 1977a).
Special studies on embryo/fetotoxicity
Rats
- Aldicarb
Using a test protocol where both the reproductive and
teratologic potential of aldicarb was evaluated, groups of pregnant
rats were fed aldicarb at dietary concentrations at dose levels of
0, 0.04, 0.2 and 1.0 mg/kg bw/day. Females from each of the dietary
groups were assigned to one of three treatment groups: (1) aldicarb
administered in the diet throughout pregnancy or until pups were
weaned; (2) aldicarb administered in the diet from day 0 to day 7 of
gestation; (3) aldicarb administered in the diet from day 5 to day
15 of gestation. Five or six females from each group were sacrificed
and examined on day 20 of pregnancy and a similar number of females
were allowed to bear, nurse and wean the pups. There were neither
gross manifestations of teratogenicity in any of the pups carried by
females administered aldicarb at dose levels of up to 1 mg/kg bw/day
nor was there apparent interference with the reproductive process by
any of the dosage regimens used in this study. The administration of
aldicarb at dose levels up to and including 1.0 mg/kg bw/day had no
apparent effect on the growth of pregnant females during the course
of the study (Weil & Carpenter, 1966a).
Pregnant Sprague-Dawley rats were given aldicarb by gavage at
levels of 0, 0.125, 0.25 or 0.5 mg/kg bw/day on gestational day 6
through day 15. Maternal toxicity was indicated, at the highest
dose, by reduced weight gain and food intake during the treatment
and post-treatment period as well as by the occurrence of three
maternal deaths and, at the two highest levels, by reduced food
consumption during treatment. Gestational parameters, including
ovarian corpora lutea, number of implantations per litter and sex
ratio were unaffected by treatment. Fetal body-weight per litter was
significantly reduced at 0.5 mg/kg bw/day. There was no increase in
the incidence of external or skeletal malformations. A significantly
increased incidence of dilated lateral ventricles with tissue
depression was observed only at 0.5 mg/kg bw/day. No increased
incidence of malformation was observed in the absence of clear
maternal toxicity nor were these malformations accompanied by more
severe malformations. The NOEL was 0.125 mg/kg bw/day for maternal
toxicity and 0.25 mg/kg bw/day for embryo-fetal toxicity and
teratogenicity (Tyl & Neeper-Bradley, 1988).
Rats
- Aldicarb metabolites
The aldicarb sulfone reproduction study incorporated a
teratology bioassay. The animals were divided into 4 groups and
orally dosed with 0, 0.6, 2.4 or 9.6 mg/kg bw/day at one of the
following time intervals of gestation: 0-20 days, 6-15 days, or 7-9
days. All animals were sacrificed before parturition (day 20) and
the fetuses examined for skeletal and visceral changes. Anomalies
were essentially non-existent and there was no indication of
structural abnormalities produced under the conditions of the study
at levels up to and including 9.6 mg/kg bw/day (Woodside et al.,
1977a).
Rabbit
- Aldicarb
A range-finding study was conducted with aldicarb in rabbits to
determine dosage levels of aldicarb for a teratology study. Pregnant
Dutch belted rabbits (5/dose) were dosed by oral gavage at 0.1,
0.25, 0.5, 0.75 or 1.0 mg/kg bw/day on days 6 to 27 of gestation.
Two does in the high-dose group and one doe in the second highest
group died during the study. Increased incidences of decreased
defecation (all except low-dose group) and soft stools (two highest
dose groups) were noted. Maternal body-weight losses or markedly
decreased maternal body-weight gain during the treatment and
gestation intervals were observed at 0.25 mg/kg bw/day and greater.
Of the uterine parameters measured, there was an increase in the
mean post-implantation loss in the high-dose group. Dosage levels of
0.1, 0.25 and 0.5 mg/kg bw/day were selected for the definitive
study (Aldridge et al., 1983).
Pregnant Dutch belted rabbits (16/dose) were dosed by oral
gavage at 0.1, 0.25, or 0.5 mg/kg bw/day on days 7 to 27 of
gestation. Signs of maternal toxicity in the treated groups included
small amount of stool, pale kidneys and hydroceles on oviducts. A
loss in body-weight occurred in the 0.25 and 0.5 mg/kg bw/day dosage
groups during gestation days 7 to 27. A decline in the number of
viable fetuses per doe was observed in all treatment groups (8.7,
5.0, 6.5 and 6.2 for the control, 0.1, 0.25 and 0.5 mg/kg bw/day
treatment groups, respectively). This can be explained by an
unusually high number of implantations in the control group (9.8,
6.1, 7.2 and 7.8 implantations per doe in the control, 0.1, 0.25 and
0.5 mg/kg bw/day treatment groups, respectively). There was no clear
dose-effect relationship. There were also no increases in
post-implantation losses (1.1, 1.1, 0.7 and 1.7 post-implantation
losses for the control, 0.1, 0.25 and 0.5 mg/kg bw/day treatment
groups, respectively) these figures falling within the historical
control range. There was no meaningful difference in any of the
other developmental parameters nor in the number of developmental
variations or malfunctions between the control and treatment groups.
Aldicarb did not produce a teratogenic response when administered
orally to rabbits by gavage at dosage levels up to 0.5 mg/kg bw/day
(Leng et al., 1983).
Special studies on genotoxicity
Results of genotoxicity tests are summarised in Tables 3 and 4
(for in vitro and in vivo tests with aldicarb) and Table 5 (for
in vitro tests with aldicarb metabolites).
Table 3: Results of in vitro genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980c)
TA98,TA100,TA1535,
TA1537,TA1538
Reverse E. coli WP2 nk nk Negative Dunkel et al. (1985)
mutation assay
Reverse S. cerevisiae nk nk Negative Guerzoni et al. (1976)
mutation assay
HGPRT forward CHO cells 1000-5000 µg/ml nk Negative Stankowski et al. (1985a)
mutation assay
SCE Human nk nk Weak Debuyst & Larebeke (1983)
mutation assay lymphocytes Positive
Cytogenetics Human 10-250 µg/ml nk Weak Cid & Matos (1984)
mutation assay lymphocytes Positive
UDS Rat hepatocytes 0.16-5000 µg/ml nk Negative Godek et al. (1984a)
DNA damage S. typhimurium > 500 µg/disc nk Positive Rashid & Mumma (1986)
TA 1538 uvrB
TA 197
nk: not known
Table 4: Results of in vivo genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Micronucleus Mouse 0.001-0.01 93.47% Negative Ivett et al. (1984)
test mg/kg bw
Micronucleus ICR mouse 0.1-0.4 99.7% Negative Ivett (1990)
test mg/kg bw
Dominant Wistar rat 0.2-0.7 99.2% Negative Weil & Carpenter (1974a)
lethal test mg/kg bw
Table 5: Results of in vitro genotoxicity assays on aldicarb metabolites
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980b)
TA98,TA100,TA1535, sulphoxide
TA1537, TA1538
Ames test S. typhimurium 100-10000 µg/plate nk Negative Godek et al. (1980a)
TA98,TA100,TA1535, sulphone
TA1537, TA1538
HGPRT forward CHO cells 500-1500 µg/ml nk Negative Stankowski et al. (1985b)
mutation assay sulphone
Cytogenetics CHO cells 50-500 µg/ml nk Negative San Sebastian et al. (1984)
mutation assay sulphone
UDS Rat hepatocytes 0.1-3000 µg/ml nk Negative Godek et al. (1984b)
sulphone
nk: not known
Special studies on skin and eye irritation and sensitization
Administration of aldicarb to the conjunctival sac of rabbits
did not produce ocular irritation or corneal damage. Ocular
irritation studies were performed at doses that were lethal without
indication of ocular damage. Furthermore, there was no evidence of
dermal irritation when aldicarb was applied to the shaved, abraded
backs of rabbits (Striegel & Carpenter, 1962).
There was no indication of a sensitization reaction induced by
aldicarb. Male guinea-pigs were administered aldicarb by multiple
subdermal applications (0.7 mg/kg body-weight) and re-administered
aldicarb three weeks later by a similar intradermal administration.
There was no suggestion of sensitization in any of the animals
tested (Pozzani & Carpenter, 1968a).
Skin sensitization to aldicarb sulfone (UC 21865, technical) or
a 75% WP formulation was evaluated in albino guinea-pigs (Hartley
Strain) using a modified Landsteiner technique. Neither substance
was determined to be a sensitizer under the conditions of this study
(Conroy & Carpenter, 1977).
Special studies on delayed neurotoxicity
Hens
- Aldicarb
Groups of 6 adult chickens were administered aldicarb as a
single oral dose of 4.5 mg/kg bw or as daily oral doses of 0, 2.25
or 4.5 mg/kg bw/day for 30 days. A positive control group, treated
with 100 mg of TOCP, was used to produce typical delayed neurotoxic
signs of poisoning. While there was some weight loss, which was
correlated with the dose of aldicarb administered, the only
neurological effects attributable to aldicarb were acute signs of
poisoning noted in the first two or three days of treatment. Neither
ataxia nor hind limb paralysis were noted over the course of the
study. Aldicarb did not induce a delayed neurotoxic syndrome similar
to that induced by certain organophosphate esters (Johnson &
Carpenter, 1966a).
Hens
- Aldicarb metabolites
A group of 40 adult white Leghorn hens were intubated to
receive 250 mg/kg bw aldicarb sulfone suspended in maize oil. Two
other groups, each with 10 hens, received either TOCP (500 mg/kg bw)
or maize oil alone, and served as positive and negative control
groups respectively. There were no neurological effects other than
acute cholinergic signs of poisoning in the TOCP dosed group. No
histological examination was performed, owing to the lack of
demonstrated neurotoxic signs. Aldicarb sulfone did not cause
delayed neurotoxic reactions under the conditions of the study
(Babish & Salerno, 1977).
Special studies on behaviour
The effects of acute administration of aldicarb and aldicarb
sulfoxide on avoidance behaviour in rats was compared to a variety
of other carbamate esters. Rats were trained and evaluated for their
ability to avoid electrical shock in standard avoidance behaviour
tests. Aldicarb and aldicarb sulfoxide were administered by
intraperitoneal injection and the rats were evaluated for their
ablity to avoid shocks over a 6 h period following administration.
The lowest beviourally effective dose was found to be 0.266 mg/kg bw
which, when compared to the acute ip LD50 value, was noted to have
a smaller ratio of behaviour effects to acute LD50 than any of the
other carbamates tested. These data suggest that the level of
aldicarb needed to produce measurable avoidance is greater (closer
to a fatal dose and less likely to be achieved at the suggested use
level) than the chemicals to which it was compared. Additionally,
the activity over the 6-h period was seen to rapidly decline again
attesting to the transient nature of the cholinesterase inhibition
(Johnson & Carpenter, 1966b).
Special studies on immune responses
Aldicarb was evaluated for its ability to modulate the immune
response in two strains of mice. The B6C3F1 mouse was chosen
because it is the strain used by the US National Toxicology Program
for immunotoxicology studies, and the hybrid Swiss Webster mouse was
included because aldicarb had been reported to suppress the splenic
plaque-forming cell response to sheep red blood cells in an inverse
dose-response fashion (Olson et al., 1987). An attempt was made to
reproduce these findings and to expand the data base using more
standardised techniques with the B6C3F1 strain. Aldicarb was
administered in the drinking water ad libitum for 34 consecutive
days to female mice of both strains at dosages ranging from 0.1 to
1000 µg/litre in 10-fold increments (equivalent to 0.04 to 364 mg/kg
bw/day). Aldicarb had no effects on body-weights or organ weights,
on numbers or types of circulating white blood cells, or on the
microscopic pathology of the thymus, spleen, liver, kidneys, or
lymph nodes. Also unaffected were the number of antibody forming
cells in the spleen and the amount of circulating antibody in the
blood. Aldicarb had no effect in either strain on in vivo host
resistance to infectious viral challenge, on the capacity of B- and
T-lymphocytes to respond to nonspecific mitogens, or on the ability
of T-lymphocytes to recognize genetically different cell types in a
mixed lymphocyte culture. In this study, subchronic exposure to
aldicarb in the drinking water of mice had no effect on any measured
immunological function or toxicological parameter (Thomas et al.,
1987).
In another study, adult female B6C3F1, mice received
distilled water only or water containing 1.0, 10 or 100 µg/litre of
aldicarb daily for 34 days. To further develop an immune profile of
the compound, following aldicarb exposure, the ability of splenic
natural killer (NK) cells as well as specifically sensitized
cytotoxic T-lymphocytes to lyse YAC-1 lymphoma and, P815 tumour
cells, respectively, was evaluated. To complement the functional
assays, the impact of aldicarb exposure on spleen cell viability,
splenic cellularity and the percentages and absolute numbers of
total T-cells, T-suppressor, T-helper and B-cells was evaluated. The
results of this study demonstrated that aldicarb did not impact upon
the functional ability of interferon-induced splenic NK cells to
lyse YAC-1 lymphoma target cells. Aldicarb had no effect on the
functional capacity of cytotoxic T-lymphocytes to recognize
allogeneic cells in vivo and undergo differentiation and lysis of
these cells in a short-term 51-Cr release assay. The numbers and
percentages of T-cell subpopulations and B-cells in the spleen also
were not significantly affected. In addition, aldicarb had no
significant effect on body-weights, on spleen cellularity or cell
viability or on absolute weights of lymphoid organs. The absence of
statistically significant effects on any of these parameters
indicated that aldicarb did not have adverse effects on the immune
system of mice (Thomas et al., 1990).
Special studies on pesticide antagonistic agents
Following acute oral administration to rats, aldicarb has been
shown to induce a strong muscarinic action at excretory, bronchial
and cardiac nerve sites. A nicotinic effect was also shown to occur
at myoneural junctions. The parasympathetic signs of poisoning were
readily reduced following atropine administration. Administration of
atropine and 2-PAM, alone, or in combination, showed that while
atropine was a more effective antidote, 2-PAM was also active. While
it has been shown that aldicarb elicits a strong muscarinic action
as well as nicotinic action at myoneural sites, the control of signs
of poisoning from both mechanisms appears to be somewhat difficult
to achieve. Atropine has been shown to be an effective antidote to
block the muscarinic effects, but decamethonium, commonly used to
block the nicotinic effects, has been shown to be somewhat
ineffective. Additional studies to influence the nicotinic actions
by such drugs as tubocurare also failed to completely eliminate
nicotinic activity. Further studies confirmed the therapeutic
effects of a variety of oximes (P2S and obidoxime) in reducing the
acute toxic signs of poisoning associated with aldicarb. (Johnson &
Sullivan, 1968a,b; Natoff & Reiff, 1973).
Special studies on pesticide interactions
Aldicarb was administered orally to male rats alone and in
combination with a series of eight organophosphate esters or one
carbamate ester, all anti-cholinesterase agents, to examine the
potential interactive or additive effects. Results of the study,
using proportions of the acute lethal dose of each material alone
and in combination with aldicarb, showed a simple additive effect
with all materials tested. Aldicarb was not found to potentiate the
acute oral toxicity of other anticholinesterase agents. Further
studies were reported on the potential interaction of aldicarb with
alpha-naphthol, aldicarb sulfoxide with aldicarb sulfone and
aldicarb sulfone with parathion administered orally and aldicarb
with alpha-naphthol or with carbaryl administered by the
intraperitoneal route. In no case were any interactions greater tban
the predicted additive effects (West & Carpenter, 1966a; Weil &
Carpenter, 1970a).
Observations in humans
Groups of 4 adult male volunteers were administered aldicarb
orally in aqueous solution at dose levels of 0.025, 0.05 or 0.1
mg/kg body-weight. Clinical signs of poisoning were recorded and
whole blood cholinesterase activity was measured up to 6 h after
administration of the sample. Total urine voided was collected and
aldicarb-excretion patterns for the initial 8 h after dosing were
evaluated. In addition, spot samples were taken at 12 and 24 h.
Acute signs of poisoning, typical of anticholinesterase agents, were
observed at the high-dose level within 1 h after administration of
aldicarb. There were no signs of poisoning observed at the 0.05
mg/kg body-weight dose level. Cholinesterase depression was observed
in all volunteers prodominantly within 1-2 h after treatment. Within
the first six hours of treatment almost all cholinesterase
depression and clinical signs of poisoning were diminished.
Examination of urinary excretion patterns showed that approximately
10% of the administered dose was excreted as carbamates (toxic
residues) within the first 8 h interval. Cholinesterase analyses
confimed the same rapid inhibition and recovery pattern with man as
had been observed in experimental animals (Haines, 1971).
In another study, two additional subjects were administered
aldicarb in water solution at dose levels of 0.05 and 0.26 mg/kg
body-weight. Acute signs of poisoning were recorded at the higher-
dose level and atropine was administered to aid recovery. No signs
of poisoning were recorded with the lower-dose level. Urinary
excretion of carbamate residues within 24 h accounted for
approximately 10% of the administered dose (Cope & Romine, 1973).
A double blind, placebo controlled study has been conducted, in
which aldicarb was given as a single oral dose to healthy male and
female subjects. The doses administered were: placebo (22 subjects -
16 males and 6 females), 0.01 mg/kg bw (8 males), 0.025 mg/kg bw (8
males and 4 females), 0.05 mg/kg bw (8 males and 4 females) and
0.075 mg/kg bw (4 males). Subjects were screened before entry by
general medical history and examination and laboratory tests
including haematology, clinical chemistry and urinalysis. Clinical
measurements were made at intervals before and after dosing. These
included vital signs, (systolic and diastolic blood pressure, pulse
rate), pulmonary function tests, pupil size, electrocardiographs,
salivation and clinical signs of nausea, vomiting, diarrhoea,
sweating, abdominal cramps, involuntary movement and slurred speech.
Samples were taken for urinalysis, clinical chemistry (including red
blood cell and plasma cholinesterase activity) and haematology
evaluation before and after dosing. Urine and blood were collected
for aldicarb analysis. The results of these latter analyses were not
reported. There were no clinically significant changes in vital
signs, pupil size, pulmonary function, ECG's, salivation, clinical
signs, clinical chemistry (apart from cholinesterase), haematology
or urinalysis in the study. Cholinesterase activity in red cells and
plasma was maximally depressed at one hour after dosing and had
recovered by 8 h in all subjects. The fall in activity was dose-
related. Only marginal depression of cholinesterase activity (< 20%)
was seen in erythrocytes from patients treated with 0.01 or 0.025
mg/kg bw and in plasma at 0.01 mg/kg bw. Depression in
cholinesterase activity > 20% was seen in erythrocytes at 0.05 and
0.075 mg/kg bw and in plasma at 0.025, 0.05 and 0.075 mg/kg bw. No
serious adverse events occurred. The minor adverse events which were
recorded were similar to those reported in other volunteer studies.
Only one subject (0.075 mg/kg bw group, actual dose 0.06 mg/kg bw)
developed symptoms which were reported to be related to aldicarb.
The NOAEL in this study should be based on depression (> 20%) in
erythrocyte cholinesterase activity and was thus 0.025 mg/kg bw
(Nimmo et al., 1992).
Fifteen male volunteers participated in a study in Panama to
evaluate human exposure in a banana plantation where Temik-15G was
applied under natural conditions. Temperatures ranged from 24 °C to
32 °C with 80% to 90% relative humidity. The workers used three
different types of hand-held applicators. Blood samples were taken
from all volunteers prior to initiating the test and immediately
following each 6 h working period for estimation of blood
cholinesterase activity. Samples were obtained in the field and
analyzed 1-2 h later in the laboratory. Only two workers showed
greater than 25% reduction in their blood cholinesterase activity
(29% and 50%). Spontaneous reversibility was evident in both cases.
The worker with 50% reduction showed 25% recovery within 3 h. Blood
samples from the other worker indicated 100% recovery 24 h later.
Results of blood analyses indicated that cholinesterase activity was
below the normal range (population) in samples collected from six
workers during the second day of the study. Cholinesterase activity
in all samples taken during the first and third day of the study
were within the normal range. No clinical signs of aldicarb-induced
intoxication were found, although one individual presented symptoms
of nausea, stomachache and headache (Union Carbide, 1979).
A series of human exposure episodes was reported occurring as a
result of a variety of field and glasshouse conditions in an effort
to assess the potential for human harm from exposure under actual
occupational conditions. In several instances, slight blood
cholinesterase depression attested to the actual exposure situation.
Exposure data, as indicated by cholinesterase depression or urinary
excretion, suggested that there was no change in the general health
of workers exposed under any of the working conditions. Although
there were acute clinical signs of poisoning there was no indication
that the workers exposed were harmed once removed from exposure
situation (Williams, 1966; Burrows et al., 1970; Wakefield et
al., 1973; Shrivastava, 1975; Pandey, 1977).
From 1966 to 1979, 133 cases of apparent over-exposure to
aldicarb formulations were reported. Of these cases, 40 were
confirmed aldicarb poisoning episodes where clinical diagnosis
and/or urinalysis for aldicarb and its metabolites were performed.
As has been the case with other carbamate insecticides, the acute
signs of toxicity are rapidly dissipated, although atropine therapy
and hospitalization have been useful therapeutic regimens (Abdalla,
1977, 1979). Several deaths have been reported, but all of these
have been attributed to suicide or gross neglect (Lee, 1984).
California reported that in 1974, 1975 and 1976 a total of 10,
14 and 13 cases of human illnesses were reported, respectively. In
these incidents, people were directly exposed to aldicarb and
illness was brought on by dermal, inhalation and in one instance,
ocular exposure. While most illnesses resulted from aldicarb
exposure in loading or applying the formulated pesticide, some
illness has been reported from the handling of plants and soils
treated with aldicarb (Peoples et al., 1977).
Several misuses of aldicarb on watermelons and cucumbers have
been reported in the literature. Goes et al. (1980) reported on a
suspected food-borne intoxication in Nebraska associated with the
consumption of aldicarb-contaminated hydroponically grown cucumbers.
Two episodes were reported (April 1977: 9 cases; July 1978: 5
cases). Neither cholinesterase determinations nor urine residue
determinations were performed in any of these patients. Four
cucumbers (two in the supermarket and two in the greenhouse were
analyzed and aldicarb content was 6.6 and 10.7 mg/kg (supermarket) 0
and 9.9 mg/kg (greenhouse). No data are available on cucumbers
actually eaten and no attempts to correlate symptoms and aldicarb
consumption were made.
Hirsch et al. (1987) reported over 300 alleged intoxications
in Canada following ingestion of aldicarb contaminated cucumbers
(residue levels up to 26 mg/kg). In only five cases involving 13
patients, were any remaining portion of the cucumbers consumed still
available for analyses.
Goldman (1990) reported on four outbreaks of aldicarb poisoning
having occurred in California and other states in the USA from 1978
to 1988.
Outbreak 1: watermelons, 1985
Outbreak 2: watermelons, 1987
Outbreak 3: cucumbers, 1988
Outbreak 4: cucumbers, 1978 (corresponds to Goes (1980)
publication).
In the first outbreak, 1376 people allegedly became ill due to
consumption of contaminated watermelons. Of this total, 308 were
considered by the authors themselves as unlikely (235) or incomplete
(73). Nevertheless, only 28 cases were supported by positive
findings of aldicarb residues in the food commodity. All the other
cases were only supported by self-diagnosis.
An epidemiological study of potential adverse health effects of
aldicarb metabolites in drinking water was performed. In two surveys
conducted on Long Island, New York, the major cohorts were selected
on the basis of aldicarb levels in their drinking water. Levels of
contamination were generally 4-12 µg/litre, but a maximum of 400
µg/litre was reported. Questionnaires to determine water and food
consumption, symptoms experienced, and diagnosed illnesses were sent
to 1035 residents of 462 households. Although the initial survey
indicated a possible association between the incidence of diarrhoea
and levels of aldicarb in the water, the follow-up study, focusing
on children, did not confirm this association. No relationship was
detected between food consumption or water source and adverse health
symptoms, and self-reported physician-diagnosed illnesses for
1974-1979 were not significantly related to levels of aldicarb in
the water (Whitlock et al., 1982).
Another survey attempted to relate self-reported symptoms
suggestive of peripheral neuropathy to aldicarb levels in drinking
water in Suffolk County, New York. The response rate was less than
20%. Responses were classified as "probably", "possibly", or
"vaguely" suggestive of a neurologic syndrome. A significant
correlation with aldicarb concentration was obtained only by
combining all three categories of response, including reports of
just one symptom or of symptoms not forming any cohesive syndrome.
The authors concluded that further study was needed (Sterman &
Varma, 1983).
A pilot epidemiological study by Fiore et al. (1986)
evaluated a wide range of clinical immunological parameters in 23
women exposed to aldicarb in their drinking water and in a
non-exposed control group. The groups did not differ in any
immunological parameters except in T8-Lymphocytes: the number was
considered as elevated in five exposed and in one control.
Observation of an elevated stimulation assay response to one of the
large number of antigens (Candida) was not considered as
toxicologically significant, nor was it attributed to aldicarb
exposure.
In a follow-up study, five women of the preceeding group and
still exposed to aldicarb were examined versus previous control
women plus women previously exposed but for whom exposure no longer
occurred (e.g., change in water supply due to addition of a charcoal
filter). According to the authors, this follow-up study confirmed
the previous results, except for increased response to Candida
antigens which was no longer present (Mirkin et al., 1990).
COMMENTS
Aldicarb is rapidly absorbed, widely distributed in the body
and rapidly excreted. Metabolism appears to be similar in all
species studied, aldicarb being rapidly metabolised to aldicarb
sulfoxide, which is more slowly degraded to aldicarb sulfone. All
metabolites are quickly eliminated from the body, 80-90% being
excreted within 24 h. Elimination was complete by the fifth day
after dosing and no bio-accumulation was seen.
Aldicarb has high acute toxicity in a wide variety of mammalian
species. Signs of toxicity are those commonly associated with
acetylcholinesterase inhibition by a carbamate insecticide:
cholinergic signs of poisoning, which are alleviated rapidly on
cessation of exposure. Aldicarb sulfoxide is a more potent inhibitor
of acetylcholinesterase than aldicarb itself, while aldicarb sulfone
is considerably less toxic than either aldicarb or the sulfoxide.
WHO has classified aldicarb as extremely hazardous (WHO, 1992).
Short-term and long-term studies have been performed in rats,
mice and dogs with aldicarb and aldicarb metabolites, both
individually and in combination. Toxicity tests employing mixtures
of aldicarb or aldicarb sulfoxide with aldicarb sulfone are of
interest because aldicarb sulfoxide and aldicarb sulfone are the
terminal residues potentially consumed by humans. Cholinesterase
depression is the most significant indicator of toxicity that can be
evaluated. However, considerable attention must be paid to the
methods of sample collection and determination of cholinesterase
activity. Continuous administration of aldicarb to the test animals
until collection of samples for analysis is important, as is rapid
analysis under carefully controlled conditions.
No-effect levels in the various studies which included
evaluation of cholinesterase inhibition are summarized in Table 6.
No distinction is made in this table for the methods of determining
cholinesterase activity.
It is now considered inappropriate to use no-adverse-effect
levels from many of the earlier repeat-dose studies for the
derivation of an ADI, because animals were not dosed for 24-48 h
prior to collection of tissue samples for measurement of
cholinesterase activity. In the most recent dog studies, which were
conducted in a manner designed to maximize detection of
cholinesterase depression, the overall NOEL was 0.02-0.03 mg/kg
bw/day, but the NOAEL (which discounts inhibition of plasma
cholinesterase only) was 0.05-0.06 mg/kg bw/day.
Results of repeat-dose studies with aldicarb demonstrate that
the method of administering the test material to the test animals
can greatly modify the apparent toxicity of aldicarb and its
metabolites. Mice, rats and dogs have tolerated daily doses equal to
the LD50 incorporated into the diet for 7 days to 2 years. Doses
which caused death in less than 2 h when administered as a bolus,
caused no death and only moderate cholinesterase depression when
given in the diet.
Two dietary carcinogenicity studies have been conducted with
aldicarb in rats and three in mice. A dermal carcinogenicity study
has also been conducted with aldicarb in mice and dietary studies
have been carried out with aldicarb sulfone in mice and aldicarb
sulfoxide in rats. Aldicarb was not carcinogenic in mice and rats.
Two reproduction studies have been conducted in rats with
aldicarb and one with aldicarb sulfone. There were no effects on
reproductive performance at doses up to 0.7 mg/kg bw/day aldicarb or
9.6 mg/kg bw/day aldicarb sulfone. Aldicarb did not display any
teratogenic potential in rats or rabbits in studies which included
maternally toxic doses.
After reviewing the available genotoxicity data, the meeting
concluded that aldicarb, aldicarb sulfoxide and aldicarb sulfone are
not genotoxic.
In a range of special studies in animals (involving delayed
neurotoxicity, behaviour, antagonistic agents and pesticide
interactions) aldicarb displayed no results which gave cause for
concern. There was no evidence of immuno-toxicity in mice in a
number of functional assays of cell-mediated immunity and in host
resistance to respiratory infection.
Epidemiological studies provided no convincing evidence that
aldicarb could significantly alter immunological function in humans.
In addition to the above epidemiological studies, studies
conducted in 1982 and 1983 attempted to correlate any potential
adverse health effects with the occurrence of aldicarb in drinking
water. Although the authors concluded that further study was needed,
there was no clear evidence that aldicarb contamination of drinking
water generally at concentrations of about 4-12 µg/litre, but at a
maximum concentration of 400 µg/litre was related to any health
effects.
The anticholinesterase potential of aldicarb has been
extensively investigated in human. These studies revealed the same
pattern of rapid cholinesterase inhibition and rapid recovery seen
in experimental animals. Transient erythrocyte cholinesterase
depression was seen at single doses of 0.05 mg/kg bw, and the NOAEL
for cholinesterase depression (discounting changes in plasma enzyme
activity) was 0.025 mg/kg bw.
A number of poisoning incidents have been reported in the
agricultural use of aldicarb, but there has been no indication that
the workers exposed were harmed once removed from the exposure
source. Although several deaths have been reported, all of these
have been attributed to suicide or gross neglect.
A number of food-borne aldicarb intoxications have been
reported in the literature. These have all been associated with
misuse and reliable quantification of the dose of aldicarb involved
has always proved difficult, if not impossible.
An ADI was allocated using a 10-fold safety factor applied to
the NOAEL for depression of erythrocyte cholinesterase activity in
human volunteers.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg bw/day (93-day dietary study)
Dog: 0.05 mg/kg bw/day (52-week study)
Human: 0.025 mg/kg bw (double-blind, placebo controlled
volunteer study)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans, including information regarding
the correlation of blood cholinesterase depression and clinical
signs and symptoms.
Table 6: Summary of no effect levels in repeat dose toxicity studies which included
cholinesterase investigations
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Mouse 7 days Aldicarb 0,0.1,0.3,0.6,1.2 0.6 Mortality
Mouse 7 days Aldicarb: 0,2,6,18,36 2 Kidney and liver weight
A. sulfone reduction, growth
1:1 depression
Rat 7 days Aldicarb 0,4,8,16 NE Mortality, growth
depression, kidney and
liver weight reduction
Rat 7 days Aldicarb 0,0.8,1.6,3.2 NE Growth depression,
kidney and liver weight
reduction
Rat 93 days Aldicarb 0,0.02,0.1,0.5 0.1 Mortality, growth
depression
Rat 2 years Aldicarb 0,0.005,0.025, >0.1 No effects seen
0.05,0.1
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Rat 7 days Aldicarb 0,0.4,0.8,1.6,3.2 0.8 Growth depression,
A. sulfoxide 0,0.4,0.8 0.4 RBC cholinesterase
A. sulfone 0,0.4,1.0,2.5,5,20 2.5 depression
Rat 29 days A. sulfoxide: 0,0.0074,0.03, 0.4 Growth depression,
A. sulfone 0.12,0.47,1.67 in brain, plasma and RBC
1:1 drinking water cholinesterase depression
Rat 6 months A. sulfoxide 0,0.125,0.25,0.5,1 0.125 Growth depression,
brain, plasma and RBC
cholinesterase depression
Rat 6 months A. sulfoxide 0,0.0625,0.125, 0.125 Plasma and RBC
0.25,0.5,1.0 cholinesterase depression
Rat 6 months A. sulfone 0,0.2,0.6,1.8, 0.6 Brain, plasma and RBC
5.4,15.2 cholinesterase depression
Rat 7 days A. oxime 0,31.25,62.5,125, 62.5 Growth depression,
250,500,1000 minor liver and kidney
changes
Dog 7 days Aldicarb 0,0.2,0.3,0.7 >0.7 No effects seen
Dog 100 days Aldicarb 0,0.2,0.3,0.7 0.3 Minor organ weight
changes
Dog 2 years Aldicarb 0,0.025,0.05 >0.1 No effects seen
0.1
Dog 2 weeks Aldicarb 0,0.022,0.068, NE Brain, plasma and RBC
cholinesterase
depression
0.192,0.609,1.42
Dog 2 weeks Aldicarb 0,0.003,0.008, 0.096 Brain, plasma and RBC
cholinesterase depression
0.027,0.096,0.28
Dog 5 weeks Aldicarb 0,0.012,0.024, >0.06 No effects seen
0.06
Dog 52 weeks Aldicarb 0,0.027,0.054, 0.054 RBC and plasma
0.131,0.241 cholinesterase depression
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Dog 3 months A. sulfoxide 0,0.0625,0.125, 0.25 Transient growth
0.25,0.5 depression
Dog 3 months A. sulfone 0,0.2,0.6, >5.4 No effects seen
1.8,5.4
Dog 1 year A. sulfone 0,0.11,0.59, 0.11 RBC and plasma
2.25 cholinesterase depression
* mg/kg bw/day equivalence, by dietary administration, unless otherwise stated.
Lowest effect level underlined.
** NOAEL's were tabulated without evaluation of methods of sample collection or method of
determination of cholinesterase activity.
NE Not established
REFERENCES
Abdallah, N.A. (1977) Alleged overexposure cases reported from use
of Temik formulations - 1966 to 1976. Unpublished report from Union
Carbide Corporation.
Abdallah, N.A. (1979) Alleged human overexposure cases reported from
use of Temik formulations - 1976 to 1979. Unpublished report from
Union Carbide Corporation.
Aldridge, D., Schardein, J.L., & Blair, M. (1983) Aldicarb
range-finding teratology study in rabbits. Unpublished report from
International Research and Development Corporation, submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Andrawes, N.R. (1977) The metabolism of (UC 21865) Sulfocarb
pesticide in the rat. Unpublished report from Union Carbide
Corporation.
Andrawes, N.R., Dorough, H.W., & Lindquist, D.A. (1967) degradation
and elimination of Temik in rats. J. Econ. Ent., 60(4): 979-987.
Andrawes, N.R. & Lee R.E. (1986) Temik brand aldicarb pesticide,
metabolism in lactating goats. Unpublished report from Union Carbide
Agricultural Products Company Inc.
Babish, J.G. & Salerno, A. (1977) Neurotoxicity evaluation of UC
21865 in white Leghorn hens (Gallus domesticus). Unpublished
report from Food and Drug Research Laboratories submitted to WHO by
Union Carbide Corporation.
Bull, D.L., Lindquist, D.A., & Coppedge, J.R. (1967) Metabolism of
2-methyl-2-(methylthio)propionaldehyde O-(methylcarbamoyl) oxime
(Temik, US-21149) in insects. J. Agric. Food Chem., 15(4):
610-616.
Burrows, I.E., Haynes, G., & Roberts, N. (1970) Determination of
exposure levels in operators to Temik during field trials.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Carpenter, C.P. (1963) Comparison of the acute toxicity of compound
21149 with several of its analogues. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Carpenter, C.P. (1969) Miscellaneous toxicity studies. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Carpenter, C.P. and Smyth, H.F. (1966) Temik l0G (10.5% Granular
formulation of compound 21149). 15-Day dermal applications to
rabbits. Unpublished Report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Chin, G.H. & Sullivan, L.J. (1968) Some biochemical considerations
of Temik pesticide. Unpublished report from Mellon Institute,
presented to the 156th ACS National Meeting, submitted to WHO by
Union Carbide Corporation.
Cid, M.G. & Matos, E. (1984) Induction of sister-chromatid exchanges
in cultured human lymphocytes by aldicarb, a carbamate pesticide.
Mutat. Res., 138: 175-179.
Coate, W.B., Hardy, R.J., Alsaker, R.D., Dawkins, B.G., Hepner,
K.E., Banos, D.A., & Mense, M.A. (1982) Subacute inhalation toxicity
study of a pesticide residue in dogs. T3, T6 and T10 type
cigarettes. Unpublished report from Hazleton Laboratories America
Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Debuyst, B. & Van Larebeke, N. (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242-243.
Dorough, H.W., Davis, R.B., & Ivie, G.W. (1970) Fate of
Temik-Carbon-14 in lactating cows during a 14-day feeding period.
J. Agr. Food Chem., 18: 135-142
Dorough, H.W. & Ivie, G.W. (1968) Temik-S-35 metabolism in a
lactating cow. J. Agric. Food Chem., 16(3): 460-464
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E. McGregor, D.,
Morteimans, K., Rosenkranz, H.S., & Simmon, V.F. (1985)
Reproducibility of microbial mutagenicity assays: II. Testing of
carcinogens and noncarcinogens in Salmonella typhimurium and
Escherichia coli. Environ. Mutagen., 7 (suppl 5): 1-248.
Field, W.E. (1979a) Acute dermal toxicity in rabbits. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Field, W.E. (1979b) Acute dermal toxicity in rats. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Fiore, M.C., Anderson, H.A., Hong, R., Golubjatnikov, R., Seiser,
J.E., Nordstrom, D., Hanrahan, L., & Belluck, D. (1986) Chronic
exposure to aldicarb-contaminated groundwater and human immune
function. Environ. Res., 41: 633-645.
Godek, E.G., Dolak, M.D., Naismith, R.W., & Matthews, R.J. (1980a)
Ames Salmonella microsome plate test. Aldicarb sulfone.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980b)
Ames Salmonella microsome plate test. Aldicarb sulfoxide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980c)
Ames Salmonella microsome plate test. TEMIK aldicarb pesticide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984a) Rat hepatocyte
primary culture/DNA repair test. Aldicarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984b) Rat hepatocyte
primary culture/DNA repair test. Aldoxycarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Goes, E.H., Savage, E.P., & Gibbons, G. (1980) Suspected foodborne
carbamate pesticide intoxications associated with ingestion of
hydroponic cucumbers. Am. J. Epidemiol., 111: 254-259.
Goldman, L.R., Beller, M., & Jackson, R.J. (1990) Aldicarb food
poisonings in California, 1985-1988: Toxicity estimates for humans.
Arch. Environ. Hlth., 45(3) 141-147.
Guerzoni, M.E., Del Cupolo, L. and Ponti, I. (1976) Attivita
mutagenica degli antiparassitari. Riv. Sci. Teen. Alim. Nutr.,
Urn. 6: 161-165.
Haines, R.G. (1971) Ingestion of aldicarb by human volunteers: a
controlled study of the effect of aldicarb on man. Unpublished
report from Union Carbide Corporation.
Hamada, N.N. (1987a) One-year chronic oral toxicity study in beagle
dogs with aldicarb sulphone technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1987b) Two-week dose range finding oral toxicity study
in beagle dogs with aldicarb technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1988) One-year chronic oral toxicity study in beagle
dogs with aldicarb technical. Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N. (1991) Subchronic toxicity study in beagle dogs with
aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Marshall,
P.M. (1985a) Two-week dose range finding study in beagle dogs -
Aldicarb sulphone (aldoxycarb). Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Williams,
L.D. (1985b) Two-week dose range finding study in beagle dogs -
Aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hicks B.W., Dorough, H.W., & Mehendale, H.M. (1972) Metabolism of
aldicarb pesticide in laying hens. J. Agric. Food Chem., 20(1):
151-156.
Hirsch, G.H., Mori, B.T., Morgan, G.B., Bennett, P.R., & Williams,
B.C. (1987) Report of illness caused by aldicarb-contaminated
cucumbers. Food Addit. Contam., 5: 155-160.
Ivett, J.L. (1990) Mutagenicity test on aldicarb technical in the
mouse bone marrow cytogenetic assay. Unpublished report from
Hazleton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Ivett, J.L., Myhr, B.C., & Lebowitz, H.D. (1984) Mutagenicity
evaluation of aldicarb technical 93.47% in the bone marrow
cytogenetic assay. Unpublished report from Litton Bionetics, Inc.
submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Johnson, H.E. & Carpenter, C.P (1966a) Temik (Technical grade
compound 21149). Demyelination potential in chickens. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Carpenter, C.P (1966b) Temik (technical grade
compound 21149). Comparative behavioural effect in rats. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Sullivan, L.J. (1968a) Studies on an effective
therapy for overdoses of temik, Temik sulfoxide and temik sulfone in
the rat. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Johnson, H.E. and Sullivan, L.J. (1968b) Temik (uc 21149) antidotal
therapy in rats following administration of multiple lethal doses.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Knaak, J.B., Tallant, M.J., & Sullivan, L.J. (1966) The metabolism
of 2-methyl-2-(methylthio)propionaldehyde o-(methylcarbamoyl) oxime
in the rat. J. Agric. Food Chem., 14(6): 573-578
Lee, M.H. & Ransdell, J.F. (1984) A farmworker death due to
pesticide toxicity: a case report. J. Toxicol. Environ. Health,
14: 239-246.
Leng, J.M., Schardein, J.L., & Blair, M. (1983) Aldicarb. Teratology
study in rabbits. Unpublished report from International Research and
Development Corporation submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Burk, J.,
Reynolds, H.T., & Osman, M.F. (1966) Metabolism of
2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyl) oxime in
Plant and Insect. J. Agric. Food Chem., 14(6): 579-584.
Mirkin, I.R., Anderson, H.A., Hanrahan, L., Hong, R., Golubjatnikov,
R., & Belluck, D. (1990) Changes in tlymphocyte distribution
associated with ingestion of aldicarb-contaminated drinking water: a
follow-up study. Environ. Res., 51: 35-50.
Mirro, E.J., DePass, L.R., & Frank, F.R. (1982) Twenty-nine day
water inclusion study in rats. Unpublished report from Bushy Run
Research Laboratory submitted to WHO by Union Carbide Corporation.
Natoff, I.L. & Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl.
Pharmacol., 25: 569-575.
NIH (1979) Bioassay of aldicarb for possible carcinogenicity. US
DHEW Pub.No. (NIH) 79-1391, 103 pages.
Nimmo, W.S., Wyld, P.J., Watson, C.E., & Watson, N (1992) A safety
and tolerability study of aldicarb at various dose levels in healthy
male and female volunteers. Unpublished report from Inveresk
Clinical Research, submitted to WHO by Rhône-Poulenc AGRO, Lyon,
France.
Nycum, J.S. & Carpenter, C.P. (1968a) Toxicity studies on the
probably "non-carbamate" plant metabolites of Temik. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Nycum, J.S. & Carpenter, C.P. (1968b) Toxicity studies on Temik and
related carbamates. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Olson, L.J., Erickson, B.J., Hinsdill, R.D., Wyman, J.A., Porter,
W.P., Binning, L.K., Bidgood, R.C., & Nordheim, E.V. (1987) Aldicarb
immunomodulation in mice: an inverse dose-response to parts per
billion levels in drinking water. Arch. environ. Contam. Toxicol.,
16: 433-439.
Pandey, S.Y. (1977) Human exposure study on Temik 10G. Unpublished
report from Union Carbide India Ltd., submitted to WHO by Union
Carbide Corporation.
Peoples, S.A., Maddy, K.T., & Smith, C.R. (1977) Human health
problems associated with Temik (Aldicarb) in California. Unpublished
report from California Department of Food and Agriculture submitted
to WHO by Union Carbide Corporation.
Pozzani, U.C. & Carpenter, C.P. (1968a) Sensitizing potential in
guinea-pigs as determined by a modified Landsteiner test.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21:
319-334.
Romine, R.R. (1973) Aldicarb pesticide. Aldicarb residues in milk
and meat of cows fed aldicarb sulfoxide and aldicarb sulfone in the
daily diet. Unpublished report from Union Carbide Corporation.
SanSebastian, J.R., Naismith, R.W., & Matthews, R.J. (1984) In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Aldoxycarb technical. Unpublished report from Pharmakon
Research International Inc. Submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Shrivastava, K.N. (1975) Determination of human exposure levels in
commercial application of ternik 10g on potatoes by gloved hand and
hand-operated applicators. Unpublished report from Union Carbide
India Ltd.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985a) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldicarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985b) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldoxycarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Sterman, A.B. & Varma, A. (1983) Evaluating human neurotoxicity of
the pesticide aldicarb: when man becomes the experimental animal.
Neurobehav. Toxicol. Teratol., 5: 493-495.
Striegel, J.A. & Carpenter, C.P. (1962) Range finding test on
compound 21149. Unpublished report from Mellon Institute of
Industrial Research submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. (1968a) The urinary excretion of C-14 equivalents of
Temik in the dog and their metabolic profile as revealed by silica
gel chromatography. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Sullivan, L.J (1968b) Urinary metabolic profiles as determined by
silica gel chromatography of urines from rats and dogs dosed with
Temik sulfone. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Sullivan, L.J (1968c) The excretion of C14 equivalents of Temik
sulfone by the rat after a single peroral dose. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. & Carpenter, C.P. (1974) Preliminary Metabolism of
14C-S-methyl aldicarb nitrile in the rat. Unpublished report from
Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Thomas, P.T., Ratajczak, H.V., Eisenberg, W.C., Furedz-Machacek, M.,
Ketels, K.V., & Barbera, P.W. (1987) Evaluation of host resistance
and immunity in mice exposed to the carbamate pesticide aldicarb.
Fund. Appl. Toxicol., 9: 82-89.
Thomas, P.T., Ratajczak, H.V., Demetral, D., Hagen, K., & Baron, R.
(1990) Aldicarb immunotoxicity: Functional analysis of cell-mediated
immunity and quantitation of lymphocyte sub-populations. Fund.
Appl. Toxicol., 15: 221-230.
Trutter, J.A. (1987a) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residues in bananas. Unpublished report
from Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Trutter, J.A. (1987b) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residue in watermelons. Unpublished
report from Hazleton Laboratories America Inc., submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Tyl, R.W. & Neeper-Bradley, T.L. (1988) Developmental toxicity
evaluation of aldicarb technical administered by gavage to CDO
(Sprague-Dawley) rats. Unpublished report from Bushy Run Research
Centre submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Union Carbide (1979) Volunteer worker's exposure monitoring study
(Panama). Unpublished Report from Union Carbide Corporation.
Wakefield, M., Orme, J.P.R., Ruston, E.A., & Andrews, L. (1973) The
detemination of levels of aldicarb in urine and face masks.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1963) Results of three months of
inclusion of Compound 21149 in the diet of rats. Unpublished Report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1964) Results of a three-generation
reproduction study on rats fed compound 21149 in their diet.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1965) Two-year feeding of compound
21149 in the diet of rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966a) Insecticide Temik. Teratogenic
potential in rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966b) Results of long-term tests for
mouse skin carcinogenicity of three process residues, one epoxide
and three compounds. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966c) Two-year feeding of compound
21149 in the diet of dogs. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968a) Temik 10G-V (10.3% granular
formulation of compound 21149). Acute and 14-day dermal applications
to rabbits. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968b) Temik sulfoxide. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968c) Temik sulfone. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1969a) Purified and technical temik.
Results of feeding in the diets of rats for one week. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1969b) 2-methyl-2-(methylsulfinyl)
propanol-1. Results of feeding in the diets of rats for one week.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970a) Temik and other materials.
Miscellaneous single dose peroral and parenteral LD50 assays and
some joint action studies. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970b) Temik, results of feeding in
the diets of rats for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970c) Temik (T) Temik sulfoxide
(TSO), Temik sulfone (ts02), 1:1 TSO-TS02. Results of feeding in the
diet of rats for 7 days. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970d) 1:1 Temik:Temik sulfone.
results of feeding in the diet of mice for 7 days. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1970e) Temik. Results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972a) Aldicarb (A), Aldicarb
sulfoxide (ASO), Aldicarb sulfone (AS02) and a 1:1 mixture of
ASO:AS02. Two-year feeding in the diet of rats. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972b) Miscellaneous toxicity studies.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972c) Aldicarb, 18-month feeding in
diet of mice. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1973) Aldicarb, seven-day inclusion in
diet of dogs. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974a) Aldicarb. Inclusion in the diet
of rats for three generations and a dominant lethal mutagenesis
test. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974b) Aldicarb Oxime (All). Results
of feeding in the diet of rats for 7 days. Unpublished report from
Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974c) Aldicarb. Inclusion in the
diets of dogs for three months. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974d) Aldicarb, 18-month feeding in
the diet of mice, study II. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974e) UC 21865; results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Cox, E.F. (1975) Aldicarb sulfoxide and aldicarb
sulfone cholinesterase inhibition results after periods of one to
fifty-six days of inclusion in the diets of rats. Unpublished report
from Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Weil, C.S., Conroy, W.J., Condra, N.I., & Cox, E.F. (1977) Aldicarb
sulphone UC 21865. 19-day rabbit skin inunction. Unpublished report
from Mellon institute submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1965) The single dose peroral toxicity
of compounds 20299, 21149, 19786 and 20047a for white Leghorn
cockerels. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1966a) Temik (Compound 21149,
Technical). Joint action with selected organic phosphate and
carbamate pesticides. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P.(1966b) Miscellaneous acute toxicity
data. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Whitlock, N.H., Schuman, S.H., & Loadholt, C.B. (1982) Executive
summary and epidemiologic survey of potential acute health effects
of aldicarb in drinking water - Suffolk County, N.Y. South Carolina
Pesticide Hazard Assessment Program Center, Medical University of
South Carolina, Charleston. Prepared for the Health Effects Branch,
Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency.
WHO (1966) Insecticide Evaluation and Testing Programme, Stage I,
Mammalian Toxicity Report (WHO). Unpublished report from Toxicology
Research Univ., Carshalton, U.K. submitted to WHO by Union Carbide
Corporation.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, F. (1966) Human exposure study in the field application of
Temik 10G on cotton. Unpublished report from Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977a) Inclusion in the
diet of rats for three generations (aldicarb sulphone), dominant
lethal mutagenesis and teratology studies. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977b) Aldicarb sulphone;
18-month feeding in the diet of mice. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
 Table 1: Acute toxicity of aldicarb
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Rat M oral corn oil 0.84 West & Carpenter (1966b)
Rat M oral corn oil 0.93 Striegel & Carpenter (1962)
Rat M oral corn oil 0.67-1.23 Carpenter (1963)
Nycum & Carpenter (1968b)
Rat F oral corn oil 0.62-1.07 Carpenter (1963)
Rat F oral gycerol:
ethanol 1.0 WHO (1966)
Rat F ip corn oil 0.71 Carpenter (1963)
Rat M+F ip corn oil 0.44 Carpenter (1963)
Rat M ip PEG 0.37-0.44 Weil & Carpenter (1970a)
Rat M ip ethanol 0.57 Johnson & Carpenter (1966b)
Rat M iv water 0.47 Weil & Carpenter (1970a)
Rat M dermal corn oil >10 Field (1979b)
Rat F dermal DMP 3.2-7.0 WHO (1966)
Rat M dermal water 38.1-44.9 Weil & Carpenter (1968a)
Mouse M oral corn oil 0.382 Weil & Carpenter (1972b)
Mouse M oral corn oil 0.5 Weil & Carpenter (1972c)
Mouse F oral cotton seed 1.5 Dorough et al. (1970)
oil
Mouse F ip cotton seed 0.3 Dorough et al. (1970)
oil
Guinea-pig oral corn oil 1.0 Nycum & Carpenter (1968b)
Rabbit oral propylene 1.3 Nycum & Carpenter (1968b)
glycol
Rabbit M dermal corn oil >10 Field (1979a)
Rabbit M dermal PEG 5.0 Striegel & Carpenter (1962)
Rabbit M dermal dry 141->200 Weil & Carpenter (1968a)
Chicken M oral 9 West & Carpenter (1965)
Table 2: Acute toxicity of aldicarb metabolites
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. nitrile rat oral 570 West & Carpenter (1966b)
A. sulfoxide rat (M) oral corn oil 0.49-1.13 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfone rat (M) oral corn oil 20-25 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfoxide mouse (M) oral corn oil 0.8-1.6 Nycum & Carpenter (1968b)
A. sulfone mouse (M) oral corn oil 25 Nycum & Carpenter (1968b)
A. sulfoxide guinea-pig oral corn oil 0.8-1.8 Nycum & Carpenter (1968b)
A. sulfone guinea-pig oral corn oil >50 Nycum & Carpenter (1968b)
A. sulfoxide rabbit oral corn oil 0.4-1.8 Nycum & Carpenter (1968b)
Table 2 (cont'd)
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. sulfone rabbit oral corn oil 75 Nycum & Carpenter (1968b)
A. sulfoxide rat (M) ip water 0.47 Weil & Carpenter (1970a)
A. sulfoxide rat (M) ip corn oil 0.71 Johnson & Carpenter (1966b)
A. sulfone rat (M) ip PEG 21.2 Weil & Carpenter (1970a)
A. sulfoxide rat (M) iv water 0.47 Weil & Carpenter (1970a)
A. sulfone rat (M) iv water 14.9 Weil & Carpenter (1970a)
A. sulfoxide rabbit dermal water >20 West & Carpenter (1966b)
A. sulfone rabbit dermal water >20 West & Carpenter (1966b)
2-methyl-2-
(methyl-sulfinyl)
propanol-1 rat oral 11 000 Weil & Carpenter (1969b)
OH-methyl A rat oral 42.9 Carpenter (1969)
A. sulfoxide oxime rat (M) oral 8060 Nycum & Carpenter (1968a)
A. sulfone oxime rat (M) oral 1590 Nycum & Carpenter (1968a)
A. sulfoxide nitrile rat (M) oral 4000 Nycum & Carpenter (1968a)
A. sulfone nitrile rat (M) oral 350 Nycum & Carpenter (1968a)
A: aldicarb
Mice
- Aldicarb/aldicarb metabolites
Groups of 3 male and 5 female mice were fed a mixture of
aldicarb and aldicarb sulfone (1:1) in the diet at dose levels of 0,
2, 6, 18, or 36 mg/kg bw/day for 7 days. No mortality was noted at
any dose level, but severe cholinergic signs of poisoning were
observed at the high-dose in males. Depression of growth was
observed at the two highest dose levels. Kidney weight was depressed
at the highest dose and liver weight was reduced substantially at 6
mg/kg bw/day and above in both males and females. The NOAEL in this
study was 2 mg/kg bw/day (Weil & Carpenter, 1970d)
Groups of 5 Charles River CD-1 mice/sex were fed aldicarb
sulfone in the diet at dose levels of 0, 0.15, 0.6, 2.4, 9.6 or 38.4
mg/kg bw/day for 7 days. Significant body-weight decrease was noted
at the highest dose but there were no significant organ weight
changes. Cholinesterase activity was not determined (Weil &
Carpenter, 1974e).
Rats
- Aldicarb
Groups of 5 rats/sex were fed aldicarb in the diet for 7 days
at dose levels of 0, 4, 8 or 16 mg/kg bw/day. Mortality was noted
predominantly at the highest dose at which all males and 2 of 5
females died. One of 5 males also died at 8 mg/kg bw/day. There was
a substantial reduction in body-weight gain noted at all dose
levels. In males, kidney weights were significantly reduced at 8
mg/kg bw/day and liver weight was depressed at 4 and 8 mg/kg bw/day.
In females, both liver and kidney weight was significantly depressed
at all dose levels (Weil & Carpenter, 1970b).
Groups of 5 rats/sex were fed aldicarb in the diet at dose
levels of 0, 0.8, 1.6 or 3.2 mg/kg bw/day for 7 days. Growth was
depressed at 1.6 mg/kg bw/day and above. There was no mortality
attributable to aldicarb. In males, liver weights were depressed in
all treatment groups. In females, liver weights were affected only
at the highest dose, but the liver to body-weight ratio was reduced
at 1.6 mg/kg bw/day and above. Kidney weights were reduced in males
at all dose levels and in females only at the highest dose.
Cholinesterase activity, measured on the day after the conclusion of
feeding, was normal at the highest dose tested with the exception of
plasma cholinesterase which was slightly reduced at the highest
level (Weil & Carpenter, 1969a).
Groups of 10 CFE rats/sex were fed aldicarb in their diet for
93 days at dose levels of 0, 0.02, 0.1 or 0.5 mg/kg bw/day. One rat
per level was killed for cholinesterase (plasma, RBC and brain)
determinations at 1, 2, 4 and 29 days of dosing. Mortality was
increased and body-weight and food consumption were decreased at the
highest level. Histopathology of selected organs was unremarkable
and there were no compound-related effects noted. There were no
consistent dose-related effects on the cholinesterase
determinations, except for plasma cholinesterase depression in both
sexes after 30 days at the highest level. There was no indication of
how soon after feeding the cholinesterase determinations were
performed, which could account for the sporadic results, together
with the small number of animals sampled at each interval. The NOAEL
in this study was 0.1 mg/kg bw/day (Weil & Carpenter, 1963).
Rats
- Aldicarb/aldicarb metabolites
Groups of 5 rats/sex were fed diets containing aldicarb at dose
levels of 0, 0.4, 0.8, 1.6 or 3.2 mg/kg bw/day, or aldicarb
sulfoxide at dose levels of 0, 0.4 or 0.8 mg/kg bw/day, or aldicarb
sulfone, at dose levels of 0, 0.4, 1.0, 2.5, 5 or 20 mg/kg bw/day,
for 7 days. With aldicarb (as with its two major carbamate
metabolites) there was a significant growth depression at the
highest dose. There were no effects noted with respect to liver or
kidney weights. The erythrocyte cholinesterase appeared to be the
most sensitive parameter with all three materials tested. The
NOAELs, based on erythrocyte cholinesterase depression or decreased
body-weight gain over the 7-day treatment period were: aldicarb -
0.8 mg/kg bw/day; aldicarb sulfoxide - 0.4 mg/kg bw/day; and
aldicarb sulfone - 2.5 mg/kg bw/day (Nycum & Carpenter, 1968b).
Groups of 5 rats/sex received in the diet, aldicarb (0.3 mg/kg
bw/day), aldicarb sulfoxide (0.4, 0.8 or 1.6 mg/kg bw/day), aldicarb
sulfone (0.6, 5 or 20 mg/kg bw/day), a 1:1 mixture of aldicarb
sulfoxide and aldicarb sulfone (1.2 mg/kg bw/day) or a control diet.
At the end of the 7 days of feeding, animals were placed on control
diets for one day after which they were sacrificed for
cholinesterase determination and for examination of liver and
kidney. A second one-week feeding trial was performed to compare the
data with other strains of rats. Aldicarb sulfoxide was fed to
groups of 5 male rats at dose levels of 0, 0.4, 0.8 or 1.6 mg/kg
bw/day and aldicarb sulfone was fed to male rats at dose levels of
0, 5 or 20 mg/kg bw/day. As might be expected with the protocol
followed in the study, cholinesterase depression was not observed in
either plasma, erythrocyte or brain at the conclusion of the study.
The second trial using both the same and a different strain of rats
was performed in an effort to explain a slight but non-significant
inhibition of erythrocyte cholinesterase activity measured in the
initial study. Over the course of this study, there were no effects
noted on erythrocyte cholinesterase activity (Weil & Carpenter,
1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.3 or 1.0 mg/kg bw/day and aldicarb sulfone at 0,
2.4 or 16.2 mg/kg bw/day. Animals were sacrificed at 1, 3, 7, 14, 28
and 56 days for cholinesterase analyses. Plasma and erythrocyte
cholinesterase activities were measured at the first three time
intervals. At the last three time intervals, plasma erythrocyte and
brain cholinesterase activities were examined. There was no
mortality, but growth was depressed at the highest dose levels with
both the sulfoxide and sulfone. Rats administered aldicarb sulfoxide
at 1.0 mg/kg bw/day had a slight but significant cholinesterase
depression during the study. There were no effects noted at 0.3
mg/kg bw/day. Cholinesterase depression was marked with the aldicarb
sulfone consistently throughout the study at the highest dose level
of 16.2 mg/kg bw/day (Weil & Cox, 1975).
Groups of 10 Wistar rats/sex were administered aldicarb
sulfoxide and aldicarb sulfone in a 1:1 ratio, in drinking water,
ad libitum, for 29 days. The drinking water levels were 0, 0.075,
0.3, 1.2, 4.8 or 19.2 mg/litre. Plasma and red blood cell
cholinesterase levels were determined after 8, 15 and 29 days of
treatment. Brain cholinesterase was measured at termination. Animals
were continuously exposed to the test substances in water until
blood samples were taken or sacrifice was performed. There was no
mortality and food and water consumption values together with body-
weight gains were significantly decreased in both sexes at the high-
dose level when compared to controls. Significant depression of
plasma, RBC and brain cholinesterase, in both sexes, occurred at the
highest dose level. The data demonstrate that a level of 4.8
mg/litre of aldicarb sulfoxide:aldicarb sulfone in water is without
effect on cholinesterase (plasma, RBC, brain) in either sex. Based
on the actual concentrations being approximately 80% of the nominal
concentrations, the NOAEL was roughly 3.8 mg/litre, equivalent to
0.4 mg/kg bw/day (Mirro et al., 1982).
Groups of 15 rats/sex were fed aldicarb sulfoxide in the diet
at dose levels of 0, 0.125, 0.25, 0.5 or 1 mg/kg bw/day for 6
months. Animals were sacrificed at 3 months and at the conclusion of
the study for cholinesterase determinations and for gross and
microscopic examination of liver and kidneys. There was no mortality
noted during the course of the study, although growth, especially in
males, at 0.25 mg/kg bw/day and above was reduced. Cholinesterase
activity was substantially reduced, at the three highest dose
levels, especially in plasma and erythrocytes of males. Gross
examination of liver and kidneys revealed no abnormalities
attributable to aldicarb. The NOAEL in this study was 0.125 mg/kg
bw/day (Weil & Carpenter, 1968b).
In an attempt to resolve the question of cholinesterase
depression and rapid recovery, groups of 5 rats/sex were fed
aldicarb sulfoxide for one week or one week plus one day of control
diets at a dose level of 1 mg/kg bw/day. When the study was
concluded (within one week), animals were sacrificed at 0 and 24 h
after the dietary feeding interval (the 24 h animals were fed
control diets). Cholinesterase depression was noted at the 0 h
sacrifice in erythrocyte and plasma preparations. Administration of
a control diet for one day (24 h sacrifice) completely reversed the
cholinesterase depression noted when animals were sacrificed without
any recovery interval (Weil & Carpenter, 1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.0625, 0.125, 0.25, 0.5 or 1.0 mg/kg bw/day for 3
and 6 months after which some of the animals were sacrificed
immediately and others were placed on a control diet for 24 h prior
to sacrifice and cholinesterase analysis. Cholinesterase activity in
the brain was unaffected by aldicarb sulfoxide. Plasma and
erythrocyte cholinesterase was substantially reduced at the 0 h
sacrifice in both sexes. Males were slightly more sensitive with
depression being noted at 0.25 mg/kg bw/day and above, while with
females depression was noted at 0.5 mg/kg bw/day and above. There
was no cholinesterase depression noted in any of the animals treated
for either 3 months or 6 months when the animals were allowed to
recover from cholinesterase depression for a one day recovery
interval. The NOAEL in this study was 0.125 mg/kg bw/day (Weil &
Carpenter, 1968b).
In a series of studies similar to those reported with aldicarb
sulfoxide, groups of rats were fed aldicarb sulfone at dietary
concentrations at dose levels of 0, 0.2, 0.6, 1.8, 5.4 or 15.2 mg/kg
bw/day for 6 months. Animals were sacrificed at 3 and 6 months for
examination of liver and kidney abnormalities and for evaluation of
cholinesterase activity. Cholinesterase determinations were made at
the end of the 3 and 6 month intervals with rats fed continuously
until sacrifice or rats were allowed to consume a control diet for
24 h prior to sacrifice and determination of cholinesterase
activity. There was no mortality over the course of the study.
Transient but significant growth depression was noted at the highest
level in the longer study. There were no effects noted with respect
to food consumption or on gross and microscopic examinations of
liver and kidneys. Plasma, erythrocyte and brain cholinesterase were
significantlv depressed at 5.4 mg/kg bw/day and above. Erythrocyte
cholinesterase depression was also noted at 1.8 mg/kg bw/day. There
was no cholinesterase depression noted at 0.6 mg/kg bw/day in any of
the tissues examined. In the study to evaluate recovery of
cholinesterase activity, when animals were allowed to equilibrate
for one day on control diets, all depressed cholinesterase values
returned to normal. The NOAEL in this study was 0.6 mg/kg bw/day
(Weil & Carpenter, 1968c).
Groups of rats were fed aldicarb oxime at dose levels of 0,
31.25, 62.5, 125, 250, 500 or 1000 mg/kg bw/day for 7 days. There
was no mortality over the course of the study. Growth was slightly
reduced at the initiation of the study at dose levels of 125 mg/kg
bw/day and above, but at the end of one week only the two highest
dose levels appeared to show a retardation in growth. Gross changes
were noted in both liver and kidneys at the two highest dose levels
in both males and females. The NOAEL in this study was 62.5 mg/kg
bw/day (Weil & Carpenter, 1974b).
Groups of 5 rats/sex were fed the hydrolytic metabolite of
aldicarb (2-methyl-2-(methylsulfinyl)propanol-1) for 7 days at dose
levels of 0, 500 or 1000 mg/kg bw/day. Growth was depressed at both
dose levels in males but only at the highest dose level in females.
In females, while growth was depressed within one day of treatment,
the animals appeared to recover during the rest of the week. There
was no apparent effect on major organs, although, in females at the
highest dose level, kidney weights were slightly depressed.
Cholinesterase activity was not measured (Weil & Carpenter, 1969b).
Rabbits
- Aldicarb
Four groups, each of 5 male rabbits with abraded skin, were
administered aldicarb 10G formulation at dose levels of 0, 5, 10 or
20 mg/kg bw/day, for 6 h per day, 5 days per week, for 15 days.
Water was added periodically during the exposure time to the
dressing containing the aldicarb treatment, simulating a condition
of excess perspiration. One additional group was administered 20
mg/kg bw/day to intact, unabraded skin with no water added to the
dressing. Animals treated with aldicarb under a dry condition with
unabraded skin showed normal weight gains and no apparent effects as
a result of the treatment. Administration of aldicarb under
conditions where the dressing was wet and the skin abraded resulted
in reduced body-weight at a dermal dose of 5 mg/kg bw/day and above.
During the interim where dermal treatment was not applied, recovery
of growth was extremely rapid. Plasma cholinesterase activity was
inhibited at the two highest dose levels. There were no other
adverse effects on haematology or clinical chemical parameters and
limited organ weight analysis and microscopic examination of several
major tissues showed no pathological effects attributable to
aldicarb. Almost identical results were also obtained in a similar
study in rats (Carpenter & Smyth, 1966; Weil & Carpenter, 1968a).
Rabbits
- Aldicarb metabolites
Aldicarb sulfone (technical and 75 WP) were administered to the
unabraded, but closely clipped, ventral surface of rabbits (6
males/group, 5 days/week for 19 applications) at dosages of 4.8
mg/kg bw/day for 75 WP and 3.5, 7.0 or 14.0 mg/kg bw/day for
technical aldicarb sulfone. No mortality, skin irritation or
cholinesterase depression were observed. However, rabbits were
removed from treatment 19 h prior to sampling for cholinesterase
analyses, which could account for the lack of cholinesterase
depression. Body-weight was significantly depressed in the high-dose
level rabbits. There were no reported treatment-related effects on
organ weights and necropsy findings were unremarkable (Weil et
al., 1977).
Dogs
- Aldicarb
Groups of 2 beagle dogs/sex were fed aldicarb at dose levels of
0, 0.2, 0.3 or 0.7 mg/kg bw/day for 7 days. There was no mortality
over the course of the study. Plasma, erythrocyte and brain
cholinesterase activities, measured one day after conclusion of the
dietary treatment, were normal. Gross liver and kidney weights and
organ to body-weight ratios were unaffected by aldicarb in the diet
(Weil & Carpenter, 1973).
Groups of 4 beagle dogs/sex were fed aldicarb in the diet for
five days per week at dose levels of 0, 0.2, 0.3 or 0.7 mg/kg bw/day
for 100 days. There was no mortality over the course of the study
and growth was comparable within all dosage groups. A slightly
decreased testes weight and a slight increase in adrenal weight were
observed at the highest level in males, but there were no effects in
females on any of the tissues and organs examined. Microscopic
analysis did not suggest any abnomalities including tissues where
gross changes had been seen to occur. Cholinesterase values, as well
as other clinical chemistry and haematology parameters, were
unaffected by the presence of aldicarb in the diet, however, the
animals had been removed from aldicarb exposure for 24 to 48 h prior
to cholinesterase analyses. The NOAEL in this study was 0.3 mg/kg
bw/day (Weil & Carpenter, 1974c).
Groups of 3 beagle dogs/sex were fed aldicarb in the diet at
dose levels of 0, 0.025, 0.05 or 0.1 mg/kg bw/day, 5 days per week
for 2 years. There was no mortality over the course of the study and
growth and food consumption data were comparable to control values.
Haematology parameters and clinical chemistry values, evaluated at
five intervals over the course of the study were normal. Plasma and
erythrocyte cholinesterase, evaluated over the course of the study,
did not differ from control values. At the conclusion of the study
brain cholinesterase, while somewhat lower at the high dose level,
was not statistically different from controls. Gross and microscopic
examination of tissues and organs showed no lesions which could be
attributable to the presence of aldicarb in the diet at dose levels
up to and including 0.1 mg/kg bw/day (Weil & Carpenter, 1966c).
A two-week range-finding study was conducted in beagle dogs to
select dose levels for a subsequent one-year dog study. Aldicarb was
fed to groups of 1 male and 1 female at dietary concentrations of 0,
1, 3, 10, 30 or 100 ppm. There was no mortality during the study,
but tremors and slight ataxia were observed at 30 and 100 ppm, and
soft faeces were noted more frequently in these dogs. Food
consumption was depressed in all treated males, particularly at the
highest dose, and slightly depressed in the 100 ppm female. Body-
weight losses were noted in the 100 ppm group and in the 30 ppm
male. Red blood cell and plasma cholinesterase inhibition exceeded
25% at doses of 3 ppm and greater. Brain cholinesterase inhibition
exceeded 25% for the 30 and 100 ppm group males (no calculation was
possible in the female groups due to presumed technical error)
(Hamada et al., 1985b).
As a clear NOEL for cholinesterase depression was not
demonstrated in the first study, a second two-week dietary dose
range-finding study was conducted to select the dose levels for a
subsequent one-year dog study. Aldicarb was fed to groups of 1 male
and 1 female at dietary concentrations of 0, 0.1, 0.3, 1, 3 or 10
ppm. There was no mortality during the study and no
treatment-related clinical signs were observed. Body-weight change
and food consumption were unaffected by treatment. Maximum plasma
and erythrocyte cholinesterase inhibition occurred immediately
following the two-hour feeding and lasted approximately 4 h after
the dosing period. Erythrocyte and plasma cholinesterase inhibition
(> 25% compared to the mean pre-study value) occurred in the 10 ppm
group on both days 7 and 14. There was no inhibition of brain
cholinesterase and no other compound-related effects noted. The
NOAEL in this study was 3 ppm, equal to 0.096 mg/kg bw/day (Hamada,
1987b).
In a five-week study, conducted in order to further investigate
the cholinesterase inhibition dose response curve, aldicarb (99.7%)
was fed at dietary concentrations of 0, 0.35, 0.7, or 2 ppm, to 3
groups of 6 beagle dogs/sex. There were no mortalities nor any
changes in body-weight gain, food consumption, clinical symptoms or
gross pathology indicative of a compound-related effect seen during
the study. Plasma cholinesterase inhibition over 20% when compared
to the control occurred only in the highest dose group. Red blood
cell cholinesterase activity inhibition was not observed in any dose
group and brain cholinesterase activity was not measured. No adverse
effects were observed at 2 ppm (equal to 0.06 mg/kg bw/day) and the
NOEL for any symptoms including plasma cholinesterase depression was
0.7 ppm (equal to 0.02 mg/kg bw/day) (Hamada, 1991).
In a 52-week oral toxicity study in beagle dogs, groups of 5
dogs/sex were fed dietary concentrations of 0, 1, 2, 5 or 10 ppm
aldicarb (95.5%) daily for 52 weeks. The study was designed to
produce maximum cholinesterase depression by limiting feeding time
to two hours per day to mimic a bolus administration of aldicarb.
Erythrocyte and plasma cholinesterase activities were measured from
blood samples collected approximately 2 h after the daily feeding
period to minimise dissociation of the carbamate-cholinesterase-
complex. Aldicarb at up to 10 ppm (equal to 0.241 mg/kg bw/day)
caused no observable effects other than inhibition of cholinesterase
activity. Inhibition of erythrocyte and brain cholinesterase
activity was restricted to dogs receiving 5 or 10 ppm. The NOEL for
plasma cholinesterase inhibition was 1 ppm, equal to 0.027 mg/kg
bw/day, while if the changes in the plasma enzyme activity are
discounted, the NOAEL was 2 ppm, equal to 0.054 mg/kg bw/day
(Hamada, 1988).
An inhalation study was conducted to determine the toxicity of
pyrolysis products of cigarettes made from tobacco pretreated with
aldicarb. Twelve beagle dogs (two animals/sex/dose), which had been
initially conditioned prior to commencement of the test to smoking
10 cigarettes per day via tracheostomy, were exposed to untreated,
low-level and high-level treated cigarettes on a schedule of two
successive cigarettes 5 times per day, 5 days per week for a total
of 19 exposure days. Whole blood cholinesterase was monitored
throughout the study with samples taken before smoking, after 4 and
after 10 cigarettes. All effects observed in this study were
apparently due to inhalation of cigarette smoke. No inhibition of
cholinesterase was seen in any treatment group (Coate et al.,
1982).
Dogs
- Aldicarb metabolites
Groups of 3 beagle dogs/sex were fed aldicarb sulfoxide in the
diet at dose levels of 0, 0.0625, 0.125, 0.25 or 0.5 mg/kg bw/day 5
days per week for 3 months. There was no mortality over the course
of the study. Slight body-weight changes were noted in many of the
dogs at the highest dose level within the first week of treatment
but thereafter the body-weight changes were similar to but lower
than control values. No effects on haematologic and blood chemistry
parameters were observed. Cholinesterase depression measured 24-48 h
after the final exposure was not observed in plasma, erythrocytes or
brain of any of the animals at the conclusion of the study. Gross
and microscopic examination of tissues and organs did not show any
adverse effects attributable to the presence of aldicarb sulfoxide.
The NOAEL in this study was 0.25 mg/kg bw/day (Weil & Carpenter,
1968b).
Groups of 3 dogs/sex were fed aldicarb sulfone in the diet at
dose levels of 0, 0.2, 0.6, 1.8 or 5.4 mg/kg bw/day 5 days per week
for 90 days. There was no mortality over the course of the study.
Slight body-weight depression was noted at the highest dose level,
although the body-weight was not statistically lower than control
levels. There were no effects noted with respect to biochemical and
haematologic parameters and gross and microscopic examination of
tissues and organs revealed no effects attributable to the presence
of aldicarb sulfone in the diet. Cholinesterase depression was not
observed, but dogs were removed from treated diet prior to
cholinesterase determinations. The NOAEL in this study was therefore
greater than 5.4 mg/kg bw/day (Weil & Carpenter, 1968c).
In a two-week range-finding study, aldicarb sulfone was fed to
six groups of 1 male and 1 female beagle dog at dietary
concentrations of 0, 3, 10, 30, 100 or 300 ppm. There was no
mortality during the study, but emesis, tremors and decreased food
consumption were observed in the high-dose group. Inhibition of
cholinesterase (> 25%) occurred at 10 ppm and over in both sexes
for plasma cholinesterase, and 100 ppm and over for red blood cell
and brain cholinesterase (Hamada et al., 1985a).
In a one-year feeding study, aldicarb sulfone was administered
to four groups of 6 beagle dogs/sex at dietary concentrations of 0,
5, 25 or 100 ppm. In this study, samples for cholinesterase
determinations were taken approximately two hours after feeding in
order to measure maximum cholinesterase inhibition. No
treatment-related clinical signs were seen. Body-weight gain was
slightly reduced in the high-dose males and females. There was a
slight decrease in the spleen weights of the mid- and high-dose
females and in the thyroid/parathyroid weight of the high-dose
females when compared to respective control values. These changes
were not seen in the males. Slight treatment-related effects were
noted in the mandibular lymph nodes of the high-dose males and
females, and adrenal cortex of the high-dose females. The NOAEL
based on depression of erythrocyte and plasma cholinesterase
activity was 25 ppm, equal to 0.54 mg/kg bw/day (Hamada, 1987a).
Monkeys
As a result of reports of alleged illness associated with
consumption of watermelons and cucumbers illegally treated or
contaminated with aldicarb in 1985, two studies were conducted to
evaluate the acute toxicity of aldicarb residues in food
commodities. Two crops were chosen for the studies: bananas as a
representative of a registered food crop in the USA, and watermelons
as a representative of a non-registered food crop from the cucurbit
family. (Suggestions had been made that some possible natural
component in cucurbits might synergise aldicarb toxicity). Bananas
were treated with approximately ten times the recommended label rate
and watermelons were treated at 13.44 kg ai/ha, to ensure adequate
residues in the fruit. Each study was performed using 3 Cynomolgus
monkeys/sex, with an additional group of 3 monkeys/sex serving as a
control (total of 12 animals per study). Following analytical
determination of actual residues in the two commodities to be
consumed, (approximately 0.3 ppm in bananas and 5 ppm in
watermelons), intake of the treated crops was adjusted with control
fruit to provide a dose of exactly 0.005 mg aldicarb/kg bw. The
animals were offered the fruit as the first meal of the day, and
consumed it immediately. Individuals were monitored for clinical
signs at regular intervals, and plasma and erythrocyte
cholinesterase activities were measured pre-dose, then at 1, 2, 4,
6, 12, 18 and 24 hours post-feeding. In both studies, there was no
evidence of acute cholinergic distress or any signs of over-exposure
in any animal. Depression of plasma cholinesterase activity was
evident within 1-4 h after ingestion and reached a maximum of 32 to
36% at 2 h after feeding (banana trial) and 36 to 37% at 1 h after
feeding (watermelon trial). Erythrocyte cholinesterase activity was
not depressed in either study. Rapid recovery of all enzyme activity
was observed. There was no indication in these studies that
components in watermelons potentiate aldicarb toxicity (Trutter,
1987a,b).
Long-term toxicity/carcinogenicity studies
Mice
- Aldicarb
Groups of 50 B6C3F1 mice/sex (25 of each sex were used as
controls) were fed aldicarb at dietary concentrations of 0, 2 or 6
ppm for 103 weeks in a carcinogenicity bioassay. A preliminary
dietary study (using 10 mice/sex fed dietary concentrations of
aldicarb of 0, 0.5, 1.0, 2.5, 5, 10, 20 or 40 ppm for 13 weeks)
showed no significant effects on microscopic examinations of the 0,
20 and 40 ppm levels. In the long-term carcinogenicity study there
was no mortality attributable to aldicarb. A variety of benign and
malignant tumours, occurring at different sites in both control and
aldicarb-treated mice, were not unusual for this strain of mice and
were evaluated to be independent of the administration of aldicarb.
Gross and microscopic examination of tissues, organs and all gross
lesions was performed and it was concluded that aldicarb was not
carcinogenic for the B6C3F1 strain of mice of either sex (NIH,
1979).
Groups of 44 CD-1 mice/sex were fed aldicarb at dose levels of
0, 0.1, 0.2, 0.4 or 0.7 mg/kg bw/day for 18 months. Mortality was
evident in males at the two highest dose levels and in females at
the three highest dosage levels during the first two and one-half
months of the study. Following this period, aldicarb was admixed
with the diet in a different manner which appeared to eliminate its
acutely toxic effects. (In the early parts of the study, aldicarb
was mixed in a dry fashion using a finely ground aldicarb
preparation. At the 2.5 month interval, aldicarb was dissolved in
acetone and the acetone-aldicarb solution was dispersed in the diet
at a more uniform rate. It was assumed that consumption of small
crystalline particles of aldicarb may have led to the high mortality
during the initial phases of the study). At the high dose level in
males there was a statistically significant increase in hepatomas
found predominantly in the survivors at the termination of the study
and an increase in lymphoid neoplasias which occured in the mice
that died. None of the male mice surviving at the end of the study
were found to have lymphoid neoplasias. There were no significant
increases in any other types of tumours at dose levels of 0.4 mg/kg
bw/day and below (Weil & Carpenter, 1972c).
Groups of 50 male CD-1 mice were fed aldicarb at dose levels of
0, 0.1, 0.3 or 0.7 mg/kg bw/day in an effort to verify the results
of the previous mouse carcinogenicity bioassay. A group of 150 mice
were used as concurrent controls with a mouse being sacrificed for
each treated animal that died during the course of the study. Diets
were prepared by dissolving aldicarb in acetone and mixing the
solution with the diet. The aldicarb was the same sample as used in
the previous study and the duration of the study was approximately
the same as in the previous trial. There was no mortality observed
in the study as a result of aldicarb in the diet. At the end of 18
months cumulative mortality at all dose levels was the same as noted
in controls. There was no effect of aldicarb on growth in any of the
groups. An examination of the animals that died during the course of
the study and those that were sacrificed at the end of 18 months was
made and the data compared with control values. There was no
significant association between aldicarb in the diet and the
formation of tumours, particularly with respect to the incidence of
hepatomas, lung adenomas, and lymphoid neoplasias. The data were
evaluated with respect to the mice that died, those that survived
the test and the total of all animals. It was concluded that the
administration of aldicarb at levels up to and including 0.7 mg/kg
bw/day for approximately 18 months did not result in a higher than
normal incidence of tumours and the inclusion of aldicarb in the
diet of CD-1 mice did not result in an increased incidence of
carcinogenic response (Weil & Carpenter, 1974d).
Groups of C3H/Hej male mice were administered aldicarb
dissolved in acetone, dermally 3 times a week for 28 months by
applying a brush full of an acetone solution to the shaved back of
the mice. For the first 2 weeks, treatment was performed 3 times a
week using a 0.25% solution in acetone. After 2 weeks this was
reduced to a twice weekly application. This dosing regimen was
maintained for 2 months and further reduced thereafter to a
concentration of 0.125% which was maintained for the rest of the
study. While there was some aldicarb-induced mortality noted over
the course of the study this mortality was not substantially
different from that noted with control applications. There were no
substantial differences with respect to the incidence or onset of
tumours. Two growths, a haemangioma and a thymoma, were noted in the
animals administered aldicarb. Neither of these internal growths was
accompanied by cutaneous papillomas or carcinomas and were
considered to be spontaneous growths unrelated to treatment.
Aldicarb, administered dermally to this sensitive species, did not
induce any incidence of malignancy (Weil & Carpenter, 1966b).
Mice
- Aldicarb metabolites
Groups of Charles River CD-1 mice (50/sex/group) were
administered 0, 0.15, 0.6, 2.4 or 9.6 mg aldicarb sulfone/kg bw/day
via the diet for 18 months. Aldicarb sulfone did not affect tumour
incidence or produce any pathological alteration in this strain of
mouse (Woodside et al., 1977b).
Rats
- Aldicarb
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0, 0.005, 0.025, 0.05 or 0.1 mg/kg bw/day. Additional
groups of 16 rats/sex were maintained for serial sacrifices at 6 and
12 months. There was no mortality, attributable to the presence of
aldicarb. Growth was normal at all dose levels as was consumption of
food and behavioural characteristics. Results of gross examination
of liver and kidney weight at 6 months and at one year did not
differ from control values. Haematologic determinations in the
highest dose level and control groups were normal. Blood and brain
cholinesterase activity, measured at 6 and 12 months, were normal.
Microscopic examination of tissues and organs for histopathologic
occurrences and neoplasms showed the incidence of lesions to be
similar in aldicarb-treated and in control groups. An apparent
no-effect-level in this study is 0.1 mg/kg bw/day (Weil & Carpenter,
1965).
Groups of 50 F344 rats/sex (25/sex for controls) were fed
aldicarb in the diet at dietary concentrations of 0, 2 or 6 ppm for
103 weeks. A preliminary study used 10 rats/sex, with dietary
concentrations of 0, 5, 10, 20, 40, 80, 160 or 320 ppm for 13 weeks.
Microscopic examinations performed on male and female rats of the 0
and 80 ppm dose levels at the conclusion of the preliminary trial
showed no significant effects. In the long-term carcinogenicity
study, there was no mortality attributable to aldicarb in the diet.
A variety of benign and malignant tumours occurring at different
sites in both control and aldicarb treated rats were not unusual for
this strain of rat and were evaluated to be independent of the
administration of aldicarb. Gross and microscopic examination of
tissues, organs and all gross lesions was performed and it was
concluded that aldicarb was not carcinogenic for the F344 strain of
rat of either sex at dietary concentrations of up to 6 ppm (NIH,
1979).
Rats
- Aldicarb/Aldicarb metabolites
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0 or 0.3 mg/kg bw/day. In addition, groups of rats were
fed aldicarb sulfoxide (0.3 or 0.6 mg/kg bw/day), aldicarb sulfone
(0.6 or 2.4 mg/kg bw/day) or a 1:1 mixture of aldicarb sulfoxide and
aldicarb sulfone (0.6 or 1.2 mg/kg bw/day). There was no significant
mortality observed over the course of the study with any of the
individual chemicals or the mixture of sulfoxide and sulfone. In the
initial phases of the study, there was a slightly higher mortality
noted in the high-dose level of aldicarb sulfoxide and in the group
receiving the combined aldicarb sulfoxide and aldicarb sulfone. A
slight increase in mortality was also noted at the latter part of
the study with aldicarb sulfoxide. Growth was slightly depressed at
the high-dose level of the sulfoxide:sulfone mixture, primarily in
males. There were no apparent effects on growth with respect to the
aldicarb, aldicarb sulfoxide or aldicarb sulfone administered alone.
Haematological values observed at various intervals over the course
of the study did not differ from controls. Cholinesterase
determinations were made periodically over the course of the study
(6, 12 and 24 months). Plasma, erythrocyte and brain cholinesterase
were examined only at a 24-h interval after animals were removed
from test diets. There was a slight depression of plasma
cholinesterase noted in males administered the high-dose level of
the combination aldicarb sulfoxide and sulfone at the 24-month
interval. A repeat of the analyses within the final week of the
study showed a slight depression in all chemical groups with respect
to plasma cholinesterase. There were no effects noted at any
interval with respect to red blood cell or brain cholinesterase. The
plasma cholinesterase depression noted at the 24-month interval was
limited to male rats. An evaluation of the incidence of tumours
suggested that there was no statistical difference between treated
and control groups. Gross and microscopic examination of tissues and
organs at various periods over the two-year test interval showed
that these sporadically distributed lesions could not be considered
to be indicative of damage induced by aldicarb, its major
metabolites, or the combination of the sulfoxide and sulfone (Weil &
Carpenter, 1972a).
Reproduction studies
Rats
- Aldicarb
Groups of rats (8 male and 16 female rats per group) were fed
aldicarb at dietary concentrations at doses of 0, 0.05 or 0.1 mg/kg
bw/day for approximately 90 days and mated to initiate a 3-
generation, one litter per generation reproduction study. Dietary
administration was continuous throughout the study. In addition to
the reproduction indices (fertility, gestation, viability and
lactation) the F3 generation was maintained for an additional
period and tissues from these animals were histologically examined
at either weaning or at 90 days of age. In all groups, the
reproduction indices from the aldicarb-treated animals were similar
to the control group. Body-weights of both male and female pups at
weaning were similar to control values as were results of gross and
microscopic examinations of tissues and organs in the F3 weanling
and 90-day old animals. In all three generations, with all criteria
examined, there were no effects of aldicarb on reproduction at a
dose level of 0.1 mg/kg body-weight (Weil & Carpenter, 1964).
Groups of rats (10 male and 20 female per group) were
administered aldicarb in the diet at doses of 0, 0.2, 0.3 or 0.7
mg/kg bw/day for 100 days prior to pairing and mated to initiate an
additional 3-generation (one litter per generation) reproduction
study. Dietary administration was continuous throughout the study. A
larger group was used for the F2 generation (15 male and 25 female
rats) as male pups of this generation were maintained on aldicarb
diets for 148 days and subjected to a (modified) dominant lethal
(mutagenesis) bioassay where they were mated with groups of
untreated virgin females for a period of 10 weeks. Each female in
the group was mated with 2 treated males and allowed to maintain
pregnancy until day 12 when they were sacrificed and examined. There
was some mortality over the course of the study which was associated
with lung infection and not as a result of aldicarb in the diet. At
the high-dose level, body-weights of both male and female F2 pups
were lower than the control values. Overall, there were no effects
on any of the reproduction indices (fertility, gestation, viability,
or lactation). Gross and microscopic examinations of the parents and
pups of the high level and control groups showed no effects
attributable to aldicarb. The dietary dominant lethal mutagenesis
bioassay showed no statistical differences between the
aldicarb-treated rats and controls with respect to early or late
fetal death or any other parameter examined (Weil & Carpenter,
1974a).
Rats
Aldicarb metabolites
Aldicarb sulfone (99.76% pure) was fed to groups of 10 male and
20 female rats at dietary concentrations at doses of 0, 0.6, 2.4 or
9.6 mg/kg bw/day for approximately 100 days. Rats were then mated to
initiate a three-generation, one litter per generation, reproduction
study. Dietary administration was continuous throughout the study.
Male rats fed 9.6 mg/kg bw/day exhibited reduced body-weights. There
were no differences from the control with regard to fertility,
gestation survival or viability indices. It was determined that
aldicarb sulfone, at levels up to and including 9.6 mg/kg bw/day,
was without adverse effects on reproduction under the conditions of
this study (Woodside et al., 1977a).
Special studies on embryo/fetotoxicity
Rats
- Aldicarb
Using a test protocol where both the reproductive and
teratologic potential of aldicarb was evaluated, groups of pregnant
rats were fed aldicarb at dietary concentrations at dose levels of
0, 0.04, 0.2 and 1.0 mg/kg bw/day. Females from each of the dietary
groups were assigned to one of three treatment groups: (1) aldicarb
administered in the diet throughout pregnancy or until pups were
weaned; (2) aldicarb administered in the diet from day 0 to day 7 of
gestation; (3) aldicarb administered in the diet from day 5 to day
15 of gestation. Five or six females from each group were sacrificed
and examined on day 20 of pregnancy and a similar number of females
were allowed to bear, nurse and wean the pups. There were neither
gross manifestations of teratogenicity in any of the pups carried by
females administered aldicarb at dose levels of up to 1 mg/kg bw/day
nor was there apparent interference with the reproductive process by
any of the dosage regimens used in this study. The administration of
aldicarb at dose levels up to and including 1.0 mg/kg bw/day had no
apparent effect on the growth of pregnant females during the course
of the study (Weil & Carpenter, 1966a).
Pregnant Sprague-Dawley rats were given aldicarb by gavage at
levels of 0, 0.125, 0.25 or 0.5 mg/kg bw/day on gestational day 6
through day 15. Maternal toxicity was indicated, at the highest
dose, by reduced weight gain and food intake during the treatment
and post-treatment period as well as by the occurrence of three
maternal deaths and, at the two highest levels, by reduced food
consumption during treatment. Gestational parameters, including
ovarian corpora lutea, number of implantations per litter and sex
ratio were unaffected by treatment. Fetal body-weight per litter was
significantly reduced at 0.5 mg/kg bw/day. There was no increase in
the incidence of external or skeletal malformations. A significantly
increased incidence of dilated lateral ventricles with tissue
depression was observed only at 0.5 mg/kg bw/day. No increased
incidence of malformation was observed in the absence of clear
maternal toxicity nor were these malformations accompanied by more
severe malformations. The NOEL was 0.125 mg/kg bw/day for maternal
toxicity and 0.25 mg/kg bw/day for embryo-fetal toxicity and
teratogenicity (Tyl & Neeper-Bradley, 1988).
Rats
- Aldicarb metabolites
The aldicarb sulfone reproduction study incorporated a
teratology bioassay. The animals were divided into 4 groups and
orally dosed with 0, 0.6, 2.4 or 9.6 mg/kg bw/day at one of the
following time intervals of gestation: 0-20 days, 6-15 days, or 7-9
days. All animals were sacrificed before parturition (day 20) and
the fetuses examined for skeletal and visceral changes. Anomalies
were essentially non-existent and there was no indication of
structural abnormalities produced under the conditions of the study
at levels up to and including 9.6 mg/kg bw/day (Woodside et al.,
1977a).
Rabbit
- Aldicarb
A range-finding study was conducted with aldicarb in rabbits to
determine dosage levels of aldicarb for a teratology study. Pregnant
Dutch belted rabbits (5/dose) were dosed by oral gavage at 0.1,
0.25, 0.5, 0.75 or 1.0 mg/kg bw/day on days 6 to 27 of gestation.
Two does in the high-dose group and one doe in the second highest
group died during the study. Increased incidences of decreased
defecation (all except low-dose group) and soft stools (two highest
dose groups) were noted. Maternal body-weight losses or markedly
decreased maternal body-weight gain during the treatment and
gestation intervals were observed at 0.25 mg/kg bw/day and greater.
Of the uterine parameters measured, there was an increase in the
mean post-implantation loss in the high-dose group. Dosage levels of
0.1, 0.25 and 0.5 mg/kg bw/day were selected for the definitive
study (Aldridge et al., 1983).
Pregnant Dutch belted rabbits (16/dose) were dosed by oral
gavage at 0.1, 0.25, or 0.5 mg/kg bw/day on days 7 to 27 of
gestation. Signs of maternal toxicity in the treated groups included
small amount of stool, pale kidneys and hydroceles on oviducts. A
loss in body-weight occurred in the 0.25 and 0.5 mg/kg bw/day dosage
groups during gestation days 7 to 27. A decline in the number of
viable fetuses per doe was observed in all treatment groups (8.7,
5.0, 6.5 and 6.2 for the control, 0.1, 0.25 and 0.5 mg/kg bw/day
treatment groups, respectively). This can be explained by an
unusually high number of implantations in the control group (9.8,
6.1, 7.2 and 7.8 implantations per doe in the control, 0.1, 0.25 and
0.5 mg/kg bw/day treatment groups, respectively). There was no clear
dose-effect relationship. There were also no increases in
post-implantation losses (1.1, 1.1, 0.7 and 1.7 post-implantation
losses for the control, 0.1, 0.25 and 0.5 mg/kg bw/day treatment
groups, respectively) these figures falling within the historical
control range. There was no meaningful difference in any of the
other developmental parameters nor in the number of developmental
variations or malfunctions between the control and treatment groups.
Aldicarb did not produce a teratogenic response when administered
orally to rabbits by gavage at dosage levels up to 0.5 mg/kg bw/day
(Leng et al., 1983).
Special studies on genotoxicity
Results of genotoxicity tests are summarised in Tables 3 and 4
(for in vitro and in vivo tests with aldicarb) and Table 5 (for
in vitro tests with aldicarb metabolites).
Table 3: Results of in vitro genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980c)
TA98,TA100,TA1535,
TA1537,TA1538
Reverse E. coli WP2 nk nk Negative Dunkel et al. (1985)
mutation assay
Reverse S. cerevisiae nk nk Negative Guerzoni et al. (1976)
mutation assay
HGPRT forward CHO cells 1000-5000 µg/ml nk Negative Stankowski et al. (1985a)
mutation assay
SCE Human nk nk Weak Debuyst & Larebeke (1983)
mutation assay lymphocytes Positive
Cytogenetics Human 10-250 µg/ml nk Weak Cid & Matos (1984)
mutation assay lymphocytes Positive
UDS Rat hepatocytes 0.16-5000 µg/ml nk Negative Godek et al. (1984a)
DNA damage S. typhimurium > 500 µg/disc nk Positive Rashid & Mumma (1986)
TA 1538 uvrB
TA 197
nk: not known
Table 4: Results of in vivo genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Micronucleus Mouse 0.001-0.01 93.47% Negative Ivett et al. (1984)
test mg/kg bw
Micronucleus ICR mouse 0.1-0.4 99.7% Negative Ivett (1990)
test mg/kg bw
Dominant Wistar rat 0.2-0.7 99.2% Negative Weil & Carpenter (1974a)
lethal test mg/kg bw
Table 5: Results of in vitro genotoxicity assays on aldicarb metabolites
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980b)
TA98,TA100,TA1535, sulphoxide
TA1537, TA1538
Ames test S. typhimurium 100-10000 µg/plate nk Negative Godek et al. (1980a)
TA98,TA100,TA1535, sulphone
TA1537, TA1538
HGPRT forward CHO cells 500-1500 µg/ml nk Negative Stankowski et al. (1985b)
mutation assay sulphone
Cytogenetics CHO cells 50-500 µg/ml nk Negative San Sebastian et al. (1984)
mutation assay sulphone
UDS Rat hepatocytes 0.1-3000 µg/ml nk Negative Godek et al. (1984b)
sulphone
nk: not known
Special studies on skin and eye irritation and sensitization
Administration of aldicarb to the conjunctival sac of rabbits
did not produce ocular irritation or corneal damage. Ocular
irritation studies were performed at doses that were lethal without
indication of ocular damage. Furthermore, there was no evidence of
dermal irritation when aldicarb was applied to the shaved, abraded
backs of rabbits (Striegel & Carpenter, 1962).
There was no indication of a sensitization reaction induced by
aldicarb. Male guinea-pigs were administered aldicarb by multiple
subdermal applications (0.7 mg/kg body-weight) and re-administered
aldicarb three weeks later by a similar intradermal administration.
There was no suggestion of sensitization in any of the animals
tested (Pozzani & Carpenter, 1968a).
Skin sensitization to aldicarb sulfone (UC 21865, technical) or
a 75% WP formulation was evaluated in albino guinea-pigs (Hartley
Strain) using a modified Landsteiner technique. Neither substance
was determined to be a sensitizer under the conditions of this study
(Conroy & Carpenter, 1977).
Special studies on delayed neurotoxicity
Hens
- Aldicarb
Groups of 6 adult chickens were administered aldicarb as a
single oral dose of 4.5 mg/kg bw or as daily oral doses of 0, 2.25
or 4.5 mg/kg bw/day for 30 days. A positive control group, treated
with 100 mg of TOCP, was used to produce typical delayed neurotoxic
signs of poisoning. While there was some weight loss, which was
correlated with the dose of aldicarb administered, the only
neurological effects attributable to aldicarb were acute signs of
poisoning noted in the first two or three days of treatment. Neither
ataxia nor hind limb paralysis were noted over the course of the
study. Aldicarb did not induce a delayed neurotoxic syndrome similar
to that induced by certain organophosphate esters (Johnson &
Carpenter, 1966a).
Hens
- Aldicarb metabolites
A group of 40 adult white Leghorn hens were intubated to
receive 250 mg/kg bw aldicarb sulfone suspended in maize oil. Two
other groups, each with 10 hens, received either TOCP (500 mg/kg bw)
or maize oil alone, and served as positive and negative control
groups respectively. There were no neurological effects other than
acute cholinergic signs of poisoning in the TOCP dosed group. No
histological examination was performed, owing to the lack of
demonstrated neurotoxic signs. Aldicarb sulfone did not cause
delayed neurotoxic reactions under the conditions of the study
(Babish & Salerno, 1977).
Special studies on behaviour
The effects of acute administration of aldicarb and aldicarb
sulfoxide on avoidance behaviour in rats was compared to a variety
of other carbamate esters. Rats were trained and evaluated for their
ability to avoid electrical shock in standard avoidance behaviour
tests. Aldicarb and aldicarb sulfoxide were administered by
intraperitoneal injection and the rats were evaluated for their
ablity to avoid shocks over a 6 h period following administration.
The lowest beviourally effective dose was found to be 0.266 mg/kg bw
which, when compared to the acute ip LD50 value, was noted to have
a smaller ratio of behaviour effects to acute LD50 than any of the
other carbamates tested. These data suggest that the level of
aldicarb needed to produce measurable avoidance is greater (closer
to a fatal dose and less likely to be achieved at the suggested use
level) than the chemicals to which it was compared. Additionally,
the activity over the 6-h period was seen to rapidly decline again
attesting to the transient nature of the cholinesterase inhibition
(Johnson & Carpenter, 1966b).
Special studies on immune responses
Aldicarb was evaluated for its ability to modulate the immune
response in two strains of mice. The B6C3F1 mouse was chosen
because it is the strain used by the US National Toxicology Program
for immunotoxicology studies, and the hybrid Swiss Webster mouse was
included because aldicarb had been reported to suppress the splenic
plaque-forming cell response to sheep red blood cells in an inverse
dose-response fashion (Olson et al., 1987). An attempt was made to
reproduce these findings and to expand the data base using more
standardised techniques with the B6C3F1 strain. Aldicarb was
administered in the drinking water ad libitum for 34 consecutive
days to female mice of both strains at dosages ranging from 0.1 to
1000 µg/litre in 10-fold increments (equivalent to 0.04 to 364 mg/kg
bw/day). Aldicarb had no effects on body-weights or organ weights,
on numbers or types of circulating white blood cells, or on the
microscopic pathology of the thymus, spleen, liver, kidneys, or
lymph nodes. Also unaffected were the number of antibody forming
cells in the spleen and the amount of circulating antibody in the
blood. Aldicarb had no effect in either strain on in vivo host
resistance to infectious viral challenge, on the capacity of B- and
T-lymphocytes to respond to nonspecific mitogens, or on the ability
of T-lymphocytes to recognize genetically different cell types in a
mixed lymphocyte culture. In this study, subchronic exposure to
aldicarb in the drinking water of mice had no effect on any measured
immunological function or toxicological parameter (Thomas et al.,
1987).
In another study, adult female B6C3F1, mice received
distilled water only or water containing 1.0, 10 or 100 µg/litre of
aldicarb daily for 34 days. To further develop an immune profile of
the compound, following aldicarb exposure, the ability of splenic
natural killer (NK) cells as well as specifically sensitized
cytotoxic T-lymphocytes to lyse YAC-1 lymphoma and, P815 tumour
cells, respectively, was evaluated. To complement the functional
assays, the impact of aldicarb exposure on spleen cell viability,
splenic cellularity and the percentages and absolute numbers of
total T-cells, T-suppressor, T-helper and B-cells was evaluated. The
results of this study demonstrated that aldicarb did not impact upon
the functional ability of interferon-induced splenic NK cells to
lyse YAC-1 lymphoma target cells. Aldicarb had no effect on the
functional capacity of cytotoxic T-lymphocytes to recognize
allogeneic cells in vivo and undergo differentiation and lysis of
these cells in a short-term 51-Cr release assay. The numbers and
percentages of T-cell subpopulations and B-cells in the spleen also
were not significantly affected. In addition, aldicarb had no
significant effect on body-weights, on spleen cellularity or cell
viability or on absolute weights of lymphoid organs. The absence of
statistically significant effects on any of these parameters
indicated that aldicarb did not have adverse effects on the immune
system of mice (Thomas et al., 1990).
Special studies on pesticide antagonistic agents
Following acute oral administration to rats, aldicarb has been
shown to induce a strong muscarinic action at excretory, bronchial
and cardiac nerve sites. A nicotinic effect was also shown to occur
at myoneural junctions. The parasympathetic signs of poisoning were
readily reduced following atropine administration. Administration of
atropine and 2-PAM, alone, or in combination, showed that while
atropine was a more effective antidote, 2-PAM was also active. While
it has been shown that aldicarb elicits a strong muscarinic action
as well as nicotinic action at myoneural sites, the control of signs
of poisoning from both mechanisms appears to be somewhat difficult
to achieve. Atropine has been shown to be an effective antidote to
block the muscarinic effects, but decamethonium, commonly used to
block the nicotinic effects, has been shown to be somewhat
ineffective. Additional studies to influence the nicotinic actions
by such drugs as tubocurare also failed to completely eliminate
nicotinic activity. Further studies confirmed the therapeutic
effects of a variety of oximes (P2S and obidoxime) in reducing the
acute toxic signs of poisoning associated with aldicarb. (Johnson &
Sullivan, 1968a,b; Natoff & Reiff, 1973).
Special studies on pesticide interactions
Aldicarb was administered orally to male rats alone and in
combination with a series of eight organophosphate esters or one
carbamate ester, all anti-cholinesterase agents, to examine the
potential interactive or additive effects. Results of the study,
using proportions of the acute lethal dose of each material alone
and in combination with aldicarb, showed a simple additive effect
with all materials tested. Aldicarb was not found to potentiate the
acute oral toxicity of other anticholinesterase agents. Further
studies were reported on the potential interaction of aldicarb with
alpha-naphthol, aldicarb sulfoxide with aldicarb sulfone and
aldicarb sulfone with parathion administered orally and aldicarb
with alpha-naphthol or with carbaryl administered by the
intraperitoneal route. In no case were any interactions greater tban
the predicted additive effects (West & Carpenter, 1966a; Weil &
Carpenter, 1970a).
Observations in humans
Groups of 4 adult male volunteers were administered aldicarb
orally in aqueous solution at dose levels of 0.025, 0.05 or 0.1
mg/kg body-weight. Clinical signs of poisoning were recorded and
whole blood cholinesterase activity was measured up to 6 h after
administration of the sample. Total urine voided was collected and
aldicarb-excretion patterns for the initial 8 h after dosing were
evaluated. In addition, spot samples were taken at 12 and 24 h.
Acute signs of poisoning, typical of anticholinesterase agents, were
observed at the high-dose level within 1 h after administration of
aldicarb. There were no signs of poisoning observed at the 0.05
mg/kg body-weight dose level. Cholinesterase depression was observed
in all volunteers prodominantly within 1-2 h after treatment. Within
the first six hours of treatment almost all cholinesterase
depression and clinical signs of poisoning were diminished.
Examination of urinary excretion patterns showed that approximately
10% of the administered dose was excreted as carbamates (toxic
residues) within the first 8 h interval. Cholinesterase analyses
confimed the same rapid inhibition and recovery pattern with man as
had been observed in experimental animals (Haines, 1971).
In another study, two additional subjects were administered
aldicarb in water solution at dose levels of 0.05 and 0.26 mg/kg
body-weight. Acute signs of poisoning were recorded at the higher-
dose level and atropine was administered to aid recovery. No signs
of poisoning were recorded with the lower-dose level. Urinary
excretion of carbamate residues within 24 h accounted for
approximately 10% of the administered dose (Cope & Romine, 1973).
A double blind, placebo controlled study has been conducted, in
which aldicarb was given as a single oral dose to healthy male and
female subjects. The doses administered were: placebo (22 subjects -
16 males and 6 females), 0.01 mg/kg bw (8 males), 0.025 mg/kg bw (8
males and 4 females), 0.05 mg/kg bw (8 males and 4 females) and
0.075 mg/kg bw (4 males). Subjects were screened before entry by
general medical history and examination and laboratory tests
including haematology, clinical chemistry and urinalysis. Clinical
measurements were made at intervals before and after dosing. These
included vital signs, (systolic and diastolic blood pressure, pulse
rate), pulmonary function tests, pupil size, electrocardiographs,
salivation and clinical signs of nausea, vomiting, diarrhoea,
sweating, abdominal cramps, involuntary movement and slurred speech.
Samples were taken for urinalysis, clinical chemistry (including red
blood cell and plasma cholinesterase activity) and haematology
evaluation before and after dosing. Urine and blood were collected
for aldicarb analysis. The results of these latter analyses were not
reported. There were no clinically significant changes in vital
signs, pupil size, pulmonary function, ECG's, salivation, clinical
signs, clinical chemistry (apart from cholinesterase), haematology
or urinalysis in the study. Cholinesterase activity in red cells and
plasma was maximally depressed at one hour after dosing and had
recovered by 8 h in all subjects. The fall in activity was dose-
related. Only marginal depression of cholinesterase activity (< 20%)
was seen in erythrocytes from patients treated with 0.01 or 0.025
mg/kg bw and in plasma at 0.01 mg/kg bw. Depression in
cholinesterase activity > 20% was seen in erythrocytes at 0.05 and
0.075 mg/kg bw and in plasma at 0.025, 0.05 and 0.075 mg/kg bw. No
serious adverse events occurred. The minor adverse events which were
recorded were similar to those reported in other volunteer studies.
Only one subject (0.075 mg/kg bw group, actual dose 0.06 mg/kg bw)
developed symptoms which were reported to be related to aldicarb.
The NOAEL in this study should be based on depression (> 20%) in
erythrocyte cholinesterase activity and was thus 0.025 mg/kg bw
(Nimmo et al., 1992).
Fifteen male volunteers participated in a study in Panama to
evaluate human exposure in a banana plantation where Temik-15G was
applied under natural conditions. Temperatures ranged from 24 °C to
32 °C with 80% to 90% relative humidity. The workers used three
different types of hand-held applicators. Blood samples were taken
from all volunteers prior to initiating the test and immediately
following each 6 h working period for estimation of blood
cholinesterase activity. Samples were obtained in the field and
analyzed 1-2 h later in the laboratory. Only two workers showed
greater than 25% reduction in their blood cholinesterase activity
(29% and 50%). Spontaneous reversibility was evident in both cases.
The worker with 50% reduction showed 25% recovery within 3 h. Blood
samples from the other worker indicated 100% recovery 24 h later.
Results of blood analyses indicated that cholinesterase activity was
below the normal range (population) in samples collected from six
workers during the second day of the study. Cholinesterase activity
in all samples taken during the first and third day of the study
were within the normal range. No clinical signs of aldicarb-induced
intoxication were found, although one individual presented symptoms
of nausea, stomachache and headache (Union Carbide, 1979).
A series of human exposure episodes was reported occurring as a
result of a variety of field and glasshouse conditions in an effort
to assess the potential for human harm from exposure under actual
occupational conditions. In several instances, slight blood
cholinesterase depression attested to the actual exposure situation.
Exposure data, as indicated by cholinesterase depression or urinary
excretion, suggested that there was no change in the general health
of workers exposed under any of the working conditions. Although
there were acute clinical signs of poisoning there was no indication
that the workers exposed were harmed once removed from exposure
situation (Williams, 1966; Burrows et al., 1970; Wakefield et
al., 1973; Shrivastava, 1975; Pandey, 1977).
From 1966 to 1979, 133 cases of apparent over-exposure to
aldicarb formulations were reported. Of these cases, 40 were
confirmed aldicarb poisoning episodes where clinical diagnosis
and/or urinalysis for aldicarb and its metabolites were performed.
As has been the case with other carbamate insecticides, the acute
signs of toxicity are rapidly dissipated, although atropine therapy
and hospitalization have been useful therapeutic regimens (Abdalla,
1977, 1979). Several deaths have been reported, but all of these
have been attributed to suicide or gross neglect (Lee, 1984).
California reported that in 1974, 1975 and 1976 a total of 10,
14 and 13 cases of human illnesses were reported, respectively. In
these incidents, people were directly exposed to aldicarb and
illness was brought on by dermal, inhalation and in one instance,
ocular exposure. While most illnesses resulted from aldicarb
exposure in loading or applying the formulated pesticide, some
illness has been reported from the handling of plants and soils
treated with aldicarb (Peoples et al., 1977).
Several misuses of aldicarb on watermelons and cucumbers have
been reported in the literature. Goes et al. (1980) reported on a
suspected food-borne intoxication in Nebraska associated with the
consumption of aldicarb-contaminated hydroponically grown cucumbers.
Two episodes were reported (April 1977: 9 cases; July 1978: 5
cases). Neither cholinesterase determinations nor urine residue
determinations were performed in any of these patients. Four
cucumbers (two in the supermarket and two in the greenhouse were
analyzed and aldicarb content was 6.6 and 10.7 mg/kg (supermarket) 0
and 9.9 mg/kg (greenhouse). No data are available on cucumbers
actually eaten and no attempts to correlate symptoms and aldicarb
consumption were made.
Hirsch et al. (1987) reported over 300 alleged intoxications
in Canada following ingestion of aldicarb contaminated cucumbers
(residue levels up to 26 mg/kg). In only five cases involving 13
patients, were any remaining portion of the cucumbers consumed still
available for analyses.
Goldman (1990) reported on four outbreaks of aldicarb poisoning
having occurred in California and other states in the USA from 1978
to 1988.
Outbreak 1: watermelons, 1985
Outbreak 2: watermelons, 1987
Outbreak 3: cucumbers, 1988
Outbreak 4: cucumbers, 1978 (corresponds to Goes (1980)
publication).
In the first outbreak, 1376 people allegedly became ill due to
consumption of contaminated watermelons. Of this total, 308 were
considered by the authors themselves as unlikely (235) or incomplete
(73). Nevertheless, only 28 cases were supported by positive
findings of aldicarb residues in the food commodity. All the other
cases were only supported by self-diagnosis.
An epidemiological study of potential adverse health effects of
aldicarb metabolites in drinking water was performed. In two surveys
conducted on Long Island, New York, the major cohorts were selected
on the basis of aldicarb levels in their drinking water. Levels of
contamination were generally 4-12 µg/litre, but a maximum of 400
µg/litre was reported. Questionnaires to determine water and food
consumption, symptoms experienced, and diagnosed illnesses were sent
to 1035 residents of 462 households. Although the initial survey
indicated a possible association between the incidence of diarrhoea
and levels of aldicarb in the water, the follow-up study, focusing
on children, did not confirm this association. No relationship was
detected between food consumption or water source and adverse health
symptoms, and self-reported physician-diagnosed illnesses for
1974-1979 were not significantly related to levels of aldicarb in
the water (Whitlock et al., 1982).
Another survey attempted to relate self-reported symptoms
suggestive of peripheral neuropathy to aldicarb levels in drinking
water in Suffolk County, New York. The response rate was less than
20%. Responses were classified as "probably", "possibly", or
"vaguely" suggestive of a neurologic syndrome. A significant
correlation with aldicarb concentration was obtained only by
combining all three categories of response, including reports of
just one symptom or of symptoms not forming any cohesive syndrome.
The authors concluded that further study was needed (Sterman &
Varma, 1983).
A pilot epidemiological study by Fiore et al. (1986)
evaluated a wide range of clinical immunological parameters in 23
women exposed to aldicarb in their drinking water and in a
non-exposed control group. The groups did not differ in any
immunological parameters except in T8-Lymphocytes: the number was
considered as elevated in five exposed and in one control.
Observation of an elevated stimulation assay response to one of the
large number of antigens (Candida) was not considered as
toxicologically significant, nor was it attributed to aldicarb
exposure.
In a follow-up study, five women of the preceeding group and
still exposed to aldicarb were examined versus previous control
women plus women previously exposed but for whom exposure no longer
occurred (e.g., change in water supply due to addition of a charcoal
filter). According to the authors, this follow-up study confirmed
the previous results, except for increased response to Candida
antigens which was no longer present (Mirkin et al., 1990).
COMMENTS
Aldicarb is rapidly absorbed, widely distributed in the body
and rapidly excreted. Metabolism appears to be similar in all
species studied, aldicarb being rapidly metabolised to aldicarb
sulfoxide, which is more slowly degraded to aldicarb sulfone. All
metabolites are quickly eliminated from the body, 80-90% being
excreted within 24 h. Elimination was complete by the fifth day
after dosing and no bio-accumulation was seen.
Aldicarb has high acute toxicity in a wide variety of mammalian
species. Signs of toxicity are those commonly associated with
acetylcholinesterase inhibition by a carbamate insecticide:
cholinergic signs of poisoning, which are alleviated rapidly on
cessation of exposure. Aldicarb sulfoxide is a more potent inhibitor
of acetylcholinesterase than aldicarb itself, while aldicarb sulfone
is considerably less toxic than either aldicarb or the sulfoxide.
WHO has classified aldicarb as extremely hazardous (WHO, 1992).
Short-term and long-term studies have been performed in rats,
mice and dogs with aldicarb and aldicarb metabolites, both
individually and in combination. Toxicity tests employing mixtures
of aldicarb or aldicarb sulfoxide with aldicarb sulfone are of
interest because aldicarb sulfoxide and aldicarb sulfone are the
terminal residues potentially consumed by humans. Cholinesterase
depression is the most significant indicator of toxicity that can be
evaluated. However, considerable attention must be paid to the
methods of sample collection and determination of cholinesterase
activity. Continuous administration of aldicarb to the test animals
until collection of samples for analysis is important, as is rapid
analysis under carefully controlled conditions.
No-effect levels in the various studies which included
evaluation of cholinesterase inhibition are summarized in Table 6.
No distinction is made in this table for the methods of determining
cholinesterase activity.
It is now considered inappropriate to use no-adverse-effect
levels from many of the earlier repeat-dose studies for the
derivation of an ADI, because animals were not dosed for 24-48 h
prior to collection of tissue samples for measurement of
cholinesterase activity. In the most recent dog studies, which were
conducted in a manner designed to maximize detection of
cholinesterase depression, the overall NOEL was 0.02-0.03 mg/kg
bw/day, but the NOAEL (which discounts inhibition of plasma
cholinesterase only) was 0.05-0.06 mg/kg bw/day.
Results of repeat-dose studies with aldicarb demonstrate that
the method of administering the test material to the test animals
can greatly modify the apparent toxicity of aldicarb and its
metabolites. Mice, rats and dogs have tolerated daily doses equal to
the LD50 incorporated into the diet for 7 days to 2 years. Doses
which caused death in less than 2 h when administered as a bolus,
caused no death and only moderate cholinesterase depression when
given in the diet.
Two dietary carcinogenicity studies have been conducted with
aldicarb in rats and three in mice. A dermal carcinogenicity study
has also been conducted with aldicarb in mice and dietary studies
have been carried out with aldicarb sulfone in mice and aldicarb
sulfoxide in rats. Aldicarb was not carcinogenic in mice and rats.
Two reproduction studies have been conducted in rats with
aldicarb and one with aldicarb sulfone. There were no effects on
reproductive performance at doses up to 0.7 mg/kg bw/day aldicarb or
9.6 mg/kg bw/day aldicarb sulfone. Aldicarb did not display any
teratogenic potential in rats or rabbits in studies which included
maternally toxic doses.
After reviewing the available genotoxicity data, the meeting
concluded that aldicarb, aldicarb sulfoxide and aldicarb sulfone are
not genotoxic.
In a range of special studies in animals (involving delayed
neurotoxicity, behaviour, antagonistic agents and pesticide
interactions) aldicarb displayed no results which gave cause for
concern. There was no evidence of immuno-toxicity in mice in a
number of functional assays of cell-mediated immunity and in host
resistance to respiratory infection.
Epidemiological studies provided no convincing evidence that
aldicarb could significantly alter immunological function in humans.
In addition to the above epidemiological studies, studies
conducted in 1982 and 1983 attempted to correlate any potential
adverse health effects with the occurrence of aldicarb in drinking
water. Although the authors concluded that further study was needed,
there was no clear evidence that aldicarb contamination of drinking
water generally at concentrations of about 4-12 µg/litre, but at a
maximum concentration of 400 µg/litre was related to any health
effects.
The anticholinesterase potential of aldicarb has been
extensively investigated in human. These studies revealed the same
pattern of rapid cholinesterase inhibition and rapid recovery seen
in experimental animals. Transient erythrocyte cholinesterase
depression was seen at single doses of 0.05 mg/kg bw, and the NOAEL
for cholinesterase depression (discounting changes in plasma enzyme
activity) was 0.025 mg/kg bw.
A number of poisoning incidents have been reported in the
agricultural use of aldicarb, but there has been no indication that
the workers exposed were harmed once removed from the exposure
source. Although several deaths have been reported, all of these
have been attributed to suicide or gross neglect.
A number of food-borne aldicarb intoxications have been
reported in the literature. These have all been associated with
misuse and reliable quantification of the dose of aldicarb involved
has always proved difficult, if not impossible.
An ADI was allocated using a 10-fold safety factor applied to
the NOAEL for depression of erythrocyte cholinesterase activity in
human volunteers.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg bw/day (93-day dietary study)
Dog: 0.05 mg/kg bw/day (52-week study)
Human: 0.025 mg/kg bw (double-blind, placebo controlled
volunteer study)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans, including information regarding
the correlation of blood cholinesterase depression and clinical
signs and symptoms.
Table 6: Summary of no effect levels in repeat dose toxicity studies which included
cholinesterase investigations
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Mouse 7 days Aldicarb 0,0.1,0.3,0.6,1.2 0.6 Mortality
Mouse 7 days Aldicarb: 0,2,6,18,36 2 Kidney and liver weight
A. sulfone reduction, growth
1:1 depression
Rat 7 days Aldicarb 0,4,8,16 NE Mortality, growth
depression, kidney and
liver weight reduction
Rat 7 days Aldicarb 0,0.8,1.6,3.2 NE Growth depression,
kidney and liver weight
reduction
Rat 93 days Aldicarb 0,0.02,0.1,0.5 0.1 Mortality, growth
depression
Rat 2 years Aldicarb 0,0.005,0.025, >0.1 No effects seen
0.05,0.1
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Rat 7 days Aldicarb 0,0.4,0.8,1.6,3.2 0.8 Growth depression,
A. sulfoxide 0,0.4,0.8 0.4 RBC cholinesterase
A. sulfone 0,0.4,1.0,2.5,5,20 2.5 depression
Rat 29 days A. sulfoxide: 0,0.0074,0.03, 0.4 Growth depression,
A. sulfone 0.12,0.47,1.67 in brain, plasma and RBC
1:1 drinking water cholinesterase depression
Rat 6 months A. sulfoxide 0,0.125,0.25,0.5,1 0.125 Growth depression,
brain, plasma and RBC
cholinesterase depression
Rat 6 months A. sulfoxide 0,0.0625,0.125, 0.125 Plasma and RBC
0.25,0.5,1.0 cholinesterase depression
Rat 6 months A. sulfone 0,0.2,0.6,1.8, 0.6 Brain, plasma and RBC
5.4,15.2 cholinesterase depression
Rat 7 days A. oxime 0,31.25,62.5,125, 62.5 Growth depression,
250,500,1000 minor liver and kidney
changes
Dog 7 days Aldicarb 0,0.2,0.3,0.7 >0.7 No effects seen
Dog 100 days Aldicarb 0,0.2,0.3,0.7 0.3 Minor organ weight
changes
Dog 2 years Aldicarb 0,0.025,0.05 >0.1 No effects seen
0.1
Dog 2 weeks Aldicarb 0,0.022,0.068, NE Brain, plasma and RBC
cholinesterase
depression
0.192,0.609,1.42
Dog 2 weeks Aldicarb 0,0.003,0.008, 0.096 Brain, plasma and RBC
cholinesterase depression
0.027,0.096,0.28
Dog 5 weeks Aldicarb 0,0.012,0.024, >0.06 No effects seen
0.06
Dog 52 weeks Aldicarb 0,0.027,0.054, 0.054 RBC and plasma
0.131,0.241 cholinesterase depression
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Dog 3 months A. sulfoxide 0,0.0625,0.125, 0.25 Transient growth
0.25,0.5 depression
Dog 3 months A. sulfone 0,0.2,0.6, >5.4 No effects seen
1.8,5.4
Dog 1 year A. sulfone 0,0.11,0.59, 0.11 RBC and plasma
2.25 cholinesterase depression
* mg/kg bw/day equivalence, by dietary administration, unless otherwise stated.
Lowest effect level underlined.
** NOAEL's were tabulated without evaluation of methods of sample collection or method of
determination of cholinesterase activity.
NE Not established
REFERENCES
Abdallah, N.A. (1977) Alleged overexposure cases reported from use
of Temik formulations - 1966 to 1976. Unpublished report from Union
Carbide Corporation.
Abdallah, N.A. (1979) Alleged human overexposure cases reported from
use of Temik formulations - 1976 to 1979. Unpublished report from
Union Carbide Corporation.
Aldridge, D., Schardein, J.L., & Blair, M. (1983) Aldicarb
range-finding teratology study in rabbits. Unpublished report from
International Research and Development Corporation, submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Andrawes, N.R. (1977) The metabolism of (UC 21865) Sulfocarb
pesticide in the rat. Unpublished report from Union Carbide
Corporation.
Andrawes, N.R., Dorough, H.W., & Lindquist, D.A. (1967) degradation
and elimination of Temik in rats. J. Econ. Ent., 60(4): 979-987.
Andrawes, N.R. & Lee R.E. (1986) Temik brand aldicarb pesticide,
metabolism in lactating goats. Unpublished report from Union Carbide
Agricultural Products Company Inc.
Babish, J.G. & Salerno, A. (1977) Neurotoxicity evaluation of UC
21865 in white Leghorn hens (Gallus domesticus). Unpublished
report from Food and Drug Research Laboratories submitted to WHO by
Union Carbide Corporation.
Bull, D.L., Lindquist, D.A., & Coppedge, J.R. (1967) Metabolism of
2-methyl-2-(methylthio)propionaldehyde O-(methylcarbamoyl) oxime
(Temik, US-21149) in insects. J. Agric. Food Chem., 15(4):
610-616.
Burrows, I.E., Haynes, G., & Roberts, N. (1970) Determination of
exposure levels in operators to Temik during field trials.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Carpenter, C.P. (1963) Comparison of the acute toxicity of compound
21149 with several of its analogues. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Carpenter, C.P. (1969) Miscellaneous toxicity studies. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Carpenter, C.P. and Smyth, H.F. (1966) Temik l0G (10.5% Granular
formulation of compound 21149). 15-Day dermal applications to
rabbits. Unpublished Report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Chin, G.H. & Sullivan, L.J. (1968) Some biochemical considerations
of Temik pesticide. Unpublished report from Mellon Institute,
presented to the 156th ACS National Meeting, submitted to WHO by
Union Carbide Corporation.
Cid, M.G. & Matos, E. (1984) Induction of sister-chromatid exchanges
in cultured human lymphocytes by aldicarb, a carbamate pesticide.
Mutat. Res., 138: 175-179.
Coate, W.B., Hardy, R.J., Alsaker, R.D., Dawkins, B.G., Hepner,
K.E., Banos, D.A., & Mense, M.A. (1982) Subacute inhalation toxicity
study of a pesticide residue in dogs. T3, T6 and T10 type
cigarettes. Unpublished report from Hazleton Laboratories America
Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Debuyst, B. & Van Larebeke, N. (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242-243.
Dorough, H.W., Davis, R.B., & Ivie, G.W. (1970) Fate of
Temik-Carbon-14 in lactating cows during a 14-day feeding period.
J. Agr. Food Chem., 18: 135-142
Dorough, H.W. & Ivie, G.W. (1968) Temik-S-35 metabolism in a
lactating cow. J. Agric. Food Chem., 16(3): 460-464
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E. McGregor, D.,
Morteimans, K., Rosenkranz, H.S., & Simmon, V.F. (1985)
Reproducibility of microbial mutagenicity assays: II. Testing of
carcinogens and noncarcinogens in Salmonella typhimurium and
Escherichia coli. Environ. Mutagen., 7 (suppl 5): 1-248.
Field, W.E. (1979a) Acute dermal toxicity in rabbits. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Field, W.E. (1979b) Acute dermal toxicity in rats. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Fiore, M.C., Anderson, H.A., Hong, R., Golubjatnikov, R., Seiser,
J.E., Nordstrom, D., Hanrahan, L., & Belluck, D. (1986) Chronic
exposure to aldicarb-contaminated groundwater and human immune
function. Environ. Res., 41: 633-645.
Godek, E.G., Dolak, M.D., Naismith, R.W., & Matthews, R.J. (1980a)
Ames Salmonella microsome plate test. Aldicarb sulfone.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980b)
Ames Salmonella microsome plate test. Aldicarb sulfoxide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980c)
Ames Salmonella microsome plate test. TEMIK aldicarb pesticide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984a) Rat hepatocyte
primary culture/DNA repair test. Aldicarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984b) Rat hepatocyte
primary culture/DNA repair test. Aldoxycarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Goes, E.H., Savage, E.P., & Gibbons, G. (1980) Suspected foodborne
carbamate pesticide intoxications associated with ingestion of
hydroponic cucumbers. Am. J. Epidemiol., 111: 254-259.
Goldman, L.R., Beller, M., & Jackson, R.J. (1990) Aldicarb food
poisonings in California, 1985-1988: Toxicity estimates for humans.
Arch. Environ. Hlth., 45(3) 141-147.
Guerzoni, M.E., Del Cupolo, L. and Ponti, I. (1976) Attivita
mutagenica degli antiparassitari. Riv. Sci. Teen. Alim. Nutr.,
Urn. 6: 161-165.
Haines, R.G. (1971) Ingestion of aldicarb by human volunteers: a
controlled study of the effect of aldicarb on man. Unpublished
report from Union Carbide Corporation.
Hamada, N.N. (1987a) One-year chronic oral toxicity study in beagle
dogs with aldicarb sulphone technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1987b) Two-week dose range finding oral toxicity study
in beagle dogs with aldicarb technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1988) One-year chronic oral toxicity study in beagle
dogs with aldicarb technical. Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N. (1991) Subchronic toxicity study in beagle dogs with
aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Marshall,
P.M. (1985a) Two-week dose range finding study in beagle dogs -
Aldicarb sulphone (aldoxycarb). Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Williams,
L.D. (1985b) Two-week dose range finding study in beagle dogs -
Aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hicks B.W., Dorough, H.W., & Mehendale, H.M. (1972) Metabolism of
aldicarb pesticide in laying hens. J. Agric. Food Chem., 20(1):
151-156.
Hirsch, G.H., Mori, B.T., Morgan, G.B., Bennett, P.R., & Williams,
B.C. (1987) Report of illness caused by aldicarb-contaminated
cucumbers. Food Addit. Contam., 5: 155-160.
Ivett, J.L. (1990) Mutagenicity test on aldicarb technical in the
mouse bone marrow cytogenetic assay. Unpublished report from
Hazleton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Ivett, J.L., Myhr, B.C., & Lebowitz, H.D. (1984) Mutagenicity
evaluation of aldicarb technical 93.47% in the bone marrow
cytogenetic assay. Unpublished report from Litton Bionetics, Inc.
submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Johnson, H.E. & Carpenter, C.P (1966a) Temik (Technical grade
compound 21149). Demyelination potential in chickens. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Carpenter, C.P (1966b) Temik (technical grade
compound 21149). Comparative behavioural effect in rats. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Sullivan, L.J. (1968a) Studies on an effective
therapy for overdoses of temik, Temik sulfoxide and temik sulfone in
the rat. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Johnson, H.E. and Sullivan, L.J. (1968b) Temik (uc 21149) antidotal
therapy in rats following administration of multiple lethal doses.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Knaak, J.B., Tallant, M.J., & Sullivan, L.J. (1966) The metabolism
of 2-methyl-2-(methylthio)propionaldehyde o-(methylcarbamoyl) oxime
in the rat. J. Agric. Food Chem., 14(6): 573-578
Lee, M.H. & Ransdell, J.F. (1984) A farmworker death due to
pesticide toxicity: a case report. J. Toxicol. Environ. Health,
14: 239-246.
Leng, J.M., Schardein, J.L., & Blair, M. (1983) Aldicarb. Teratology
study in rabbits. Unpublished report from International Research and
Development Corporation submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Burk, J.,
Reynolds, H.T., & Osman, M.F. (1966) Metabolism of
2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyl) oxime in
Plant and Insect. J. Agric. Food Chem., 14(6): 579-584.
Mirkin, I.R., Anderson, H.A., Hanrahan, L., Hong, R., Golubjatnikov,
R., & Belluck, D. (1990) Changes in tlymphocyte distribution
associated with ingestion of aldicarb-contaminated drinking water: a
follow-up study. Environ. Res., 51: 35-50.
Mirro, E.J., DePass, L.R., & Frank, F.R. (1982) Twenty-nine day
water inclusion study in rats. Unpublished report from Bushy Run
Research Laboratory submitted to WHO by Union Carbide Corporation.
Natoff, I.L. & Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl.
Pharmacol., 25: 569-575.
NIH (1979) Bioassay of aldicarb for possible carcinogenicity. US
DHEW Pub.No. (NIH) 79-1391, 103 pages.
Nimmo, W.S., Wyld, P.J., Watson, C.E., & Watson, N (1992) A safety
and tolerability study of aldicarb at various dose levels in healthy
male and female volunteers. Unpublished report from Inveresk
Clinical Research, submitted to WHO by Rhône-Poulenc AGRO, Lyon,
France.
Nycum, J.S. & Carpenter, C.P. (1968a) Toxicity studies on the
probably "non-carbamate" plant metabolites of Temik. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Nycum, J.S. & Carpenter, C.P. (1968b) Toxicity studies on Temik and
related carbamates. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Olson, L.J., Erickson, B.J., Hinsdill, R.D., Wyman, J.A., Porter,
W.P., Binning, L.K., Bidgood, R.C., & Nordheim, E.V. (1987) Aldicarb
immunomodulation in mice: an inverse dose-response to parts per
billion levels in drinking water. Arch. environ. Contam. Toxicol.,
16: 433-439.
Pandey, S.Y. (1977) Human exposure study on Temik 10G. Unpublished
report from Union Carbide India Ltd., submitted to WHO by Union
Carbide Corporation.
Peoples, S.A., Maddy, K.T., & Smith, C.R. (1977) Human health
problems associated with Temik (Aldicarb) in California. Unpublished
report from California Department of Food and Agriculture submitted
to WHO by Union Carbide Corporation.
Pozzani, U.C. & Carpenter, C.P. (1968a) Sensitizing potential in
guinea-pigs as determined by a modified Landsteiner test.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21:
319-334.
Romine, R.R. (1973) Aldicarb pesticide. Aldicarb residues in milk
and meat of cows fed aldicarb sulfoxide and aldicarb sulfone in the
daily diet. Unpublished report from Union Carbide Corporation.
SanSebastian, J.R., Naismith, R.W., & Matthews, R.J. (1984) In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Aldoxycarb technical. Unpublished report from Pharmakon
Research International Inc. Submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Shrivastava, K.N. (1975) Determination of human exposure levels in
commercial application of ternik 10g on potatoes by gloved hand and
hand-operated applicators. Unpublished report from Union Carbide
India Ltd.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985a) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldicarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985b) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldoxycarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Sterman, A.B. & Varma, A. (1983) Evaluating human neurotoxicity of
the pesticide aldicarb: when man becomes the experimental animal.
Neurobehav. Toxicol. Teratol., 5: 493-495.
Striegel, J.A. & Carpenter, C.P. (1962) Range finding test on
compound 21149. Unpublished report from Mellon Institute of
Industrial Research submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. (1968a) The urinary excretion of C-14 equivalents of
Temik in the dog and their metabolic profile as revealed by silica
gel chromatography. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Sullivan, L.J (1968b) Urinary metabolic profiles as determined by
silica gel chromatography of urines from rats and dogs dosed with
Temik sulfone. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Sullivan, L.J (1968c) The excretion of C14 equivalents of Temik
sulfone by the rat after a single peroral dose. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. & Carpenter, C.P. (1974) Preliminary Metabolism of
14C-S-methyl aldicarb nitrile in the rat. Unpublished report from
Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Thomas, P.T., Ratajczak, H.V., Eisenberg, W.C., Furedz-Machacek, M.,
Ketels, K.V., & Barbera, P.W. (1987) Evaluation of host resistance
and immunity in mice exposed to the carbamate pesticide aldicarb.
Fund. Appl. Toxicol., 9: 82-89.
Thomas, P.T., Ratajczak, H.V., Demetral, D., Hagen, K., & Baron, R.
(1990) Aldicarb immunotoxicity: Functional analysis of cell-mediated
immunity and quantitation of lymphocyte sub-populations. Fund.
Appl. Toxicol., 15: 221-230.
Trutter, J.A. (1987a) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residues in bananas. Unpublished report
from Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Trutter, J.A. (1987b) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residue in watermelons. Unpublished
report from Hazleton Laboratories America Inc., submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Tyl, R.W. & Neeper-Bradley, T.L. (1988) Developmental toxicity
evaluation of aldicarb technical administered by gavage to CDO
(Sprague-Dawley) rats. Unpublished report from Bushy Run Research
Centre submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Union Carbide (1979) Volunteer worker's exposure monitoring study
(Panama). Unpublished Report from Union Carbide Corporation.
Wakefield, M., Orme, J.P.R., Ruston, E.A., & Andrews, L. (1973) The
detemination of levels of aldicarb in urine and face masks.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1963) Results of three months of
inclusion of Compound 21149 in the diet of rats. Unpublished Report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1964) Results of a three-generation
reproduction study on rats fed compound 21149 in their diet.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1965) Two-year feeding of compound
21149 in the diet of rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966a) Insecticide Temik. Teratogenic
potential in rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966b) Results of long-term tests for
mouse skin carcinogenicity of three process residues, one epoxide
and three compounds. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966c) Two-year feeding of compound
21149 in the diet of dogs. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968a) Temik 10G-V (10.3% granular
formulation of compound 21149). Acute and 14-day dermal applications
to rabbits. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968b) Temik sulfoxide. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968c) Temik sulfone. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1969a) Purified and technical temik.
Results of feeding in the diets of rats for one week. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1969b) 2-methyl-2-(methylsulfinyl)
propanol-1. Results of feeding in the diets of rats for one week.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970a) Temik and other materials.
Miscellaneous single dose peroral and parenteral LD50 assays and
some joint action studies. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970b) Temik, results of feeding in
the diets of rats for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970c) Temik (T) Temik sulfoxide
(TSO), Temik sulfone (ts02), 1:1 TSO-TS02. Results of feeding in the
diet of rats for 7 days. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970d) 1:1 Temik:Temik sulfone.
results of feeding in the diet of mice for 7 days. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1970e) Temik. Results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972a) Aldicarb (A), Aldicarb
sulfoxide (ASO), Aldicarb sulfone (AS02) and a 1:1 mixture of
ASO:AS02. Two-year feeding in the diet of rats. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972b) Miscellaneous toxicity studies.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972c) Aldicarb, 18-month feeding in
diet of mice. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1973) Aldicarb, seven-day inclusion in
diet of dogs. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974a) Aldicarb. Inclusion in the diet
of rats for three generations and a dominant lethal mutagenesis
test. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974b) Aldicarb Oxime (All). Results
of feeding in the diet of rats for 7 days. Unpublished report from
Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974c) Aldicarb. Inclusion in the
diets of dogs for three months. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974d) Aldicarb, 18-month feeding in
the diet of mice, study II. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974e) UC 21865; results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Cox, E.F. (1975) Aldicarb sulfoxide and aldicarb
sulfone cholinesterase inhibition results after periods of one to
fifty-six days of inclusion in the diets of rats. Unpublished report
from Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Weil, C.S., Conroy, W.J., Condra, N.I., & Cox, E.F. (1977) Aldicarb
sulphone UC 21865. 19-day rabbit skin inunction. Unpublished report
from Mellon institute submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1965) The single dose peroral toxicity
of compounds 20299, 21149, 19786 and 20047a for white Leghorn
cockerels. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1966a) Temik (Compound 21149,
Technical). Joint action with selected organic phosphate and
carbamate pesticides. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P.(1966b) Miscellaneous acute toxicity
data. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Whitlock, N.H., Schuman, S.H., & Loadholt, C.B. (1982) Executive
summary and epidemiologic survey of potential acute health effects
of aldicarb in drinking water - Suffolk County, N.Y. South Carolina
Pesticide Hazard Assessment Program Center, Medical University of
South Carolina, Charleston. Prepared for the Health Effects Branch,
Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency.
WHO (1966) Insecticide Evaluation and Testing Programme, Stage I,
Mammalian Toxicity Report (WHO). Unpublished report from Toxicology
Research Univ., Carshalton, U.K. submitted to WHO by Union Carbide
Corporation.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, F. (1966) Human exposure study in the field application of
Temik 10G on cotton. Unpublished report from Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977a) Inclusion in the
diet of rats for three generations (aldicarb sulphone), dominant
lethal mutagenesis and teratology studies. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977b) Aldicarb sulphone;
18-month feeding in the diet of mice. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Table 1: Acute toxicity of aldicarb
Species Sex Route Vehicle LD50 Reference
(mg/kg bw)
Rat M oral corn oil 0.84 West & Carpenter (1966b)
Rat M oral corn oil 0.93 Striegel & Carpenter (1962)
Rat M oral corn oil 0.67-1.23 Carpenter (1963)
Nycum & Carpenter (1968b)
Rat F oral corn oil 0.62-1.07 Carpenter (1963)
Rat F oral gycerol:
ethanol 1.0 WHO (1966)
Rat F ip corn oil 0.71 Carpenter (1963)
Rat M+F ip corn oil 0.44 Carpenter (1963)
Rat M ip PEG 0.37-0.44 Weil & Carpenter (1970a)
Rat M ip ethanol 0.57 Johnson & Carpenter (1966b)
Rat M iv water 0.47 Weil & Carpenter (1970a)
Rat M dermal corn oil >10 Field (1979b)
Rat F dermal DMP 3.2-7.0 WHO (1966)
Rat M dermal water 38.1-44.9 Weil & Carpenter (1968a)
Mouse M oral corn oil 0.382 Weil & Carpenter (1972b)
Mouse M oral corn oil 0.5 Weil & Carpenter (1972c)
Mouse F oral cotton seed 1.5 Dorough et al. (1970)
oil
Mouse F ip cotton seed 0.3 Dorough et al. (1970)
oil
Guinea-pig oral corn oil 1.0 Nycum & Carpenter (1968b)
Rabbit oral propylene 1.3 Nycum & Carpenter (1968b)
glycol
Rabbit M dermal corn oil >10 Field (1979a)
Rabbit M dermal PEG 5.0 Striegel & Carpenter (1962)
Rabbit M dermal dry 141->200 Weil & Carpenter (1968a)
Chicken M oral 9 West & Carpenter (1965)
Table 2: Acute toxicity of aldicarb metabolites
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. nitrile rat oral 570 West & Carpenter (1966b)
A. sulfoxide rat (M) oral corn oil 0.49-1.13 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfone rat (M) oral corn oil 20-25 Weil & Carpenter (1970a)
Nycum & Carpenter (1968b)
A. sulfoxide mouse (M) oral corn oil 0.8-1.6 Nycum & Carpenter (1968b)
A. sulfone mouse (M) oral corn oil 25 Nycum & Carpenter (1968b)
A. sulfoxide guinea-pig oral corn oil 0.8-1.8 Nycum & Carpenter (1968b)
A. sulfone guinea-pig oral corn oil >50 Nycum & Carpenter (1968b)
A. sulfoxide rabbit oral corn oil 0.4-1.8 Nycum & Carpenter (1968b)
Table 2 (cont'd)
Chemical Species Route Vehicle LD50 Reference
(sex) (mg/kg bw)
A. sulfone rabbit oral corn oil 75 Nycum & Carpenter (1968b)
A. sulfoxide rat (M) ip water 0.47 Weil & Carpenter (1970a)
A. sulfoxide rat (M) ip corn oil 0.71 Johnson & Carpenter (1966b)
A. sulfone rat (M) ip PEG 21.2 Weil & Carpenter (1970a)
A. sulfoxide rat (M) iv water 0.47 Weil & Carpenter (1970a)
A. sulfone rat (M) iv water 14.9 Weil & Carpenter (1970a)
A. sulfoxide rabbit dermal water >20 West & Carpenter (1966b)
A. sulfone rabbit dermal water >20 West & Carpenter (1966b)
2-methyl-2-
(methyl-sulfinyl)
propanol-1 rat oral 11 000 Weil & Carpenter (1969b)
OH-methyl A rat oral 42.9 Carpenter (1969)
A. sulfoxide oxime rat (M) oral 8060 Nycum & Carpenter (1968a)
A. sulfone oxime rat (M) oral 1590 Nycum & Carpenter (1968a)
A. sulfoxide nitrile rat (M) oral 4000 Nycum & Carpenter (1968a)
A. sulfone nitrile rat (M) oral 350 Nycum & Carpenter (1968a)
A: aldicarb
Mice
- Aldicarb/aldicarb metabolites
Groups of 3 male and 5 female mice were fed a mixture of
aldicarb and aldicarb sulfone (1:1) in the diet at dose levels of 0,
2, 6, 18, or 36 mg/kg bw/day for 7 days. No mortality was noted at
any dose level, but severe cholinergic signs of poisoning were
observed at the high-dose in males. Depression of growth was
observed at the two highest dose levels. Kidney weight was depressed
at the highest dose and liver weight was reduced substantially at 6
mg/kg bw/day and above in both males and females. The NOAEL in this
study was 2 mg/kg bw/day (Weil & Carpenter, 1970d)
Groups of 5 Charles River CD-1 mice/sex were fed aldicarb
sulfone in the diet at dose levels of 0, 0.15, 0.6, 2.4, 9.6 or 38.4
mg/kg bw/day for 7 days. Significant body-weight decrease was noted
at the highest dose but there were no significant organ weight
changes. Cholinesterase activity was not determined (Weil &
Carpenter, 1974e).
Rats
- Aldicarb
Groups of 5 rats/sex were fed aldicarb in the diet for 7 days
at dose levels of 0, 4, 8 or 16 mg/kg bw/day. Mortality was noted
predominantly at the highest dose at which all males and 2 of 5
females died. One of 5 males also died at 8 mg/kg bw/day. There was
a substantial reduction in body-weight gain noted at all dose
levels. In males, kidney weights were significantly reduced at 8
mg/kg bw/day and liver weight was depressed at 4 and 8 mg/kg bw/day.
In females, both liver and kidney weight was significantly depressed
at all dose levels (Weil & Carpenter, 1970b).
Groups of 5 rats/sex were fed aldicarb in the diet at dose
levels of 0, 0.8, 1.6 or 3.2 mg/kg bw/day for 7 days. Growth was
depressed at 1.6 mg/kg bw/day and above. There was no mortality
attributable to aldicarb. In males, liver weights were depressed in
all treatment groups. In females, liver weights were affected only
at the highest dose, but the liver to body-weight ratio was reduced
at 1.6 mg/kg bw/day and above. Kidney weights were reduced in males
at all dose levels and in females only at the highest dose.
Cholinesterase activity, measured on the day after the conclusion of
feeding, was normal at the highest dose tested with the exception of
plasma cholinesterase which was slightly reduced at the highest
level (Weil & Carpenter, 1969a).
Groups of 10 CFE rats/sex were fed aldicarb in their diet for
93 days at dose levels of 0, 0.02, 0.1 or 0.5 mg/kg bw/day. One rat
per level was killed for cholinesterase (plasma, RBC and brain)
determinations at 1, 2, 4 and 29 days of dosing. Mortality was
increased and body-weight and food consumption were decreased at the
highest level. Histopathology of selected organs was unremarkable
and there were no compound-related effects noted. There were no
consistent dose-related effects on the cholinesterase
determinations, except for plasma cholinesterase depression in both
sexes after 30 days at the highest level. There was no indication of
how soon after feeding the cholinesterase determinations were
performed, which could account for the sporadic results, together
with the small number of animals sampled at each interval. The NOAEL
in this study was 0.1 mg/kg bw/day (Weil & Carpenter, 1963).
Rats
- Aldicarb/aldicarb metabolites
Groups of 5 rats/sex were fed diets containing aldicarb at dose
levels of 0, 0.4, 0.8, 1.6 or 3.2 mg/kg bw/day, or aldicarb
sulfoxide at dose levels of 0, 0.4 or 0.8 mg/kg bw/day, or aldicarb
sulfone, at dose levels of 0, 0.4, 1.0, 2.5, 5 or 20 mg/kg bw/day,
for 7 days. With aldicarb (as with its two major carbamate
metabolites) there was a significant growth depression at the
highest dose. There were no effects noted with respect to liver or
kidney weights. The erythrocyte cholinesterase appeared to be the
most sensitive parameter with all three materials tested. The
NOAELs, based on erythrocyte cholinesterase depression or decreased
body-weight gain over the 7-day treatment period were: aldicarb -
0.8 mg/kg bw/day; aldicarb sulfoxide - 0.4 mg/kg bw/day; and
aldicarb sulfone - 2.5 mg/kg bw/day (Nycum & Carpenter, 1968b).
Groups of 5 rats/sex received in the diet, aldicarb (0.3 mg/kg
bw/day), aldicarb sulfoxide (0.4, 0.8 or 1.6 mg/kg bw/day), aldicarb
sulfone (0.6, 5 or 20 mg/kg bw/day), a 1:1 mixture of aldicarb
sulfoxide and aldicarb sulfone (1.2 mg/kg bw/day) or a control diet.
At the end of the 7 days of feeding, animals were placed on control
diets for one day after which they were sacrificed for
cholinesterase determination and for examination of liver and
kidney. A second one-week feeding trial was performed to compare the
data with other strains of rats. Aldicarb sulfoxide was fed to
groups of 5 male rats at dose levels of 0, 0.4, 0.8 or 1.6 mg/kg
bw/day and aldicarb sulfone was fed to male rats at dose levels of
0, 5 or 20 mg/kg bw/day. As might be expected with the protocol
followed in the study, cholinesterase depression was not observed in
either plasma, erythrocyte or brain at the conclusion of the study.
The second trial using both the same and a different strain of rats
was performed in an effort to explain a slight but non-significant
inhibition of erythrocyte cholinesterase activity measured in the
initial study. Over the course of this study, there were no effects
noted on erythrocyte cholinesterase activity (Weil & Carpenter,
1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.3 or 1.0 mg/kg bw/day and aldicarb sulfone at 0,
2.4 or 16.2 mg/kg bw/day. Animals were sacrificed at 1, 3, 7, 14, 28
and 56 days for cholinesterase analyses. Plasma and erythrocyte
cholinesterase activities were measured at the first three time
intervals. At the last three time intervals, plasma erythrocyte and
brain cholinesterase activities were examined. There was no
mortality, but growth was depressed at the highest dose levels with
both the sulfoxide and sulfone. Rats administered aldicarb sulfoxide
at 1.0 mg/kg bw/day had a slight but significant cholinesterase
depression during the study. There were no effects noted at 0.3
mg/kg bw/day. Cholinesterase depression was marked with the aldicarb
sulfone consistently throughout the study at the highest dose level
of 16.2 mg/kg bw/day (Weil & Cox, 1975).
Groups of 10 Wistar rats/sex were administered aldicarb
sulfoxide and aldicarb sulfone in a 1:1 ratio, in drinking water,
ad libitum, for 29 days. The drinking water levels were 0, 0.075,
0.3, 1.2, 4.8 or 19.2 mg/litre. Plasma and red blood cell
cholinesterase levels were determined after 8, 15 and 29 days of
treatment. Brain cholinesterase was measured at termination. Animals
were continuously exposed to the test substances in water until
blood samples were taken or sacrifice was performed. There was no
mortality and food and water consumption values together with body-
weight gains were significantly decreased in both sexes at the high-
dose level when compared to controls. Significant depression of
plasma, RBC and brain cholinesterase, in both sexes, occurred at the
highest dose level. The data demonstrate that a level of 4.8
mg/litre of aldicarb sulfoxide:aldicarb sulfone in water is without
effect on cholinesterase (plasma, RBC, brain) in either sex. Based
on the actual concentrations being approximately 80% of the nominal
concentrations, the NOAEL was roughly 3.8 mg/litre, equivalent to
0.4 mg/kg bw/day (Mirro et al., 1982).
Groups of 15 rats/sex were fed aldicarb sulfoxide in the diet
at dose levels of 0, 0.125, 0.25, 0.5 or 1 mg/kg bw/day for 6
months. Animals were sacrificed at 3 months and at the conclusion of
the study for cholinesterase determinations and for gross and
microscopic examination of liver and kidneys. There was no mortality
noted during the course of the study, although growth, especially in
males, at 0.25 mg/kg bw/day and above was reduced. Cholinesterase
activity was substantially reduced, at the three highest dose
levels, especially in plasma and erythrocytes of males. Gross
examination of liver and kidneys revealed no abnormalities
attributable to aldicarb. The NOAEL in this study was 0.125 mg/kg
bw/day (Weil & Carpenter, 1968b).
In an attempt to resolve the question of cholinesterase
depression and rapid recovery, groups of 5 rats/sex were fed
aldicarb sulfoxide for one week or one week plus one day of control
diets at a dose level of 1 mg/kg bw/day. When the study was
concluded (within one week), animals were sacrificed at 0 and 24 h
after the dietary feeding interval (the 24 h animals were fed
control diets). Cholinesterase depression was noted at the 0 h
sacrifice in erythrocyte and plasma preparations. Administration of
a control diet for one day (24 h sacrifice) completely reversed the
cholinesterase depression noted when animals were sacrificed without
any recovery interval (Weil & Carpenter, 1970c).
Groups of 5 rats/sex were fed aldicarb sulfoxide in the diet at
dose levels of 0, 0.0625, 0.125, 0.25, 0.5 or 1.0 mg/kg bw/day for 3
and 6 months after which some of the animals were sacrificed
immediately and others were placed on a control diet for 24 h prior
to sacrifice and cholinesterase analysis. Cholinesterase activity in
the brain was unaffected by aldicarb sulfoxide. Plasma and
erythrocyte cholinesterase was substantially reduced at the 0 h
sacrifice in both sexes. Males were slightly more sensitive with
depression being noted at 0.25 mg/kg bw/day and above, while with
females depression was noted at 0.5 mg/kg bw/day and above. There
was no cholinesterase depression noted in any of the animals treated
for either 3 months or 6 months when the animals were allowed to
recover from cholinesterase depression for a one day recovery
interval. The NOAEL in this study was 0.125 mg/kg bw/day (Weil &
Carpenter, 1968b).
In a series of studies similar to those reported with aldicarb
sulfoxide, groups of rats were fed aldicarb sulfone at dietary
concentrations at dose levels of 0, 0.2, 0.6, 1.8, 5.4 or 15.2 mg/kg
bw/day for 6 months. Animals were sacrificed at 3 and 6 months for
examination of liver and kidney abnormalities and for evaluation of
cholinesterase activity. Cholinesterase determinations were made at
the end of the 3 and 6 month intervals with rats fed continuously
until sacrifice or rats were allowed to consume a control diet for
24 h prior to sacrifice and determination of cholinesterase
activity. There was no mortality over the course of the study.
Transient but significant growth depression was noted at the highest
level in the longer study. There were no effects noted with respect
to food consumption or on gross and microscopic examinations of
liver and kidneys. Plasma, erythrocyte and brain cholinesterase were
significantlv depressed at 5.4 mg/kg bw/day and above. Erythrocyte
cholinesterase depression was also noted at 1.8 mg/kg bw/day. There
was no cholinesterase depression noted at 0.6 mg/kg bw/day in any of
the tissues examined. In the study to evaluate recovery of
cholinesterase activity, when animals were allowed to equilibrate
for one day on control diets, all depressed cholinesterase values
returned to normal. The NOAEL in this study was 0.6 mg/kg bw/day
(Weil & Carpenter, 1968c).
Groups of rats were fed aldicarb oxime at dose levels of 0,
31.25, 62.5, 125, 250, 500 or 1000 mg/kg bw/day for 7 days. There
was no mortality over the course of the study. Growth was slightly
reduced at the initiation of the study at dose levels of 125 mg/kg
bw/day and above, but at the end of one week only the two highest
dose levels appeared to show a retardation in growth. Gross changes
were noted in both liver and kidneys at the two highest dose levels
in both males and females. The NOAEL in this study was 62.5 mg/kg
bw/day (Weil & Carpenter, 1974b).
Groups of 5 rats/sex were fed the hydrolytic metabolite of
aldicarb (2-methyl-2-(methylsulfinyl)propanol-1) for 7 days at dose
levels of 0, 500 or 1000 mg/kg bw/day. Growth was depressed at both
dose levels in males but only at the highest dose level in females.
In females, while growth was depressed within one day of treatment,
the animals appeared to recover during the rest of the week. There
was no apparent effect on major organs, although, in females at the
highest dose level, kidney weights were slightly depressed.
Cholinesterase activity was not measured (Weil & Carpenter, 1969b).
Rabbits
- Aldicarb
Four groups, each of 5 male rabbits with abraded skin, were
administered aldicarb 10G formulation at dose levels of 0, 5, 10 or
20 mg/kg bw/day, for 6 h per day, 5 days per week, for 15 days.
Water was added periodically during the exposure time to the
dressing containing the aldicarb treatment, simulating a condition
of excess perspiration. One additional group was administered 20
mg/kg bw/day to intact, unabraded skin with no water added to the
dressing. Animals treated with aldicarb under a dry condition with
unabraded skin showed normal weight gains and no apparent effects as
a result of the treatment. Administration of aldicarb under
conditions where the dressing was wet and the skin abraded resulted
in reduced body-weight at a dermal dose of 5 mg/kg bw/day and above.
During the interim where dermal treatment was not applied, recovery
of growth was extremely rapid. Plasma cholinesterase activity was
inhibited at the two highest dose levels. There were no other
adverse effects on haematology or clinical chemical parameters and
limited organ weight analysis and microscopic examination of several
major tissues showed no pathological effects attributable to
aldicarb. Almost identical results were also obtained in a similar
study in rats (Carpenter & Smyth, 1966; Weil & Carpenter, 1968a).
Rabbits
- Aldicarb metabolites
Aldicarb sulfone (technical and 75 WP) were administered to the
unabraded, but closely clipped, ventral surface of rabbits (6
males/group, 5 days/week for 19 applications) at dosages of 4.8
mg/kg bw/day for 75 WP and 3.5, 7.0 or 14.0 mg/kg bw/day for
technical aldicarb sulfone. No mortality, skin irritation or
cholinesterase depression were observed. However, rabbits were
removed from treatment 19 h prior to sampling for cholinesterase
analyses, which could account for the lack of cholinesterase
depression. Body-weight was significantly depressed in the high-dose
level rabbits. There were no reported treatment-related effects on
organ weights and necropsy findings were unremarkable (Weil et
al., 1977).
Dogs
- Aldicarb
Groups of 2 beagle dogs/sex were fed aldicarb at dose levels of
0, 0.2, 0.3 or 0.7 mg/kg bw/day for 7 days. There was no mortality
over the course of the study. Plasma, erythrocyte and brain
cholinesterase activities, measured one day after conclusion of the
dietary treatment, were normal. Gross liver and kidney weights and
organ to body-weight ratios were unaffected by aldicarb in the diet
(Weil & Carpenter, 1973).
Groups of 4 beagle dogs/sex were fed aldicarb in the diet for
five days per week at dose levels of 0, 0.2, 0.3 or 0.7 mg/kg bw/day
for 100 days. There was no mortality over the course of the study
and growth was comparable within all dosage groups. A slightly
decreased testes weight and a slight increase in adrenal weight were
observed at the highest level in males, but there were no effects in
females on any of the tissues and organs examined. Microscopic
analysis did not suggest any abnomalities including tissues where
gross changes had been seen to occur. Cholinesterase values, as well
as other clinical chemistry and haematology parameters, were
unaffected by the presence of aldicarb in the diet, however, the
animals had been removed from aldicarb exposure for 24 to 48 h prior
to cholinesterase analyses. The NOAEL in this study was 0.3 mg/kg
bw/day (Weil & Carpenter, 1974c).
Groups of 3 beagle dogs/sex were fed aldicarb in the diet at
dose levels of 0, 0.025, 0.05 or 0.1 mg/kg bw/day, 5 days per week
for 2 years. There was no mortality over the course of the study and
growth and food consumption data were comparable to control values.
Haematology parameters and clinical chemistry values, evaluated at
five intervals over the course of the study were normal. Plasma and
erythrocyte cholinesterase, evaluated over the course of the study,
did not differ from control values. At the conclusion of the study
brain cholinesterase, while somewhat lower at the high dose level,
was not statistically different from controls. Gross and microscopic
examination of tissues and organs showed no lesions which could be
attributable to the presence of aldicarb in the diet at dose levels
up to and including 0.1 mg/kg bw/day (Weil & Carpenter, 1966c).
A two-week range-finding study was conducted in beagle dogs to
select dose levels for a subsequent one-year dog study. Aldicarb was
fed to groups of 1 male and 1 female at dietary concentrations of 0,
1, 3, 10, 30 or 100 ppm. There was no mortality during the study,
but tremors and slight ataxia were observed at 30 and 100 ppm, and
soft faeces were noted more frequently in these dogs. Food
consumption was depressed in all treated males, particularly at the
highest dose, and slightly depressed in the 100 ppm female. Body-
weight losses were noted in the 100 ppm group and in the 30 ppm
male. Red blood cell and plasma cholinesterase inhibition exceeded
25% at doses of 3 ppm and greater. Brain cholinesterase inhibition
exceeded 25% for the 30 and 100 ppm group males (no calculation was
possible in the female groups due to presumed technical error)
(Hamada et al., 1985b).
As a clear NOEL for cholinesterase depression was not
demonstrated in the first study, a second two-week dietary dose
range-finding study was conducted to select the dose levels for a
subsequent one-year dog study. Aldicarb was fed to groups of 1 male
and 1 female at dietary concentrations of 0, 0.1, 0.3, 1, 3 or 10
ppm. There was no mortality during the study and no
treatment-related clinical signs were observed. Body-weight change
and food consumption were unaffected by treatment. Maximum plasma
and erythrocyte cholinesterase inhibition occurred immediately
following the two-hour feeding and lasted approximately 4 h after
the dosing period. Erythrocyte and plasma cholinesterase inhibition
(> 25% compared to the mean pre-study value) occurred in the 10 ppm
group on both days 7 and 14. There was no inhibition of brain
cholinesterase and no other compound-related effects noted. The
NOAEL in this study was 3 ppm, equal to 0.096 mg/kg bw/day (Hamada,
1987b).
In a five-week study, conducted in order to further investigate
the cholinesterase inhibition dose response curve, aldicarb (99.7%)
was fed at dietary concentrations of 0, 0.35, 0.7, or 2 ppm, to 3
groups of 6 beagle dogs/sex. There were no mortalities nor any
changes in body-weight gain, food consumption, clinical symptoms or
gross pathology indicative of a compound-related effect seen during
the study. Plasma cholinesterase inhibition over 20% when compared
to the control occurred only in the highest dose group. Red blood
cell cholinesterase activity inhibition was not observed in any dose
group and brain cholinesterase activity was not measured. No adverse
effects were observed at 2 ppm (equal to 0.06 mg/kg bw/day) and the
NOEL for any symptoms including plasma cholinesterase depression was
0.7 ppm (equal to 0.02 mg/kg bw/day) (Hamada, 1991).
In a 52-week oral toxicity study in beagle dogs, groups of 5
dogs/sex were fed dietary concentrations of 0, 1, 2, 5 or 10 ppm
aldicarb (95.5%) daily for 52 weeks. The study was designed to
produce maximum cholinesterase depression by limiting feeding time
to two hours per day to mimic a bolus administration of aldicarb.
Erythrocyte and plasma cholinesterase activities were measured from
blood samples collected approximately 2 h after the daily feeding
period to minimise dissociation of the carbamate-cholinesterase-
complex. Aldicarb at up to 10 ppm (equal to 0.241 mg/kg bw/day)
caused no observable effects other than inhibition of cholinesterase
activity. Inhibition of erythrocyte and brain cholinesterase
activity was restricted to dogs receiving 5 or 10 ppm. The NOEL for
plasma cholinesterase inhibition was 1 ppm, equal to 0.027 mg/kg
bw/day, while if the changes in the plasma enzyme activity are
discounted, the NOAEL was 2 ppm, equal to 0.054 mg/kg bw/day
(Hamada, 1988).
An inhalation study was conducted to determine the toxicity of
pyrolysis products of cigarettes made from tobacco pretreated with
aldicarb. Twelve beagle dogs (two animals/sex/dose), which had been
initially conditioned prior to commencement of the test to smoking
10 cigarettes per day via tracheostomy, were exposed to untreated,
low-level and high-level treated cigarettes on a schedule of two
successive cigarettes 5 times per day, 5 days per week for a total
of 19 exposure days. Whole blood cholinesterase was monitored
throughout the study with samples taken before smoking, after 4 and
after 10 cigarettes. All effects observed in this study were
apparently due to inhalation of cigarette smoke. No inhibition of
cholinesterase was seen in any treatment group (Coate et al.,
1982).
Dogs
- Aldicarb metabolites
Groups of 3 beagle dogs/sex were fed aldicarb sulfoxide in the
diet at dose levels of 0, 0.0625, 0.125, 0.25 or 0.5 mg/kg bw/day 5
days per week for 3 months. There was no mortality over the course
of the study. Slight body-weight changes were noted in many of the
dogs at the highest dose level within the first week of treatment
but thereafter the body-weight changes were similar to but lower
than control values. No effects on haematologic and blood chemistry
parameters were observed. Cholinesterase depression measured 24-48 h
after the final exposure was not observed in plasma, erythrocytes or
brain of any of the animals at the conclusion of the study. Gross
and microscopic examination of tissues and organs did not show any
adverse effects attributable to the presence of aldicarb sulfoxide.
The NOAEL in this study was 0.25 mg/kg bw/day (Weil & Carpenter,
1968b).
Groups of 3 dogs/sex were fed aldicarb sulfone in the diet at
dose levels of 0, 0.2, 0.6, 1.8 or 5.4 mg/kg bw/day 5 days per week
for 90 days. There was no mortality over the course of the study.
Slight body-weight depression was noted at the highest dose level,
although the body-weight was not statistically lower than control
levels. There were no effects noted with respect to biochemical and
haematologic parameters and gross and microscopic examination of
tissues and organs revealed no effects attributable to the presence
of aldicarb sulfone in the diet. Cholinesterase depression was not
observed, but dogs were removed from treated diet prior to
cholinesterase determinations. The NOAEL in this study was therefore
greater than 5.4 mg/kg bw/day (Weil & Carpenter, 1968c).
In a two-week range-finding study, aldicarb sulfone was fed to
six groups of 1 male and 1 female beagle dog at dietary
concentrations of 0, 3, 10, 30, 100 or 300 ppm. There was no
mortality during the study, but emesis, tremors and decreased food
consumption were observed in the high-dose group. Inhibition of
cholinesterase (> 25%) occurred at 10 ppm and over in both sexes
for plasma cholinesterase, and 100 ppm and over for red blood cell
and brain cholinesterase (Hamada et al., 1985a).
In a one-year feeding study, aldicarb sulfone was administered
to four groups of 6 beagle dogs/sex at dietary concentrations of 0,
5, 25 or 100 ppm. In this study, samples for cholinesterase
determinations were taken approximately two hours after feeding in
order to measure maximum cholinesterase inhibition. No
treatment-related clinical signs were seen. Body-weight gain was
slightly reduced in the high-dose males and females. There was a
slight decrease in the spleen weights of the mid- and high-dose
females and in the thyroid/parathyroid weight of the high-dose
females when compared to respective control values. These changes
were not seen in the males. Slight treatment-related effects were
noted in the mandibular lymph nodes of the high-dose males and
females, and adrenal cortex of the high-dose females. The NOAEL
based on depression of erythrocyte and plasma cholinesterase
activity was 25 ppm, equal to 0.54 mg/kg bw/day (Hamada, 1987a).
Monkeys
As a result of reports of alleged illness associated with
consumption of watermelons and cucumbers illegally treated or
contaminated with aldicarb in 1985, two studies were conducted to
evaluate the acute toxicity of aldicarb residues in food
commodities. Two crops were chosen for the studies: bananas as a
representative of a registered food crop in the USA, and watermelons
as a representative of a non-registered food crop from the cucurbit
family. (Suggestions had been made that some possible natural
component in cucurbits might synergise aldicarb toxicity). Bananas
were treated with approximately ten times the recommended label rate
and watermelons were treated at 13.44 kg ai/ha, to ensure adequate
residues in the fruit. Each study was performed using 3 Cynomolgus
monkeys/sex, with an additional group of 3 monkeys/sex serving as a
control (total of 12 animals per study). Following analytical
determination of actual residues in the two commodities to be
consumed, (approximately 0.3 ppm in bananas and 5 ppm in
watermelons), intake of the treated crops was adjusted with control
fruit to provide a dose of exactly 0.005 mg aldicarb/kg bw. The
animals were offered the fruit as the first meal of the day, and
consumed it immediately. Individuals were monitored for clinical
signs at regular intervals, and plasma and erythrocyte
cholinesterase activities were measured pre-dose, then at 1, 2, 4,
6, 12, 18 and 24 hours post-feeding. In both studies, there was no
evidence of acute cholinergic distress or any signs of over-exposure
in any animal. Depression of plasma cholinesterase activity was
evident within 1-4 h after ingestion and reached a maximum of 32 to
36% at 2 h after feeding (banana trial) and 36 to 37% at 1 h after
feeding (watermelon trial). Erythrocyte cholinesterase activity was
not depressed in either study. Rapid recovery of all enzyme activity
was observed. There was no indication in these studies that
components in watermelons potentiate aldicarb toxicity (Trutter,
1987a,b).
Long-term toxicity/carcinogenicity studies
Mice
- Aldicarb
Groups of 50 B6C3F1 mice/sex (25 of each sex were used as
controls) were fed aldicarb at dietary concentrations of 0, 2 or 6
ppm for 103 weeks in a carcinogenicity bioassay. A preliminary
dietary study (using 10 mice/sex fed dietary concentrations of
aldicarb of 0, 0.5, 1.0, 2.5, 5, 10, 20 or 40 ppm for 13 weeks)
showed no significant effects on microscopic examinations of the 0,
20 and 40 ppm levels. In the long-term carcinogenicity study there
was no mortality attributable to aldicarb. A variety of benign and
malignant tumours, occurring at different sites in both control and
aldicarb-treated mice, were not unusual for this strain of mice and
were evaluated to be independent of the administration of aldicarb.
Gross and microscopic examination of tissues, organs and all gross
lesions was performed and it was concluded that aldicarb was not
carcinogenic for the B6C3F1 strain of mice of either sex (NIH,
1979).
Groups of 44 CD-1 mice/sex were fed aldicarb at dose levels of
0, 0.1, 0.2, 0.4 or 0.7 mg/kg bw/day for 18 months. Mortality was
evident in males at the two highest dose levels and in females at
the three highest dosage levels during the first two and one-half
months of the study. Following this period, aldicarb was admixed
with the diet in a different manner which appeared to eliminate its
acutely toxic effects. (In the early parts of the study, aldicarb
was mixed in a dry fashion using a finely ground aldicarb
preparation. At the 2.5 month interval, aldicarb was dissolved in
acetone and the acetone-aldicarb solution was dispersed in the diet
at a more uniform rate. It was assumed that consumption of small
crystalline particles of aldicarb may have led to the high mortality
during the initial phases of the study). At the high dose level in
males there was a statistically significant increase in hepatomas
found predominantly in the survivors at the termination of the study
and an increase in lymphoid neoplasias which occured in the mice
that died. None of the male mice surviving at the end of the study
were found to have lymphoid neoplasias. There were no significant
increases in any other types of tumours at dose levels of 0.4 mg/kg
bw/day and below (Weil & Carpenter, 1972c).
Groups of 50 male CD-1 mice were fed aldicarb at dose levels of
0, 0.1, 0.3 or 0.7 mg/kg bw/day in an effort to verify the results
of the previous mouse carcinogenicity bioassay. A group of 150 mice
were used as concurrent controls with a mouse being sacrificed for
each treated animal that died during the course of the study. Diets
were prepared by dissolving aldicarb in acetone and mixing the
solution with the diet. The aldicarb was the same sample as used in
the previous study and the duration of the study was approximately
the same as in the previous trial. There was no mortality observed
in the study as a result of aldicarb in the diet. At the end of 18
months cumulative mortality at all dose levels was the same as noted
in controls. There was no effect of aldicarb on growth in any of the
groups. An examination of the animals that died during the course of
the study and those that were sacrificed at the end of 18 months was
made and the data compared with control values. There was no
significant association between aldicarb in the diet and the
formation of tumours, particularly with respect to the incidence of
hepatomas, lung adenomas, and lymphoid neoplasias. The data were
evaluated with respect to the mice that died, those that survived
the test and the total of all animals. It was concluded that the
administration of aldicarb at levels up to and including 0.7 mg/kg
bw/day for approximately 18 months did not result in a higher than
normal incidence of tumours and the inclusion of aldicarb in the
diet of CD-1 mice did not result in an increased incidence of
carcinogenic response (Weil & Carpenter, 1974d).
Groups of C3H/Hej male mice were administered aldicarb
dissolved in acetone, dermally 3 times a week for 28 months by
applying a brush full of an acetone solution to the shaved back of
the mice. For the first 2 weeks, treatment was performed 3 times a
week using a 0.25% solution in acetone. After 2 weeks this was
reduced to a twice weekly application. This dosing regimen was
maintained for 2 months and further reduced thereafter to a
concentration of 0.125% which was maintained for the rest of the
study. While there was some aldicarb-induced mortality noted over
the course of the study this mortality was not substantially
different from that noted with control applications. There were no
substantial differences with respect to the incidence or onset of
tumours. Two growths, a haemangioma and a thymoma, were noted in the
animals administered aldicarb. Neither of these internal growths was
accompanied by cutaneous papillomas or carcinomas and were
considered to be spontaneous growths unrelated to treatment.
Aldicarb, administered dermally to this sensitive species, did not
induce any incidence of malignancy (Weil & Carpenter, 1966b).
Mice
- Aldicarb metabolites
Groups of Charles River CD-1 mice (50/sex/group) were
administered 0, 0.15, 0.6, 2.4 or 9.6 mg aldicarb sulfone/kg bw/day
via the diet for 18 months. Aldicarb sulfone did not affect tumour
incidence or produce any pathological alteration in this strain of
mouse (Woodside et al., 1977b).
Rats
- Aldicarb
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0, 0.005, 0.025, 0.05 or 0.1 mg/kg bw/day. Additional
groups of 16 rats/sex were maintained for serial sacrifices at 6 and
12 months. There was no mortality, attributable to the presence of
aldicarb. Growth was normal at all dose levels as was consumption of
food and behavioural characteristics. Results of gross examination
of liver and kidney weight at 6 months and at one year did not
differ from control values. Haematologic determinations in the
highest dose level and control groups were normal. Blood and brain
cholinesterase activity, measured at 6 and 12 months, were normal.
Microscopic examination of tissues and organs for histopathologic
occurrences and neoplasms showed the incidence of lesions to be
similar in aldicarb-treated and in control groups. An apparent
no-effect-level in this study is 0.1 mg/kg bw/day (Weil & Carpenter,
1965).
Groups of 50 F344 rats/sex (25/sex for controls) were fed
aldicarb in the diet at dietary concentrations of 0, 2 or 6 ppm for
103 weeks. A preliminary study used 10 rats/sex, with dietary
concentrations of 0, 5, 10, 20, 40, 80, 160 or 320 ppm for 13 weeks.
Microscopic examinations performed on male and female rats of the 0
and 80 ppm dose levels at the conclusion of the preliminary trial
showed no significant effects. In the long-term carcinogenicity
study, there was no mortality attributable to aldicarb in the diet.
A variety of benign and malignant tumours occurring at different
sites in both control and aldicarb treated rats were not unusual for
this strain of rat and were evaluated to be independent of the
administration of aldicarb. Gross and microscopic examination of
tissues, organs and all gross lesions was performed and it was
concluded that aldicarb was not carcinogenic for the F344 strain of
rat of either sex at dietary concentrations of up to 6 ppm (NIH,
1979).
Rats
- Aldicarb/Aldicarb metabolites
Groups of 20 rats/sex were fed aldicarb for 2 years at dose
levels of 0 or 0.3 mg/kg bw/day. In addition, groups of rats were
fed aldicarb sulfoxide (0.3 or 0.6 mg/kg bw/day), aldicarb sulfone
(0.6 or 2.4 mg/kg bw/day) or a 1:1 mixture of aldicarb sulfoxide and
aldicarb sulfone (0.6 or 1.2 mg/kg bw/day). There was no significant
mortality observed over the course of the study with any of the
individual chemicals or the mixture of sulfoxide and sulfone. In the
initial phases of the study, there was a slightly higher mortality
noted in the high-dose level of aldicarb sulfoxide and in the group
receiving the combined aldicarb sulfoxide and aldicarb sulfone. A
slight increase in mortality was also noted at the latter part of
the study with aldicarb sulfoxide. Growth was slightly depressed at
the high-dose level of the sulfoxide:sulfone mixture, primarily in
males. There were no apparent effects on growth with respect to the
aldicarb, aldicarb sulfoxide or aldicarb sulfone administered alone.
Haematological values observed at various intervals over the course
of the study did not differ from controls. Cholinesterase
determinations were made periodically over the course of the study
(6, 12 and 24 months). Plasma, erythrocyte and brain cholinesterase
were examined only at a 24-h interval after animals were removed
from test diets. There was a slight depression of plasma
cholinesterase noted in males administered the high-dose level of
the combination aldicarb sulfoxide and sulfone at the 24-month
interval. A repeat of the analyses within the final week of the
study showed a slight depression in all chemical groups with respect
to plasma cholinesterase. There were no effects noted at any
interval with respect to red blood cell or brain cholinesterase. The
plasma cholinesterase depression noted at the 24-month interval was
limited to male rats. An evaluation of the incidence of tumours
suggested that there was no statistical difference between treated
and control groups. Gross and microscopic examination of tissues and
organs at various periods over the two-year test interval showed
that these sporadically distributed lesions could not be considered
to be indicative of damage induced by aldicarb, its major
metabolites, or the combination of the sulfoxide and sulfone (Weil &
Carpenter, 1972a).
Reproduction studies
Rats
- Aldicarb
Groups of rats (8 male and 16 female rats per group) were fed
aldicarb at dietary concentrations at doses of 0, 0.05 or 0.1 mg/kg
bw/day for approximately 90 days and mated to initiate a 3-
generation, one litter per generation reproduction study. Dietary
administration was continuous throughout the study. In addition to
the reproduction indices (fertility, gestation, viability and
lactation) the F3 generation was maintained for an additional
period and tissues from these animals were histologically examined
at either weaning or at 90 days of age. In all groups, the
reproduction indices from the aldicarb-treated animals were similar
to the control group. Body-weights of both male and female pups at
weaning were similar to control values as were results of gross and
microscopic examinations of tissues and organs in the F3 weanling
and 90-day old animals. In all three generations, with all criteria
examined, there were no effects of aldicarb on reproduction at a
dose level of 0.1 mg/kg body-weight (Weil & Carpenter, 1964).
Groups of rats (10 male and 20 female per group) were
administered aldicarb in the diet at doses of 0, 0.2, 0.3 or 0.7
mg/kg bw/day for 100 days prior to pairing and mated to initiate an
additional 3-generation (one litter per generation) reproduction
study. Dietary administration was continuous throughout the study. A
larger group was used for the F2 generation (15 male and 25 female
rats) as male pups of this generation were maintained on aldicarb
diets for 148 days and subjected to a (modified) dominant lethal
(mutagenesis) bioassay where they were mated with groups of
untreated virgin females for a period of 10 weeks. Each female in
the group was mated with 2 treated males and allowed to maintain
pregnancy until day 12 when they were sacrificed and examined. There
was some mortality over the course of the study which was associated
with lung infection and not as a result of aldicarb in the diet. At
the high-dose level, body-weights of both male and female F2 pups
were lower than the control values. Overall, there were no effects
on any of the reproduction indices (fertility, gestation, viability,
or lactation). Gross and microscopic examinations of the parents and
pups of the high level and control groups showed no effects
attributable to aldicarb. The dietary dominant lethal mutagenesis
bioassay showed no statistical differences between the
aldicarb-treated rats and controls with respect to early or late
fetal death or any other parameter examined (Weil & Carpenter,
1974a).
Rats
Aldicarb metabolites
Aldicarb sulfone (99.76% pure) was fed to groups of 10 male and
20 female rats at dietary concentrations at doses of 0, 0.6, 2.4 or
9.6 mg/kg bw/day for approximately 100 days. Rats were then mated to
initiate a three-generation, one litter per generation, reproduction
study. Dietary administration was continuous throughout the study.
Male rats fed 9.6 mg/kg bw/day exhibited reduced body-weights. There
were no differences from the control with regard to fertility,
gestation survival or viability indices. It was determined that
aldicarb sulfone, at levels up to and including 9.6 mg/kg bw/day,
was without adverse effects on reproduction under the conditions of
this study (Woodside et al., 1977a).
Special studies on embryo/fetotoxicity
Rats
- Aldicarb
Using a test protocol where both the reproductive and
teratologic potential of aldicarb was evaluated, groups of pregnant
rats were fed aldicarb at dietary concentrations at dose levels of
0, 0.04, 0.2 and 1.0 mg/kg bw/day. Females from each of the dietary
groups were assigned to one of three treatment groups: (1) aldicarb
administered in the diet throughout pregnancy or until pups were
weaned; (2) aldicarb administered in the diet from day 0 to day 7 of
gestation; (3) aldicarb administered in the diet from day 5 to day
15 of gestation. Five or six females from each group were sacrificed
and examined on day 20 of pregnancy and a similar number of females
were allowed to bear, nurse and wean the pups. There were neither
gross manifestations of teratogenicity in any of the pups carried by
females administered aldicarb at dose levels of up to 1 mg/kg bw/day
nor was there apparent interference with the reproductive process by
any of the dosage regimens used in this study. The administration of
aldicarb at dose levels up to and including 1.0 mg/kg bw/day had no
apparent effect on the growth of pregnant females during the course
of the study (Weil & Carpenter, 1966a).
Pregnant Sprague-Dawley rats were given aldicarb by gavage at
levels of 0, 0.125, 0.25 or 0.5 mg/kg bw/day on gestational day 6
through day 15. Maternal toxicity was indicated, at the highest
dose, by reduced weight gain and food intake during the treatment
and post-treatment period as well as by the occurrence of three
maternal deaths and, at the two highest levels, by reduced food
consumption during treatment. Gestational parameters, including
ovarian corpora lutea, number of implantations per litter and sex
ratio were unaffected by treatment. Fetal body-weight per litter was
significantly reduced at 0.5 mg/kg bw/day. There was no increase in
the incidence of external or skeletal malformations. A significantly
increased incidence of dilated lateral ventricles with tissue
depression was observed only at 0.5 mg/kg bw/day. No increased
incidence of malformation was observed in the absence of clear
maternal toxicity nor were these malformations accompanied by more
severe malformations. The NOEL was 0.125 mg/kg bw/day for maternal
toxicity and 0.25 mg/kg bw/day for embryo-fetal toxicity and
teratogenicity (Tyl & Neeper-Bradley, 1988).
Rats
- Aldicarb metabolites
The aldicarb sulfone reproduction study incorporated a
teratology bioassay. The animals were divided into 4 groups and
orally dosed with 0, 0.6, 2.4 or 9.6 mg/kg bw/day at one of the
following time intervals of gestation: 0-20 days, 6-15 days, or 7-9
days. All animals were sacrificed before parturition (day 20) and
the fetuses examined for skeletal and visceral changes. Anomalies
were essentially non-existent and there was no indication of
structural abnormalities produced under the conditions of the study
at levels up to and including 9.6 mg/kg bw/day (Woodside et al.,
1977a).
Rabbit
- Aldicarb
A range-finding study was conducted with aldicarb in rabbits to
determine dosage levels of aldicarb for a teratology study. Pregnant
Dutch belted rabbits (5/dose) were dosed by oral gavage at 0.1,
0.25, 0.5, 0.75 or 1.0 mg/kg bw/day on days 6 to 27 of gestation.
Two does in the high-dose group and one doe in the second highest
group died during the study. Increased incidences of decreased
defecation (all except low-dose group) and soft stools (two highest
dose groups) were noted. Maternal body-weight losses or markedly
decreased maternal body-weight gain during the treatment and
gestation intervals were observed at 0.25 mg/kg bw/day and greater.
Of the uterine parameters measured, there was an increase in the
mean post-implantation loss in the high-dose group. Dosage levels of
0.1, 0.25 and 0.5 mg/kg bw/day were selected for the definitive
study (Aldridge et al., 1983).
Pregnant Dutch belted rabbits (16/dose) were dosed by oral
gavage at 0.1, 0.25, or 0.5 mg/kg bw/day on days 7 to 27 of
gestation. Signs of maternal toxicity in the treated groups included
small amount of stool, pale kidneys and hydroceles on oviducts. A
loss in body-weight occurred in the 0.25 and 0.5 mg/kg bw/day dosage
groups during gestation days 7 to 27. A decline in the number of
viable fetuses per doe was observed in all treatment groups (8.7,
5.0, 6.5 and 6.2 for the control, 0.1, 0.25 and 0.5 mg/kg bw/day
treatment groups, respectively). This can be explained by an
unusually high number of implantations in the control group (9.8,
6.1, 7.2 and 7.8 implantations per doe in the control, 0.1, 0.25 and
0.5 mg/kg bw/day treatment groups, respectively). There was no clear
dose-effect relationship. There were also no increases in
post-implantation losses (1.1, 1.1, 0.7 and 1.7 post-implantation
losses for the control, 0.1, 0.25 and 0.5 mg/kg bw/day treatment
groups, respectively) these figures falling within the historical
control range. There was no meaningful difference in any of the
other developmental parameters nor in the number of developmental
variations or malfunctions between the control and treatment groups.
Aldicarb did not produce a teratogenic response when administered
orally to rabbits by gavage at dosage levels up to 0.5 mg/kg bw/day
(Leng et al., 1983).
Special studies on genotoxicity
Results of genotoxicity tests are summarised in Tables 3 and 4
(for in vitro and in vivo tests with aldicarb) and Table 5 (for
in vitro tests with aldicarb metabolites).
Table 3: Results of in vitro genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980c)
TA98,TA100,TA1535,
TA1537,TA1538
Reverse E. coli WP2 nk nk Negative Dunkel et al. (1985)
mutation assay
Reverse S. cerevisiae nk nk Negative Guerzoni et al. (1976)
mutation assay
HGPRT forward CHO cells 1000-5000 µg/ml nk Negative Stankowski et al. (1985a)
mutation assay
SCE Human nk nk Weak Debuyst & Larebeke (1983)
mutation assay lymphocytes Positive
Cytogenetics Human 10-250 µg/ml nk Weak Cid & Matos (1984)
mutation assay lymphocytes Positive
UDS Rat hepatocytes 0.16-5000 µg/ml nk Negative Godek et al. (1984a)
DNA damage S. typhimurium > 500 µg/disc nk Positive Rashid & Mumma (1986)
TA 1538 uvrB
TA 197
nk: not known
Table 4: Results of in vivo genotoxicity assays on aldicarb
Test System Test Object Concentration Purity Results Reference
Micronucleus Mouse 0.001-0.01 93.47% Negative Ivett et al. (1984)
test mg/kg bw
Micronucleus ICR mouse 0.1-0.4 99.7% Negative Ivett (1990)
test mg/kg bw
Dominant Wistar rat 0.2-0.7 99.2% Negative Weil & Carpenter (1974a)
lethal test mg/kg bw
Table 5: Results of in vitro genotoxicity assays on aldicarb metabolites
Test System Test Object Concentration Purity Results Reference
Ames test S. typhimurium 50-5000 µg/plate nk Negative Godek et al. (1980b)
TA98,TA100,TA1535, sulphoxide
TA1537, TA1538
Ames test S. typhimurium 100-10000 µg/plate nk Negative Godek et al. (1980a)
TA98,TA100,TA1535, sulphone
TA1537, TA1538
HGPRT forward CHO cells 500-1500 µg/ml nk Negative Stankowski et al. (1985b)
mutation assay sulphone
Cytogenetics CHO cells 50-500 µg/ml nk Negative San Sebastian et al. (1984)
mutation assay sulphone
UDS Rat hepatocytes 0.1-3000 µg/ml nk Negative Godek et al. (1984b)
sulphone
nk: not known
Special studies on skin and eye irritation and sensitization
Administration of aldicarb to the conjunctival sac of rabbits
did not produce ocular irritation or corneal damage. Ocular
irritation studies were performed at doses that were lethal without
indication of ocular damage. Furthermore, there was no evidence of
dermal irritation when aldicarb was applied to the shaved, abraded
backs of rabbits (Striegel & Carpenter, 1962).
There was no indication of a sensitization reaction induced by
aldicarb. Male guinea-pigs were administered aldicarb by multiple
subdermal applications (0.7 mg/kg body-weight) and re-administered
aldicarb three weeks later by a similar intradermal administration.
There was no suggestion of sensitization in any of the animals
tested (Pozzani & Carpenter, 1968a).
Skin sensitization to aldicarb sulfone (UC 21865, technical) or
a 75% WP formulation was evaluated in albino guinea-pigs (Hartley
Strain) using a modified Landsteiner technique. Neither substance
was determined to be a sensitizer under the conditions of this study
(Conroy & Carpenter, 1977).
Special studies on delayed neurotoxicity
Hens
- Aldicarb
Groups of 6 adult chickens were administered aldicarb as a
single oral dose of 4.5 mg/kg bw or as daily oral doses of 0, 2.25
or 4.5 mg/kg bw/day for 30 days. A positive control group, treated
with 100 mg of TOCP, was used to produce typical delayed neurotoxic
signs of poisoning. While there was some weight loss, which was
correlated with the dose of aldicarb administered, the only
neurological effects attributable to aldicarb were acute signs of
poisoning noted in the first two or three days of treatment. Neither
ataxia nor hind limb paralysis were noted over the course of the
study. Aldicarb did not induce a delayed neurotoxic syndrome similar
to that induced by certain organophosphate esters (Johnson &
Carpenter, 1966a).
Hens
- Aldicarb metabolites
A group of 40 adult white Leghorn hens were intubated to
receive 250 mg/kg bw aldicarb sulfone suspended in maize oil. Two
other groups, each with 10 hens, received either TOCP (500 mg/kg bw)
or maize oil alone, and served as positive and negative control
groups respectively. There were no neurological effects other than
acute cholinergic signs of poisoning in the TOCP dosed group. No
histological examination was performed, owing to the lack of
demonstrated neurotoxic signs. Aldicarb sulfone did not cause
delayed neurotoxic reactions under the conditions of the study
(Babish & Salerno, 1977).
Special studies on behaviour
The effects of acute administration of aldicarb and aldicarb
sulfoxide on avoidance behaviour in rats was compared to a variety
of other carbamate esters. Rats were trained and evaluated for their
ability to avoid electrical shock in standard avoidance behaviour
tests. Aldicarb and aldicarb sulfoxide were administered by
intraperitoneal injection and the rats were evaluated for their
ablity to avoid shocks over a 6 h period following administration.
The lowest beviourally effective dose was found to be 0.266 mg/kg bw
which, when compared to the acute ip LD50 value, was noted to have
a smaller ratio of behaviour effects to acute LD50 than any of the
other carbamates tested. These data suggest that the level of
aldicarb needed to produce measurable avoidance is greater (closer
to a fatal dose and less likely to be achieved at the suggested use
level) than the chemicals to which it was compared. Additionally,
the activity over the 6-h period was seen to rapidly decline again
attesting to the transient nature of the cholinesterase inhibition
(Johnson & Carpenter, 1966b).
Special studies on immune responses
Aldicarb was evaluated for its ability to modulate the immune
response in two strains of mice. The B6C3F1 mouse was chosen
because it is the strain used by the US National Toxicology Program
for immunotoxicology studies, and the hybrid Swiss Webster mouse was
included because aldicarb had been reported to suppress the splenic
plaque-forming cell response to sheep red blood cells in an inverse
dose-response fashion (Olson et al., 1987). An attempt was made to
reproduce these findings and to expand the data base using more
standardised techniques with the B6C3F1 strain. Aldicarb was
administered in the drinking water ad libitum for 34 consecutive
days to female mice of both strains at dosages ranging from 0.1 to
1000 µg/litre in 10-fold increments (equivalent to 0.04 to 364 mg/kg
bw/day). Aldicarb had no effects on body-weights or organ weights,
on numbers or types of circulating white blood cells, or on the
microscopic pathology of the thymus, spleen, liver, kidneys, or
lymph nodes. Also unaffected were the number of antibody forming
cells in the spleen and the amount of circulating antibody in the
blood. Aldicarb had no effect in either strain on in vivo host
resistance to infectious viral challenge, on the capacity of B- and
T-lymphocytes to respond to nonspecific mitogens, or on the ability
of T-lymphocytes to recognize genetically different cell types in a
mixed lymphocyte culture. In this study, subchronic exposure to
aldicarb in the drinking water of mice had no effect on any measured
immunological function or toxicological parameter (Thomas et al.,
1987).
In another study, adult female B6C3F1, mice received
distilled water only or water containing 1.0, 10 or 100 µg/litre of
aldicarb daily for 34 days. To further develop an immune profile of
the compound, following aldicarb exposure, the ability of splenic
natural killer (NK) cells as well as specifically sensitized
cytotoxic T-lymphocytes to lyse YAC-1 lymphoma and, P815 tumour
cells, respectively, was evaluated. To complement the functional
assays, the impact of aldicarb exposure on spleen cell viability,
splenic cellularity and the percentages and absolute numbers of
total T-cells, T-suppressor, T-helper and B-cells was evaluated. The
results of this study demonstrated that aldicarb did not impact upon
the functional ability of interferon-induced splenic NK cells to
lyse YAC-1 lymphoma target cells. Aldicarb had no effect on the
functional capacity of cytotoxic T-lymphocytes to recognize
allogeneic cells in vivo and undergo differentiation and lysis of
these cells in a short-term 51-Cr release assay. The numbers and
percentages of T-cell subpopulations and B-cells in the spleen also
were not significantly affected. In addition, aldicarb had no
significant effect on body-weights, on spleen cellularity or cell
viability or on absolute weights of lymphoid organs. The absence of
statistically significant effects on any of these parameters
indicated that aldicarb did not have adverse effects on the immune
system of mice (Thomas et al., 1990).
Special studies on pesticide antagonistic agents
Following acute oral administration to rats, aldicarb has been
shown to induce a strong muscarinic action at excretory, bronchial
and cardiac nerve sites. A nicotinic effect was also shown to occur
at myoneural junctions. The parasympathetic signs of poisoning were
readily reduced following atropine administration. Administration of
atropine and 2-PAM, alone, or in combination, showed that while
atropine was a more effective antidote, 2-PAM was also active. While
it has been shown that aldicarb elicits a strong muscarinic action
as well as nicotinic action at myoneural sites, the control of signs
of poisoning from both mechanisms appears to be somewhat difficult
to achieve. Atropine has been shown to be an effective antidote to
block the muscarinic effects, but decamethonium, commonly used to
block the nicotinic effects, has been shown to be somewhat
ineffective. Additional studies to influence the nicotinic actions
by such drugs as tubocurare also failed to completely eliminate
nicotinic activity. Further studies confirmed the therapeutic
effects of a variety of oximes (P2S and obidoxime) in reducing the
acute toxic signs of poisoning associated with aldicarb. (Johnson &
Sullivan, 1968a,b; Natoff & Reiff, 1973).
Special studies on pesticide interactions
Aldicarb was administered orally to male rats alone and in
combination with a series of eight organophosphate esters or one
carbamate ester, all anti-cholinesterase agents, to examine the
potential interactive or additive effects. Results of the study,
using proportions of the acute lethal dose of each material alone
and in combination with aldicarb, showed a simple additive effect
with all materials tested. Aldicarb was not found to potentiate the
acute oral toxicity of other anticholinesterase agents. Further
studies were reported on the potential interaction of aldicarb with
alpha-naphthol, aldicarb sulfoxide with aldicarb sulfone and
aldicarb sulfone with parathion administered orally and aldicarb
with alpha-naphthol or with carbaryl administered by the
intraperitoneal route. In no case were any interactions greater tban
the predicted additive effects (West & Carpenter, 1966a; Weil &
Carpenter, 1970a).
Observations in humans
Groups of 4 adult male volunteers were administered aldicarb
orally in aqueous solution at dose levels of 0.025, 0.05 or 0.1
mg/kg body-weight. Clinical signs of poisoning were recorded and
whole blood cholinesterase activity was measured up to 6 h after
administration of the sample. Total urine voided was collected and
aldicarb-excretion patterns for the initial 8 h after dosing were
evaluated. In addition, spot samples were taken at 12 and 24 h.
Acute signs of poisoning, typical of anticholinesterase agents, were
observed at the high-dose level within 1 h after administration of
aldicarb. There were no signs of poisoning observed at the 0.05
mg/kg body-weight dose level. Cholinesterase depression was observed
in all volunteers prodominantly within 1-2 h after treatment. Within
the first six hours of treatment almost all cholinesterase
depression and clinical signs of poisoning were diminished.
Examination of urinary excretion patterns showed that approximately
10% of the administered dose was excreted as carbamates (toxic
residues) within the first 8 h interval. Cholinesterase analyses
confimed the same rapid inhibition and recovery pattern with man as
had been observed in experimental animals (Haines, 1971).
In another study, two additional subjects were administered
aldicarb in water solution at dose levels of 0.05 and 0.26 mg/kg
body-weight. Acute signs of poisoning were recorded at the higher-
dose level and atropine was administered to aid recovery. No signs
of poisoning were recorded with the lower-dose level. Urinary
excretion of carbamate residues within 24 h accounted for
approximately 10% of the administered dose (Cope & Romine, 1973).
A double blind, placebo controlled study has been conducted, in
which aldicarb was given as a single oral dose to healthy male and
female subjects. The doses administered were: placebo (22 subjects -
16 males and 6 females), 0.01 mg/kg bw (8 males), 0.025 mg/kg bw (8
males and 4 females), 0.05 mg/kg bw (8 males and 4 females) and
0.075 mg/kg bw (4 males). Subjects were screened before entry by
general medical history and examination and laboratory tests
including haematology, clinical chemistry and urinalysis. Clinical
measurements were made at intervals before and after dosing. These
included vital signs, (systolic and diastolic blood pressure, pulse
rate), pulmonary function tests, pupil size, electrocardiographs,
salivation and clinical signs of nausea, vomiting, diarrhoea,
sweating, abdominal cramps, involuntary movement and slurred speech.
Samples were taken for urinalysis, clinical chemistry (including red
blood cell and plasma cholinesterase activity) and haematology
evaluation before and after dosing. Urine and blood were collected
for aldicarb analysis. The results of these latter analyses were not
reported. There were no clinically significant changes in vital
signs, pupil size, pulmonary function, ECG's, salivation, clinical
signs, clinical chemistry (apart from cholinesterase), haematology
or urinalysis in the study. Cholinesterase activity in red cells and
plasma was maximally depressed at one hour after dosing and had
recovered by 8 h in all subjects. The fall in activity was dose-
related. Only marginal depression of cholinesterase activity (< 20%)
was seen in erythrocytes from patients treated with 0.01 or 0.025
mg/kg bw and in plasma at 0.01 mg/kg bw. Depression in
cholinesterase activity > 20% was seen in erythrocytes at 0.05 and
0.075 mg/kg bw and in plasma at 0.025, 0.05 and 0.075 mg/kg bw. No
serious adverse events occurred. The minor adverse events which were
recorded were similar to those reported in other volunteer studies.
Only one subject (0.075 mg/kg bw group, actual dose 0.06 mg/kg bw)
developed symptoms which were reported to be related to aldicarb.
The NOAEL in this study should be based on depression (> 20%) in
erythrocyte cholinesterase activity and was thus 0.025 mg/kg bw
(Nimmo et al., 1992).
Fifteen male volunteers participated in a study in Panama to
evaluate human exposure in a banana plantation where Temik-15G was
applied under natural conditions. Temperatures ranged from 24 °C to
32 °C with 80% to 90% relative humidity. The workers used three
different types of hand-held applicators. Blood samples were taken
from all volunteers prior to initiating the test and immediately
following each 6 h working period for estimation of blood
cholinesterase activity. Samples were obtained in the field and
analyzed 1-2 h later in the laboratory. Only two workers showed
greater than 25% reduction in their blood cholinesterase activity
(29% and 50%). Spontaneous reversibility was evident in both cases.
The worker with 50% reduction showed 25% recovery within 3 h. Blood
samples from the other worker indicated 100% recovery 24 h later.
Results of blood analyses indicated that cholinesterase activity was
below the normal range (population) in samples collected from six
workers during the second day of the study. Cholinesterase activity
in all samples taken during the first and third day of the study
were within the normal range. No clinical signs of aldicarb-induced
intoxication were found, although one individual presented symptoms
of nausea, stomachache and headache (Union Carbide, 1979).
A series of human exposure episodes was reported occurring as a
result of a variety of field and glasshouse conditions in an effort
to assess the potential for human harm from exposure under actual
occupational conditions. In several instances, slight blood
cholinesterase depression attested to the actual exposure situation.
Exposure data, as indicated by cholinesterase depression or urinary
excretion, suggested that there was no change in the general health
of workers exposed under any of the working conditions. Although
there were acute clinical signs of poisoning there was no indication
that the workers exposed were harmed once removed from exposure
situation (Williams, 1966; Burrows et al., 1970; Wakefield et
al., 1973; Shrivastava, 1975; Pandey, 1977).
From 1966 to 1979, 133 cases of apparent over-exposure to
aldicarb formulations were reported. Of these cases, 40 were
confirmed aldicarb poisoning episodes where clinical diagnosis
and/or urinalysis for aldicarb and its metabolites were performed.
As has been the case with other carbamate insecticides, the acute
signs of toxicity are rapidly dissipated, although atropine therapy
and hospitalization have been useful therapeutic regimens (Abdalla,
1977, 1979). Several deaths have been reported, but all of these
have been attributed to suicide or gross neglect (Lee, 1984).
California reported that in 1974, 1975 and 1976 a total of 10,
14 and 13 cases of human illnesses were reported, respectively. In
these incidents, people were directly exposed to aldicarb and
illness was brought on by dermal, inhalation and in one instance,
ocular exposure. While most illnesses resulted from aldicarb
exposure in loading or applying the formulated pesticide, some
illness has been reported from the handling of plants and soils
treated with aldicarb (Peoples et al., 1977).
Several misuses of aldicarb on watermelons and cucumbers have
been reported in the literature. Goes et al. (1980) reported on a
suspected food-borne intoxication in Nebraska associated with the
consumption of aldicarb-contaminated hydroponically grown cucumbers.
Two episodes were reported (April 1977: 9 cases; July 1978: 5
cases). Neither cholinesterase determinations nor urine residue
determinations were performed in any of these patients. Four
cucumbers (two in the supermarket and two in the greenhouse were
analyzed and aldicarb content was 6.6 and 10.7 mg/kg (supermarket) 0
and 9.9 mg/kg (greenhouse). No data are available on cucumbers
actually eaten and no attempts to correlate symptoms and aldicarb
consumption were made.
Hirsch et al. (1987) reported over 300 alleged intoxications
in Canada following ingestion of aldicarb contaminated cucumbers
(residue levels up to 26 mg/kg). In only five cases involving 13
patients, were any remaining portion of the cucumbers consumed still
available for analyses.
Goldman (1990) reported on four outbreaks of aldicarb poisoning
having occurred in California and other states in the USA from 1978
to 1988.
Outbreak 1: watermelons, 1985
Outbreak 2: watermelons, 1987
Outbreak 3: cucumbers, 1988
Outbreak 4: cucumbers, 1978 (corresponds to Goes (1980)
publication).
In the first outbreak, 1376 people allegedly became ill due to
consumption of contaminated watermelons. Of this total, 308 were
considered by the authors themselves as unlikely (235) or incomplete
(73). Nevertheless, only 28 cases were supported by positive
findings of aldicarb residues in the food commodity. All the other
cases were only supported by self-diagnosis.
An epidemiological study of potential adverse health effects of
aldicarb metabolites in drinking water was performed. In two surveys
conducted on Long Island, New York, the major cohorts were selected
on the basis of aldicarb levels in their drinking water. Levels of
contamination were generally 4-12 µg/litre, but a maximum of 400
µg/litre was reported. Questionnaires to determine water and food
consumption, symptoms experienced, and diagnosed illnesses were sent
to 1035 residents of 462 households. Although the initial survey
indicated a possible association between the incidence of diarrhoea
and levels of aldicarb in the water, the follow-up study, focusing
on children, did not confirm this association. No relationship was
detected between food consumption or water source and adverse health
symptoms, and self-reported physician-diagnosed illnesses for
1974-1979 were not significantly related to levels of aldicarb in
the water (Whitlock et al., 1982).
Another survey attempted to relate self-reported symptoms
suggestive of peripheral neuropathy to aldicarb levels in drinking
water in Suffolk County, New York. The response rate was less than
20%. Responses were classified as "probably", "possibly", or
"vaguely" suggestive of a neurologic syndrome. A significant
correlation with aldicarb concentration was obtained only by
combining all three categories of response, including reports of
just one symptom or of symptoms not forming any cohesive syndrome.
The authors concluded that further study was needed (Sterman &
Varma, 1983).
A pilot epidemiological study by Fiore et al. (1986)
evaluated a wide range of clinical immunological parameters in 23
women exposed to aldicarb in their drinking water and in a
non-exposed control group. The groups did not differ in any
immunological parameters except in T8-Lymphocytes: the number was
considered as elevated in five exposed and in one control.
Observation of an elevated stimulation assay response to one of the
large number of antigens (Candida) was not considered as
toxicologically significant, nor was it attributed to aldicarb
exposure.
In a follow-up study, five women of the preceeding group and
still exposed to aldicarb were examined versus previous control
women plus women previously exposed but for whom exposure no longer
occurred (e.g., change in water supply due to addition of a charcoal
filter). According to the authors, this follow-up study confirmed
the previous results, except for increased response to Candida
antigens which was no longer present (Mirkin et al., 1990).
COMMENTS
Aldicarb is rapidly absorbed, widely distributed in the body
and rapidly excreted. Metabolism appears to be similar in all
species studied, aldicarb being rapidly metabolised to aldicarb
sulfoxide, which is more slowly degraded to aldicarb sulfone. All
metabolites are quickly eliminated from the body, 80-90% being
excreted within 24 h. Elimination was complete by the fifth day
after dosing and no bio-accumulation was seen.
Aldicarb has high acute toxicity in a wide variety of mammalian
species. Signs of toxicity are those commonly associated with
acetylcholinesterase inhibition by a carbamate insecticide:
cholinergic signs of poisoning, which are alleviated rapidly on
cessation of exposure. Aldicarb sulfoxide is a more potent inhibitor
of acetylcholinesterase than aldicarb itself, while aldicarb sulfone
is considerably less toxic than either aldicarb or the sulfoxide.
WHO has classified aldicarb as extremely hazardous (WHO, 1992).
Short-term and long-term studies have been performed in rats,
mice and dogs with aldicarb and aldicarb metabolites, both
individually and in combination. Toxicity tests employing mixtures
of aldicarb or aldicarb sulfoxide with aldicarb sulfone are of
interest because aldicarb sulfoxide and aldicarb sulfone are the
terminal residues potentially consumed by humans. Cholinesterase
depression is the most significant indicator of toxicity that can be
evaluated. However, considerable attention must be paid to the
methods of sample collection and determination of cholinesterase
activity. Continuous administration of aldicarb to the test animals
until collection of samples for analysis is important, as is rapid
analysis under carefully controlled conditions.
No-effect levels in the various studies which included
evaluation of cholinesterase inhibition are summarized in Table 6.
No distinction is made in this table for the methods of determining
cholinesterase activity.
It is now considered inappropriate to use no-adverse-effect
levels from many of the earlier repeat-dose studies for the
derivation of an ADI, because animals were not dosed for 24-48 h
prior to collection of tissue samples for measurement of
cholinesterase activity. In the most recent dog studies, which were
conducted in a manner designed to maximize detection of
cholinesterase depression, the overall NOEL was 0.02-0.03 mg/kg
bw/day, but the NOAEL (which discounts inhibition of plasma
cholinesterase only) was 0.05-0.06 mg/kg bw/day.
Results of repeat-dose studies with aldicarb demonstrate that
the method of administering the test material to the test animals
can greatly modify the apparent toxicity of aldicarb and its
metabolites. Mice, rats and dogs have tolerated daily doses equal to
the LD50 incorporated into the diet for 7 days to 2 years. Doses
which caused death in less than 2 h when administered as a bolus,
caused no death and only moderate cholinesterase depression when
given in the diet.
Two dietary carcinogenicity studies have been conducted with
aldicarb in rats and three in mice. A dermal carcinogenicity study
has also been conducted with aldicarb in mice and dietary studies
have been carried out with aldicarb sulfone in mice and aldicarb
sulfoxide in rats. Aldicarb was not carcinogenic in mice and rats.
Two reproduction studies have been conducted in rats with
aldicarb and one with aldicarb sulfone. There were no effects on
reproductive performance at doses up to 0.7 mg/kg bw/day aldicarb or
9.6 mg/kg bw/day aldicarb sulfone. Aldicarb did not display any
teratogenic potential in rats or rabbits in studies which included
maternally toxic doses.
After reviewing the available genotoxicity data, the meeting
concluded that aldicarb, aldicarb sulfoxide and aldicarb sulfone are
not genotoxic.
In a range of special studies in animals (involving delayed
neurotoxicity, behaviour, antagonistic agents and pesticide
interactions) aldicarb displayed no results which gave cause for
concern. There was no evidence of immuno-toxicity in mice in a
number of functional assays of cell-mediated immunity and in host
resistance to respiratory infection.
Epidemiological studies provided no convincing evidence that
aldicarb could significantly alter immunological function in humans.
In addition to the above epidemiological studies, studies
conducted in 1982 and 1983 attempted to correlate any potential
adverse health effects with the occurrence of aldicarb in drinking
water. Although the authors concluded that further study was needed,
there was no clear evidence that aldicarb contamination of drinking
water generally at concentrations of about 4-12 µg/litre, but at a
maximum concentration of 400 µg/litre was related to any health
effects.
The anticholinesterase potential of aldicarb has been
extensively investigated in human. These studies revealed the same
pattern of rapid cholinesterase inhibition and rapid recovery seen
in experimental animals. Transient erythrocyte cholinesterase
depression was seen at single doses of 0.05 mg/kg bw, and the NOAEL
for cholinesterase depression (discounting changes in plasma enzyme
activity) was 0.025 mg/kg bw.
A number of poisoning incidents have been reported in the
agricultural use of aldicarb, but there has been no indication that
the workers exposed were harmed once removed from the exposure
source. Although several deaths have been reported, all of these
have been attributed to suicide or gross neglect.
A number of food-borne aldicarb intoxications have been
reported in the literature. These have all been associated with
misuse and reliable quantification of the dose of aldicarb involved
has always proved difficult, if not impossible.
An ADI was allocated using a 10-fold safety factor applied to
the NOAEL for depression of erythrocyte cholinesterase activity in
human volunteers.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 0.1 mg/kg bw/day (93-day dietary study)
Dog: 0.05 mg/kg bw/day (52-week study)
Human: 0.025 mg/kg bw (double-blind, placebo controlled
volunteer study)
Estimate of acceptable daily intake for humans
0-0.003 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans, including information regarding
the correlation of blood cholinesterase depression and clinical
signs and symptoms.
Table 6: Summary of no effect levels in repeat dose toxicity studies which included
cholinesterase investigations
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Mouse 7 days Aldicarb 0,0.1,0.3,0.6,1.2 0.6 Mortality
Mouse 7 days Aldicarb: 0,2,6,18,36 2 Kidney and liver weight
A. sulfone reduction, growth
1:1 depression
Rat 7 days Aldicarb 0,4,8,16 NE Mortality, growth
depression, kidney and
liver weight reduction
Rat 7 days Aldicarb 0,0.8,1.6,3.2 NE Growth depression,
kidney and liver weight
reduction
Rat 93 days Aldicarb 0,0.02,0.1,0.5 0.1 Mortality, growth
depression
Rat 2 years Aldicarb 0,0.005,0.025, >0.1 No effects seen
0.05,0.1
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Rat 7 days Aldicarb 0,0.4,0.8,1.6,3.2 0.8 Growth depression,
A. sulfoxide 0,0.4,0.8 0.4 RBC cholinesterase
A. sulfone 0,0.4,1.0,2.5,5,20 2.5 depression
Rat 29 days A. sulfoxide: 0,0.0074,0.03, 0.4 Growth depression,
A. sulfone 0.12,0.47,1.67 in brain, plasma and RBC
1:1 drinking water cholinesterase depression
Rat 6 months A. sulfoxide 0,0.125,0.25,0.5,1 0.125 Growth depression,
brain, plasma and RBC
cholinesterase depression
Rat 6 months A. sulfoxide 0,0.0625,0.125, 0.125 Plasma and RBC
0.25,0.5,1.0 cholinesterase depression
Rat 6 months A. sulfone 0,0.2,0.6,1.8, 0.6 Brain, plasma and RBC
5.4,15.2 cholinesterase depression
Rat 7 days A. oxime 0,31.25,62.5,125, 62.5 Growth depression,
250,500,1000 minor liver and kidney
changes
Dog 7 days Aldicarb 0,0.2,0.3,0.7 >0.7 No effects seen
Dog 100 days Aldicarb 0,0.2,0.3,0.7 0.3 Minor organ weight
changes
Dog 2 years Aldicarb 0,0.025,0.05 >0.1 No effects seen
0.1
Dog 2 weeks Aldicarb 0,0.022,0.068, NE Brain, plasma and RBC
cholinesterase
depression
0.192,0.609,1.42
Dog 2 weeks Aldicarb 0,0.003,0.008, 0.096 Brain, plasma and RBC
cholinesterase depression
0.027,0.096,0.28
Dog 5 weeks Aldicarb 0,0.012,0.024, >0.06 No effects seen
0.06
Dog 52 weeks Aldicarb 0,0.027,0.054, 0.054 RBC and plasma
0.131,0.241 cholinesterase depression
Table 6 (cont'd)
Species Duration Test Material Dosages* NOAEL** Effects at higher doses
Dog 3 months A. sulfoxide 0,0.0625,0.125, 0.25 Transient growth
0.25,0.5 depression
Dog 3 months A. sulfone 0,0.2,0.6, >5.4 No effects seen
1.8,5.4
Dog 1 year A. sulfone 0,0.11,0.59, 0.11 RBC and plasma
2.25 cholinesterase depression
* mg/kg bw/day equivalence, by dietary administration, unless otherwise stated.
Lowest effect level underlined.
** NOAEL's were tabulated without evaluation of methods of sample collection or method of
determination of cholinesterase activity.
NE Not established
REFERENCES
Abdallah, N.A. (1977) Alleged overexposure cases reported from use
of Temik formulations - 1966 to 1976. Unpublished report from Union
Carbide Corporation.
Abdallah, N.A. (1979) Alleged human overexposure cases reported from
use of Temik formulations - 1976 to 1979. Unpublished report from
Union Carbide Corporation.
Aldridge, D., Schardein, J.L., & Blair, M. (1983) Aldicarb
range-finding teratology study in rabbits. Unpublished report from
International Research and Development Corporation, submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Andrawes, N.R. (1977) The metabolism of (UC 21865) Sulfocarb
pesticide in the rat. Unpublished report from Union Carbide
Corporation.
Andrawes, N.R., Dorough, H.W., & Lindquist, D.A. (1967) degradation
and elimination of Temik in rats. J. Econ. Ent., 60(4): 979-987.
Andrawes, N.R. & Lee R.E. (1986) Temik brand aldicarb pesticide,
metabolism in lactating goats. Unpublished report from Union Carbide
Agricultural Products Company Inc.
Babish, J.G. & Salerno, A. (1977) Neurotoxicity evaluation of UC
21865 in white Leghorn hens (Gallus domesticus). Unpublished
report from Food and Drug Research Laboratories submitted to WHO by
Union Carbide Corporation.
Bull, D.L., Lindquist, D.A., & Coppedge, J.R. (1967) Metabolism of
2-methyl-2-(methylthio)propionaldehyde O-(methylcarbamoyl) oxime
(Temik, US-21149) in insects. J. Agric. Food Chem., 15(4):
610-616.
Burrows, I.E., Haynes, G., & Roberts, N. (1970) Determination of
exposure levels in operators to Temik during field trials.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Carpenter, C.P. (1963) Comparison of the acute toxicity of compound
21149 with several of its analogues. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Carpenter, C.P. (1969) Miscellaneous toxicity studies. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Carpenter, C.P. and Smyth, H.F. (1966) Temik l0G (10.5% Granular
formulation of compound 21149). 15-Day dermal applications to
rabbits. Unpublished Report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Chin, G.H. & Sullivan, L.J. (1968) Some biochemical considerations
of Temik pesticide. Unpublished report from Mellon Institute,
presented to the 156th ACS National Meeting, submitted to WHO by
Union Carbide Corporation.
Cid, M.G. & Matos, E. (1984) Induction of sister-chromatid exchanges
in cultured human lymphocytes by aldicarb, a carbamate pesticide.
Mutat. Res., 138: 175-179.
Coate, W.B., Hardy, R.J., Alsaker, R.D., Dawkins, B.G., Hepner,
K.E., Banos, D.A., & Mense, M.A. (1982) Subacute inhalation toxicity
study of a pesticide residue in dogs. T3, T6 and T10 type
cigarettes. Unpublished report from Hazleton Laboratories America
Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Debuyst, B. & Van Larebeke, N. (1983) Induction of sister-chromatid
exchanges in human lymphocytes by aldicarb, thiofanox and methomyl.
Mutat. Res., 113: 242-243.
Dorough, H.W., Davis, R.B., & Ivie, G.W. (1970) Fate of
Temik-Carbon-14 in lactating cows during a 14-day feeding period.
J. Agr. Food Chem., 18: 135-142
Dorough, H.W. & Ivie, G.W. (1968) Temik-S-35 metabolism in a
lactating cow. J. Agric. Food Chem., 16(3): 460-464
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E. McGregor, D.,
Morteimans, K., Rosenkranz, H.S., & Simmon, V.F. (1985)
Reproducibility of microbial mutagenicity assays: II. Testing of
carcinogens and noncarcinogens in Salmonella typhimurium and
Escherichia coli. Environ. Mutagen., 7 (suppl 5): 1-248.
Field, W.E. (1979a) Acute dermal toxicity in rabbits. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Field, W.E. (1979b) Acute dermal toxicity in rats. Unpublished
report from CDC Research, Inc. submitted to WHO by Union Carbide
Corporation.
Fiore, M.C., Anderson, H.A., Hong, R., Golubjatnikov, R., Seiser,
J.E., Nordstrom, D., Hanrahan, L., & Belluck, D. (1986) Chronic
exposure to aldicarb-contaminated groundwater and human immune
function. Environ. Res., 41: 633-645.
Godek, E.G., Dolak, M.D., Naismith, R.W., & Matthews, R.J. (1980a)
Ames Salmonella microsome plate test. Aldicarb sulfone.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980b)
Ames Salmonella microsome plate test. Aldicarb sulfoxide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Dolak, M.C., Naismith, R.W., & Matthews, R.J. (1980c)
Ames Salmonella microsome plate test. TEMIK aldicarb pesticide.
Unpublished report from Pharmakon Laboratories submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984a) Rat hepatocyte
primary culture/DNA repair test. Aldicarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Godek, E.G., Naismith, R.W., & Matthews, R.J. (1984b) Rat hepatocyte
primary culture/DNA repair test. Aldoxycarb technical. Unpublished
report from Pharmakon Research International Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Goes, E.H., Savage, E.P., & Gibbons, G. (1980) Suspected foodborne
carbamate pesticide intoxications associated with ingestion of
hydroponic cucumbers. Am. J. Epidemiol., 111: 254-259.
Goldman, L.R., Beller, M., & Jackson, R.J. (1990) Aldicarb food
poisonings in California, 1985-1988: Toxicity estimates for humans.
Arch. Environ. Hlth., 45(3) 141-147.
Guerzoni, M.E., Del Cupolo, L. and Ponti, I. (1976) Attivita
mutagenica degli antiparassitari. Riv. Sci. Teen. Alim. Nutr.,
Urn. 6: 161-165.
Haines, R.G. (1971) Ingestion of aldicarb by human volunteers: a
controlled study of the effect of aldicarb on man. Unpublished
report from Union Carbide Corporation.
Hamada, N.N. (1987a) One-year chronic oral toxicity study in beagle
dogs with aldicarb sulphone technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1987b) Two-week dose range finding oral toxicity study
in beagle dogs with aldicarb technical. Unpublished report from
Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Hamada, N.N. (1988) One-year chronic oral toxicity study in beagle
dogs with aldicarb technical. Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N. (1991) Subchronic toxicity study in beagle dogs with
aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Marshall,
P.M. (1985a) Two-week dose range finding study in beagle dogs -
Aldicarb sulphone (aldoxycarb). Unpublished report from Hazleton
Laboratories America Inc., submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Hamada, N.N., Hagan, W.H., Vargas, K.V., Alsaker, R.D., & Williams,
L.D. (1985b) Two-week dose range finding study in beagle dogs -
Aldicarb technical. Unpublished report from Hazleton Laboratories
America Inc., submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Hicks B.W., Dorough, H.W., & Mehendale, H.M. (1972) Metabolism of
aldicarb pesticide in laying hens. J. Agric. Food Chem., 20(1):
151-156.
Hirsch, G.H., Mori, B.T., Morgan, G.B., Bennett, P.R., & Williams,
B.C. (1987) Report of illness caused by aldicarb-contaminated
cucumbers. Food Addit. Contam., 5: 155-160.
Ivett, J.L. (1990) Mutagenicity test on aldicarb technical in the
mouse bone marrow cytogenetic assay. Unpublished report from
Hazleton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Ivett, J.L., Myhr, B.C., & Lebowitz, H.D. (1984) Mutagenicity
evaluation of aldicarb technical 93.47% in the bone marrow
cytogenetic assay. Unpublished report from Litton Bionetics, Inc.
submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Johnson, H.E. & Carpenter, C.P (1966a) Temik (Technical grade
compound 21149). Demyelination potential in chickens. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Carpenter, C.P (1966b) Temik (technical grade
compound 21149). Comparative behavioural effect in rats. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Johnson, H.E. & Sullivan, L.J. (1968a) Studies on an effective
therapy for overdoses of temik, Temik sulfoxide and temik sulfone in
the rat. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
Johnson, H.E. and Sullivan, L.J. (1968b) Temik (uc 21149) antidotal
therapy in rats following administration of multiple lethal doses.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Knaak, J.B., Tallant, M.J., & Sullivan, L.J. (1966) The metabolism
of 2-methyl-2-(methylthio)propionaldehyde o-(methylcarbamoyl) oxime
in the rat. J. Agric. Food Chem., 14(6): 573-578
Lee, M.H. & Ransdell, J.F. (1984) A farmworker death due to
pesticide toxicity: a case report. J. Toxicol. Environ. Health,
14: 239-246.
Leng, J.M., Schardein, J.L., & Blair, M. (1983) Aldicarb. Teratology
study in rabbits. Unpublished report from International Research and
Development Corporation submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Metcalf, R.L., Fukuto, T.R., Collins, C., Borck, K., Burk, J.,
Reynolds, H.T., & Osman, M.F. (1966) Metabolism of
2-methyl-2-(methylthio)-propionaldehyde O-(methylcarbamoyl) oxime in
Plant and Insect. J. Agric. Food Chem., 14(6): 579-584.
Mirkin, I.R., Anderson, H.A., Hanrahan, L., Hong, R., Golubjatnikov,
R., & Belluck, D. (1990) Changes in tlymphocyte distribution
associated with ingestion of aldicarb-contaminated drinking water: a
follow-up study. Environ. Res., 51: 35-50.
Mirro, E.J., DePass, L.R., & Frank, F.R. (1982) Twenty-nine day
water inclusion study in rats. Unpublished report from Bushy Run
Research Laboratory submitted to WHO by Union Carbide Corporation.
Natoff, I.L. & Reiff, B. (1973) Effect of oximes on the acute
toxicity of anticholinesterase carbamates. Toxicol. Appl.
Pharmacol., 25: 569-575.
NIH (1979) Bioassay of aldicarb for possible carcinogenicity. US
DHEW Pub.No. (NIH) 79-1391, 103 pages.
Nimmo, W.S., Wyld, P.J., Watson, C.E., & Watson, N (1992) A safety
and tolerability study of aldicarb at various dose levels in healthy
male and female volunteers. Unpublished report from Inveresk
Clinical Research, submitted to WHO by Rhône-Poulenc AGRO, Lyon,
France.
Nycum, J.S. & Carpenter, C.P. (1968a) Toxicity studies on the
probably "non-carbamate" plant metabolites of Temik. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Nycum, J.S. & Carpenter, C.P. (1968b) Toxicity studies on Temik and
related carbamates. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Olson, L.J., Erickson, B.J., Hinsdill, R.D., Wyman, J.A., Porter,
W.P., Binning, L.K., Bidgood, R.C., & Nordheim, E.V. (1987) Aldicarb
immunomodulation in mice: an inverse dose-response to parts per
billion levels in drinking water. Arch. environ. Contam. Toxicol.,
16: 433-439.
Pandey, S.Y. (1977) Human exposure study on Temik 10G. Unpublished
report from Union Carbide India Ltd., submitted to WHO by Union
Carbide Corporation.
Peoples, S.A., Maddy, K.T., & Smith, C.R. (1977) Human health
problems associated with Temik (Aldicarb) in California. Unpublished
report from California Department of Food and Agriculture submitted
to WHO by Union Carbide Corporation.
Pozzani, U.C. & Carpenter, C.P. (1968a) Sensitizing potential in
guinea-pigs as determined by a modified Landsteiner test.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Rashid, K.A. & Mumma, R.O. (1986) Screening pesticides for their
ability to damage bacterial DNA. J. Environ. Sci. Health, B21:
319-334.
Romine, R.R. (1973) Aldicarb pesticide. Aldicarb residues in milk
and meat of cows fed aldicarb sulfoxide and aldicarb sulfone in the
daily diet. Unpublished report from Union Carbide Corporation.
SanSebastian, J.R., Naismith, R.W., & Matthews, R.J. (1984) In
vitro chromosome aberration analysis in Chinese hamster ovary
cells (CHO). Aldoxycarb technical. Unpublished report from Pharmakon
Research International Inc. Submitted to WHO by Rhône-Poulenc AGRO,
Lyon, France.
Shrivastava, K.N. (1975) Determination of human exposure levels in
commercial application of ternik 10g on potatoes by gloved hand and
hand-operated applicators. Unpublished report from Union Carbide
India Ltd.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985a) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldicarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Stankowski, L.F., Naismith, R.W., & Matthews, R.J. (1985b) CHO/HGPRT
mammalian cell forward gene mutation assay. Aldoxycarb. Unpublished
report from Pharmakon Research International, Inc. submitted to WHO
by Rhône-Poulenc AGRO, Lyon, France.
Sterman, A.B. & Varma, A. (1983) Evaluating human neurotoxicity of
the pesticide aldicarb: when man becomes the experimental animal.
Neurobehav. Toxicol. Teratol., 5: 493-495.
Striegel, J.A. & Carpenter, C.P. (1962) Range finding test on
compound 21149. Unpublished report from Mellon Institute of
Industrial Research submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. (1968a) The urinary excretion of C-14 equivalents of
Temik in the dog and their metabolic profile as revealed by silica
gel chromatography. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Sullivan, L.J (1968b) Urinary metabolic profiles as determined by
silica gel chromatography of urines from rats and dogs dosed with
Temik sulfone. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Sullivan, L.J (1968c) The excretion of C14 equivalents of Temik
sulfone by the rat after a single peroral dose. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Sullivan, L.J. & Carpenter, C.P. (1974) Preliminary Metabolism of
14C-S-methyl aldicarb nitrile in the rat. Unpublished report from
Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Thomas, P.T., Ratajczak, H.V., Eisenberg, W.C., Furedz-Machacek, M.,
Ketels, K.V., & Barbera, P.W. (1987) Evaluation of host resistance
and immunity in mice exposed to the carbamate pesticide aldicarb.
Fund. Appl. Toxicol., 9: 82-89.
Thomas, P.T., Ratajczak, H.V., Demetral, D., Hagen, K., & Baron, R.
(1990) Aldicarb immunotoxicity: Functional analysis of cell-mediated
immunity and quantitation of lymphocyte sub-populations. Fund.
Appl. Toxicol., 15: 221-230.
Trutter, J.A. (1987a) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residues in bananas. Unpublished report
from Hazleton Laboratories America Inc., submitted to WHO by Rhône-
Poulenc AGRO, Lyon, France.
Trutter, J.A. (1987b) Acute oral toxicity in cynomolgus monkeys:
aldicarb sulfoxide/sulfone residue in watermelons. Unpublished
report from Hazleton Laboratories America Inc., submitted to WHO by
Rhône-Poulenc AGRO, Lyon, France.
Tyl, R.W. & Neeper-Bradley, T.L. (1988) Developmental toxicity
evaluation of aldicarb technical administered by gavage to CDO
(Sprague-Dawley) rats. Unpublished report from Bushy Run Research
Centre submitted to WHO by Rhône-Poulenc AGRO, Lyon, France.
Union Carbide (1979) Volunteer worker's exposure monitoring study
(Panama). Unpublished Report from Union Carbide Corporation.
Wakefield, M., Orme, J.P.R., Ruston, E.A., & Andrews, L. (1973) The
detemination of levels of aldicarb in urine and face masks.
Unpublished report from Huntingdon Research Centre submitted to WHO
by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1963) Results of three months of
inclusion of Compound 21149 in the diet of rats. Unpublished Report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1964) Results of a three-generation
reproduction study on rats fed compound 21149 in their diet.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1965) Two-year feeding of compound
21149 in the diet of rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966a) Insecticide Temik. Teratogenic
potential in rats. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966b) Results of long-term tests for
mouse skin carcinogenicity of three process residues, one epoxide
and three compounds. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1966c) Two-year feeding of compound
21149 in the diet of dogs. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968a) Temik 10G-V (10.3% granular
formulation of compound 21149). Acute and 14-day dermal applications
to rabbits. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968b) Temik sulfoxide. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1968c) Temik sulfone. Results of
feeding in the diet of rats for six months and dogs for three
months. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1969a) Purified and technical temik.
Results of feeding in the diets of rats for one week. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1969b) 2-methyl-2-(methylsulfinyl)
propanol-1. Results of feeding in the diets of rats for one week.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970a) Temik and other materials.
Miscellaneous single dose peroral and parenteral LD50 assays and
some joint action studies. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970b) Temik, results of feeding in
the diets of rats for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970c) Temik (T) Temik sulfoxide
(TSO), Temik sulfone (ts02), 1:1 TSO-TS02. Results of feeding in the
diet of rats for 7 days. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1970d) 1:1 Temik:Temik sulfone.
results of feeding in the diet of mice for 7 days. Unpublished
report from Mellon Institute submitted to WHO by Union Carbide
Corporation.
Weil, C.S. & Carpenter, C.P. (1970e) Temik. Results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972a) Aldicarb (A), Aldicarb
sulfoxide (ASO), Aldicarb sulfone (AS02) and a 1:1 mixture of
ASO:AS02. Two-year feeding in the diet of rats. Unpublished report
from Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972b) Miscellaneous toxicity studies.
Unpublished report from Mellon Institute submitted to WHO by Union
Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1972c) Aldicarb, 18-month feeding in
diet of mice. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1973) Aldicarb, seven-day inclusion in
diet of dogs. Unpublished report from Mellon Institute submitted to
WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974a) Aldicarb. Inclusion in the diet
of rats for three generations and a dominant lethal mutagenesis
test. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974b) Aldicarb Oxime (All). Results
of feeding in the diet of rats for 7 days. Unpublished report from
Mellon Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974c) Aldicarb. Inclusion in the
diets of dogs for three months. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974d) Aldicarb, 18-month feeding in
the diet of mice, study II. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Carpenter, C.P. (1974e) UC 21865; results of feeding in
the diet of mice for 7 days. Unpublished report from Mellon
Institute submitted to WHO by Union Carbide Corporation.
Weil, C.S. & Cox, E.F. (1975) Aldicarb sulfoxide and aldicarb
sulfone cholinesterase inhibition results after periods of one to
fifty-six days of inclusion in the diets of rats. Unpublished report
from Carnegie-Mellon Institute of Research submitted to WHO by Union
Carbide Corporation.
Weil, C.S., Conroy, W.J., Condra, N.I., & Cox, E.F. (1977) Aldicarb
sulphone UC 21865. 19-day rabbit skin inunction. Unpublished report
from Mellon institute submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1965) The single dose peroral toxicity
of compounds 20299, 21149, 19786 and 20047a for white Leghorn
cockerels. Unpublished report from Mellon Institute submitted to WHO
by Union Carbide Corporation.
West, J.S. & Carpenter, C.P. (1966a) Temik (Compound 21149,
Technical). Joint action with selected organic phosphate and
carbamate pesticides. Unpublished report from Mellon Institute
submitted to WHO by Union Carbide Corporation.
West, J.S. & Carpenter, C.P.(1966b) Miscellaneous acute toxicity
data. Unpublished report from Mellon Institute submitted to WHO by
Union Carbide Corporation.
Whitlock, N.H., Schuman, S.H., & Loadholt, C.B. (1982) Executive
summary and epidemiologic survey of potential acute health effects
of aldicarb in drinking water - Suffolk County, N.Y. South Carolina
Pesticide Hazard Assessment Program Center, Medical University of
South Carolina, Charleston. Prepared for the Health Effects Branch,
Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency.
WHO (1966) Insecticide Evaluation and Testing Programme, Stage I,
Mammalian Toxicity Report (WHO). Unpublished report from Toxicology
Research Univ., Carshalton, U.K. submitted to WHO by Union Carbide
Corporation.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, F. (1966) Human exposure study in the field application of
Temik 10G on cotton. Unpublished report from Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977a) Inclusion in the
diet of rats for three generations (aldicarb sulphone), dominant
lethal mutagenesis and teratology studies. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
Woodside, M.D., Weil, C.S., & Cox, E.F. (1977b) Aldicarb sulphone;
18-month feeding in the diet of mice. Unpublished report from
Carnegie Mellon institute submitted to WHO by Union Carbide
Corporation.
